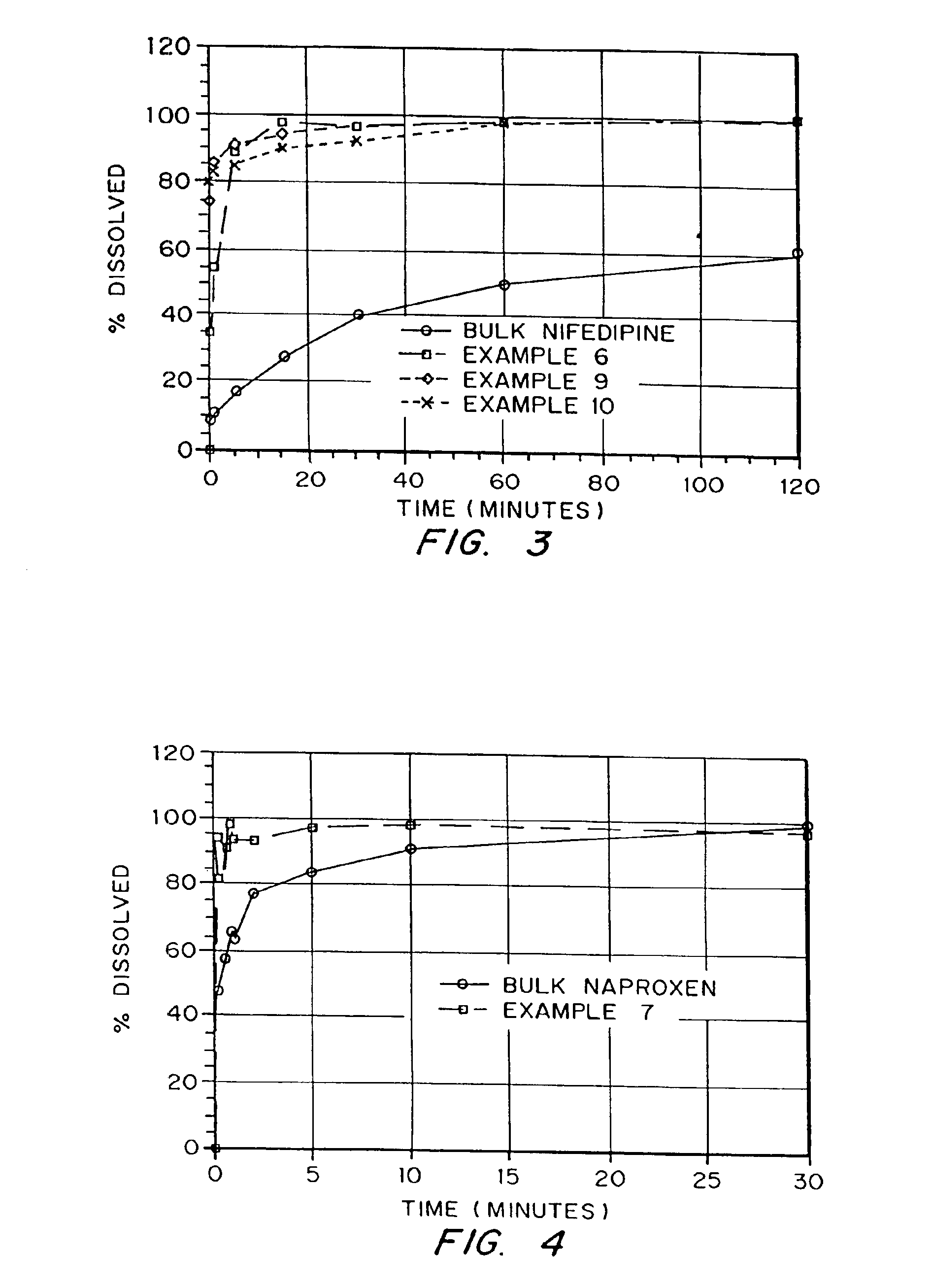
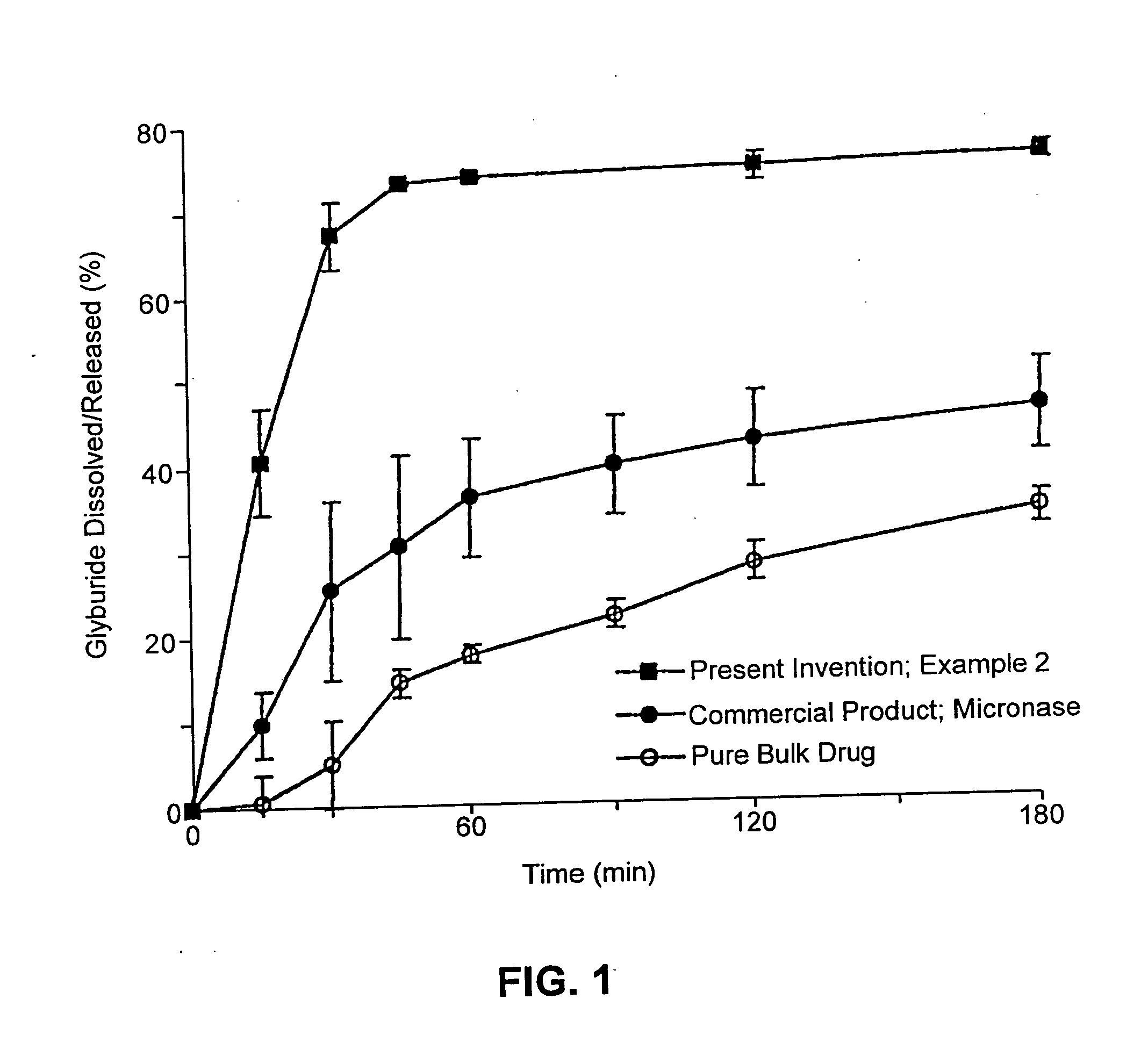
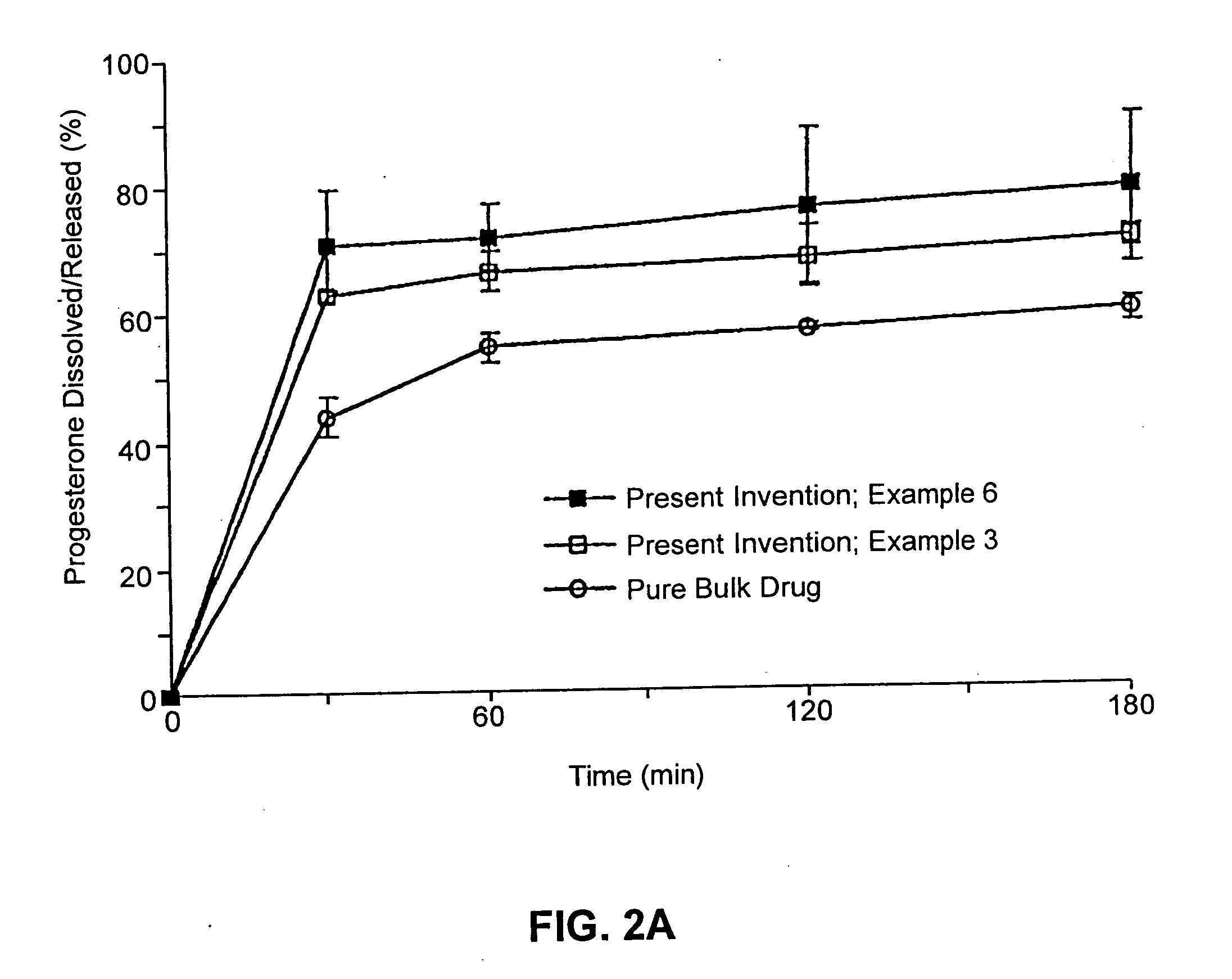
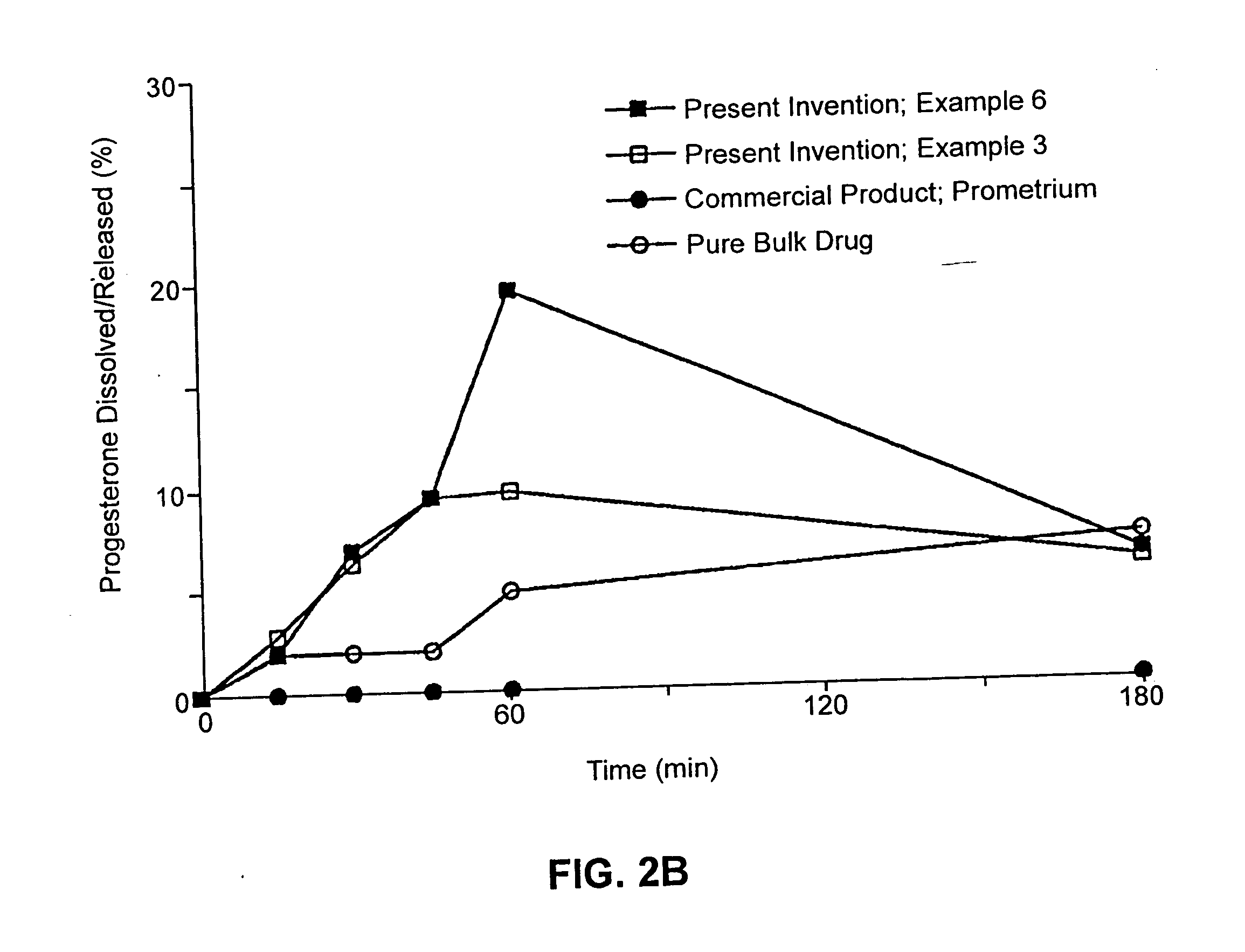
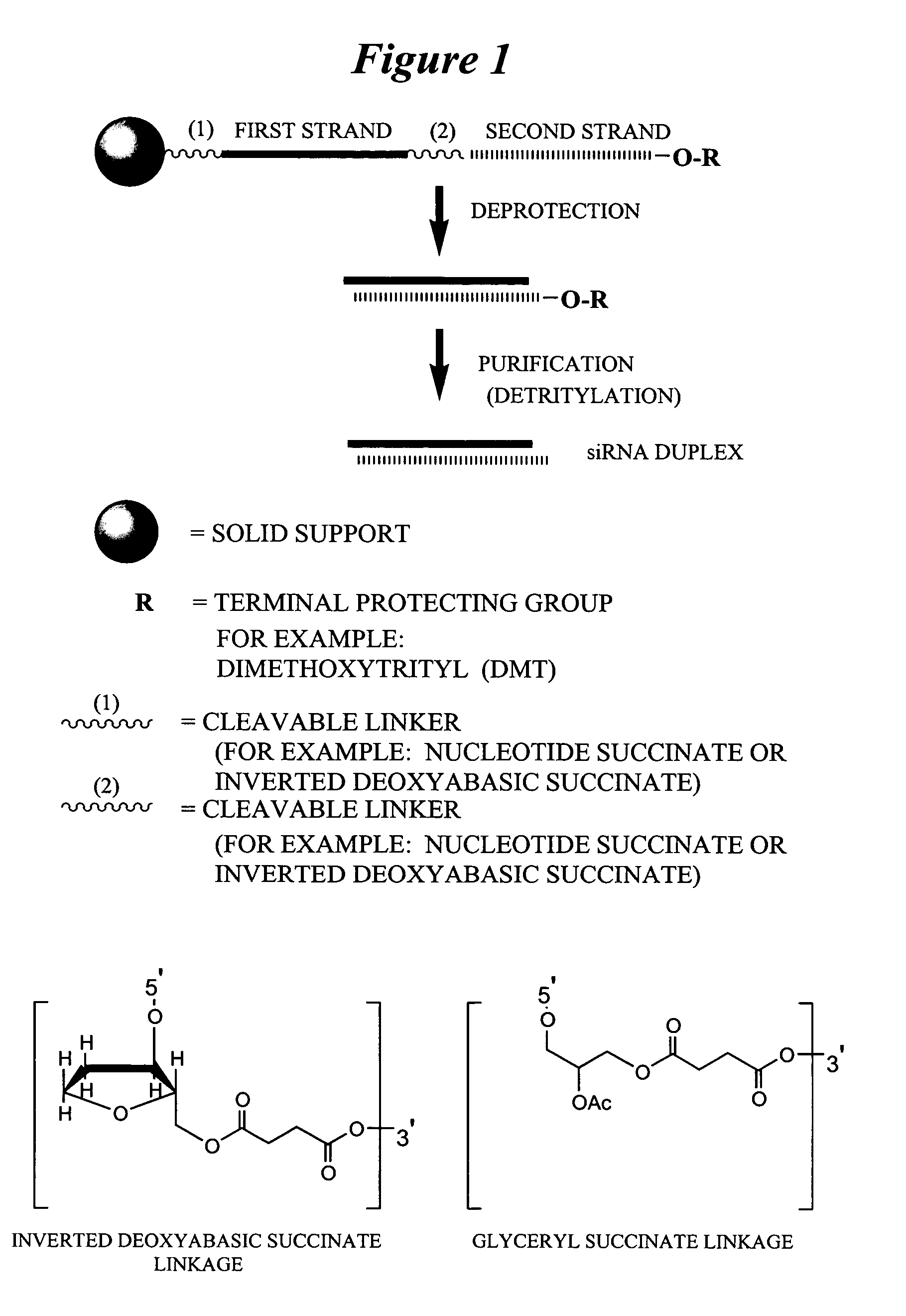
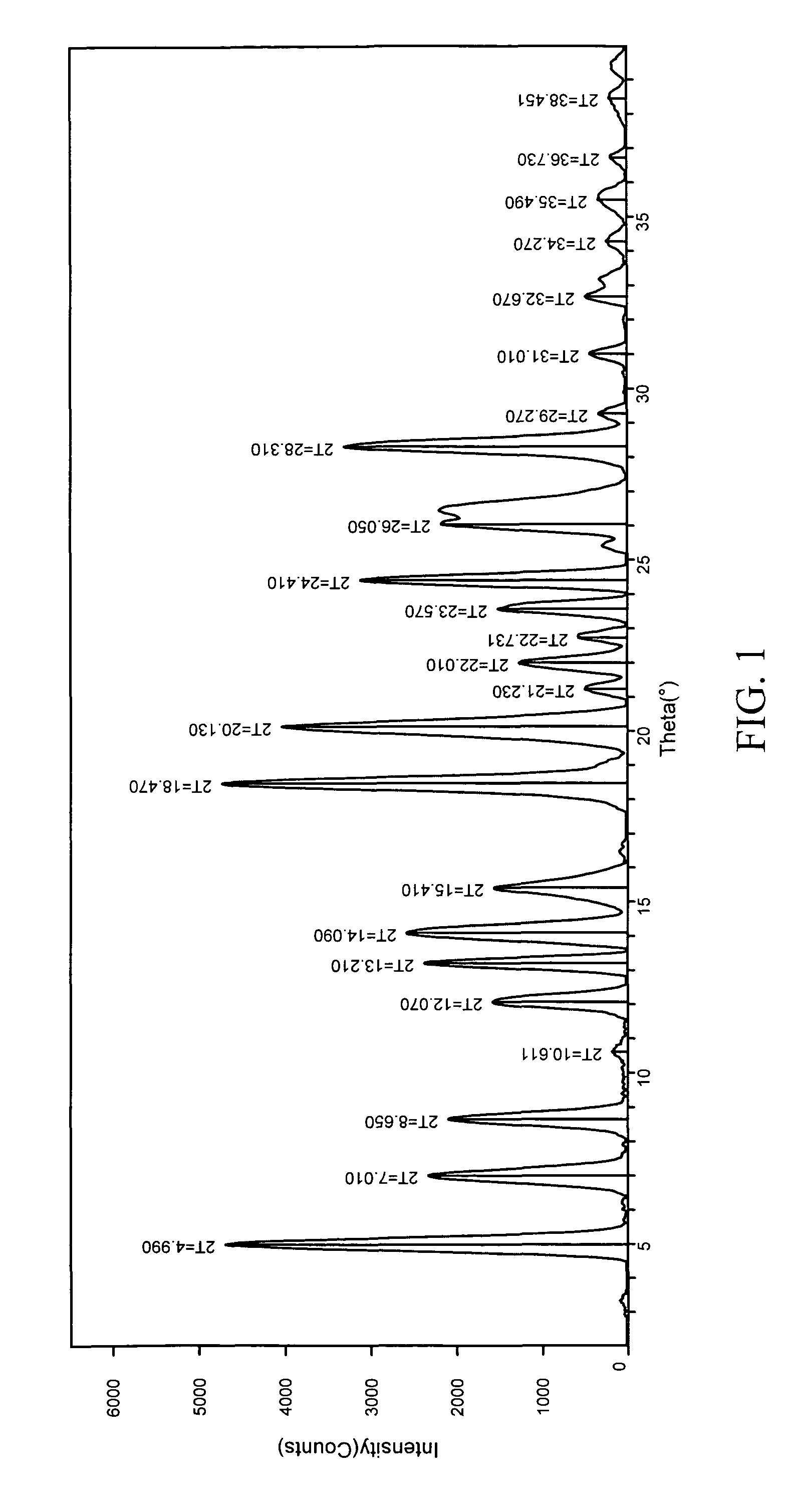
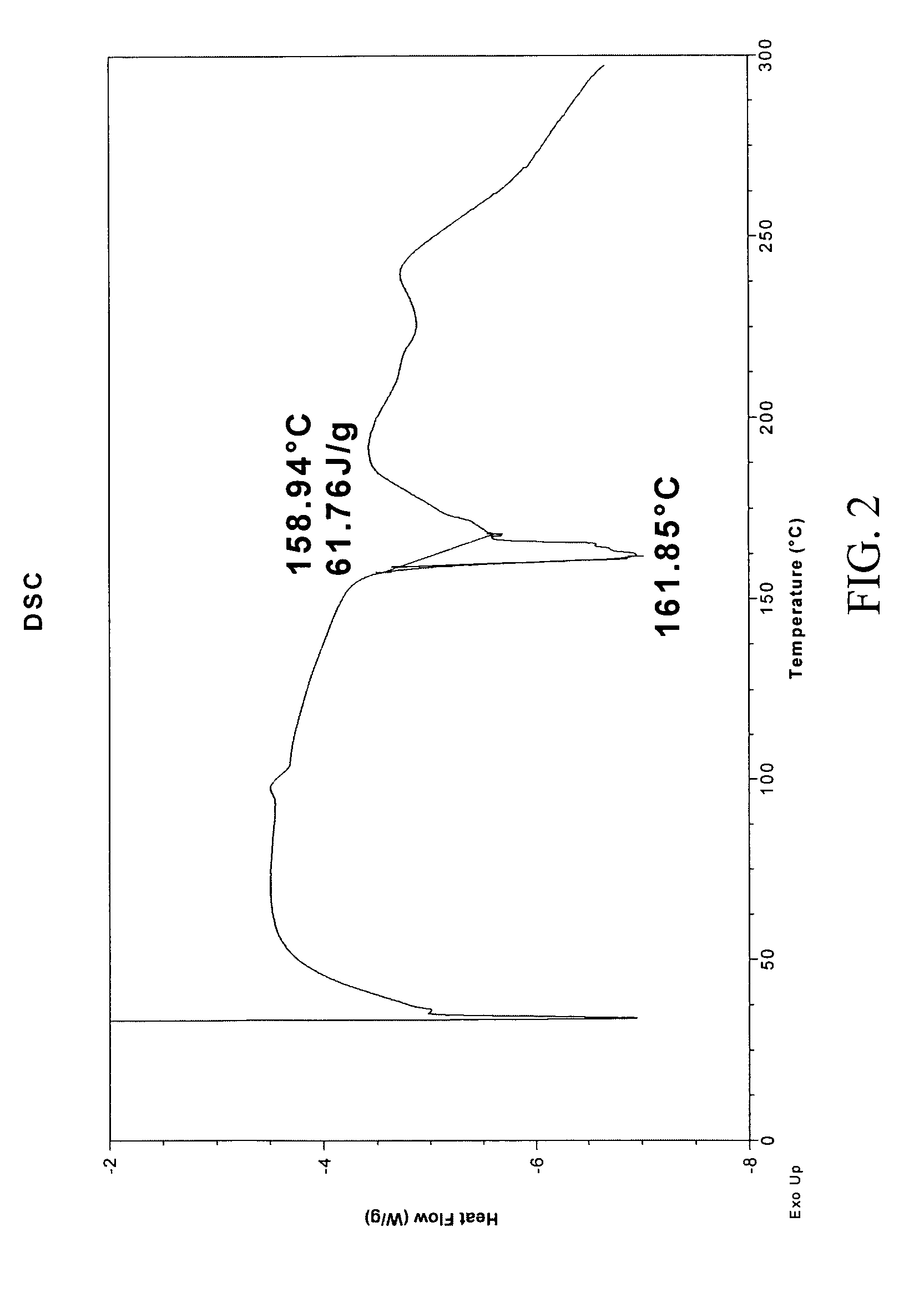
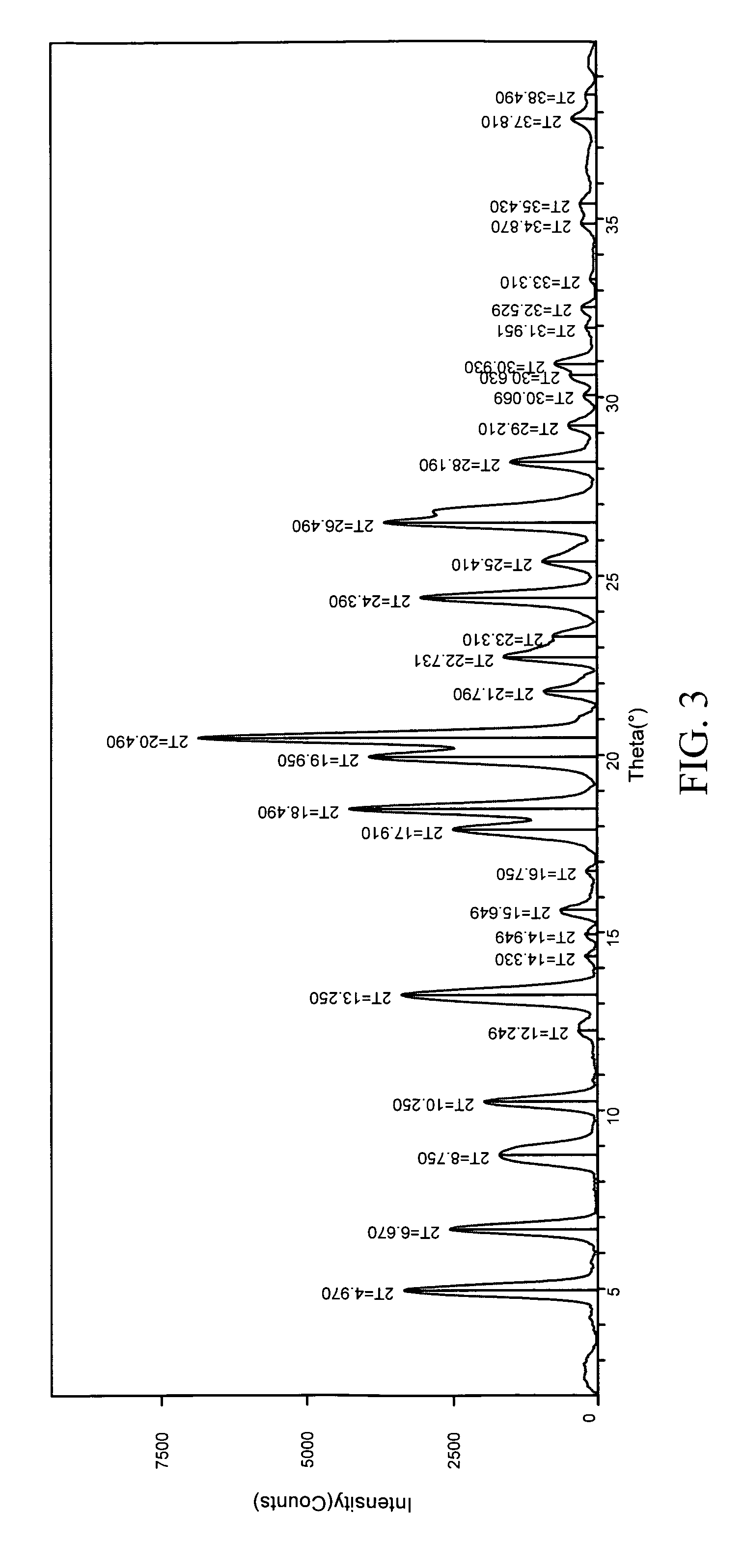
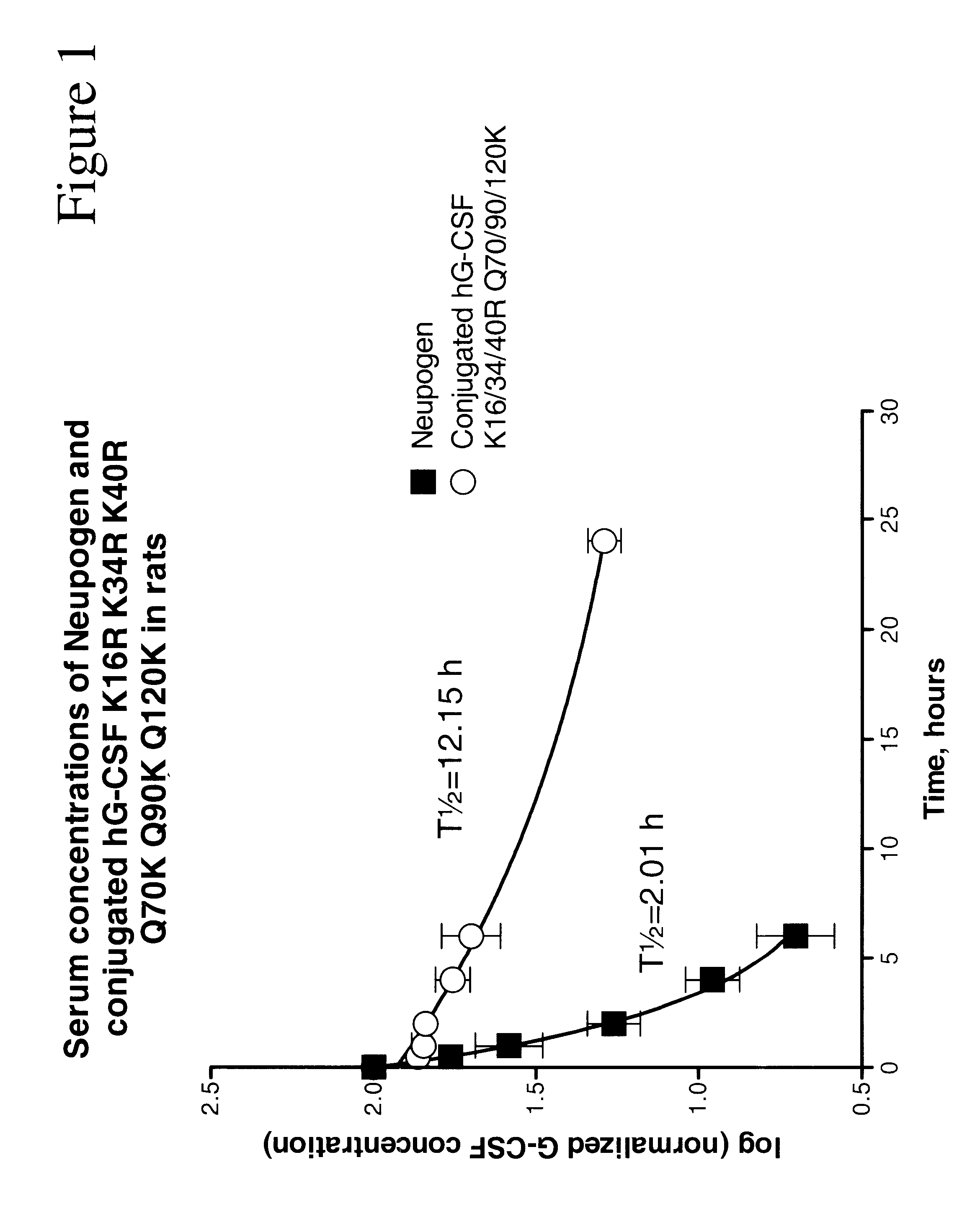
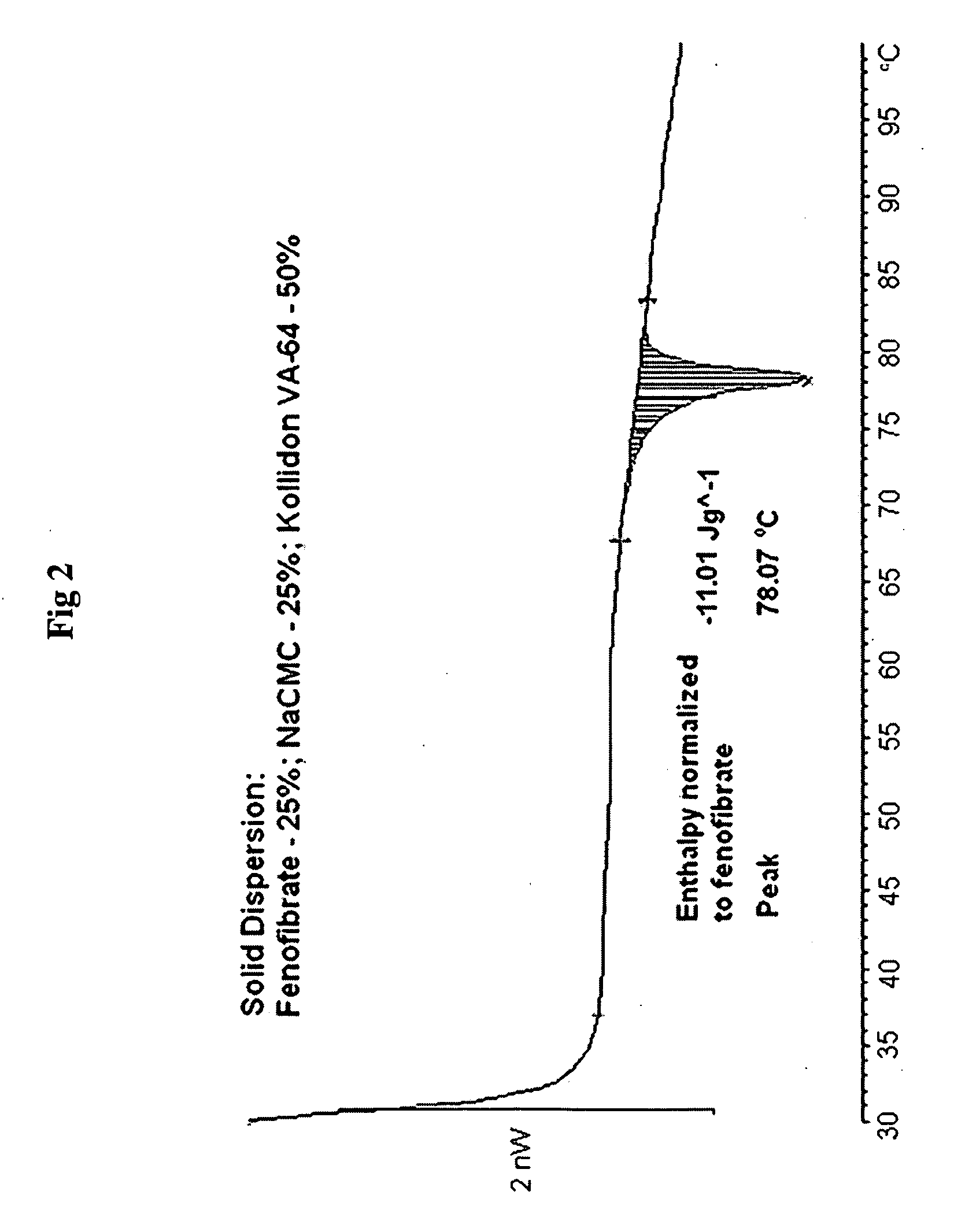
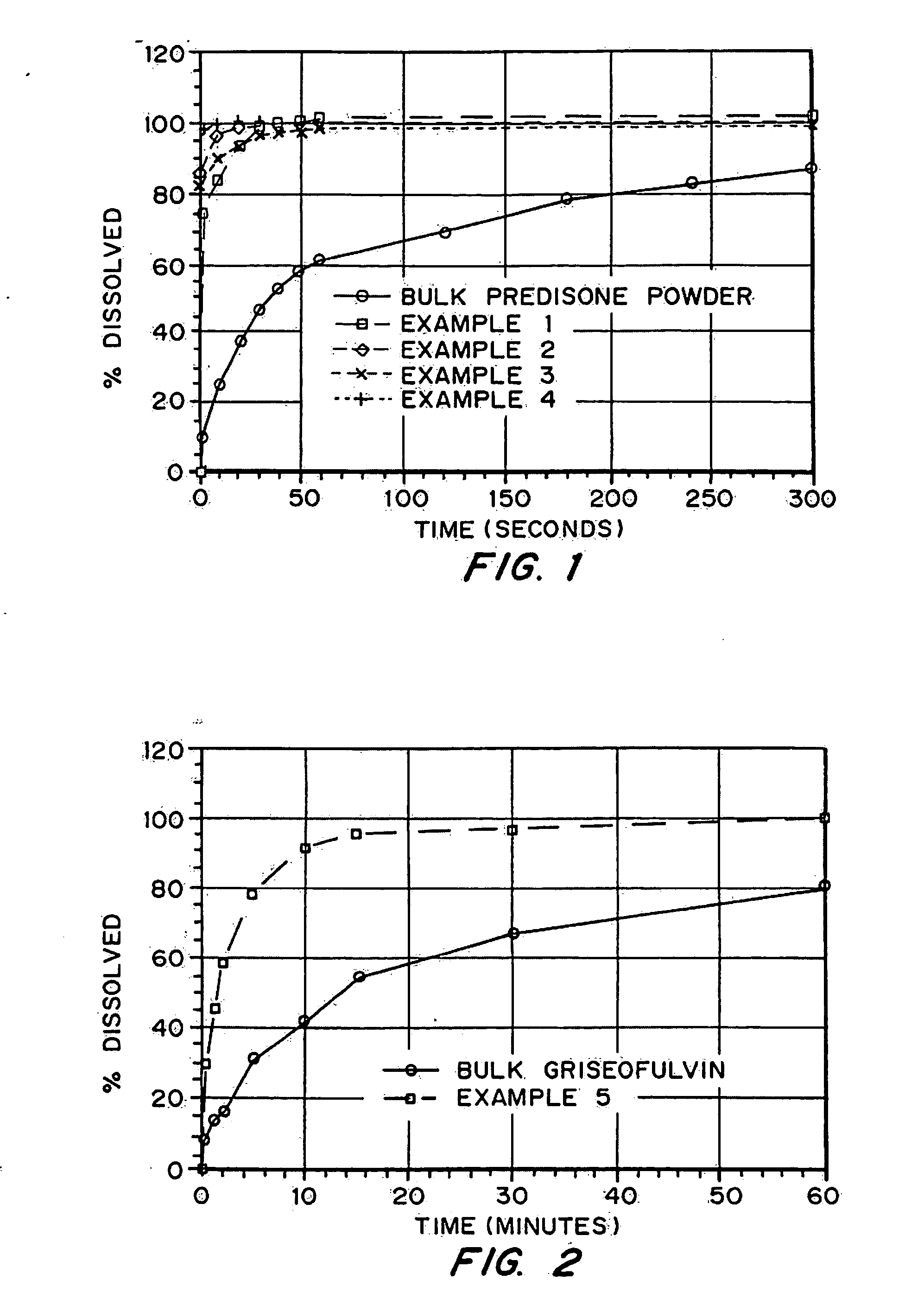
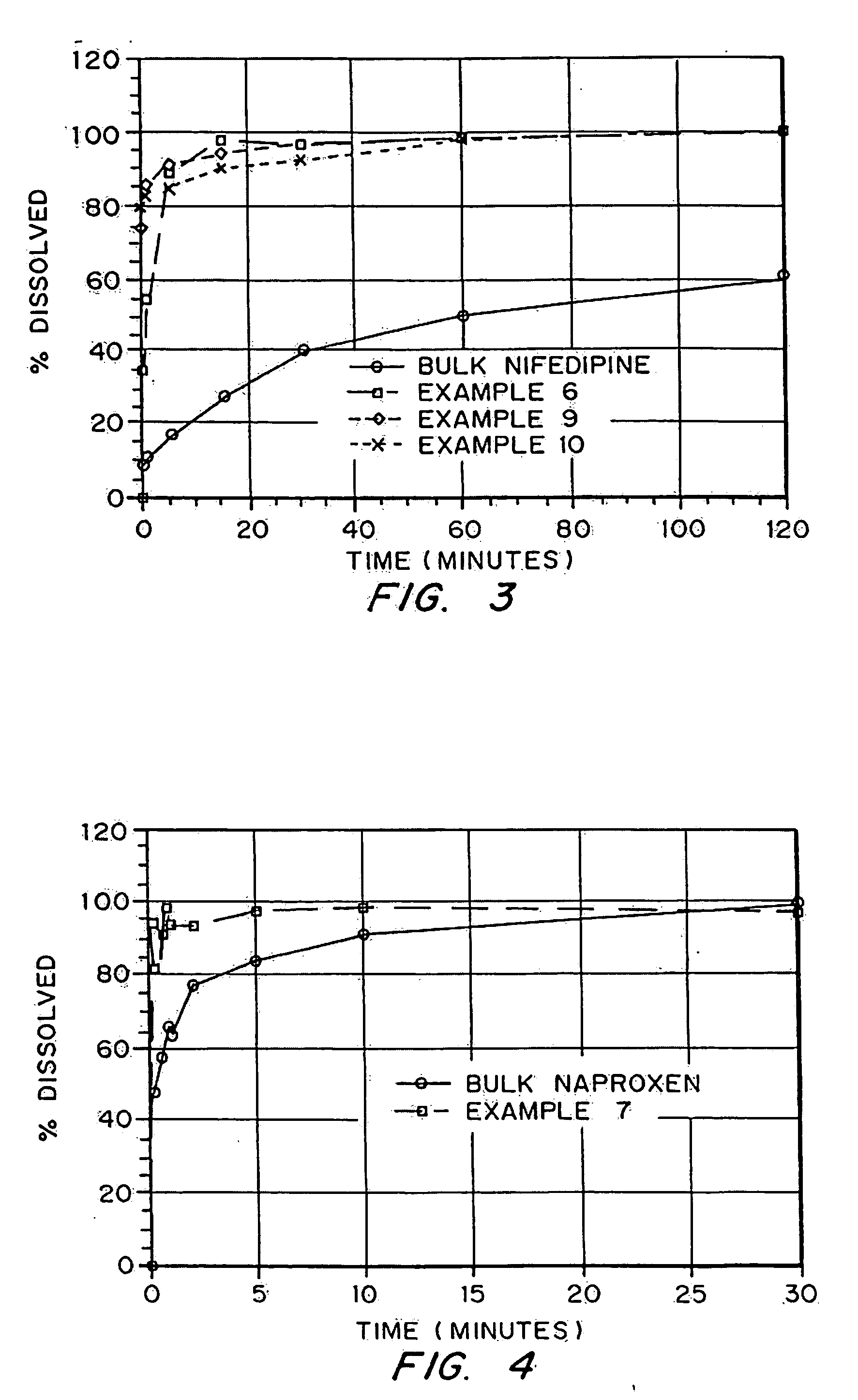
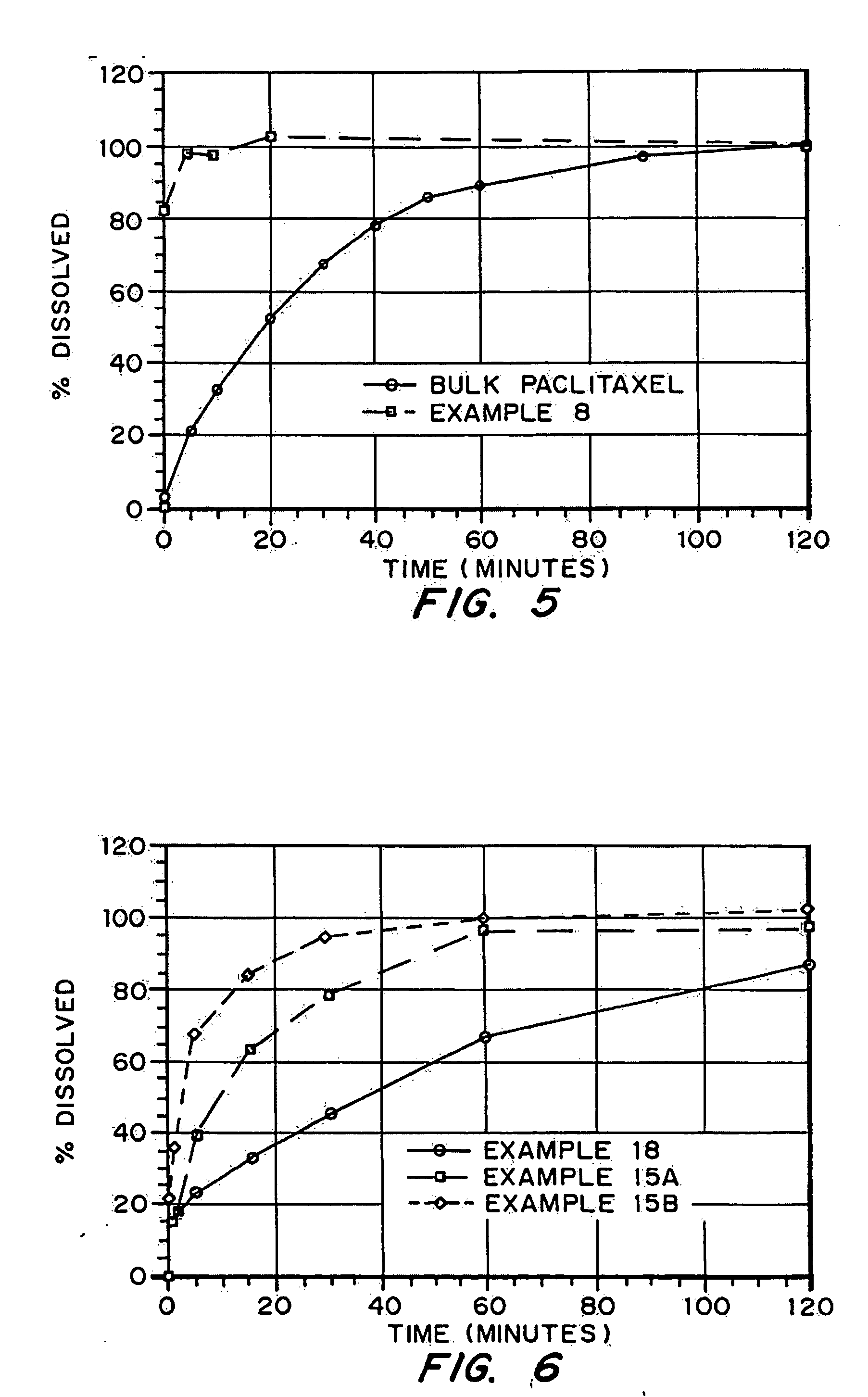
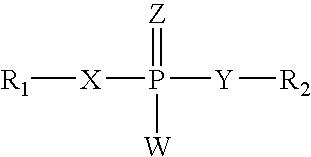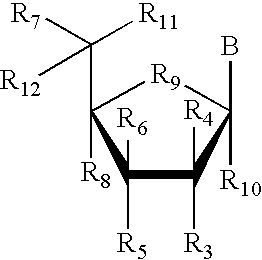Patents
Literature
18109results about How to "Improve bioavailability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
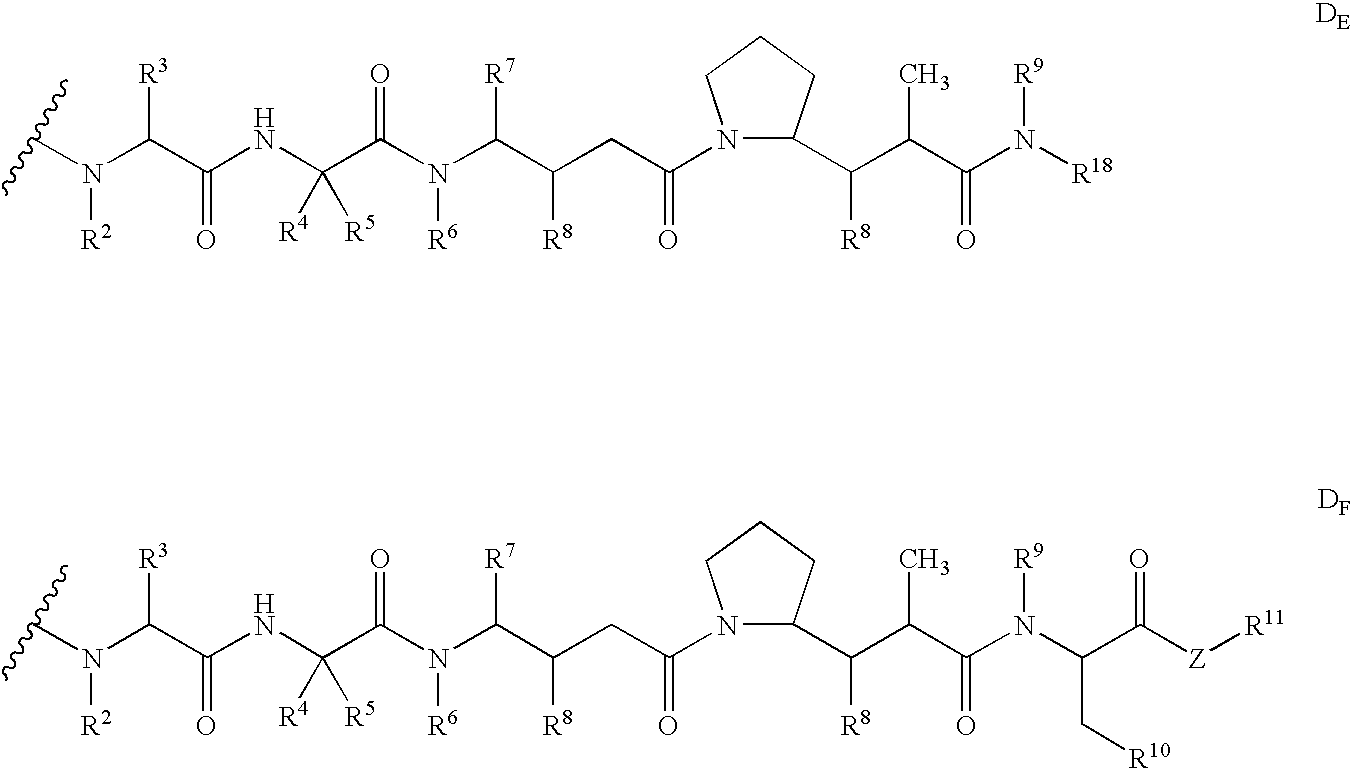
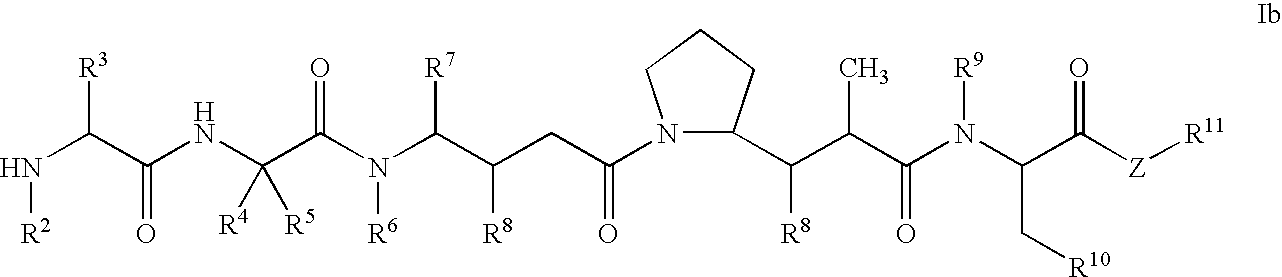
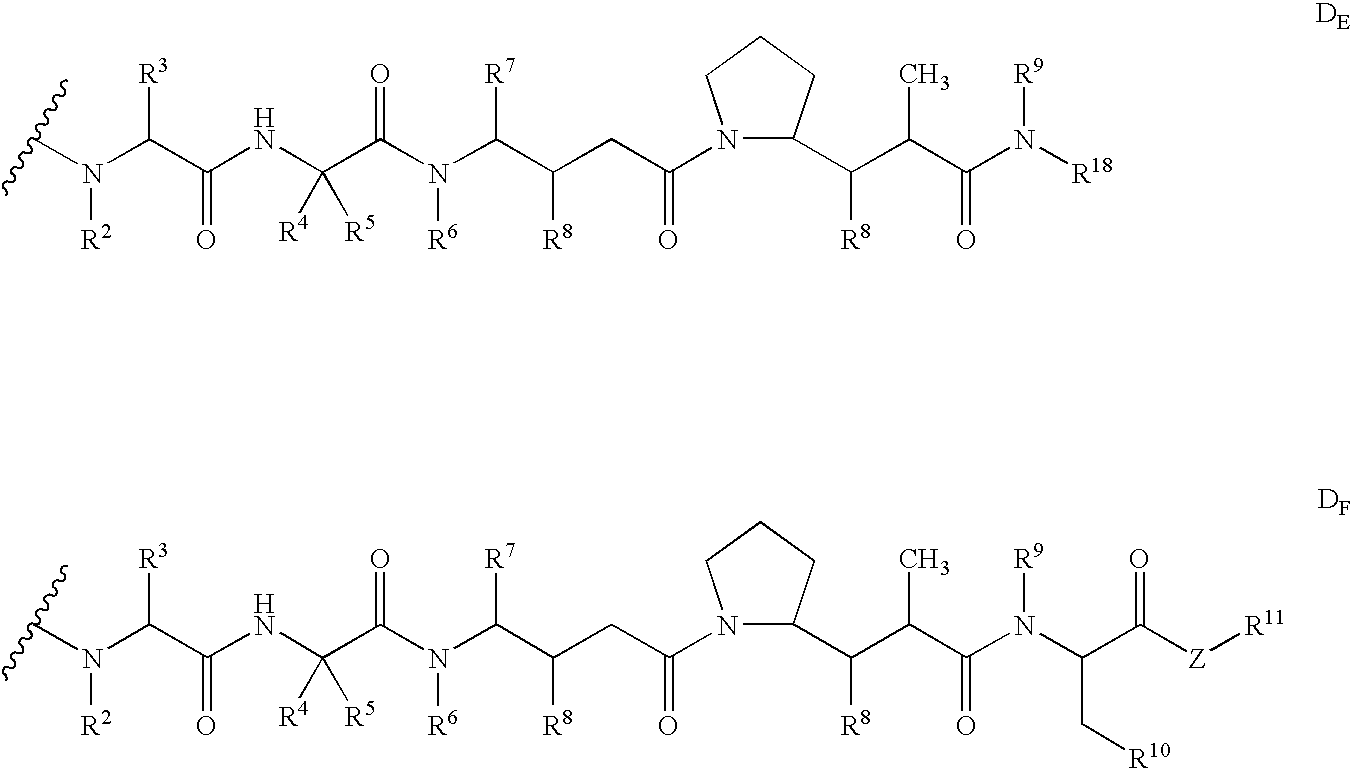
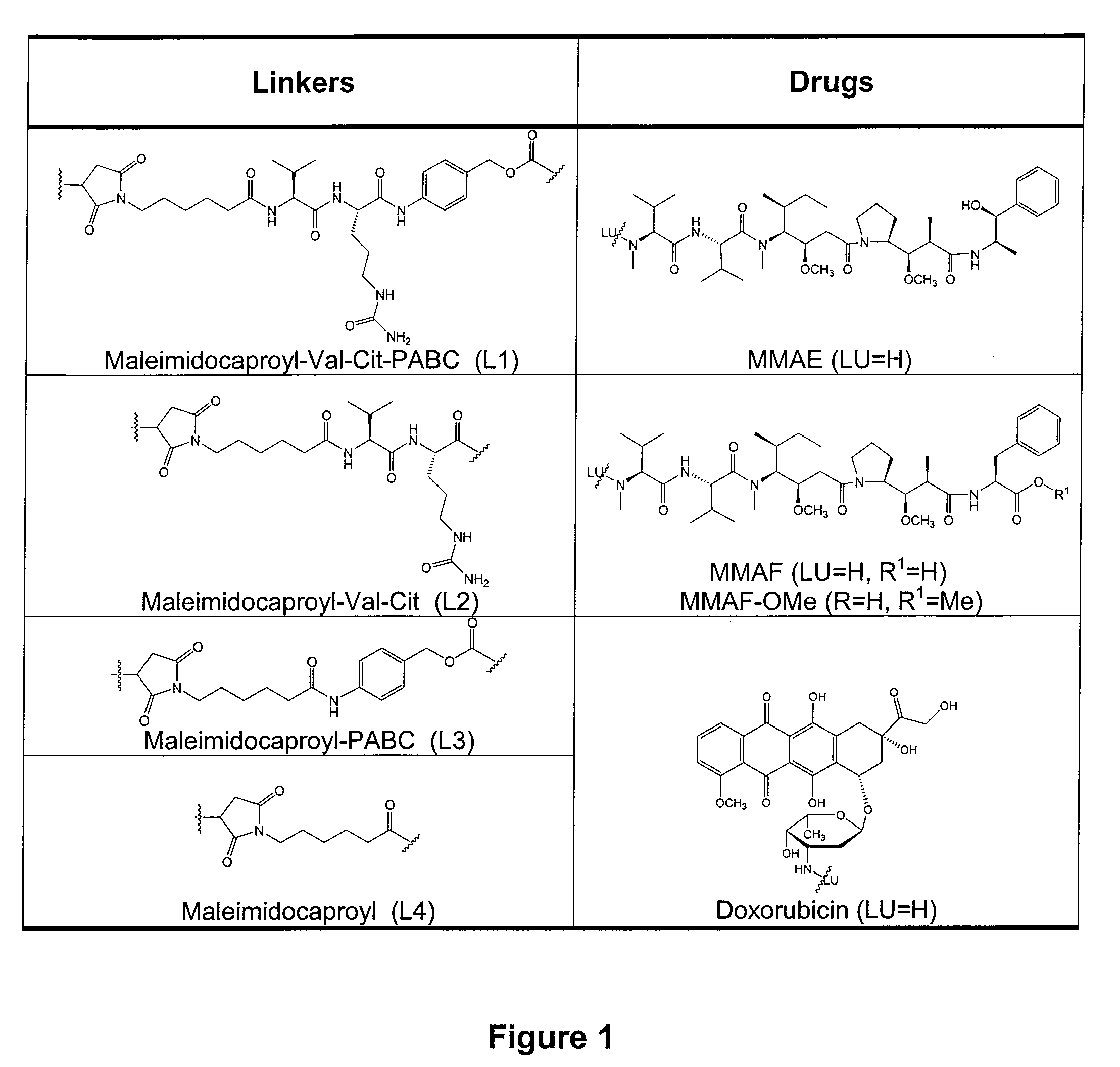
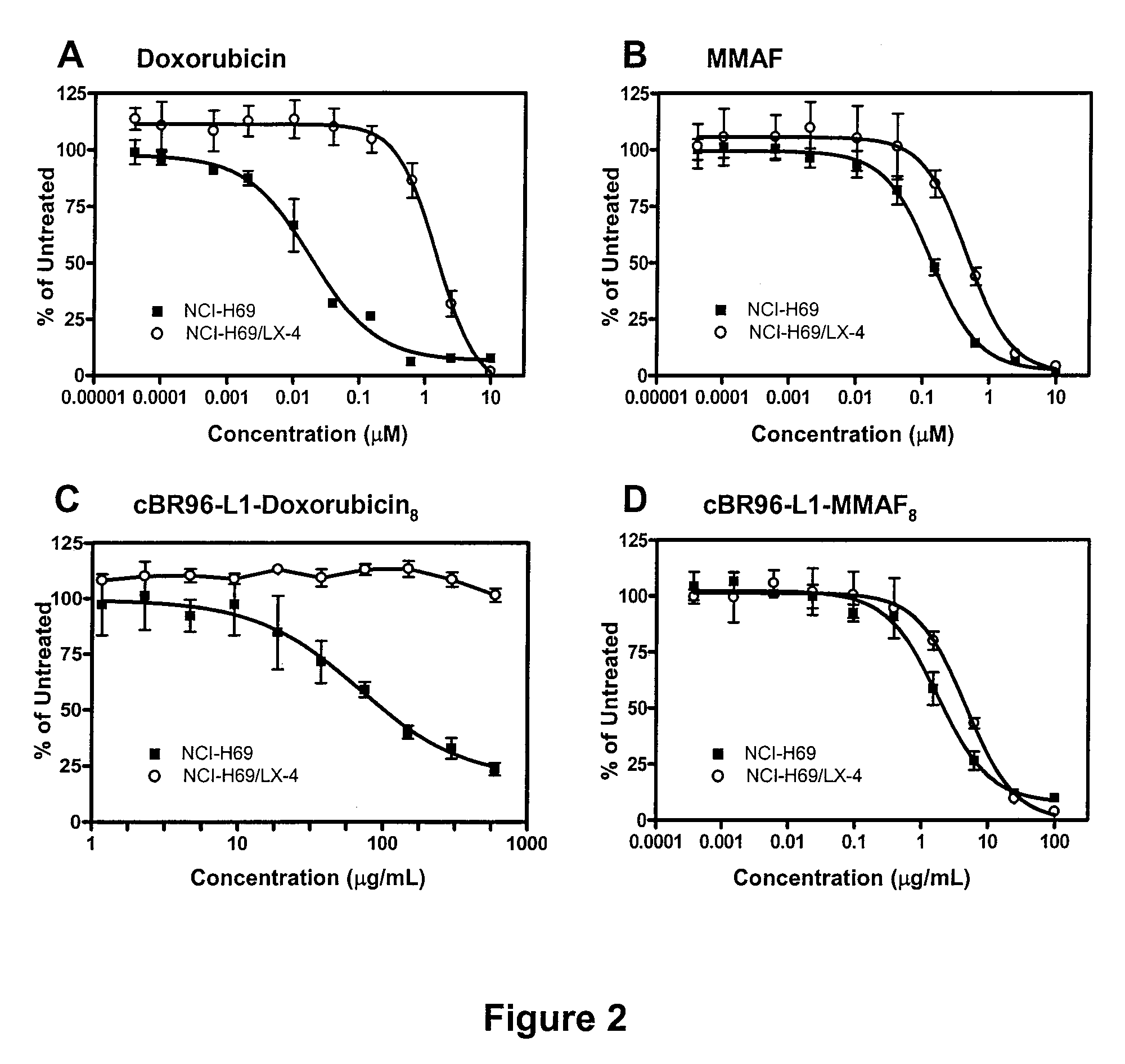
Monomethylvaline compounds capable of conjugation to ligands
ActiveUS20050238649A1Improve bioavailabilityImprove compoundAntibacterial agentsBiocideD-norephedrineBiochemistry
Owner:SEAGEN INC
Oral transmucosal drug dosage using solid solution
