Patents
Literature
1592 results about "Dilator" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dilator or dilatator is a medical term with a number of uses, including...
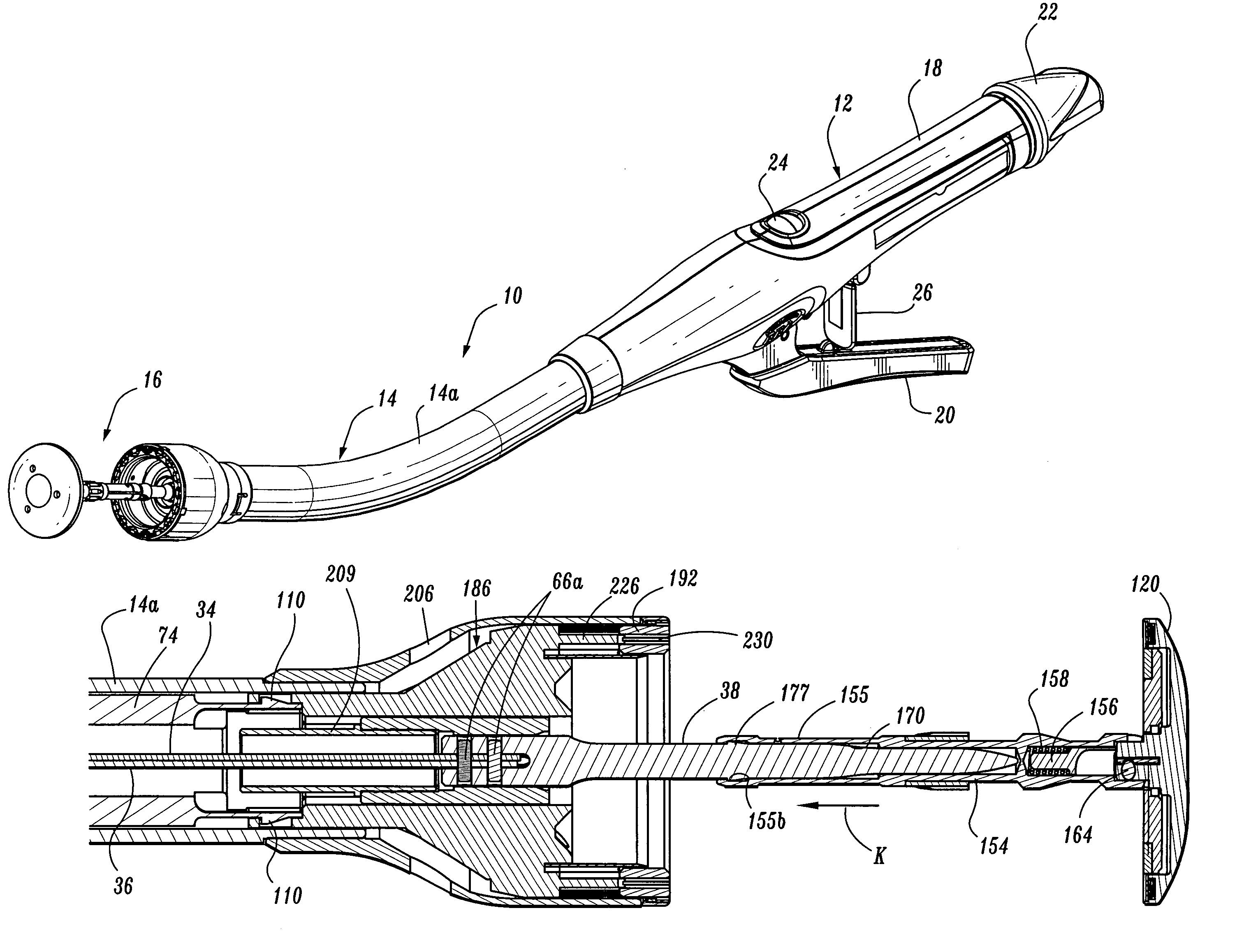
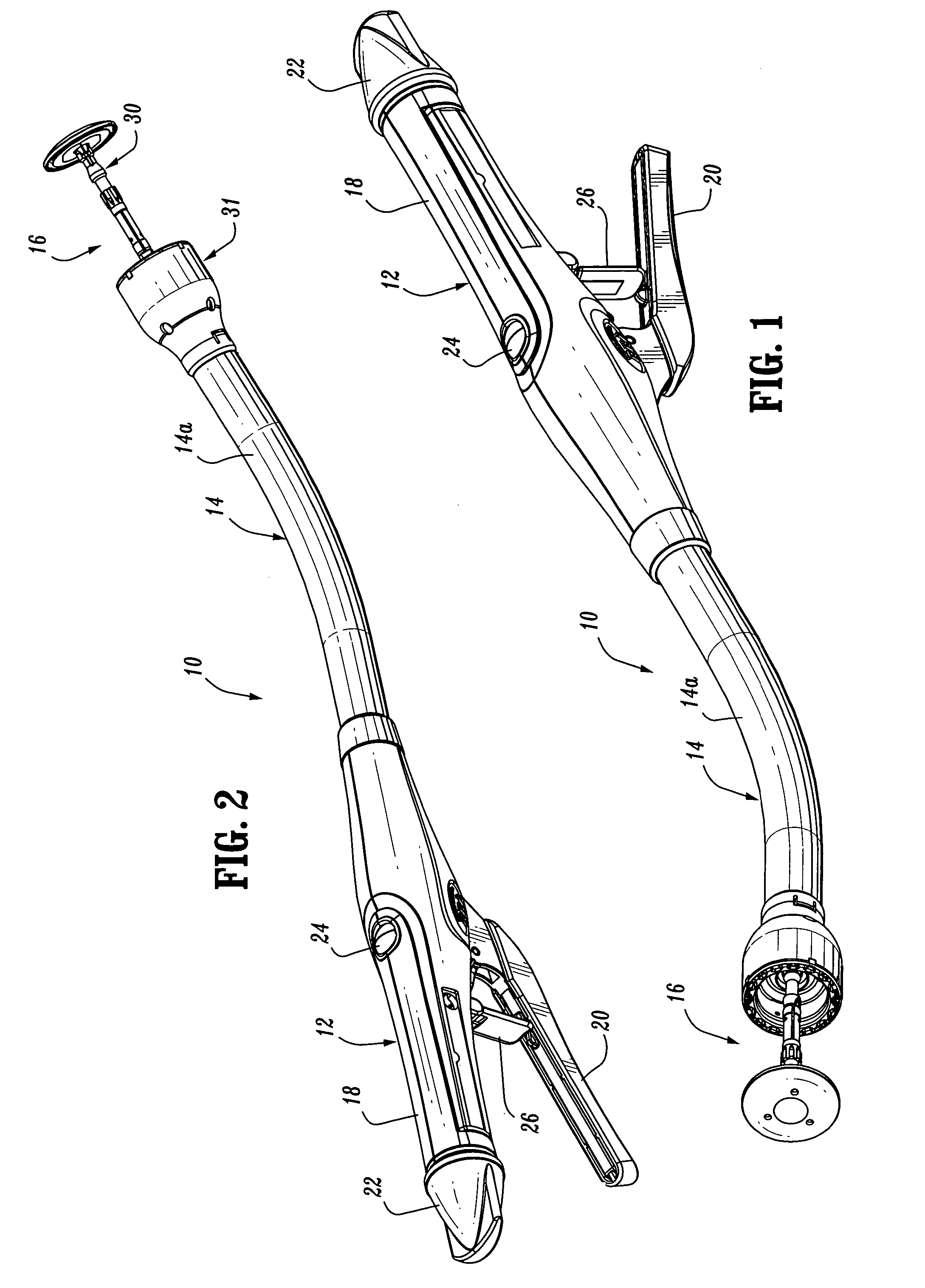
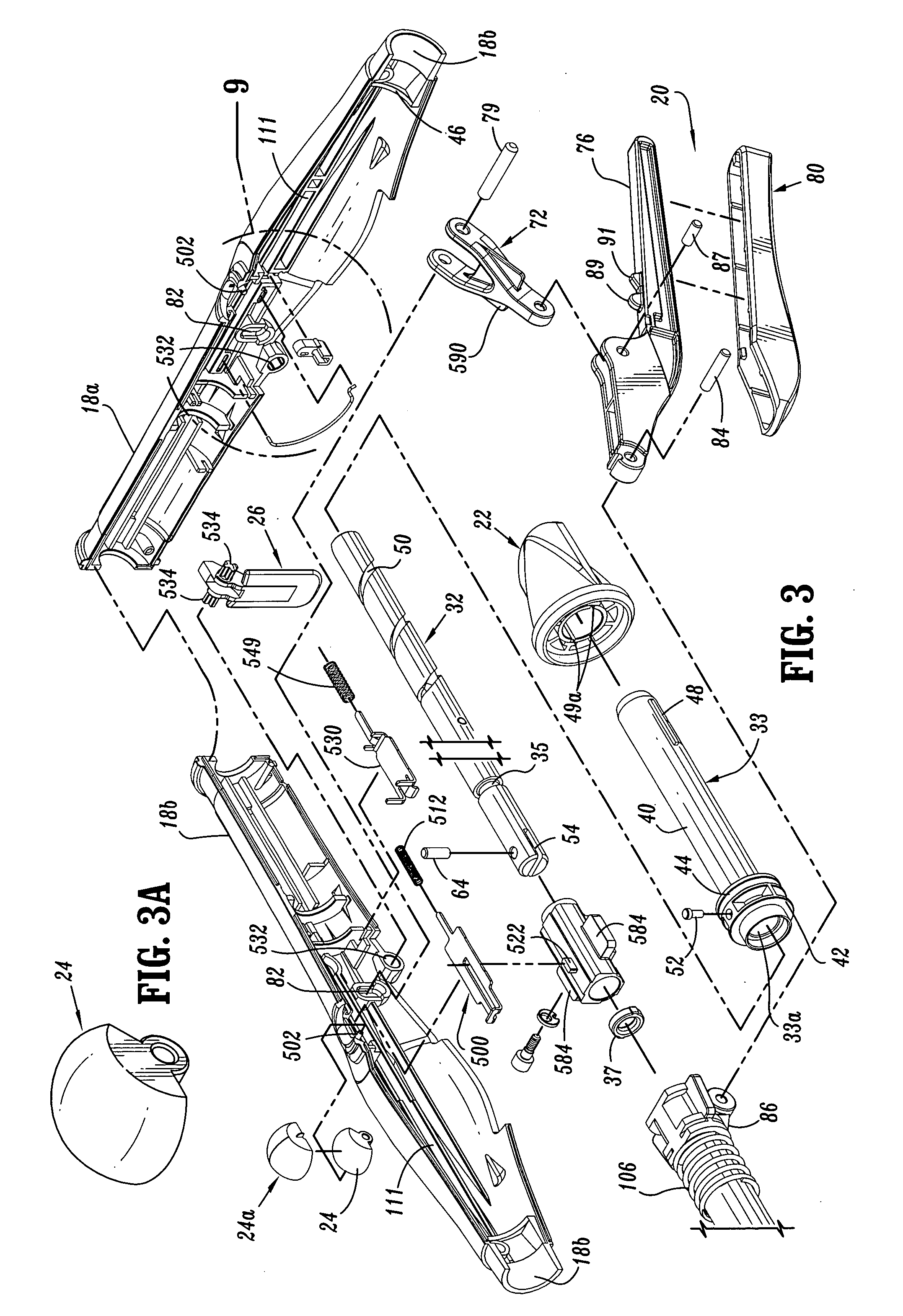
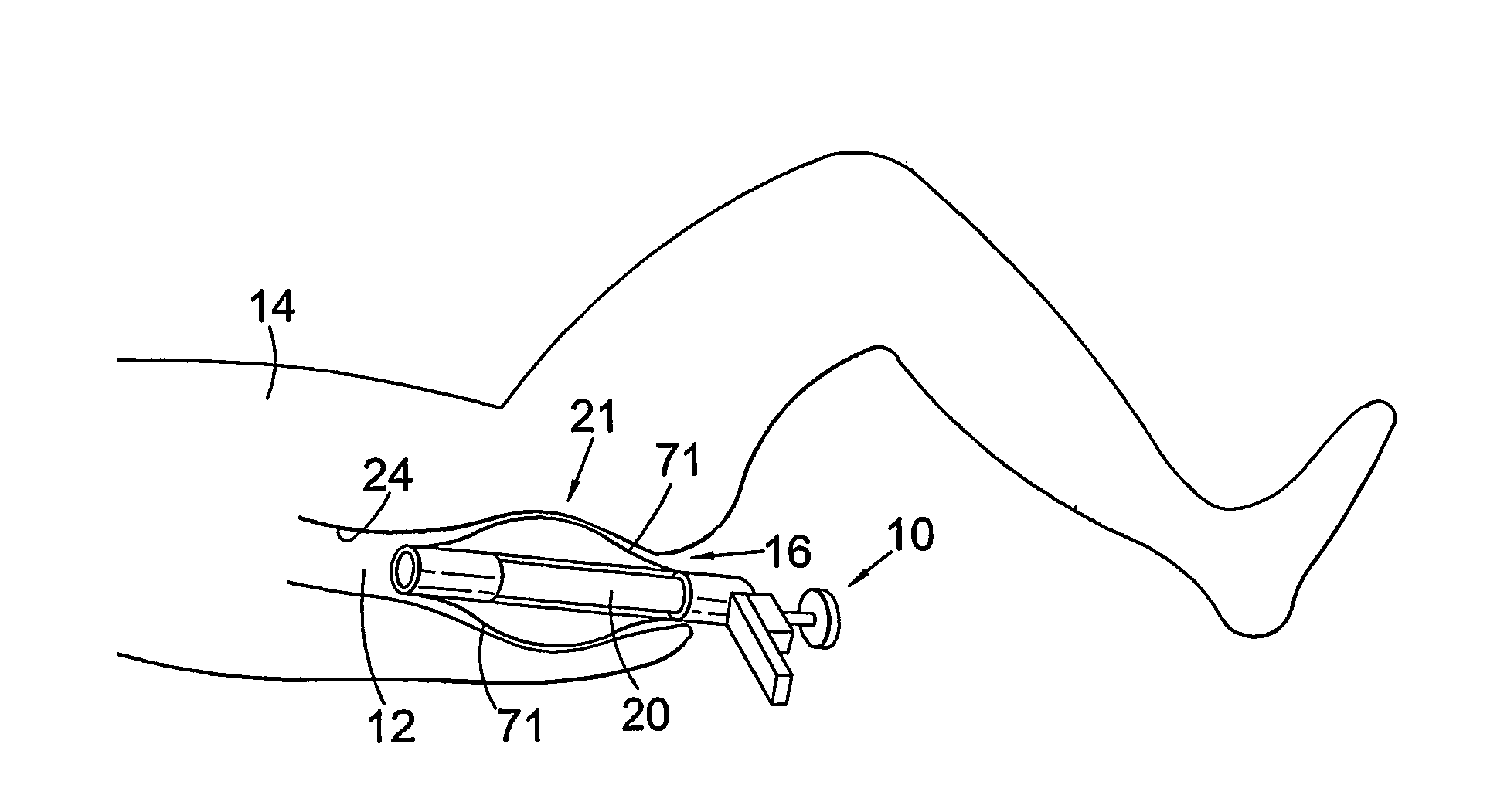
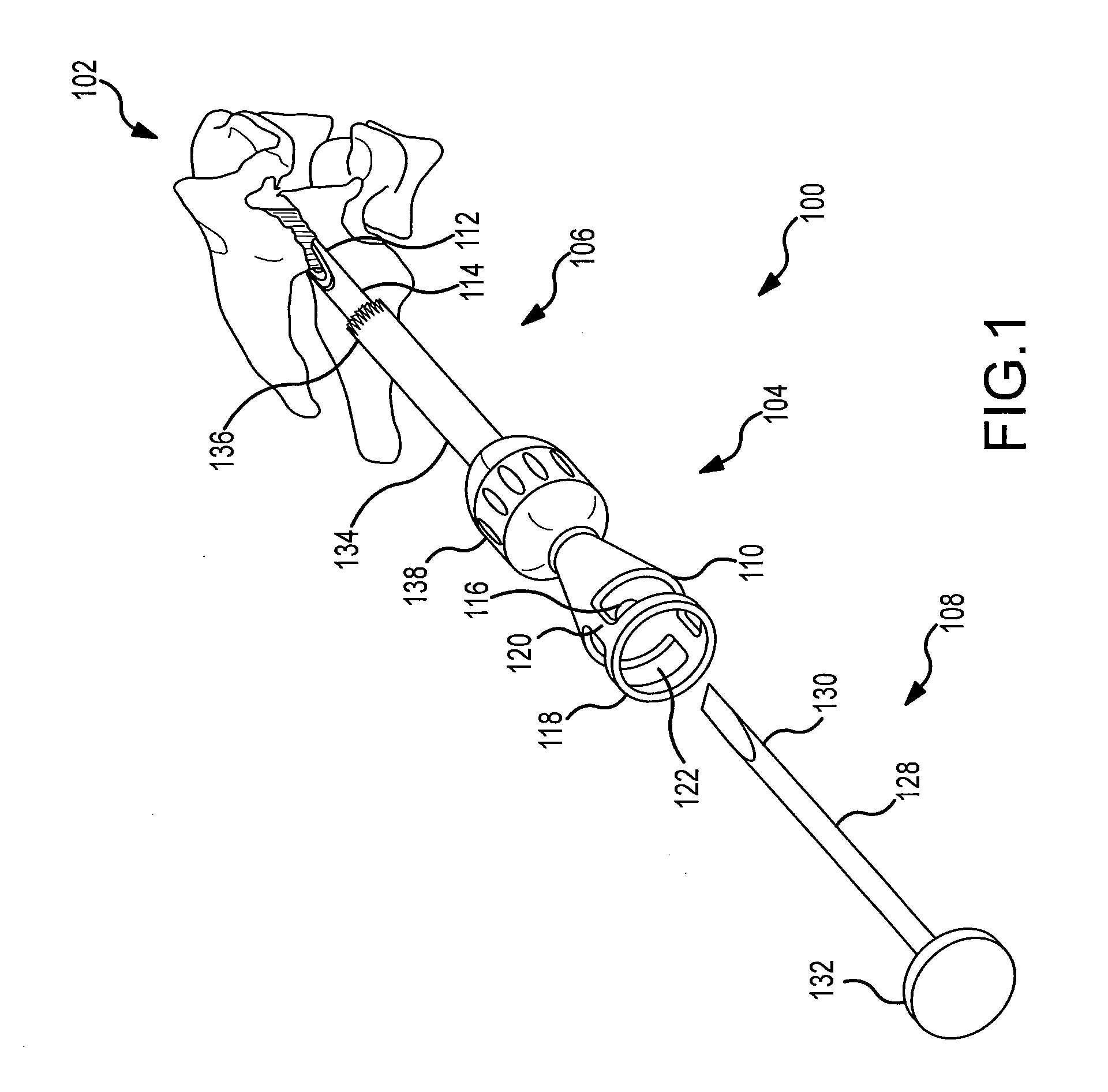
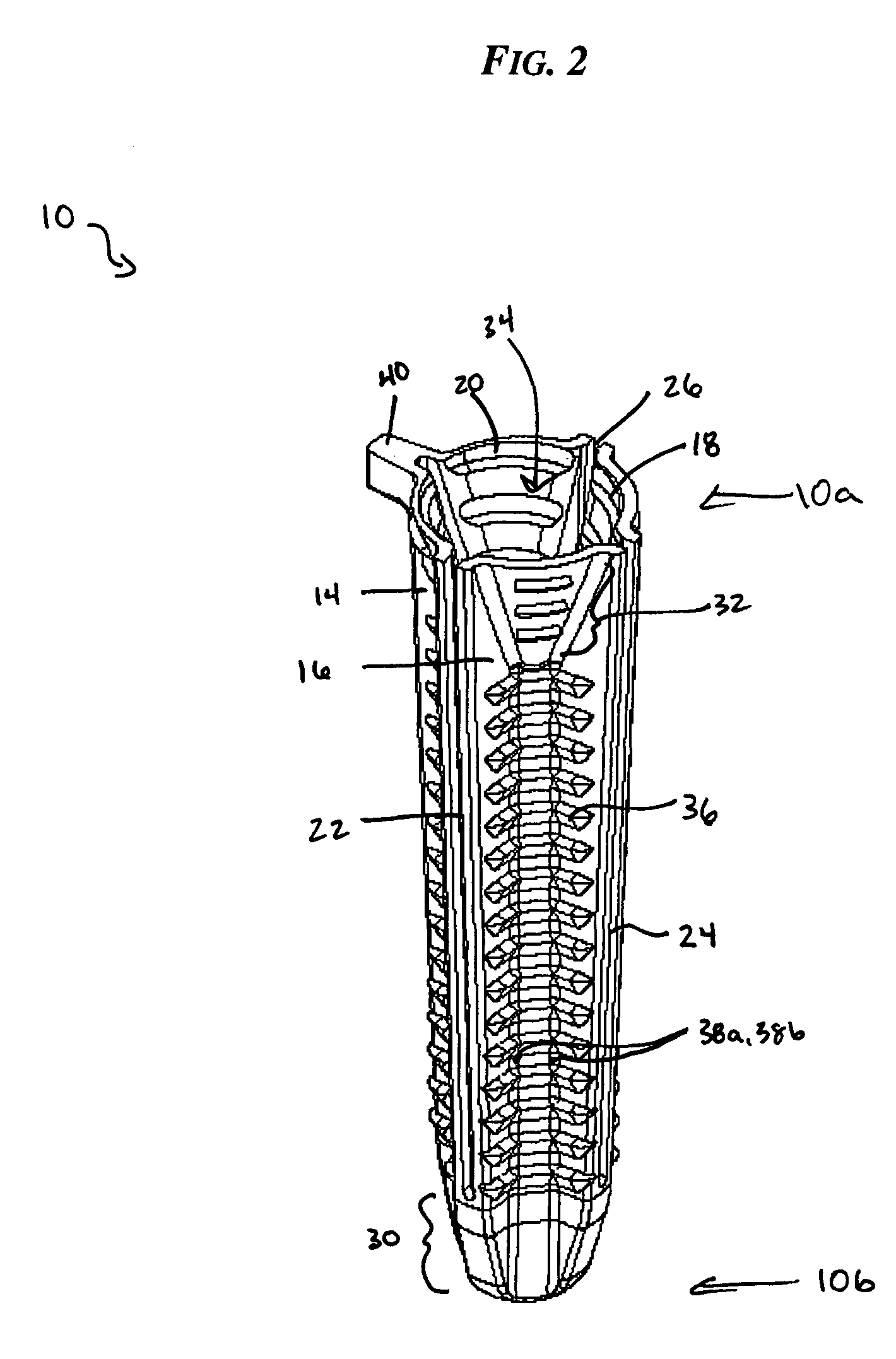
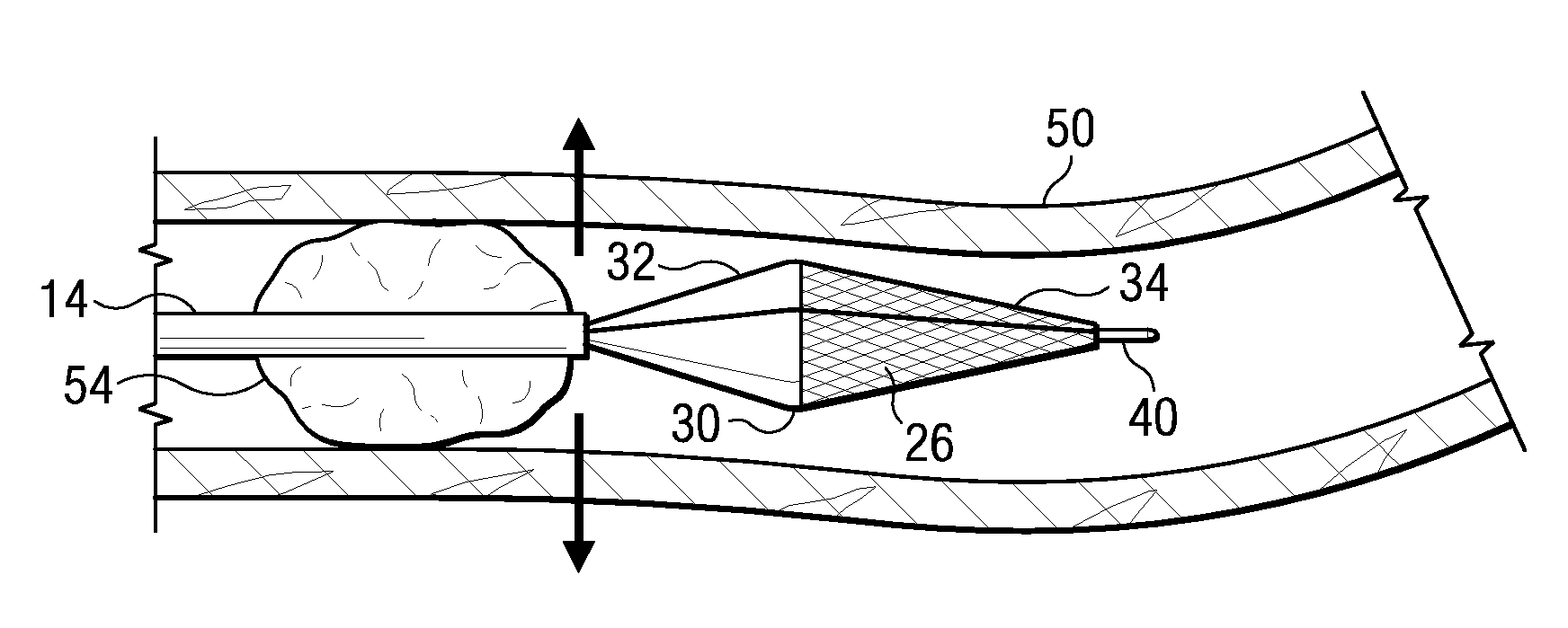
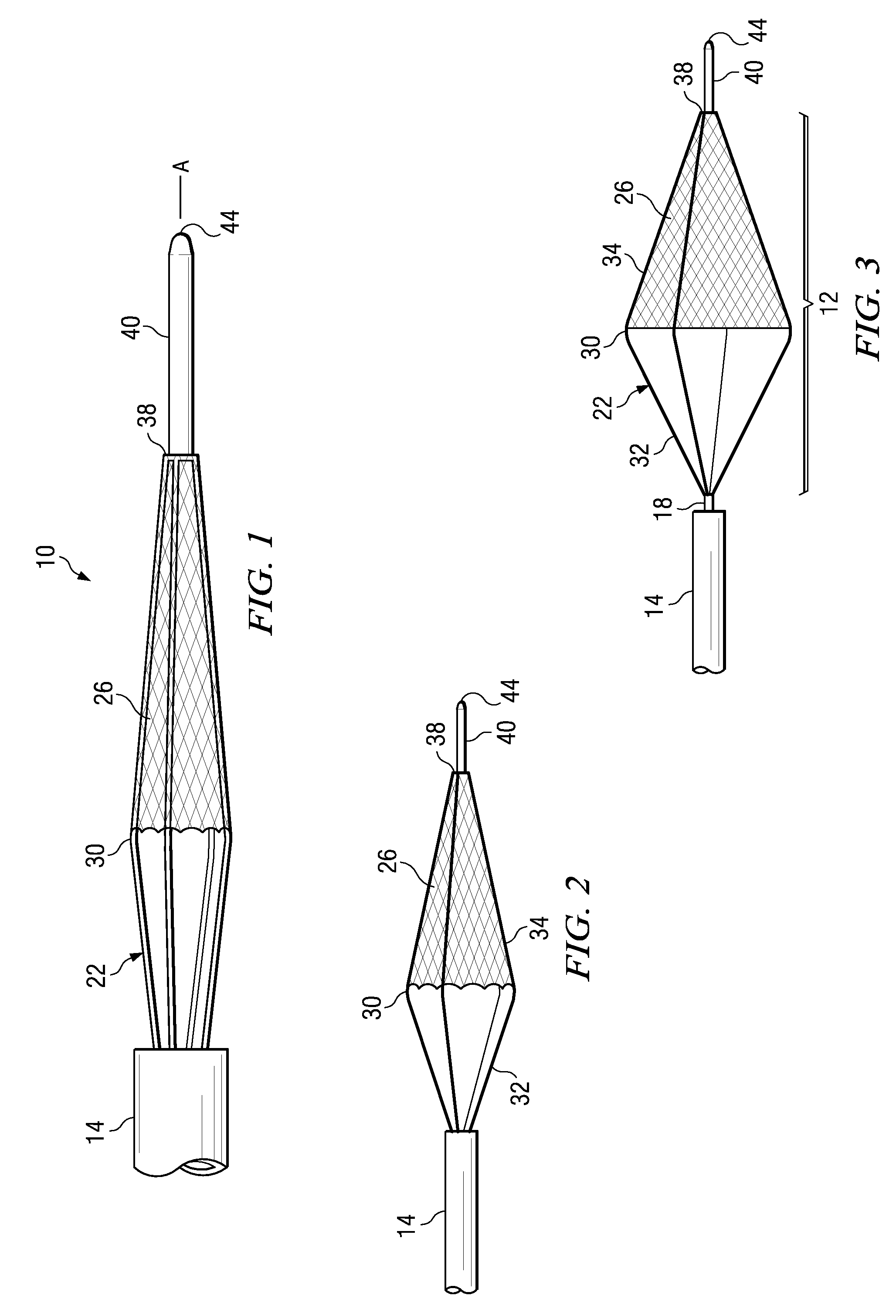
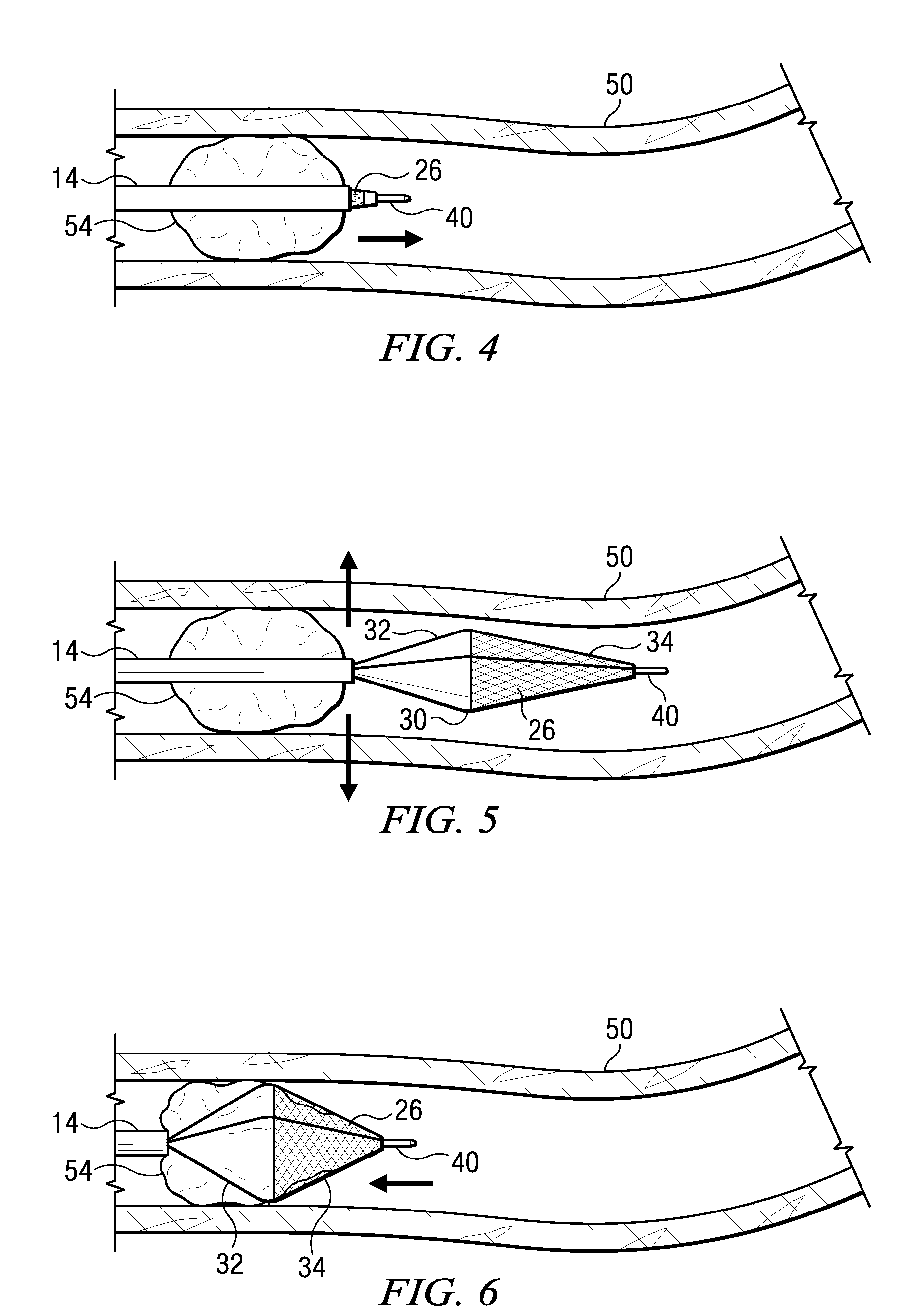
Surgical stapling device
ActiveUS7168604B2Enhanced advantageReduce firing forceSuture equipmentsStapling toolsDilatorSurgical site
A surgical stapling device is disclosed for the treatment of internal hemorrhoids. The surgical stapling device includes a handle portion, an elongated body portion and a head portion including an anvil assembly and a shell assembly. The head portion includes an anvil assembly including a tiltable anvil which will tilt automatically after the device has been fired and unapproximated. The tiltable anvil provides a reduced anvil profile to reduce trauma during removal of the device after the anastomoses procedure has been performed. The anvil assembly of the stapling device may include an approximation mechanism having an anvil retainer including an elongated distal extension dimensioned to be telescopingly received within a longitudinal bore of an anvil center rod of the anvil assembly. The elongated distal extension is of a length to provide telescopic engagement with the anvil center rod without obstructing visualization of the surgical site. A kit including a surgical instrument having a removable anvil assembly and an anvil assembly insertion handle is also disclosed. The kit may also include a speculum, an anal dialator and / or an obturator.
