Patents
Literature
252 results about "Peritoneal cavity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The peritoneal cavity is a potential space between the parietal peritoneum (the peritoneum that surrounds the abdominal wall) and visceral peritoneum (the peritoneum that surrounds the internal organs). Both the parietal and visceral peritonea are not different but the same peritoneum given two names depending on their function/location. It is one of the spaces derived from the coelomic cavity of the embryo, the others being the pleural cavities around the lungs and the pericardial cavity around the heart.
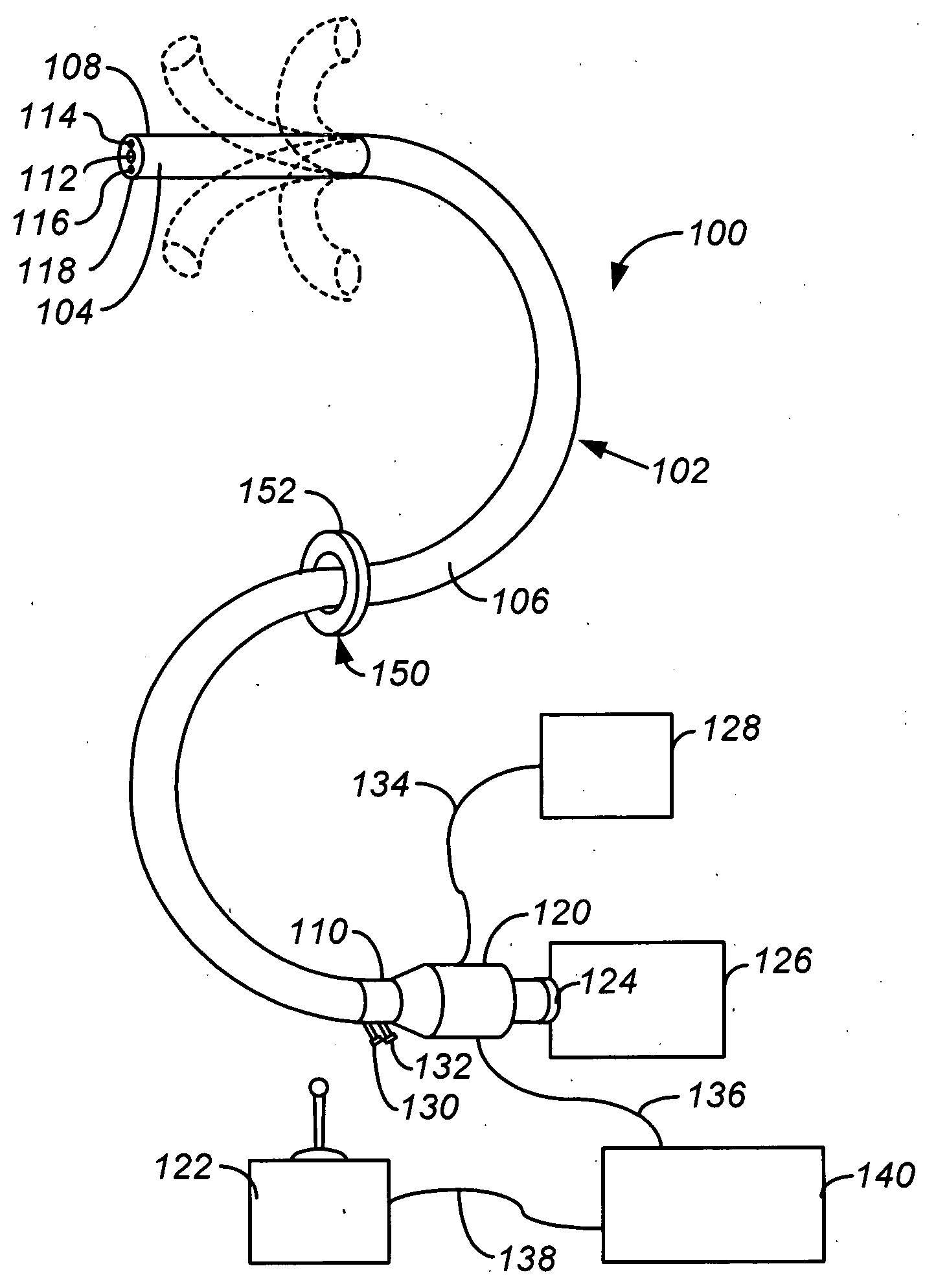
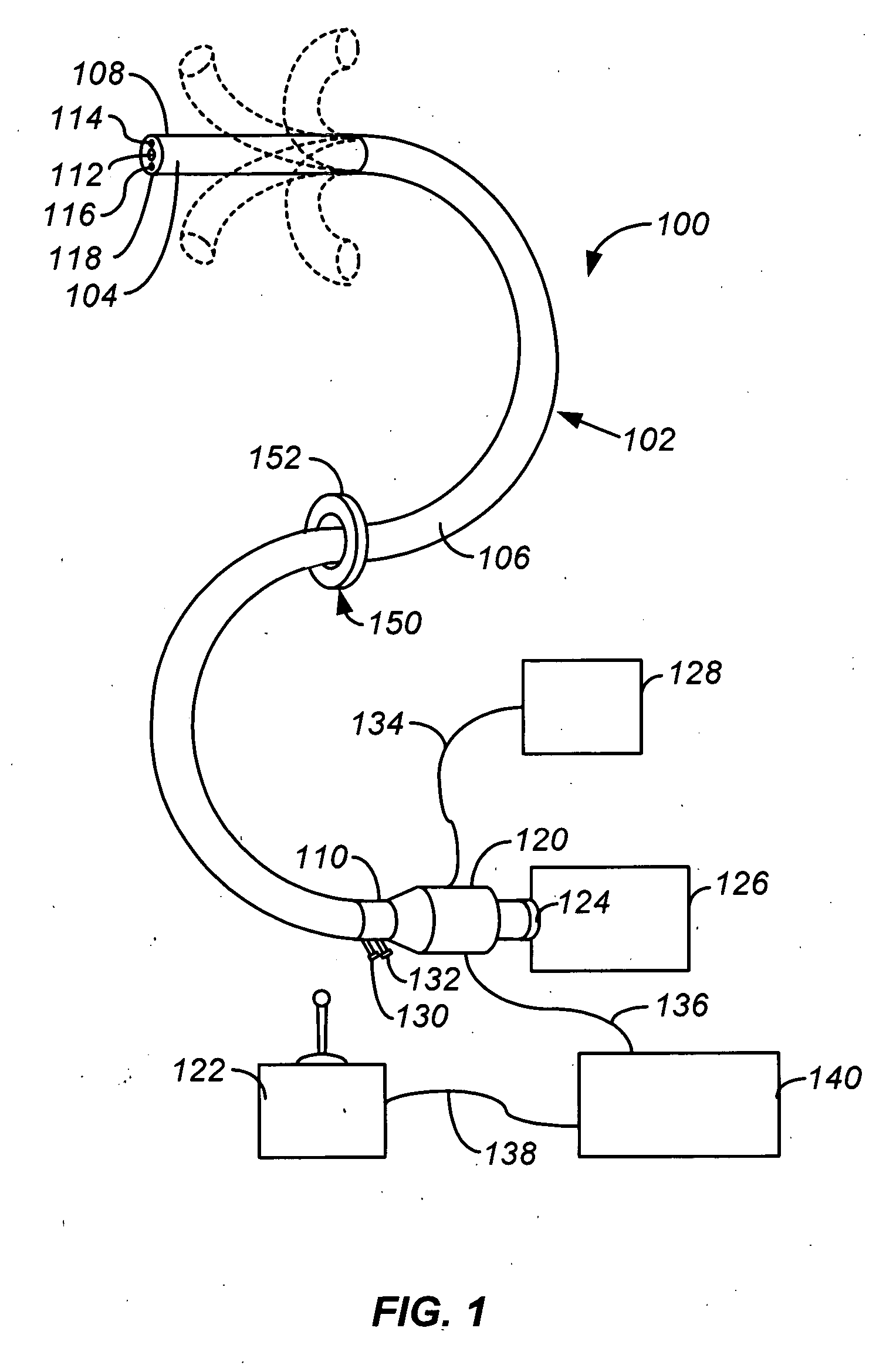
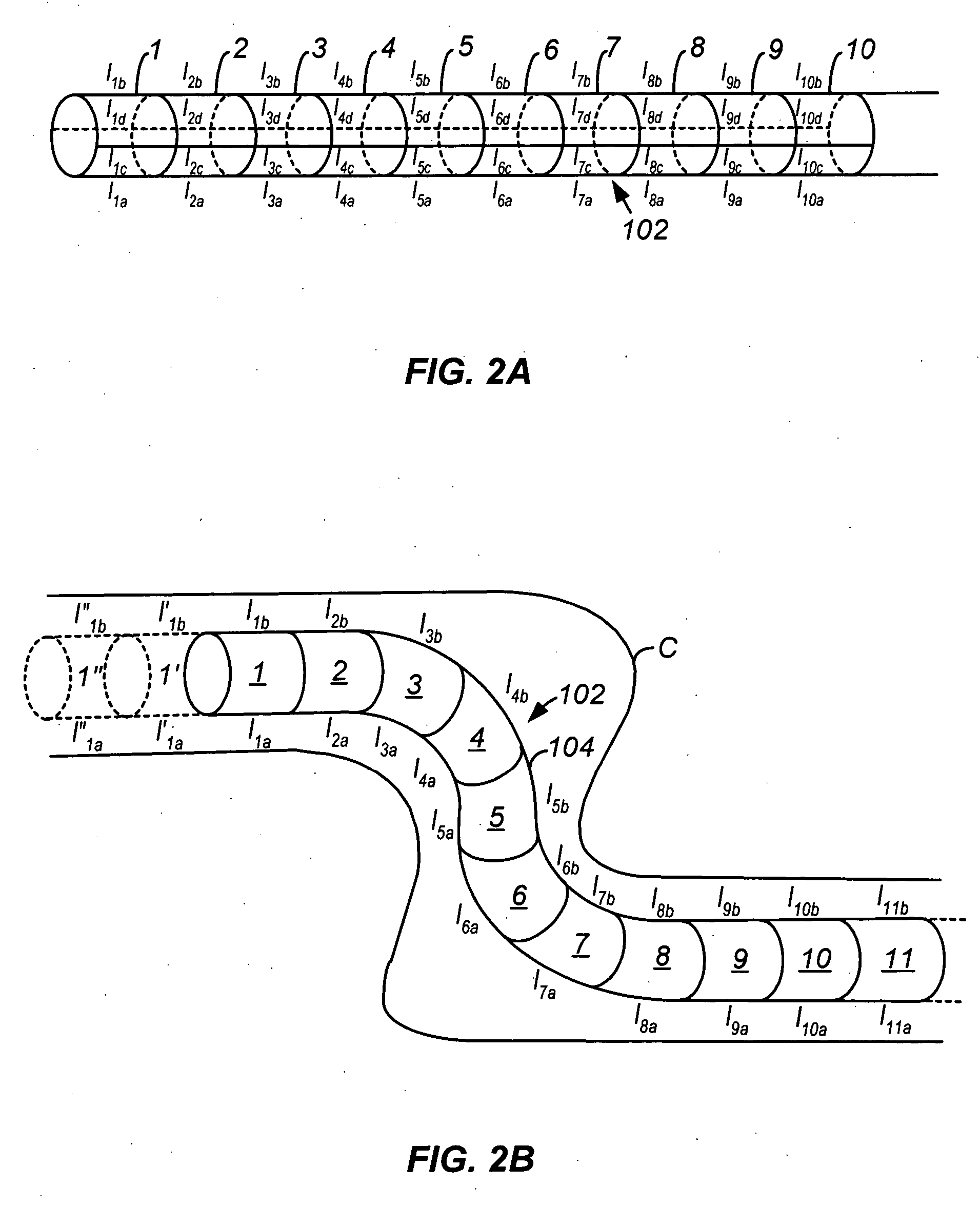
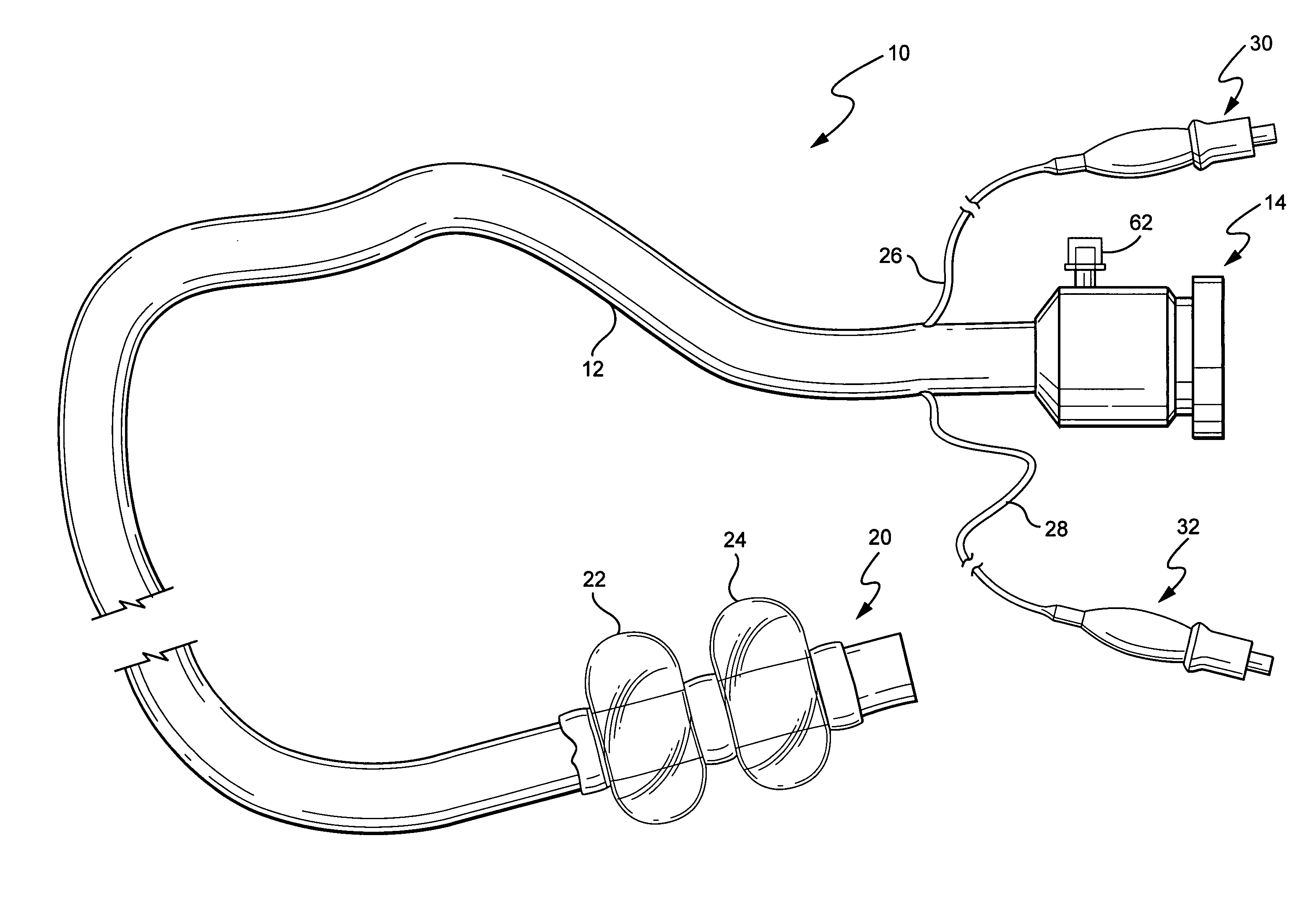
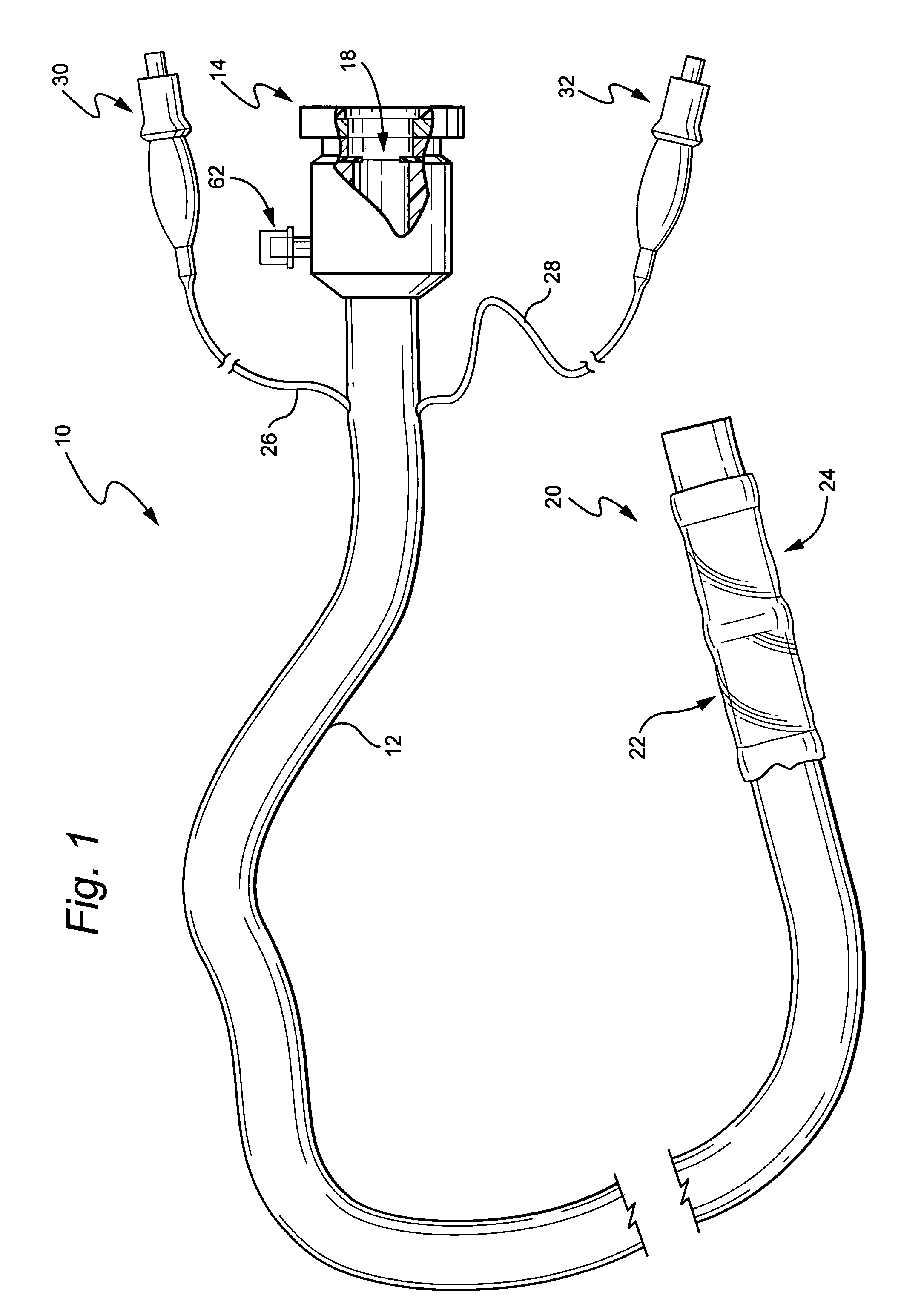
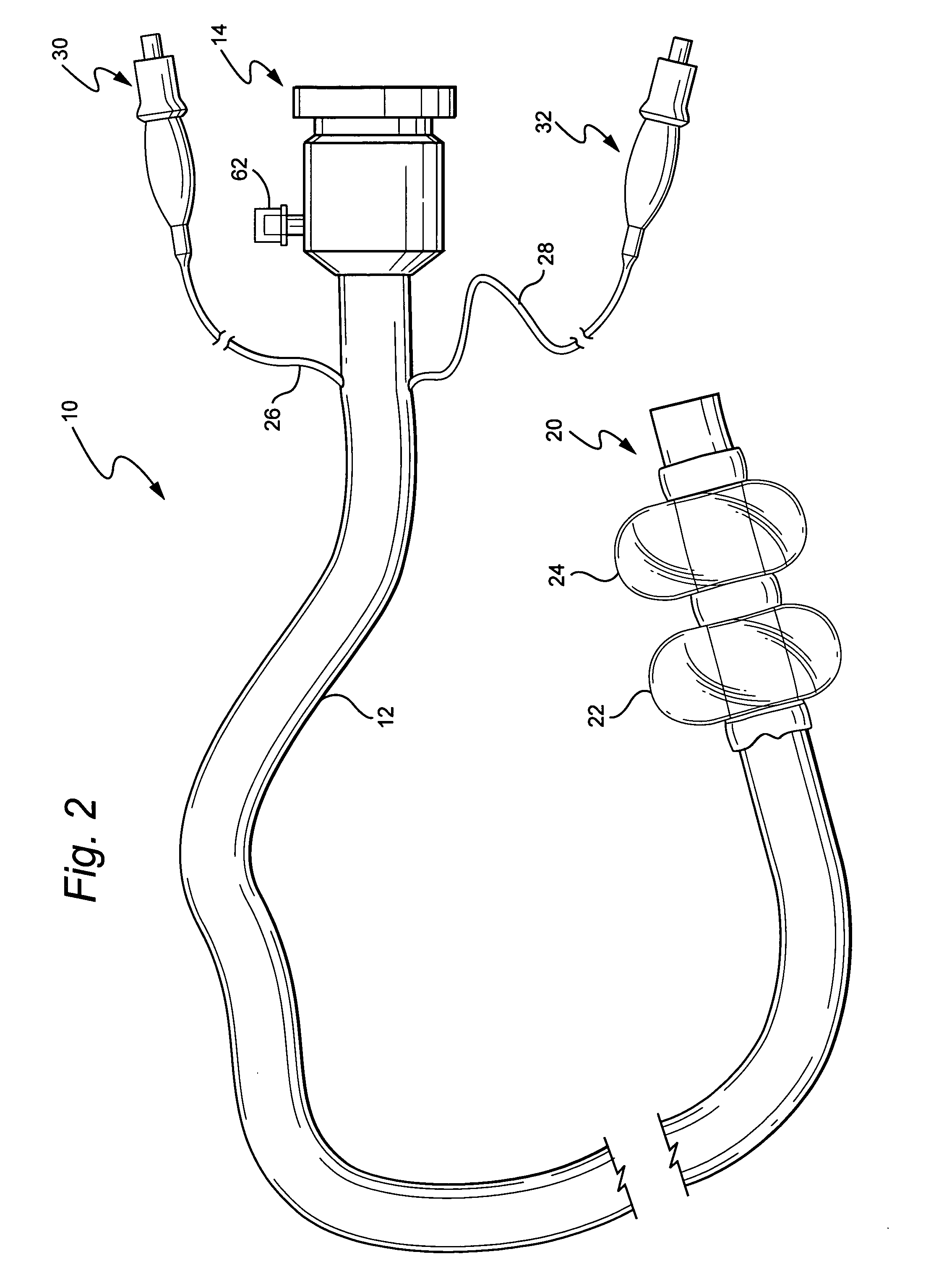
Electrosurgical instrument
InactiveUS7278994B2Lower impedanceReduced effectivenessCannulasDiagnosticsGynecologyPeritoneal cavity
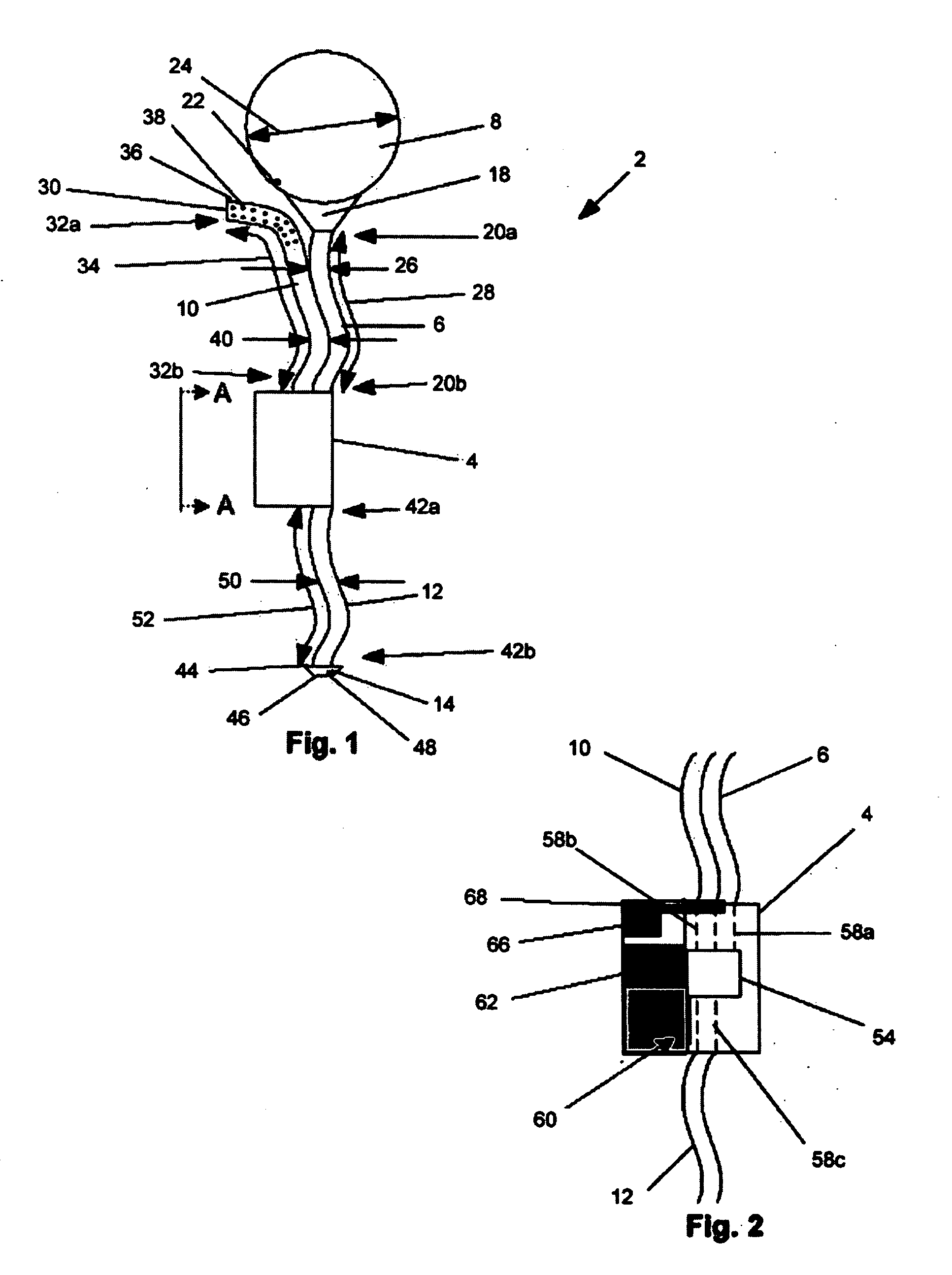
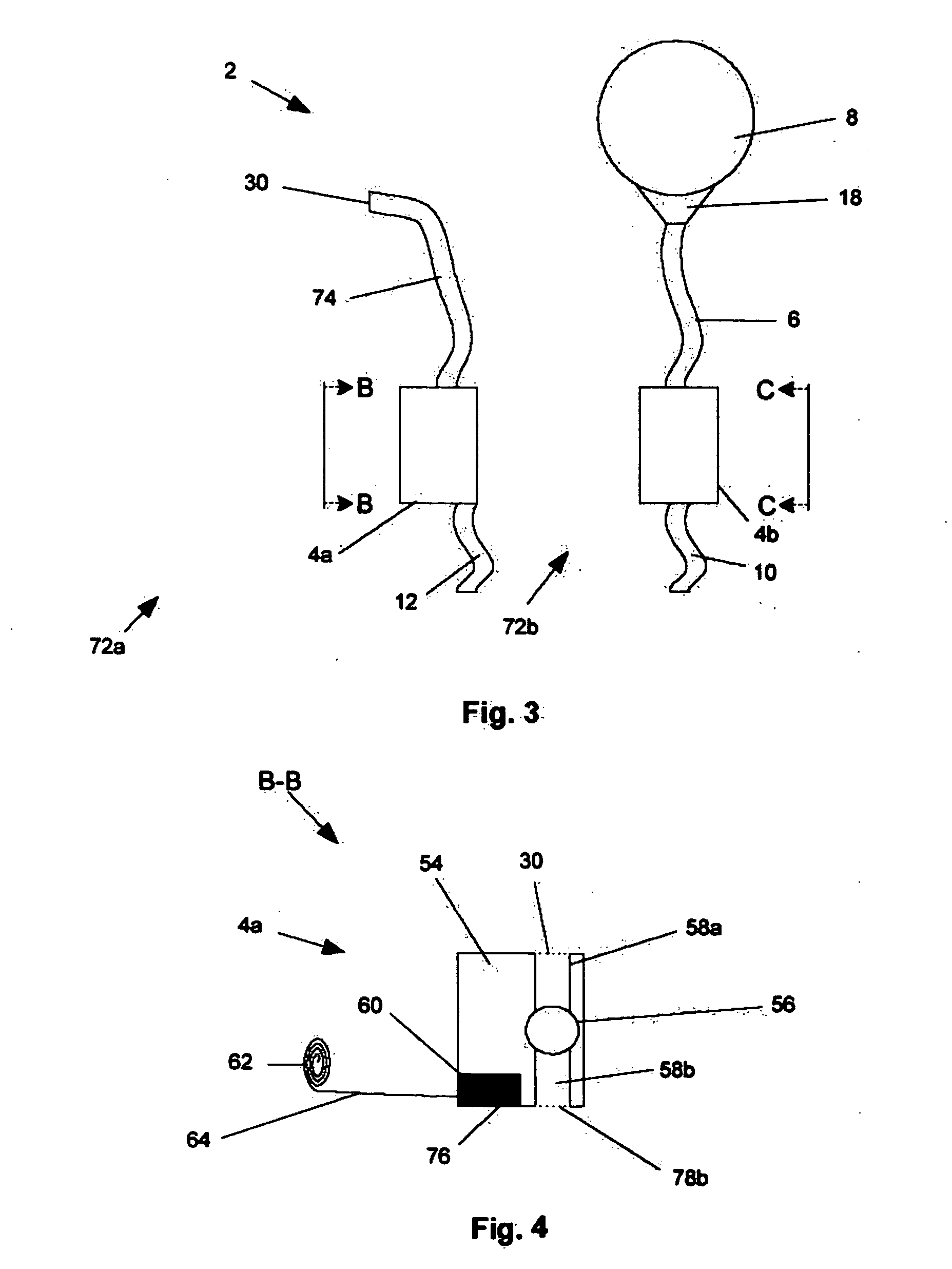
A system and method are disclosed for removing a uterus using a fluid enclosure inserted in the peritoneal cavity of a patient so as to enclose the uterus. The fluid enclosure includes a distal open end surrounded by an adjustable loop, that can be tightened, a first proximal opening for inserting an electrosurgical instrument into the fluid enclosure, and a second proximal opening for inserting an endoscope. The loop is either a resilient band extending around the edge of the distal open end or a drawstring type of arrangement that can be tightened and released. The fluid enclosure is partially inserted into the peritoneal cavity of a patient in a deflated condition and then manipulated within the peritoneal cavity over the body and fundus of the uterus to the level of the uterocervical junction. The loop is tightened around the uterocervical junction, after which the enclosure is inflated using a conductive fluid. The loop forms a pressure seal against the uterocervical junction to contain the conductive fluid used to fill the fluid enclosure. Endoscopically inserted into the fluid enclosure is an electrosurgical instrument that is manipulated to vaporize and morcellate the fundus and body of the uterus. The fundus and body tissue that is vaporized and morcellated is then removed from the fluid enclosure through the shaft of the instrument, which includes a hollow interior that is connected to a suction pump The fundus and body are removed after the uterus has been disconnected from the tissue surrounding uterus.
