Patents
Literature
288 results about "Bacterial endotoxin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bacterial Endotoxin Definition. Lipopolysaccharides (LPS), also known as lipoglycans and endotoxins, Endotoxins are part of the outer membrane of the cell wall of Gram-negative bacteria.
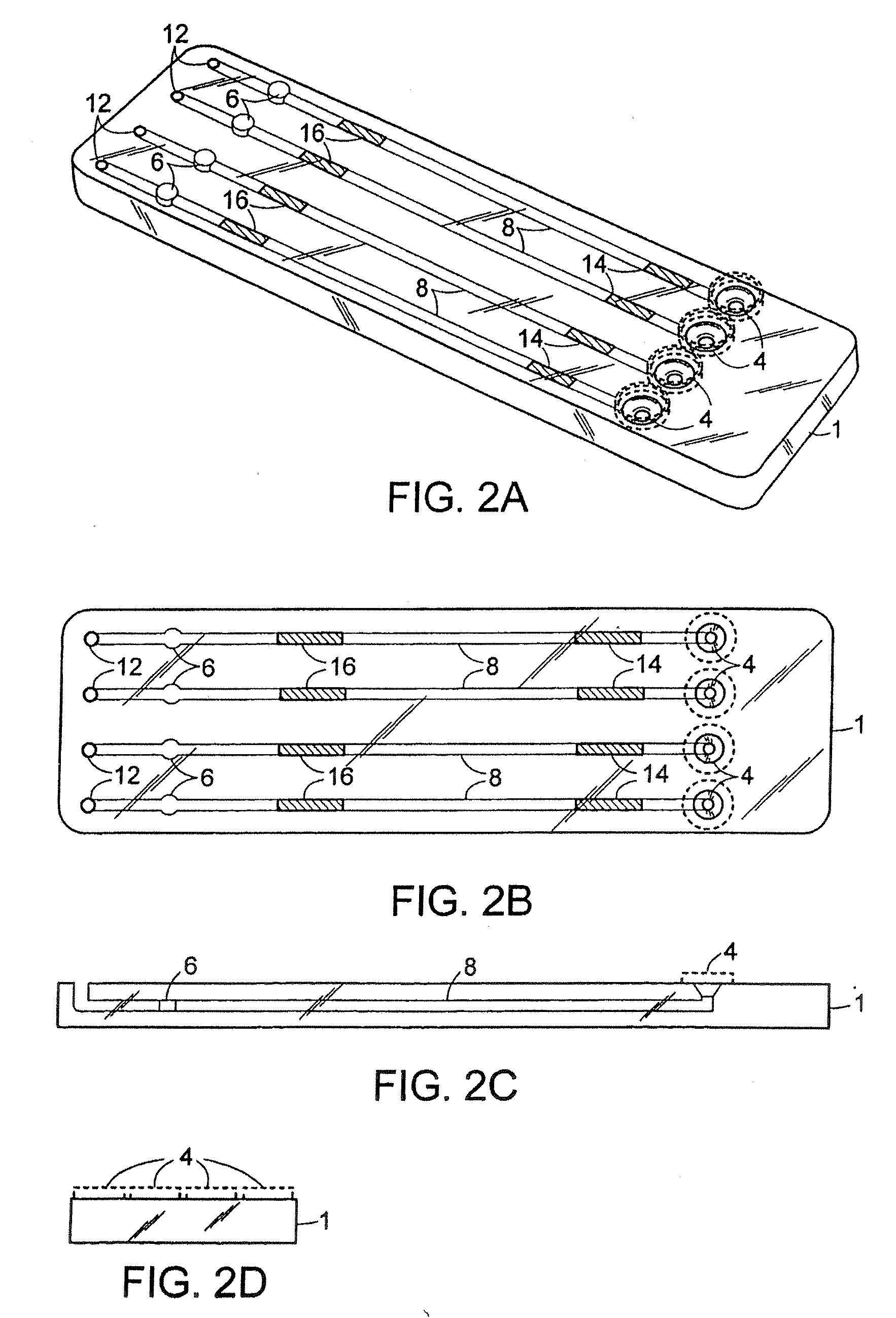
Methods and compositions for the detection of microbial contaminants
ActiveUS7329538B2Bioreactor/fermenter combinationsBiological substance pretreatmentsMicroorganismCell lysates
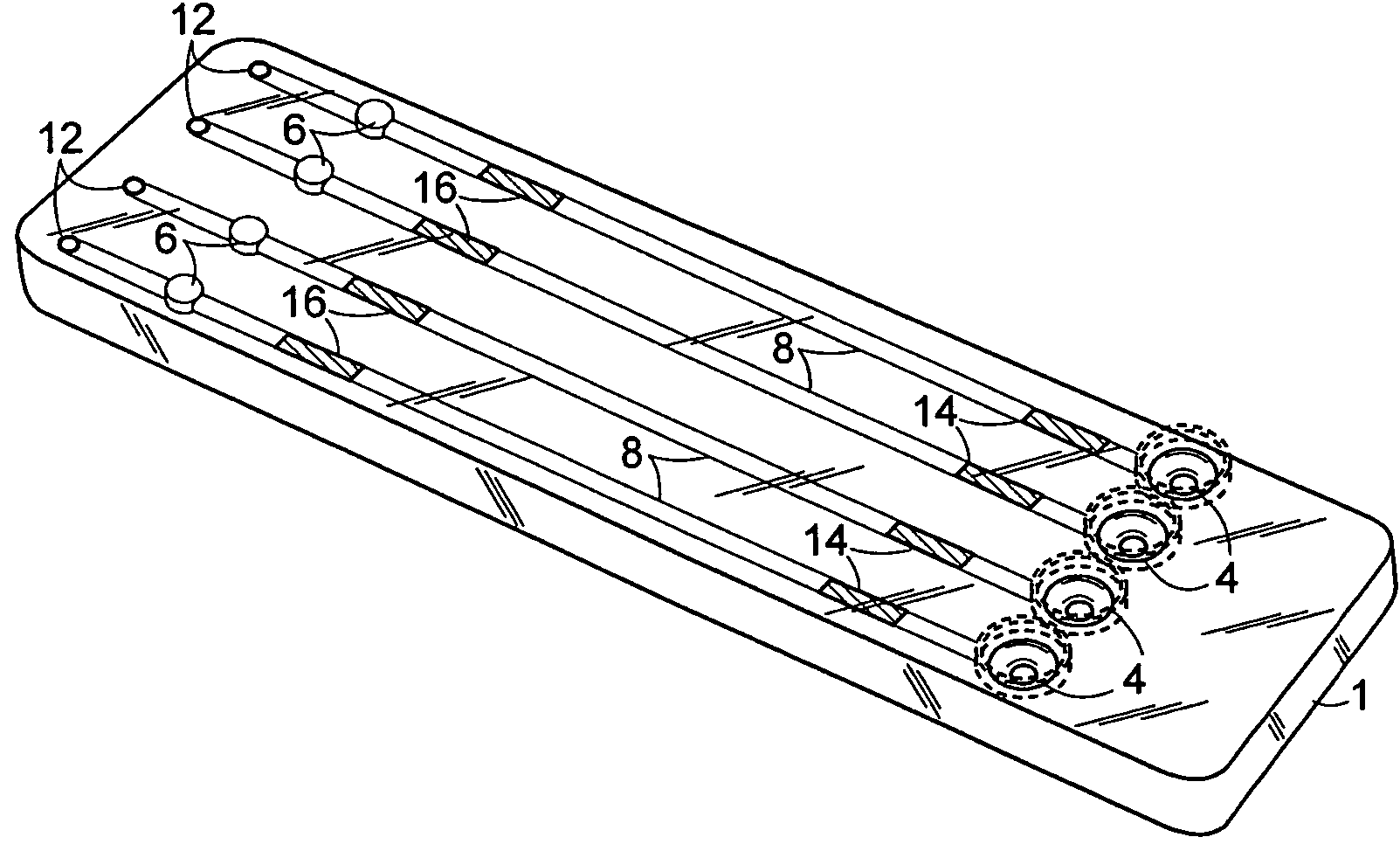
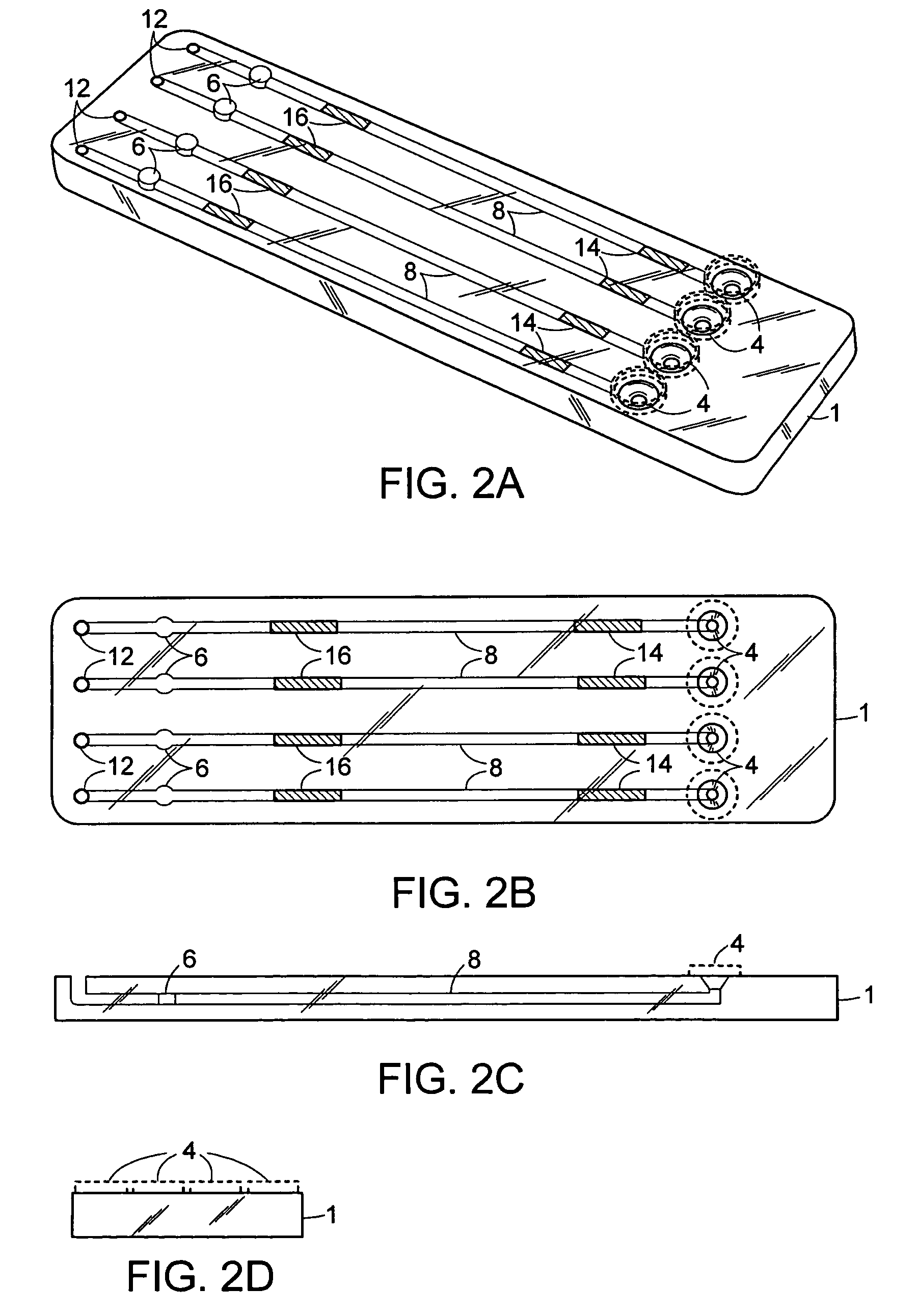
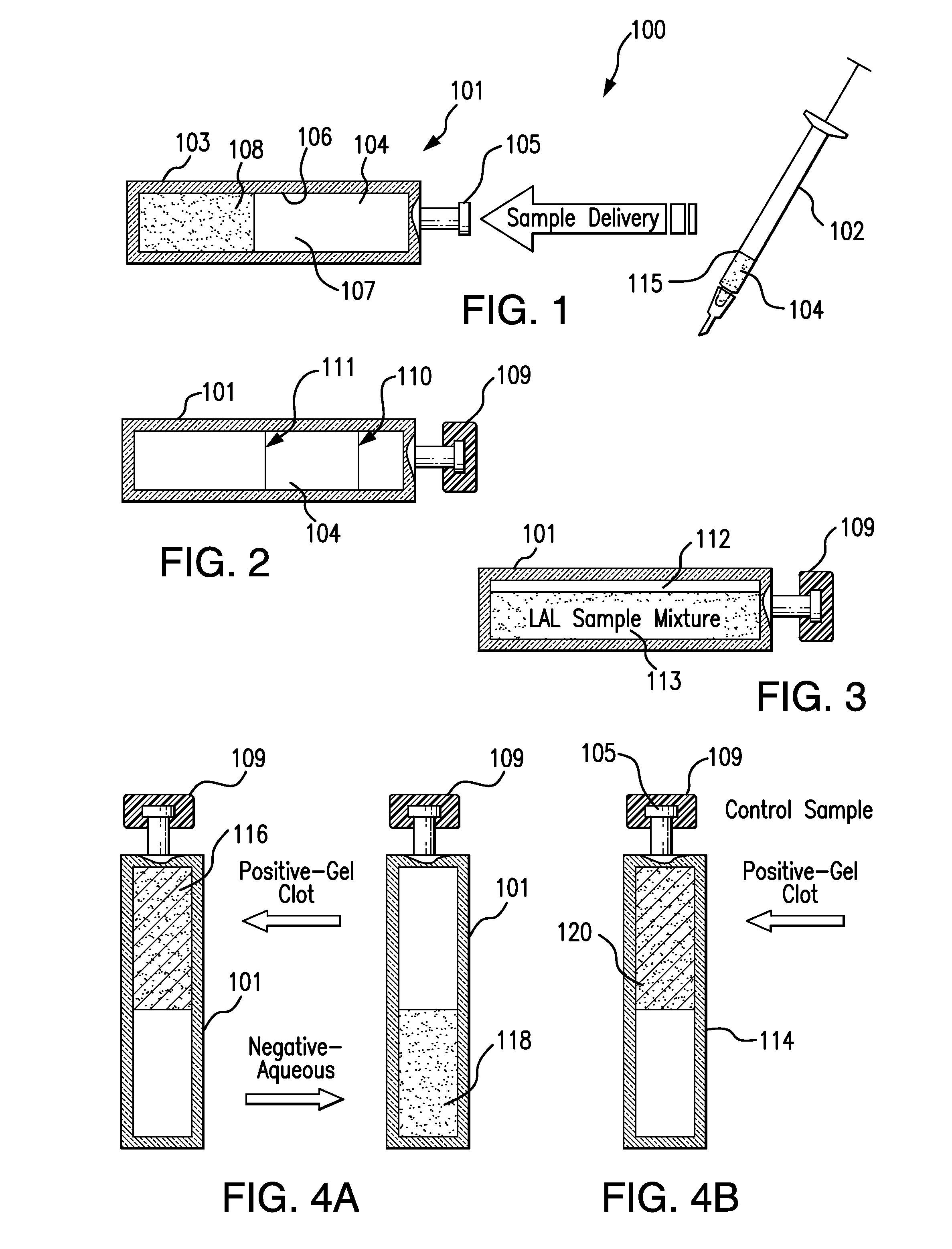
The invention provides methods and compositions for the detection and / or quantification of a microbial contaminant, for example, a bacterial endotoxin or a glucan, in a sample. In particular, the invention provides a test cartridge useful in the practice of hemocyte lysate-based assays for the detection and / or quantification of a microbial contaminant in a sample. In addition, the invention provides methods of making and using such cartridges. In addition, the invention provides a rapid, sensitive, multi-step kinetic hemocyte lysate-based assay for the detection and / or quantification of a microbial contaminant in a sample. In addition, the invention provides a glucan-specific lysate that can be used in a variety of assay formats, including, for example, a test cartridge, optionally configured to perform a kinetic assay.