Owner:TYCO HEALTHCARE GRP LP
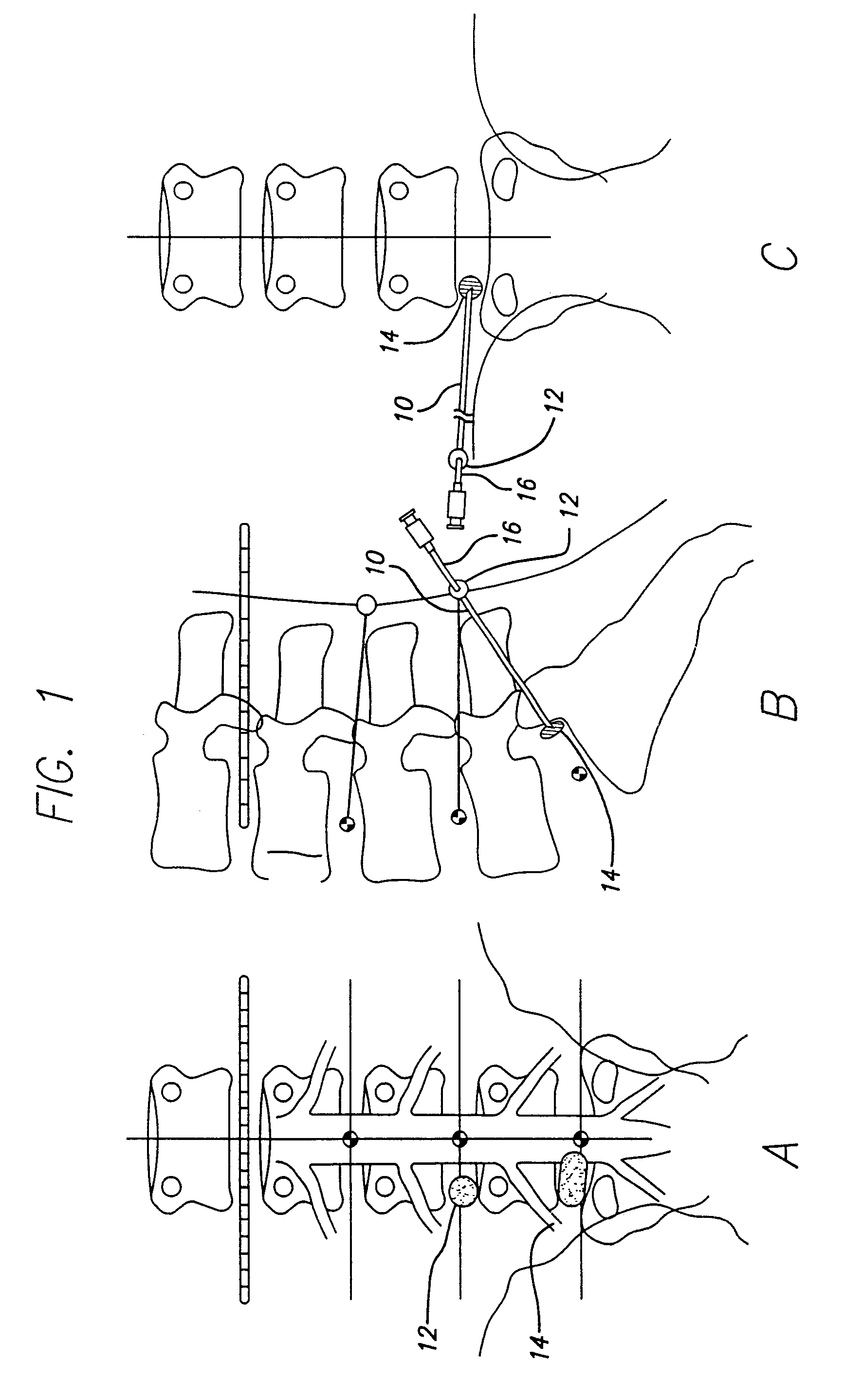
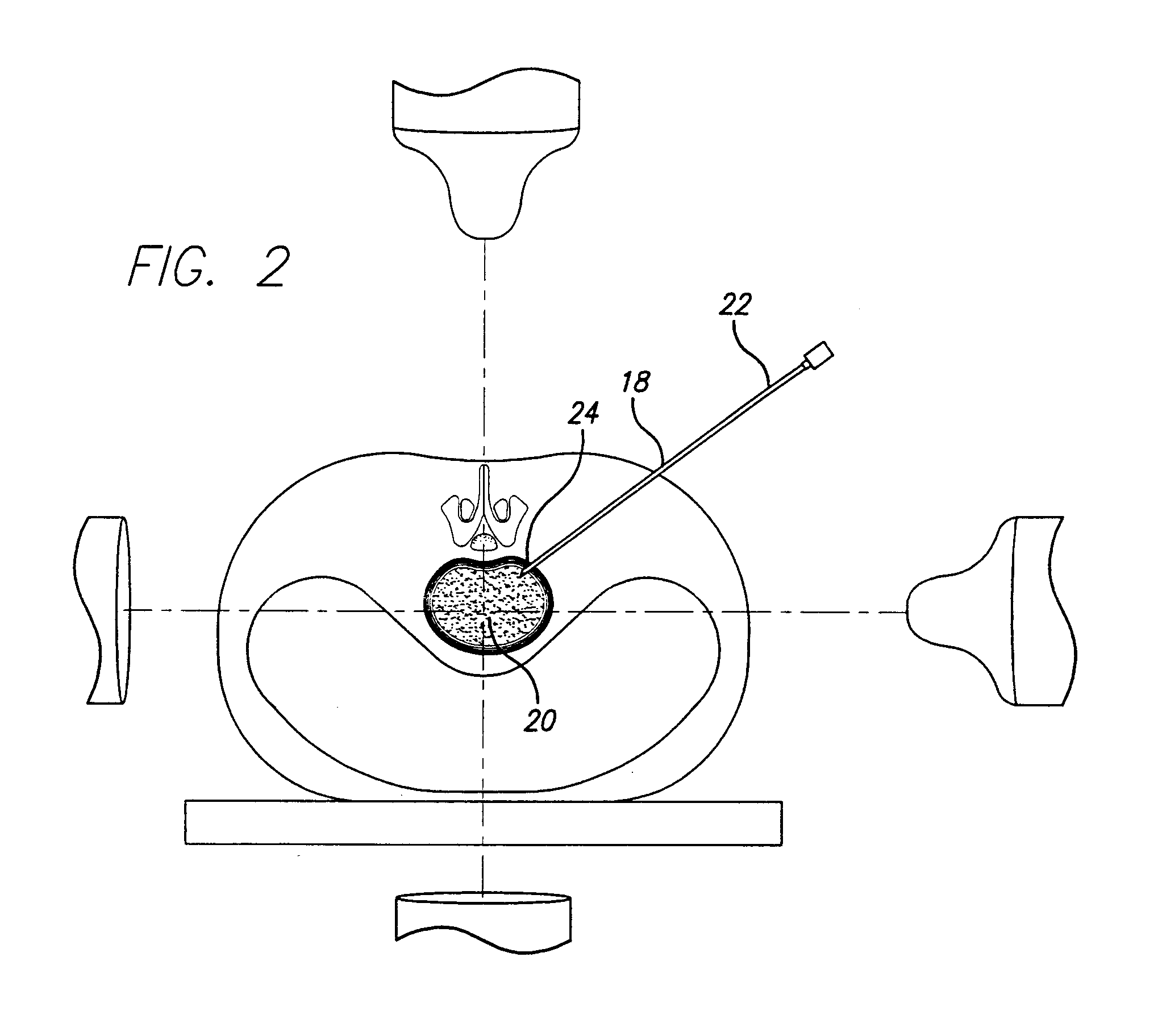
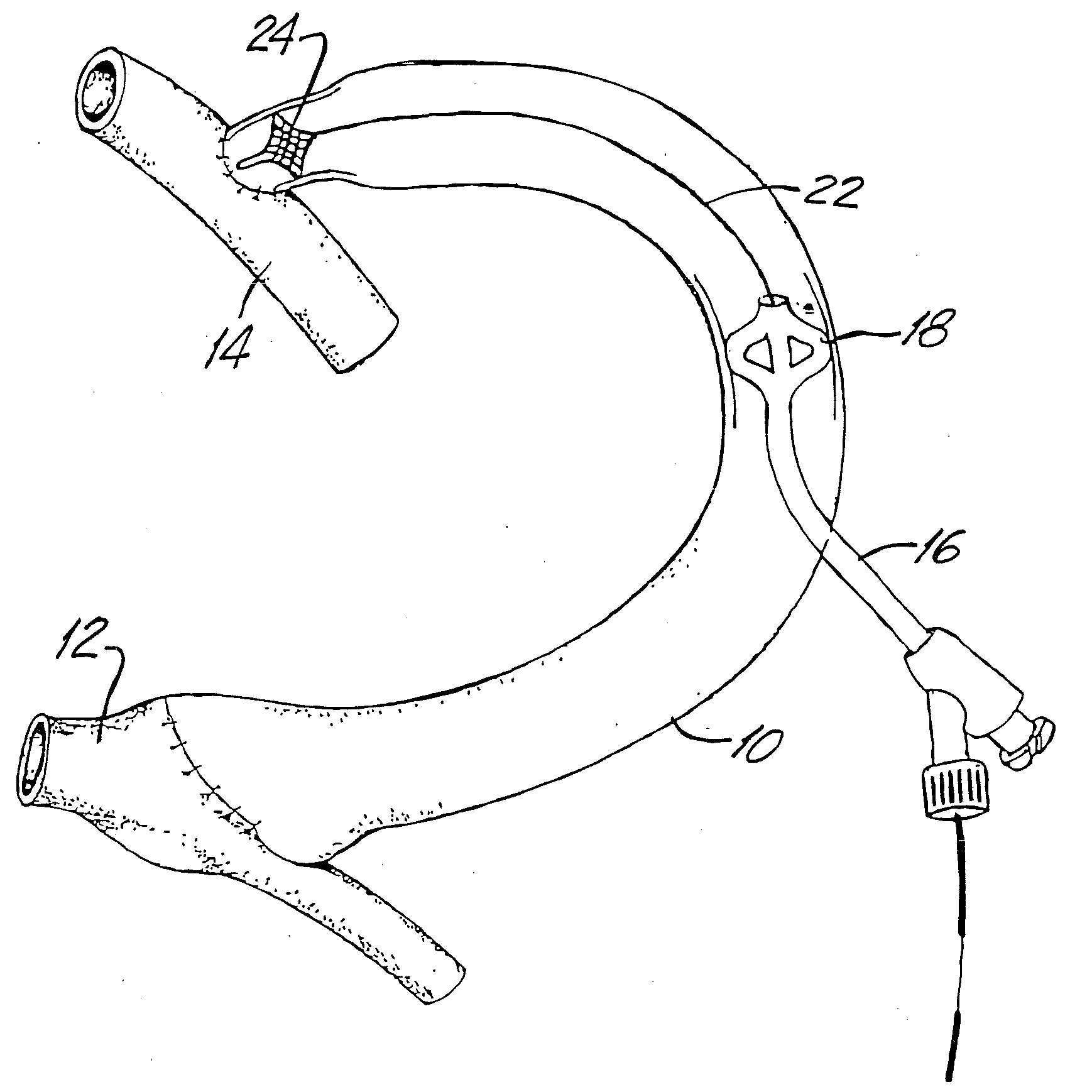
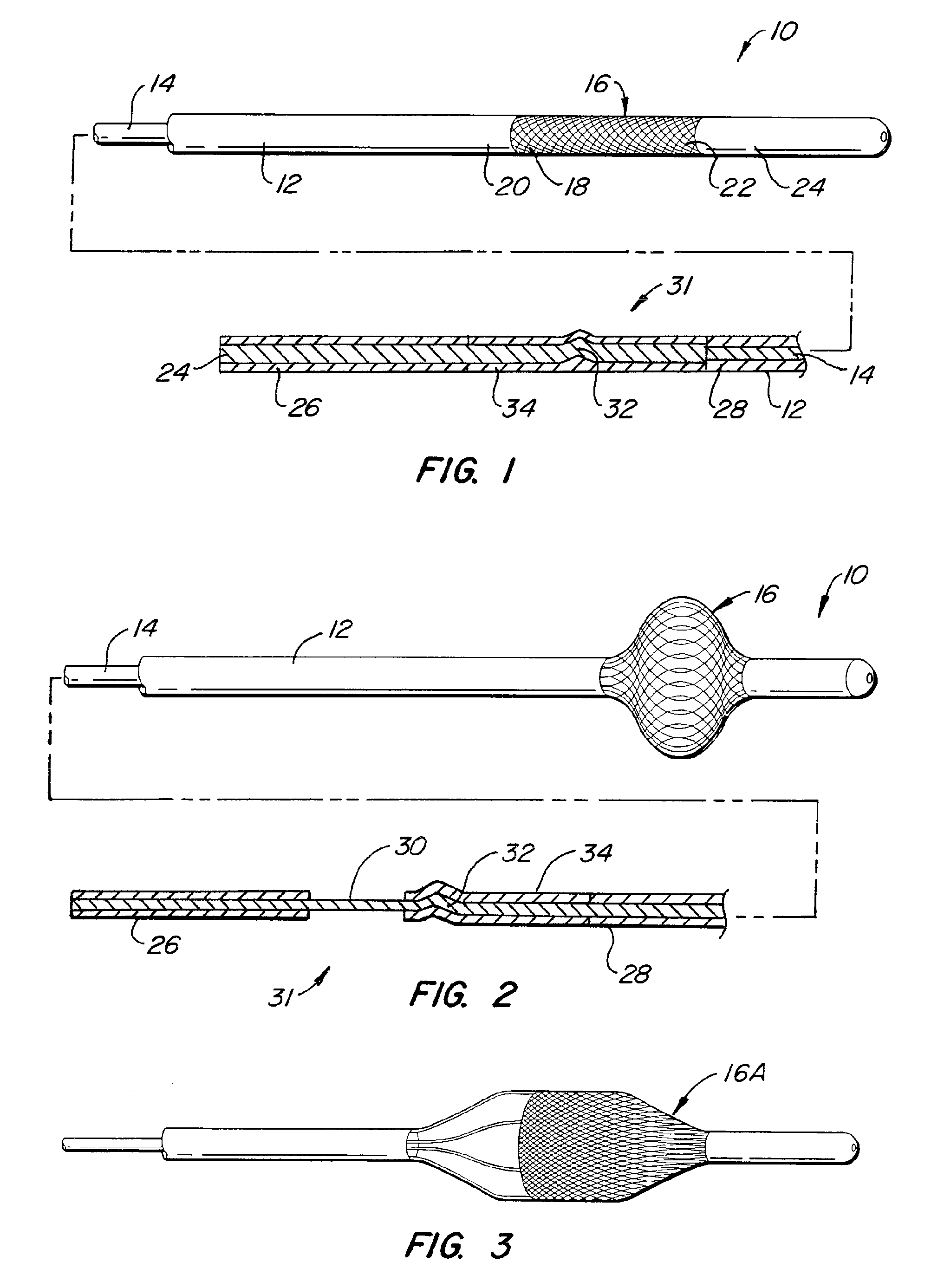
Rectal expander
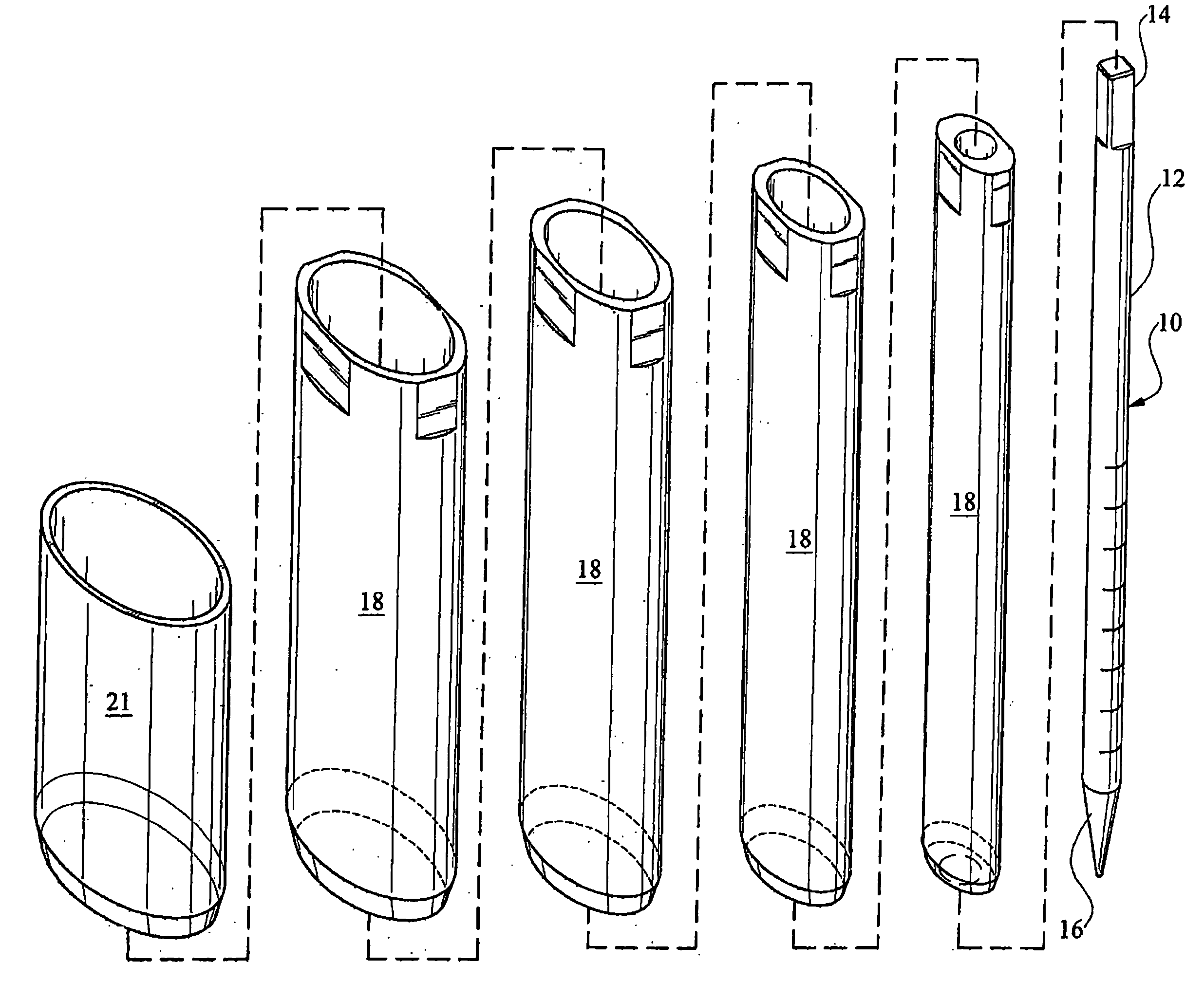
There is disclosed medical apparatus of the type for use in surgery such as transanal endoscopic microsurgery, as well as methods of providing access to, inspecting and enabling surgery within a body passage. In one embodiment of the invention, medical apparatus in the form of a rectal expander (10) is disclosed, the expander (10) being adapted for location at least partly within a body passage such as the rectum (12) of a patient (14), the expander (10) having a leading end (18) and an access area in the form of an opening (20) for access from the expander (10) into the rectum (12), at least part of the opening (20) being spaced from the leading end (18), and the expander (10) being controllably movable between collapse and expansion positions, for expanding the rectum (12).
