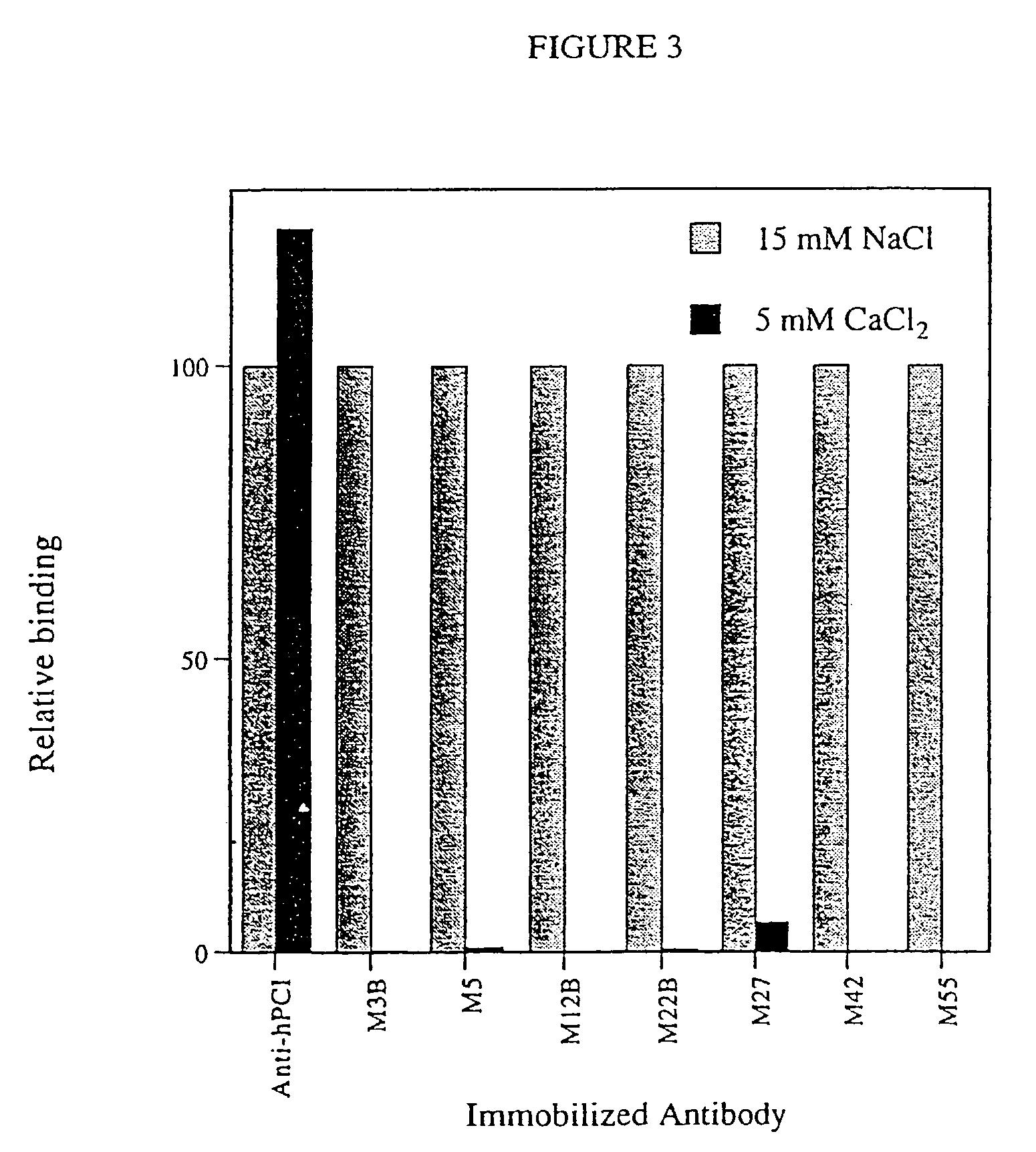
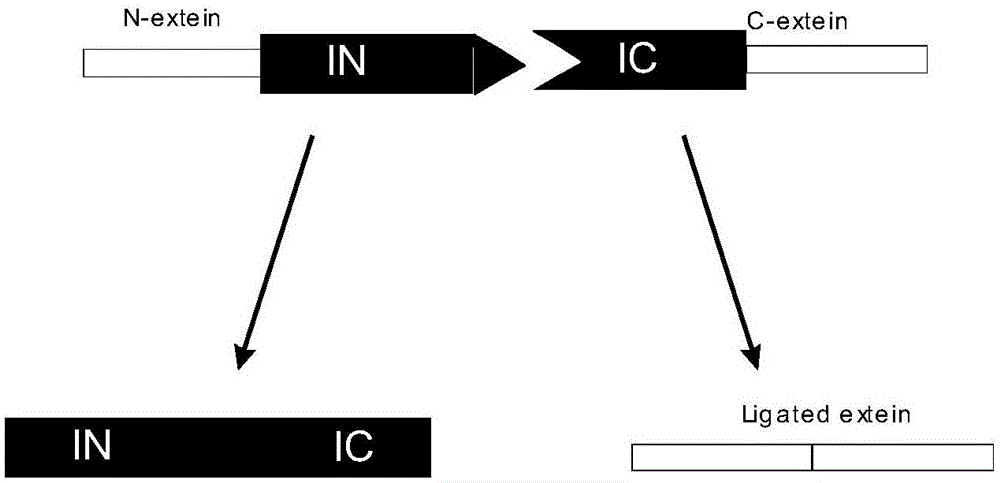
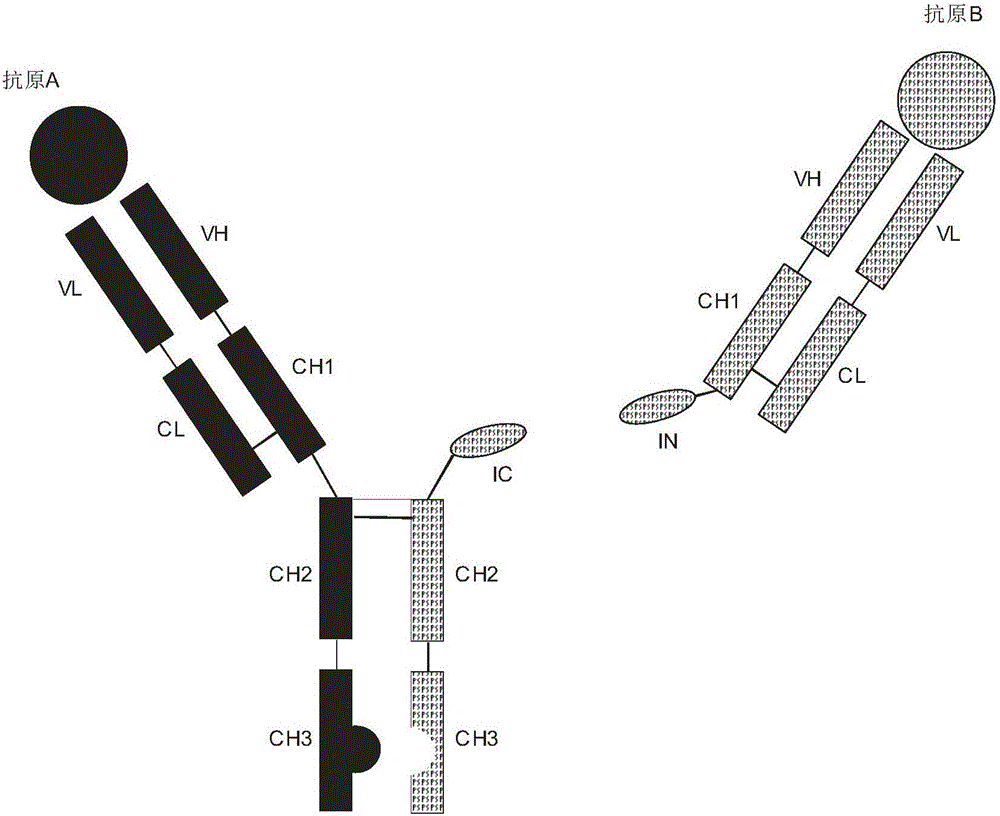
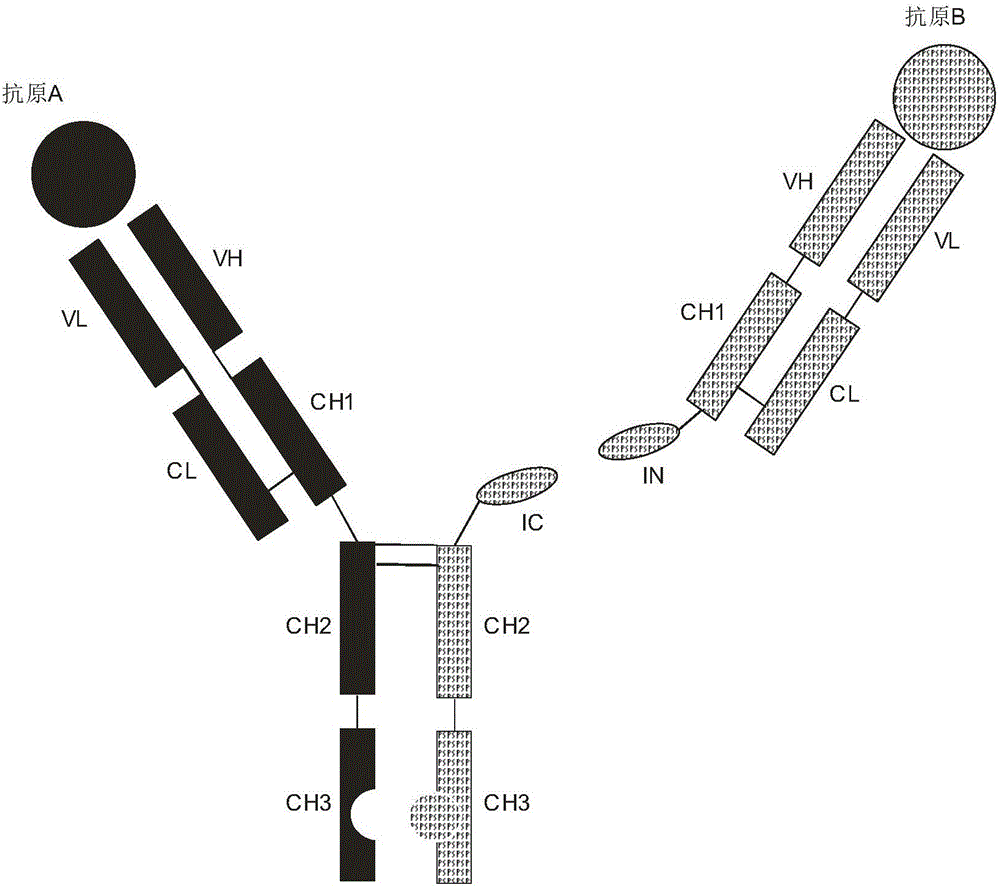
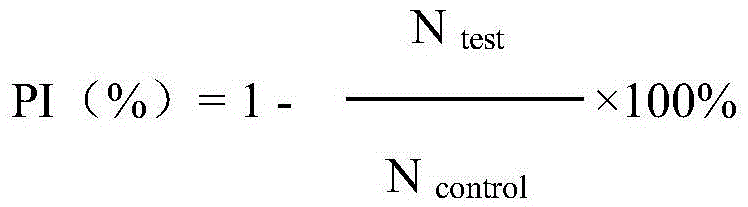Patents
Literature
116 results about "Affinity chromatography" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Affinity chromatography is a method of separating biochemical mixture based on a highly specific interaction between antigen and antibody, enzyme and substrate, receptor and ligand, or protein and nucleic acid. It is a type of chromatographic laboratory technique used for purifying biological molecules within a mixture by exploiting molecular properties. Protein could be eluted by ligand solution Biological macromolecules, such as enzymes and other proteins, interact with other molecules with high specificity through several different types of bonds and interaction. Such interactions include hydrogen bonding, ionic interaction, disulfide bridges, hydrophobic interaction, and more. The high selectivity of affinity chromatography is caused by allowing the desired molecule to interact with the stationary phase and be bound within the column in order to be separated from the undesired material which will not interact and elute first. The molecules no longer needed are first washed away with a buffer while the desired proteins are let go in the presence of the eluting solvent (of higher salt concentration). This process creates a competitive interaction between the desired protein and the immobilized stationary molecules, which eventually lets the now highly purified proteins be released.