Owner:CHARLES RIVER LAB INC
Compositions and methods for treatment of autoimmune and allergic diseases
InactiveUS20110143994A1Reduce the possibilityPreventing”, “prophylaxis”Senses disorderNervous disorderEpitopeAutoimmune responses
The present invention provides improved methods and compositions for treating and preventing autoimmune and allergic diseases. More specifically the invention relates to new immuno-modulating complexes which are fusion proteins comprising mutant subunits of bacterial endotoxins, a peptide capable of binding to a specific cellular receptor, and one or more epitopes associated with an autoimmune or allergic disease.
Owner:TOLERANZIA
Removal of bacterial endotoxin in a protein solution by immobilized metal affinity chromatography
InactiveUS6942802B2Ion-exchange process apparatusIon-exchanger regenerationProtein solutionBacteroides
The present invention relates to the purification of polypeptides and the removal of endotoxin via immobilized metal affinity chromatography (IMAC). More specifically, the invention relates to methods for removing bacterial endotoxin in a protein solution. In specific embodiments, the invention relates to the elimination of endotoxin from Moraxella catarrhalis outer membrane proteins.
Owner:WYETH HOLDINGS CORP
Cell Free Biosynthesis of High-Quality Nucleic Acid and Uses Thereof
InactiveUS20080305142A1Short timeSmall volumeAntibacterial agentsOrganic active ingredientsCell freeRestriction enzyme digestion
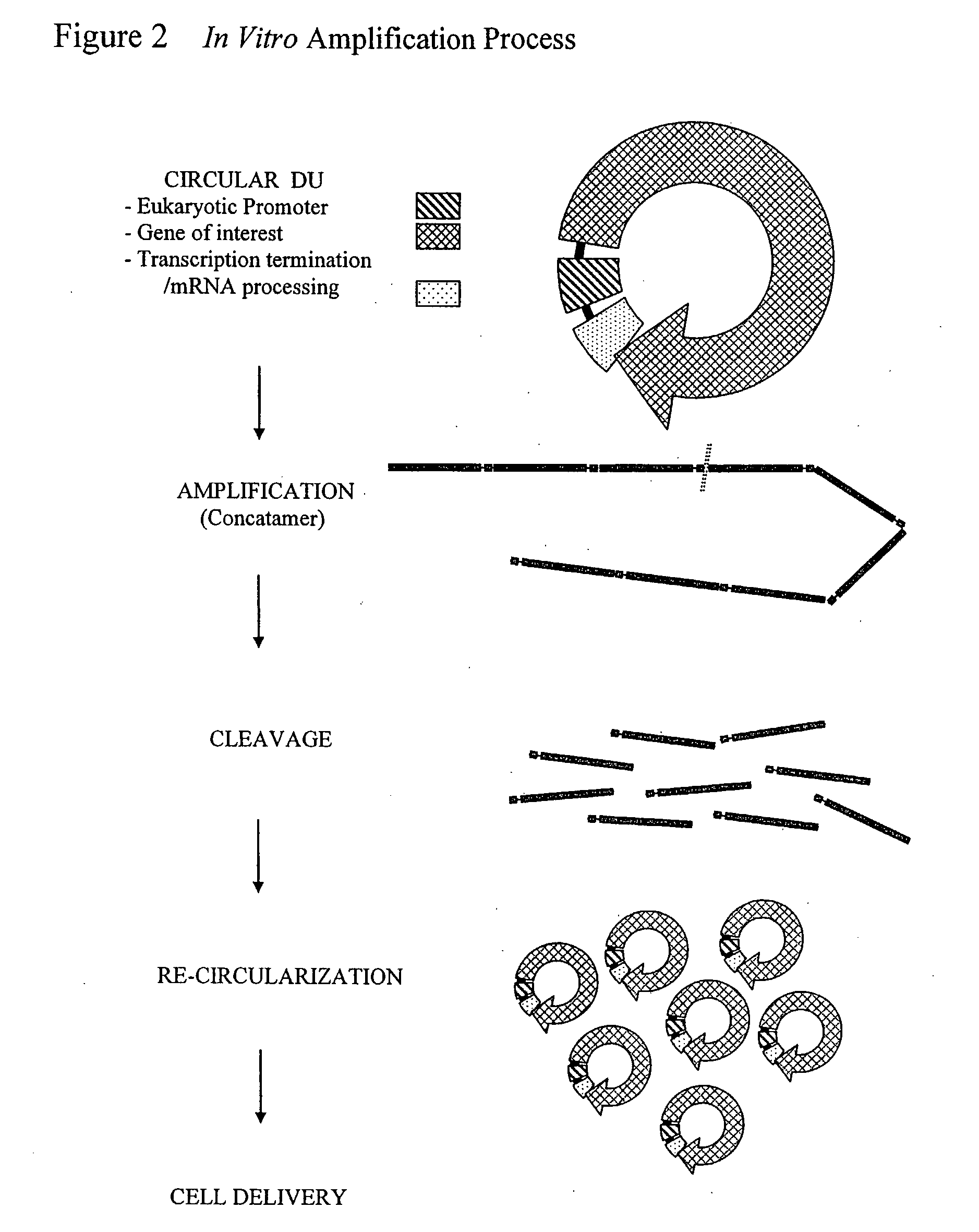
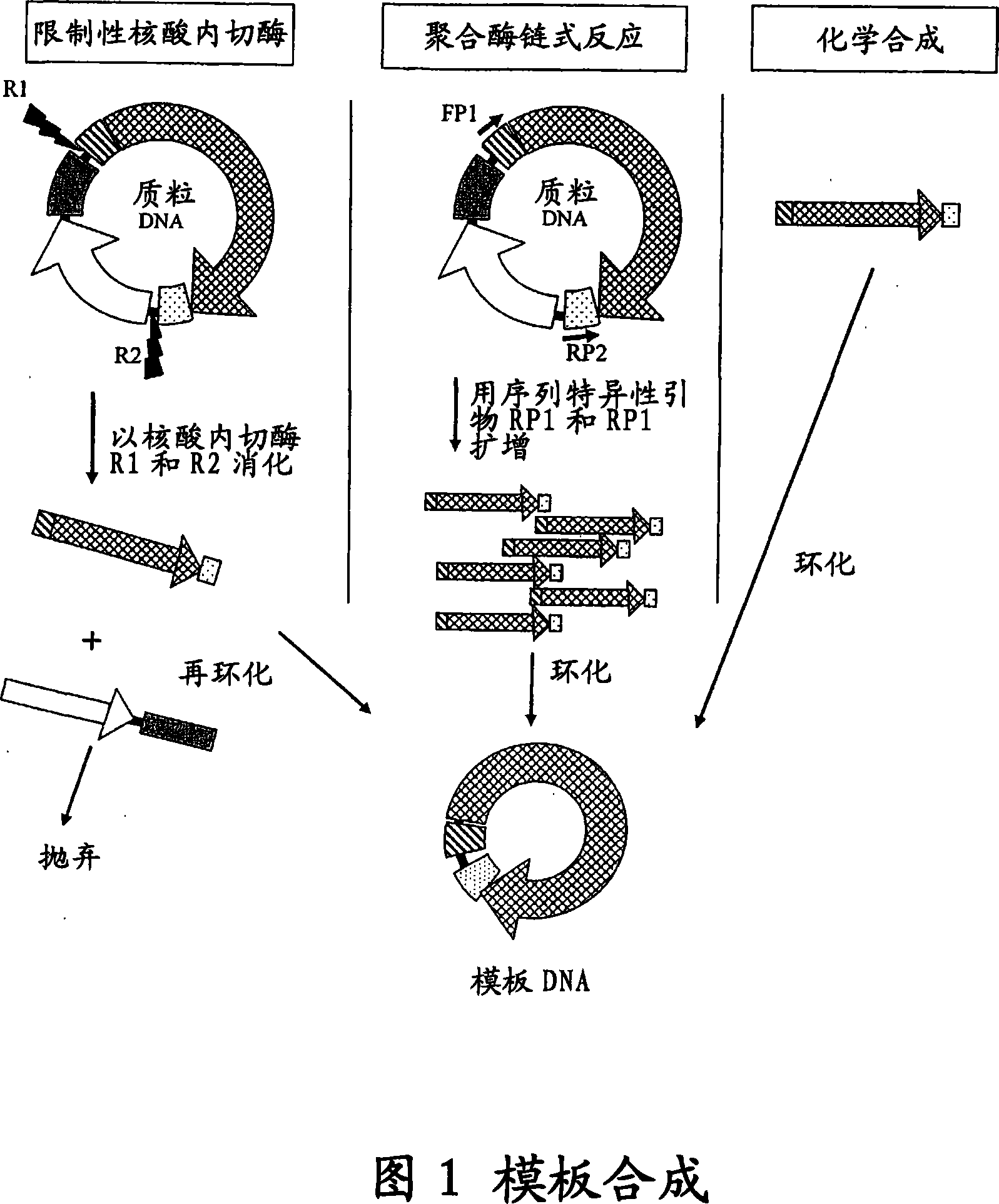
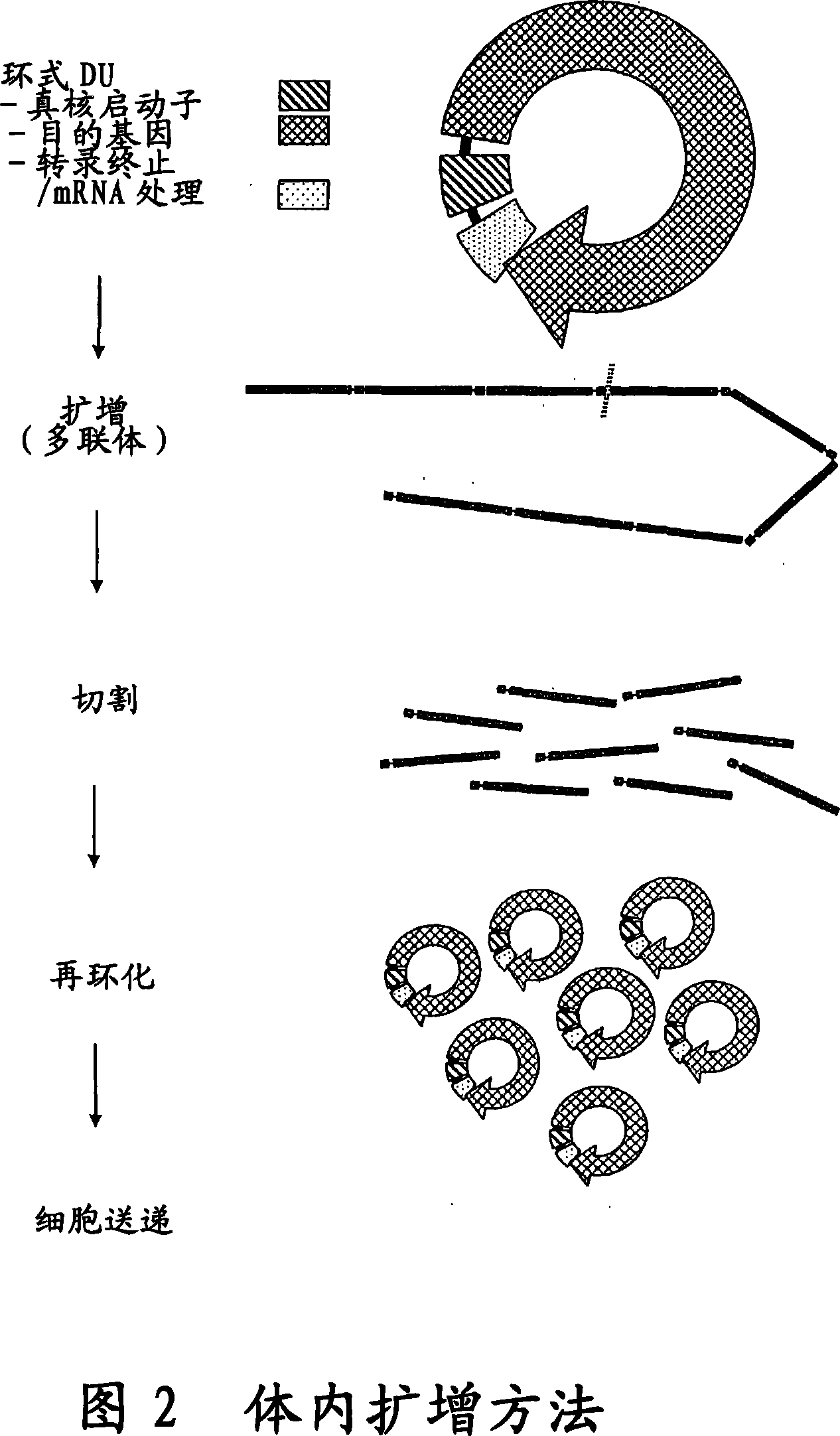
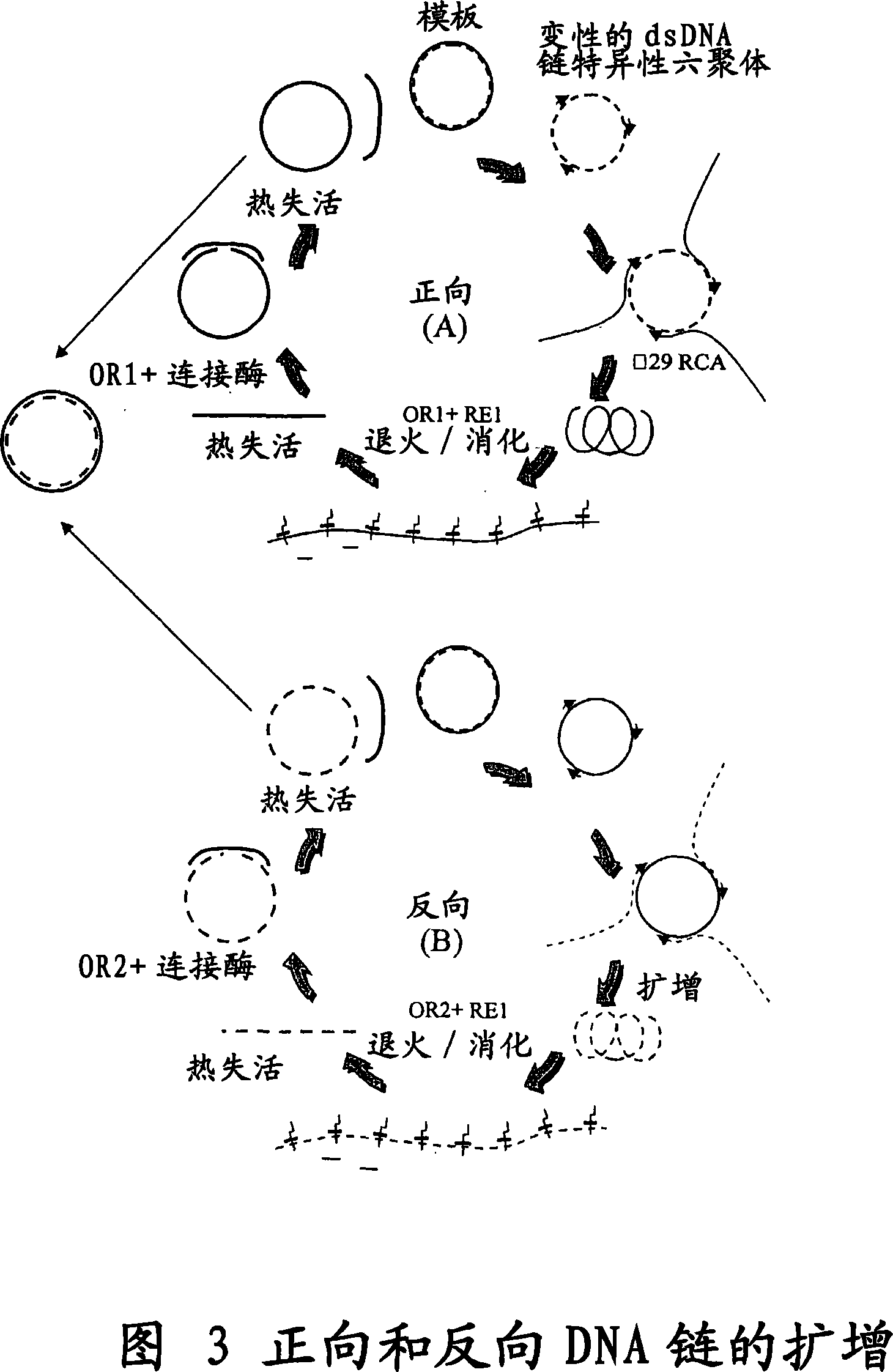
The invention provides an improved cell free amplification method capable of producing large quantities of therapeutic-quality nucleic acids and methods of using the synthesized nucleic acid in research, therapeutic and other applications—The methods combine several different state-of-the-art procedures and coordinate their applications to affordably synthesize nucleic acids for therapeutic purposes. It combines in vitro rolling circle amplification, high fidelity polymerases, high affinity primers, and streamlined template specifically designed for particular applications. For expression purposes, the templates contain an expression cassette including a