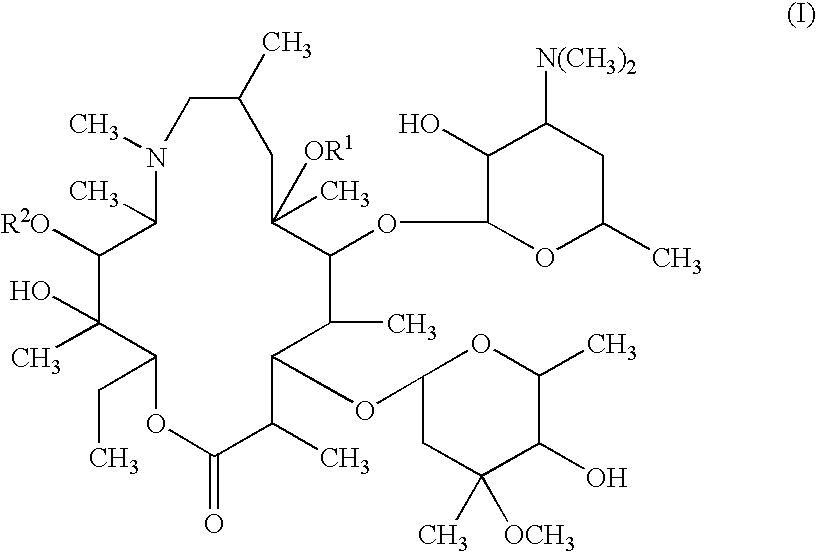
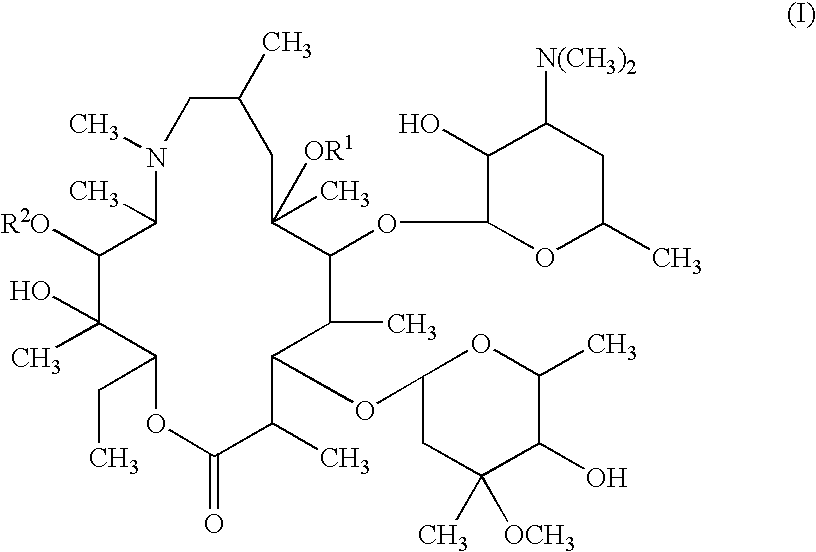
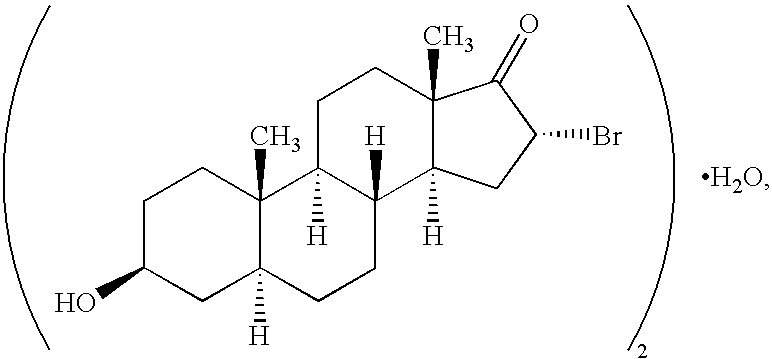
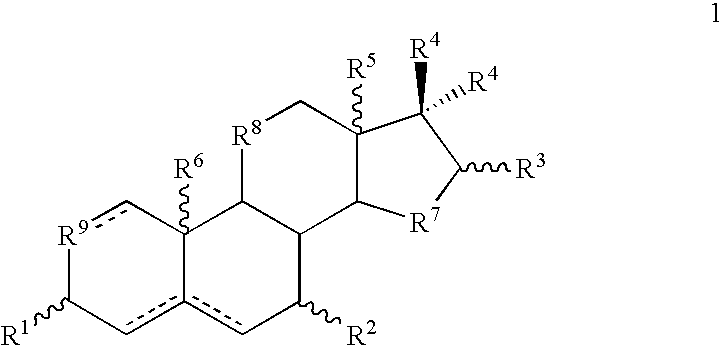
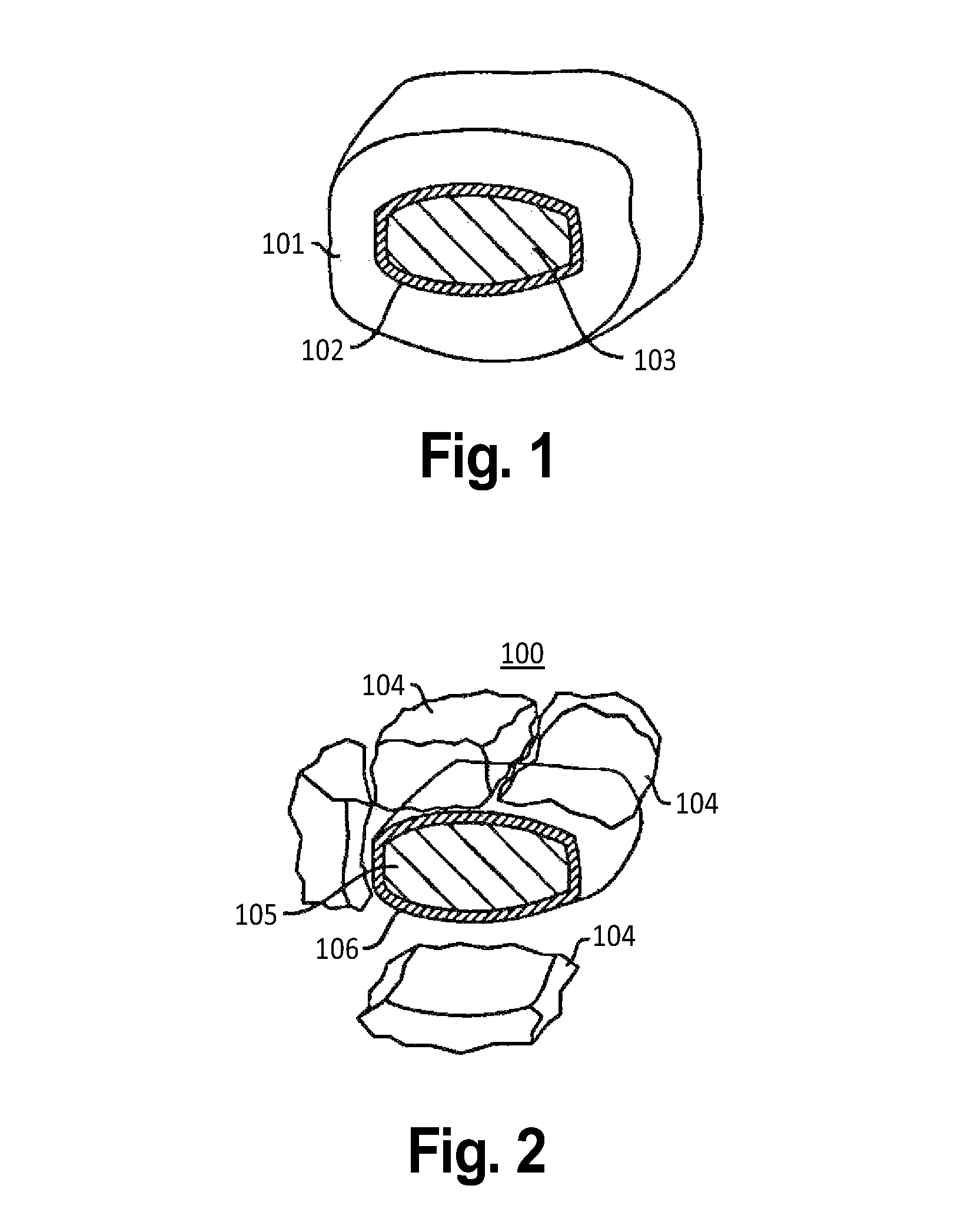
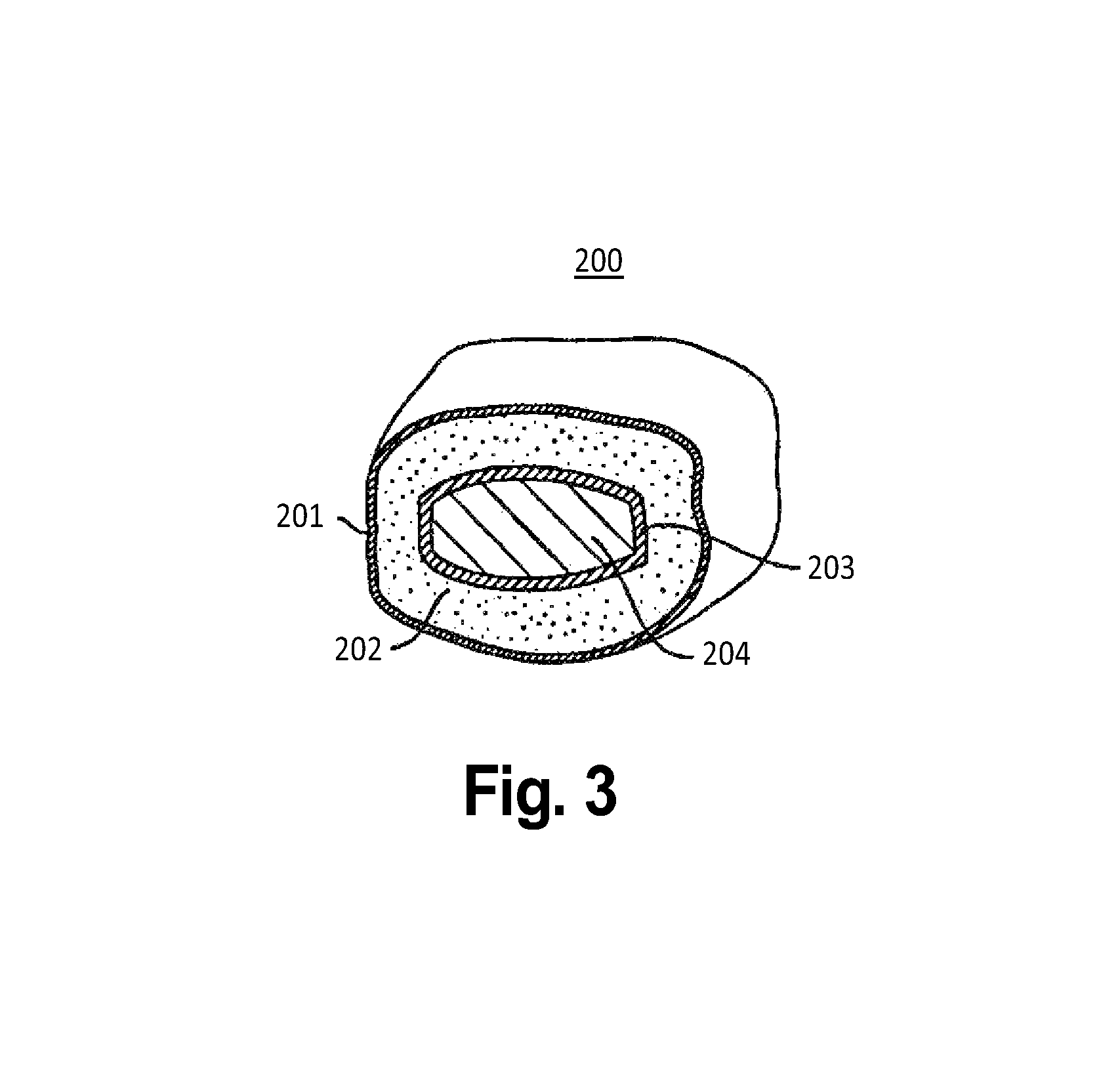
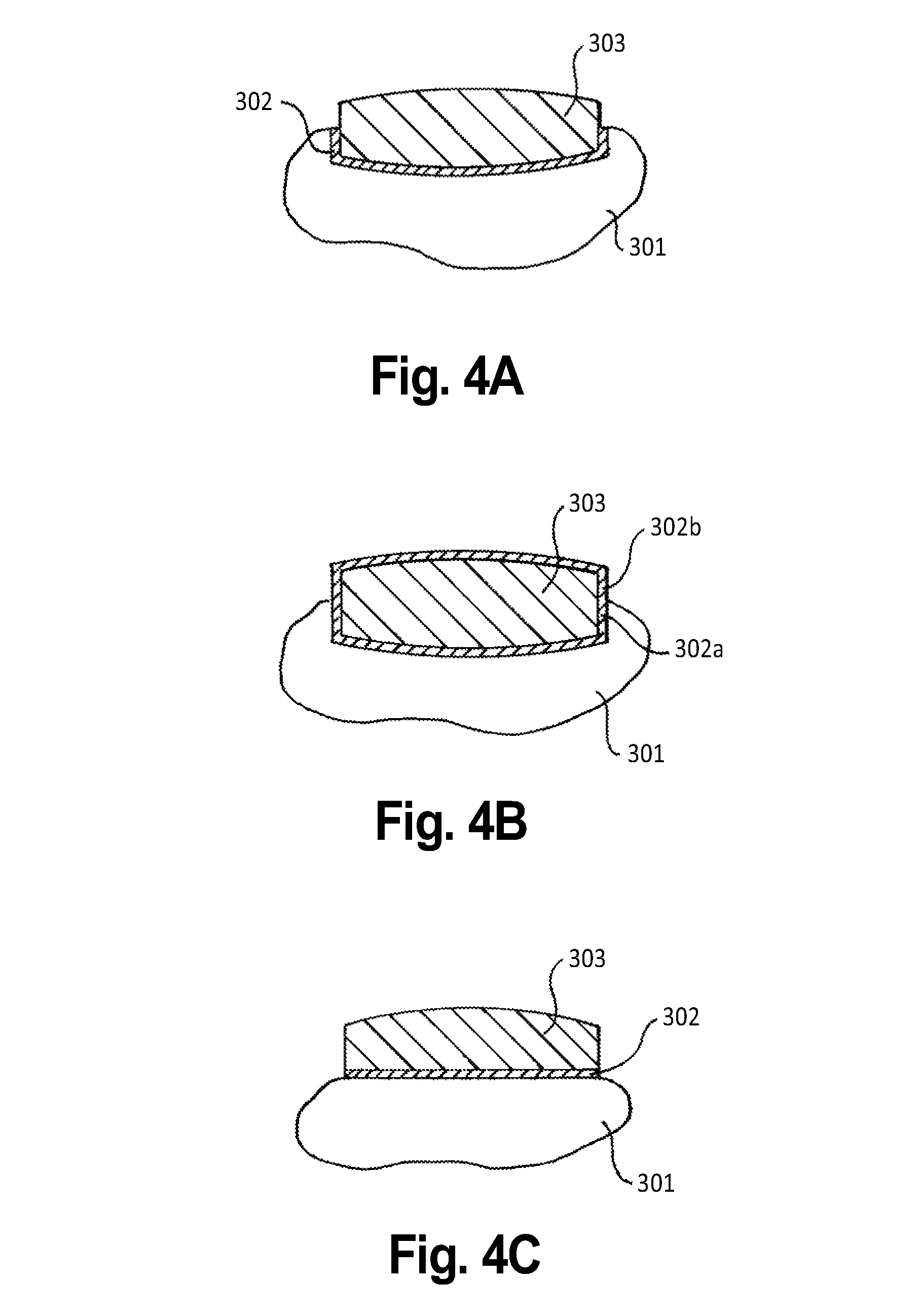
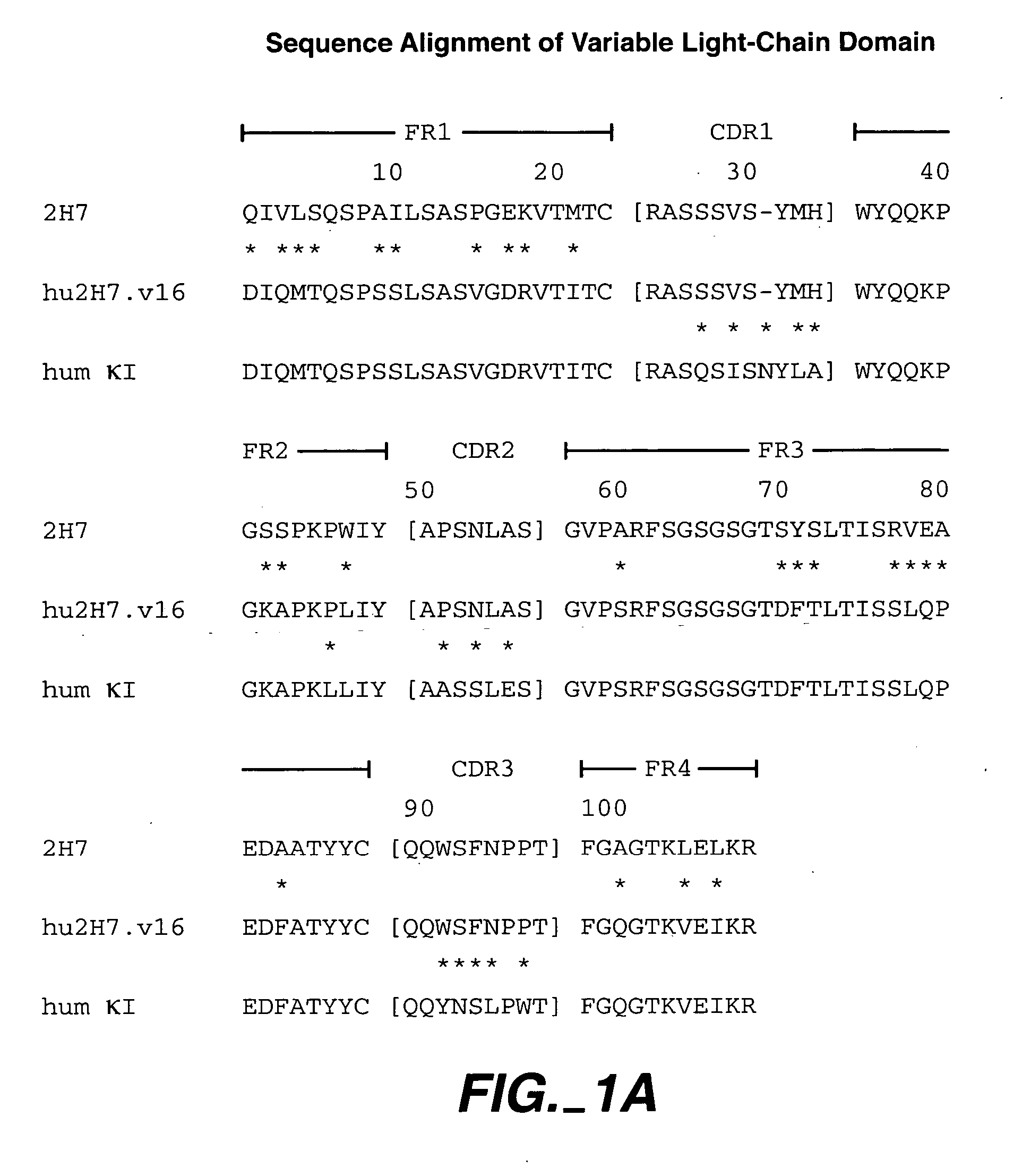
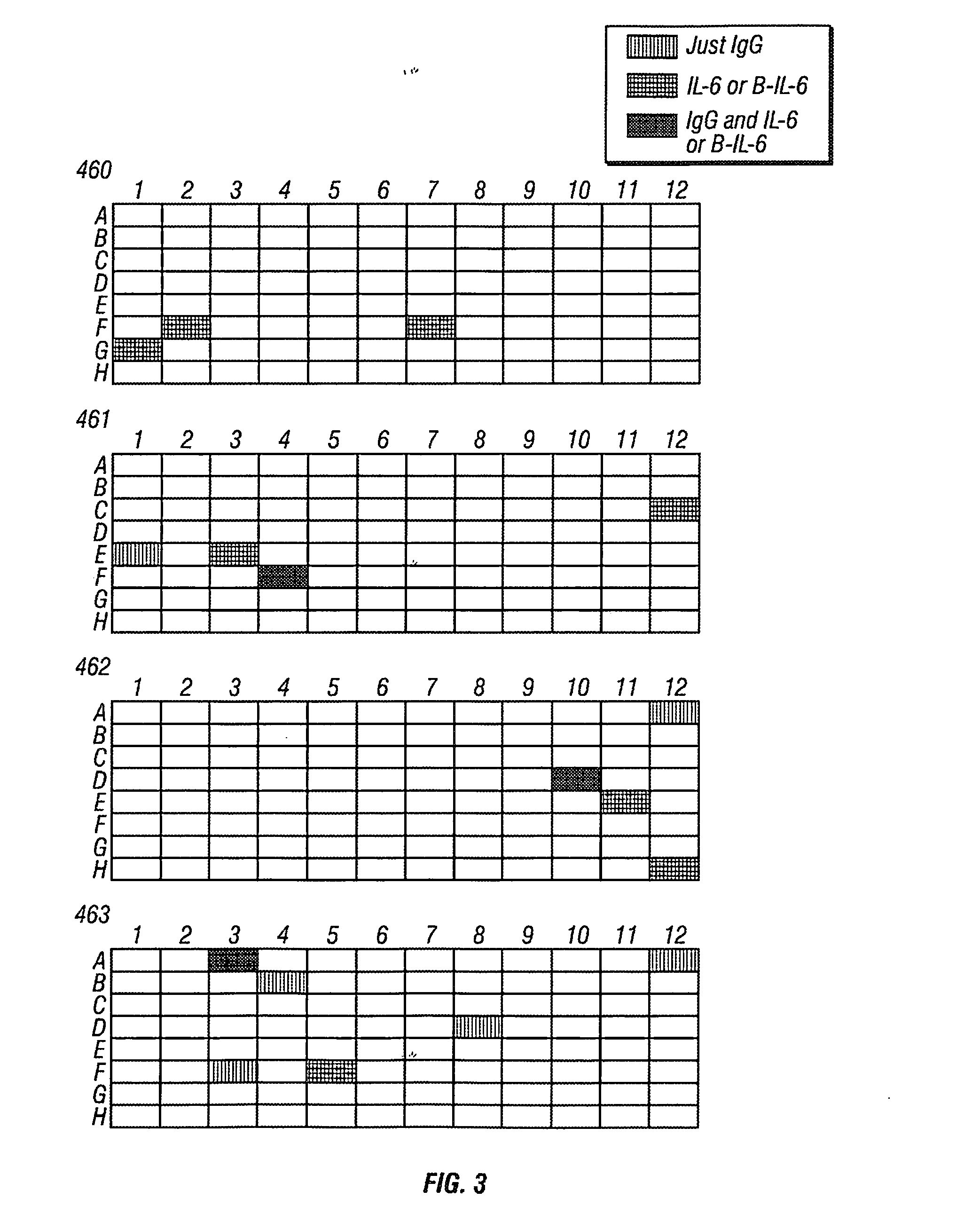
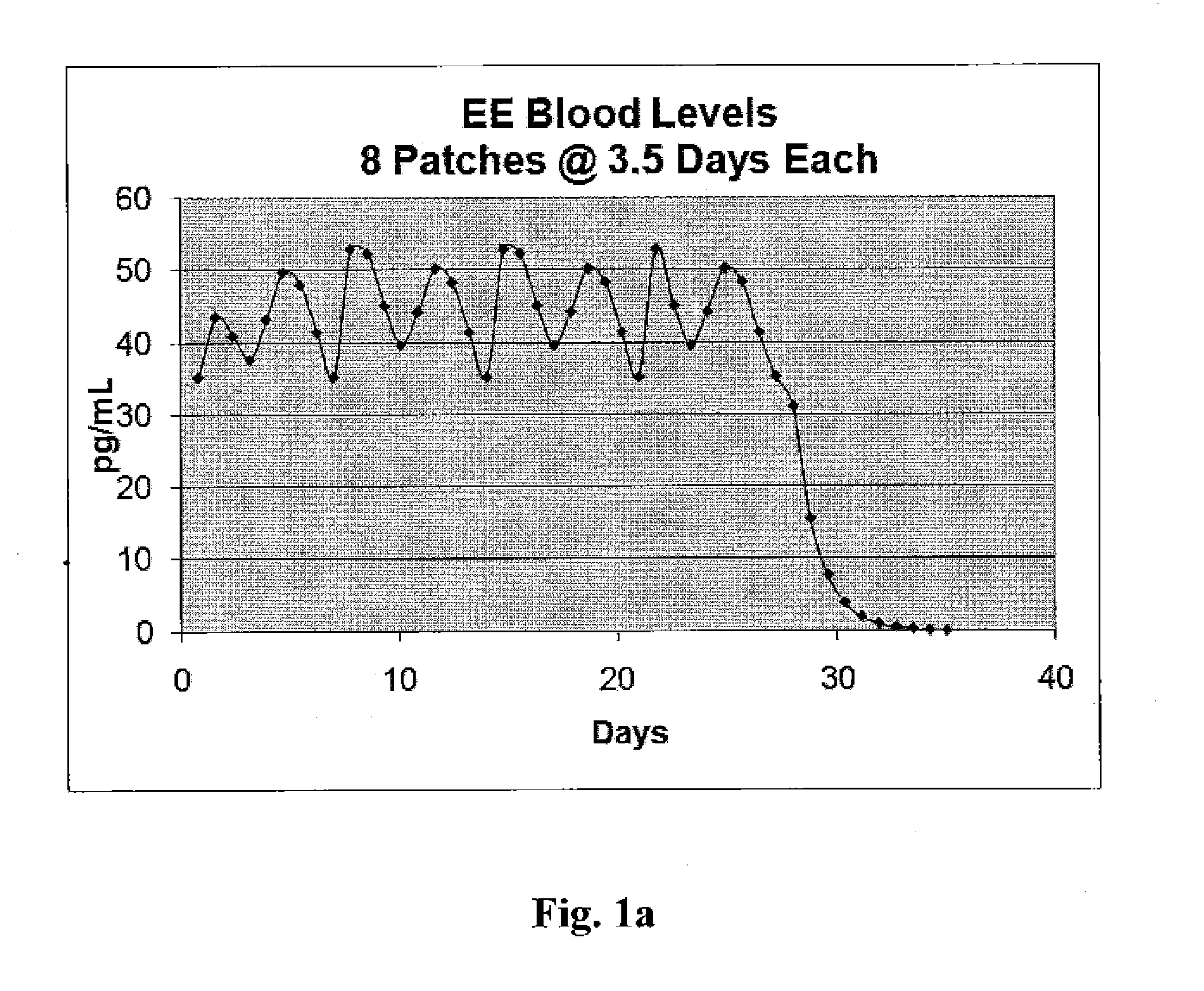
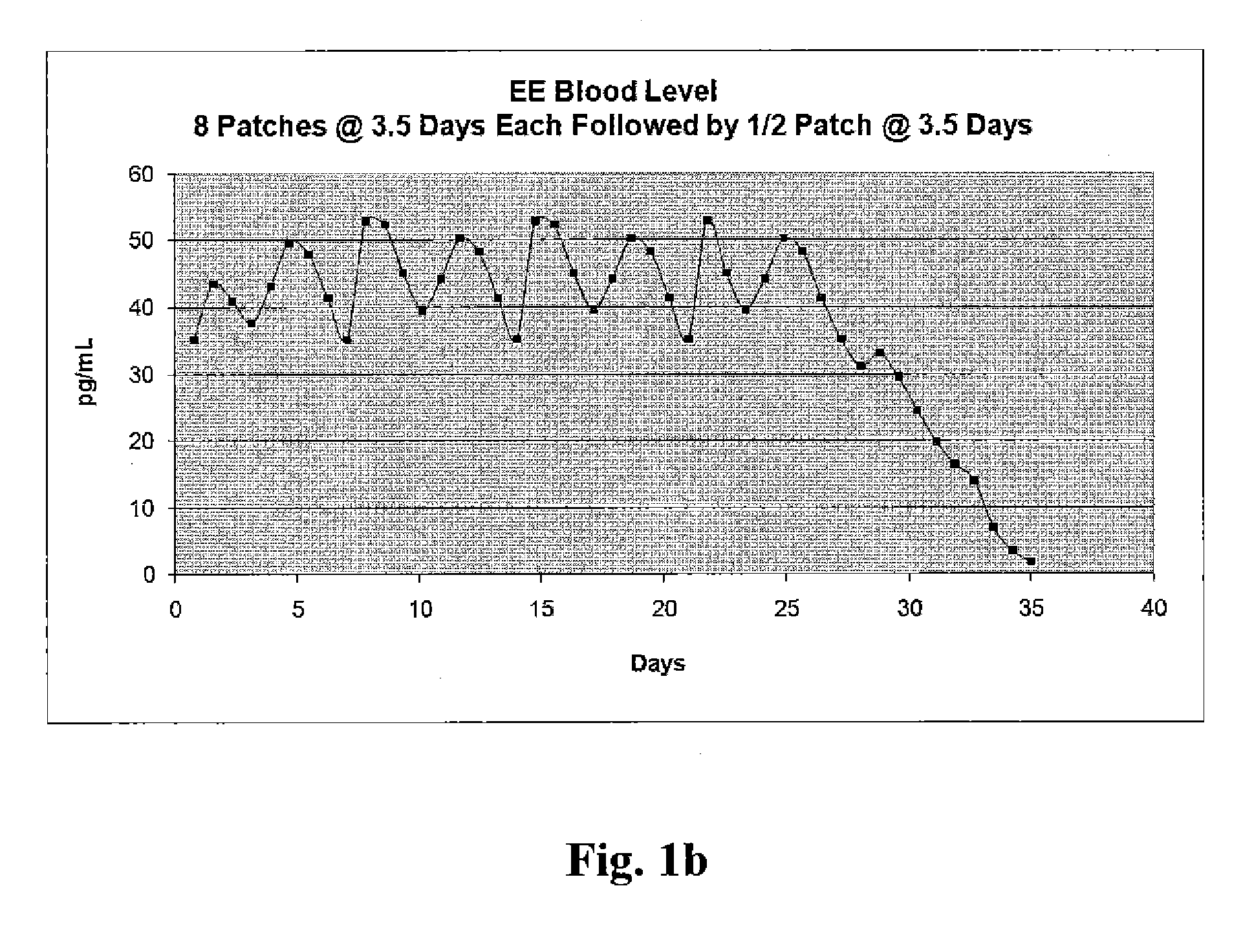
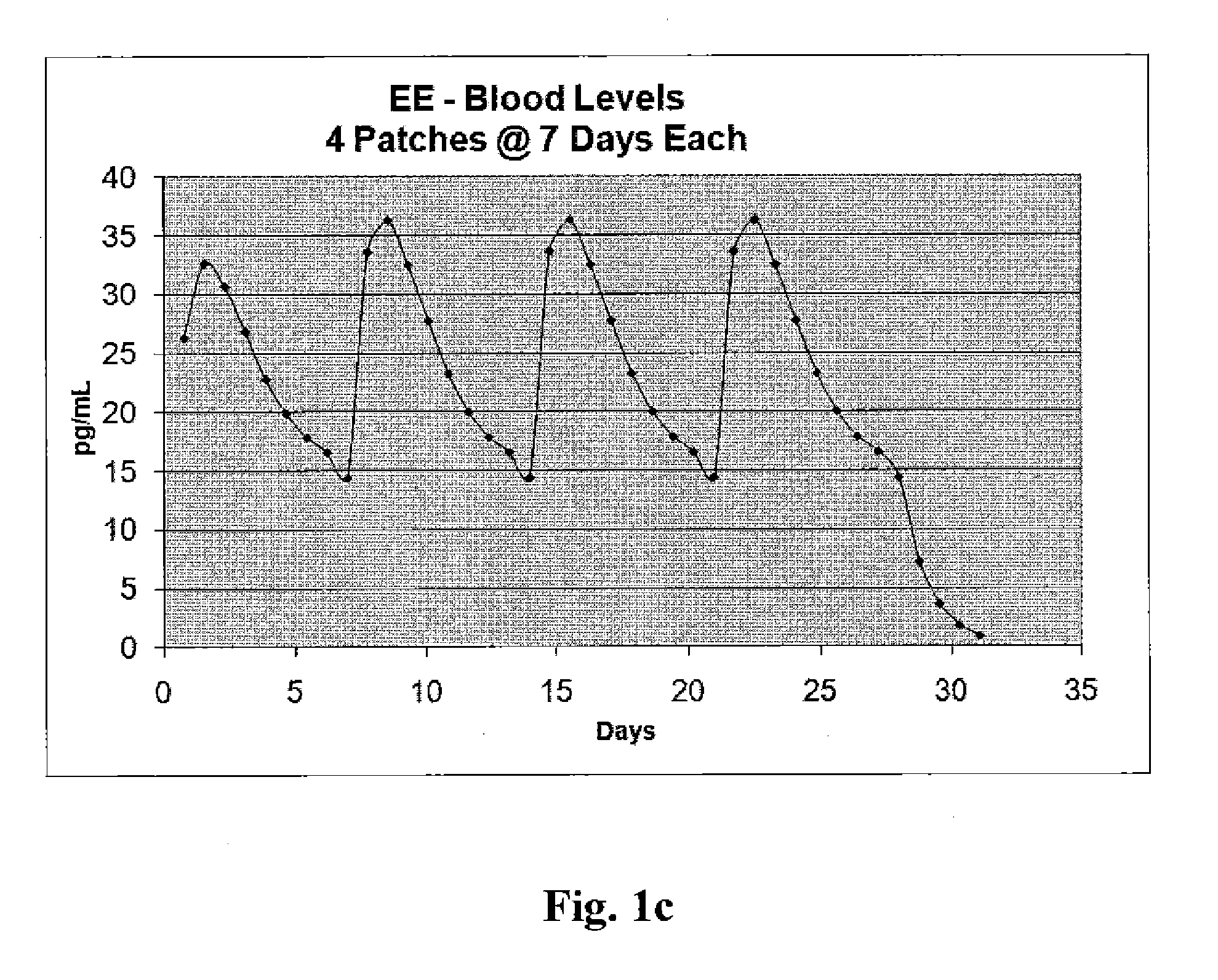
Patents
Literature
671 results about "Dosing regimen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
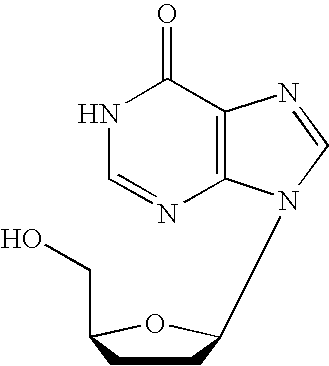
Dosage regimen. The schedule of doses of a therapeutic agent per unit of time, including: the time between doses (e.g., every 6 hours) or the time when the dose(s) are to be given (e.g., at 8 a.m. and 4 p.m. daily), and the amount of a medicine (e.g., number of capsules) to be given at each specific time.