Owner:UNIVERSITY OF DUNDEE
Minimally invasive apparatus for implanting a sacral stimulation lead
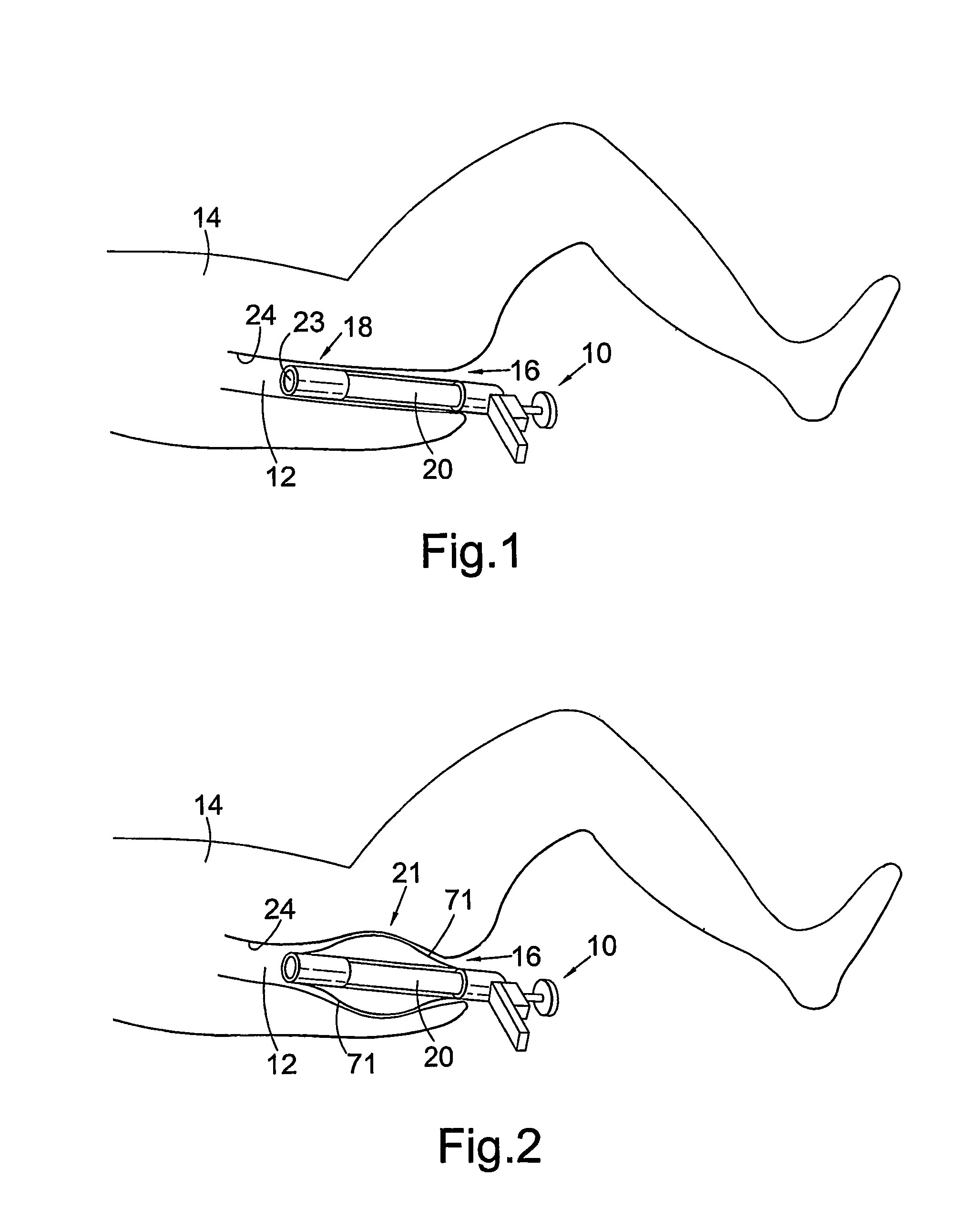
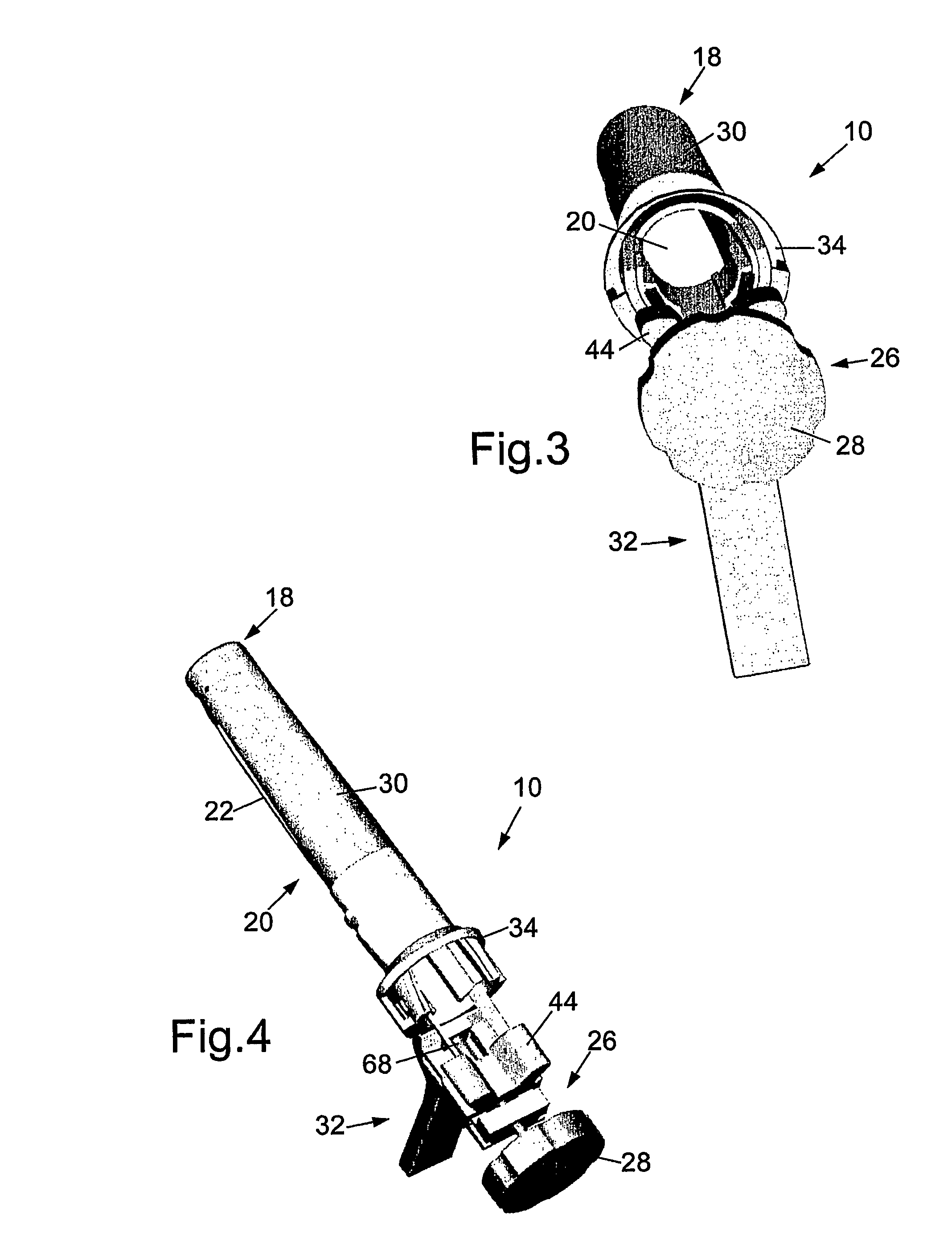
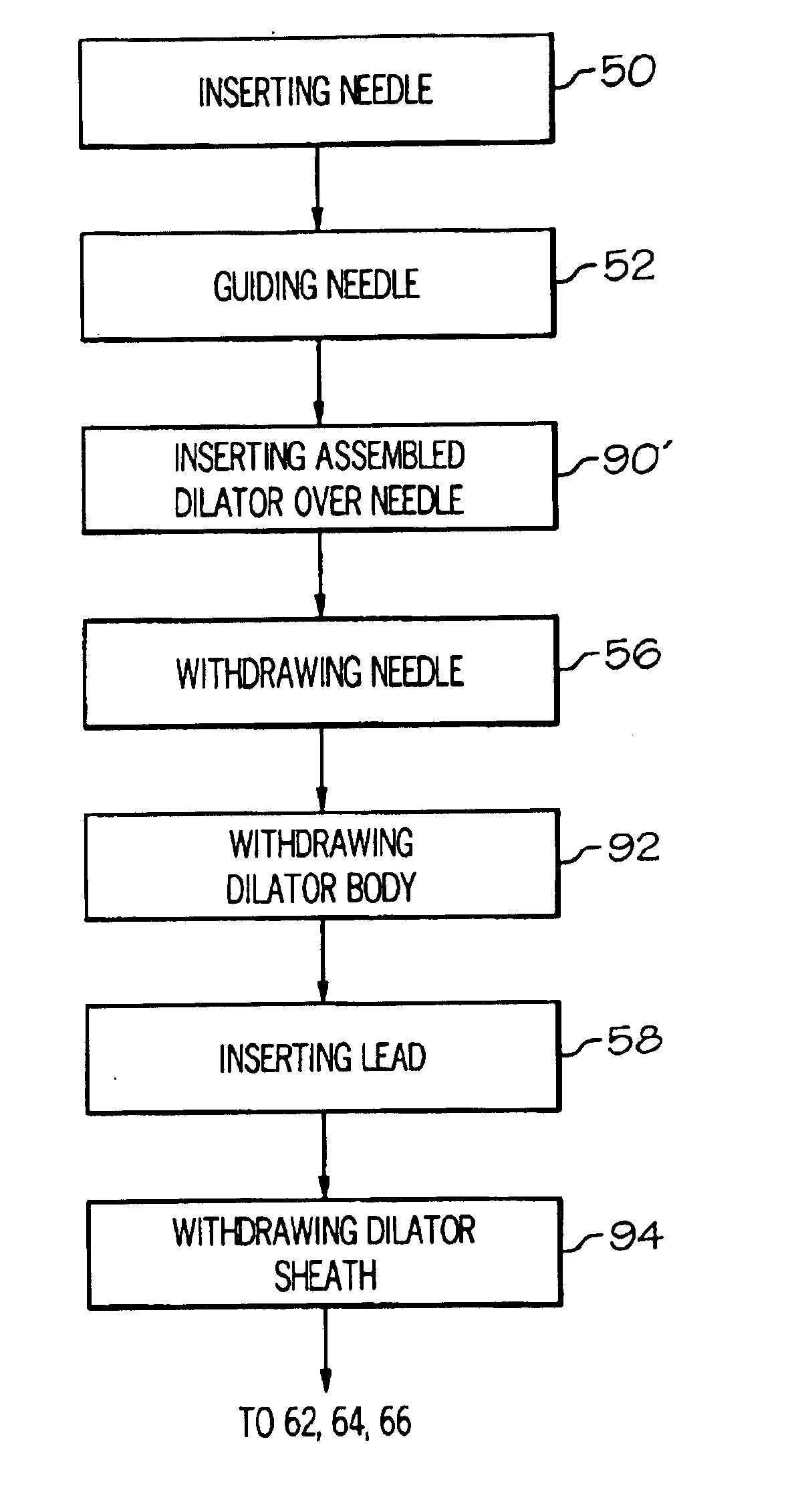
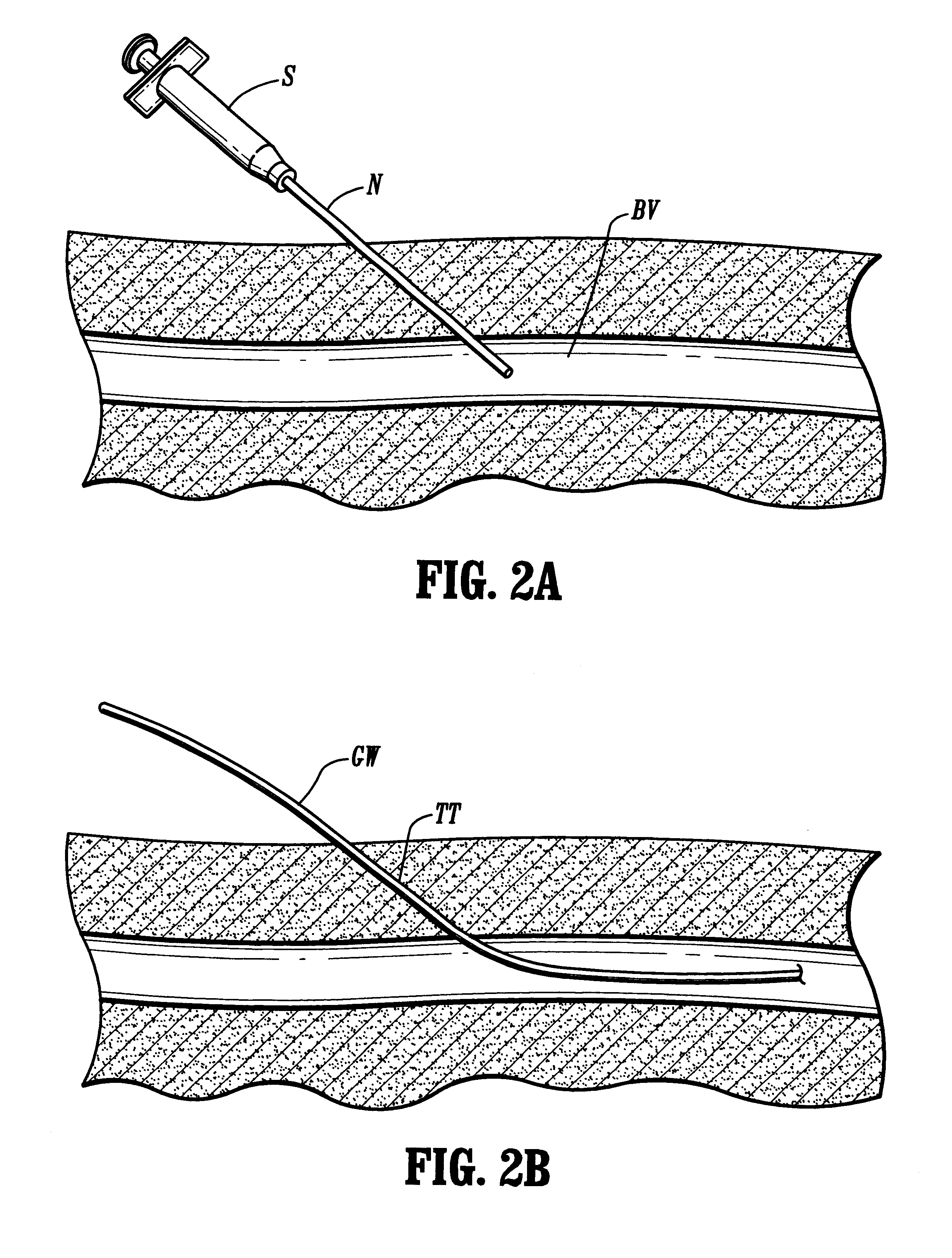
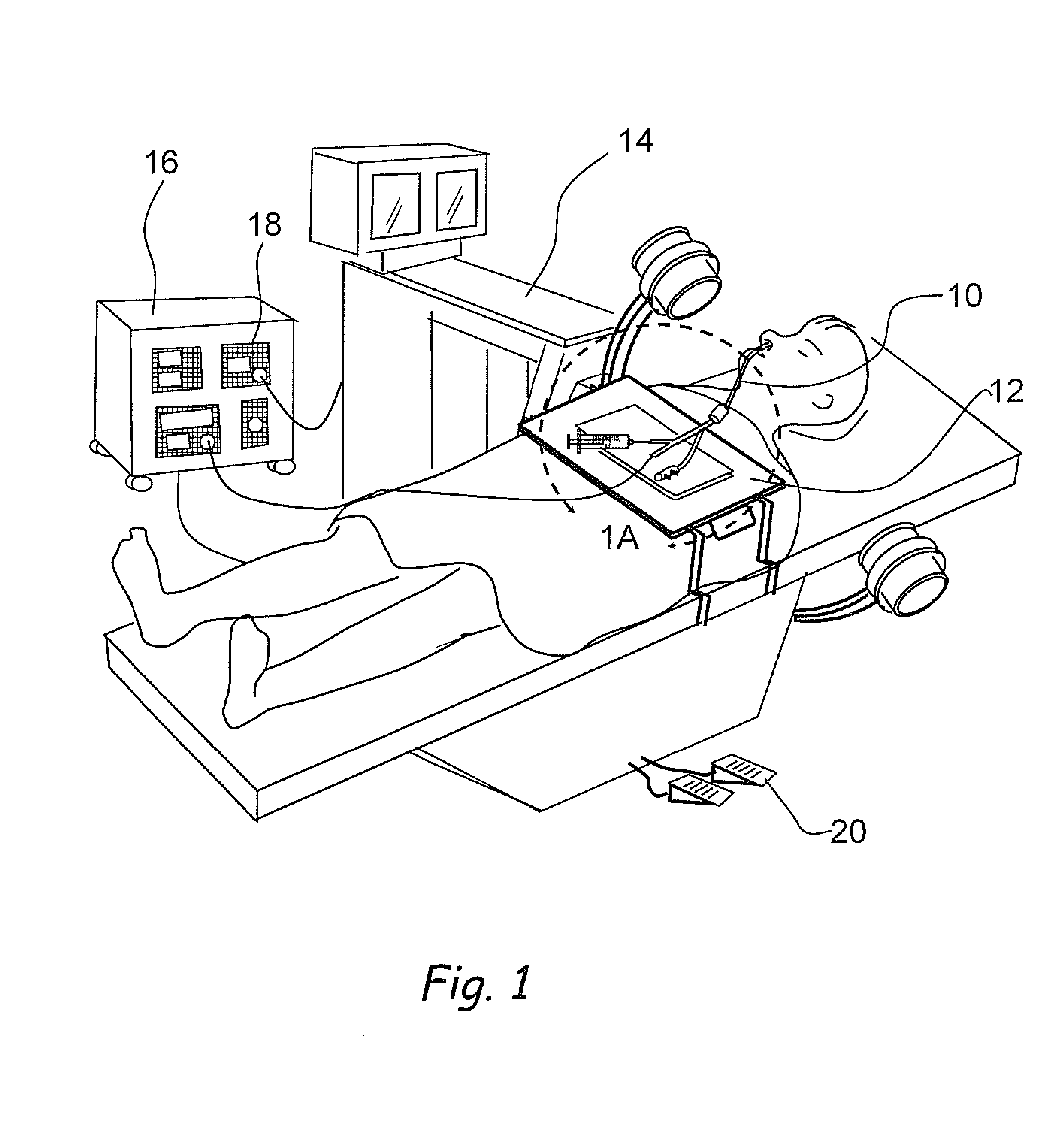
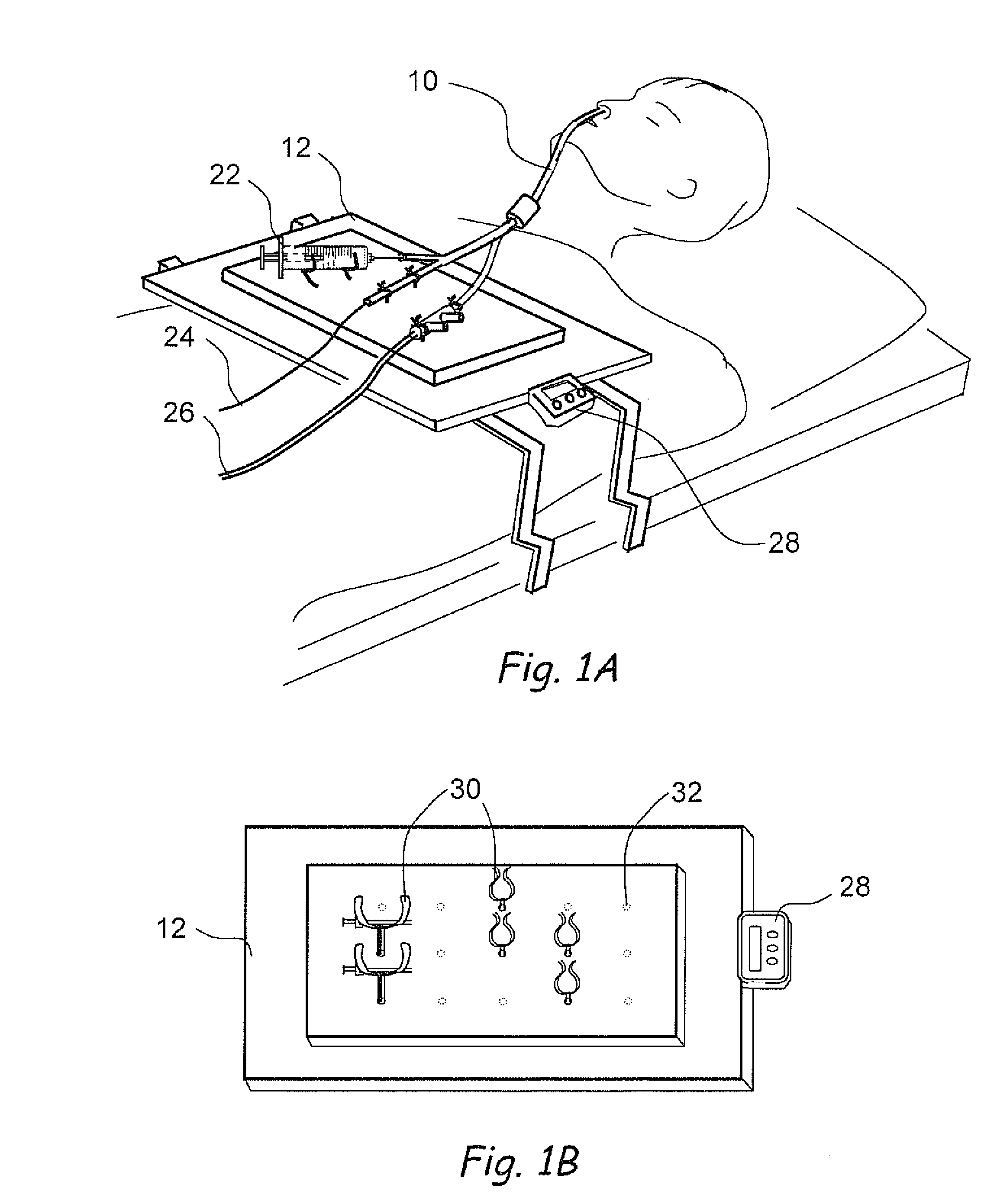
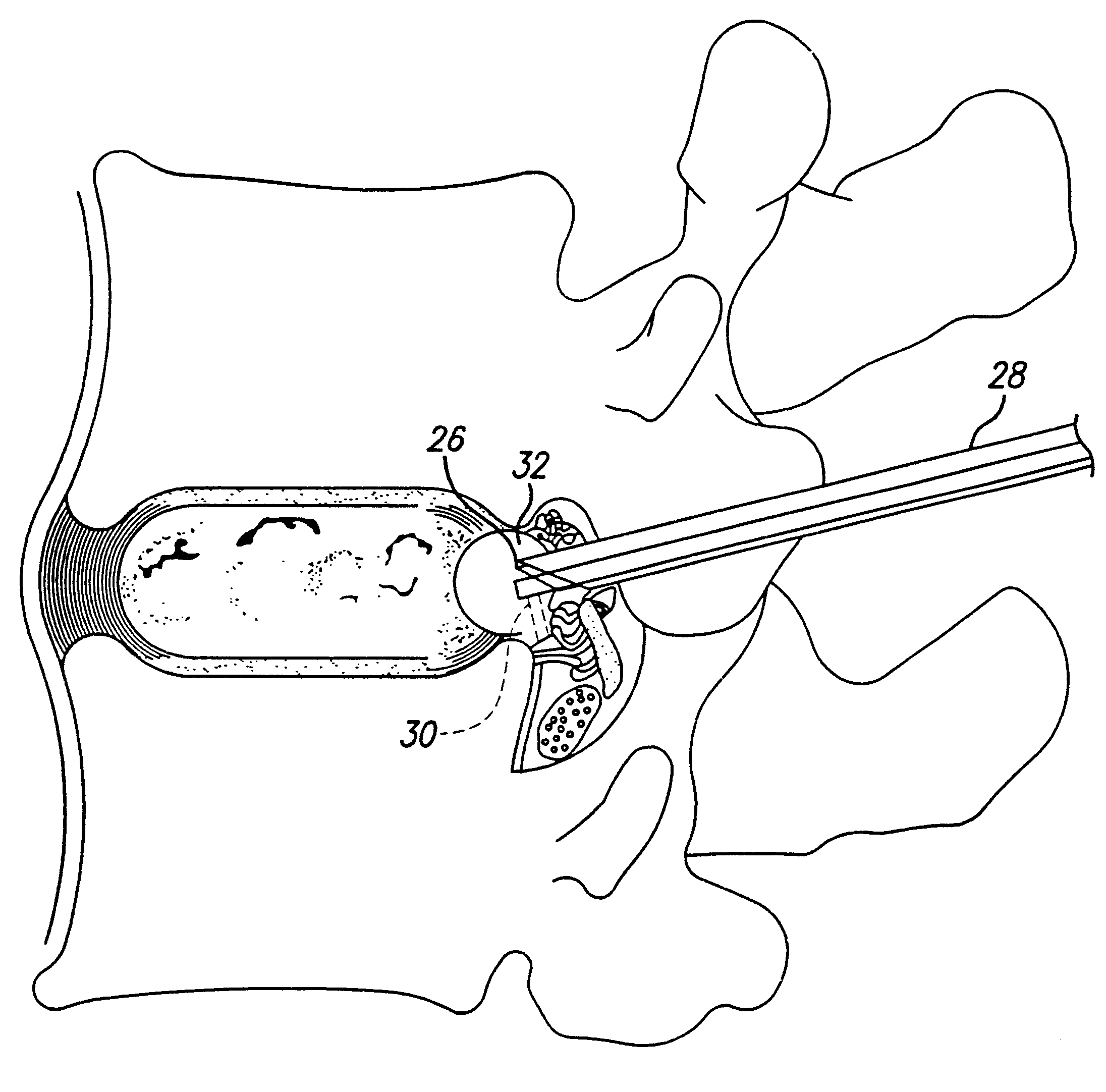
Methods and apparatus for implanting a stimulation lead in a patient's sacrum to deliver neurostimulation therapy that can reduce patient surgical complications, reduce patient recovery time, and reduce healthcare costs. A surgical instrumentation kit for minimally invasive implantation of a sacral stimulation lead through a foramen of the sacrum in a patient to electrically stimulate a sacral nerve comprises a needle and a dilator and optionally includes a guide wire. The needle is adapted to be inserted posterior to the sacrum through an entry point and guided into a foramen along an insertion path to a desired location. In one variation, a guide wire is inserted through a needle lumen, and the needle is withdrawn. The insertion path is dilated with a dilator inserted over the needle or over the guide wire to a diameter sufficient for inserting a stimulation lead, and the needle or guide wire is removed from the insertion path. The dilator optionally includes a dilator body and a dilator sheath fitted over the dilator body. The stimulation lead is inserted to the desired location through the dilator body lumen or the dilator sheath lumen after removal of the dilator body, and the dilator sheath or body is removed from the insertion path. If the clinician desires to separately anchor the stimulation lead, an incision is created through the entry point from an epidermis to a fascia layer, and the stimulation lead is anchored to the fascia layer. The stimulation lead can be connected to the neurostimulator to delivery therapies to treat pelvic floor disorders such as urinary control disorders, fecal control disorders, sexual dysfunction, and pelvic pain.
Owner:MEDTRONIC INC +1
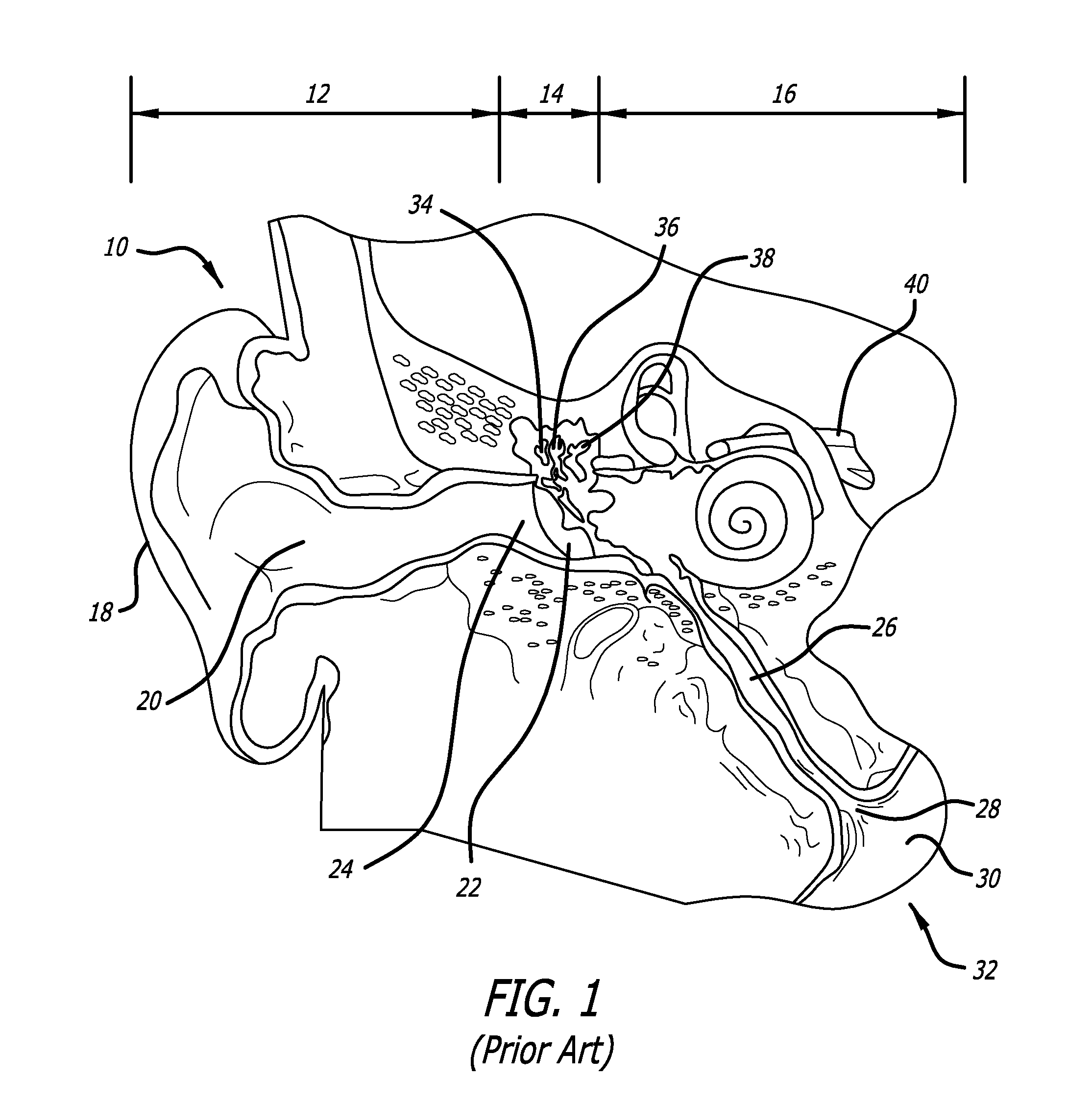
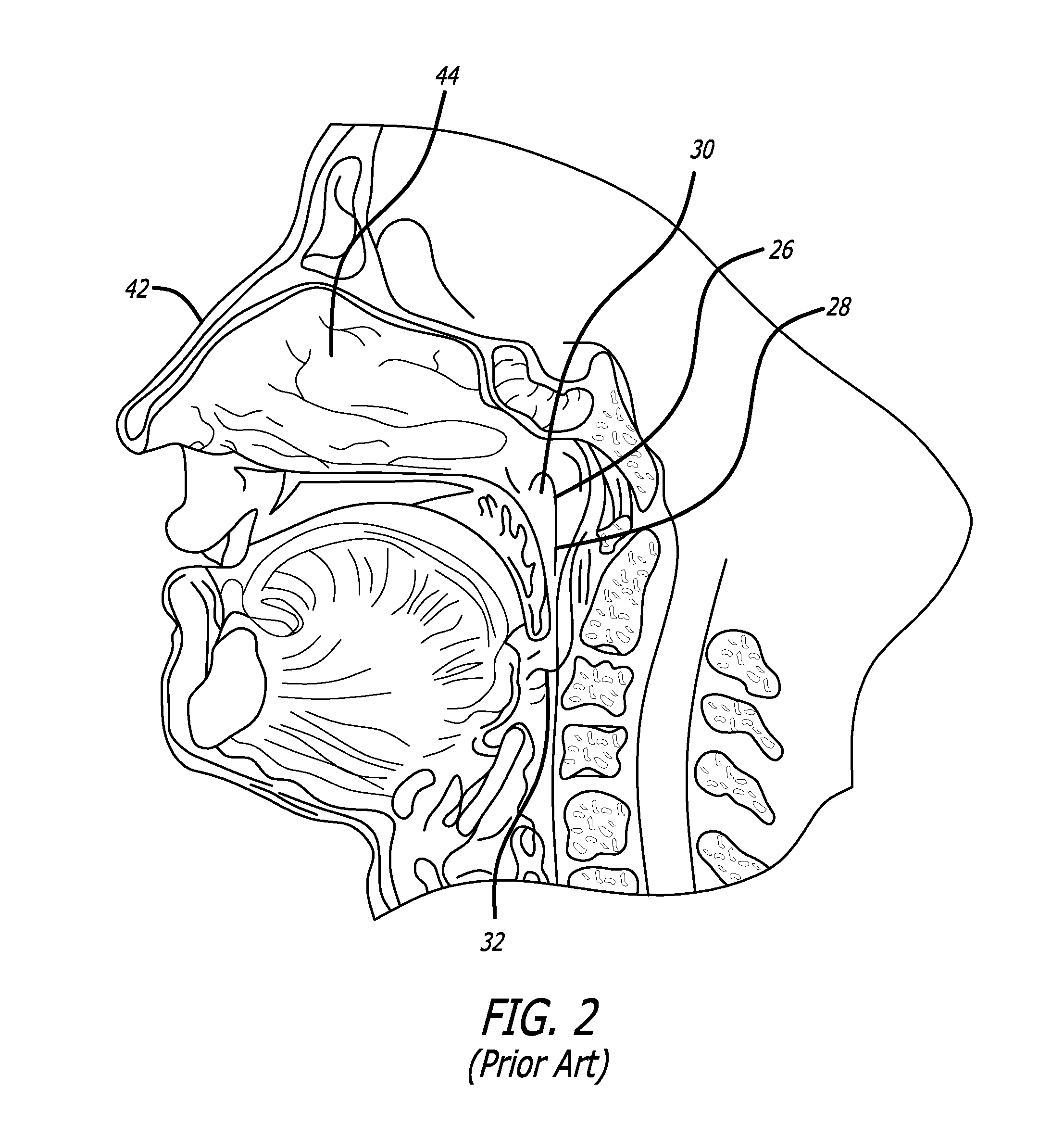
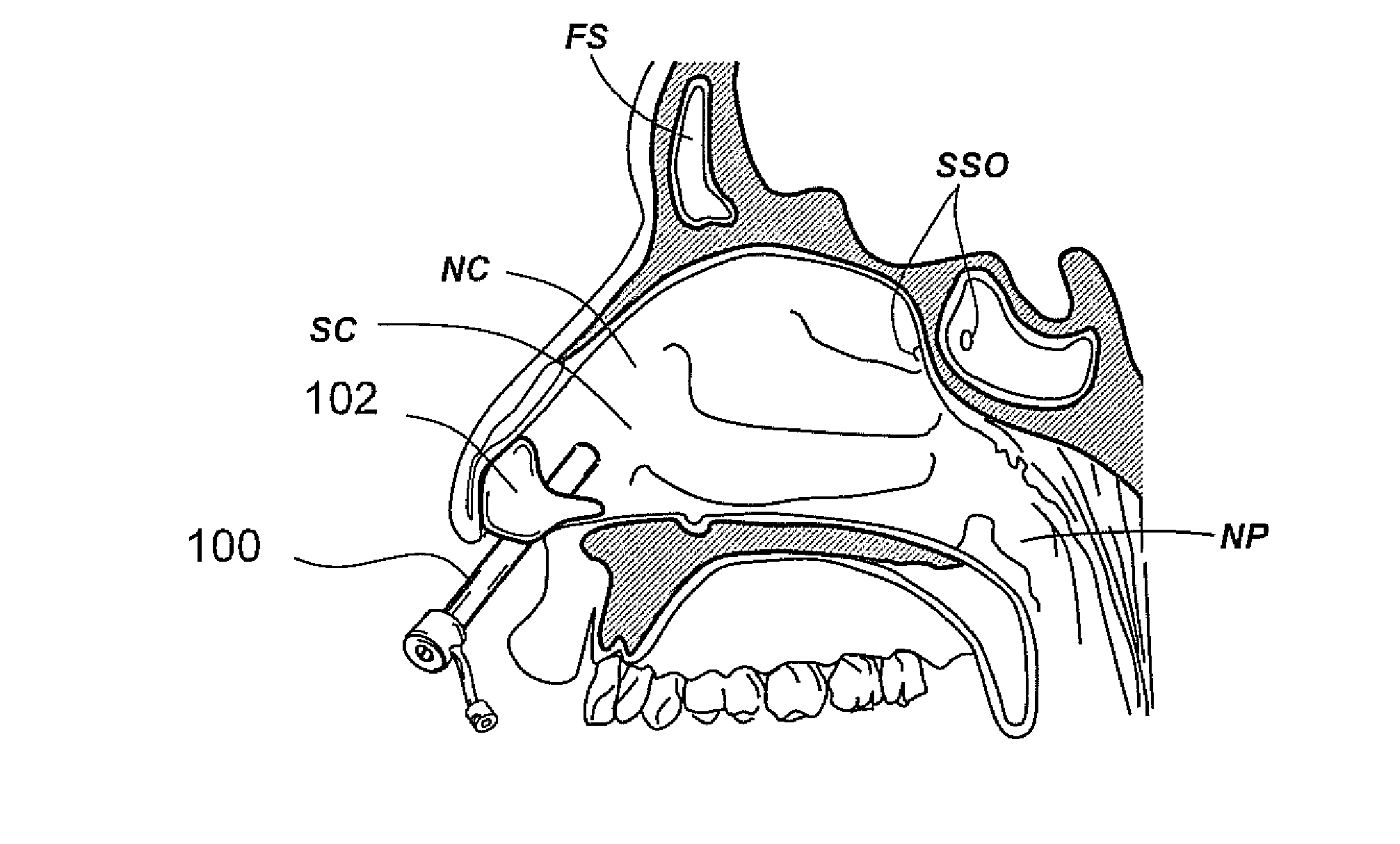
Devices, systems and methods for treating disorders of the ear, nose and throat
Sinusitis, mucocysts, tumors, infections, hearing disorders, choanal atresia, fractures and other disorders of the paranasal sinuses, Eustachian tubes, Lachrymal ducts and other ear, nose, throat and mouth structures are diagnosed and / or treated using minimally invasive approaches and, in many cases, flexible catheters as opposed to instruments having rigid shafts. Various diagnostic procedures and devices are used to perform imaging studies, mucus flow studies, air / gas flow studies, anatomic dimension studies and endoscopic studies. Access and occluding devices may be used to facilitate insertion of working devices such asendoscopes, wires, probes, needles, catheters, balloon catheters, dilation catheters, dilators, balloons, tissue cutting or remodeling devices, suction or irrigation devices, imaging devices, sizing devices, biopsy devices, image-guided devices containing sensors or transmitters, electrosurgical devices, energy emitting devices, devices for injecting diagnostic or therapeutic agents, devices for implanting devices such as stents, substance eluting or delivering devices and implants, etc.
