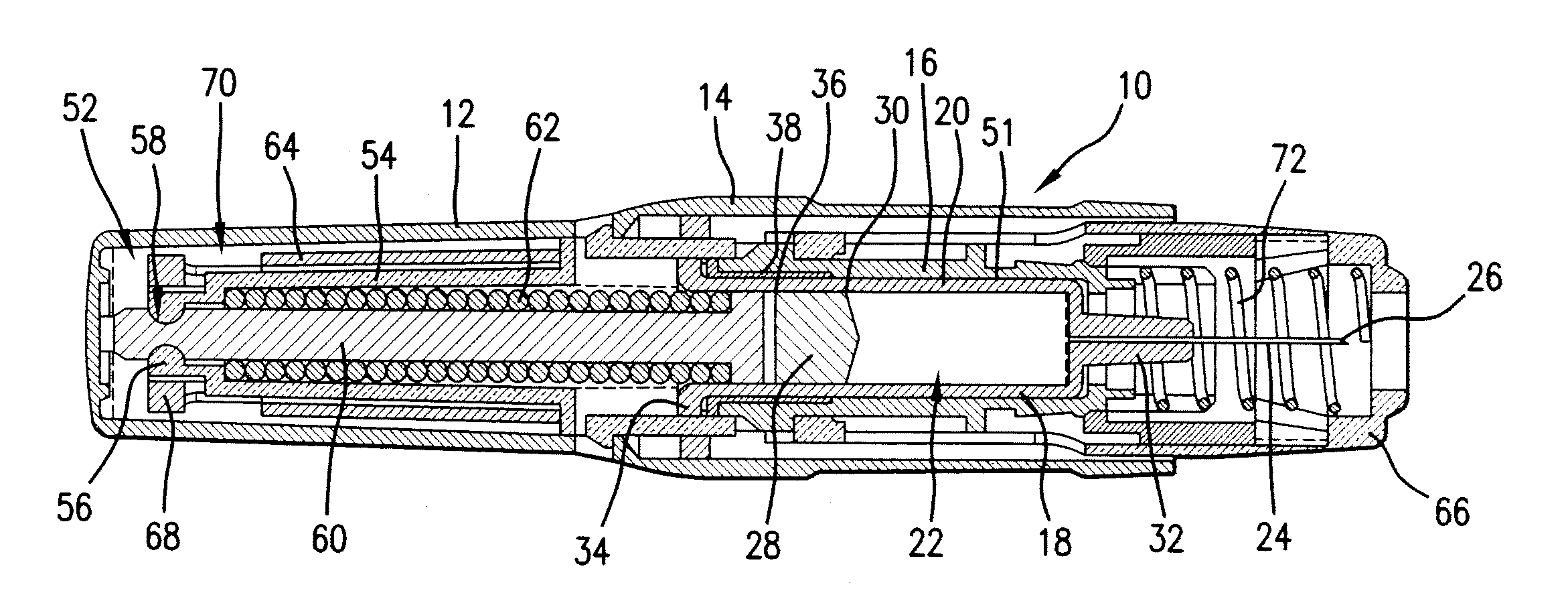
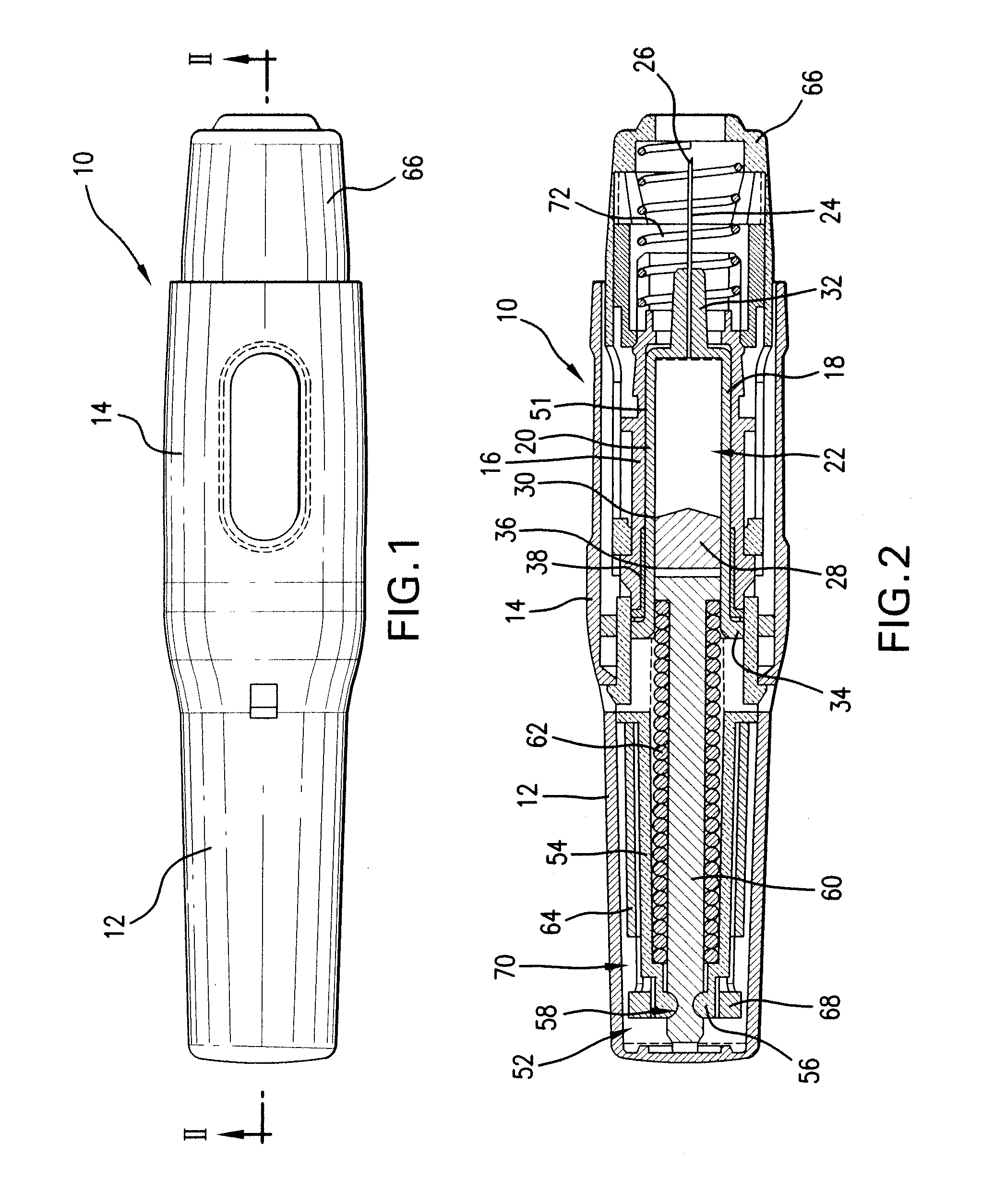
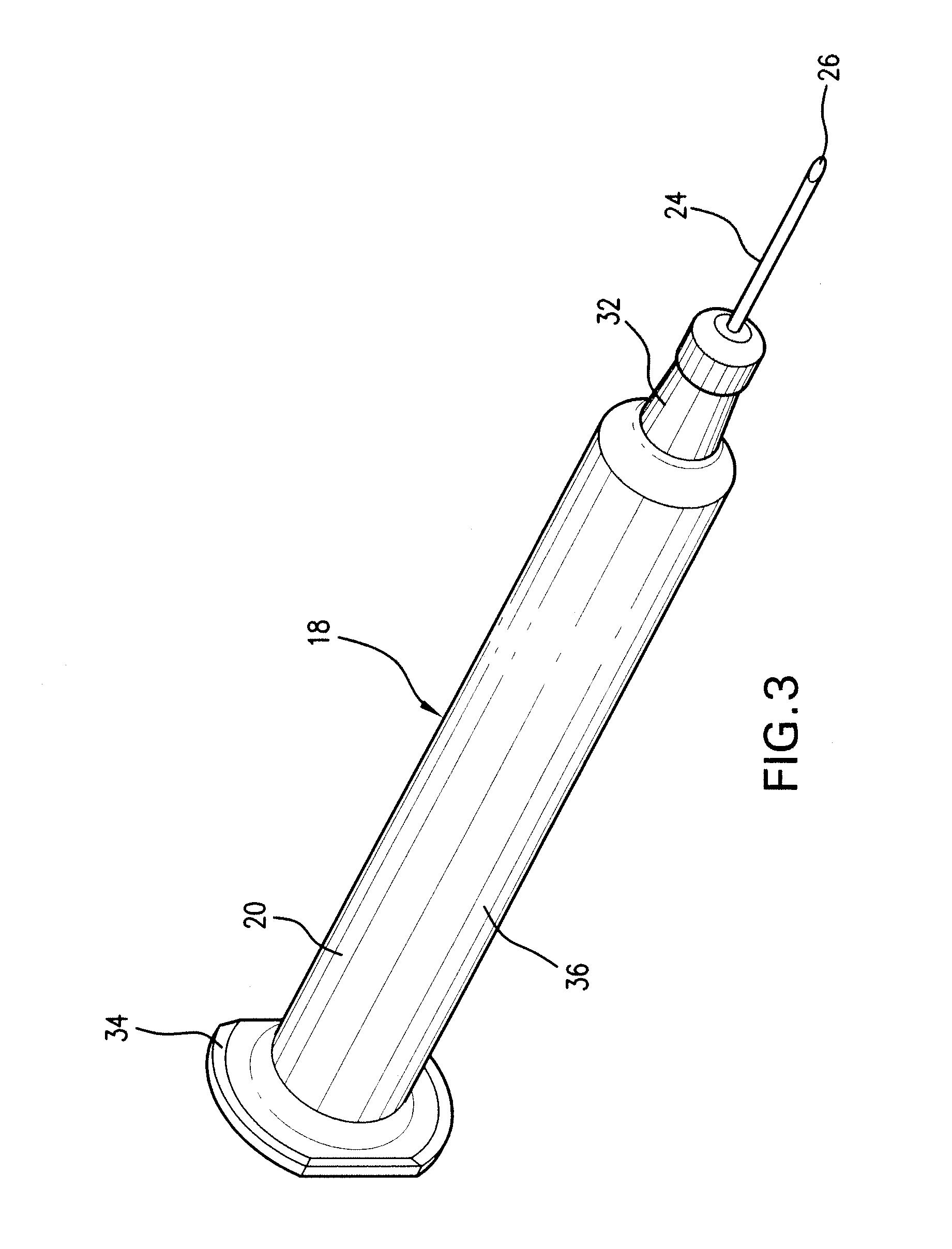
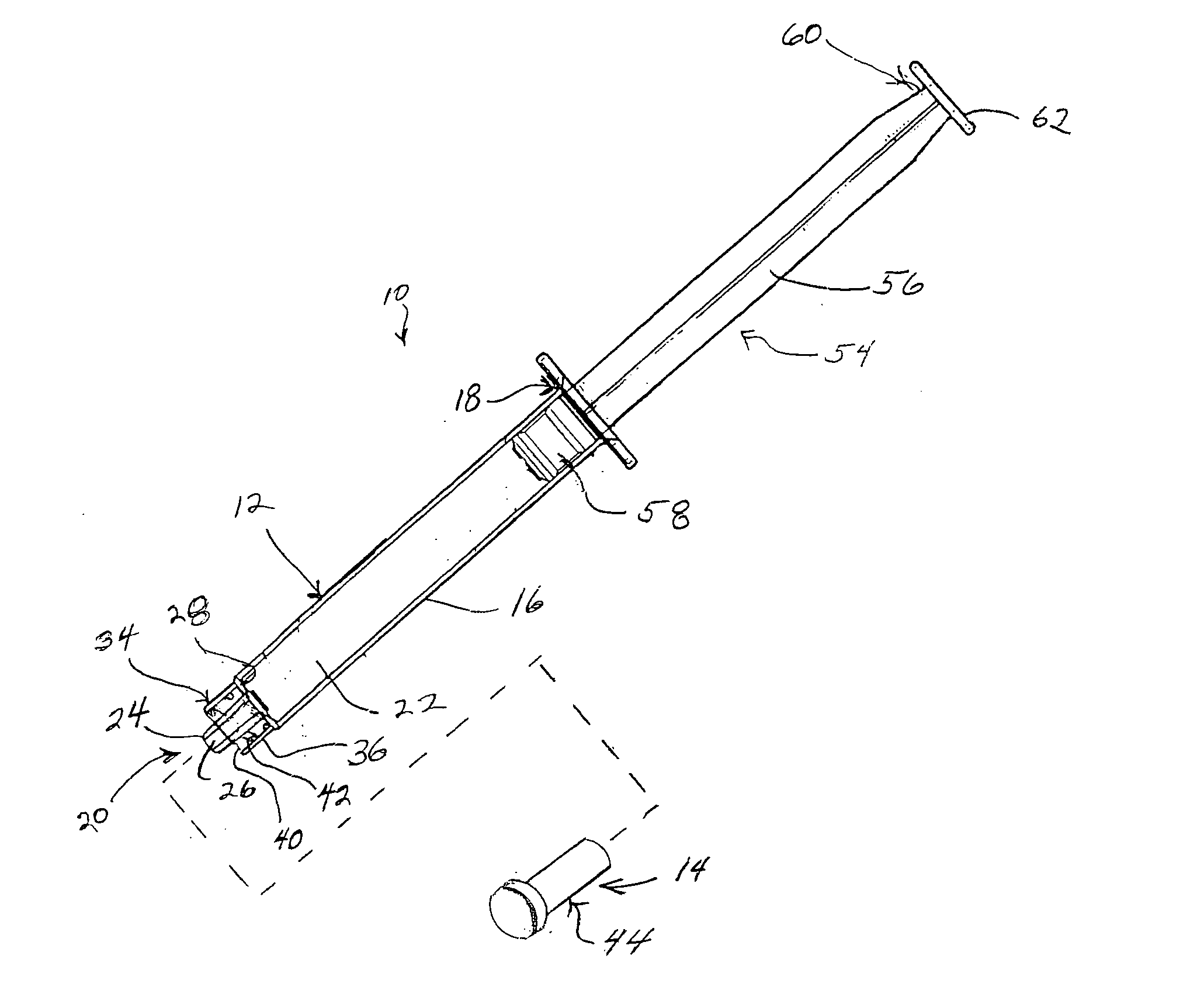
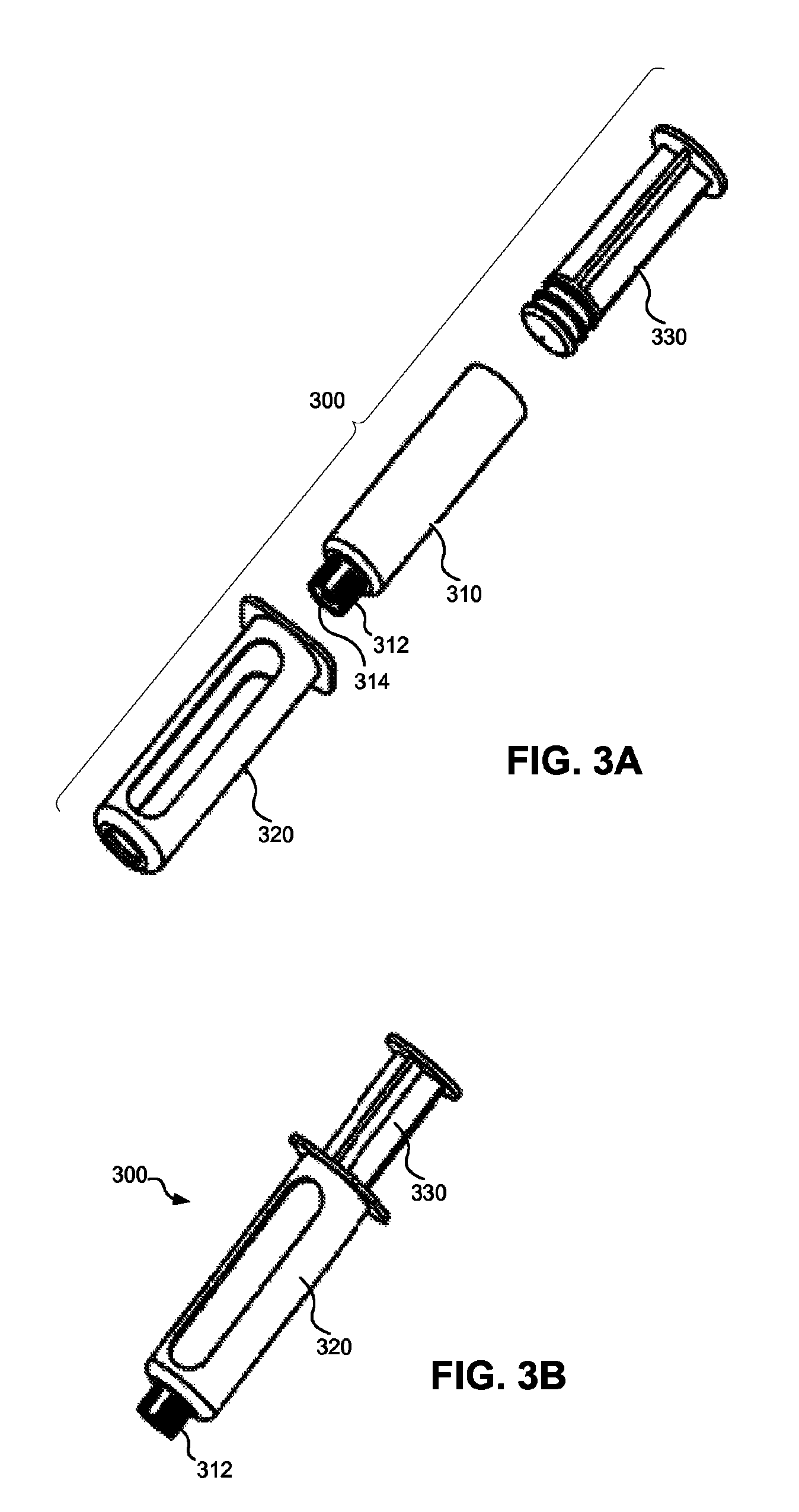
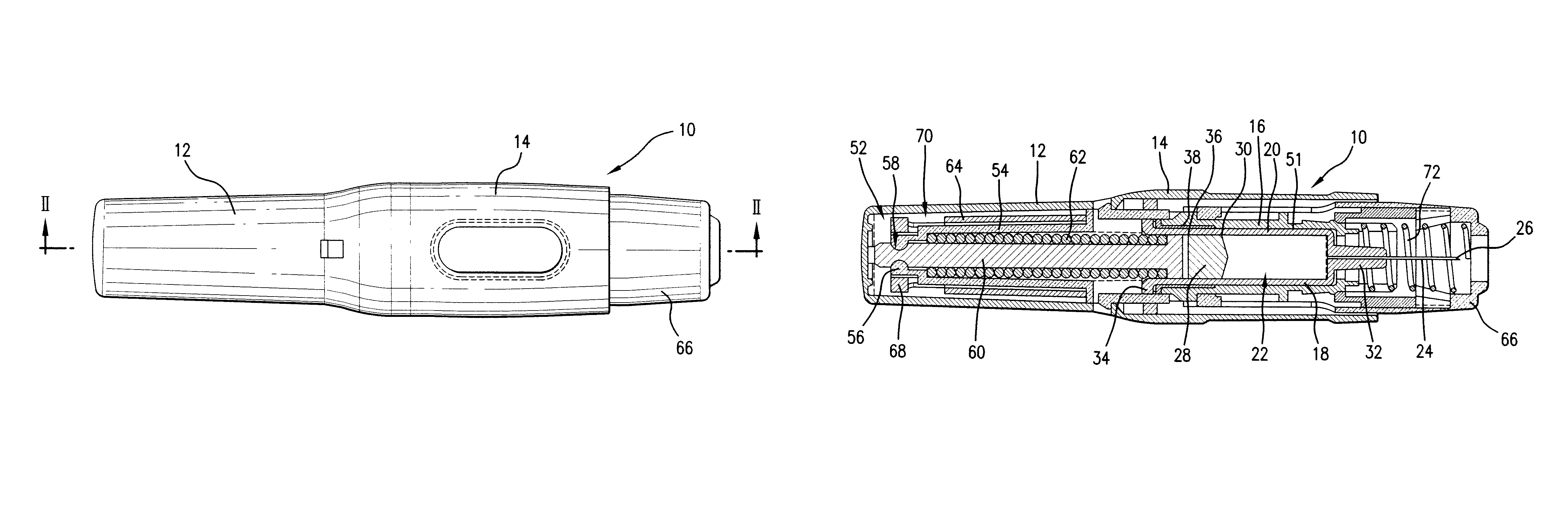
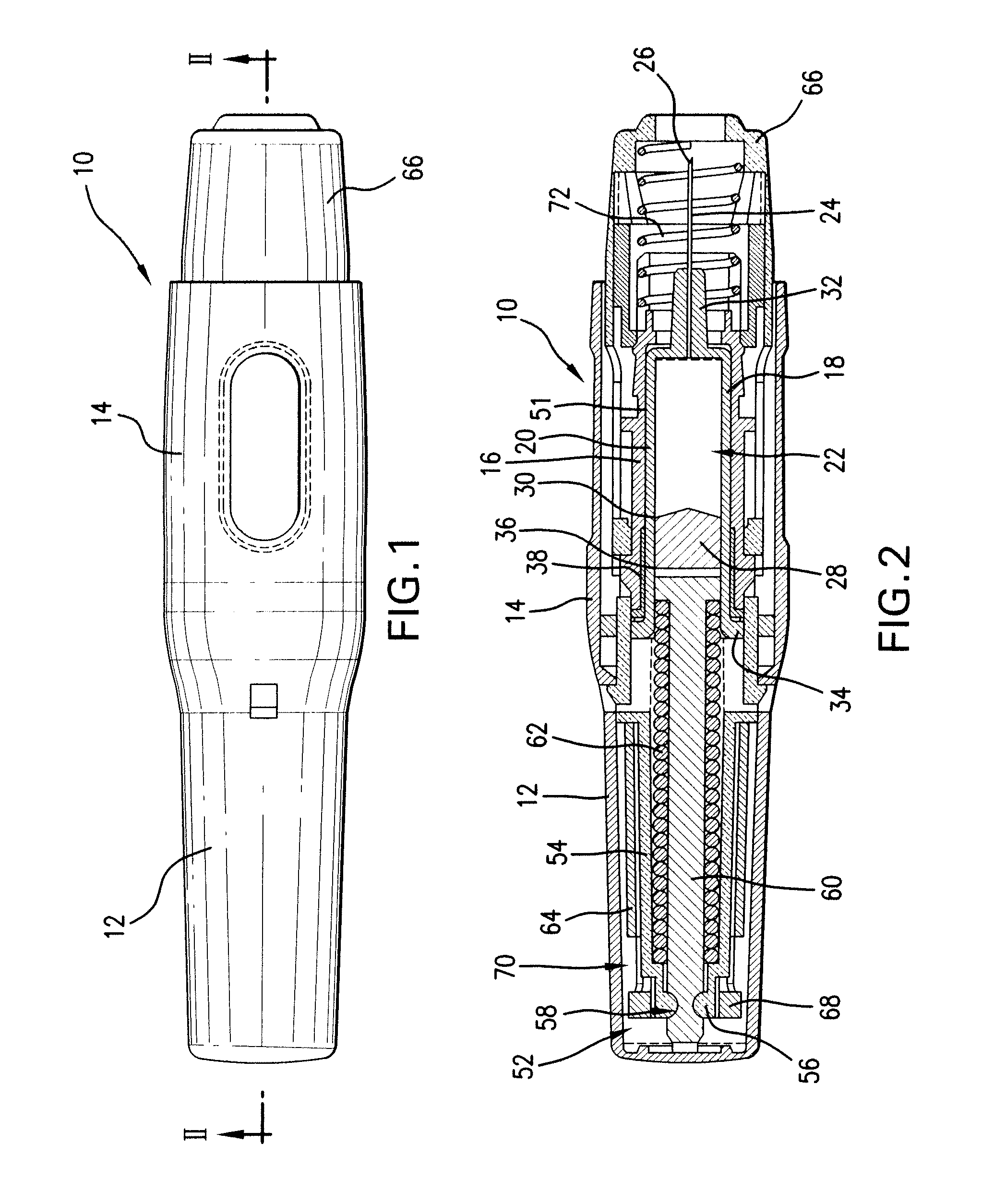
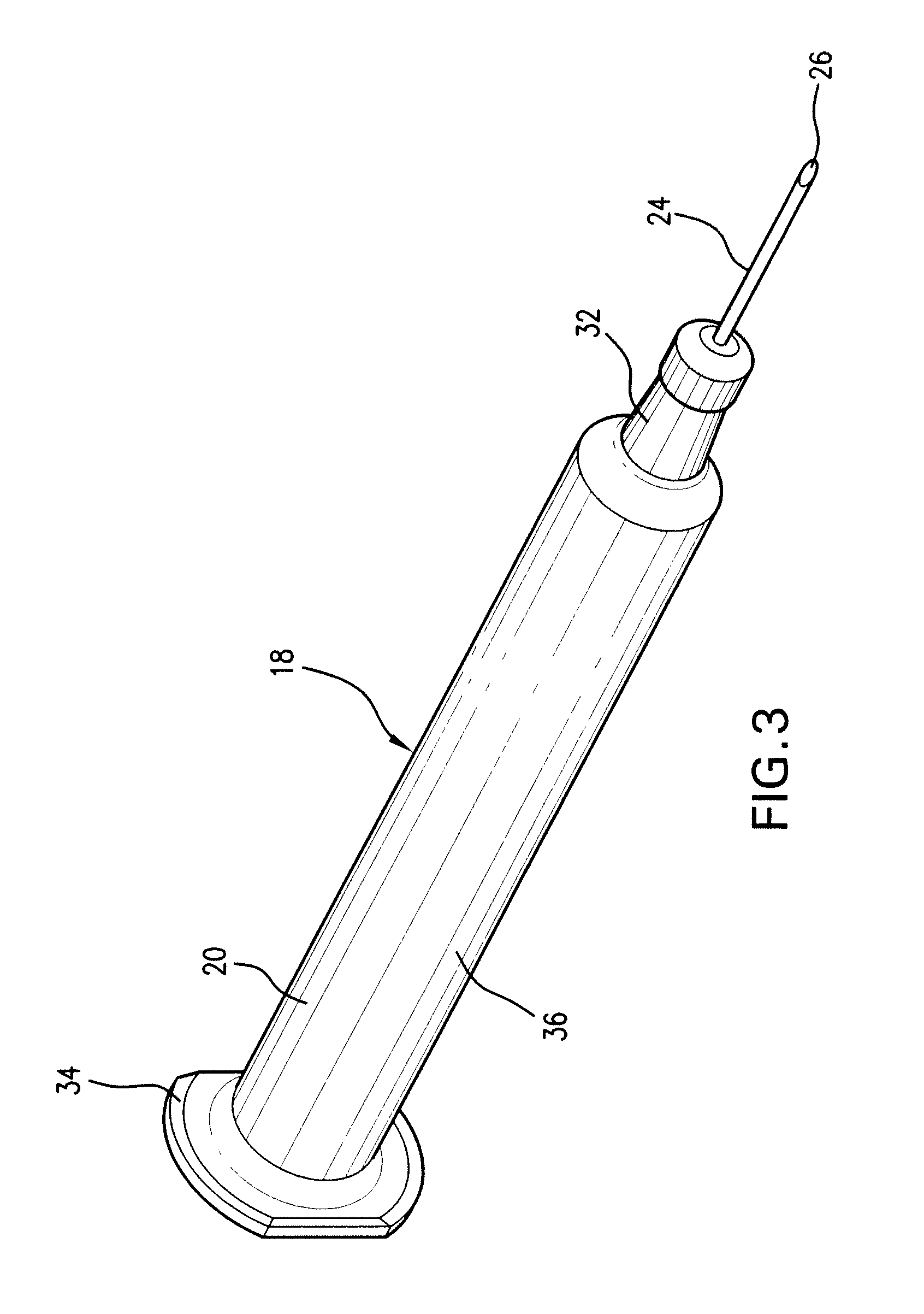
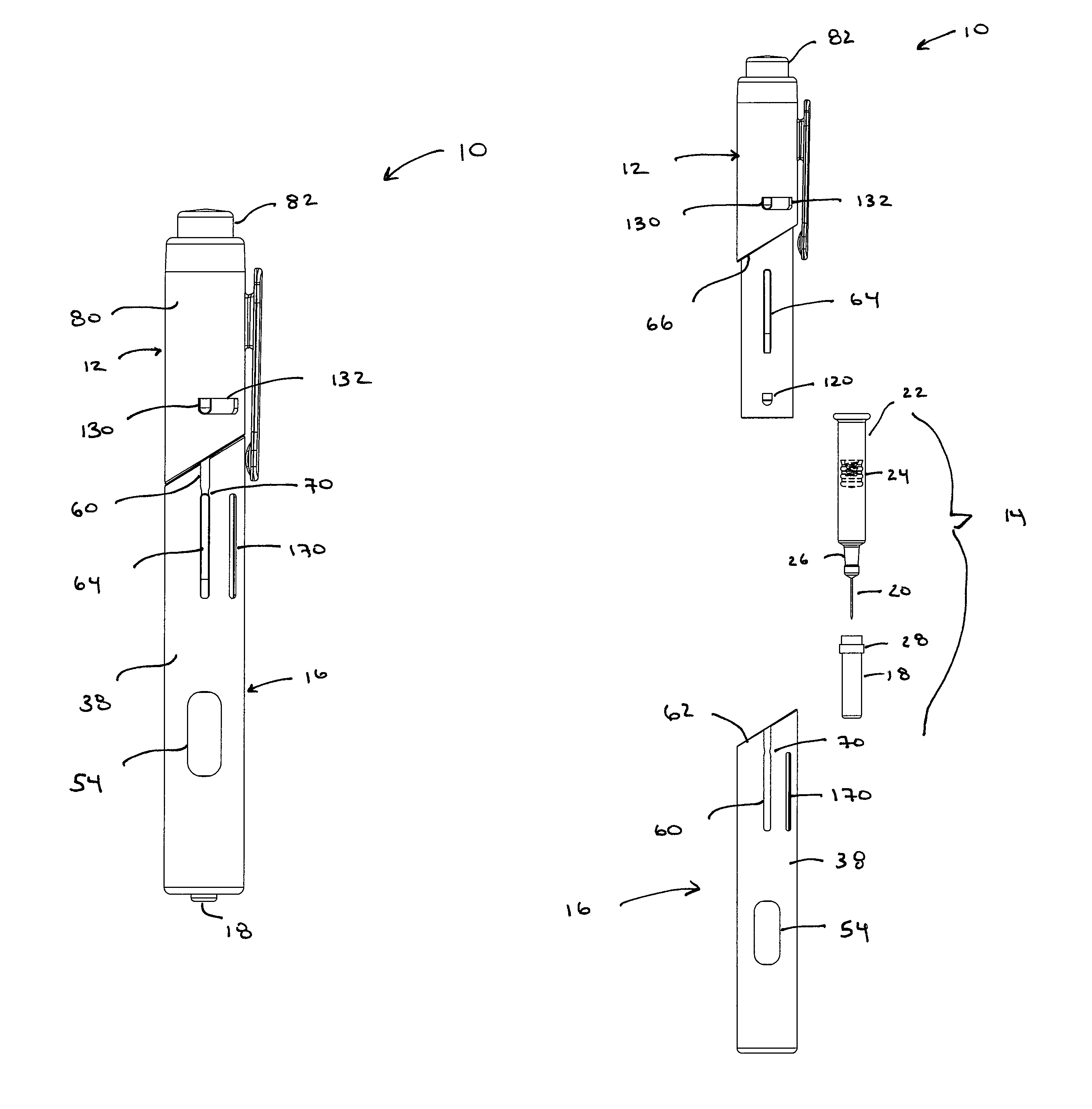
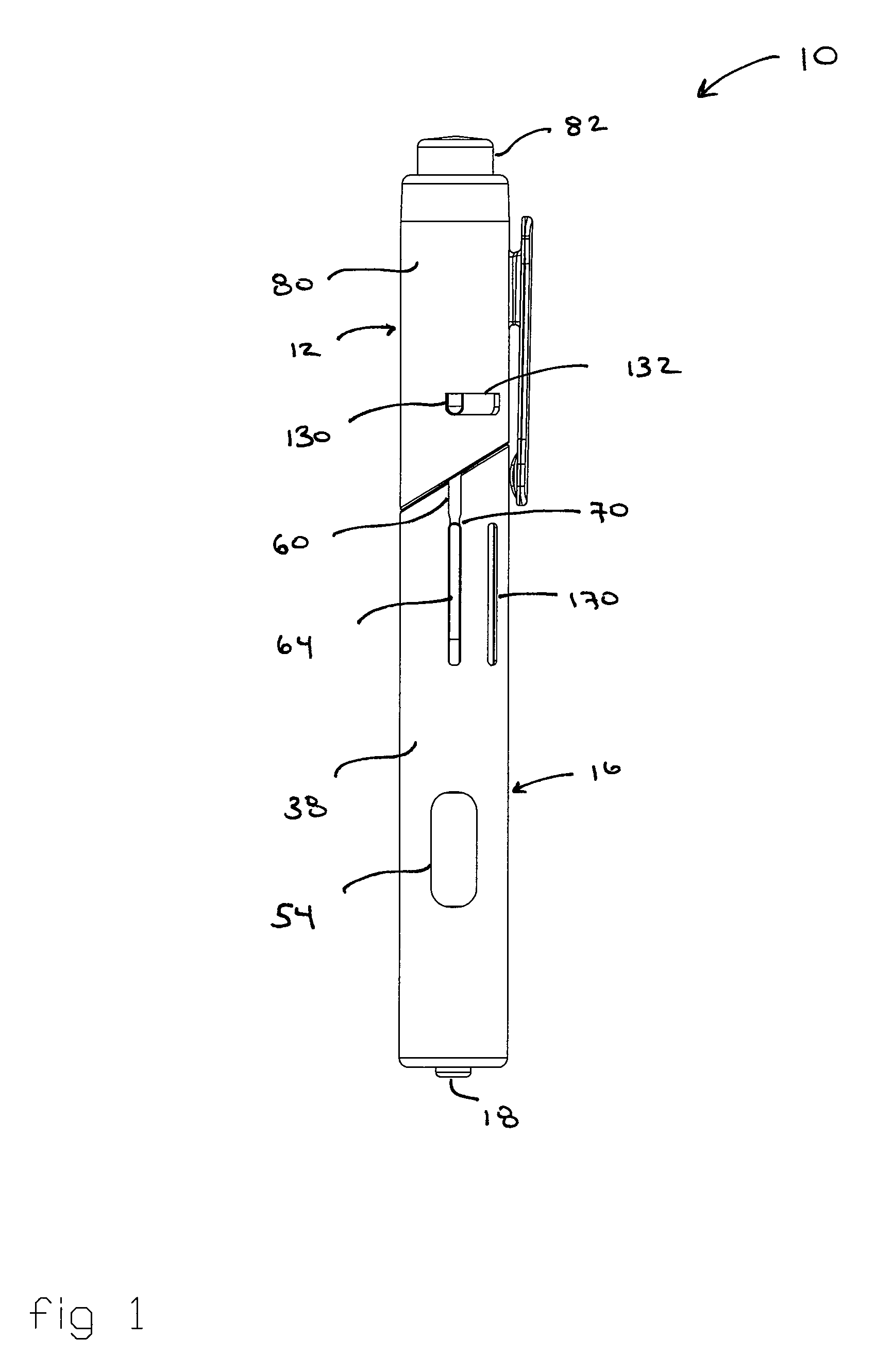
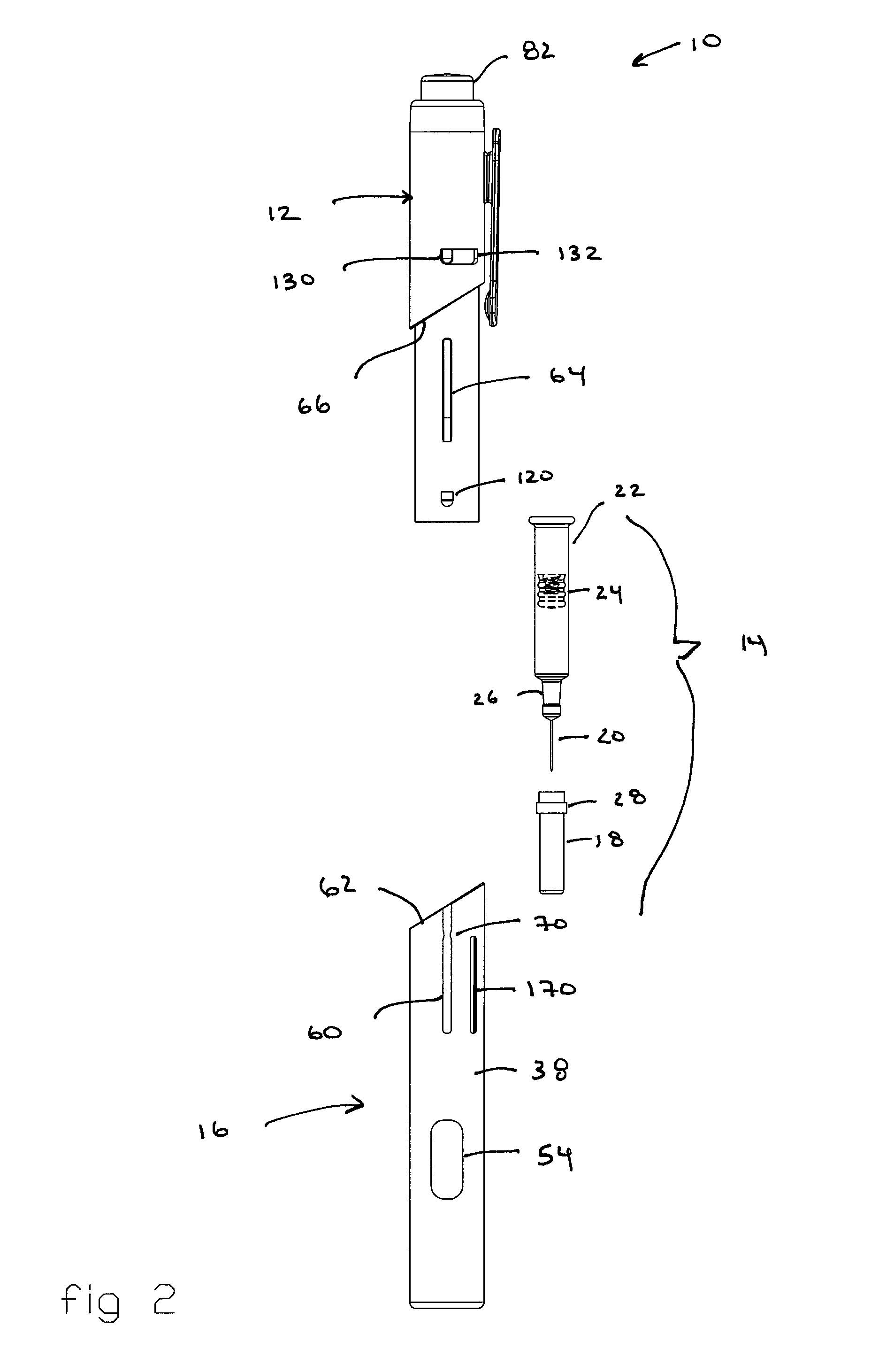
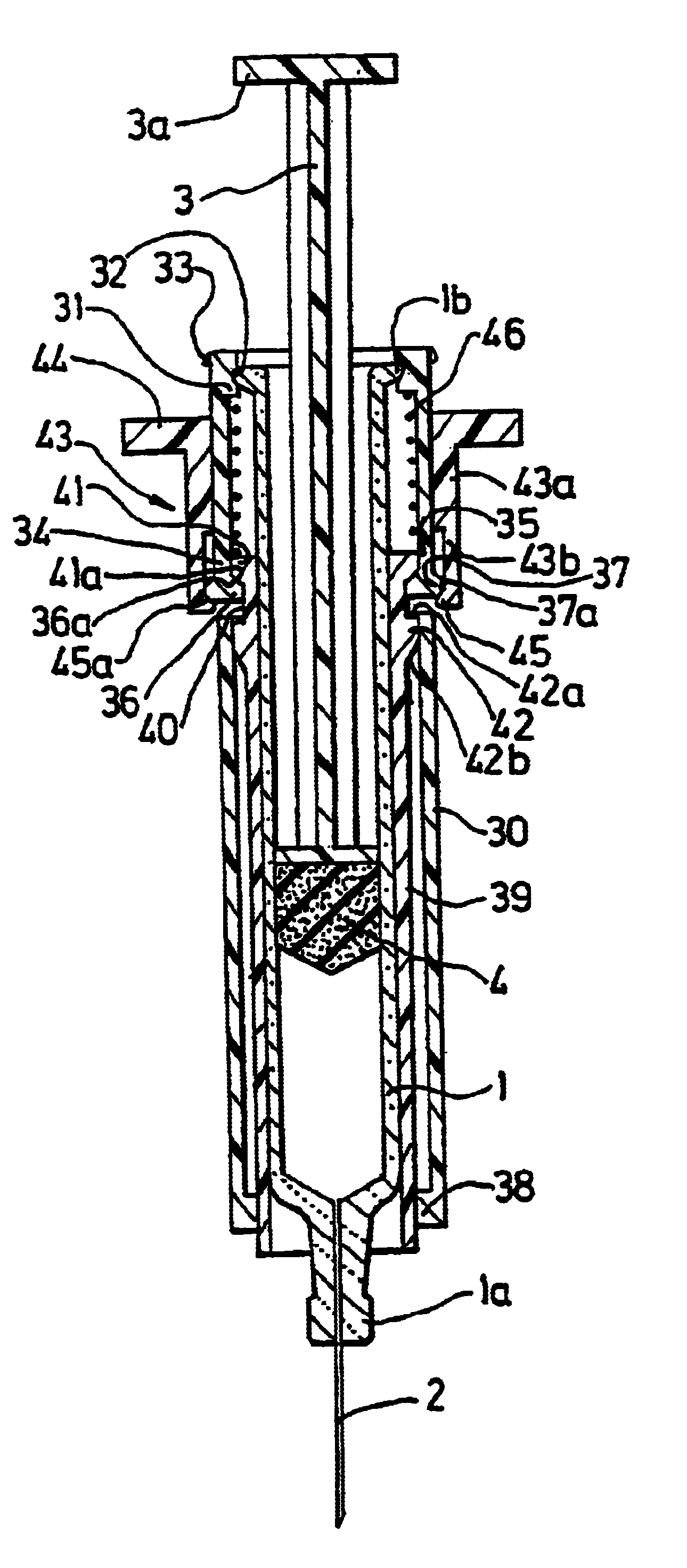
