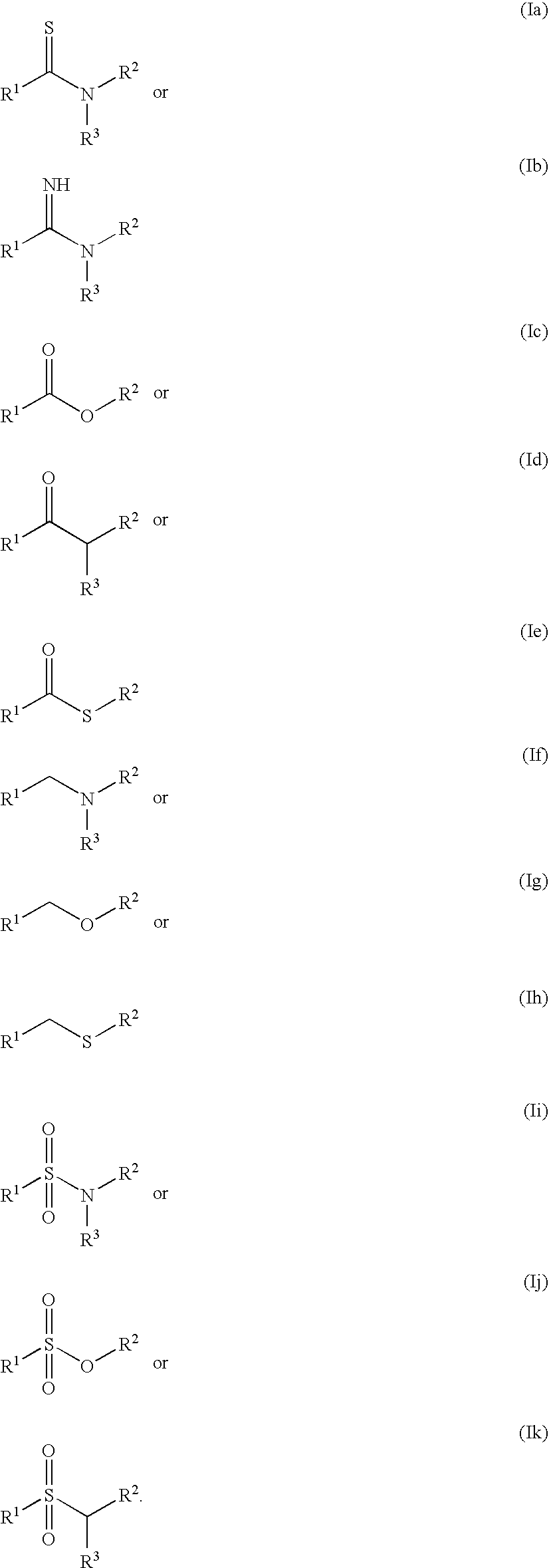
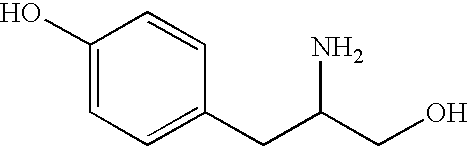
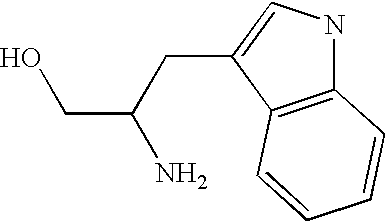
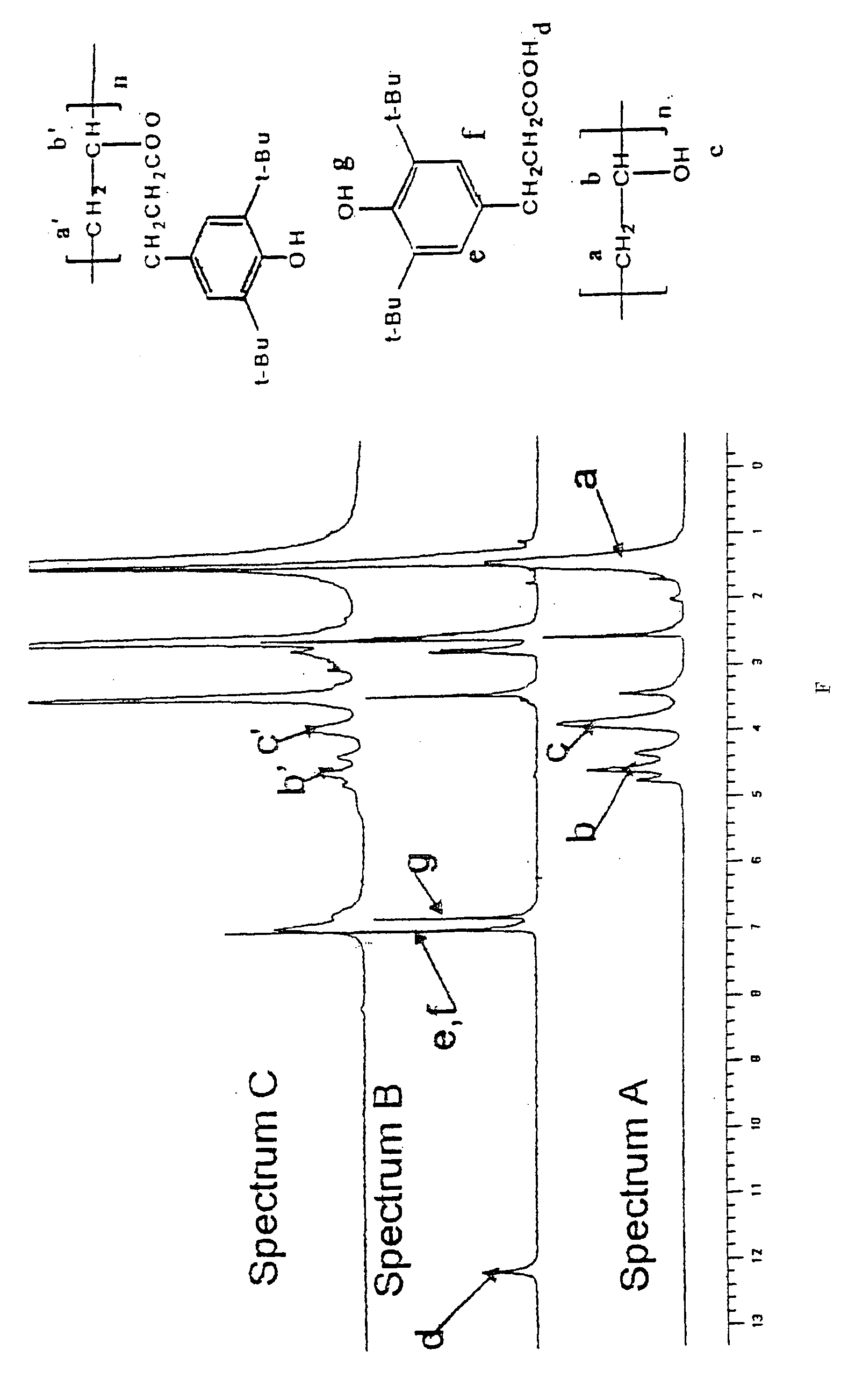
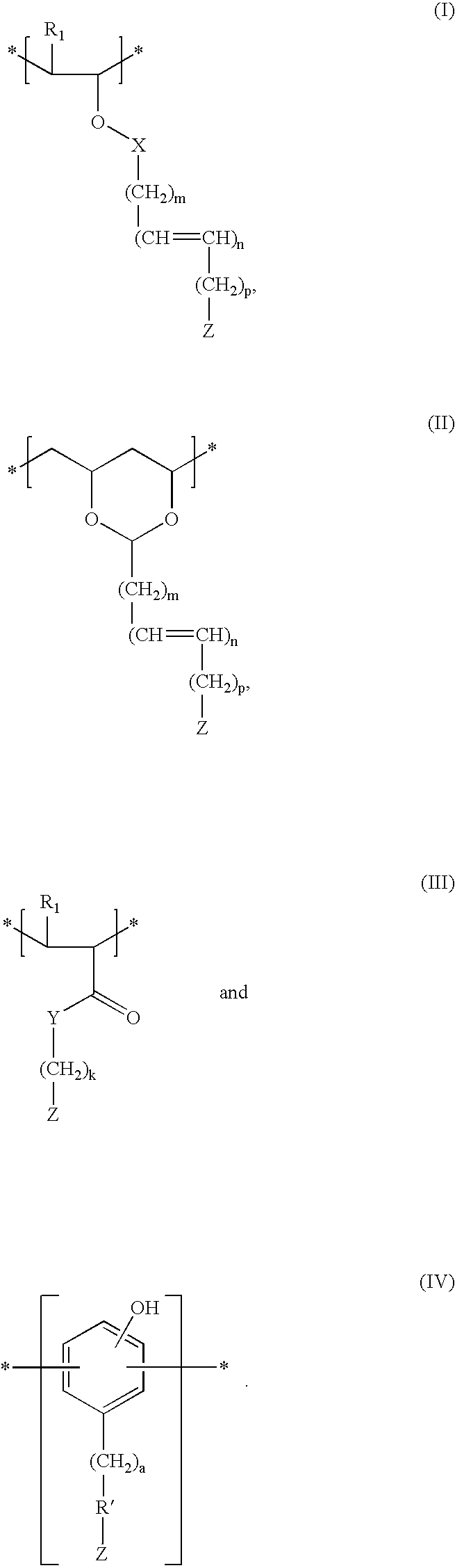
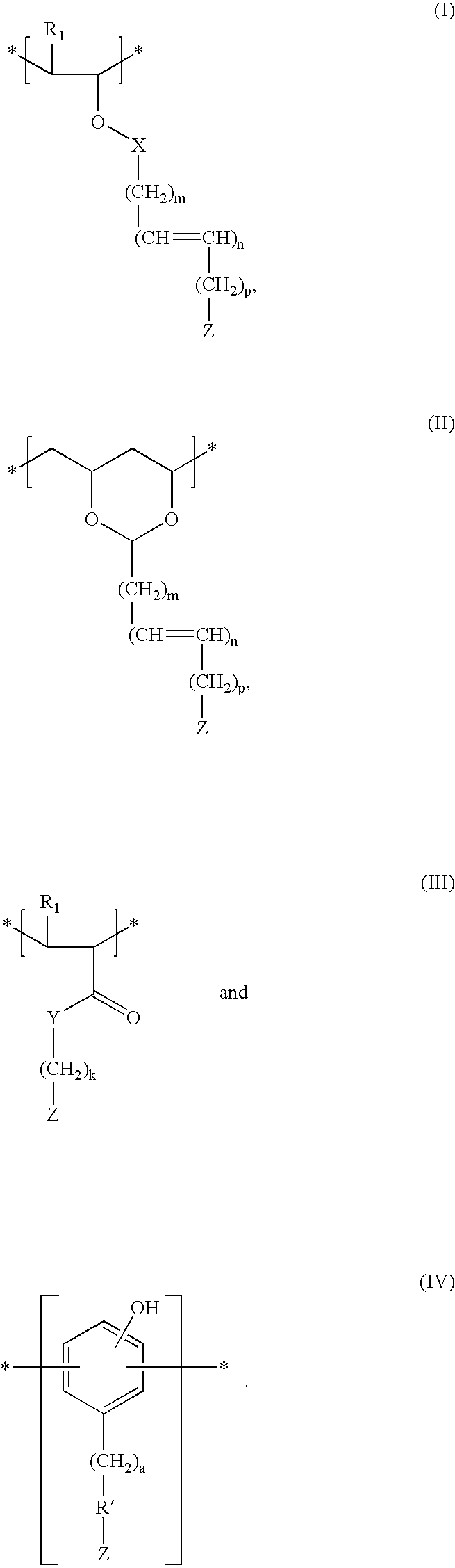
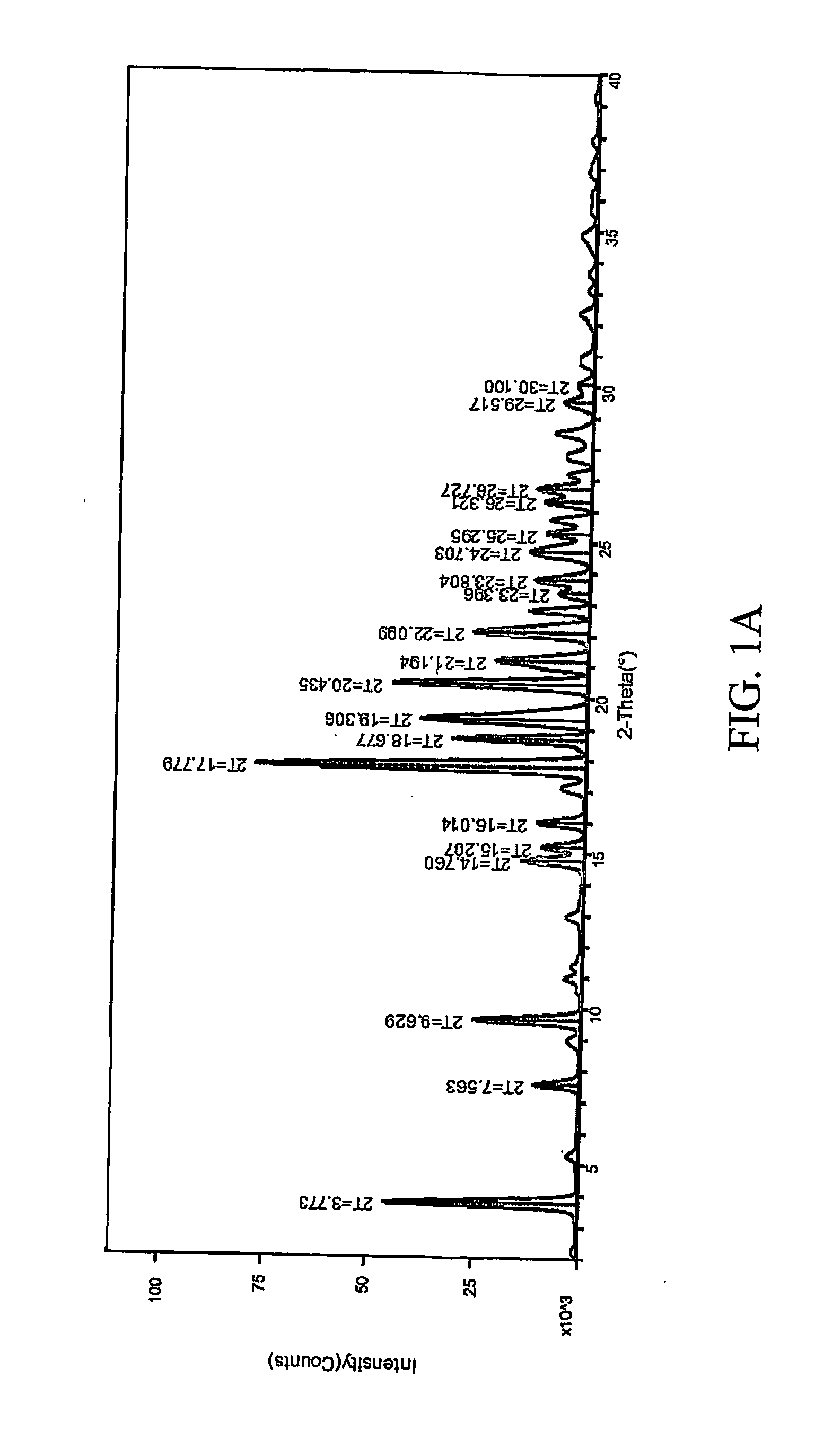
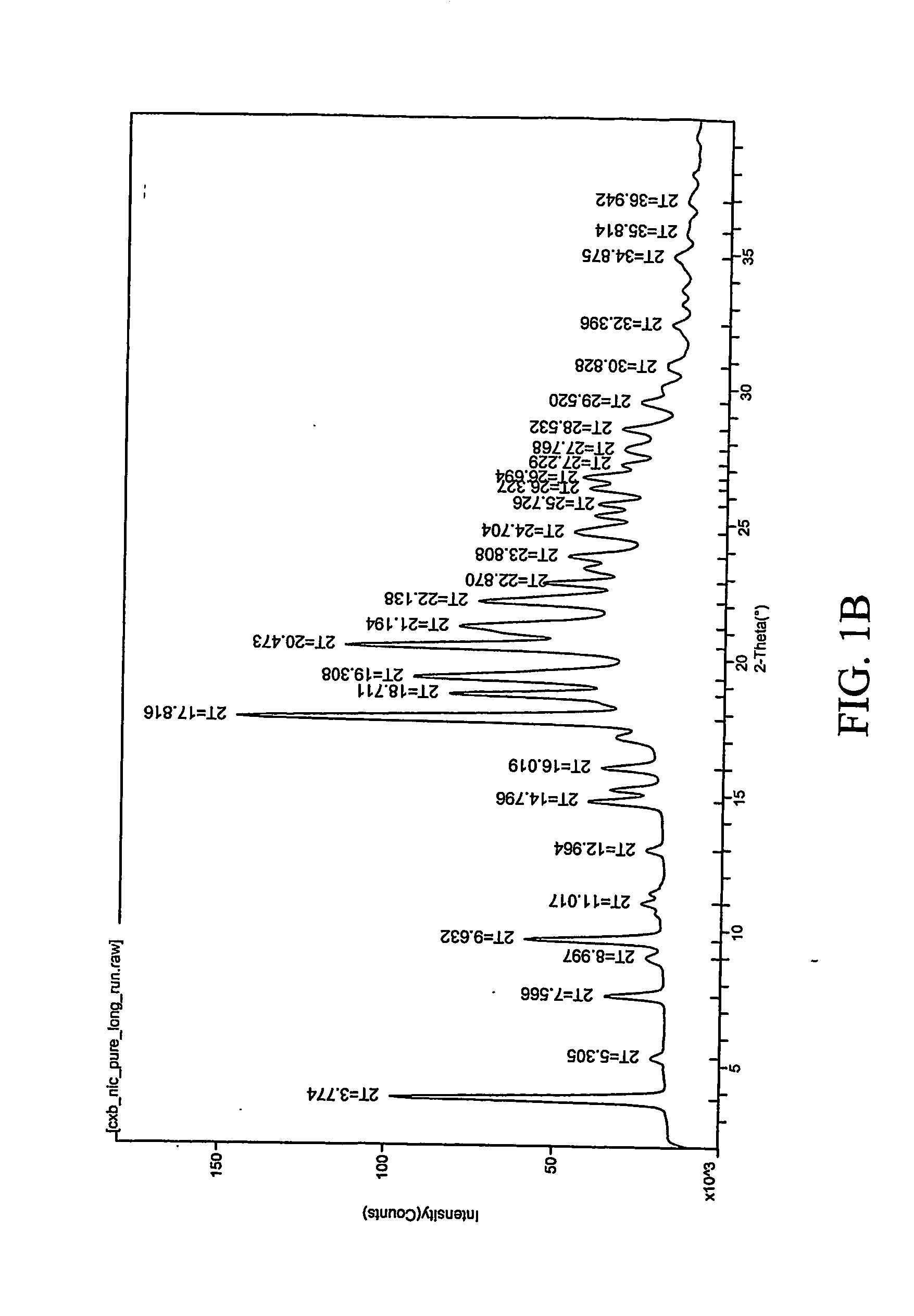
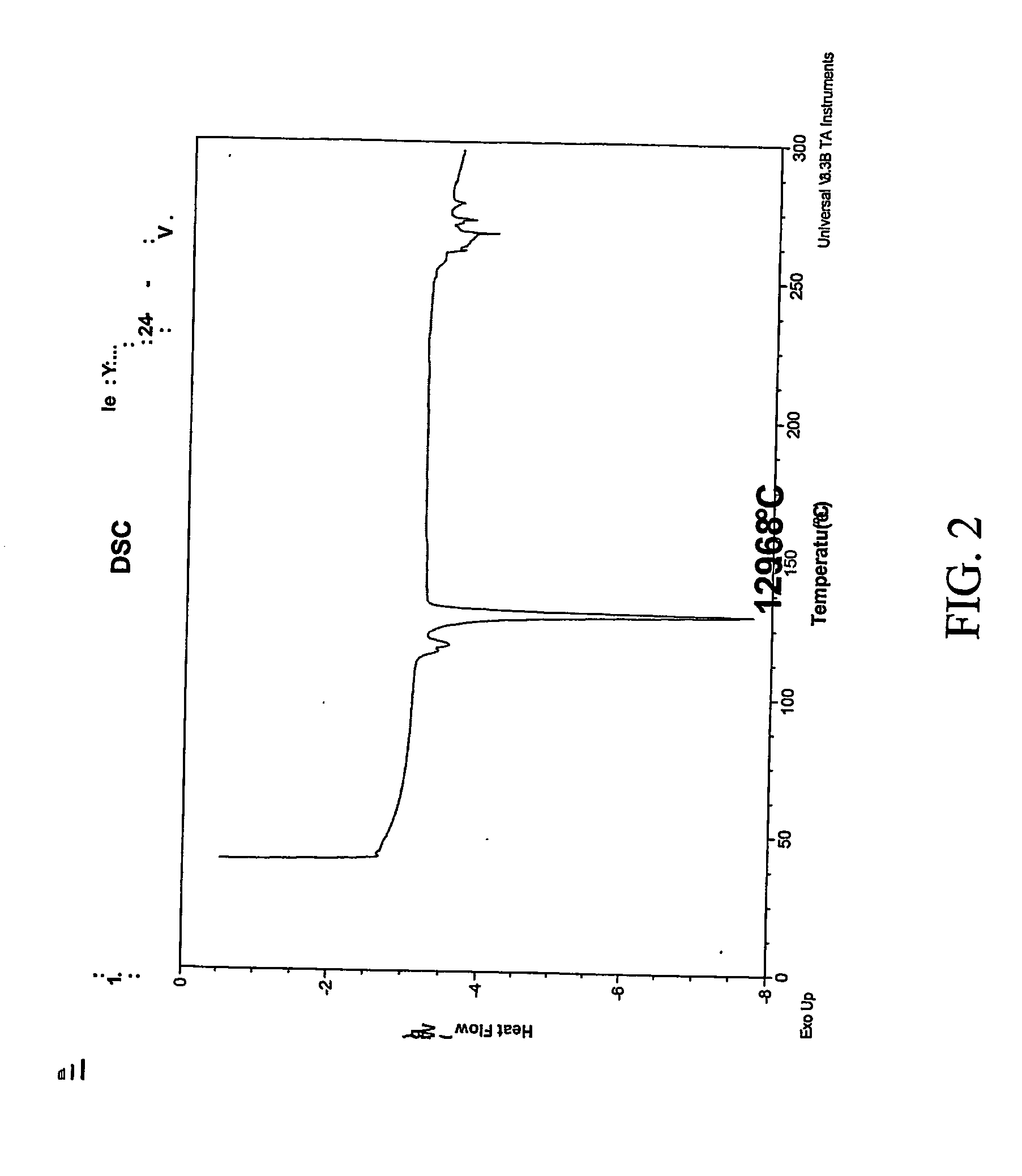
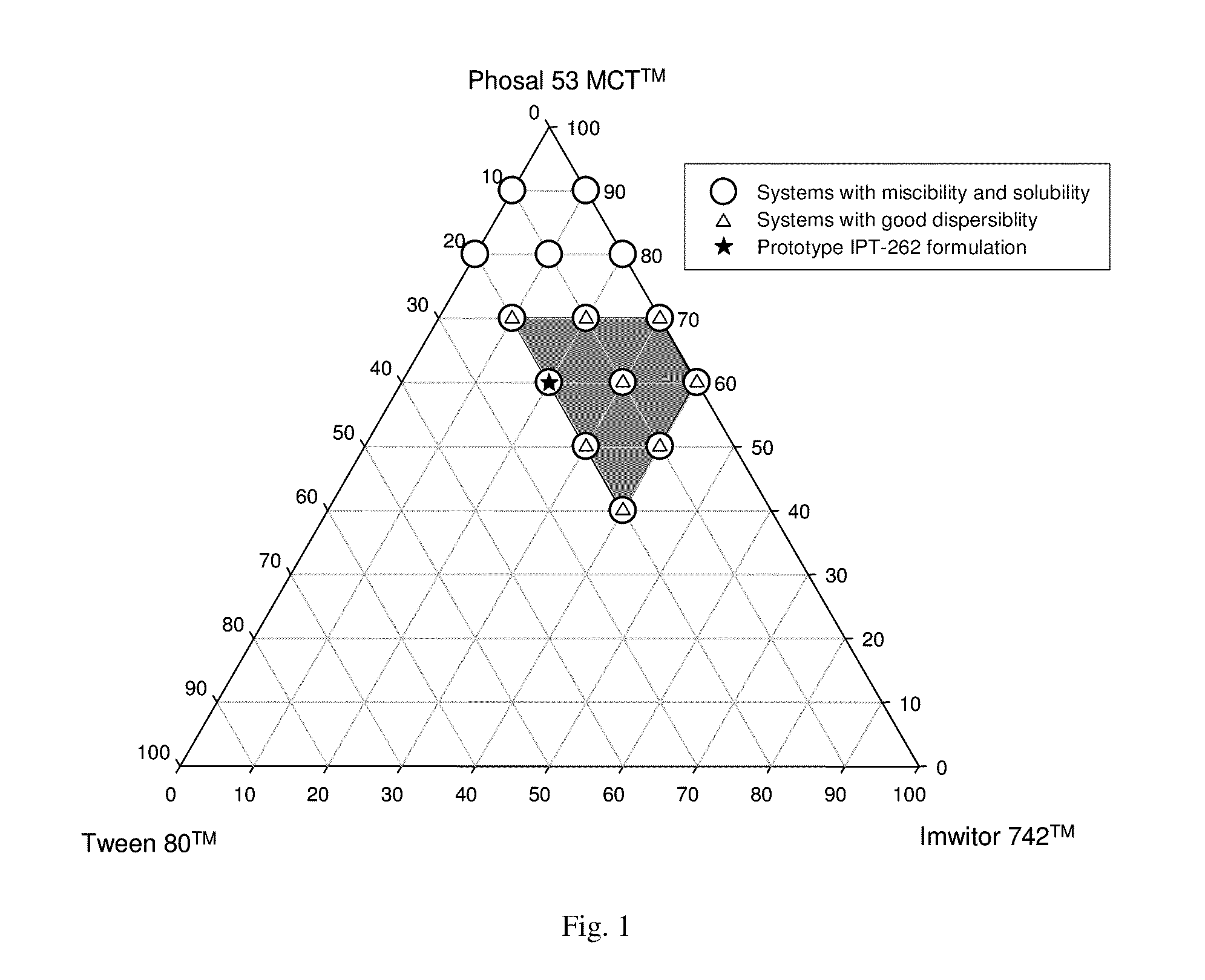
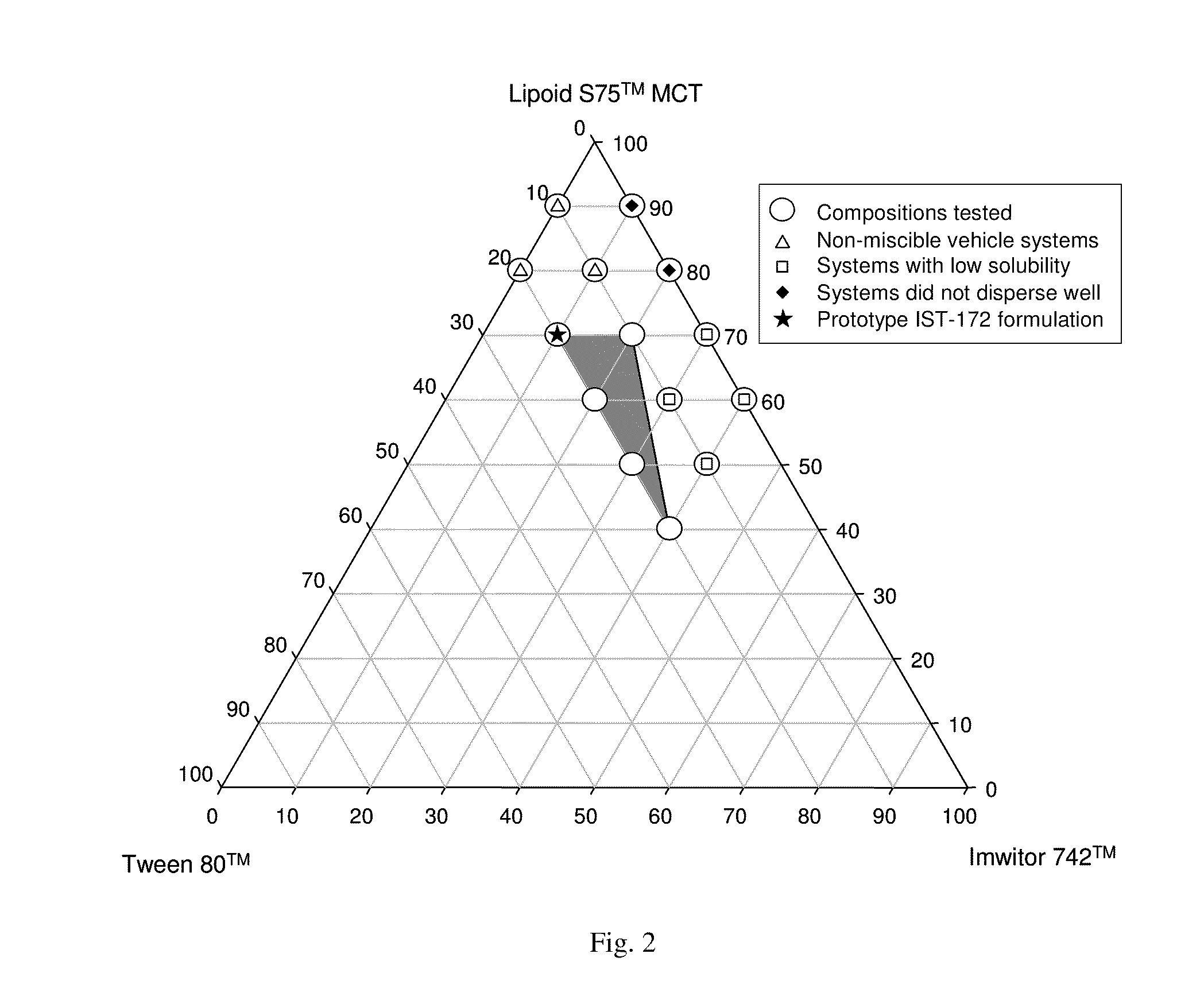
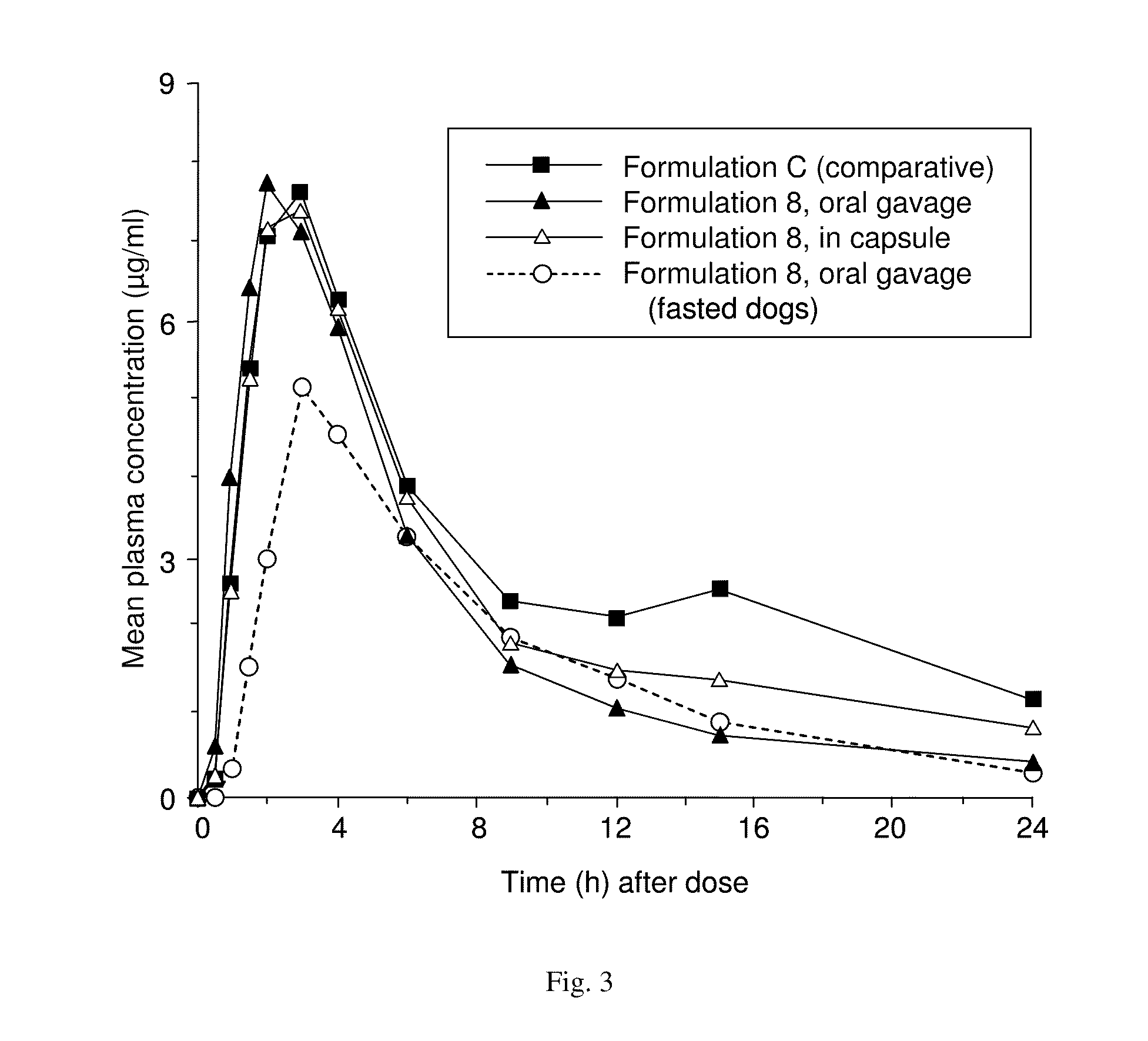