Owner:ACCLARENT INC
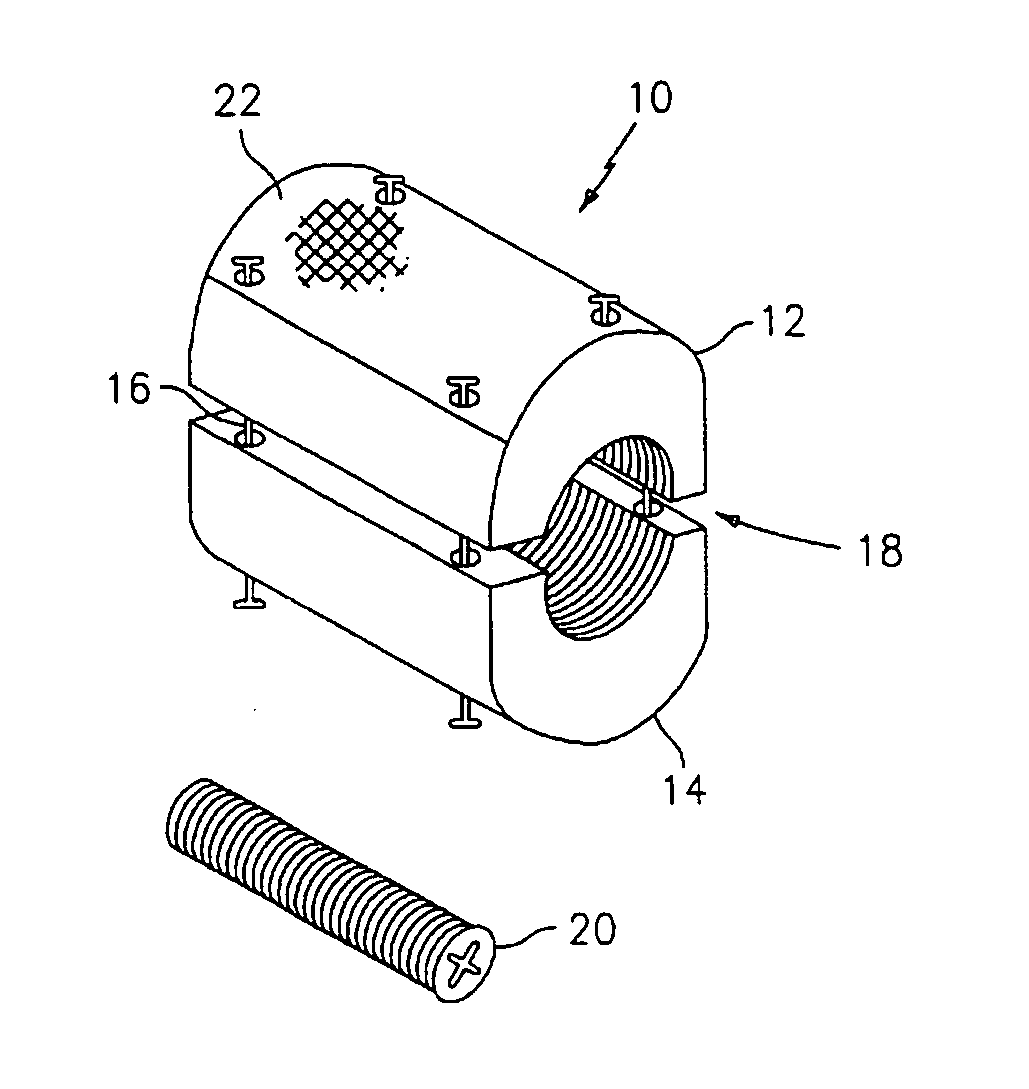
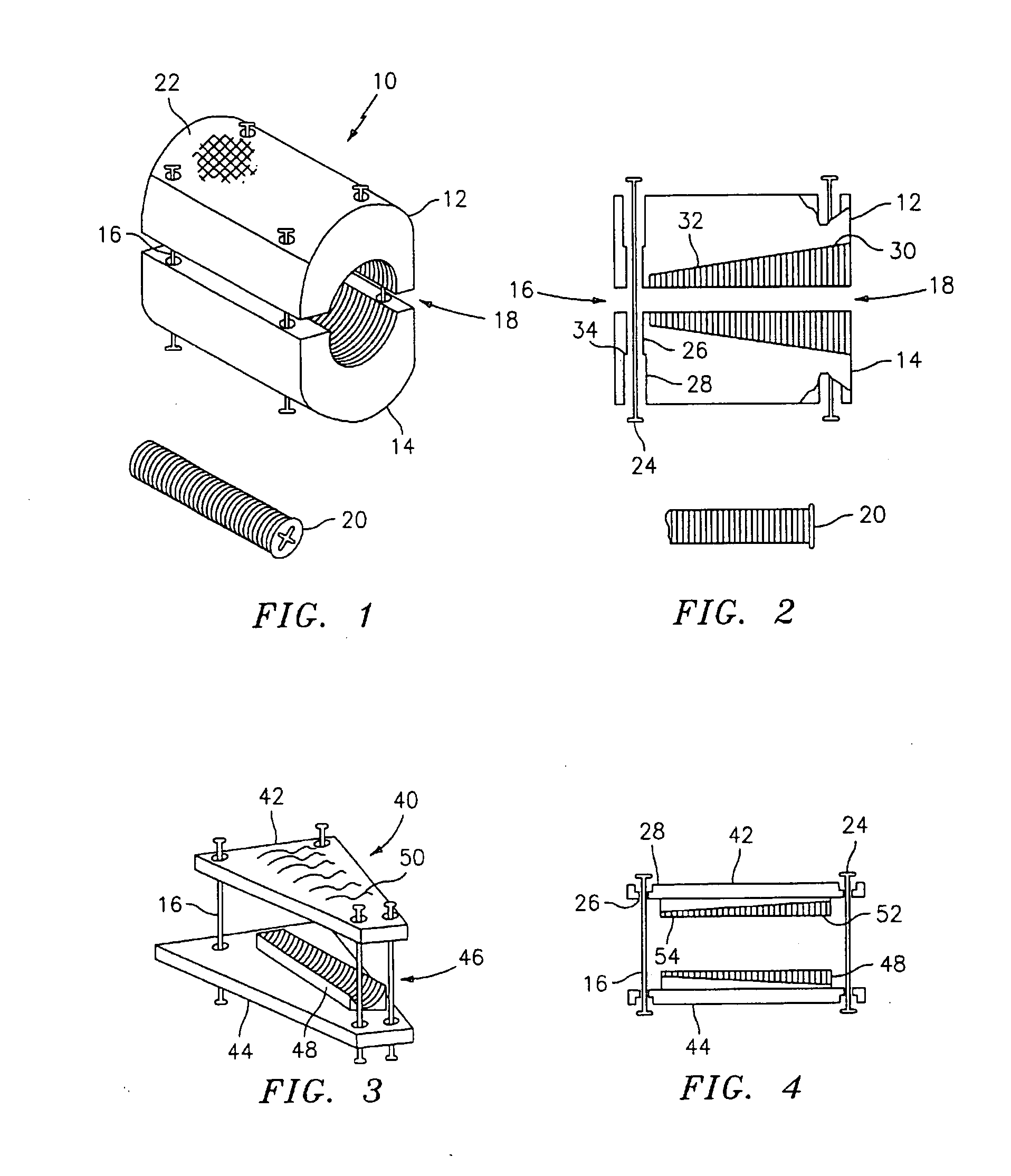
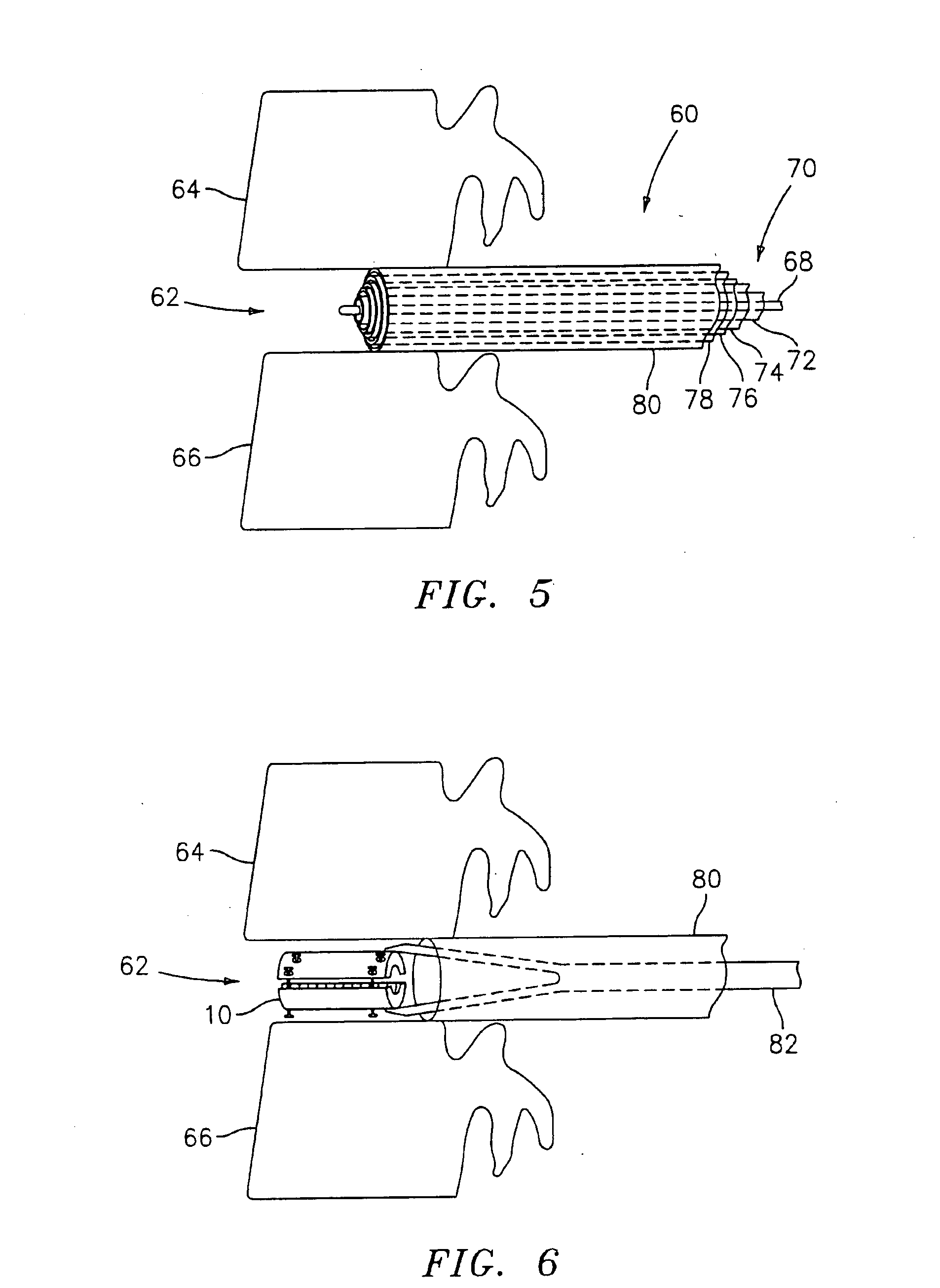
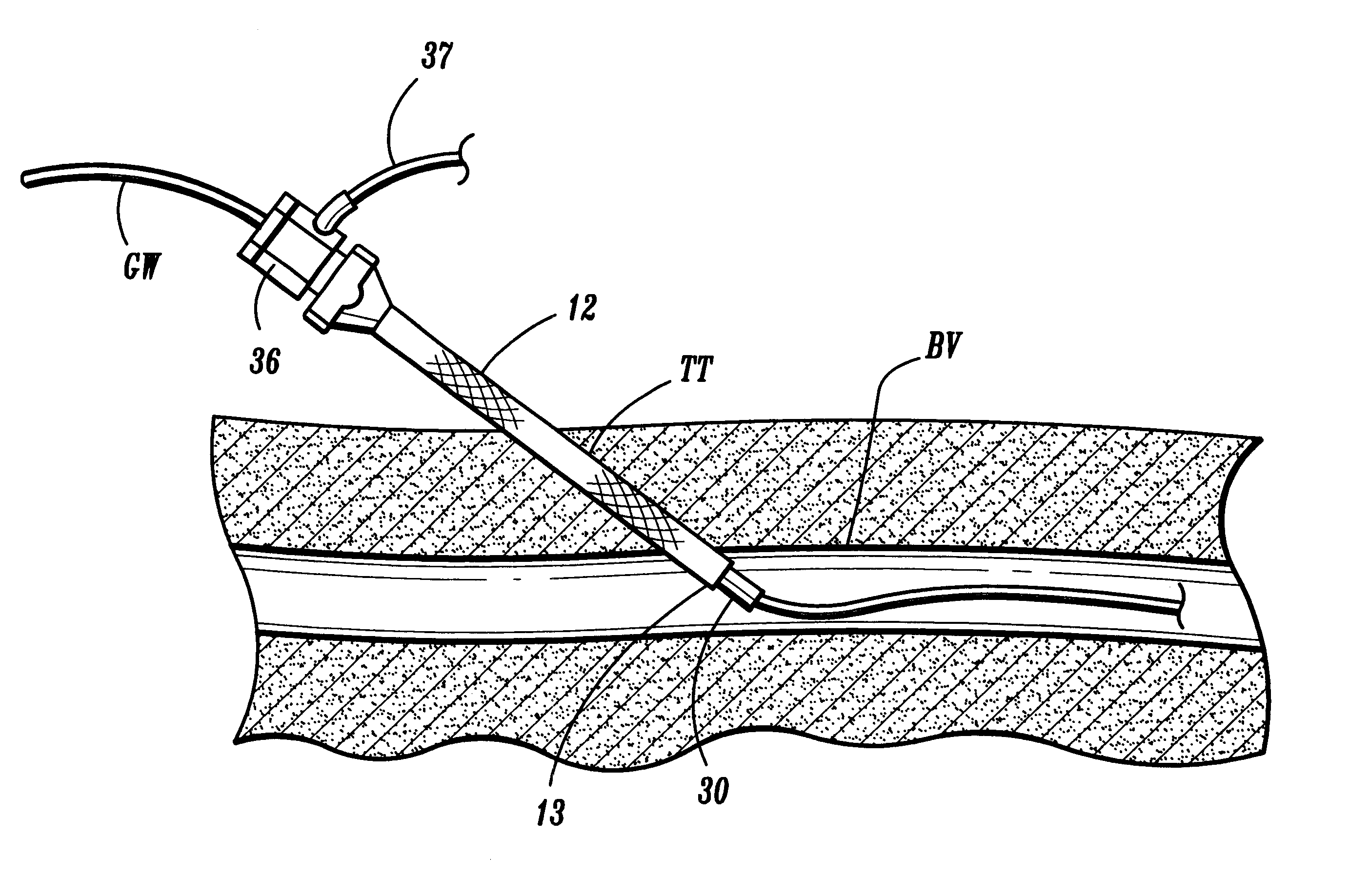
Expandable trans-septal sheath
InactiveUS20060135962A1Inhibit bindingAvoid interferenceGuide needlesEar treatmentAccess routeDilator
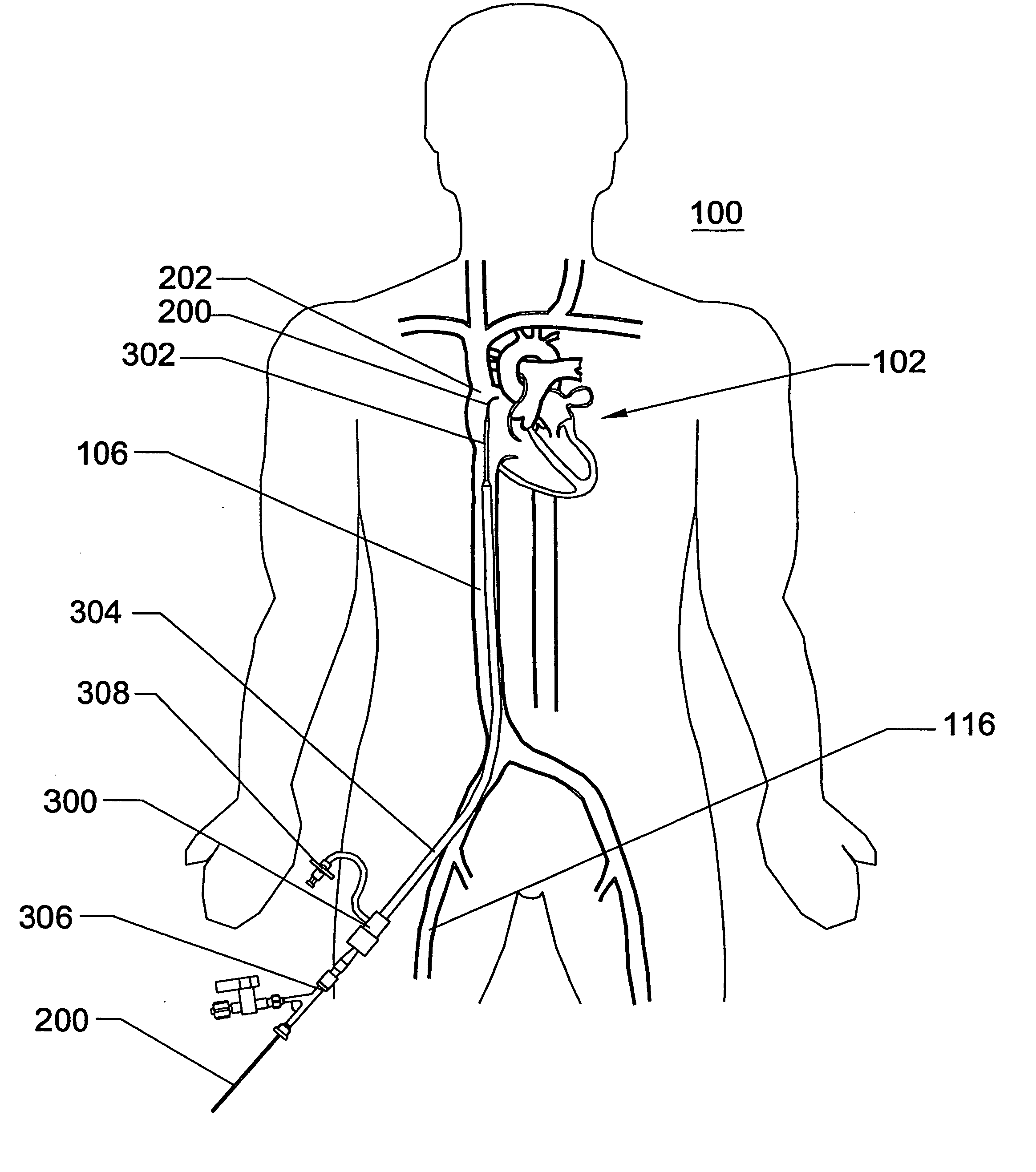
Disclosed is an expandable transluminal sheath, for introduction into the body while in a first, low cross-sectional area configuration, and subsequent expansion of at least a part of the distal end of the sheath to a second, enlarged cross-sectional configuration. The sheath is configured for use in the vascular system. The access route is through the inferior vena cava to the right atrium, where a trans-septal puncture, followed by advancement of the catheter is completed. The distal end of the sheath is maintained in the first, low cross-sectional configuration during advancement through the atrial septum into the left atrium. The distal end of the sheath is expanded using a radial dilator. In one application, the sheath is utilized to provide access for a diagnostic or therapeutic procedure such as electrophysiological mapping of the heart, radio-frequency ablation of left atrial tissue, placement of atrial implants, valve repair, or the like.
Owner:ONSET MEDICAL CORP
Non cannulated dilators
A non-cannulated dilator that is designed with a rigid elongated solid body and with a judiciously configured tip is utilized as the first dilator of a series of dilators which are inserted into the body of a patient for minimally invasive spinal surgery and is made from a solid elongated body, that could be round, ovoid or other cross sectional configuration whose diameter is greater than one and a half (1½) millimeter, and that includes a tool engaging end portion at the proximal end is sufficiently rigid so as not to bend and has a pointed shaped insertion end portion at the distal end with the point of the pointed end being discreetly blunted and utilized in a surgical procedure as a replacement of the typical guide wire and is characterized as providing a “fee” to the surgeon as it penetrates through the tissue and muscle of the patient as it proceeds toward the target.
Owner:DEPUY SPINE INC (US) +1
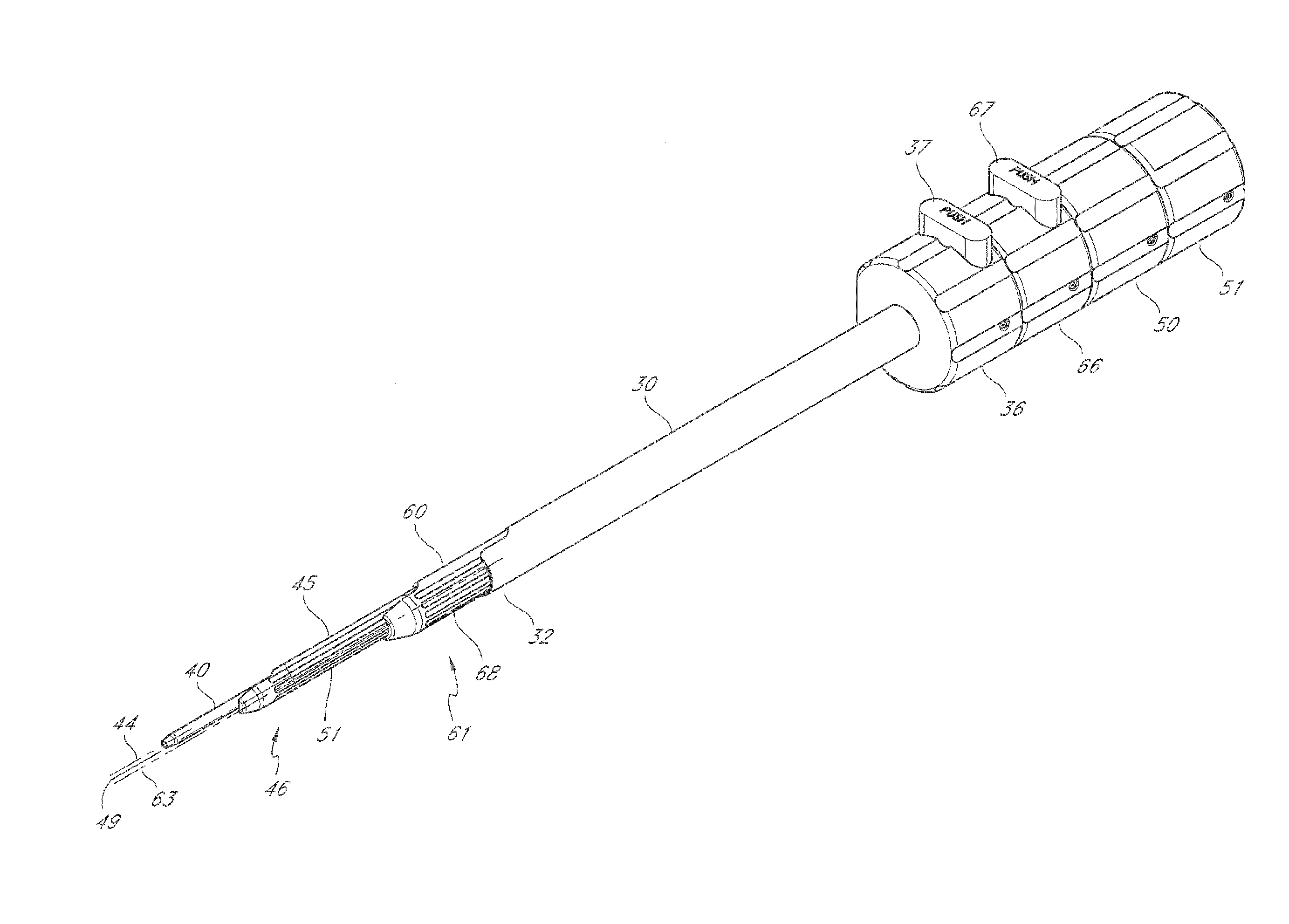
Instrumentation and procedure for implanting spinal implant devices
An instrumentation set may include insertion instruments for forming an implant between bone structures. The insertion instruments may include a spreader and a separator. The bone structures may be vertebrae. Implant members may be attached to the spreader and positioned between the bone structures. The separator may be inserted into the spreader to establish a desired separation distance between the implant members. Connectors may be inserted into the implant members to join the implant members together and form the implant. The insertion instruments may be removed. A seater may be used to set the position of the connectors relative to the implant members to inhibit disassembly of the implant.