eukaryotic promoter, the coding sequence for the gene of interest, and a eukaryotic termination sequence. Following amplification, concatamers are subsequently processed according to their intended use and may include: restriction enzyme digestion for the production of short expression cassettes (SECs); ligation steps to circularize the SEC (CNAs); and / or supercoiling steps to produce sCNAs. The final product contains nearly non-detectable levels of bacterial endotoxin.
Owner:STAR BIOLOGICS
Kit For Sampling And Detection Of Endotoxin In Aqueous Solution
InactiveUS20150276742A1Rapid and simple and cost-effectiveEasy and fast testingMicrobiological testing/measurementBiological material analysisDialysis fluidAqueous solution
The present invention relates to a simple, rapid, and cost-effective test kit for detecting bacterial endotoxin in aqueous solutions, such as water for dialysis or dialysate, using an endotoxin-activatable clotting agent-based gel clot assay which can be applied at ambient temperature. The present invention also relates to a method of detecting endotoxin in aqueous solution with use of the test kit.
Owner:FRESENIUS MEDICAL CARE HLDG INC
Kit for detecting fungus (1-3)-beta-D glucan in human body fluid and application method of kit
ActiveCN102866255AAchieve qualitativeEasy to detectTransmissivity measurementsBiological testingZymogenHuman body
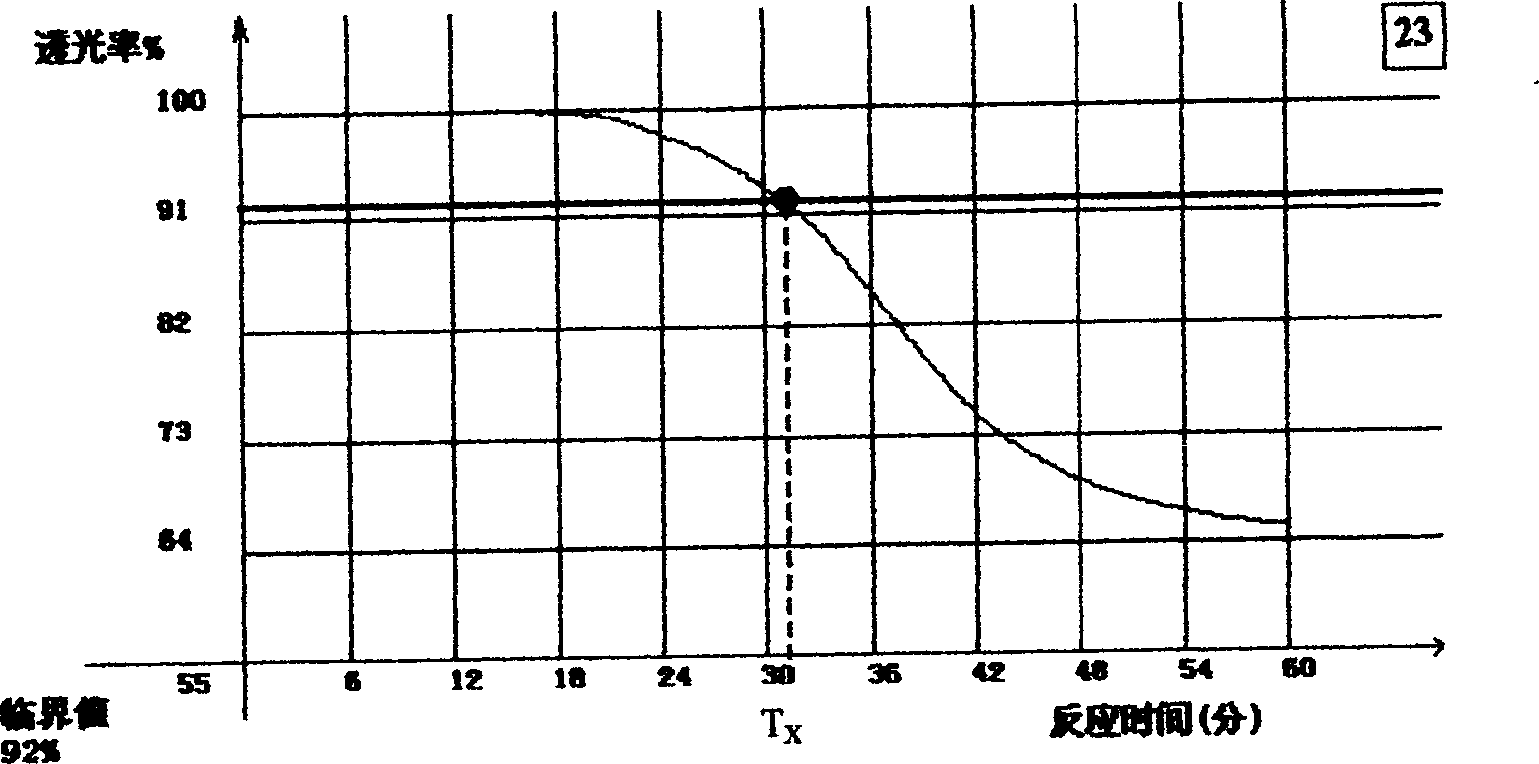
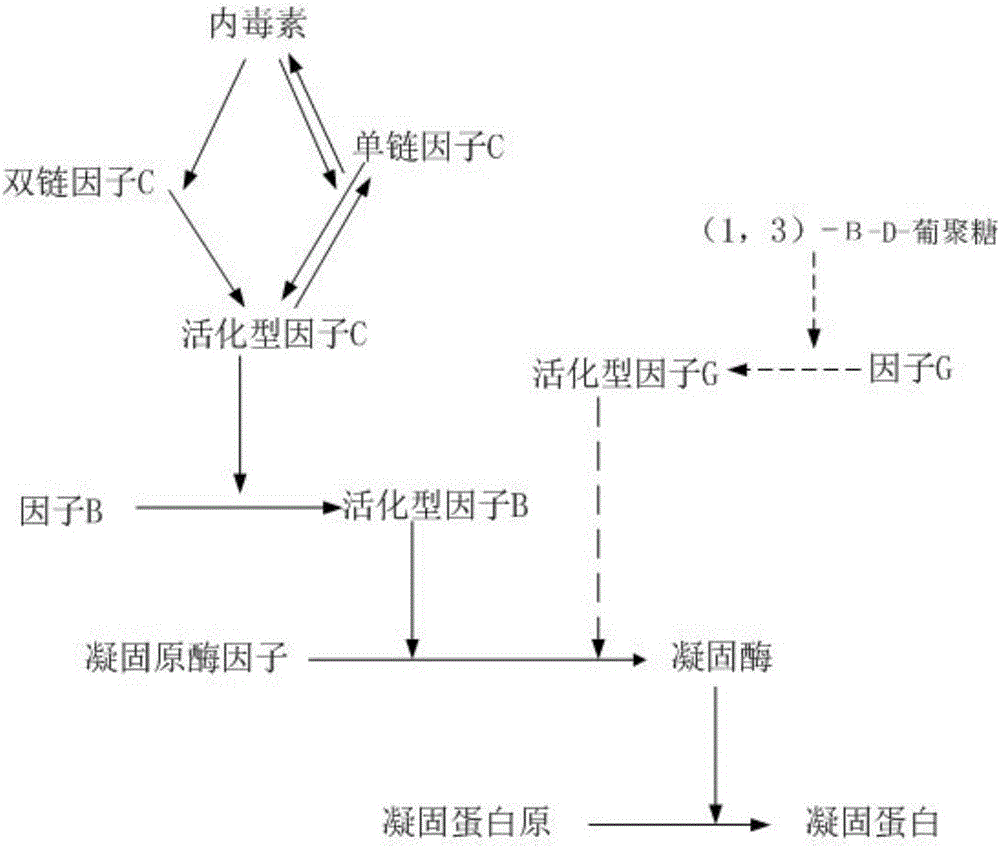
The invention provides a kit for detecting a fungus (1-3)-beta-D glucan in human body fluid. The kit can be industrially produced, and is accurate and high in speed and specificity. The kit is characterized by comprising a main reactant, a body fluid treating agent, an alkaline diluent, an acidic diluent, sterile water, lyophilized serum and a (1-3)-beta-D glucan quality control substance, wherein the main reactant contains a tachypleus tridentatus blood cell G factor, coagulating zymogen and an activator. A detection method is convenient, rapid, safe and high in specificity, the (1-3)-beta-D glucan in body fluid samples of 10 persons can be detected within 80 minutes, a discrimination coincidence rate reaches 100 percent, and the fungus (1-3)-beta-D glucan and bacterial endotoxin can be discriminated within the same reaction time.
