Patents
Literature
9767 results about "Body fluid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Body fluids, bodily fluids, or biofluids are liquids within the human body. In lean healthy adult men, the total body water is about 60% (60-67%) of the total body weight; it is usually slightly lower in women. The exact percentage of fluid relative to body weight is inversely proportional to the percentage of body fat. A lean 70 kg (160 pound) man, for example, has about 42 (42-47) liters of water in his body.
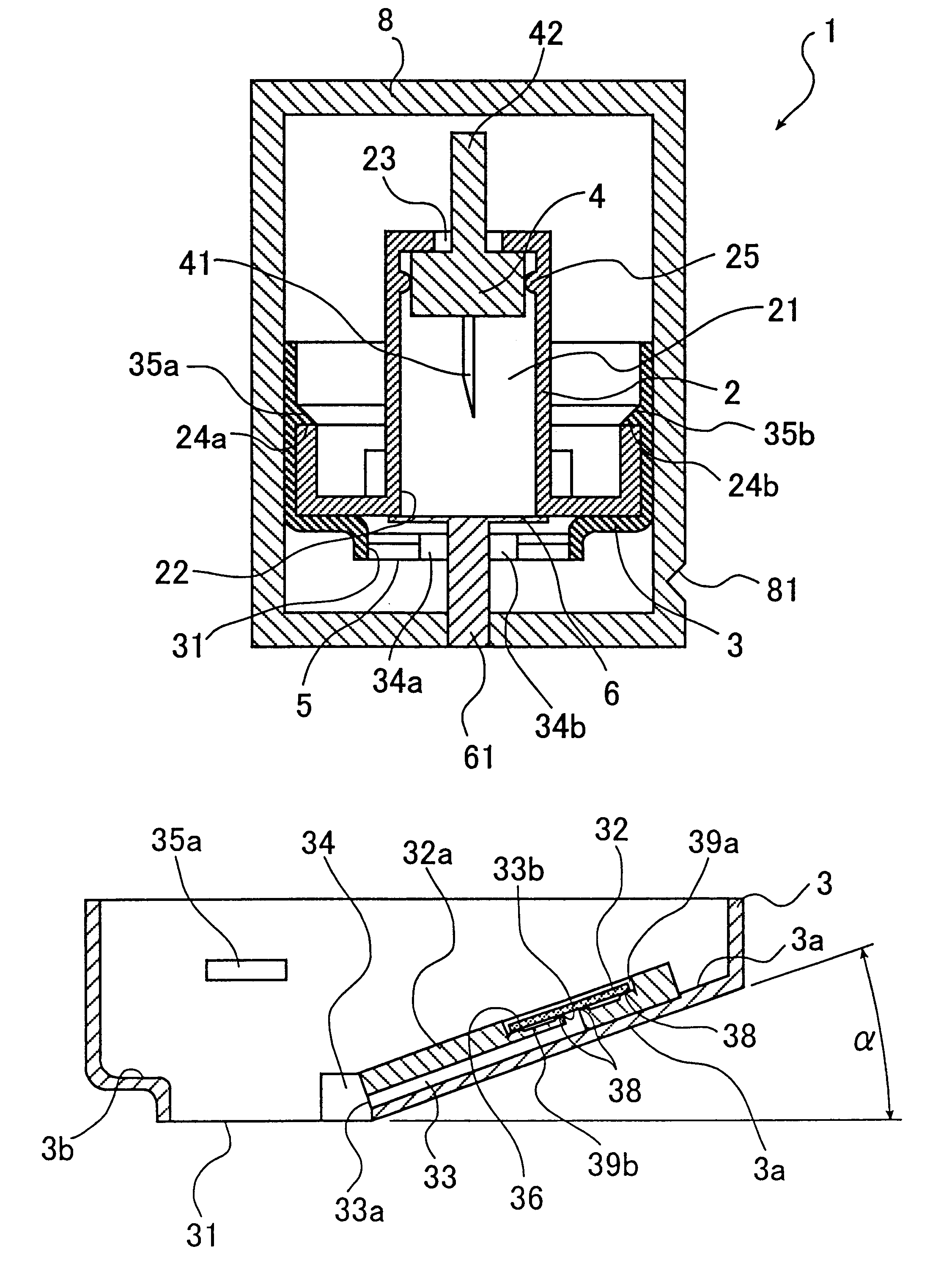
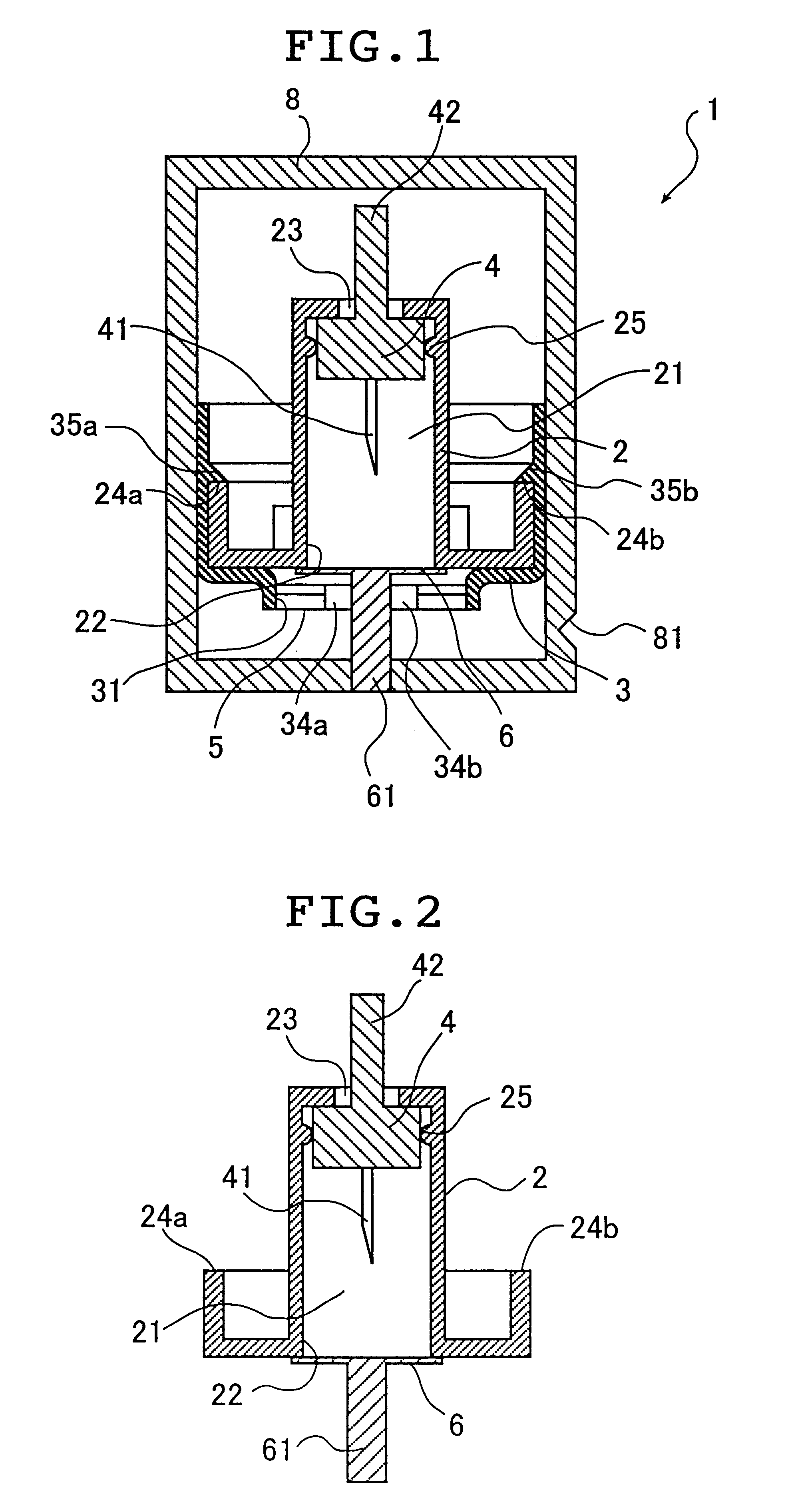
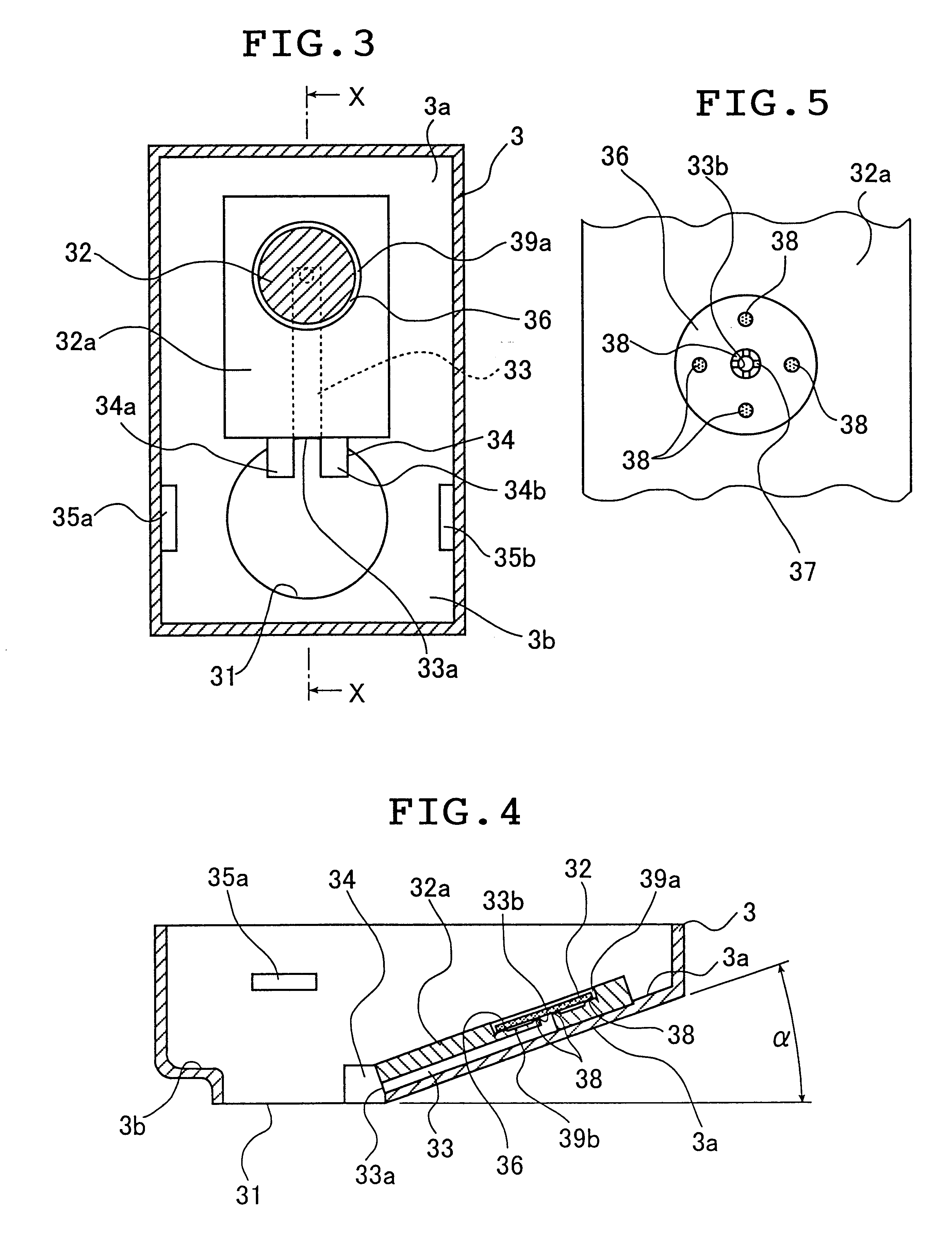
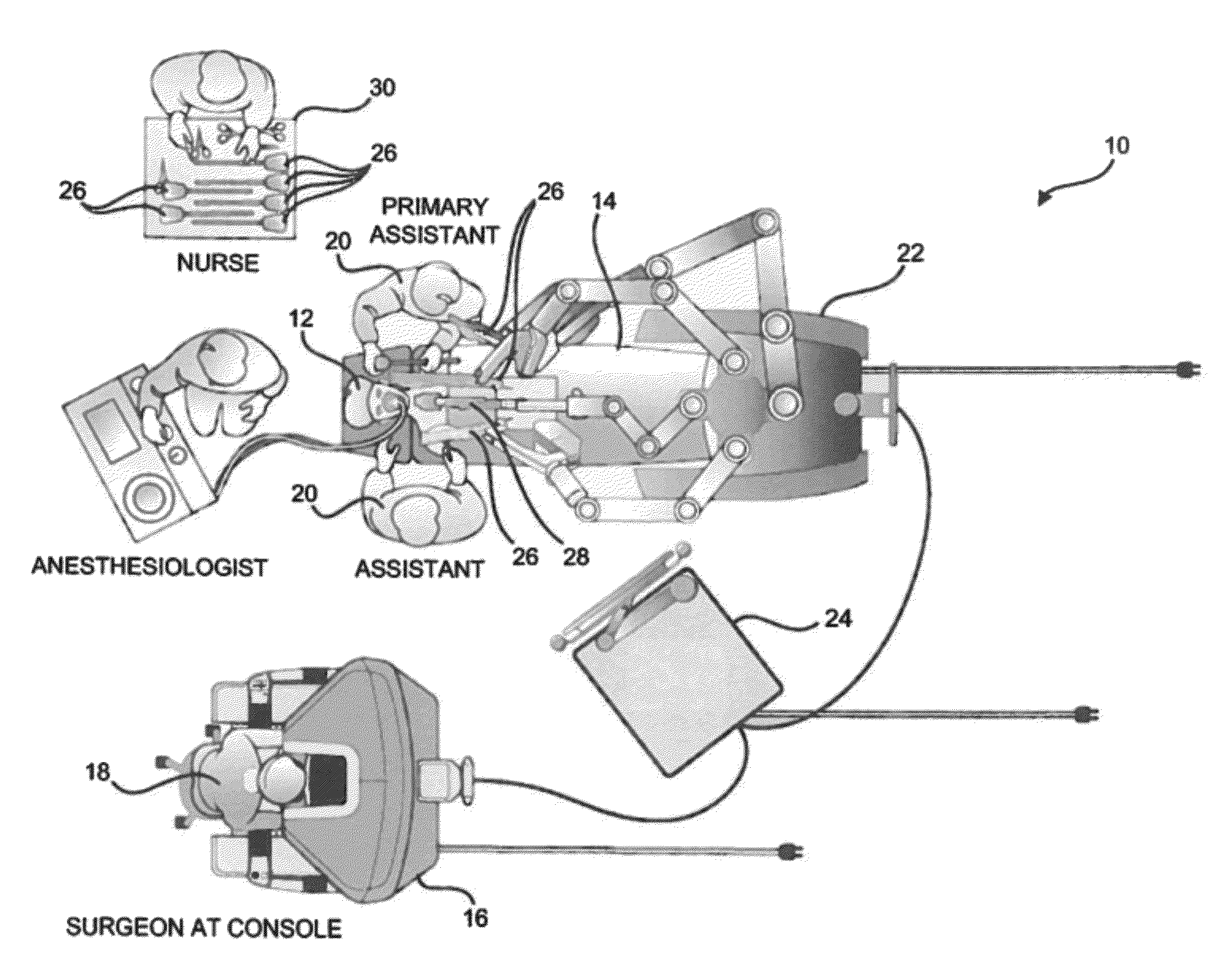
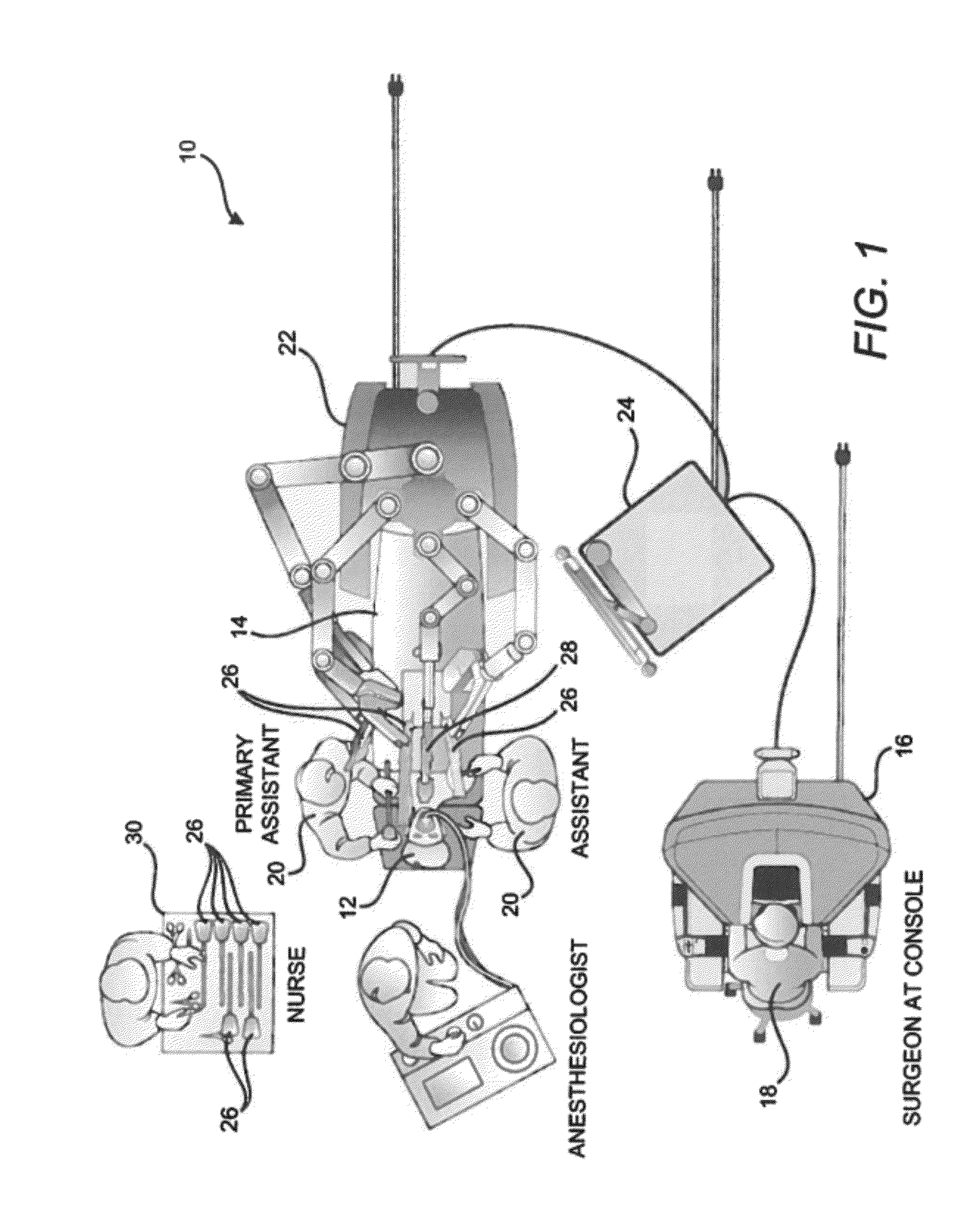
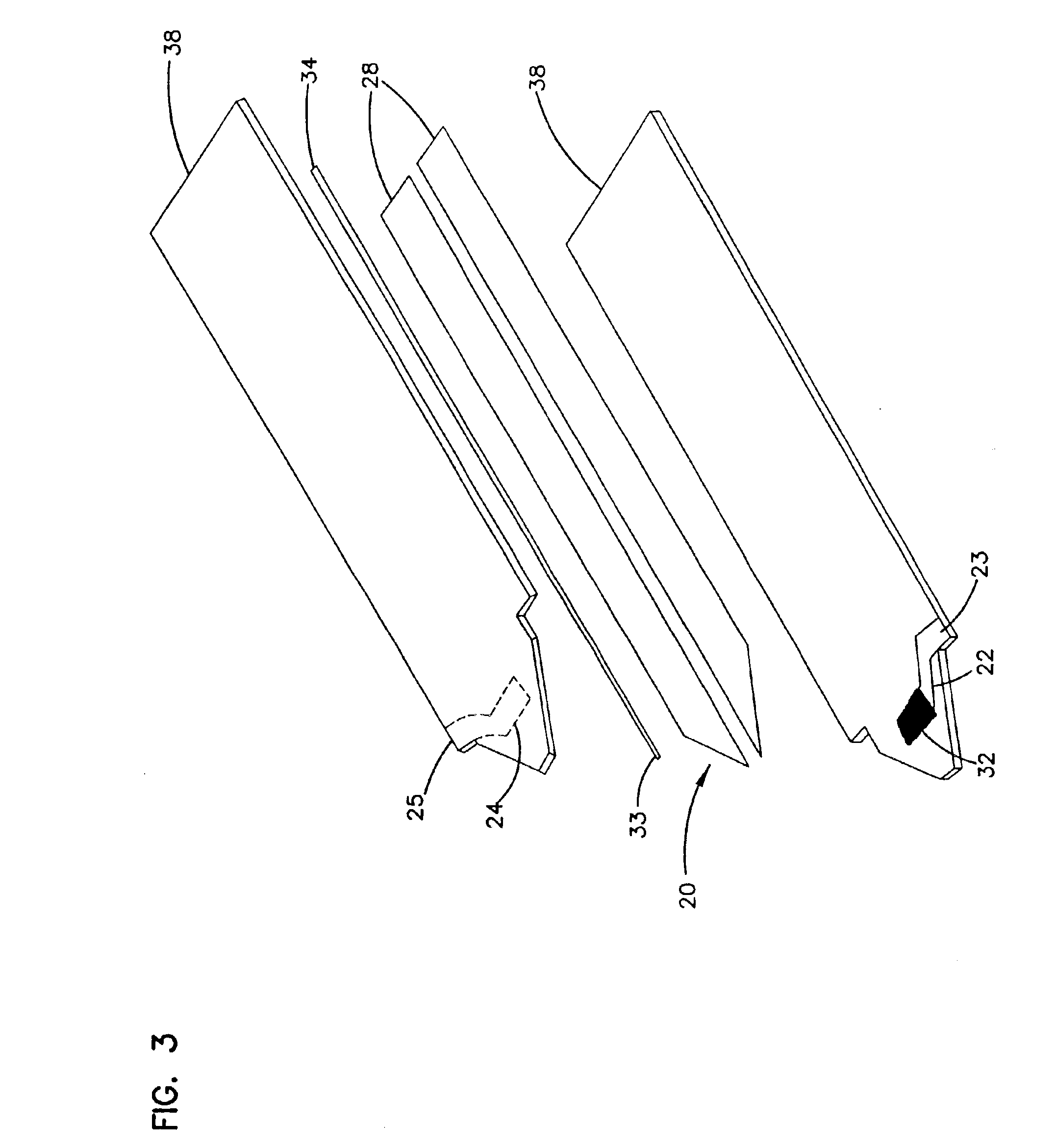
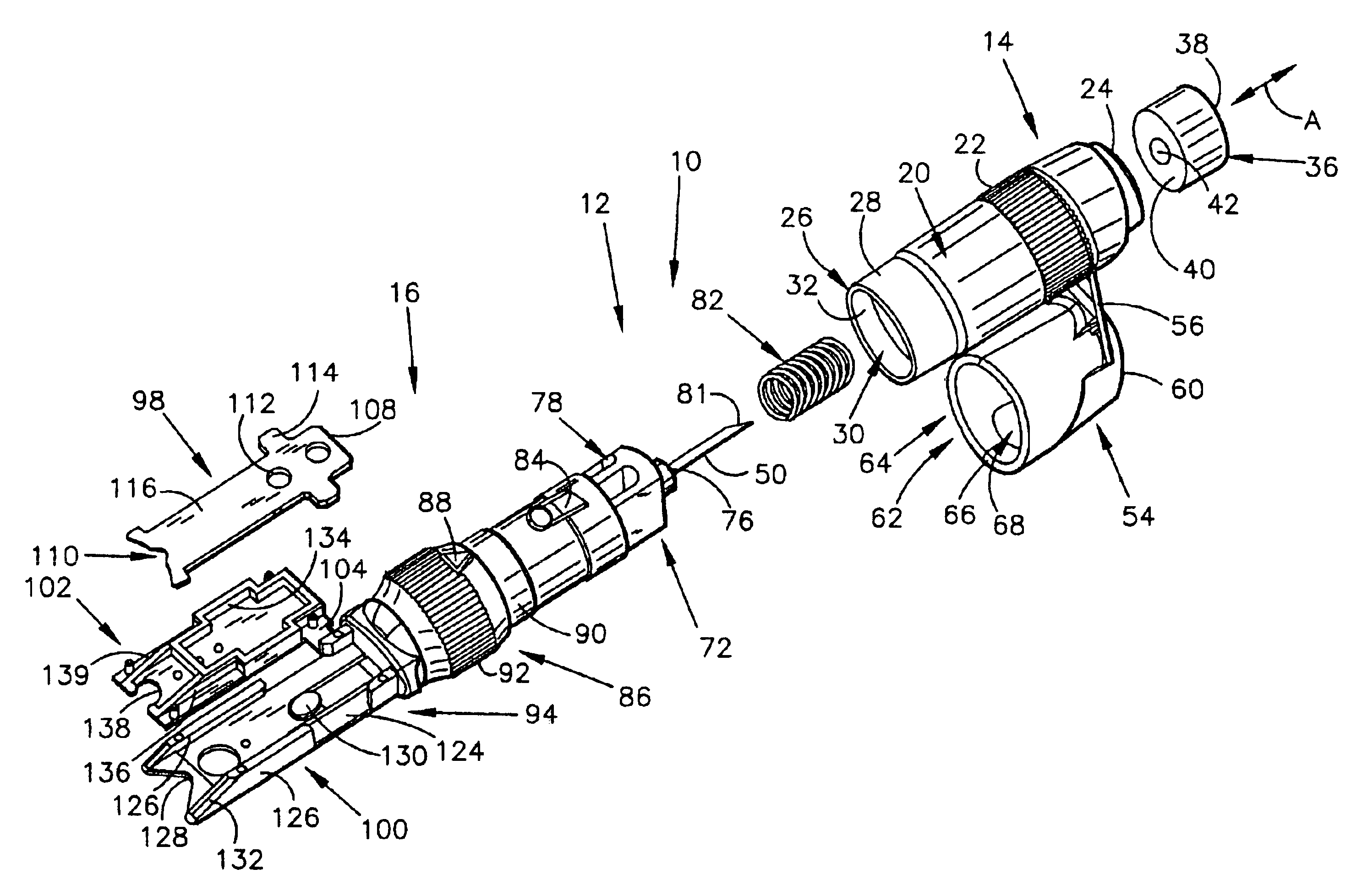
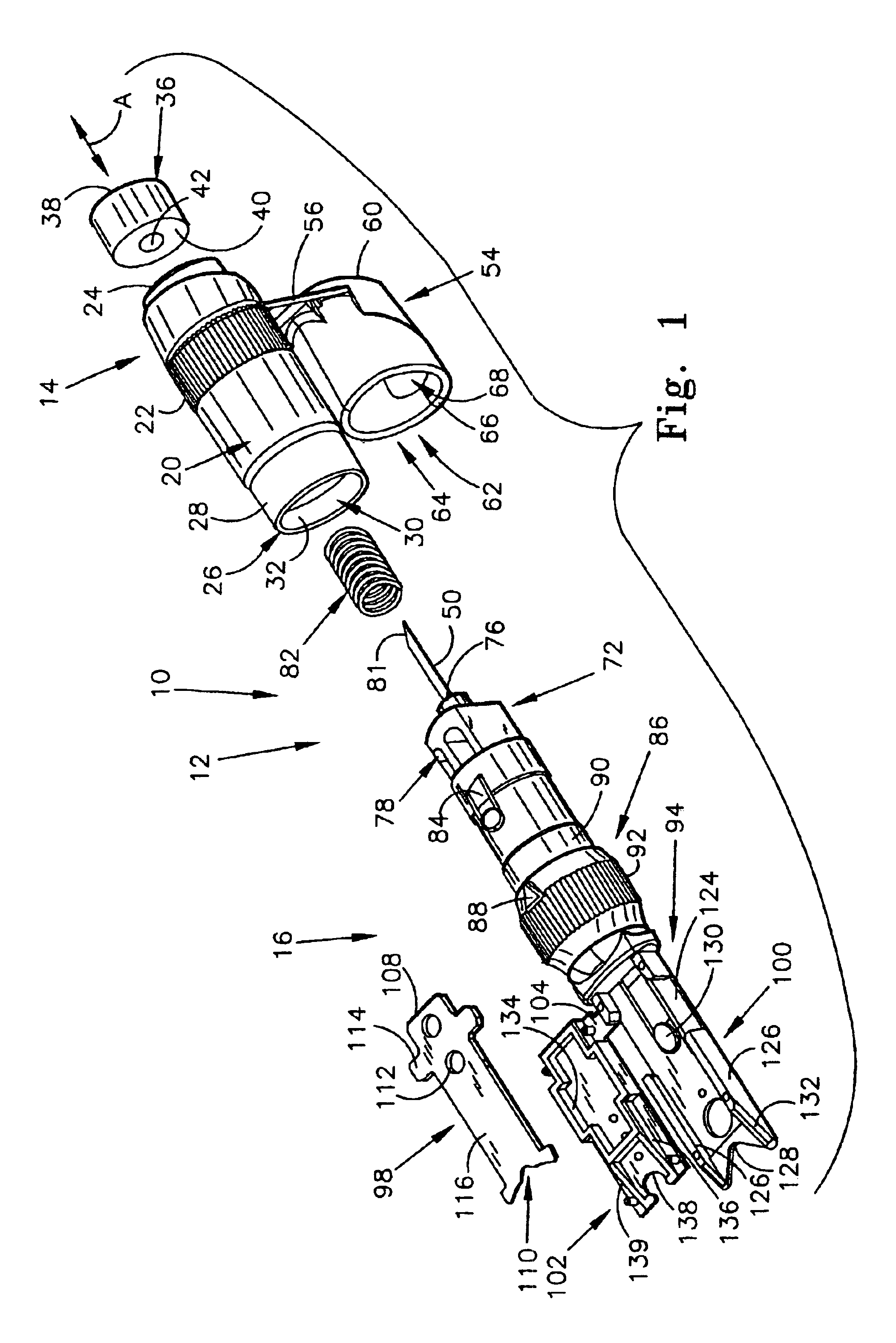
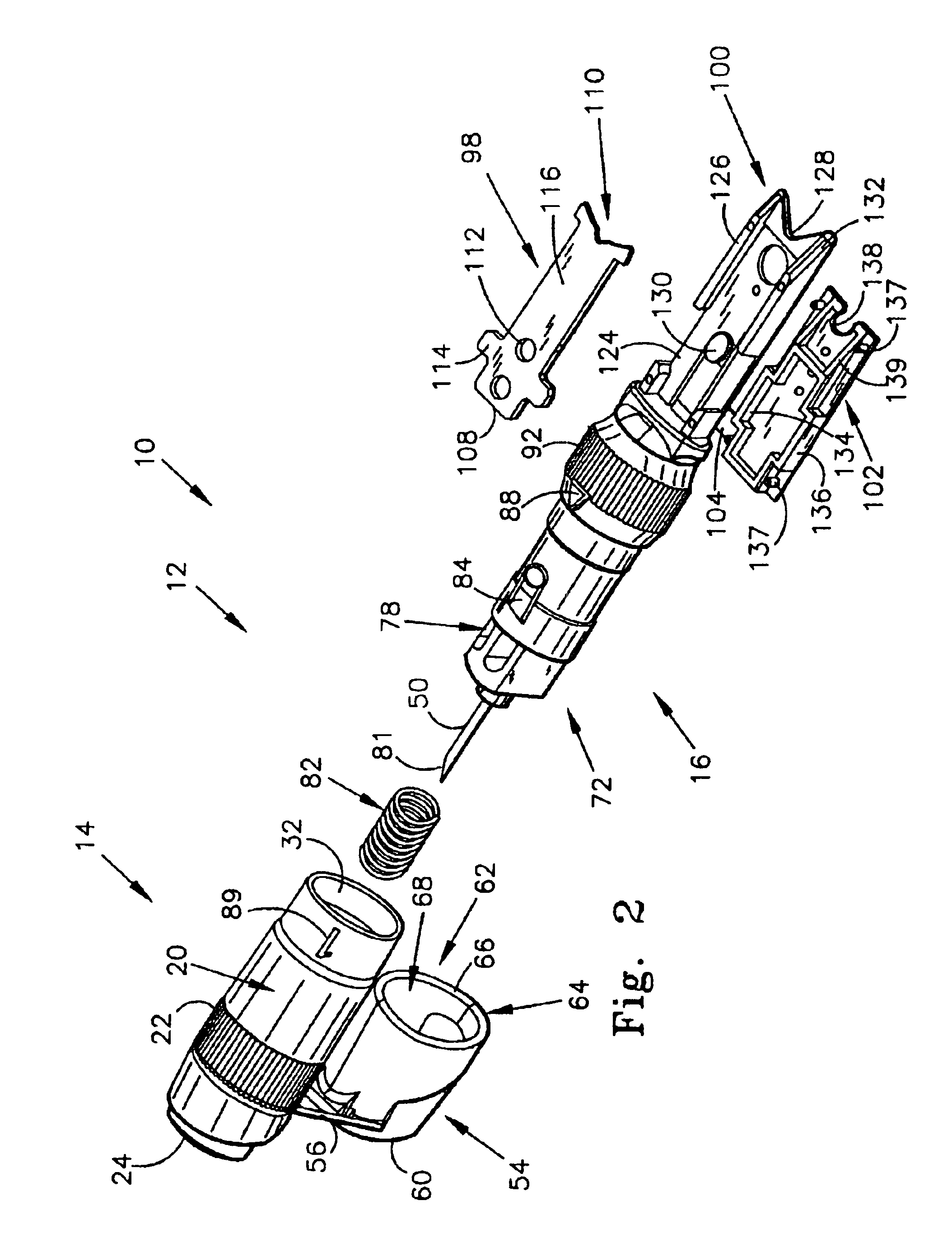
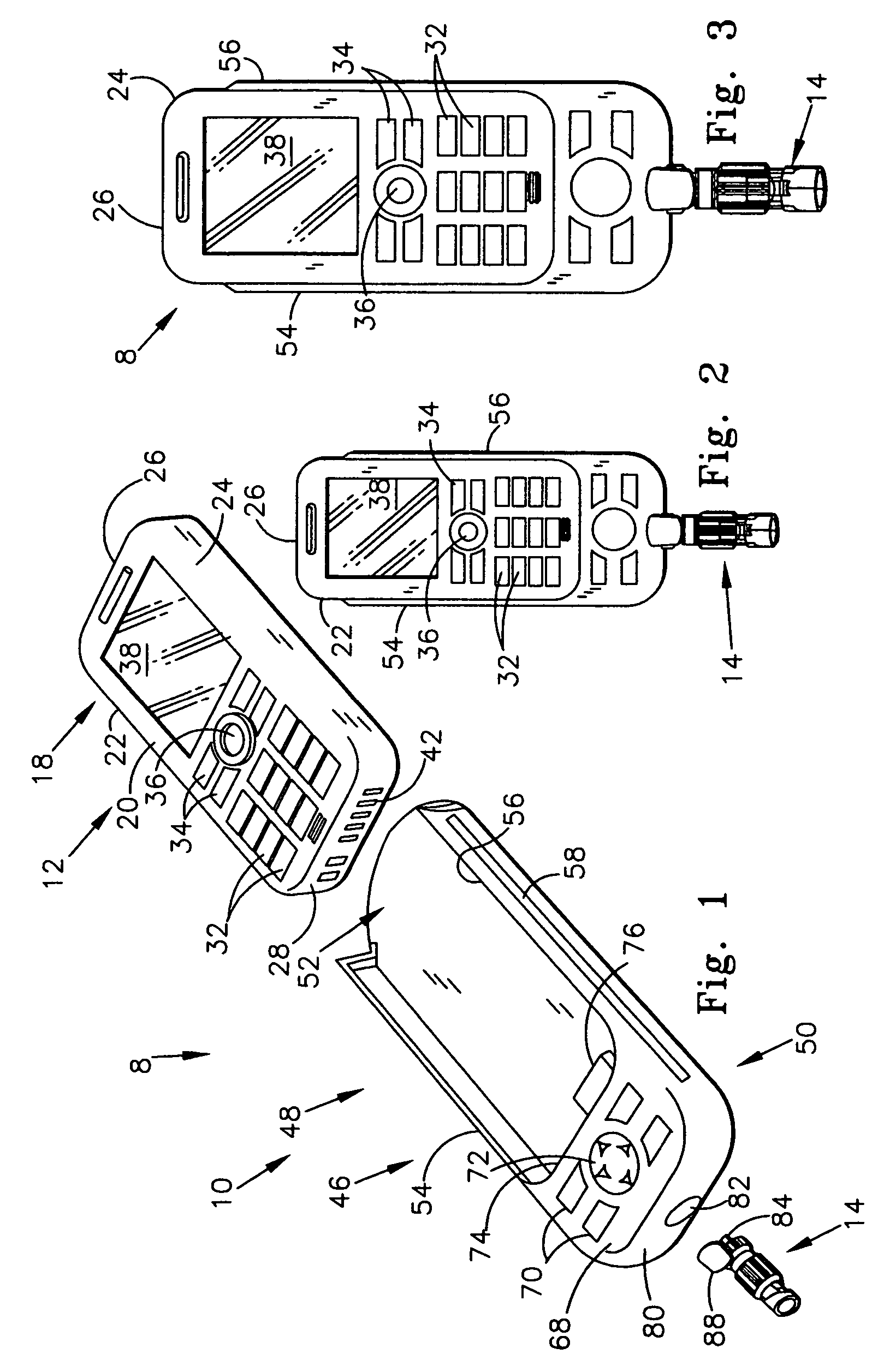
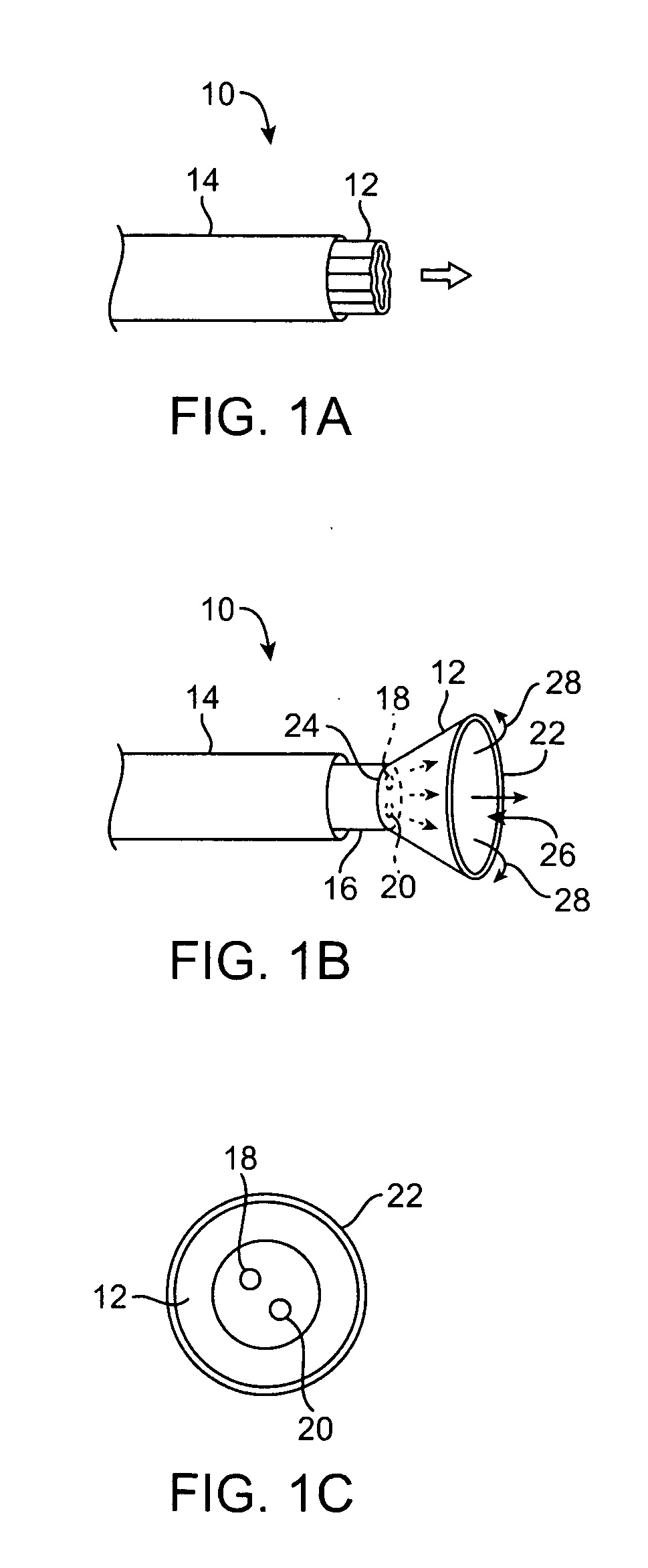
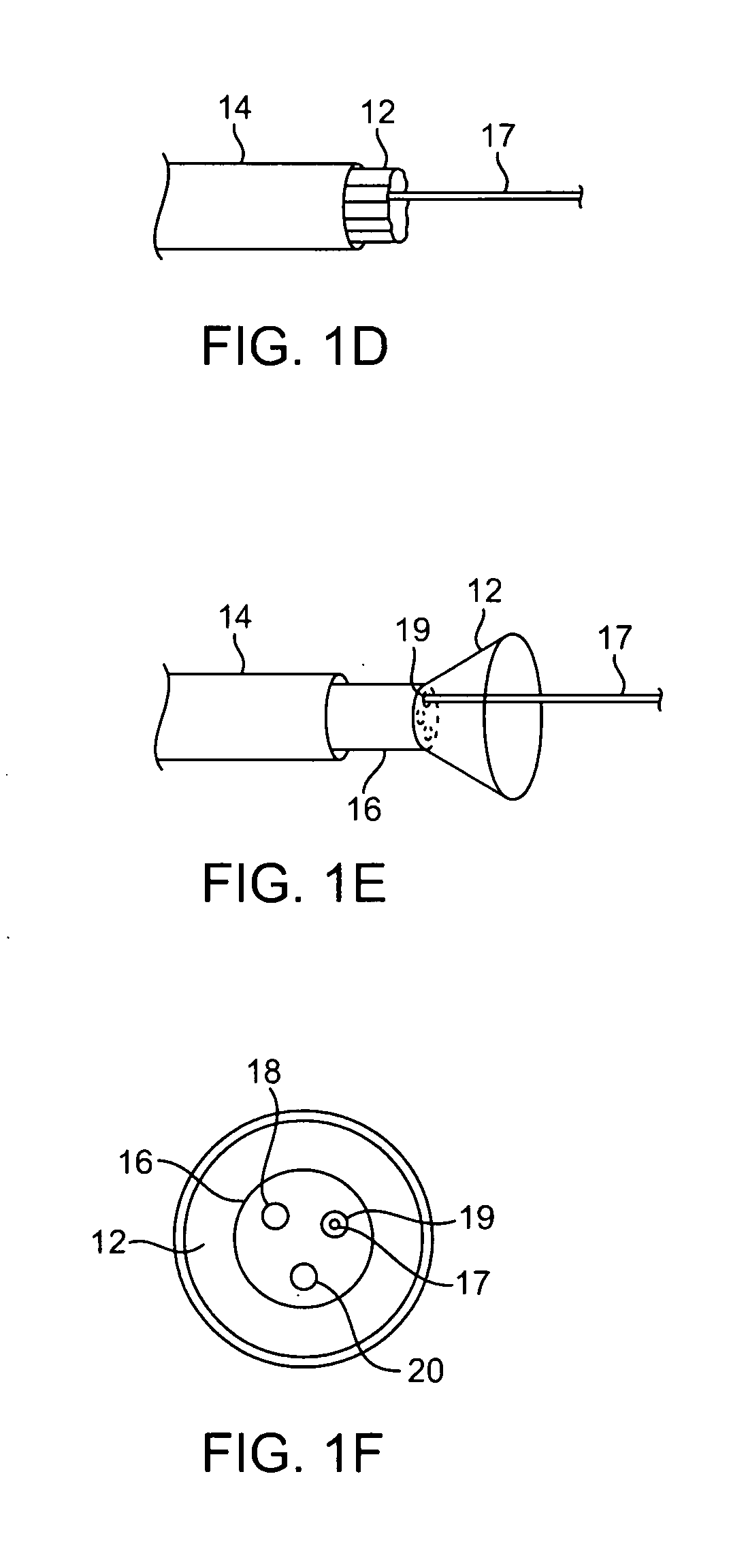
Seals and sealing methods for a surgical instrument having an articulated end effector actuated by a drive shaft
Owner:INTUITIVE SURGICAL OPERATIONS INC
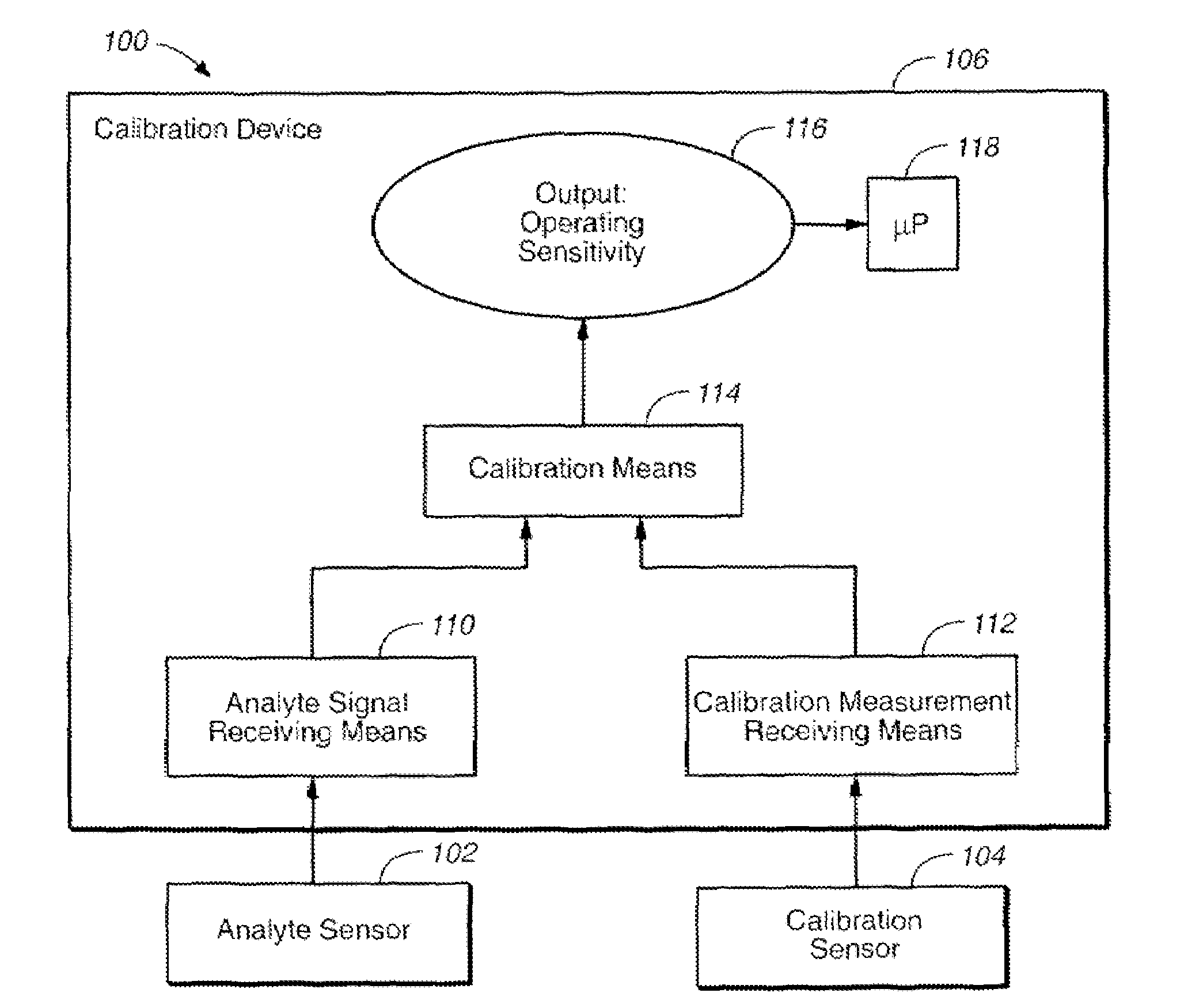
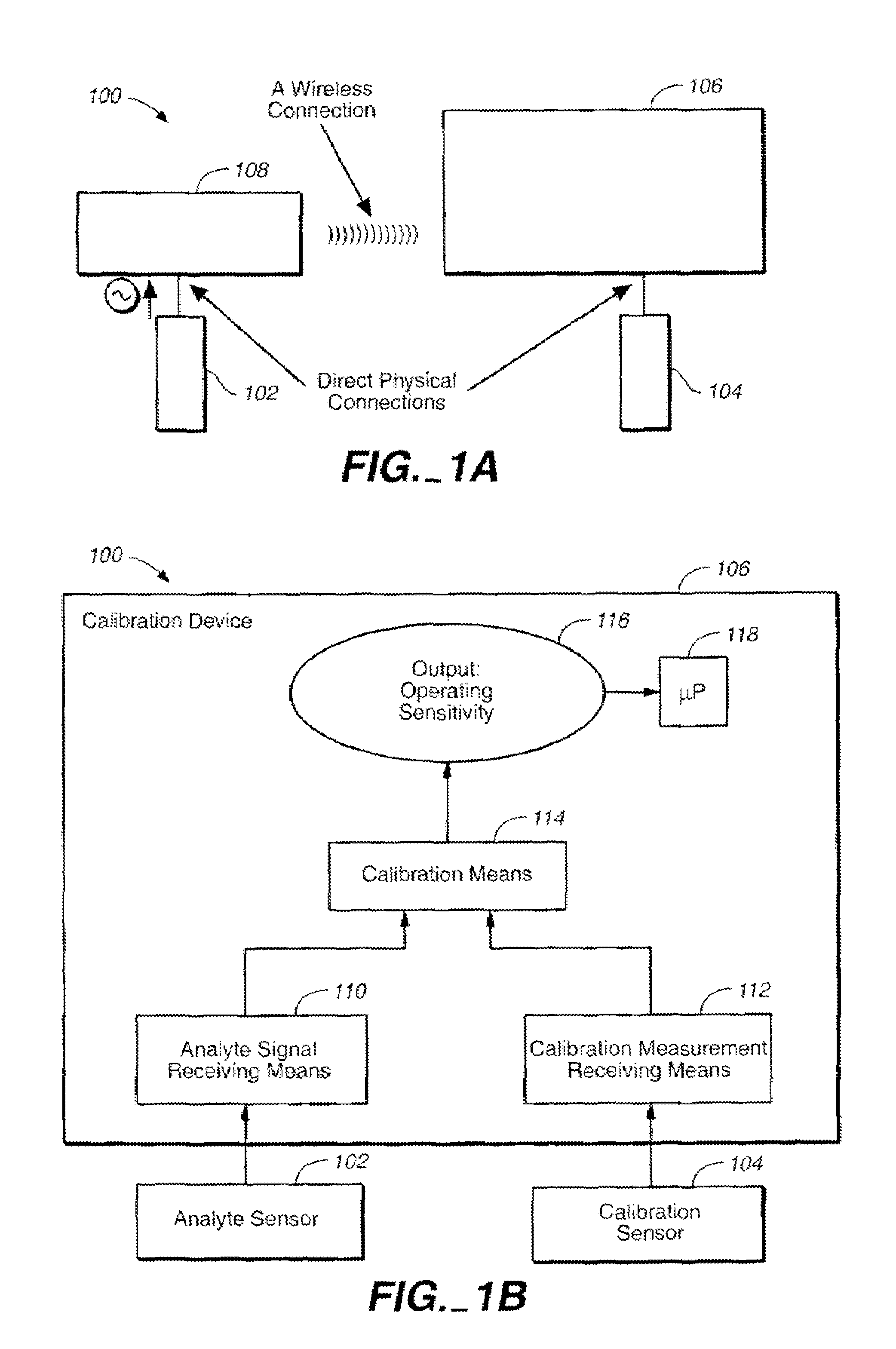
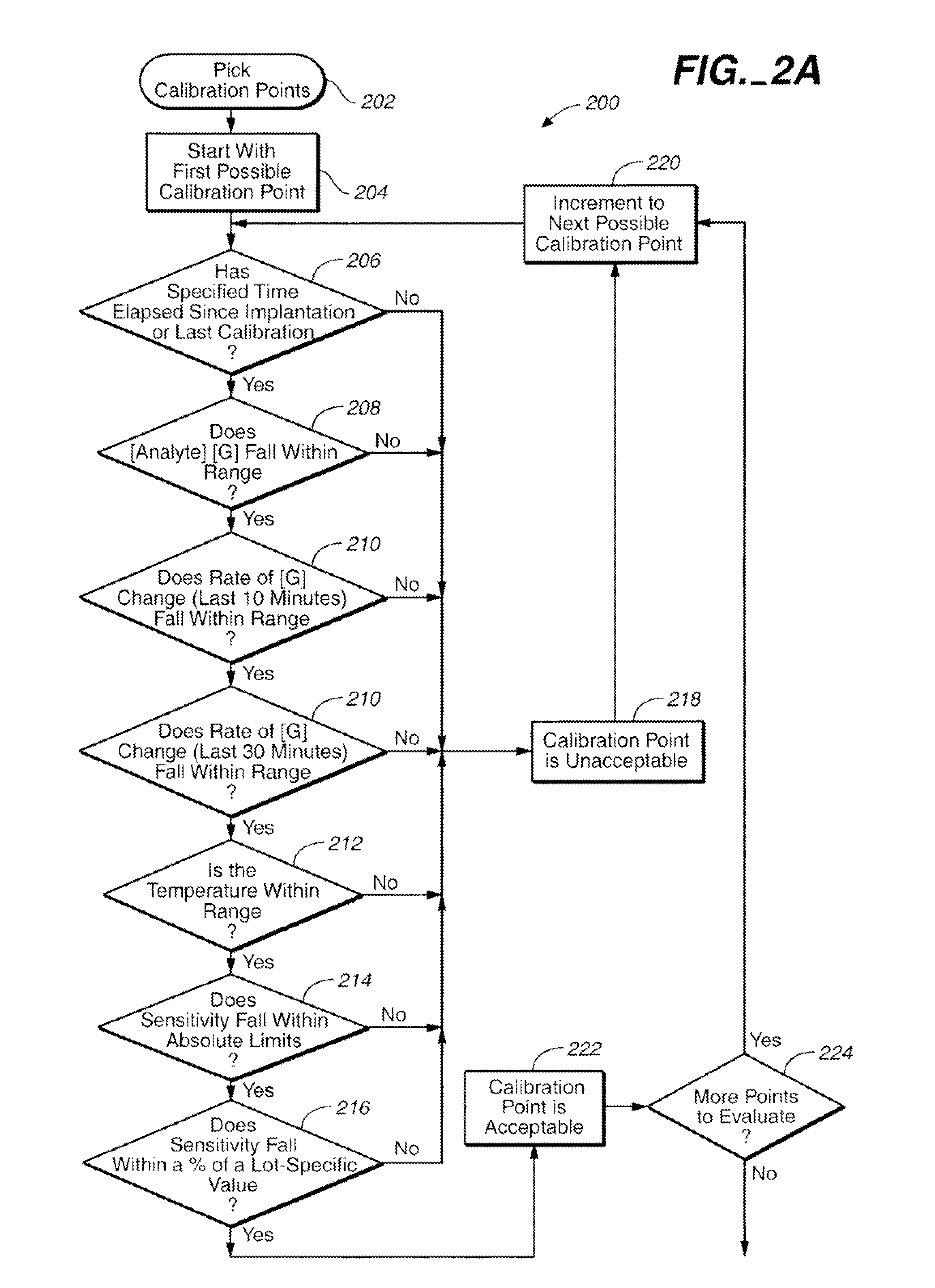