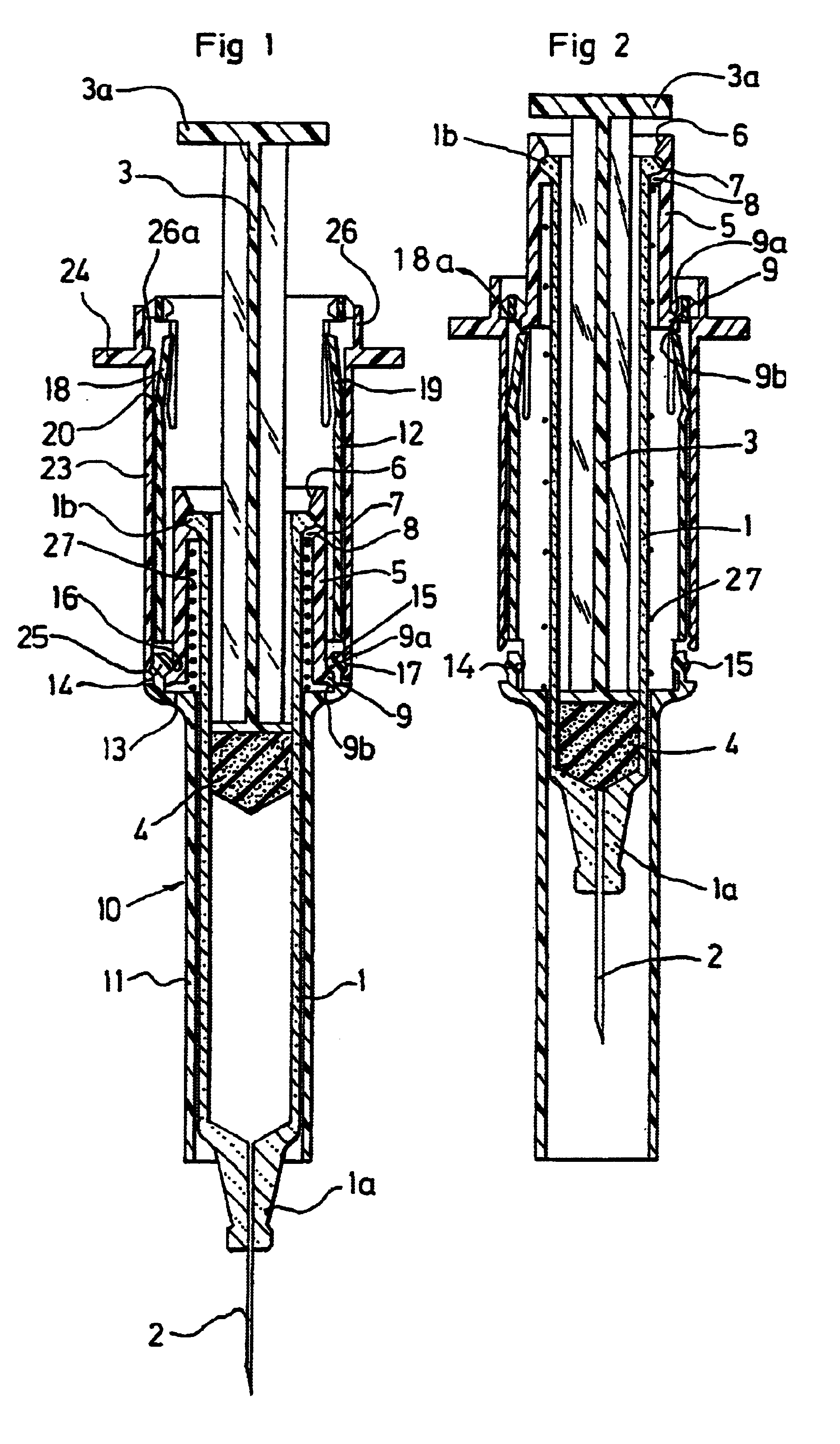
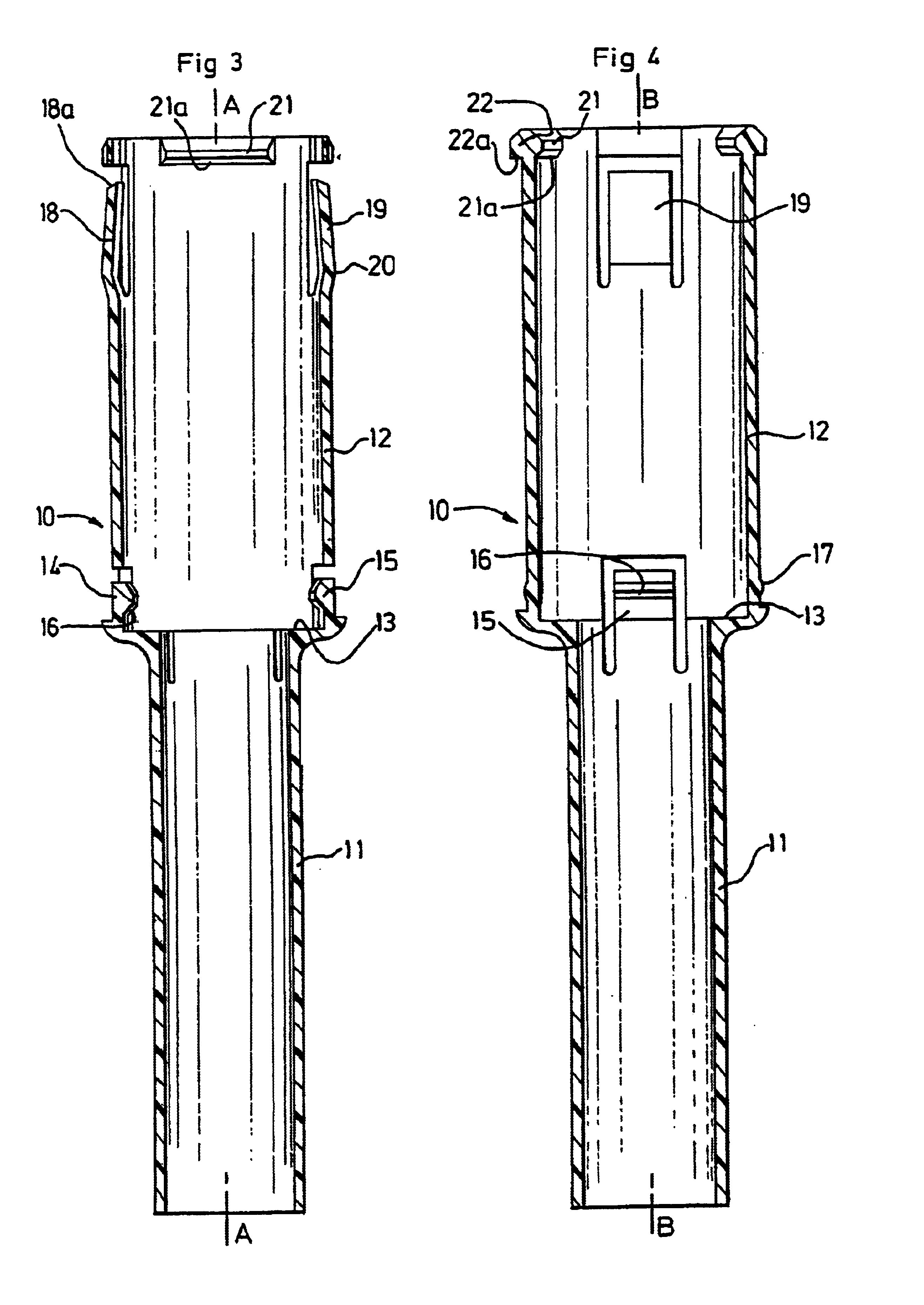
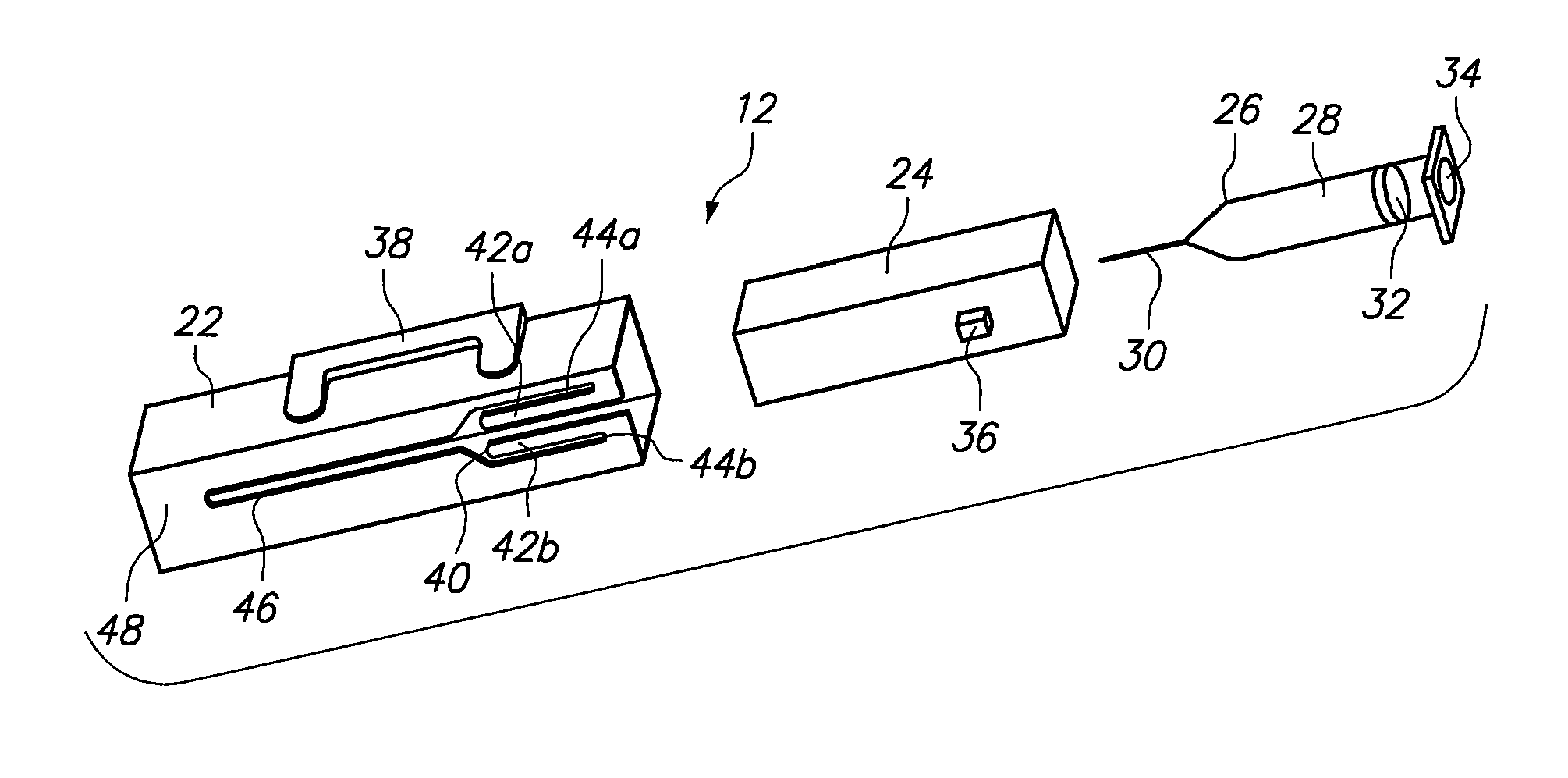
Patents
Literature
324 results about "Prefilled Syringe" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The use of prefilled syringes is expected to accelerate over the coming decades. For the pharmaceutical company, the advantages of prefilled syringes are minimizing drug waste; increasing product life span and enhancing level of market share are some of driving market demand.