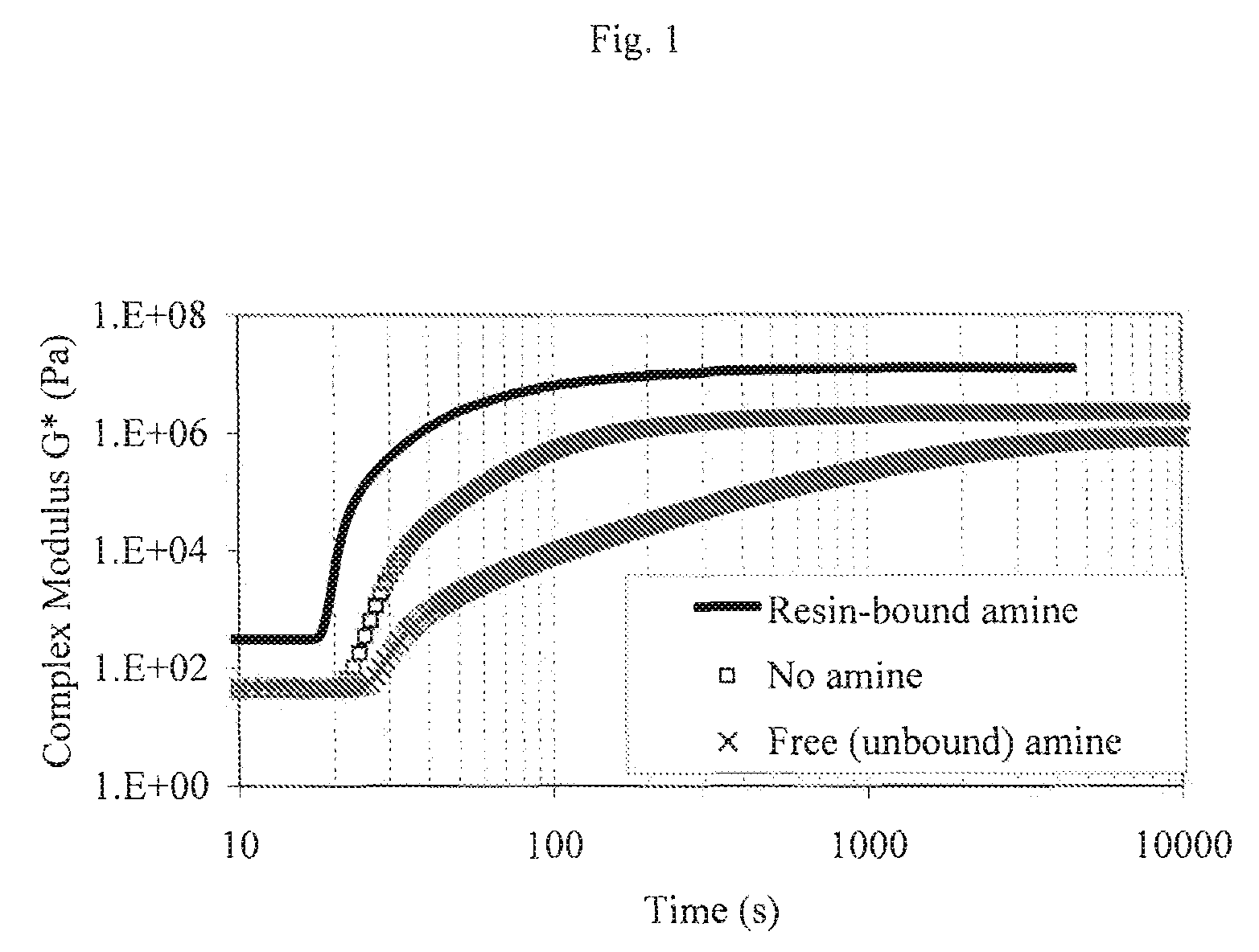
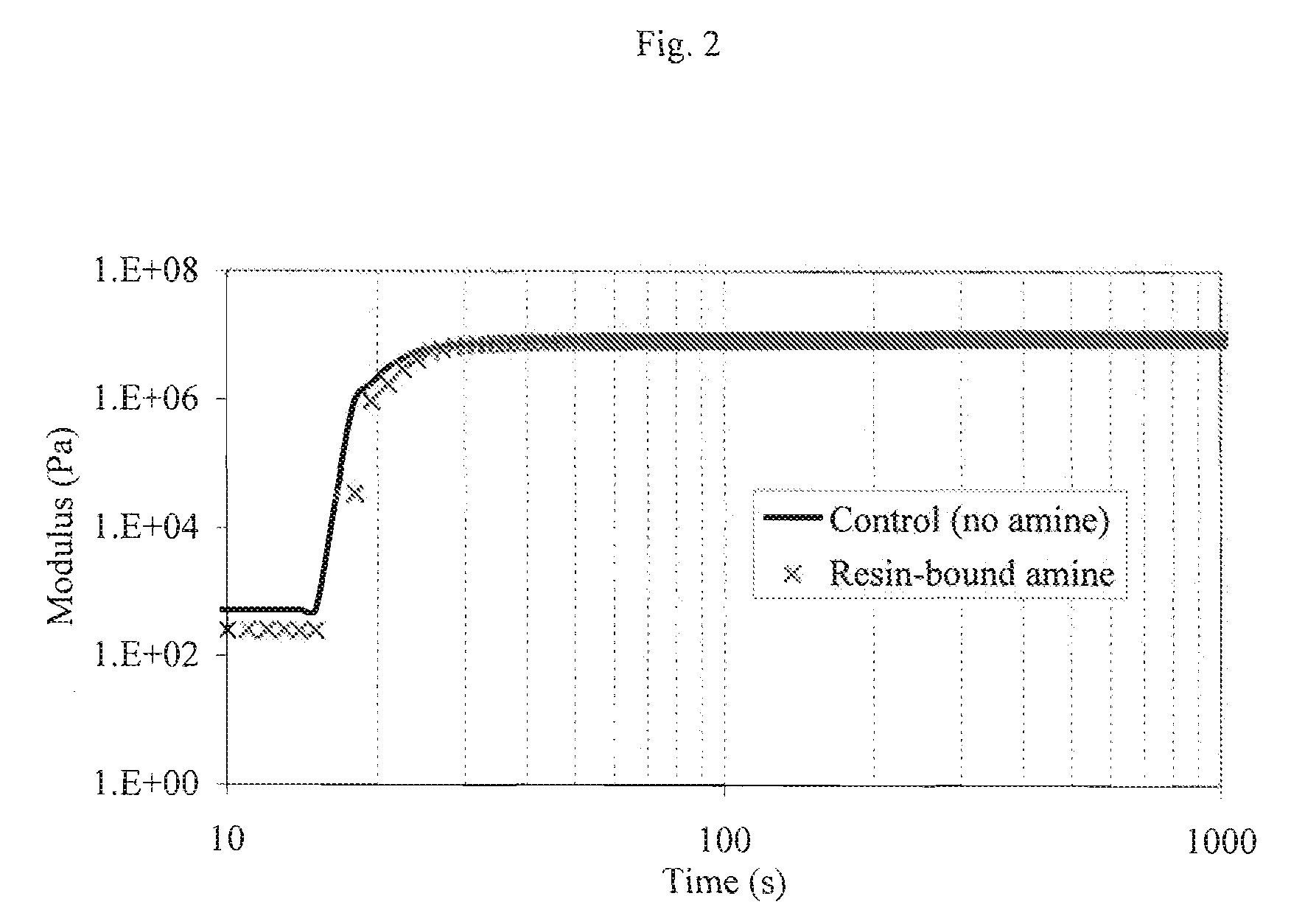
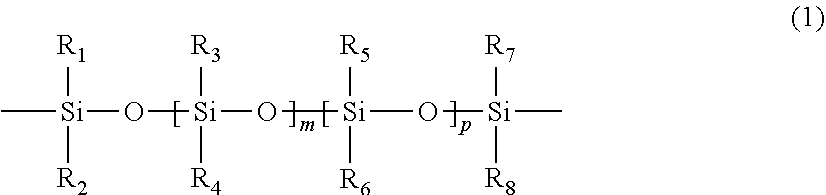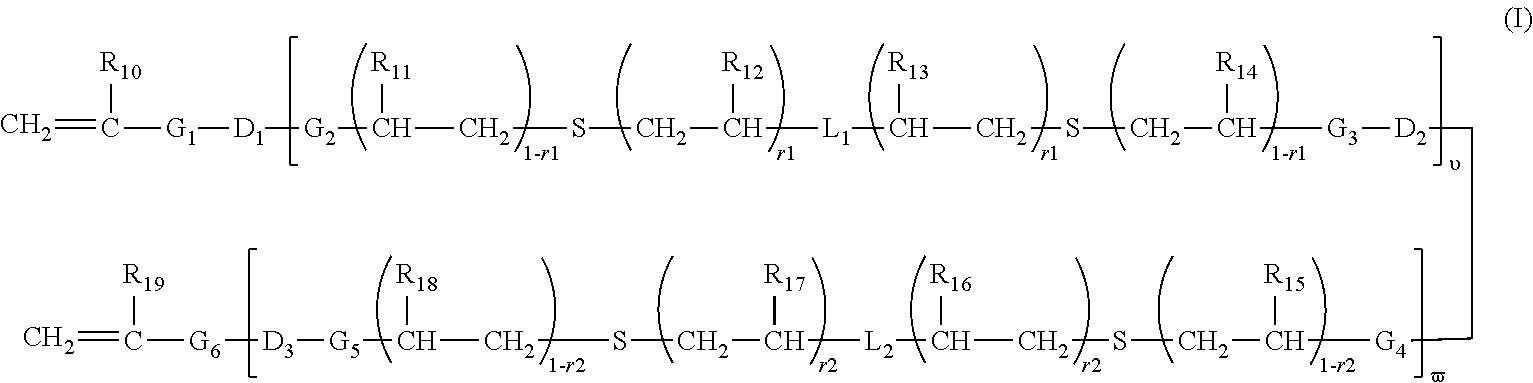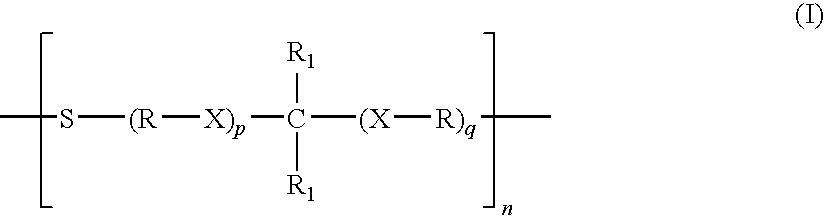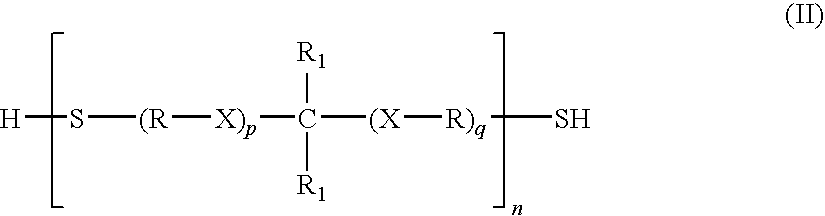Patents
Literature
2932 results about "Thioether" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
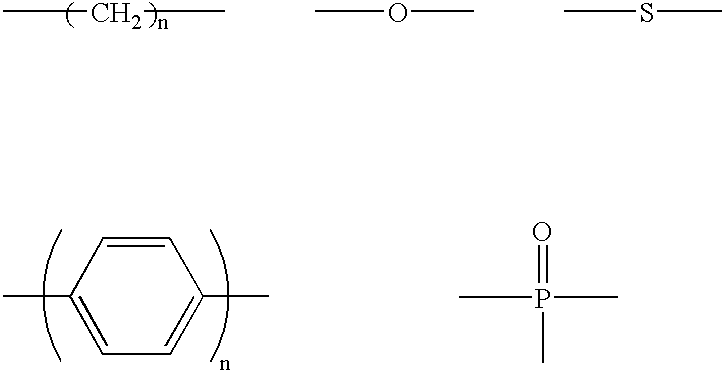
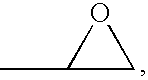
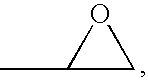
An organic sulfide (British English sulphide) or thioether is a functional group in organosulfur chemistry with the connectivity C–S–C as shown on right. Like many other sulfur-containing compounds, volatile sulfides have foul odors. A sulfide is similar to an ether except that it contains a sulfur atom in place of the oxygen. The grouping of oxygen and sulfur in the periodic table suggests that the chemical properties of ethers and sulfides are somewhat similar, though the extent to which this is true in practice varies depending on the application.