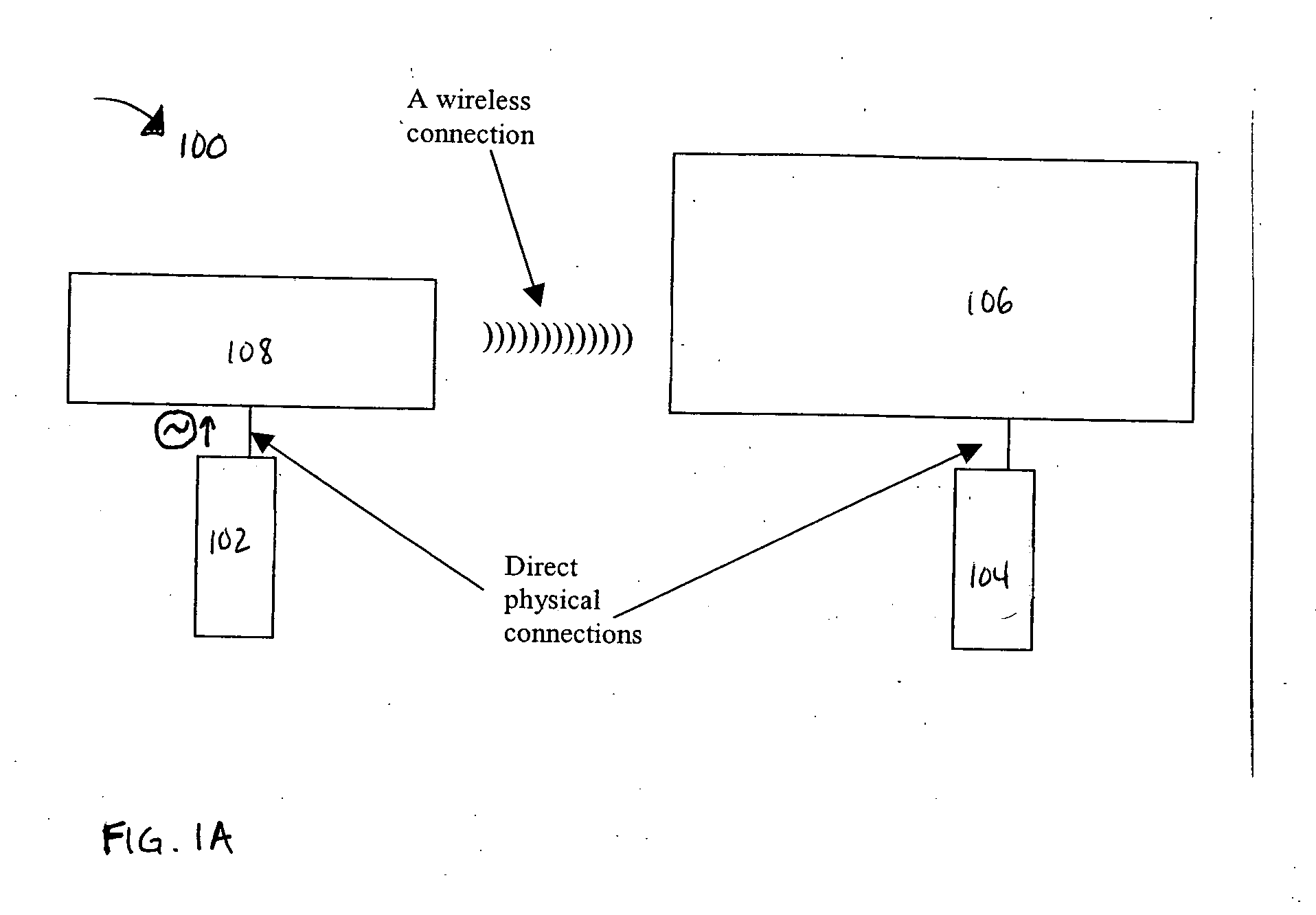
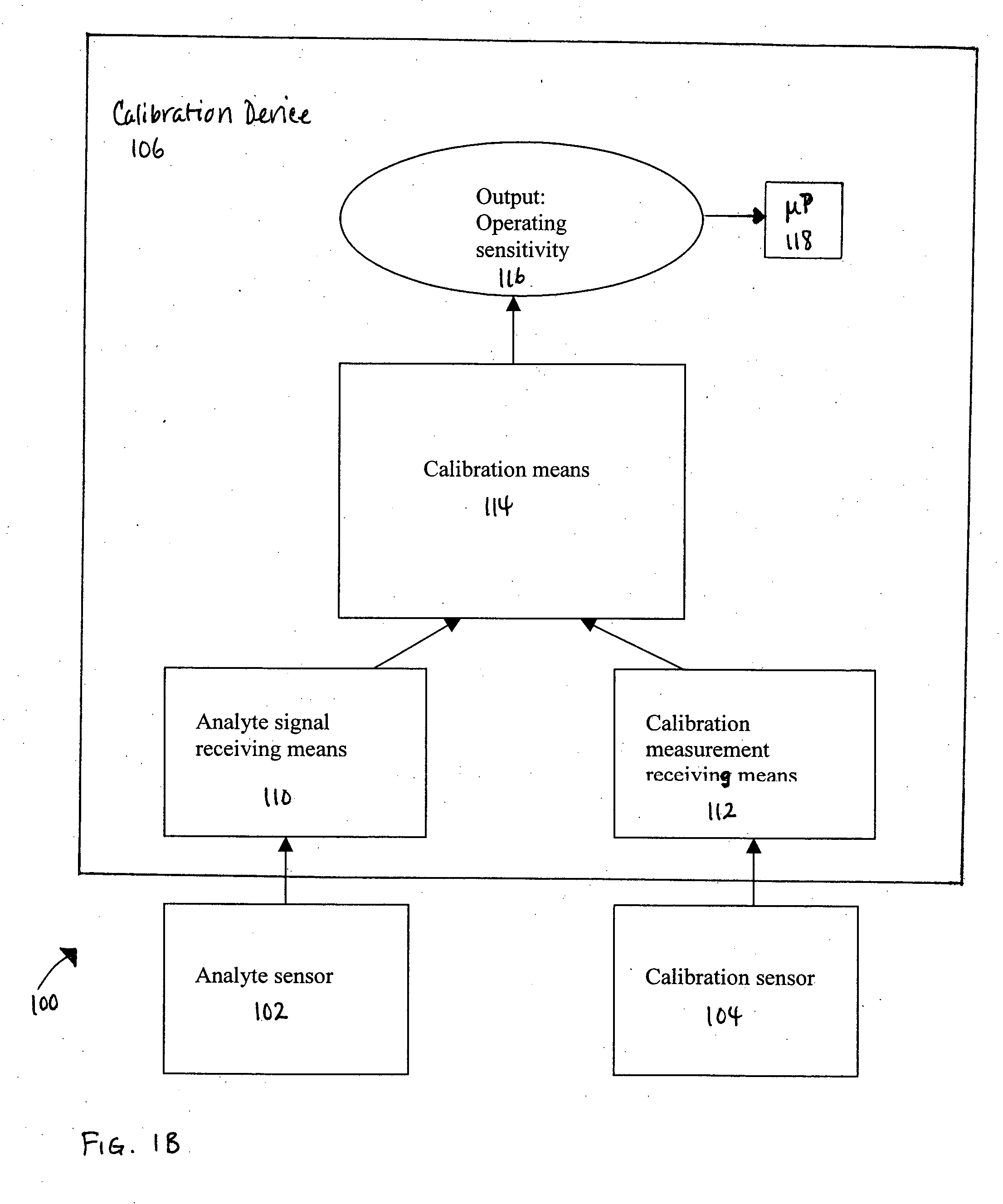
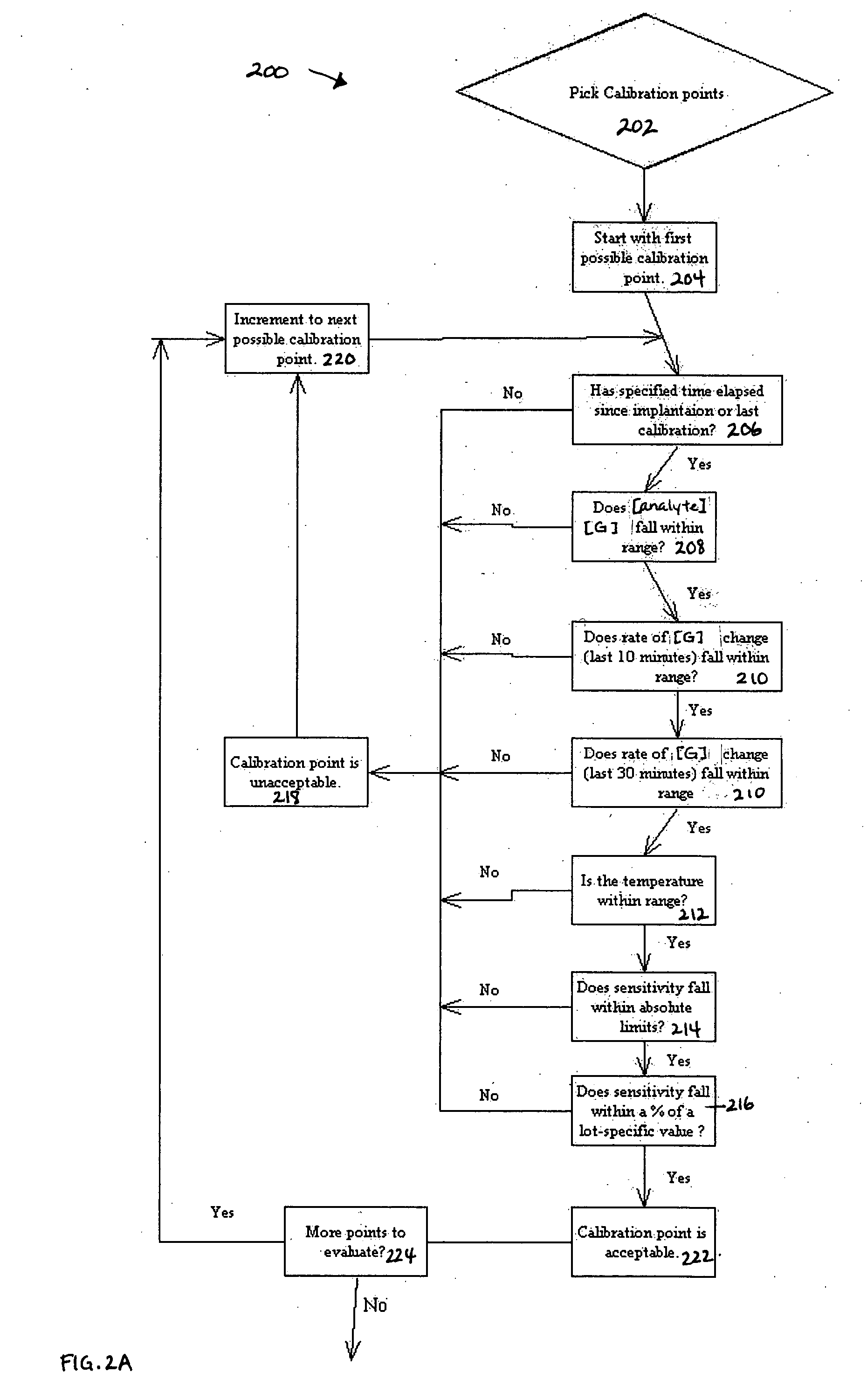
Method of calibrating an analyte-measurement device, and associated methods, devices and systems
ActiveUS7299082B2Improve mobilityAvoid high pressureMicrobiological testing/measurementCatheterMeasurement deviceAnalyte
Owner:ABBOTT DIABETES CARE INC
A method of calibrating an analyte-measurement device, and associated methods, devices and systems
ActiveUS20050239154A1Improve mobilityAvoid high pressureMicrobiological testing/measurementCatheterMeasurement deviceAnalyte
The invention relates to a method for calibrating an analyte-measurement device that is used to evaluate a concentration of analyte in bodily fluid at or from a measurement site in a body. The method involves measuring a concentration, or calibration concentration, of an analyte in blood from an “off-finger” calibration site, and calibrating the analyte-measurement device based on that calibration concentration. The invention also relates to a device, system, or kit for measuring a concentration of an analyte in a body, which employs a calibration device for adjusting analyte concentration measured in bodily fluid based on an analyte concentration measured in blood from an “off-finger” calibration site.
