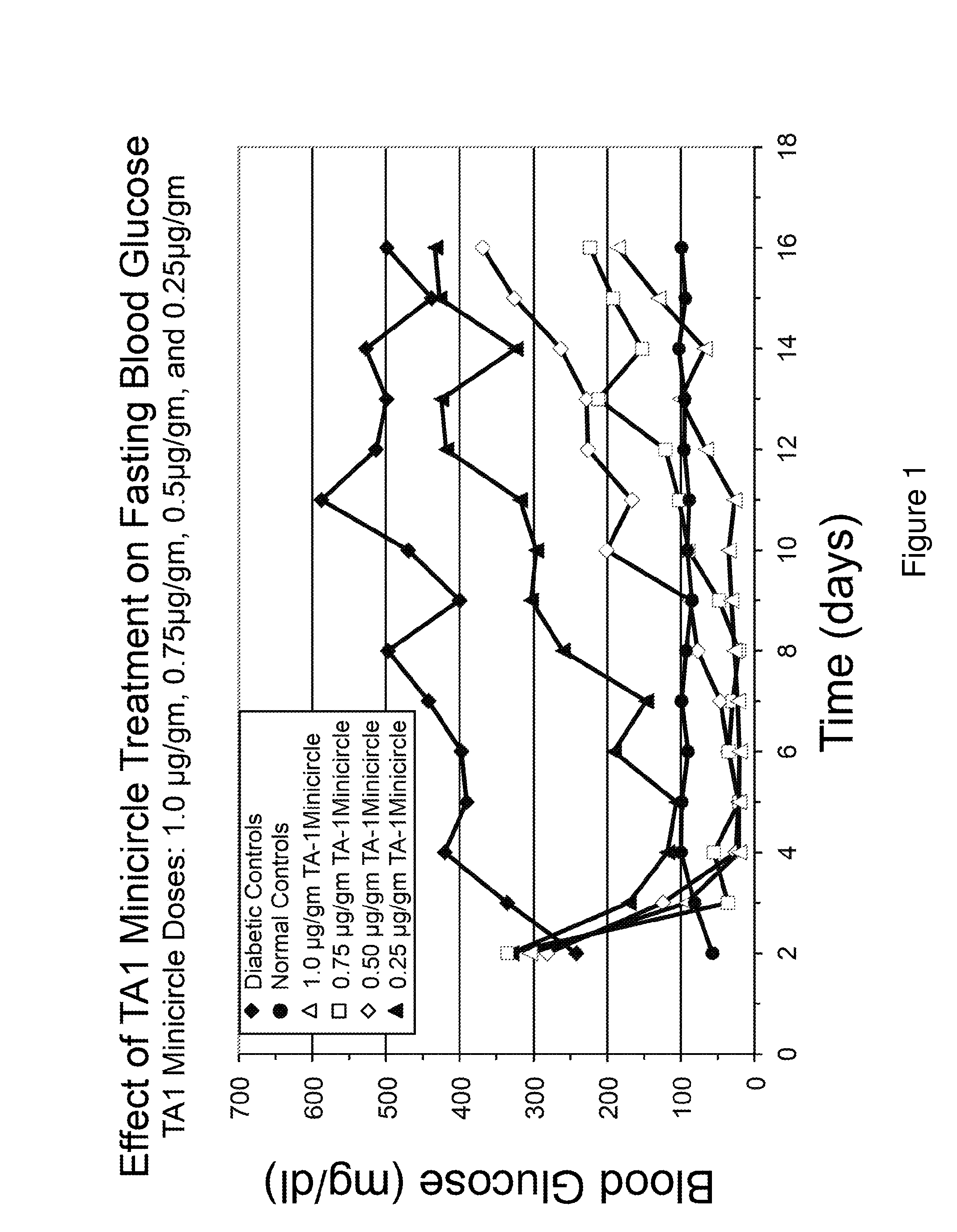