Owner:GYRUS MEDICAL LTD
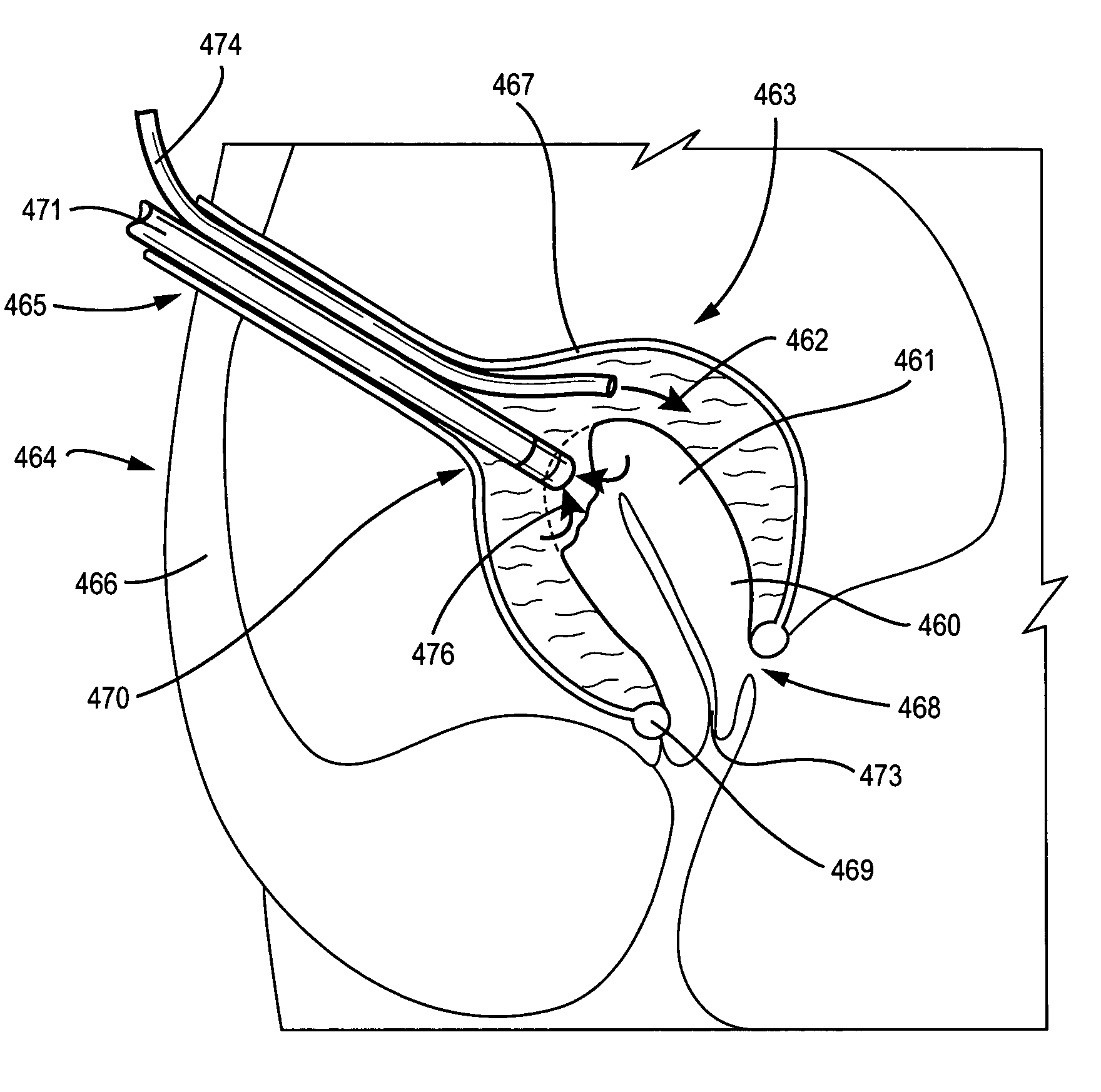
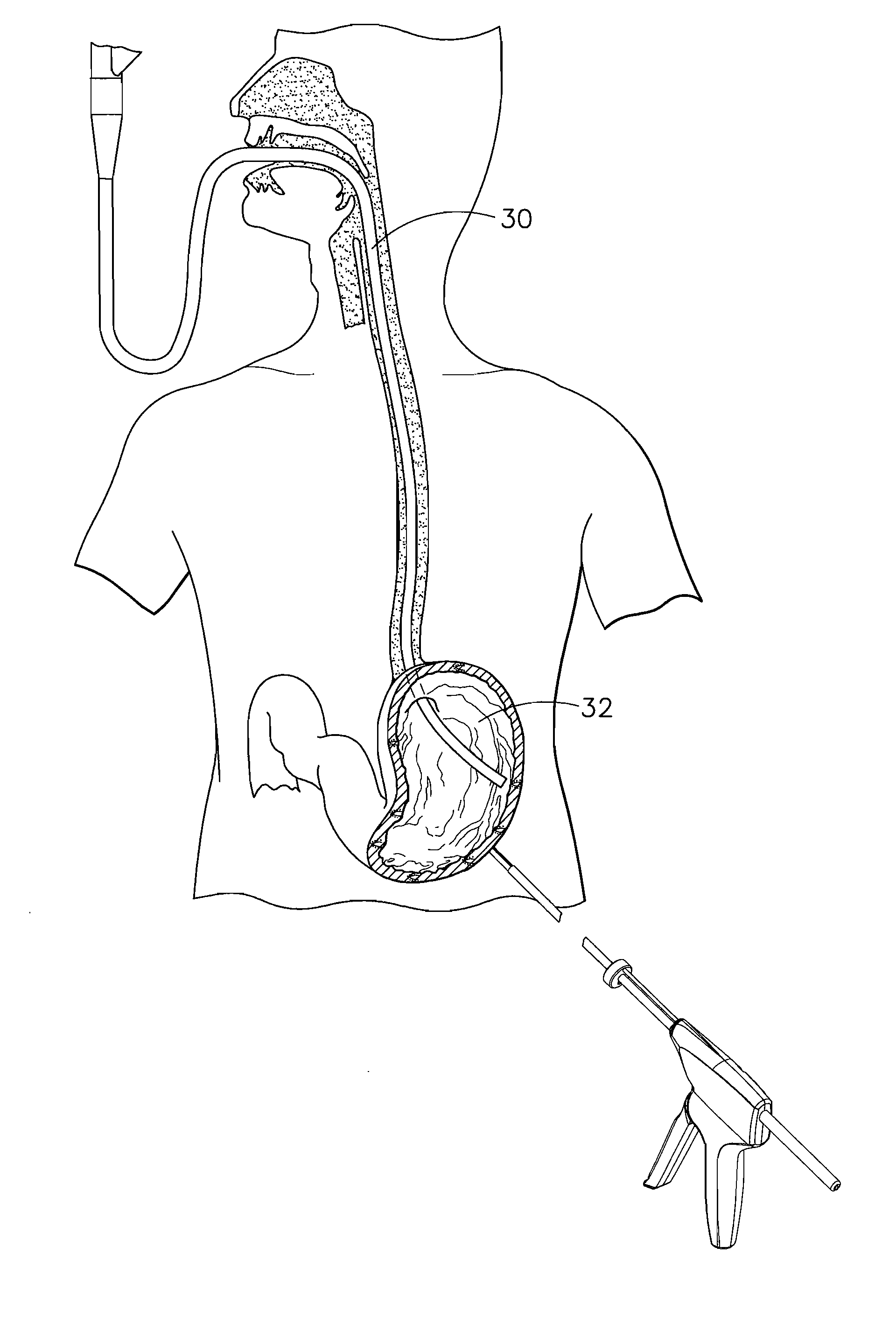
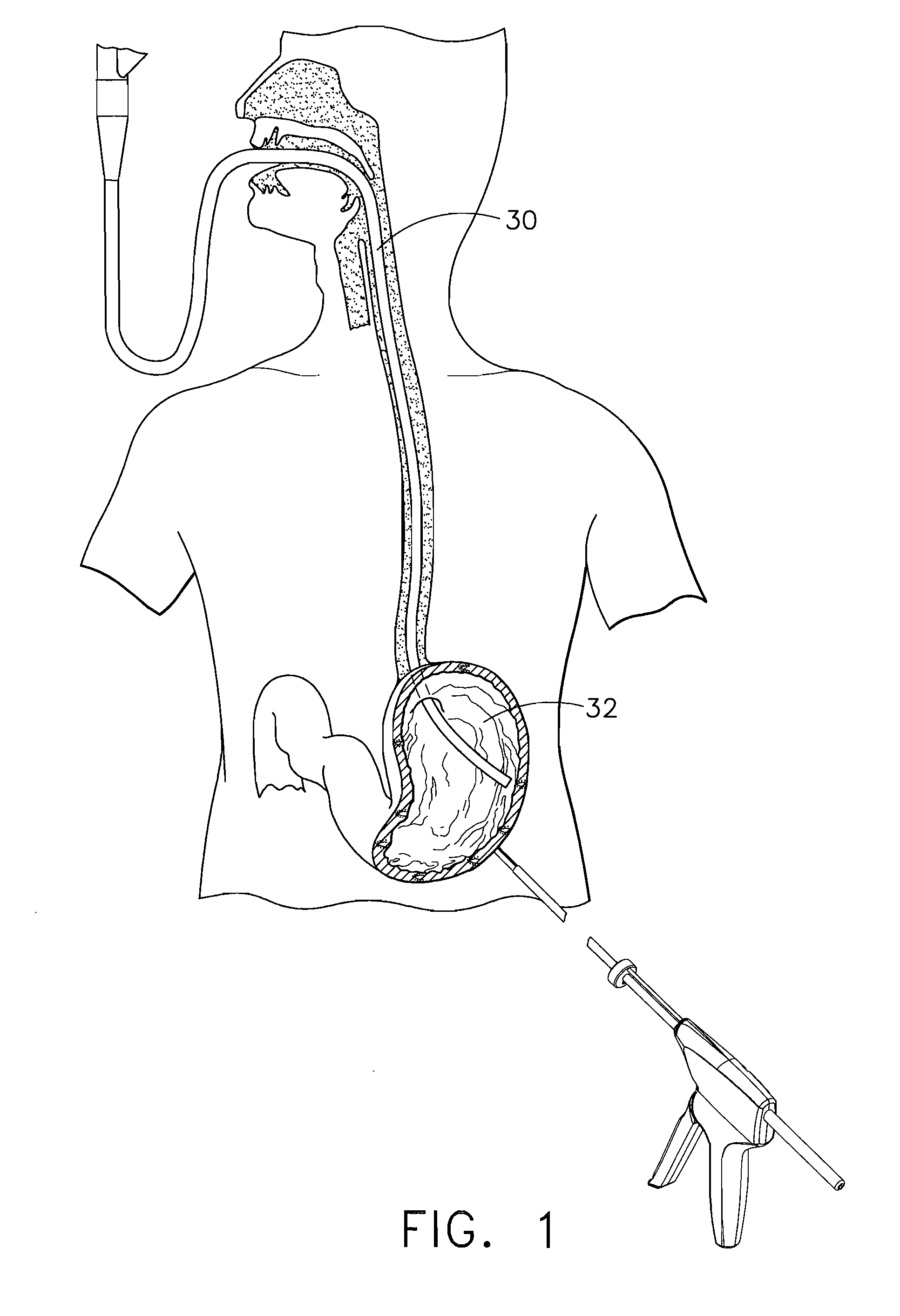
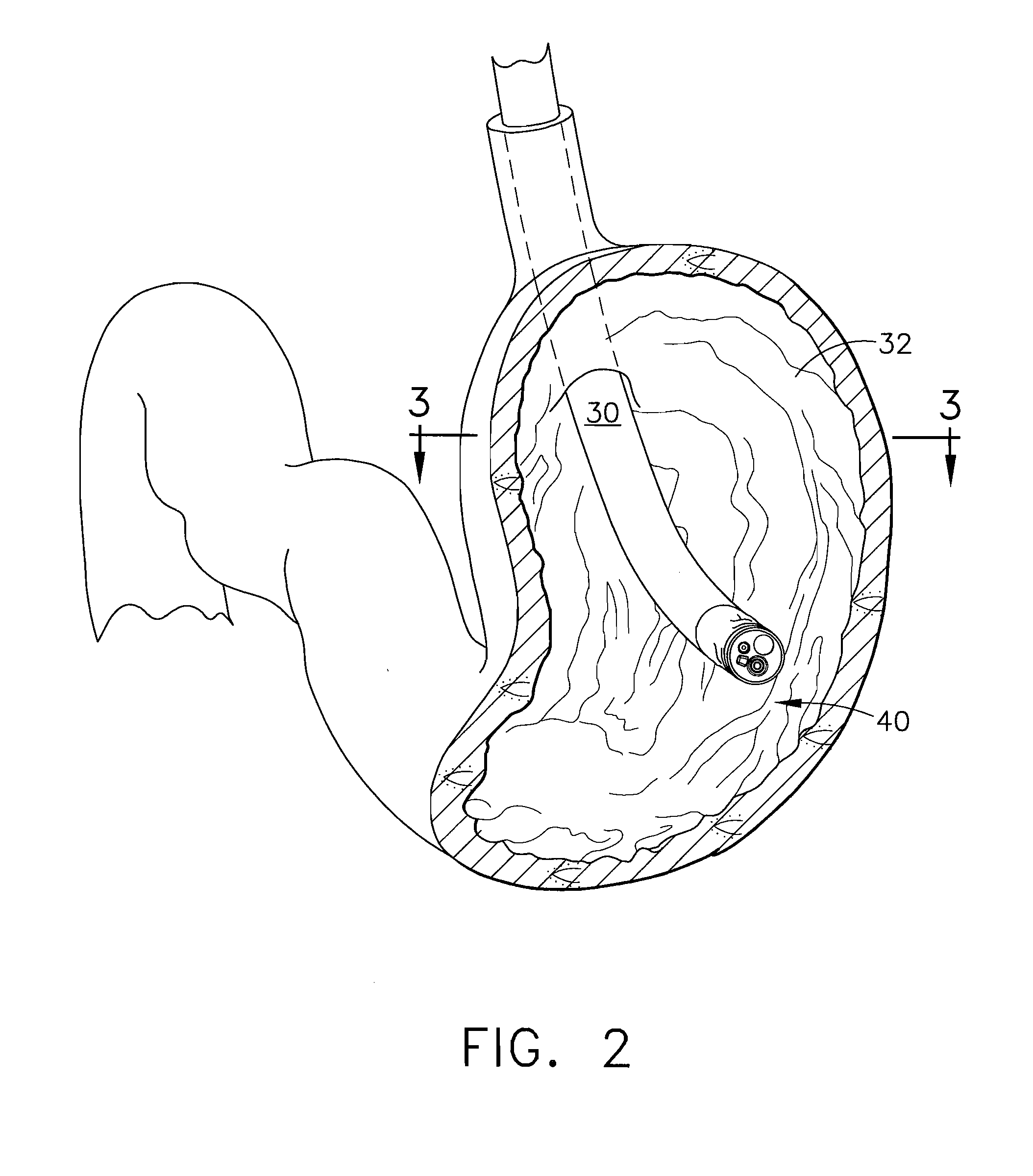
Methods and devices for diagnostic and therapeutic interventions in the peritoneal cavity
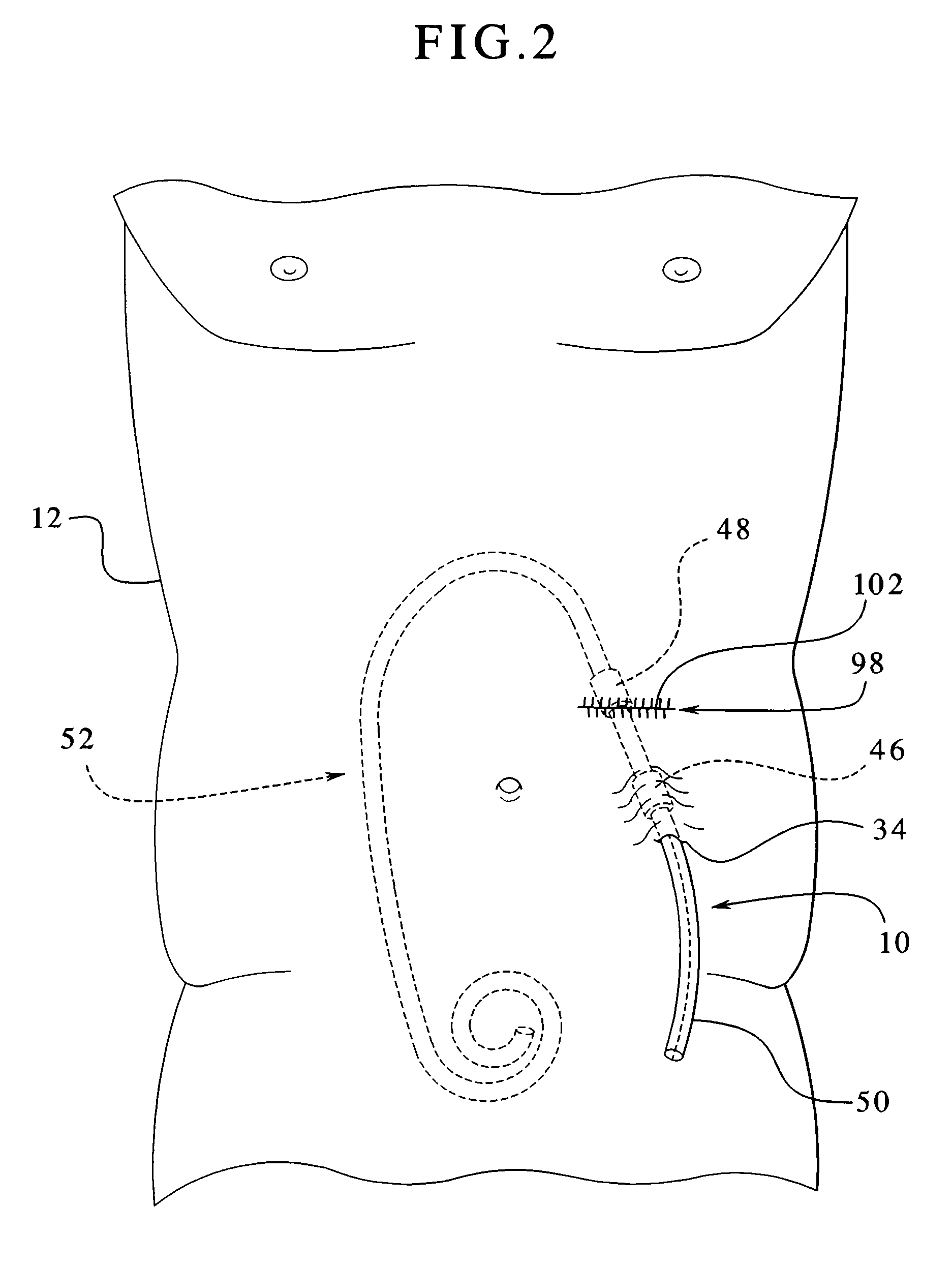
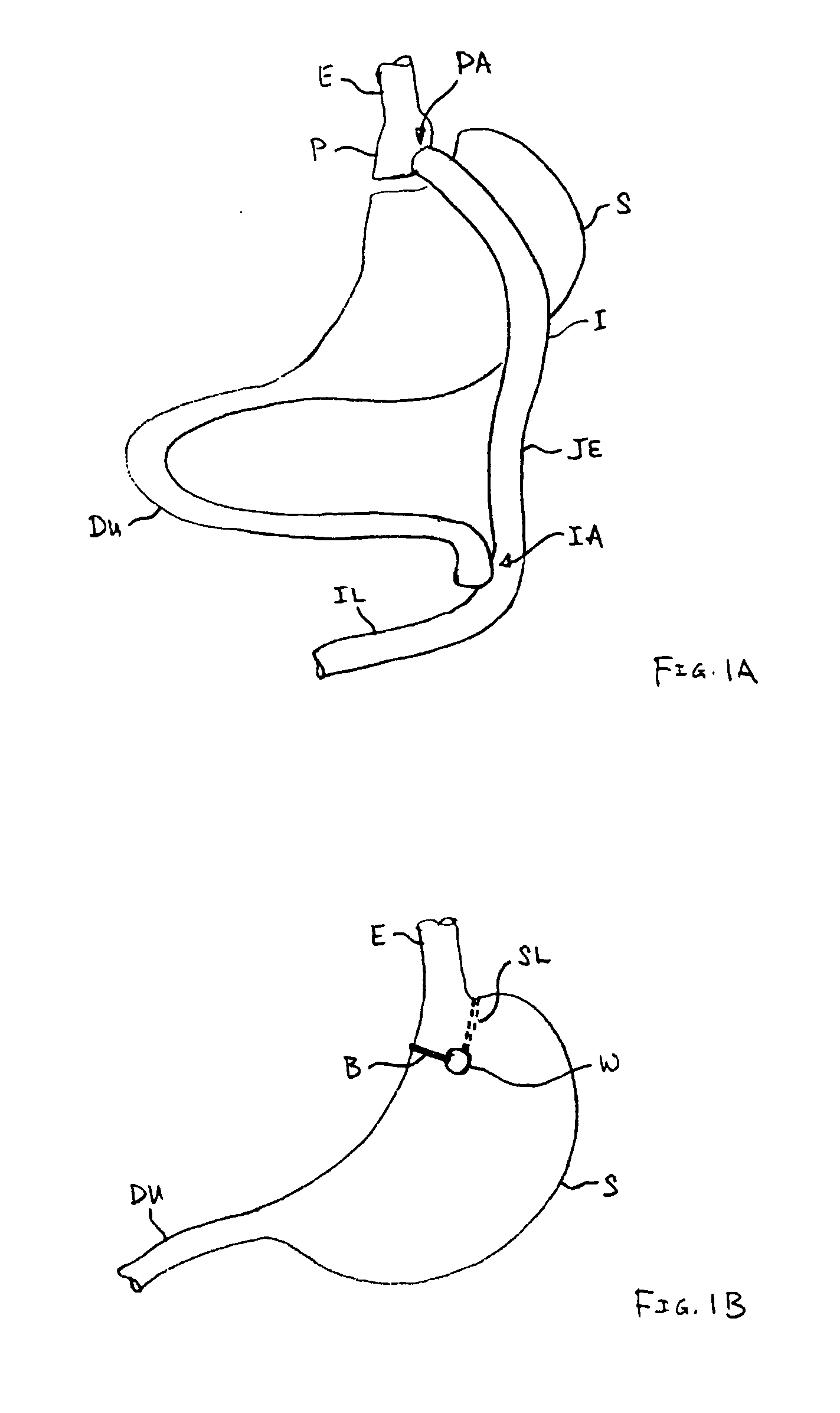
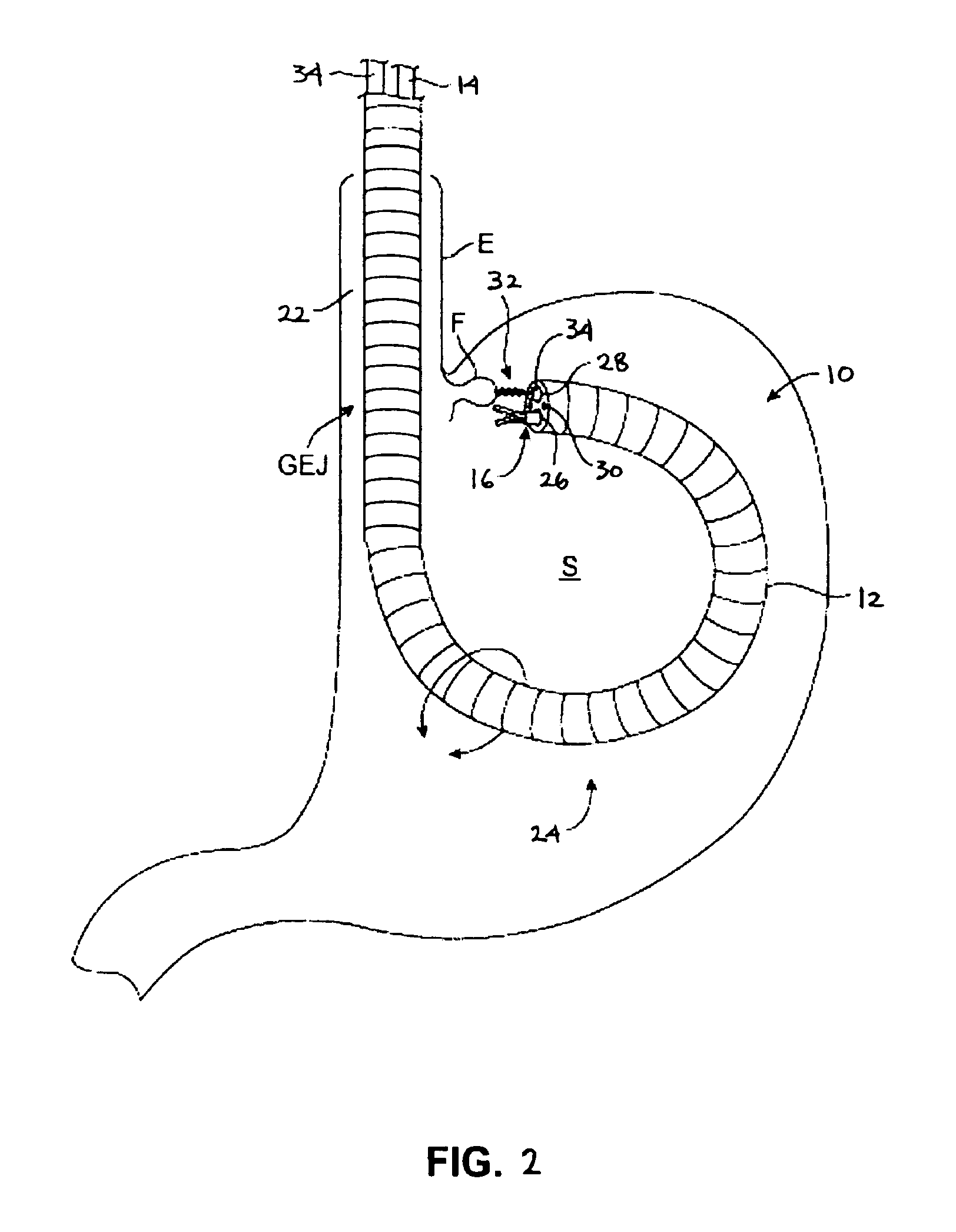
A novel approach to diagnostic and therapeutic interventions in the peritoneal cavity is described. More specifically, a technique for accessing the peritoneal cavity via the wall of the digestive tract is provided so that examination of and / or a surgical procedure in the peritoneal cavity can be conducted via the wall of the digestive tract with the use of a flexible endoscope. As presently proposed, the technique is particularly adapted to transgastric peritoneoscopy. However, access in addition or in the alternative through the intestinal wall is contemplated and described as well. Transgastric and / or transintestinal peritoneoscopy will have an excellent cosmetic result as there are no incisions in the abdominal wall and no potential for visible post-surgical scars or hernias.
