Patents
Literature
1660 results about "Inner/Lumen Diameter" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
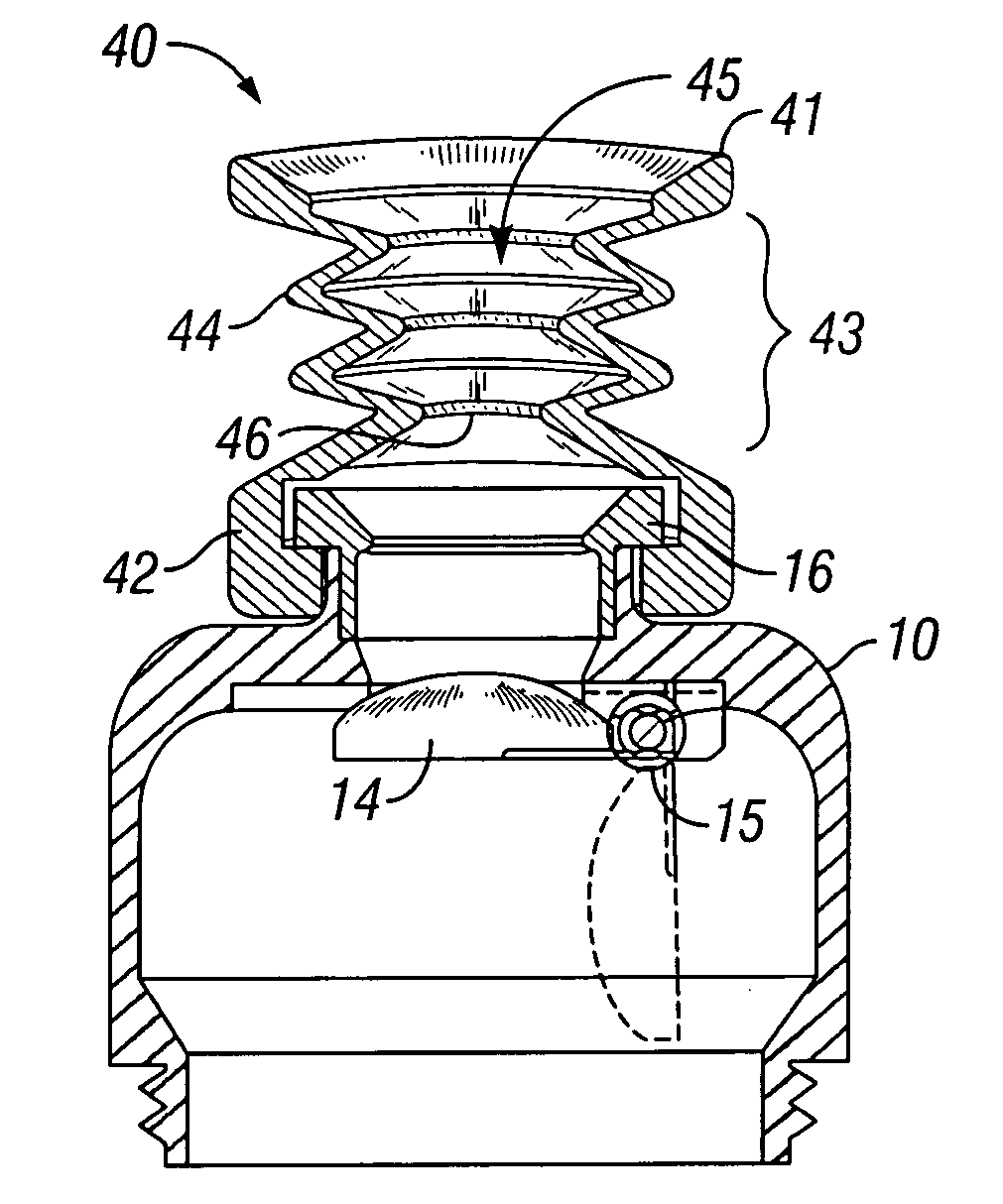
Variable compression surgical fastener cartridge
InactiveUS20090255976A1Great compressive forceSuture equipmentsStapling toolsBiomedical engineeringSurgical Fasteners
A surgical fastener applying apparatus comprising a cartridge body including a tissue contacting surface including a plurality of fastener retention slots arranged in a plurality of rows including at least an inner row and an outer row, a plurality of surgical fasteners disposed in the inner and outer rows and configured such that a plurality of fasteners disposed in the inner row have a first diameter that is less than a second diameter of a plurality of surgical fasteners disposed in the outer row, and an anvil having an inner row and an outer row of depressions for forming the fasteners. A plurality of pushers are operably associated with the plurality of surgical fasteners, the pushers configured to eject an associated surgical fastener towards the respective depression in the anvil such that upon formation of a corresponding surgical fastener, the surgical fasteners ejected from the inner row provide a greater compressive force to tissue than the surgical fasteners ejected from the outer row.
Owner:TYCO HEALTHCARE GRP LP
Circular stapler buttress
A buttress for use with circular surgical staplers that does not require adhesive to securely fasten the buttress to the stapler. Following cutting and stapling by the circular stapler, the buttress has an adaptive opening through its central region with a diameter smaller than the outer diameter of the stapler anvil. Because of relief features built into the buttress, the stapler anvil may be pulled through the buttress material without causing permanent alteration to the buttress. These relief features may be provided regardless of whether the buttress is made of inelastic or elastic materials. The buttress is generally circular in shape with an outer diameter sized to coincide with the outer diameter of the stapler body staple compression surface and the outer diameter of the anvil compression surface of a circular stapler with which it is used. Prior to surgical use, the buttress is attached to the stapler with disruptable portions extending from outer perimetal areas of the buttress.
Owner:WL GORE & ASSOC INC
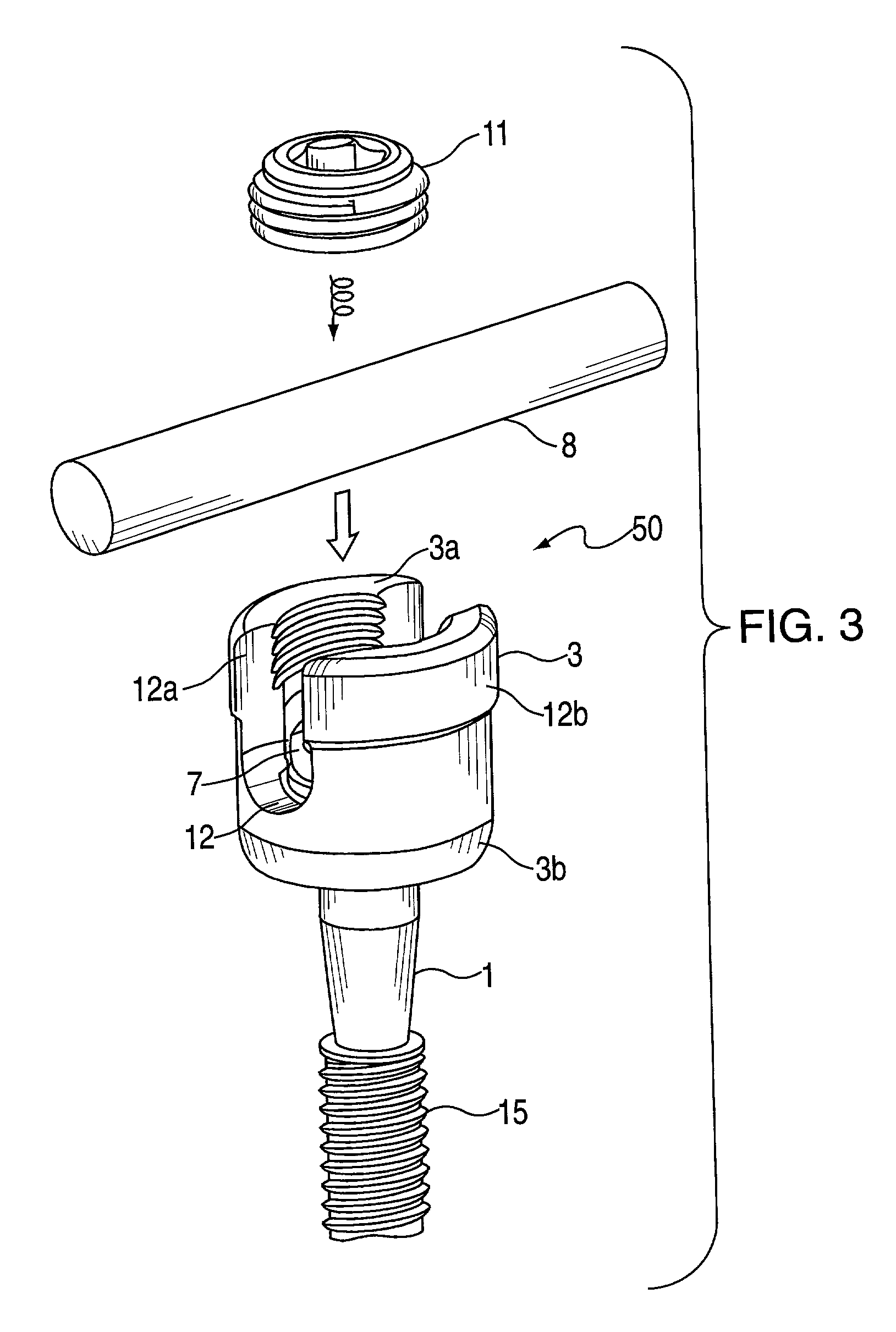
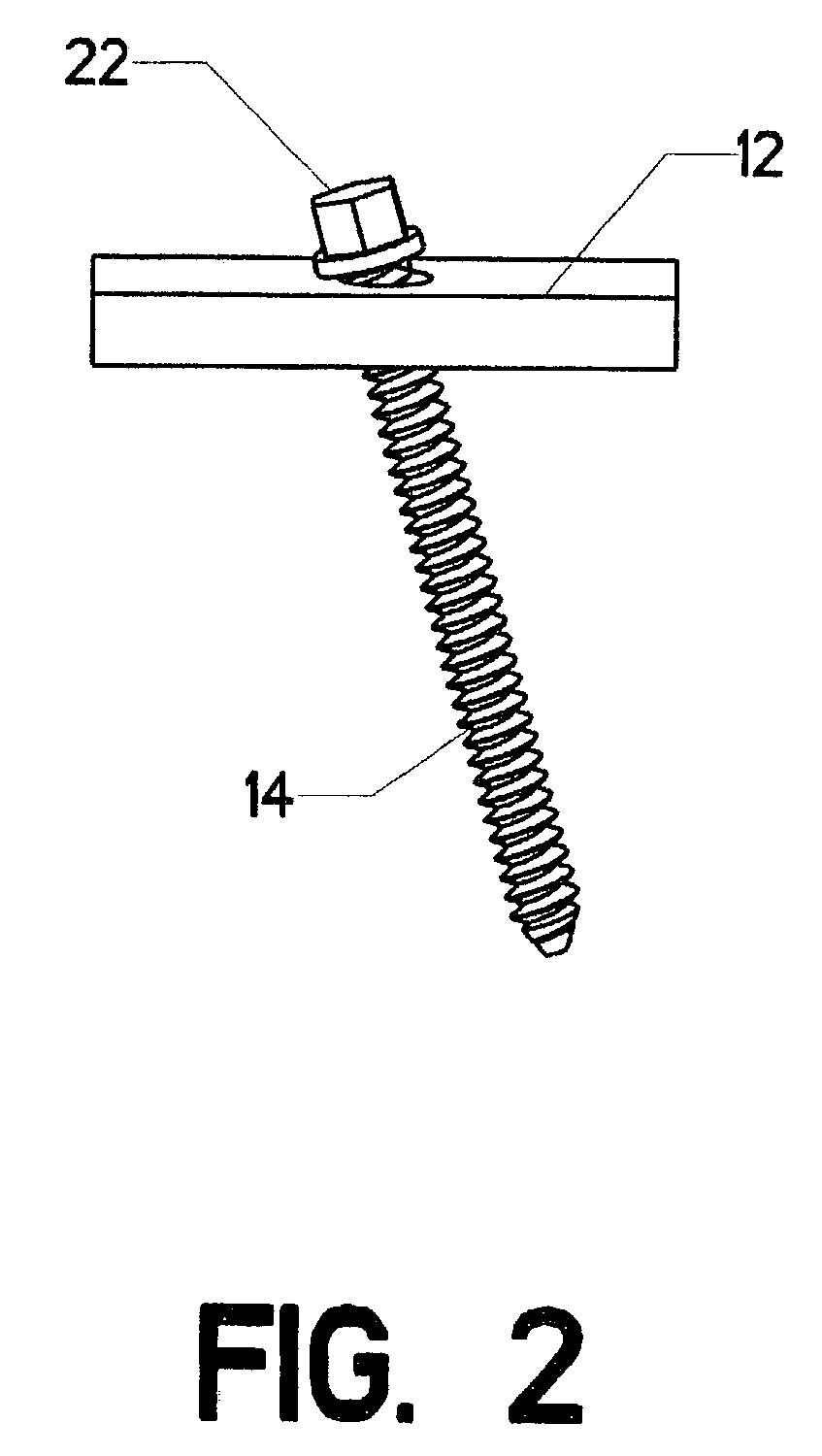
Surgical screw system and related methods
A surgical screw system for use with implantation rods includes a screw member, a receiver member, a pressure cap and a locking device. The screw member has a shaft and a head with a spherical undersurface and a conical tapered recess. The receiver member has an upper and lower portion, a u-shaped rod receiving channel and an axial bore. The u-shaped channel has two lateral legs at the upper portion and forms an opening leading to the axial bore. The axial bore near the lower portion includes an inwardly conical tapered surface which has a diameter larger than the shaft of the screw member but smaller than the head of the screw member. The conical surface forms a support upon which the spherical undersurface of the head of the screw member rests when the screw member is guided through the bore of the lower portion of the receiver member. The pressure cap is positioned within the axial bore and situated upon the head of the screw member. The locking device is designed for securing the rod within the u-shaped channel of the receiver member by applying a tightening torque upon the rod when positioned within the opening and the bore near the upper portion of the receiver member.
