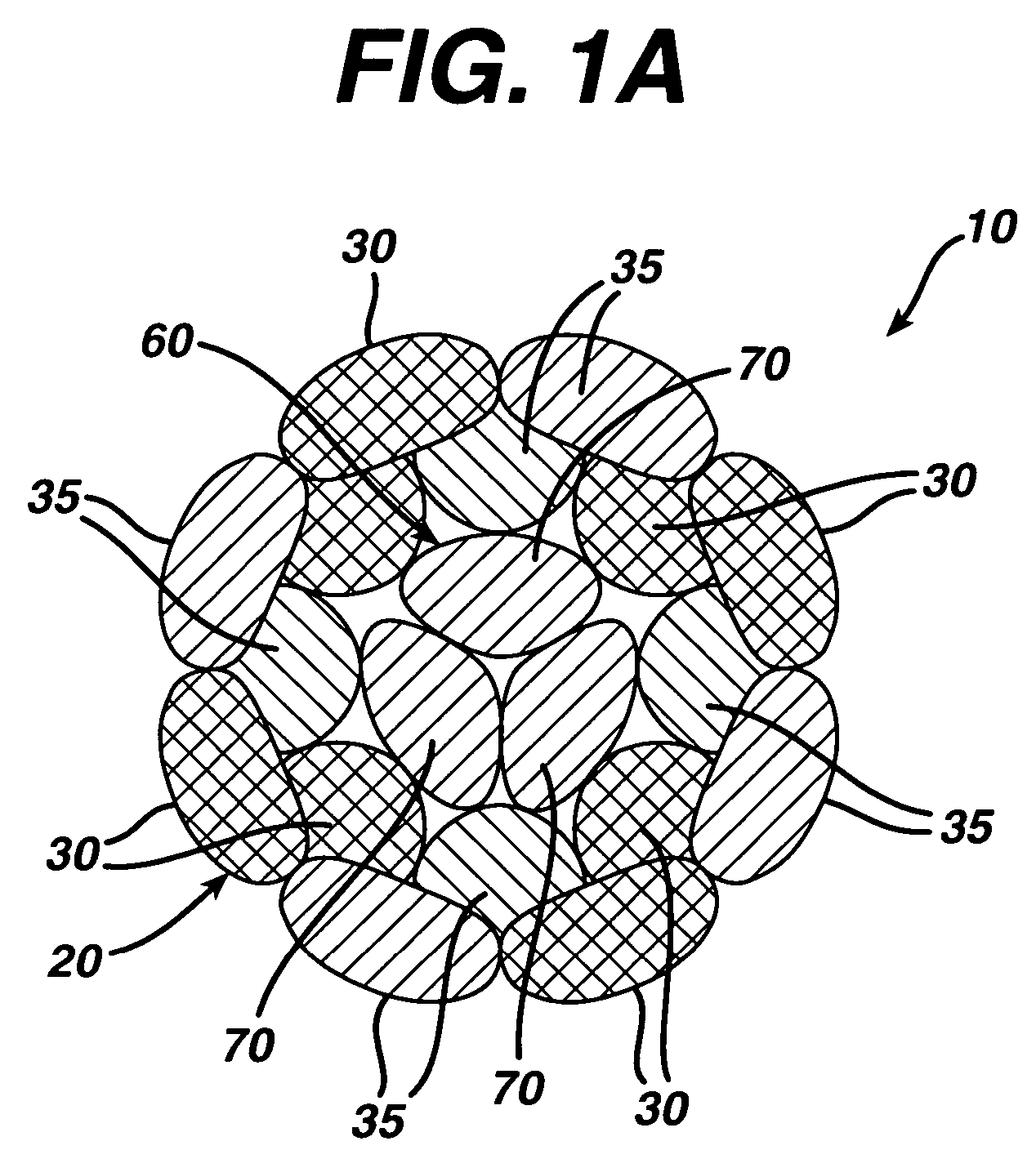
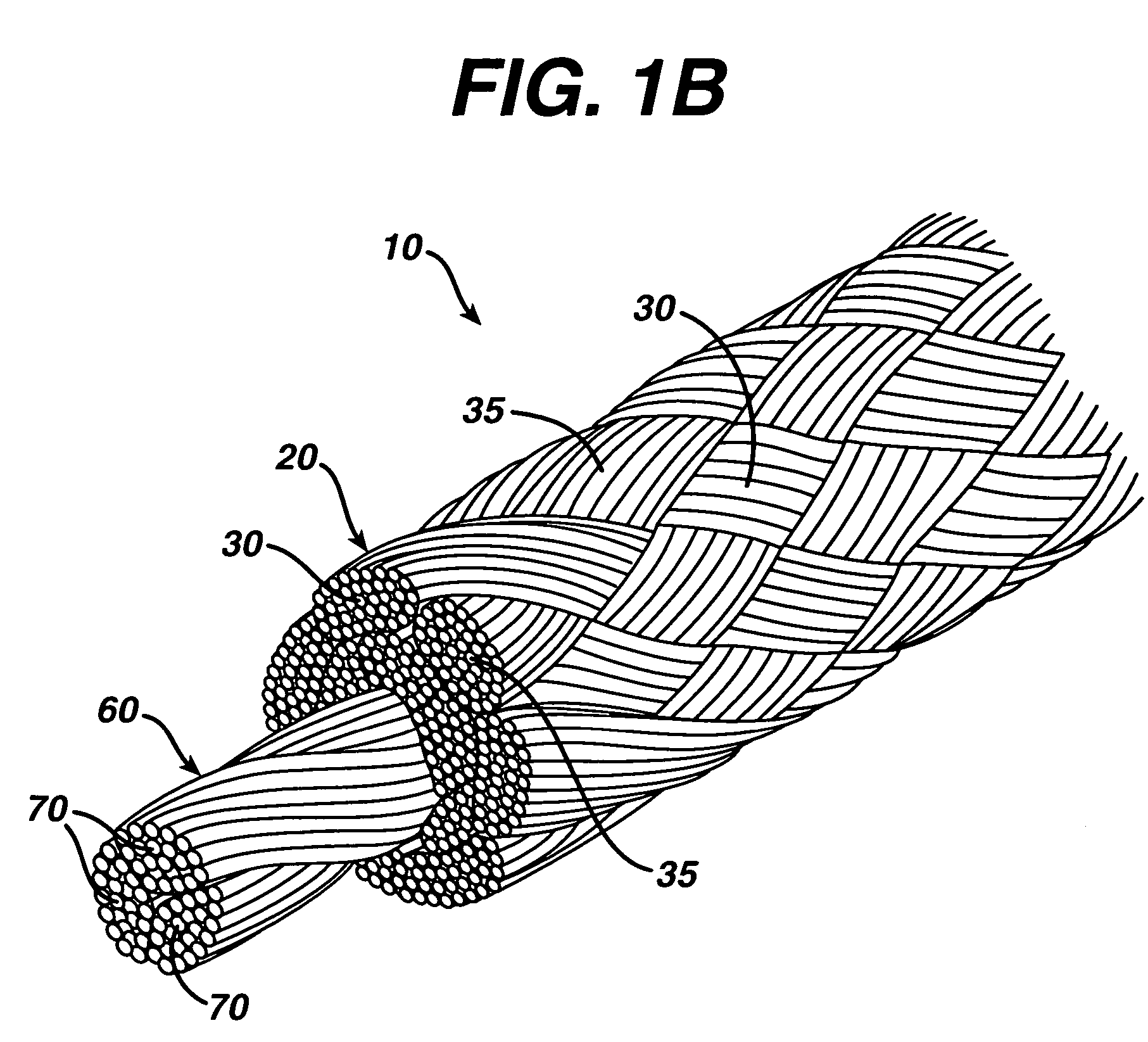
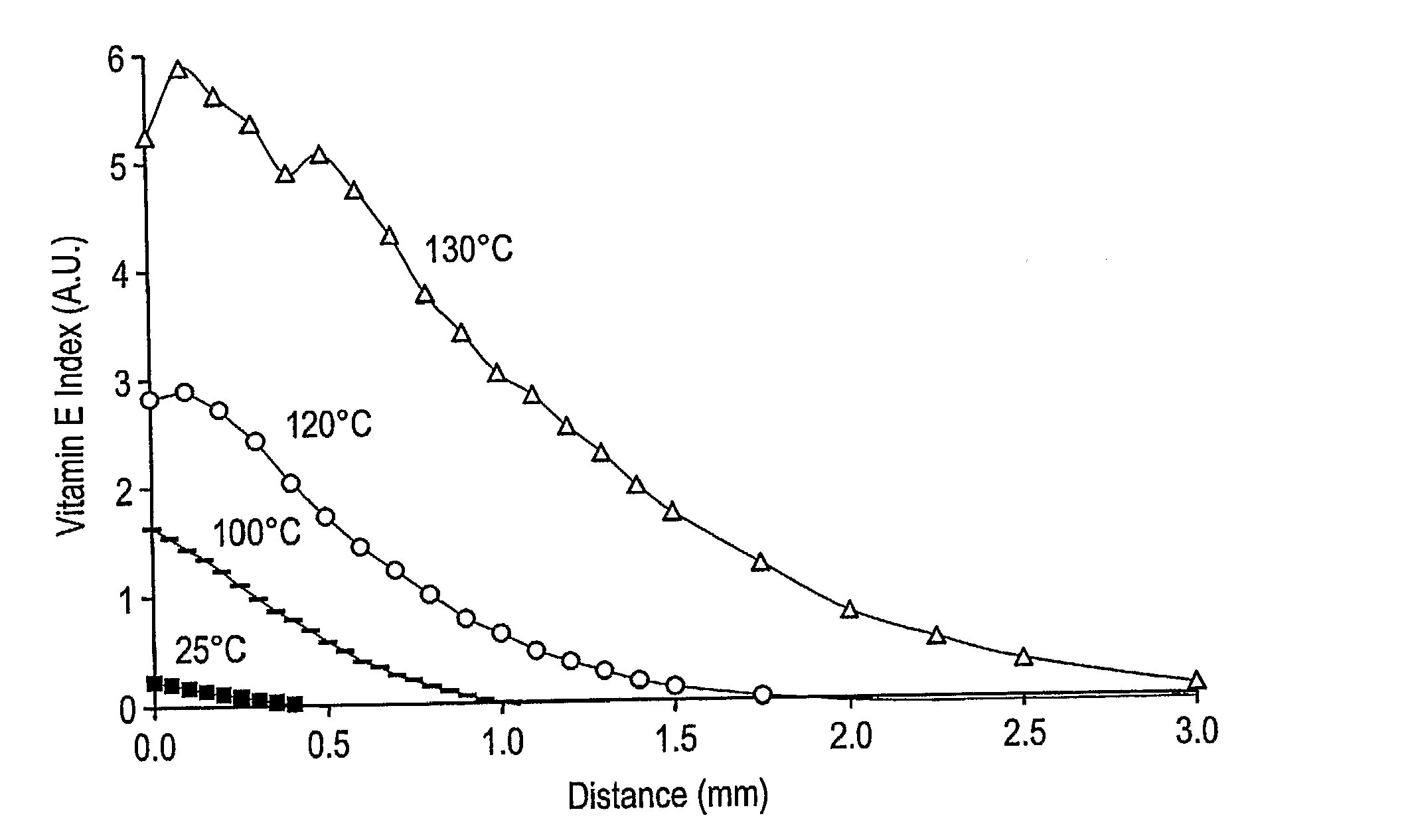
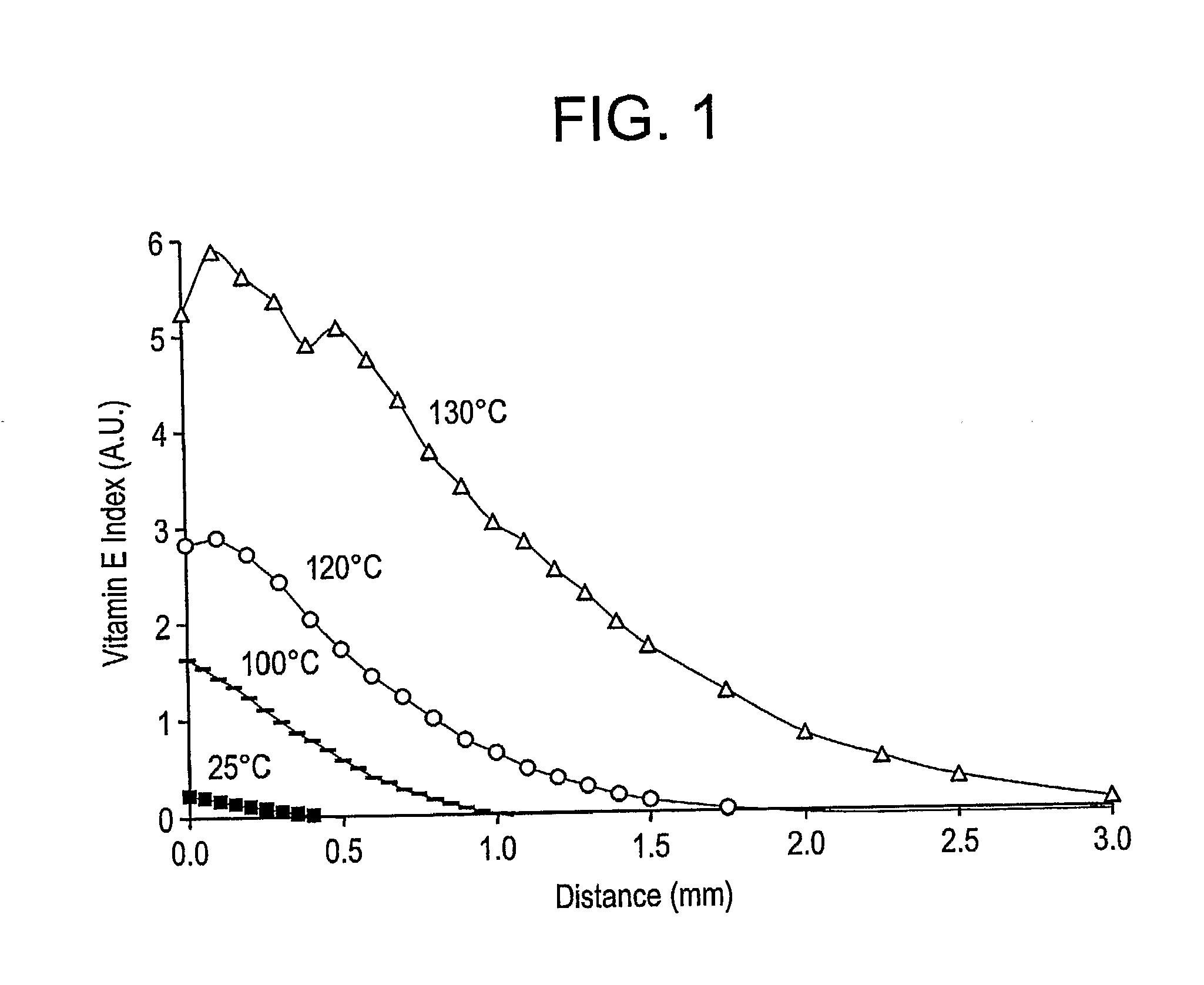
Patents
Literature
2057 results about "Ultra-high-molecular-weight polyethylene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ultra-high-molecular-weight polyethylene (UHMWPE, UHMW) is a subset of the thermoplastic polyethylene. Also known as high-modulus polyethylene, (HMPE), it has extremely long chains, with a molecular mass usually between 3.5 and 7.5 million amu. The longer chain serves to transfer load more effectively to the polymer backbone by strengthening intermolecular interactions. This results in a very tough material, with the highest impact strength of any thermoplastic presently made.
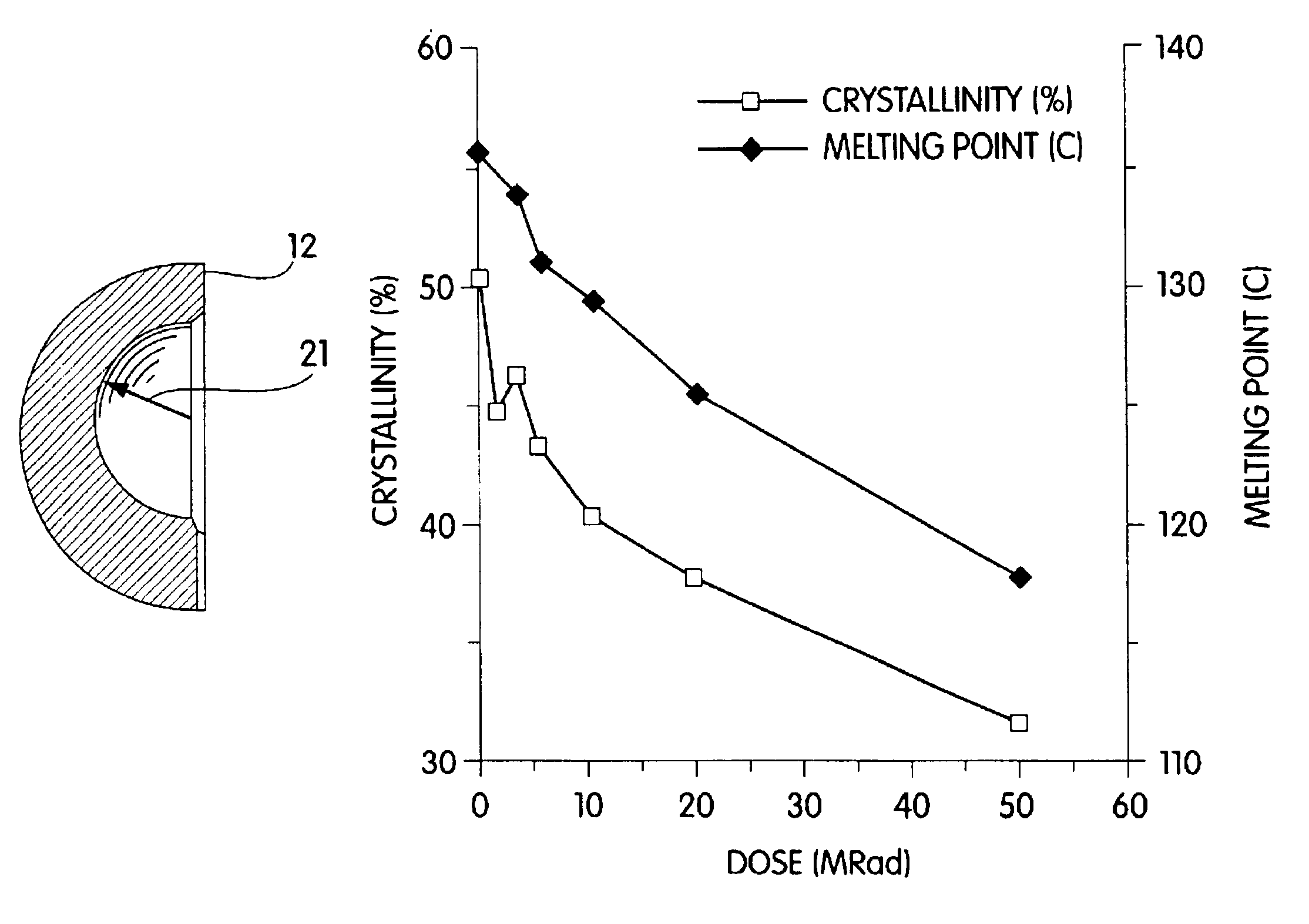
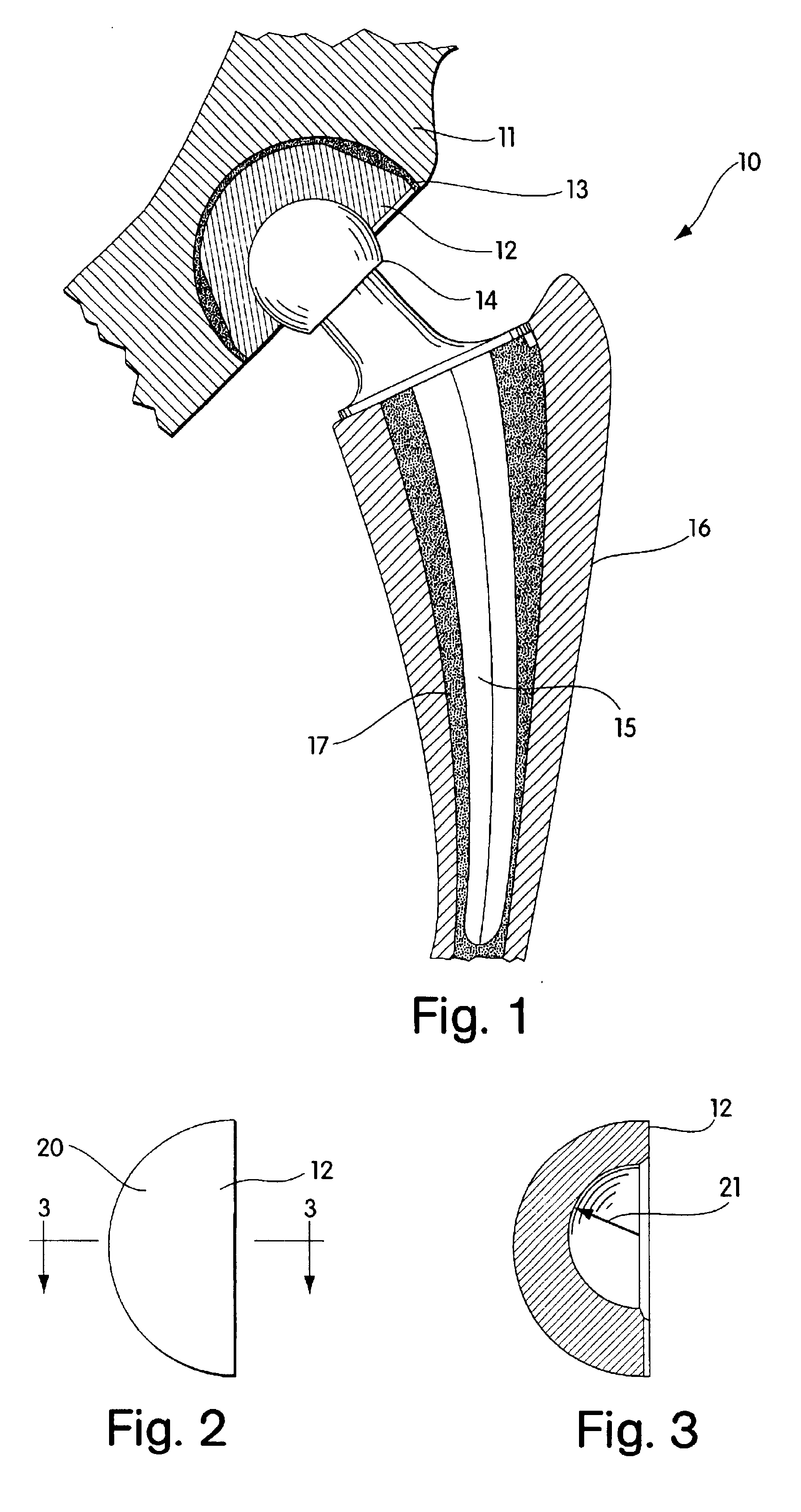
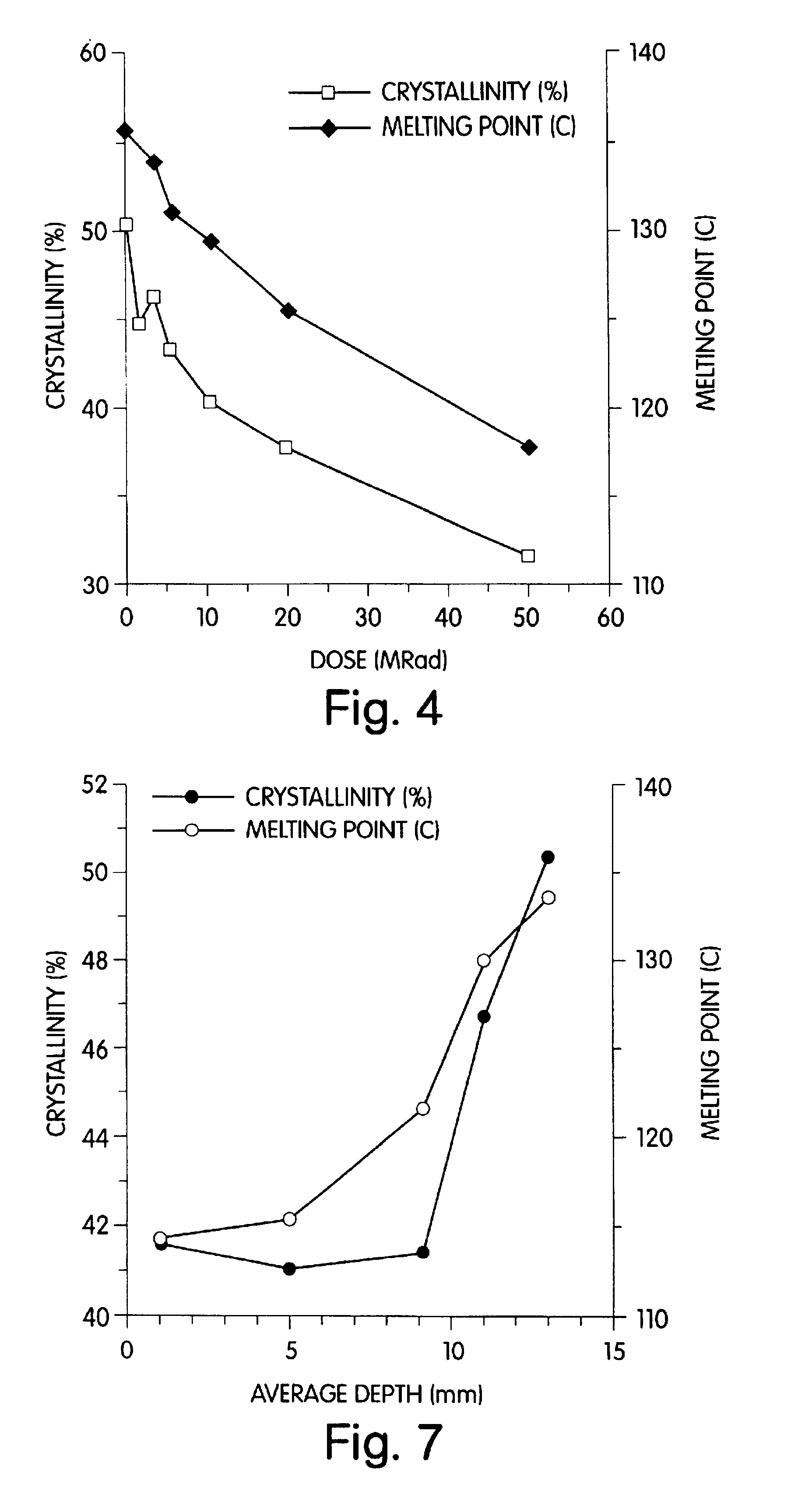
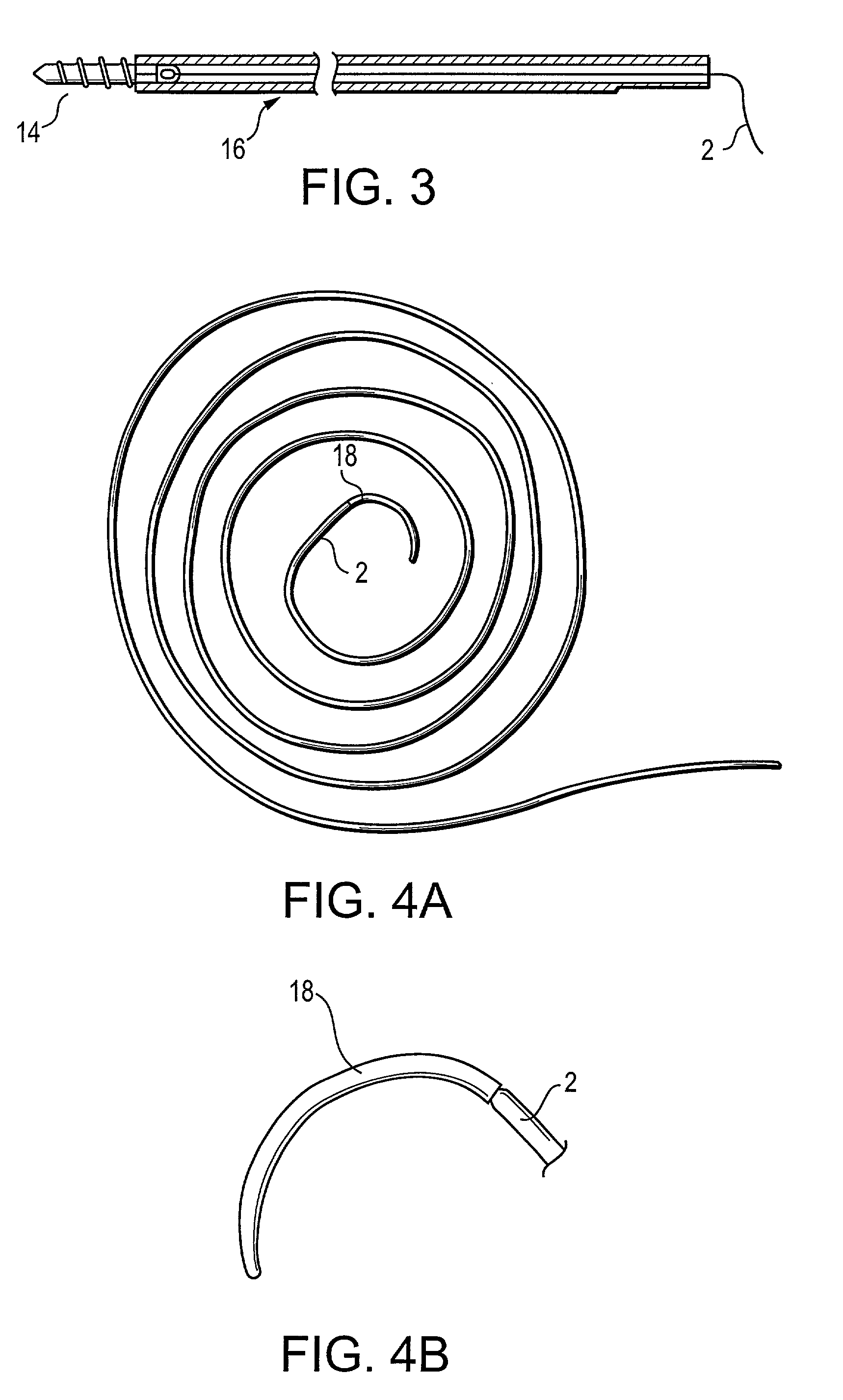
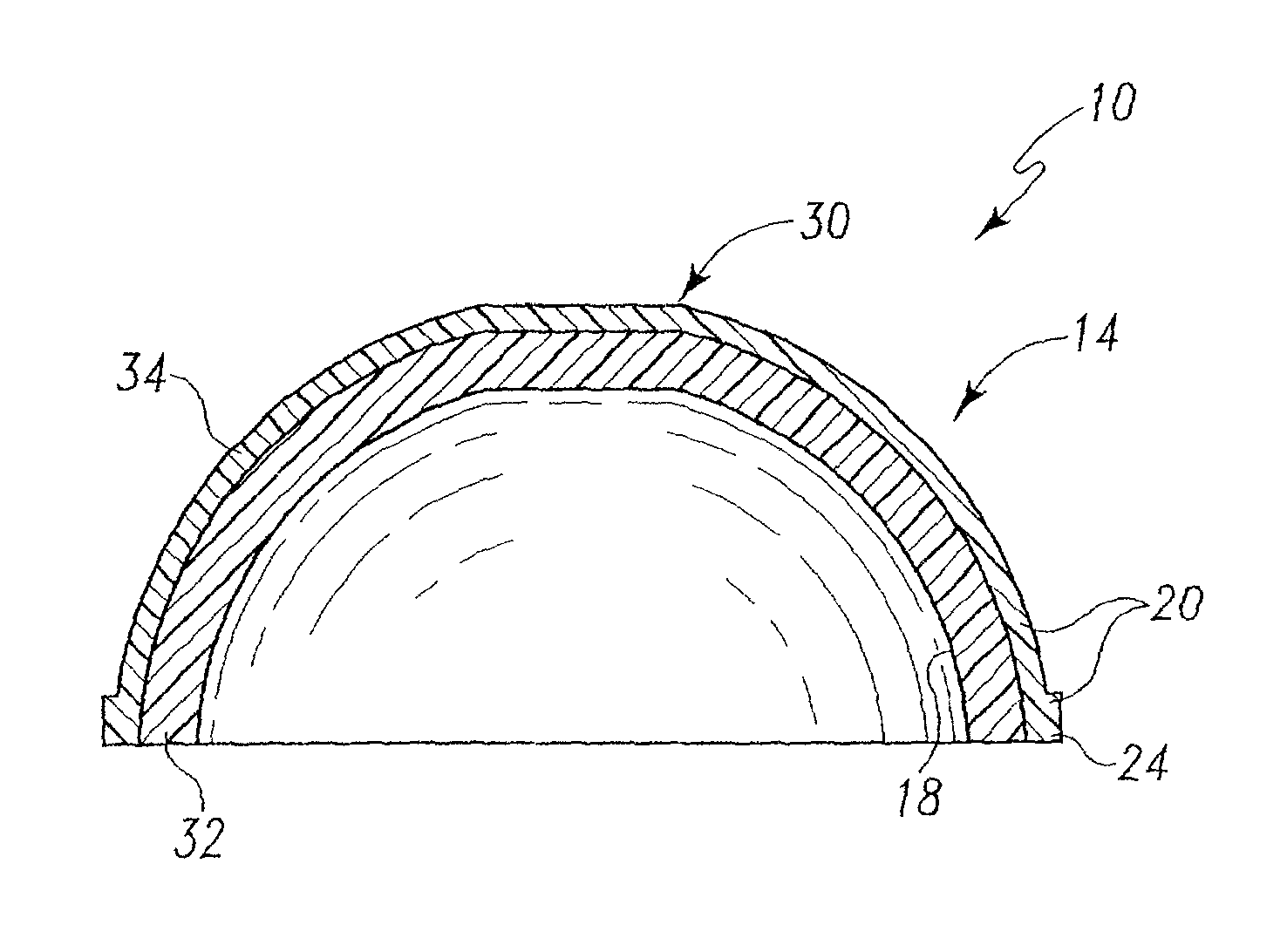