Owner:湛江博康海洋生物有限公司
Method of removing endotoxin from vaccines
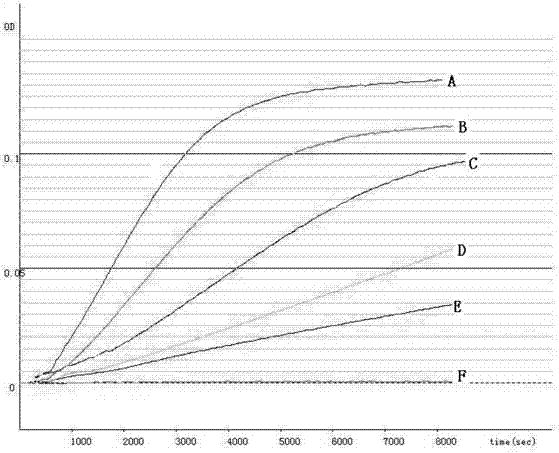
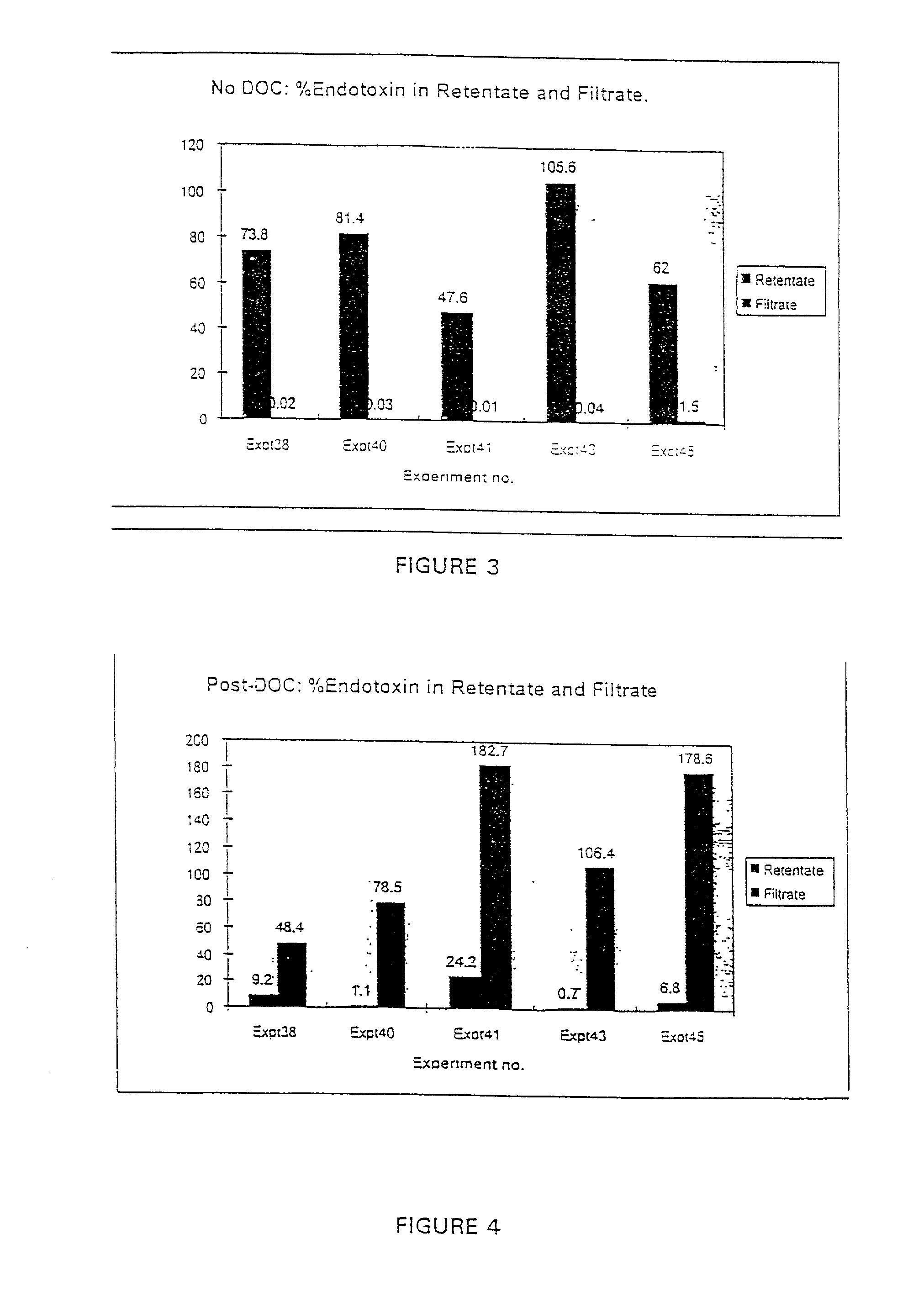
InactiveUS7226775B2Effectively scaledSsRNA viruses negative-sensePeptide/protein ingredientsBacteroidesMedicine
A method of removing bacterial endotoxin from a pharmaceutical process solution is disclosed. In one embodiment, the method comprises treating the solution with a surfactant effective to dissociate the endotoxin from a pharmaceutical drug or vaccine substance in the solution, and then filtering the solution through a molecular cut-off filter having a pore size effective to retain the pharmaceutical drug or vaccine substance but allow the dissociated bacterial endotoxin to pass therethrough.
Owner:MEDEVA EURO
Preparation and application of detection kit for blood bacterial endotoxin
ActiveCN102901726AAchieve qualitativeEasy to detectMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationBlood collectionLysis
The invention provides a specific limulus reagent and a preparation method thereof. The preparation method comprises steps of blood collection, cell separation, cell lysis, cell wall separation, addition of an activator and a G factor inhibitor, so as to prepare a semi-finished product. The invention further provides a detection kit for blood bacterial endotoxin, wherein the kit contains the specific limulus reagent, and further comprises a humoral treatment agent I, a humoral treatment agent II, bacterial endotoxin controls and inspection water. The specific limulus reagent and the detection kit provided by the invention can qualitatively identify a gel reaction of the limulus reagent with the bacteria endotoxin and glucan type substances, thereby improving reliability of the detection results of the blood bacterial endotoxin, and realizing quantitative detection for the blood bacterial endotoxin. By using the specific limulus reagent and the detection kit, the detection of the blood bacterial endotoxin is characterized by high sensitivity, reliable detection result and high detection efficiency.
Owner:湛江博康海洋生物有限公司
Freeze-dried vaccine protective agent, freeze-dried varicella attenuated live vaccine and preparation methods of freeze-dried vaccine protective agent and freeze-dried varicella attenuated live vaccine
ActiveCN104258404ALess prone to allergic reactionsExtensive and neat lesionsAntiviralsPharmaceutical non-active ingredientsArginineVaccine Production
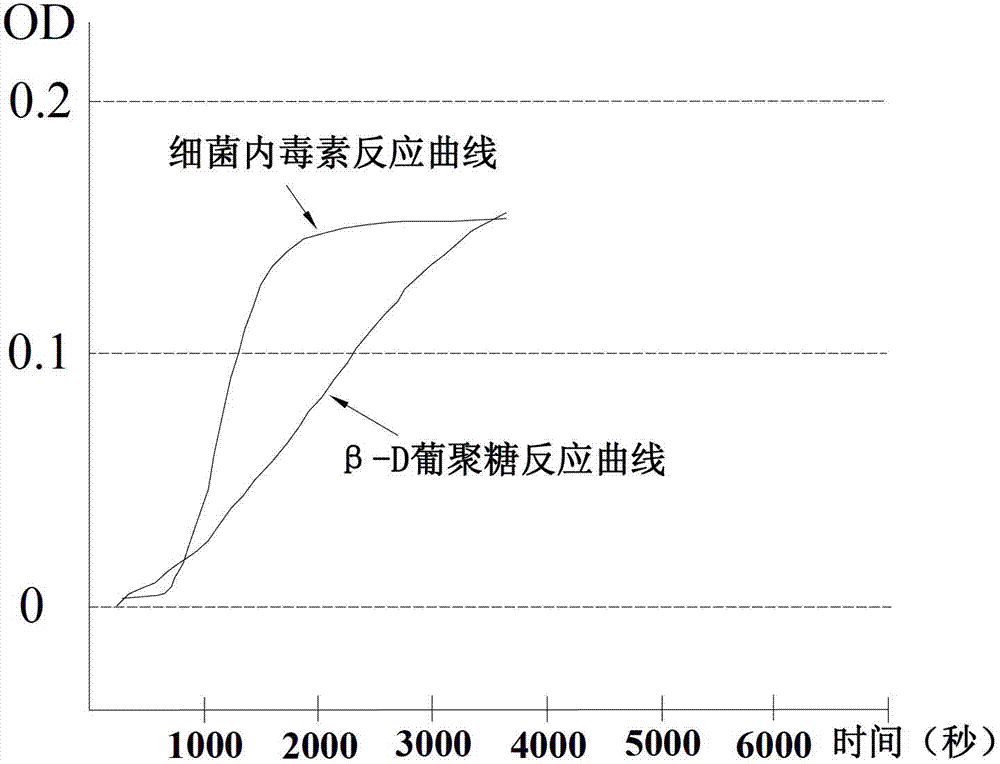
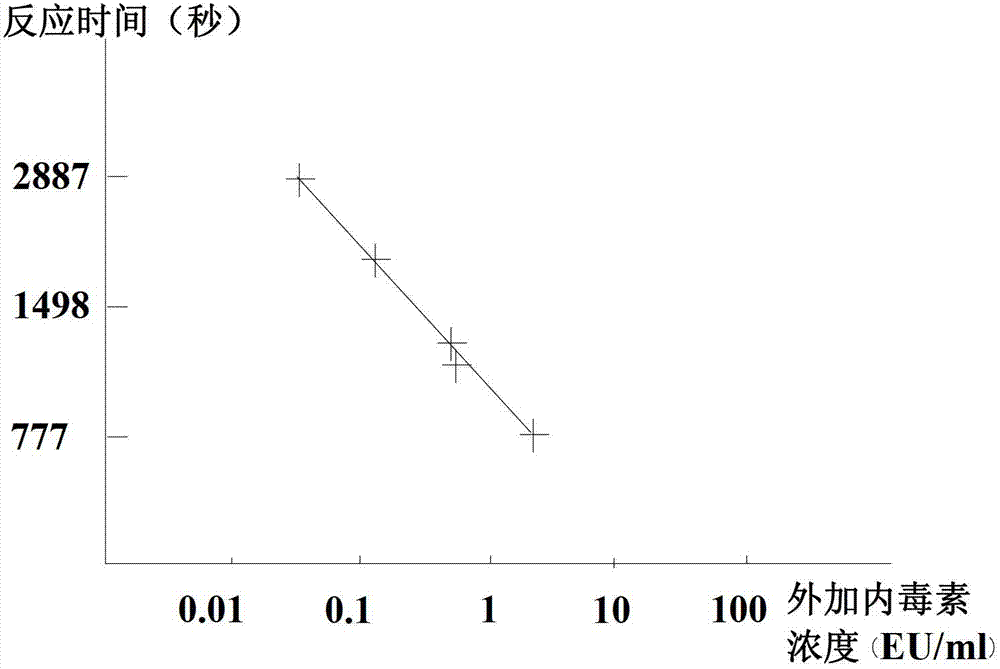
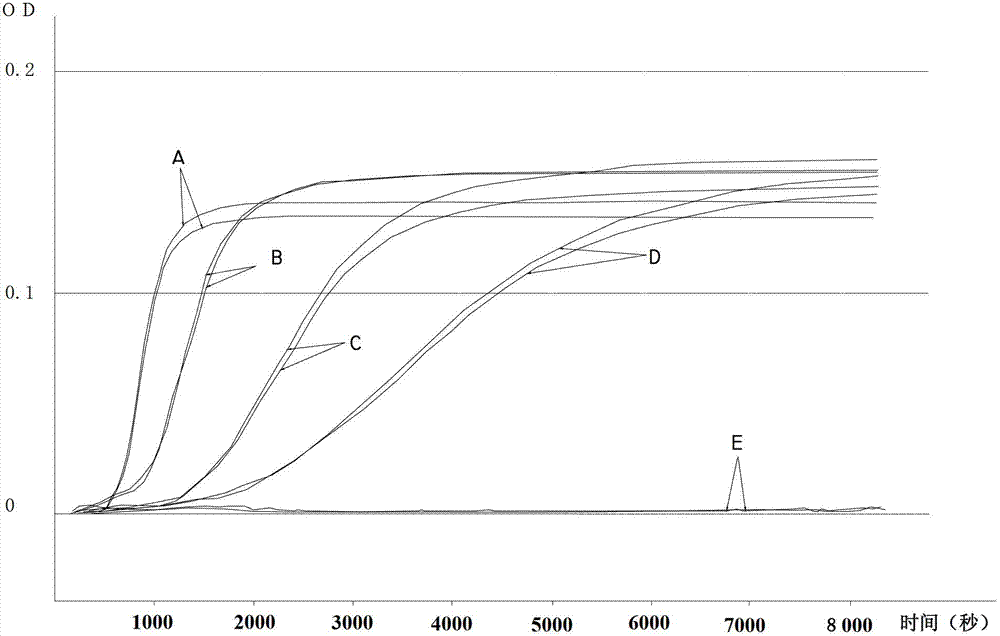
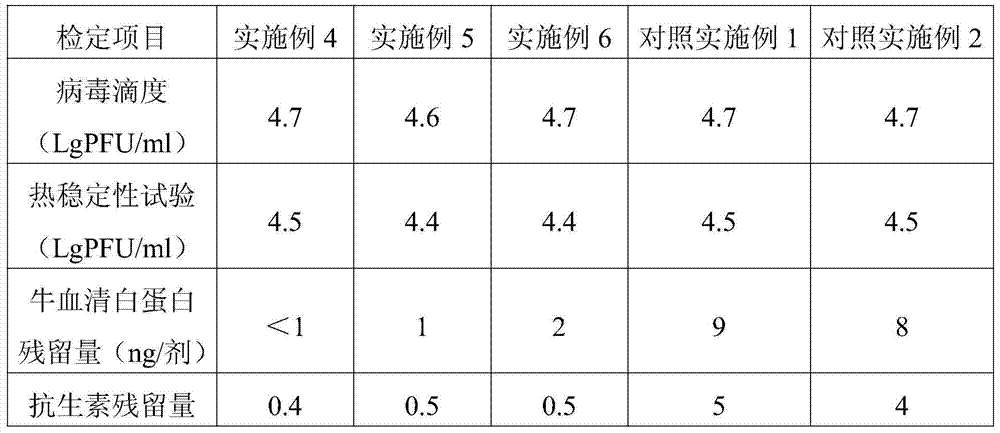
The invention discloses a freeze-dried vaccine protective agent, a freeze-dried varicella attenuated live vaccine and preparation methods of the freeze-dried vaccine protective agent and the freeze-dried varicella attenuated live vaccine, belongs to the field of vaccine production and preparation processes, and solves the problem that an existing freeze-dried vaccine protective agent of a varicella vaccine contains gelatin, or contains dextranum and still contains human serum albumin even if the gelatin is removed, so that a potential hazard still exists in the safety of medication of the vaccine. The freeze-dried vaccine protective agent contains mycose, sodium glutamate, urea, L-arginine and a 199 culture medium, and does not contain macromolecular allergen ingredients such as gelatin, dextranum and human serum albumin; the bacterial endotoxin content is low; the varicella vaccine prepared by using the protective agent is low in endotoxin content and residual content of bovine serum albumin and antibiotics, good in stability, safe and effective; the main point is direct infection after passage; the cells are cleaned in the next day after infection, and are replaced with a serum-free maintenance fluid; the operation is simple; the cost is low; regular and uniform formation of cytopathy is facilitated; and the residual content of the bovine serum albumin can be greatly lowered.