Owner:BLACKSTONE MEDICAL
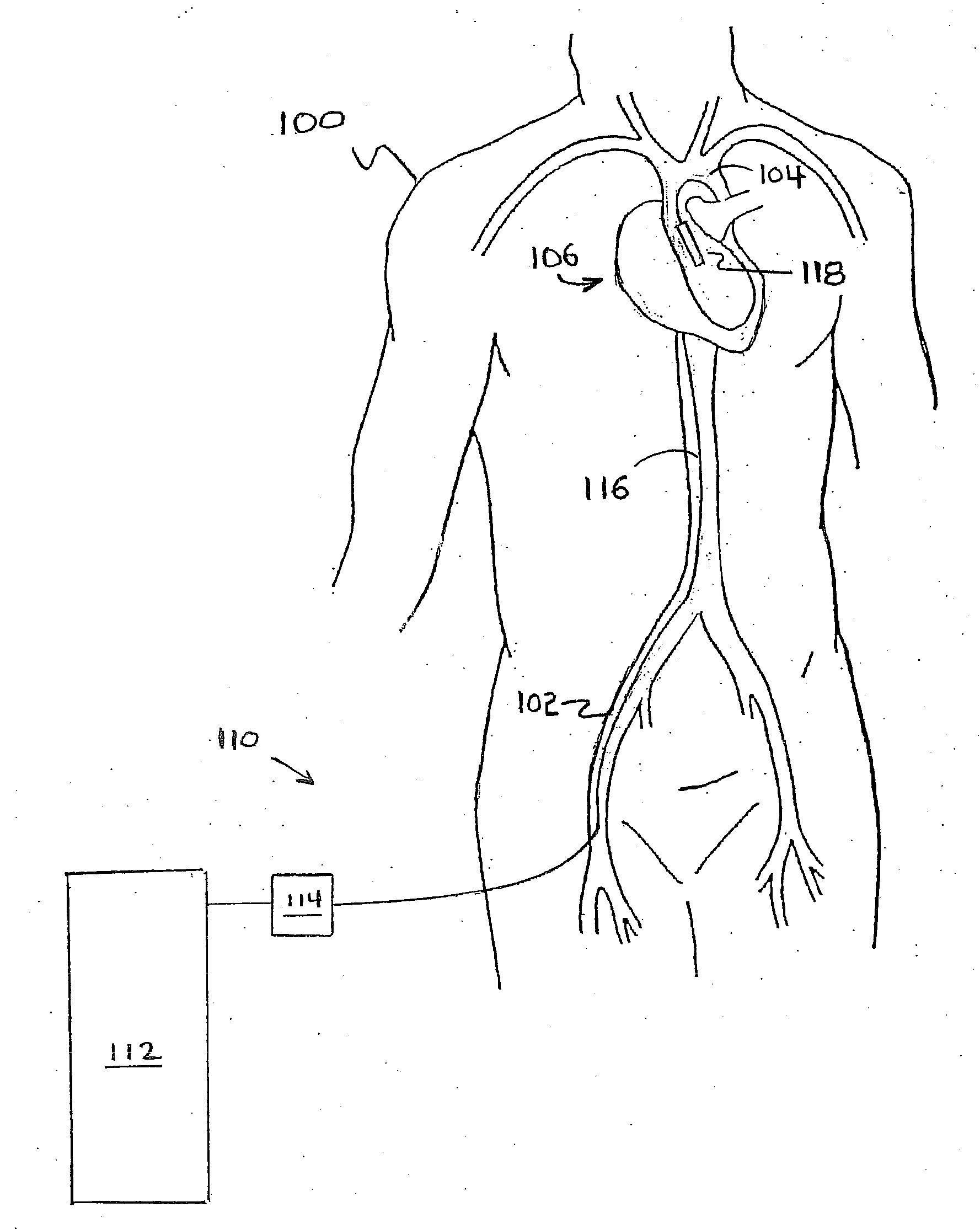
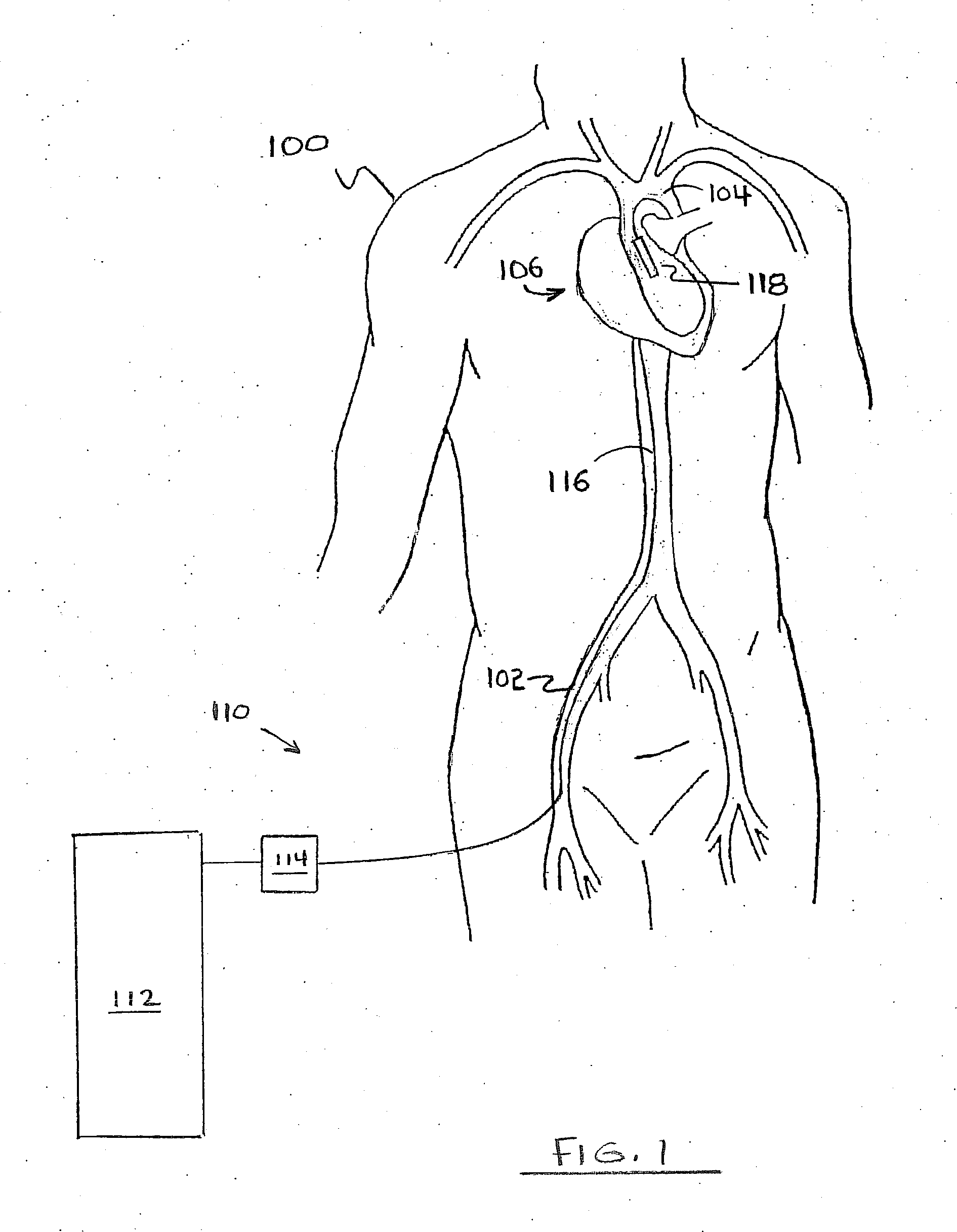
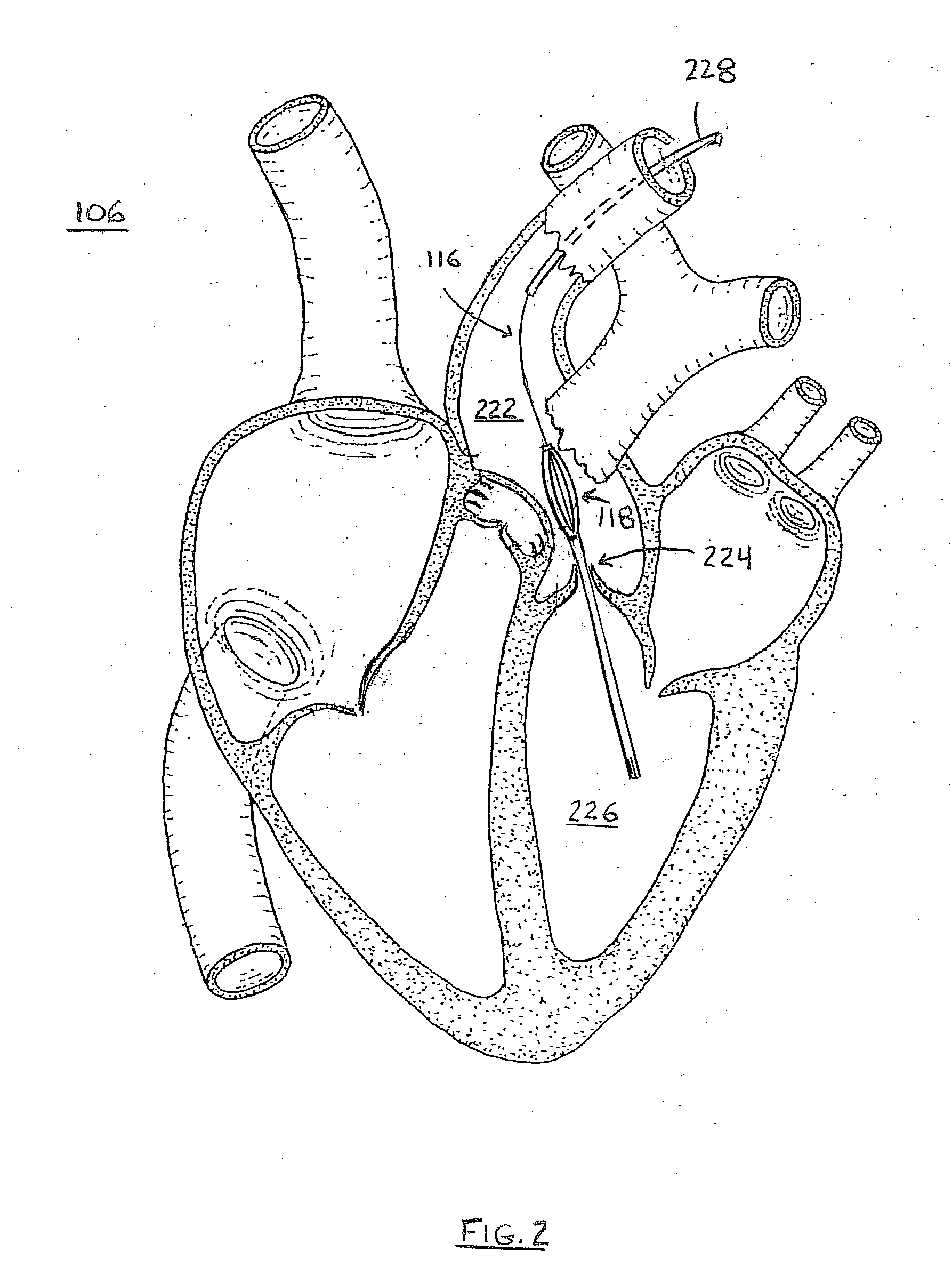
Method for Deployment of a Medical Device
InactiveUS20080132748A1Reduce bending loadMinimizing contact pressureControl devicesBlood pumpsAortic archMedical device
A method for deploying a cardiac-assist device is disclosed. In accordance with the illustrative embodiment, an introducing tube, such as a catheter, sheath, or the like is inserted into the vascular system and advanced beyond the aortic arch. The cardiac-assist device is then inserted into the tube, advanced through it, and deployed from its distal end. In some embodiments, the diameter of the cardiac-assist device expands when it deploys from the distal end of the tube.
Owner:MEDICAL VALUE PARTNERS
Easily placeable and removable wound retractor
InactiveUS20060149137A1Easy to placeGood for scrollingCannulasSurgical needlesSurgical departmentBody tissue
The invention is directed to a surgical wound retractor for retracting and sealing an incision and forming a functional opening or channel through which a surgical procedure may be executed. The wound retractor provides a path for a surgeon to insert his hand and / or instruments through the opening formed by the wound retractor. The wound retractor is sized and configured to be easily placed through a small incision and removed without further insult to the body tissue adjacent to the incision. The wound retractor is adapted to dilate a surgical wound incision to a desired diameter, and comprises a first ring having a diameter greater than the desired diameter of the wound incision and being adapted for disposition interiorly of the wound incision; a second ring having an annular axis and a diameter greater than the desired diameter of the wound incision and being adapted for disposition exteriorly of the wound incision; and a flexible sleeve disposed in a generally cylindrical form between the first ring and the second ring, the first ring having at least one notch to facilitate folding or collapsing of the first ring during insertion and removal of the first ring through the wound incision. The first ring may further comprise a second notch disposed on an opposing end of the first notch to further facilitate folding or collapsing of the first ring. With this aspect, the first ring is folded by squeezing between the first and second notches during insertion and removal of the retractor from the incision. In another aspect, the wound retractor may further comprise a tether having a length, a first end attached to the first ring interiorly of the sheath, and a second end disposed outside of the wound incision, wherein the tether facilitates removal of the first ring by pulling on the second end to retrieve the first ring through the wound incision.