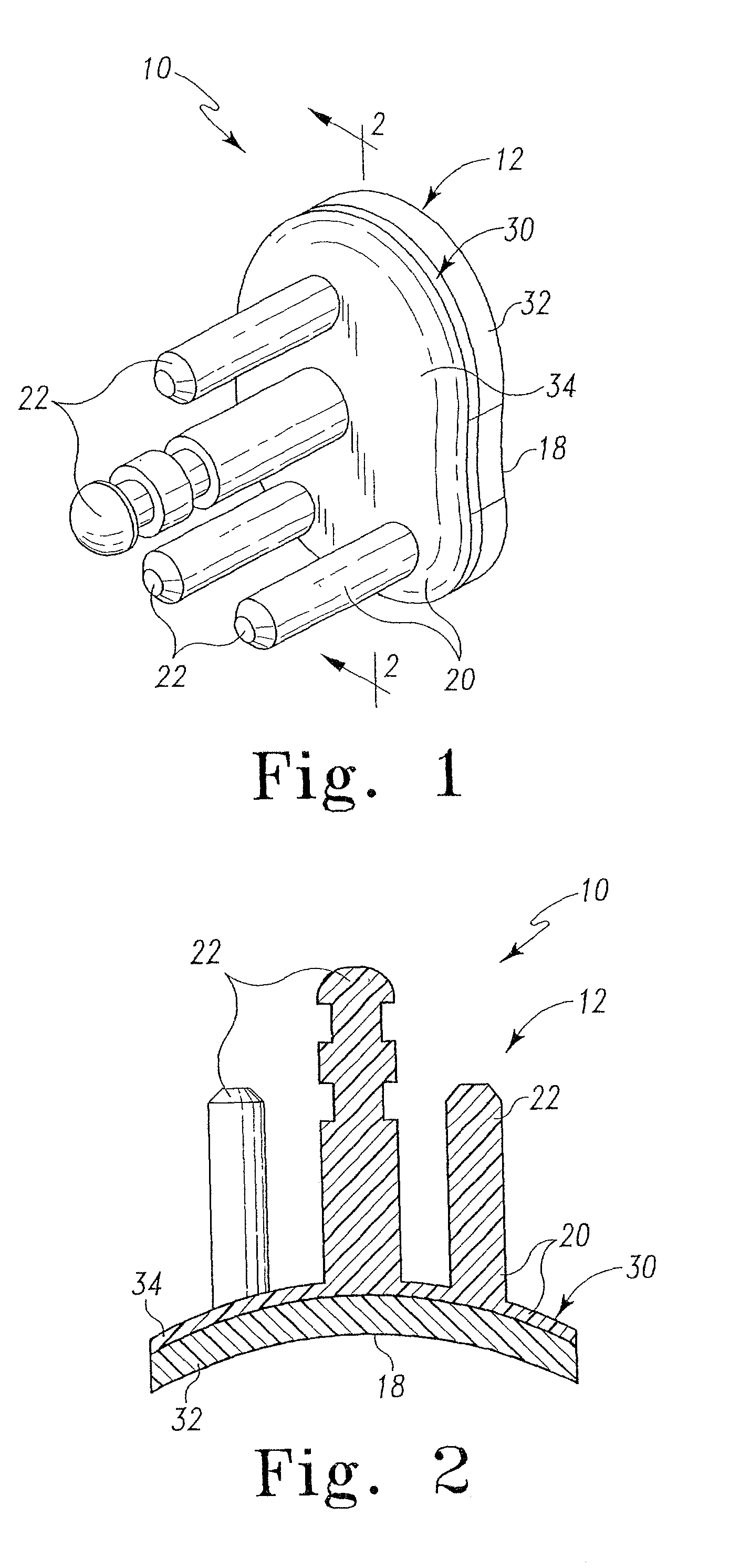
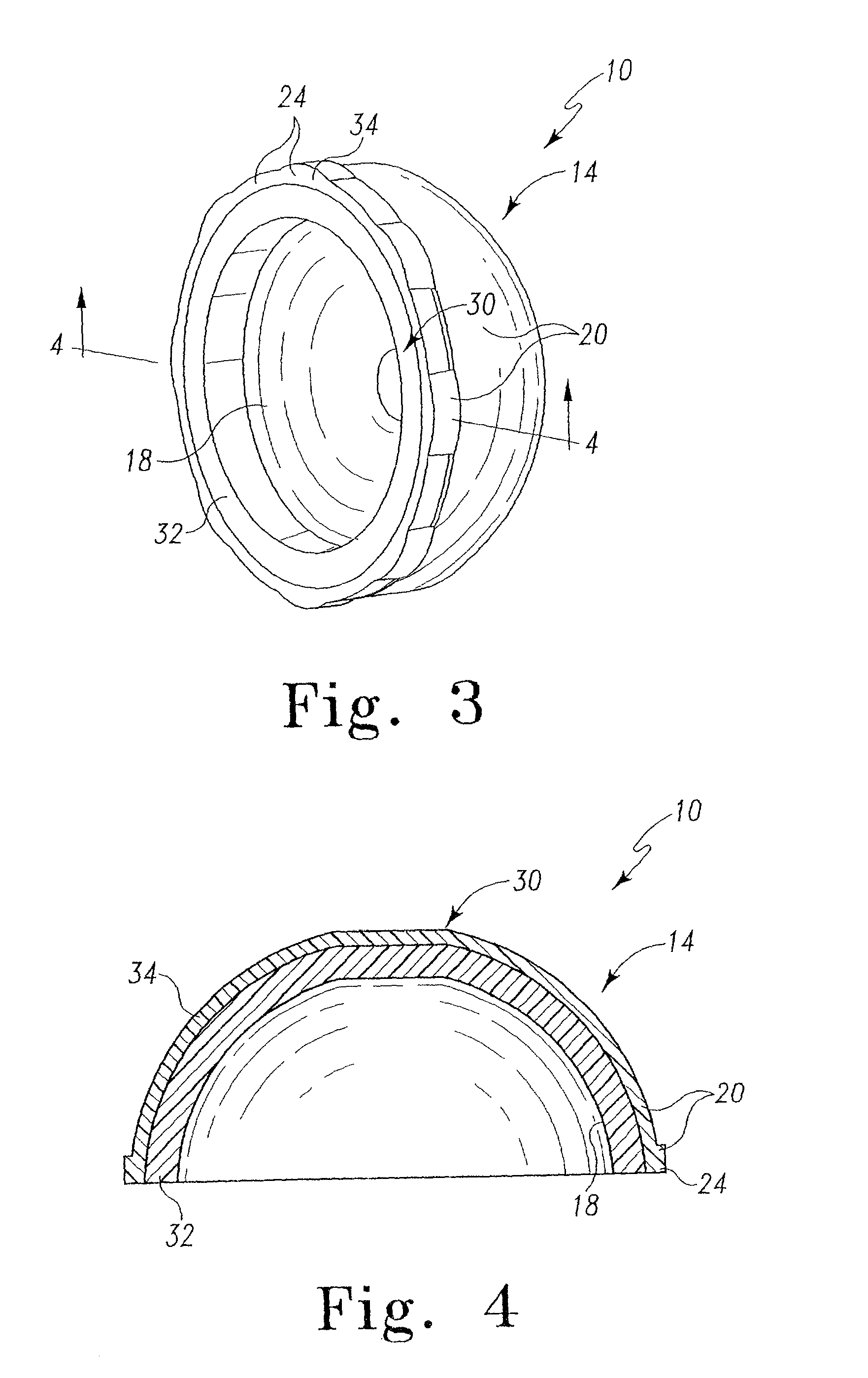
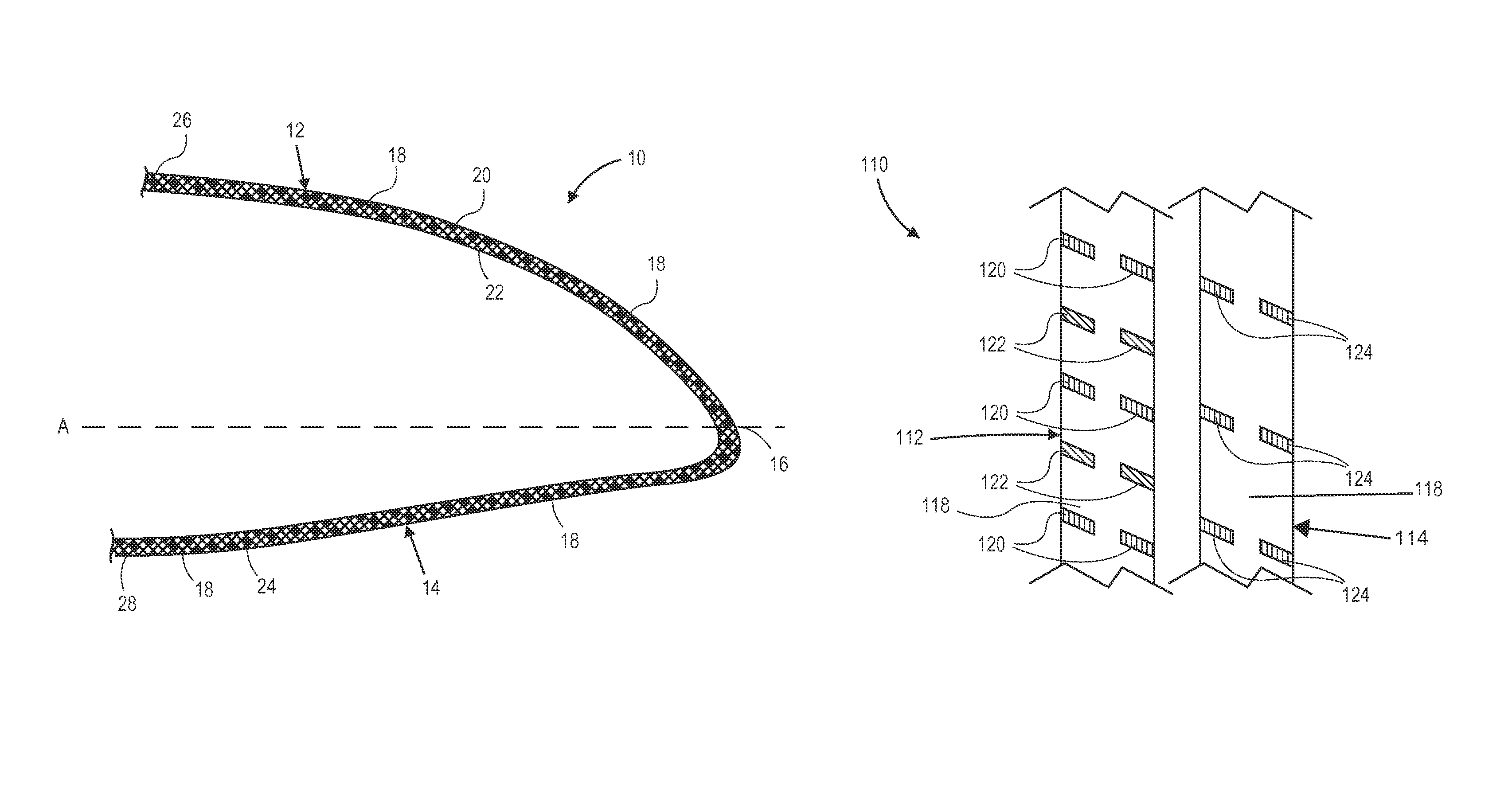
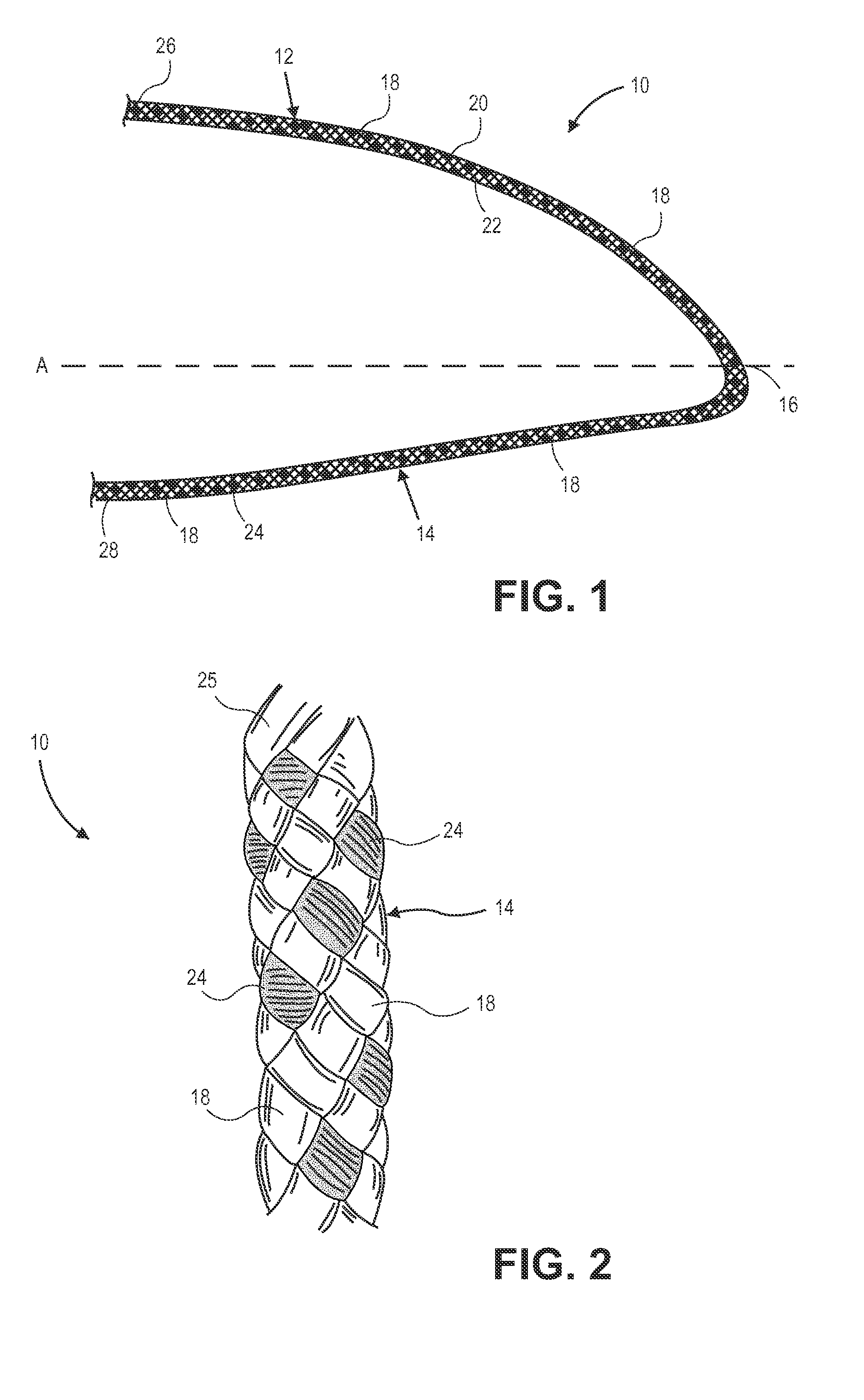
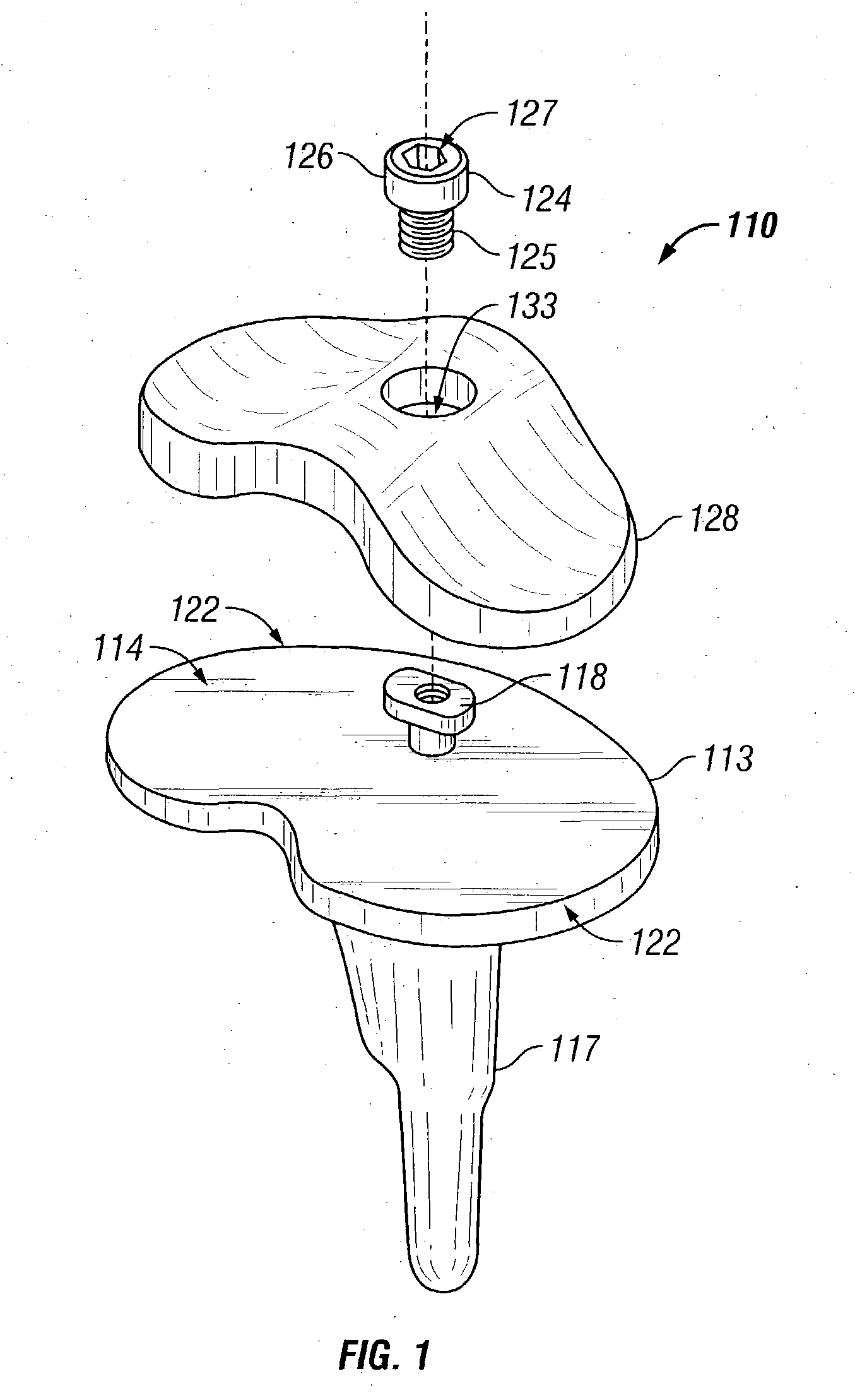
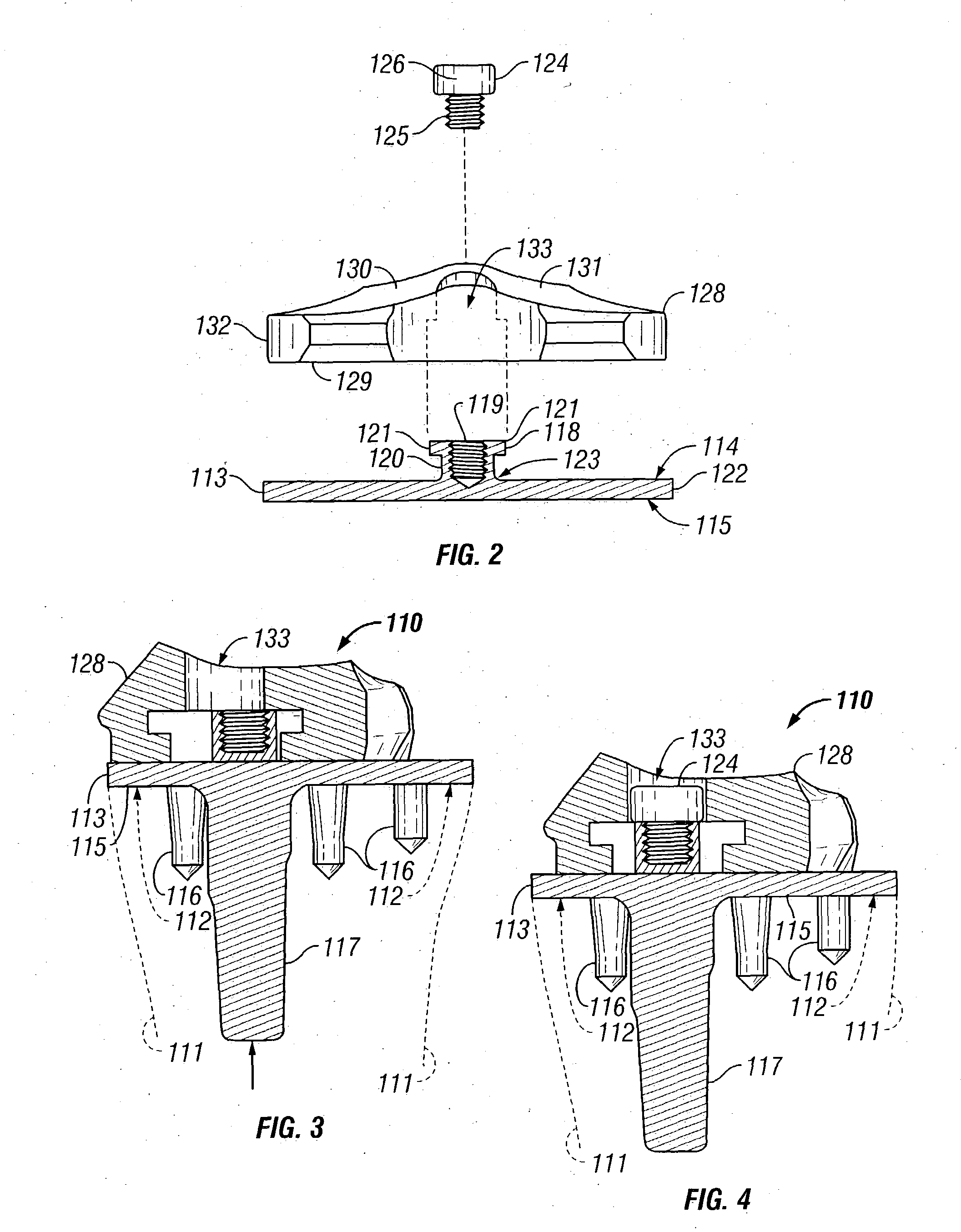
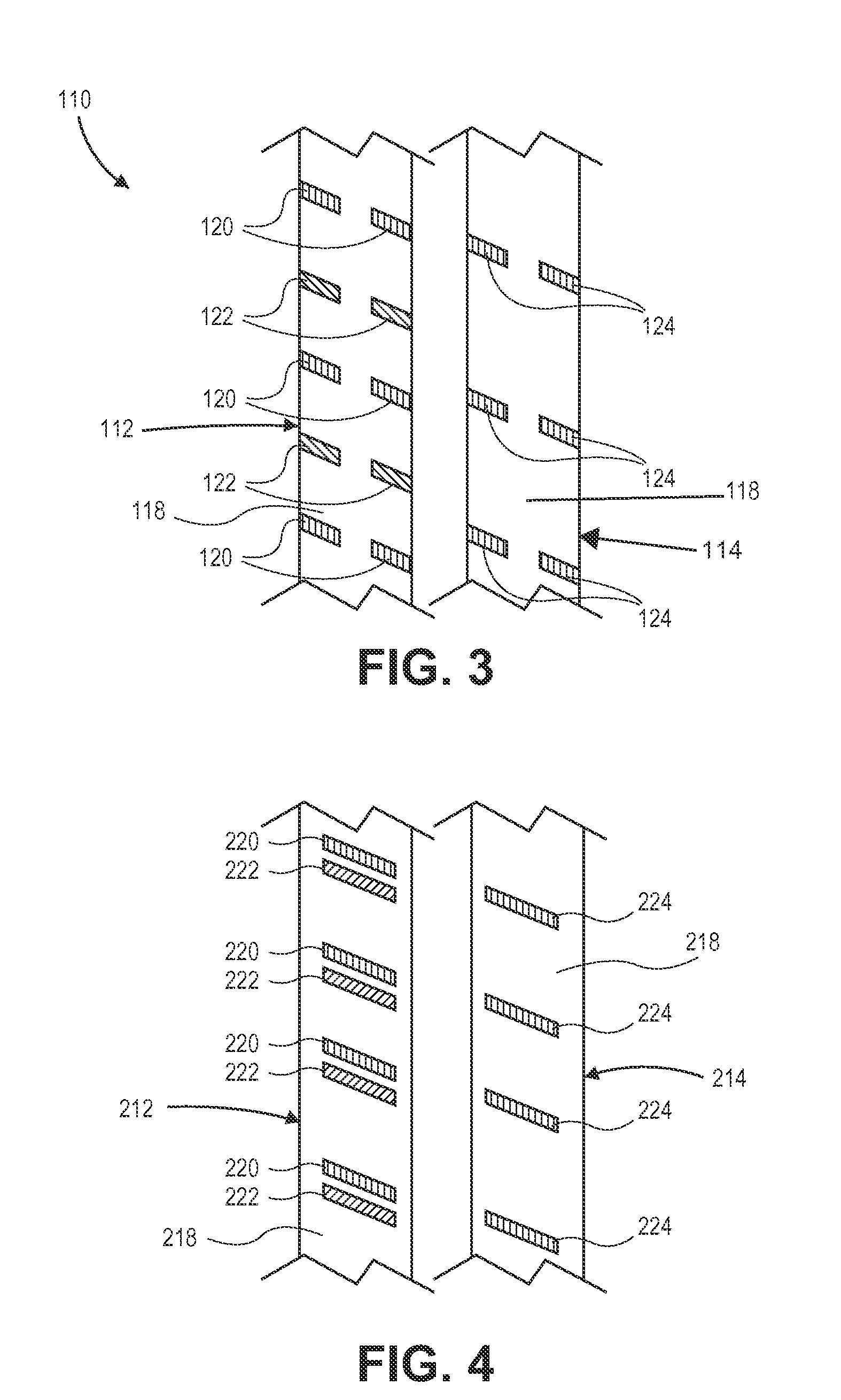
Radiation and melt treated ultra high molecular weight polyethylene prosthetic device and method
InactiveUS6641617B1Reduce productionReduce osteolysis and inflammatory reactionBone implantJoint implantsOxidation resistantPeriprosthetic
A medical prosthesis for use within the body which is formed of radiation treated ultra high molecular weight polyethylene having substantially no detectable free radicals, is described. Preferred prostheses exhibit reduced production of particles from the prosthesis during wear of the prosthesis, and are substantially oxidation resistant. Methods of manufacture of such devices and material used therein are also provided.