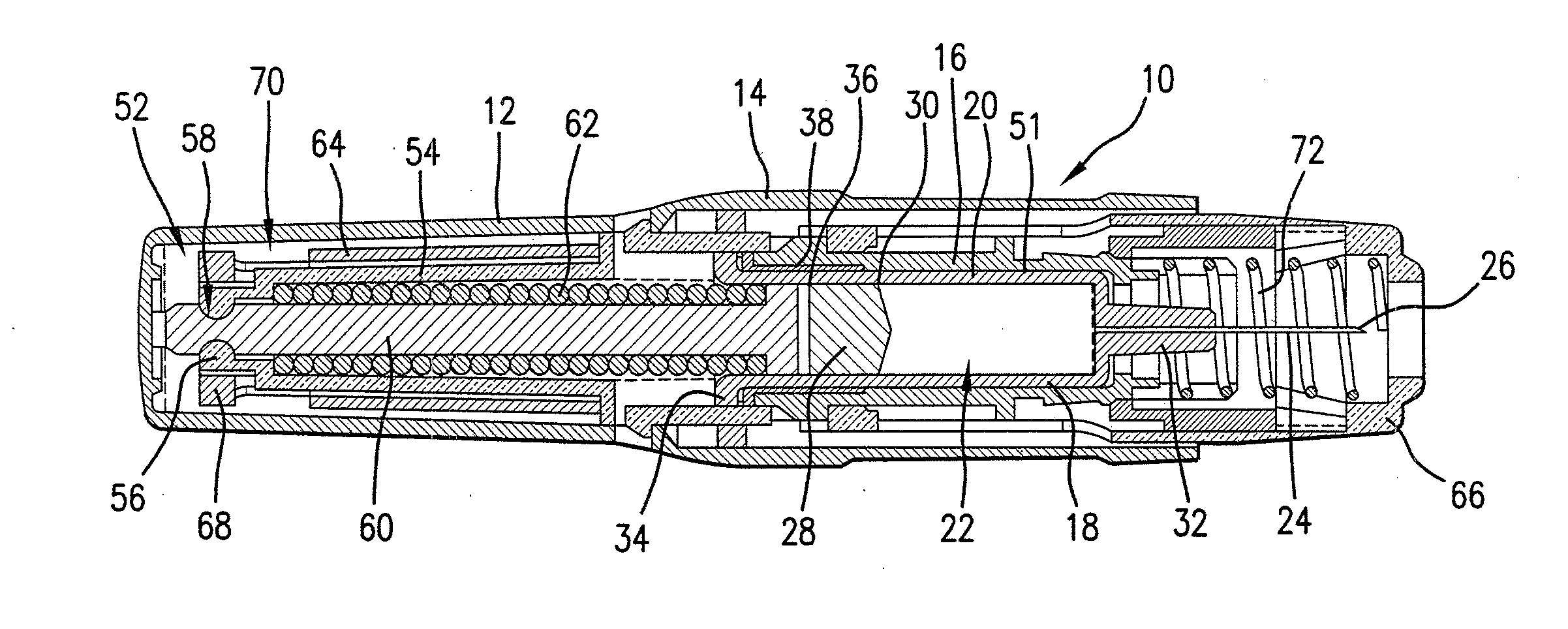
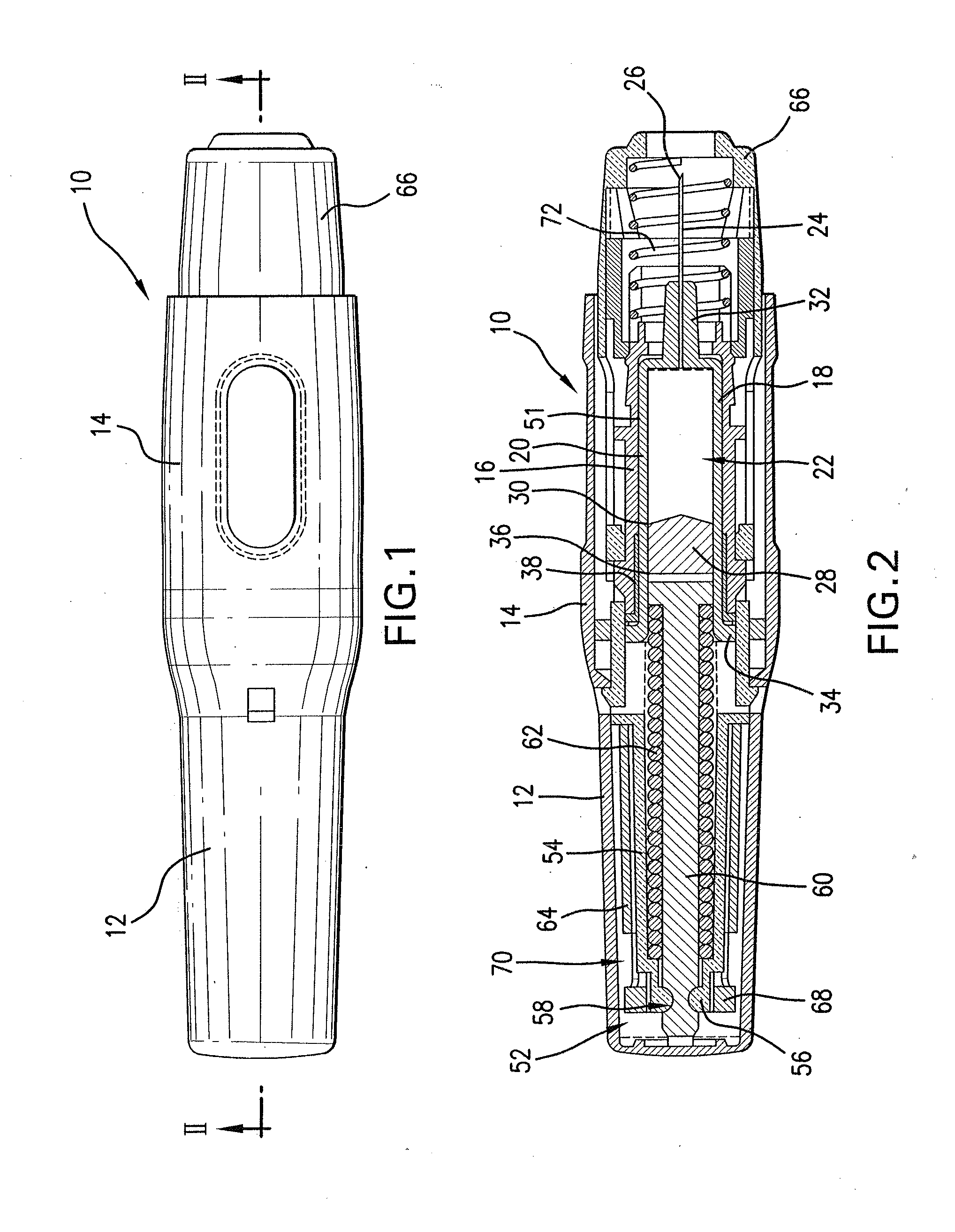
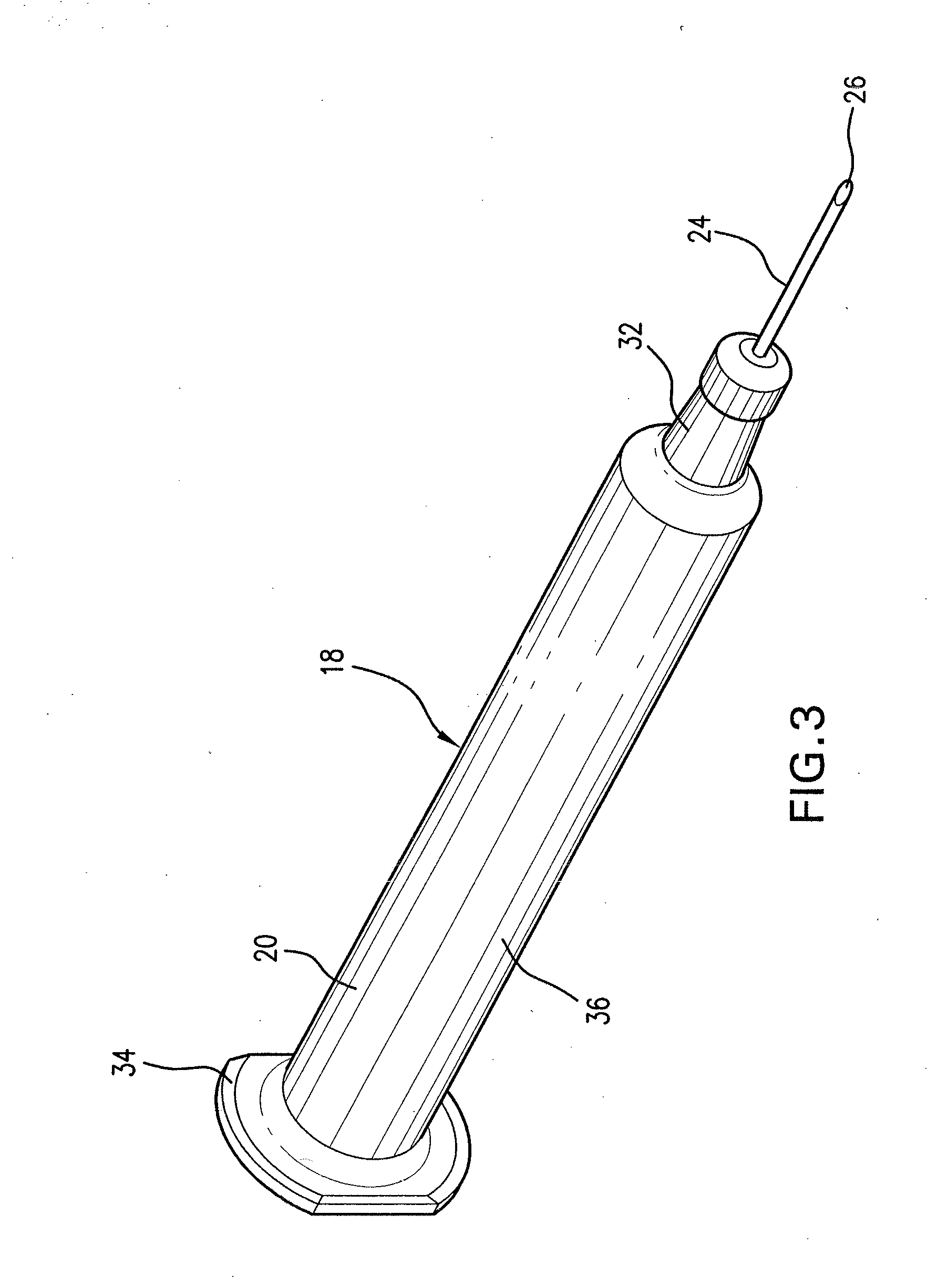
Automatic injection device
ActiveUS20100160894A1Easy to useReduce anxietyPeptide/protein ingredientsAntipyreticHypodermoclysisSubcutaneous injection
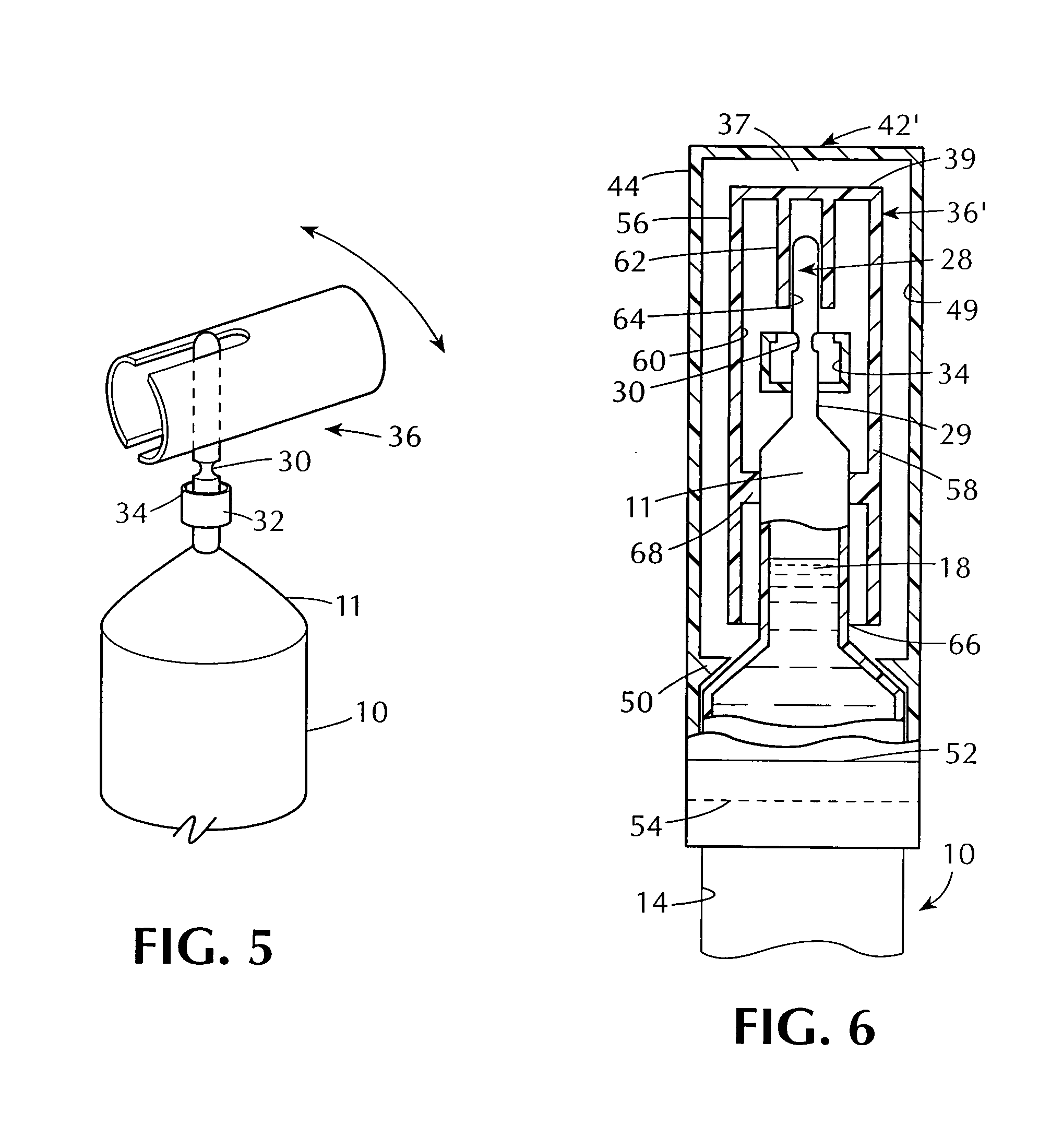
The invention provides an automatic injection device for providing a subcutaneous injection of a substance into a user, comprising: a housing having an open first end and a second end; a syringe movably disposed in the housing, the syringe including a barrel portion for holding the substance, a hollow needle in fluid communication with the barrel portion for ejecting the substance from the syringe, and a bung for sealing the barrel portion and selectively applying pressure to the substance to force the substance through the hollow needle; a plunger for first moving the syringe towards the first end such that the needle projects from the first end and subsequently applying pressure to the bung, the plunger including a rod connected at a first end to the bung, a compressible expanded central portion and a flange between a second end of the rod and the compressible expanded central portion; and a biasing mechanism for biasing the plunger towards the first open end of the housing, the biasing mechanism disposed about the second end of the rod between the flange and the second end of the housing. The present invention also provides methods and kits for using an automatic injection device, and methods and kits for promoting an automatic injection device comprising a medication based on advantageous properties of the device as compared to a pre-filled syringe. The invention also provides methods and kits for training a recipient on use of the automatic injection device.
Owner:ABBVIE BIOTECHNOLOGY LTD
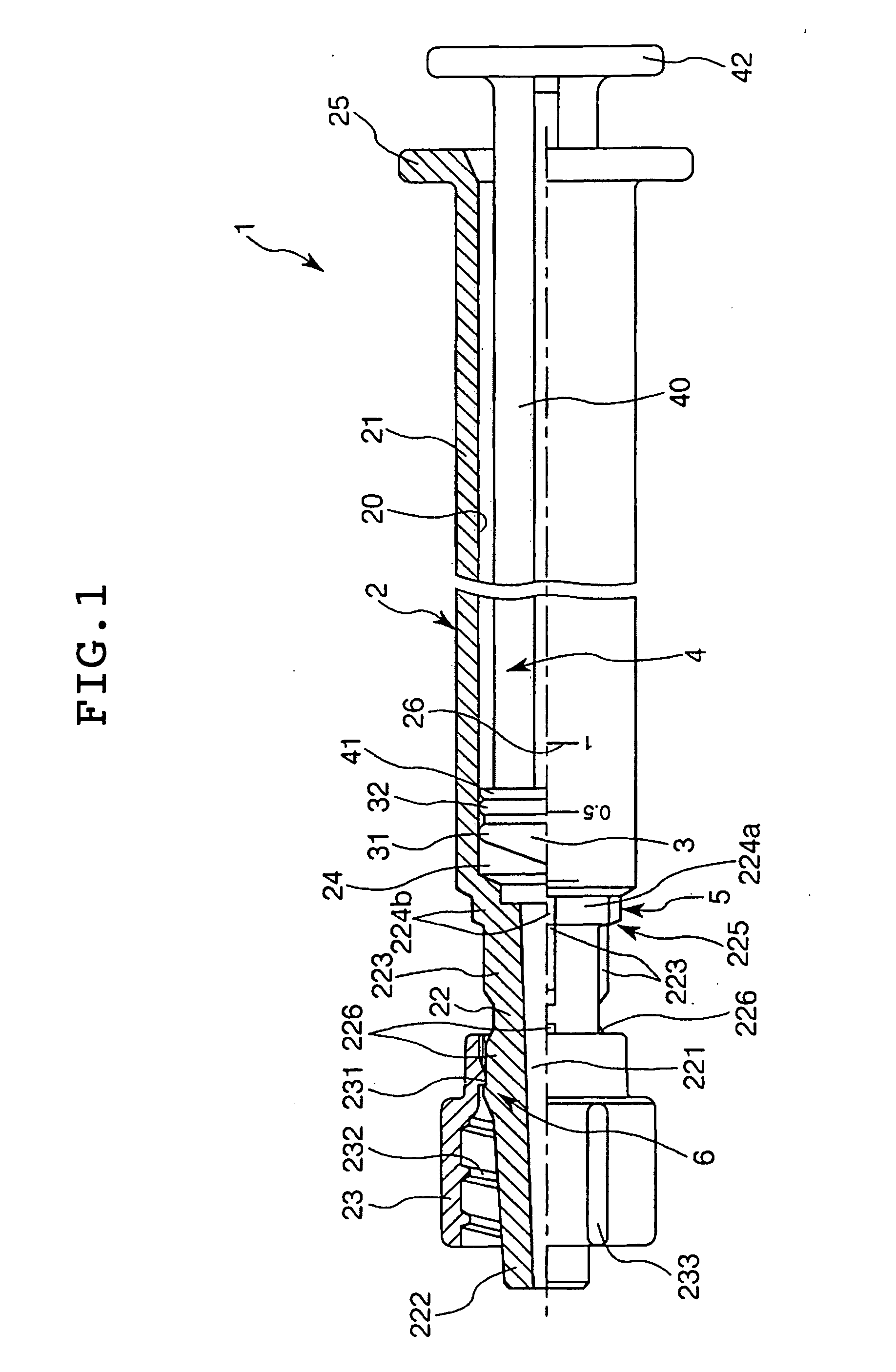
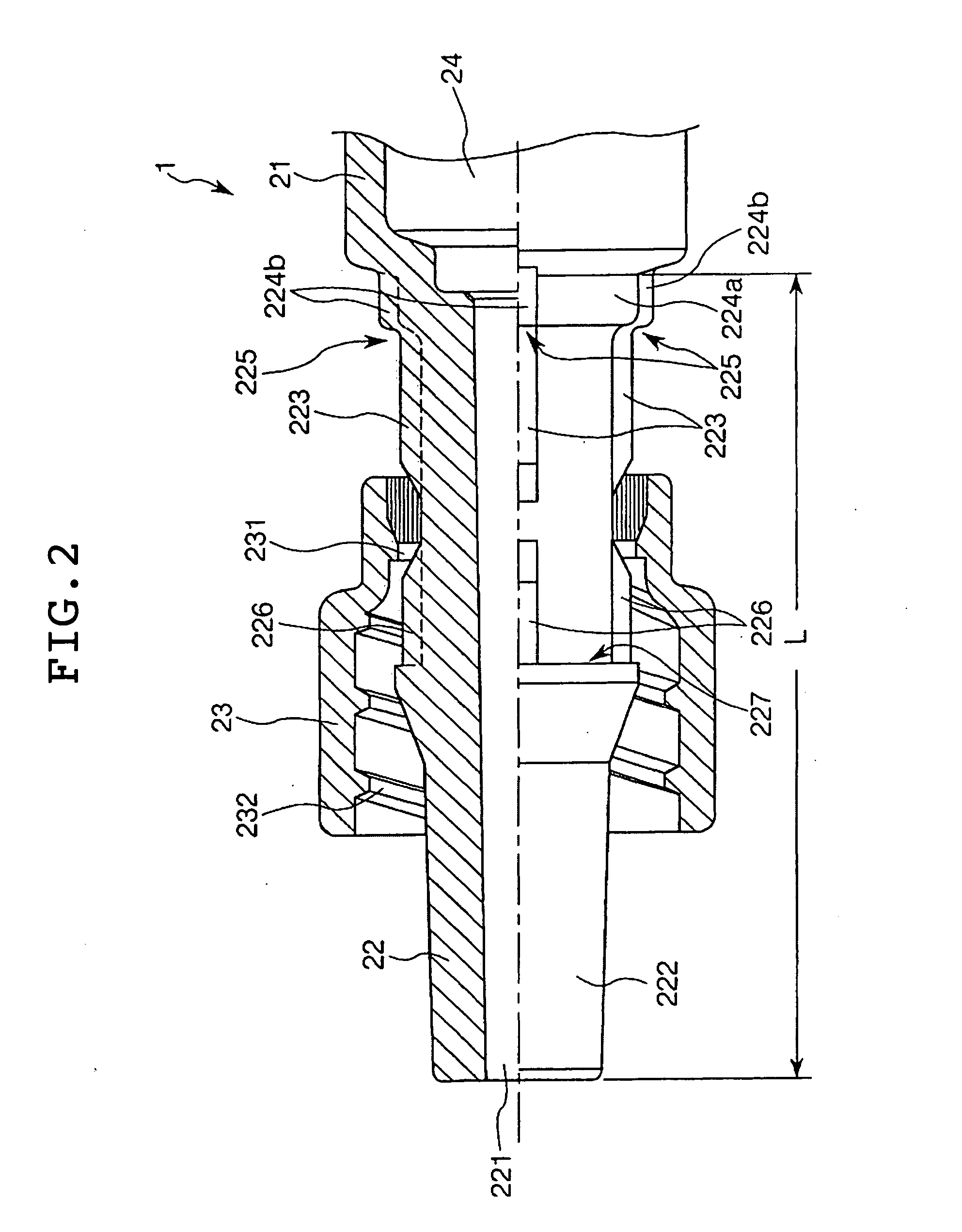
Sealing stopper for a syringe and a prefilled syringe
There can be provided a sealing stopper for a syringe, having very high sealing property and sliding property, and a prefilled syringe using this sealing stopper and capable of preserving a medicament for a long time and operating in easy and precise manner during injecting. This syringe is also excellent in sanitary and operating property during a step of formulation or preservation of a medicament. In this sealing stopper for a syringe, a surface of the rubber body is laminated with a tetrafluoroethylene resin film or ultra-high molecular weight polyethylene film having an average roughness Ra on the central line of the surface in a range of at most 0.05 mu m and a kinematic friction coefficient of at most 0.2.
Owner:DAIKYO SEIKO LTD
Powerhead of a power injection system
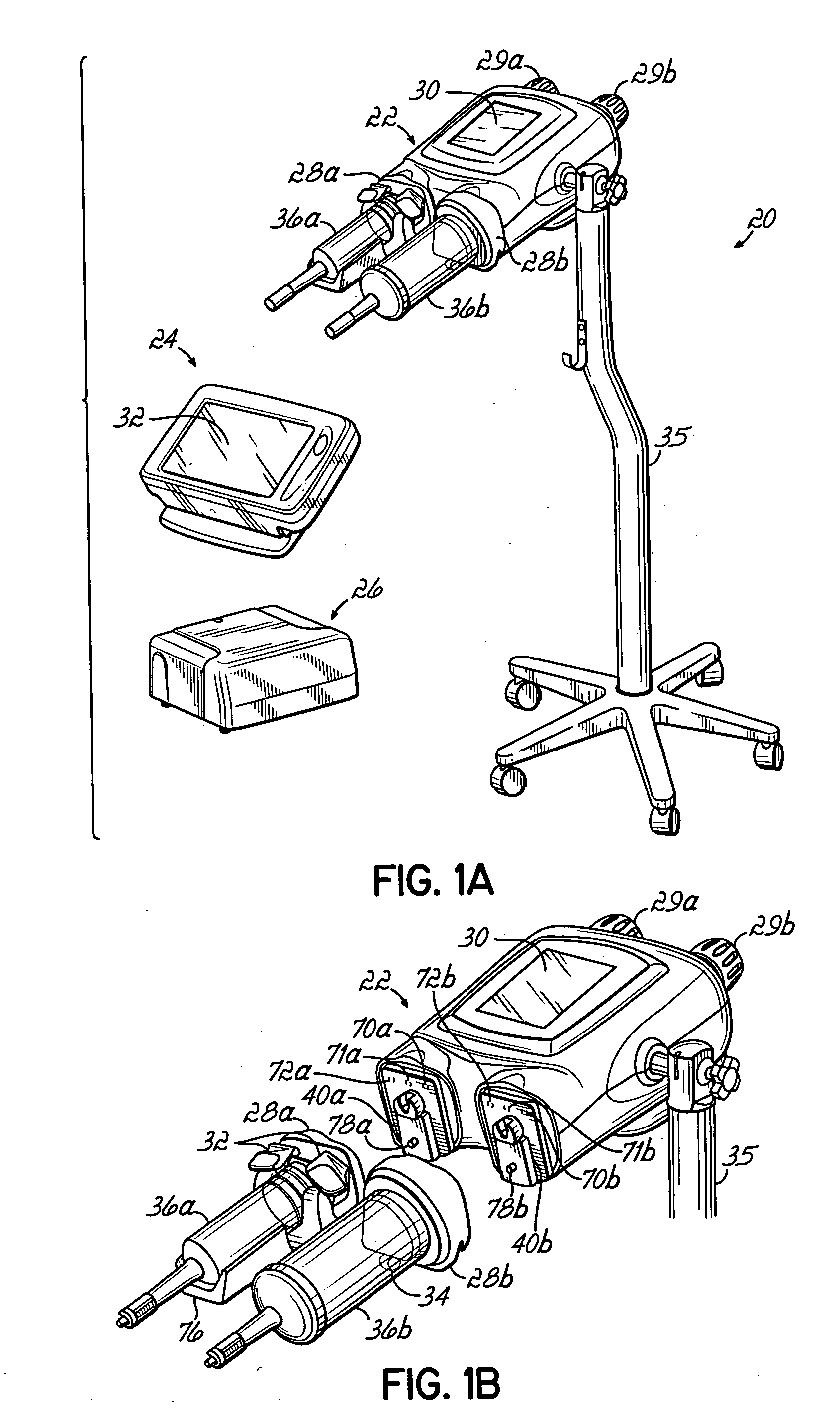
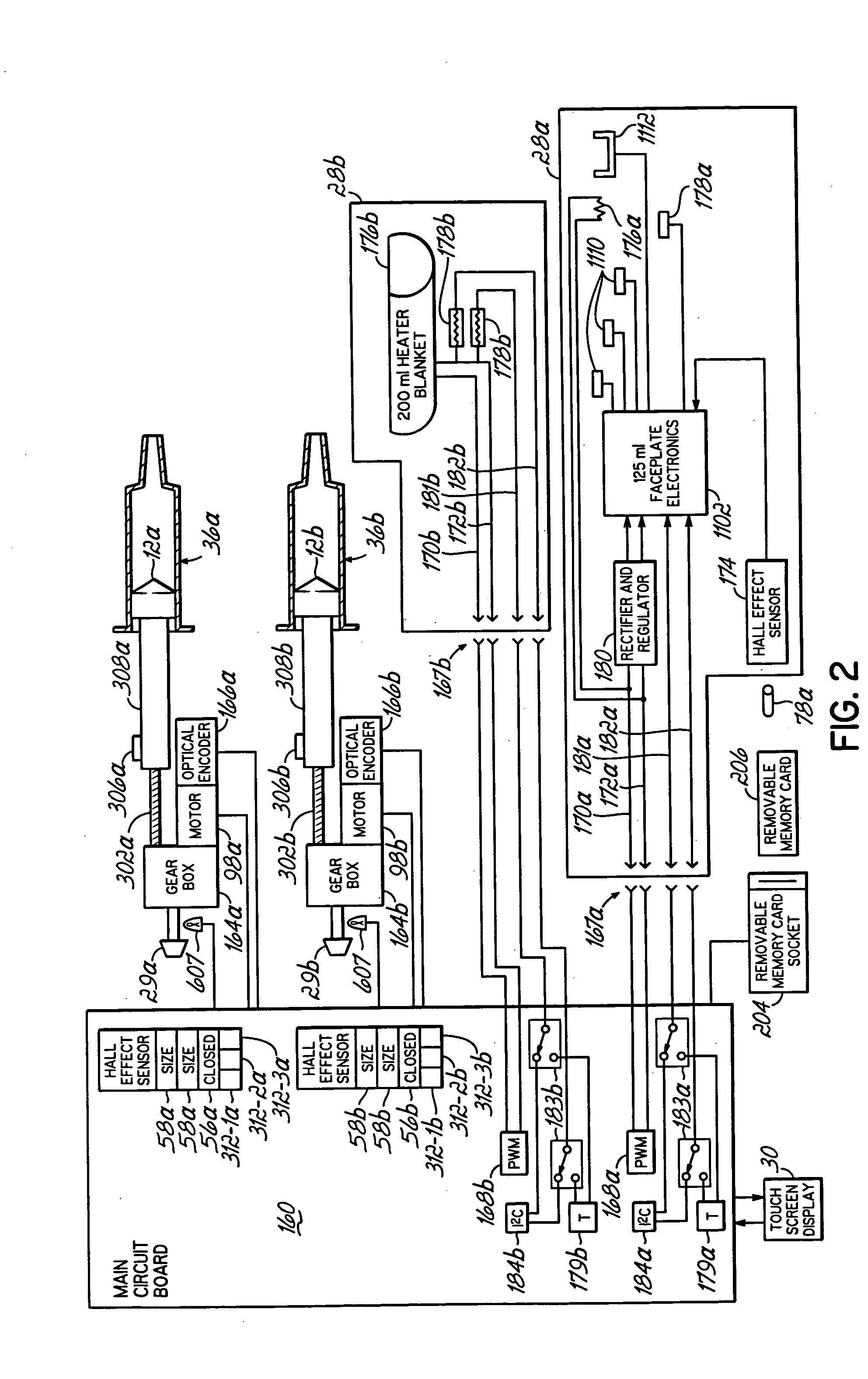
InactiveUS20060079765A1Increase powerImprove accuracyMedical devicesIntravenous devicesEngineeringPassword protection
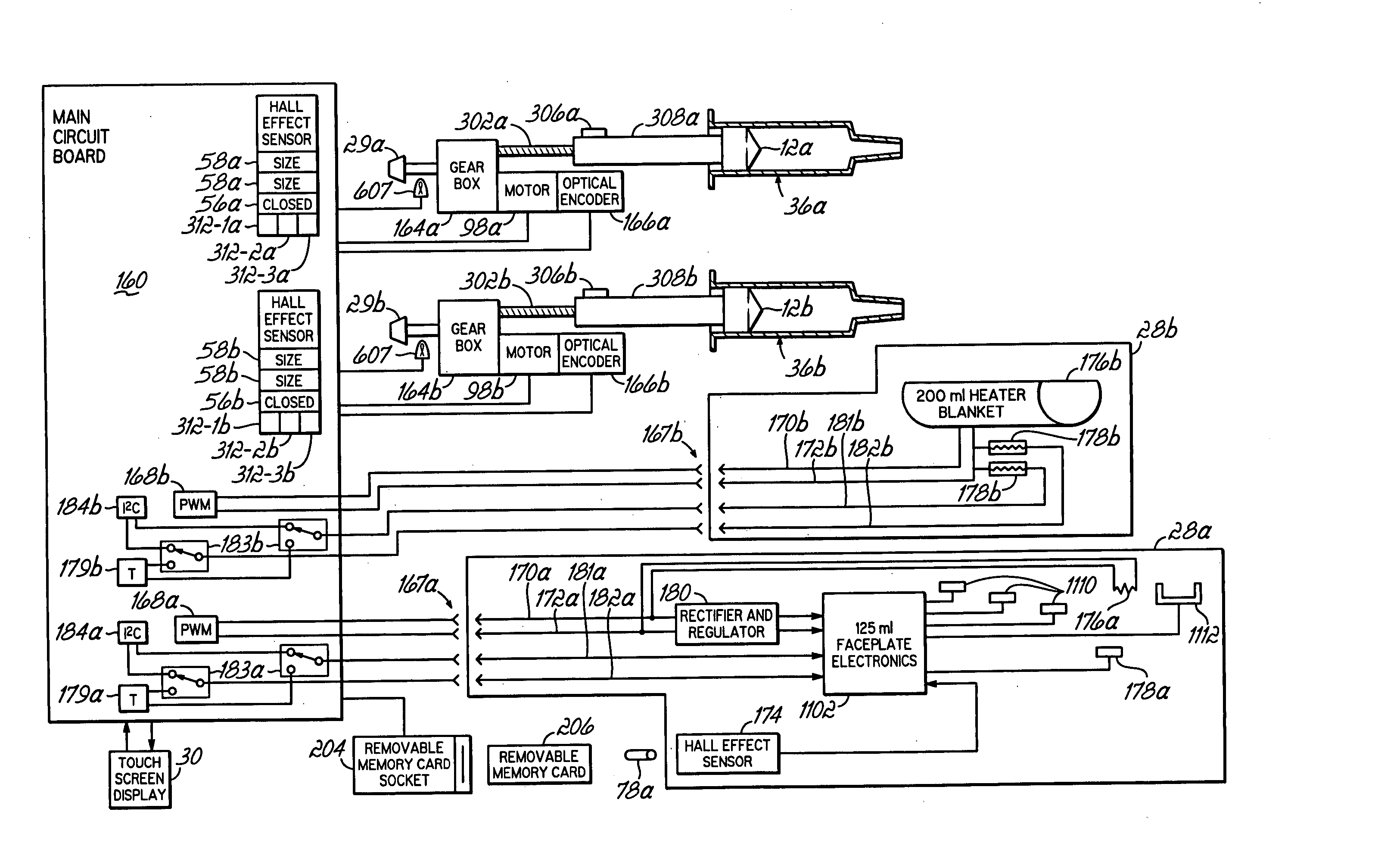
A contrast media injection system includes detects the absolute position of the syringe ram using a non-contact sensor. A series of magnets and Hall-Effect sensors may be used or an opto-reflective system. Illuminated knobs that are connected to the drive mechanism for the syringe ram rotate with the drive and provide visual feedback on operation through the illumination. Analog Hall-Effect sensors are used to determine the presence or absence of magnets that identify the type of faceplate being used. The faceplates include control electronics, connected to the powerhead through connectors, which may be interchangeably used by the two faceplates. The faceplate electronics include detectors for automatically detecting the capacity of pre-filled syringes. Additional features include using historical data to provide optimum pressure limit values during an injection protocol, a removable memory device for storing and transferring information such as injection protocols and injector statistics, and password protection of such protocols.
Owner:LIEBEL FLARSHEIM CO
Automatic injection and retraction devices for use with pre-filled syringe cartridges
ActiveUS20070135767A1Safe disposalEasy to disassembleAmpoule syringesAutomatic syringesHypodermic needleEngineering
An automatic injection and retraction device having a longitudinal axis is provided that includes an injection assembly, a retraction assembly, and a pre-filled syringe cartridge. The injection assembly has an activation-prevention feature moveable between an on position and an off position. The retraction assembly has a needle guard that is removable in a direction along the longitudinal axis upon application of a removal force. The pre-filled syringe cartridge has a hypodermic needle with a needle sheath thereon. The retraction and injection assemblies are secured to one another so that the pre-filled syringe cartridge is in the retraction assembly with the needle sheath secured to the needle guard. The retraction and injection assemblies are configured so that, upon application of a twisting torque to the injection and retraction assemblies, the activation-prevention feature moves from the on position to the off position simultaneous with applying the removal force to the needle guard.
Owner:WEST PHARMA SERVICES OF DELAWARE
Multi-chamber, sequential dose dispensing syringe
A valve assembly is disclosed which effectively partitions a syringe into proximal and distal chambers to provide a multi-chamber, sequentially dispensing syringe apparatus. The valve assembly may be effectively used with a variety of standard, currently available commercial syringes and pre-filled syringes. Incorporated in the valve assembly is a valved stopper having a valve (which may be a slit valve), an impact sensor which opens the valve upon impact between the valve assembly and internal distal end of the syringe and a gas separator which separates liquid from gas disposed in the proximal chamber to assure gas is not delivered therefrom. The valve assembly is displaced as a plunger of the syringe is displaced via communication through fluid in the proximal chamber of the syringe. The actuator has a latching feature which latches the valve to an open state after being opened by the impact sensor. The gas separator has a proximally disposed orifice which facilitates priming. The valve assembly may be made from two parts. One part, a valved stopper, may be molded from the same rubber based material used in syringe plunger stoppers. The second part, the valve actuator, may be injection molded as a single part. A synthetic resinous material which is compatible with manufacture of living hinges my be used for the second part. Multiple valve assemblies may be used in a single syringe barrel.
