Freeze-dried vaccine protective agent, freeze-dried varicella attenuated live vaccine and preparation methods of freeze-dried vaccine protective agent and freeze-dried varicella attenuated live vaccine
A technology of freeze-dried protective agent and live attenuated vaccine, which is applied in the field of vaccine production and preparation technology, can solve the problems of hidden safety hazards in vaccine administration, and achieve the effects of low bovine serum albumin residue, stable virus titer, and less allergic reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
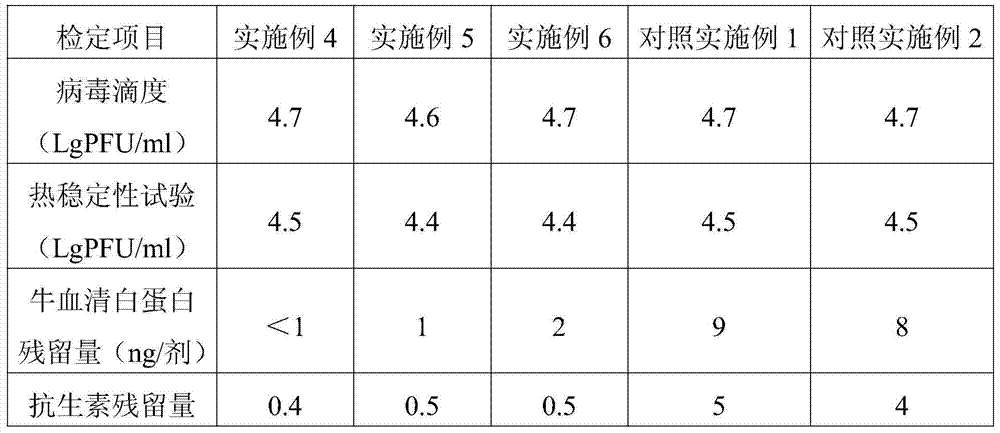
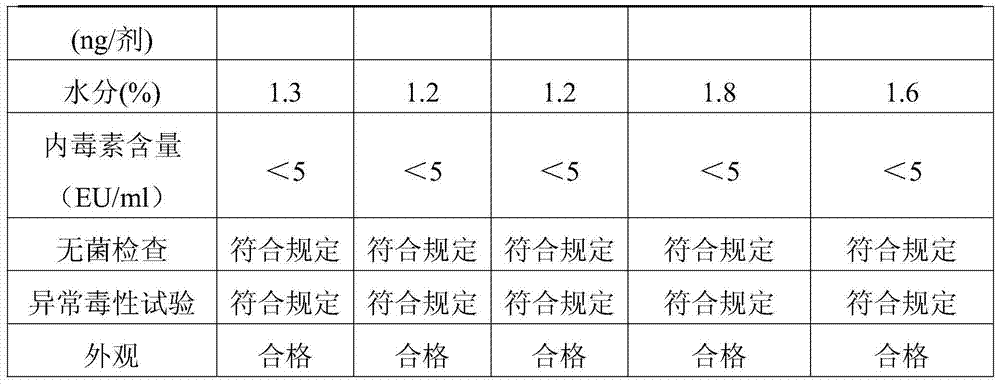
Examples
Embodiment 1
[0026] Example 1 Preparation of vaccine lyoprotectant
[0027]According to the proportion of trehalose 75g / l, sodium glutamate 10g / l, urea 5g / l, L-arginine 2g / l, 199 culture medium 9.9g / l, weigh each material, fully dissolve in appropriate amount of sterile injection After watering, dilute to volume, use 1N hydrochloric acid to adjust the pH value to 7.3, sterilize and filter to get ready; store at 2-8°C for later use, and the validity period is 12 months.
Embodiment 2
[0028] Example 2 Preparation of vaccine lyoprotectant
[0029] According to the ratio of trehalose 50g / l, sodium glutamate 15g / l, urea 8g / l, L-arginine 2g / l, 199 culture medium 9.9g / l, weigh each material, fully dissolve in appropriate amount of sterile injection After watering, dilute to volume, use 1N hydrochloric acid to adjust the pH value to 7.2, sterilize and filter to get ready; store at 2-8°C for later use, and the validity period is 12 months.
Embodiment 3
[0030] Example 3 Preparation of Vaccine Lyoprotectant
[0031] According to the proportion of trehalose 100g / l, sodium glutamate 5g / l, urea 2g / l, L-arginine 2g / l, 199 culture medium 9.9g / l, weigh each material, fully dissolve in appropriate amount of sterile injection After watering, dilute to volume, use 1N hydrochloric acid to adjust the pH value to 7.4, sterilize and filter to get ready; store at 2-8°C for later use, and the validity period is 12 months.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com