Owner:APPL MEDICAL RESOURCES CORP
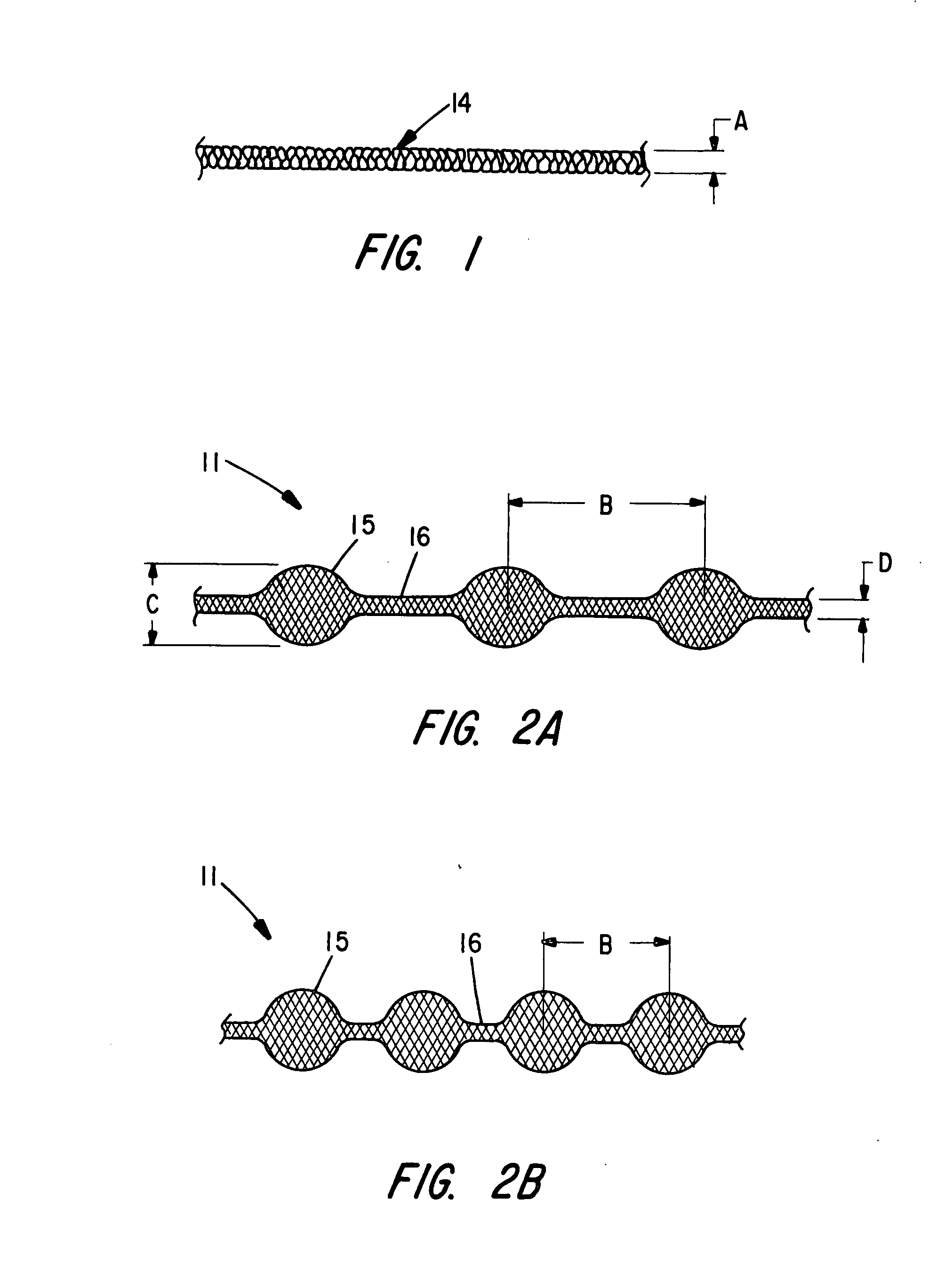
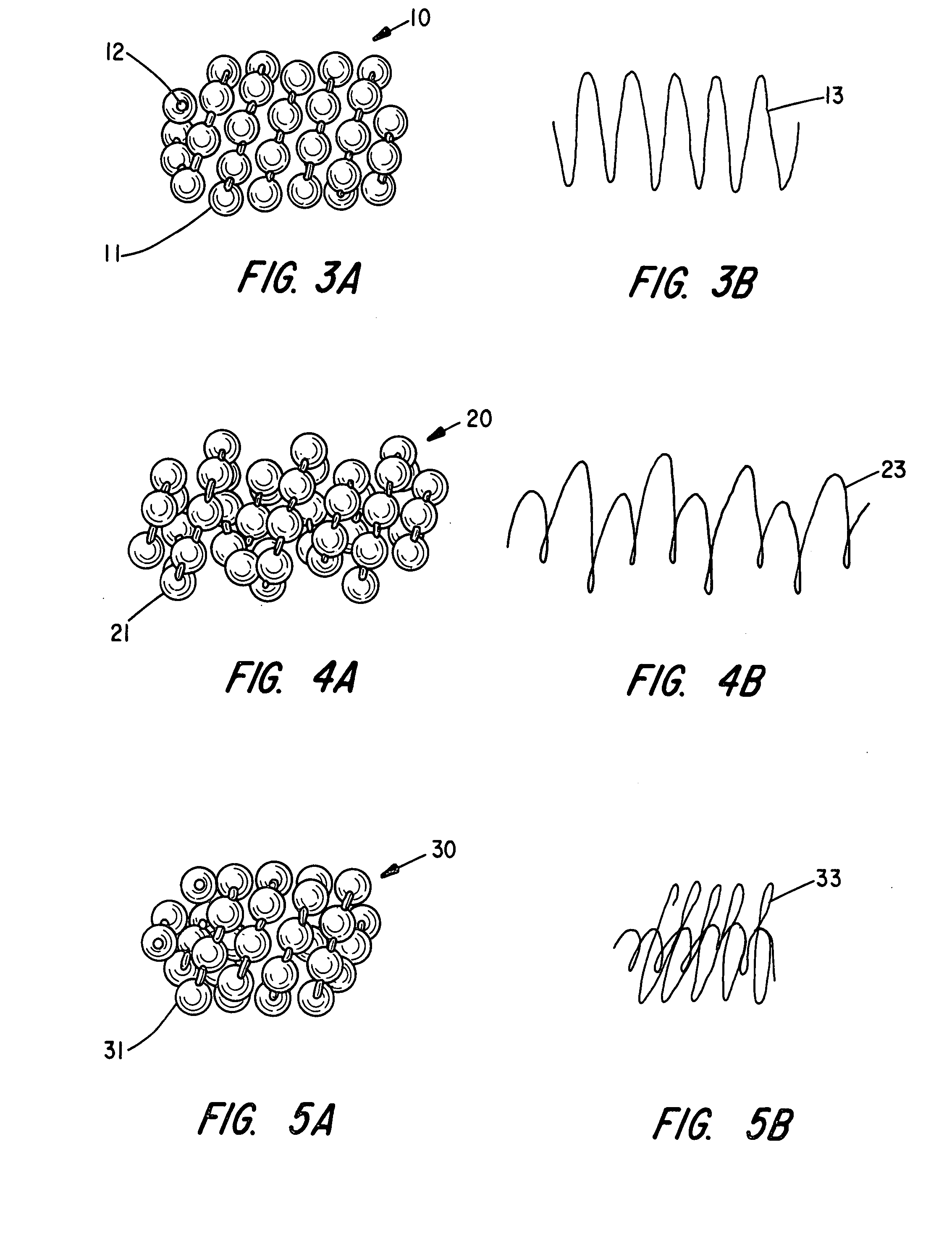
Braided occlusion device having repeating expanded volume segments separated by articulation segments
InactiveUS20090025820A1Occlude a vessel, channel, lumen, or cavity quicklyMeet high volumeWire articlesDilatorsBody organsVolume filling
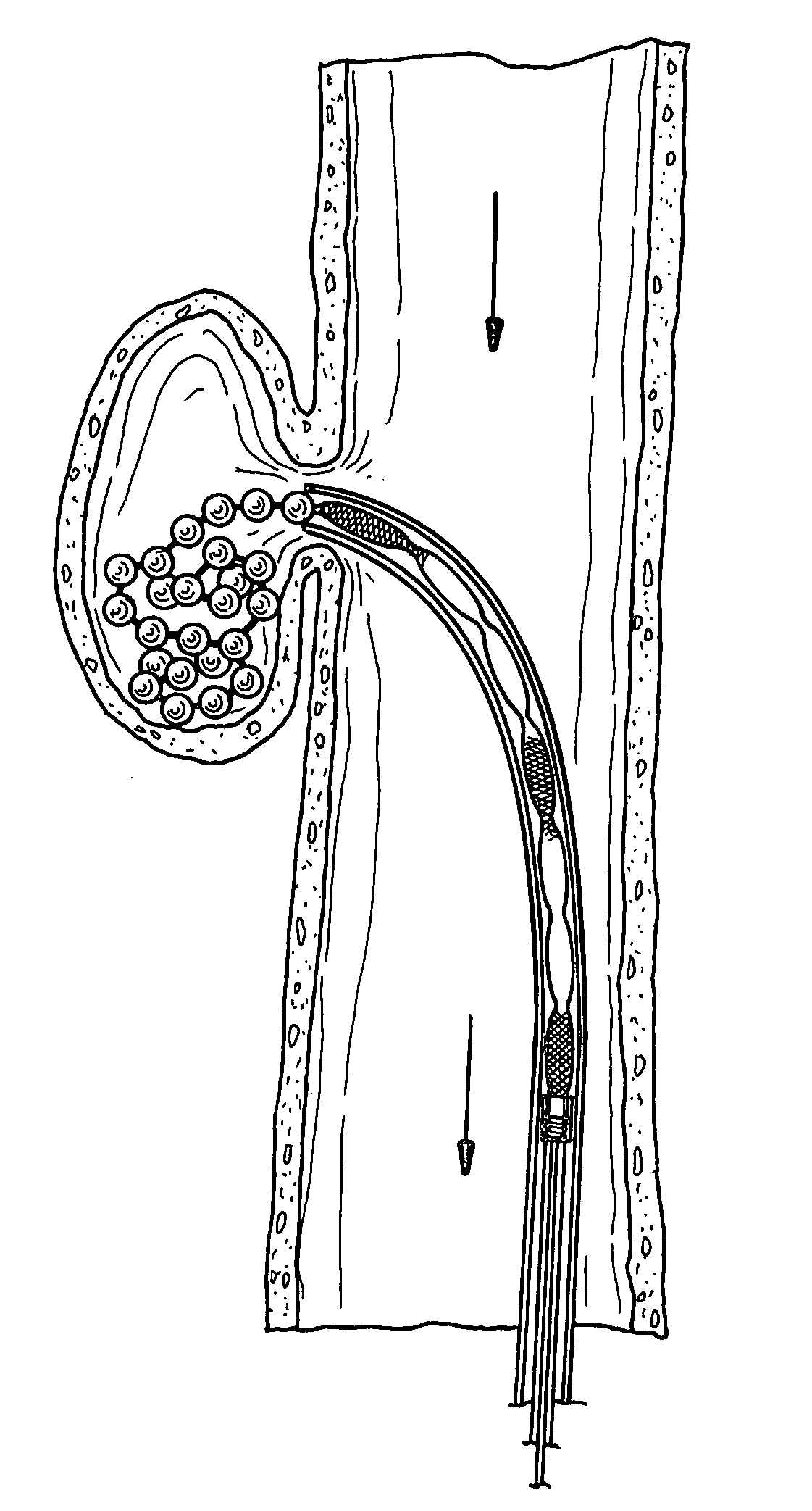
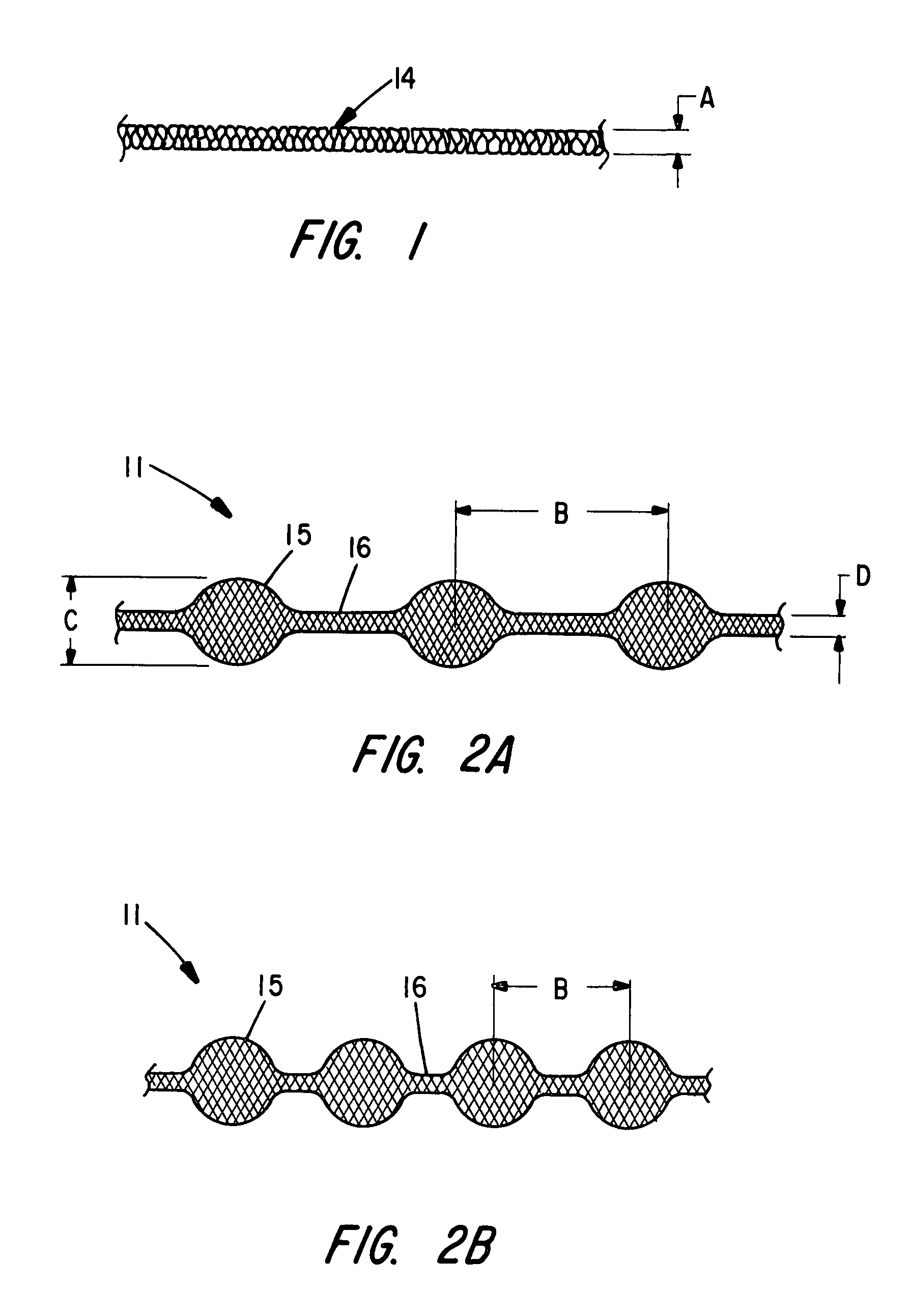
The present invention provides a flexible, low profile vascular occlusion device having large volume filling capability and high metal content for fast occlusion, of the type fabricated from braided tubular metal fabric having an expanded preset configuration and an elongated collapsed reduced diameter configuration for delivery through a catheter to a treatment site and shaped to create an occlusion of an abnormal opening in a body organ or vessel, the woven metal fabric having a memory property whereby the medical device tends to return to said expanded preset configuration when unconstrained. The device further includes a first shape formed from the braided tubular fabric consisting of a repeating pattern of expanded volume segments separated by small diameter articulation segments and a second overall device shape comprised of the first shape formed about itself in various shapes to occlude a vessel. In one embodiment a shaping wire contained coaxially within the tubular braid, provides or assists in the formation of the second overall device shape.
Owner:ST JUDE MEDICAL CARDILOGY DIV INC
Systems And Methods For Removing Obstructive Matter From Body Lumens And Treating Vascular Defects
InactiveUS20120101510A1Increase blood flowHigh trafficMulti-lumen catheterCannulasMedicineDistal portion
Systems and methods for removing obstructions from, delivering implantable devices or substances in or near and / or restoring flow through body lumens, such as blood vessel lumens. A catheter having a proximal portion of a first diameter and a distal portion of a second diameter (smaller than the first diameter) is advanced into a body lumen. The distal portion of the catheter is caused to expand to a diameter that is larger than the second diameter but no larger than the first diameter. A working device is then advanced out of the distal end of the catheter and used to remove obstructive matter, deliver an implantable device or substance and / or restore flow. The distal portion can be reduced in diameter prior to removal from the body.
Owner:TYCO HEALTHCARE GRP LP
Systems and methods for compartmental replacement in a knee
A knee replacement system provides a knee implant that may be used to more accurately replicate the diameter of a natural knee. In one embodiment, a patellofemoral component is connected to the posterior portion of a condylar component by screws that pass through the femur allowing the patellofemoral component and the condylar component to be torqued against opposing sides of the femur. Two additional screws are used to connect the patellofemoral component to an anterior portion of the condylar component. A gap may be left between the patellofemoral component and the anterior portion of the condylar component if needed to provide precise replication of the diameter of the natural knee from the patellofemoral component to the condylar component.
Owner:DEPUY PROD INC
System and method for removal of material from a blood vessel using a small diameter catheter
InactiveUS20070118165A1Increase flexibilityMaximize supportCannulasExcision instrumentsDistal portionThrombus
This invention provides a small diameter snare device and device for thrombus removal consisting of a hollow, elongate, thin-walled outer sheath constructed from polymer, at least at a distal part thereof. A single central core wire extends through the entire length of the sheath. The outer diameter of the core wire is sized close to the inner diameter of the sheath while allowing for axial sliding, in order to maximize the support to the body portion of the snare device. The distal end of the core wire has a tapered section of reduced diameter or cross section to provide a “guidewire-like” flexibility to the distal portion of the device. A second wire (or wires) of about fifty percent or less of the inner diameter of the sheath is (are) shaped to form a snare loop or radially extended tool tip that is attached to the distal most portion of the central core wire. The snare loop is typically circular or oval shaped and can also be multiplanar (for example, a twisted, figure eight shape) so as to increase the ability to ensnare and capture objects. The tool tip can be a planar or multi-planar, proximally directed structure that, when deployed, is adapted to engage the thrombus from behind after piercing the thrombus with the sheath while the tool tip is retracted.