Method for preparing high purity chymotrypsin
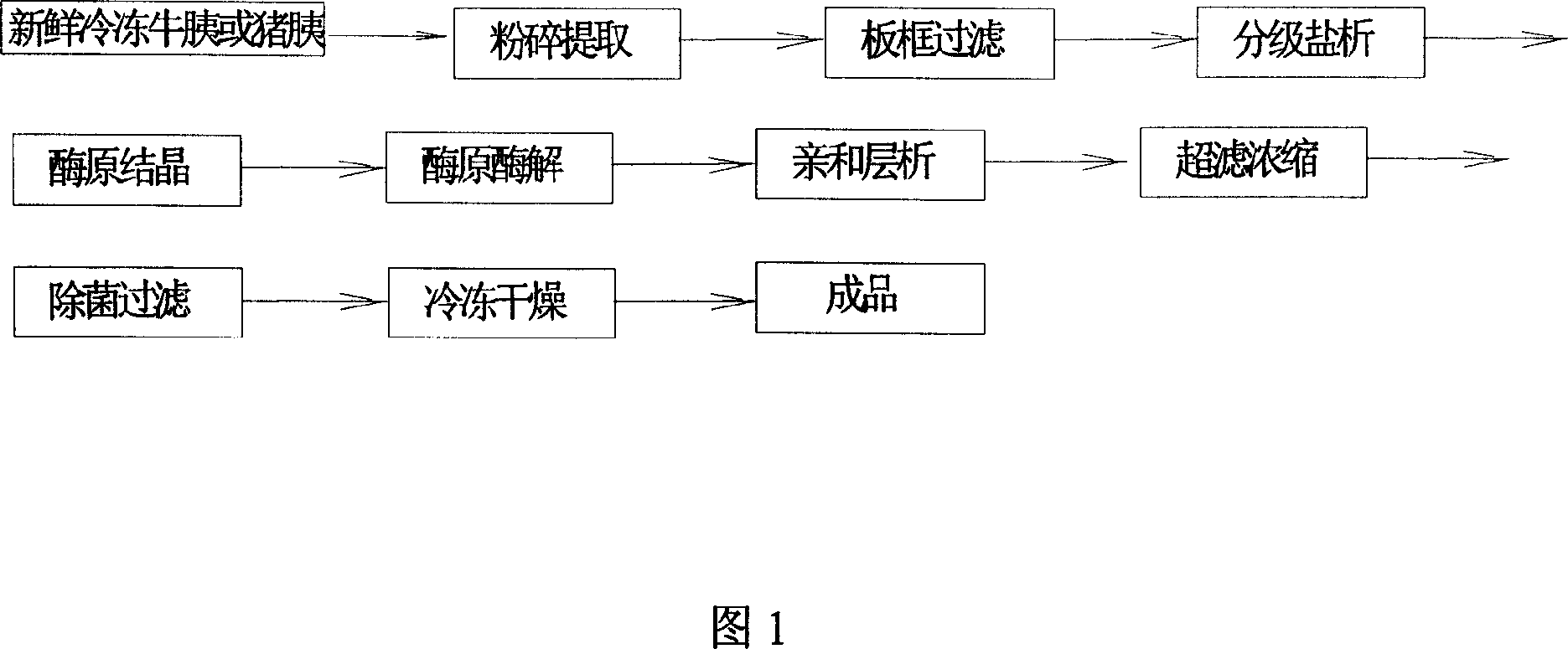
The affinity chromatographic process for producing high purity chymotrypsin includes the following technological steps: freezing fresh ox or pig pancreas as the material, crushing and extracting protein, stepped salting out and crystallizing zymogen, enzymolyzing to obtain coarse product, affinity chromatographic separating and purifying, ultrafiltering and concentrating sterilizing, and vacuum freeze drying to obtain product. Compared with available technology, the present invention has the advantages of suitability for large scale produce, high efficiency, specificity, low cost and high product quality.
Owner:宁波林叶生物科技有限公司
Stimulus responsive affinity chromatographic material and separation/purification method
InactiveUS20040039177A1Easy to produceIon-exchange process apparatusOther chemical processesProduction ratePurification methods
A separation material characterized by supporting, in its surface, a linear or crosslinked stimulus responsive polymer to which a substance A having specific affinity for a target substance and a low-molecular substance B having specific affinity for the substance A have been bonded. Such a separation material can increase the recovery of a target substance to be purified and do not require desalting and hapten sugar-eliminating operations and, in addition, have high productivity.
Owner:GE HEALTHCARE BIO SCI CORP
Selective bond reduction in microfluidic devices
InactiveUS20120184046A1Selectively reducing bondingOvercome limitationsValve arrangementsComponent separationPlanar substrateEngineering
The invention overcomes the limitations described for the bonding of structured layers by providing a method for selectively reducing the bonding of materials. In its most generic form, the invention uses a bonding technique in combination with a printing method for modifying or covering at least one portion of a surface to either fully or partially prevent localised bonding. The structuring process may act upon the layers either before or after the bonding of the layers. The invention overcomes the limitations described in the application of affinity chromatography by providing a planar substrate with discrete optical detection flow cells that contain porous material and have connecting microchannels for fluid delivery and / or removal, and a method for making the same.
Owner:ATKIN MICAH
Affinity chromatography matrix
InactiveUS20120149875A1High binding capacityEasy to eluteSolid sorbent liquid separationPeptide preparation methodsChemistryAffinity chromatography
The present invention relates to a method of separating one or more immunoglobulin containing proteins from a liquid. The method includes first contacting the liquid with a separation matrix comprising ligands immobilised to a support; allowing the immunoglobulin containing proteins to adsorb to the matrix by interaction with the ligands; followed by an optional step of washing the adsorbed immunoglobulin containing proteins; and recovering said immunoglobulin containing proteins by contacting the matrix with an eluent which releases the proteins. The method improves upon previous separation methods being that each of the ligands consists essentially of a monomer or dimer SpA or protein Z or a functional variant thereof.
Owner:GE HEALTHCARE BIO SCI CORP
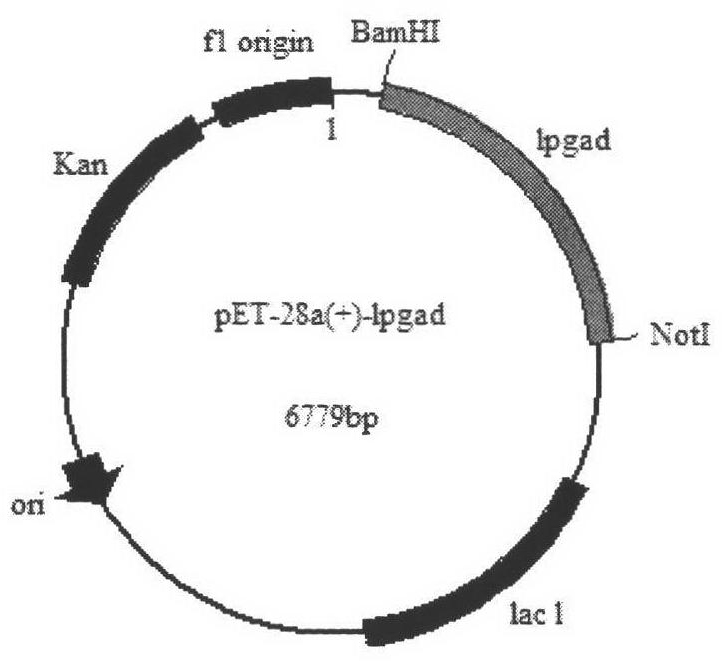
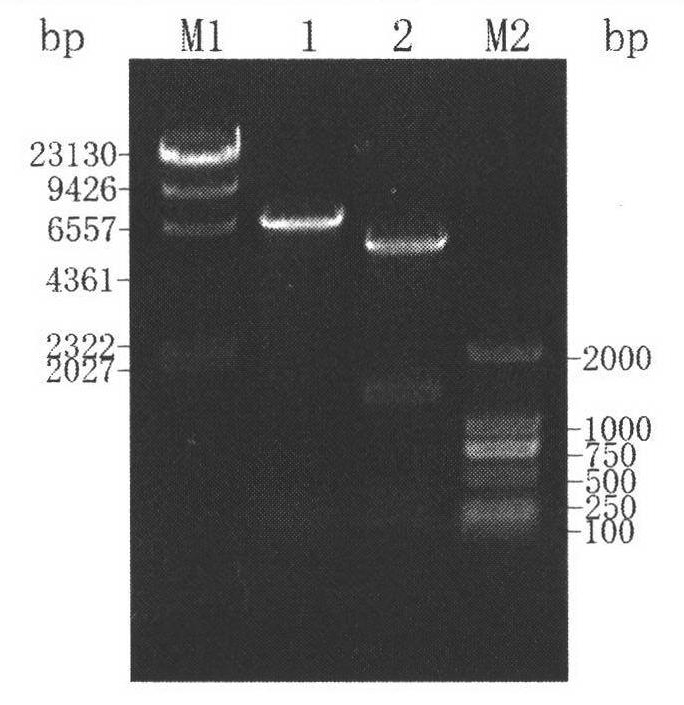
Construction method and application of high-yield gamma-aminobutyric acid recombinant escherichia coli/pET-28a-1pgad