Owner:ZIMMER BIOMET SPINE INC
Curved dilator and method
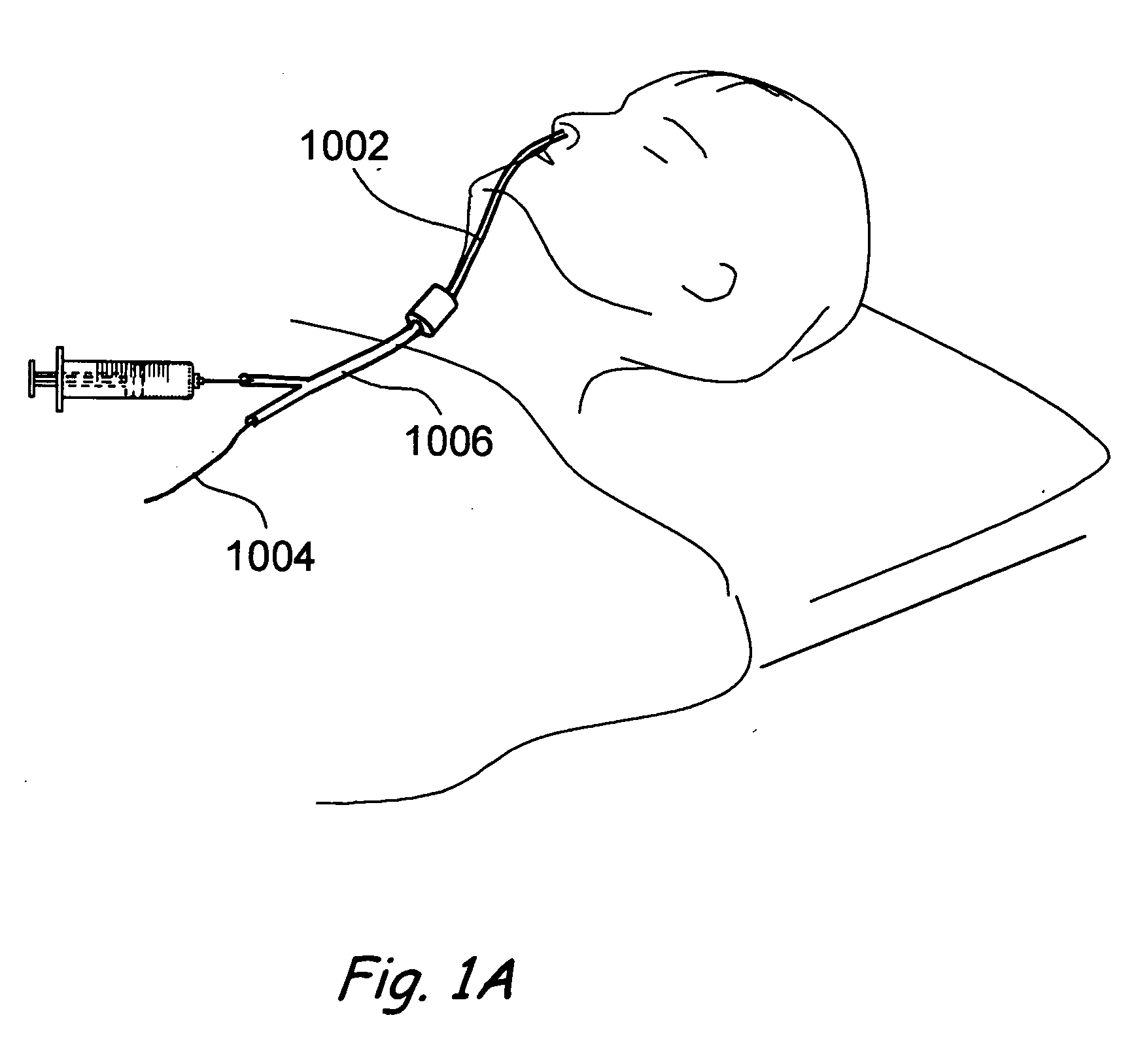
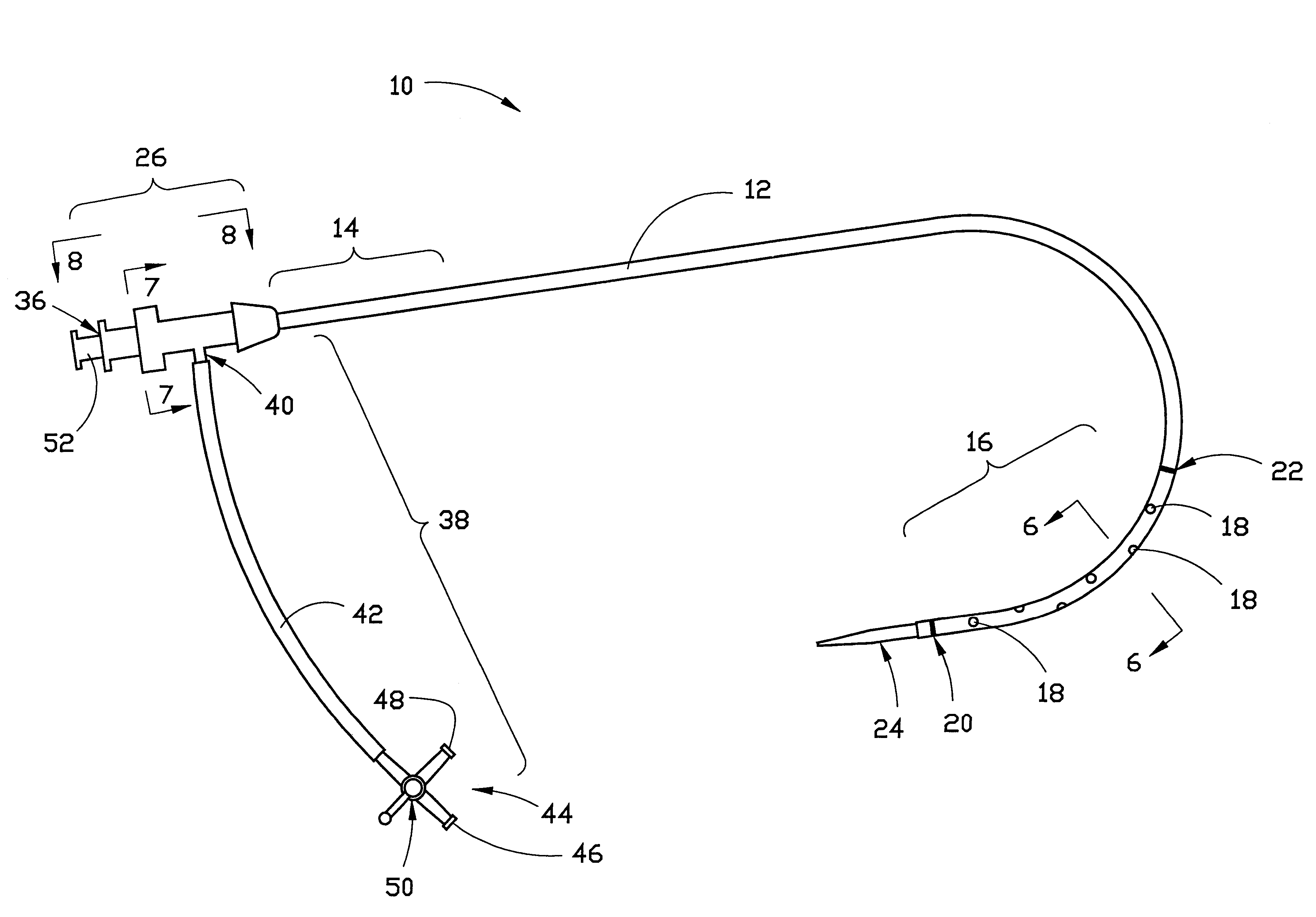
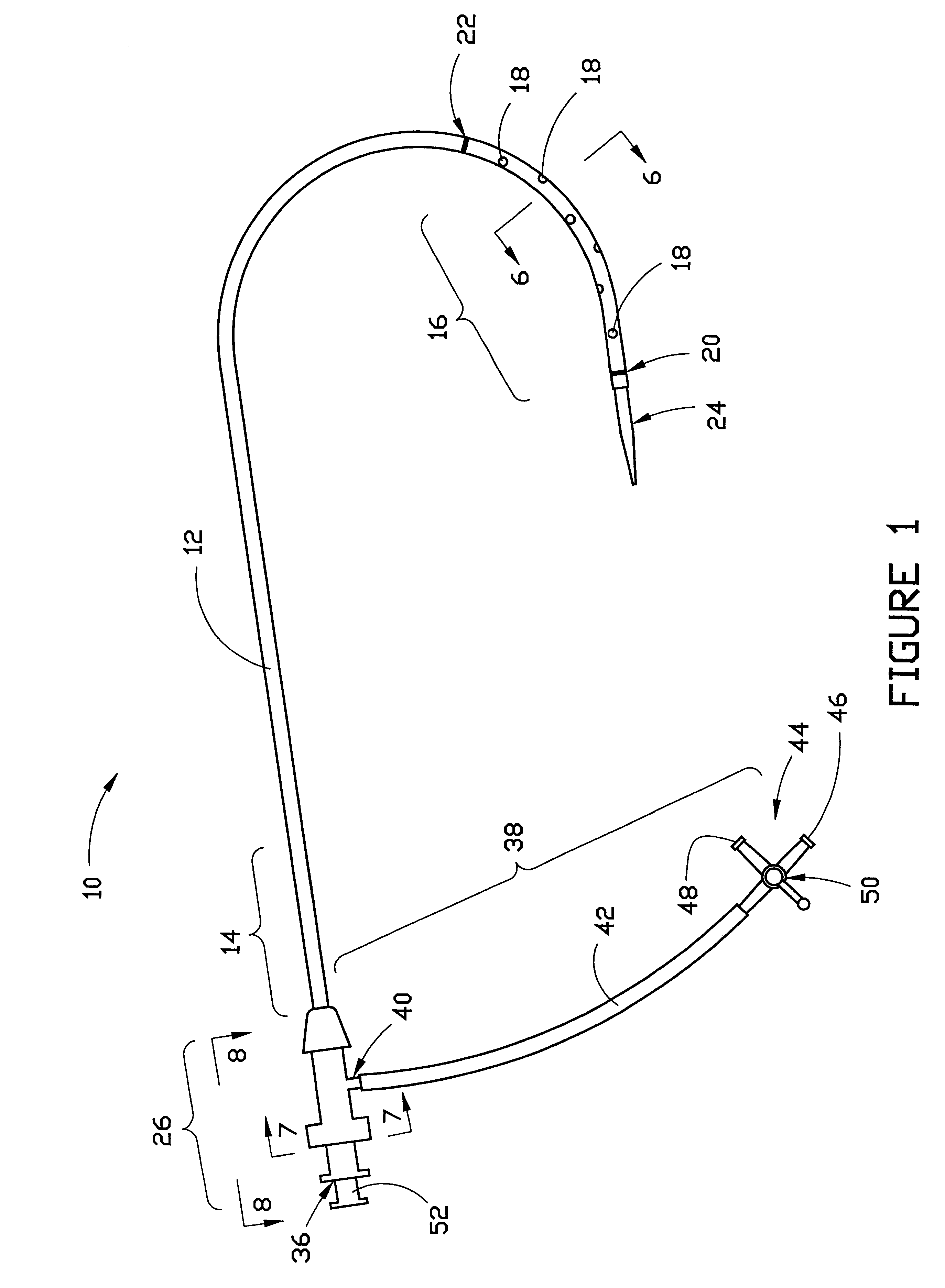
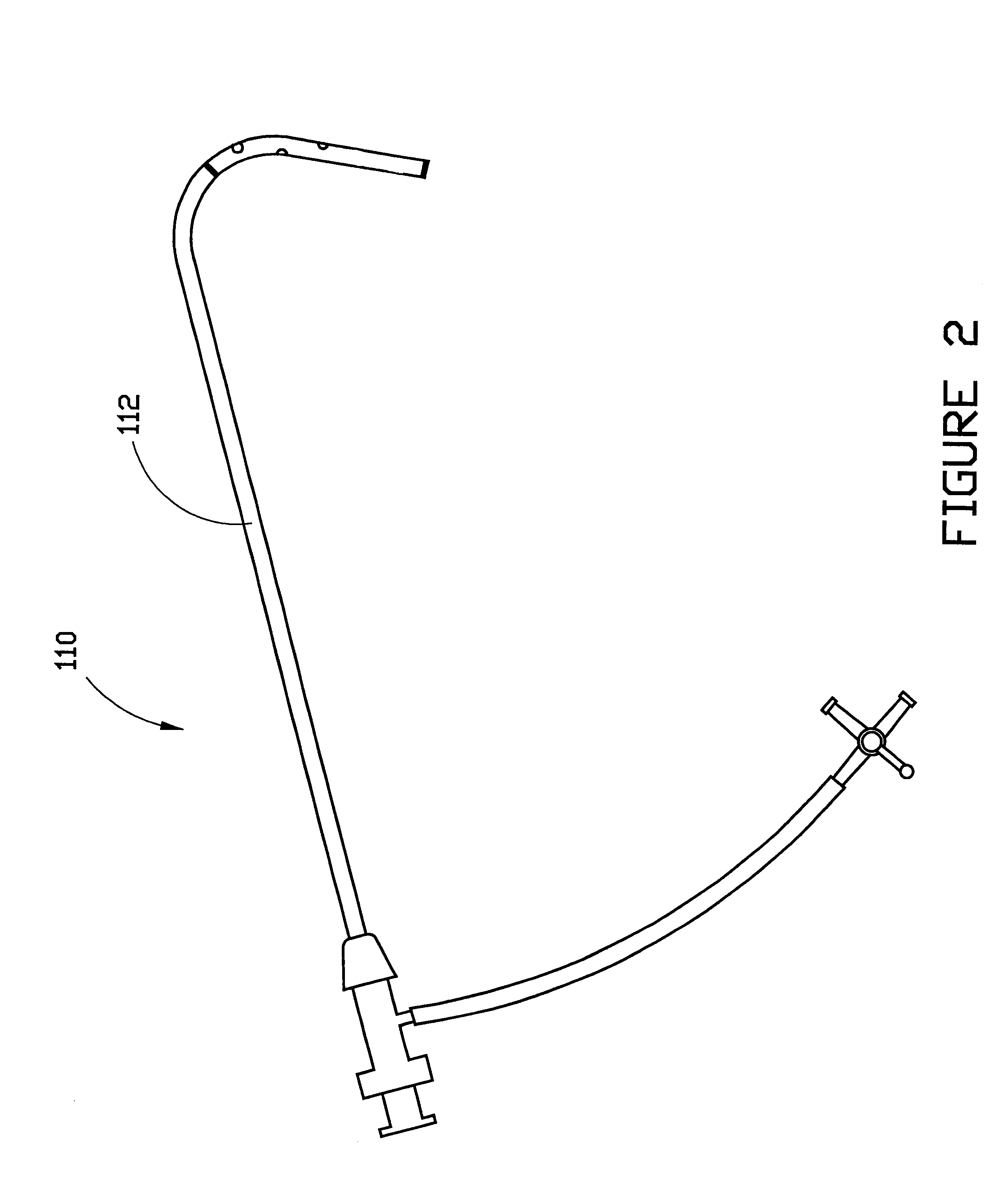
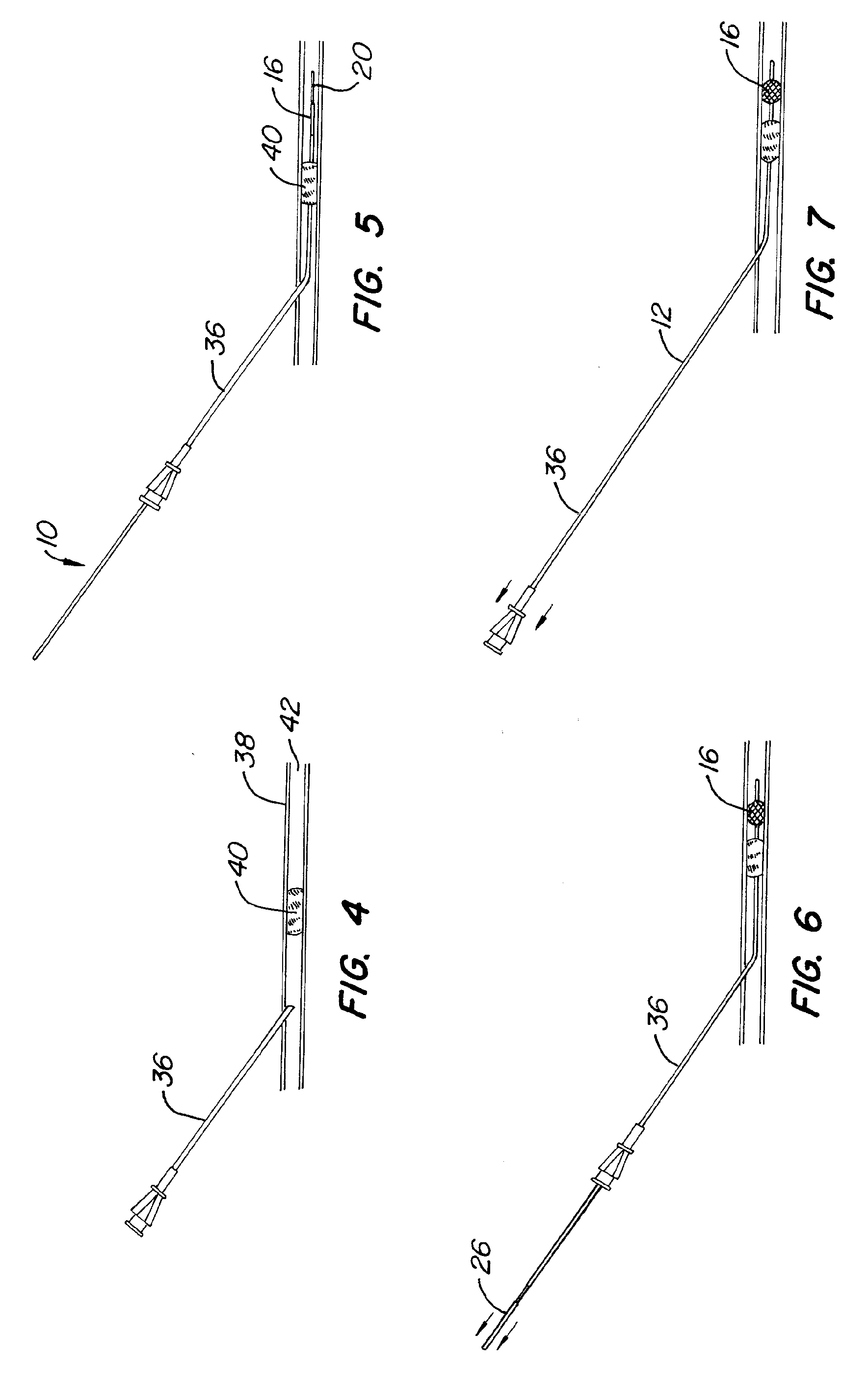
The present invention generally relates to a dilator with a curved tip. The curved tip is machined through a specific range of diameters so that a physician can establish the diameter of the opening with the dilator inserted. The angle of the dilator also allows the physician to access the interspinous ligament through a minimally invasive opening in the patients back, minimizing the trauma caused to surrounding body tissue.
Owner:MEDTRONIC EURO SARL
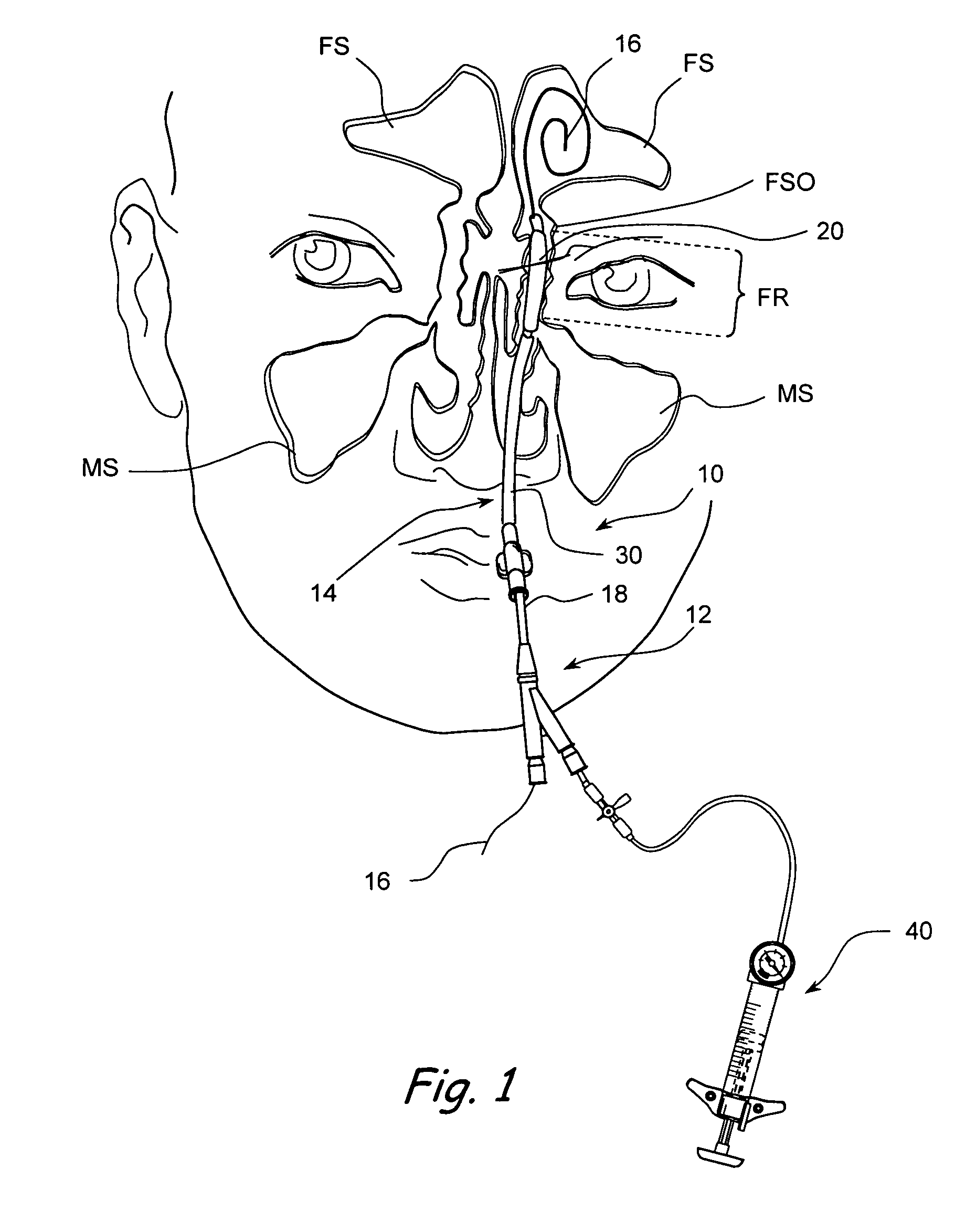
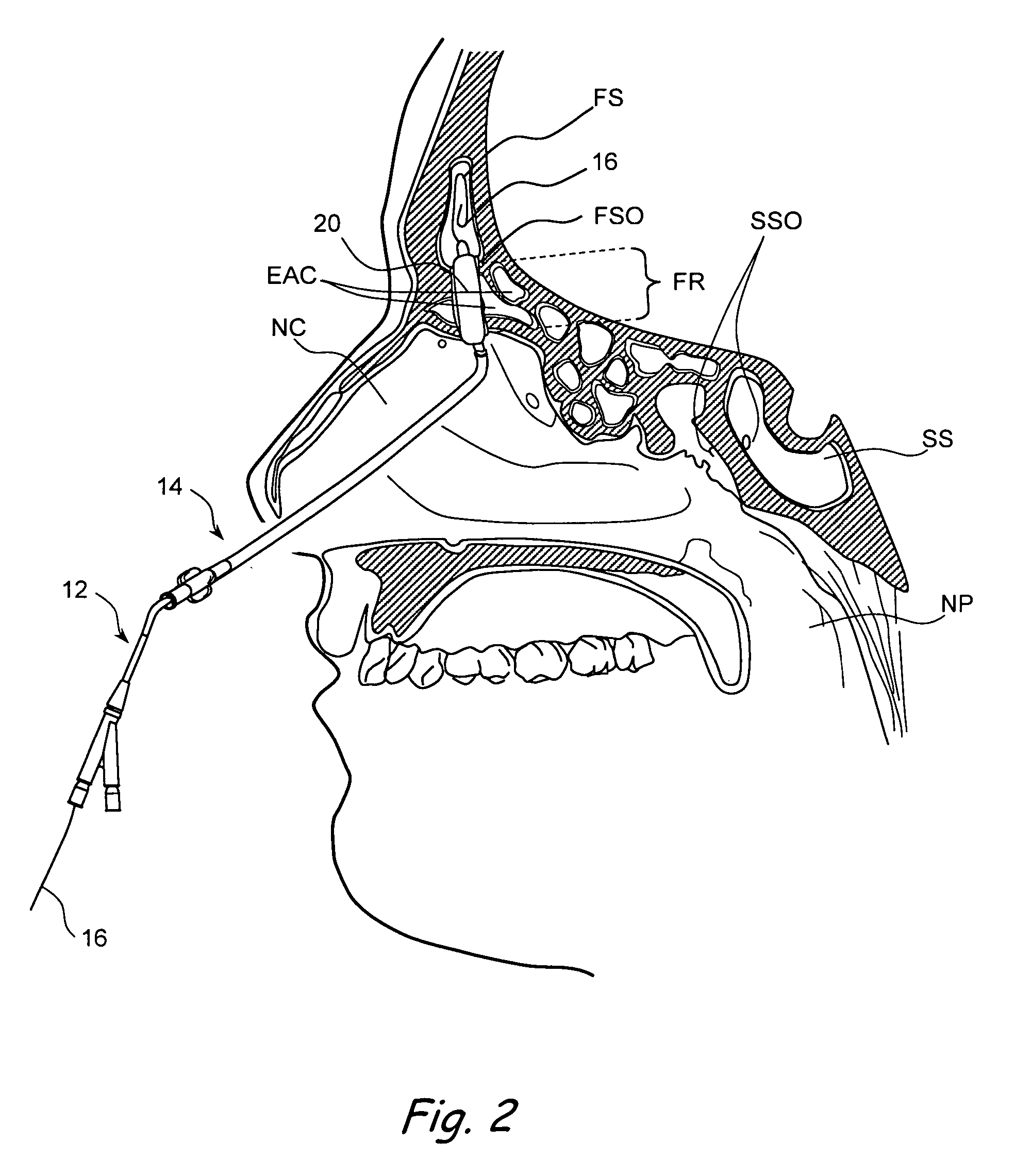
Devices, systems and methods useable for treating frontal sinusitis
Devices, systems and methods wherein a dilator, such as a balloon or other expandable member, is positionable within the frontal sinus ostium and adjacent frontal recess and useable to dilate the frontal sinus ostium and substantially all of the frontal sinus recess without requiring repositioning and repeated re-expansion of the dilator. One balloon catheter device of the invention comprises a catheter body that is less than about 50 cm in length (and in some embodiments less than 25 cm in length and a semi-compliant or non-compliant balloon on the catheter body. The balloon may have a working length of about 12 mm to about 30 mm and a width at its widest point when fully inflated of about 2 mm to about 7 mm. Such balloon may be constructed to withstand inflation pressures of about 12 atmospheres. In some embodiments, the dilator is advanced through or over a guide (e.g., guidewire or guide catheter) that has a preformed shape.
Owner:ACCLARENT INC
Method and System for Treating Target Tissue Within the Eustachian Tube
InactiveUS20100274188A1Reduce edemaPromote wettingElectrotherapyEar treatmentNasal passageNasal passages
A method for dilating a Eustachian tube of a patient is disclosed. In one embodiment, the method may involve advancing a dilation device through a nasal passage of the patient to position a dilator of the device at least partially in a Eustachian tube of the patient, expanding the dilator to an expanded configuration to dilate a portion of the Eustachian tube, collapsing the dilator, and removing the dilation device from the patient. The dilated portion of the Eustachian tube remains at least partially dilated after removal of the device.
Owner:ACCLARENT INC
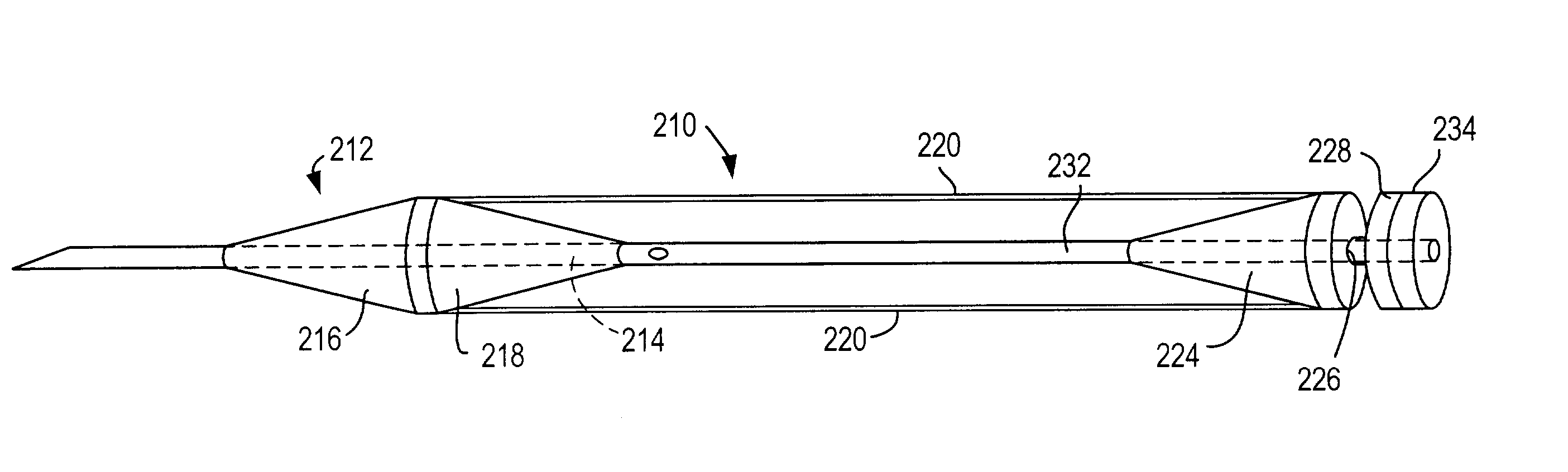
Vascular access device
ActiveUS7025746B2Reduce in quantityEasy accessGuide needlesInfusion syringesVascular Access DevicesDilator
A vascular access system comprises a needle having a distal tip and a proximal fitting section and a dilator having a solid distal section connected to a solid proximal section and an annular recess therebetween, the dilator having a passageway so that the dilator is coaxially positioned around the needle. The needle has at least one opening positioned proximal to its distal tip so that, when the distal end of the needle enters a blood vessel, blood enters the annular recess to show the operator that access has been achieved. Optionally, a sheath having a distal tip, a proximal fitting section, and a lumen sufficient to coaxially fit over the dilator.
Owner:YALE UNIV
Configured and sized cannula
A dilator retractor and the dilators that are used for minimally invasive spinal surgery or other surgery are configured to accommodate the anatomical structure of the patient as by configuring the cross sectional area in an elliptical shape, or by forming a funnel configuration with the wider end at the proximate end. In some embodiments the distal end is contoured to also accommodate the anatomical structure of the patient so that a cylindrically shaped, funnel shaped, ovoid shaped dilator retractor can be sloped or tunneled to accommodate the bone structure of the patient or provide access for implants. The dilator retractor is made with different lengths to accommodate the depth of the cavity formed by the dilators.