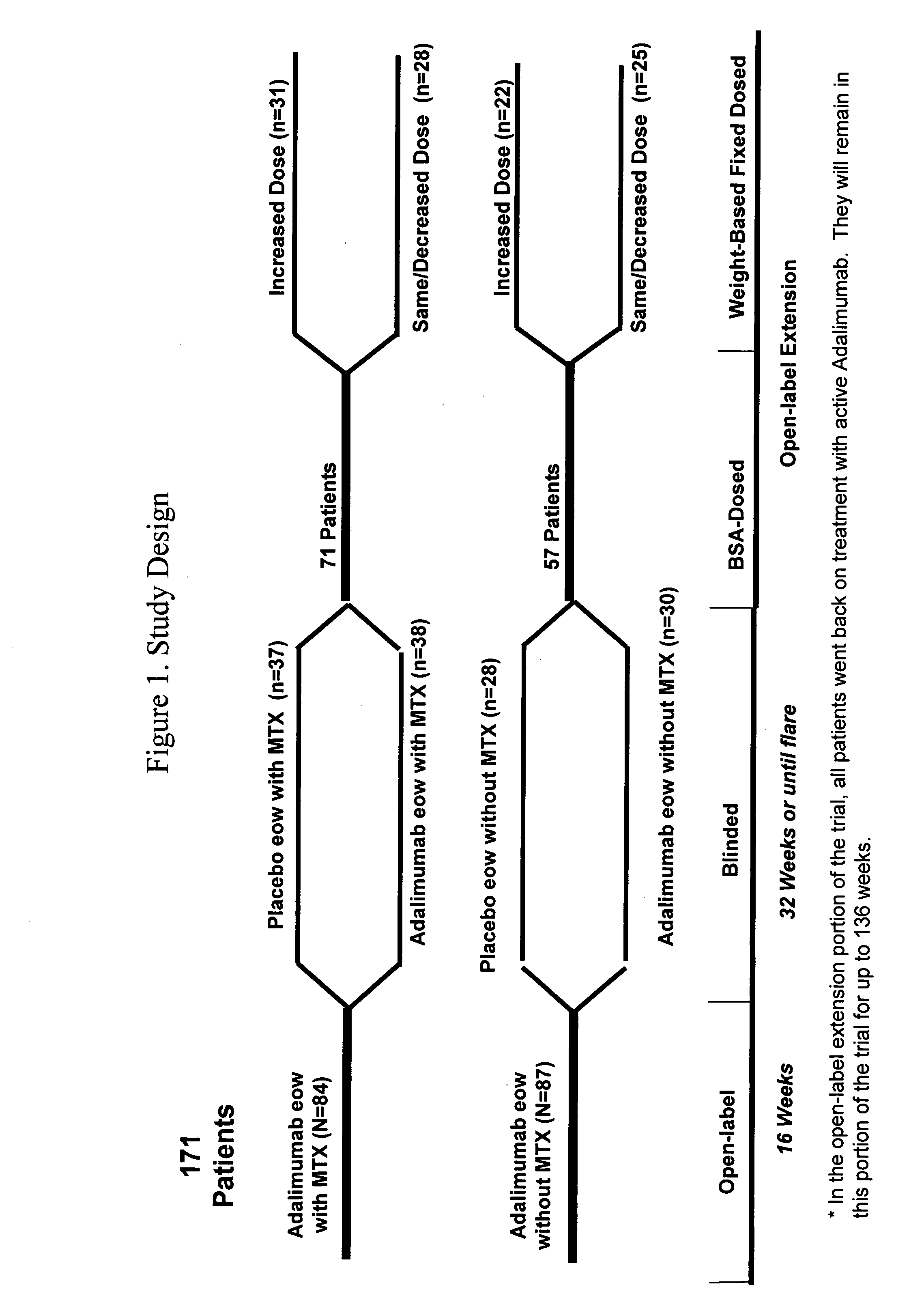
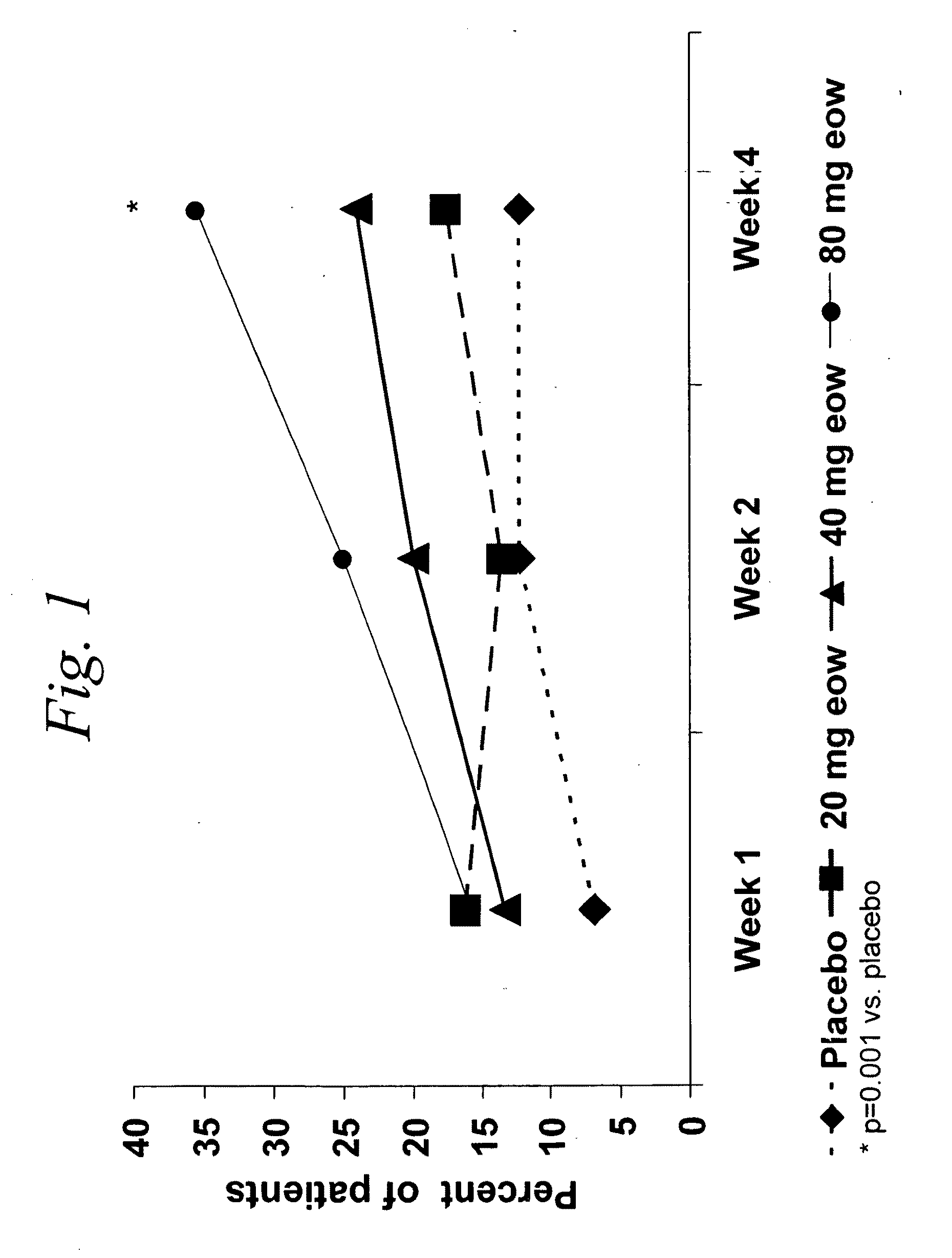
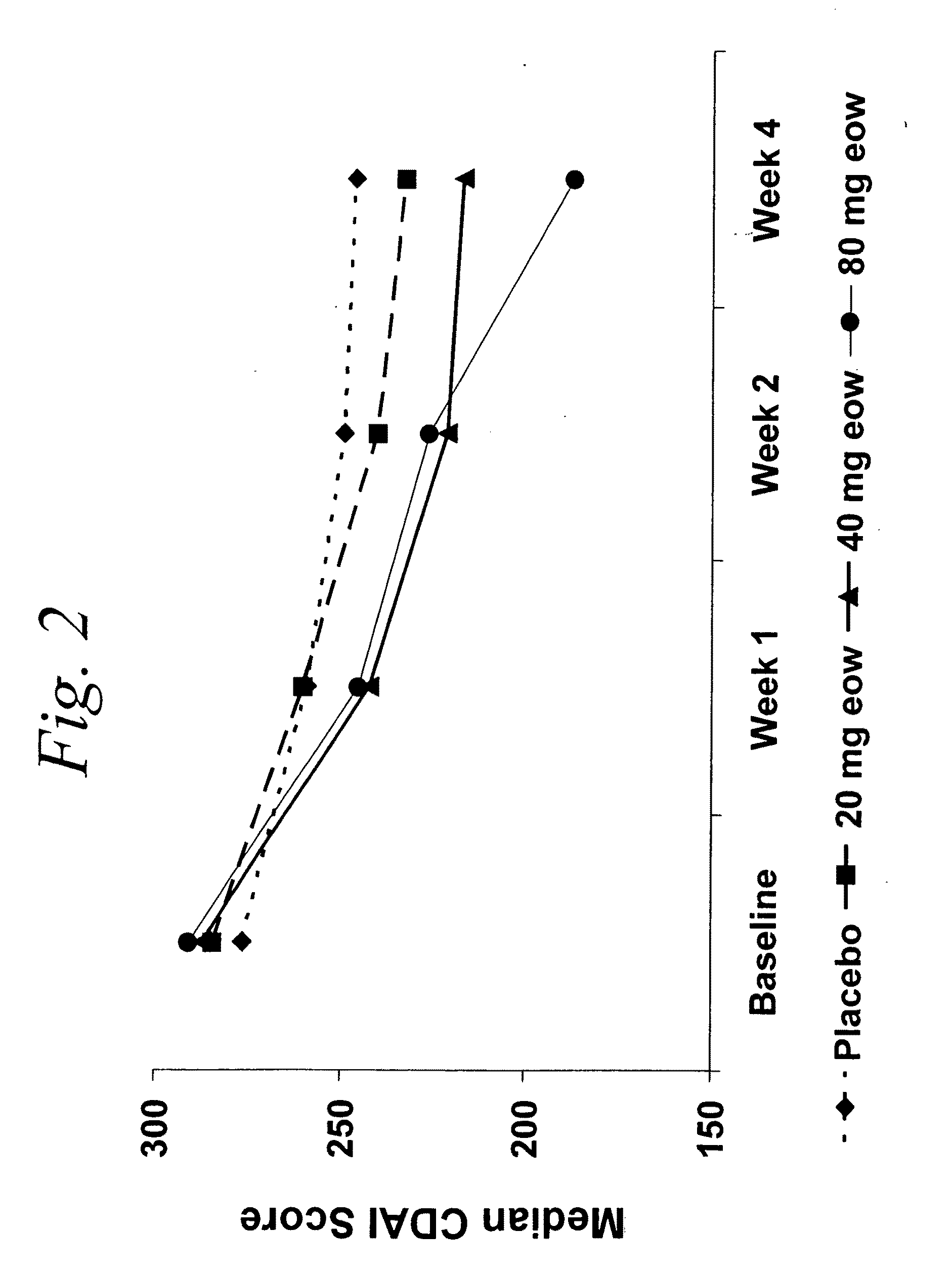
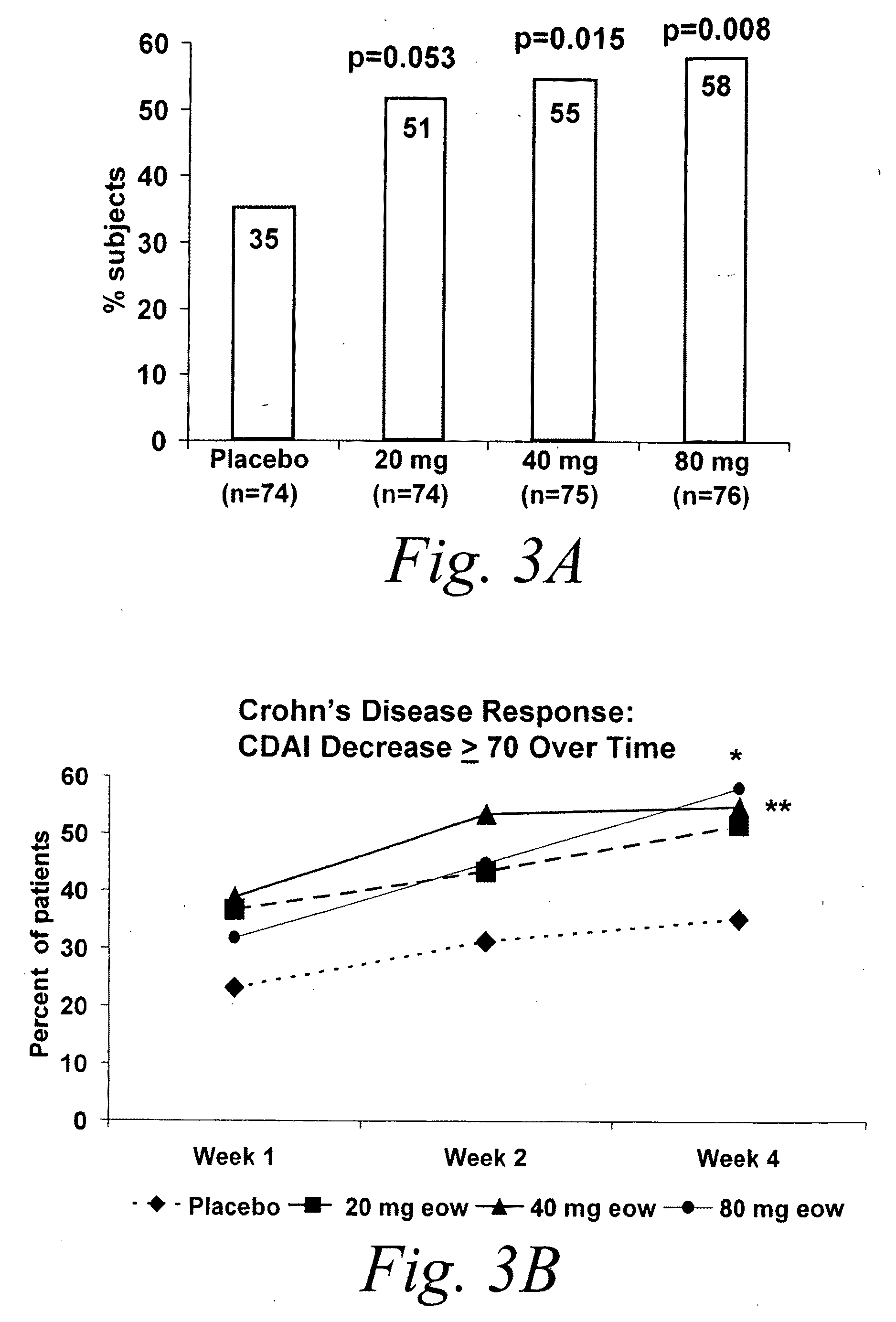
Multiple-variable dose regimen for treating TNFalpha-related disorders
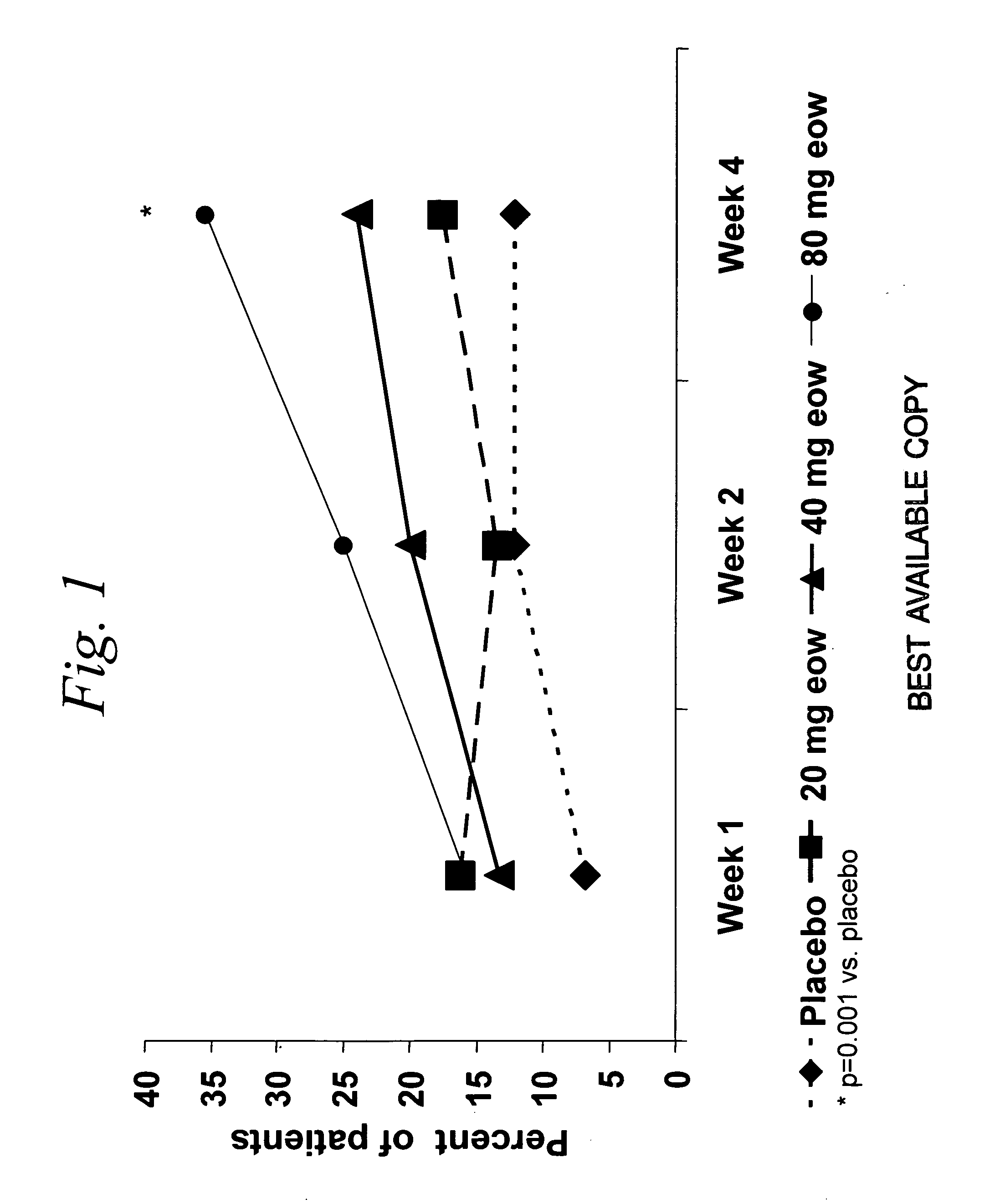
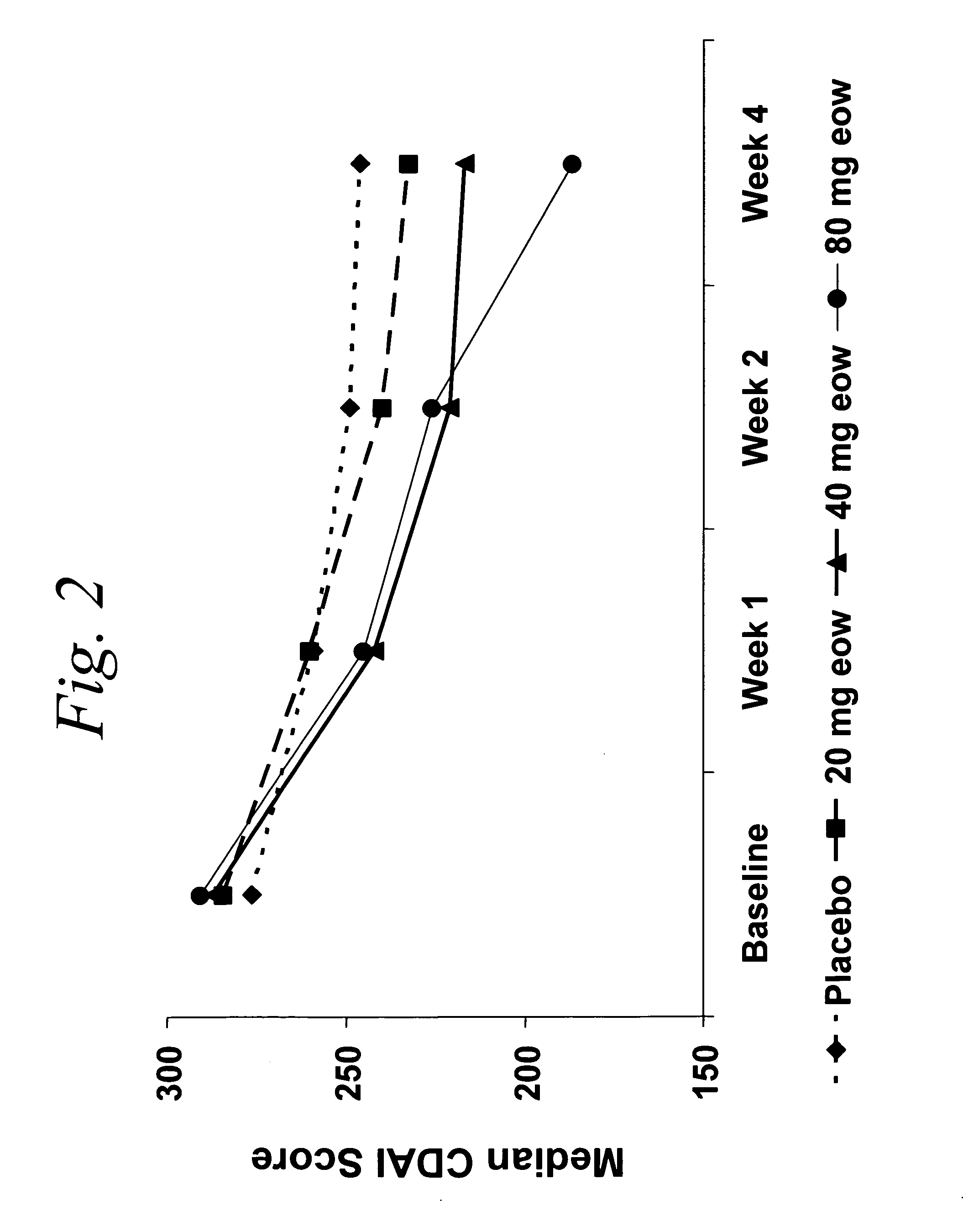
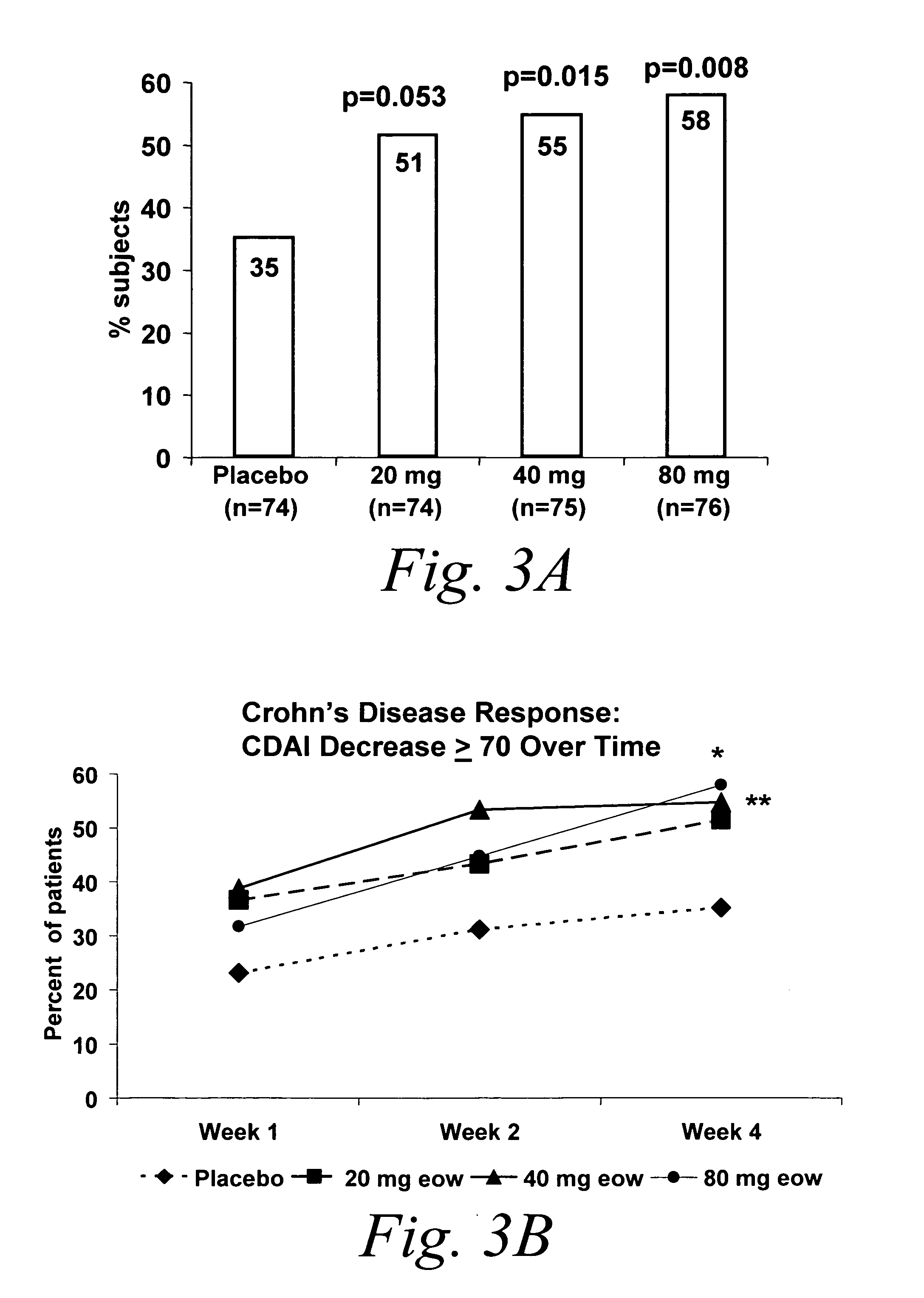
ActiveUS20060009385A1Reduce decreaseIncrease the areaBiocideOrganic active ingredientsDosing regimenRegimen
Multiple-variable dose methods for treating TNFα-related disorders, including Crohn's disease and psoriasis, comprising administering TNFα inhibitors, including TNFα antibodies, are described. Multiple-variable dose methods include administration of a TNF-inhibitor in an induction or loading phase followed by administration of the agent in a maintenance or treatment phase, wherein the TNF-inhibitor is administered in a higher dosage during the induction phase.