Owner:陈安明
Method for detecting anhydrous dextrose bacteria endotoxin content by spectrophotometry
InactiveCN101387647AExplain carefullyInterference test fastMaterial analysis by observing effect on chemical indicatorLuminous intensityTurbidity
The invention relates to a method for using luminous intensity to test the bacterial endotoxin content of glucosum anhydricum, belonging to the analytical chemistry technical field. The technical scheme is that: using luminous intensity to test the reaction time from the reaction of limulus polyphemus agent and the endotoxin to the turbidity of the mixture reaching a preset absorbency; finding the endotoxin content of the object glucosum anhydricum sample. According to the scheme, the method dilutes an endotoxin operation standard sample of known content into operation standard sample series of different contents, tests the times of different endotoxin content standards reaching the preset absorbency, and draws a standard curve; and tests the time of the object glucosum anhydricum sample reaching the preset value, to find the content of the endotoxin of the glucosum anhydricum sample.
Owner:XIWANG GROUP
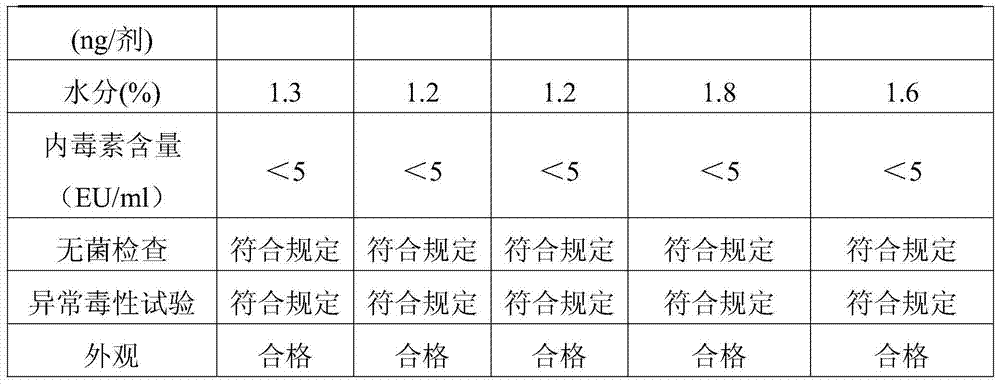
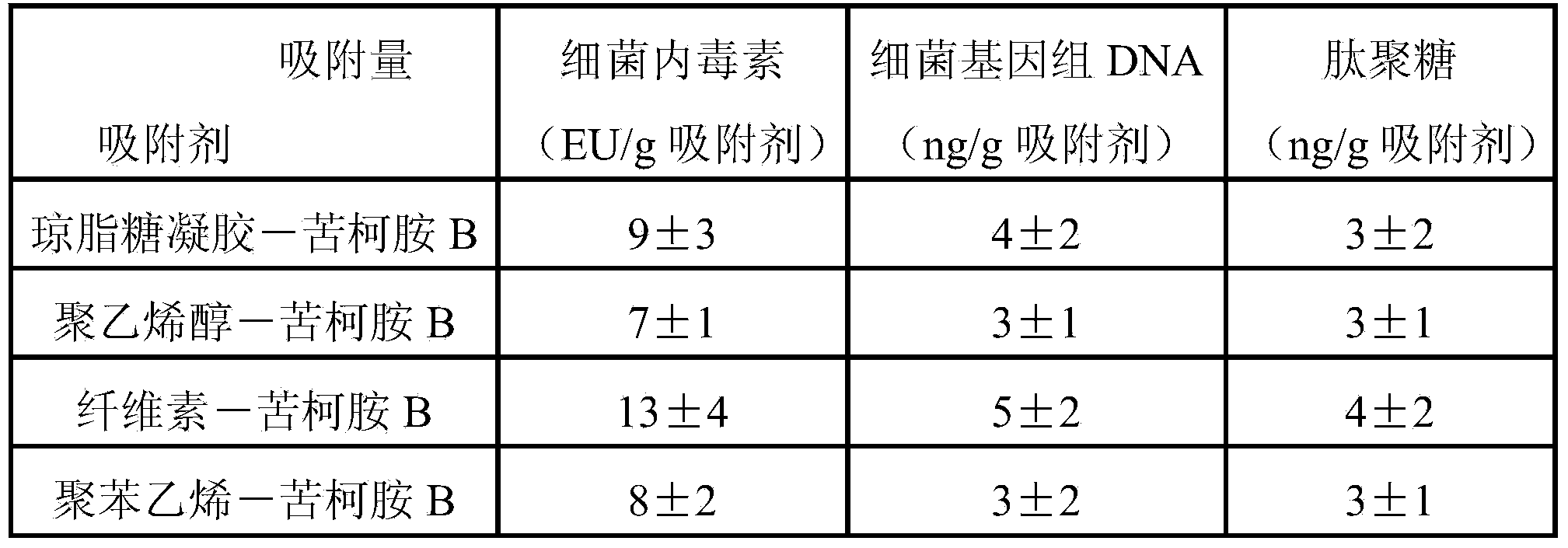
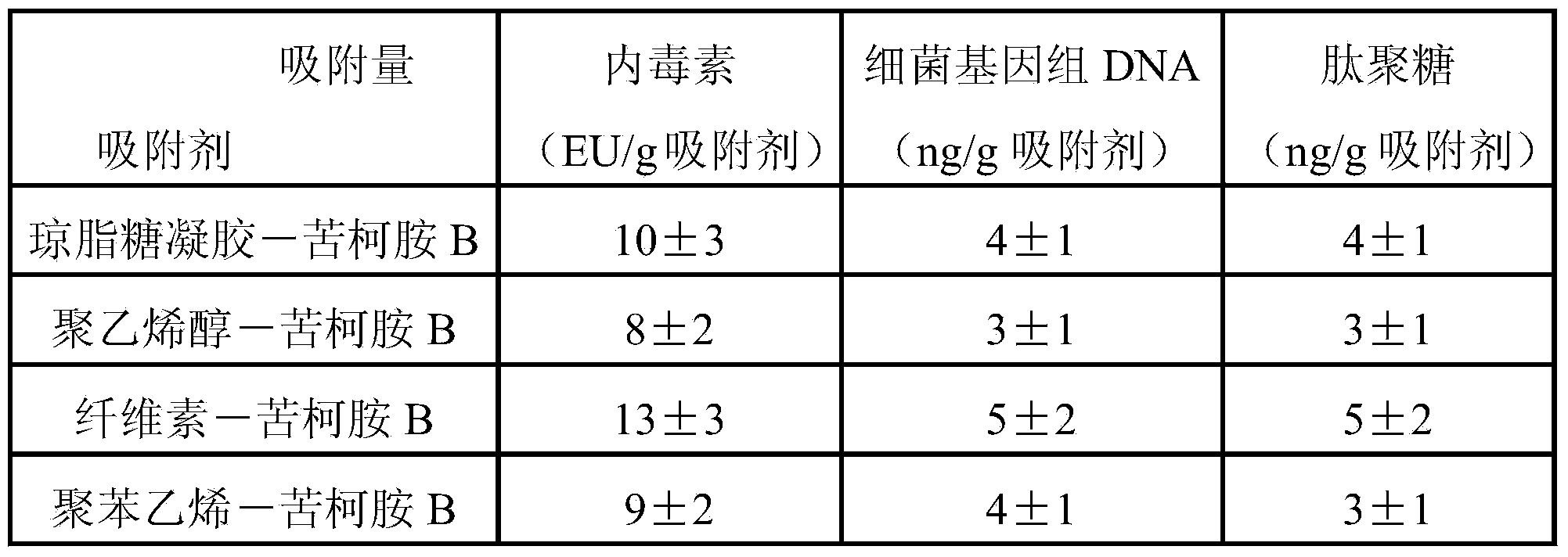
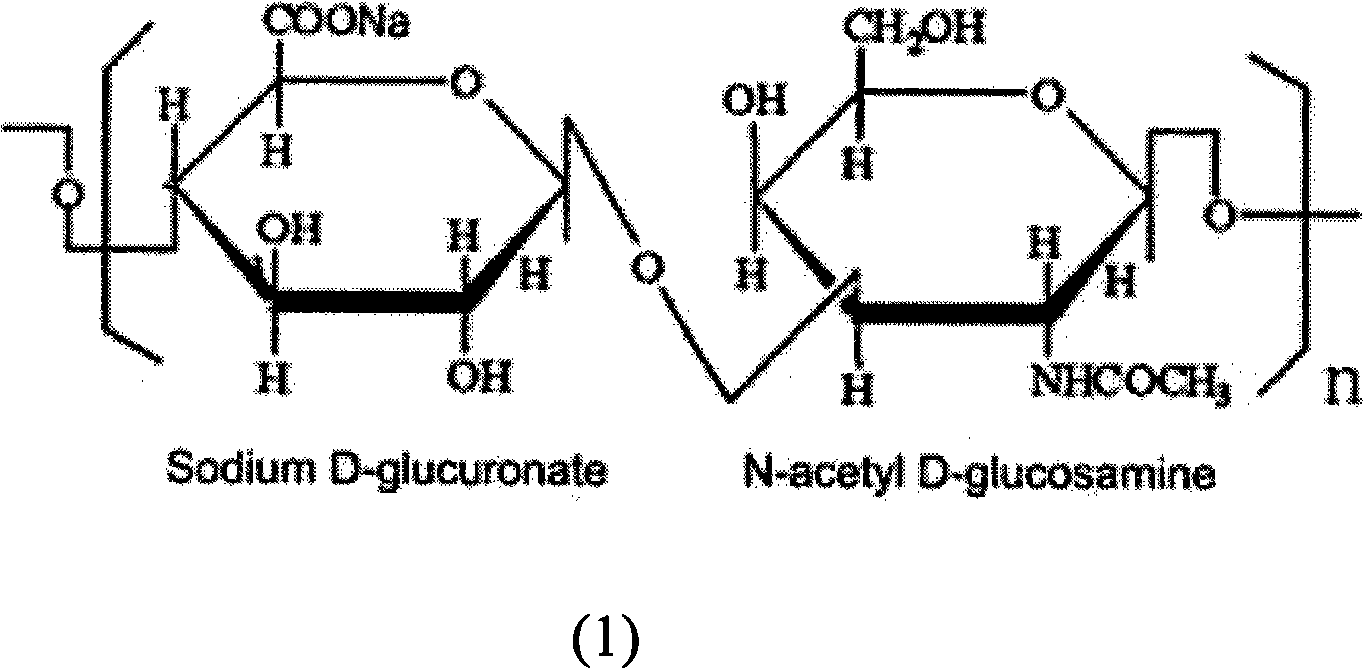
Adsorbent for removing bacterial endotoxin, DNA and peptidoglycan and preparation method and use
ActiveCN103769060ASolve the problem of only adsorbing bacterial endotoxinImprove adsorption capacityOther blood circulation devicesOther chemical processesClinical valuePeptidoglycan
The invention relates to an adsorbent for removing bacterial endotoxin, DNA and peptidoglycan. The adsorbent is connected with the surface of a carrier with good blood compatibility. The adsorbent is capable of effectively adsorbing bacterial endotoxin, DNA and peptidoglycan from blood of human body so as to achieve the aims of removing pathogene related molecular inducing the pyemia and exerting the effect of treating the pyemia, and the adsorbent has an important clinical value.
Owner:CHONGQING ZHENGBO BIOTECH CO LTD
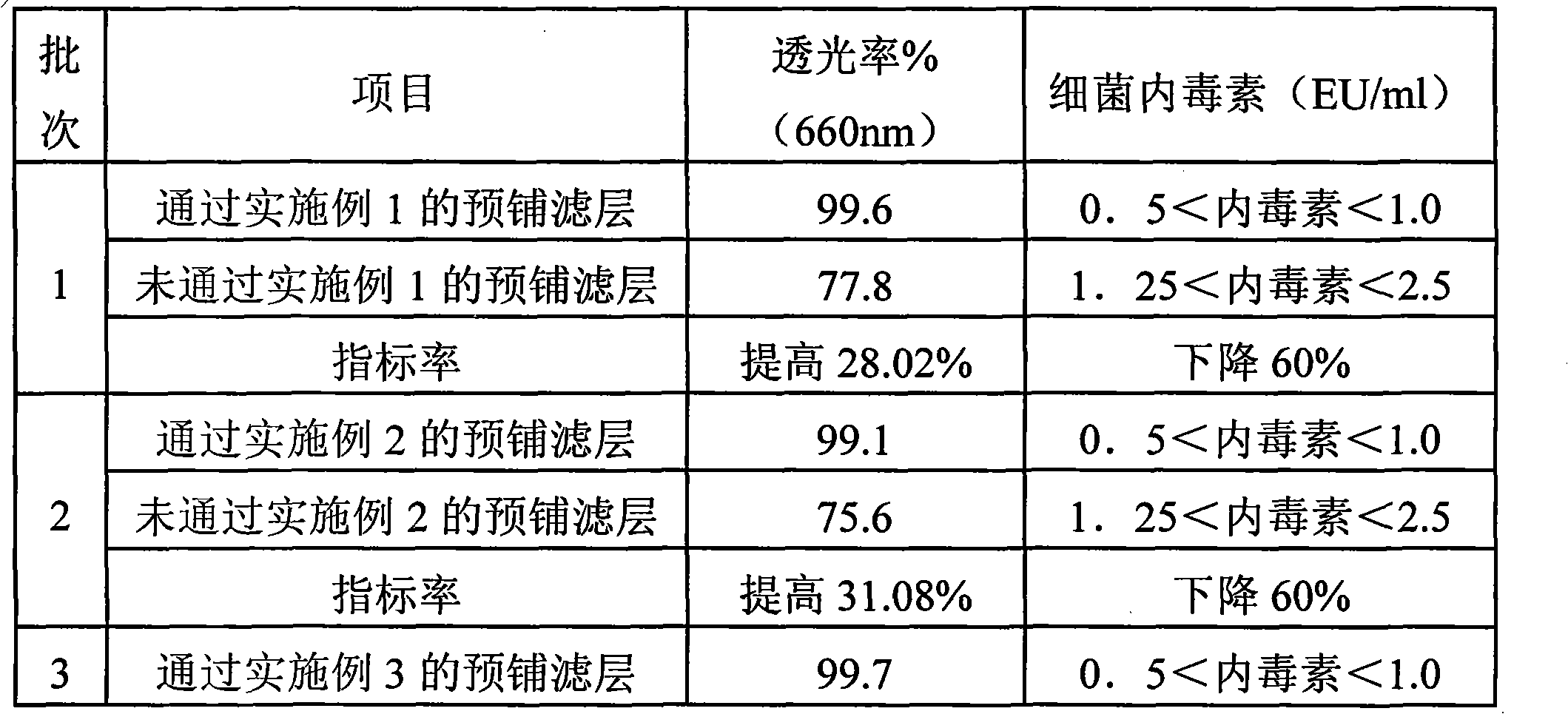
Method for purifying hyaluronic acid by pre-laying filter aid
ActiveCN101935362AIncrease filter aidImprove adsorption capacityFiltration separationPurification methodsFiltration
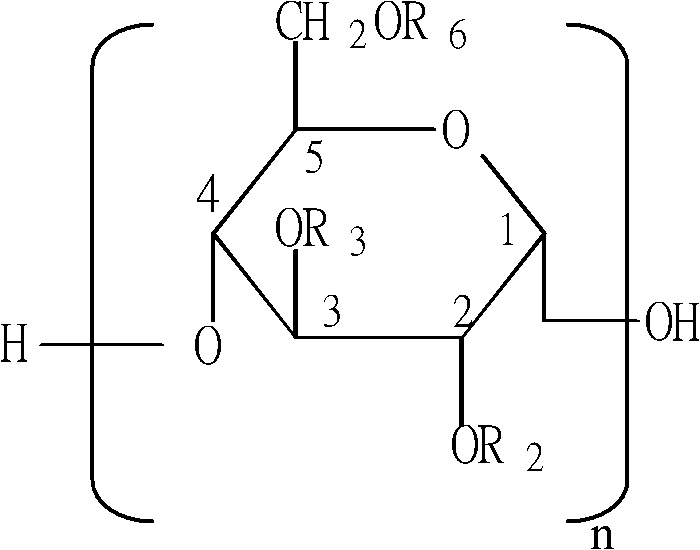
The invention relates to hyaluronic acid, in particular to method for purifying hyaluronic acid by pre-laying filter aid. In the step of kieselguhr and active carbon absorption and filtration, after the kieselguhr is pre-laid on a titanium bar filter as a filter layer, the enzymolysis solution or fermentation solution is filtered. When the method is used for preparing hyaluronic acid, the filtration assisting and absorbing effects of the kieselguhr are increased, the light transmittance of the filtrate can be improved, the bacterial endotoxin content of medicinal liquid is lowered effectively, and the impurities introduced to the medicinal liquid with the use of the kieselguhr are avoided. Compared with the overseas plate type coarse filtering method, the method reduces production cost, achieves the same effect and is particularly suitable for industrial production.
