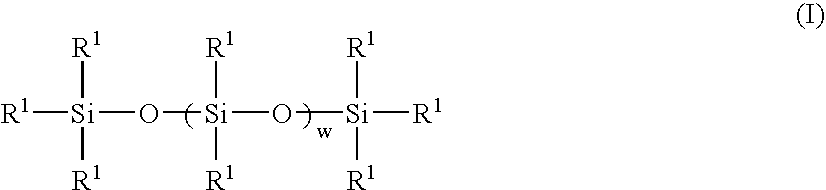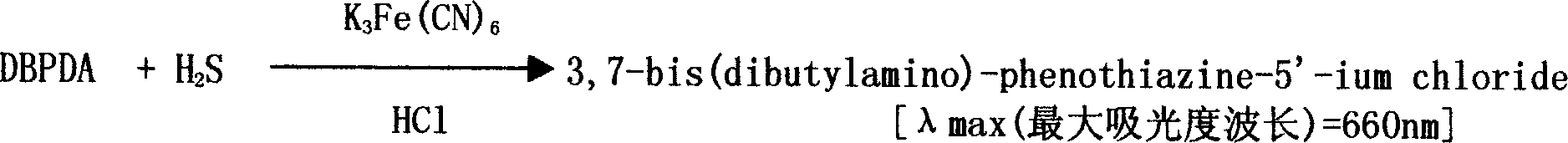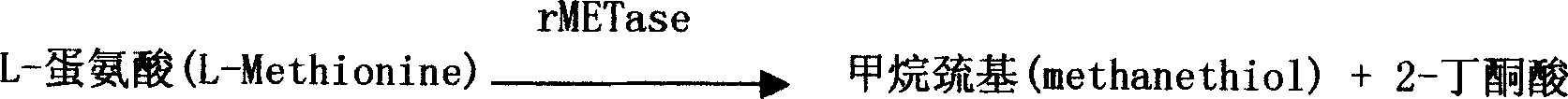Patents
Literature
17701 results about "Ketone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
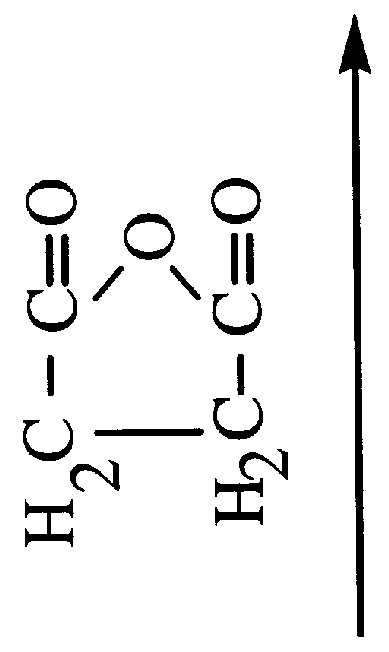
In chemistry, a ketone /ˈkiːtoʊn/ is a functional group with the structure RC(=O)R', where R and R' can be a variety of carbon-containing substituents. Ketones and aldehydes are simple compounds that contain a carbonyl group (a carbon-oxygen double bond). They are considered "simple" because they do not have reactive groups like −OH or −Cl attached directly to the carbon atom in the carbonyl group, as in carboxylic acids containing −COOH. Many ketones are known and many are of great importance in industry and in biology. Examples include many sugars (ketoses) and the industrial solvent acetone, which is the smallest ketone.
Process for producing oxime
InactiveUS7161036B2Speed up the conversion processHigh selectivityOrganic compound preparationOrganic chemistry methodsKetoneAmmonia
A process for producing an oxime is provided, wherein the process comprises the step of reacting a ketone, hydrogen peroxide and ammonia in the presence of a crystalline titanosilicate having MWW structure under the condition that the ammonia concentration in the liquid portion of the reaction mixture is about 1% by weight or more. By the process, an ammoximation reaction of the ketone can be carried out with a high conversion of the ketone and a high selectivity to the oxime corresponding to the ketone, thereby producing the oxime with a high yield.
Owner:SUMITOMO CHEM CO LTD
Method of treating androgen independent prostate cancer