Owner:ABBOTT DIABETES CARE INC
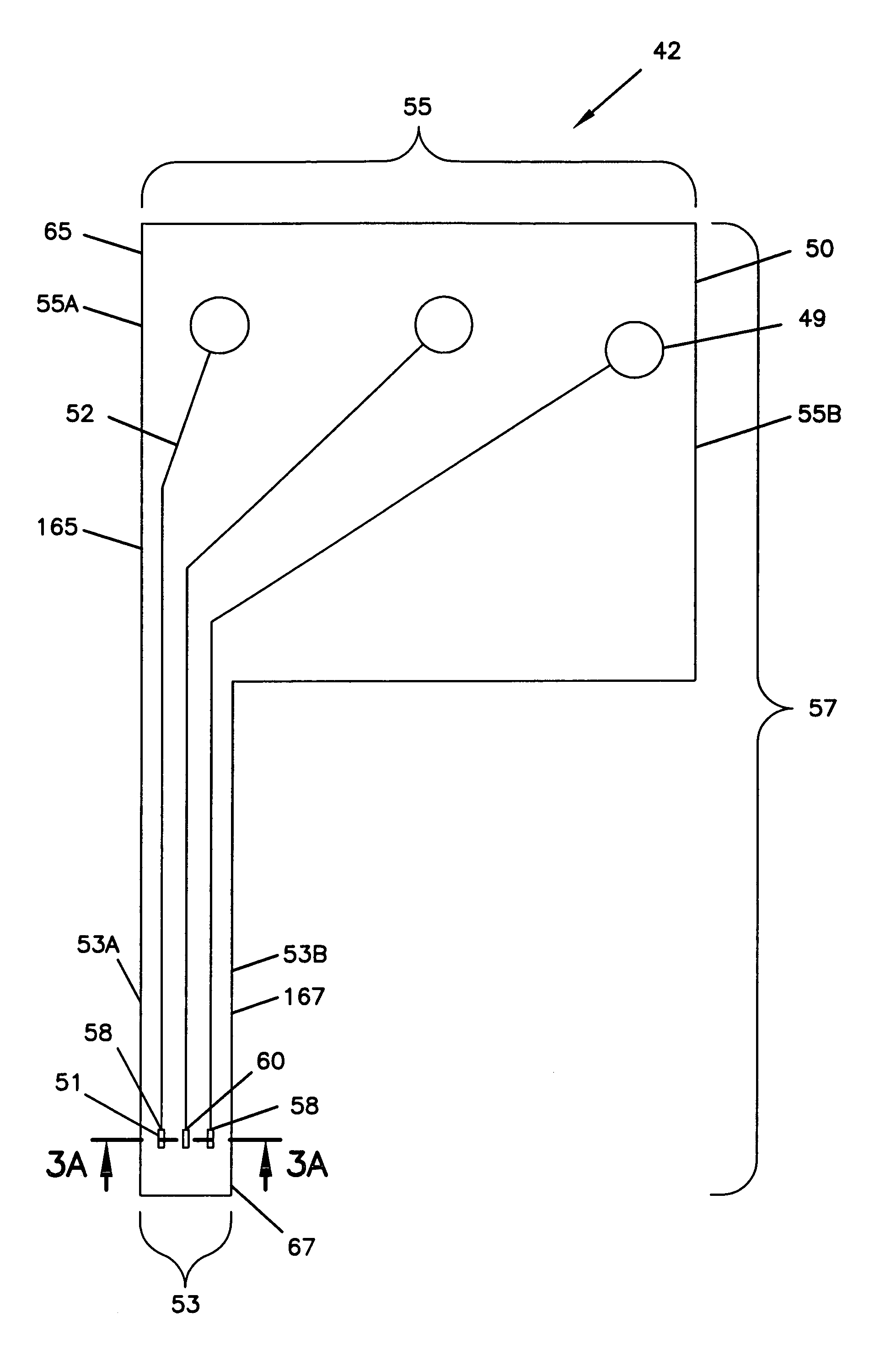
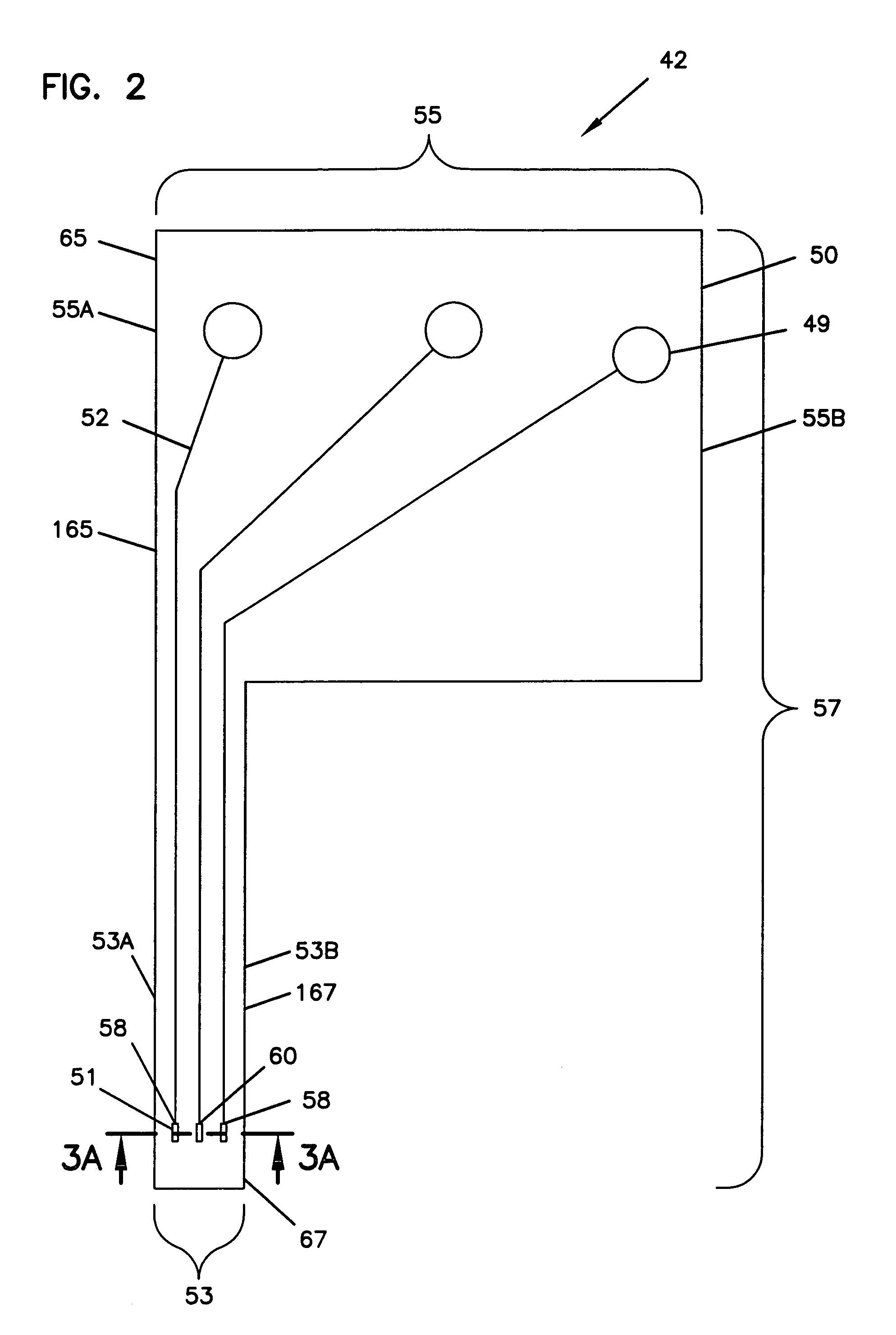
Electrochemical analyte sensor
InactiveUS7003340B2Avoid and reduce corrosionRotary clutchesMicrobiological testing/measurementElectrolysisAnalyte
An electrochemical analyte sensor formed using conductive traces on a substrate can be used for determining and / or monitoring a level of analyte in in vitro or in vivo analyte-containing fluids. For example, an implantable sensor may be used for the continuous or automatic monitoring of a level of an analyte, such as glucose, lactate, or oxygen, in a patient. The electrochemical analyte sensor includes a substrate and conductive material disposed on the substrate, the conductive material forming a working electrode. In some sensors, the conductive material is disposed in recessed channels formed in a surface of the sensor. An electron transfer agent and / or catalyst may be provided to facilitate the electrolysis of the analyte or of a second compound whose level depends on the level of the analyte. A potential is formed between the working electrode and a reference electrode or counter / reference electrode and the resulting current is a function of the concentration of the analyte in the body fluid.