ActiveCN102367432AHigh expressionThe high expression level is controlled by the metabolic state of the bacteria, the expression levelBacteriaMicroorganism based processesEscherichia coliGlutamate decarboxylase
The invention relates to a construction method and an application of high-yield gamma-aminobutyric acid recombinant escherichia coli / pET-28a-1pgad, in particular to a genetic engineering bacterium construction method, recombinant enzyme enzymology property study and an application to the conversion of L-glutamic acid for producing gamma amino butyric acid (GABA), which belongs to the technical field of biology in the fermentation engineering. Firstly, lactobacillus plantarum GB 01-21 glutamic acid decarboxylase (GAD) genes are obtained through polymerase chain reaction (PCR) amplification, recombinant plasmids pET-28a-1pgad are constructed, in addition, the successful expression is realized in E.coli BL21(ED3), secondly, Ni column affiliation chromatography purification is adopted on crude enzyme liquid for obtaining recombinant GAD, in addition, the enzymology property of the recombinant GAD is primarily studied for guiding the optimization of conversion conditions, finally, conversion experiments are carried out on a 5L fermentation tank, the GABA accumulation concentration can reach 204.5g / L, the mol conversion rate is 97.92 percent, and good foundation is made on the further industrial application.
Owner:JIANGNAN UNIV
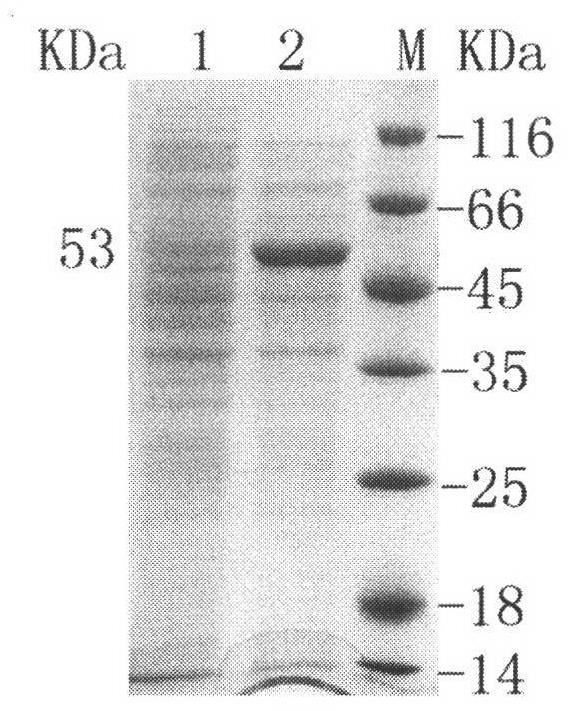
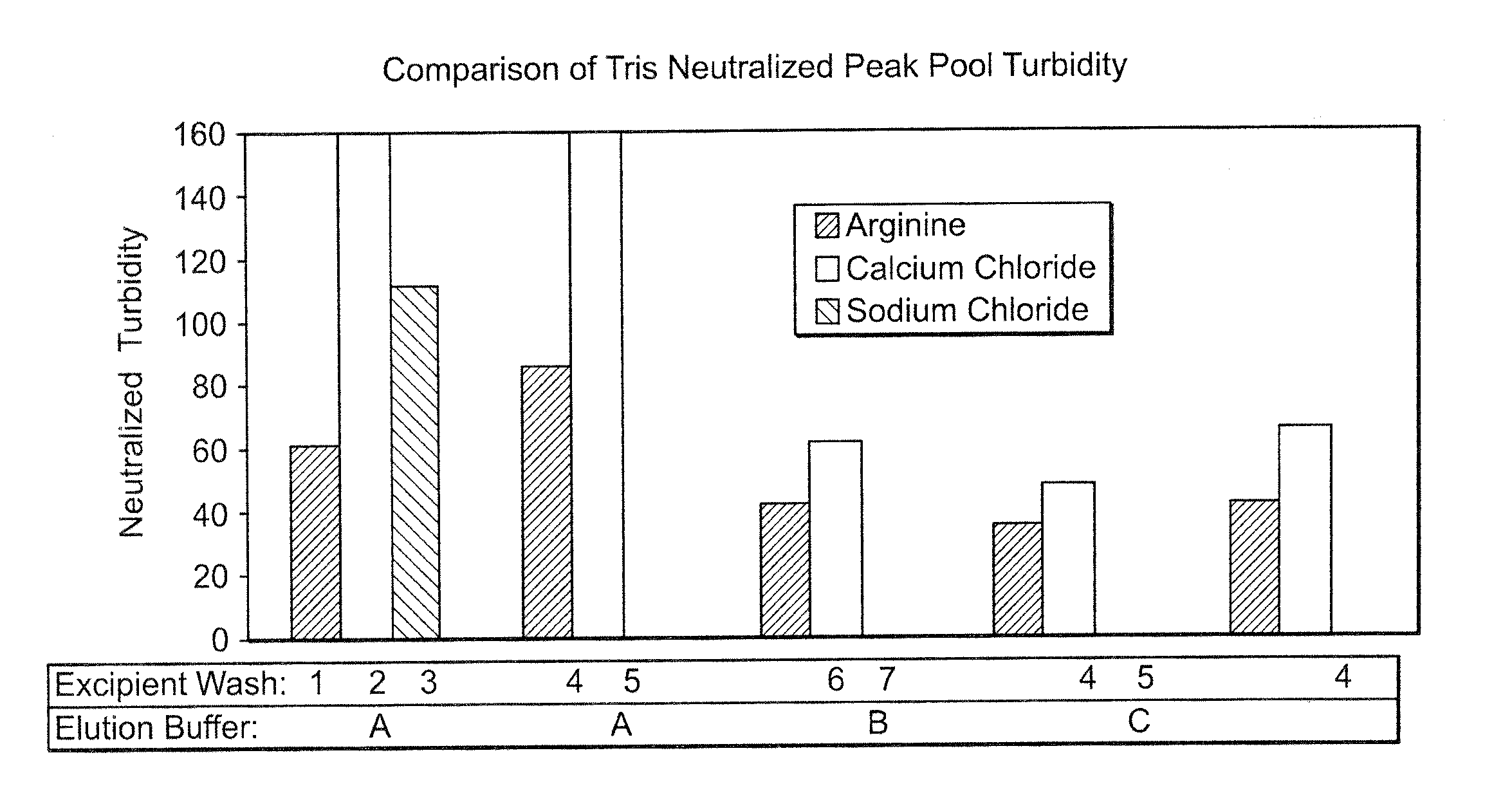
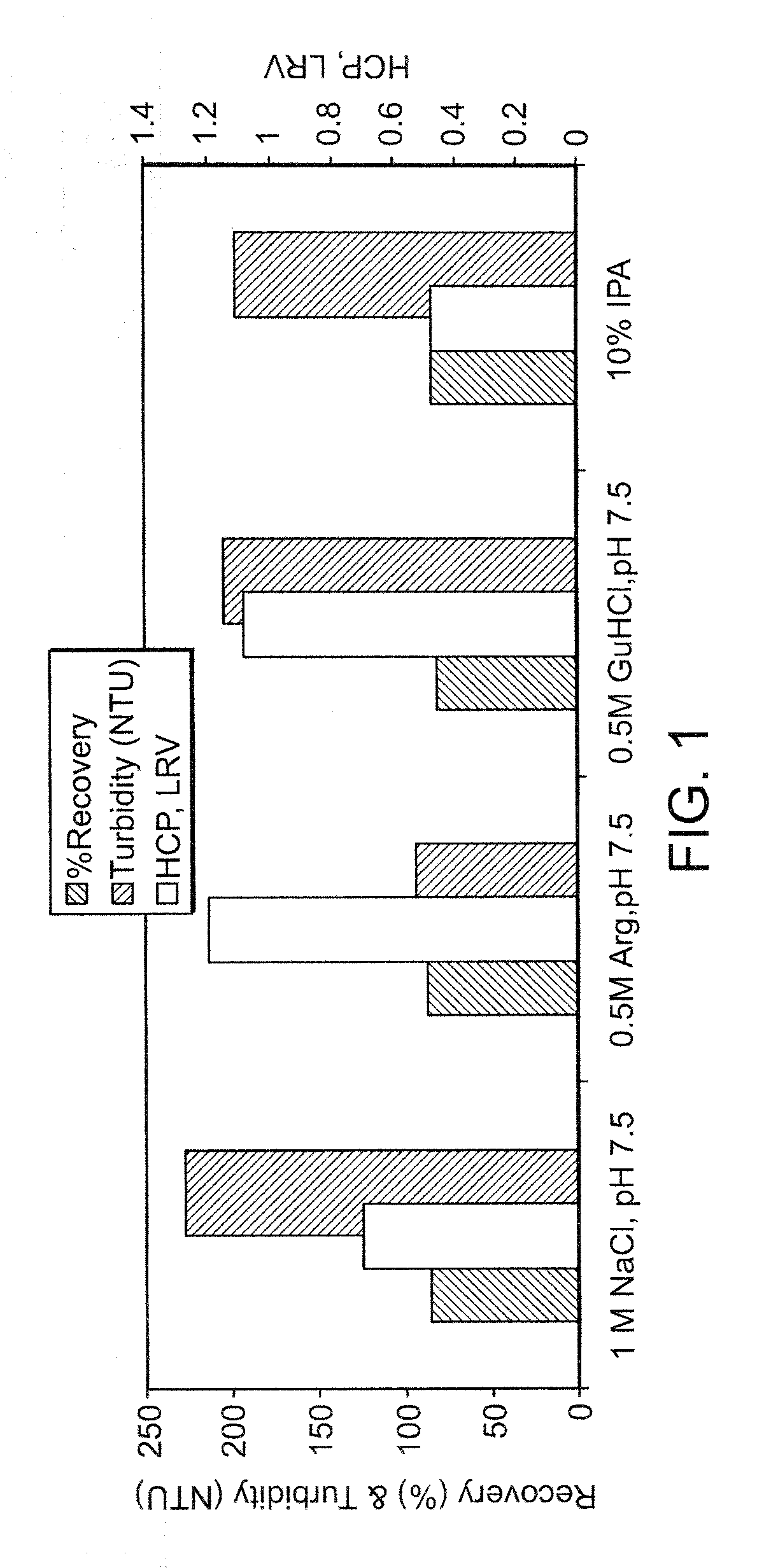
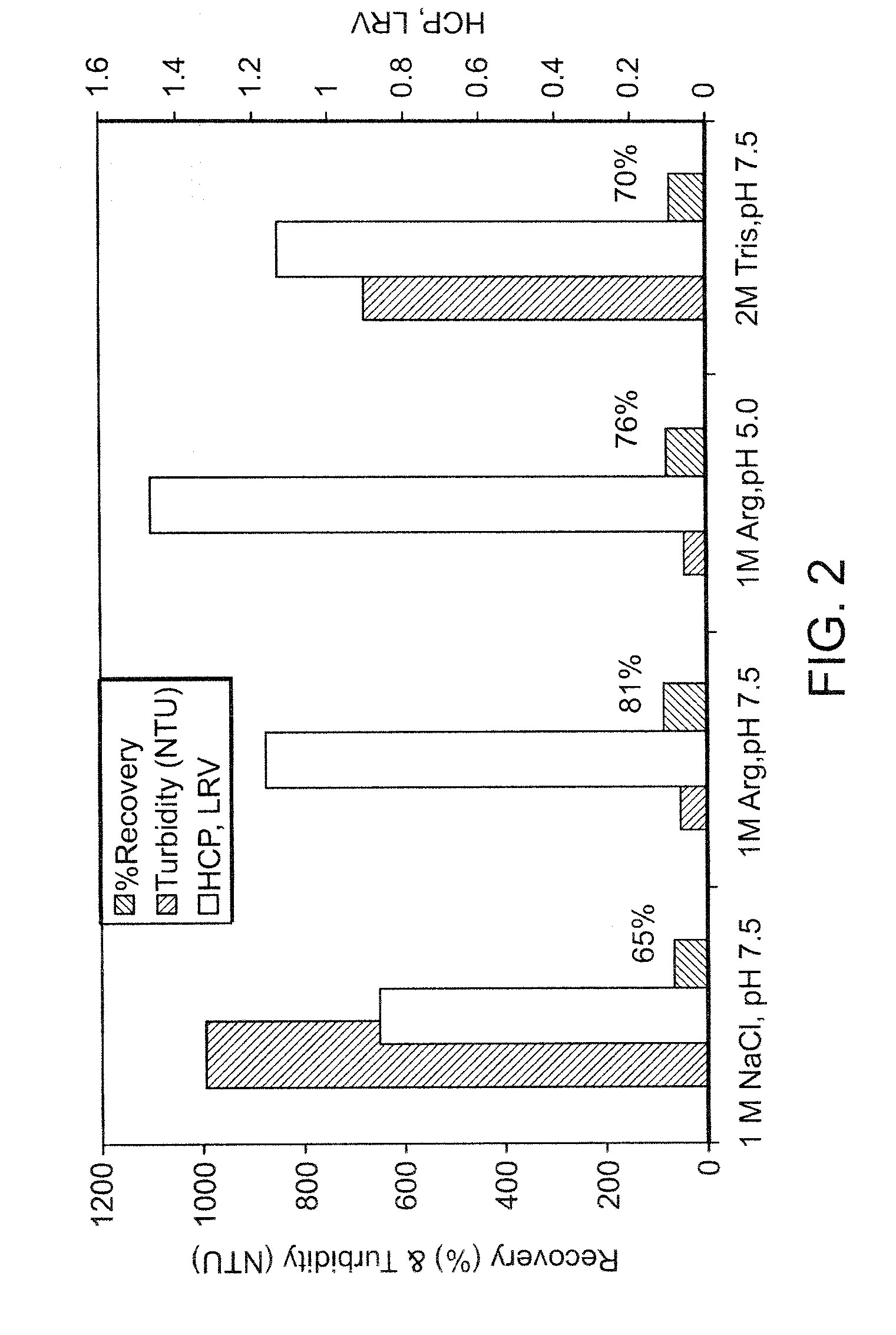
Arginine wash in protein purification using affinity chromotography
ActiveUS20080064861A1Reduce turbidityRaise the ratioAntibody ingredientsPeptide preparation methodsArginineTurbidity
The invention relates to methods for isolating a product and / or reducing turbidity and / or impurities from a load fluid comprising the product and one or more impurities by passing the load fluid through a medium, followed by at least one wash solution comprising arginine, and collecting the product using an elution solution. The invention further relates to a product prepared using a method as described herein.
Owner:WYETH LLC
Preparation of recombinant polypeptide by intein with trans-splicing function
InactiveCN101884910AAvoid pollutionLow costIon-exchange process apparatusOther chemical processesCross-linkProtein insertion
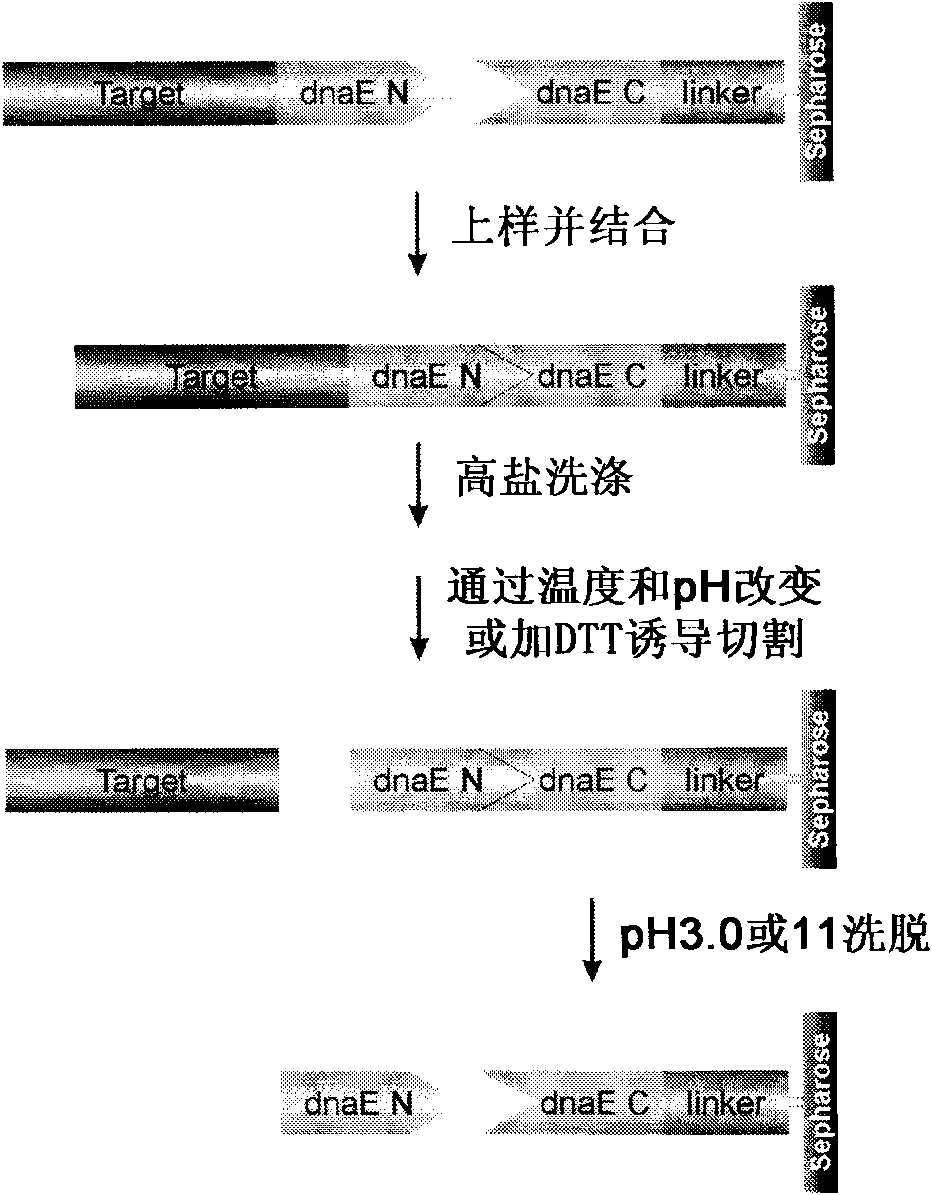
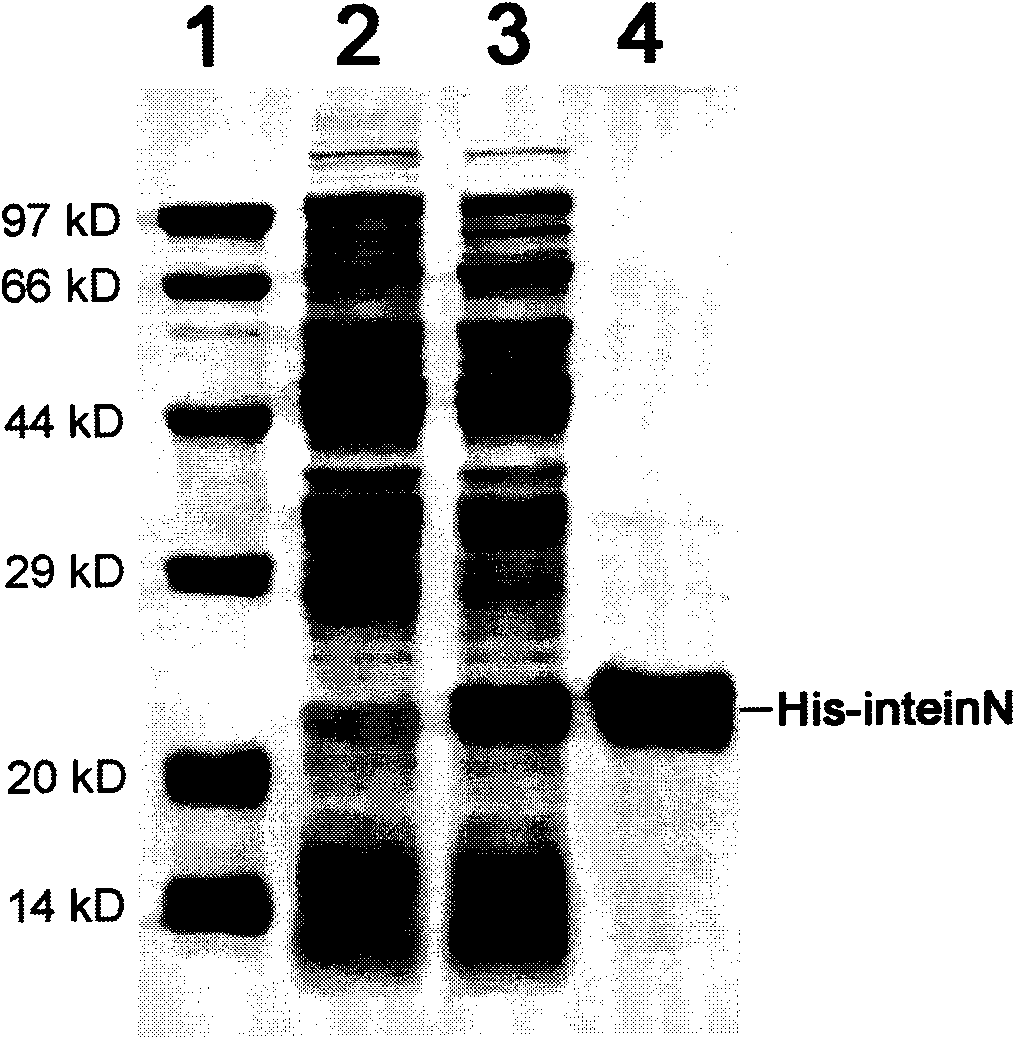
The invention provides preparation of recombinant polypeptide by an intein with a trans-splicing function, wherein the N-end splicing structure domain and the C-end splicing structure domain of the intein with the trans-splicing function can be specifically bound together. One splicing structure domain (the N- or C-end splicing structure domain) of the intein is taken as carrier protein to carry out fusion expression with target polypeptide; the other splicing structure domain (the C- or N-end splicing structure domain) is cross-linked with a supporting medium to prepare an affinity column for adsorbing and purifying the obtained fusion protein containing the target polypeptide; impurities in a fusion protein sample are removed by increasing the salinity of washing liquid in the affinity chromatographic column; and trans-splicing of the intein is induced by changing the pH and the temperature of the chromatographic column or adding a chemical reagent containing a sulfydryl group so as to release the target polypeptide from the fusion protein and purify the target polypeptide at the same time.