The present invention is directed to a method treating prostate cancer. The method comprises administering to a patient in need thereof at least one compound selected from N-methyl-Δ3,3′-dihydroindole-2,2′ diketone; N-1-(β-D-O-triacetyl-xylopranosyl)-Δ3,3′-dihydroindole-2,2′ diketone; and N-1-(β-D-O-triacetyl-xylopranosyl)-N′-methyl-Δ3,3′-dihydroindole-2,2′ diketone. Preferably the compound is in an amount sufficient to inhibit growth, invasion, and / or metastasis of prostate cancer cells.
Owner:NATROGEN THERAPEUTICS INT
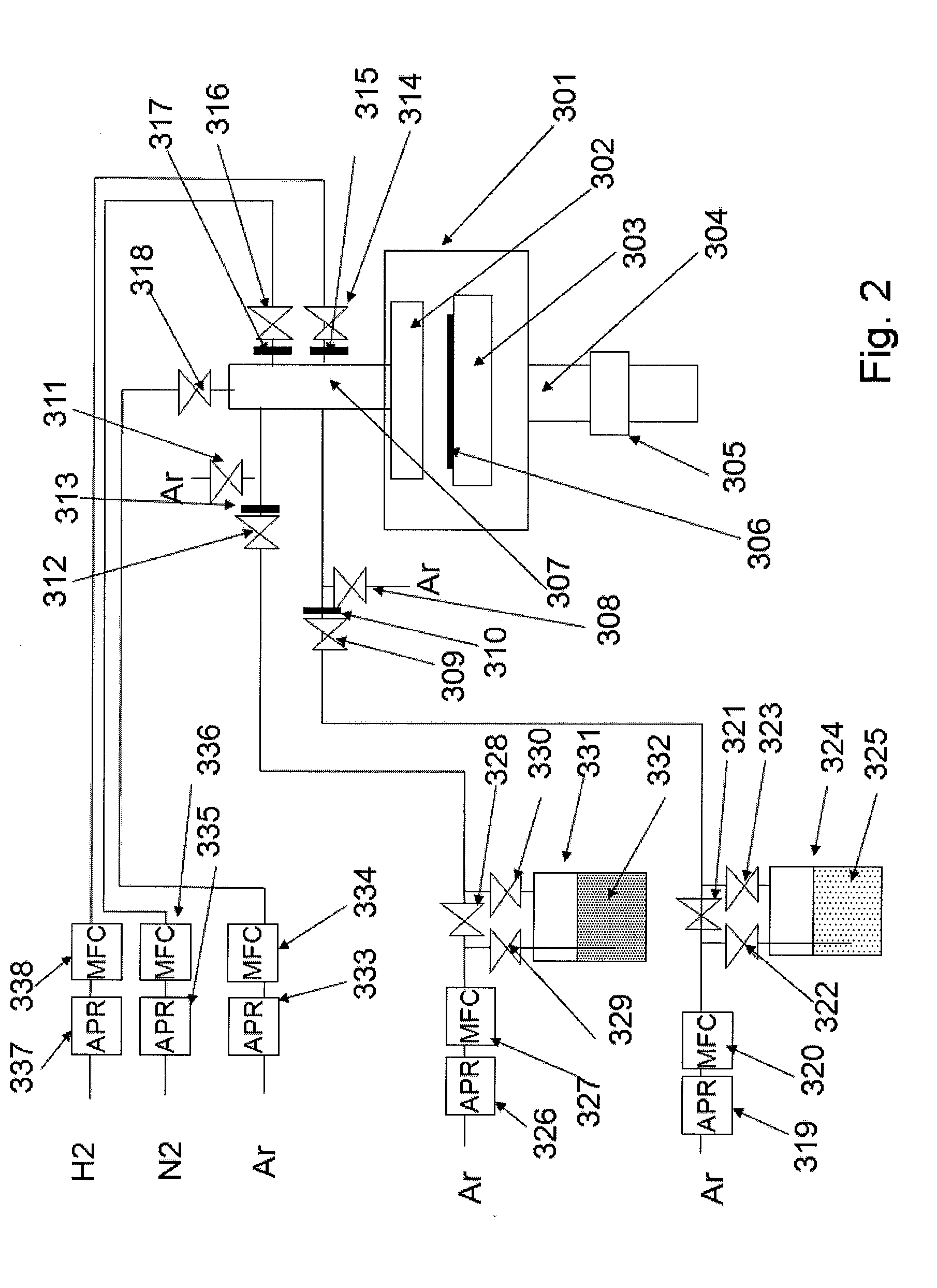
Precursor source mixtures
A precursor source mixture useful for CVD or ALD of a film comprising: at least one precursor composed of an element selected from the group consisting of Li, Na, K, Rb, Cs, Fr, Be, Mg, Ti, Zr, Hf, Sc, Y, La, V, Nb, Ta, Cr, Mo, W, Mn, Re, Fe, Ru, Os, Co, Rh, Ir, Ni, Pd, Pt, Cu, Ag, Au, Zn, Cd, Hg, B, Al, Ga, In, Tl, Si, Ge, Sn, Pb, As, P, Sb and Bi, to which is bound at least one ligand selected from the group consisting of hydride, alkyl, alkenyl, cycloalkenyl, aryl, alkyne, carbonyl, amido, imido, hydrazido, phosphido, nitrosyl, nitryl, nitrate, nitrile, halide, azide, alkoxy, siloxy, silyl, and halogenated, sulfonated or silyated derivatives thereof, which is dissolved, emulsified or suspended in an inert liquid selected from the group consisting of aliphatic hydrocarbons, aromatic hydrocarbons, alcohols, ethers, aldehydes, ketones, acids, phenols, esters, amines, alkylnitrile, halogenated hydrocarbons, silyated hydrocarbons, thioethers, amines, cyanates, isocyanates, thiocyanates, silicone oils, nitroalkyl, alkylnitrate, and mixtures thereof. The precursor source mixture may be a solution, emulsion or suspension and may consist of a mixture of solid, liquid and gas phases which are distributed throughout the mixture.
