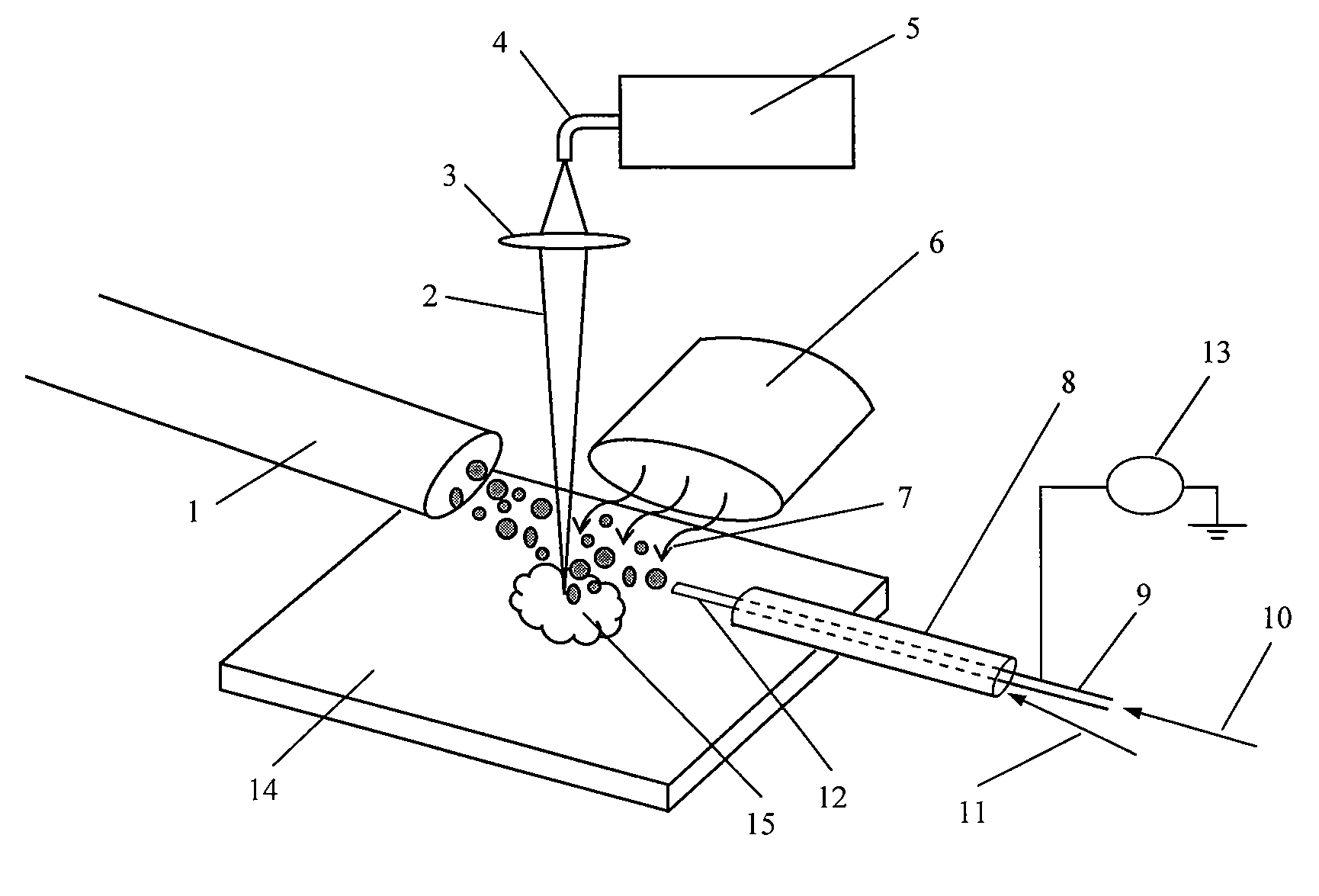
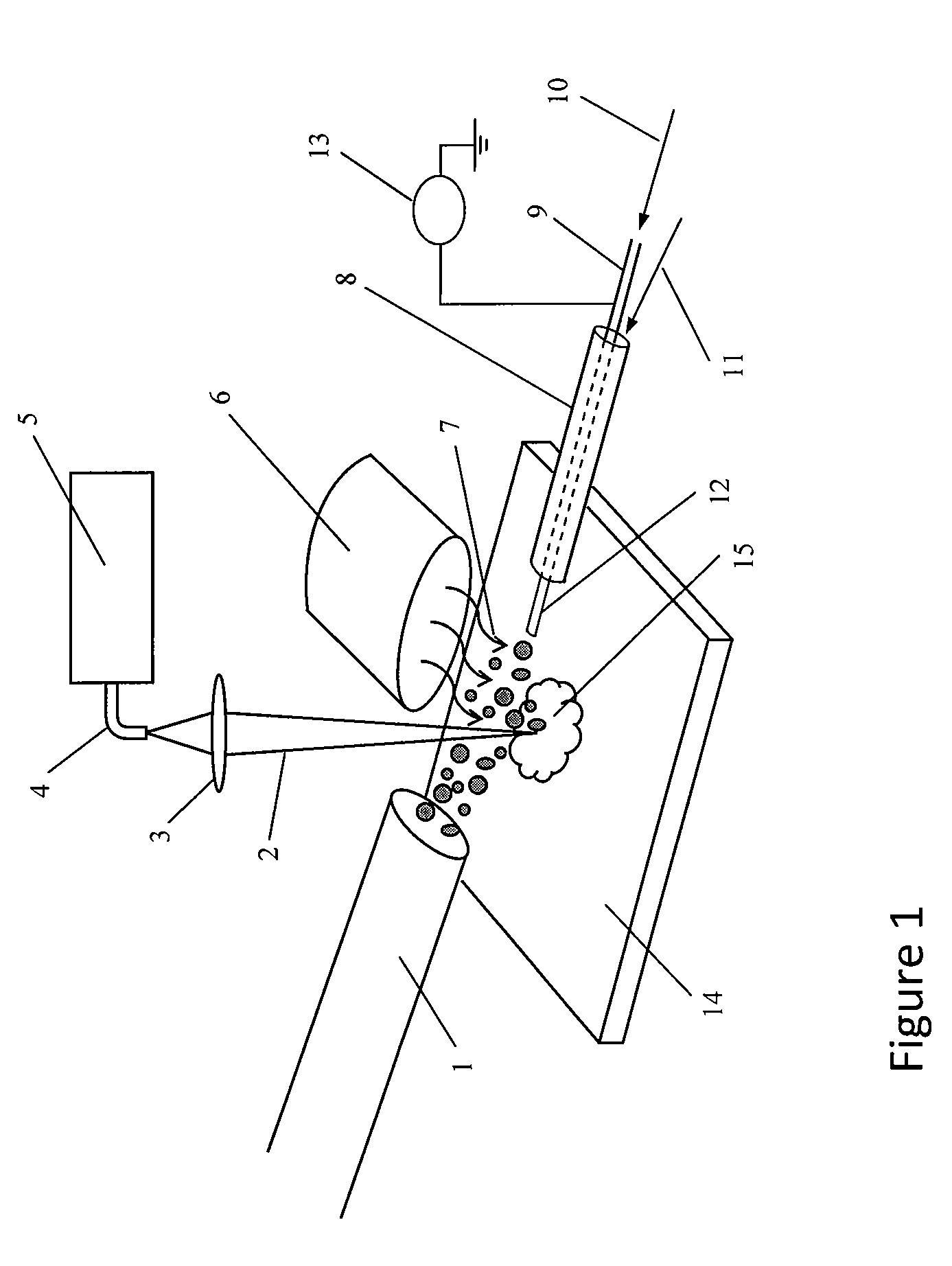
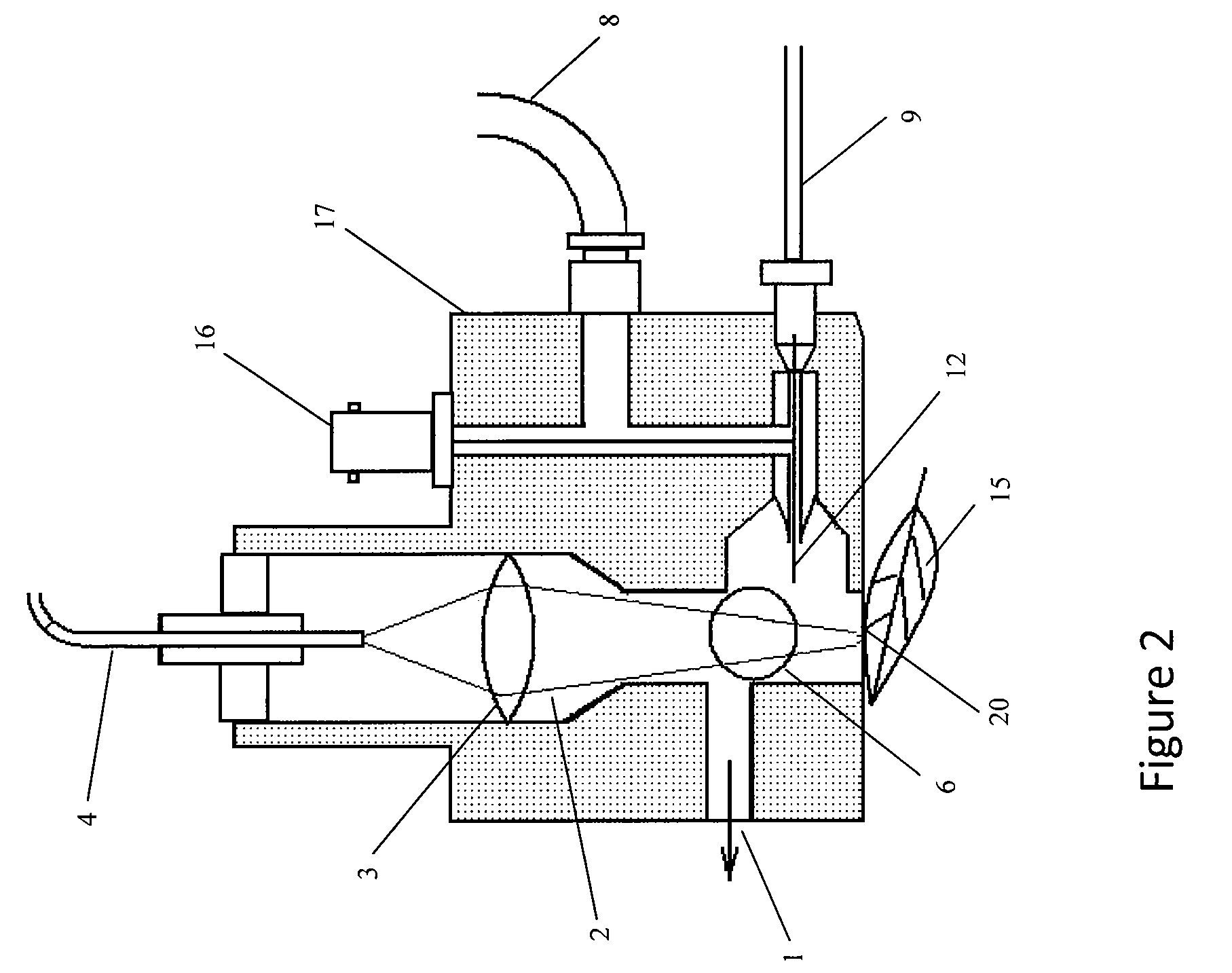
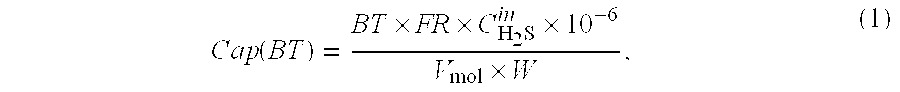Patents
Literature
8435 results about "Desorption" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
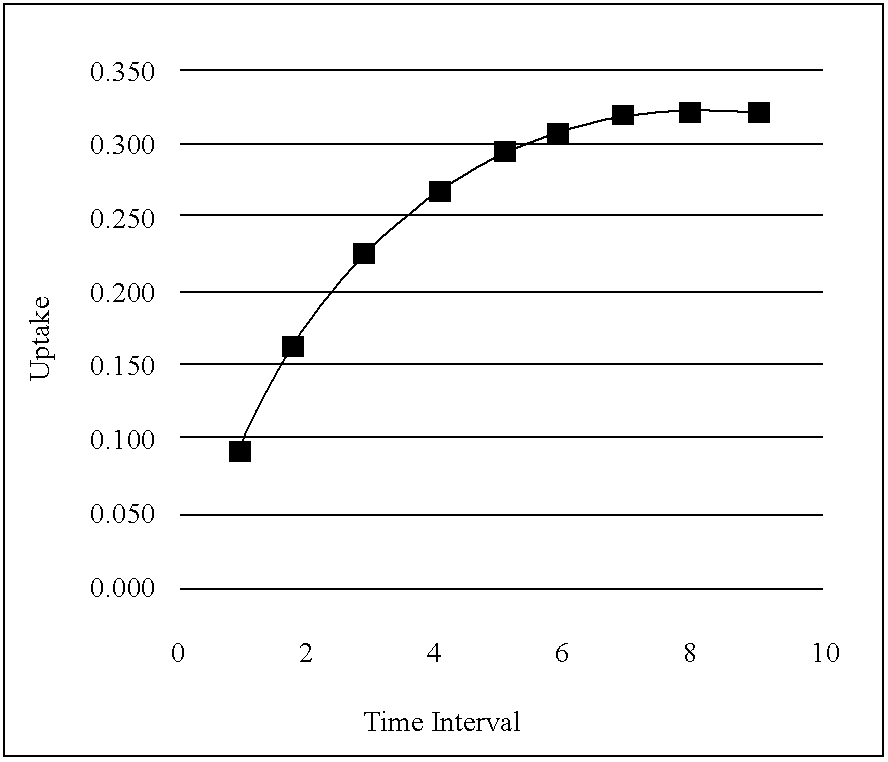
Desorption is a phenomenon whereby a substance is released from or through a surface. The process is the opposite of sorption (that is, either adsorption or absorption). This occurs in a system being in the state of sorption equilibrium between bulk phase (fluid, i.e. gas or liquid solution) and an adsorbing surface (solid or boundary separating two fluids). When the concentration (or pressure) of substance in the bulk phase is lowered, some of the sorbed substance changes to the bulk state.
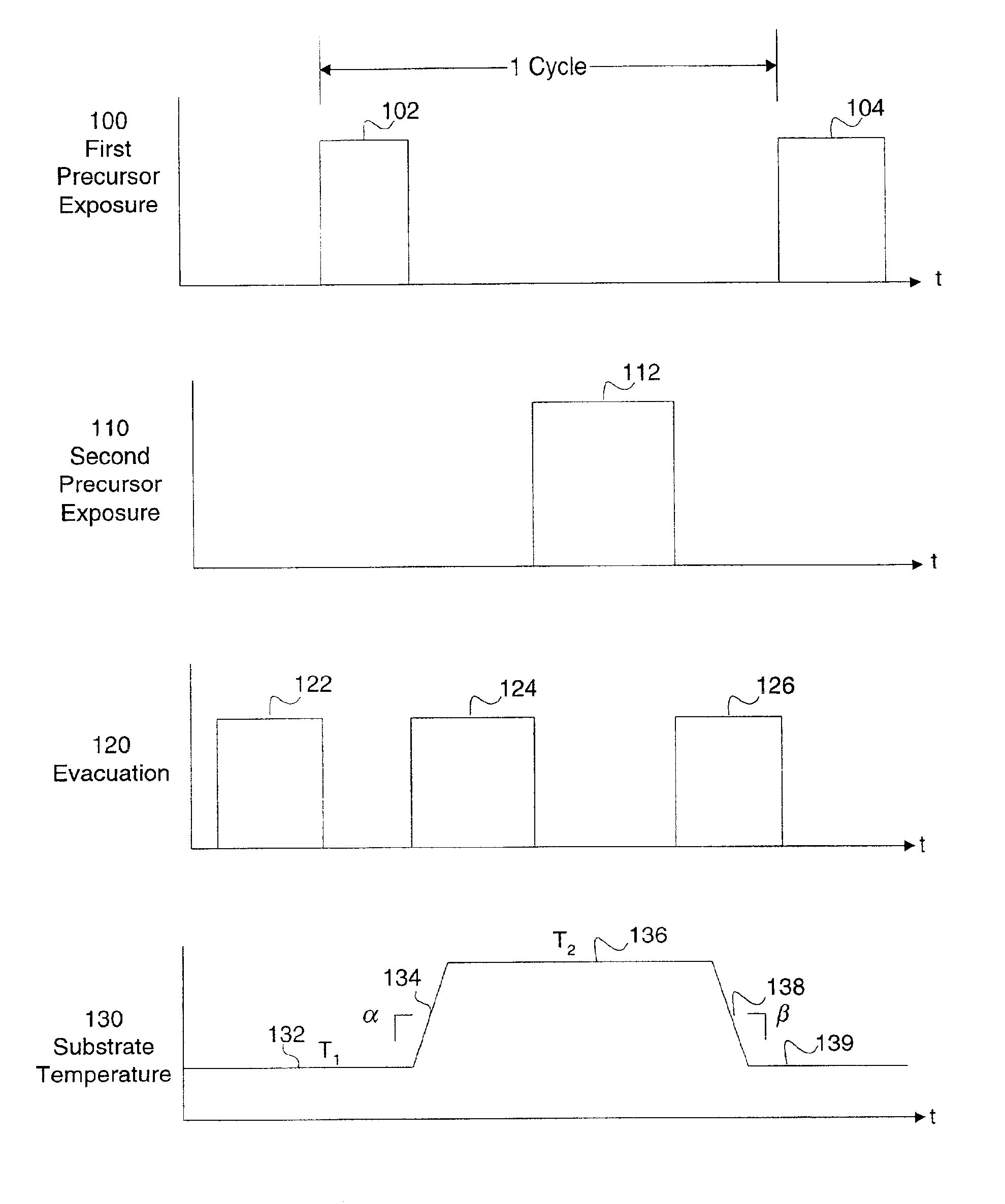
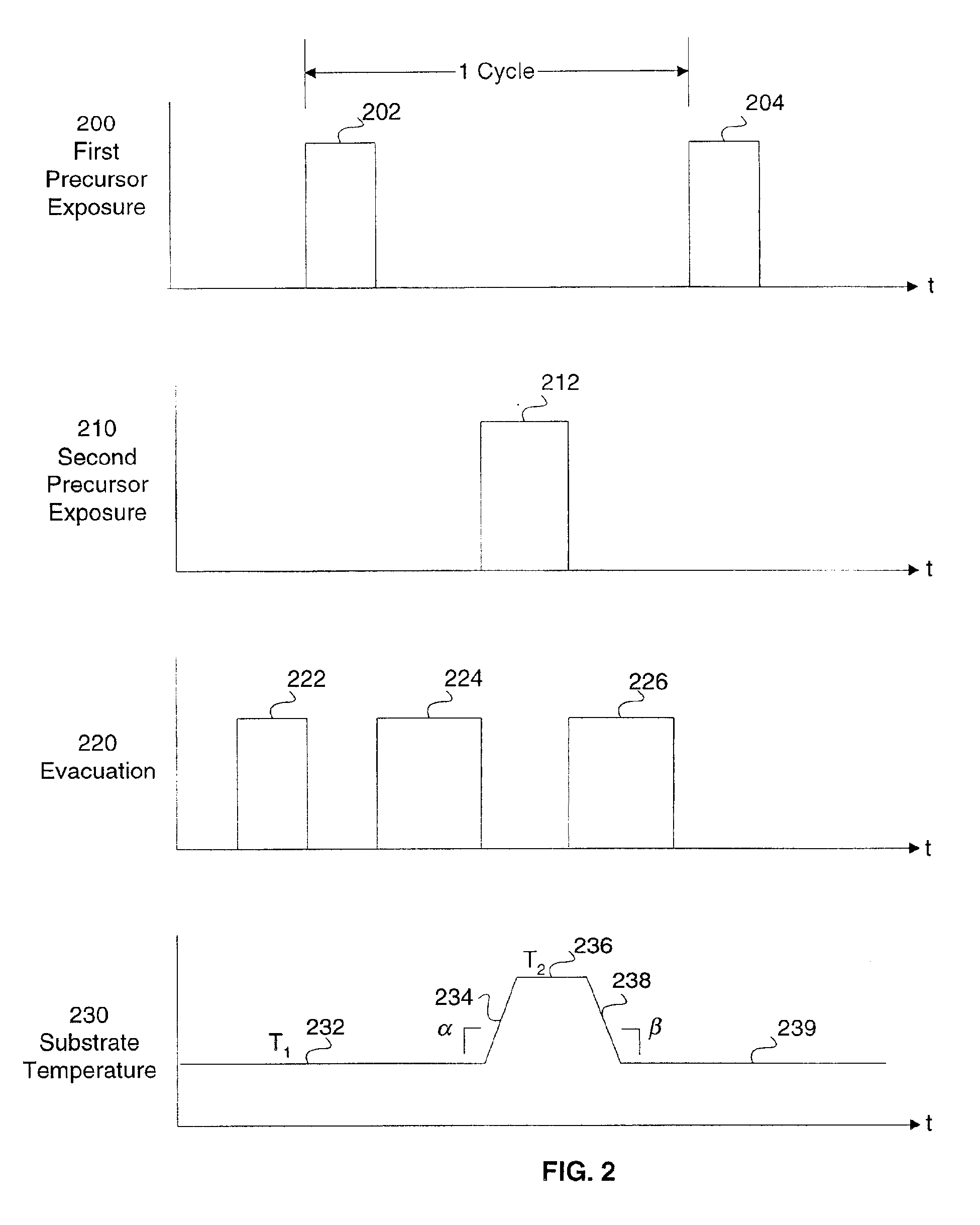
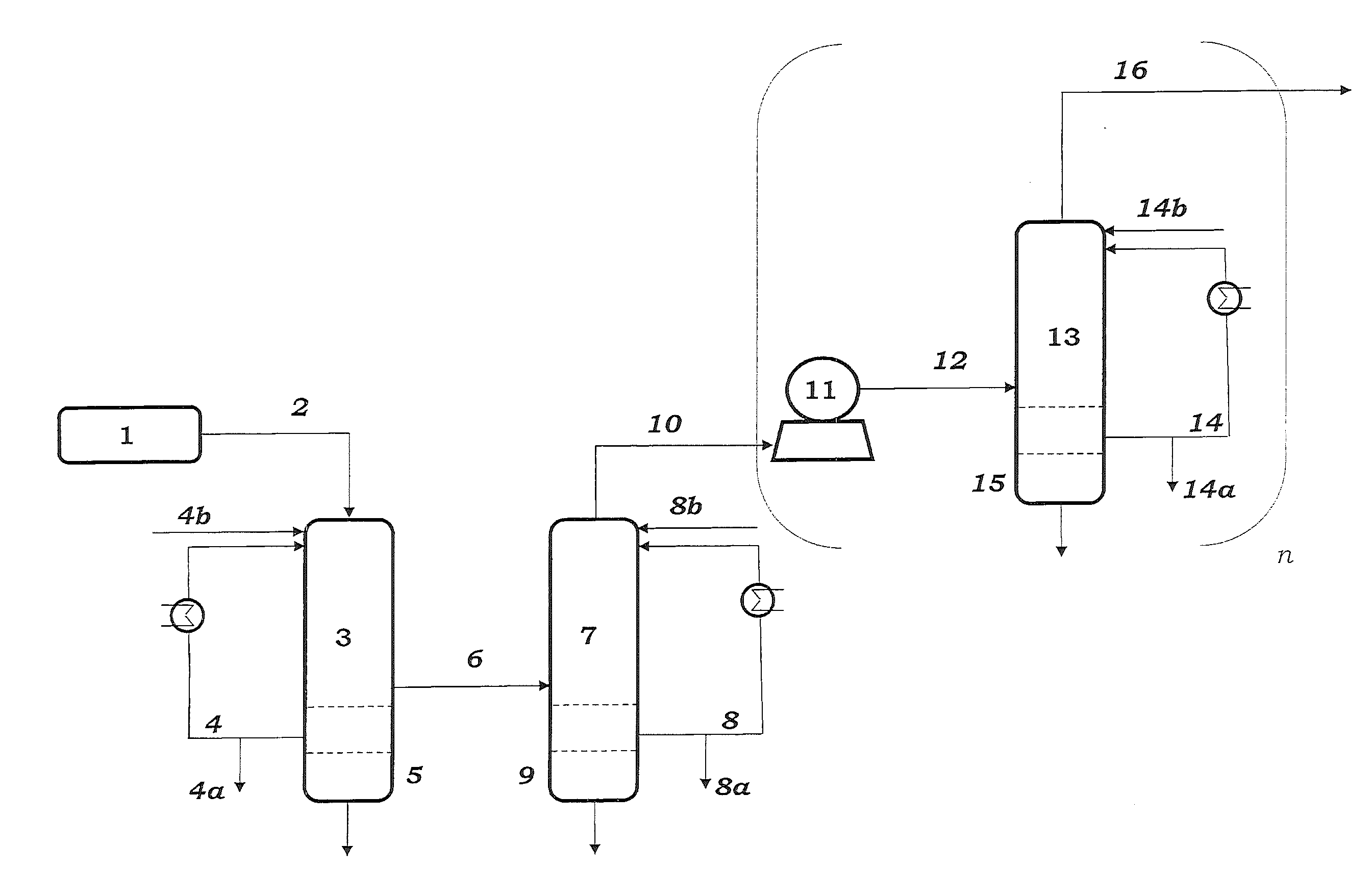
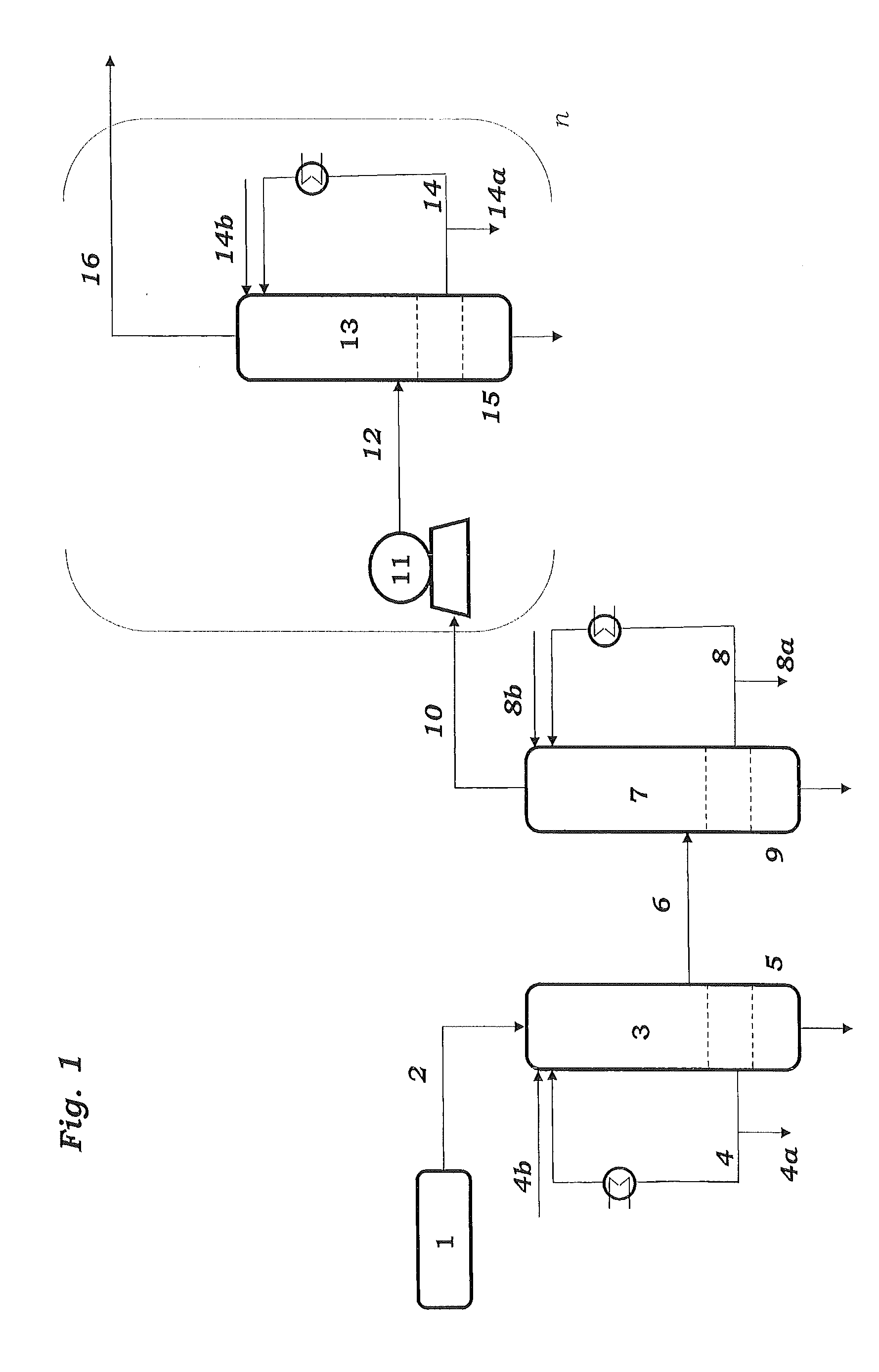
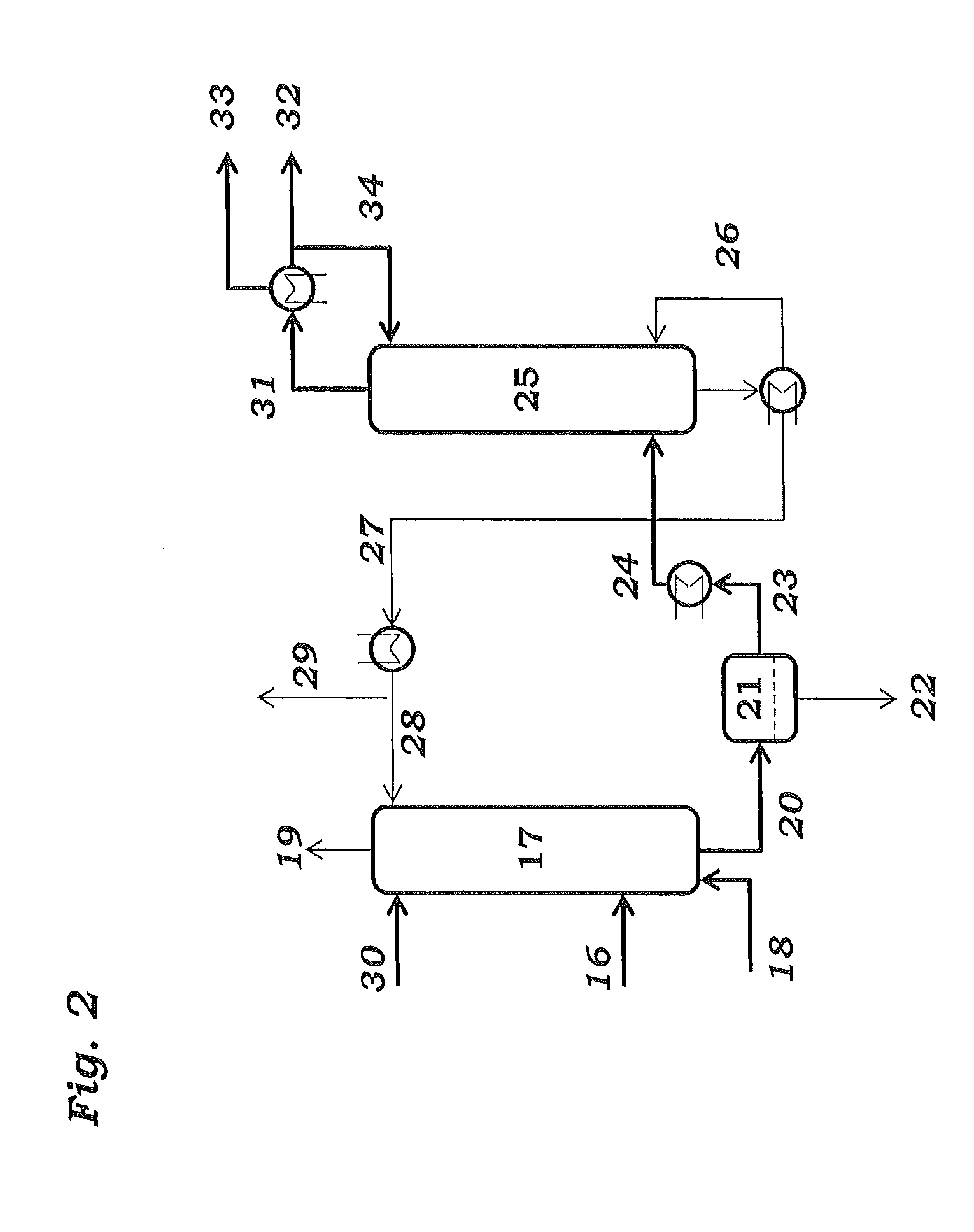
Method and apparatus for improved temperature control in atomic layer deposition
InactiveUS6878402B2Electric discharge heatingVacuum evaporation coatingTemperature controlThermal state
A system and method for that allows one part of an atomic layer deposition (ALD) process sequence to occur at a first temperature while allowing another part of the ALD process sequence to occur at a second temperature. In such a fashion, the first temperature can be chosen to be lower such that decomposition or desorption of the adsorbed first reactant does not occur, and the second temperature can be chosen to be higher such that comparably greater deposition rate and film purity can be achieved. Additionally, the invention relates to improved temperature control in ALD to switch between these two thermal states in rapid succession. It is emphasized that this abstract is provided to comply with rules requiring an abstract. It is submitted with the understanding that it will not be used to interpret or limit the scope or meaning of the claims.
