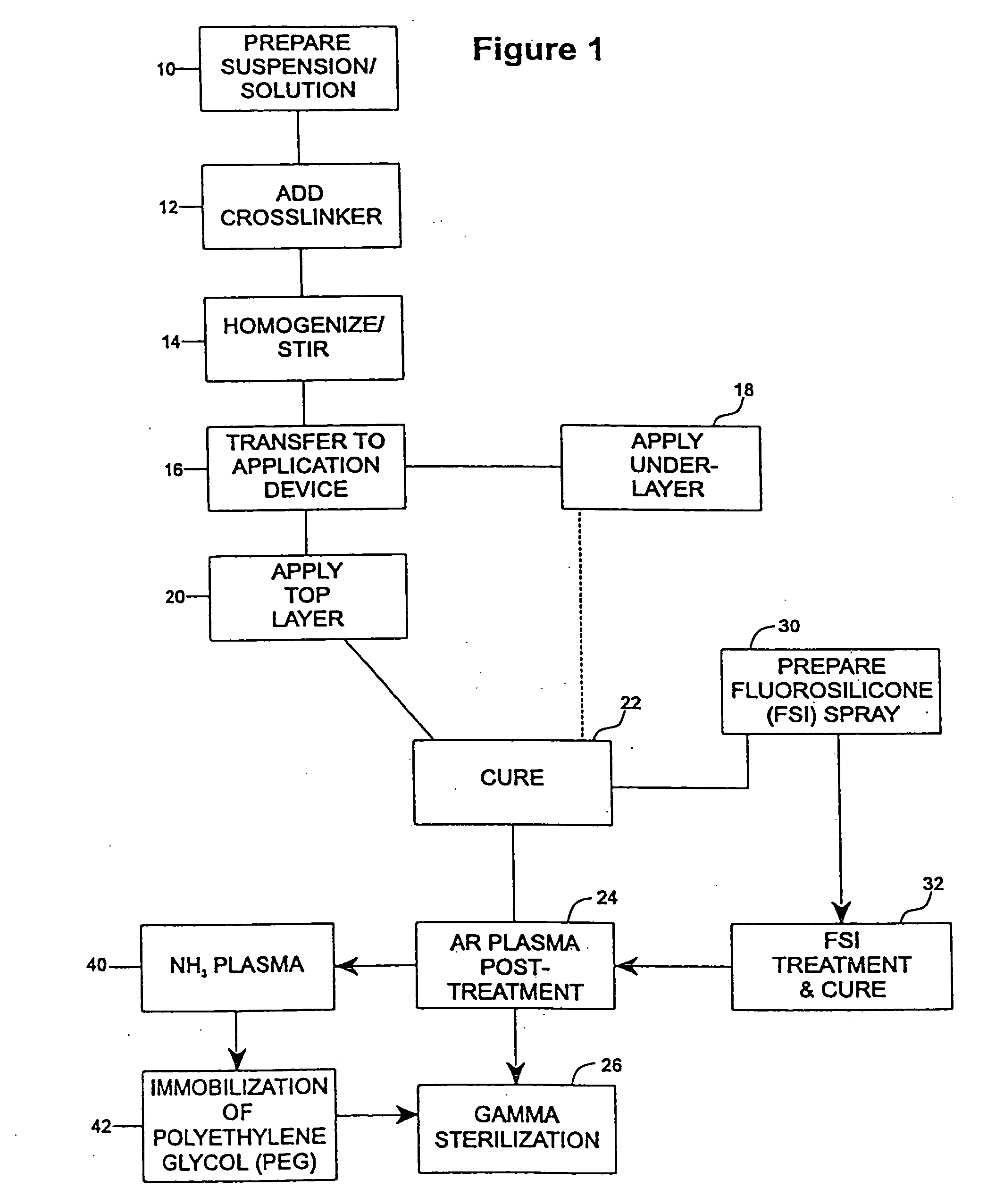
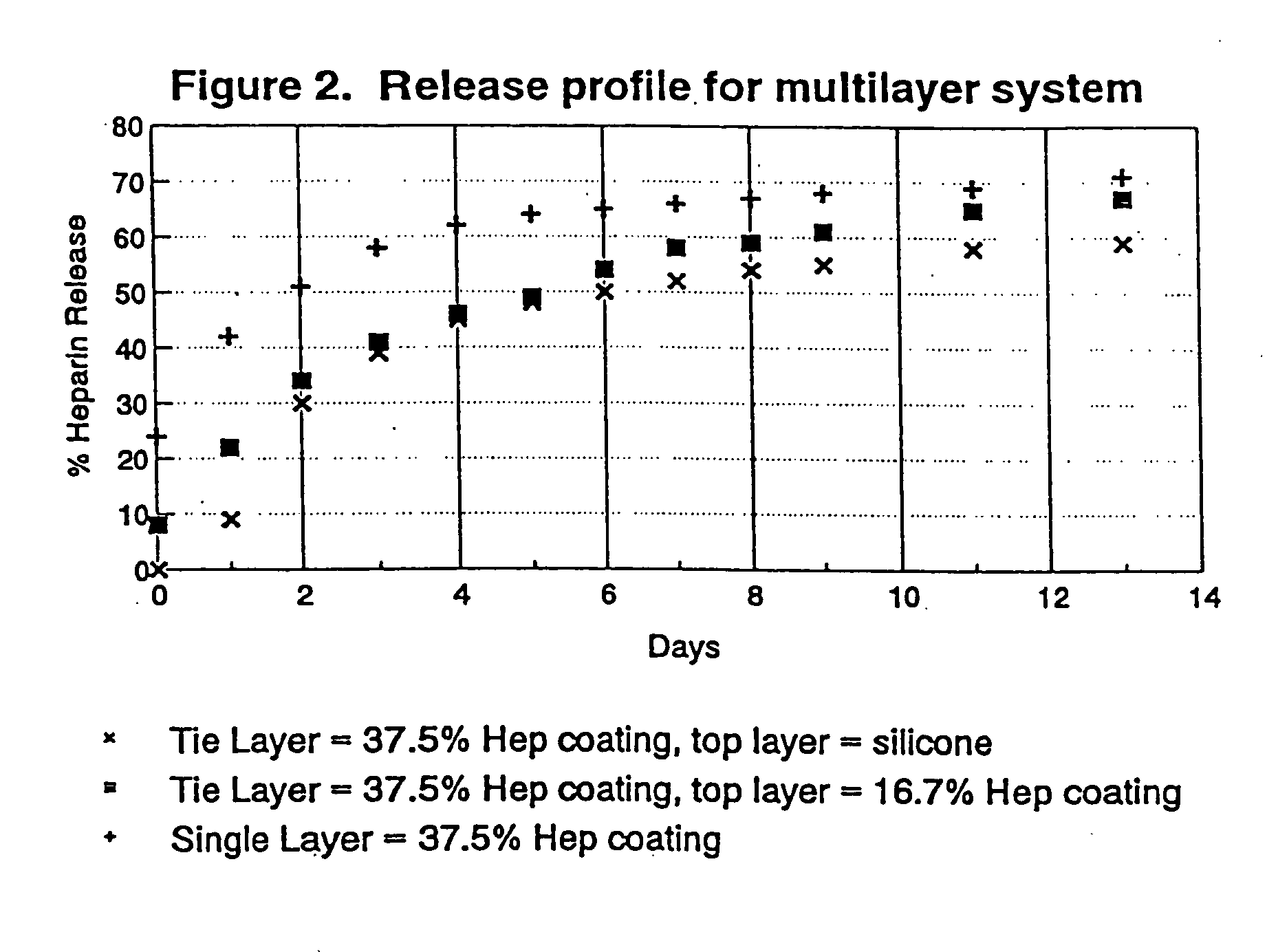
Patents
Literature
1584 results about "Heparin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to keep IV catheters open and flowing freely.
Microneedle device for extraction and sensing of bodily fluids
InactiveUS7344499B1Simple wayMinimal and no damageAdditive manufacturing apparatusMicroneedlesMetaboliteIrritation
Microneedle devices are provided for controlled sampling of biological fluids in a minimally-invasive, painless, and convenient manner. The microneedle devices permit in vivo sensing or withdrawal of biological fluids from the body, particularly from or through the skin or other tissue barriers, with minimal or no damage, pain, or irritation to the tissue. The microneedle device includes one or more microneedles, preferably in a three-dimensional array, a substrate to which the microneedles are connected, and at least one collection chamber and / or sensor in communication with the microneedles. Preferred embodiments further include a means for inducing biological fluid to be drawn through the microneedles and into the collection chamber for analysis. In a preferred embodiment, this induction is accomplished by use of a pressure gradient, which can be created for example by selectively increasing the interior volume of the collection chamber, which includes an elastic or movable portion engaged to a rigid base. Preferred biological fluids for withdrawal and / or sensing include blood, lymph, interstitial fluid, and intracellular fluid. Examples of analytes in the biological fluid to be measured include glucose, cholesterol, bilirubin, creatine, metabolic enzymes, hemoglobin, heparin, clotting factors, uric acid, carcinoembryonic antigen or other tumor antigens, reproductive hormones, oxygen, pH, alcohol, tobacco metabolites, and illegal drugs.
Owner:GEORGIA TECH RES CORP +1
Bioactive coating composition and methods
InactiveUS6921811B2Promotes desired biologicalPromotes therapeutic effectSuture equipmentsOrganic active ingredientsArylBody fluid
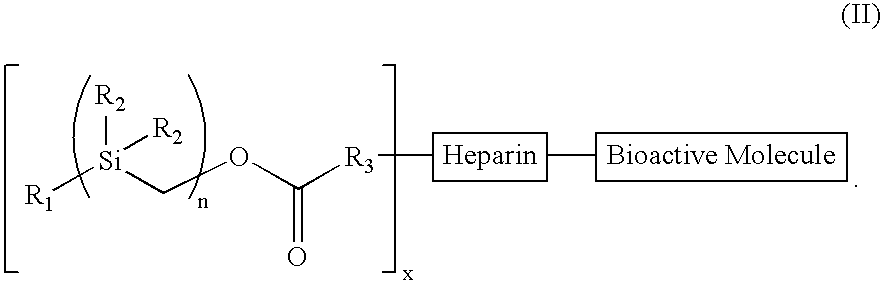
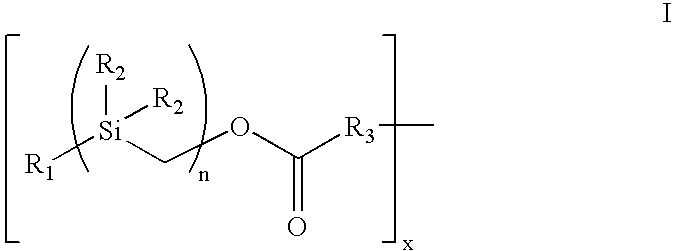
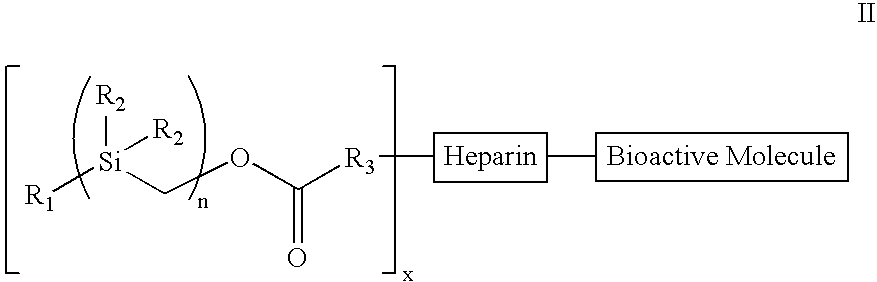