Owner:INFUSIVE TECH LLC
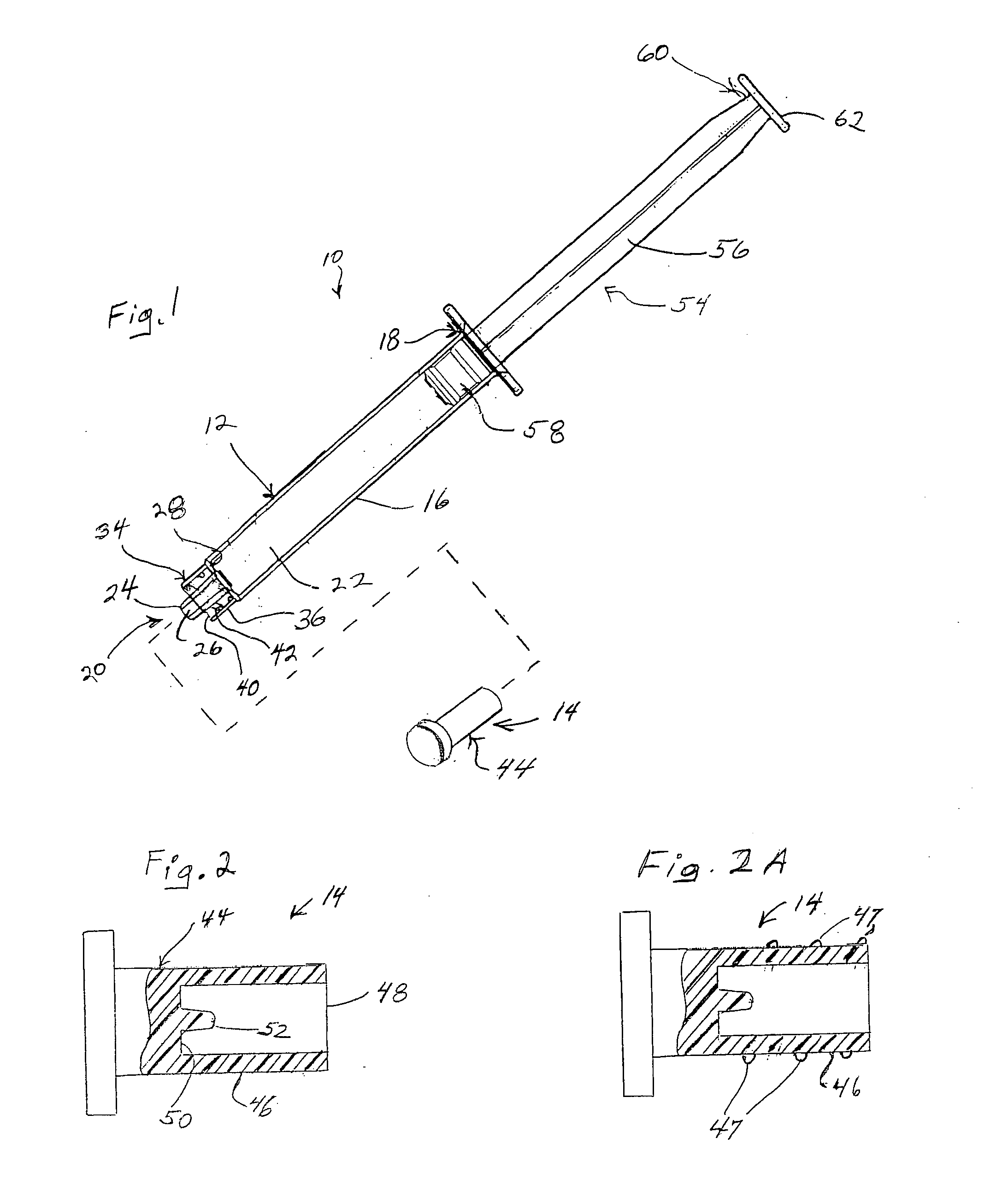
Prefilled syringe jet injector
ActiveUS20080154200A1Prevent backflowImprove distributionAmpoule syringesJet injection syringesEngineeringPrefilled Syringe
A jet injector that includes a prefilled syringe. The syringe includes a fluid chamber that contains a medicament. The syringe also has an injection-assisting needle, and a plunger is movable within the fluid chamber. A housing is configured for allowing insertion of the needle to a penetration depth. An energy source is configured for biasing the plunger to produce an injecting pressure in the medicament in the fluid chamber of between about 80 and 1000 p.s.i. to jet inject the medicament from the fluid chamber through the needle to an injection site.
Owner:ANTARES PHARMA INC
Low extractable, thermoplastic syringe and tip cap
InactiveUS20050075611A1Low extractable levelWithout loss of flexible and resilient propertyAmpoule syringesLavatory sanitoryThermoplastic elastomerPrefilled Syringe
A prefilled syringe and syringe assembly having a syringe and a tip cap are produced from materials that do not interfere with the substance contained in the syringe and enable long term storage. The tip cap is made from a blend of a cyclic olefin polymer or copolymer and a thermoplastic elastomer. The thermoplastic elastomer is blended with the cyclic olefin copolymer in an amount so that the normally stiff and hard cyclic olefin copolymer is flexible and resilient to effectively seal and couple to the tip of a prefilled syringe.
Owner:BECTON DICKINSON & CO
Interfacing a prefilled syringe with an infusion pump to fill the infusion pump
A prefilled syringe may be interfaced with and used to fill an infusion pump with therapeutic liquid. The dispensing end of the prefilled syringe may be coupled (either directly or indirectly using a syringe cap) to a syringe coupling region of the infusion pump, for example, using a threaded engagement or snap fit engagement. As the dispensing end of the prefilled syringe is coupled to the syringe coupling region of the pump housing, a needle passes through the pump housing and / or a needle passage region of the dispensing end such that the prefilled syringe is fluidly coupled to a reservoir in the pump. In various embodiments, the needle may be located in the syringe coupling region or in a syringe cap coupled to the dispensing end of the prefilled syringe.
Owner:INSULET CORP
Prefilled syringe jet injector
ActiveUS8021335B2Prevent backflowImprove distributionAmpoule syringesJet injection syringesInjection pressureInjection site
A jet injector that includes a prefilled syringe. The syringe includes a fluid chamber that contains a medicament. The syringe also has an injection-assisting needle, and a plunger is movable within the fluid chamber. A housing is configured for allowing insertion of the needle to a penetration depth. An energy source is configured for biasing the plunger to produce an injecting pressure in the medicament in the fluid chamber of between about 80 and 1000 p.s.i. to jet inject the medicament from the fluid chamber through the needle to an injection site.
Owner:ANTARES PHARMA INC
Subcutaneous and intradermal patch infusers
The device infuses a compound subcutaneously or intradermally over an extended period of time. The delivery is from a conventional prefilled syringe with a staked cannula with the device adhered to the injection site and the syringe positioned substantially parallel to the injection site. The cannula is formed by a mechanism integral to the device to provide the cannula distal section substantially perpendicular to the syringe axis. The compound delivery is based on springs or other mechanisms. The approach offers a low cost mechanical system for the infusion of high volume, viscous compounds. The activation of the patch infuser required a few simple steps by the user. A single lever is used to trigger all device functions.
Owner:SID TECH LLC
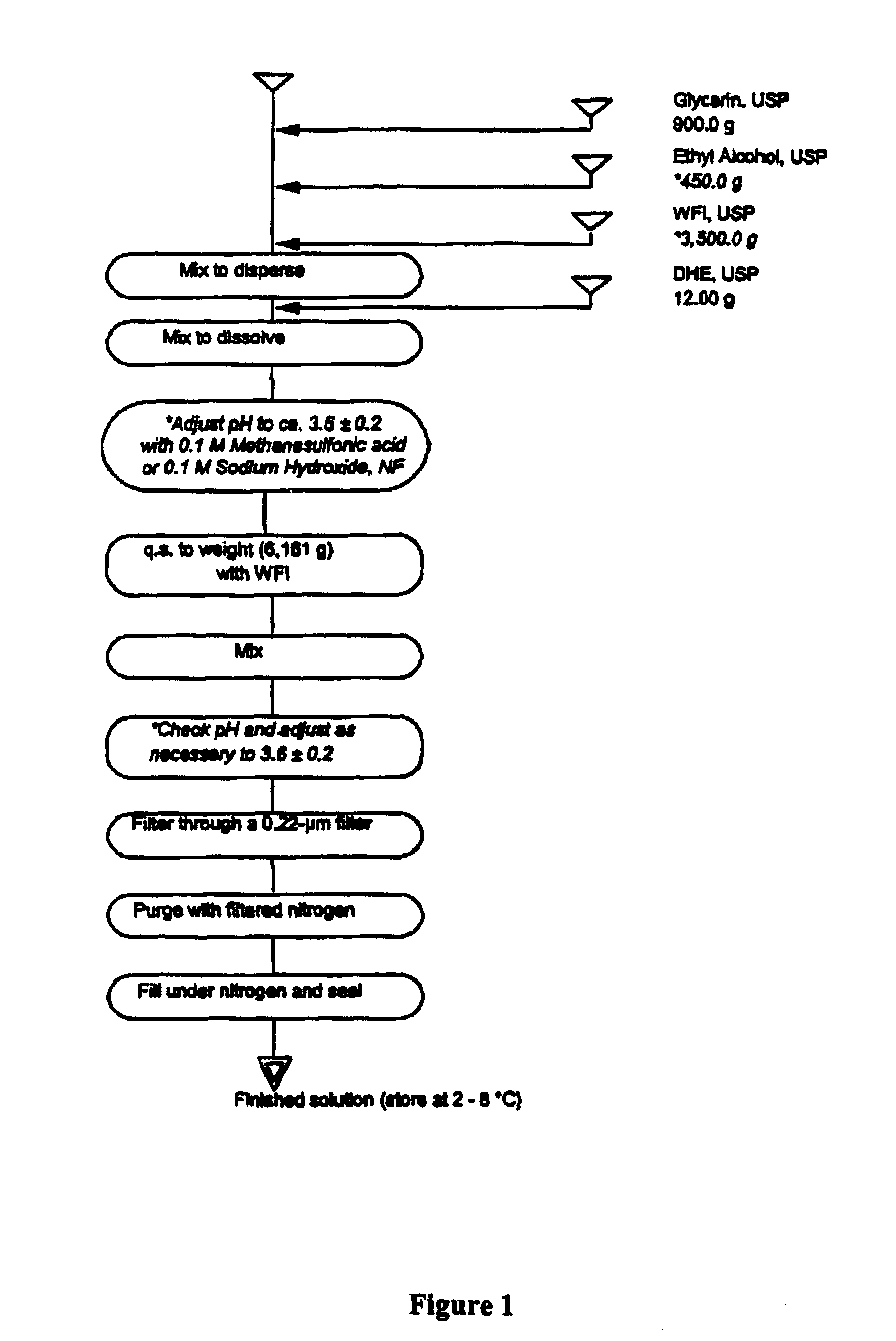
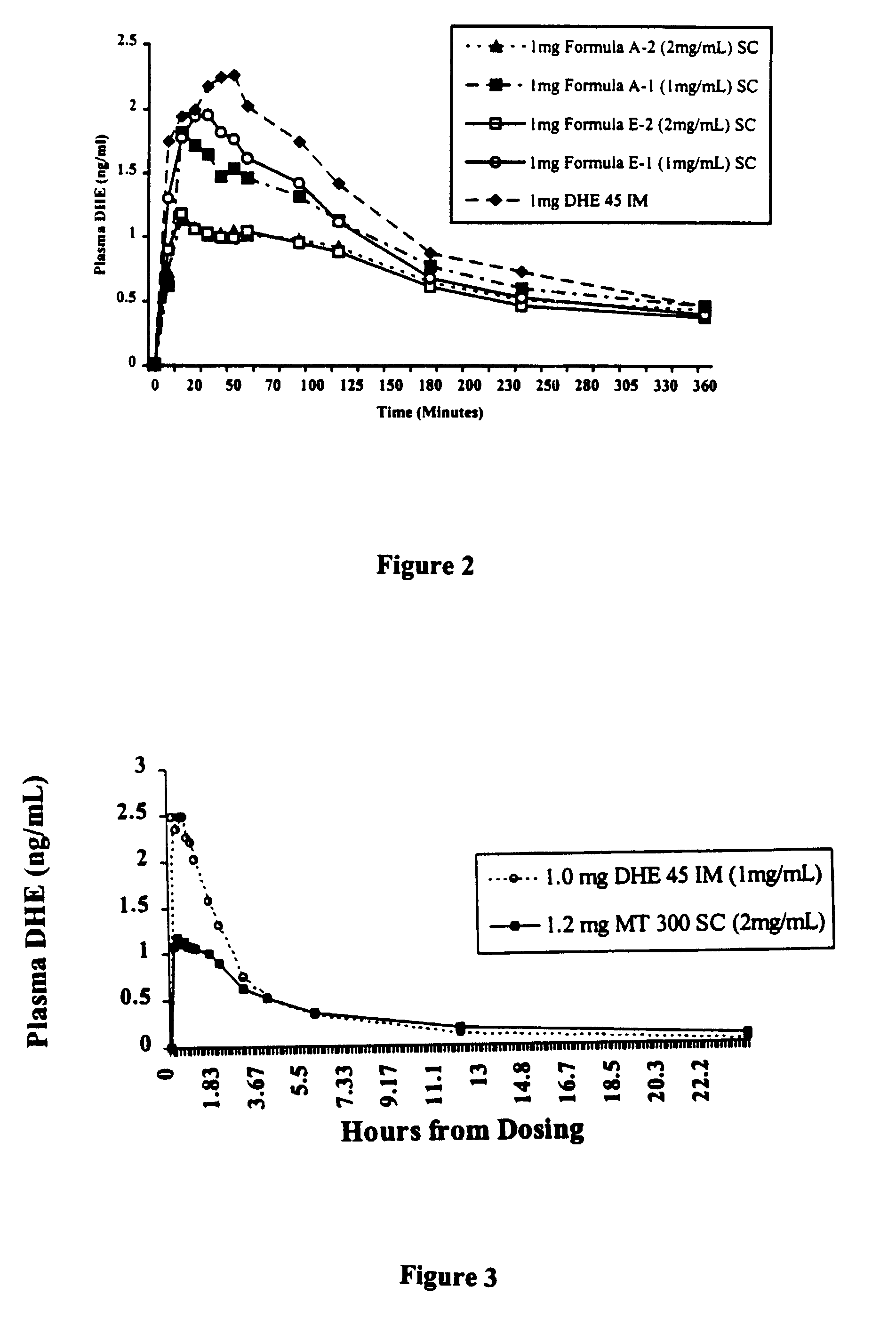
High potency dihydroergotamine compositions
InactiveUS7060694B2Improvement of side effectsEliminate side effectsBiocideNervous disorderDihydroergotamineHeadache severe
The present invention is directed to improved formulations for dihydroergotamine in which the drug is present at a concentration of at least 2.9 mM. The invention encompasses methods for using these formulations in treating patients for migraine headaches and the packaging of formulation into prefilled syringes for self-administration by patients.