Owner:ABBOTT DIABETES CARE INC
Lancet device having capillary action
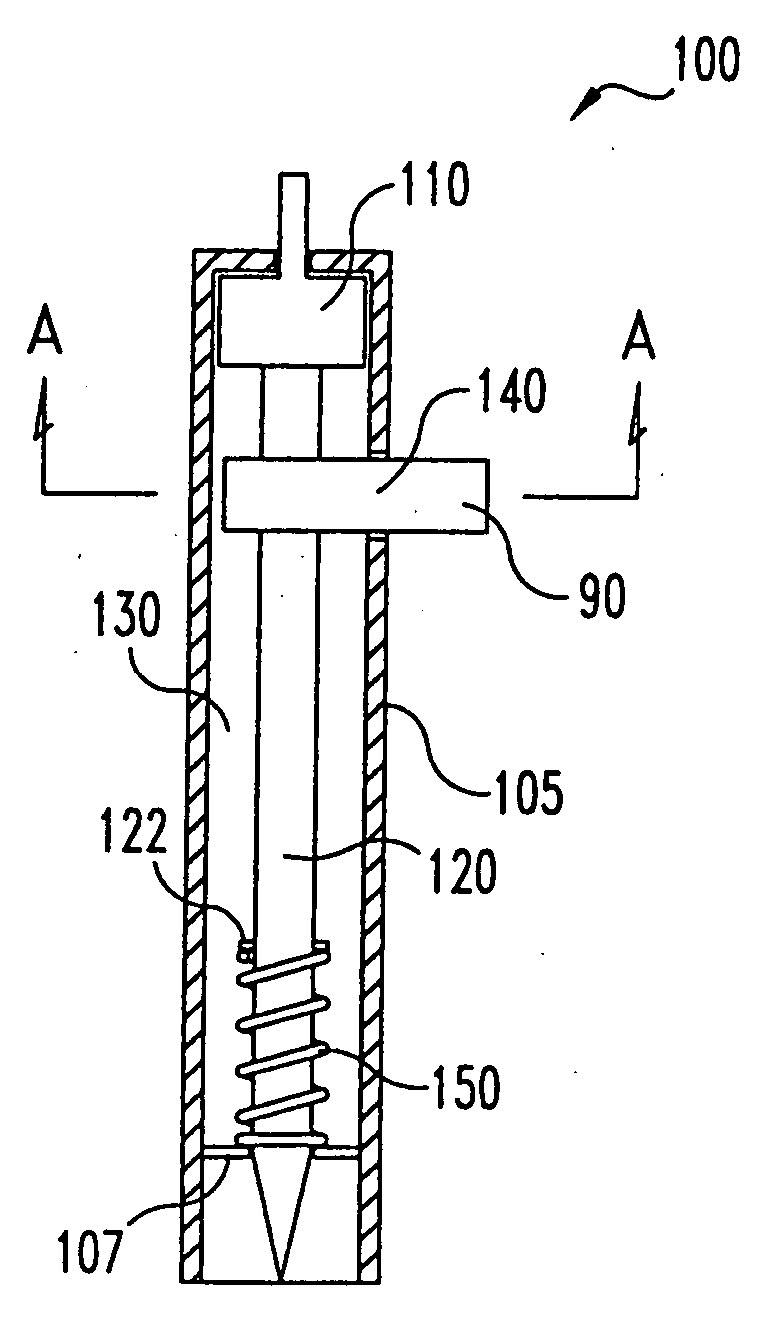
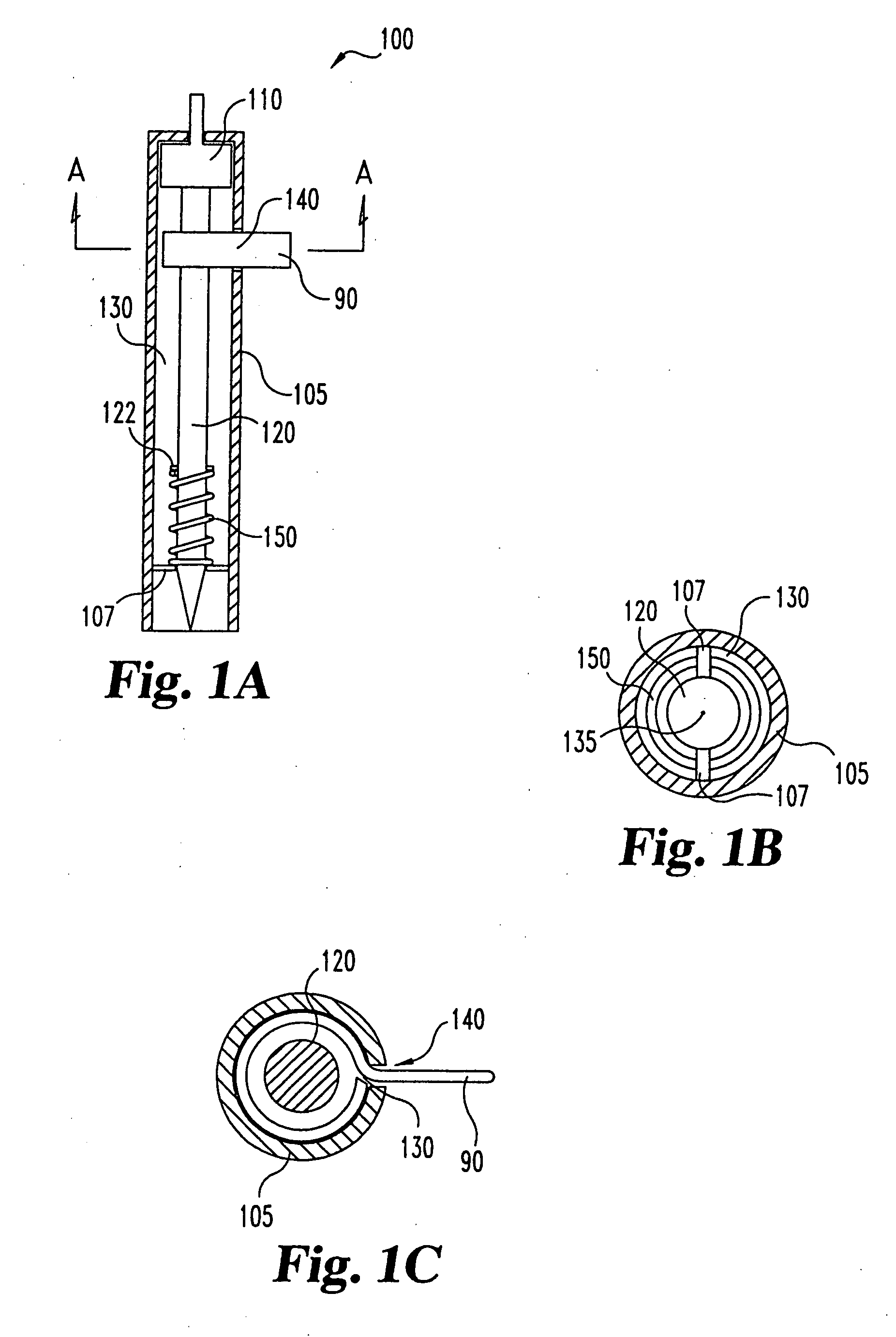
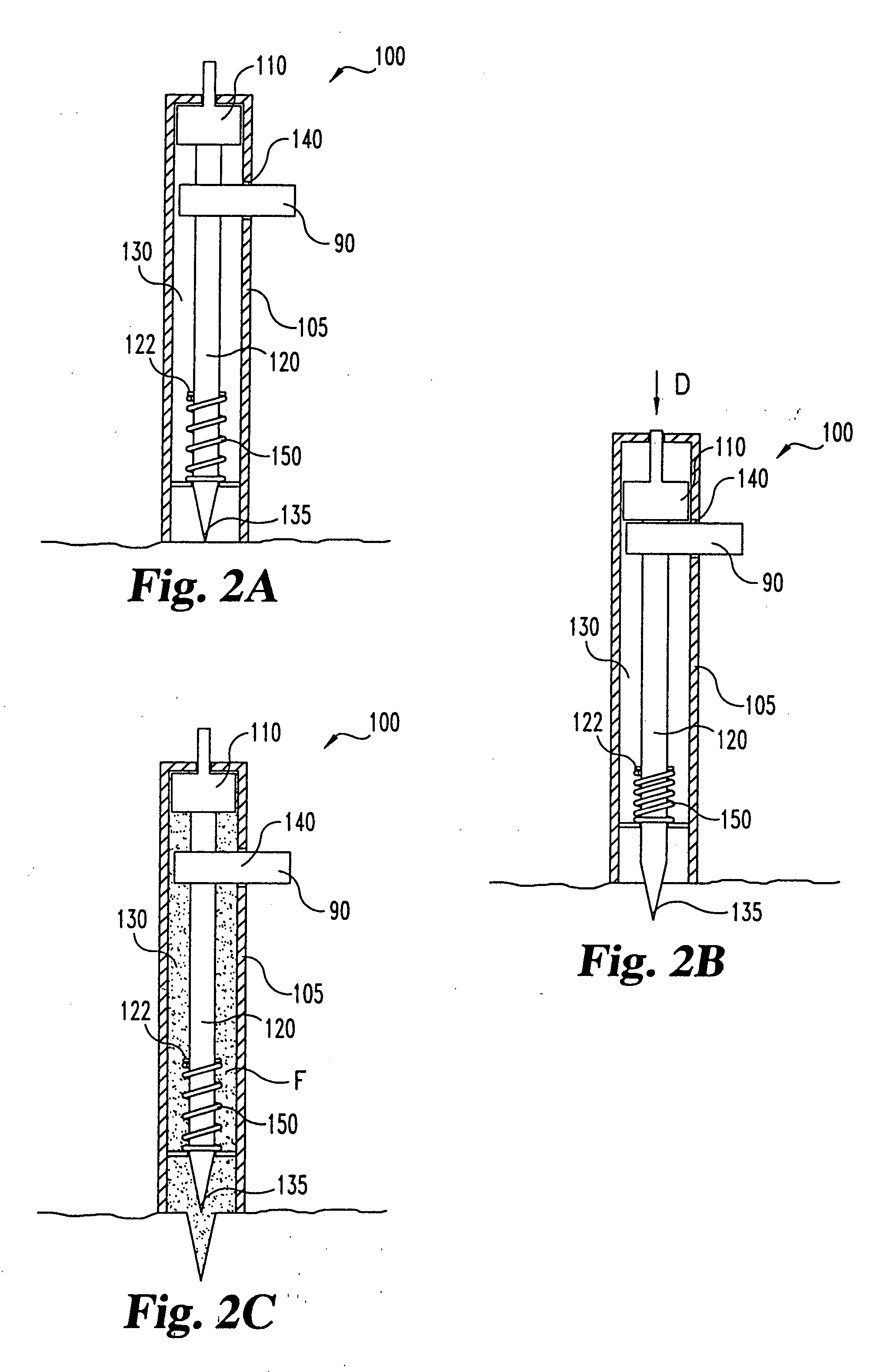
InactiveUS20050004494A1Material minimizationReduced structureSamplingSurgeryVisual inspectionBody fluid
A device for sampling body fluid, the device comprising, a main body, a lancet disposed within the main body, a carrier disposed within the main body fixedly attached to the lancet, a biasing means in communication with the lancet and the carrier, an annular space disposed within the main body adjacent the lancet, and a means for measuring a body fluid. Wherein the means for measuring the body fluid may include micro-porous test strips, an electronic testing device, an optical / reflectance testing measuring device, or a visual inspection.
Owner:ROCHE DIABETES CARE INC
Method and apparatus for a multi-use body fluid sampling device with analyte sensing
A device for use with a penetrating member driver to penetrate tissue is provided. A plurality of penetrating members are coupled to a single cartridge and are operatively couplable to the penetrating member driver. The penetrating members are movable to extend radially outward from the cartridge to penetrate tissue. A plurality of analyte sensors are coupled to the single cartridge and are positioned on the cartridge to receive body fluid from a wound in the tissue created by the penetrating member.
Owner:SANOFI AVENTIS DEUT GMBH
Method and apparatus for body fluid sampling and analyte sensing
InactiveUS20060241666A1Improve depth accuracyImprove cutting efficiencyIncision instrumentsBagsAnalyteEngineering
The method comprises providing a lancing device comprising a penetrating member (68) driver having a position sensor (74) and a processor (60) that can determine the relative position and velocity of the penetrating member (18, 72) based on measuring relative position of the penetrating member with respect to time; providing a predetermined velocity control trajectory based on a model of the driver (68) and a model of tissue to be contacted. Furthermore, a feedforward control is able to maintain penetrating member velocity along the trajectory.
Owner:PELIKAN TECH INC
In vivo biosensor apparatus and method of use
InactiveUS6673596B1Less can be administeredCost-effective administration of drugBioreactor/fermenter combinationsBiological substance pretreatmentsIn vivoGenetically engineered
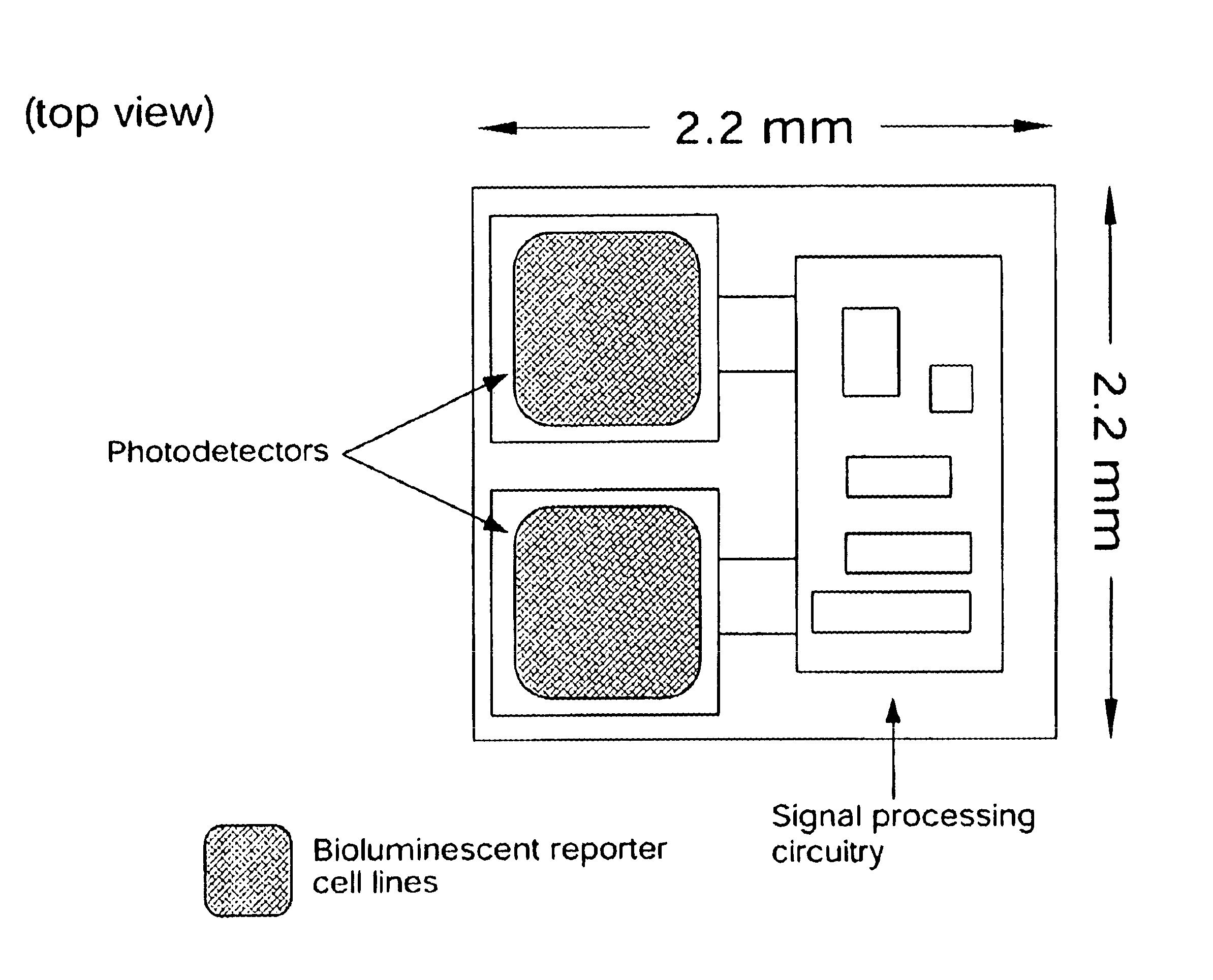
Disclosed are bioluminescent bioreporter integrated circuit devices that detect selected analytes in fluids when implanted in the body of an animal. The device comprises a bioreporter that has been genetically engineered to contain a nucleic acid segment that comprises a cis-activating response element that is responsive to the selected substance operably linked to a gene encoding a bioluminescent reporter polypeptide. In preferred embodiments, the target analyte is glucose, glucagons, or insulin. Exposure of the bioreporter to the target substance causes the response element to up-regulate the nucleic acid sequence encoding the reporter polypeptide to produce a luminescent response that is detected and quantitated. In illustrative embodiments, the bioreporter device is encapsulated on an integrated circuit that is capable of detecting the emitted light, processing the resultant signal, and then remotely reporting the results. Also disclosed are controlled drug delivery systems capable of being directly or indirectly controlled by the detection device that provide drugs such as insulin to the animal in reponse to the amount of target analyte present in the body fluids.
Owner:UNIV OF TENNESSEE RES FOUND +1
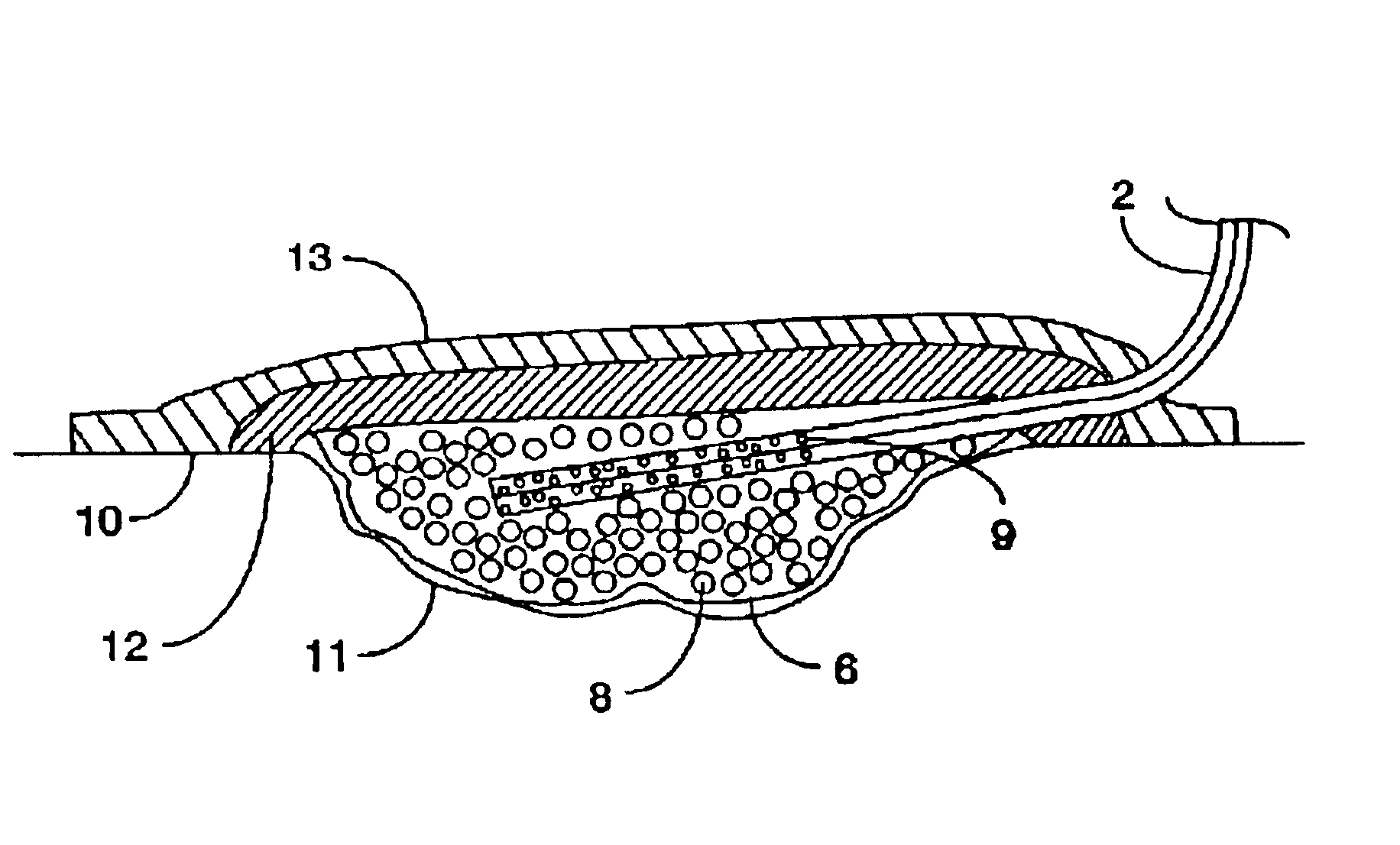
Closed wound drainage system
A portable closed wound drainage system that uses a pouch shaped dressing which is inserted into a wound. At least a portion of the outer surface of the pouch is porous to allow exudates to enter. Exudates are removed from the pouch by flexible tubing which is secured inside the pouch at one end, and secured at the other end to a portable drain / suction unit. The pouch contains porous material, and may optionally contain beads and fillers which are antibacterial in nature. The tubing can have a single or multi-lumen structure with perforations in the side walls of the end of the tube that is inserted in the pouch to allow body fluids to enter laterally. The portable drain / suction unit is preferably a portable battery powered device. The pouch and the tube are sealed by a flexible sealing material which is applied to the outer surface of the skin around the periphery of the pouch and the tubing as it exits the pouch. This sealing material is preferably a hydro-colloid, a silicone, or a lyogel, such as a hydrogel, which are easily deformable. A cosmetic cover sheet is attached to the patient's skin over the closed wound drainage system.