Owner:VASCULAR SOLUTIONS
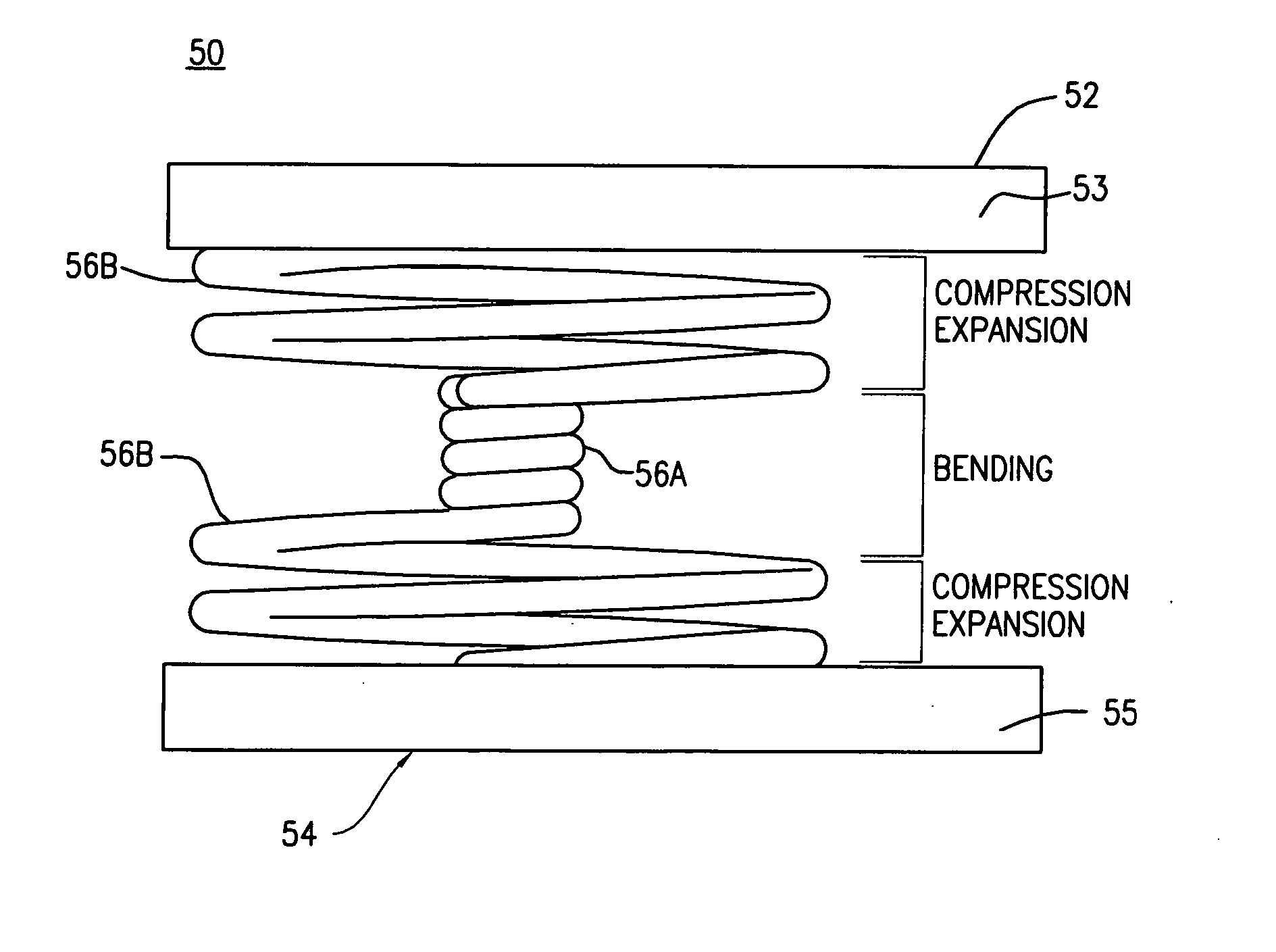
Posterior lumbar intervertebral stabilizer
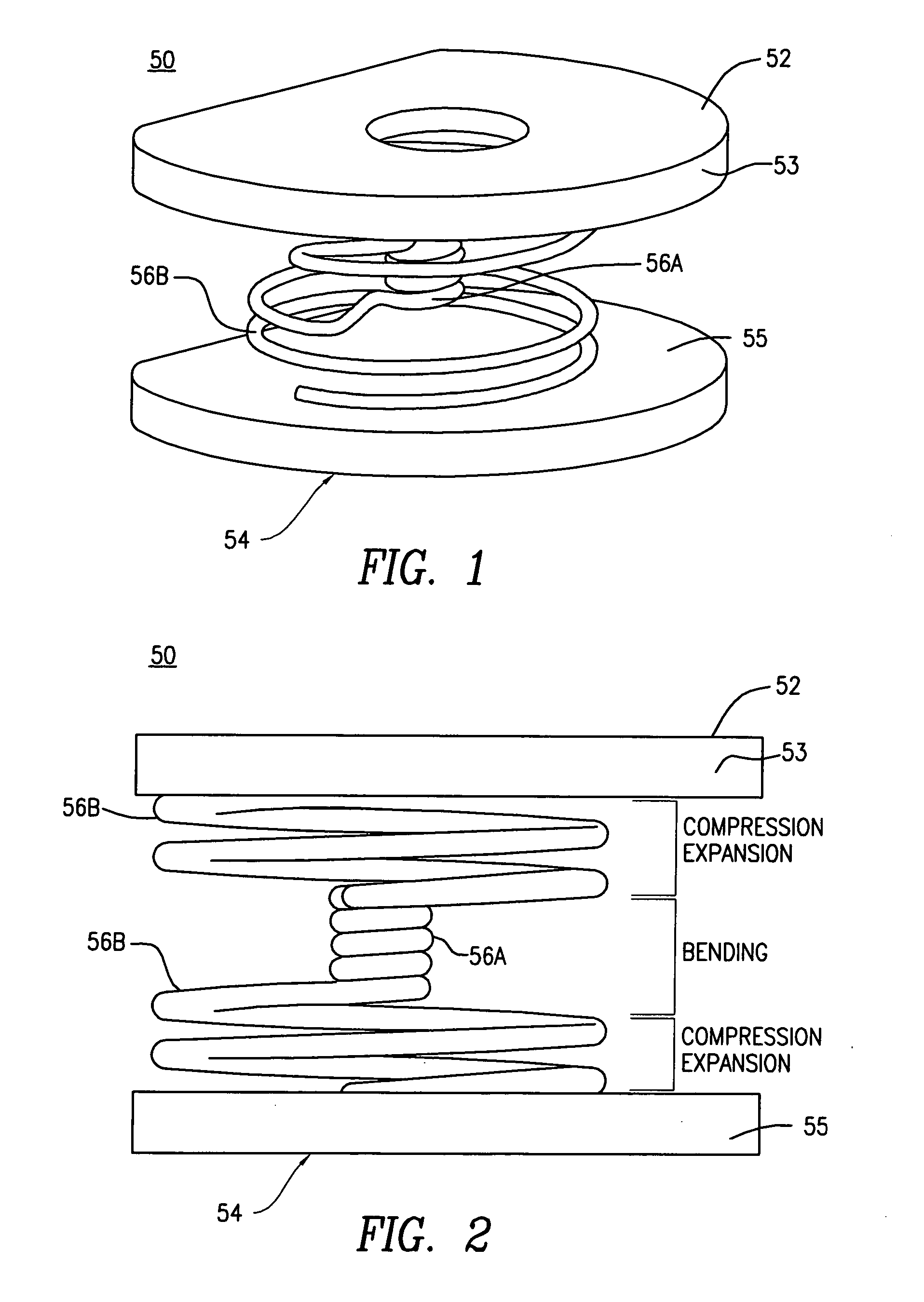
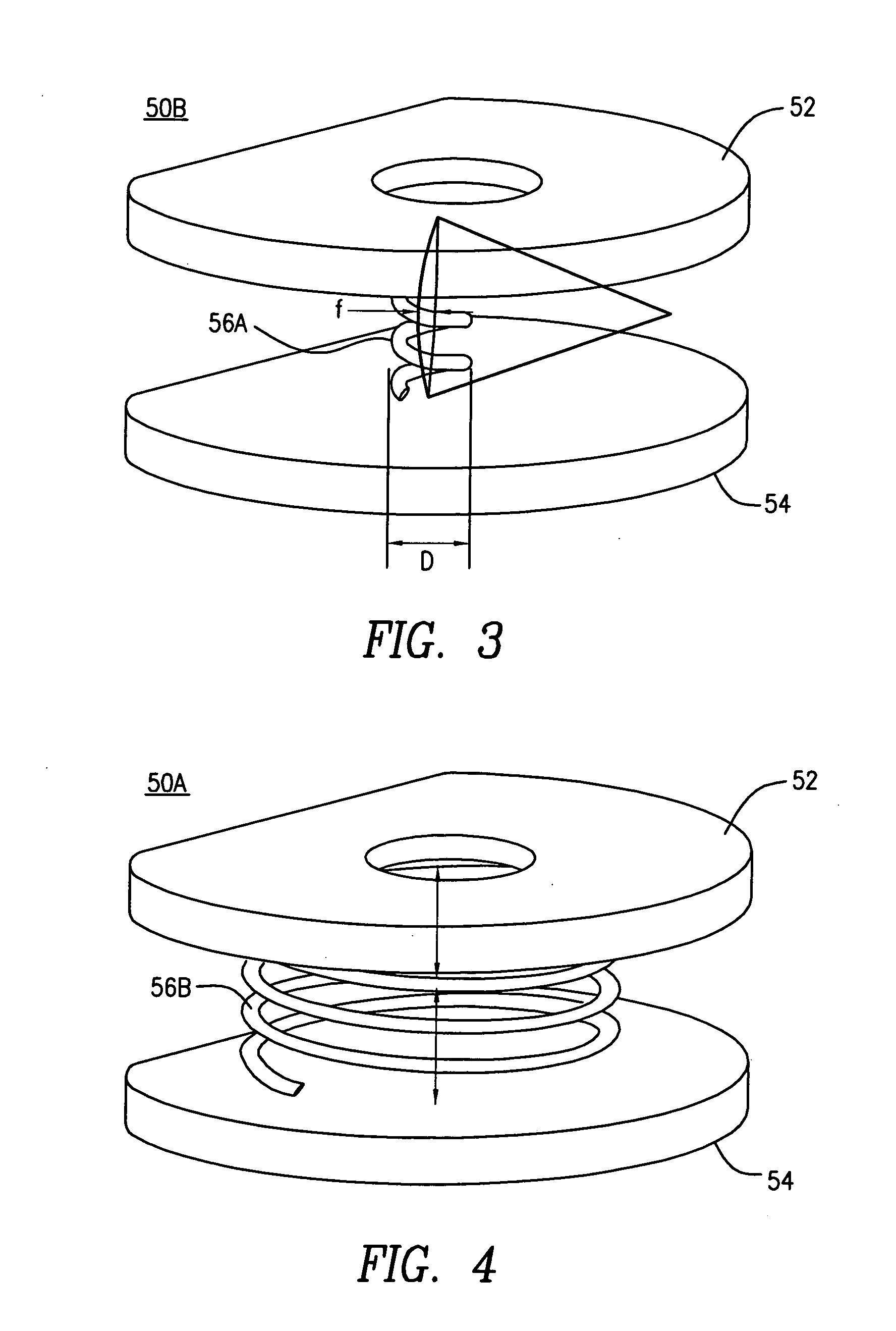
A posterior intervertebral stabilizer includes: a first stabilizing element having: (i) a first surface operable to engage an endplate of a first vertebral bone of a spine, and (ii) a second surface spaced apart from the first surface and operable to engage an endplate of an adjacent second vertebral bone of the spine; a second stabilizing element having: (i) a first surface operable to engage an endplate of the first vertebral bone of the spine, and (ii) a second surface spaced apart from the first surface and operable to engage an endplate of the adjacent second vertebral bone of the spine; and respective spring elements, each including helical coils, disposed between the respective first and second surfaces of the first and second stabilizing elements, each operable to provide a reactive force in response to compression loads from the first and second vertebral bones, wherein at least some diameters of respective turns of the respective helical coils differ.
Owner:CARDO MEDICAL
Braided occlusion device having repeating expanded volume segments separated by articulation segments
InactiveUS8361138B2Occlude a vessel, channel, lumen, or cavity quicklyMeet high volumeDilatorsOcculdersBody organsMedical device
Owner:ST JUDE MEDICAL CARDILOGY DIV INC
Method and apparatus for providing a relative location indication during a surgical procedure
An orthopedic guidewire includes an elongate guidewire body having longitudinally spaced proximal and distal guidewire ends. An engaging feature is located at the distal guidewire end and is configured to selectively engage a bone surface. At least one of a variable diameter and a variable stiffness are along a portion of the guidewire body spaced apart from the engaging feature. A method of providing a relative location indication during a surgical procedure utilizing the orthopedic guidewire is also included.
