Patents
Literature
609 results about "Rectum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
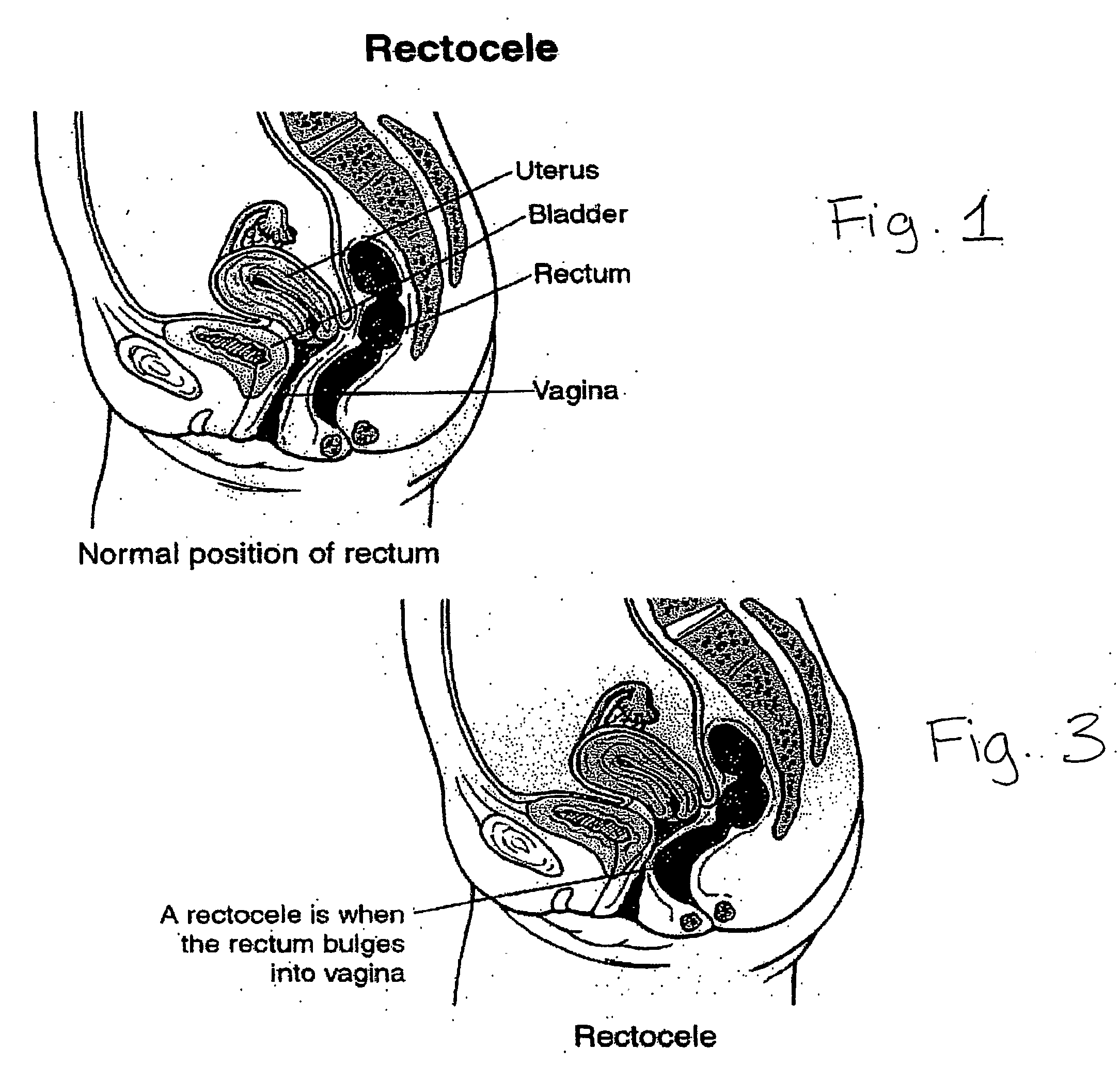
The rectum is the final straight portion of the large intestine in humans and some other mammals, and the gut in others. The adult human rectum is about 12 centimetres (4.7 in) long, and begins at the rectosigmoid junction, the end of the sigmoid colon, at the level of the third sacral vertebra or the sacral promontory depending upon what definition is used. Its caliber is similar to that of the sigmoid colon at its commencement, but it is dilated near its termination, forming the rectal ampulla. It terminates at the level of the anorectal ring (the level of the puborectalis sling) or the dentate line, again depending upon which definition is used. In humans, the rectum is followed by the anal canal which is about 4 centimetres (1.6 in) long, before the gastrointestinal tract terminates at the anal verge. The word rectum comes from the Latin rectum intestinum, meaning straight intestine.
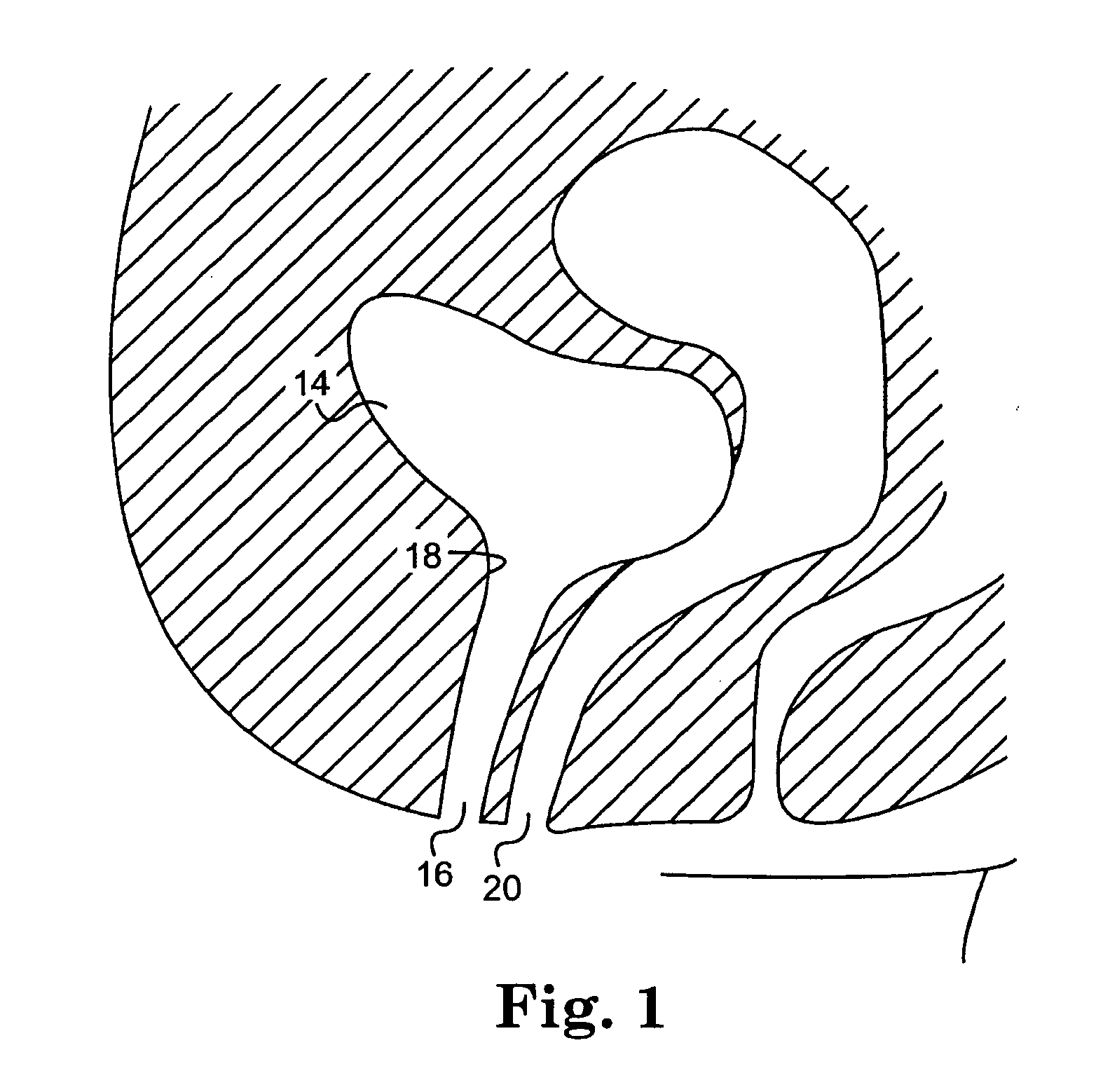
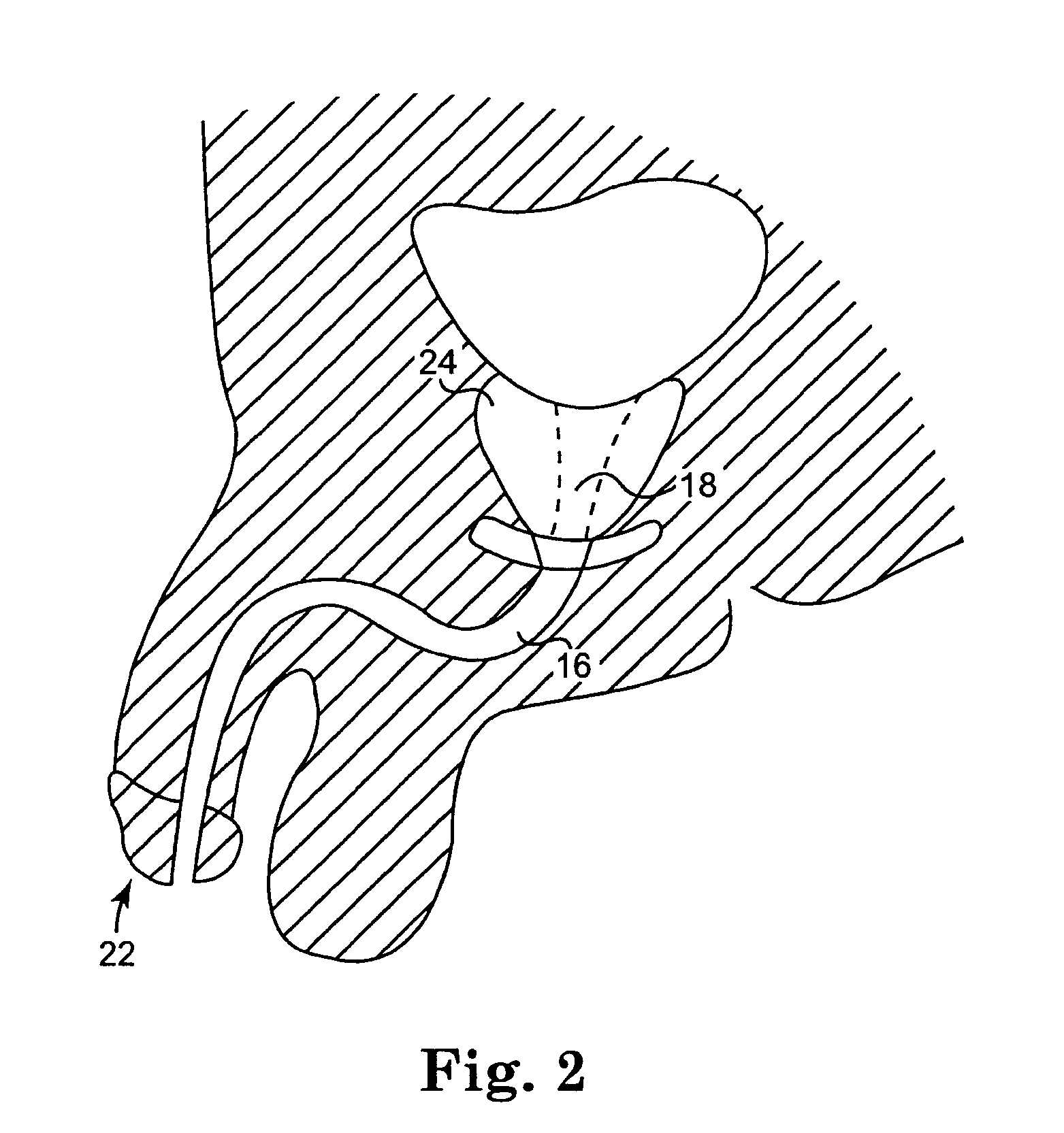
Trans-rectum universal ports
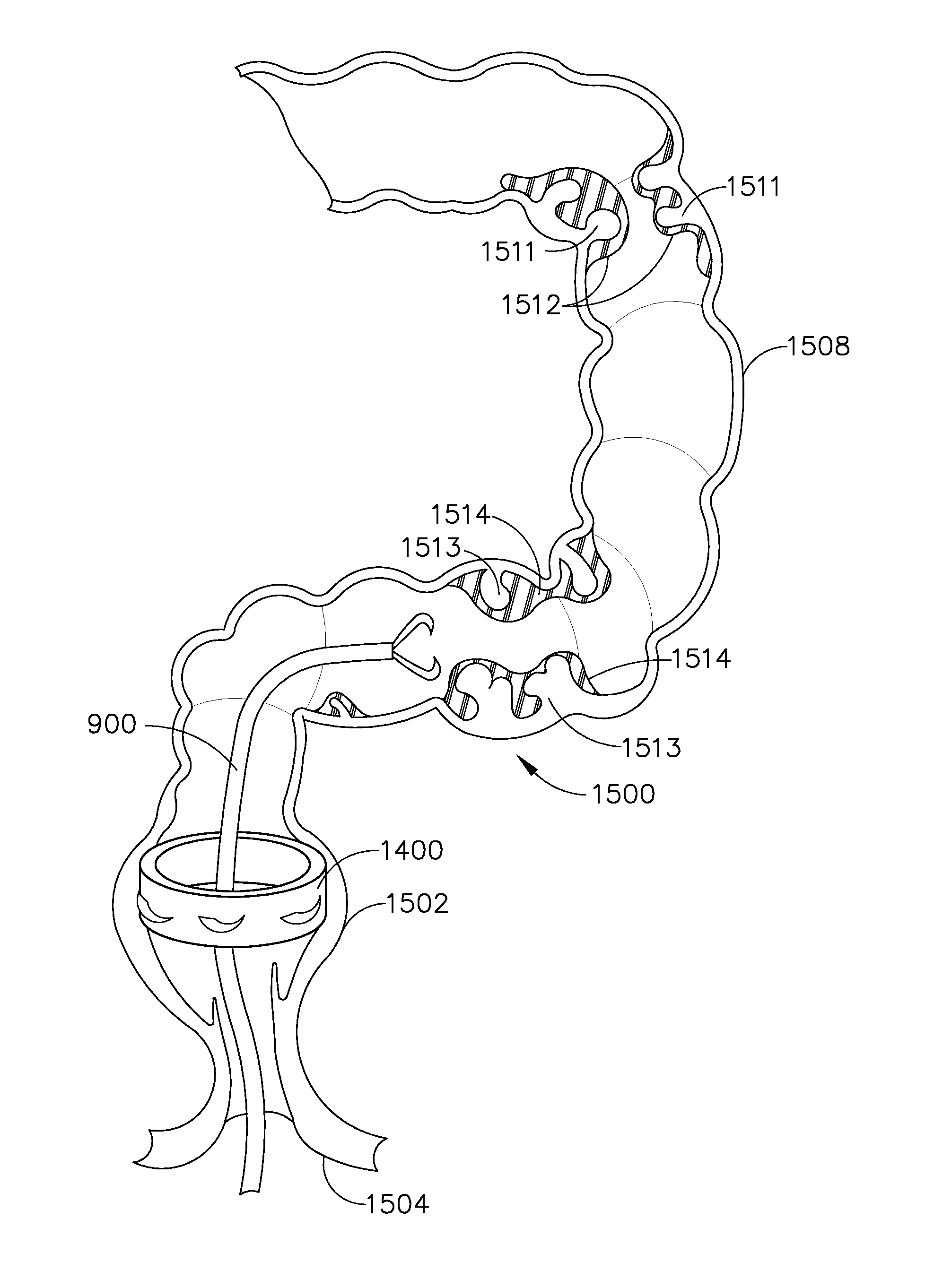
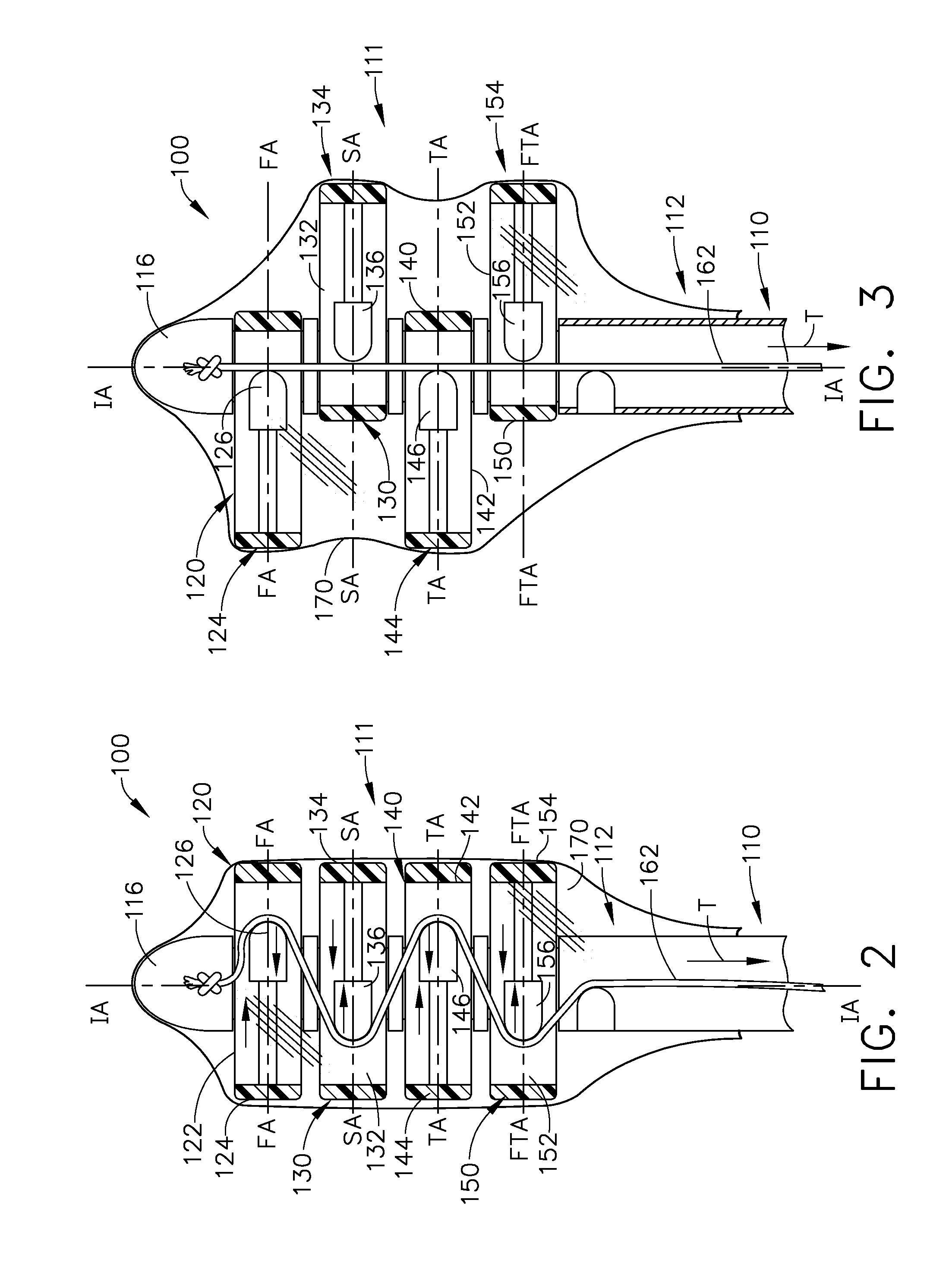
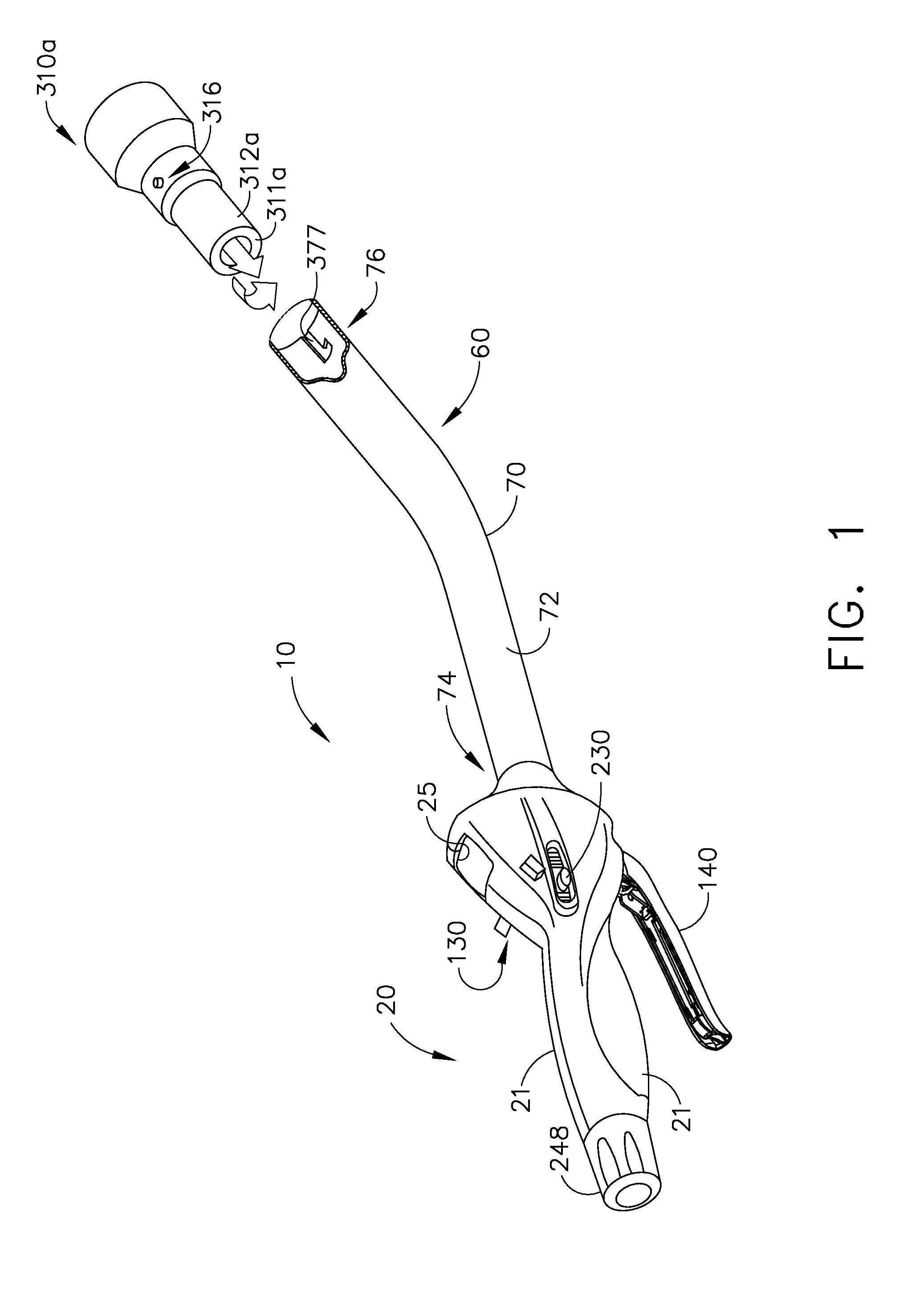
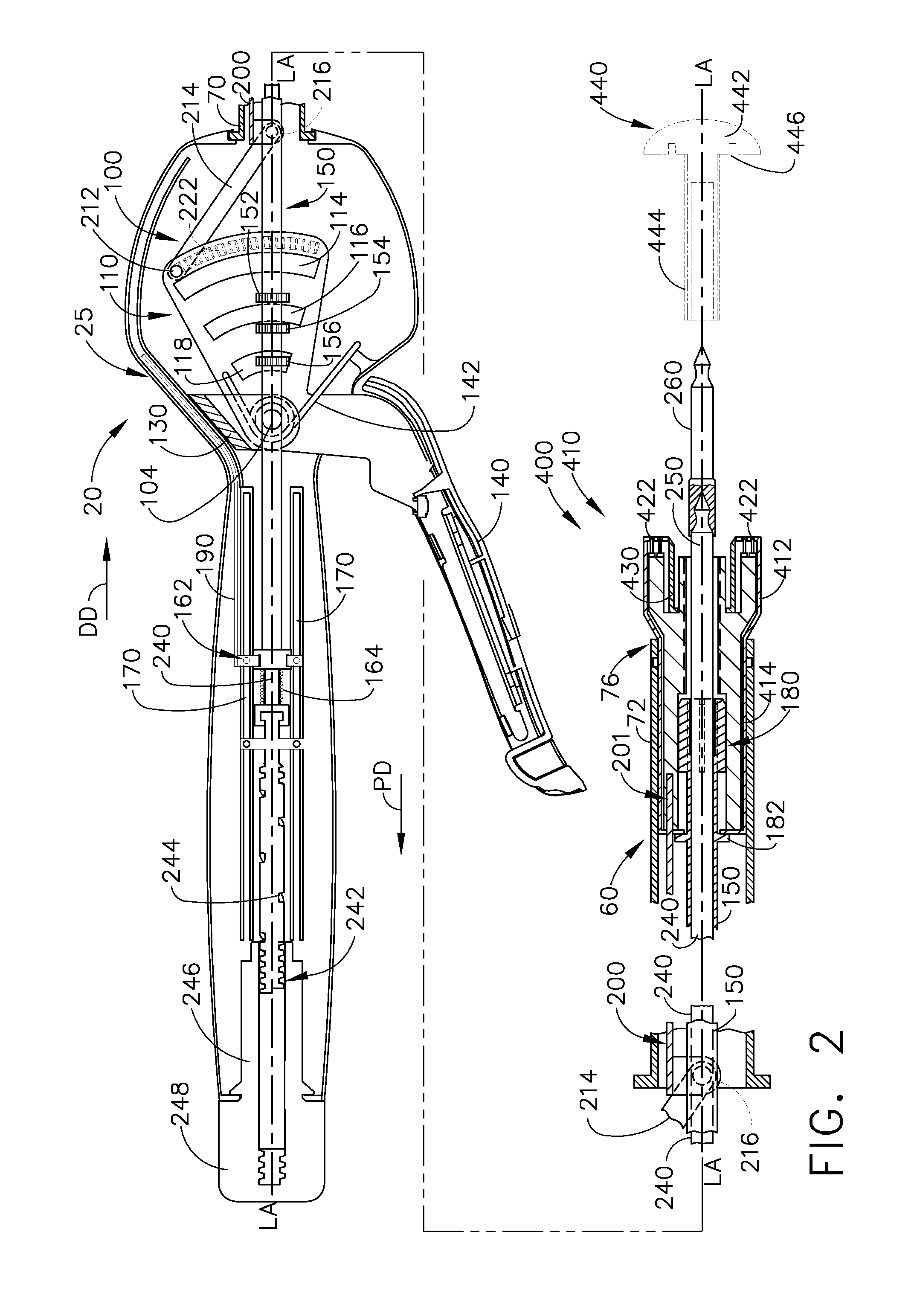
Various universal ports for installation into a colon are disclosed. Various embodiments include expandable port bodies that may employ selectively deployable tissue-engaging barbs for securing the port in position within the colon. Some embodiments also employ tissue cutting members that may be deployed to sever the colon. Some embodiments also employ a flexible sleeve that may extend from the port out through the anus to provide a passageway for passing surgical instruments and into and out of the rectum and also for removing severed specimens therefrom.