Precursor source mixtures
A precursor source mixture useful for CVD or ALD of a film comprising: at least one precursor composed of an element selected from the group consisting of Li, Na, K, Rb, Cs, Fr, Be, Mg, Ti, Zr, Hf, Sc, Y, La, V, Nb, Ta, Cr, Mo, W, Mn, Re, Fe, Ru, Os, Co, Rh, Ir, Ni, Pd, Pt, Cu, Ag, Au, Zn, Cd, Hg, B, Al, Ga, In, Tl, Si, Ge, Sn, Pb, As, P, Sb and Bi, to which is bound at least one ligand selected from the group consisting of hydride, alkyl, alkenyl, cycloalkenyl, aryl, alkyne, carbonyl, amido, imido, hydrazido, phosphido, nitrosyl, nitryl, nitrate, nitrile, halide, azide, alkoxy, siloxy, silyl, and halogenated, sulfonated or silyated derivatives thereof, which is dissolved, emulsified or suspended in an inert liquid selected from the group consisting of aliphatic hydrocarbons, aromatic hydrocarbons, alcohols, ethers, aldehydes, ketones, acids, phenols, esters, amines, alkylnitrile, halogenated hydrocarbons, silyated hydrocarbons, thioethers, amines, cyanates, isocyanates, thiocyanates, silicone oils, nitroalkyl, alkylnitrate, and mixtures thereof. The precursor source mixture may be a solution, emulsion or suspension and may consist of a mixture of solid, liquid and gas phases which are distributed throughout the mixture.
Owner:GLOBALFOUNDRIES INC
Production of tetravalent antibodies
The present invention relates to a novel process for the preparation of biologically active antibody dimers in a pharmaceutically acceptable composition. The dimers can be composed of two antibody molecules having the same antigen binding specificity and linked through reducible, disulfide, or a non-reducible thioether, bond (homodimer). Alternatively, the dimers can be composed of two different antibody molecules having binding specificity for two distinct antigens (heterodimer). These dimers are useful for inducing hyper-cross-linking of membrane antigens. The present invention further relates to the use of biologically active antibody dimers for the preferential killing or inhibition of selected cell populations in the treatment of diseases such as cancer and autoimmune disorders.
Owner:BIOGEN INC
Pharmaceutical co-crystal compositions
A pharmaceutical composition comprising a co-crystal of an API and a co-crystal former; wherein the API has at least one functional group selected from ether, thioether, alcohol, thiol, aldehyde, ketone, thioketone, nitrate ester, phosphate ester, thiophosphate ester, ester, thioester, sulfate ester, carboxylic acid, phosphonic acid, phosphinic acid, sulfonic acid, amide, primary amine, secondary amine, ammonia, tertiary amine, sp2 amine, thiocyanate, cyanamide, oxime, nitrile diazo, organohalide, nitro, s-heterocyclic ring, thiophene, n-heterocyclic ring, pyrrole, o-heterocyclic ring, furan, epoxide, peroxide, hydroxamic acid, imidazole, pyridine and the co-crystal former has at least one functional group selected from amine, amide, pyridine, imidazole, indole, pyrrolidine, carbonyl, carboxyl, hydroxyl, phenol, sulfone, sulfonyl, mercapto and methyl thio, such that the API and co-crystal former are capable of co-crystallizing from a solution phase under crystallization conditions.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES +2
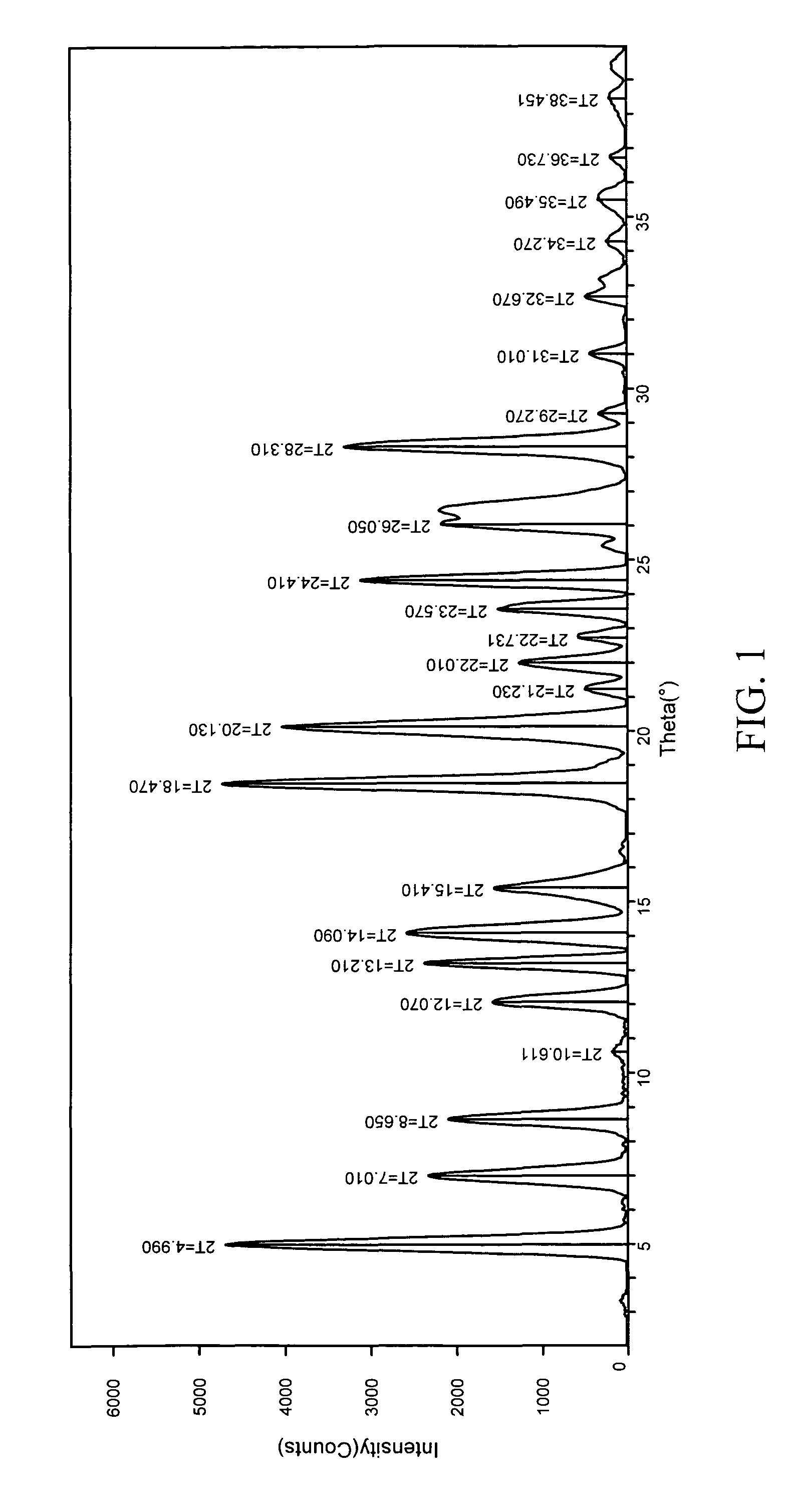
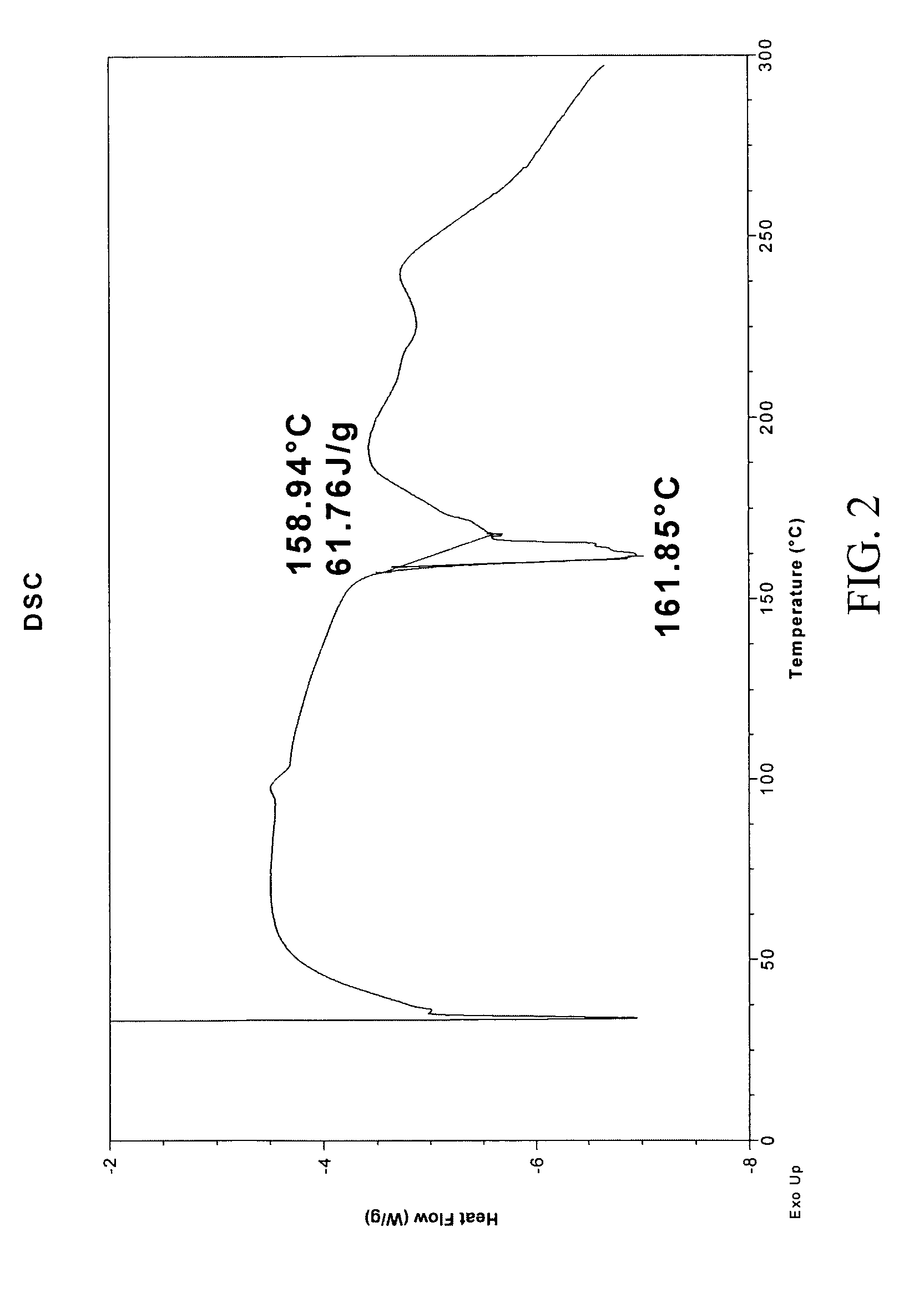
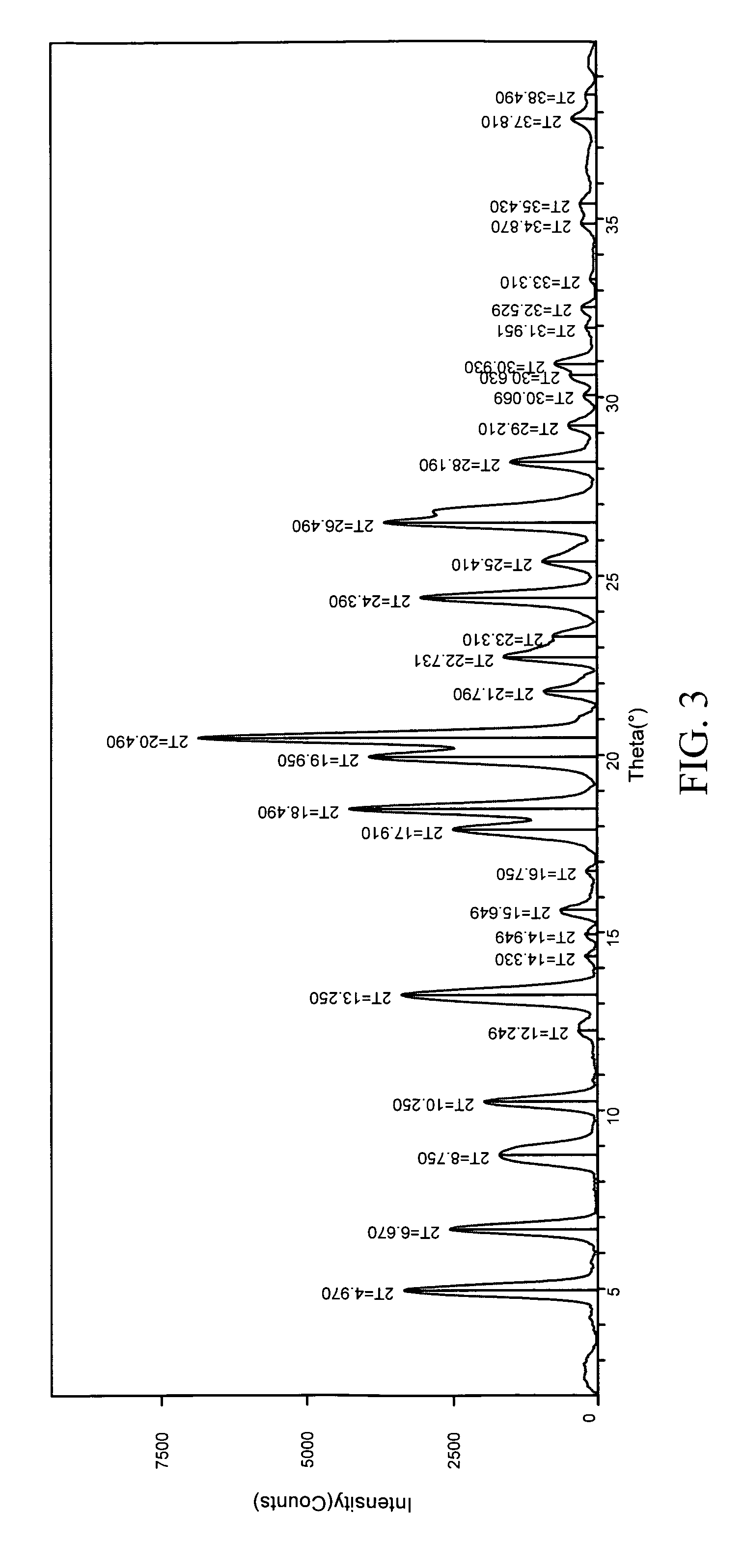
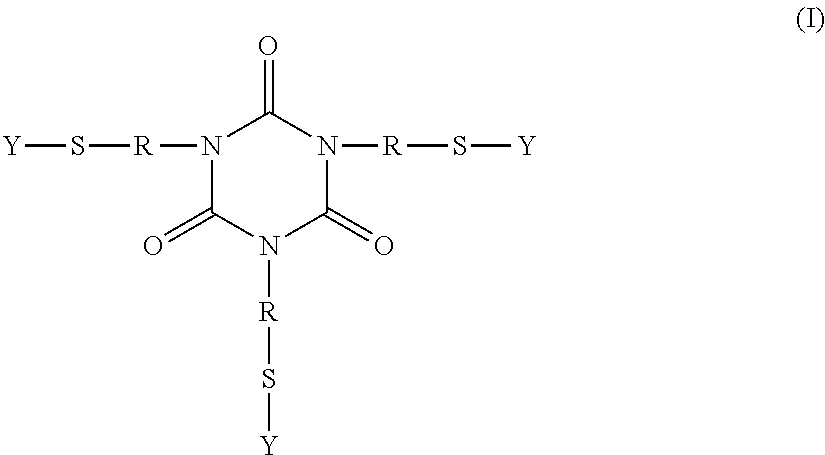
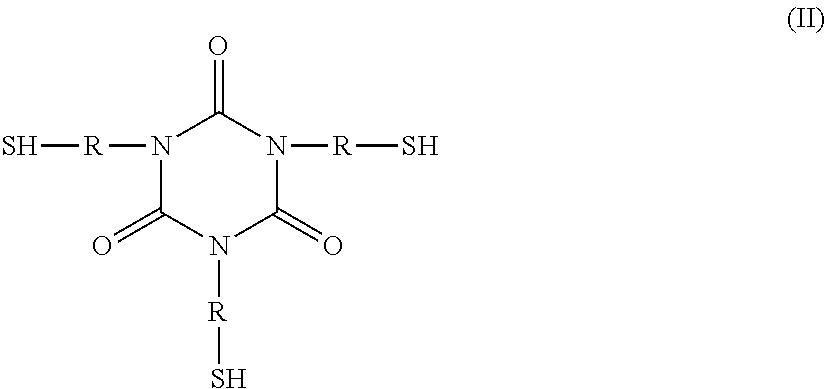
Polythioether polymers, methods for preparation thereof, and compositions comprising them
Disclosed are polythioethers that are the reaction product of reactants that include: a) an isocyanurate-containing trithiol; b) a polythiol different from (a); and c) a diene. Also disclosed are compositions, such as sealant compositions, that include such polythioethers.
