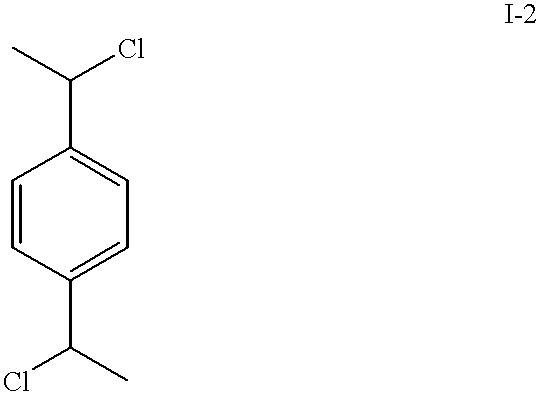
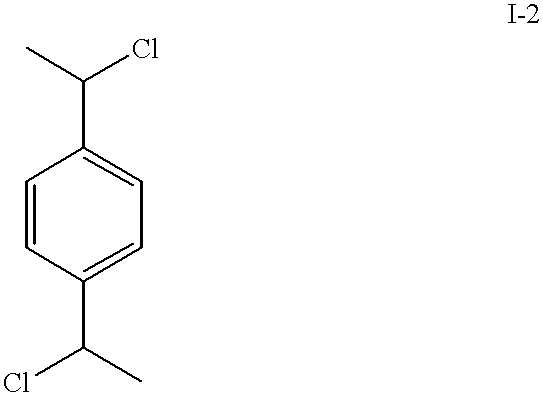
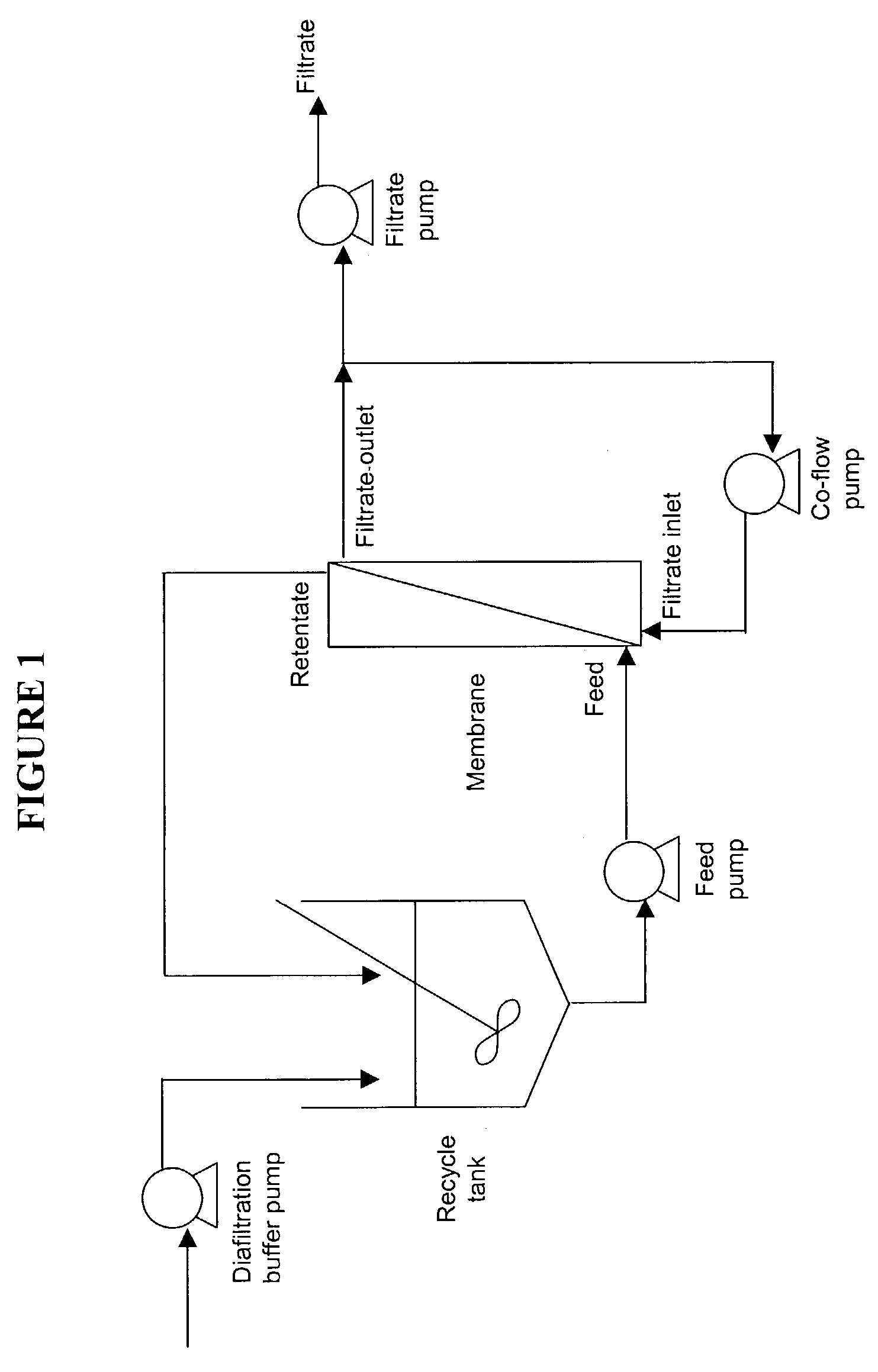
Patents
Literature
56027results about "Component separation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
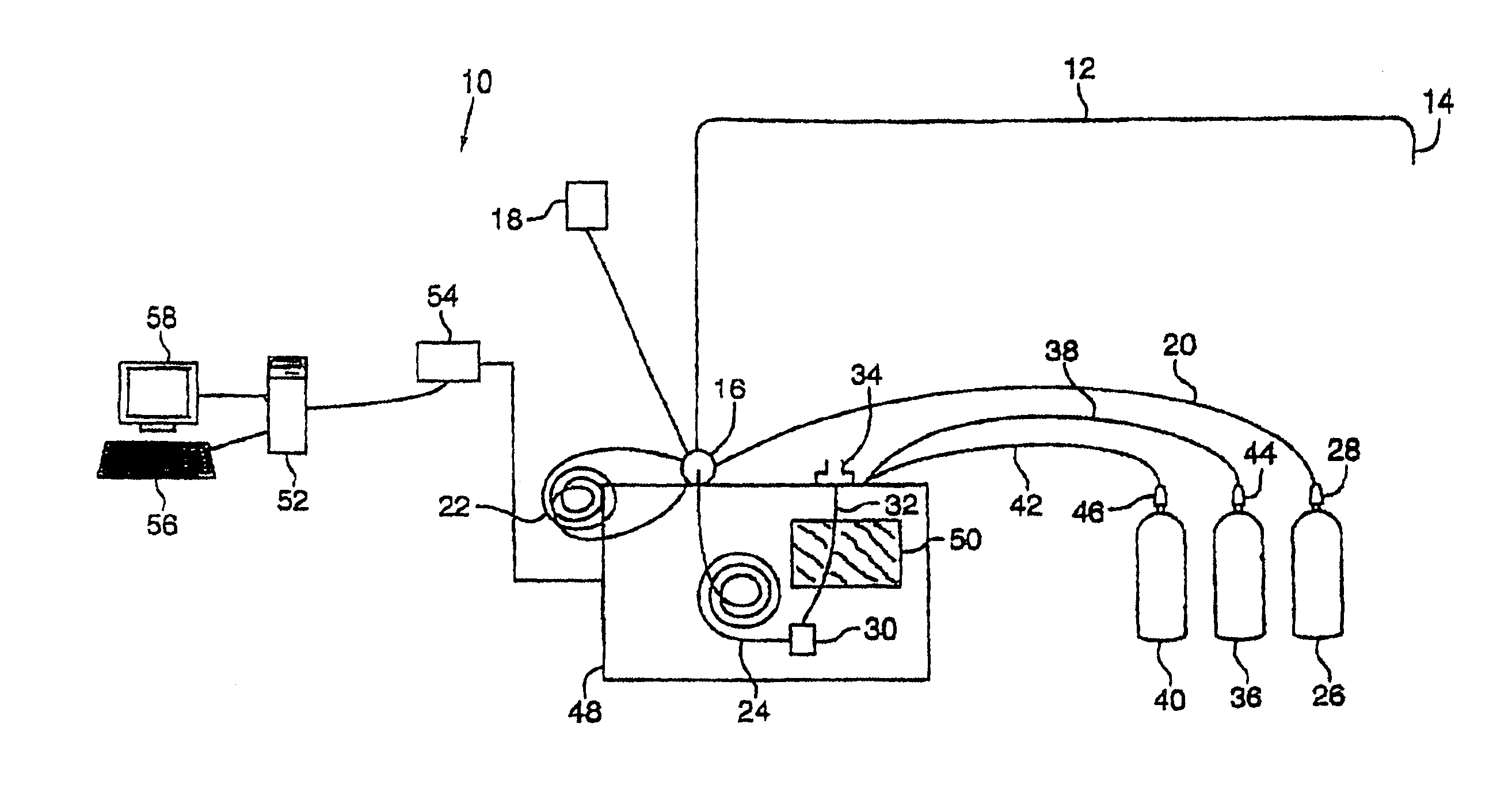
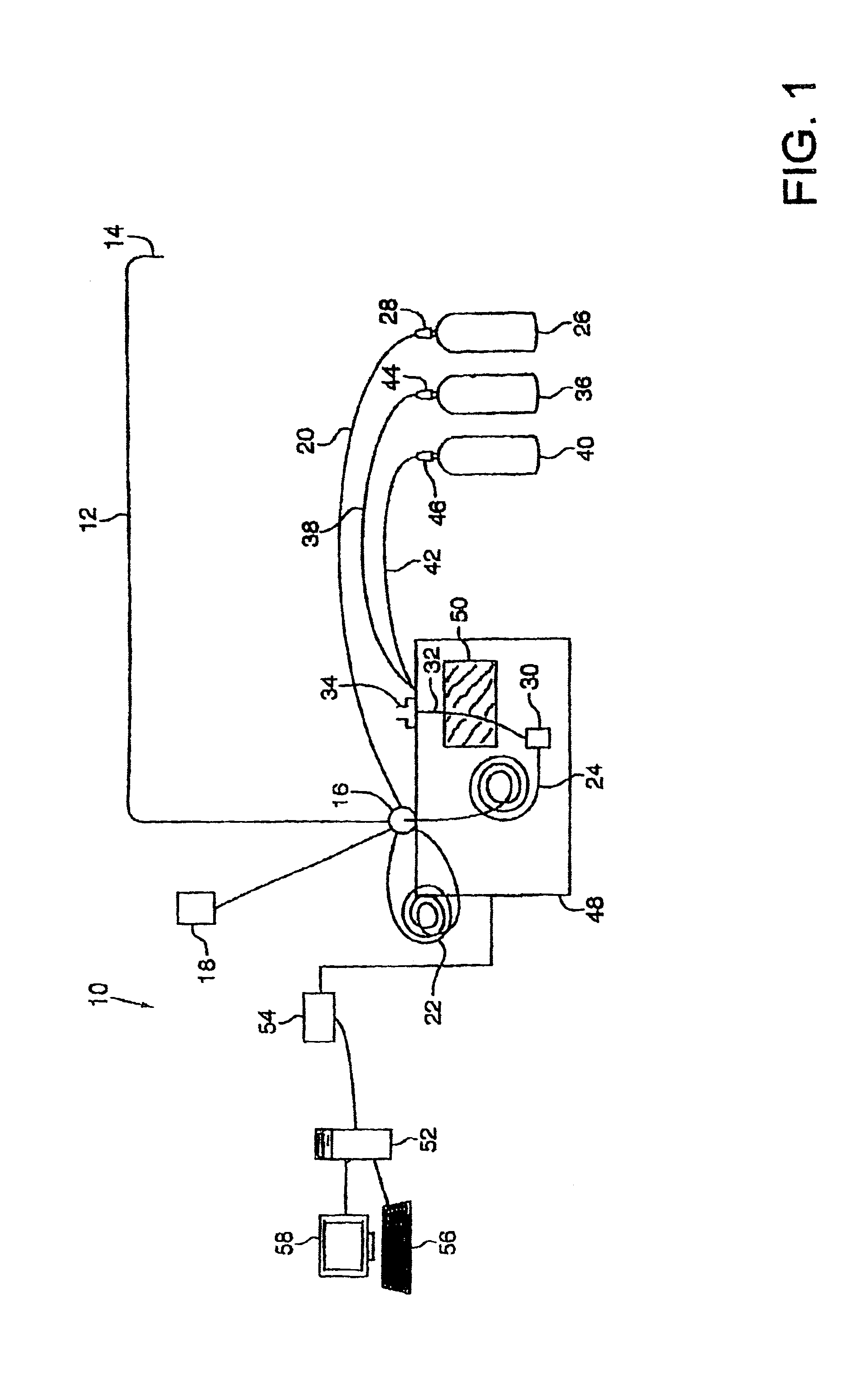
Robust system for screening mail for biological agents
InactiveUS6887710B2Lower the thresholdReduce riskAnalysing fluids using sonic/ultrasonic/infrasonic wavesLiquid dispersion analysisParticulatesEngineering
Items of mail are rapidly processed in a mail sampling system to determine if the mail is contaminated with a chemical or biological agent. The mail sampling system maintains a negative pressure in a containment chamber and includes a triggering sampler that makes a threshold determination regarding possible contamination, and a detecting sampler that obtains a sample for more detailed analysis in response to a signal from the triggering sampler. A sample of particulates collected from an item of mail is either removed for analysis or analyzed in the system to identify a contaminating agent. Optionally, the system includes an archiving sampler, which archives samples for subsequent processing and analysis, and a decontamination system, which is activated to decontaminate the mail if needed.