Owner:GLOBALFOUNDRIES INC
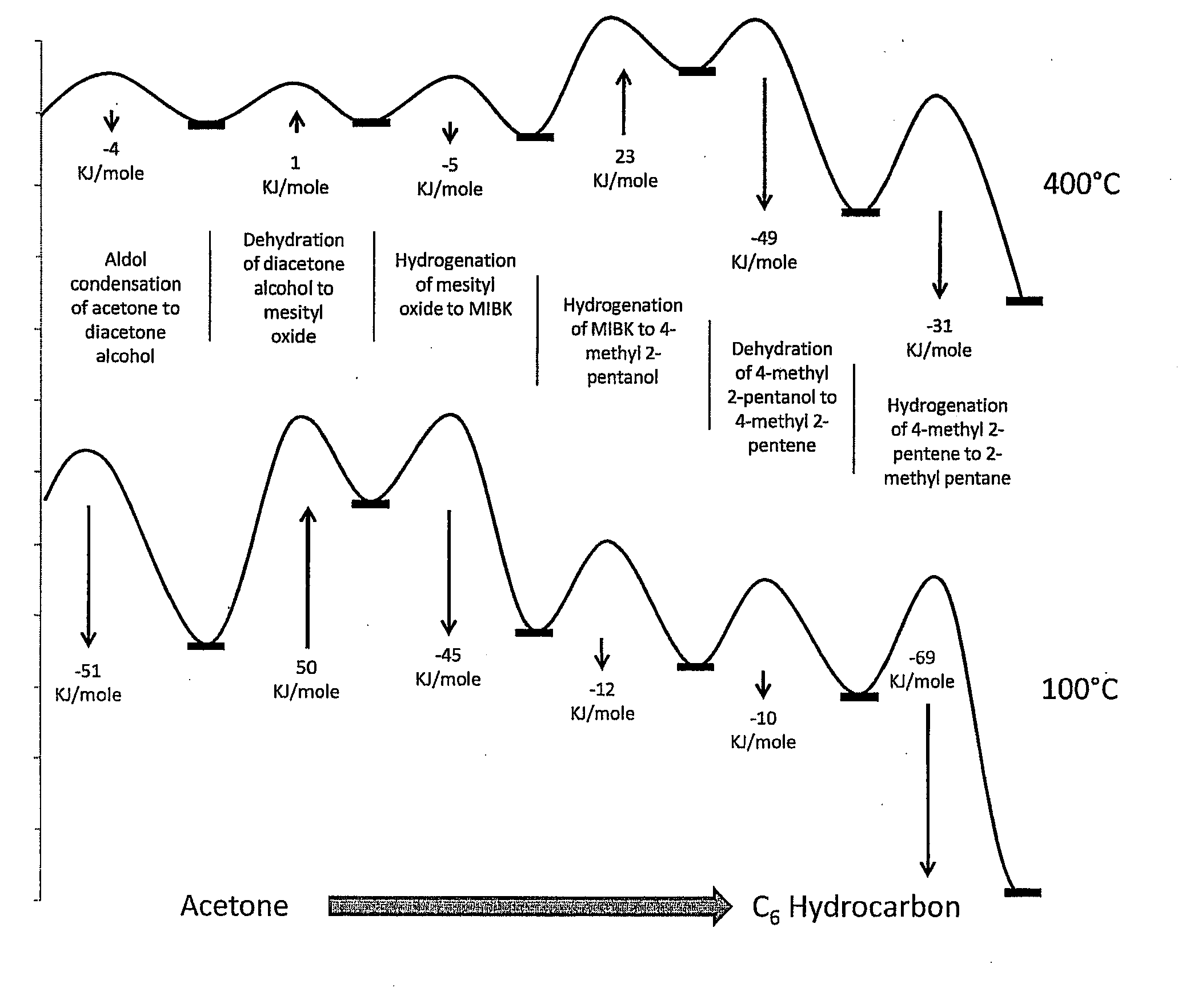
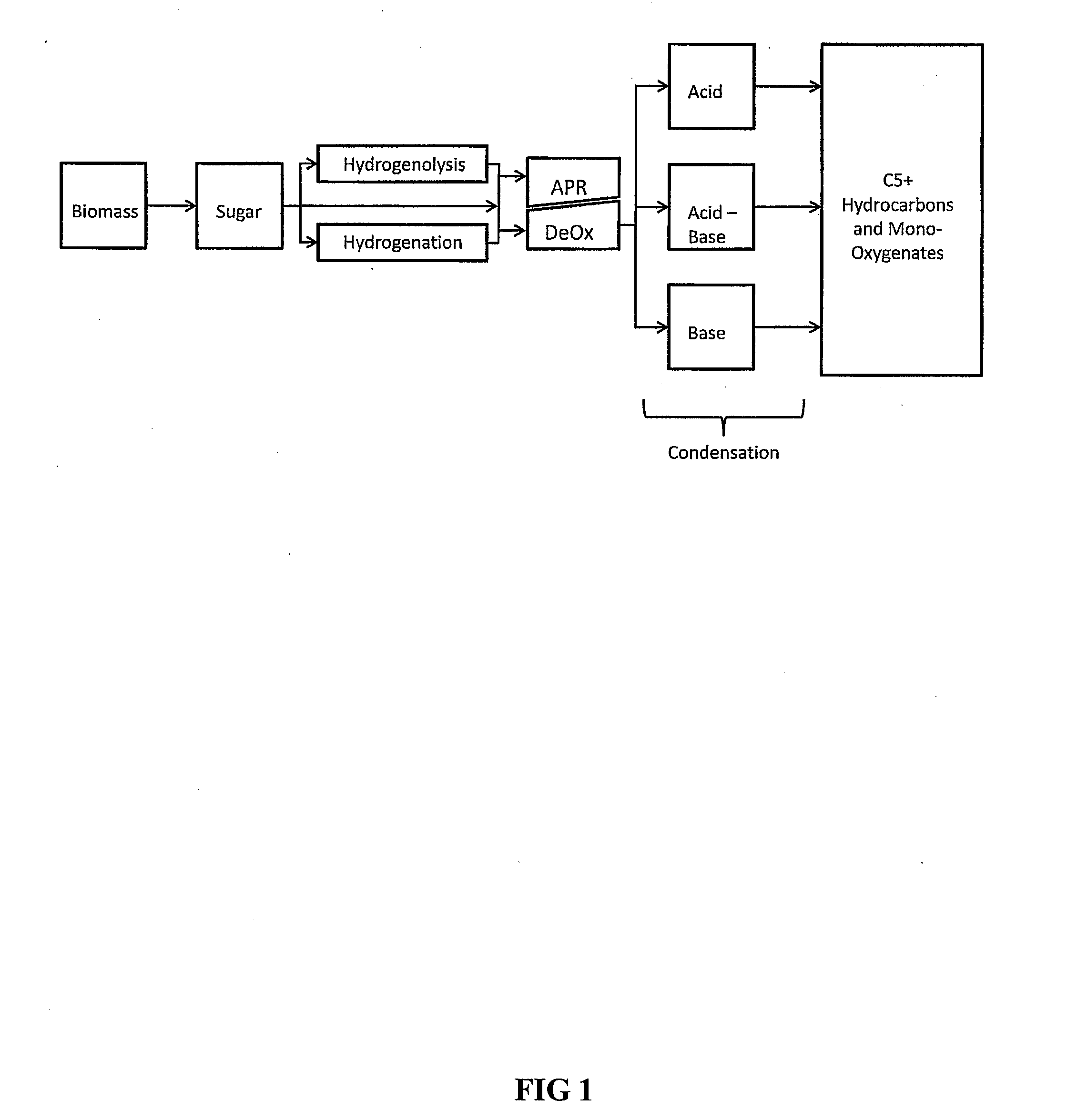
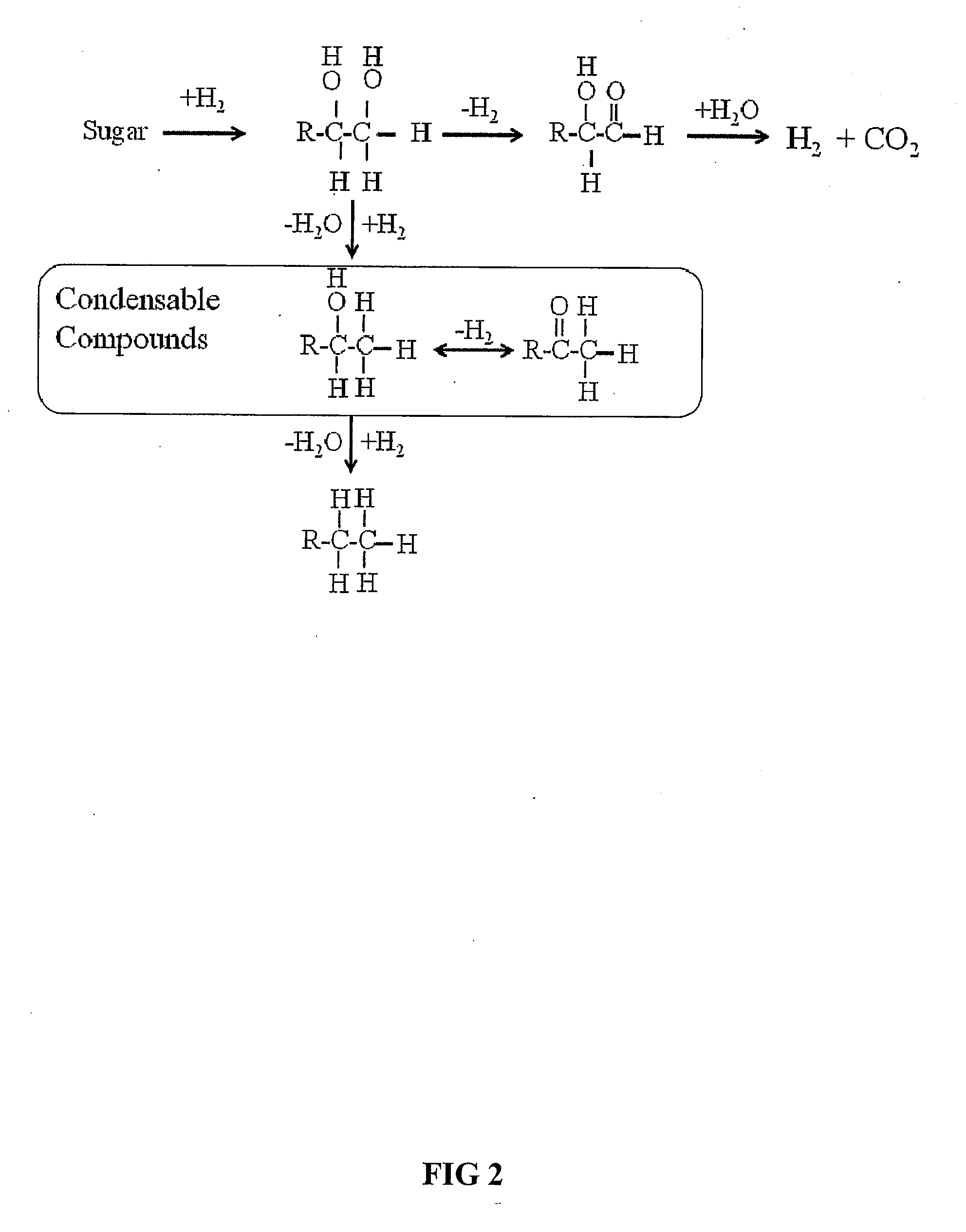
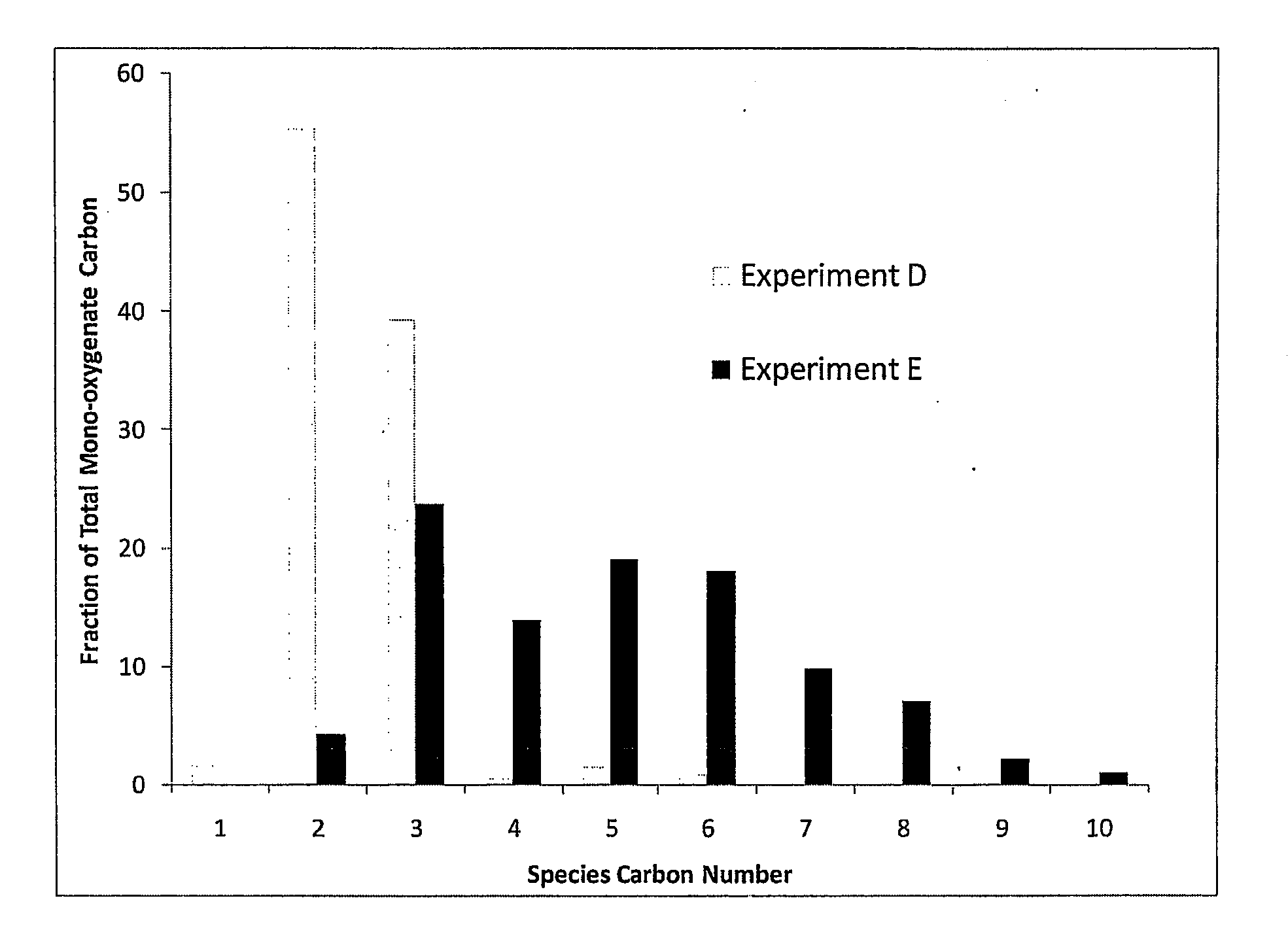
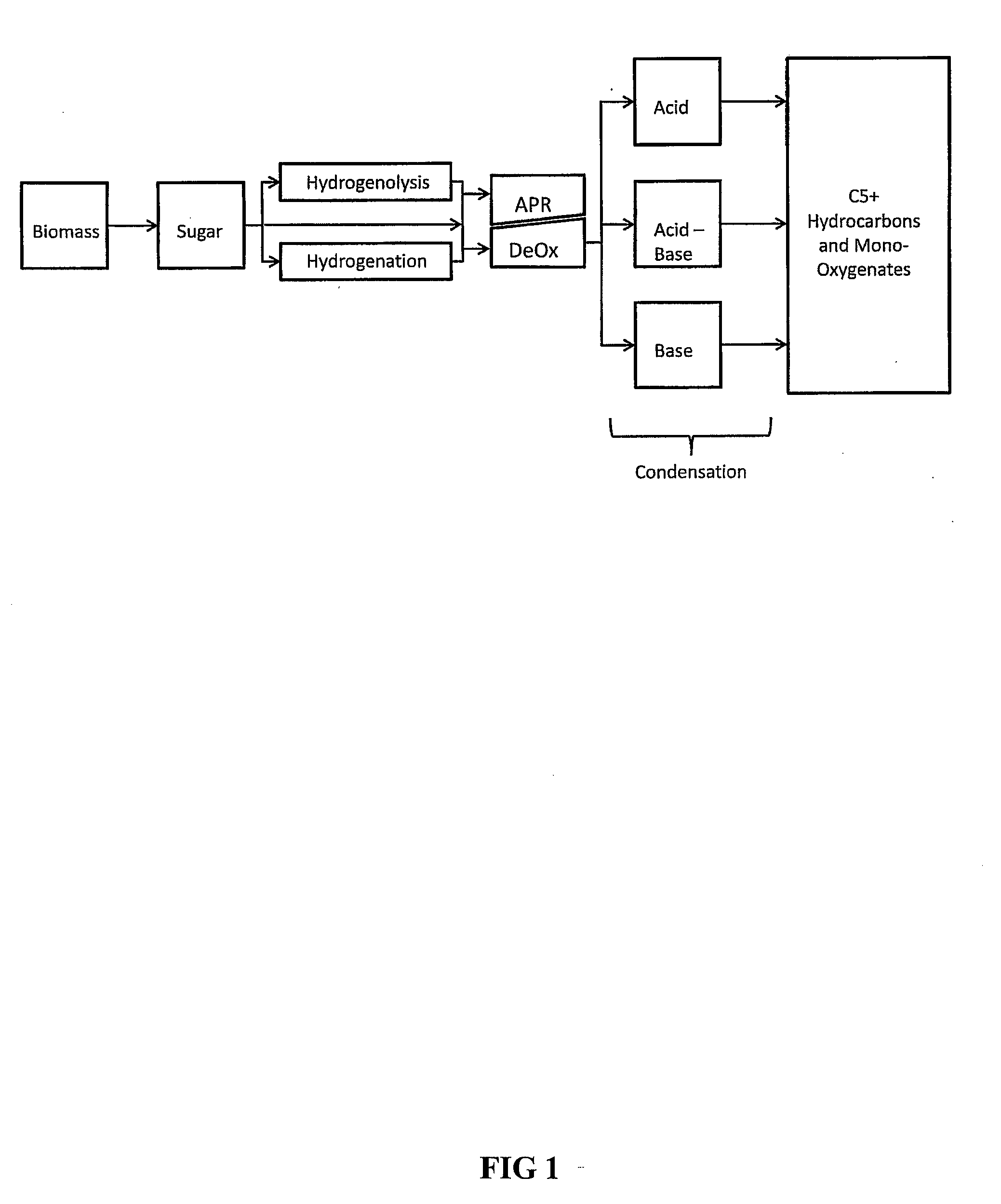
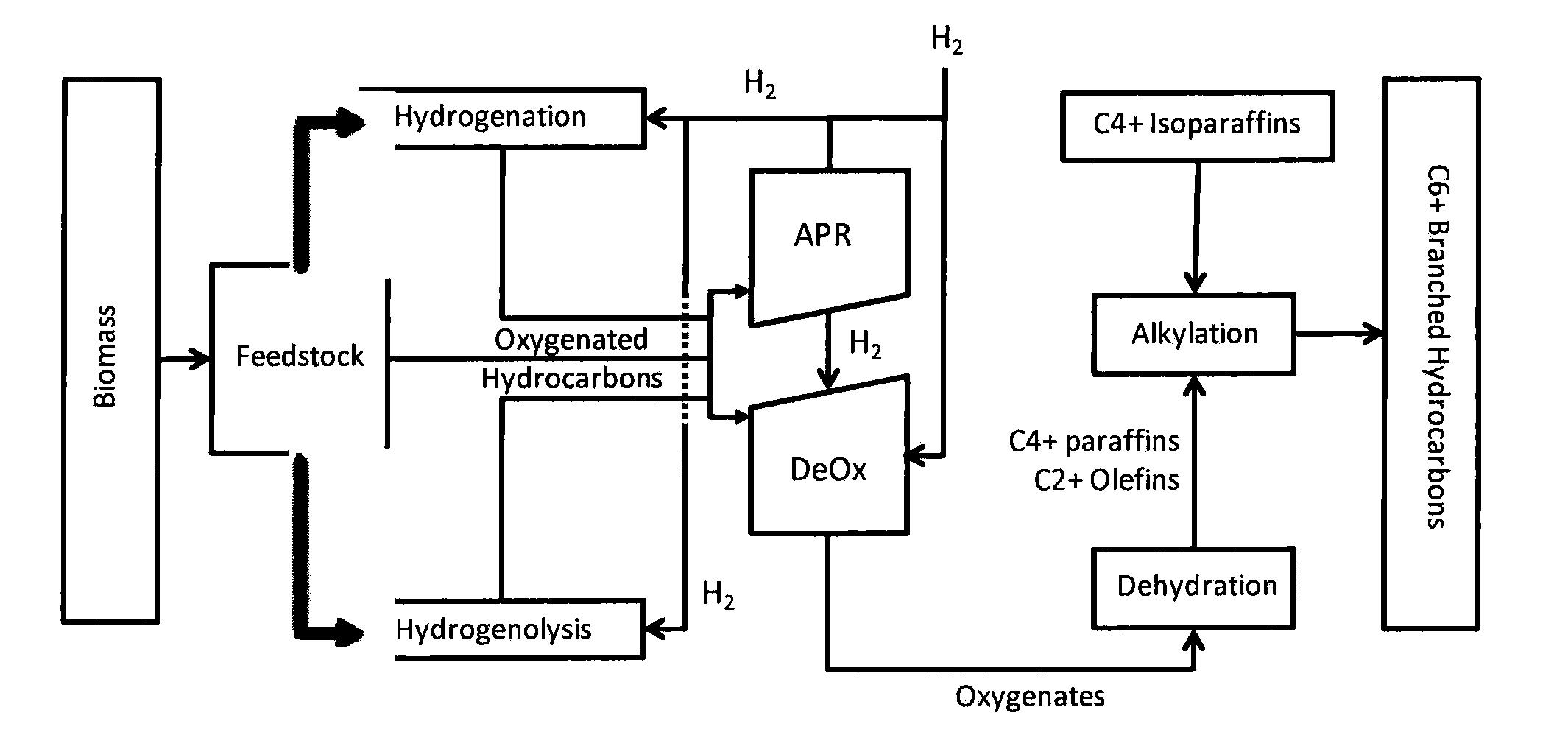
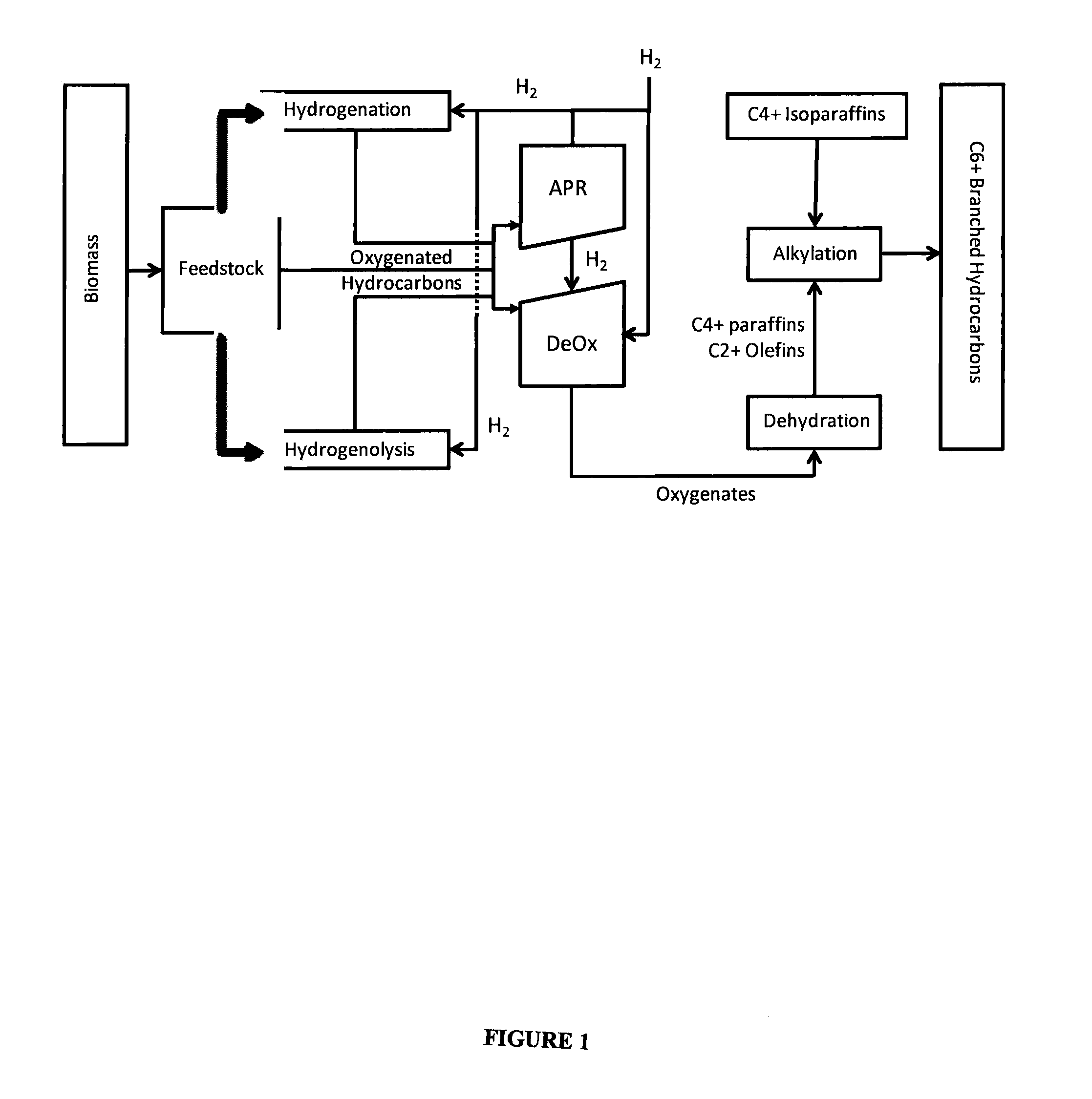
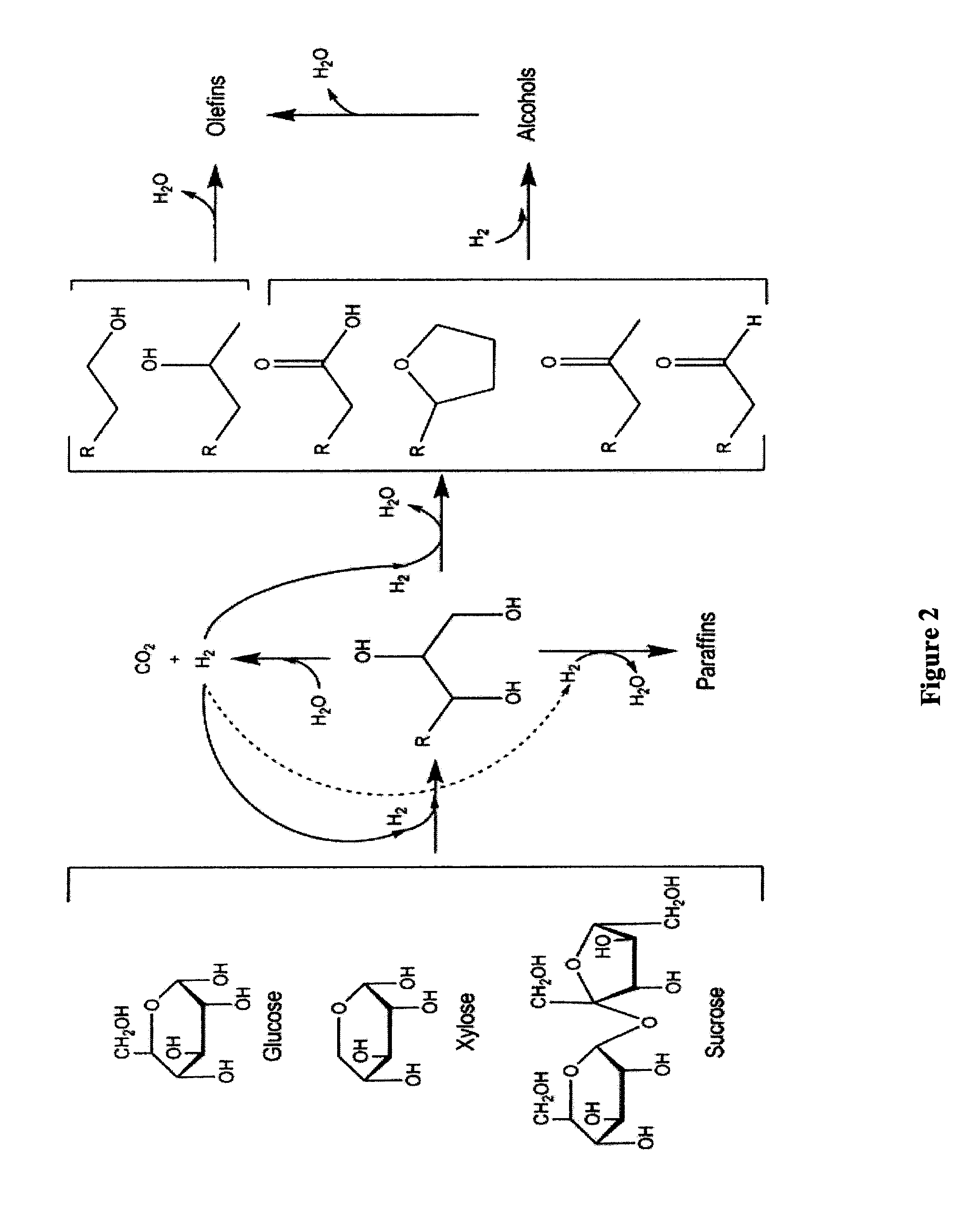
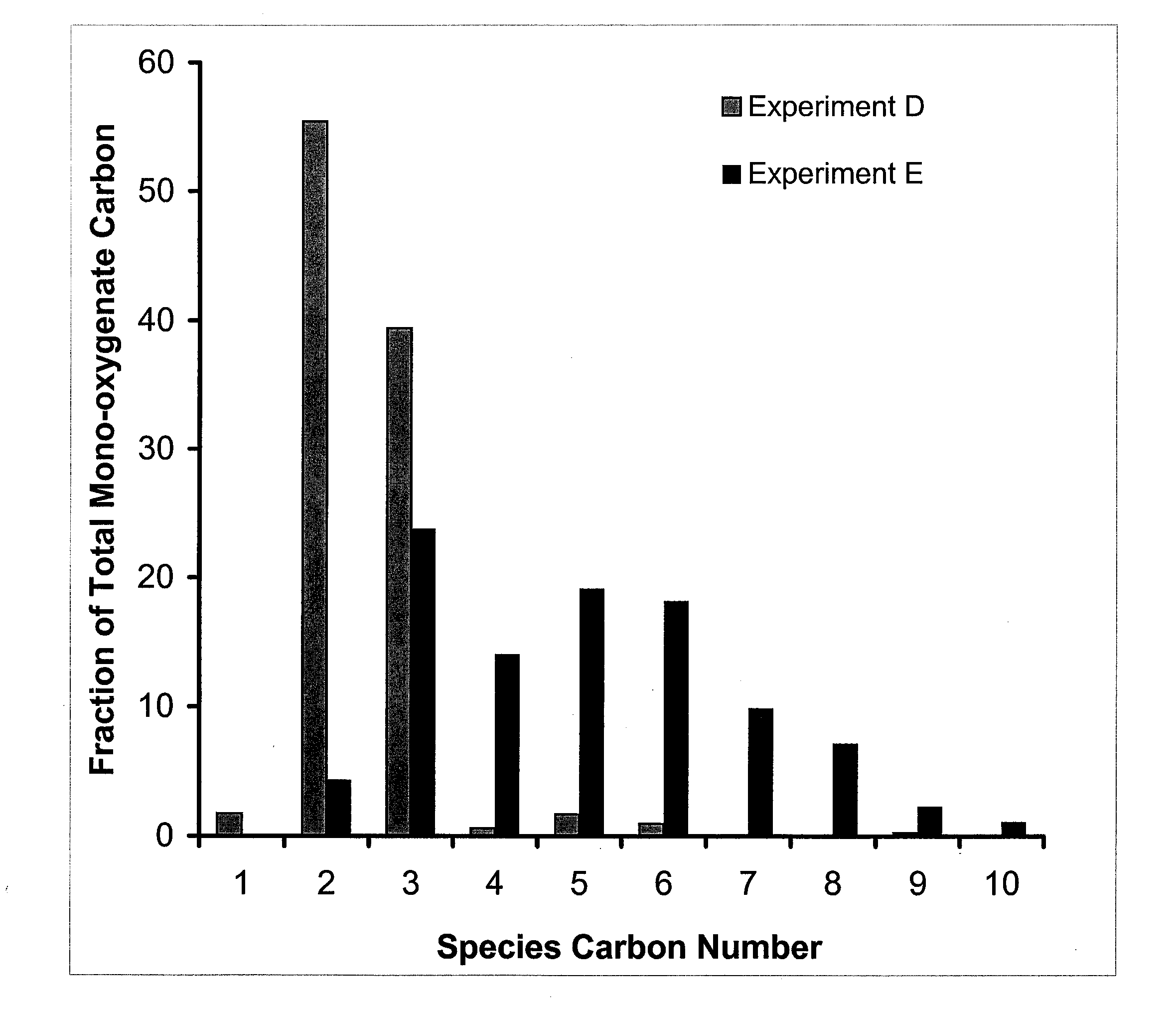
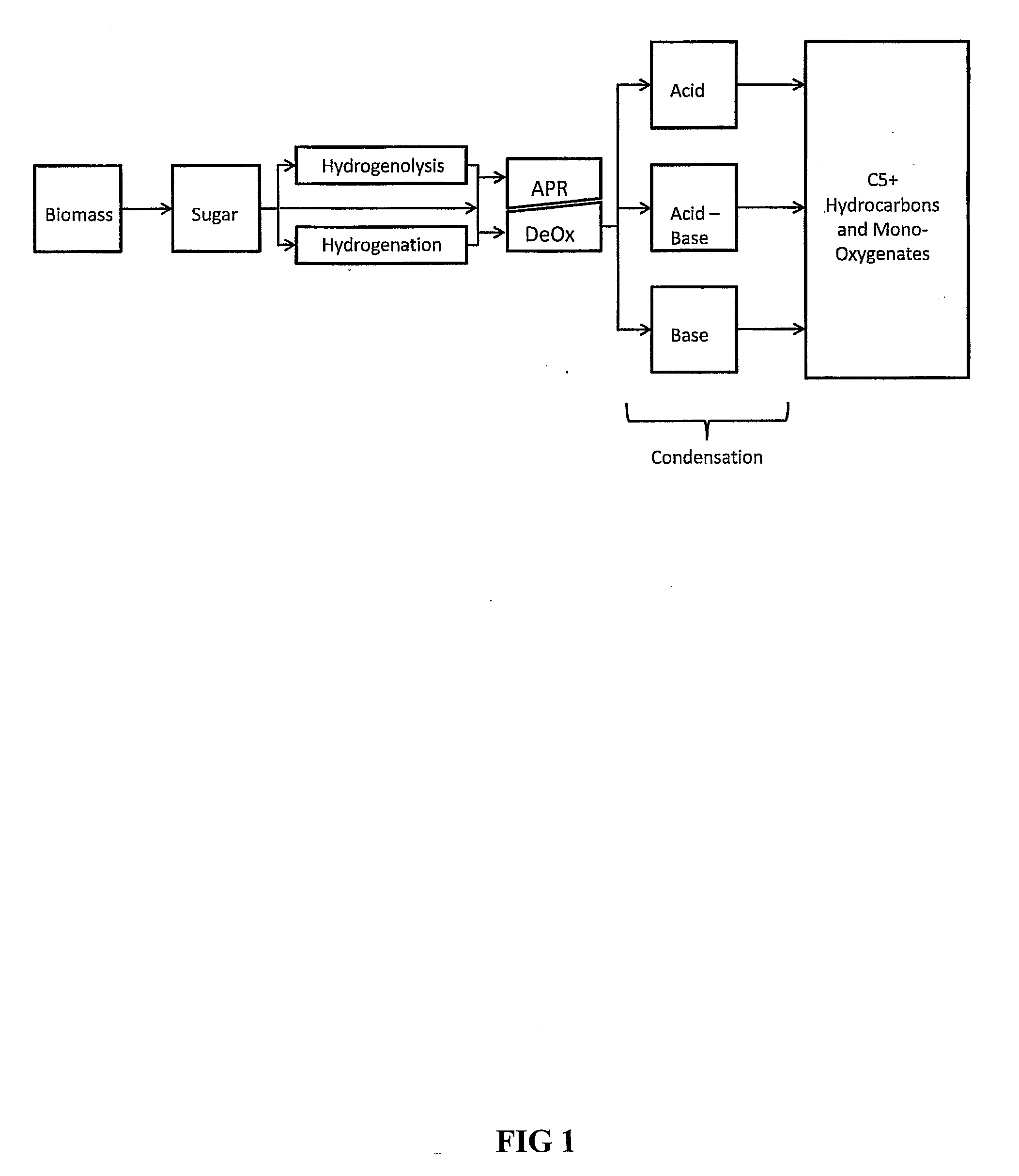
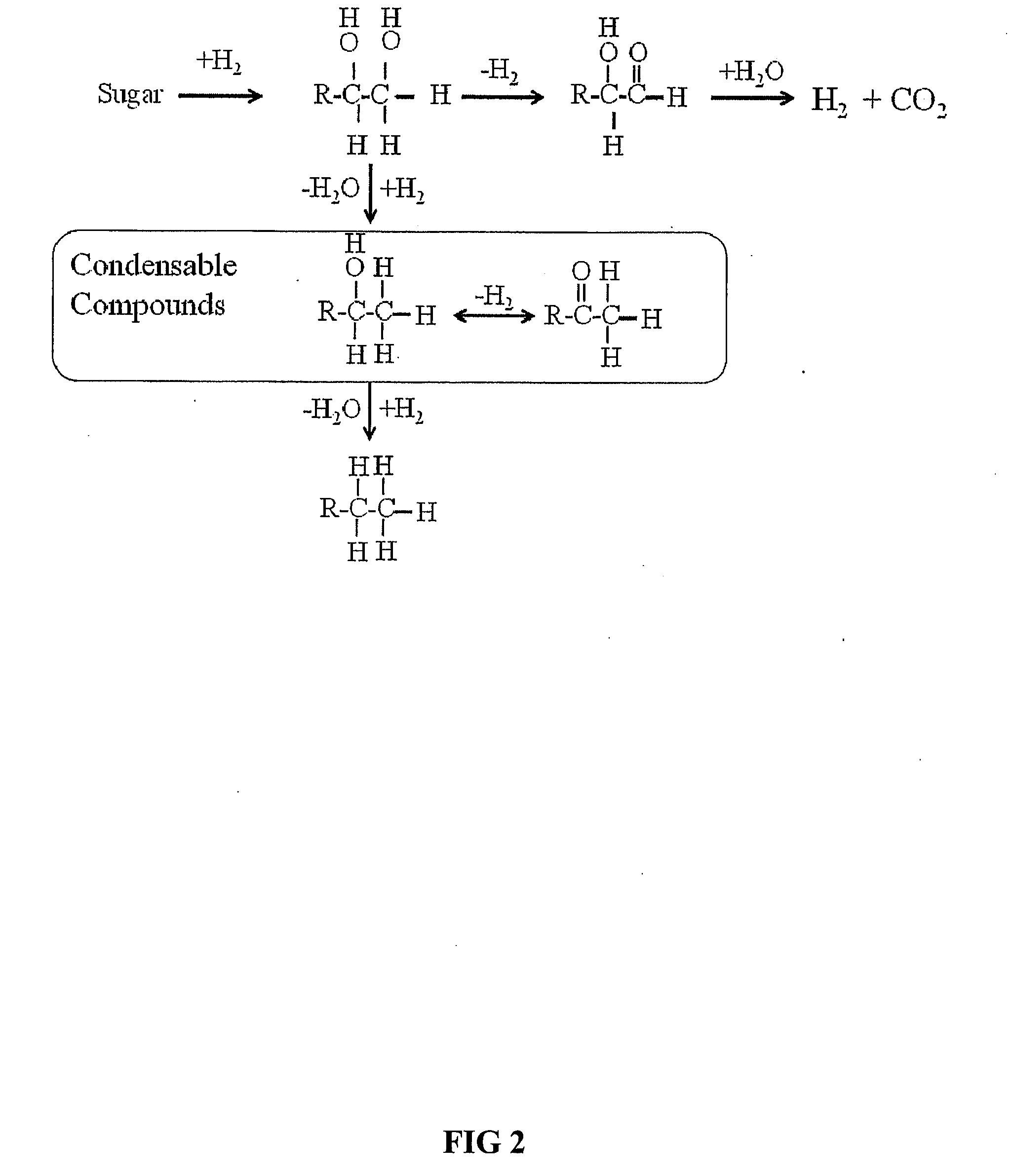
Synthesis of liquid fuels and chemicals from oxygenated hydrocarbons
ActiveUS20080216391A1Organic compound preparationHydrocarbon from oxygen organic compoundsFuranLiquid fuel
Processes and reactor systems are provided for the conversion of oxygenated hydrocarbons to hydrocarbons, ketones and alcohols useful as liquid fuels, such as gasoline, jet fuel or diesel fuel, and industrial chemicals. The process involves the conversion of mono-oxygenated hydrocarbons, such as alcohols, ketones, aldehydes, furans, carboxylic acids, diols, triols, and / or other polyols, to C4+ hydrocarbons, alcohols and / or ketones, by condensation. The oxygenated hydrocarbons may originate from any source, but are preferably derived from biomass.
Owner:VIRENT
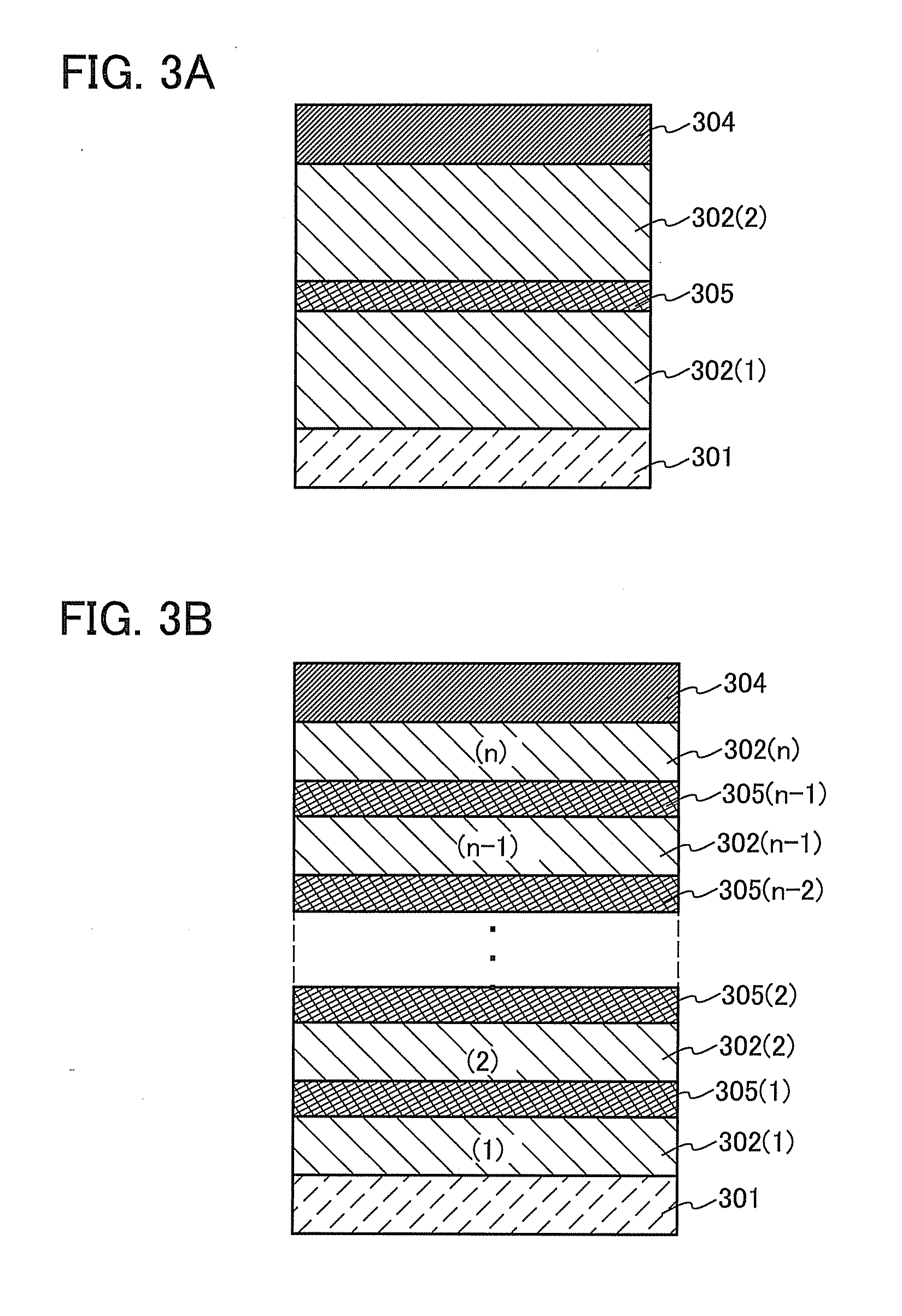
Organometallic Complex, Light-Emitting Element, Light-Emitting Device, Electronic Device, and Lighting Device
ActiveUS20130165653A1Improve emission efficiencyHigh color purityGroup 5/15 element organic compoundsGroup 3/13 element organic compoundsNitrogenKetone