Owner:THE CLEVELAND CLINIC FOUND
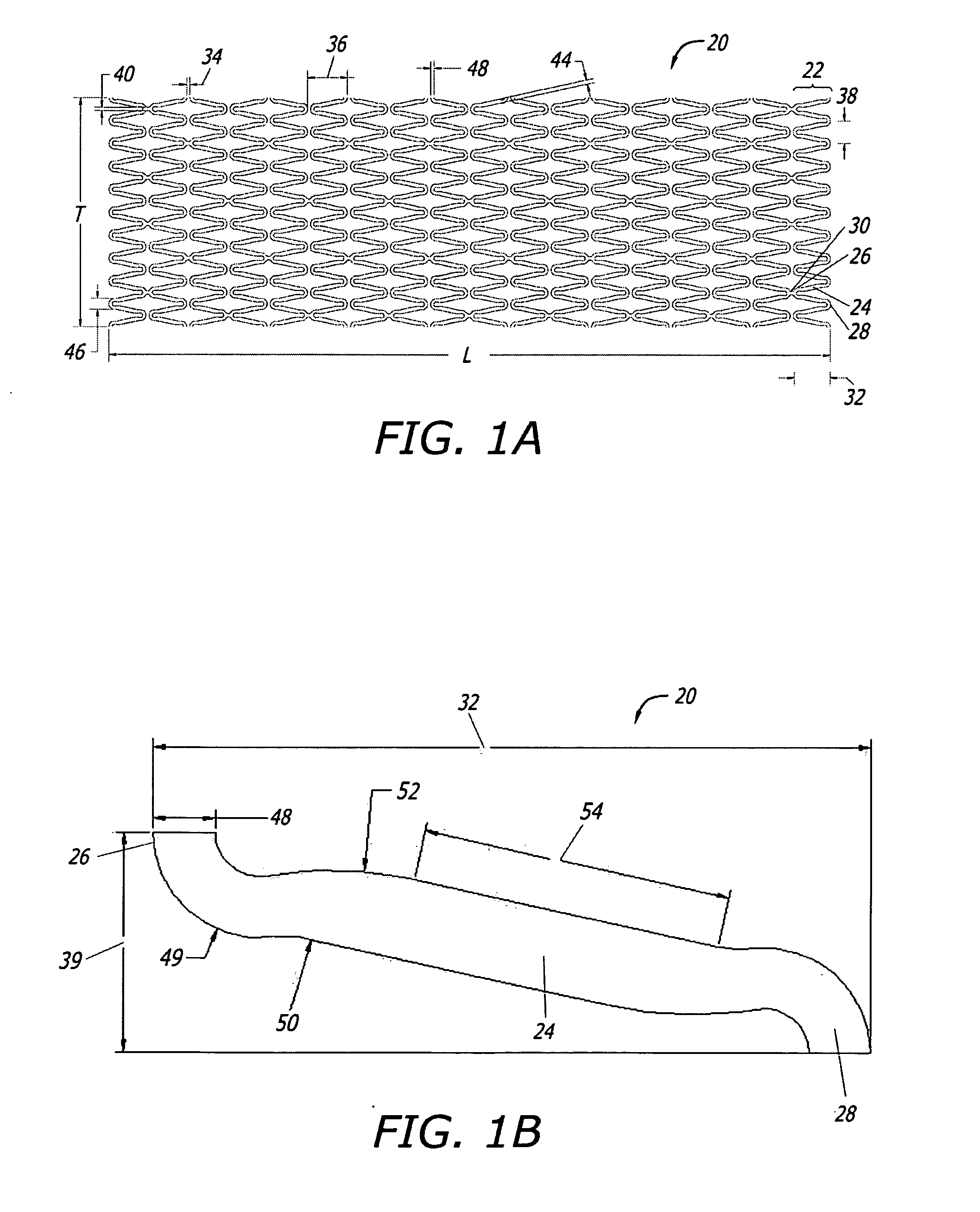
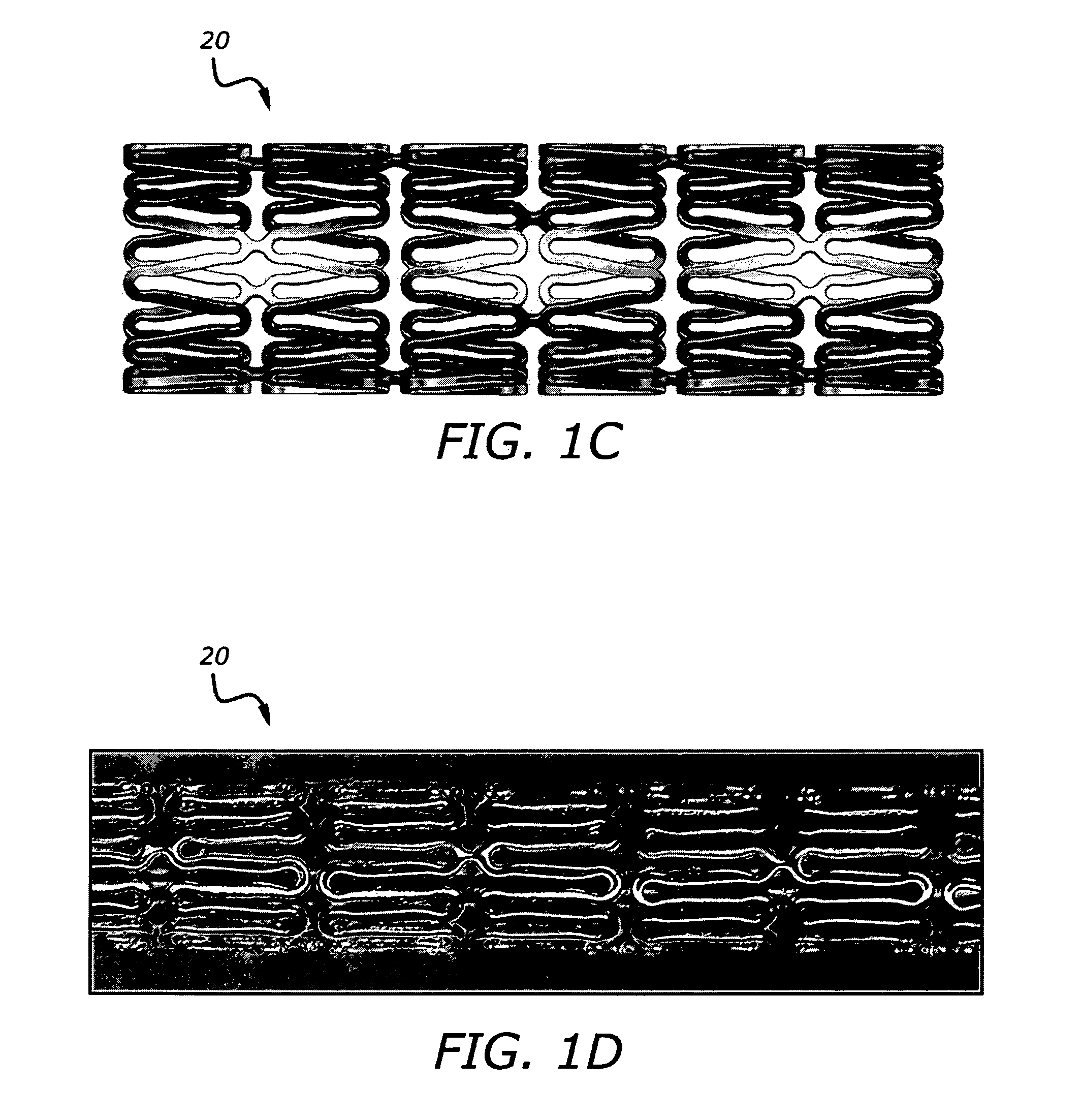
Implantable and lumen-supporting stents and related methods of manufacture and use
InactiveUS20090105809A1Improve expansion propertiesImprove featuresStentsBlood vesselsVisibilityLateral bending
An implantable stent includes multiple circumferential segments that surround a bore and are connected in series along a length to form a tubular wall. Multiple adjacent alternating opposite facing crowns arranged along each segment's circumference are bridged by struts. The struts include a series of staggered arcuate edges with limited flats to provide a limited region of maximum width between significantly extended reducing diameter tapers at either end where they transition into the crowns. Connections between adjacent segments are wider and stiffer than the struts and strut-crown transitions in the segments. The crowns include inner and outer radii with off-set centers along a common axis to provide medial crown peaks along the axis that are wider than the narrowed crown shoulders on either side of the axis and from which the tapered struts extend. Material strain and flexure along the stent during lateral bending is distributed mainly within the segments, e.g. along the struts or crowns, versus at the connections between segments. Material strain and deformation during radial expansion is principally concentrated at the crown shoulders and tapered transition region with the struts. Particular closed-open-closed arrangements along the stent length are disclosed, though with fewer stent connections in the relatively “closed” end-portions along the stent than are provided by other typically “open” cell stents in prior use. Enhanced combinations of performance characteristics are provided regarding visibility, trackability, expansion characteristics, fatigue failures, coating integrity, and local drug delivery from the stent.
Owner:MEDLOGICS DEVICE CORP
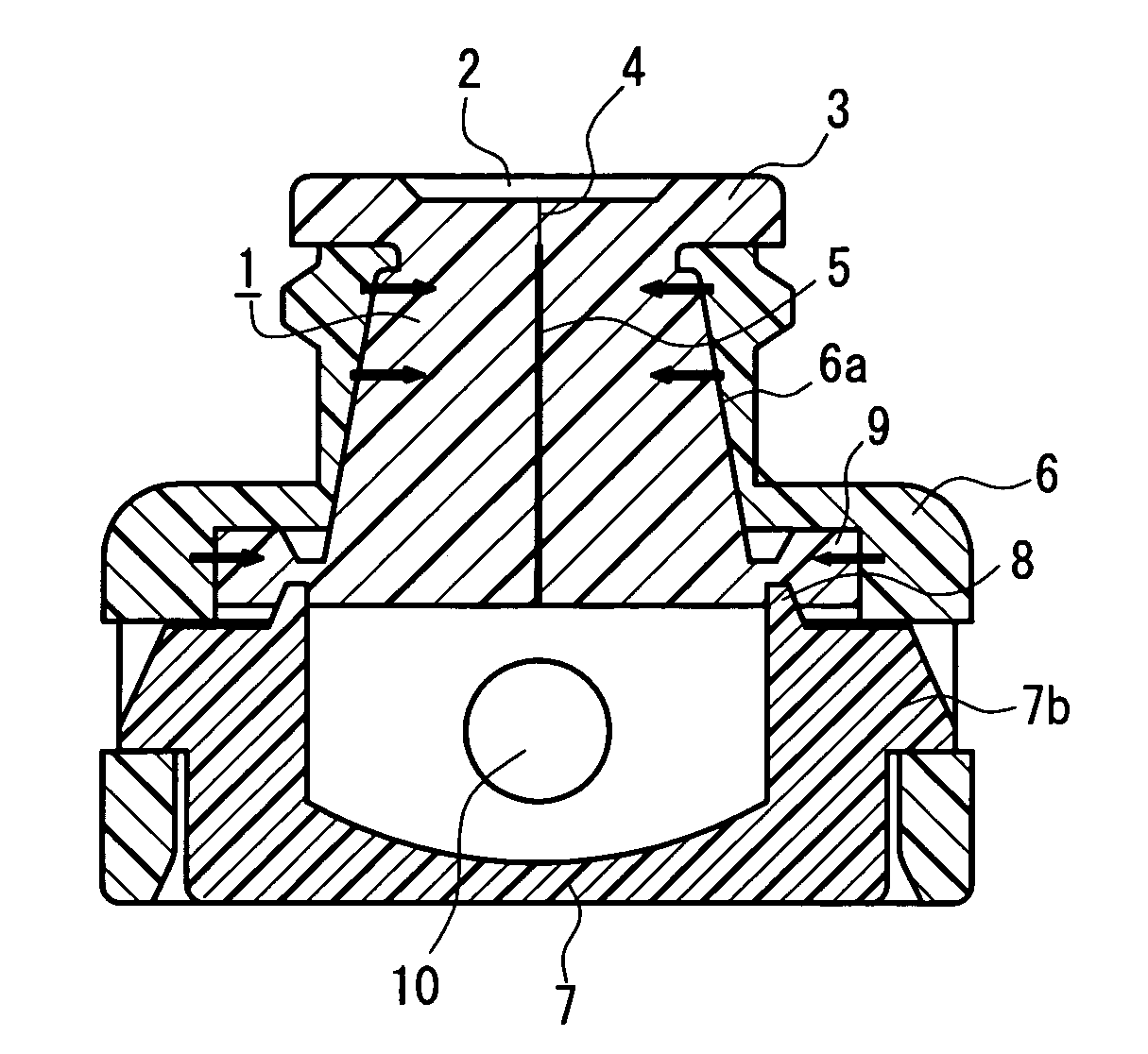
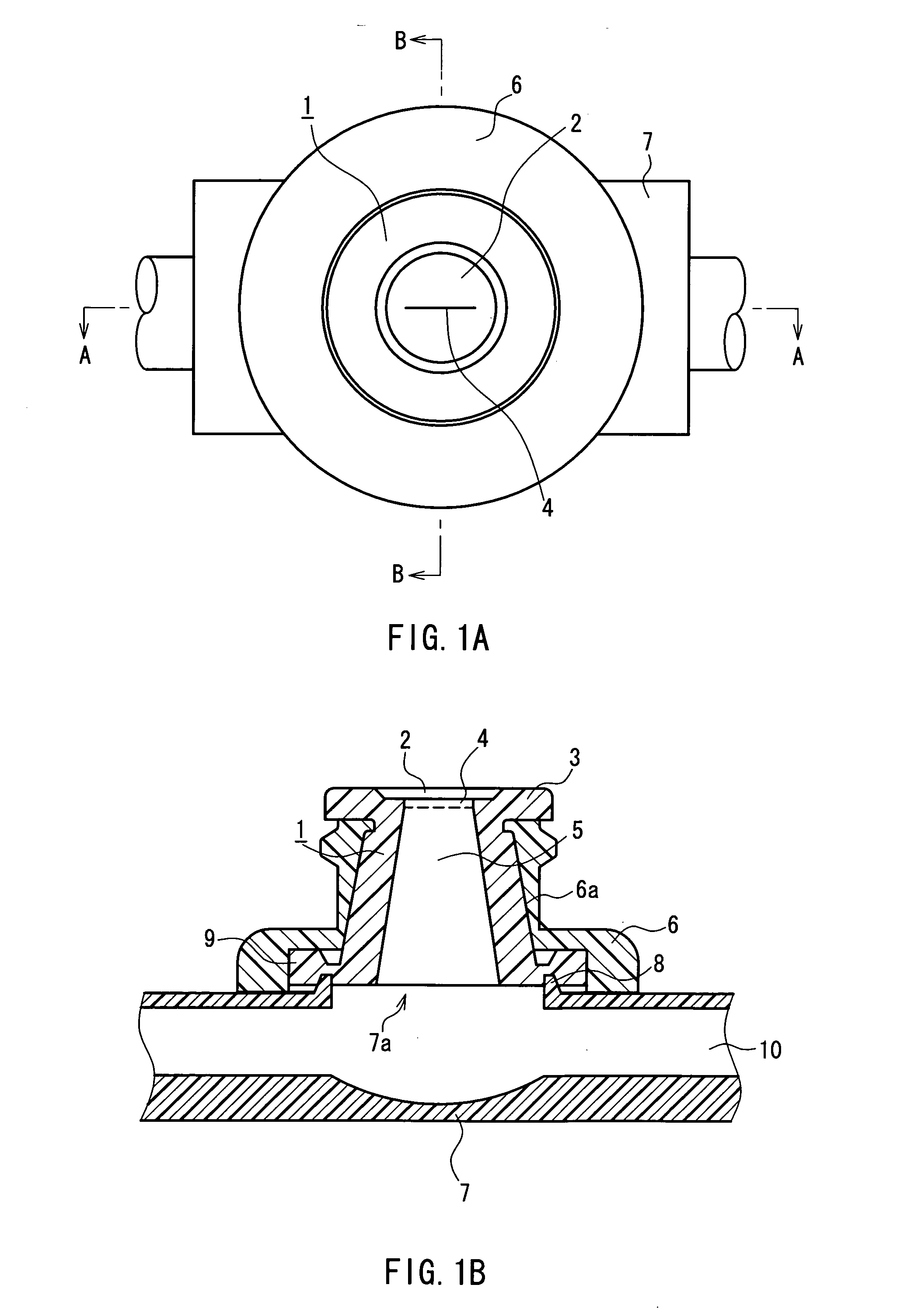
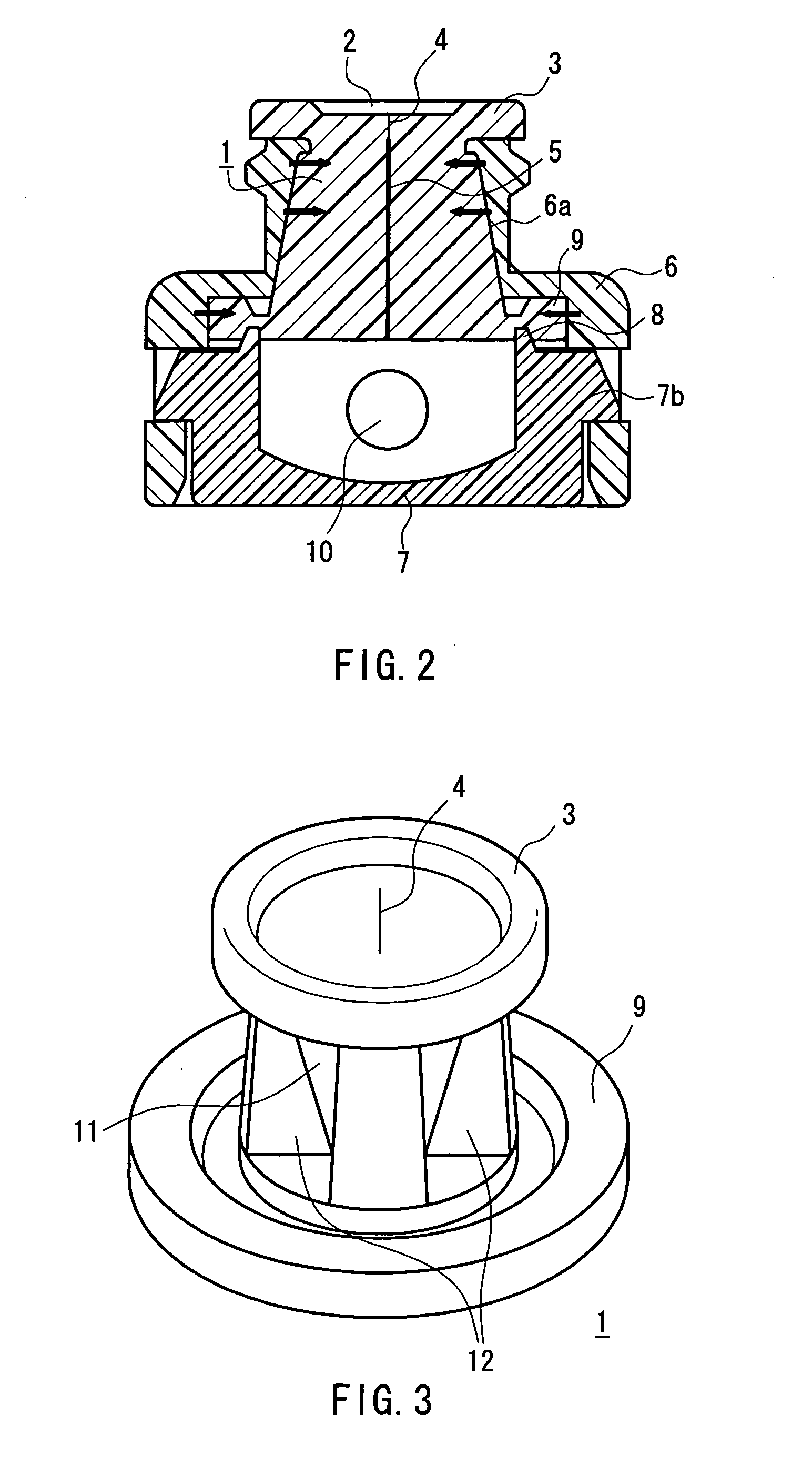
Needleless port and method of manufacturing the same
A septum (1) is held in the cavity of a cover (6) and mounted on a pedestal (7) forming part of a flow channel, and comprises a main body having a through passageway and compression ribs formed in the sides thereof. In the main body, the vertical diameter of the cross section orthogonal to the through passageway is longer than the transverse diameter, and the through passageway includes a vertical diameter direction slit (4) formed in the vicinity of the outer end surface of the main body, and a hole (5) that is formed in a region extending from the slit to the inner end surface of the main body and whose cross section is spindle-shaped having a longer axis extending in the vertical diameter direction. The compression ribs are formed in the opposite sides of the main body in the transverse diameter direction, and the cavity of the cover is of circular cross section whose diameter is shorter than the outer surface spacing between the compression ribs. With the septum mounted, a space is defined between part of the surface of the main body and the inner wall surface of the cover. Further, the hole is closed by a compressive force acting on the septum from the inner wall surface of the cover through the compression ribs. The through passageway in the septum hardly forms a dead space that would cause a residual liquid therein, and the slit in the septum surface hardly opens even at the time of pressurization.