Owner:PRC DE SOTO INT INC
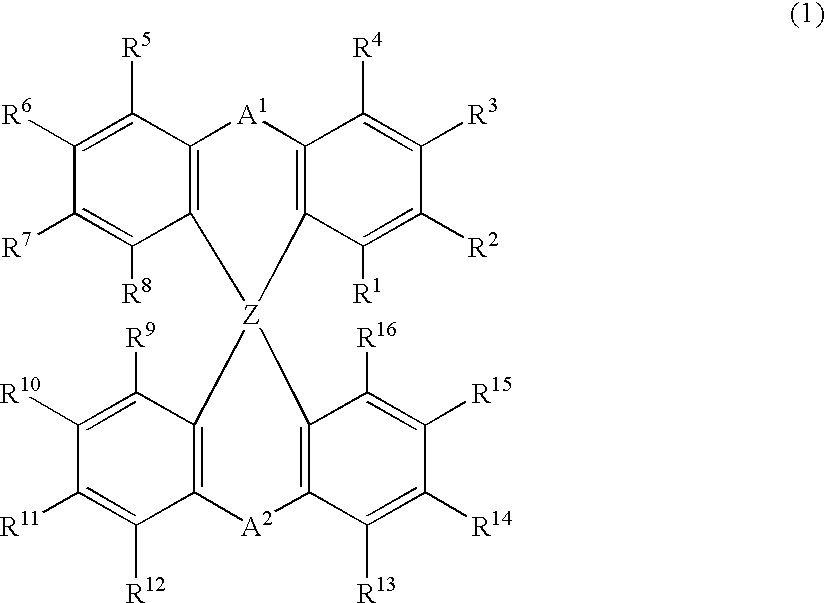
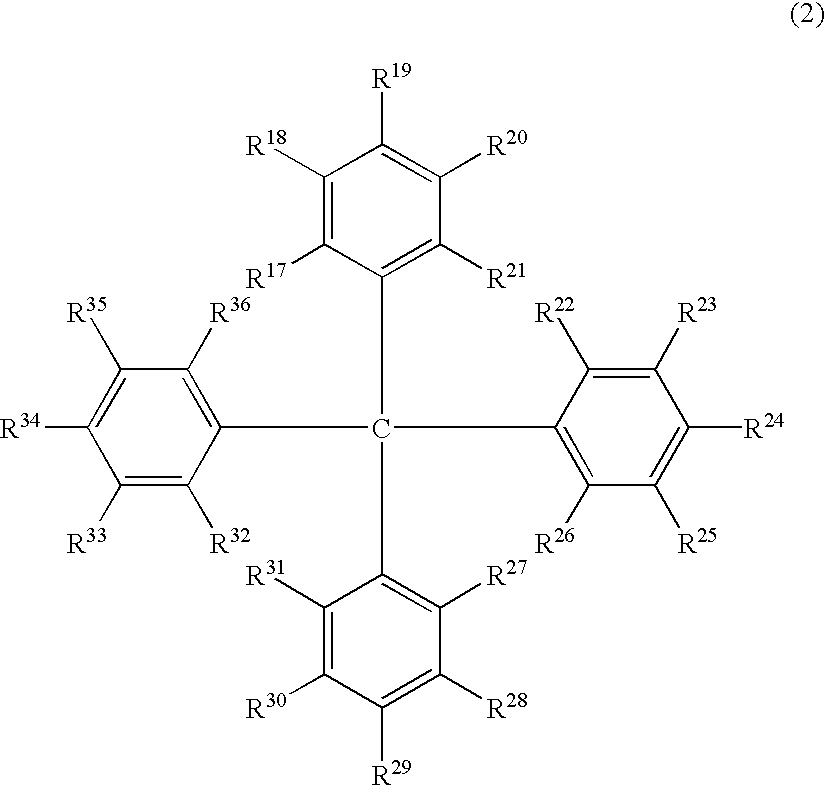
Luminescent element material and luminescent element comprising the same
The light emitting device of the present invention relates to a light emitting device which is characterized in that it is a device with an emissive substance present between an anode and cathode, and which emits light by means of electrical energy, and said device has a least one type of compound denoted by (a) to (d) below. (a) A compound having a plurality of 1,7-phenanthroline skeletal structures (b) A benzoquinoline derivative (c) A spiro compound represented by general formula (1) A1 and A2 are each selected from single bonds, substituted or unsubstituted alkyl chains, ether chains, thioether chains, ketone chains and substituted or unsubstituted amino chains. However, A1<> A2. Z represents carbon or silicon. R1 to R16 are each selected from hydrogen, alkyl group, cycloalkyl group, aralkyl group, alkenyl group, cycloalkenyl group, alkynyl group, hydroxyl group, mercapto group, alkoxy group, alkylthio group, aryl ether group, aryl thioether group, aryl group, heterocyclic group, halogen, haloalkane, haloalkene, haloalkyne, cyano group, aldehyde group, carbonyl group, carboxyl group, ester group, carbamoyl group, amino group, nitro group, silyl group, siloxanyl group and a cyclic structure formed with an adjacent substituent. (d) A tetraphenylmethane derivative represented by general formula (2) R17 to R36 are each selected from hydrogen, alkyl group, cycloalkyl group, aralkyl group, alkenyl group, cycloalkenyl group, alkynyl group, hydroxyl group, mercapto group, alkoxy group, alkylthio group, aryl ether group, aryl thioether group, aryl group, heterocyclic group, halogen, haloalkane, haloalkene, haloalkyne, cyano group, aldehyde group, carbonyl group, carboxyl group, ester group, carbamoyl group, amino group, nitro group, silyl group, siloxanyl group and a cyclic structure formed with an adjacent substituent. However, at least one of R17 to R36 is selected from substituents represented by general formula (3). -X-Ar (3) X is a single bond or is selected from the following, and Ar denotes a condensed aromatic ring or heteroaromatic ring. In the case where X is phosphorus oxide, then Ar represents an aromatic hydrocarbon or heteroaromatic ring. n is an natural number.
Owner:TORAY IND INC
Polymerizable chain-extended polysiloxanes with pendant hydrophilic groups
The invention provide a class of chain-extended polysiloxane crosslinkers which comprises (1) at least two polysiloxane segments, wherein each pair of adjacent polysiloxane segments is linked by one divalent organic radical which includes at least one pendant hydrophilic group (hydroxyl and / or carboxyl groups) or at least one dangling hydrophilic polymer chain and a di-thioether linkage —S-DR-S— in which DR is a divalent organic radical; and (2) two terminal ethylenically unsaturated groups. The present invention is also related to a polymer comprising crosslinking units derived from chain-extended polysiloxane crosslinker of the invention and to ophthalmic lenses comprising such a polymer.
Owner:ALCON INC
Pharmaceutical co-crystal compositions
InactiveUS20070026078A1Improve solubilityLow hygroscopicityBiocidePowder deliveryThioketoneHydroxamic acid
A pharmaceutical composition comprising a co-crystal of an API and a co-crystal former; wherein the API has at least one functional group selected from ether, thioether, alcohol, thiol, aldehyde, ketone, thioketone, nitrate ester, phosphate ester, thiophosphate ester, ester, thioester, sulfate ester, carboxylic acid, phosphonic acid, phosphinic acid, sulfonic acid, amide, primary amine, secondary amine, ammonia, tertiary amine, sp2 amine, thiocyanate, cyanamide, oxime, nitrile diazo, organohalide, nitro, s-heterocyclic ring, thiophene, n-heterocyclic ring, pyrrole, o-heterocyclic ring, furan, epoxide, peroxide, hydroxamic acid, imidazole, pyridine and the co-crystal former has at least one functional group selected from amine, amide, pyridine, imidazole, indole, pyrrolidine, carbonyl, carboxyl, hydroxyl, phenol, sulfone, sulfonyl, mercapto and methyl thio, such that the API and co-crystal former are capable of co-crystallizing from a solution phase under crystallization conditions.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES +2
Polymerizable chain-extended polysiloxanes with pendant hydrophilic groups
The invention provide a class of chain-extended polysiloxane crosslinkers which comprises (1) at least two polysiloxane segments, wherein each pair of adjacent polysiloxane segments is linked by one divalent organic radical which includes at least one pendant hydrophilic group (hydroxyl and / or carboxyl groups) or at least one dangling hydrophilic polymer chain and a di-thioether linkage —S-DR—S— in which DR is a divalent organic radical; and (2) two terminal ethylenically unsaturated groups. The present invention is also related to a polymer comprising crosslinking units derived from chain-extended polysiloxane crosslinker of the invention and to ophthalmic lenses comprising such a polymer.
Owner:ALCON INC
Composite of aluminum alloy and resin composition and process for producing the same
InactiveCN1717323ASynthetic resin layered productsThin material handlingPolytetramethylene terephthalateSurface roughness
The invention relates to a complex, which is characterized in that the complex consists of an aluminium alloy shape and a thermoplastic resin combination; the roughness of surface of the complex is more than 5 to 50 Mu m; the surface is provided with the aluminium alloy shape with minute recessed parts or raised parts, the diameter of which is less than 1 Mu m; the aluminium alloy shape is fixed through the recessed parts or raised parts; with the longitudinal and transverse average coefficientof linear expansion ranging from 2X<-5> DEG C<-1> to 4X<-5> DEG C<-1>, the thermoplastic resin combination is primarily made of polybutylene terephthalate resin or polyphenyl thioether; as the thermoplastic resin combination is not peeled off easily from the aluminium alloy shape, the invention has the advantages of metal enclosers and synthetic resin structures in electronic machines and electric appliances. The production efficiency is high, and the complex can be produced on large scale with shape and structure to be designed freely.