Owner:CILAG GMBH INT
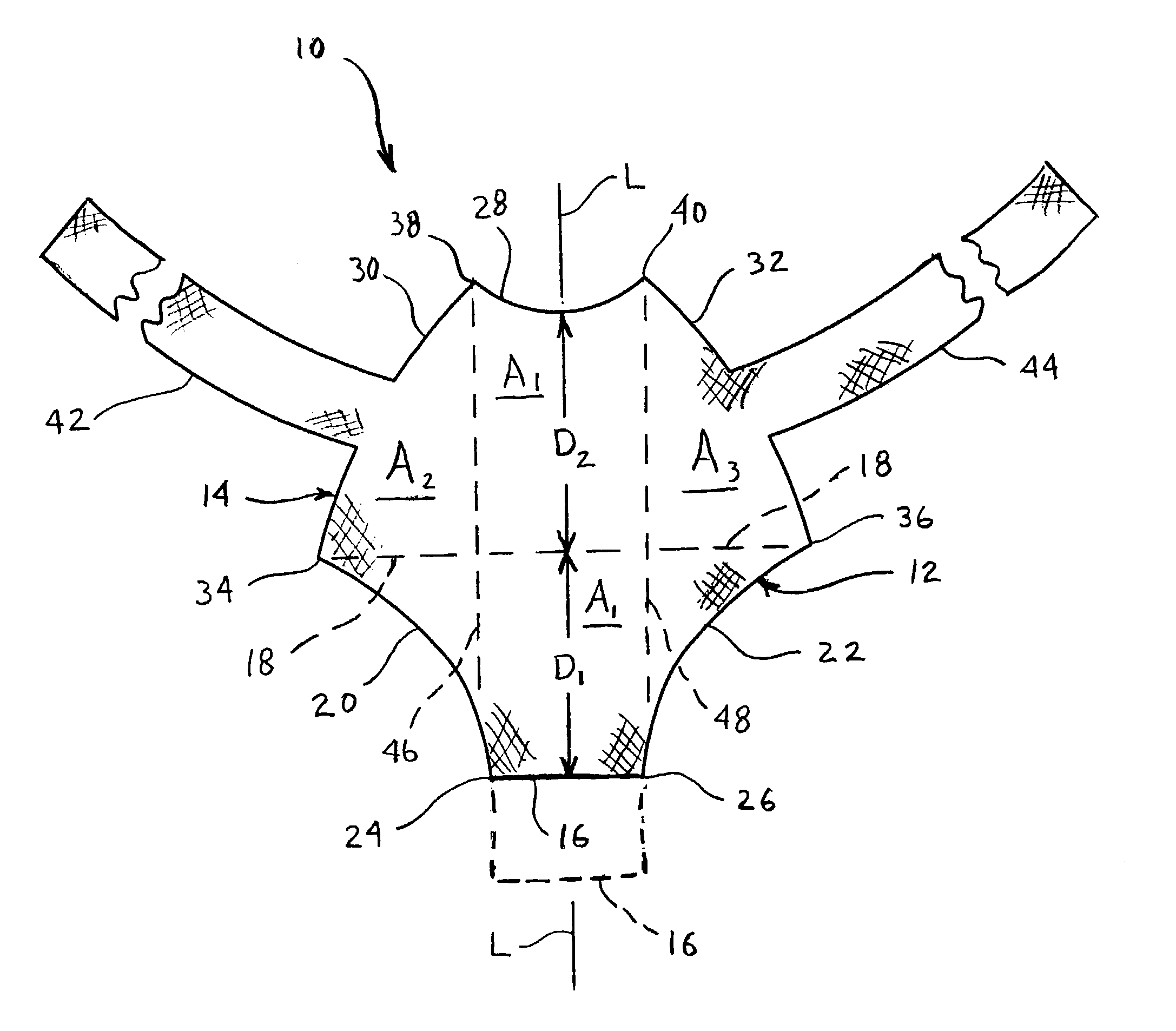
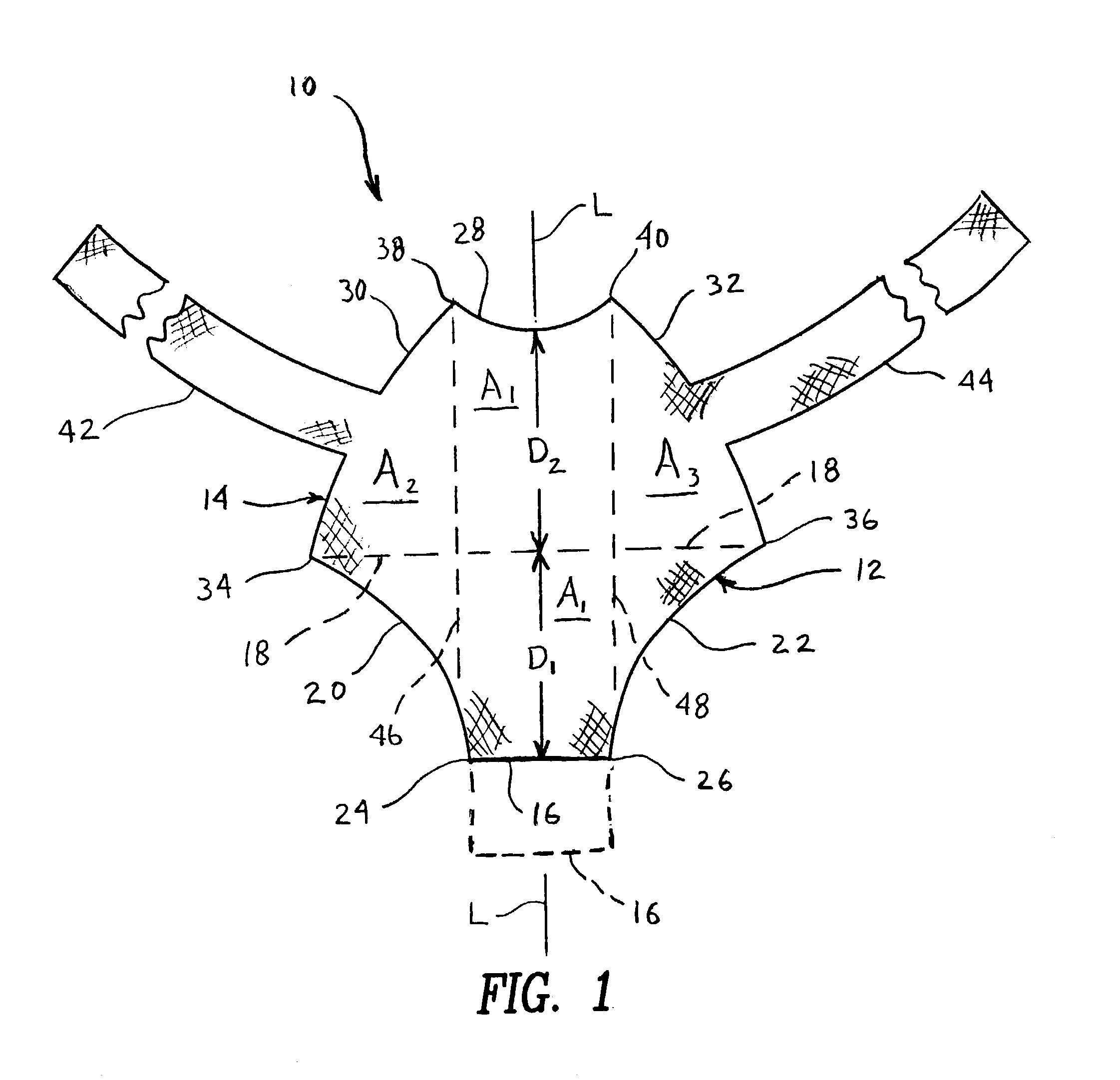
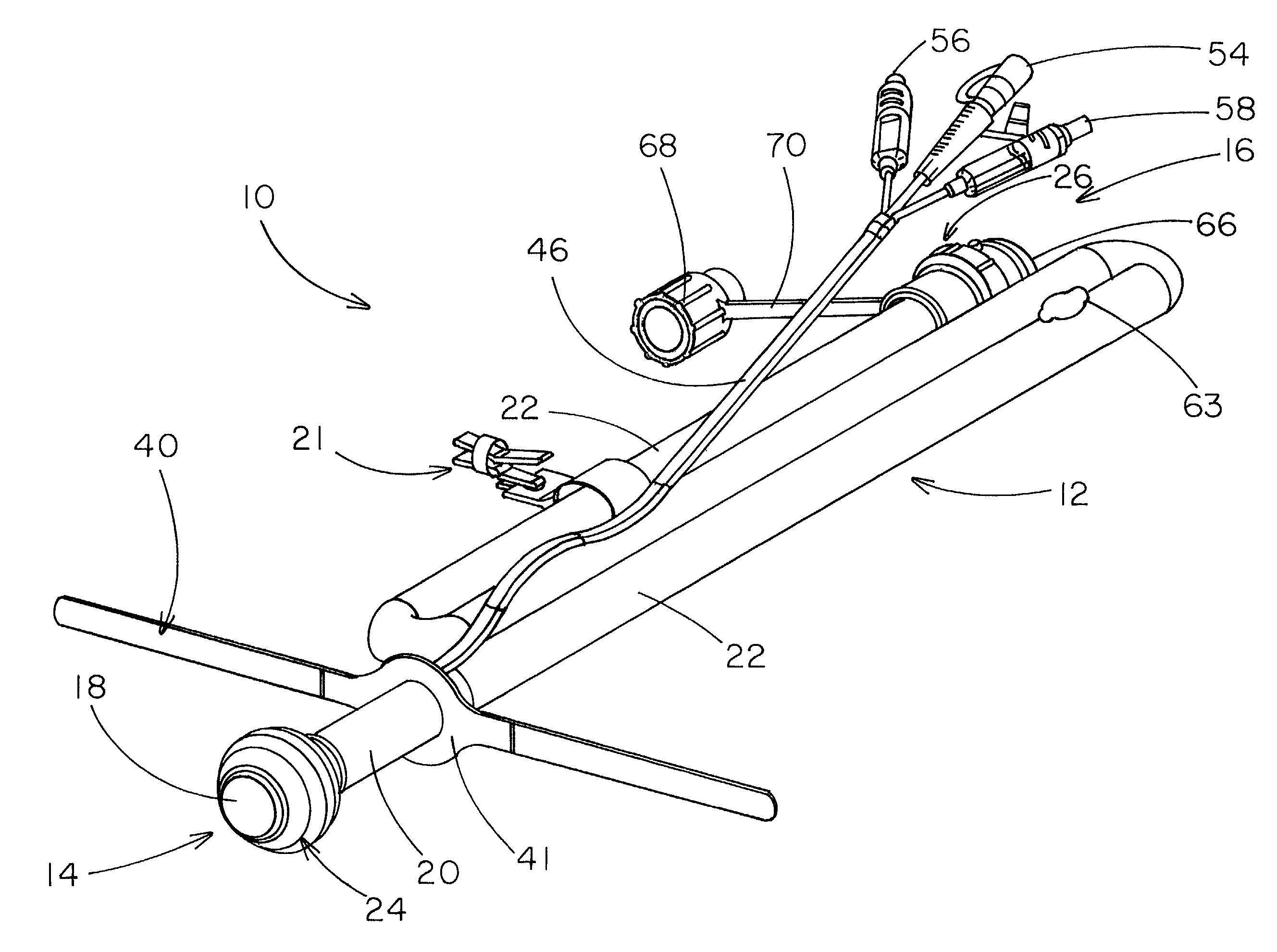
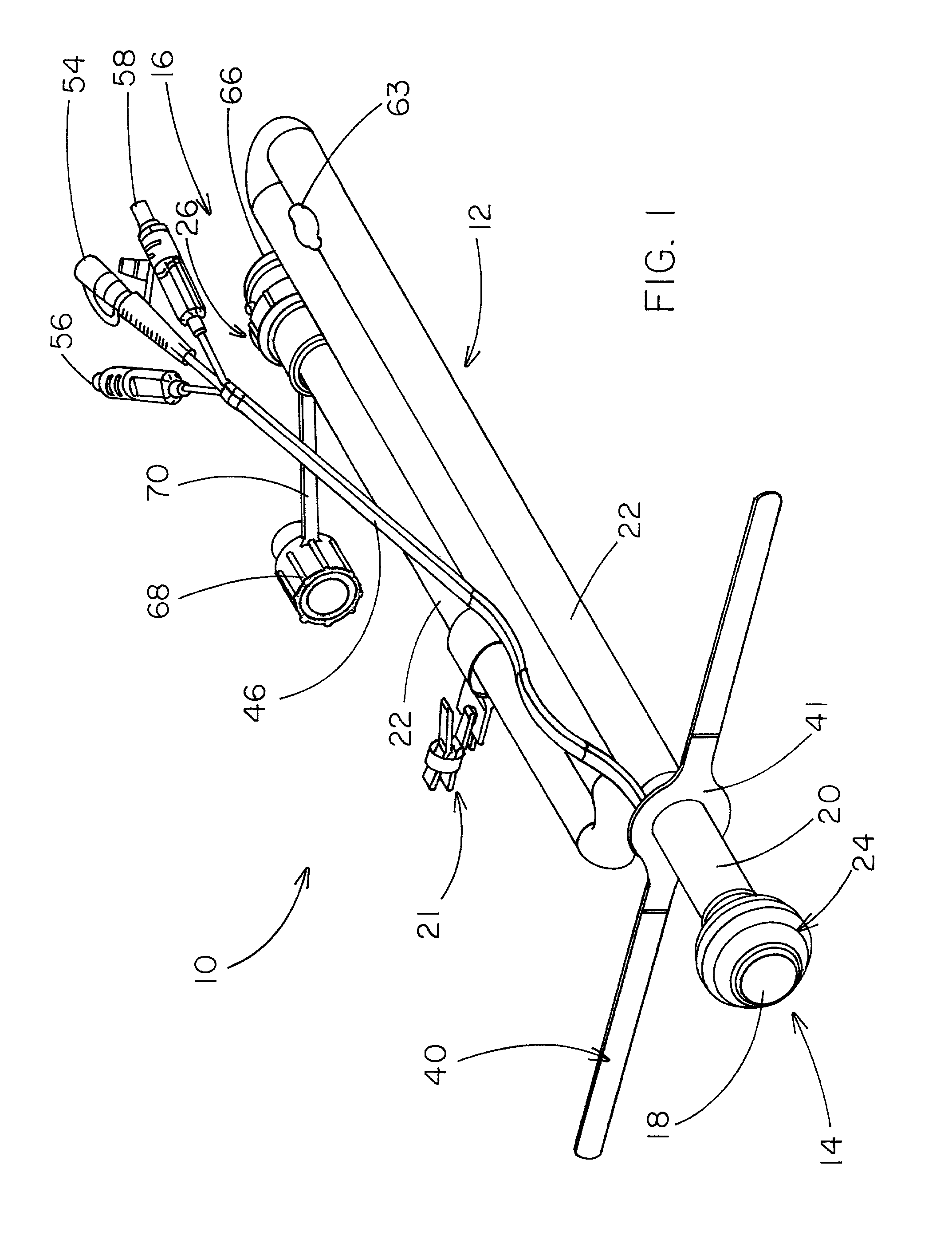
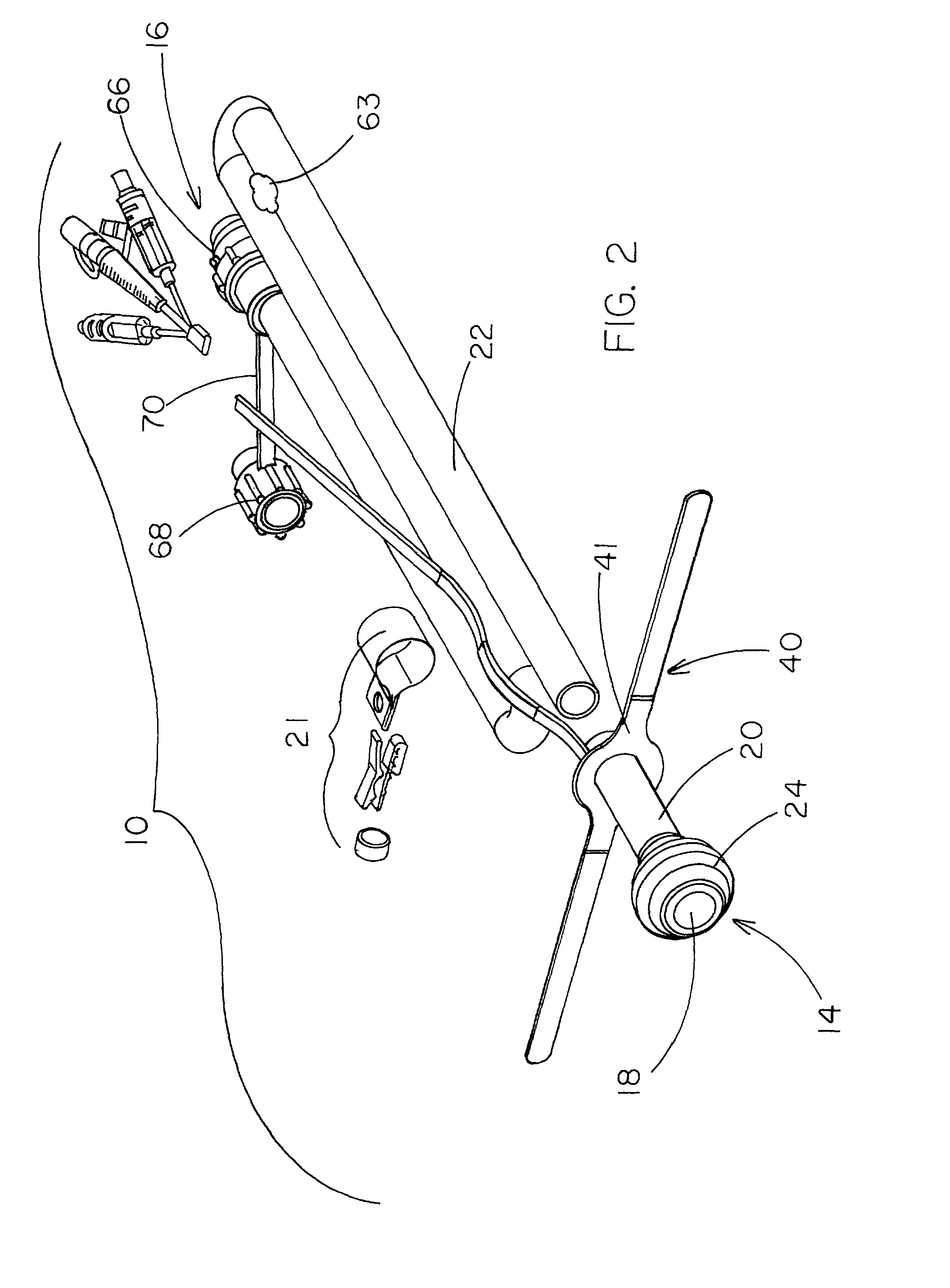
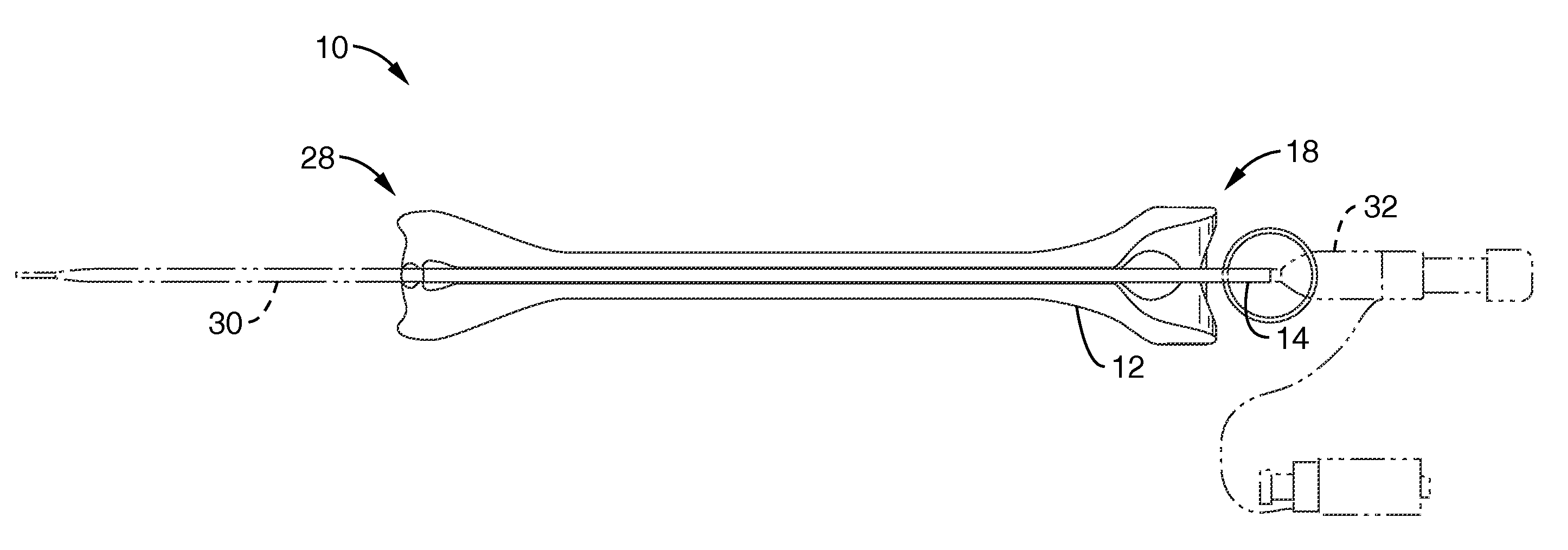
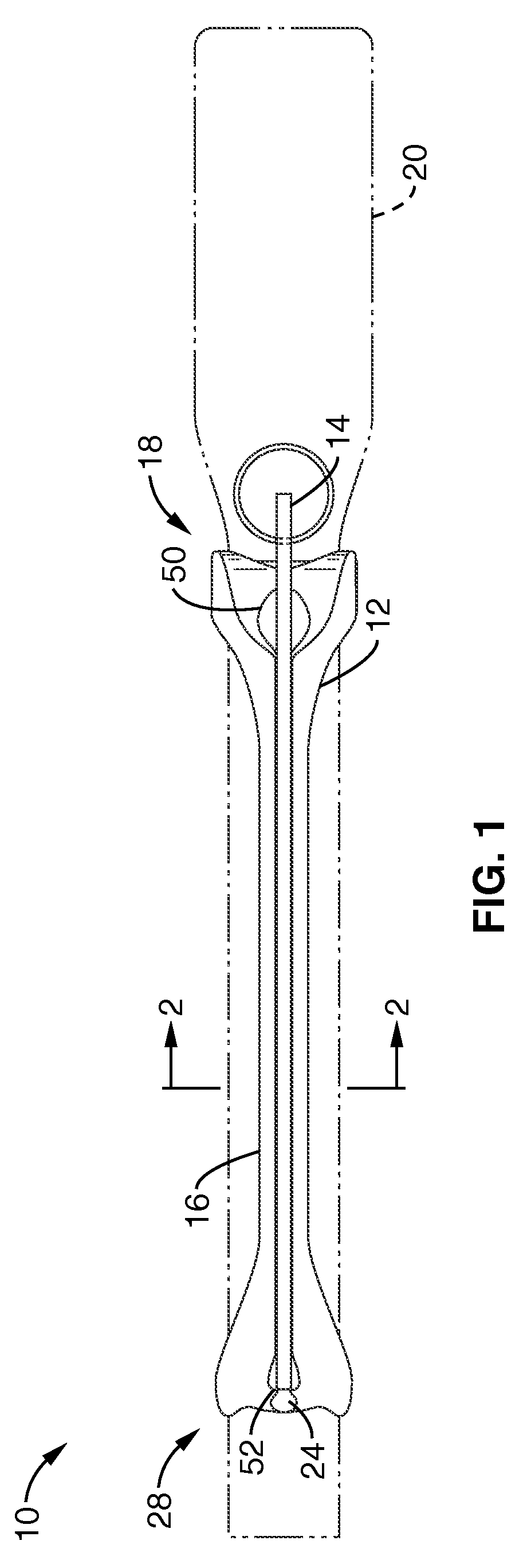
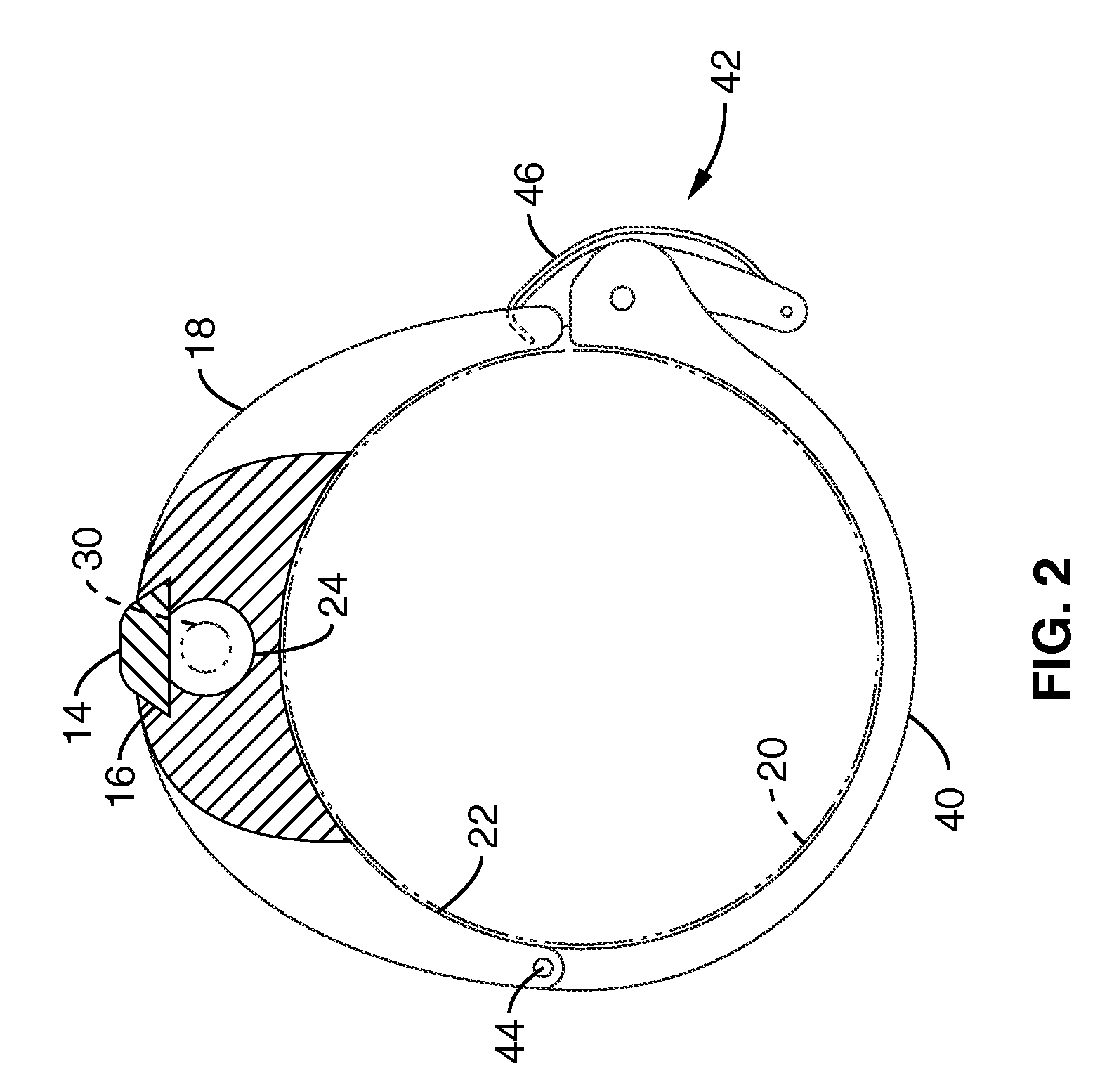
Rectal expander
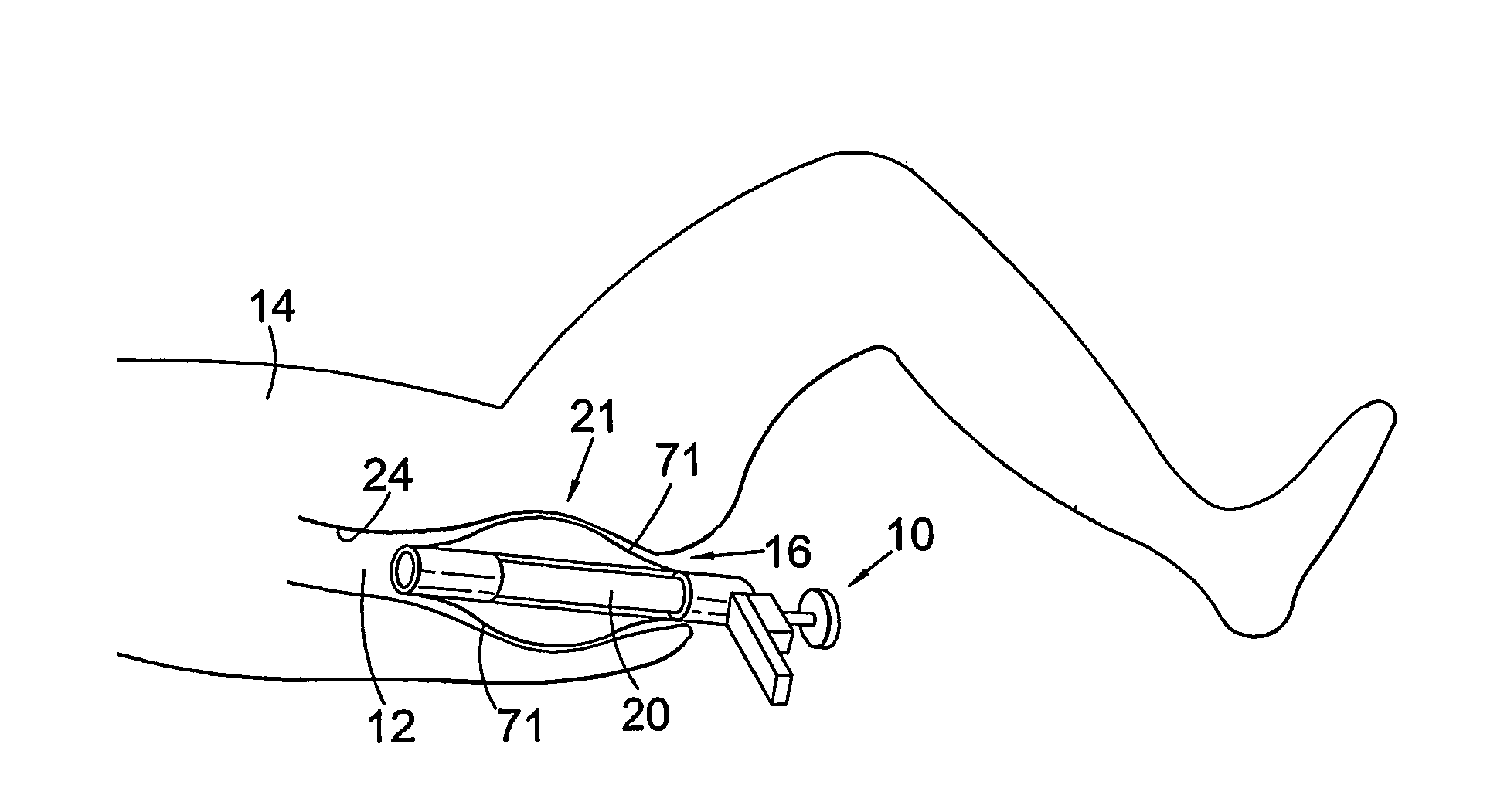
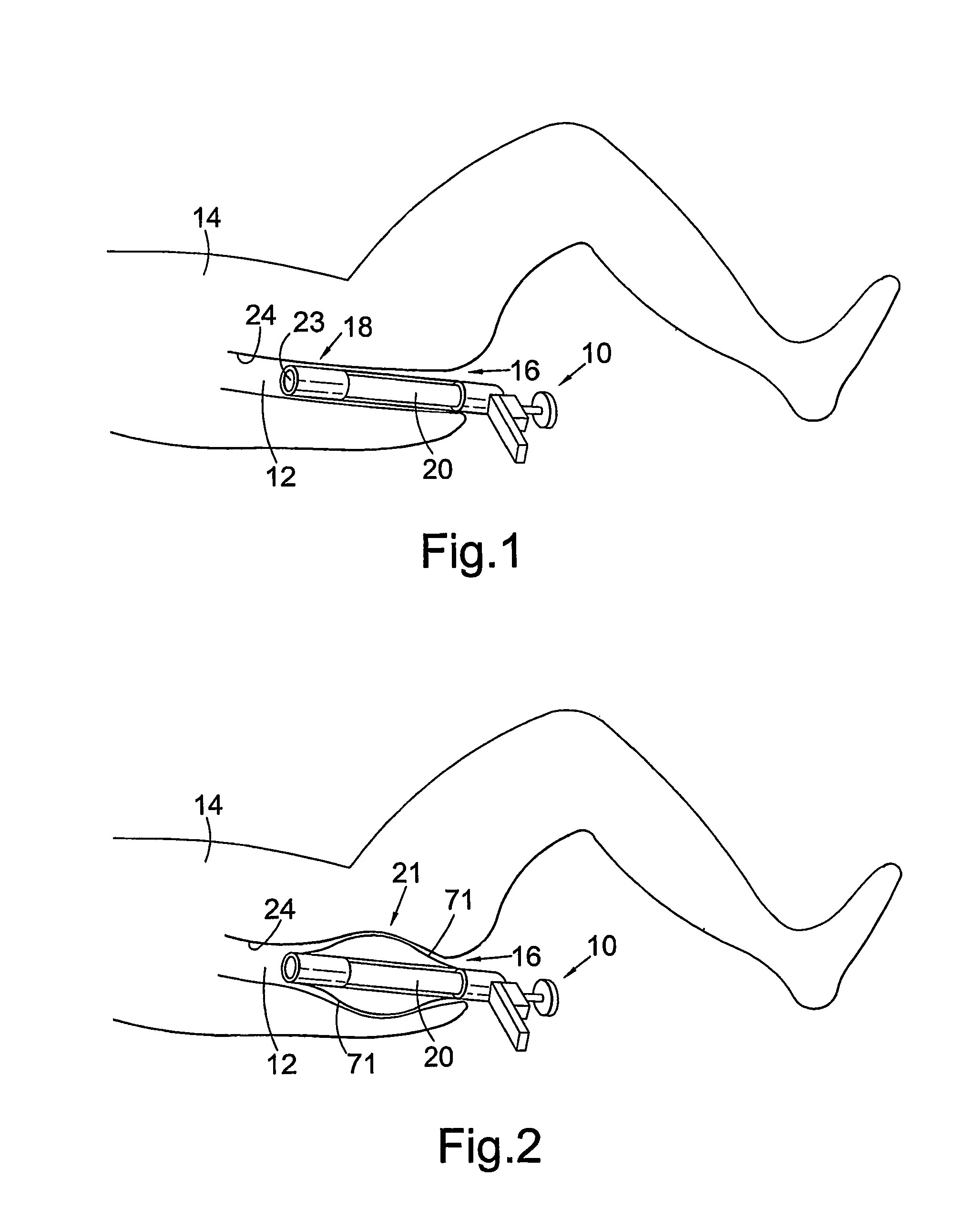
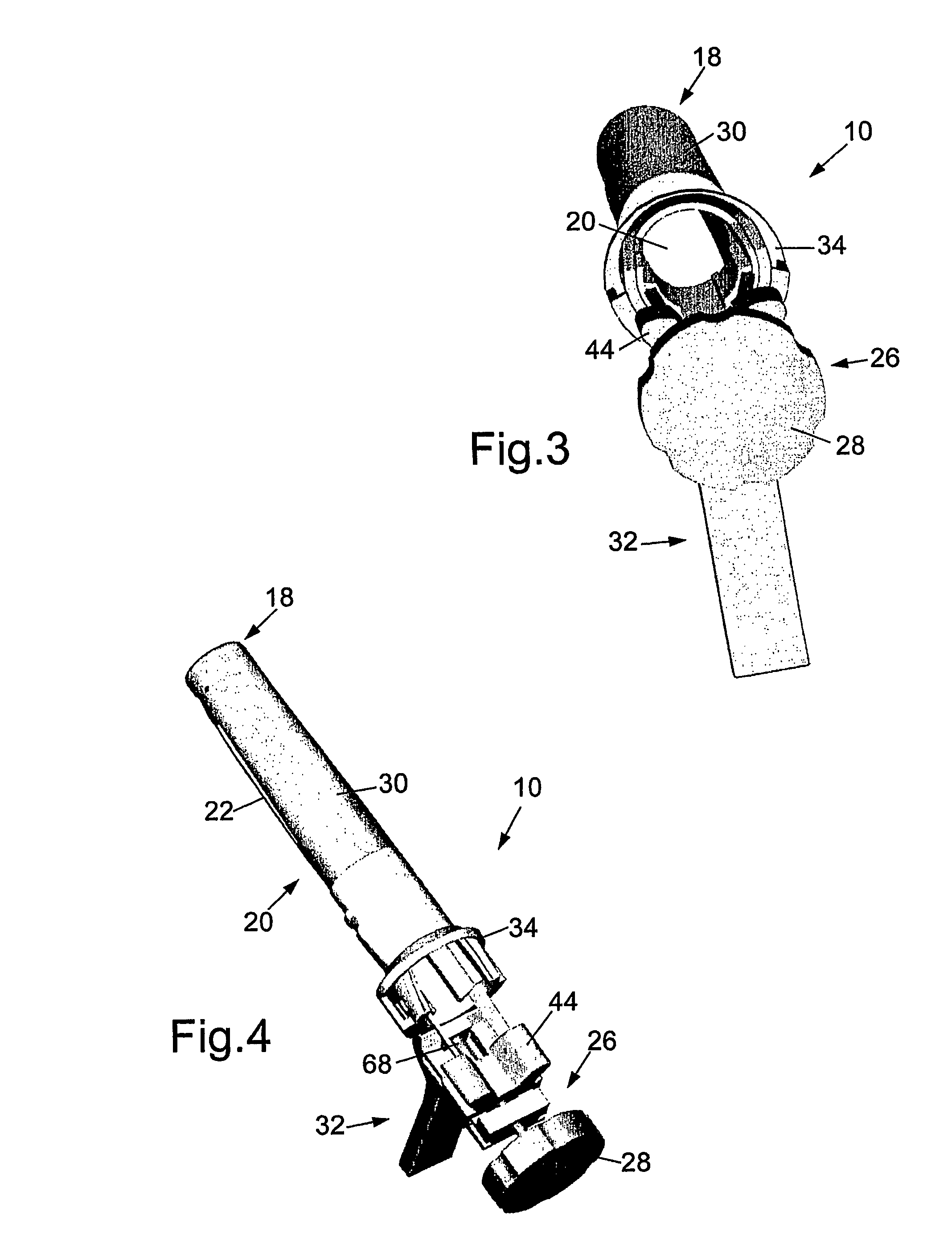
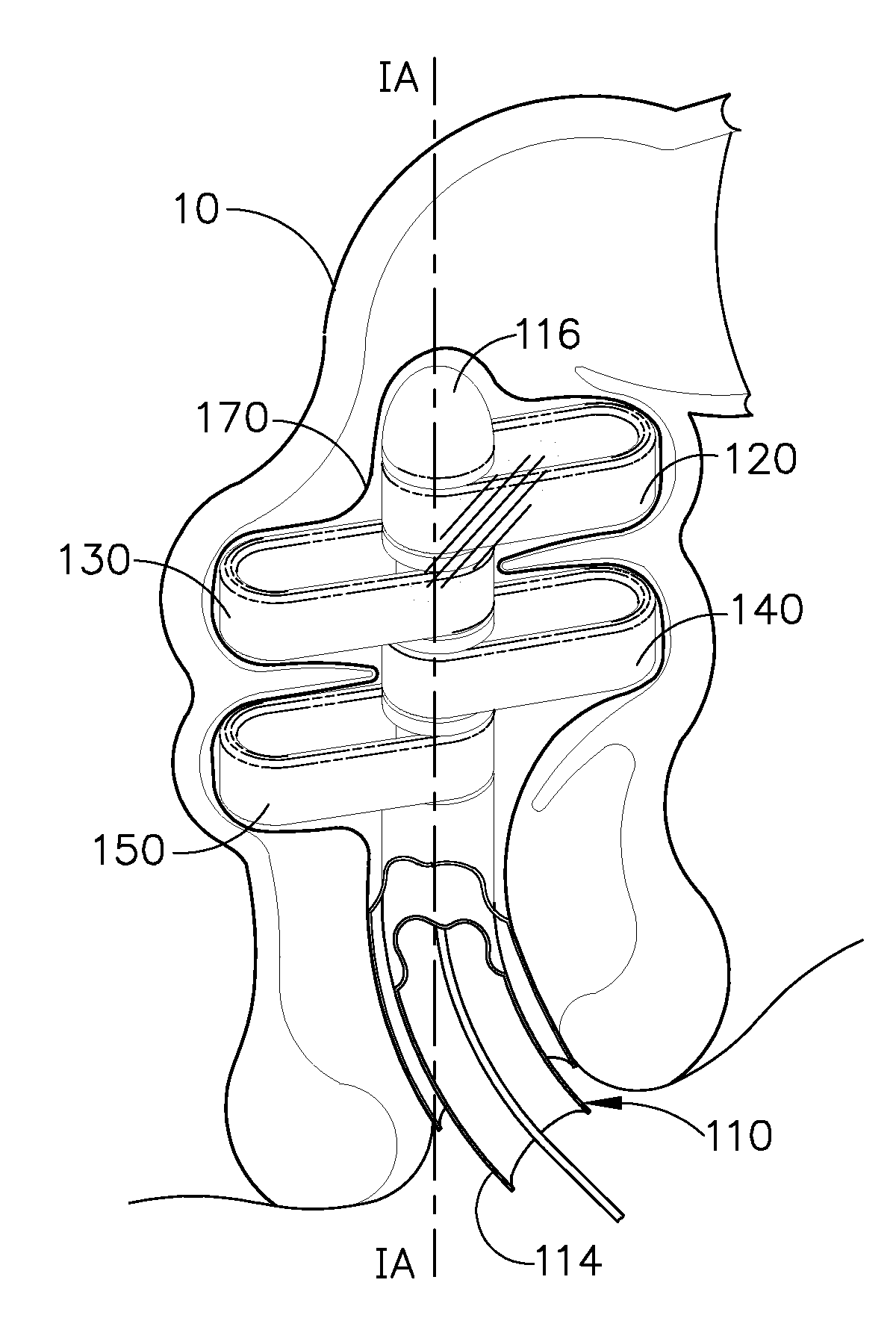
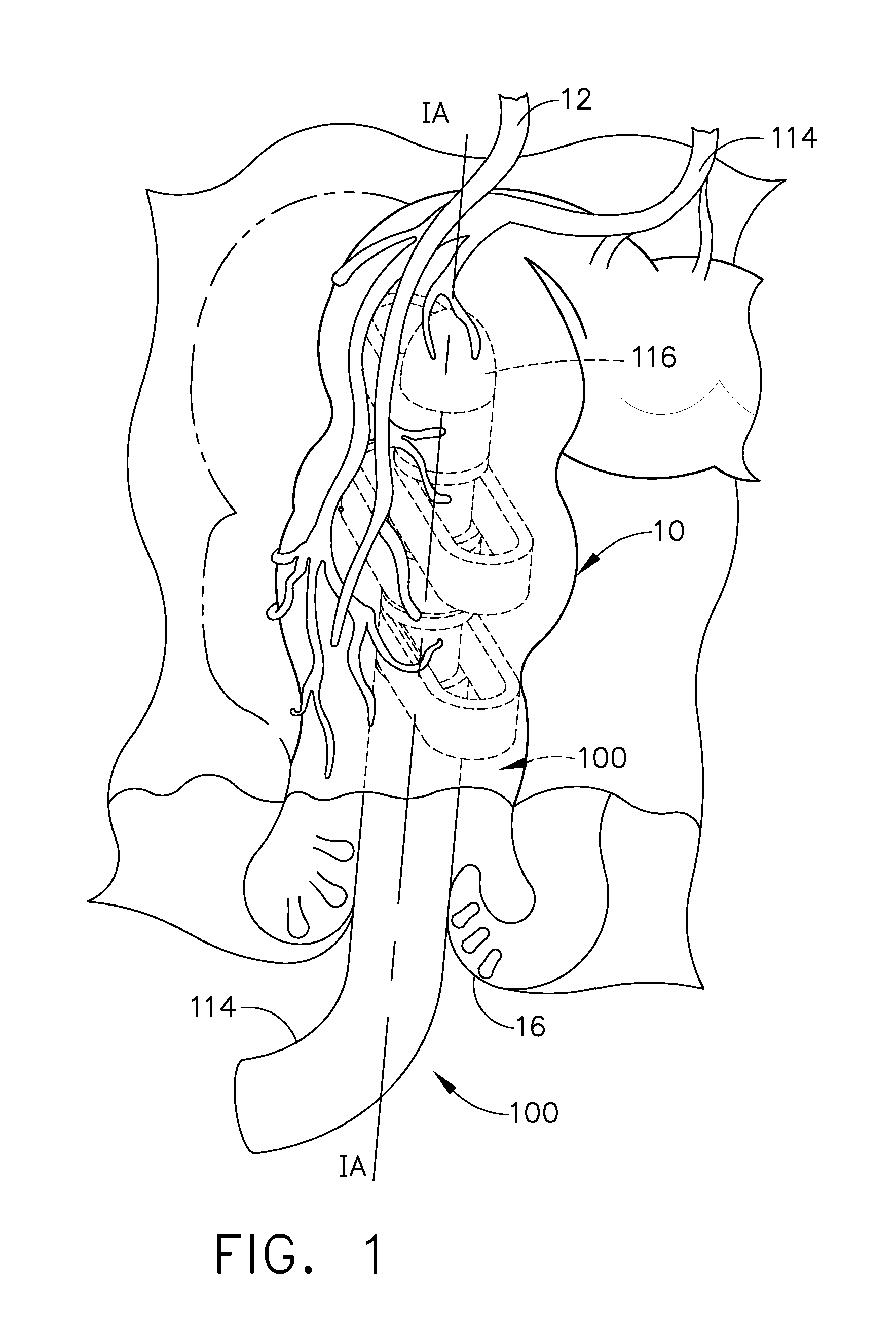
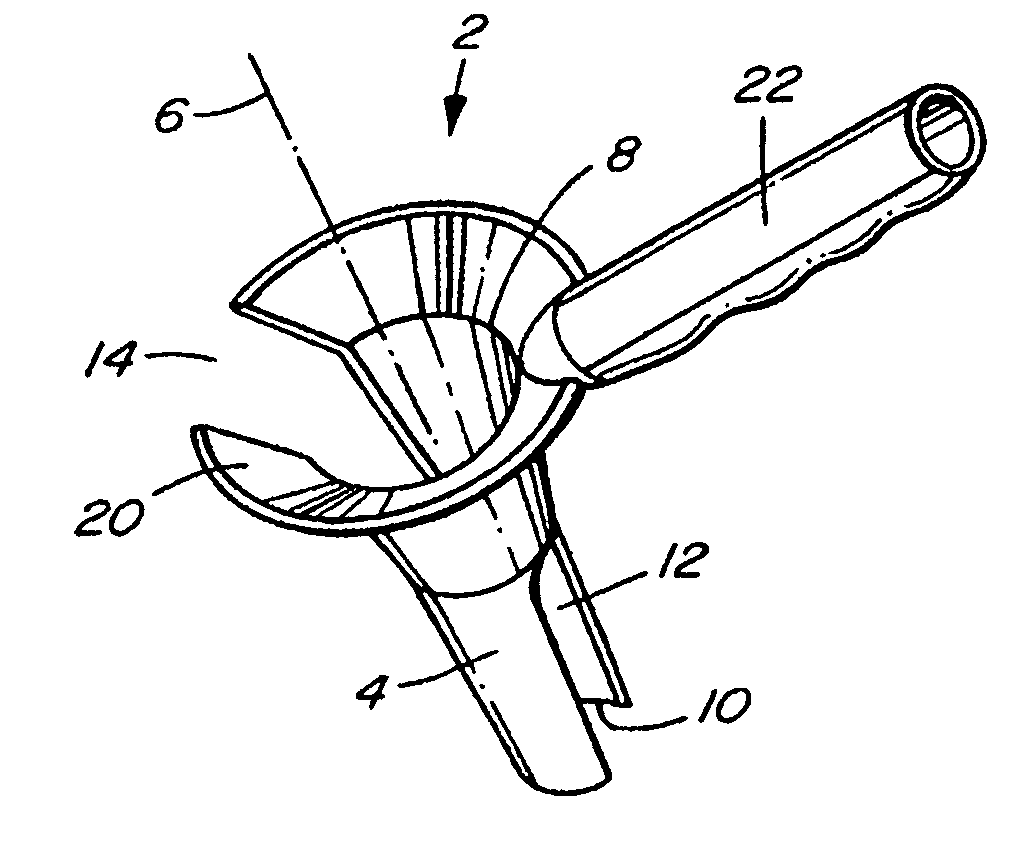
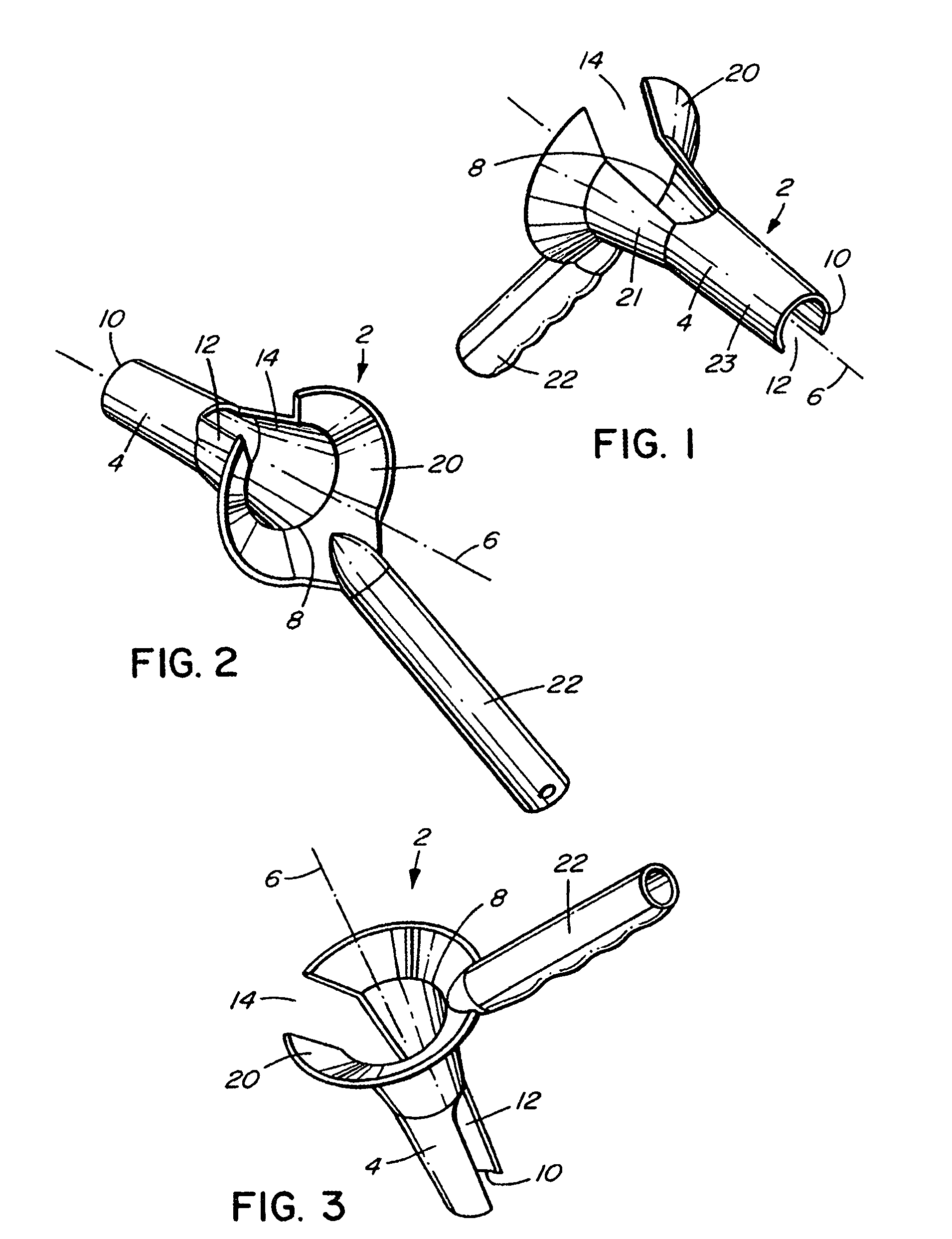
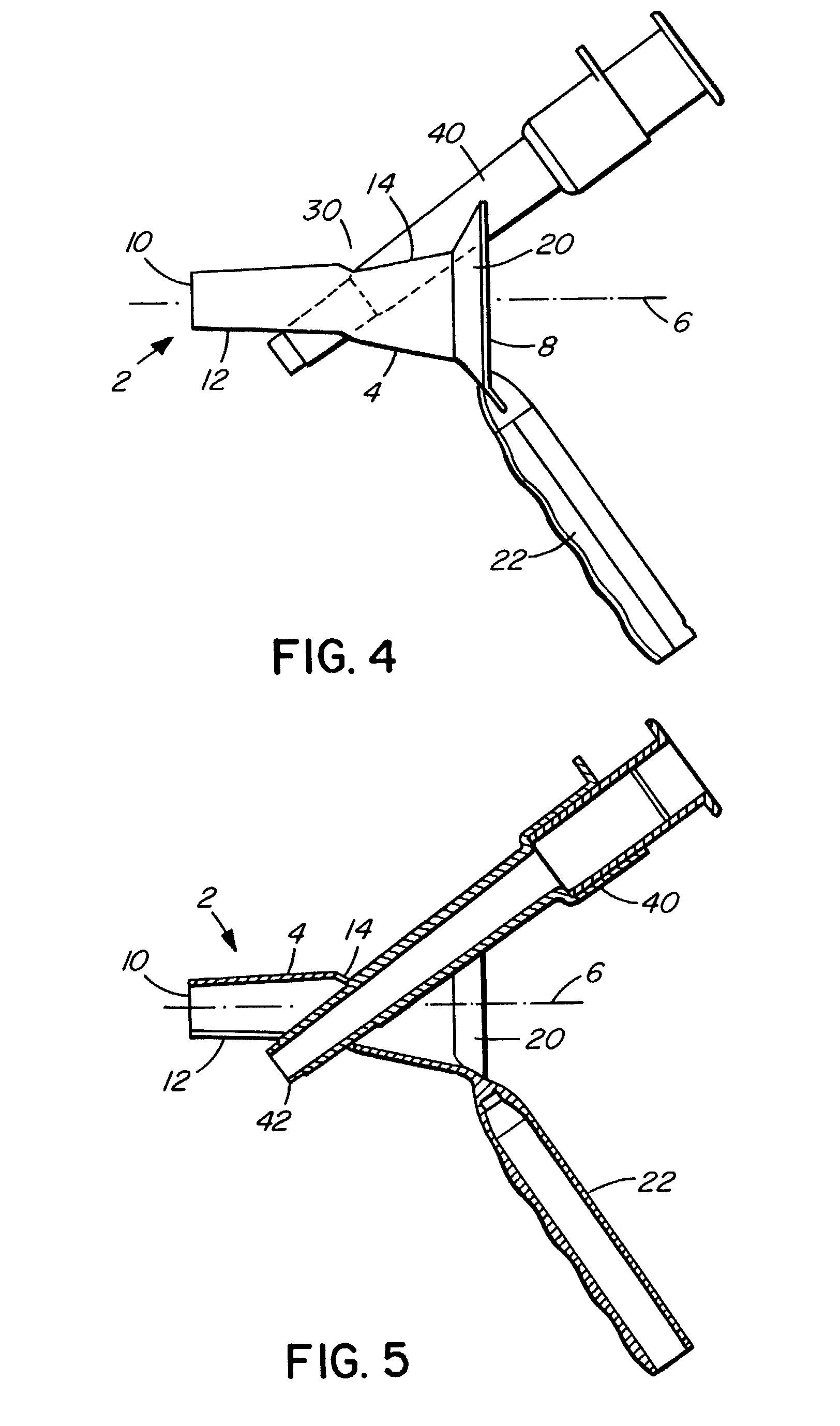
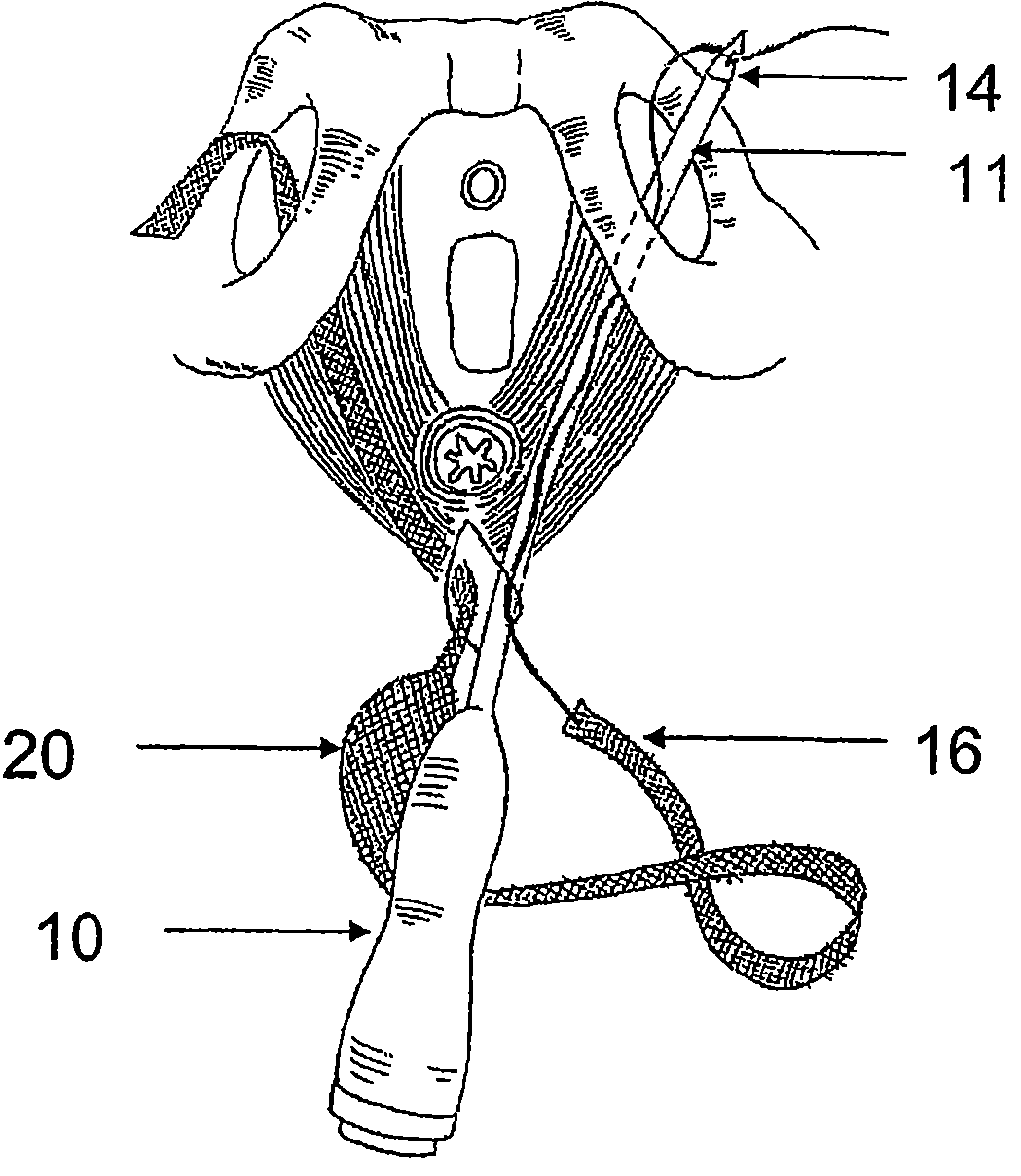
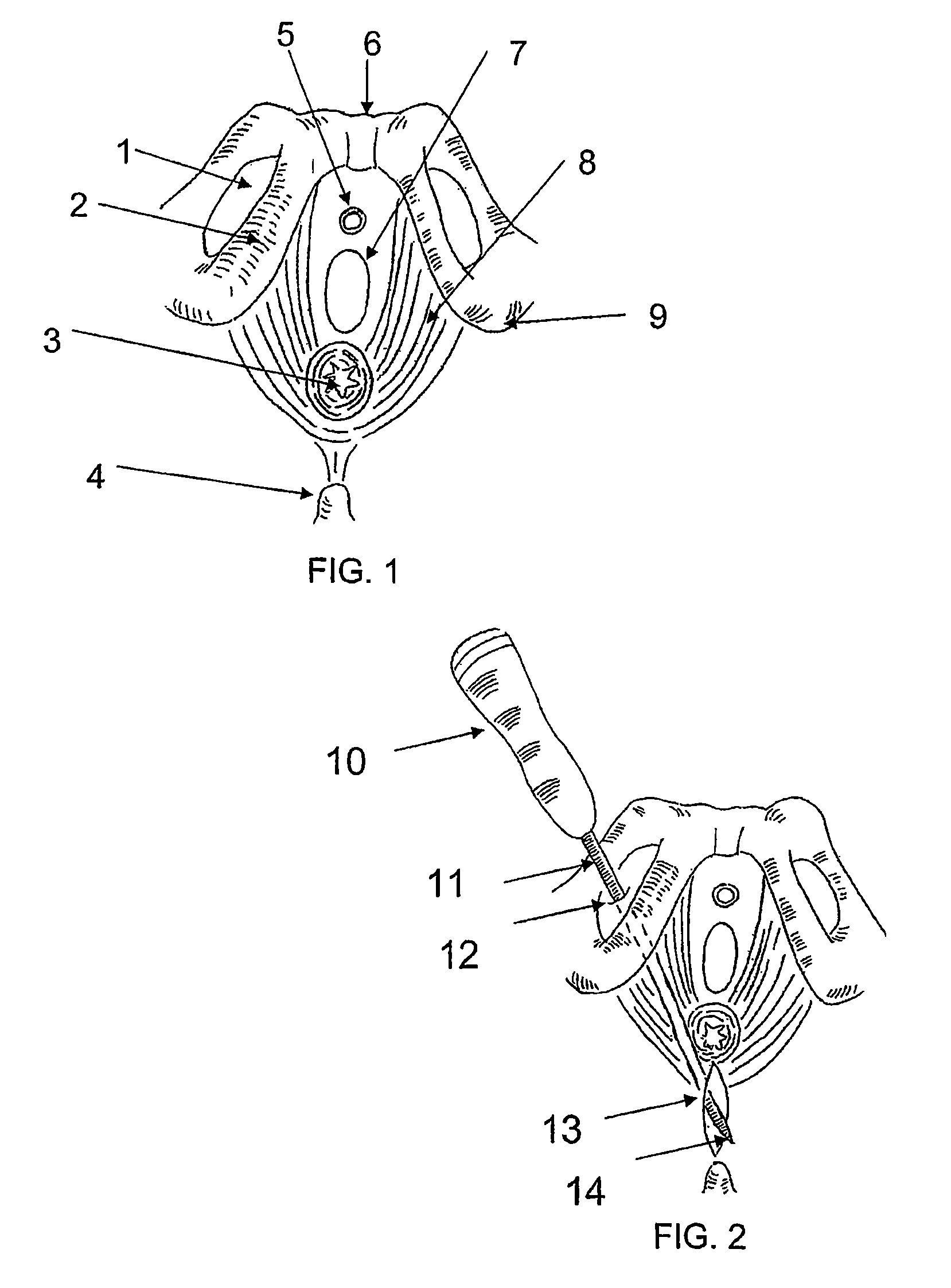
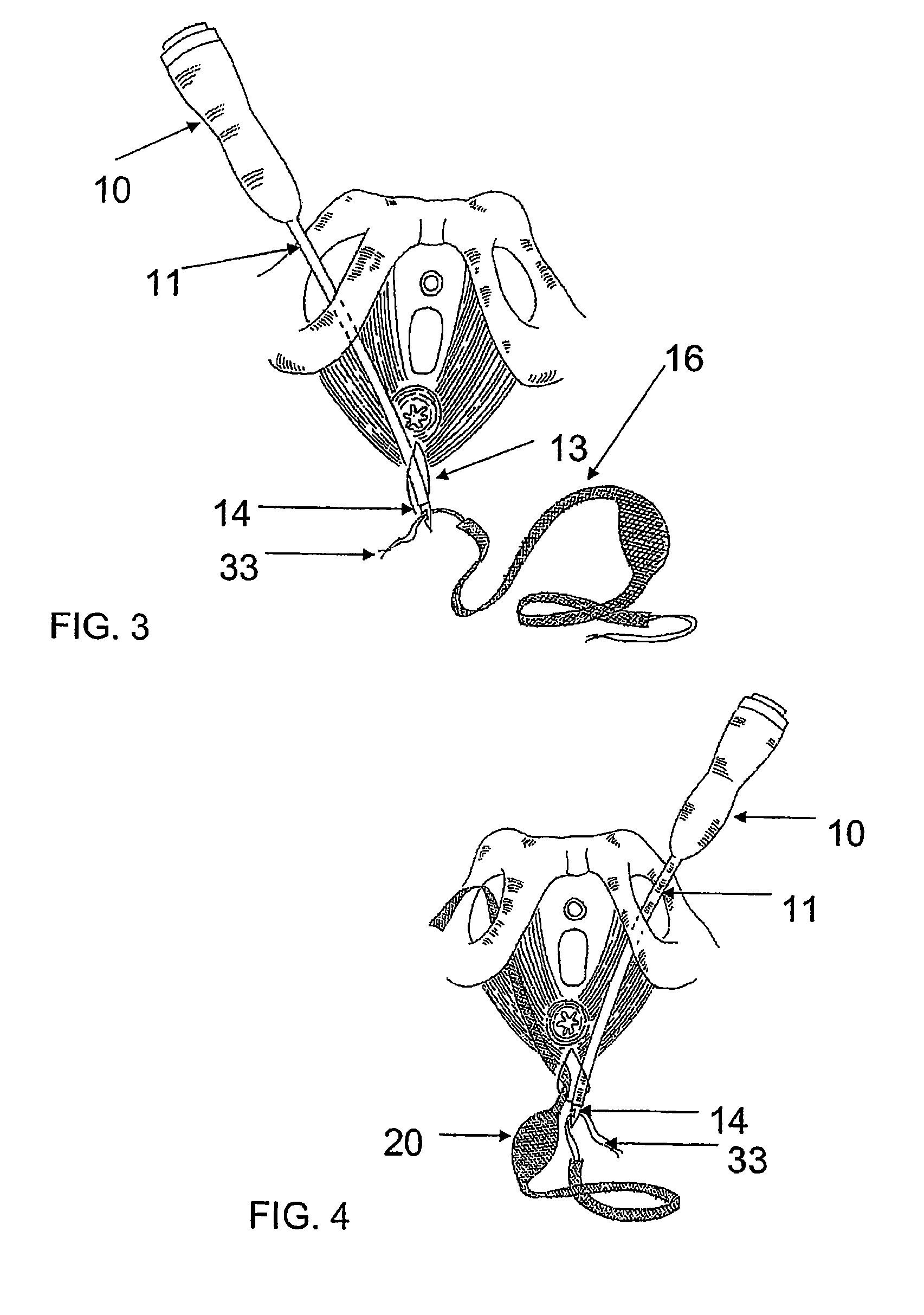
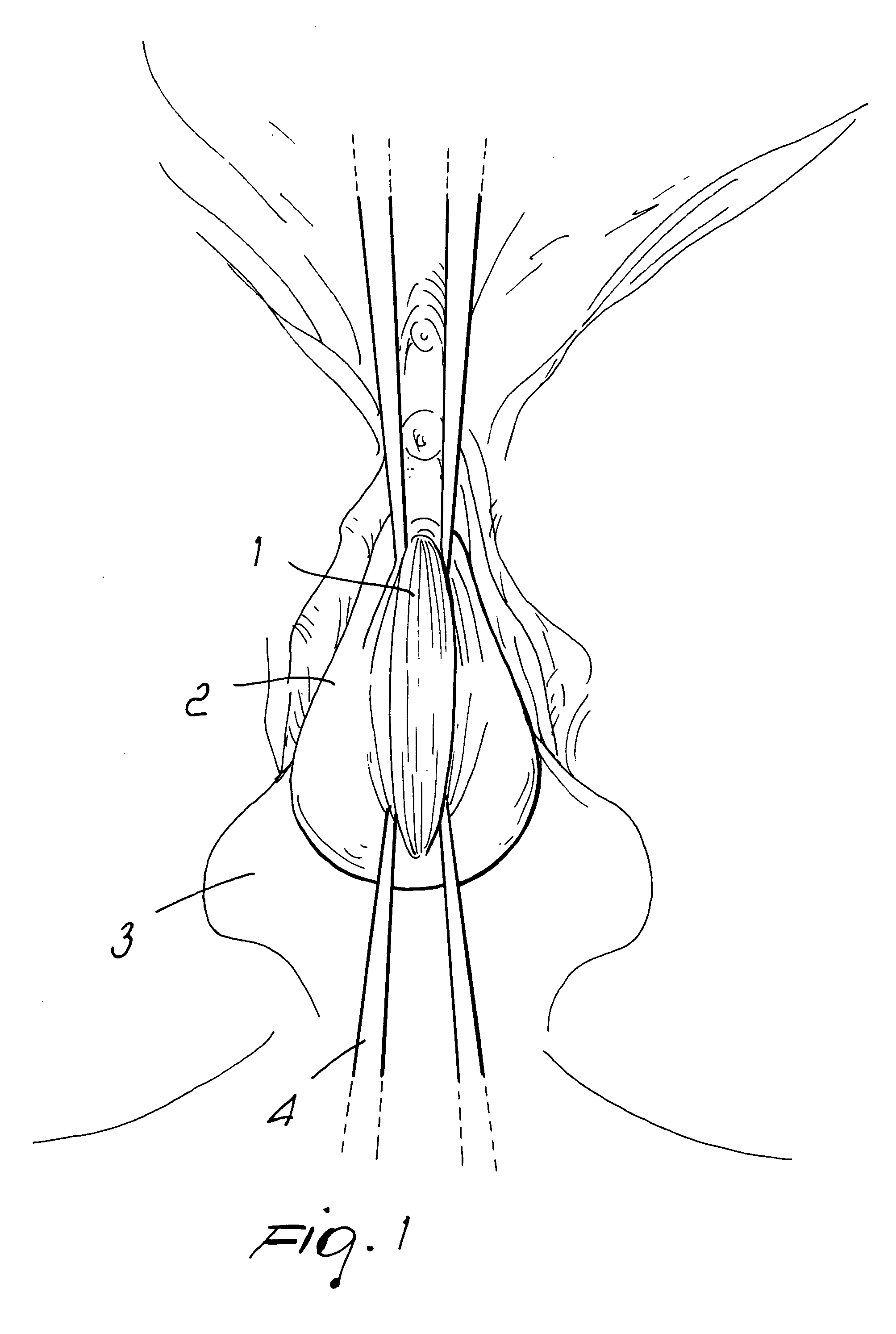
