Patents
Literature
399 results about "Injection site" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
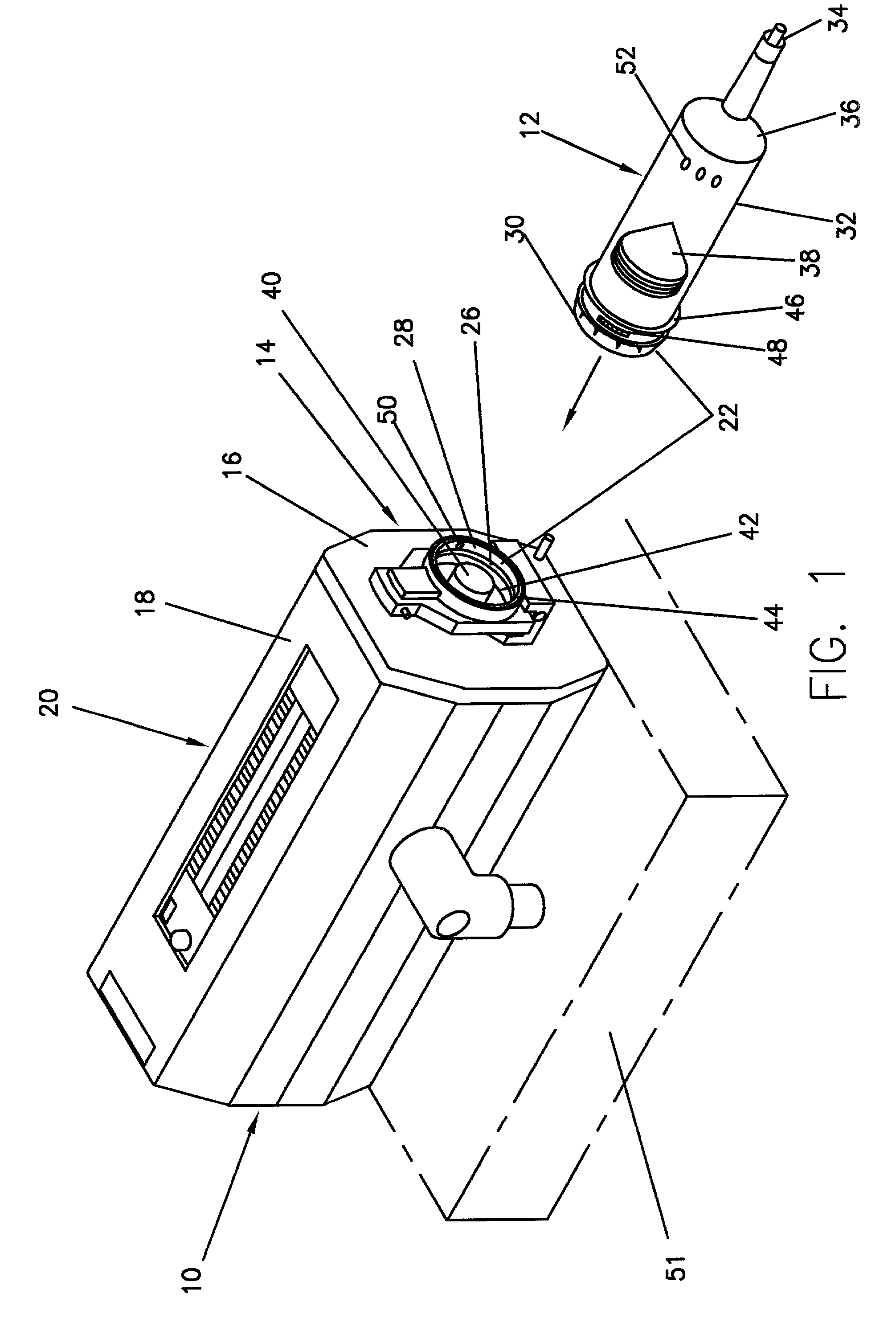
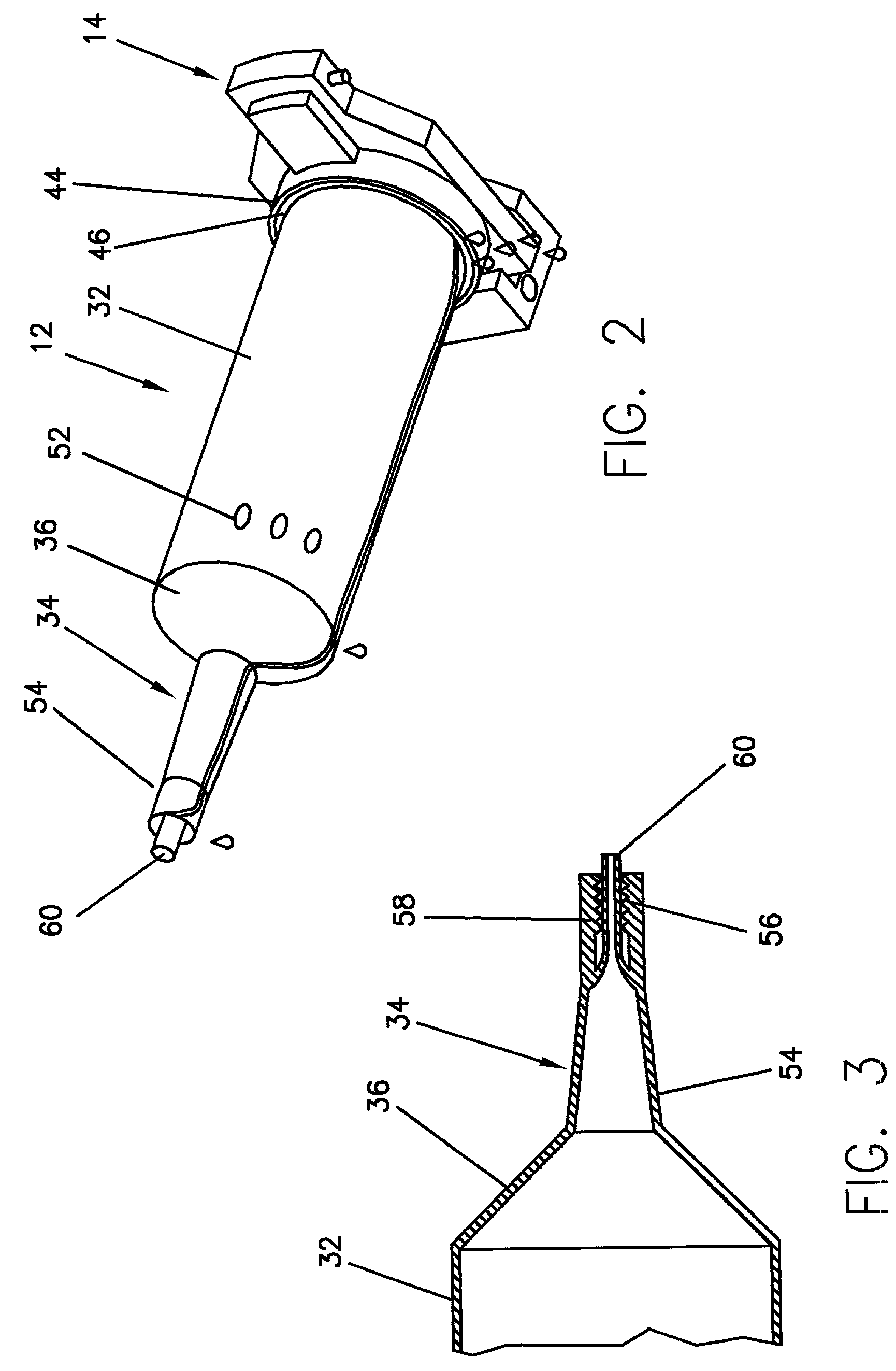
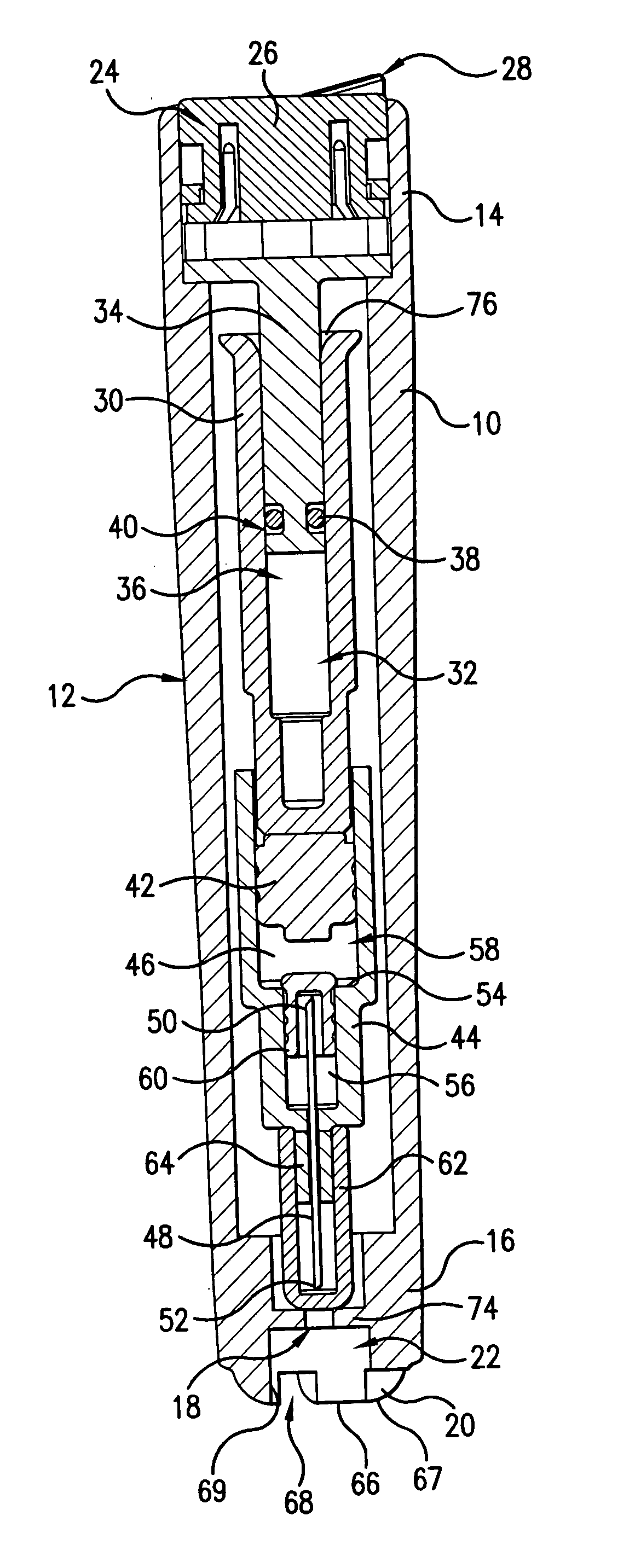
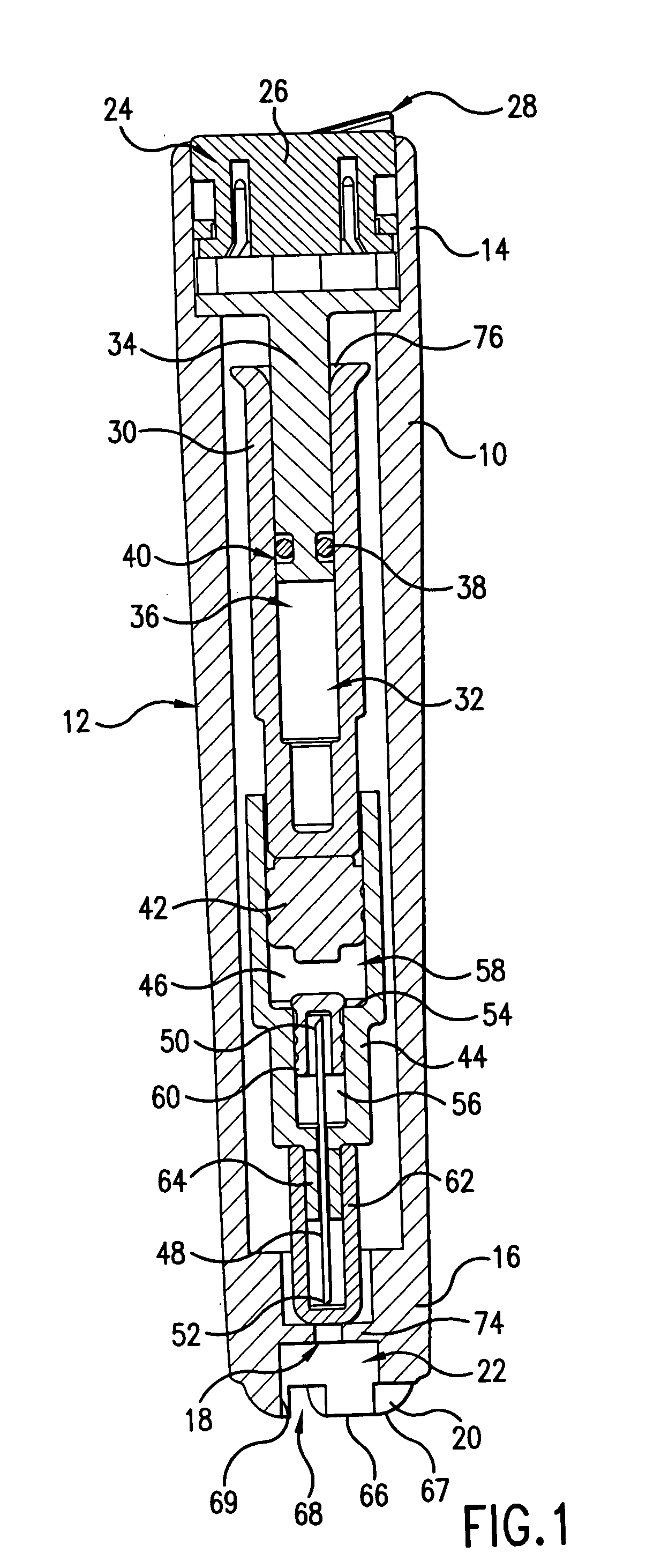
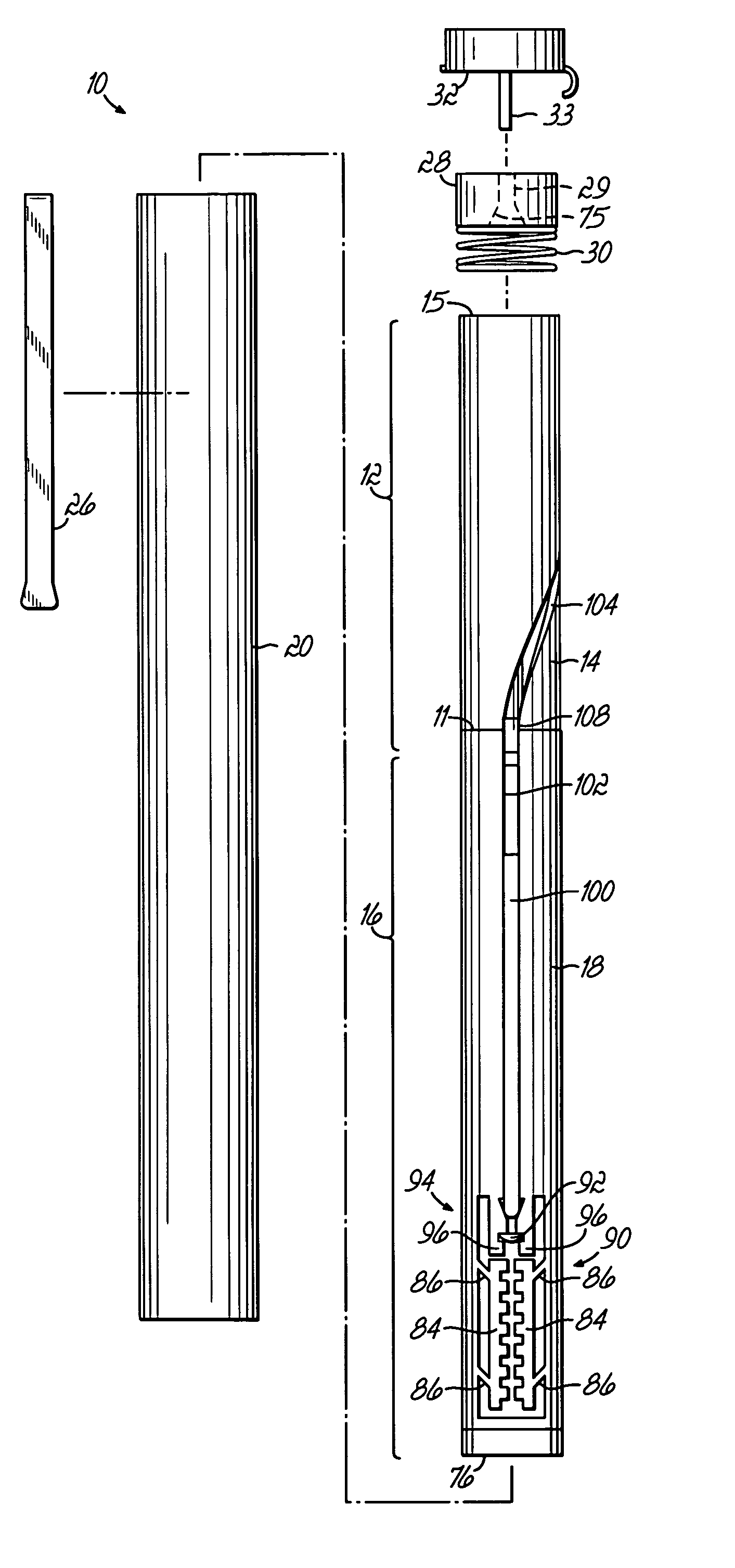
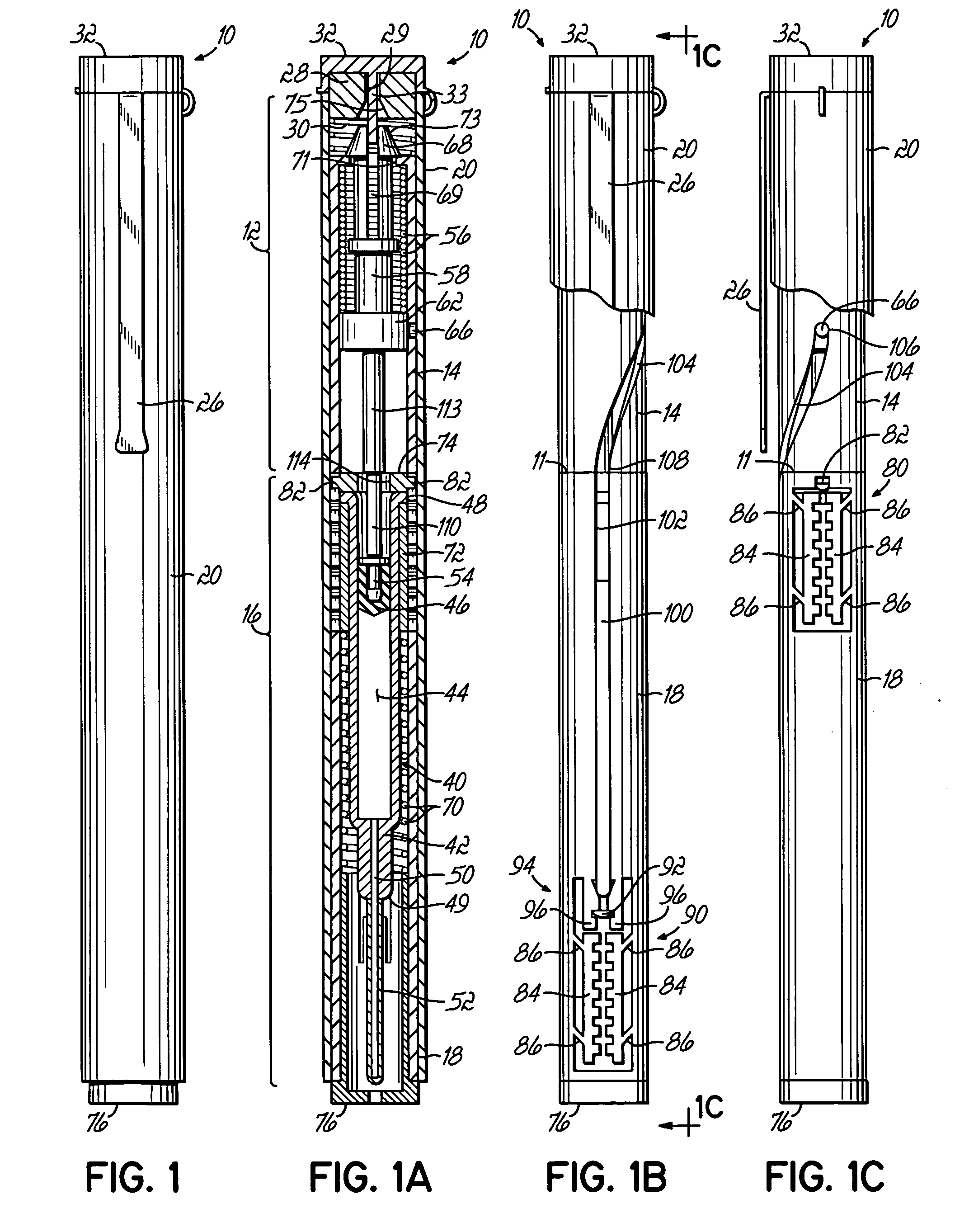
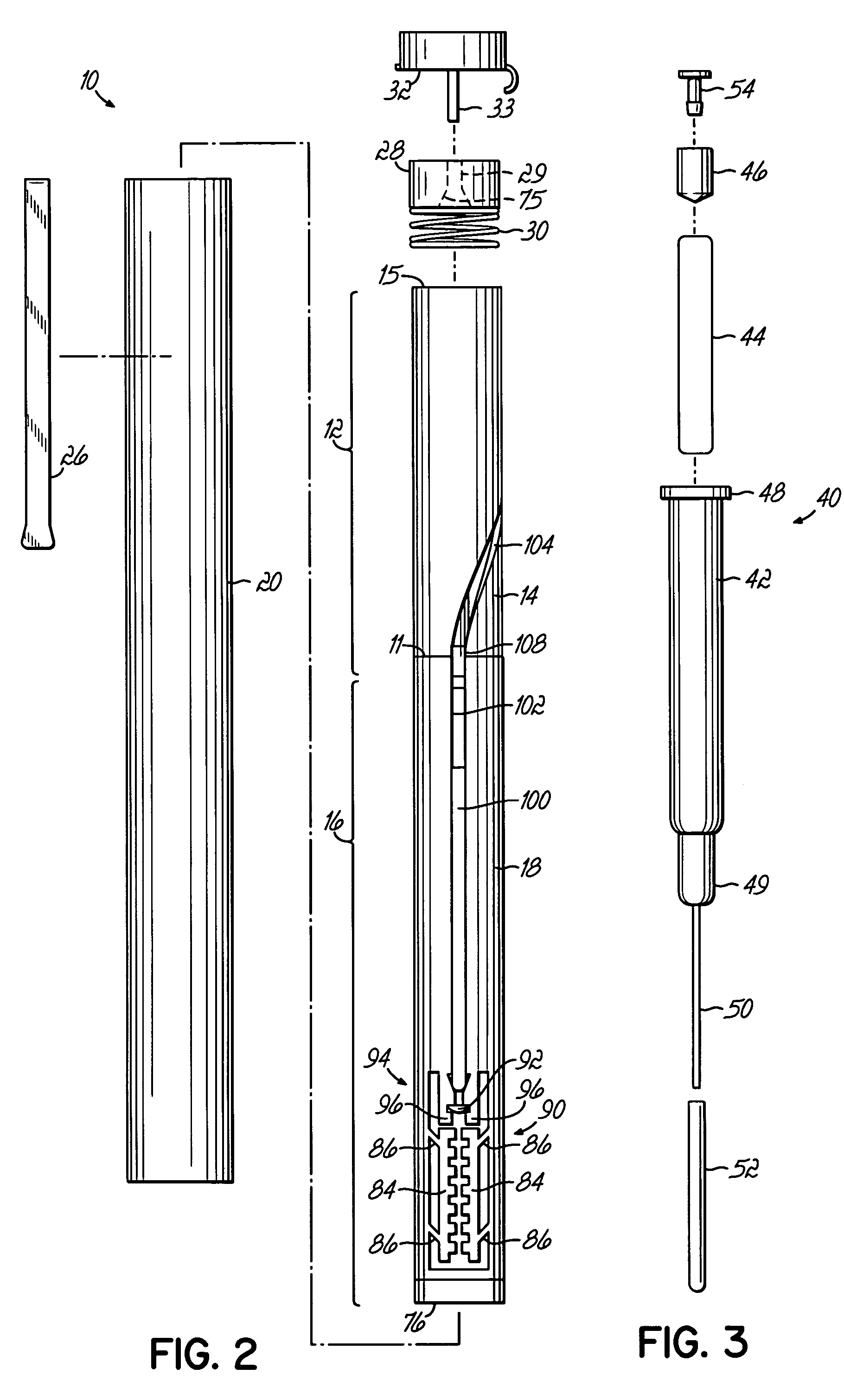
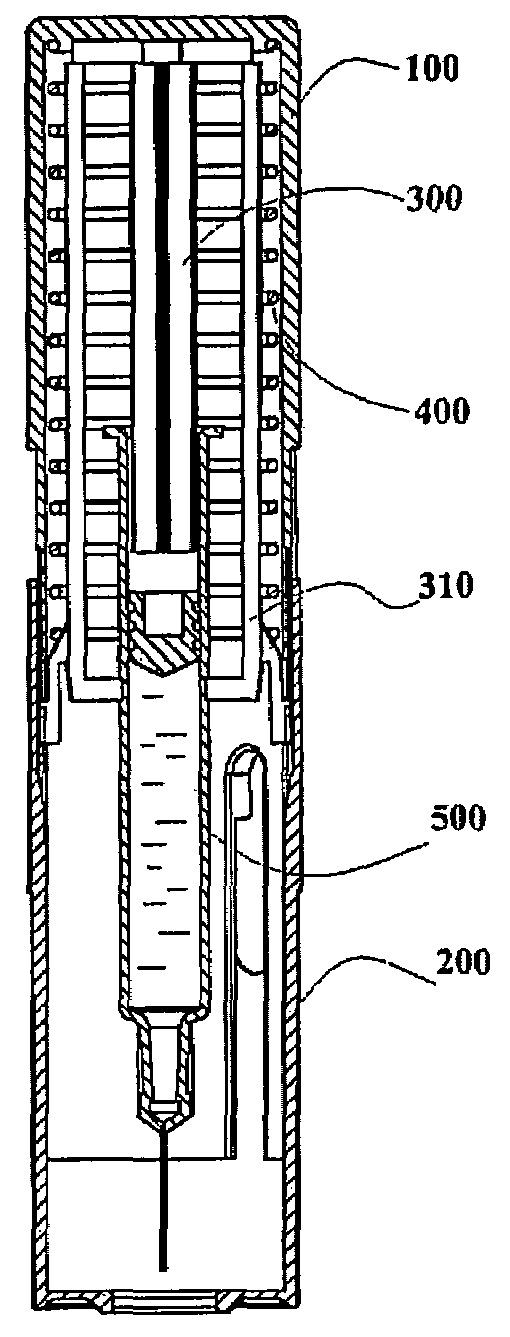
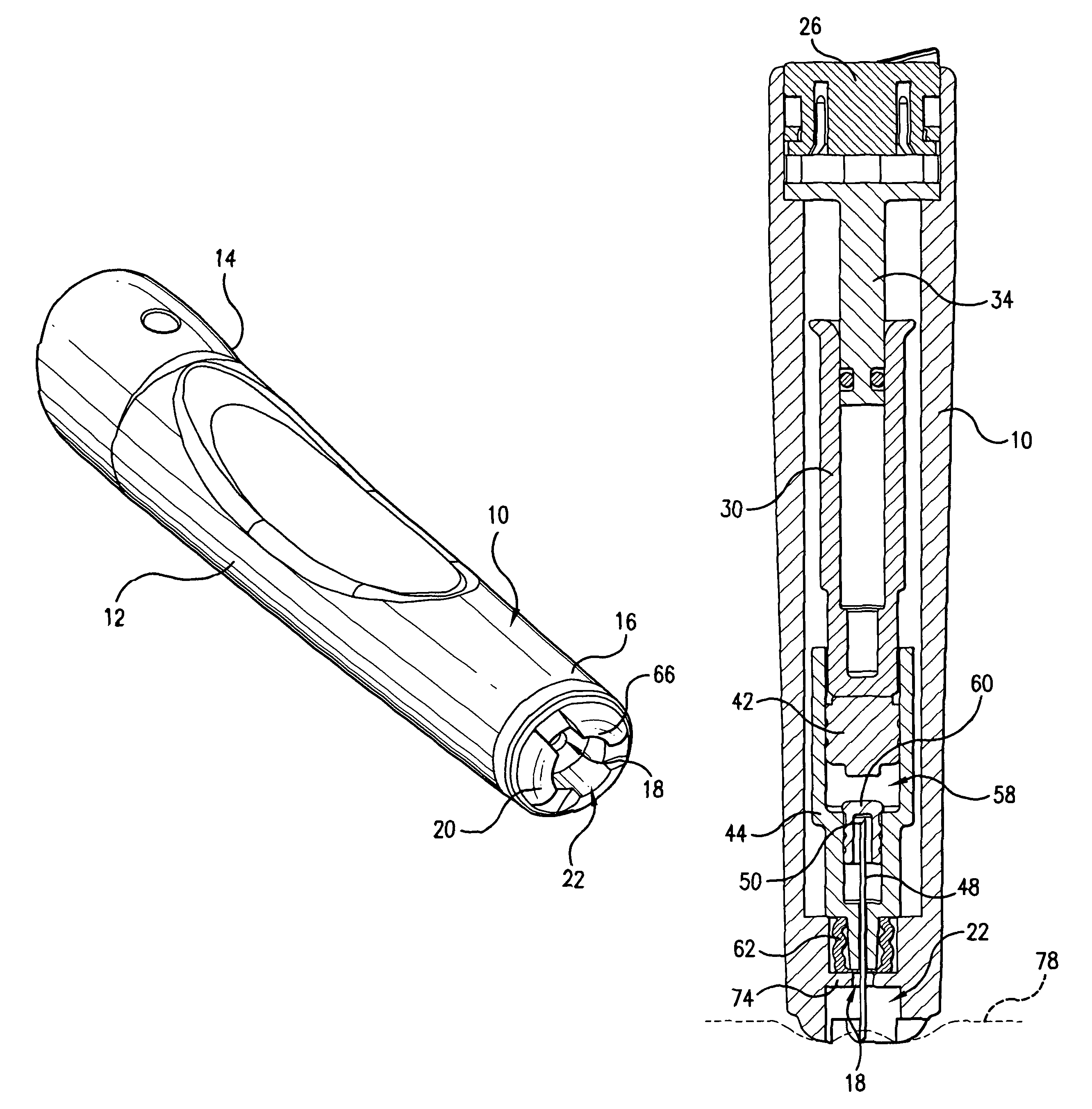
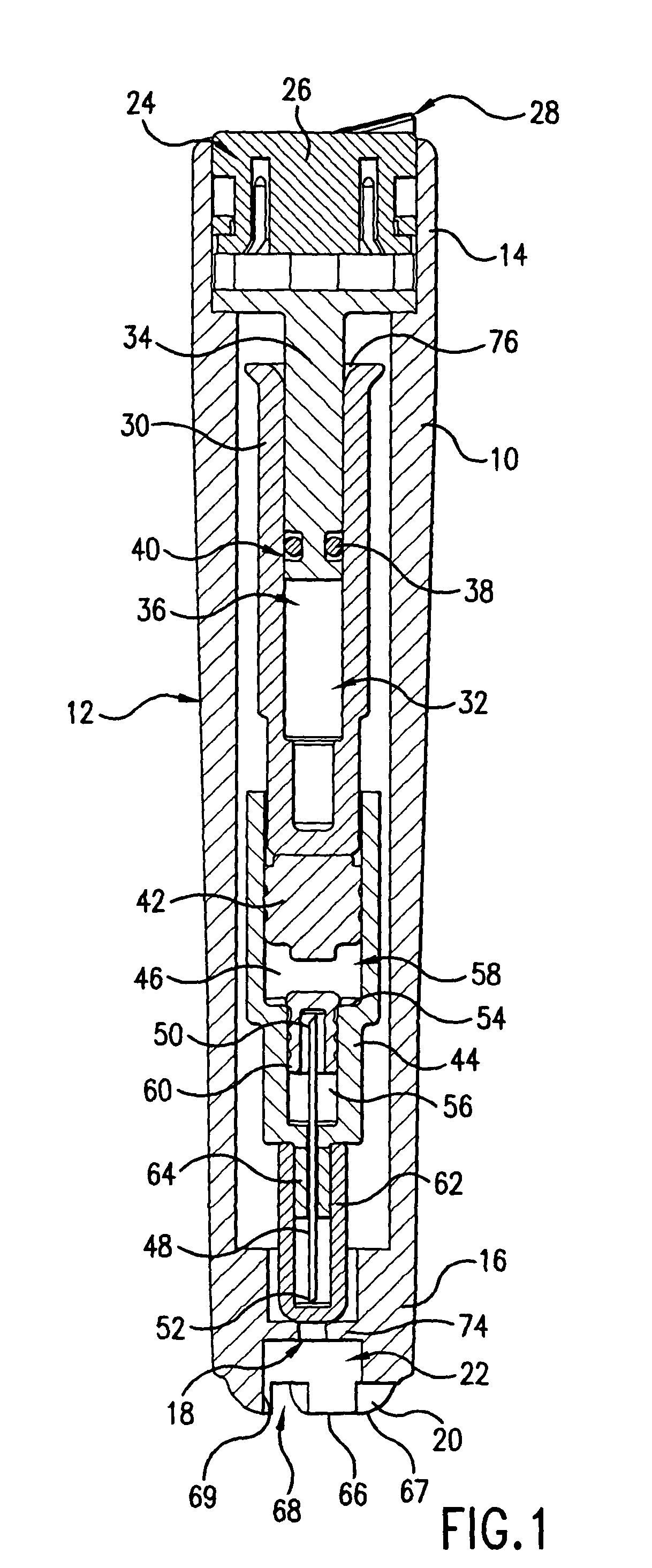
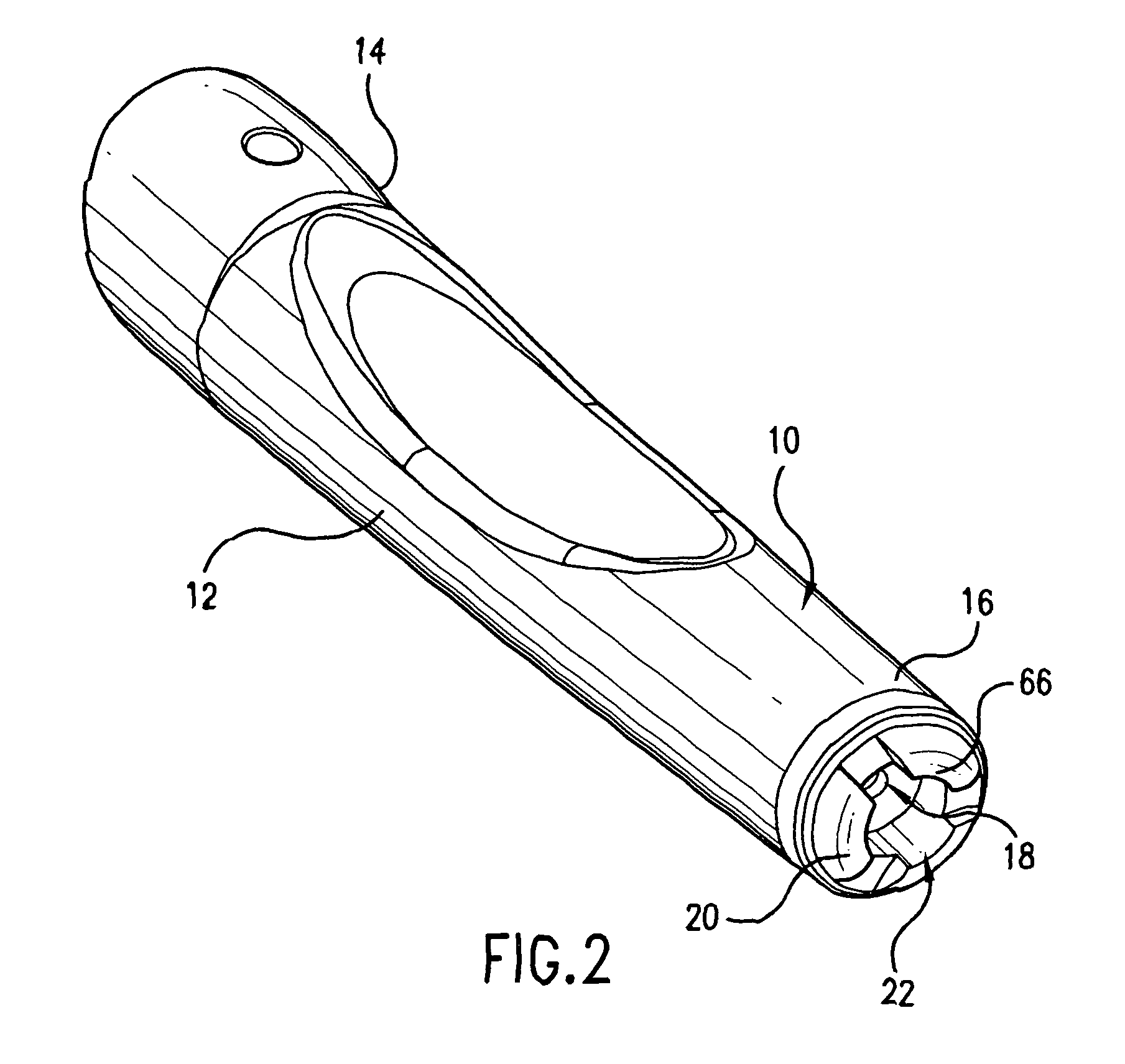