Owner:FLIR DETECTION
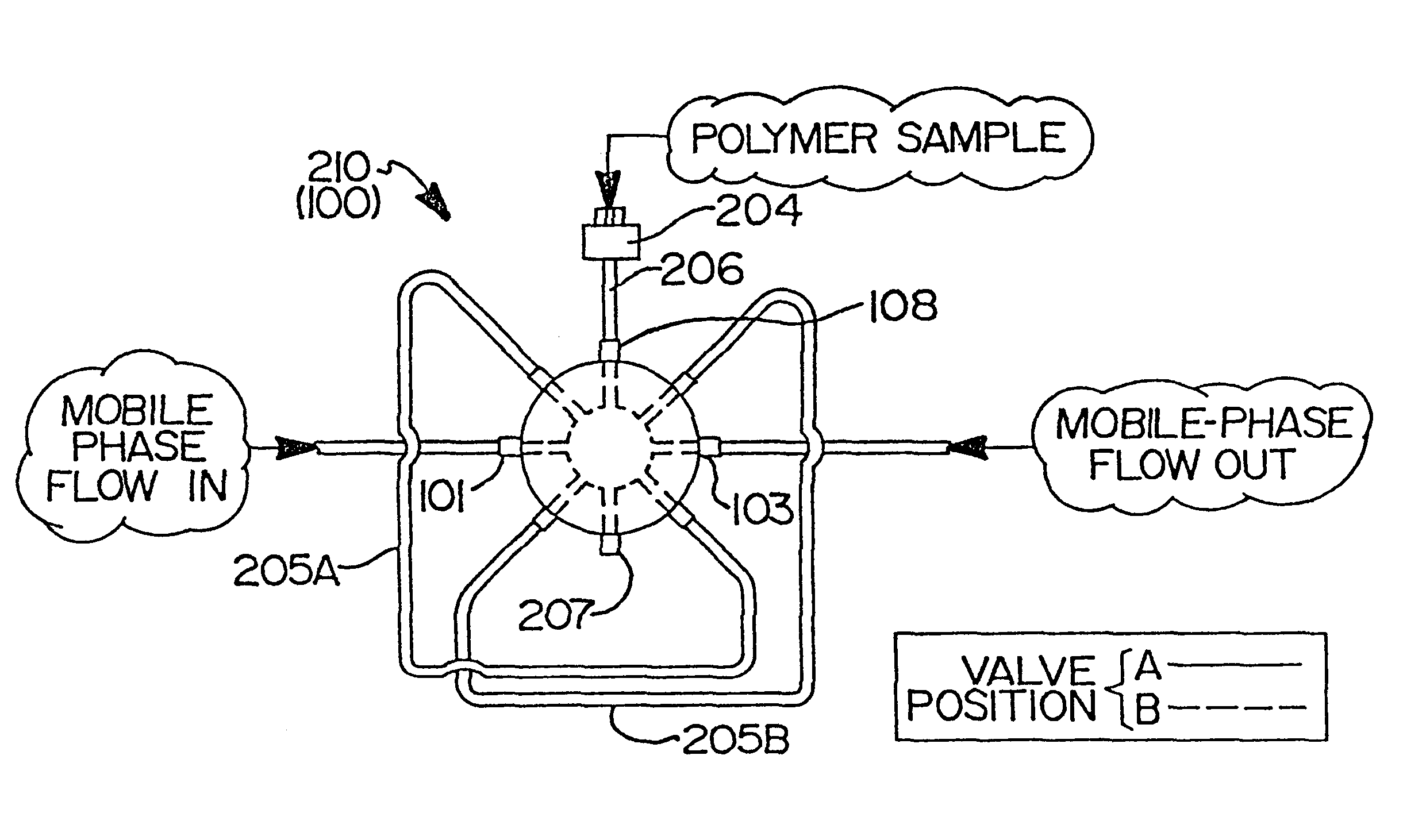
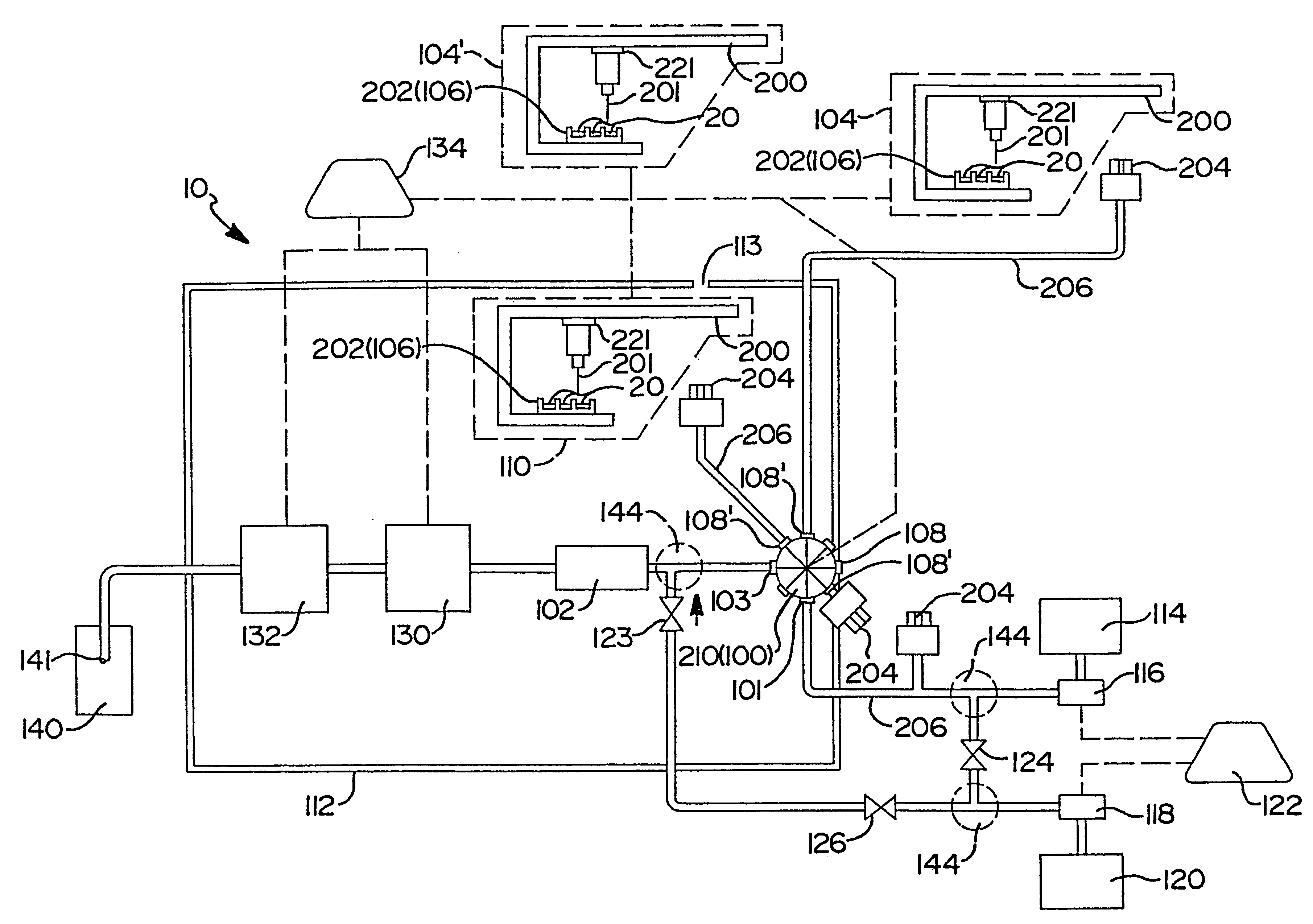
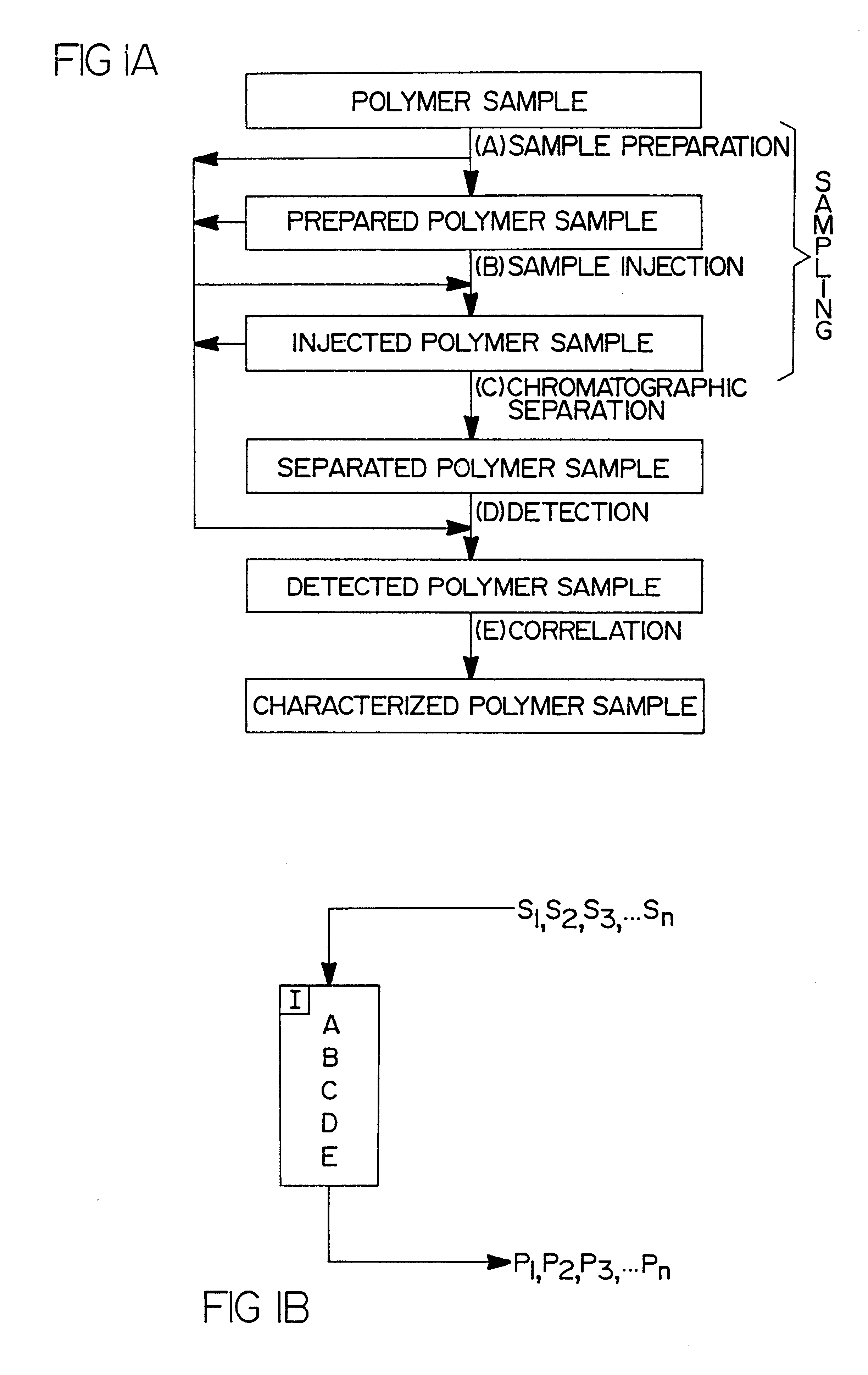
Flow-injection analysis and variable-flow light-scattering methods and apparatus for characterizing polymers
InactiveUS6175409B1Avoid backlogImprove throughputSequential/parallel process reactionsSamplingFlow injection analysisPolymer
Rapid characterization and screening of polymer samples to determine average molecular weight, molecular weight distribution and other properties is disclosed. Rapid flow characterization systems and methods, including liquid chromatography and flow-injection analysis systems and methods are preferably employed. High throughput, automated sampling systems and methods, high-temperature characterization systems and methods, and rapid, indirect calibration compositions and methods are also disclosed. The described methods, systems, and devices have primary applications in combinatorial polymer research and in industrial process control.
Owner:INTERMOLECULAR
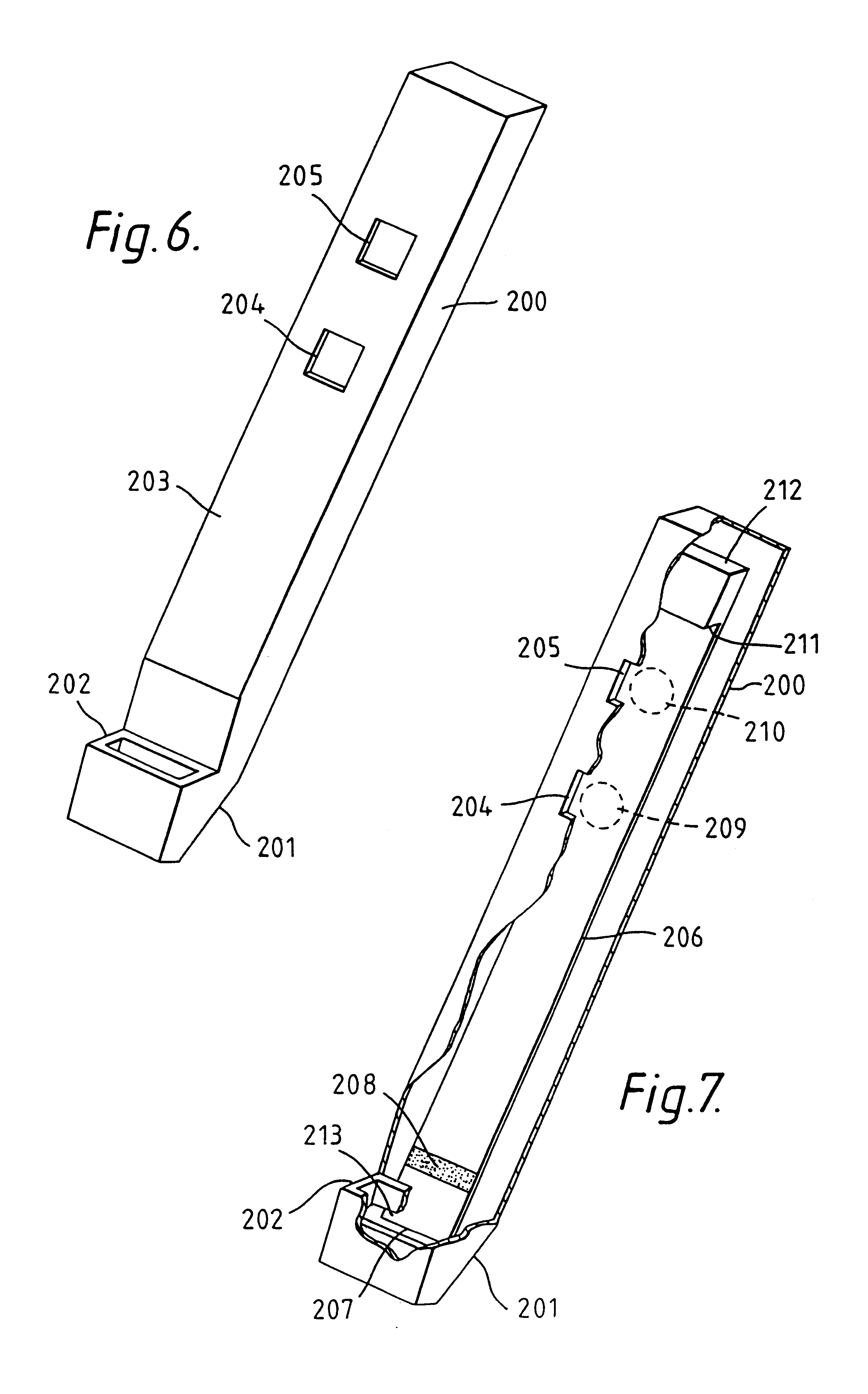
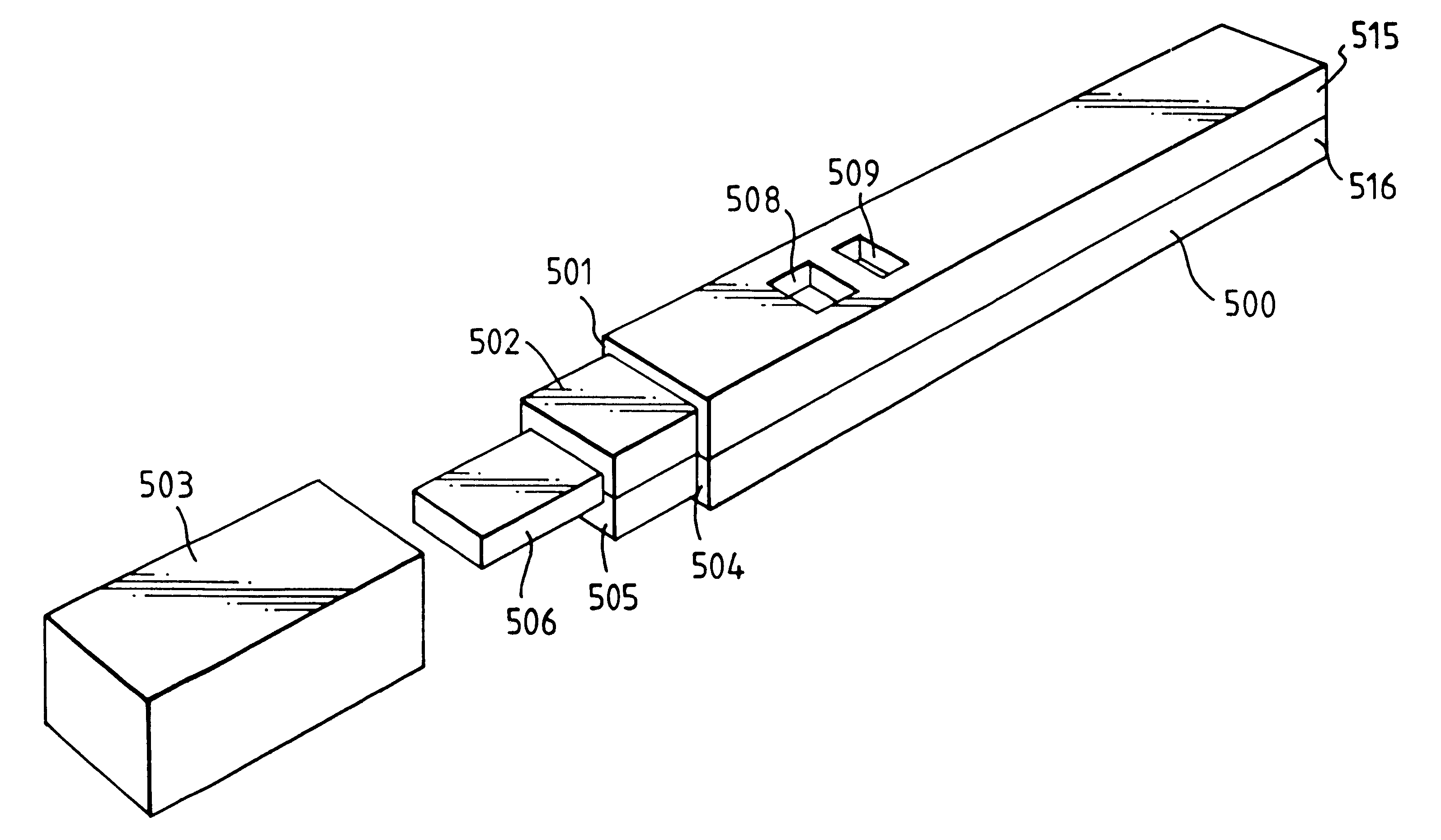
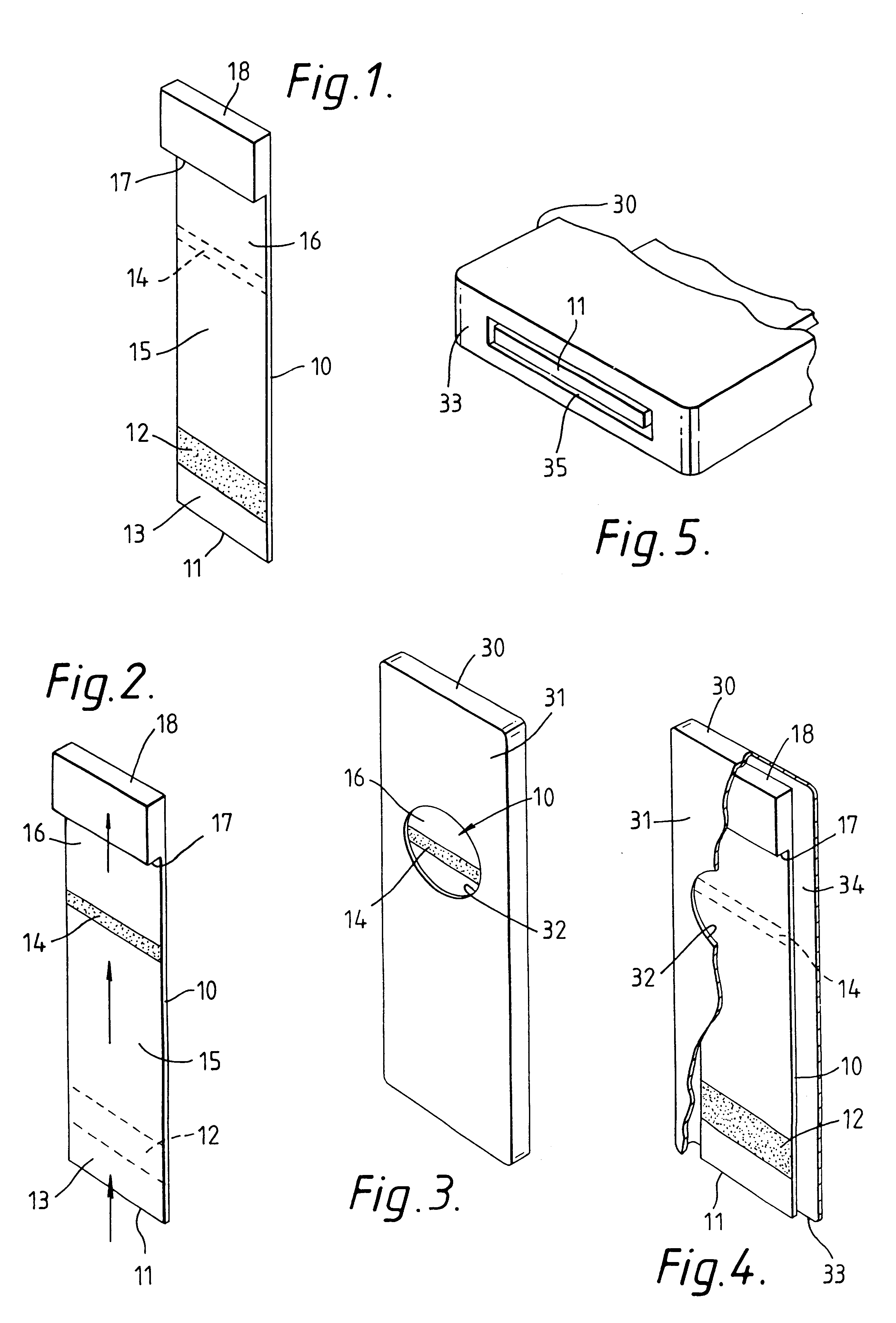
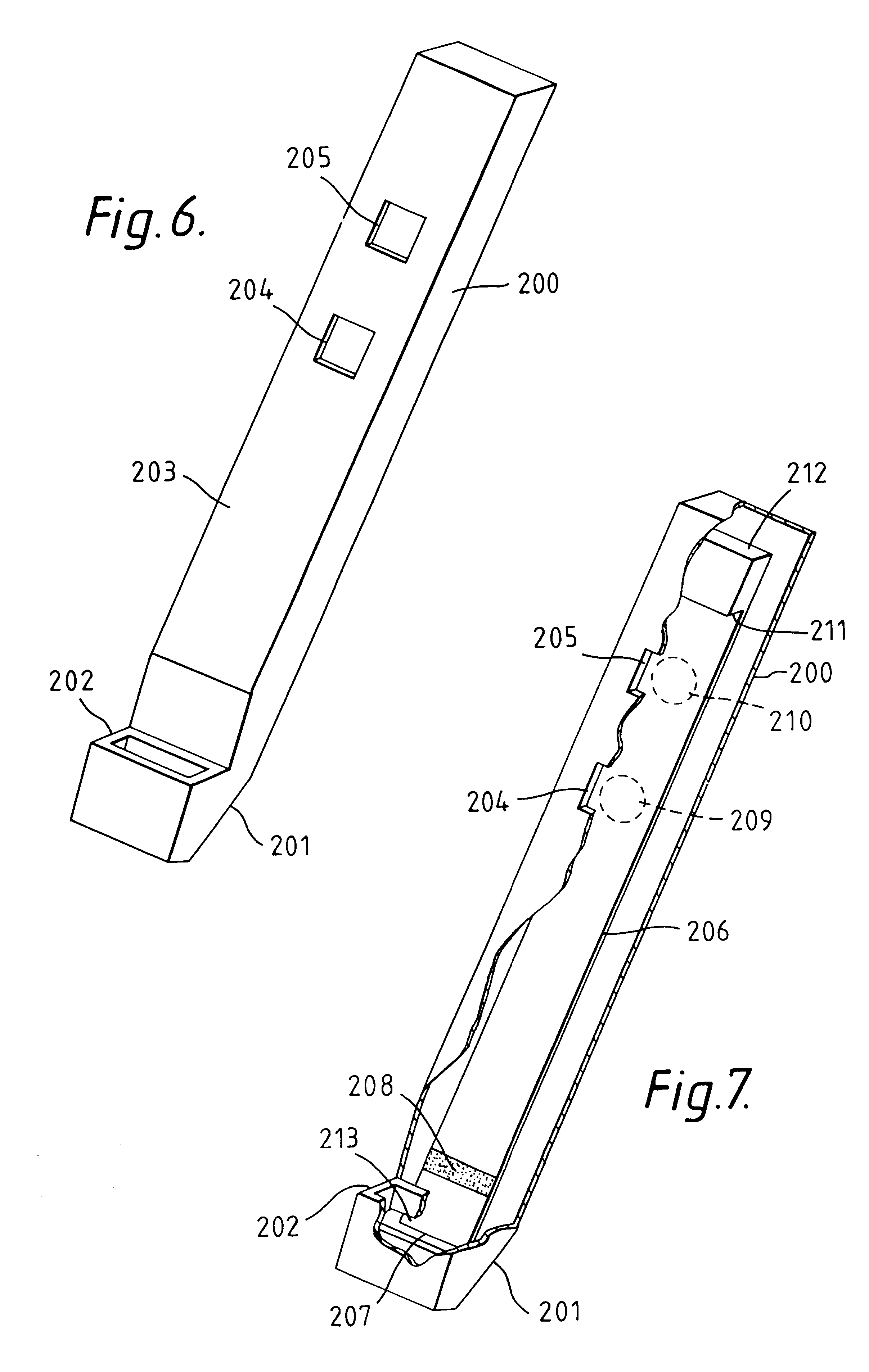
Capillary immunoassay and device therefor comprising mobilizable particulate labelled reagents
InactiveUS6228660B1Improve completenessAnalysis using chemical indicatorsComponent separationParticulatesAnalyte
An analytical test device useful for example in pregnancy testing, comprises a hollow casing (500) constructed of moisture-impervious solid material, such as plastics materials, containing a dry porous carrier (510) which communicates indirectly with the exterior of the casing via a bibulous sample receiving member (506) which protrudes from the casing such that a liquid test sample can be applied to the receiving member and permeate therefrom to the porous carrier, the carrier containing in a first zone a labelled specific binding reagent is freely mobile within the porous carrier when in the moist state, and in a second zone spatially distinct from the first zone unlabelled specific binding reagent for the same analyte which unlabelled reagent is permanently immobilized on the carrier material and is therefore not mobile in the moist state, the two zones being arranged such that liquid sample applied to the porous carrier can permeate via the first zone into the second zone, and the device incorporating means, such as an aperture (508) in the casing, enabling the extent (if any) to which the labelled reagent becomes bound in the second zone to be observed. Preferably the device includes a removable cap for the protruding bibulous member.