Owner:NANJING UNIV
Fusion protein with anti-tumor, anti-inflammation and oculopathy-treatment functions and preparation method and application thereof
ActiveCN105418769AInhibition of newbornsImprove ductilitySenses disorderPeptide/protein ingredientsTumor angiogenesisHalf-life
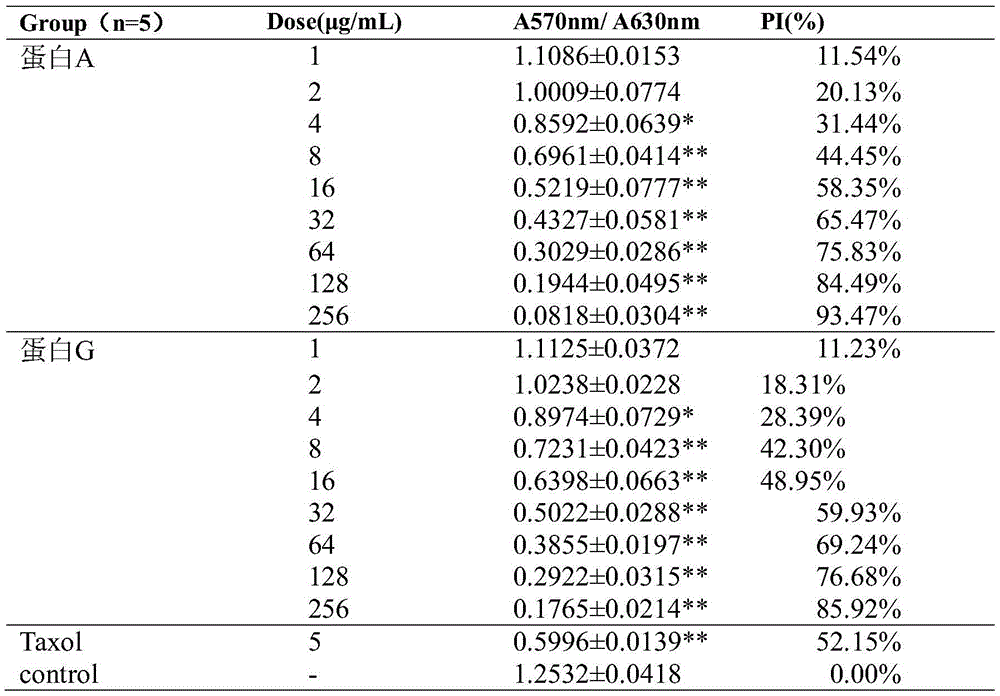
The invention discloses fusion protein with anti-tumor, anti-inflammation and oculopathy-treatment functions and a preparation method and application thereof, which belong to the technical field of biological pharmacy. Specifically, the invention relates to the fusion protein of an integrin blocking agent, which has the function of inhibiting tumor angiogenesis and has the integrin affinity and the combining capacity. A rigid (R) or flexible (F) linker is adopted to fuse two polypeptides, so as to respectively obtain protein A and protein G, the medicine effect can be increased, the half-life period can be extended, the stability can be enhanced, and the fusion protein has the characteristics of strong action effect, low toxicity and the like and can be used for preventing and treating solid tumors, all kinds of inflammation and angiogenesis oculopathy. The fusion protein structurally comprises two angiogenesis inhibiting polypeptides and the amino acid linker between the two angiogenesis inhibiting polypeptides, and the fusion protein is expressed in colibacillus or eukaryocyte by using a genetic engineering method and is obtained through separation and purification of a GST (Glutathione S Transferase) affinity column.
Owner:CHINA PHARM UNIV
Integrated system for liquid separation and electrospray ionization
ActiveUS9459240B2Easy temperature controlAmbient temperature is stableComponent separationIon sources/gunsESI mass spectrometryElectricity
The present invention relates to an integrated system for liquid separation, such as LC, CE, affinity chromatography, and ion exchange chromatography. A preferred aspect relates to a specialized protection means for protecting the fragile electrospray needle when not in use. Another preferred aspect relates to a specialized electrical contact means for applying voltage to the electrospray needle. The invention comprises an integrated system for liquid separation and electrospray ionization comprising: a separation column; and an electrospray emitter connected with the separation column. In one aspect there is a retractable protective sleeve (8) for covering and supporting the electrospray emitter (2) along at least a portion of its axis. In another aspect there is an electrically conducting sheath surrounding the emitter and providing an electrical connection.
Owner:PROXEON BIOSYST
Preparation method of immune nanometer gold test paper strip for fast detecting furazolidone
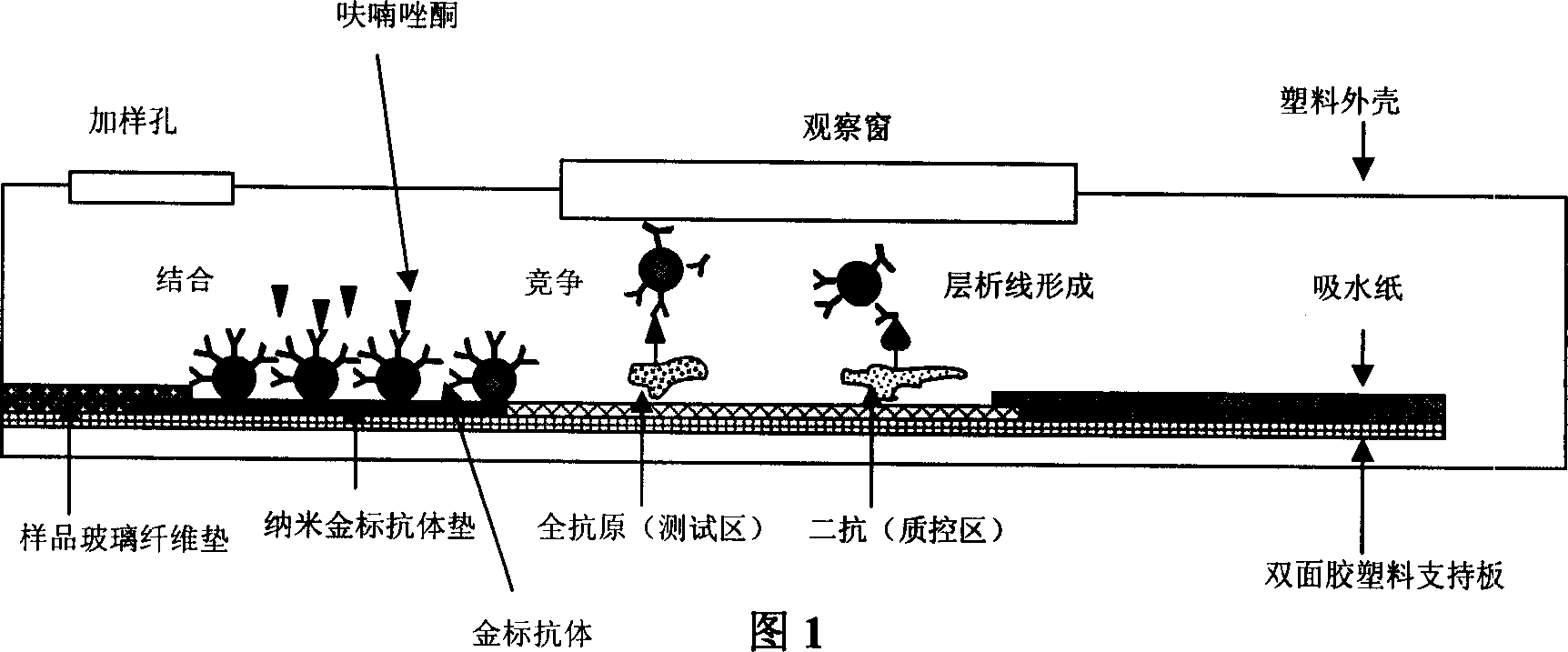
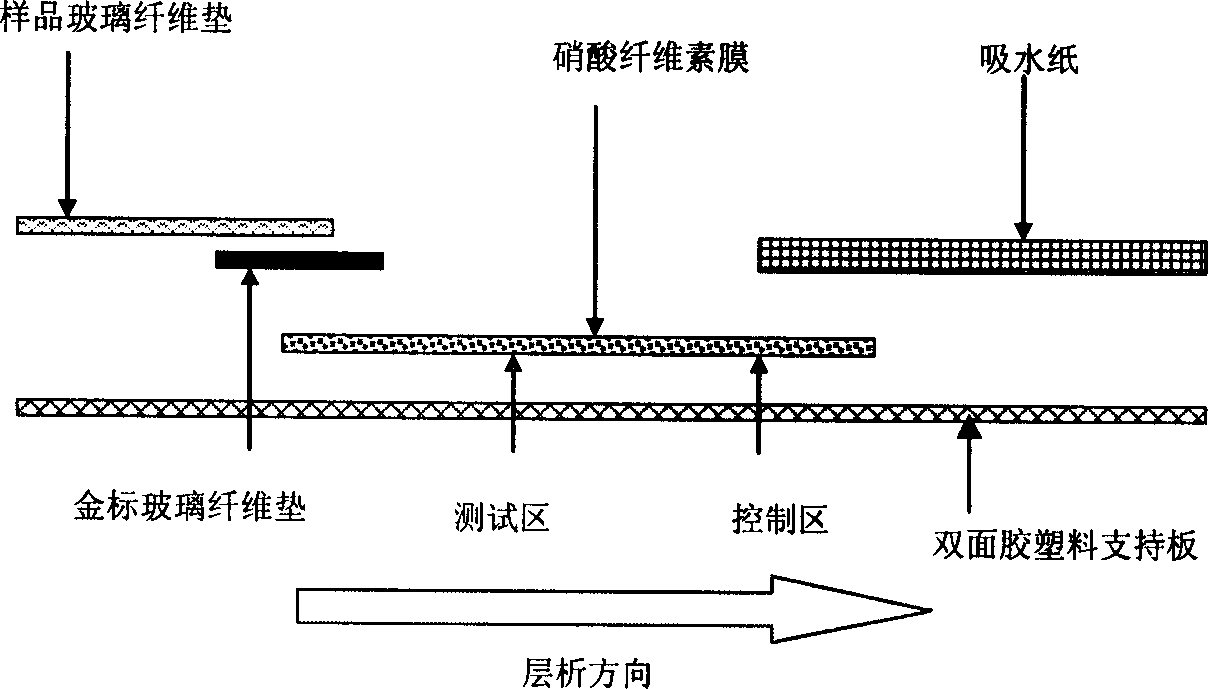
InactiveCN1793926AHigh specificityShorten detection timeMaterial analysis by observing effect on chemical indicatorPolyclonal antibodiesAntibody Affinity Chromatography
A method for preparing immune nanogold test paper used to quickly detect furazolidone includes synthesizing coupled matter of flurazolidone ¿C cattle seralbumin and coupled matter of flurazolidone ¿C catalase ; using coupled matter of flurazolidone ¿C cattle seralbumin as immune total antigen; making immune experiment on rabbit to obtain multiple clone antibody of rabbit resistance furazolidone; preparing nanogold reagent being used to label said antibody; fixing IgG antibody and coupled matter of furazolidone ¿C catalase on nitrocellulose film; sealing unreacted active group; using above ¿C prepared film, antibody and paper as well as sample pad to form said test paper.
Owner:SHANGHAI JIAO TONG UNIV
Antibodies binding a gamma carboxyglutamic acid displaying epitope
InactiveUS7439025B2Quick selectionComponent separationHybrid cell preparationTissue extractsImmunoprecipitation
Antibodies are described, which are suitable for identification of γ-carboxyglutamic acid (Gla), said antibodies having an ability of identifying Gla residues in proteins and / or peptides, while not reacting with glutamic (Glu) residues in corresponding proteins and / or peptides. Methods for the preparation and identification of said antibodies as well as methods for detection of Gla in biological fluids, tissue extracts, tissue specimens, in which methods said antibodies are used, are also described. There is also described the use of said antibodies for immunopurification of Gla-containing proteins or peptides by, for instance, immunoprecipitation or immunoaffinity chromatography.