Owner:SHANGHAI JINGFENG PHARMA
Preparation of tetravalent wheel shaped virus inactivated vaccine and application
ActiveCN1686540APrevent infectious diseasesViral antigen ingredientsAntiviralsBacteroidesRotavirus RNA
A deactivated tetravalent rotavirus vaccine for preventing the infantile rotavirus infections diseases is prepared from the calf kidney cells digested and dispersed by pancreatin or cultured Vero cells through inoculating rotaviruses G1, G2, G3 and G4, culturing in non-serum culture liquid D-MEM, concentrating, purifying, deactivating, mixing and adding aluminium hydroxide.
Owner:LANZHOU INST OF BIOLOGICAL PROD
Methods and Compositions for the Detection of Microbial Contaminants
ActiveUS20080020422A1Microbiological testing/measurementBiological material analysisMicroorganismDextran
The invention provides methods and compositions for the detection and / or quantification of a microbial contaminant, for example, a bacterial endotoxin or a glucan, in a sample. In particular, the invention provides a test cartridge useful in the practice of hemocyte lysate-based assays for the detection and / or quantification of a microbial contaminant in a sample. In addition, the invention provides methods of making and using such cartridges. In addition, the invention provides a rapid, sensitive, multi-step kinetic hemocyte lysate-based assay for the detection and / or quantification of a microbial contaminant in a sample. In addition, the invention provides a glucan-specific lysate that can be used in a variety of assay formats, including, for example, a test cartridge, optionally configured to perform a kinetic assay.
Owner:CHARLES RIVER LAB INC
Method for producing recombinant human granulocyte colony stimulating factor
ActiveCN1814779ASimple production methodProduction method is stablePeptide preparation methodsCytokines/lymphokines/interferonsInclusion bodiesIon exchange
This invention relates to a method for producing recombined human granular leukocyte colony stimulating factors including: fermenting, breaking bactrriums, extracting occlusion bodies, chromatographing with molecular sieves, renaturating, exchanging anions, exchanging cations to get the raw fluid, in which, BL21 is selected as the host bacterium and the engineering bacterium type is got in high expression volume, quick reproduction and stable passage, several purification steps greatly reduce the residural toxin in the bacterium and the specific activity is increased greatly, a dialysis method is applied for the renaturation to reduce the concentration of the denaturalization agent steadily so the renaturation rate is at high level.
Owner:山东泉港药业有限公司
Shuanghuanglian traditional Chinese medicine toothpaste and preparation method thereof
ActiveCN102274139AGood colorGood characterCosmetic preparationsToilet preparationsAnti virusToothpaste
The invention provides Chinese medicinal (honeysuckle, baikal skullcap root and forsythia) toothpaste and a preparation method thereof. Each 100 grams of Chinese medicinal toothpaste contains 0.03 to 10 grams of honeysuckle extract, 0.03 to 10 grams of baikal skullcap root extract and 0.03 to 10 grams of weeping forsythia extract. The Chinese medicinal toothpaste has strong anti-inflammatory, antibacterial, anti-virus, immunity regulating, free radical clearing and anti-oxidation effects. The baikal skullcap root can inhibit caries bacteria, can effectively degrade bacterial endotoxin, and can be used for preventing and treating peridentitis; and the honeysuckle has antibacterial effect of different degree on most pathogenic bacteria, and the use of the honeysuckle for local medication isan effective means for preventing and treating oral diseases. The baikal skullcap root, the honeysuckle and the forsythia have the effects of clearing heat, detoxifying and dispelling wind and heat from the body; and the three Chinese medicinal extracts used together have synergistic effect, and can effectively inhibit the propagation of bacteria which generate peculiar smell in the oral cavity. Oral bacterial sensitivity test proves that the Chinese medicinal toothpaste has obvious effect of inhibiting related pathogenic bacteria in bacterial plaque of the gingival part.
Owner:HARBIN PHARM GROUP SANJING PHARMACEUTICAL CO LTD
Improved broad-spectrum endonuclease and industrial production method thereof
ActiveCN105985968AIntermediate processing steps are safeAvoid digestionFungiHydrolasesBiotechnologyFreeze-drying
The invention relates to DNA which achieves efficient expression in yeast cells and is used for coding improved broad-spectrum endonuclease, the improved broad-spectrum endonuclease which is coded by the DNA, and a method for constructing the DNA and the broad-spectrum endonuclease by virtue of genetic engineering technology and protein engineering technology; and the invention also relates to an industrial fermentation method for producing the improved broad-spectrum endonuclease. By virtue of the industrial fermentation method, the DNA, which is used for coding the improved broad-spectrum endonuclease, is expressed in eukaryotic host yeast cells, and the produced improved broad-spectrum endonuclease is high in specific activity and yield; therefore, the problems of the prior art that yield is low and an operation of conducting purifying is difficult. In addition, the improved broad-spectrum endonuclease disclosed by the invention, which is free from bacterial endotoxin, is conducive to application of medical and bio-engineering fields. Meanwhile, the improved broad-spectrum endonuclease disclosed by the invention can be also prepared into a dosage form of freeze-dried powder, so that the broad-spectrum endonuclease, as a finished product, is more convenient for transportation, preservation and industrial application.
Owner:GPROAN BIOTECH (SUZHOU) INC
Lyophilized powder injection of glutathione and preparation process thereof
InactiveCN1970075AQuality improvementQuality is easy to controlPowder deliveryTripeptide ingredientsDry weightFreeze-drying
The invention discloses a reduced-type glutathione freeze dried injection and making technique, which is characterized by the following: loading 95.0%-105.0% reduced-type glutathione (C10H17N3O6S) to display white block and powder; testing nitrosoferric sodium cyanide to display red; keeping the consistent reserving time of main peak of sample solution as comparison sample solution with pH value at 5.0-6.5; making dried weight not over 5.0% and the content of relative material not more than 4%; limiting the content of bacterial endotoxin less than 0.125EU; making other items satisfy the standard.
Owner:SHANDONG LUYE PHARMA CO LTD
Cell free biosynthesis of high-quality nucleic acid and uses thereof
InactiveCN101103122ASimple purification stepsImprove fidelityFermentationCell freeRestriction enzyme digestion
The invention provides an improved cell free amplification method capable of producing large quantities of therapeutic-quality nucleic acids and methods of using the synthesized nucleic acid in research, therapeutic and other applications- The methods combine several different state-of-the-art procedures and coordinate their applications to affordably synthesize nucleic acids for therapeutic purposes. It combines in vitro rolling circle amplification, high fidelity polymerases, high affinity primers, and streamlined template specifically designed for particular applications. For expression purposes, the templates contain an expression cassette including a eukaryotic promoter, the coding sequence for the gene of interest, and a eukaryotic termination sequence. Following amplification, concatamers are subsequently processed according to their intended use and may include: restriction enzyme digestion for the production of short expression cassettes (SECs); ligation steps to circularize the SEC (CNAs); and / or supercoiling steps to produce sCNAs. The final product contains nearly non-detectable levels of bacterial endotoxin.
Owner:CYTOGENIX INC
Veterinary traditional Chinese medicine for preventing and treating fish diseases
InactiveCN103989852APromote growth and developmentEffective prevention and treatmentAntibacterial agentsAnthropod material medical ingredientsGinkgo bilobaRheum officinale
The invention relates to a veterinary traditional Chinese medicine for treating fish diseases. The medicine is characterized by comprising the following components in parts by weight: 5-10 parts of folium isatidis, 4-8 parts of coptis chinensis, 6-12 parts of folium cortex eucommiae, 2-6 parts of Chinese Gall, 3-7 parts of acorus calamus, 8-15 parts of gingko leaf, 2-6 parts of pomegranate bark, 4-10 parts of scutellaria baicalensis, 8-15 parts of felwort, 3-8 parts of radix sophorae flavescentis, 6-12 parts of radix bupleuri, 5-10 parts of rheum officinale and 4-9 parts of common andrographis herb. The compound traditional Chinese medicine is synergetic and complementary in medicinal materials, has the effects of resisting bacteria, virus, bacterial endotoxin, fungus and parasite, improving immunity and promoting fish body growth and development, can simultaneously act on multiple targets of multiple diseases of fish, can be used for effectively preventing and treating hemorrhagic disease, septicemia, sepsis and other diseases caused by pathogenic bacterium / or bacterial products (such as bacterial endotoxin), parasite, virus and the like, and has remarkable preventing and treating effects on fish composite infectious diseases caused by multiple infectious agents.
Owner:湖州天健兽药有限公司
Kit for removing bacterial endotoxin in biological product, method thereof, and preparation method of biological product
ActiveCN104370997AEasy to removeDoes not affect biological activitySerum albuminMicrobiological testing/measurementEndotoxin removalPotassium
The invention discloses a kit for removing bacterial endotoxin in a biological product, a method for removing the bacterial endotoxin in the biological product by using the kit, and a preparation method of the endotoxin removed biological product. The kit includes an anionic surfactant and a potassium salt. The methods commonly comprise the following steps: fully combining the anionic surfactant with the endotoxin in the biological product to form a conjugate, adding the potassium salt to precipitate the conjugate, filtering to remove the obtained precipitate in order to obtain an endotoxin removed biological product solution, and separating the biological product from the biological product solution. The kit can be used to simply and efficiently remove the endotoxin in the biological product and has a low cost, and the endotoxin removal method has the advantages of easy operation, no influences on the bioactivity of the biological product, and only use of compounds allowed in the medicine industry. The content of the endotoxin in the biological product prepared by using the methods accords with clinic pharmacy standards, and the loss of active substances is less.
Owner:陈辉
Deporteinnized calf serum injection and its preparing method
InactiveCN1899307AHigh content of active ingredientsHigh purityPowder deliveryNervous disorderSide effectCurative effect
The present invention discloses a kind of deproteinized calf serum injection and its preparation process. Of the deproteinized calf serum injection, each milliliter contains solid 0.035-0.055 g, free amino acid 0.6-1.5 mg, and polypeptide 0.4-3.0 mg. The injection of the present invention has bacterial endotoxin content lower than 1 EU, and has pH value of 6.0-8.0. The injection has high effective component content, high bioactivity, high purity and low impurity content. Tests show that the injection of the present invention has high clinical curative effect, no any toxic side effect and no allergic reaction.
Owner:赵红梅
Method for removing bacterial endotoxins in cephalosporin antibiotics by utilizing macroporous adsorption resins
The invention discloses a method for removing bacterial endotoxins in cephalosporin antibiotics by utilizing macroporous adsorption resins, comprising the following steps: firstly filtering the cephalosporin antibiotics solution containing the bacterial endotoxins and removing solid insoluble matters; then utilizing the pretreated macroporous adsorption resins to carry out static adsorption or dynamic adsorption on the cephalosporin antibiotics solution; and finally crystallizing, drying and grinding the solution after adsorption treatment, thus obtaining the product with bacterial endotoxins in accordance with the quality standard. The method has the advantages of high bacterial endotoxins removal rate, good selectivity, easy desorption, repeated use, low fluid resistance and easy magnification.
Owner:东瑞(南通)医药科技有限公司
Homologous system model method for quantitatively detecting bacterial endotoxin of blood
InactiveCN1469124AEliminate distractionsEnhanced inhibitory effectColor/spectral properties measurementsBiological testingInterference factorSystematic deviation
The present invention relates to homologous system model method for quantitatively detecting bacterial endotoxin of blood, and aims at solving the problem of difference or interference caused by different system property. In the system model method, one new concept is proposed for solving the BET interference of the sample, i. e., all the unkown interference factors are considered as systematic deviation. Once the systematic deviation is eliminated, the said BET interference of the sample will be eliminated. In some reaction system, digital model is established and sample is detected in the same system, the difference caused by different system property will be eliminated.