Owner:ABBVIE BIOTECHNOLOGY LTD
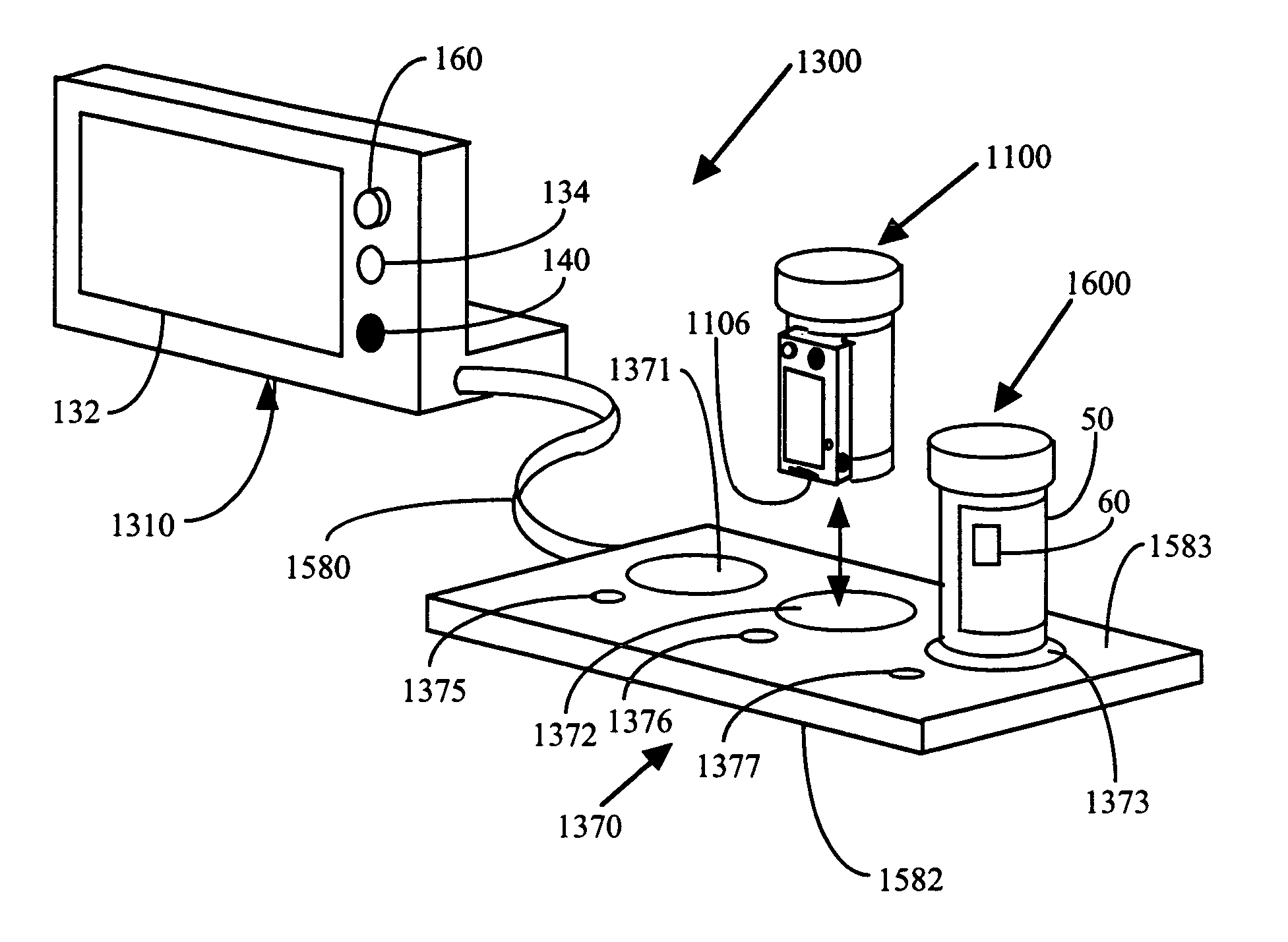
Method and system for monitoring and analyzing compliance with internal dosing regimen
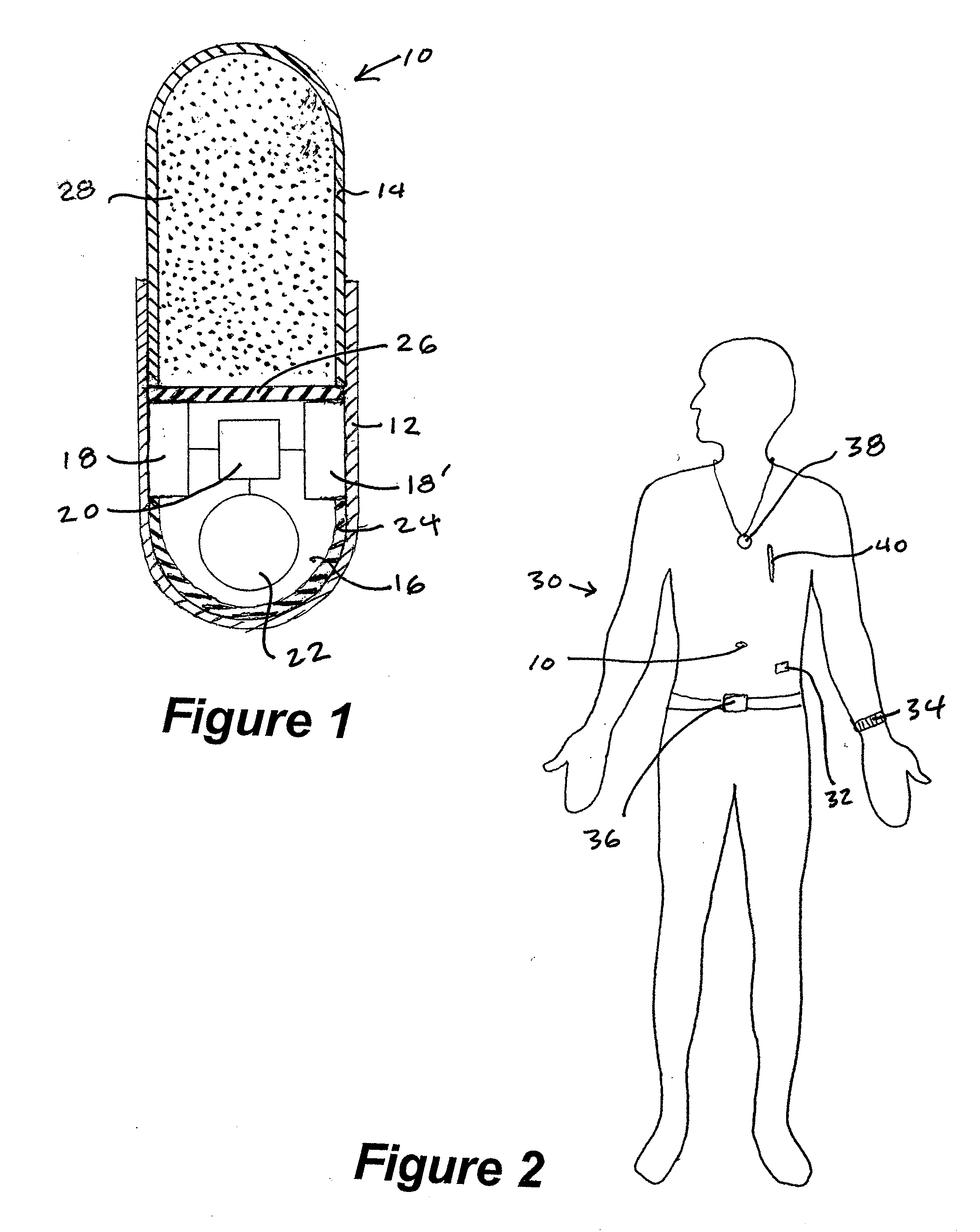
InactiveUS20070237719A1Ultrasonic/sonic/infrasonic diagnosticsCompounds screening/testingDosing regimenMedicine
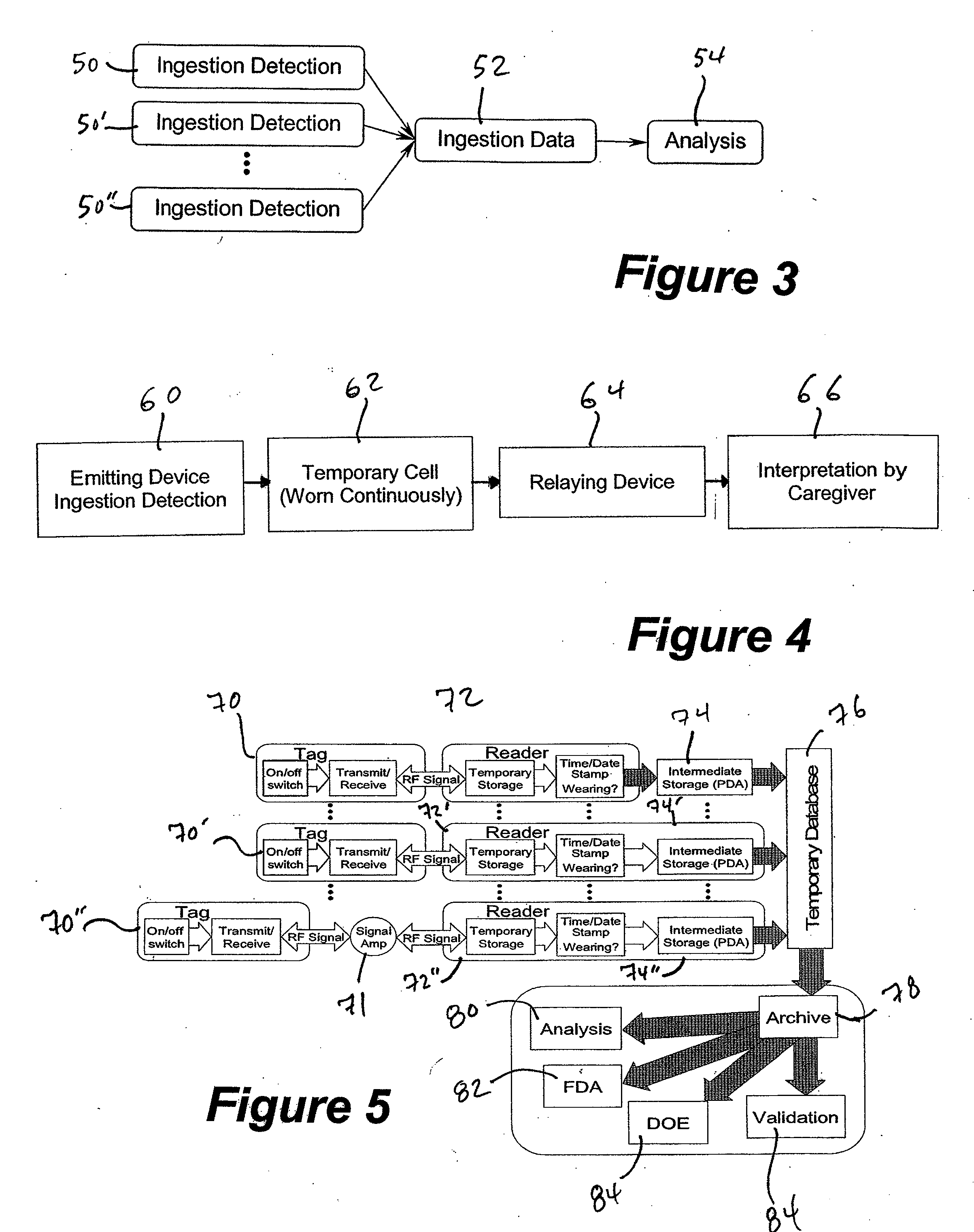
A method and system for monitoring and analyzing compliance with an internal dosing regimen prescribed to be taken in multiple dose forms includes the steps of detecting internalization of a first dose form to generate a first data point, detecting internalization of a second dose form to generate a second data point, and analyzing the first data point and the second data point. The step of analyzing the first and second data points generates a metric of a variety of possible metric types. The first and second dose forms may be two of any plural number of sequentially-internalized dose forms which generate a like number of sequential data points. Subsequent internalizations of dose forms result in at least a like number of data points being generated. To effect the disclosed method a system is provided which includes at least two dose forms, a time stamp identifier operatively associated with each dose form, a receiving device for receiving the time stamp identifier data, and an analyzer for analyzing the received data,
Owner:DOW GLOBAL TECH LLC
Interactive medication container
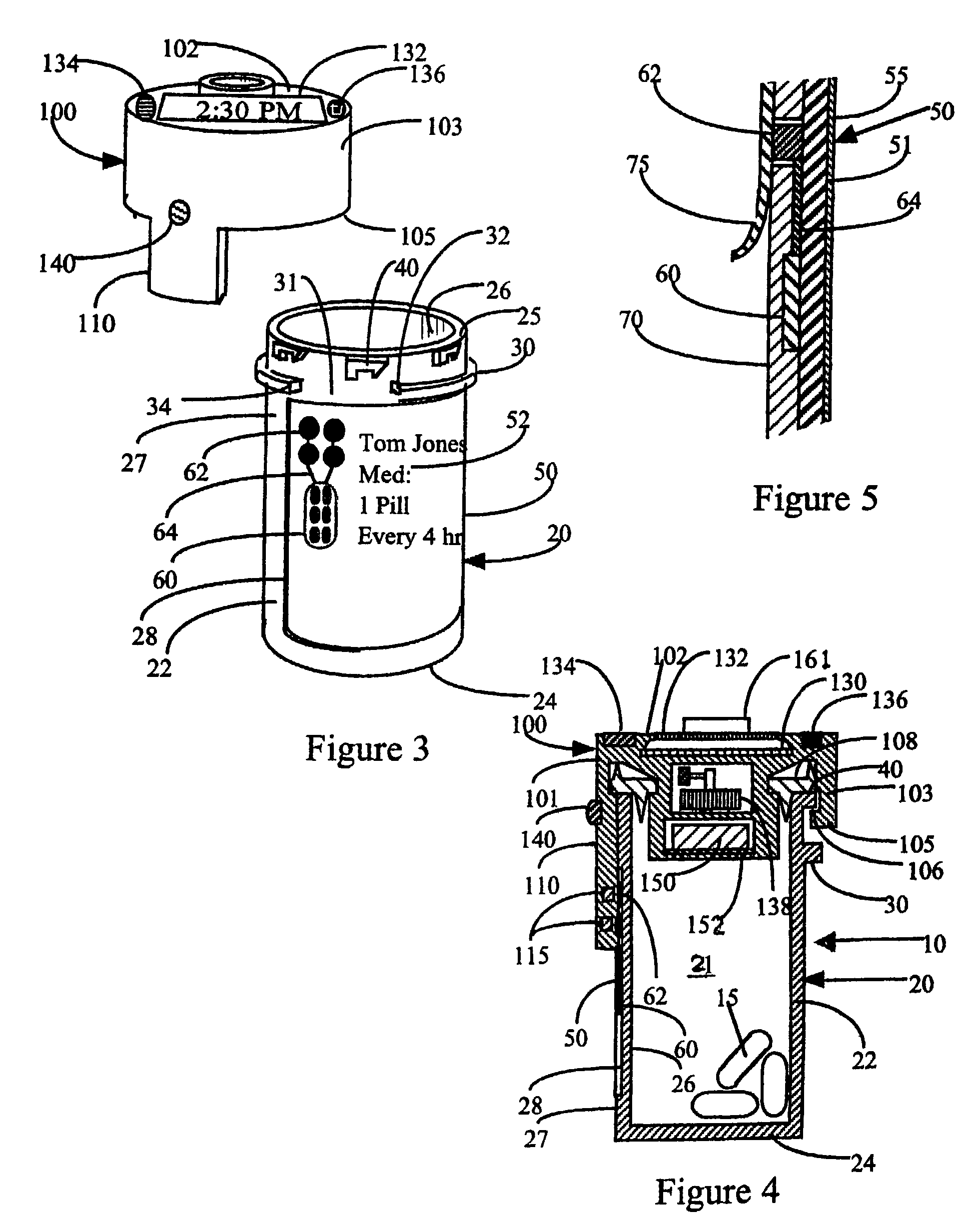
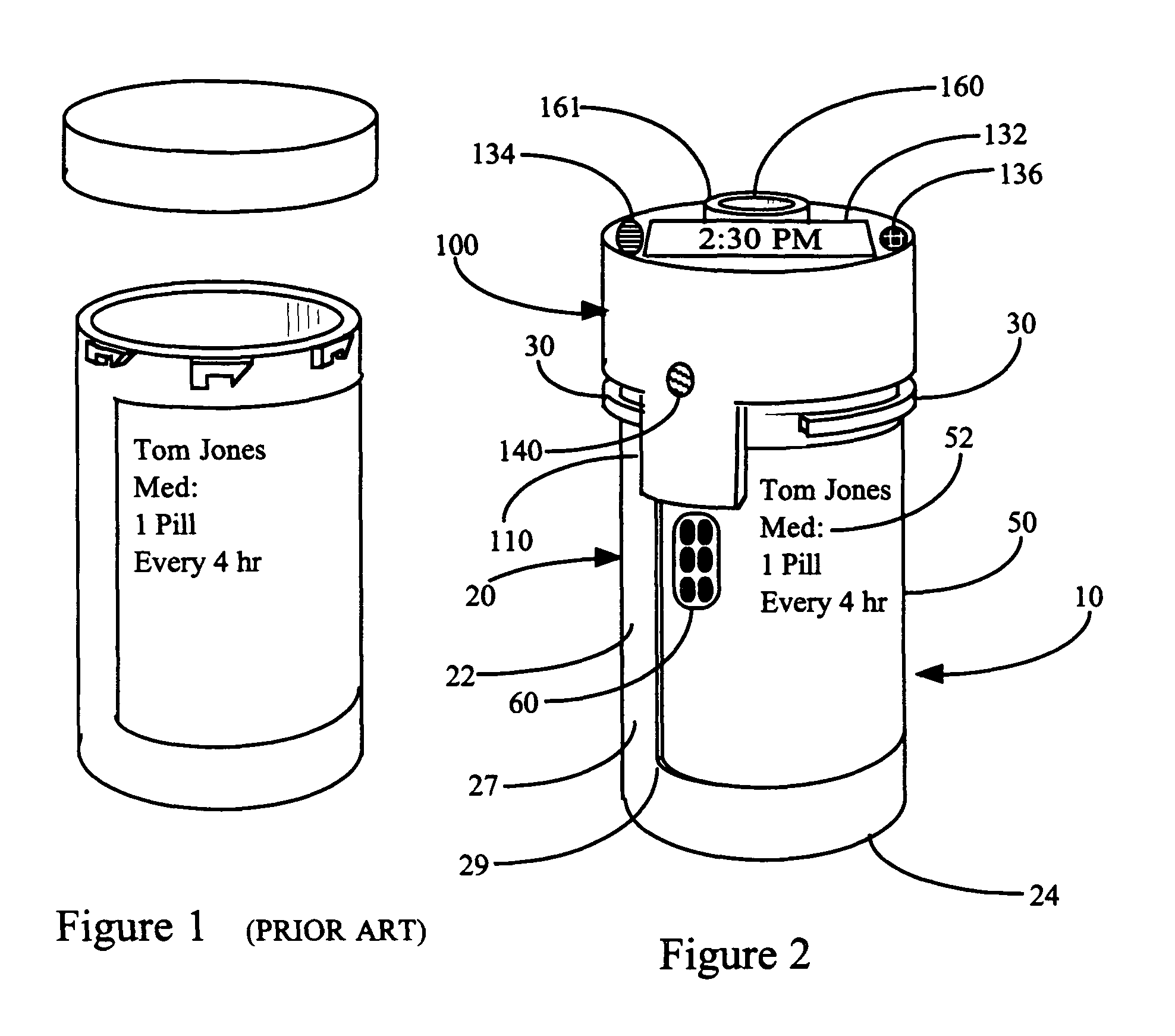
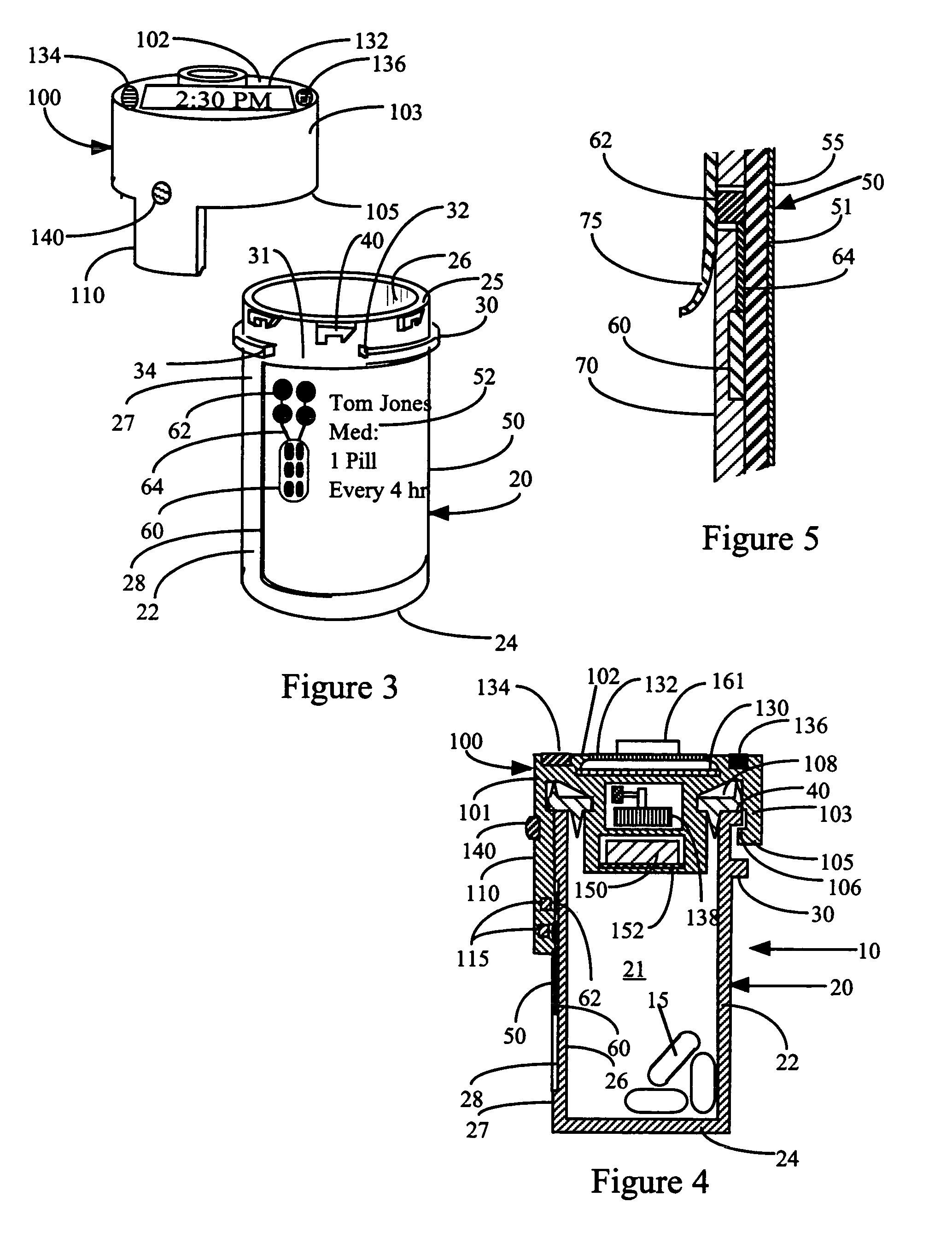
InactiveUS7715277B2Food safetySafe dosingTime indicationDrug and medicationsDosing regimenPatient input
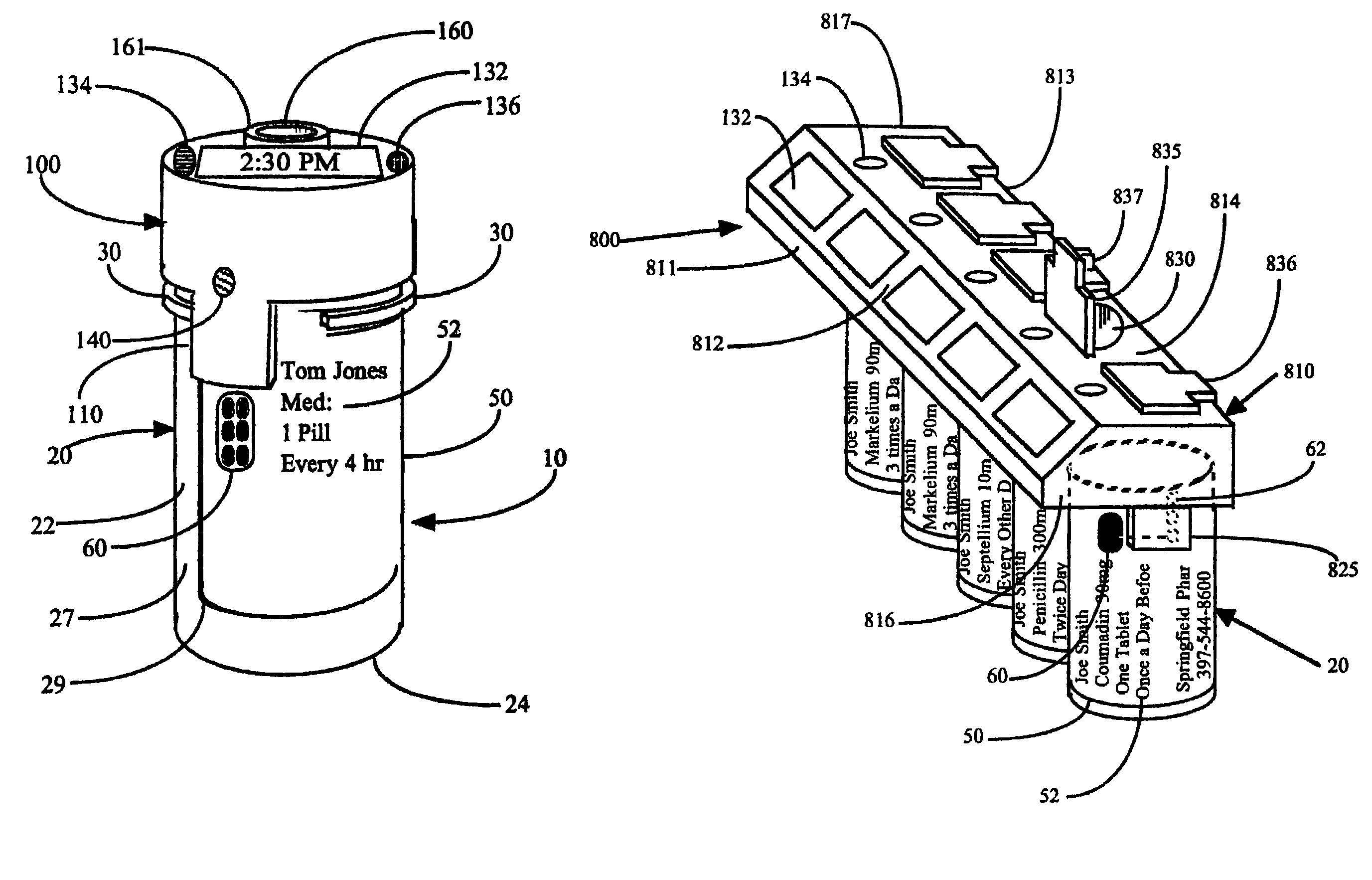
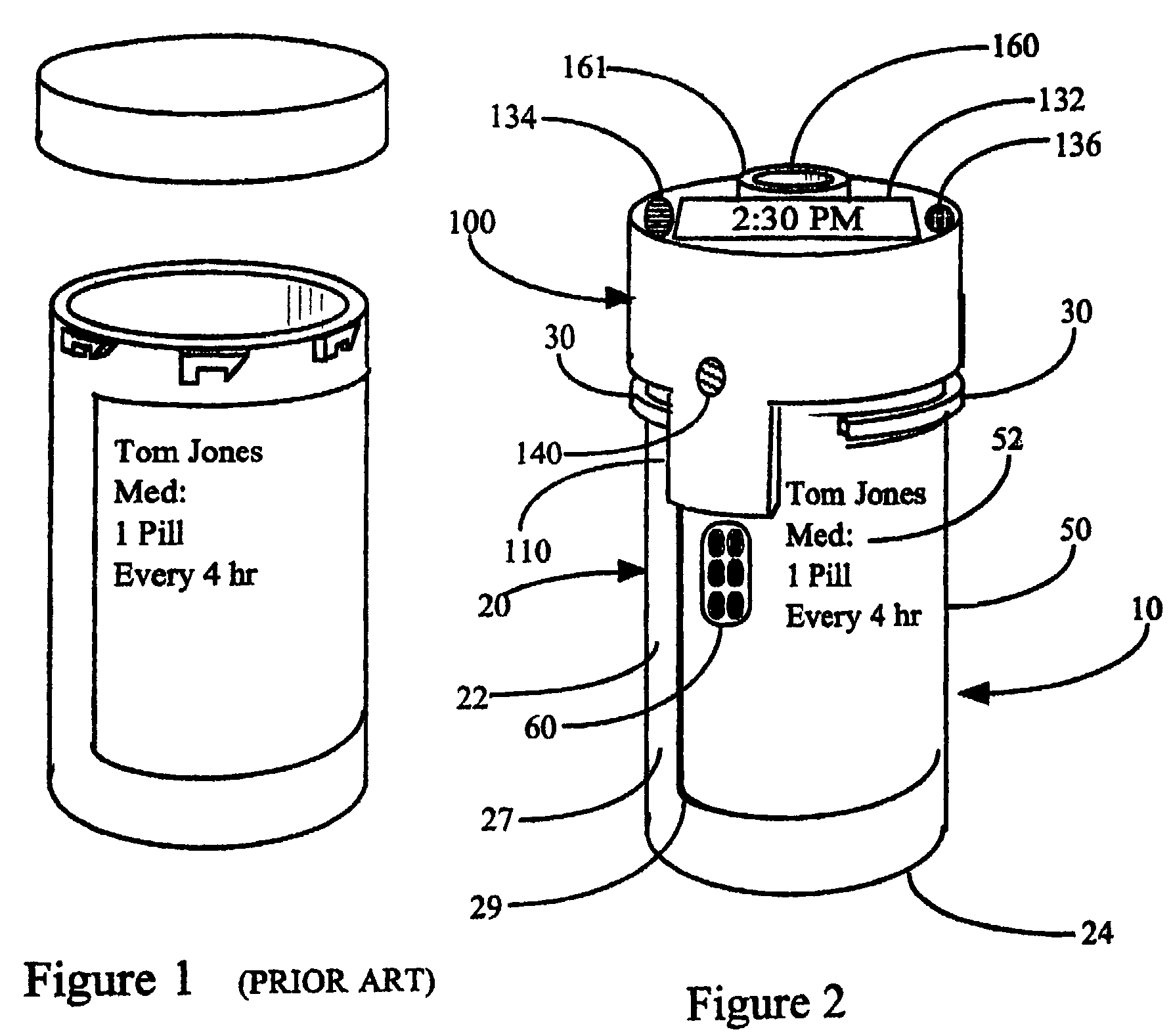
This invention relates to an interactive medication container or console that hold or otherwise organizes one or more medication vials or containers. Each vial has a memory strip containing medication and prescription information. Each vial can also include a reminder unit that is attached to and portable with the individual vials. The console or reminder unit reads the information strip of the vial and communicates this information to or interacts with a patient to remind them to take the medication. The medication container or reminder unit also gathers or tracks information such as consumption time, quantity remaining, patient feedback, and contraindication information. The medication container or reminder unit interacts with the patient by displaying questions or receiving and recording input from the patient before, during or after a dose of medication is taken. The patient input can be used to modify the dosing regimen for future doses of medication. The medication container reorders medication when the quantity remaining reaches a threshold level. Contraindication information in the memory strip is downloaded to a personal home computer or a hospital or nursing home computer.