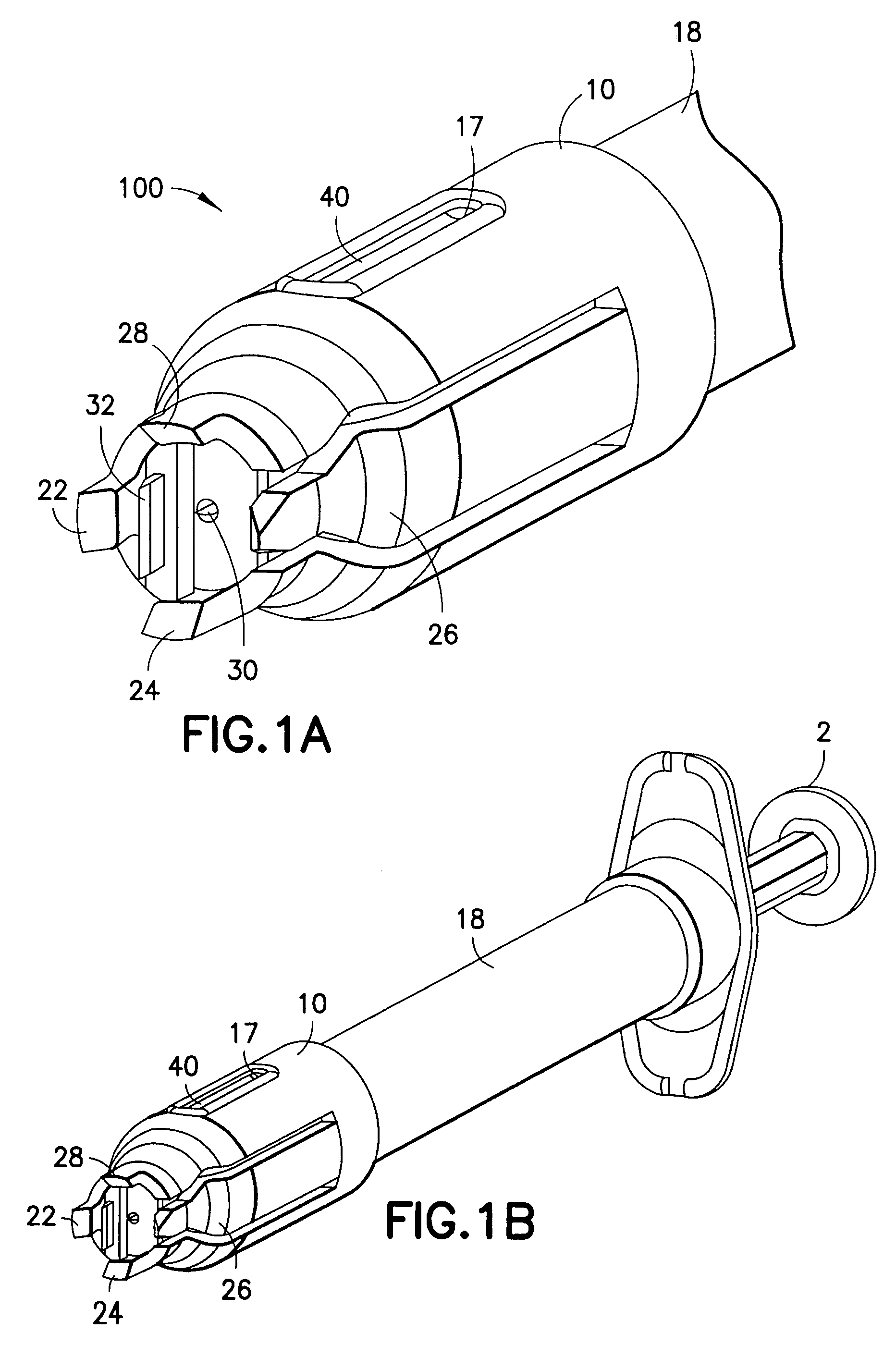
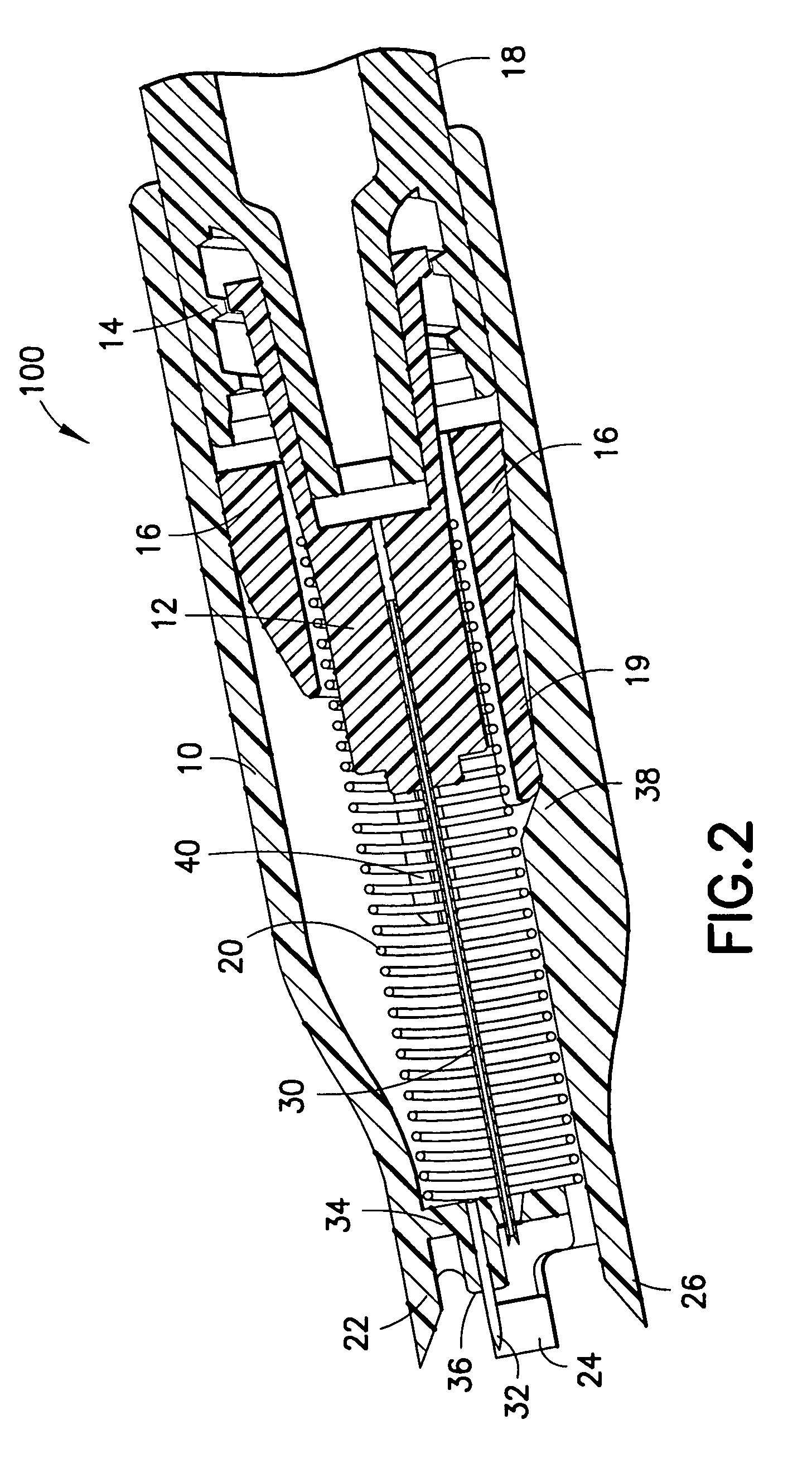
Device for an injector
InactiveUS20050101919A1Improve functionalityEasy to useAutomatic syringesInfusion needlesInjection siteEngineering
The present invention relates to an injection device comprising a generally elongated tubular housing, a syringe containing medicament and having a needle, a needle shield slidably arranged to the housing and protruding a distance outside the front end of the housing, a plunger arranged to act on the syringe and a pre-tensioned drive member arranged the drive the plunger, characterized in that it comprises radially acting holding elements capable of releasably holding the plunger, axially acting actuator elements connected to the needle shield and capable of releasably locking the holding elements and axially acting activator elements capable of releasing the holding elements from the actuator elements have when the needle shield and the actuator elements has moved axially a certain distance due to that injection device has been placed against an injection site.