Owner:POZEN INC
Drug solution preparing kit
InactiveUS20090326506A1Easy to handleReduce pressureDiagnosticsSurgeryDrugs solutionAerosol dispersion
To provide a drug solution preparing kit which can be handled with ease in a substantially aseptic manner, and has no risk that a leakage of a drug solution such as a splash and a dispersion of an aerosol to an ambient environment occurs upon preparation of the drug solution There is provided a drug solution preparing kit including a pre-filled syringe and a transfusing tool, wherein the pre-filled syringe includes a sealing member which seals the tip end and can not be removed from the tip end, and that the transfusing tool includes a one-way valve which can discharge only gas from the system in an irreversible manner, and a filter which is provided so as to adjoin to the second communication channel with respect to the one-way valve.
Owner:NIPRO CORP
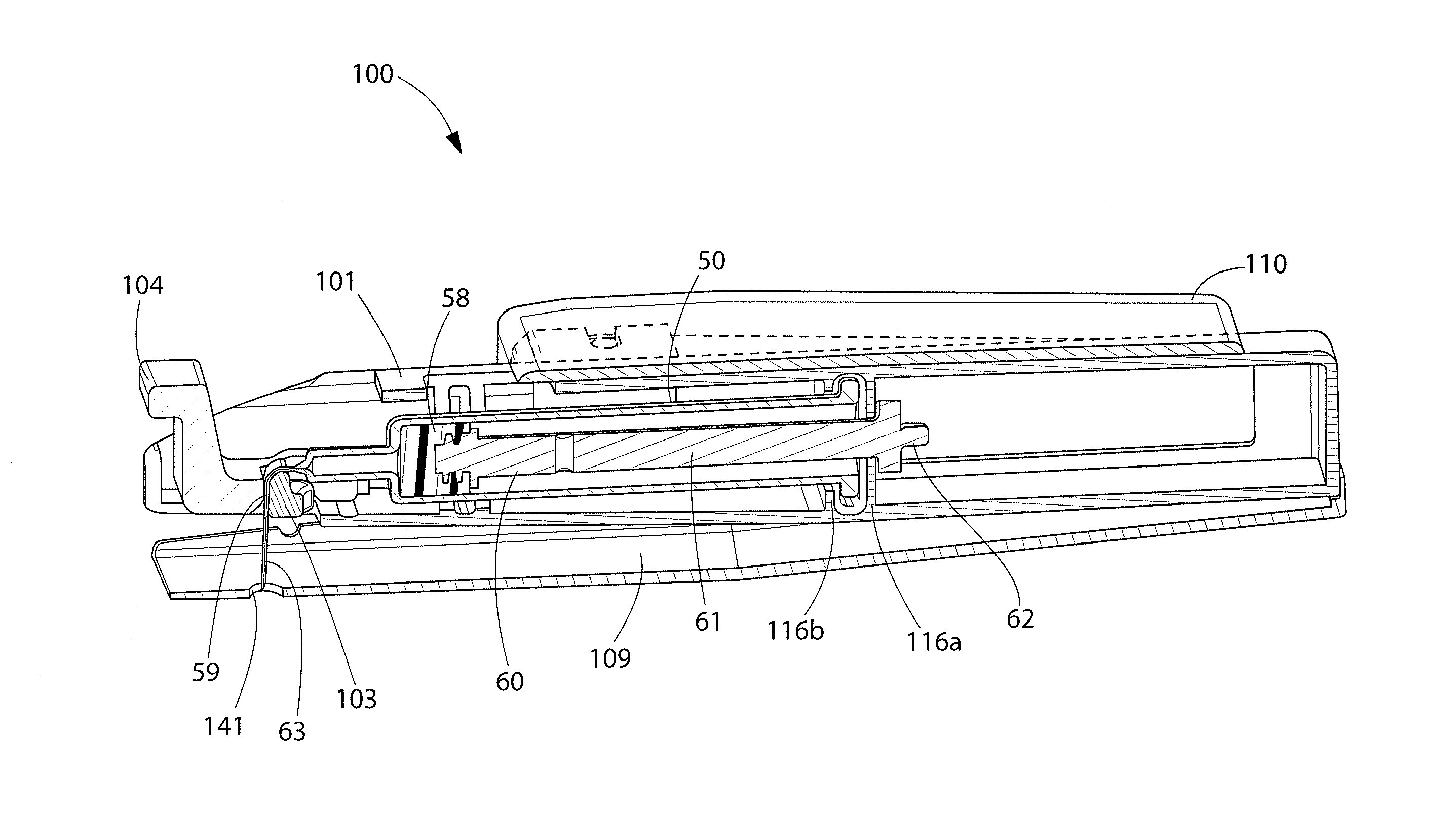
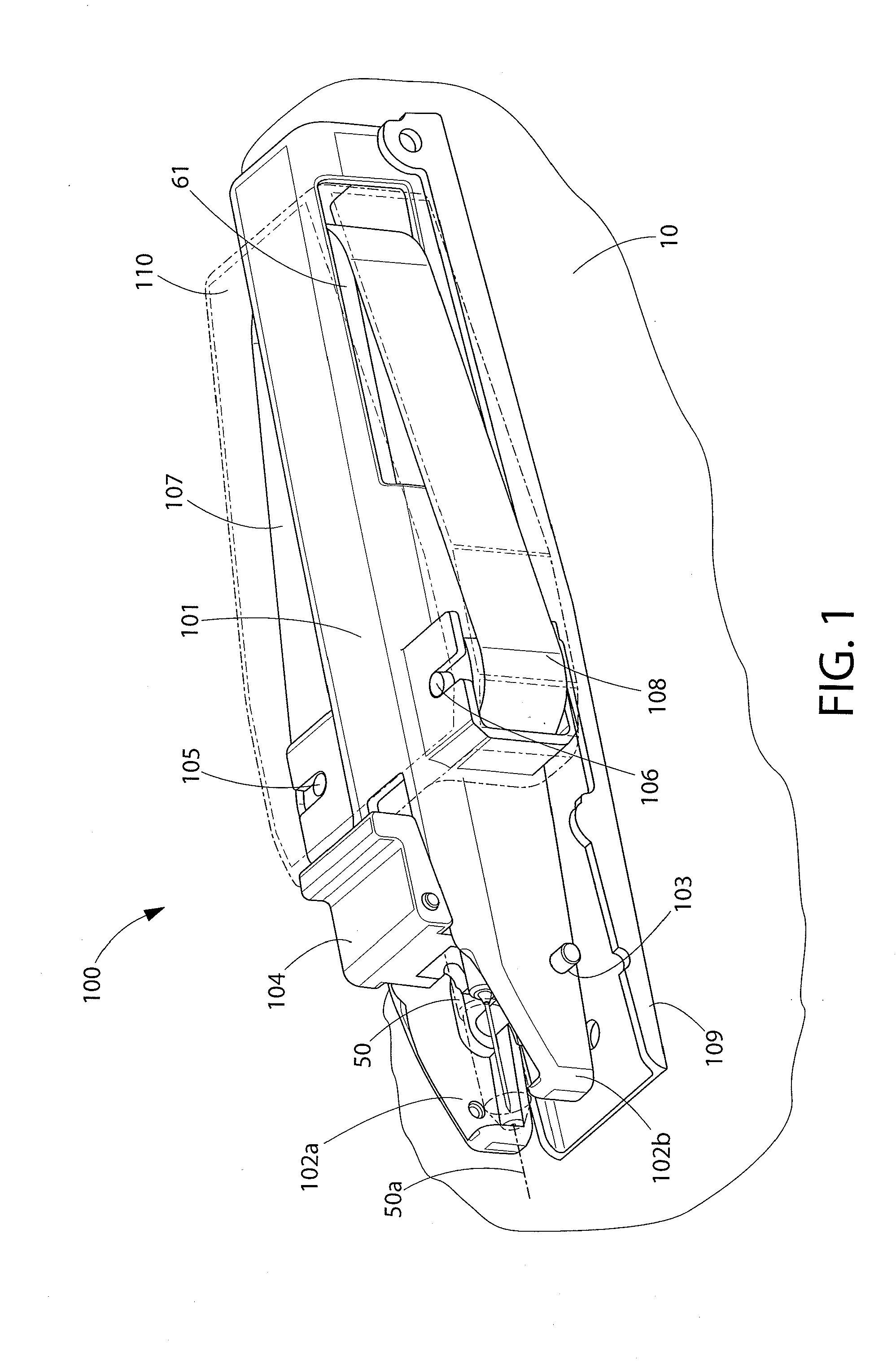
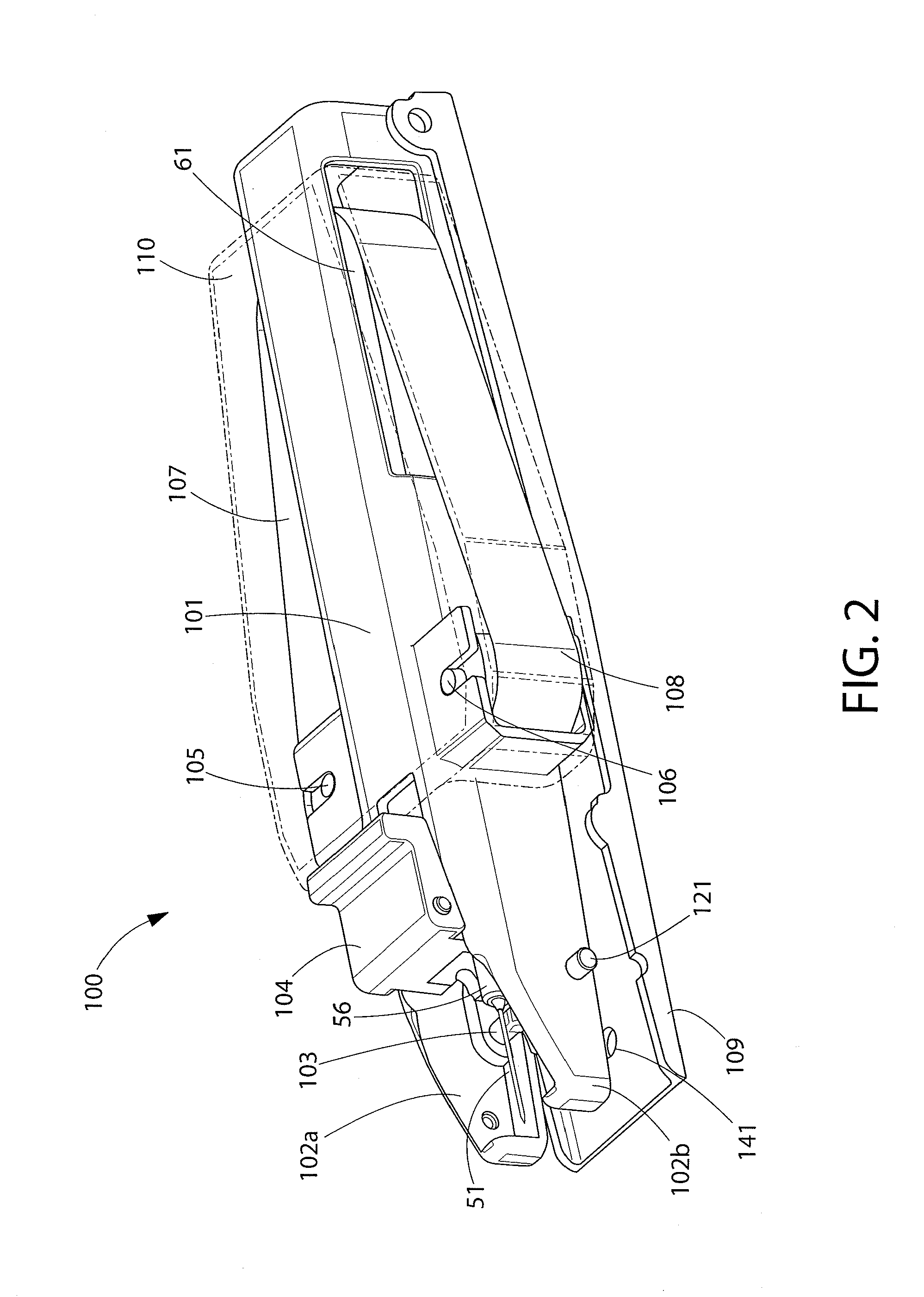
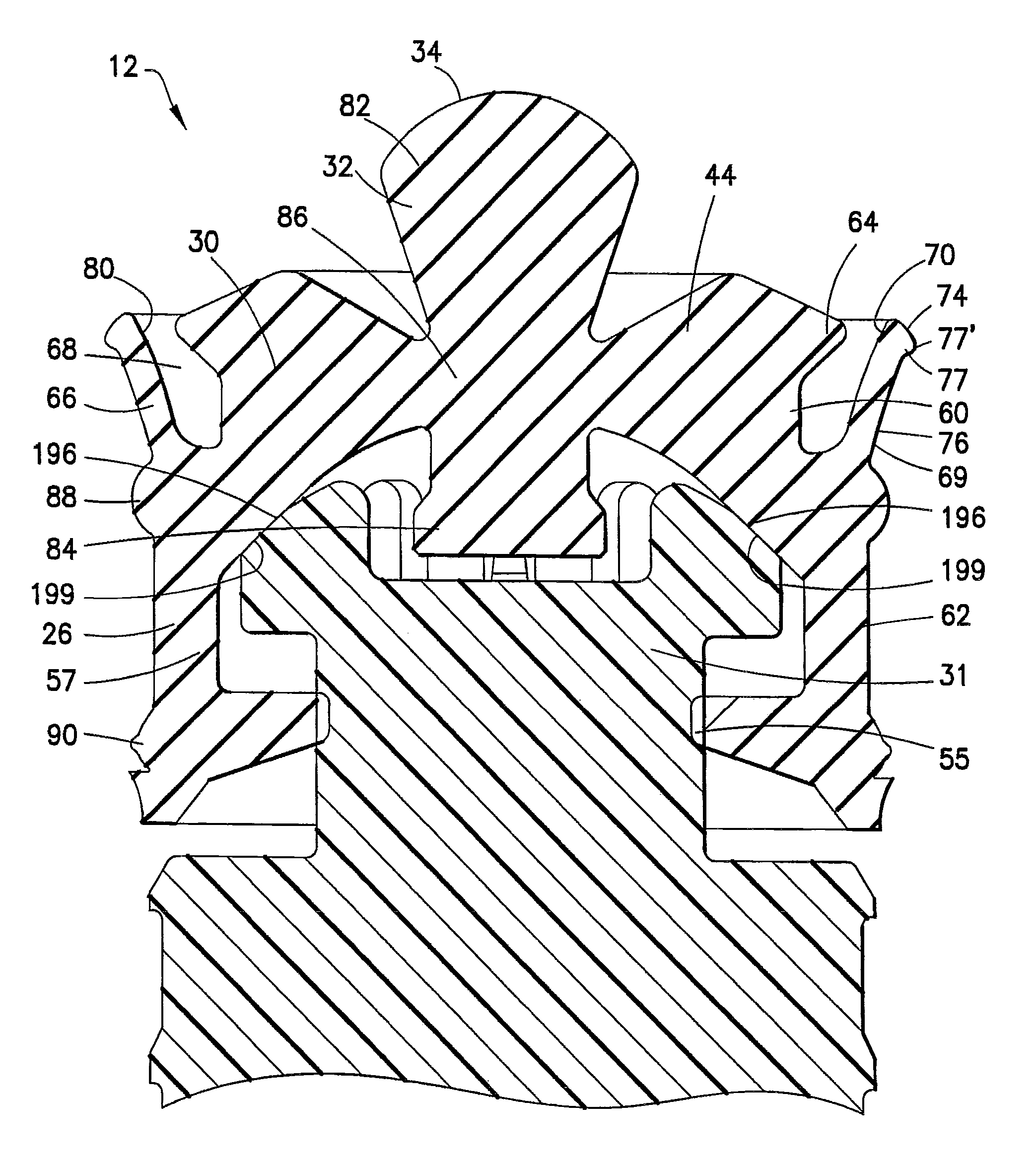
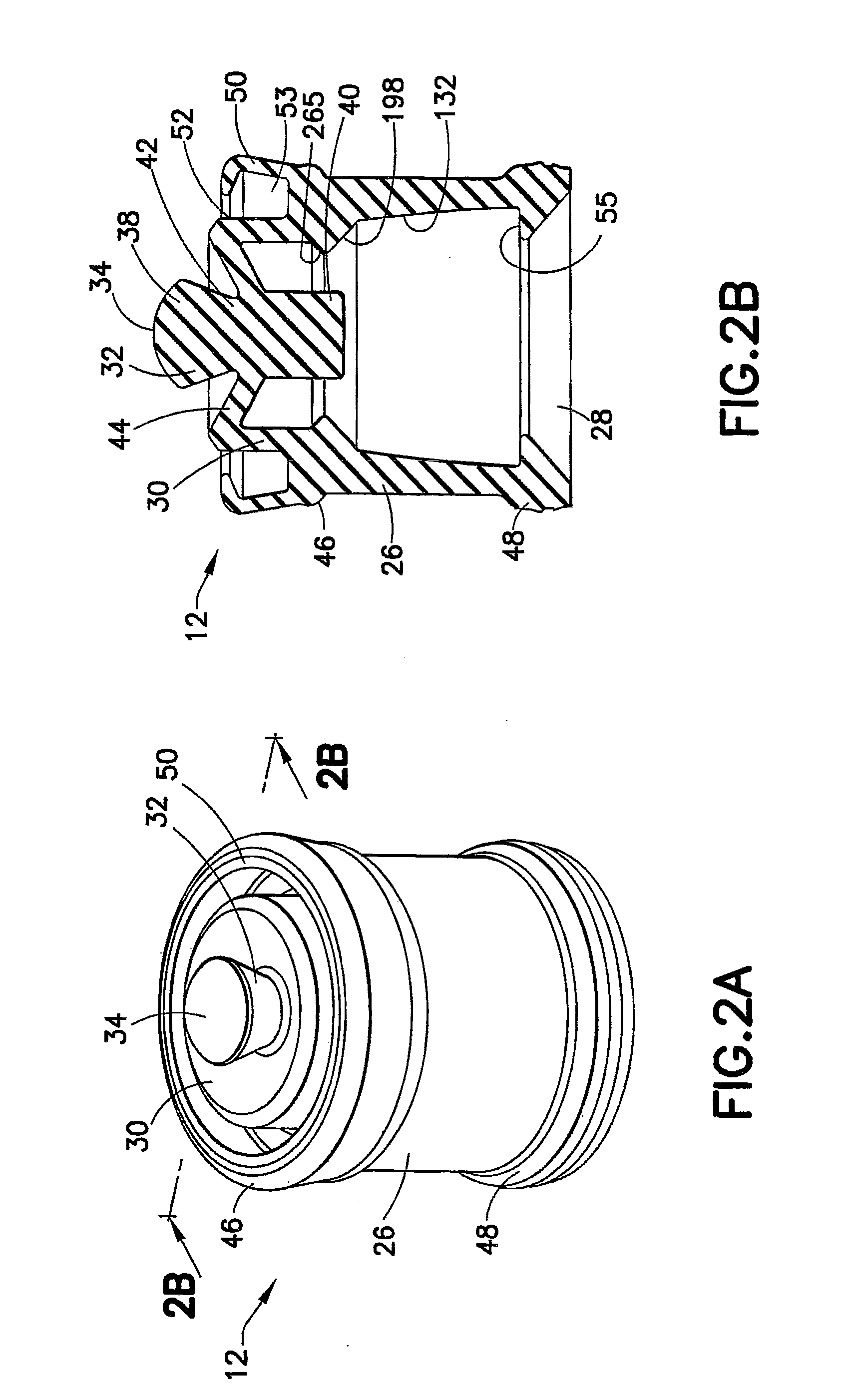
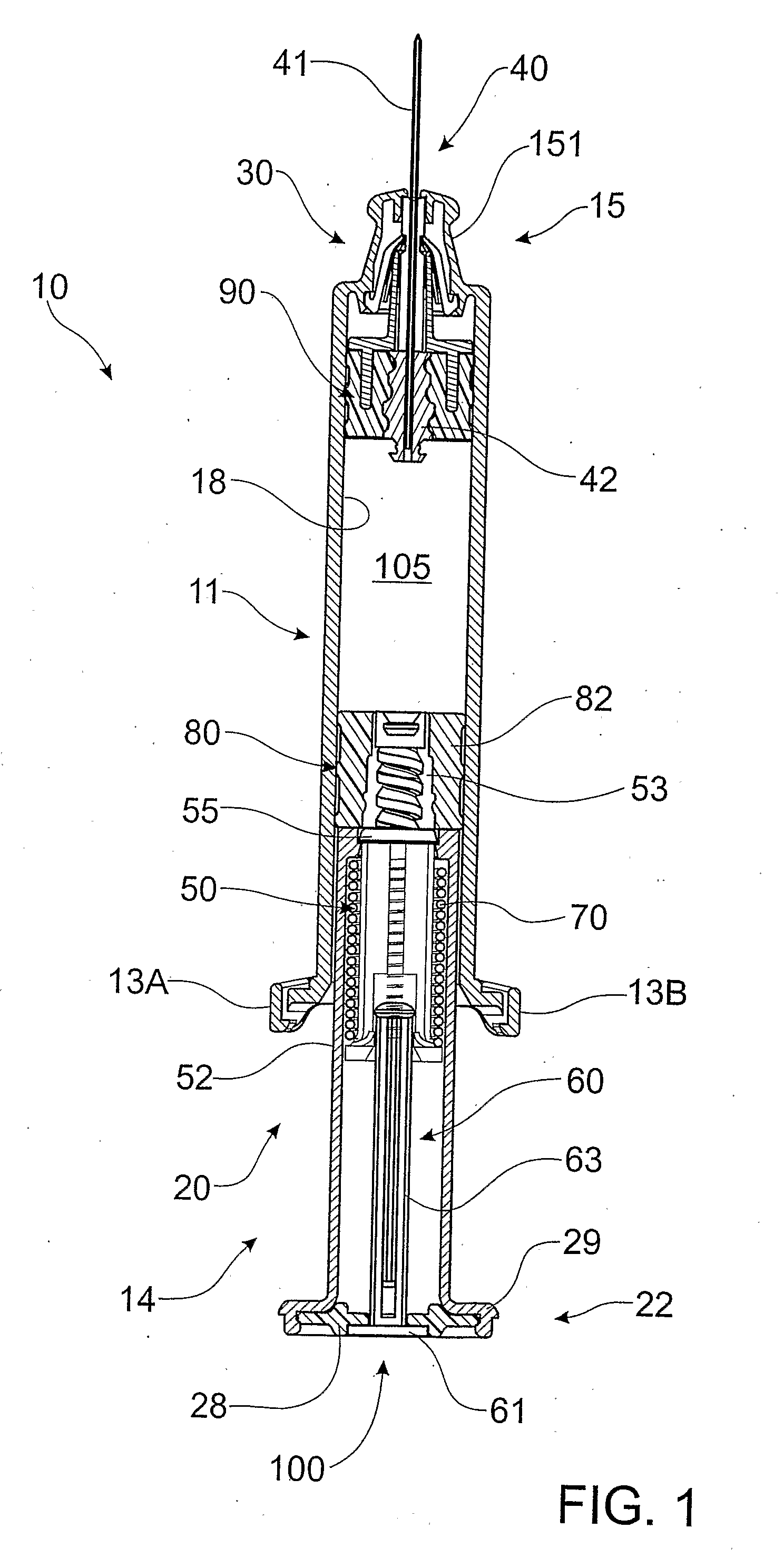
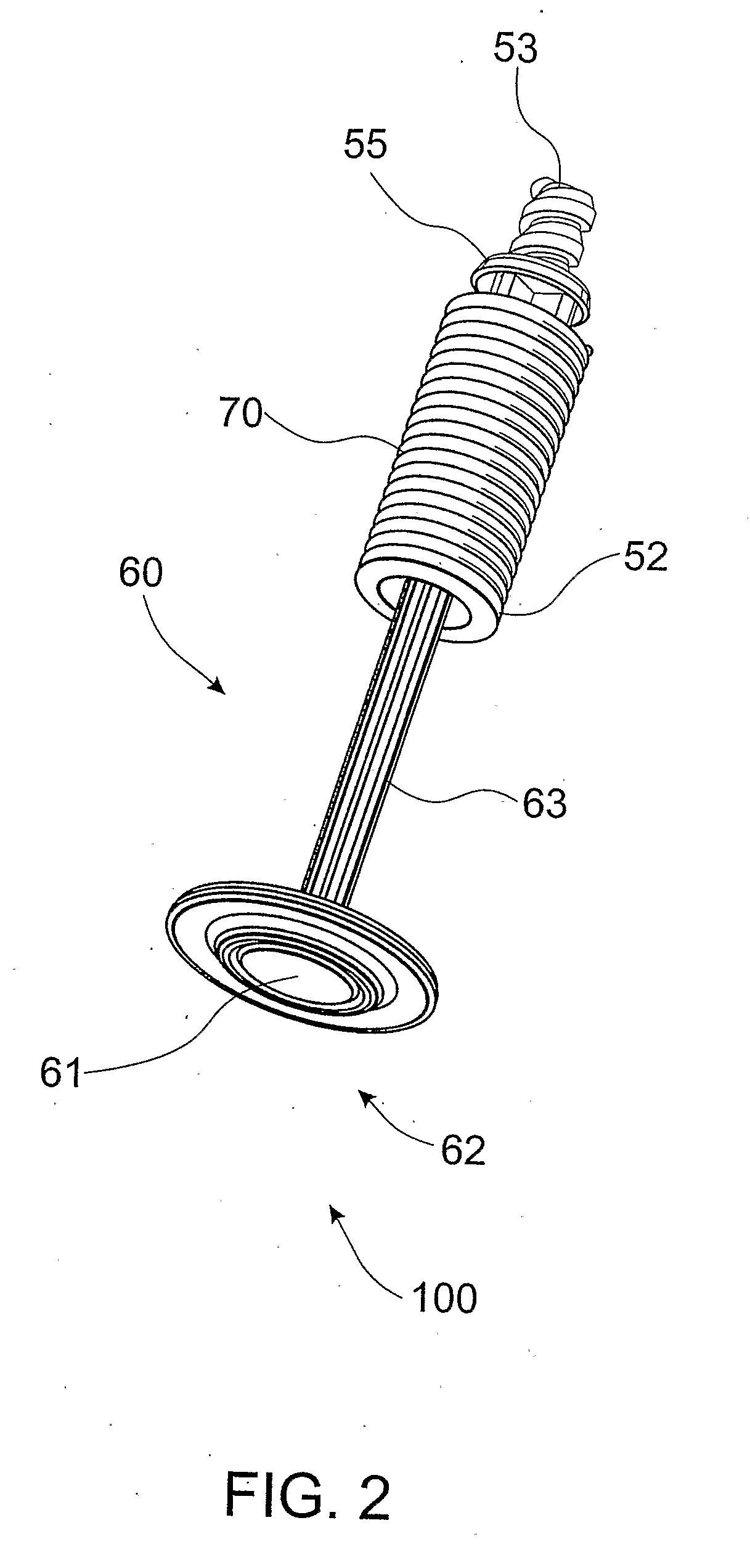
Positive displacement stopper for a pre-filled syringe
ActiveUS20080300550A1Prevent leakageEffectively and consistently reducing and eliminating refluxInfusion syringesIntravenous devicesPrefilled SyringeNose
A stopper adapted for attachment with a plunger rod for use within a syringe barrel is disclosed. The stopper includes a main body defining an open rearward end and a closed front end. The open rearward end is adapted to receive a front forward end attachment portion of the plunger rod. The stopper also includes a core member integrally formed with said main body adjacent the closed front end. The core member includes a nose portion having a profile adapted to create a positive seal with an outlet opening of such syringe barrel.
Owner:BECTON DICKINSON & CO
Pre-filled syringe including an oxygen absorber
ActiveUS20120143144A1Improve easeImprove effectivenessAmpoule syringesAutomatic syringesAutoinjectorOxygen
The invention provides a syringe or autoinjector for dispensing a drug comprising a rigid syringe body containing the drug, an oxygen absorbing material, a separating element between the drug and oxygen absorbing material to prevent the drug from contacting the oxygen absorbing material but which allows oxygen to pass from the drug to the oxygen absorbing material, and an oxygen impermeable container enclosing both the drug and the oxygen absorbing material, wherein the oxygen impermeable container partially or fully forms the rigid syringe body or is held within the rigid syringe body.
Owner:OVAL MEDICAL TECH
Lockable safety shield assembly for a prefillable syringe
InactiveUS6193696B1Easy to assemblePromote activationInfusion syringesMedical devicesEngineeringPrefilled Syringe
A lockable safety shield assembly for a prefillable syringe is provided. The design of the lockable safety shield assembly enhances pharmaceutical manufacturers' ease of assembling the various components as part of its filling or processing of the prefillable syringes in normal practice, while at the same time minimizes difficulties in mating parts made from different materials. A tube is placed around the outside surface of the syringe barrel and affixed thereto. A collar is provided on the tube adjacent the distal end of the syringe barrel. A safety shield is axially slidable over the tube between a retracted position, wherein the distal end of the piercing element associated with the prefillable syringe is exposed, and an extended position, wherein the safety shield is locked to the collar to protectively cover the distal end of the piercing element. The safety shield includes locking structure configured so that the shield can be easily fitted over the tube. The locking structure includes at least one deflectable arm provided on the body of the shield. The deflectable arm includes a proximal end deflectable towards the interior of the shield. A stop member is provided on the interior of a shield in spaced relation to the proximal end of the deflectable arm. A ring is axially slidable over the shield to deflect the arm towards the interior of the shield to activate the locking structure. The safety shield is slid distally by an end user such that the collar is lockingly retained between the stop member and the proximal end of the deflectable arm. The collar, the stop member, and the deflectable arm may be configured to provide tactile as well as audible indication of locking to the end user.