Owner:SOUTHWEST TECH INNOVATIONS
Interactive medication container
InactiveUS7978564B2Patient compliance is goodLow costMechanical clocksCoin-freed apparatus detailsPatient inputDosing regimen
This invention relates to an interactive medication container or console that hold or otherwise organizes one or more medication vials or containers. Each vial has a memory strip containing medication and prescription information. Each vial can also include a reminder unit that is attached to and portable with the individual vials. The console or reminder unit reads the information strip of the vial and communicates this information to or interacts with a patient to remind them to take the medication. The medication container or reminder unit also gathers or tracks information such as consumption time, quantity remaining, patient feedback, and contraindication information. The medication container or reminder unit interacts with the patient by displaying questions or receiving and recording input from the patient before, during or after a dose of medication is taken. The patient input can be used to modify the dosing regimen for future doses of medication. The medication container reorders medication when the quantity remaining reaches a threshold level. Contraindication information in the memory strip is downloaded to a personal home computer or a hospital or nursing home computer.
Owner:SOUTHWEST TECH INNOVATIONS +2
Methods for treating juvenile idiopathic arthritis
ActiveUS20090258018A1Raise countIncrease the number ofOrganic active ingredientsPharmaceutical delivery mechanismDosing regimenMedicine
The invention provides methods and compositions for the treatment of juvenile idiopathic arthritis (JIA) where a TNFα inhibitor, such as a human TNFα antibody, or antigen-binding portion thereof, is used to treat JIA. In particular, the invention is directed to methods and compositions relating to a fixed dosing regimen for treating JIA with a TNFα inhibitor.
Owner:ABBVIE BIOTECHNOLOGY LTD
Multiple-variable dose regimen for treating TNFa-related disorders
InactiveUS20090304682A1Reducing remissionReducing signOrganic active ingredientsPeptide/protein ingredientsDosing regimenRegimen
Owner:ABBVIE BIOTECHNOLOGY LTD
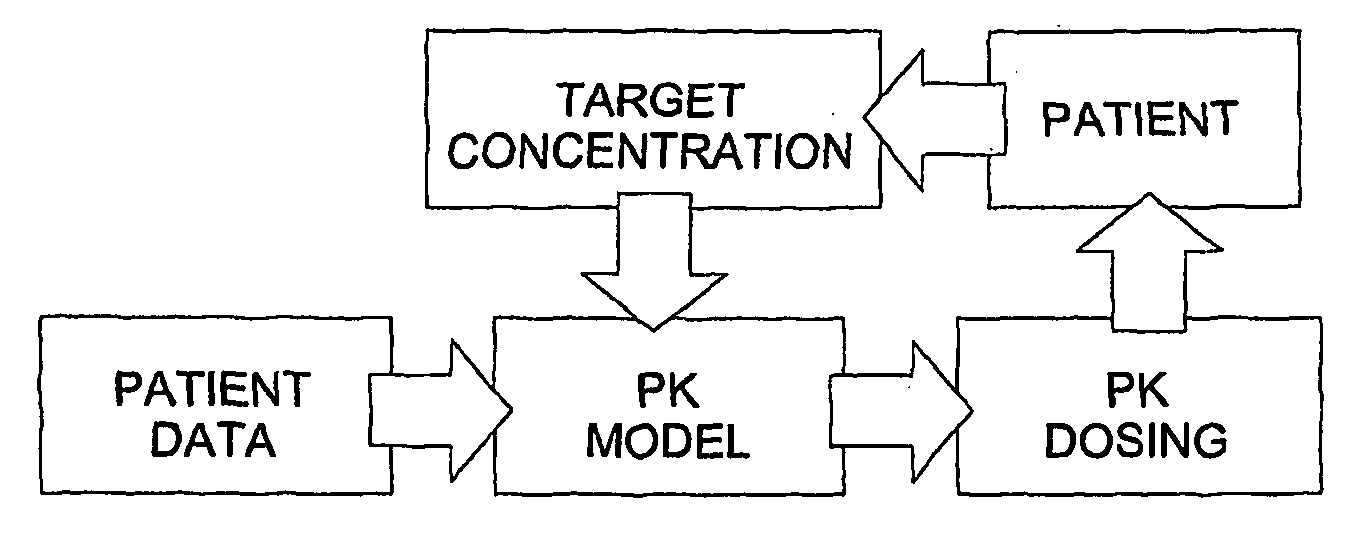
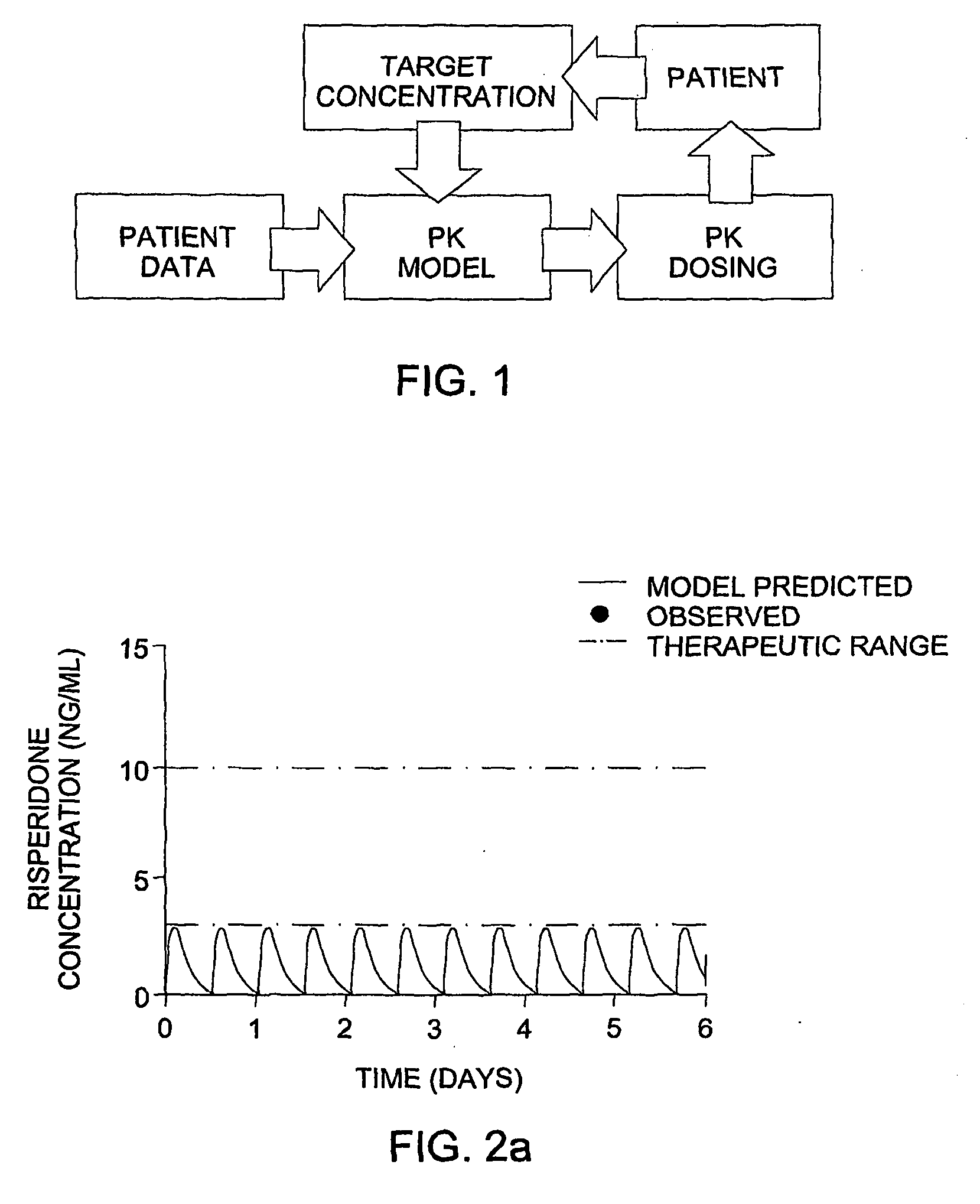
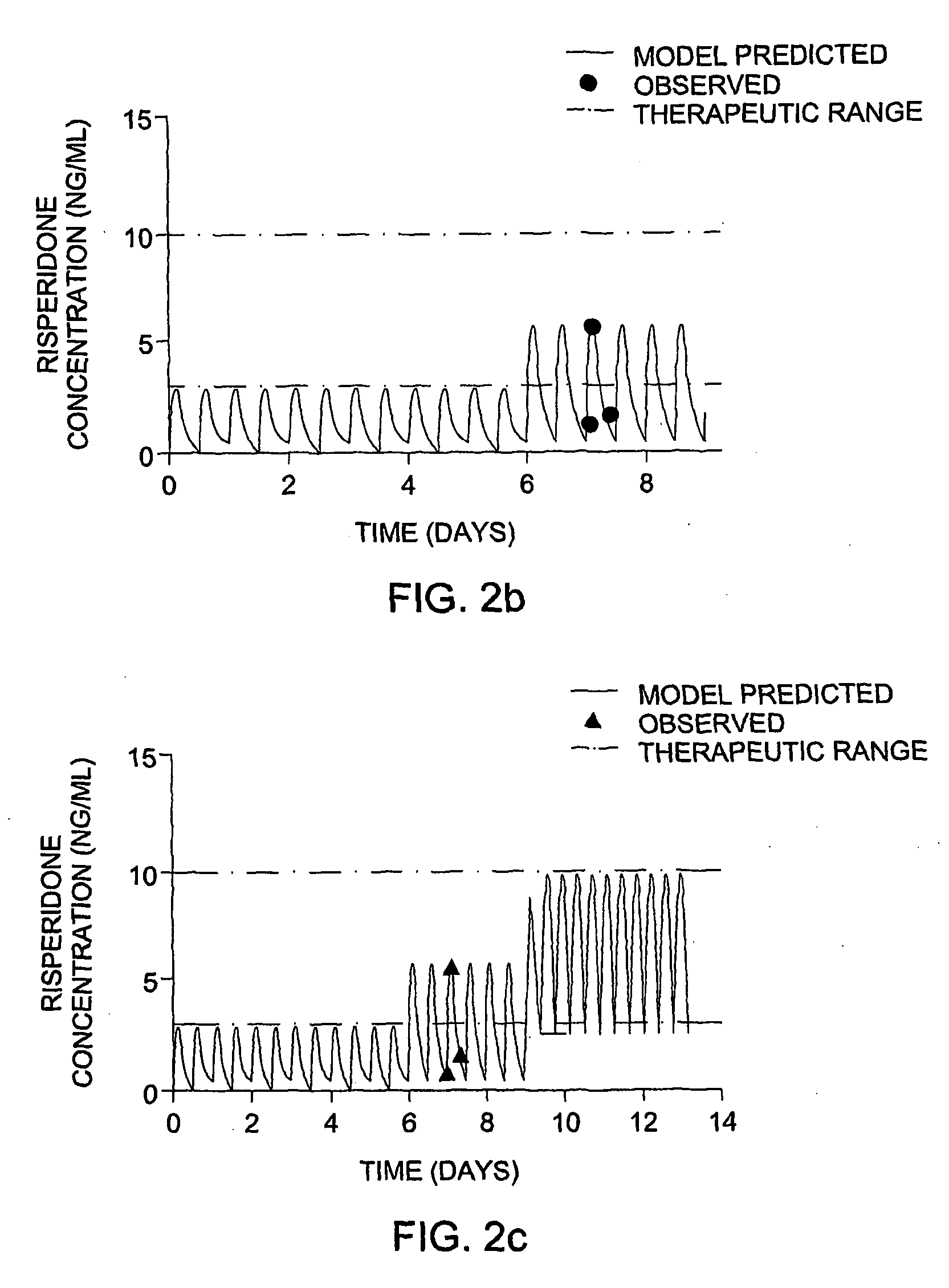
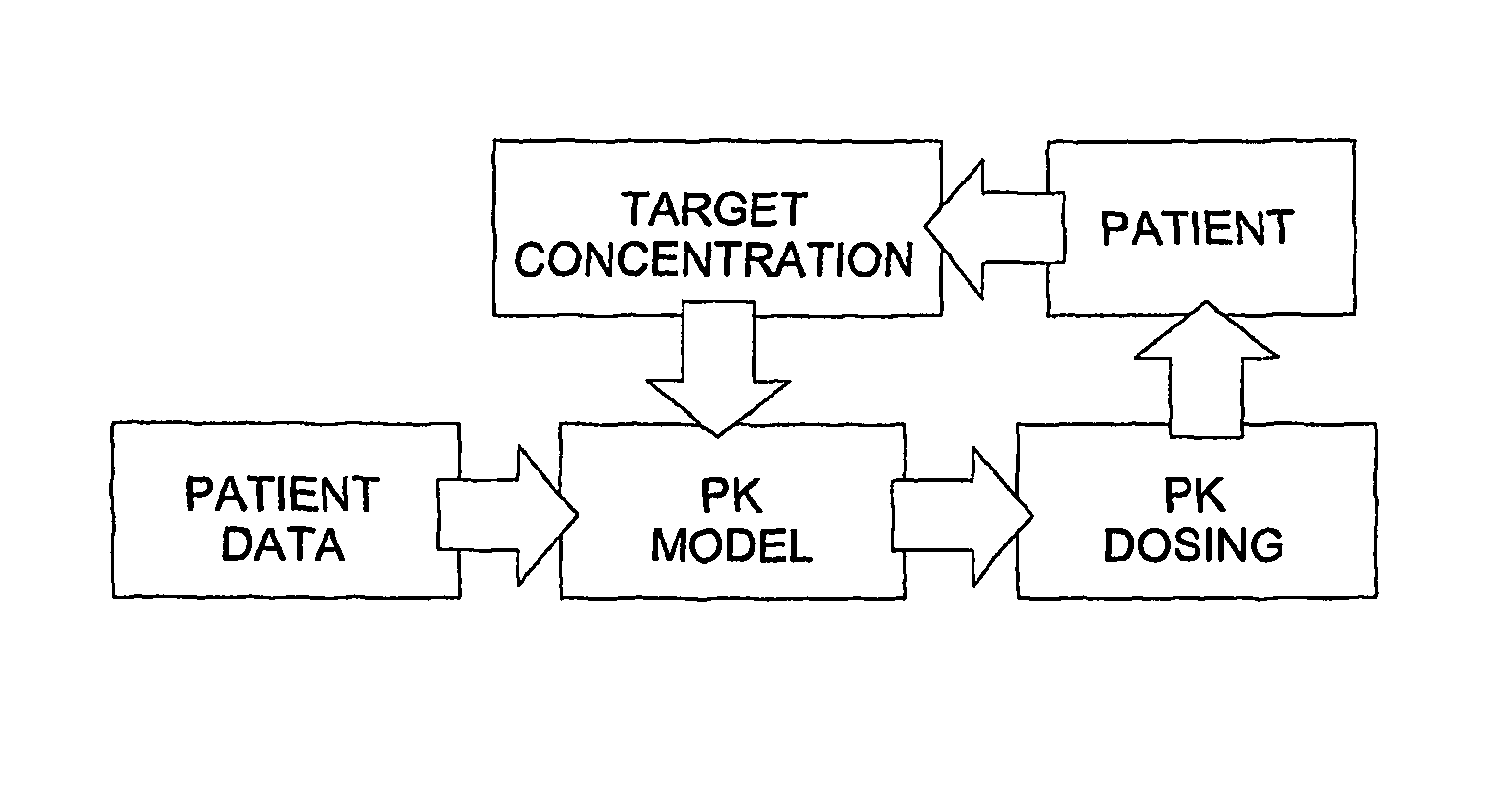
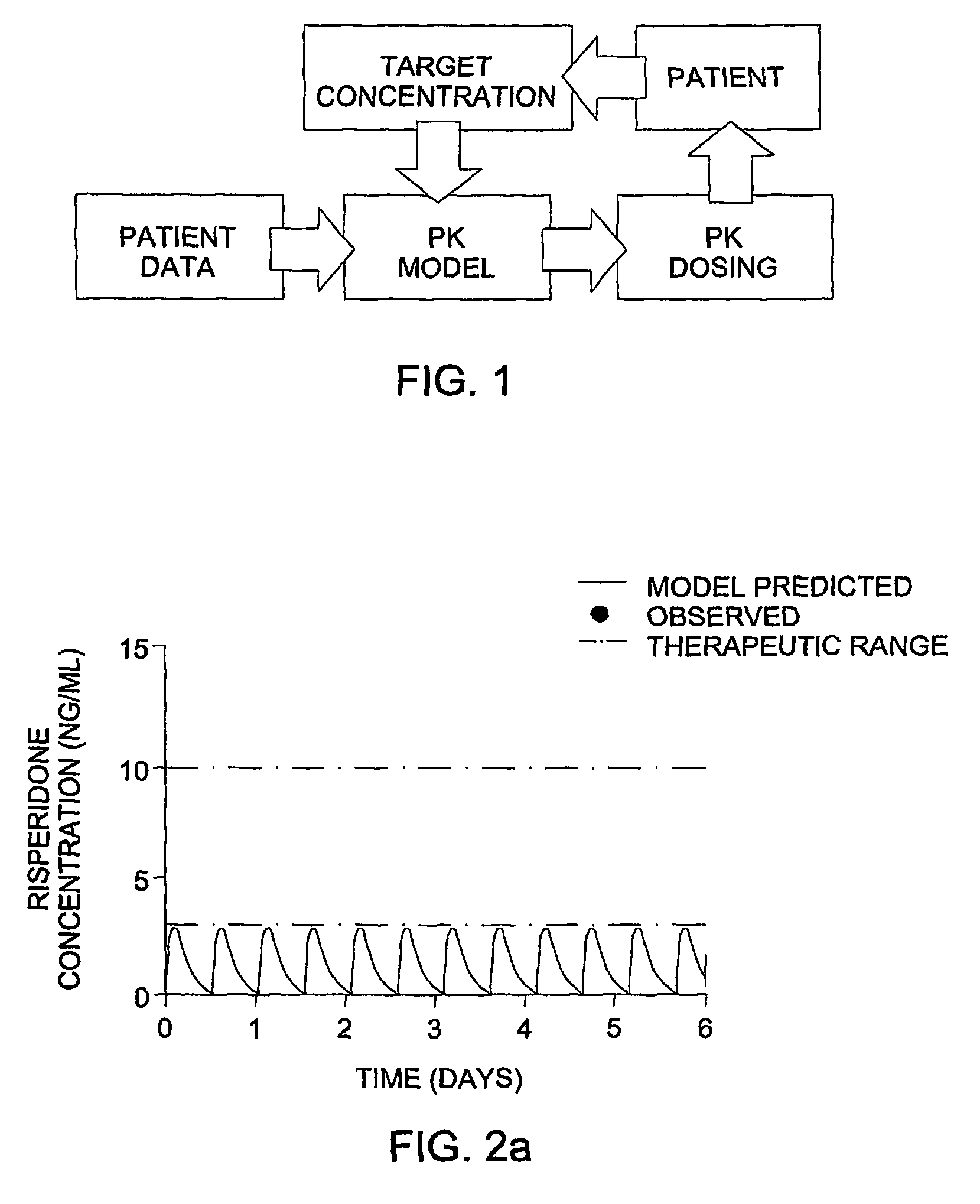
Optimization and Individualization of Medication Selection and Dosing
ActiveUS20090171697A1Easy to understandEasy to recommendationDrug and medicationsBiostatisticsPersonalizationDosing regimen
The invention provides population models, methods, and algorithms for targeting a dosing regimen or compound selection to an individual patient. The methods and algorithms of the invention utilize population models that incorporate genotype information for genes encoding drug metabolizing enzymes for one or more compounds of interest. The methods allow integration of genotype information for one or more genes encoding a drug metabolizing enzyme, particularly a cytochrome P450 gene with patient data. The methods allow integration of genotype information and the effect of one or more compounds on one or more drug metabolizing enzymes. The methods allow iterative feedback of drug metabolizing data obtained from a patient into the process of generating a dosage regimen recommendation for a compound of interest for an individual patient.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Topical treatment or prevention of ocular infections
The topical application of an azalide antibiotic such as azithromycin to the eye is useful in treating or preventing ocular infections. In one embodiment, the azalide antibiotic is supplied to the eye in a depot for sustained release. A more convenient dosing regimen can also be provided by the use of an appropriate depot. Furthermore, a composition containing a combination of medicaments is also provided.
Owner:INSITE VISION
Pharmaceutical compositions and treatment methods
InactiveUS6667299B1Efficient transportReduce yieldAntibacterial agentsOrganic active ingredientsRegimenKetone
The invention provides compositions comprising, 16alpha-bromo-3beta-hydroxy-5alpha-androstan-17-one hemihydrate and one or more excipients, typically wherein the composition comprises less than about 3% water. The compositions are useful to make improved pharmaceutical formulations. The invention also provides methods of intermittent dosing of steroid compounds such as analogs of 16alpha-bromo-3beta-hydroxy-5alpha-androstan-17-one and compositions useful in such dosing regimens. The invention further provides compositions and methods to inhibit pathogen (viral) replication, ameliorate symptoms associated with immune dysregulation and to modulate immune responses in a subject using certain steroids and steroid analogs. The invention also provides methods to make and use these immunomodulatory compositions and formulations.