The present invention provides a bioactive coating composition, method and devices for bodily fluid-contacting surfaces. The coating comprises a complex of Formula II: wherein R1 is an C1-18alkyl or C6-32aryl group, each R2 is independently selected from the group consisting of C1-18alkyl and C6-32aryl, R3 is N or O, n is a number from 1 to 10, and x in a number from 1 to about 30, directly bound to a heparin-activity molecule via covalent bonding, with one or more bioactive molecules bound to the heparin-activity molecule. The bioactive molecule may be an adhesive molecule such as fibronectin, a growth factor such as basic fibroblast growth factor, or any other bioactive molecule that binds, by any mechanism, to a heparin-activity molecule
Owner:BIOSURFACE ENG TECH
Process for preparing surface modification substances
InactiveUS6461665B1Improved nonthrombogenic activitySufficient non-thrombogenicitySuture equipmentsGlovesSurface modificationSurface modified
The present invention relates to a process for preparing surface modifications having an improved antithrombogenic activity, whereby the improvement is achieved by treating heparin at a temperature above 40° C. or a pH in the rage of 9-14 or in contact with nucleophilic catalysts before attaching said heparin to the surface to be modified.
Owner:CARMEDA AB
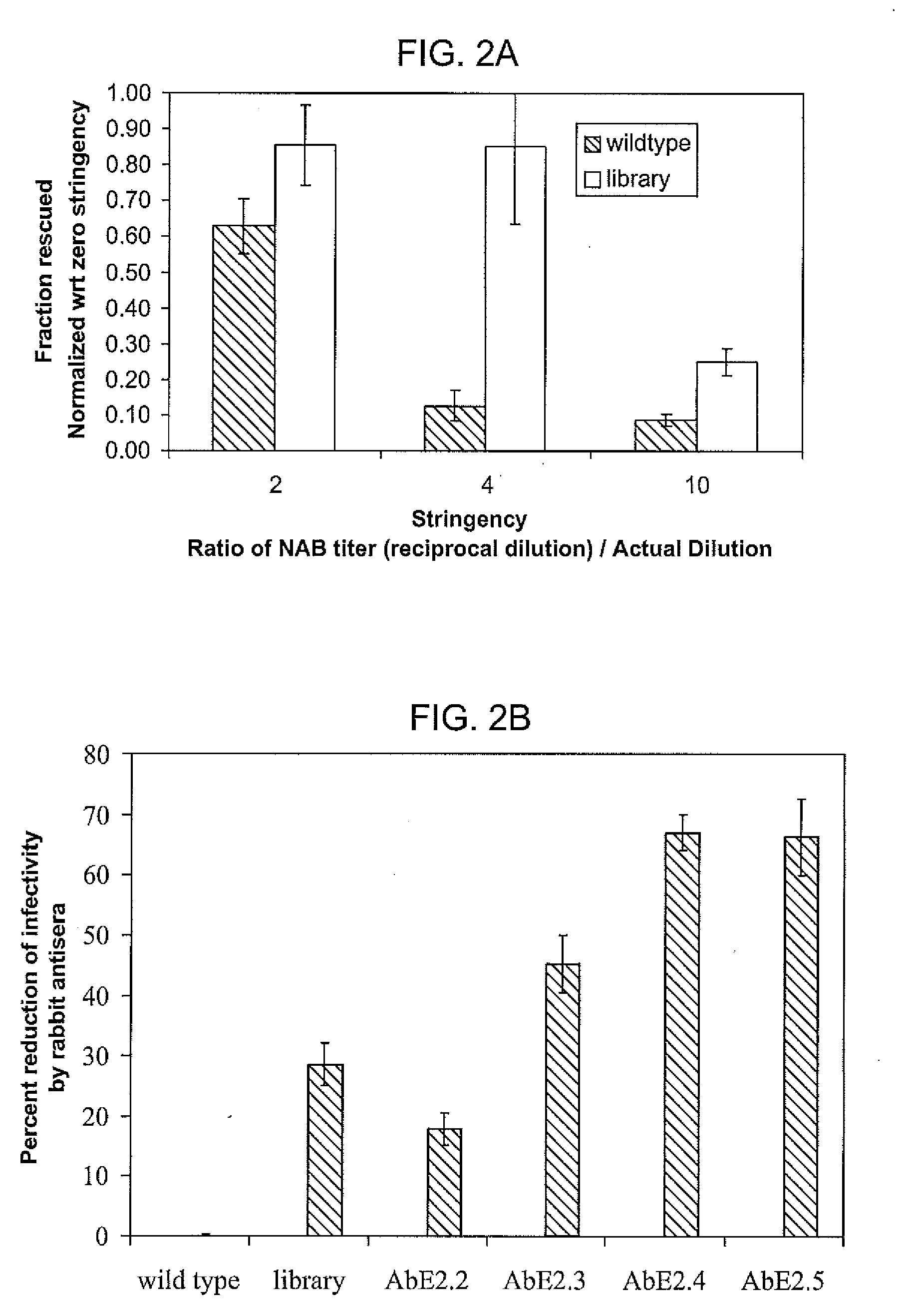
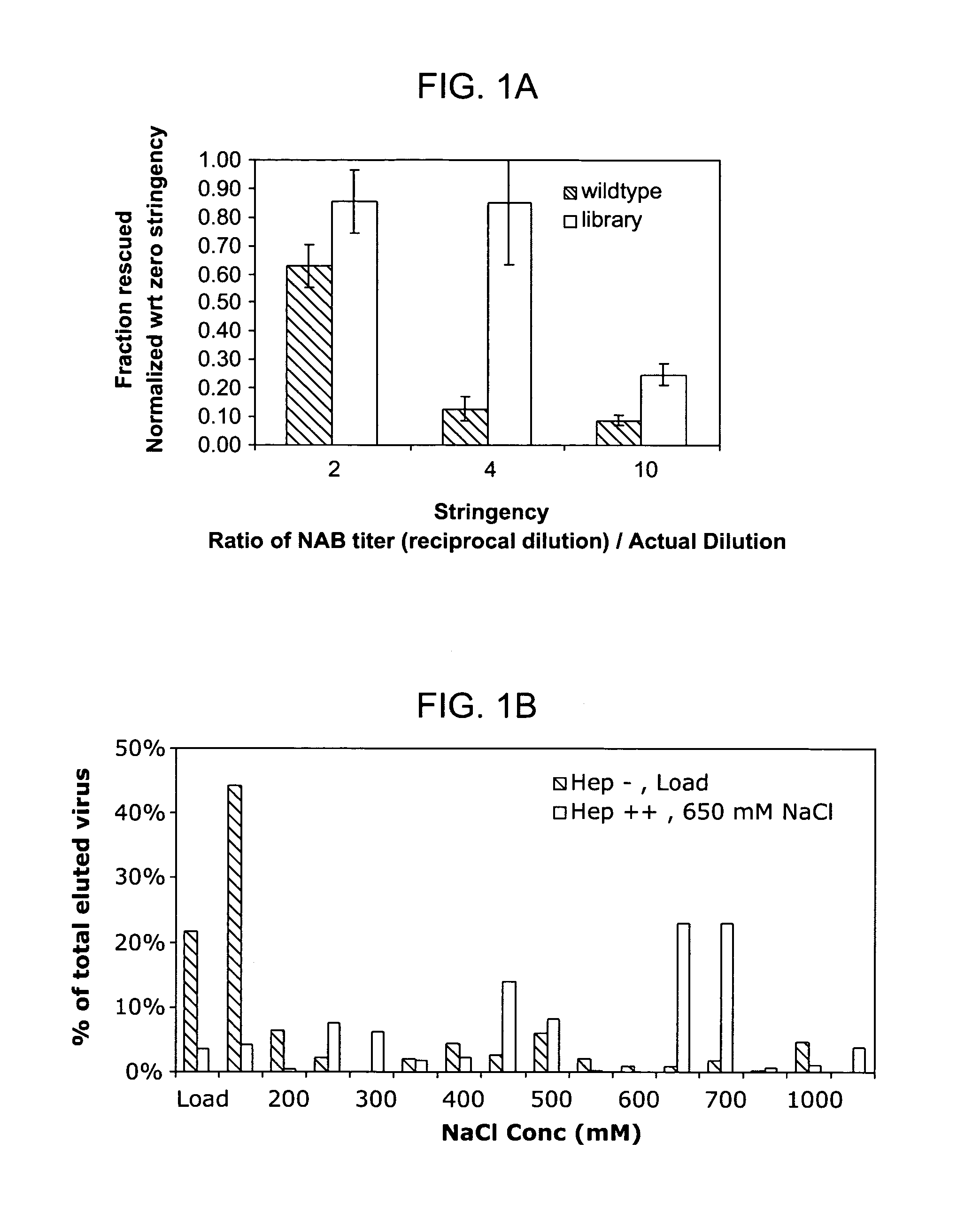
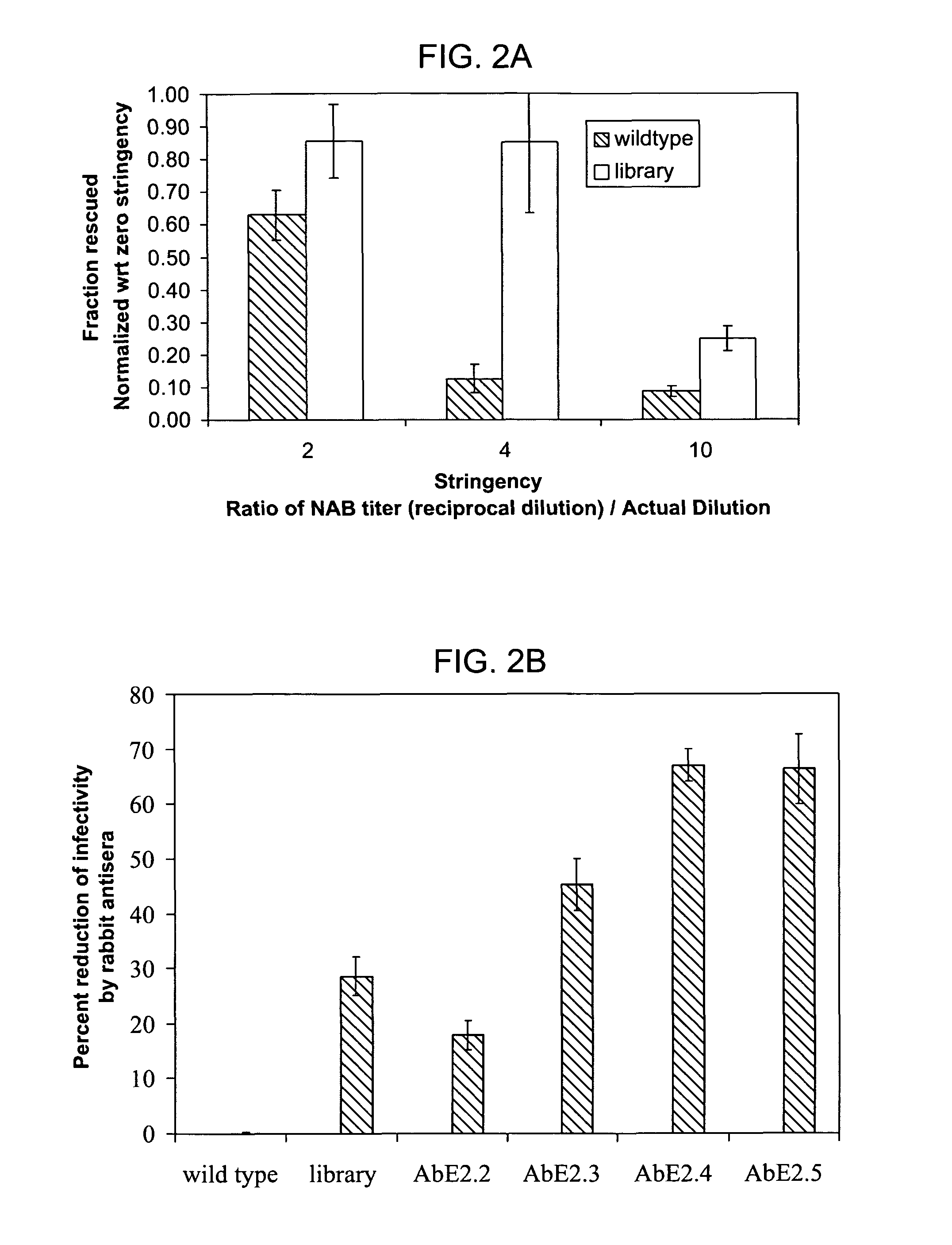
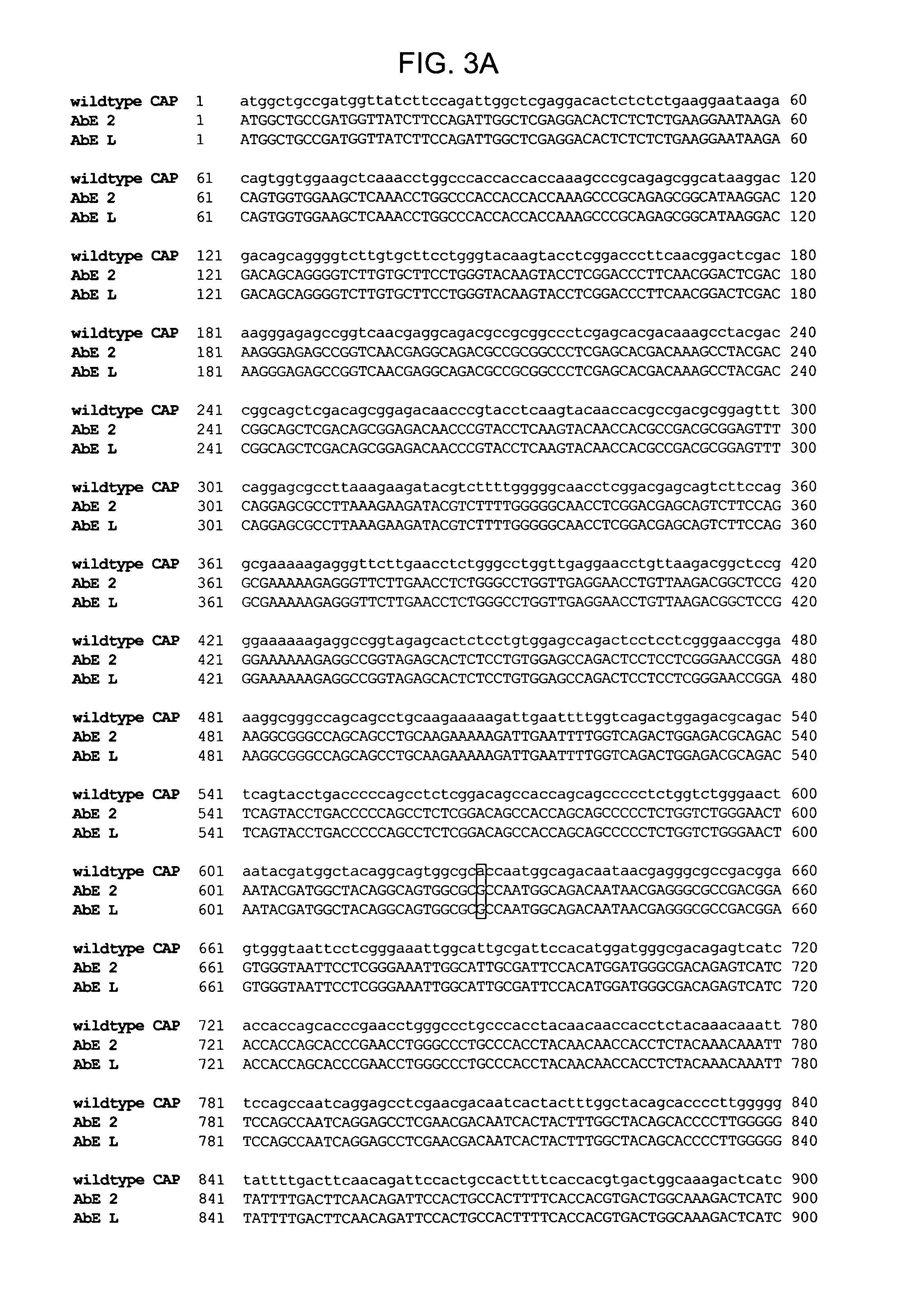
Mutant adeno-associated virus virions and methods of use thereof
ActiveUS20090202490A1Reduce the binding forceAltered infectivityOrganic active ingredientsBiocideCell type specificNeutralizing antibody
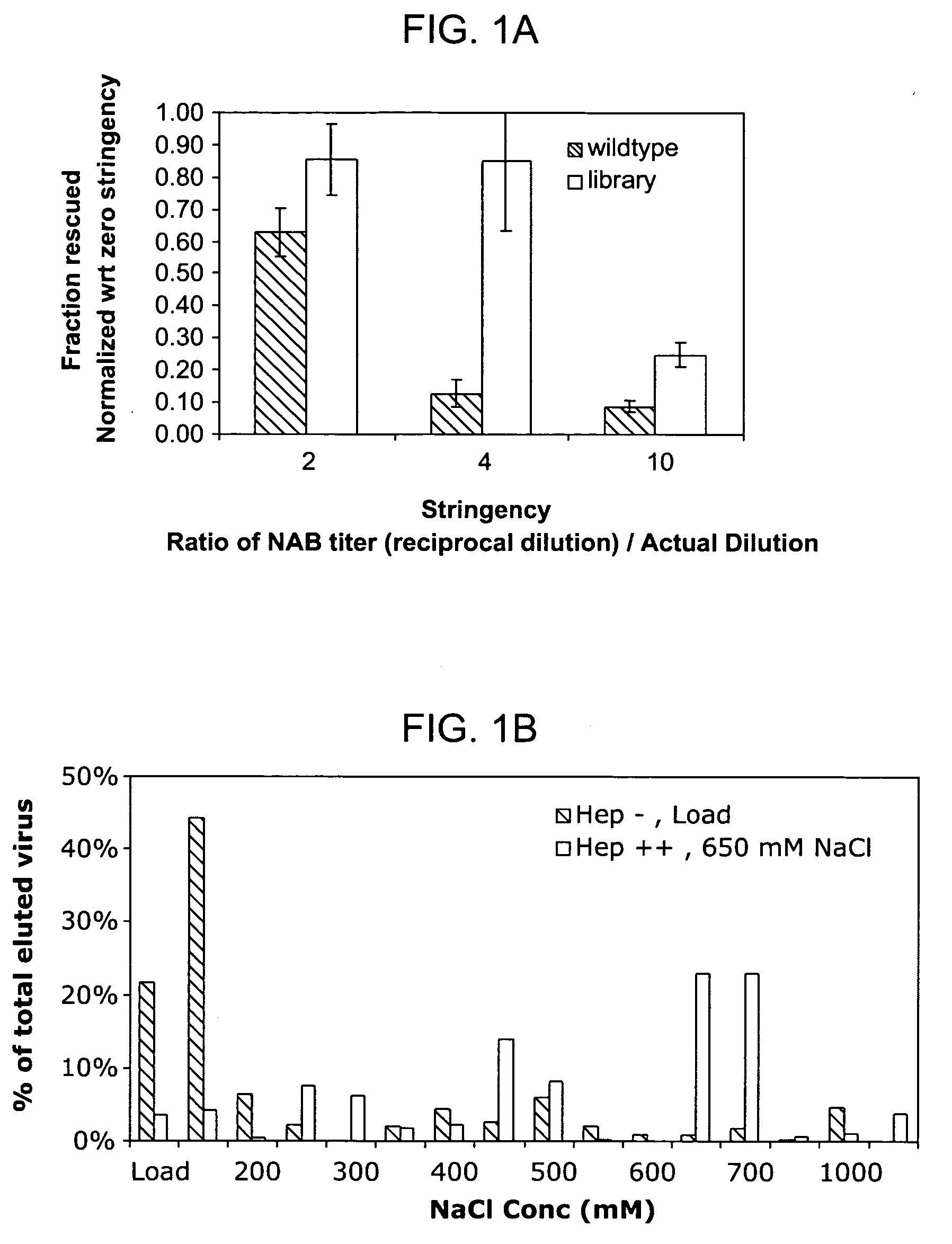
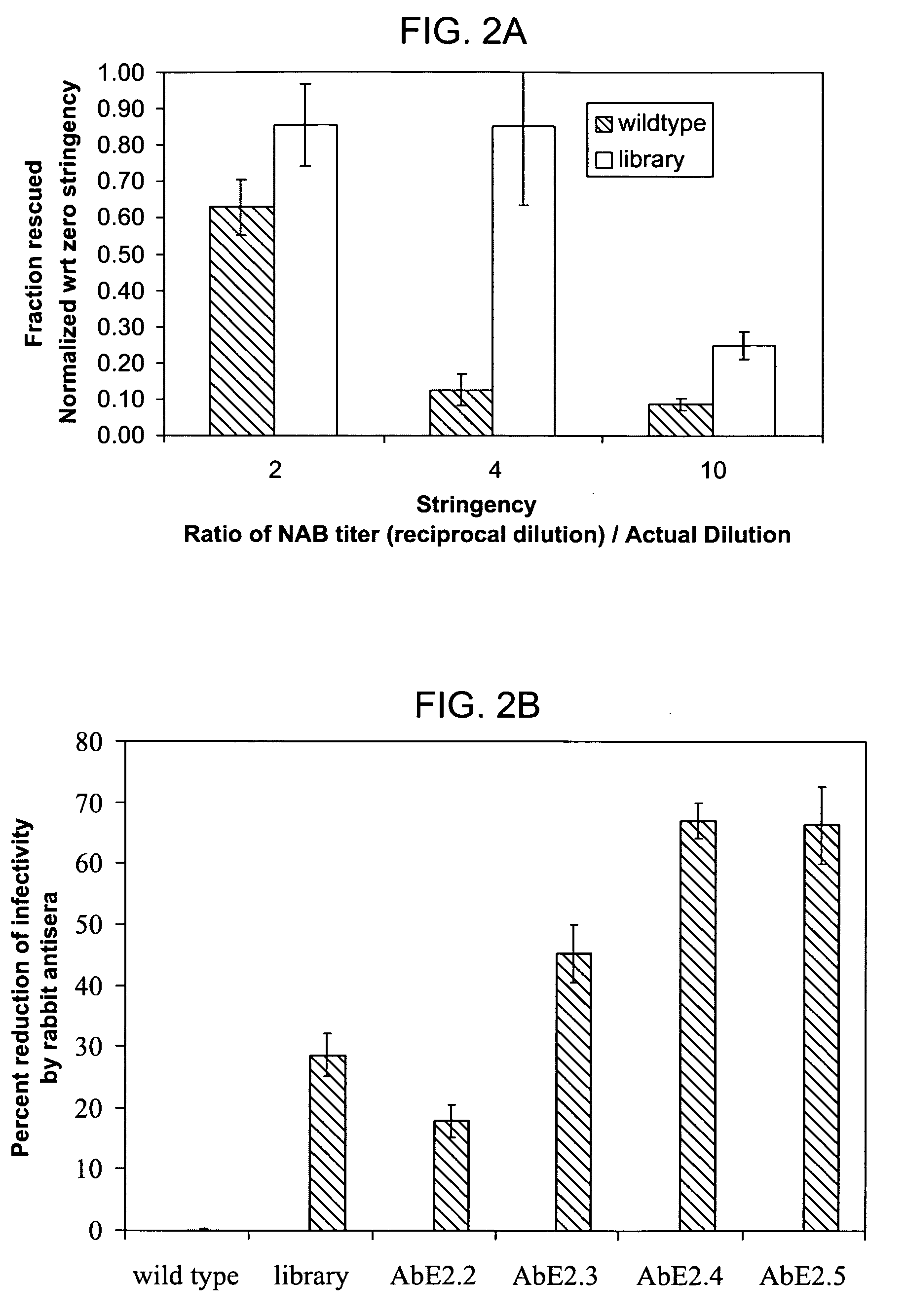
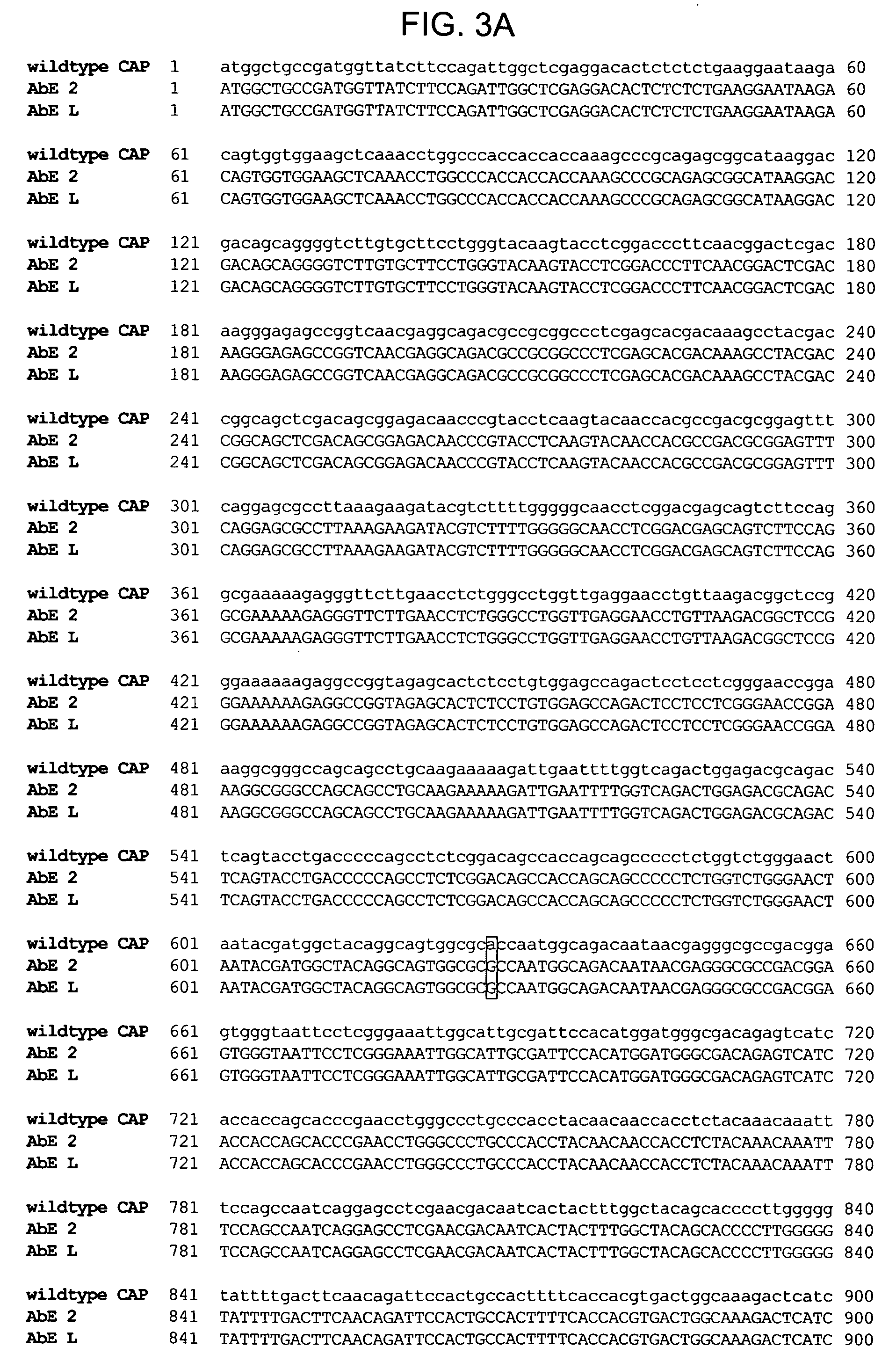
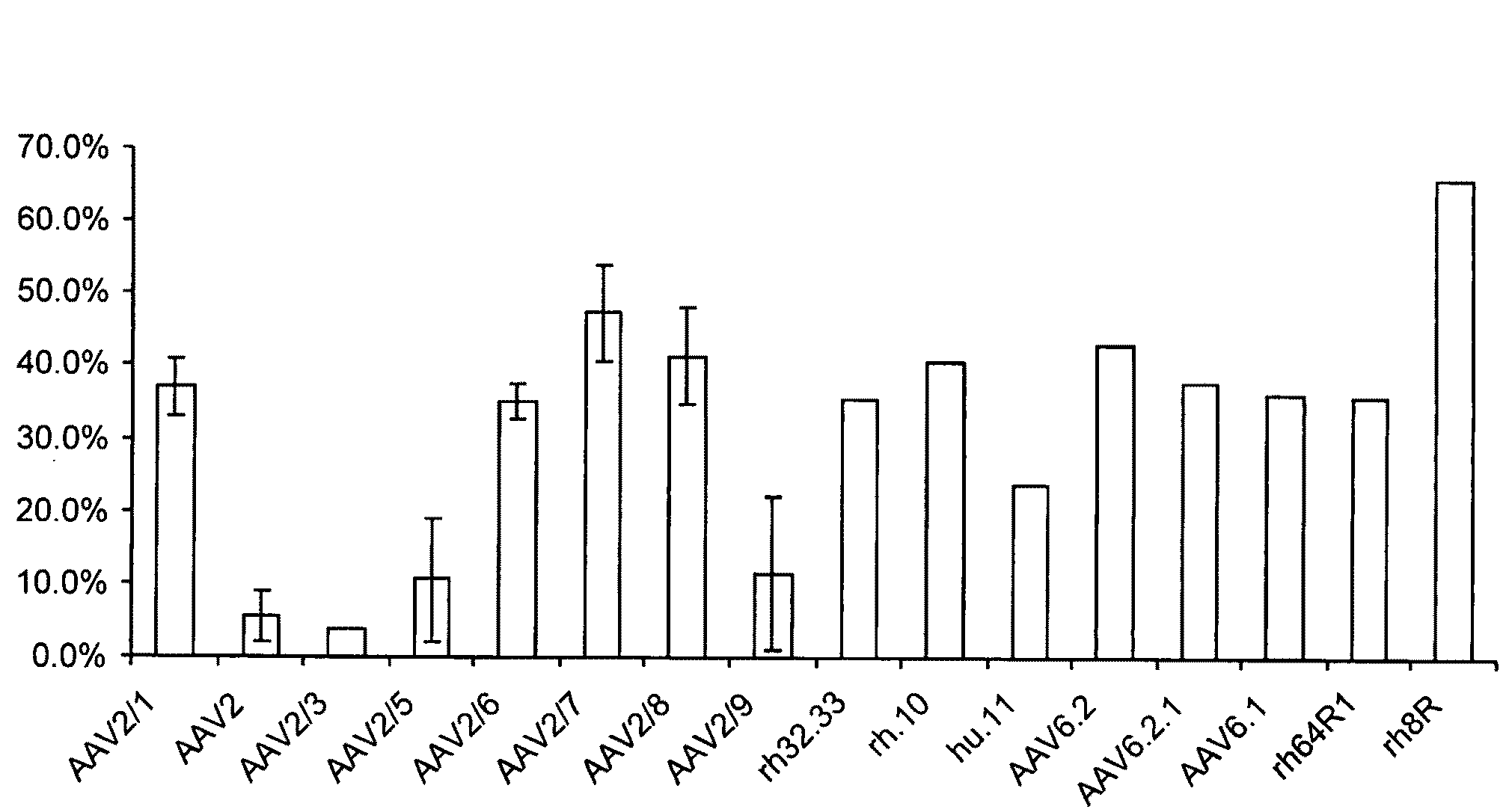
The present invention provides mutant adeno-associated virus (AAV) that exhibit altered capsid properties, e.g., reduced binding to neutralizing antibodies in serum and / or altered heparin binding and / or altered infectivity of particular cell types. The present invention further provides libraries of mutant AAV comprising one or more mutations in a capsid gene. The present invention further provides methods of generating the mutant AAV and mutant AAV libraries, and compositions comprising the mutant AAV. The present invention further provides recombinant AAV (rAAV) virions that comprise a mutant capsid protein. The present invention further provides nucleic acids comprising nucleotide sequences that encode mutant capsid proteins, and host cells comprising the nucleic acids. The present invention further provides methods of delivering a gene product to an individual, the methods generally involving administering an effective amount of a subject rAAV virion to an individual in need thereof.