Owner:APOLLO ENDOSURGERY INC
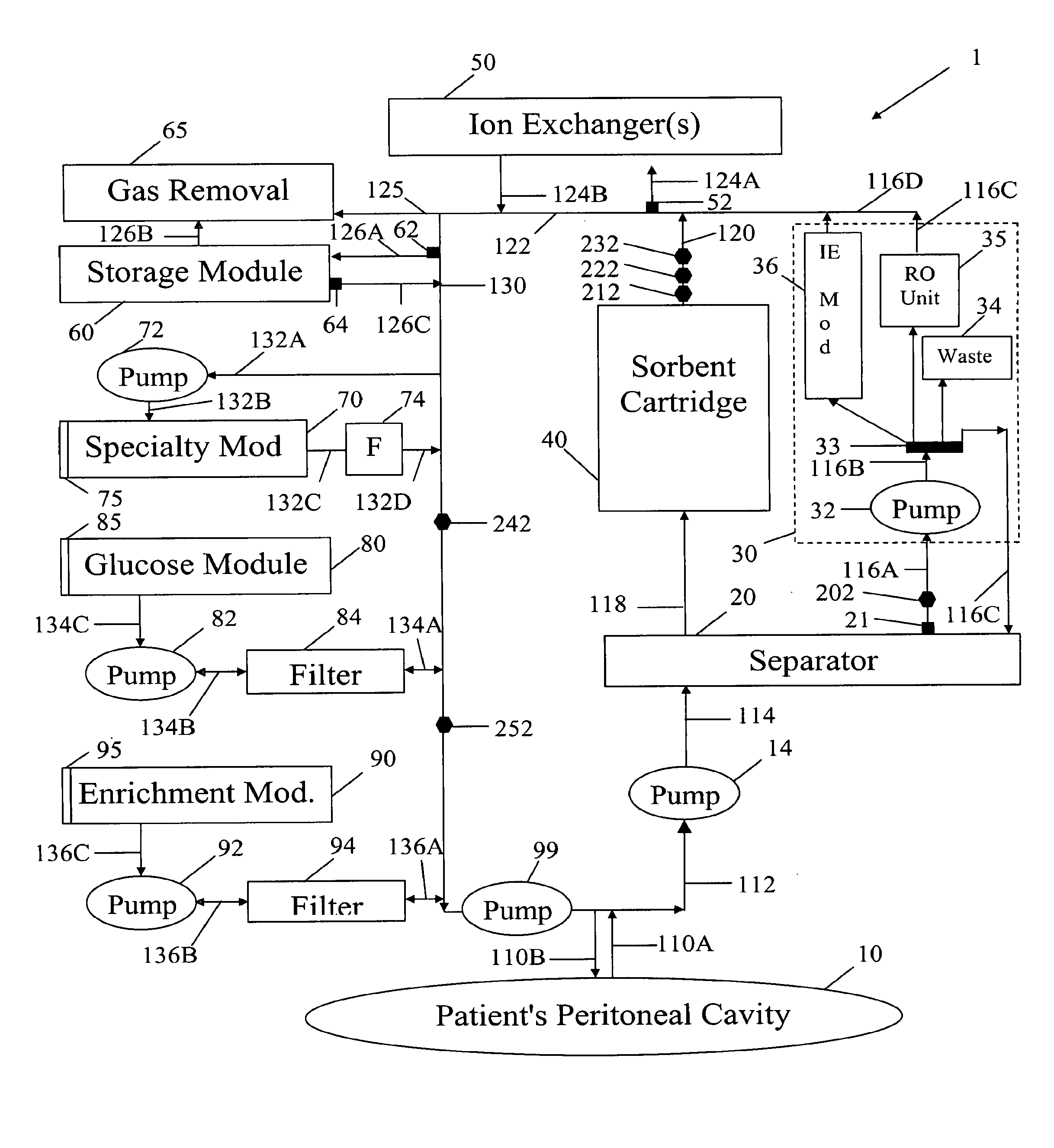
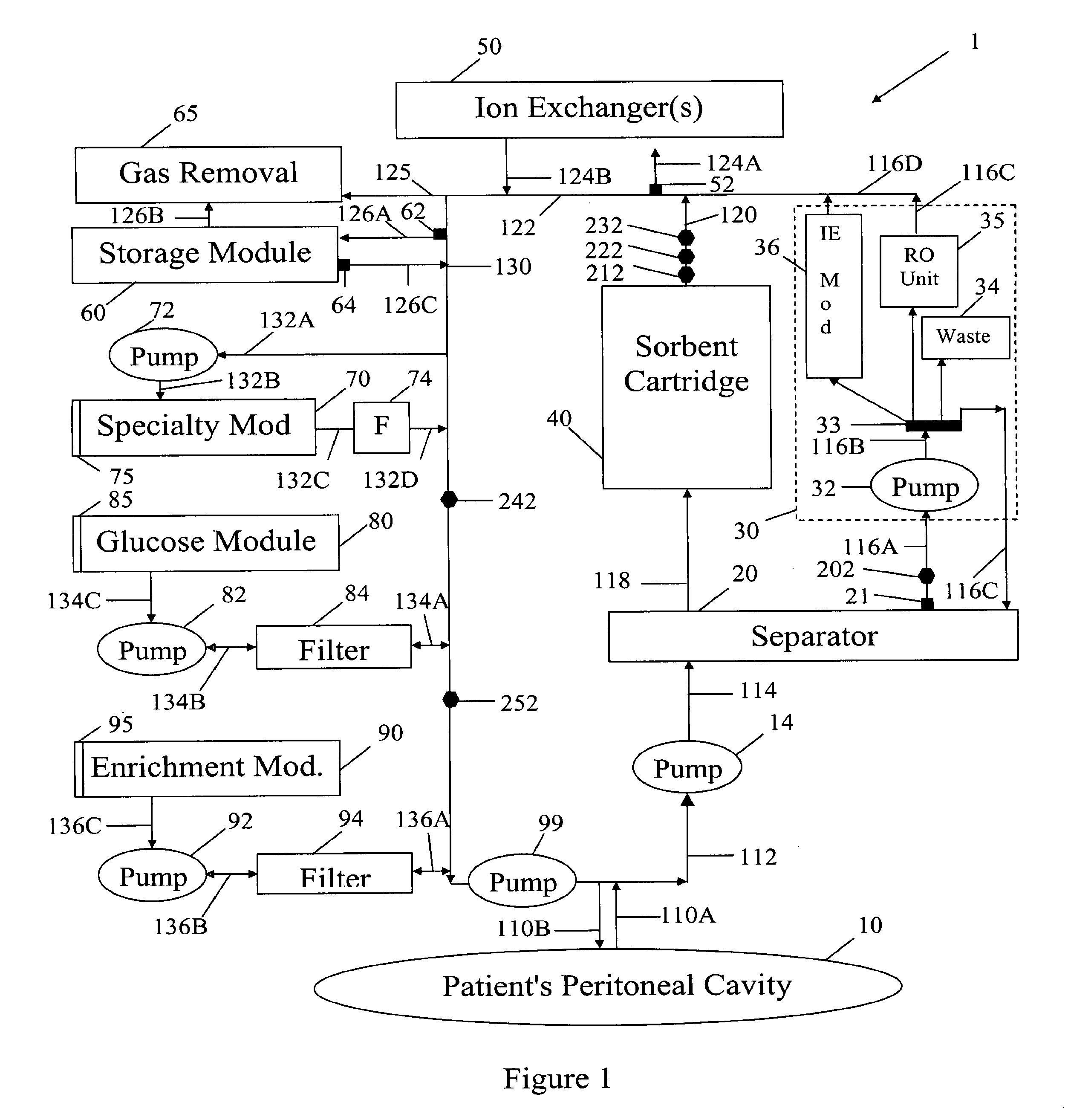
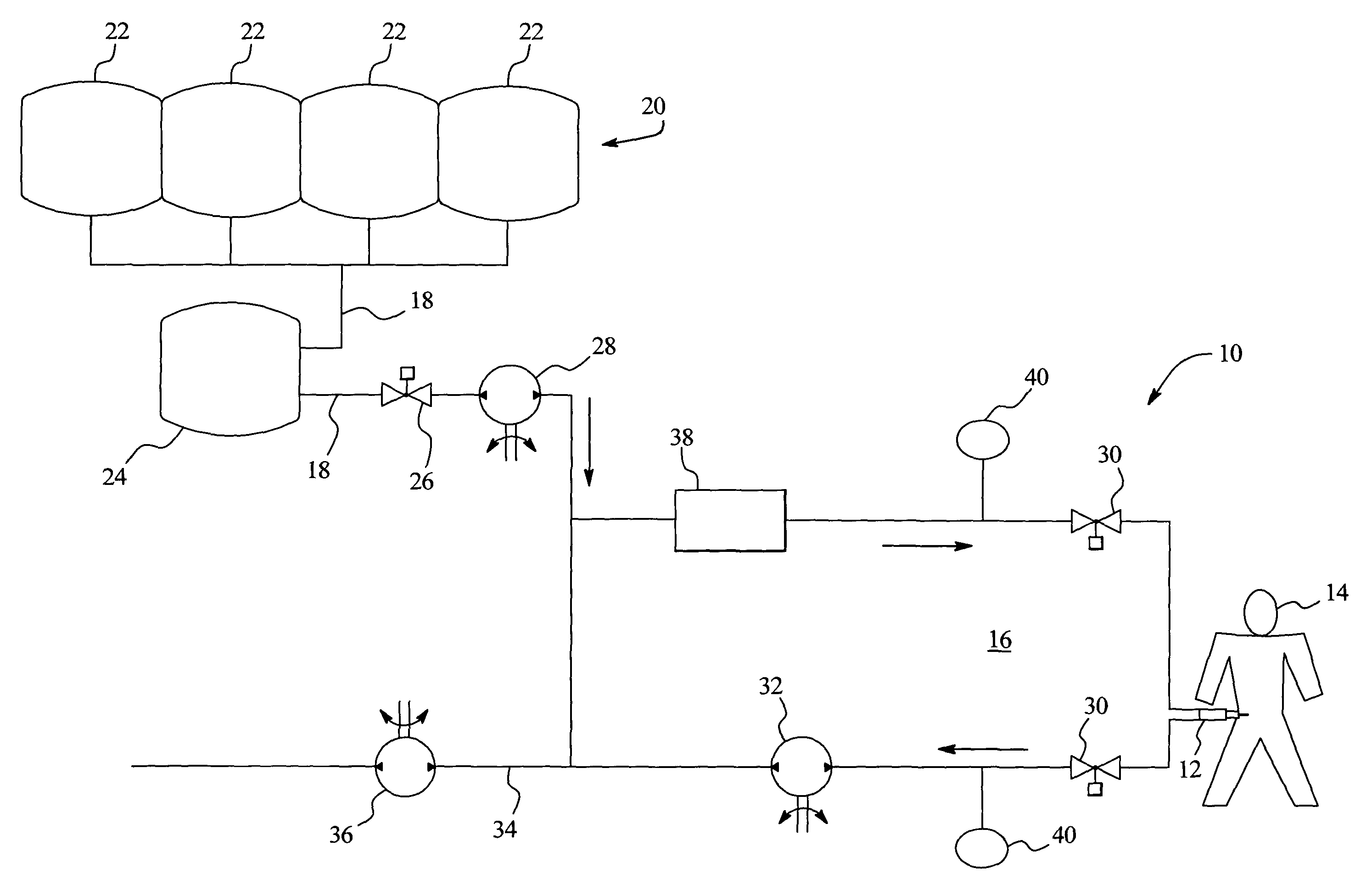
Systems and methods for performing peritoneal dialysis
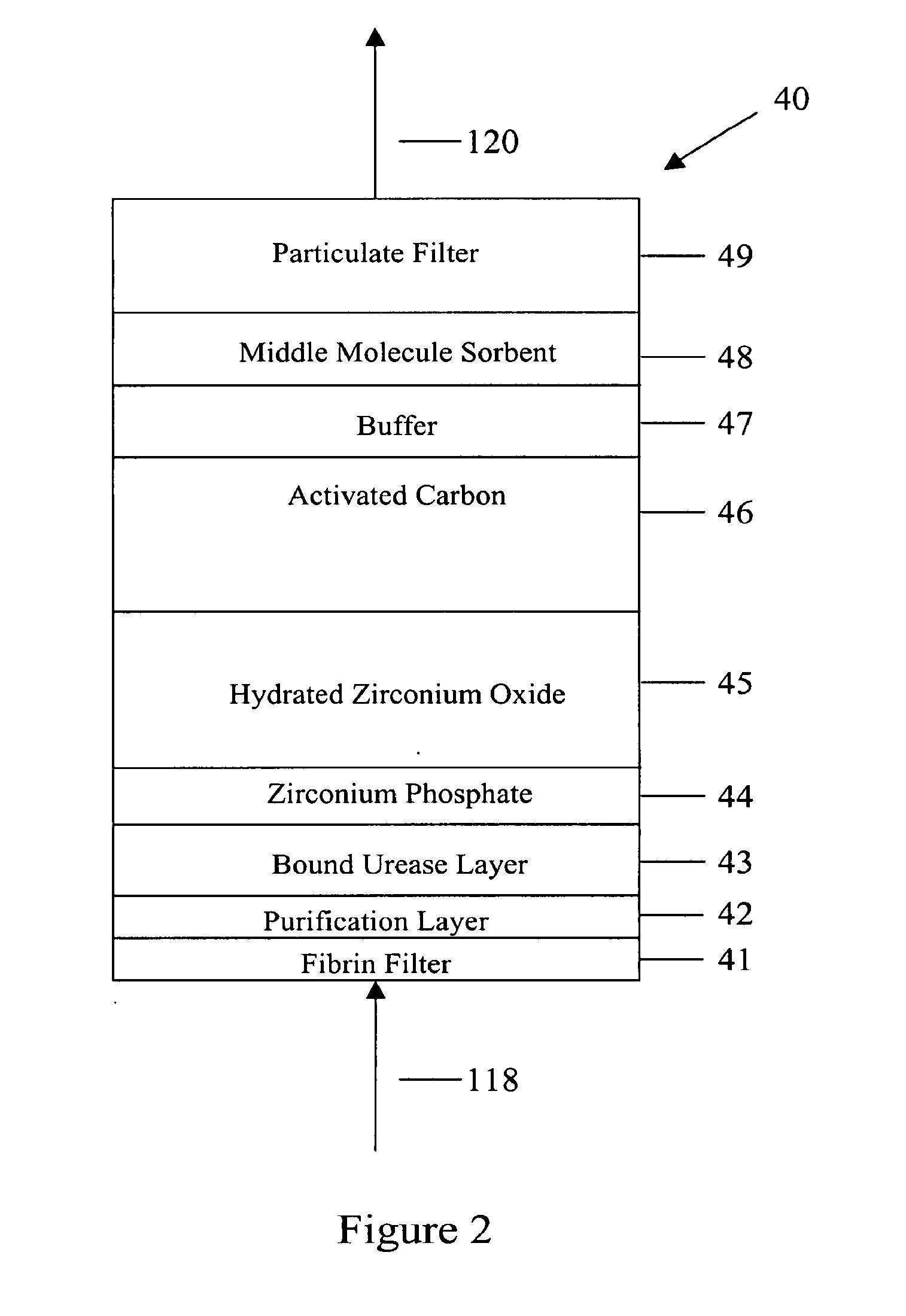
ActiveUS7867214B2Strengthen the systemImprove methodSolvent extractionIon-exchanger regenerationMetabolic wasteSorbent
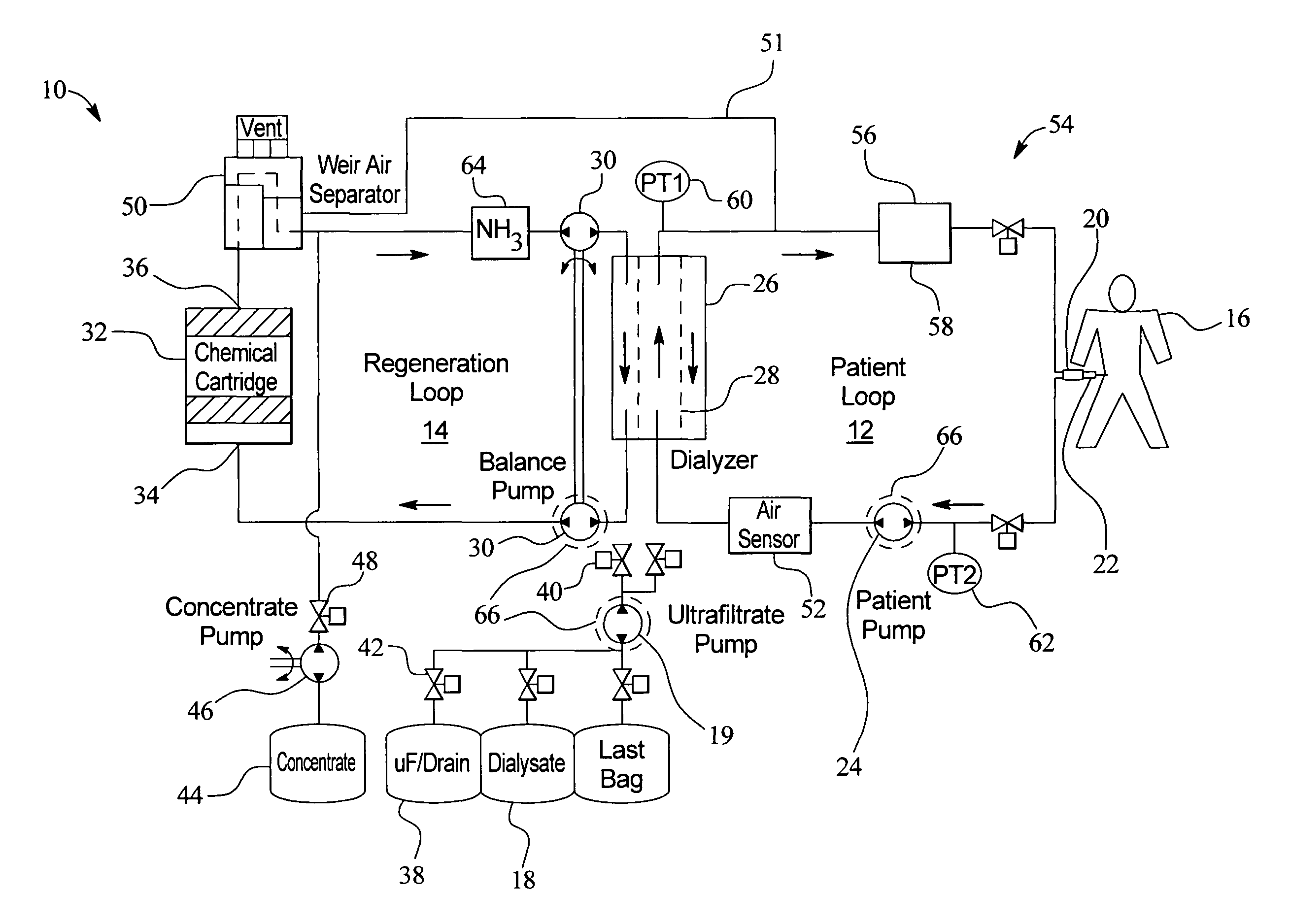
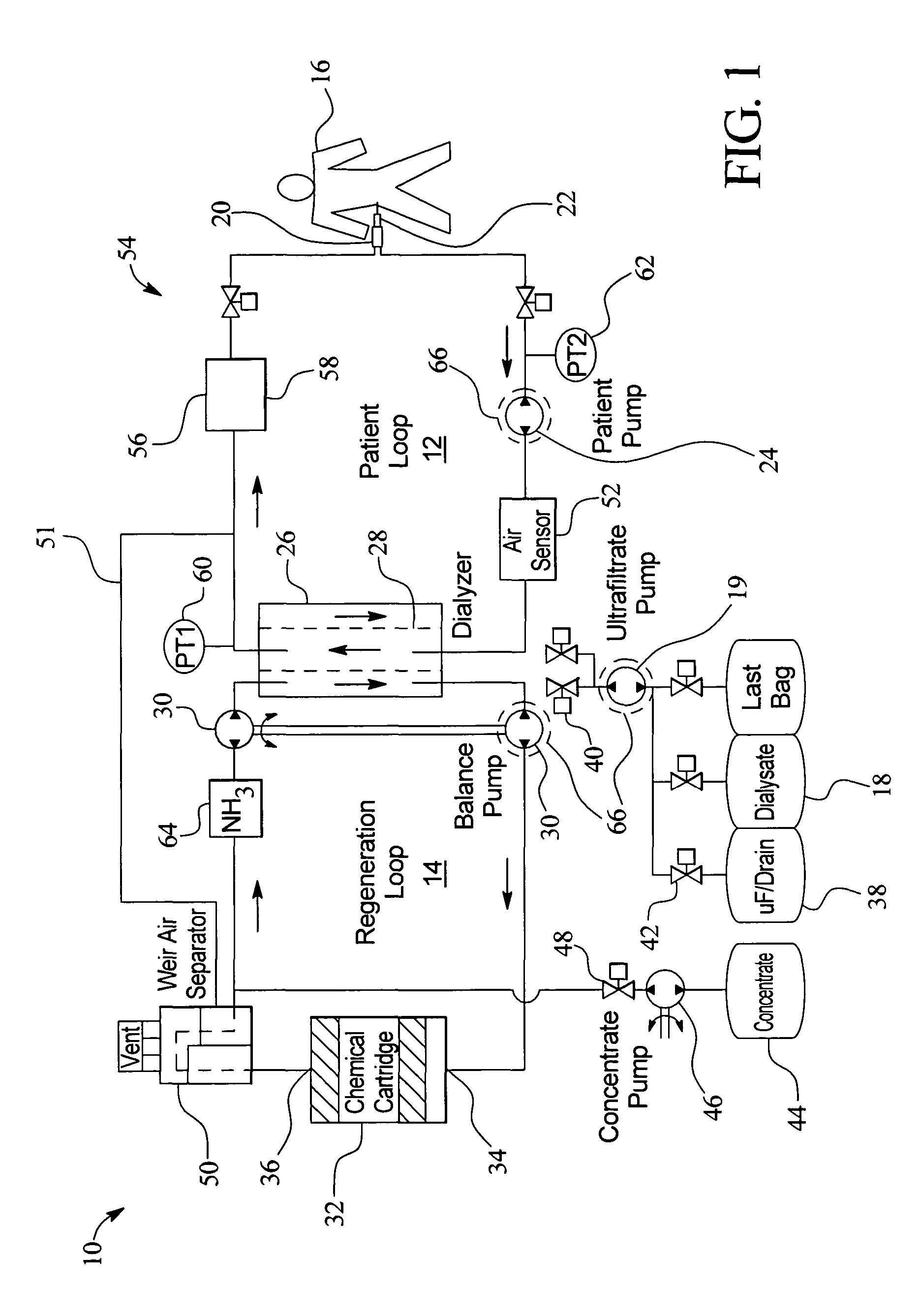
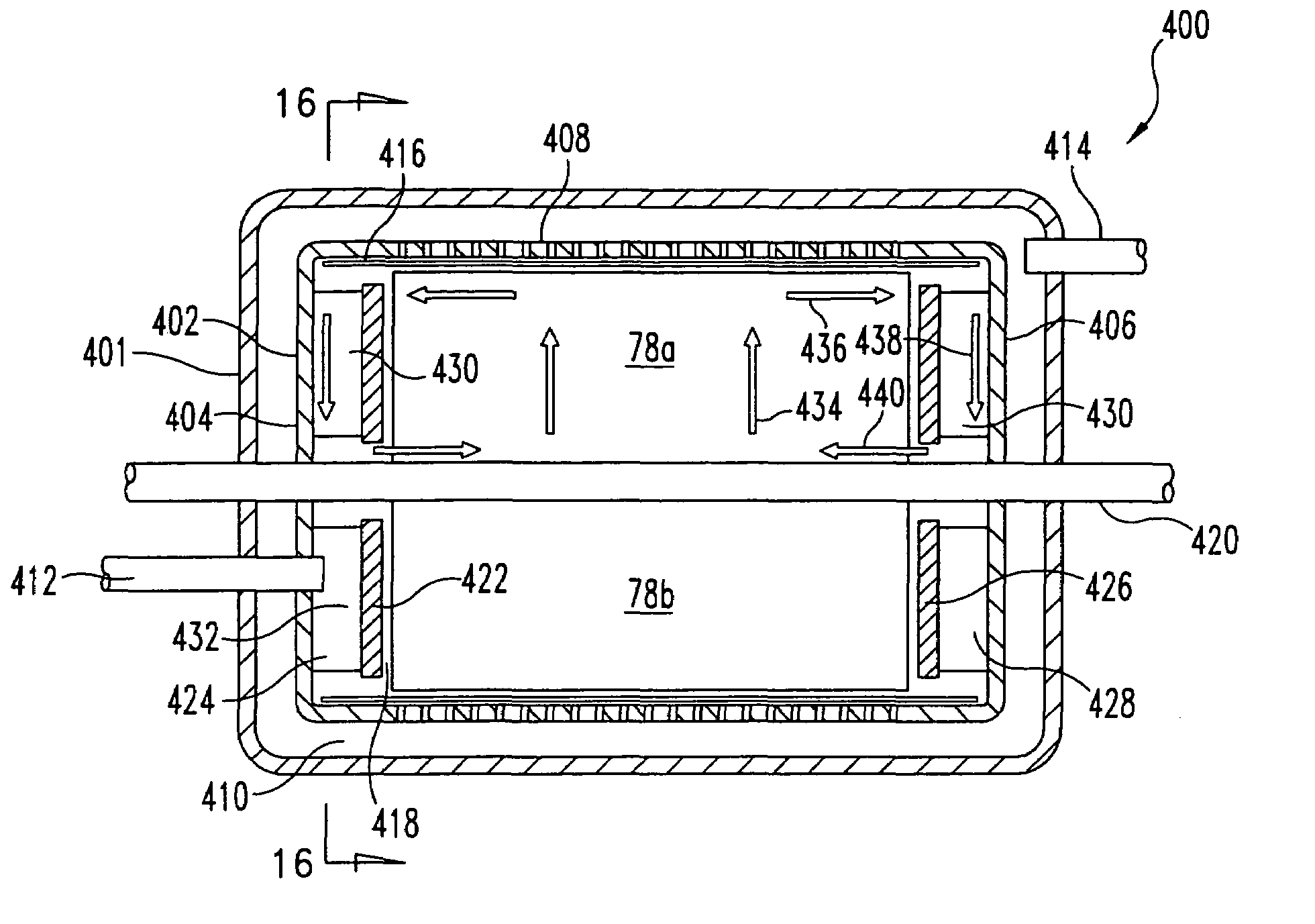
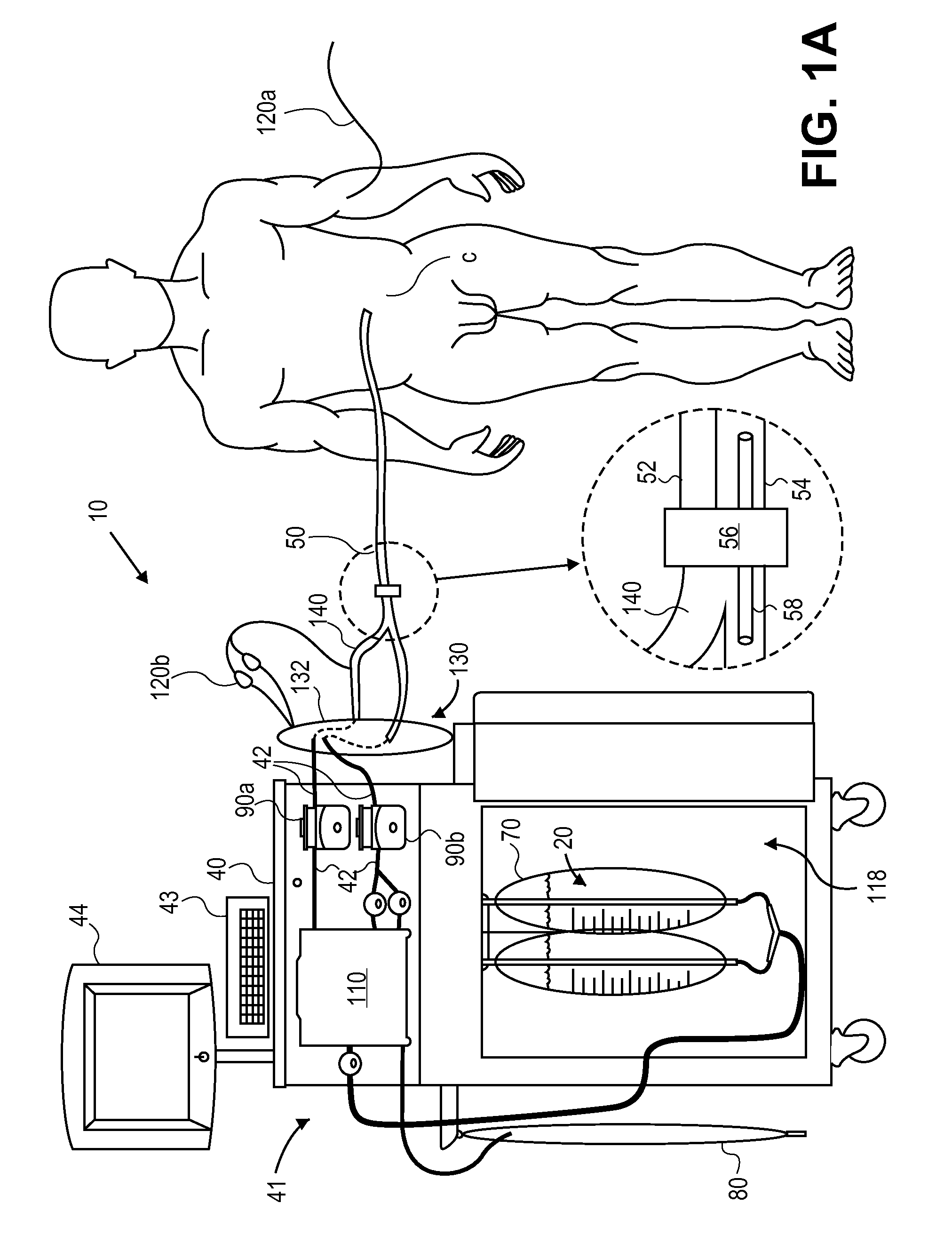
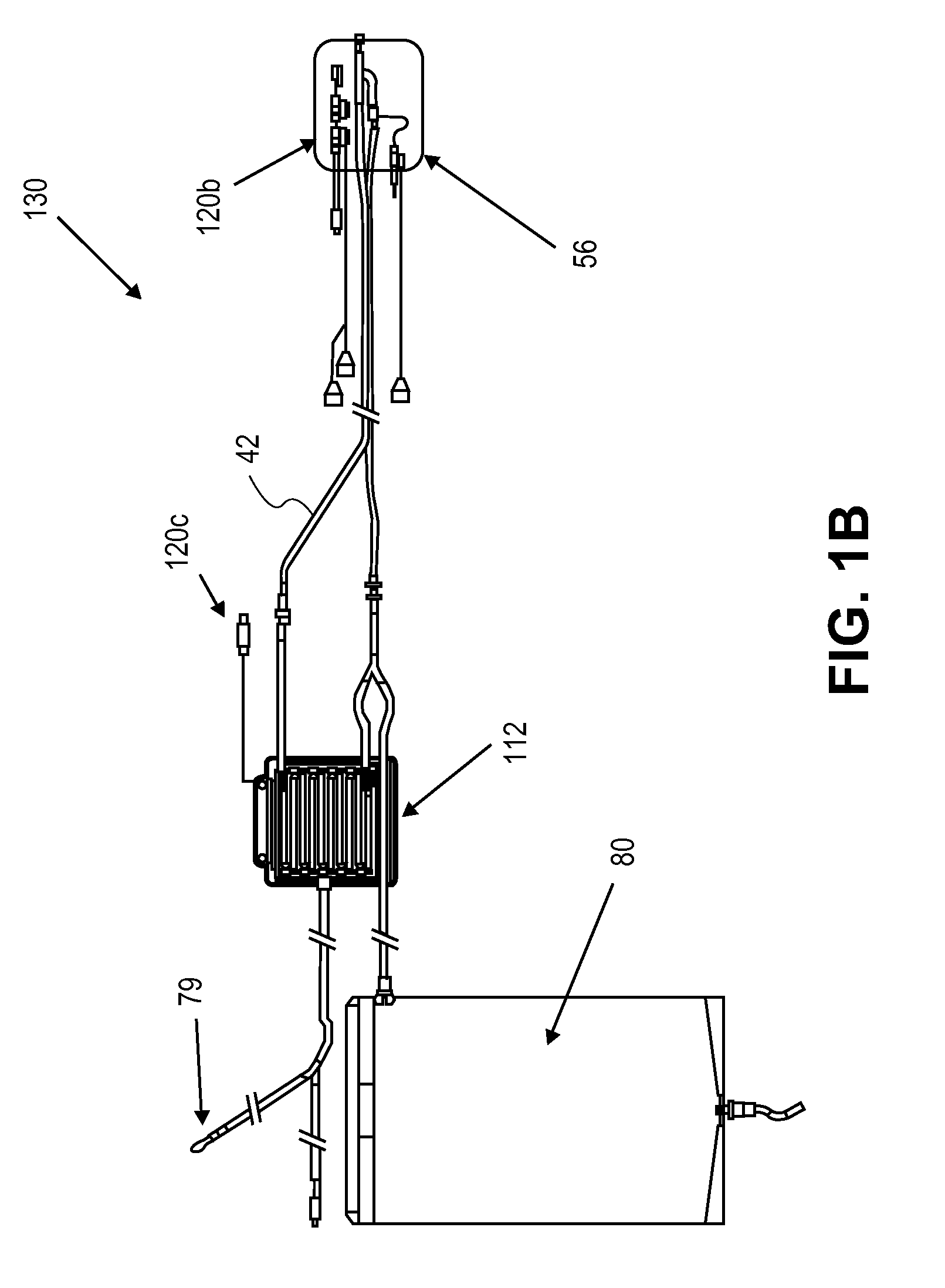
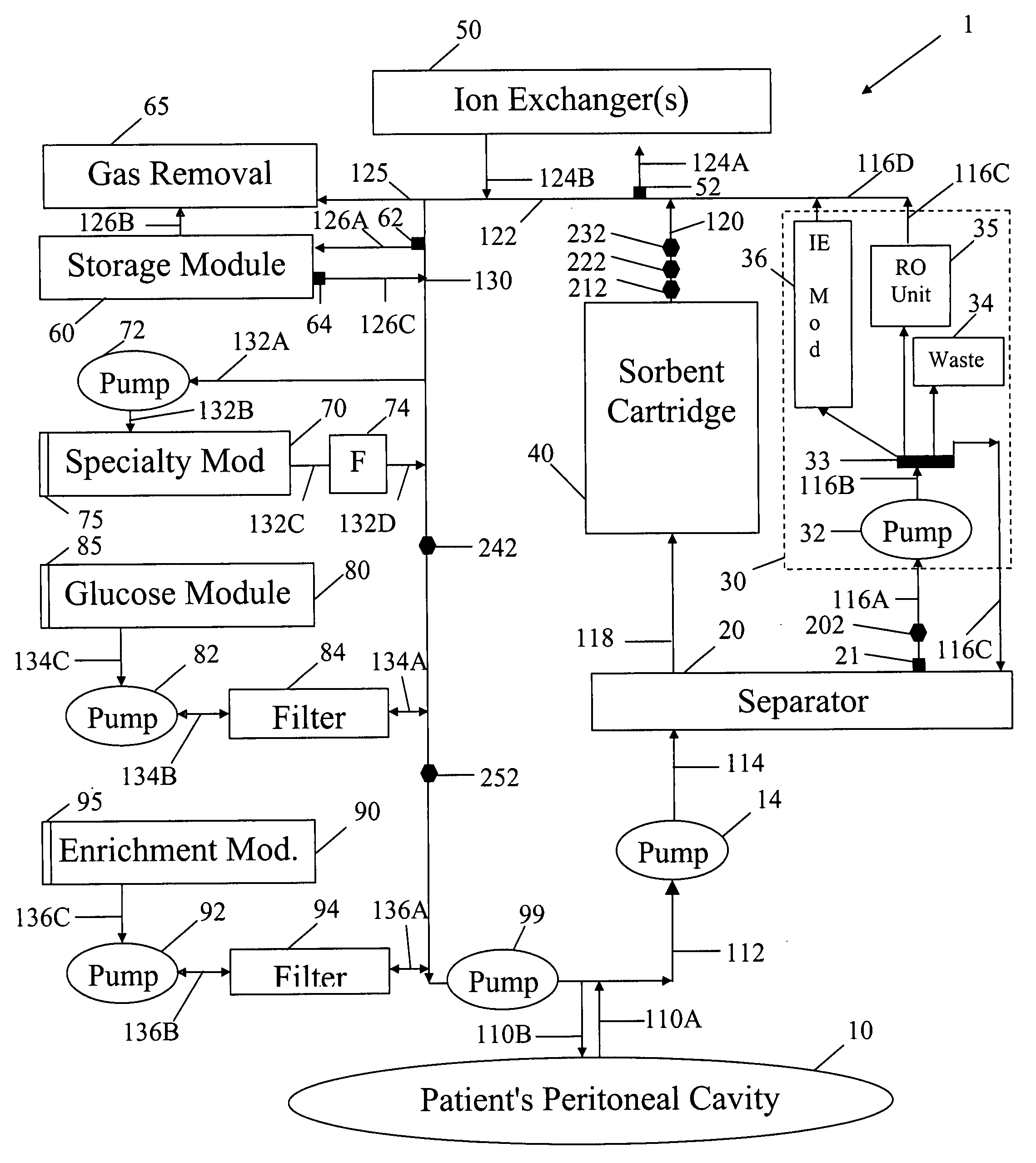
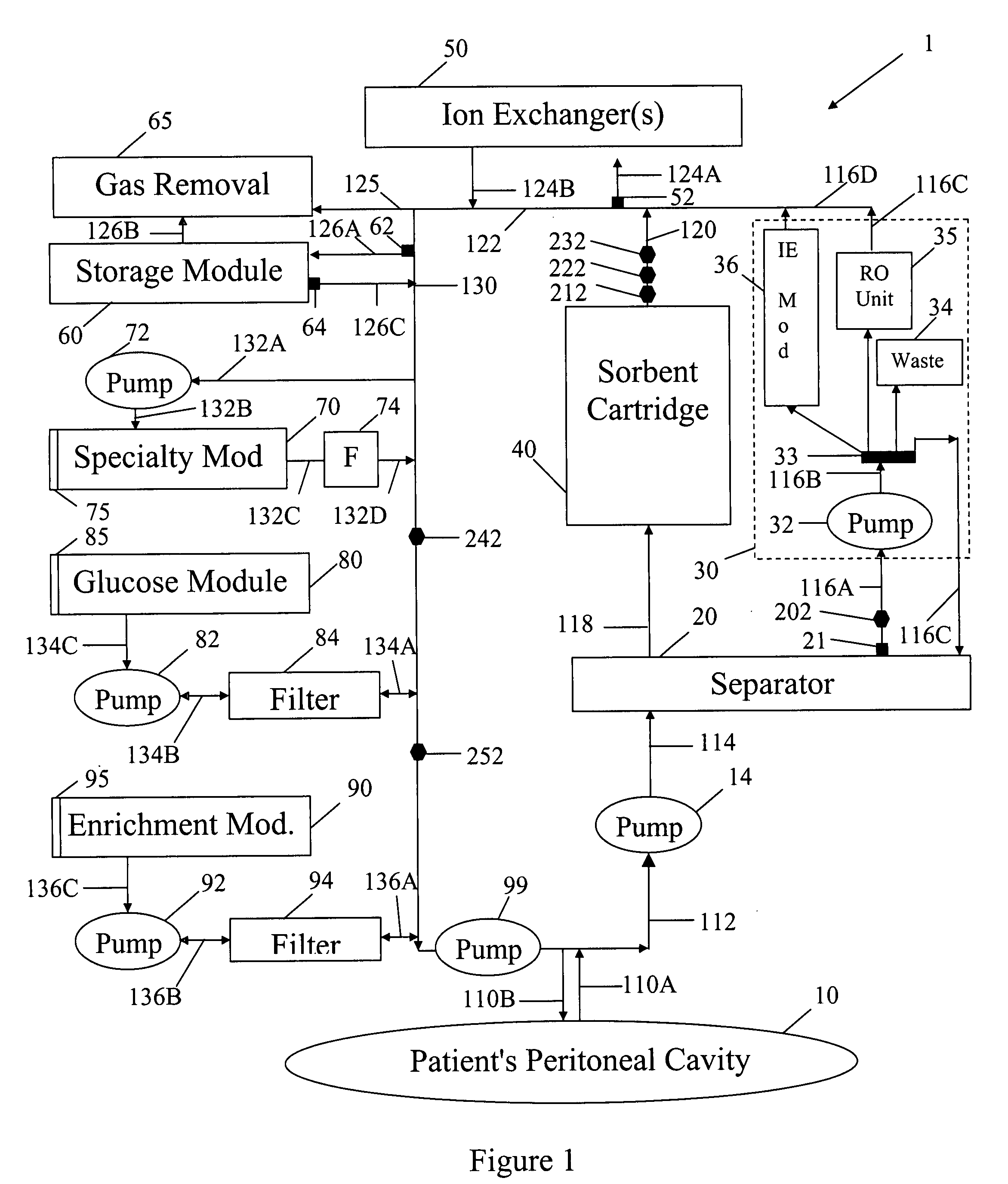
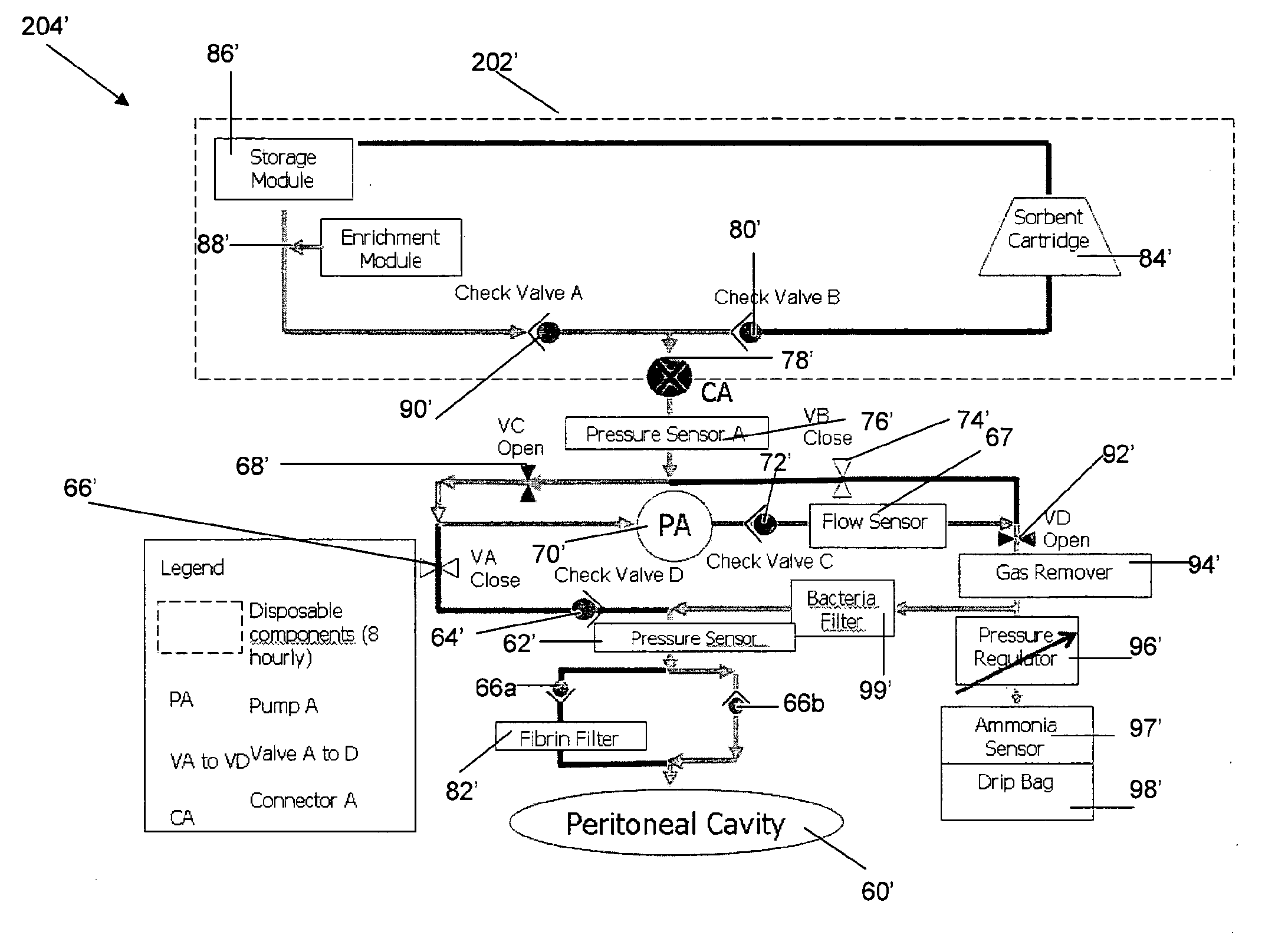
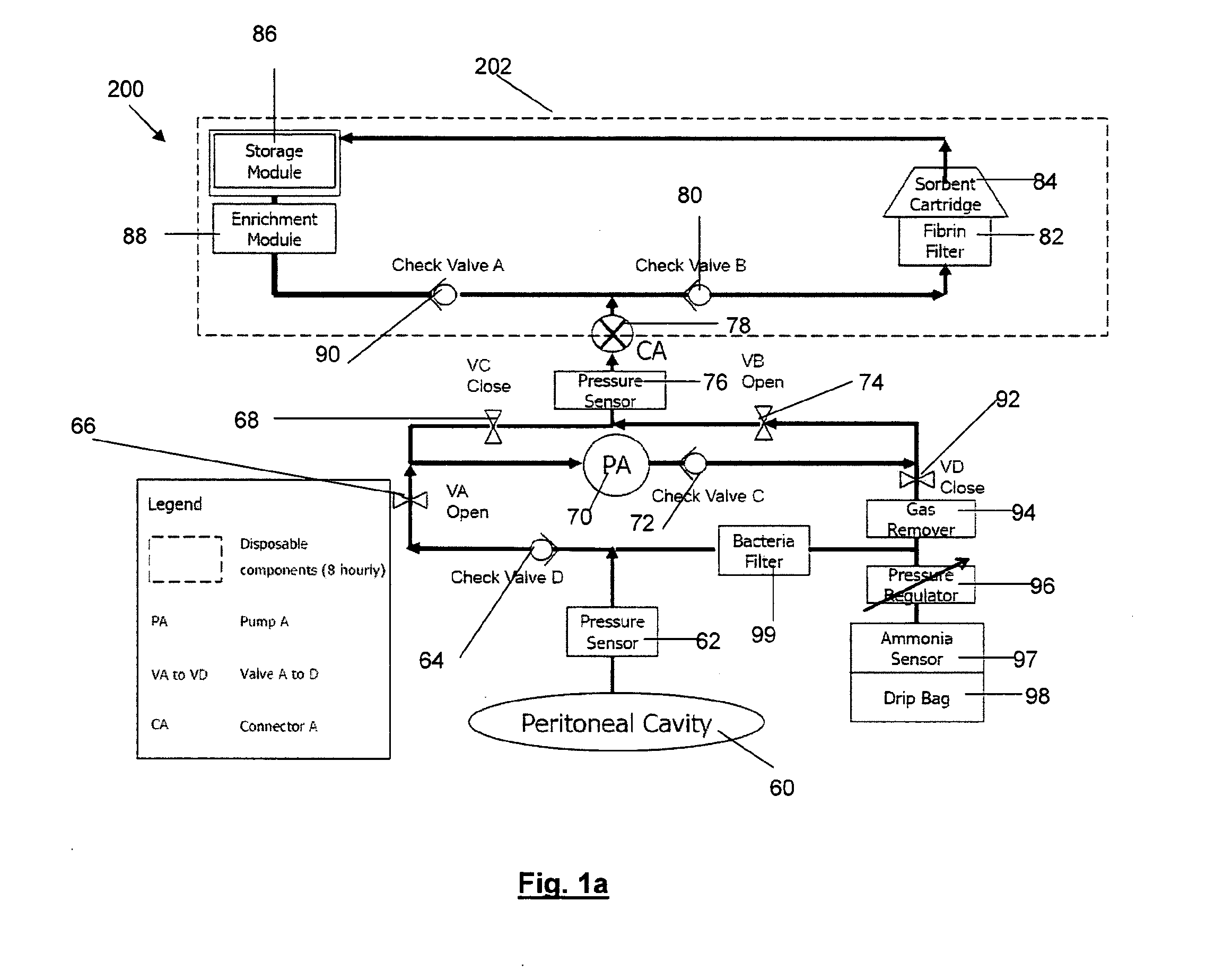
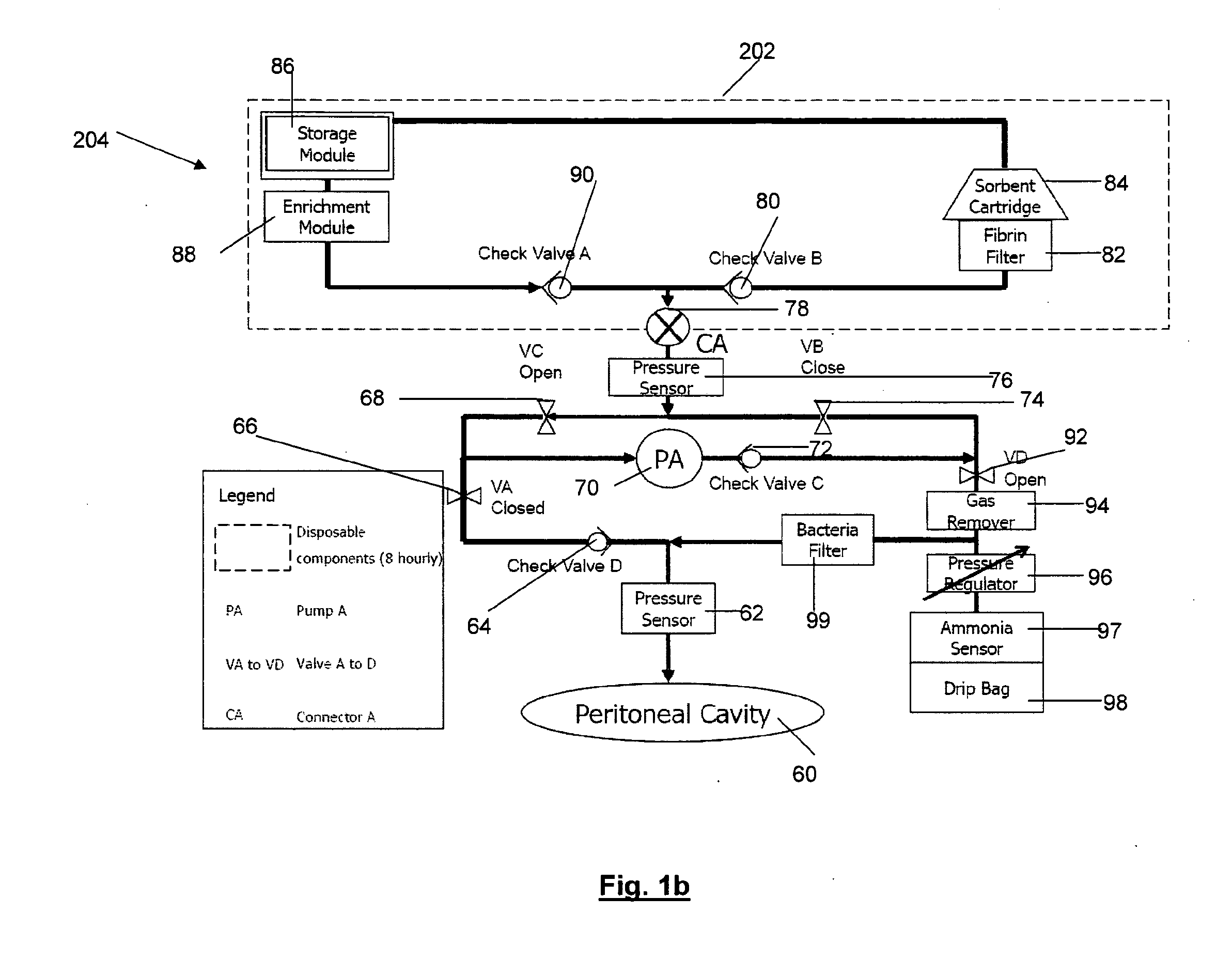
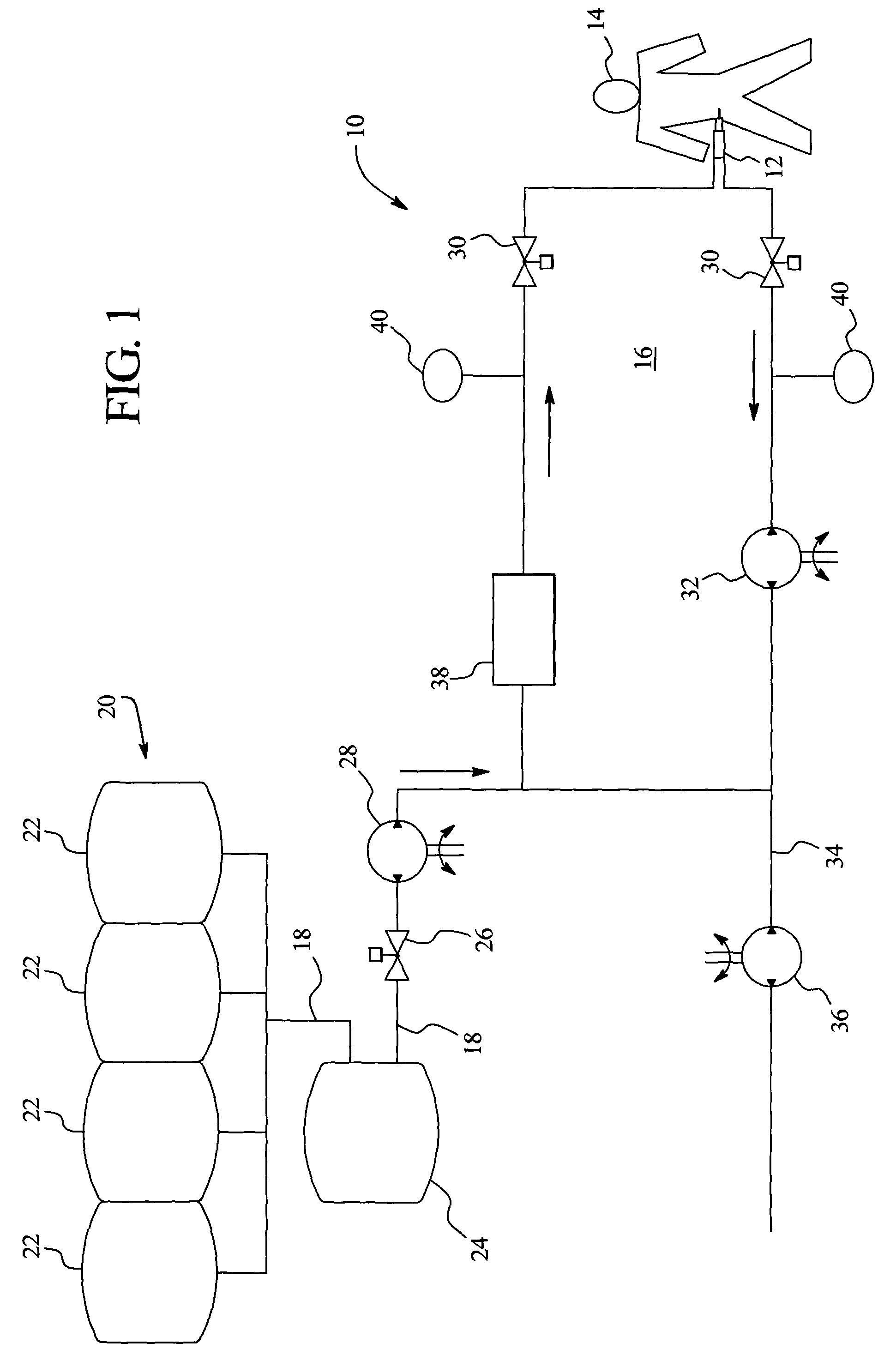
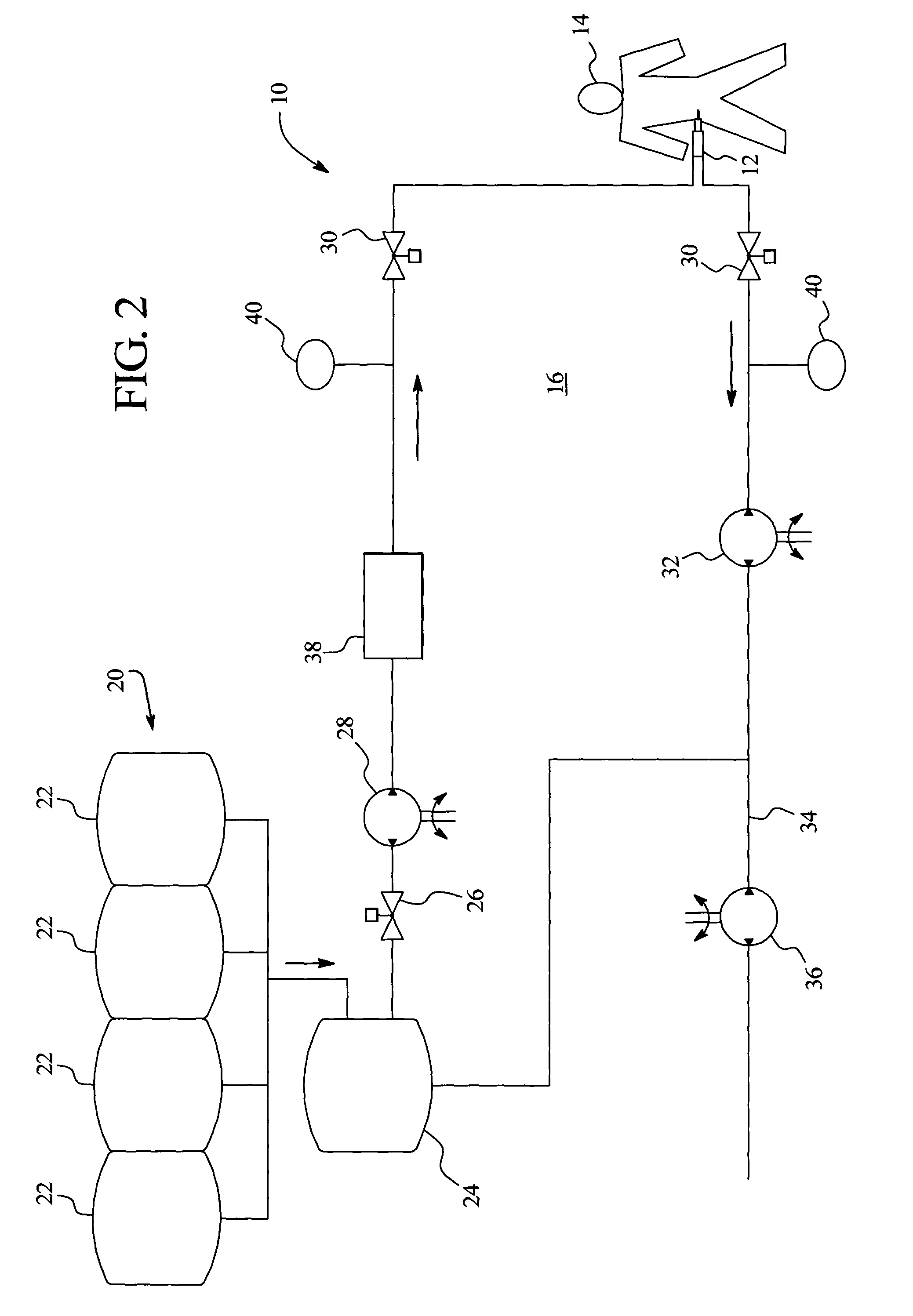
In a peritoneal dialysis embodiment of the present invention, spent dialysate from the patient's peritoneal cavity passes, along a patient loop, through a dialyzer having a membrane that separates waste components from the spent dialysate, wherein the patient loop returns fresh dialysate to the patient's peritoneal cavity. The waste components are carried away in a second regeneration loop to a regeneration unit or sorbent cartridge, which absorbs the waste components. The regeneration unit removes undesirable components in the dialysate that were removed from the patient loop by the dialyzer, for example, excess water (ultrafiltrate or UF), toxins and metabolic wastes. Desirable components can be added to the dialysate by the system, such as glucose and electrolytes. The additives assist in maintaining the proper osmotic gradients in the patient to perform dialysis and provide the necessary compounds to the patient.
Owner:BAXTER INT INC
Instrument assisted abdominal access
InactiveUS20070123840A1Precise positioningIncrease the diameterSuture equipmentsSurgical needlesStomach wallsAbdominal wall
Instrument assisted abdominal access methods and apparatus are described herein. A shape-lockable elongate body can be advanced endoluminally in a flexible state into the stomach, where an opening is created through the stomach wall. The opening can be created endoluminally or by incising instruments placed through the abdominal wall. The elongate body can be transitioned to a rigid state prior to, during, or after advancement into the patient and is passed through the opening into the peritoneal cavity. To assist in the passage of the elongate body, a helical tissue engager can be advanced into the peritoneal cavity and temporarily anchored into a tissue wall. By pulling the helical tissue engager proximally and pushing the elongate body over a flexible length of the engager, the elongate body can be brought into the peritoneal cavity