Owner:NEURMEDIX +2
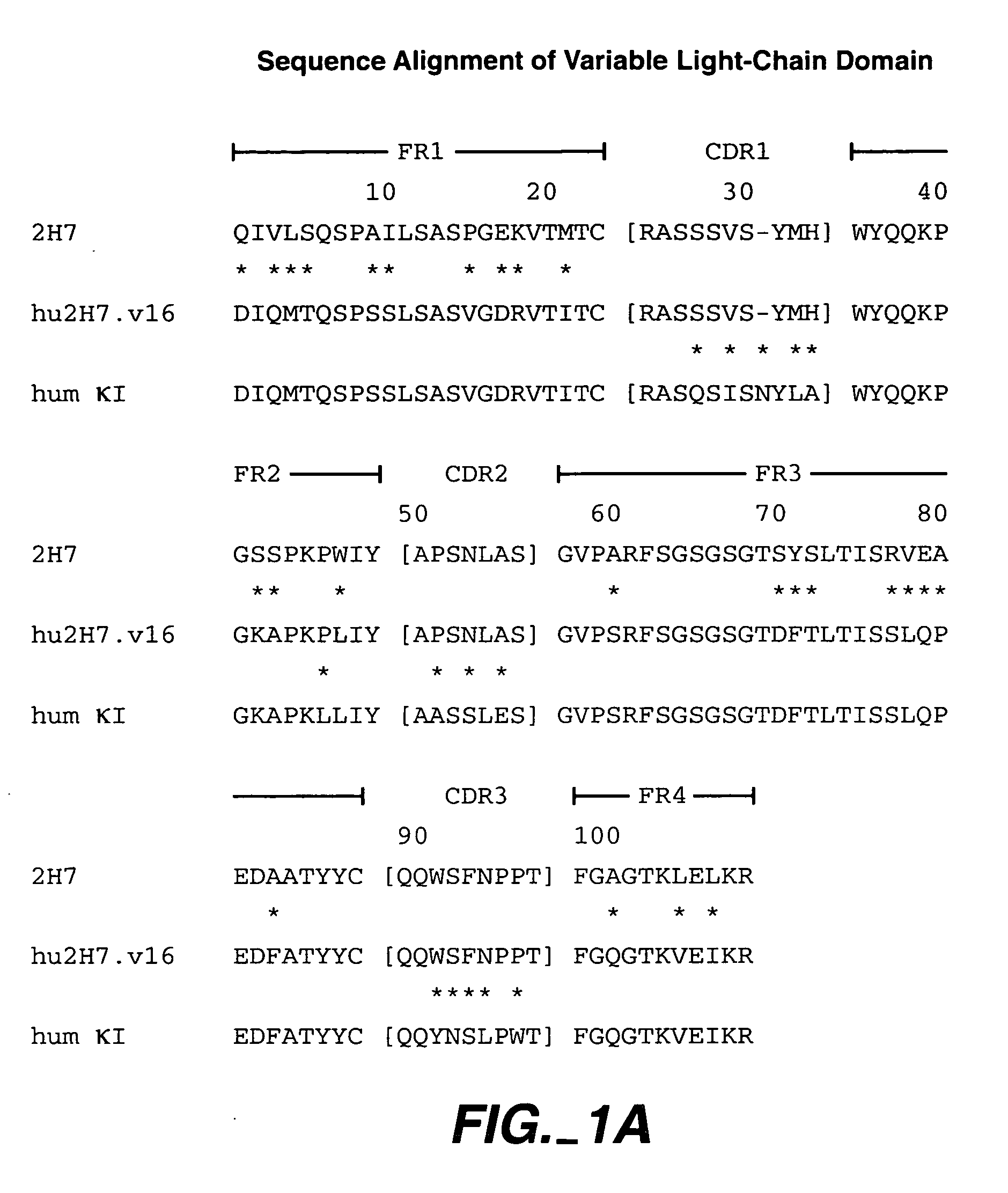
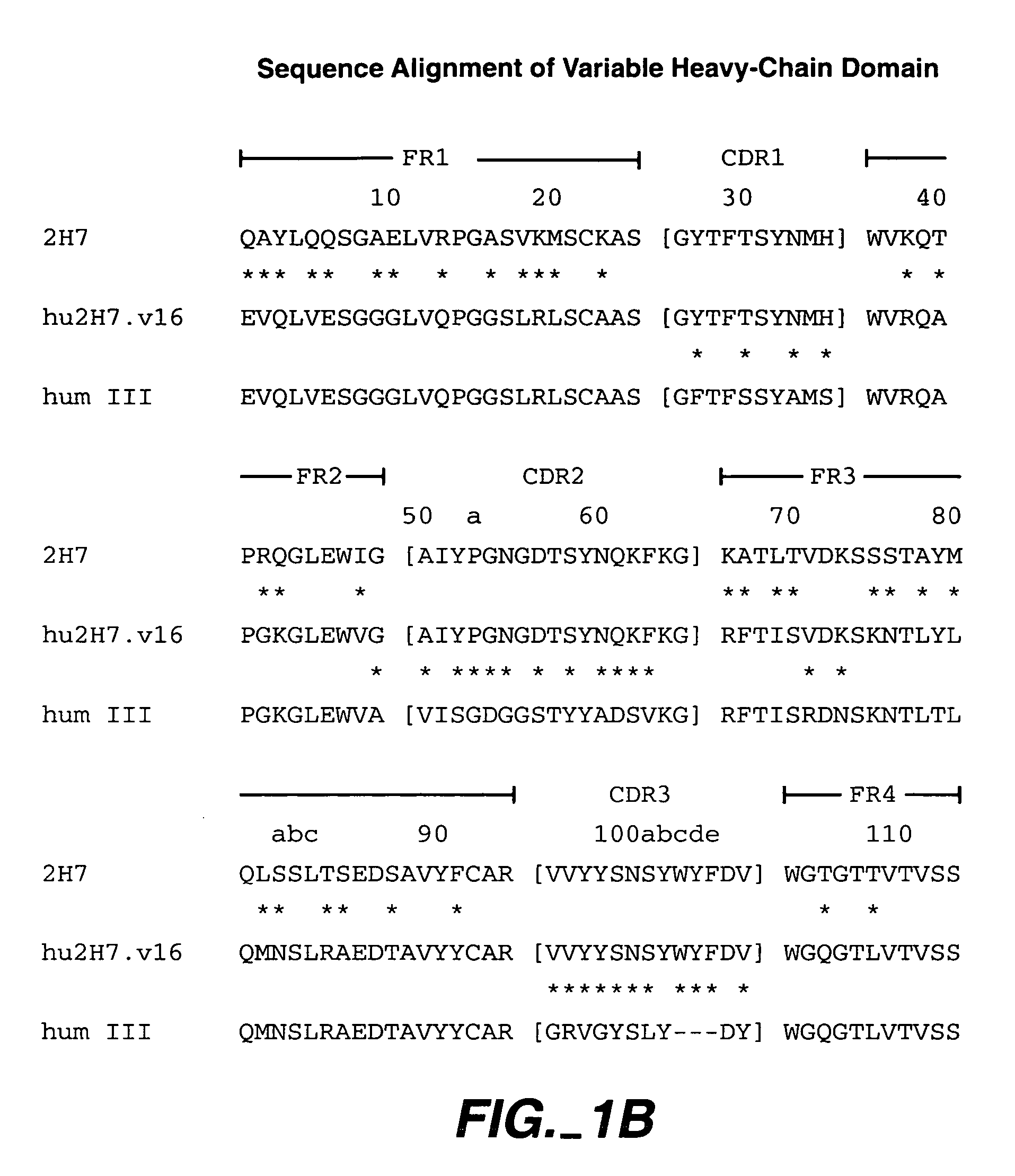
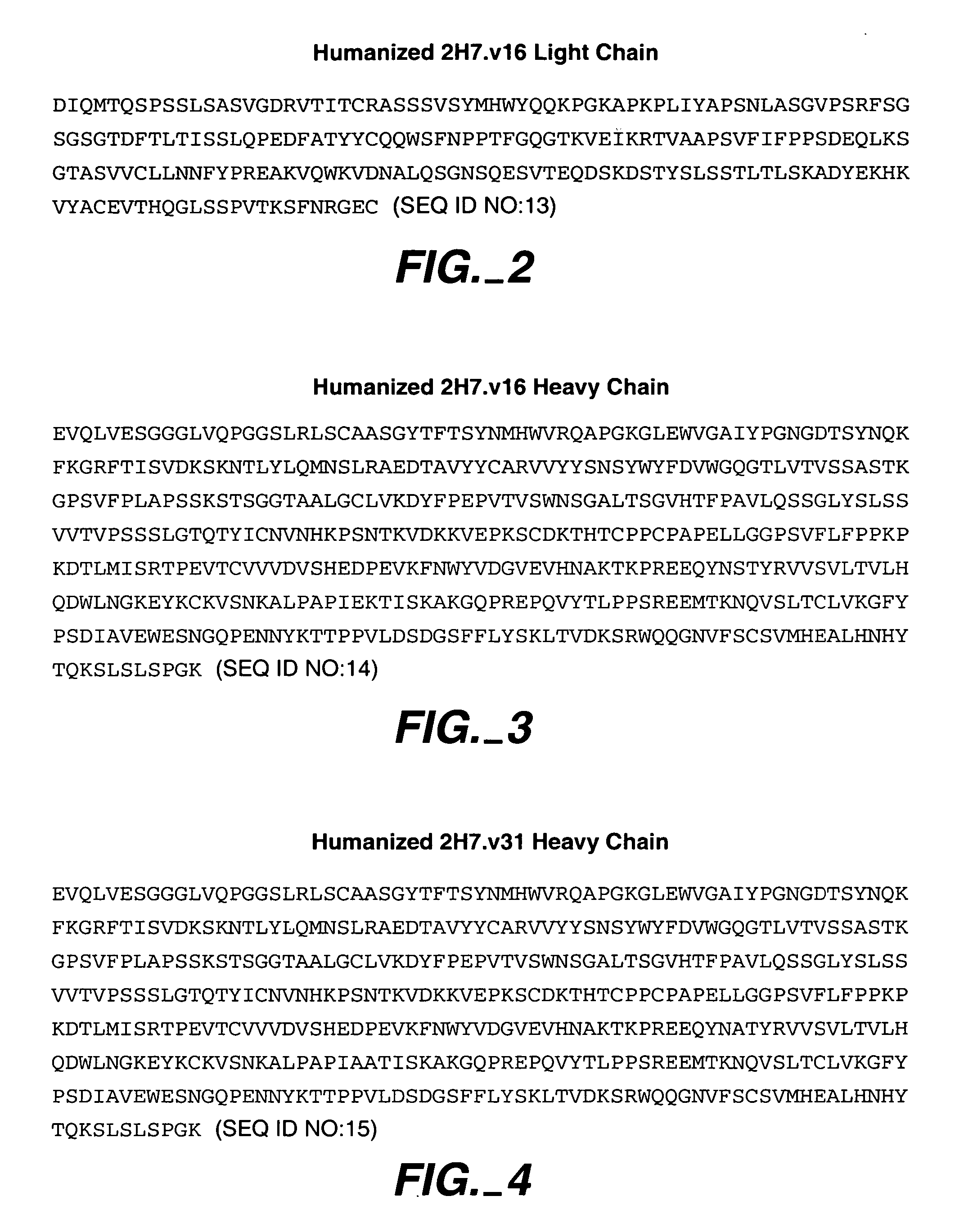
Method for treating multiple sclerosis
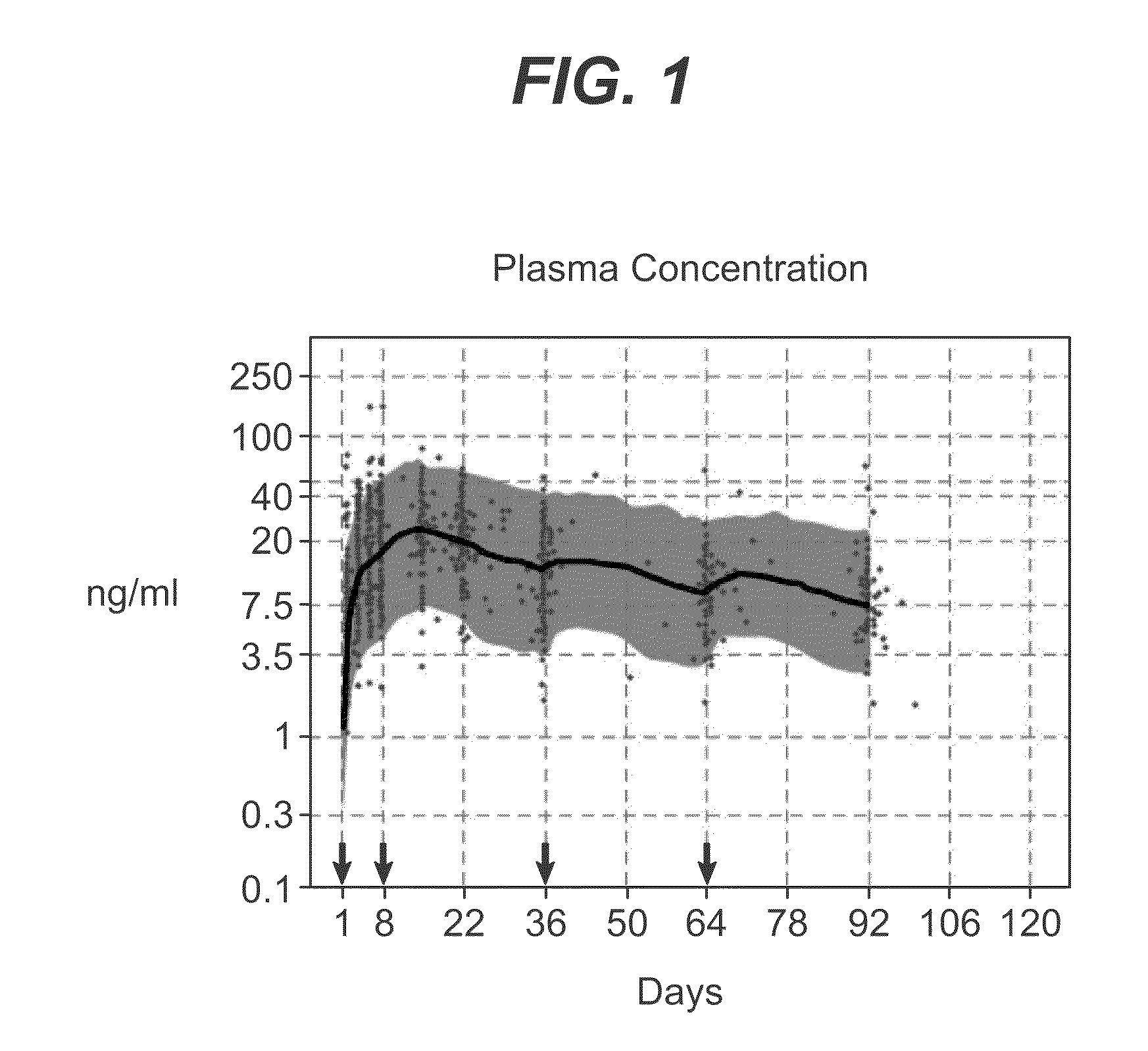
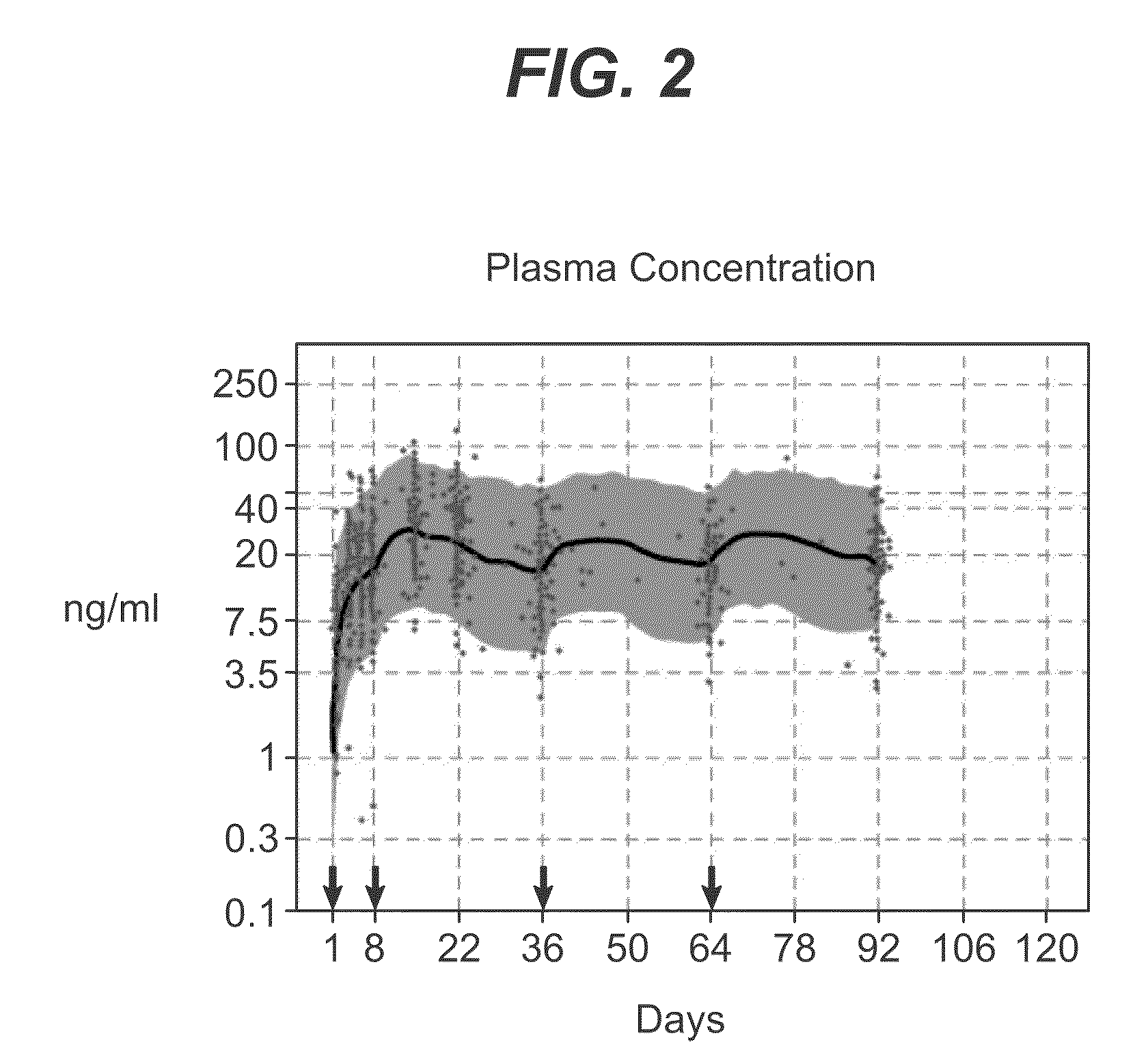
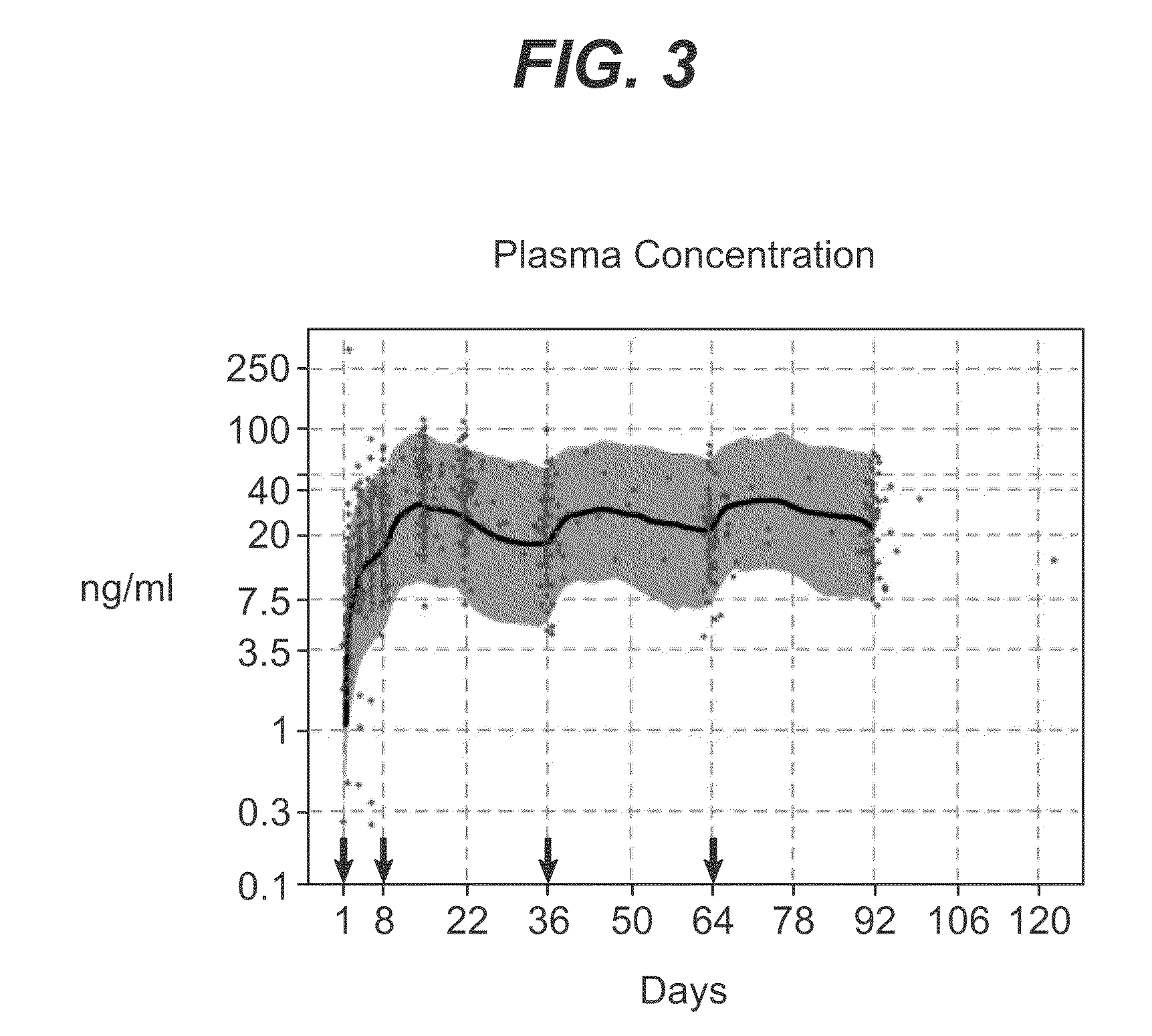
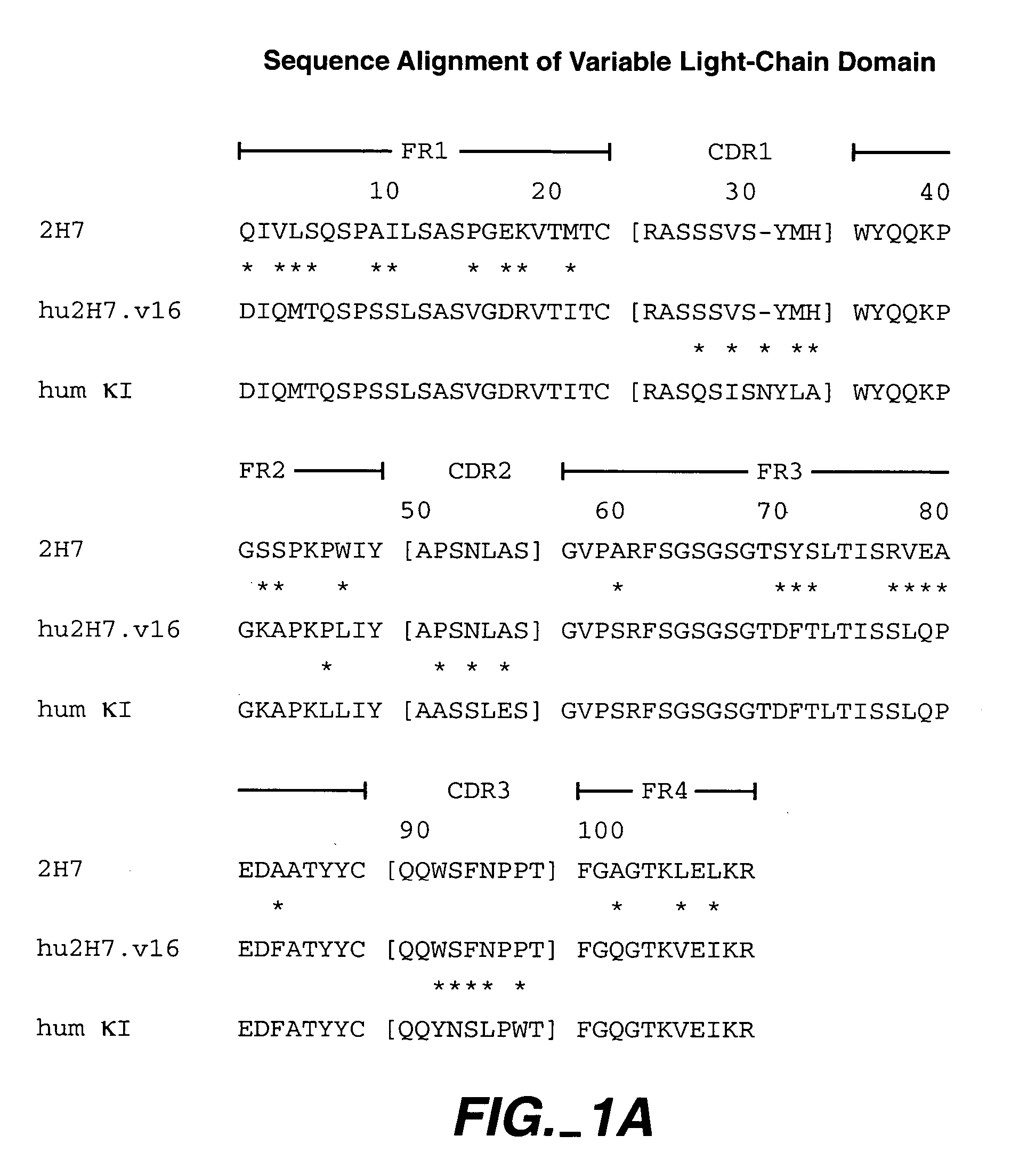
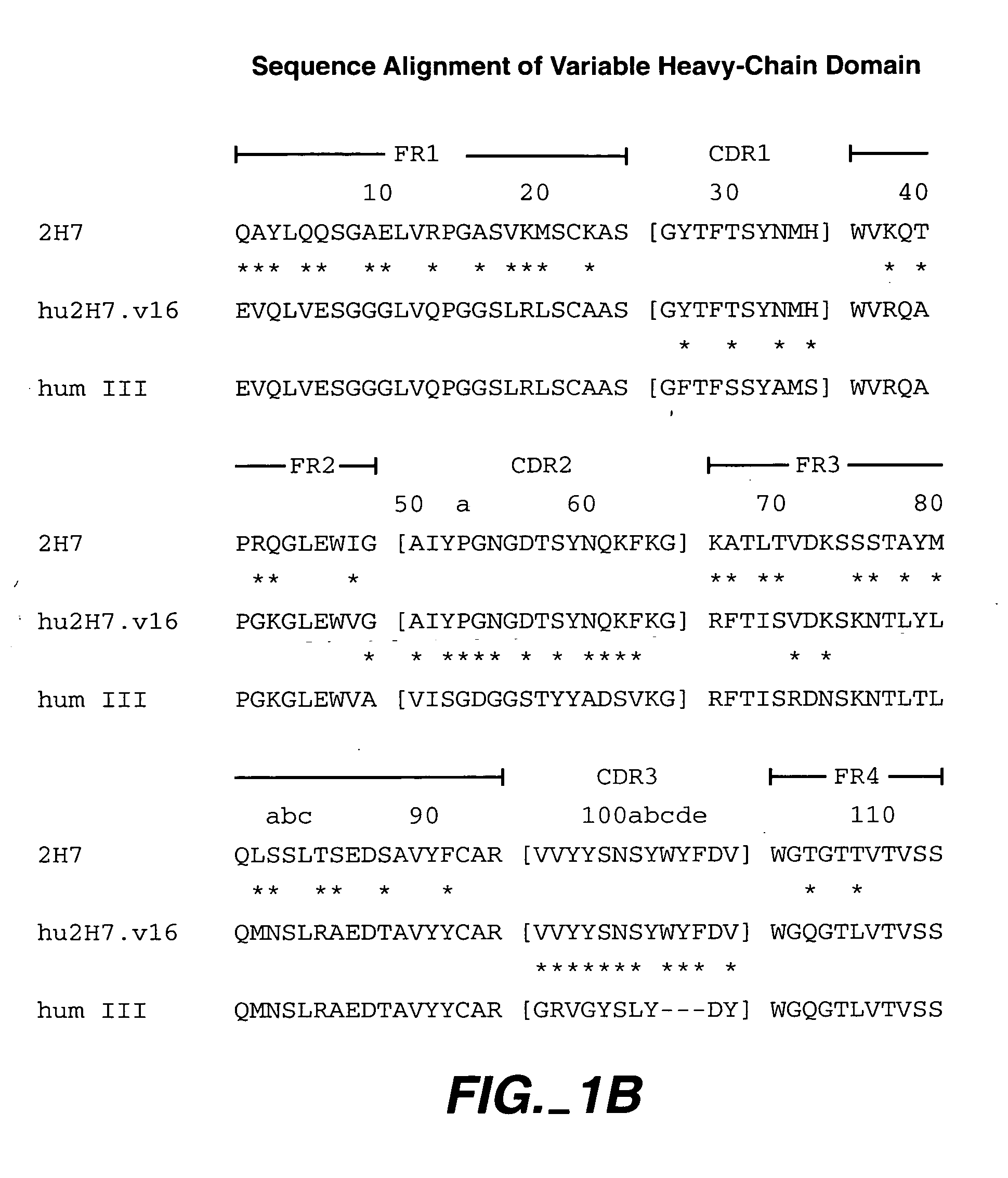
Methods for treating multiple sclerosis (MS) with a CD20 antibody using special dosing regimens and protocols are described. Articles of manufacture for use in such methods are also described.
Owner:GENENTECH INC
Dosing regimen associated with long acting injectable paliperidone esters
The present invention provides a method of treating patients in need of treatment with long acting injectable paliperidone palmitate formulations.
Owner:JANSSEN PHARMA NV
Method for treating lupus
InactiveUS20060024295A1Reduce and minimize needAvoid much side effectAntipyreticAnalgesicsSurface markerRegimen
A method of treating lupus in a subject eligible for treatment is provided involving administering an effective amount of an antibody that binds to a B-cell surface marker to the subject to provide an initial exposure and a subsequent exposure to the antibody within certain dosing regimens and an article of manufacture therefor.