Owner:湛江安度斯生物有限公司
A clean production method of medium molecular weight hydroxyethyl starch
The invention provides a clean production method of medium molecular weight hydroxyethyl starch, in particular to a clean production method in the refining process of chemical raw materials hydroxyethyl starch 200 / 0.5 and hydroxyethyl starch 130 / 04 used for plasma expanders. Bacterial endotoxin is the key toxic substance leading to clinical infusion pyrogenic reaction. The bacterial endotoxin in the raw material drug of hydroxyethyl starch exceeds the limit, which can easily lead to the limit of bacterial endotoxin in the final product of the preparation, hydroxyethyl starch injection, and the unqualified rate increases. The method of the invention is controlled by necessary process conditions such as clean air spray drying under clean conditions, microbial control under high temperature conditions, and on-line cleaning and disinfection of equipment, so that the quality of the bacterial endotoxin index of the obtained final product is obviously better than the current national drug quality standards. At the same time, it provides the necessary conditions for the direct preparation method of raw materials without microfiltration, which is helpful for the qualification of the insoluble particle index of the preparation, shortens the process flow of the final preparation, and reduces the probability of pollution.
Owner:WUHAN HUST LIFE SCI & TECH
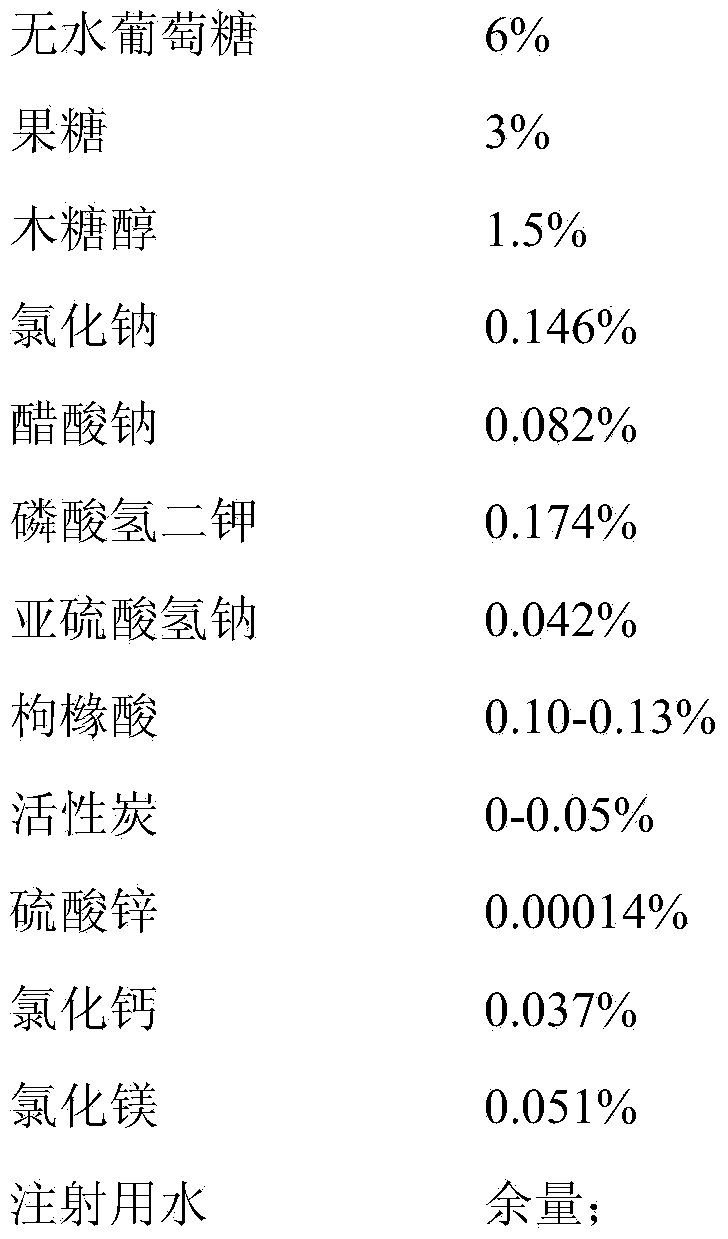
Mixed carbohydrate-electrolyte injection and preparation method thereof
ActiveCN103405473AAvoid the risk of introducing impurities such as metal ionsSimple processOrganic active ingredientsInorganic phosphorous active ingredientsHydroxymethylfurfuralPharmaceutical formulation
The invention belongs to the field of a pharmaceutical preparation, and particularly relates to a mixed carbohydrate-electrolyte injection and a preparation method thereof. A pH modifier of the injection is citric acid, and pH is 4.0-4.4. By controlling the dosage range of the pH modifier, the pH range of the finished product can meet the specification only by regulating the pH value for one time in the preparation process. In addition, according to the method, activated carbon is added only in the step, and the activated carbon is not added in the preparation process, so that the risk of introducing metal ions and other impurities through the activated carbon can be effectively avoided, and toxins in bacteria of the finished product are qualified. According to the method, the technology is further simplified, the production time and cost can be shortened and lowered, and the sterilized product has no crystallization or precipitation phenomenon. After being stored for 24 months, the 5-hydroxymethyl-furfural range of the injection is within 0.03-0.05, and the impurities are controlled at the extremely low level; after being stored for 24 months, the injection almost has no character change and is still colorless clear liquid compared with the injection which is stored for zero day.
Owner:SHANDONG QIDU PHARMA
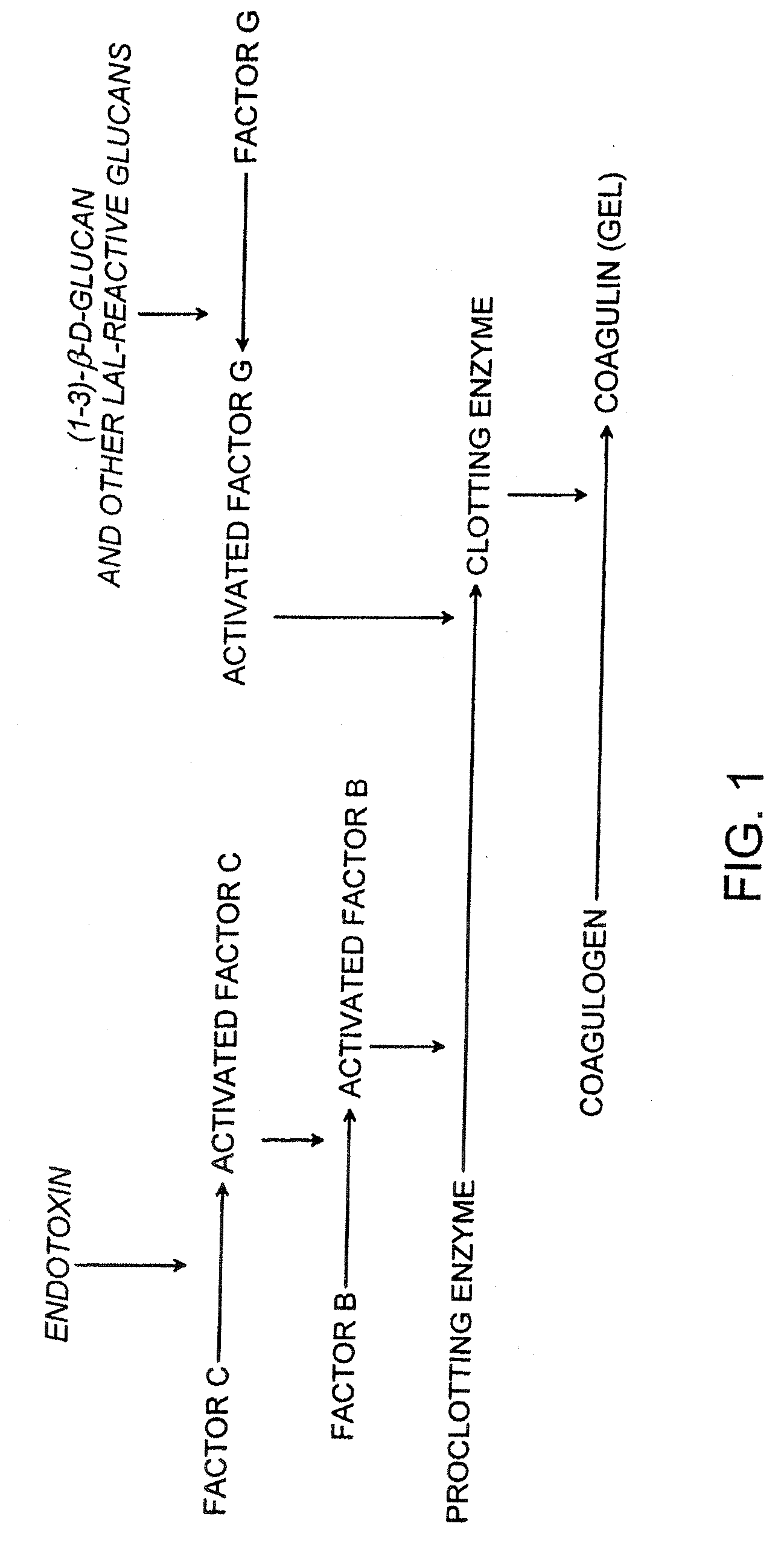
Recombinant limulus three-factor reagent and method for detecting endotoxin with same
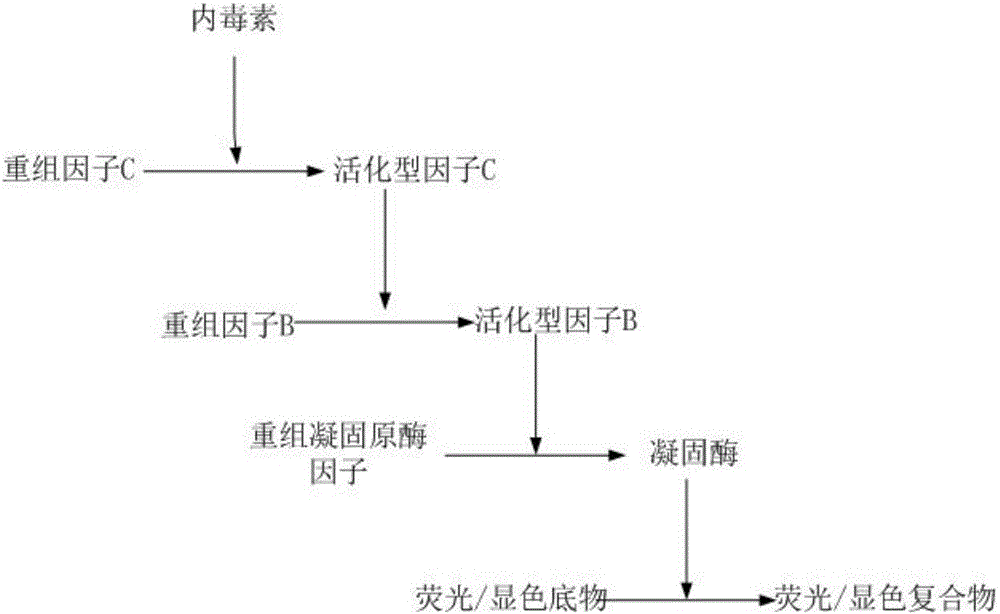
ActiveCN105866080AEfficient and sensitive detectionSolve the problem of a sharp decrease in the number ofFluorescence/phosphorescenceBacteroidesLimulus factor C
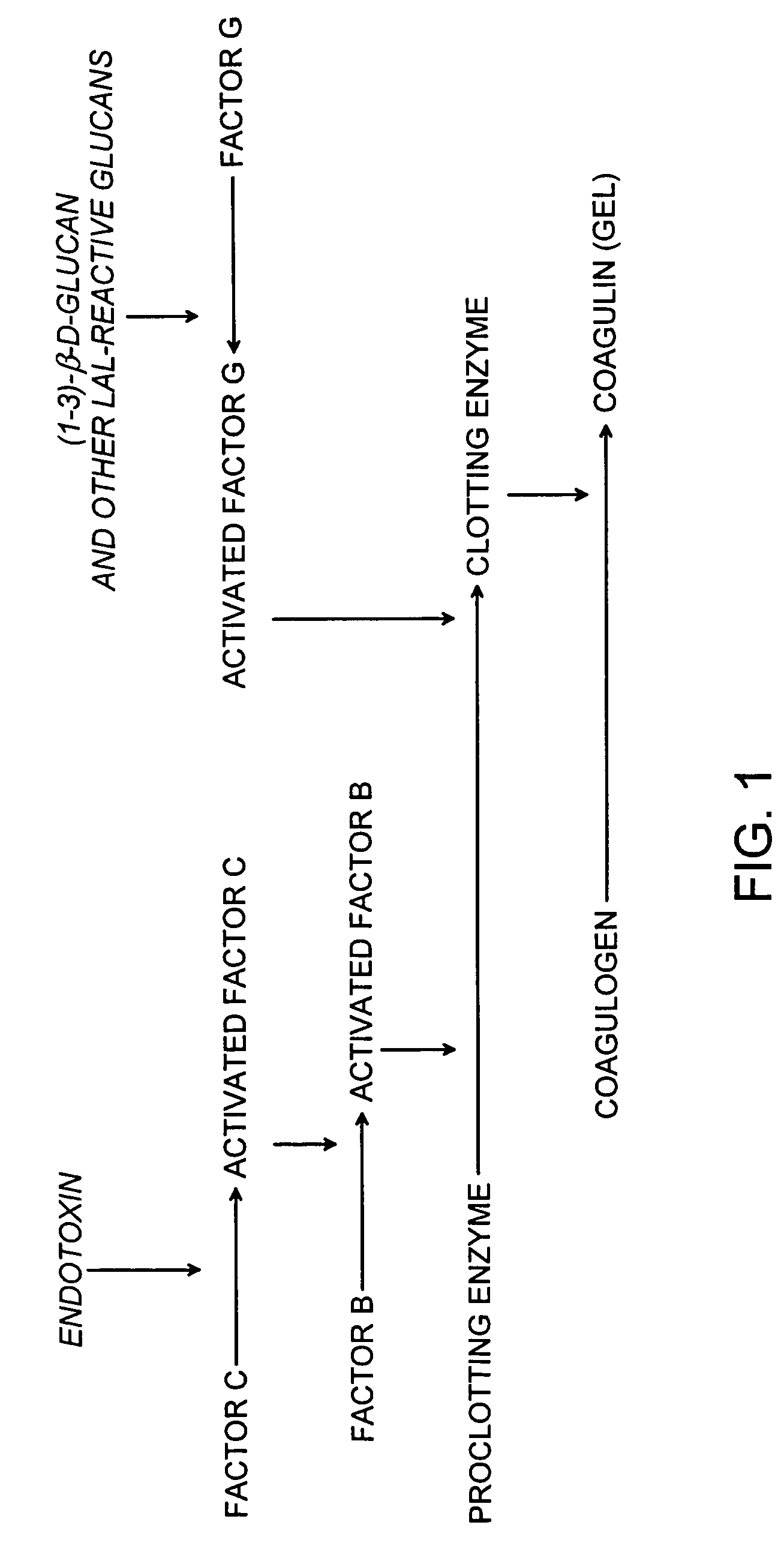
The invention discloses a recombinant limulus three-factor reagent which comprises the following components: endotoxin-free water, a heat-resource-free Tris buffer liquid, Tween-20, a fluorescent substrate, a recombinant limulus factor C, a recombinant limulus factor B and a recombinant limulus proclotting enzyme factor. The invention provides the recombinant limulus three-factor reagent and a method for detecting endotoxin with the same, three recombinant limulus factors (the proclotting enzyme factor, the recombinant limulus factor B and the recombinant limulus factor C) can be expressed by using an insect baculovirus expression system, the reagent can be used for detecting bacterial endotoxin, and by adopting the method, bypass interference of a factor G can be eliminated, so that the possibility of false positive caused by glucan is effectively avoided; moreover, the defects that the number of limulus is limited and different batches of limulus reagents are not stable are avoided, and thus the reagent can be used as a novel endotoxin detection reagent.