Owner:CENTPULSE ORTHOPEDICS +1
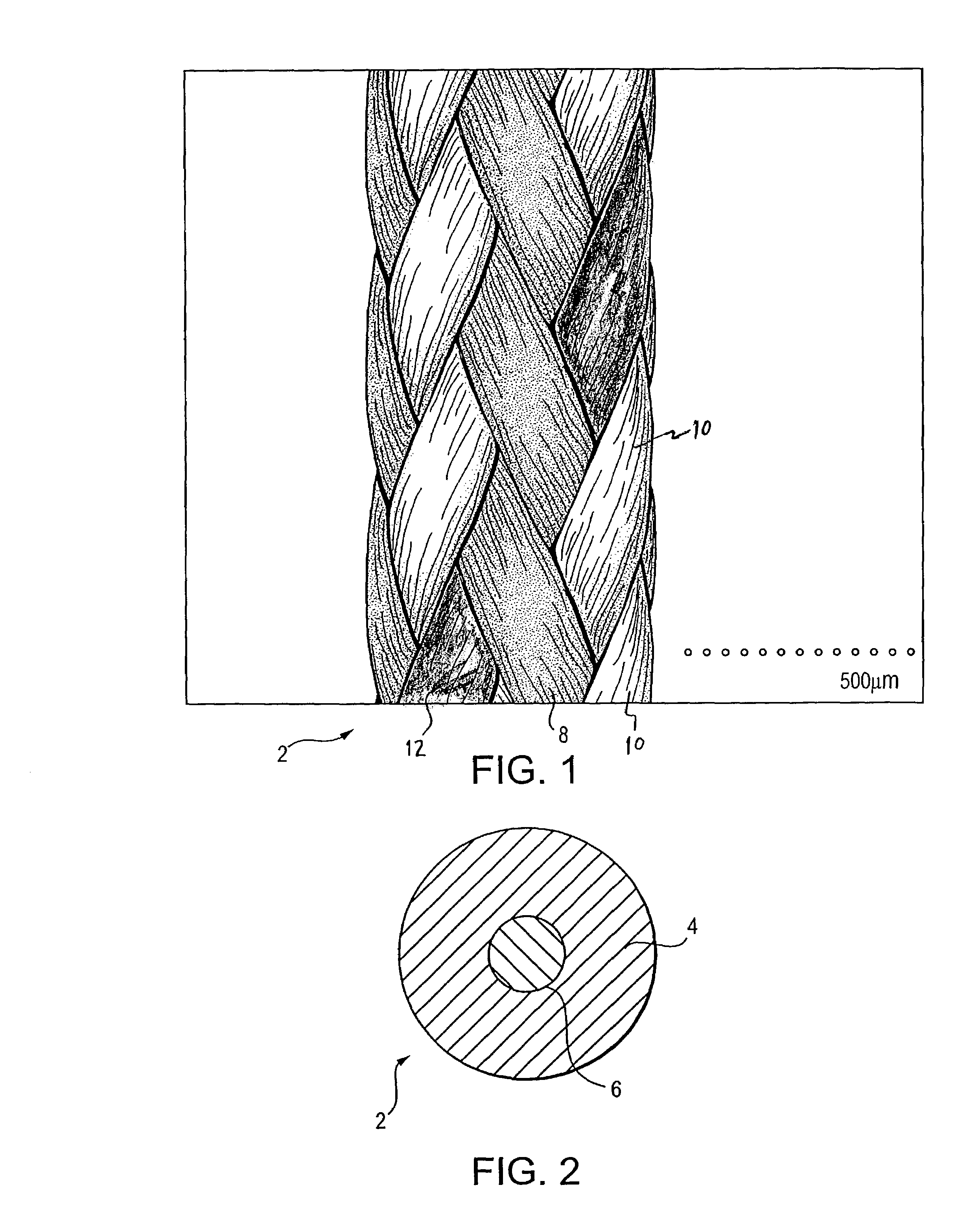
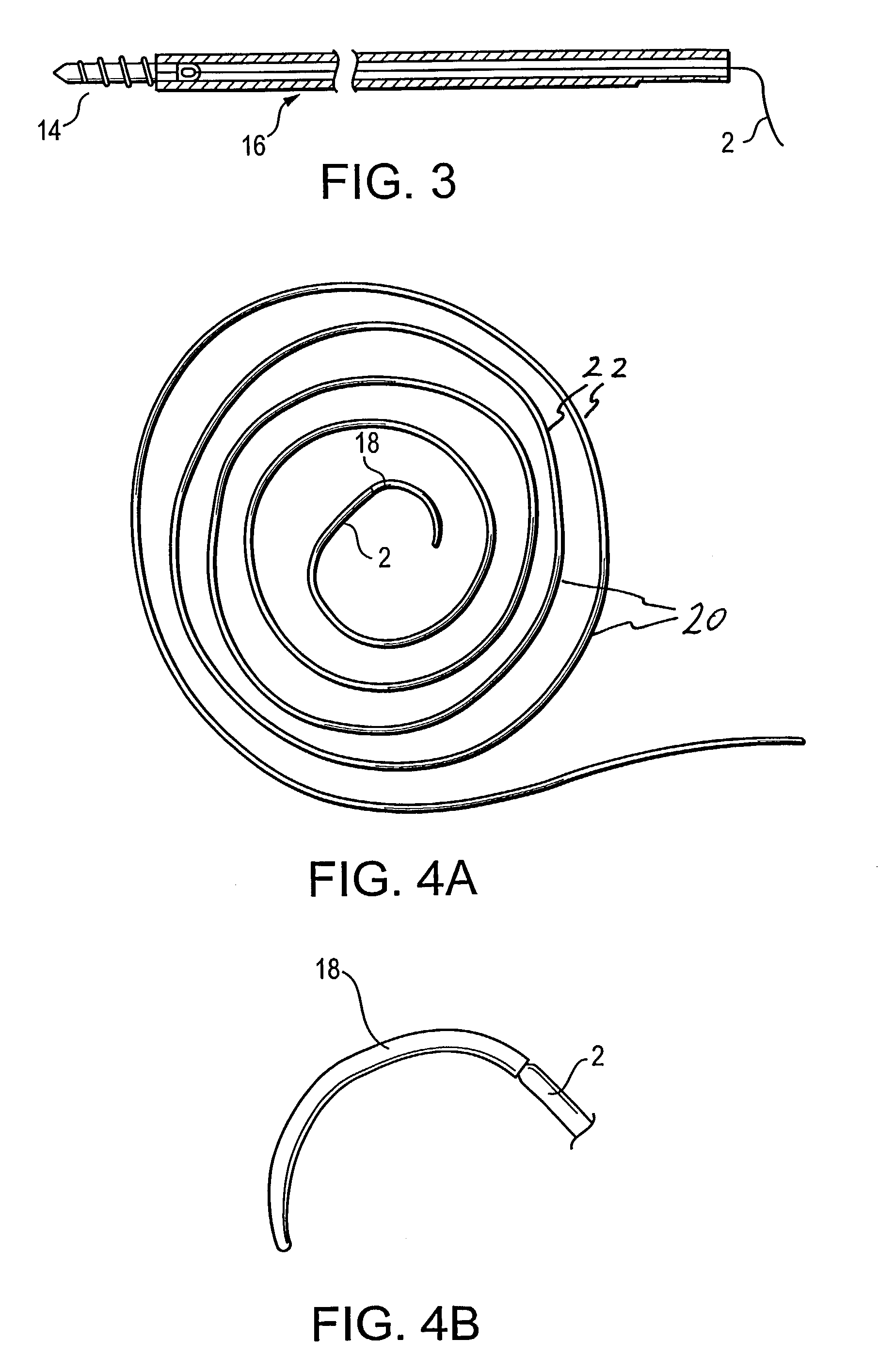
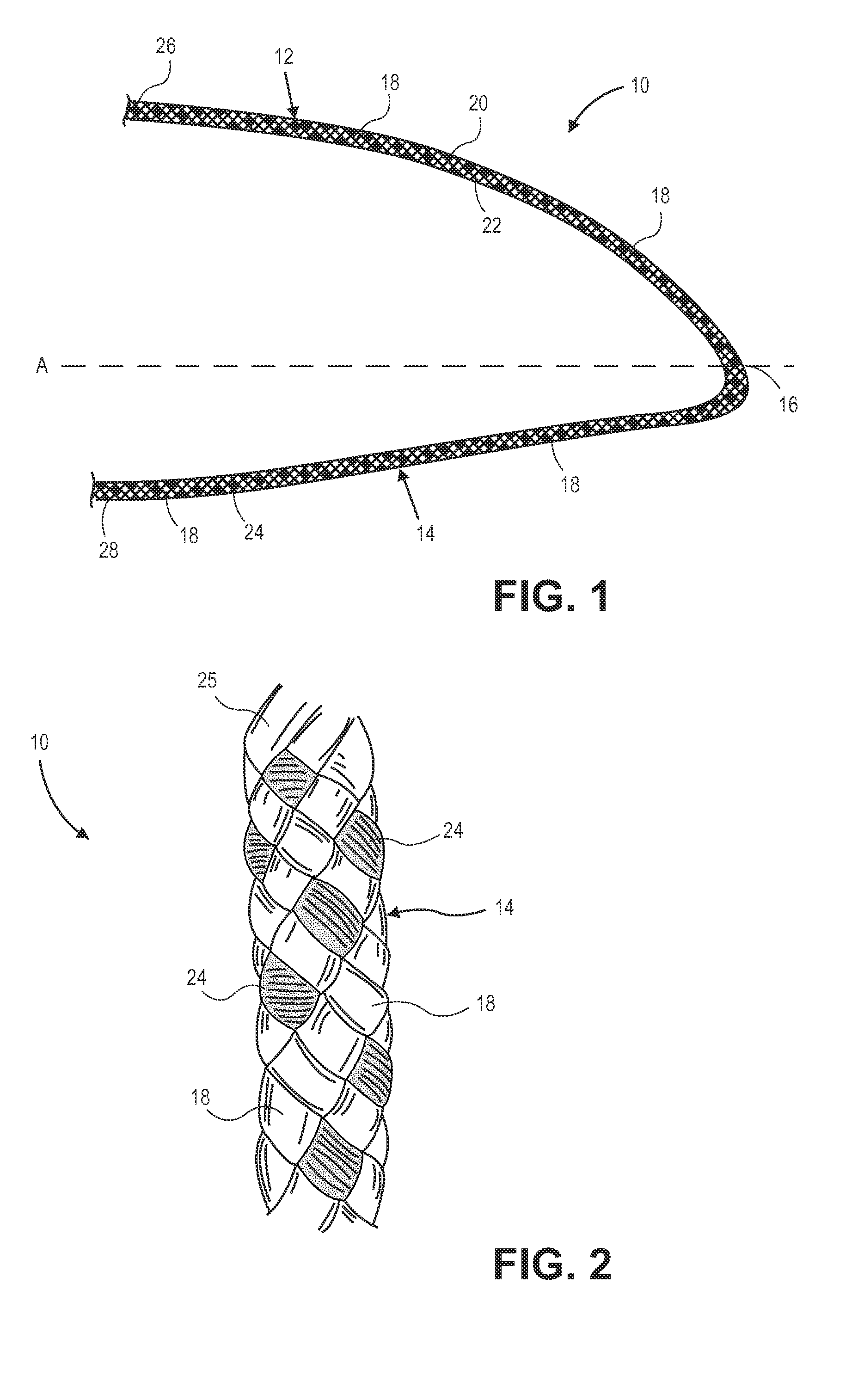
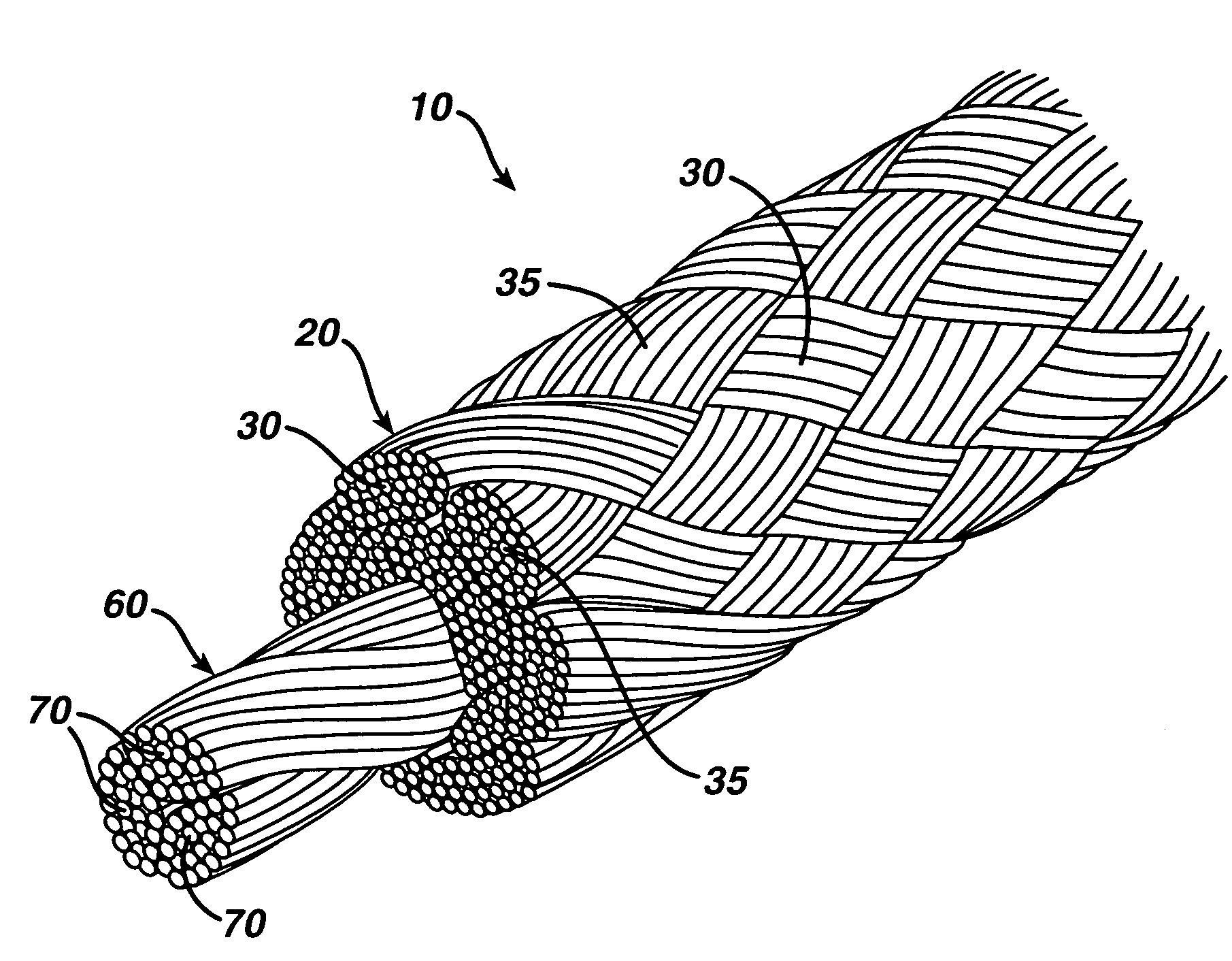
High strength suture with colored trace at one end
InactiveUS6994719B2High strengthImproved tie down characteristicSuture equipmentsDiagnosticsPolyesterEngineering
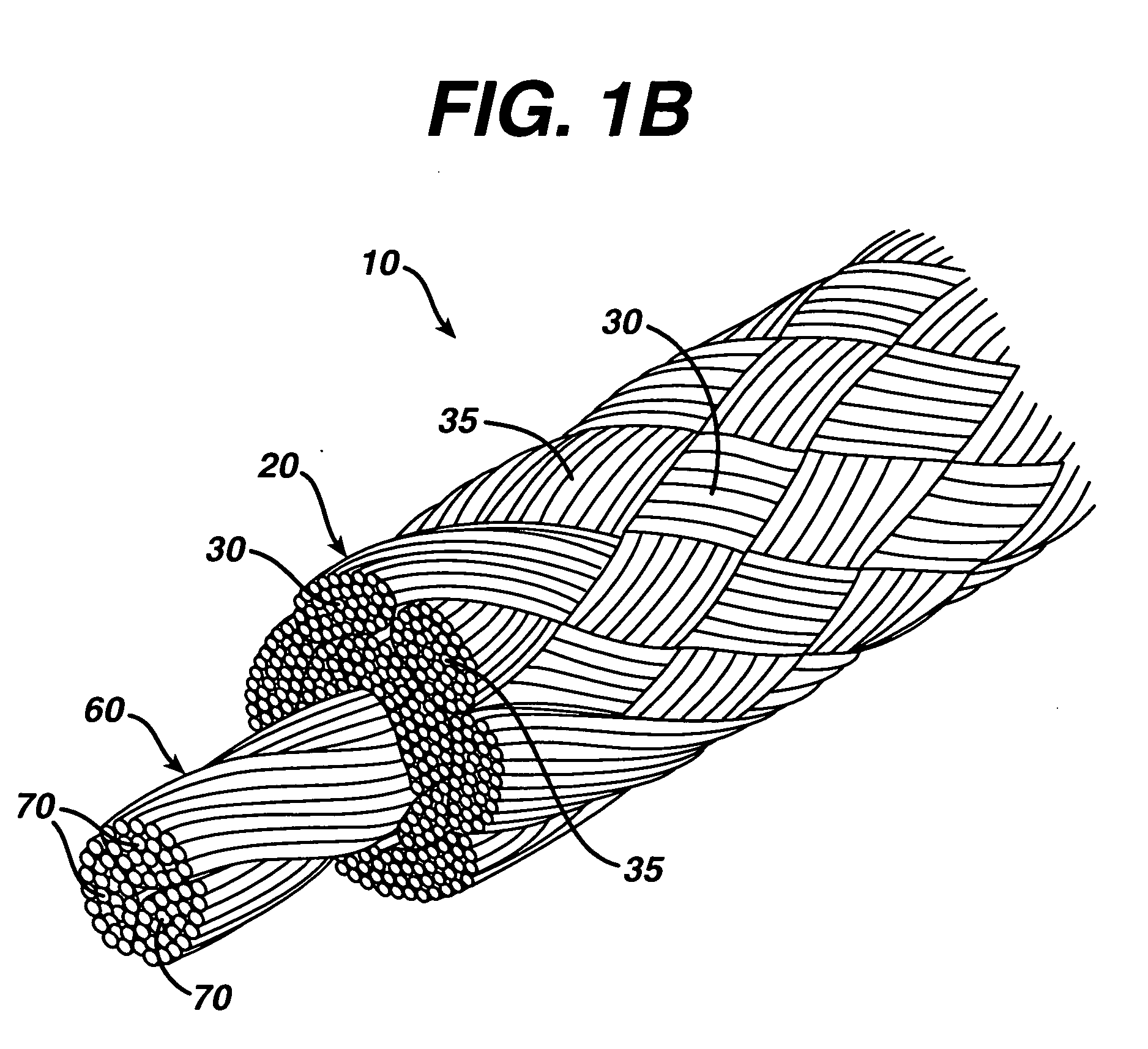
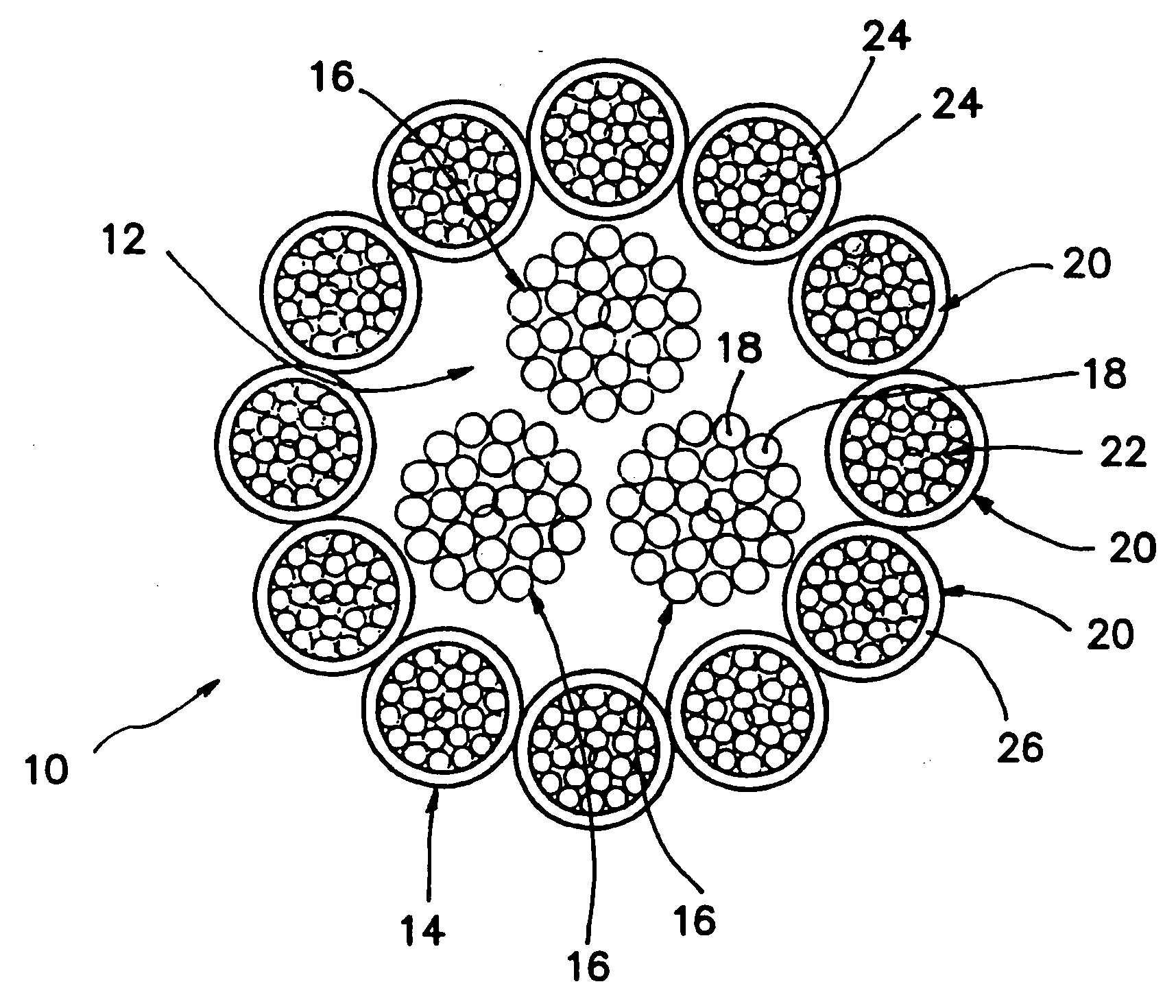
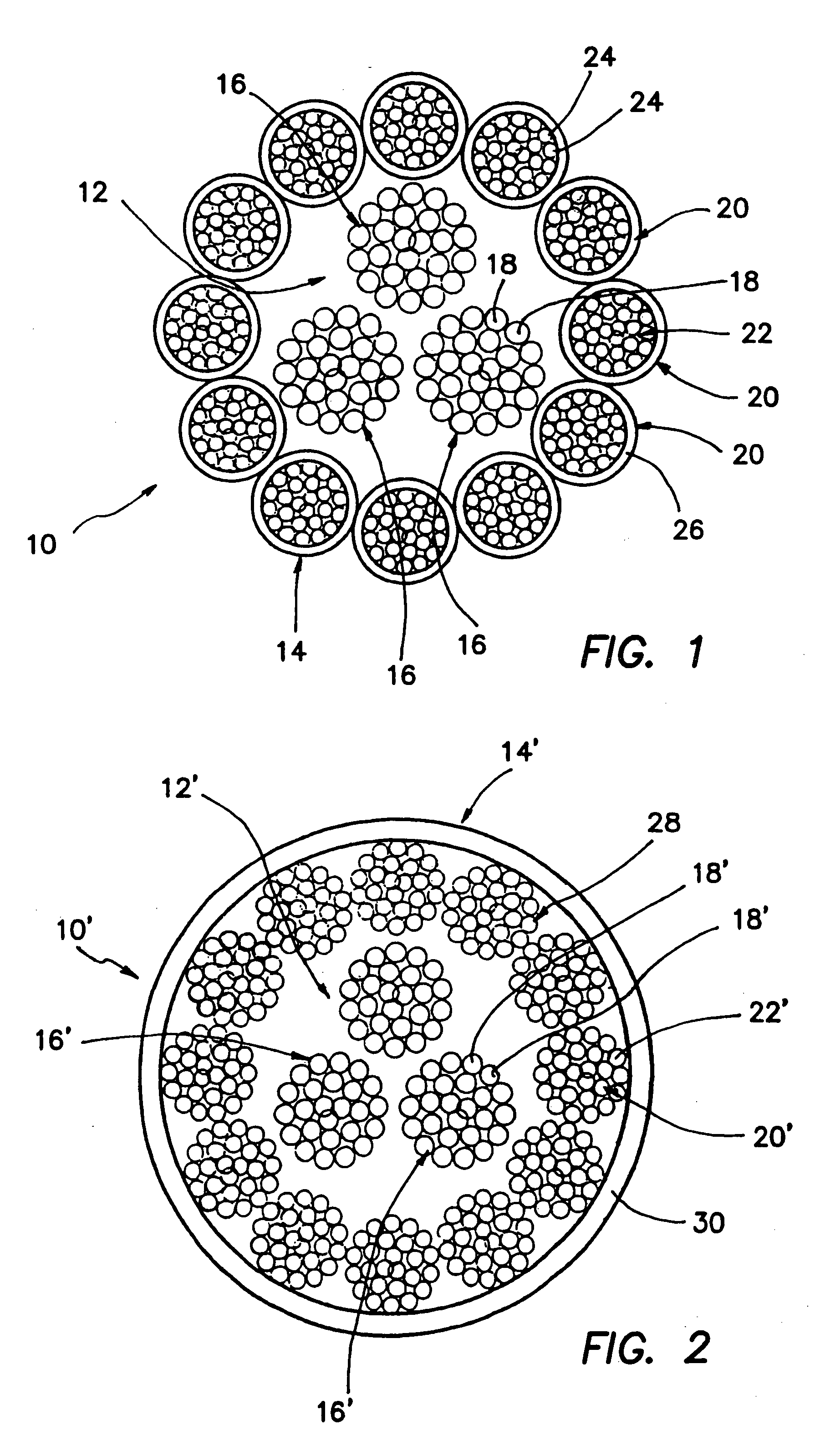
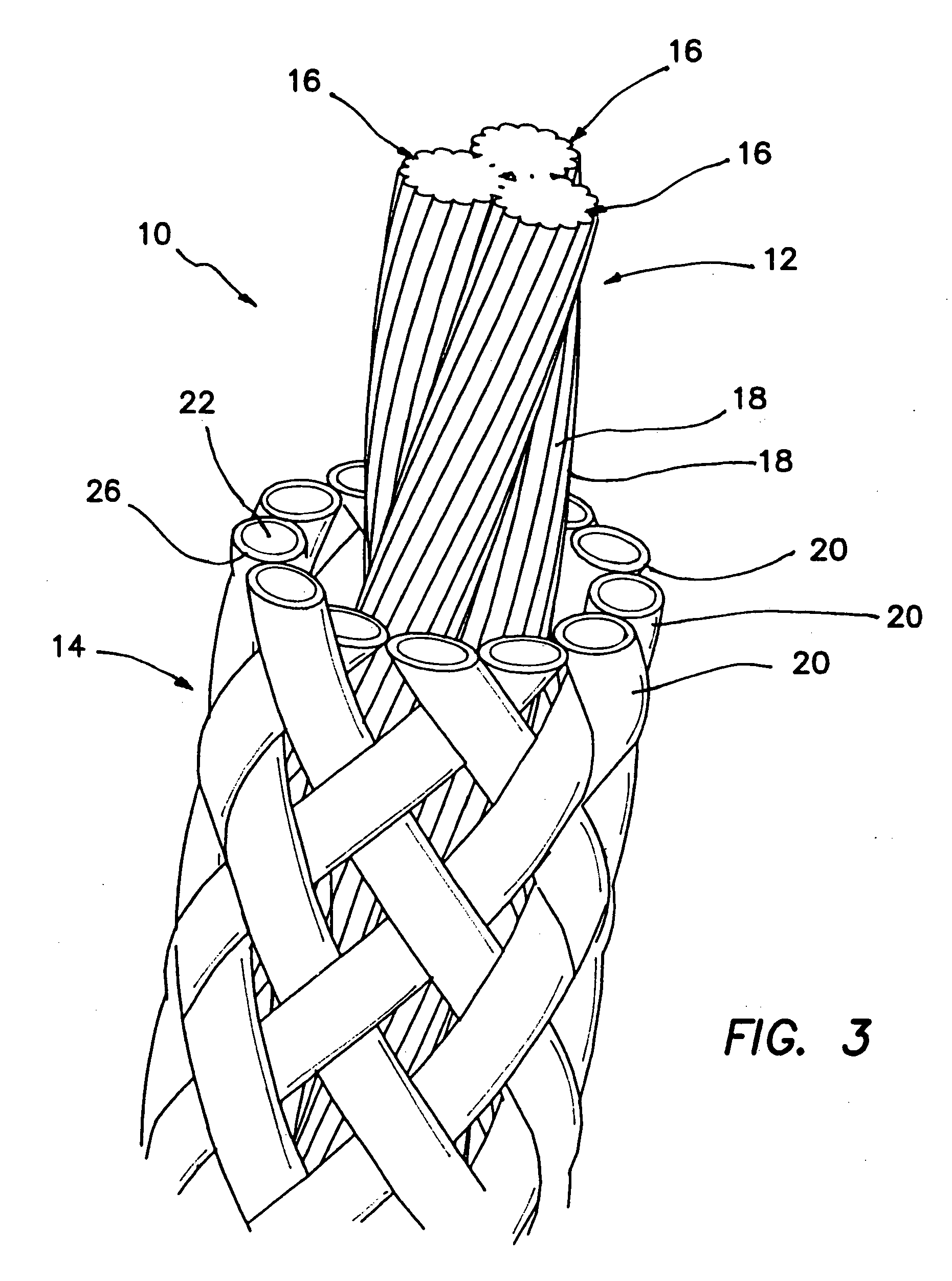
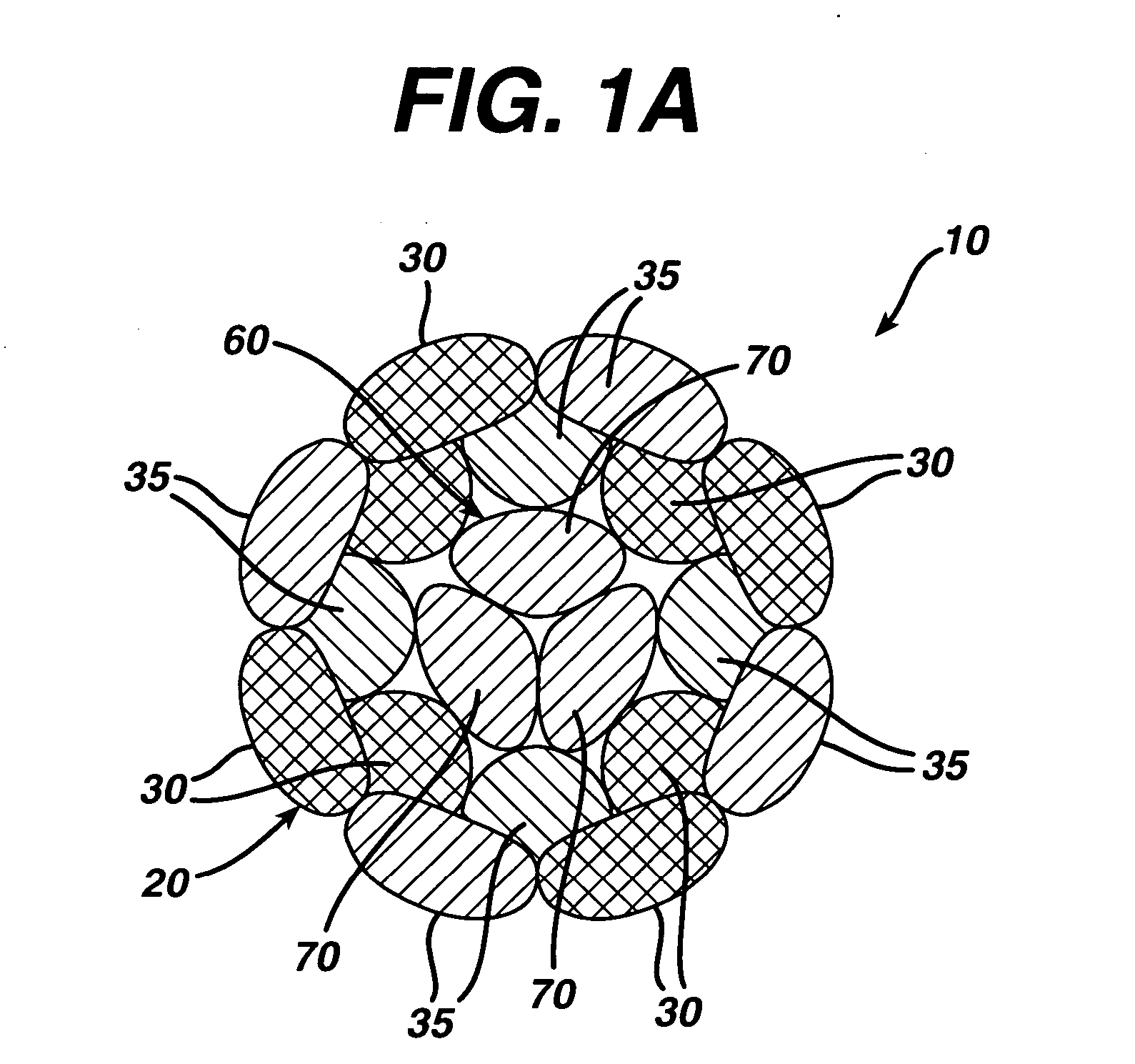
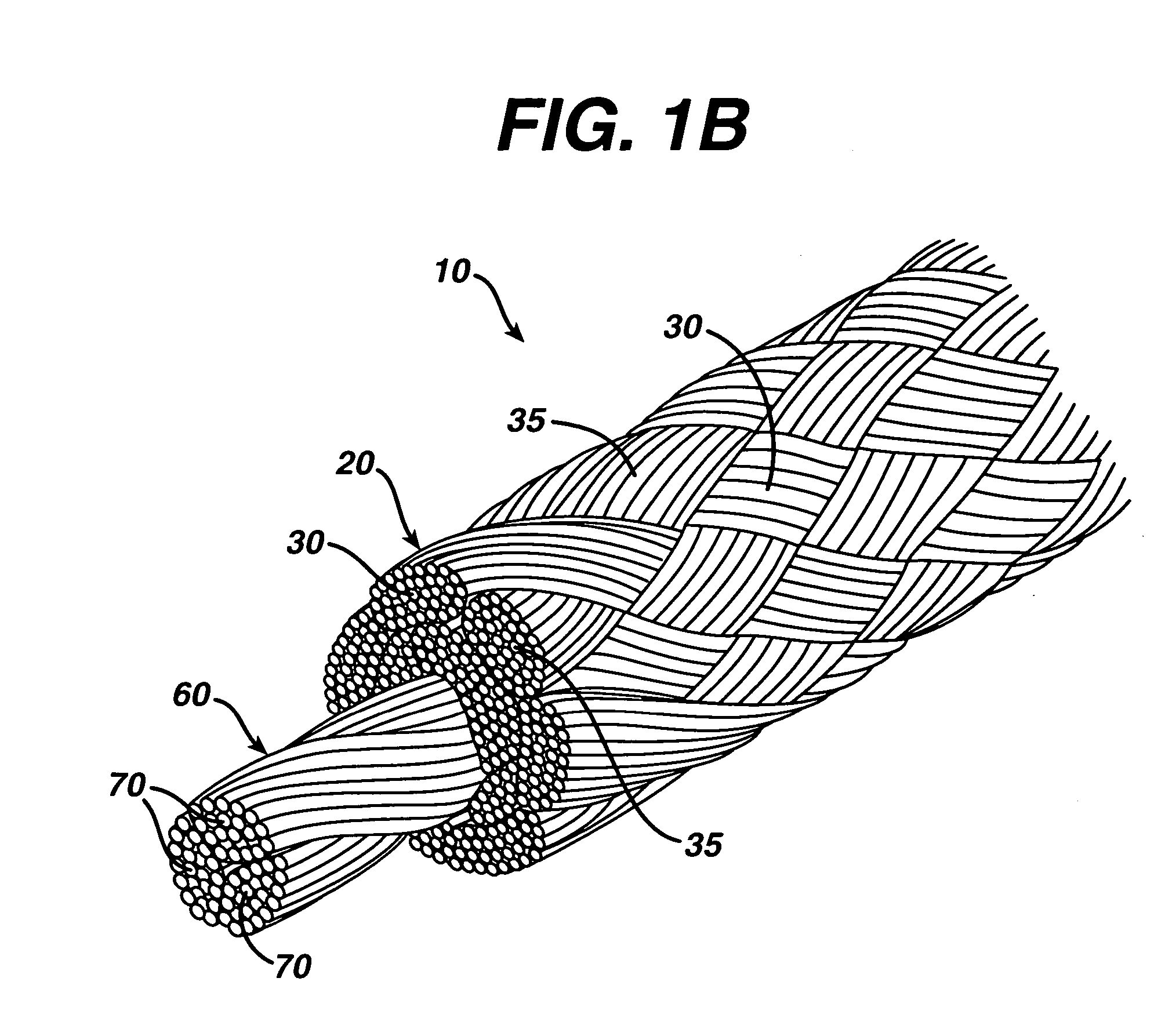
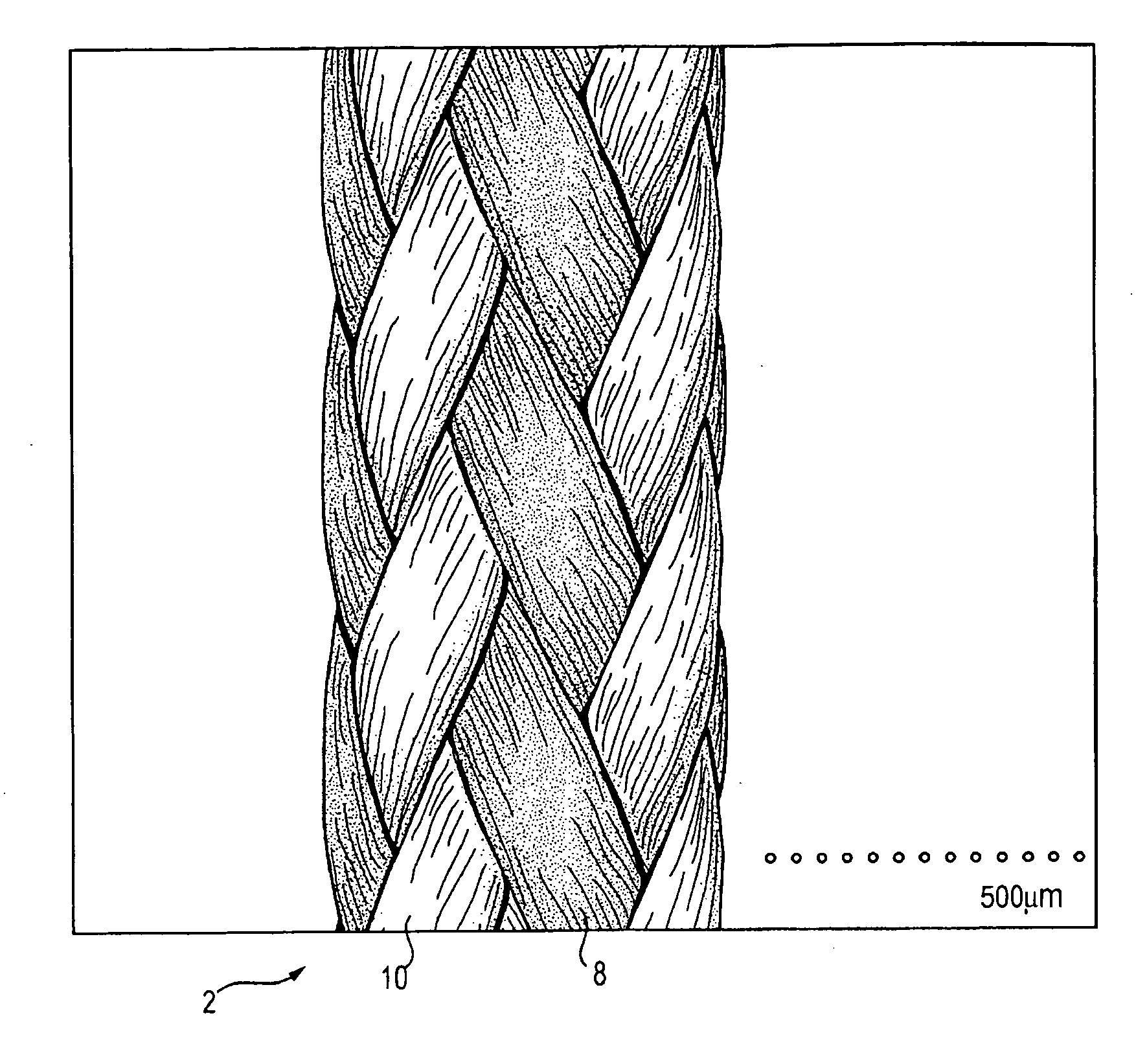
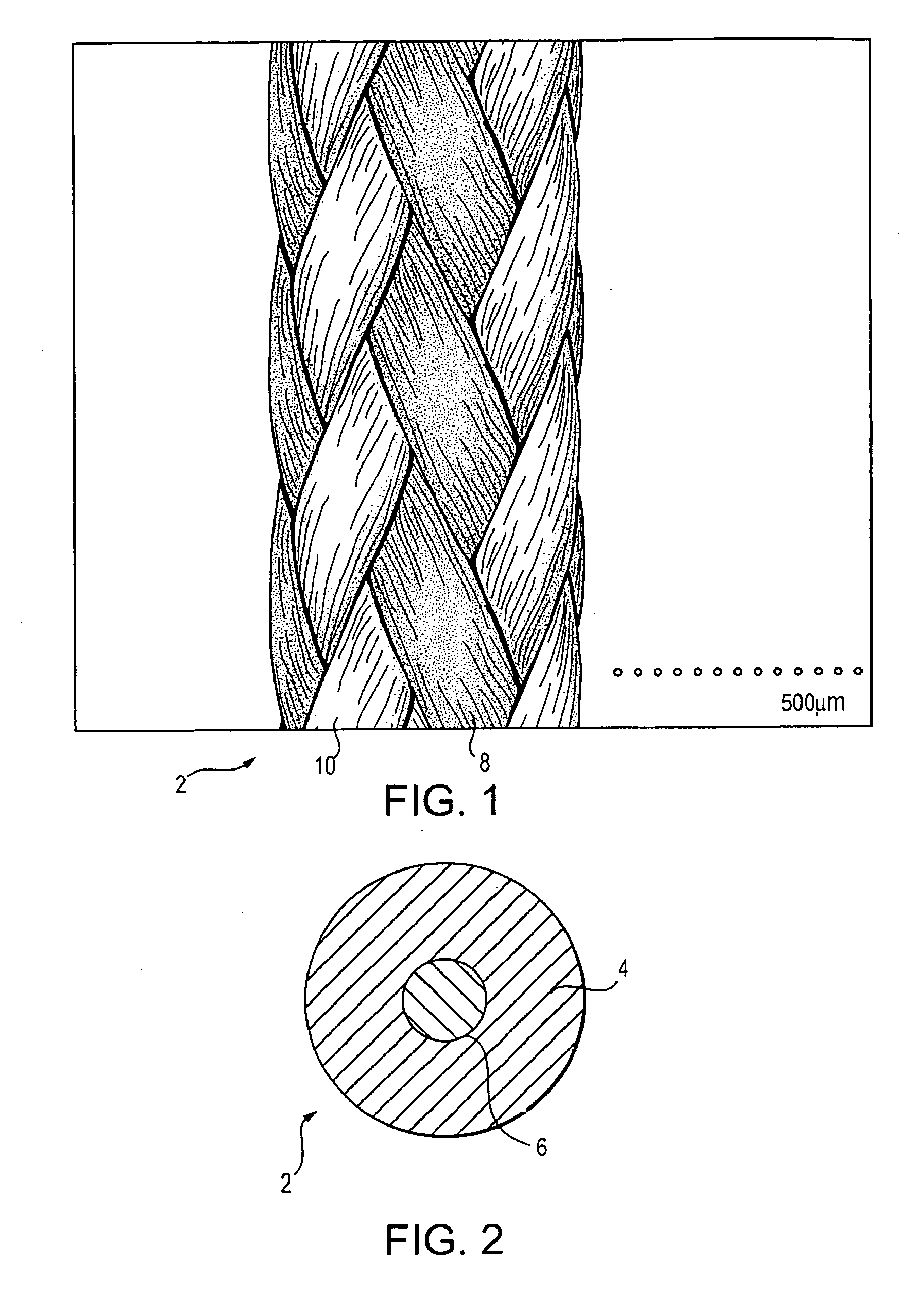
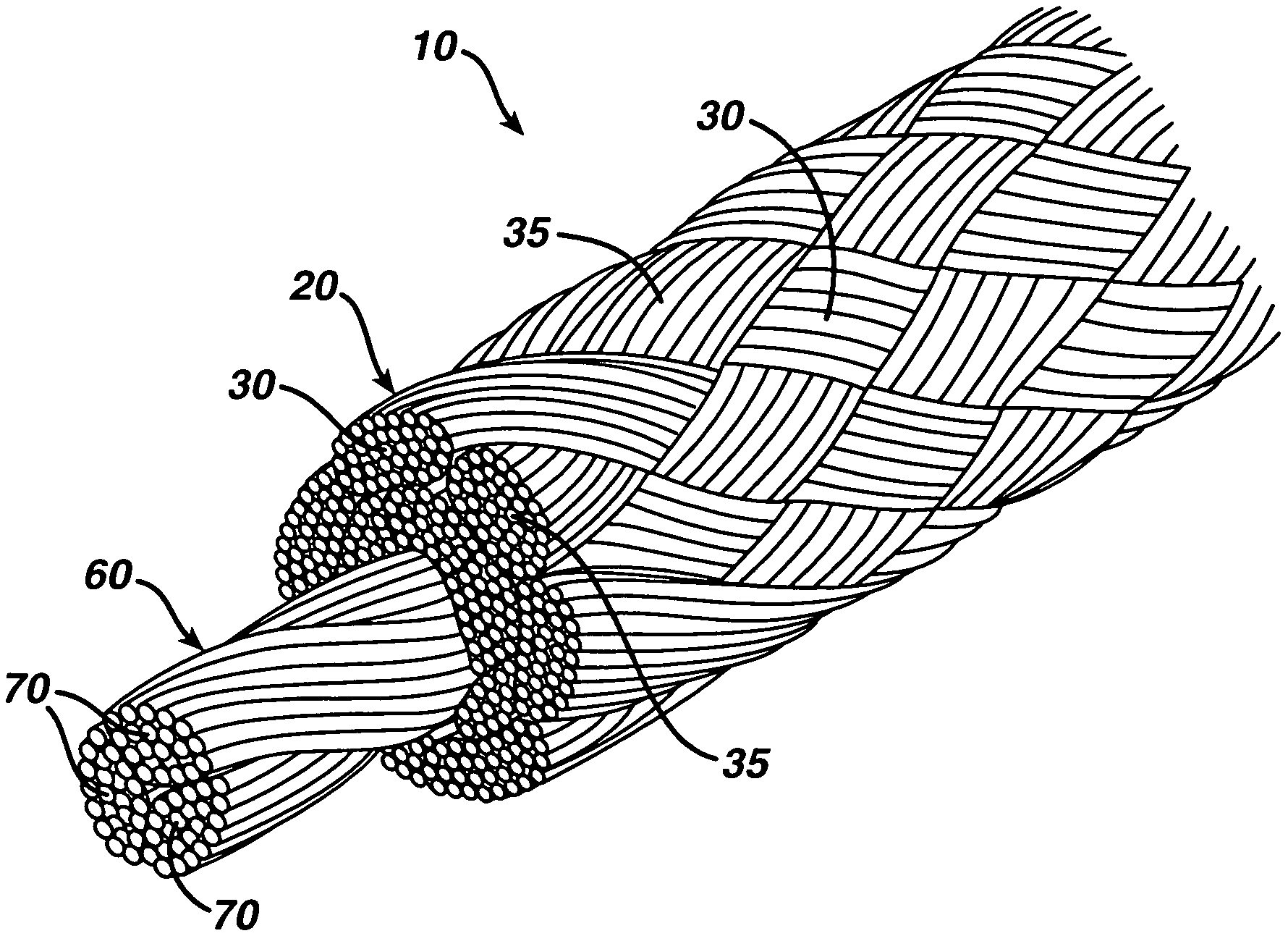
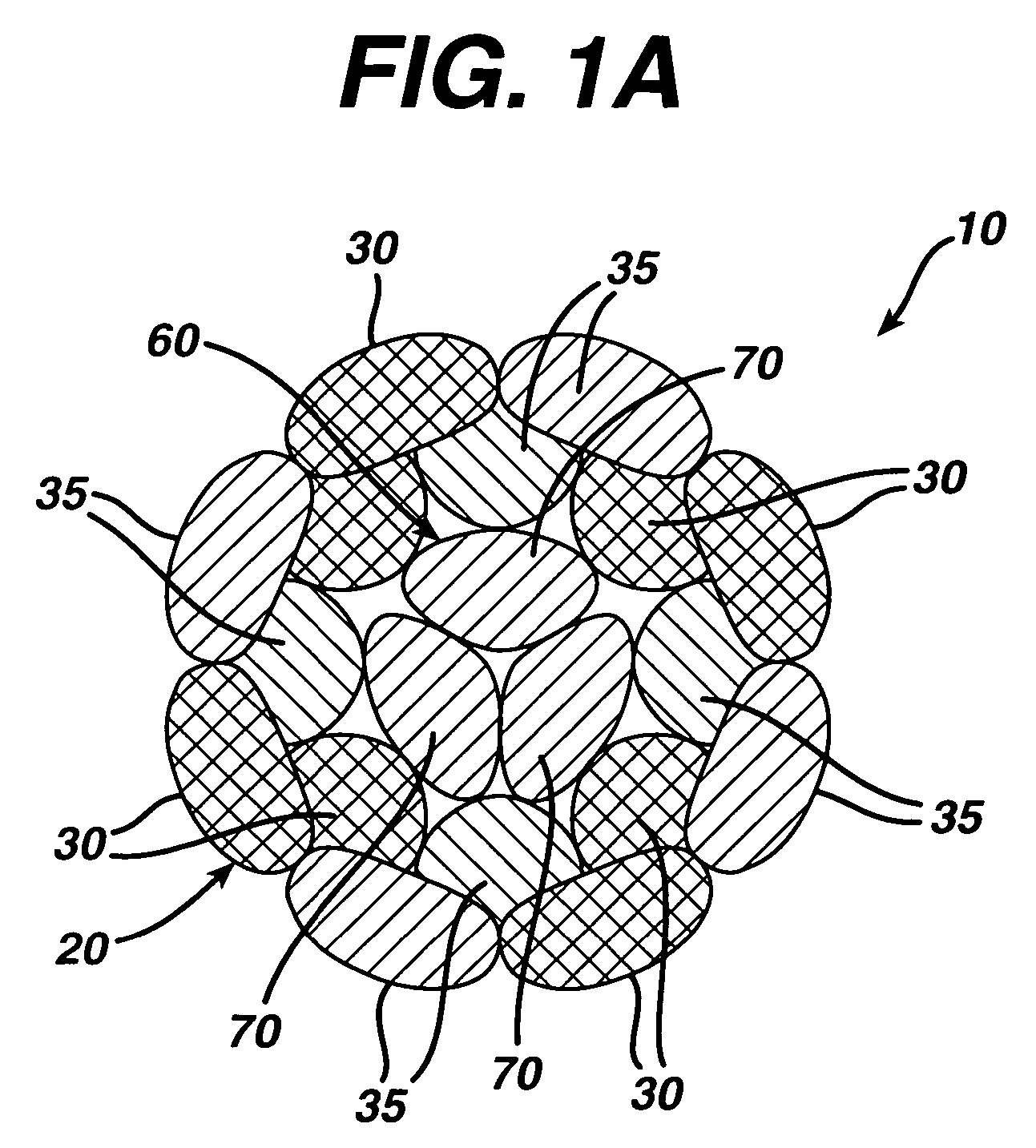
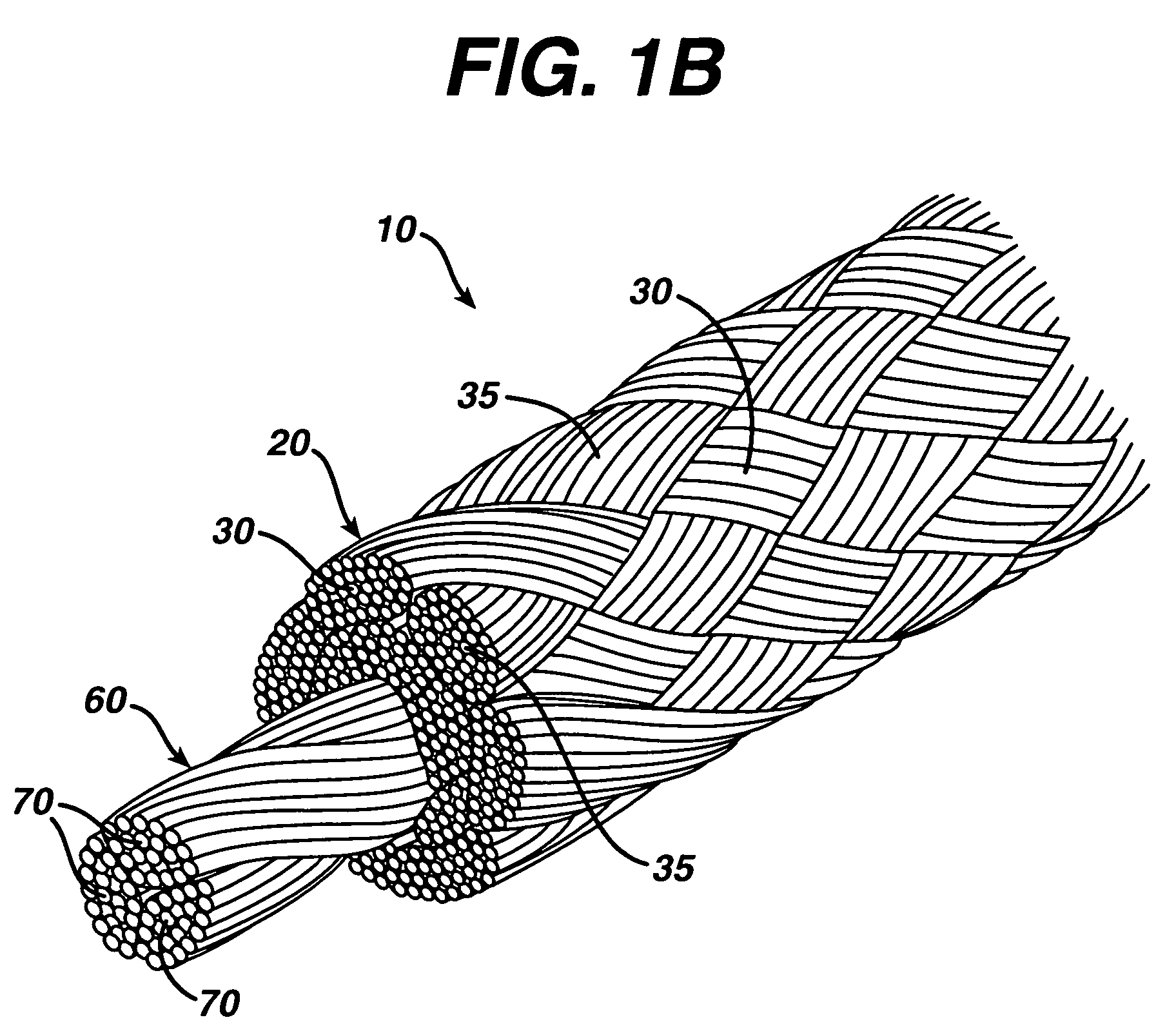
A high strength abrasion resistant surgical suture material with improved tie down characteristics is color coded for visualization and identification purposes. The suture features a multifilament cover formed of strands of ultra high molecular weight long chain polyethylene braided with polyester, nylon or a bioabsorbable material. Selected nylon fibers in the cover are provided in a color contrasting with the other cover fibers to provide an identifiable trace. The cover surrounds a core formed of twisted strands of ultrahigh molecular weight polyethylene. The suture, provided in a #2 size, has the strength of #5 Ethibond, is ideally suited for most orthopedic procedures, and can be attached to a suture anchor or a curved needle. The identifiable trace preferably is provided along one half of the length of the suture, so that when the suture is loaded onto a suture anchor, for example, the two legs of the length of suture on either side of the suture anchor can be readily identified.
Owner:ARTHREX INC
Sealing stopper for a syringe and a prefilled syringe
There can be provided a sealing stopper for a syringe, having very high sealing property and sliding property, and a prefilled syringe using this sealing stopper and capable of preserving a medicament for a long time and operating in easy and precise manner during injecting. This syringe is also excellent in sanitary and operating property during a step of formulation or preservation of a medicament. In this sealing stopper for a syringe, a surface of the rubber body is laminated with a tetrafluoroethylene resin film or ultra-high molecular weight polyethylene film having an average roughness Ra on the central line of the surface in a range of at most 0.05 mu m and a kinematic friction coefficient of at most 0.2.