Owner:BIOMEDICA DIAGNOSTICS INC +1
Expression and preparation methods for bivalent bispecific antibody and hybrid protein
ActiveCN106397599AExtended half-lifeThe structure is complete and stableHybrid immunoglobulinsPolypeptide with affinity tagEucaryotic cellIntein
The invention discloses expression and preparation methods for a bivalent bispecific antibody and a hybrid protein and belongs to the technical field of biology. According to the methods, the aim of preparing products, i.e., the bivalent bispecific antibody and the immune hybrid protein thereof are prepared through expressing all parts of the bivalent bispecific antibody and the immune hybrid protein thereof separately in appropriate procaryotic or eucaryotic cell systems, carrying out high-performance affinity-chromatography purification and separation, and then, carrying out splicing in vitro under the condition of intein splicing. The methods have high production efficiency, are wide in applicable application range and facilitate the separation and purification of the products. The products prepared by using the methods have biological activities, i.e., bivalent bispecific immunoaffinity and cytotoxicity. The methods are novel methods for preparing biological drugs for directional attack on cancers or other diseases.
Owner:SHANGHAI JIAO TONG UNIV +2
Production method of recombinant fusion protein of human serum albumin-interferon alpha 2b
InactiveCN1807646AReduce preprocessingConvenient purification workFungiFermentationHigh densityIon exchange
The invention discloses a purified rHSA-IFN alpha2b producing method of pichia fermentation liquor from expressing and restructuring albuminar-interferon alpha2b anastomosing protein, which comprise the following steps: fermenting and expressing high density pichia engineering bacteria of protein gene by integrated albuminar-interferon alpha2b anastomosing; combinating and purifying sequentially fermentation liquor with rHSA-IFN alpha2b with affinity column chromatography, hydrophobic column chromatography, ion exchange column chromatography and gel column chromatography; Getting high-purity medicinal recombination albuminar- interferon alpha2b anastomosing protein by large scale preparing. The invention operates simply and convenience.
Owner:HANGZHOU JIUYUAN GENE ENG
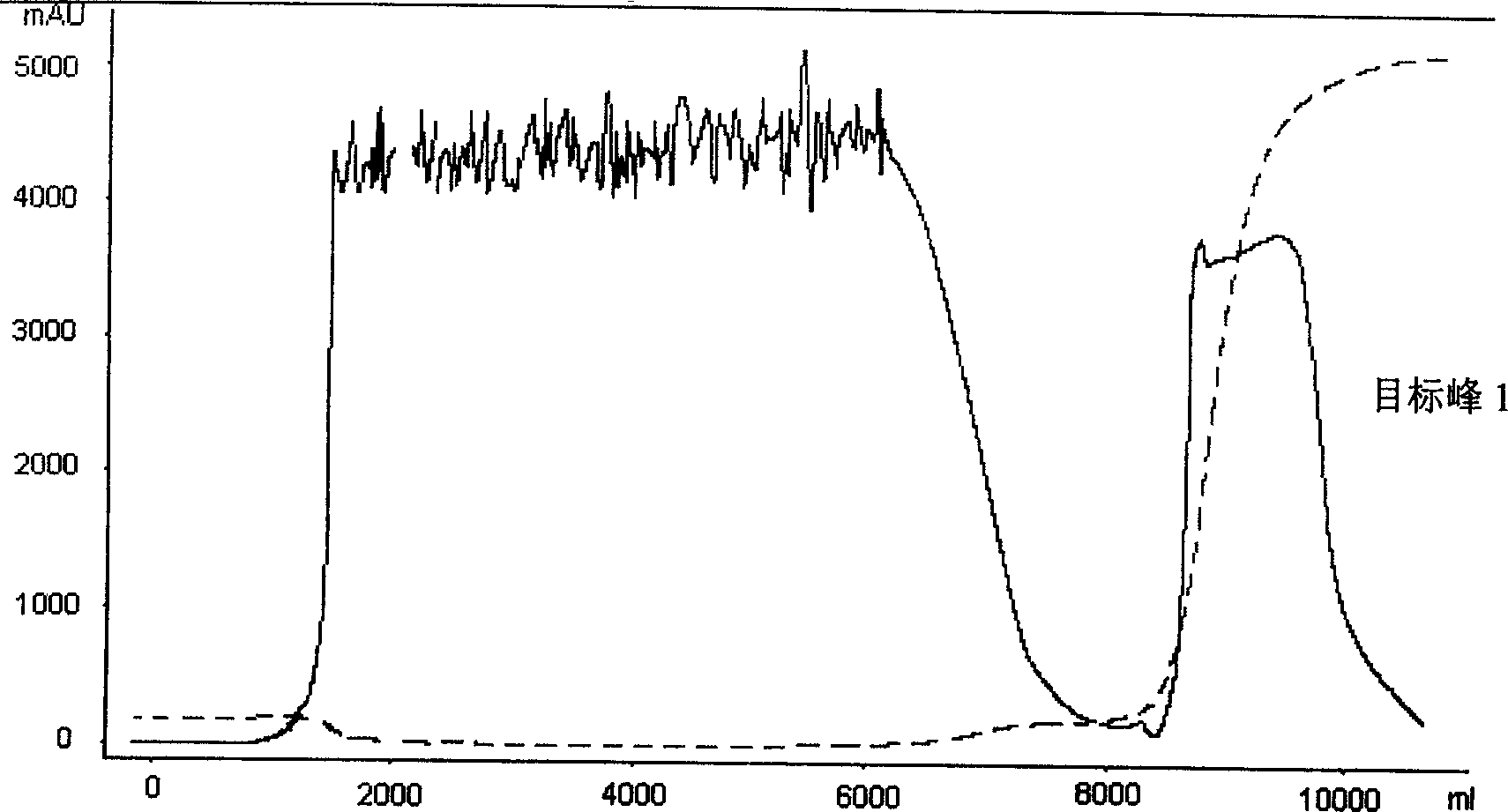
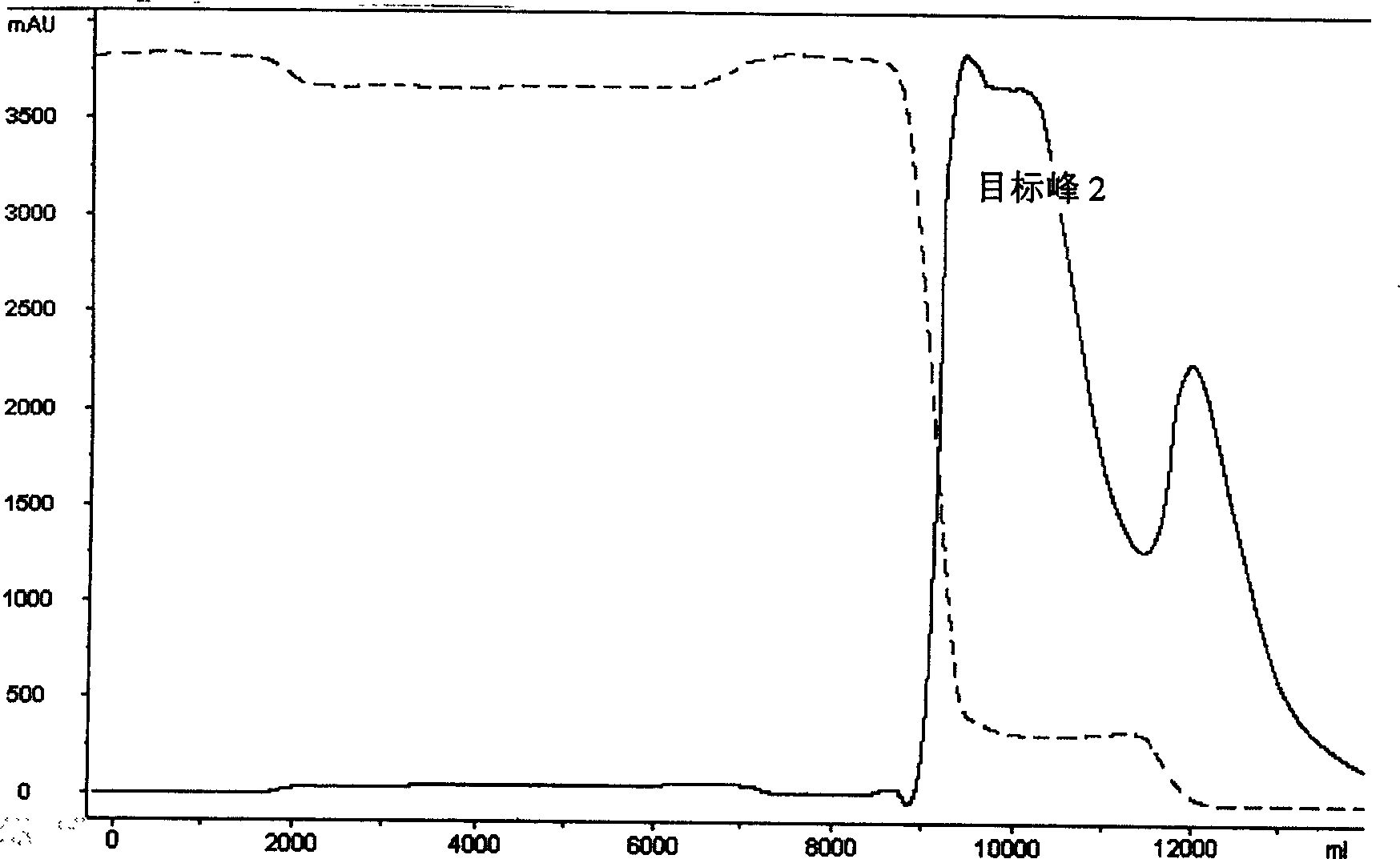
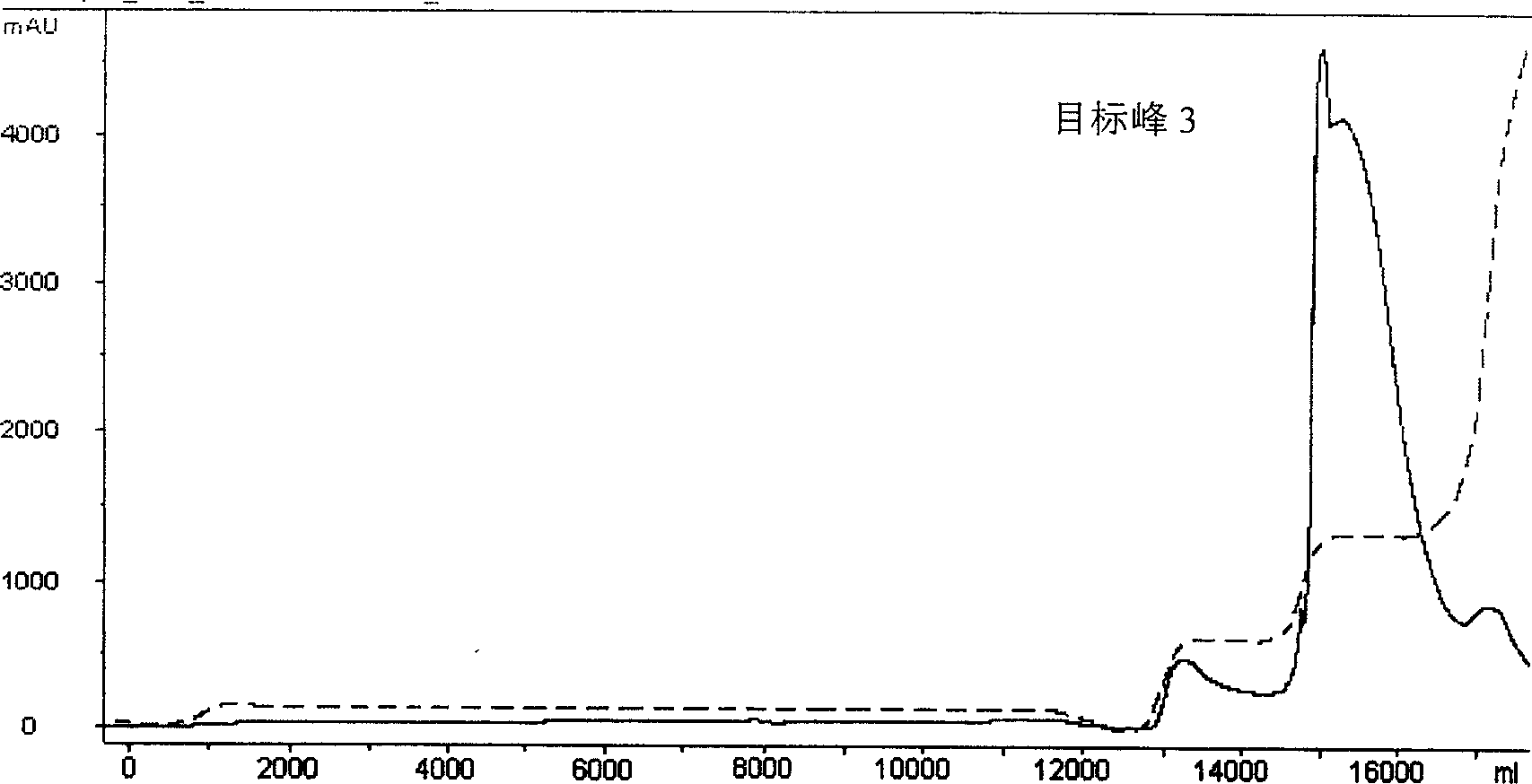
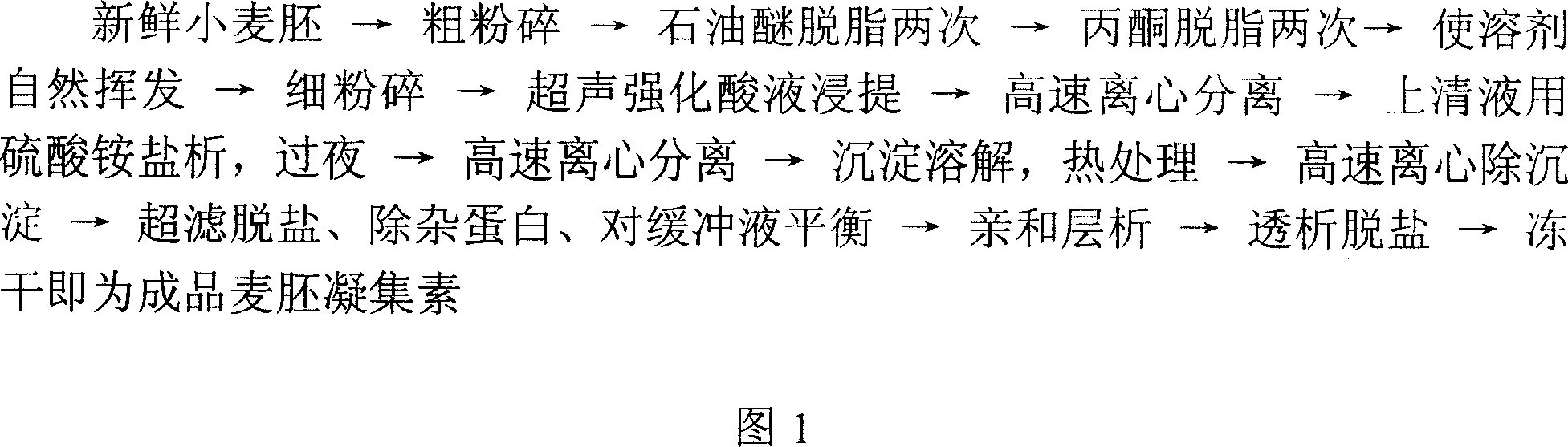