As a novel substance having a novel skeleton, an organometallic complex with high emission efficiency which achieves improved color purity by a reduction of half width of an emission spectrum is provided. One embodiment of the present invention is an organometallic complex in which a β-diketone and a six-membered heteroaromatic ring including two or more nitrogen atoms inclusive of a nitrogen atom that is a coordinating atom are ligands. In General Formula (G1), X represents a substituted or unsubstituted six-membered heteroaromatic ring including two or more nitrogen atoms inclusive of a nitrogen atom that is a coordinating atom. Further, R1 to R4 each represent a substituted or unsubstituted alkyl group having 1 to 6 carbon atoms.
Owner:SEMICON ENERGY LAB CO LTD
Red phosphorescent compound and organic electroluminescent device using the same
ActiveUS20070224450A1Desirable chromaticity coordinate characteristicSolve low luminous efficiencyDischarge tube luminescnet screensGroup 8/9/10/18 element organic compoundsOrganic electroluminescenceKetone
Disclosed herein is a red phosphorescent compound represented by Formula 1 below:whereinisR1 is selected from the group consisting of C1-C4 alkyl and C1-C4 alkoxy; R2, R3, R4 and R5 are independently selected from the group consisting ofhydrogen, C1-C4 alkyl and C1-C4 alkoxy; andis selected from the group consisting of 2,4-pentanedione, 2,2,6,6,-tetramethylheptane-3,5-dione, 1,3-propanedione, 1,3-butanedione, 3,5-heptanedione, 1,1,1-trifluoro-2,4-pentanedione, 1,1,1,5,5,5-hexafluoro-2,4-pentanedione and 2,2-dimethyl-3,5-hexanedione.Further disclosed herein is an organic electroluminescent (EL) device using the red phosphorescent compound.