Owner:DAIKYO SEIKO LTD
High strength suture with absorbable core and suture anchor combination
ActiveUS20050149118A1Improved absorption profileReducing knot profile of knotSuture equipmentsSurgical needlesYarnMedicine
A novel high tensile strength semi-absorbable composite suture with minimized non-absorbable mass. The suture has a core made from a bioabsorbable polymer. The core is covered by a braided sheath. The braided sheath is made from an absorbable yarn and a bioabsorbable yarn. The bioabsorbable yarn is made from a least one filament of a bioabsorbable polymer. The nonabsorbable yarn is made from at least one filament of ultra high molecular weight polyethylene.
Owner:DEPUY SYNTHES PROD INC
Optimized suture braid
A high strength abrasion resistant surgical suture material with industry standard knot tying characteristics and color marking characteristics includes a core formed of a plurality of twisted fibers of a first material, surrounded by a braided cover made from fibers of a second material different than the first material. The first material is preferably ultrahigh molecular weight polyethylene and the second material is preferably a polymeric material having good knot-tying characteristics.
Owner:ARTHROCARE
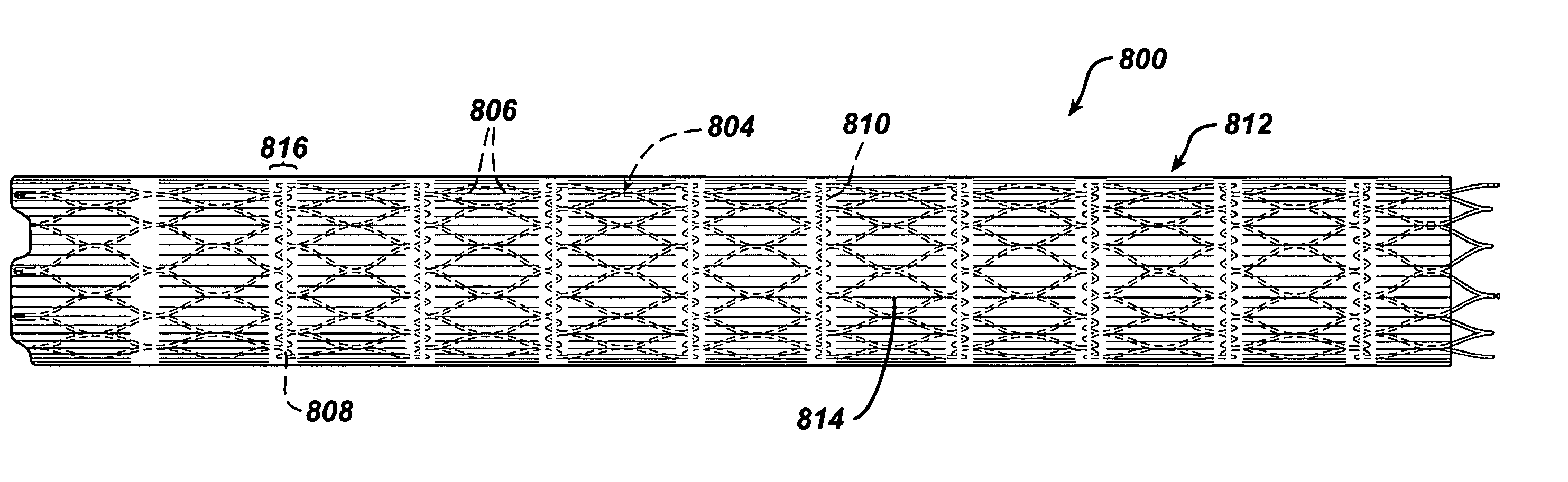
Endovascular graft with differentiable porosity along its length
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device such as a stent-graft. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. A stent-graft fabricated from a thin-walled, high strength material provides for a more durable and lower profile endoprosthesis. The stent-graft comprises one or more stent segments covered with a fabric formed by the weaving, knitting or braiding of a biocompatible, high tensile strength, abrasion resistant, highly durable yarn such as ultra high molecular weight polyethylene. The one or more stent segments may be balloon expandable or self-expanding. The fabric may be attached to the stent segments utilizing any number of known materials and techniques. In addition, the pore size of the graft material may be varied.