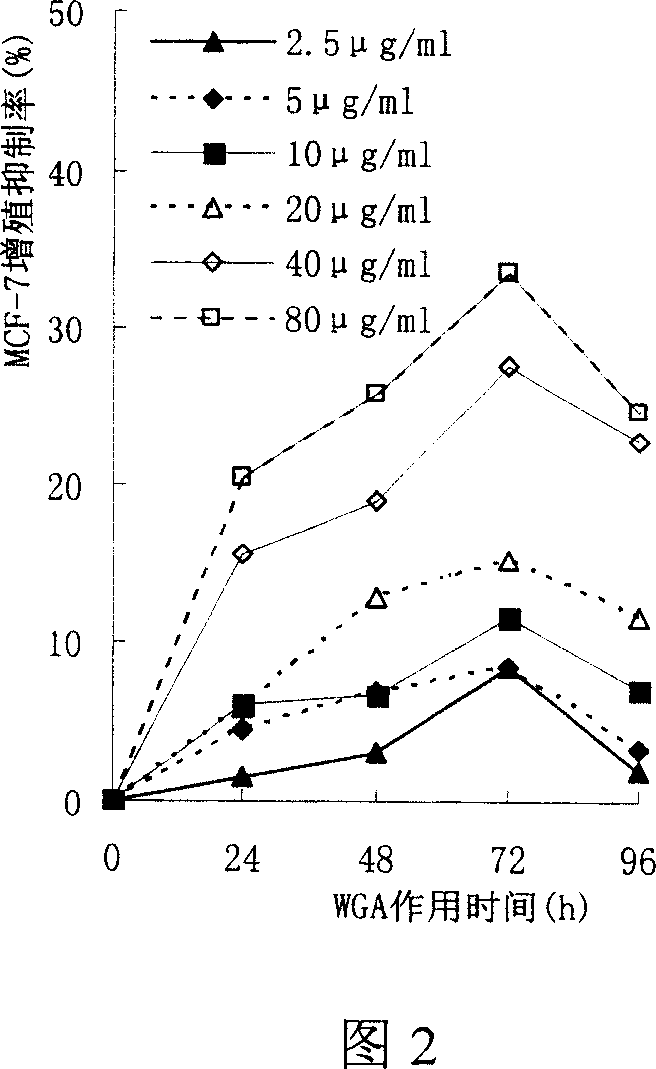
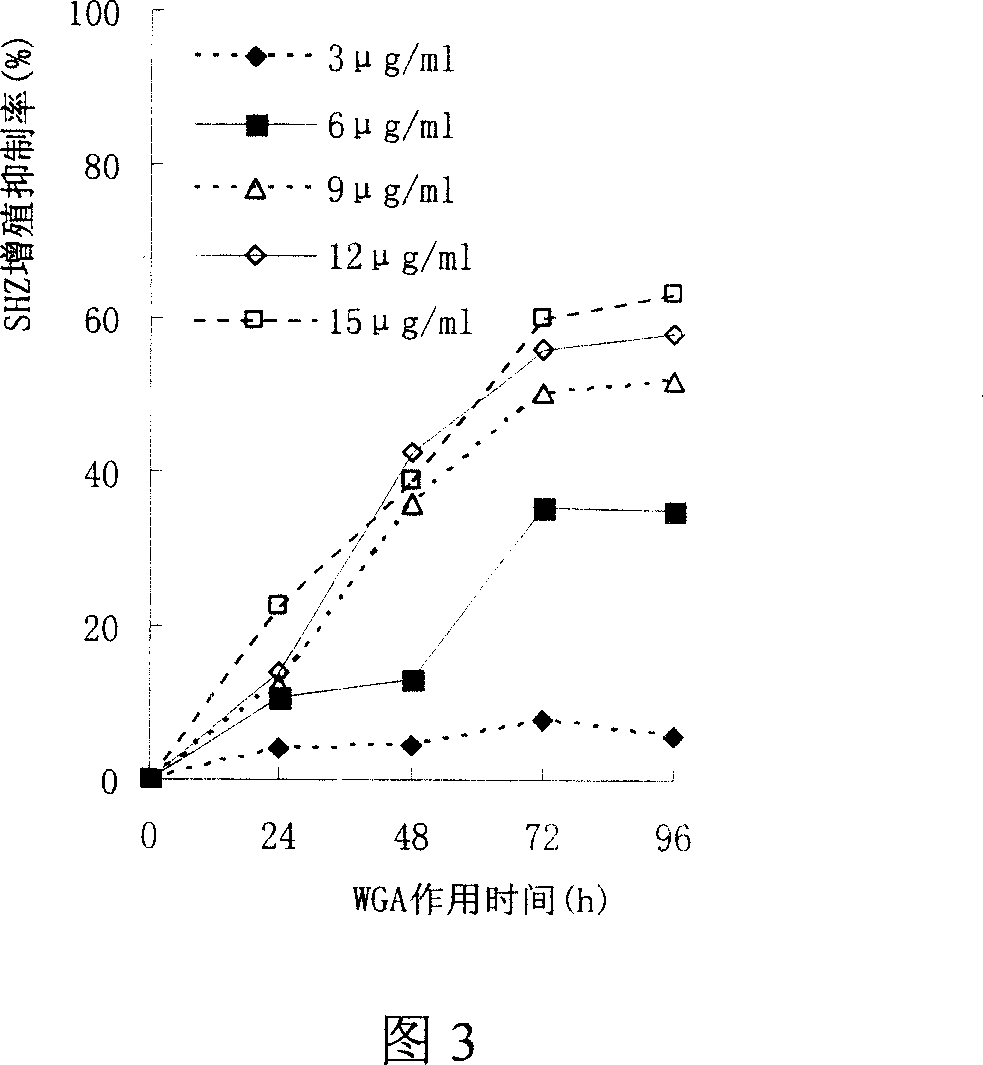
Preparation method of wheat germ agglutinin and its application in inhibiting mammary gland cancer activity
InactiveCN1948337AImprove the utilization value of deep processingHigh yieldPeptide/protein ingredientsPeptide preparation methodsFreeze-dryingRed blood cell
This invention relates to the preparation of wheat germ agglutinin (WGA) and its application in restraining breast cancer's activity. It belongs to a field of wheat by-product material's deep processing technology. The wheat germ was comminuted, degreased,deep comminuted, super sound intensified acid extracted, salting out, heat treated, ultrafiltrated and affinity chromatographed, then the composition which have red cell agglutinating activity was desalted and freeze dried, and at last WGA is gained. Cell culture determines that the WGA has depressant effect on rat breast cancer cell strain SHZ and trypsin dependent form human breast cancer cell strain MCF-7. This invention's technology design is scientific and reasonable, manipulate is convenient and procedure is not polluted. The invention provides groundwork for exploiting drug of restraining cancer, and can enhance the utilization value of wheat germ deep processing and related industry's economic returns.
Owner:JIANGNAN UNIV
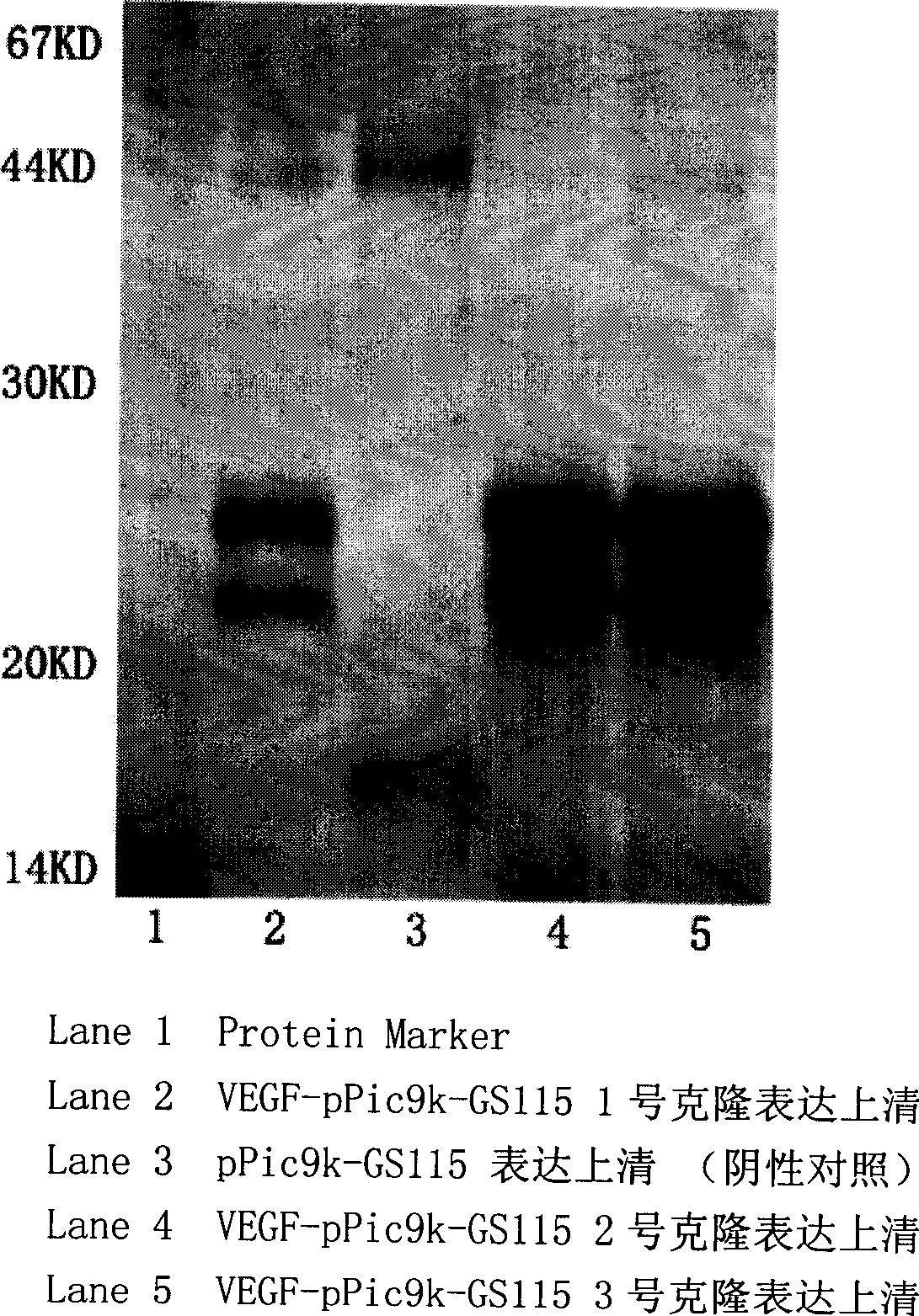
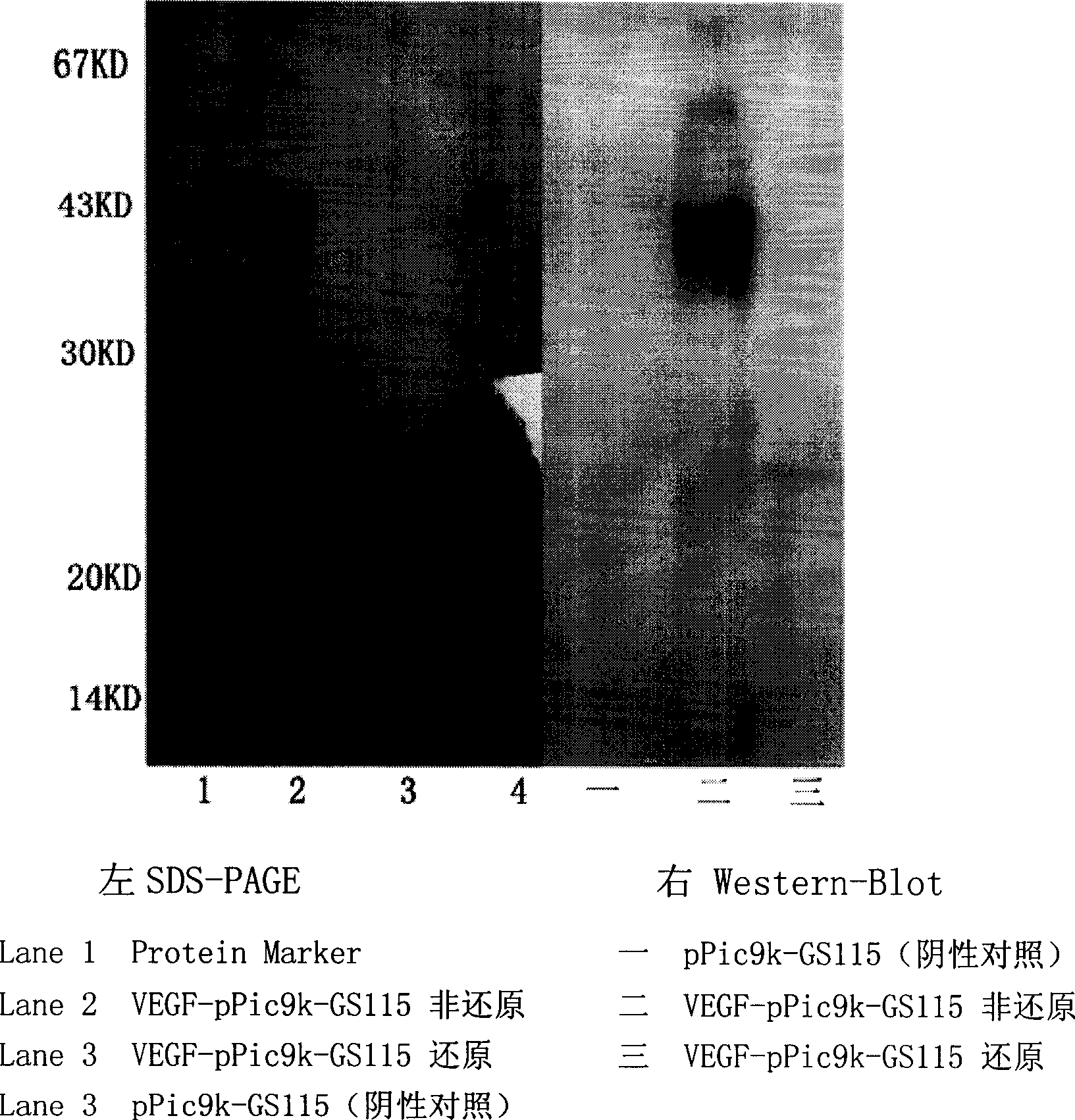
Separation and purification of recombinant human blood vessel endothelia cell growth factor, chemical labeling in vitro and use thereof
The invention relates to the field of biotechnology. A vascular endothelial cell grow factor (VEGF) plays an important function in the regeneration and hyperplasia of blood vessels; but the VEGF in the body has low content and a short half life; therefore, sufficient endogenous VEGF protein is hardly extracted to meet the ever growing experimental study and clinical requirements. The invention discloses a method for economically and conveniently preparing, extracting, purifying and recombining the VEGF protein of a human body through a recombination gene engineering and immunity affinity column method. The invention also discloses a method for carrying out the in-vitro non-radioactive chemical coupling label processing of the purified VEGF protein. The VEGF protein (including the protein having no label or having the chemical coupling label) can be widely applied to experimental study and clinical diagnosis and treatment.