Owner:SHL MEDICAL
Syringe plunger sensing mechanism for a medical injector
ActiveUS7553294B2Easily and readily and securely fastenedQuick uninstallMedical devicesPressure infusionInjection siteBiomedical engineering
Embodiments of an injector, syringe, syringe interface, syringe adapter, and piston / plunger assembly for an injector (of contrast medium, for example) are described. Preferably, the syringe is adapted to engage a syringe interface mechanism such that the syringe may be connected to an injector without regard to any particular orientation of the syringe to the injector or to the piston / plunger assembly. The injector piston or drive member includes a sensing pin positioned at a forward end thereof to sense contact with the syringe plunger during advancement of the piston or drive member to contact the plunger. The piston can be advanced automatically upon connection of, for example, the syringe to the injector, or piston advance can be automated to occur after operator initiation. The injector may include or be adapted to accommodate two syringes, which for example provide for simultaneous injection of fluid into separate injection sites on a patient.
Owner:BAYER HEALTHCARE LLC
Intradermal injector
InactiveUS20050033234A1Avoid back pressureAvoid insufficient lengthAmpoule syringesAutomatic syringesInjection siteSkin contact
An injection device that comprises a chamber configured for containing a substance to be injected and a needle operatively associated with the chamber and having a length sufficient to deliver the substance to an intradermal injection site. A collar surrounds the needle, defining a collar cavity. The collar also has a peripheral forward skin-contacting surface that surrounds and is radially spaced from the needle and injection site by an area that is sufficiently large to allow a patient's skin to move into the collar cavity to properly position the needle for intradermal delivery of the substance to the injection site to allow spread of the injected substance under the skin while inhibiting or preventing backpressure within the skin from forcing the substance out through the injection site.
Owner:ANTARES PHARMA
Needle insertion systems and methods
A housing may have a needle, plunger, and bias mechanism supported within the housing by a tab configured to retain the plunger in position and to allow the plunger to move under bias force imparted by the bias mechanism to move the needle to an insert position when the tab is removed. A base and a structure may be configured for relative movement there between and may be adapted to be secured to a user with a cannula extending through a body of the structure into the user during use of a medical device. A housing may be adapted to be secured to a user to support a medical device operable with an insertion needle, the housing having a magnifying material for increasing visibility of an injection site.
Owner:MEDTRONIC MIMIMED INC
Automatic injection device
An injector device comprising a body containing a syringe with a needle and plunger, a drive spring coupled with the syringe and operable, when released, to drive the syringe forward to inject the needle and subsequently to dispense a dosage from the syringe, a housing containing the body and drive spring, and a release apparatus coupled with the housing. The drive spring is initially locked in an unreleased position, and the body is slidable with respect to the housing and configured for sliding upward in the housing when the injector device is pressed down at an injection site to engage the release apparatus and release the drive spring for delivering a dosage.
Owner:PA2008
Injecting apparatus
ActiveUS7717877B2Facilitates automatic insertionAmpoule syringesAutomatic syringesCaregiver personInjection site
An injector is automatic in that the needle is inserted into the injection site (e.g., a patient's skin) with user or caregiver assistance, the delivery is automatically initiated upon needle insertion, and the needle is retracted automatically after the end of delivery. Preferably the needle is not seen by the user prior to, during or after injection. Prior to and after injection, the needle is hidden in the device so as to avoid any potential injury or health risk to the user or health care provider. The injector includes a housing and a shield arranged to slide relative to the housing and a driver moving during drug delivery. The housing and shield form a cartridge enclosure. The cartridge is shielded and locked after delivery is completed. A needle-locking mechanism can be used in any number of pen-like injectors or safety needles.
Owner:WEST PHARMA SERVICES OF DELAWARE
Methods and devices for improving delivery of a substance to skin
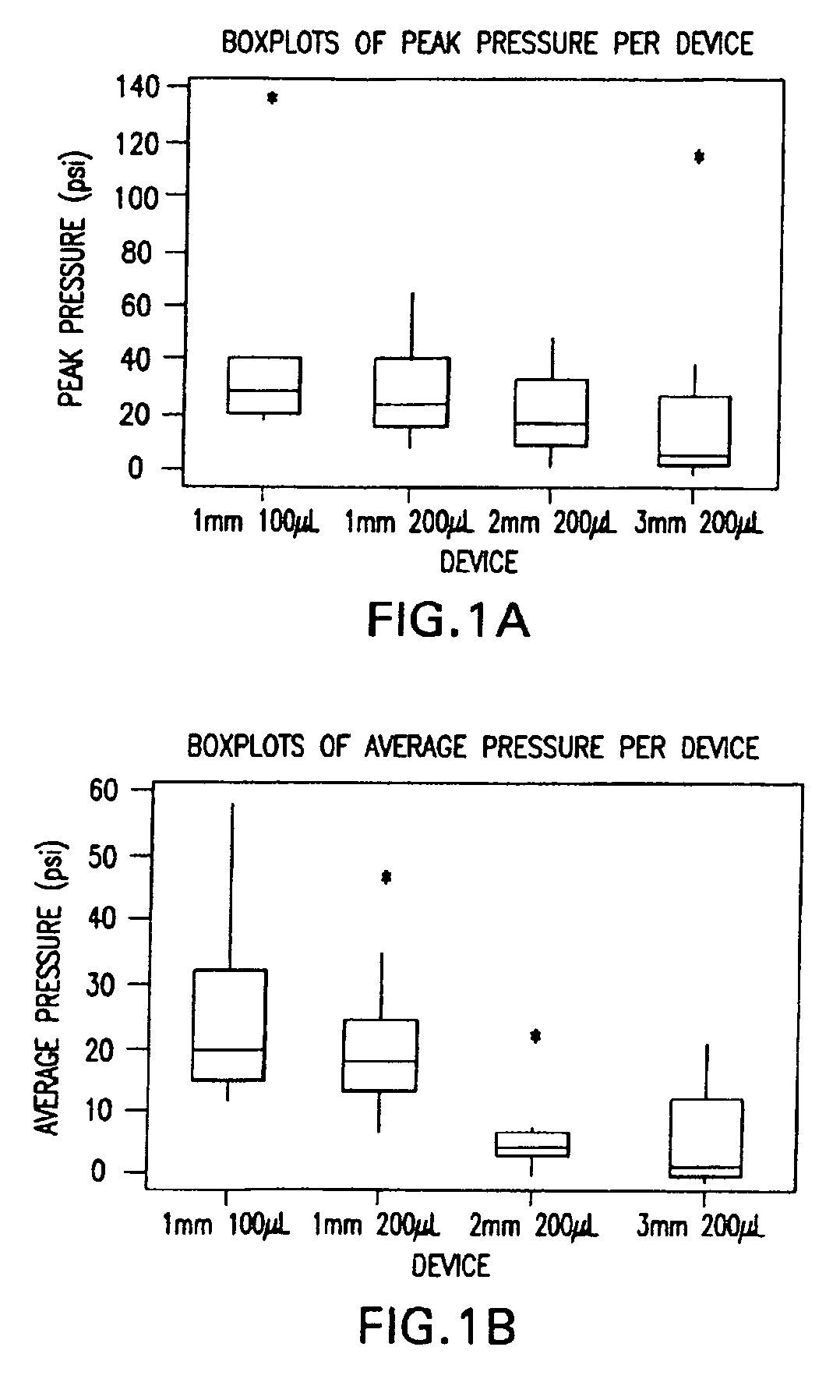
InactiveUS20050256499A1Efficacy is alteredImprove delivery capabilitiesMedical devicesPressure infusionDelivery PerformanceInjection site
A method of delivery of a substance to a human subject's skin comprising deposition into a specific compartment of the skin, wherein the delivery occurs at a controlled rate and pressure. The methods of the invention provide accurate deposition of s pre-selected volume of the substance, e.g., greater than 90% of the pre-selected volume. The methods of the invention encompass varying one or more parameters including but not limited to configurations of the delivery device, volume, pressure, and flow rate of delivery, to enhance the efficacy of delivery of the substance to the human skin. Substances delivered in accordance with the methods of the invention result in a more efficacious deposition of the substance into the targeted compartment, improved delivery performance, i.e., completeness of delivery as measured by quantification of the substance not delivered or the amount of the substance leaked out from the injection site, and enhanced safety as measured by the occurrence of minimal adverse cutaneous events at the site of injection.
Owner:BECTON DICKINSON & CO
Automatic Injector
An injector is automatic in that the needle insertion into the injection site (e.g., a patient's skin) is triggered by the user or caregiver, the insertion is automatic, the following delivery is automatically initiated upon needle insertion, and the needle is shielded automatically after the end of delivery. Preferably the user does not see the needle prior to, during, or after injection. The injector includes a proximal and distal housings and a needle shield arranged to slide on the distal housing. The injector has a driver arranged to move the syringe to cause the needle penetration, to inject the drug, and subsequently to release the shield. The syringe needle is shielded and the shield locked automatically after delivery is completed. The patient does not experience any injector force on the tissue during injection. The injector has built in patient safety features.
Owner:TSALS IZRAIL +1
Devices and methods for interstitial injection of biologic agents into tissue
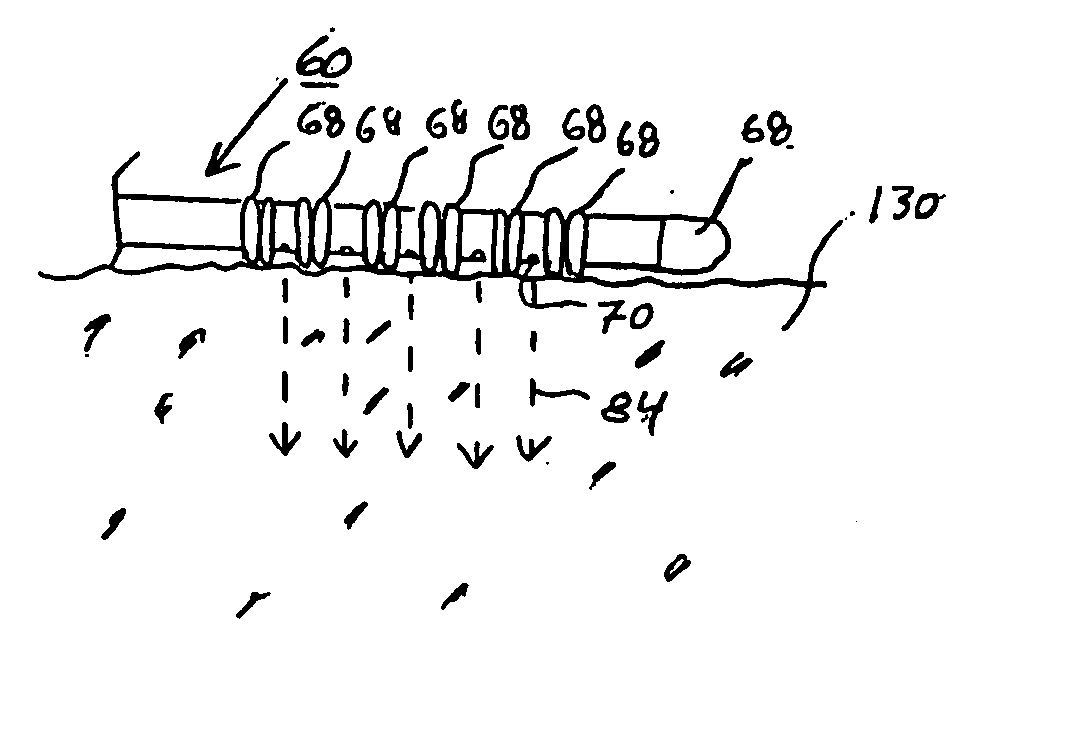
The current invention discloses a method for treating infracted / ischemic injury to a myocardium by injecting a substance into the myocardium. The injected substance helps to prevent negative adaptive remodeling by providing mechanical reinforcement or mechanical reinforcement combined with biological therapy. A number of substances for injection are disclosed, including multi component substances such as platelet gel, and other substances. The substances disclosed may contain additives to augment / enhance the desired effects of the injection. The invention also discloses devices used to inject the substances. The devices can include means for ensuring needles do not penetrate beyond a desired depth into the myocardium. The devices can also include needles having multiple lumens such that the components of the platelet gel will be combined at the injection site and begin polymerization in the myocardium.
Owner:MEDTRONIC INC
Injecting apparatus
ActiveUS20070112310A1Facilitates automatic insertionAmpoule syringesAutomatic syringesInjection siteDrug delivery
Owner:WEST PHARMA SERVICES OF DELAWARE
Methods and devices for improving delivery of a substance to skin
InactiveUS20090124997A1Efficacy is alteredImprove delivery capabilitiesMedical devicesPressure infusionDelivery PerformanceHuman skin
A method of delivery of a substance to a human subject's skin comprising deposition into a specific compartment of the skin, wherein the delivery occurs at a controlled rate and pressure. The methods of the invention provide accurate deposition of s pre-selected volume of the substance, e.g., greater than 90% of the pre-selected volume. The methods of the invention encompass varying one or more parameters including but not limited to configurations of the delivery device, volume, pressure, and flow rate of delivery, to enhance the efficacy of delivery of the substance to the human skin. Substances delivered in accordance with the methods of the invention result in a more efficacious deposition of the substance into the targeted compartment, improved delivery performance, i.e., completeness of delivery as measured by quantification of the substance not delivered or the amount of the substance leaked out from the injection site, and enhanced safety as measured by the occurrence of minimal adverse cutaneous events at the site of injection.