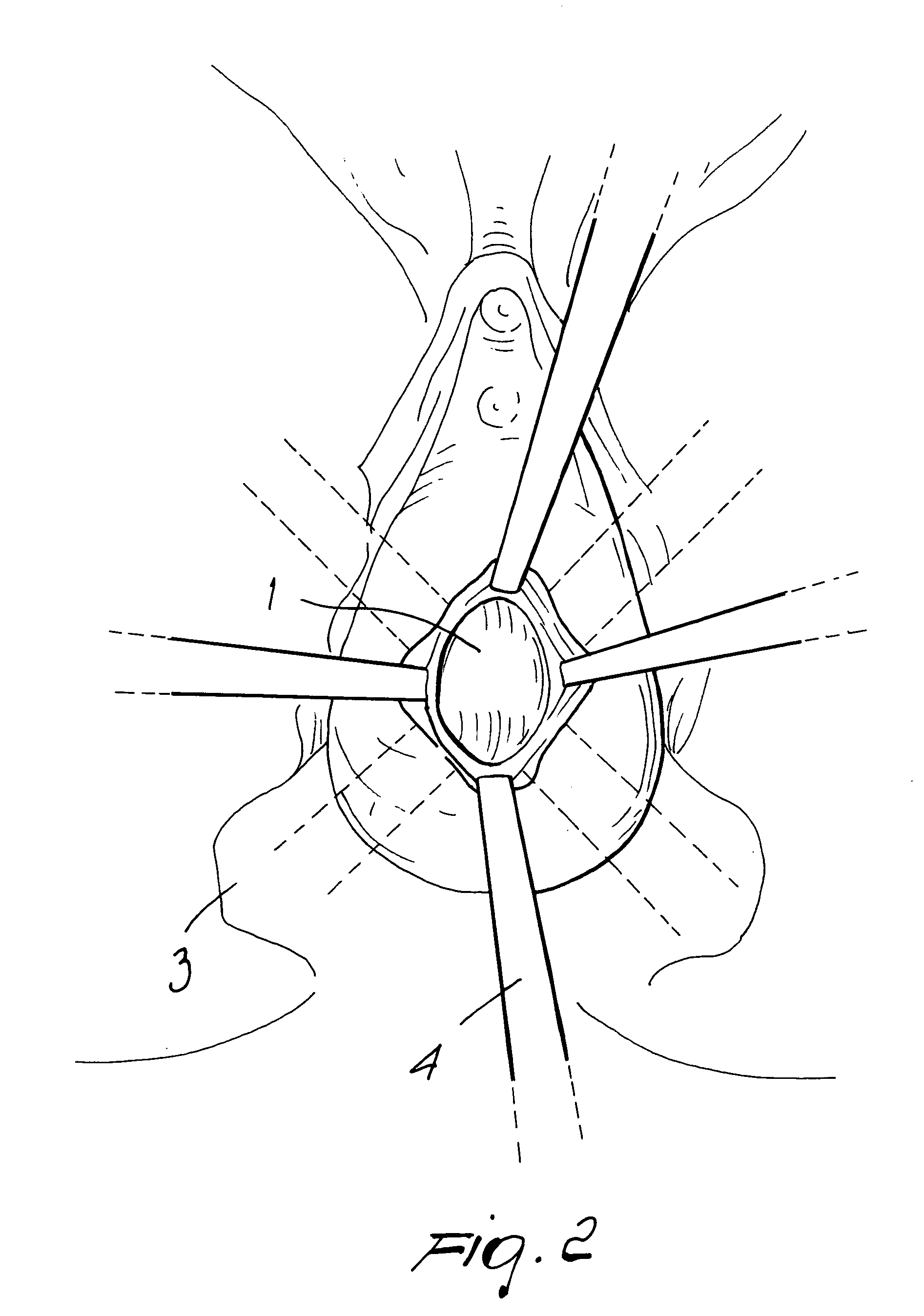
There is disclosed medical apparatus of the type for use in surgery such as transanal endoscopic microsurgery, as well as methods of providing access to, inspecting and enabling surgery within a body passage. In one embodiment of the invention, medical apparatus in the form of a rectal expander (10) is disclosed, the expander (10) being adapted for location at least partly within a body passage such as the rectum (12) of a patient (14), the expander (10) having a leading end (18) and an access area in the form of an opening (20) for access from the expander (10) into the rectum (12), at least part of the opening (20) being spaced from the leading end (18), and the expander (10) being controllably movable between collapse and expansion positions, for expanding the rectum (12).