InactiveUS6264981B1High dissolution rateEasy to usePharmaceutical non-active ingredientsPill deliverySolid solutionPharmaceutical formulation
The present invention is directed toward formulation and method for oral transmucosal delivery of a pharmaceutical. The invention provides a drug formulation comprising a solid pharmaceutical agent in solid solution with a dissolution agent. The formulation is administered into a patient's oral cavity, delivering the pharmaceutical agent by absorption through a patient's oral mucosal tissue. The formulation and method provide for improved oral mucosal delivery of the pharmaceutical agent.
Owner:CEPHALON INC
Porous drug matrices and methods of manufacture thereof
InactiveUS6932983B1Lower the volumePrevent precipitationPowder deliveryNanotechDrugs solutionWater soluble drug
Drugs, especially low aqueous solubility drugs, are provided in a porous matrix form, preferably microparticles, which enhances dissolution of the drug in aqueous media. The drug matrices preferably are made using a process that includes (i) dissolving a drug, preferably a drug having low aqueous solubility, in a volatile solvent to form a drug solution, (ii) combining at least one pore forming agent with the drug solution to form an emulsion, suspension, or second solution, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of drug. The pore forming agent can be either a volatile liquid that is immiscible with the drug solvent or a volatile solid compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. The resulting porous matrix has a faster rate of dissolution following administration to a patient, as compared to non-porous matrix forms of the drug. In a preferred embodiment, microparticles of the porous drug matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
System for repairing inter-vertebral discs
InactiveUS6428576B1Reduce incidenceReduce morbidityJoint implantsSpinal implantsAdverse effectBiomedical engineering
A method of ameliorating the adverse effects of a defect in the annulus fibrosus by applying a curable bio-compatible material to the defect, and curing that material, in situ, into a cross linked visco-elastic solid polymer adhering to remaining annulus fibrosus and thereby closing said defect. Alternatively, the bio-compatible material may be cross linked immediately before insertion into the annulus fibrosus.