Owner:TAISEI PLAS CO LTD
Low temperature liquid polythioether polymers
Owner:PRC DE SOTO INT INC
Tricyclic arylamine containing polymers and electronic devices therefrom
InactiveUS20040127666A1Improve conductivityImprove efficiencySolid-state devicesSemiconductor/solid-state device manufacturingArylMedicinal chemistry
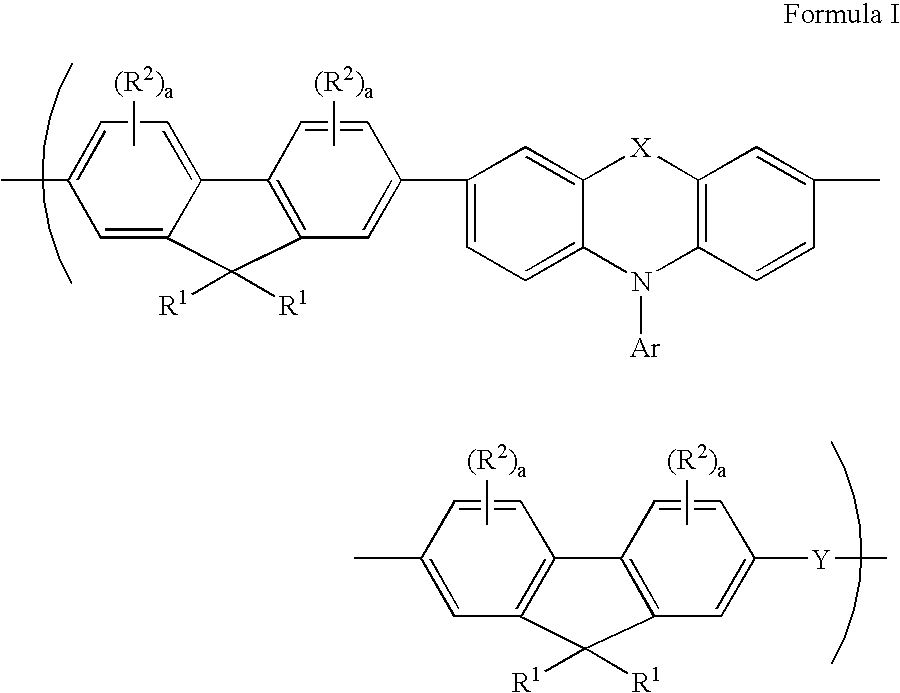
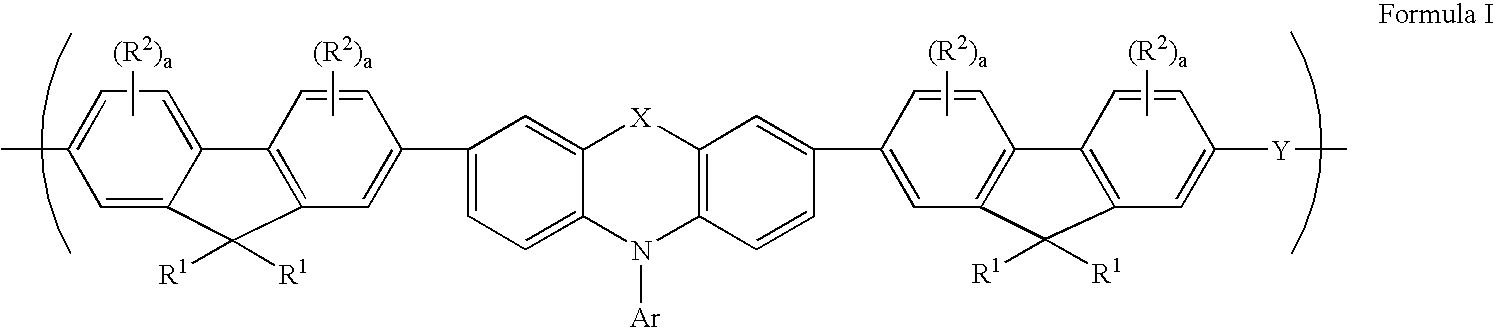
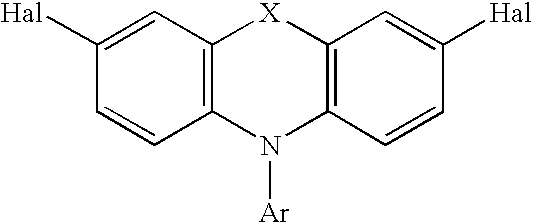
A composition comprising a polymer comprising a repeat unit of Formula I.: wherein R<1 >is independently in each occurrence H, C1-40 hydrocarbyl or C3-40 hydrocarbyl containing one or more S, N, O, P or Si atoms, or both of R<1 >together with the 9-carbon on the fluorene, may form a C5-20 ring structure which may contain one or more S, N, or O atoms; R2 is independently in each occurrence C1-20 hydrocarbyl, C1-20 hydrocarbyloxy, C1-20 thioether, C1-20 hydrocarbyloxycarbonyl, C1-20 hydrocarbylcarbonyloxy, or cyano; a is independently in each occurrence, 0 or 1; X is O, S, SO2, C(R<3>)2 or N-R<3 >wherein R<3 >is aryl or substituted aryl of C6 to C40, aralkyl of C6 to C24, or alkyl of C1 to C24. Preferably R<3 >is aryl of C6 to C24 and more preferably R<3 >is an alkylated aryl group of C6 to C24; Ar is an aryl or heteroaryl group of C6 to C40 or substituted aryl or heteroaryl group of C6 to C40, preferably C6-C24, and most preferably C6-C14. Y is a conjugated moiety that can vary in each occurrence of the repeat unit.
Owner:DOW GLOBAL TECH LLC
Hydrosilylation catalysts and silicone compositions using the same
InactiveUS6140446AShort pot lifeReduce concentrationMetal/metal-oxides/metal-hydroxide catalystsHydrosilylationSiloxane
A hydrosilylation catalyst is provided wherein a platinum catalyst is enclosed in a heat-fusible compound having a melting point of 40-200 DEG C. and containing an aliphatic unsaturated bond, carbonyl, carboxyl or thioether radical in a molecule. The catalyst is blended in organo-polysiloxane to form a silicone composition having both shelf stability and fast-curing capability.
Owner:SHIN ETSU CHEM IND CO LTD
Molecules comprising linked organic moieties as flavor modifiers for comestible compositions
InactiveUS20060257543A1Enhancing savory tasteIncrease sweet tasteFood preparationMonosodium glutamatePharmacy medicine
The inventions disclosed herein relate to genuses of non-naturally occurring small molecule compounds which comprise two or optionally three organic moieties of limited size “linked” by certain structurally related “linker” functional groups. Suitable linker groups include ester, amine, ether, keto, imino, thioamide, thioether, sulfonamide, sulfonate ester, sulfone, guanidine, and thiourea groups. The compounds are capable, when contacted with comestible food or drinks or pharmaceutical compositions, at concentrations preferably on the order of about 100 ppm or lower, of serving as savory (“umami”) or sweet taste modifiers, savory or sweet flavoring agents and savory or sweet flavor enhancers, for use in foods, beverages, and other comestible or orally administered medicinal products or compositions, optionally in the presence of or in mixtures with conventional flavoring agents such as monosodium glutamate or known natural or artificial sweeteners.
Owner:SENOMYX INC
Ketoxime ester photoinitiator
ActiveCN101565472AImprove applicabilityApplication performance (good sensitivityOrganic compound preparationPhotomechanical apparatusCarbazoleMethyl benzene
The invention relates to a ketoxime ester photoinitiator, in particular to a ketoxime ester photoinitiator for a photo-curing material. The ketoxime ester photoinitiator has a chemical structural formula as the right, wherein in a R1 structure, n is an integer of between 0 and 5; m is an integer of between 1 and 6; R2 is methyl, phenyl, substituted phenyl, benzyl or substituted benzyl; and R3 is diphenyl sulfide group, substituted diphenyl sulfide group, carbazole group or substituted carbazole group. The ketoxime ester photoinitiator solves the problems of poor application performance and poor thermal stability of the prior OXE-1 ketoxime ester photoinitiator.
Owner:CHANGZHOU TRONLY NEW ELECTRONICS MATERIALS
Absorbing liquid for eliminating sulfide from gas mixture
InactiveCN1421264AReduce processing stepsEconomical desulfurization methodDispersed particle separationSulfur preparation/purificationSulfolaneMorpholine
The present invention belongs to gas producing technology. The absorbing liquid for eliminating sulfide from gas mixture includes main absorbent comprising steric hindrance amine and N-methyl diethanolamine; cosolvent of one or several of sulfolane, N-methyl pyrrolidine, polyglycol dialkyl ether, morpholine and its derivative; catalyst of one or several of C2-C12 alkanolamine, piperazine and its derivative, quinoline, urea and metal phthalocyanine complex. Using the absorbing liquid can eliminate H2S, COS, mercaptan, thioether and other sulfide in less steps.
Owner:江苏蓝电环保股份有限公司
Thioethers, methods for their preparation, and compositions including such thioethers
Disclosed are thioethers, methods for preparing such thioethers, and curable compositions, such as coating and sealant compositions, that include such thioethers. The thioethers can be the reaction product of (a) an alpha, omega dihalo organic compound, (b) a metal hydrosulfide, and (c) a metal hydroxide.
Owner:PRC DE SOTO INT INC
Method for anion-exchange adsorption and anion-exchangers
InactiveUS6702943B1Reduced ligand contentSolve insufficient capacityIon-exchanger regenerationOrganic anion exchangersHydrogenDesorption
A method for the removal of a substance carrying a negative charge and being present in an aqueous liquid (I). The method comprises the steps of: (i) contacting the liquid with a matrix carrying a plurality of ligands comprising a positively charged structure and a hydrophobic structure, and (ii) desorbing the substance. The characterizing feature is that (I) each of said ligands together with a spacer has the formula: -SP-[Ar-R1-N<+>(R2R3R4)] where (A) [Ar-R1-N<+>(R2R3R4)] represents a ligand a) Ar is an aromatic ring, b) R1 is [(L)nR'1]m where n and m are integers selected amongst zero or 1; L is amino nitrogen, ether oxygen or thioether sulphur; R'1 is a linker selected among 1) hydrocarbon groups; 2) -C(=NH)-; c) R2-4 are selected among hydrogen and alkyls; (B) SP is a spacer providing a carbon or a heteroatom directly attached to Ar-R1-N<+>(R2R3R4); (C)-represents that SP replaces a hydrogen in (Ar-R1-N<+>(R2R3R4); (D)-represents binding to the matrix; and (II) desorption. There is also described (a) anion-exchangers having high breakthrough capacities, (b) a screening method and (c) a desalting protocol.
Owner:CYTIVA BIOPROCESS R&D AB
Post-coupling synthetic approach for polymeric antioxidants
A method of preparing an antioxidant polymer includes forming or obtaining a first polymer having reactive pendant groups, where the first polymer does not include cyclic anhydride repeat units, and derivatizing the first polymer with an antioxidant. Another method of preparing an antioxidant polymer includes forming or obtaining a first polymer having reactive pendant groups and derivatizing the first polymer with an antioxidant, where the antioxidant is attached to the first polymer by an acetal, amide, amine, carbamate, carbonate, ester, ether or thioether linkage or by a carbon-carbon bond. The invention is also directed to polymers that are generally prepared by these methods, compositions that include such polymers and methods of using such polymers.
Owner:UNIVERSITY OF MASSACHUSETTS LOWELL
Pharmaceutical co-crystal compositions of drugs such as carbamazepine, celecoxib, olanzapine, itraconazole, topiramate, modafinil, 5-fluorouracil, hydrochlorothiazide, acetaminophen, aspirin, flurbiprofen, phenytoin and ibuprofen
A pharmaceutical composition comprising a co-crystal of an API and a co-crystal former; wherein the API has at least one functional group selected from ether, thioether, alcohol, thiol, aldehyde, ketone, thioketone, nitrate ester, phosphate ester, thiophosphate ester, ester, thioester, sulfate ester, carboxylic acid, phosphinic acid, phosphonic acid, sulfonic acid, amide, primary amine, secondary amine, ammonia, tertiary amine, imine, thiocyanate, cyanamide, oxime, nitrile diazo, organohalide, nitro, S-heterocyclic ring, thiophene, N-heterocyclic ring, pyrrole, 0-heterocyclic ring, furan, epoxide, peroxide, hydroxamic acid, imidazole, pyridine and the co-crystal former has at least one functional group selected from amine, amide, pyridine, imidazole, indole, pyrrolidine, carbonyl, carboxyl, hydroxyl, phenol, sulfone, sulfonyl, mercapto and methyl thio, such that the API and co-crystal former are capable of co-crystallizing from a solution phase under crystallization conditions.
Owner:UNIV OF SOUTH FLORIDA +3
Absorption-biological treatment method for malodorous gas
The invention relates to an absorption and biological treatment method of malodorous waste gas. The malodorous waste gas enters a spray absorption tower to have an absorbing, humidifying and cooling function, and then enters into a biological dripping and filtrating device and contacts with a biological film attached to the surface of the filler in the process of rising to be degraded into odorless compounds, and then the purified gas is discharged. The residence time of a vacant bed is 11 to 45 seconds. Part of the waste water produced when spraying and absorbing can be used as biological circulating water, which can be reused again as spraying water after the biochemical treatment. The absorption and biological treatment method of the invention can effectively remove the odor polluting matters such as organic amine, ammonia gas, hydrogen sulfide, mercaptan type, dimethyl sulfide (DMS), dimethyl disulfide (DMDS), styrene, volatile organic compounds (VOC), dimethyl trisulfide, benzylamine, carbon disulfide, carbonyl sulfide, etc. and all kinds of malodorous smells, most of which are poisonous and a plurality of which are carcinogens. The deodorization effect can reach 99 percent, which can discharge by reaching the standard and can be applied to a deodorization and purification treatment of the large-capacity matters polluted by different malodorous gas with low concentration.