Owner:UNIVERSITY OF DUNDEE
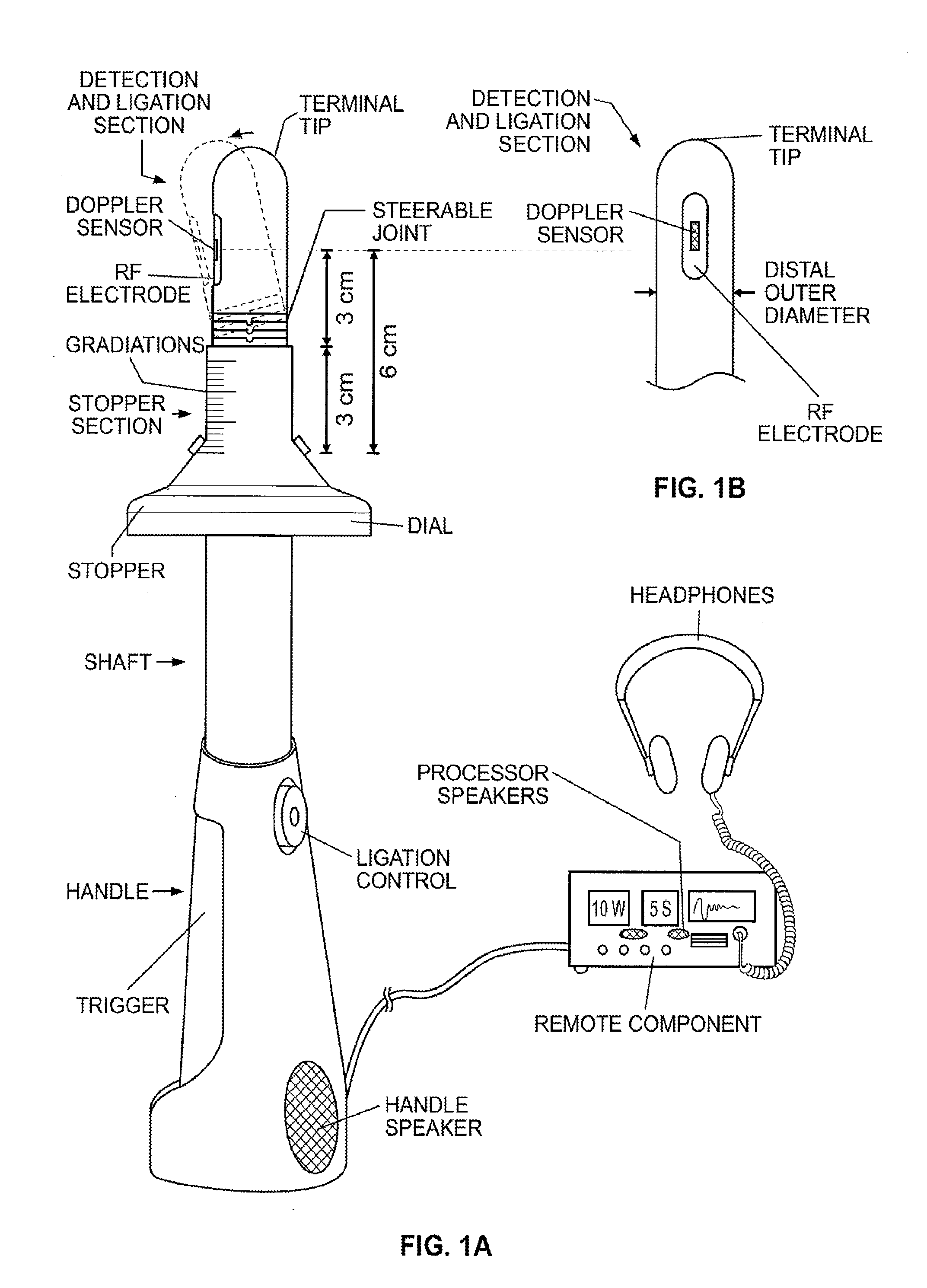
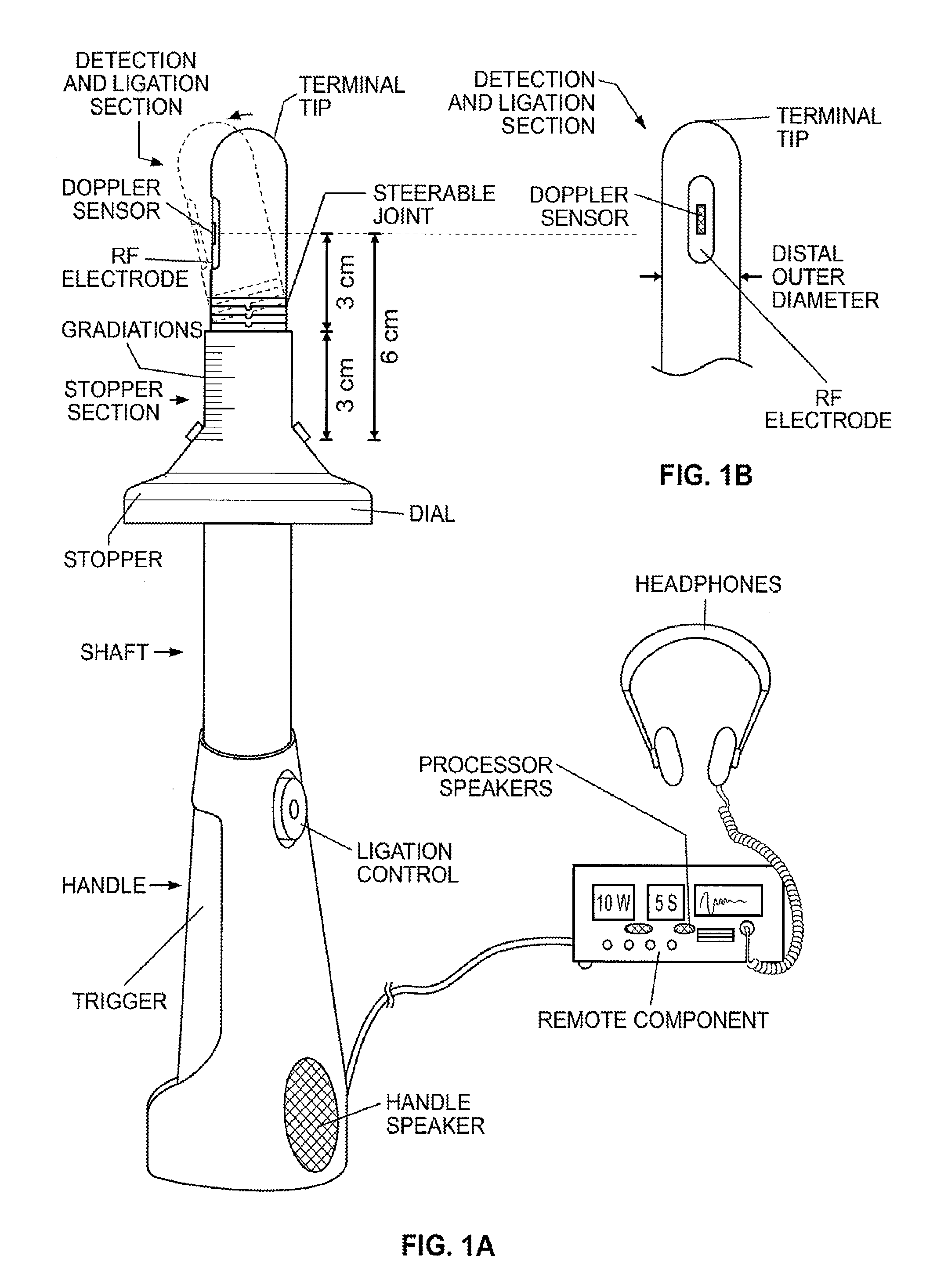
Ablation of rectal and other internal body structures
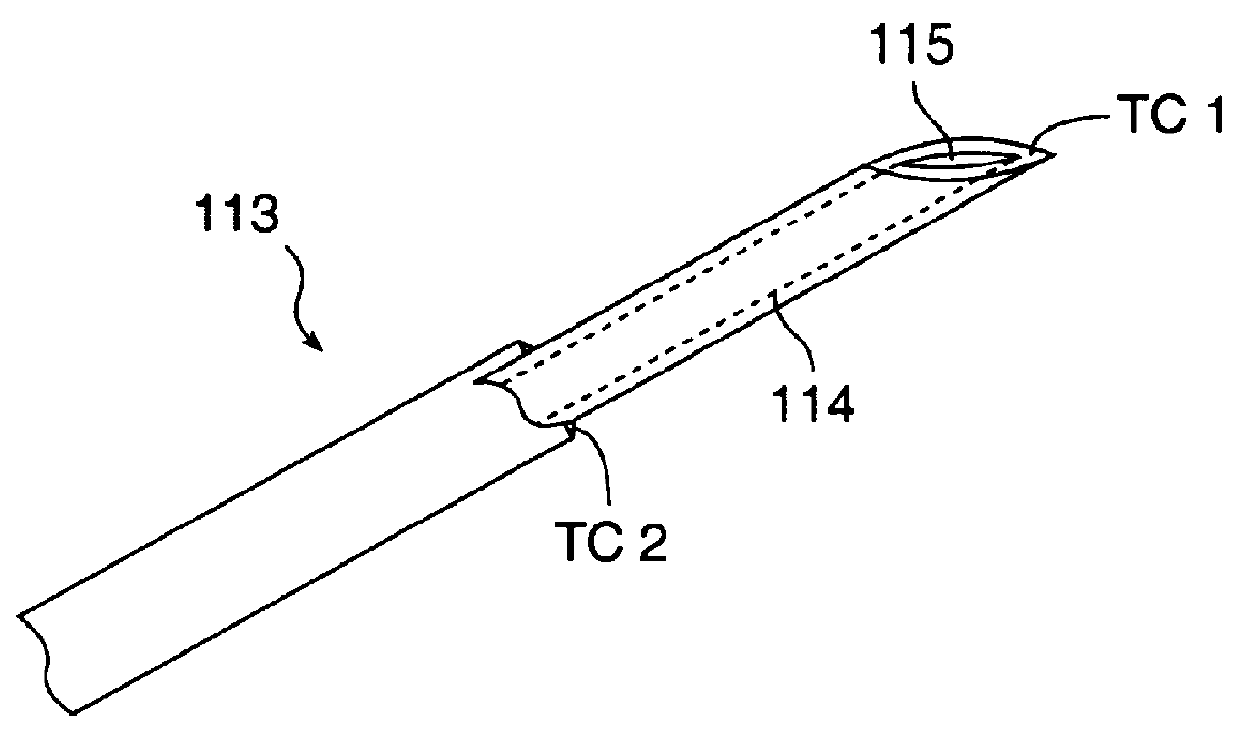
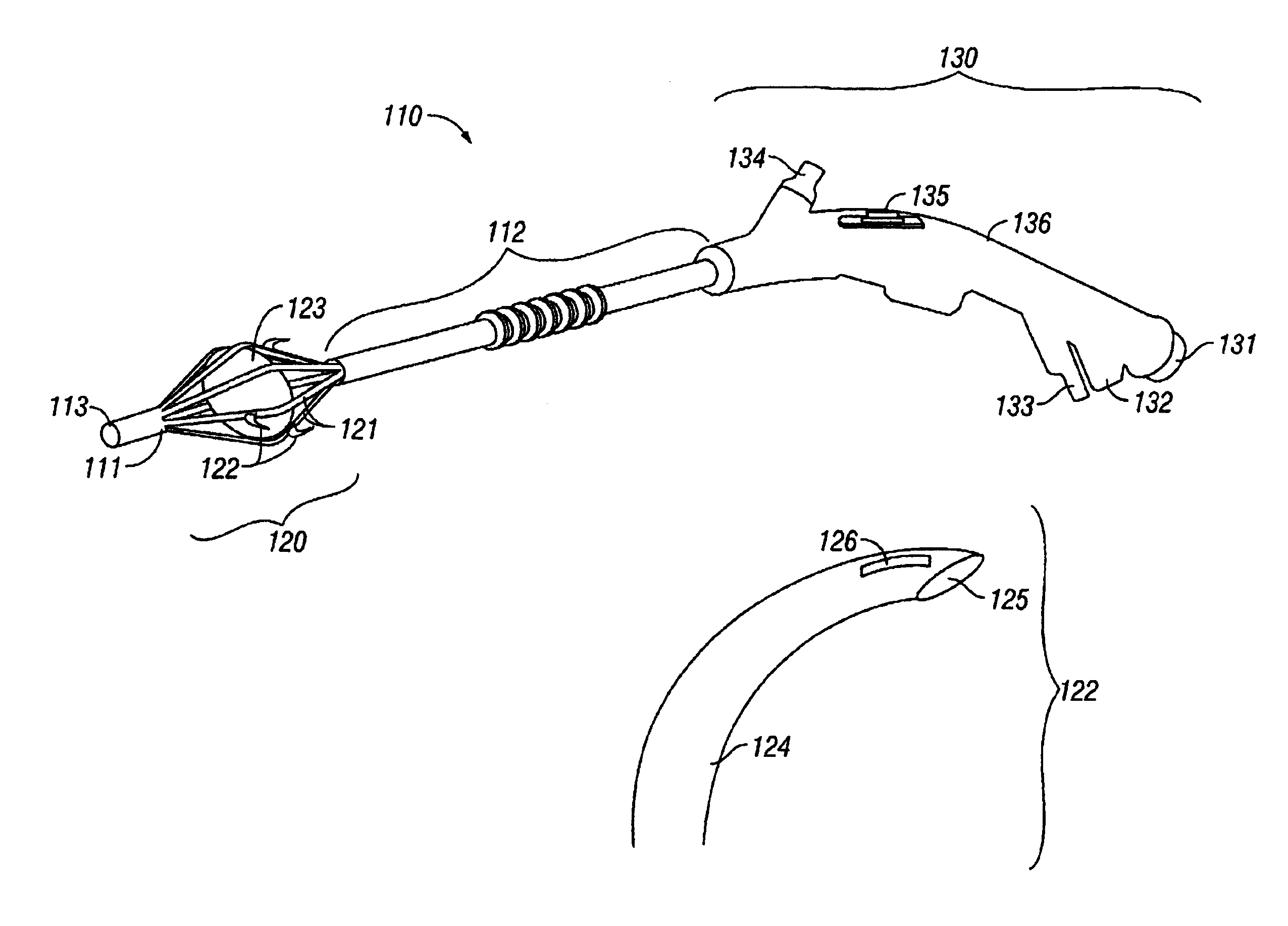
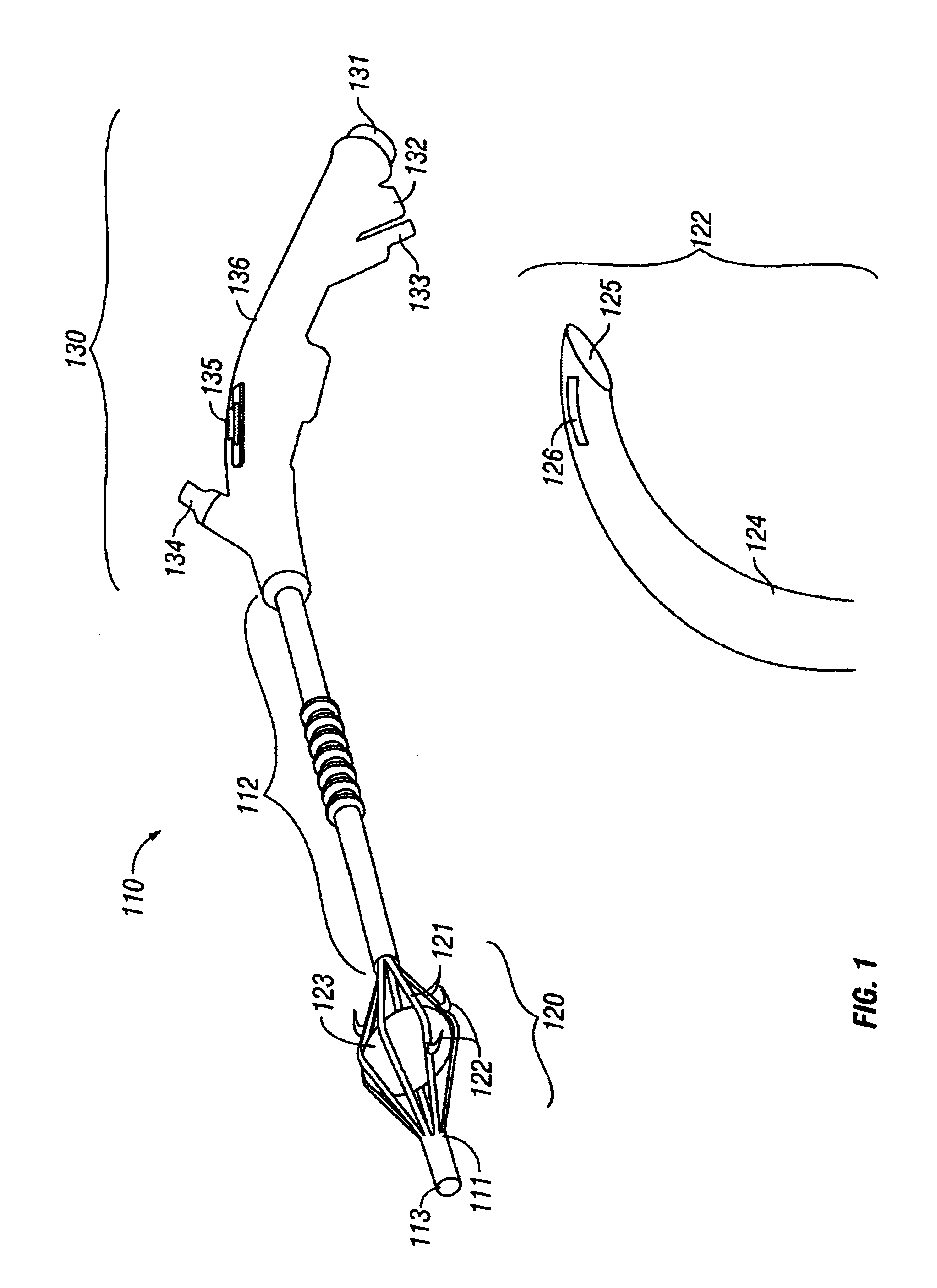
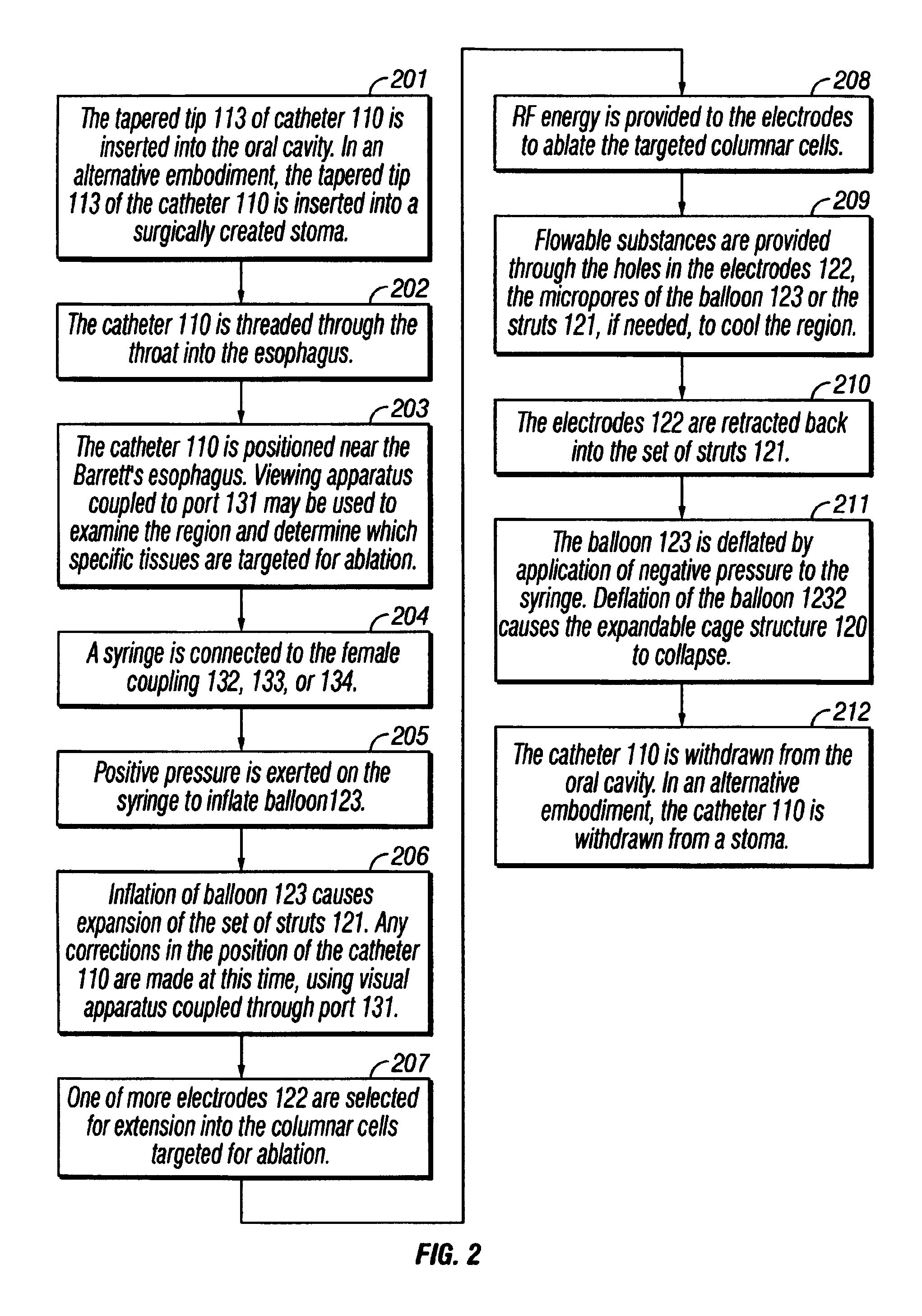
The invention provides an apparatus and system for ablation of body structures or tissue in the region of the rectum. A catheter is inserted into the rectum, and an electrode is disposed thereon for emitting energy. The environment for an ablation region is isolated or otherwise controlled by blocking gas or fluid using a pair of inflatable balloons at upstream and downstream locations. Inflatable balloons also serve to anchor the catheter in place. A plurality of electrodes are disposed on the catheter and at least one such electrode is selected and advanced out of the catheter to penetrate and ablate selected tissue inside the body in the region of the rectum. The electrodes are coupled to sensors to determine control parameters of the body structure or tissue, and which are used by feedback technique to control delivery of energy for ablation or fluids for cooling or hydration. The catheter includes an optical path disposed for coupling to an external view piece, so as to allow medical personnel to view or control positioning of the catheter and operation of the electrodes. The catheter is disposed to deliver flowable substances for aiding in ablation, or for aiding in repair of tissue, such as collagen or another substance for covering lesions or for filling fissures. The flowable substances are delivered using at least one lumen in the catheter, either from at least one hole in the catheter, from an area of the catheter covered by a microporous membrane, or from microporous balloons.
Owner:VIDACARE
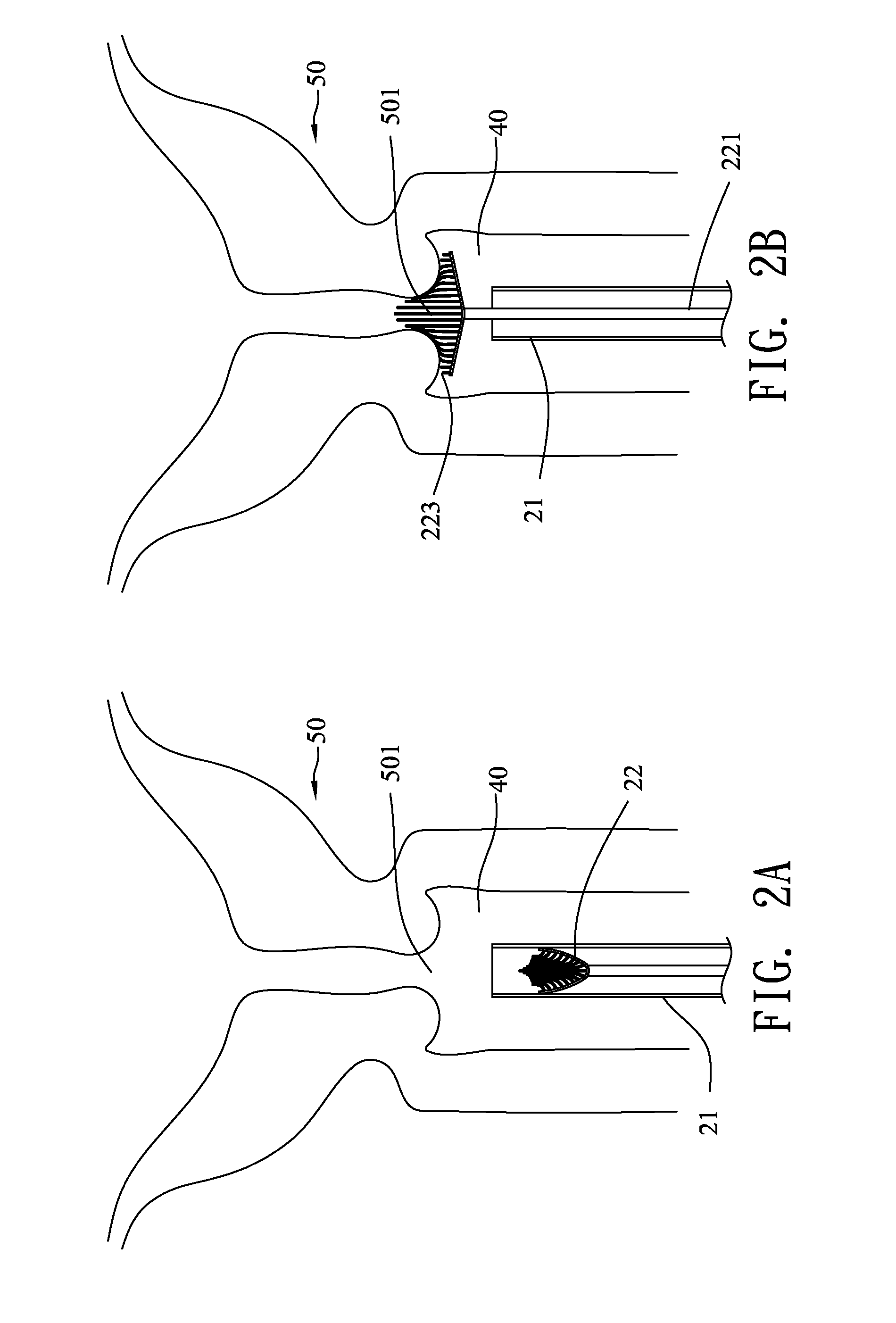
Treatment of tissue in sphincters, sinuses and orifices
Owner:NOVASYS MEDICAL
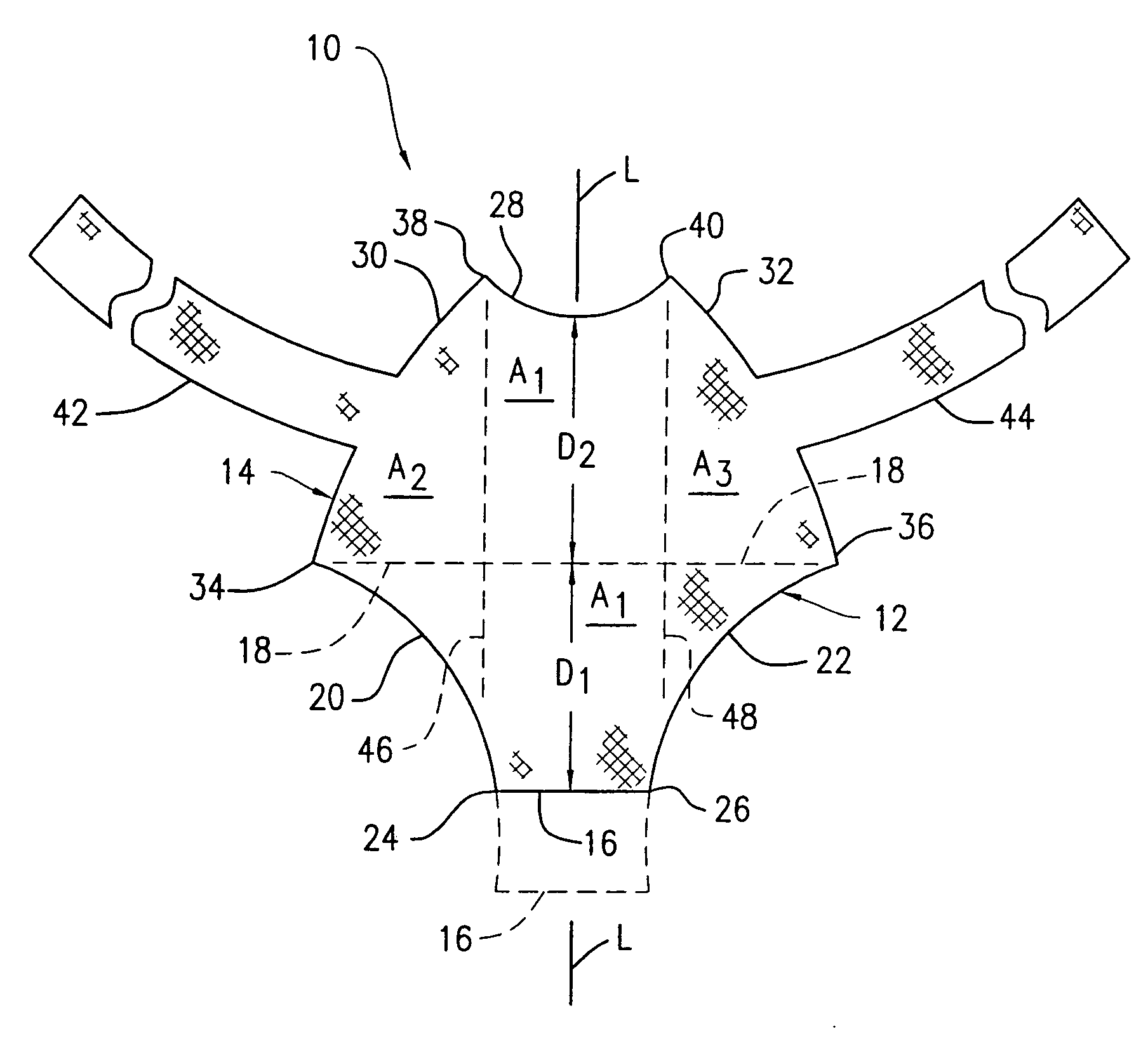
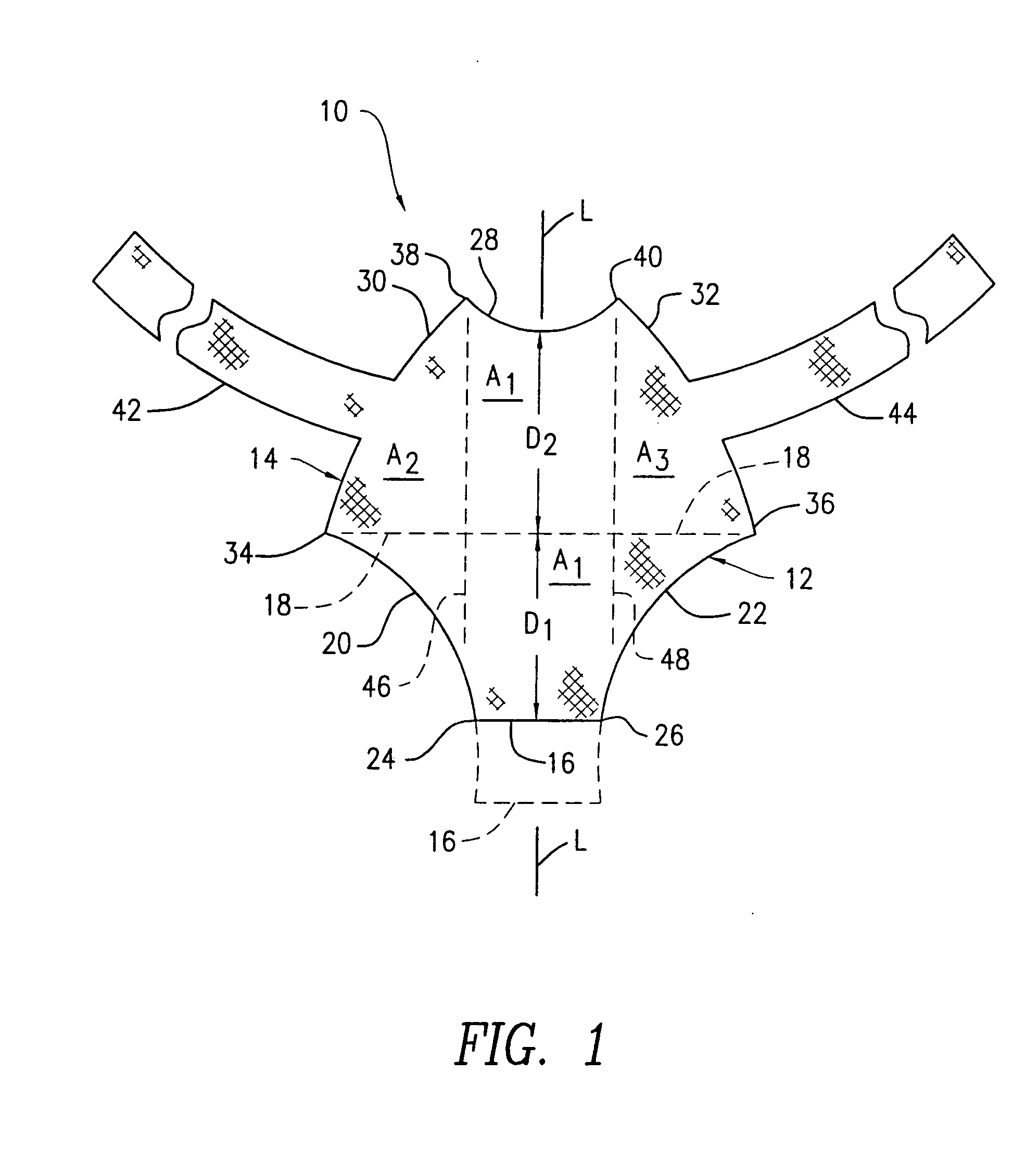
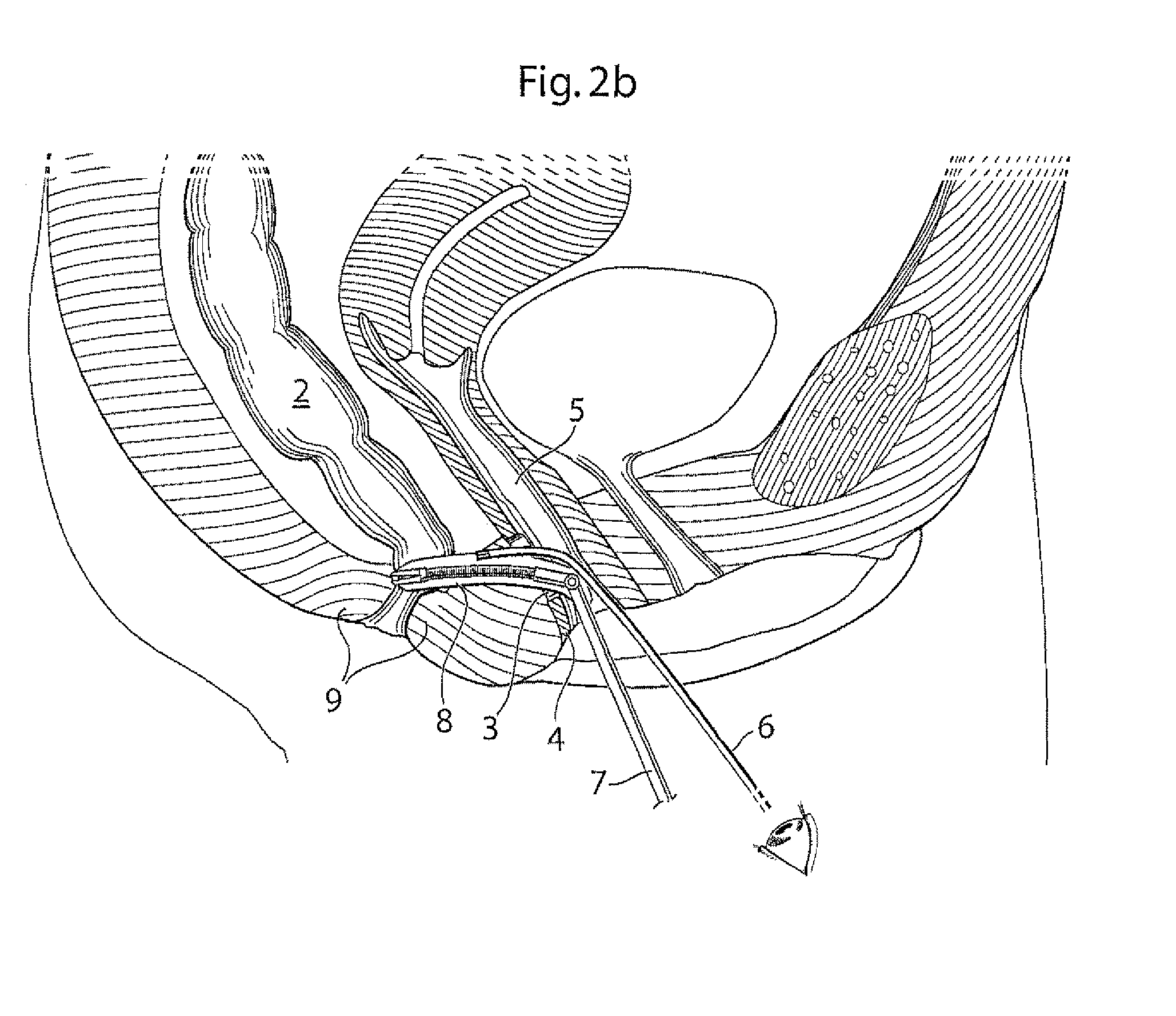
Method and apparatus for treating pelvic organ prolapses in female patients
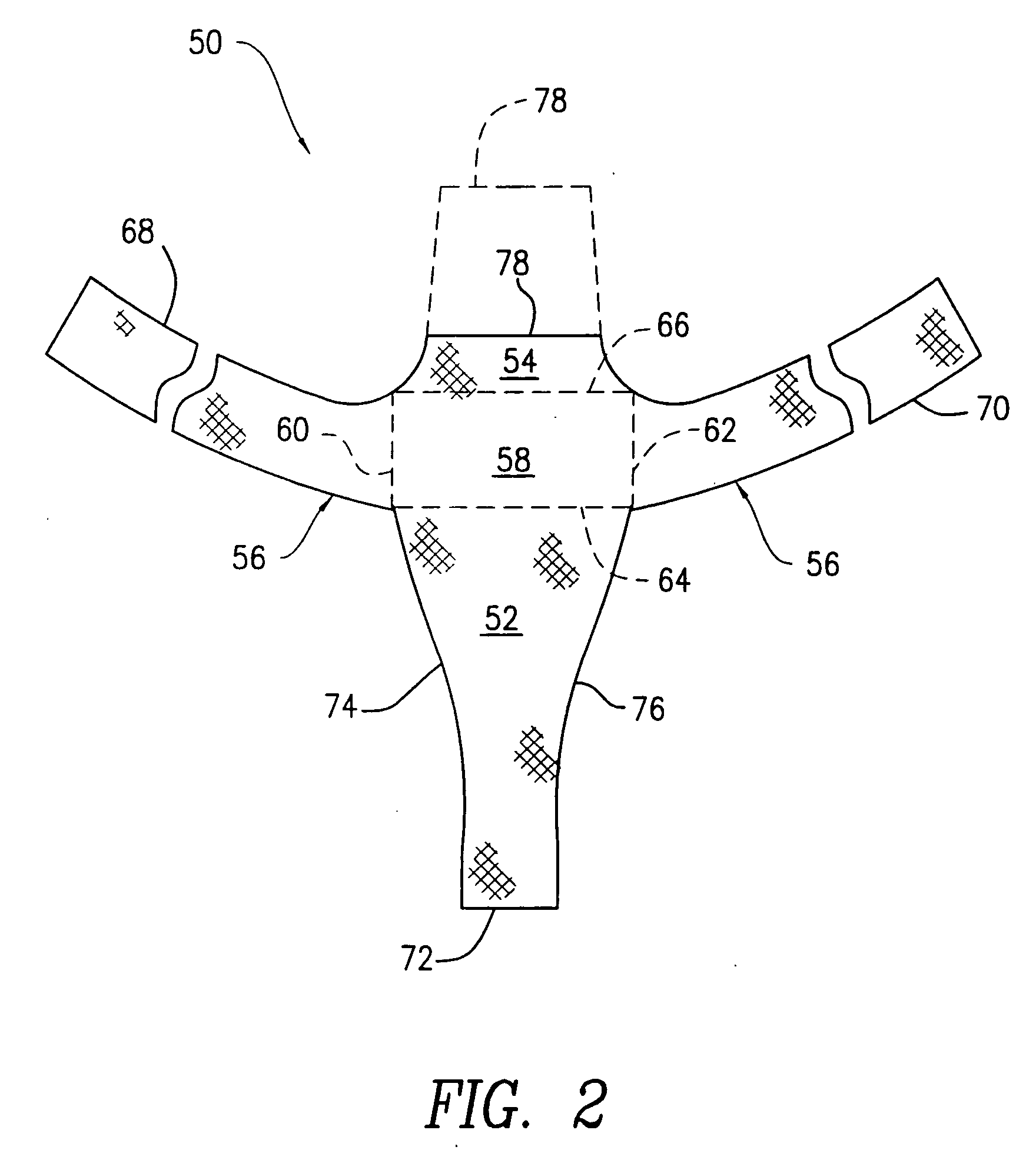
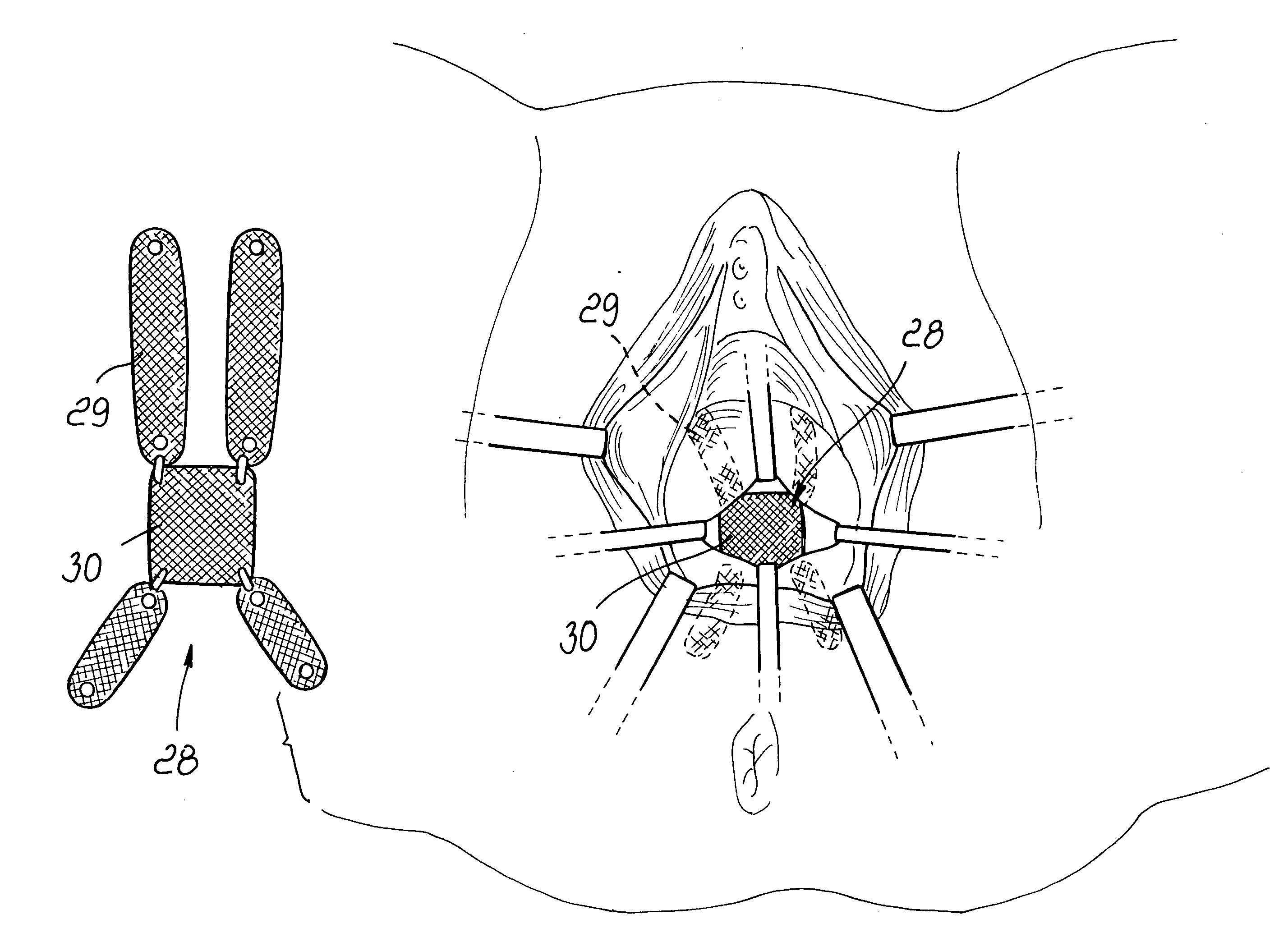
An anterior implant adapted to treat central and lateral cystoceles present in a female patient includes laterally extending stabilizing straps for supporting the implant between the patient's bladder and vagina independently of the patient's arcus tendineous fascia pelvis. Rectocele and hysterocele repairs can be carried out using a single posterior implant which, like the anterior implant, is provided with laterally extending stabilizing straps for supporting the implant between the patient's rectum and vagina.