Owner:NOVELLUS SYSTEMS
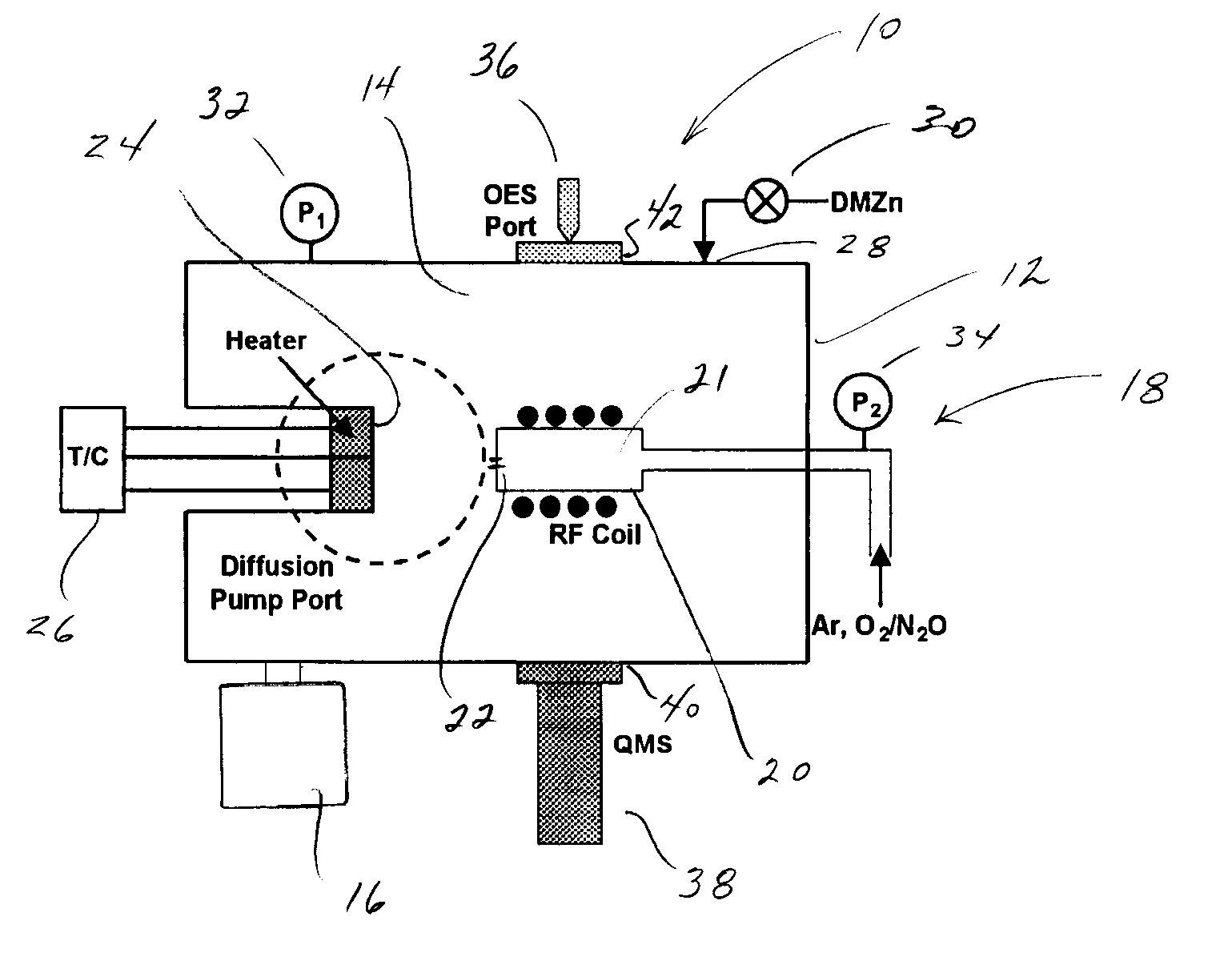
High Vacuum Plasma-Assisted Chemical Vapor Deposition System
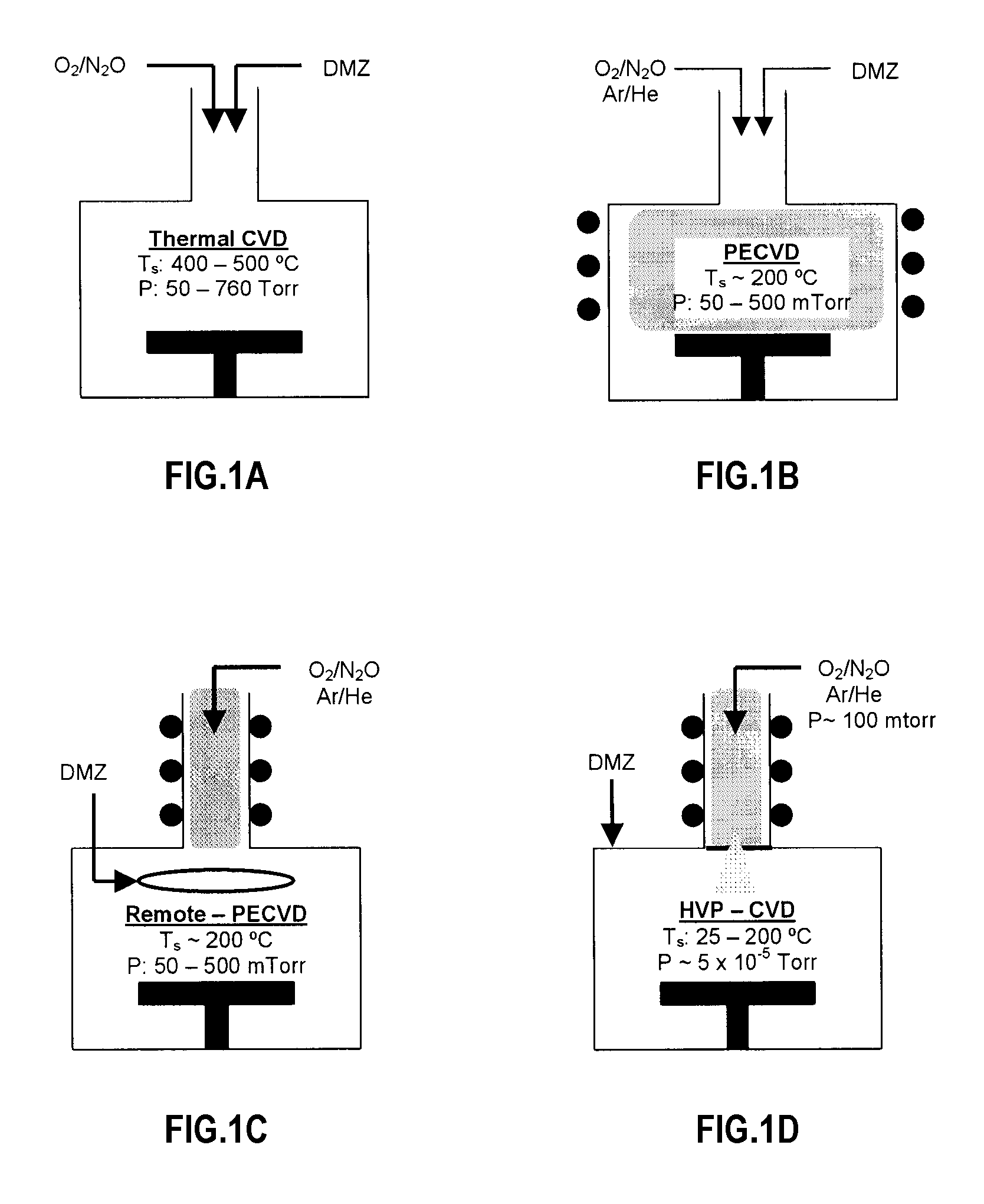
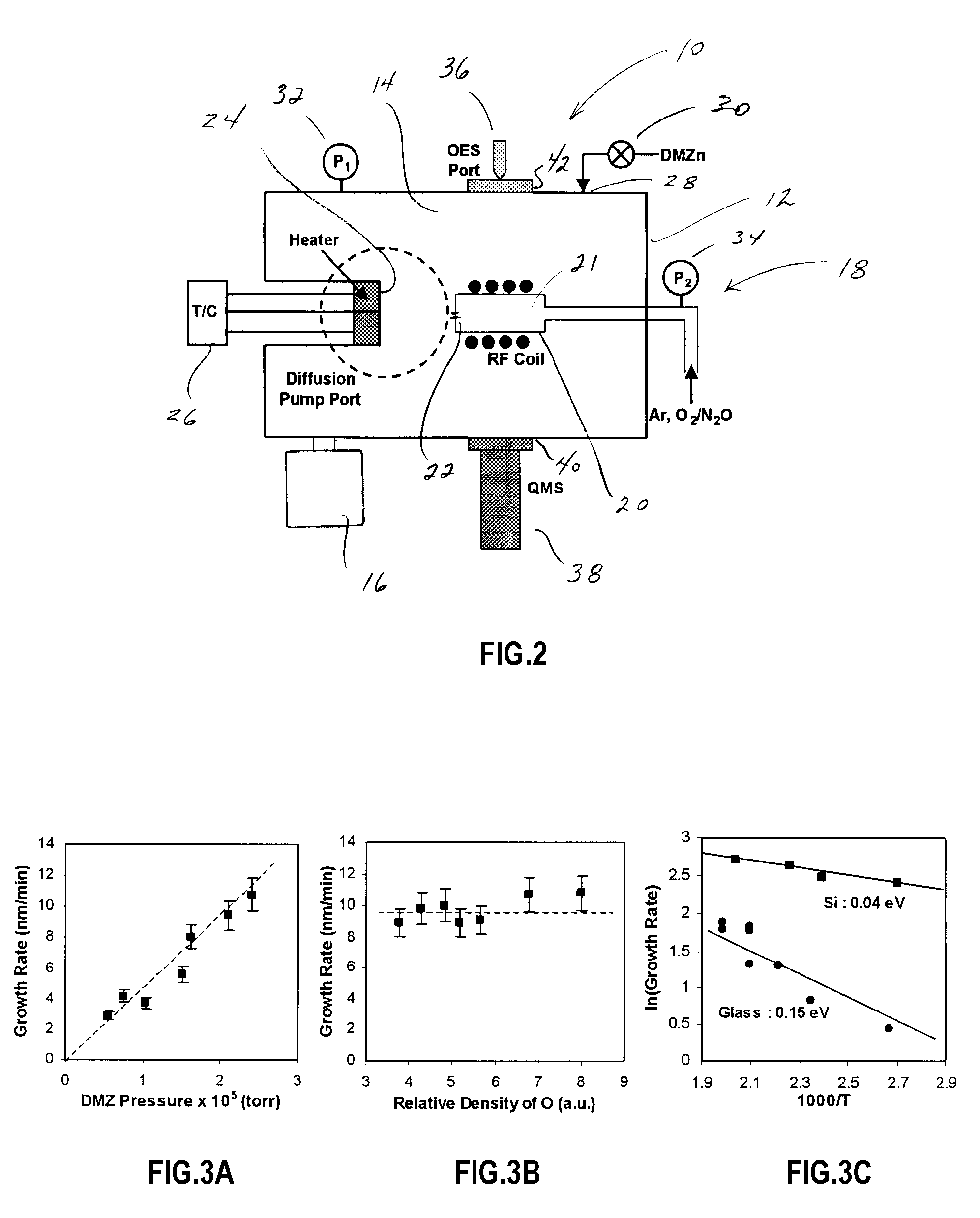
The invention is directed to a novel approach to thin film synthesis that is described as high vacuum plasma-assisted chemical vapor deposition (HVP-CVD). In one application of HVP-CVD, atomic oxygen and organometallic precursors are simultaneously introduced into a high vacuum chamber. Gas-phase chemistry is eliminated or substantially eliminated in the collisionless or substantially collisionless environment, allowing the surface chemistry between atomic oxygen and the precursor(s) to be interrogated directly. In preliminary work it has been observed that the presence of atomic oxygen greatly accelerates the desorption of organic ligands, facilitating oxide formation. The prominent advantages of the HVP-CVD include reduced substrate temperature, significant rates, inherent uniformity, facilitated doping, and the ability to directly study these processes in-situ with high vacuum diagnostics that are not compatible with conventional CVD technologies.
Owner:COLORADO SCHOOL OF MINES
Bottom gate type thin film transistor, method of manufacturing the same, and display apparatus
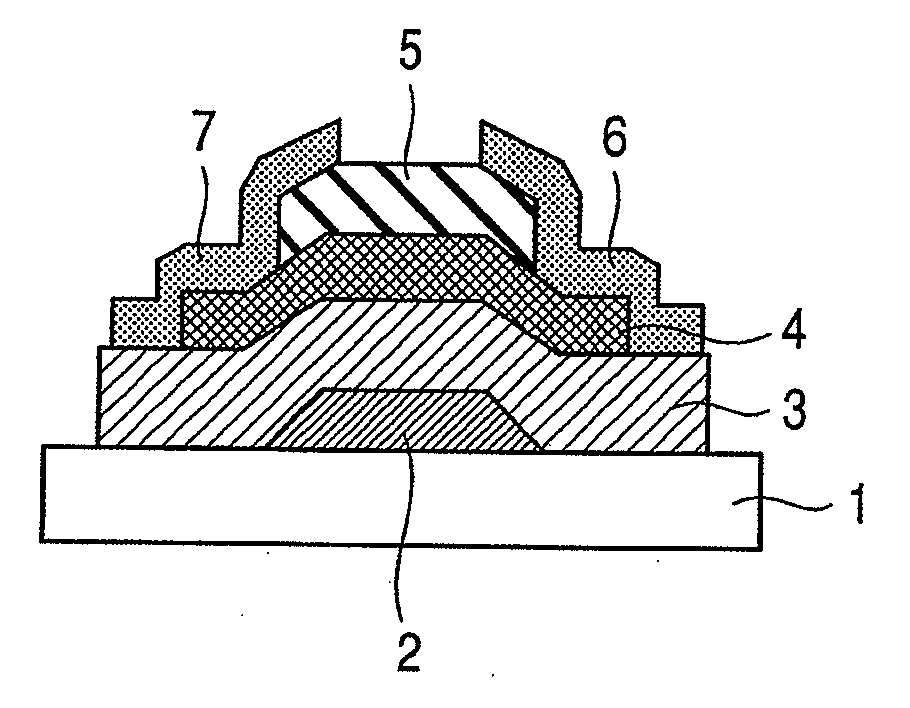
InactiveUS8148721B2Optimise total massImprove batch productivityTransistorElectroluminescent light sourcesDesorptionBottom gate
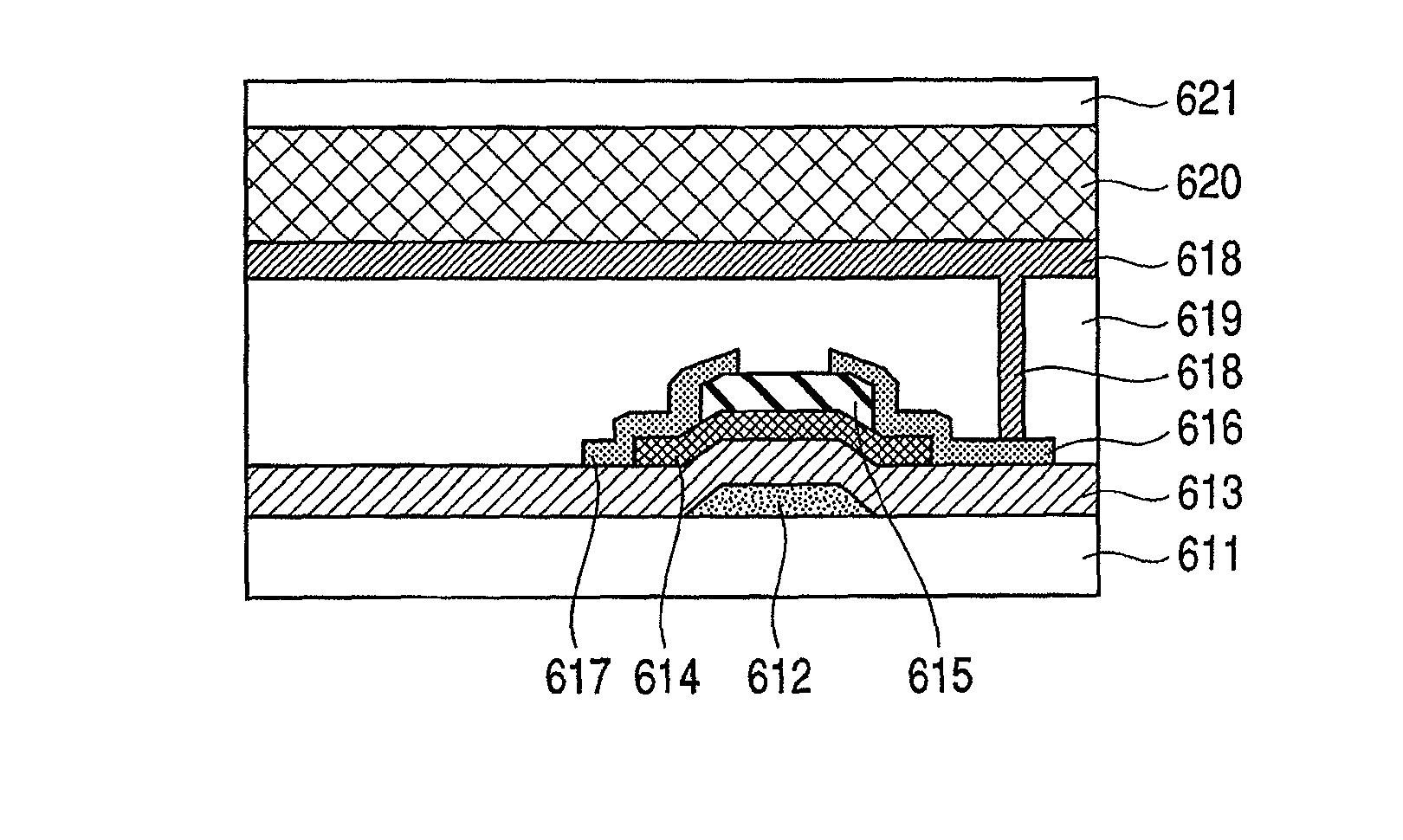
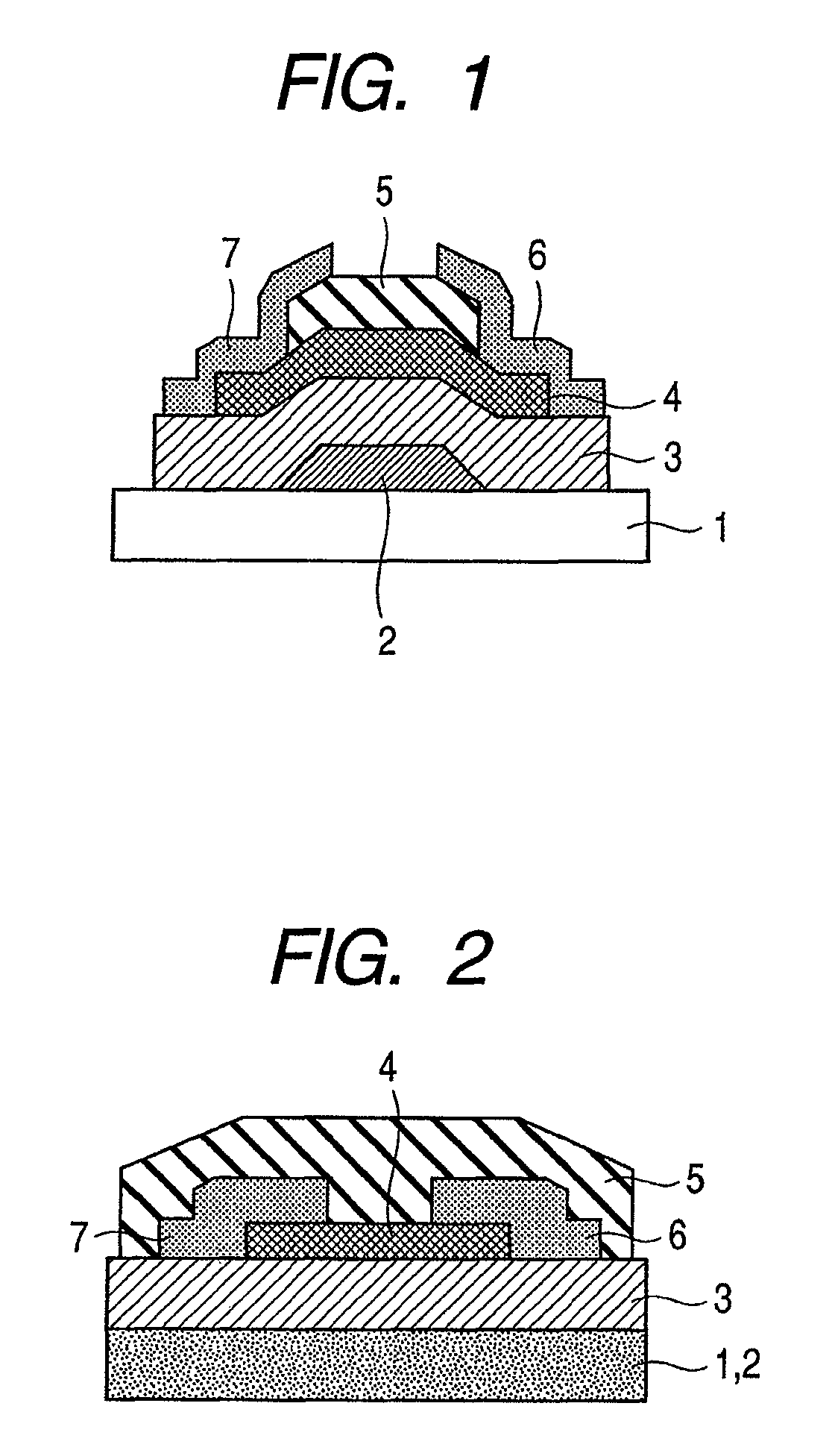
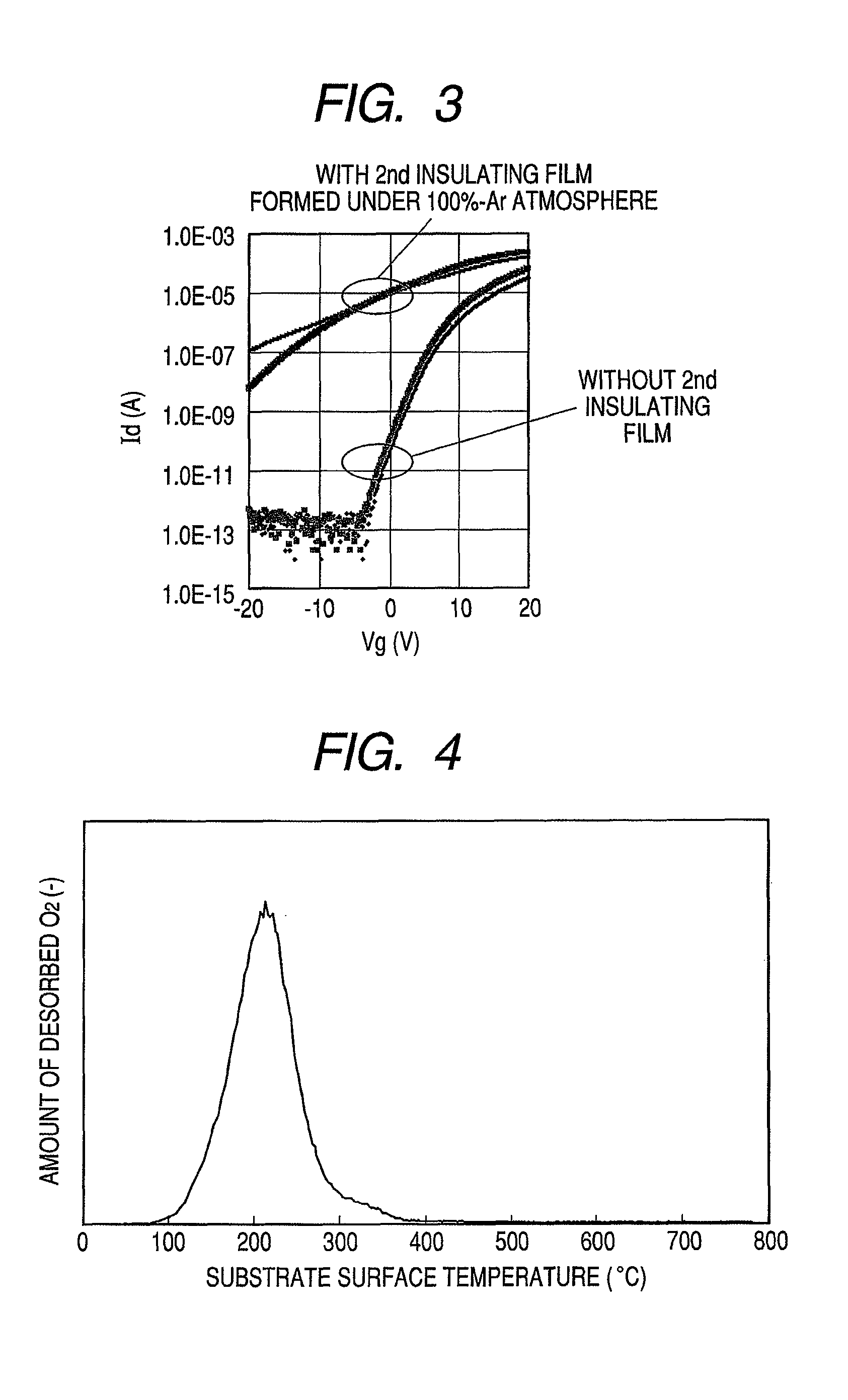
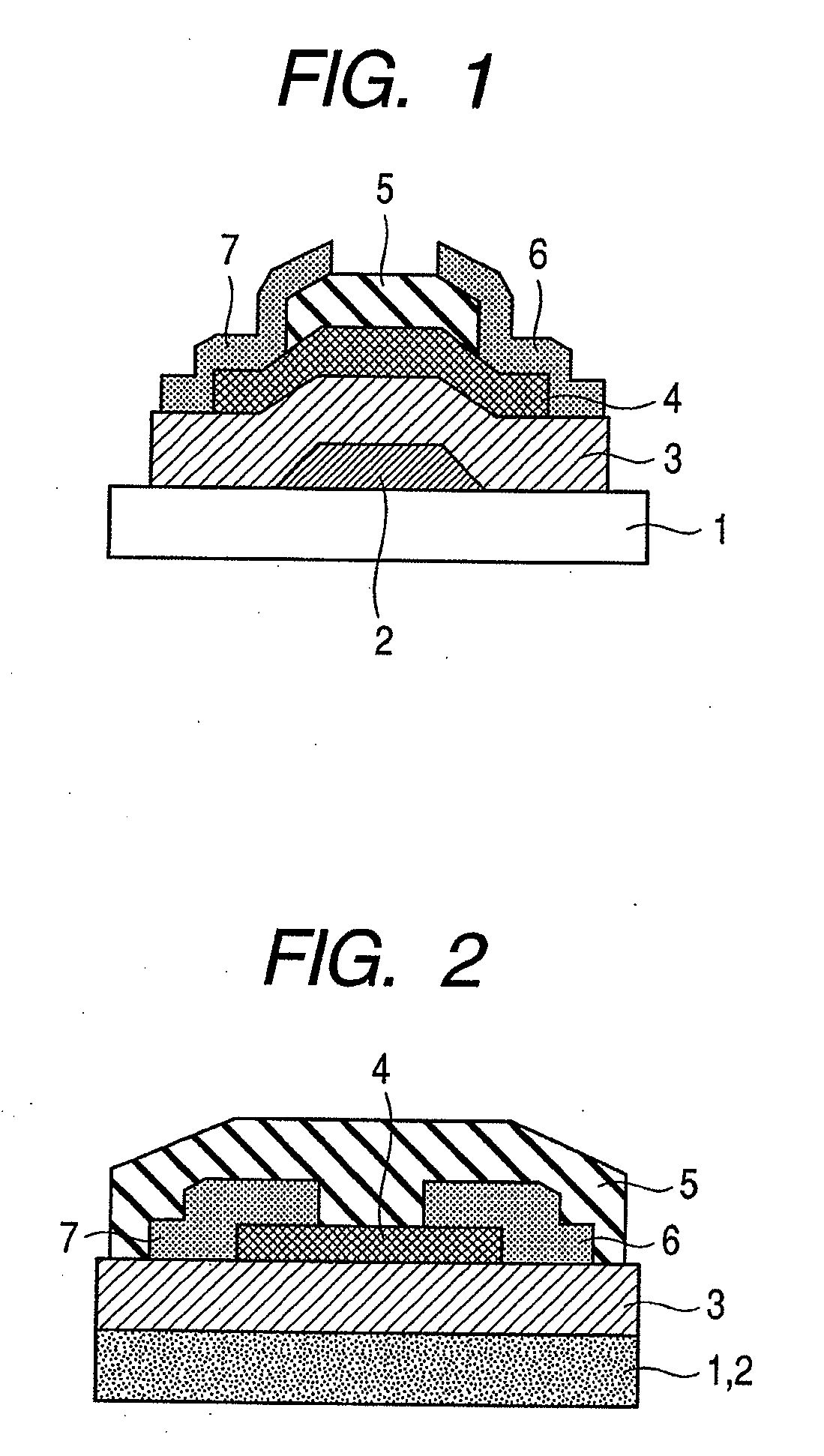
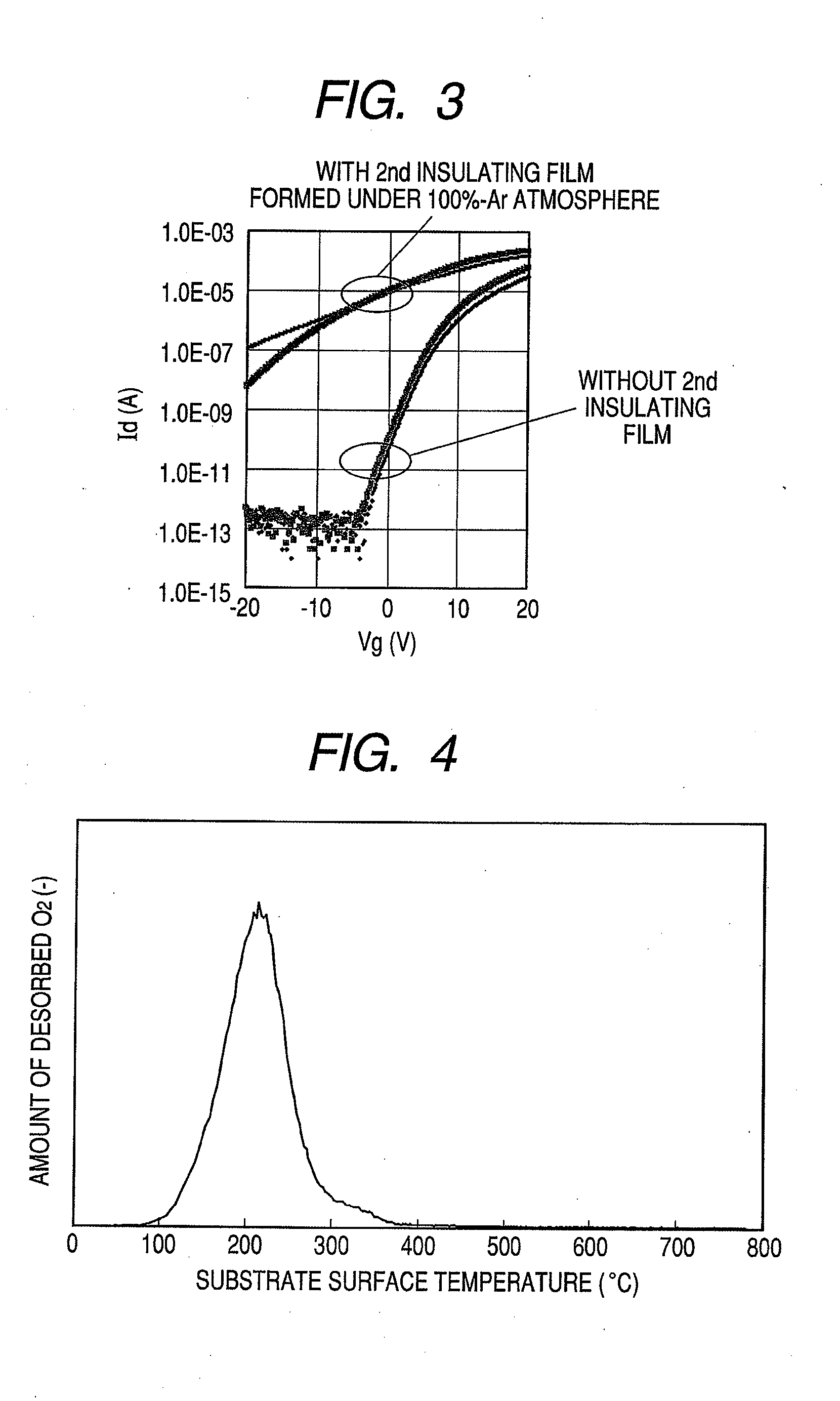
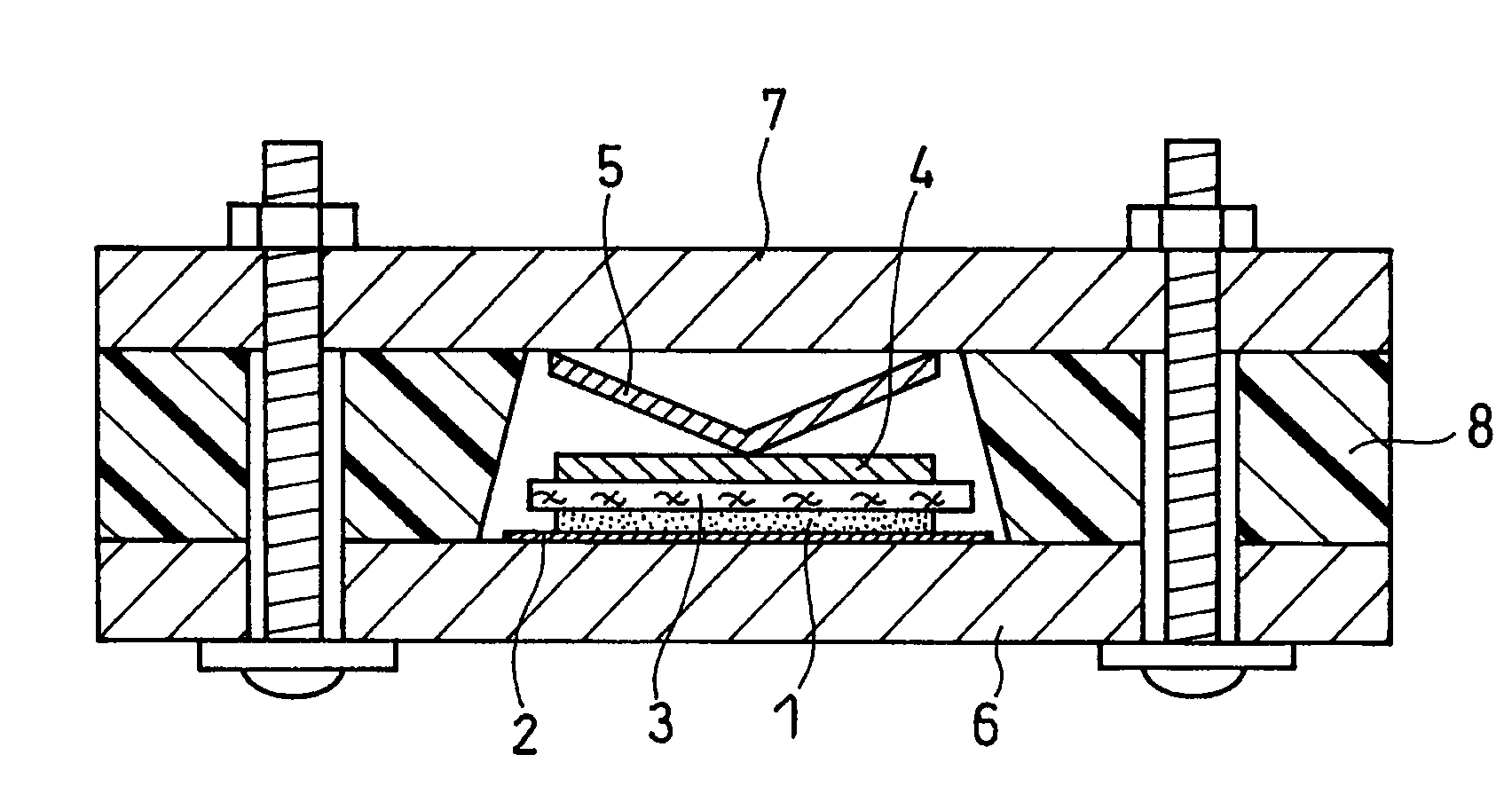
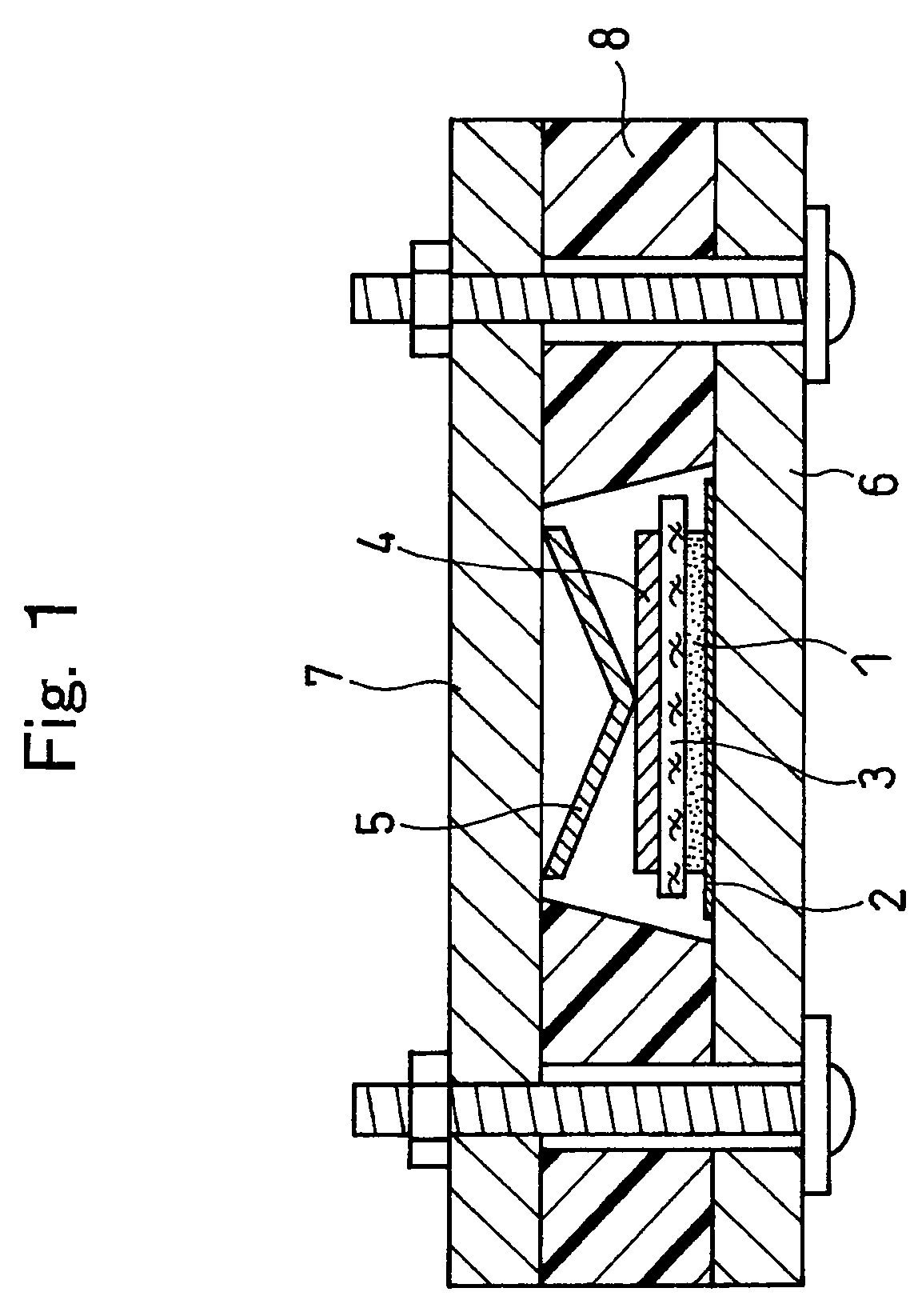
Provided is a bottom gate type thin film transistor including on a substrate (1) a gate electrode (2), a first insulating film (3) as a gate insulating film, an oxide semiconductor layer (4) as a channel layer, a second insulating film (5) as a protective layer, a source electrode (6), and a drain electrode (7), in which the oxide semiconductor layer (4) includes an oxide including at least one selected from the group consisting of In, Zn, and Sn, and the second insulating film (5) includes an amorphous oxide insulator formed so as to be in contact with the oxide semiconductor layer (4) and contains therein 3.8×1019 molecules / cm3 or more of a desorbed gas observed as oxygen by temperature programmed desorption mass spectrometry.
Owner:CANON KK
Absorbent articles with distribution materials positioned underneath storage material