Owner:GENENTECH INC
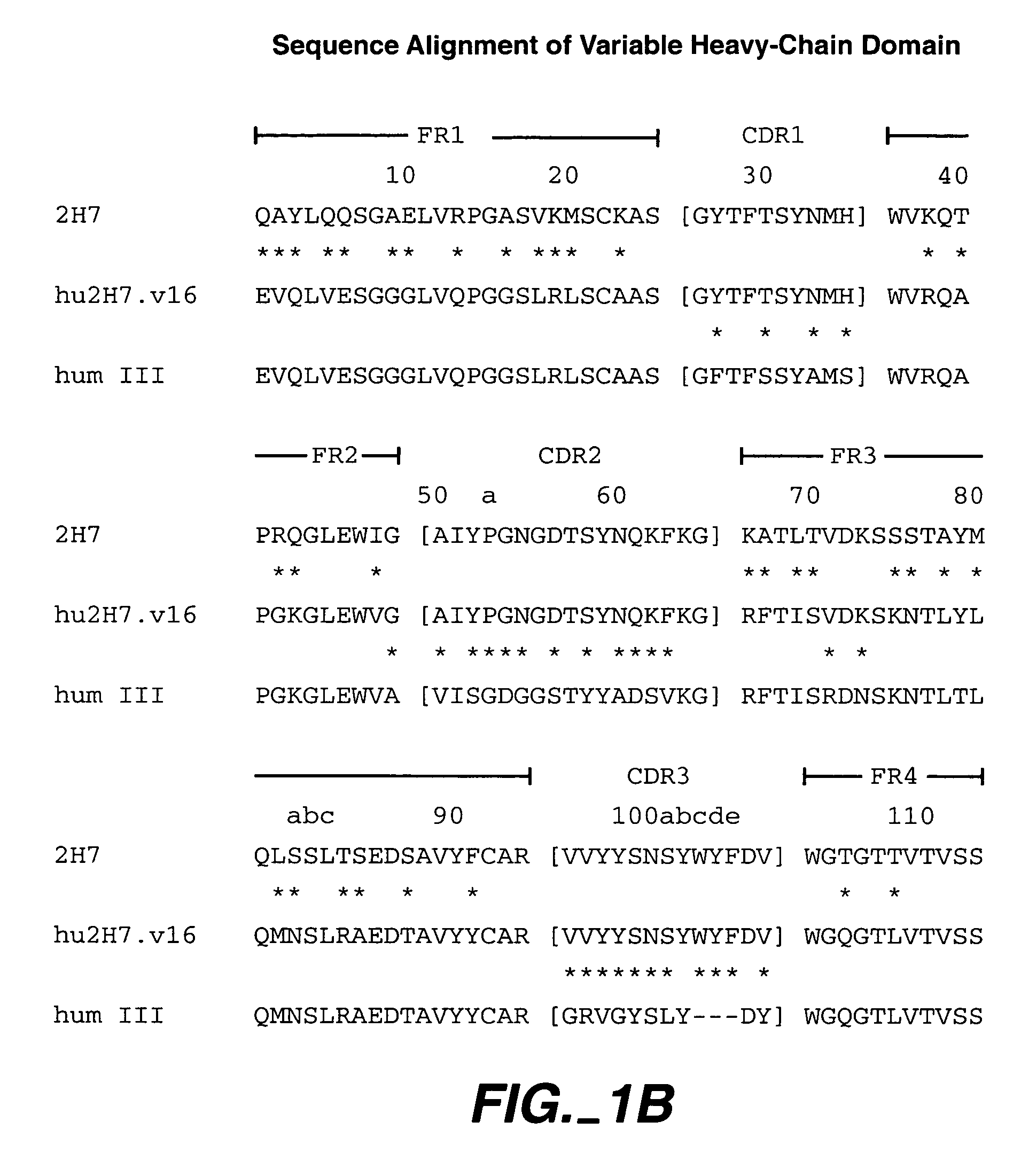
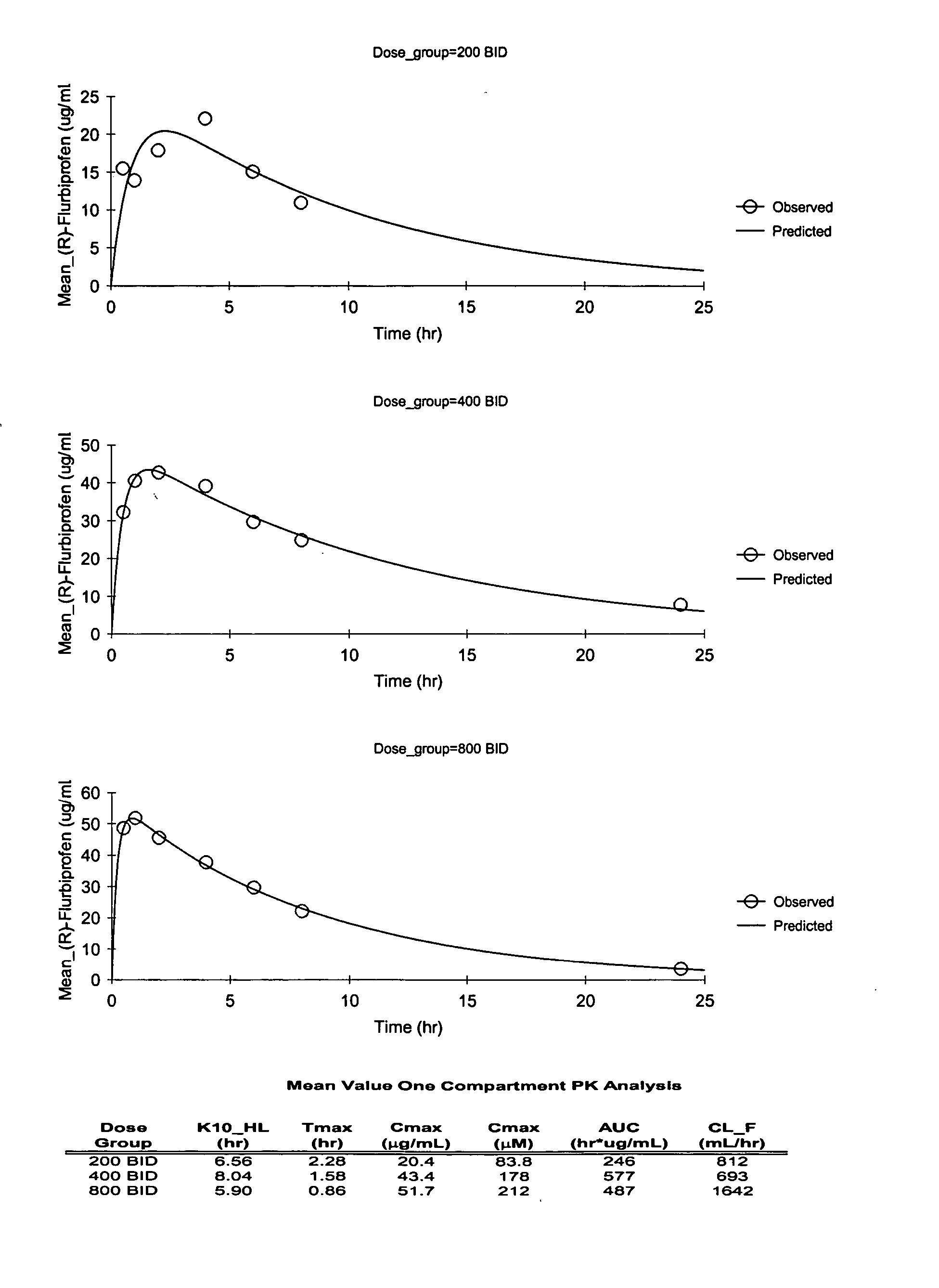
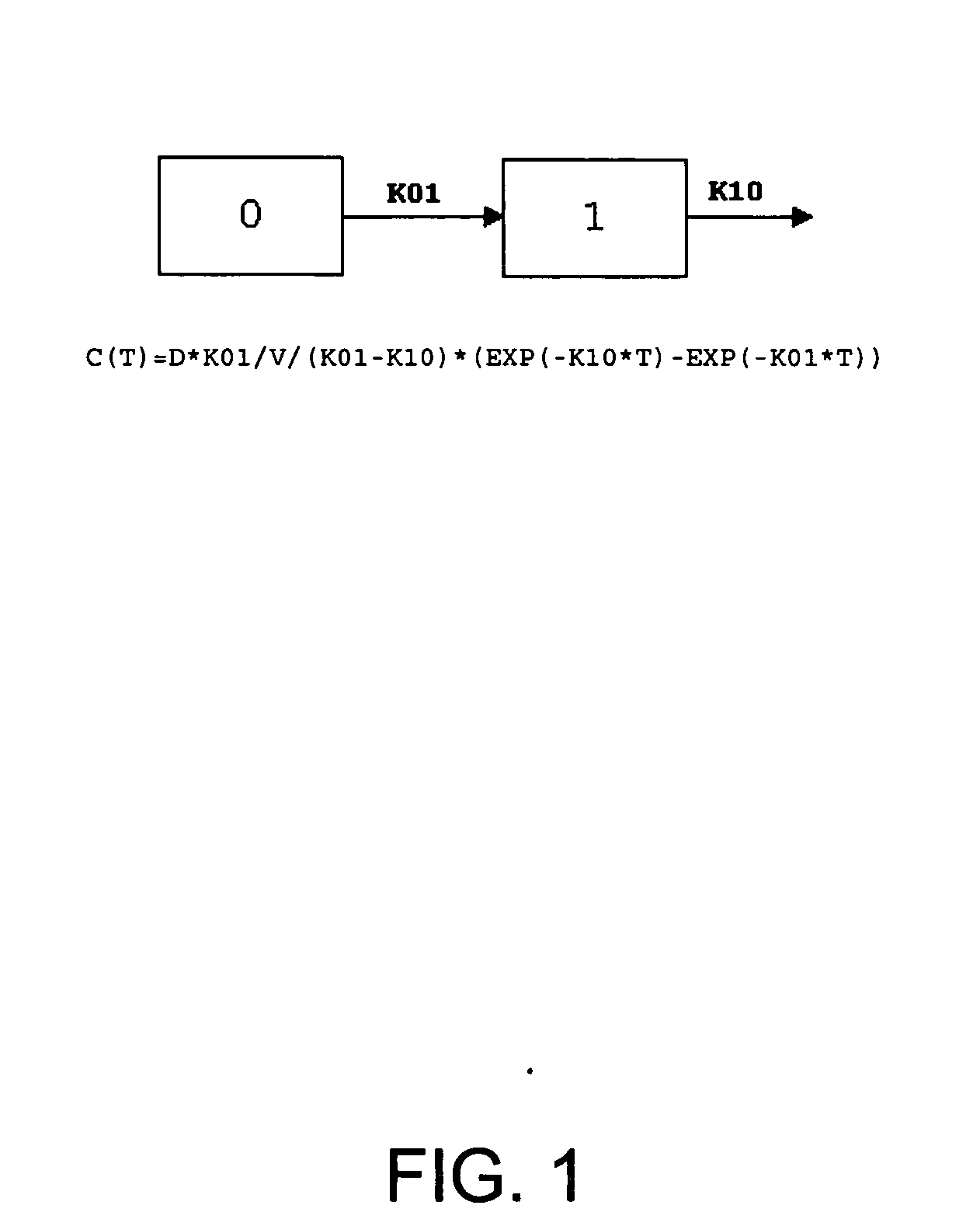
Pharmaceutical methods, dosing regimes and dosage forms for the treatment of Alzheimer's disease
InactiveUS20050042284A1Improving and lessening rate of declineReduction of the decline in saidBiocideNervous disorderDosing regimenGuideline
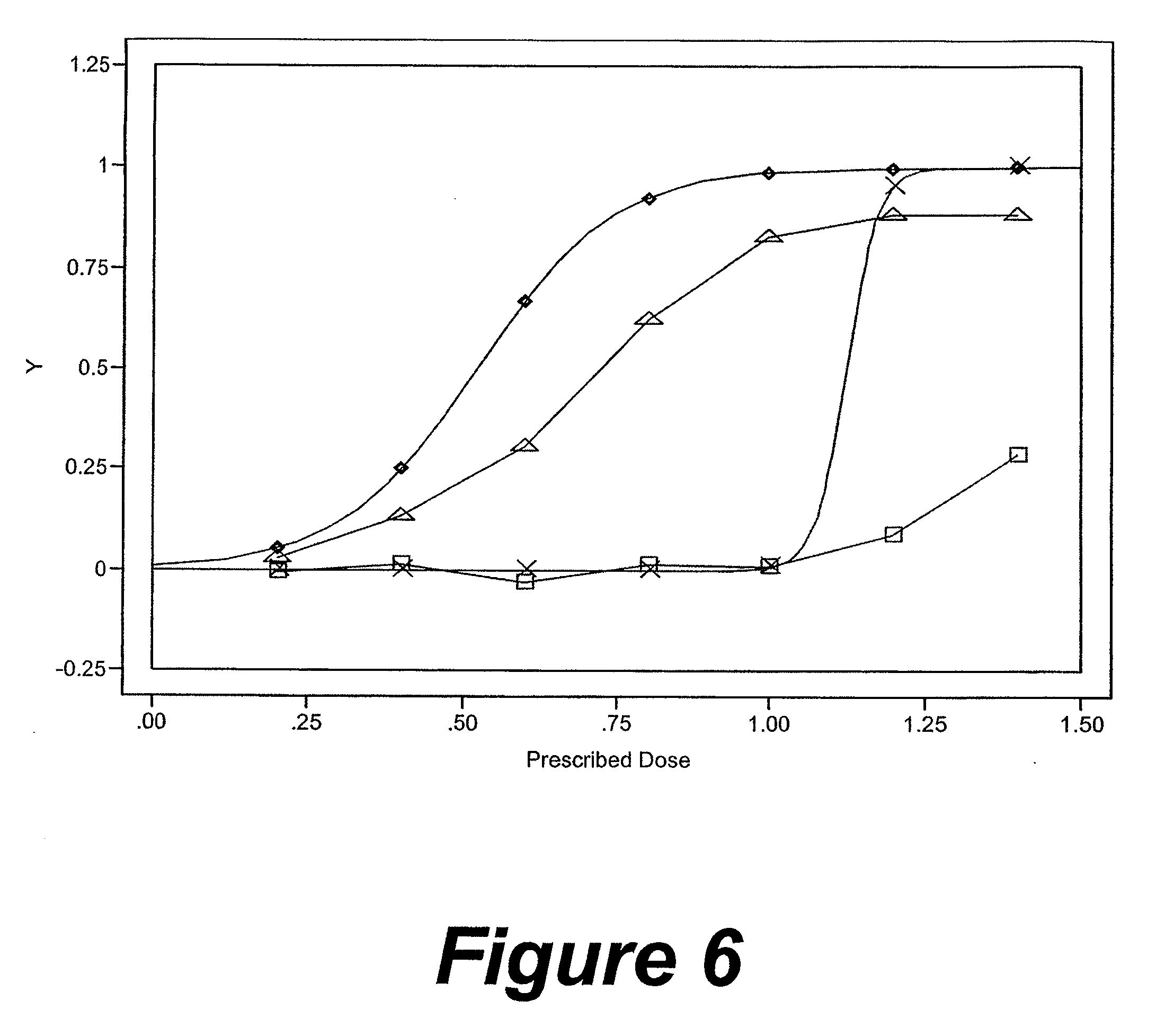
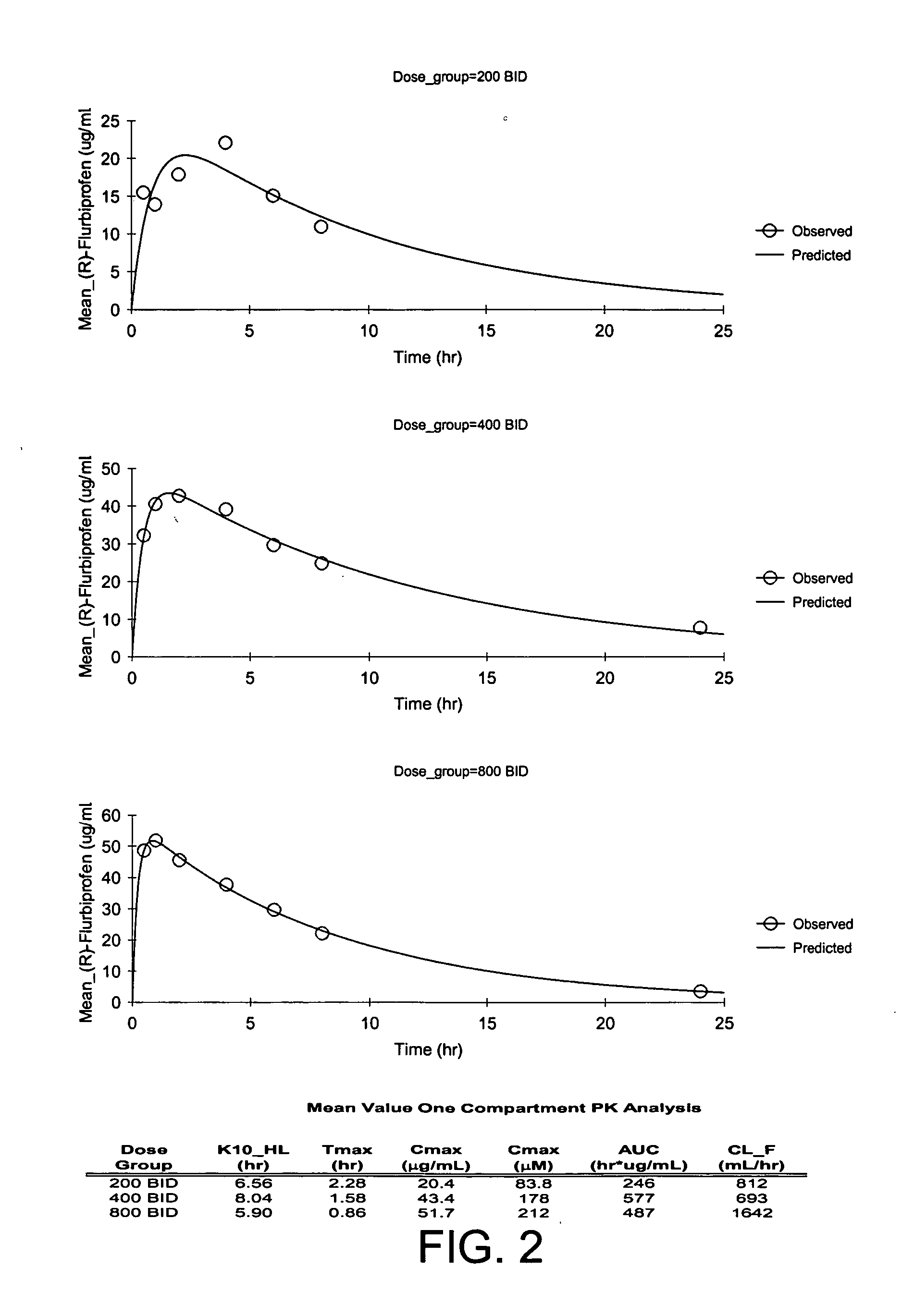
In general, the invention relates to a pharmaceutical dose having R-flurbiprofen as the active ingredient that upon oral administration of a single dose to a fasting subject provides a Cmax of about 30-95 μg per mL. When the dose is administered to an individual having mild-to-moderate Alzheimer's disease (or desiring protection against Alzheimer's disease) twice daily for at least 4 months according to the described guidelines, an improvement or lessening in decline of cognitive function as characterized by cognition tests is observed in the patient. The composition of the invention is formulated with one or more pharmaceutically acceptable excipients, salts or carriers.
Owner:MYRIAD GENETICS
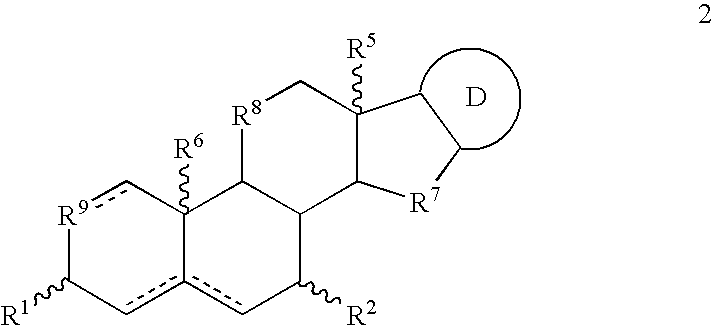
Dosing regimen
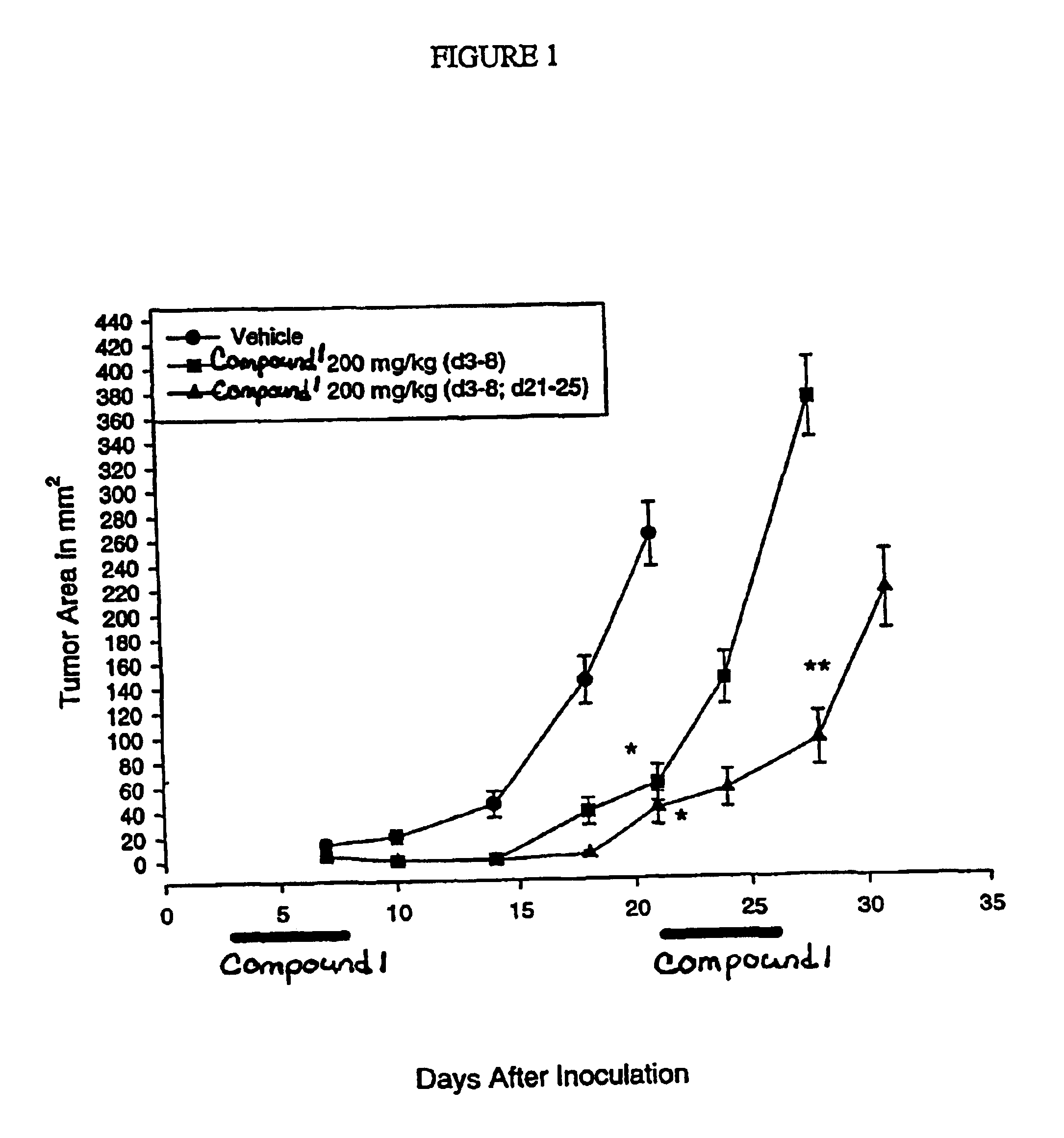
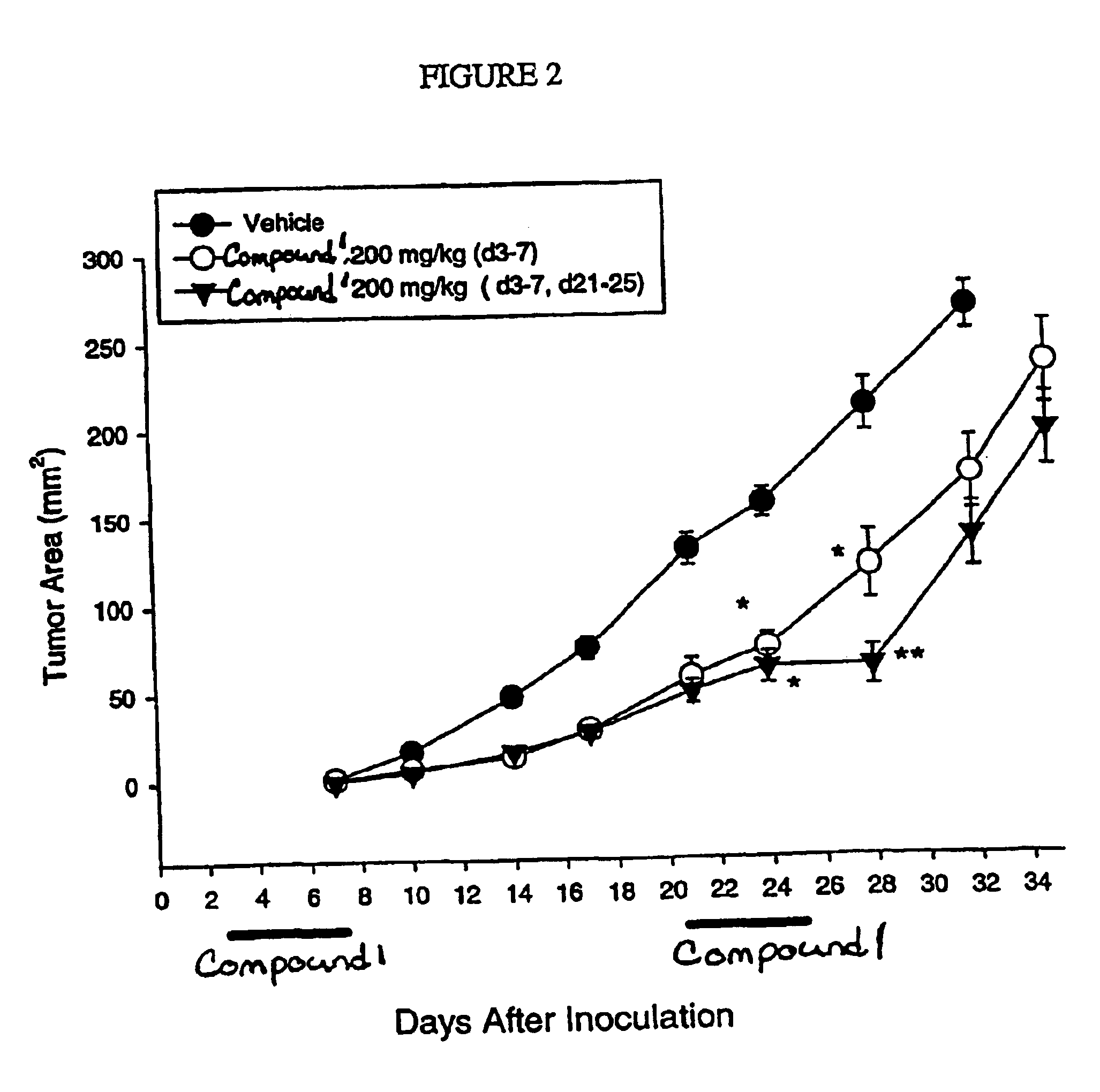
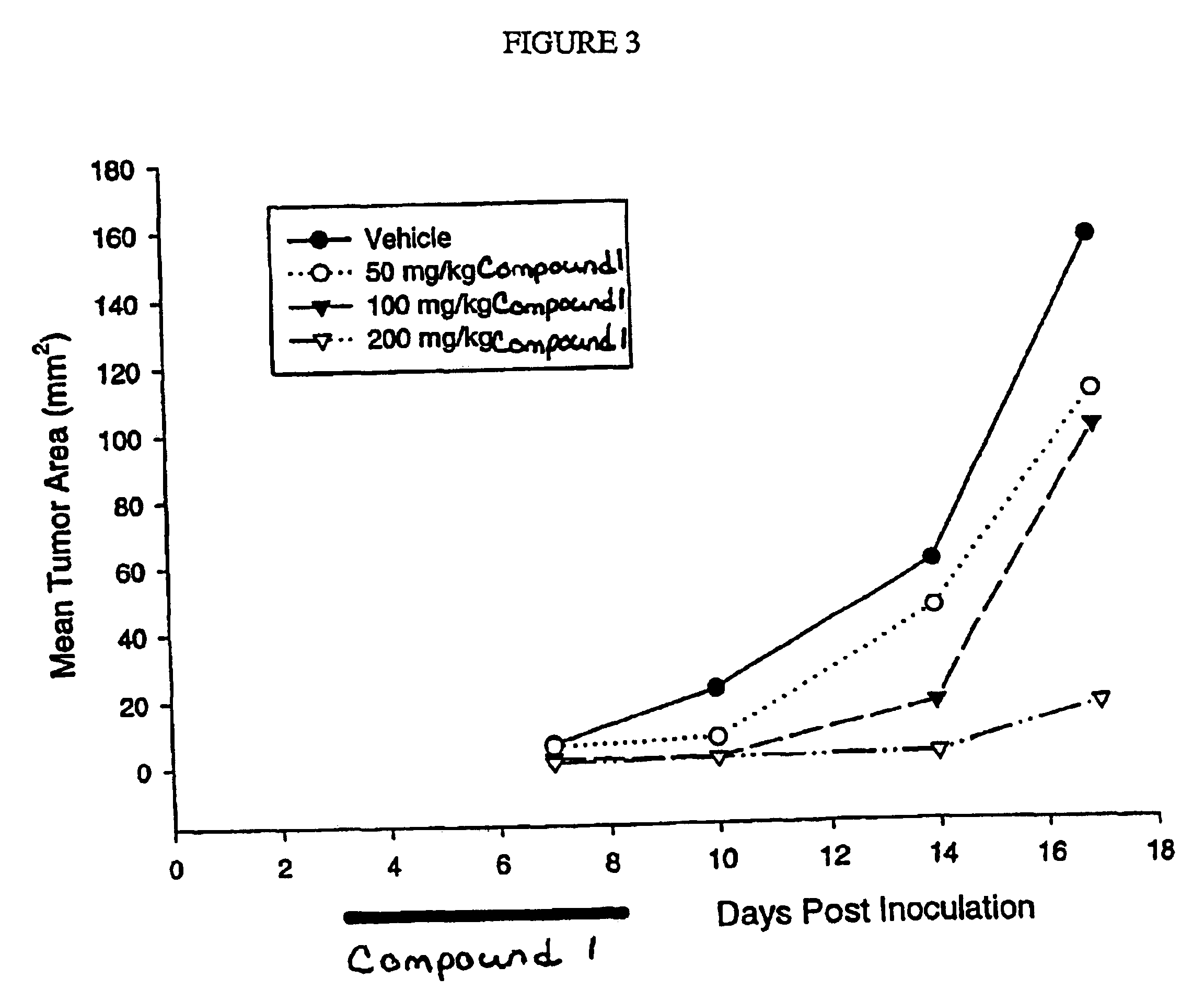
InactiveUS6838467B2Inhibit tumor growthSustained antitumor effectBiocideOrganic chemistryDosing regimenFarnesyl Transferase Inhibitor
This invention relates to a method of treatment and dosing regimen for treating mammalian tumors by the discontinuous administration of a farnesyl transferase inhibitor over an abbreviated one to five day dosing schedule.
Owner:JANSSEN PHARMA NV
Combination tablet with chewable outer layer
ActiveUS8404275B2Improve the level ofAbsorbed more rapidlyBiocideAnimal repellantsDosing regimenSide effect
A pharmaceutical composition in the form of a combination tablet is described. The tablet has a rapidly absorbed component that enters the circulation by traversing the buccal mucosa, oral mucosa and combinations thereof, and a more slowly absorbed component that is swallowed. The therapeutic agent in the swallowed portion is absorbed across the gastric mucosa. The combination tablet may be modified, by varying the specific combinations of excipients, fillers, and the like to effect distinct release rates. In addition, the rapid and slow components may have identical or different therapeutic agents depending on the application to a specific medical condition. One embodiment of the combination tablet includes a prostaglandin inhibitor in the rapidly absorbed component in order to mitigate the side effects of immediate release niacin that is in the slow absorbing component. Such combination compositions will increase patient compliance with various dosing regimens due to the resultant decrease in the number of tablets that a patient would need to take on a daily basis.
Owner:VITALS
Method for treating vasculitis
A method of treating anti-neutrophil cytoplasmic antibodies-associated vasculitis (ANCA-associated vasculitis) in a patient eligible for treatment is provided involving administering an antagonist that binds to a B-cell surface marker, such as CD20 antibody, to the patient in a dose of about 400 mg to 1.3 grams at a frequency of one to three doses within a period of about one month. Another method of treating ANCA-associated vasculitis in a subject eligible for treatment is provided involving administering an effective amount of an antibody that binds to a B-cell surface marker to the subject to provide an initial exposure and a subsequent exposure to the antibody within certain dosing regimens. Further provided are articles of manufacture useful for such methods.
Owner:GENENTECH INC
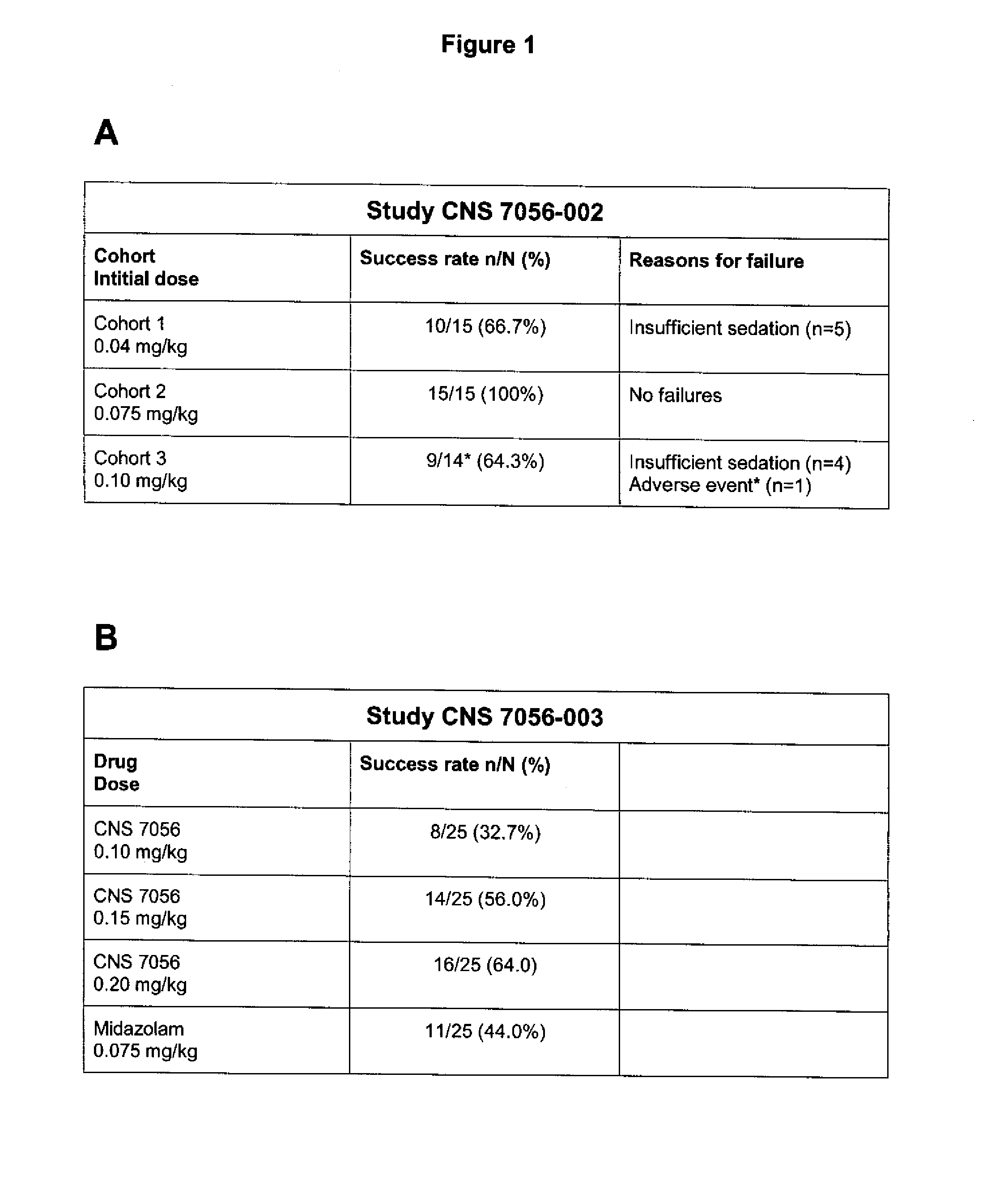
Dosing regimen for sedation with CNS 7056 (remimazolam)
ActiveUS20140080815A1Good sedative effectSafe and convenientBiocideNervous disorderDosing regimenRegimen
The invention relates to a dosing regimen for sedation with the fast-acting benzodiazepine CNS 7056 in combination with an opioid, in particular fentanyl, whereas CNS 7056 is given in a dose of 2 to 10 mg, preferably between 4 and 9 mg and most preferably between 5 and 8 mg.