Owner:USGI MEDICAL
Sorbent reactor for extracorporeal blood treatment systems, peritoneal dialysis systems, and other body fluid treatment systems
InactiveUS7169303B2Facilitate homogeneous suspensionReduce probabilitySolvent extractionHaemofiltrationFluid balancePeritoneal dialysis
Systems and methods for extracorporeal processing of blood or other body fluid for the treatment of conditions, such as sepsis, autoimmune disease, or toxemia related to kidney failure, liver failure, or drug overdose are provided. In an extracorporeal treatment system, a fraction of a body fluid is passed into a treatment fluid, at least a portion of which is then passed through a sorbent suspension reactor for treatment by a sorbent suspension. The treatment fluid circuit can be maintained at a fixed volume, which enables accurate fluid balance between the patient and the extracorporeal circuit. Some or all of the treatment fluid, optionally also containing nutrients and / or therapeutic agents, is returned to the patient. In a peritoneal dialysis system, dialysate is passed into a patient's peritoneal cavity, recovered from the cavity, passed through a sorbent suspension reactor in accordance with the invention, and returned to the cavity.
Owner:HEMOCLEANSE TECH
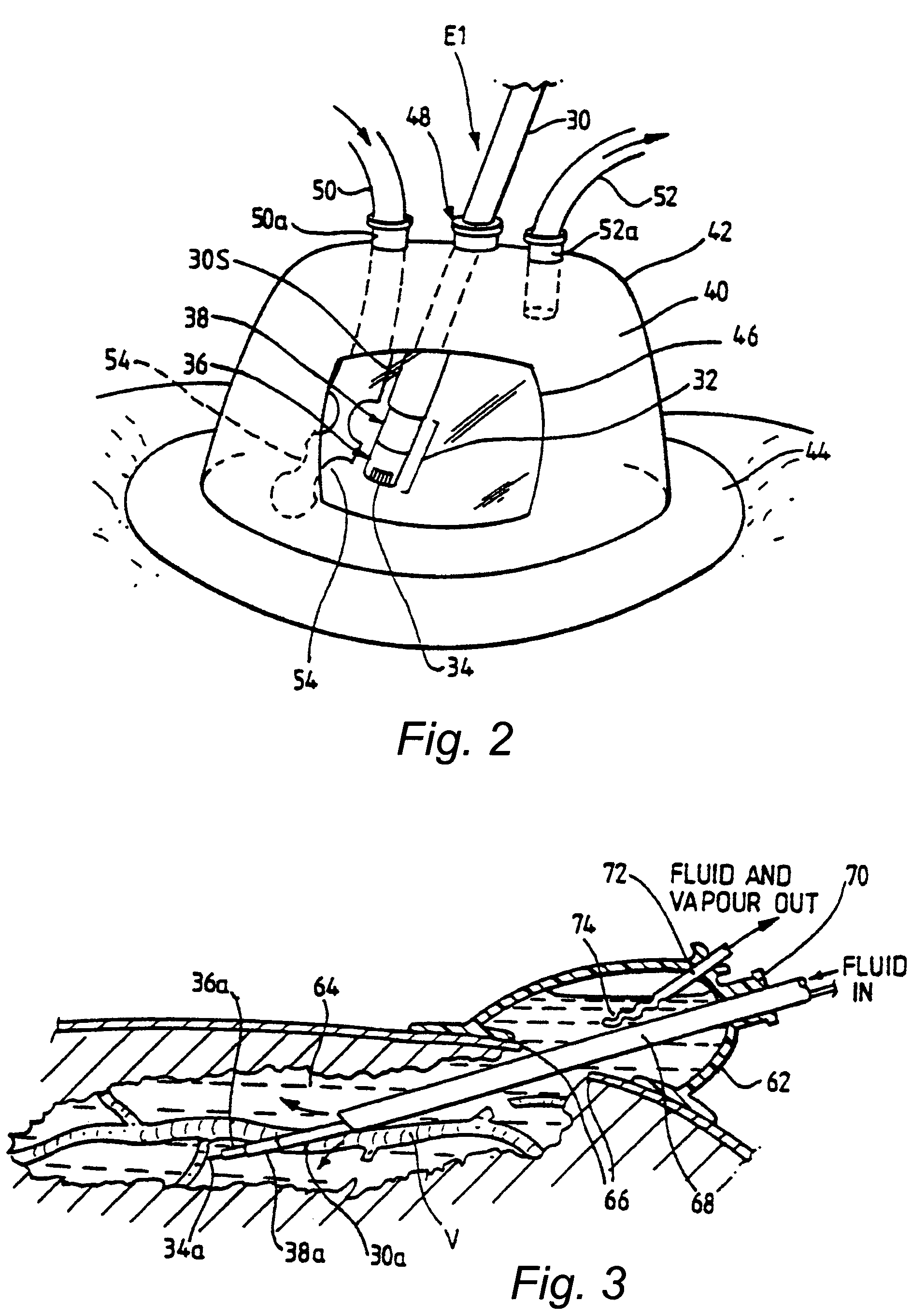
Method and Apparatus for Inducing Therapeutic Hypothermia
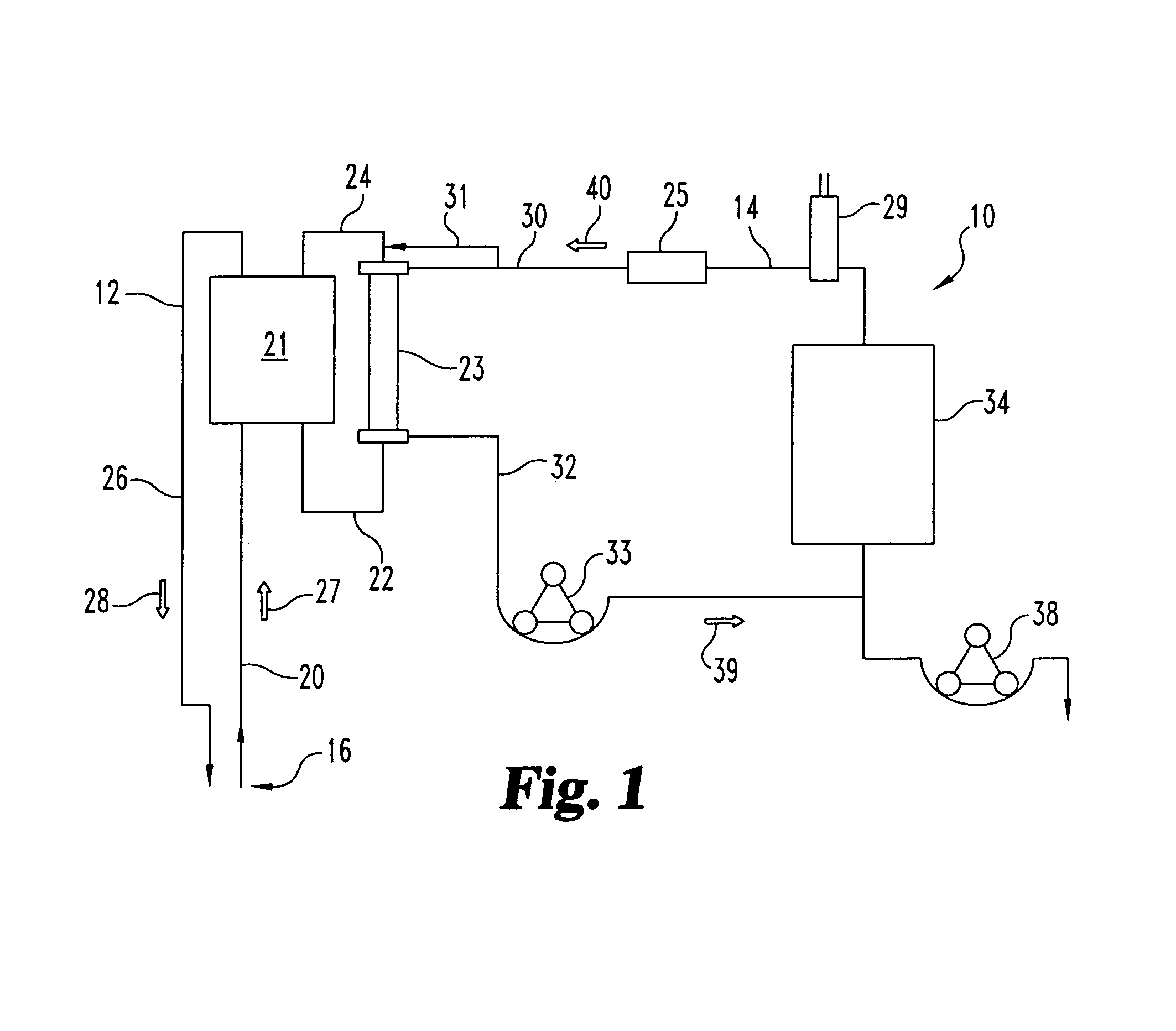
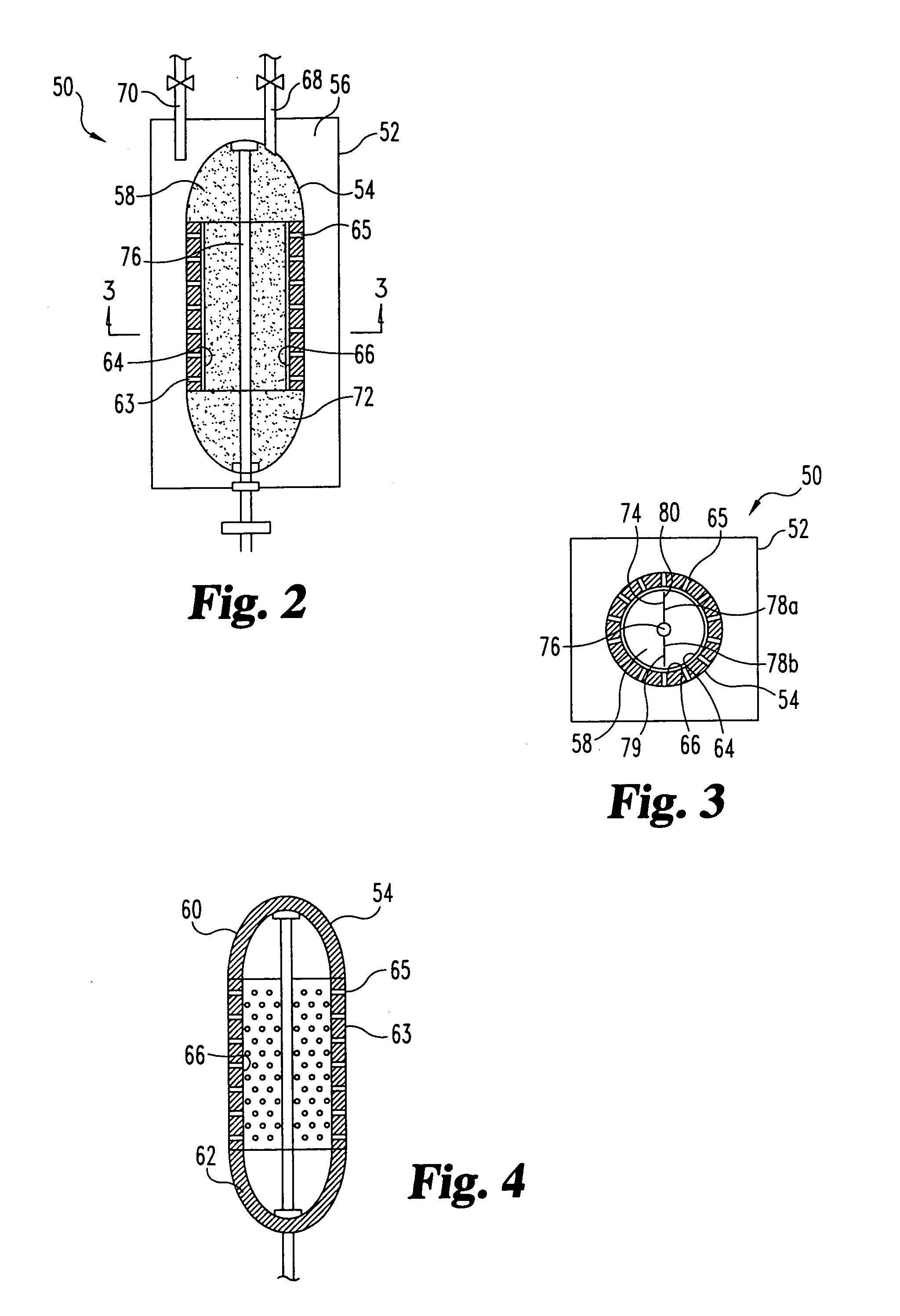
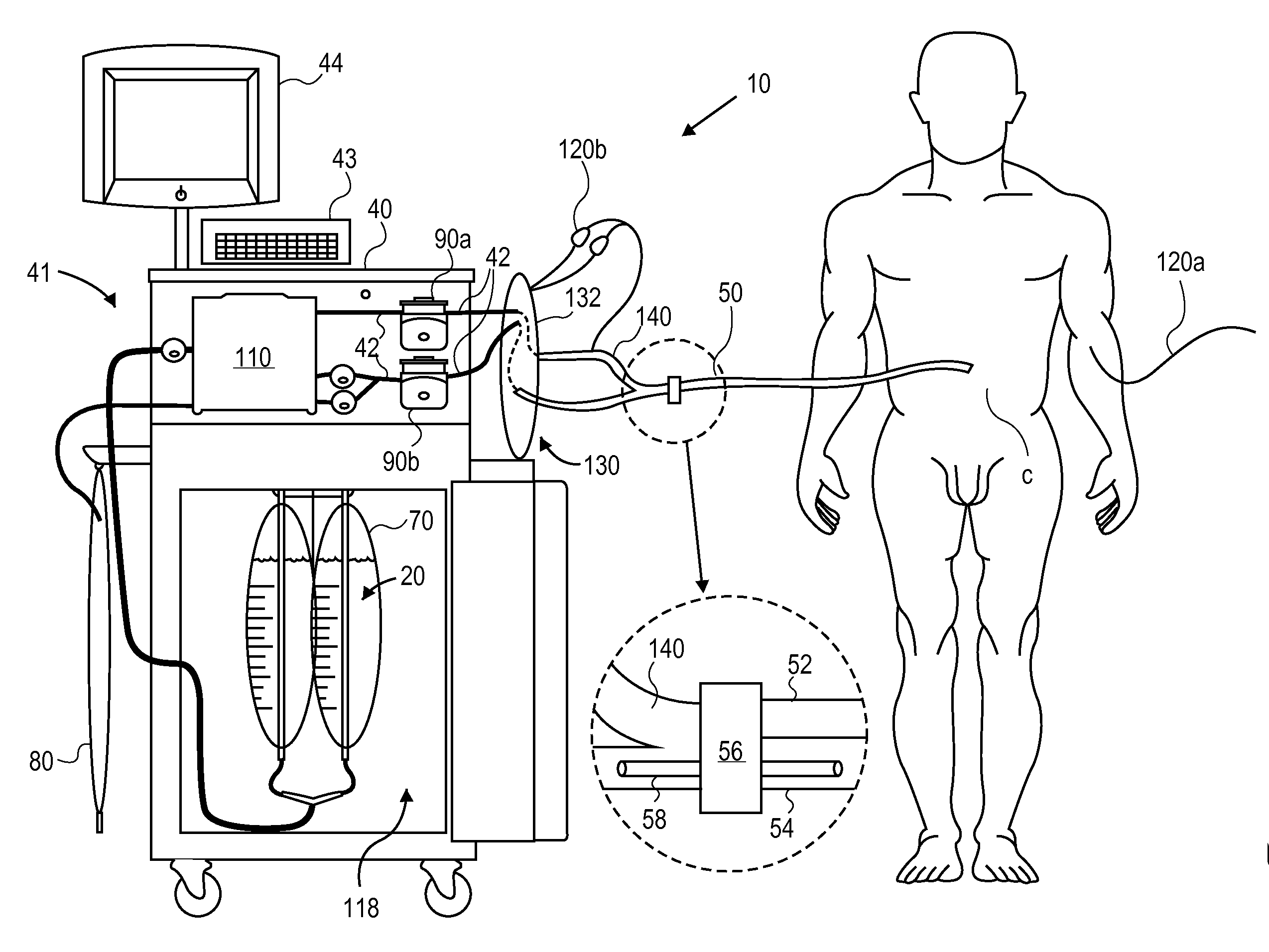
Methods and apparatus for delivering therapeutic hypothermia to a patient are provided which may include any number of features. One feature is a hypothermia system comprising a fluid source, a heat exchanger assembly, a catheter in fluid communication with the fluid source, and a pump system configured to infuse hypothermic fluid into a patient cavity and extract hypothermic fluid from the patient cavity. The hypothermia system can infuse and extract fluid automatically from the patient cavity. In one embodiment, the patient cavity is a peritoneal cavity. A safe access device to gain access to the patient cavity is also provided.