Owner:CORDIS CORP
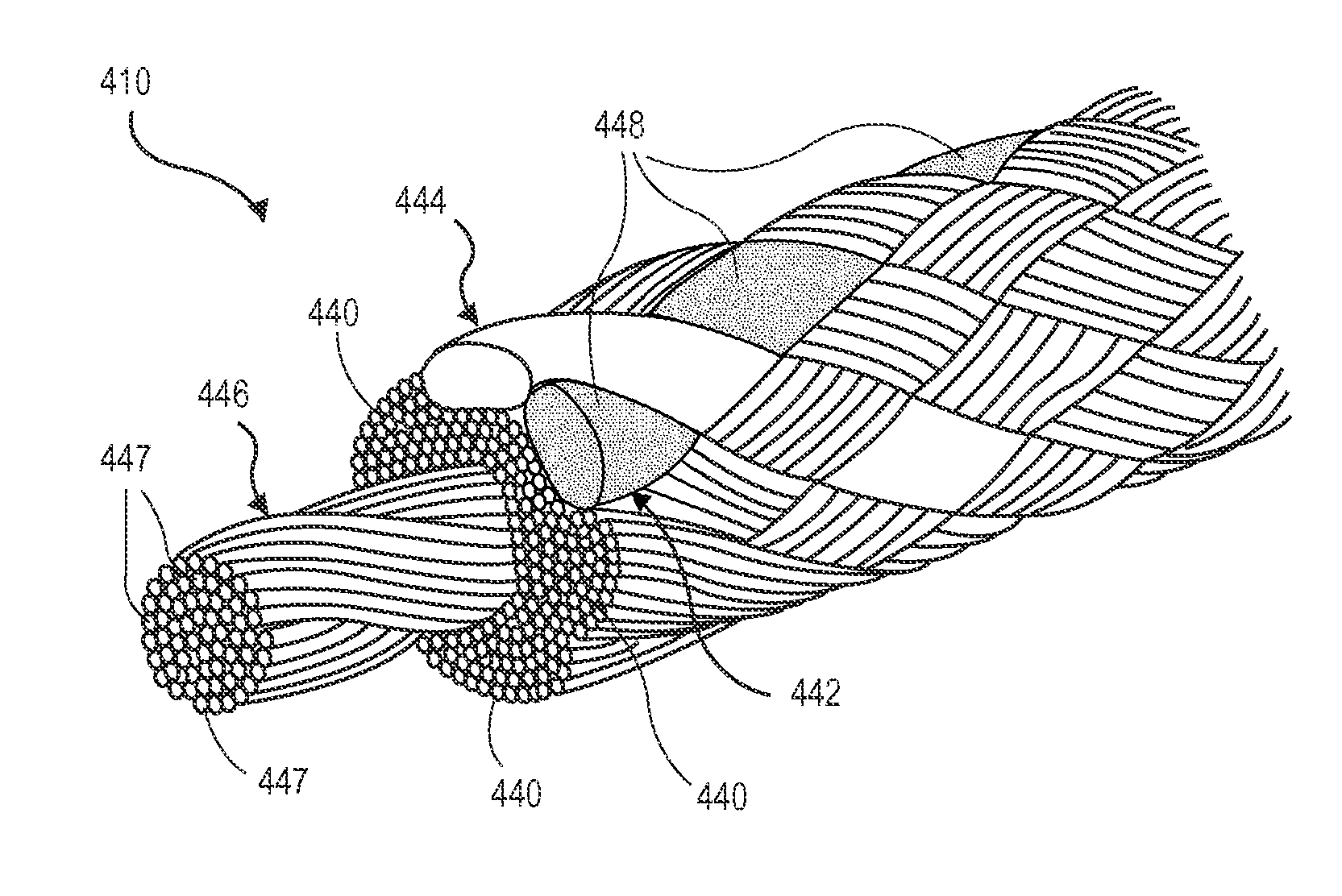
Ballistic-resistant panel including high modulus ultra high molecular weight polyethylene tape
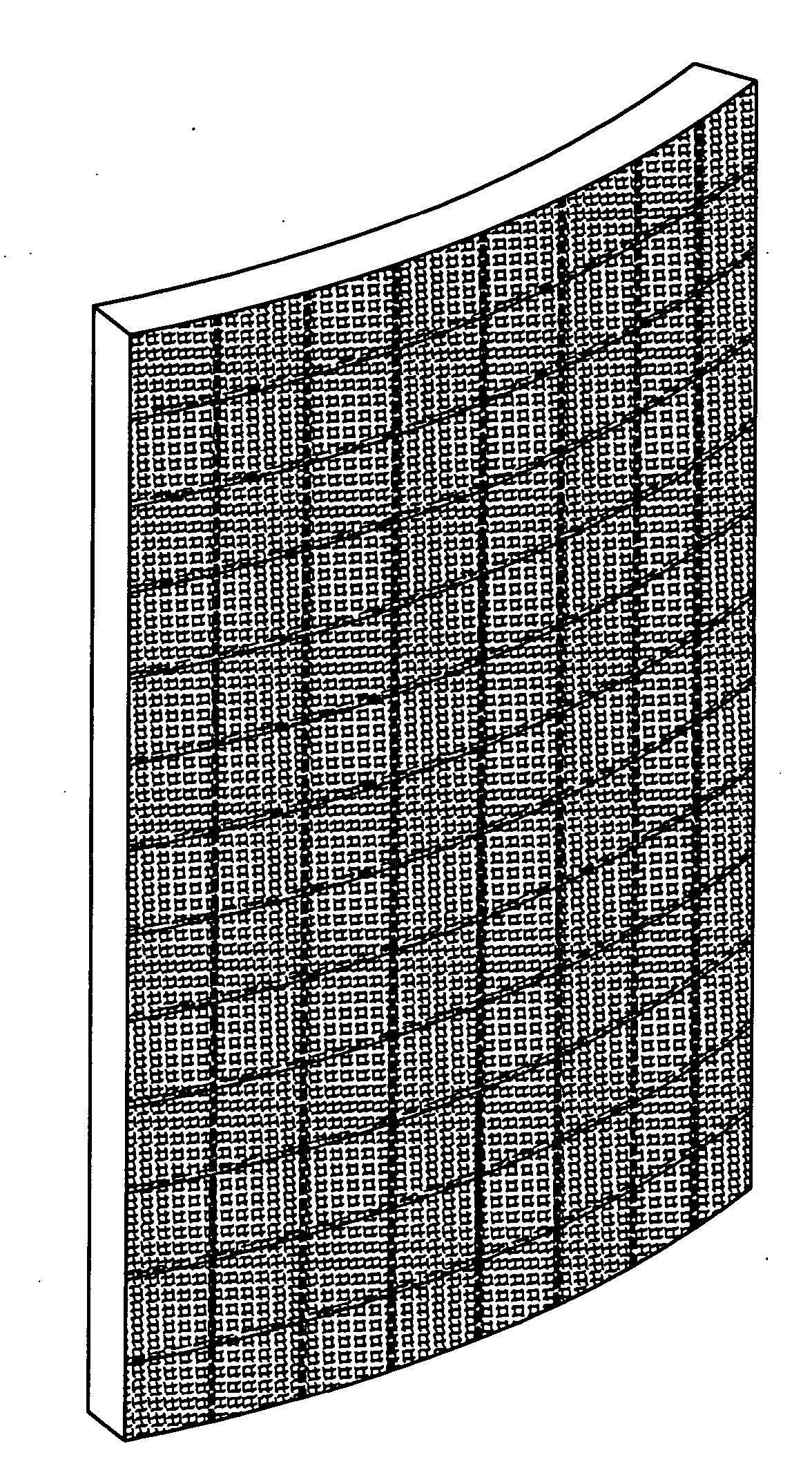
A ballistic-resistant panel in which the entire panel or a strike-face portion thereof is formed of a plurality of sheets of high modulus high molecular weight polyethylene tape. The sheets of high modulus polyethylene tape can be in the form of cross-plied laminated layers of tape strips or a woven fabric of tape strips. The strips of UHMWPE tape include a width of at least one inch and a modulus of greater than 1400 grams per denier. The ballistic-resistant panel may include a backing layer of conventional high modulus fibers embedded in resin. A wide variety of adhesives were found acceptable for bonding the cross-plied layers of high modulus polyethylene tape together for forming the ballistic-resistant panels of the present invention.
Owner:DUPONT SAFETY & CONSTR INC
High strength suture with coating and colored trace
A high strength abrasion resistant surgical suture material with improved tie down characteristics is color coded for visualization and identification purposes. The suture features a multifilament cover formed of strands of ultra high molecular weight long chain polyethylene braided with polyester, nylon or a bioabsorbable material. Selected nylon fibers in the cover are provided in a color contrasting with the other cover fibers to provide an identifiable trace. The cover surrounds a core formed of twisted strands of ultrahigh molecular weight polyethylene. The suture, provided in a #2 size, has the strength of #5 Ethibond, is ideally suited for most orthopedic procedures, and can be attached to a suture anchor or a curved needle.
Owner:ARTHREX
Composite prosthetic bearing having a crosslinked articulating surface and method for making the same
InactiveUS7819925B2Promote oxidationEasy to wearBone implantSynthetic resin layered productsProsthesisCrosslinked polymers
An implantable prosthetic bearing is constructed of a composite material having a first layer and second layer. The first layer has an articulating surface defined therein, whereas the second layer has a engaging surface defined therein for engaging either another prosthetic component or the bone itself The first layer of the implantable prosthetic bearing is constructed of crosslinked polymer such as Ultra-High Molecular Weight Polyethylene, whereas the second layer of the implantable prosthetic bearing is constructed of polymer such as Ultra-High Molecular Weight Polyethylene that is either non-crosslinked or crosslinked to a lesser degree than the first layer. In such a manner, the first layer possesses mechanical properties which are advantageous in regard to the articulating surface (e.g., enhanced wear and oxidation resistance), whereas the second layer possesses mechanical properties which are advantageous in regard to the engaging surface (e.g., high ductility, toughness, and creep resistance). A method of making a prosthetic bearing is also disclosed.
Owner:DEPUY SYNTHES PROD INC
Colored suture construction
A colored suture includes an elongate woven braid of filaments including one or more ends made of an ultra high molecular weight polyethylene (UHMWPE). The suture also includes second and third ends which can be colorable or dyeable before or after incorporation into the elongate woven braid. This invention provides surgeons with improved recognition of suture ends in surgery by construction of a bi-colored co-braid with at least two ends of different color schemes or patterns braided into a UHMWPE construction. One of the colored ends runs continuously from one end of the suture to the other end. The other colored end can be colored only on one half of the end. This provides a suture with two distinguishable ends, while still maintaining a continuous line of color along the length of the suture.
Owner:TELEFLEX MEDICAL INC
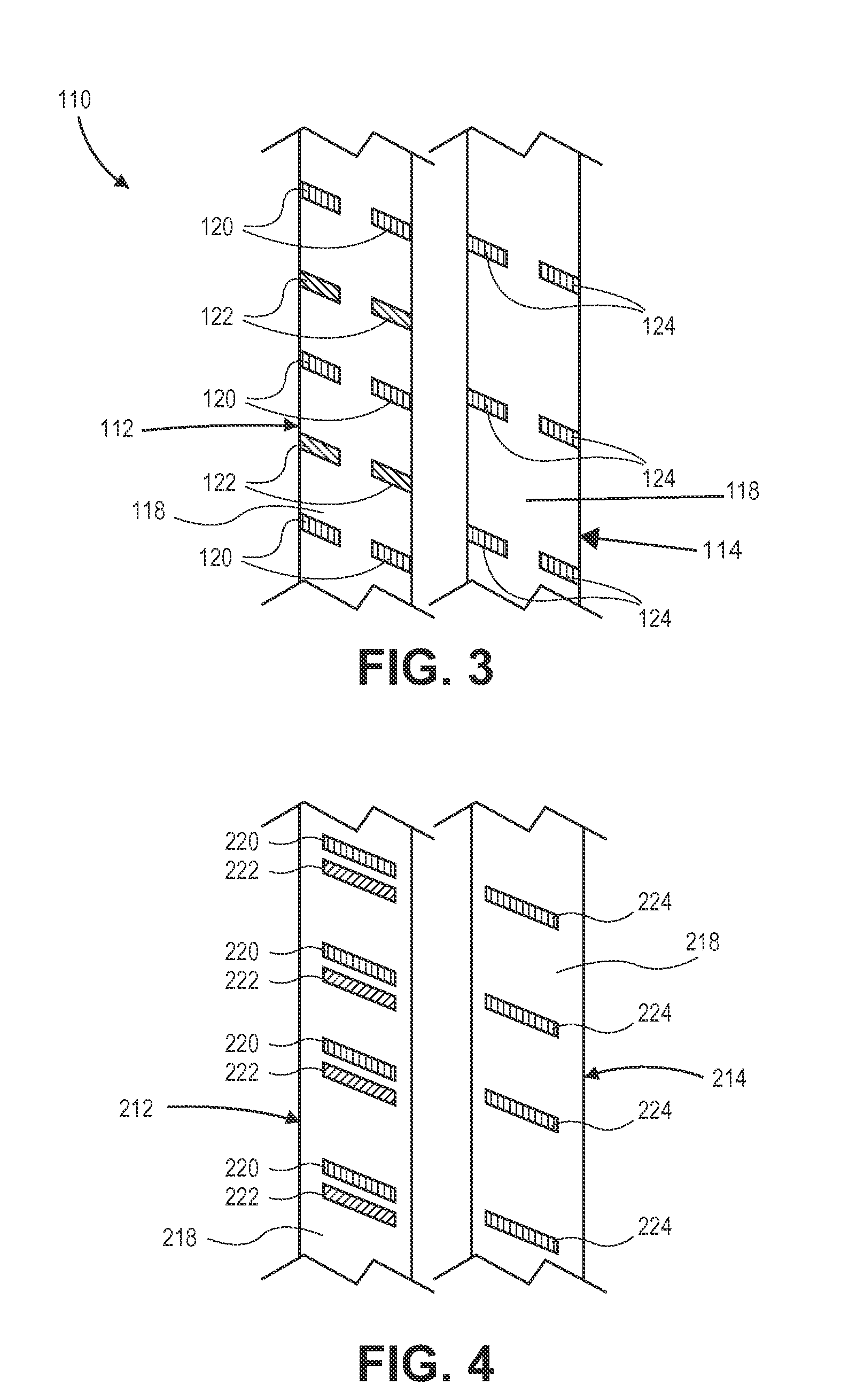
Mobile bearing tibial base prosthetic devices employing oxidized zirconium surfaces
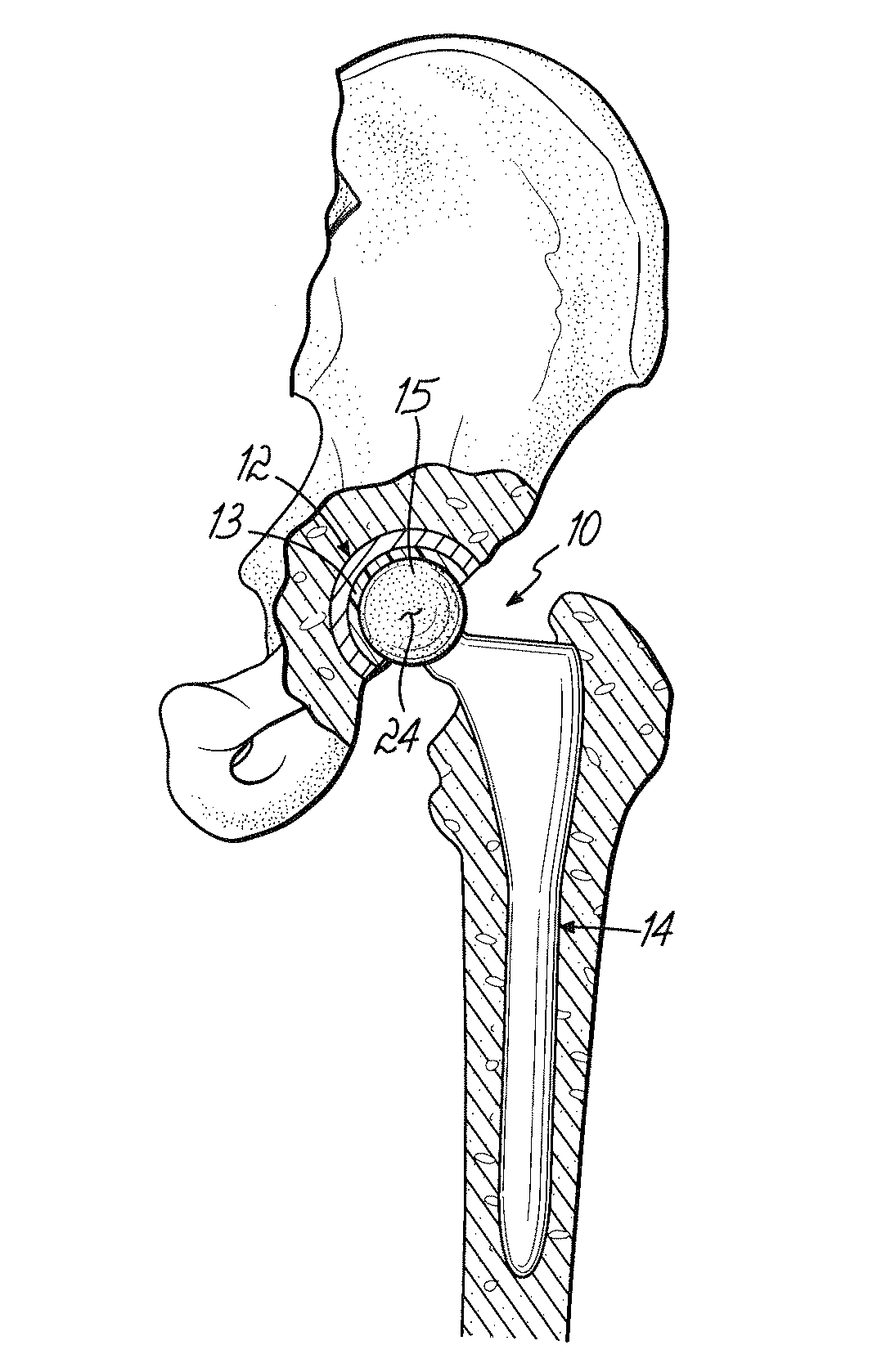
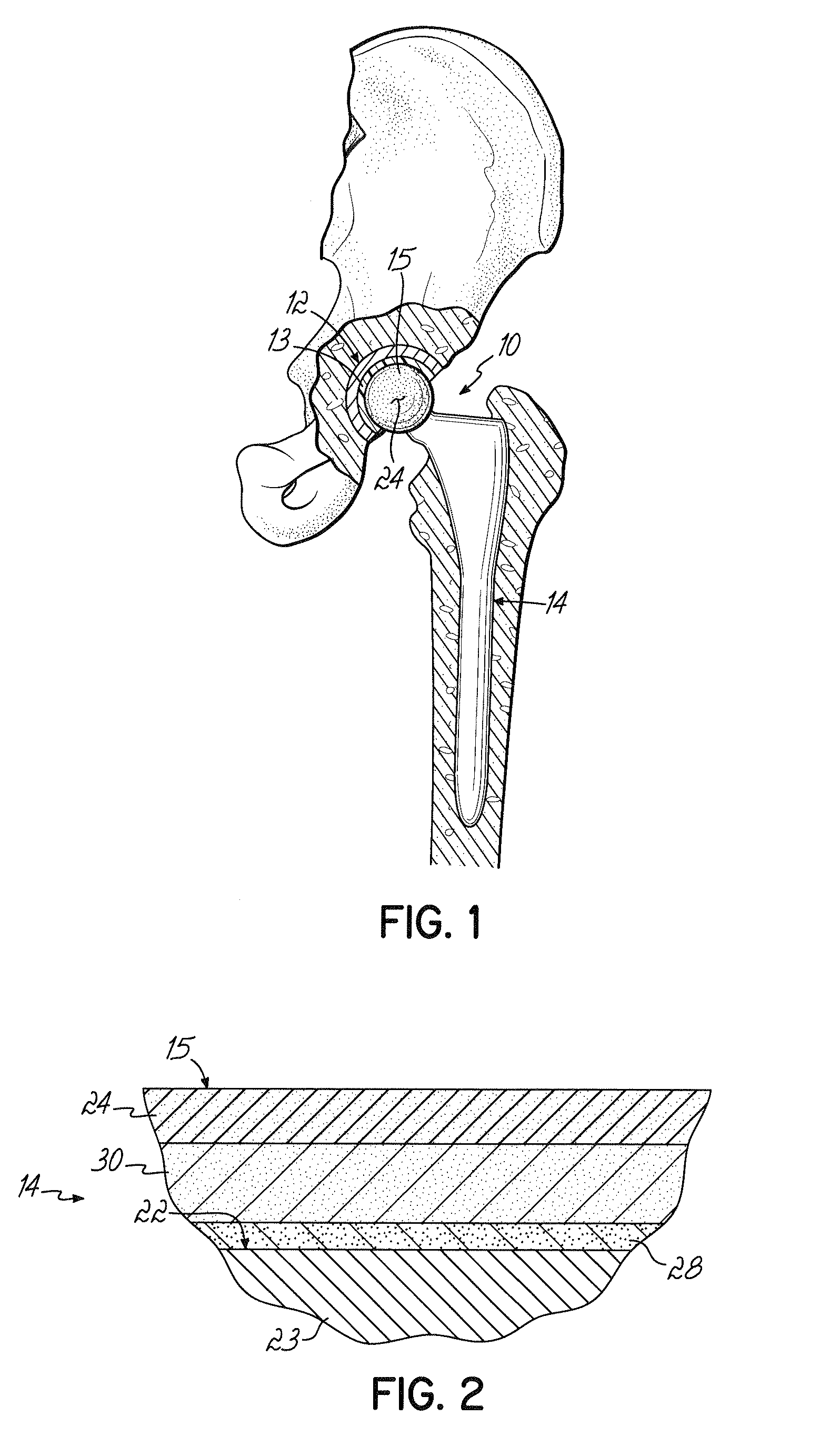
An orthopedic implant with a diffusion-hardened surface on non-load bearing areas of the implant for interaction with non-load bearing surfaces of a polymeric bio-compatible material, such as UHMWPE (ultra-high molecular weight polyethylene). The orthopedic implant is a mobile-bearing knee prosthetic and system where a coating of oxidized zirconium is formed on the post of the tibial tray of the prosthetic for interaction with an opening of a polymeric tibial insert. The diffusion-hardened surface of the orthopedic implant provides a strengthened post and reduction in wear in the opening of the polymeric insert.
Owner:SMITH & NEPHEW INC
Colored Suture Construction
A colored suture includes an elongate woven braid of filaments including one or more ends made of an ultra high molecular weight polyethylene (UHMWPE). The suture also includes second and third ends which can be colorable or dyeable before or after incorporation into the elongate woven braid. This invention provides surgeons with improved recognition of suture ends in surgery by construction of a bi-colored co-braid with at least two ends of different color schemes or patterns braided into a UHMWPE construction. One of the colored ends runs continuously from one end of the suture to the other end. The other colored end can be colored only on one half of the end. This provides a suture with two distinguishable ends, while still maintaining a continuous line of color along the length of the suture.
Owner:TELEFLEX MEDICAL INC
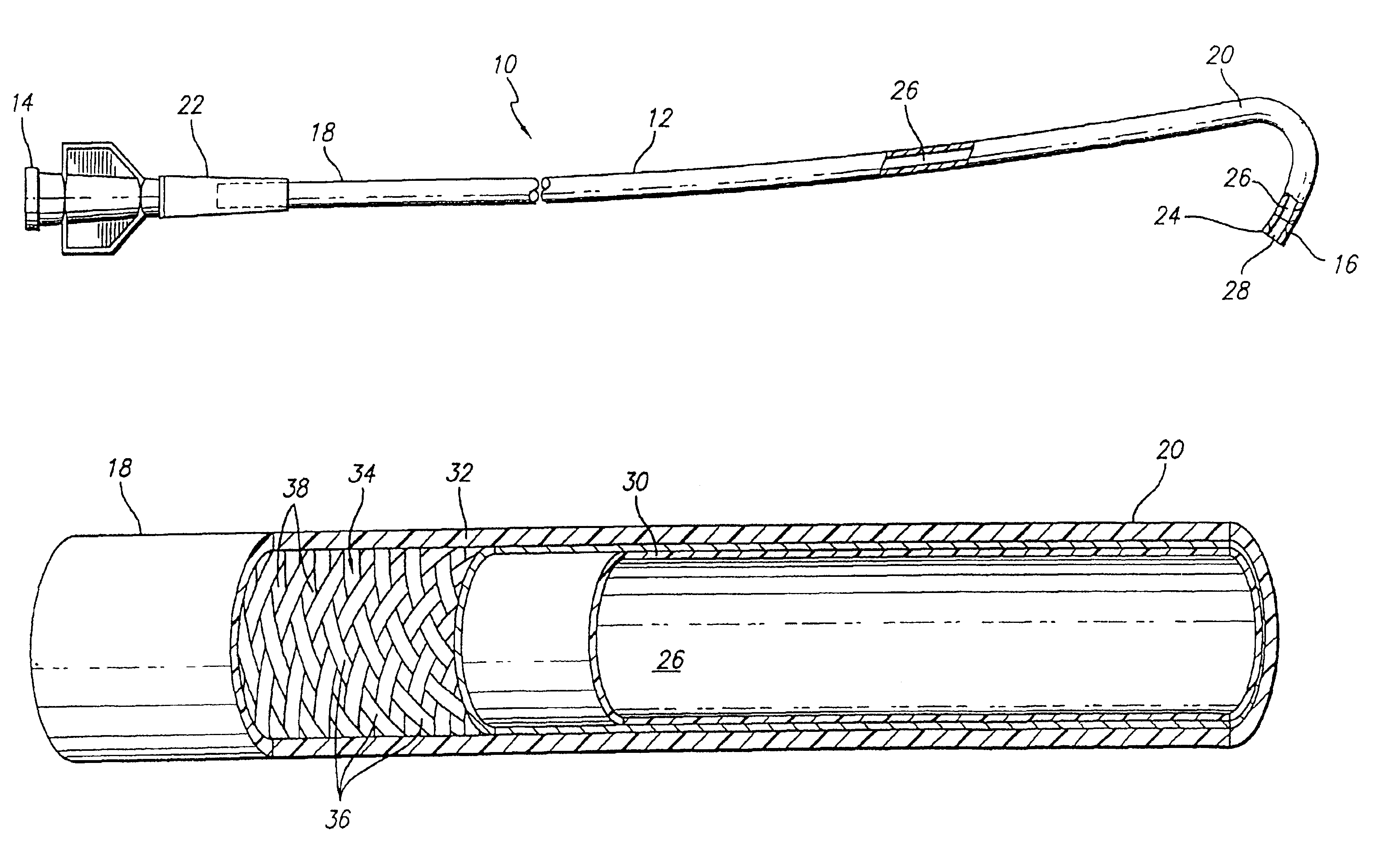
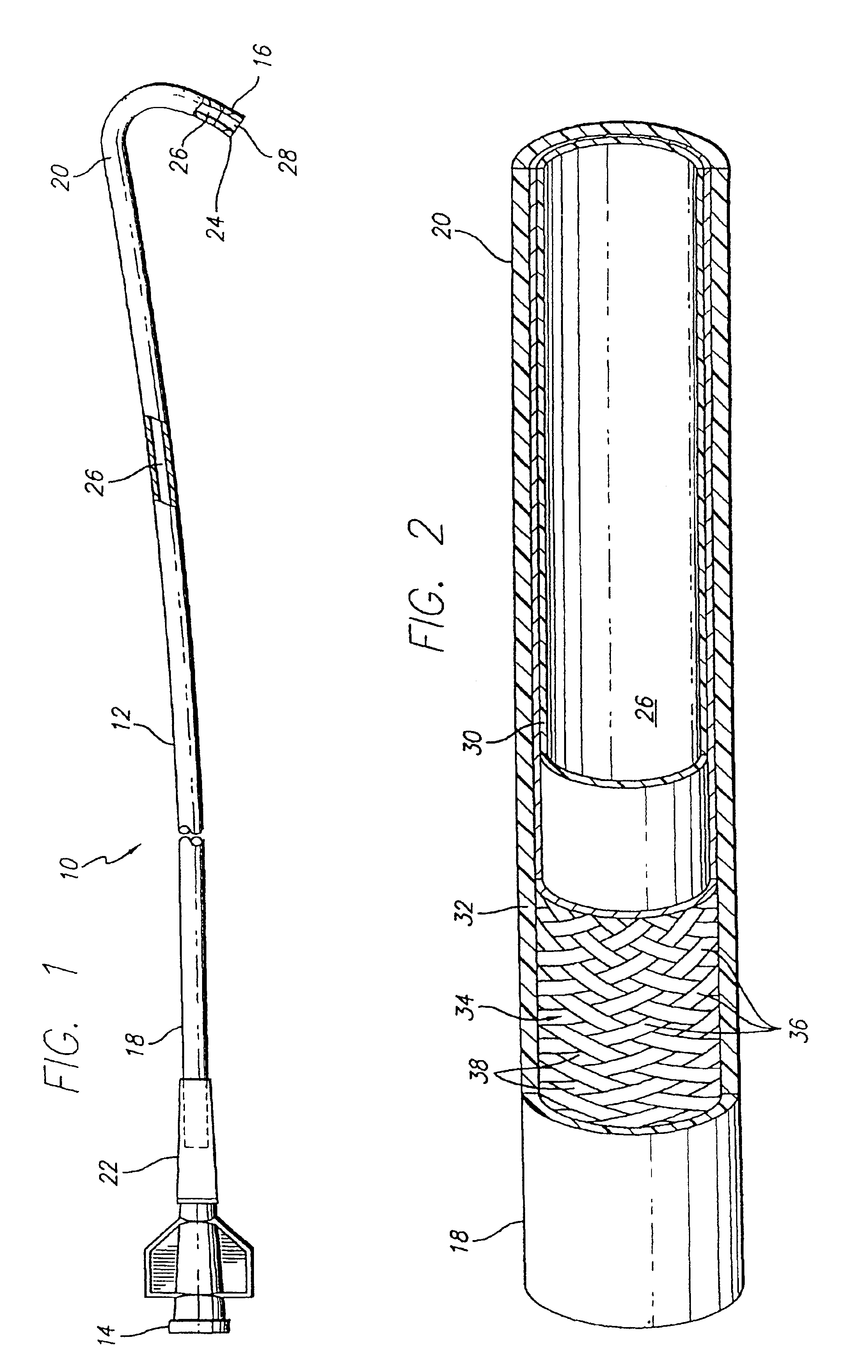
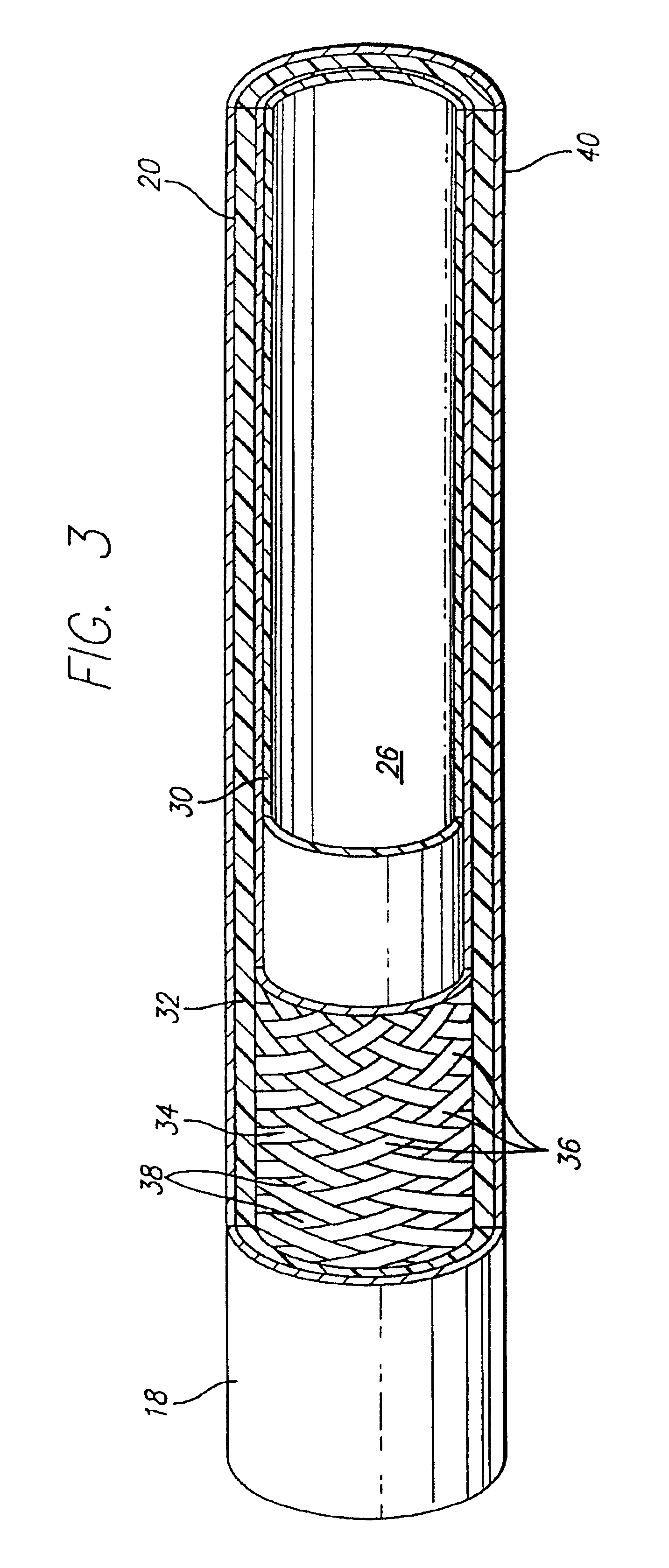
Expanded UHMWPE for guiding catheter liners and other lubricious coatings
An intraluminal catheter, such as a guiding catheter, employed for intravascular procedures and having an inner liner formed of expanded Ultra High Molecular Weight Polyethylene (UHMWPE) is disclosed. The expanded UHMWPE is microporous and has an oriented microstructure structure characterized by nodes interconnected by fibrils. The inner liner formed of expanded UHMWPE is very thin to maximize the inner lumen diameter and has excellent mechanical properties.