Owner:RGT UNIV OF CALIFORNIA +1
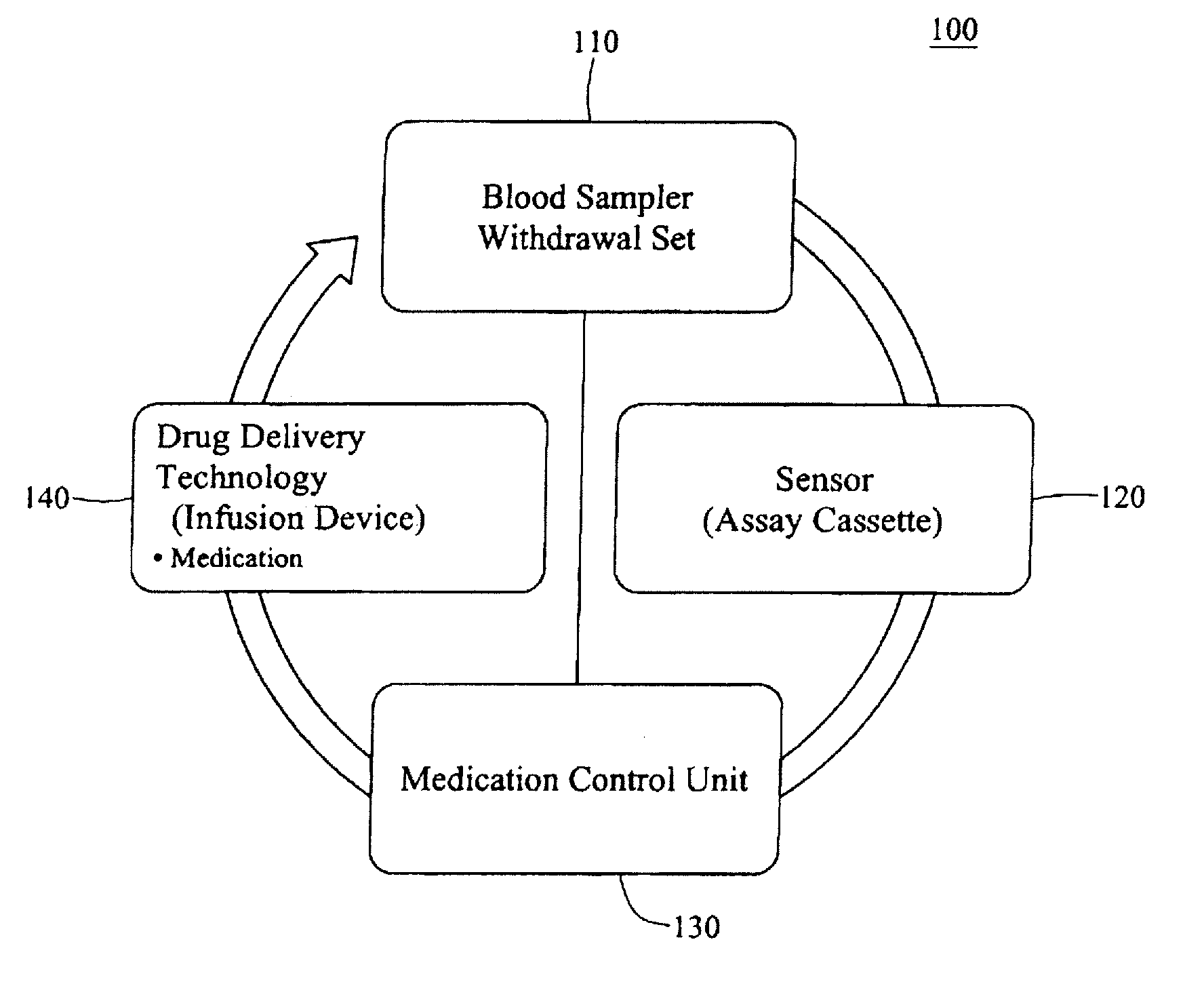
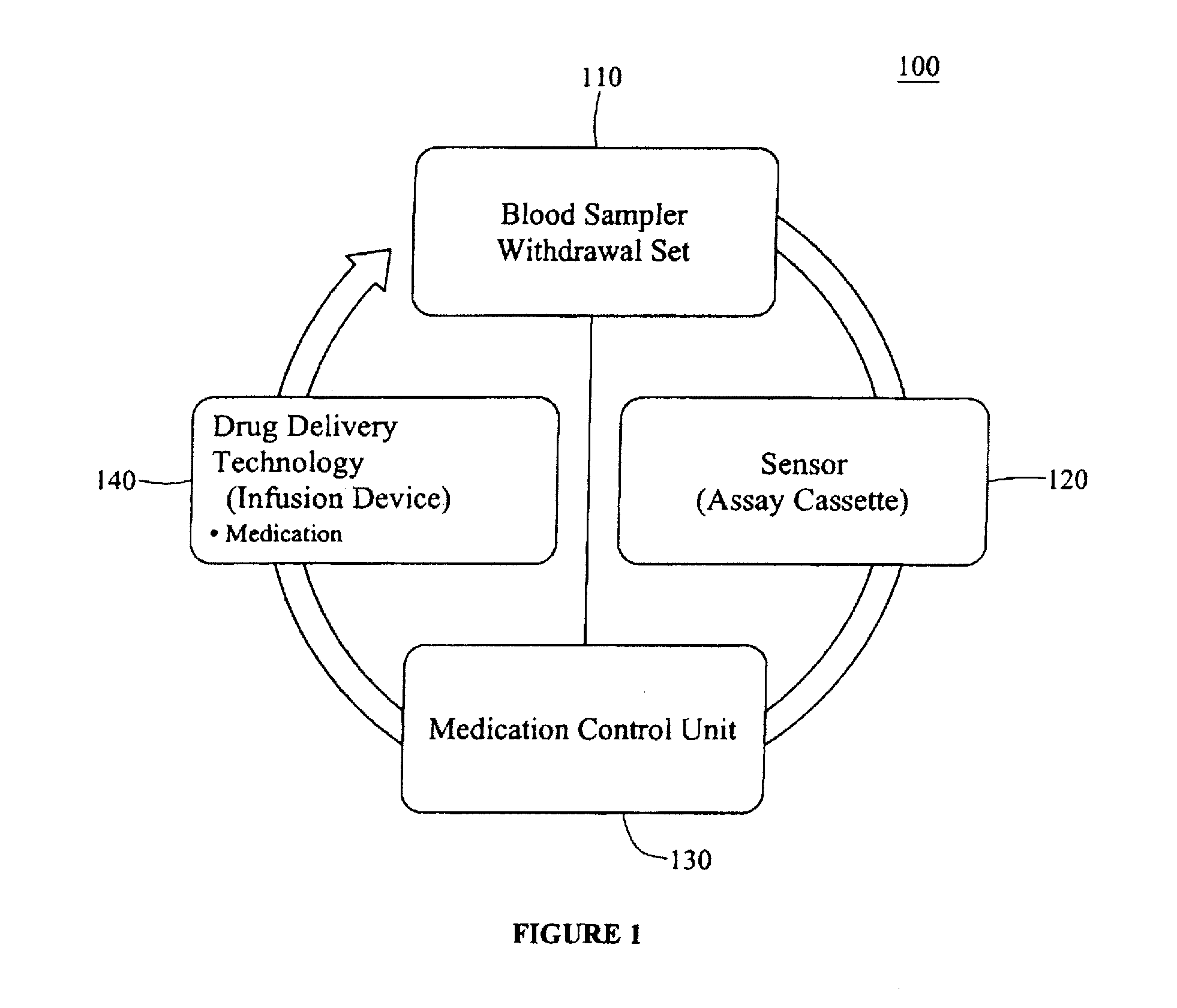
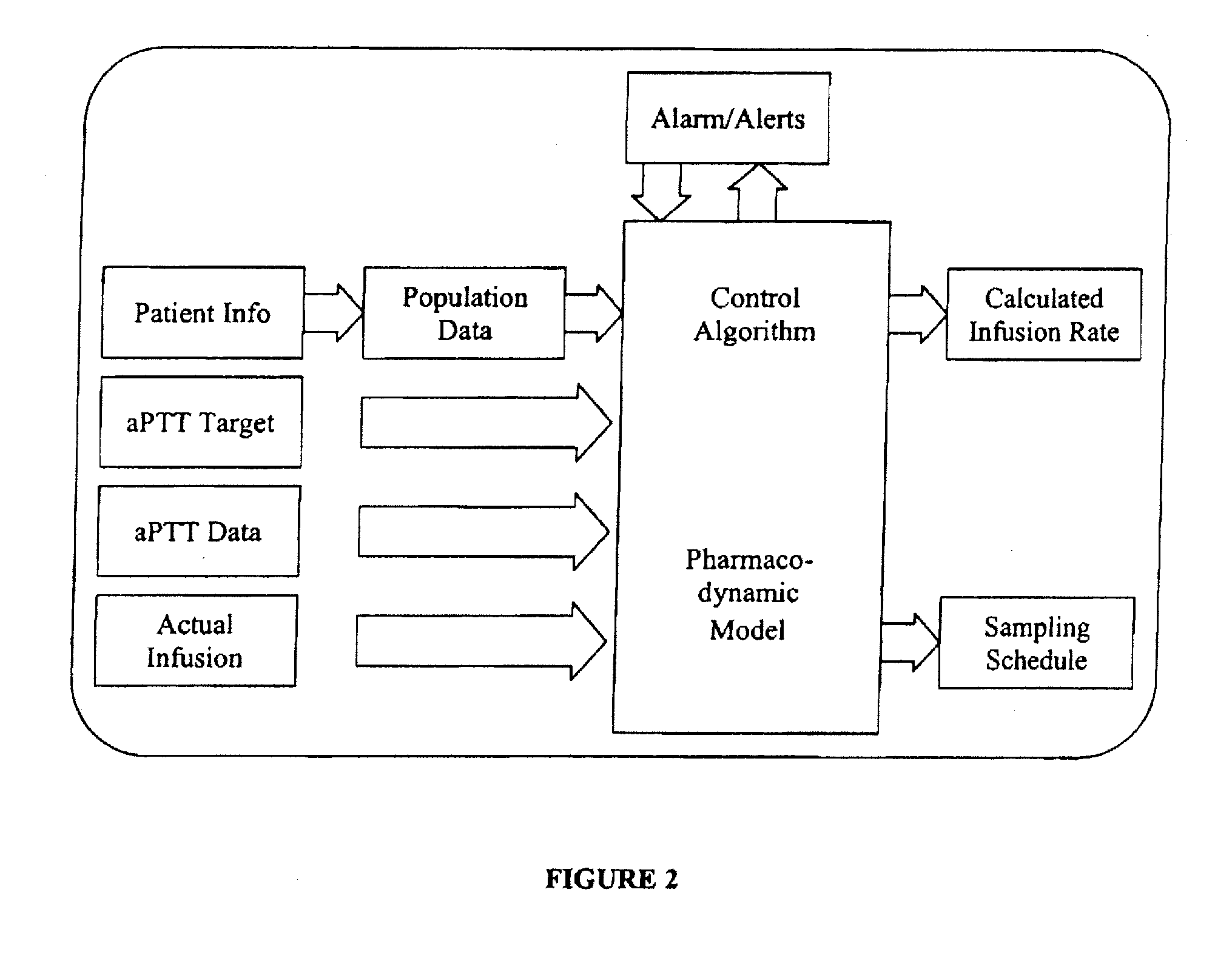
Integrated patient management and control system for medication delivery
InactiveUS20100273738A1Quality improvementOrganic active ingredientsDrug and medicationsPatient modelMeasurement device
An integrated patient monitoring and control system is provided which includes a closed-loop control system for monitoring and adjusting the heparin infusion rate for a patient. The system includes a processor which uses a dynamic patient model that is continuously adjusted based on the patient's aPTT measurements to calculate an optimal heparin infusion rate to achieve an operator-input aPTT target range. The processor also includes a forecasting model to calculate the optimum sample time interval for measuring the patient's aPTT to calculate a new infusion rate. An automated sampling system, which includes a storage device for storing a series of assay devices, an advancement mechanism for moving the assay devices to a sample area, and a measurement device for analyzing a sample dispensed on the assay, is provided. The sampling system is used to repeatedly measure the patient's aPTT according to the sample time interval determined by the processor.
Owner:AUTOMEDICS MEDICAL SYST
Mutant adeno-associated virus virions and methods of use thereof
ActiveUS20050053922A1Reduce the binding forceAltered infectivityAntibacterial agentsVirusesReassortant VirusesNeutralizing antibody
The present invention provides mutant adeno-associated virus (AAV) that exhibit altered capsid properties, e.g., reduced binding to neutralizing antibodies in serum and / or altered heparin binding and / or altered infectivity of particular cell types. The present invention further provides libraries of mutant AAV comprising one or more mutations in a capsid gene. The present invention further provides methods of generating the mutant AAV and mutant AAV libraries, and compositions comprising the mutant AAV. The present invention further provides recombinant AAV (rAAV) virions that comprise a mutant capsid protein. The present invention further provides nucleic acids comprising nucleotide sequences that encode mutant capsid proteins, and host cells comprising the nucleic acids. The present invention further provides methods of delivering a gene product to an individual, the methods generally involving administering an effective amount of a subject rAAV virion to an individual in need thereof.
Owner:INTEGRATIVE GENE THERAPEUTICS +1
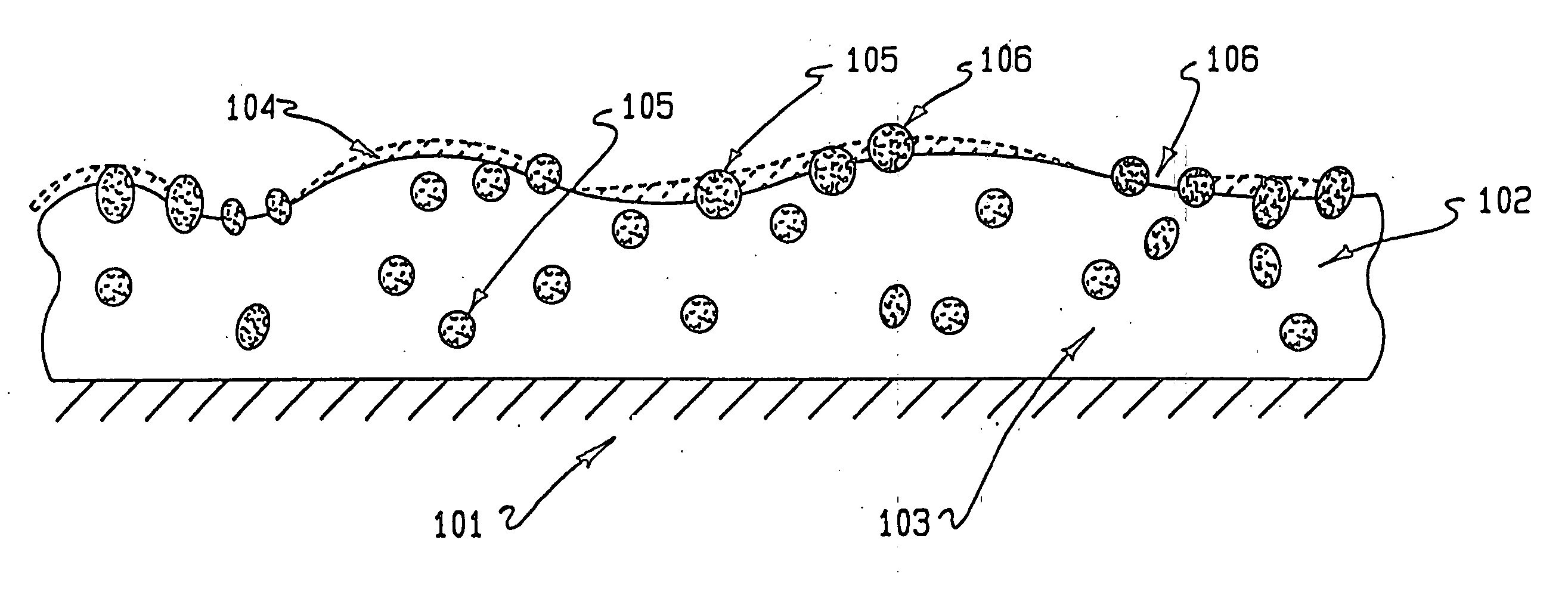
Compositions comprising porous articles and uses in implantable medical devices
A composition comprising a first polymer having pores, nanoparticles dispersed within the pores of the first polymer, the nanoparticles comprising a second polymer and at least one pharmaceutically active agent dispersed in the second polymer, and heparin covalently bonded to at least one of the first and second polymer.
Owner:SAHAJANAND TECHNOLOGIES PRIVATE LTD
Method of using hydroxycarboxylic acids or related compounds for treating skin changes associated with intrinsic and extrinsic aging
A composition comprising an amphoteric or pseudo-amphoteric agent and a polyhydroxy alpha hydroxyacid existing as a free acid, lactone, or salt, and isomeric or non-isomeric forms thereof is provided. The amphoteric or pseudo-amphoteric agent can be selected from amino acids, dipeptides, aminoaldonic acid, aminouronic acid, lauryl aminoproplyglycine, aminoaldaric acid, neuraminic acid desulfated heparin, deacetylated hyaluronic acid, hyalobiuronic acid, chondrosine, deacetylated chondroitin, creatine, creatinine, hydroxyproline, homocysteine, homocystine, homoserine, ornithine, citrulline, phosphatidylserine, and sphingomyelin. The composition may contain other additives, including cosmetic or pharmaceutical agents for topical treatment of dermatological disorders.
Owner:TRISTRATA TECH
Composition and method for preparing biocompatible surfaces
ActiveUS20050244453A1Reduced throughput timeEfficient and cost-effectiveBiocideOrganic active ingredientsPre irradiationActive agent
The invention provides methods and compositions for providing biocompatible surfaces to medical articles. In particular the invention provides biocompatible coatings with heparin activity. In some aspects, the biocompatible coatings of the invention are able to release a bioactive agent. The coatings can be formed using biostable or biodegradable polymeric material and photoreactive groups. The invention also provides methods for improving the quality of bioactive agent-containing coatings by performing pre-irradiation of biocompatible coating compositions.