Owner:CONVATEC LTD
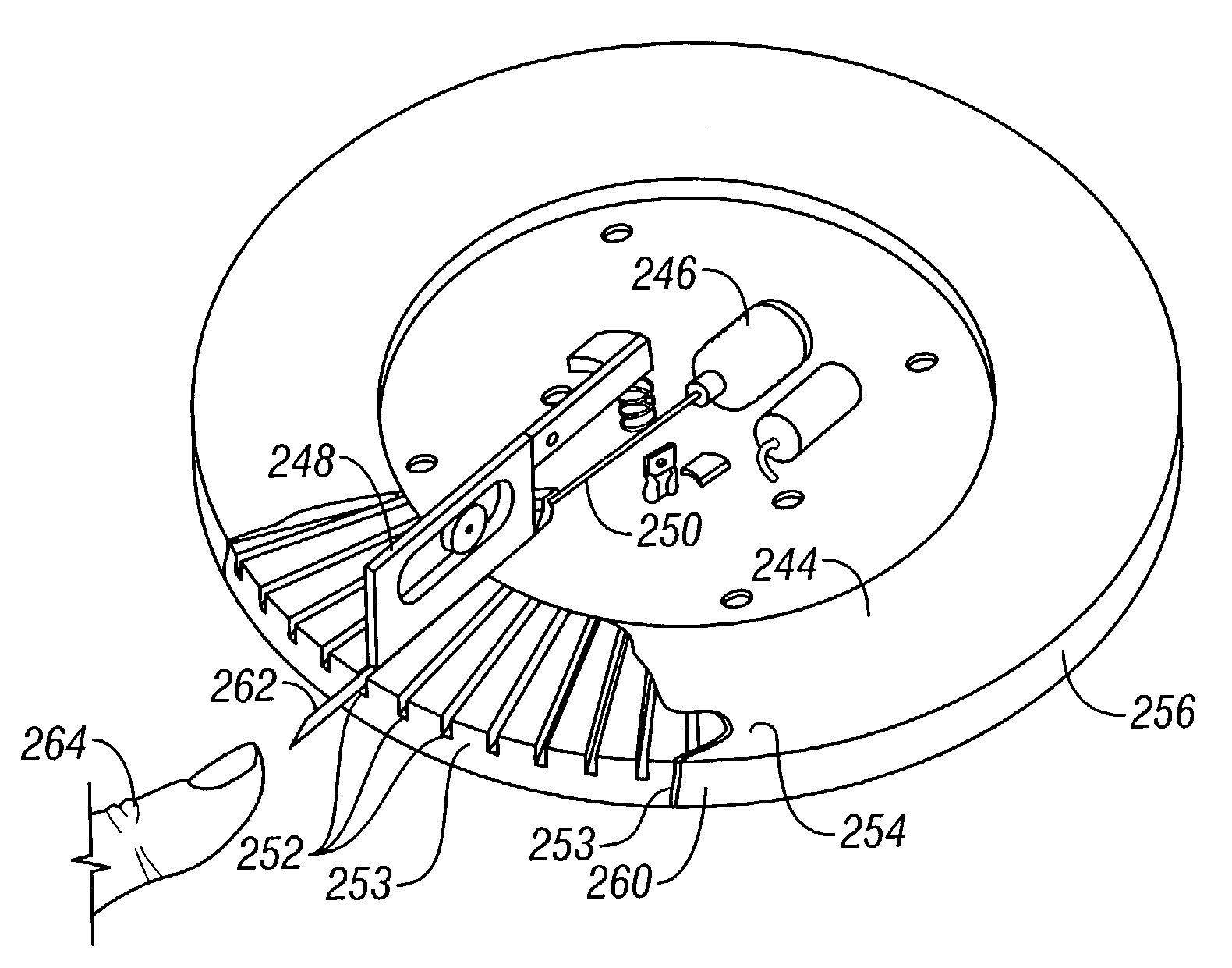
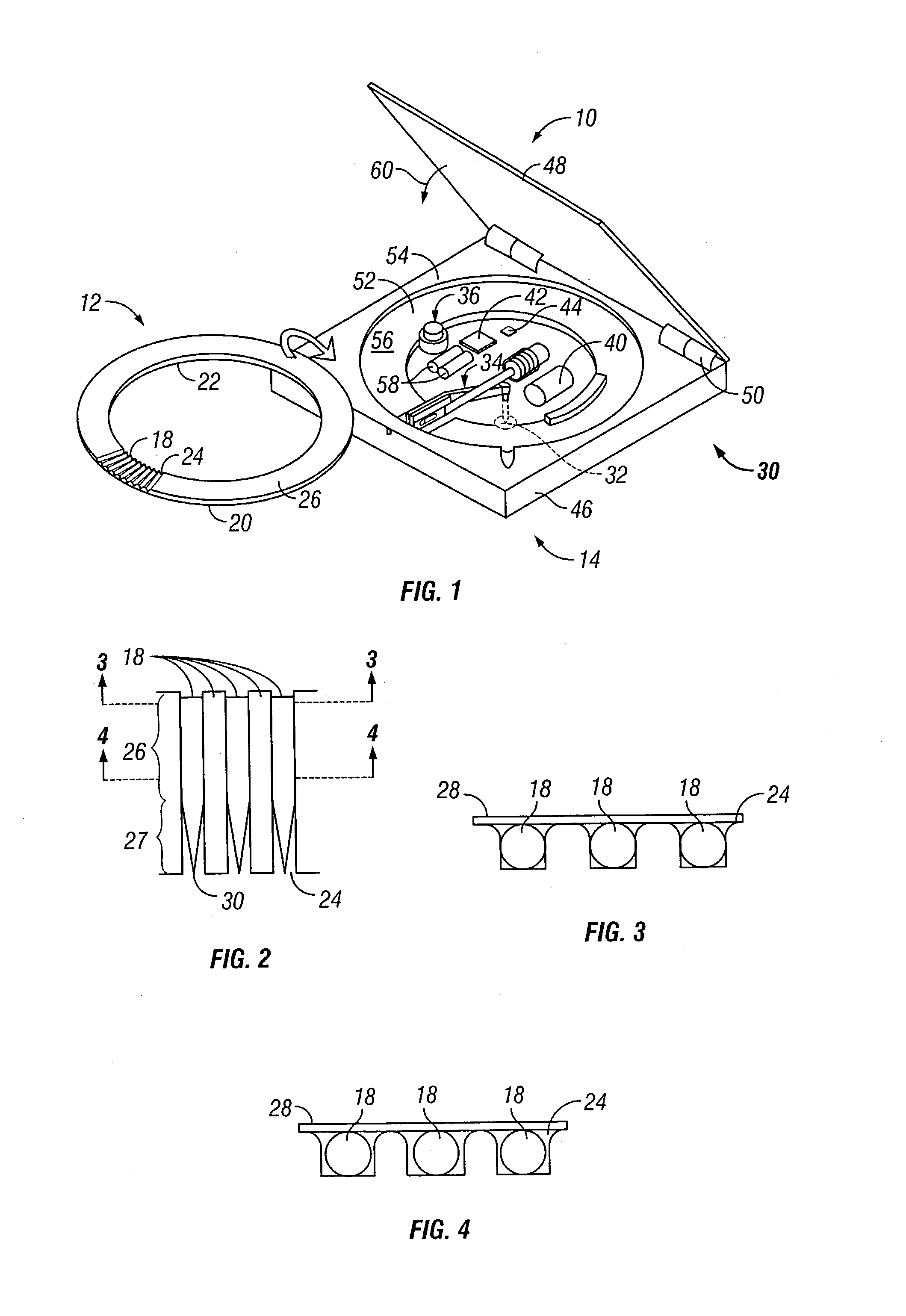
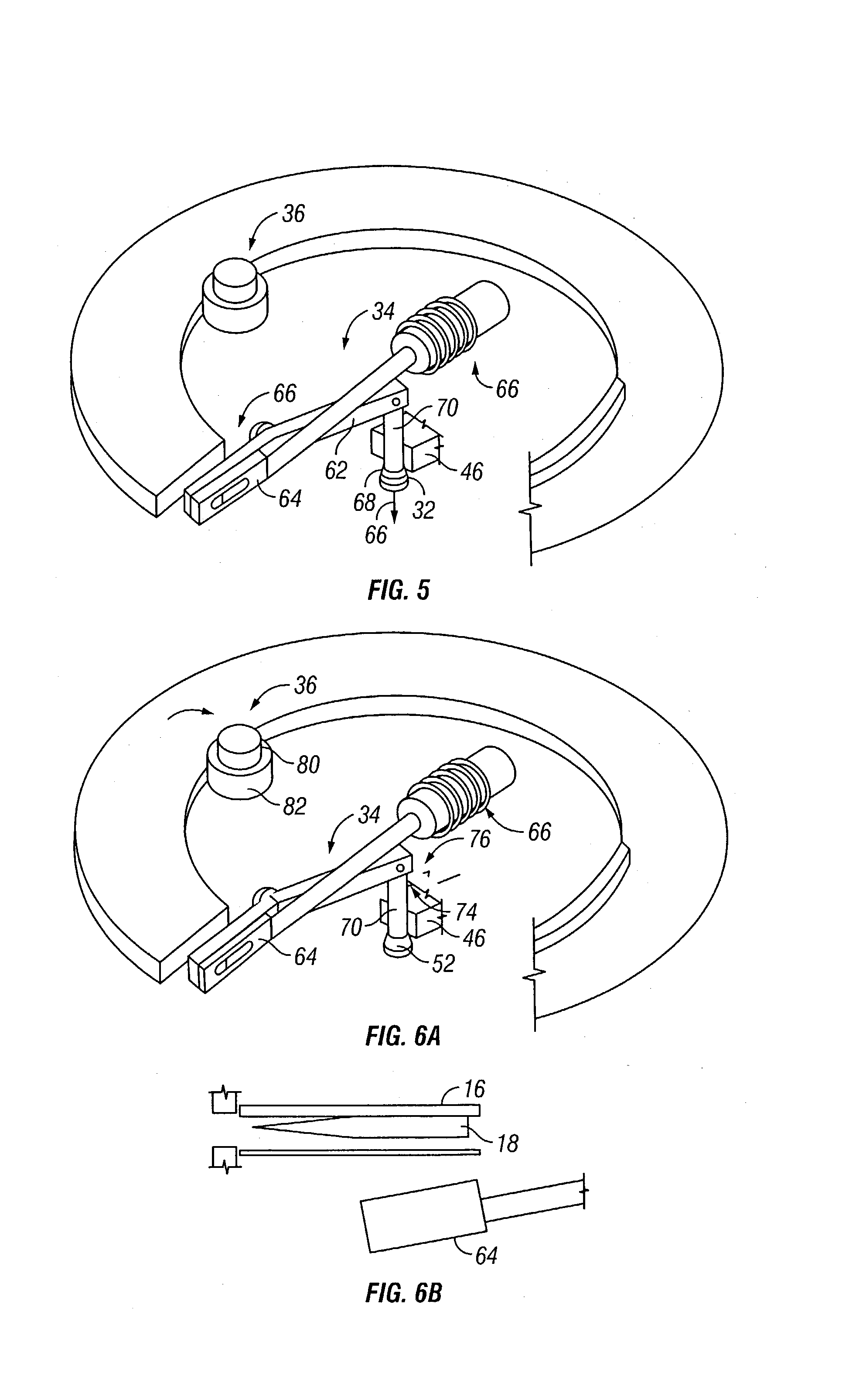
Assembly having lancet and means for collecting and detecting body fluid
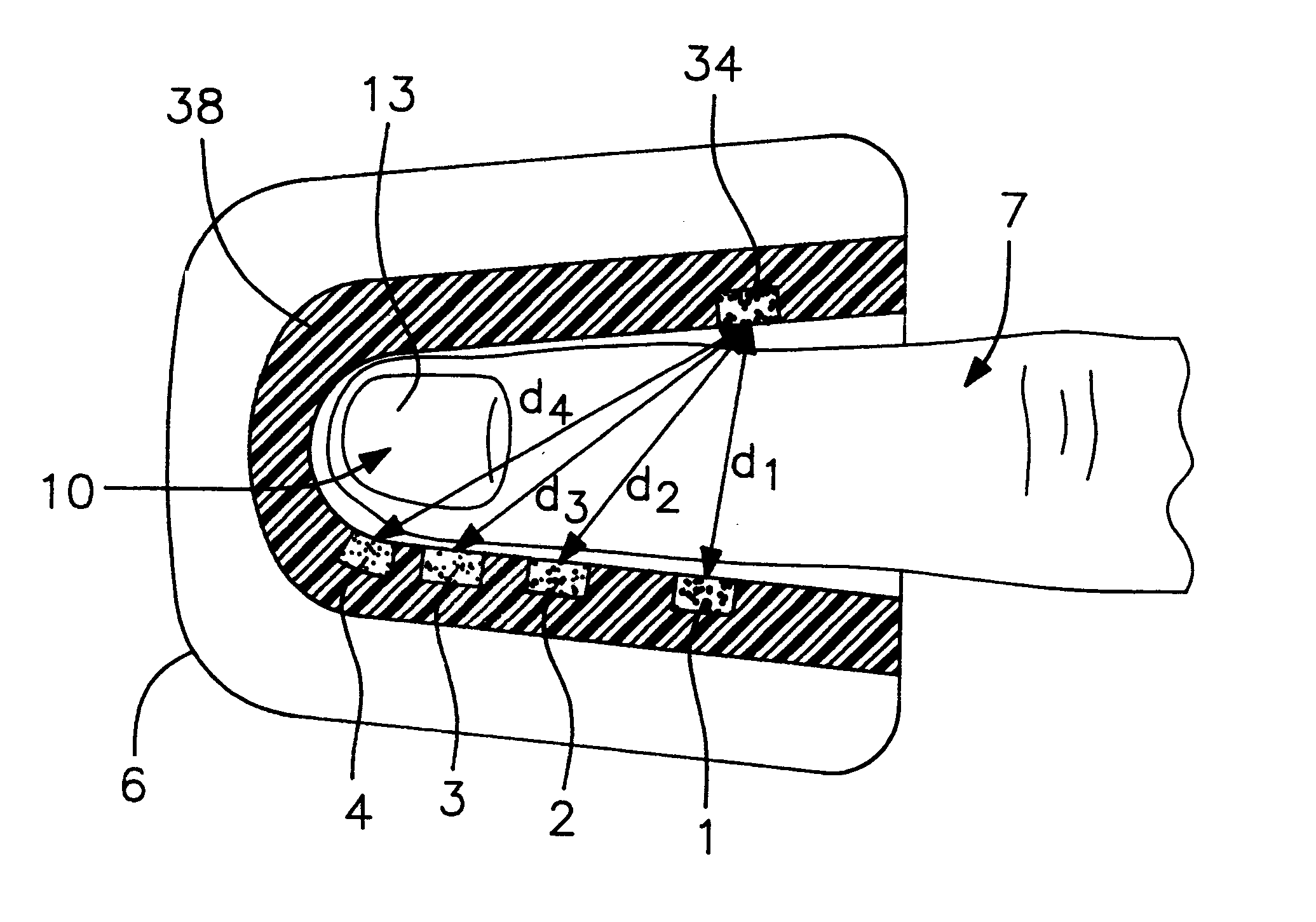
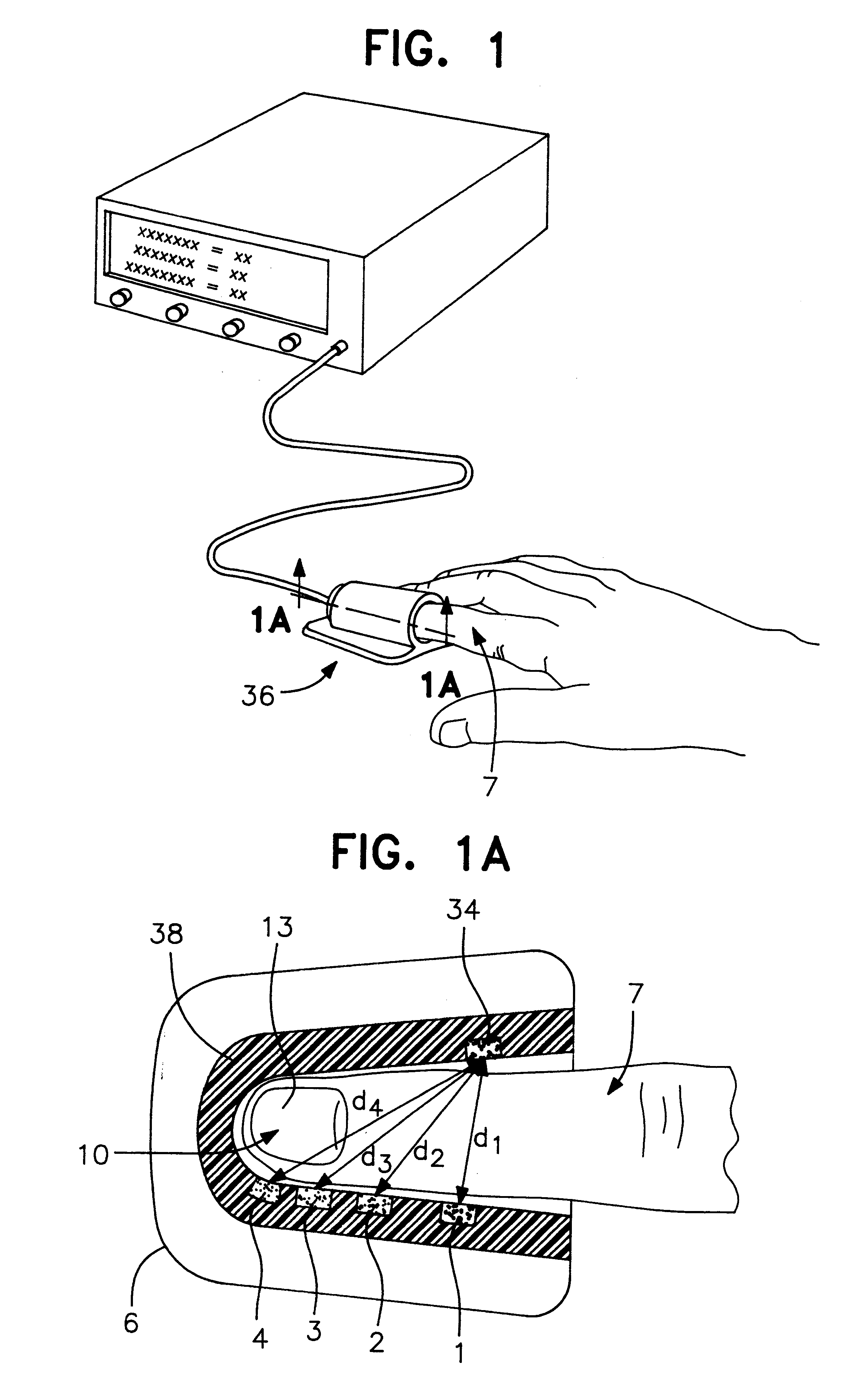
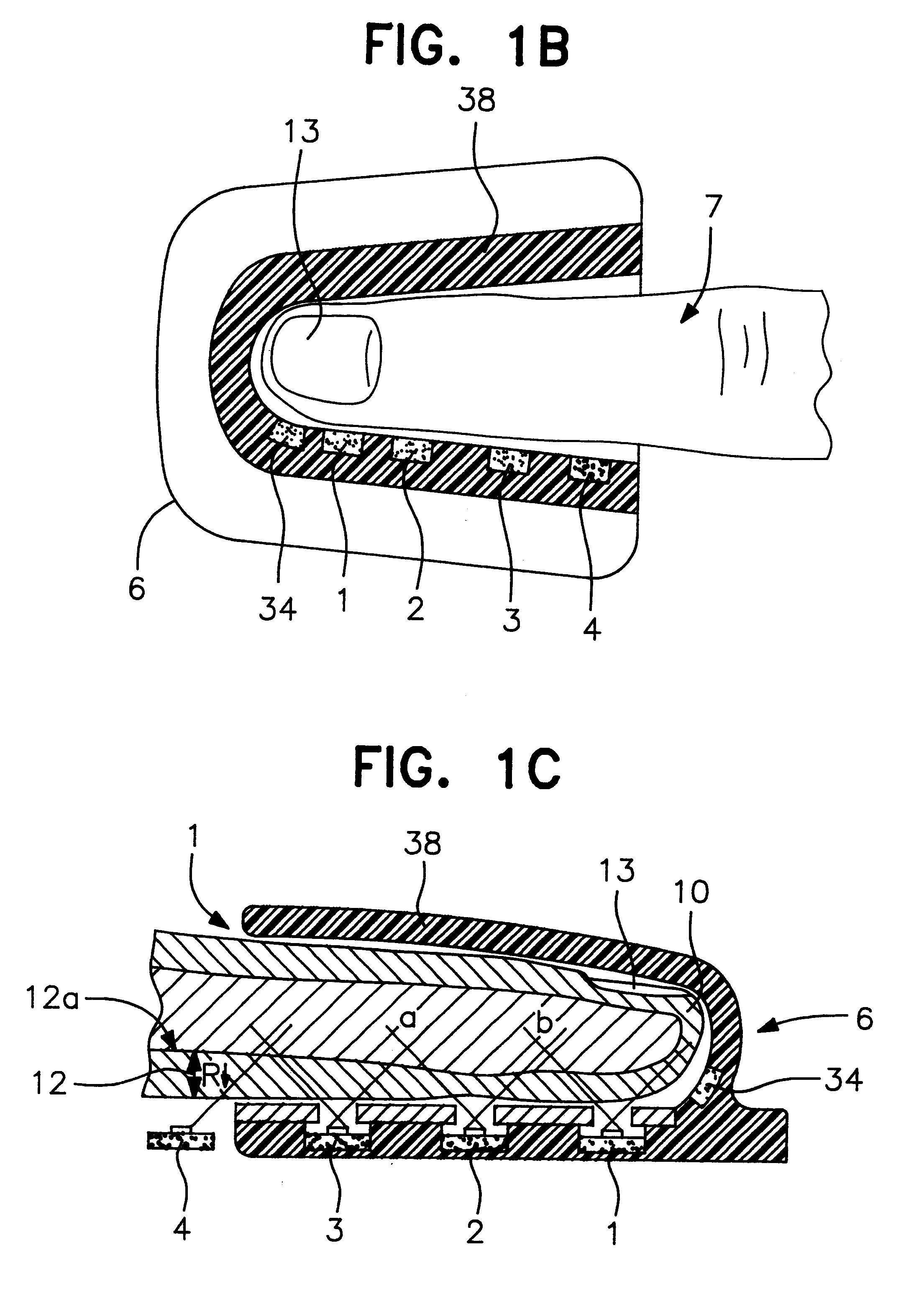
An assembly to be detachably mounted on a body fluid monitoring system is provided. The assembly has a lancet and a device for collecting and detecting a body fluid. The lancet has a puncture needle. In this assembly, the puncture needle is maintained in sterilized conditions until its use, and the sterilization can be conducted with no adverse effects on the detection device. A readily sterilizable lancet unit and a body fluid-collecting and detecting unit adapted for use in such an assembly as well as a body fluid-monitoring system including such an assembly are also provided. The assembly comprises a first housing having a sleeve which movably accommodates the lancet in its interior, and a second housing having the body fluid detection device. The first housing and the second housing share an opening. The lancet is sterilized before the assembly. The body fluid-collecting and detecting section has a body fluid guide on the periphery of the inlet.
Owner:TERUMO KK
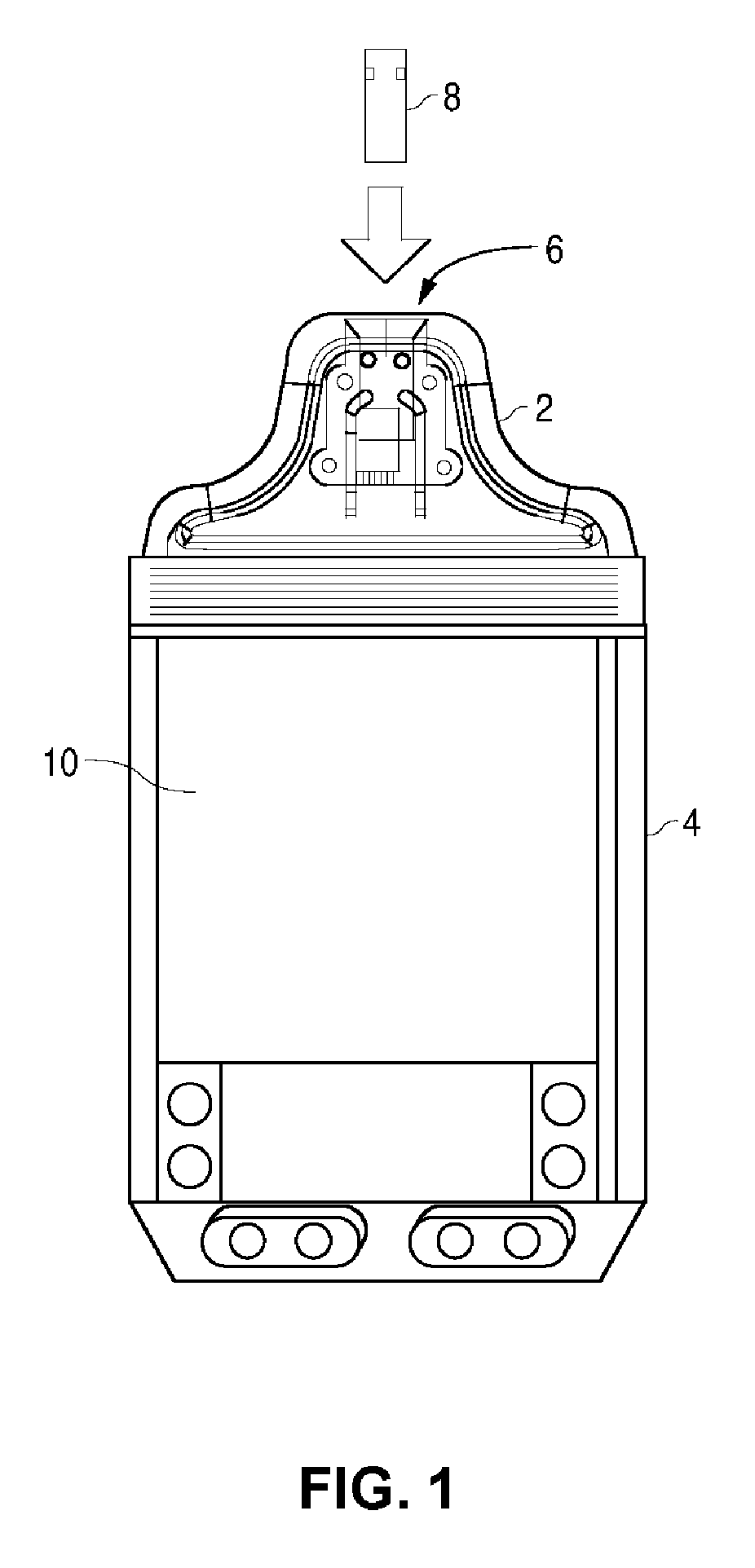
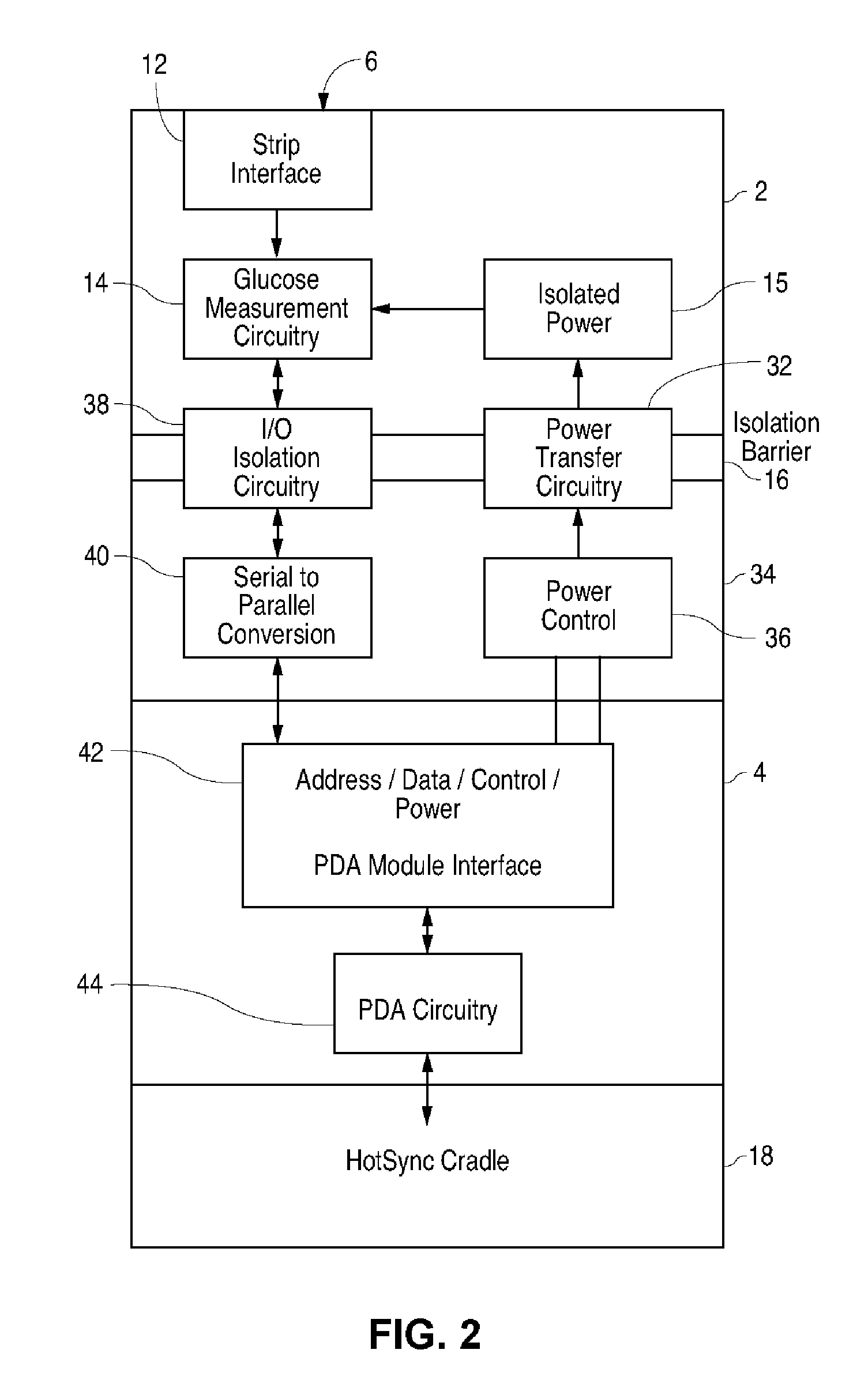
Blood glucose tracking apparatus and methods
ActiveUS20050239156A1Easy and precise applicationEfficient use ofBioreactor/fermenter combinationsBiological substance pretreatmentsHand heldComputer module
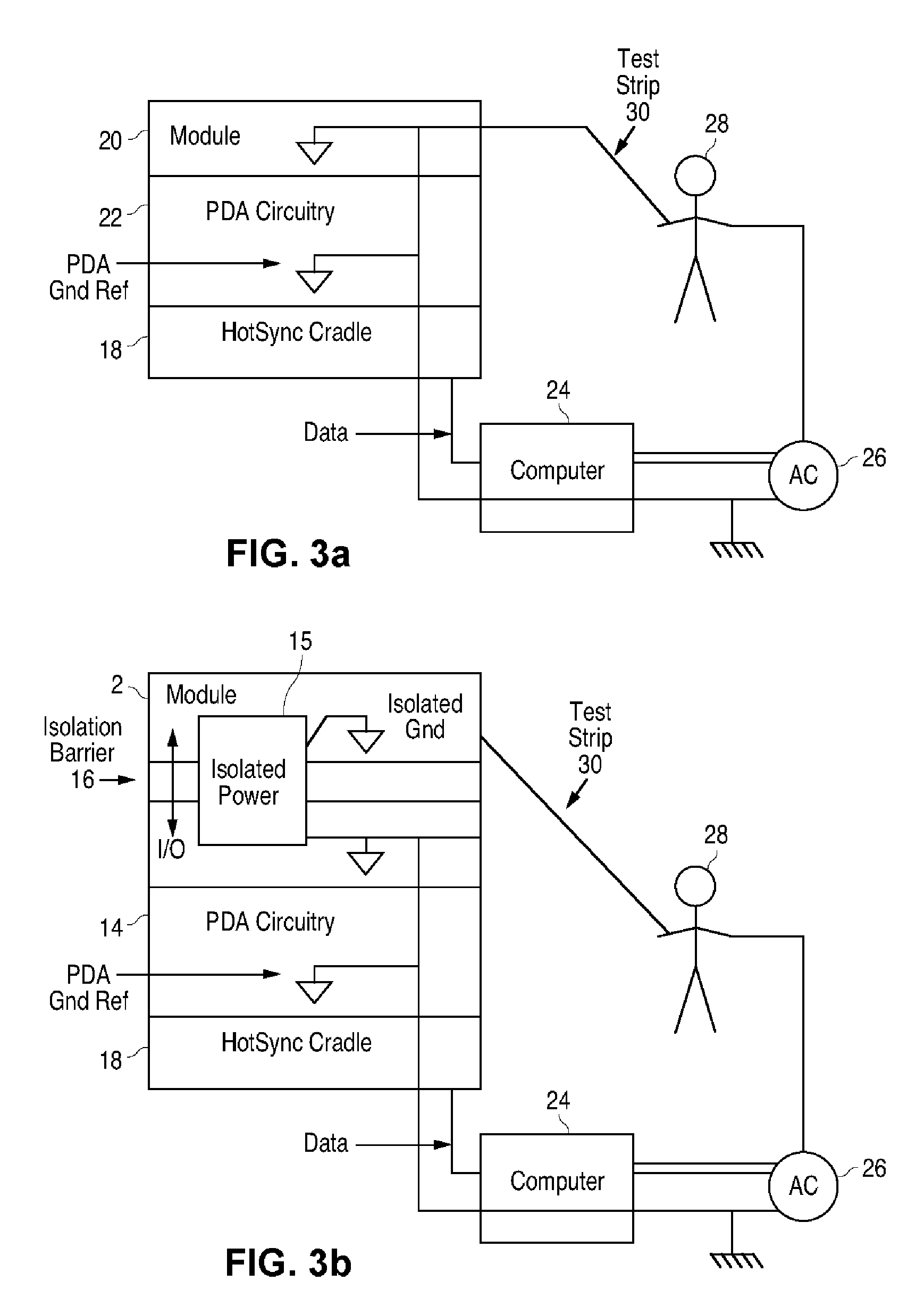
A measurement module for glucose testing includes a glucose testing measurement module housing, a test strip receptacle formed in the housing, and a connector portion formed in the housing and shaped to permit mechanical removable attachment of the housing to a hand-held computer. Electronics determine the amount of glucose present in a sample of body fluid, when the test strip is positioned in the receptacle and the body fluid is placed on a test strip, and communicate the glucose amount to the hand-held computer via the connector portion.