Owner:CONOPCO INC D B A UNILEVER
Capillary immunoassay and device therefor comprising mobilizable particulate labelled reagents
InactiveUS6187598B1Improve completenessBioreactor/fermenter combinationsBiological substance pretreatmentsPlastic materialsCapillary Tubing
An analytical test device useful for example in pregnancy testing, comprises a hollow casing (500) constructed of moisture-impervious solid material, such as plastics materials, containing a dry porous carrier (510) which communicates indirectly with the exterior of the casing via a bibulous sample receiving member (506) which protrudes from the casing such that a liquid test sample can be applied to the receiving member and permeate therefrom to the porous carrier, the carrier containing in a first zone a labelled specific binding reagent is freely mobile within the porous carrier when in the moist state, and in a second zone spatially distinct from the first zone unlabelled specific binding reagent for the same analyte which unlabelled reagent is permanently immobilised on the carrier material and is therefore not mobile in the moist state, the two zones being arranged such that liquid sample applied to the porous carrier can permeate via the first zone into the second zone, and the device incorporating means, such as an aperture (508) in the casing, enabling the extent (if any) to which the labelled reagent becomes bound in the second zone to be observed. Preferably the device includes a removable cap for the protruding bibulous member.
Owner:INVERNESS SWITZERLAND GMBH
Targeted delivery of active/bioactive and perfuming compositions
Described are controlled, time-release microparticulate active and bioactive compositions (including perfuming compositions) for targeted delivery to surfaces such as skin, hair and fabric and the environment proximate thereto, where the active and bioactive materials have a calculated log10P values of between 1 and 8 (P being the n-octanol-water partition coefficient). Such compositions include the active or bioactive material in single phase, solid solution in a wax or polymer matrix also having coated thereon and / or containing a compatible surfactant. Also described are processes and apparatus for preparing such compositions and processes for using same. Furthermore, certain component(s) of the aforementioned compositions in combination with one another are novel, and other components have novel uses in increasing fragrance substantivity, particularly in hair care preparations such as hair gels and shampoos.
Owner:INTERNATIONAL FLAVORS & FRAGRANCES
High-temperature characterization of polymers
InactiveUS6260407B1Avoid backlogImprove throughputSequential/parallel process reactionsComponent separationElutionChromatography column
Rapid characterization and screening of polymer samples to determine average molecular weight, molecular weight distribution and other properties is disclosed. Rapid flow characterization systems and methods, including liquid chromatography and flow-injection analysis systems and methods are preferably employed. High throughput, automated sampling systems and methods, high-temperature characterization systems and methods, and rapid, indirect calibration compositions and methods are also disclosed. In preferred high-temperature embodiments, the polymer sample is maintained at a temperature of not less than about 75° C. during sample preparation, loading into a liquid chromatography or flow-injection analysis system, injection into a mobile phase of a liquid chromatography or flow-injection analysis system, and / or elution from chromatographic column. The described methods, systems, and device have primary applications in combinatorial polymer research and in industrial process control.
Owner:INTERMOLECULAR
Non-invasive detection of fetal genetic traits
InactiveUS20050164241A1Facilitates non-invasive detectionComponent separationOther chemical processesPregnancyNon invasive
Blood plasma of pregnant women contains fetal and (generally>90%) maternal circulatory extracellular DNA. Most of said fetal DNA contains ≦500 base pairs, said maternal DNA having a greater size. Separation of circulatory extracellular DNA of <500 base pairs results in separation of fetal from maternal DNA. A fraction of a blood plasma or serum sample of a pregnant woman containing, due to size separation (e.g. by chromatography, density gradient centrifugation or nanotechnological methods), extracellular DNA substantially comprising ≦500 base pairs is useful for non-invasive detection of fetal genetic traits (including the fetal RhD gene in pregnancies at risk for HDN; fetal Y chromosome-specific sequences in pregnancies at risk for X chromosome-linked disorders; chromosomal aberrations; hereditary Mendelian genetic disorders and corresponding genetic markers; and traits decisive for paternity determination) by e.g. PCR, ligand chain reaction or probe hybridization techniques, or nucleic acid arrays.
Owner:SEQUENOM INC
Rapid characterization of polymers
InactiveUS6406632B1More separatedHigh sample throughputSequential/parallel process reactionsSamplingFluid phasePhysical chemistry
Rapid characterization and screening of polymer samples to determine average molecular weight, molecular weight distribution and other properties is disclosed. Rapid flow characterization systems and methods, including liquid chromatography and flow-injection analysis systems and methods are preferably employed. High throughput, automated sampling systems and methods, high-temperature characterization systems and methods, and rapid, indirect calibration compositions and methods are also disclosed. The described methods, systems, and devices have primary applications in combinatorial polymer research and in industrial process control.
Owner:INTERMOLECULAR
Apparatus for preparing a solid phase microparticulate composition
InactiveUS6042792ALow costImproved substantivityCosmetic preparationsComponent separationWaxMicroparticle
Described are controlled, time-release microparticulate active and bioactive compositions (including perfuming compositions) for targeted delivery to services such as skin, hair and fabric and the environment proximate thereto, where the active and bioactive materials have a calculated log10P values of between 1 and 8 (P being the n-octanol-water partition coefficient). Such compositions include the active or bioactive material in single phase, solid solution in a wax or polymer matrix also having coated thereon and / or containing a compatible surfactant. Also described are processes and apparatus for preparing such compositions and processes for using same. Furthermore, certain component(s) of the aforementioned compositions in combination with one another are novel, and other components have novel uses in increasing fragrance substantivity.
Owner:INTERNATIONAL FLAVORS & FRAGRANCES
Indirect calibration of polymer characterization systems
InactiveUS6294388B1Avoid backlogImprove throughputIon-exchange process apparatusSamplingPolymer characterizationFlow injection analysis
Rapid characterization and screening of polymer samples to determine average molecular weight, molecular weight distribution and other properties is disclosed. Rapid flow characterization systems and methods, including liquid chromatography and flow-injection analysis systems and methods are preferably employed. High throughput, automated sampling systems and methods, high-temperature characterization systems and methods, and rapid, indirect calibration compositions and methods are also disclosed. The described methods, systems, and devices have primary applications in combinatorial polymer research and in industrial process control.
Owner:INTERMOLECULAR
Parallel high-performance liquid chromatography with post-separation treatment
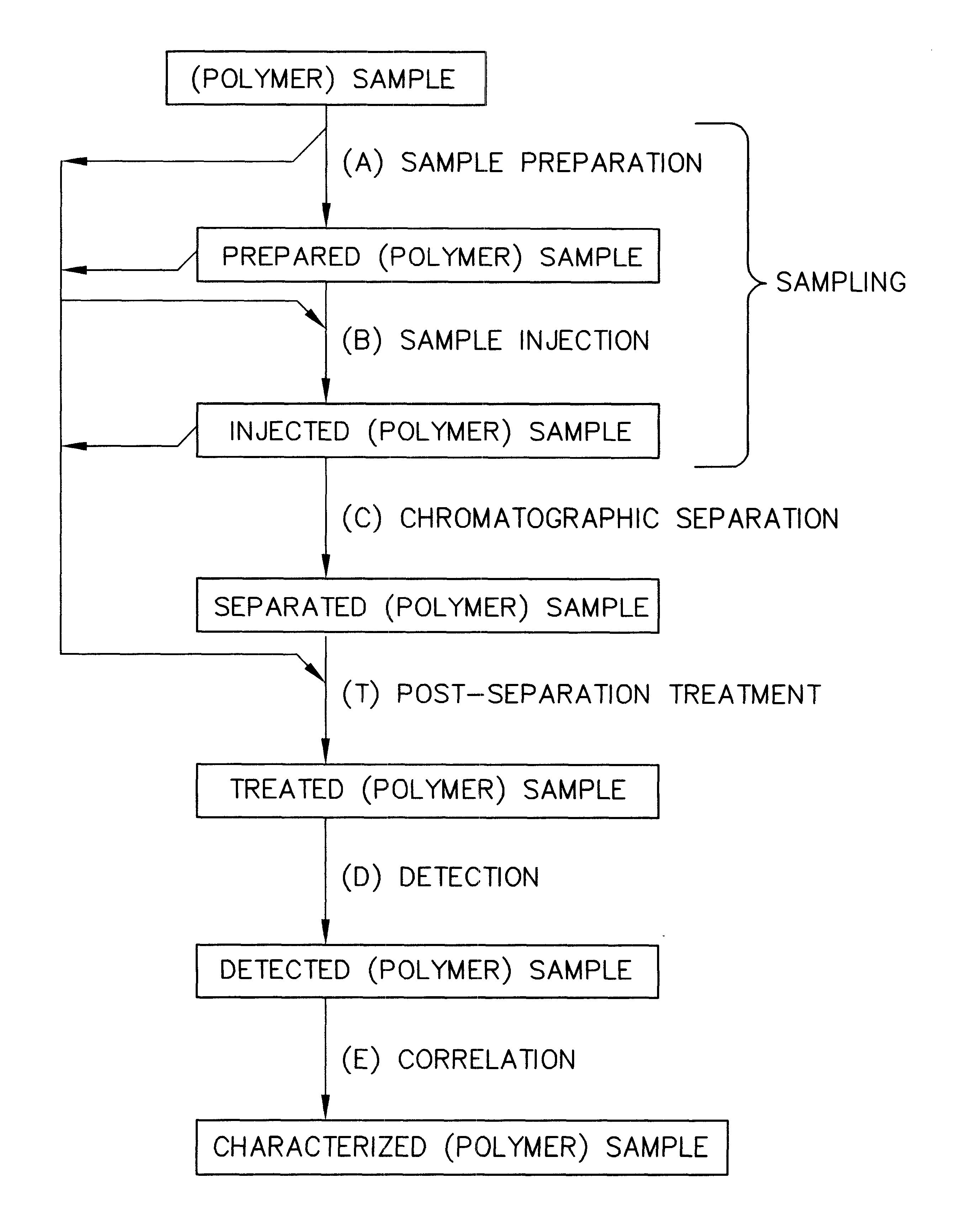
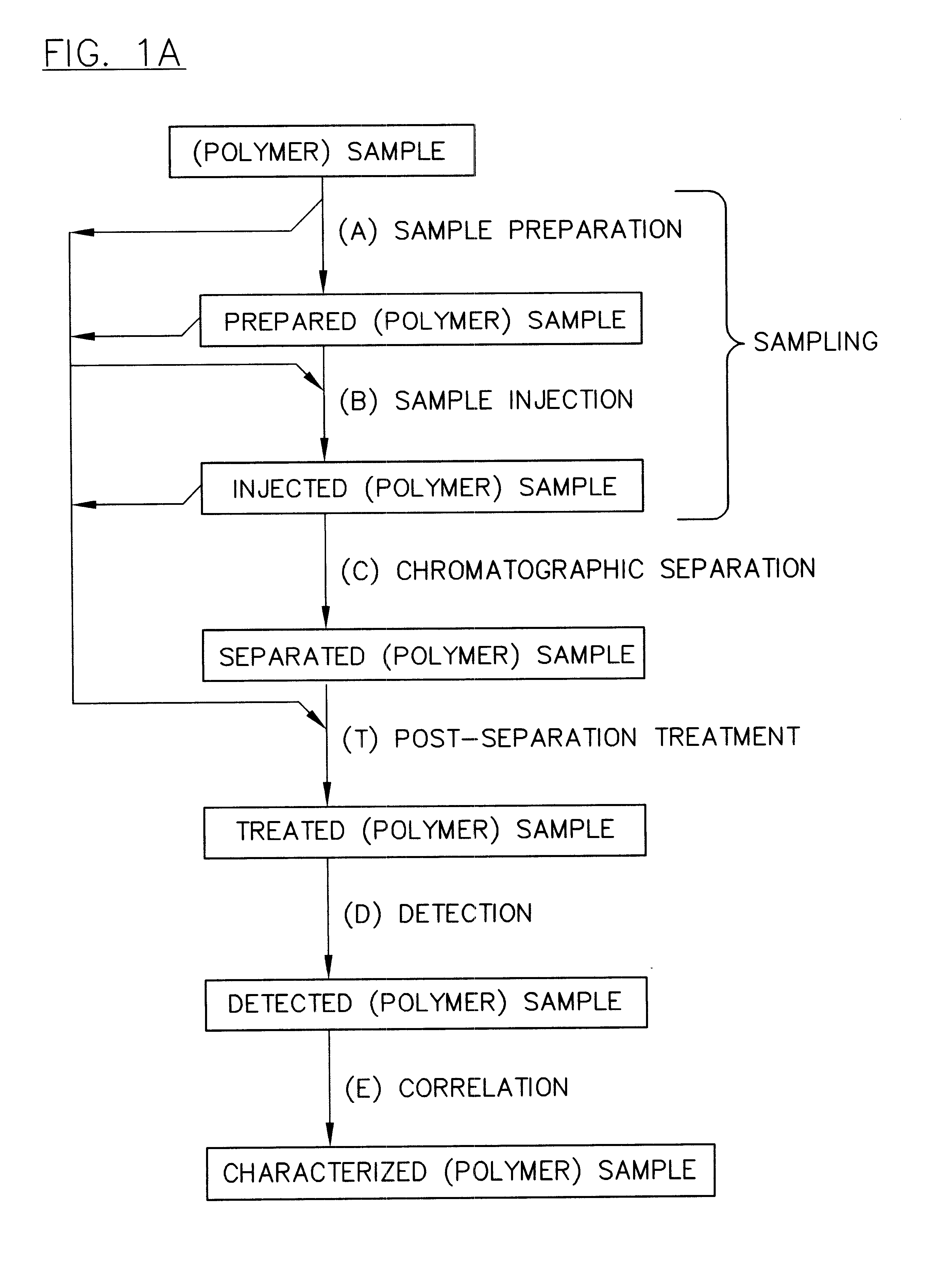
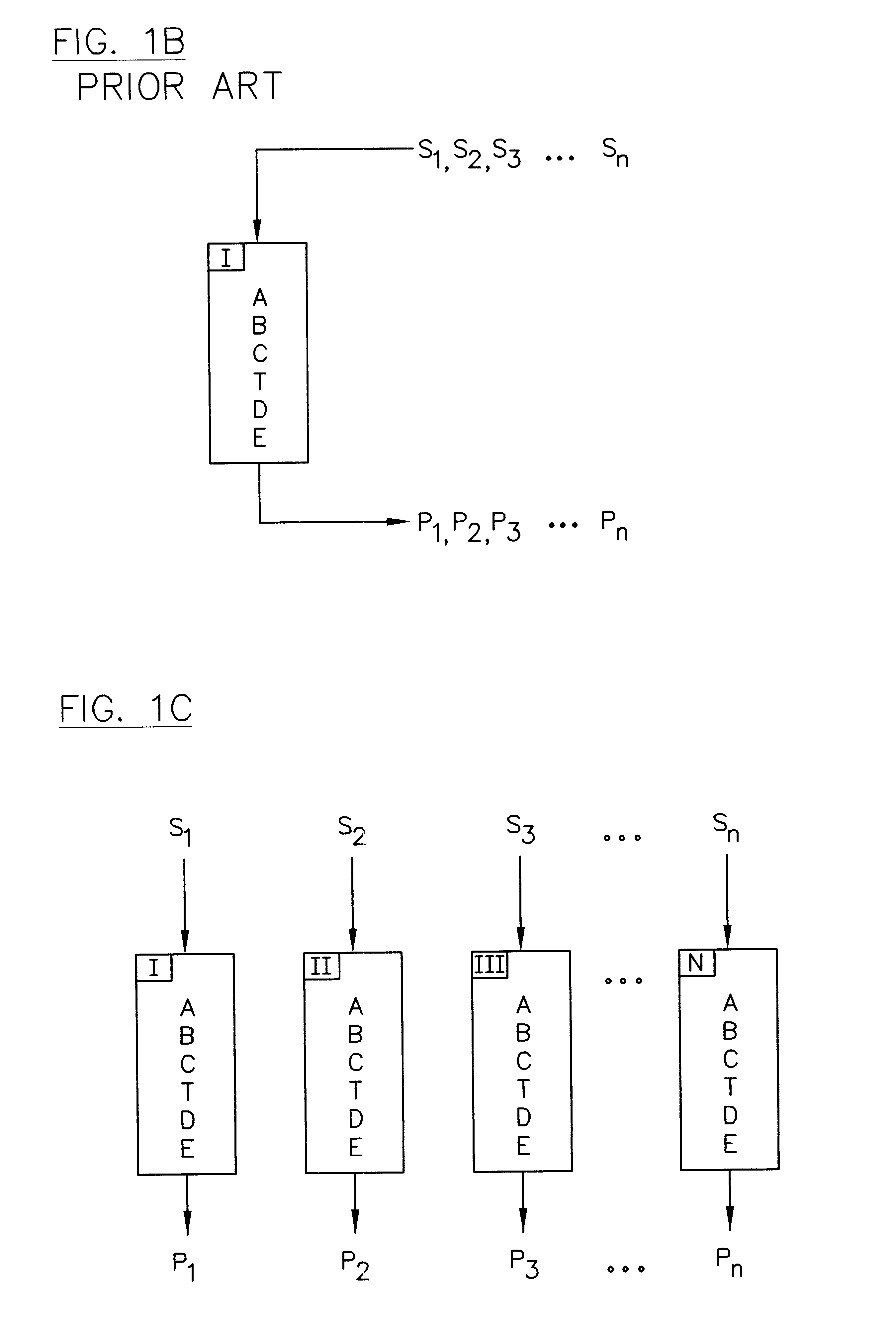
InactiveUS6436292B1High sample throughputEasy to findIon-exchange process apparatusSamplingChromatographic separationCombinatorial synthesis
High-performance liquid chromatography (HPLC) methods and systems are disclosed that combine parallel chromatographic separation of a plurality of samples with a detection technique that involves post-separation treatment of the plurality of samples to enhance one or more properties of the sample or of a component thereof, followed by detection of the one or more enhanced properties. Selective, tunable detection schemes are achievable, and are particularly advantageous as applied in connection with combinatorial chemistry, combinatorial material science and more particularly, combinatorial synthesis and screening of polymeric materials.