Owner:BECTON DICKINSON & CO
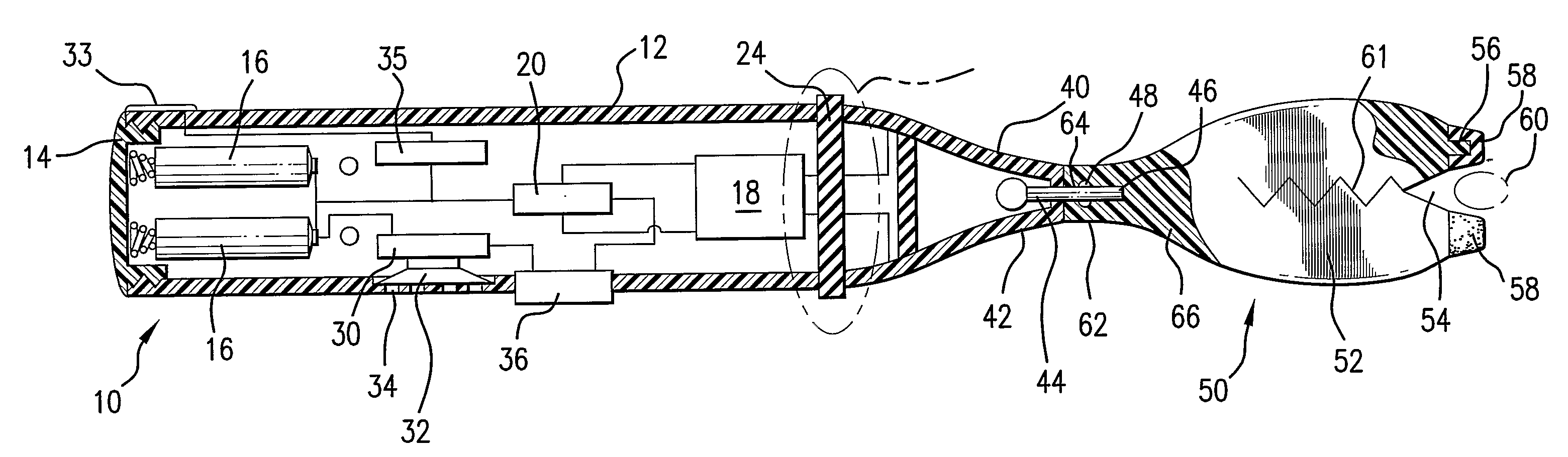
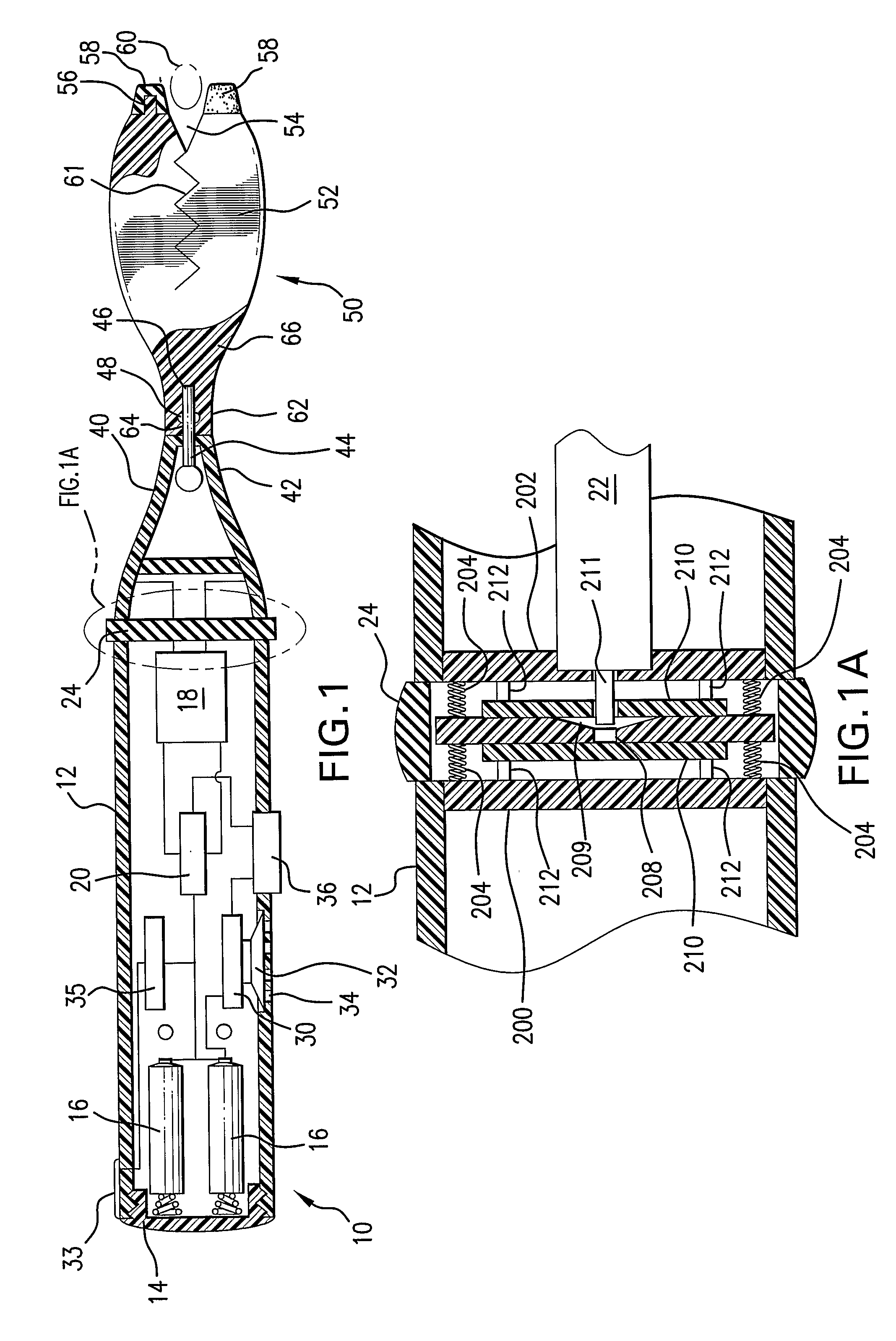
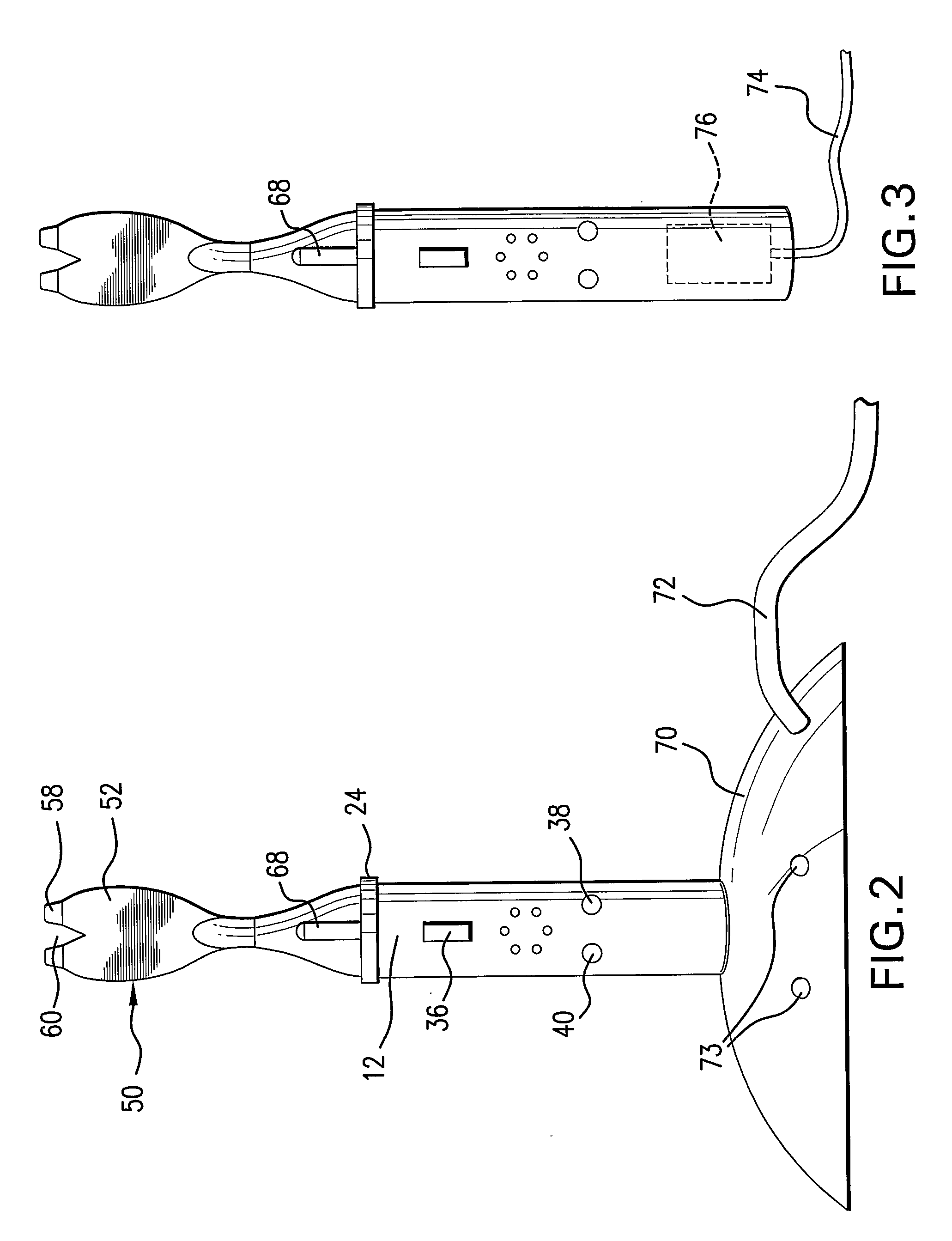
Prefilled syringe jet injector
ActiveUS8021335B2Prevent backflowImprove distributionAmpoule syringesJet injection syringesInjection pressureInjection site
A jet injector that includes a prefilled syringe. The syringe includes a fluid chamber that contains a medicament. The syringe also has an injection-assisting needle, and a plunger is movable within the fluid chamber. A housing is configured for allowing insertion of the needle to a penetration depth. An energy source is configured for biasing the plunger to produce an injecting pressure in the medicament in the fluid chamber of between about 80 and 1000 p.s.i. to jet inject the medicament from the fluid chamber through the needle to an injection site.
Owner:ANTARES PHARMA INC
Sanitized tubing termination method and assembly
InactiveUS20050147525A1Easy and inexpensive to make in quantityDead animal preservationLavatory sanitoryInjection siteCoupling
A catheter termination assembly includes a catheter connection of the type having a coupling stem at one end, a catheter connection nipple at the opposite end, an axial lumen extending between those ends, and a transverse enlargement between the stem and the nipple. The assembly also includes cup-like end cap, the end cap having an end wall, and a skirt extending from the end wall. The skirt is sized to engage around the enlargement and interfitting surfaces on the enlargement and interior wall of the skirt enable the skirt to be releasably coupled to the enlargement so as to define a fluid-tight chamber encircling the stem. The end cap has a fluid injection site by which a sanitizing agent may be present in or introduced into the chamber so as to immerse the stem in the sterilizing agent. A method of sanitizing a tubing connector fitted with the end cap is also disclosed.
Owner:MED CATH CONDUITS
Apparatus for Injecting a Pharmaceutical
InactiveUS20080269692A1Easy to operateProvide usageInfusion syringesInfusion needlesInjection siteCam
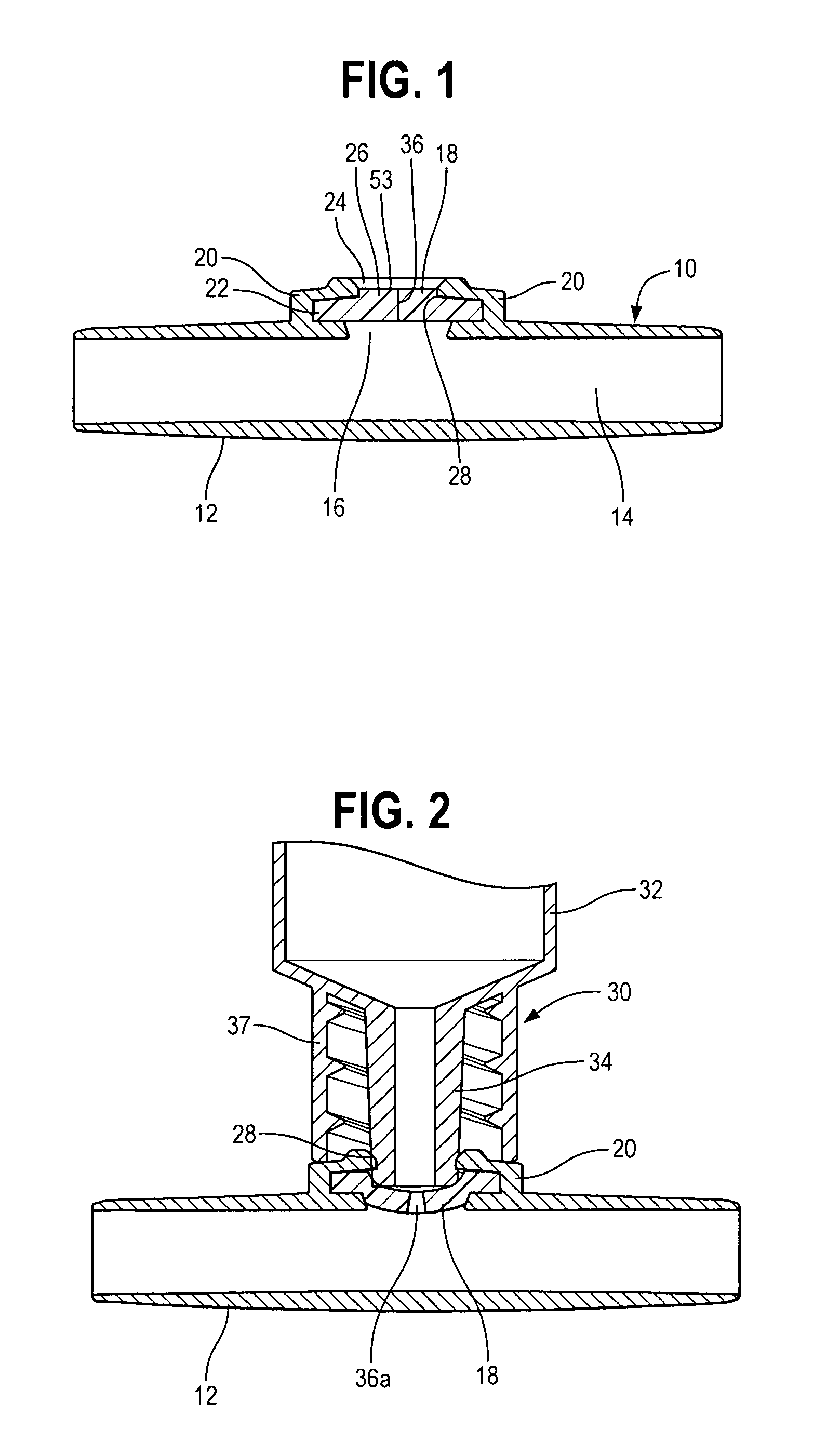
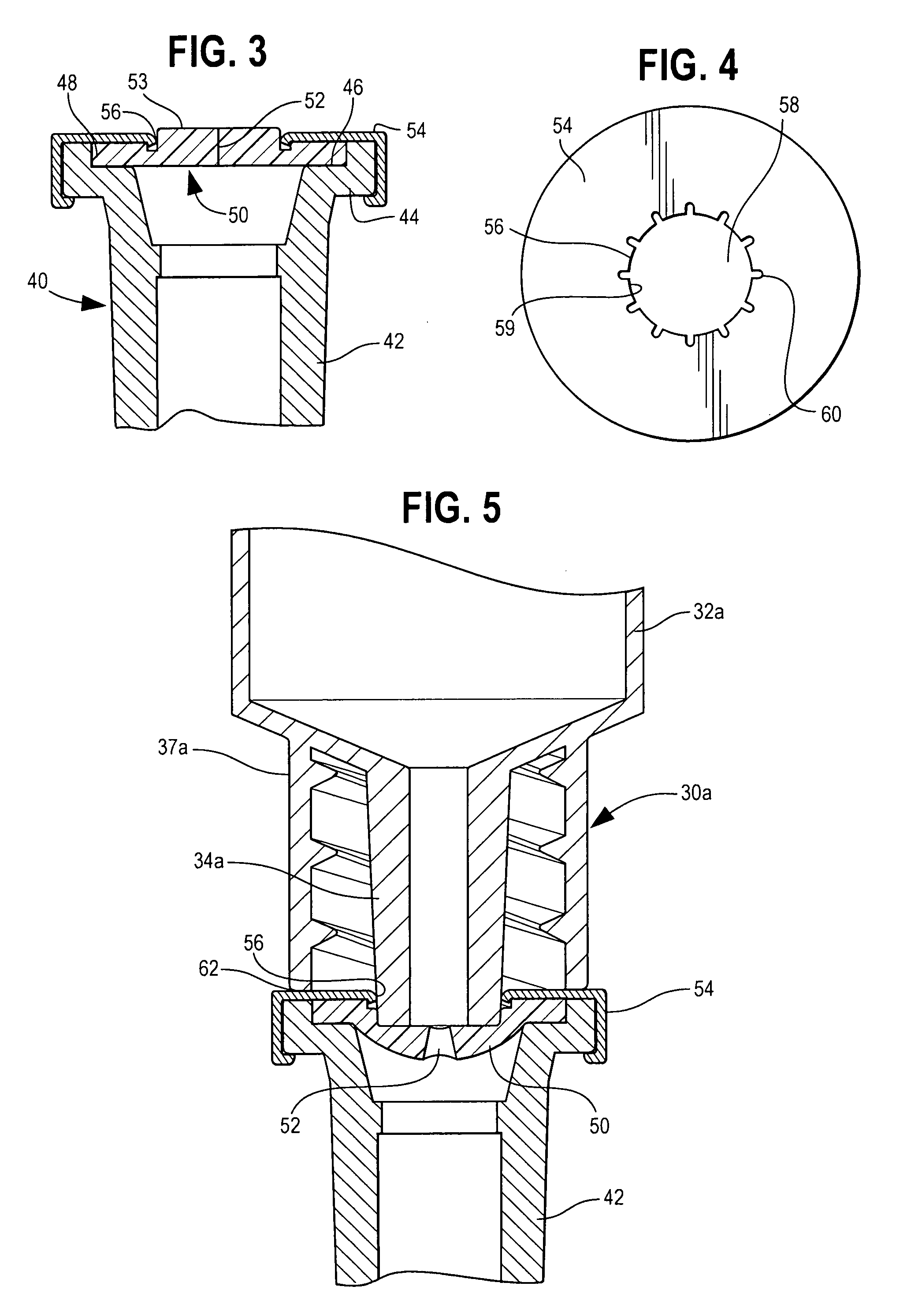
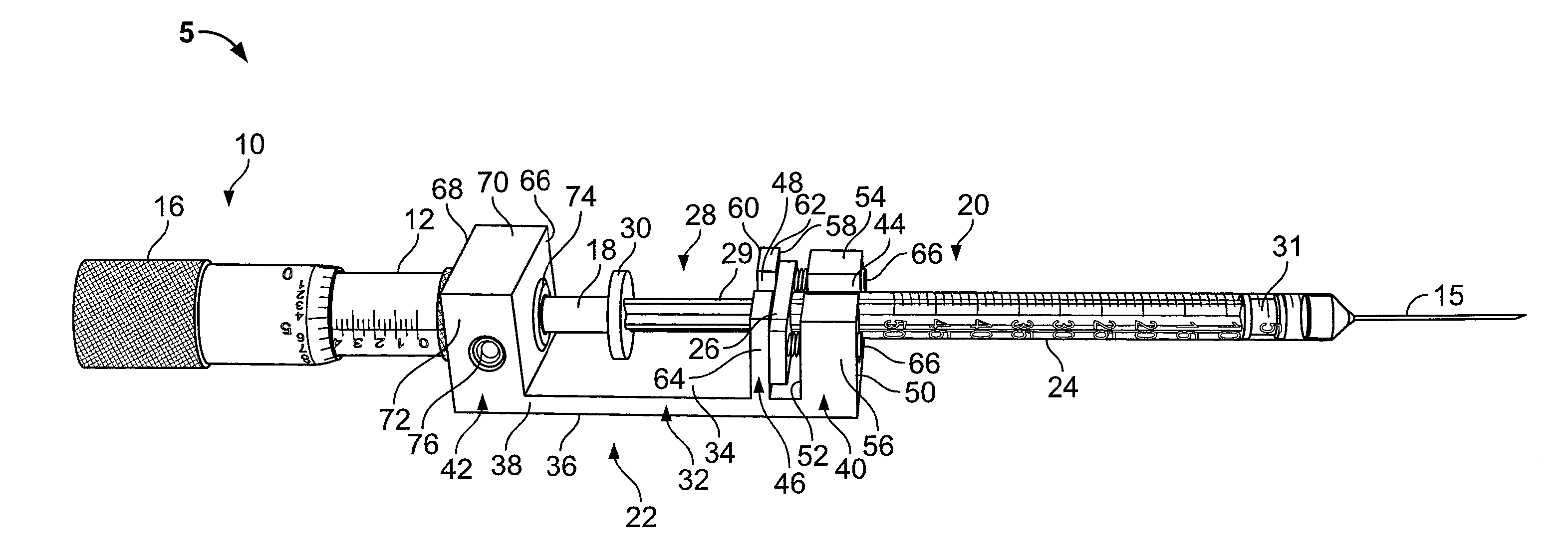
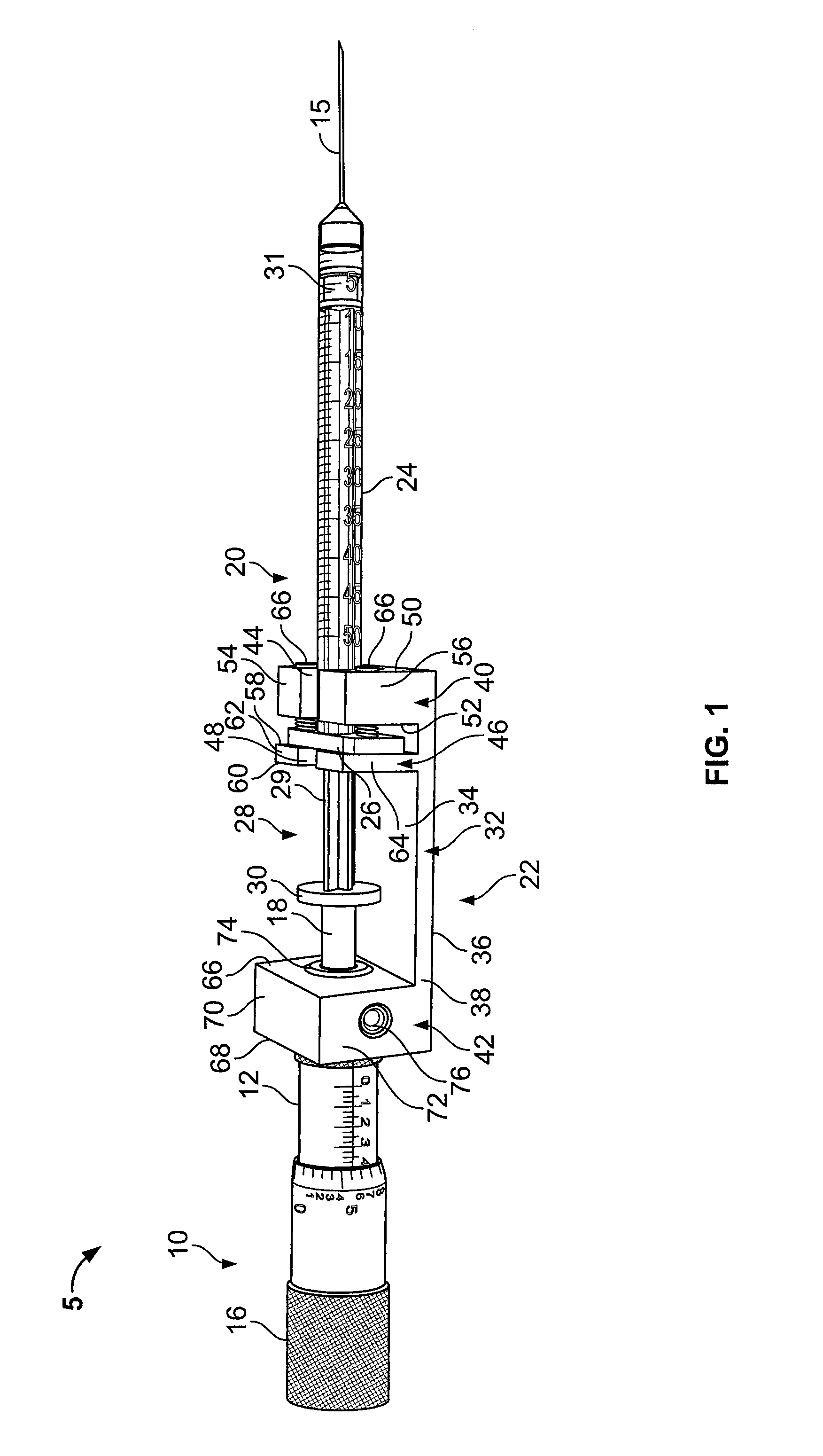
A pharmaceutical delivery apparatus including a housing (22), a syringe assembly, and a needle (44) cap. The syringe assembly is plungeable relative to the housing from a first position, at which its needle tip is disposed within the housing, to a second position, at which its needle tip projects from the housing beyond the proximal end for insertion into an injection site. A base (46) of the needle cap is exposed at the housing proximal end to be manually grippable for cap removal. A needle cap stem is upstanding from the base and sized and configured to insert through an opening is the housing proximal end to cover the needle tip when the syringe assembly is disposed in the first position. The needle cap base further includes a plurality of distally projecting cams (54, 258) located radially outward of the stem. The cams are fittable within slots (40, 240) in the housing proximal end when the cap is fully mounted to the apparatus.
Owner:ELI LILLY & CO
Automatic injector with needle cover
ActiveUS20090270804A1Inhibition releaseAvoid accidental activationAmpoule syringesAutomatic syringesInjection siteBiological activation
An auto-injector automatically dispenses a predetermined dose of medicament upon activation. The auto-injector includes a needle cover operative to engage an injection site and activate the injector. The needle cover is configured to move from a locked retracted position prior to a medicament dispensing operation to a locked extended position after the medicament dispensing operation. The non-removable needle cover prevents contact with the needle both before and after the medicament dispensing operation.
Owner:MYLAN SPECIALTY
Self-administration injection system
Self-injection system allows a user to inject a drug from a cartridge carrying unique identification information, into any one of a plurality of injection sites. Tissue at each injection site is associated with at least one injection parameter, such as flow-rate, that is different for each site. A scanner reads the identification information of the cartridge and cooperates with a central processing unit to determine the validity of the drug in order to permit an injection procedure to commence. The central processing unit has a memory for storing the different injection parameters and controls a drive unit for driving fluid from the cartridge and through a needle into the selected tissue, at the injection parameter that is associated with the user selected tissue for the injection.