Owner:PAUZA KEVIN +1
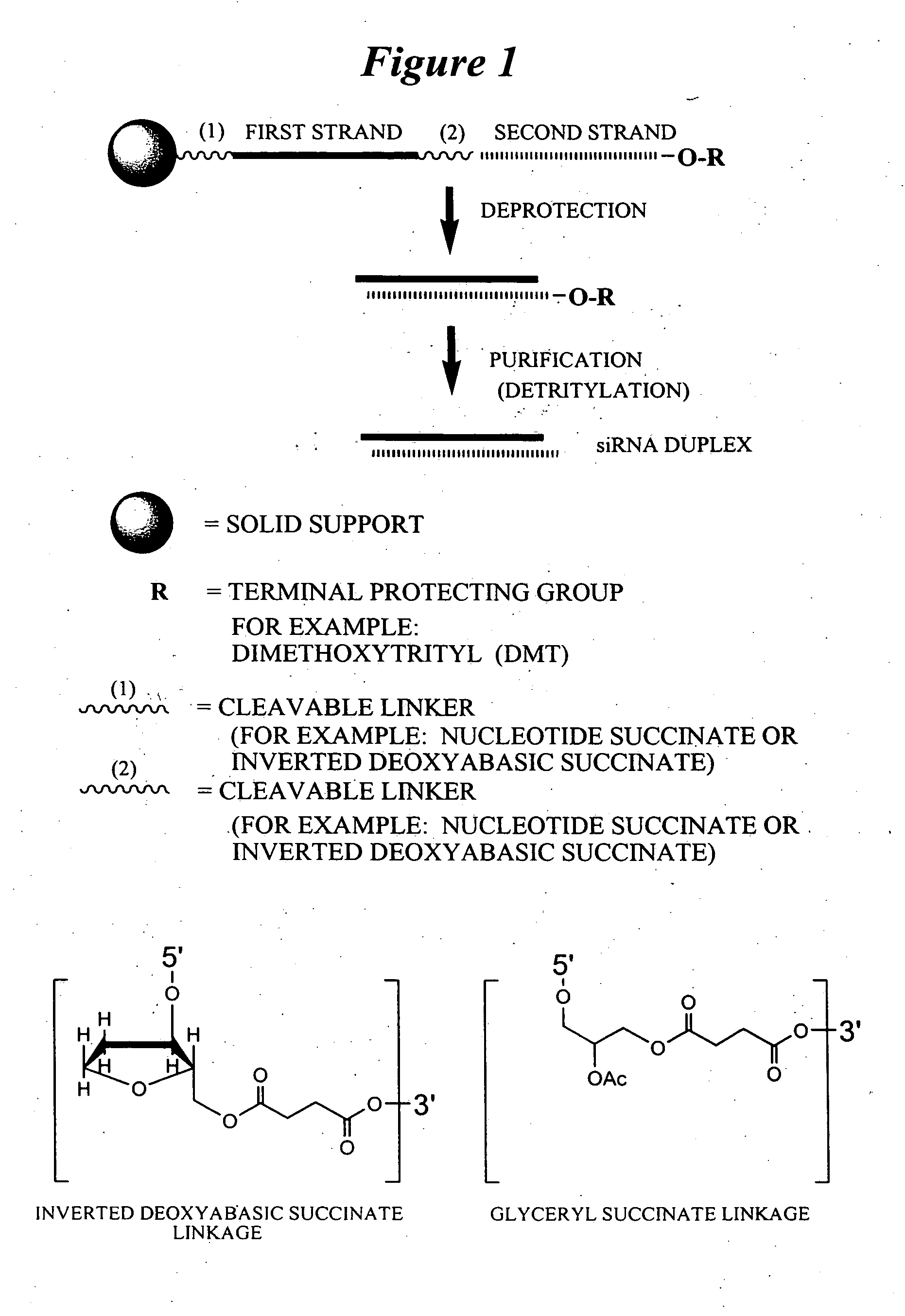
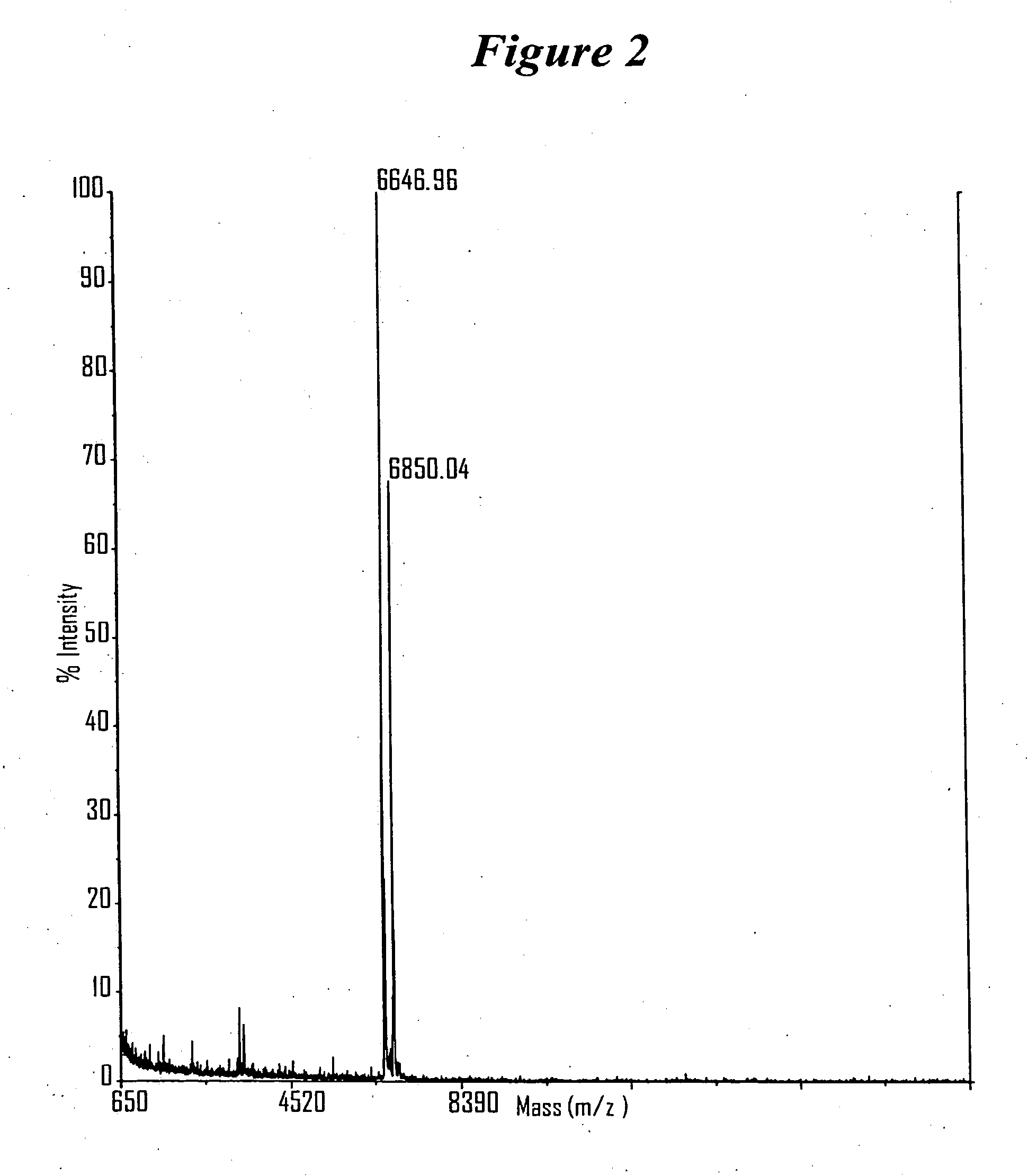
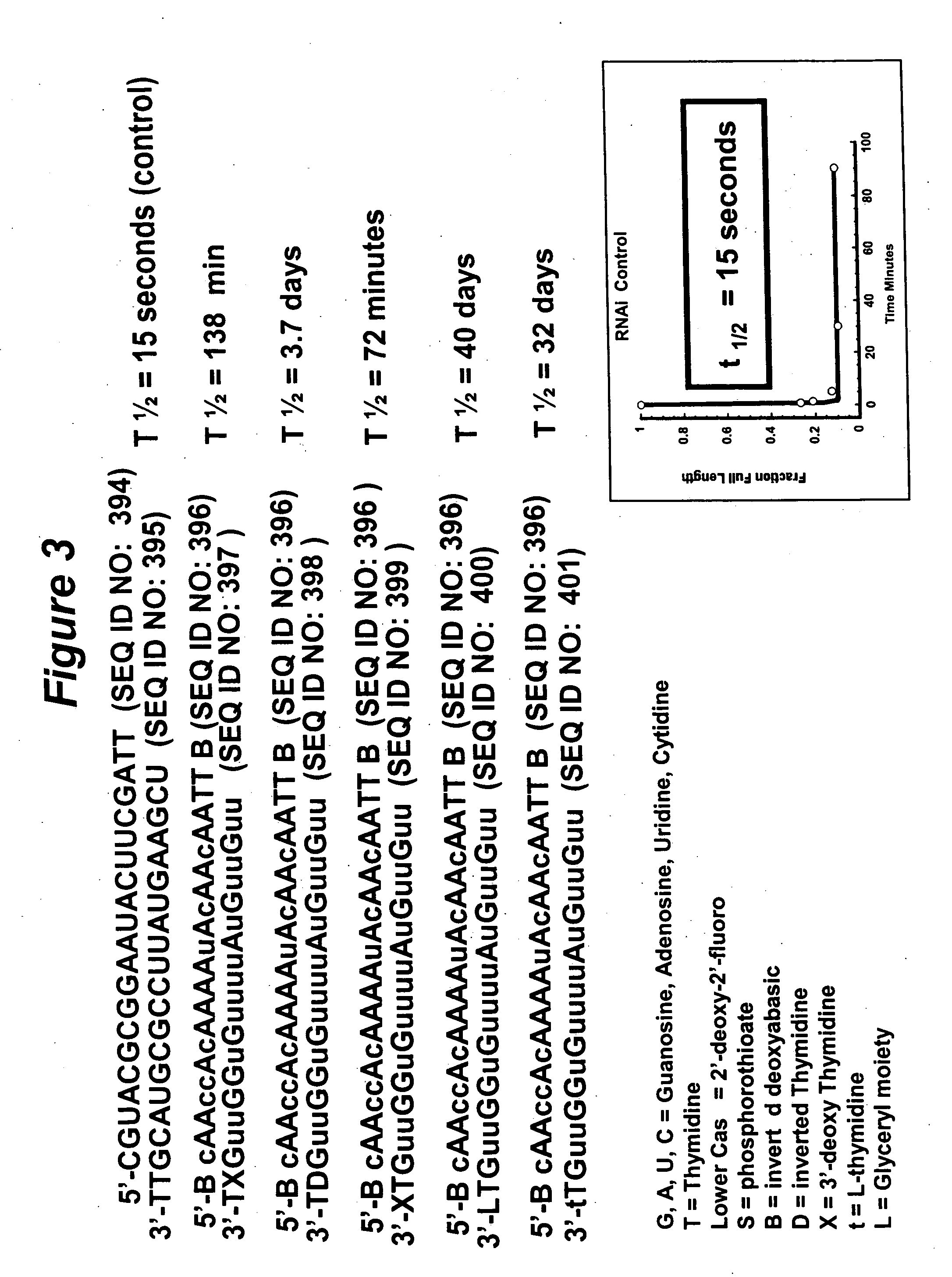
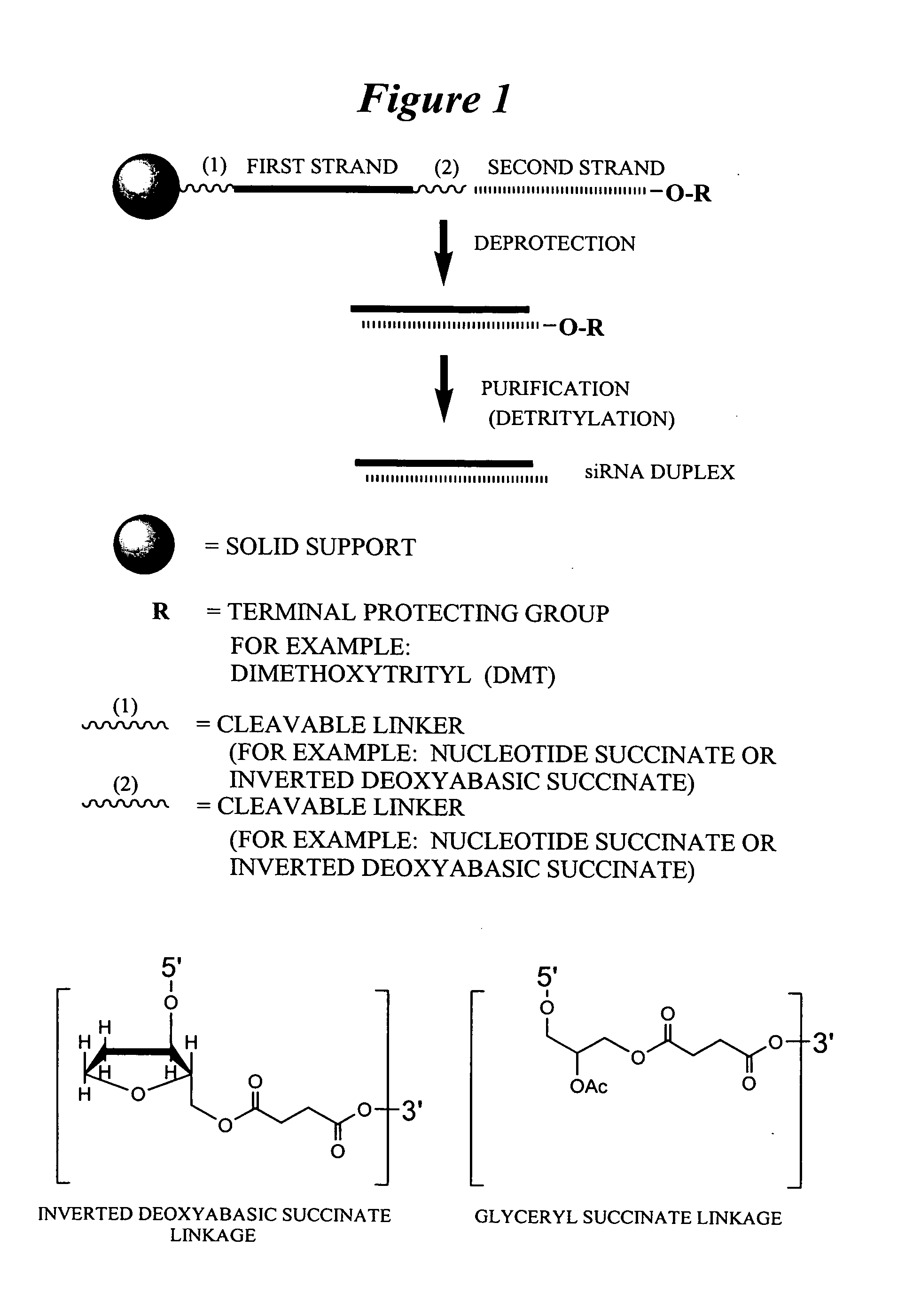
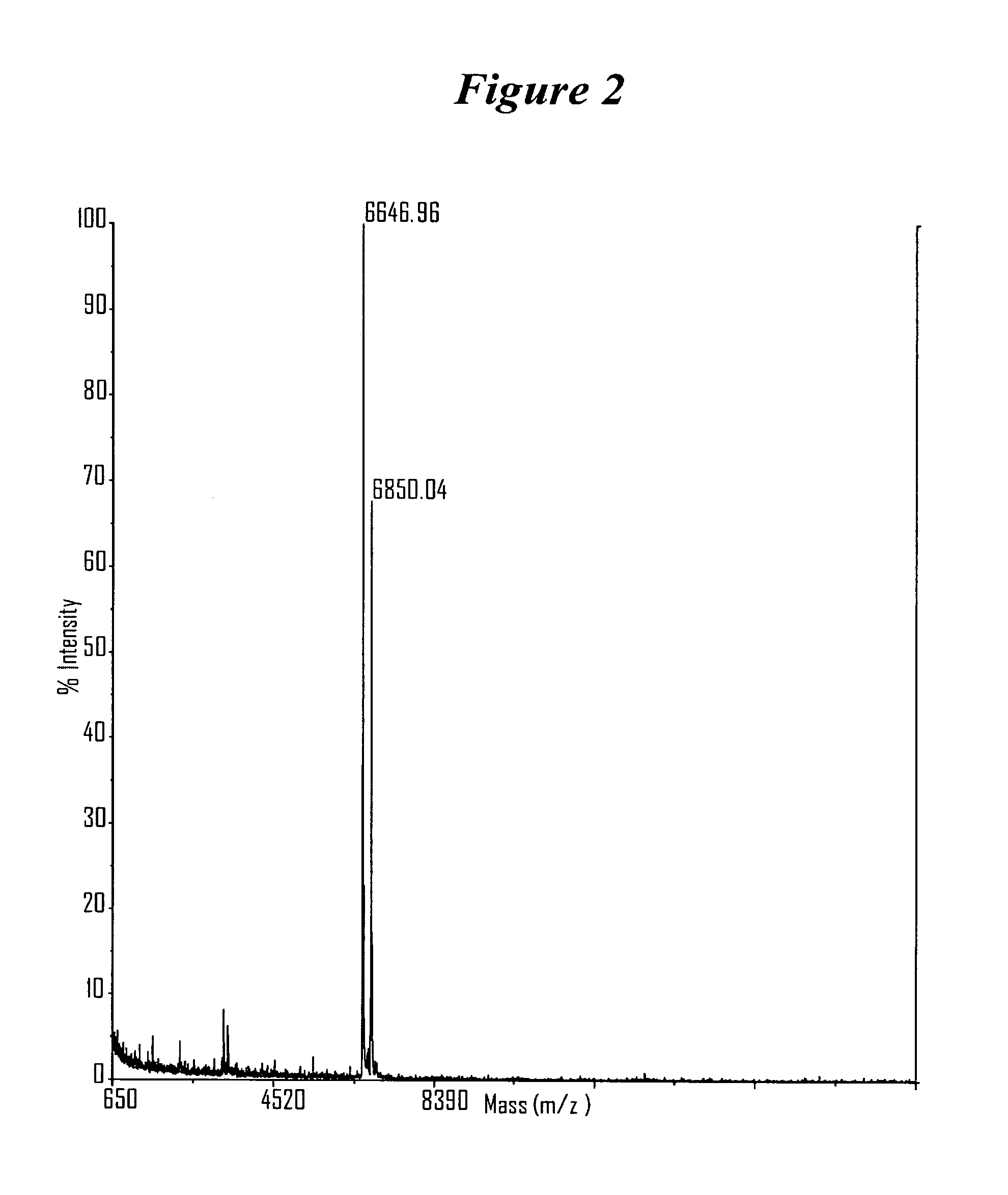
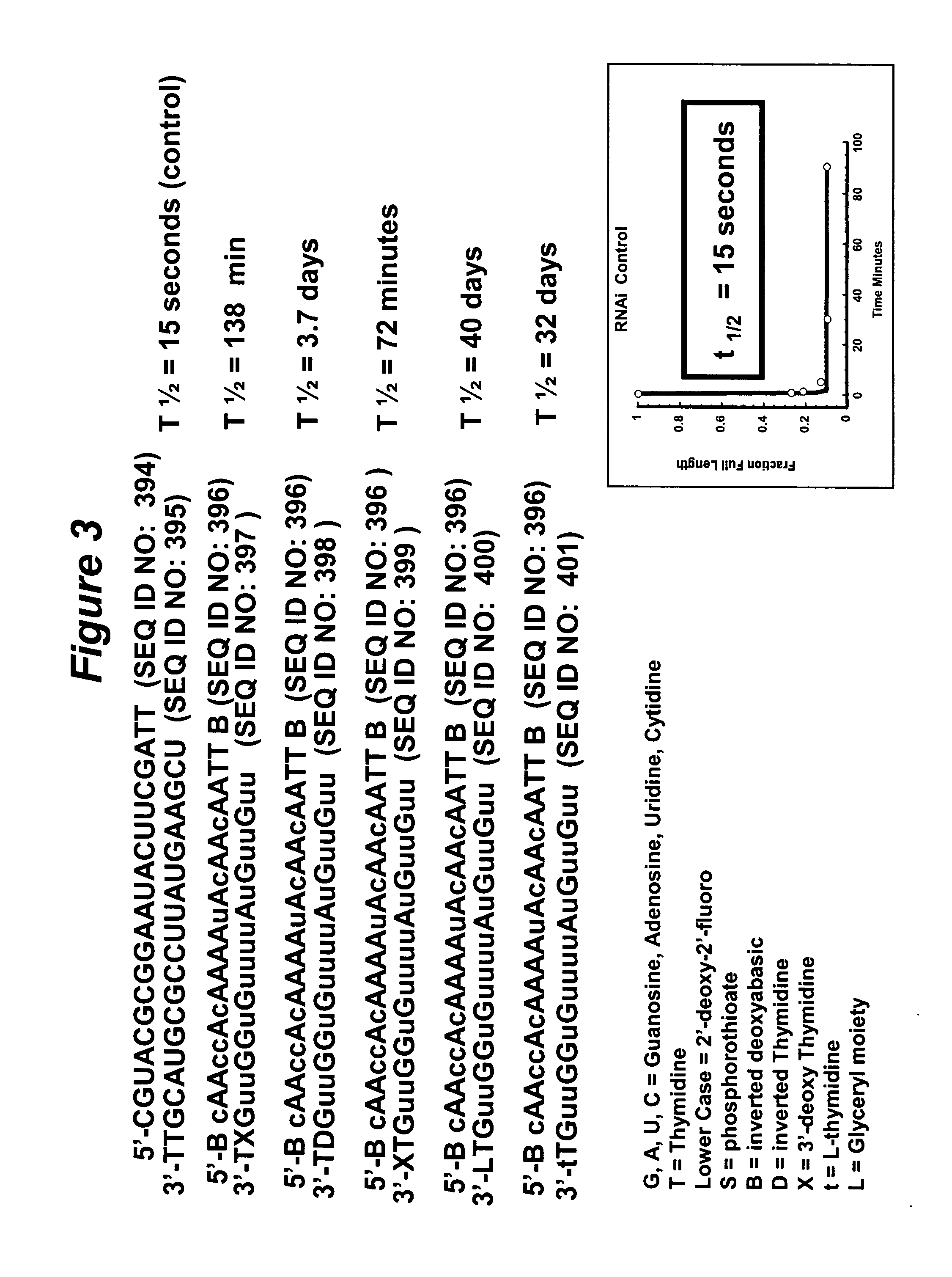
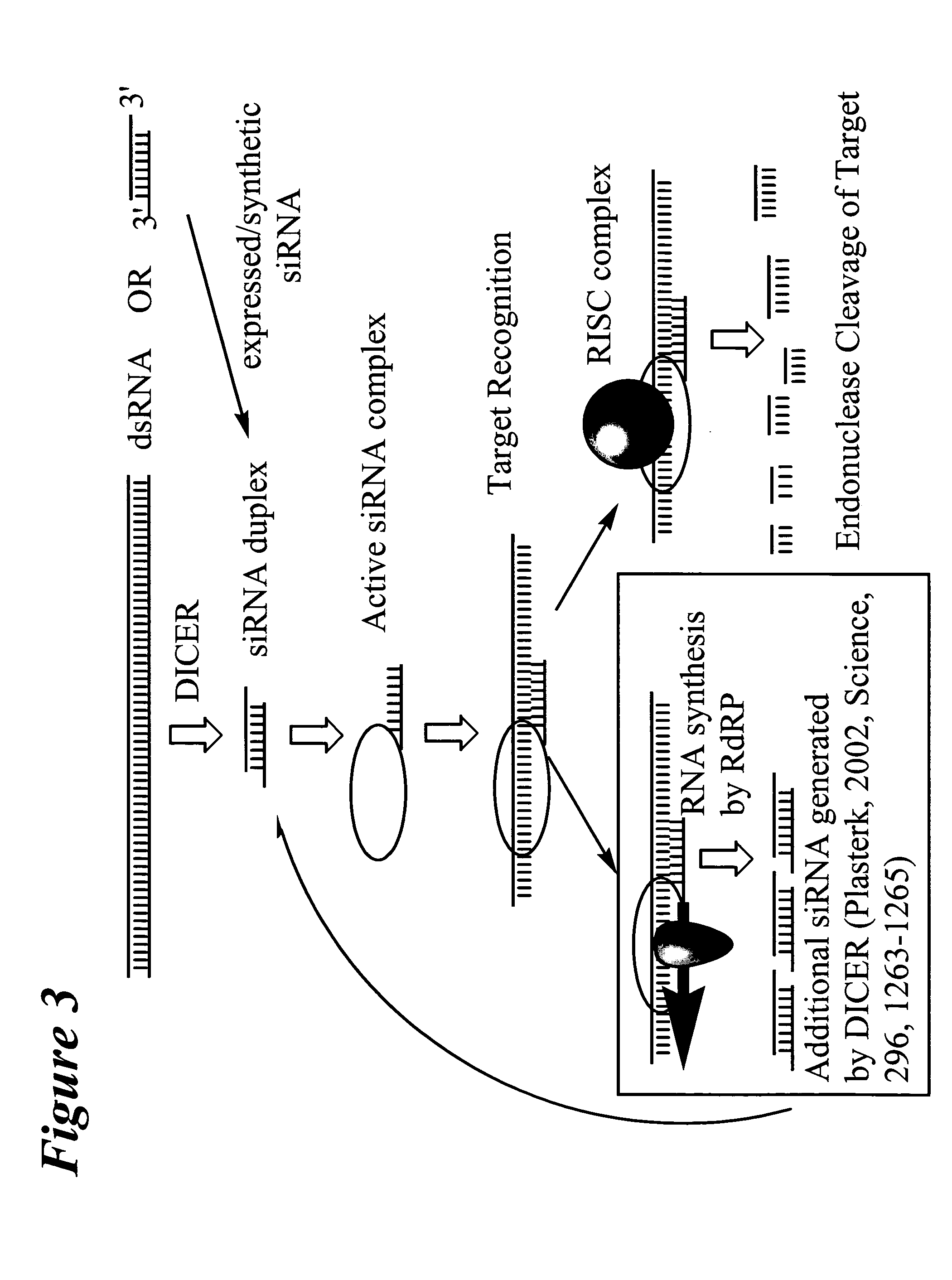
RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA)
InactiveUS20050020525A1Improve various propertyModulate its functionCompounds screening/testingSugar derivativesDouble strandOrganism
The present invention concerns methods and reagents useful in modulating gene expression in a variety of applications, including use in therapeutic, diagnostic, target validation, and genomic discovery applications. Specifically, the invention relates to synthetic chemically modified small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules capable of mediating RNA interference (RNAi) against target nucleic acid sequences. The small nucleic acid molecules are useful in the treatment of any disease or condition that responds to modulation of gene expression or activity in a cell, tissue, or organism.