Owner:JMS CO LTD
Minimally invasive pedicle screw and guide support
A minimally invasive orthopedic bone attachment system comprising a pedicle screw and guide support sleeve for insertion of the screw. The pedicle screw is a self-boring, self-tapping integral screw having a distal sharply pointed end for guiding the insertion of the screw as well as forming a borehole for the self-tapping threads of the screw. The proximal end of the screw provides a cylindrical extension which can include a recess for receiving a rotational drive tool or wrench. The length of the threads and the type of threads provided in the screw are designed for the type of orthopedic surgery that is intended. A relatively thin hollow support sleeve including an internal threaded section in the distal end is provided to match the threads and the length of the threaded portion of the pedicle screw. The proximal end of the sleeve includes a central passageway having an internal diameter that can receive the extension portion of the screw and also guide and receive a drive device for the screw. A retainer clip associated with circumferential slots spacedly indexed along the longitudinal surface of the drive device can be used to limit the depth of the screw upon insertion as well as to lock the drive device as an assembly with the screw and sleeve to form the attachment system. A small diameter passageway can be provided along the longitudinal axis of the assembly extending from the proximal end of the drive device through the screw to exit through the pointed distal end of the screw. A thin rigid rod having a point at the distal end is slidably positioned within the narrow passageway. The proximal end of the road includes a cap. A flange surface on the under portion of the cap limits the travel of the rod through the assembly. The length of the rod is determined so that it equals the length of the assembly plus an additional dimension corresponding to the anticipated installed depth of the screw. Prior to insertion of the screw, the rod is driven into the bone through the assembly. The location of the rod is ascertained by an image guidance system to determine the correct final projected location of the screw upon installation.
Owner:WONG DAVID A
Apparatus and methods for treating bone
ActiveUS20070055274A1Increased radialIncreasing diameter of coilInternal osteosythesisSpinal implantsFiberBobbin
Implants and methods for bone treatment, preferably minimally invasive treatment, including repositioning of vertebrae may comprise insertion of a bobbin having a wire, string, thread or band, coiled around the bobbin. During coiling, the diameter of the bobbing / band complex may increase. Such increase in diameter can push against the inner side of the endplates of the vertebral body, and augment the vertebral body to its original height. The implant may also take the form of a coiled sleeve which when inserted into the vertebral body is uncoiled. The force of the uncoiling sleeve pushes against the inner side o the endplates of the vertebral body, restoring the vertebral body to its original height. The implant may also take the form of fibrous masses comprised of a thread or other relatively thin structure, for example a fiber or strand, of any biocompatible material having desired characteristics, for example a shape memory alloy, titanium, stainless steel, another metal or metal alloy, a ceramic, a composite or any combination thereof. The, strand, thread or other fiber may be coiled, woven, matted, tangled or otherwise formed into a wool-like mass or body having a desired configuration. Expansion of the expandable member within the vertebral body or other bone may reposition the fractured bone to a desired height and augment the bone to maintain the desired height. A bone cement or other filler can be added to further treat and stabilize the vertebral body or other bone.
Owner:SYNTHES USA
Valve assembly including diameter reduction structure for trocar
InactiveUS7025747B2Convenient introductionSmall sizeCannulasInfusion syringes% diameter reductionVALVE PORT
A valve assembly and diameter reduction structure for trocar employing a movable diameter reduction structure. The diameter reduction structure is positioned in proximity to a first seal and includes a plurality of stand off members movable between a first position, a second position, and a third position. The diameter reduction structure in the first position reduces the likelihood of the first seal losing its integrity by limiting excessive off-axis and angular movements of small diameter surgical instruments. When large diameter surgical instruments are positioned through the trocar, the diameter reduction structure pivots to accommodate the passage of larger diameter surgical instruments without any operational adjustments.
Owner:TYCO HEALTHCARE GRP LP
Magnetic devices and applications for medical/surgical procedures and methods for using same
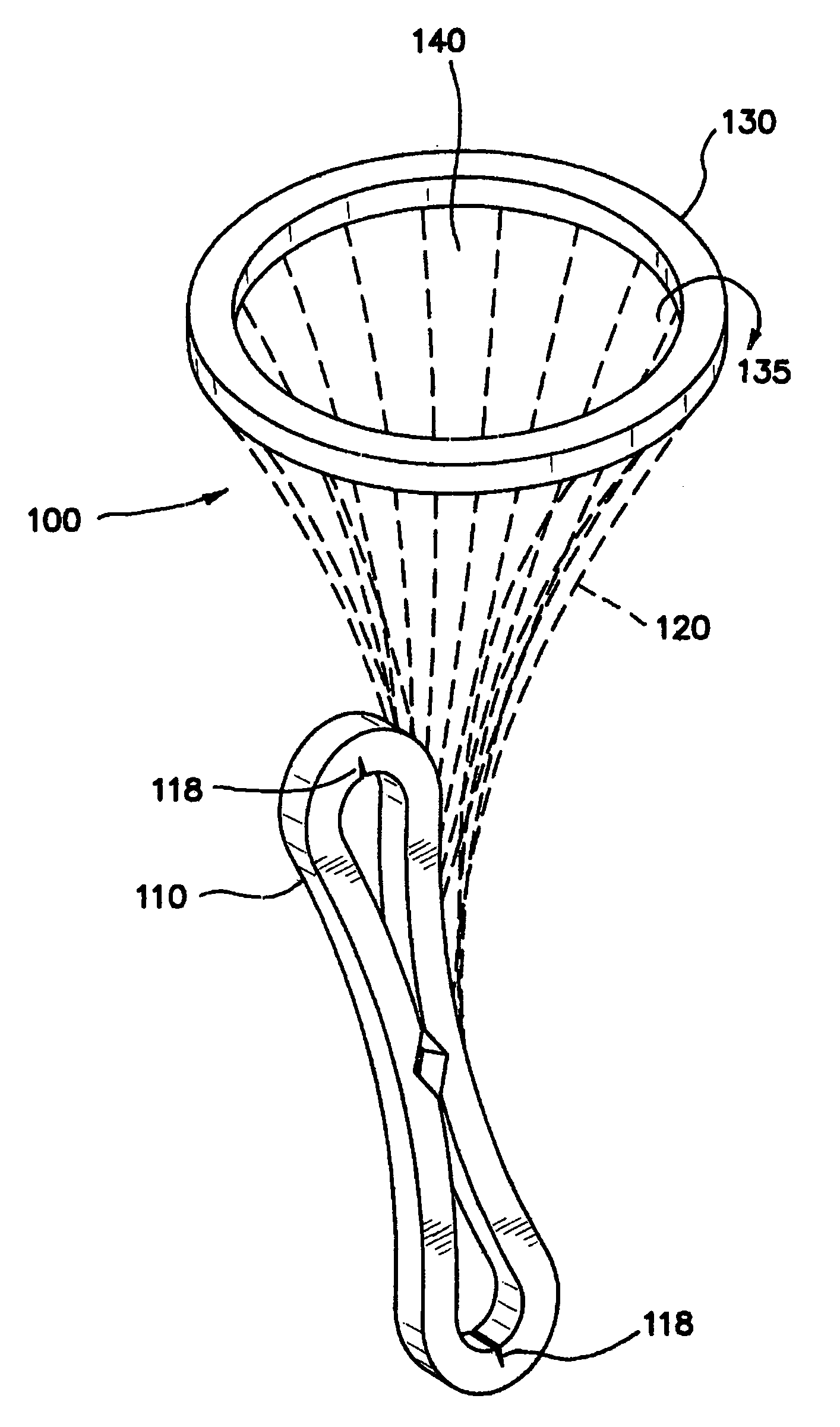
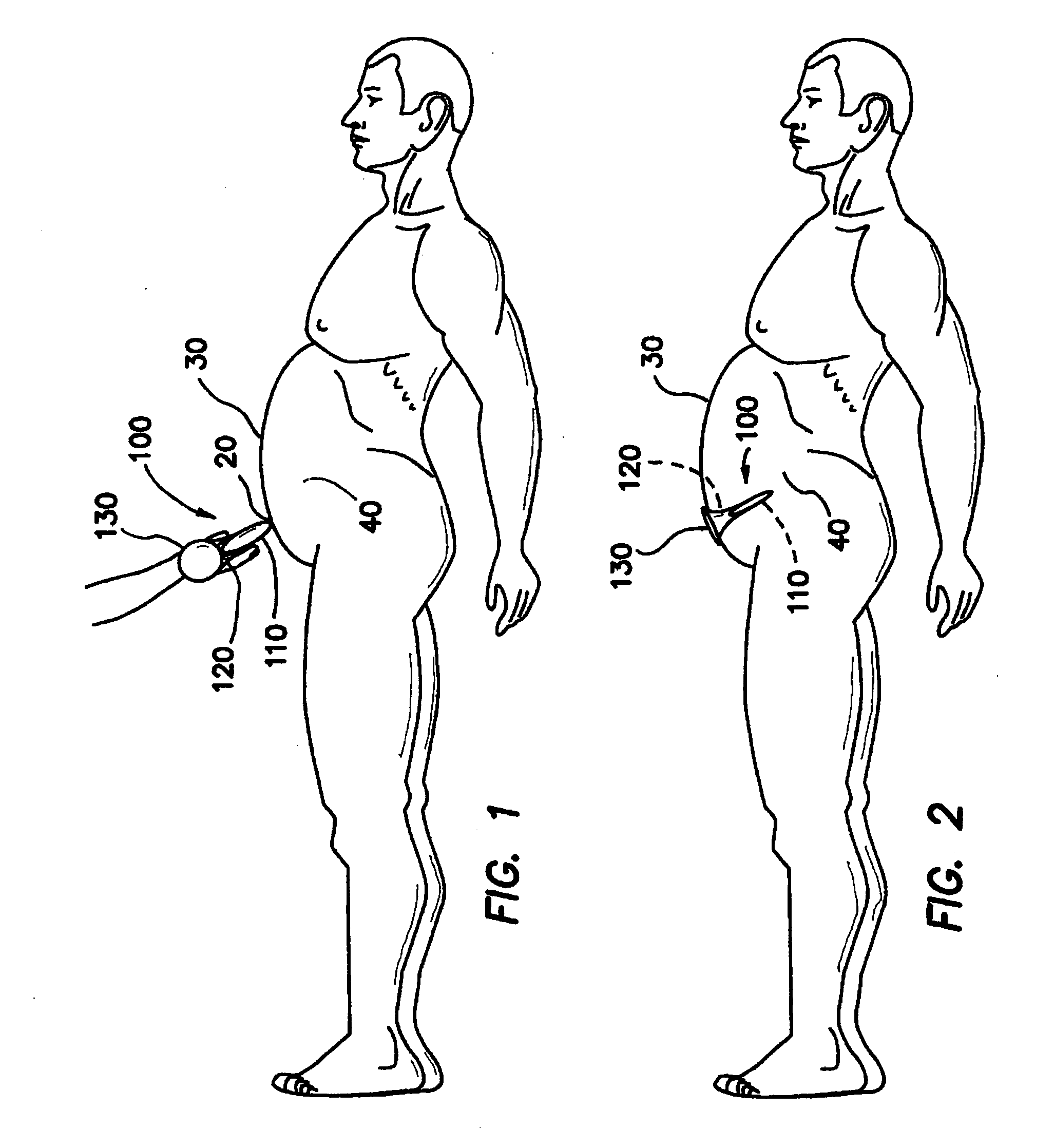
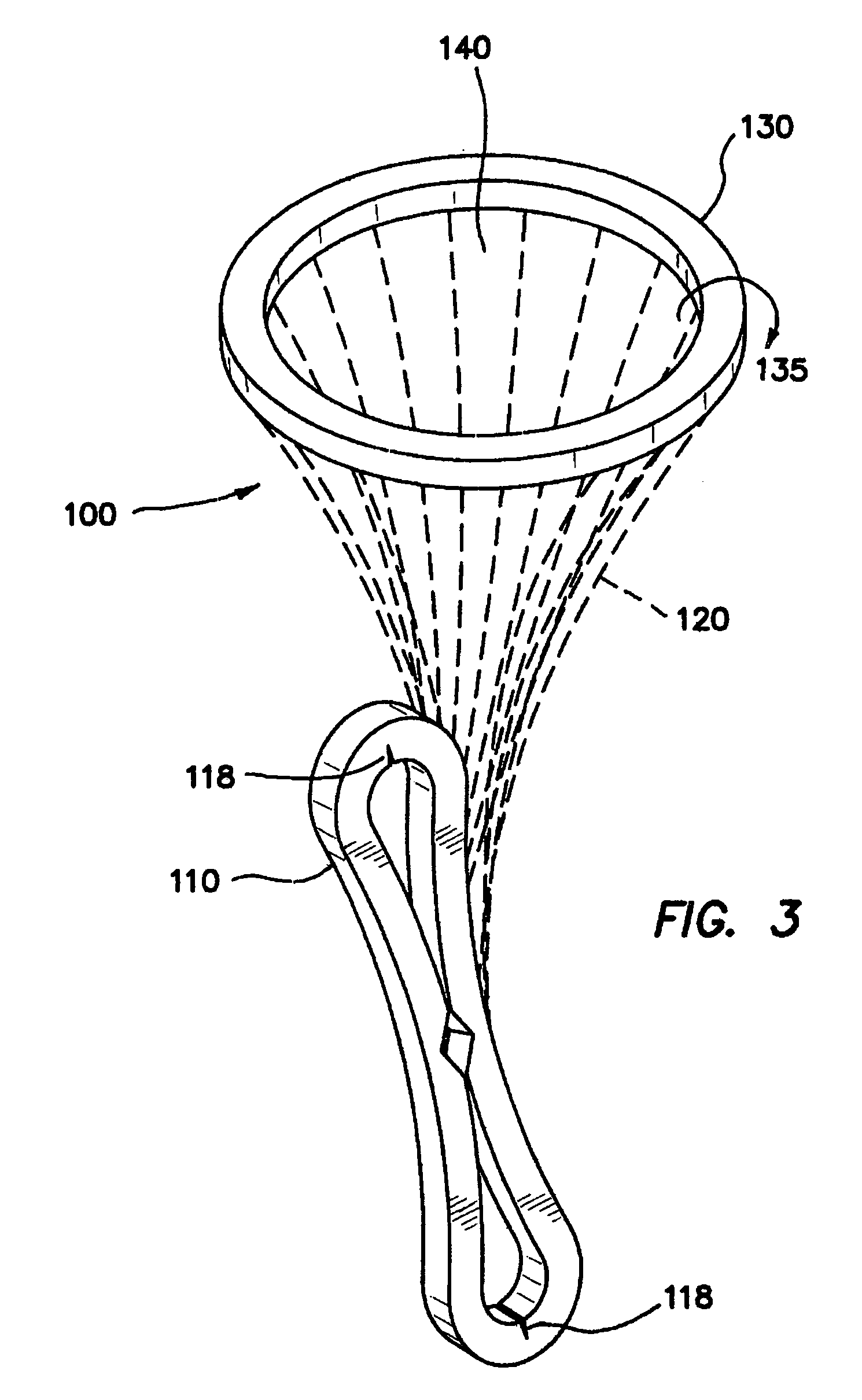
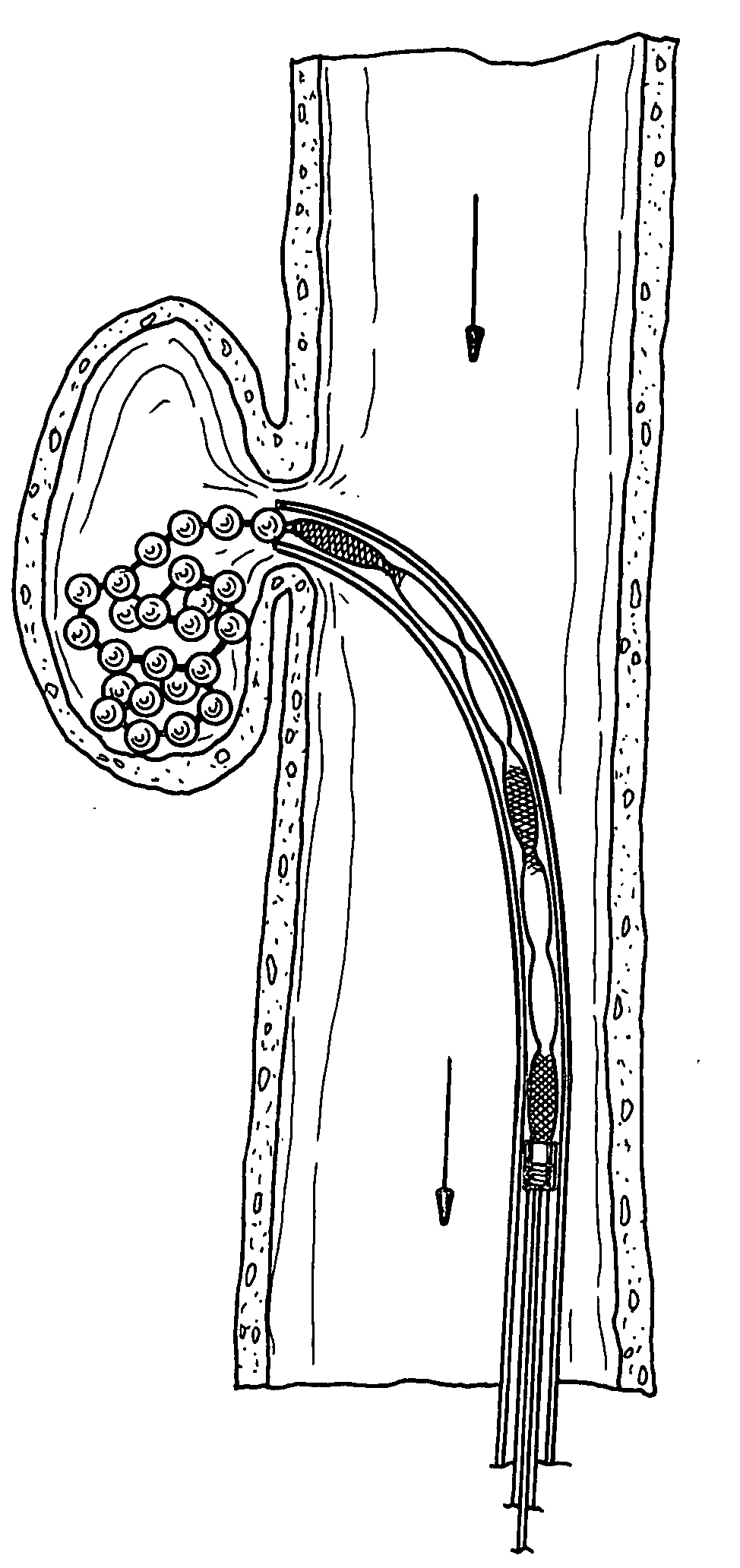
InactiveUS20070142780A1Facilitate manipulationEasy alignmentCannulasInfusion syringesEngineeringReducer
One embodiment of the invention comprises a trocar and a reducer cap that magnetically attaches to the trocar. The trocar and the cap each include a magnetic member, at least one of which is a first magnet, and the other of which is either a second magnet or a non-magnetized magnetically permeable member. Including a magnet of sufficient strength in the trocar and / or the cap will create a magnetic field that automatically holds a surgical instrument having a magnetically permeable member at its tip in axial alignment with the cap or trocar lumen. Introduction of the surgical instrument into the lumen can be further facilitated by providing the trocar or cap lumen with a funnel-shaped opening. A lumen seal can be provided by one or more compliant toroidal seal members that expand radially inwardly when compressed axially by the magnetic attraction between the cap and trocar. The alignment feature is particularly advantageous when incorporated in a mini-trocar having a lumen on the order of 1-3 mm in diameter. In that case, a trocar cap can be a small disc magnetically attracted to the trocar to cover the lumen. Magnetic aligning devices according to the invention can be used internally of a patient or transdermally. Another embodiment of the invention is an ostium plug with a lumen therethrough that can be used in tubal sterilization. The plug is permanently implanted in the patient, but a cap is coupled magnetically to the proximal end of the plug to permit reopening of the lumen when desired.
Owner:VAN LUE VETERINARY SURGICAL
Devices and methods for facilitating fluid transport
ActiveUS20070078358A1Easy to transportShorten the timeDiagnostic recording/measuringSensorsFluid transportEngineering
Arrangements are provided including a base having a bore disposed therein extending from a first surface of the base through a second surface of the base, a fluid transport tube having a first end, a second end opposite the first end, and a lumen having an inner diameter, at least the second end of the tube being received within the bore of the base, and at least one fluid transport enhancing groove having at least a first section disposed in the second surface of the base and in fluid communication with the bore.