An absorbent article having an ultimate fluid storage region and a fluid distribution region, positioned between the ultimate storage region and the garment oriented surface of the article, in fluid communication with the ultimate fluid storage region, the ultimate fluid storage region includes a material which has: (1) a Capillary Sorption Desorption Capacity at 100 cm (CSDC 100) of at least 10 g / g; (2) a Capillary Sorption Desorption Capacity at 0 cm (CSDC 0) higher than the CSDC 100; (3) a Loosely Bound Liquid Capacity (LBLC); and (4) a Capillary Sorption Desorption Release Height when 50% of the LBLC are released (CSDRH 50) less than 60 cm. Further, the liquid distribution layer material has a Capillary Sorption Absorption Height at 30% of its maximum capacity (CSAH 30) of at least 35 cm. Distribution material can be foam materials, particularly those derived from high internal phase water-in-oil emulsions.
Owner:THE PROCTER & GAMBLE COMPANY
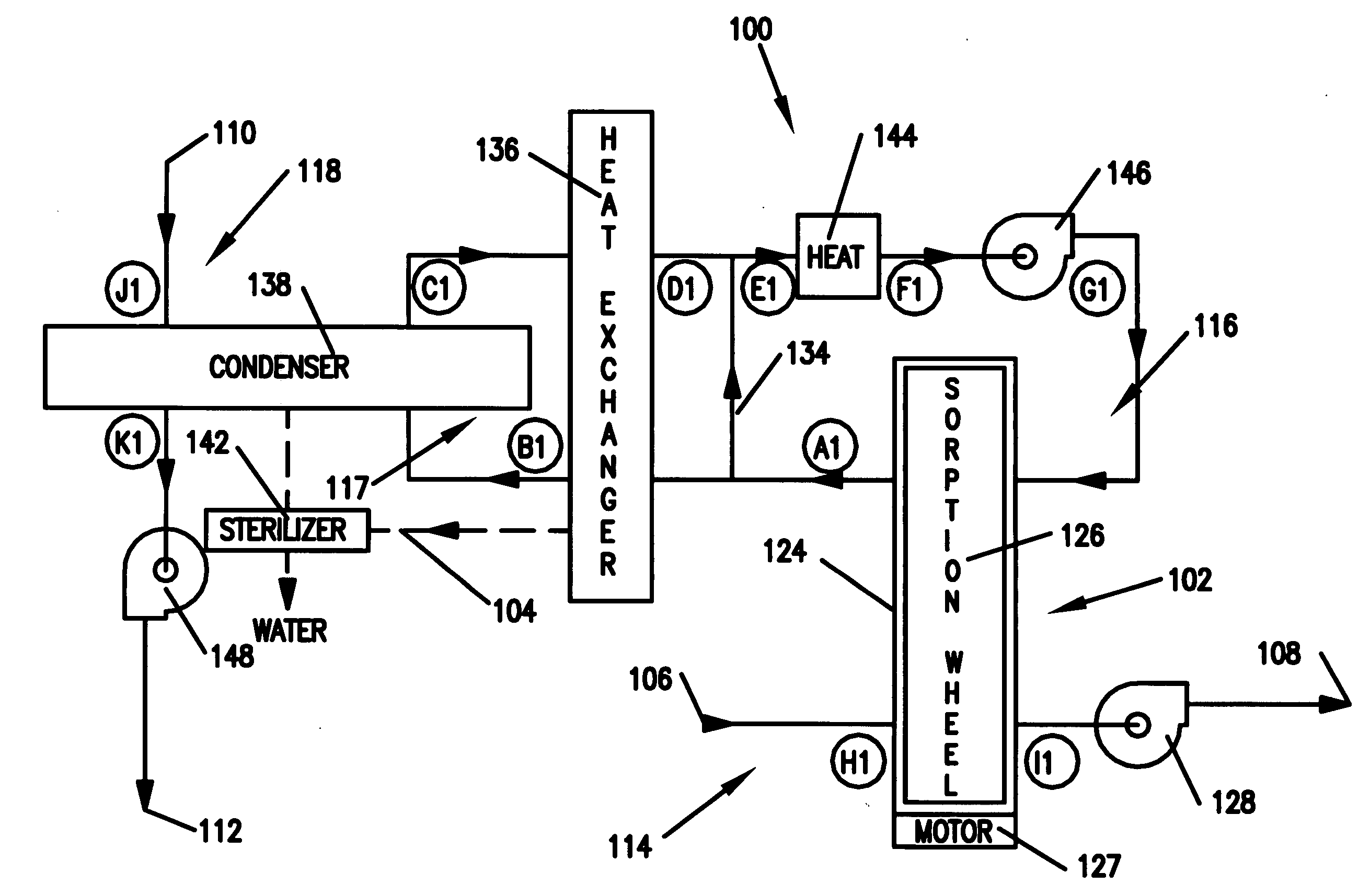
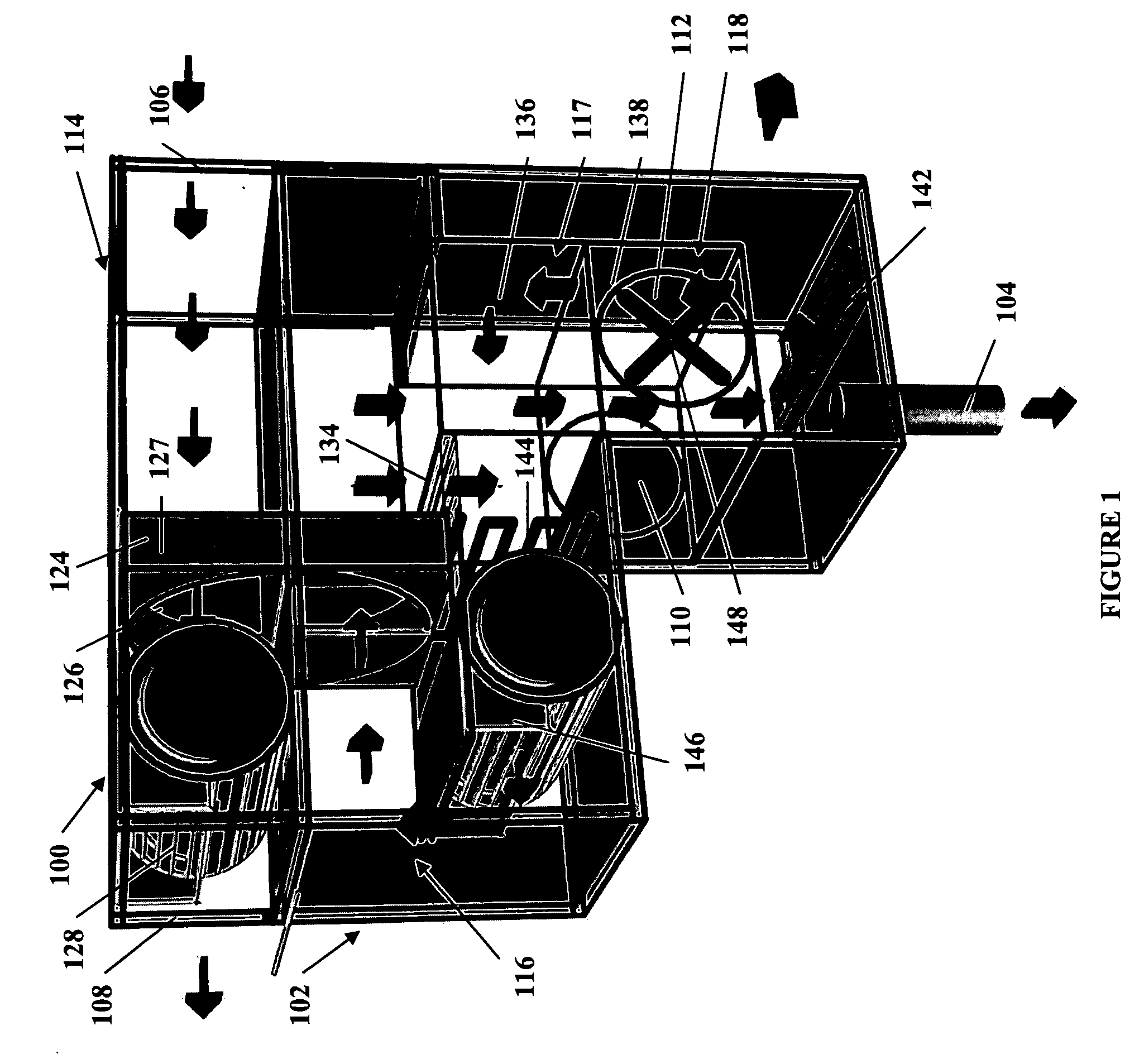
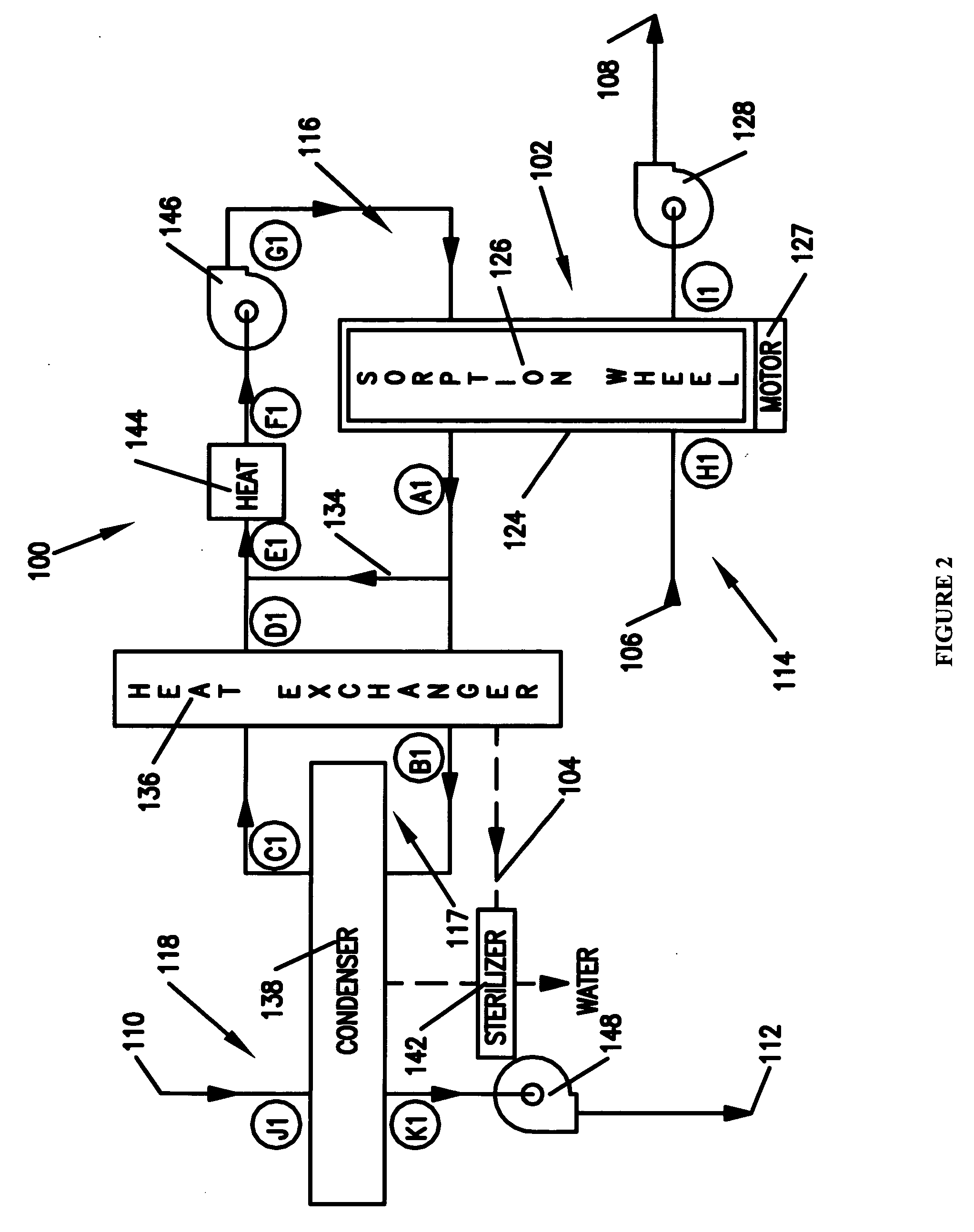
Method and apparatus for producing potable water from air including severely arid and hot climates
Methods and apparatus for extracting liquid water from ambient air, including ambient air in severely arid and hot climates, are described. An example apparatus uses a sorption-desorption-condensation cycle using a sorption wheel to extract moisture from ambient air and concentrate the water vapor driven off from the sorption material in a circulating gas, with condensation of liquid water from the circulating gas.
Owner:EPLEE DUSTIN M +1
Bottom gate type thin film transistor, method of manufacturing the same, and display apparatus
InactiveUS20100051936A1Excels in mass productionOptimise total massTransistorElectroluminescent light sourcesDesorptionBottom gate
Provided is a bottom gate type thin film transistor including on a substrate (1) a gate electrode (2), a first insulating film (3) as a gate insulating film, an oxide semiconductor layer (4) as a channel layer, a second insulating film (5) as a protective layer, a source electrode (6), and a drain electrode (7), in which the oxide semiconductor layer (4) includes an oxide including at least one selected from the group consisting of In, Zn, and Sn, and the second insulating film (5) includes an amorphous oxide insulator formed so as to be in contact with the oxide semiconductor layer (4) and contains therein 3.8×1019 molecules / cm3 or more of a desorbed gas observed as oxygen by temperature programmed desorption mass spectrometry.