Owner:MILESTONE SCIENTIFIC INC
Low-loss multi-lumen injection apparatus
InactiveUS20070073267A1Reduce residual lossInfusion syringesMedical devicesDrug reservoirInjection site
Injection devices, systems and methods are disclosed for injecting two or more medicaments to a patient at a single injection site while preferably minimizing any mixing of the medicaments prior to delivery to the patient. The invention can also be used to sequentially deliver the medicaments to the patient in a repetitive manner. For example, the injection apparatus can sequentially provide a first medicament and then a second medicament to the patient during a first injection procedure. During a second injection procedure, the injection apparatus can again sequentially provide the first medicament and the second medicament to the patient either at the injection site of the first injection procedure or at a different injection site. Multi-lumen manifolds are disclosed for coupling to conventional drug ampoules, to permit the user to sequentially delivery different medicaments via a single skin penetration and to reduce losses associated with usage. Systems including multiple drug reservoirs and filling adaptors are also disclosed.
Owner:MILE CREEK CAPITAL
Injection site for male luer or other tubular connector
ActiveUS7025744B2Decreased blood flowReduce turbulenceInfusion devicesMedical devicesInjection siteEngineering
A medical device has an interior for containment of fluids; an opening into the interior, an elastomeric wall comprising a fixedly placed, flexible barrier across the opening; and a retention wall positioned adjacent to a peripheral portion of the elastomeric wall. The retention wall defines a central opening and has a generally rigid retention zone surrounding the central opening, to engage and retain a connector tube which is advanced into the central opening to displace the elastomeric wall and to open a flow aperture through the elastomeric wall, for flow through the wall and connector tube. One of the retention zone and connector tube are made of a material of sufficient hardness that material of the other engaging member is deformed by engagement therewith, which increases the strength of retention between the retention wall and the connector tube.
Owner:B BRAUN MEDICAL
Apparatus and methods for delivering fluid and material to a subject
The present invention provides fluid and material delivery methods and devices for practicing the methods. The invention provides a method of delivering cellular material comprising injecting the cellular material into a subject such that the injected cells retain their inherent morphologic characteristics upon injection. The method comprises the steps of aspirating the cellular material into a fluid delivery device which incorporates a syringe arrangement. The cellular material is aspirated into the main body of the syringe until the desired amount of a material has filled the syringe body. The needle of the fluid delivery device is then inserted into the skin of a subject at an angle about parallel to the skin until a desired depth has been reached. The cellular material is then injected in the subject until the desired volume of material has been injected. The needle of the device is then rotated approximately 45 to 90 degrees and the needle is removed from the injection site.
Owner:ADERANS RES INST
Automatic injector with needle cover
ActiveUS8048035B2Increase surface areaRelieve pressureAmpoule syringesAutomatic syringesInjection siteBiological activation
An auto-injector automatically dispenses a predetermined dose of medicament upon activation. The auto-injector includes a needle cover operative to engage an injection site and activate the injector. The needle cover is configured to move from a locked retracted position prior to a medicament dispensing operation to a locked extended position after the medicament dispensing operation. The non-removable needle cover prevents contact with the needle both before and after the medicament dispensing operation.