Owner:SIMA THERAPEUTICS ICN
RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (SiNA)
InactiveUS20050032733A1Improve various propertyModulate its functionCompounds screening/testingSpecial deliveryBiological bodyNucleic acid sequencing
The present invention concerns methods and reagents useful in modulating gene expression in a variety of applications, including use in therapeutic, diagnostic, target validation, and genomic discovery applications. Specifically, the invention relates to synthetic chemically modified small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules capable of mediating RNA interference (RNAi) against target nucleic acid sequences. The small nucleic acid molecules are useful in the treatment of any disease or condition that responds to modulation of gene expression or activity in a cell, tissue, or organism.
Owner:SIRNA THERAPEUTICS INC
RNA interference mediated inhibition of B-cell CLL/Lymphoma-2 (BCL-2) gene expression using short interfering nucleic acid (siNA)
InactiveUS20050176025A1Improves various propertyImprove the immunityCompounds screening/testingSpecial deliveryAutoimmune conditionAutoimmune disease
This invention relates to compounds, compositions, and methods useful for modulating BCL2 gene expression using short interfering nucleic acid (siNA) molecules. This invention also relates to compounds, compositions, and methods useful for modulating the expression and activity of other genes involved in pathways of BCL2 gene expression and / or activity by RNA interference (RNAi) using small nucleic acid molecules. In particular, the instant invention features small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules and methods used to modulate the expression of BCL2 genes (e.g., BCL2, BCL-XL, BCL2-L1, MCL-1 CED-9, BAG-1, E1B-194 and / or BCL-A1). The small nucleic acid molecules are useful in the treatment of cancer, malignant blood disease, polycytemia vera, idiopathic myelofibrosis, essential thrombocythemia, myelodysplastic syndromes, autoimmune disease, viral infection, and proliferative diseases and conditions
Owner:SIRNA THERAPEUTICS INC
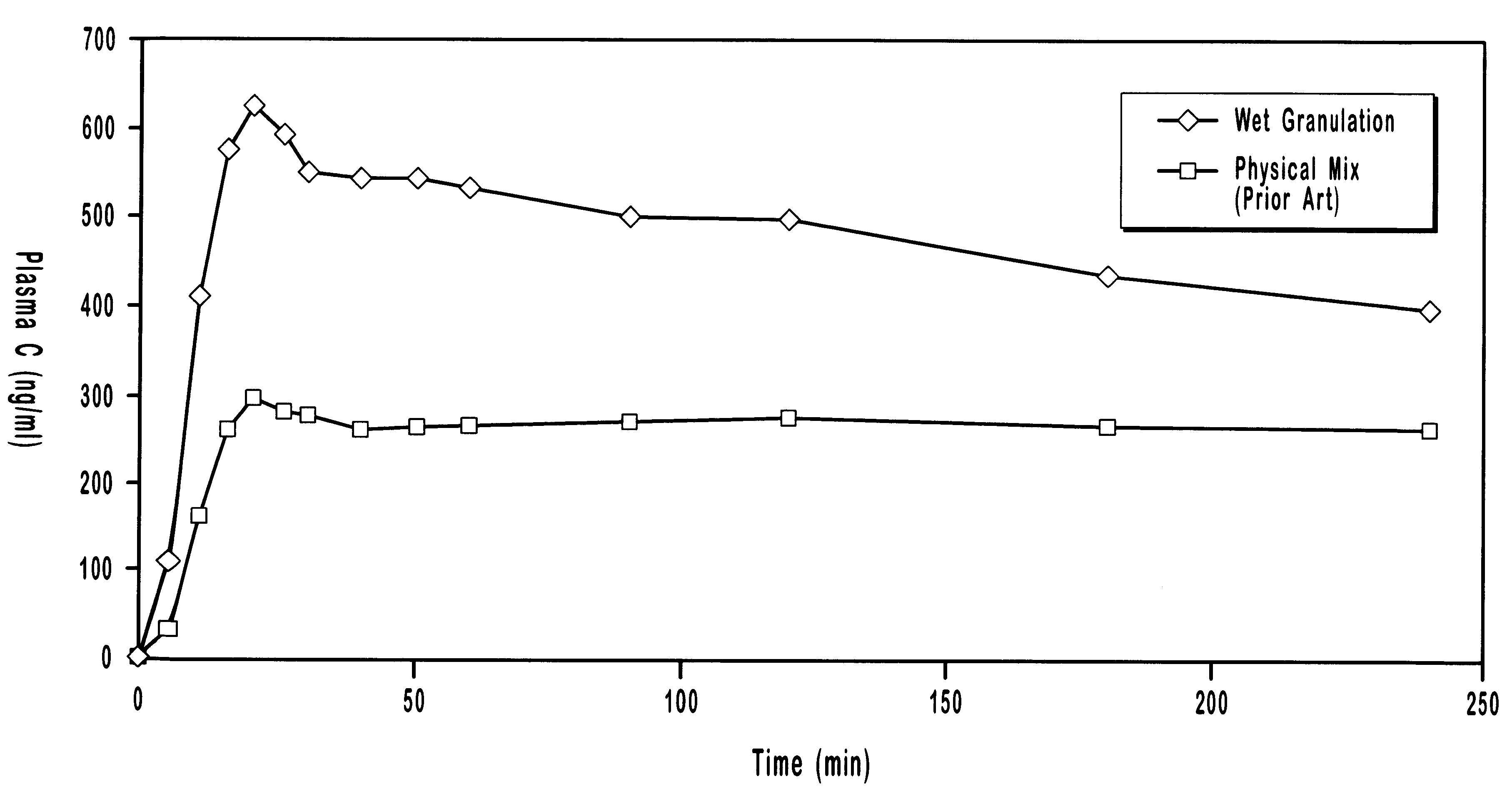
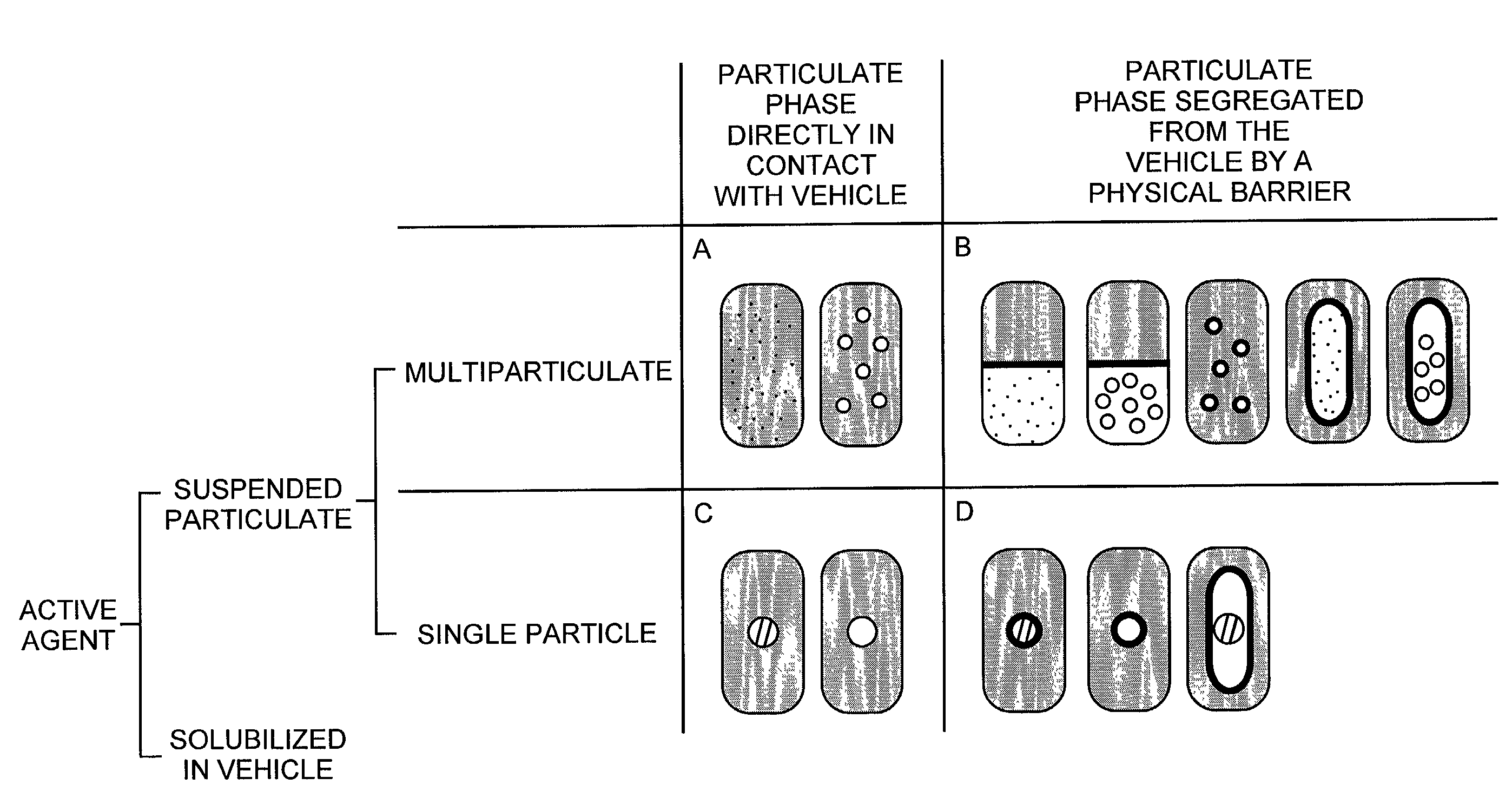
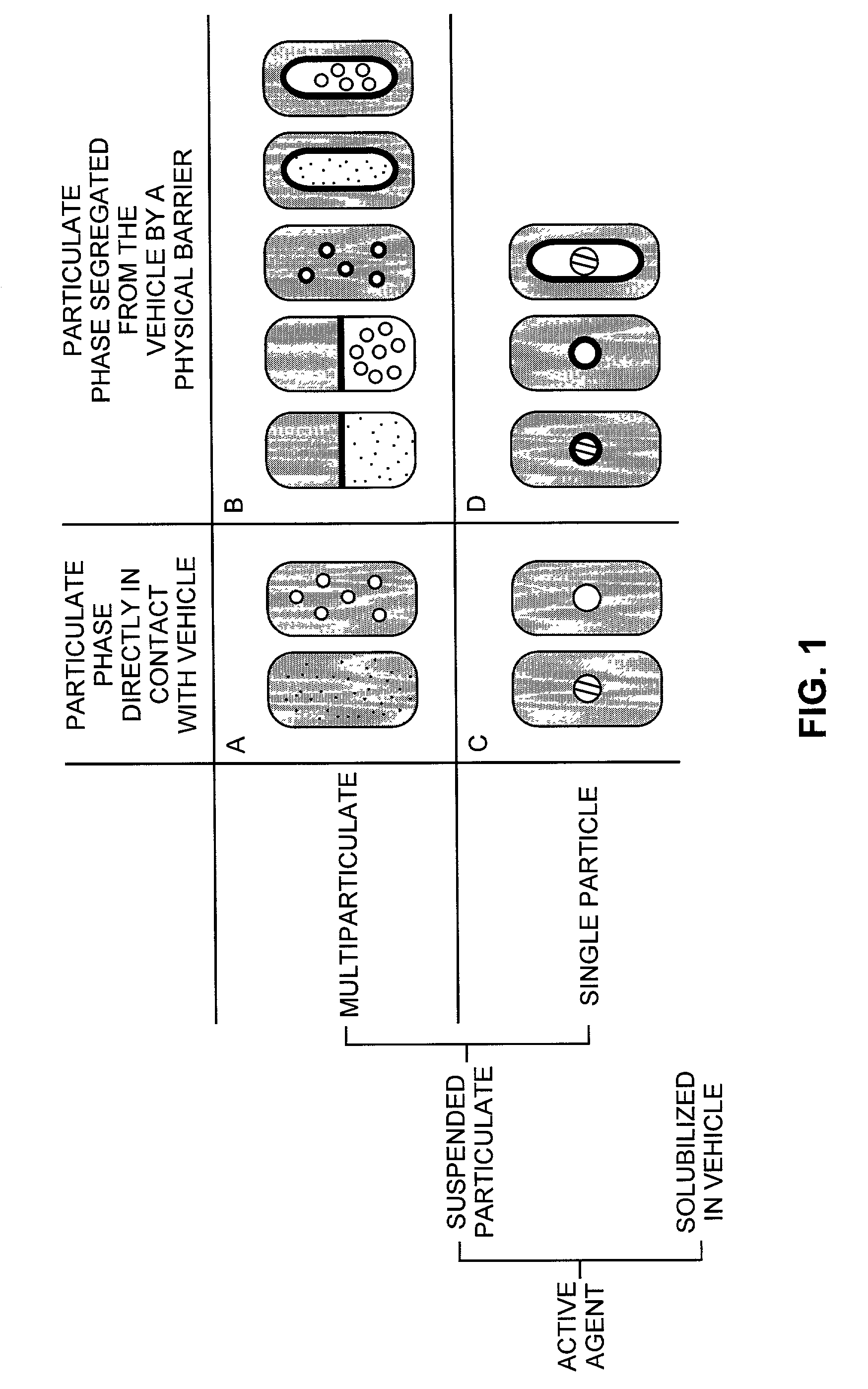
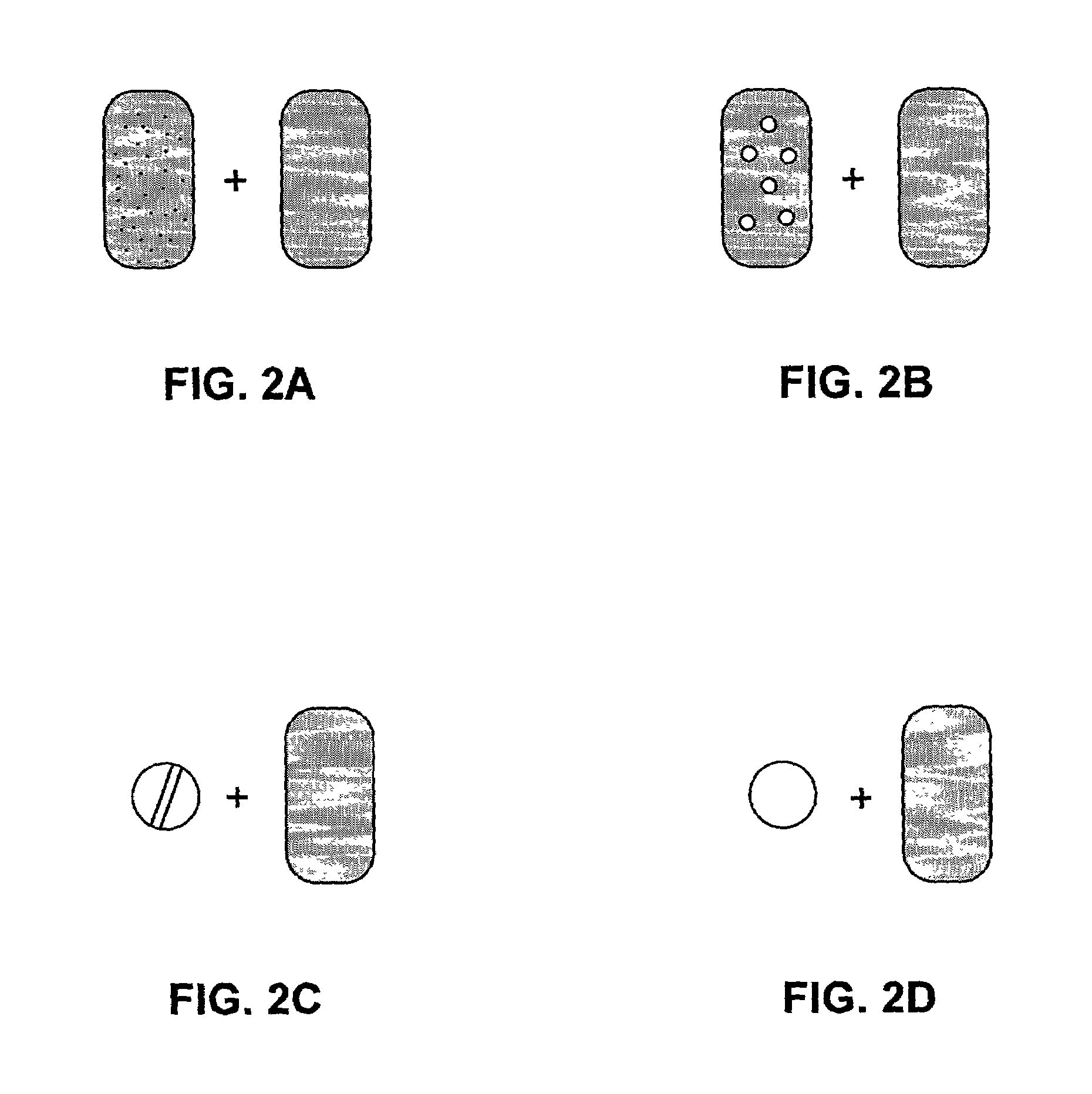
Pharmaceutical formulations and systems for improved absorption and multistage release of active agents
InactiveUS7374779B2Improve bioavailabilityLow variabilityPowder deliveryOrganic active ingredientsActive agentFast release
The present invention pertains to pharmaceutical formulations and systems for delivery of active agents, wherein a first fraction of an active agent is suspended in a vehicle and a second fraction of active agent is solubilized in the vehicle, with the suspended fraction representing about 5 wt. % to about 80 wt. % of the active agent and the second fraction representing about 20 wt. % to about 95 wt. % of the active agent. One or more additional active agents, which may be fully solubilized, partially solubilized, or suspended, may also be present. The first and second fractions of the active agent may or may not have different release profiles. Generally, a significant fraction of the solubilized drug will release rapidly, providing for rapid onset, while the suspended drug may be formulated for delayed and / or sustained release.
Owner:LIPOCINE
Solid carriers for improved delivery of active ingredients in pharmaceutical compositions
InactiveUS20060034937A1Good chemical stabilityPromote absorptionGranular deliveryMicrocapsulesDiagnostic agentMedicine
The present invention provides solid pharmaceutical compositions for improved delivery of a wide variety of pharmaceutical active ingredients contained therein or separately administered. In one embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate. The encapsulation coat can include different combinations of pharmaceutical active ingredients, hydrophilic surfactant, lipophilic surfactants and triglycerides. In another embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier being formed of different combinations of pharmaceutical active ingredients, hydrophilic surfactants, lipophilic surfactants and triglycerides. The compositions of the present invention can be used for improved delivery of hydrophilic or hydrophobic pharmaceutical active ingredients, such as drugs, nutritional agents, cosmeceuticals and diagnostic agents.
Owner:LIPOCINE
RNA interference mediated inhibition of wingless gene expression using short interfering nucleic acid (siNA)
InactiveUS20050130181A1Improves various propertyImprove the immunityCompounds screening/testingSpecial deliveryWnt genesFhit gene
This invention relates to compounds, compositions, and methods useful for modulating wingless (WNT) gene expression using short interfering nucleic acid (siNA) molecules. This invention also relates to compounds, compositions, and methods useful for modulating the expression and activity of other genes involved in pathways of WNT gene expression and / or activity by RNA interference (RNAi) using small nucleic acid molecules. In particular, the instant invention features small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules and methods used to modulate the expression of WNT genes such as WNT3A and WNT7A.
Owner:SIRNA THERAPEUTICS INC
RNA interference mediated inhibition of TNF and TNF receptor gene expression using short interfering nucleic acid (siNA)
InactiveUS20050227935A1Improves various propertyImprove the immunitySugar derivativesGenetic material ingredientsTumor necrosis factor receptorDouble strand
This invention relates to compounds, compositions, and methods useful for modulating tumor necrosis factor and / or tumor necrosis factor receptor gene expression using short interfering nucleic acid (siNA) molecules. This invention also relates to compounds, compositions, and methods useful for modulating the expression and activity of other genes involved in pathways of tumor necrosis factor and / or tumor necrosis factor receptor gene expression and / or activity by RNA interference (RNAi) using small nucleic acid molecules. In particular, the instant invention features small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules and methods used to modulate the expression of tumor necrosis factor and / or tumor necrosis factor receptor genes, (TNF and / or TNF receptor).