Owner:VELOMEDIX
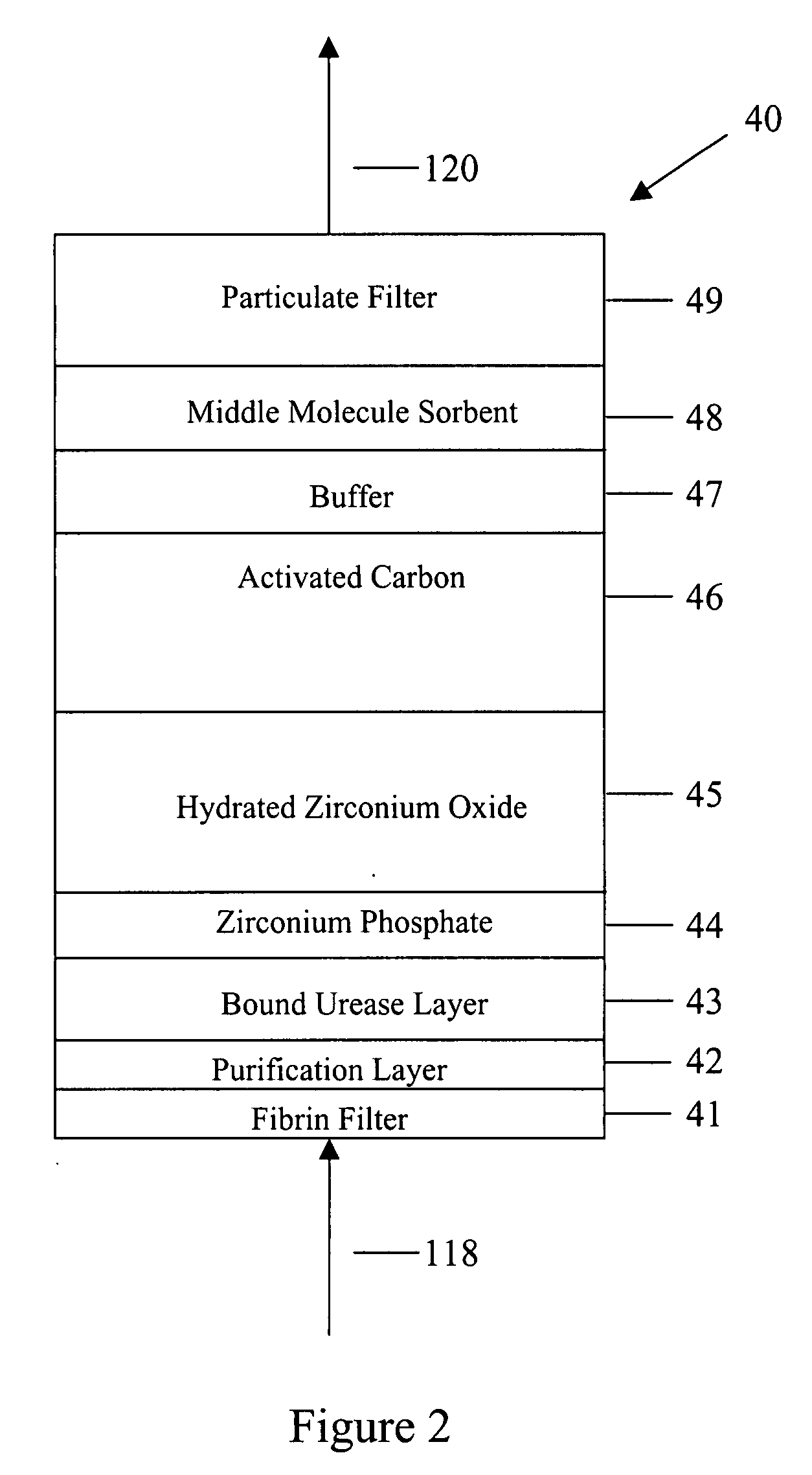
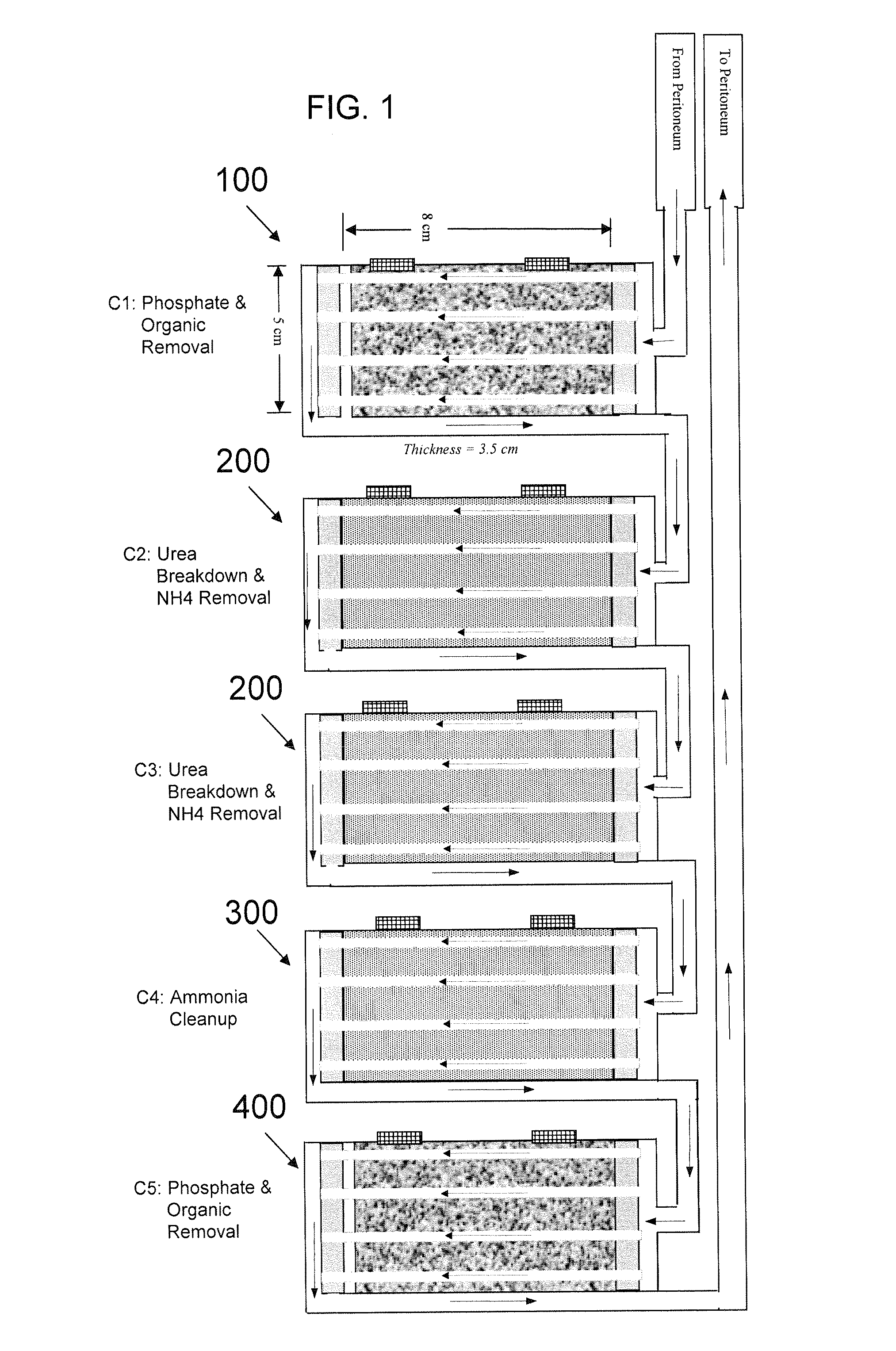
Peritoneal dialysis methods and apparatus
ActiveUS20070179431A1Increase percentageImprove wear resistancePeritoneal dialysisProtein compositionPeritoneal fluid
A peritoneal-based (“bloodless”) artificial kidney processes peritoneal fluid without need for additional fluids (“waterless”). Fluid is separated into a protein-rich stream and a protein-free stream. The protein-rich stream is regenerated using a sorbent assembly, and its protein composition can be modified by removal of selected protein(s) (“dialysate-pheresis”). It is then reconstituted with additives and returned into the peritoneal cavity, thereby reducing protein-loss and providing oncotic-pressure for ultrafiltration. The protein-free stream is used to produce free water, and an alkaline or acid fluid for optimization of the composition of the regenerated stream. The unused protein-free stream can be used to “reverse flush” the separator to maintain its patency and the excess discarded for fluid-balance regulation. Compared to prior art, immobilization of urease allows more protein rich fluid to be regenerated and re-circulated into the peritoneal cavity for toxin removal and allows practicable development of portable and wearable artificial kidneys.
Owner:RGT UNIV OF CALIFORNIA +1
Methods and devices for diagnostic and therapeutic interventions in the peritoneal cavity
A novel approach to diagnostic and therapeutic interventions in the peritoneal cavity is described. More specifically, a technique for accessing the peritoneal cavity via the wall of the digestive tract is provided so that examination of and / or a surgical procedure in the peritoneal cavity can be conducted via the wall of the digestive tract with the use of a flexible endoscope. As presently proposed, the technique is particularly adapted to transgastric peritoneoscopy. However, access in addition or in the alternative through the intestinal wall is contemplated and described as well. Transgastric and / or transintestinal peritoneoscopy will have an excellent cosmetic result as there are no incisions in the abdominal wall and no potential for visible post-surgical scars or hernias.
Owner:APOLLO ENDOSURGERY INC
Apparatus and methods for facilitating treatment of tissue via improved delivery of energy based and non-energy based modalities
InactiveUS20050020901A1Avoid excessive forceAccurately advancedEndoscopesCatheterThoracic structureAutomatic control
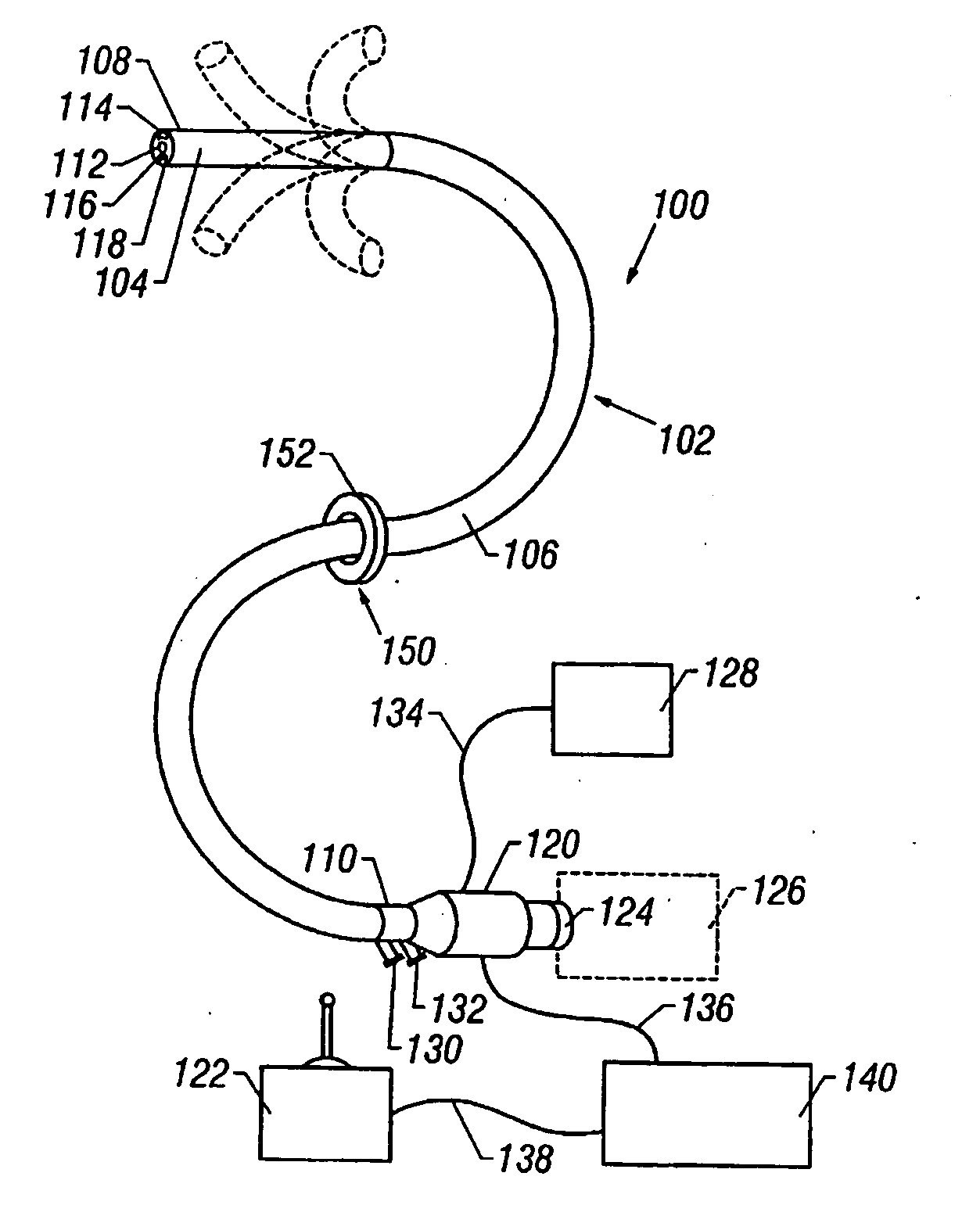
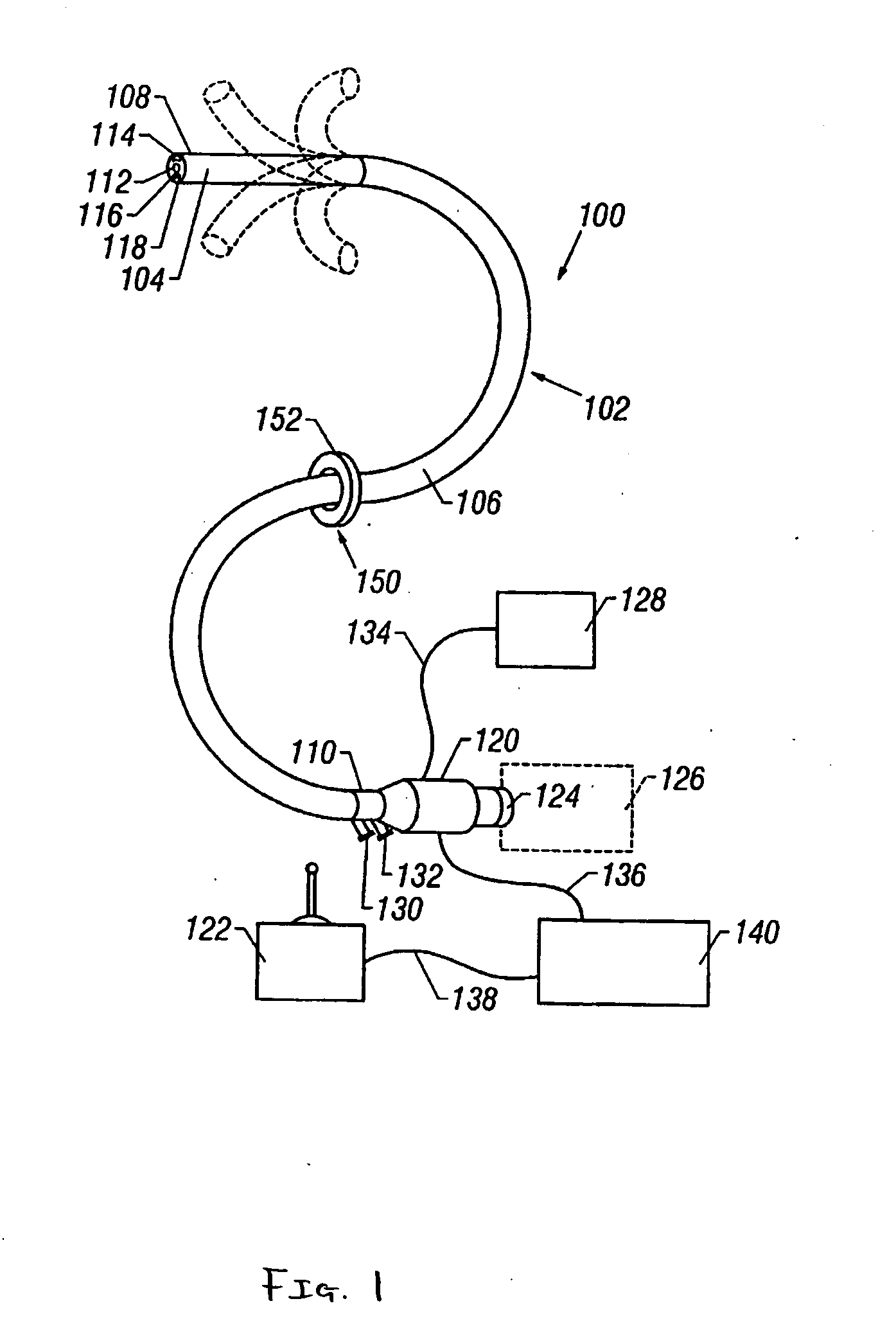
Methods and apparatus for accessing and treating regions of the body are disclosed herein. Using an endoscopic device having an automatically controllable proximal portion and a selectively steerable distal portion, the device generally may be advanced into the body through an opening. The distal portion is selectively steered to assume a selected curve along a desired path within the body which avoids contact with tissue while the proximal portion is automatically controlled to assume the selected curve of the distal portion. The endoscopic device can then be used for accessing various regions of the body which are typically difficult to access and treat through conventional surgical techniques because the device is unconstrained by “straight-line” requirements. Various applications can include accessing regions of the brain, thoracic cavity, including regions within the heart, peritoneal cavity, etc., which are difficult to reach using conventional surgical procedures.