Owner:SUN YAT SEN UNIV
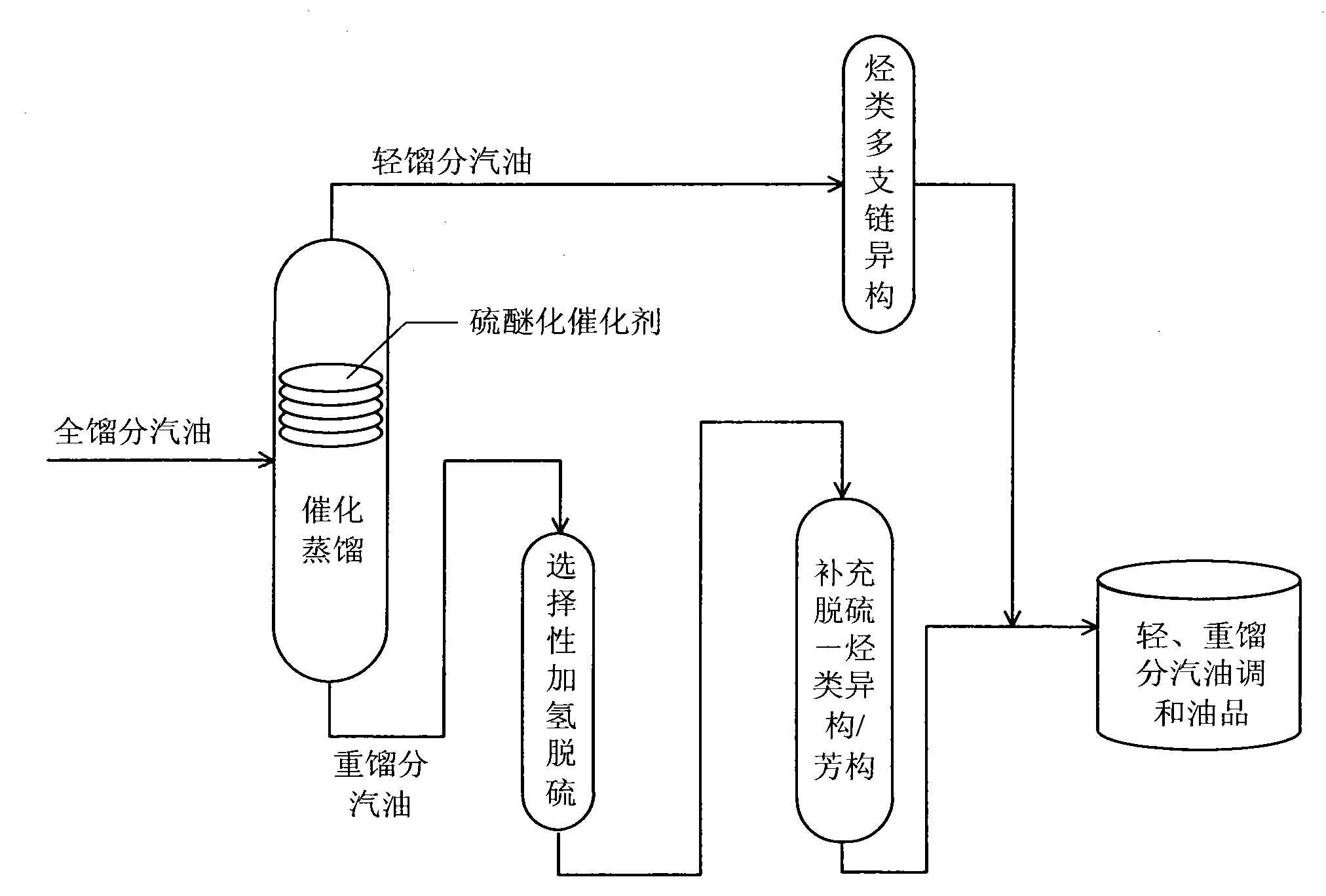
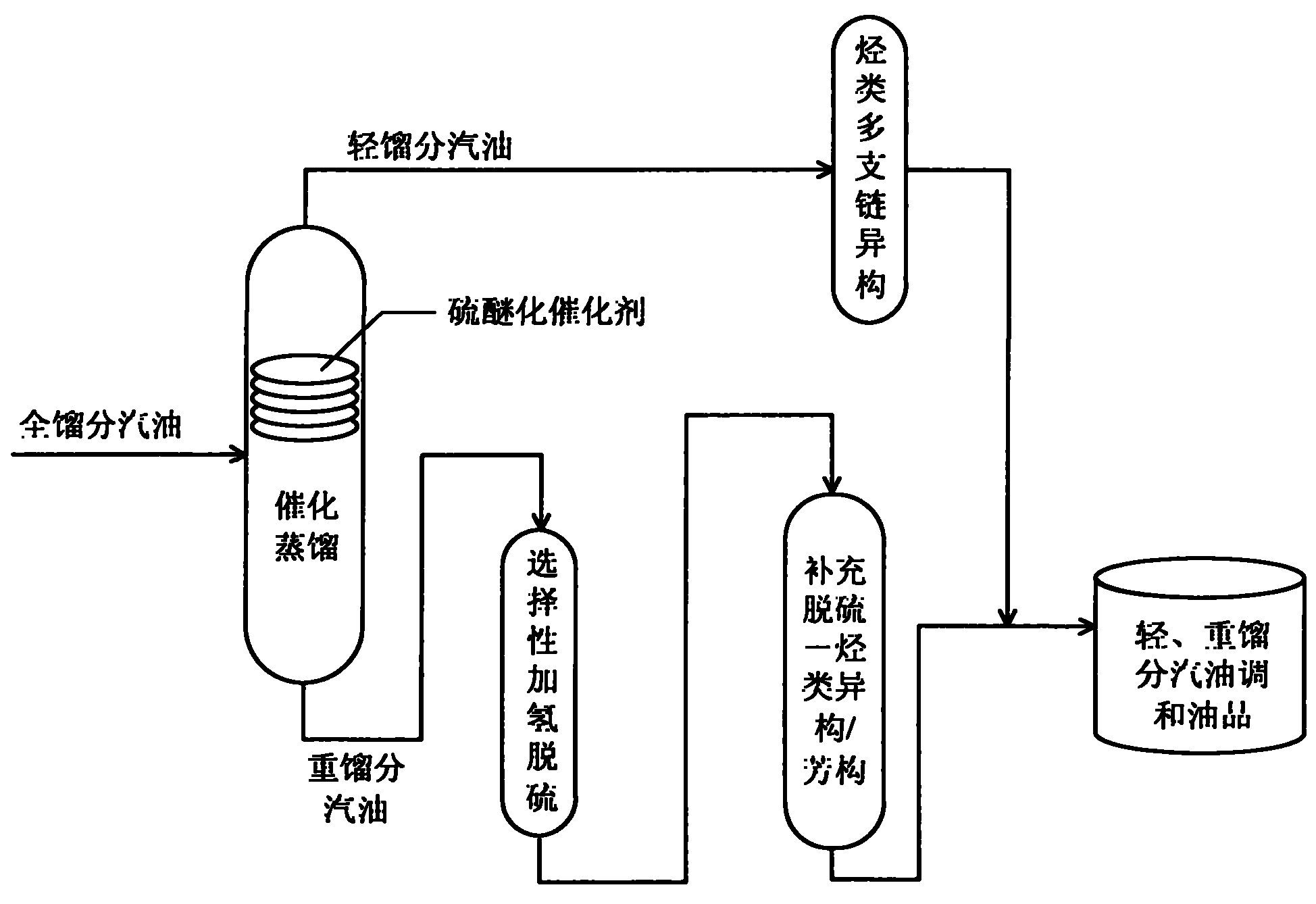
Production method for ultra-low sulfur and high-octane number gasoline
ActiveCN101885985ATake advantage ofIncrease temperatureTreatment with hydrotreatment processesIsomerizationHydrodesulfurization
The invention relates to a production method for ultra-low sulfur and high-octane number gasoline. The method comprises the following steps of: filling a poor-quality full range gasoline raw material in a reaction distillation column to contact the material with a sulfoether catalyst to perform a sulfur ether reaction and fraction cutting so that low-boiling point sulfides, such as thiol and thiophene, are converted into high-boiling point sulfoether which is then transferred into heavy fraction gasoline, wherein the cutting fractionation temperature of light fraction gasoline and the heavy fraction gasoline is 50 to 90 DEG C; contacting the light fraction gasoline with a hydrocarbon highly branched isomerization catalyst; contacting the heavy fraction gasoline with a selective hydrodesulfurization catalyst and a desulfurization-hydrocarbon isomerization / aromatization catalyst; and mixing the treated light fraction gasoline and the heavy fraction gasoline to obtain the ultra-low sulfur and high-octane number gasoline. The method is suitable for modifying poor-quality gasoline, can reach better desulfurization and olefin reduction effects on ultra-high sulfur and high-olefin poor-quality catalytic gasoline, and can maintain or increase the octane number of the product and keep a higher product yield after reaction.
Owner:CHINA UNIV OF PETROLEUM (BEIJING)
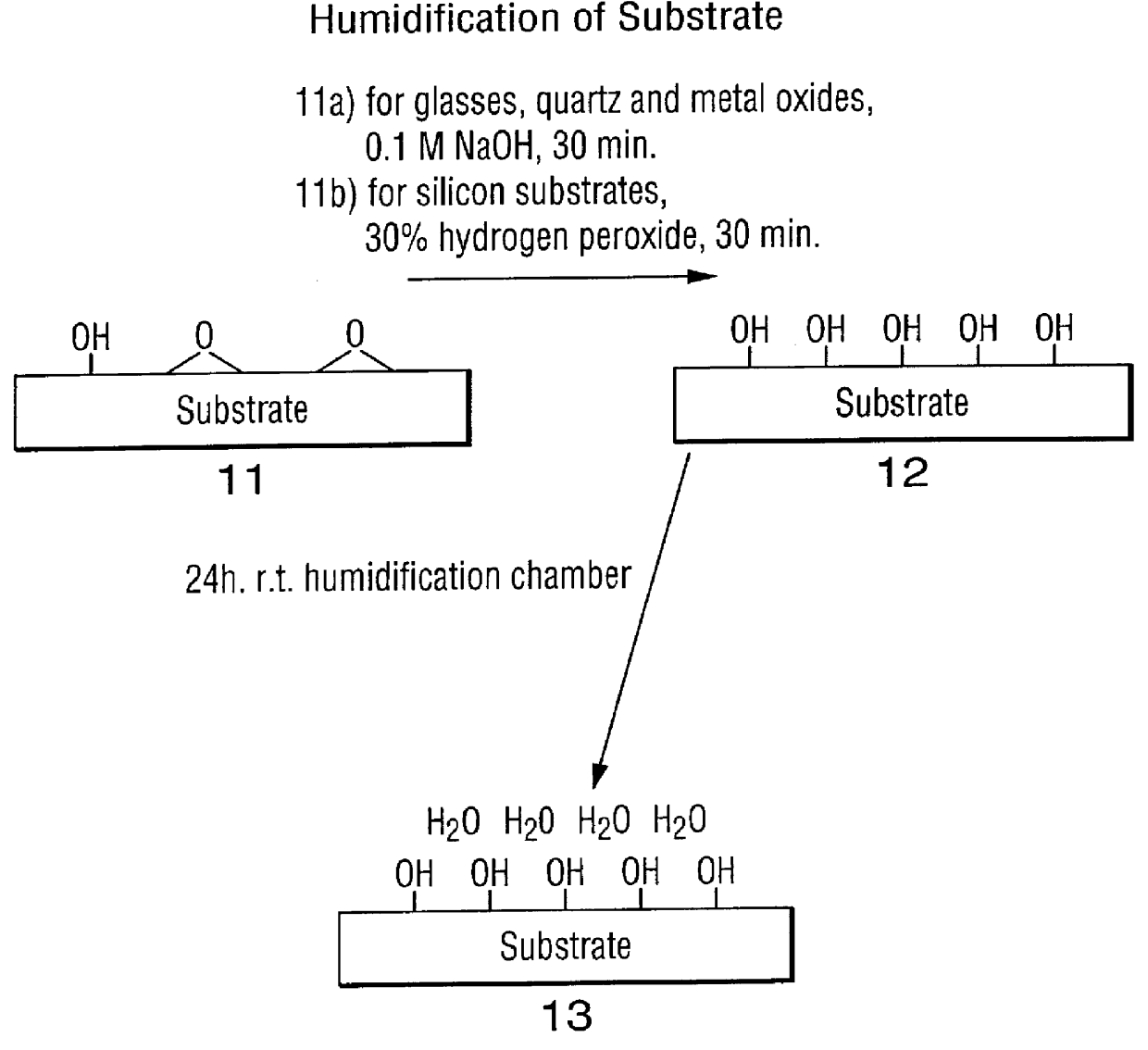
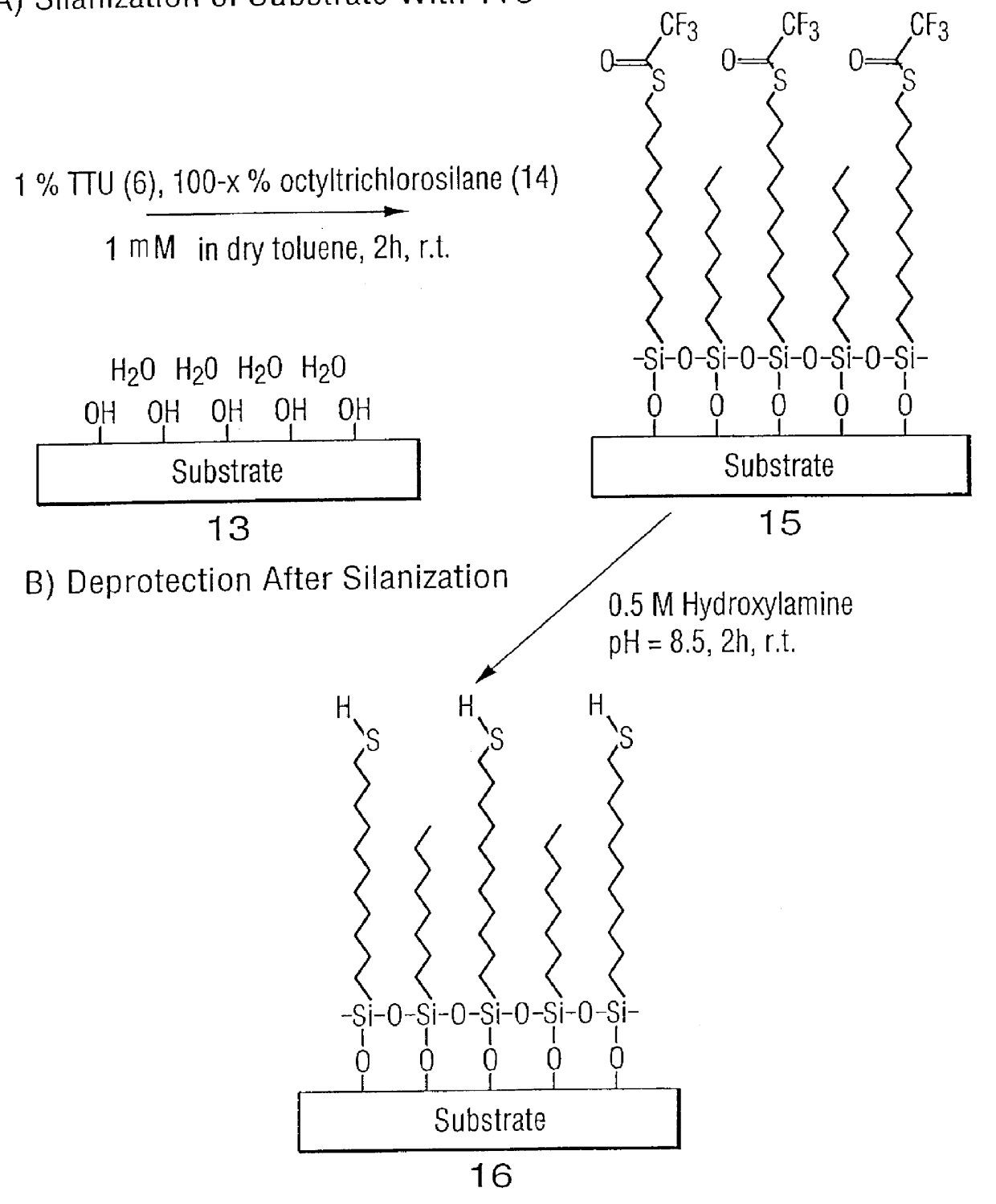
High surface density covalent immobilization of oligonucleotide monolayers
InactiveUS6159695AIncrease surface densityHigh sensitivityBioreactor/fermenter combinationsMaterial nanotechnologySilanesHigh surface
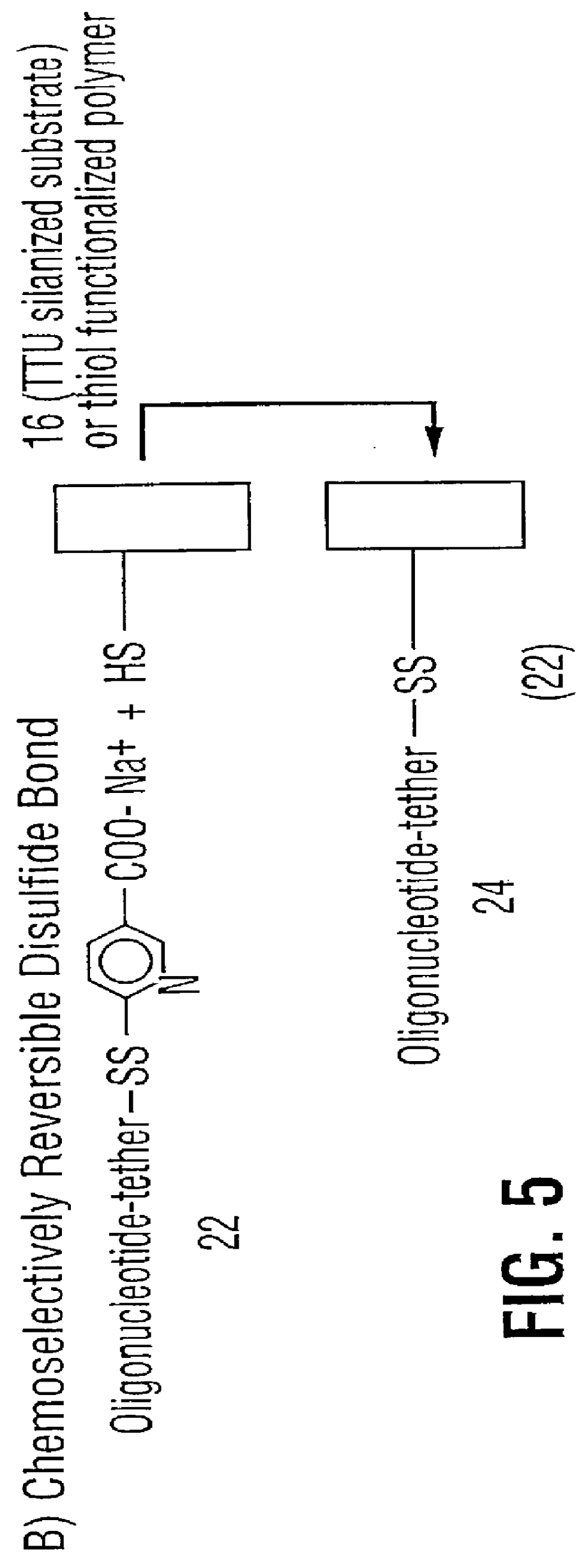
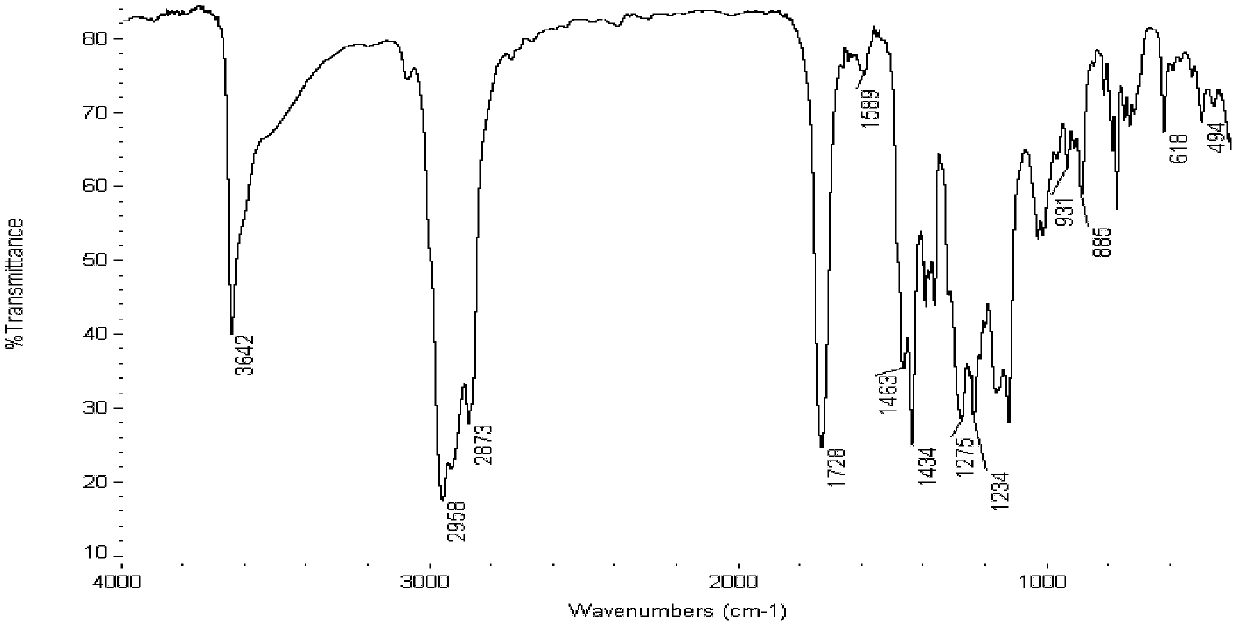
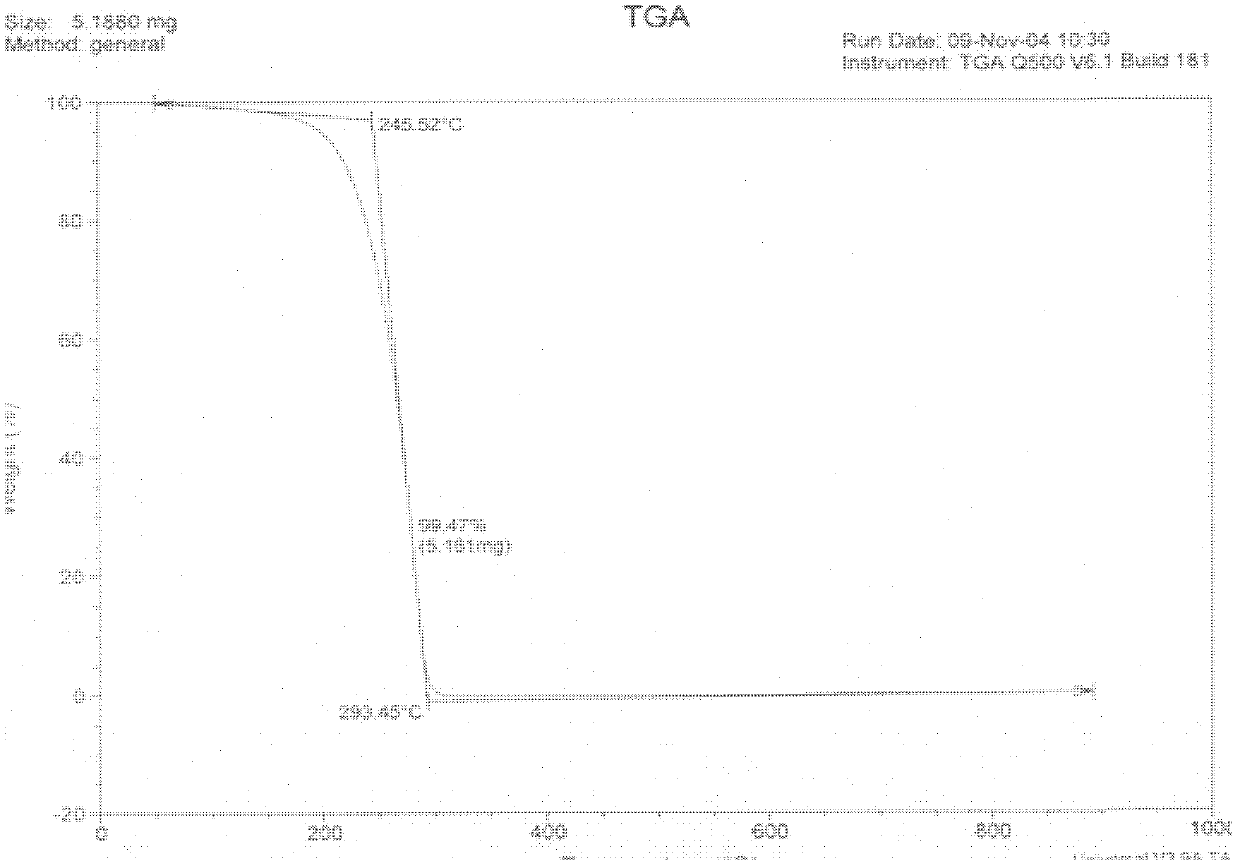
Oligonucleotides and other biomolecules are immobilized in high density on solid substrates through covalent forces using either a permanent thioether bond, or a chemoselectively reversible disulfide bond to a surface thiol. Substrates which have hydroxyl groups on their surfaces can be first silanized with a trichlorosilane containing 2-20 carbon atoms in its hydrocarbon backbone, terminating in a protected thiol group. The oligonucleotides or other biomolecules are first connected to a tether consisting of a hydrocarbon or polyether chain of 2-20 units in length which terminates in a thiol group. This thiol may be further modified with a halobenzylic-bifunctional water soluble reagent which allows the conjugate to be immobilized onto the surface thiol group by a permanent thioether bond. Alternatively, the oligonucleotide-tether-thiol group can be converted to a pyridyldisulfide functionality which attaches to the surface thiol by a chemoselectively reversible disulfide bond. The permanently bound oligonucleotides are immobilized in high density compared to other types of thiol functionalized silane surface and to the avidin-biotin method.
Owner:SENSORCHEM INT
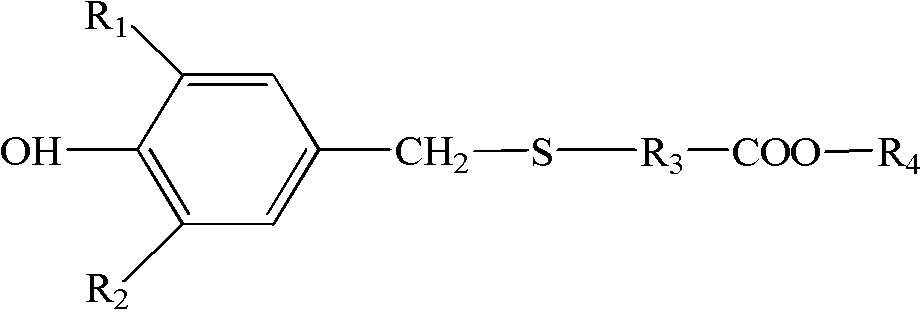
Thioether-containing hindered phenol antioxidant and preparation method thereof
InactiveCN103320198AImprove antioxidant capacityAchieve synergistic effectAdditivesSulfide preparationThioetherChemistry
The present invention provides a thioether bond structure-containing hindered phenol antioxidant and a preparation method thereof, wherein screen phenol, thiol ester and formaldehyde in a reaction medium are subjected to a reaction under a certain condition in the presence of a catalyst to obtain a thioether bond-containing hindered phenol antioxidant compound, wherein the compound has an excellent antioxidation performance in hydrogenation base oil, and can be adopted as a lubricating agent antioxidant to be separately used or is compounded with other additives to be used, and the antioxidant is applicable for internal combustion engine oils and industrial lubricating oils.
Owner:PETROCHINA CO LTD
Formulation for oral administration of apoptosis promoter
InactiveUS20100297194A1Improve oral bioavailabilityConvenient route of administrationOrganic active ingredientsBiocideDiseaseAntioxidant
An orally deliverable pharmaceutical composition comprises as a sole or first active ingredient a compound of Formula I defined herein or a pharmaceutically acceptable salt thereof, for example ABT-263 free base or ABT-263 bis-HCl salt, dispersed, in a free base equivalent amount of at least about 2.5% by weight of the composition, in a pharmaceutically acceptable carrier; wherein said active ingredient is in solid-state form and / or the composition further comprises, dispersed in the carrier, a pharmaceutically acceptable heavier-chalcogen antioxidant in an amount effective to inhibit oxidation of the active ingredient at a thioether linkage thereof. The composition is suitable for oral administration to a subject in need thereof for treatment of a disease characterized by overexpression of one or more anti-apoptotic Bcl-2 family proteins, for example cancer.
Owner:ABBOTT LAB INC +1
Thioethers, methods for their preparation, and compositions including such thioethers
Disclosed are thioethers, methods for preparing such thioethers, and curable compositions, such as coating and sealant compositions, that include such thioethers. The thioethers can be the reaction product of (a) an alpha, omega dihalo organic compound, (b) a metal hydrosulfide and (c) a metal hydroxide.
Owner:PRC DE SOTO INT INC