Owner:CANON KK
Fuel vapor handling apparatus and diagnostic apparatus thereof
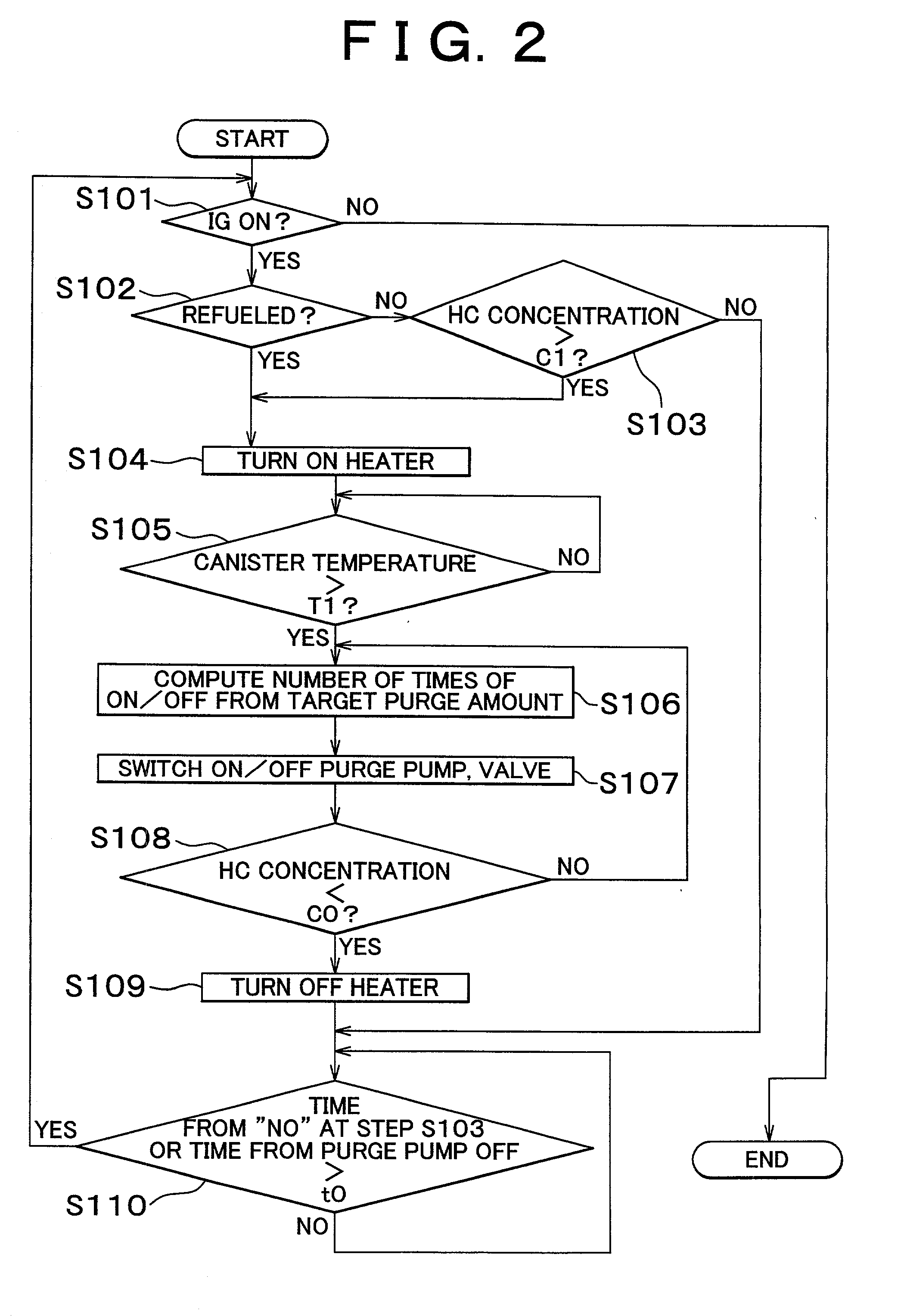
InactiveUS20020162457A1Desorption of fuel is facilitatedFuel can be purged efficientlyNon-fuel substance addition to fuelFuel injection apparatusDesorptionVaporization
A fuel vapor handling apparatus supplies a purging air to a canister by using a purge pump and purges fuel desorbed from the canister into an intake pipe. A controller intermittently operates the purge so that the canister internal temperature recovers from a reduced level caused by the latent heat of vaporization of fuel during an operating period of the purge pump. Therefore, desorption of fuel from the canister during an operating period is facilitated. Since the actual operating time of the purge pump is reduced, the life of a motor that is a power unit of the purge pump becomes longer.
Owner:TOYOTA JIDOSHA KK +1
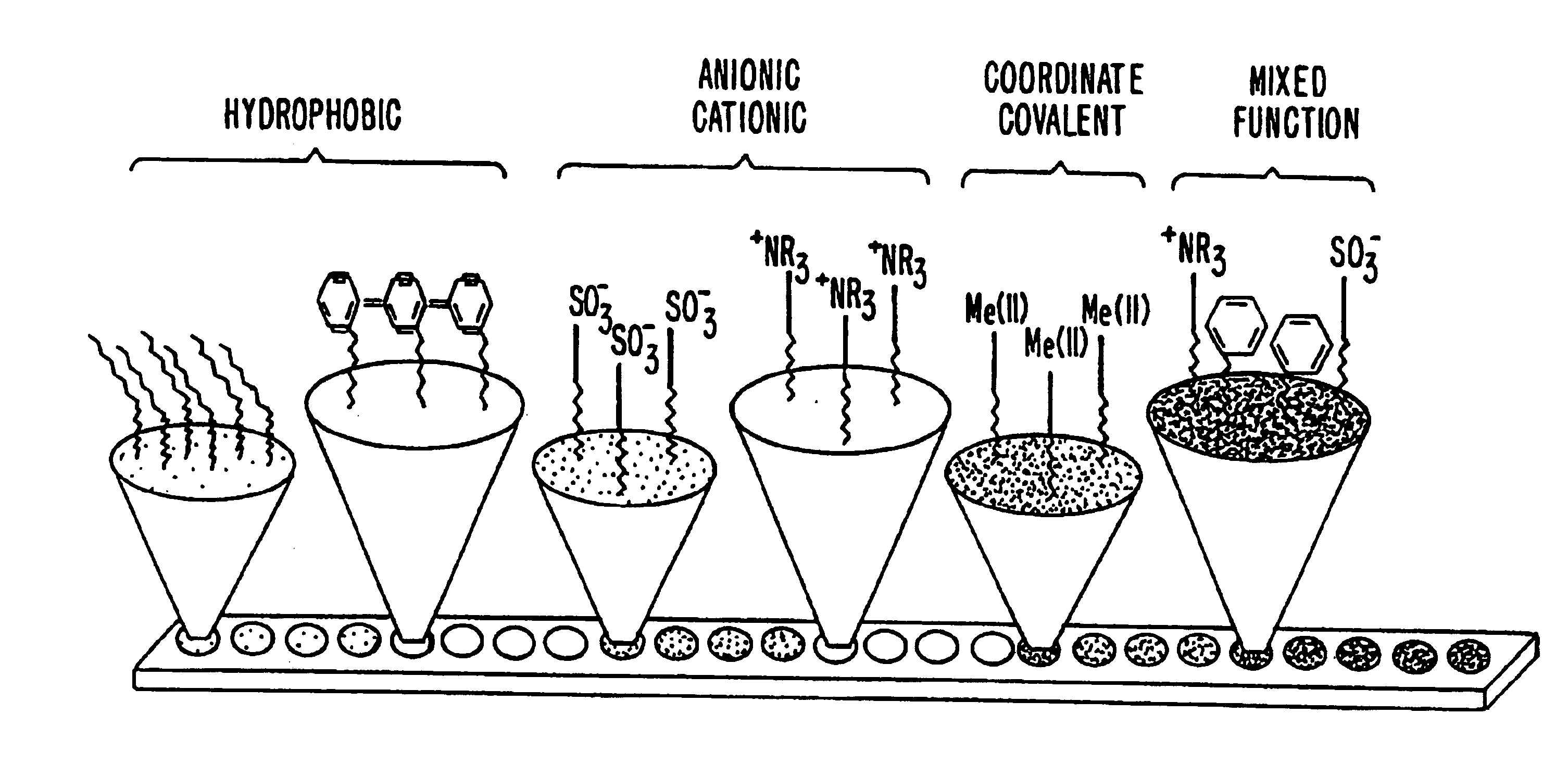
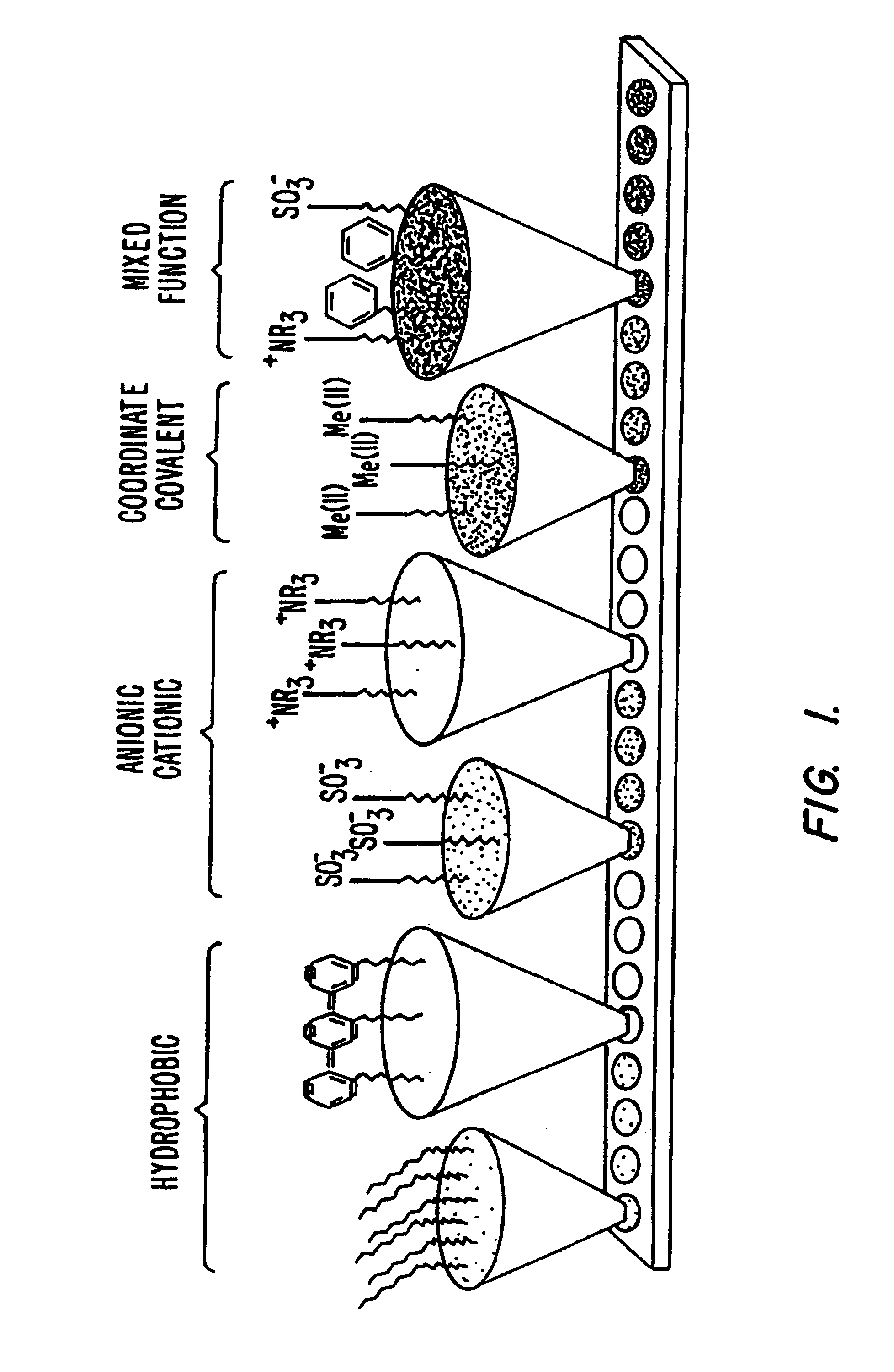
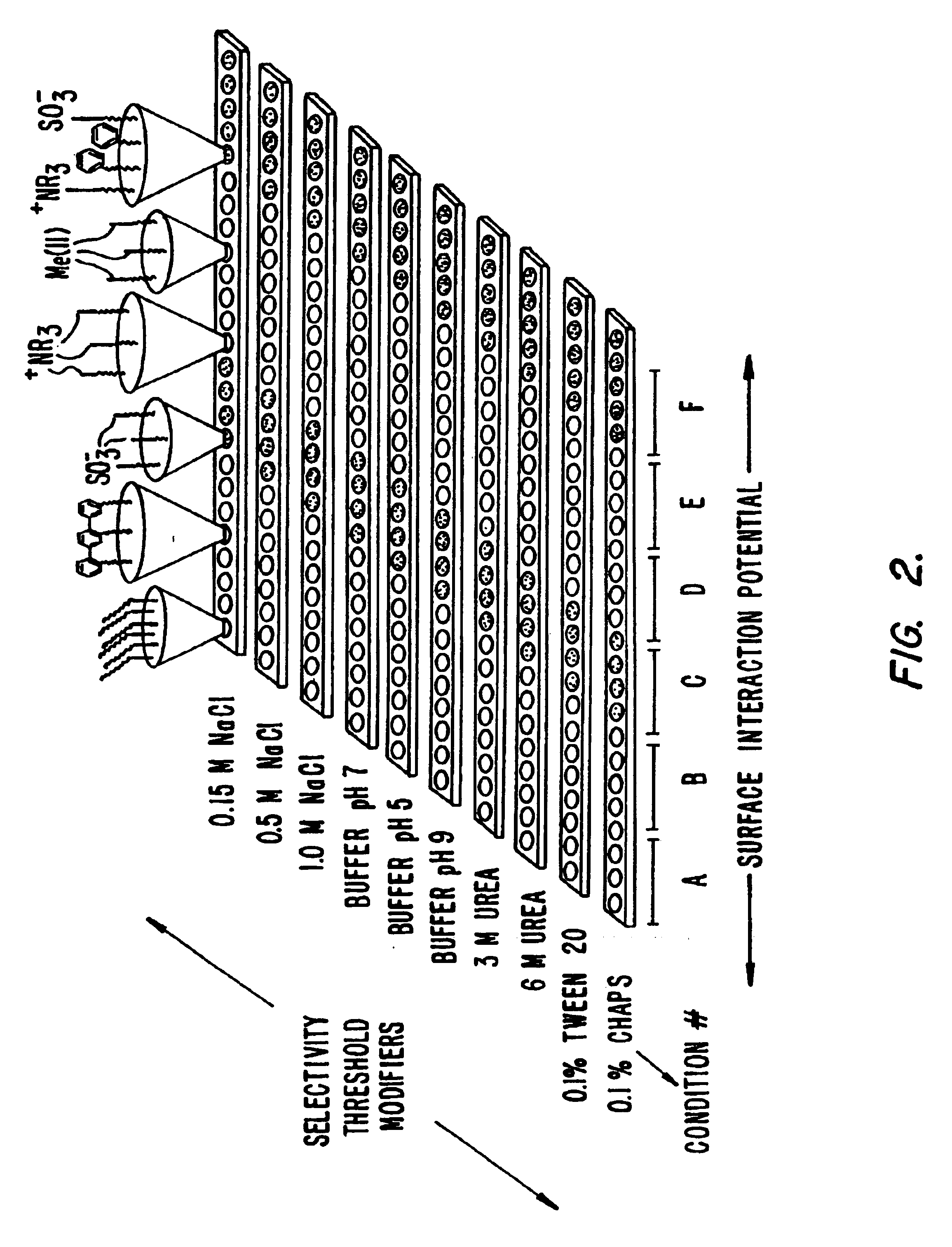
Surface-enhanced affinity capture for desorption and detection of analytes
InactiveUS6027942AFacilitate desorption/ionizationAccurately determineTime-of-flight spectrometersComponent separationAnalyteDesorption
This invention relates generally to a mass spectrometer probe and a method of using said probe for desorption and ionization of analytes. The sample probe comprises an affinity reagent on the probe surface, wherein the affinity reagent is capable of selectively binding an analyte. An analyte bound to the affinity reagent can be desorbed by a high energy source and detected in the mass spectrometer. The probe and methods are useful in detection and analysis of macromolecules such as proteins or other biomolecules.
Owner:HUTCHENS T WILLIAM
Absorbent structures comprising fluid storage members with improved ability to dewater acquisition/distribution members
InactiveUS6551295B1Effectively and efficiently dewateredImprove distributionOther chemical processesBaby linensAbsorption capacityDesorption
The present invention is an absorbent structure to be used in absorbent articles, having at least a first region for acquisition / distribution of fluid and a second region for storage of fluid. The first region can contain materials which have a relatively high capillary desorption pressure, as the materials in the second region exhibit a sufficiently high capillary absorption pressure so as to still efficiently drain the first region.The first region material has a Capillary Sorption Desorption Height (CSDH 90) of more than 40 cm and the second region material satisfies at least one of following requirements:(a) an absorption capacity of at least 15 g / g at 35 cm in the capsorption test;(b) an absorption capacity of at least 15 g / g at 0 cm in the capsorption test and an absorption efficiency of at least 55% at 40 cm;(c) a Capillary Sorption Absorption height at 50% of its capacity at 0 cm absorption height (CSAH 50) of at least 35 cm in the capsorption test.
Owner:THE PROCTER & GAMBLE COMPANY
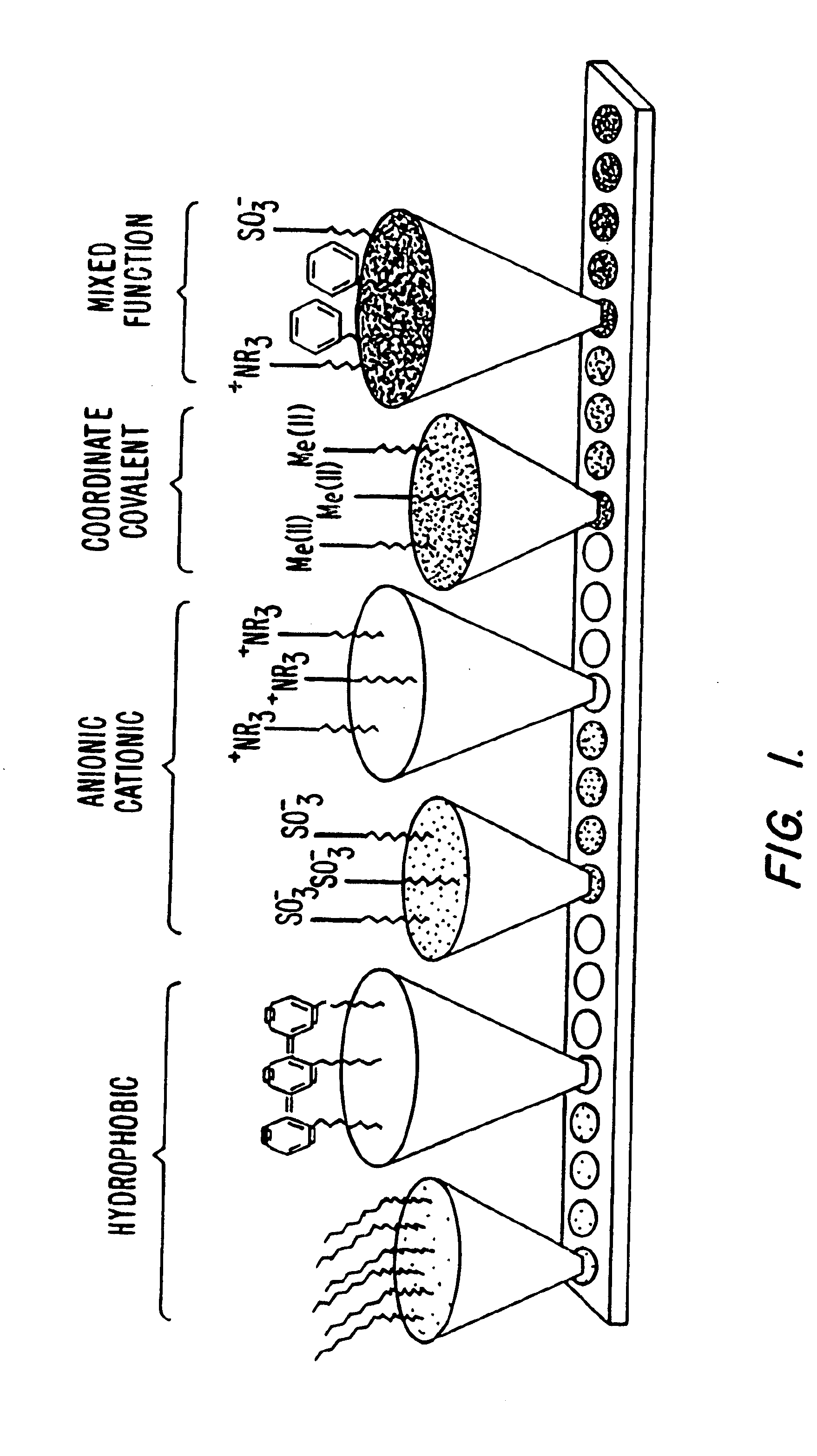
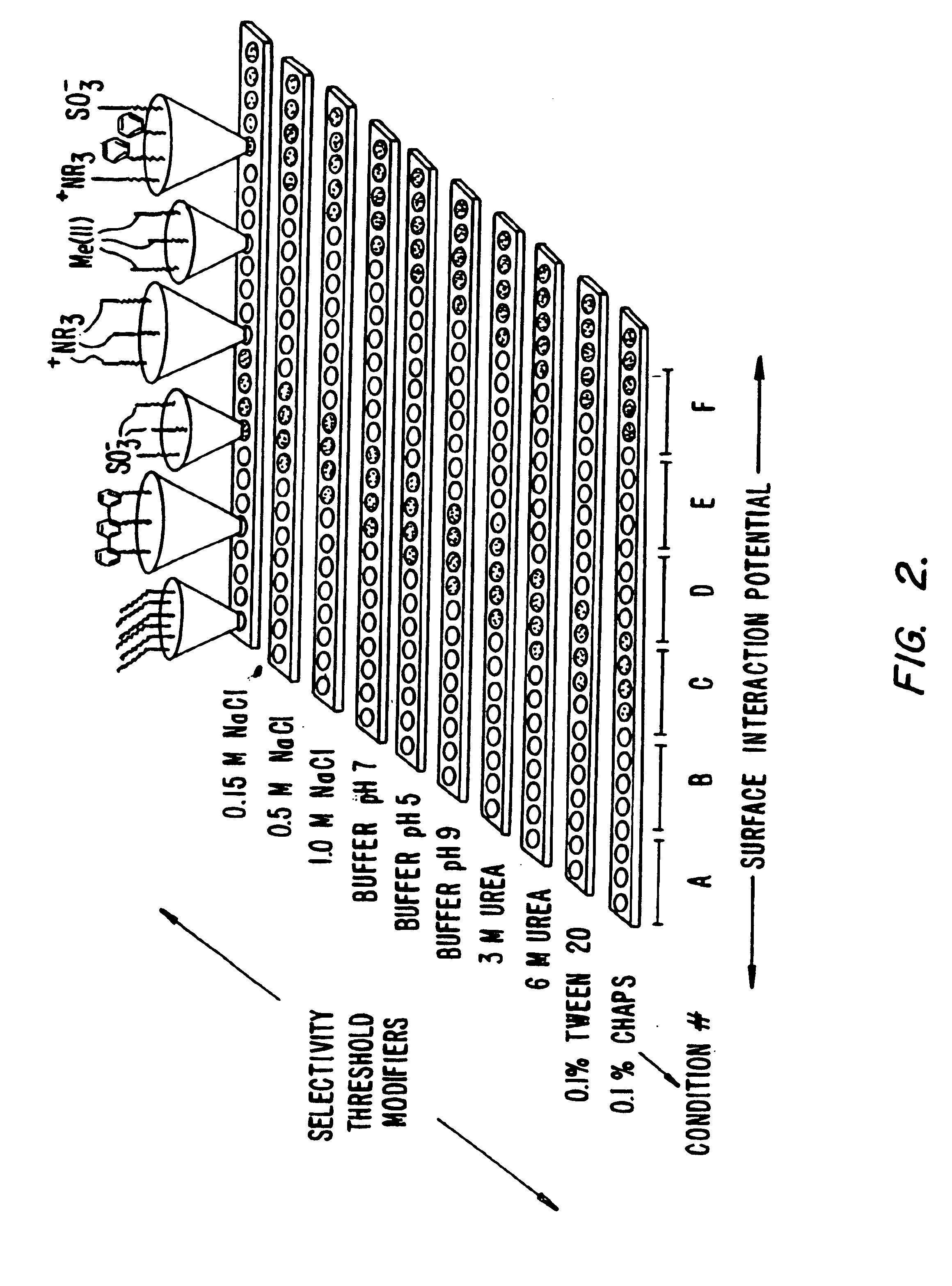
Retentate chromatography and protein chip arrays with applications in biology and medicine
InactiveUS6844165B2Easy to testHigh information resolutionBioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteDesorption
This invention provides methods of retentate chromatography for resolving analytes in a sample. The methods involve adsorbing the analytes to a substrate under a plurality of different selectivity conditions, and detecting the analytes retained on the substrate by desorption spectrometry. The methods are useful in biology and medicine, including clinical diagnostics and drug discovery.
Owner:BIO RAD LAB INC
Sensor instrument system including method for detecting analytes in fluids
ActiveUS8449824B2Novel and uniqueSimple designLaboratory glasswaresNanosensorsTransceiverHydrogen selectivity
A sensor instrument system for detecting and identifying analytes in fluids of a region contains a local sensor instrument and remote central station. The instrument includes a core technology employing a single sensor having two electrodes operated by an electrical frequency sweeping to generate two sets of patterned electrical information from a single measurement, a data transmission module and a GPS receiver module. The central station connects to a network means connected to a plurality of local receiving sites equipped with including the respective transceivers, so that the local analyte electrical information and geographic position information transmitted by the instrument can be wirelessly and remotely received and processed by the central station. The core technology further includes a reference sensor for eliminating background influence, a temperature programming to control adsorption and desorption of analytes, and usage of all kinds of adsorbent materials having chemical selectivities including the hydrogen selectivity to enhance detection and identification of analytes in fluids.
Owner:SUN YIZHONG
Apparatus for thermal swing adsorption and thermally-enhanced pressure swing adsorption
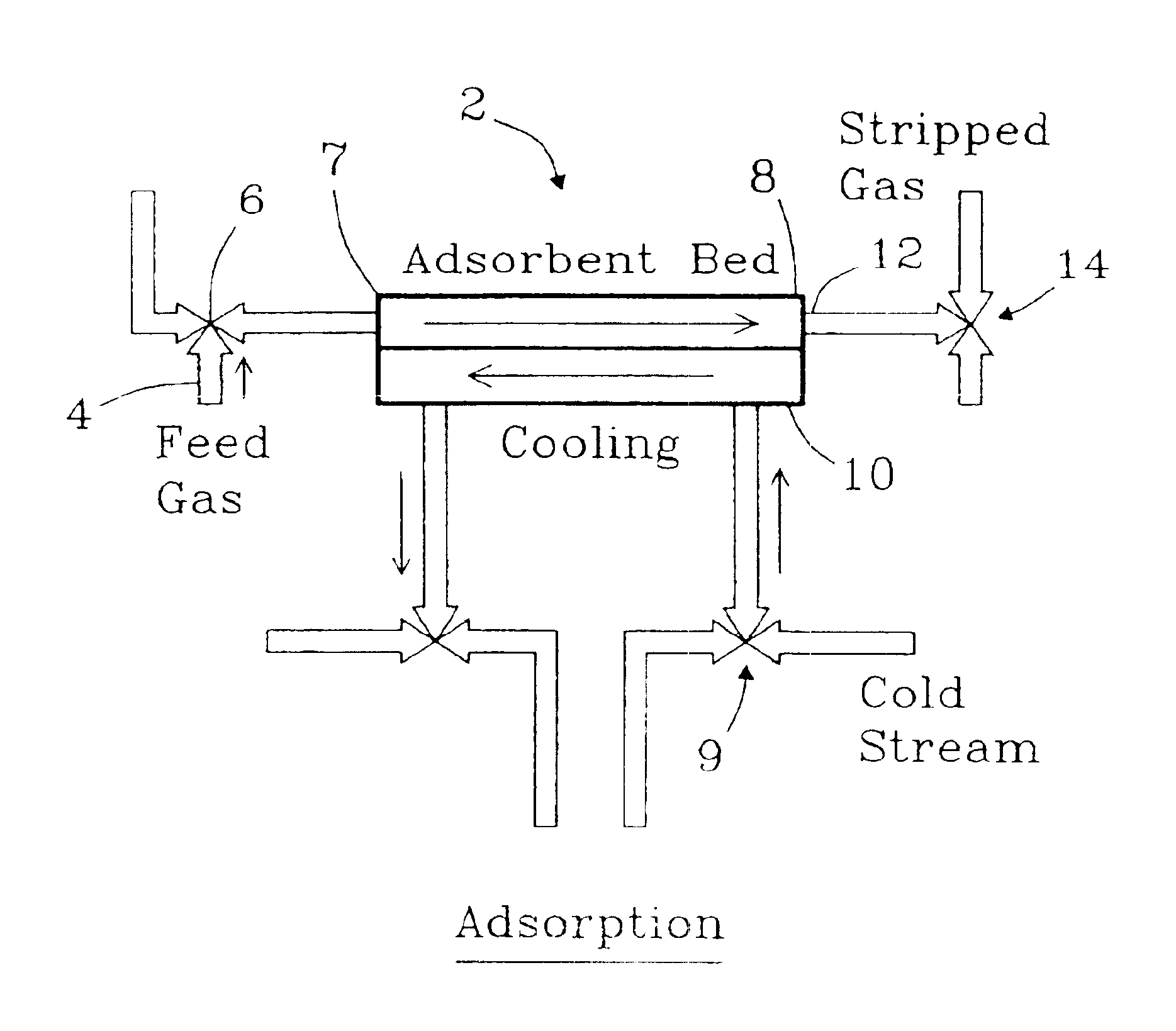
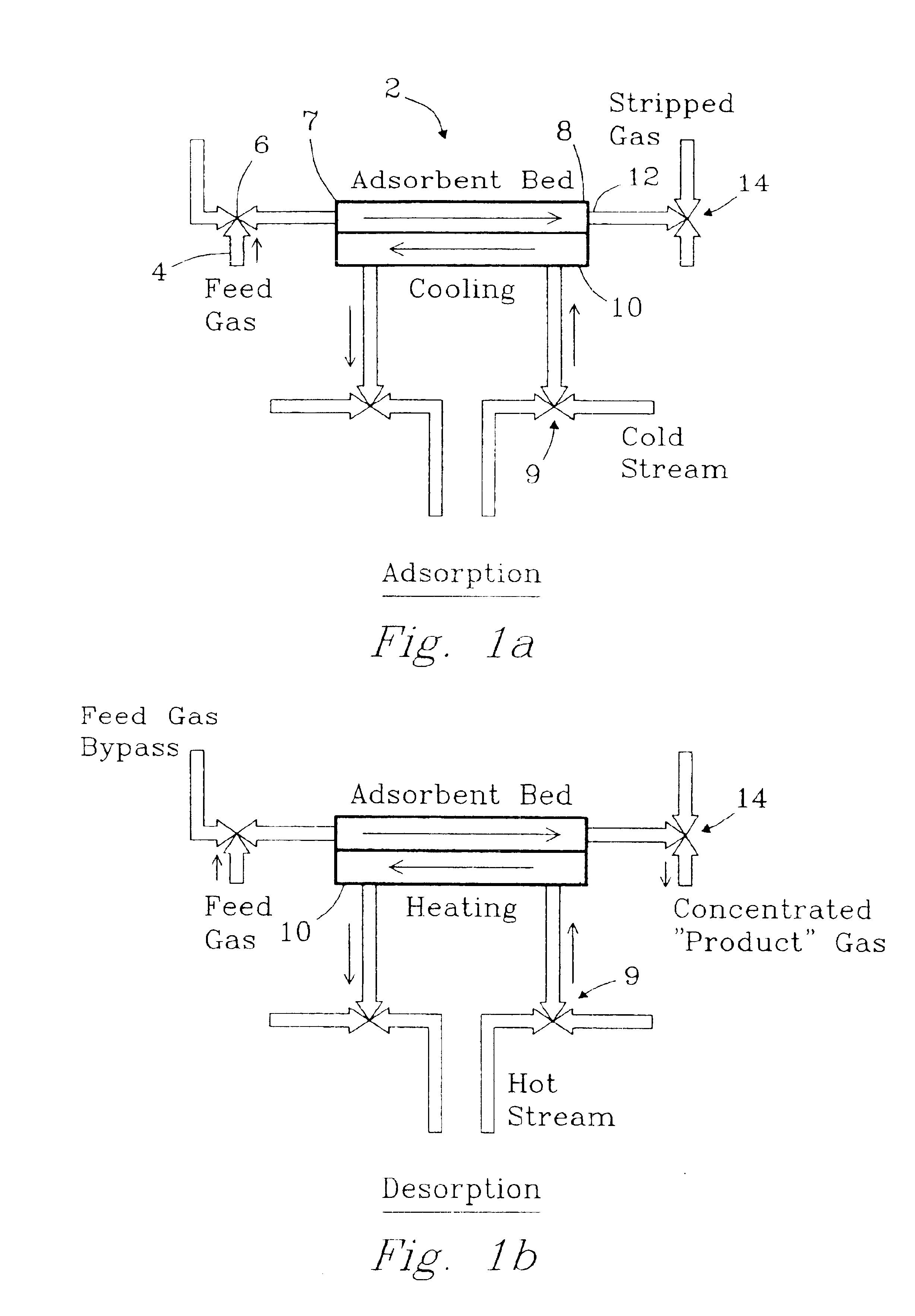
The present invention provides compact adsorption systems that are capable of rapid temperature swings and rapid cycling. Novel methods of thermal swing adsorption and thermally-enhanced pressure swing adsorption are also described. In some aspects of the invention, a gas is passed through the adsorbent thus allowing heat exchangers to be very close to all portions of the adsorbent and utilize less space. In another aspect, the adsorption media is selectively heated, thus reducing energy costs. Methods and systems for gas adsorption / desorption having improved energy efficiency with capability of short cycle times are also described. Advantages of the invention include the ability to use (typically) 30-100 times less adsorbent compared to conventional systems.