Owner:ABBOTT CARDIOVASCULAR
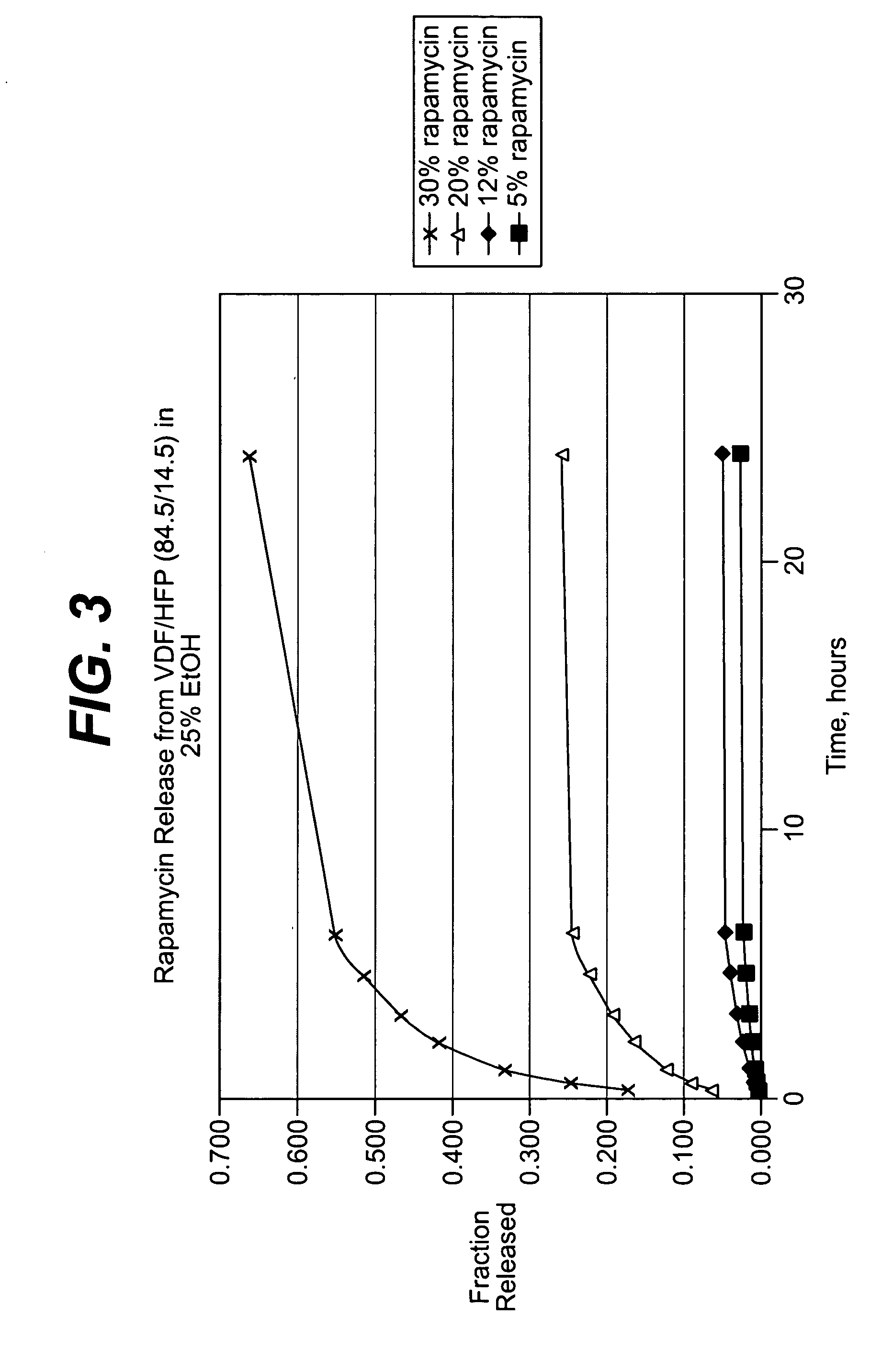
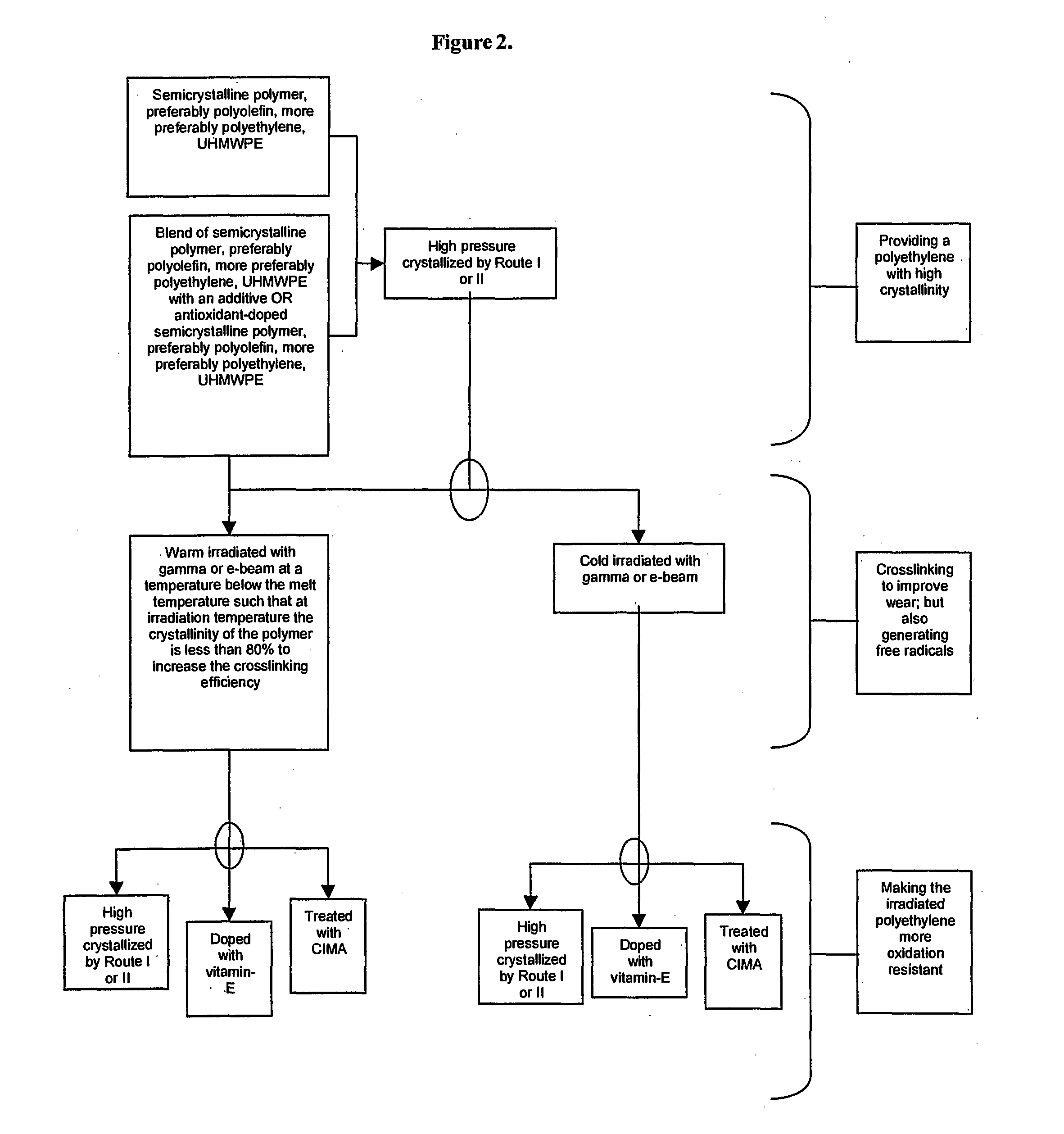
Methods for making oxidation resistant polymeric material
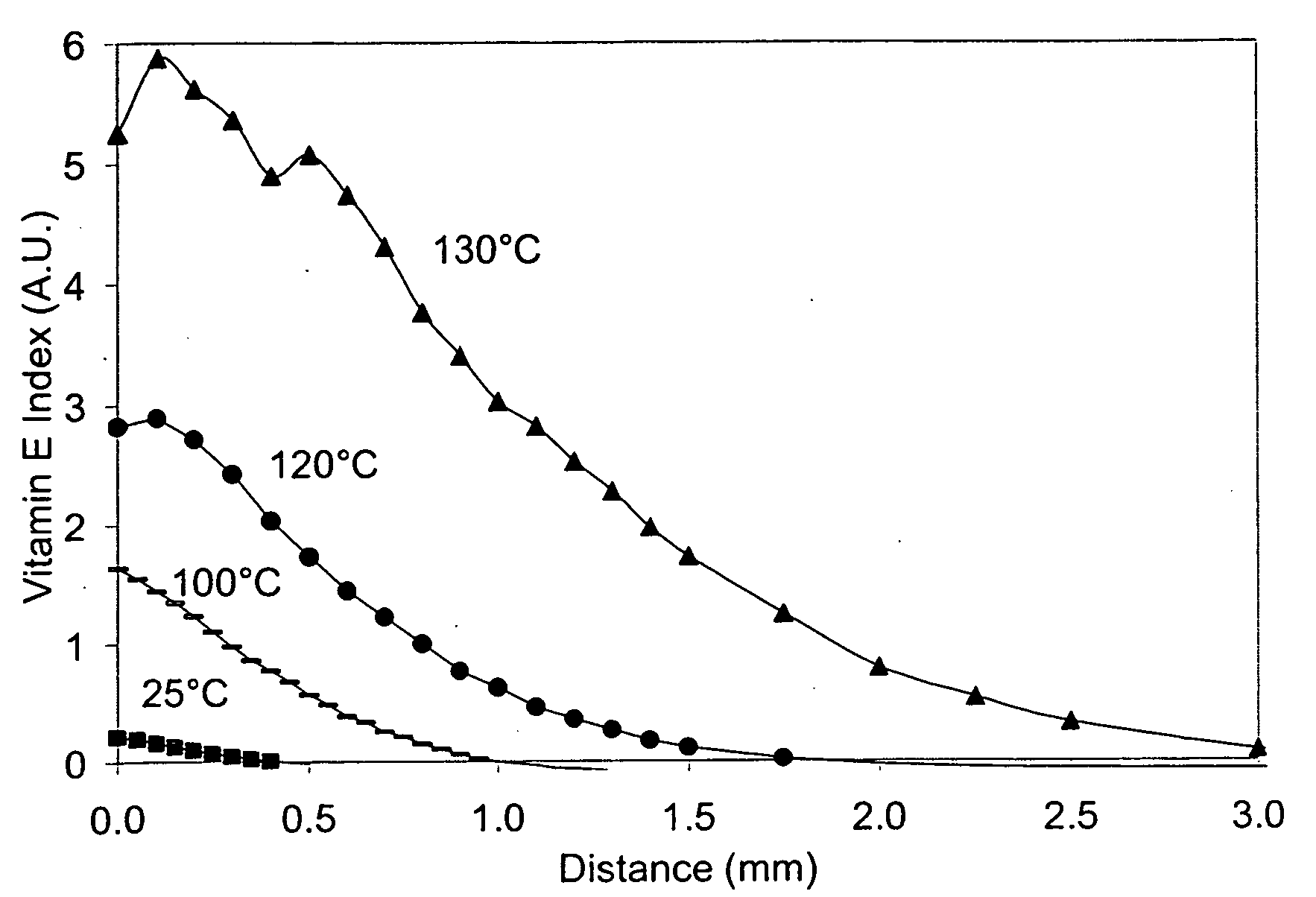
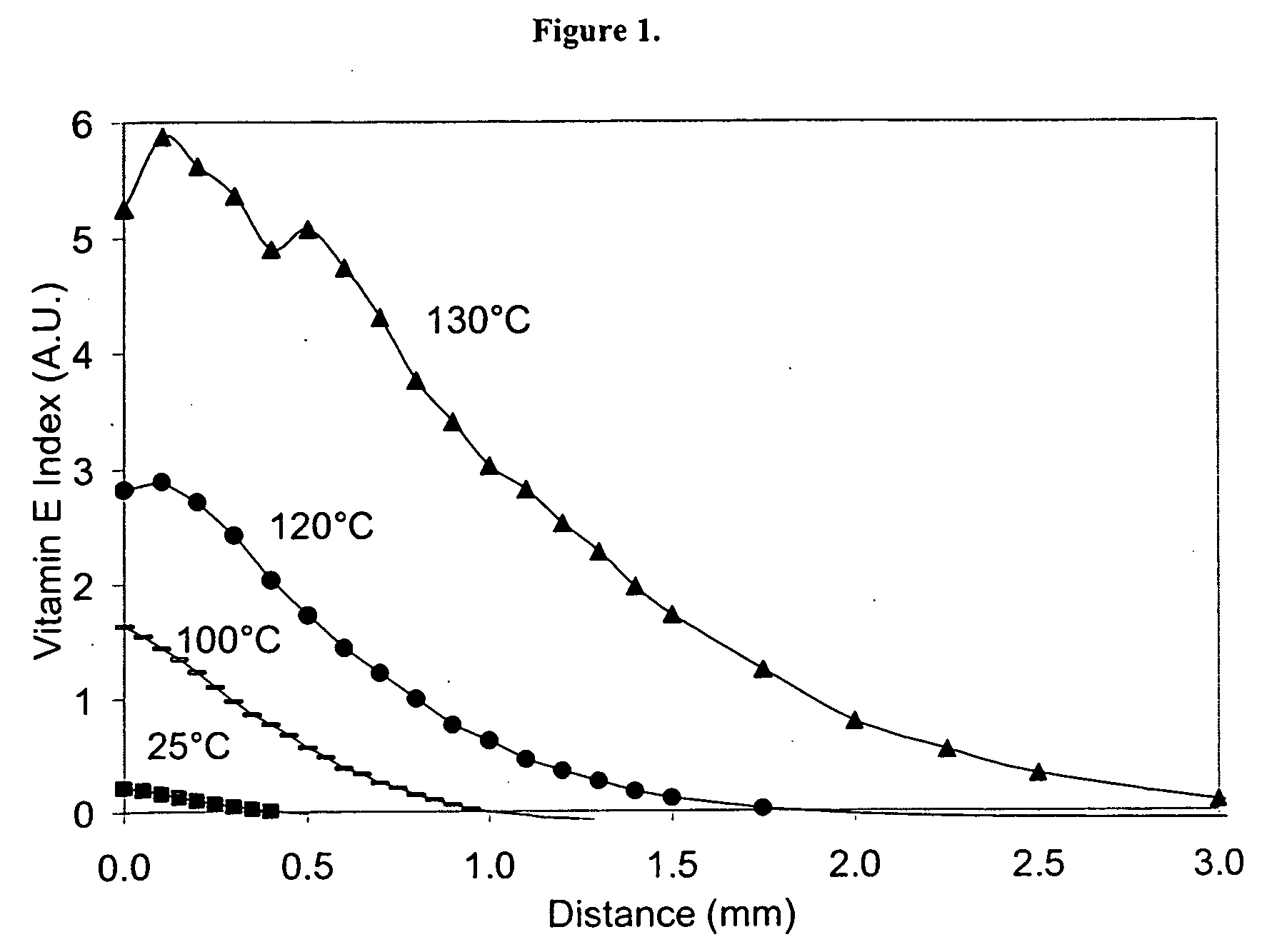
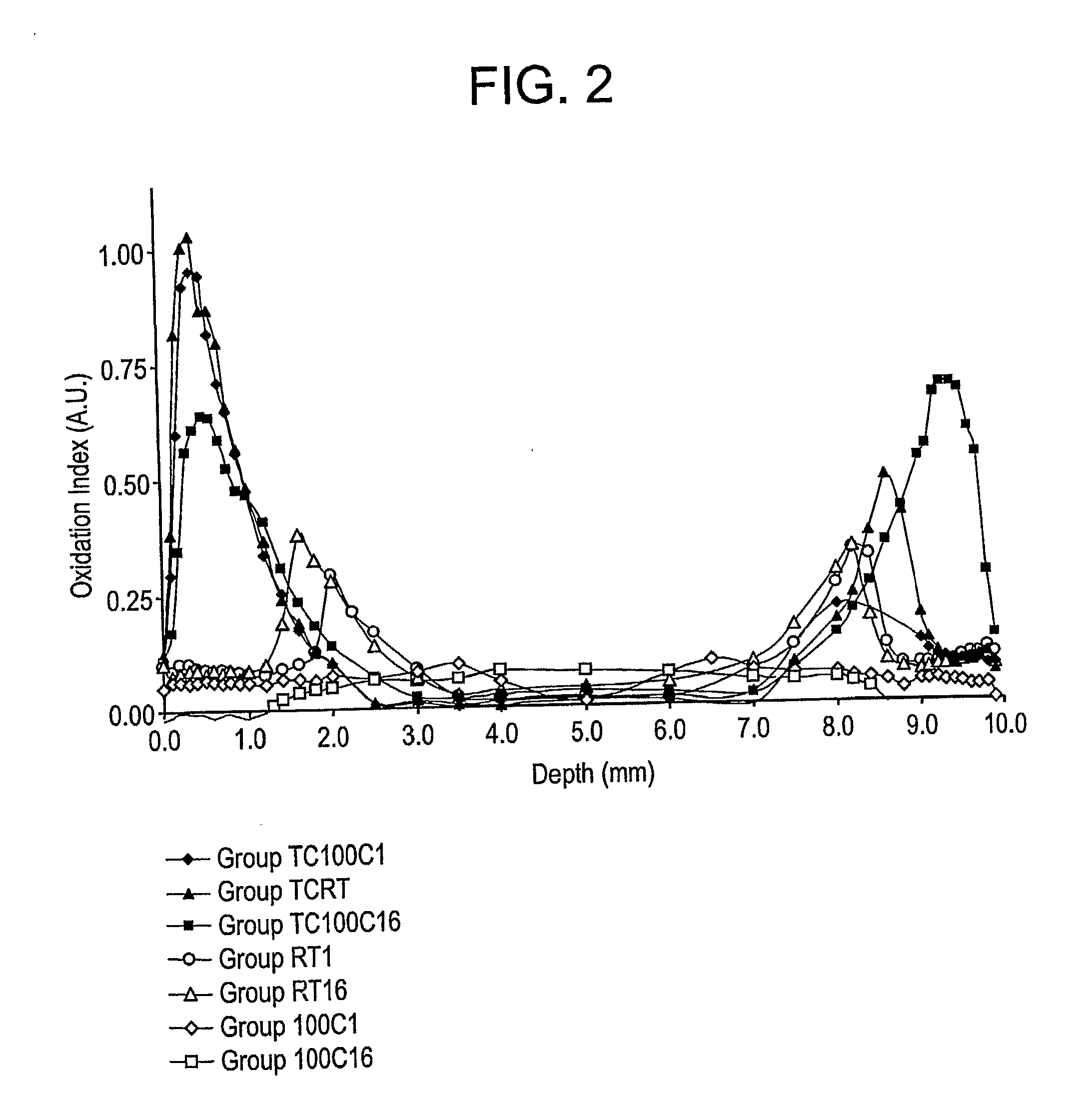
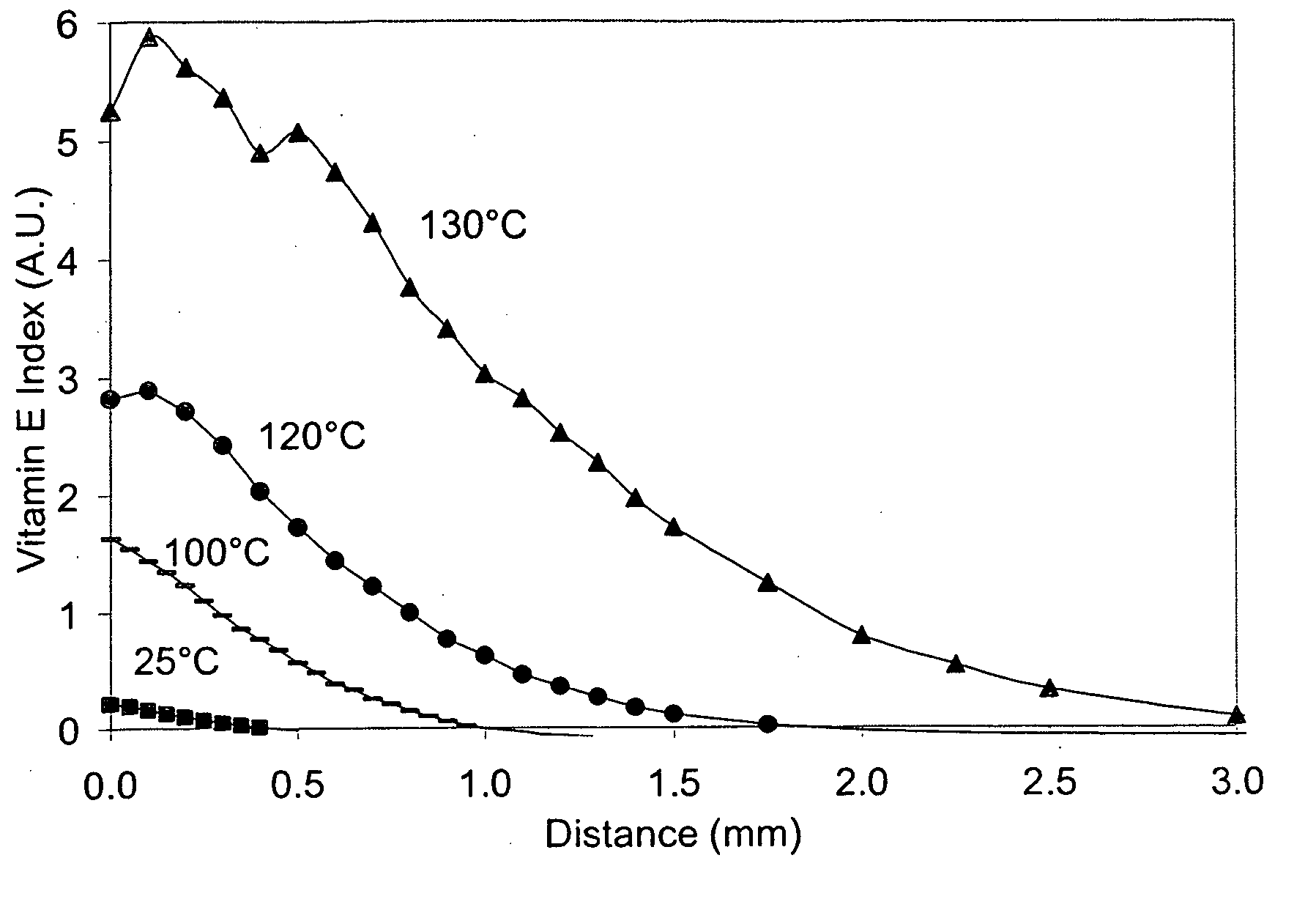
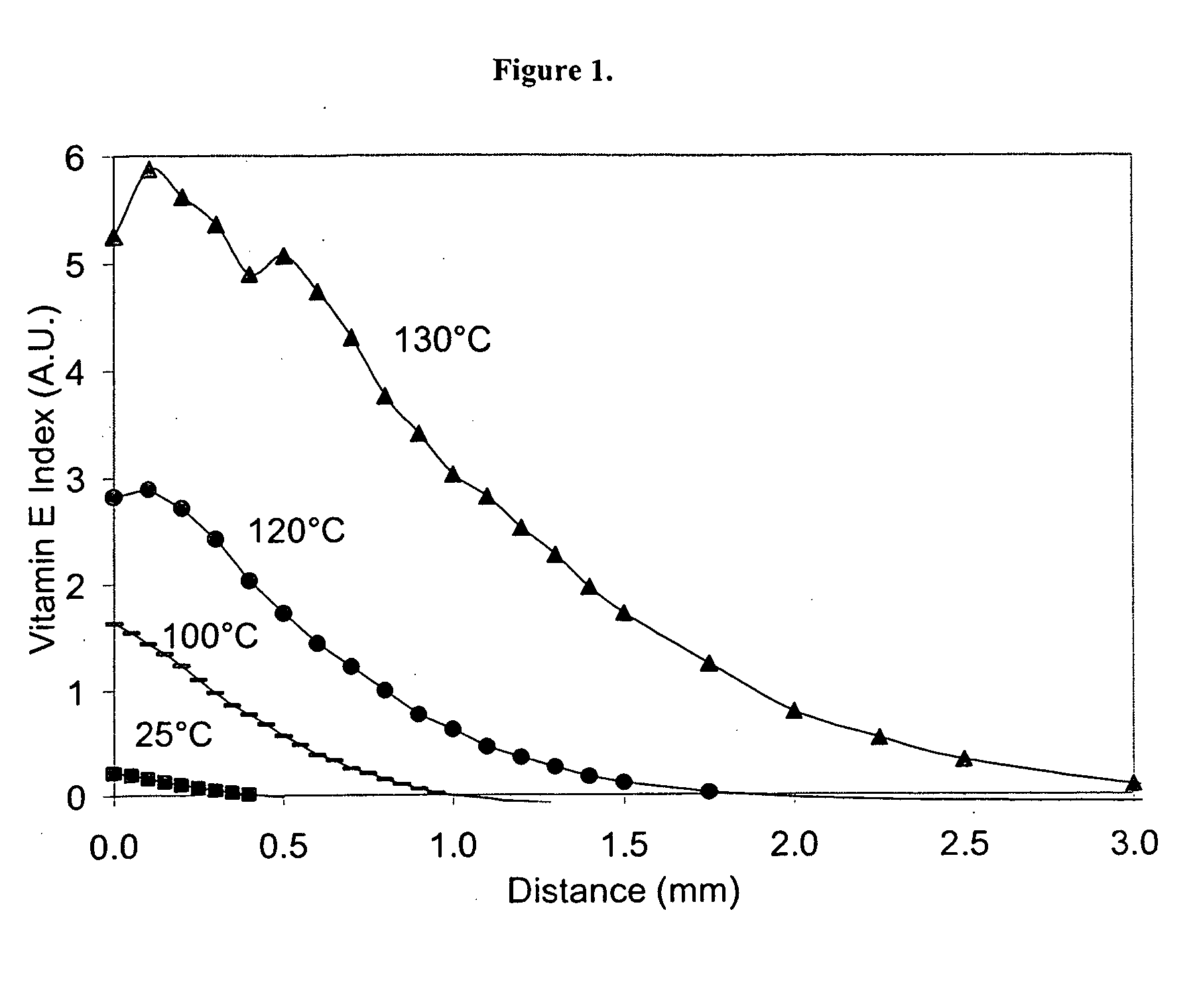
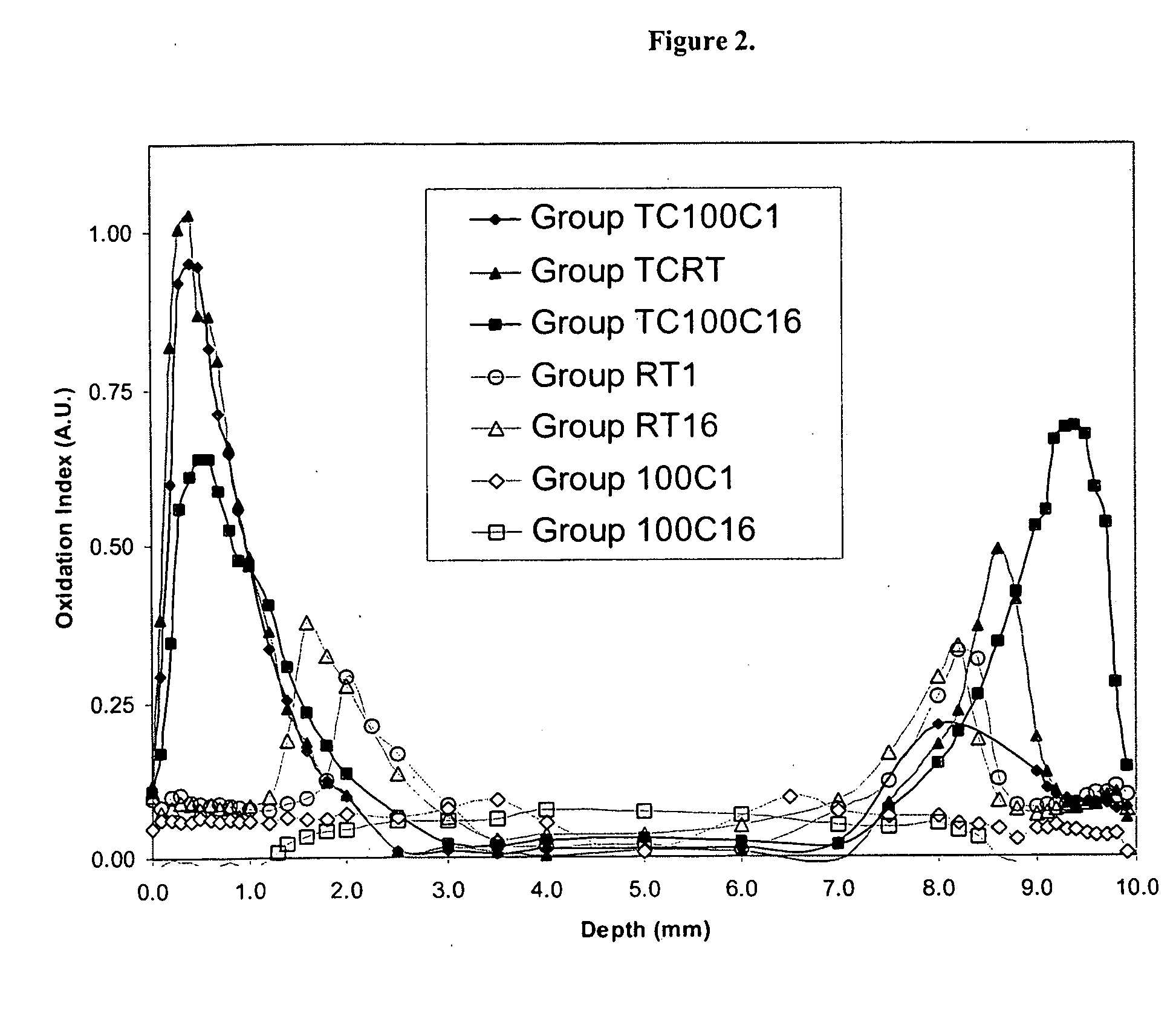
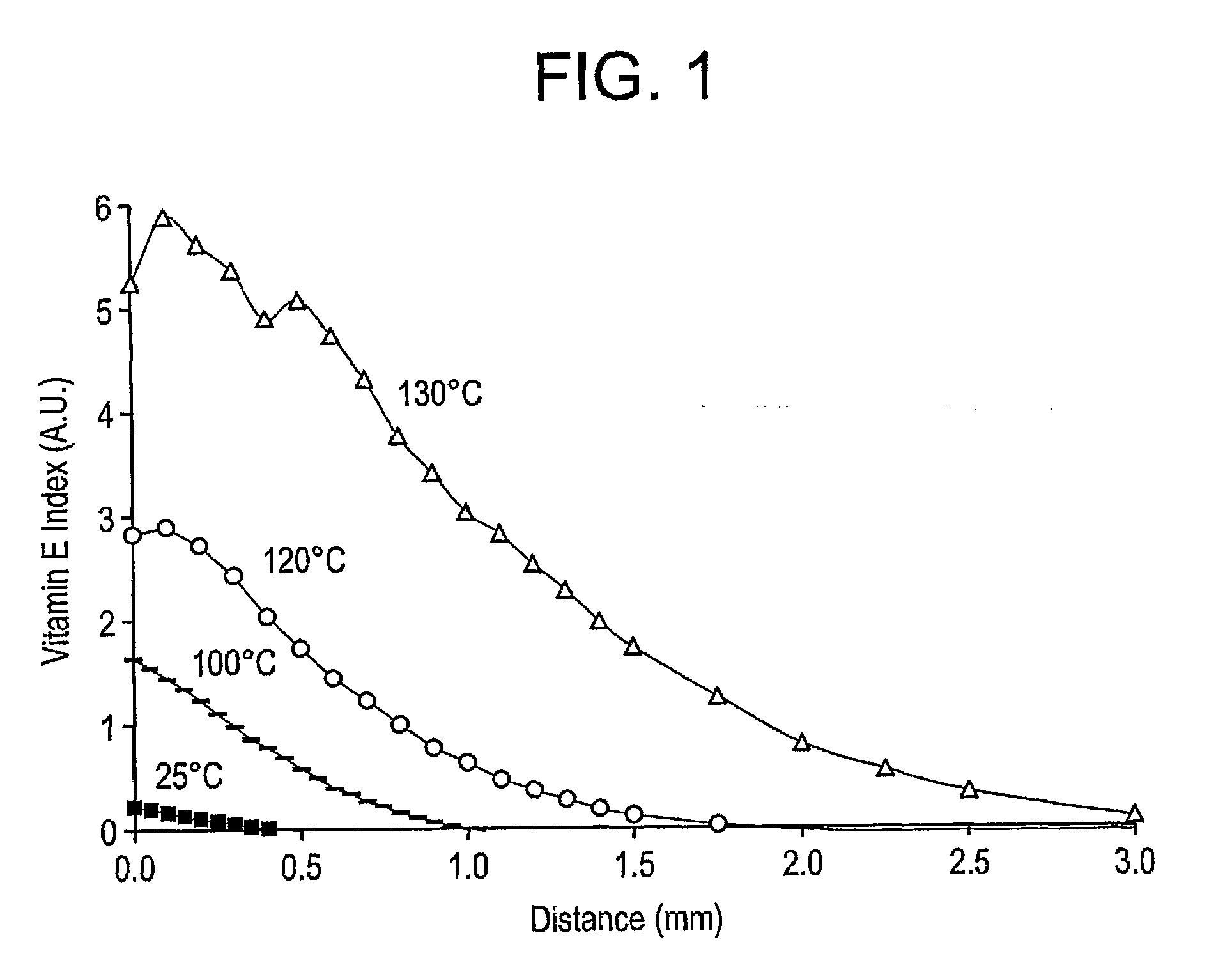
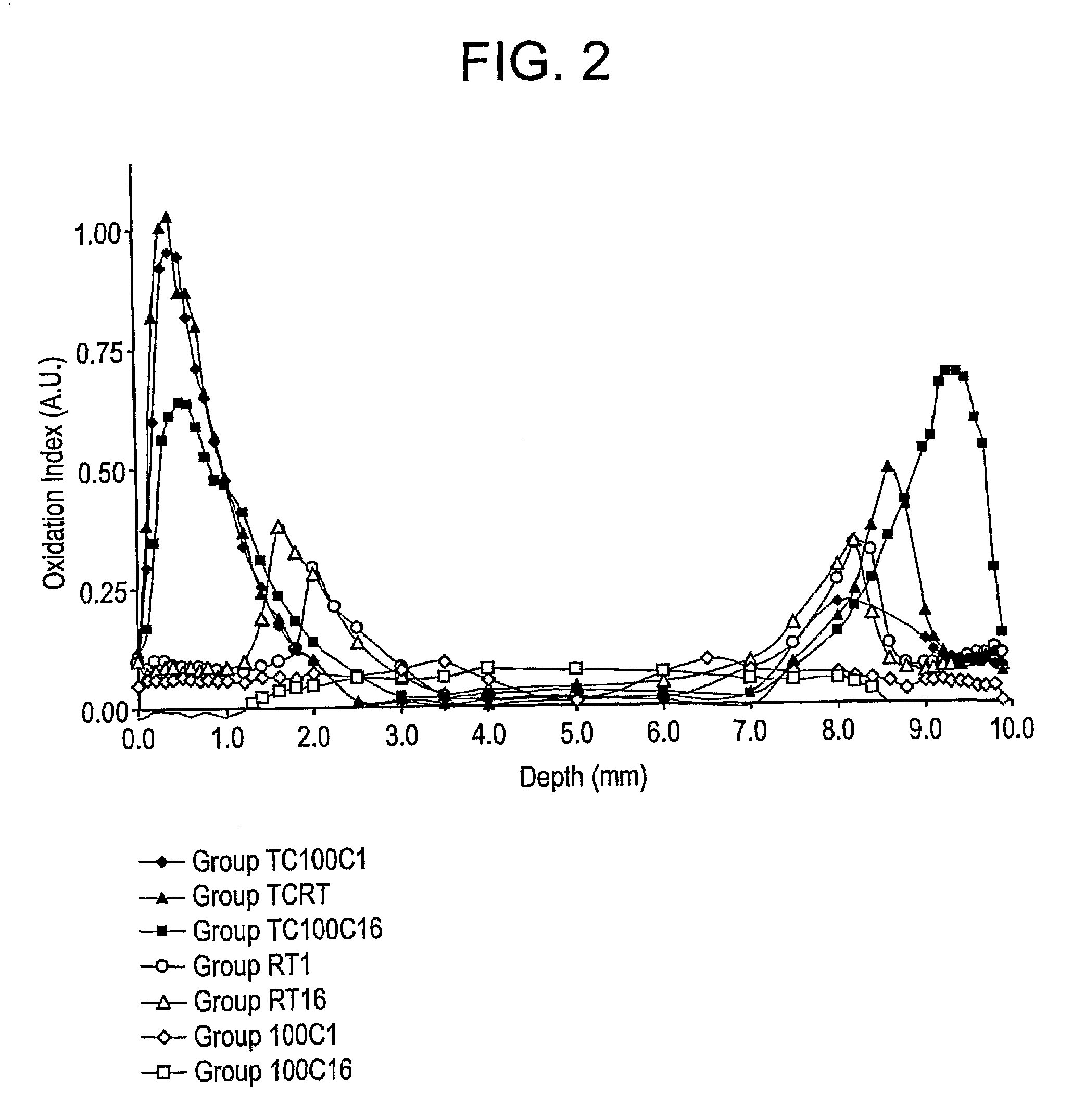
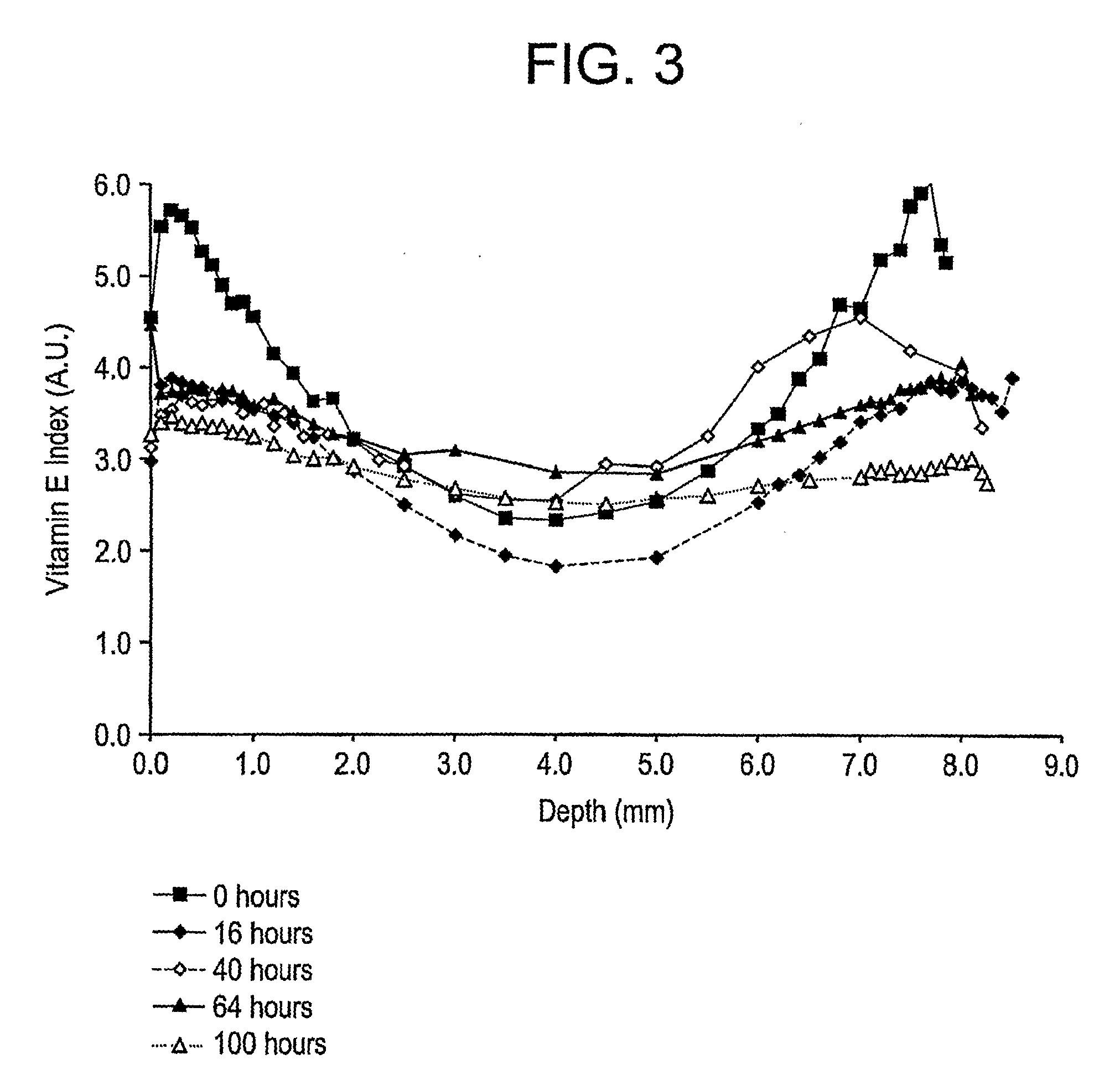
The present invention relates to methods for making oxidation resistant medical devices that comprise polymeric materials, for example, ultra-high molecular weight polyethylene (UHMWPE). The invention also provides methods of making antioxidant-doped medical implants, for example, doping of medical devices containing cross-linked UHMWPE with vitamin E by diffusion and materials used therein.
Owner:MURATOGLU ORHUN K +1
High strength suture with absorbable core
ActiveUS20050149119A1Improved absorption profileReducing knot profile of knotSuture equipmentsSurgical needlesYarnMedicine
A novel high tensile strength semi-absorbable composite suture with minimized non-absorbable mass. The suture has a core made from a bioabsorbable polymer. The core is covered by a braided sheath. The braided sheath is made from an absorbable yarn and a bioabsorbable yarn. The bioabsorbable yarn is made from a least one filament of a bioabsorbable polymer. The nonabsorbable yarn is made from at least one filament of ultra high molecular weight polyethylene.
Owner:DEPUY SYNTHES PROD INC
Titanium alloy with oxidized zirconium for a prosthetic implant