Owner:ABBOTT DIABETES CARE INC
Microneedle device for extraction and sensing of bodily fluids
InactiveUS7344499B1Simple wayMinimal and no damageAdditive manufacturing apparatusMicroneedlesMetaboliteIrritation
Microneedle devices are provided for controlled sampling of biological fluids in a minimally-invasive, painless, and convenient manner. The microneedle devices permit in vivo sensing or withdrawal of biological fluids from the body, particularly from or through the skin or other tissue barriers, with minimal or no damage, pain, or irritation to the tissue. The microneedle device includes one or more microneedles, preferably in a three-dimensional array, a substrate to which the microneedles are connected, and at least one collection chamber and / or sensor in communication with the microneedles. Preferred embodiments further include a means for inducing biological fluid to be drawn through the microneedles and into the collection chamber for analysis. In a preferred embodiment, this induction is accomplished by use of a pressure gradient, which can be created for example by selectively increasing the interior volume of the collection chamber, which includes an elastic or movable portion engaged to a rigid base. Preferred biological fluids for withdrawal and / or sensing include blood, lymph, interstitial fluid, and intracellular fluid. Examples of analytes in the biological fluid to be measured include glucose, cholesterol, bilirubin, creatine, metabolic enzymes, hemoglobin, heparin, clotting factors, uric acid, carcinoembryonic antigen or other tumor antigens, reproductive hormones, oxygen, pH, alcohol, tobacco metabolites, and illegal drugs.
Owner:GEORGIA TECH RES CORP +1
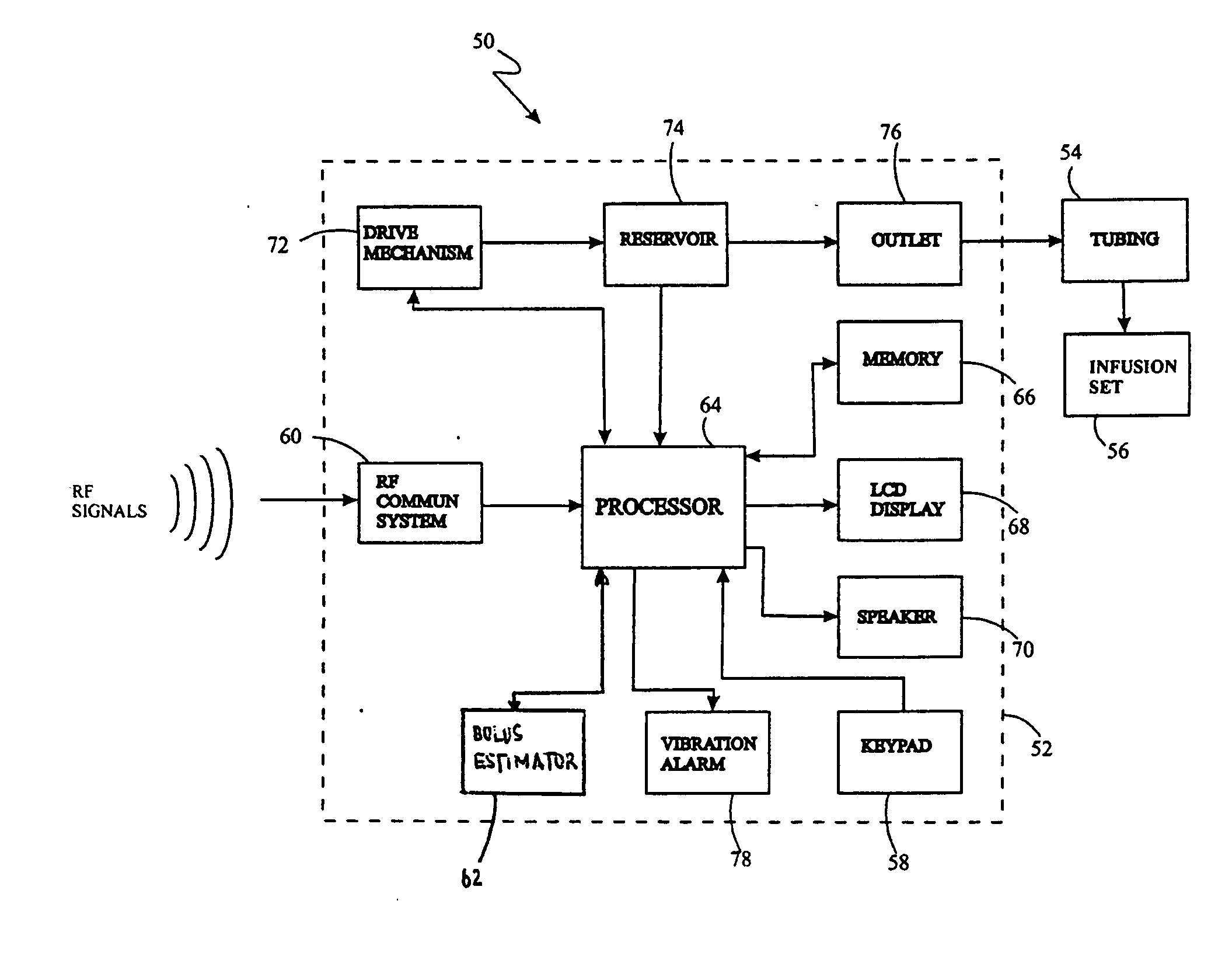
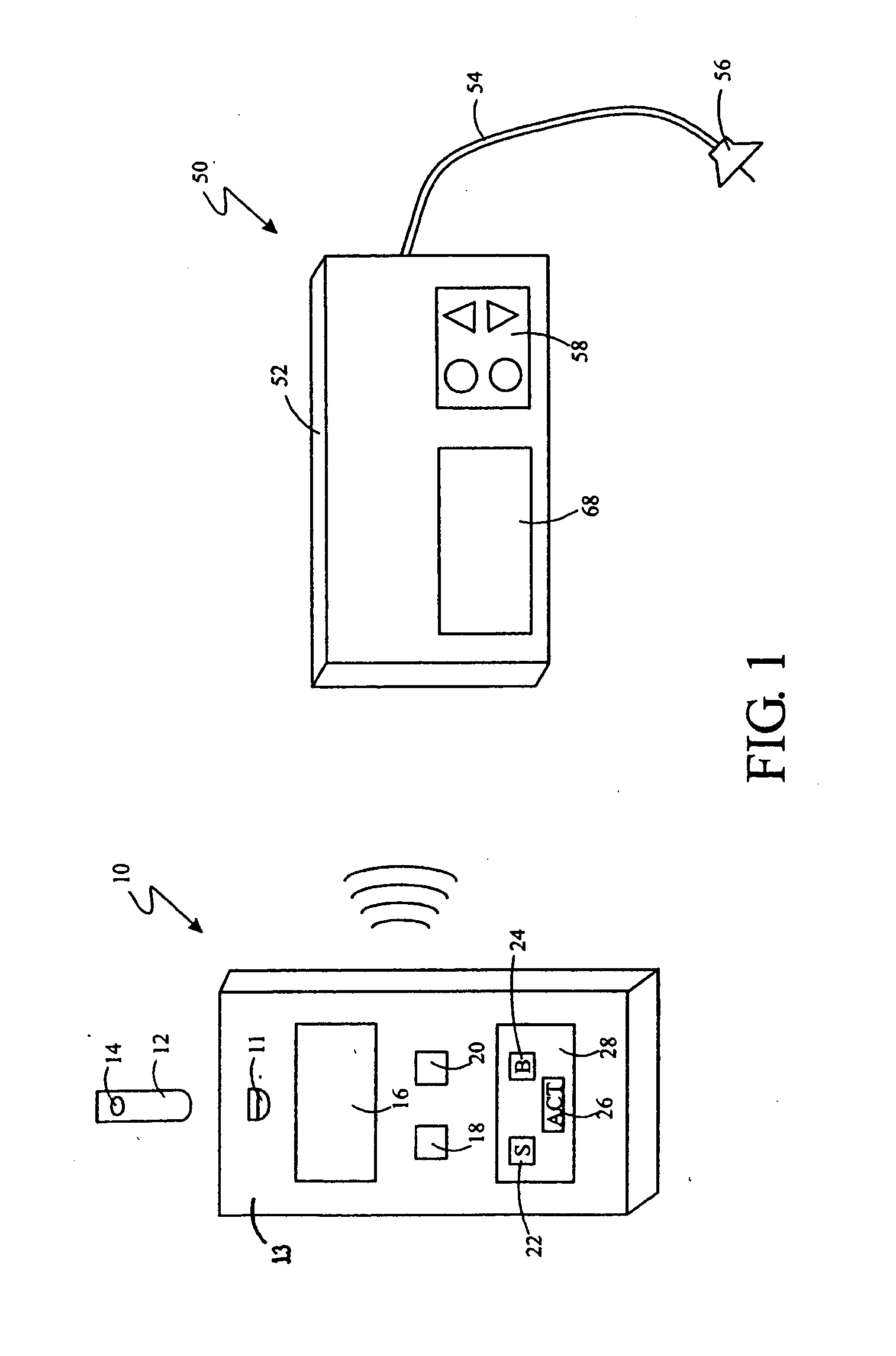
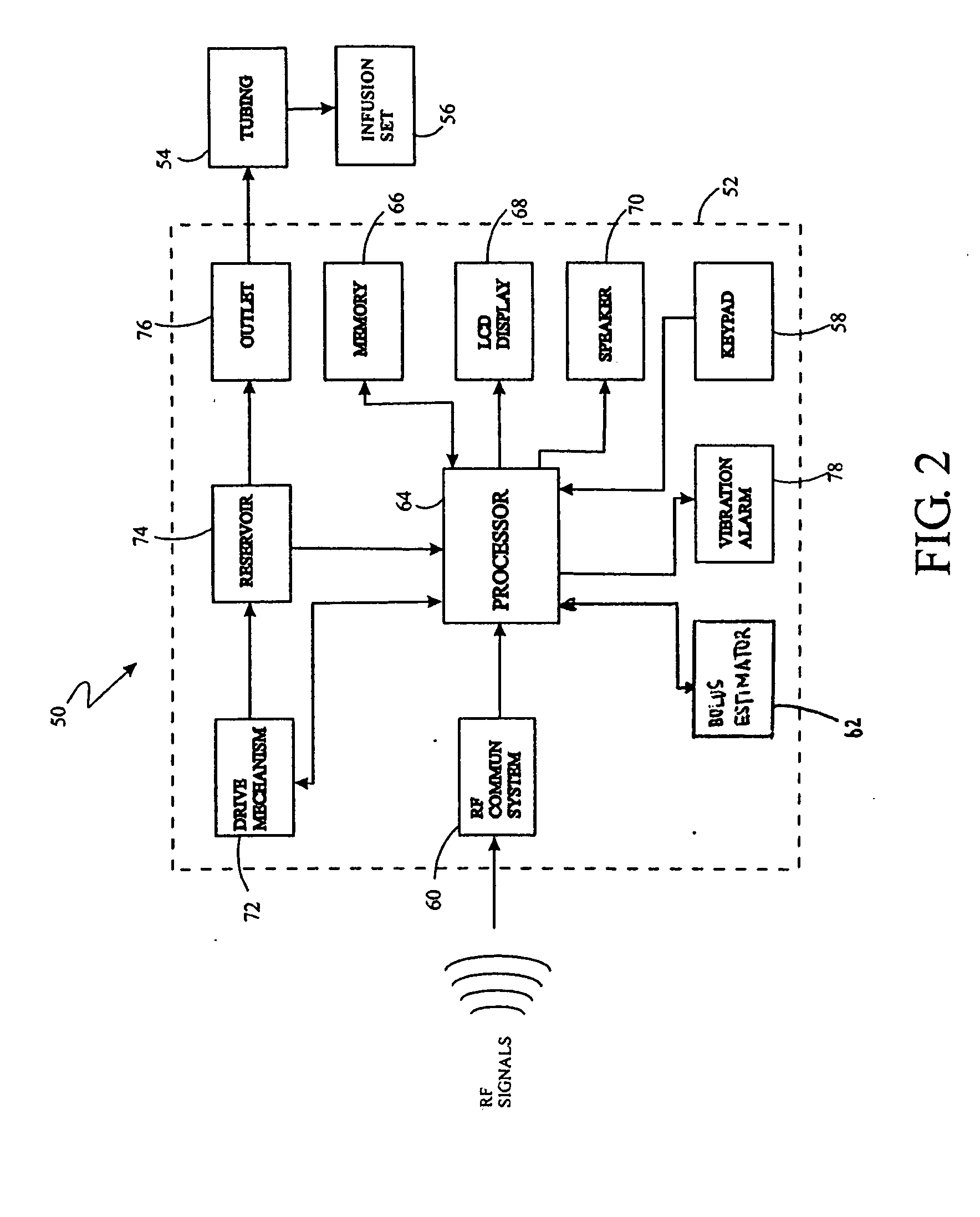
System for providing blood glucose measurements to an infusion device
An infusion system includes an infusion device and a sensing device. The infusion system may further include a characteristic determining device. The infusion device also includes a communication system for transmitting to and receiving communications from the sensing device or a computer. The sensing device may sense an analyte of a bodily fluid of the user. The analyte may be calibrated using data from the infusion device and from a characteristic determining device. The system may be set up to automatically call for assistance when analytes reach a certain level.
Owner:MEDTRONIC MIMIMED INC
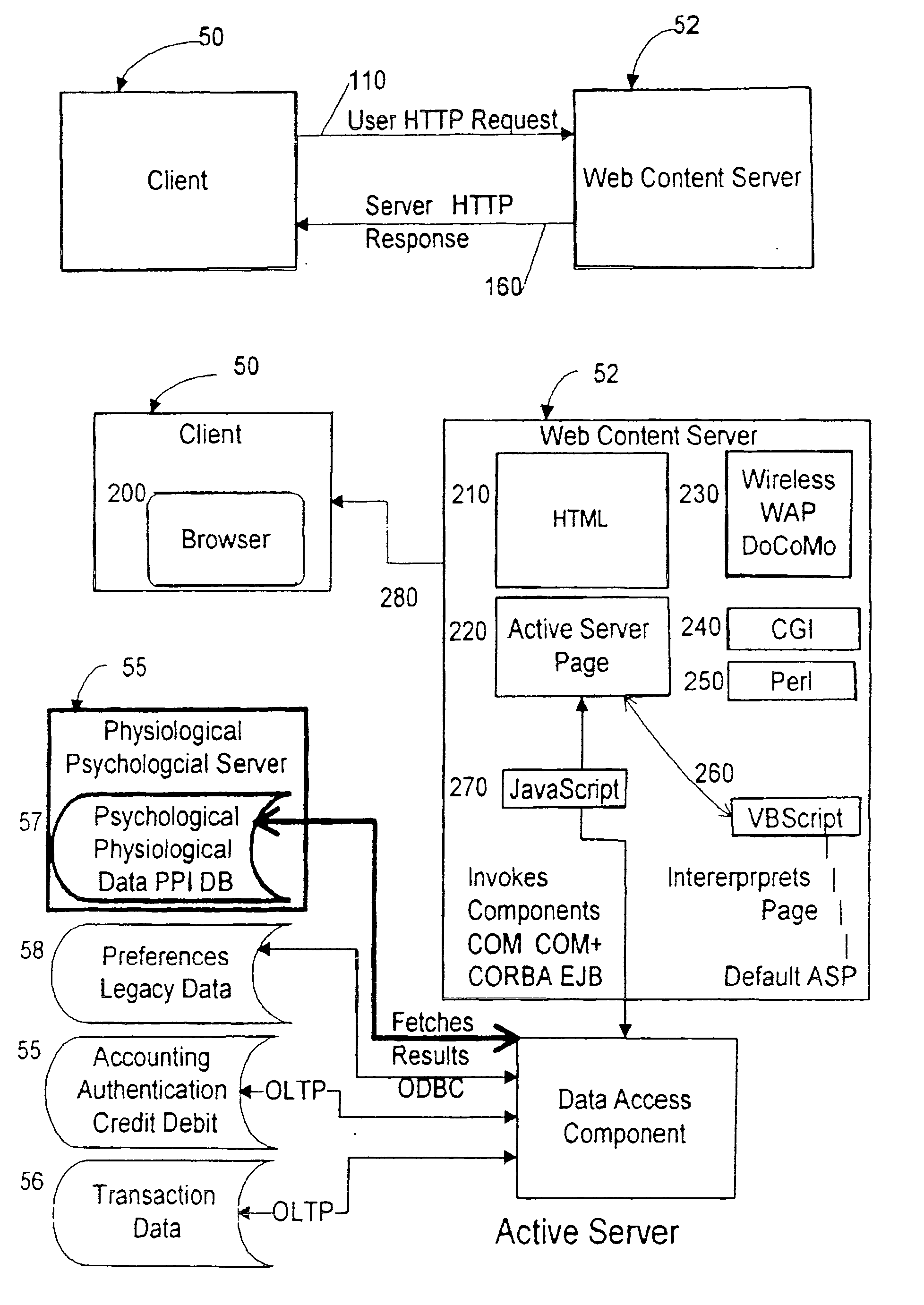
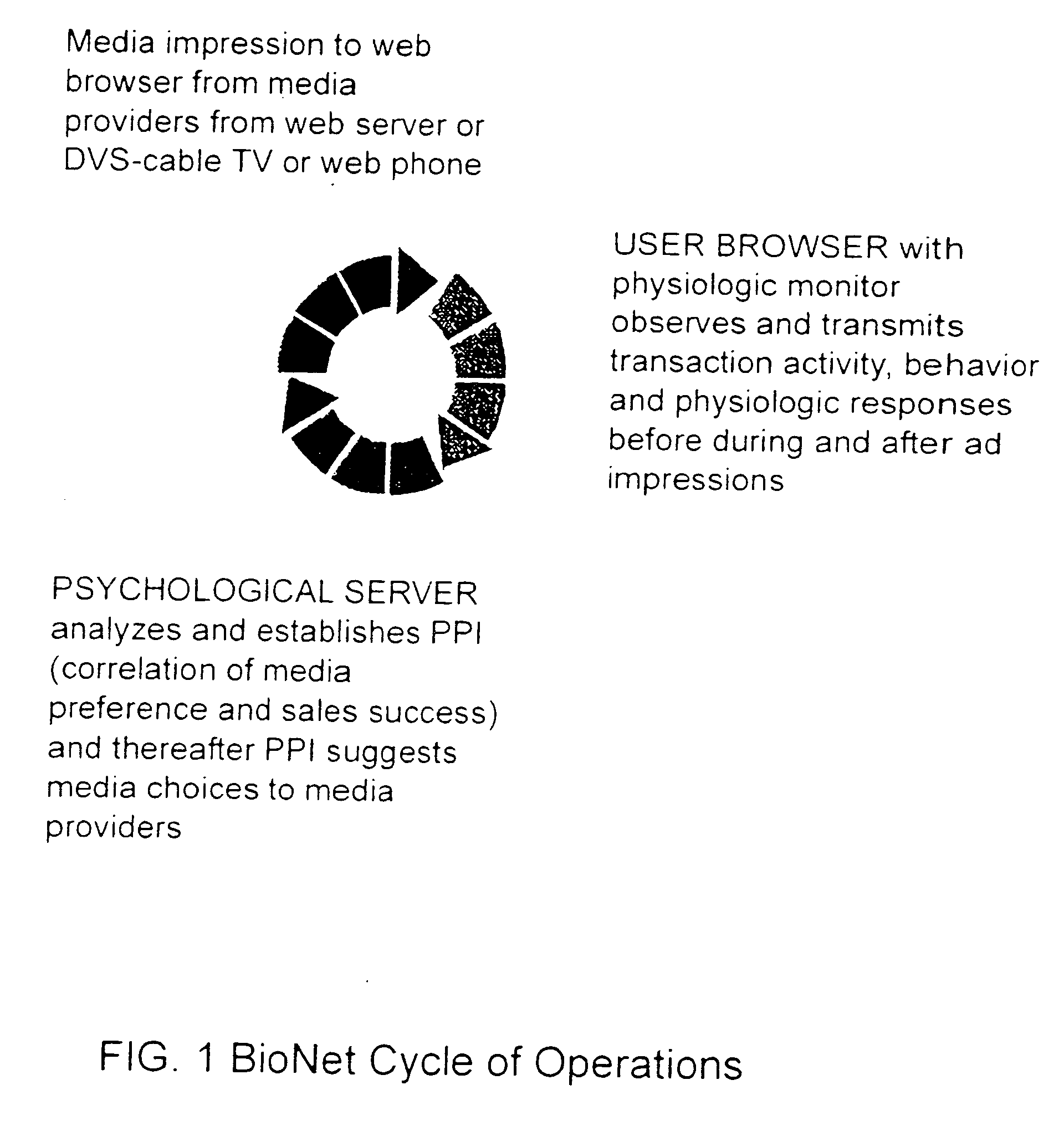
Input device for web content manager responsive to browser viewers' psychological preferences, behavioral responses and physiological stress indicators
InactiveUS20060293921A1Easy and efficient to manufactureLow costDiagnostic recording/measuringSensorsPulse oximetersBehavioral response
Owner:MCCARTHY JOHN +1
Blood glucose tracking apparatus and methods
ActiveUS20050277164A1Easy and precise applicationEfficient use ofBioreactor/fermenter combinationsBiological substance pretreatmentsHand heldComputer module
Owner:ABBOTT DIABETES CARE INC
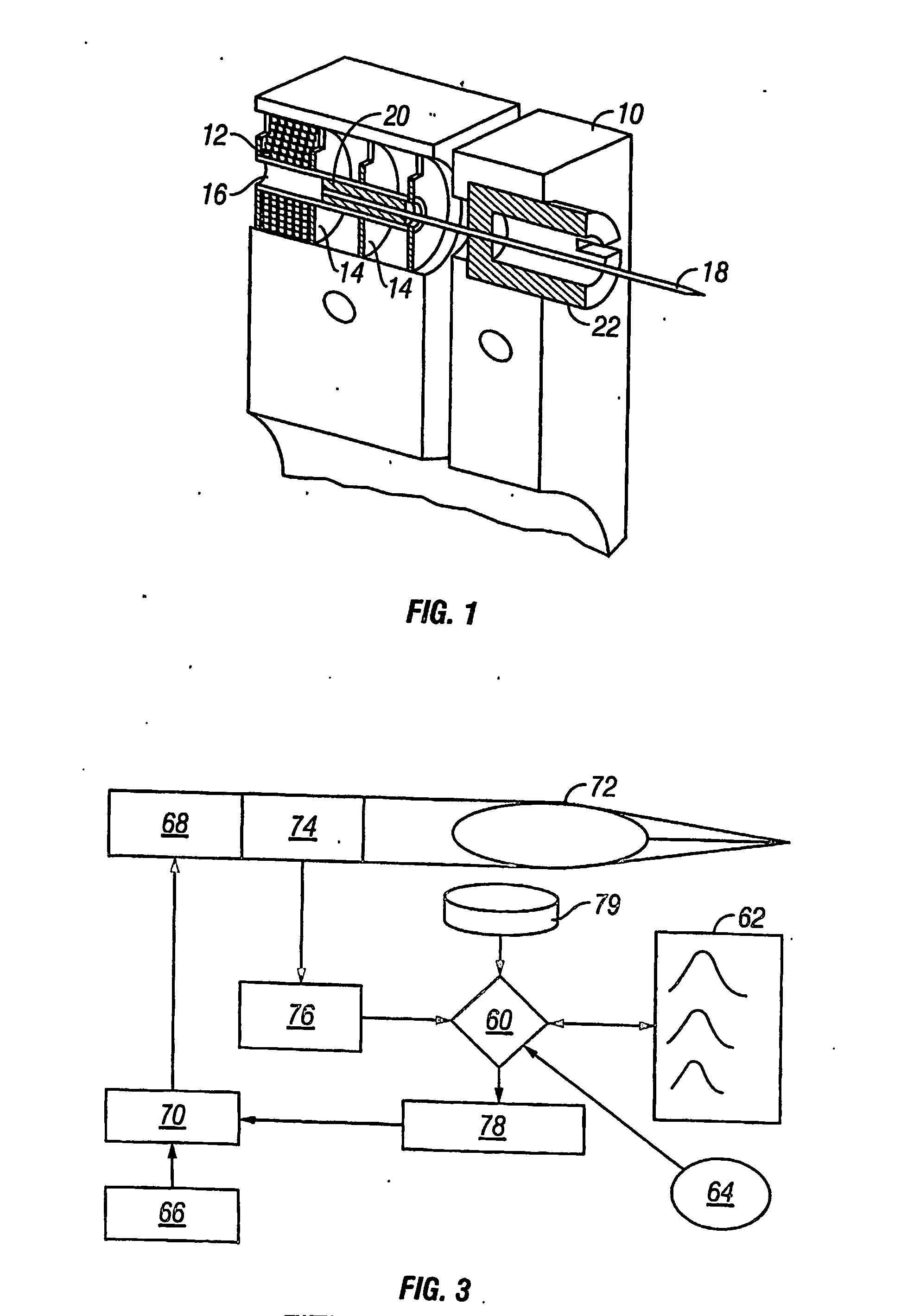
Method and apparatus for non-invasive blood constituent monitoring
InactiveUS6181958B1Repeatable and reliableEasy to implementSensorsBlood characterising devicesNon invasiveHemoglobin G Szuhu
A system for determining a biologic constituent including hematocrit transcutaneously, noninvasively and continuously. A finger clip assembly includes including at least a pair of emitters and a photodiode in appropriate alignment to enable operation in either a transmissive mode or a reflectance mode. At least one predetermined wavelength of light is passed onto or through body tissues such as a finger, earlobe, or scalp, etc. and attenuation of light at that wavelength is detected. Likewise, the change in blood flow is determined by various techniques including optical, pressure, piezo and strain gage methods. Mathematical manipulation of the detected values compensates for the effects of body tissue and fluid and determines the hematocrit value. If an additional wavelength of light is used which attenuates light substantially differently by oxyhemoglobin and reduced hemoglobin, then the blood oxygen saturation value, independent of hematocrit may be determined. Further, if an additional wavelength of light is used which greatly attenuates light due to bilirubin (440 nm) or glucose (1060 nm), then the bilirubin or glucose value may also be determined. Also how to determine the hematocrit with a two step DC analysis technique is provided. Then a pulse wave is not required, so this method may be utilized in states of low blood pressure or low blood flow.