Owner:MYLAN SPECIALTY
Optical detection of intravenous infiltration
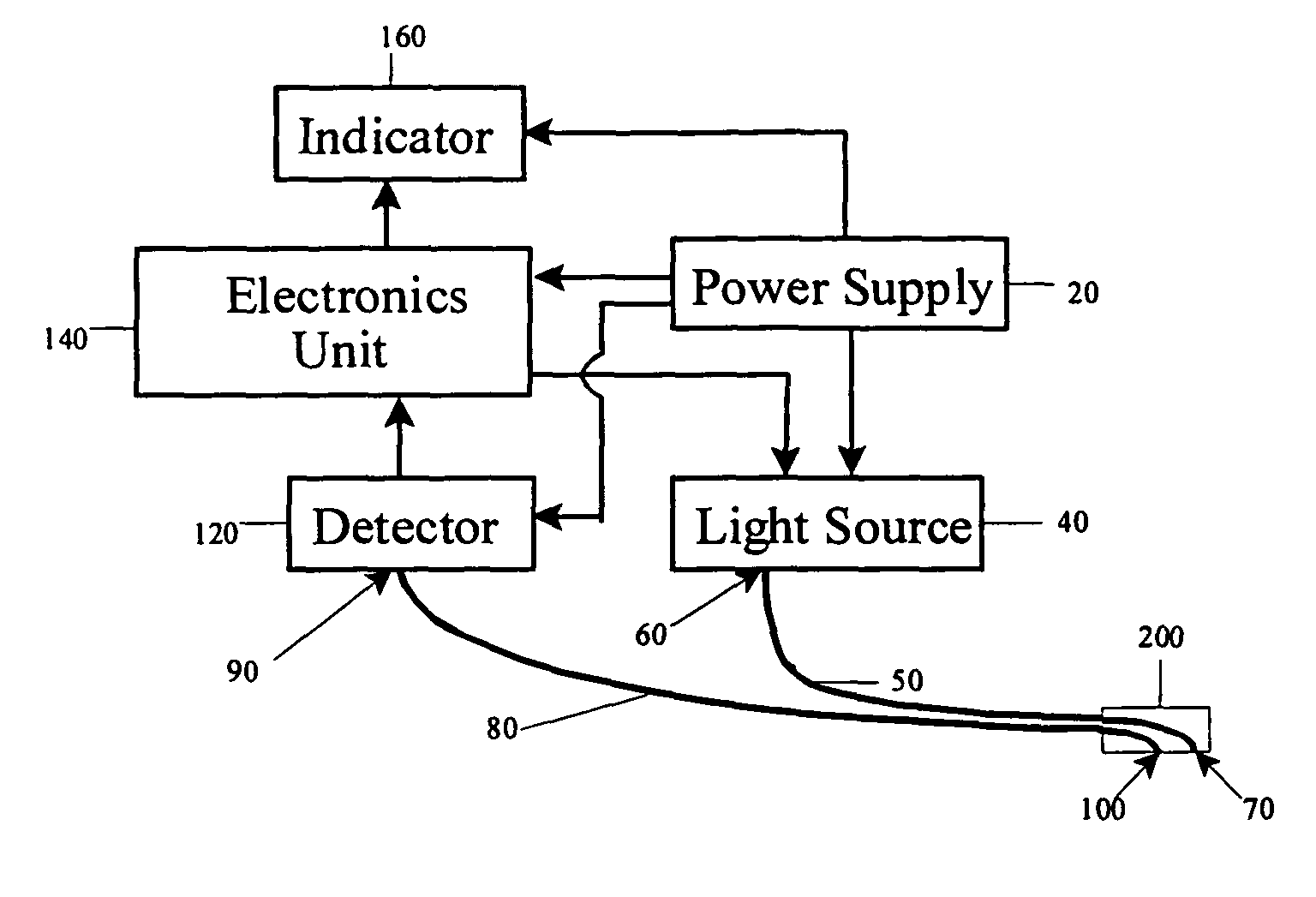
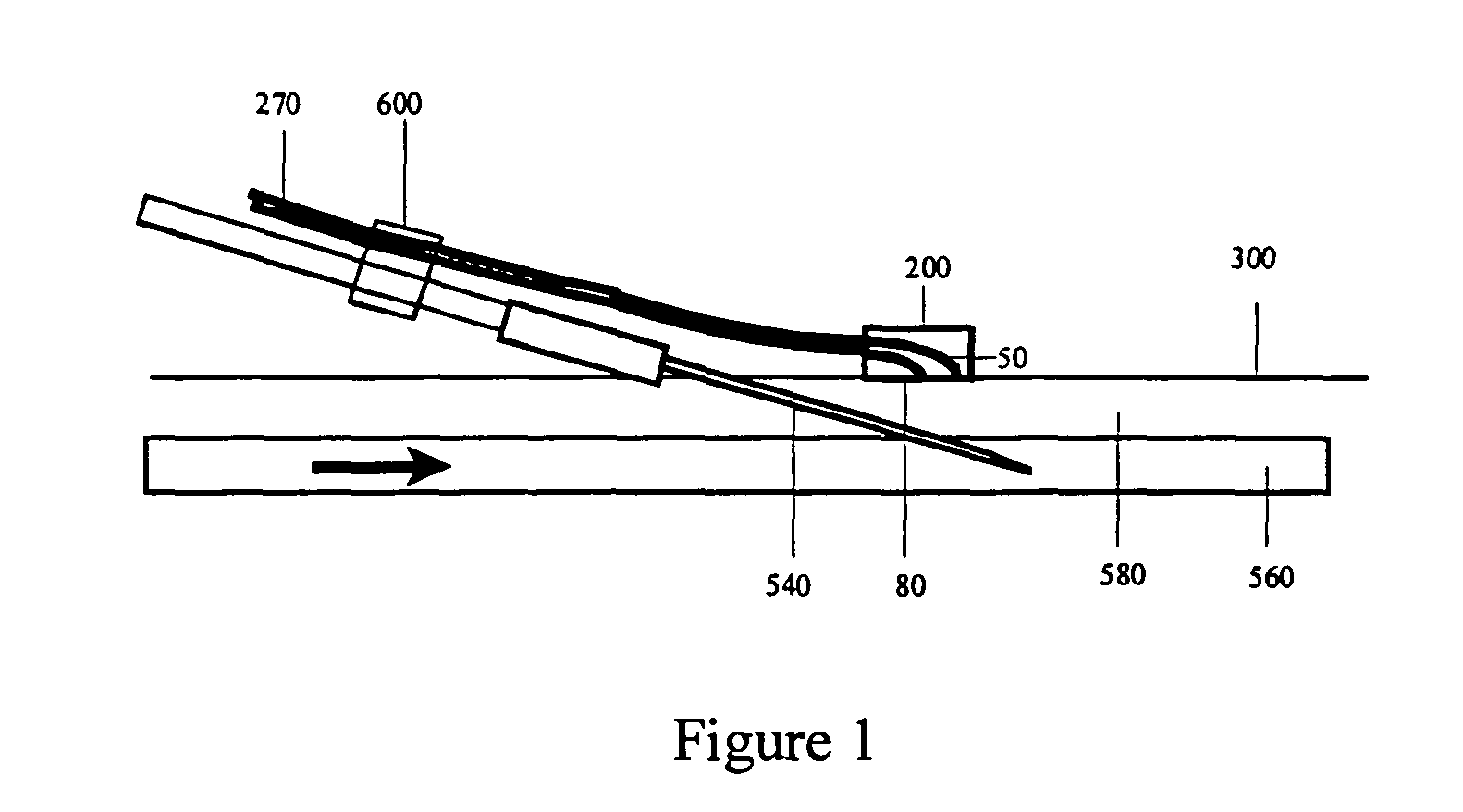
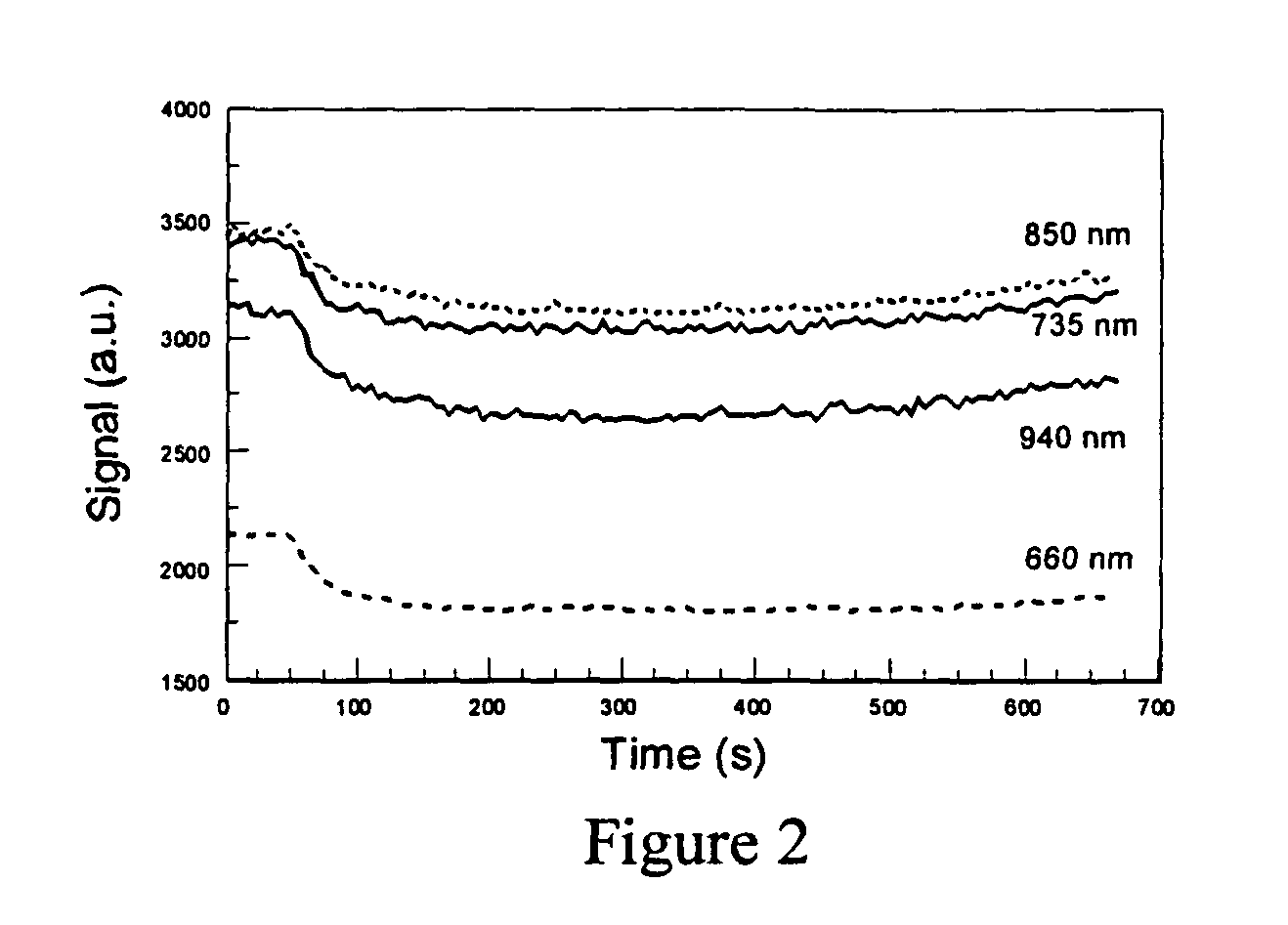
An intravenous infiltration detection apparatus for monitoring intravenous failures, which applies an optical method coupled with fiber optics and algorithms for tissue optics to provide a means for noninvasive detection of intravenous infiltration surround the site of IV injection. In the invention, the tissue surrounding the injection site is exposed to a single-wavelength of electromagnetic radiation, and light is collected with only one detector. Changes in the relative intensity of the radiation reflected, scattered, diffused or otherwise emitted provide a means for monitoring infiltration. The invention provides routine, automated, continuous, and real-time monitoring for patients undergoing IV therapy.
Owner:IVWATCH
Intradermal injector
InactiveUS8162886B2Avoid back pressureAvoid insufficient lengthAmpoule syringesAutomatic syringesInjection siteSkin contact
An injection device that comprises a chamber configured for containing a substance to be injected and a needle operatively associated with the chamber and having a length sufficient to deliver the substance to an intradermal injection site. A collar surrounds the needle, defining a collar cavity. The collar also has a peripheral forward skin-contacting surface that surrounds and is radially spaced from the needle and injection site by an area that is sufficiently large to allow a patient's skin to move into the collar cavity to properly position the needle for intradermal delivery of the substance to the injection site to allow spread of the injected substance under the skin while inhibiting or preventing backpressure within the skin from forcing the substance out through the injection site.
Owner:ANTARES PHARMA INC
Microstream injector
InactiveUS20070043320A1Quick distinctionPrevent accidental contactJet injection syringesMicroneedlesNeedle Free InjectionInjection site
The present invention provides a micromachined or microsized component-based needleless injector for delivering a dose of a liquid formulation containing a biologically active agent to tissue by way of a high pressure liquid microstream that penetrates the skin and deposits the agent at an optimal depth in the tissue. The device is appropriate for subcutaneous, intramuscular, or mucosal injection sites, as well as intracellular injection. Embodiments include micromachined or microsized components such as valves, jets, and MEMS pumps. Some embodiments of the invention are modular, with interchangeable parts, other embodiments are integrated in a unitary design. Embodiments with a unitary design are typically single use, however modular features also create embodiments with components that provide multiple instances of use.
Owner:KENANY SAAD AL
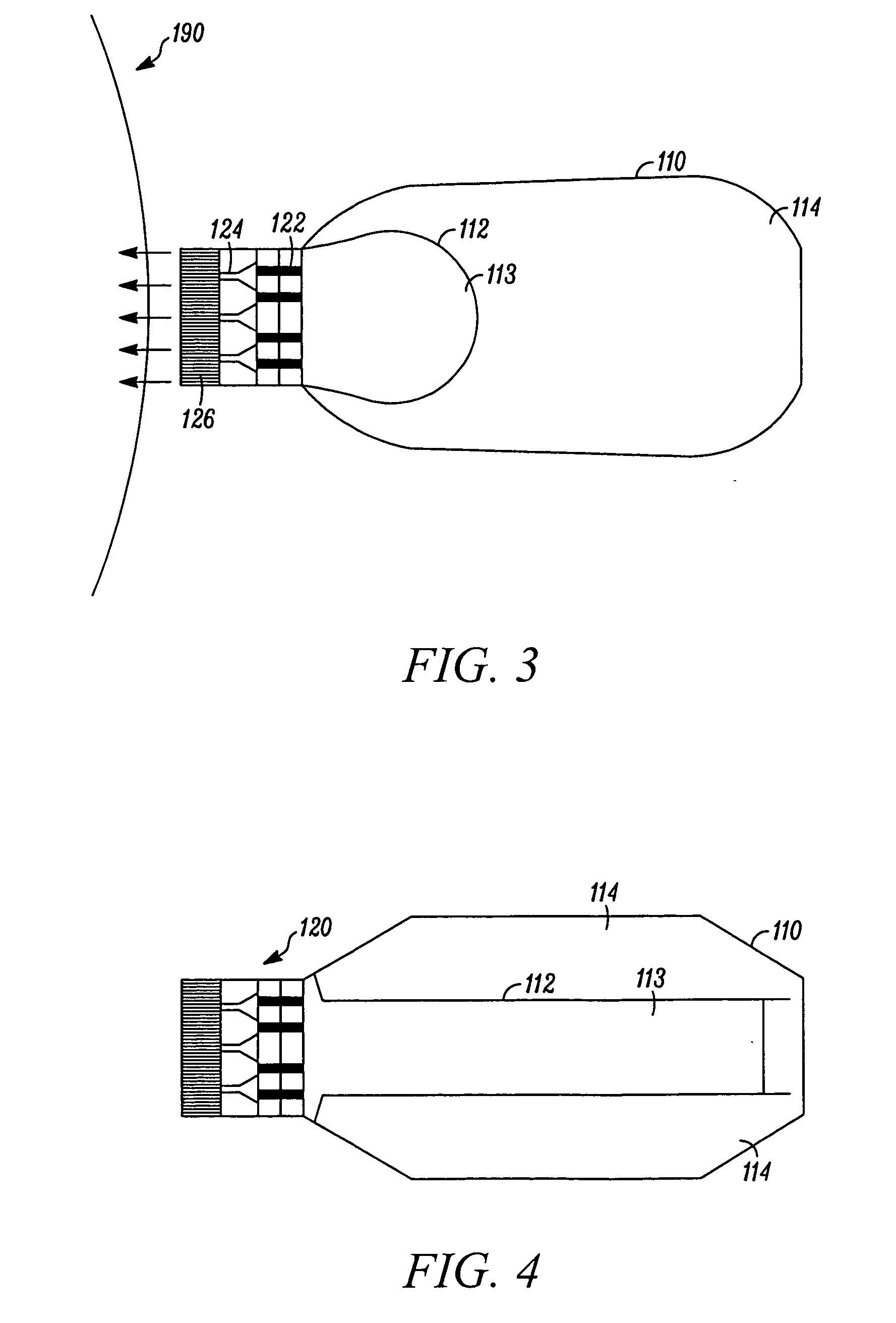
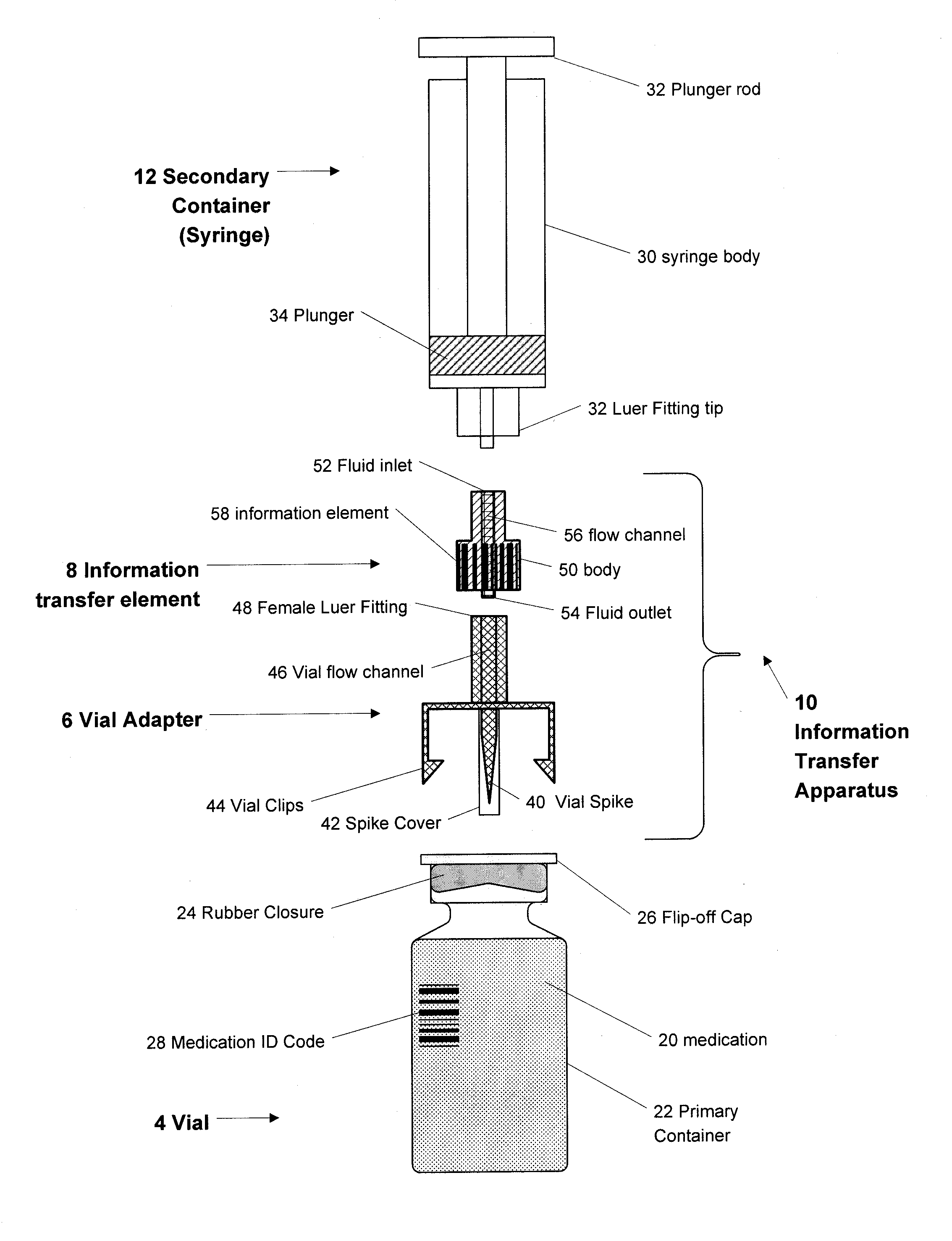
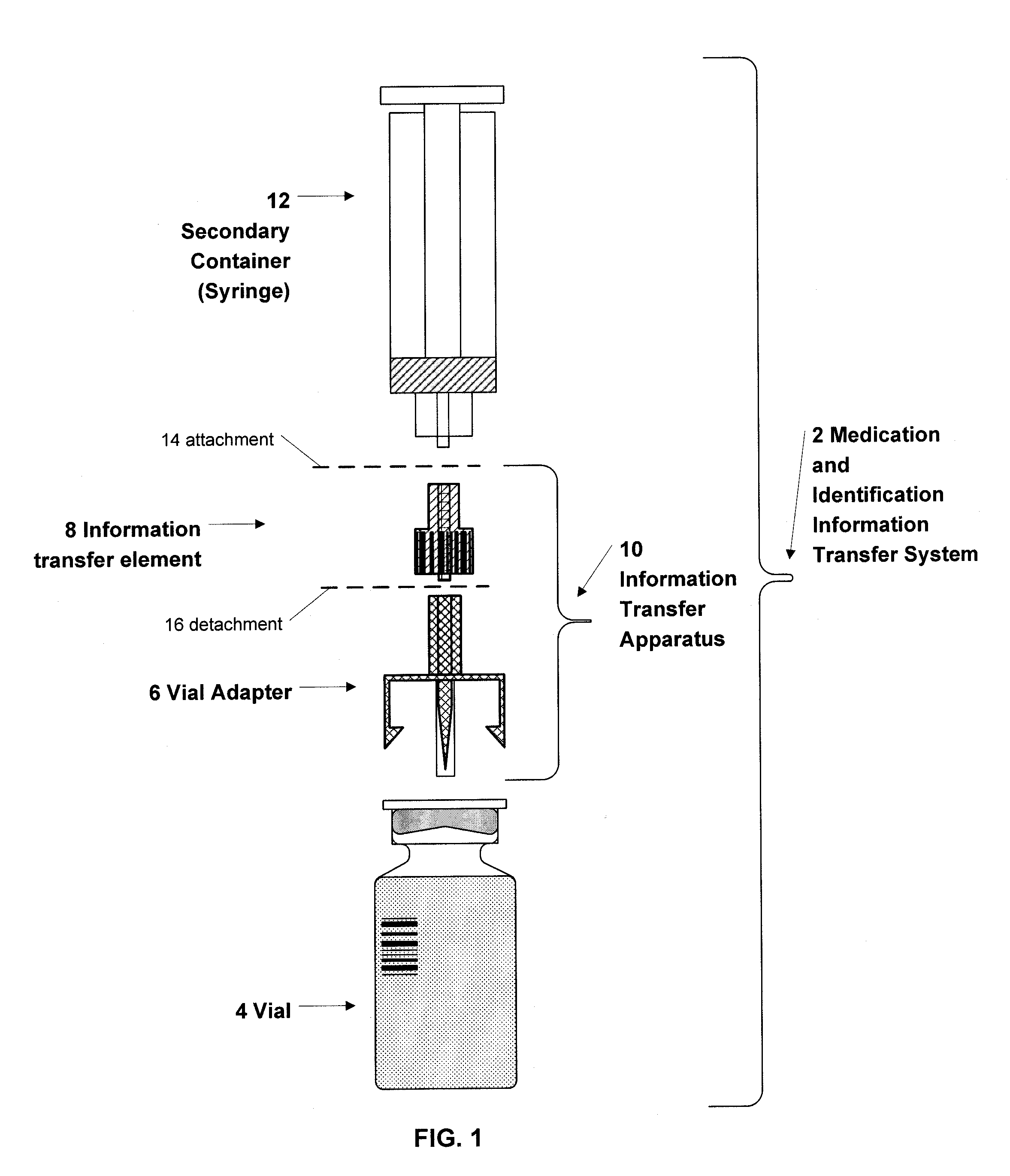
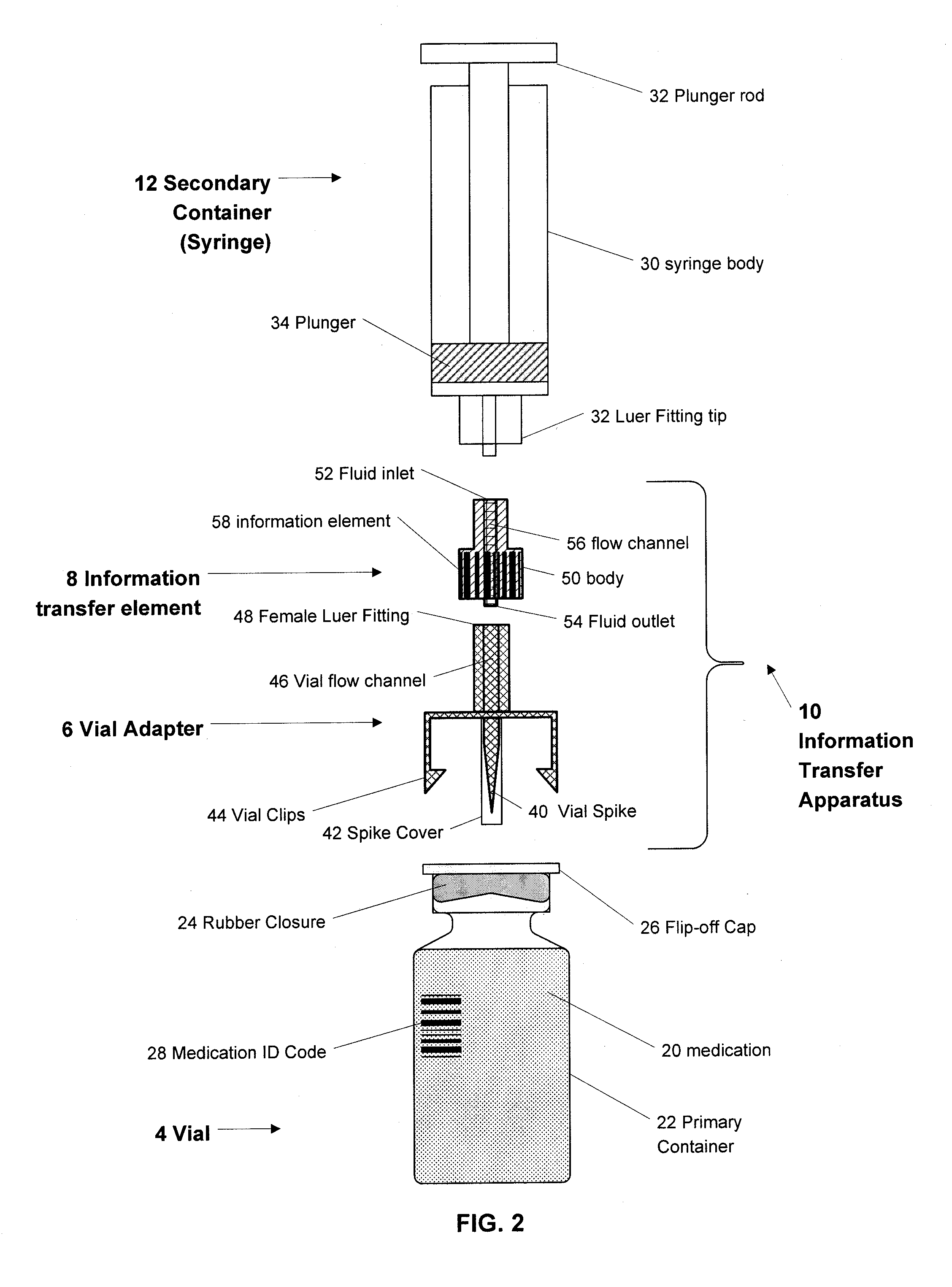
Medication and identification information transfer apparatus
A medication and identification information transfer system is provided that includes a medication vial, a secondary medication container (syringe) and a medication information transfer apparatus. The medication information transfer apparatus, when coupled to a vial, can transfer information indicative of the contents of the vial to an intelligent injection site. The medication information transfer apparatus has a shape and size enabling it to be connected to a vial adapter for removal of medication from the vial transfer it to a syringe for delivery to an injection site while simultaneously transferring information about the medication in the vial to the injection site. In some implementations, the medication injection site can be placed on a fluid delivery line for infusion into a patient. Related apparatus, systems, and kits are also disclosed.
Owner:CRISI MEDICAL SYST
Injectable depot compositions and it's process of preparation
InactiveUS20100015195A1Facilitated releaseSimplifying available daily dosage regimenPowder deliveryBiocideInjection siteActive agent
Novel injectable depot compositions are provided comprising at least one active agent(s) optionally with one or more pharmaceutically acceptable excipient(s) in the form of a multi-component system preferably comprising at least two components which when administered to a subject in need thereof forms an in situ gel depot or implant at the site of injection upon contact with body fluids. Also described are process for preparation of such compositions and method of using such compositions.
Owner:PANACEA BIOTEC
Systems and methods for RF ablation using jet injection of a conductive fluid
InactiveUS20050049583A1Reduce effective resistivityAccurate and deep lesionSurgical instruments for heatingRf ablationInjection site