Owner:FREESLATE
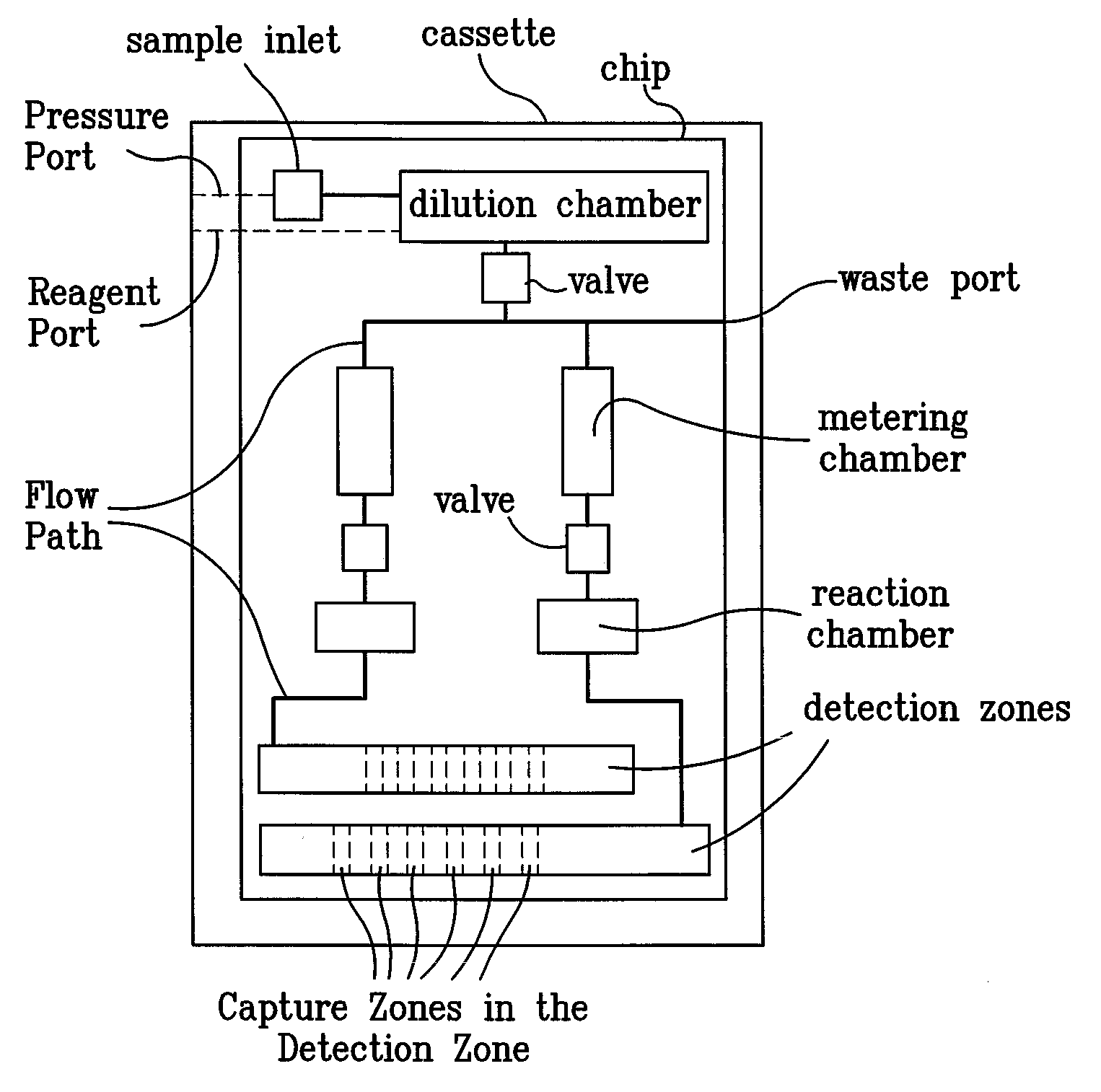
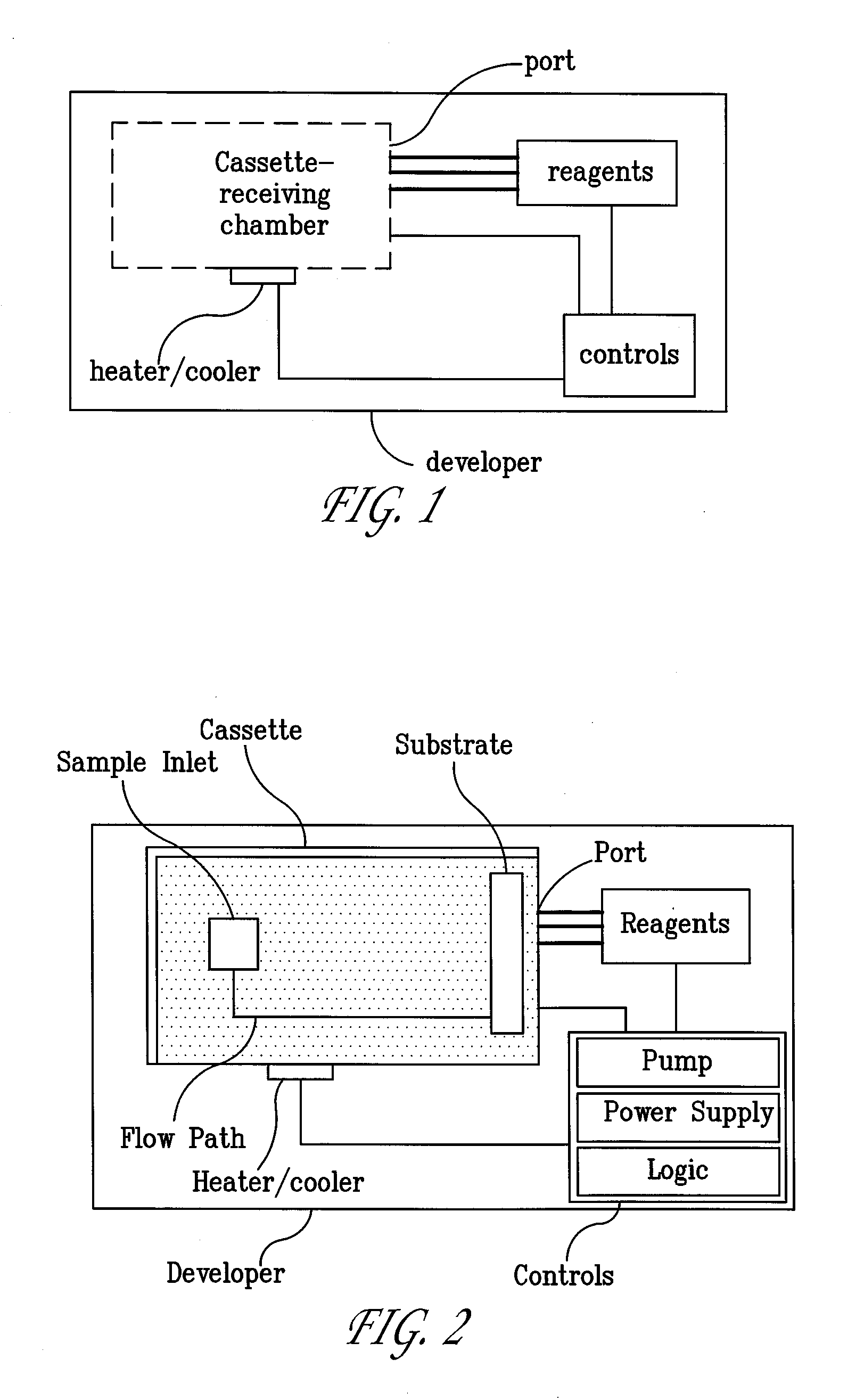
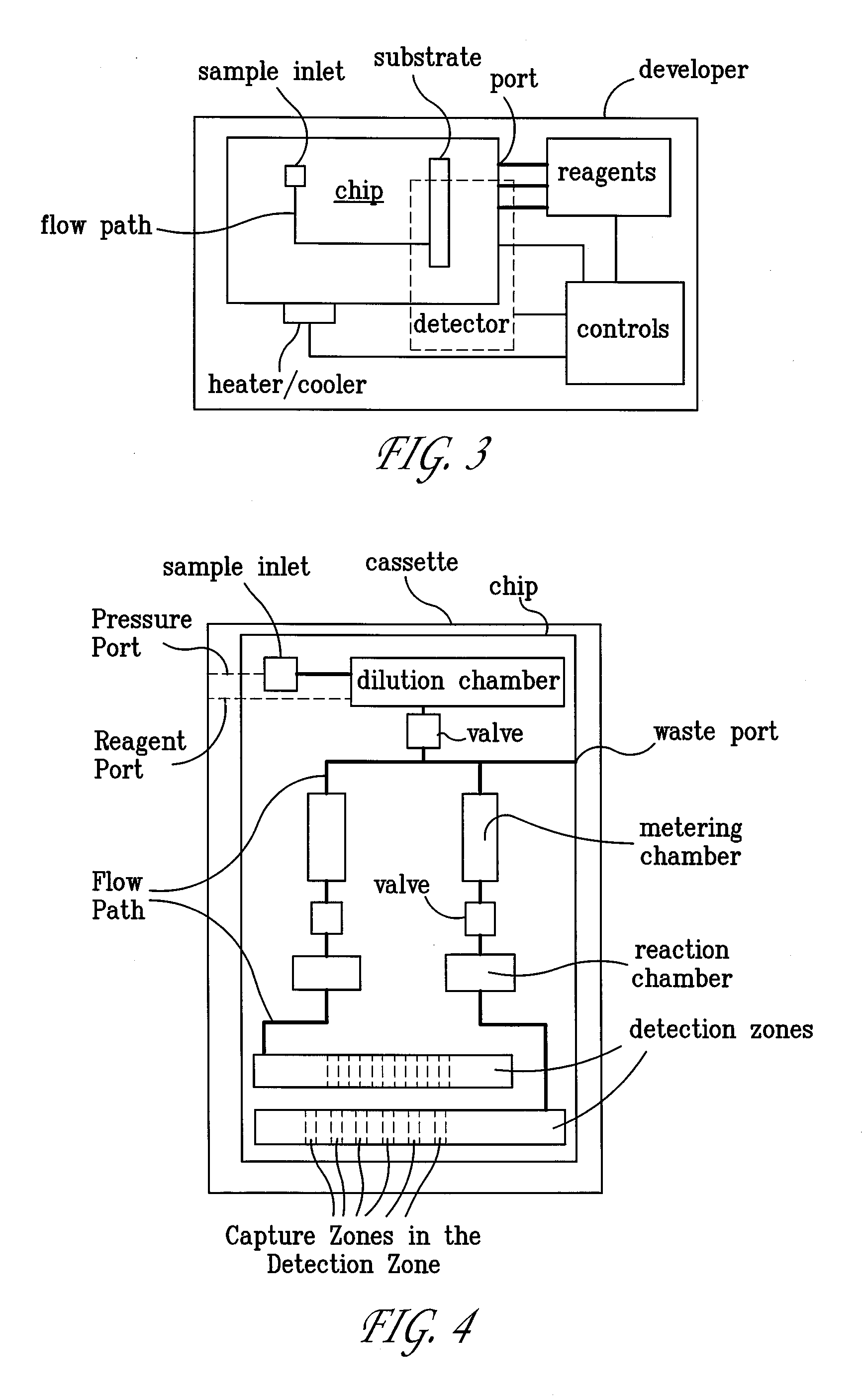
Systems and Methods For Testing using Microfluidic Chips
InactiveUS20080280285A1Easy to controlReduce materialBioreactor/fermenter combinationsHeating or cooling apparatusAntigenRNA Sequence
Disclosed are methods, devices and systems for biological and chemical sample processing using microfluidic chips. The disclosed microfluidic chips contain at least two detection zones for interacting with pre-selected RNA sequences, DNA sequences, antibodies, or antigens to determine their presence in the sample. Systems are also described comprising a cassette having at least one port and a sample inlet in fluid communication with a detection zone for interacting with pre-selected RNA sequences, DNA sequences, antibodies, or antigens, or mixtures thereof, if present, in a sample. Methods for concurrent testing of at least two of RNA, DNA, antibody, and antigen in a sample are also described, as are methods for testing for pre-selected pathogens and microfluidic methods.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
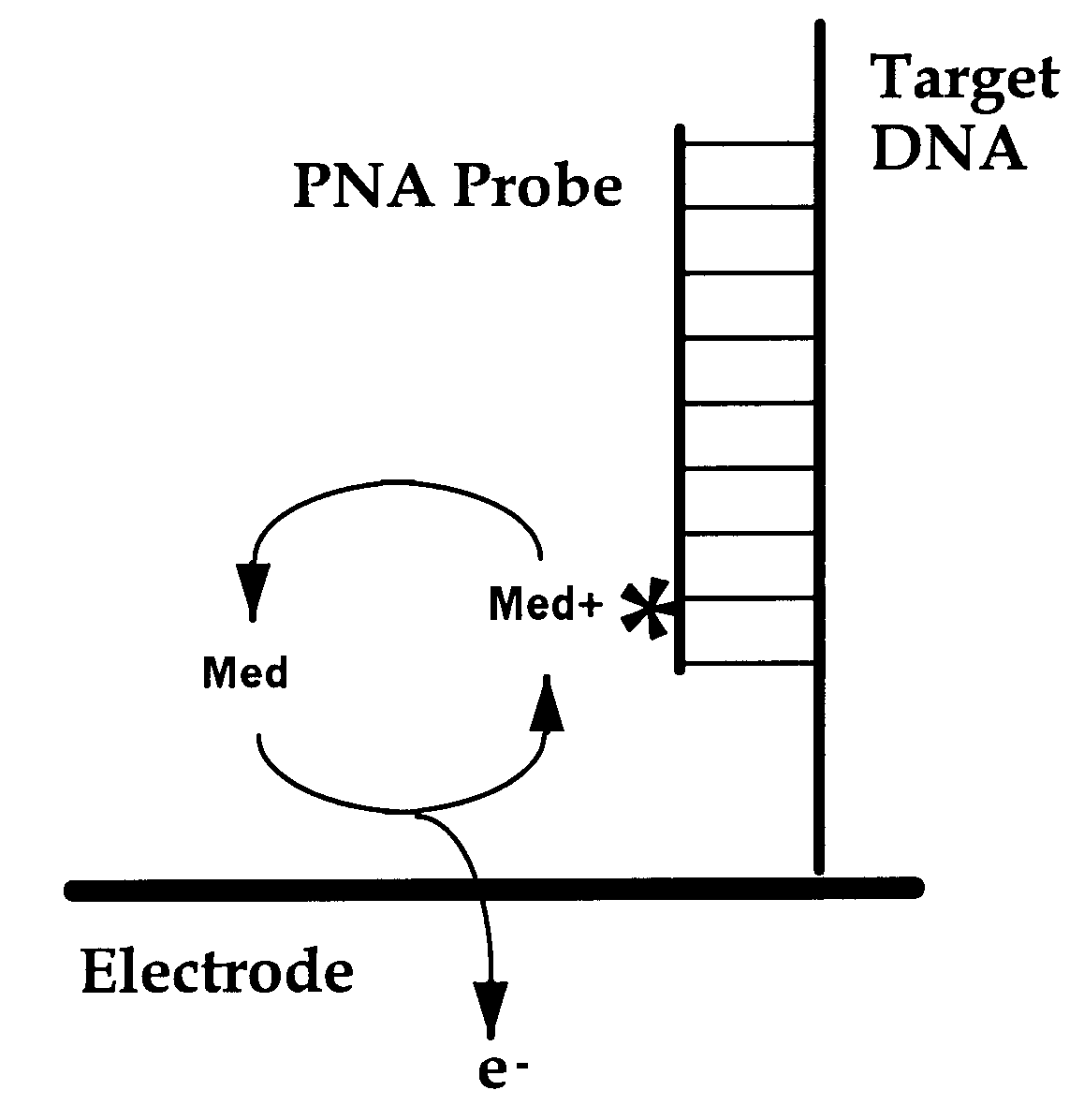
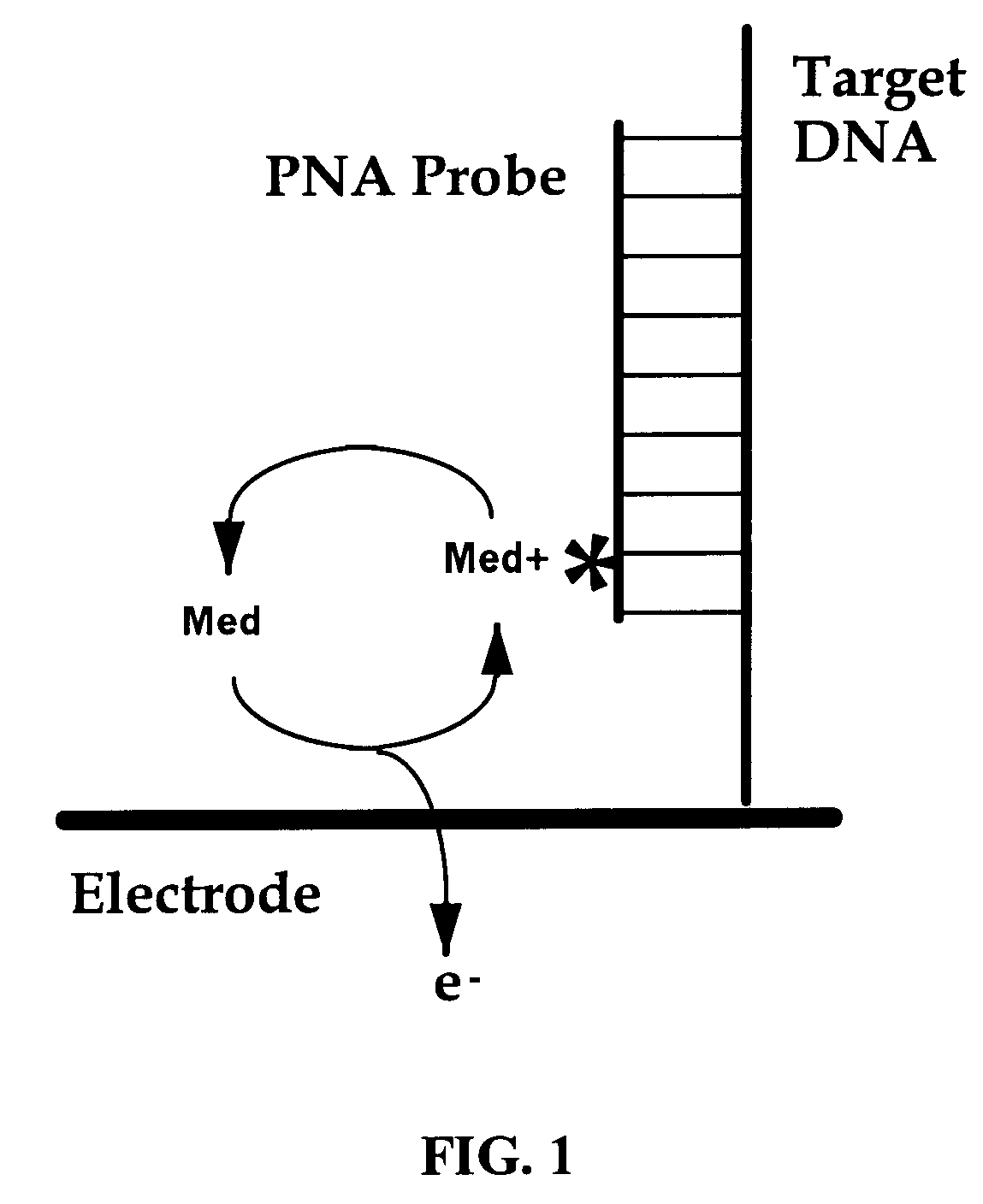
Electrochemical detection of nucleic acid sequences
InactiveUS6391558B1Quick checkComponent separationMicrobiological testing/measurementNucleic acid detectionNucleic acid sequencing
An electrochemical detection system which specifically detects selected nucleic acid segments is described. The system utilizes biological probes such as nucleic acid or peptide nucleic acid probes which are complementary to and specifically hybridize with selected nucleic acid segments in order to generate a measurable current when an amperometric potential is applied. The electrochemical signal can be quantified.