Owner:HEMA METRICS
Seals and sealing methods for a surgical instrument having an articulated end effector actuated by a drive shaft
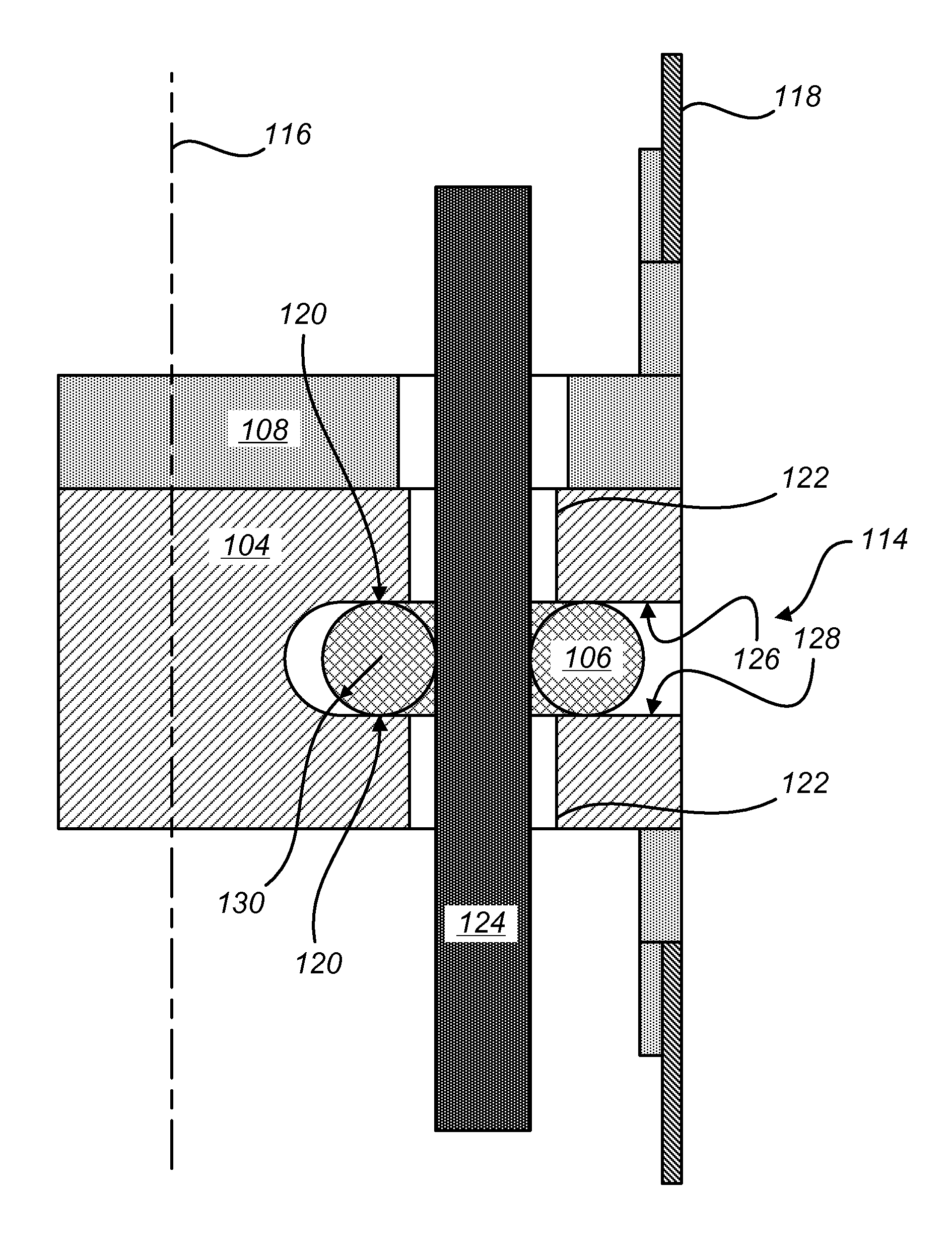
Sealing assemblies and methods are disclosed for sealing a surgical instrument having an internal drive shaft subject to lateral displacement. A sealing assembly includes a rigid portion shaped to interface with an instrument shaft of the surgical instrument. A laterally oriented slot is open at a radially perimeter location and configured to receive an o-ring seal via the perimeter location. Apertures are disposed on opposing sides of the slot and open to the slot. The apertures are configured to receive the drive shaft there through and are larger than the drive shaft to accommodate lateral displacement of the drive shaft. The slot includes opposing internal sides spaced to interface with opposed axial surfaces of the o-ring seal. The seal inhibits axial transmission of an insufflated gas and / or bodily fluids while accommodating lateral displacement of the drive shaft.
Owner:INTUITIVE SURGICAL OPERATIONS INC
Indwelling heat exchange catheter and method of using same
A catheter is adapted to exchange heat with a body fluid, such as blood, flowing in a body conduit, such as a blood vessel. The catheter includes a shaft with a heat exchange region disposed at its distal end. This region may include hollow fibers which are adapted to receive a remotely cooled heat exchange fluid preferably flowing in a direction counter to that of the body fluid. The hollow fibers enhance the surface area of contact, as well as the mixing of both the heat exchange fluid and the body fluid. The catheter can be positioned to produce hypothermia in a selective area of the body or alternatively positioned to systemically cool the entire body system.
Owner:ZOLL CIRCULATION +1
Methods of determining concentration of glucose
InactiveUS7058437B2Lower the volumePrecise processMicrobiological testing/measurementMaterial analysis by electric/magnetic meansAnalyteMedicine
A region of skin, other than the fingertips, is stimulated. After stimulation, an opening is created in the skin (e.g., by lancing the skin) to cause a flow of body fluid from the region. At least a portion of this body fluid is transported to a testing device where the concentration of analyte (e.g., glucose) in the body fluid is then determined. It is found that the stimulation of the skin provides results that are generally closer to the results of measurements from the fingertips, the traditional site for obtaining body fluid for analyte testing.
Owner:ABBOTT DIABETES CARE INC
Indwelling heat exchange catheter and method of using same
A catheter is adapted to exchange heat with a body fluid, such as blood, flowing in a body conduit, such as a blood vessel. The catheter includes a shaft with a heat exchange region disposed at its distal end. This region may include at least one balloon which is adapted to receive a remotely cooled heat exchange fluid preferably flowing in a direction counter to that of the body fluid. Embodiments including multiple balloons enhance the surface area of contact, and the mixing of both the heat exchange and the body fluid. The catheter can be positioned to produce hypothermia in a selective area of the body without cooling the entire body system. It is of particular advantage in brain surgeries where stroke, trauma or cryogenic tumors can best be addressed under hypothermic conditions.
Owner:ZOLL CIRCULATION +1
Consolidated body fluid testing device and method
A body fluid testing device includes a body member and a tissue penetrator carried by the body member. A test strip holder is carried by the body member, and a test strip is carried by the test strip holder. The test strip is capable of receiving a body fluid thereon and processing the body fluid into a form suitable for yielding test results relating to the content of the body fluid.
Owner:KLOEPFER DR HANS
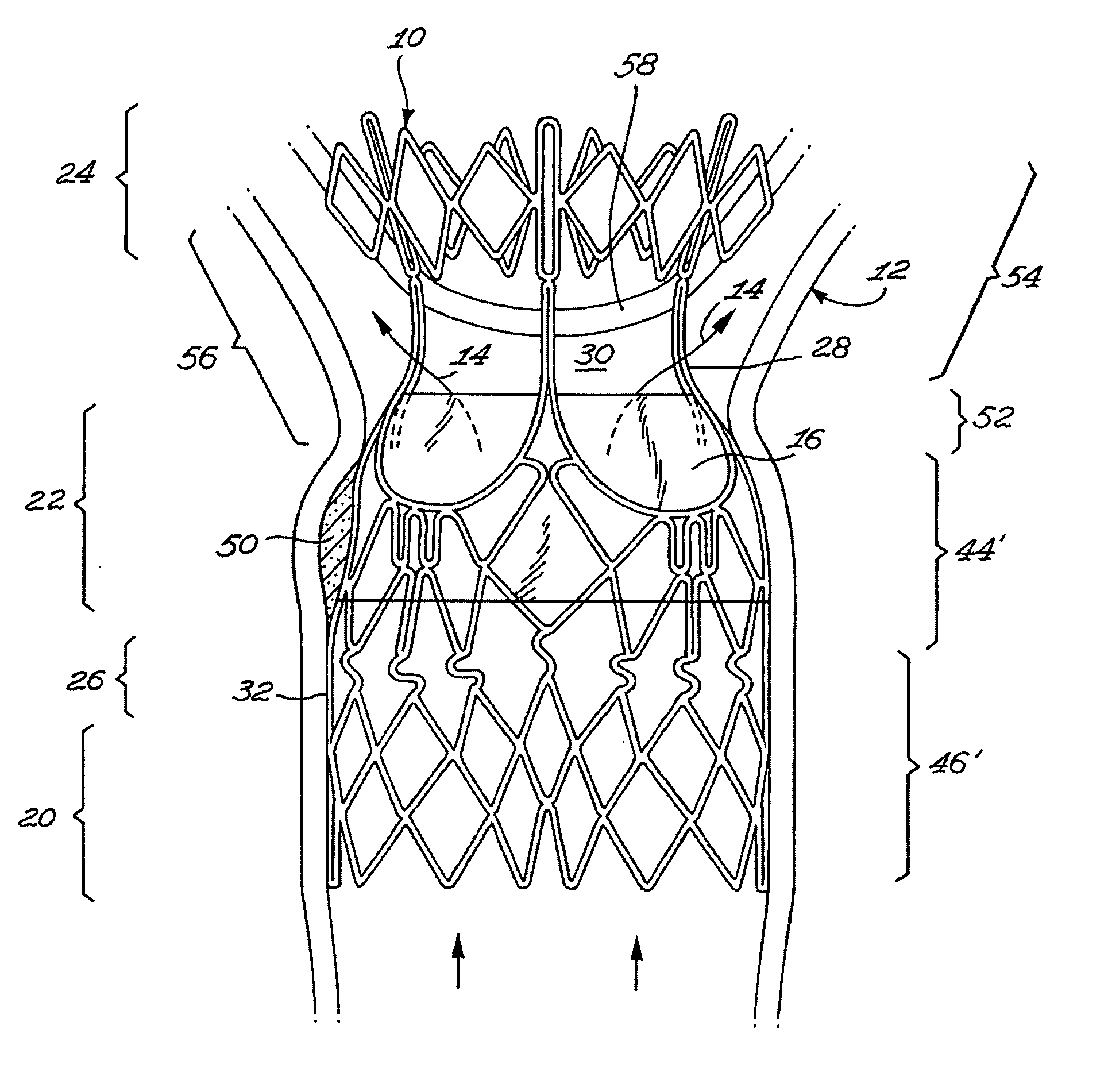
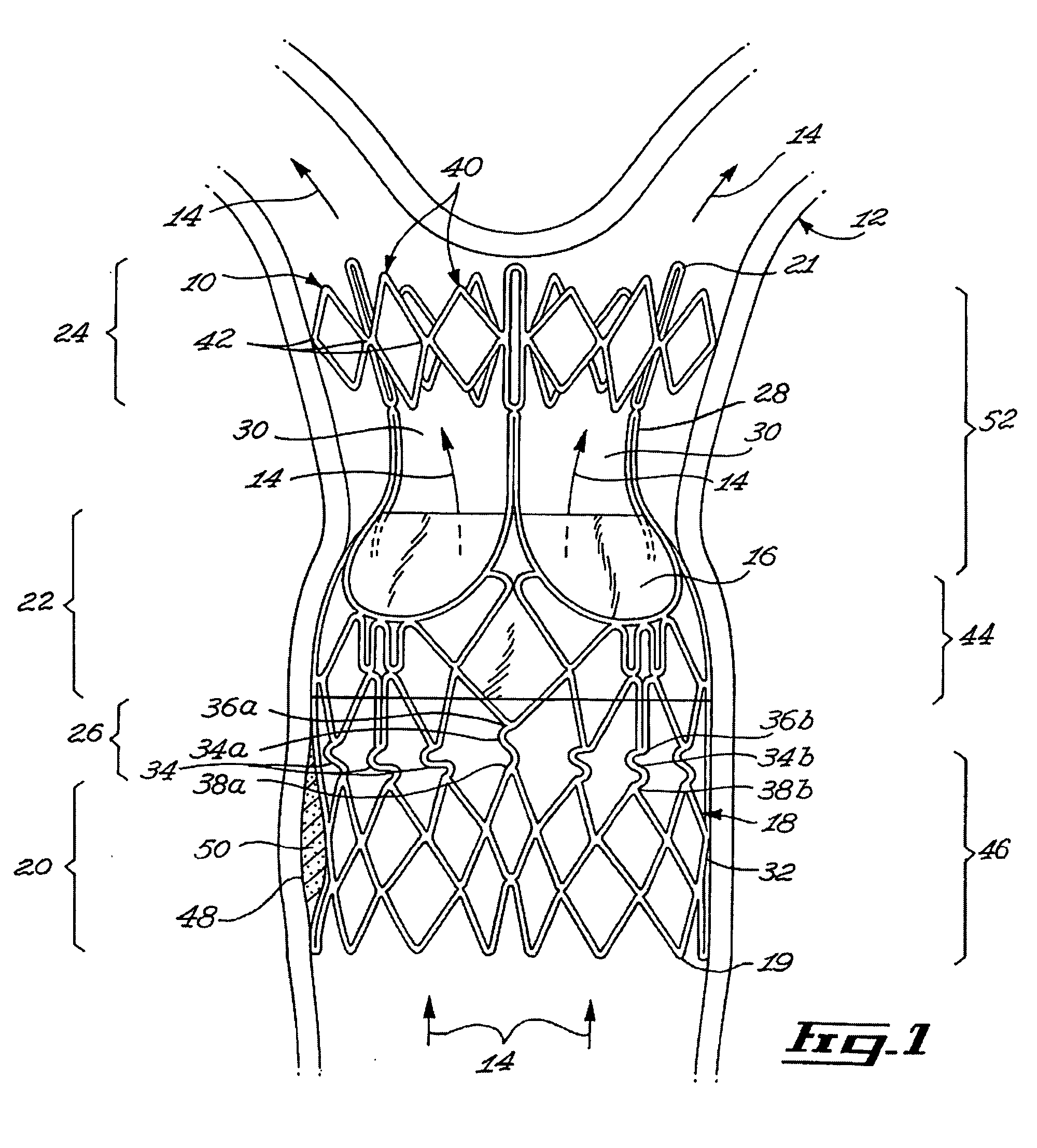
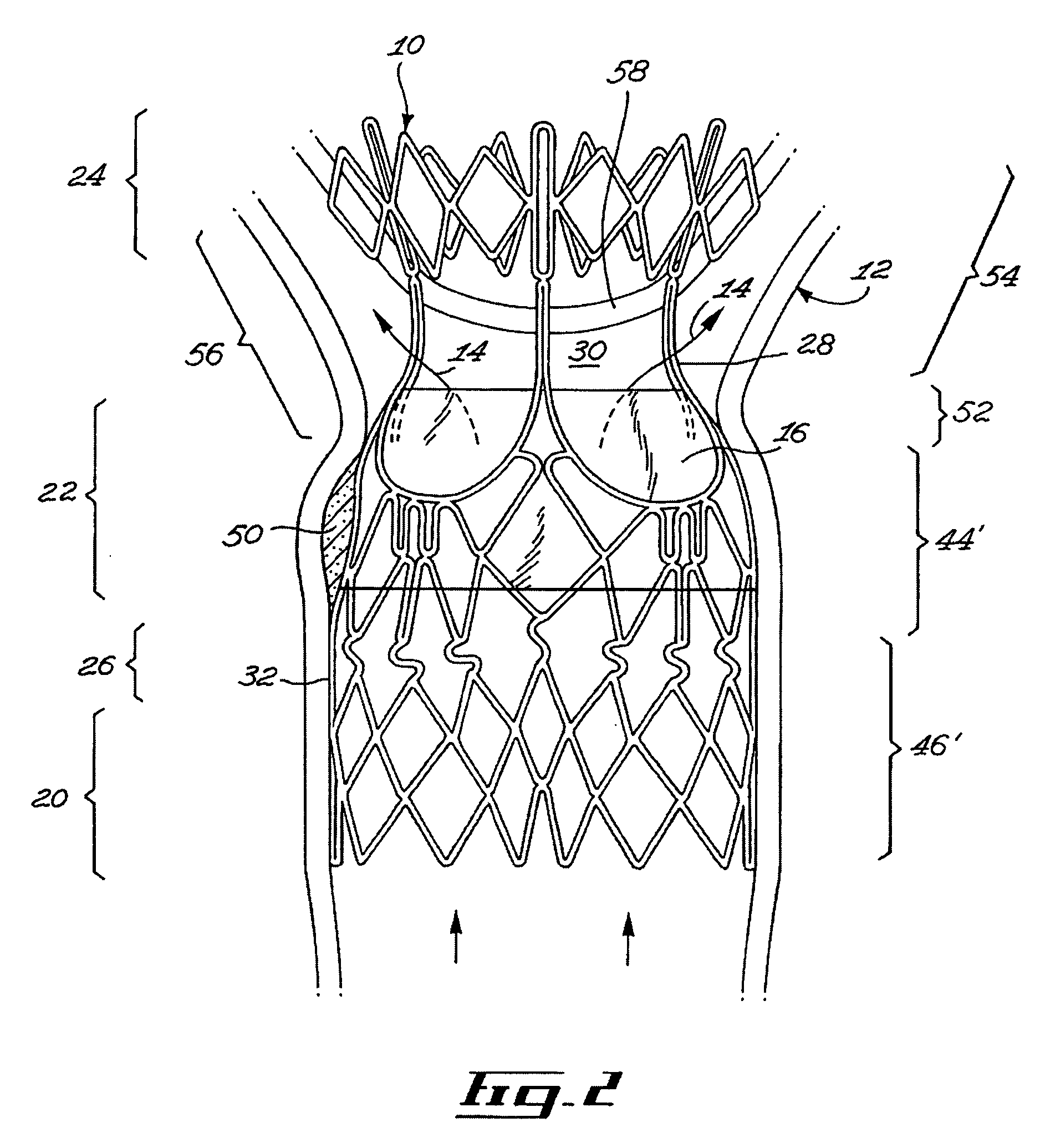
Stent valve and method of using same
A stent valve insertable in a body vessel containing a body fluid. The stent valve includes: a valve for at least partially controlling the flow of the body fluid in the body vessel; and a scaffold, the scaffold including : an anchoring section for anchoring the scaffold to said body vessel and a valve supporting section supporting the valve. The scaffold is substantially radially expandable from a scaffold retracted configuration to a scaffold expanded configuration. The valve supporting section is expandable over a greater range of radial expansion than the anchoring section.
Owner:BAYLIS MEDICAL
Body fluid testing component for simultaneous analyte detection
ActiveUS20060222567A1Reduce duplicationLow costMaterial analysis by observing effect on chemical indicatorDiagnostic recording/measuringCamera lensAnalyte
An analyte testing device is provided for use with a mobile processing device having a camera with a lens, a processor for processing an image captured by the lens. The analyte testing device comprises a casing and a test strip positioner. The test strip positioner positions an analyte containing test strip adjacent to the camera lens to permit the camera to capture an image of the analyte containing test strip. A light source is disposed within the casing. The light source is positioned within the casing to illuminate the analyte containing test strip to facilitate the capture of the image of the test strip. Software is contained within the mobile processing device for performing a quantitative analysis of at least one analyte from the captured image, and providing an output of the results of the quantitative analysis.
Owner:4A MEDICOM +1
Device and method for in vitro determination of analyte concentrations within body fluids
InactiveUS6989891B2Withdrawing sample devicesVaccination/ovulation diagnosticsSpectral bandsCell wall
A reagentless whole-blood analyte detection system that is capable of being deployed near a patient has a source capable of emitting a beam of radiation that includes a spectral band. The whole-blood system also has a detector in an optical path of the beam. The whole-blood system also has a housing that is configured to house the source and the detector. The whole-blood system also has a sample element that is situated in the optical path of the beam. The sample element has a sample cell and a sample cell wall that does not eliminate transmittance of the beam of radiation in the spectral band.