Owner:XIAMEN BIOENDO TECH CO LTD
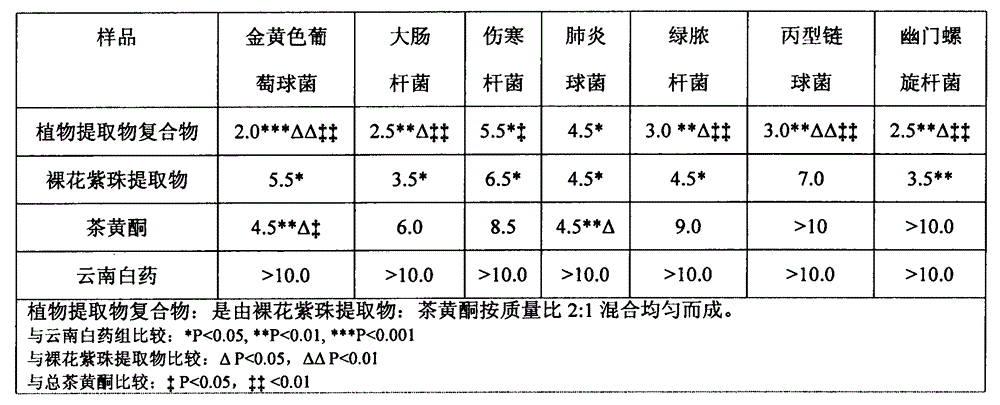
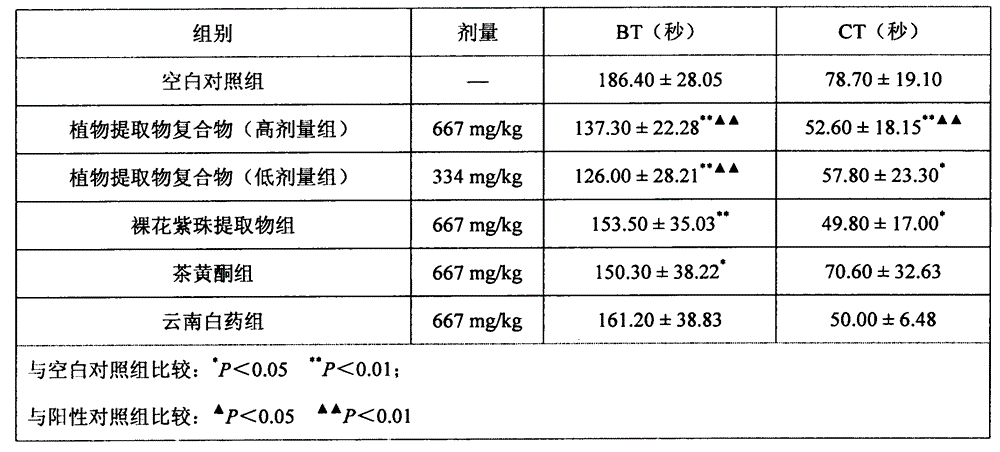
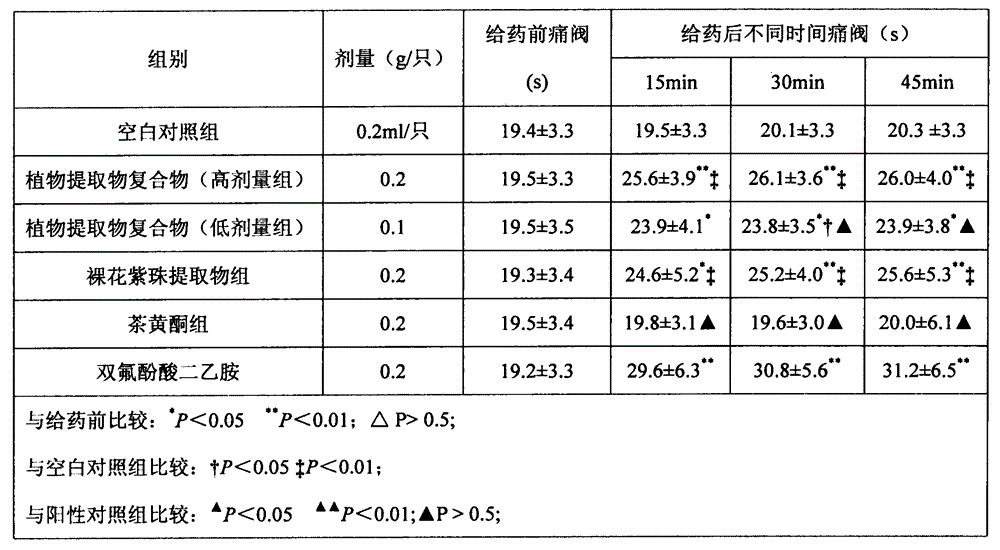
Plant extract compound capable of inhibiting bacteria, resisting inflammation, stopping bleeding and relieving pain and application of plant extract compound
ActiveCN104435379AEasy to produceEasy to useAntibacterial agentsCosmetic preparationsCallicarpa nudifloraTherapeutic effect
The invention discloses a plant extract compound capable of inhibiting bacteria, resisting inflammation, stopping bleeding and relieving pain and an application of the plant extract compound. The plant extract compound is prepared from callicarpa nudiflora extract and flavonoids according to a mass ratio of (1-5) to (5-1). The callicarpa nudiflora extract has the functions of resisting inflammation, inhibiting bacteria, resisting viruses, stopping blood, relieving pain, adjusting immunity, removing free radicals and resisting oxidation; the callicarpa nudiflora extract has an effect of resisting most of pathogenic bacteria to different extents. The flavonoids have the effects of inhibiting dental caries, effectively degrading bacterial endotoxin, and preventing and treating periodontitis. The antibacterial, anti-inflammatory, analgesia and hemostasis experiments prove that the plant extract compound is exact in therapeutic effect, natural, healthy and easy to produce; the use effect of the plant extract compound is obviously superior to that of the components of oral cavity cleaning products. Therefore, the plant extract compound has a good market development prospect.
Owner:弘海融合发展深圳有限公司
Method for detecting bacterial endotoxin in citric acid raw material
InactiveCN104232739ASolve inspection problemsEnsure safetyMicrobiological testing/measurementInjection productToxin
The invention discloses a method for detecting bacterial endotoxin in a citric acid raw material. The method comprises the following steps of firstly carrying out interference test to determine the sensitivity of tachypleus amebocyte lysate selected to detect the bacterial endotoxin in the citric acid raw material; and then diluting the to-be-detected citric acid raw material to prepare a to-be-detected solution, adding the tachypleus amebocyte lysate determined in the interference test and detecting the bacterial endotoxin. By virtue of the method for detecting bacterial endotoxin in the citric acid raw material, which is disclosed by the invention, the problem of detection of the bacterial endotoxin in the citric acid raw material for a newly developed injection product is solved; the quality of a sterile product is controlled at the source and the method is of great significance for ensuring the safety of the injection product. Since the method has a theoretical basis of biochemistry, the method is more scientific; molecular and biochemical reactions of an enzyme are applied and the method has the advantages of high specificity, strong reproducibility, high accuracy and sensitivity, no need of special instrument and equipment and strong practicability and is simple and quick to operate.
Owner:CHINA OTSUKA PHARM CO LTD
Method for detecting content of bacterial endotoxin of bromhexine hydrochloride raw material
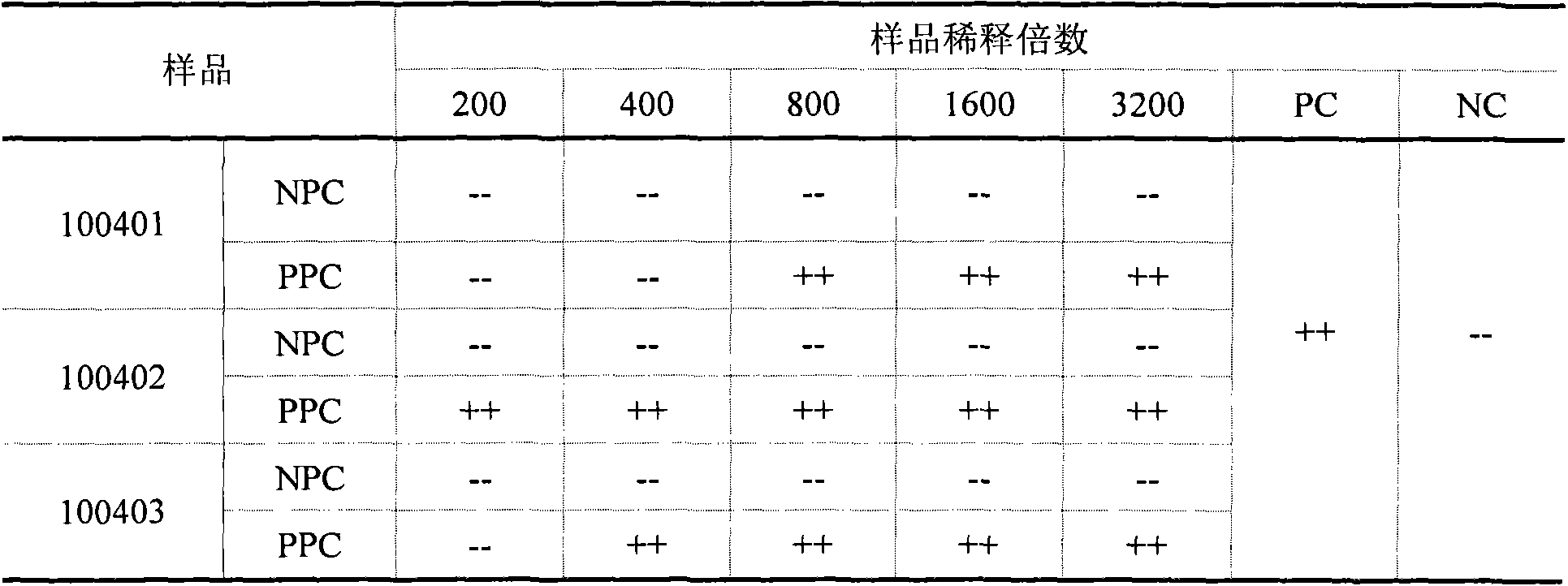
InactiveCN103837673AAccurate contentReduce inspection costsPreparing sample for investigationBiological testingInterference factorLimit value
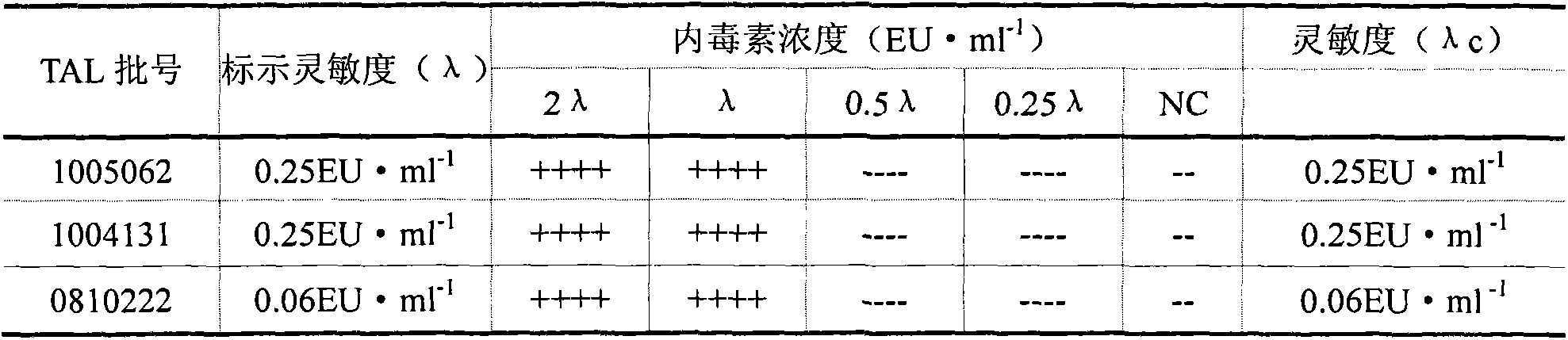
The invention aims at providing a sensitive and reliable method, and particularly relates to a method for quickly detecting the content of bacterial endotoxin of a bromhexine hydrochloride raw material. The method comprises the following steps of measuring bromhexine hydrochloride powder, dissolving the bromhexine hydrochloride powder in DMSO (dimethyl sulfoxide) to have the pre-interference experiment and find out the maximal dilution times; preparing a bromhexine hydrochloride test solution according to 1600 times of the maximal dilution times to have the interference experiment with two TAL (tachypleus amebocyte lysate) with the sensitivity of 0.25EU / ml from two different factories, finally confirming whether the interference factor exists or not, and determining the limit value of the bacterial endotoxin; diluting the bromhexine hydrochloride solution according to the non-interfered maximal valid dilution times, and then examining the bacterial endotoxin.
Owner:张嵩
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com