Owner:LG DISPLAY CO LTD
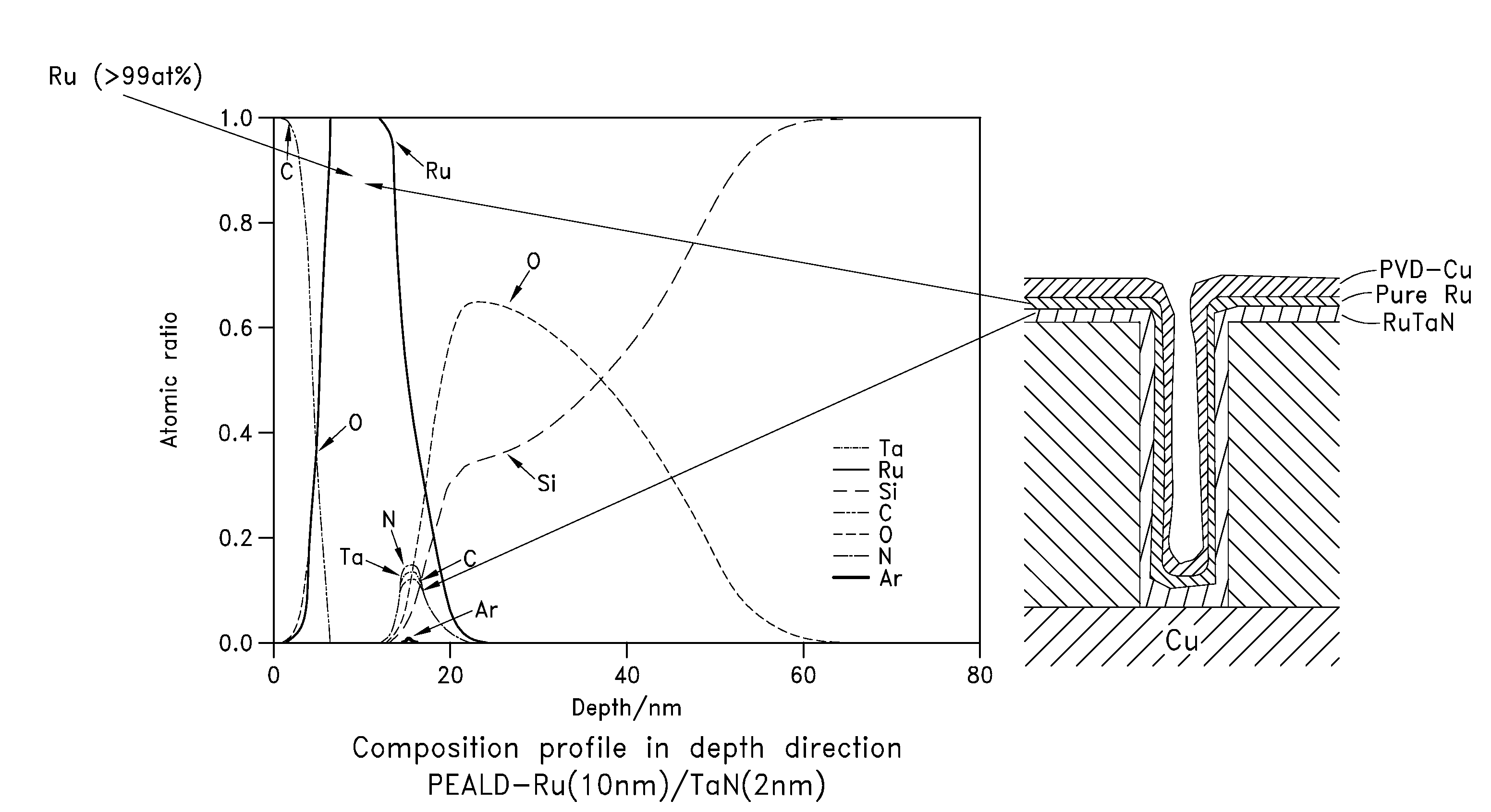
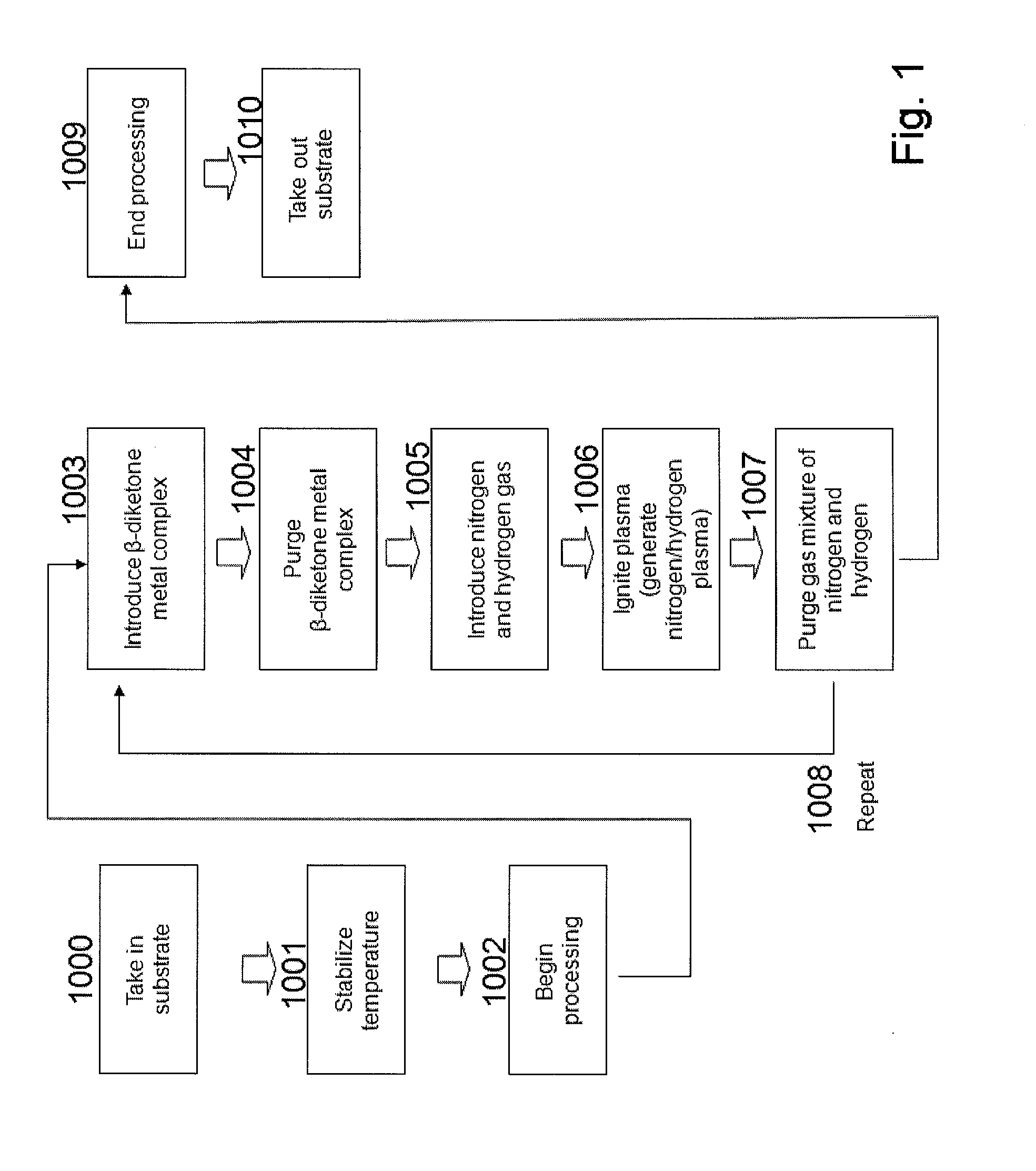
Method for forming metal film by ald using beta-diketone metal complex
ActiveUS20100092696A1Improve shielding effectRapid and reliable mannerVacuum evaporation coatingPretreated surfacesDiketoneHydrogen
A method of forming a single-metal film on a substrate by plasma ALD includes: contacting a surface of a substrate with a β-diketone metal complex in a gas phase; exposing molecule-attached surface to a nitrogen-hydrogen mixed plasma; and repeating the above steps, thereby accumulating atomic layers to form a single-metal film on the substrate.
Owner:ASM JAPAN
Photoresist cross-linker and photoresist composition comprising the same
InactiveUS6368773B1Enhance the imageAdequate resultOrganic chemistryPhotosensitive materialsCarboxylic acidKetone
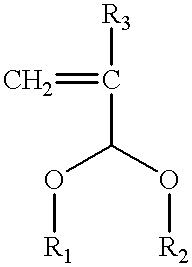
The present invention relates to a cross-linker for photoresist compositions which is suitable for a photolithography process using KrF (248 mn), ArF (193 mn), E-beam, ion beam or EUV light sources. Preferred cross-linkers, according to the present invention, comprise a copolymer of (i) a compound represented by following Chemical Formula 1 and / or (ii) one or more compound(s) selected from the group consisting of acrylic acid, methacrylic acid and maleic anhydride. ##STR1## wherein, R.sub.1 and R.sub.2 individually represent straight or branched C.sub.1-10 alkyl, straight or branched C.sub.1-10 ester, straight or branched C.sub.1-10 ketone, straight or branched C.sub.1-10 carboxylic acid, straight or branched C.sub.1-10 acetal, straight or branched C.sub.1-10 alkyl including at least one hydroxyl group, straight or branched C.sub.1-10 ester including at least one hydroxyl group, straight or branched C.sub.1-10 ketone including at least one hydroxyl group, straight or branched C.sub.1-10 carboxylic acid including at least one hydroxyl group, and straight or branched C.sub.1-10 acetal including at least one hydroxyl group; and R.sub.3 represents hydrogen or methyl.
Owner:HYUNDAI ELECTRONICS IND CO LTD
Synthesis of liquid fuels and chemicals from oxygenated hydrocarbons
ActiveUS20080300435A1Oxygen-containing compound preparationLiquid hydrocarbon mixture productionFuranLiquid fuel
Processes and reactor systems are provided for the conversion of oxygenated hydrocarbons to hydrocarbons, ketones and alcohols useful as liquid fuels, such as gasoline, jet fuel or diesel fuel, and industrial chemicals. The process involves the conversion of mono-oxygenated hydrocarbons, such as alcohols, ketones, aldehydes, furans, carboxylic acids, diols, triols, and / or other polyols, to C4+ hydrocarbons, alcohols and / or ketones, by condensation. The oxygenated hydrocarbons may originate from any source, but are preferably derived from biomass.
Owner:VIRENT
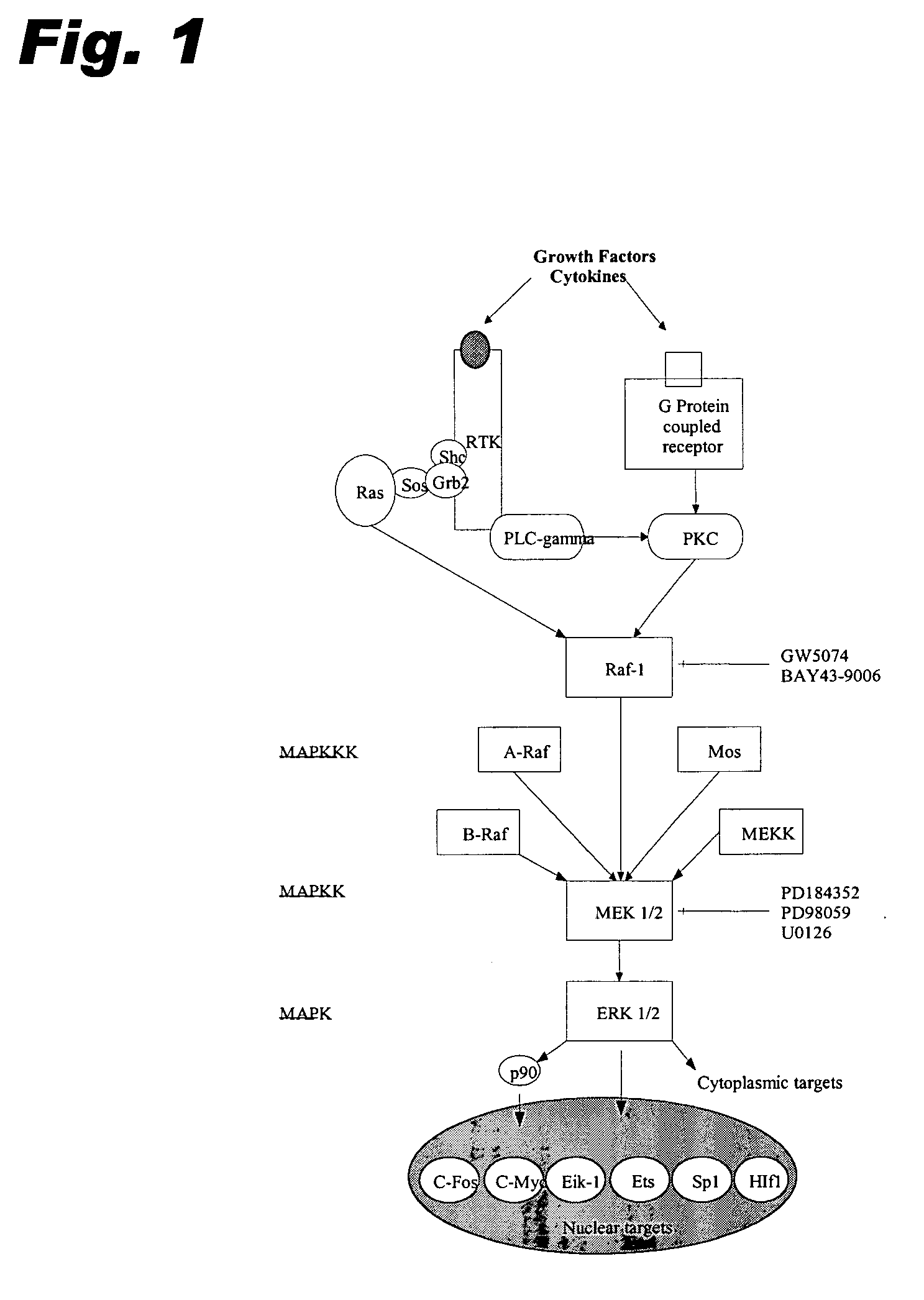
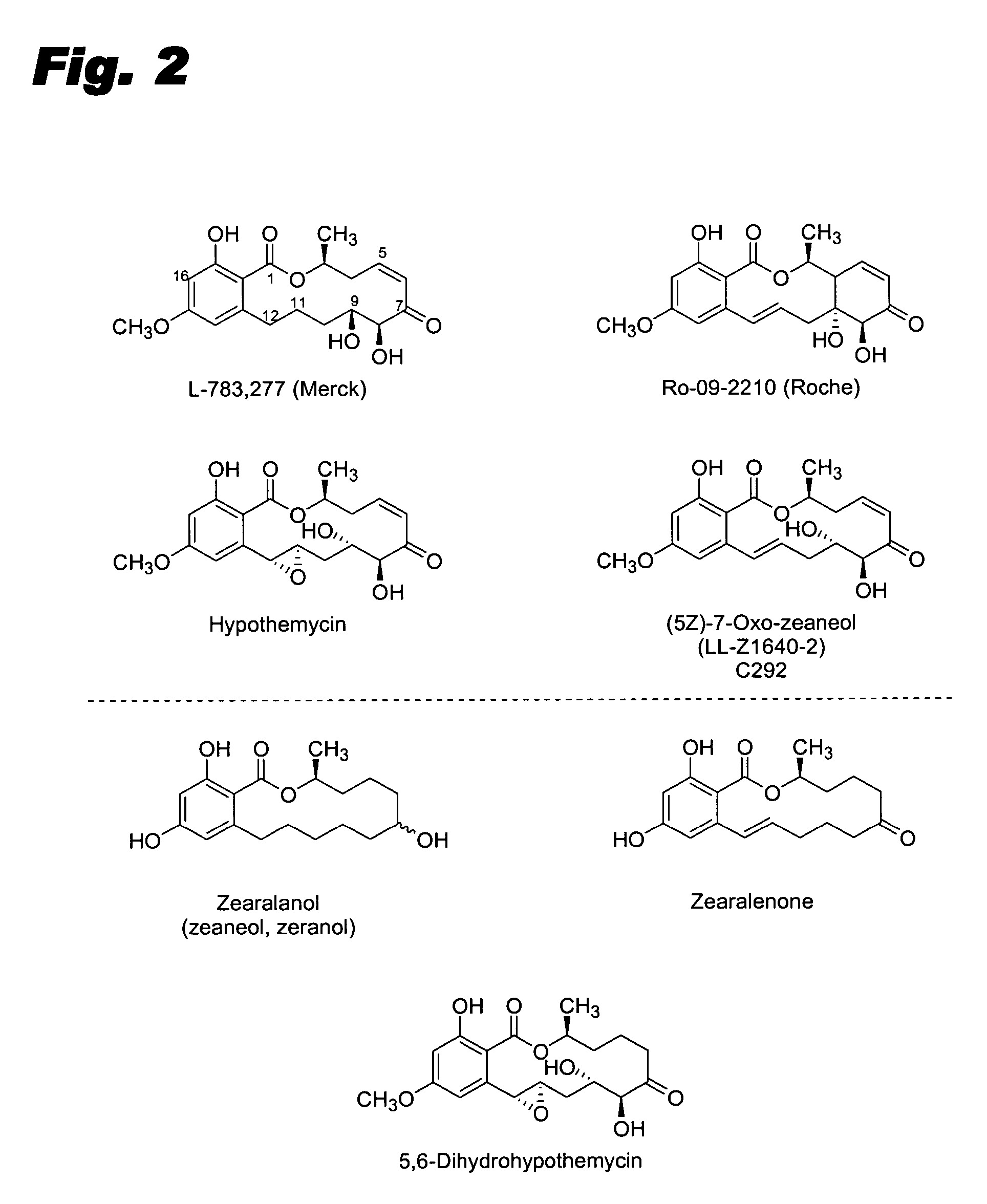
Specific kinase inhibitors
Resorcylic acid lactones having a C5-C6 cis double bond and a ketone at C7 and other compounds capable of Michael adduct formation are potent and stable inhibitors of a subset of protein kinases having a specific cysteine residue in the ATP binding site.
Owner:KOSAN BIOSCI
Pro-fragrances
InactiveUS6861402B1Enhanced perfume longevityCosmetic preparationsOrganic chemistryFlavorAdditive ingredient
The present invention relates to fragrance delivery systems which comprise: A) from about 0.01% by weight of a pro-fragrance component which comprises pro-fragrances or pro-accords selected from at least two of the following: i) aldehyde and ketone releasing pro-fragrances, preferably an oxazolidine pro-fragrance; ii) β-amino pro-fragrances; and iii) orthoester pro-accords; and B) the balance carries and others adjunct ingredients.