Owner:MAGELLAN DIAGNOSTICS
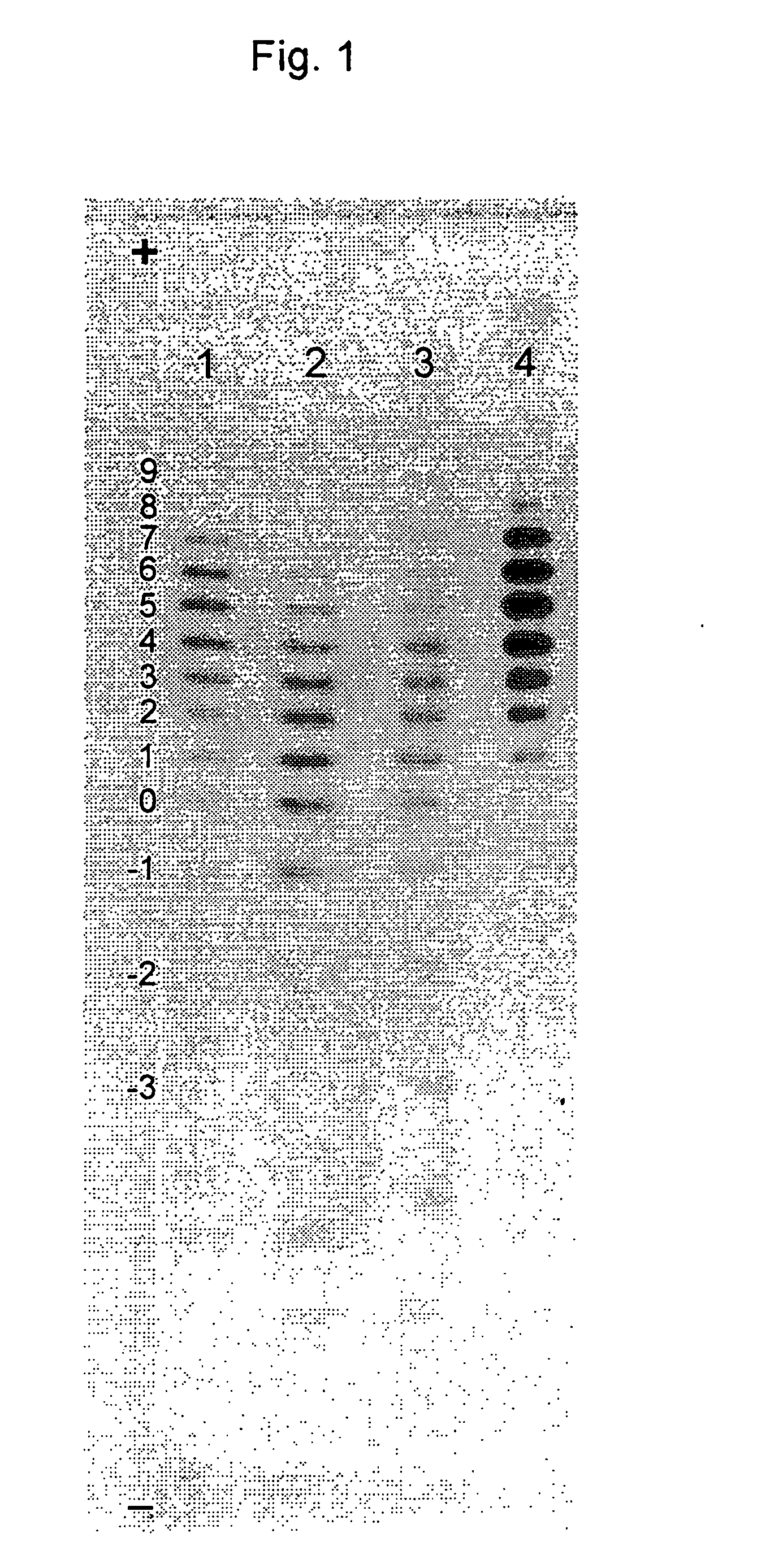
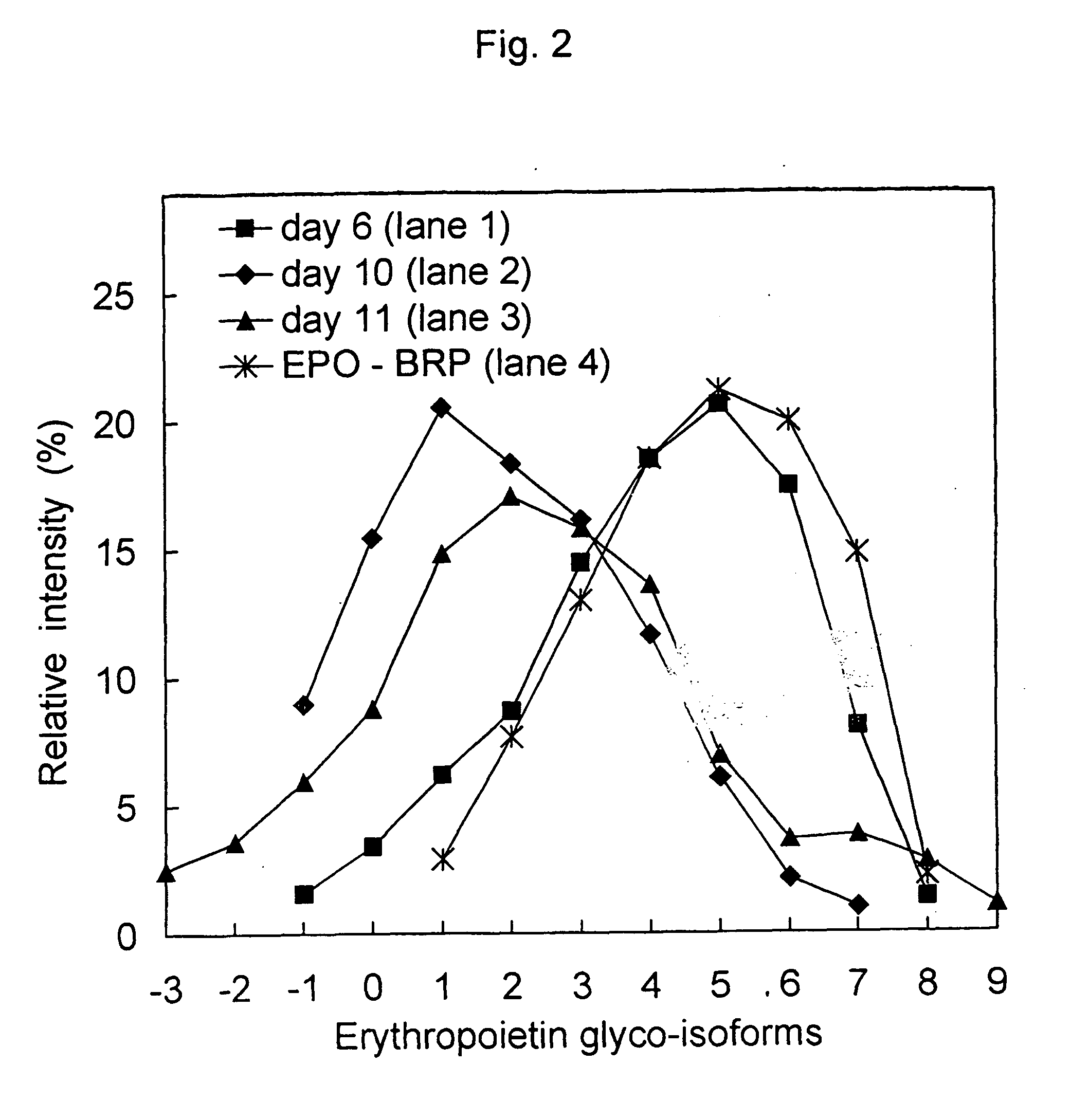
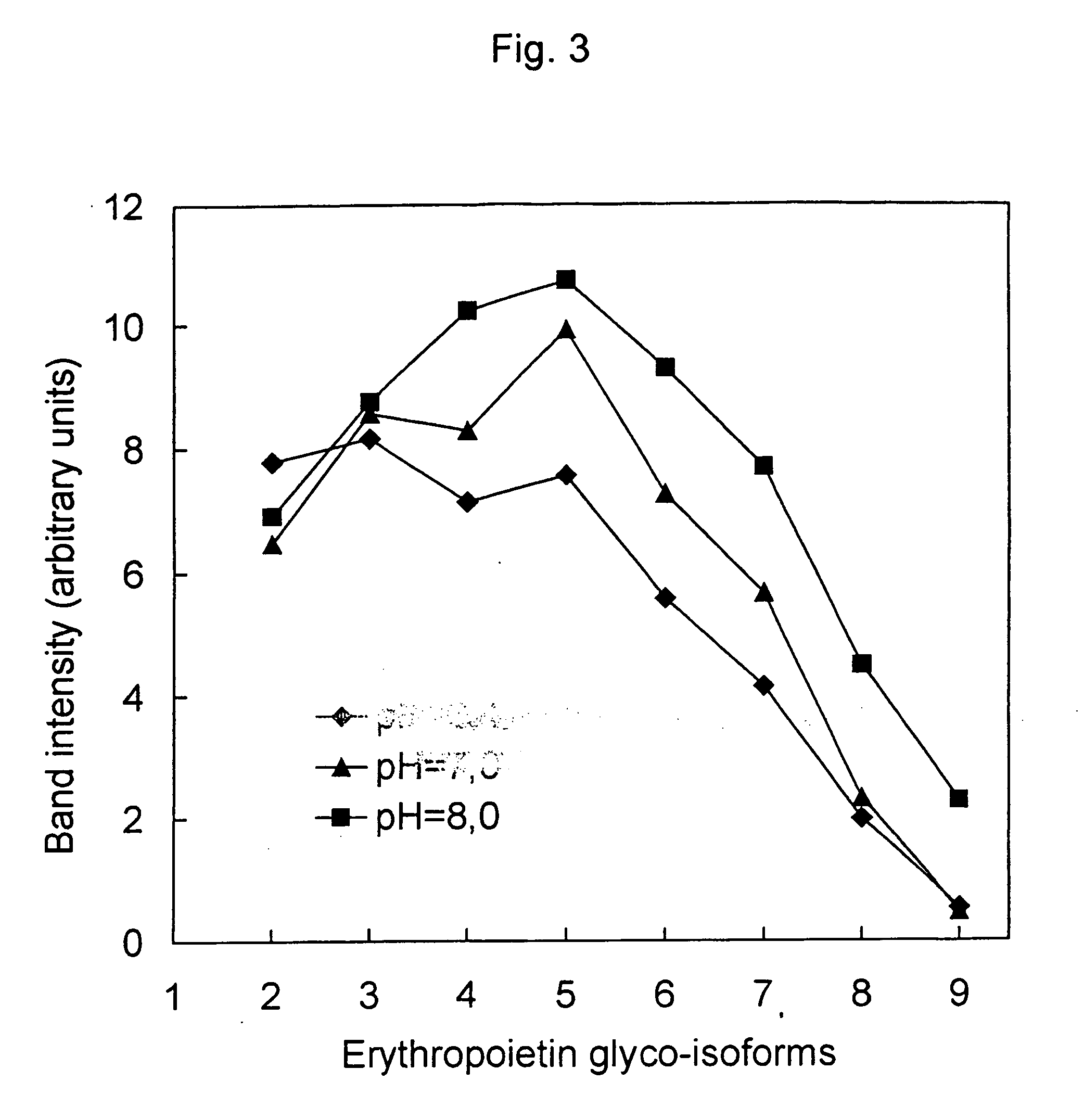
Process for the preparation of a desired erythropoietin glyco-isoform profile
InactiveUS20050153879A1High and uniform product specificityImprove product qualityPeptide/protein ingredientsComponent separationFiltrationRed blood cell
The present invention provides a process for the production of erythropoietin (EPO) with high purity and with a desired profile of EPO glycol-isoforms by using a combination of specific chromatographic steps in such a manner that the starting EPO glycol-isoform profile is changed or modified. The applied chromatographic steps includes at least (a) dye affinity chromatography, and (b) hydrophobic chromatography and / or (c) anion-exchange chromatography. In a preferred embodiment, the process further includes (d) gel filtration chromatography. The present invention also provides a process for the determination of erythropoietin (EPO) glycol-isoform profile in an EPO containing composition.
Owner:SVETINA MONICA +4
Sensor platform using a horizontally oriented nanotube element
Sensor platforms and methods of making them are described, and include platforms having horizontally oriented sensor elements comprising nanotubes or other nanostructures, such as nanowires. Under certain embodiments, a sensor element has an affinity for an analyte. Under certain embodiments, such a sensor element comprises one or more pristine nanotubes, and, under certain embodiments, it comprises derivatized or functionalized nanotubes. Under certain embodiments, a sensor is made by providing a support structure; providing a collection of nanotubes on the structure; defining a pattern within the nanotube collection; removing part of the collection so that a patterned collection remains to form a sensor element; and providing circuitry to electrically sense the sensor's electrical characterization. Under certain embodiments, the sensor element comprises pre-derivatized or pre-functionalized nanotubes. Under certain embodiments, sensor material is derivatized or functionalized after provision on the structure or after patterning. Under certain embodiments, a large-scale array includes multiple sensors.