A method of preparing and ablating heart tissue includes a pre-treatment step of delivering a jet of conductive fluid to a portion of heart tissue. The conductive fluid is delivered using an injection device such as a hand-held jet injector, probe, or catheter. After injecting the heart tissue with the conductive fluid, RF energy is delivered to the site of the injection to form a lesion. The method enables a physician to produce large and accurately placed lesions within the treated tissue.
Owner:SCI MED LIFE SYST
Intravitreal injection device and method
A coordinated cutting and spreading mechanism within a syringe dilator sub-assembly is applied to an eye surface during an intravitreal injection to provide an access window free of the conjunctival layer and through which an injection needle can be inserted. The system and method comprises a dilator sub-assembly including both the cutting and spreading mechanism and the intravitreal injection needle, for use with a conventional syringe. The dilator sub-assembly includes a number of projections to secure points of the surface of the conjunctival layer, a cutting member to incise the conjunctival layer, and at least one deflectable projection to move during the intravitreal injection, spreading the incision, and creating a window opening in the conjunctival layer through which the intravitreal injection needle then enters. Upon removal of the device from the injection site, the deflectable projection is released and the window opening in the conjunctival layer is closed.
Owner:BEAVER VISITEC INT US
Injection devices that provide reduced outflow of therapeutic agents and methods of delivering therapeutic agents
Injection devices are provided, which reduce potential outflow of therapeutic agents from an injection site. Devices are provided having at least a first lumen containing one or more therapeutic agents and a second lumen containing a second material for injection into tissue. Other devices are provided having an inner lumen with an injection needle to inject a therapeutic agent and an outer lumen that provides a vacuum seal between the injection needle and the needle track. Further provided are methods of delivering a therapeutic agent to tissue.
Owner:BOSTON SCI SCIMED INC
Apparatus and Method for Reducing Pain During Skin Puncturing Procedures
ActiveUS20080255483A1Easily and inexpensively utilizedFree from painTooth pluggers/hammersSurgeryInjection siteHand held
A handheld instrument for minimizing pain during administration by injection of a liquid, such as, an anesthetic that has a main body, a vibration unit mounted in the main body when initiated to cause the main body to vibrate, and a detachable tip cantilever mounted on the main body to vibrate with it, the tip having a free end characterized by a bifurcation to form two spaced projections defining a space between them, whereby the spaced projections can be placed in proximity to, adjacent to and bracketing a preselected injection site on a human or animal and the tissue at said preselected injection site and vibrated while an injection is given.
Owner:BING INNOVATIONS
Diagnostic imaging of lymph structures
InactiveUS6444192B1Improve visualizationReadily takenUltrasonic/sonic/infrasonic diagnosticsNanotechSurgical operationInjection site
In accordance with the present invention, there are provided methods for identifying the sentinel lymph node in a drainage field for a tissue or organ in a subject. In select embodiments, the invention allows for the identification of the first or sentinel lymph node that drains the tissue or organ, particularly those tissues associated with neoplastic or infectious diseases and disorders, and within the pertinent lymph drainage basin. Once the drainage basin from the tissue or organ, i.e., the sentinel lymph node, is identified, a pre-operative or intraoperative mapping of the affected lymphatic structure can be carried out with a contrast agent. Identification of the first or sentinel lymph node, on the most direct drainage pathway in the drainage field, can be accomplished by a variety of imaging techniques, including ultrasound, MRI, CT, nuclear and others. Moreover, once the lymphatic structure is identified as being associated with neoplastic or infectious diseases and disorders, the affected lymphatic structure can be removed surgically or by a suitable minimally invasive procedure to allow pathological analysis to be performed to determine whether certain diseases or disorders exist, without resort to more radical lymphadenectomy. Further, the agent can be made to carry diagnostic or therapeutic probes to be activated and / or delivered to the injection site or any part of the lymphatic pathway downstream from the injection site.
Owner:RGT UNIV OF CALIFORNIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com