Owner:ETHICON INC
Closure device and method
ActiveUS20120059394A1Prevent prolapseEasy to implementSuture equipmentsCannulasHemorrhoidsUltrasound
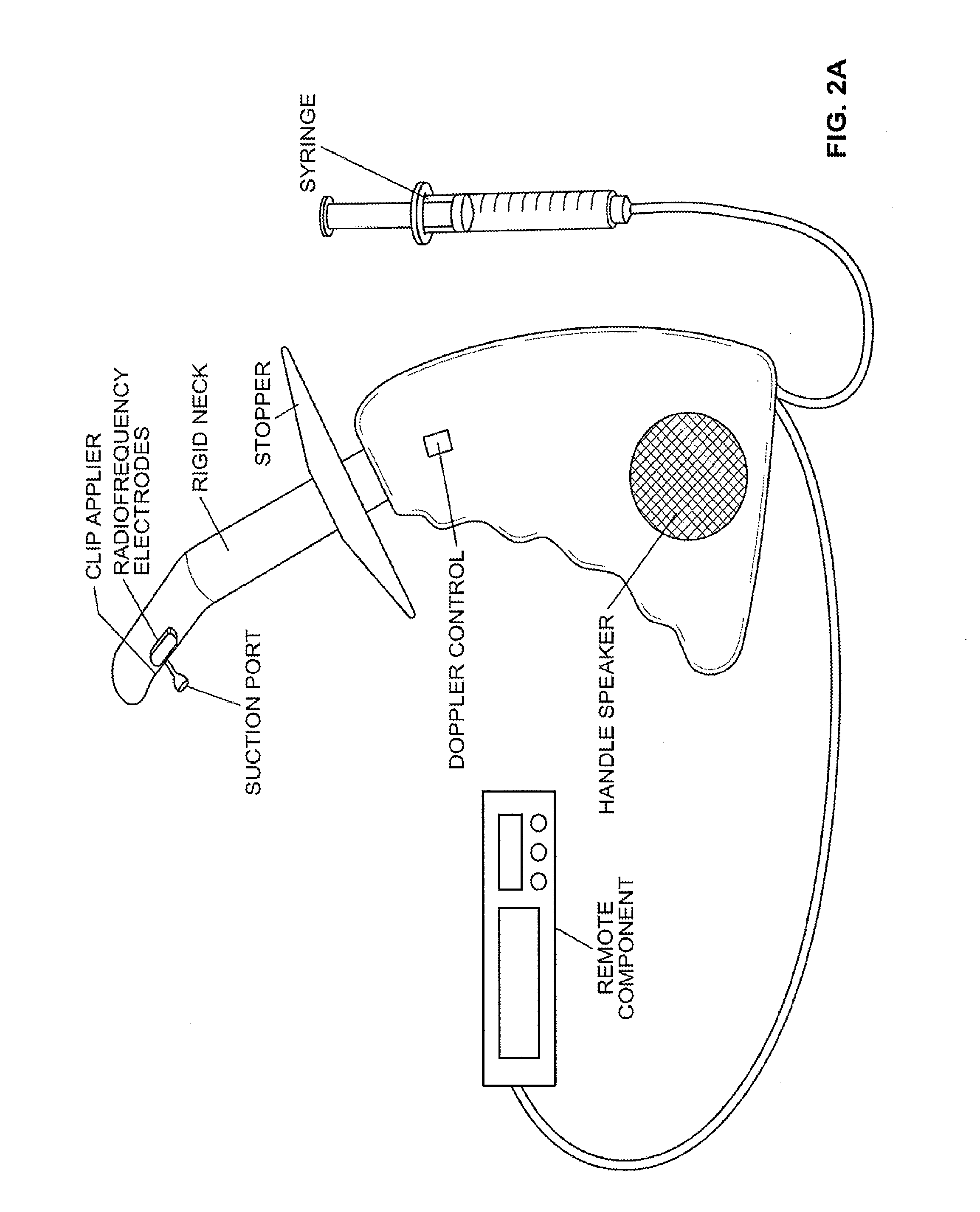
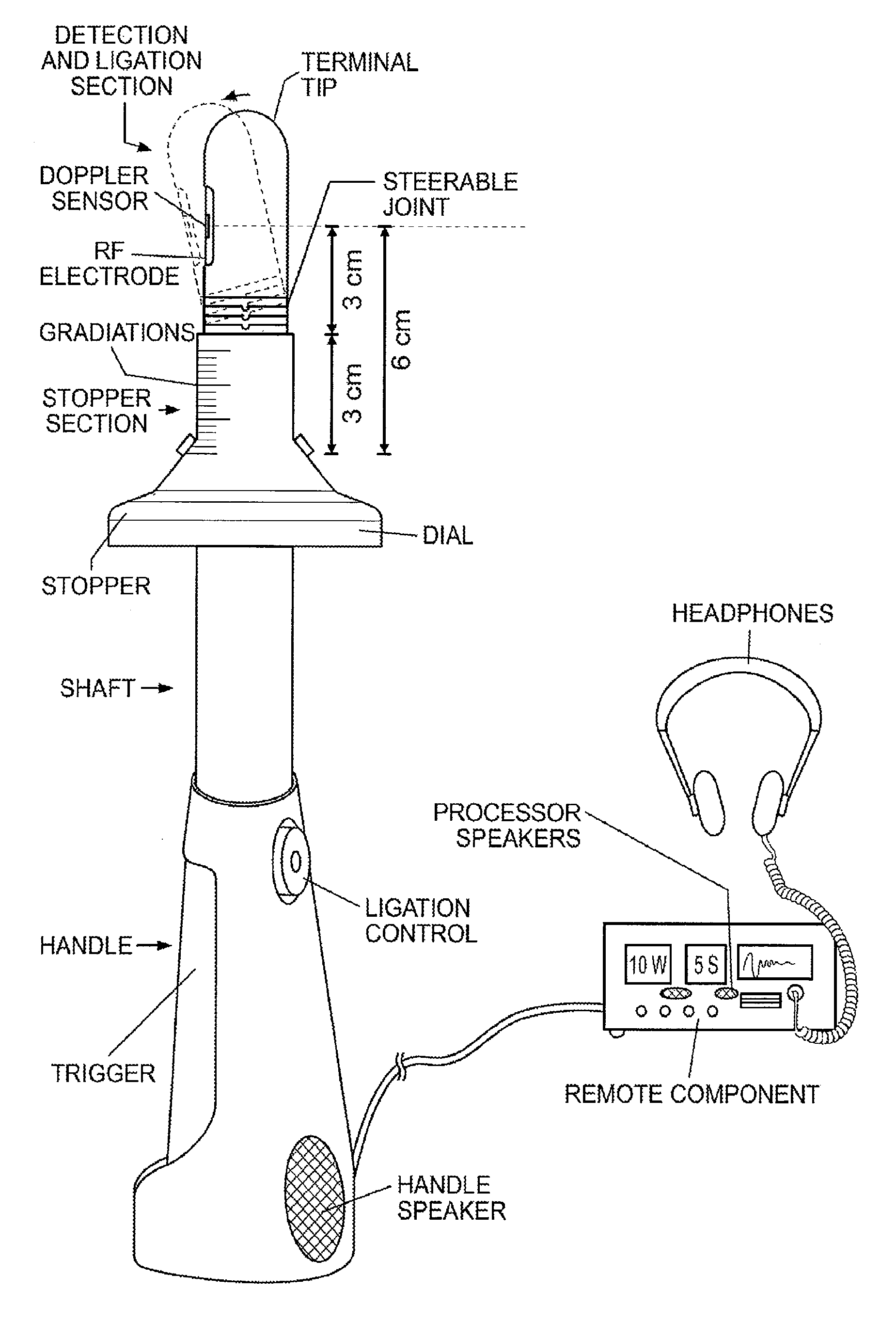
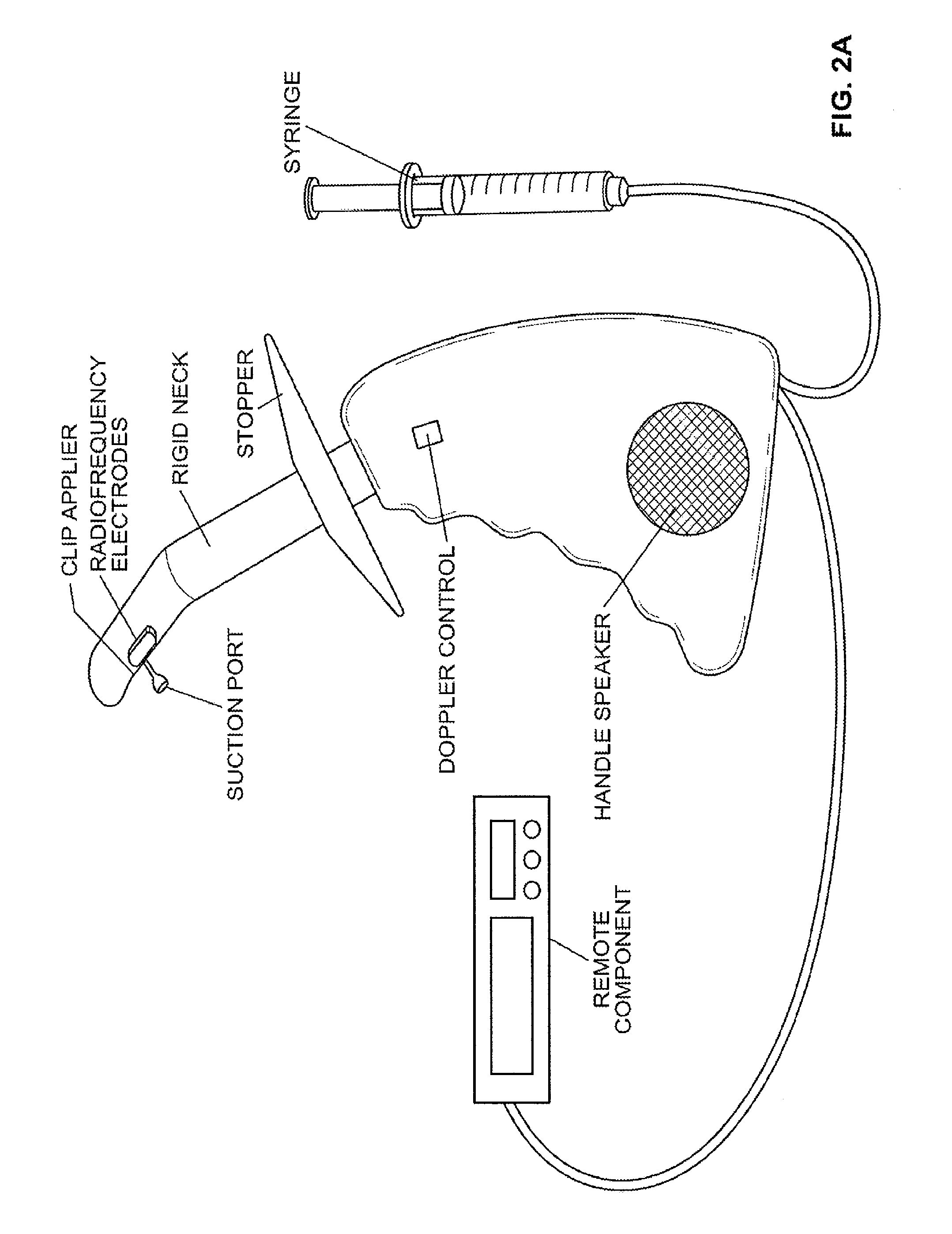
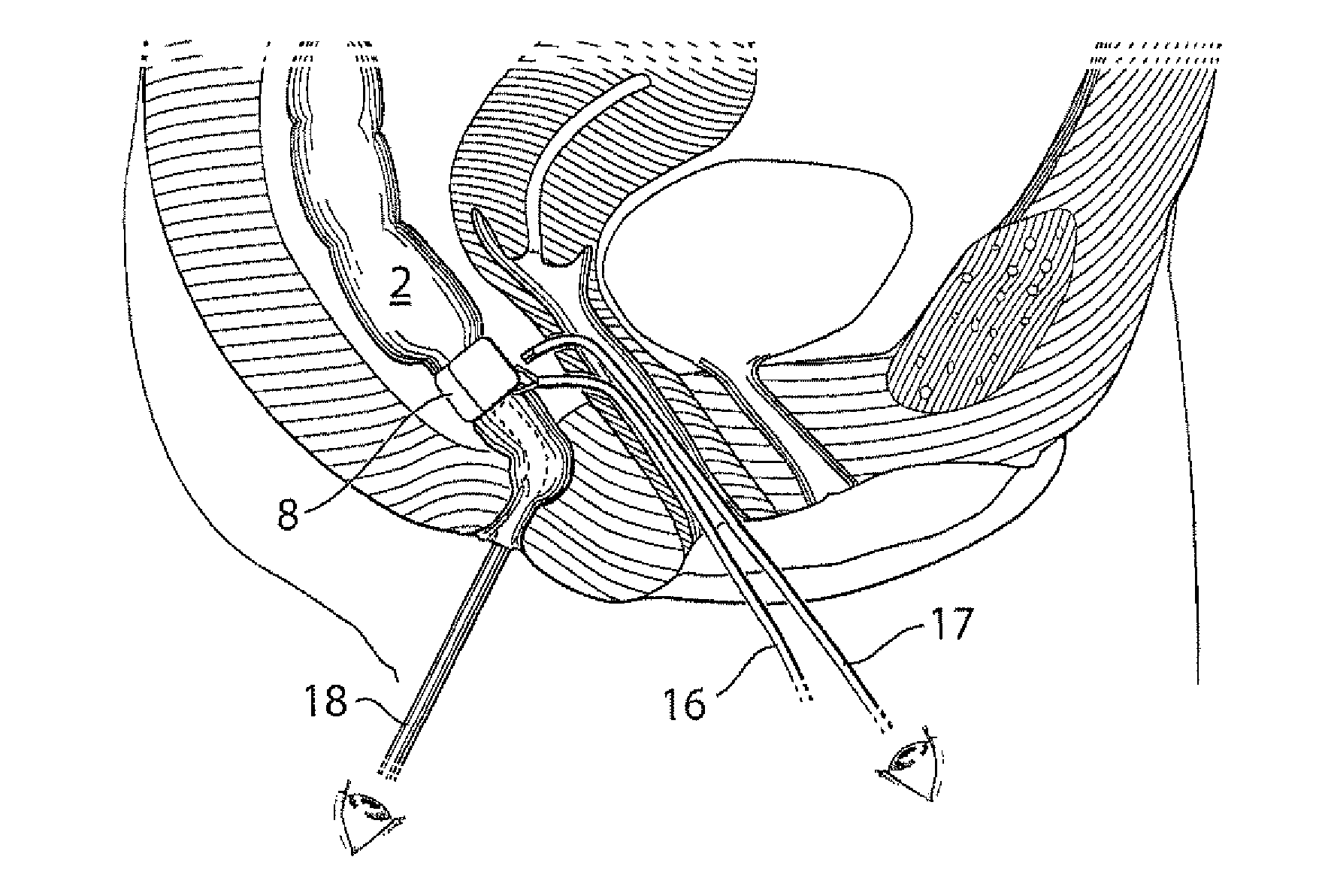
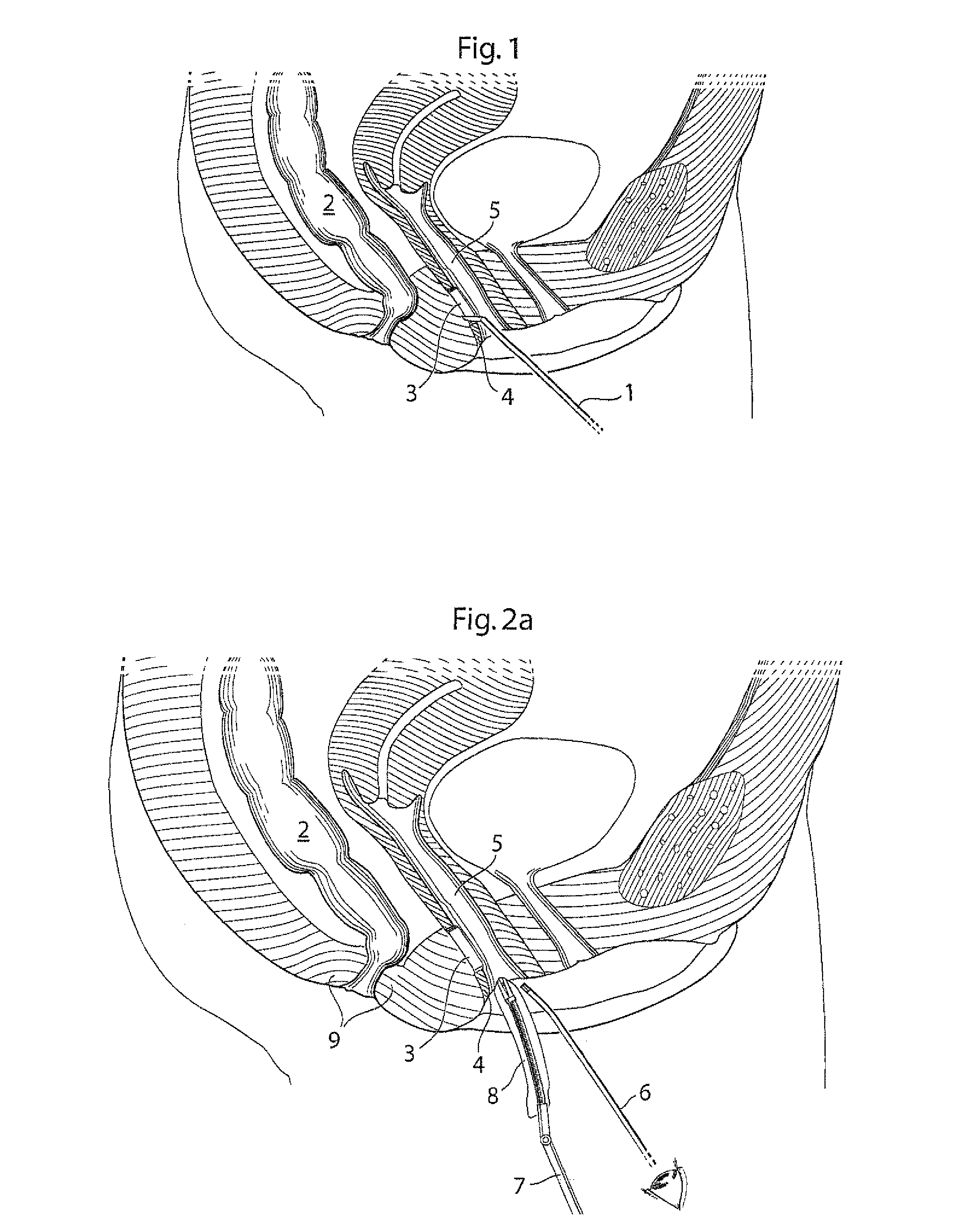
Devices and methods for treatment of prolapsed hemorrhoidal arteries is disclosed. The devices can identify the hemorrhoid and ligate the artery without causing significant pain or distension of the rectum. The artery can be identified with ultrasound. The ligation can be performed using energy and / or mechanical structures, such as clips or rubber bands.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Rectal manipulation devices
Tissue manipulation devices are disclosed. In various forms, the devices include tissue manipulation arms that are arranged in a position suitable to enable the device to be inserted into the colon then, upon application of at least one actuation motion thereto, at least some of the tissue manipulation arms are moved to deployed positions wherein they contact corresponding portions of the colon to thereby expand the colon. Various devices are actuatable by various forms of actuation forces. In various embodiments, the tissue manipulation arms may be movable along corresponding axes that are transverse to an insertion axis and may also be rotated about the insertion axis as well as be moved in directions that are substantially parallel to the insertion axis.
Owner:CILAG GMBH INT
Anoscope
An anoscope for use with a medical instrument insertable within the interior of the anoscope. The anoscope comprises a tubular member having a longitudinal axis with a proximal end and a distal end. The distal end is insertable into the rectum of a patient while the proximal end remains outside the body. A first longitudinal slot extends from the distal end toward the proximal end, and a second longitudinal slot extends from the proximal end toward the distal end. The first and second longitudinal slots are separated by about 180° on the surface of the tubular member. The first longitudinal slot is adapted to permit access to a site to be treated within the rectum and the second longitudinal slot is adapted to accommodate pivoting of the medical instrument at an angle to the longitudinal axis of the tubular member to improve access to the site to be treated.