Owner:DEPUY SYNTHES PROD INC
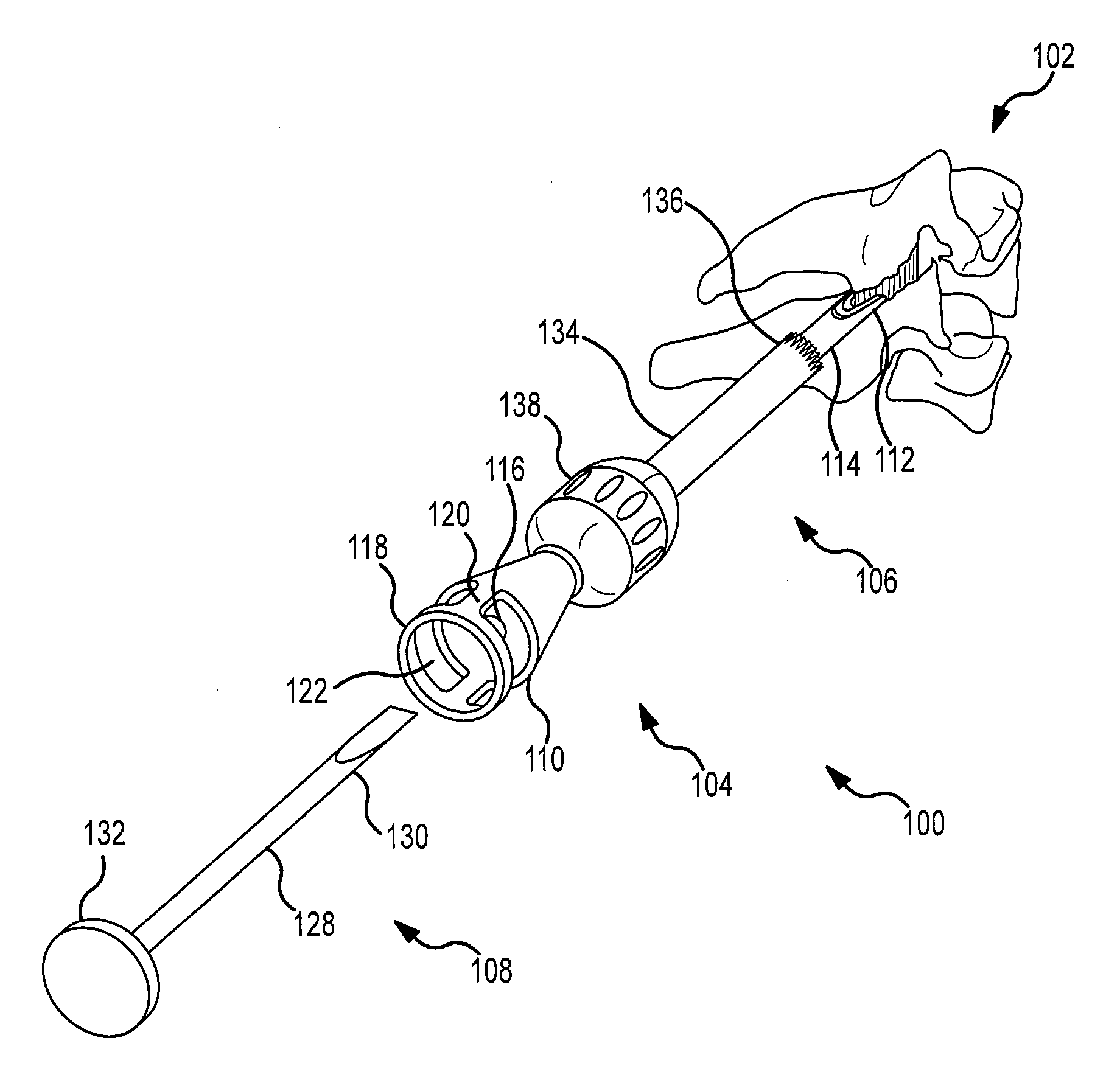
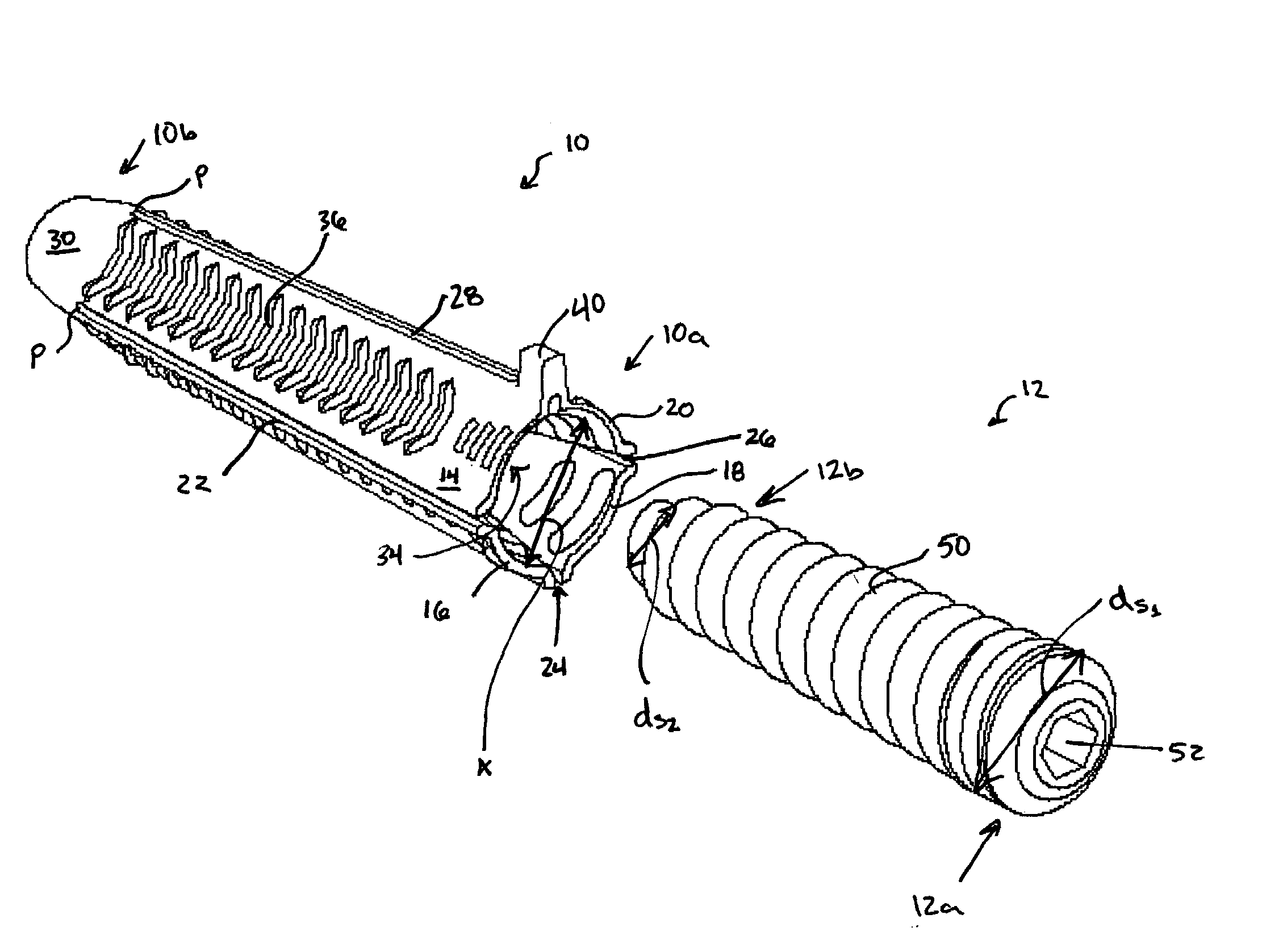
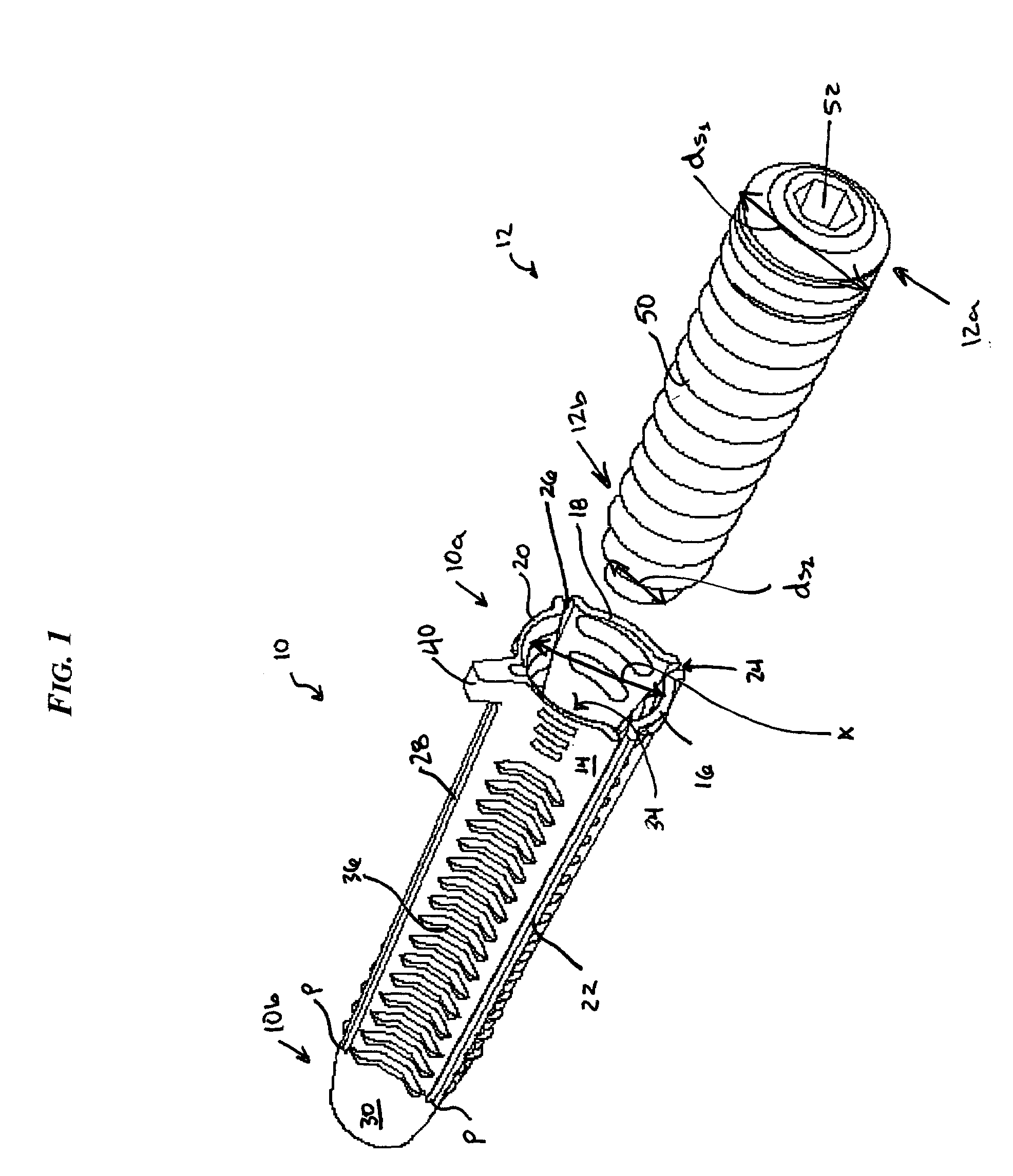
Facet joint implants and delivery tools
A spinal joint distraction system is disclosed and may include a delivery device, a driver assembly, and an internal actuator, where the driver assembly is adapted to be hold an implant and be sleevably inserted into the delivery device and the internal actuator is adapted to advance an implant distractor to distract the implant, the system also including an implant, a chisel, an injector, a gripping tool, and a dilator set. Several embodiments of an implant are disclosed as well a method of placing an implant.
Owner:PROVIDENCE MEDICAL TECH
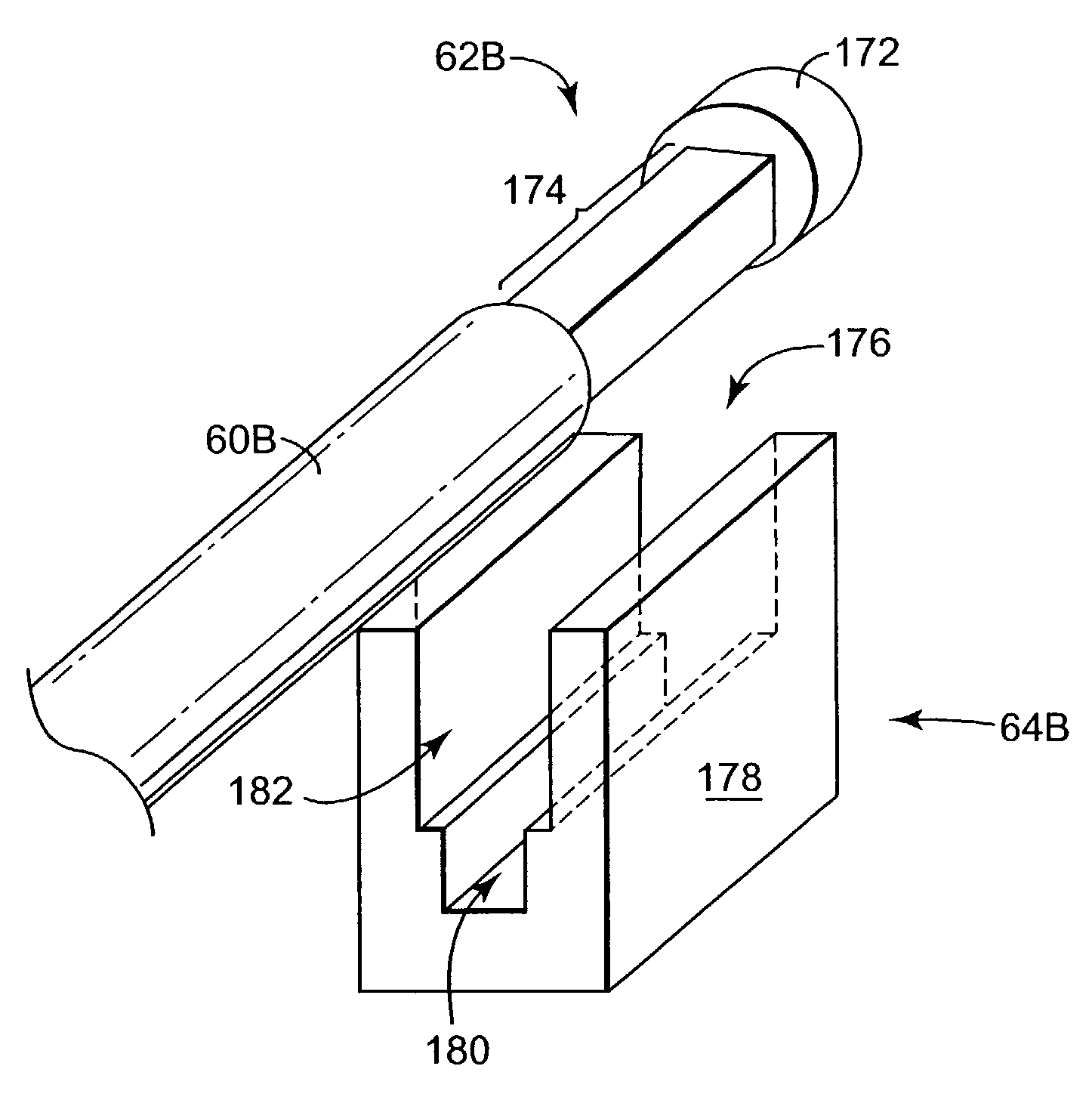
Flexible tibial sheath
ActiveUS7309355B2Prevent over-insertionWeak elasticitySuture equipmentsBone implantBone tunnelDilator
A radially expandable sheath is provided having a substantially closed distal end with at least two sidewalls extending proximally therefrom and defining a central lumen. Each sidewall can have a substantially concave outer surface adapted to seat a graft member, and each side wall is at least partially separated by a longitudinally oriented slot that extends from a proximal end along a substantial length of each sidewall. The slot preferably terminates at a position just proximal to the distal end. The device can also include a sheath expander that is adapted to be disposed in the central lumen of the radially expandable sheath and that is configured to flex the sidewalls to radially expand the sheath so as to fix a graft member within a bone tunnel.
Owner:DEPUY SYNTHES PROD INC
Mechanical embolectomy device and method
An embolectomy device includes a catheter and an elongated shaft positioned in and moveable within the catheter. The shaft has proximal and distal end portions, and a retrieval portion between the proximal and distal end portions. The retrieval portion has a plurality of legs having proximal and distal end portions. Portions of the legs are movable laterally outward from a long axis of the elongated shaft when the retrieval portion is at least partially moved out of the catheter. A retrieval net can be fixed to portions of the legs for engaging a clot or other occlusion within a body canal. An expander can be provided for moving portions of an occlusion toward canal walls to open a lumen. A method for removing clots and other occlusions from body canals is also disclosed.
Owner:RAZACK NASSER
Device for lumbar surgery
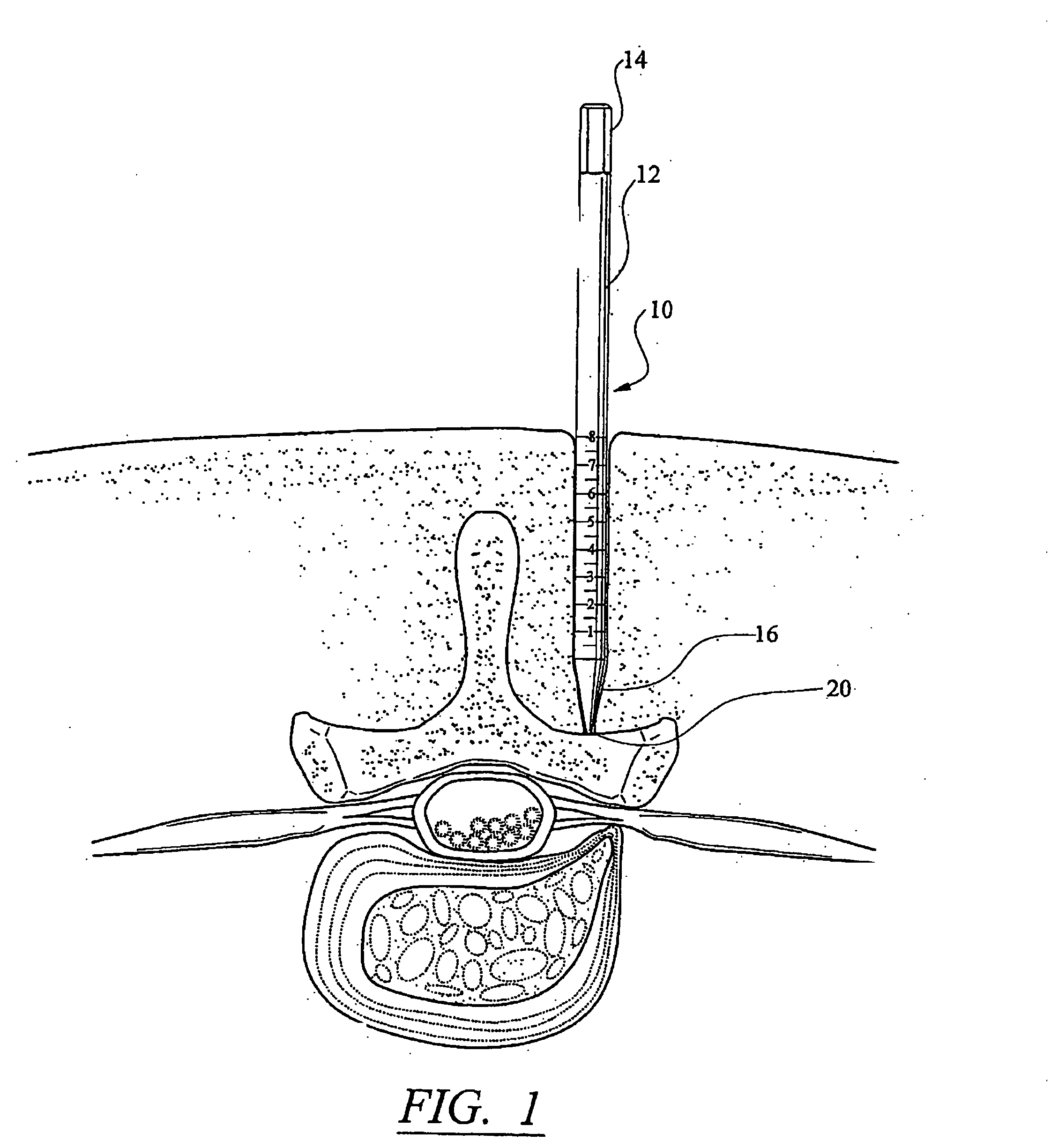
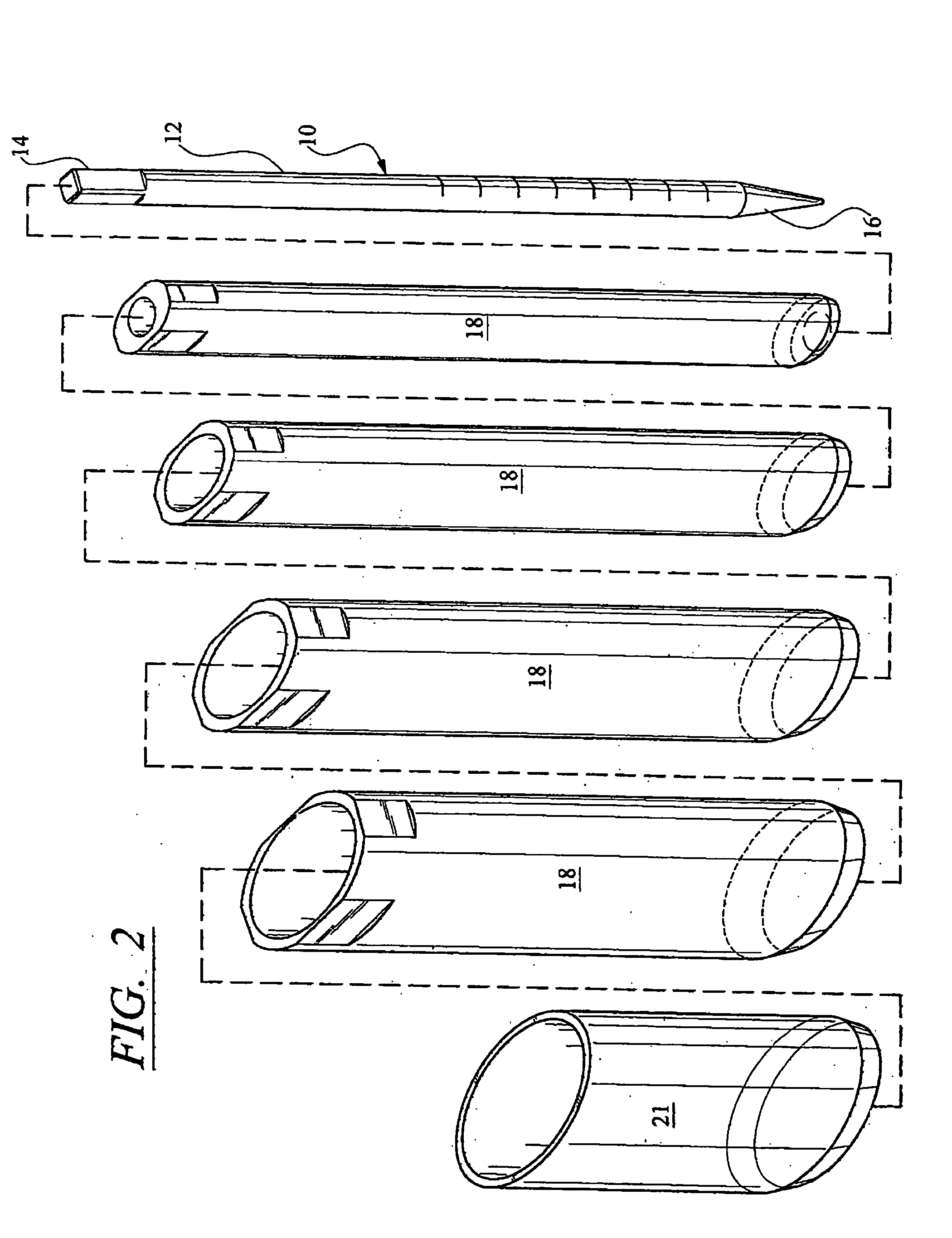
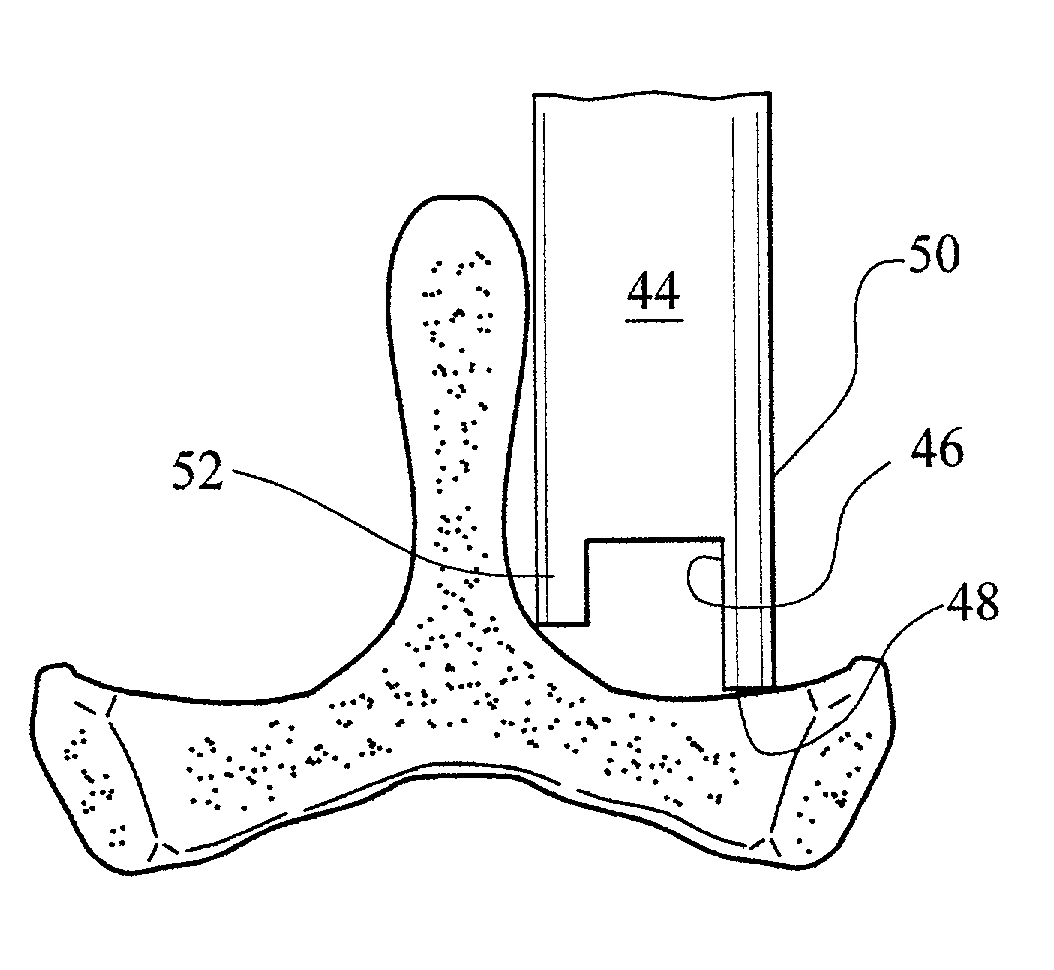
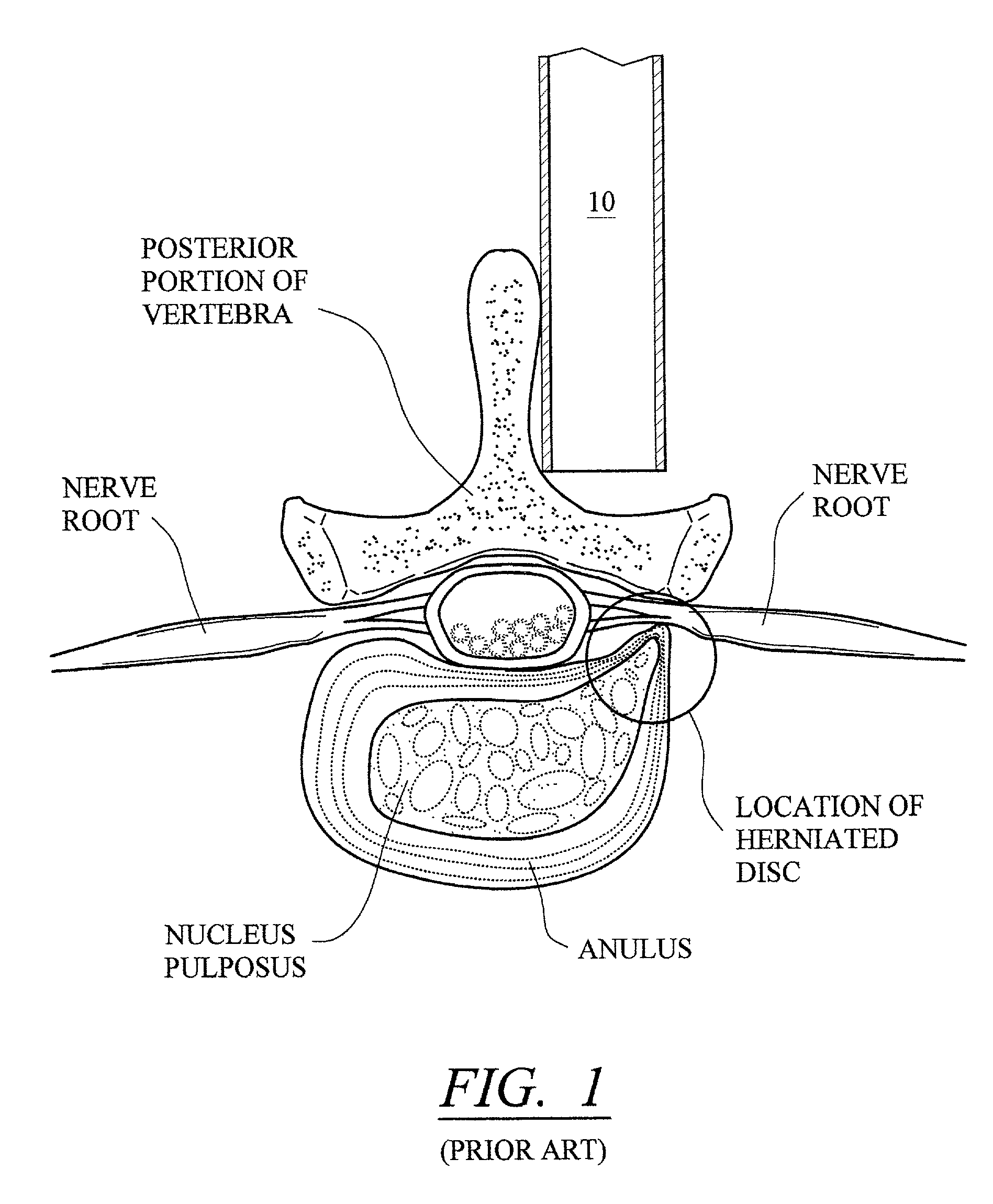
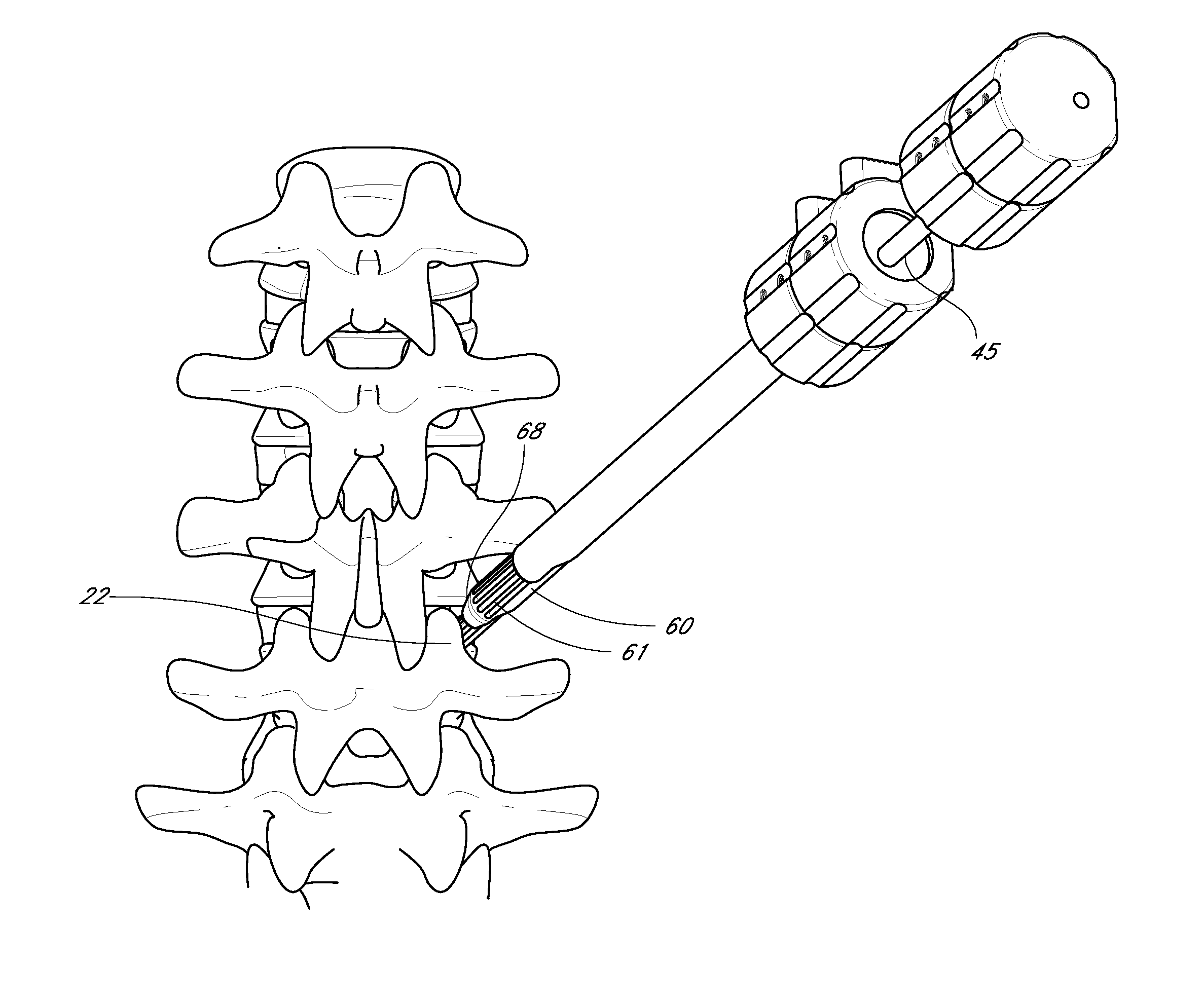
InactiveUS20050171541A1Shorten recovery timeReduces the trauma to the patientInternal osteosythesisBone implantDilatorIntervertebral space
A method for performing percutaneous interbody fusion is disclosed. The method includes the steps of inserting a guide needle posteriorly to the disc space, inserting a dilator having an inner diameter slightly larger than the outer diameter of the guide needle over the guide needle to the disc space to enlarge the disc space, and successively passing a series of dilators, each having an inner diameter slightly larger than the outer diameter of the previous dilator, over the previous dilator to the disc space the gradually and incrementally increase the height of the disc space. Once the desired disc height is achieved, the guide needle and all the dilators, with the exception of the outermost dilator, are removed. An expandible intervertebral disc spacer is then passed through the remaining dilator and positioned in the disc space. The disc spacer is expanded to the required disc height, and then a bone matrix is passed through the dilator to fill the disc space. The dilator is then removed. An expandible intervertebral disc spacer is also disclosed, having a tapered bore that causes greater expansion of one end of the spacer with respect to the other. A kit for performing the percutaneous interbody fusion procedure is also disclosed.