Owner:NANTERO
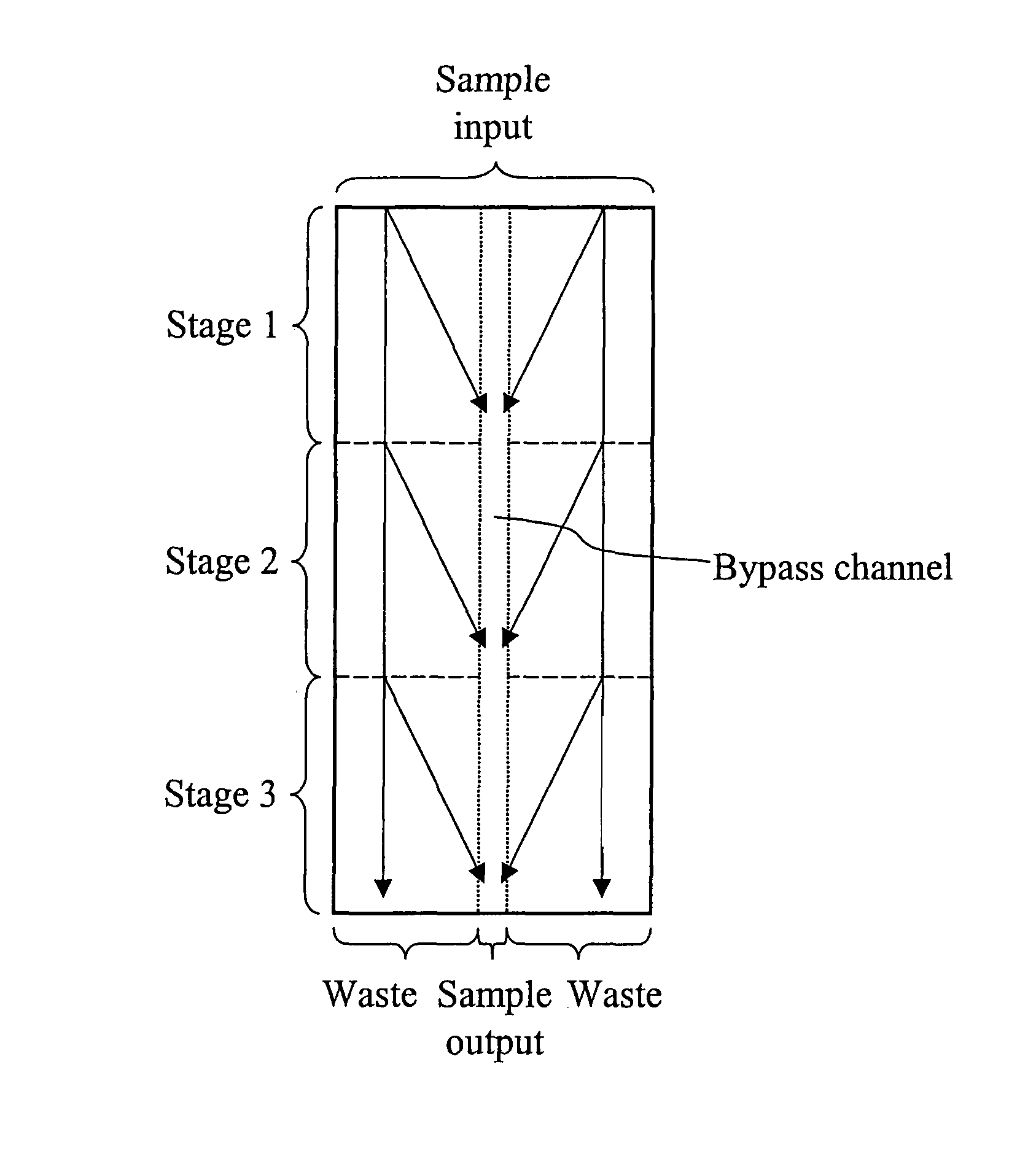
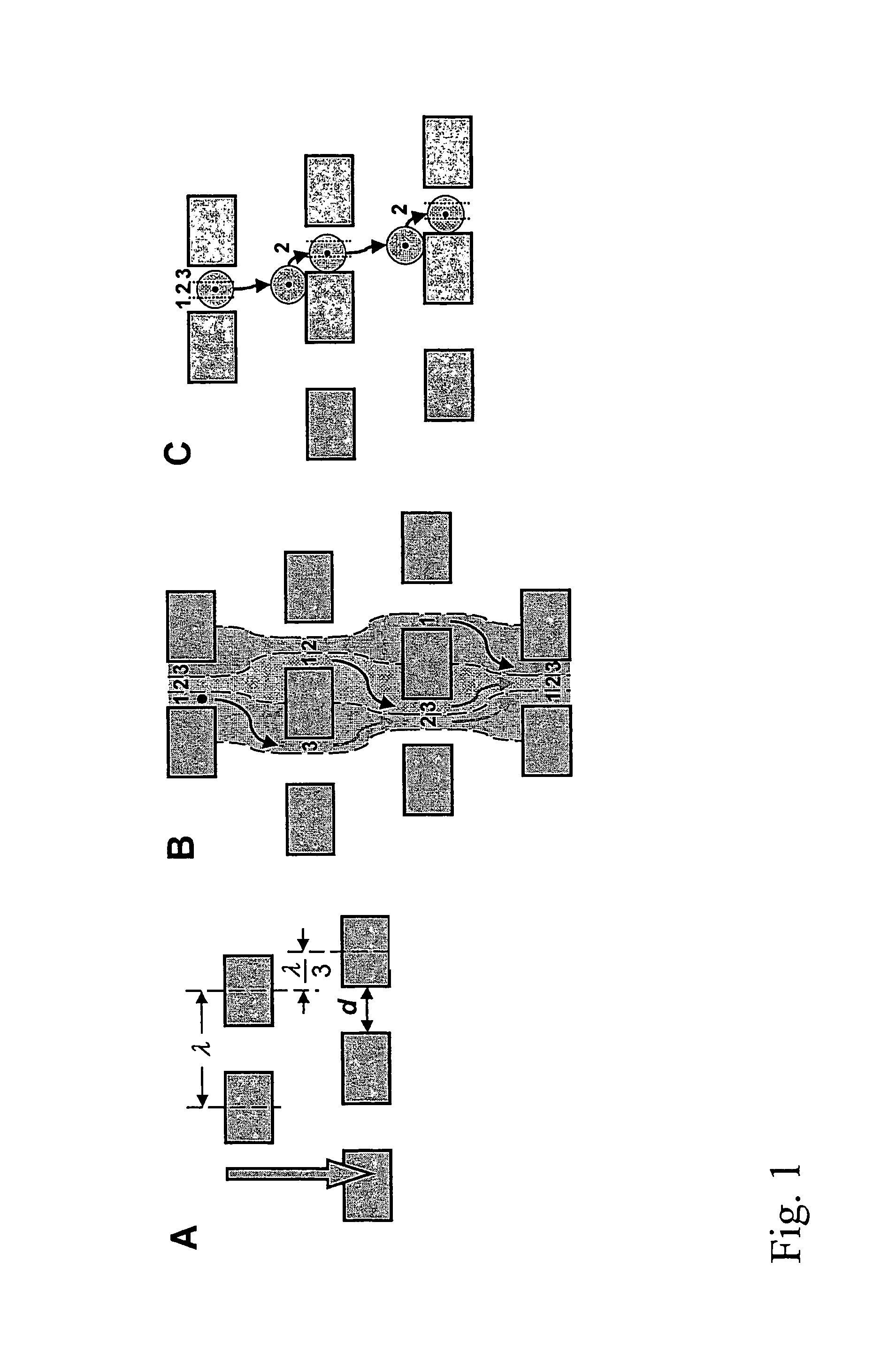
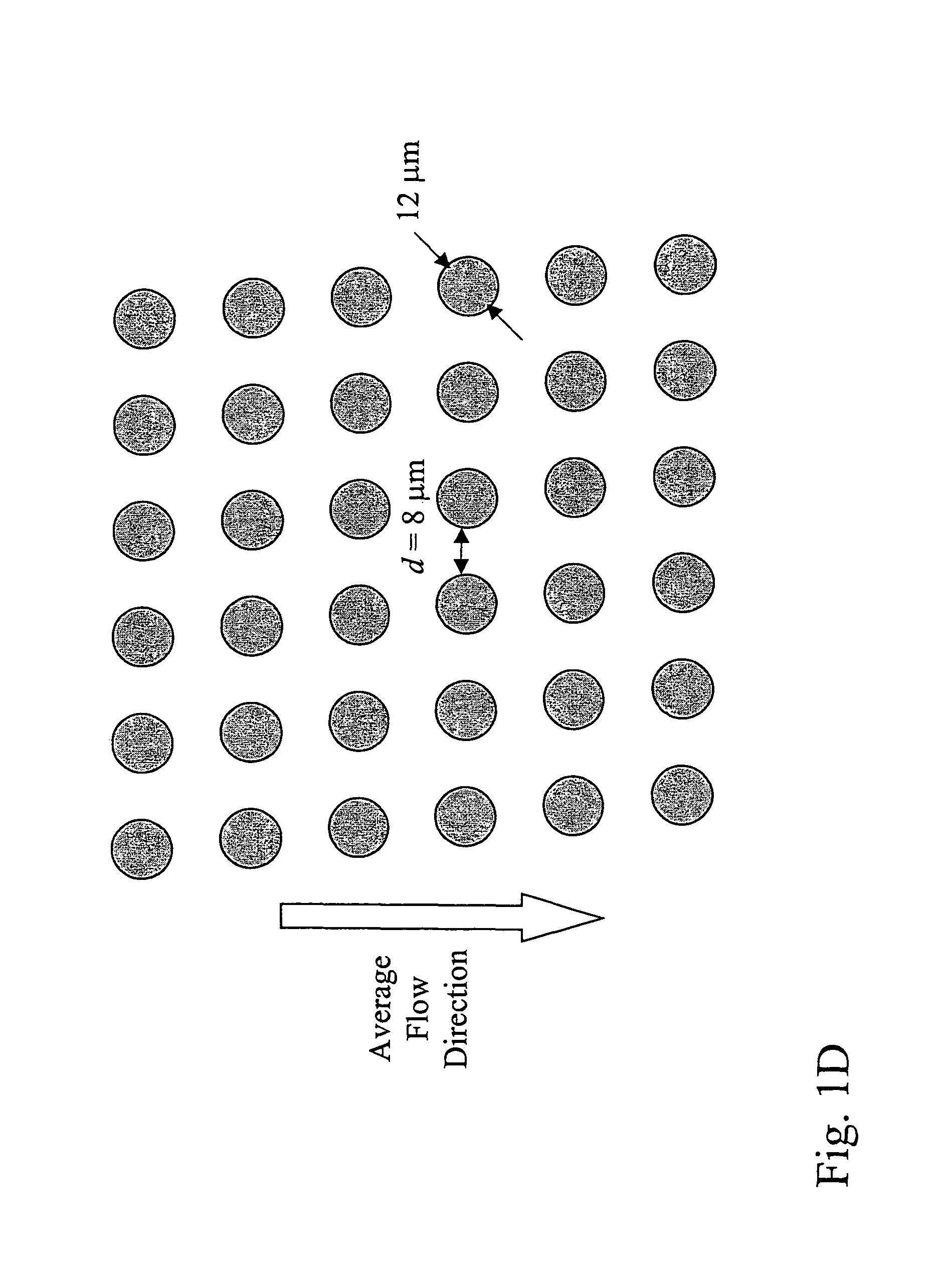
Devices and methods for enrichment and alteration of cells and other particles
ActiveUS20070026381A1Increase volumeReduced deformabilityBioreactor/fermenter combinationsBiological substance pretreatmentsCellular componentLysis
The invention features devices and methods for the deterministic separation of particles. Exemplary methods include the enrichment of a sample in a desired particle or the alteration of a desired particle in the device. The devices and methods are advantageously employed to enrich for rare cells, e.g., fetal cells, present in a sample, e.g., maternal blood and rare cell components, e.g., fetal cell nuclei. The invention further provides a method for preferentially lysing cells of interest in a sample, e.g., to extract clinical information from a cellular component, e.g., a nucleus, of the cells of interest. In general, the method employs differential lysis between the cells of interest and other cells (e.g., other nucleated cells) in the sample.
Owner:THE GENERAL HOSPITAL CORP +1
Microfluidic sample delivery devices, systems, and methods
ActiveUS7303727B1Improve throughputResidue reductionParticle separator tubesComponent separationSpray nozzleMass spectrometry
Methods and apparatus for delivering fluidic materials to sample destinations, including mass spectrometers for analysis are provided. In preferred embodiments, sample aliquots are electrosprayed from tapered spray tips of capillary elements into the orifices of mass spectrometric inlet systems. In certain embodiments, fluidic samples are orthogonally sprayed from capillary elements or other fluid conduits, whereas in other embodiments samples are sprayed after devices are rotated or otherwise translocated from sample sources to sample destinations. In still other embodiments, samples are sprayed from flexed or deflected capillary elements at selected sample destinations.
Owner:CAPLIPER LIFE SCI INC
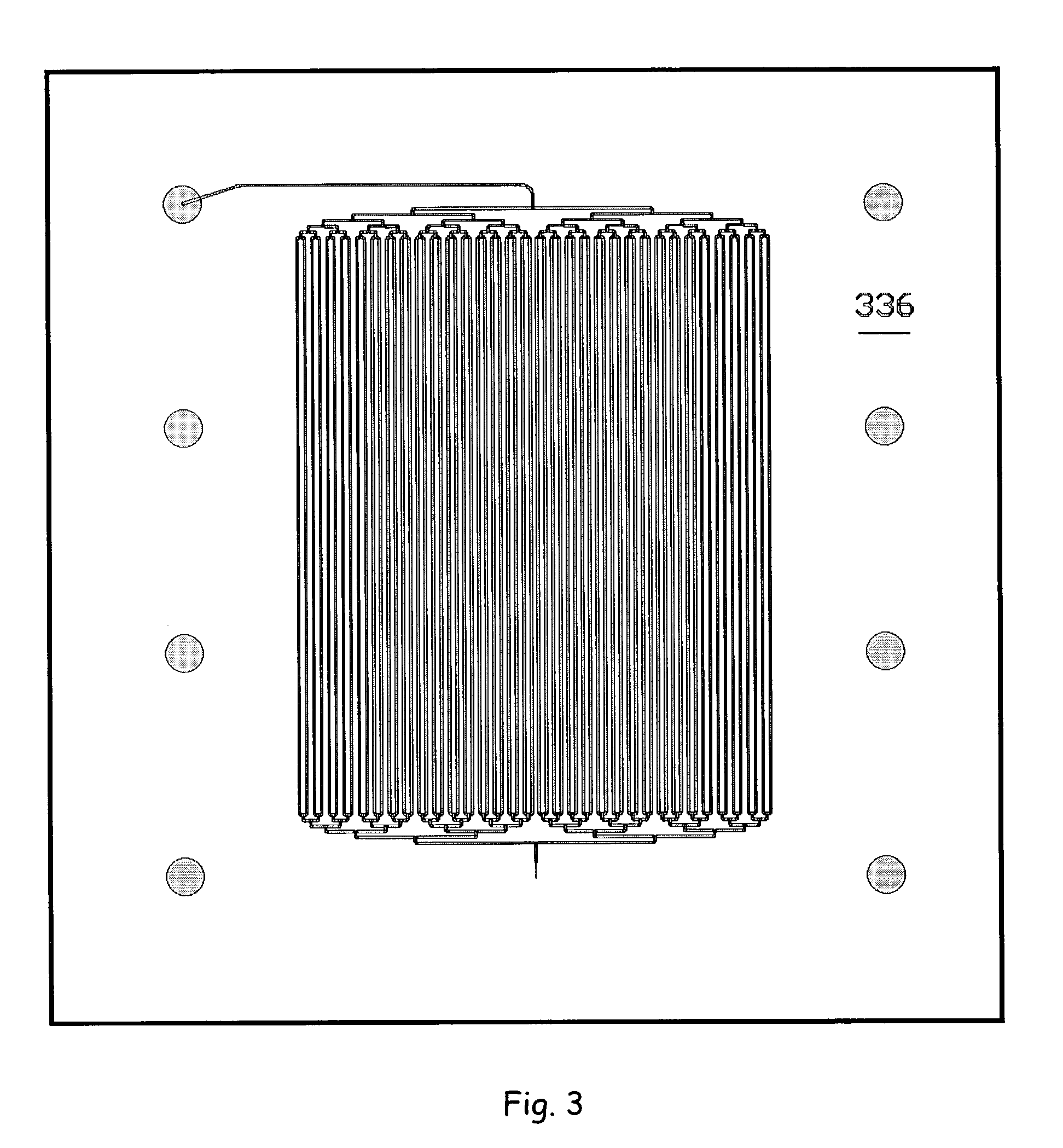
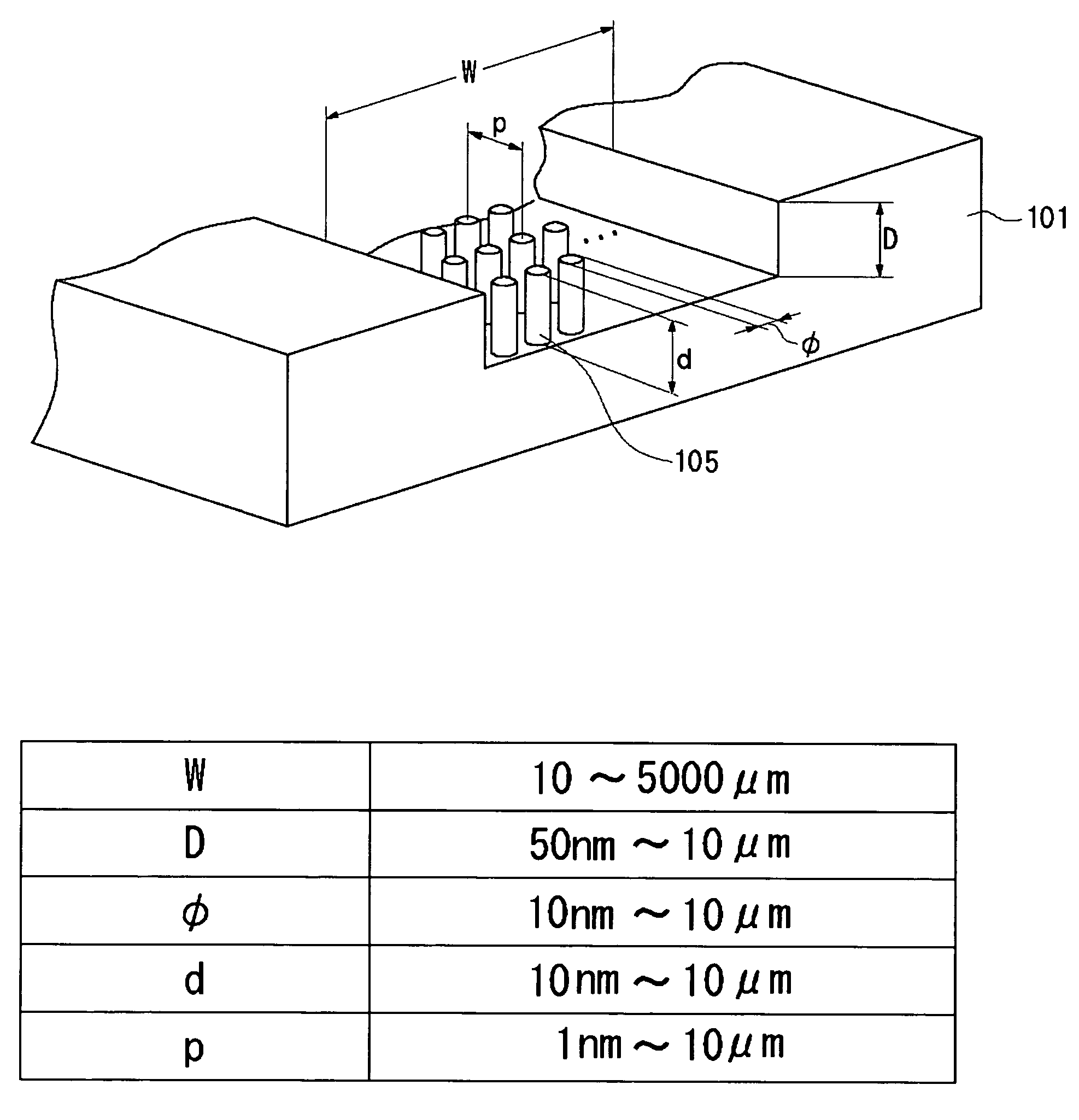
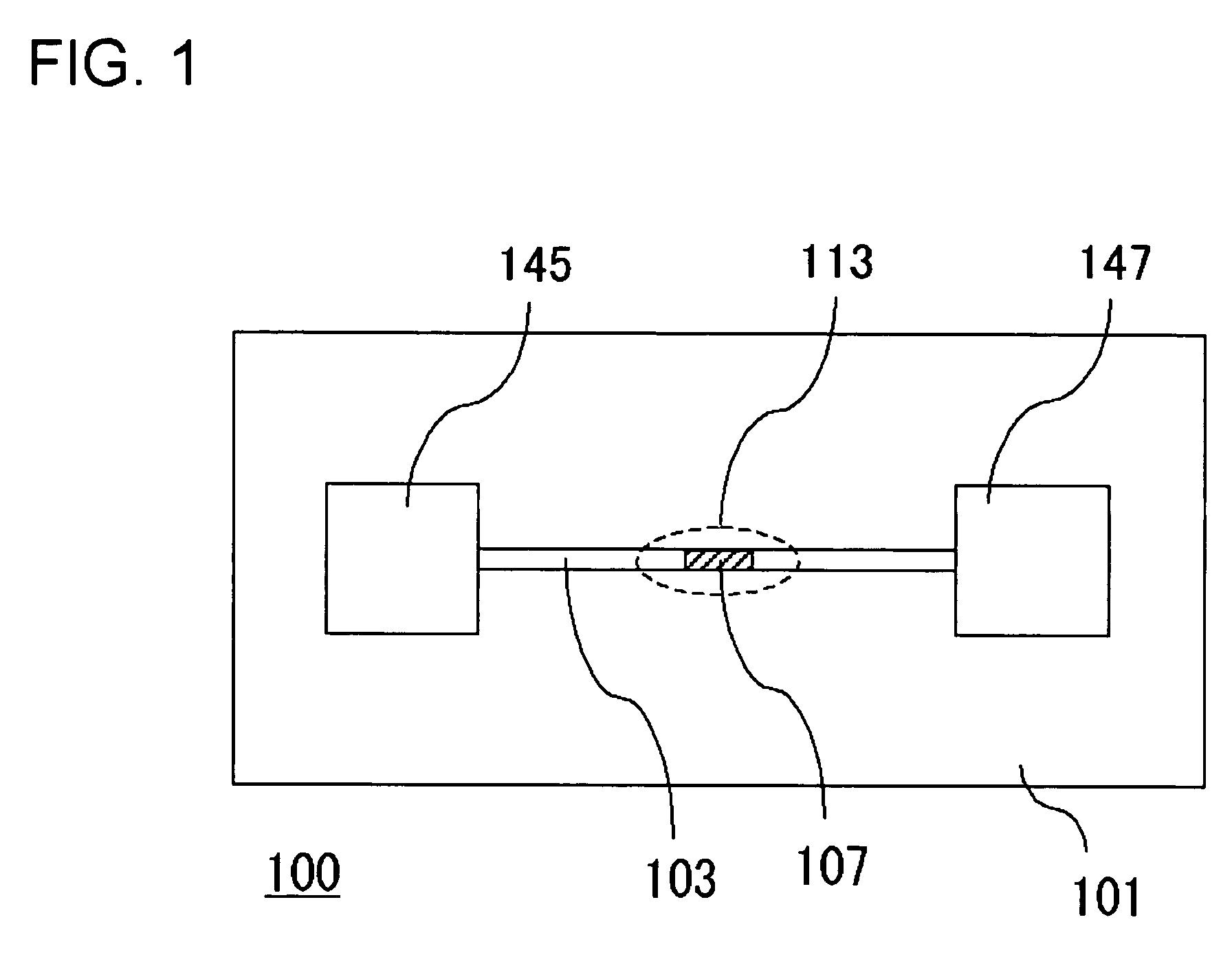
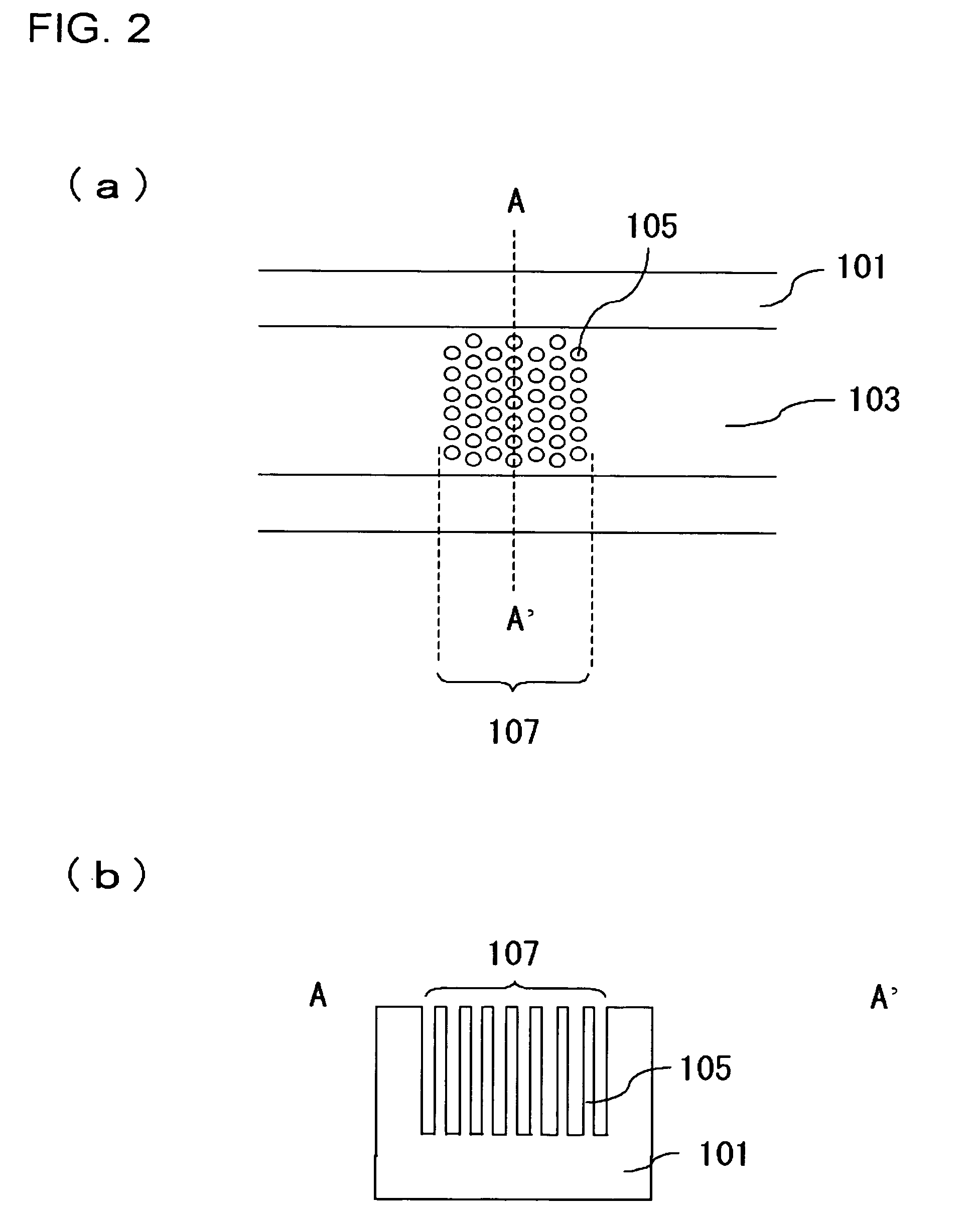
Separation apparatus and separation method
InactiveUS20060000772A1Improve efficiencyHigh activityIon-exchange process apparatusSemi-permeable membranesBuffer solutionAnalytical chemistry