Owner:SIRNA THERAPEUTICS INC
Powdered protein compositions and methods of making same
InactiveUS20090226530A1Easy to moveWide concentration rangeAntibacterial agentsPowder deliveryBiotechnologyProtein composition
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Dosage form comprising liquid formulation
InactiveUS6174547B1Improve oral bioavailabilityImprove bioavailabilityCapsule deliveryEmulsion deliveryPharmaceutical formulationDosage form
Owner:ENCINAL PHARMA INVESTMENTS
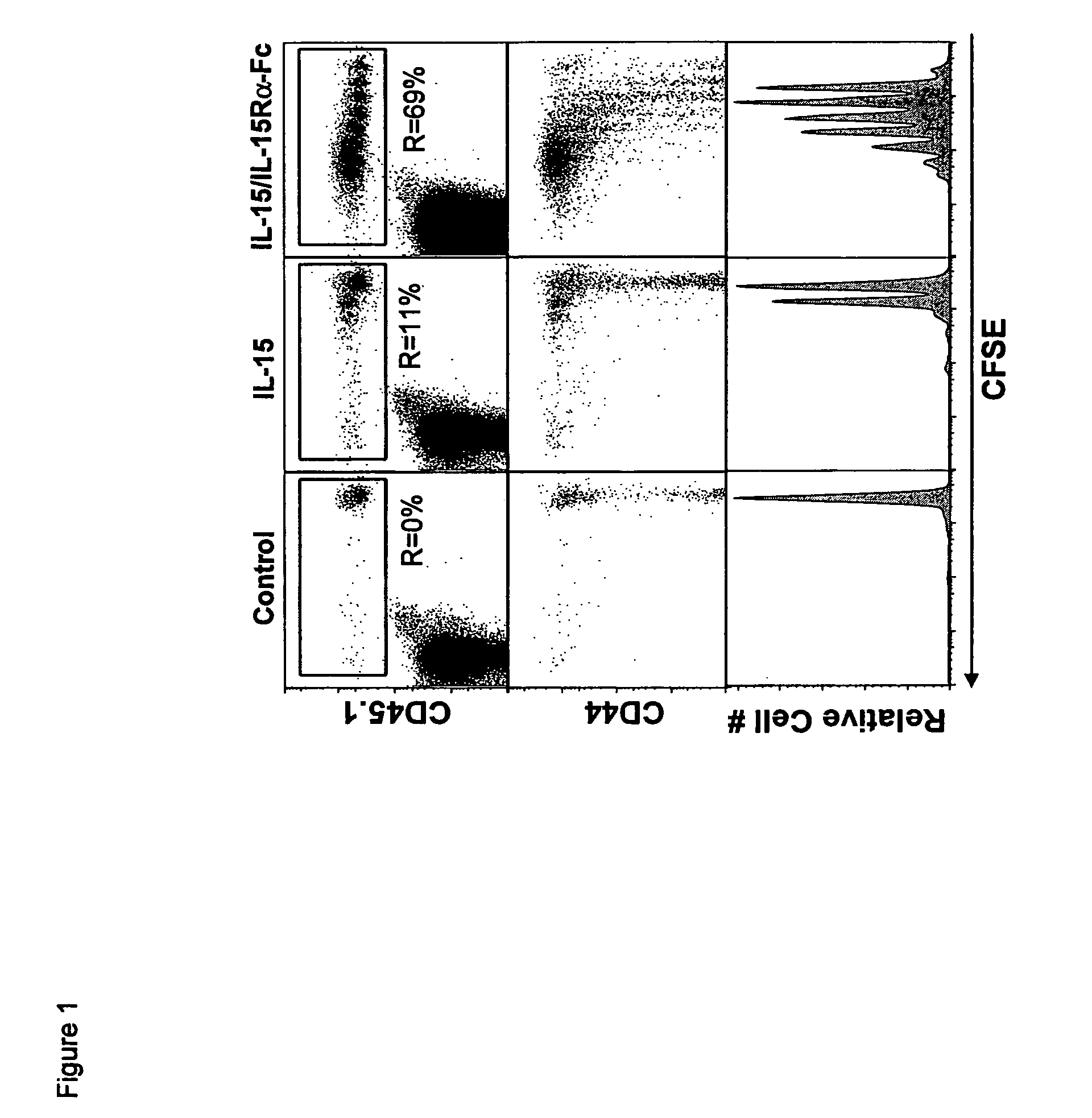
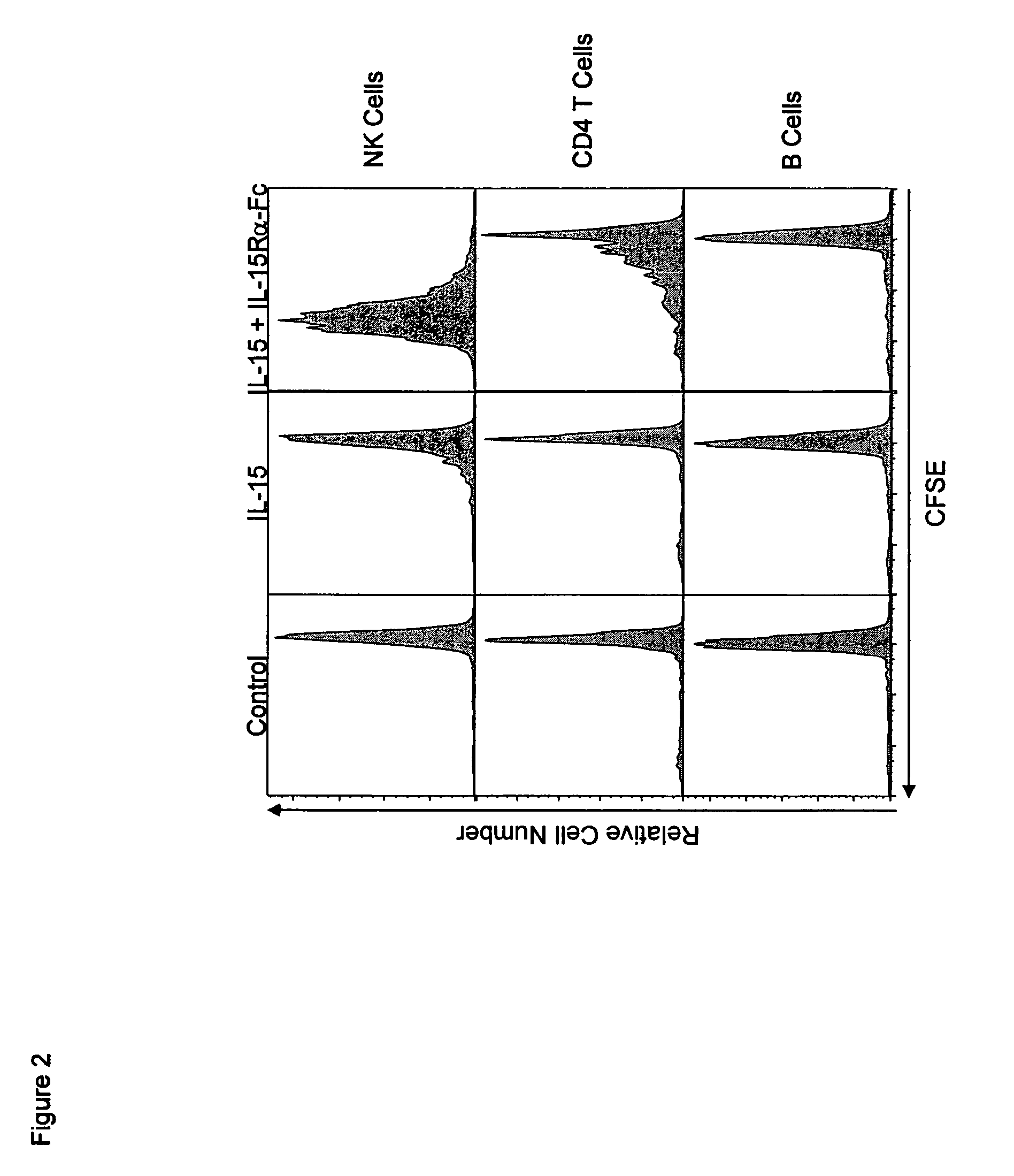
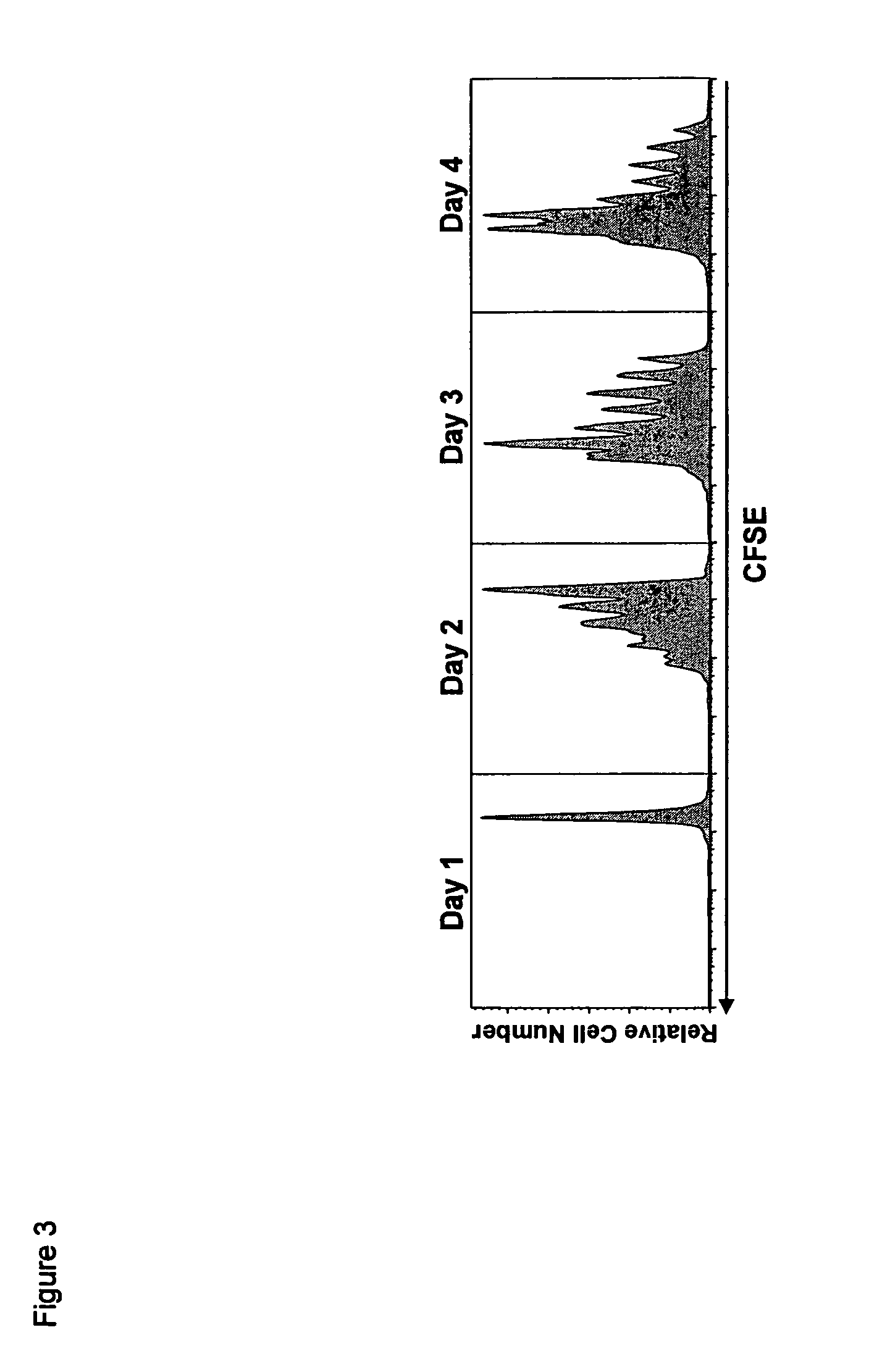
Compositions and methods for immunomodulation in an organism using IL-15 and soluble IL-15Ra
ActiveUS8124084B2Extended half-lifeImprove bioavailabilityPeptide/protein ingredientsAntibody mimetics/scaffoldsBiological bodyVaccination
The present invention relates to a therapeutic polypeptide and methods for its creation and use for modulating an immune response in a host organism in need thereof. In particular, the invention relates to the administration to an organism in need thereof, of an effective amount of a pre-coupled polypeptide complex comprising a lymphokine polypeptide portion, for example IL-15 (SEQ ID NO: 5, 6), IL-2 (SEQ ID NO: 10, 12) or combinations of both, and an interleukin receptor polypeptide portion, for example IL-15Ra (SEQ ID NO: 7, 8), IL-2Ra (SEQ ID NO: 9, 11) or combinations of both, for augmenting the immune system in, for example, cancer, SCID, AIDS, or vaccination; or inhibiting the immune system in, for example, rheumatoid arthritis, or Lupus. The therapeutic complex of the invention surprisingly demonstrates increased half-life, and efficacy in vivo.
Owner:UNIV OF CONNECTICUT
Inhibition of irritating side effects associated with use of a topical ophthalmic medication
InactiveUS20050004074A1Reduce severityEffective deliveryPowder deliveryBiocideCyclodextrin derivativeCyclodextrin Derivatives
This invention relates to a method of reducing an irritating or adverse side effect associated with the topical use of an active ophthalmic drug comprising incorporating an effective amount of a cyclodextrin or cyclodextrin derivative into a formulation to complex the active drug such that the concentration of the free active drug is reduced below a tolerable threshold, and incorporating an effective amount of a viscosity increasing agent in said formulation such that the bioavailability of said drug is high enough to be therapeutically effective, wherein the cyclodextrin or cyclodextrin derivative is not required to solubilize the active drug. Another aspect of this invention relates to topical ophthalmic formulations comprising an active drug, a cyclodextrin or cyclodextrin derivative, and a viscosity-enhancing agent, in effective amounts as stated above.
Owner:ALLERGAN INC
Antibody drug conjugate metabolites
ActiveUS7750116B1Improve drug bioavailabilityImprove bioavailabilityPeptide/protein ingredientsImmunoglobulinsMetaboliteWilms' tumor
Methods of treating a refractory or drug resistant cancer, cell proliferative disorder and tumor cells are provided. Also provided are antibody drug conjugate metabolites.
Owner:SEAGEN INC
Double-Stranded Ribonucleic Acid with Increased Effectiveness in an Organism
ActiveUS20070275465A1Improve stabilityGreat to enzymatic digestionSugar derivativesMicrobiological testing/measurementSingle strandDouble strand
The invention concerns a method for the targeted selection of a double-stranded ribonucleic acid (dsRNA) consisting of two single strands that exhibits increased effectiveness in inhibiting the expression of a target gene by means of RNA interference, whereby the sequences of the single strands of the dsRNA are selected in such a way that on both ends of the dsRNA the last complementary nucleotide pair is a G-C, or at least two of the last four complementary nucleotide pairs are G-C pairs; whereby the dsRNA exhibits a single-stranded overhang consisting of 1 to 4 unpaired nucleotides at the first end, and no overhang at the second end; whereby the unpaired nucleotide of the single-stranded overhang that is directly adjacent to the last complementary nucleotide pair contains a purine base.
Owner:ALNYLAM PHARM INC
Method and Device for Ophthalmic Administration of Active Pharmaceutical Ingredients
InactiveUS20080233053A1Efficient transferImprove bioavailabilityOrganic active ingredientsPeptide/protein ingredientsAdditive ingredientBioavailability
Disclosed is the use of a mist of a pharmaceutical composition for ophthalmic delivery of a protein or peptide active pharmaceutical ingredient, a related method of treatment and a device useful in implementing the use and method. Disclosed is also the use of a mist for ophthalmic delivery of a pharmaceutical composition including a highly irritating penetration enhancer and an ophthalmically acceptable carrier, a related method of treatment and a device useful in implementing the use and method. Disclosed is also a device for ophthalmic administration configured to direct a mist of a pharmaceutical composition to the eye only when the eye is open. Disclosed is also a self-sterilizing device for ophthalmic administration. Disclosed is also a device and a method for increasing the bioavailability of an ophthalmically administered API in a pharmaceutical composition.
Owner:PHARMALIGHT
Effervescent green tea extract formulation
InactiveUS6299925B1Fast absorptionMaintain good propertiesAntibacterial agentsPre-extraction tea treatmentNatural productAdditive ingredient
A solid state water soluble formulation in granular or tablet form is provided. The formulation is a natural products formulation containing a green tea plant extract in combination with other ingredients which create an effervescent liquid composition upon dispensing the formulation in a liquid. The liquid form of administration, as well as the effervescent properties of the dissolved formulation increase bioavailability of the advantageous components of the green tea plants such as Polyphenols, by increasing absorption speed and amount in the human body. The formulation may include additional components such as, other plant extracts, vitamins, ionic minerals, and other substances purported to be of a health benefit.
Owner:XEL HERBACEUTICALS INC
High-throughput formation, identification, and analysis of diverse solid-forms
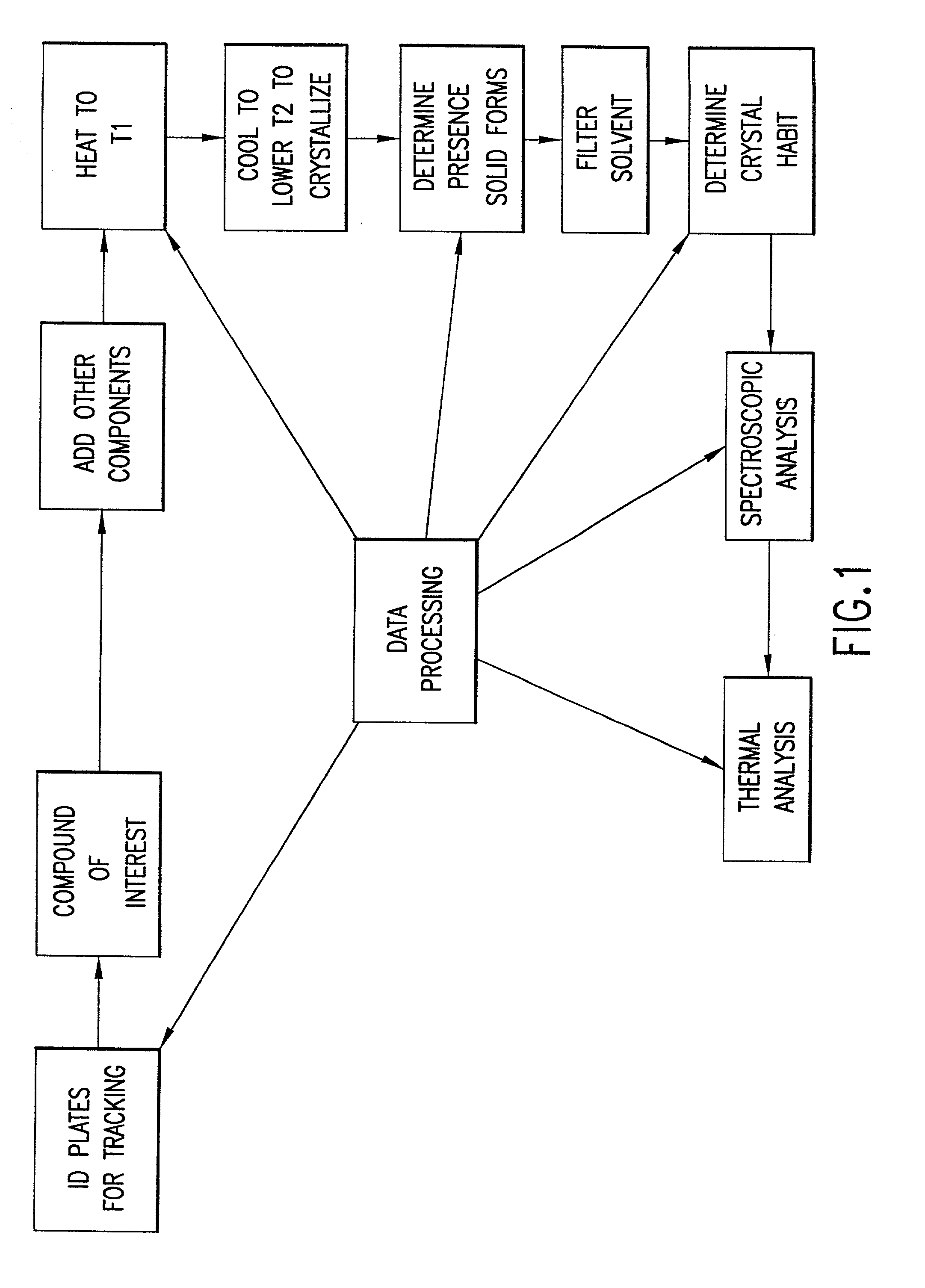
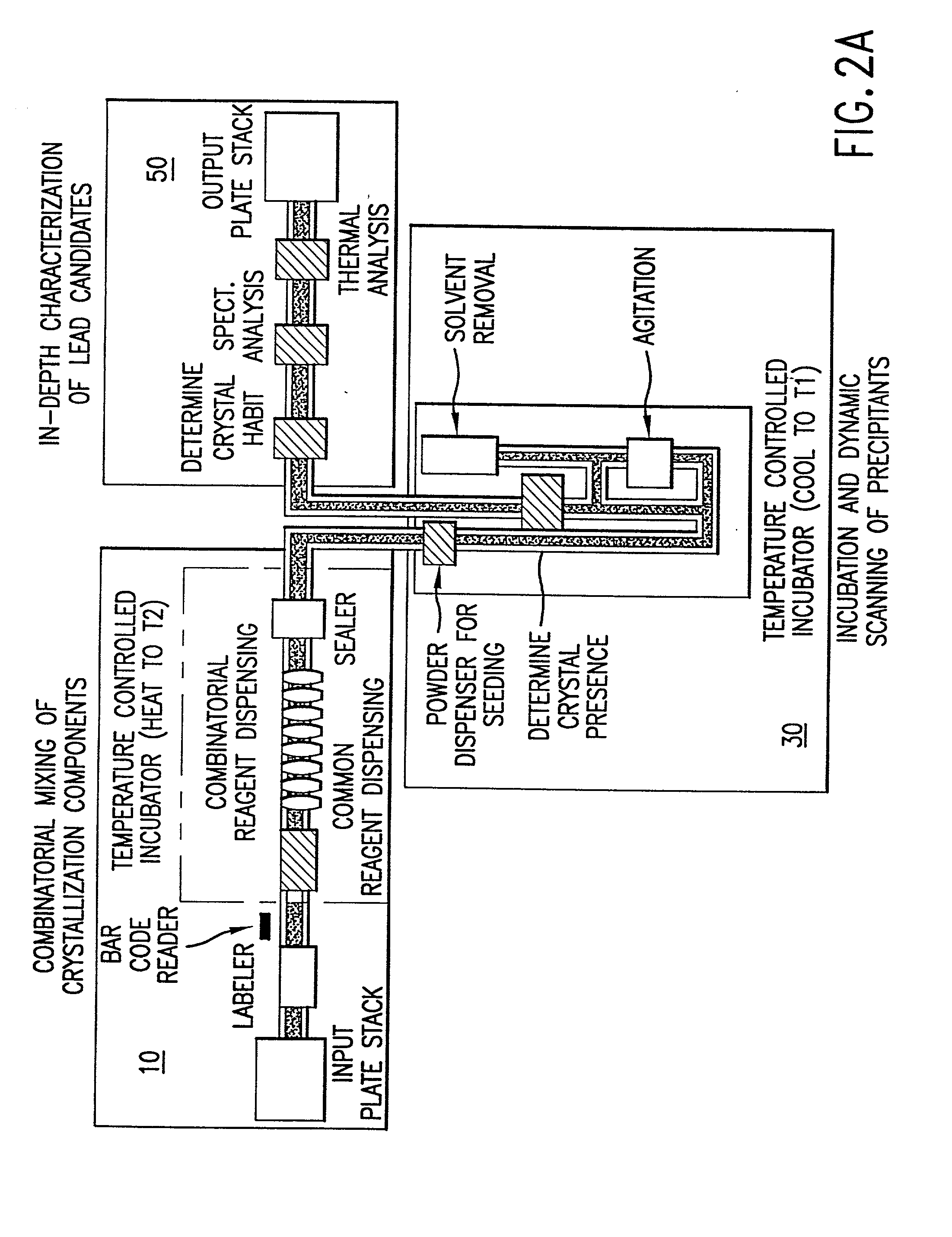
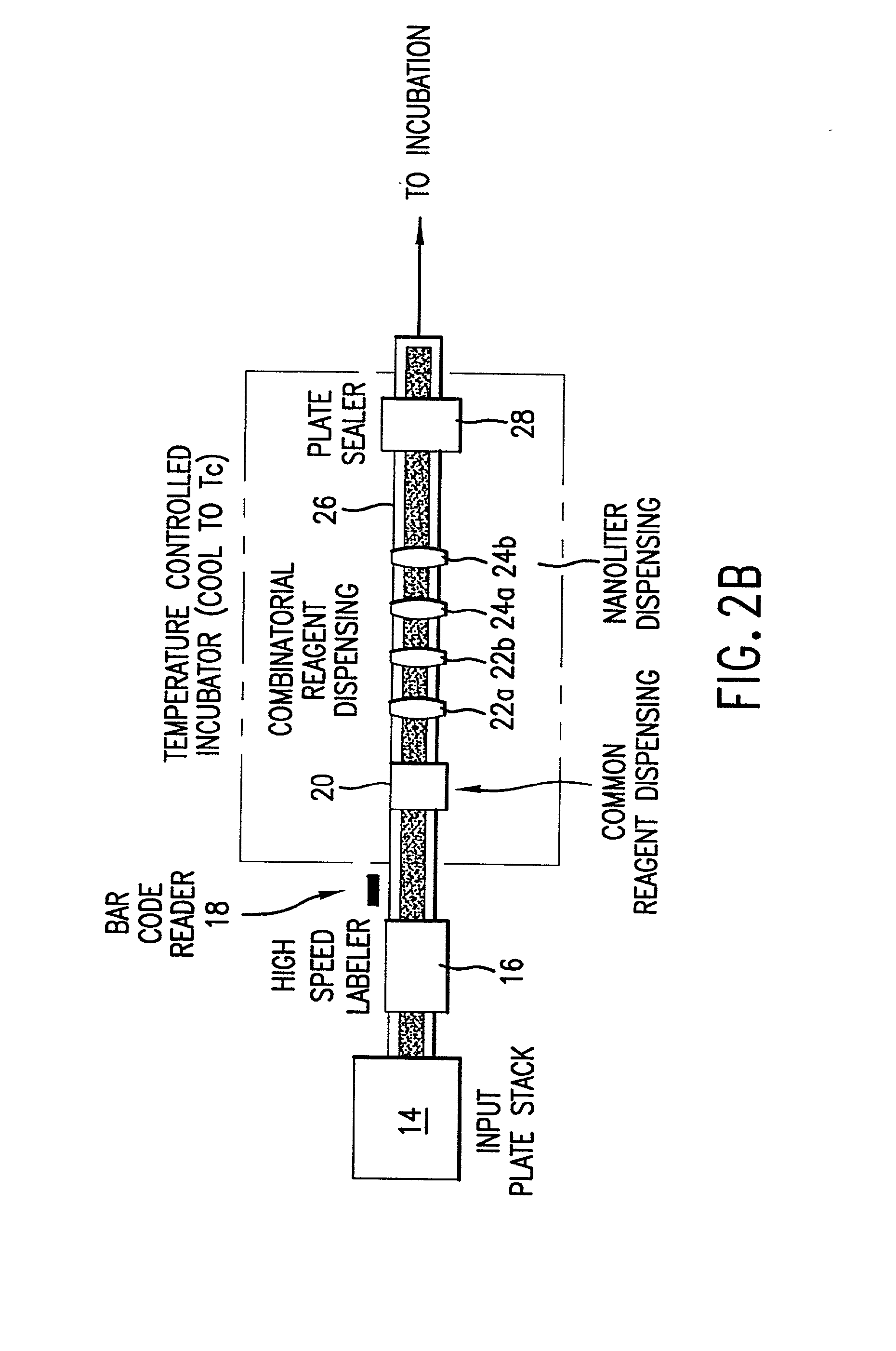
InactiveUS20020048610A1Cost-effectiveImprove bioavailabilitySequential/parallel process reactionsFrom normal temperature solutionsSolubilitySolid mass
The invention concerns arrays of solid-forms of substances, such as compounds and rapid-screening methods therefor to identify solid-forms, particularly of pharmaceuticals, with enhanced properties. Such properties include improved bioavailability, solubility, stability, delivery, and processing and manufacturing characteristics. The invention relates to a practical and cost-effective method to rapidly screen hundreds to thousands of samples in parallel. The invention further provides methods for determining the conditions and / or ranges of conditions required to produce crystals with desired compositions, particle sizes, habits, or polymorphic forms. In a further aspect, the invention provides high-throughput methods to identify sets of conditions and / or combinations of components compatible with particular solid-forms, for example, conditions and / or components that are compatible with advantageous polymorphs of a particular pharmaceutical.