Owner:BOEHM FR H JR +1
System and method for establishing vascular access
Systems, kits, and methods for establishing vascular access are described. A system may include a needle, a radially expandable sleeve and a dilator. The methods include creating an initial tissue tract to a target blood vessel with a radially expandable sleeve mounted on a needle. Upon removal of the needle, the dilator is then passed through the radially expandable sleeve to effect radial expansion of the sleeve. Use of the sleeve reduces the risk of injuring tissue surrounding the tissue tract by lessening the axial forces imparted to the tissue. Kits comprise at least the radially expandable sleeve together with instructions for use.
Owner:TYCO HEALTHCARE GRP LP
Use of mechanical dilator devices to enlarge ostia of paranasal sinuses and other passages in the ear, nose, throat and paranasal sinuses
Sinusitis and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches with flexible or rigid instruments. Various methods and devices are used for remodeling or changing the shape, size or configuration of a sinus ostium or duct or other anatomical structure in the ear, nose or throat; implanting a device, cells or tissues; removing matter from the ear, nose or throat; delivering diagnostic or therapeutic substances or performing other diagnostic or therapeutic procedures. Introducing devices (e.g., guide catheters, tubes, guidewires, elongate probes, other elongate members) may be used to facilitate insertion of working devices (e.g. catheters e.g. balloon catheters, guidewires, tissue cutting or remodeling devices, devices for implanting elements like stents, electrosurgical devices, energy emitting devices, devices for delivering diagnostic or therapeutic agents, substance delivery implants, scopes etc.) into the paranasal sinuses or other structures in the ear, nose or throat. Specific devices (e.g., tubular guides, guidewires, balloon catheters, tubular sheaths) are provided as are methods for manufacturing and using such devices to treat disorders of the ear, nose or throat.
Owner:ACCLARENT INC
Combination sheath and catheter for cardiovascular use
InactiveUS6245045B1Easy and inexpensive to manufactureGuide needlesInfusion syringesVascular diseaseDilator
A vascular interventional device may be introduced over a guidewire into a vessel of the cardiovascular system of a patient. This device includes a hollow, flexible tube having a proximal end and a distal end that is adapted to selectively engage a target vessel of the cardiovascular system of the patient. This tube also includes a lumen that is continuous from the proximal to the distal end, and has an end hole in the distal end that is in fluid communication with the lumen. A plurality of side holes are provided near the distal end of the tube, each of which is in continuous fluid communication with the lumen. The device also includes a hollow vessel dilator that is adapted for insertion into and through the tube and over the guidewire. The dilator has an inside diameter that is slightly larger than the guidewire and an outside diameter that is slightly smaller than the diameter of the lumen of the tube. The distal end of the dilator is adapted to accommodate vascular entry over the guidewire, and the dilator is adapted to dilate the vessel to accept the tube. The device also includes a hub at the proximal end of the tube. The hub includes an end port through which a second interventional device having an outside diameter smaller than the diameter of the lumen may be introduced into the lumen of the tube. The hub also includes a side port through which a fluid agent may be injected for delivery through the lumen and out the end hole and side holes of the tube. A sealing mechanism is also provided in the hub to prevent air from entering the tube and blood and other fluids from leaking out of the tube through the hub. A pair of vascular interventional devices, at least one of which is constructed according to the invention, may be utilized to treat or study a cardiovascular condition, or to measure the blood pressure across a vascular segment.
Owner:STRATIENKO ALEXANDER ANDREW
Method and apparatus for endoscopic spinal surgery
A method of performing percutaneous transforaminal endoscopic lumbar surgery on a patient, includes the steps of creating an opening in the patient's skin, passing at least one tubular cannula through the opening so as to create a soft tissue tunnel, placing a semi-tubular spreader over the at least one tubular cannula inside the soft tissue tunnel, placing a flat blade spreader into an opening formed by the semi-tubular spreader, dilating the opening by spreading apart blades of the flat blade spreader, inserting bone grafts through the opening and into an intervertebral space of the patient.
Owner:TSOU PAUL M
Method and apparatus for minimally invasive insertion of intervertebral implants
ActiveUS8518087B2Convenient introductionImprove protectionCannulasSurgical needlesIntervertebral discDilator
Owner:DEPUY SYNTHES PROD INC
Method and apparatus for minimally invasive insertion of intervertebral implants
ActiveUS8394129B2Convenient introductionImprove protectionCannulasSurgical needlesIntervertebral discDilator
A dilation introducer for orthopedic surgery is provided for minimally invasive access for insertion of an intervertebral implant. The dilation introducer may be used to provide an access position through Kambin's triangle from a posterolateral approach. A first dilator tube with a first longitudinal axis is provided. A second dilator tube may be introduced over the first, advanced along a second longitudinal axis parallel to but offset from the first. A third dilator tube may be introduced over the second, advanced along a third longitudinal axis parallel to but offset from both the first and the second. An access cannula may be introduced over the third dilator tube. With the first, second, and third dilator tubes removed, surgical instruments may pass through the access cannula to operate on an intervertebral disc and / or insert an intervertebral implant.
Owner:DEPUY SYNTHES PROD INC
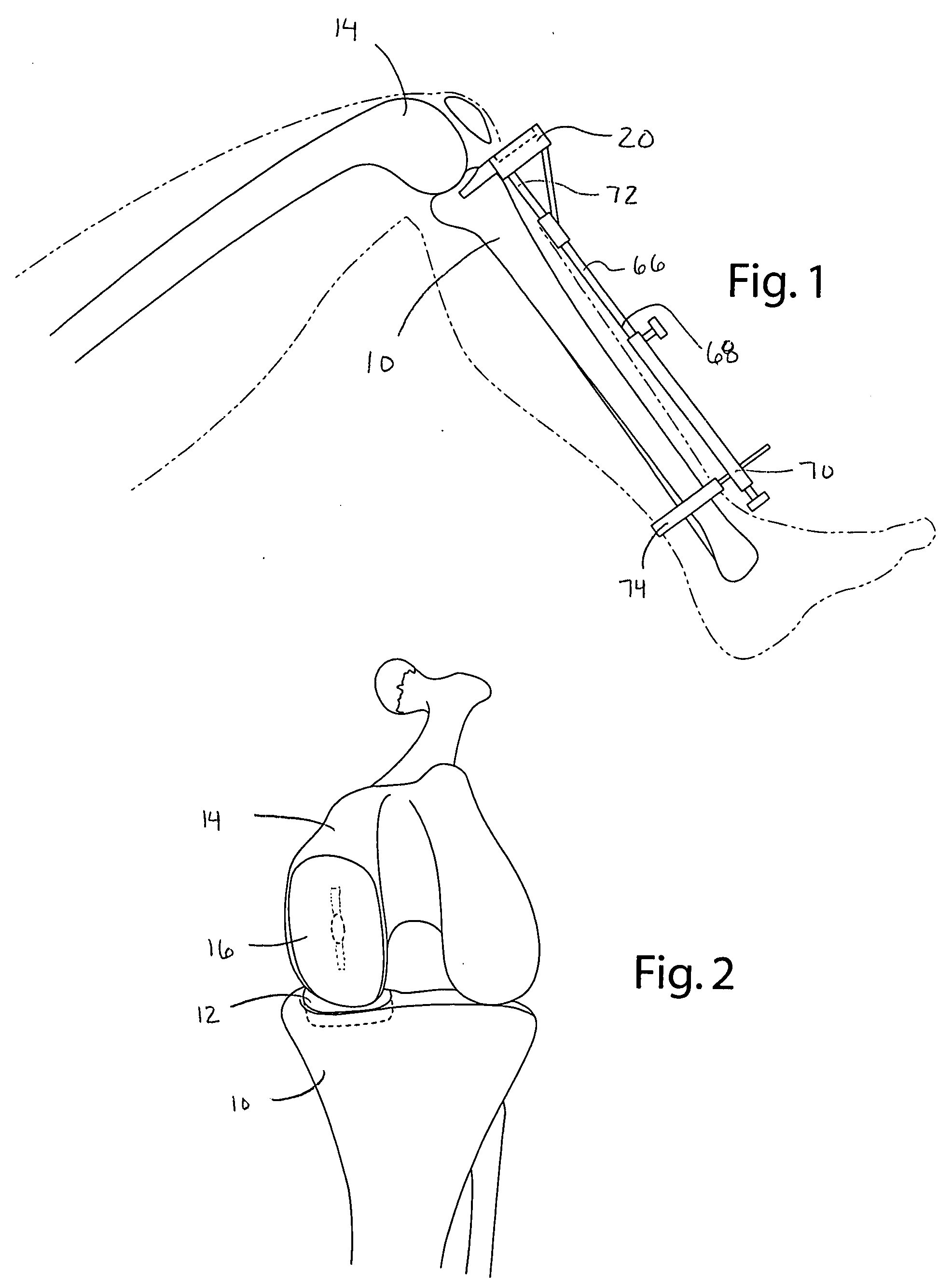
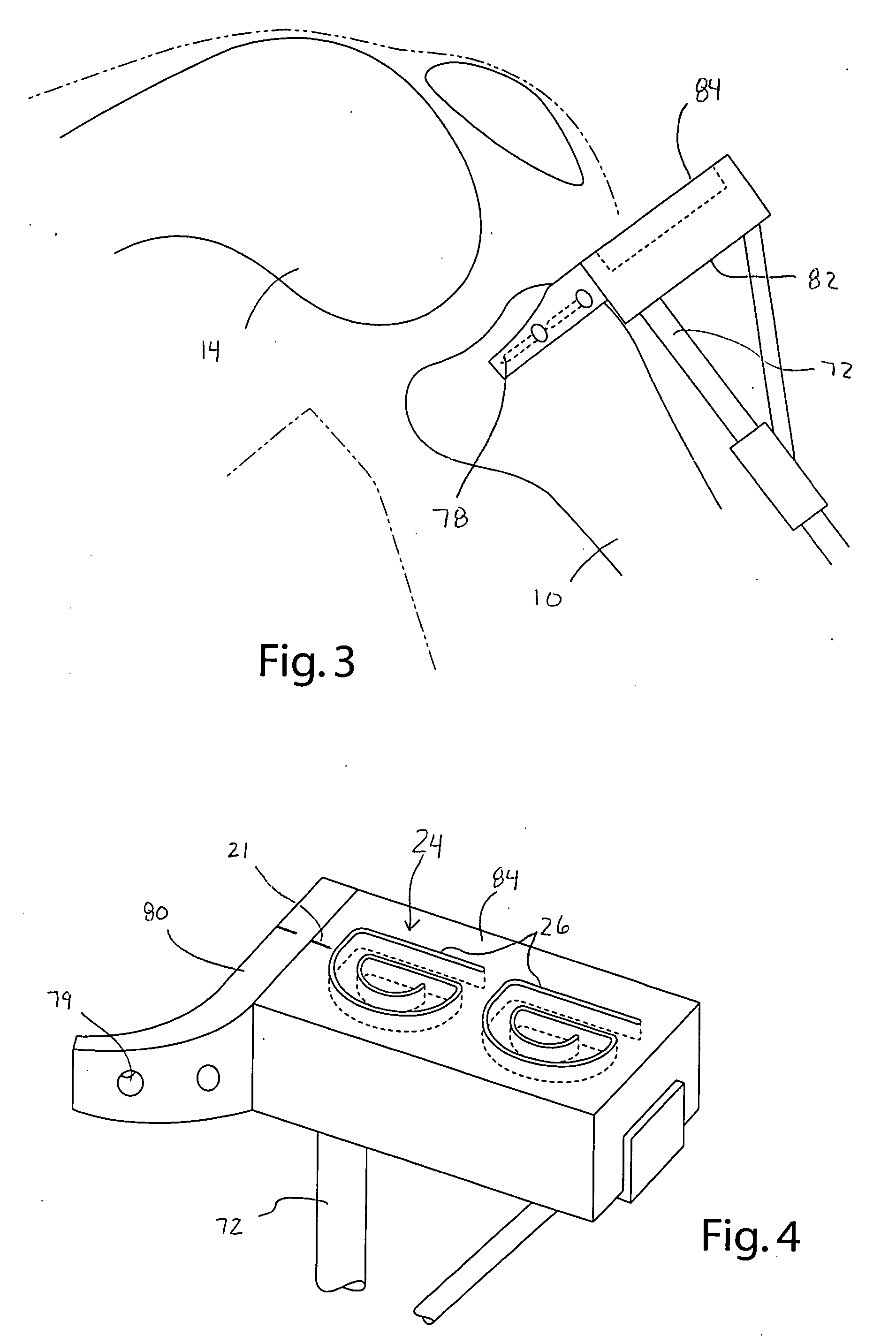
Instrumentation and method for prosthetic knee
InactiveUS20050192588A1Shorten study timeImprove usabilityDiagnosticsSurgical sawsTibial boneFemoral bone
Instrumentation for guiding a surgeon in performing a unicompartmental knee replacement includes a tibial block having a guide, and a milling tool adapted to engage the guide, such that the surgeon can mill the desired tibial bone bed by directing the milling tool along the guide. Further including a femoral jig having a cutting slot, a guide, and a milling tool adapted to engage the guide, such that a surgeon can cut a portion of the femur through the slot, and mill the femoral bone bed by directing the milling tool about the guide. A femoral trial removal clamp facilitates in removing the femoral trial prosthesis, and a spreader compression clamp aids in compressing the final prostheses as the bone cement cures.
Owner:GARCIA DANIEL X
Sling delivery system and method of use
InactiveUS6971986B2Structural damageReduce the amount requiredSuture equipmentsIncision instrumentsDiseaseSide effect
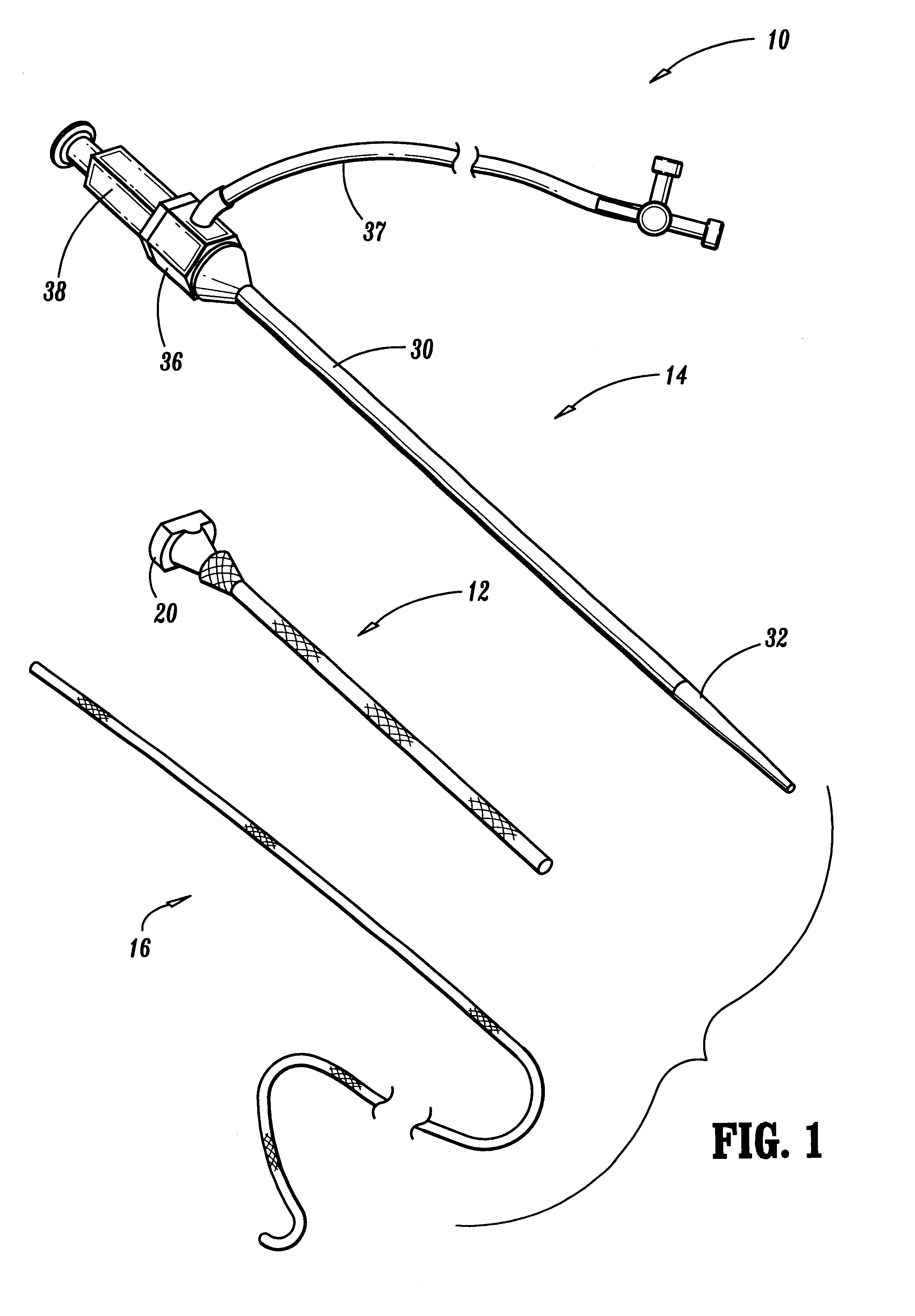
An apparatus and method of use are disclosed to treat urological disorders. The biocompatible device includes a handle, needle, dilator and sling assembly configured to be minimally invasive and provide sufficient support to the target site. In addition, the configuration of the sling assembly also allows the sling to be adjusted during and / or after implantation. The device and treatment procedure are highly effective and produce little to no side effects or complications. Further, operative risks, pain, infections and post operative stays are reduced, thereby improving patient quality of life.
Owner:STASKIN DAVID MD DR
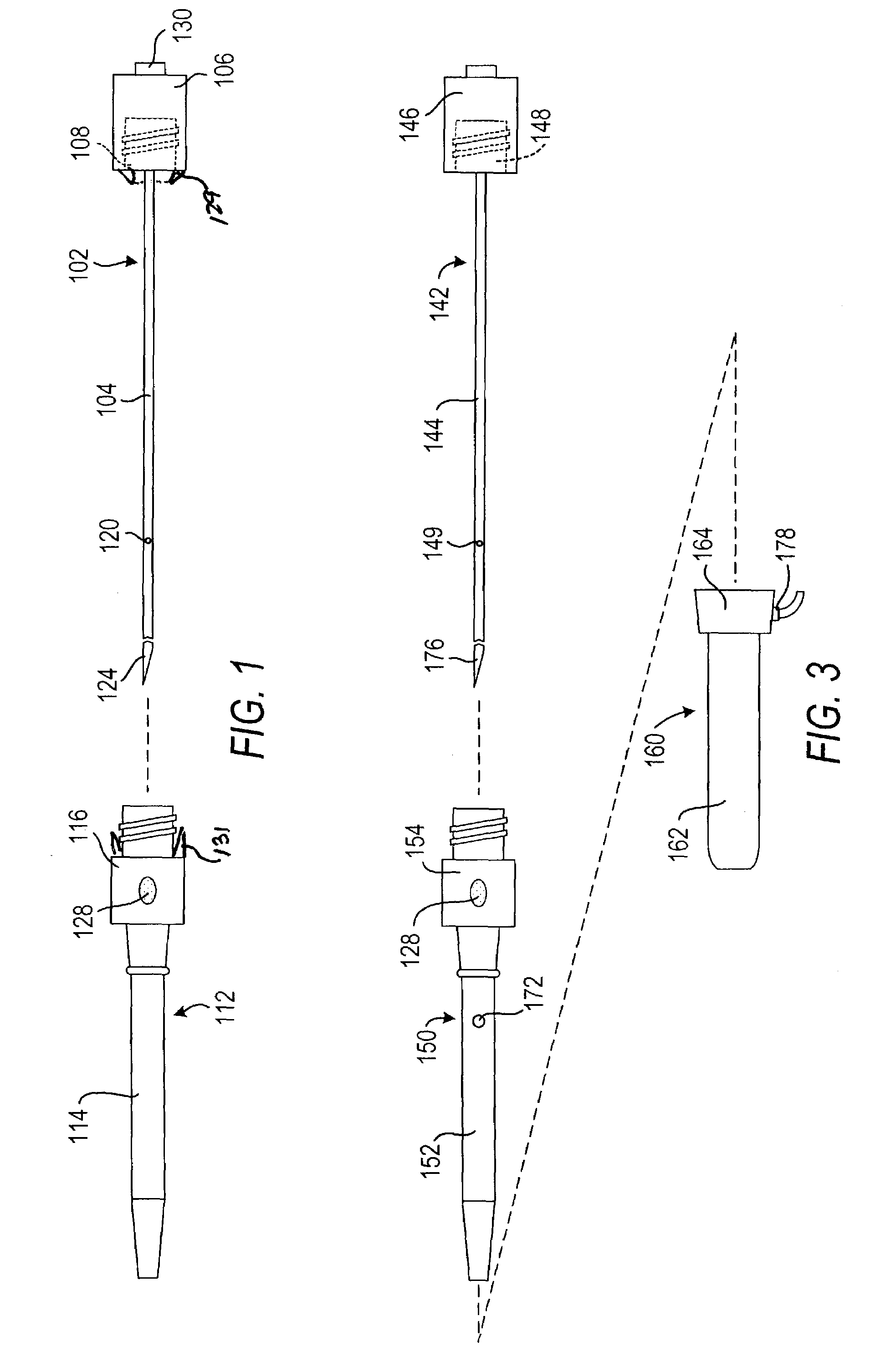
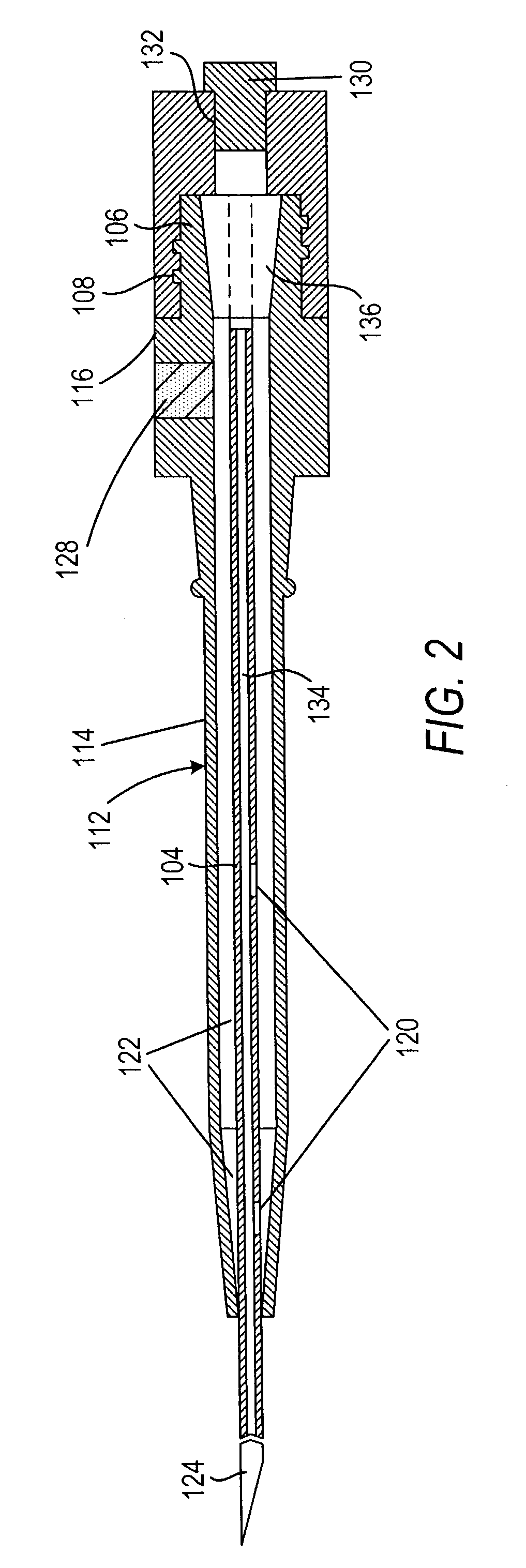
Cardiovascular sheath/catheter
InactiveUS6595959B1Convenient treatmentFacilitate studyGuide needlesInfusion syringesVascular diseaseDilator
A vascular interventional device may be introduced over a guidewire into a vessel of the cardiovascular system of a patient. This device includes a hollow, flexible tube having a proximal end and a distal end that is adapted to selectively engage a target vessel of the cardiovascular system of the patient. This tube also includes a lumen that is continuous from the proximal to the distal end, and has an end hole in the distal end that is in fluid communication with the lumen. The device also includes a hollow vessel dilator that is adapted for insertion into and through the tube and over the guidewire. The dilator has an inside diameter that is slightly larger than the guidewire and an outside diameter that is slightly smaller than the diameter of the lumen of the tube. The distal end of the dilator is adapted to accommodate vascular entry over the guidewire, and the dilator is adapted to dilate the vessel to accept the tube. The device also includes a hub at the proximal end of the tube. The hub includes an end port through which a second interventional device having an outside diameter smaller than the diameter of the lumen may be introduced into the lumen of the tube. The hub also includes a side port through which a fluid agent may be injected for delivery through the lumen and out the end hole of the tube. A sealing mechanism is also provided in the hub to prevent air from entering the tube and blood and other fluids from leaking out of the tube through the hub. A pair of vascular interventional devices, at least one of which is constructed according to the invention, may be utilized to treat or study a cardiovascular condition, or to measure the blood pressure across a vascular segment.