Owner:BATTELLE MEMORIAL INST
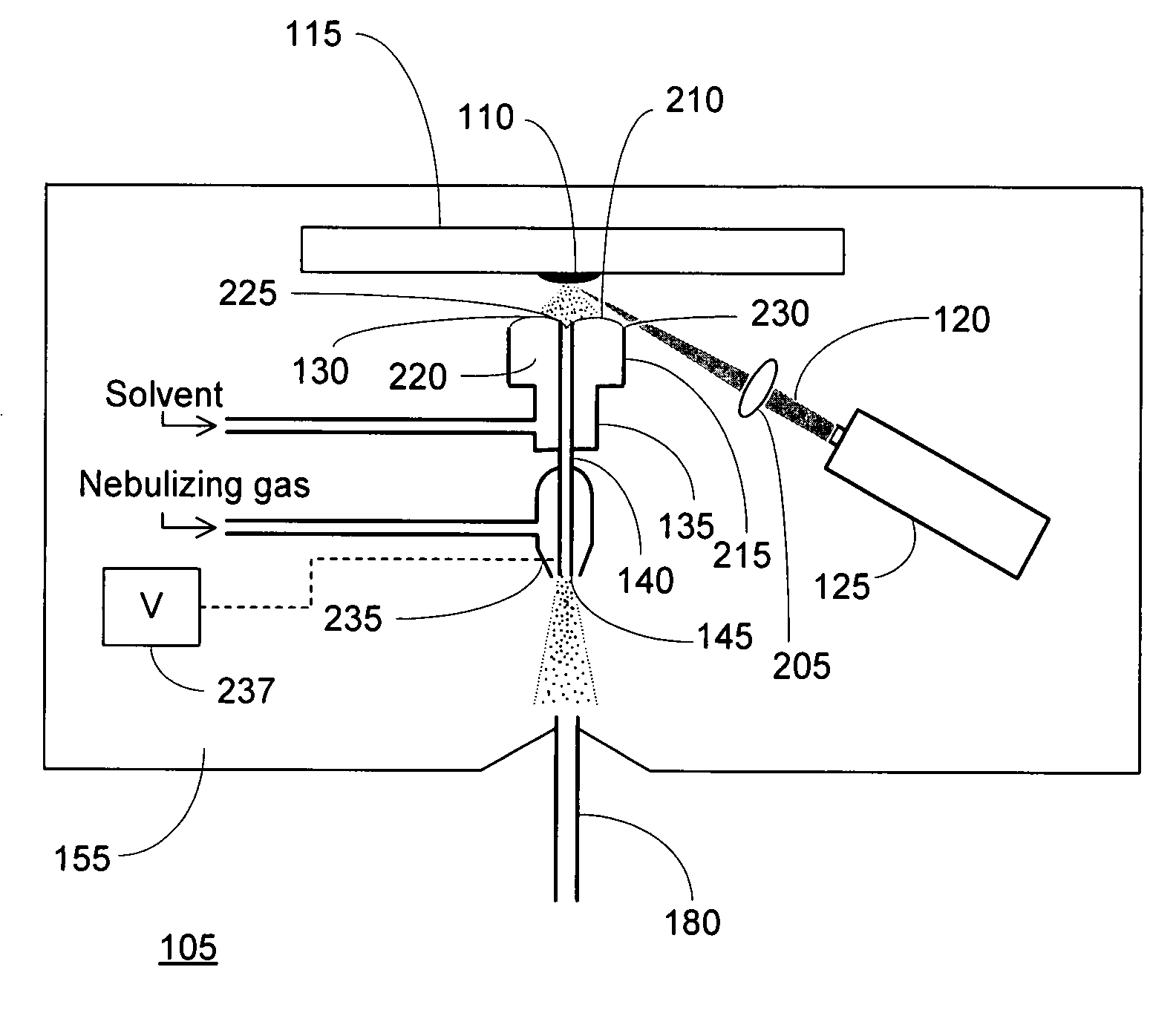
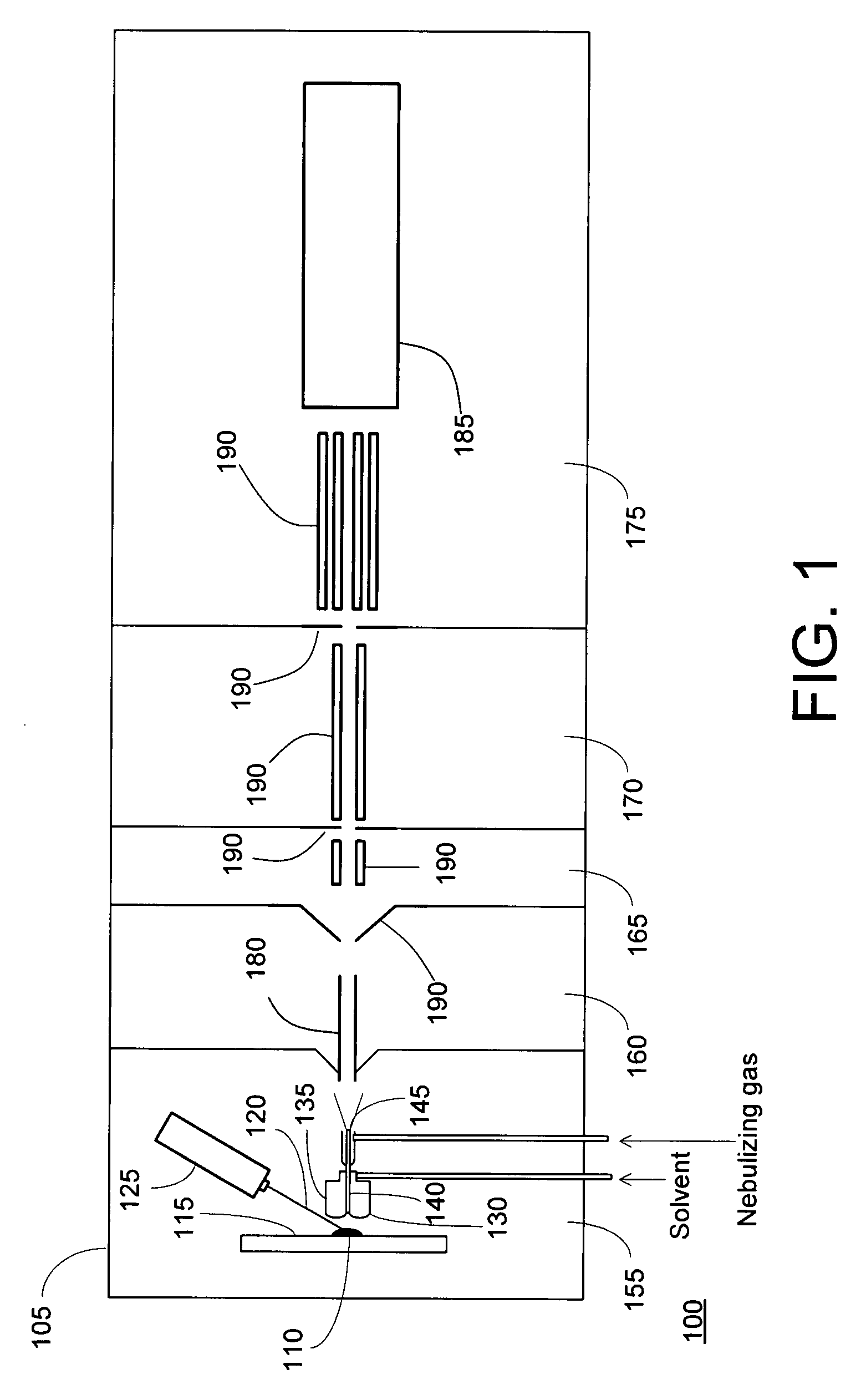
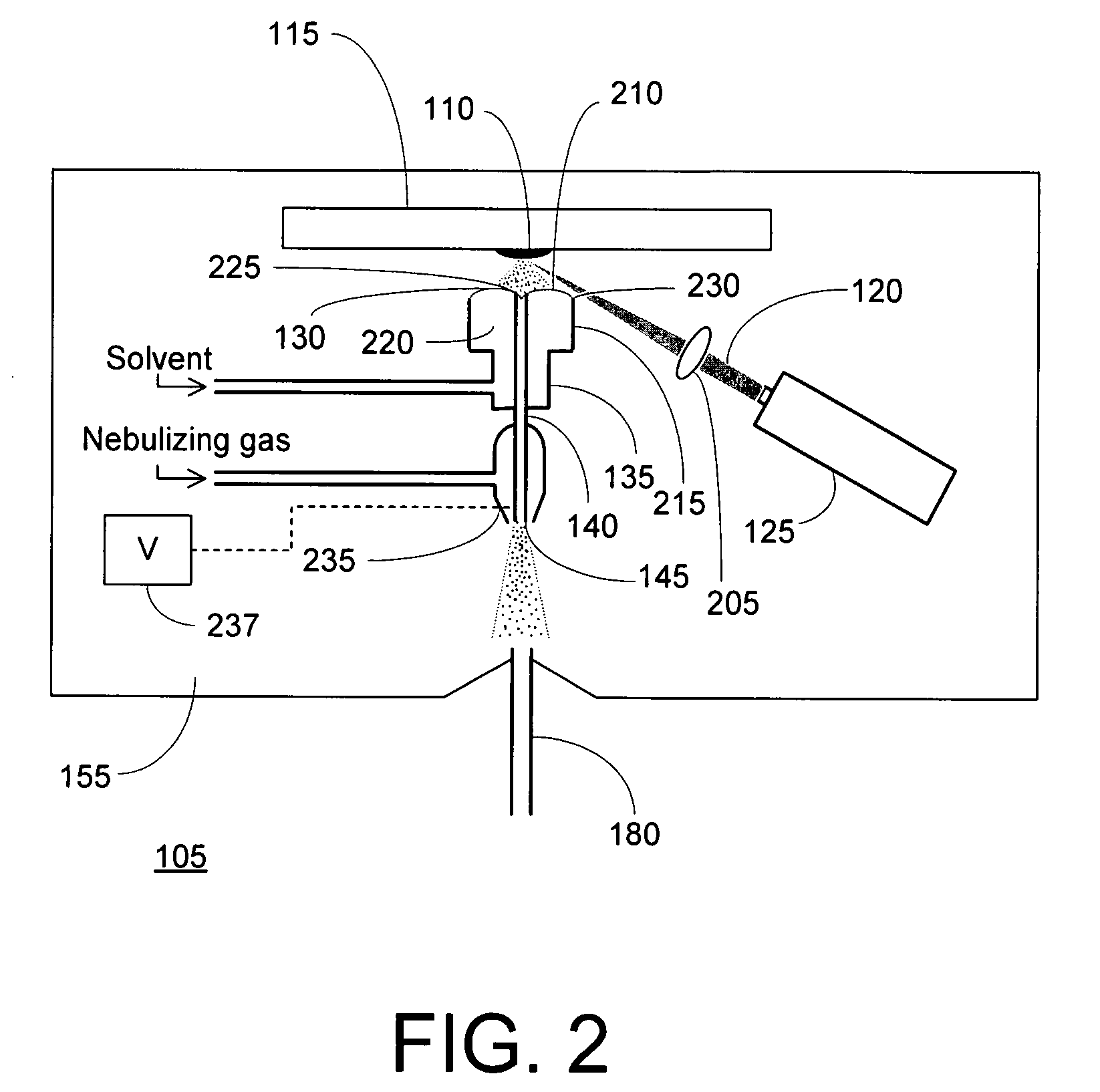
Laser desorption - electrospray ion (ESI) source for mass spectrometers
InactiveUS20080272294A1Minimize dilutionMaterial analysis by optical meansIon sources/gunsDesorptionElectrospray ionization
An ion source is disclosed for forming multiply-charged analyte ions from a solid sample. A beam of pulsed radiation is directed onto a portion of the sample to desorb analyte molecules. A retaining structure holding a solvent volume is positioned proximate the sample. Desorbed analyte molecules contact a free surface of the solvent and pass into solution. The solution is then conveyed through an outlet passageway to an electrospray apparatus, which introduces a spray of charged solvent droplets into an ionization chamber.
Owner:THERMO FINNIGAN
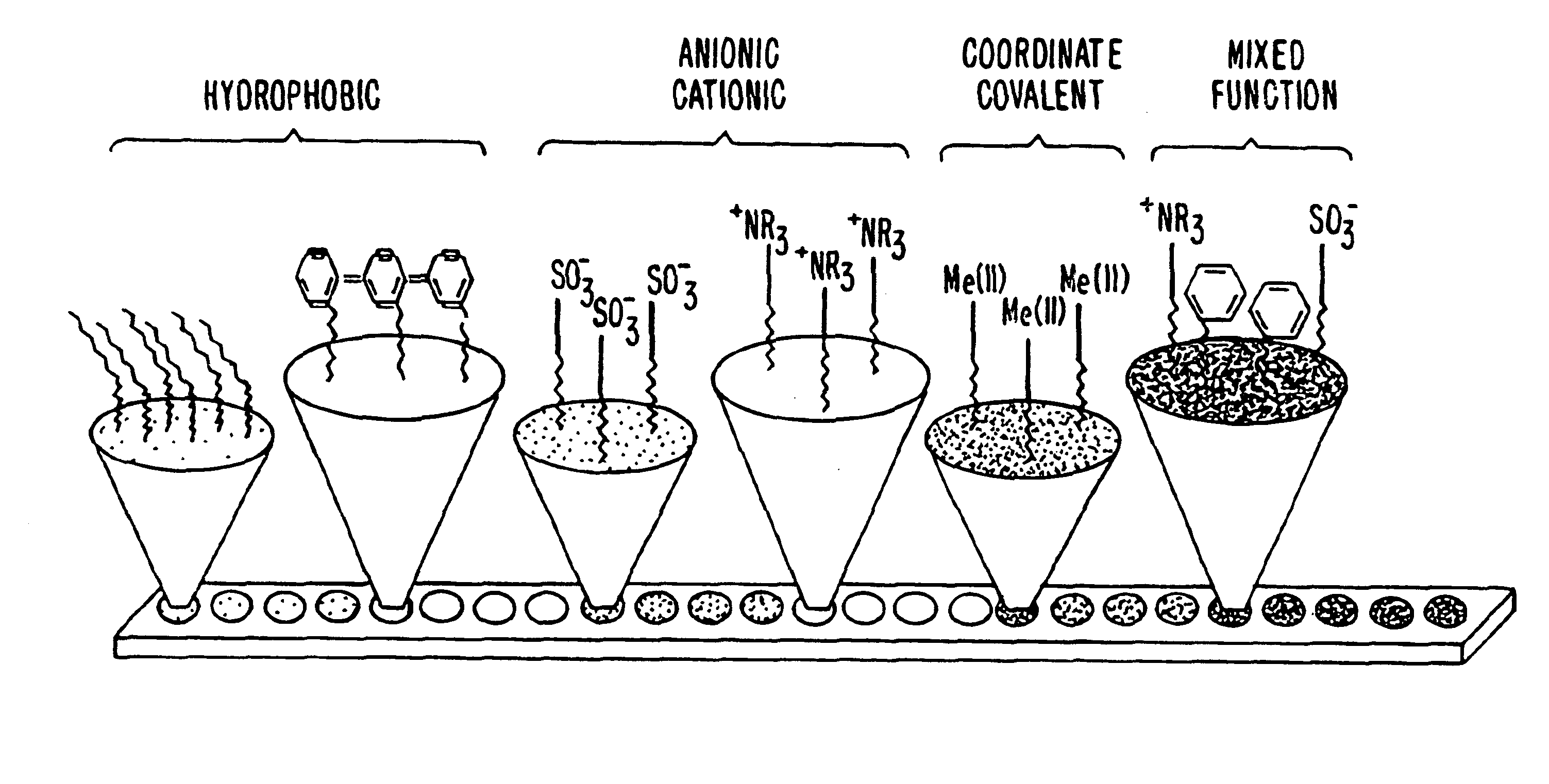
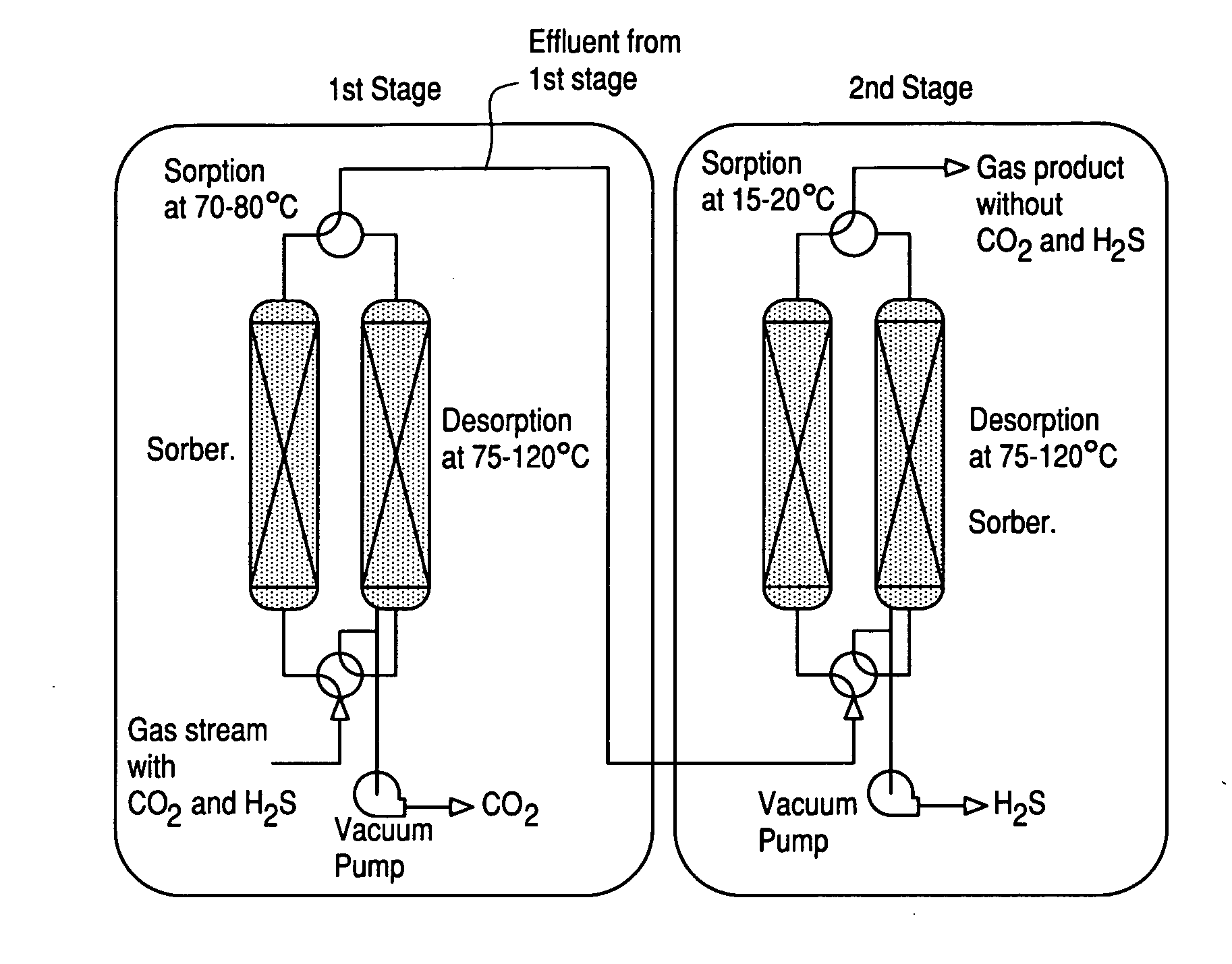
Novel sorbents and purification and bulk separation of gas streams
InactiveUS20080264254A1Large capacityLittle and no corrosive effectNitrous oxide captureGas treatmentSorbentDesorption
Porous-material-supported polymer sorbents and process for removal of undesirable gases such as H2S, COS, CO2, N2O, NO, NO2, SO2, SO3, HCl, HF, HCN, NH3, H2O, C2H5OH, CH3OH, HCHO, CHCl3, CH2Cl2, CH3Cl, CS2, C4H4S, CH3SH, and CH3—S—CH3 from various gas streams such as natural gas, coal / biomass gasification gas, biogas, landfill gas, coal mine gas, ammonia syngas, H2 and oxo-syngas, Fe ore reduction gas, reformate gas, refinery process gases, indoor air, fuel cell anode fuel gas and cathode air are disclosed. The sorbents have numerous advantages such as high breakthrough capacity, high sorption / desorption rates, little or no corrosive effect and are easily regenerated. The sorbents may be prepared by loading H2S—, COS—, CO2—, N2O, NO—, NO2—, SO2—, SO3—, HCl—, HF—, HCN—, NH3—, H2O—, C2H5OH—, CH3OH—, HCHO—, CHCl3—, CH2Cl2—, CH3Cl—, CS2—, C4H4S—, CH3SH—, CH3—S—CH3-philic polymer(s) or mixtures thereof, as well as any one or more of H2S—, COS—, CO2—, N2O, NO—, NO2—, SO2—, SO3—, HCl—, HF—, HCN—, NH3—, H2O—, C2H5OH—, CH3OH—, HCHO—, CHCl3—, CH2Cl2—, CH3Cl—, CS2—, C4H4S—, CH3SH—, CH3—S—CH3-philic compound(s) or mixtures thereof on to porous materials such as mesoporous, microporous or macroporous materials. The sorbents may be employed in processes such as one-stage and multi-stage processes to remove and recover H2S, COS, CO2, N2O, NO, NO2, SO2, SO3, HCl, HF, HCN, NH3, H2O, C2H5OH, CH3OH, HCHO, CHCl3, CH2Cl2, CH3Cl, CS2, C4H4S, CH3SH and CH3—S—CH3 from gas streams by use of, such as, fixed-bed sorbers, fluidized-bed sorbers, moving-bed sorbers, and rotating-bed sorbers.
Owner:PENN STATE RES FOUND +1
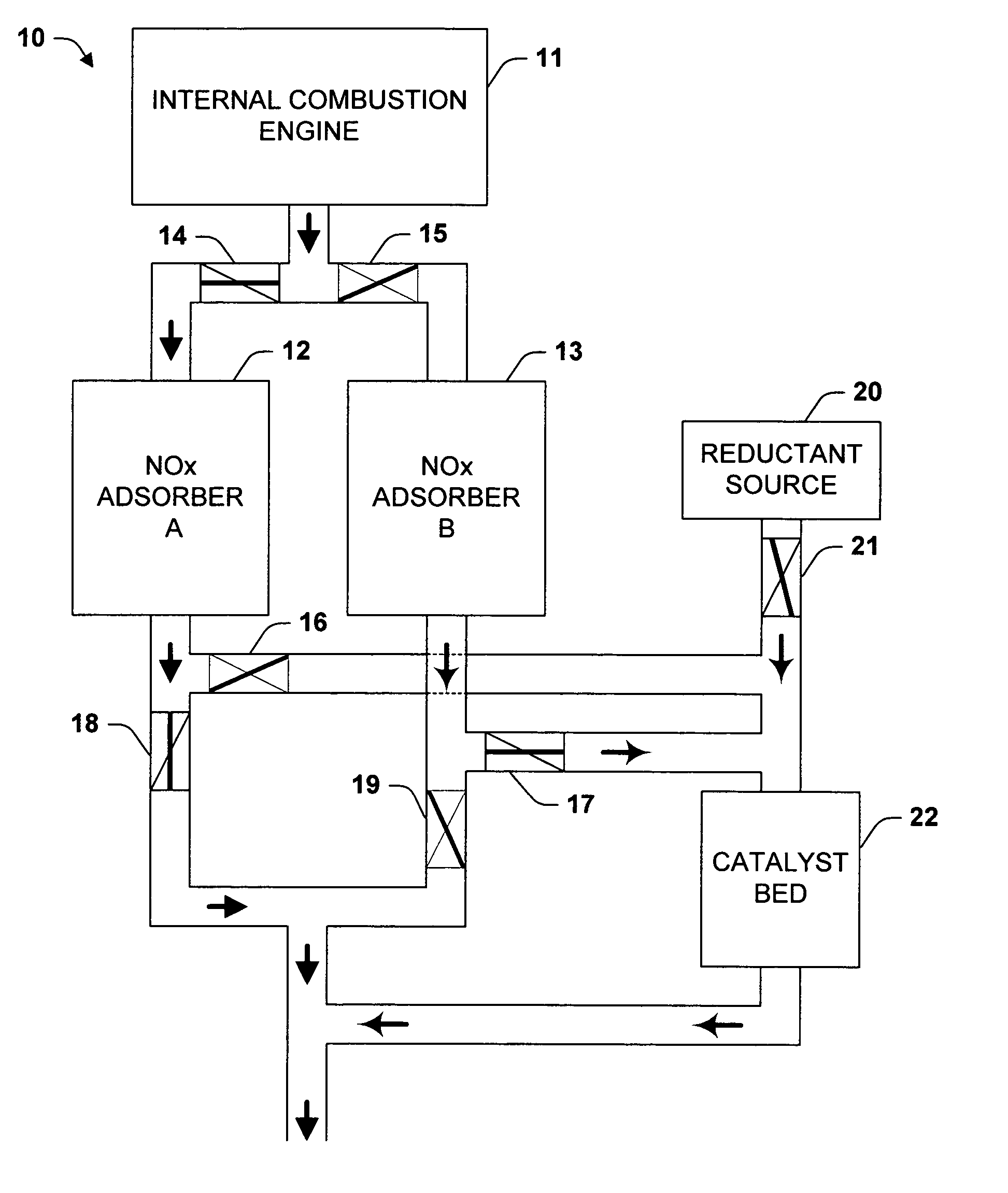
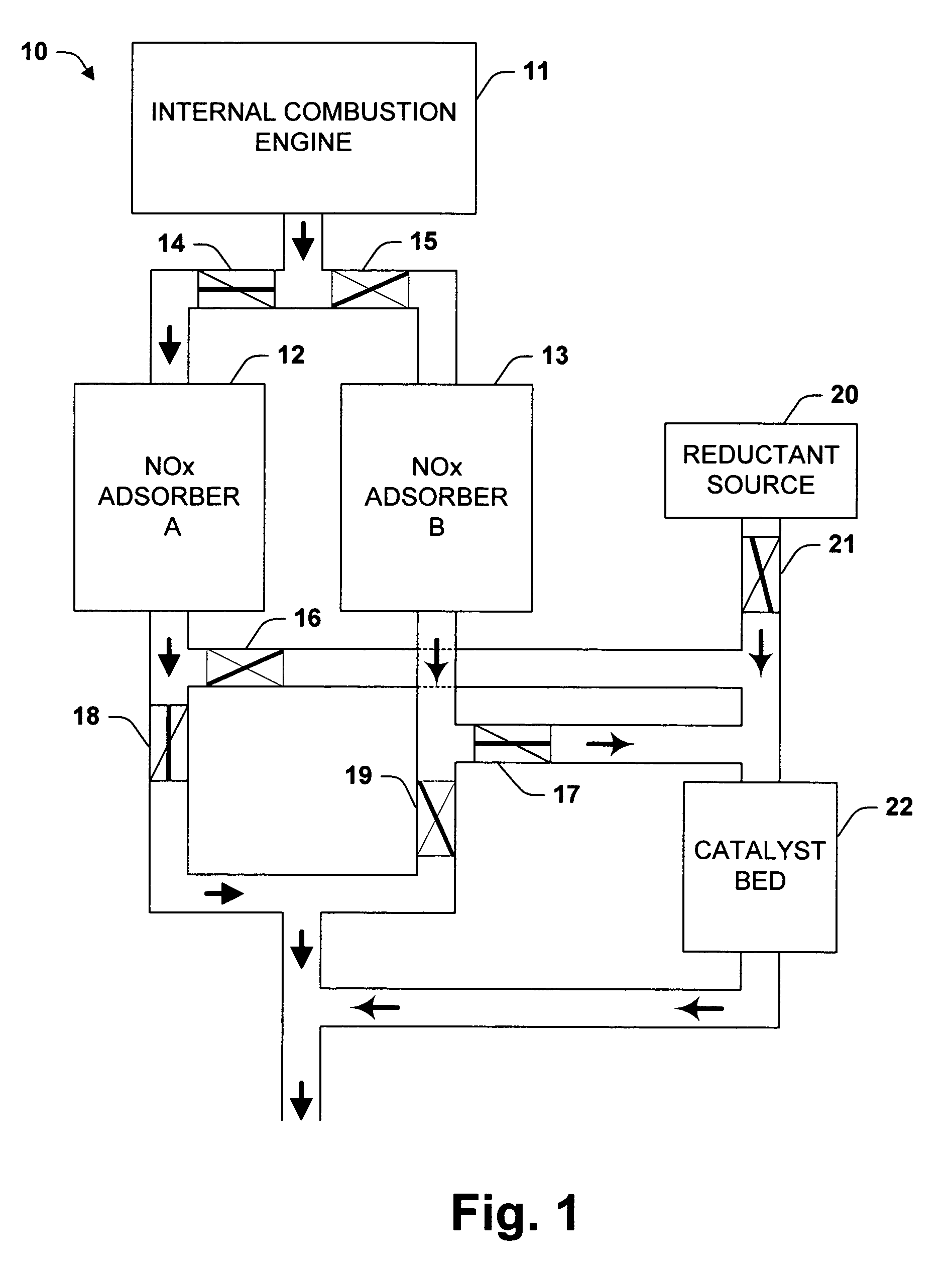
Temperature swing adsorption and selective catalytic reduction NOx removal system
InactiveUS7117669B2Simpler and more replacedEasy to useMolecular sieve catalystsInternal combustion piston enginesSyngasDesorption
The invention relates to systems for removing NOx from exhaust. In one aspect of the invention, after adsorption, an NOx adsorber is isolated from the main exhaust flow and desorption induced by raising the temperature. The desorbed NOx is combined with a reductant and reduced over a catalyst. Preferably, the reductant is syn gas produced in an on-board reformer. The catalyst need never be exposed to the main exhaust flow, which is particularly advantageous for catalysts sensitive to water, oxygen, or sulfur. In another aspect of the invention, a recirculating flow is induced through an NOx adsorber during a regeneration cycle. Recirculation can induce greater desorption at a given temperature, provide a source of heat for the adsorber, and allow a higher conversion rate with a fixed amount of catalyst. A further aspect of the invention relates to a vehicle-mounted adsorbers with provisions for heating.