Owner:THE PROCTER & GAMBLE COMPANY
Synthesis of liquid fuels from biomass
ActiveUS20100076233A1Hydrocarbon by metathesis reactionLiquid hydrocarbon mixture productionFuranAlkane
Processes and reactor systems are provided for the conversion of oxygenated hydrocarbons to paraffins useful as liquid fuels. The process involves the conversion of water soluble oxygenated hydrocarbons to oxygenates, such as alcohols, furans, ketones, aldehydes, carboxylic acids, diols, triols, and / or other polyols, followed by the subsequent conversion of the oxygenates to paraffins by dehydration and alkylation. The oxygenated hydrocarbons may originate from any source, but are preferably derived from biomass.
Owner:VIRENT
Water absorbent resin composition and production method thereof
InactiveUS20050288182A1Promote absorptionInhibitionOther chemical processesBaby linensCross-linkAbsorption capacity
The water absorbent resin composition and the production method thereof according to the present invention are characterized by including: water absorbent resin particles having an internal cross-linked structure obtained by polymerizing a water-soluble unsaturated monomer; a nitrogenous ketone compound (A) (containing no carboxyl group) having a structure represented by formula (1); and a bivalent and / or trivalent and / or tetravalent water-soluble metal salt, wherein a total amount of the nitrogenous ketone compound (A) and the bivalent and / or trivalent and / or tetravalent water-soluble metal salt ranges from 0.01 to 100 parts by mass with respect to 100 parts by mass of the water absorbent resin particles, thereby providing a water absorbent resin composition, having an excellent absorption capacity represented by a centrifuge retention capacity (CRC), an absorbency against pressure of 4.83 kPa (AAP) etc., having excellent liquid permeability and liquid diffusion properties, having excellent fluidity at the time of moisture absorption, having an excellent damage resistance property, effectively suppressing occurrence of dusts, hardly bringing about permeation of added metal compounds into water absorbent resin particles, hardly bringing about segregation of added metal compounds.
Owner:NIPPON SHOKUBAI CO LTD
Fragrance pro-accords and aldehyde and ketone fragrance libraries
InactiveUS7018978B2Extend your lifeEasy to prepareCosmetic preparationsOrganic chemistryFlavorOxazolidine E
The present invention relates to novel heterocyclic pro-fragrances, preferably oxazolidines, tertahydro-1,3-oxazines, thiazolidines, or tetrahydro-1,3-thiazines, more preferably oxazolidines, or tertahydro-1,3-oxazines, most preferably oxazolidines, which are capable of sustained release of fragrance raw material ketones and aldehydes and to fragrance delivery systems which comprise said pro-fragrances.
Owner:THE PROCTER & GAMBLE COMPANY
Biosensor having improved hematocrit and oxygen biases
ActiveUS7501053B2Accurate responseDesensitizationImmobilised enzymesBioreactor/fermenter combinationsQuinoneManganese
A biosensor that utilizes a mediator, i.e., an isomer of phenanthroline quinone, 1,10-phenanthroline-5,6-dione, and a metal ion, such as manganese, with an enzyme dependent upon NAD(P)+, such as, for example, glucose dehydrogenase, for improving the hematocrit bias and oxygen bias of biosensors. The electrodes of the biosensors employing this mediator and a metal ion provide an accurate clinical response over a hematocrit range that ranges from about 20% to about 70% and over an oxygen tension range that ranges from about 1 kPa to about 20 kPa.
Owner:ABBOTT DIABETES CARE INC
Pharmaceutical co-crystal compositions
A pharmaceutical composition comprising a co-crystal of an API and a co-crystal former; wherein the API has at least one functional group selected from ether, thioether, alcohol, thiol, aldehyde, ketone, thioketone, nitrate ester, phosphate ester, thiophosphate ester, ester, thioester, sulfate ester, carboxylic acid, phosphonic acid, phosphinic acid, sulfonic acid, amide, primary amine, secondary amine, ammonia, tertiary amine, sp2 amine, thiocyanate, cyanamide, oxime, nitrile diazo, organohalide, nitro, s-heterocyclic ring, thiophene, n-heterocyclic ring, pyrrole, o-heterocyclic ring, furan, epoxide, peroxide, hydroxamic acid, imidazole, pyridine and the co-crystal former has at least one functional group selected from amine, amide, pyridine, imidazole, indole, pyrrolidine, carbonyl, carboxyl, hydroxyl, phenol, sulfone, sulfonyl, mercapto and methyl thio, such that the API and co-crystal former are capable of co-crystallizing from a solution phase under crystallization conditions.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES +2
Synthesis of liqiud fuels and chemicals from oxygenated hydrocarbons
ActiveUS20080300434A1Oxygen-containing compound preparationHydrocarbon purification/separationFuranCarboxylic acid
Processes and reactor systems are provided for the conversion of oxygenated hydrocarbons to hydrocarbons, ketones and alcohols useful as liquid fuels, such as gasoline, jet fuel or diesel fuel, and industrial chemicals. The process involves the conversion of mono-oxygenated hydrocarbons, such as alcohols, ketones, aldehydes, furans, carboxylic acids, diols, triols, and / or other polyols, to C4+ hydrocarbons, alcohols and / or ketones, by condensation. The oxygenated hydrocarbons may originate from any source, but are preferably derived from biomass.
Owner:VIRENT
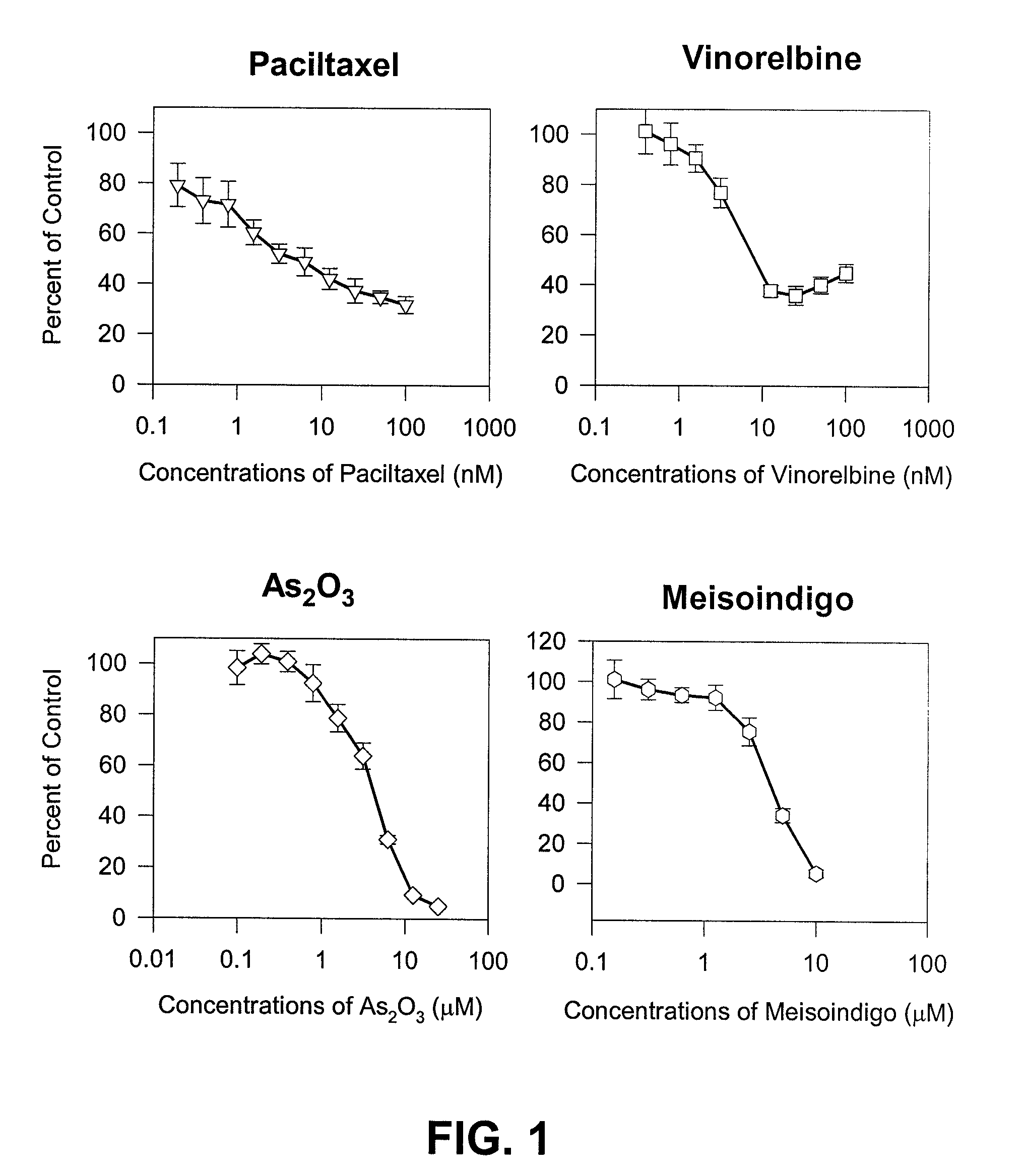
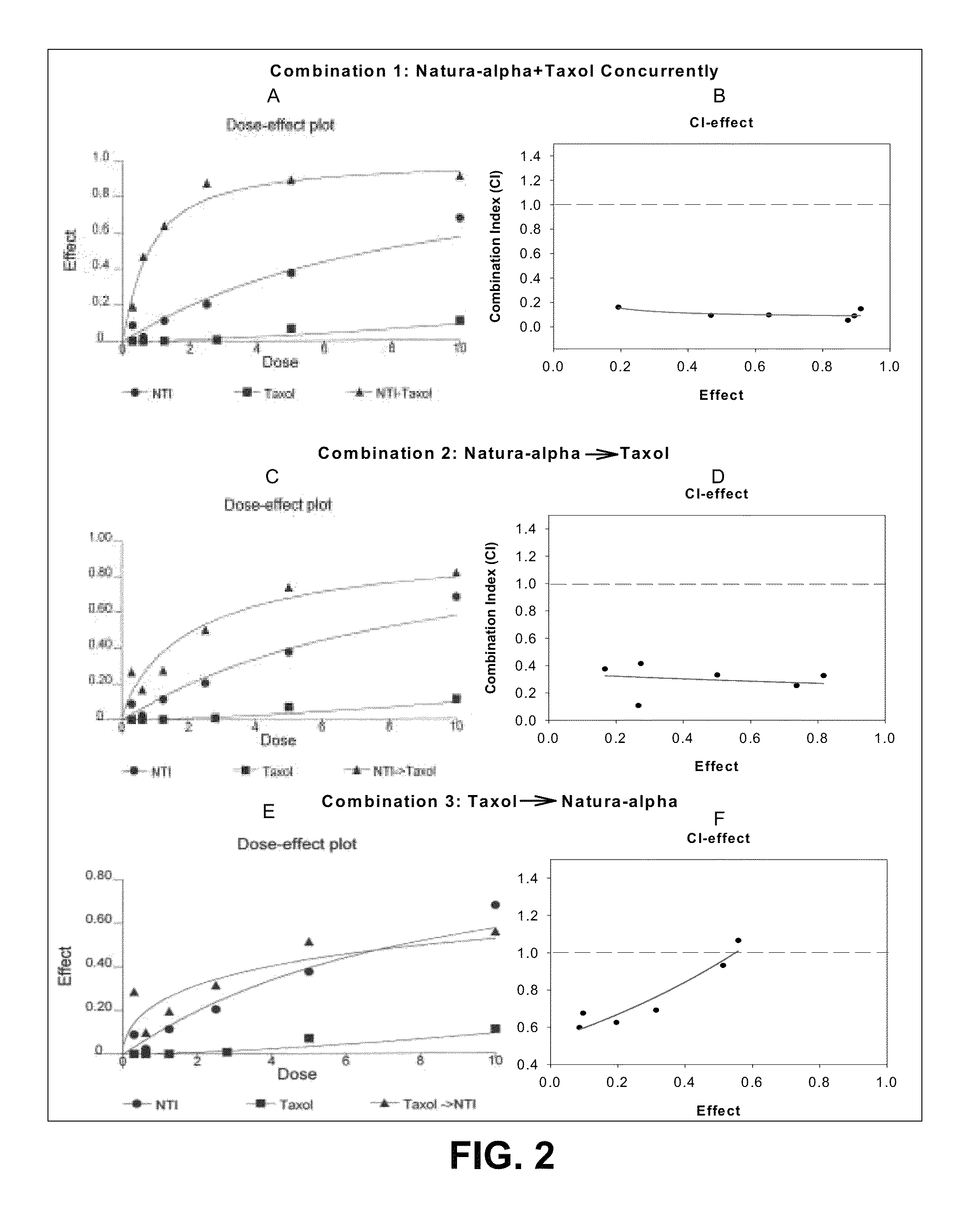
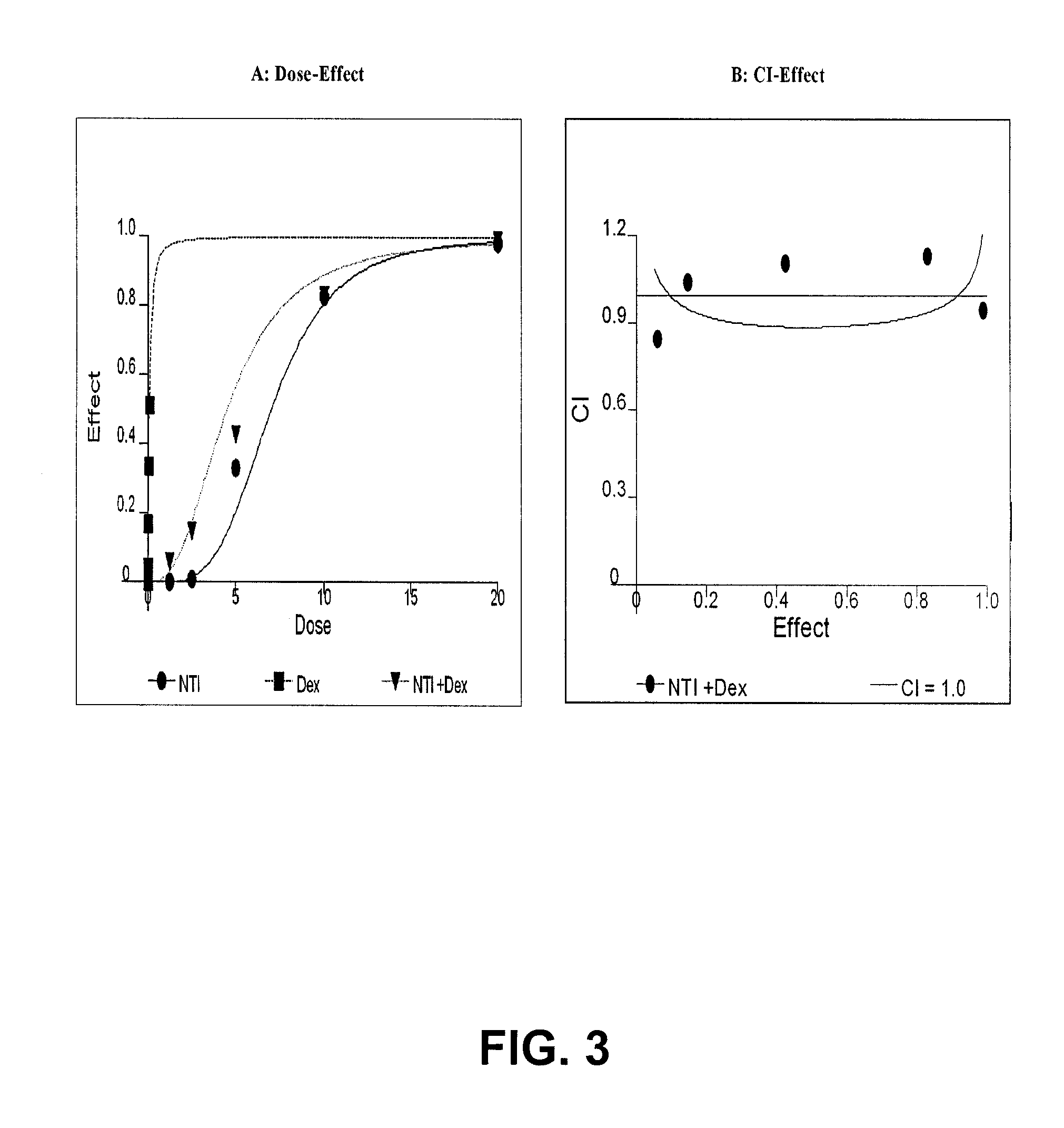
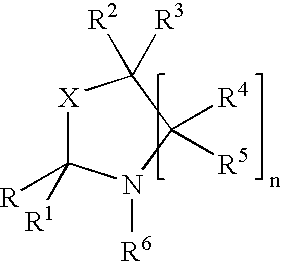
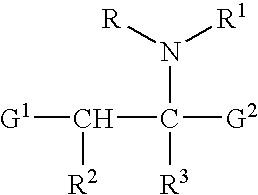
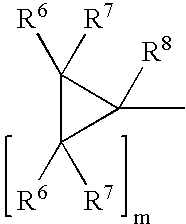
(+)-2-[1-(3-Ethoxy-4-methoxyphenyl)-2-methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione: methods of using and compositions thereof
Stereomerically pure (+)-2-[1-(3-Ethoxy-4-methoxyphenyl)-2-methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione, substantially free of its (−) isomer, and prodrugs, metabolites, polymorphs, salts, solvates, hydrates, and clathrates thereof are discussed. Also discussed are methods of using and pharmaceutical compositions comprising the (+) enantiomer of 2-[1-(3-Ethoxy-4-methoxyphenyl)-2-methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione are disclosed. The methods include methods of treating and / or preventing disorders ameliorated by the reduction of levels of TNF-α or the inhibition of PDE4.