Owner:STRATIENKO ALEXANDER A
Dilation introducer for orthopedic surgery
InactiveUS20050256525A1Lowering chance damageInvasive surgical procedureOrganic active ingredientsGenetic material ingredientsDilatorBone tissue
The dilation introducer has a locked assembled configuration for placement of the dilation introducer against a patient's tissue to be treated, and an unlocked, collapsed configuration for dilating the patient's soft tissue down to tissue to be treated. Dilator tubes are successively released and advanced to progressively expand the patient's soft tissue down to the bone tissue to be treated. The dilator tubes and a guide insert may include spikes for engaging bone tissue. The dilation introducer may include a light emitter disposed in a dilator tube. A telescoping expander sleeve is also provided.
Owner:INTERVENTIONAL SPINE
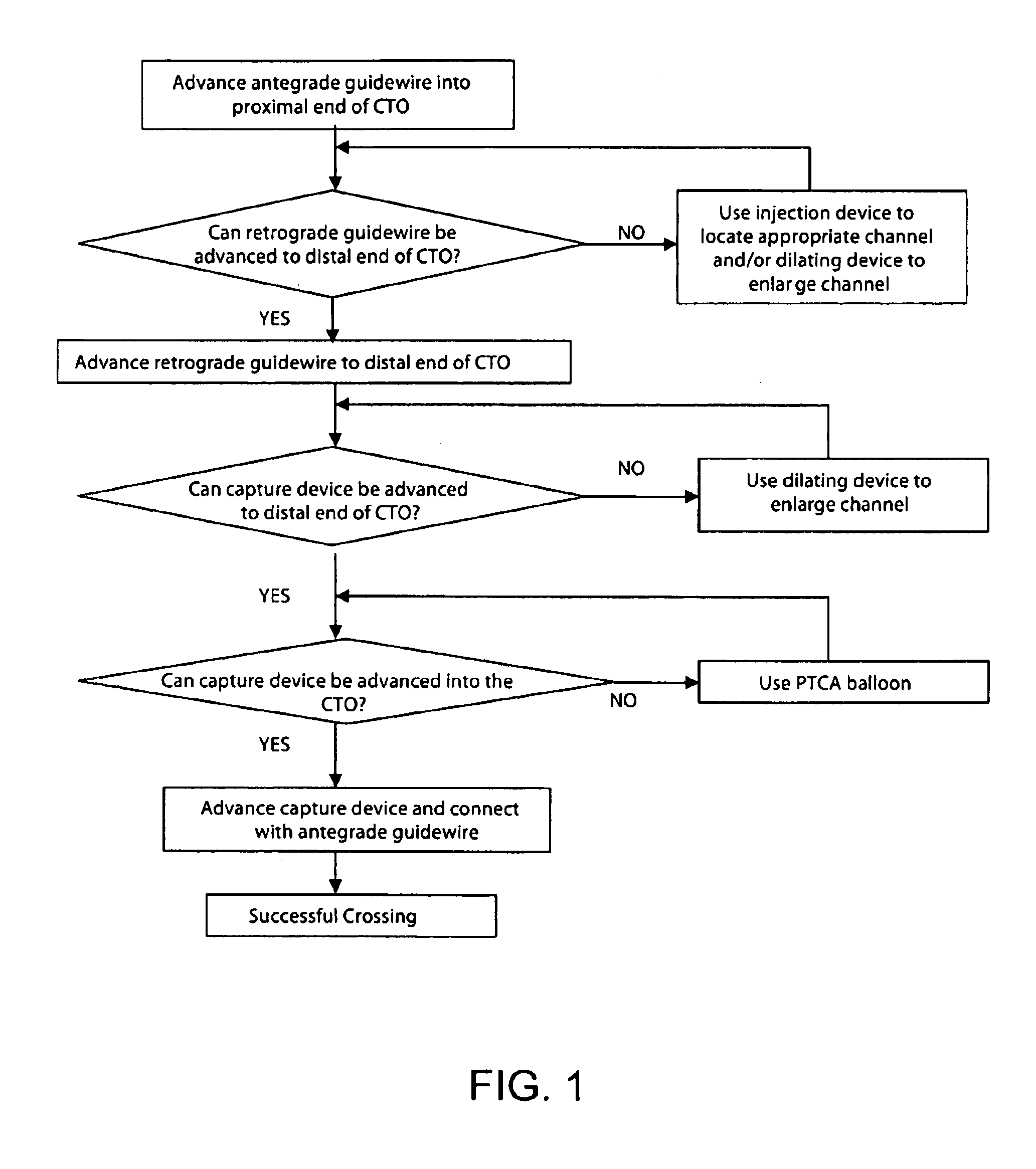
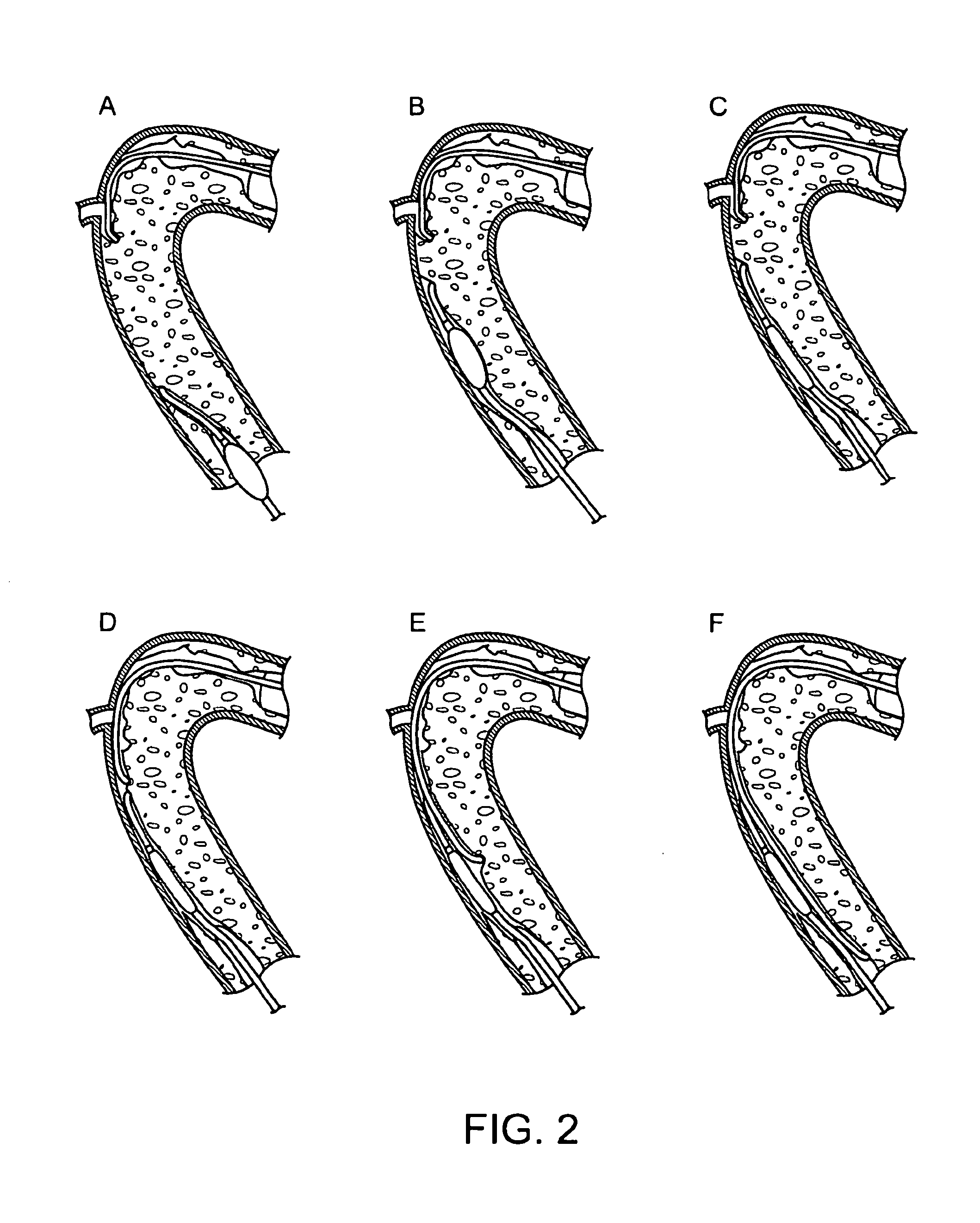
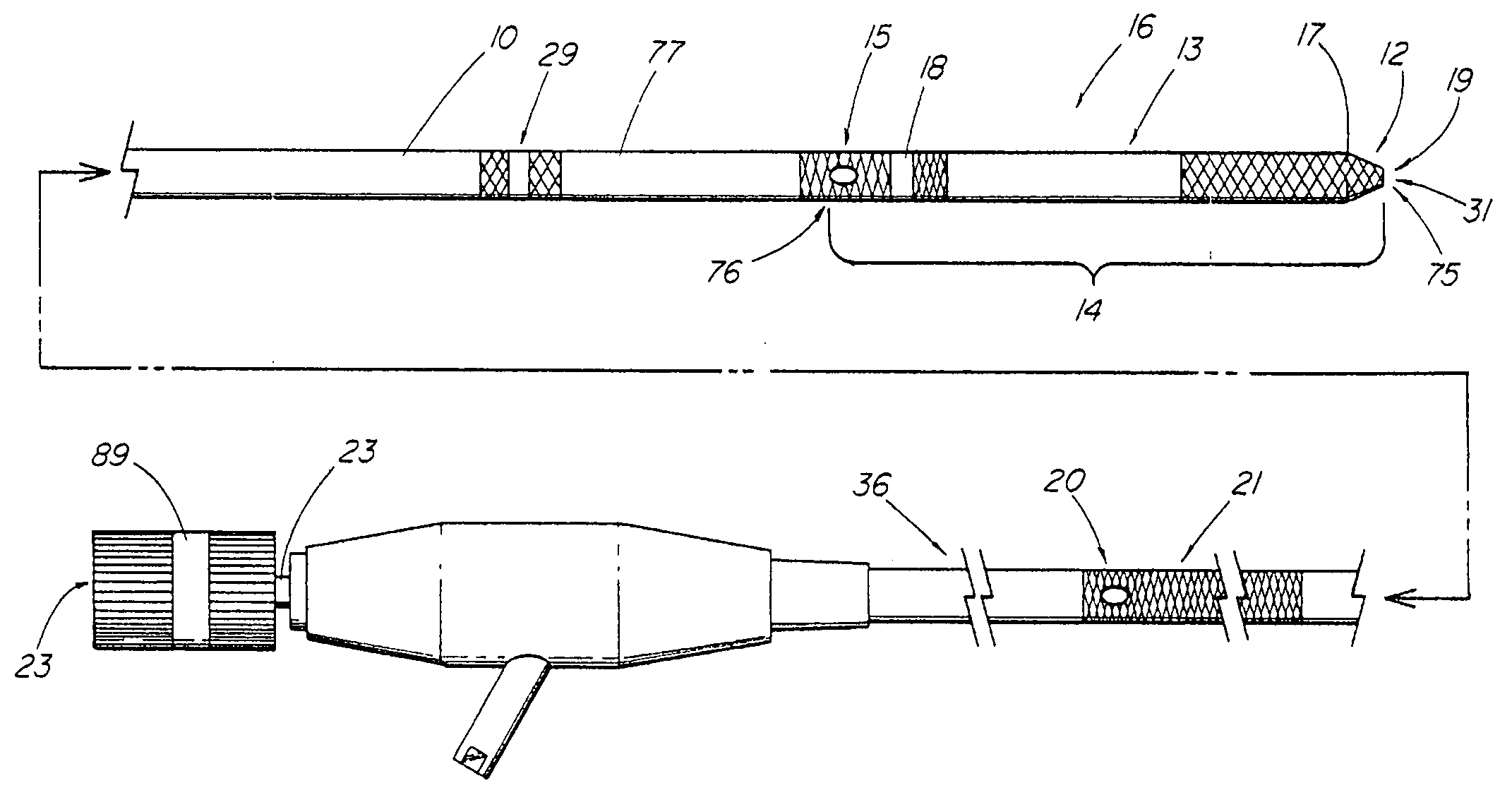
Recanalizing occluded vessels using controlled antegrade and retrograde tracking
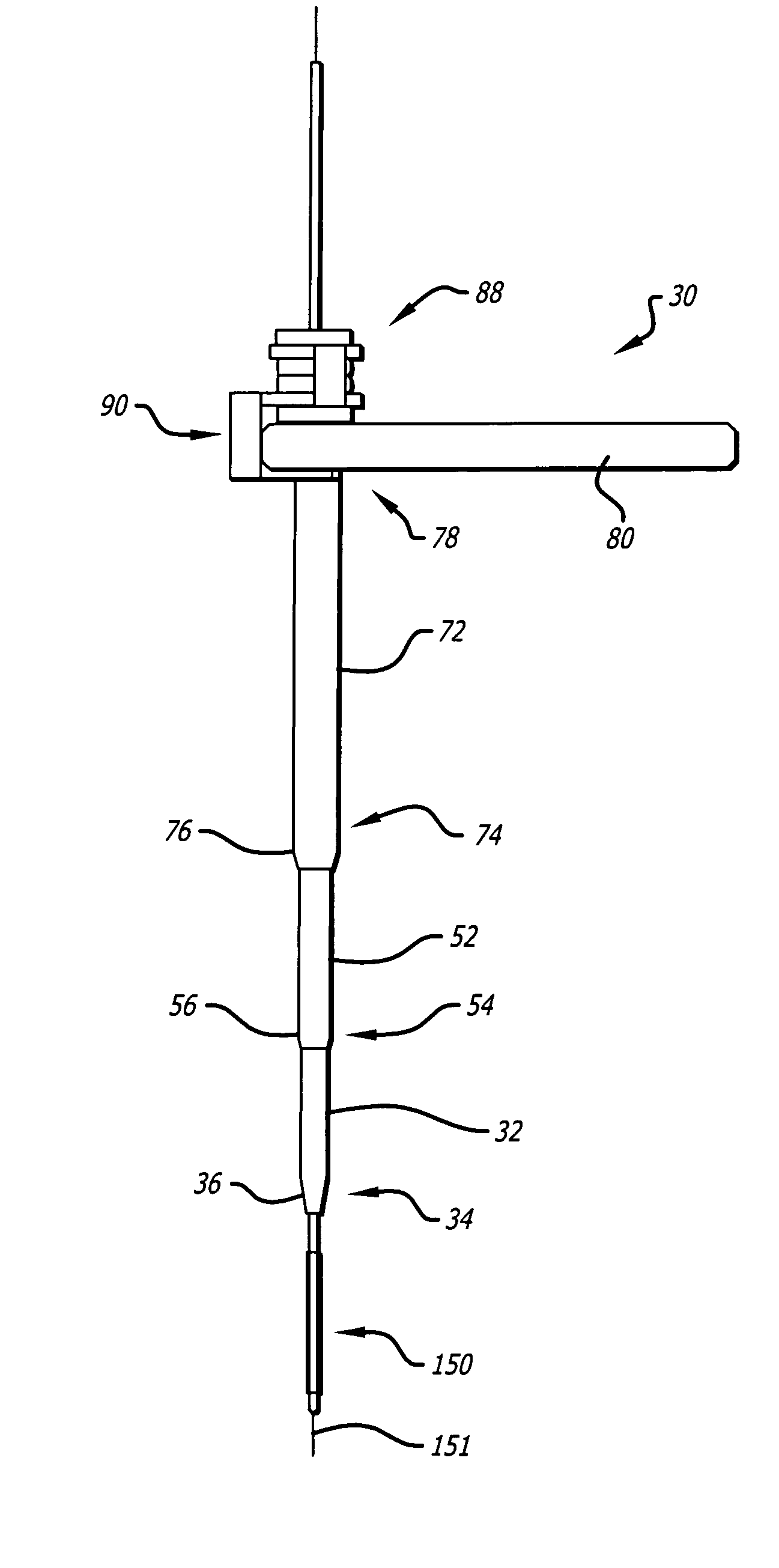
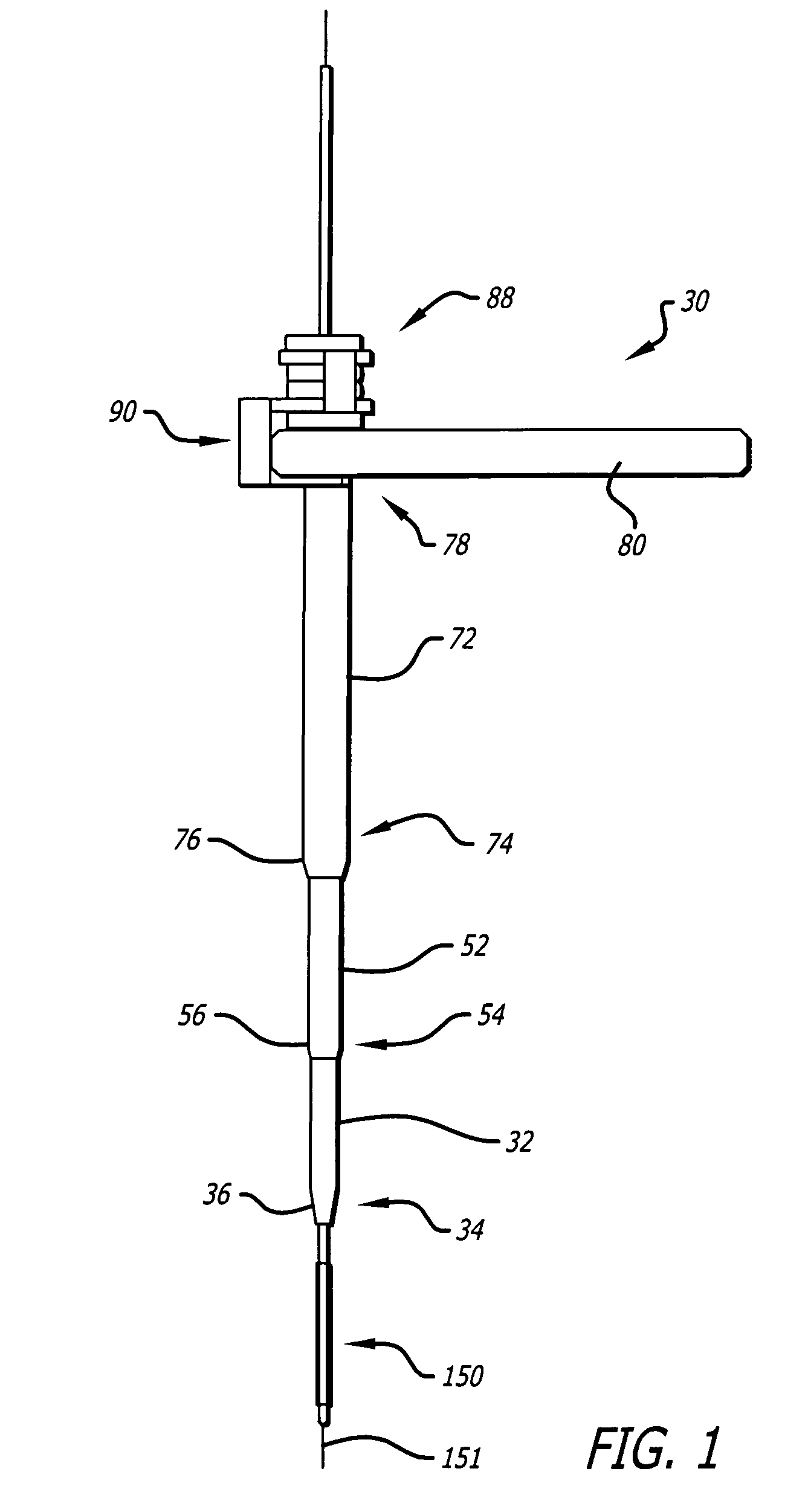
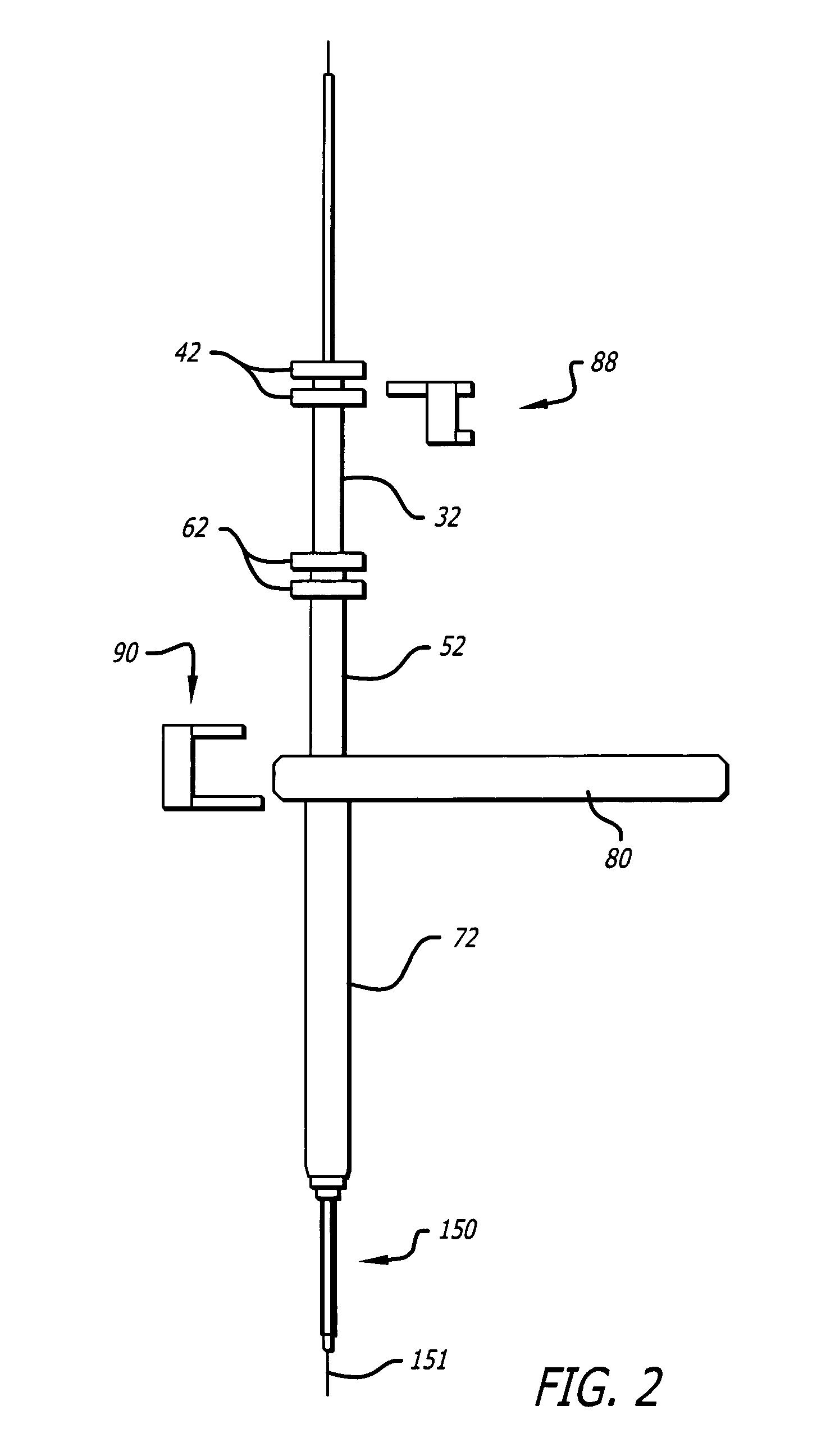
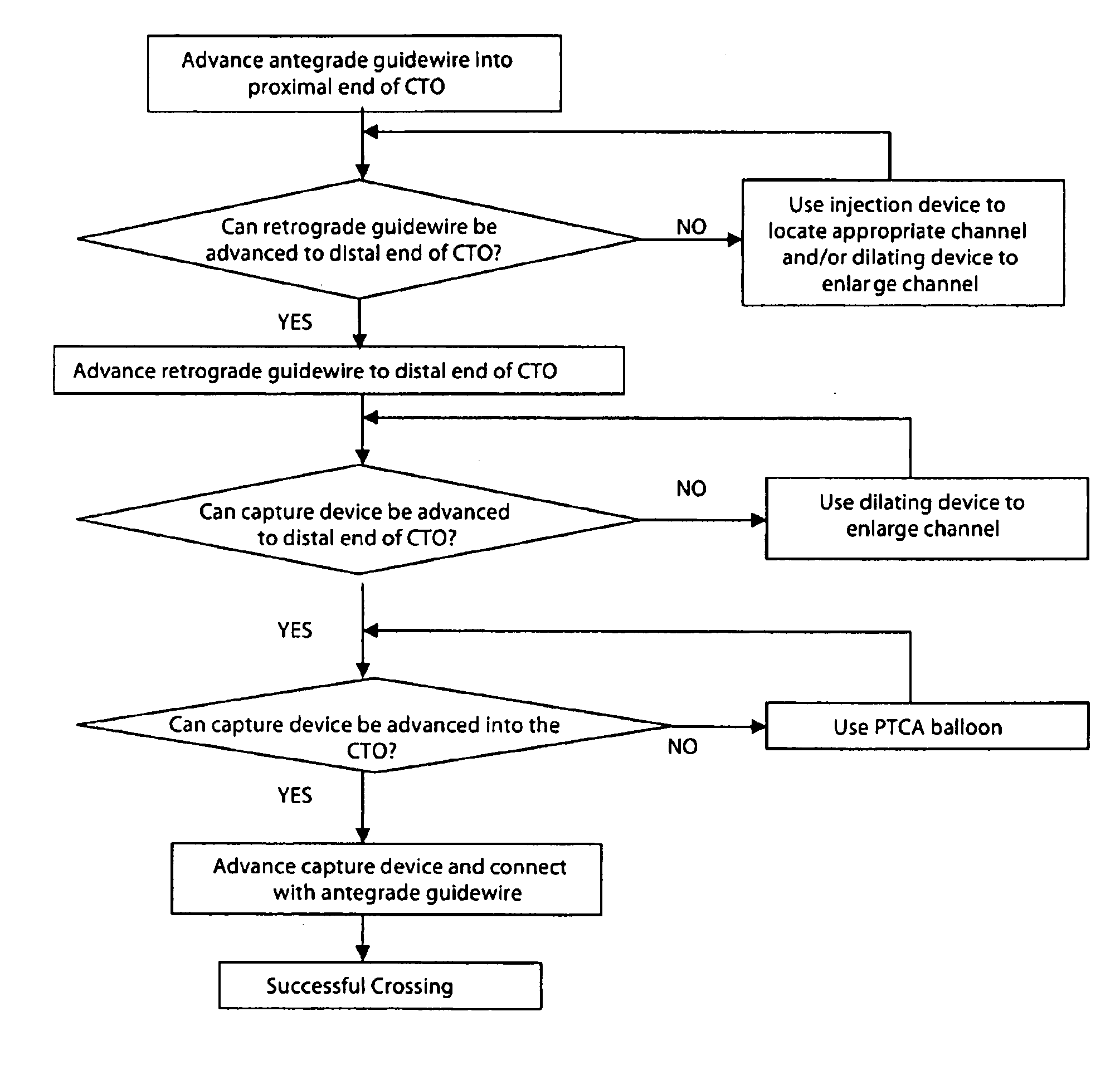
A method and systems for treating chronic total occlusions, particularly those that are difficult to treat, is disclosed. In this approach, recanalizing the CTO is achieved using a combined antegrade and retrograde approach. The proximal end of the occlusion is penetrated using an antegrade wire, using a traditional approach. Using collateral vessels, the distal end of the occlusion is crossed in a retrograde fashion and by appropriately maneuvering each member, a continuous channel is created. Additional elements such as capture devices, dilators and injection catheters are also disclosed.
Owner:RETROVASCULAR
System and method for introducing multiple medical devices
ActiveUS20050059990A1Timely controlSatisfy safety performance requirementsStentsGuide needlesDilatorBiliary tract
A method and apparatus for introducing a first elongate medical device and short wire guide that are coupled together into a work site and remotely disconnecting them within the work site such that a secondary device comprising a catheter member can be introduced over the wire guide to the work site, and / or a second wire guide can be introduced to the work site via a passageway of the primary access device. A separating member may be provided to remotely separate the wire guide from the elongate medical device. A system of indicia, such as radiopaque or viewable markers, permits the operator to monitor the relative alignment of the devices within the work site to determine when uncoupling has occurred. In one example of the method, a wire guide and primary access device (e.g., a sphincterotome) is coupled to the wire guide and introduced via a duodenoscope into the biliary system. After performing a first medical operation, the devices are uncoupled with the wire guide being left within the biliary system such that a secondary access device, such as a balloon, biopsy device, stent delivery catheter, dilator, etc., can be introduced to perform a second medical operation without a traditional over-the-wire exchange being required. In another example of the method, a prosthesis, such as a valve or stent, is placed within the work site coupled to a wire guide which is remotely disconnected within the work site and a secondary device, such as a dilation balloon or second prosthesis, is introduced into the work site after the first delivery system is removed.
Owner:COOK MEDICAL TECH LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com