Owner:CRH MEDICAL CORP
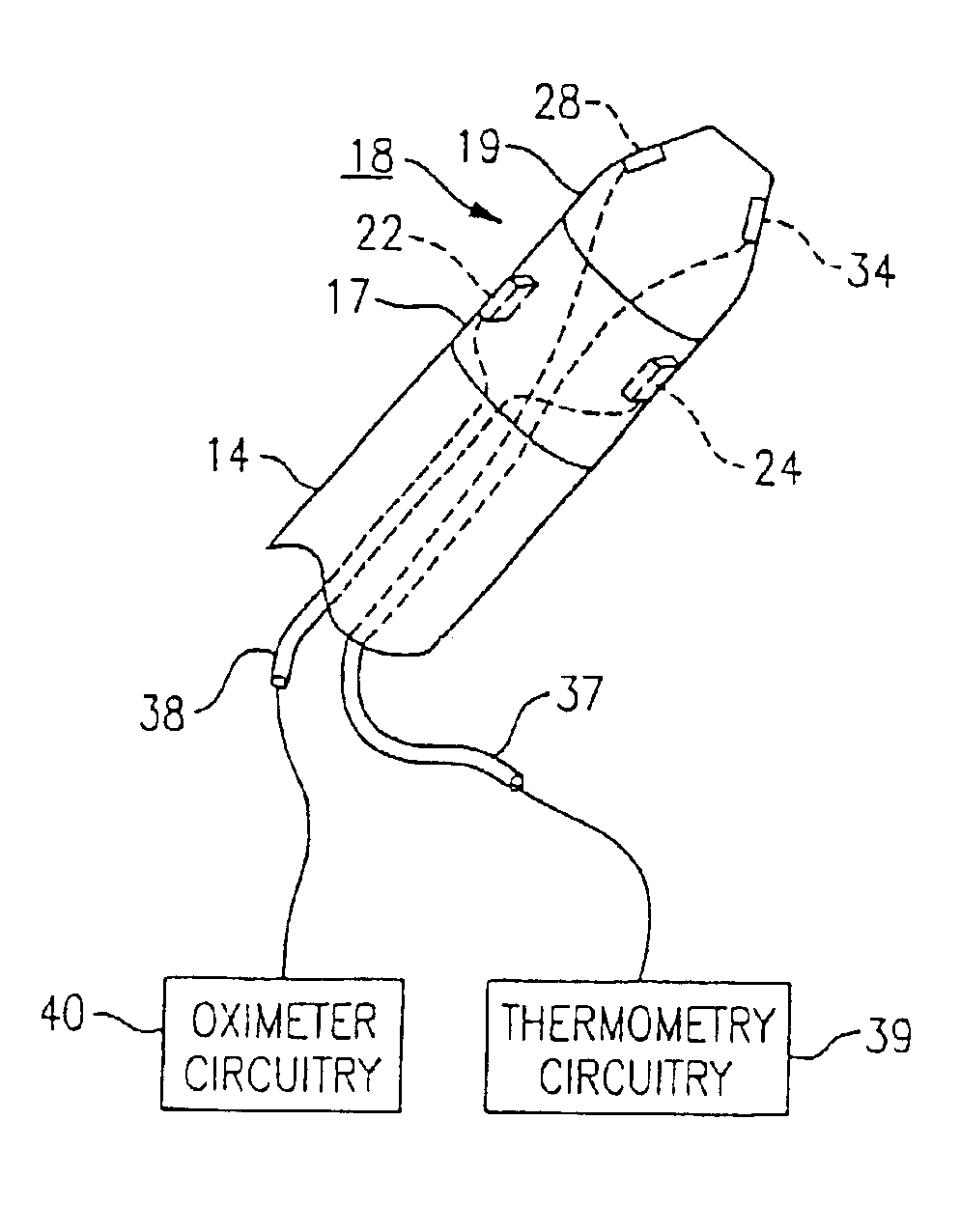
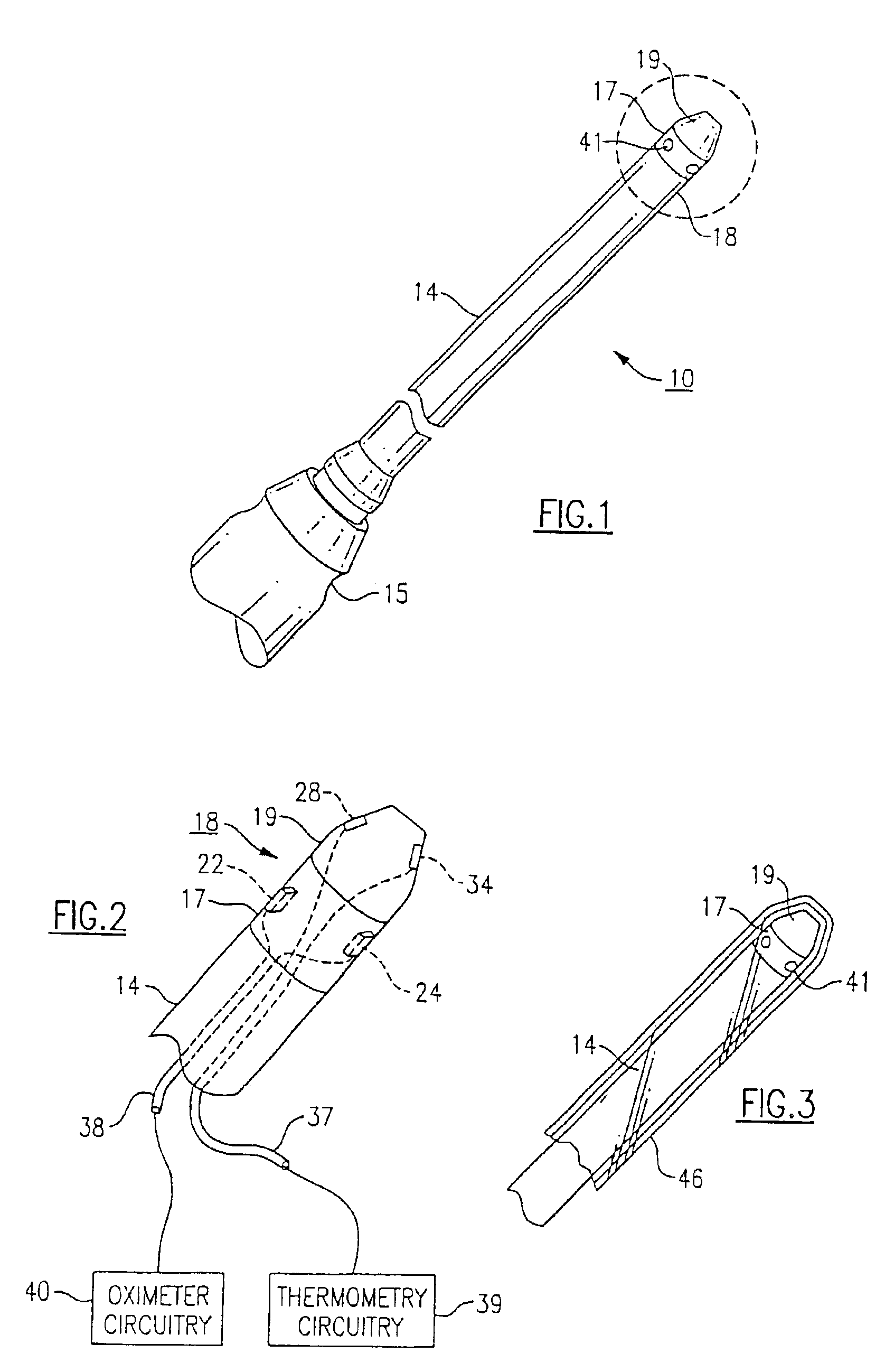
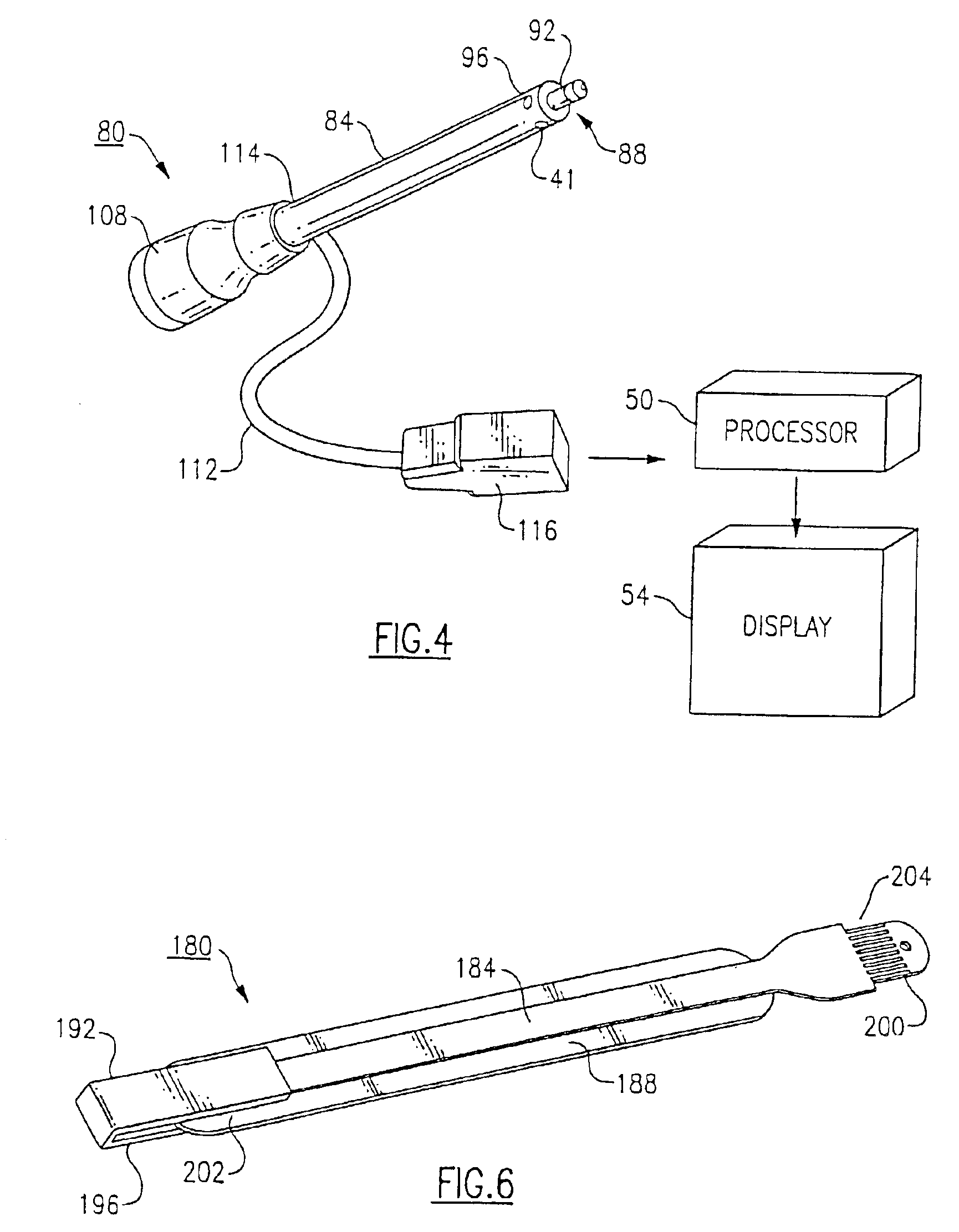
Combination SPO2/temperature measuring apparatus
InactiveUS6850789B2Hasten overall measurement timeAvoid thermal effectsRadiation pyrometryThermometers using electric/magnetic elementsMedicineAxilla
A medical diagnostic instrument includes at least one blood oxygen saturation sensor and at least one temperature sensor. The sensors are being capable of measuring blood oxygen saturation and temperature simultaneously after insertion of a probe portion of the instrument relative to a defined body site, such as the axilla, rectum, or sublingual pocket of a patient. At least a portion of the probe portion of the instrument is disposable.
Owner:WELCH ALLYN INC
Trans-rectum universal ports
Various universal ports for installation into a colon are disclosed. Various embodiments include expandable port bodies that may employ selectively deployable tissue-engaging barbs for securing the port in position within the colon. Some embodiments also employ tissue cutting members that may be deployed to sever the colon. Some embodiments also employ a flexible sleeve that may extend from the port out through the anus to provide a passageway for passing surgical instruments and into and out of the rectum and also for removing severed specimens therefrom.
Owner:CILAG GMBH INT
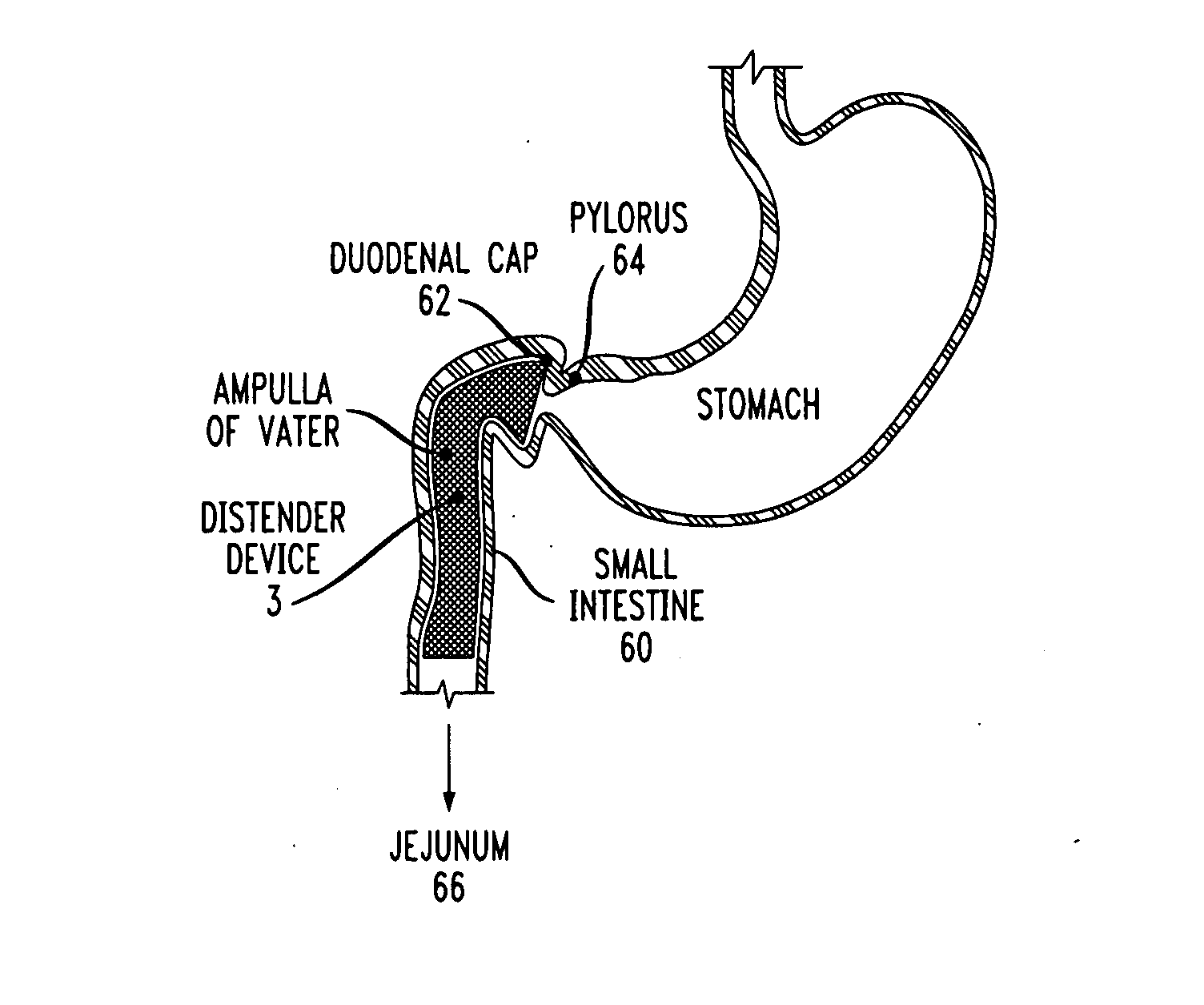
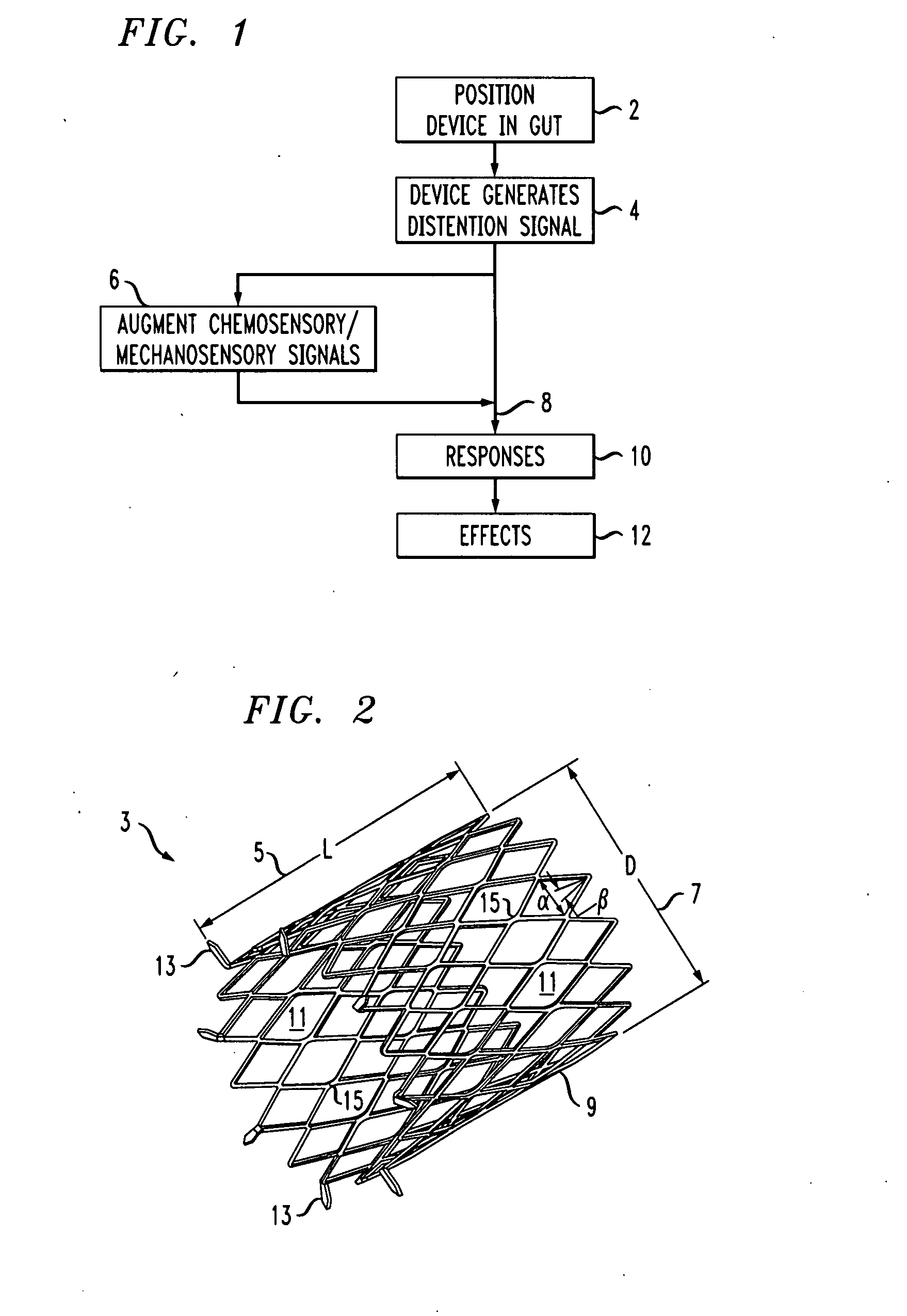
Distender device and method for treatment of obesity and metabolic and other diseases
A gastrointestinal implant device is positioned in a patient's small intestine or rectum and produces an outward force that itself produces a distension signal which is a therapeutically useful neural or humoral signal that evokes satiogenic or weight loss effects by itself. The device may advantageously be placed in the duodenum adjacent the pylorus or in the jejunum, ileum or rectum. The distension signals may amplify chemosensory or mechanosensory signals such as enteroendocrine secretions within the patient. The device may be a mesh and include a low material density that allows for unrestricted chyme absorption within the small intestine and unrestricted chyme flow through the gastrointestinal system. A method includes inserting the device into the patient then either retrieving the device after treatment is complete or allowing a device formed of a biodegradable material to degrade in time after treatment is complete.
Owner:ADVANCED NEUROMODULATION SYST INC
Apparatus for Posterior Pelvic Floor Repair
InactiveUS20090012353A1Provide supportProtect from harmSuture equipmentsAnti-incontinence devicesPelvic diaphragm muscleImproved method
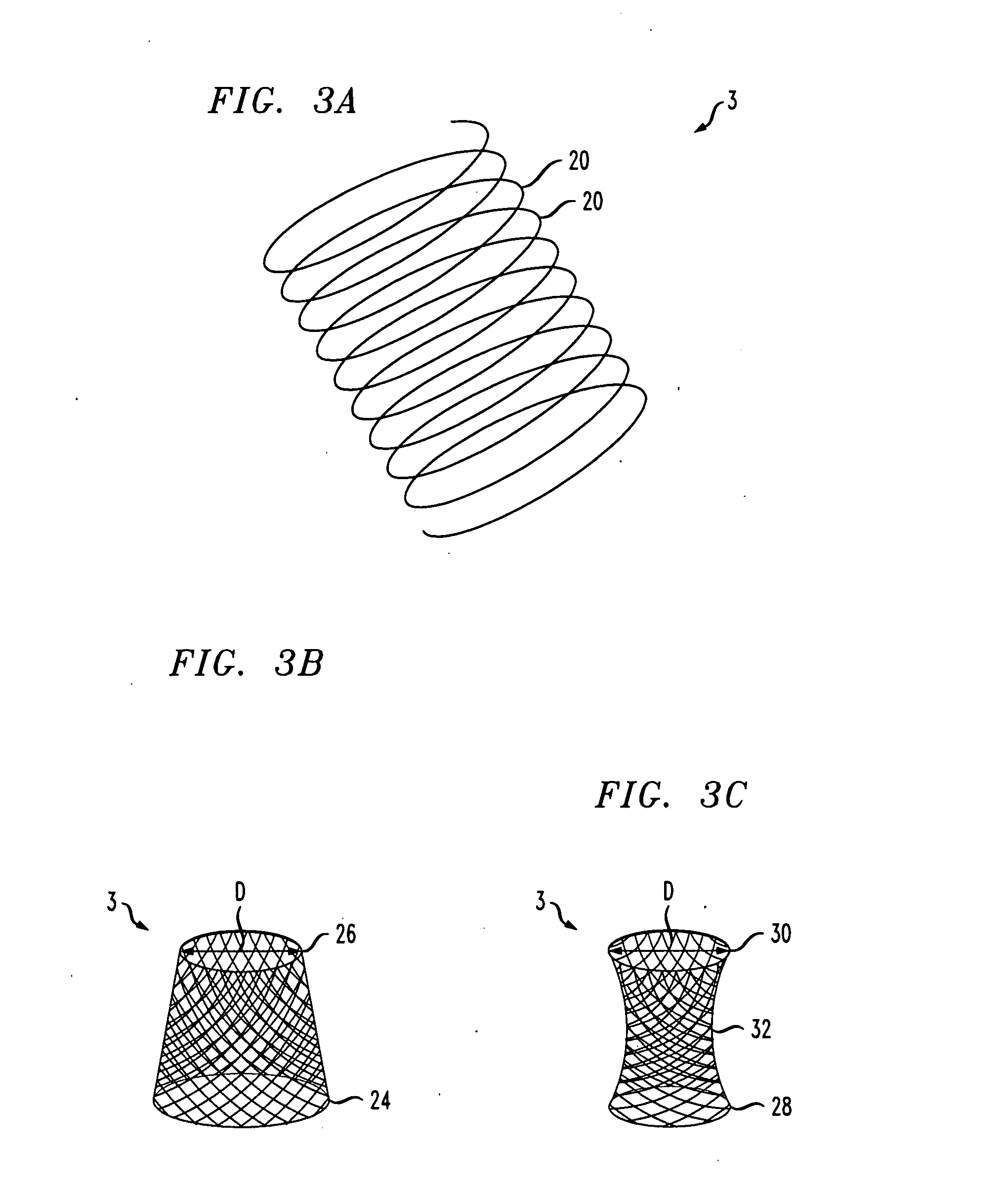
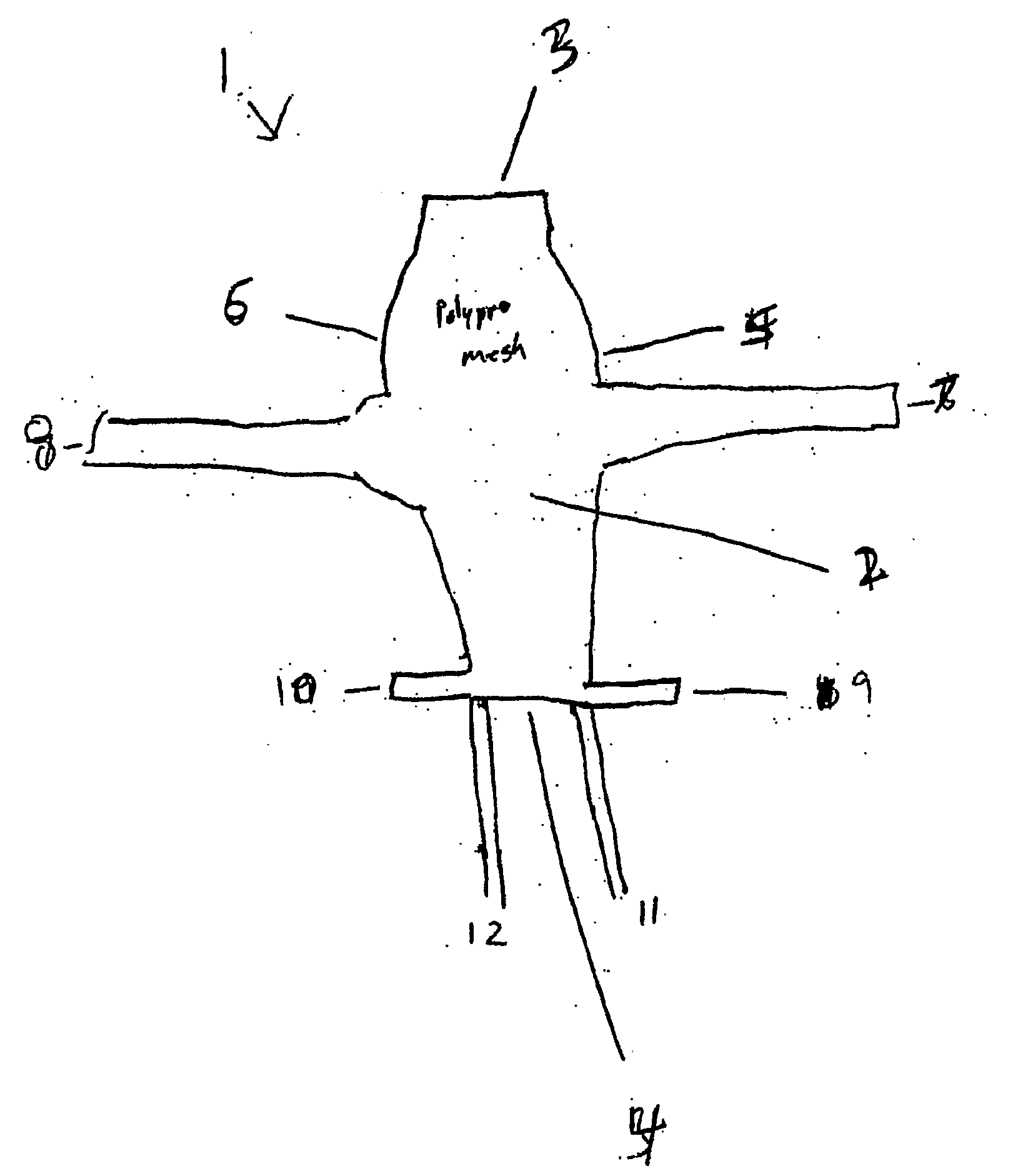
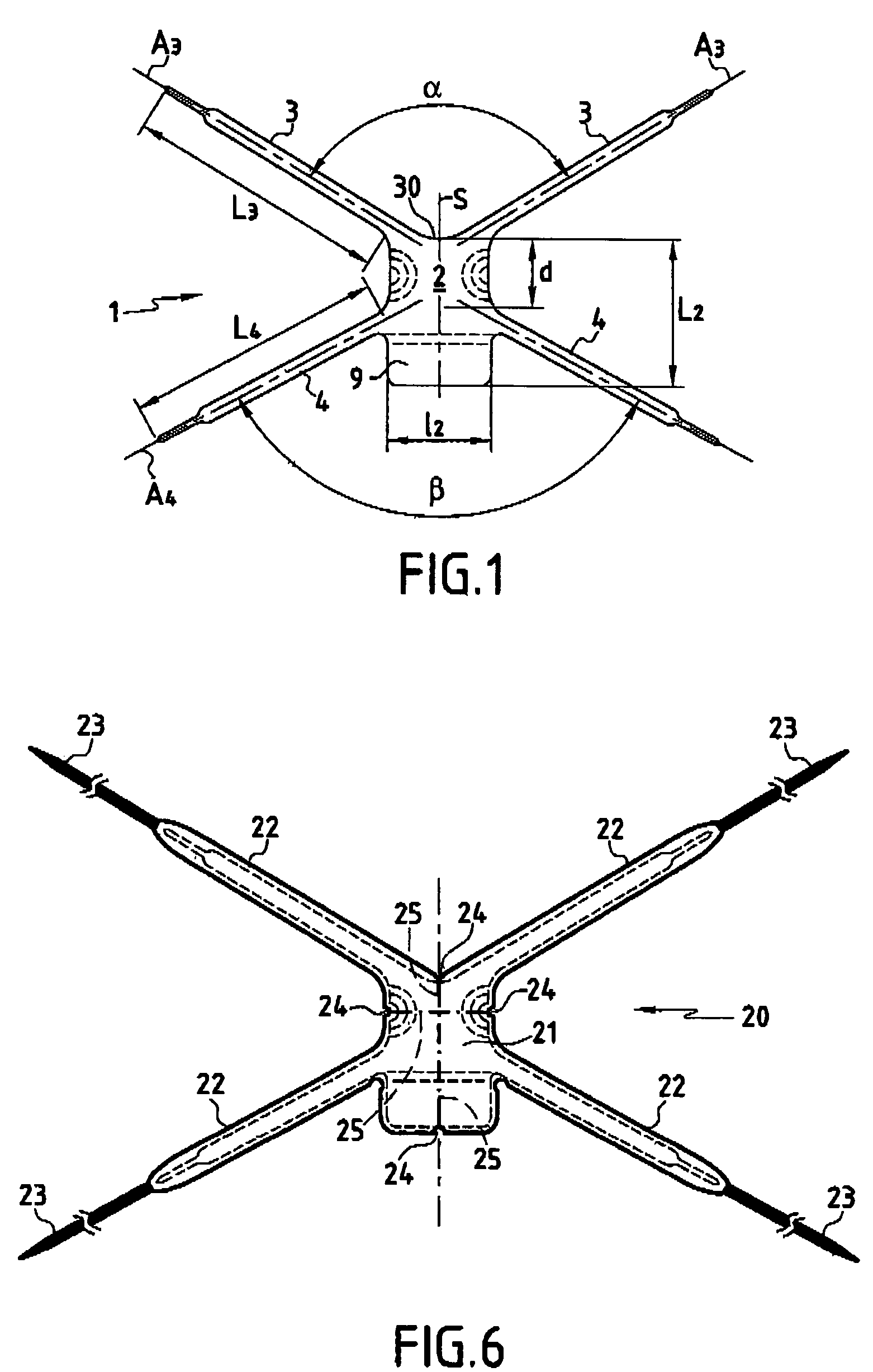
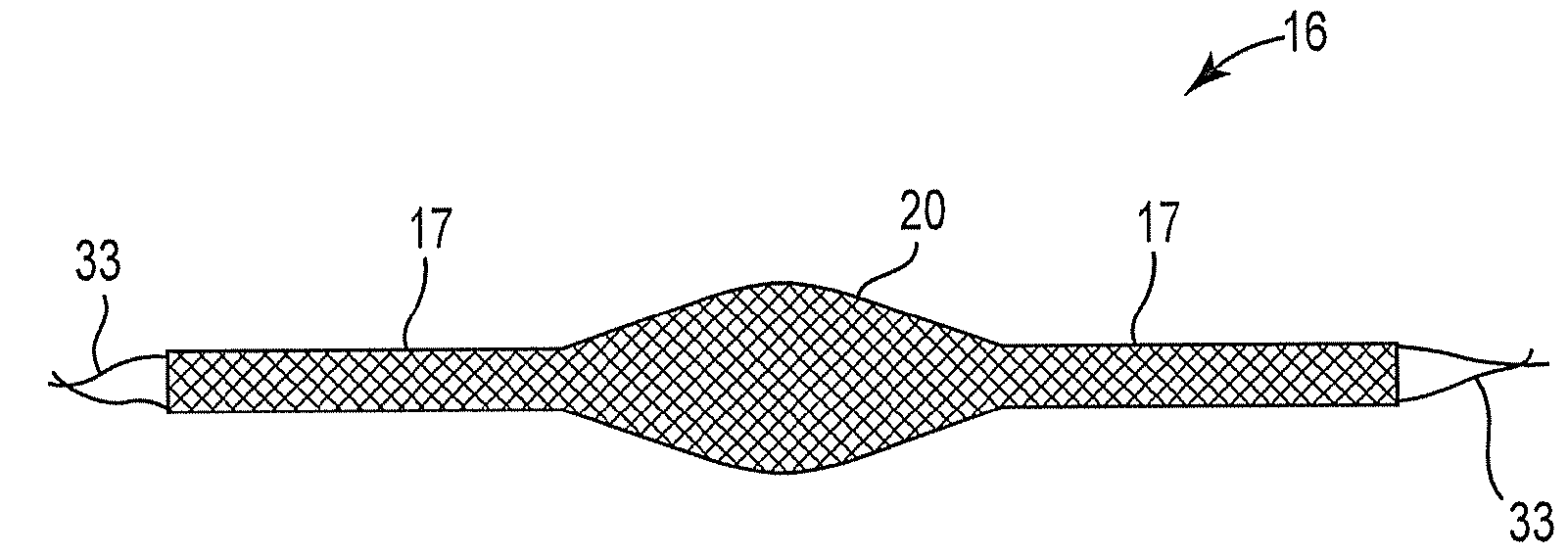
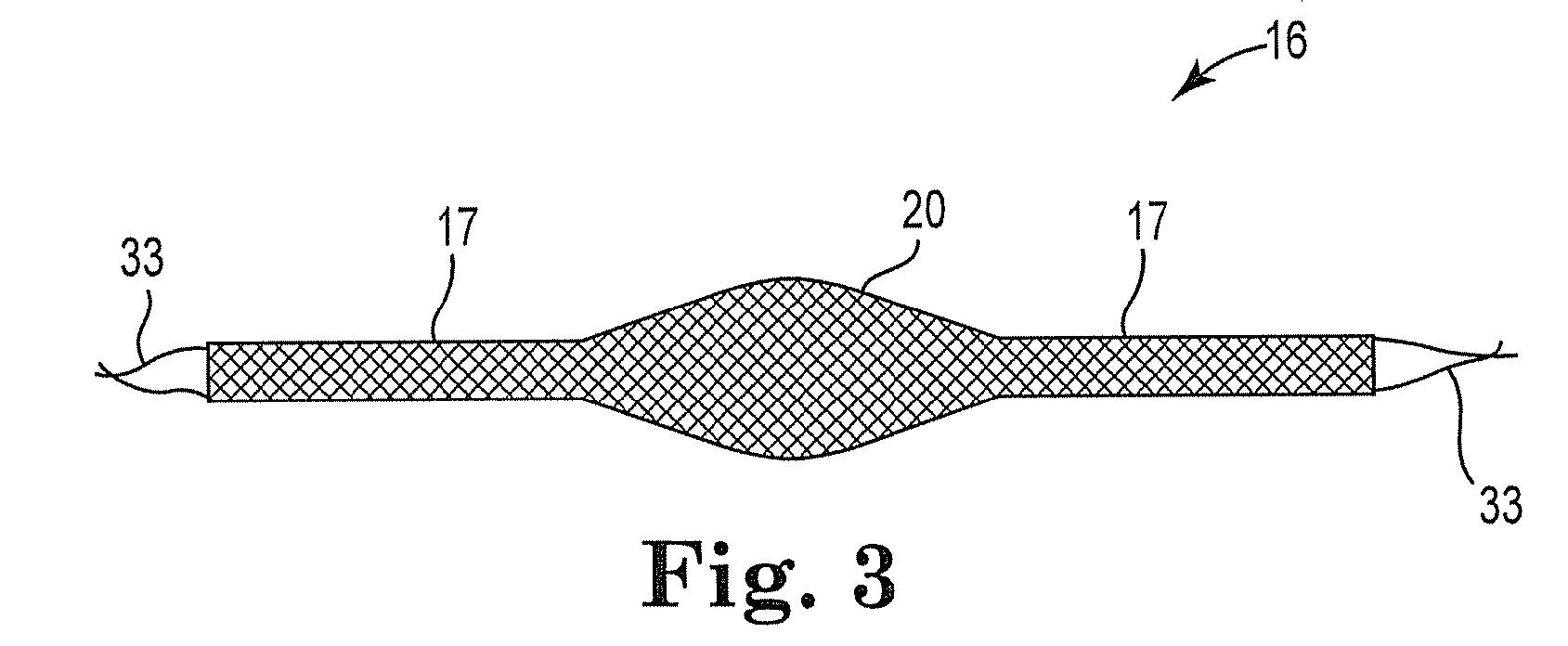
Improved methods and apparatuses for treatment of posterior pelvic floor repair, including rectocele and related pelvic organ prolapse, are provided. A specialized mesh (3) having a shape for convenient placement to treat rectocele by providing both level 2 and level 3 support. Appropriate devices for introducing such a mesh implant are also disclosed.