Owner:OPTISCAN BIOMEDICAL
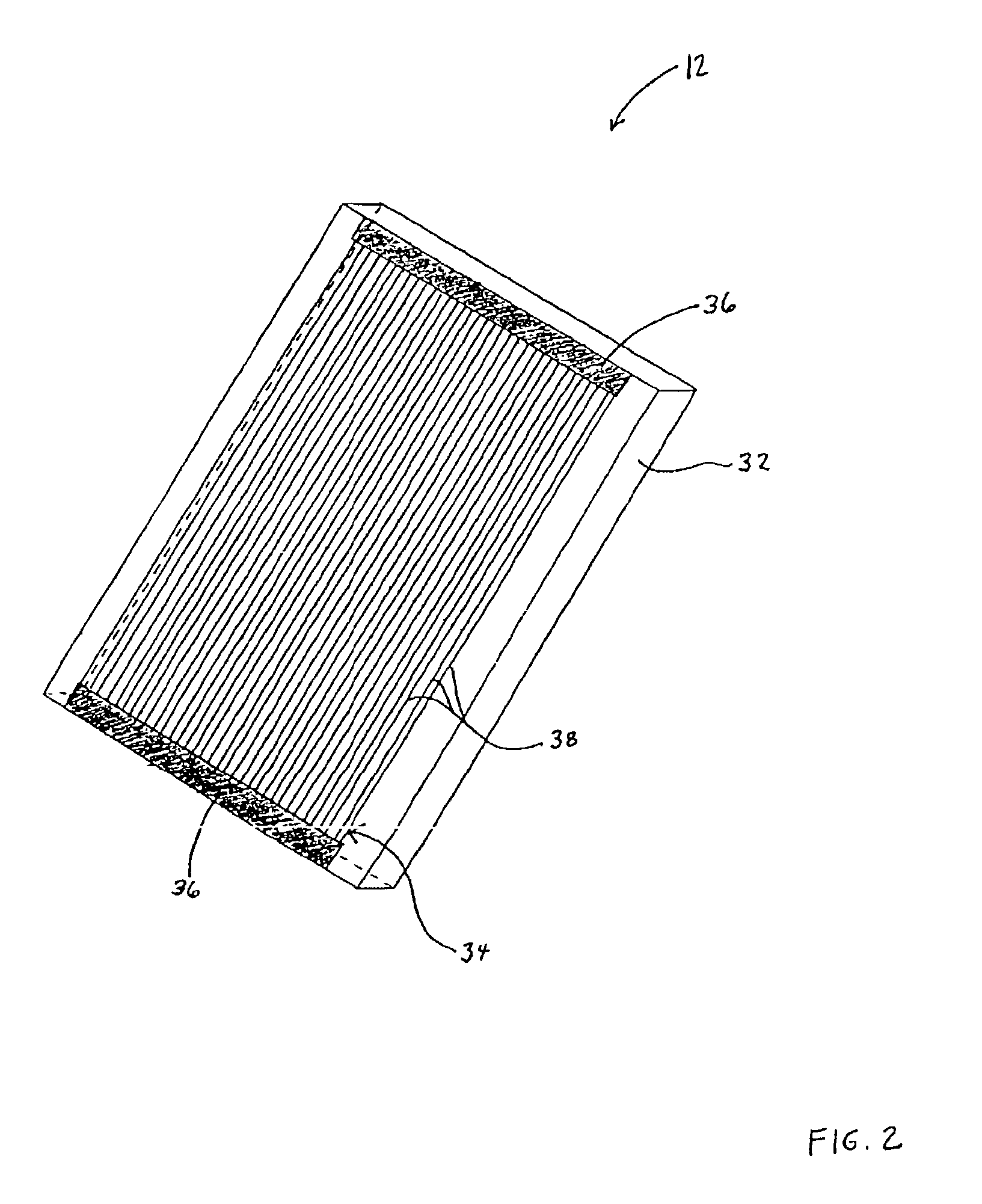
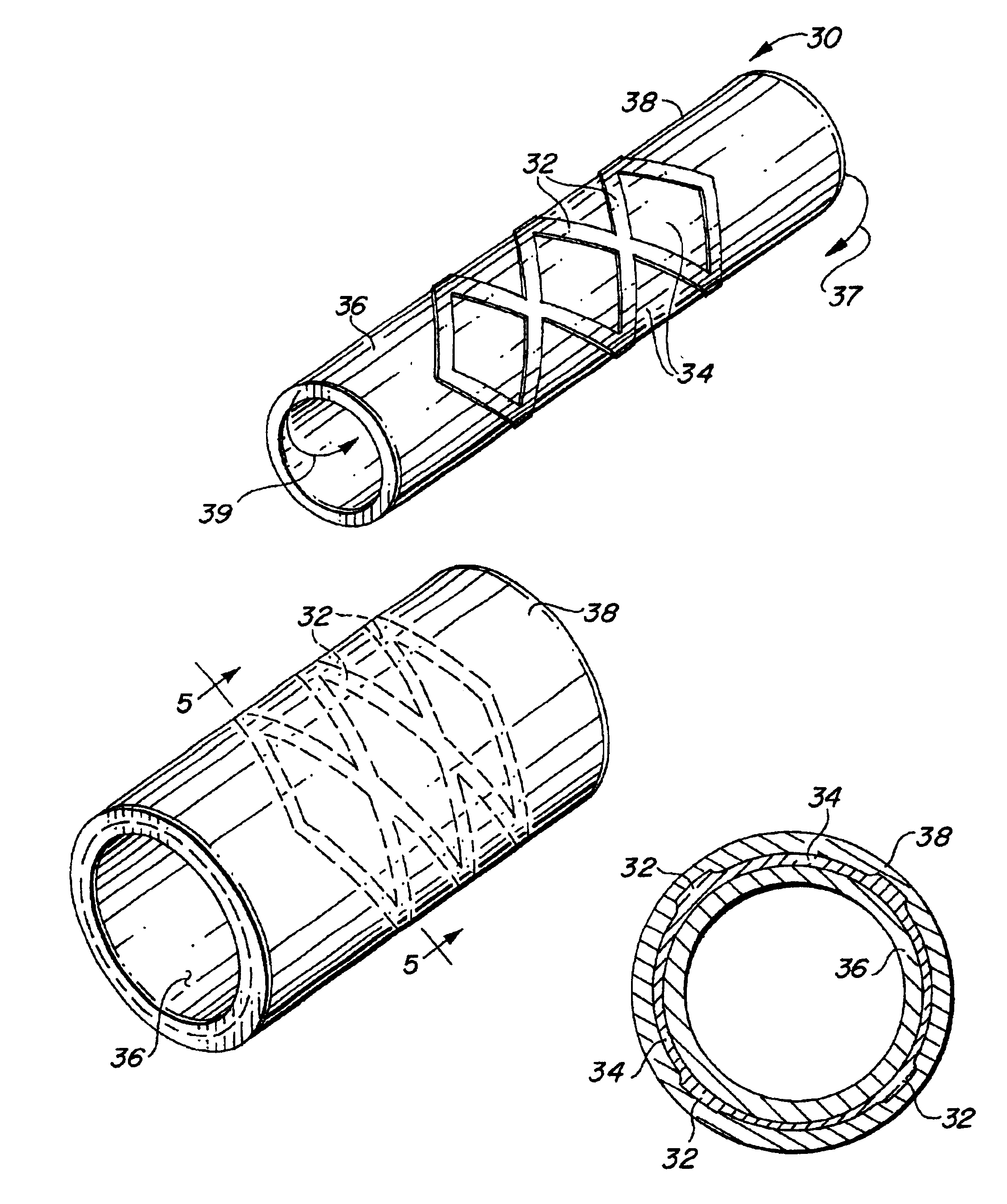
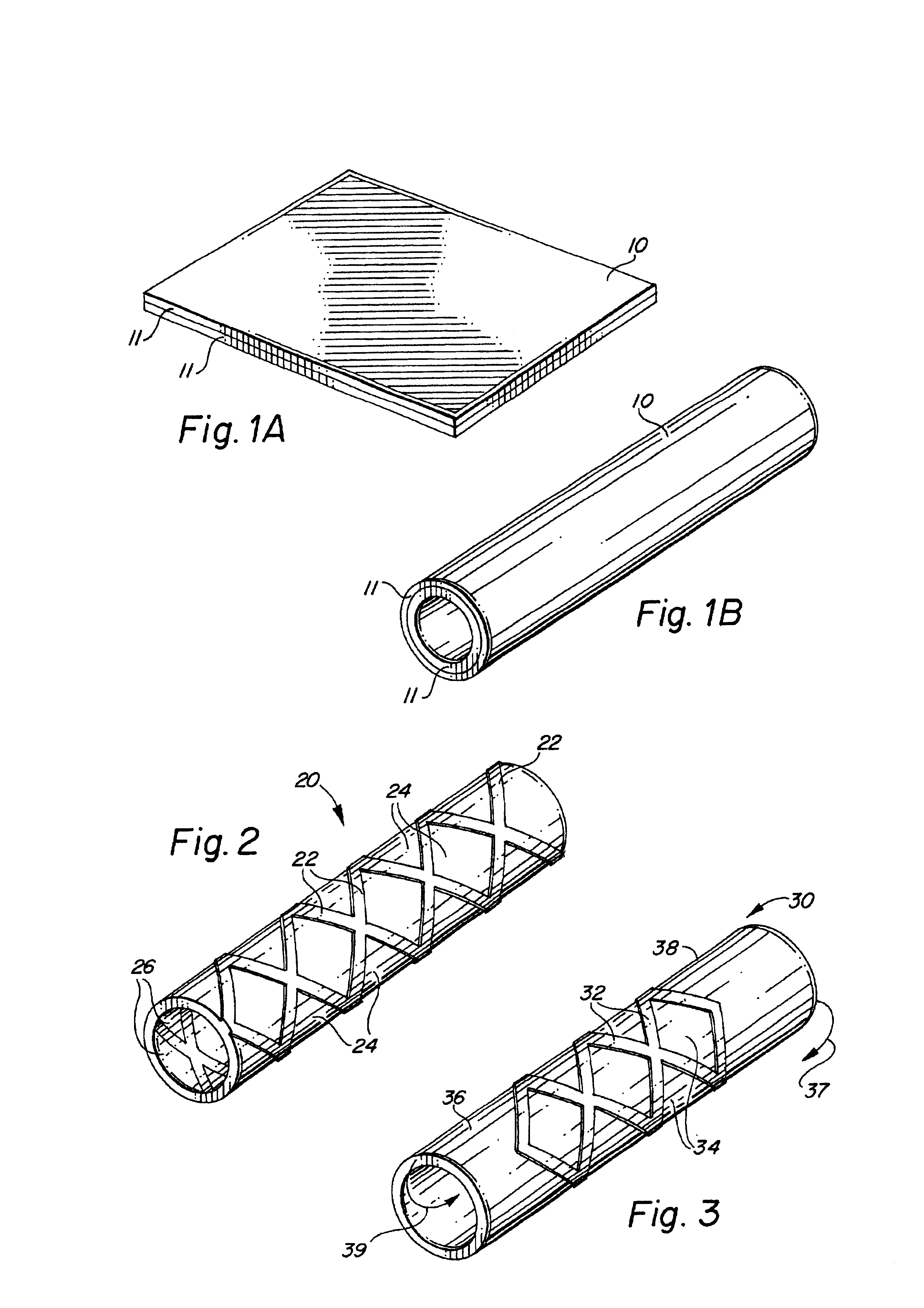
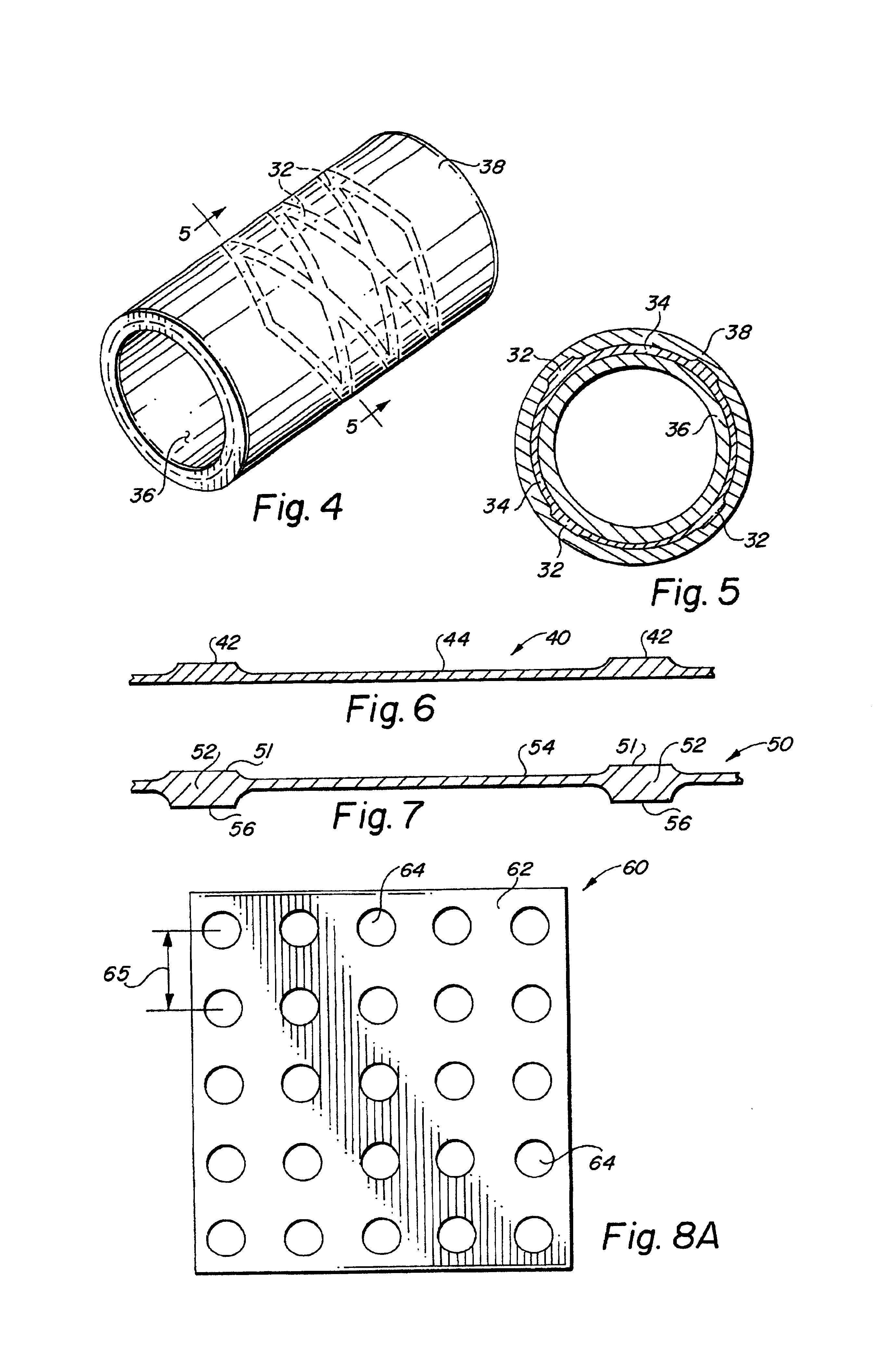
Self-supporting laminated films, structural materials and medical devices manufactured therefrom and methods of making same
Metal foils, wires, and seamless tubes with increased mechanical strength are provided. As opposed to wrought materials that are made of a single metal or alloy, these materials are made of two or more layers forming a laminate structure. Laminate structures are known to increase mechanical strength of sheet materials such as wood and paper products and are used in the area of thin films to increase film hardness, as well as toughness. Laminate metal foils have not been used or developed because the standard metal forming technologies, such as rolling and extrusion, for example, do not lend themselves to the production of laminate structures. Vacuum deposition technologies can be developed to yield laminate metal structures with improved mechanical properties. In addition, laminate structures can be designed to provide special qualities by including layers that have special properties such as superelasticity, shape memory, radio-opacity, corrosion resistance etc. Examples of articles which may be made by the inventive laminate structures include implantable medical devices that are fabricated from the laminated deposited films and which present a blood or body fluid and tissue contact surface that has controlled heterogeneities in material constitution. An endoluminal stent-graft and web-stent that is made of a laminated film material deposited and etched into regions of structural members and web regions subtending interstitial regions between the structural members. An endoluminal graft is also provided which is made of a biocompatible metal or metal-like material. The endoluminal stent-graft is characterized by having controlled heterogeneities in the stent material along the blood flow surface of the stent and the method of fabricating the stent using vacuum deposition methods.
Owner:VACTRONIX SCI LLC
Method and device for monitoring loss of body fluid and dislodgment of medical instrument from body
InactiveUS20050038325A1Unobstructed viewOther blood circulation devicesMedical devicesFistula needlesHaemodialysis machine
A method of alerting medical personnel of a problem during hemodialysis includes providing an active, fail-to-safe site sensor for a fistula needle at an access site during hemodialysis; and automatically alerting medical personnel of a problem during hemodialysis using the active, fail-to-safe site sensor during at least the following: failing of the active, fail-to-safe site sensor; insufficient powering to the active, fail-to-safe site sensor; partial fistula needle dislodging from the access site; and complete needle dislodging from the access site.
Owner:BRADLEY JON MOLL RODNEY L MOLL & ANN E MOLL FAMILY TRUST
Tissue visualization and manipulation system
Tissue visualization and manipulation systems are described herein. Such a system may include a deployment catheter and an attached imaging hood deployable into an expanded configuration. In use, the imaging hood is placed against or adjacent to a region of tissue to be imaged in a body lumen that is normally filled with an opaque bodily fluid such as blood. A translucent or transparent fluid, such as saline, can be pumped into the imaging hood until the fluid displaces any blood, thereby leaving a clear region of tissue to be imaged via an imaging element in the deployment catheter. Additionally, any number of therapeutic tools can also be passed through the deployment catheter and into the imaging hood for treating the tissue region of interest.
Owner:INTUITIVE SURGICAL OPERATIONS INC
Sensors
ActiveUS20070108048A1Immobilised enzymesBioreactor/fermenter combinationsBody fluidBiomedical engineering
The subject invention provides devices and methods for the analysis of a body fluid. Embodiments include sensors that are sample-fillable by contacting a corner of the sensor to a sample.
Owner:ABBOTT DIABETES CARE INC
Devices, systems and methods for extracting bodily fluid and monitoring an analyte therein
ActiveUS7258673B2Little painSlight discomfortWithdrawing sample devicesEvaluation of blood vesselsAnalyteD-Glucose
An interstitial fluid (ISF) extraction device includes a penetration member configured for penetrating a target site of a user's skin layer and, subsequently, residing in the user's skin layer and extracting an ISF sample therefrom and at least three concentrically-arranged pressure rings, each adapted for applying pressure to the user's skin layer in the vicinity of the target site while the penetration member is residing in the user's skin layer. In addition, the ISF extraction device is configured such that (i) the pressure rings apply pressure in an oscillating manner with asymmetric deployment and retraction cycles and (ii) only one of the at least three concentrically-arranged pressure rings is deployed at a time, thereby mitigating an ISF glucose lag of the ISF sample extracted by the penetration member.
Owner:LIFESCAN IP HLDG LLC
Intralumenal contact sensor
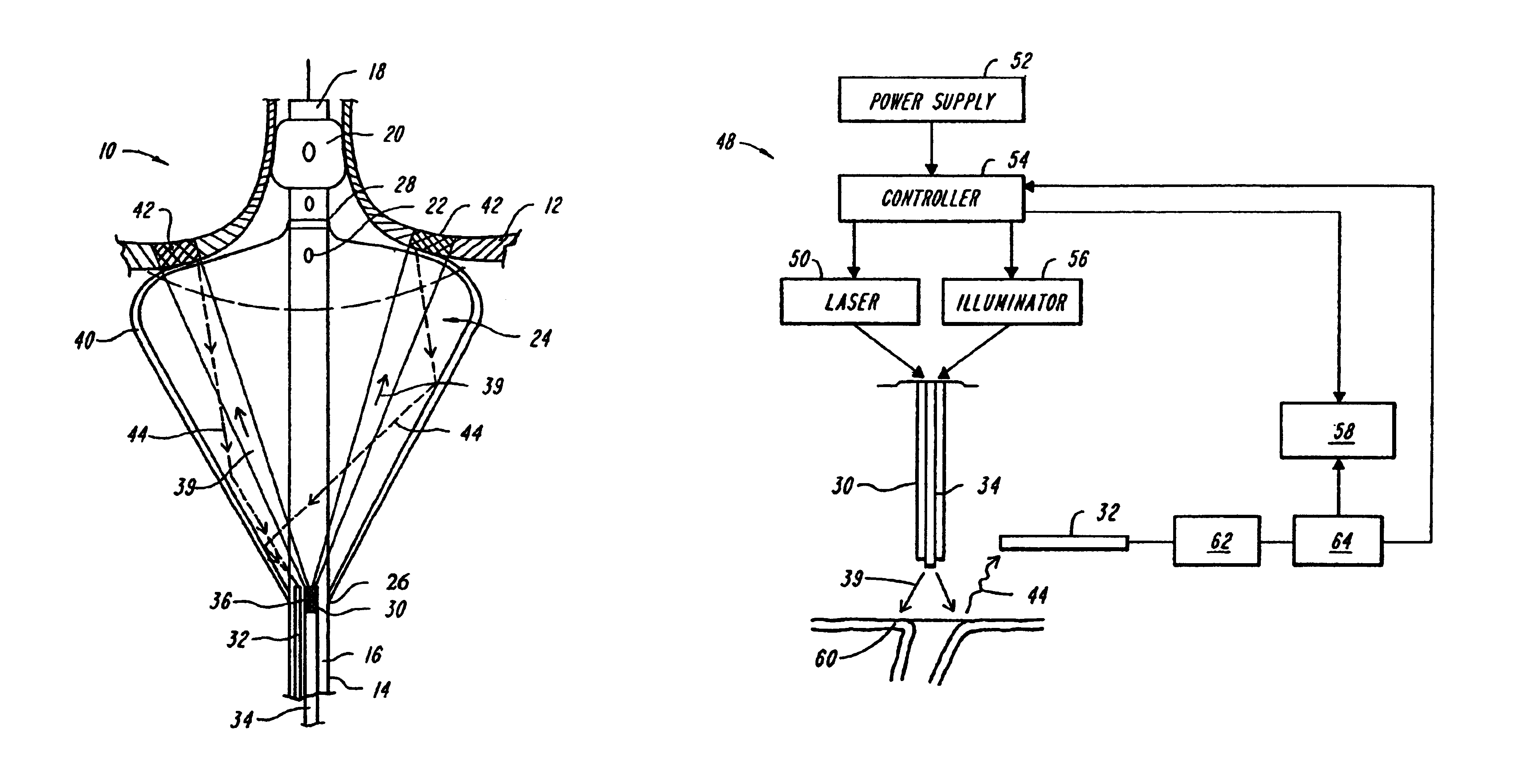
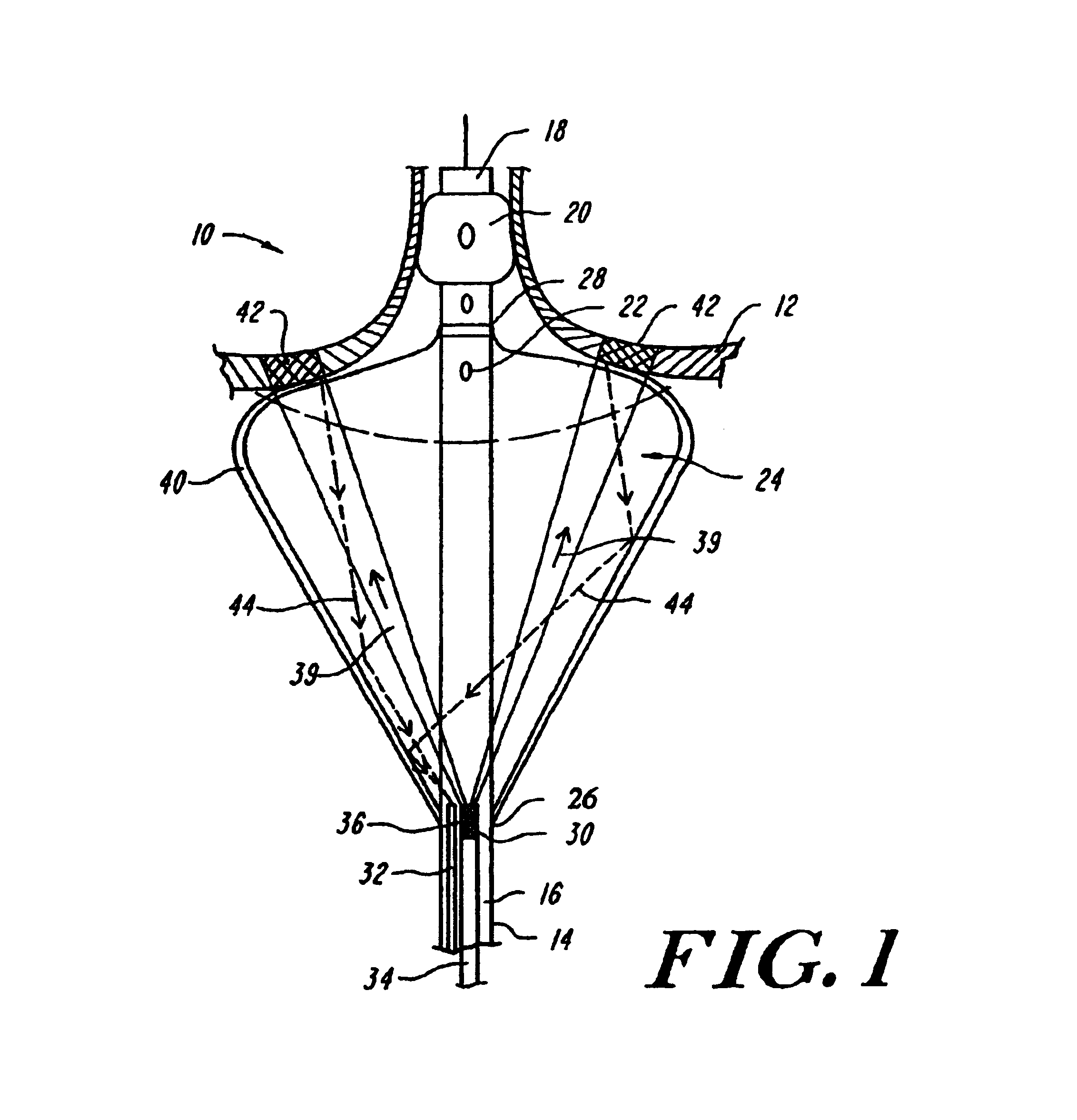
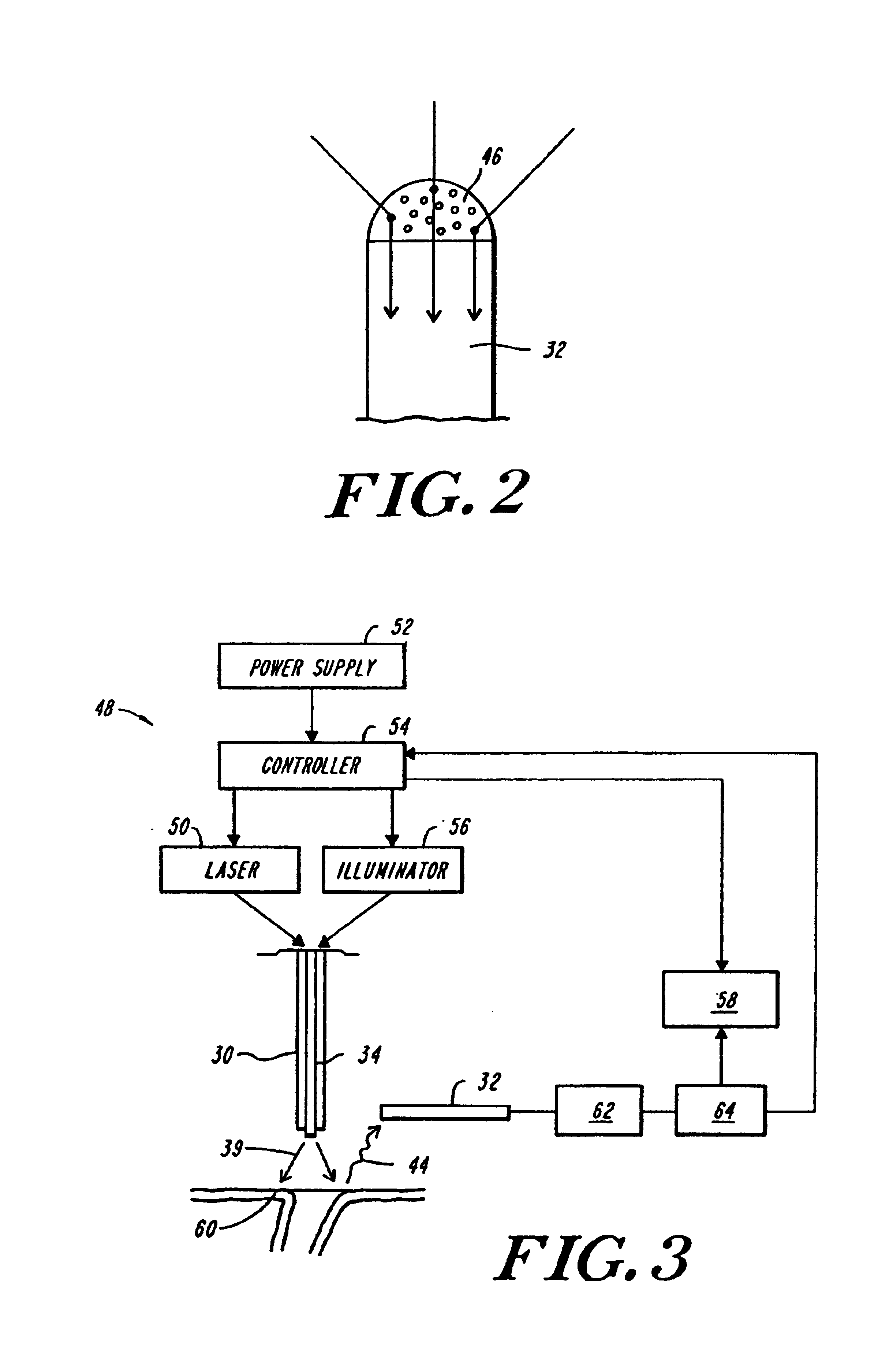
InactiveUS6942657B2Eliminate aberrant wave conductionReduce lossDiagnosticsCatheterLight treatmentLaser light
An apparatus and method for phototherapy are described in which laser light or other radiation is projected from within a catheter, through a balloon member, and toward the surface of tissue. The light reflected from body fluids or the tissue surface is captured by a collecting device located within the catheter, e.g., within the balloon member, and the intensity of the reflected light is ascertained. The apparatus and method provides for accurately positioning the apparatus against the tissue treatment site.
Owner:CARDIOFOCUS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com