Owner:SUZHOU STAINWEI BIOTECH INC
Recombinant human pancreas kininogenase
The present invention relates to one kind of recombinant kallidinogenase and its preparation process. The recombinant kallidinogenase is produced by means of molecular biological technology and with host cell for expressing recombinant protein, and is purified through an affinity chromatographic process. It has several clinical uses and less side effects.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Method for detecting human tumor antigen P 185 Her-2 and its application in diagnosing tumor
InactiveCN1397803AStrong specificityImprove purification efficiencyPeptide preparation methodsRecovery/purificationNeoplasm diagnosisHuman tumor
A dual-antibody sandwith ELISA detecting method for human soluble p185HER-2 antigen features that the purified and coated P185HER-2 monoclonal antibody is bound to solid supporter, the specimen containing soluble p185HER-2, the purified standard p185HER-2 antigen and the coated antibody are incubated, and another purified and coated p185HER-2 monoclonal antibody marked by horseradish perioxidase is used as detecting antibody. It can be used for serum diagnosis of tumor.
Owner:UNIV OF SCI & TECH OF CHINA
Chitin enzyme and application thereof
The invention aims at providing a novel chitin enzyme with a high-efficiency degradation rate. The high-activity soluble chitin enzyme is obtained through cloning a gene, which is used for coding thechitin enzyme, in a genome of streptomyces albolongus ATCC (American Type Culture Collection) 27414, constructing a recombinant vector and transferring the recombinant vector into escherichia coli BL21 (DE3) to carry out inducible expression. Through Ni column affinity chromatography, the chitin pure enzyme is purified and obtained; the enzymatic property and hydrolysis mechanism of the chitin pure enzyme are researched; the optimum temperature and optimum pH (potential of Hydrogen) of the enzyme are 55 DEG C and 5.0 respectively; the specific enzyme activity is 66.2U / mg; a main product of degraded chitin is chitobiose, and the chitin enzyme is expected to be applied to the preparation of a chitooligosaccharide, and has potential application value.
Owner:OCEAN UNIV OF CHINA
Preparation and application of dmt monoclonal antibody immunoaffinity column supported by chitosan
InactiveCN102258987AHigh purityRich sourcesOther chemical processesPreparing sample for investigationVeterinary DrugsAffinity chromatography
The invention discloses a preparation method and use of a DMT monoclonal antibody immunoaffinity column with CTS as a vector, which belong to the technical field of immunoaffinity chromatography and veterinary medicine residue detection. In the invention, the immunoaffinity column specifically absorbs DMT is prepared by filling purified a monoclonal antibody, which is secreted by a hybridoma cellline, and an immunoaffinity adsorbent, which is obtained by crosslinking CTS into spheres, into a solid phase extraction tube. The immunoaffinity column prepared by the invention can be specifically bound with the DMT; the maximum binding capacity is about 1,792ng of DMT per mL of gel; and when 90 percent methanol and water solution is used as eluent, the average standard recovery rate is 86.85 to 96.95 percent. The immunoaffinity column can be used for quickly detecting DMT.
Owner:JIANGSU UNIV
Methods of producing interachain fluorophore-quencher FRET-aptamers and assays
InactiveUS20060257914A1Maximize sensitivityMaximize specificitySugar derivativesMicrobiological testing/measurementPolymerase LSingle strand
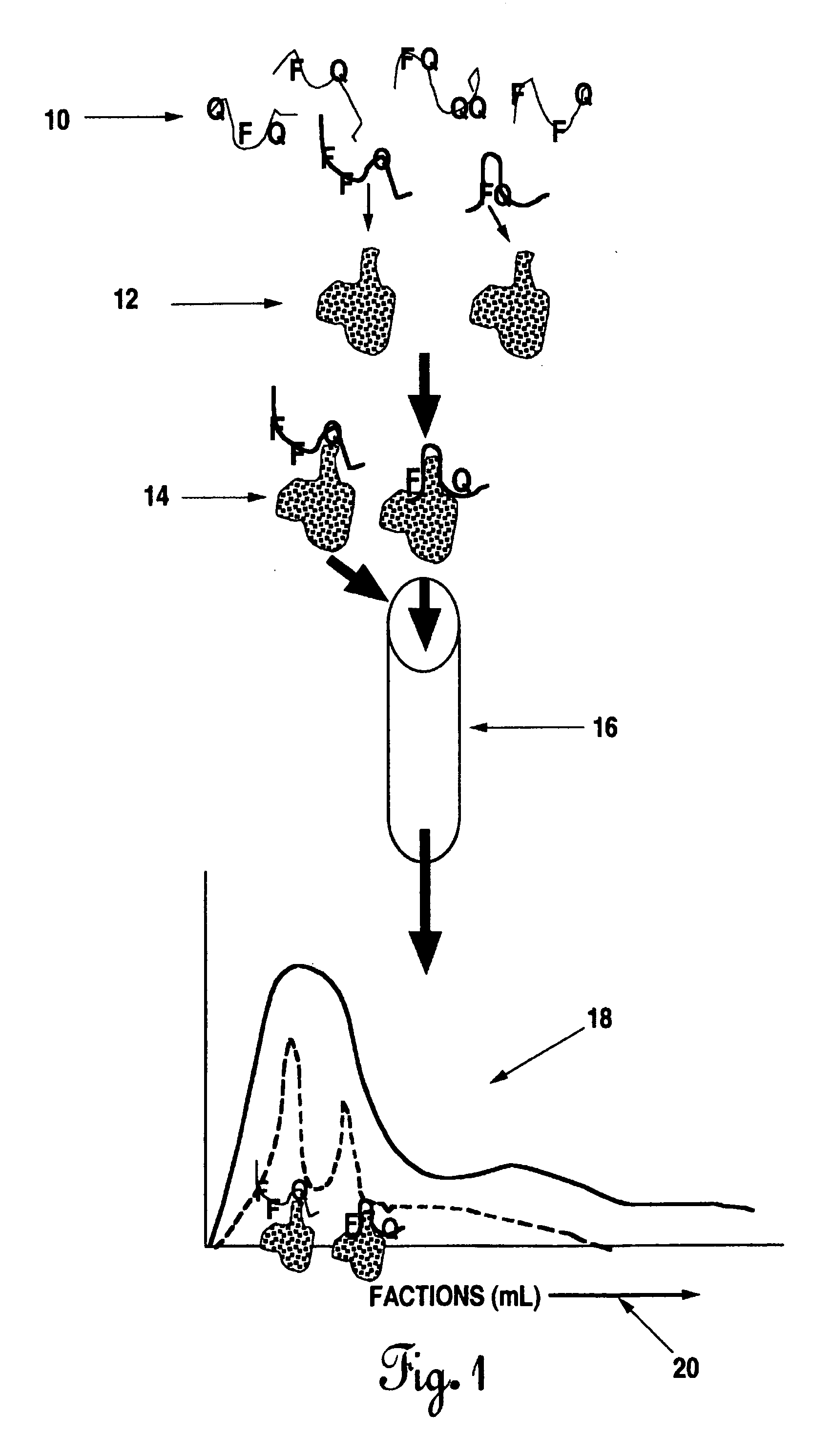
The present invention describes methods for the production and use of single chain (single-stranded) fluorescence resonance energy transfer (“FRET”) DNA or RNA aptamers containing fluorophores (F) and quenchers (Q) at various loci within their structures, such that when its specific matching analyte is bound and the FRET-aptamers are excited by specific wavelengths of light, the fluorescence intensity of the system is modulated (increased or decreased) in proportion to the amount of analyte added. F and Q are covalently linked to nucleotide triphosphates (NTPs), which are incorporated by various nucleic acid polymerases such as Taq polymerase during the polymerase chain reaction (PCR) and then selected by affinity chromatographic, size-exclusion or molecular sieving, and fluorescence techniques. Further separation of related FRET-aptamers can be achieved by ion-pair reverse phase high performance liquid chromatography (HPLC) or other types of chromatography. Finally, FRET-aptamer structures and the specific locations of F and Q within FRET-aptamer structures are determined by digestion with exonucleases and mass spectral nucleotide sequencing analysis.
Owner:CIBUSDX INC
Method for finding protein interaction with baculovirus PCNA (Proliferating Cell Nuclear Antigen)
InactiveCN103713037AEasy to findQuick searchVirus peptidesMaterial analysis by electric/magnetic meansProliferating cell nuclear antigenMass spectrography
The invention discloses a method for finding a protein interaction with baculovirus PCNA (Proliferating Cell Nuclear Antigen). The method is characterized by comprising the following steps of cloning full-length DNA of an autographa californica nuclear polyhedrosis virus (AcMNPV) PCNA gene by utilizing the means such as molecular cloning, and DNA sequencing, purifying the full-length DNA, coupling the full-length DNA to an affinity column, and fining the interaction protein by adopting the mass spectrography. The method can be used for finding and functionally studying interaction protein of the baculovirus PCNA.