A channel (103) is formed in a substrate (101), and a portion of the channel (103) is provided with a separating portion (107). A number of pillars are formed in the separating portion (107), and an adsorptive substance layer having an adsorptive substance, which exhibits a specific interaction for a specific substance, immobilized on the surface thereof, is formed. Once a sample is introduced into the channel (103), the specific substance is adsorbed on the adsorptive substance layer to be separated from other components. After washing the inside of the channel (103) with a buffer solution, the specific substance is desorbed from the adsorptive substance layer by flowing a eluting solution through the channel (103) and the specific substance is recovered.
Owner:NEC CORP
Devices and methods for enrichment and alteration of cells and other particles
ActiveUS8021614B2Increases the hydrodynamic radius of a particleIncrease volumeBioreactor/fermenter combinationsBiological substance pretreatmentsCellular componentClinical information
The invention features devices and methods for the deterministic separation of particles. Exemplary methods include the enrichment of a sample in a desired particle or the alteration of a desired particle in the device. The devices and methods are advantageously employed to enrich for rare cells, e.g., fetal cells, present in a sample, e.g., maternal blood and rare cell components, e.g., fetal cell nuclei. The invention further provides a method for preferentially lysing cells of interest in a sample, e.g., to extract clinical information from a cellular component, e.g., a nucleus, of the cells of interest. In general, the method employs differential lysis between the cells of interest and other cells (e.g., other nucleated cells) in the sample.
Owner:THE GENERAL HOSPITAL CORP +1
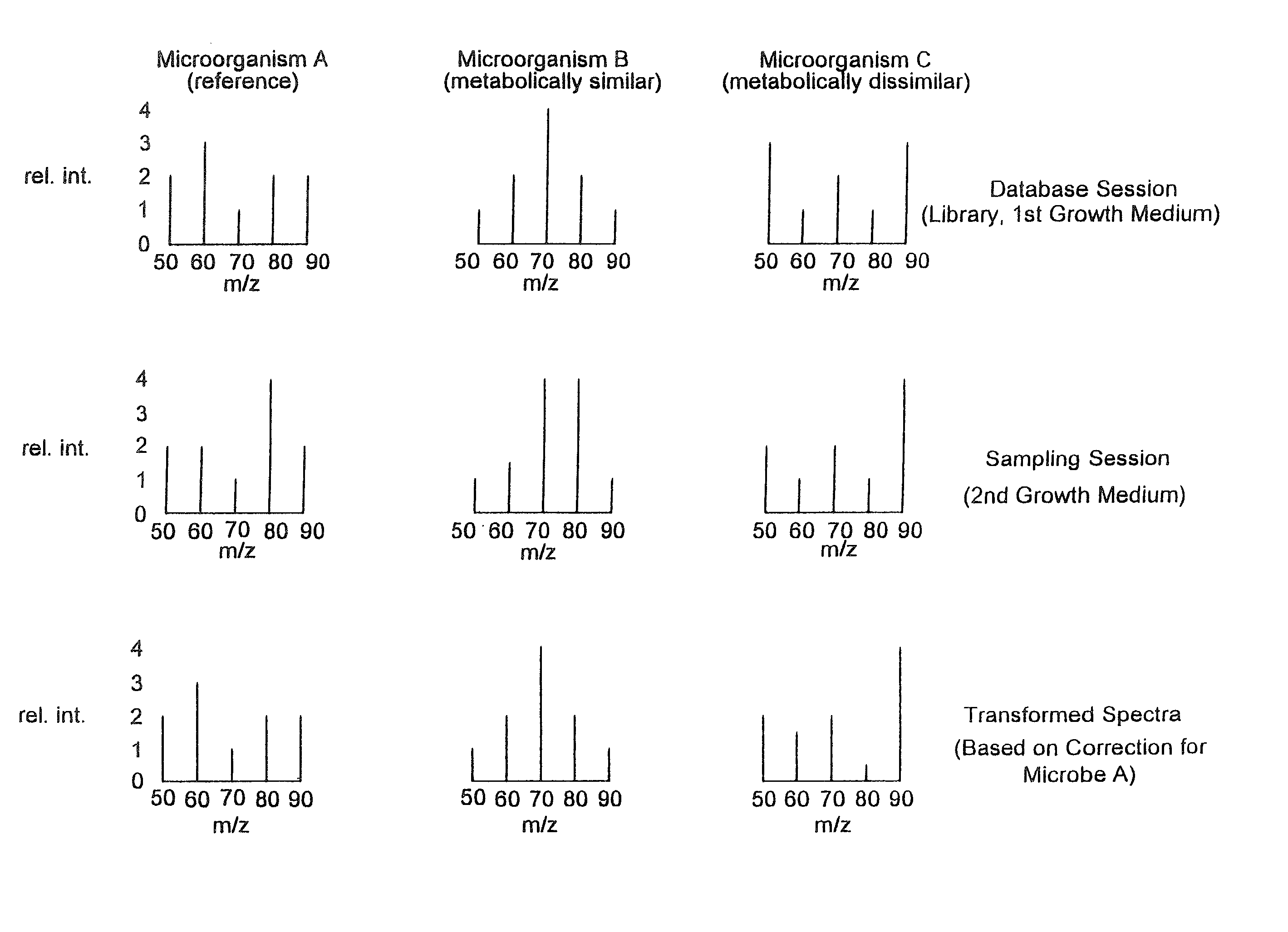
Microbial identification databases
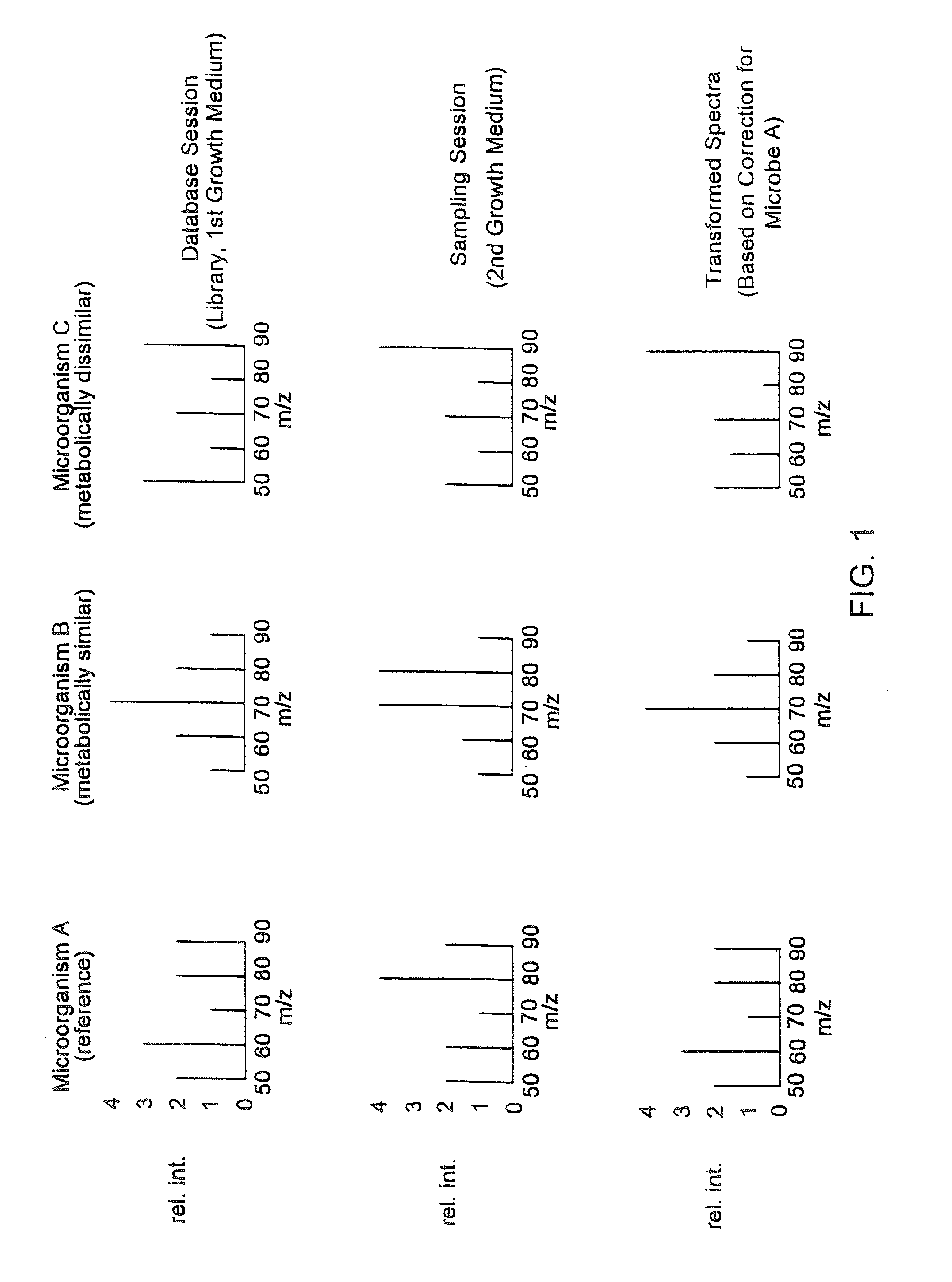
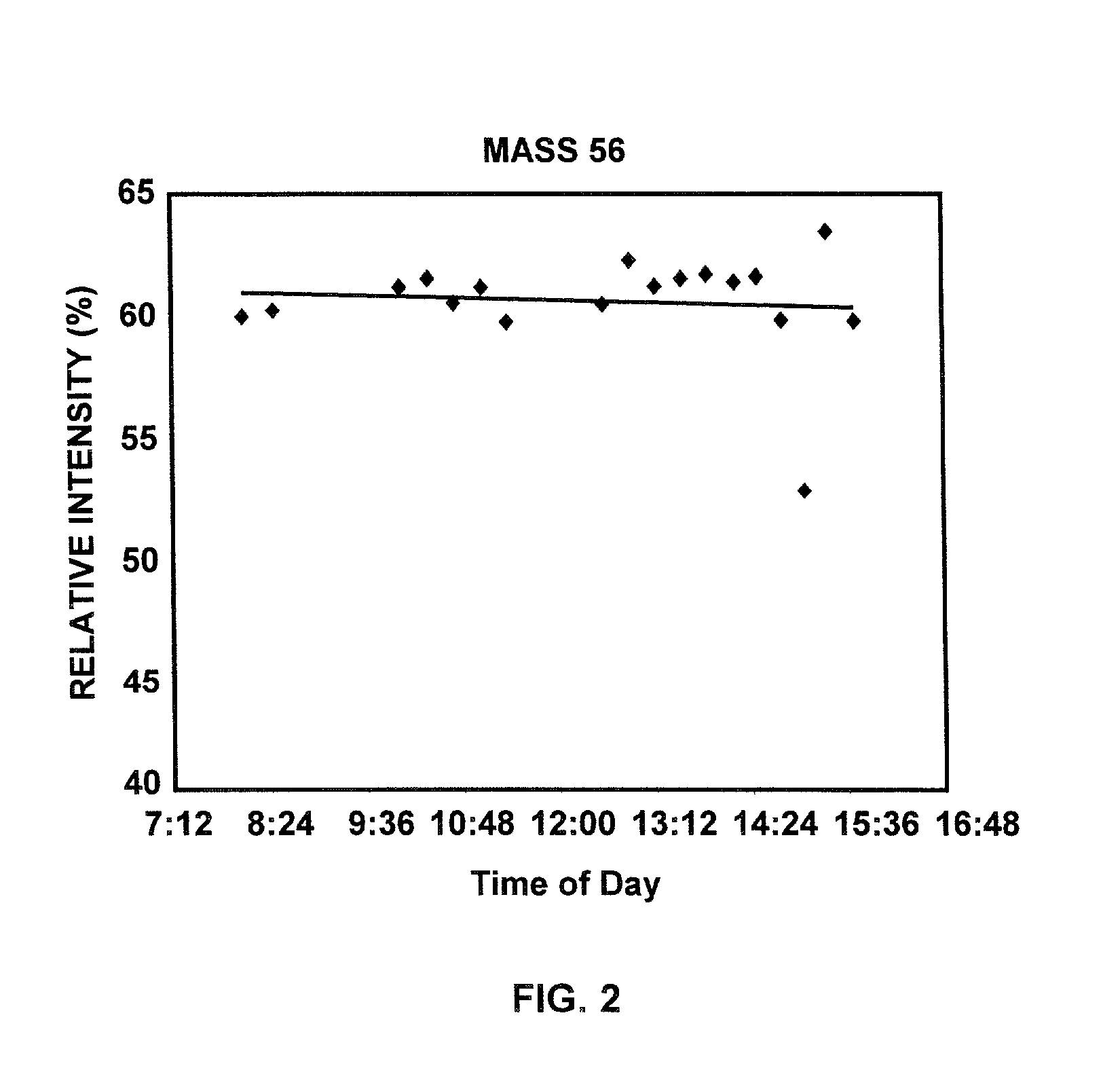
InactiveUS20020138210A1Easy mappingComponent separationBiological testingMicroorganismMass spectral database
Methods of compensating for drift in fingerprint spectra of microorganisms caused by changes in their environment are disclosed. These methods of compensating for drift permit identification of microorganisms from their fingerprint spectra regardless of the environment from which the microorganisms are obtained. Furthermore, the disclosed methods may be used to construct coherent databases of fingerprint spectra that may be expanded even though the standard database conditions are no longer experimentally achievable. In particular embodiments, methods of compensating for drift in pyrolysis mass spectra, constructing coherent pyrolysis mass spectral databases, and identifying bacteria from their pyrolysis mass spectra are disclosed.