Owner:SURMODICS INC
Scalable Production Method for AAV
ActiveUS20090275107A1Reduce and eliminate bindingHigh yieldGenetic therapy composition manufactureVirus peptidesLysisBinding site
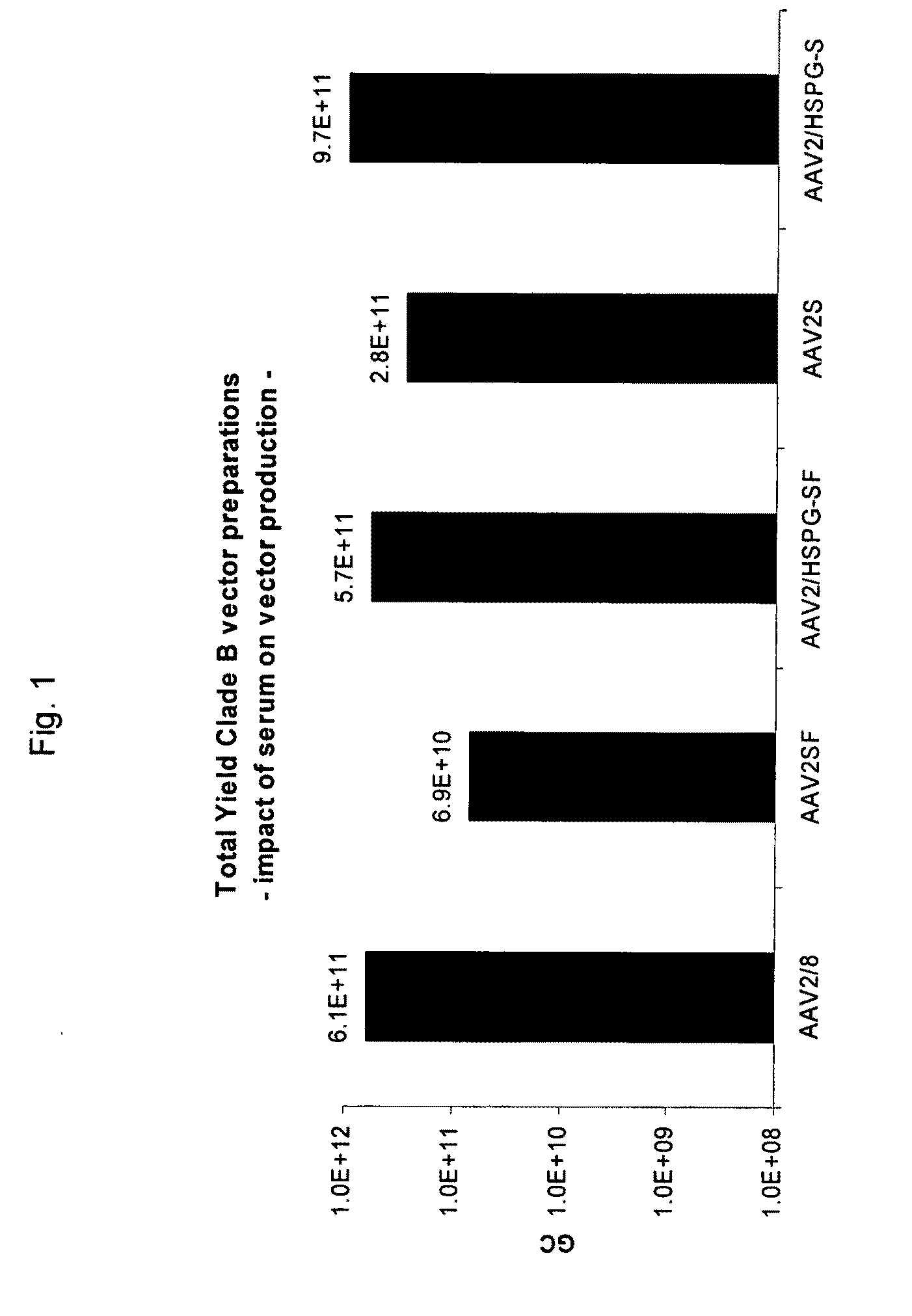
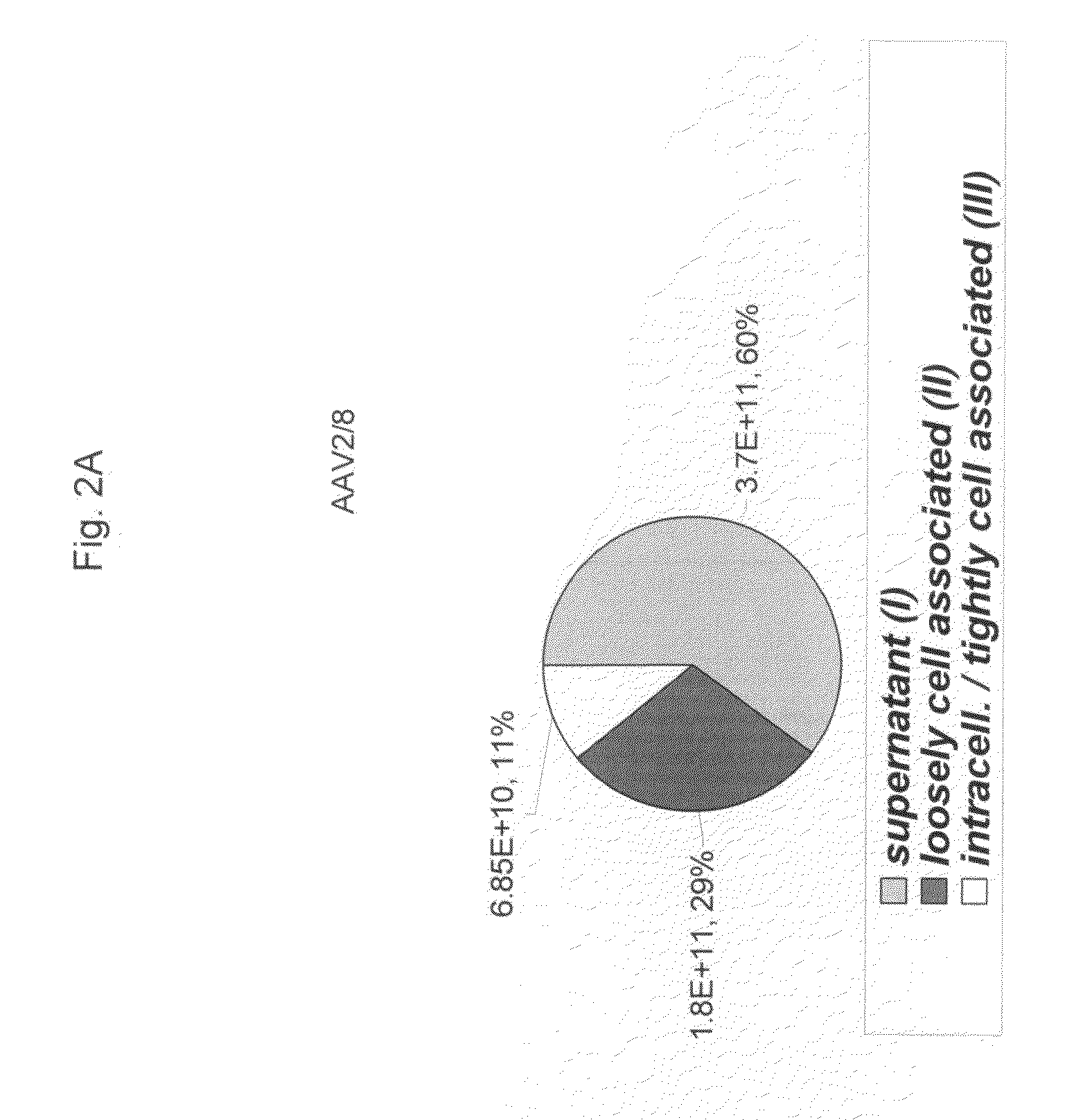
A method for producing AAV, without requiring cell lysis, is described. The method involves harvesting AAV from the supernatant. For AAV having capsids with a heparin binding site, the method involves modifying the AAV capsids and / or the culture conditions to ablate the binding between the AAV heparin binding site and the cells, thereby allowing the AAV to pass into the supernatant, i.e., media. Thus, the method of the invention provides supernatant containing high yields of AAV which have a higher degree of purity from cell membranes and intracellular materials, as compared to AAV produced using methods using a cell lysis step.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Measurement of bioactive hepcidins
ActiveUS20060019339A1Enhancing innate immunityAntibacterial agentsBiocideMicroorganismVertebrate Animals
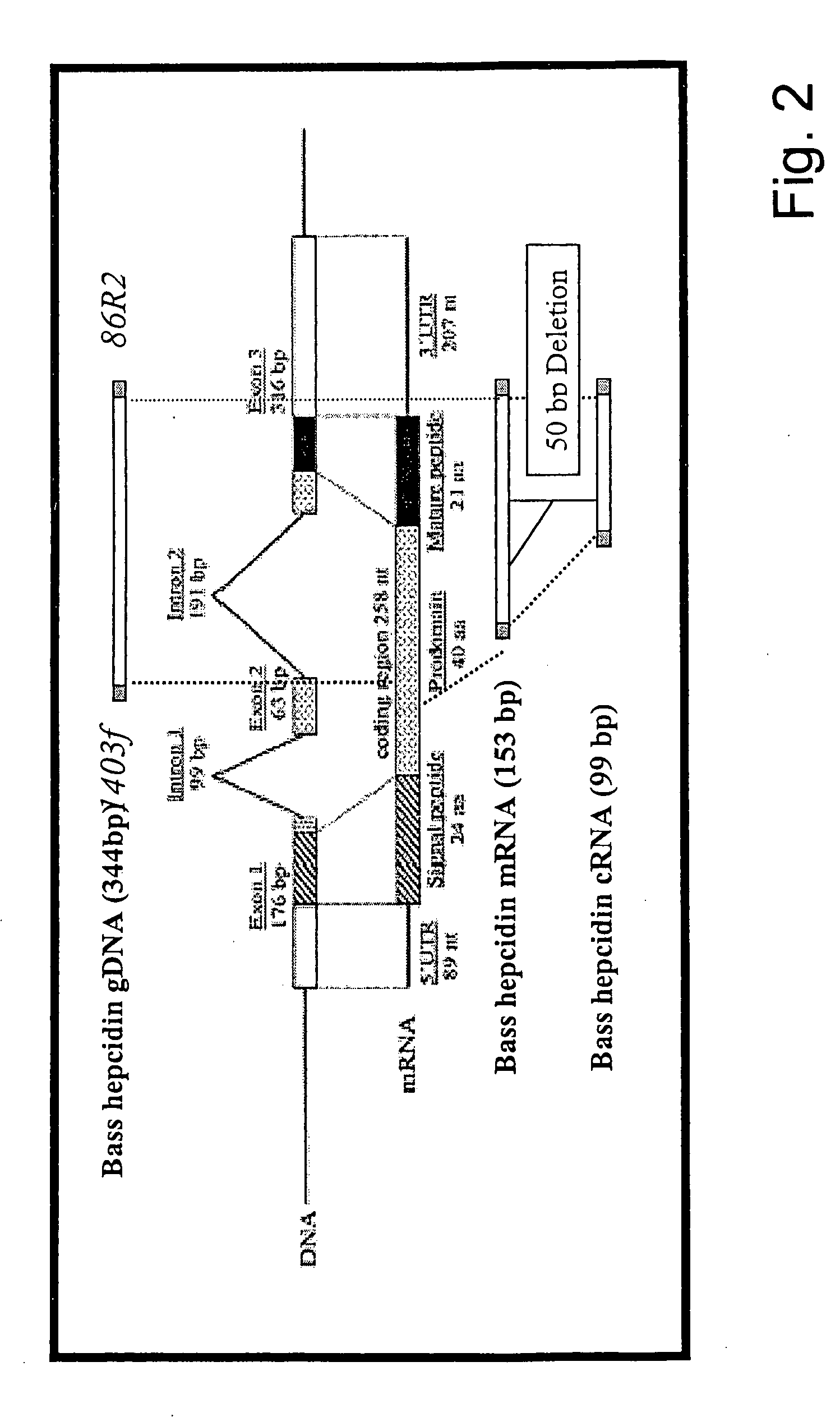
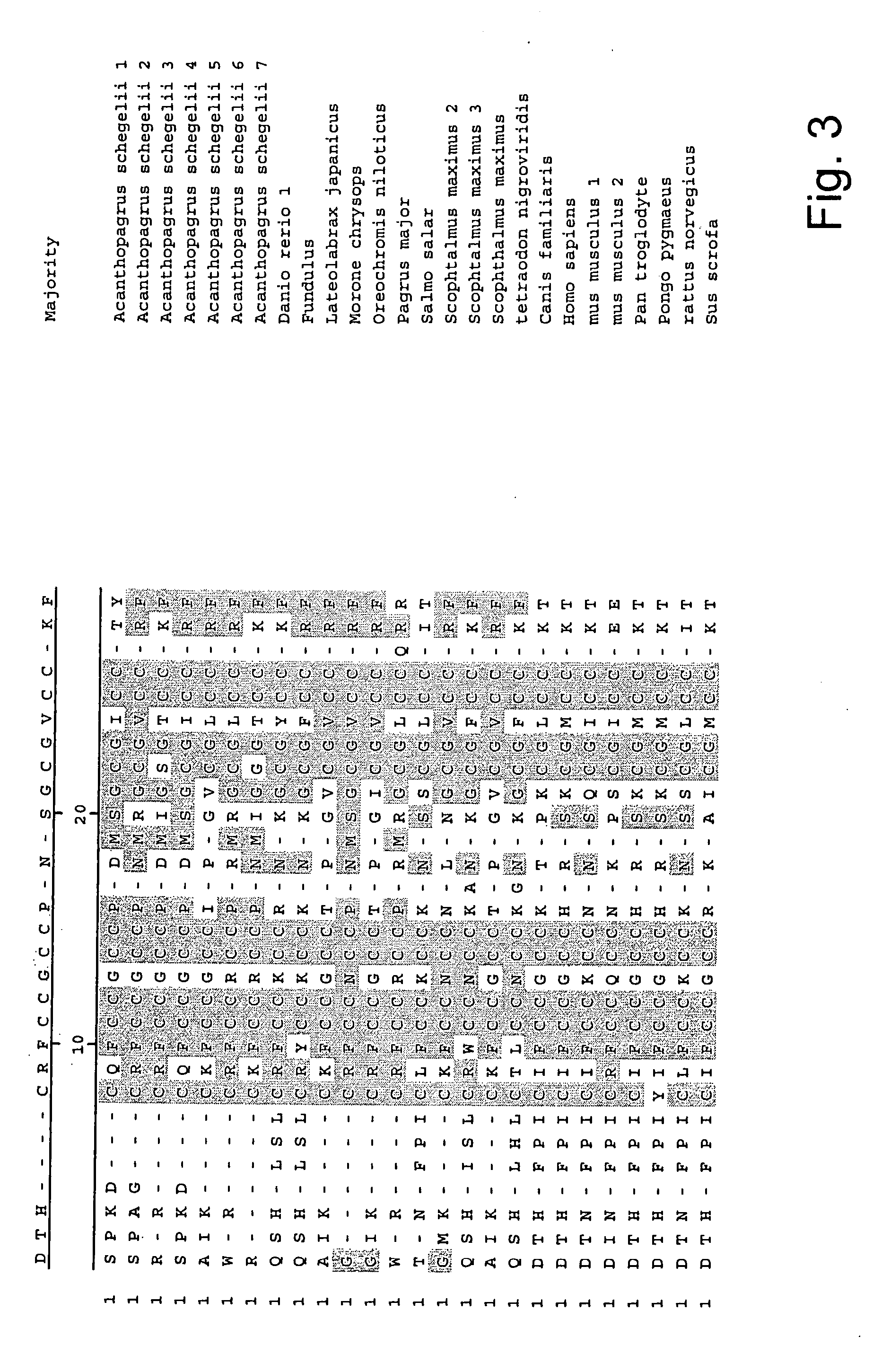
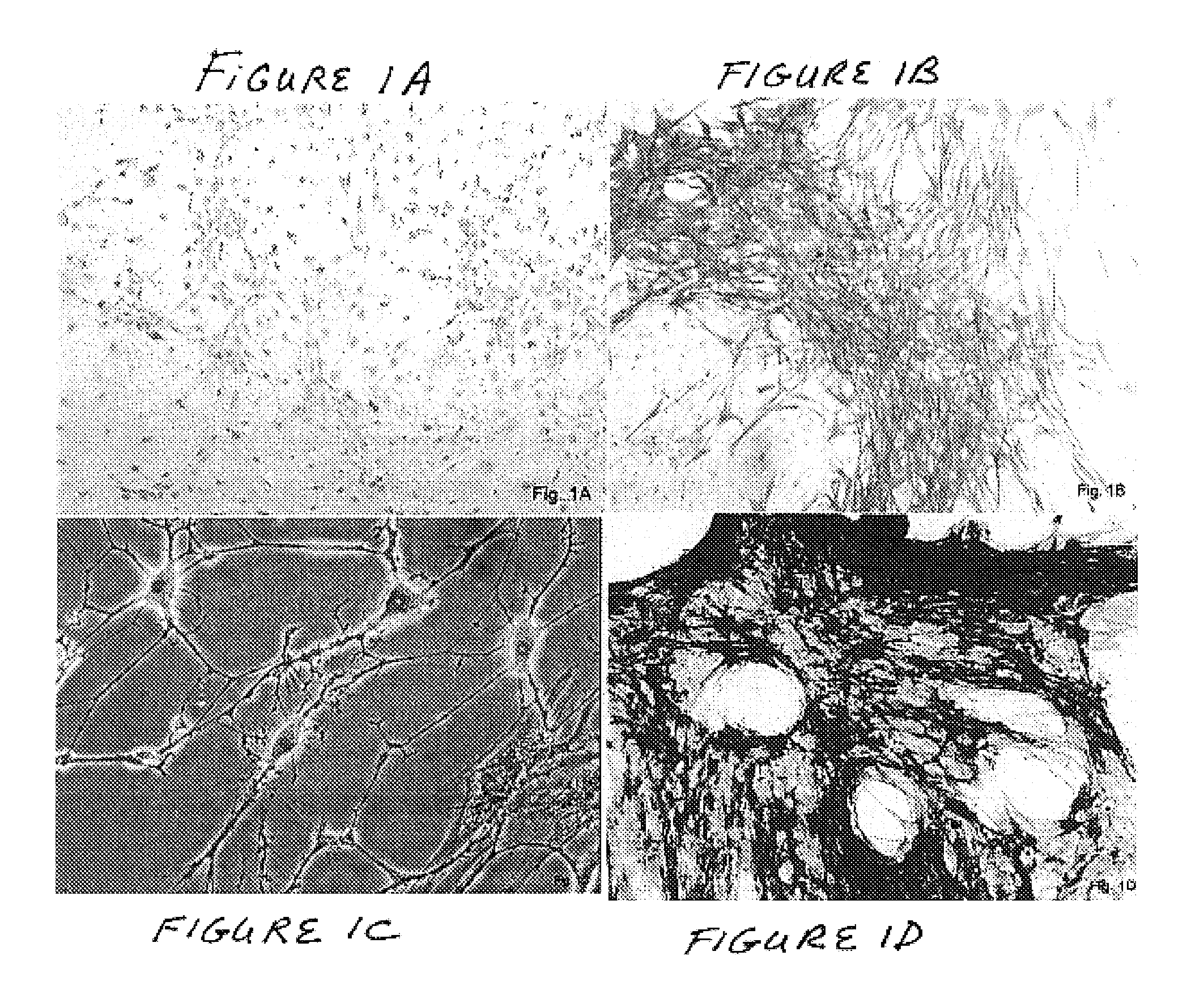
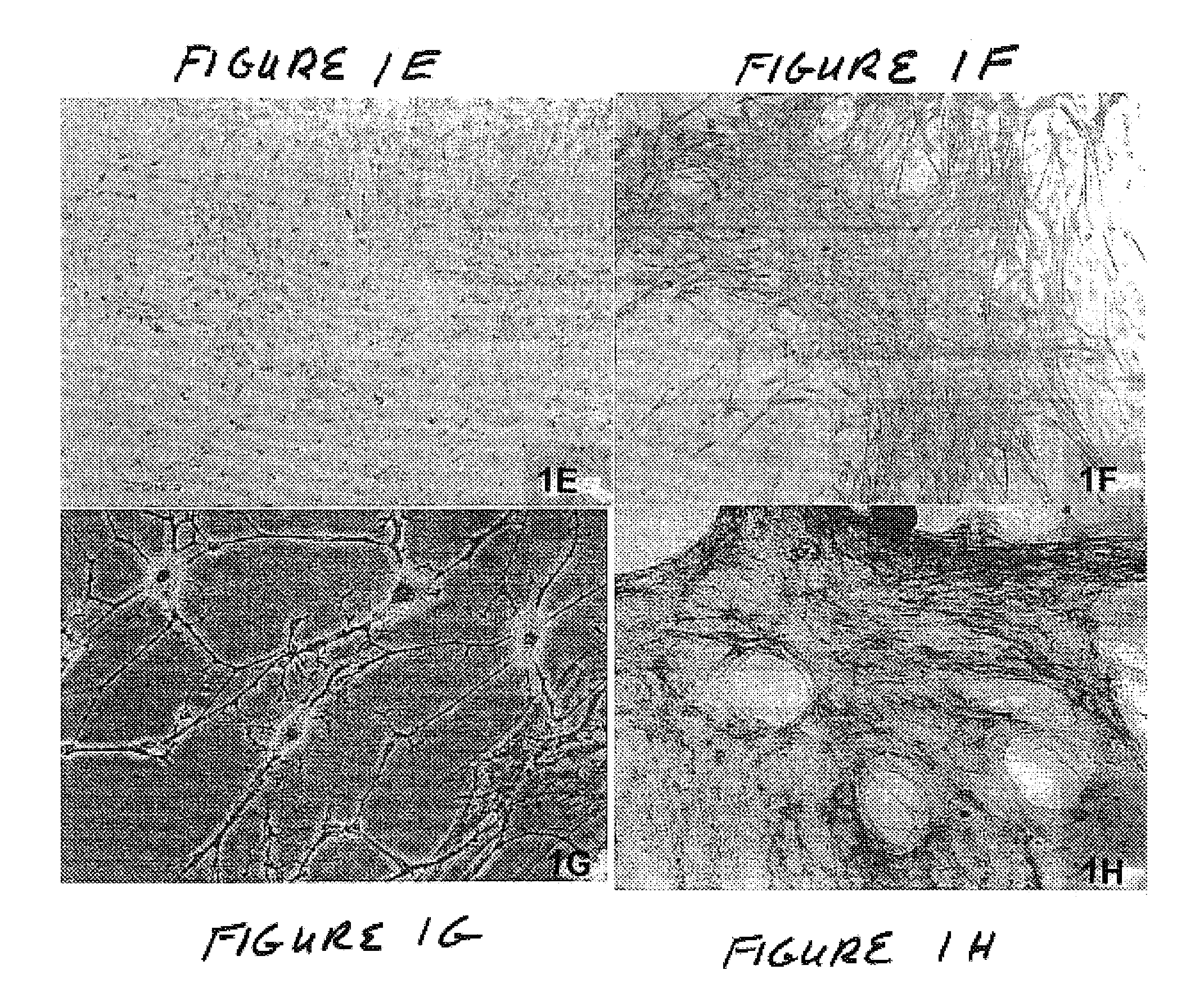
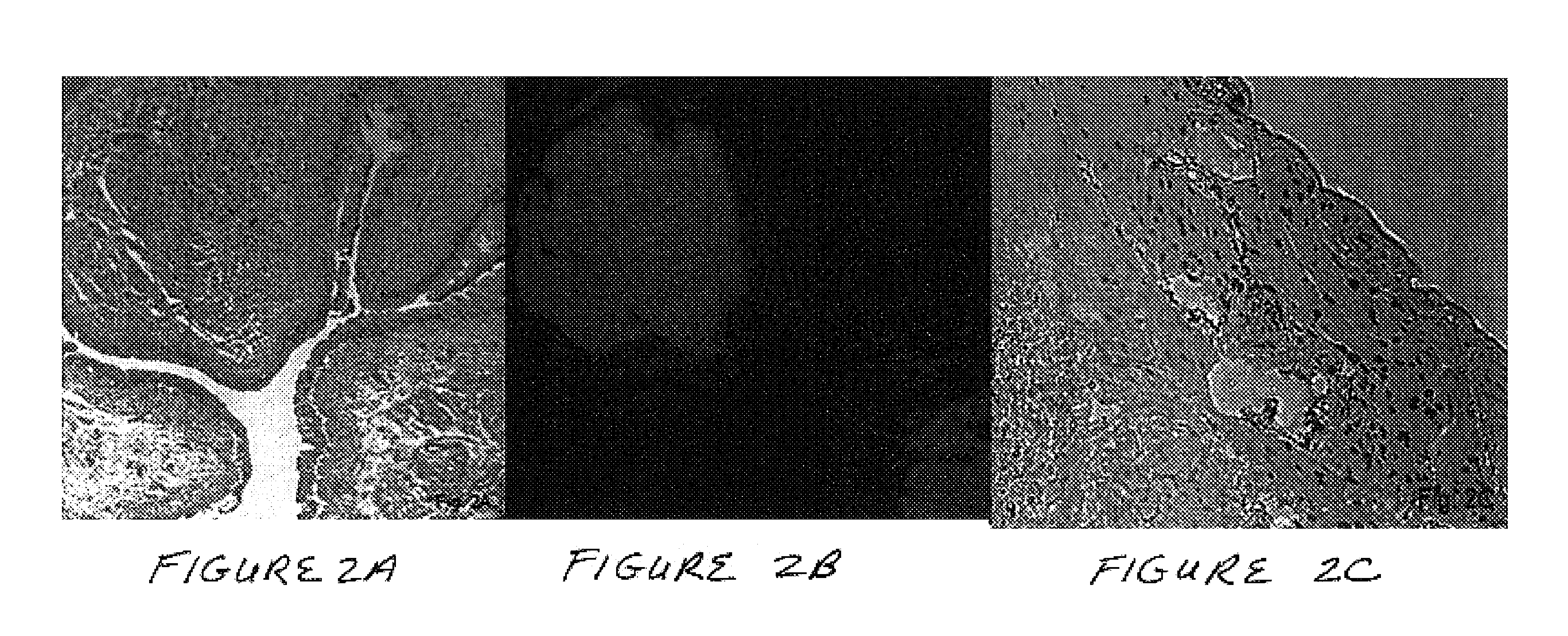
The present invention concerns a method for the oxidative refolding of a hepcidin polypeptide to a form that is mature, bioactive and folded as in the native configuration and molecular mass; a method for measuring the level of native, bioactive hepcidin in a vertebrate animal; a method for measuring the level of hepcidin gene expression in a vertebrate animal; and a method for regulating the production of native, bioactive hepcidin in a vertebrate animal in vivo. The present invention also concerns an antibody or fragment thereof that specifically binds to a continuous, discontinuous, and / or conformational epitope of a mature and bioactive hepcidin folded as in the native configuration; and a pharmaceutical composition that includes the antibody or a hepcidin polypeptide and that provides antimicrobial, agonistic, or antagonistic activities in vivo in a vertebrate animal.