Owner:AMGEN INC
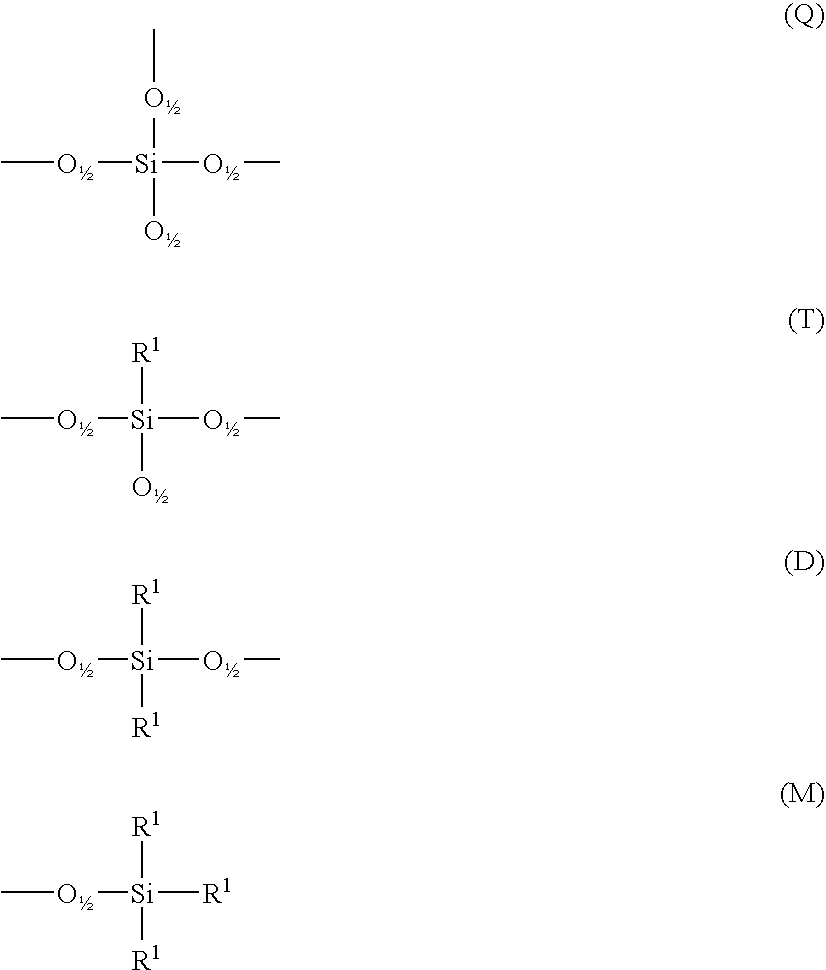
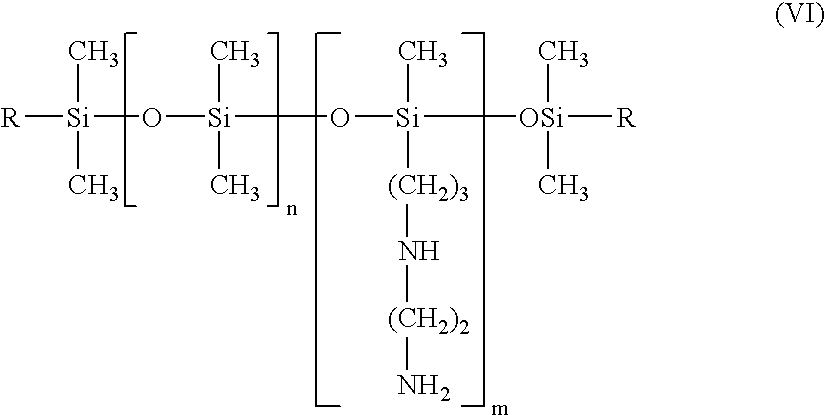
Perfumed liquid laundry detergent compositions with functionalized silicone fabric care agents
InactiveUS20060003913A1Organic detergent compounding agentsNon-surface-active detergent compositionsLiquid laundry detergentNitrogen
The invention is directed to aqueous liquid laundry detergent compositions for cleaning and imparting fabric care benefits to fabrics laundered therewith and to methods for preparing such compositions. Such compositions comprise (A) at least one textile-cleaning surfactant; (B) droplets of miscible silicones comprising both a polarly-functionalized, preferably nitrogen-containing amino or ammonium functionalized, polysiloxane component and a nitrogen-free non-functionalized or non-polarly-functionalized polysiloxane component; and (C) a perfume component comprising fragrant aldehydes and / or ketones or a pro-perfume capable of providing such aldheyde and / or ketone perfume materials in situ. Incorporation of a polarly-functionalized polysiloxane fabric care agent into liquid laundry detergent compositions by miscibly combining it with a non-functionalized or non-polarly functionalized polysiloxane minimizes the undesirable interaction such polarly-functionalized silicone material might otherwise have with aldehyde and / or ketone perfume compounds.
Owner:THE PROCTER & GAMBLE COMPANY
Metal-oxide films from small molecules for lithographic applications
Metal-oxide films for lithographic applications are provided. The films are formed from compositions comprising metal-oxide precursor compounds including metals and metalloids other than silicon. These films are easily produced and can be modified with a variety of ligands, including alkoxides, phenoxides, carboxylates, beta-diketones, and beta-ketoesters.
Owner:BREWER SCI
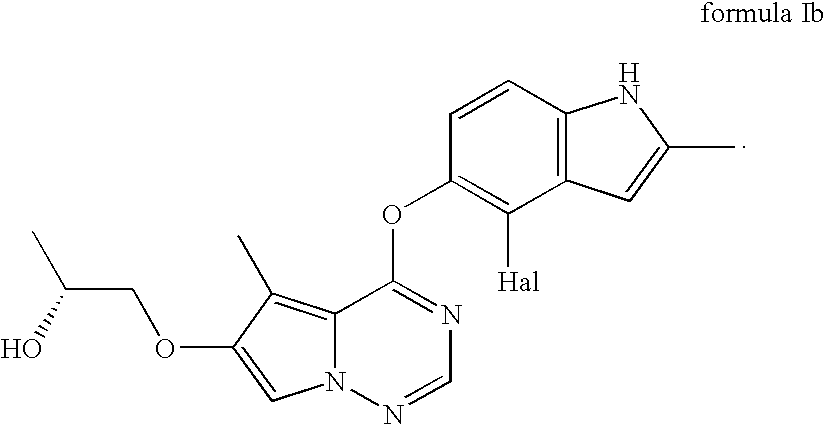
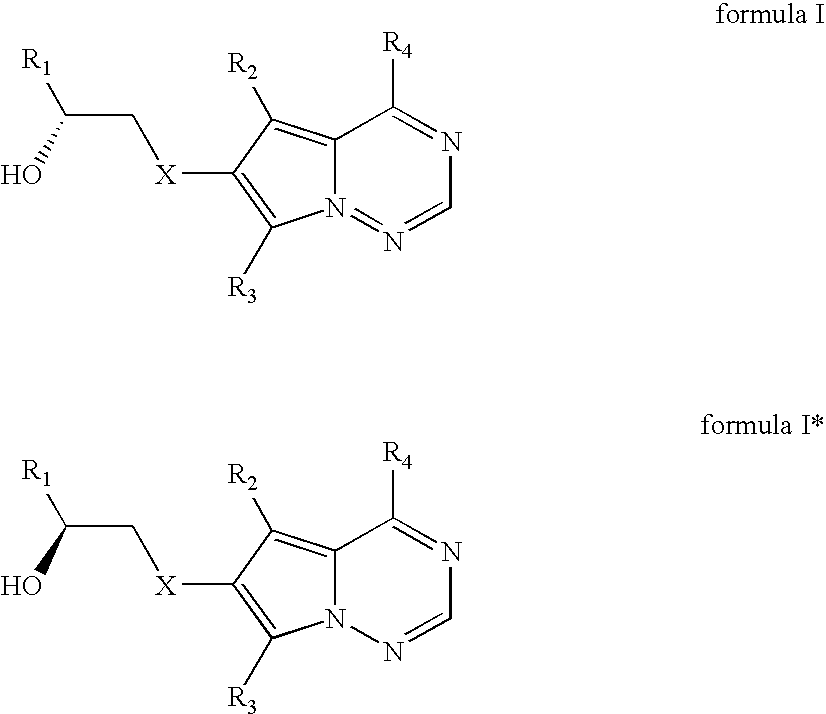
Stereoselective reduction process for the preparation of pyrrolotriazine compounds
The invention relates to a process for the enzymatic, stereoselective reduction of ketone compounds to provide chiral alcohols, for example the compound of formula Ib:
Owner:BRISTOL MYERS SQUIBB CO
Analyte test system for determining the concentration of an analyte in a physiological or aqueous fluid
InactiveUS20050196747A1Cheap productionReliable resultsBioreactor/fermenter combinationsBiological substance pretreatmentsSmall sampleQuality control system
This invention provides a device for determining the concentration of an analyte like glucose, cholesterol, free fatty acids, triglycerides, proteins, ketones, phenylalanine or enzymes, in a physiological or aqueous fluid like blood, serum, plasma, saliva, urine, interstitial and / or intra-cellular fluid, the device having an integrated calibration and quality control system suitable for dry reagent test strips with a very small sample volume of about 0.5 μL based on to a new sample distribution system. The production of the inventive analyte test element involves only a small number of uncomplicated production steps enabling an inexpensive production of the strips.
Owner:EGOMEDICAL SWISS
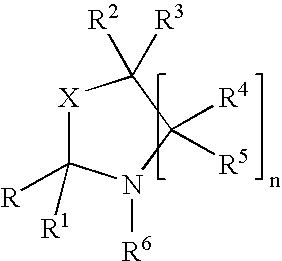
Luminescent element material and luminescent element comprising the same
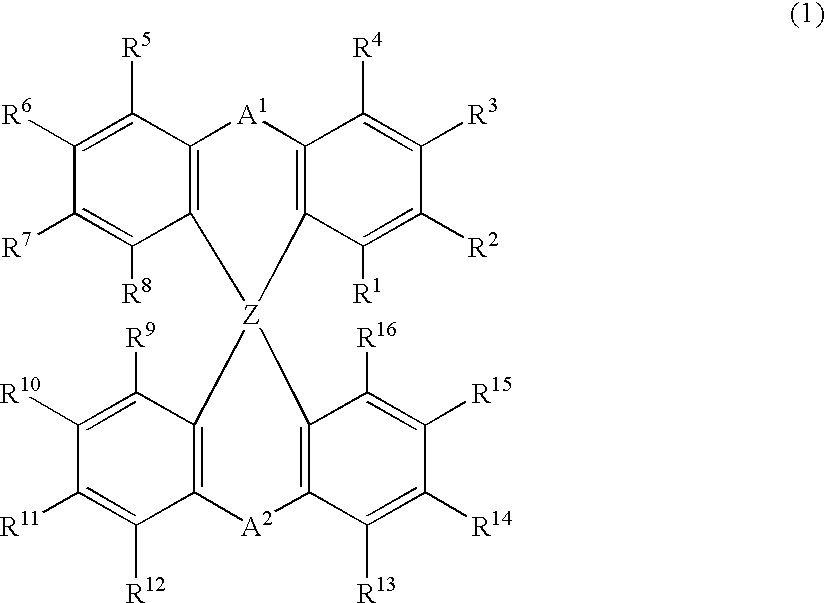
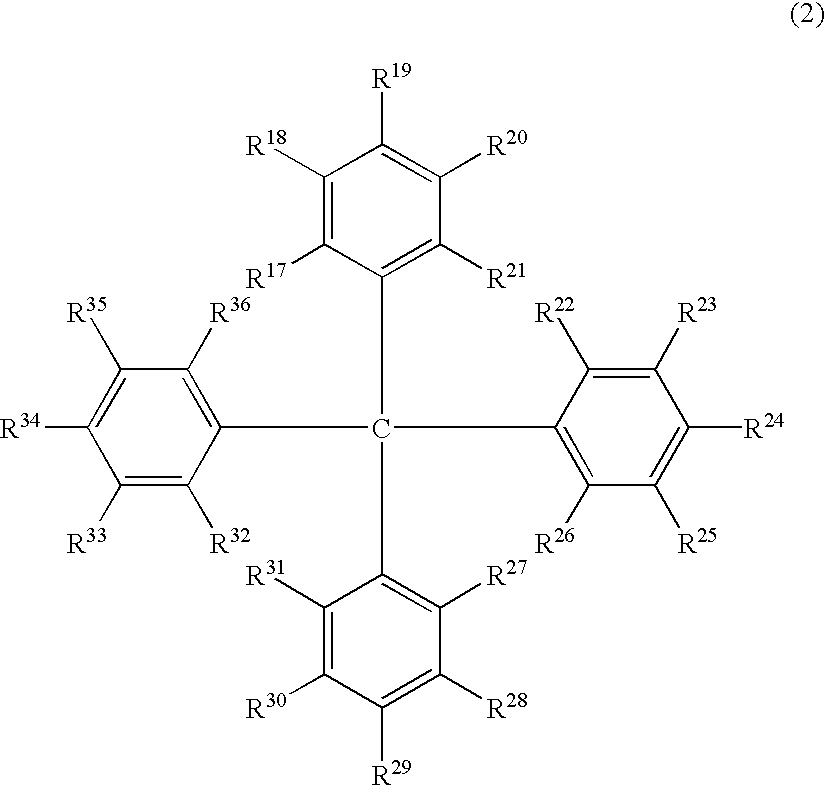
The light emitting device of the present invention relates to a light emitting device which is characterized in that it is a device with an emissive substance present between an anode and cathode, and which emits light by means of electrical energy, and said device has a least one type of compound denoted by (a) to (d) below. (a) A compound having a plurality of 1,7-phenanthroline skeletal structures (b) A benzoquinoline derivative (c) A spiro compound represented by general formula (1) A1 and A2 are each selected from single bonds, substituted or unsubstituted alkyl chains, ether chains, thioether chains, ketone chains and substituted or unsubstituted amino chains. However, A1<> A2. Z represents carbon or silicon. R1 to R16 are each selected from hydrogen, alkyl group, cycloalkyl group, aralkyl group, alkenyl group, cycloalkenyl group, alkynyl group, hydroxyl group, mercapto group, alkoxy group, alkylthio group, aryl ether group, aryl thioether group, aryl group, heterocyclic group, halogen, haloalkane, haloalkene, haloalkyne, cyano group, aldehyde group, carbonyl group, carboxyl group, ester group, carbamoyl group, amino group, nitro group, silyl group, siloxanyl group and a cyclic structure formed with an adjacent substituent. (d) A tetraphenylmethane derivative represented by general formula (2) R17 to R36 are each selected from hydrogen, alkyl group, cycloalkyl group, aralkyl group, alkenyl group, cycloalkenyl group, alkynyl group, hydroxyl group, mercapto group, alkoxy group, alkylthio group, aryl ether group, aryl thioether group, aryl group, heterocyclic group, halogen, haloalkane, haloalkene, haloalkyne, cyano group, aldehyde group, carbonyl group, carboxyl group, ester group, carbamoyl group, amino group, nitro group, silyl group, siloxanyl group and a cyclic structure formed with an adjacent substituent. However, at least one of R17 to R36 is selected from substituents represented by general formula (3). -X-Ar (3) X is a single bond or is selected from the following, and Ar denotes a condensed aromatic ring or heteroaromatic ring. In the case where X is phosphorus oxide, then Ar represents an aromatic hydrocarbon or heteroaromatic ring. n is an natural number.