Owner:PAION UK
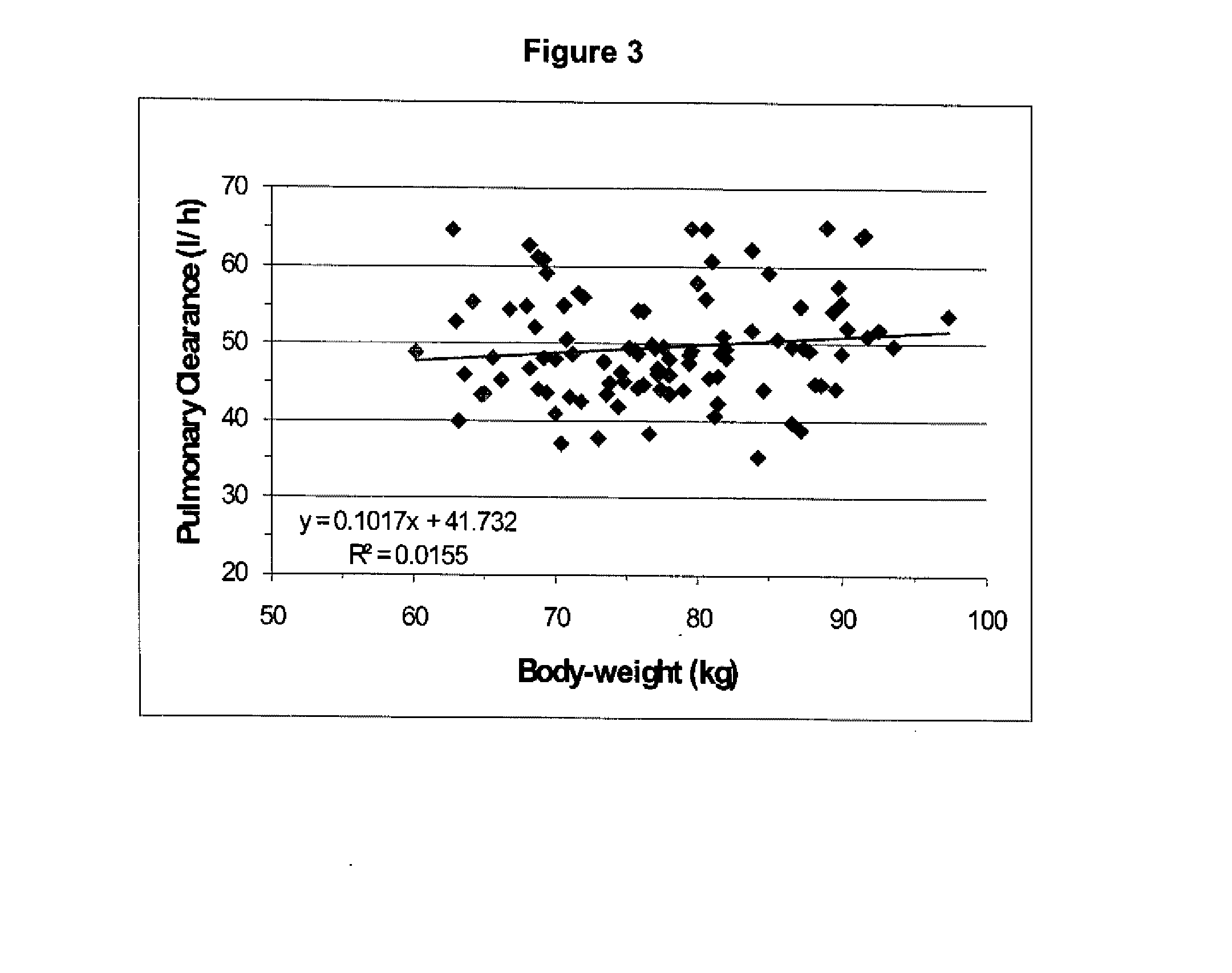
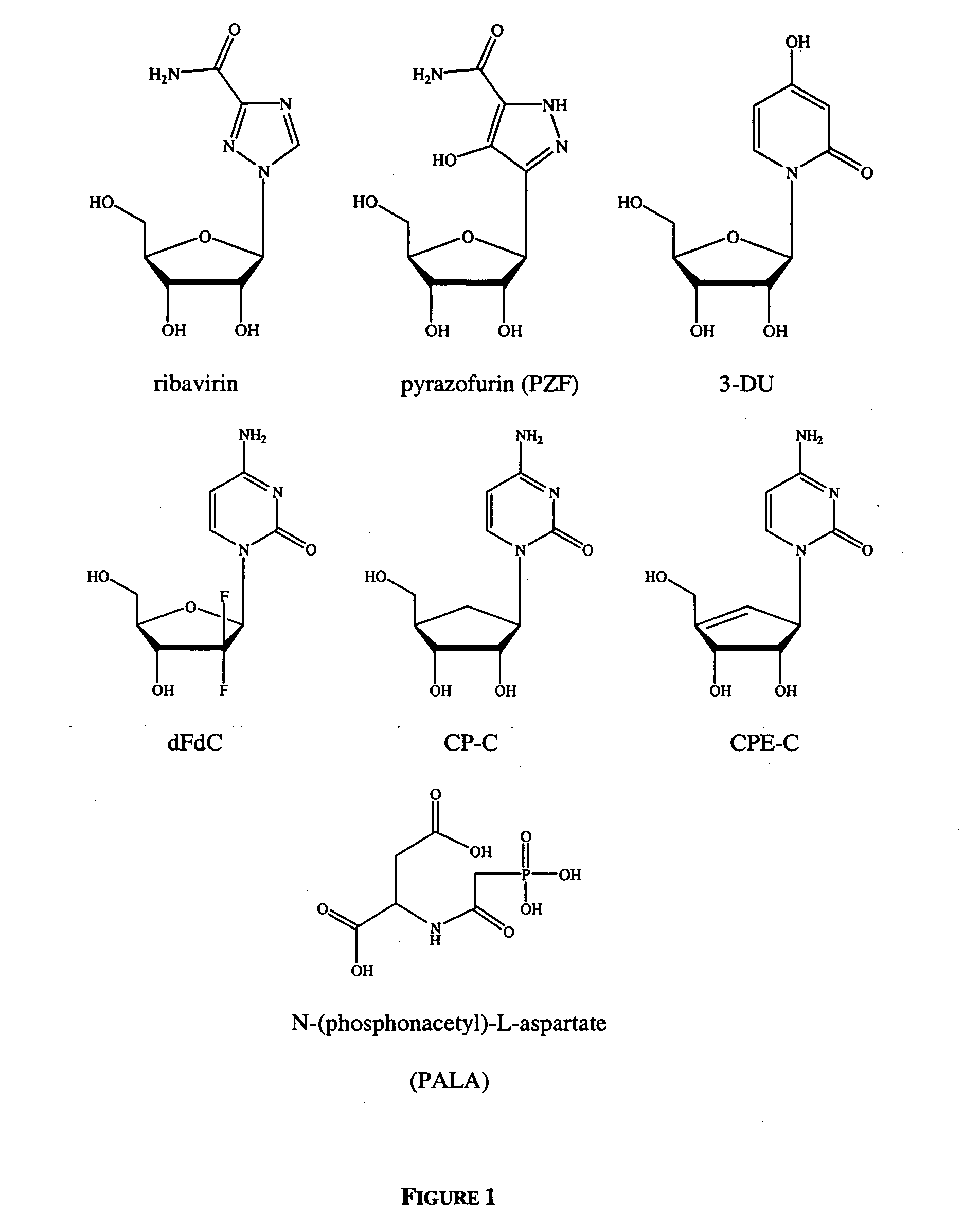
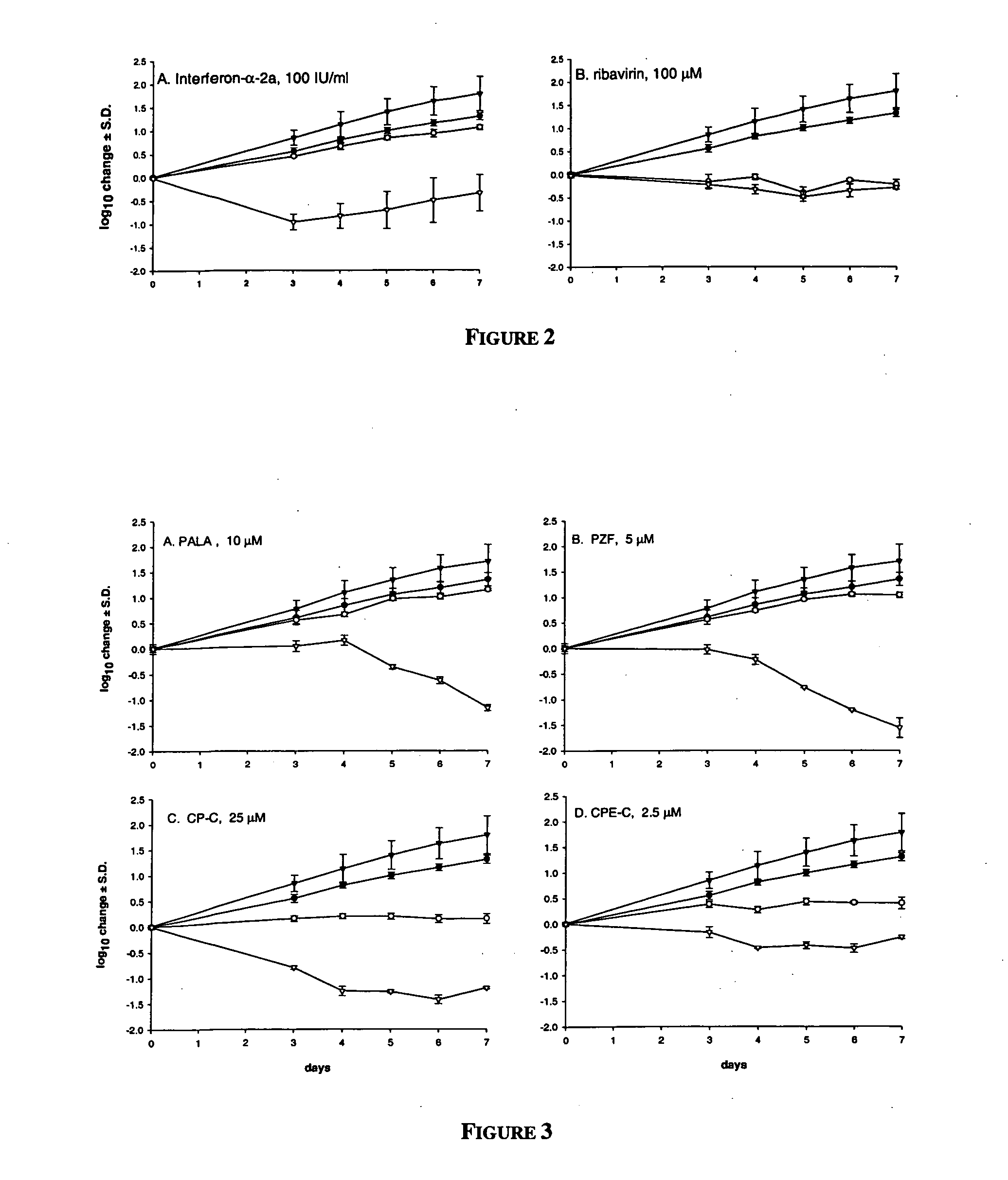
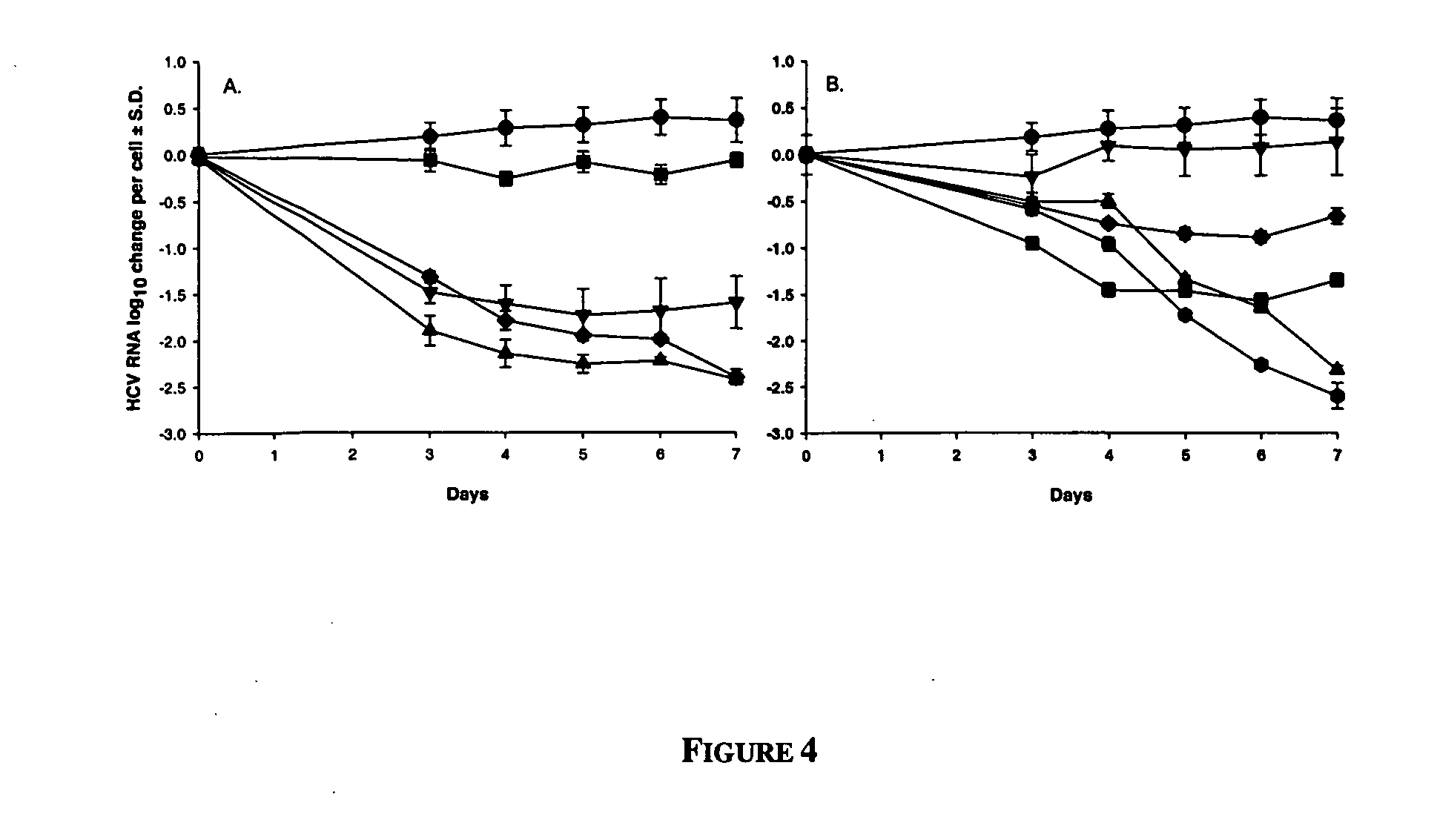
Dosing regimen for Flaviviridae therapy
An anti-hepatitis C agent which is an anti-metabolite to the host and cannot be administered on a daily or chronic basis as is usual in anti-viral therapy (referred to below as an “anti-HCV anti-metabolite”), can be administered using a traditional anti-cancer dosing regimen (for example via intravenous or parenteral injection), over a period of one, two, three, four, five, six, or seven days followed by cessation of therapy until rebound of the viral load is noted. This dosing regimen runs counter to conventional antiviral experience, wherein effective agents are usually administered over at least fourteen days of sustained therapy, and typically on an indefinite daily basis.
Owner:PHARMASSET
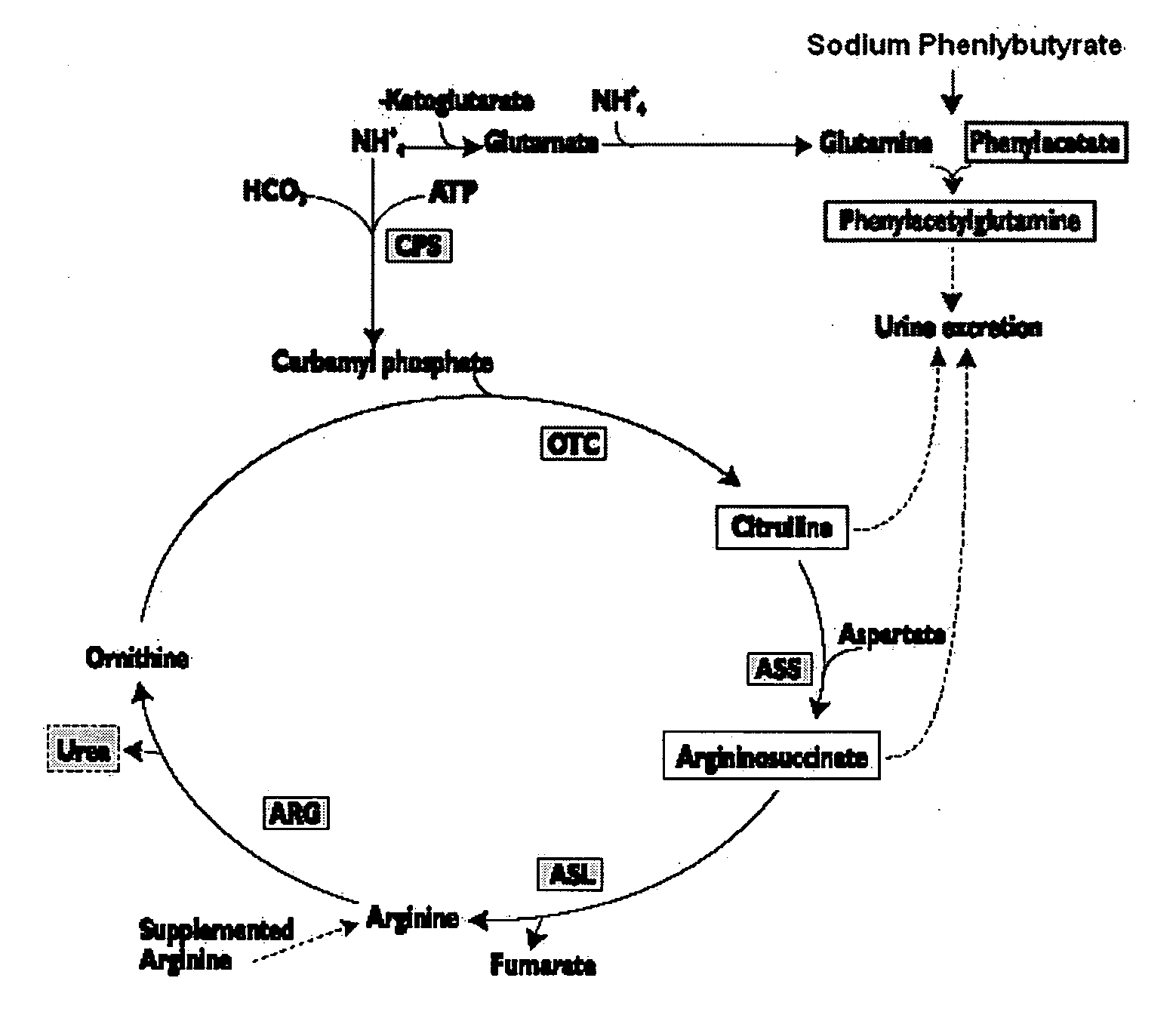
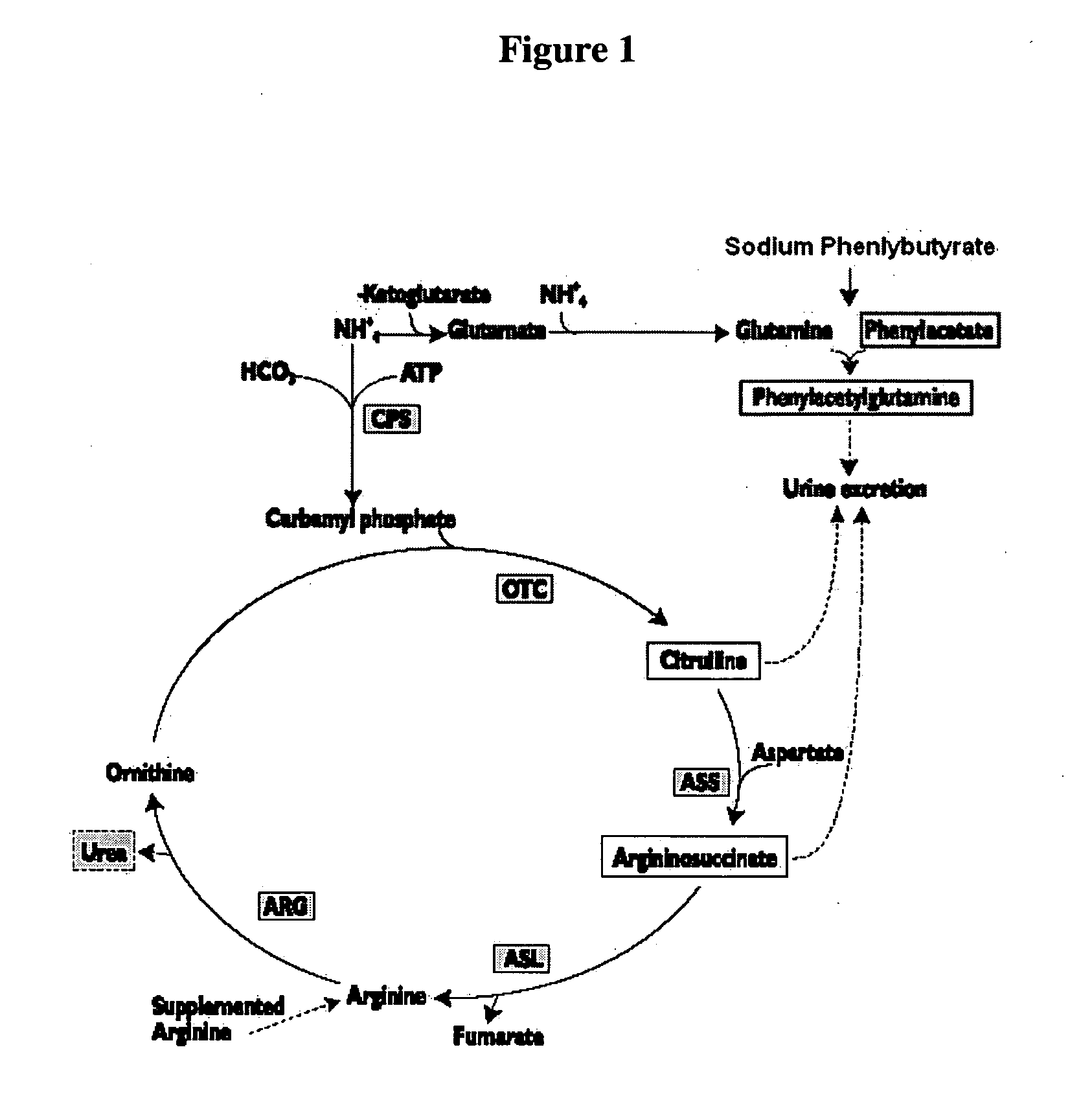
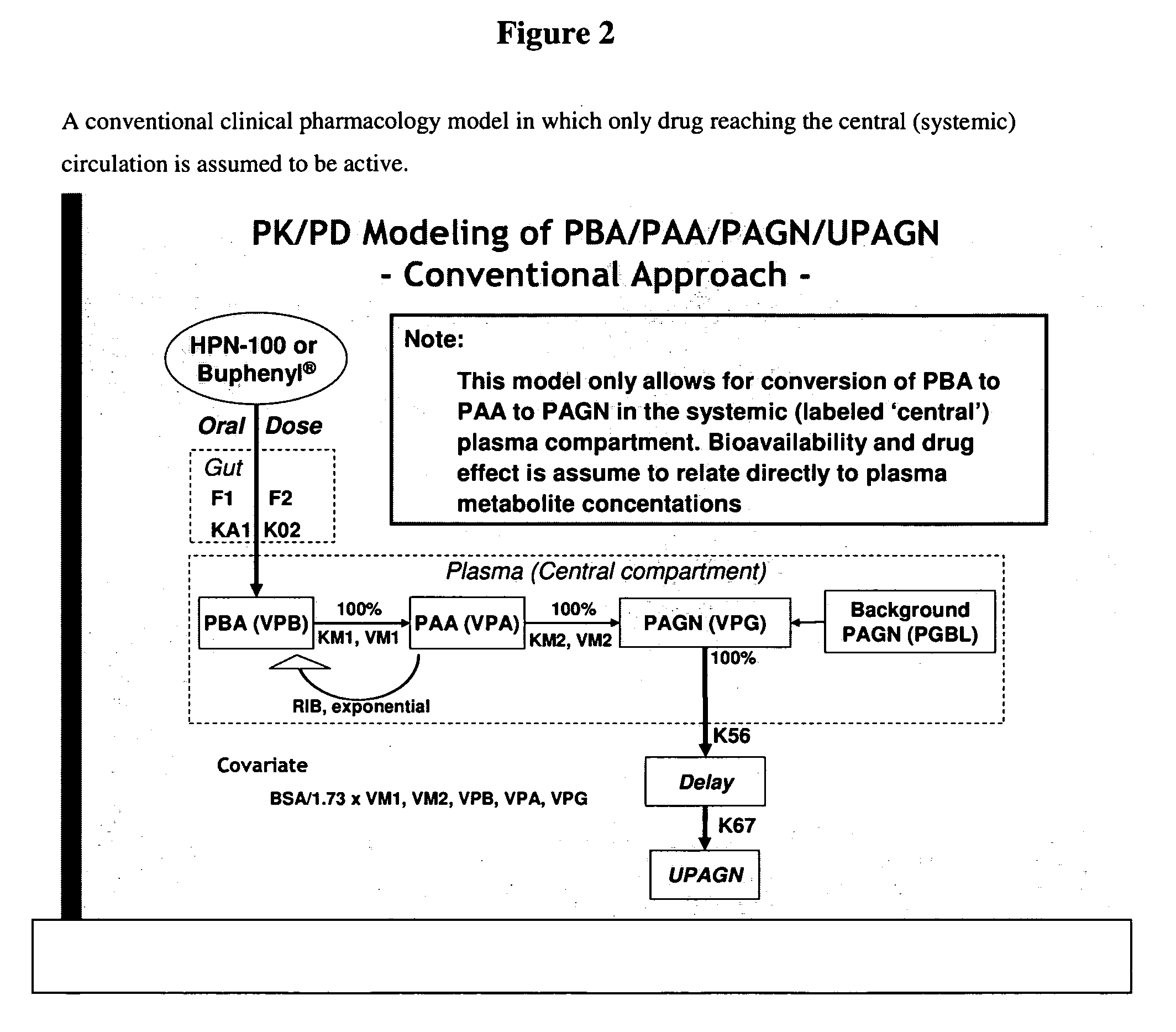
Methods of treatment using ammonia-scavenging drugs
The invention provides a method for determining a dose and schedule and making dose adjustments of PBA prodrugs used to treat nitrogen retention states, or ammonia accumulation disorders, by measuring urinary excretion of phenylacetylglutamine and / or total urinary nitrogen. The invention provides methods to select an appropriate dosage of a PBA prodrug based on the patient's dietary protein intake, or based on previous treatments administered to the patient. The methods are applicable to selecting or modifying a dosing regimen for a subject receiving an orally administered ammonia scavenging drug.
Owner:HORIZON THERAPEUTICS LLC
Therapeutic regimens for administering drug combinations
InactiveUS20050112199A1Good curative effectIncrease the length of timePowder deliveryOrganic active ingredientsDosing regimenRegimen
Owner:COMBINATORX
Methods for the Treatment of Autoimmune Disorders Using Immunosuppressive Monoclonal Antibodies with Reduced Toxicity
ActiveUS20080095766A1Reduce the possibilityIncreasing concentration of antibodySenses disorderNervous disorderDosing regimenInsulin dependent diabetes
The present invention provides methods of treating, preventing, slowing the progression of, or ameliorating the symptoms of T cell mediated immunological diseases, particularly autoimmune diseases (e.g., autoimmune diabetes (i.e. type 1 diabetes or insulin-dependent diabetes mellitus (IDDM)) and multiple sclerosis) through the use of anti-human CD3 antibodies. The antibodies of the invention of the invention are preferably used in low dose dosing regimens, chronic dosing regimens or regimens that involve redosing after a certain period of time. The methods of the invention provide for administration of antibodies that specifically bind the epsilon subunit within the human CD3 complex. Such antibodies modulate the T cell receptor / alloantigen interaction and, thus, regulate the T cell mediated cytotoxicity associated with autoimmune disorders. Additionally, the methods of the invention provide for use of anti-human CD3 antibodies modified such that they exhibit reduced or eliminated effector function and T cell activation as compared to non-modified anti-human CD3 antibodies.
Owner:PROVENTION BIO INC
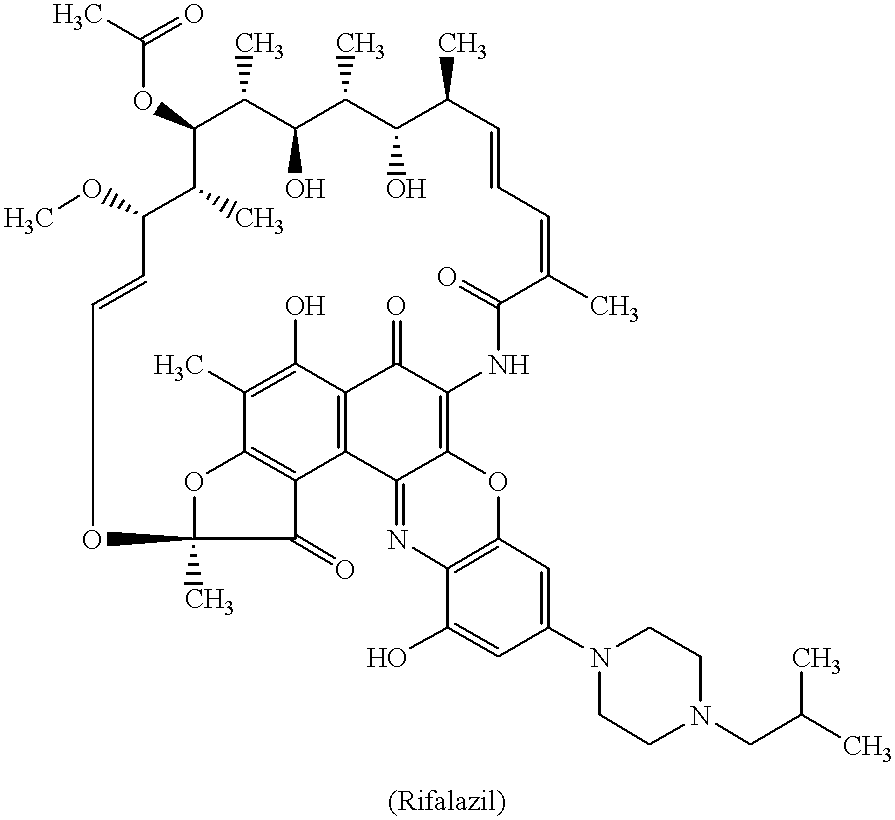
Method for treatment of bacterial infections with once or twice-weekly administered rifalazil
A method for treatment of bacterial infections with rifalazil administered once-weekly or twice-weekly. A method for treatment of tuberculosis caused by Mycobacterium tuberculosis, infections caused by Mycobacterium avium complex, infections caused by Chlamydia pneumoniae and infections caused by Helicobacter pylori by administering to a patient suffering from the bacterial infection 1-100 mg of rifalazil once or twice a week. In this dose regimen, the treatment is fast, efficacious and eliminates undesirable secondary symptoms observed with daily doses of 1-50 mg of rifalazil.