A prosthetic device having a generally fixed member formed from a low friction material such as ultra-high molecular weight polyethylene and an articulating titanium member, which includes an articular bearing surface. The articular surface is a zirconium oxide layer formed by applying a coating of zirconium onto the titanium member and heating this in an oxygen-containing environment. This causes the zirconium to oxidize and further causes the zirconium to migrate into the titanium member forming a titanium zirconium diffusion layer, which prevents delamination.
Owner:ZIMMER INC
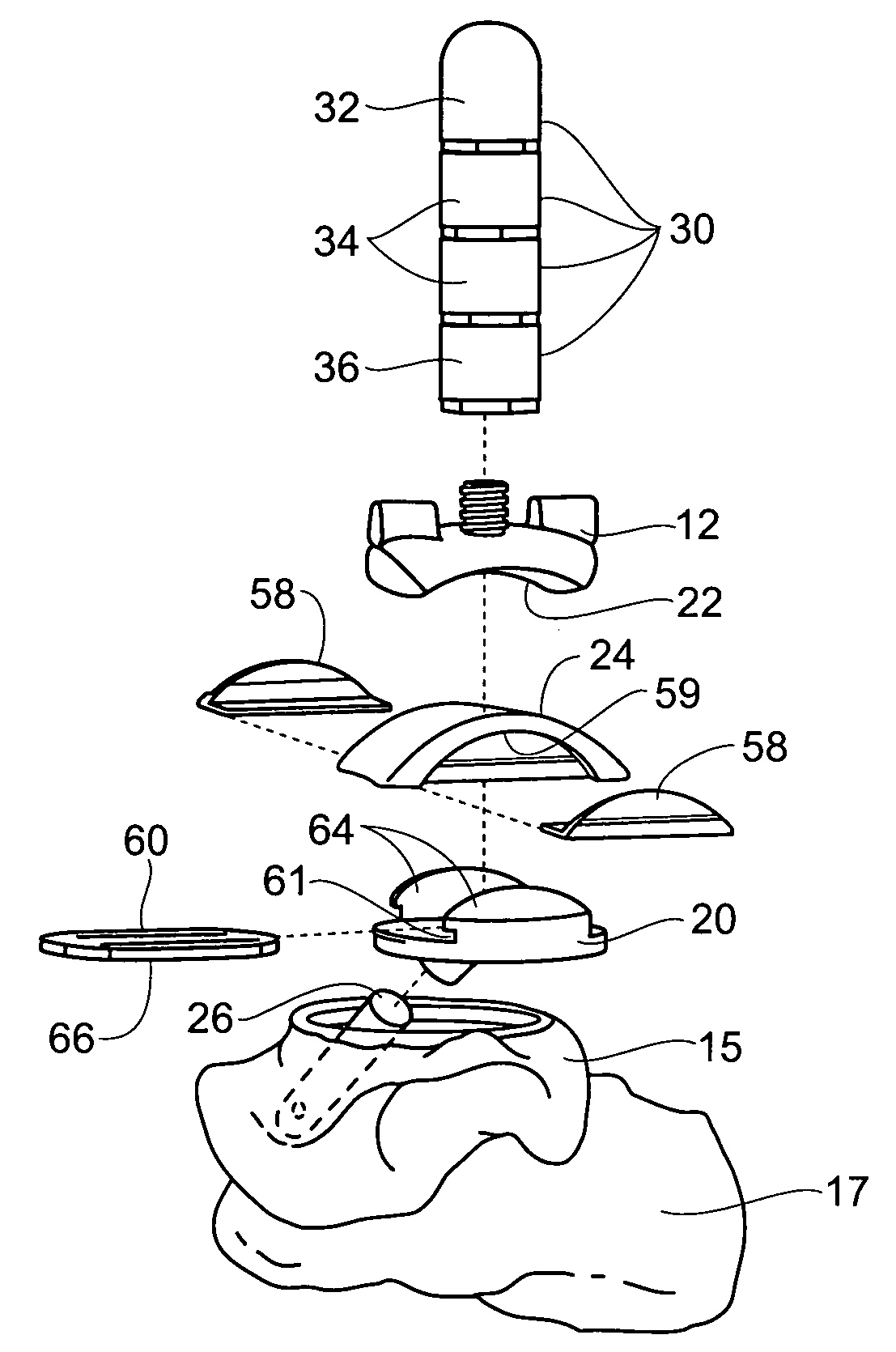
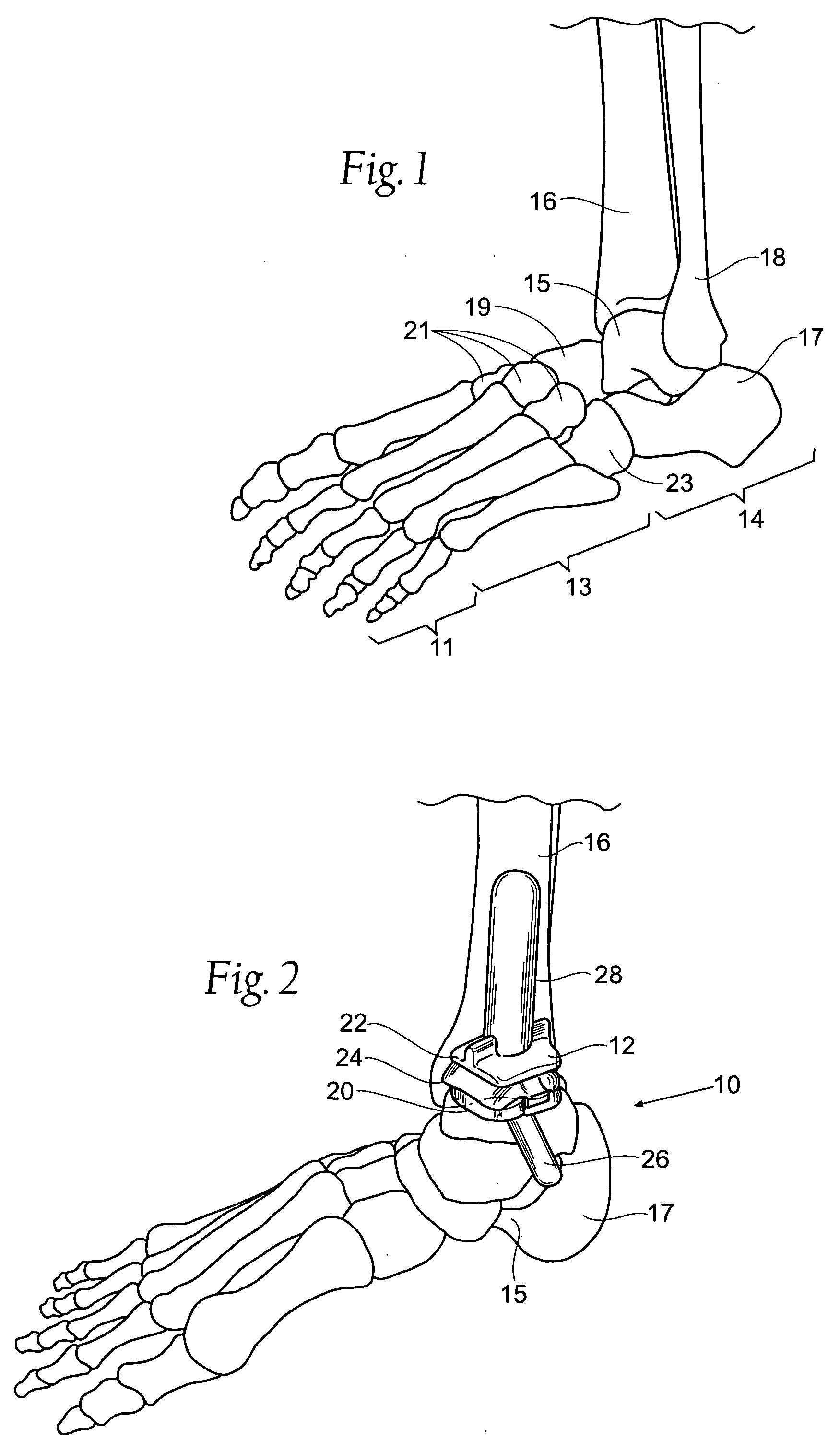
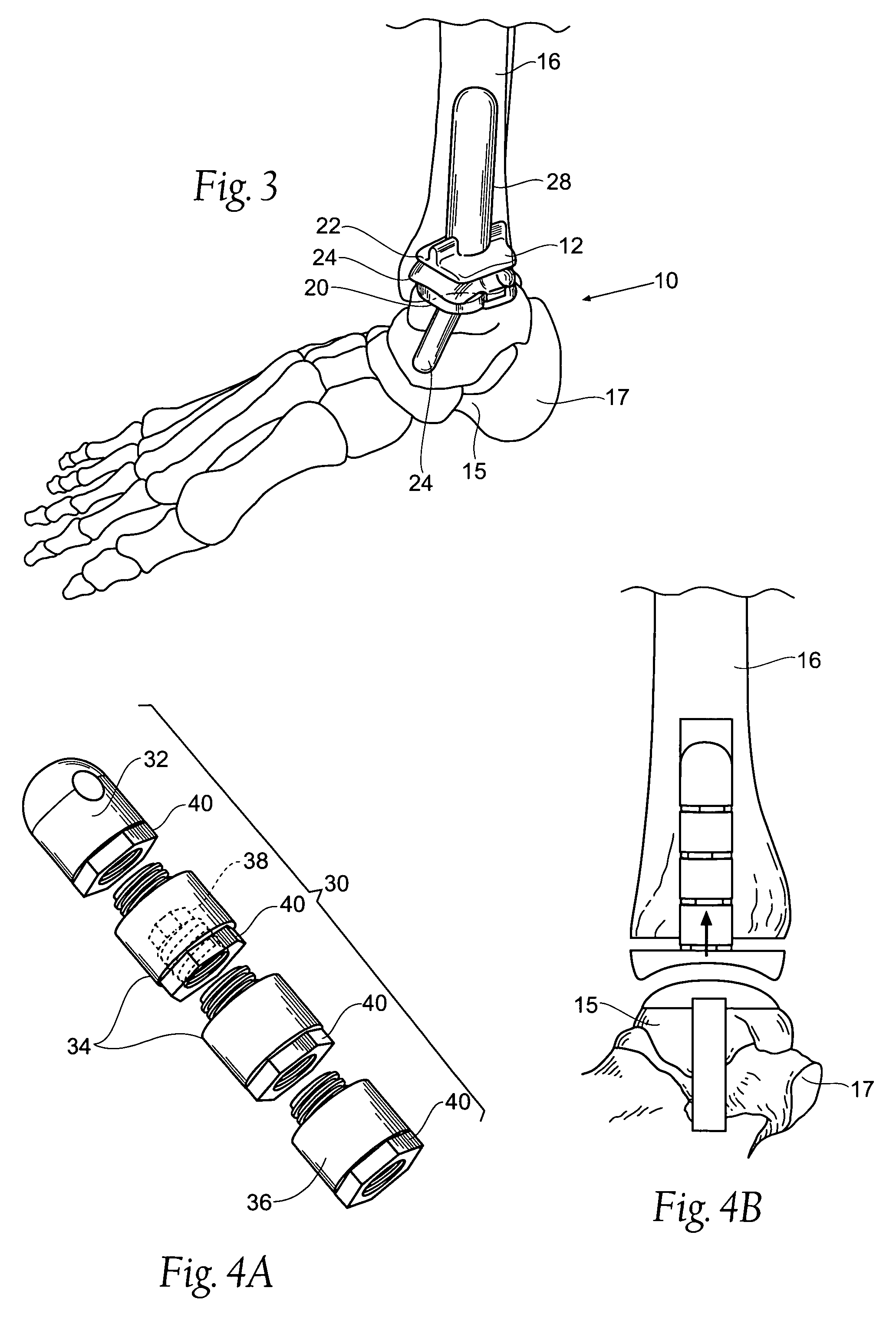
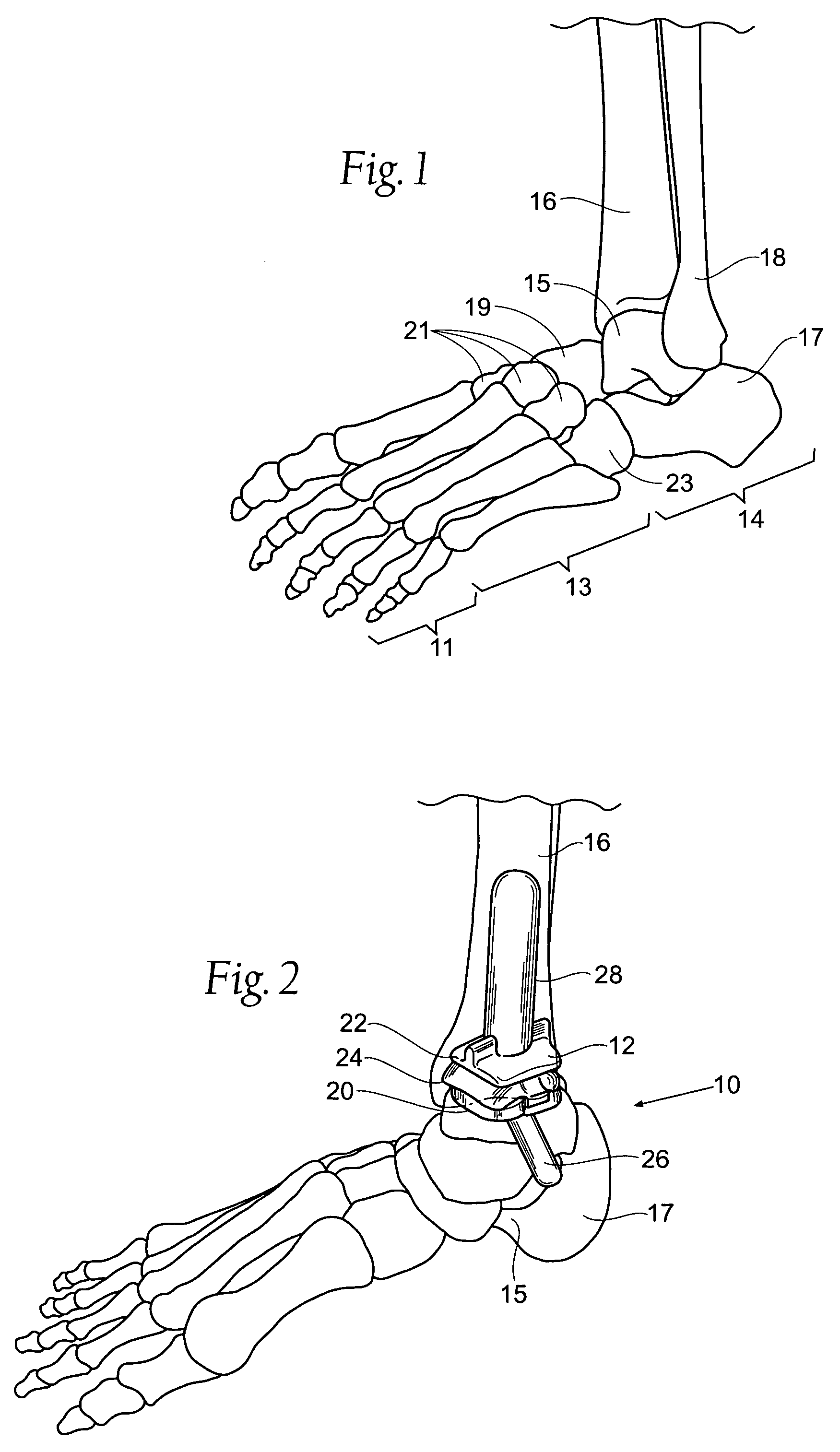
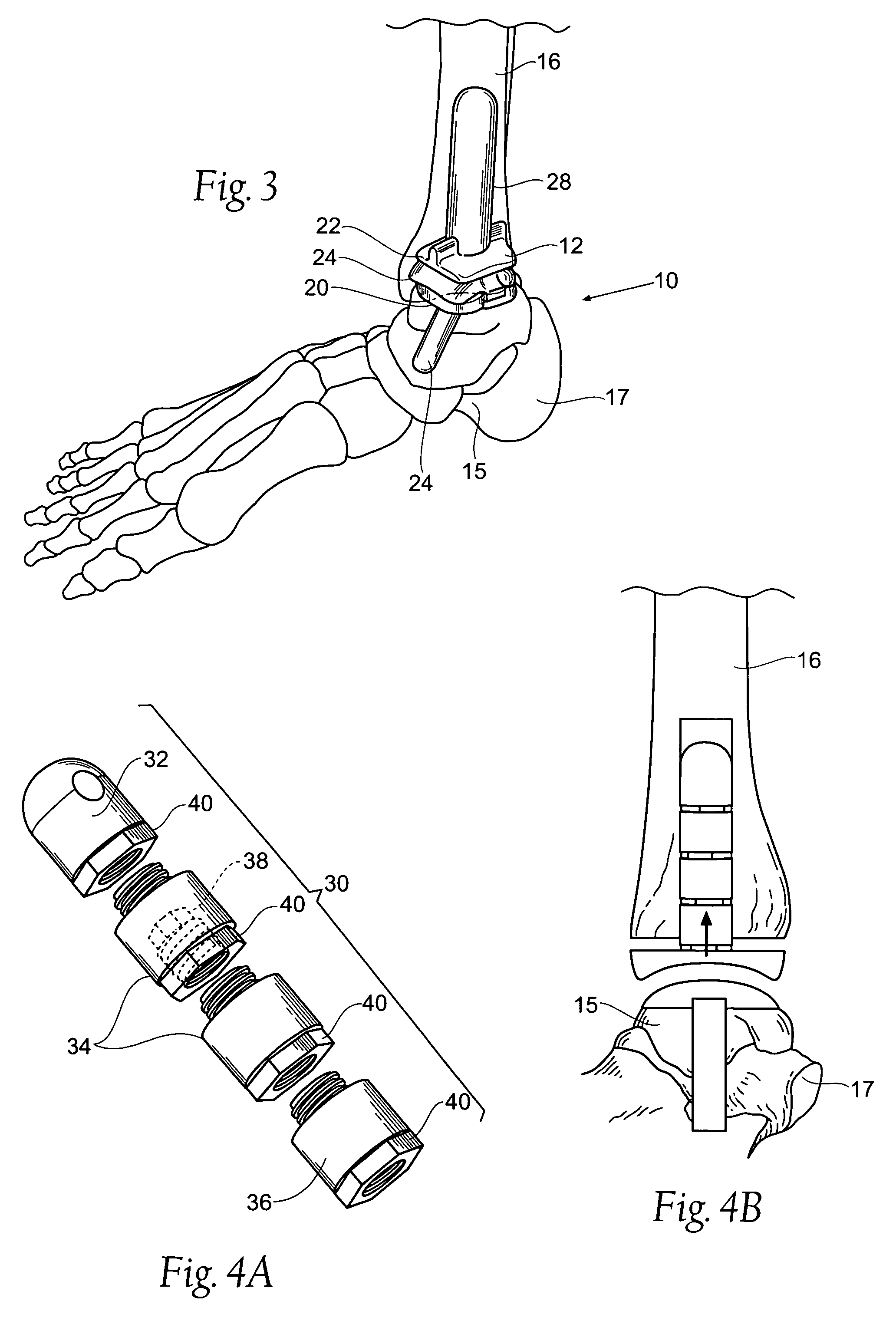
Ankle replacement system
ActiveUS20060229730A1Improved long-term resultPrecise positioningWrist jointsAnkle jointsCalcaneusAnkle joint replacement
A prosthesis suited for orthopedic implantation possesses a multi-piece stem component that supports an artificial joint surface that can articulate with another artificial joint surface in various ways. The prosthesis can be assembled in a snap fit and / or interlocking fashion that provides positive locking means without the use of screws or other fasteners. The prosthesis can accommodate fitment of a plastic joint surface made, e.g., from ultra high molecular weight polyethylene. The prosthesis is well suited for use in an ankle replacement system that can be installed using minimally invasive intramedullary guidance established with respect to the major axis of the tibia by minimally invasive access through the calcaneus, through an incision in the bottom of the foot. The prosthesis makes possible the installation of a total ankle system using minimally invasive anterior access to the ankle joint for making bony cuts and to install prosthesis components.