Owner:INTRINSIC LIFESCIENCES LLC
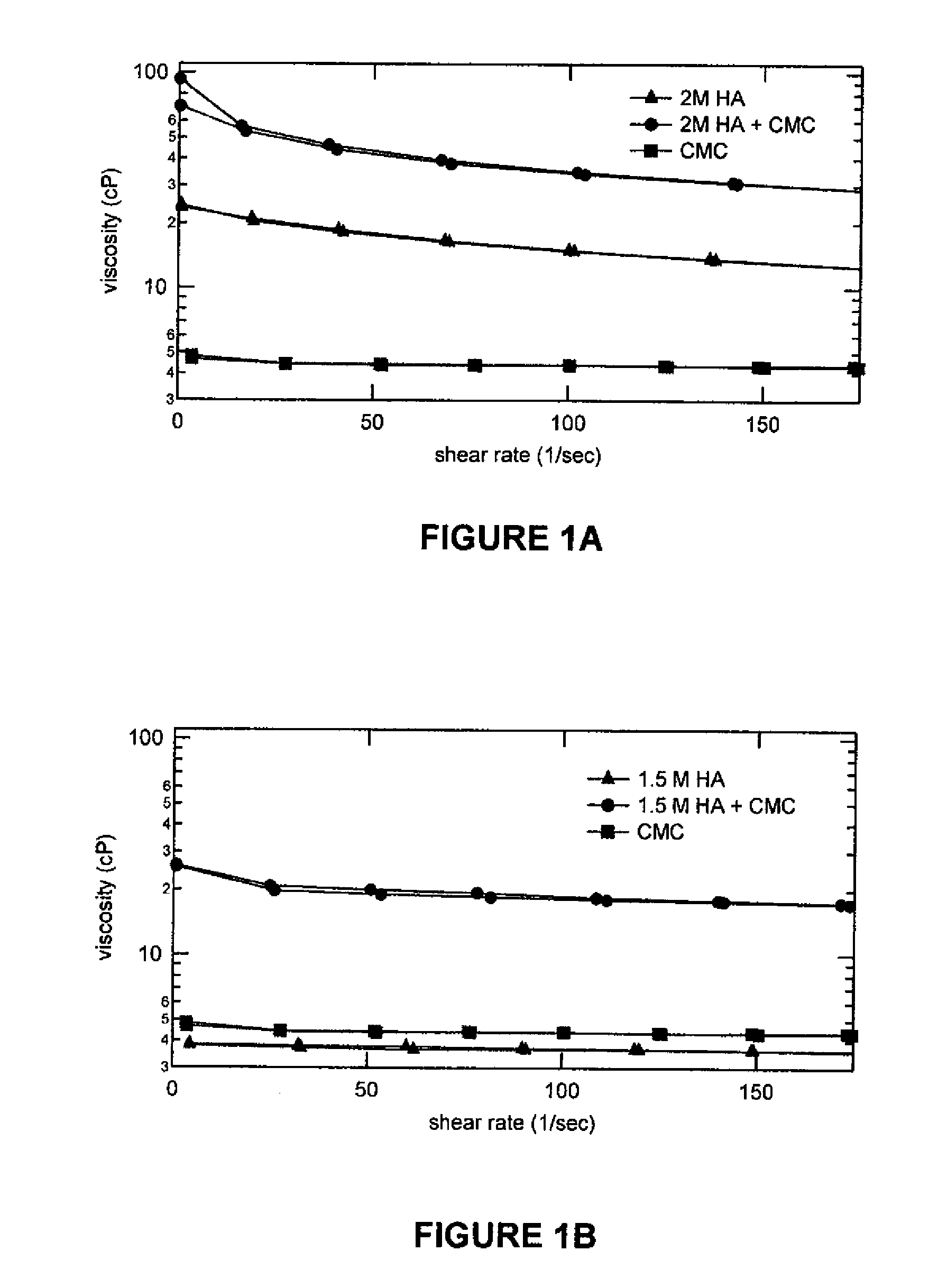
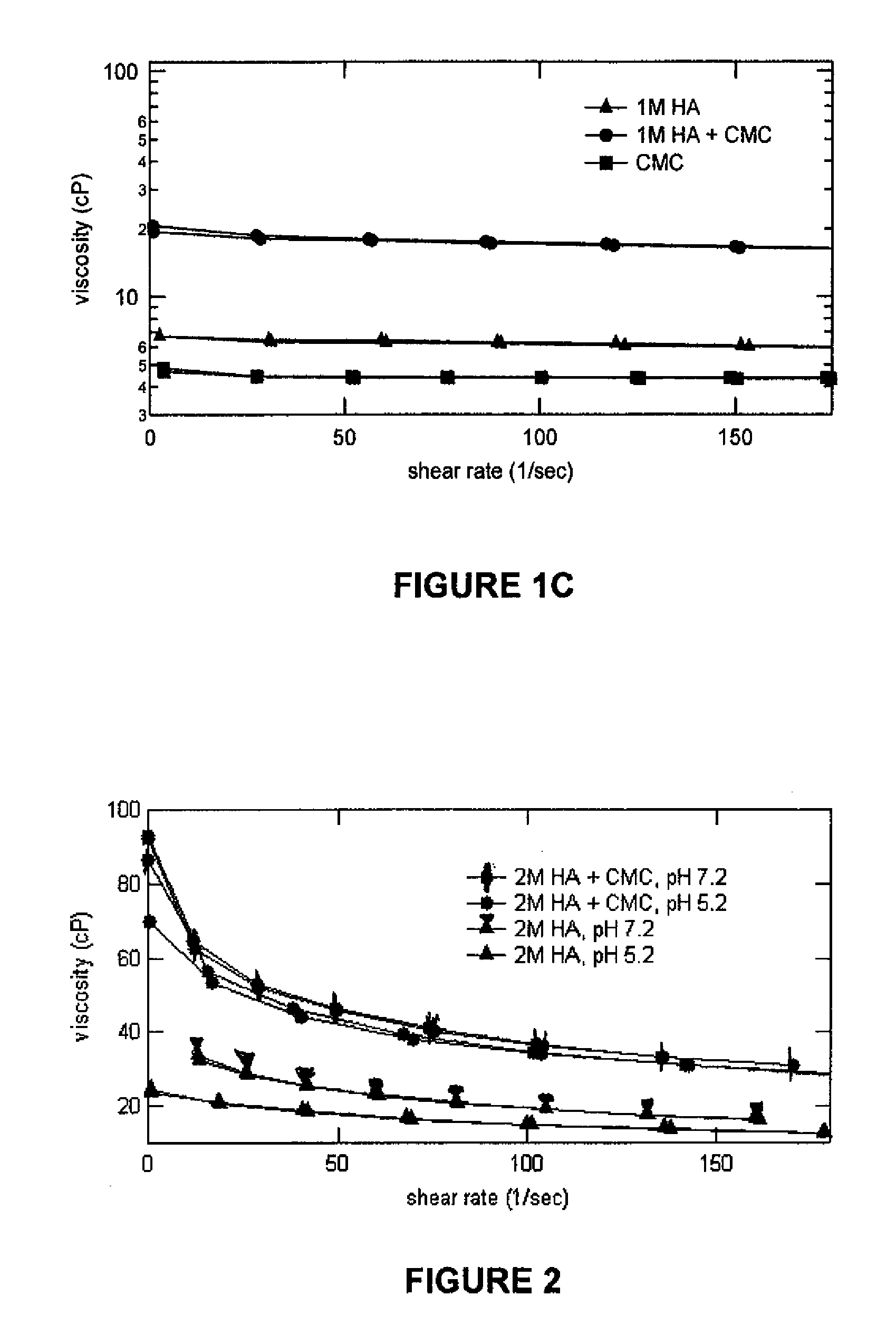
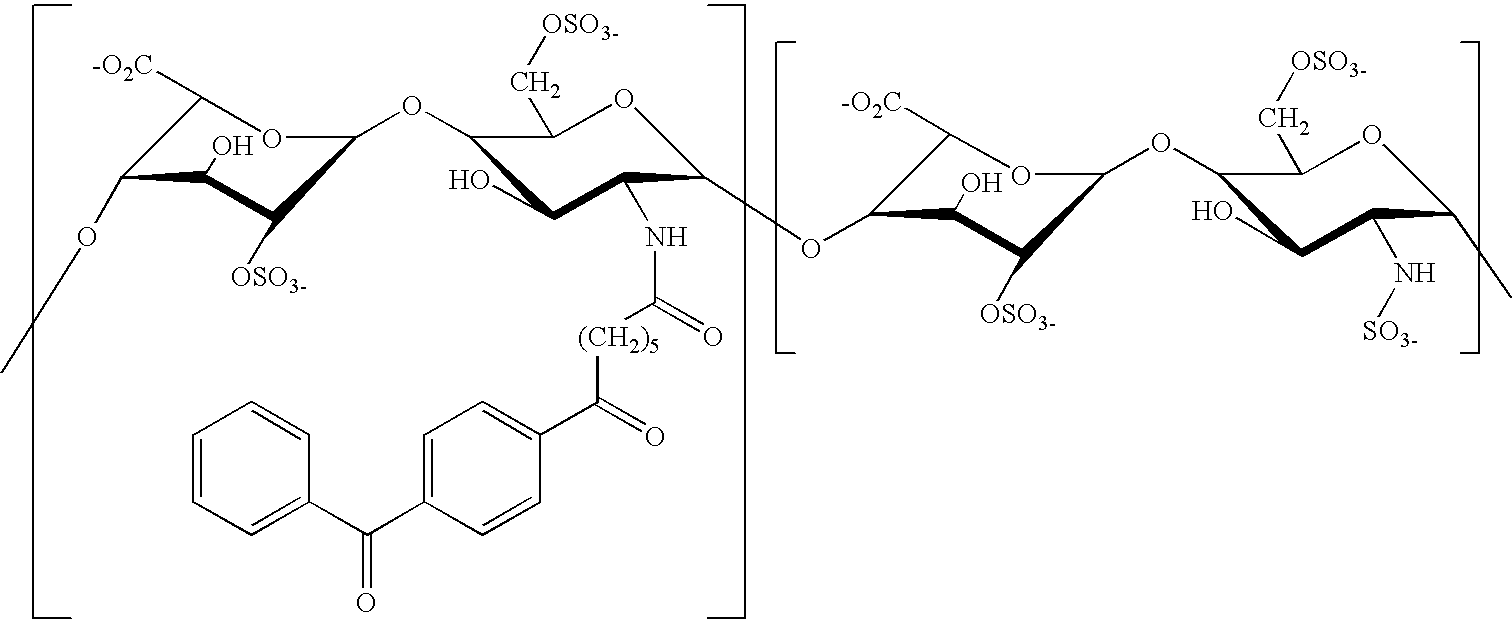
Stabilized Glycosaminoglycan Preparations and Related Methods
Compositions comprising a glycosaminoglycan (e.g., a hyaluronan, hyaluronic acid, hyaluronate, sodium hyaluronate, dermatan sulfate, karatan sulfate, chondroitin 6-sulfate, heparin, etc.) in combination with at least one component selected from; i) polyglycols (e.g., polyethylene glycol), ii) long chain hydroxy polyanionic polysaccharides (e.g., dextran, sodium alginate, alginic acid, propylene glycol alginate, carboxymethyl cellulose and carboxyethyl cellulose, hydroxyl ethyl starch, hydroxyl propyl methyl cellulose, hydroxy propyl ethyl cellulose, hydroxy propyl cellulose, methyl cellulose, polylysine, polyhistidine, polyhydroxy proline, poly ornithine, polyvinyl pyrolidone, polyvinyl alcohol, chitosan, etc.) and iii) long chain Nitrogen containing polymers (e.g., Polylysine, Polyvinylpyrrolidone, and polyvinyl alcohol). The invention also includes methods for using such compositions (e.g., as substance delivery materials, tissue fillers or bulking agents, as moistening or hydrating agents, etc.)
Owner:S K PHARMA INC
Method for modifying a medical implant surface for promoting tissue growth
InactiveUS20080091234A1Without excessive fibrosisIncreased riskCoatingsSurgical veterinaryBioabsorbable polymerPolyhydroxybutyrate
Disclosed is an occluder for closing an intracardiac defect, such as a patent foramen ovale (PFO), and a method for making the same. The occluder includes a frame and at least one scaffold which are formed from a bioabsorbable polymer, such as poly-4-hydroxybutyrate. The surface of the frame and scaffold are textured to promote cell attachment. Texturing of the surface can be achieved by any number of mechanical or chemical procedures. The device is coated with collagen and heparin which are covalently bound to the surface of the device. The occluder provides improved defect closure compared to other septal occluders known in the art. In particular, the occluder described is specifically designed to improve host cell attachment to and tissue ingrowth over the device when implanted in a patient as compared to the level of host cell attachment and tissue ingrowth achieved with other implantable devices made of bioabsorbable polymers.
Owner:WL GORE & ASSOC INC
Methods and compositions for smooth muscle reconstruction
This invention also provides a purified or isolated population of ADSCs that can differentiate into a cell of the leiomyogenic lineage, e.g., smooth muscle or skeletal muscle. In yet another aspect, the population additionally can be differentiated into a lineage selected from the group consisting of osteogenic, adipogenic, chondrogenic, myogenic and neuronal. This invention further provides a composition comprising a substantially homogeneous expanded population of smooth muscle cells. This invention provides a composition comprising a substantially homogeneous expanded population of skeletal muscle cells. Also provided herein is an isolated composition comprising a purified adipose-derived stem cell (ADSC) or progeny of said ADSC and an effective amount of laminin or heparin, effective to induce leiomyogenic differentiation. Diagnositic and therapeutic uses for these compositions are provided herein.
Owner:RGT UNIV OF CALIFORNIA
Diagnostic methods for diseases by screening for hepcidin in human or animal tissues, blood or body fluids; monoclonal antibodies specific to human hepcidin and associated uses therefor
ActiveUS20070224186A1Modulate bindingUseful in treatmentPeptide/protein ingredientsAntipyreticAssayMonoclonal antibody
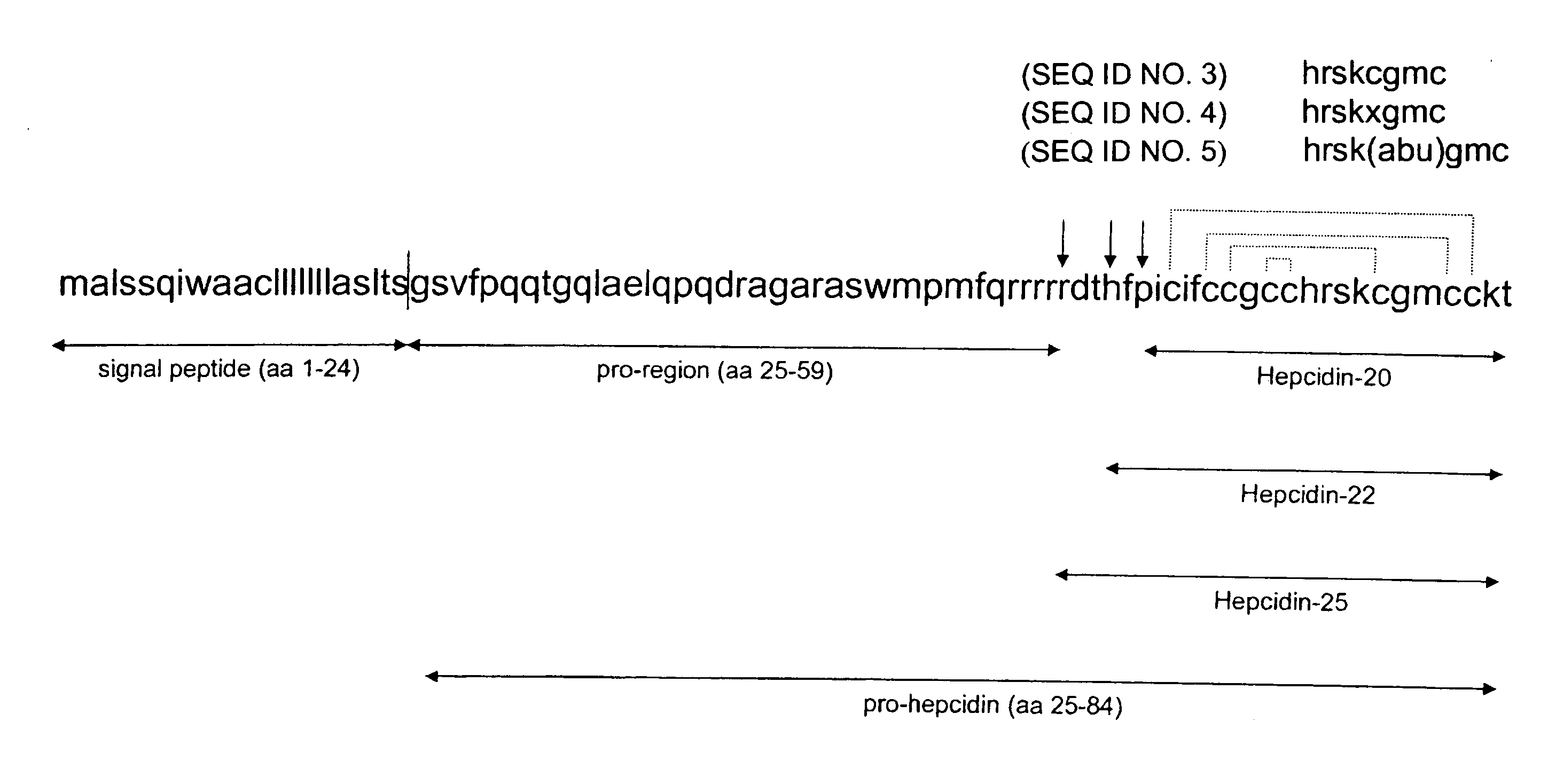
The present invention concerns antibodies specific for the C-terminus of human hepcidin, and related methods and kits for diagnosing and / or treating a disease condition characterized by non-physiological levels of hepcidin protein, including prohepcidin and fragments thereof, comprising obtaining a tissue or fluid sample from a subject; contacting the sample with an antibody or fragment thereof that specifically binds to a polypeptide corresponding to the amino acid sequence between and including amino acids 60 and 84, or, in another embodiment, amino acids 74 and 81, as aligned with the human pre-pro-hepcidin precursor protein, and quantifying the pro-hepcidin and / or mature hepcidin level using an assay based on binding of the antibody and the polypeptide; wherein the non-physiological level of prohepcidin / mature hepcidin is indicative of the disease condition. The present invention also concerns diagnostic methods and kits for applications in genetic technological approaches, such as for overexpressing or downregulating hepcidin.