Owner:KANEKA CORP
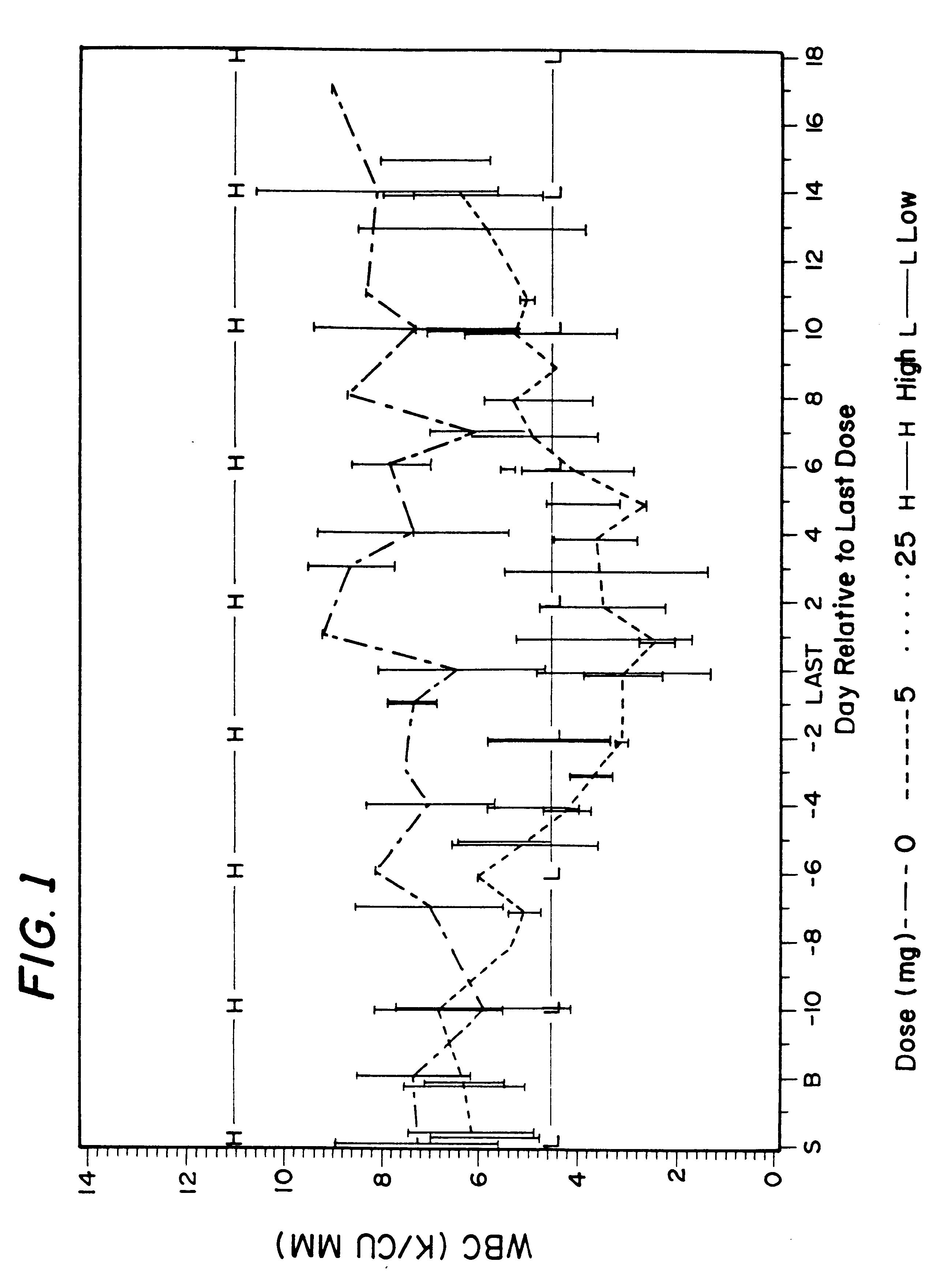
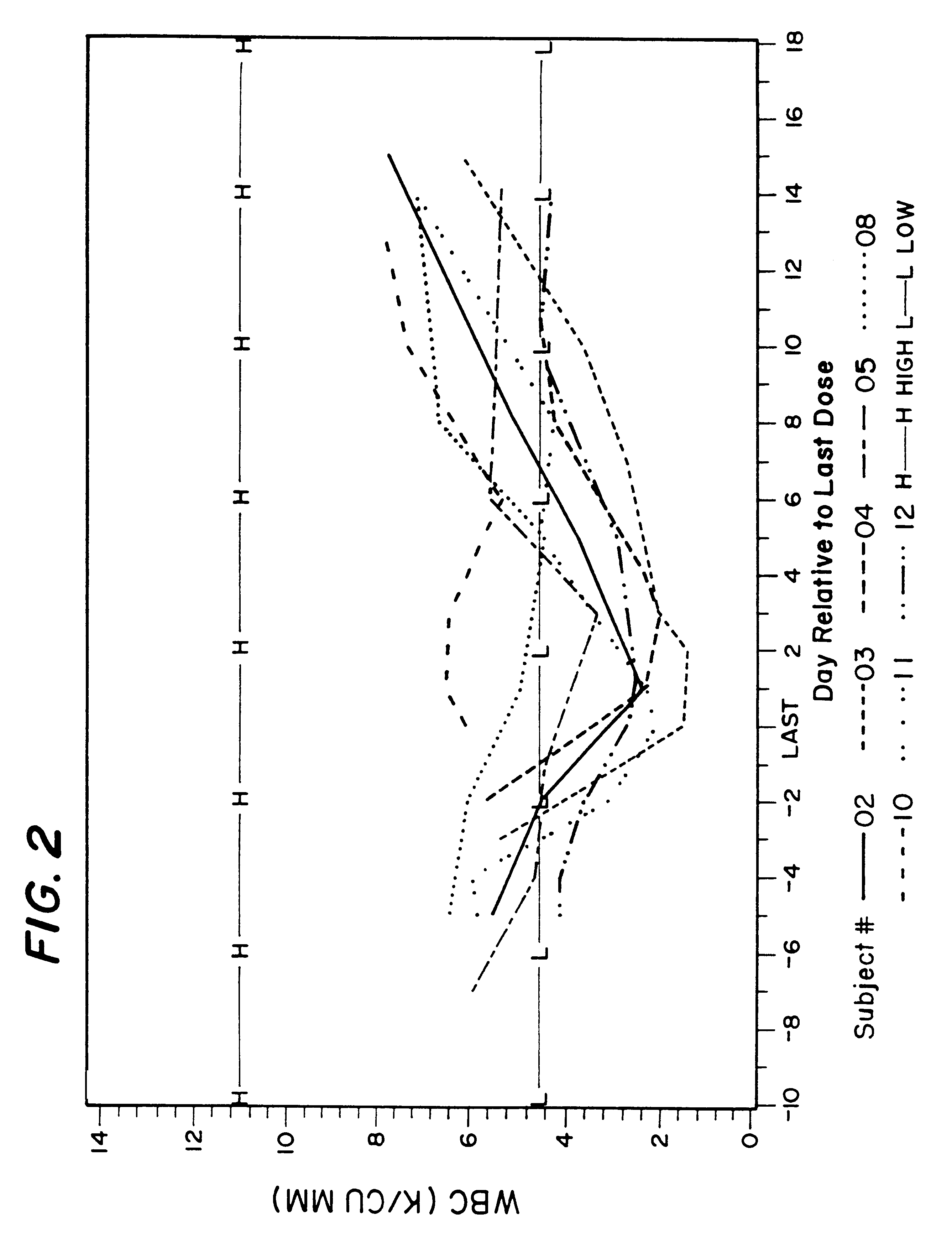
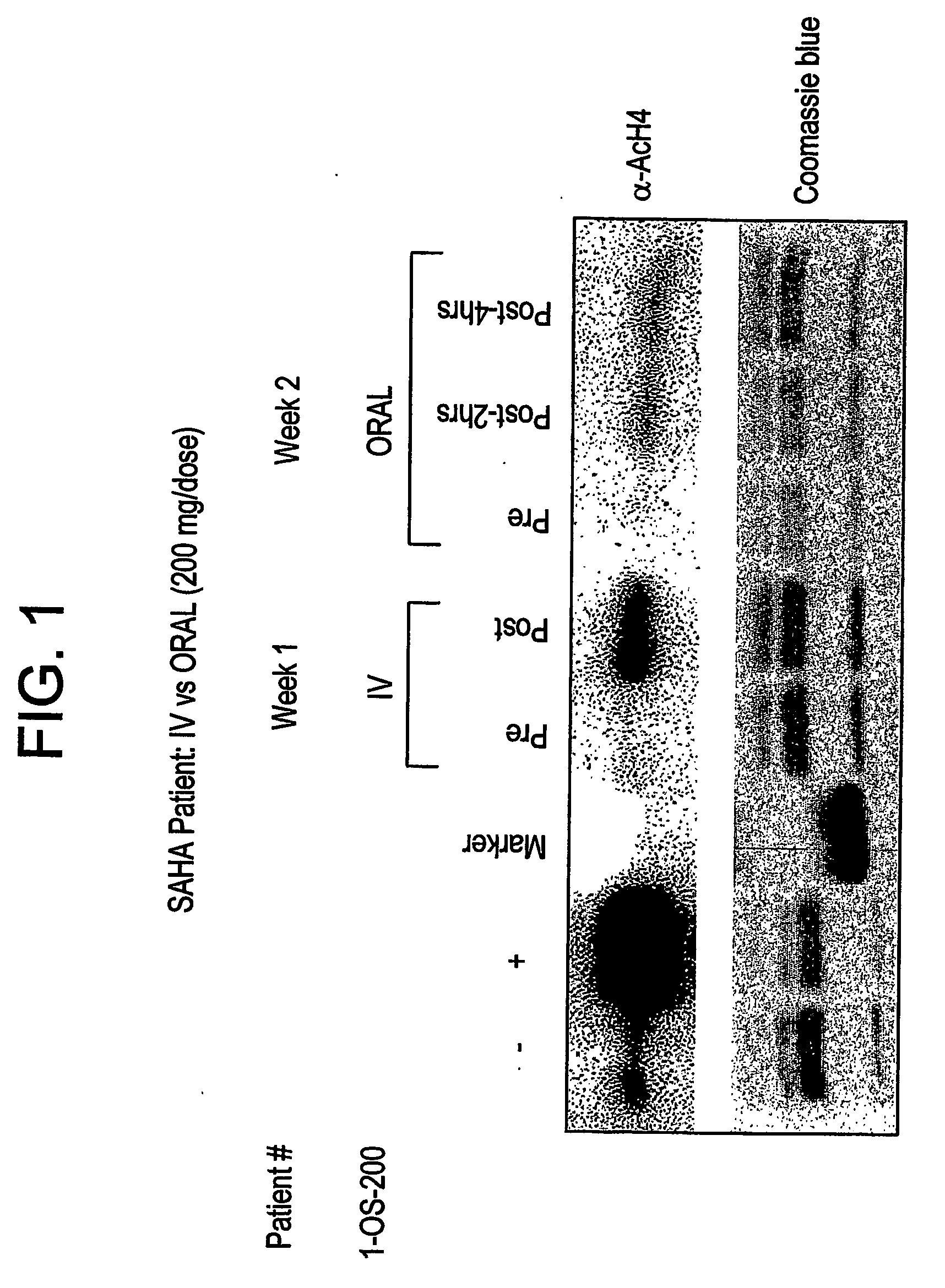
Methods of treating cancer with hdac inhibitors
InactiveUS20070060614A1Better pharmacokinetic profileImprove bioavailabilityBiocideAnimal repellantsDosing regimenOncology
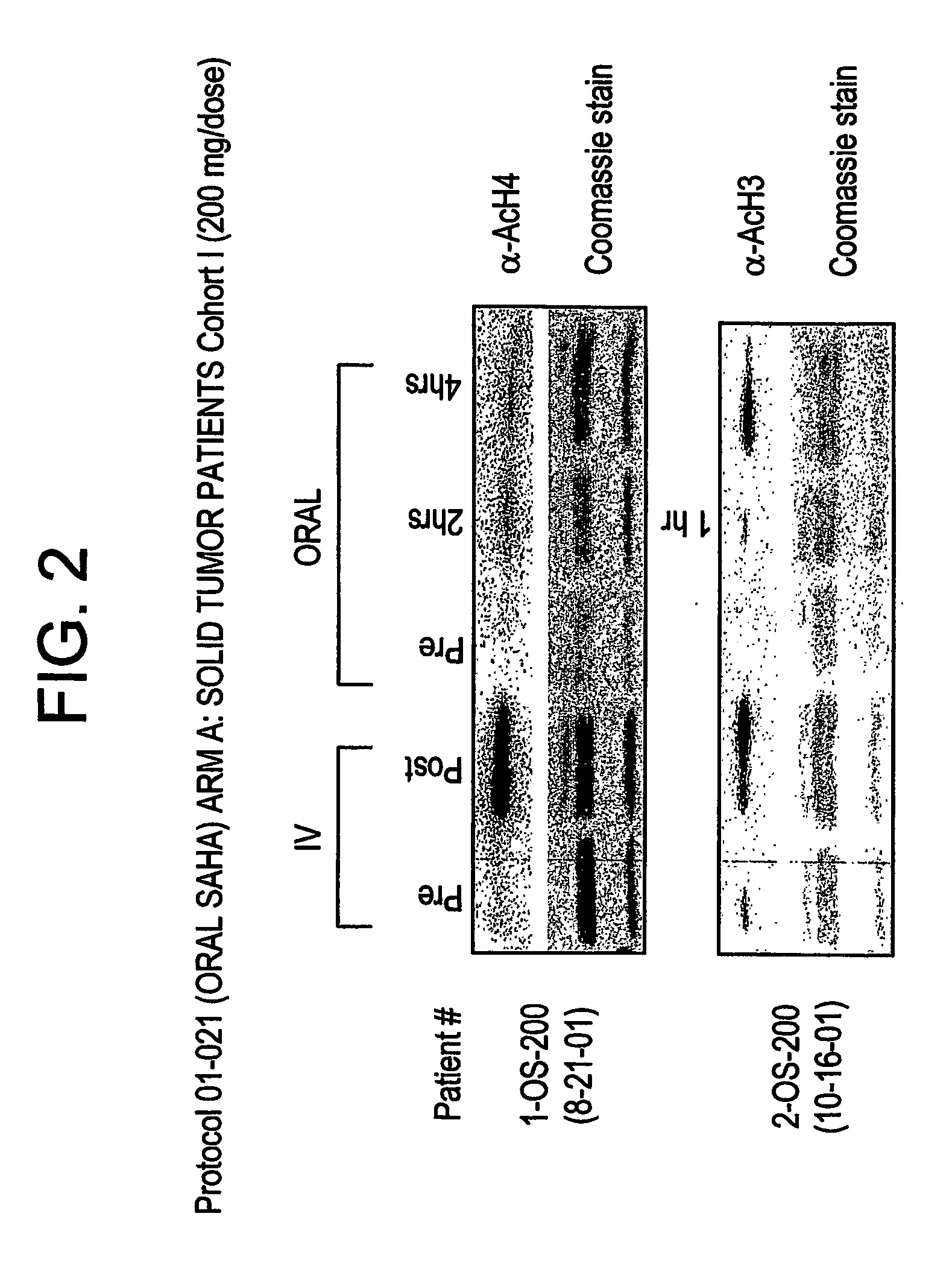
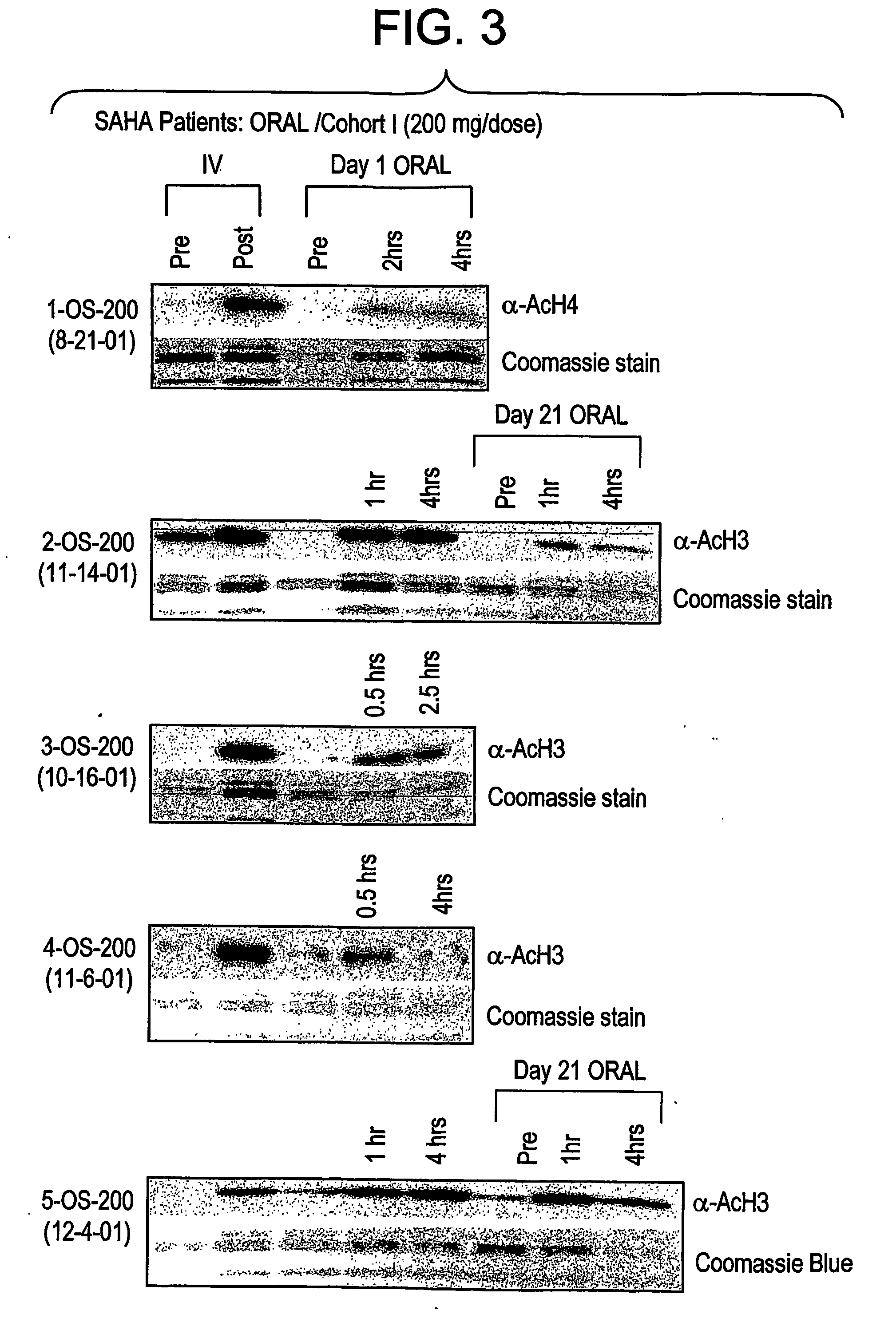
The present invention relates to methods of treating cancers, e.g., mesothelioma or lymphoma. More specifically, the present invention relates to methods of treating mesothelioma or diffuse large B-cell lymphoma (DLBCL), by administration of pharmaceutical compositions comprising HDAC inhibitors, e.g., suberoylanilide hydroxamic acid (SAHA). The oral formulations of the pharmaceutical compositions have favorable pharmacokinetic profiles such as high bioavailability and surprisingly give rise to high blood levels of the active compounds over an extended period of time. The present invention further provides a safe, daily dosing regimen of these pharmaceutical compositions, which is easy to follow, and which results in a therapeutically effective amount of the HDAC inhibitors in vivo.
Owner:MERCK HDAC RESEARCH LLC +1
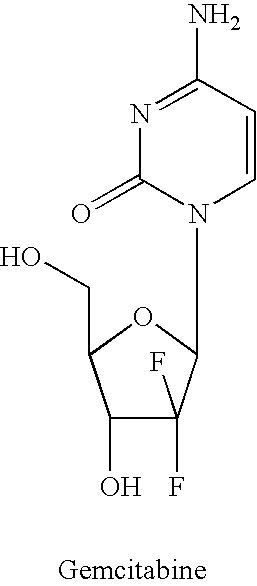
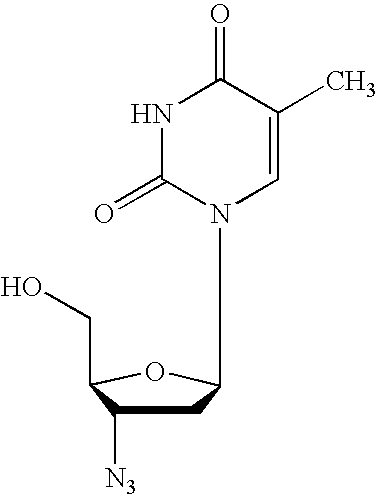
Dosing regimen for gemcitabine HCV therapy
InactiveUS20030225029A1Reduce viral loadRapid and large in viral loadBiocideSugar derivativesDosing regimenHepatitis c viral
A dosage regiment for the treatment of a Flaviviridae infection, including a hepatitis C viral infection, that includes administering gemcitabine (or its salt, prodrug or derivative, as described herein) in a dosage range of approximately 50 mg / m<2 >to about 1300 mg / m<2 >per day for between one and seven days (e.g. 1, 2, 3, 4, 5, 6, or 7 days) followed by cessation of therapy. Viral load is optionally monitored over time, and after cessation, viral rebound is monitored. Therapy is not resumed unless a significant viral load is again observed, and then therapy for 1-7 days and more preferred, 1, 2 or 3 days, is repeated. This therapy can be continued indefinitely to monitor and maintain the health of the patient.
Owner:PHARMASSET
Dosing regimen
Methods and kits are provided enabling a twice daily dosing regimen that achieves daily patient blood levels of active pharmaceutical ingredient comparable to a dosing regimen requiring the same active ingredient to be administered three times a day.
Owner:ELAN PHRMA INT LTD
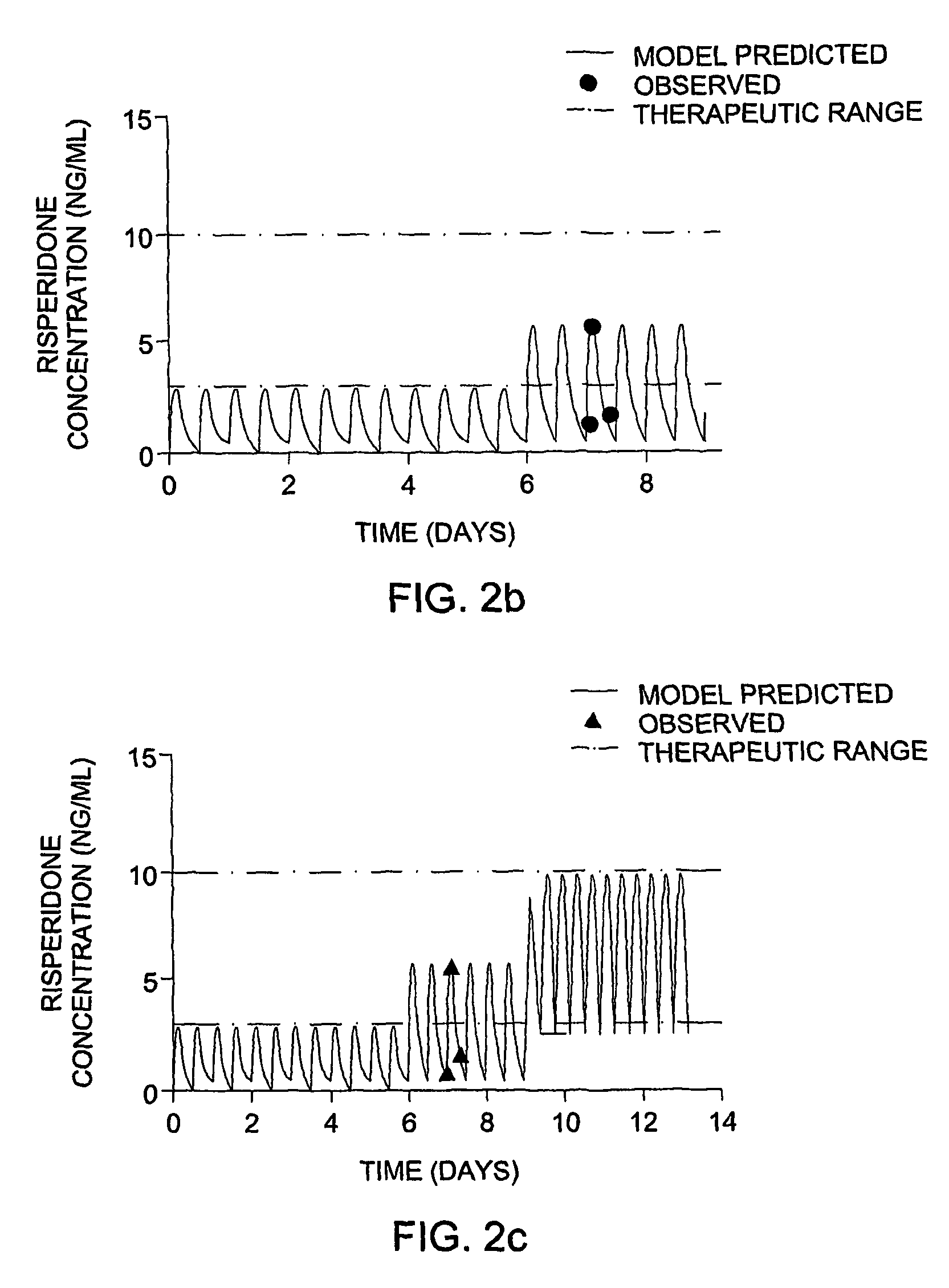
Methods and dosage forms for controlled delivery of paliperidone and risperidone
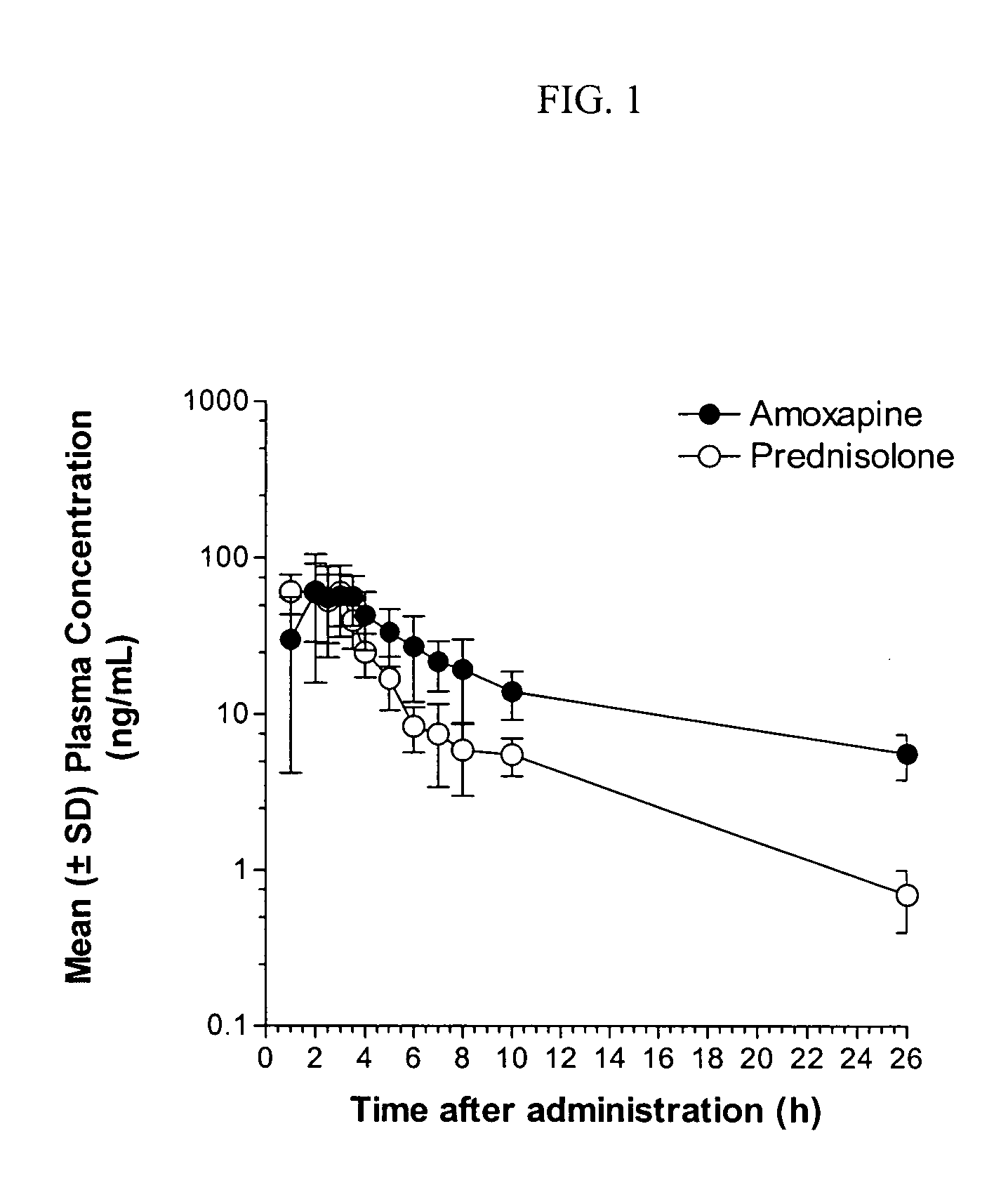
Dosage forms and methods for providing a substantially ascending rate of release of paliperidone or risperidone are provided. The sustained release dosage forms provide therapeutically effective average steady-state plasma paliperidone or risperidone concentrations when administered once per day. This once-a-day dosing regimen results in only one peak plasma paliperidone or risperidone concentration occurrence in each 24 hour period. In addition, the peak plasma paliperidone or risperidone concentration occurs at a later time following dose administration and exhibits a lesser magnitude than the peak plasma paliperidone or risperidone concentration that occurs following administration of paliperidone or risperidone in an immediate-release dosage form.
Owner:ALZA CORP
Methods of therapy for cancers characterized by overexpression of the HER2 receptor protein
InactiveUS7306801B2Promotes therapeutic responseImproved therapeutic responsePowder deliveryPeptide/protein ingredientsDosing regimenAnti her2
Methods for treating a subject with a cancer that is characterized by overexpression of HER2 receptor protein using a combination of interleukin-2 (IL-2) or variant thereof and at least one anti-HER2 antibody or fragment thereof are provided. These anti-tumor agents are administered as two separate pharmaceutical compositions, one containing IL-2 (or variant thereof), the other containing at least one anti-HER2 antibody (or fragment thereof), according to a dosing regimen. Administering of these two agents together potentiates the effectiveness of the anti-HER2 antibody alone, resulting in a positive therapeutic response that is improved with respect to that observed with this anti-tumor agent.
Owner:HEALTH RES INC +1
Antibodies to il-6 and use thereof
ActiveUS20130034554A1Reduce riskIncrease apoptosisCompounds screening/testingAntipyreticEGFR inhibitorsAnti-coagulants
Owner:VITAERIS INC +1
Transdermal delivery
ActiveUS20120021041A1Reduce riskPreventing pregnancyOrganic active ingredientsBiocideDosing regimenGynecology
Dosing regimen for transdermal delivery of hormones comprising a 28 day treatment cycle with a fixed treatment interval and a fixed rest interval.
Owner:AGILE THERAPEUTICS
Optimization and individualization of medication selection and dosing
ActiveUS8589175B2Easy to understandEasy to recommendationDrug and medicationsBiostatisticsPersonalizationDosing regimen
The invention provides population models, methods, and algorithms for targeting a dosing regimen or compound selection to an individual patient. The methods and algorithms of the invention utilize population models that incorporate genotype information for genes encoding drug metabolizing enzymes for one or more compounds of interest. The methods allow integration of genotype information for one or more genes encoding a drug metabolizing enzyme, particularly a cytochrome P450 gene with patient data. The methods allow integration of genotype information and the effect of one or more compounds on one or more drug metabolizing enzymes. The methods allow iterative feedback of drug metabolizing data obtained from a patient into the process of generating a dosage regimen recommendation for a compound of interest for an individual patient.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com