Owner:TORAY IND INC
Pharmaceutical compositions and treatment methods
InactiveUS6667299B1Efficient transportReduce yieldAntibacterial agentsOrganic active ingredientsRegimenKetone
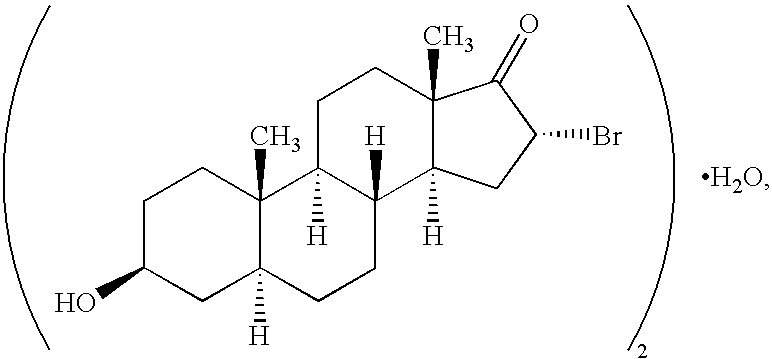
The invention provides compositions comprising, 16alpha-bromo-3beta-hydroxy-5alpha-androstan-17-one hemihydrate and one or more excipients, typically wherein the composition comprises less than about 3% water. The compositions are useful to make improved pharmaceutical formulations. The invention also provides methods of intermittent dosing of steroid compounds such as analogs of 16alpha-bromo-3beta-hydroxy-5alpha-androstan-17-one and compositions useful in such dosing regimens. The invention further provides compositions and methods to inhibit pathogen (viral) replication, ameliorate symptoms associated with immune dysregulation and to modulate immune responses in a subject using certain steroids and steroid analogs. The invention also provides methods to make and use these immunomodulatory compositions and formulations.
Owner:NEURMEDIX +2
Systematic evolution of ligands exponential enrichment: blended selex
InactiveUS6083696AHigh affinityDesired effectSugar derivativesMicrobiological testing/measurementAla-Ala-Pro-ValKetone
A method is described for generating blended nucleic acid ligands containing non-nucleic acid functional units. Specifically, a SELEX identified RNA ligand to the integrin gpIIbIIIa is conjugated to the peptide Gly-Arg-Gly-Asp-Thr-Pro (SEQ ID NO:1). This blended RNA ligand inhibits the biological activity of gpIIbIIIa with high specificity. Also described is a single-stranded DNA ligand to elastase coupled to N-methoxysuccinyl-Ala-Ala-Pro-Val-chloromethyl (SEQ ID NO:3) ketone. This elastase blended nucleic acid ligand inhibits the biological activity of elastase.
Owner:GILEAD SCI INC
Carbon black composition and usage thereof
InactiveUS20130029183A1Good dispersionInhibition formationPigmenting treatmentMagnetic materials for record carriersCyclohexanoneOrganic solvent
An aspect of the present invention relates to a carbon black composition, which comprises carbon black; an organic tertiary amine selected from the group consisting of an aliphatic tertiary monoamine and an alicyclic tertiary amine; and at least one organic solvent selected from the group consisting of methyl ethyl ketone, cyclohexanone, isophorone, and ethanol.
Owner:SOITEC SA +1
Method and kit for investigating humotype semi-cystinol by enzyme biochemical reaction
InactiveCN1693879AMicrobiological testing/measurementColor/spectral properties measurementsChemical reactionFluorescence
The invention is an enzyme biologic and chemical reaction measure method to mensurate homotype cysteine in a biologic sample. Cysteine can lose its ammonia and be changed into alpha-4-ketone acid, ammonia and sulfureted hydrogen by L-ovi-ammonia acid and Y-dispeling enzyme. Sulfureted hydrogen and fluorescence cpd.DMPD2HCL can create blue product-sub-armour blue whose degree of absorbing light is 670nm, in the situation of Fe3+ and acid. Thus, people can mensurate the chroma of homotype cysteine in a biologic sample by knowing this degree. This method can measure the chroma of homotype cysteine(3-1000 uMs). The invention also involves a reagent box used for implementing the method mentioned above. The box uses liquid double reagents, and needs less quantity of samples(25 microlitre or serum or plasm);In addition, the respond time is short, and the operation is easy. Thus, this method is suitable for a great deal of examinations. The reagent box costs less than other types.
Owner:ZHEJIANG YAKE SCI & TECH +1
Antimicrobial coating compositions
ActiveUS20100137472A1Reduce complicationsSuitable for useAntifouling/underwater paintsPretreated surfacesAlkaneCetrimide
Antimicrobial compositions and methods are disclosed. The antimicrobial compositions are particularly useful in providing antimicrobial capability to a wide-range of medical devices. In one aspect, the invention relates to a mild solvent coating using acrylate-type mild solution coating. These compositions include rheological modifiers as necessary. The compositions also include antimicrobial agents, which may be selected from a wide array of agents. Representative antimicrobial agents include cetyl pyridium chloride, cetrimide, alexidine, chlorexidine diacetate, benzalkonium chloride, and o-phthalaldehyde. Additionally, the compositions comprise one or more suitable mild solvents, such as a low molecular weight alcohol, alkane, ketone, and combinations thereof.
Owner:BECTON DICKINSON & CO
Ketone assay
InactiveUS20030175993A1Easy to useLow costMaterial analysis by observing effect on chemical indicatorVaccination/ovulation diagnosticsAnalyteKetone
The present invention relates to analyte detection test systems, including test systems for the oral detection of analytes in saliva. The present invention also provides compositions and methods for storing multiple assay tests and compositions and methods for measuring the concentration of analytes in a sample.
Owner:TORANTO ANTHONY +2
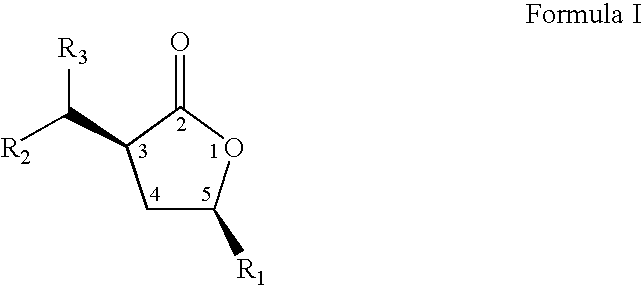
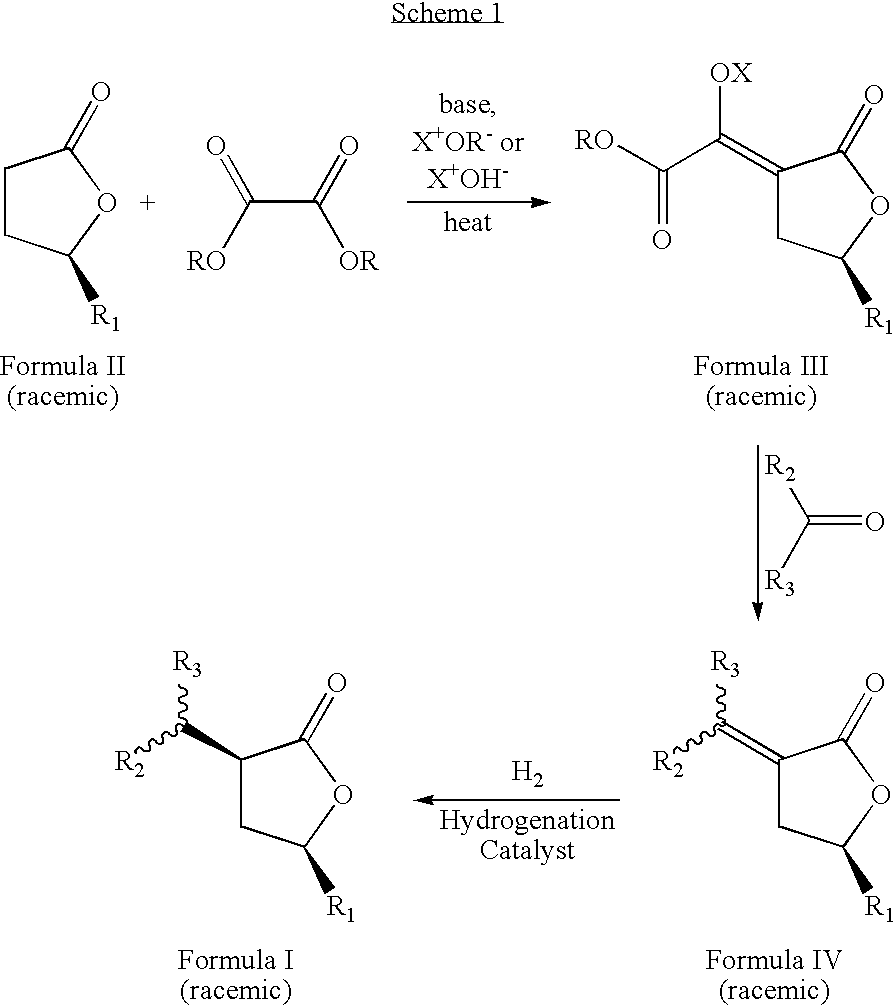
Cis-3,5-disubstituted-dihydro-furan-2-ones and the preparation and use thereof
The present invention relates to an improved process to prepare cis-3-dihydrocarbylmethano-5-hydrocarbyidihydro-furan-2-ones. The present invention also relates to novel compositions of matter comprising enantiomerically pure cis-3-dihydrocarbylmethano-5-hydrocarbyldihydro-furan-2-ones, being the (3S,5S), (3R,5R), (3S,5R), or (3R,5S) optically pure isomers, and a new, more cost efficient process to prepare said optically pure isomers.
Owner:EI DU PONT DE NEMOURS & CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
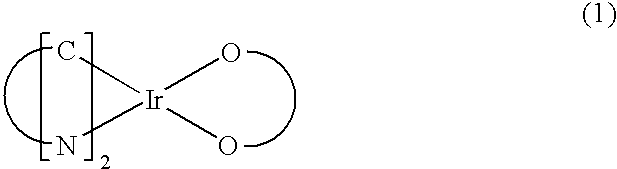
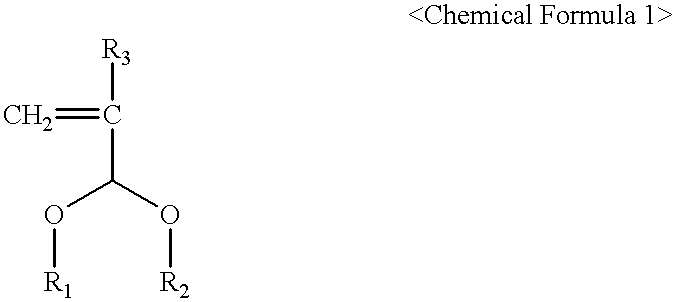
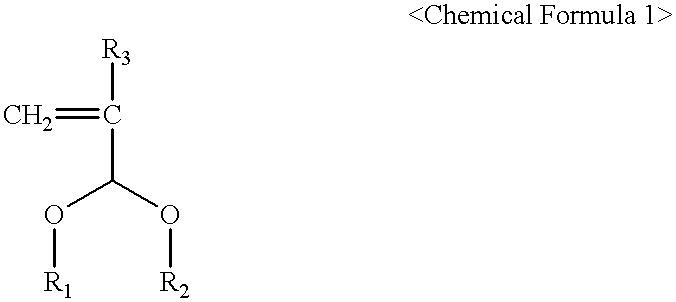


![(+)-2-[1-(3-Ethoxy-4-methoxyphenyl)-2-methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione: methods of using and compositions thereof (+)-2-[1-(3-Ethoxy-4-methoxyphenyl)-2-methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione: methods of using and compositions thereof](https://images-eureka.patsnap.com/patent_img/96aea4cd-d328-4f40-a36e-7bd9b2c52804/US06962940-20051108-D00001.png)
![(+)-2-[1-(3-Ethoxy-4-methoxyphenyl)-2-methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione: methods of using and compositions thereof (+)-2-[1-(3-Ethoxy-4-methoxyphenyl)-2-methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione: methods of using and compositions thereof](https://images-eureka.patsnap.com/patent_img/96aea4cd-d328-4f40-a36e-7bd9b2c52804/US06962940-20051108-D00002.png)
![(+)-2-[1-(3-Ethoxy-4-methoxyphenyl)-2-methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione: methods of using and compositions thereof (+)-2-[1-(3-Ethoxy-4-methoxyphenyl)-2-methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione: methods of using and compositions thereof](https://images-eureka.patsnap.com/patent_img/96aea4cd-d328-4f40-a36e-7bd9b2c52804/US06962940-20051108-C00001.png)