Owner:BECTON DICKINSON & CO
Palm-controlled injectors
InactiveUS20120253314A1Easy to carryReduce the possibilityAutomatic syringesMedical devicesPrefilled SyringeBiomedical engineering
A palm-controlled device is disclosed for injection of a substance into an organism. The device includes a palm-receiving surface for receiving a palm of a hand, the palm receiving surface being shaped so that the palm is substantially parallel to a surface of an injection site of the organism while operating the device. The palm receiving surface can also be shaped so that the palm is substantially perpendicular to a surface of the injection site. The device also includes an injector having at least one pre-filled syringe, the palm-receiving surface being cooperative with the injector such that when pressure is applied to the palm-receiving surface, the injector is actuated so as to inject contents of the at least one pre-filled syringe. Actuation of the injector is by gradual pressure; not sudden motion. Single or multiple doses of same or different medications can be included in a single injector.
Owner:HARISH ZIV
Automatic injection and retraction devices for use with pre-filled syringe cartridges
An automatic injection and retraction device having a longitudinal axis is provided that includes an injection assembly, a retraction assembly, and a pre-filled syringe cartridge. The injection assembly has an activation-prevention feature moveable between an on position and an off position. The retraction assembly has a needle guard that is removable in a direction along the longitudinal axis upon application of a removal force. The pre-filled syringe cartridge has a hypodermic needle with a needle sheath thereon. The retraction and injection assemblies are secured to one another so that the pre-filled syringe cartridge is in the retraction assembly with the needle sheath secured to the needle guard. The retraction and injection assemblies are configured so that, upon application of a twisting torque to the injection and retraction assemblies, the activation-prevention feature moves from the on position to the off position simultaneous with applying the removal force to the needle guard.
Owner:WEST PHARMA SERVICES OF DELAWARE
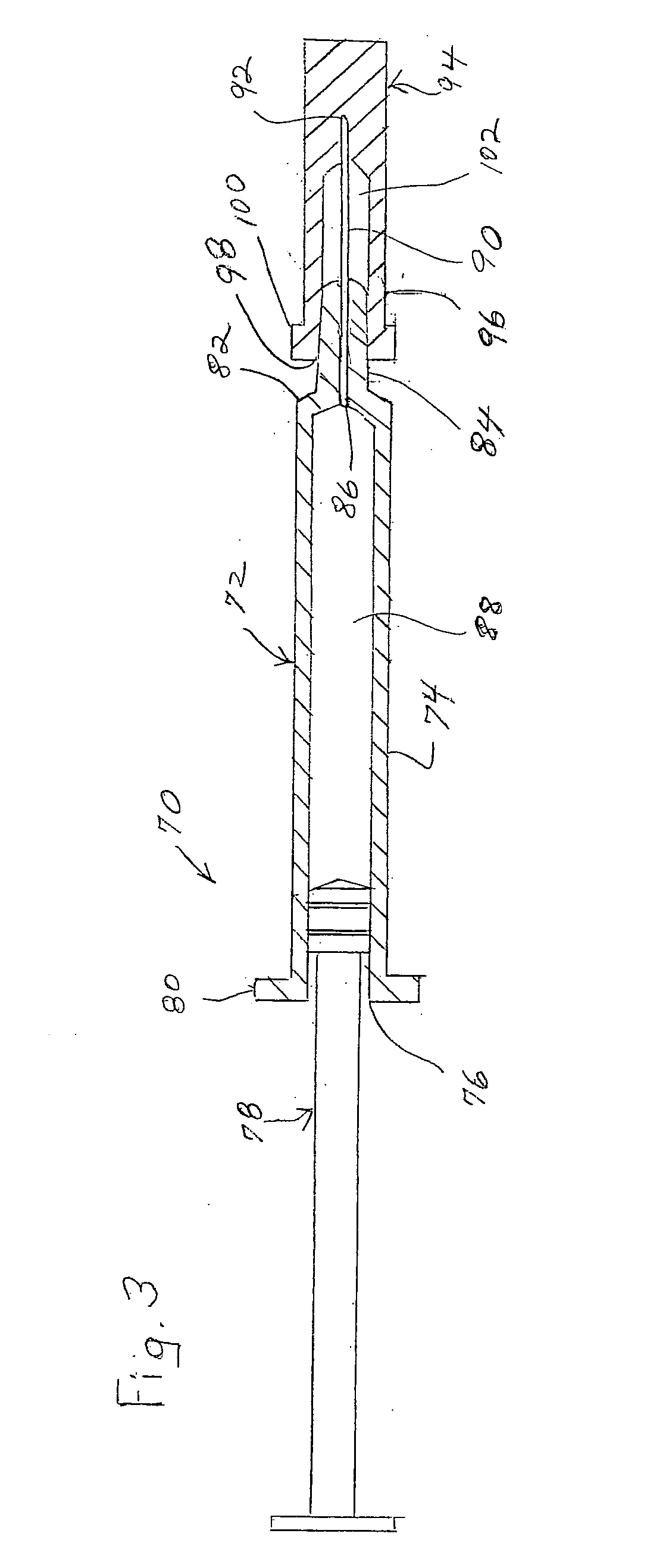
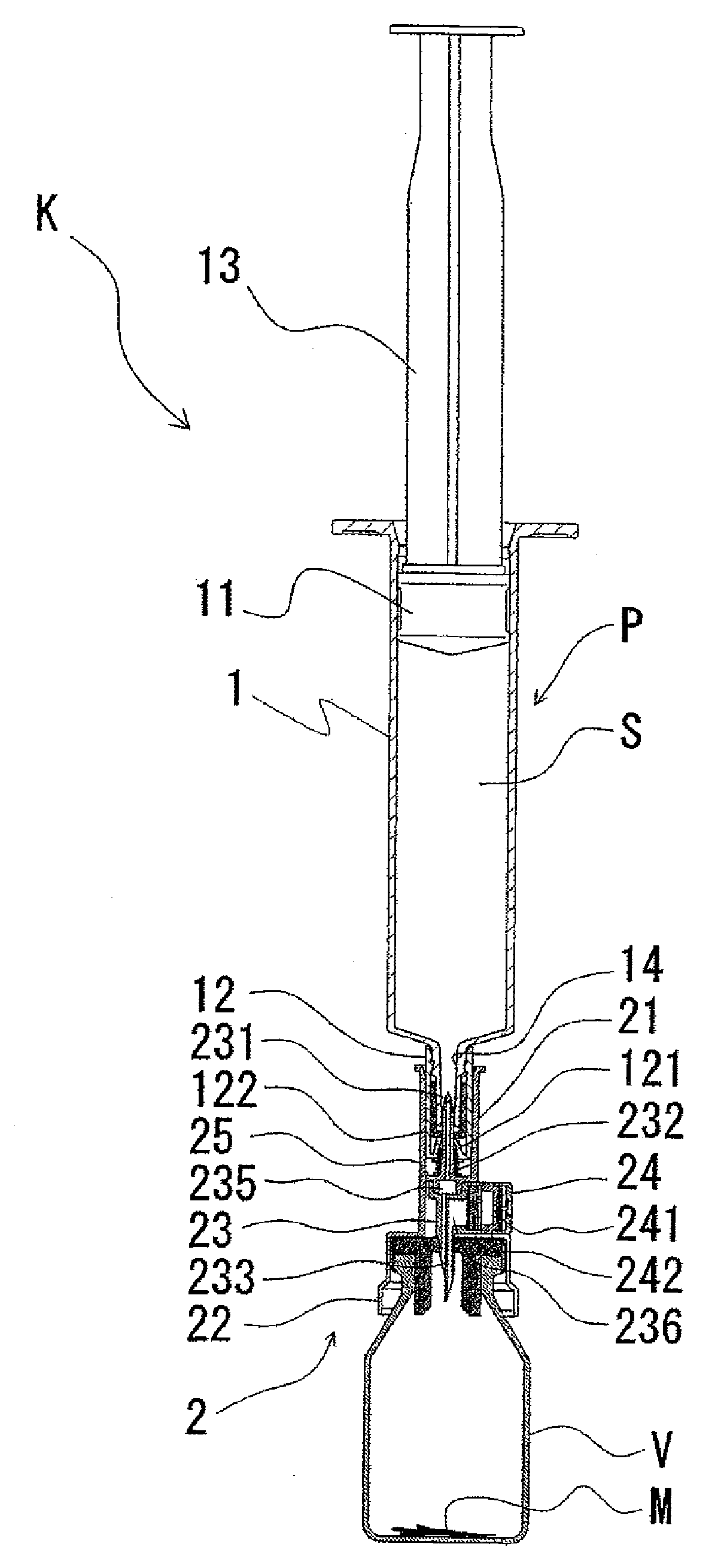
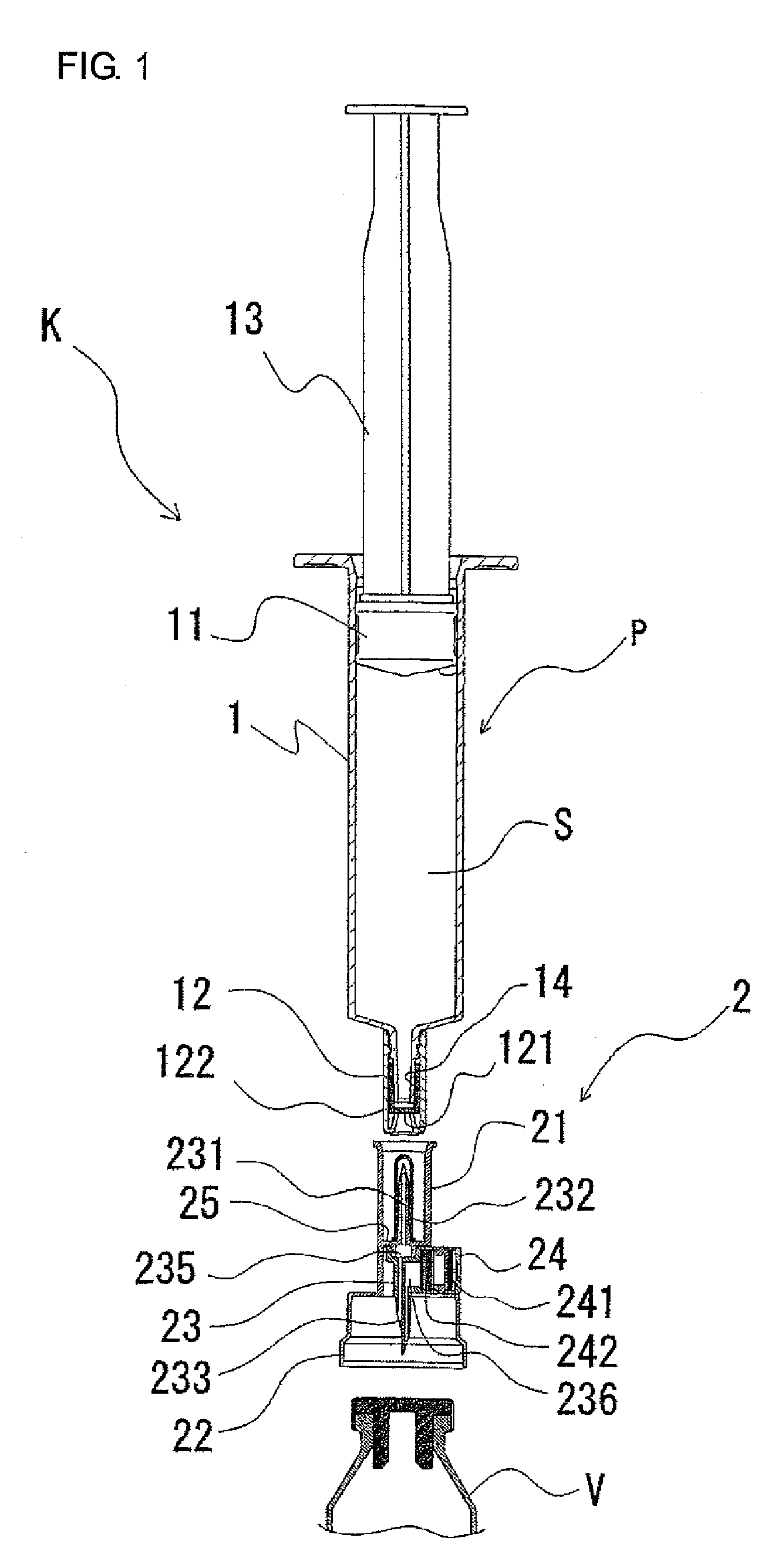
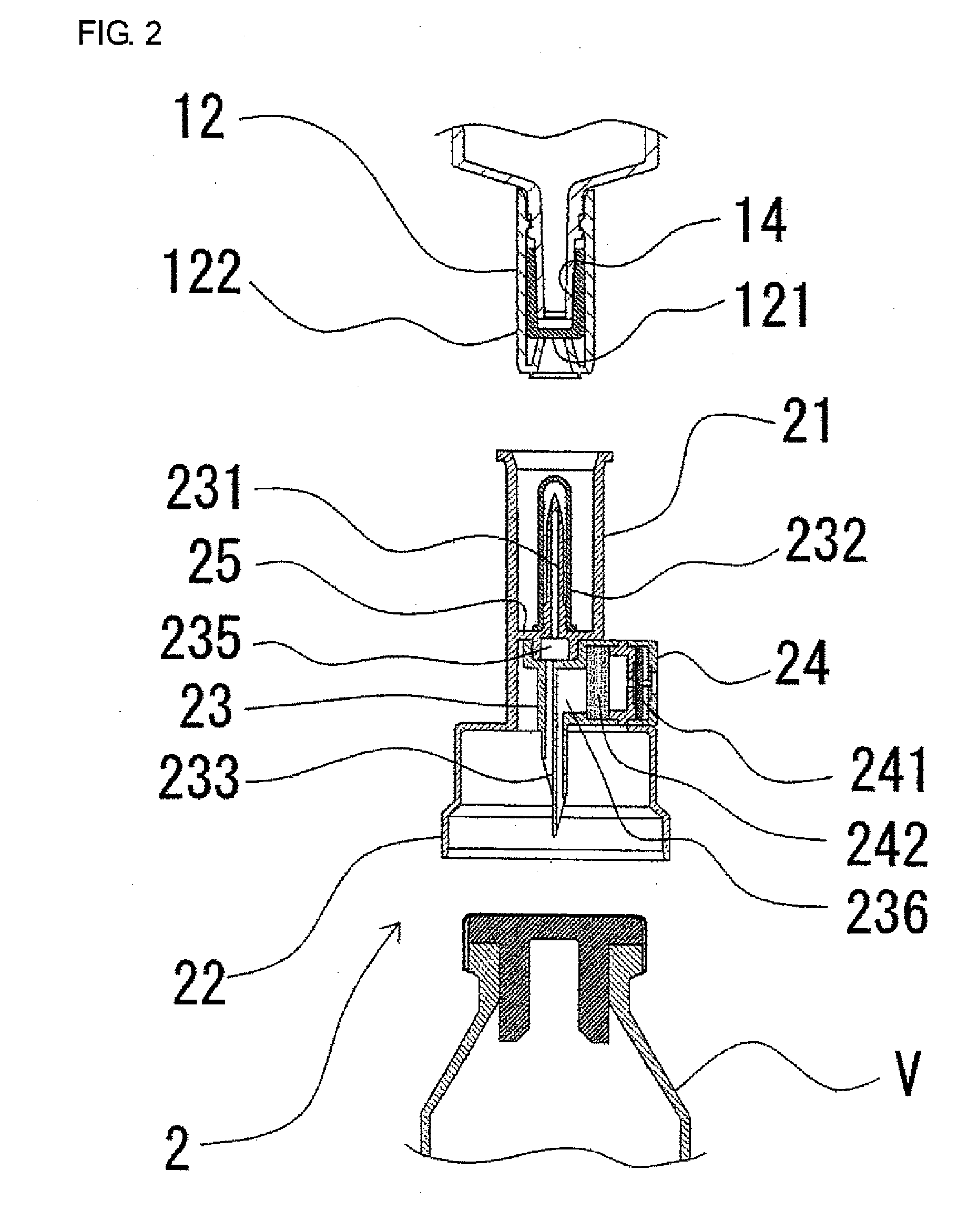
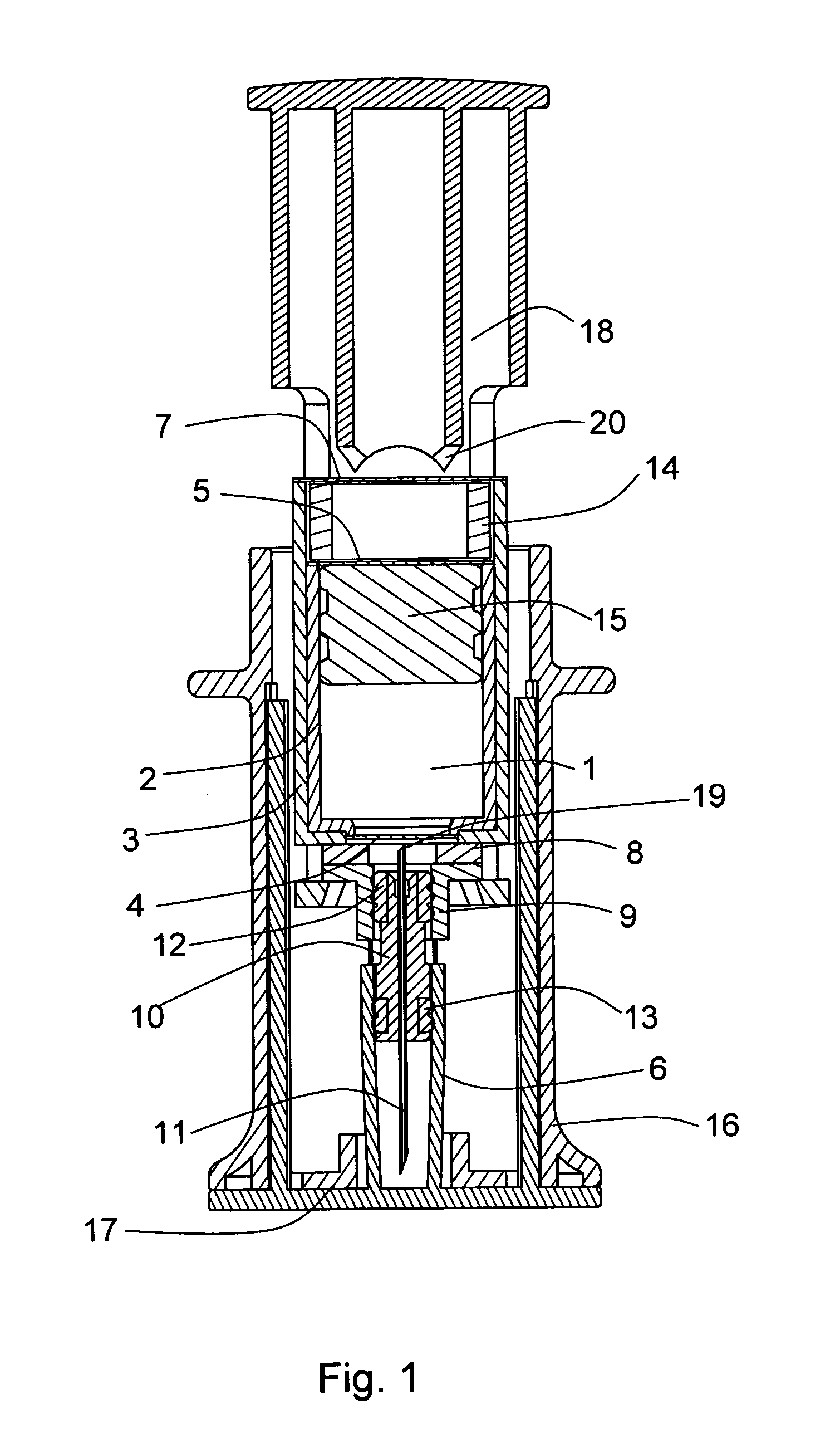
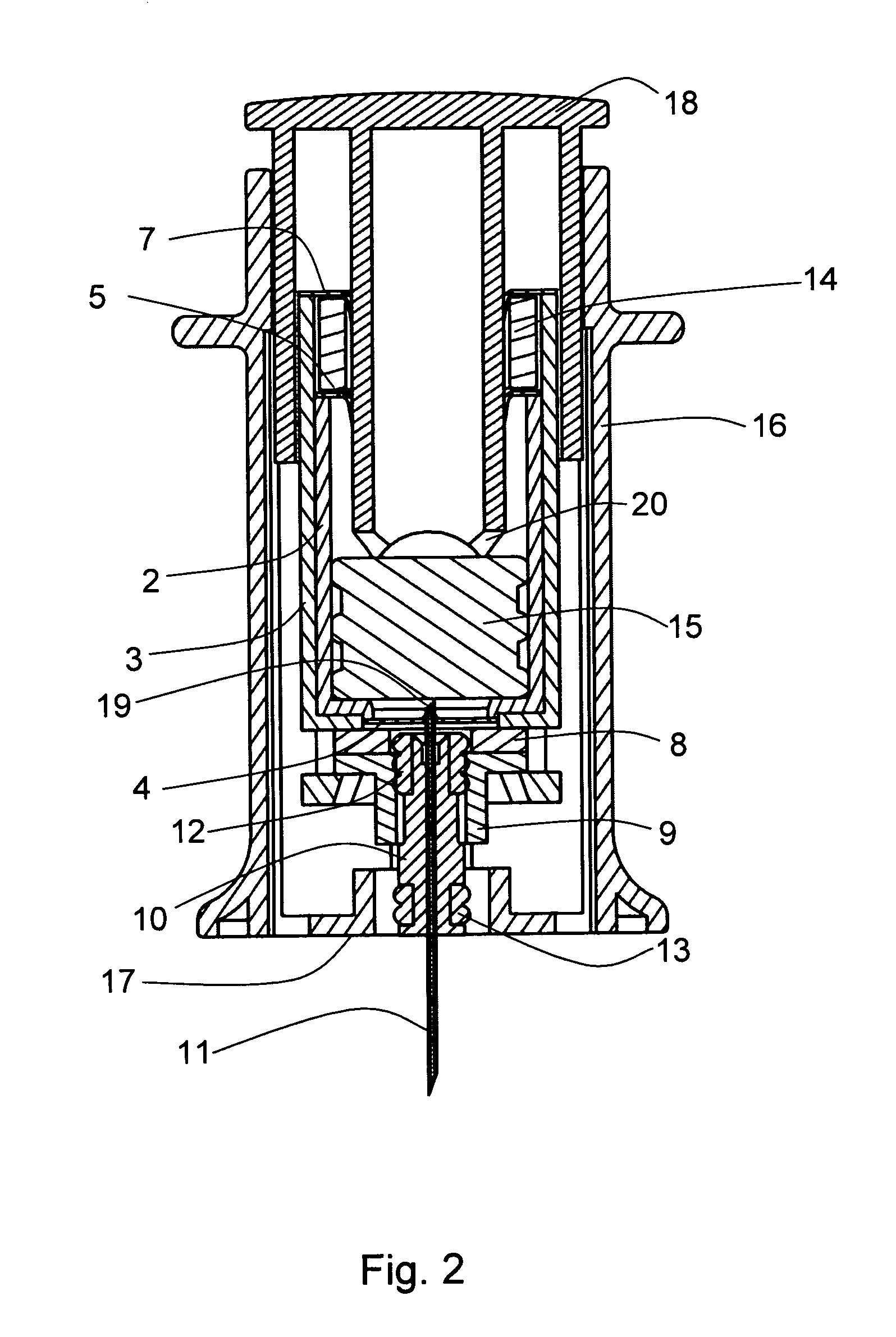
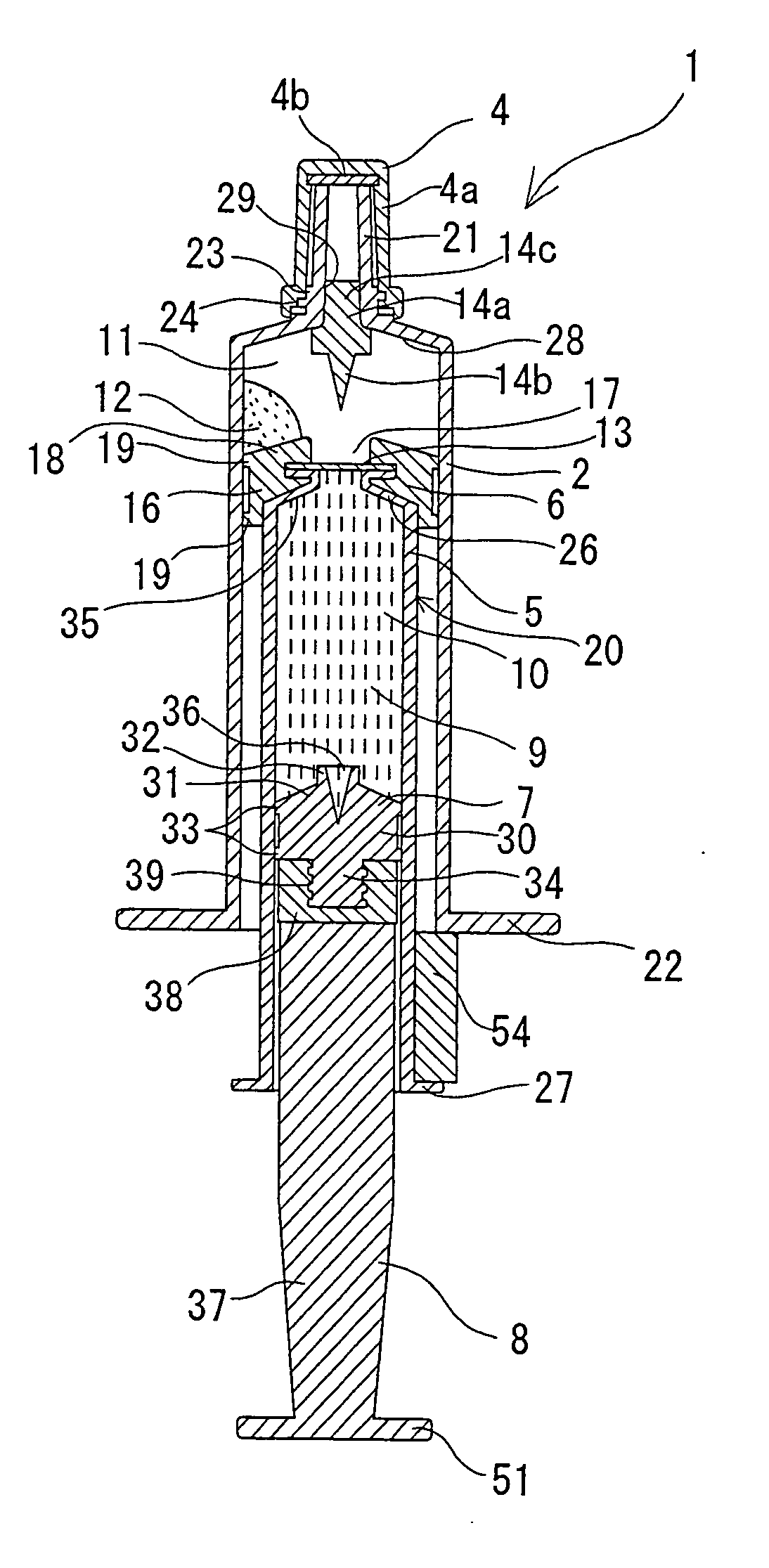
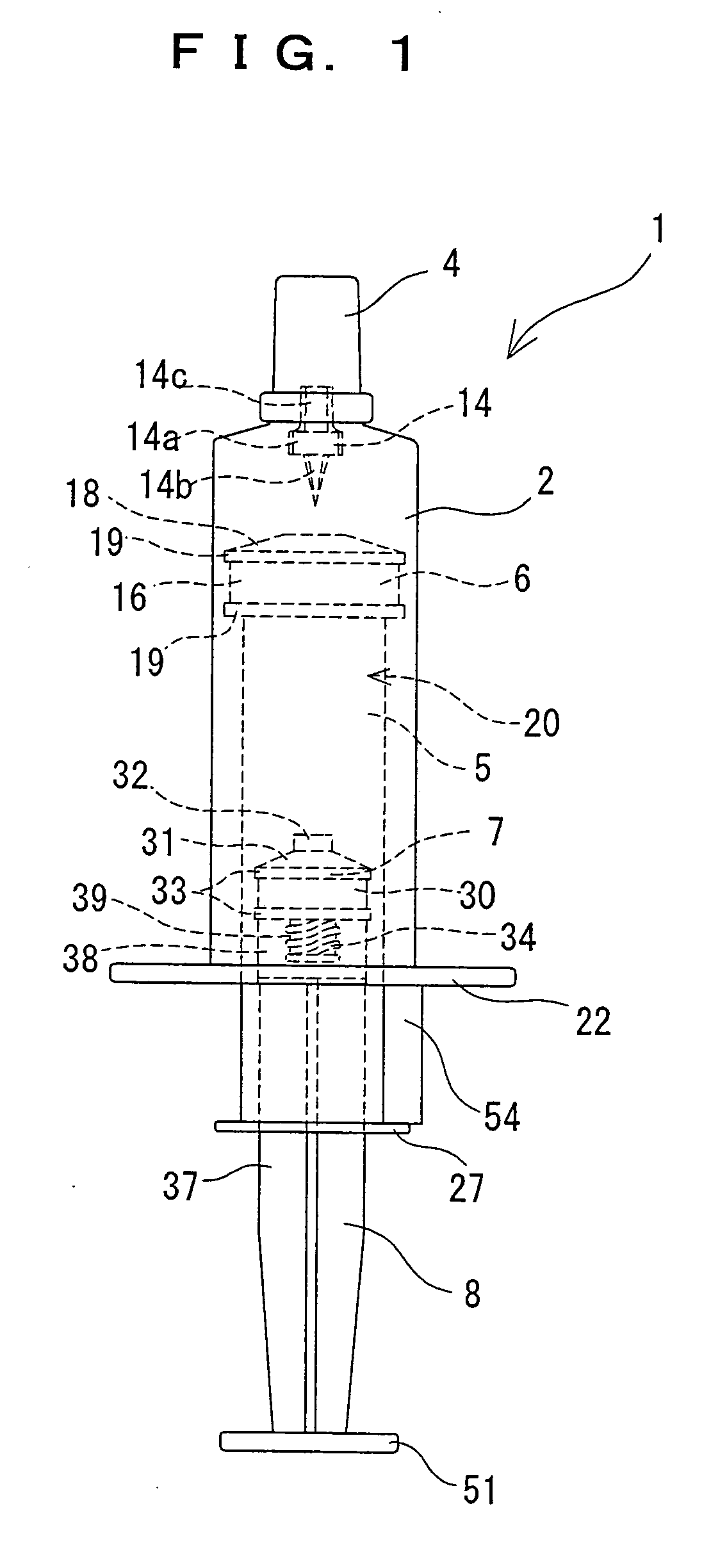
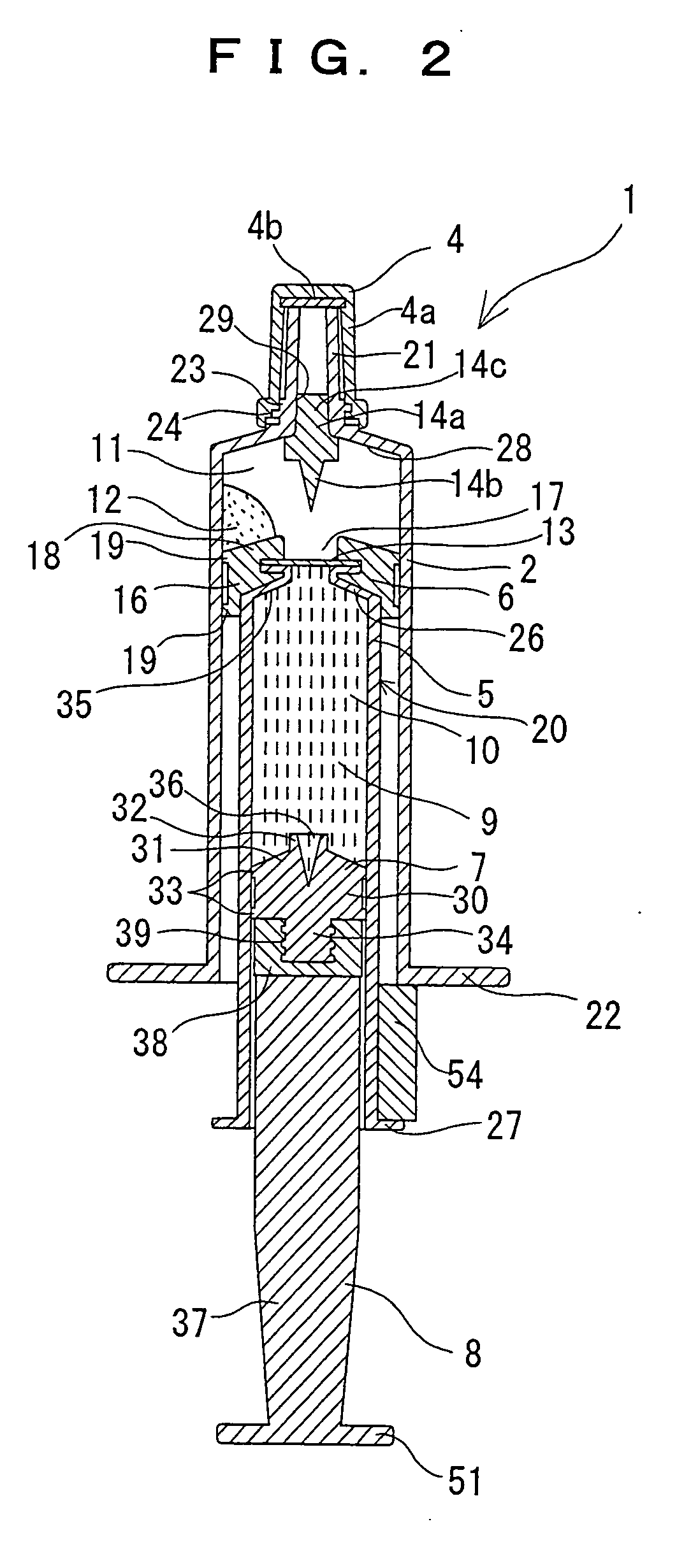
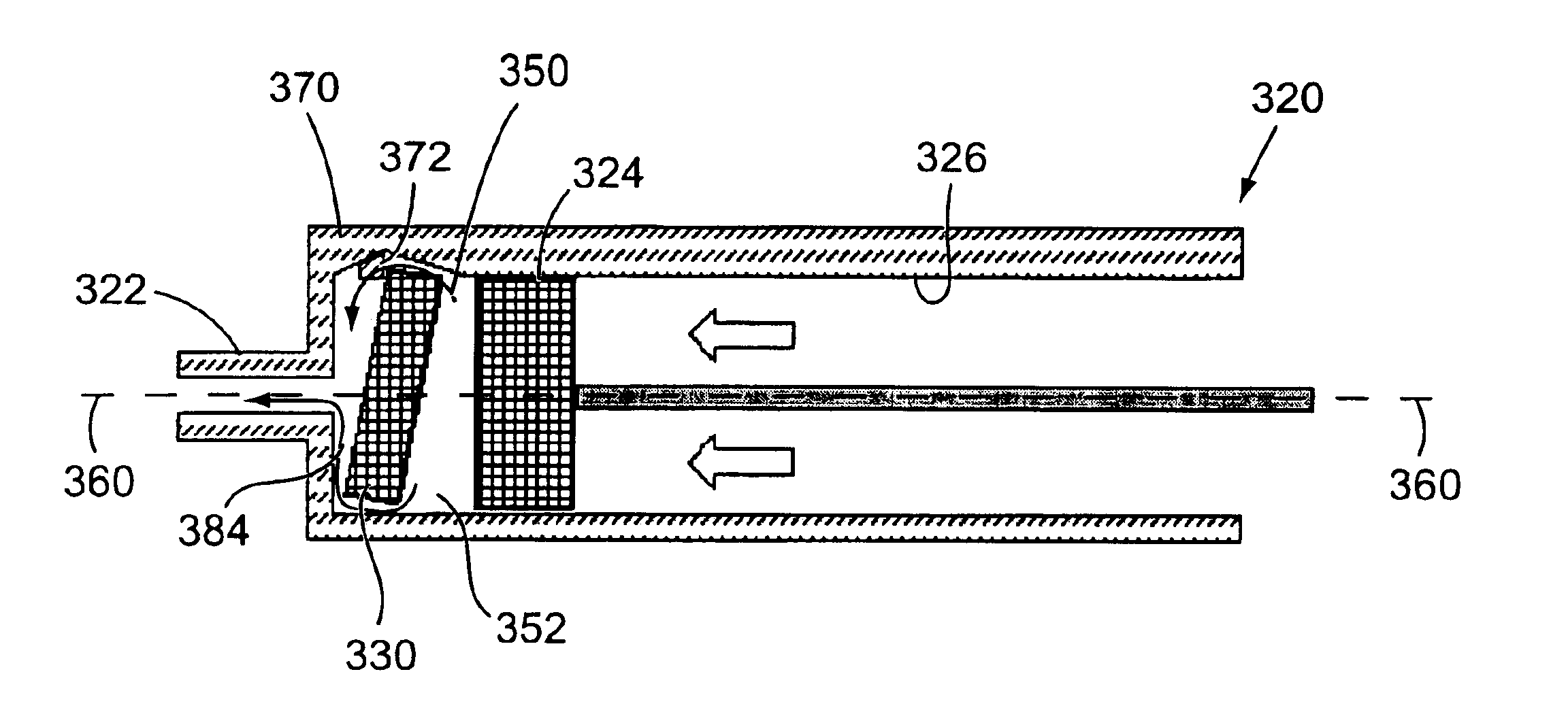
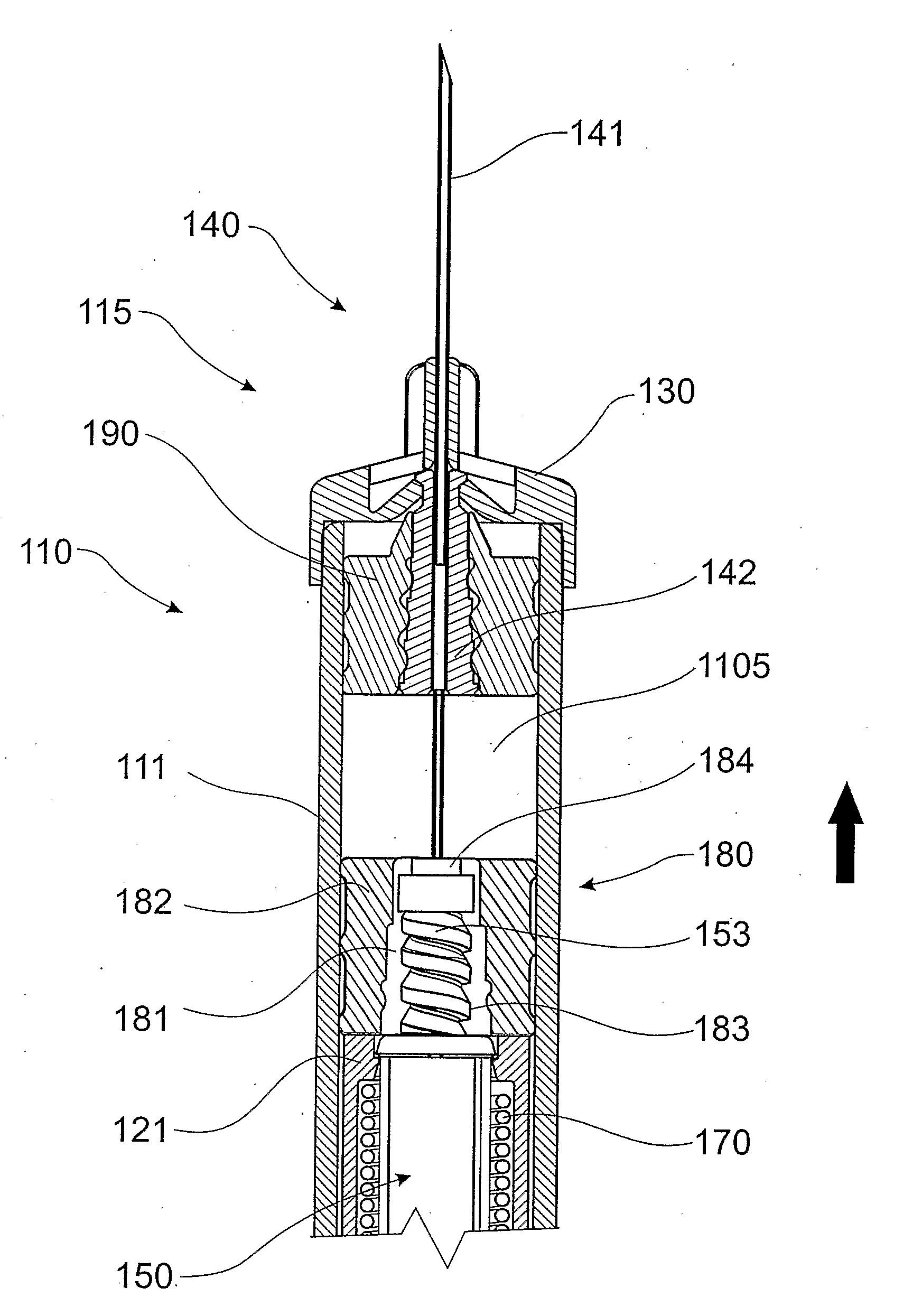
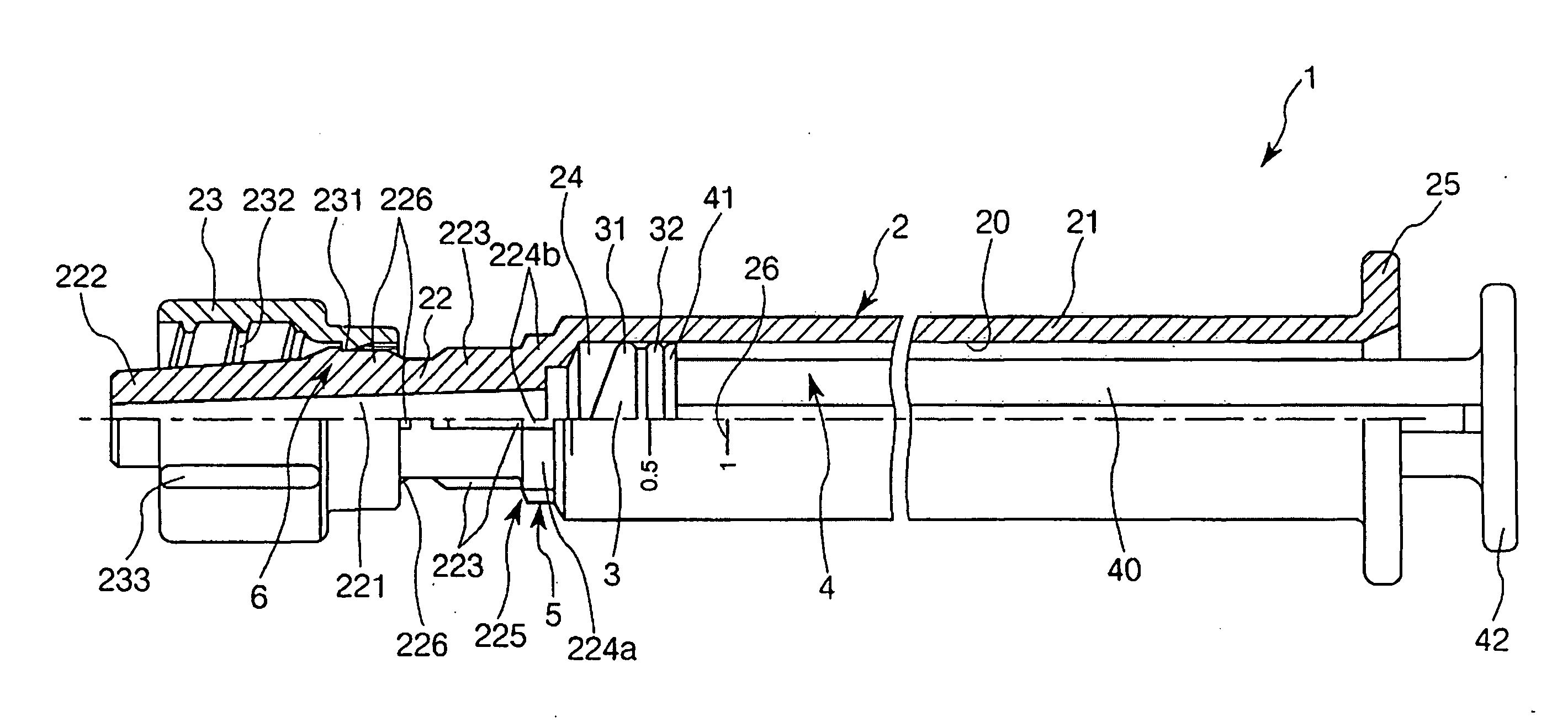
Two-chamber pre-filled syringe
A two-chamber type pre-filled syringe (1) includes an outer cylinder (2) having a projected portion (14b) for breaking use provided inside the outer cylinder (2); an inner cylinder assemblage (20) having an inner cylinder (5), an annular first gasket (6) provided in the vicinity of a front-end portion of the inner cylinder (5), and a sealing member (13) which airtightly seals a front-end portion of the inner cylinder (5) and can be broken by the projected portion (14b) for breaking use; a second gasket (7) accommodated inside the inner cylinder (5); a plunger (8) mounted at a rear-end portion of the second gasket (7); a first accommodation portion (9); a second accommodation portion (11); a medicine-dissolving liquid (10) accommodated in the first accommodation portion (9); and a powdery or frozen dry medicine (12) accommodated inside the second accommodation portion (11).
Owner:TERUMO KK
Stoppers Used in Pre-filled Syringes
ActiveUS20110034882A1Simple moldingIncrease contact pressureInfusion syringesIntravenous devicesEngineeringPrefilled Syringe
A stopper adapted for attachment with a plunger rod for use within a syringe barrel is disclosed. The stopper includes a main body defining an open rearward end and a closed front end. The open rearward end is adapted to receive a front forward end attachment portion of the plunger rod. The stopper also includes a core member integrally formed with the main body adjacent the closed front end. The core member includes a nose portion having a conical tip configured for entering an outlet opening of the syringe barrel. The closed front end of the stopper has a profile configured to cooperate with an internal surface of the syringe barrel wall to prevent reflux and reduce dead space within the barrel.