Owner:DRG INTERNATIONAL INC
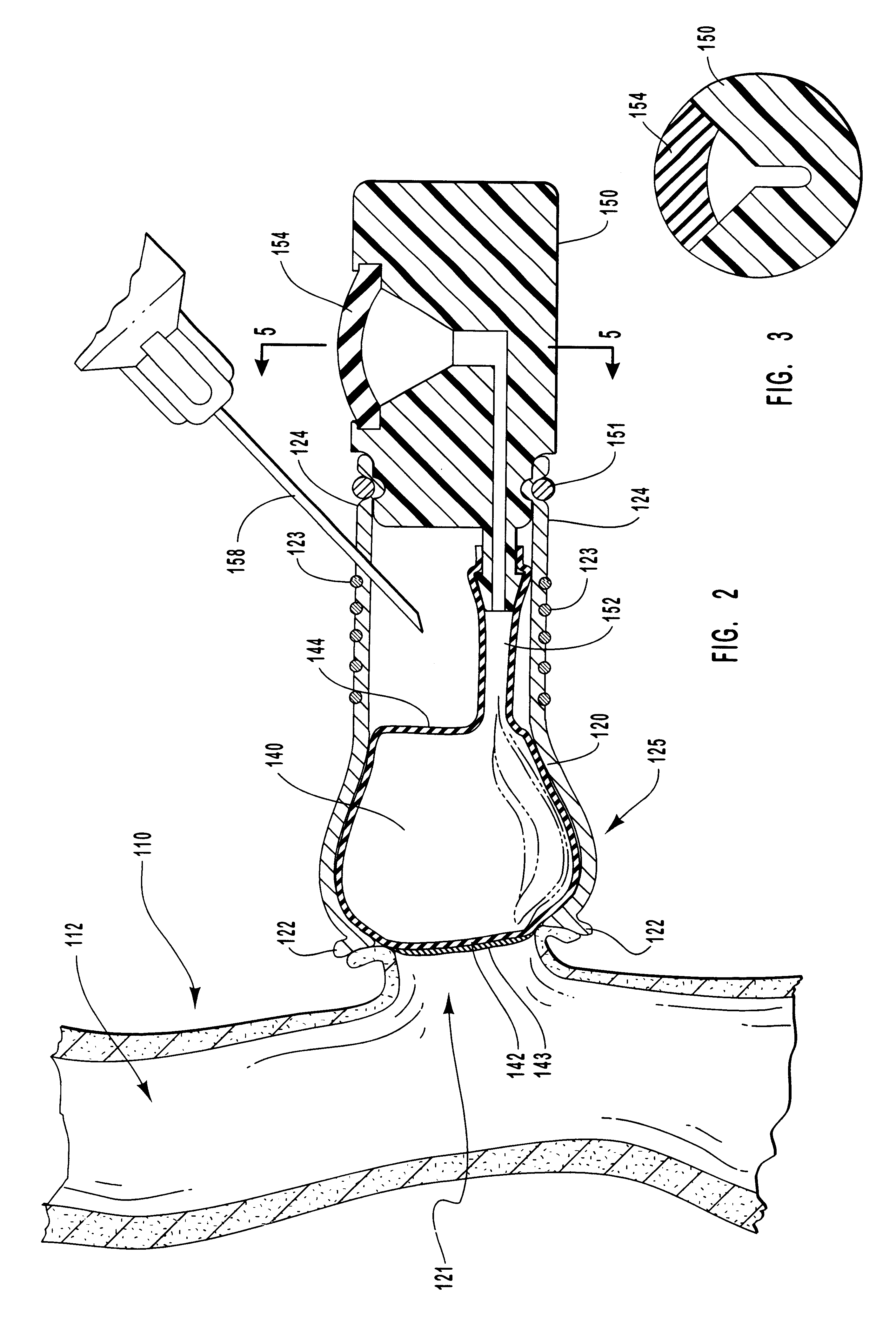
Vascular occlusal balloons and related vascular access devices and systems
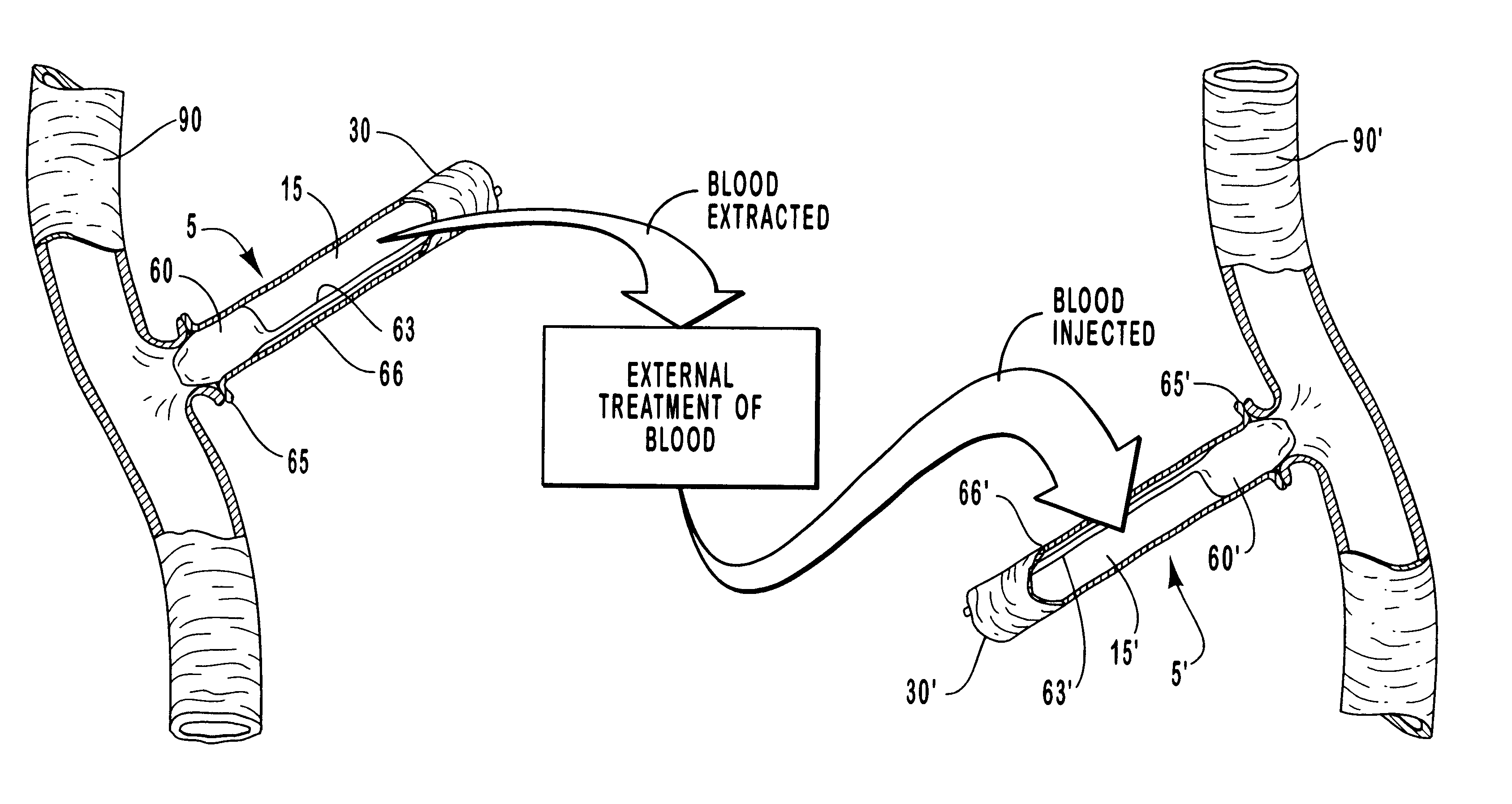
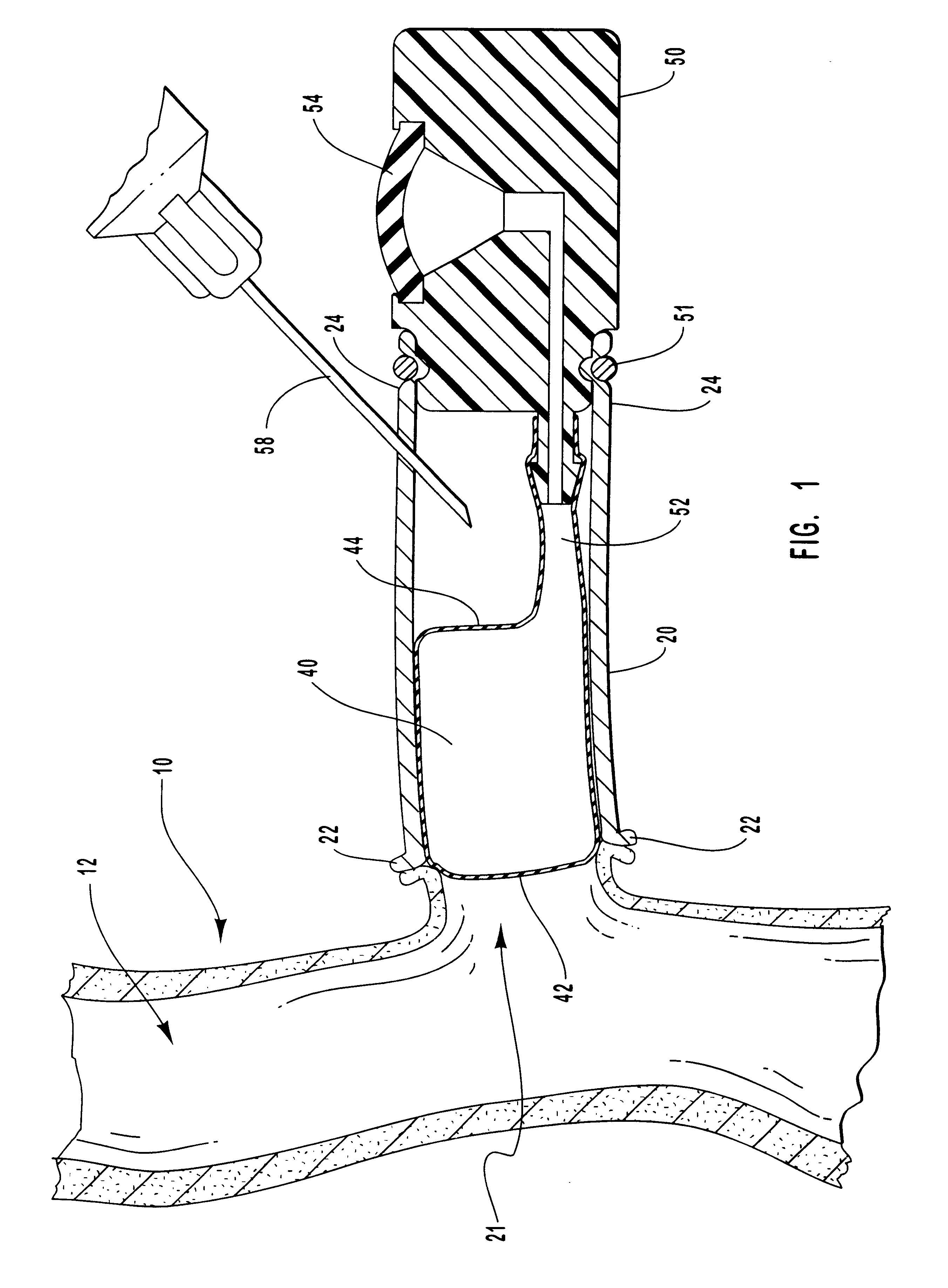
InactiveUS20010007931A1Avoid harmful effectsImprove accessibilityStentsBalloon catheterVeinVascular Access Devices
Vascular access systems and devices for facilitating repeated access to a blood vessel for the external treatment of blood, such as dialysis, and in intra-venous administration of medicines, such as heparin, for extended periods of time. The vascular access systems comprise an anastomosis graft vessel, an occlusal balloon, and a port device for accessing the occlusal balloon. Occlusal balloons can be nonpermeable or permeable to drive an osmotic gradient and to deliver agents, such as heparin, into the blood stream. In addition, occlusal balloons can adopt a distended and a collapsed configuration, the latter allowing for blood flow through the anastomosis graft vessel.
Owner:VITAL ACCESS CORP
Non-thrombogenic and anti-thrombogenic polymers
InactiveUS6096798AEasy to attachReducing the thrombin-antithrombin complex concentrationCosmetic preparationsImpression capsPolymer scienceThrombogenicity
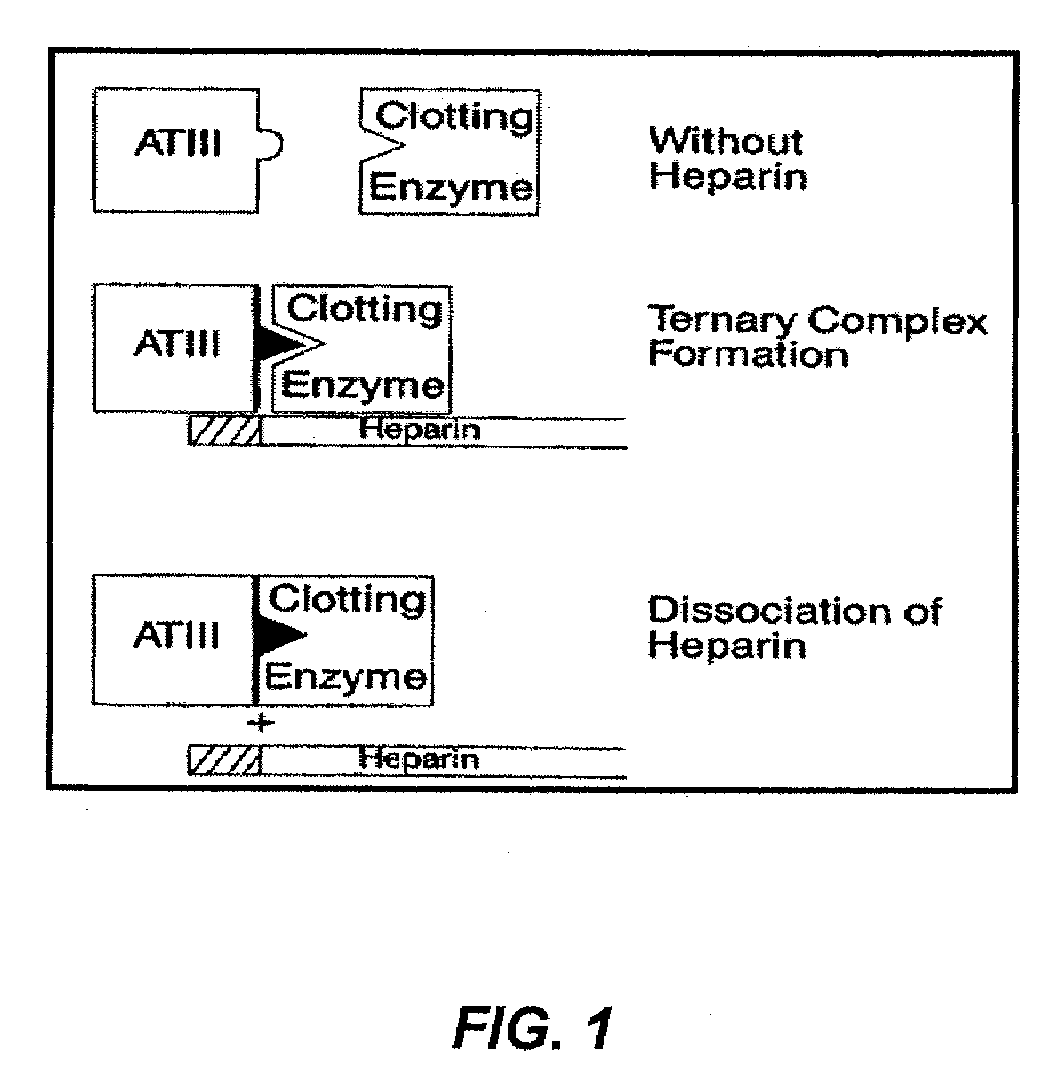
PCT No. PCT / GB97 / 01173 Sec. 371 Date Apr. 30, 1999 Sec. 102(e) Date Apr. 30, 1999 PCT Filed Apr. 30, 1997 PCT Pub. No. WO97 / 41164 PCT Pub. Date Nov. 6, 1997Polymers having non-thrombogenic properties can be prepared by copolymerizing monomers of at least three classes selected from (a) monomers having sulphate groups, (b) monomers having sulphonate groups, (c) monomers having sulphamate groups, (d) monomers having polyoxyalkylene ether groups, and (e) monomers having zwitterionic groups. The polymers can additionally be provided with anti-thrombogenic properties by including an additional comonomer having a pendant heparin (or hirudin, warfarin or hyaluronic acid) group. The polymers can be used as coating materials for medical devices, such as tubing or connectors, in order to provide them with non-thrombogenic, and optionally anti-thrombogenic, properties.
Owner:BIOINTERACTIONS
Coating Employing an Anti-Thrombotic Conjugate
InactiveUS20090018646A1Few stepsImprove versatilityStentsOrganic active ingredientsActive agentSide chain
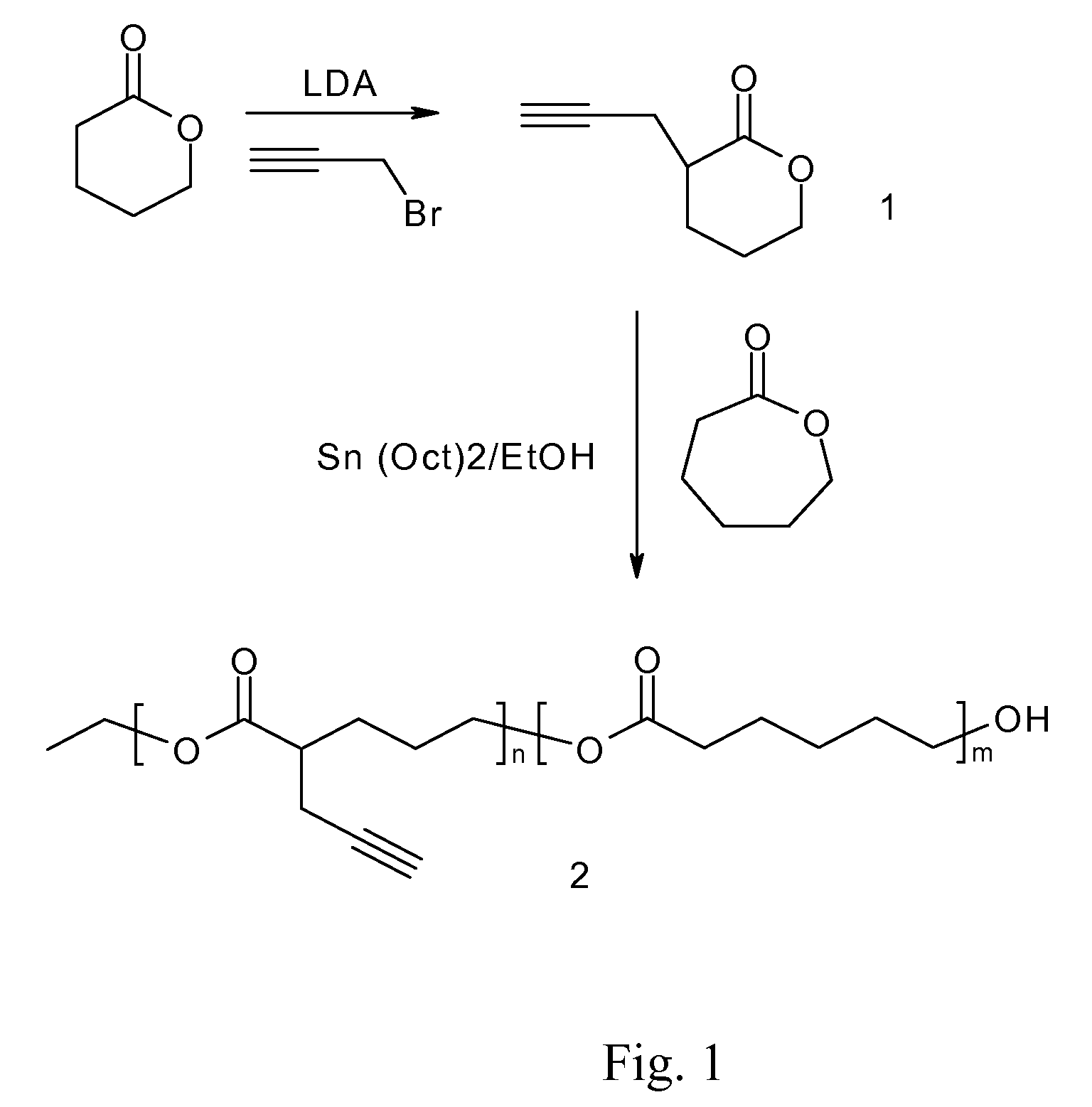
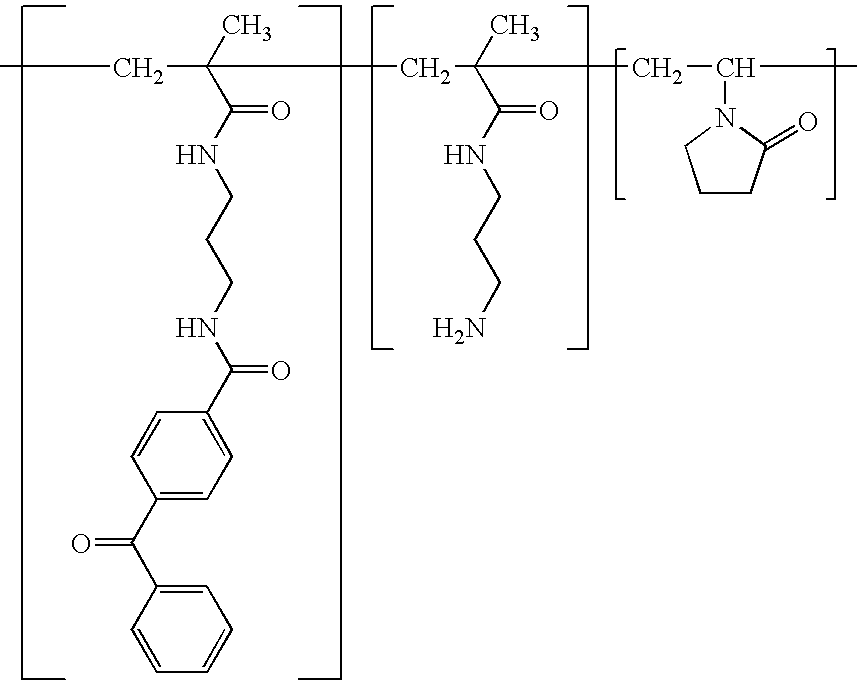
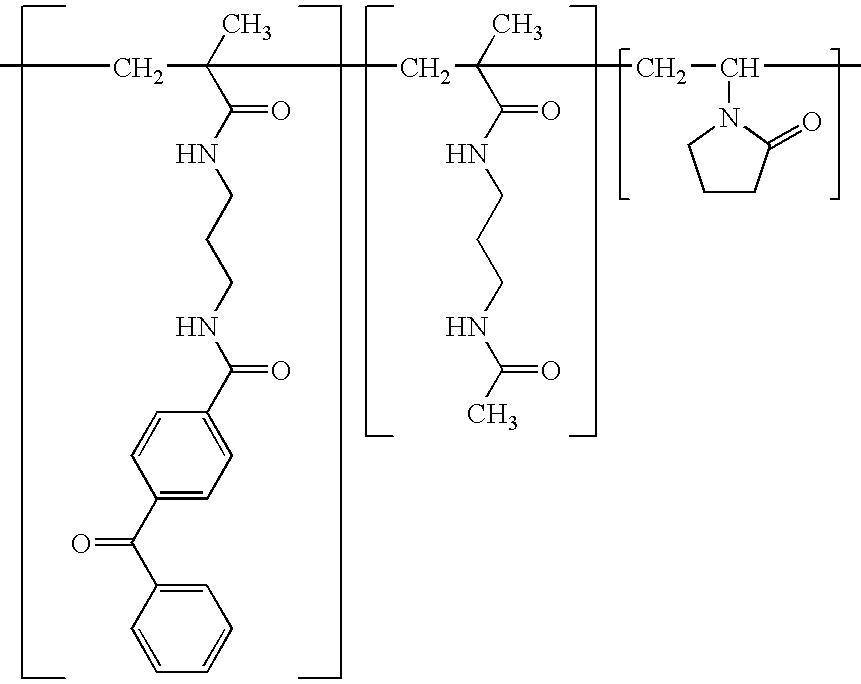
A biodegradable antithrombotic conjugate having heparin and other anti-thrombotic moieties are introduced as side chains to the polymer backbone modified by click chemistry. Various bioabsorbable monomers and dimers such as valerolactone may be used in the monomer derivation, homo- and co-polymerization, and the conjugation with a biologically active molecule by click chemistry. A coating comprising a biocompatible and bioabsorbable polymer anti-thrombotic conjugate is applied to at least a portion of an implantable device to prevent or reduce the formation of thrombosis on the surface of the implantable device. A first or sub-layer of the coating is prepared by mixing a polymeric material and a biologically active agent with a solvent, thereby forming a homogeneous solution. A second or outer layer comprising the present anti-thrombotic conjugate may be applied over the inner drug-containing layers using, for example, a dip coating or spray coating process.