Owner:BEYER ROGER D
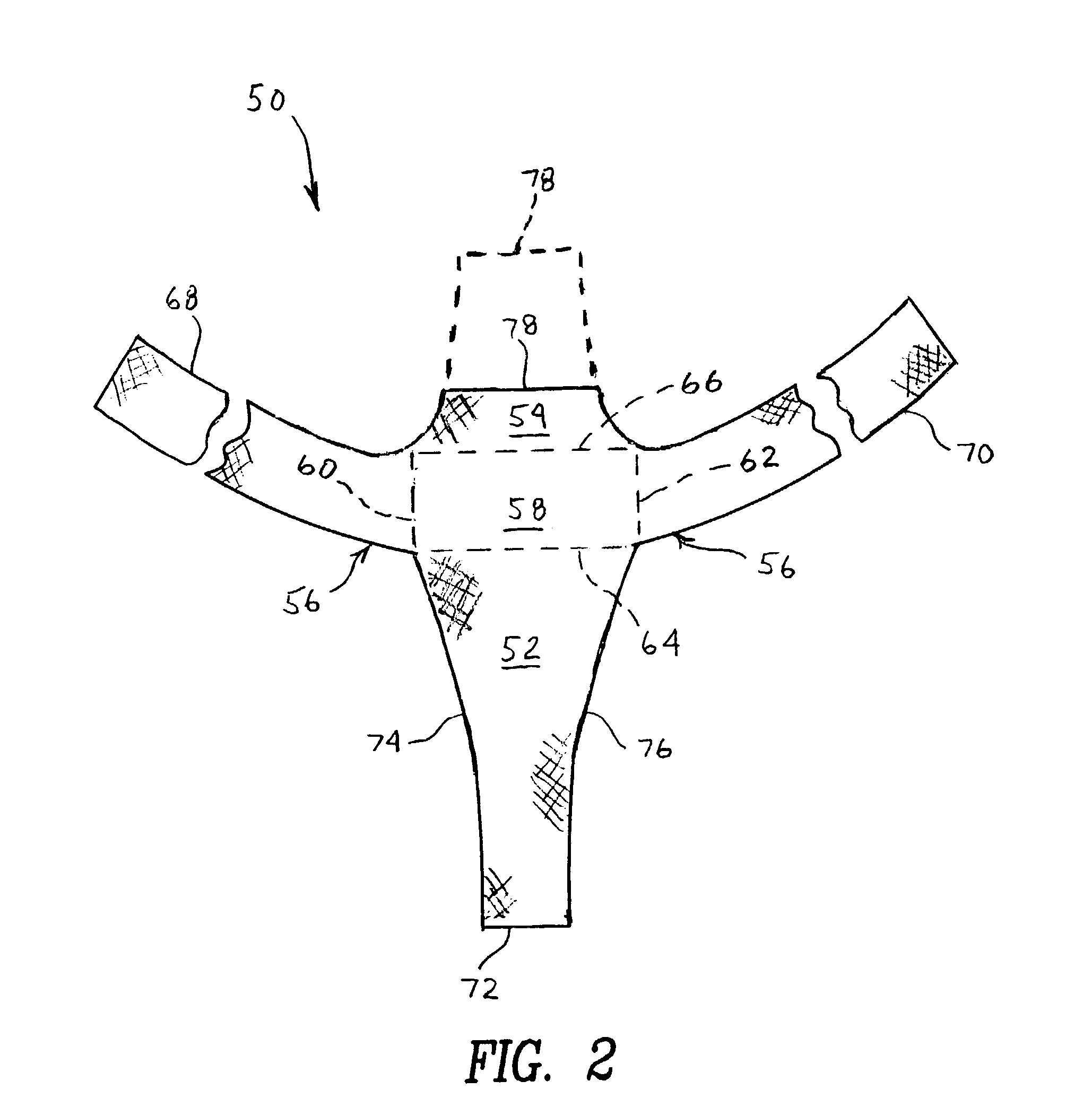
Implant for treating rectocele and a device for putting said implant into place
InactiveUS7588598B2Good stability of implantationMinimize traumaAnti-incontinence devicesSurgical needlesFiberPolyester
An implant for treating rectocele and / or prolapsus of the vaginal fornix is thin and flexible and includes a support body from which there extend at least two upper suspension stabilizers disposed on either side of a sagittal plane and two lower suspension stabilizers disposed on either side of the sagittal plane. The implant may be constructed from a suitable biocompatible material such as a woven synthetic material, or a knitted material of polypropylene or polyester fibers.
Owner:COLOPLAST AS
Method and articles for treatment of stress urinary incontinence
ActiveUS20100010631A1Minimal complicationMinimal painAnti-incontinence devicesMedical devicesUrethraPelvic diaphragm muscle
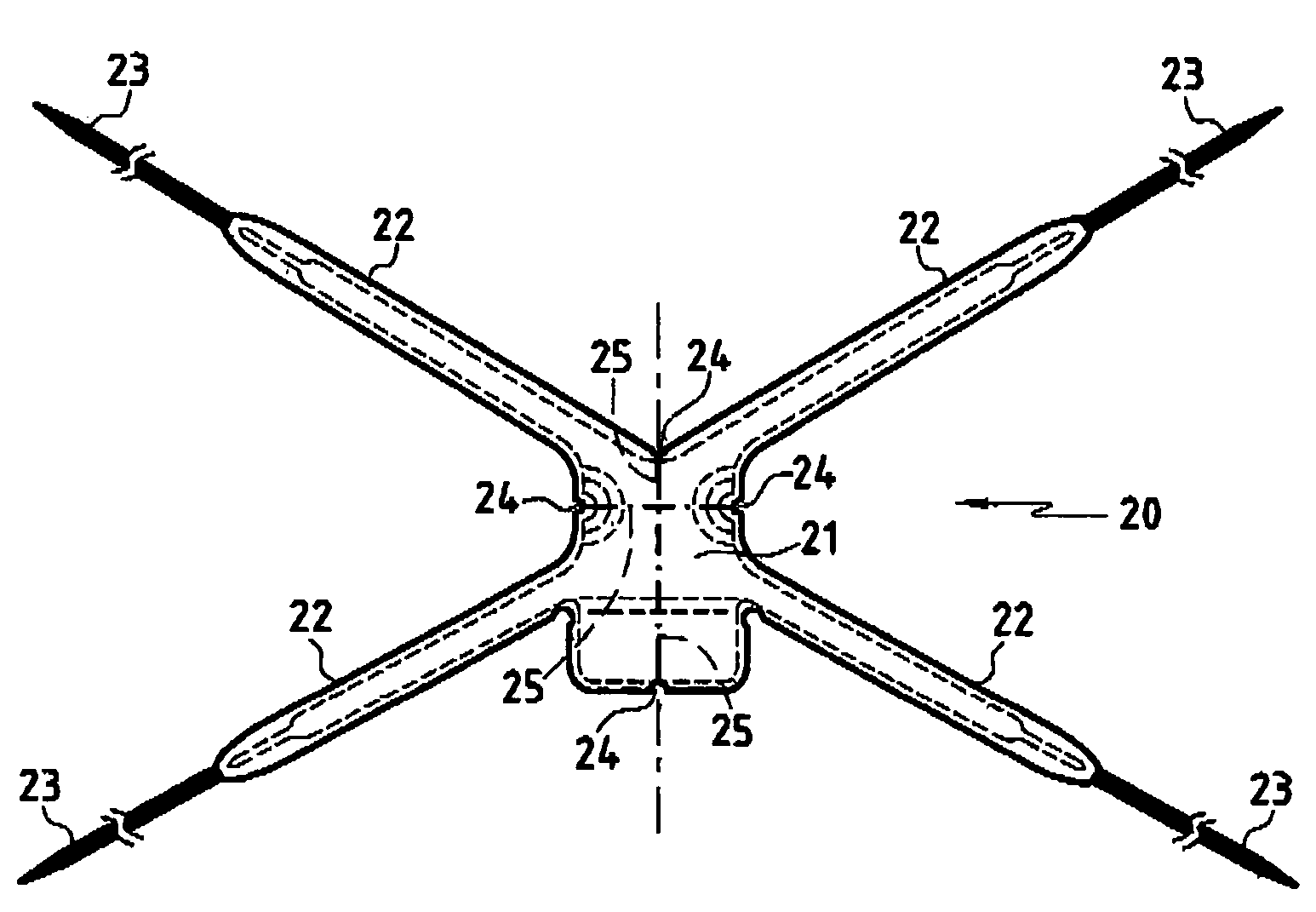
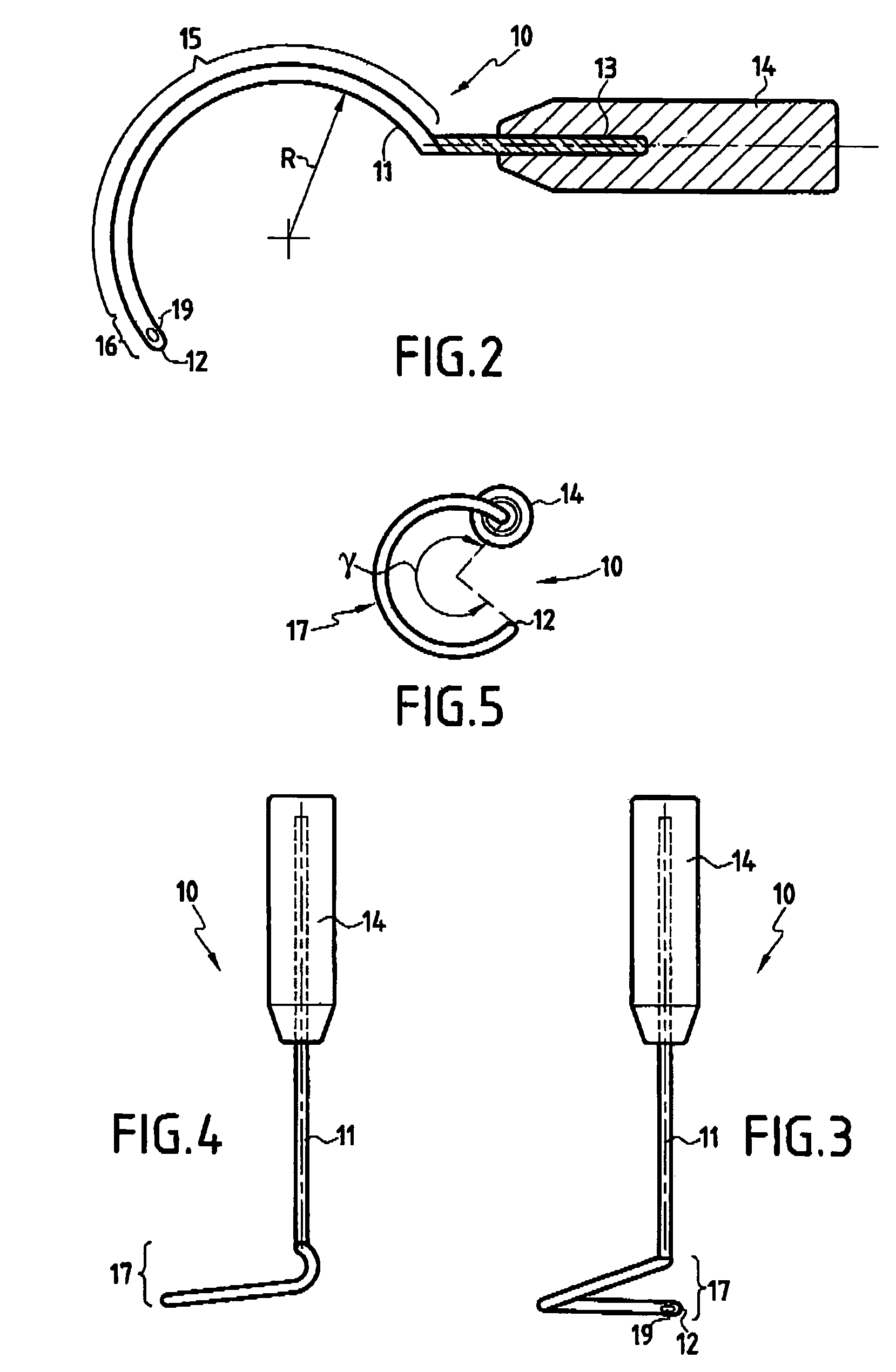
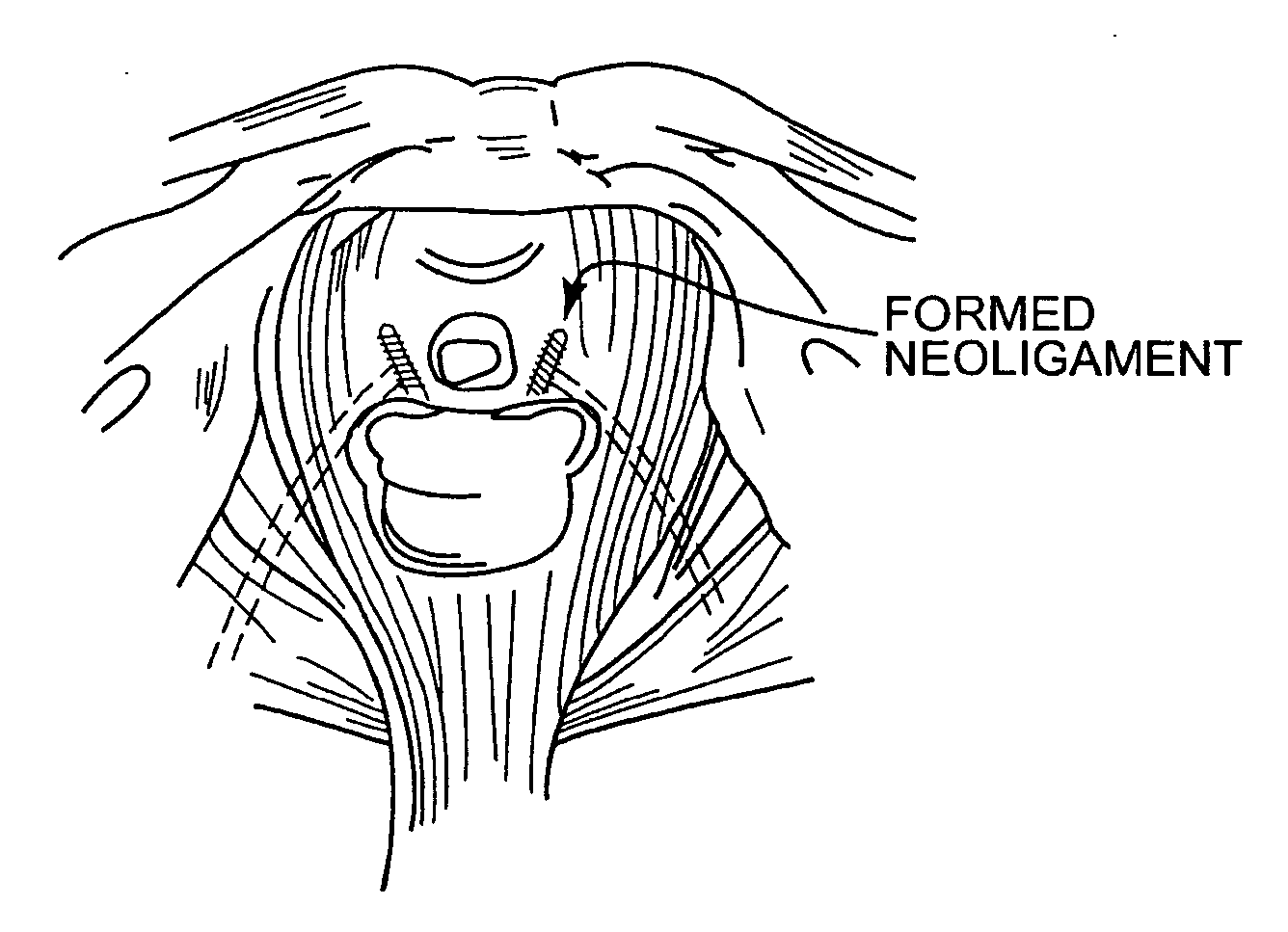
Improved methods and apparatuses for treatment of incontinence or pelvic organ prolapse are provided. A neo ligament for increasing the supportive function of the tissues supporting the urethra, bladder neck, or rectum is disclosed. Methods of using such a neoligament to treat incontinence are disclosed. Additionally, a self tensioning elastic tissue bolster for support of the tissues that support the urethra, rectum, and bladder neck are disclosed. A method of treating incontinence by injecting proliferative agents into supportive structures is disclosed. Methods and apparatus for transvaginal and transperitieai pelvic floor bolster treatment of incontinence and bladder pillar systems are also disclosed.
Owner:BOSTON SCI SCIMED INC
Method and apparatus for treating pelvic organ prolapses in female patients
An anterior implant adapted to treat central and lateral cystoceles present in a female patient includes laterally extending stabilizing straps for supporting the implant between the patient's bladder and vagina independently of the patient's arcus tendineous fascia pelvis. Rectocele and hysterocele repairs can be carried out using a single posterior implant which, like the anterior implant, is provided with laterally extending stabilizing straps for supporting the implant between the patient's rectum and vagina.
Owner:ETHICON INC
System and method for treatment of anal incontinence and pelvic organ prolapse
ActiveUS7794385B2Facilitate minimally-invasive treatment of anal incontinenceSuture equipmentsAnti-incontinence devicesThighFecal incontinence
Using a sling that includes a central portion and at least two arms extending from the central portion, a method of treating anal incontinence may include positioning the central portion posteriorly to the rectum and / or anus of a subject, and extending each arm of the sling to a respective obturator region. Using a sling having the same or similar structure, a method of treating pelvic organ prolapse may include positioning the central portion beneath the ano-rectum of a subject, and extending each arm of the sling to a respective thigh incision near the obturator region.
Owner:ROSENBLATT ASSOC
Compounds as rearranged during transfection (RET) inhibitors
This invention relates to novel compounds which are inhibitors of the Rearranged during Transfection (RET) kinase, to pharmaceutical compositions containing them, to processes for their preparation, and to their use in therapy, alone or in combination, for the normalization of gastrointestinal sensitivity, motility and / or secretion and / or abdominal disorders or diseases and / or treatment related to diseases related to RET dysfunction or where modulation of RET activity may have therapeutic benefit including but not limited to all classifications of irritable bowel syndrome (IBS) including diarrhea-predominant, constipation-predominant or alternating stool pattern, functional bloating, functional constipation, functional diarrhea, unspecified functional bowel disorder, functional abdominal pain syndrome, chronic idiopathic constipation, functional esophageal disorders, functional gastroduodenal disorders, functional anorectal pain, inflammatory bowel disease, proliferative diseases such as non-small cell lung cancer, hepatocellular carcinoma, colorectal cancer, medullary thyroid cancer, follicular thyroid cancer, anaplastic thyroid cancer, papillary thyroid cancer, brain tumors, peritoneal cavity cancer, solid tumors, other lung cancer, head and neck cancer, gliomas, neuroblastomas, Von Hippel-Lindau Syndrome and kidney tumors, breast cancer, fallopian tube cancer, ovarian cancer, transitional cell cancer, prostate cancer, cancer of the esophagus and gastroesophageal junction, biliary cancer, adenocarcinoma, and any malignancy with increased RET kinase activity.
Owner:GLAXOSMITHKLINE INTPROP DEV LTD
Endometriosis treatment protocol
The endometriosis treatment protocol provides for administering to a female patient in need of treatment for endometriosis a pharmaceutical composition in a form suitable for vaginal or rectal delivery having a pharmaceutically effective amount of an aromatase inhibitor, which may be either a steroid or non-steroidal. The pharmaceutical composition may be formed as a vaginal suppository, a rectal suppository, a vaginal gel, a rectal gel, a vaginal cream or a rectal cream. The pharmaceutical composition may optionally have pharmaceutically effective amounts of progesterone and calcitriol, and may be administered in combination with an oral COX-2 inhibitor. Alternatively, the pharmaceutical composition comprises an aromatase inhibitor administered vaginally or rectally and is administered in combination with oral calcitriol and the oral COX-2 inhibitor. The aromatase inhibitor is either steroidal or non-steroidal.
Owner:SHIPPEN EUGENE R
Closure device and method
ActiveUS9232947B2Easy to implementPrevent prolapseSuture equipmentsInternal osteosythesisHemorrhoidsUltrasound
Devices and methods for treatment of prolapsed hemorrhoidal arteries is disclosed. The devices can identify the hemorrhoid and ligate the artery without causing significant pain or distension of the rectum. The artery can be identified with ultrasound. The ligation can be performed using energy and / or mechanical structures, such as clips or rubber bands.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Self-expanding device for the gastrointestinal or urogenital area
InactiveUS20060142794A1Lower the volumeLose weightSurgeryDilatorsIntestinal structureReproductive tract
Devices for treatments of diseases and disorders associated with the gastrointestinal tract, especially the stomach, or urinogenital tract are described herein. Initially, the device is in a temporary form which is suitable for oral or rectal administration. After exposure to a stimulus, such as a temperature or pH change, the device changes shape to a permanent form, which allows it to become mechanically fixed in the stomach, esophagus or intestine. In one embodiment, the device is used to reduce the volume of the stomach, esophagus or intestine without interfering with the flow of the food through the gastrointestinal tract. The device may be used to help overweight patients lose weight and to deliver drugs to treat disorders and diseases in the in the stomach or intestine. The devices are manufactured from a stimuli-sensitive polymeric material, which is biocompatible and primarily adapted to the mechanical properties and geometry in the area to which it is applied. In the preferred embodiment, the material is a shape memory polymer. Depending on the desired application, the polymer may be either biodegradable or non-degradable.
Owner:MINEMOSCIENCE GMBH
Bowel management system
Owner:HOLLISTER INCORPORAED
Medical probe
A probe system for electro-stimulation and bio-feedback training of muscles in the pelvic floor region, in particular for pelvic floor physiotherapy and diagnosis, includes a probe having a probe body which is insertable into a vagina or a rectum, and a plurality of electrodes which are positioned at several locations along the length and around the circumference on the outer surface of the probe, the probe system further includes a control unit, operationally coupled to the probe, adapted for receiving EMG signals from each of the electrodes and for processing each of the signals for mapping the response of the muscles in the pelvic floor region.
Owner:NOVUQARE PELVIC HEALTH BV
Foldable Brush Self-sampling Device
InactiveUS20080188769A1Easy constructionInexpensive materialsSurgical needlesVaccination/ovulation diagnosticsSelf samplingNasal Cavity Epithelium
A foldable brush self-sampling device for collecting of cell specimens from a body cavity, such as cervix, endometrium, rectum, nasal cavity and fauces, for diagnostic purposes, allows a user to collect cell specimens in the privacy of home. The device includes a sheath and a brush bar. The brush bar would fold into the sheath, which is with smaller diameter. The user inserts the sheath into the cavity easily and pushes the brush bar to expose the brush elements. After insertion of the sheath and push of the brush bar, the brush bar is rotated to obtain the specimen from the body cavity and then retracted in order to return the sheath.
Owner:LU LI CHENG
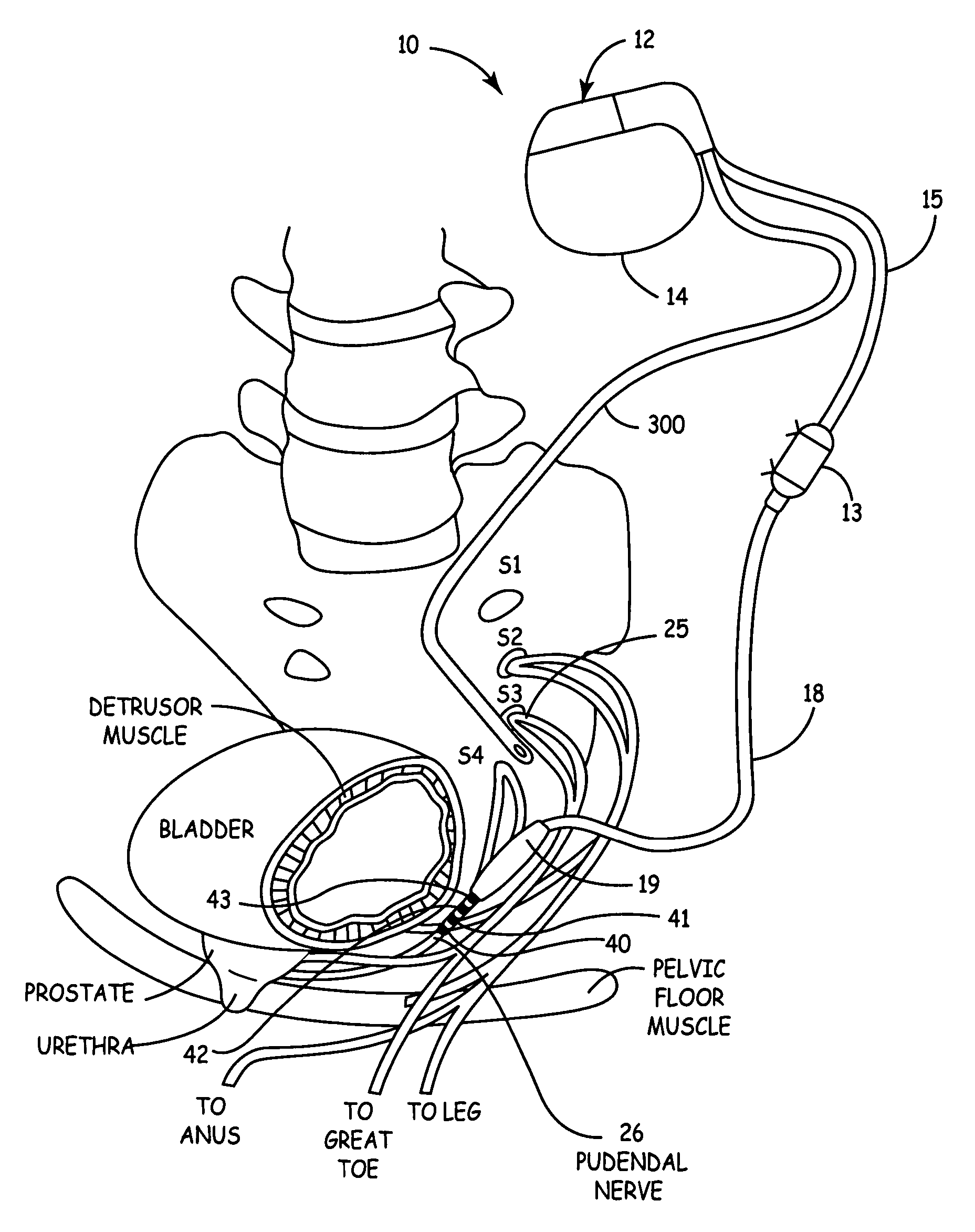
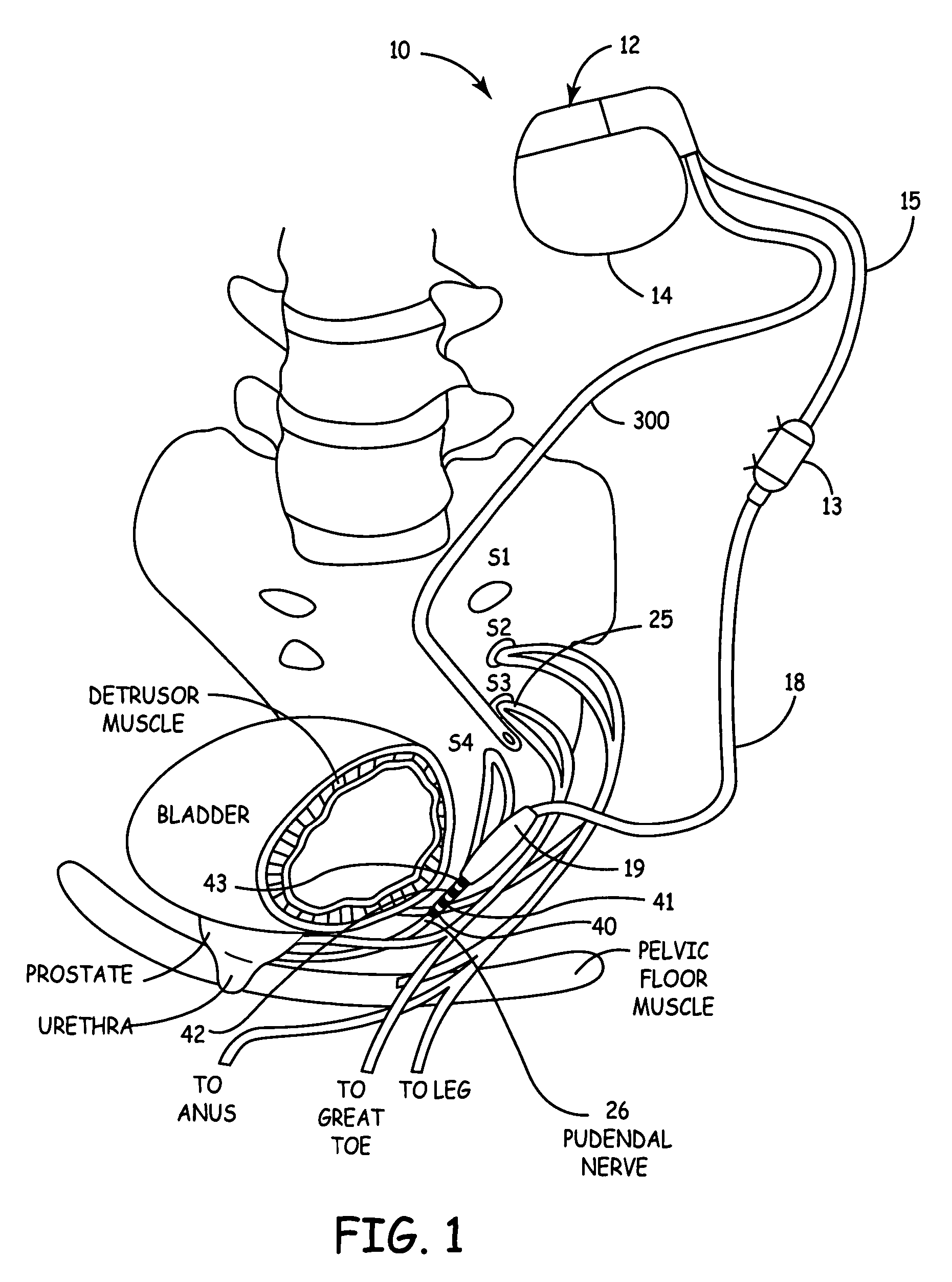
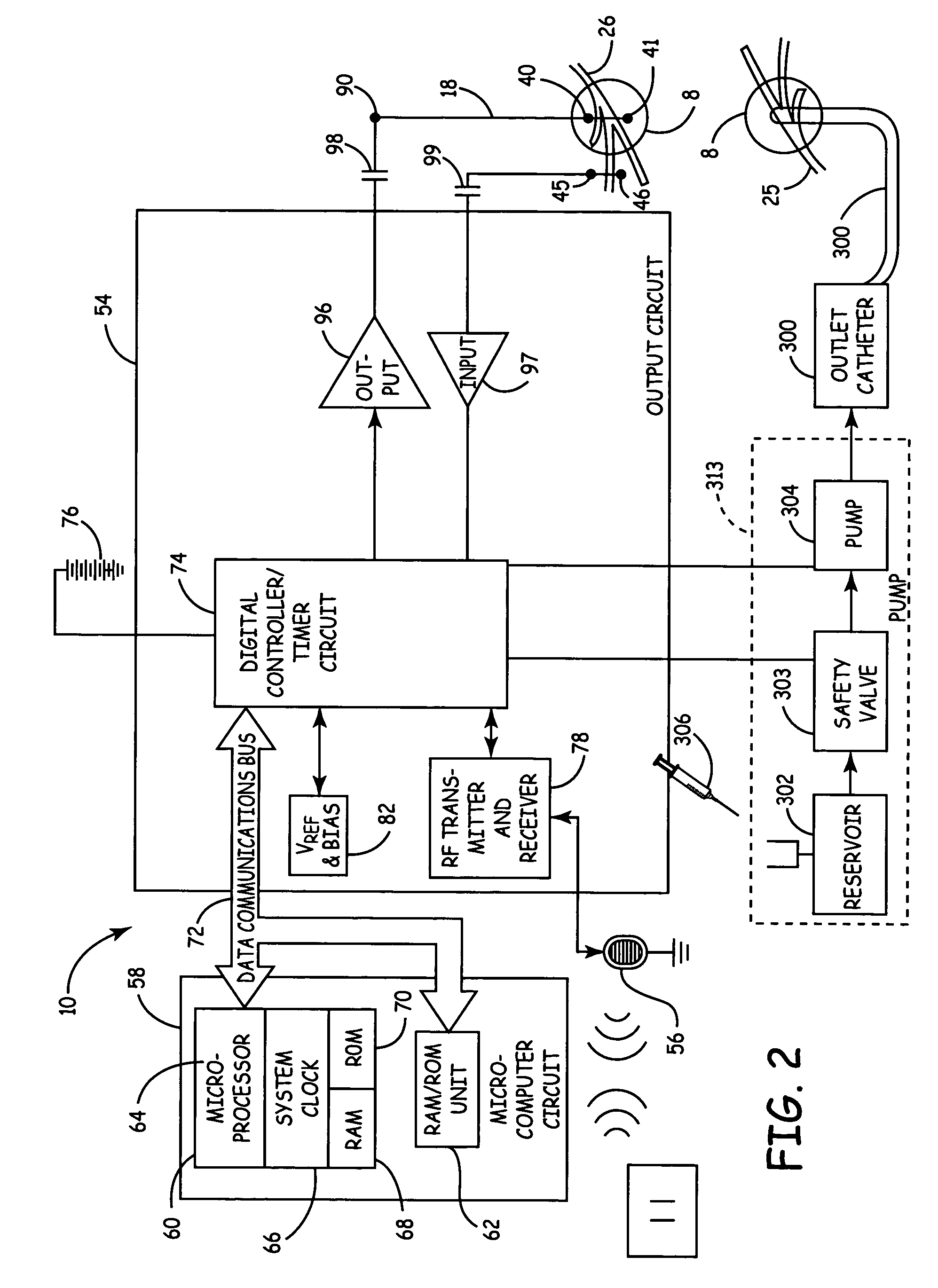
Method, system and device for treating disorders of the pelvic floor by delivering drugs to various nerves or tissues
InactiveUS7427280B2EfficaciousMore delivery of drugElectrotherapyMedical devicesDiseaseLigament structure
Described are implantable devices and methods for treating various disorders of the pelvic floor by delivering one or more drugs to a patient's hypogastric nerve or branches or portions thereof, prostatic plexus nerve or branches or portions thereof, sacral splanchnic nerve or branches or portions thereof, pelvic splanchnic nerve or branches or portions thereof, prostate or branches or portions thereof, pelvic floor, colon or branches or portions thereof, bladder or portions thereof, vagina or portions thereof, anus or portions thereof, external anal sphincter or portions thereof, urethra or portions thereof, penile dorsal nerve or portions thereof, inferior rectal nerves or branches or portions thereof, perineal nerves or branches or portions thereof, scrotal nerves or branches or portions thereof, scrotum or portions thereof, Alcock's Canal or branches or portions thereof, sacro-tuberous ligament or branches or portions thereof, ischial tuberosity or branches or portions thereof, greater sciatic foramen or branches or portions thereof, or lesser sciatic foramen or branches or portions thereof. One, two or more drug delivery regimes are provided on a continuous, alternating, intermittent or other basis to one or more selected nerves, nerve portions or tissues in an amount and manner effective to treat a number of disorders, including, but not limited to, urinary and / or fecal voiding dysfunctions such as constipation, incontinence disorders such as urge frequency and urinary retention disorders, sexual dysfunctions such as orgasmic and erectile dysfunction, pelvic pain, prostatitis, prostatalgia and prostatodynia.