Owner:MILLENNIUM PHARMA INC +1
Pharmaceutical co-crystal compositions
A pharmaceutical composition comprising a co-crystal of an API and a co-crystal former; wherein the API has at least one functional group selected from ether, thioether, alcohol, thiol, aldehyde, ketone, thioketone, nitrate ester, phosphate ester, thiophosphate ester, ester, thioester, sulfate ester, carboxylic acid, phosphonic acid, phosphinic acid, sulfonic acid, amide, primary amine, secondary amine, ammonia, tertiary amine, sp2 amine, thiocyanate, cyanamide, oxime, nitrile diazo, organohalide, nitro, s-heterocyclic ring, thiophene, n-heterocyclic ring, pyrrole, o-heterocyclic ring, furan, epoxide, peroxide, hydroxamic acid, imidazole, pyridine and the co-crystal former has at least one functional group selected from amine, amide, pyridine, imidazole, indole, pyrrolidine, carbonyl, carboxyl, hydroxyl, phenol, sulfone, sulfonyl, mercapto and methyl thio, such that the API and co-crystal former are capable of co-crystallizing from a solution phase under crystallization conditions.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES +2
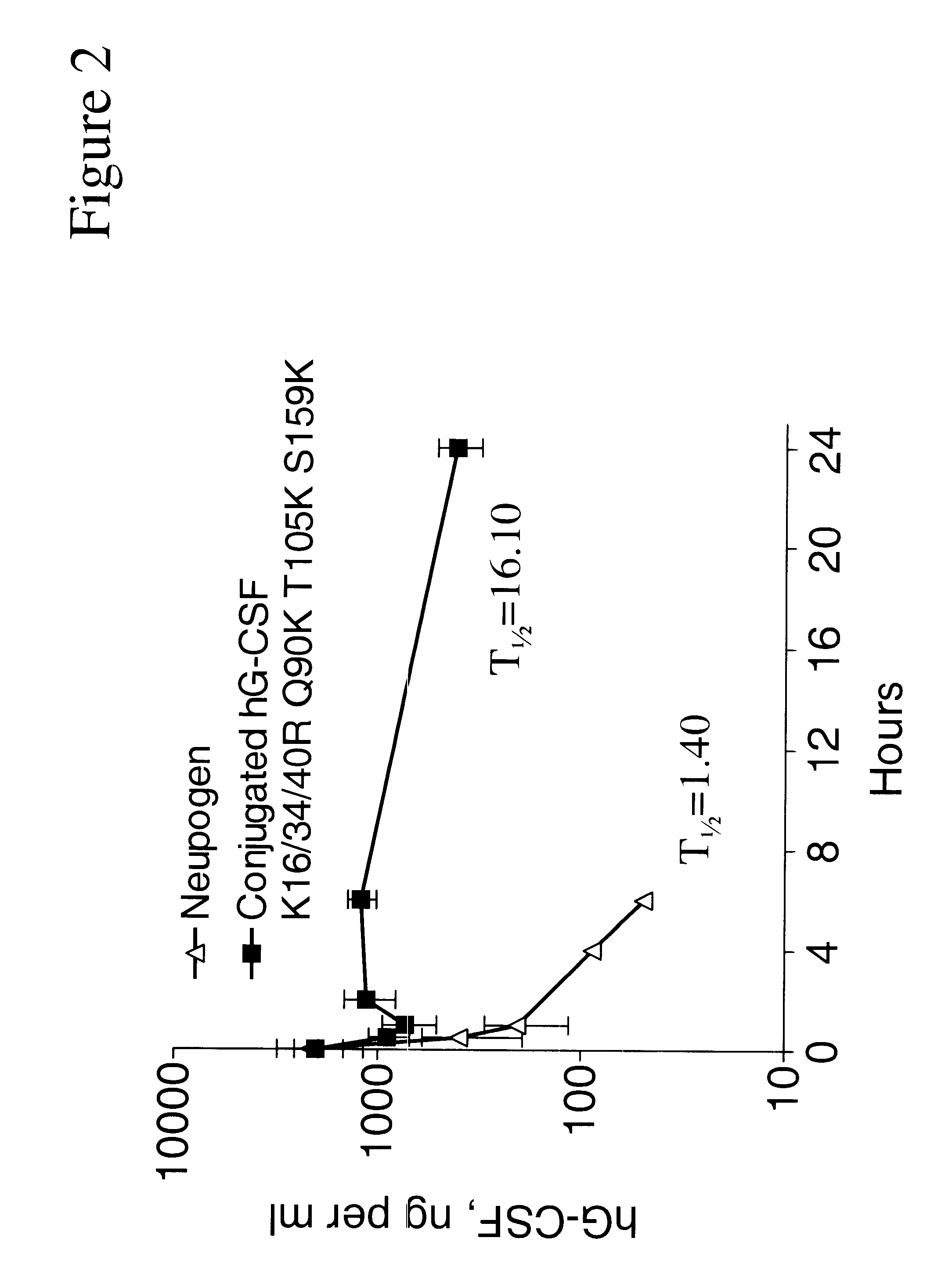
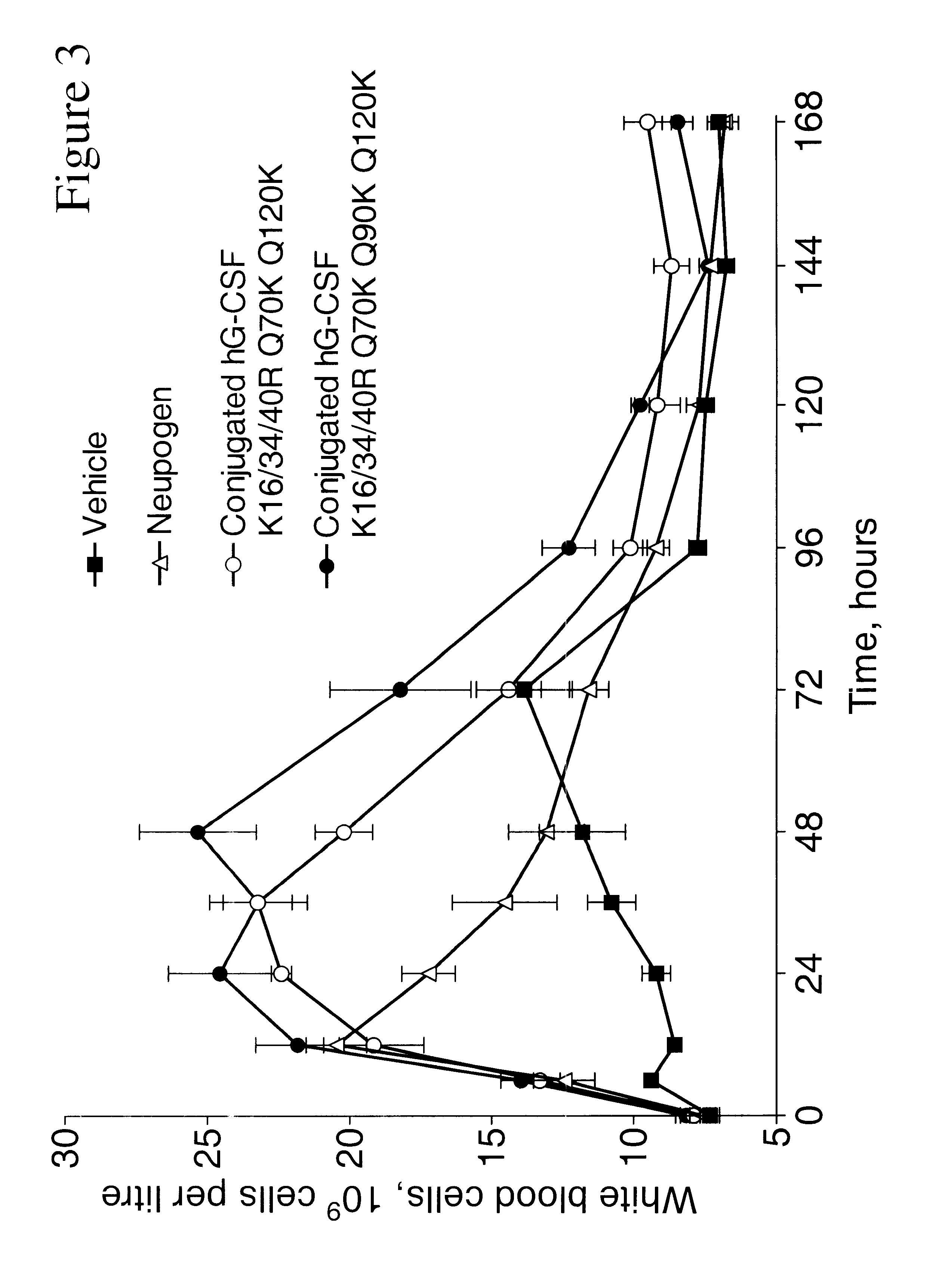
G-CSF conjugates
InactiveUS6555660B2Improved propertyReduced in vitroBiocidePeptide/protein ingredientsHalf-lifePolyethylene glycol
The invention relates to polypeptide conjugates comprising a polypeptide exhibiting G-CSF activity and having an amino acid sequence that differs from the amino acid sequence of human G-CSF in at least one specified introduced and / or removed amino acid residue comprising an attachment group for a non-polypeptide moiety, and having at least one non-polypeptide moiety attached to an attachment group of the polypeptide. The attachment group may e.g. be a lysine, cysteine, aspartic acid or glutamic acid residue or a glycosylation site, and the non-polypeptide moiety may e.g. be a polymer such as polyethylene glycol or an oligosaccharide. The conjugate, which has a reduced in vitro bioactivity compared to hG-CSF, has one or more improved properties such as increased biological half-life and increased stimulation of neutrophils.
Owner:MAXYGEN
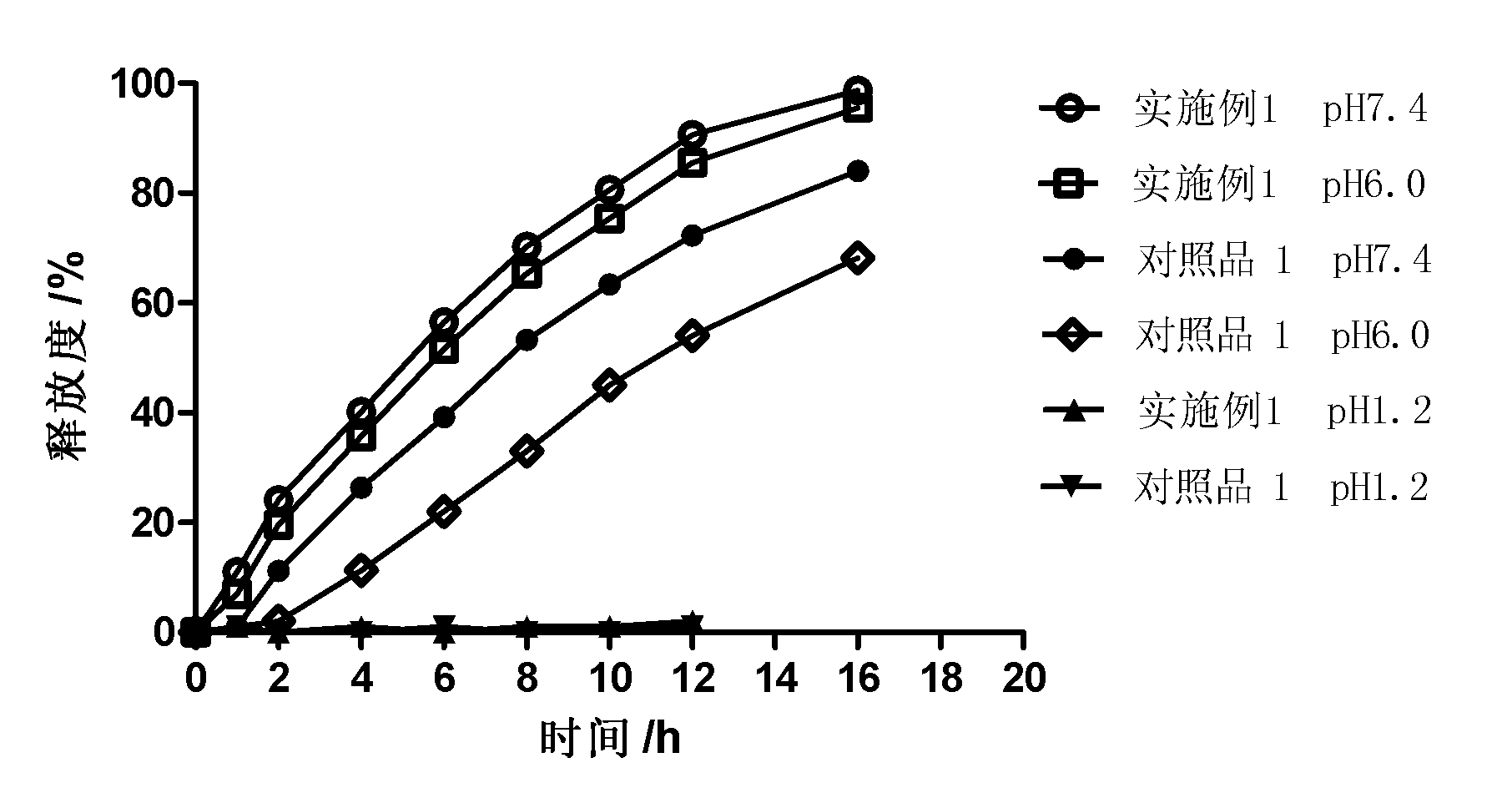
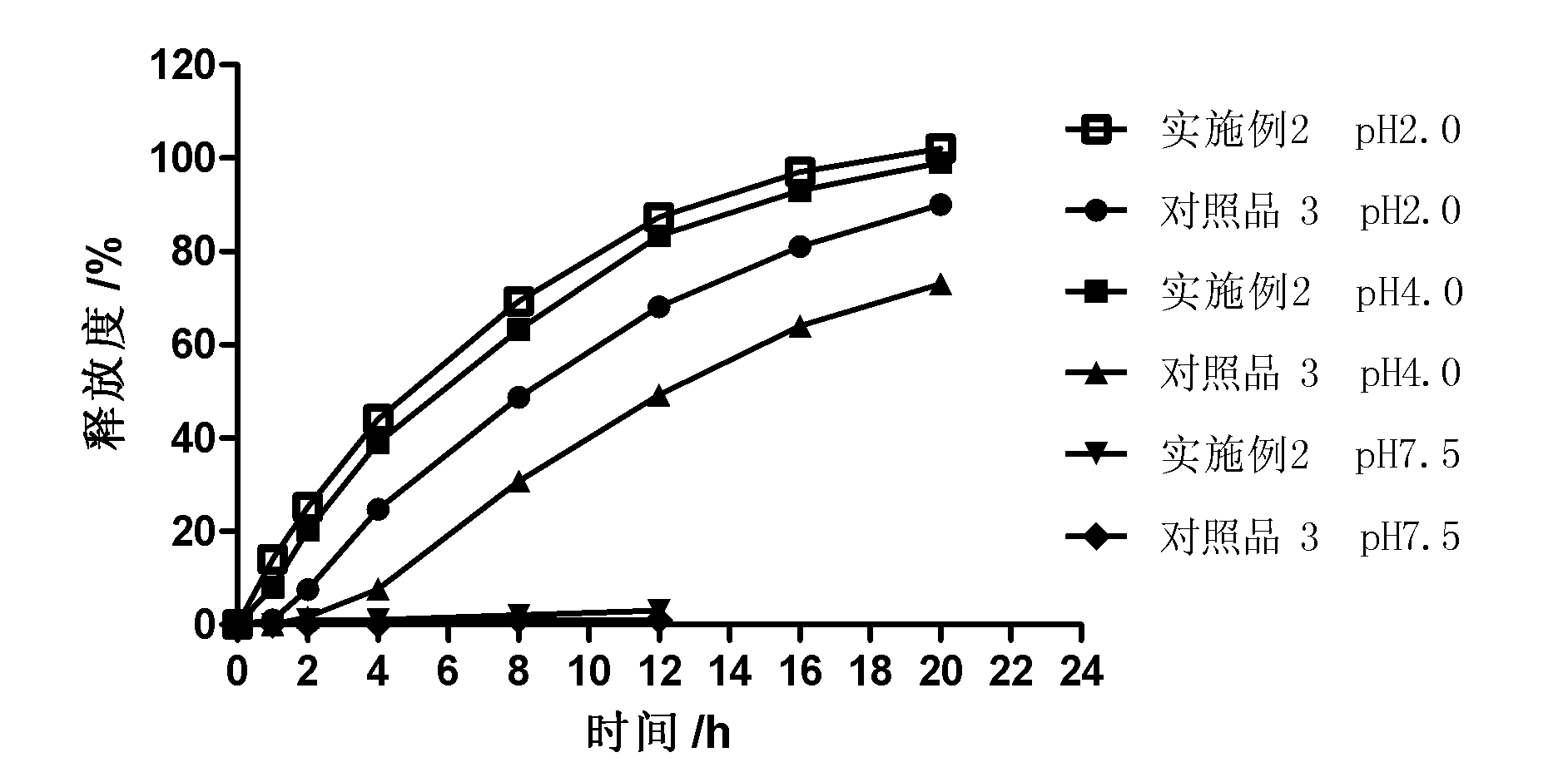
Controlled release preparation
InactiveCN101987081AImprove stabilityRelease impact mitigationInorganic non-active ingredientsSuppositories deliveryParticulatesChemical reaction
The invention discloses a controlled release preparation with improved performance. The controlled release preparation comprises a core containing medicament and a controlled release film covering the outside of the core and being almost insoluble in water as well as stomach and intestines digestive juice. The controlled release film comprises particulate matters of a water soluble medicinal additive, the water-soluble medicinal additive is covered by a polymer film which can be soluble in the stomach and / or intestines digestive juice but almost insoluble in water, the polymer and the medicinal additive can not produce chemical reaction or can produce chemical reaction but do not produce water-insoluble non-gaseous products and the pharmaceutically unacceptable products, and the amount of the polymer is no more 700% of that of the medicinal additive. The invention also discloses a preparation method of the controlled release preparation. The controlled release preparation has the advantages of improved medicament release reproducibility, reduced medicament release lag time, accelerated medicament release and improved bioavailability, can realize located controlled release, delayed controlled release and interval type or pulse type controlled release of the medicament in the gastrointestinal tract, and the like.