Owner:INTUITY MEDICAL INC
Internal compression tourniquet catheter system and method for wound track navigation and hemorrhage control
An internal compression tourniquet catheter system and method for controlling hemorrhage from wounds, particularly penetrating wounds. Its construction includes an inflatable member constructed of very thin, flexible, biocompatible, and nonelastic and puncture resistant material such that when deflated it lies flat and can be wrapped around the catheter shaft, which passes within and has a lumen to inflate it, to minimize overall diameter when deflated for insertion into the tissue track created by the wounding agent. The inflatable member is of large potential volume enabling full inflation with near zero internal pressure when unconstrained externally. When positioned within a wound track and inflated, the gas or liquid injected into the balloon lumen creates pressure because its expansion is constrained by the tissues of the wound, and that pressure is transmitted directly to the surrounding tissue of the wound track. The pressure exerted on the tissue can be precisely measured and controlled, automatically if appropriate, such that sufficient pressure is applied to tamponade bleeding, but not damage tissue. Since the balloon is of large potential volume, it can easily expand to fill and compress small, large, and irregular wound tracks and can successfully tamponade wounds that smaller, elastic balloon catheters would be unable to tamponade. The catheter system includes means to assist insertion into the wound track, including a rounded or bulbous exploring tip and an internal stylet with an external orientable handle. The distal end of the catheter-stylet assembly can be bent slightly to allow following a curved or irregular wound track, such wound track navigation further assisted by twisting the stylet handle to orient the bent catheter tip within the wound track.
Owner:CARDIOCOMMAND
Expanded UHMWPE for guiding catheter liners and other lubricious coatings
An intraluminal catheter, such as a guiding catheter, employed for intravascular procedures and having an inner liner formed of expanded Ultra High Molecular Weight Polyethylene (UHMWPE) is disclosed. The expanded UHMWPE is microporous and has an oriented microstructure structure characterized by nodes interconnected by fibrils. The inner liner formed of expanded UHMWPE is very thin to maximize the inner lumen diameter and has excellent mechanical properties.
Owner:ABBOTT CARDIOVASCULAR
Centering catheter
A catheter with at least one centering device attached near a distal end of the catheter. The centering device has at least two struts extending between a proximal end and a distal end. The centering device has a variable diameter and tends to center the distal end of the catheter, steering the catheter away from the vessel wall during insertion through the vasculature and toward the treatment site. The centering catheter may facilitate access to tortuous anatomy by preventing the catheter tip from catching on irregularities in the lumenal surface. The centering catheter may also facilitate uniform stent expansion by stabilizing the catheter during stent deployment.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Translation dilator and stand alone vascular guide catheter
Systems and methods for delivering implantable devices, catheters, or substances in or near and / or restoring flow through body lumens, such as blood vessel lumens are described. A catheter having a proximal portion of a first diameter and a distal portion of a second diameter (smaller than the first diameter) is advanced into a body lumen. The distal portion of the catheter is caused to expand to a diameter that is larger than the second diameter but no larger than the first diameter. A working device is then advanced out of the distal end of the catheter and used to remove obstructive matter, deliver an implantable device or substance and / or restore flow. The distal portion can be reduced in diameter prior to removal from the body. A stand alone, guide catheter is also disclosed possessing high resistance to kinking even with a very thin wall.
Owner:TYCO HEALTHCARE GRP LP
Trocar seal
InactiveUS20050209608A1Increase the diameterUniform diameterEar treatmentCannulasSurgical deviceGeneral surgery
A seal is disclosed for installation on the proximal end of a trocar to seal on surgical instruments having varying diameters. The seal has a general hourglass shape with an upper portion, a bottom portion and a sealing portion interposed between the upper and bottom portions. The bottom portion is formed for mating engagement with the access port at the proximal end of the trocar. A passage exists between the upper and bottom portions, and the diameter is expandable from 2 mm to about 13 mm. Various configurations of such a seal are disclosed.
Owner:COOPERSURGICAL INC
Expandable cerebrovascular sheath and method of use
Disclosed is an expandable transluminal sheath, for introduction into the body while in a first, small cross-sectional area configuration, and subsequent expansion of at least a part of the distal end of the sheath to a second, enlarged cross-sectional configuration. The sheath is configured for use in the upper vascular system and has utility in the introduction and removal of therapeutic or diagnostic microcatheters. The access route is through the femoral arteries or the iliac arteries to the cerebrovasculature. The distal end of the sheath is maintained in the first, low cross-sectional configuration during advancement to the cerebrovasculature. The distal end of the sheath is subsequently expanded using a radial dilatation device, which is removed prior to the introduction of microcatheters. The sheath can be inserted in a first, small cross-sectional configuration, be expanded diametrically to a second, larger cross-sectional configuration, and then be reduced to a diametrically smaller size for removal.
Owner:ONSET MEDICAL CORP
Method for removing orthopaedic hardware
A method for gaining access to and removing mechanical fasteners such as pedicle screws. A small arthroscopic incision is made over the location of the fastener. A guide wire is then placed into the incision and attached to the fastener. A series of progressively enlarging dilator tubes are slipped into the incision to progressively enlarge its diameter. A screw extractor tube is placed into the incision, over the largest dilator tube. The screw extractor tube is advanced until its lower extreme surrounds the head of the fastener. The dilator tubes are then removed. At this point, the screw extractor tube holds open the incision and provides access to the fastener through its hollow interior. A specially designed extractor tool is inserted into the screw extractor tube. An arthroscope is preferably also inserted through the screw extractor tube so that the surgeon can visualize the process. Jaws on the end of the extractor tool are clamped to the fastener's head. The extractor tool is then rotated to back out the fastener. Once the fastener is freed from its installed position, the extractor tool withdraws it through the screw extractor tube. Other conventional processes—such as debridement—may then be performed through the screw extractor tube. Finally, the surgeon withdraws the screw extractor tube and closes the small incision.