Owner:HEALTH & HUMAN SERVICES THE GOVERNMENT OF THE US SEC THE DEPT
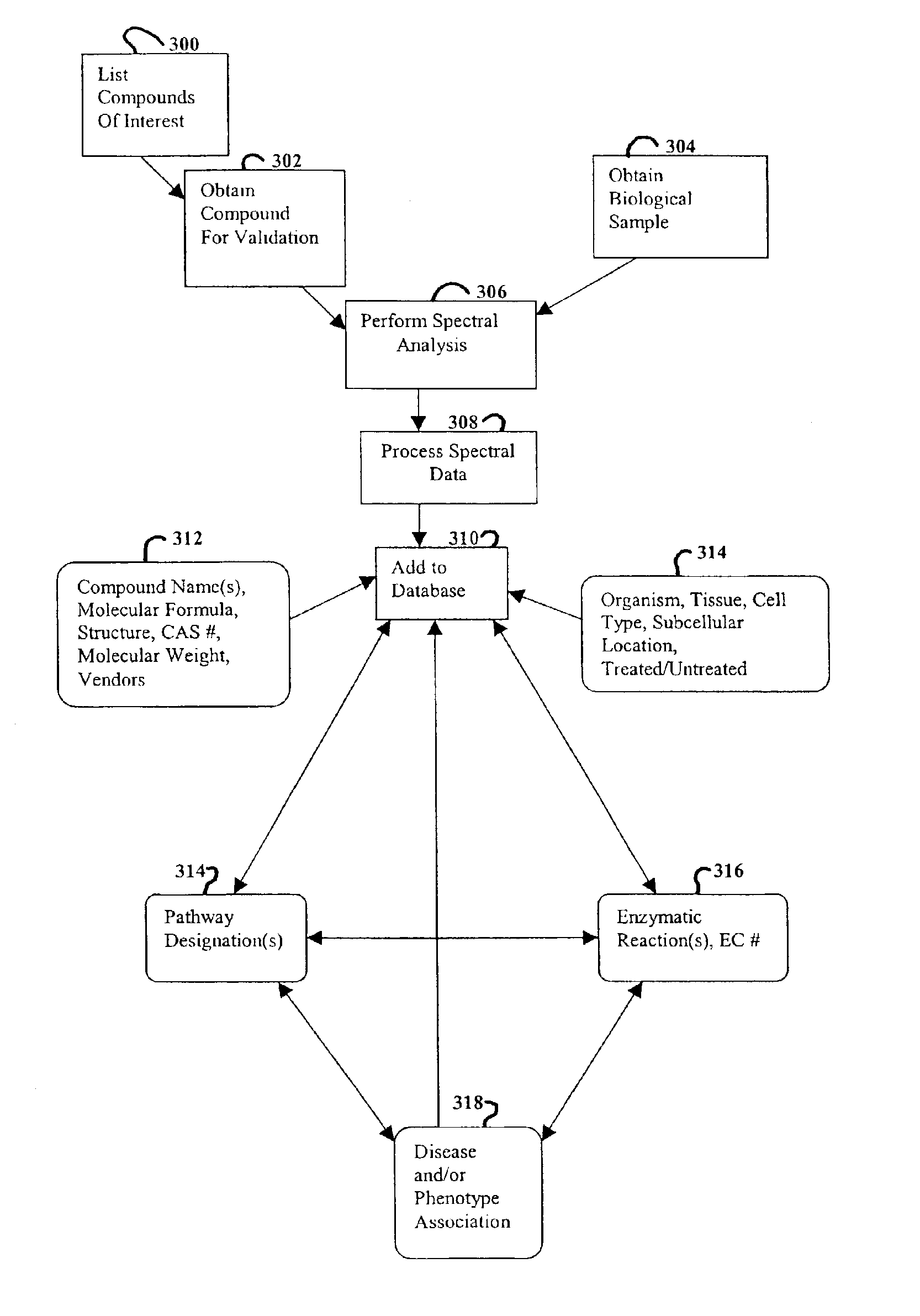
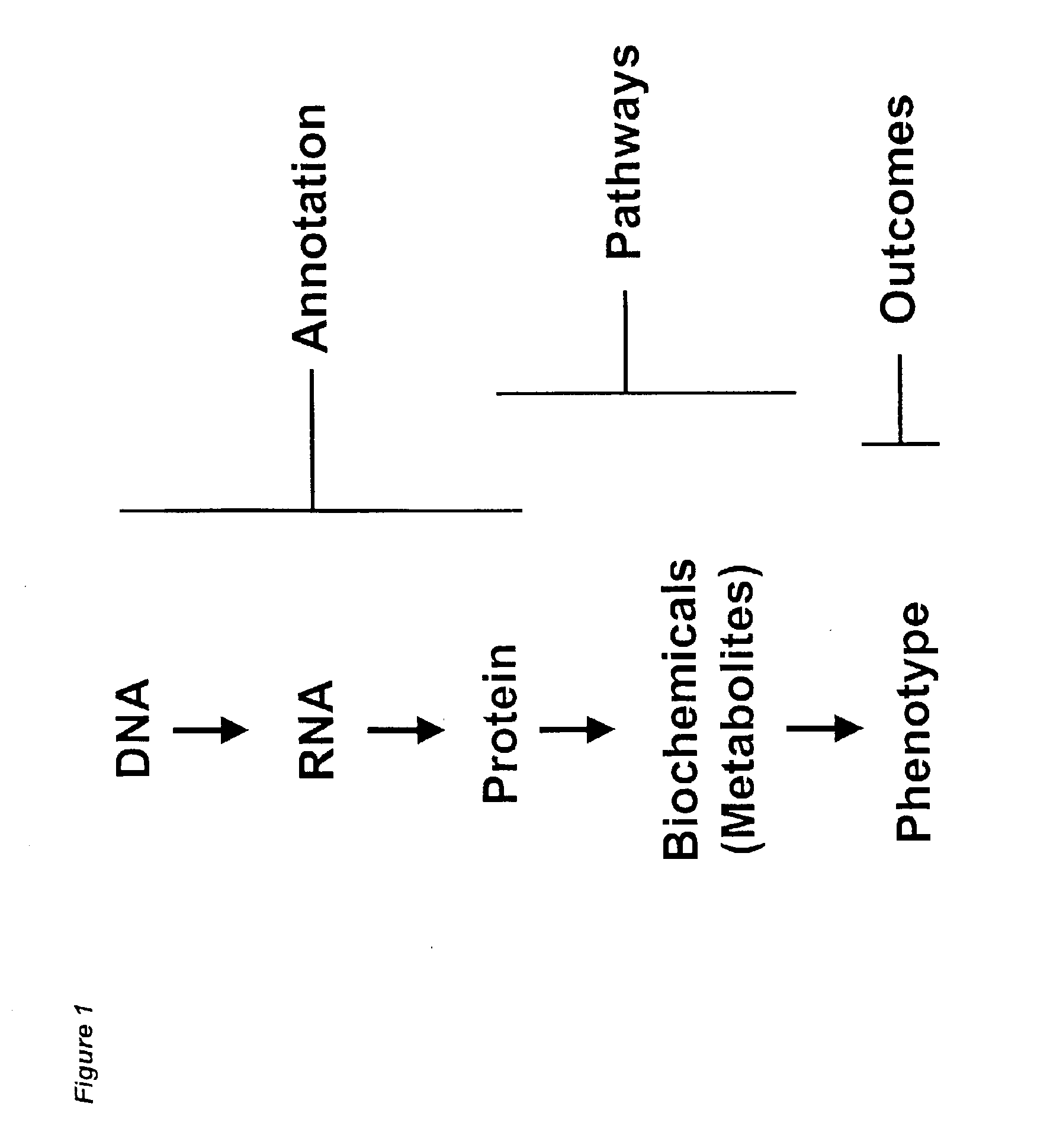
Methods and systems for analyzing complex biological systems
The present invention provides methods and systems for organizing complex and disparate data. More specifically, the present invention provides methods and systems for organizing complex and disparate data into coherent data sets. Coherent data sets resulting from the methods and systems of the present invention serve as models for biological systems. Methods and systems for integrating data and creating coherent data sets are useful for numerous biological applications, such as, for example, determining gene function, identifying and validating drug and pesticide targets, identifying and validating drug and pesticide candidate compounds, profiling drug and pesticide compounds, producing a compilation of health or wellness profiles, determining compound site(s) of action, identifying unknown samples, and numerous other applications in the agricultural, pharmaceutical, forensic, and biotechnology industries.
Owner:METABOLON
Nucleoside phosphoramidates
Disclosed herein are nucleoside phosphoramidates and their use as agents for treating viral diseases. These compounds are inhibitors of RNA-dependent 5 RNA viral replication and are useful as inhibitors of HCV NS5B polymerase, as inhibitors of HCV replication and for treatment of hepatitis C infection in mammals.
Owner:GILEAD SCI INC
Automated sampling methods for rapid characterization of polymers
InactiveUS6265226B1Avoid backlogImprove throughputSequential/parallel process reactionsComponent separationFlow injection analysisPolymer
Rapid characterization and screening of polymer samples to determine average molecular weight, molecular weight distribution and other properties is disclosed. Rapid flow characterization systems and methods, including liquid chromatography and flow-injection analysis systems and methods are preferably employed. High throughput, automated sampling systems and methods, high-temperature characterization systems and methods, and rapid, indirect calibration compositions and methods are also disclosed. The described methods, systems, and devices have primary applications in combinatorial polymer research and in industrial process control.
Owner:INTERMOLECULAR
Configurable Microfluidic Substrate Assembly
InactiveUS20080047836A1Improve design flexibilitySludge treatmentComponent separationEngineeringBiomedical engineering
A microfluidic substrate assembly includes a substrate body having at least one fluid inlet port. At least one microscale fluid flow channel in the substrate is in fluid communication with the inlet port for transport of a fluid to be tested. The substrate body also has a plurality of sockets, with each of one or sockets configured to receive an operative component. At least one socket is in communication with the microscale fluid flow channel.
Owner:PROTASIS CORP
Non-invasive detection of fetal genetic traits
ActiveUS20080071076A1Facilitates non-invasive detectionSugar derivativesOther chemical processesPregnancyNon invasive
Blood plasma of pregnant women contains fetal and (generally>90%) maternal circulatory extracellular DNA. Most of said fetal DNA contains .Itoreq.500 base pairs, said maternal DNA having a greater size. Separation of circulatory extracellular DNA of .Itoreq.500 base pairs results in separation of fetal from maternal DNA. A fraction of a blood plasma or serum sample of a pregnant woman containing, due to size separation (e.g. by chromatography, density gradient centrifugation or nanotechnological methods), extracellular DNA substantially comprising .Itoreq.500 base pairs is useful for non-invasive detection of fetal genetic traits (including the fetal RhD gene in pregnancies at risk for HDN; fetal Y chromosome-specific sequences in pregnancies at risk for X chromosome-linked disorders; chromosomal aberrations; hereditary Mendelian genetic disorders and corresponding genetic markers; and traits decisive for paternity determination) by e.g. PCR, ligand chain reaction or probe hybridization techniques, or nucleic acid arrays.
Owner:SEQUENOM INC
Electrochemical detector integrated on microfabricated capilliary electrophoresis chips
InactiveUS6045676AMinimizes the effect of interference from applied electrophoresis fieldsAccurately and conveniently placed, robust and sensitiveCellsFatty/oily/floating substances removal devicesElectrochemical detectorCapillary electrophoresis
A microfabricated capillary electrophoresis chip which includes an integral thin film electrochemical detector for detecting molecules separated in the capillary.
Owner:RGT UNIV OF CALIFORNIA
Non-affinity purification of proteins
InactiveUS7323553B2Chromatographic cation exchangersOther chemical processesProtein purificationAntibody Affinity Chromatography
The present invention relates to a method for protein purification that involves the combination of non-affinity chromatography with HPTFF.
Owner:GENENTECH INC
Reduction of migration shift assay interference
ActiveUS20050170362A1Easy to separateReduce distractionsComponent separationMicrobiological testing/measurementHigh concentrationAssay
This invention provides methods and compositions, e.g., to reduce interference from non-specific binding sample constituents in a migration shift assay. Interference due to non-specific binding of sample constituents to an affinity substance (e.g., an affinity molecule or a conjugate of an affinity molecule and a charged carrier molecule) is prevented by, e.g., binding the constituents to charged polymers such as heparin sulfate. The present invention also provides methods to concentrate an analyte of interest with high concentration and to detect the analyte with high sensitivity, and further to optimize the reaction conditions for easily concentrating the analyte. Such objects of the present invention are attained, for example, by concentrating a complex of the analyte and a conjugate which is formed by contacting the analyte in a sample with an affinity molecule bound to a charged carrier molecule such as DNA.
Owner:CAPLIPER LIFE SCI INC +1
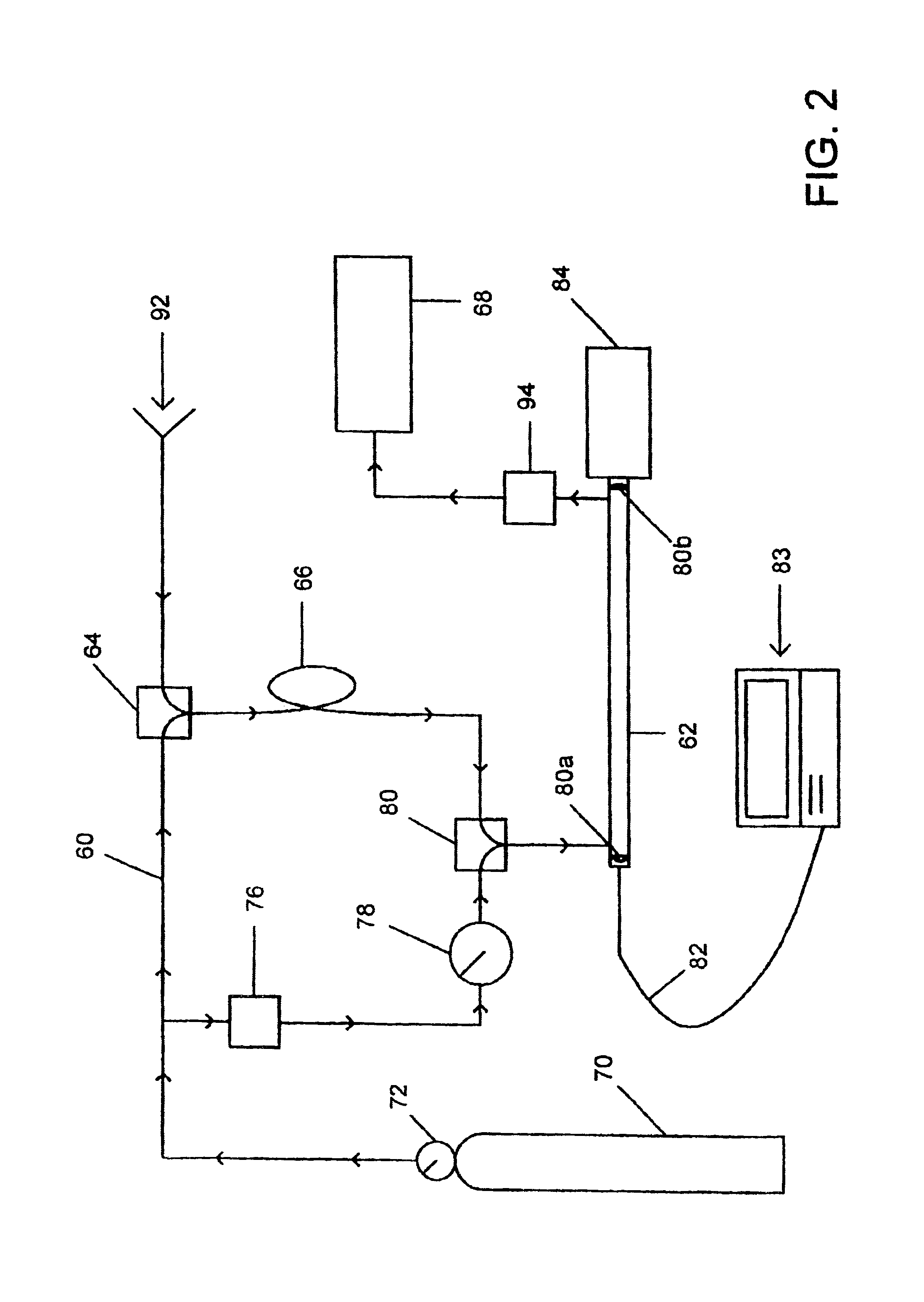
Method and apparatus for sample analysis
InactiveUS6865926B2Easy to operateAnalysing fluids using sonic/ultrasonic/infrasonic wavesComponent separationParticulatesGas analysis
Methods and systems for analyzing samples, such as gas samples, are described. One method comprises providing a gas sample, increasing pressure applied to the gas sample to compress the sample to a smaller volume and provide a pneumatically focused gas sample, and analyzing the pneumatically focused gas sample using any of a variety of analytical techniques. Also disclosed are systems for gas analysis, including systems for analysis of pneumatically focused, and thereby concentrated, gas samples and for analysis of particulate matter in gas samples. Analytical systems constructed within personal computer cases also are disclosed.
Owner:PORTLAND STATE UNIV
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com