Owner:EATON CORP
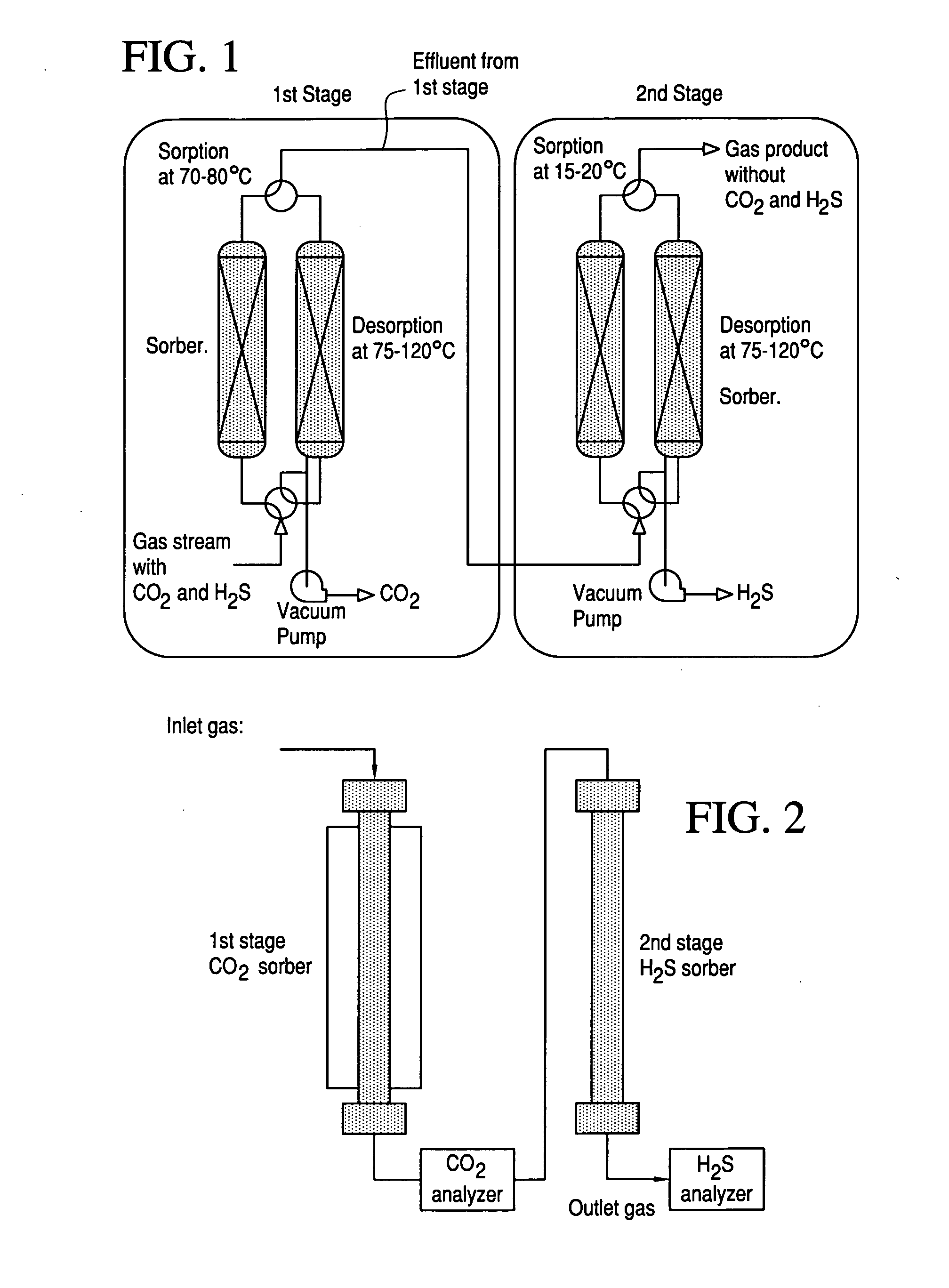
Systems and Methods for Acid Gas Removal
InactiveUS20110217218A1Reduce and eliminate needReduce investmentGas treatmentDispersed particle separationDesorptionProduct gas
A method and system for the selective removal of CO2 and / or H2S from a gaseous stream containing one or more acid gases. In particular, a system and method for separating CO2 and / or H2S from a gas mixture containing an acid gas using an absorbent solution and one or more ejector venturi nozzles in flow communication with one or more absorbent contactors. The method involves contacting a gas mixture containing at least one acid gas with the absorbent solution under conditions sufficient to cause absorption of at least a portion of said acid gas. The absorbent contactors operate in co-current flow and are arranged in a counter-current configuration to increase the driving force for mass transfer. Monoliths can be used that operate in a Taylor flow or slug flow regime. The absorbent solution is treated under conditions sufficient to cause desorption of at least a portion of the acid gas.
Owner:EXXON RES & ENG CO
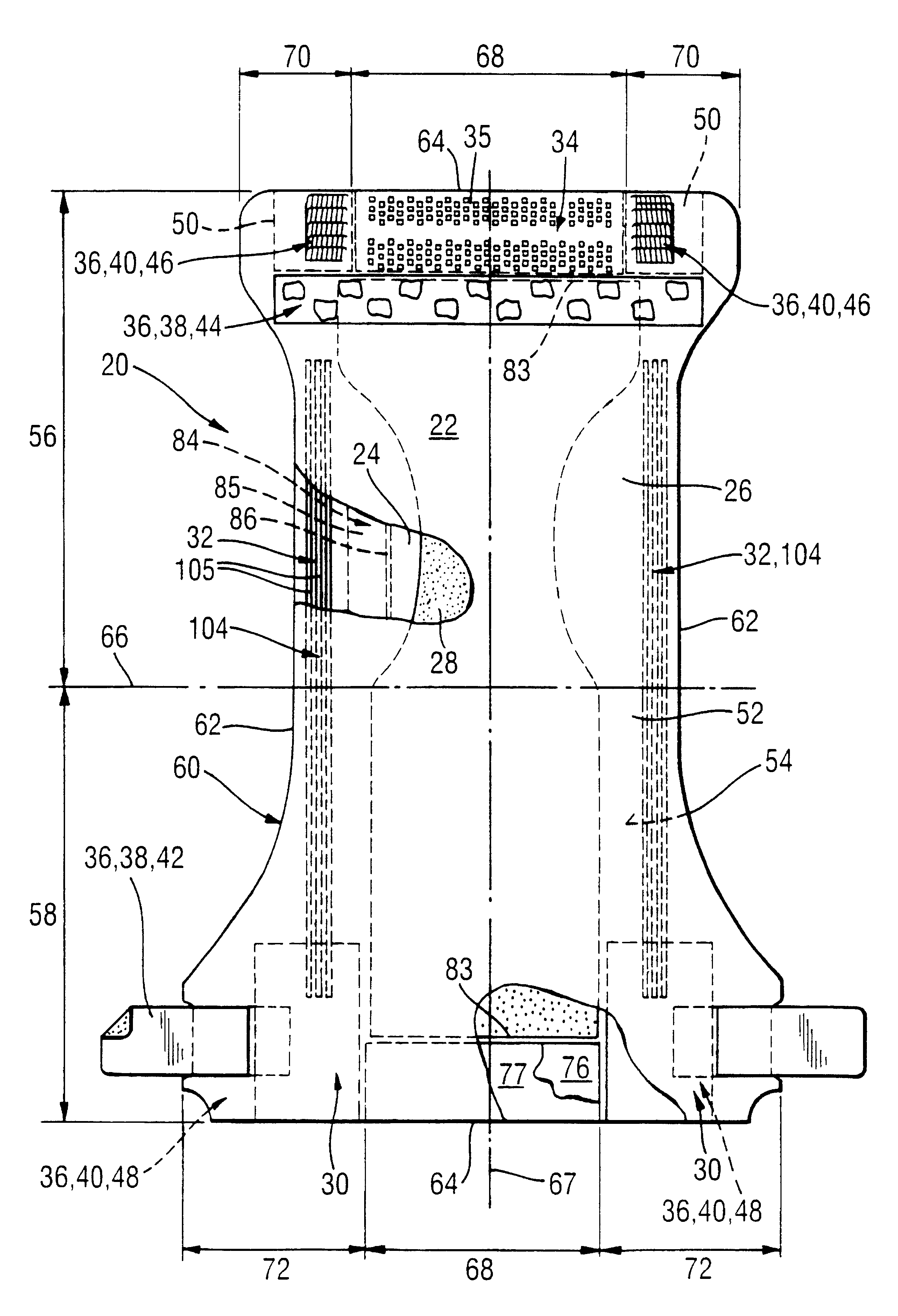
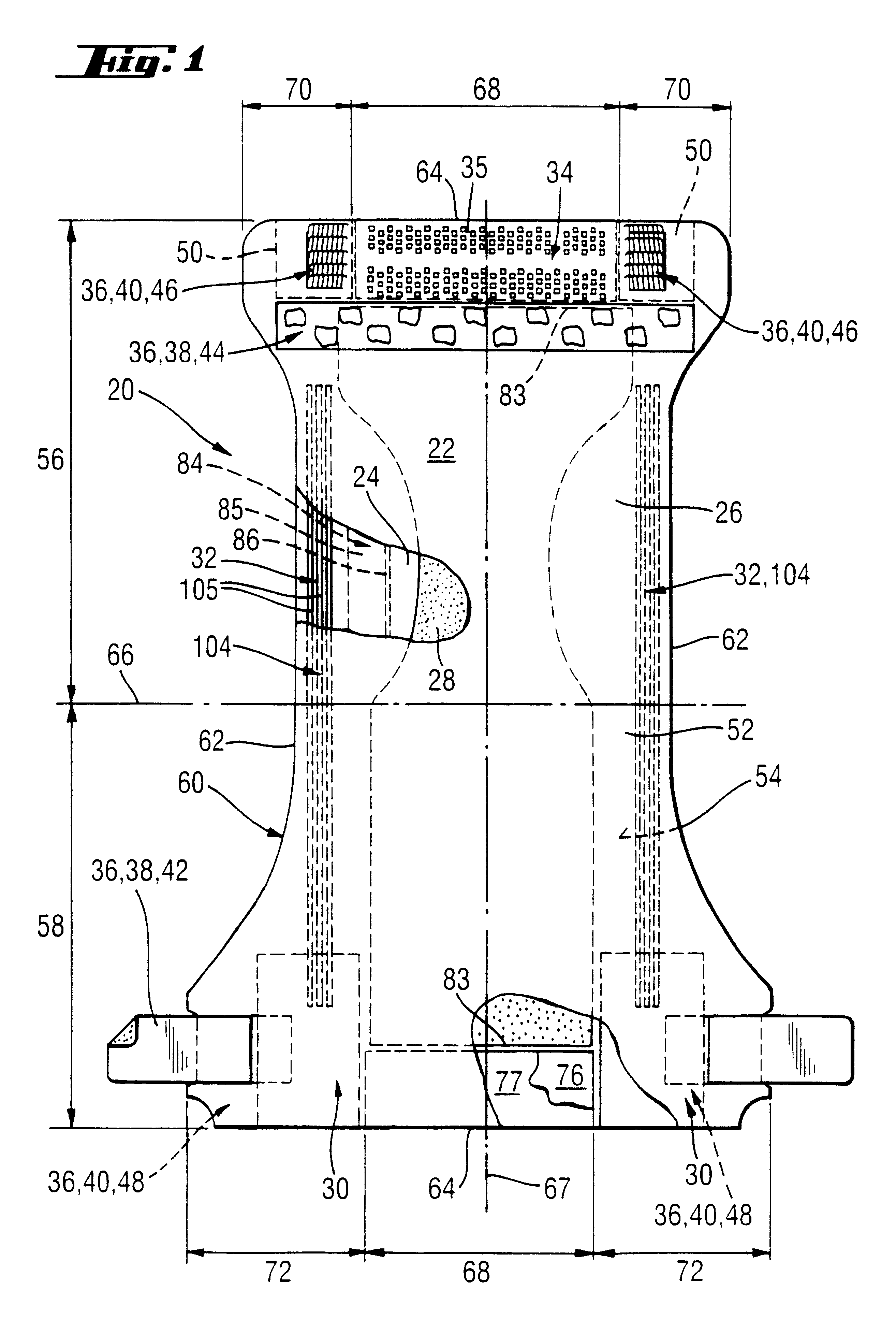
Thin film transistor and display device
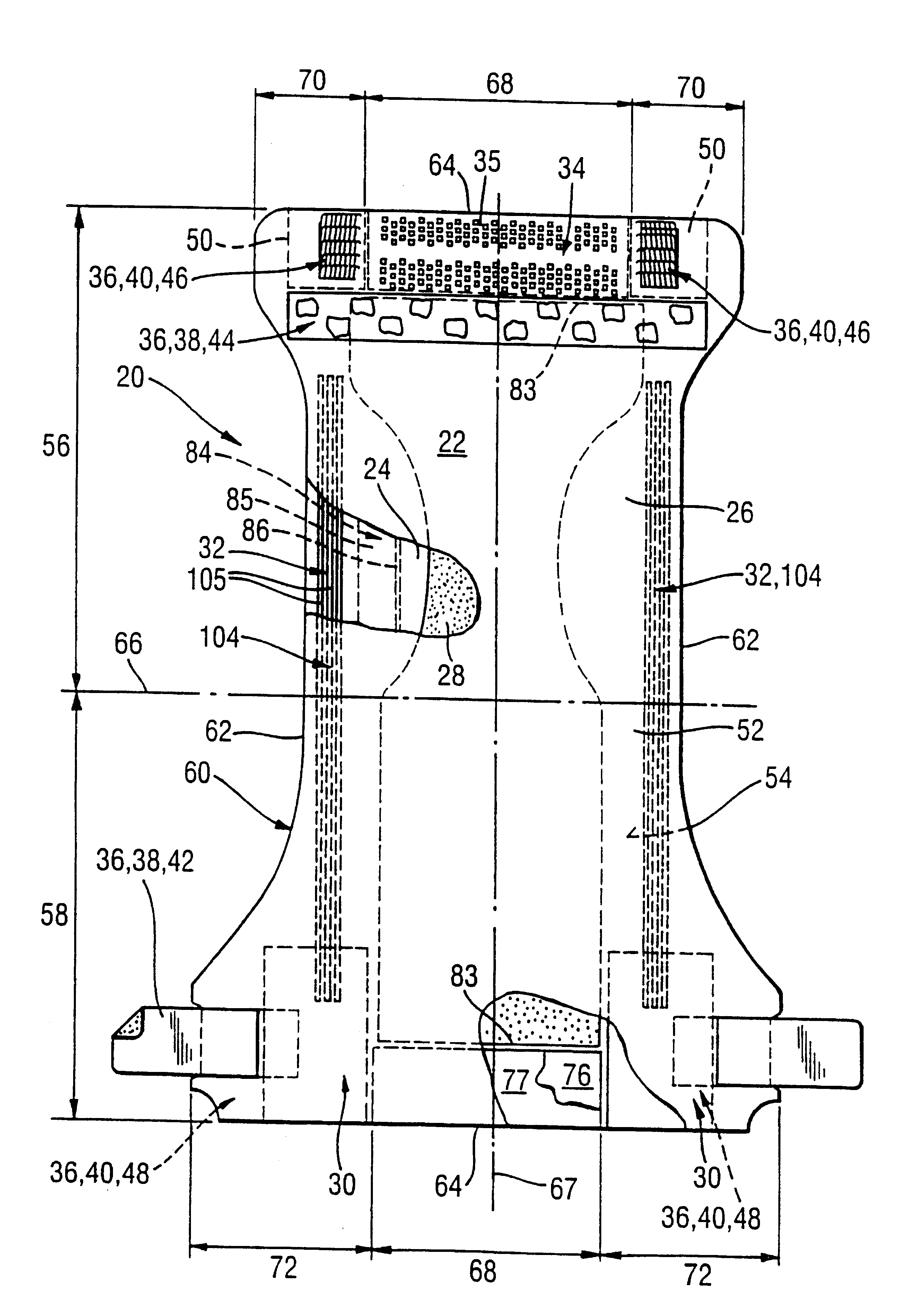
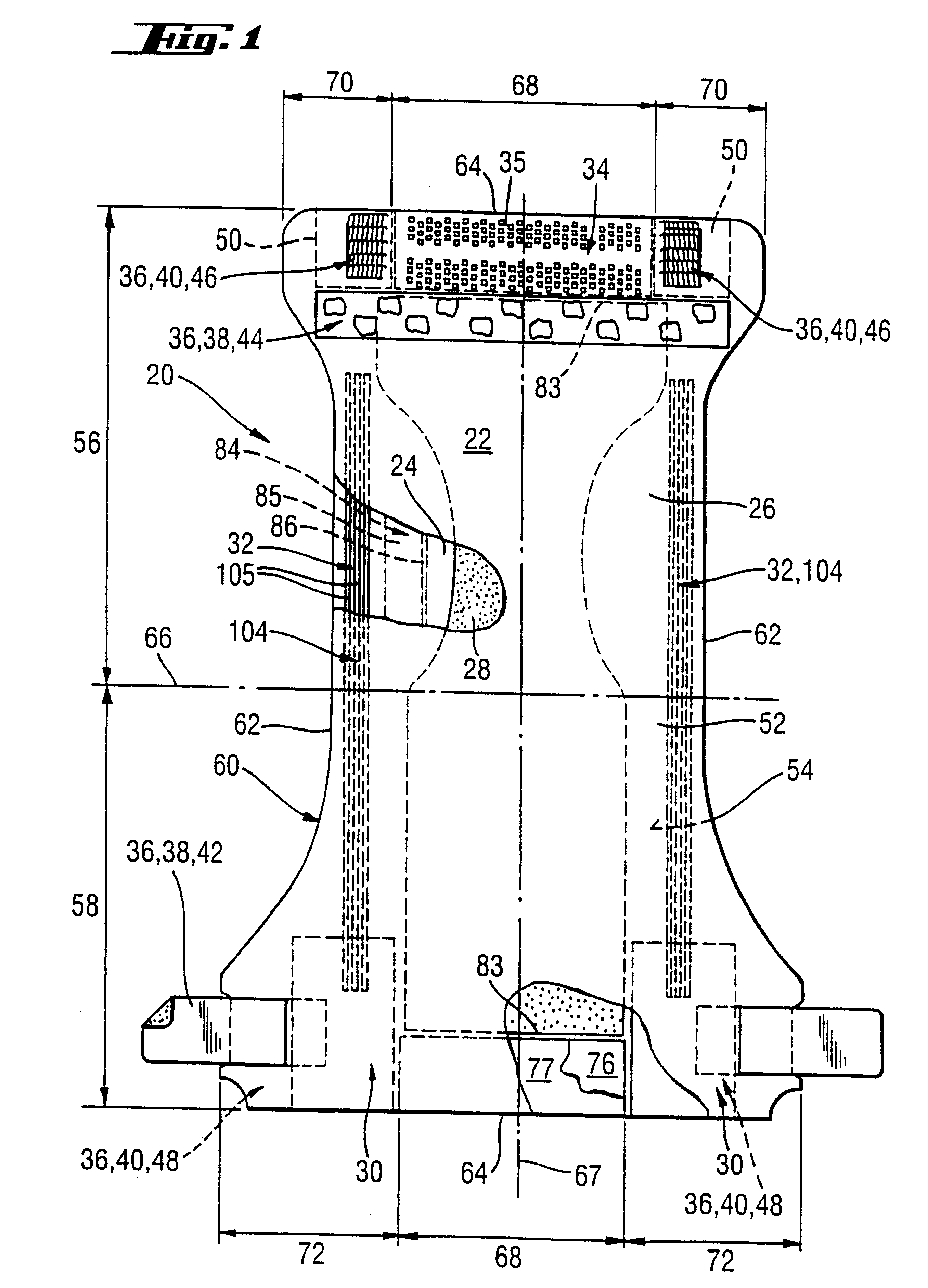
ActiveUS20110180802A1Inhibition of desorptionShorten the timeSolid-state devicesSemiconductor devicesDesorptionDisplay device
Provided are a thin film transistor that is capable of suppressing desorption of oxygen and others from an oxide semiconductor layer, and reducing the time to be taken for film formation, and a display device provided therewith. A gate insulation film 22, a channel protection layer 24, and a passivation film 26 are each in the laminate configuration including a first layer 31 made of aluminum oxide, and a second layer 32 made of an insulation material including silicon (Si). The first and second layers 31 and 32 are disposed one on the other so that the first layer 31 comes on the side of an oxide semiconductor layer 23. The oxide semiconductor layer 23 is sandwiched on both sides by the first layers 31 made of aluminum oxide, thereby suppressing desorption of oxygen and others, and stabilizing the electrical characteristics of a TFT 20. Moreover, since the second layer 32 is made of an insulation material including silicon (Si), the time to be taken for film formation can be reduced compared with a single layer made of aluminum oxide.
Owner:JOLED INC
Process for removing a target gas from a mixture of gases by thermal swing adsorption
The separation of a target gas from a mixture of gases using a thermal swing adsorption process wherein a thermal wave is used, primarily in the desorption step. The process of this invention enables one to separately remove multiple contaminants from a treated gaseous stream.