Owner:MEDITRON DEVICES
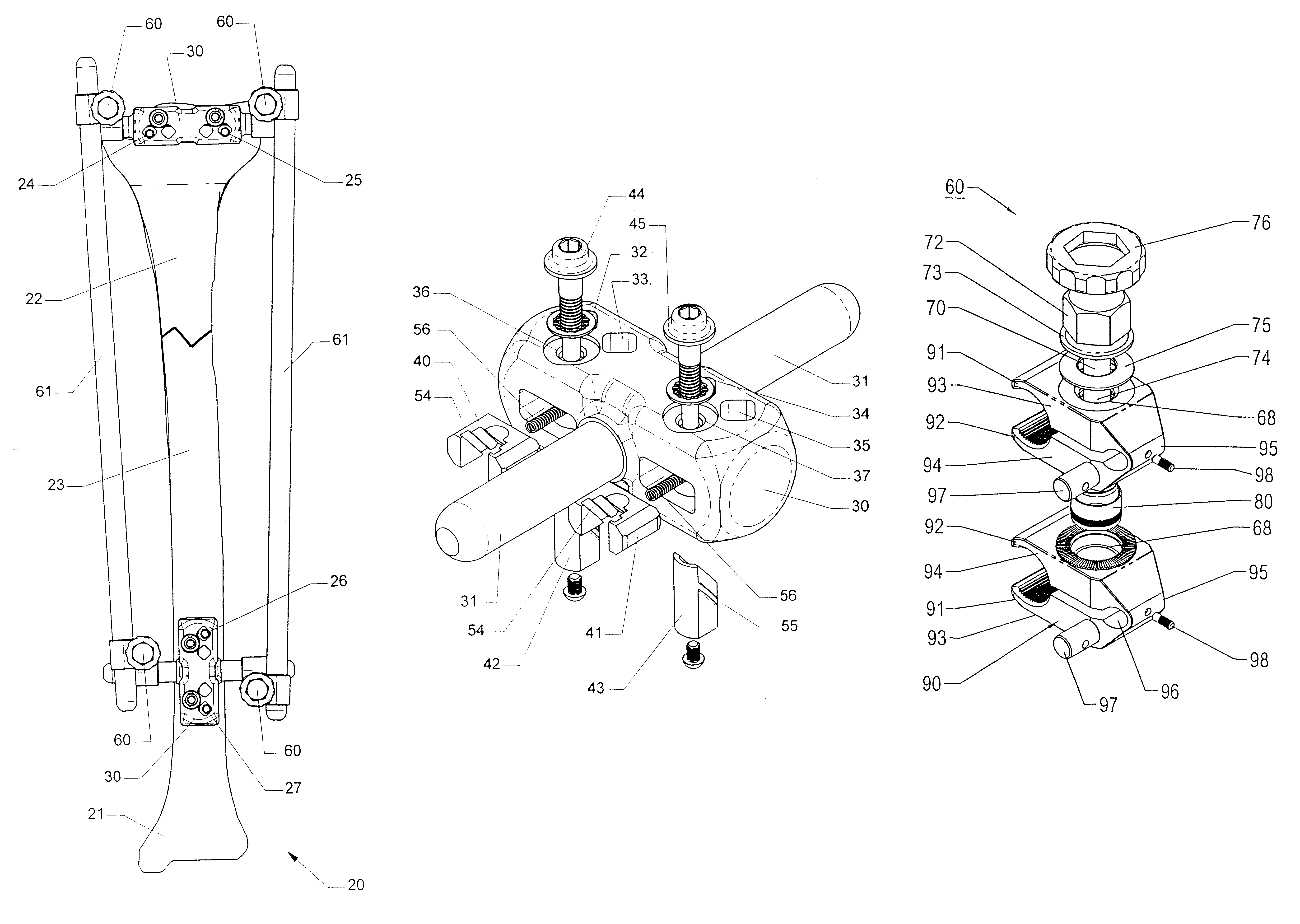
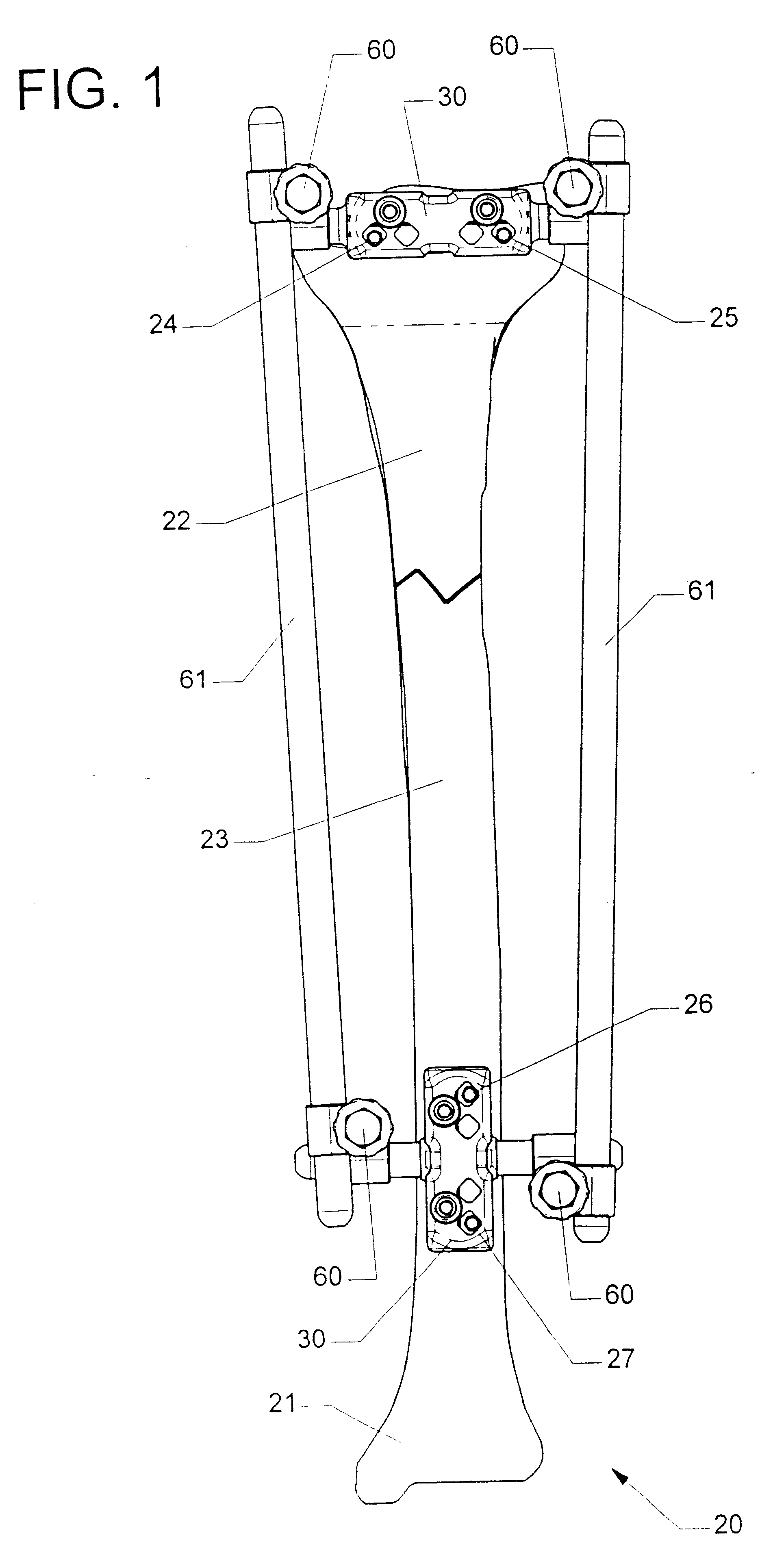
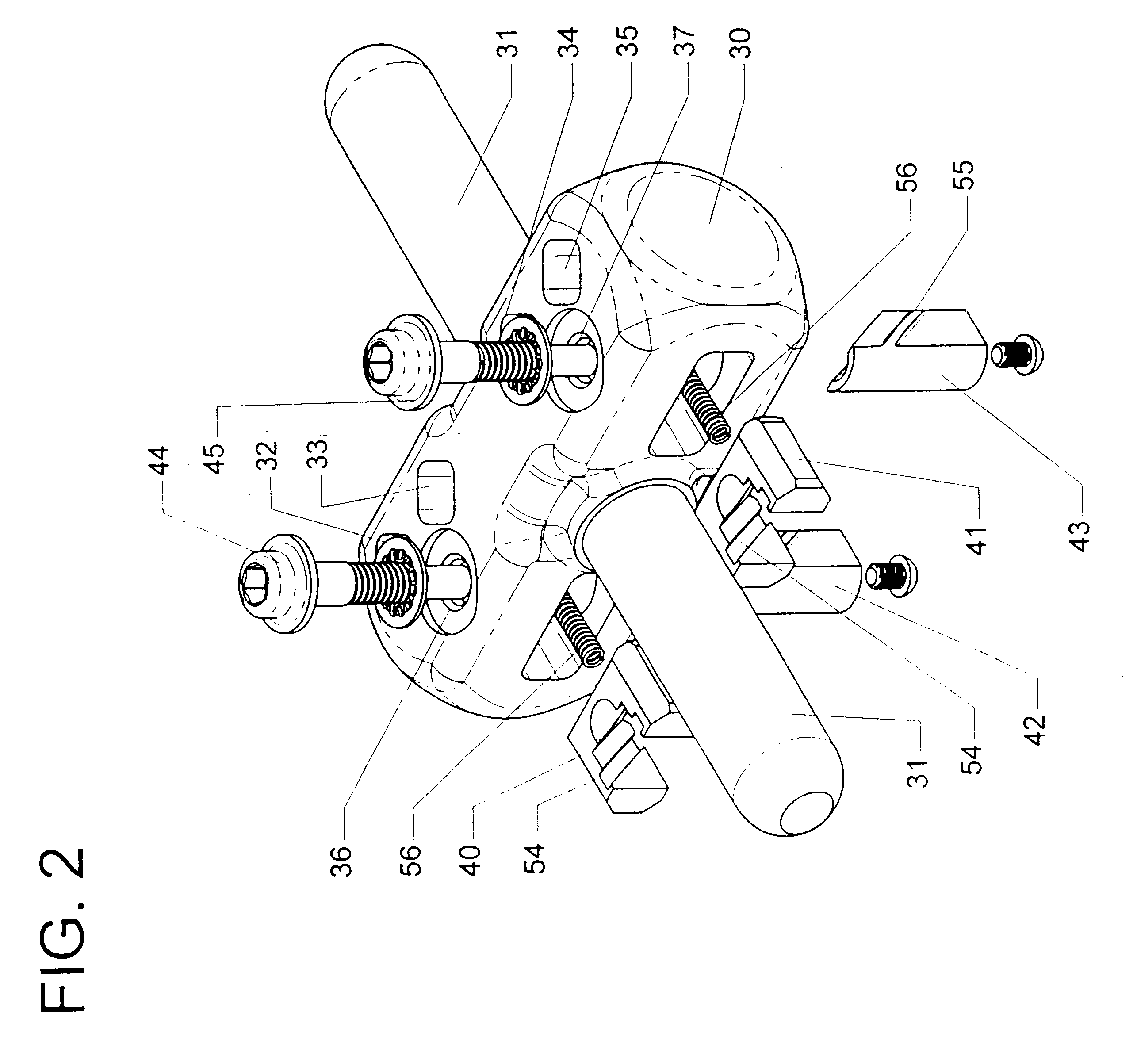
Adjustable bone stabilizing frame system
InactiveUS6613049B2Easy and fast assemblyEasy constructionInvalid friendly devicesFractureEngineeringIliac screw
By providing components securable to anchor pins or screws of different diameters as well as providing clamps which hold associated pins in any position during adjustments, an external fixation or adjustable frame structure is provided which is capable of being quickly and easily assembled in any desired configuration. In the present invention, the frame structure is retained in any assembled configuration in order to allow final adjustments to be made, prior to the final securement of the frame assembly in the precisely desired configuration by closure of each clamp member. In this way, an entire frame assembly is capable of being constructed, adjusted, and readjusted in order to assure each component is oriented in the precisely desired position prior to final closure of the clamping members. In one preferred embodiment, the clamping members employed in the frame structure of the present invention incorporate friction pins internally mounted in each clamp which engages the rod member once this rod is inserted into the jaws of the clamp. In this way, any rod member inserted into the clamping jaws contacts the surface of the jaws and the friction pin, preventing the rod member from sliding or moving relative to the clamp. In addition, by incorporating a uniquely constructed, moving wedge plate that is adjustably engageable with any cooperating anchor pin, secure affixation of the mounting member with the anchor pins of any diameter is easily achieved, regardless of the orientation configuration, or diameter of the anchor pin.
Owner:ZIMMER INC
Expandable intraluminal endoprosthesis
An expandable intraluminal endoprosthesis including a tubular member (1) a first diameter which permits intraluminal delivery of the member into a lumen of a body passageway, particularly a blood vessel. The tubular member (1) is capable of acquiring a second, expanded and deformed diameter upon the application from the interior of the tubular member of a radially outwardly extending force, which second diameter is variable and dependent on the amount of the force applied to the tubular member. Such a tubular member may be expanded and deformed to expand the lumen of the body passageway. The wall of the tubular member includes a substantially continuous structure (2) of mutually staggered undulations. This structure has been separated from a tube wall and exhibits at least one pattern which advances substantially helically along a longitudinal axis of the tubular member. Connection elements within the structure connect adjacent undulations to each other. These connection elements are an integral extension of the undulations thereby interconnected.
Owner:ORBUSNEICH MEDICAL PTE LTD
Occlusion Device
ActiveUS20150313605A1Minimization requirementsReduce riskDilatorsDiagnostic markersMedicineBlood vessel
Provided herein is an occlusion device for intrasaccular implantation and / or vascular occlusion comprising: (a) a substantially solid marker having a proximal end, and a distal end; and (b) a low profile resilient mesh body attached to the distal end of the marker, the body having a delivery shape and a deployed shape capable of conforming to aneurysm walls; wherein the body has a diameter greater than a diameter of an aneurysm to be treated. Also provided herein is a kit comprising the occlusion device disclosed herein and a means for delivery thereof. Methods of manufacture and use of the occlusion device disclosed herein are also disclosed.
Owner:CERUS ENDOVASCULAR
Method for securing a glenoid component to a scapula
A medical procedure includes providing a prosthesis which includes (i) a body portion having a rear surface and a bearing surface, and (ii) an anchor peg including a shaft extending from the rear surface of the body portion, wherein the anchor peg includes a plurality of outwardly extending fins secured to the shaft, and further wherein the plurality of outwardly extending fins each possesses a first diameter. The medical procedure further includes creating an anchor hole in a natural bone, the anchor hole possessing a second diameter which is less than the first diameter. In addition, the method procedure includes positioning the anchor peg within the anchor hole so that each of the plurality of outwardly extending fins deforms so as to possess (i) a concave side which faces an open end of the anchor hole, and (ii) a convex side that faces a closed end of the anchor hole.
Owner:DEPUY PROD INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com