Owner:INTUITIVE SURGICAL OPERATIONS INC
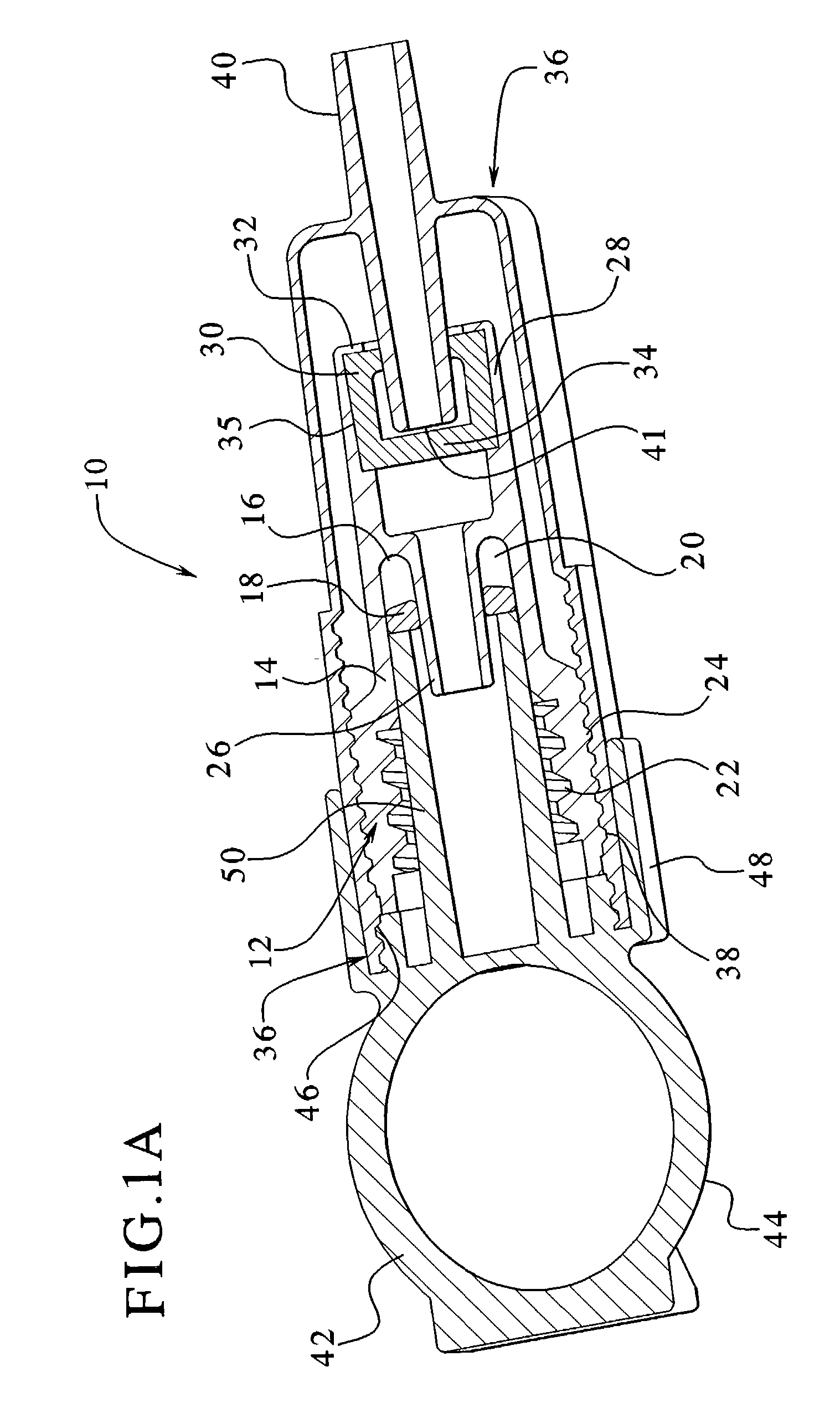
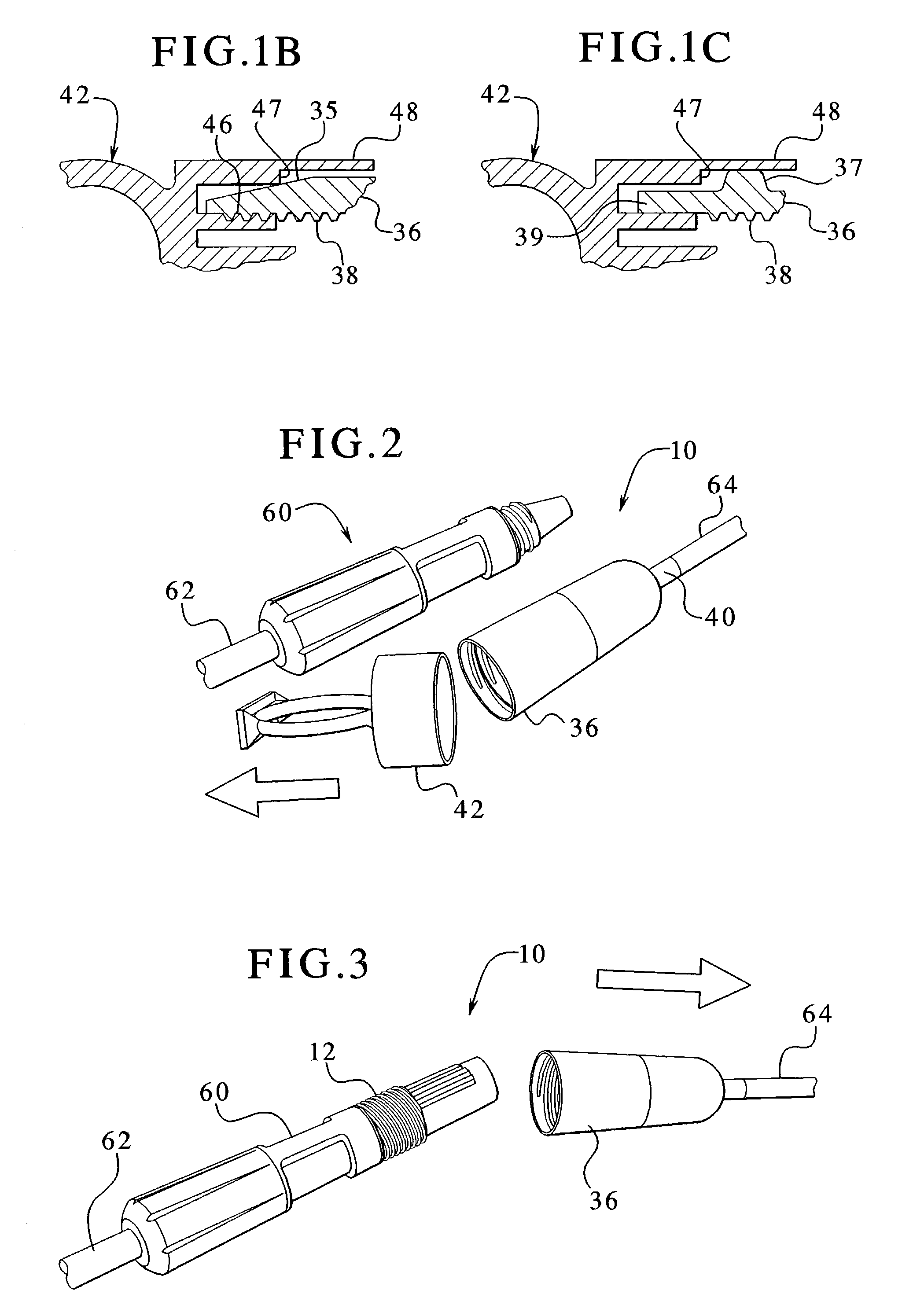
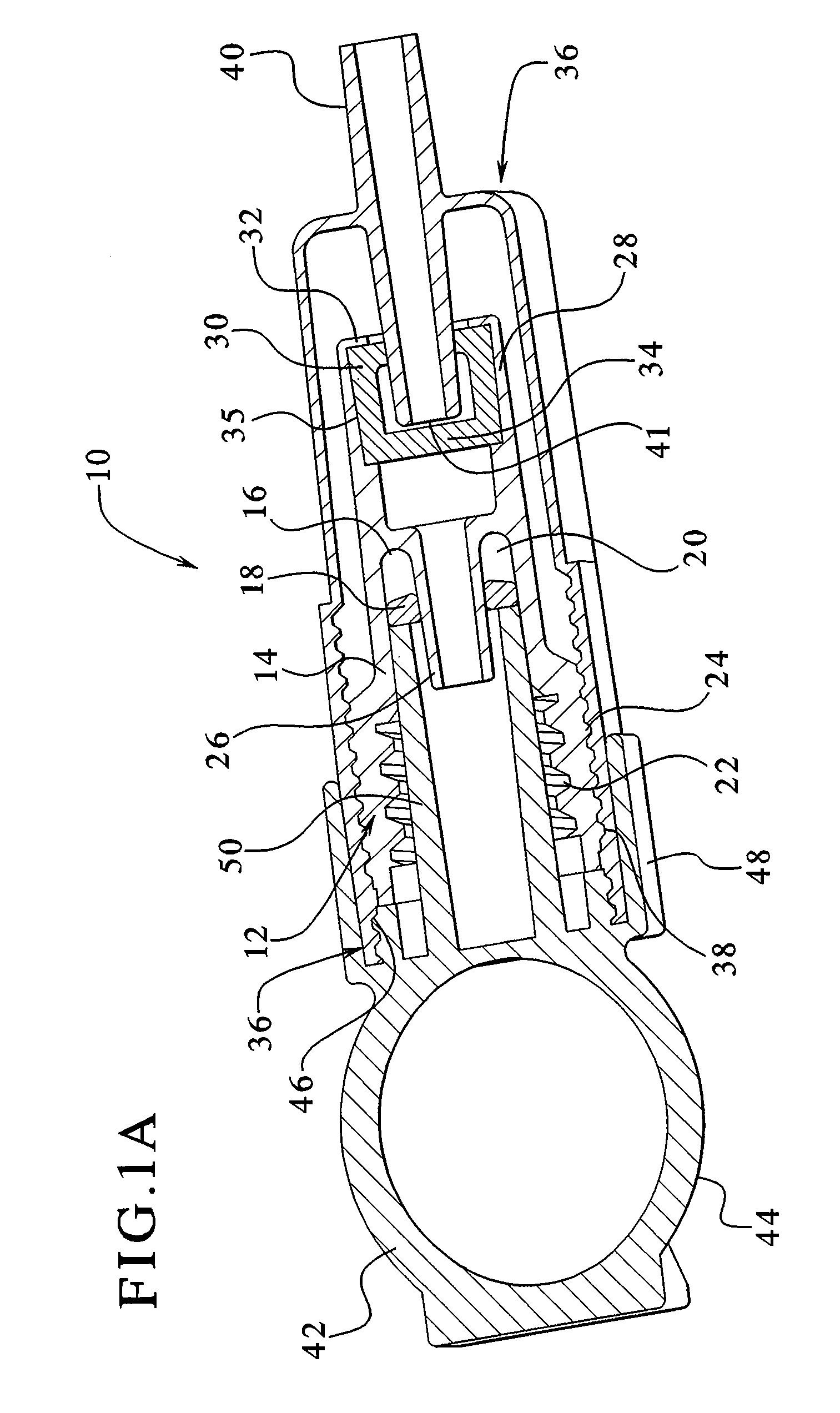
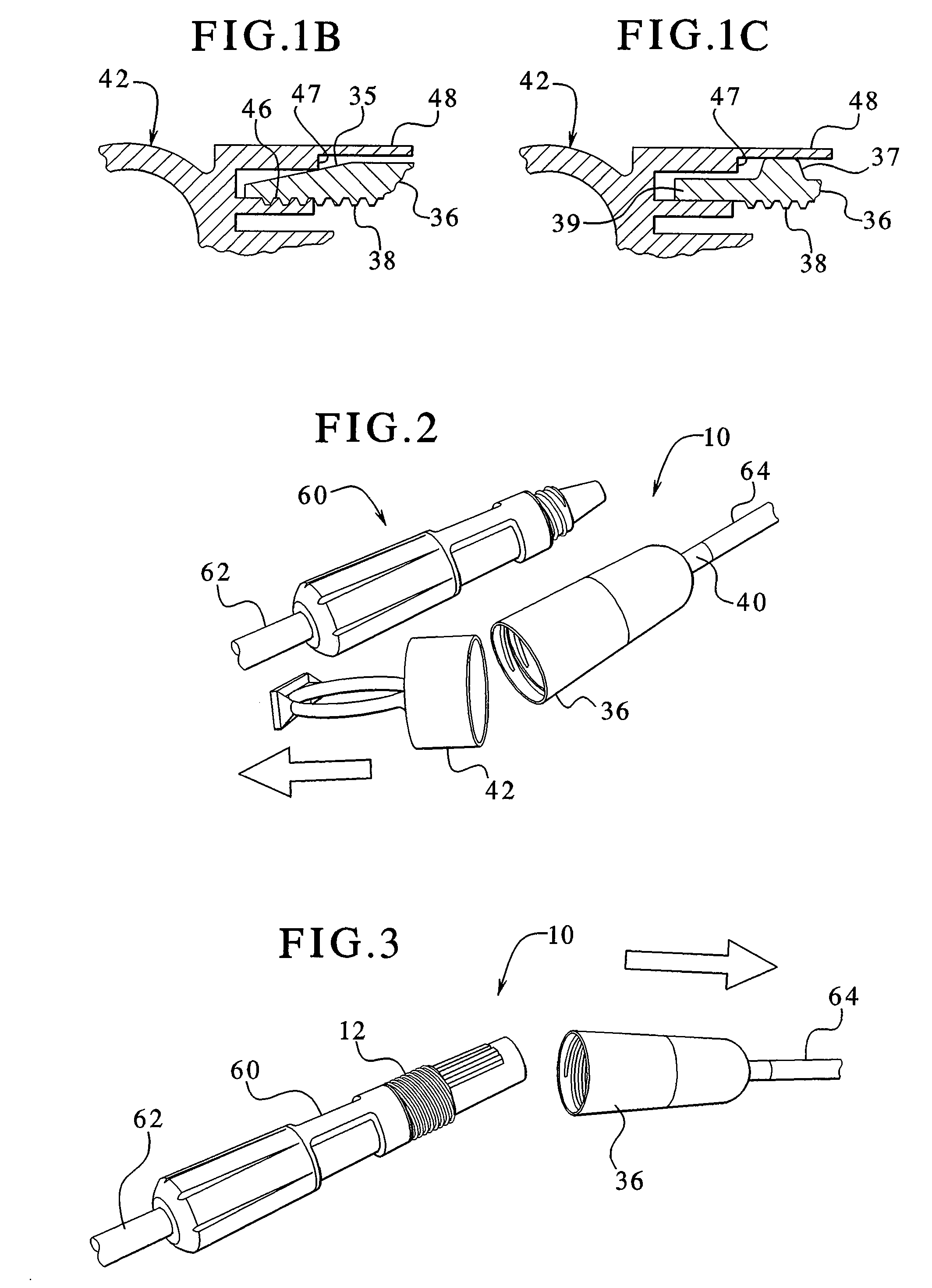
Dialysis connector and cap having an integral disinfectant
ActiveUS7198611B2Eliminate needReduce the possibilityMedical devicesCatheterDisinfectantPeritoneal cavity
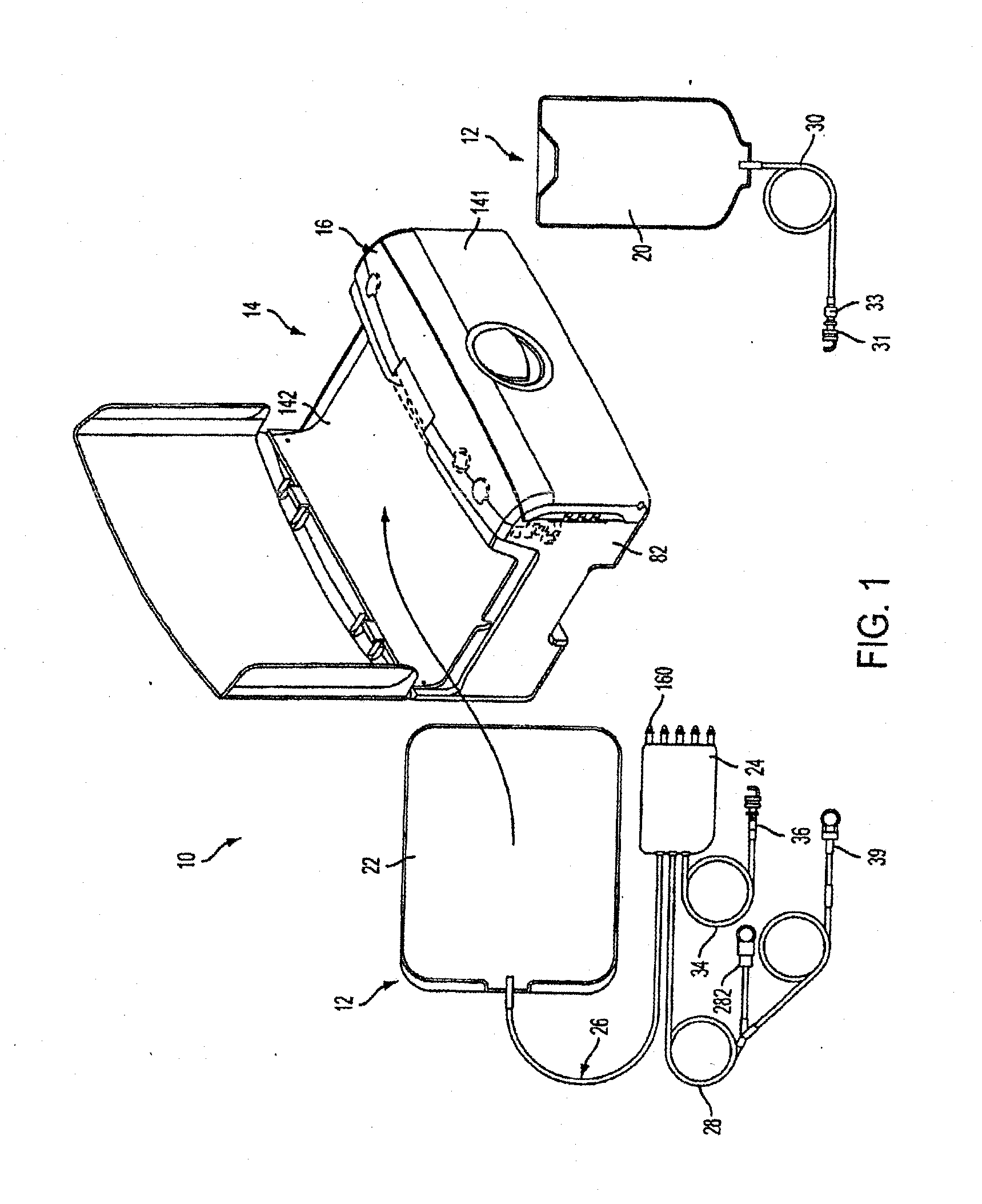
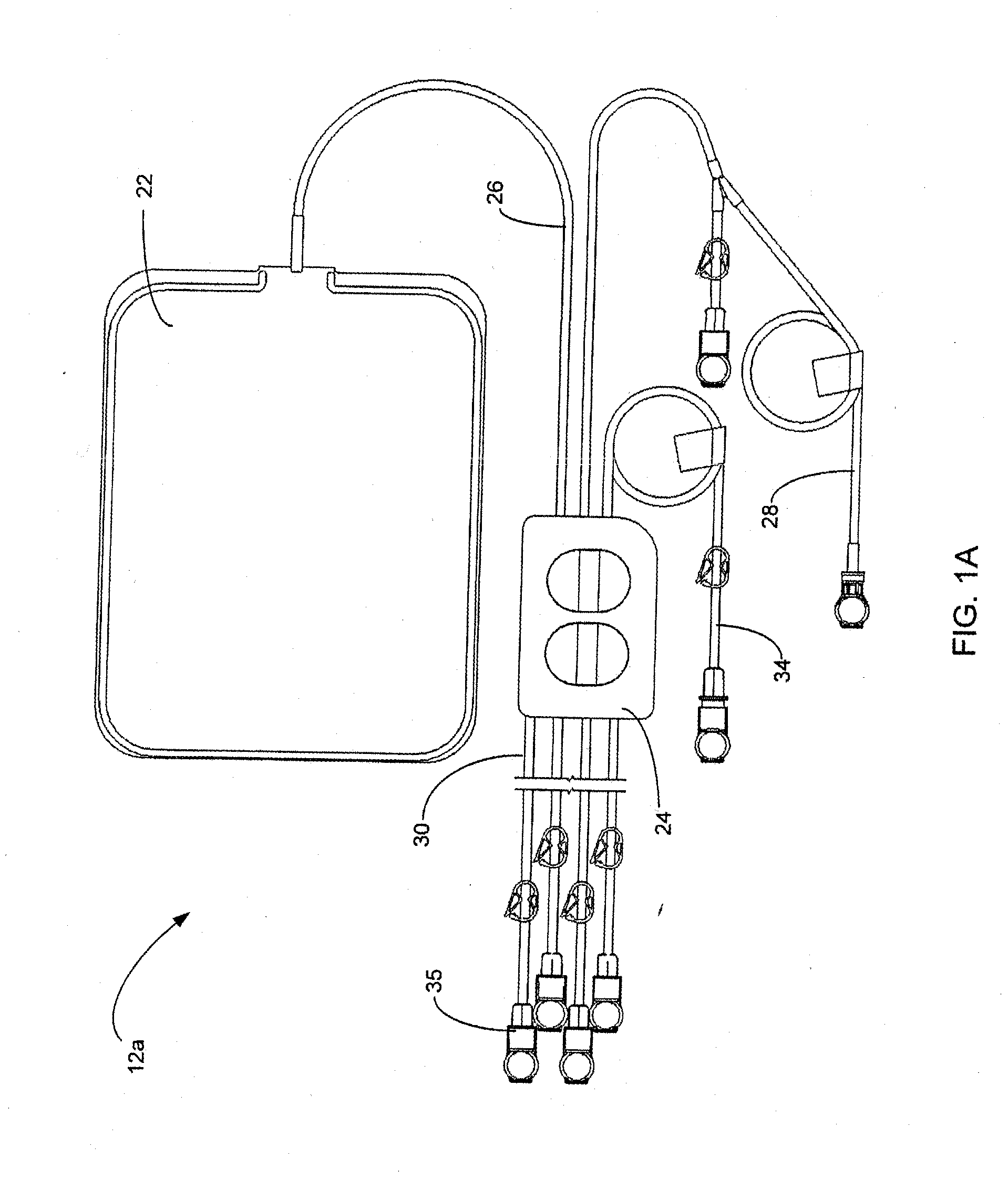
Apparatuses and methods for providing sterile connection during, for example, dialysis therapy. The present invention provides a connector and a cap therefore that easily and readily attaches to a dialysate container and a catheter inserted into a patient's peritoneal cavity. The connector and the cap enable the dialysate to readily transport between the container and the peritoneal cavity while minimizing the potential of contamination therein due to, for example, handling during use. The connector includes a shell that encloses a cap. The cap houses a slit septum and also includes a sealed disinfectant within an interior receptacle. When the catheter or catheter set attaches to the cap, the seal breaks and the disinfectant spreads over the threads between the catheter set and the cap.
Owner:BAXTER HEALTHCARE SA +1
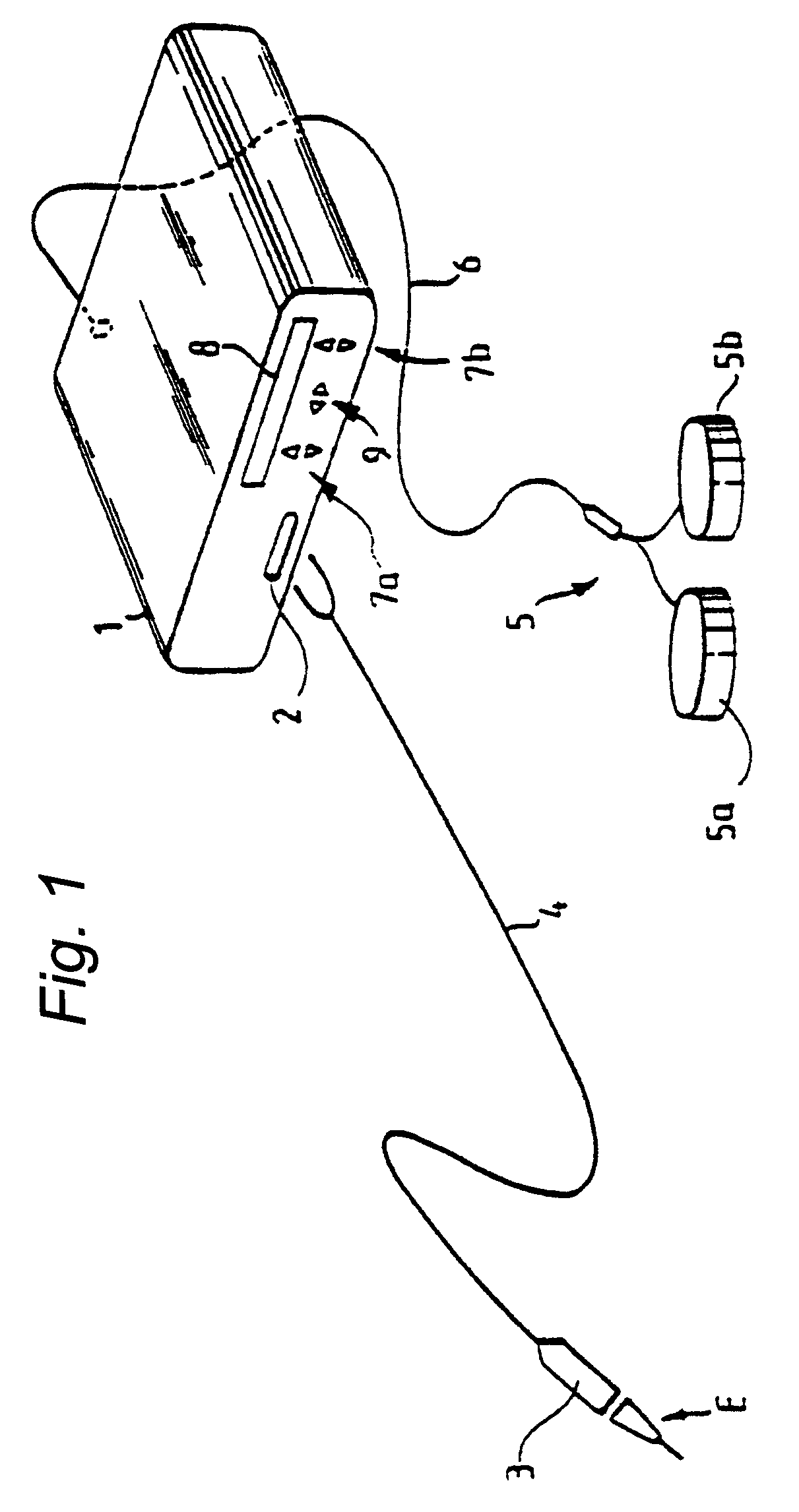
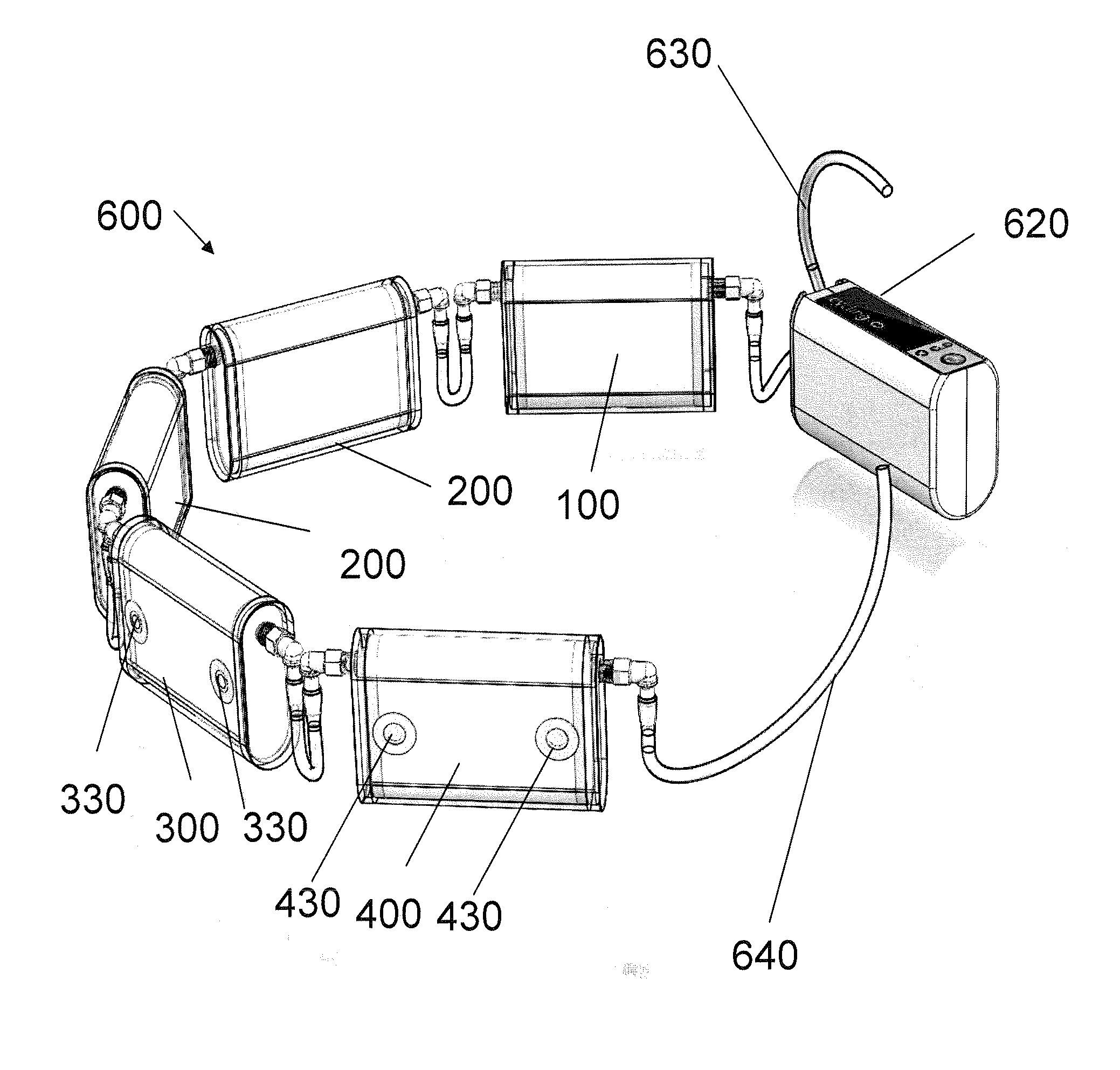
Portable Peritoneal Dialysis System
ActiveUS20100114012A1Comfortable to wearComfortable to carryMedical devicesDialysisSimple Organic CompoundsMetabolite
A portable peritoneal dialysis system for a patient includes an inlet port for providing inflow to the patient's peritoneal cavity, an outlet port for providing outflow from the patient's peritoneal cavity, and a volume of dialysate for flow into and out of the patient's peritoneal cavity, thereby removing from the dialysate uremic waste metabolites that have diffused into the dialysate. The portable peritoneal dialysis system also includes a closed liquid flow loop, including a pump, for flowing the dialysate into and out of the patient's peritoneal cavity, and an organic- and phosphate-removing stage, including at least one replaceable cartridge in the closed liquid flow loop, the cartridge containing material for removing organic compounds and phosphate from dialysate removed from the patient's peritoneal cavity. The portable peritoneal dialysis system further includes a urea- and ammonia-removing stage, including at least one replaceable cartridge in the closed liquid flow loop, the cartridge containing material for removing urea and ammonia from dialysate removed from the patient's peritoneal cavity, the material being packed around semi-permeable hollow fibers with interior fiber walls that reject cations, thereby retaining cations in the dialysate.
Owner:FRESENIUS MEDICAL CARE HLDG INC
Sorbent reactor for extracorporeal blood treatment systems, peritoneal dialysis systems, and other body fluid treatment systems
InactiveUS20050006296A1Facilitate homogeneous suspensionReduce probabilityWater treatment parameter controlSemi-permeable membranesFluid balancePeritoneal dialysis
Systems and methods for extracorporeal processing of blood or other body fluid for the treatment of conditions, such as sepsis, autoimmune disease, or toxemia related to kidney failure, liver failure, or drug overdose are provided. In an extracorporeal treatment system, a fraction of a body fluid is passed into a treatment fluid, at least a portion of which is then passed through a sorbent suspension reactor for treatment by a sorbent suspension. The treatment fluid circuit can be maintained at a fixed volume, which enables accurate fluid balance between the patient and the extracorporeal circuit. Some or all of the treatment fluid, optionally also containing nutrients and / or therapeutic agents, is returned to the patient. In a peritoneal dialysis system, dialysate is passed into a patient's peritoneal cavity, recovered from the cavity, passed through a sorbent suspension reactor in accordance with the invention, and returned to the cavity.
Owner:HEMOCLEANSE TECH
Flow system of a dialysis device and a portable dialysis device
ActiveUS20110184340A1Prevent leakagePrevent spillageSolid sorbent liquid separationAnion exchangersCatheterPeritoneal cavity
There is provided a flow system of a dialysis device including a dialysate conduit which is capable of being in fluid communication with the peritoneal cavity of a patient's body and of being in fluid communication with a flow path, the flow path allowing dialysate to flow from a patient's body to a sorbent capable of removing contaminants within the dialysate in an outflow mode and in an inflow mode returning the dialysate substantially free of contaminants to the patient's body. The device also includes a pump for moving the dialysate along the flow path in both the outflow mode and inflow mode and a plurality of valves disposed along the flow path. There is also provided a portable dialysis device.
Owner:TEMASEK POLYTECHNIC
Dialysis implant and methods of use
ActiveUS20060058731A1Prevent excessive bladder pressureLow and high pressureMedical devicesCatheterPeritoneal dialysisRenal Failures
A device and methods for treating renal failure are disclosed. One embodiment of the device is an implantable peritoneal dialysis device. When in use, the device can have a semi-permeable reservoir implanted in the peritoneal cavity. The reservoir can receive blood waste and drain through one or more conduits, via a pump, to the biological bladder. Solids and / or a solution benefiting dialysis can be pumped to the reservoir and / or implanted in the peritoneal cavity.
Owner:SEQUANA MEDICAL NV
Automatic exchanger for peritoneal dialysis
InactiveUS6293921B1Avoid misuseMinimize the possibilityPeritoneal dialysisTube connectorsPeritoneal dialysis fluidEngineering
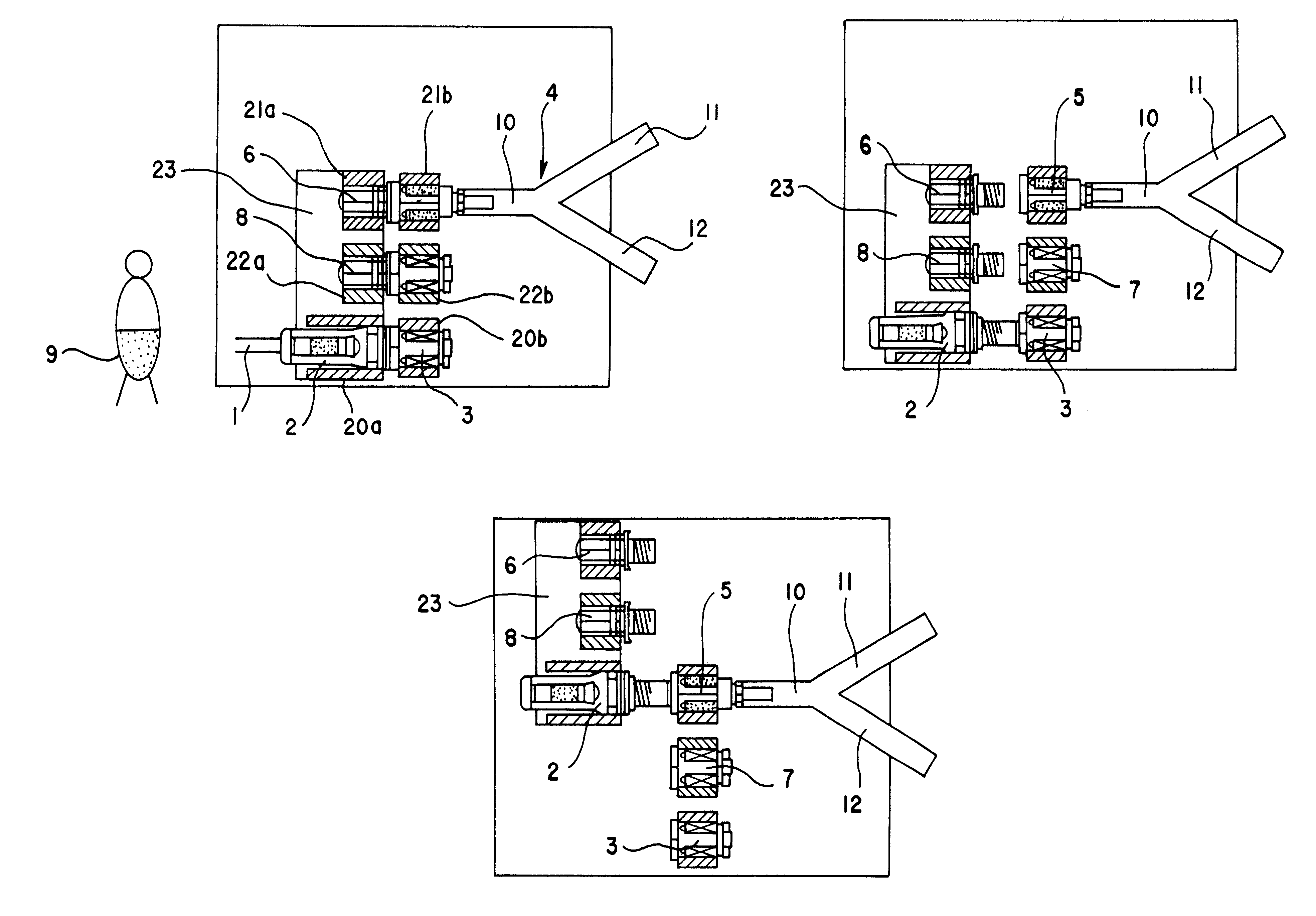
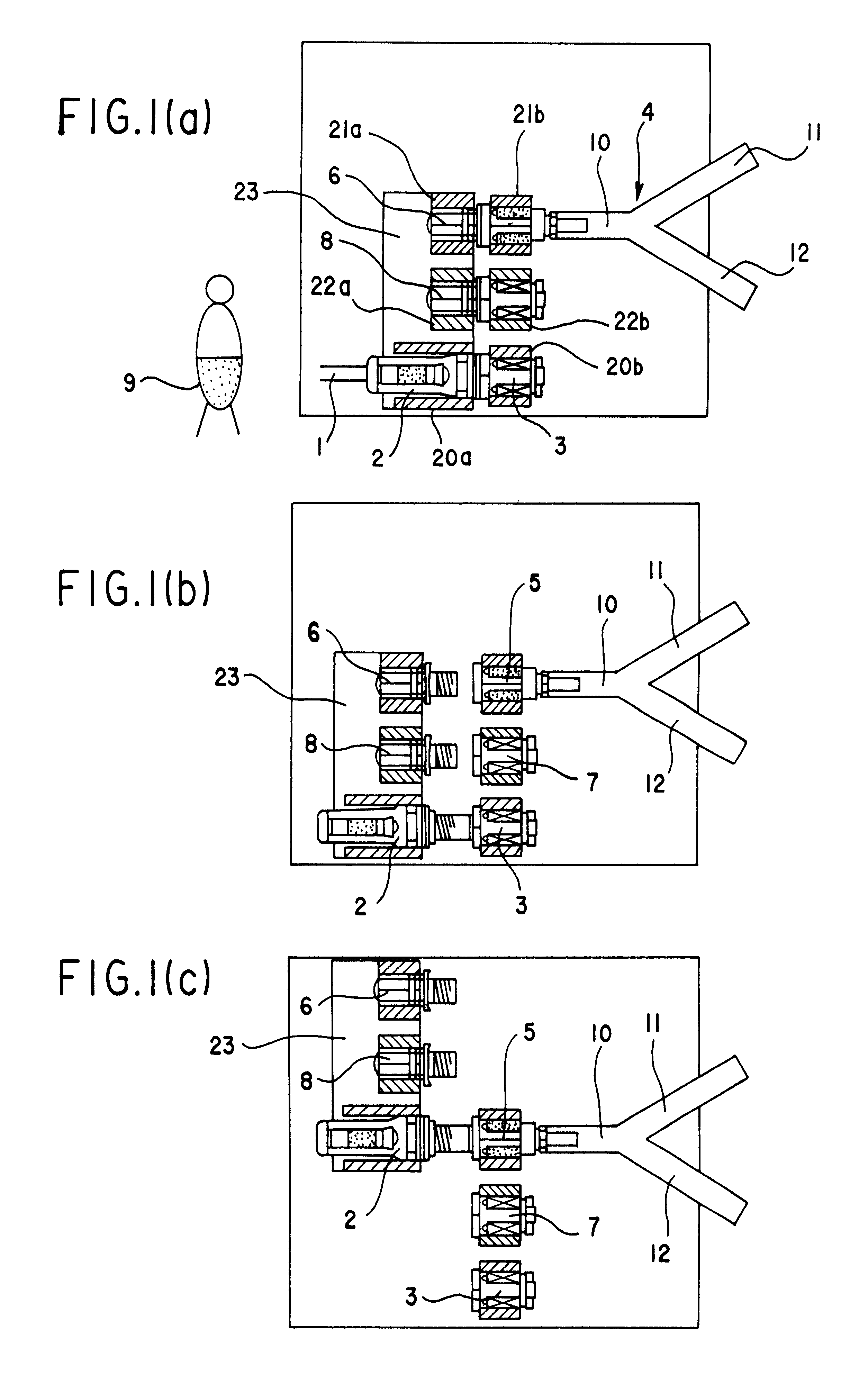
An automatic exchanger apparatus for peritoneal dialysis fluids is provided having a dialysis fluid bag and a drained fluid bag and arranged for connecting and disconnecting between the end of a peritoneal dialysis circuit equipped with a branching point and the end of a tube extending from a patient to drain the waste dialysis fluid from the cavity of the patient and fill the peritoneal cavity of the patient with a fresh peritoneal dialysis fluid for exchange, and in particular comprises: means A, B, and C for carrying out respectively a step (A) of connecting the end of the patient side tube to the end of the peritoneal dialysis circuit, a step (B) of delivering and draining the waste fluid, and a step (C) of, disconnecting the two ends and connecting the end of the patient side tube to its shut-off member, arranged for carrying out their respective steps (A) and (C) automatically; and a controlling means for controlling the respectively means to execute their respectively steps in a sequence. The apparatus is simple in the construction while carrying out, with much ease, the connection and disconnection of the tubes and the exchange of the fluids including a waste and a fresh supply one, hence avoiding operational errors of the operator and minimizing the possibility of infection and contamination.