Owner:EXXON RES & ENG CO
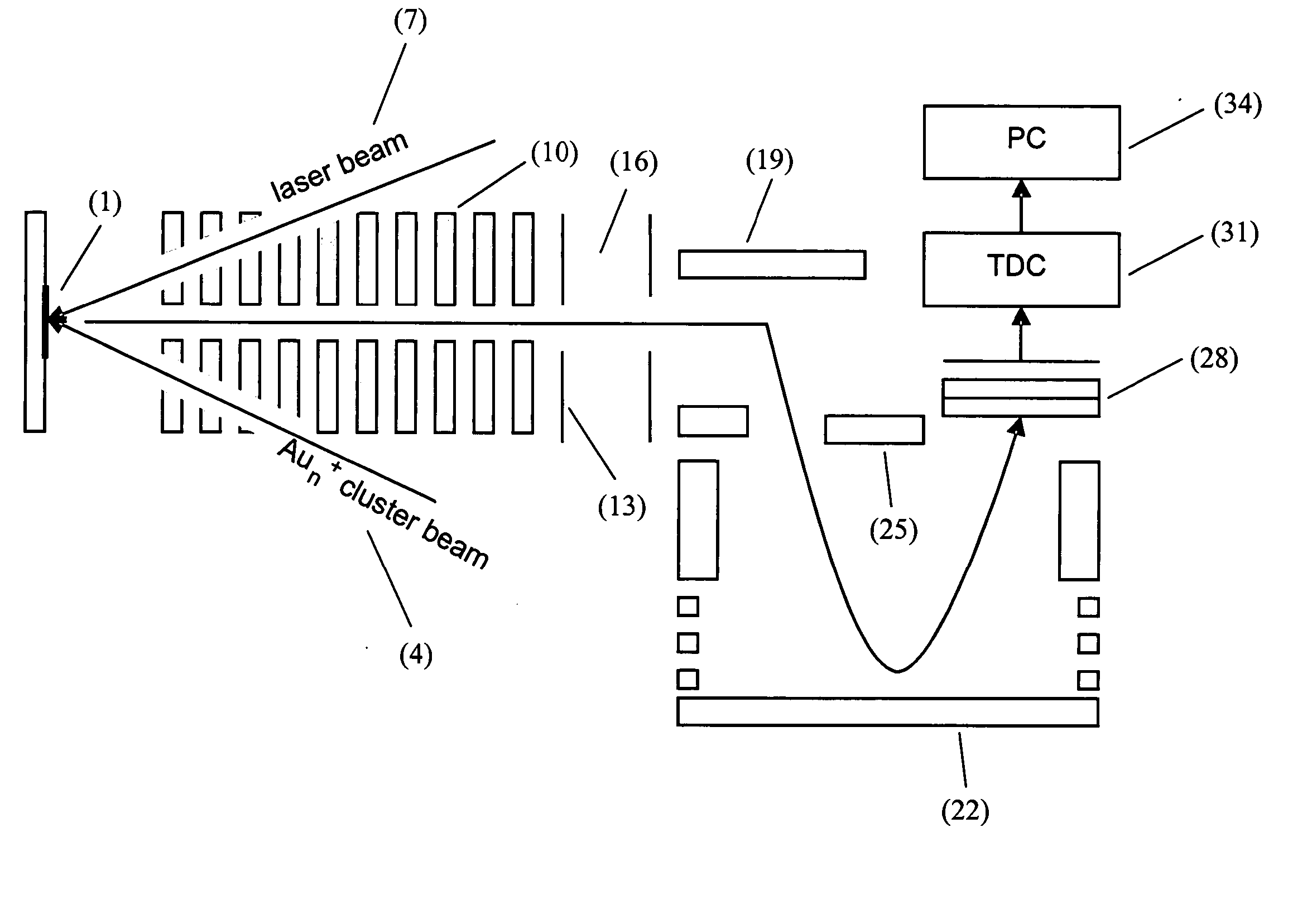
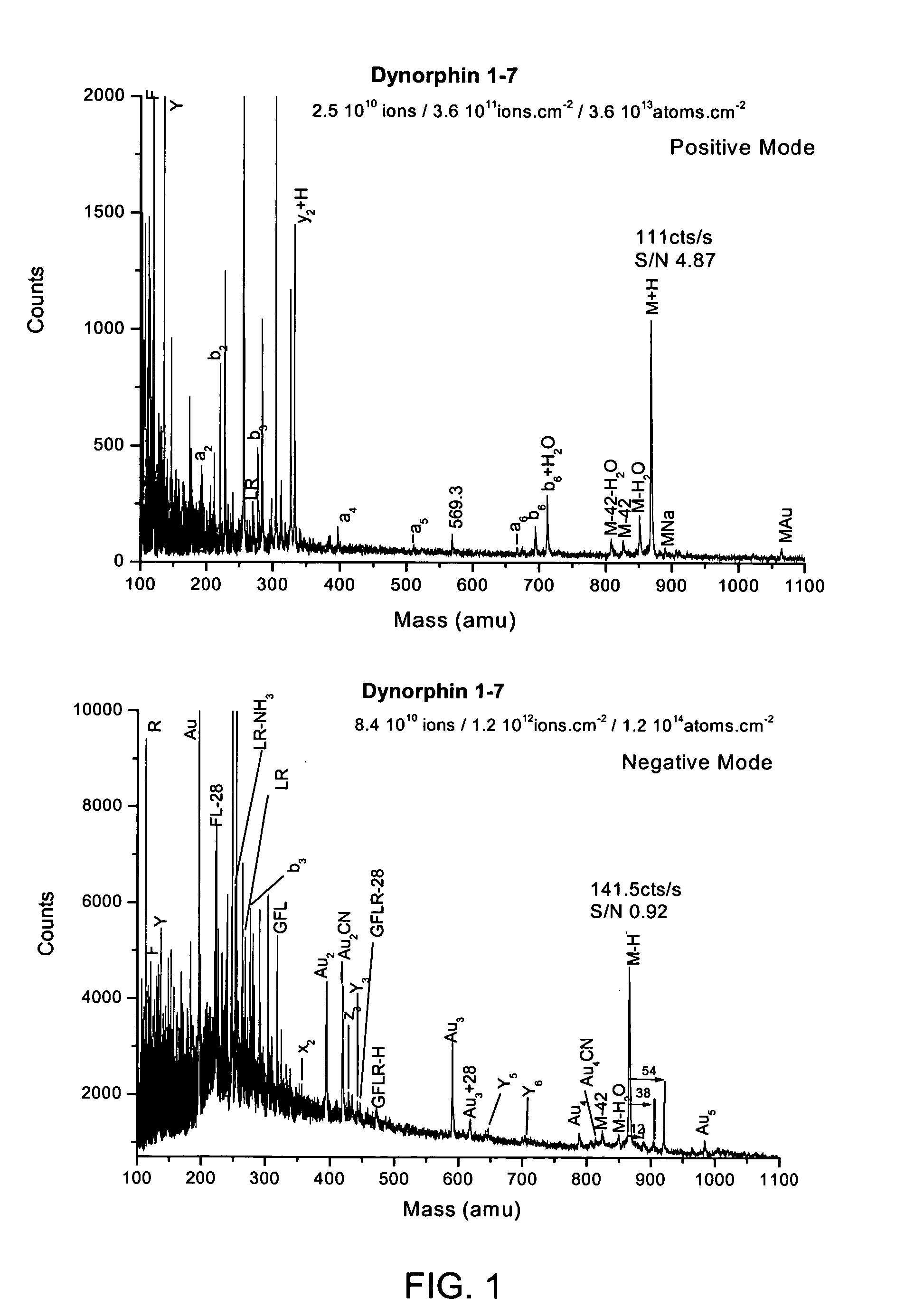
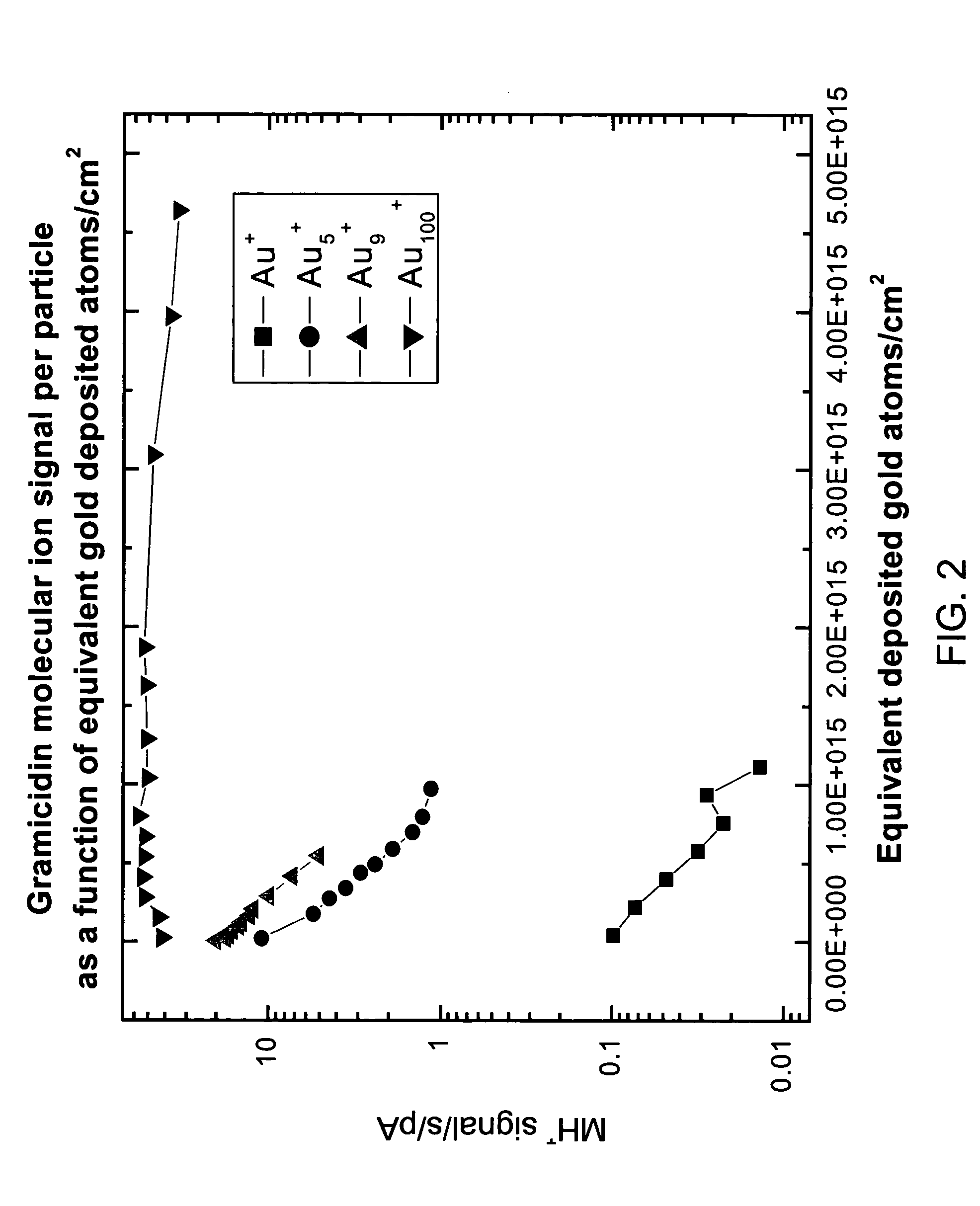
Gold implantation/deposition of biological samples for laser desorption three dimensional depth profiling of tissues
ActiveUS20050035284A1Time-of-flight spectrometersSamples introduction/extractionDesorptionIon implantation
The present invention enhances the laser desorption of biological molecular ions from surfaces by creating a surface localized MALDI particle matrix by ion implantation of low energy ionized clusters (gold, aluminum, etc.) or chemically derivatized clusters into the near surface region of the sample. MALDI analysis of the intact biomolecules on the surface or within a narrow subsurface region defined by the implantation range of the ions can then be performed by laser desorption into a mass spectrometer or, in a preferred embodiment, into a combined ion mobility orthogonal time of flight mass spectrometer.
Owner:IONWERKS
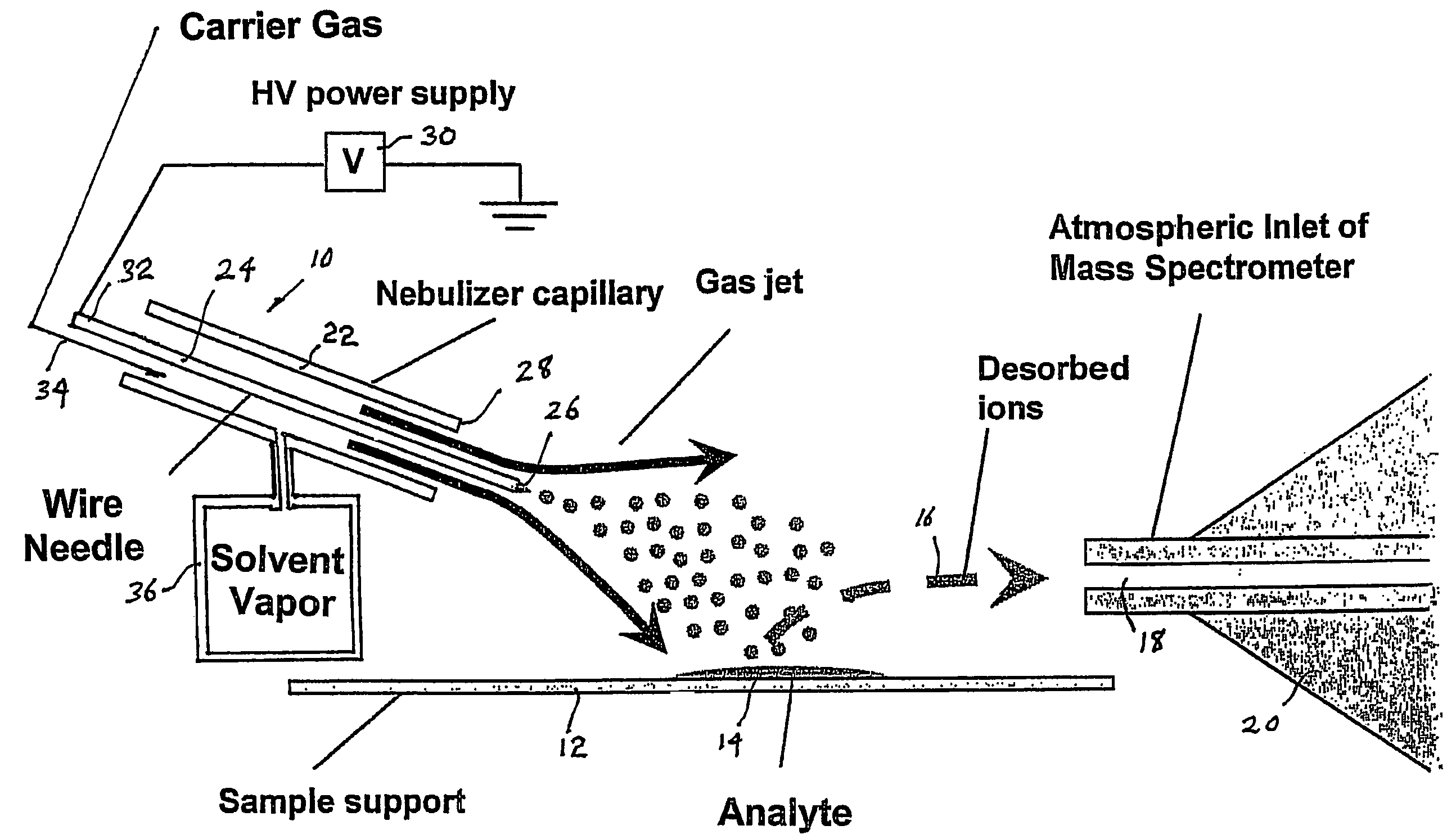
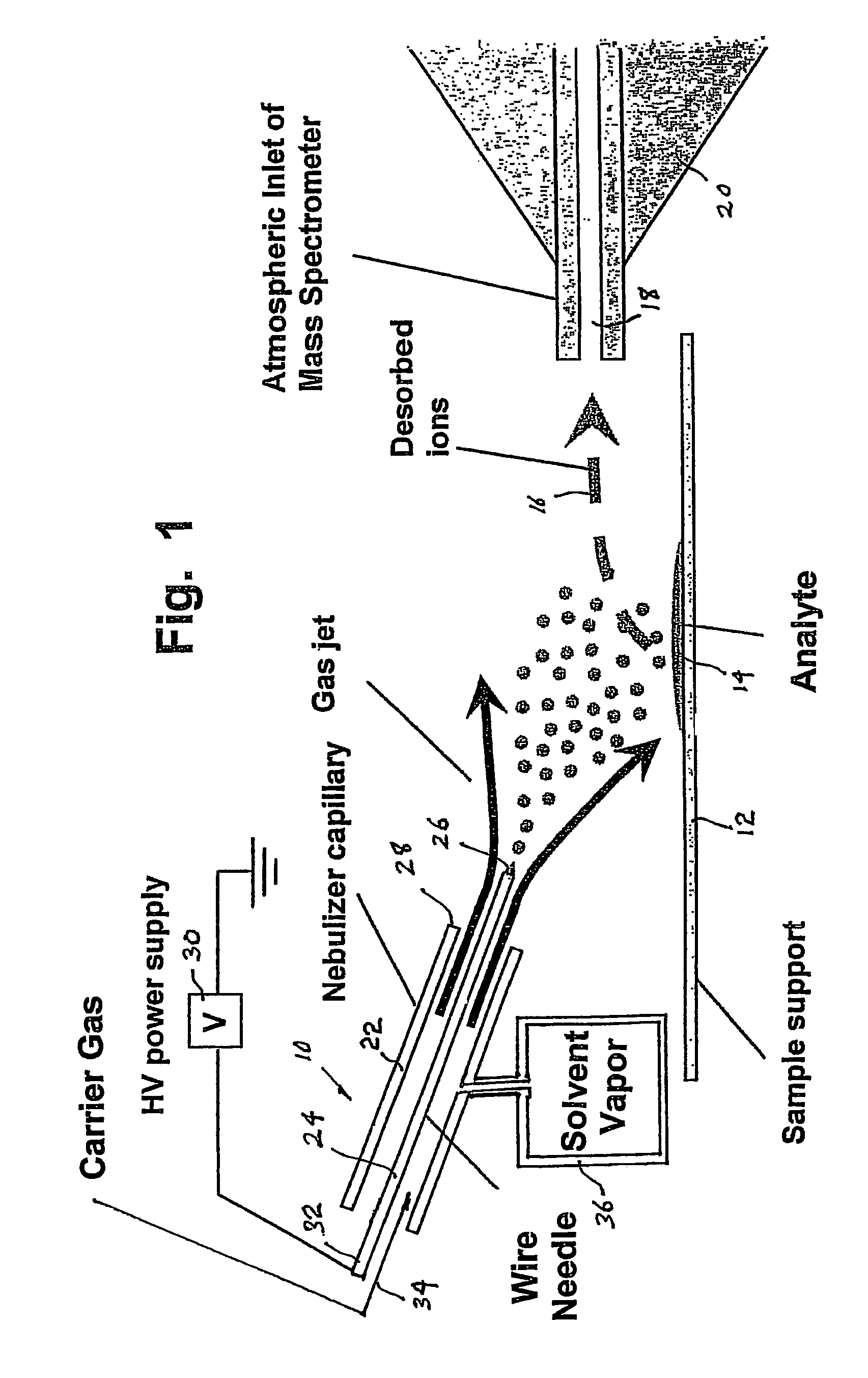
Method and system for desorption atmospheric pressure chemical ionization
ActiveUS7544933B2Minimizing chanceHigh sensitivityMaterial analysis by optical meansIon sources/gunsSolvent vaporDesorption
A desorption atmospheric pressure chemical ionization (DAPCI) system delivers a primary ion beam composed of an inert, high velocity gas and solvent ions to a surface to effect desorption and ionization of both volatile and non-volatile species present on surfaces. A electrode having a tapered tip is connected to a high voltage power supply. The tapered tip projects outward from a capillary carrying a high-speed flow of gas. A vapor of a solvent is mixed into the annular gas flow surrounding the needle. The gaseous solvent vapor is ionized in close proximity to the tapered tip by virtue of the high voltage applied to the electrode. The high-speed flow of gas and solvent vapor ions extending outward from the capillary is directed toward a substrate on which an analyte of interest may have been deposited. The solvent vapor ions can blanket the surface of the analyte causing a static charge build up that facilitates ion desorption and additionally can provide positive ion adducts of the analyte freed from the substrate surface that can be directed toward an atmospheric intake of a mass spectrometer or other instrument capable of studying the analyte.
Owner:PURDUE RES FOUND INC
Method and apparatus for ion manipulation using mesh in a radio frequency field
ActiveUS20110192969A1Better wayEasy to buildTime-of-flight spectrometersIsotope separationIon chromatographyDesorption
Ion manipulation systems include ion repulsion by an RF field penetrating through a mesh. Another comprises trapping ions in a symmetric RF field around a mesh. The system uses macroscopic parts, or readily available fine meshes, or miniaturized devices made by MEMS, or flexible PCB methods. One application is ion transfer from gaseous ion sources with focusing at intermediate and elevated gas pressures. Another application is the formation of pulsed ion packets for TOF MS within trap array. Such trapping is preferably accompanied by pulsed switching of RF field and by gas pulses, preferably formed by pulsed vapor desorption. Ion guidance, ion flow manipulation, trapping, preparation of pulsed ion packets, confining ions during fragmentation or exposure to ion to particle reactions and for mass separation are disclosed. Ion chromatography employs an ion passage within a gas flow and through a set of multiple traps with a mass dependent well depth.
Owner:LECO CORPORATION
Catalyst system for controlled polymerization
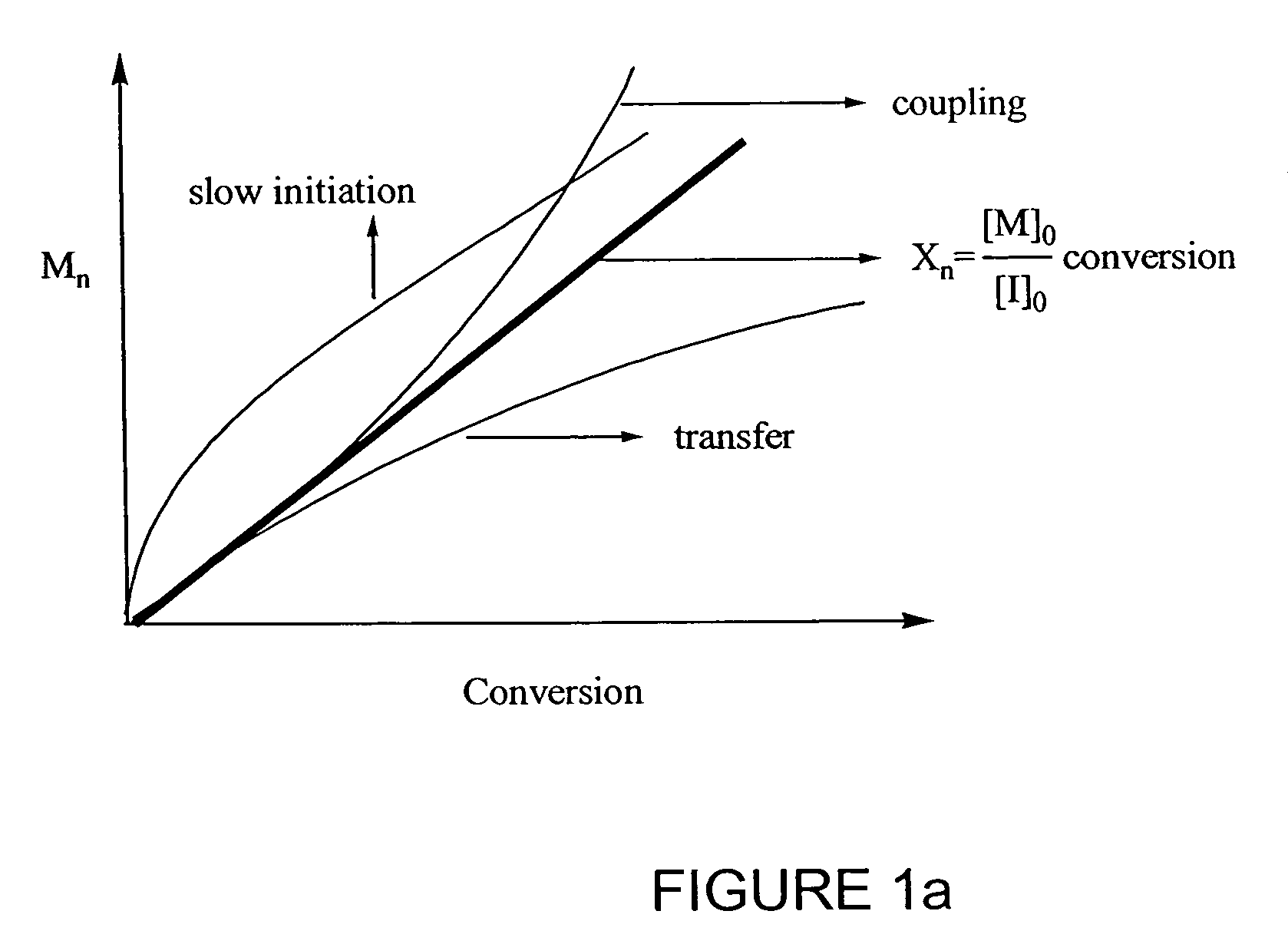
InactiveUS7157530B2Organic-compounds/hydrides/coordination-complexes catalystsDesorptionReducing agent
Owner:CARNEGIE MELLON UNIV
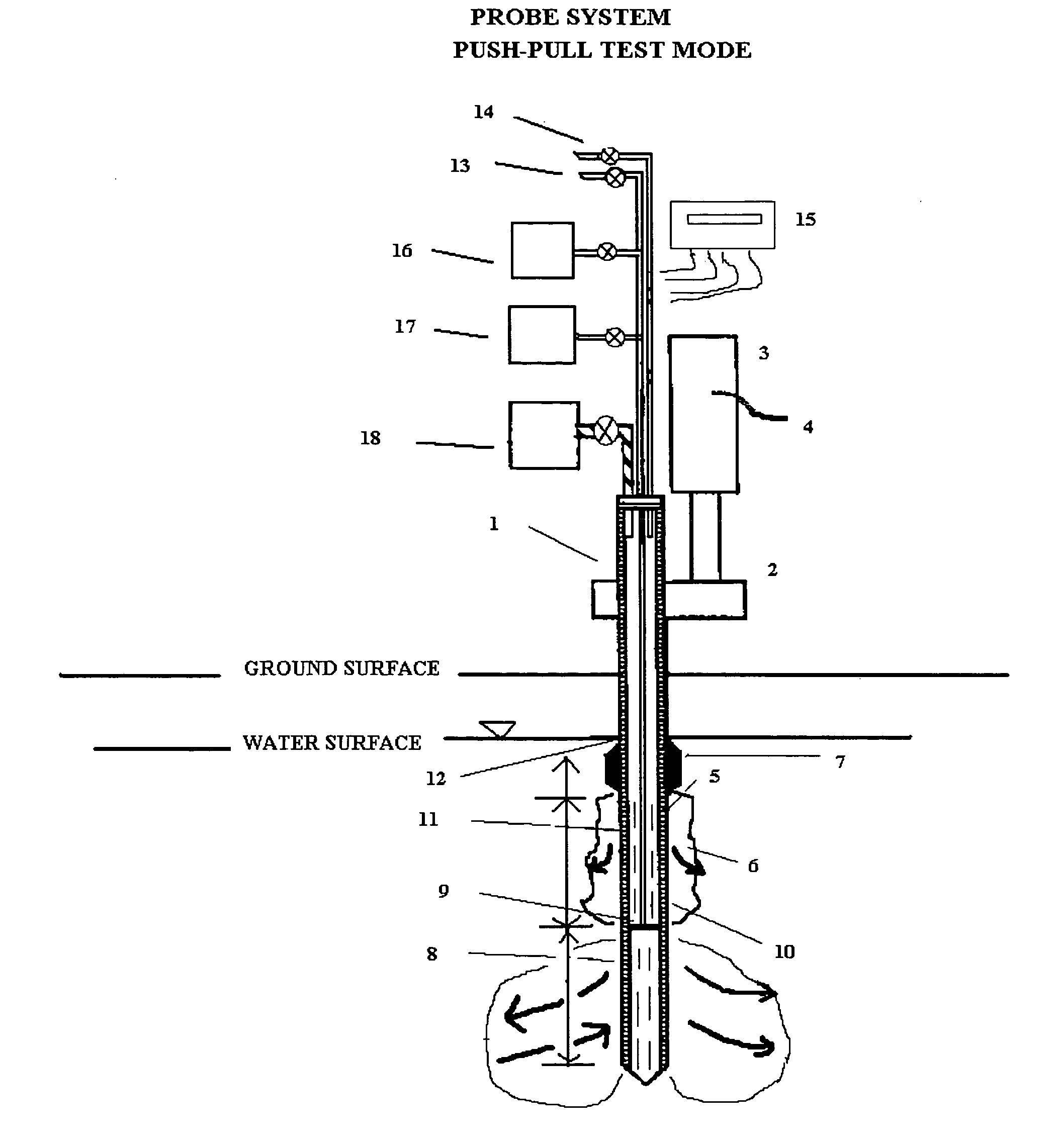
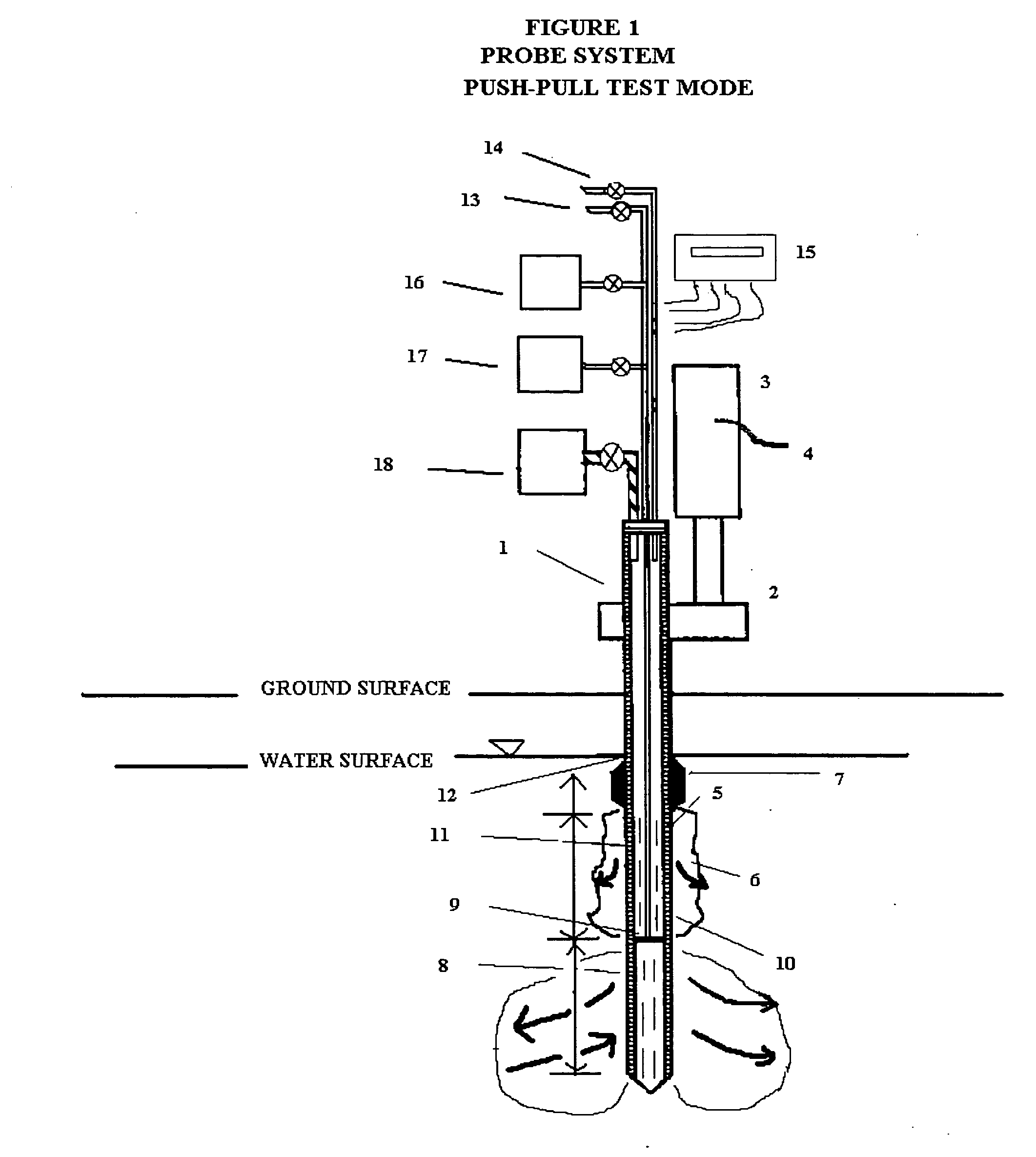
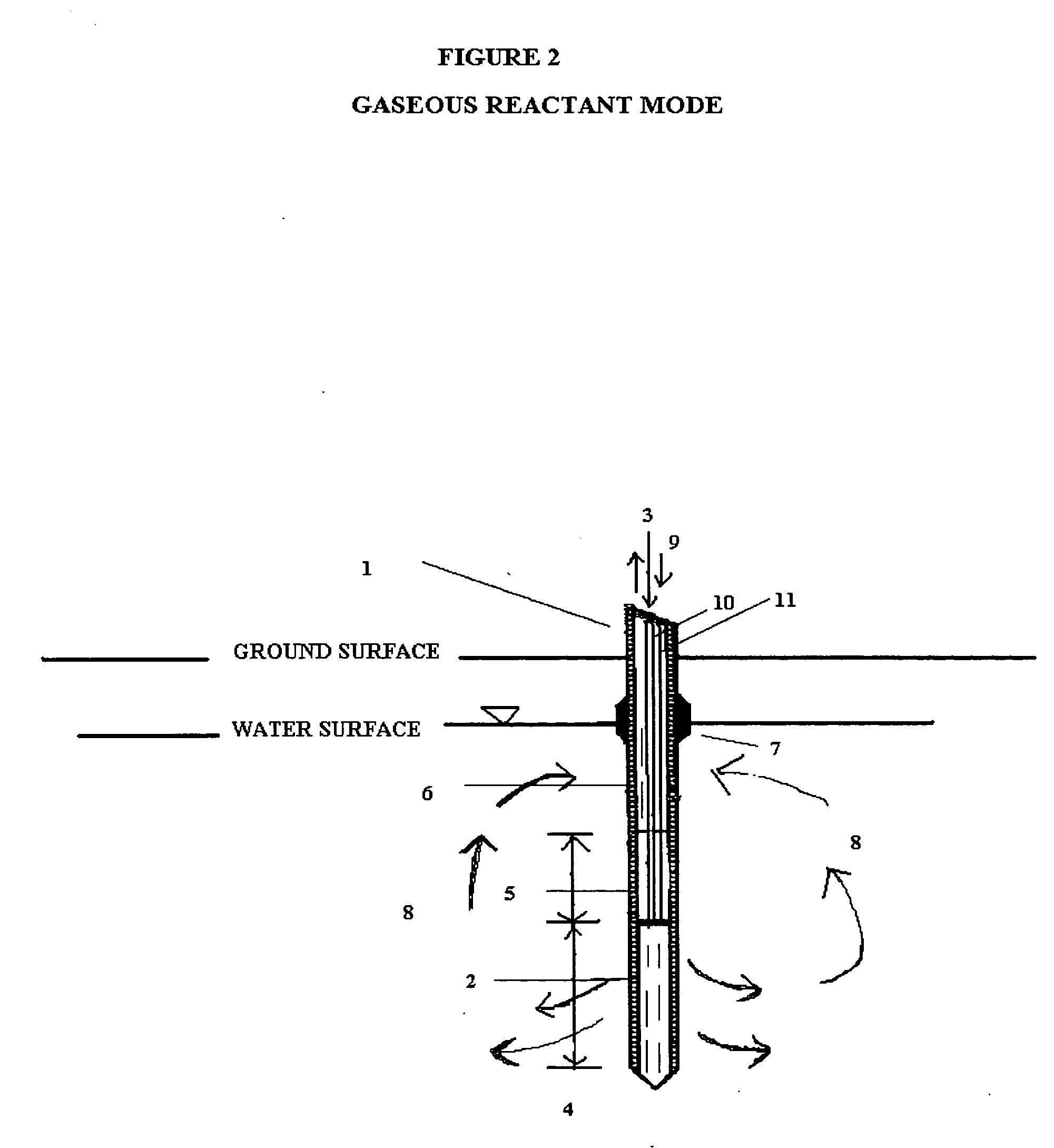
In situ remedial alternative and aquifer properties evaluation probe system
InactiveUS20060046297A1Prevent travelFaster and more easily automatedMicrobiological testing/measurementEarth material testingSoil gasPush technology
In general, the purpose of the probe system is to provide improved rapid field methods using re-designed direct push technology (DPT) and “push-pull testing” concepts to evaluate in situ chemical, biochemical, surfactant, adsorptive media, and leaching and fixation remediation technologies for hazardous subsurface contaminant(s). The probe system and methods described here when applied to a hazardous waste site being considered for in situ remediation of contaminants (organic or inorganic) by the listed treatment technologies will yield information that greatly reduces the uncertainty with regards to treatment effectiveness for the in situ soil, groundwater, and contaminant(s) conditions affecting dosage requirements and reaction rate(s) for various reactants. The probe system described here is multi-purpose in that it was designed: 1) to measure the relative permeability of the subsurface soil and groundwater to a liquid or gas ejectant, 2) to recover soil gas, soil, or groundwater samples for contaminant analyses, 3) to measure the chemical dosage and reaction, dissolution, adsorption, desorption, leaching, or fixation rate of a reactant such as a chemical or biochemical oxidant, metallic or bimetallic dehalogenating agent, surfactant or emulsifier solution, adsorbent media regenerant, leaching or fixation reagent that is injected into the matrix and withdrawn during a push-pull test, 4) to perform combinations of the above, 5) to measure the in situ adsorption capacity of adsorbent media and subsequently measure the effectiveness of regenerant(s) for the adsorbent media, and (6) to measure the effectiveness of a treated soil column for inorganic contaminant(s) leaching or fixation. In addition to being an in situ remedial alternatives evaluation tool, the probe system can be used as a reactant(s) delivery device after the specific remedial technology has been selected.