Owner:CORDIS CORP
Macromolecular drug complexes having improved stability and therapeutic use of the same
InactiveUS20050147581A1Safe and easy and effective deliveryAlleviate and eliminate and reverse complicationPowder deliveryOrganic active ingredientsDiseaseSomatotropic hormone
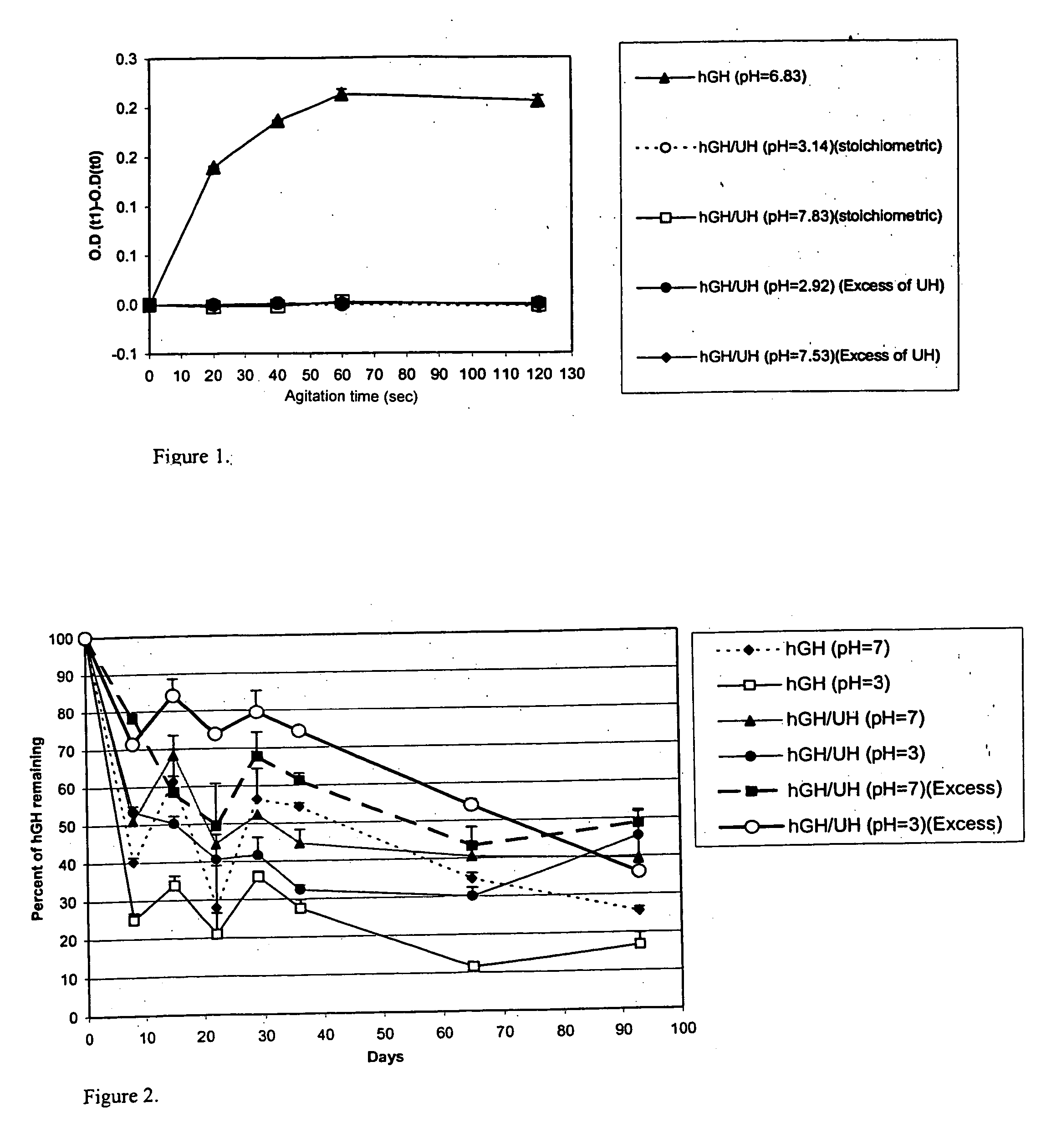
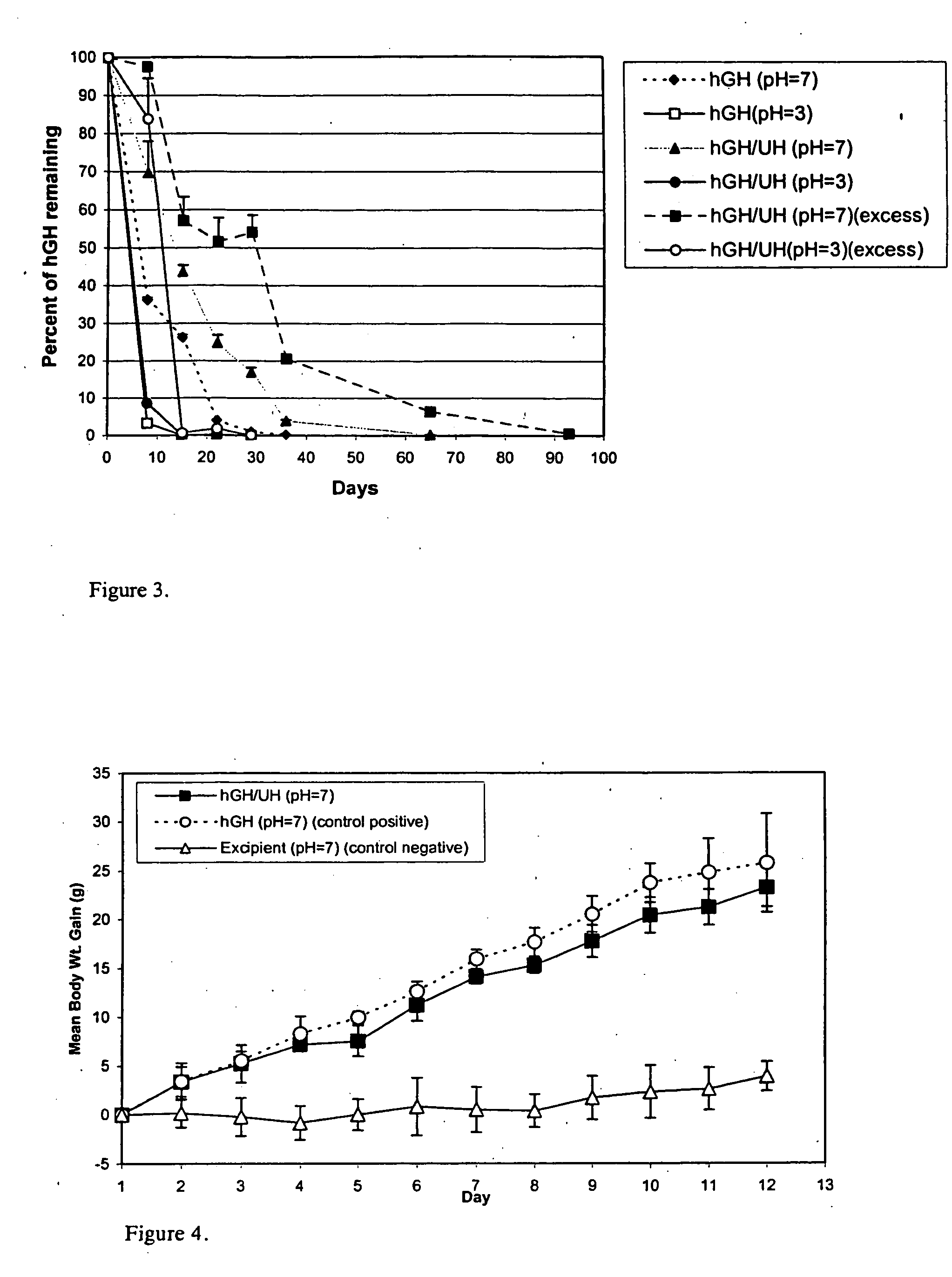
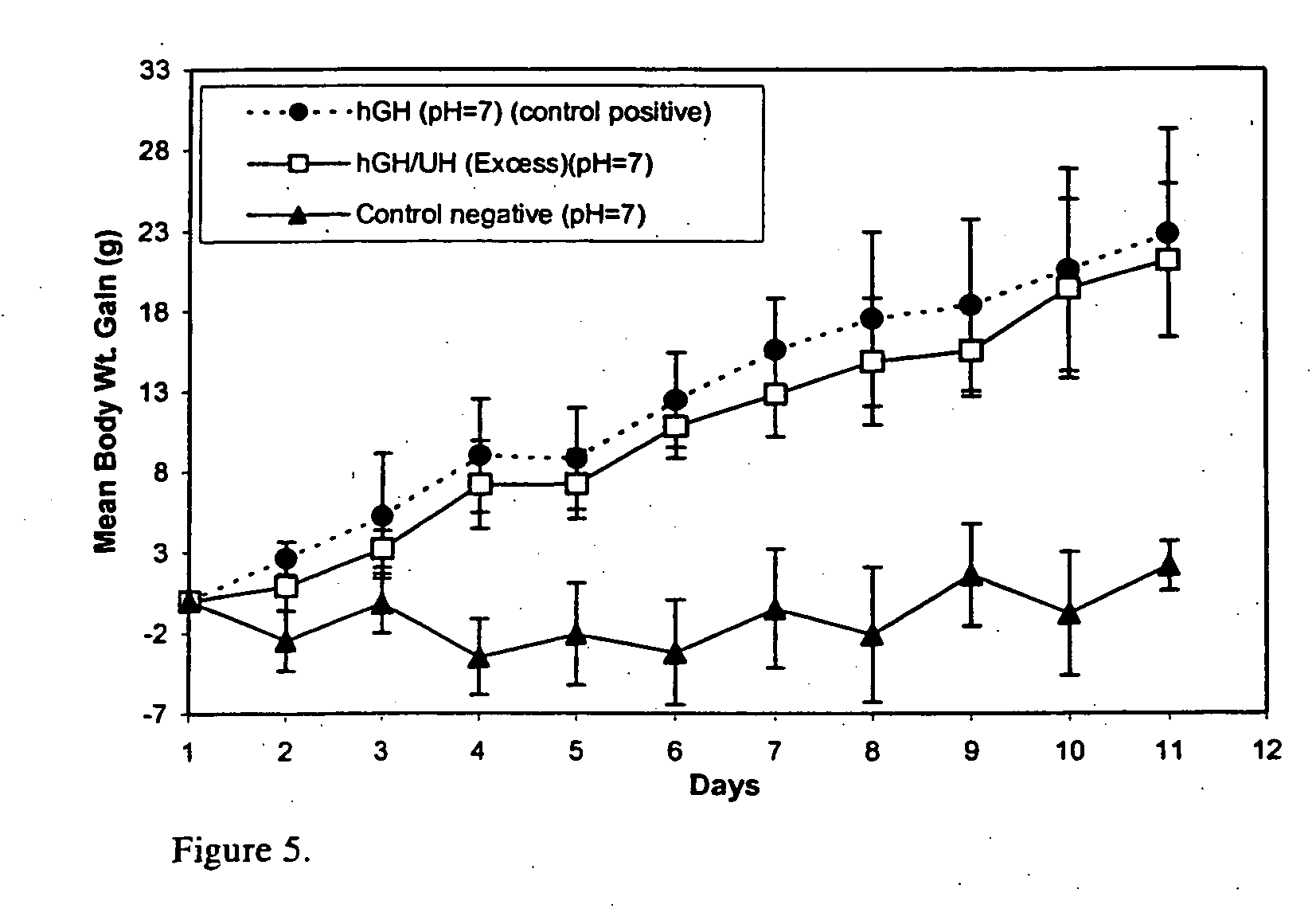
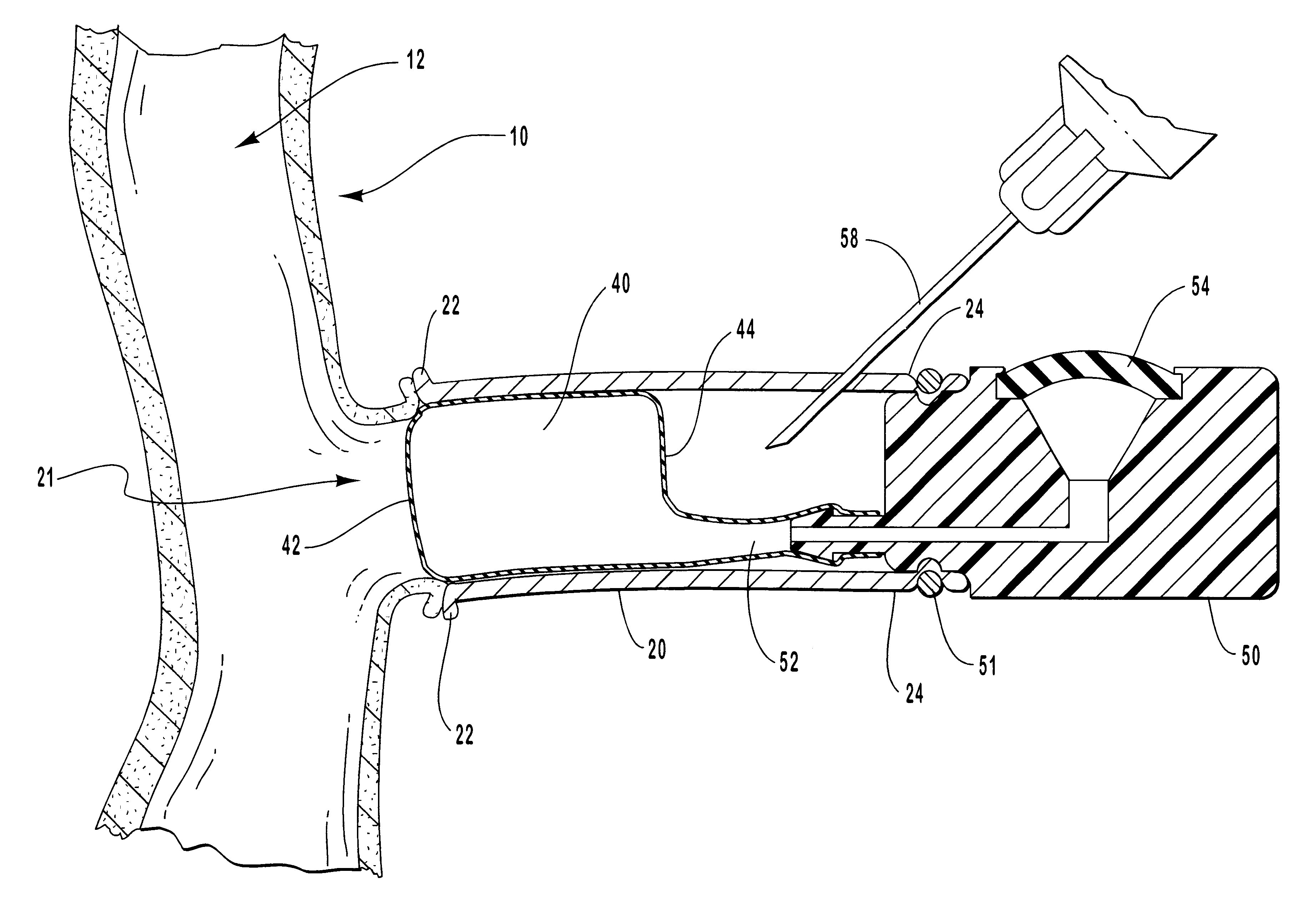
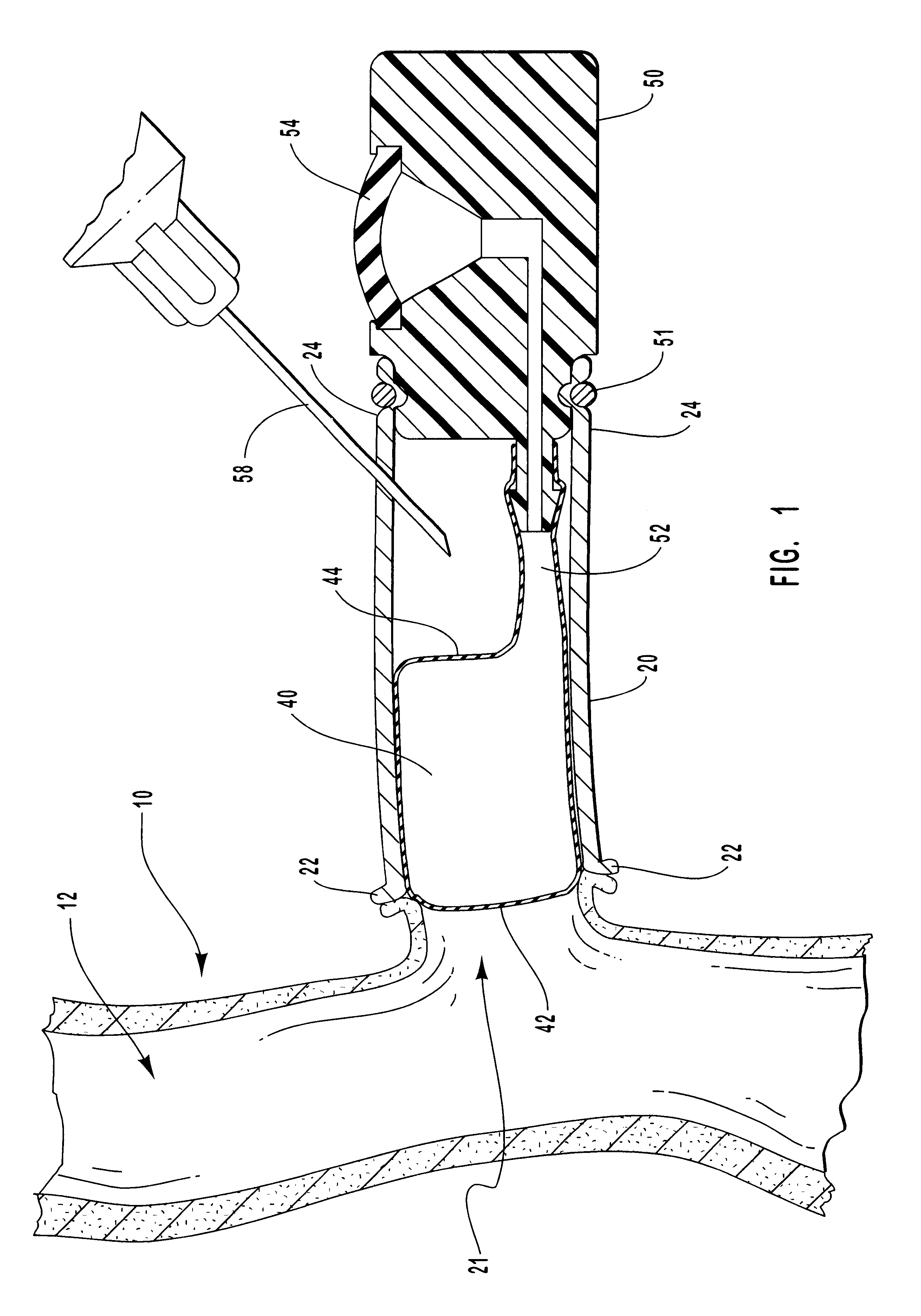
Macromolecular drug complexes containing a protein therapeutic, like human growth hormone, and an excess stoichiometric molar amount of a polymer, like heparin, and compositions containing the same, are disclosed. Compositions containing the macromolecular drug complexes are administered, including via pulmonary delivery, to individuals suffering from a disease or condition, and the complexes release the protein therapeutic, in vivo, to treat the disease or condition.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Vascular access devices and systems
InactiveUS6656151B1Vascular deterioration can be seriously acceleratedReduces and even avoids errorStentsBalloon catheterVascular Access DevicesBlood Vessel Tissue
Vascular access systems and devices for facilitating repeated access to a blood vessel. These systems and devices can be used in external treatment of blood, such as dialysis, and in intra-venous administration of medicines, such as heparin, for extended periods of time, while avoiding deleterious effects such as those derived from repeated puncturing of the blood vessel tissues or exposure of such tissues to abnormal fluid flows. The vascular access systems comprise an anastomosis graft vessel, an occlusal balloon, and a port device for accessing the occlusal balloon. Occlusal balloons can be self-contained, they can rely on osmosis, and they can serve as the support of an agent to which the blood stream is exposed, either by transport or by mere contact. In addition, occlusal balloons can adopt a distended and a collapsed configuration, the latter allowing for blood flow through the anastomosis graft vessel.
Owner:VITAL ACCESS CORP
Coatings for implantable medical devices
A composition comprising at least one polymer covalently bonded to heparin, and at least one pharmaceutically active agent other than heparin dispersed within the at least one polymer.
Owner:SAHAJANAND MEDICAL TECH PVT
Cell-mediated delivery and targeted erosion of noncovalently crosslinked hydrogels
Owner:UNIVERSITY OF DELAWARE
Vascular occlusal balloons and related vascular access devices and systems
InactiveUS6663590B2Avoid harmful effectsImprove accessibilityStentsBalloon catheterVeinVascular Access Devices
Vascular access systems and devices for facilitating repeated access to a blood vessel for the external treatment of blood, such as dialysis, and in intra-venous administration of medicines, such as heparin, for extended periods of time. The vascular access systems comprise an anastomosis graft vessel, an occlusal balloon, and a port device for accessing the occlusal balloon. Occlusal balloons can be nonpermeable or permeable to drive an osmotic gradient and to deliver agents, such as heparin, into the blood stream. In addition, occlusal balloons can adopt a distended and a collapsed configuration, the latter allowing for blood flow through the anastomosis graft vessel.