Owner:MEDTRONIC INC
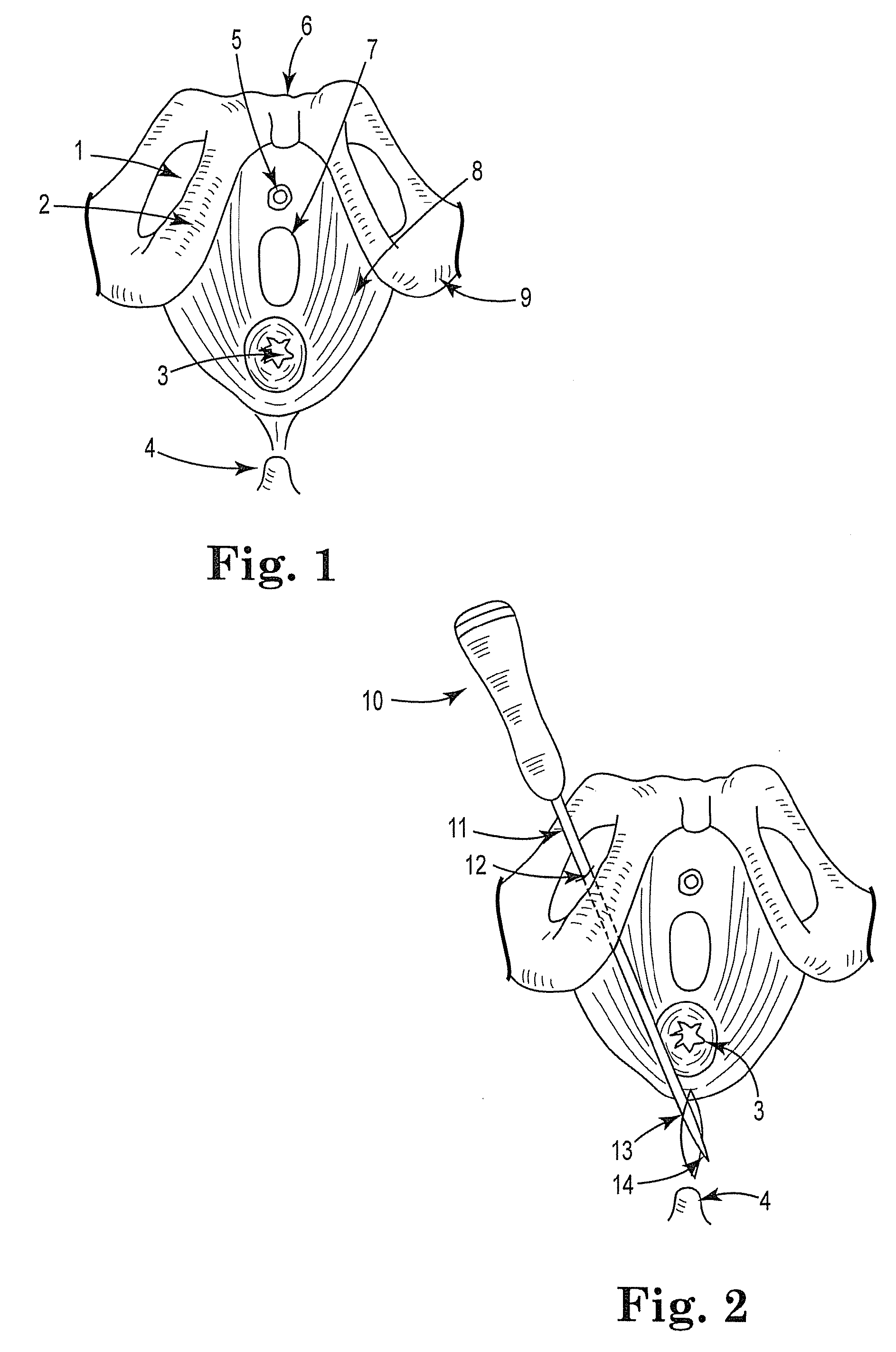
Surgical procedure for correcting cystocele and rectocele
InactiveUS20090105526A1Minimal invasivenessRapid and less painfulAnti-incontinence devicesLower poleSurgical department
A surgical procedure for correcting cystocele, comprising the steps of:identifying a center of the cystocele;performing an infiltration of the cystocele with physiological solution;performing a longitudinal incision;identifying the pubocervical fascia and performing a longitudinal incision therein;defining four paravesical tunnels directed toward the upper and lower poles of the obturator foramina bilaterally;introducing a mesh with four articulated arms, each arm being introduced in a corresponding tunnel;suturing the longitudinal incision.
Owner:PIROLI TORELLI DONATO +1
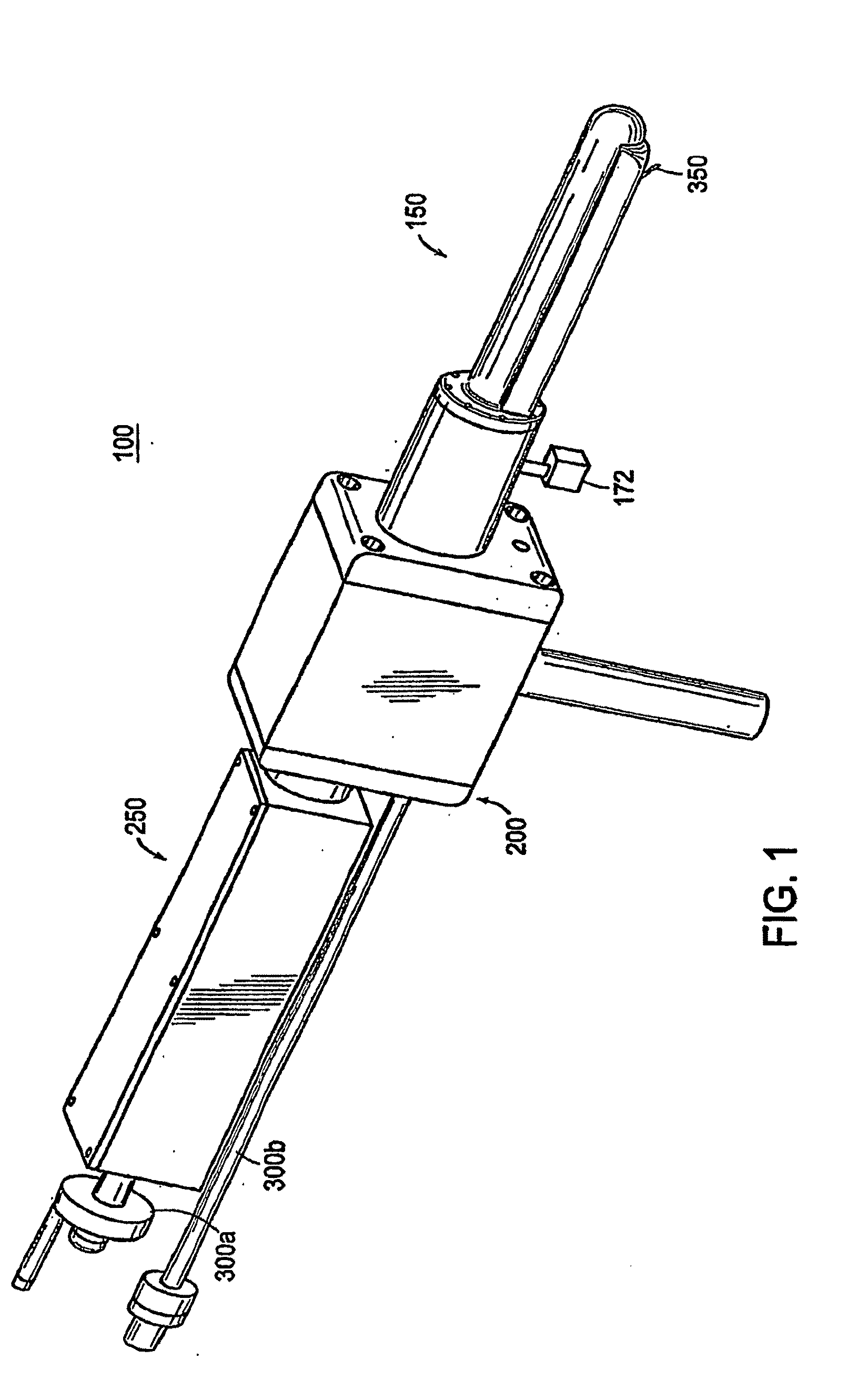
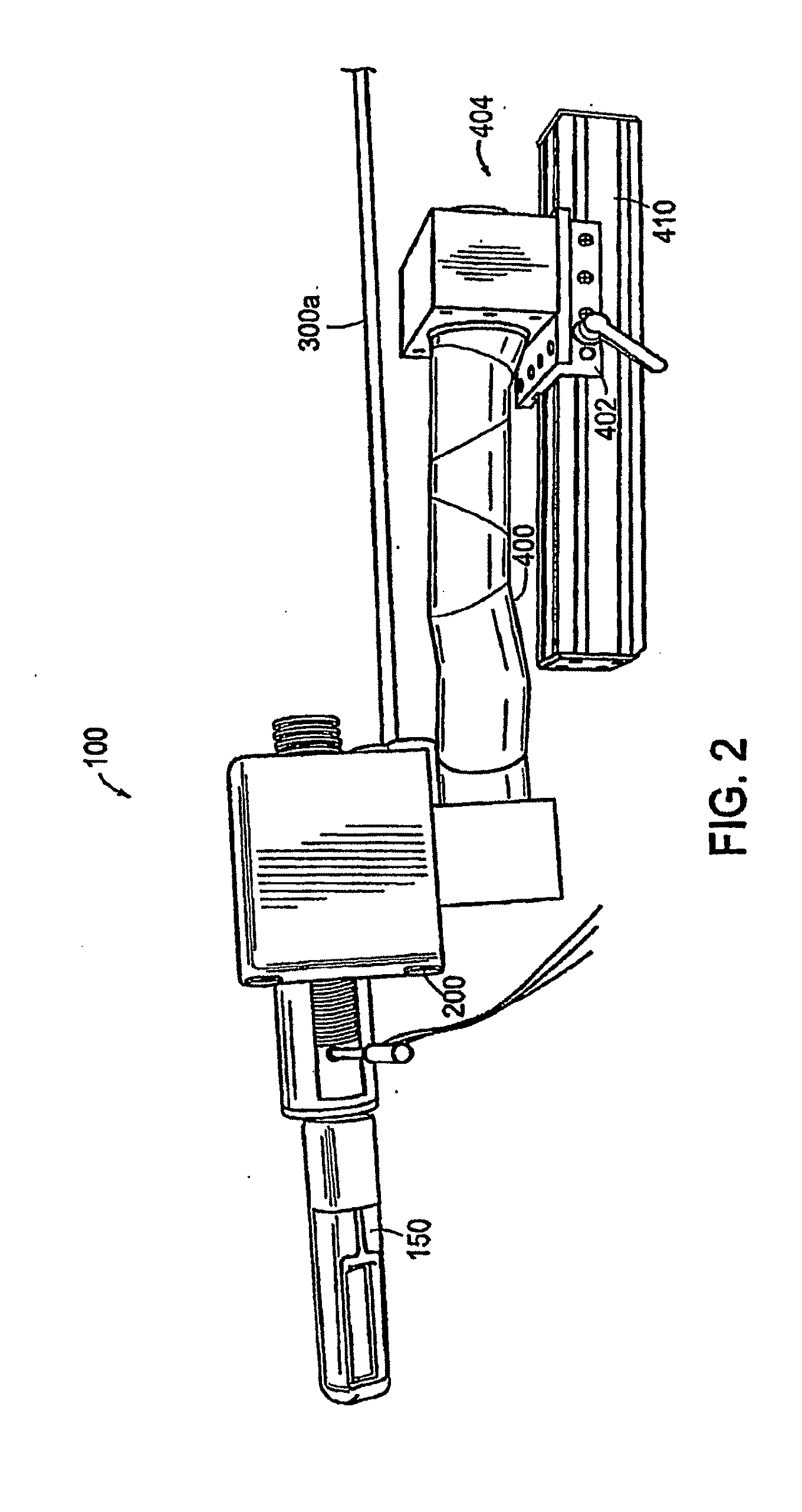
Apparatus for insertion of a medical device within a body during a medical imaging process and devices and methods related thereto
ActiveUS20100056900A1Minimizes motion and deformationEasy accessSurgical navigation systemsVaccination/ovulation diagnosticsX-rayMedical treatment
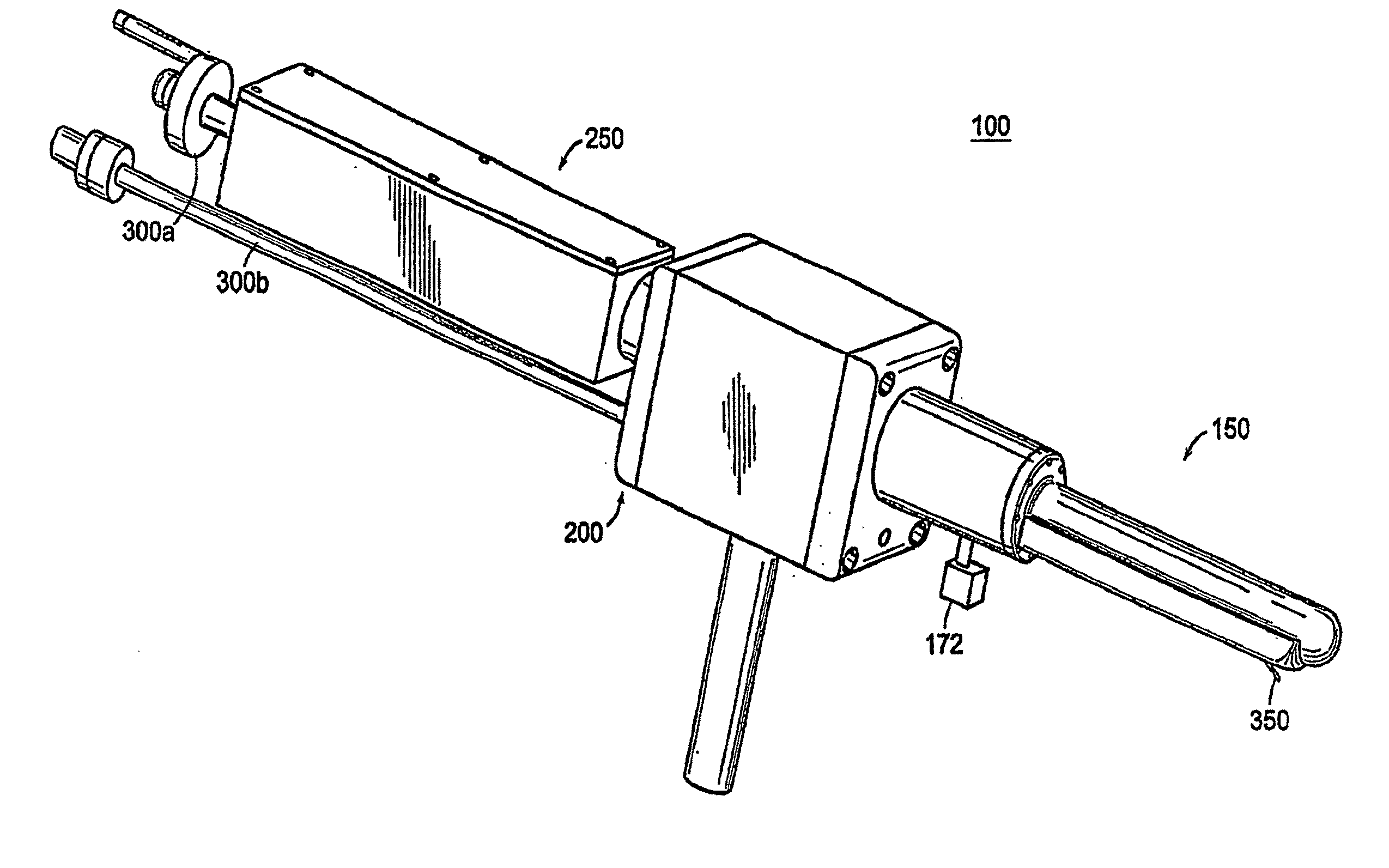
A device, system, and method for entering a medical device such as a needle into the body inside a medical imager such as a MRI scanner, CT, X-ray fluoroscopy, and ultrasound imaging, from within a body cavity (such as the rectum, vagina, or laparoscopically accessed cavity). A three degree-of-freedom mechanical device translates and rotates inside the cavity and enters a needle into the body, and steers the needle to a target point selected by the user. The device is guided by real-time images from the medical imager. Networked computers process the medical images and enable the clinician to control the motion of the mechanical device that is operated within the imager, outside of the imager or remotely from outside the imager.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Vaginal operation method for the treatment of anal incontinence in women
ActiveUS20110015474A1Reduce riskLow placementSuture equipmentsAnti-incontinence devicesFecal incontinenceVagina
There is disclosed a method for treating anal incontinence in women. The method comprises accessing the rectum or colon or anus though an incision in the vagina and implanting a powered restriction device on the rectum, colon or anal sphincter. There are also disclosed methods for energizing and controlling the restriction device.
Owner:FORSELL PETER
Treatment of anal incontinence and defecatory dysfunction
A method of treating anal incontinence in a patient comprises providing a sling having a central portion and first and second arms, creating a subcutaneous tunnel between a first buttock incision and a second buttock incision in the patient, mechanically widening the subcutaneous tunnel to create a pocket for the central portion of the sling, grasping the first arm of the sling and pulling the sling through the subcutaneous tunnel such that the central portion of the sling rests underneath the ano-rectum, inserting an introducer needle through a first thigh incision formed in the patient and advancing the introducer needle through the first buttock incision, pulling the first sling arm through the first thigh incision, inserting the introducer needle through a second thigh incision formed in the patient and advancing the introducer needle through the second buttock incision, and pulling the second sling arm through the second thigh incision.
Owner:AMS RES CORP +1
Sonographically guided transvaginal or transrectal pelvic abscess drainage using trocar method and biopsy guide attachment
ActiveUS7981041B2Avoid relative motionUltrasonic/sonic/infrasonic diagnosticsSurgical needlesUltrasound probeInstrumentation
A needle biopsy guide system is disclosed for attachment to an endoluminal ultrasound probe or like sonographic instrument. The device includes a biopsy-guide attachment that allows for trocar catheter placement for abscess drainage or like procedures, using the transvaginal or transrectal route under sonographic control. The device has a base portion, which is attachable to an ultrasound probe. A removable retainer is provided that slides into the base unit to hold a biopsy needle in place. A physician may locate the target area in the body with the ultrasound probe, insert the biopsy needle into the target area, and then remove the insert (retainer) from the base unit and ultrasound probe, and leave the biopsy needle in place in the body.
Owner:RGT UNIV OF CALIFORNIA
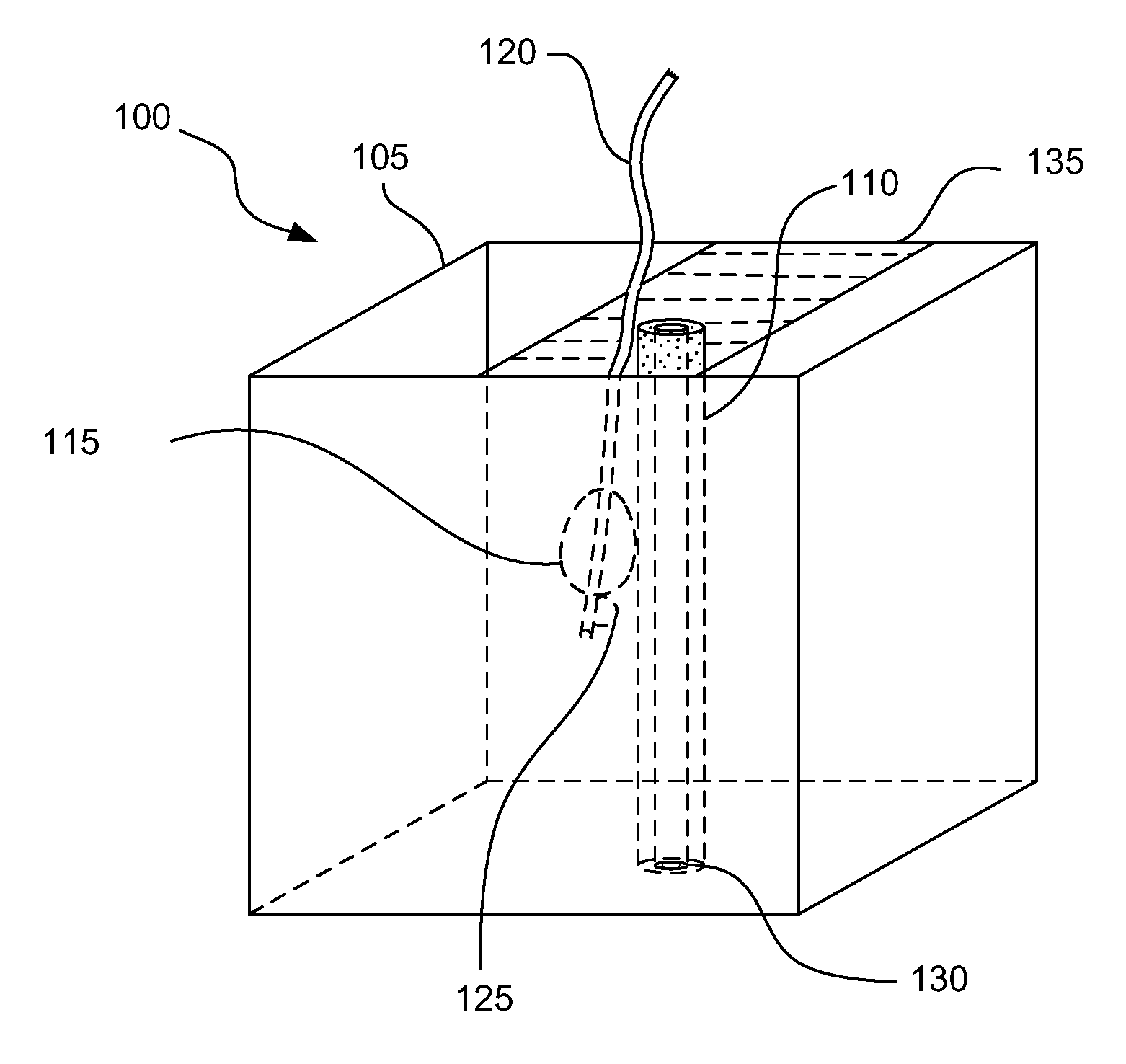
Prostate brachytherapy simulator
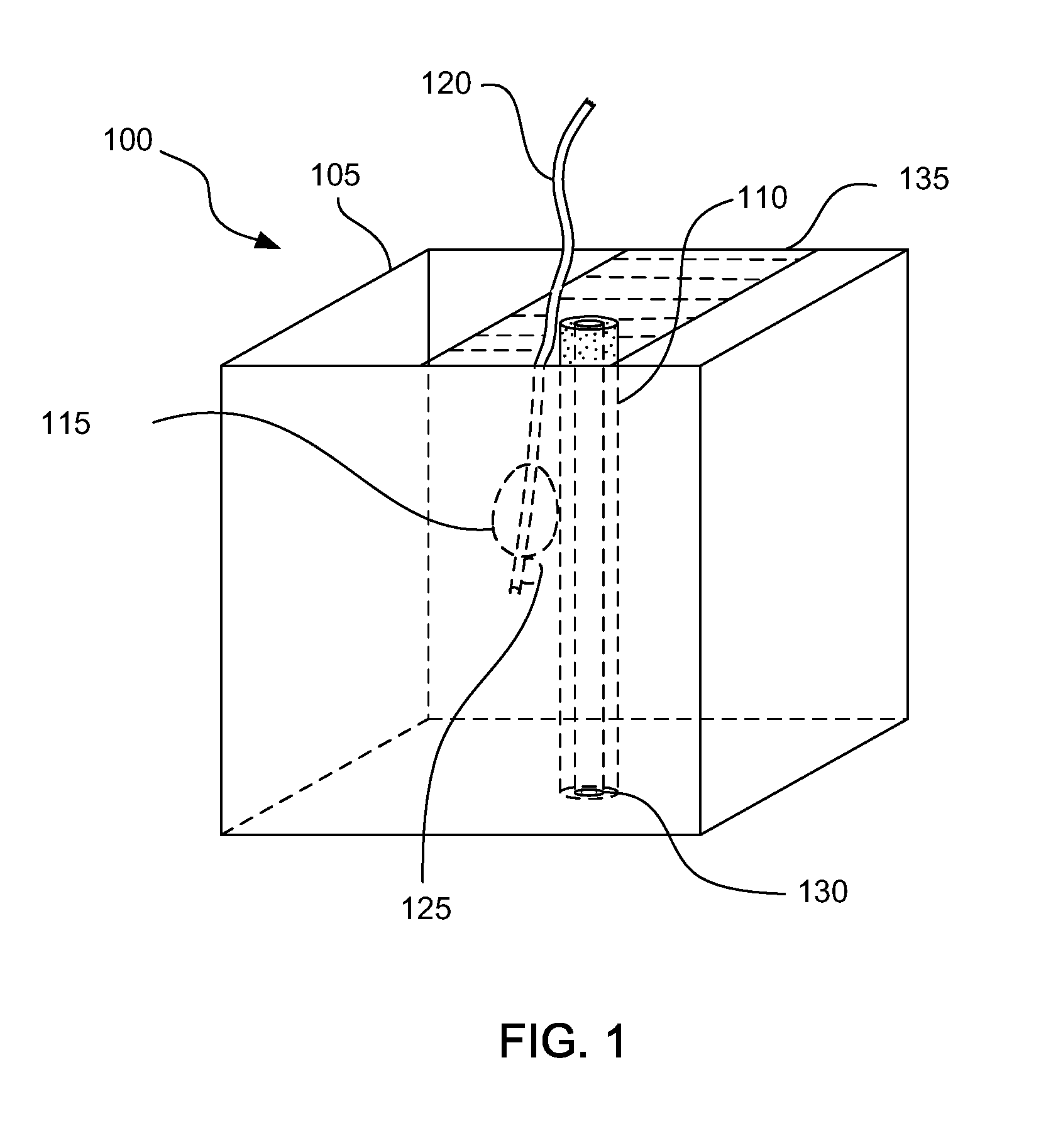
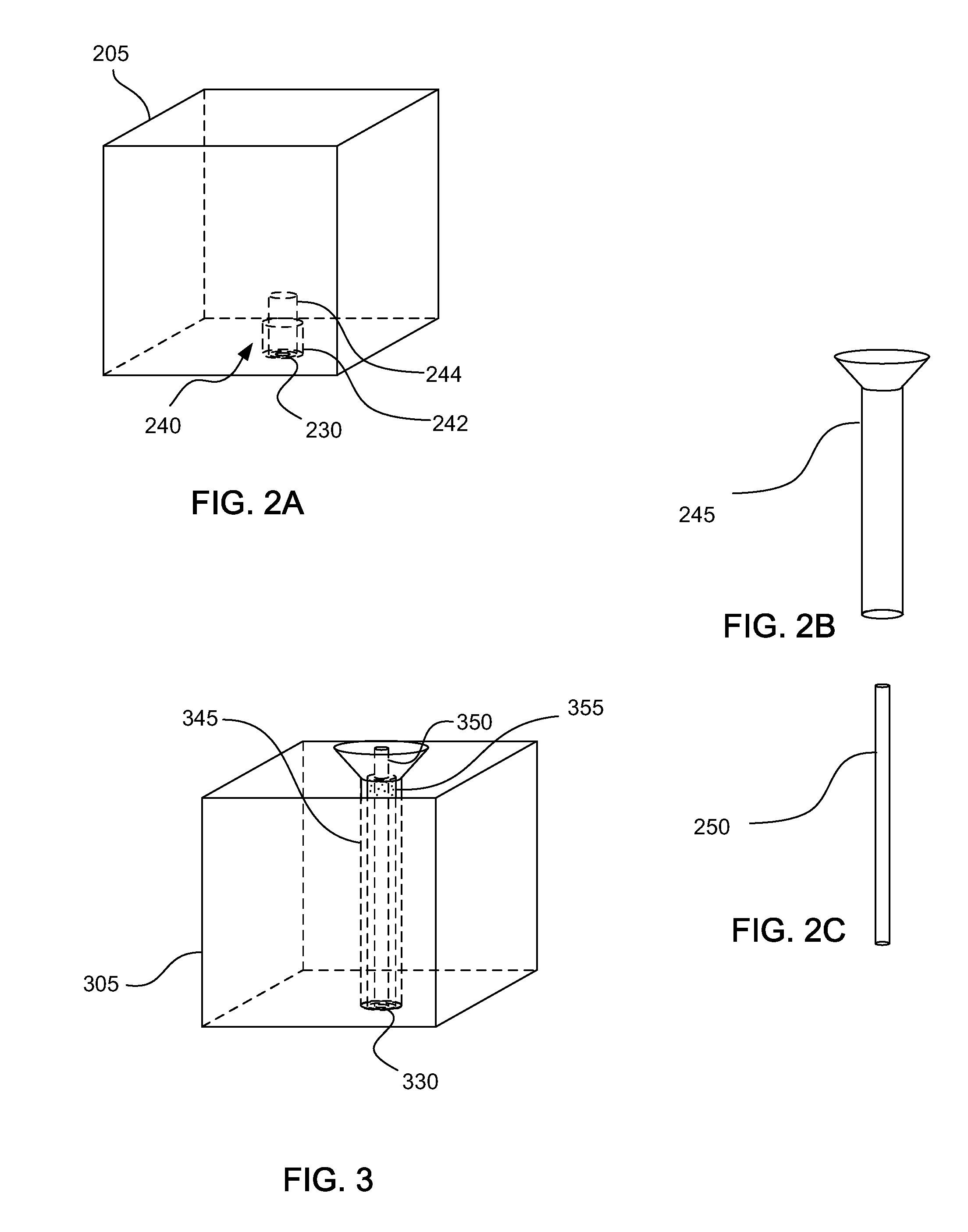
Methods for forming a prostate brachytherapy simulator are provided. A method comprises forming a molded rectum model between tubes secured to a base component of a container. The base component has a first open-bottomed cup that opens to an exterior of the container and a second cup that contains the first open-bottomed cup. The method also comprises forming a molded prostate model from recyclable materials. Additionally, the method comprises placing the prostate model relative to the rectum model. The method also comprises providing a gel-based environment that surrounds the rectum model and the prostate model within a container.
Owner:BRACHYTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com