Owner:BECTON DICKINSON & CO
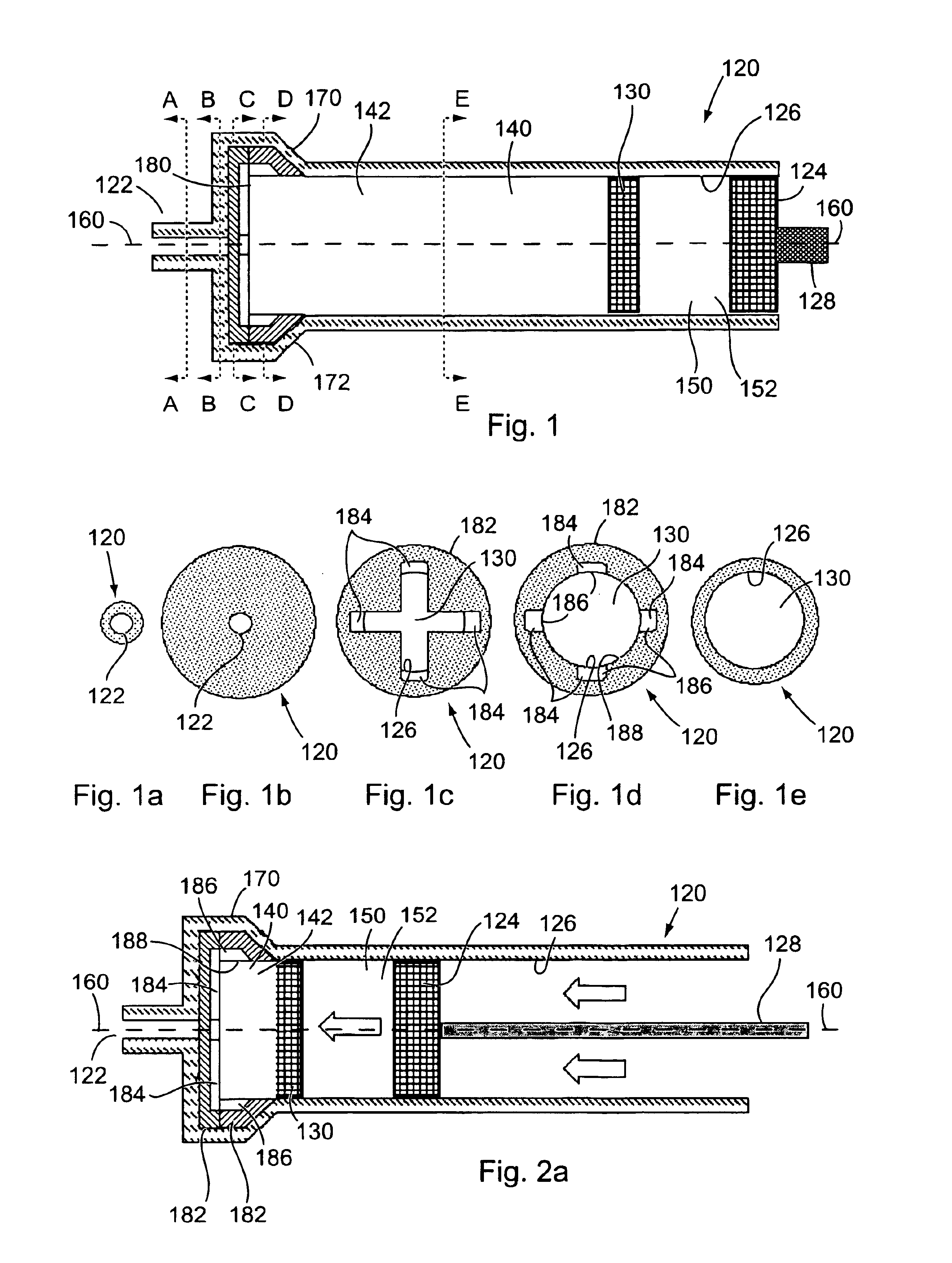
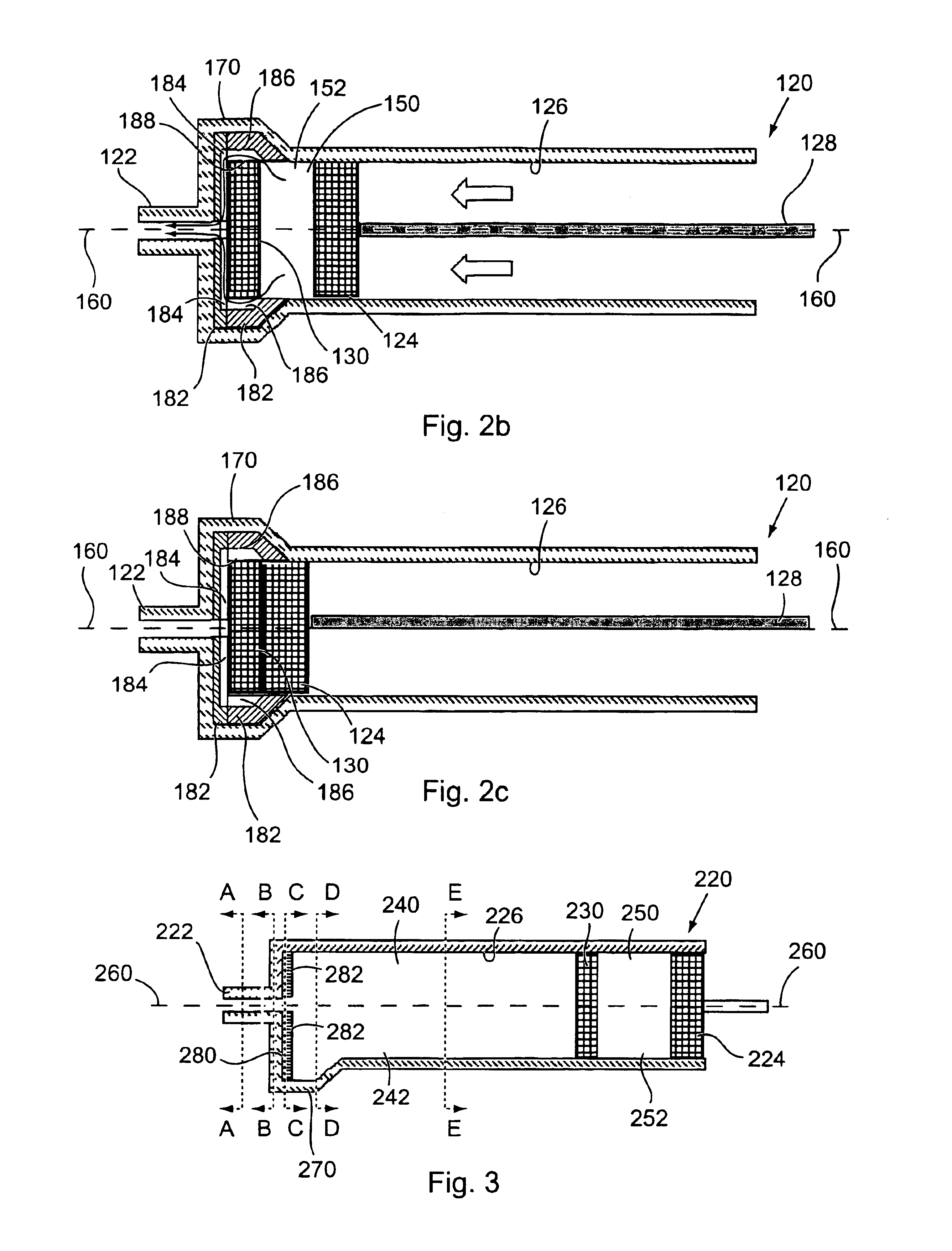
Method and apparatus for sequential delivery of multiple injectable substances stored in a prefilled syringe
A syringe, which may be pre-filled, and a method of using the syringe is disclosed to provide sequential injection of two different fluids without mixing thereof. The syringe comprises in a first version a plunger and an intermediate disc sealingly disposed within a generally cylindrical barrel, with an outlet portion distal of the barrel, the outlet portion being generally larger in diameter so that as the disc is advanced into it far enough to substantially inject the first fluid the disc loses sealing engagement and the second fluid is then expelled around the disc and through the syringe opening. Baffles are provided within the outlet portion to prevent the disc from inadvertently sealing the opening, and channels may be provided around the baffles. In a second version a bag is provided adjacent the plunger, with the first fluid being contained within the barrel and the second fluid being contained within the bag, and at least one barb is provided in the bottom of the syringe to burst the bag after the first fluid is expelled to thereby expel the second fluid.
Owner:BAE KYONGTAE T
Disposable injection device
InactiveUS6918889B1Eliminate disadvantagesReduce riskAmpoule syringesInfusion needlesRelative displacementEngineering
A single-use injection device, includes a pre-filled syringe (1), a syringe body (5) which is integral with the said syringe, and a protective case (10), the syringe body and protective case being provided with elements (9, 14, 15, 21) for relative locking in translation, which permit their relative displacement between a position of injection and a position of protection after use. The injection device includes a locking ring (23), which is provided with digital support elements (24), and locking units (25), which can lock the syringe body (5) and the protective case (10), in their position of injection, during injection, and can be displaced axially, such as to permit relative displacement of the syringe body and protective case, towards their position of protection after use, under the effect of the action of resilient member (27), only when the plunger (4) has reached its end of travel.
Owner:SANOFI SA
System and method for an injection using a syringe needle
Owner:AVANT MEDICAL CORP
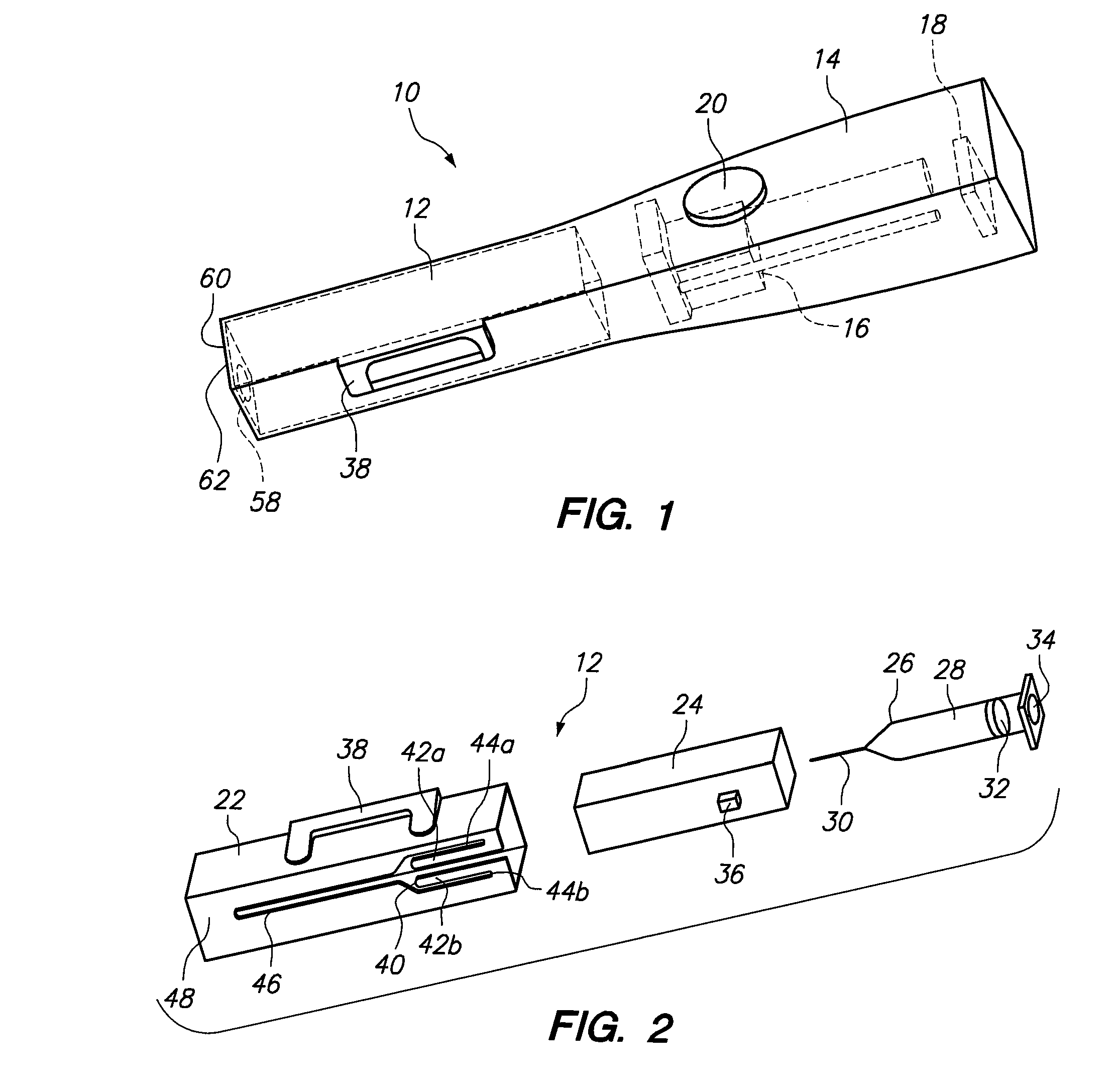
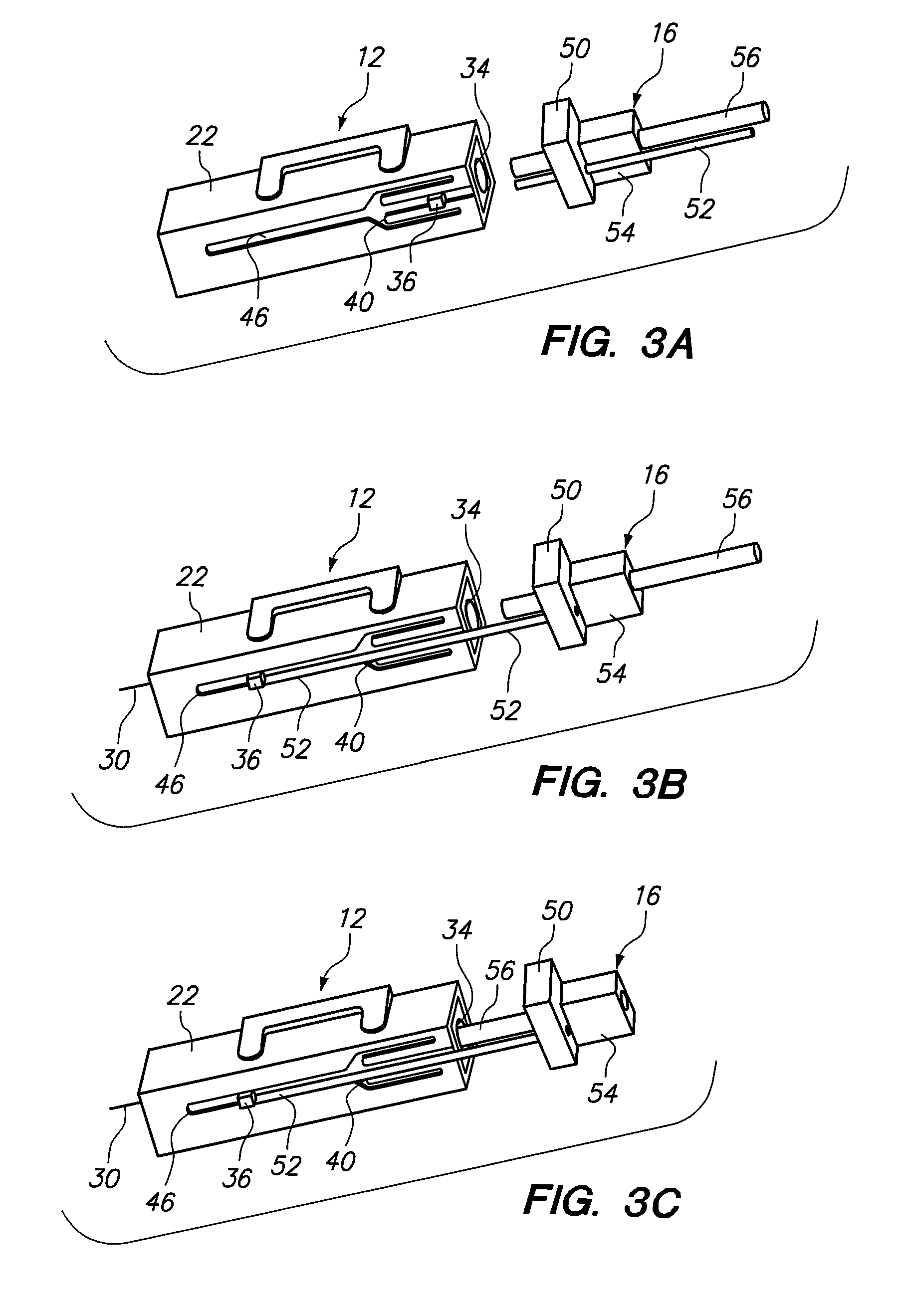
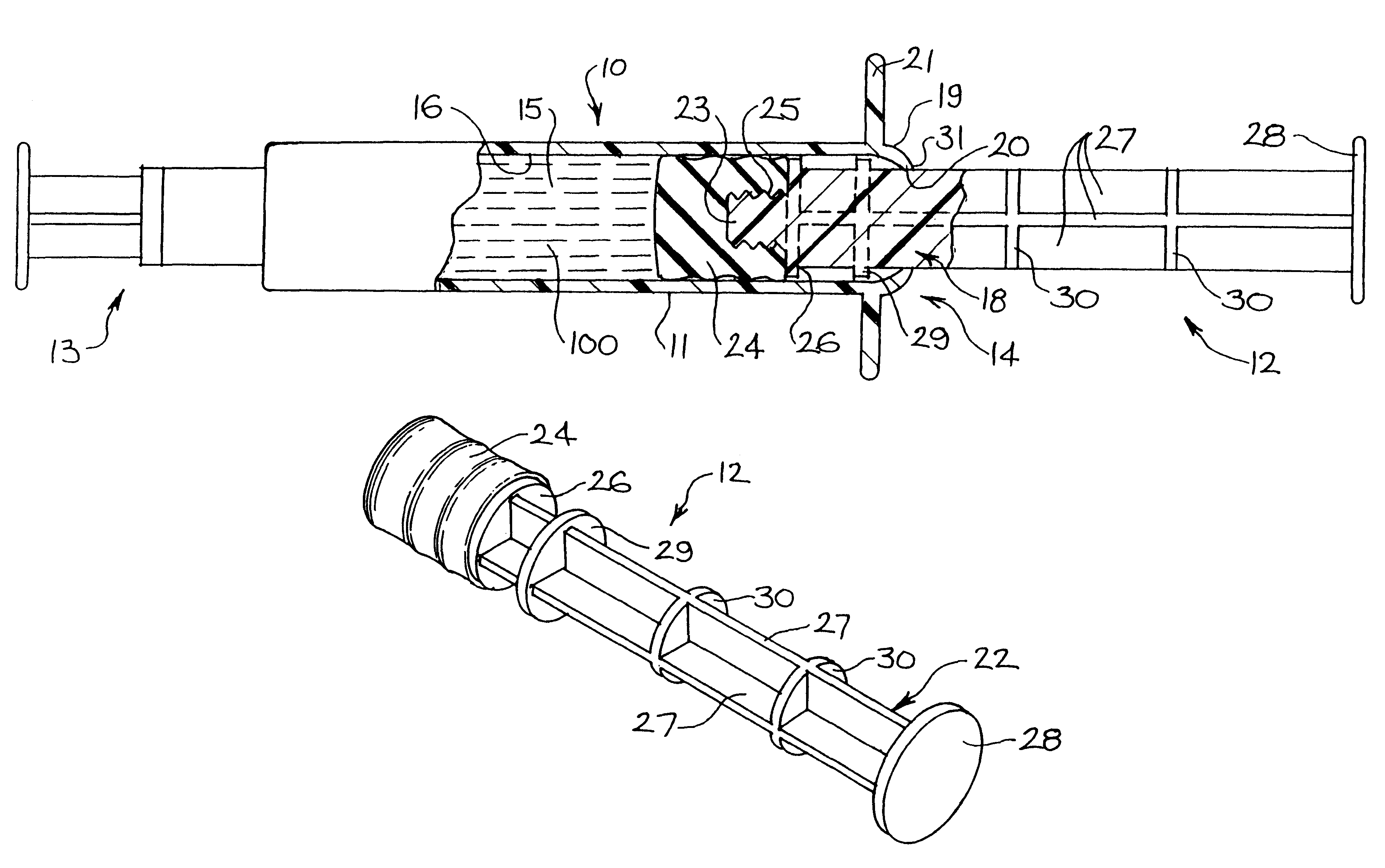
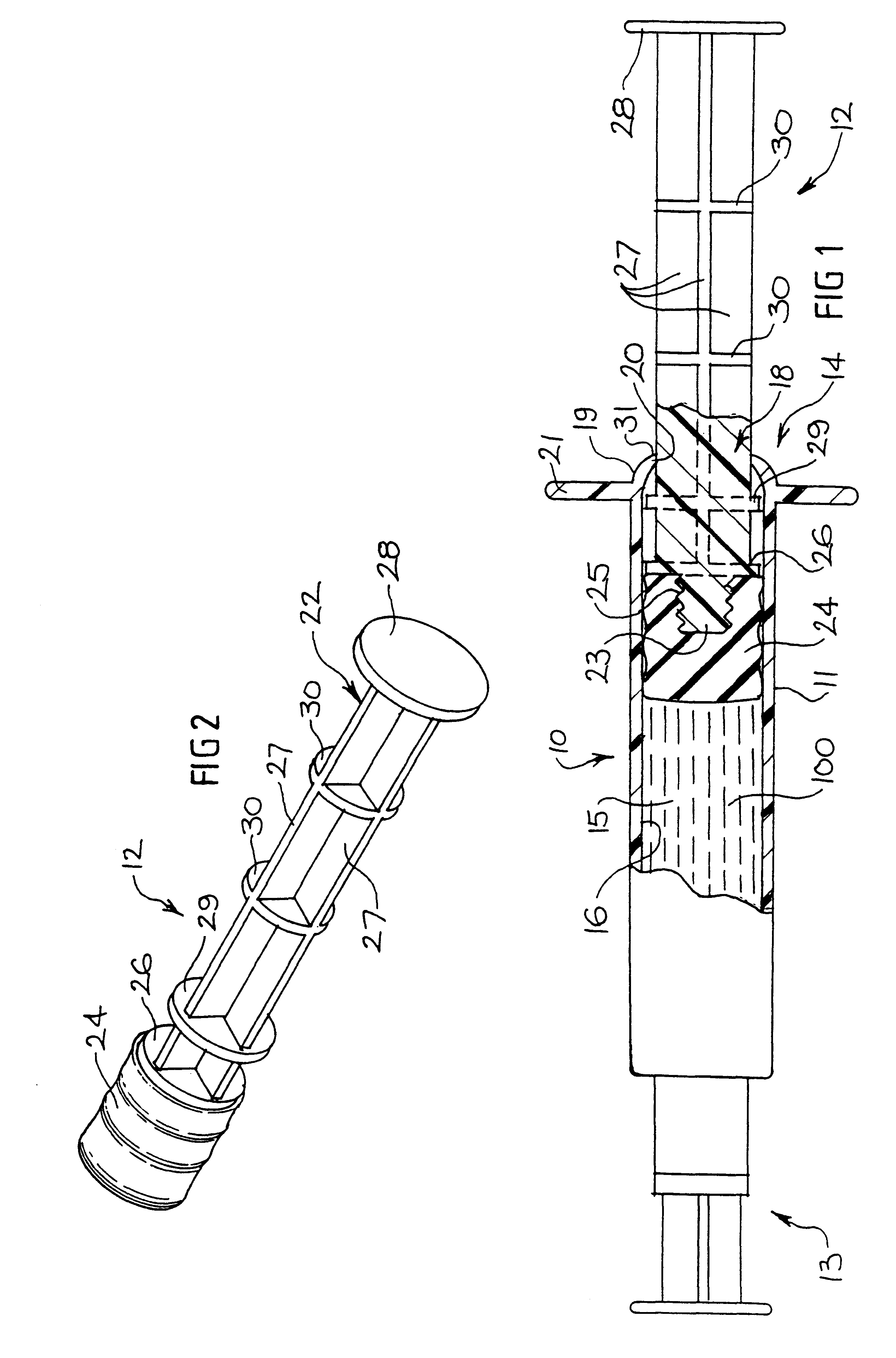
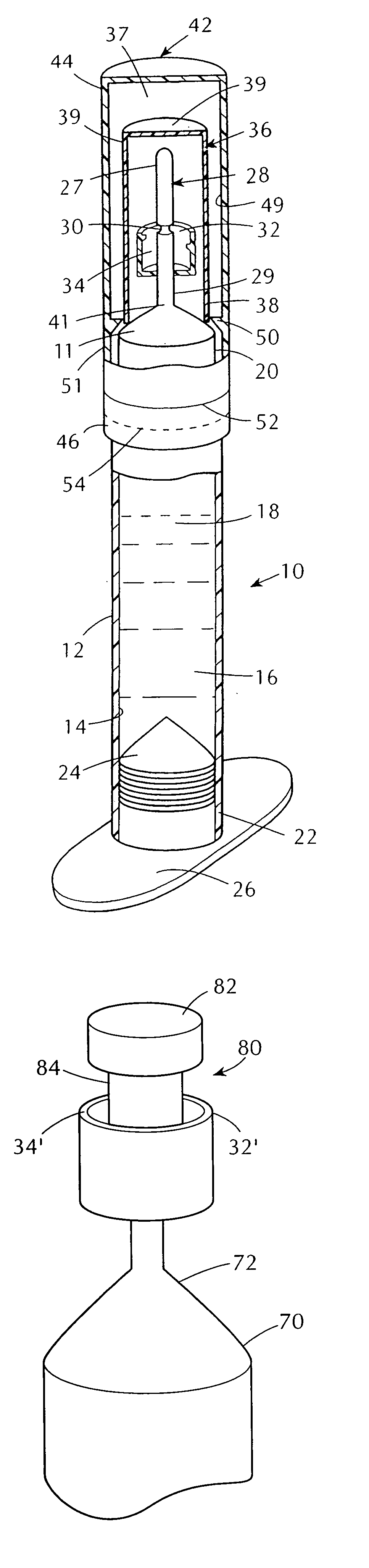
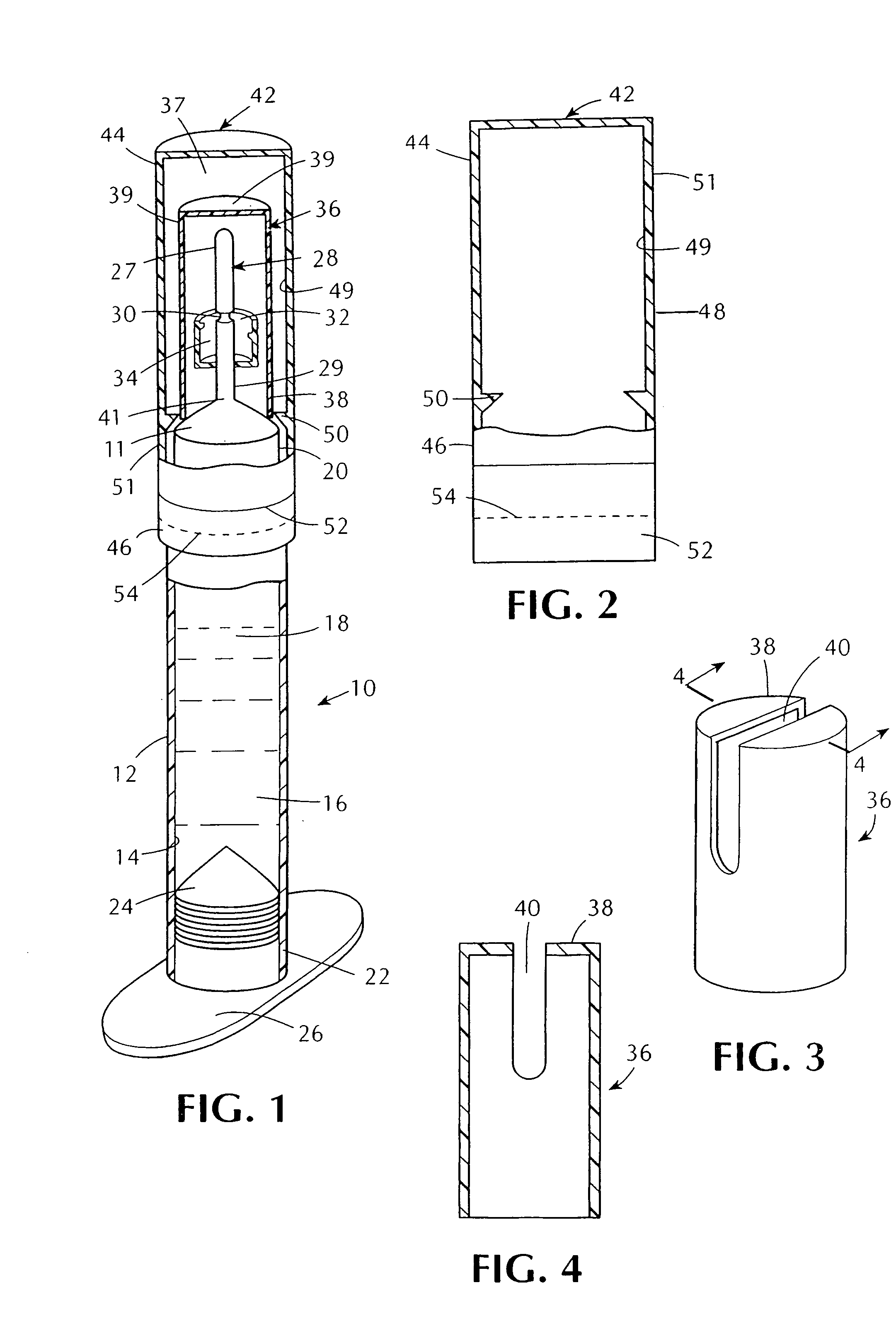
Tamper evident syringe design
A plastic prefilled syringe (10) including a syringe body (11) and a plunger assembly (12), the syringe body (11) having opposed first (13) and second (14) ends and an inner wall (16) defining a cylindrical chamber (15) which contains an injectable solution (100), the first end (13) of the syringe body (11) being sealed by a closure and the second end (14) incorporating an opening (18), the plunger assembly (12) including a plunger shaft (22) extending through said opening (18) and a stopper (24) secured at an end of said shaft (22) within said chamber, the plunger assembly (12) being movable within the chamber with the stopper (24) being operable to seal the opening (18) wherein the plunger assembly (12) includes barrier means (26 and 29) on said shaft (22) the barrier means (26 and 29) being adapted, in conjunction with a part of the syringe body (16 and 19), to inhibit access to the injectable solution (100) through the opening (18).