Owner:VITAL ACCESS CORP
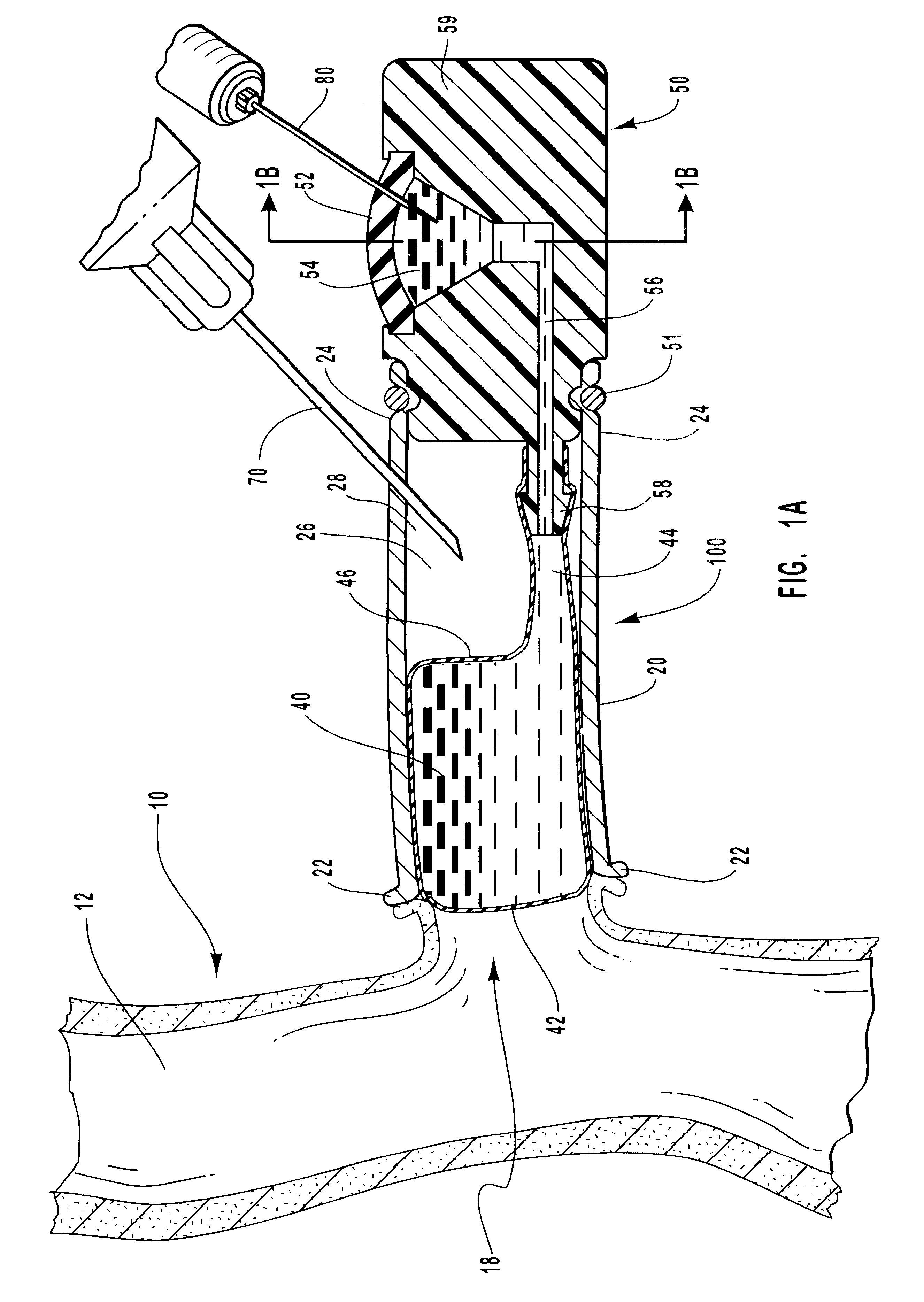
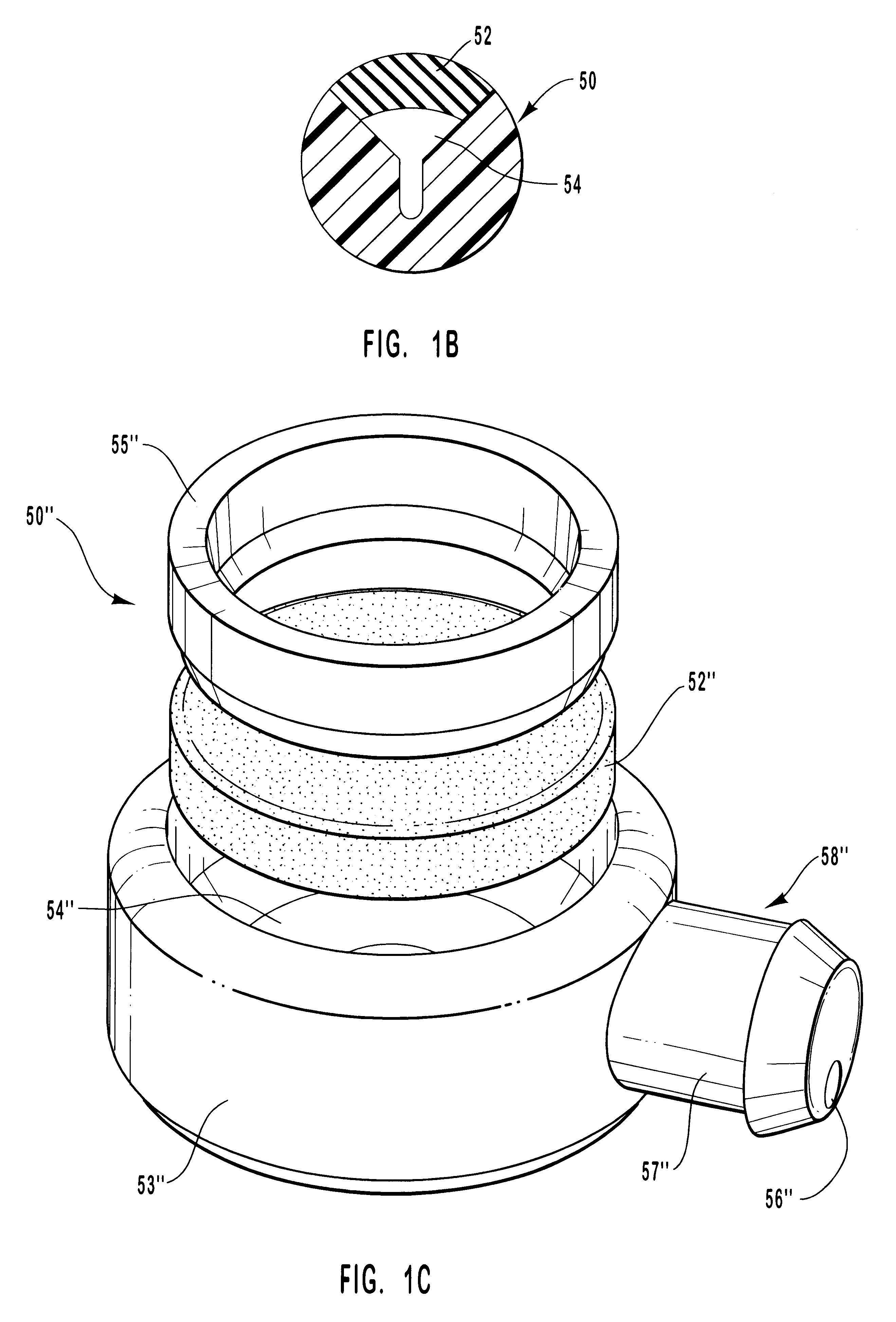
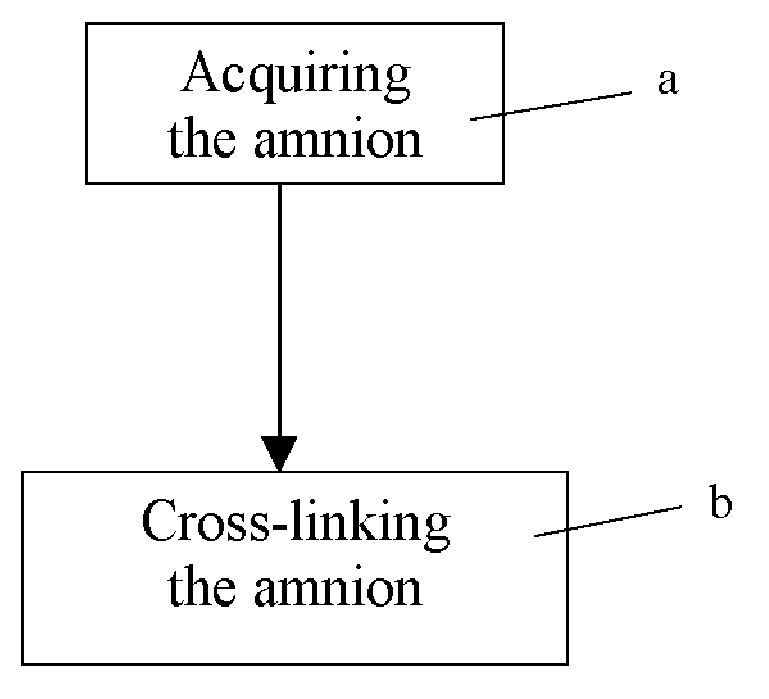
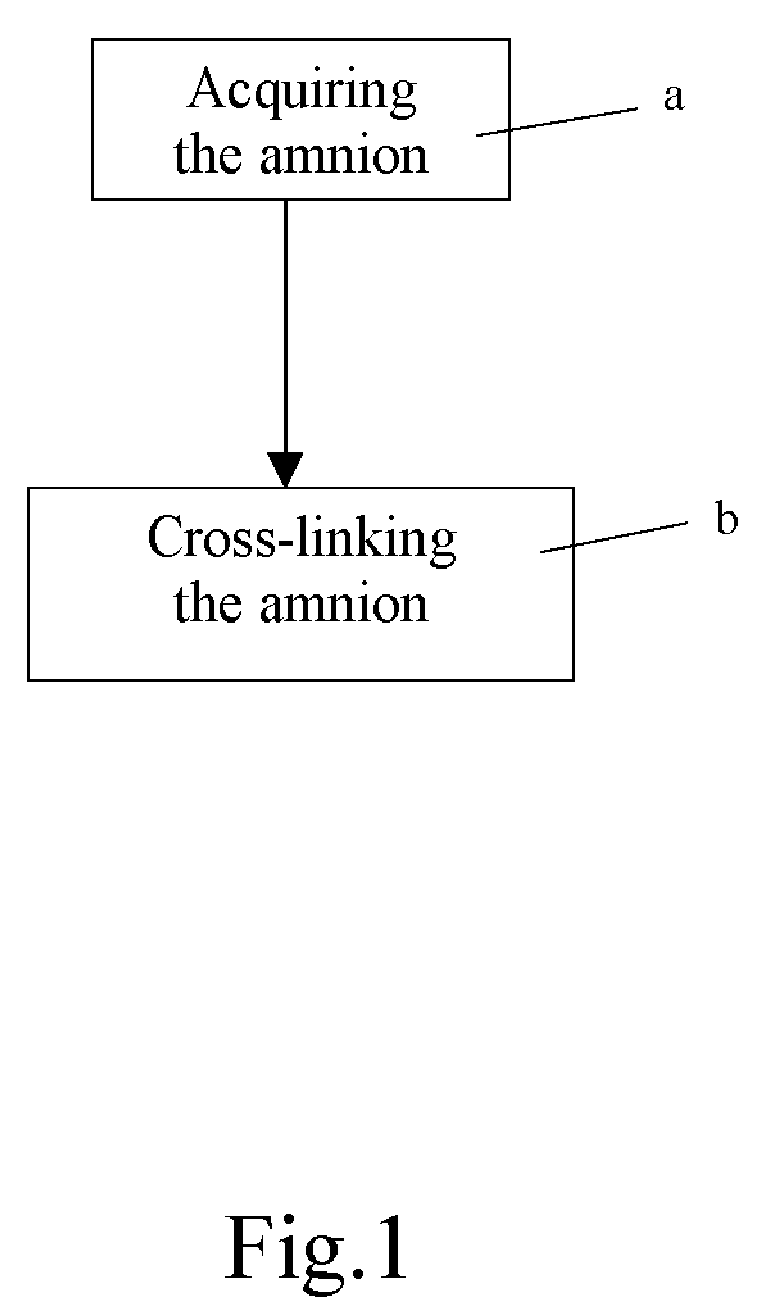
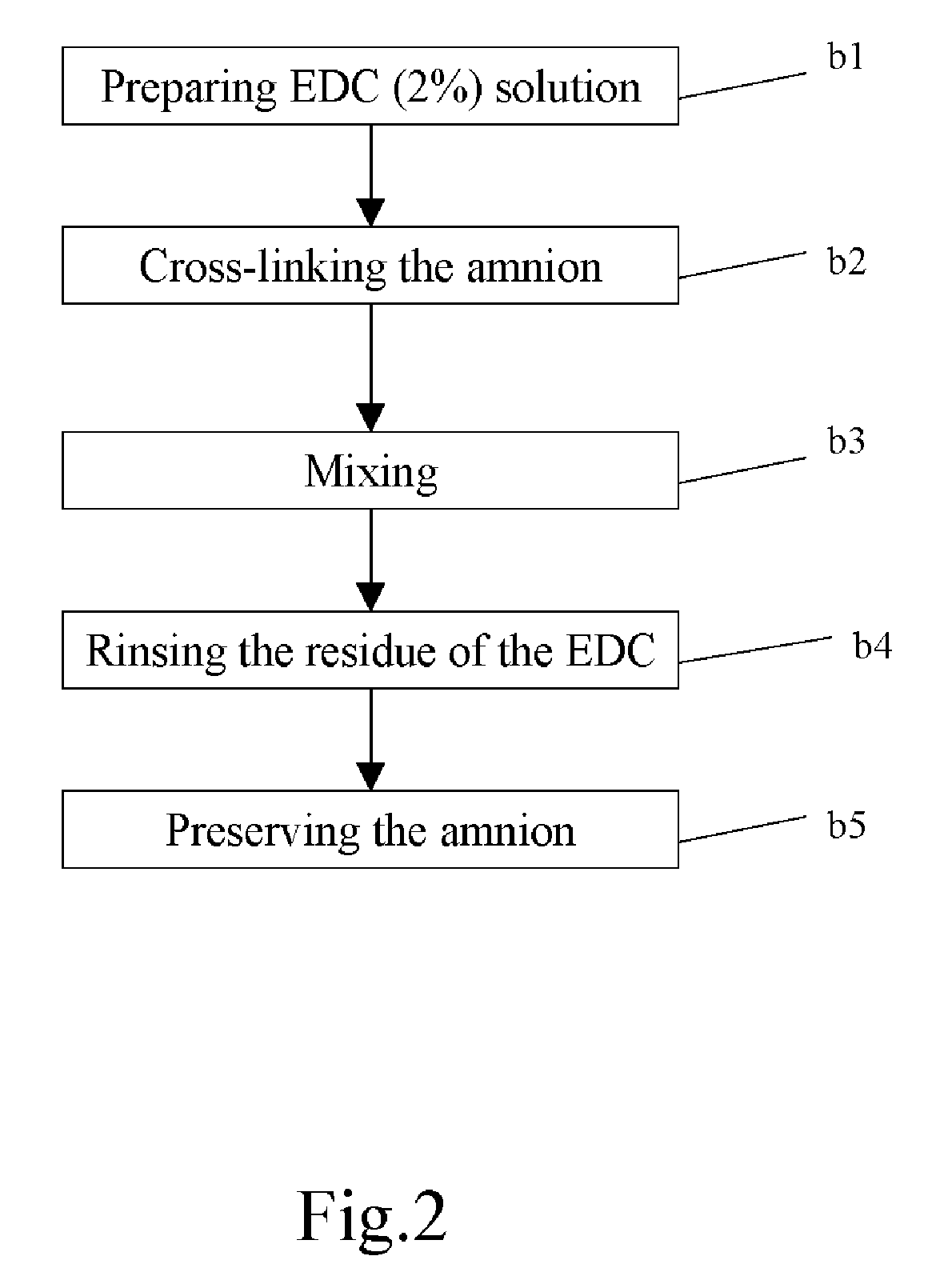
Method of cross-linking amnion to be an improved biomedical material
InactiveUS20080233552A1Reduce cell proliferationMore resistantMammal material medical ingredientsDead animal preservationCross-linkDisease
The present invention discloses a method of cross-linking amnion to be an improved biomedical material. The present invention adopts the amnion cross-linked by EDC (N-(3-dimethylaminopropyl)-N′-ethyl-carbodiimide HCI) or NHS (N-hydroxysuccinimide), and the cross-linked amnion not only has more resistance to protease, but also binds specific extracellular matrix (ECM) such as heparin by using cross-linked functional group. Further, by using the affinity of the ECM with specific growth factors, the amnion can be an efficient carrier for specific growth factor. Hence, some specific diseases may be treated.
Owner:CHANG GUNG UNIVERSITY
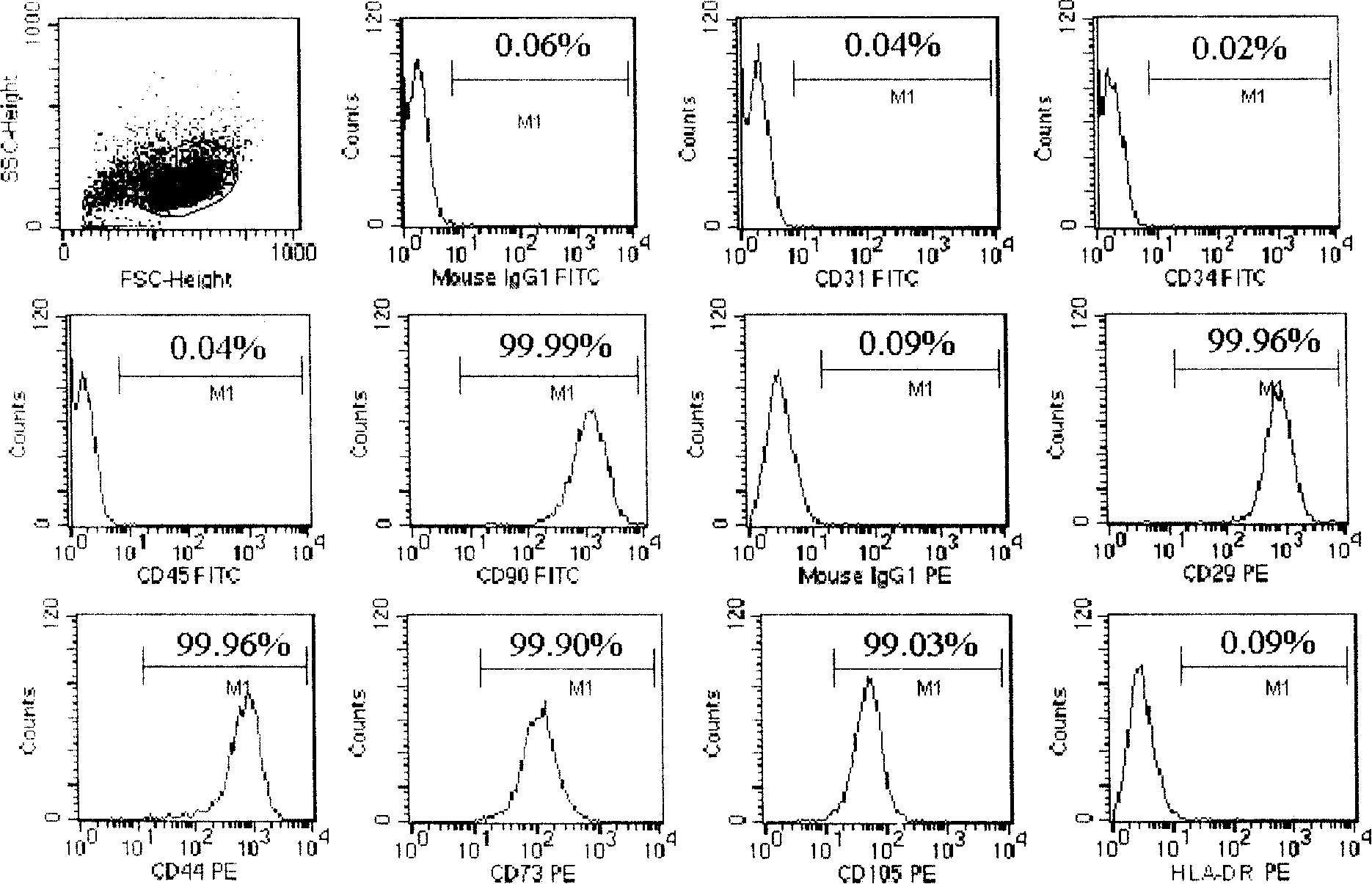
Serum-free culture medium for mesenchymal stem cells
ActiveCN102827807AAvoid instabilityClear chemical compositionSkeletal/connective tissue cellsCell phenotypeSodium bicarbonate
The invention relates to the field of biology, and discloses a serum-free culture medium which essentially comprises an IMDM (Iscove Modified Dulbecco Medium), L-glutamine, sodium bicarbonate, Hepes, recombinant human insulin, recombinant human transferrin, recombinant human albumin, 2-mercaptoethanol, protocatechuic acid, lipid, amino acid, vitamins, trace elements, Pluronic F-68, hydrocortisone, vitamin C, bonding amine or recombinant human fibronectin, progesterone, putrescine, heparin, serotonin, epidermal growth factors (EGFs), b-fibroblast growth factors (FGF), platelet derive growth factor (PDGF)-BB and insulin-like growth factor (IGF)-I. The serum-free culture medium is clear in chemical components, free from animal sources and serum and safe and ideal in cell cultivation and avoids the doped animal components and unstable batches, and the results of the cultured mesenchymal stem cells show that the total cellular score, the cell phenotype and the secretory cell factors are normal, so that the serum-free culture medium has good industrial application prospect.
Owner:内蒙古干细胞医学工程技术研究中心
Composition and method for preparing biocompatible surfaces
InactiveUS20060216324A1Reduced activityChallenge can be overcomeImpression capsSurgeryPre irradiationBiocompatible coating
The invention provides methods and compositions for providing biocompatible surfaces to medical articles. In particular the invention provides biocompatible coatings with heparin activity. In some aspects, the biocompatible coatings of the invention are able to release a bioactive agent. The coatings can be formed using biostable or biodegradable polymeric material and photoreactive groups. The invention also provides methods for improving the quality of bioactive agent-containing coatings by performing pre-irradiation of biocompatible coating compositions.
Owner:STUCKE SEAN M +3
Methods for external treatment of blood
InactiveUS6595941B1Avoid harmful effectsImprove accessibilityStentsBalloon catheterBlood Vessel TissueBlood processing
Vascular access systems, devices and methods for facilitating repeated access to a blood vessel. These systems, devices and methods can be used in external blood treatment, such as dialysis, and in intra-venous administration of medicines, such as heparin, for extended periods of time, while avoiding deleterious effects such as those derived from repeated puncturing of the blood vessel tissues or exposure of such tissues to abnormal fluid flows. The vascular access systems comprise an anastomosis graft vessel, an occlusal device, such as an occlusal balloon, and a port device for accessing the occlusal device. Occlusal devices can be self-contained, they can rely on osmosis, and they can serve as the support of an agent to which the blood stream is exposed, either by transport or by mere contact. In addition, occlusal devices can adopt a distended and a collapsed configuration, the latter allowing for blood flow through the anastomosis graft vessel.
Owner:VITAL ACCESS CORP
Mutant adeno-associated virus virions and methods of use thereof
ActiveUS9441244B2Altered capsid propertiesReduce the binding forceAntibacterial agentsVirusesNucleotideCell type specific
The present invention provides mutant adeno-associated virus (AAV) that exhibit altered capsid properties, e.g., reduced binding to neutralizing antibodies in serum and / or altered heparin binding and / or altered infectivity of particular cell types. The present invention further provides libraries of mutant AAV comprising one or more mutations in a capsid gene. The present invention further provides methods of generating the mutant AAV and mutant AAV libraries, and compositions comprising the mutant AAV. The present invention further provides recombinant AAV (rAAV) virions that comprise a mutant capsid protein. The present invention further provides nucleic acids comprising nucleotide sequences that encode mutant capsid proteins, and host cells comprising the nucleic acids. The present invention further provides methods of delivering a gene product to an individual, the methods generally involving administering an effective amount of a subject rAAV virion to an individual in need thereof.
Owner:INTEGRATIVE GENE THERAPEUTICS +1
Mesenchyme stem cell preserving fluid and use thereof
ActiveCN101210232AImprove survival rateReduce adhesionDead animal preservationTissue culturePhosphate ionCell mass
The invention discloses a preservation solution for mesenchymal stem cells and application thereof. The cell preservation solution contains human albumin and heparin as the main components, and other auxiliary reagents such as human cytokine, phosphate ion, metal ions or monosaccharide are contained in a buffer solution for preserving human mesenchymal stem cells. The preservation solution can keep high survival rate of human mesenchymal stem cells in transportation process, reduce adhesion between cells and between the cell and the inner wall of a container, and reduce the possible occurrence of cell mass embolism in blood vessel while clinically infusing human mesenchymal stem cells. The mesenchymal stem cells can be maintained in a state of single-cell suspension at an environment temperature of 4 to 15 DEG C for 24 h, thus greatly enlarging the clinic application range of the human mesenchymal stem cells. The components used in the solution accord with the clinic application, and can meet the requirement for clinic use of the human mesenchymal stem cells.
Owner:TIANJIN AMCELLGENE ENG
Drug coating with topcoat
InactiveUS20050187611A1Long release timeReduced burst effectStentsSurgeryThrombogenicityDrug coating
A coating and method for a coating an implantable device or prostheses are disclosed. The coating includes an undercoat of polymeric material containing an amount of biologically active material, particularly heparin, dispersed herein. The coating further includes a topcoat which covers less than the entire surface of the undercoat and wherein the topcoat comprises a polymeric material substantially free of pores and porosigens. The polymeric material of the topcoat can be a biostable, biocompatible material which provides long term non-thrombogenicity to the device portion during and after release of the biologically active material.
Owner:BOSTON SCI SCIMED INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com