Owner:钟术光
RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA)
InactiveUS20070004664A1Improve various propertyModulating gene expressionBiocideOrganic active ingredientsBiological bodyNucleic acid sequencing
The present invention concerns methods and reagents useful in modulating gene expression in a variety of applications, including use in therapeutic, diagnostic, target validation, and genomic discovery applications. Specifically, the invention relates to synthetic chemically modified small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules capable of mediating RNA interference (RNAi) against target nucleic acid sequences. The small nucleic acid molecules are useful in the treatment of any disease or condition that responds to modulation of gene expression or activity in a cell, tissue, or organism.
Owner:SIRNA THERAPEUTICS INC
Methods and devices for improving delivery of a substance to skin
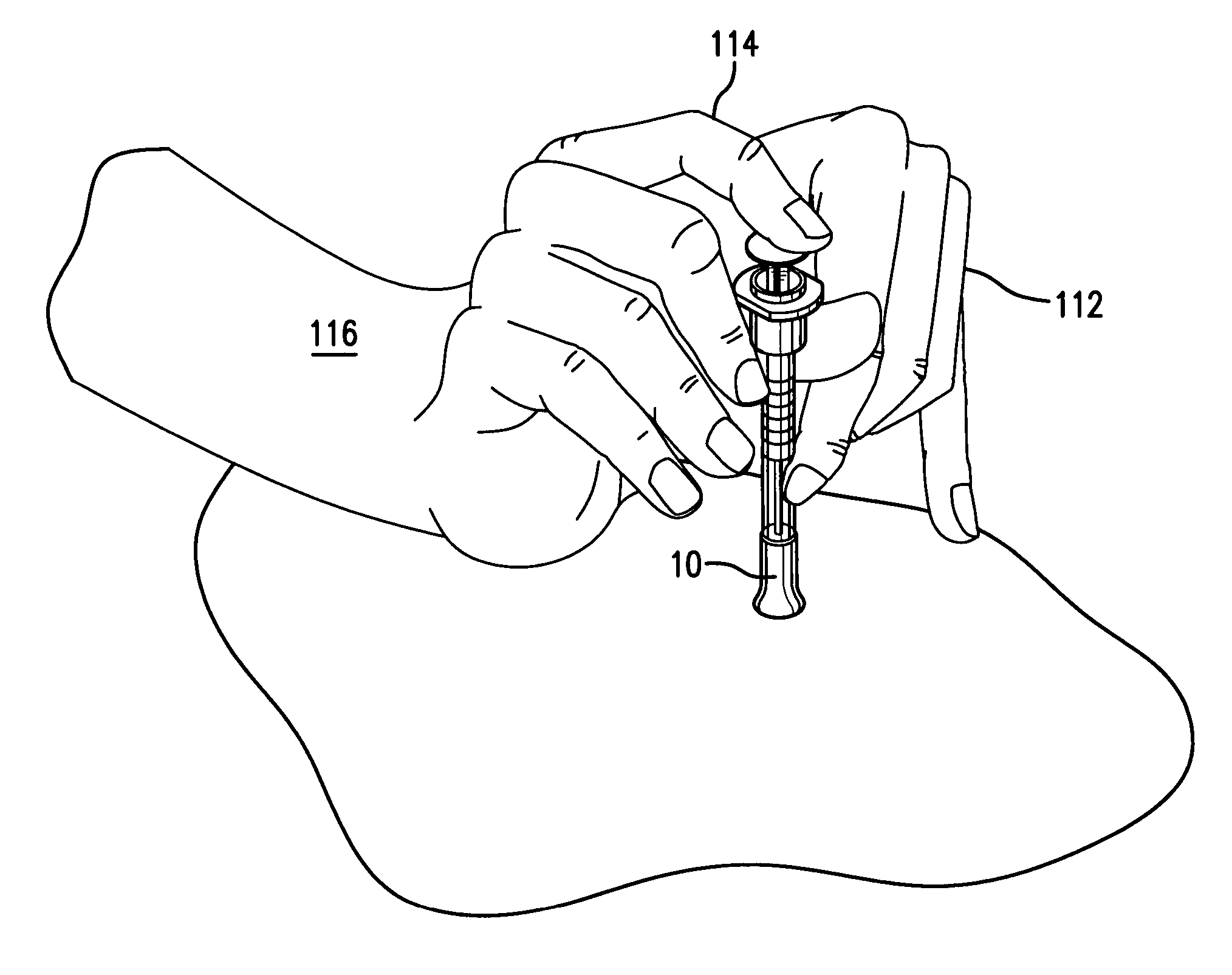
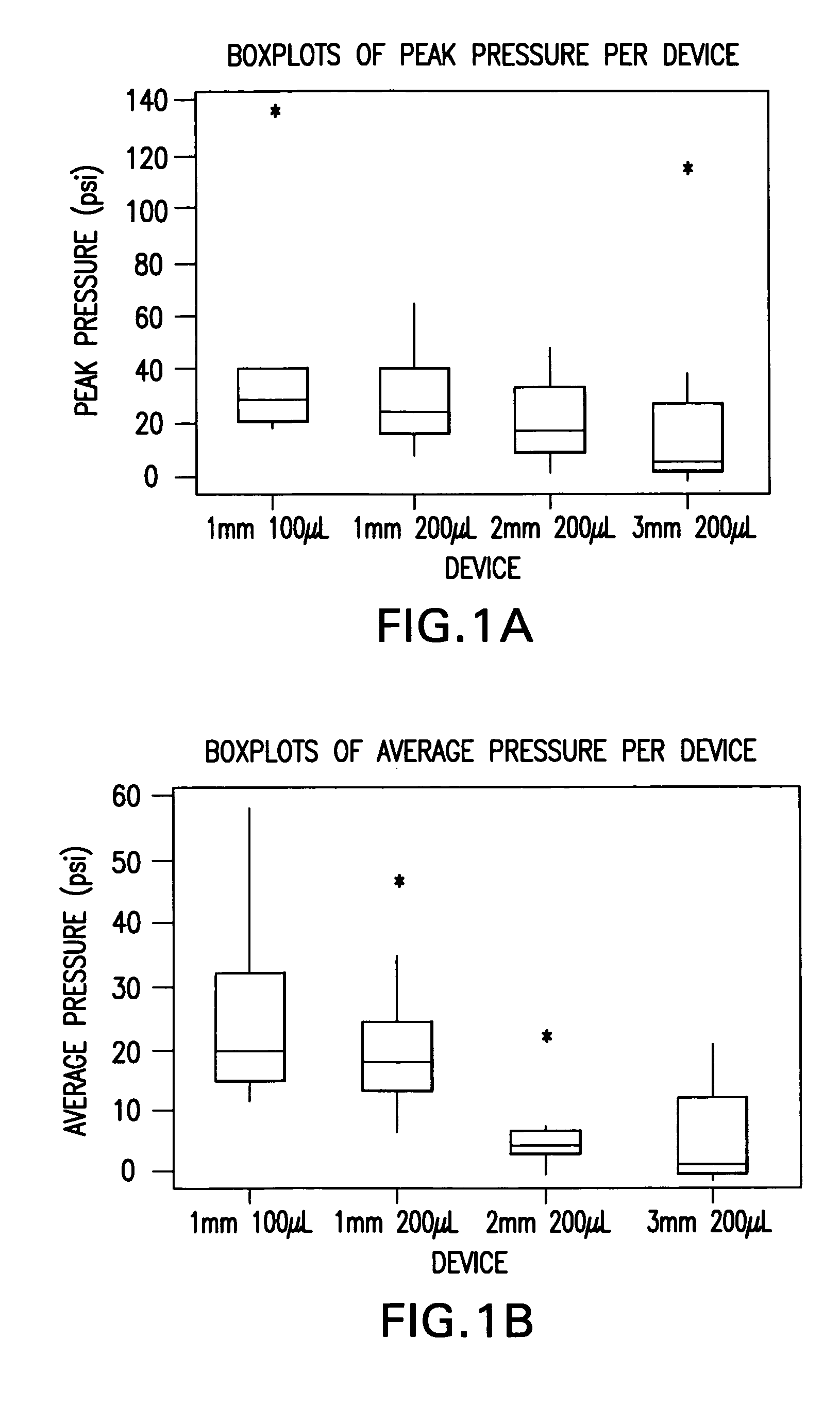
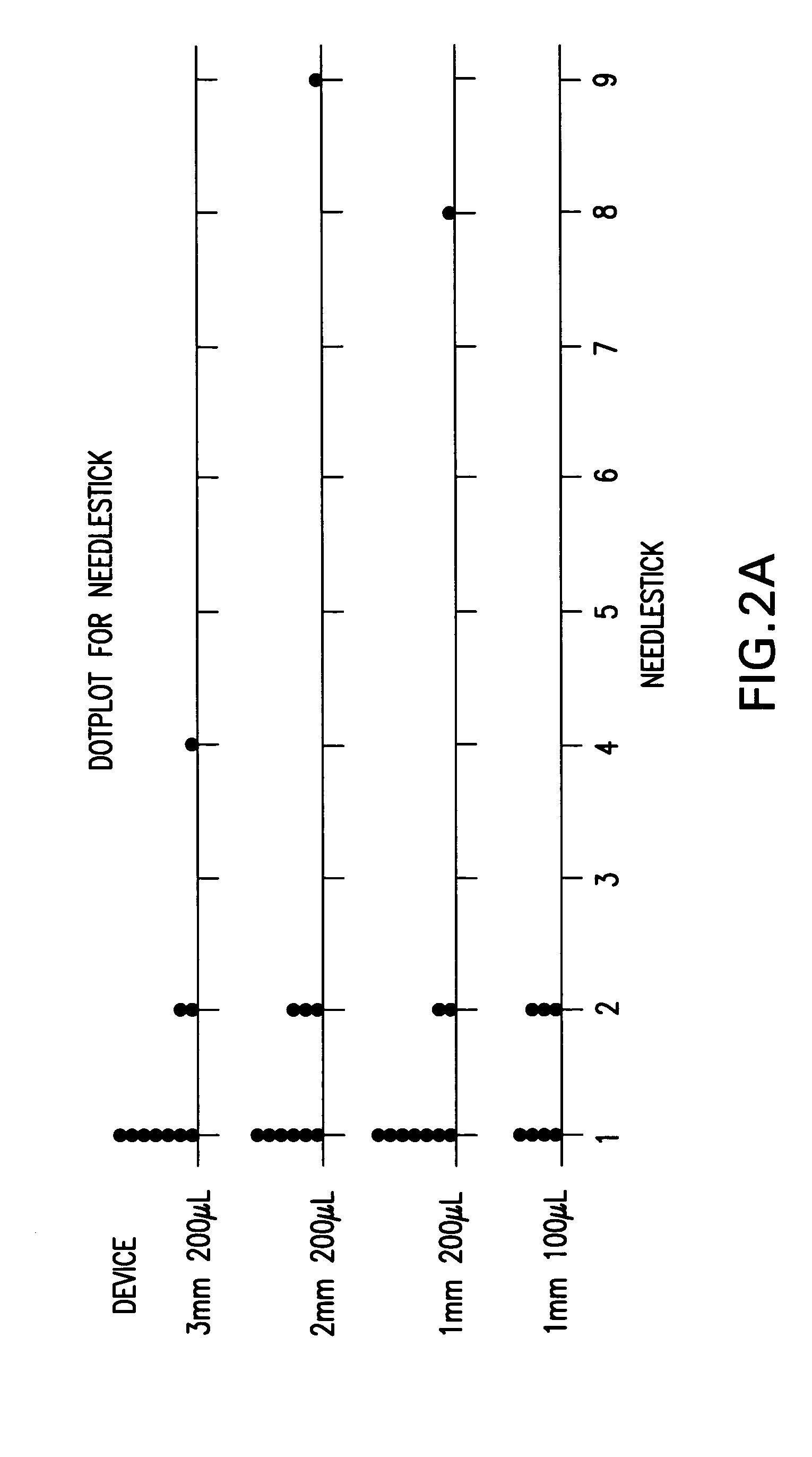
InactiveUS20050256499A1Efficacy is alteredImprove delivery capabilitiesMedical devicesPressure infusionDelivery PerformanceInjection site
A method of delivery of a substance to a human subject's skin comprising deposition into a specific compartment of the skin, wherein the delivery occurs at a controlled rate and pressure. The methods of the invention provide accurate deposition of s pre-selected volume of the substance, e.g., greater than 90% of the pre-selected volume. The methods of the invention encompass varying one or more parameters including but not limited to configurations of the delivery device, volume, pressure, and flow rate of delivery, to enhance the efficacy of delivery of the substance to the human skin. Substances delivered in accordance with the methods of the invention result in a more efficacious deposition of the substance into the targeted compartment, improved delivery performance, i.e., completeness of delivery as measured by quantification of the substance not delivered or the amount of the substance leaked out from the injection site, and enhanced safety as measured by the occurrence of minimal adverse cutaneous events at the site of injection.
Owner:BECTON DICKINSON & CO
Compositions and methods for intraocular delivery of fibronectin scaffold domain proteins
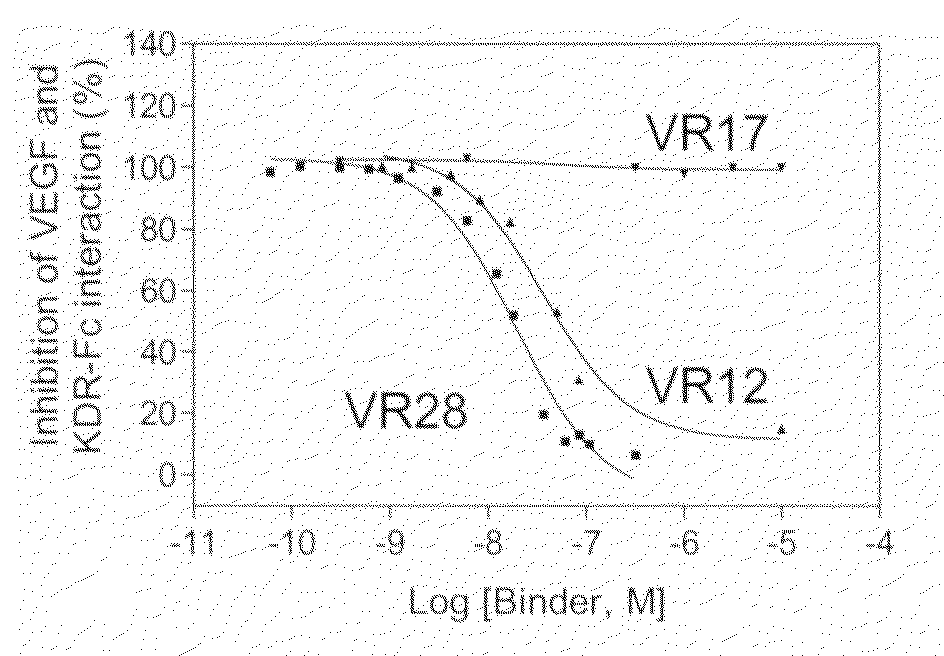
InactiveUS20080220049A1Easy to optimizeImprove bioavailabilitySenses disorderPeptide/protein ingredientsMedicineFibronectin
The present disclosure relates to novel sustained-release intraocular drug delivery systems and improvements in the treatment of retinopathies. In particular, fibronectin scaffold domain proteins that selectively inhibit VEGFR-2 are contemplated.
Owner:BRISTOL MYERS SQUIBB CO
Compositions comprising lipophilic active compounds and method for their preparation
ActiveUS20090098200A1Quick releaseImprove bioavailabilityPowder deliveryBiocideHydrophilic polymersCompound (substance)
Compositions are provided comprising a lipophilic active compound, e.g., a human or veterinary drug or a nutraceutical, interwoven with a polymeric matrix formed by two or more polymers, wherein one of the polymers is an amphiphilic polymer and the other polymer is either an amphiphilic polymer with a different hydrophobic-hydrophilic balance or a hydrophilic polymer, and the active lipophilic compound has modified physicochemical properties. The composition forms colloidal nanodispersion upon contact with aqueous media.
Owner:SOLUBEST
Porous drug matrices and methods of manufacture thereof
InactiveUS20050048116A1Fast dissolutionHigh dissolution ratePowder deliveryGranular deliveryDrugs solutionMicroparticle
Drugs, especially low aqueous solubility drugs, are provided in a porous matrix form, preferably microparticles, which enhances dissolution of the drug in aqueous media. The drug matrices preferably are made using a process that includes (i) dissolving a drug, preferably a drug having low aqueous solubility, in a volatile solvent to form a drug solution, (ii) combining at least one pore forming agent with the drug solution to form an emulsion, suspension, or second solution and hydrophilic or hydrophobic excipients that stabilize the drug and inhibit crystallization, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of drug. Hydrophobic or hydrophilic excipients may be selected to stabilize the drug in crystalline form by inhibiting crystal growth or to stabilize the drug in amorphous form by preventing crystallization. The pore forming agent can be either a volatile liquid that is immiscible with the drug solvent or a volatile solid-compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. The resulting porous matrix has a faster rate of dissolution following administration to a patient, as compared to non-porous matrix forms of the drug. In a preferred embodiment, microparticles of the porous drug matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
Intra-dermal delivery of biologically active agents
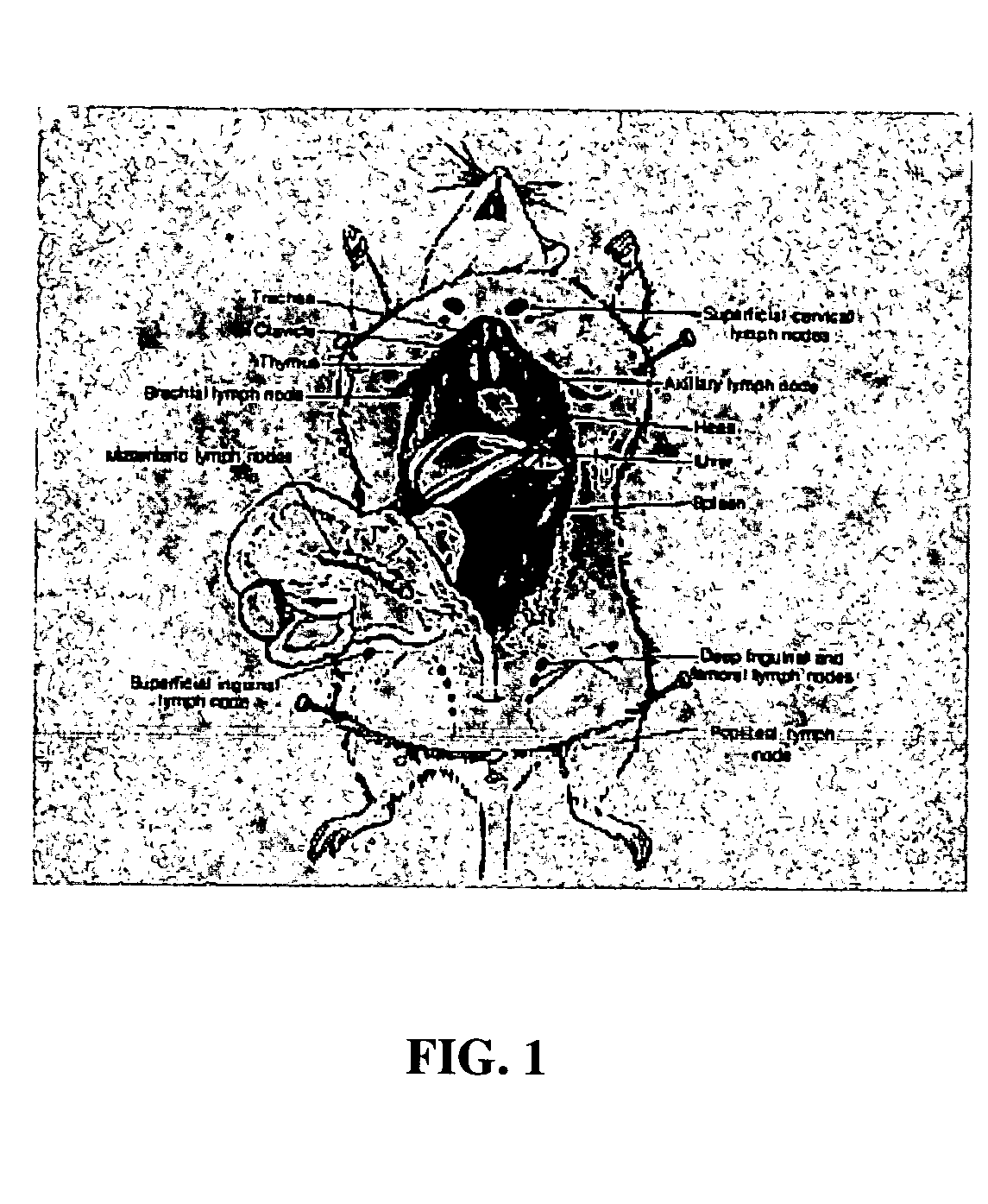
InactiveUS20050163711A1Rapid uptakeFast shippingMetabolism disorderDigestive systemLymphatic vesselDiagnostic agent
The present invention relates to methods and devices for delivering one or more biologically active agents, particularly a diagnostic agent to the intradermal compartment of a subject's skin. The present invention provides an improved method of delivery of biologically active agents in that it provides among other benefits, rapid uptake into the local lymphatics, improved targeting to a particular tissue, improved bioavailability, improved tissue bioavailability, improved tissue specific kinetics, improved deposition of a pre-selected volume of the agent to be administered, and rapid biological and pharmacodynamics and biological and pharmacokinetics. This invention provides methods for rapid transport of agents through lymphatic vasculature accessed by intradermal delivery of the agent. Methods of the invention are particularly useful for delivery of diagnostic agents.
Owner:BECTON DICKINSON & CO
Delivery of tetrahydrocannabinol
InactiveUS20070104741A1Avoiding hepatic first-pass metabolismGood choiceBiocideNervous disorderChylomicronTG - Triglyceride
A self-emulsifying drug delivery system to improve dissolution, stability, and bioavailability of drug compounds of dronabinol or other cannabinoids. The drug compound(s) are dissolved in an oily medium (e.g. triglycerides and / or mixed glycerides and / or free fatty acids containing medium and / or long chain saturated, mono-unsaturated, and / or poly-unsaturated free fatty acids) together with at least one surfactant. The surfactant promotes self-emulsification, thereby promoting targeted chylomicron delivery and optimal bioavailability to a mammalian intestinal lumen. A dosage form can optionally include co-solvents, anti-oxidants, viscosity modifying agents, cytochrome P450 metabolic inhibitors, P-GP efflux inhibitors, and amphiphilic / non-amphiphilic solutes to induce semi-solid formation for targeted release rates.
Owner:MURTY PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com