Owner:JMS CO LTD
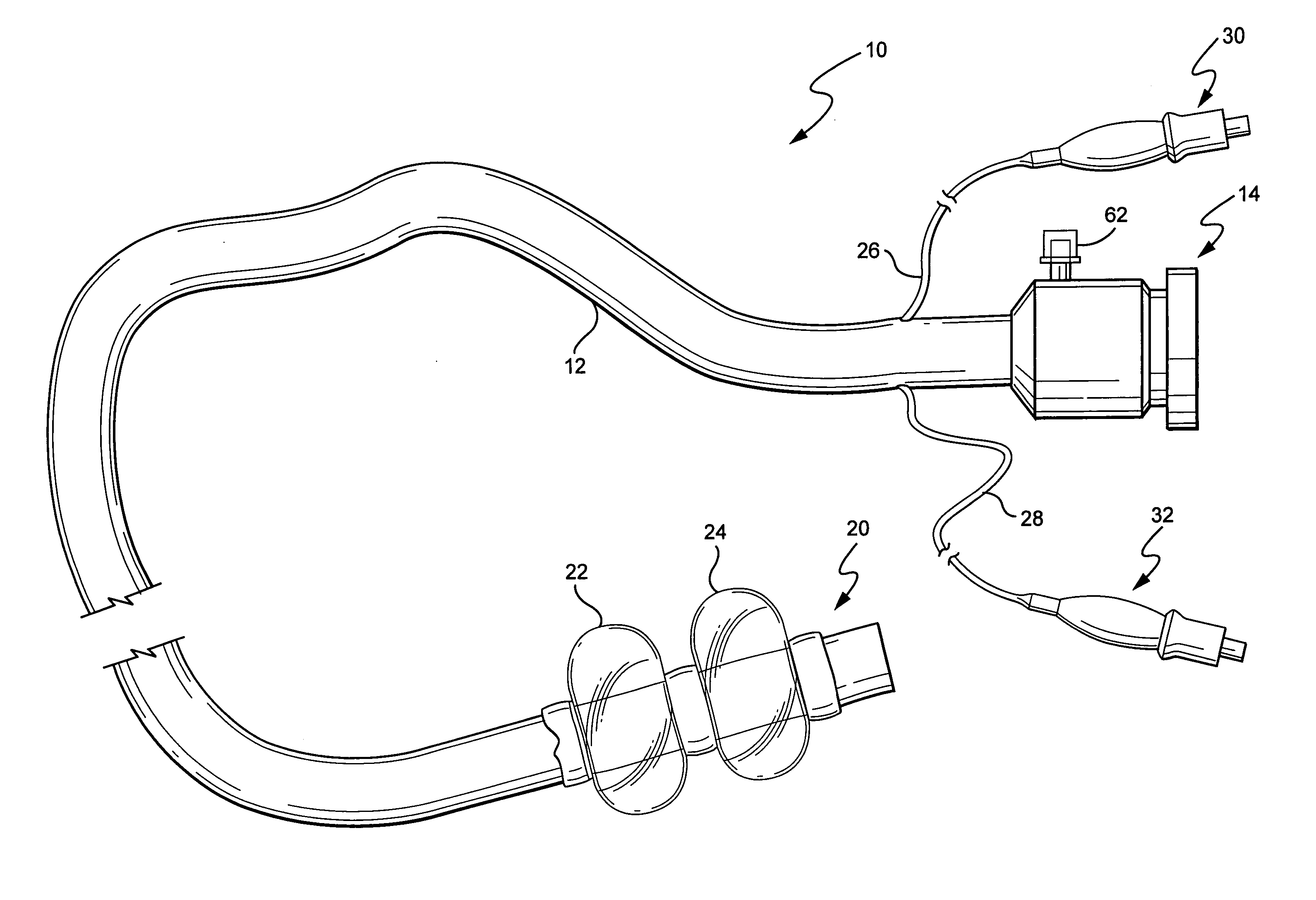
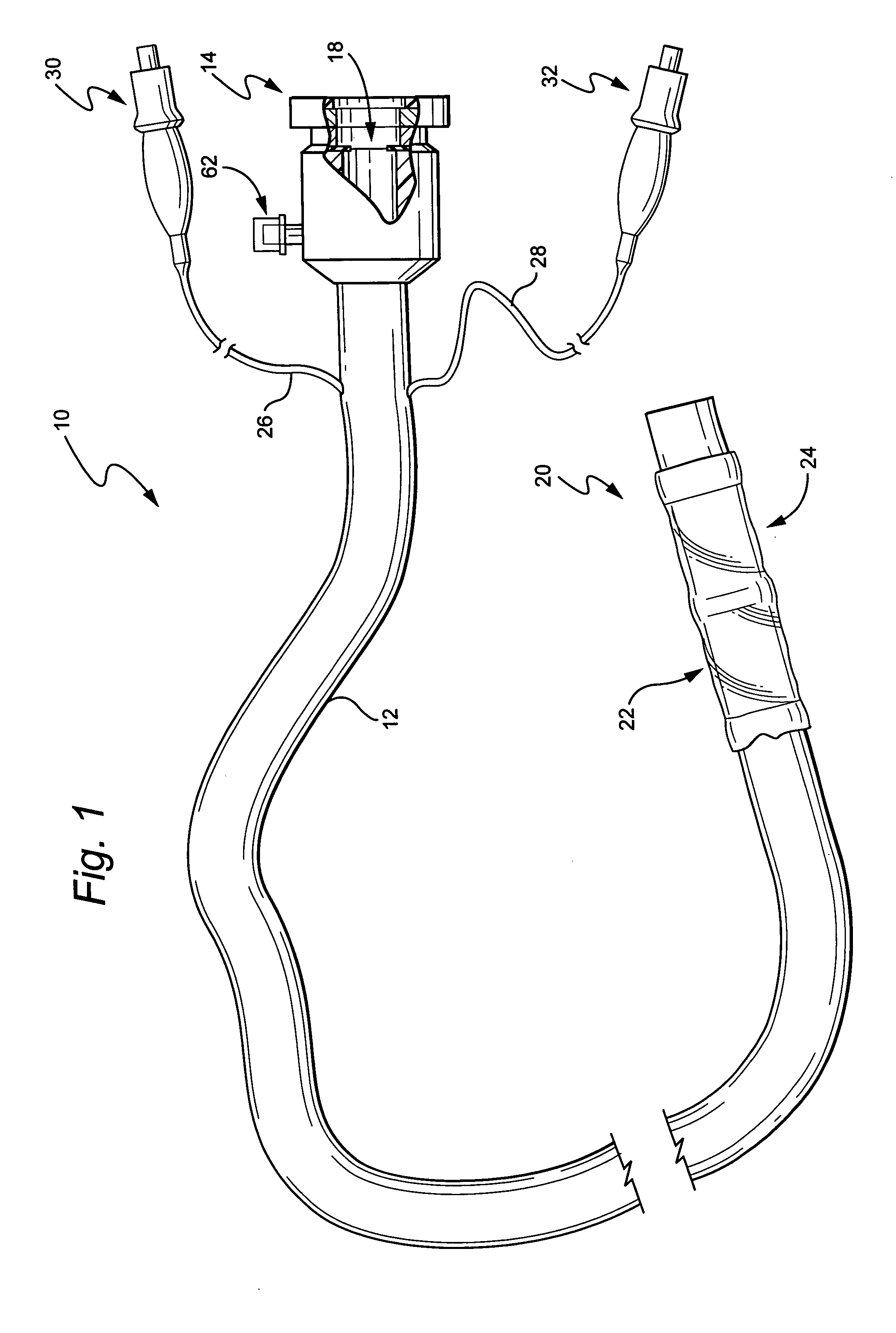
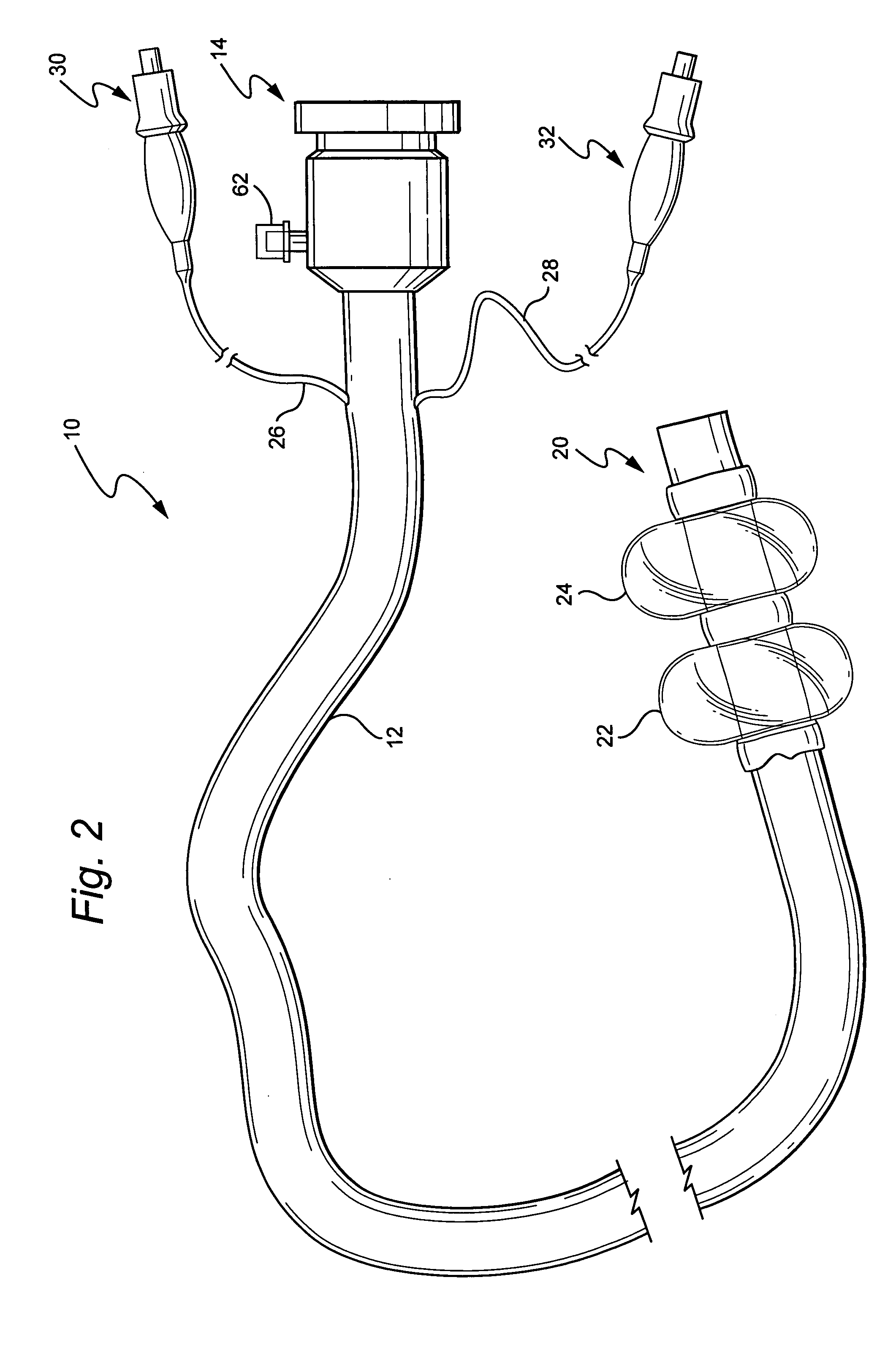
Medical treatment system and methods using a plurality of fluid lines
ActiveUS20130184638A1Reduce trafficIncrease distanceMedical devicesOptical detectionControl systemPeritoneal cavity
A medical treatment system, such as peritoneal dialysis system, may include control and other features to enhance patient comfort and ease of use. For example, a peritoneal dialysis system may include a control system that can adjust the volume of fluid infused into the peritoneal cavity to prevent the intraperitoneal fluid volume from exceeding a pre-determined amount. The control system can adjust by adding one or more therapy cycles, allowing for fill volumes during each cycle to be reduced. The control system may continue to allow the fluid to drain from the peritoneal cavity as completely as possible before starting the next therapy cycle. The control system may also adjust the dwell time of fluid within the peritoneal cavity during therapy cycles in order to complete a therapy within a scheduled time period. The cycler may also be configured to have a heater control system that monitors both the temperature of a heating tray and the temperature of a bag of dialysis fluid in order to bring the temperature of the dialysis fluid rapidly to a specified temperature, with minimal temperature overshoot.
Owner:DEKA PROD LLP
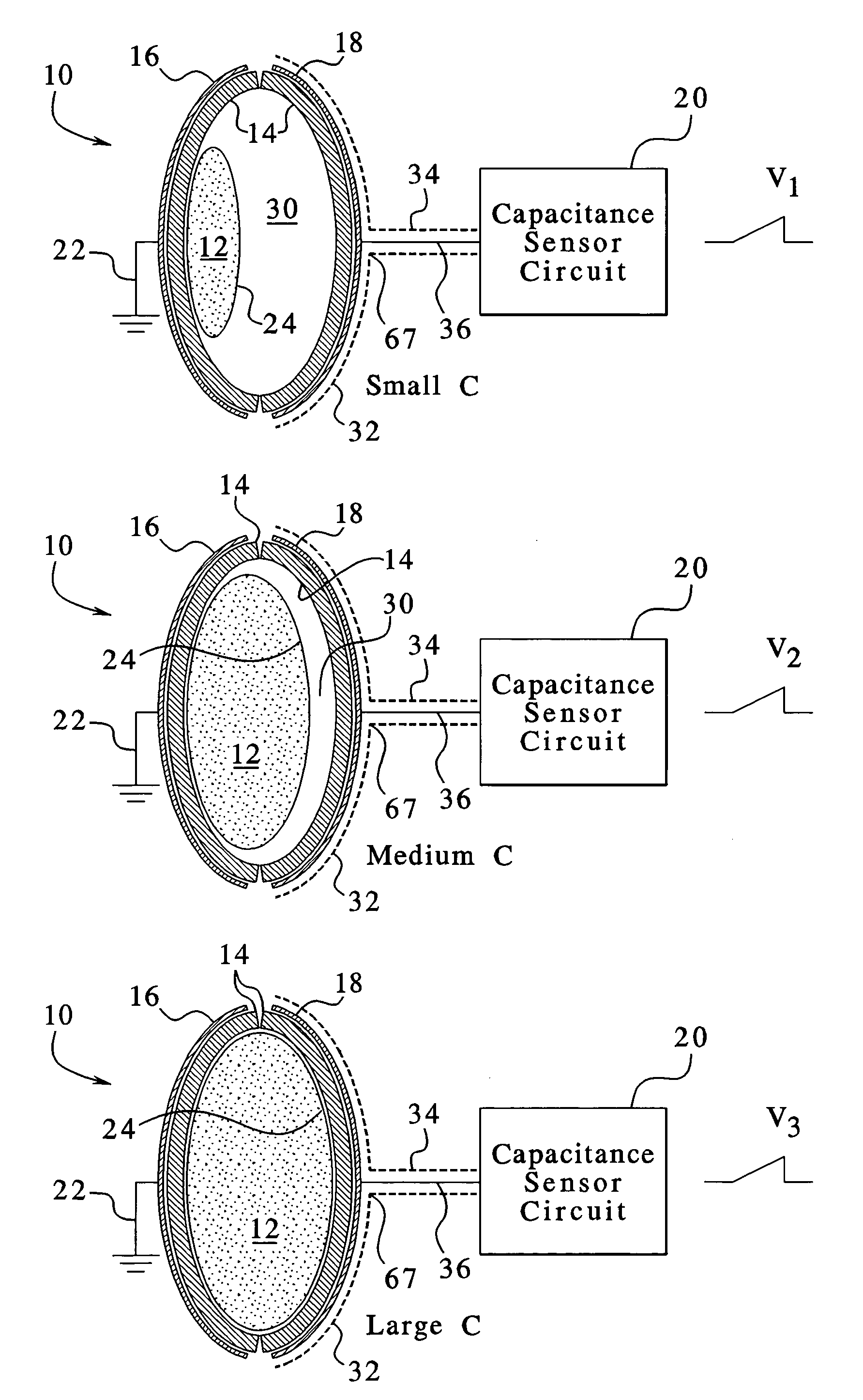
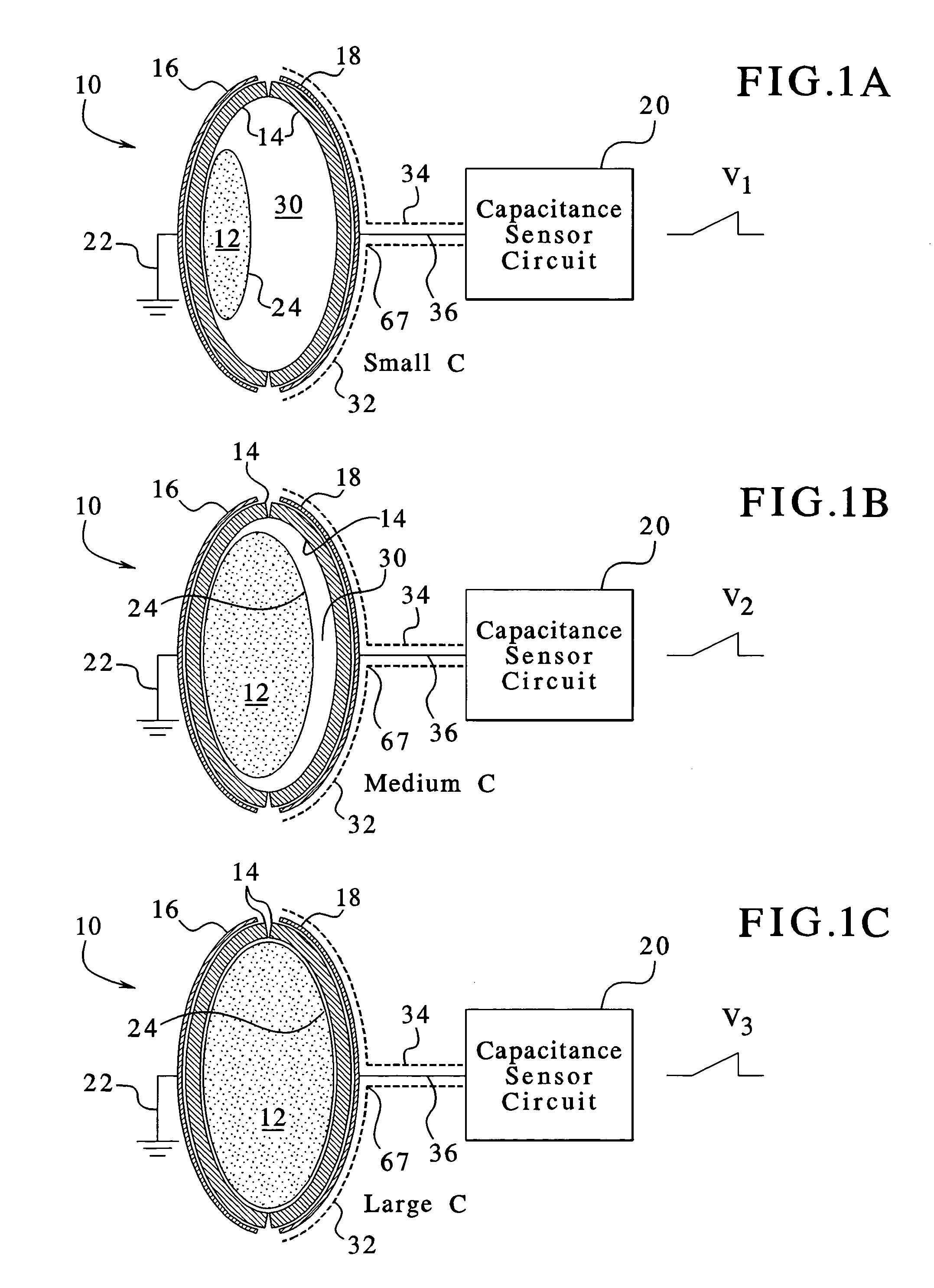
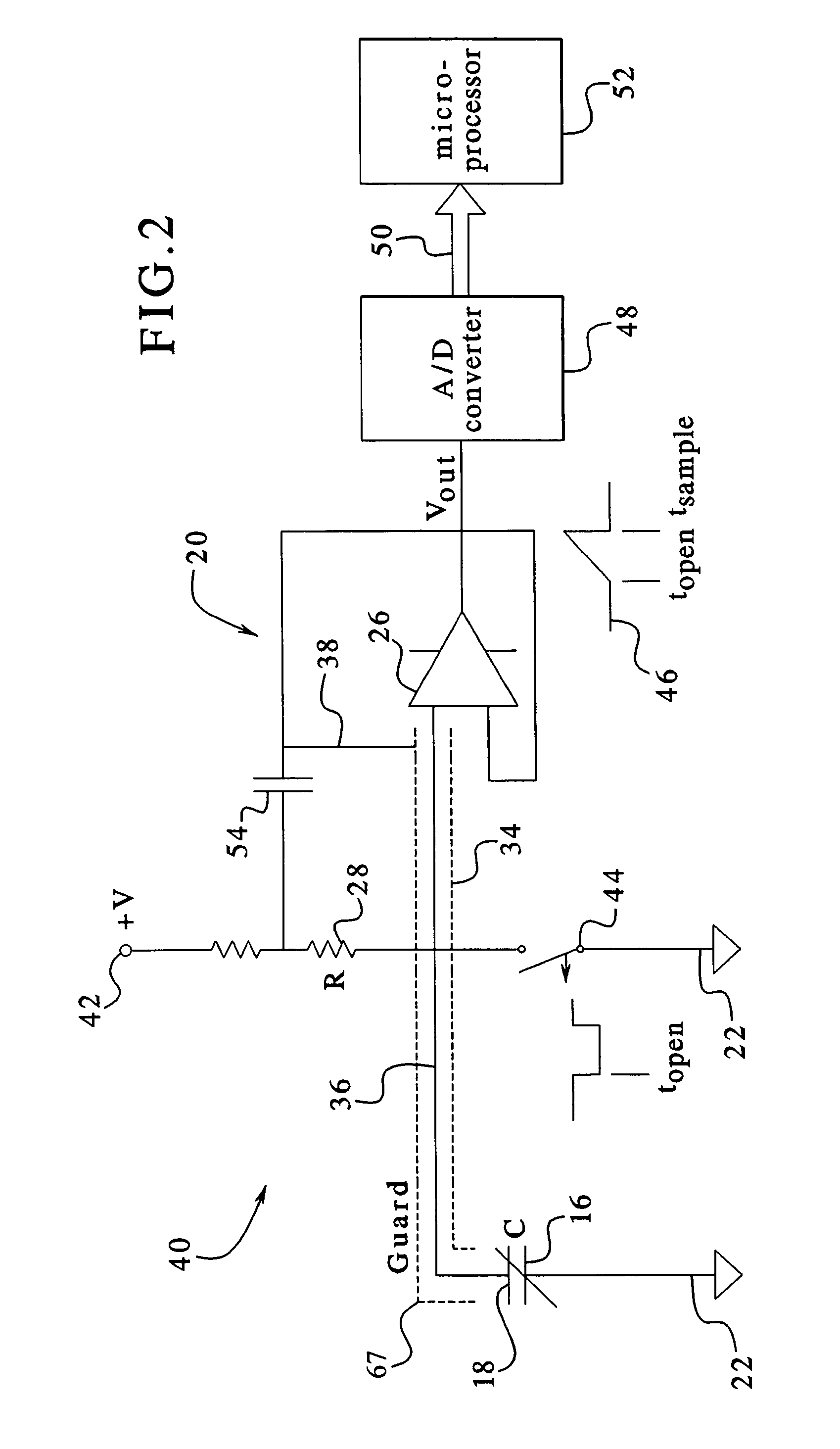
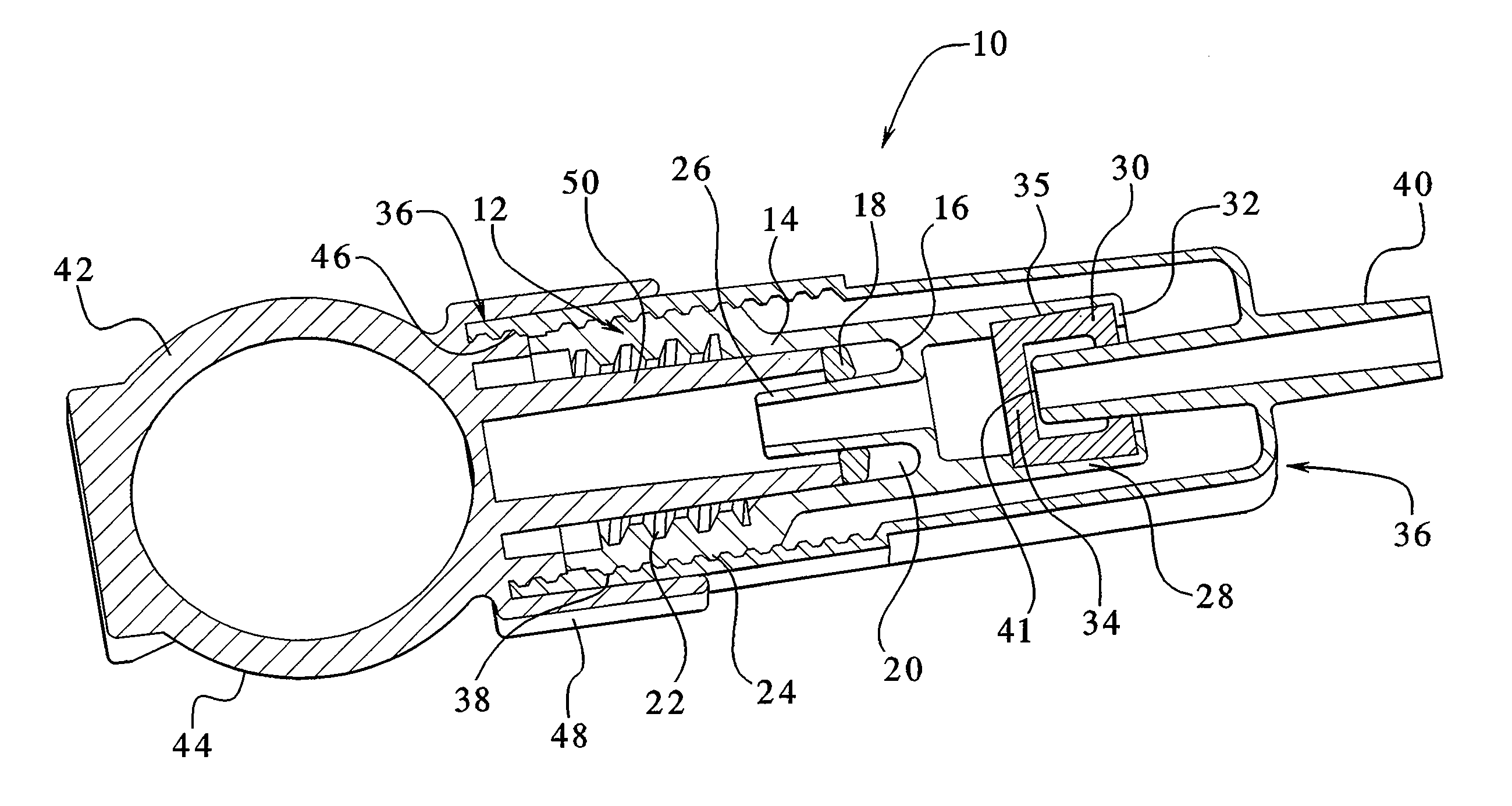
Capacitance fluid volume measurement
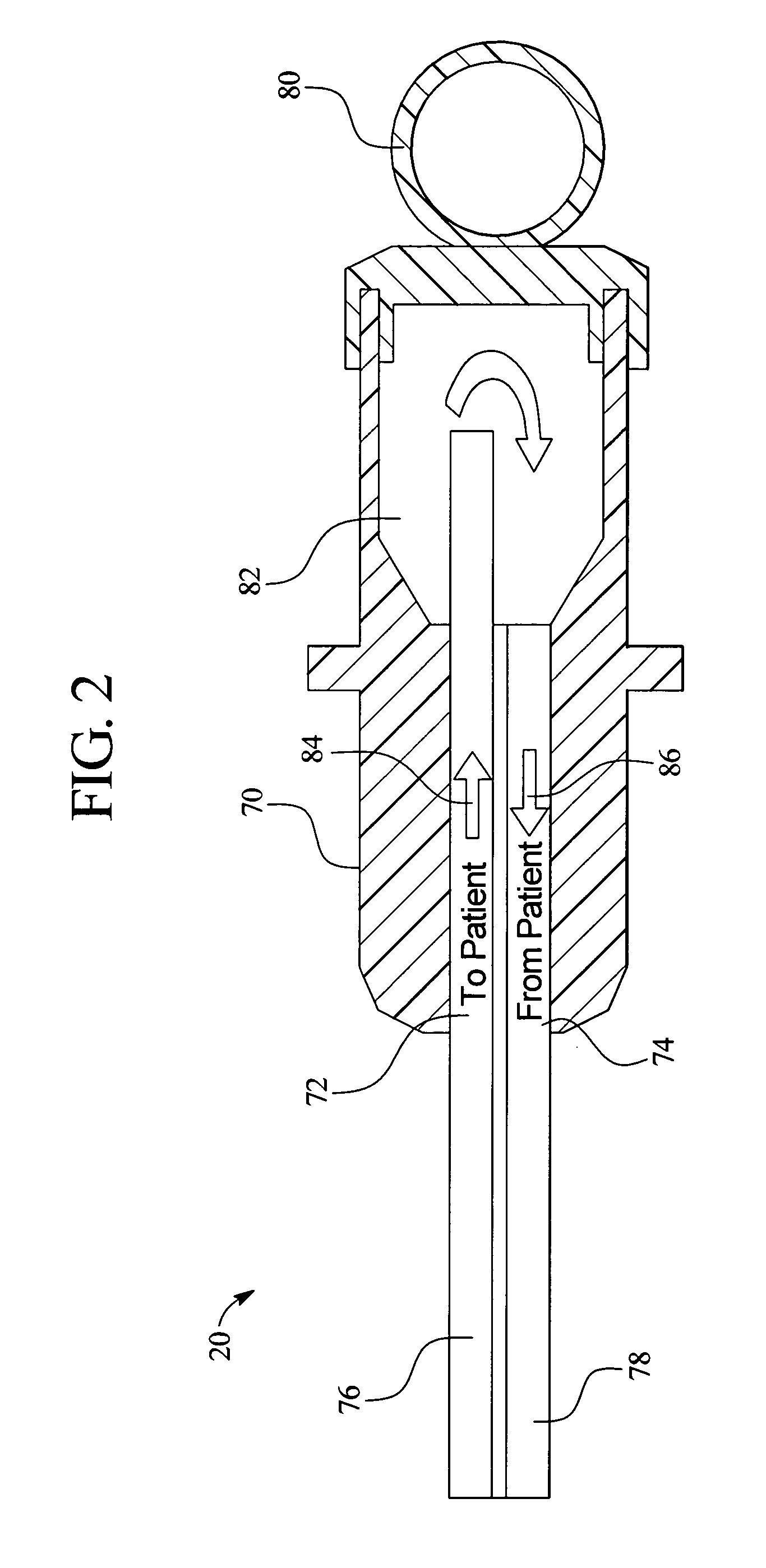
InactiveUS7107837B2Simple systemSimple methodVolume/mass flow measurementMedical devicesPump chamberEngineering
A fluid volumetric pumping system has a fluid pump and capacitor plates disposed around a pump chamber of the fluid pump. The capacitance between the capacitor plates changes as the volume of fluid in the pump chamber changes. An electrical circuit measures a change in the capacitance between the plates and outputs a signal indicative of the volume of fluid in the pump chamber. The pump having the capacitance sensor of the present invention fluidly connects to a patient. In an embodiment, a peritoneal dialysis system provides dialysate to the patient via the pump, and the capacitance sensor measures the volume of dialysate supplied to and drained from a peritoneal cavity of the patient.