Owner:INBONE TECH
Ankle replacement system
ActiveUS7534246B2Function maximizationMaximize longevityWrist jointsAnkle jointsArticular surfacesAnkle joint replacement
A prosthesis suited for orthopedic implantation possesses a multi-piece stem component that supports an artificial joint surface that can articulate with another artificial joint surface in various ways. The prosthesis can be assembled in a snap fit and / or interlocking fashion that provides positive locking means without the use of screws or other fasteners. The prosthesis can accommodate fitment of a plastic joint surface made, e.g., from ultra high molecular weight polyethylene. The prosthesis is well suited for use in an ankle replacement system that can be installed using minimally invasive intramedullary guidance established with respect to the major axis of the tibia by minimally invasive access through the calcaneus, through an incision in the bottom of the foot. The prosthesis makes possible the installation of a total ankle system using minimally invasive anterior access to the ankle joint for making bony cuts and to install prosthesis components.
Owner:INBONE TECH
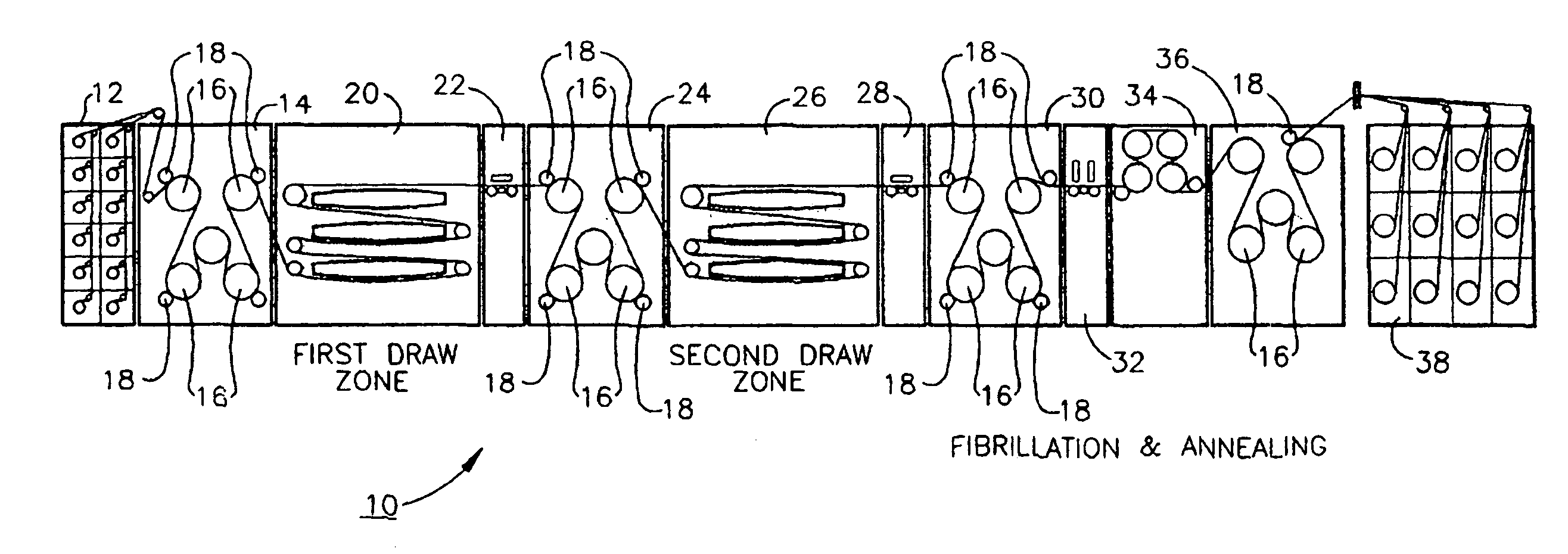
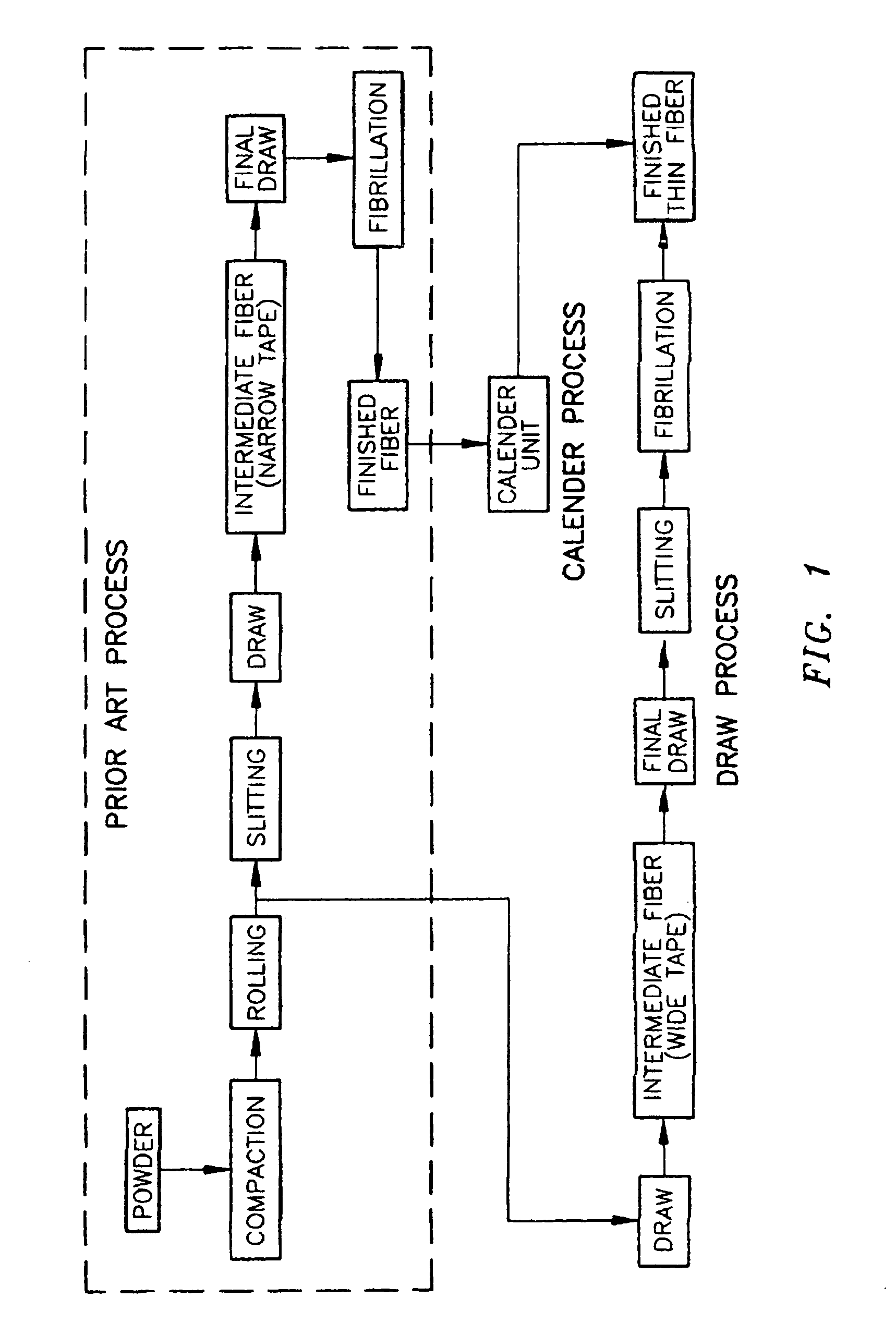
Ultra high molecular weight polyethylene fibers
InactiveUS6951685B1Easy knittingSynthetic resin layered productsDomestic articlesDental flossingFiber
A method for the production of films / fibers of UHMWPE below 3 mils and preferably about 2 mils in thickness. The process involves calendering and / or drawing the materials of the prior art under careful tension control at a temperature above the melting point of the UHMWPE material. Before and after subsequent slitting, and / or fibrillation, UV resistance treatments, etc the thin films / fibers thus produced find use in such diverse applications as personal armor, dental floss, and sails for sail boats.
Owner:EI DU PONT DE NEMOURS & CO
High strength suture with silk trace
InactiveUS20050055051A1High strengthImproved tie down characteristicSuture equipmentsPolyesterSuture anchors
A high strength abrasion resistant surgical suture material with improved tie down characteristics and tissue compliance is color coded for visualization and identification purposes. The suture features a multifilament jacket formed of braided strands of ultra high molecular weight polyethylene and polyester, with silk fibers included in a color contrasting with the other jacket fibers to provide an identifiable trace. In one embodiment, the braided jacket surrounds a core formed of twisted strands of ultrahigh molecular weight polyethylene. The suture, provided in a No. 2 size, has the strength of No. 5 Ethibond, is ideally suited for most orthopedic procedures, and can be attached to a suture anchor or a curved needle. The identifiable silk trace preferably may be provided along one half of the length of the suture, so that when the suture is loaded onto a suture anchor, for example, the two legs of the length of suture on either side of the suture anchor can be readily distinguished.
Owner:ARTHREX
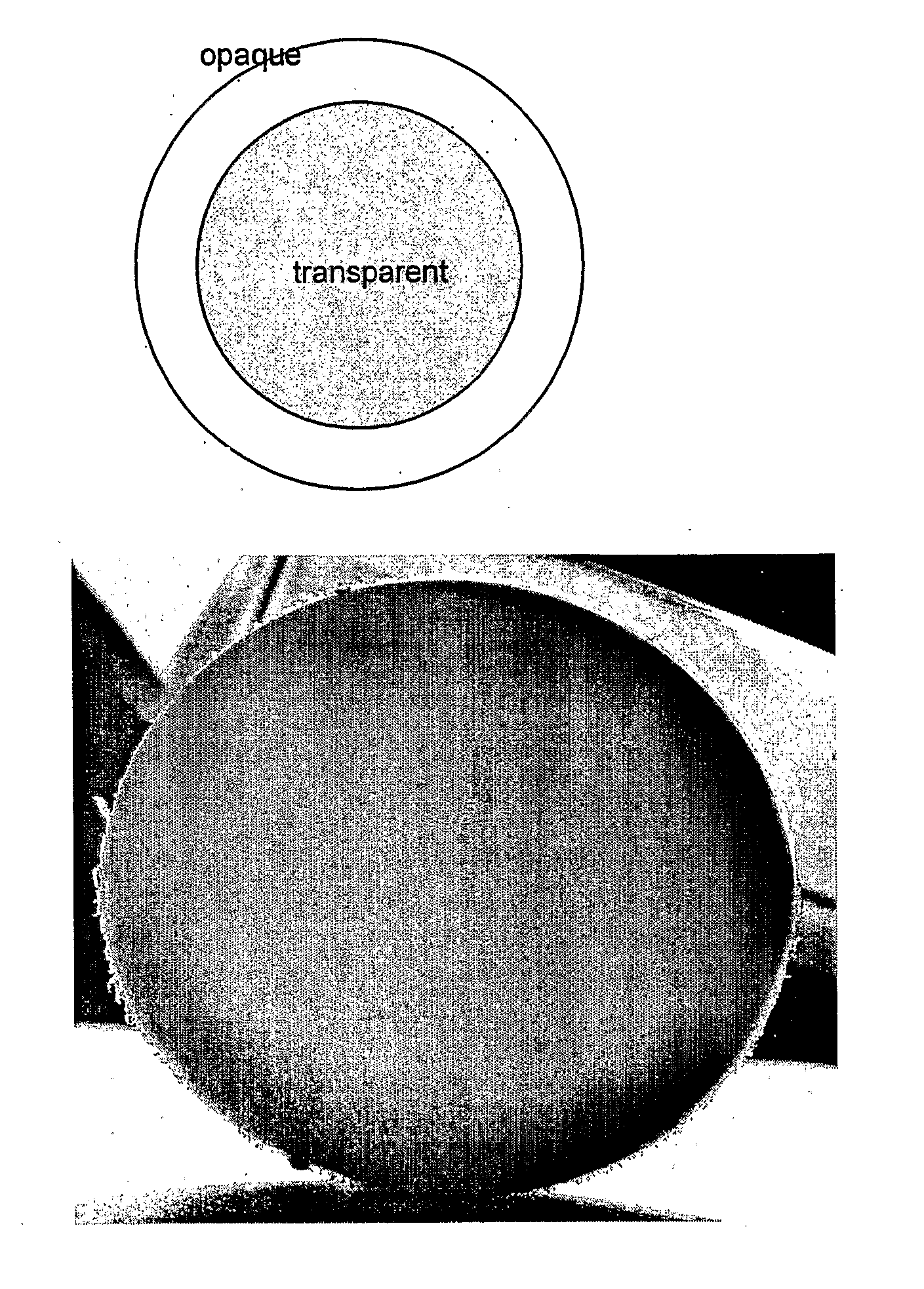
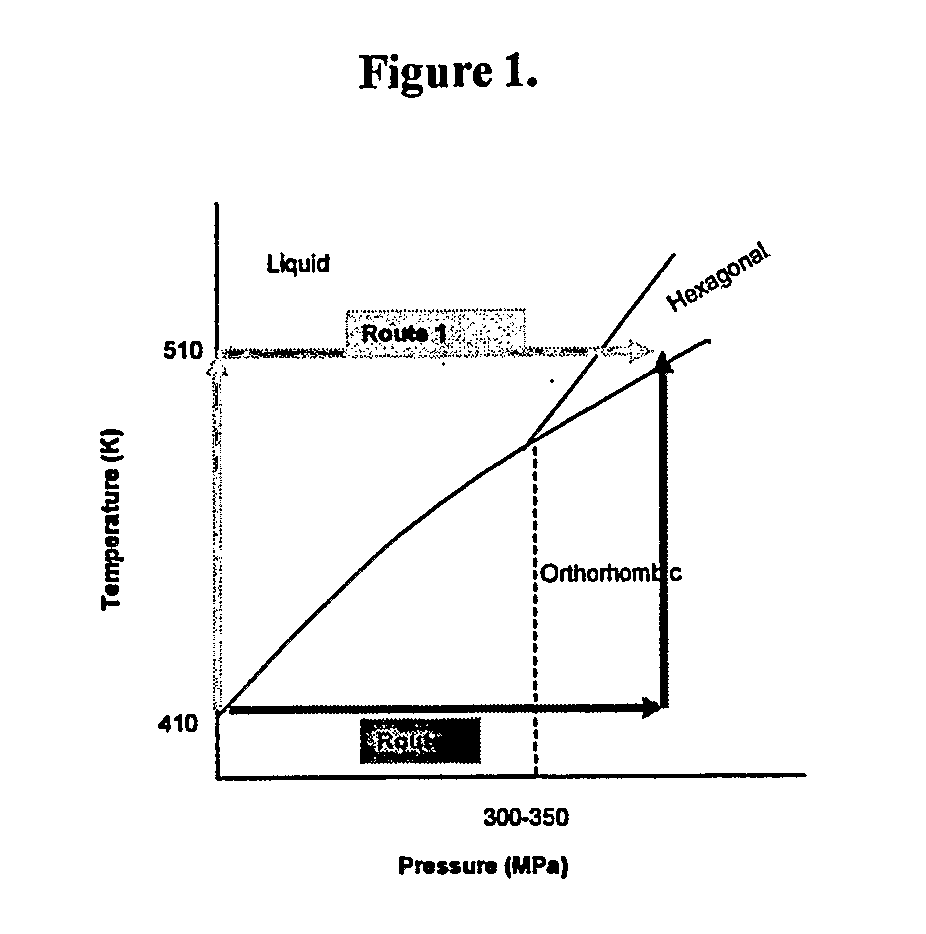
Highly Crystalline Cross-Linked Oxidation-Resistant Polyethylene
ActiveUS20070265369A1High crystallinityImprove the immunityPowder deliveryImpression capsCrystallographyCross-link
The present invention relates to methods for making highly crystalline cross-linked polymeric material, for example, highly crystalline cross-linked ultra-high molecular weight polyethylene (UHMWPE). The invention also provides methods of making antioxidant-doped highly crystalline cross-linked polymeric material using high pressure and high temperature crystallization processes, medical implants made thereof, and materials used therein.
Owner:THE GENERAL HOSPITAL CORP
High strength suture with absorbable core
ActiveUS7329271B2Improved absorption profileReducing knot profile of knotSuture equipmentsSurgical needlesYarnMedicine
Owner:DEPUY SYNTHES PROD INC
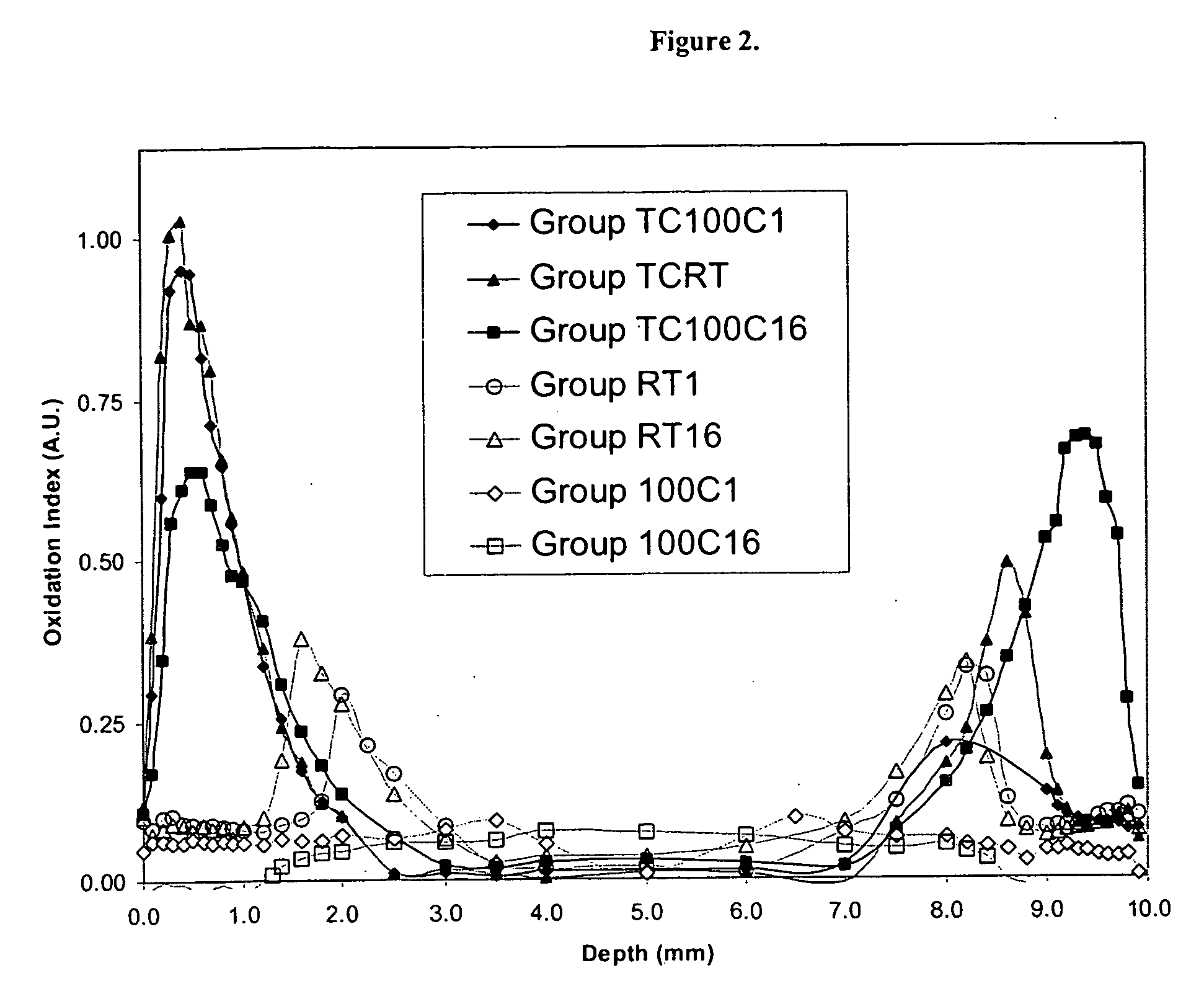
Methods for making oxidation resistant polymeric material
The present invention relates to methods for making oxidation resistant medical devices that comprise polymeric materials, for example, ultra-high molecular weight polyethylene (UHMWPE). The invention also provides methods of making antioxidant-doped medical implants, for example, doping of medical devices containing cross-linked UHMWPE with vitamin E by diffusion, post-doping annealing, and materials used therein.
Owner:THE GENERAL HOSPITAL CORP +1
Methods for making oxidation resistant polymeric material
InactiveUS20050194722A1Improve uniformityFacilitated DiffusionStentsConductive materialCross-linkAntioxidant
The present invention relates to methods for making oxidation resistant medical devices that comprise polymeric materials, for example, ultra-high molecular weight polyethylene (UHMWPE). The invention also provides methods of making antioxidant-doped medical implants, for example, doping of medical devices containing cross-linked UHMWPE with vitamin E by diffusion and materials used therein.
Owner:CAMBRIDGE POLYMER GROUP
High strength suture formed of UHMWPE and PBT
InactiveUS20070135840A1High strengthImproved tie down characteristicSuture equipmentsBraidFiberPolytetramethylene terephthalate
A high strength abrasion resistant surgical suture material with improved tie down characteristics is color coded for visualization and identification purposes. The suture features a multifilament cover formed of strands of ultra high molecular weight long chain polyethylene braided with polybutylene terephthalate. Selected fibers in the cover may be provided in a color contrasting with the other cover fibers to provide an identifiable trace. The cover surrounds a core formed of twisted strands of ultrahigh molecular weight polyethylene. The suture, provided in a #2 size, has the strength of #5 Ethibond, is ideally suited for most orthopedic procedures, and can be attached to a suture anchor or a curved needle.
Owner:ARTHREX
Medical device formed of ultrahigh molecular weight polyolefin
Medical devices having at least a component, such as a catheter balloon, stent cover and vascular graft, formed of ultrahigh molecular weight polyolefin, such as ultrahigh molecular weight polyethylene. The device component is formed from ultrahigh molecular weight polyethylene that has been processed so that it is microporous and has an oriented node and fibril structure. The device component expands compliantly at low strains and are substantially less compliant at higher strains. The invention also comprises methods for making such medical devices, including the steps of compacting a polyethylene powder and deforming it to impart the oriented structure.
Owner:ABBOTT CARDIOVASCULAR
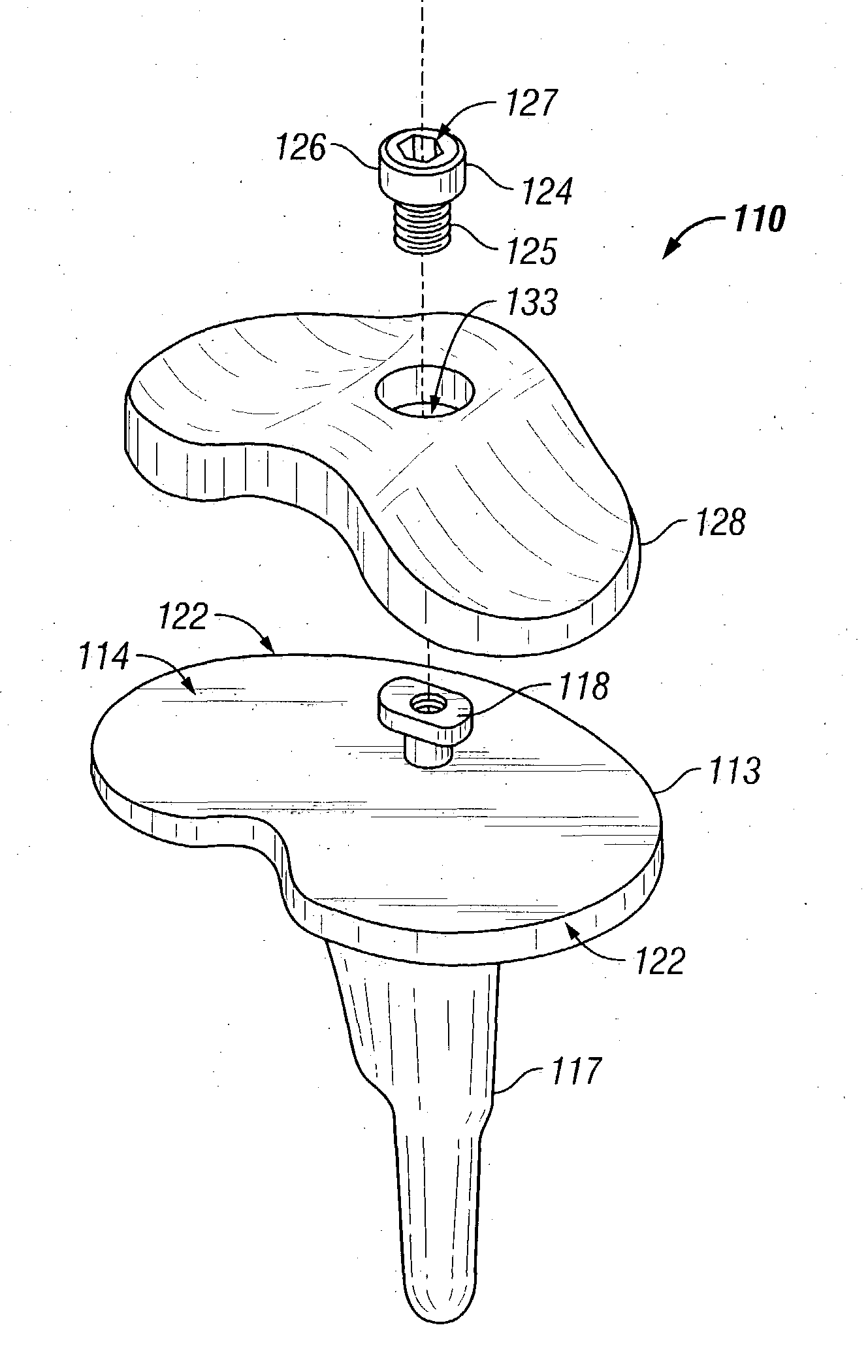
Sigmoid notch implant
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
High strength suture with absorbable core and suture anchor combination
ActiveUS7357810B2Improved absorption profileReducing knot profile of knotSuture equipmentsSurgical needlesYarnMedicine
Owner:DEPUY SYNTHES PROD INC
Multilayered polyethylene material and ballistic resistant articles manufactured therefrom
The present invention relates to polyethylene material that has a plurality of unidirectionally oriented polyethylene monolayers cross-plied and compressed at an angle to one another, each polyethylene monolayer composed of ultra high molecular weight polyethylene and essentially devoid of resins. The present invention further relates to ballistic resistant articles that include or incorporate the inventive polyethylene material and to methods of preparing the material and articles incorporating same.
Owner:AVIENT PROTECTIVE MATERIALS BV
Method For Making Oxidation Resistant Polymeric Material
ActiveUS20080119582A1Facilitated DiffusionLavatory sanitoryJoint implantsCross-linkOxidation resistant
The present invention relates to methods for making oxidation resistant medical devices that comprise polymeric materials, for example, ultra-high molecular weight polyethylene (UHMWPE). The invention also provides methods of making antoxidant-doped medical implants, for example, doping of medical devices containing cross-linked UHMWPE with vitamin E by diffusion, post-doping annealing, and materials used therein.
Owner:THE GENERAL HOSPITAL CORP +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com