Owner:OXYTEC LLC
Retentate chromatography and protein chip arrays with applications in biology and medicine
InactiveUS6881586B2Rapid and multi-dimensional characterizationHigh resolutionBioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteDesorption
This invention provides methods of retentate chromatography for resolving analytes in a sample. The methods involve adsorbing the analytes to a substrate under a plurality of different selectivity conditions, and detecting the analytes retained on the substrate by desorption spectrometry. The methods are useful in biology and medicine, including clinical diagnostics and drug discovery.
Owner:BIO RAD LAB INC
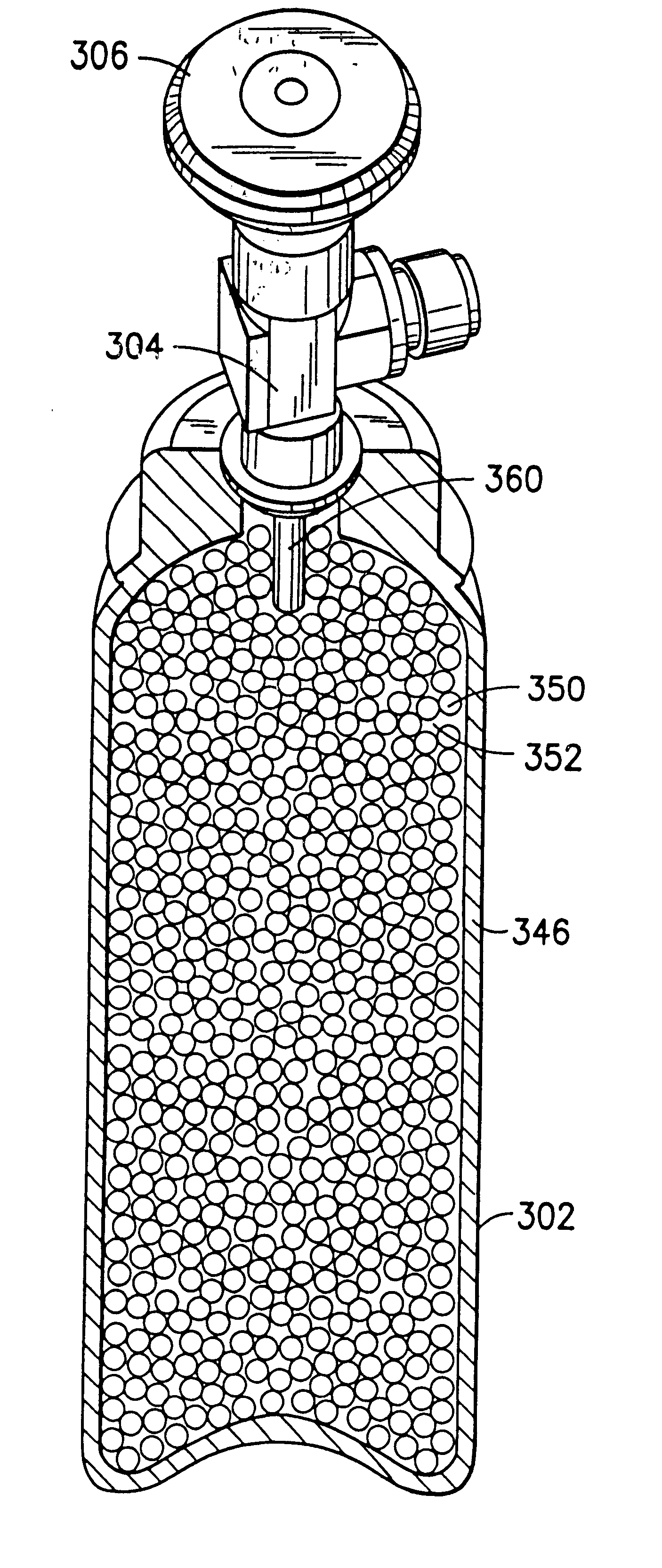
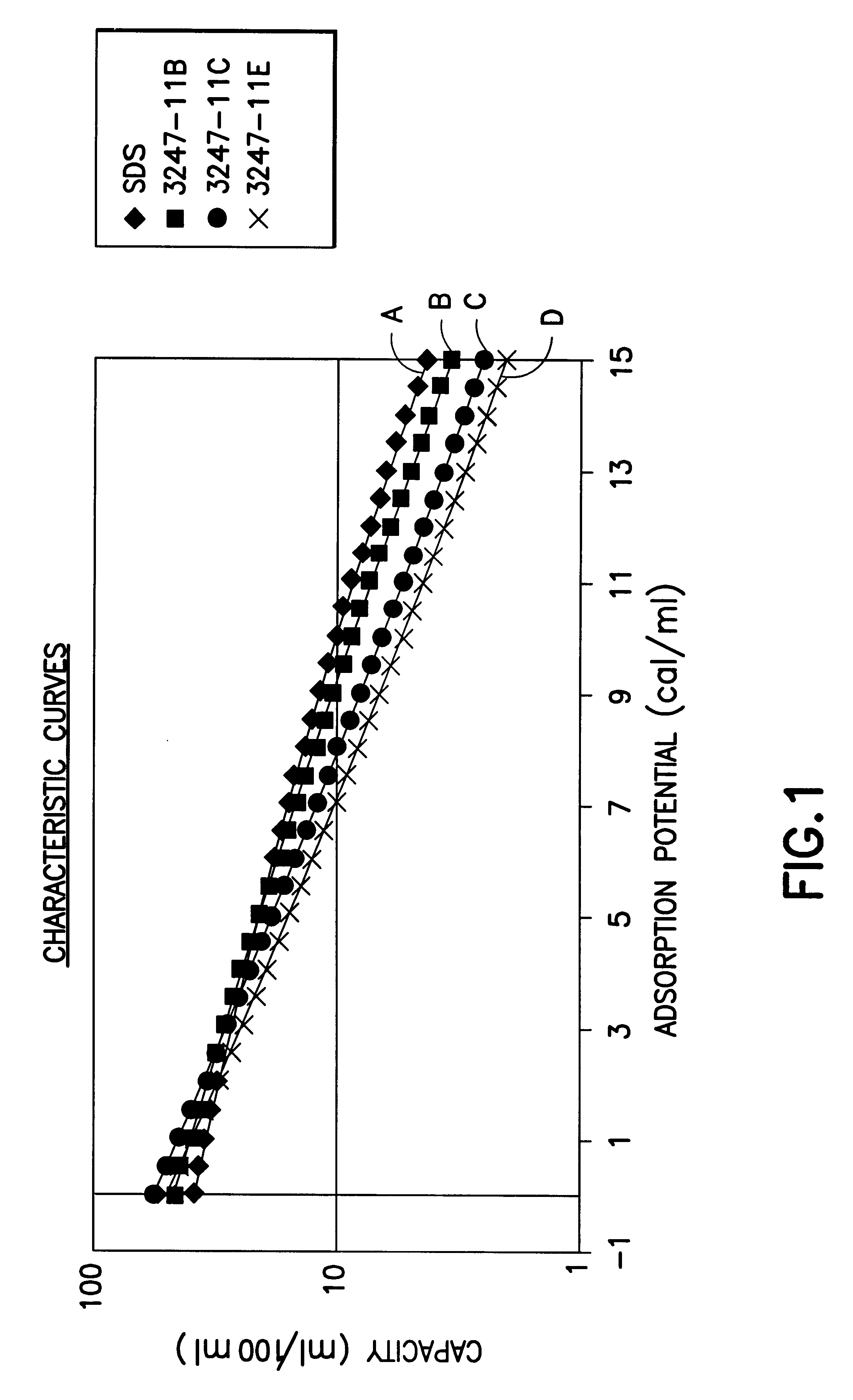
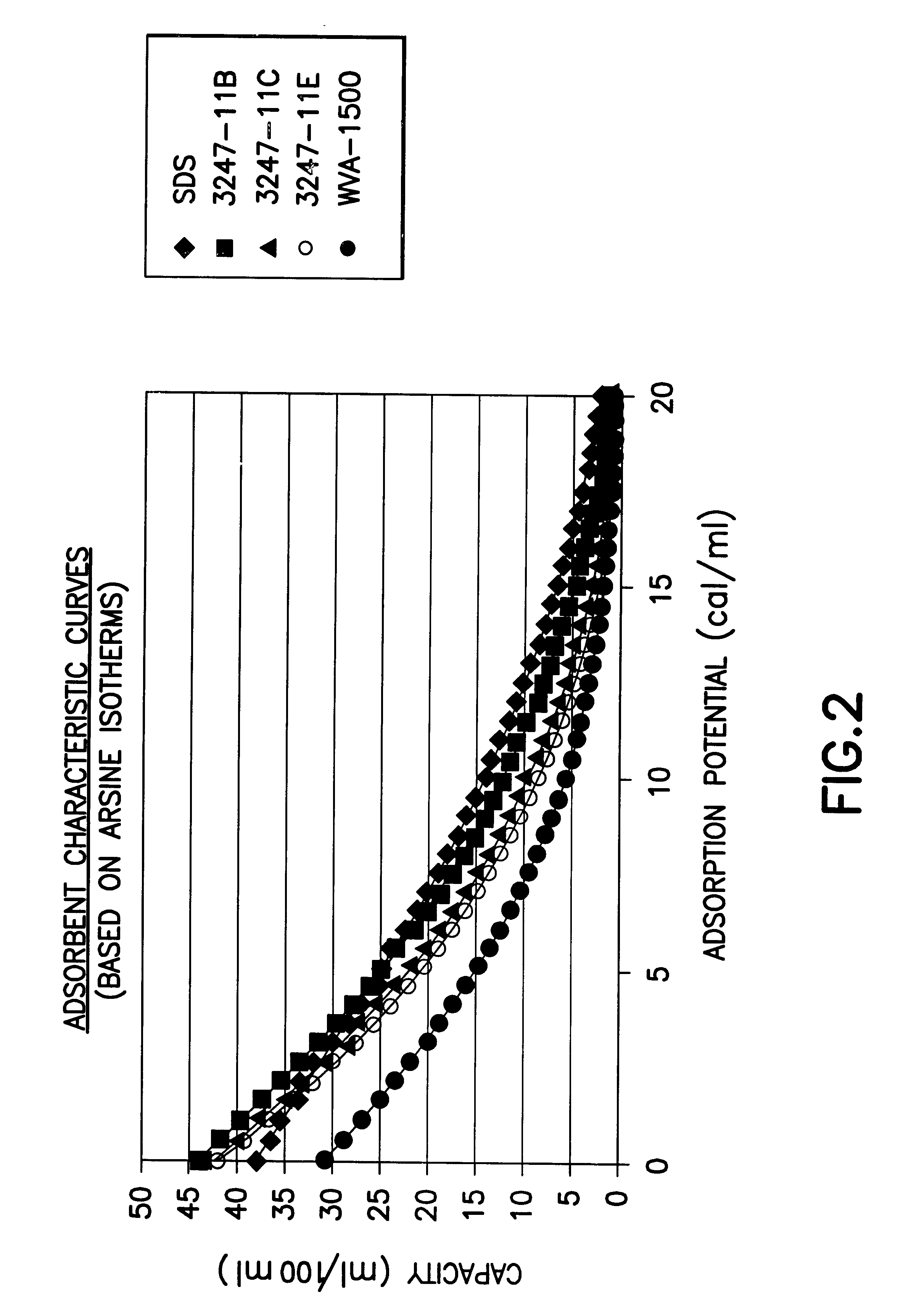
Fluid storage and delivery system utilizing low heels carbon sorbent medium
InactiveUS6592653B2Reduces sufficiencyImprove adsorption capacityGas treatmentOther chemical processesDesorptionSorbent
A fluid storage and dispensing system including a vessel containing a low heel carbon sorbent having fluid adsorbed thereon, with the system arranged to effect desorption of the fluid from the sorbent for dispensing of fluid on demand. The low heel carbon sorbent preferably is characterized by at least one of the following characteristics: (i) Heel, measured for gaseous arsine (AsH3) at 20° C. at 20 Torr, of not more than 50 grams AsH3 per liter of bed of the sorbent material; (ii) Heel, measured for gaseous boron trifluoride (BF3) at 20° C. at 20 Torr, of not more than 20 grams boron trifloride per liter of bed of the sorbent material; (iii) Heel, measured for gaseous germanium tetrafluoride (GeF4) at 20° C. at 20 Torr, of not more than 250 grams AsH3 per liter of bed of the sorbent material; (iv) Heel, measured for gaseous arsenic pentafluoride (AsF5) at 20° C. at 20 Torr, of not more than 700 grams AsF5 per liter of bed of the sorbent material; (v) Heel, measured for gaseous trimethyl silane (3MS) at 20° C. at 20 Torr, of not more than 160 grams 3MS per liter of bed of the sorbent material; and (vi) Heel, measured for gaseous ethane (C2H4) at 21° C. at 25 Torr, of not more than 10 grams ethane per liter of bed of the sorbent material.
Owner:ENTEGRIS INC
Mutation analysis using mass spectrometry
InactiveUS6503710B2Rapid and economic sample preparationAccurate massSamplingSugar derivativesChemical treatmentFree form
The invention presents a method for examining genetic material (deoxyribonucleic acid, DNA) to detect the presence of pre-known mutations, especially single nucleotide polymorphisms (SNP), using mass spectrometry with ionization by matrix-assisted laser desorption (MALDI). The invention uses nucleoside triphosphates with modified sites for the method of primer extension in a duplicating, enzymatic reaction and at least partially removal of primers from the extension product, in combination with product neutralization by chemical treatment of the modified sites, so that the resulting DNA products can be, by using special matrix materials, preferredly ionized in an adduct-free form over other constituents in the reaction solution without any further cleaning. The method is particularly suitable for simultaneous identification of several mutations by multiplexing.
Owner:BRUKER DALTONIK GMBH
Active material for non-aqueous electrolyte secondary battery and manufacturing method therefore
ActiveUS20080268347A1Increase capacityReduces electron conductivity and lithium diffusing abilityElectrode manufacturing processesNon-aqueous electrolyte accumulatorsDesorptionX-ray
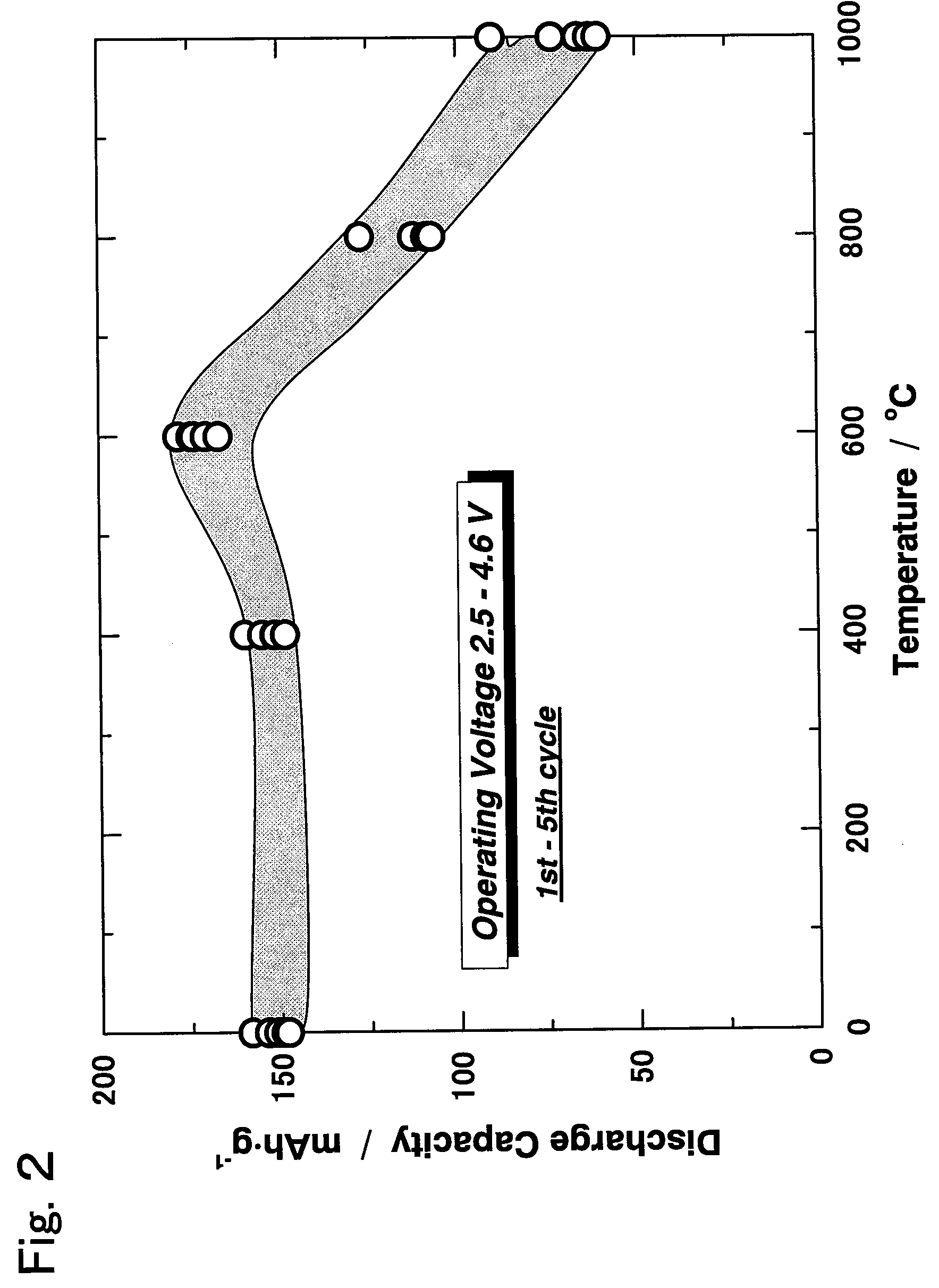
An active material for a non-aqueous electrolyte secondary battery including a lithium-containing transition metal oxide containing nickel and manganese and having a closest-packed structure of oxygen, wherein an atomic ratio MLi / MT between the number of moles of lithium MLi and the number of moles of transition metal Mt contained in the lithium-containing transition metal oxide is greater than 1.0; the lithium-containing transition metal oxide has a crystal structure attributed to a hexagonal system, and the X-ray diffraction image of the crystal structure has a peak P003 attributed to the (003) plane and a peak P104 attributed to the (104) plane; an integrated intensity ratio I003 / I104 between the peak P003 and the peak P104 varies reversibly within a range from 0.7 to 1.5 in association with absorption and desorption of lithium by the lithium-containing transition metal oxide; and the integrated intensity ratio varies linearly and continuously.
Owner:PUBLIC UNIVERSITY CORPORATION OSAKA CITY UNIVERSITY +1
Ion source
ActiveUS20110049352A1Improve ionization efficiencyEfficient processingMaterial analysis by optical meansIon sources/gunsElectricityAnalyte
This invention relates to a desorpton / ionization source operated under ambient conditions for direct analysis of solid or liquid samples on a surface. The source comprises of a laser desorption system and a UV / electrospray combined ionization system. The source is suitable for simultaneously ionizing samples with different polarity in a complex mixture. At the same time, the compact design of the source with multiple channels can maintain the level of local concentration of the analyte ions inside the source for higher efficiency of sample ionization and introduction.
Owner:SHIMADZU RES LAB SHANGHAI
Process for Preparing Butadiene by Oxidative Dehydrogenation of N-Butenes with Monitoring of the Peroxide Content During Work-Up of the Product
InactiveUS20140200381A1Analysis using chemical indicatorsHydrocarbon by hydrogenationWater vaporDesorption
The invention relates to a process for preparing butadiene from n-butenes, which comprises the following steps:A) provision of a feed gas stream a comprising n-butenes;B) introduction of the feed gas stream a comprising n-butenes and an oxygen-comprising gas into at least one dehydrogenation zone and oxidative dehydrogenation of n-butenes to butadiene, giving a product gas stream b comprising butadiene, unreacted n-butenes, water vapor, oxygen, low-boiling hydrocarbons, possibly carbon oxides and possibly inert gases;C) cooling and compression of the product gas stream b in at least one cooling stage and at least one compression stage, with the product gas stream b being brought into contact with a circulated coolant to give at least one condensate stream c1 comprising water and a gas stream c2 comprising butadiene, n-butenes, water vapor, oxygen, low-boiling hydrocarbons, possibly carbon oxides and possibly inert gases;D) separation of incondensable and low-boiling gas constituents comprising oxygen, low-boiling hydrocarbons, possibly carbon oxides and possibly inert gases as gas stream d2 from the gas stream c2 by absorption of the C4-hydrocarbons comprising butadiene and n-butenes in a circulated absorption medium, giving an absorption medium stream loaded with C4-hydrocarbons and the gas stream d2, and subsequent desorption of the C4-hydrocarbons from the loaded absorption medium stream to give a C4 product gas stream d1;E) separation of the C4 product stream d1 by extractive distillation using a solvent which is selective for butadiene into a stream e1 comprising butadiene and the selective solvent and a stream e2 comprising n-butenes;F) distillation of the stream e1 comprising butadiene and the selective solvent to give a stream f1 consisting essentially of the selective solvent and a stream f2 comprising butadiene;where samples are taken from the circulated coolant in step C) and / or the circulated absorption medium in step D) and the peroxide content of the samples taken is determined by means of iodometry, differential scanning calorimetry (DSC) or microcalorimetry.
Owner:BASF AG
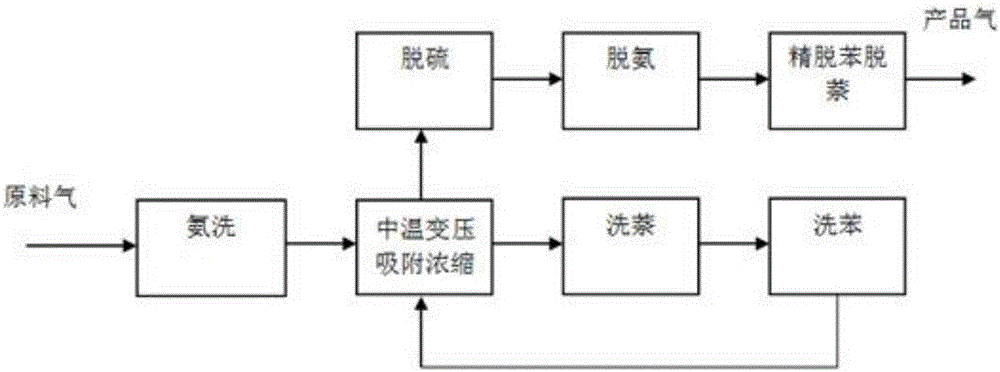
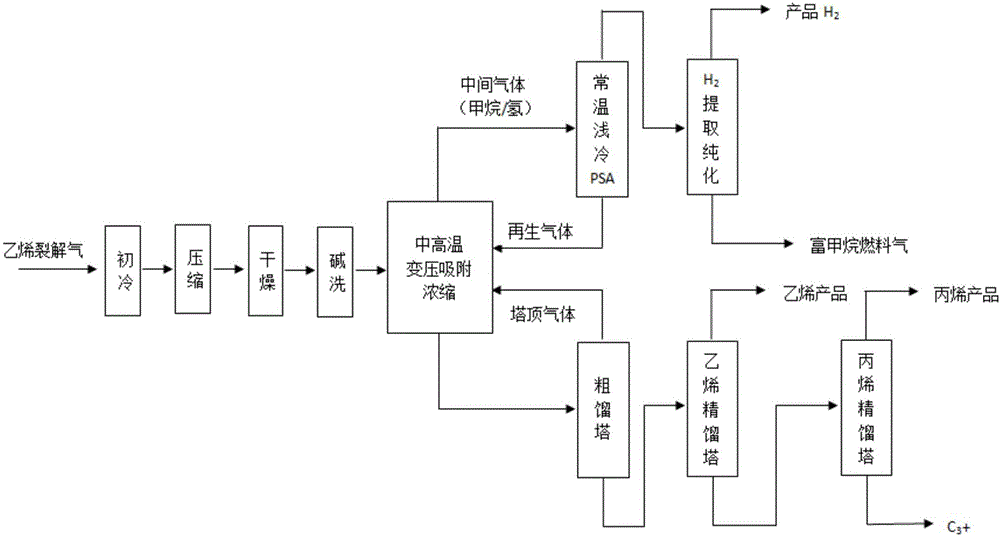
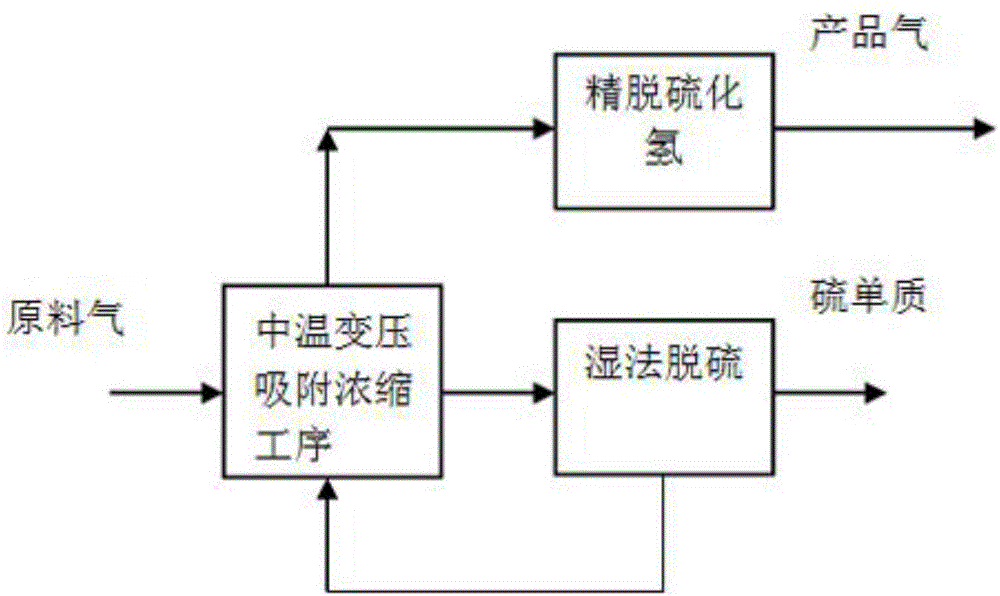
Full-temperature-range pressure swing adsorption gas separation, refinement and purification method
ActiveCN105749699AReduce energy consumptionBroaden the scope of adsorption separation applicationsSolidificationLiquefactionEnergy gradientPurification methods
The invention discloses a full-temperature-range pressure swing adsorption gas separation, refinement and purification method.By means of the difference of the temperatures and pressures of different raw material gases and the difference of the adsorption separation coefficients and physical chemistry properties of all components in the raw material gases in the temperature range of 80-200 DEG C and the pressure range of 0.03-4.0 MPa, the adsorption or desorption regeneration operation of the pressure swing adsorption circulation process is adjusted by coupling all separation methods, the adsorption theory that the pressure or temperature swing adsorption separation process is only limited to the adsorption and desorption regeneration circulation operation through pressure or temperature changes is expanded, and therefore all raw material gases are separated, refined and purified by achieving the energy gradient utilization in the gas separation, refinement and purification process and achieving the circulation operation, where adsorption, desorption and regeneration are easily matched and balanced, in the moderate to low cold and moderate to high temperature pressure swing adsorption separation process, and it is changed that a traditional adsorption method is only limited to the auxiliary effect of refinement and purification, and adsorption becomes the basic separation unit operation just as important as refinement, absorption and extraction separation.
Owner:SICHUAN TECHAIRS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com