Owner:BAXTER INT INC +1
Dialysis connector and cap having an integral disinfectant
InactiveUS20070106205A1Eliminate needReduce the possibilityMedical devicesCatheterDisinfectantPeritoneal cavity
Owner:BAXTER INT INC +1
Peritoneal Dialysis Methods and Apparatus
ActiveUS20100217181A1Increase percentageImprove wear resistancePeritoneal dialysisProtein compositionPeritoneal fluid
A peritoneal-based (“bloodless”) artificial kidney processes peritoneal fluid without need for additional fluids (“waterless”). Fluid is separated into a protein-rich stream and a protein-free stream. The protein-rich stream is regenerated using a sorbent assembly, and its protein composition can be modified by removal of selected protein(s) (“dialysate-pheresis”). It is then reconstituted with additives and returned into the peritoneal cavity, thereby reducing protein-loss and providing oncotic-pressure for ultrafiltration. The protein-free stream is used to produce free water, and an alkaline or acid fluid for optimization of the composition of the regenerated stream. The unused protein-free stream can be used to “reverse flush” the separator to maintain its patency and the excess discarded for fluid-balance regulation. Compared to prior art, immobilization of urease allows more protein rich fluid to be regenerated and re-circulated into the peritoneal cavity for toxin removal and allows practicable development of portable and wearable artificial kidneys.
Owner:RGT UNIV OF CALIFORNIA +1
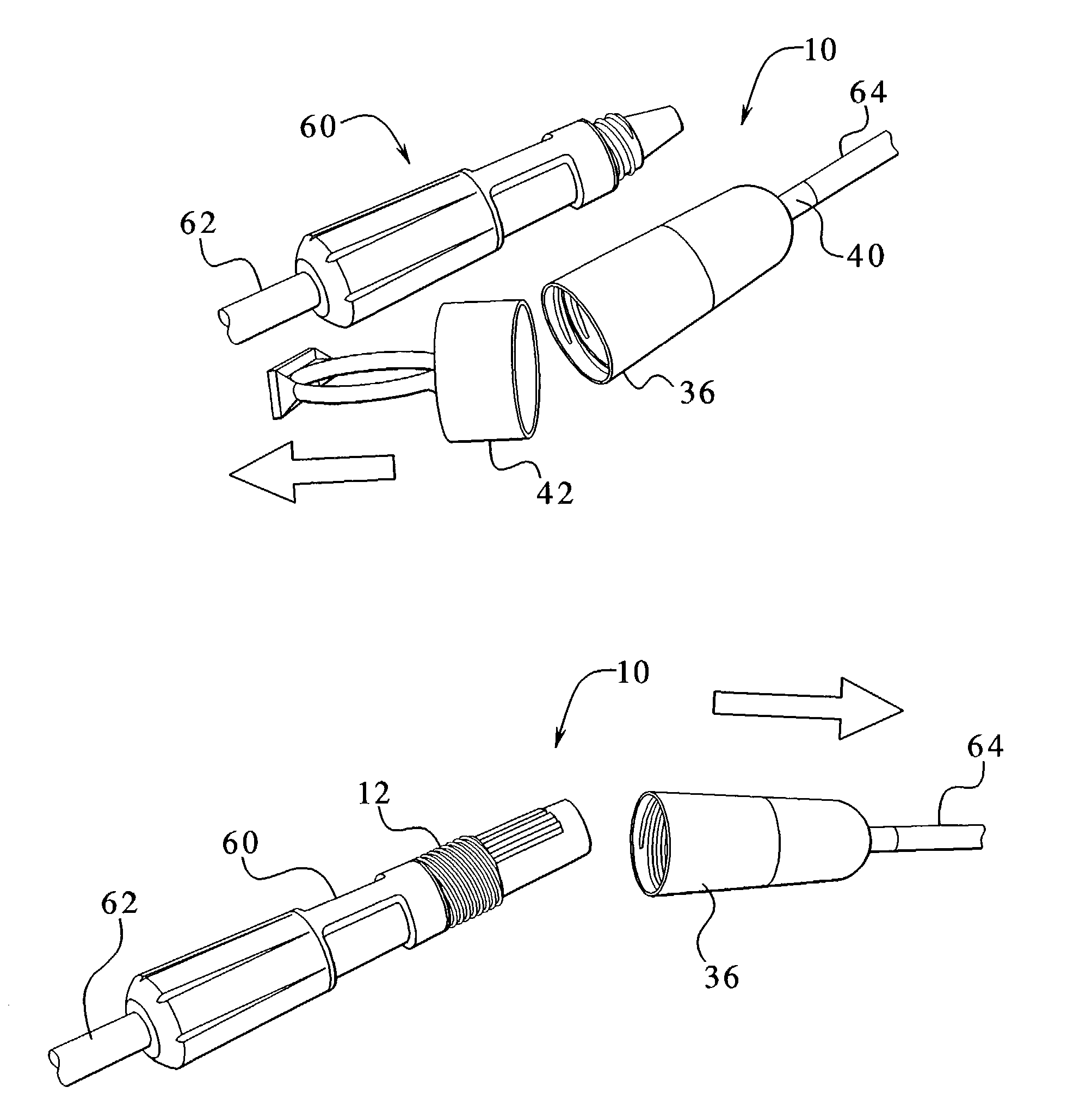
Medical treating instrument
InactiveUS7297106B2Easy to switchReduce the overall heightCannulasDiagnosticsSurgical treatmentForceps
There is provided a surgical treatment instrument in which the mode can be easily switched between a treatment under a pneumoperitoneum condition and a treatment outside the peritoneal cavity, and forceps can be manipulated under the pneumoperitoneum condition, and when taking out a cancer-affected part, an incision in the abdominal wall is protected, and the incision is free from infection. The surgical treatment instrument includes a tubular portion which has a first fixing member provided at a near end-side open portion thereof, and also has a second fixing member provided at a remote end-side open portion thereof. At least two tension belts are mounted on the second fixing member, and fixing means for adjusting the length of the tension belts to position the first fixing member is provided at the first fixing member.
Owner:SUMITOMO BAKELITE CO LTD
Methods and devices for diagnostic and therapeutic interventions in the peritoneal cavity
A novel approach to diagnostic and therapeutic interventions in the peritoneal cavity is described. More specifically, a technique for accessing the peritoneal cavity via the wall of the digestive tract is provided so that examination of and / or a surgical procedure in the peritoneal cavity can be conducted via the wall of the digestive tract with the use of a flexible endoscope. As presently proposed, the technique is particularly adapted to transgastric peritoneoscopy. However, access in addition or in the alternative through the intestinal wall is contemplated and described as well. Transgastric and / or transintestinal peritoneoscopy will have an excellent cosmetic result as there are no incisions in the abdominal wall and no potential for visible post-surgical scars or hernias.
Owner:APOLLO ENDOSURGERY INC
Systems and methods for performing peritoneal dialysis
ActiveUS7922686B2Strengthen the systemImprove methodPeritoneal dialysisProsthesisCycle rateContinuous flow
Systems and methods for providing multiple pass continuous flow dialysis therapy are provided. The present invention includes a fluid circuit connected to a patient via a catheter thereby defining a fluid loop along which a therapy fluid including a dialysate can be continuously circulated into, through and out of a peritoneal cavity of a patient to remove a therapeutically effective amount of excess water and solutes including uremic toxins. The feed rate and discharge rate of therapy fluid into the fluid loop can be controllably regulated in proportion to the circulation rate of fluid in the fluid loop such that the therapy fluid can pass a multiple number of times along the fluid loop prior to discharge.
Owner:BAXTER INT INC +1
Peritoneal dialysis catheters
InactiveUS6976973B1Well mixedMinimize shuntingMedical devicesCatheterPeritoneal dialysis catheterCatheters dialysis
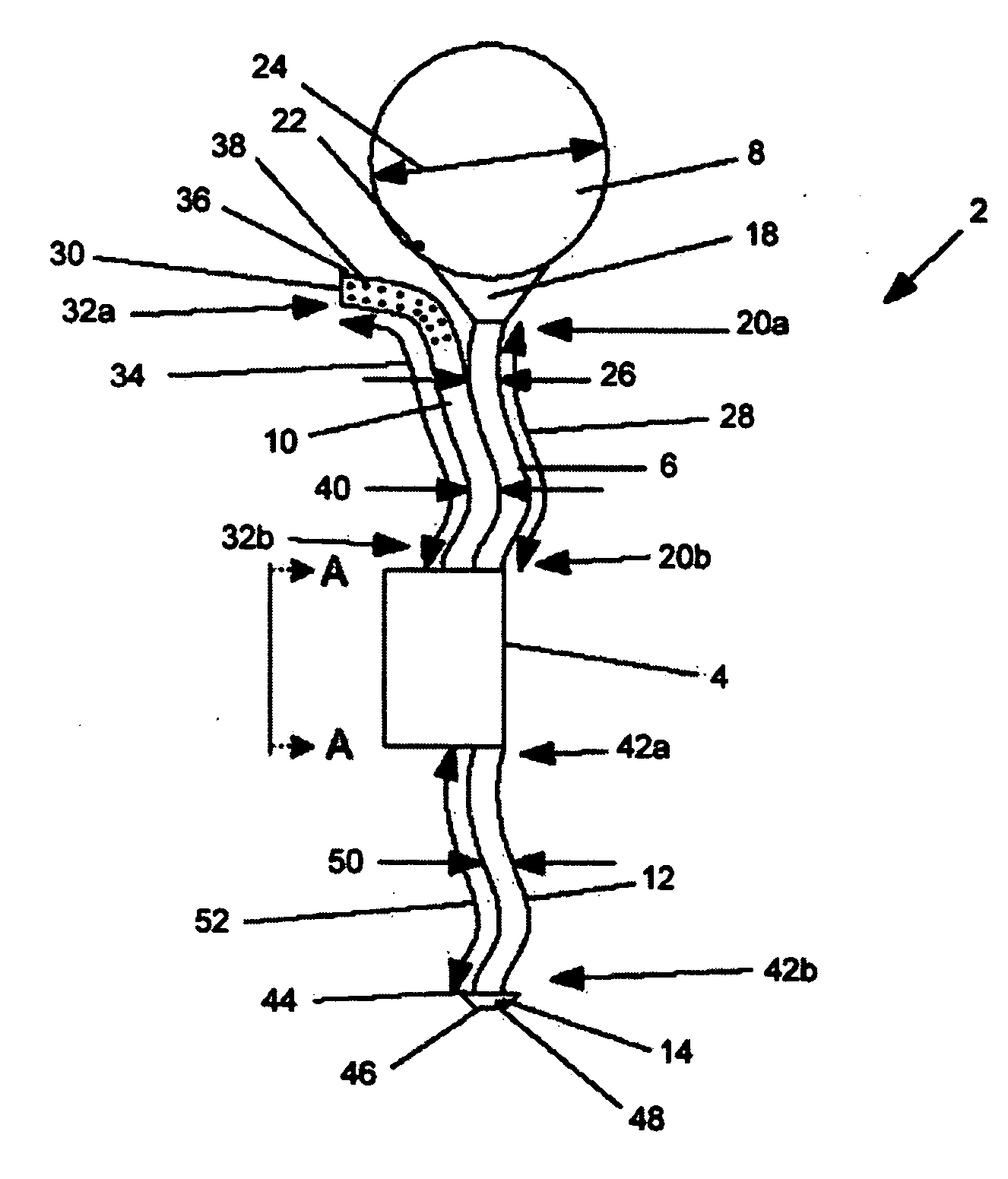
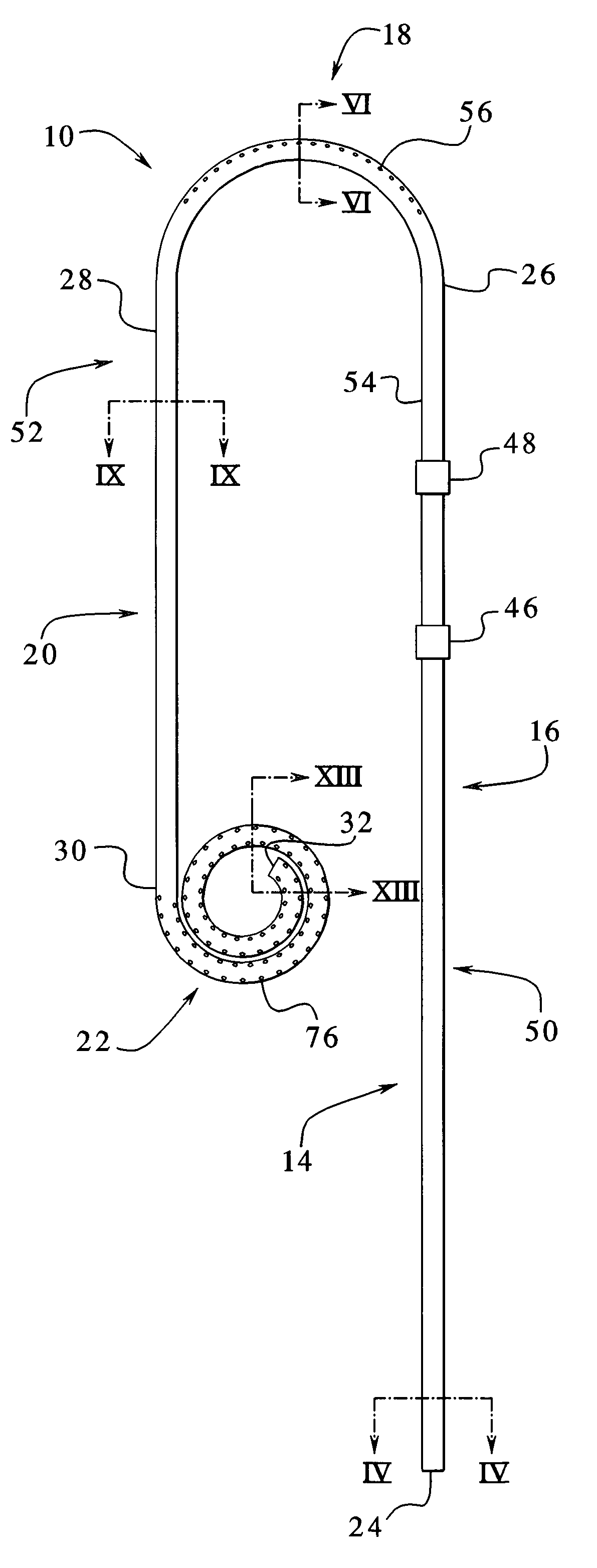
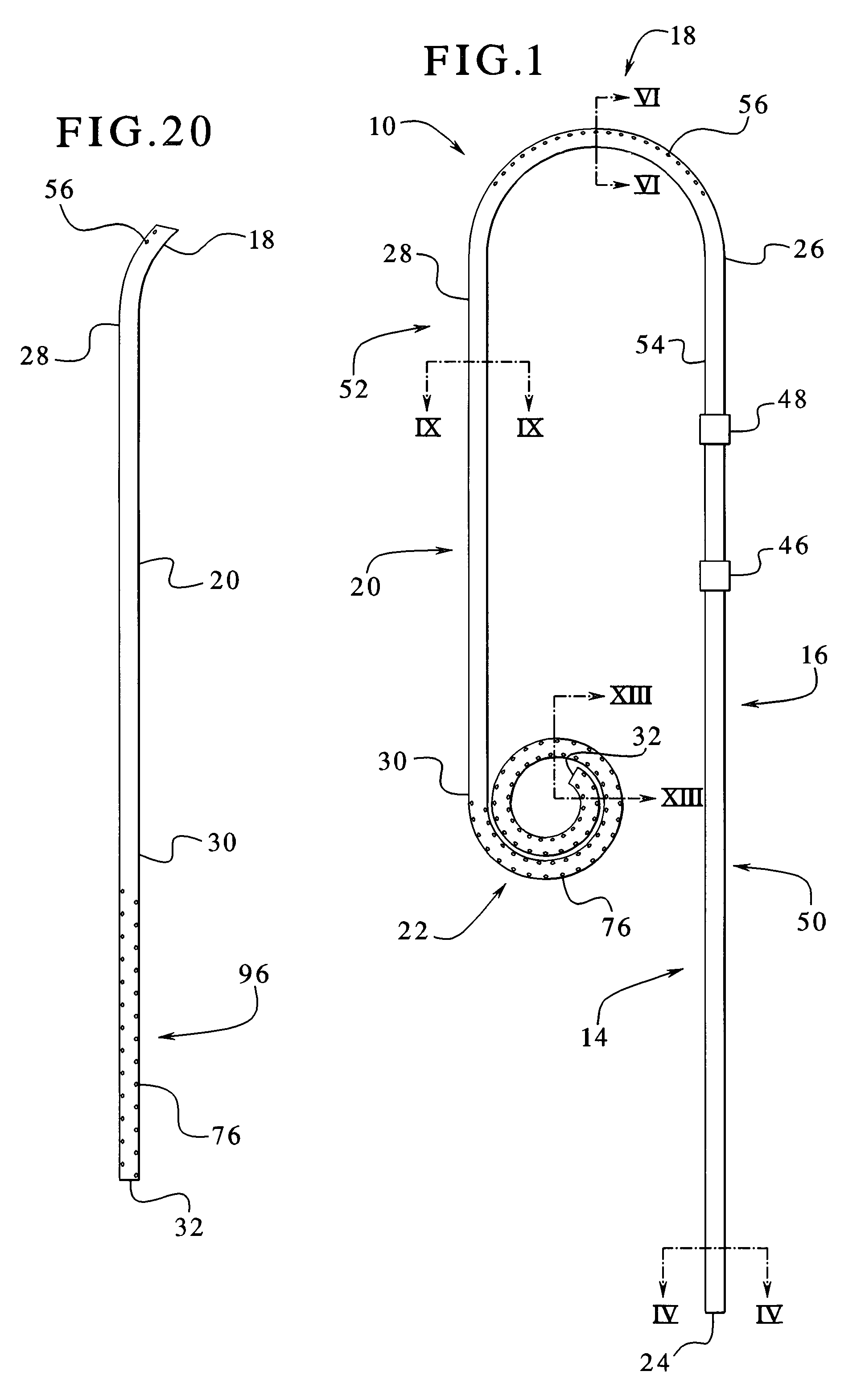
A catheter suitable for use in performing peritoneal dialysis. The catheter is a dual lumen catheter which allows for continuous flow peritoneal dialysis. Dialysate flows through the catheter into the patient via one lumen and simultaneously flows through the catheter out of the patient via the second lumen. The dual lumen dialysis catheter has a flexible tube which has an implantable portion extending from an external patient portion. Both of the lumens have openings in the external patient portion for connecting to a supply and drain of dialysate, respectively. The implantable portion has a preformed curved segment which has an outlet for the first lumen to flow dialysate into the patient's peritoneal cavity. The implantable portion has an opening for the second lumen at the distal end to flow dialysate out of the peritoneal cavity and removal from the patient. The catheter facilitates mixing of fresh and spent dialysate inside the peritoneal cavity by inflowing fresh dialysate into the cavity at a location substantially separated from the cavity outflow location, and by directing the inflow of dialysate into the cavity opposite the cavity outflow location.
Owner:BAXTER INT INC
Tumor targeting drug-loaded particles
A composition for delivering a tumor therapeutic agent to a patient includes a fast-release formulation of a tumor apoptosis inducing agent, a slow-release formulation of a tumor therapeutic agent, and a pharmaceutically acceptable carrier. An apoptosis-inducing agent in a pharmaceutically acceptable carrier may be administered before or concomitantly therewith. Nanoparticles or microparticles (e.g., cross-linked gelatin) of the therapeutic agent (e.g., paclitaxel) also may be used. The nanoparticles or microparticles may be coated with a bioadhesive coating. Microspheres that agglomerate to block the entrance of the lymphatic ducts of the bladder to retard clearance of the microparticles through the lymphatic system also may be employed. This invention also uses drug-loaded gelatin and poly(lactide-co-glycolide) (PLGA) nanoparticles and microparticles to target drug delivery to tumors in the peritoneal cavity, bladder tissues, and kidneys.
Owner:AU JESSIE L S +1
Methods and apparatus for revision of obesity procedures
ActiveUS20070175488A1Lower the volumeReduce the overall diameterSuture equipmentsDiagnosticsStomaPeritoneal cavity
Methods and apparatus for the endoluminal revision of previously performed obesity procedures which have failed are described. One or more endoluminal instruments may be advanced per-orally into the previously formed failed pouch where a number of different procedures can be performed. One or more tissue folds can be formed and secured to reduce the size of the pouch, or the stoma connecting the pouch to the intestinal tract can be reduced in size using endoluminally deployed tissue anchors. These procedures can be performed entirely from within the pouch lumen or upon the exterior surface of the pouch via transgastric entry of the instruments into the peritoneal cavity of a patient. Alternatively, the interior tissue within the pouch can be injured or sclerosed to shrink the pouch lumen. In another alterative, a length of the Roux limb can be shortened endoluminally to create a malabsorptive region.
Owner:USGI MEDICAL
Hybrid endoscopic/laparoscopic method for forming serosa to serosa plications in a gastric cavity
ActiveUS20090024163A1Lower the volumeLimiting available food capacitySuture equipmentsCannulasVia incisionFood consumption
A method for treating obesity by reducing the volume and / or alter the functioning of the gastric cavity to limit food consumption and induce early satiety. The method includes accessing the cavity endoscopically to visualize the interior of the cavity. The peritoneal cavity is accessed through a small incision in the abdominal wall. Using endoscopic visualization, suture anchoring devices are introduced into the peritoneal cavity through the incision and deployed from the exterior surface through the gastric cavity wall. Suture from pairs of the anchoring devices is drawn through the gastric cavity wall and tightened outside of the cavity to form a serosa to serosa plication. Any number of plications may be formed in the cavity wall and the contacting tissue within a plication may be treated to promote healing and a more durable bond.
Owner:ETHICON ENDO SURGERY INC
Medical treatment system and methods using a plurality of fluid lines
ActiveUS20130165847A1Prevent slight movementReduce noiseFlexible member pumpsPipe heating/coolingControl systemPeritoneal cavity
A medical treatment system, such as peritoneal dialysis system, may include control and other features to enhance patient comfort and ease of use. For example, a peritoneal dialysis system may include a control system that can adjust the volume of fluid infused into the peritoneal cavity to prevent the intraperitoneal fluid volume from exceeding a pre-determined amount. The control system can adjust by adding one or more therapy cycles, allowing for fill volumes during each cycle to be reduced. The control system may continue to allow the fluid to drain from the peritoneal cavity as completely as possible before starting the next therapy cycle. The control system may also adjust the dwell time of fluid within the peritoneal cavity during therapy cycles in order to complete a therapy within a scheduled time period. The cycler may also be configured to have a heater control system that monitors both the temperature of a heating tray and the temperature of a bag of dialysis fluid in order to bring the temperature of the dialysis fluid rapidly to a specified temperature, with minimal temperature overshoot.
Owner:DEKA PROD LLP
Methods and apparatus for accessing and treating regions of the body
InactiveUS20050165276A1Avoid excessive forceAccurately advancedEndoscopesLaproscopesThoracic structureAutomatic control
Methods and apparatus for accessing and treating regions of the body are disclosed herein. Using an endoscopic device having an automatically controllable proximal portion and a selectively steerable distal portion, the device generally may be advanced into the body through an opening. The distal portion is selectively steered to assume a selected curve along a desired path within the body which avoids contact with tissue while the proximal portion is automatically controlled to assume the selected curve of the distal portion. The endoscopic device can then be used for accessing various regions of the body which are typically difficult to access and treat through conventional surgical techniques because the device is unconstrained by “straight-line” requirements. Various applications can include accessing regions of the brain, thoracic cavity, including regions within the heart, peritoneal cavity, etc., which are difficult to reach using conventional surgical procedures.
Owner:INTUITIVE SURGICAL
Hypothermia Devices and Methods
ActiveUS20090076573A1Reduce the amount requiredLower body temperatureMedical devicesTherapeutic coolingPeritoneal cavityFeedback control
A method of providing hypothermia to a patient including the steps of inserting a fluid delivery member into a peritoneal cavity of the patient; delivering hypothermia fluid from a fluid source into the peritoneal cavity through the delivery member; and limiting fluid pressure within the peritoneal cavity without providing feedback control to the fluid source. The invention also provides an apparatus for practicing the method.
Owner:THERANOVA LLC
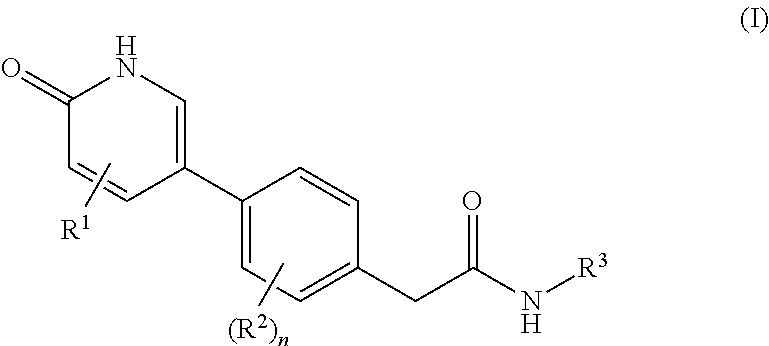
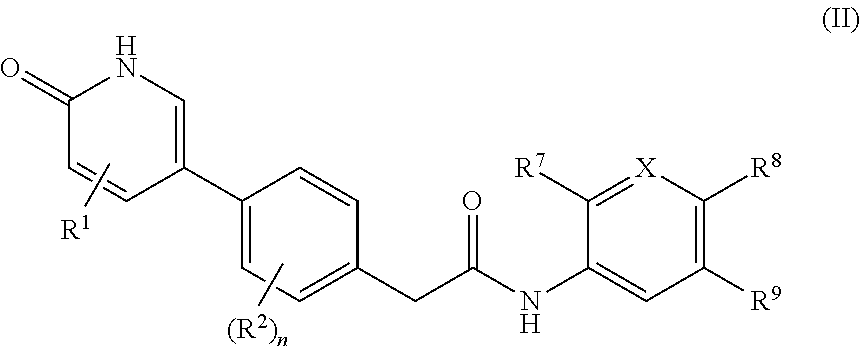
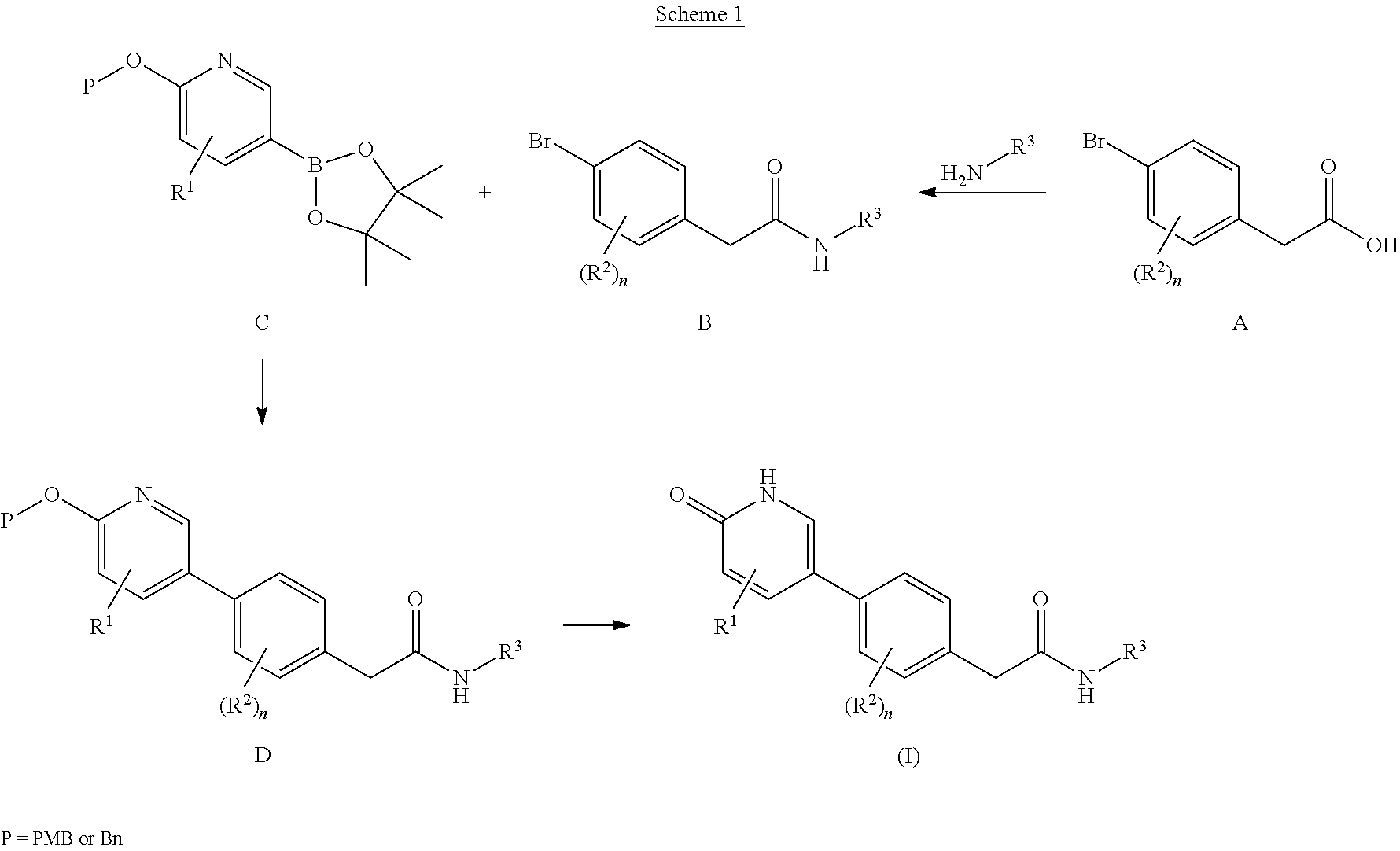
Compounds as rearranged during transfection (RET) inhibitors
This invention relates to novel compounds which are inhibitors of the Rearranged during Transfection (RET) kinase, to pharmaceutical compositions containing them, to processes for their preparation, and to their use in therapy, alone or in combination, for the normalization of gastrointestinal sensitivity, motility and / or secretion and / or abdominal disorders or diseases and / or treatment related to diseases related to RET dysfunction or where modulation of RET activity may have therapeutic benefit including but not limited to all classifications of irritable bowel syndrome (IBS) including diarrhea-predominant, constipation-predominant or alternating stool pattern, functional bloating, functional constipation, functional diarrhea, unspecified functional bowel disorder, functional abdominal pain syndrome, chronic idiopathic constipation, functional esophageal disorders, functional gastroduodenal disorders, functional anorectal pain, inflammatory bowel disease, proliferative diseases such as non-small cell lung cancer, hepatocellular carcinoma, colorectal cancer, medullary thyroid cancer, follicular thyroid cancer, anaplastic thyroid cancer, papillary thyroid cancer, brain tumors, peritoneal cavity cancer, solid tumors, other lung cancer, head and neck cancer, gliomas, neuroblastomas, Von Hippel-Lindau Syndrome and kidney tumors, breast cancer, fallopian tube cancer, ovarian cancer, transitional cell cancer, prostate cancer, cancer of the esophagus and gastroesophageal junction, biliary cancer, adenocarcinoma, and any malignancy with increased RET kinase activity.
Owner:GLAXOSMITHKLINE INTPROP DEV LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com