Owner:ASTRA PHARMA PTY LTD
Controlled retraction syringe and plunger therefor
ActiveUS20090093759A1Not compromising safety featureReduces and minimizes blood splatteringInfusion needlesPrefilled SyringeBiomedical engineering
A retractable syringe (11) and plunger (20) therefore are provided, whereby the plunger comprises a controlling means (62) which facilitates control of the rate of retraction of the retractable needle. By controlling the rate of needle retraction, the likelihood of blood spattering is reduced, thereby improving the user-friendliness and appeal of the retractable syringe. Typically, the syringe is a prefilled syringe. Following plunger depression, a symmetrical ejector member (92) releases the retractable needle (40) from a retaining member (30) to thereby allow retraction of the retractable needle. Needle retraction is facilitated by a biasing means (70), such as a spring or other compressible and / or de-compressible device. The plunger comprises a plunger housing (21), in which is located the controlling means. The controlling means may be a pneumatic air space (324) inside the plunger housing which automatically acts against the retracting needle to thereby control or regulate the rate of retraction.
Owner:UN HOLDINGS LLC
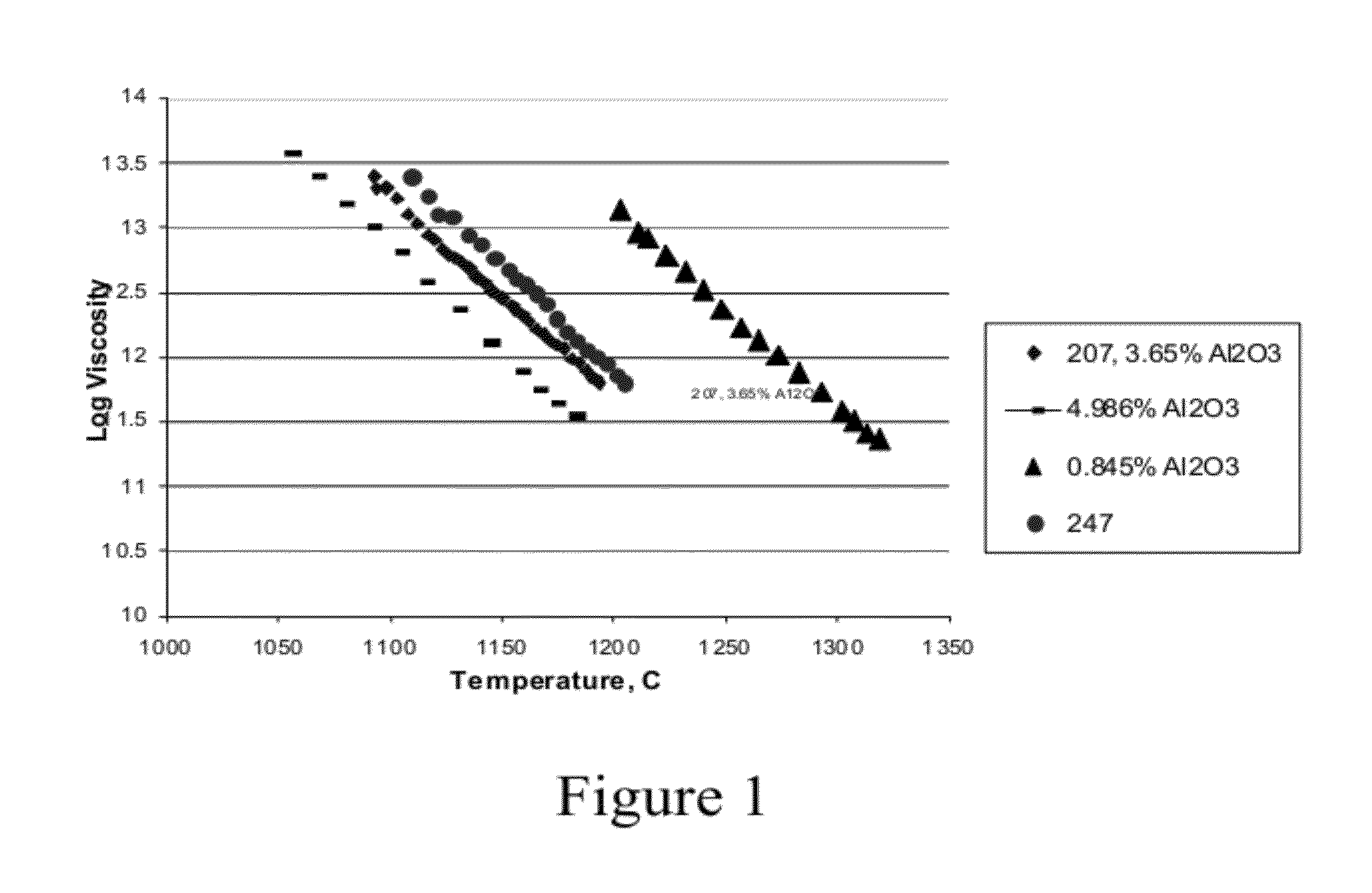
Fused quartz tubing for pharmaceutical packaging
InactiveUS20120148770A1Reduce softeningLow working point temperatureLayered productsPharmaceutical containersRare earthWorking temperature
A high silica glass composition comprising about 82 to about 99.9999 wt. % SiO2 and from about 0.0001 to about 18 wt. % of at least one dopant selected from Al2O3, CeO2, TiO2, La2O3, Y2O3, Nd2O3, other rare earth oxides, and mixtures of two or more thereof. The glass composition has a working point temperature ranging from 600 to 2,000° C. These compositions exhibit stability similar to pure fused quartz, but have a moderate working temperature to enable cost effective fabrication of pharmaceutical packages. The glass is particularly useful as a packaging material for pharmaceutical applications, such as, for example pre-filled syringes, ampoules and vials.
Owner:MOMENTIVE PERFORMANCE MATERIALS INC
Prefilled syringe jet injector
ActiveUS20120004608A1Prevent backflowImprove distributionAmpoule syringesJet injection syringesEngineeringPrefilled Syringe
Owner:ANTARES PHARMA INC
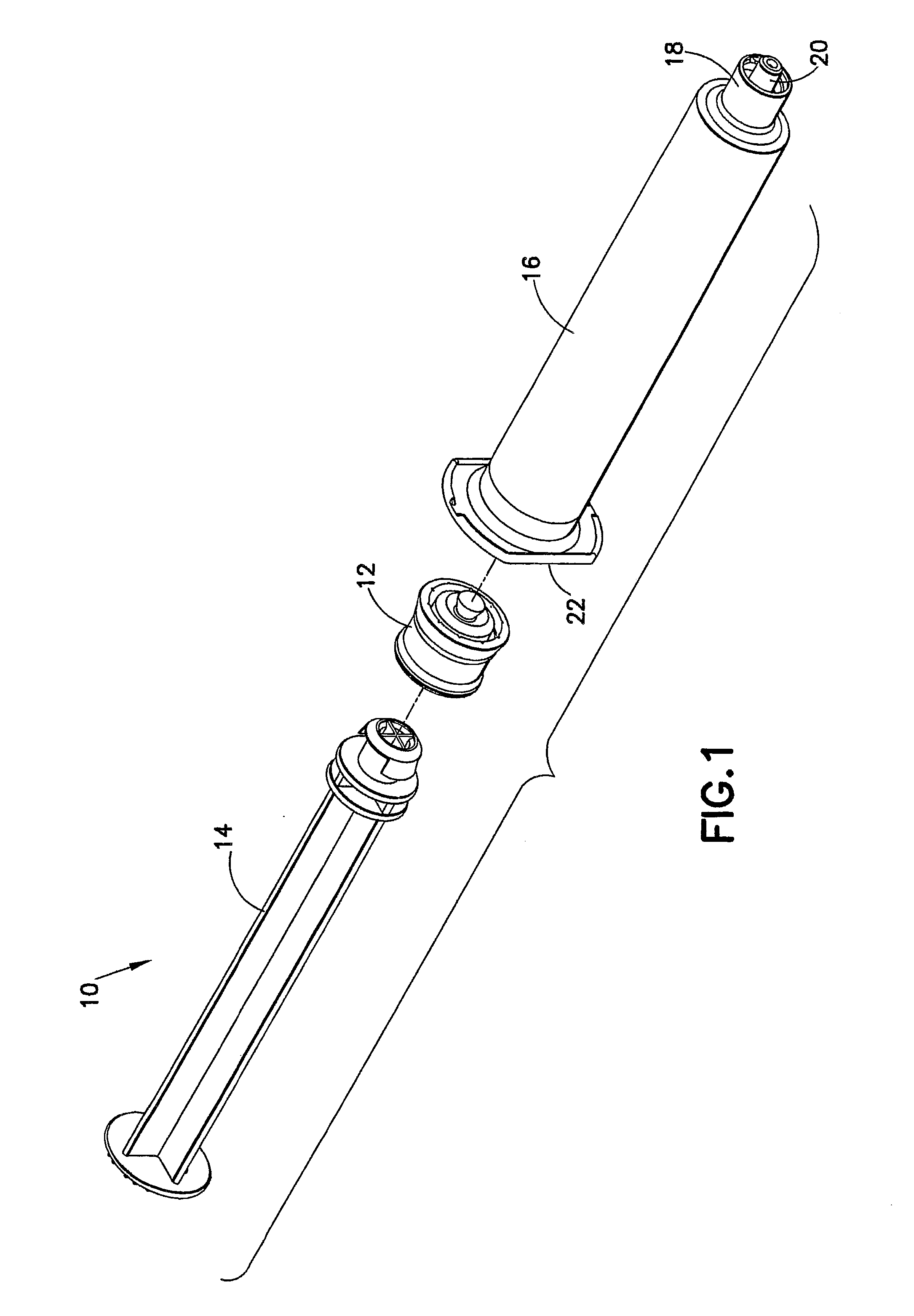
Syringe, cap and method of producing prefilled syringe
InactiveUS20060106349A1Easy and assured connectionInfusion syringesTube connectorsEngineeringPrefilled Syringe
A first female connector and a second female connector that has on its outer periphery a screw portion engaging a female screw can be alternatively connected to the top of a syringe. A mouth portion with a smaller diameter than a barrel portion of an outer hollow-cylinder main body is provided at the top of an outer hollow-cylinder. A lock adapter is provided on the outer periphery of the mouth portion. The lock adapter is rotatable about the mouth portion and axially movable in an axial direction of the mouth portion. The lock adapter can be moved to a position at the base of the mouth portion where the operation of fitting a male taper portion formed at the top of the mouth portion into a bore portion of the first female connector is not interrupted. The lock adapter can be fixed to the mouth portion at the position to which the adapter is moved.
Owner:TERUMO KK
Tamper evident syringe barrel
A prefilled syringe of cartridge barrel having a tamper evident closure. Tamper evidence is provided by: a frangible tip seal which is integral with the tapered tip of the barrel; a frangible tip cap covering the tip, the tip seal and a portion of the distal end of the barrel; and an overwrap covering the frangible tip cap and the distal end of the barrel sealed to the distal end of the barrel by a tamper evident seal. The tapered tip is optionally equipped with a luer collar to receive an external luer connector. Alternatively, the tapered tip can be provided with a bore therethrough which can be closed with an elastomeric plug and the external connector can be a tubing conduit.
Owner:BRACCO DIAGNOSTICS
Syringe needle sheath
ActiveUS7935087B2Prevent and minimize riskHinder withdrawal of the plungerInfusion needlesEngineeringPrefilled Syringe
A syringe is provided which comprises a barrel (11) and a needle sheath (30) slidably located within the barrel, a plunger (20) with a plunger seal (23), a needle, a needle sheath (30) and a sheath seal (50), arranged so that depression of the plunger forces the needle sheath to move into a position where it covers or at least partly encloses the needle after delivery of fluid contents of the syringe. The needle sheath may be manually actuated or may be actuated by decompression of a spring. The syringe may further comprise a plunger disabling means operable to impede, prevent or otherwise hinder withdrawal of the plunger during or following depression of the plunger. The syringe may be a pre-filled syringe or a syringe which is filled by a user.
Owner:UN HOLDINGS LLC
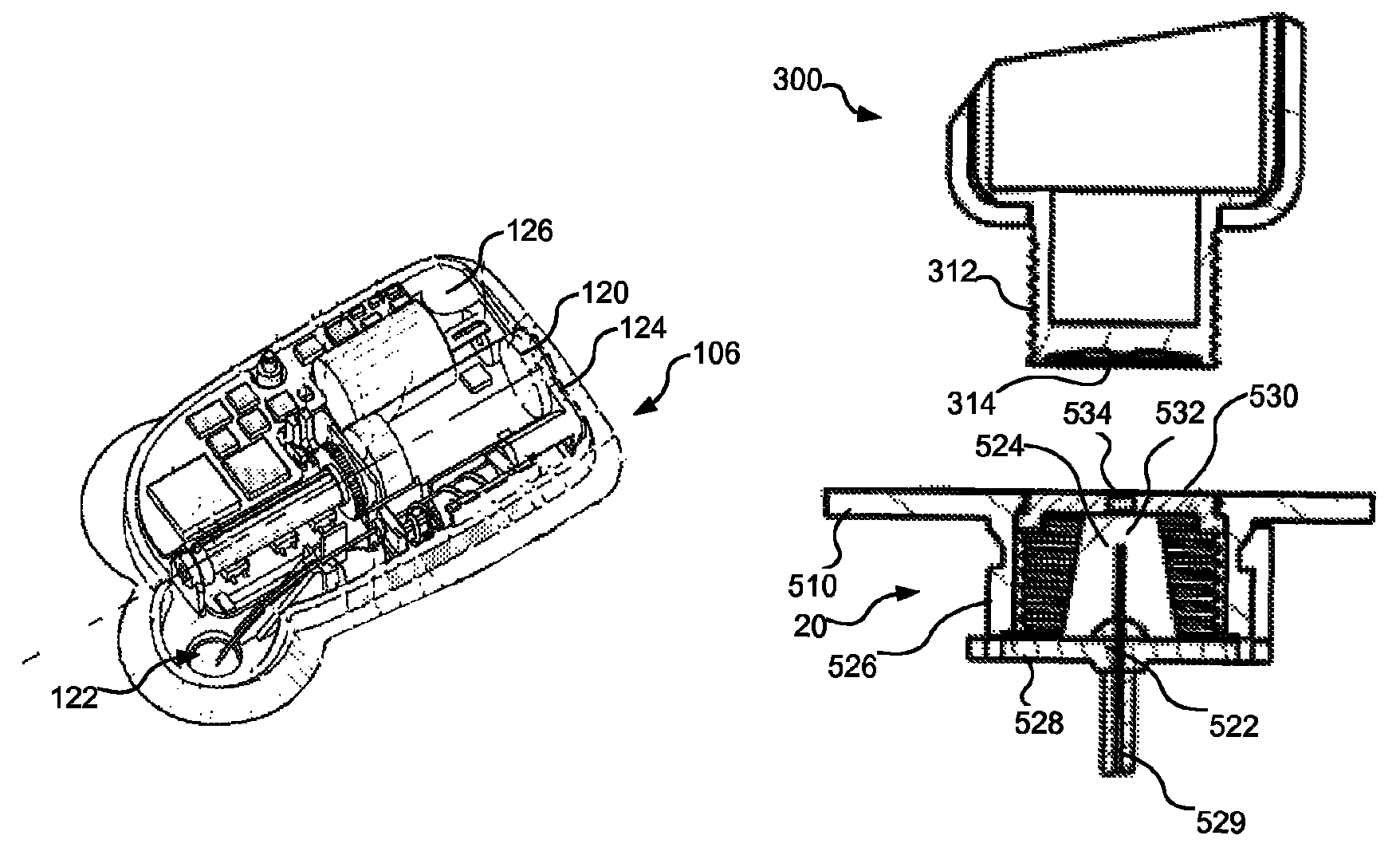
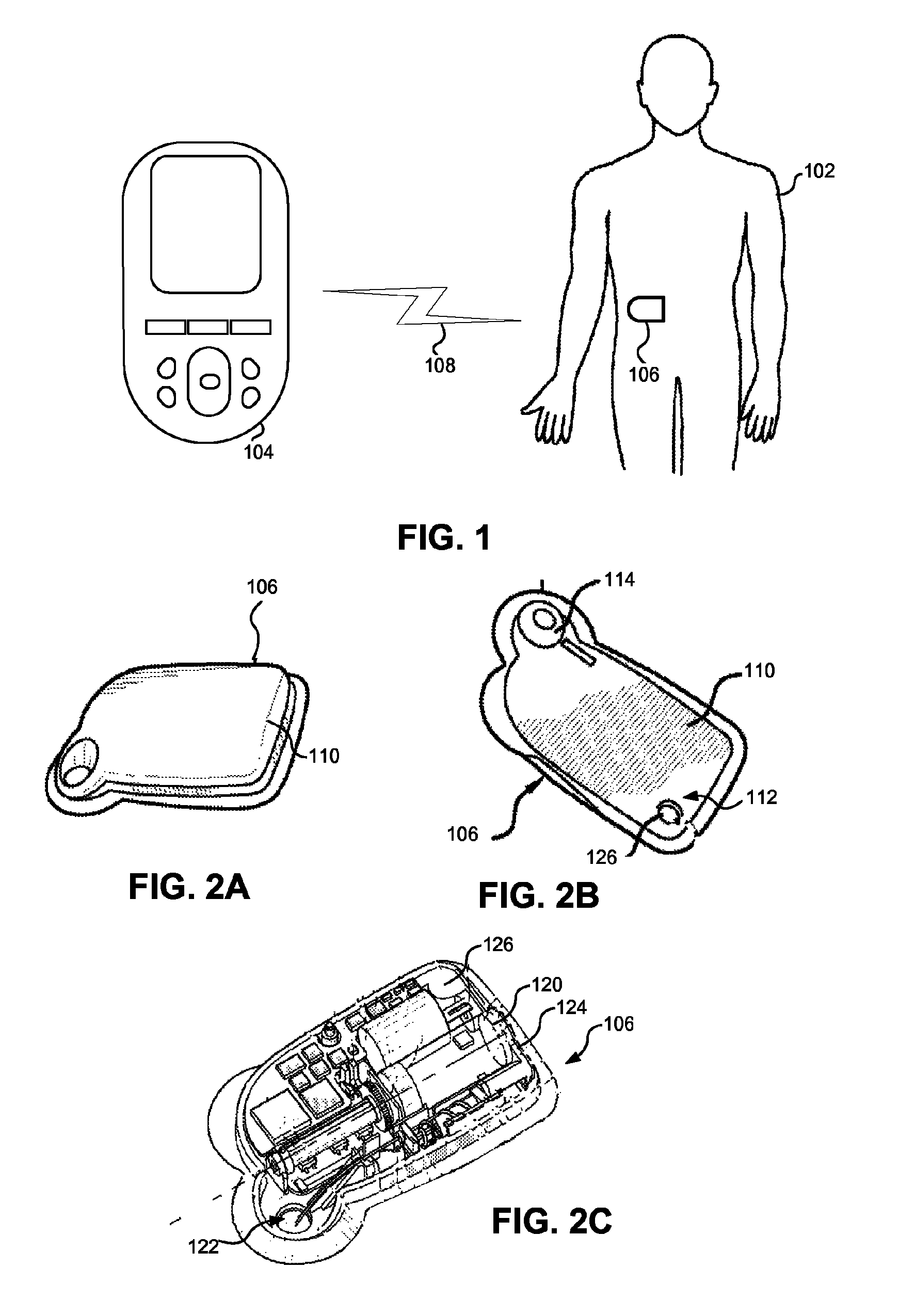
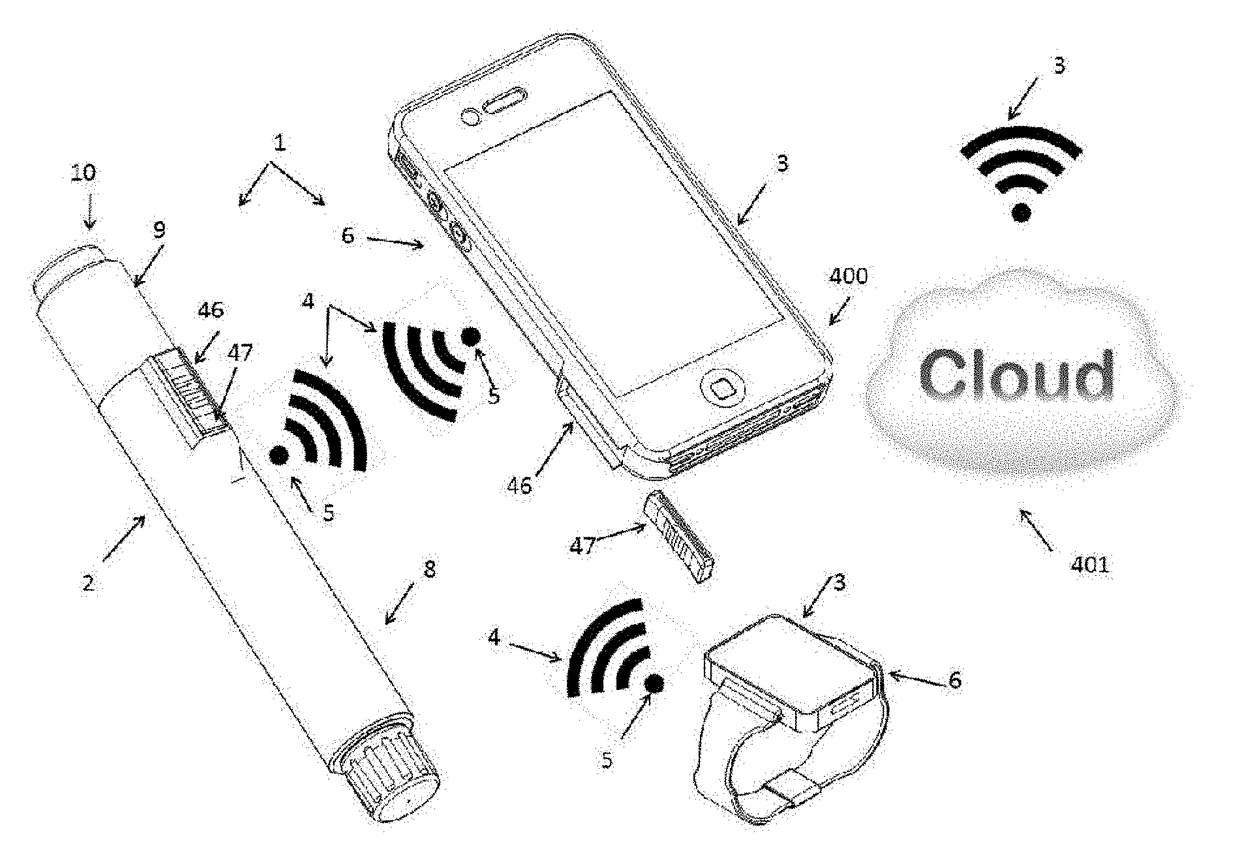
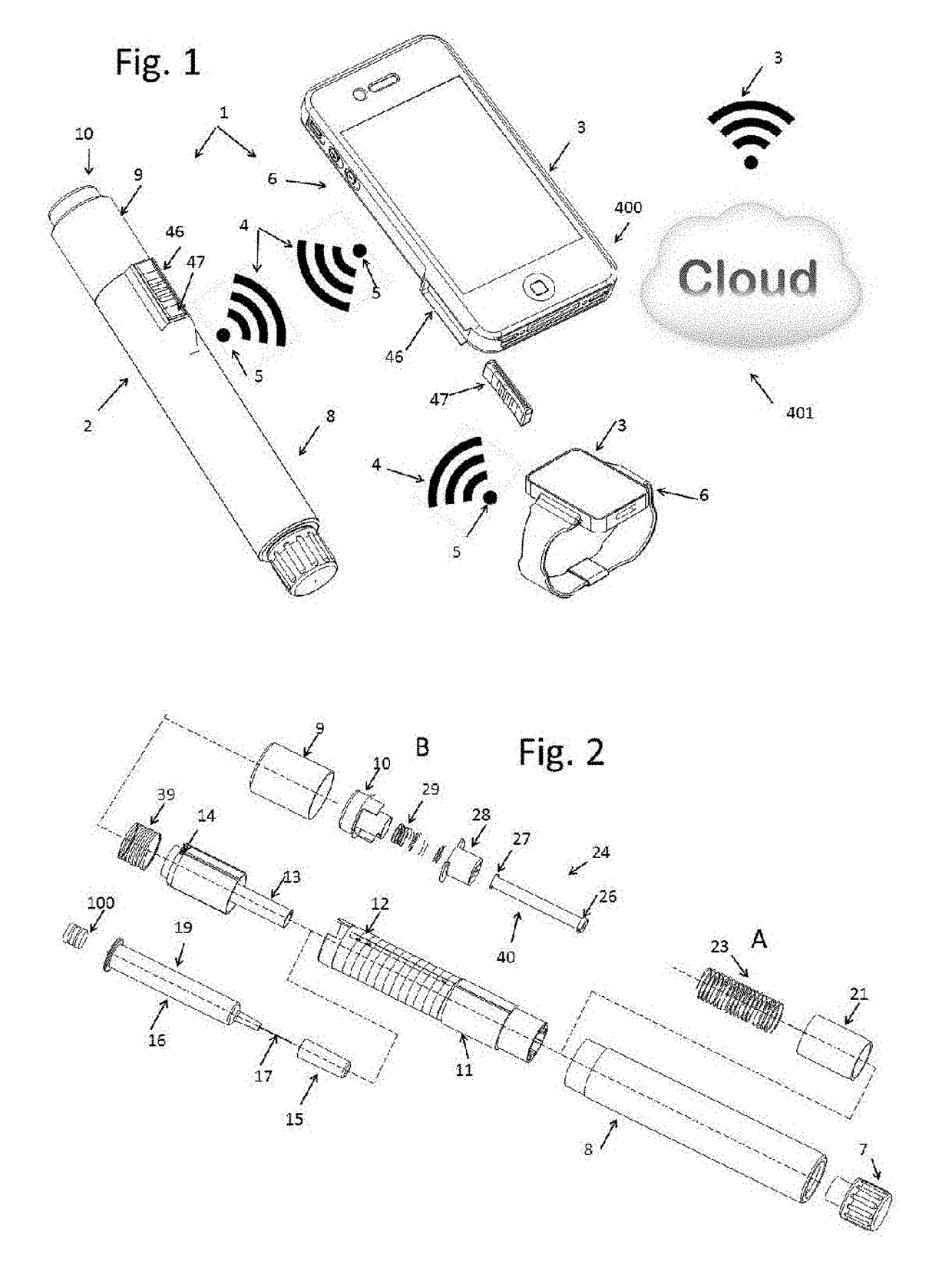
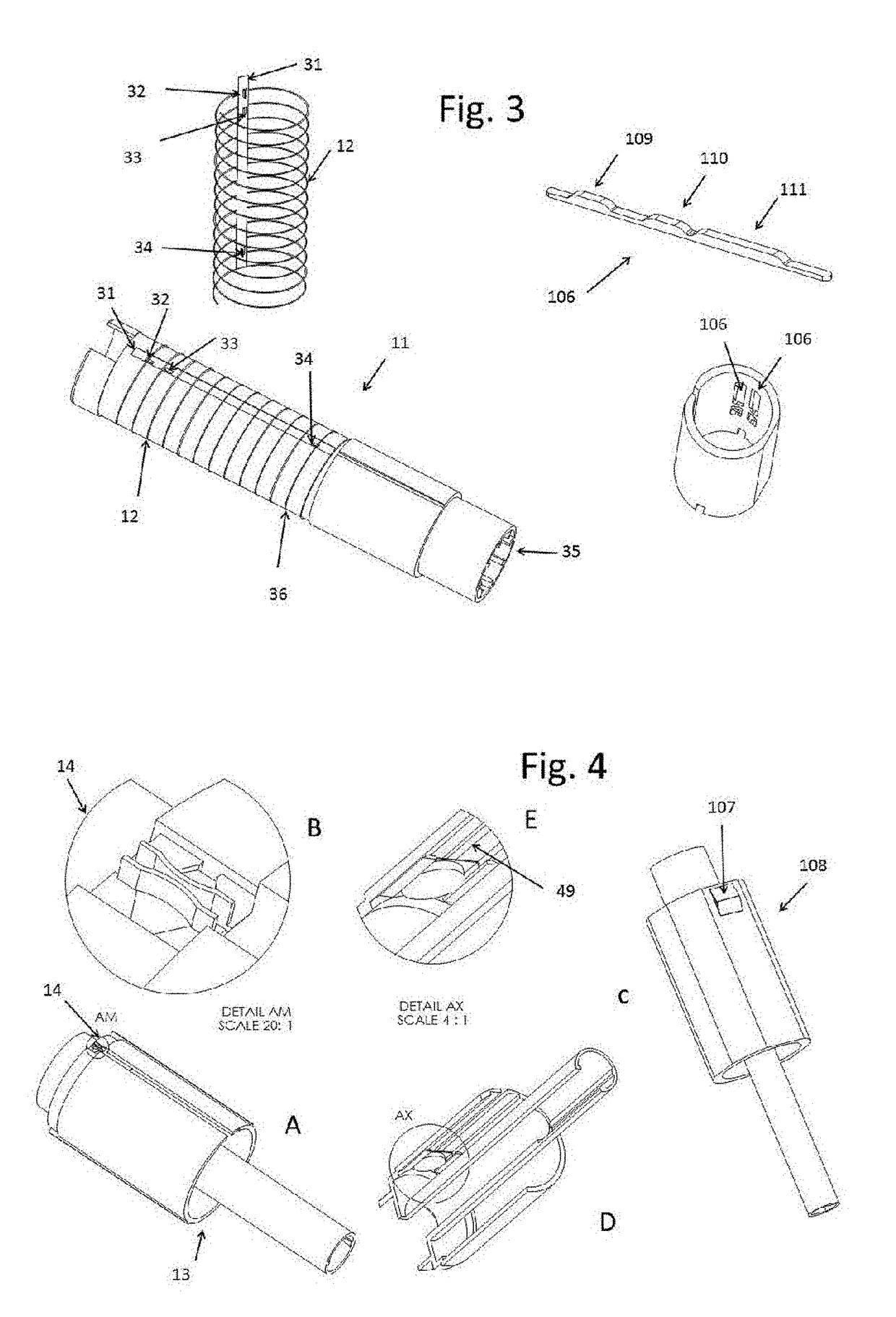
Injection System
InactiveUS20170274149A1Convenient treatmentImprove patient engagementTransmission systemsDrug and medicationsEngineeringPrefilled Syringe
The present invention provides apparatus and systems for enabling and monitoring injection of medicinal fluids into a subject. The invention includes a housing for a prefilled syringe, the housing incorporating means for generating and sending signals as parts of the syringe cooperate to deliver the fluids. The signals may be transmitted from a transmitter on the housing as radiowaves to a receiver, which may be conveniently located on a spaced device, such as a mobile phone. The mobile phone or other receiving device incorporates computer software to receive, store the signals from the transmitter as a record of the injection process. The computer software may include information about the medicine, the injection process, and other information relevant to a treatment regime. In some cases, the receiving device may transmit information stored by the computer software for storage and analysis.
Owner:MEDAXOR
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com