Dark blue organic light-emitting material and preparation method and application thereof
InactiveCN110790782AHigh color purityImprove stabilitySilicon organic compoundsSolid-state devicesBond energyULTRAMARINE BLUE
The invention discloses a dark blue organic light-emitting material and a preparation method and application thereof. The dark blue organic light-emitting material contains a structural unit disclosedin the invention, wherein, M is B or Bi; X is O, S or NR4; R1-R4 are independently selected from connecting bonds or groups obtained from H-H, H-F, H-O-H, H-S-H, H-CN, saturated hydrocarbons, unsaturated hydrocarbons, fluorinated hydrocarbons, heterocyclic compounds, organoboron, organosilicone, alcohols, mercaptans, ethers, thioethers, phenols, thiophenol, aldehydes, ketones, amines, amides, nitriles or sulfones losing one or more H; R1-R3 are located at any substitution position on rings of the structural unit where R1-R3 are located, and the bond energy between the ring where R3 is locatedand M is greater than or equal to the bond energy between the ring where R2 is located and M. The dark blue organic light-emitting material containing the B / Bi-N main body structure has very narrow light-emitting spectrum and TADF properties; the color purity is high, and the stability is good.
Owner:PEKING UNIV SHENZHEN GRADUATE SCHOOL
Lubricant composition for gasoline engine
ActiveCN102690711AMeet performance requirementsImprove antioxidant capacityAdditivesAlkaline earth metalAntioxidant
The invention relates to a lubricant composition for a gasoline engine. The lubricant composition contains: by mass, less than or equal to 0.08% of phosphorus and less than or equal to 0.9% of sulfate ash. The lubricant composition comprises A) a composite antioxidant comprising at least ingredients of 1, an alkylated diphenylamine antioxidant, 2, a thiophenolic ester antioxidant and 3, an unessential phenolic thioether antioxidant, B) a polyisobutylene succinimide ashless dispersant and / or a boronized polyisobutylene succinimide dispersant, C) one or more alkaline earth metal salicylate cleaning agents, D) at least one zinc dialkyl dithiophosphate, E) one or more oil-soluble organic molybdenum friction modifiers, F) one or more ashless friction modifiers, and G) a main amount of lubricating base oil. The lubricant composition for a gasoline engine has synergistic effect of the additives, can satisfy antioxidation requirements of a high-grade gasoline engine lubricant, and has excellent piston cleanliness, wear resistance and a viscosity increasing control capacity.
Owner:CHINA PETROLEUM & CHEM CORP +1
Propylene polymer, propylene block copolymer, process for preparing said polymer and said block copolymer, and propylene polymer composition
InactiveUS20020006993A1High crystallinityLong mesochainLayered productsPolymer scienceHindered amine light stabilizers
Disclosed are a propylene polymer having a high crystallinity of a boiled heptane-insoluble component contained therein, a high stereoregularity and an extremely long mesochain (continuous propylene units wherein directions of alpha-methyl carbons are the same as each other), and a process for preparing said polymer. Also disclosed are a propylene block copolymer containing a crystalline polypropylene portion having a high crystallinity of a boiled heptane-insoluble component contained therein, a high stereoregularity and an extremely long mesochain, and a process for preparing said copolymer. Further disclosed is a propylene polymer composition comprising the above propylene polymer or propylene block copolymer and at least one stabilizer selected from a phenol type stabilizer, an organophosphite type stabilizer, a thioether type stabilizer, a hindered amine type stabilizer and a metallic salt of higher aliphatic acid. The propylene polymer of the invention is excellent in rigidity, heat resistance and moisture resistance, and can be favorably used for sheet, film, filament, injection molded product, blow molded product, etc. The propylene block polymer of the invention is well-balanced between rigidity, heat resistance and moisture resistance, and can be favorably used for sheet, filament, injection molded product, blow molded product, etc. The propylene polymer composition of the invention has excellent properties of the propylene polymer or the propylene block copolymer, and moreover is excellent in heat stability during the molding stage, long-term heat stability and weathering resistance. The propylene polymer composition can be favorably used for sheet, film, filament, injection molded product, blow molded product, etc.
Owner:MITSUI CHEM INC
High toughness packing reinforced polyphenyl thioether composite material and its preparation method
Owner:KINGFA SCI & TECH CO LTD +1
Acrylated Urethanes, Processes for Making the Same and Curable Compositions Including the Same
InactiveUS20090012202A1Polyureas/polyurethane adhesivesPolyurea/polyurethane coatingsAlcoholCarbamate
The present invention is directed to acrylated urethanes including the reaction product of: (1)(a) at least one urethane having at least two isocyanate groups and at least one acrylate group; and (b) at least one alcohol compound having at least two hydroxyl groups; or (2) (a) at least one isocyanate functional urethane which is the reaction product of at least one alcohol compound selected from the group consisting of amino alcohols, thioether alcohols, phosphino alcohols and mixtures thereof and at least one polyisocyanate; and (b) at least one hydroxy-functional material having at least one acrylate group; curable compositions including the same and processes for making the same.
Owner:HENKEL IP & HOLDING GMBH
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com