Owner:JIANGSU UNIV
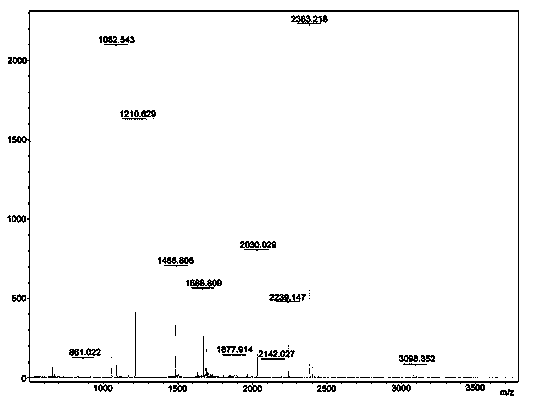
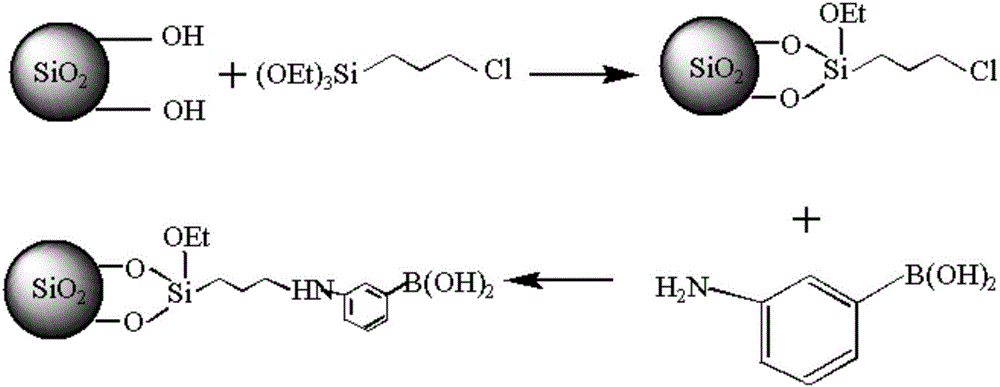
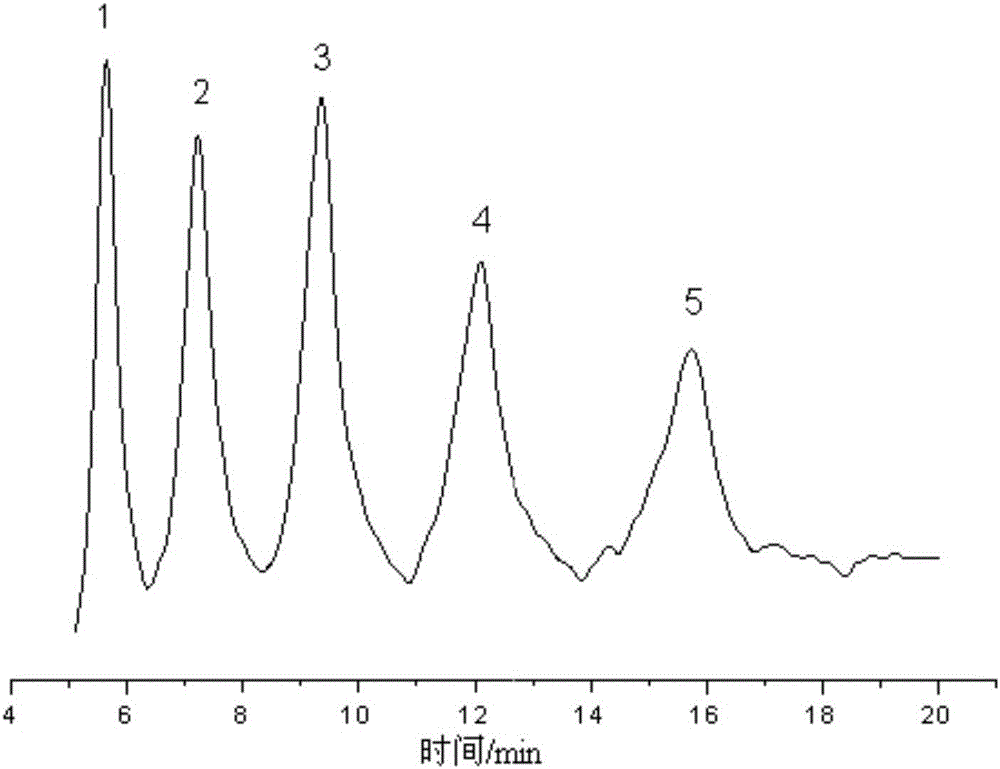
Preparation method of benzene boric acidification affinity chromatography stationary phase for chitosan oligosaccharide separation
ActiveCN106076298ANovel structureEasy to separateSugar derivativesOther chemical processesStationary phaseSilica gel
The invention relates to a preparation method of a chromatography stationary phase for chitosan oligosaccharide separation. The invention aims to solve the problems of a small number of chromatographic columns for chitosan oligosaccharide separation, unsatisfactory separation effect, and difficult acquisition of high purity single-component chitosan oligosaccharide. The method consists of: 1. preparing activated silica gel; 2. preparing chloro silica gel; 3. preparing benzene boric acidification silica gel, i.e. the benzene boric acidification affinity chromatography stationary phase for chitosan oligosaccharide separation. The invention provides the separation method of benzene boric acidification affinity chromatography stationary phase for chitosan oligosaccharide separation.
Owner:HARBIN INST OF TECH
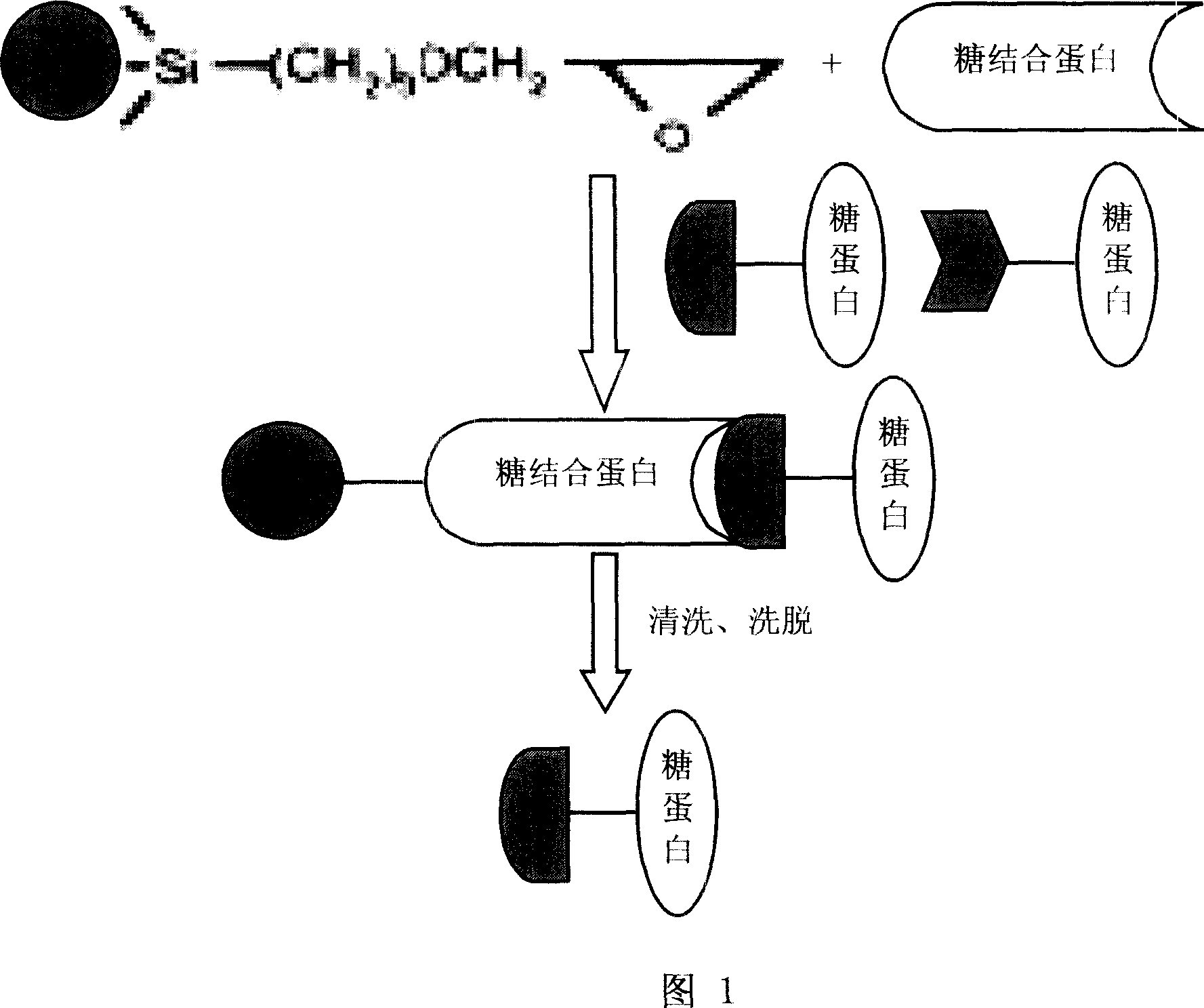
Method for enriching and purifying glycosylation protein
ActiveCN1958599ASimple methodHigh recovery rateHydrolasesPeptide preparation methodsLow affinityEpoxy
This invention discloses a method for enriching and purifying glycosylated protein. The immobilization of agglutinin comprises: adding epoxy ethyl derived magnetic particles into a centrifugal tube, performing immobilization reaction, washing, sealing, washing repeatedly to immobilize agglutinin onto epoxy ethyl derived magnetic particles, adding buffer and storing. The separation and purification of glycosylated protein comprises: immobilizing prepared agglutinin onto epoxy ethyl derived magnetic particles, adding standard glycosylated protein for adequate coupling, washing repeatedly, eluting to obtain enriched and purified glycosylated protein, loading into a dialyzing bag, and desalting. This invention solves the problems of complex enriching and separation process, low recovery rate and high cost faced by background technology. This invention has such advantages as simple process, high recovery rate, low affinity chromatographic column cost, and convenient usage.
Owner:SHANXI LIFEGEN
Removal of bacterial endotoxin in a protein solution by immobilized metal affinity chromatography
InactiveUS20050186218A1Reduce concentrationProtein recovery is greatIon-exchange process apparatusBacterial antigen ingredientsProtein solutionEndotoxin removal
The present invention relates to the purification of polypeptides and the removal of endotoxin via immobilized metal affinity chromatography (IMAC). More specifically, the invention relates to methods for removing bacterial endotoxin in a protein solution. In specific embodiments, the invention relates to the elimination of endotoxin from Moraxella catarrhalis outer membrane proteins.
Owner:WYETH HOLDINGS CORP
Recombinant scorpion toxin, its soluble expression and purification
InactiveCN1817902AStable purificationEasy to purifyPeptide preparation methodsFermentationChemistryToxin
Recombinant-scorpion entomotoxin rBmKIT, its soluble expression and purification are disclosed. The procedure is carried out by solvation expressing for BmK ITcDNA and a protein self-shearing element, fusing nucleotide sequence with 6 histidine coded on 3'end of BmK IT cDNA, breaking fermented broth of engineering bacterium by bacterium, protein extracting, self-shearing by fused protein, affinity chromatographing, concentrating sampler, high-temperature treating, gel filter chromatographing and purifying to obtain final product. It is soluble and simple.
Owner:SHANXI UNIV
Method for preparing specific Immunoglobulin of cystvirus and adenovirus in respiratory tract
InactiveCN1485342AImmunoglobulins against animals/humansImmunoglobulins against virusesProtective antigenSaccharum
A method of preparing immunoglobins with high valence and high specialty against respiratory syncytial virus and adenovirus. It comprises culturing respiratory syncytial virus and adenovirus with Hep-2 cell strain, Hala Strain, preparing virus antigen, or preparing protective antigens according to sequences of respiratory syncytial virus and adenovirus by the genetic engineering technology; then,centrifuging the prepared virus antigens and getting purified antigens, or concentrating antigens by 20úÑ sucrose, or purifying antigens by chromatographic absorbing columns, screening high-valent immunoglobins against respiratory syncytial virus and adenovirus, and neutralizing virus in viro; finally extracting immunoglobins from bloods containing high-valent immunoglobins against respiratory syncytial virus and adenovirus by a low-temperature alcohol method or ion exchanging chromatography and so on.
Owner:SHANDONG BAIYI PHARMA
Scorpion peptide for treating arrhythmia and its preparing process and application
InactiveCN1379042AObvious antiarrhythmic effectIncrease productionPeptide/protein ingredientsFermentationSolubilityEscherichia coli
An anti-arrhythmia scorpion peptide whose amino acid sequence is disclosed is prepared through designing PCR upstream primer containing enteric kinase cut site, using the PCR to amplify anti-arrhythmia peptide gene fragment through limited enzymatic cut of endoenzyme, clonining to pGX-5x-1 expression carrier, transferring to colibacillus BL21 to obtain recombinant colibacillus BL21 (pGEX / BmK1M); after inducing by IPTG, through purifying by glutathion transferase affinity chromatographic column, expressing the resultant fusion protein by enteric kinase and desalting to obtain the said peptide.Its advantages are high anti-arrhythmia activity, high solubility and high output.
Owner:WUHAN UNIV
Preparation method of citrinin immunoaffinity column
InactiveCN103157296AHigh purityIncrease concentrationSerum immunoglobulinsOther chemical processesCarrier proteinSolid phase extraction
The invention discloses a preparation method of a citrinin immunoaffinity column, belongs to the technical field of immunoaffinity chromatography and biotoxin detection, and discloses the preparation method of the citrinin immunoaffinity column. Immunogen is prepared by connecting citrinin and carrier protein through a formaldehyde condensation method. An immune animal gets an anti-citrinin antibody. Cupric embedded ionic polymer is prepared in a mass polymerization method. Immunoaffinity adsorbent which is prepared by adsorbing the polymer to the anti-citrinin antibody is filled into the citrinin immunoaffinity column which is prepared in a solid phase extraction pipe. The citrinin immunoaffinity column which is prepared in the preparation method of the citrinin immunoaffinity column is capable of combining with the citrinin in a specific mode, and cirtrinin solution with high purity and concentration is obtained through purification and enrichment. The maximum combination volume of the citrinin immunoaffinity column which is prepared in the preparation method of the citrinin immunoaffinity column is 597 ng, citrinin fortified recovery in a sample is 83.7% to 93.2%, and the recovery rate is no lower than 80% when the citrinin immunoaffinity column is repeatedly used for three times. The preparation method of the citrinin immunoaffinity column can be applied to rapid detection of the citrinin in fermented food.
Owner:SHANDONG NORMAL UNIV
Ligands for antibody and Fc-fusion protein purification by affinity chromotography IV
InactiveUS9745339B2Good chemical stabilityLow production costSerum immunoglobulinsOther chemical processesAntibody fragmentsBiochemistry
The present invention relates to the use, for affinity purification of an antibody or an fragment of an antibody, of a ligand-substituted matrix comprising a support material and at least one ligand covalently bonded to the support material, the ligand being represented by formula (I)L-(Sp)v-Ar1—Am—Ar2 (I)wherein L, SP, Ar1, AM, Ar2 and v are defined herein.
Owner:NOVALIX DEUT GMBH
Filler for affinity chromatography
ActiveUS9162161B2Increase capacityIncrease resistanceIon-exchanger regenerationImmunoglobulinsEpoxyAffinity chromatography
Provided is a filler for affinity chromatography having a high dynamic binding capacity for proteins and having excellent alkali resistance and storage stability. The filler for affinity chromatography of the present invention comprises a porous particle consisting of a copolymer of 40 parts to 99.5 parts by mass of (M-1) a methacryloyl group-containing vinyl monomer that contains a hydroxyl group and does not contain an epoxy group, 0.5 parts to 30 parts by mass of (M-2) an epoxy group-containing vinyl monomer, 0 parts to 59.5 parts by mass of (M-3) a methacryloyl group-containing vinyl monomer which is other than the monomers (M-1) and (M-2), and 0 parts to 25 parts by mass of (M-4) a vinyl monomer other than the monomers (M-1), (M-2) and (M-3) (with the proviso that the total amount of the contents of (M-1), (M-2), (M-3) and (M-4) is 100 parts by mass); ring-opened epoxy group obtainable by ring-opening of epoxy group that is contained in the copolymer; and ligand that is bound to the porous particle.
Owner:JSR CORPORATIOON
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com