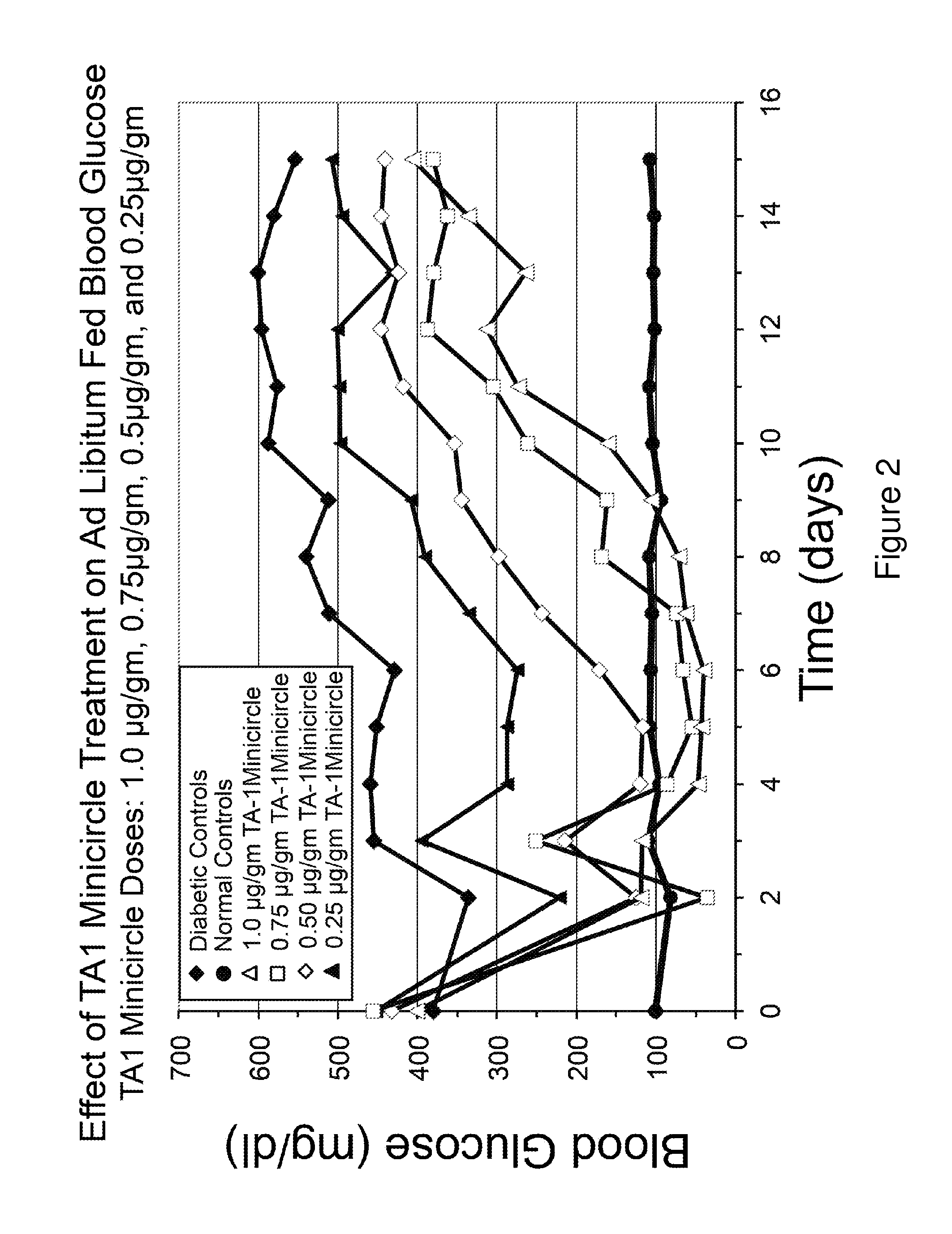
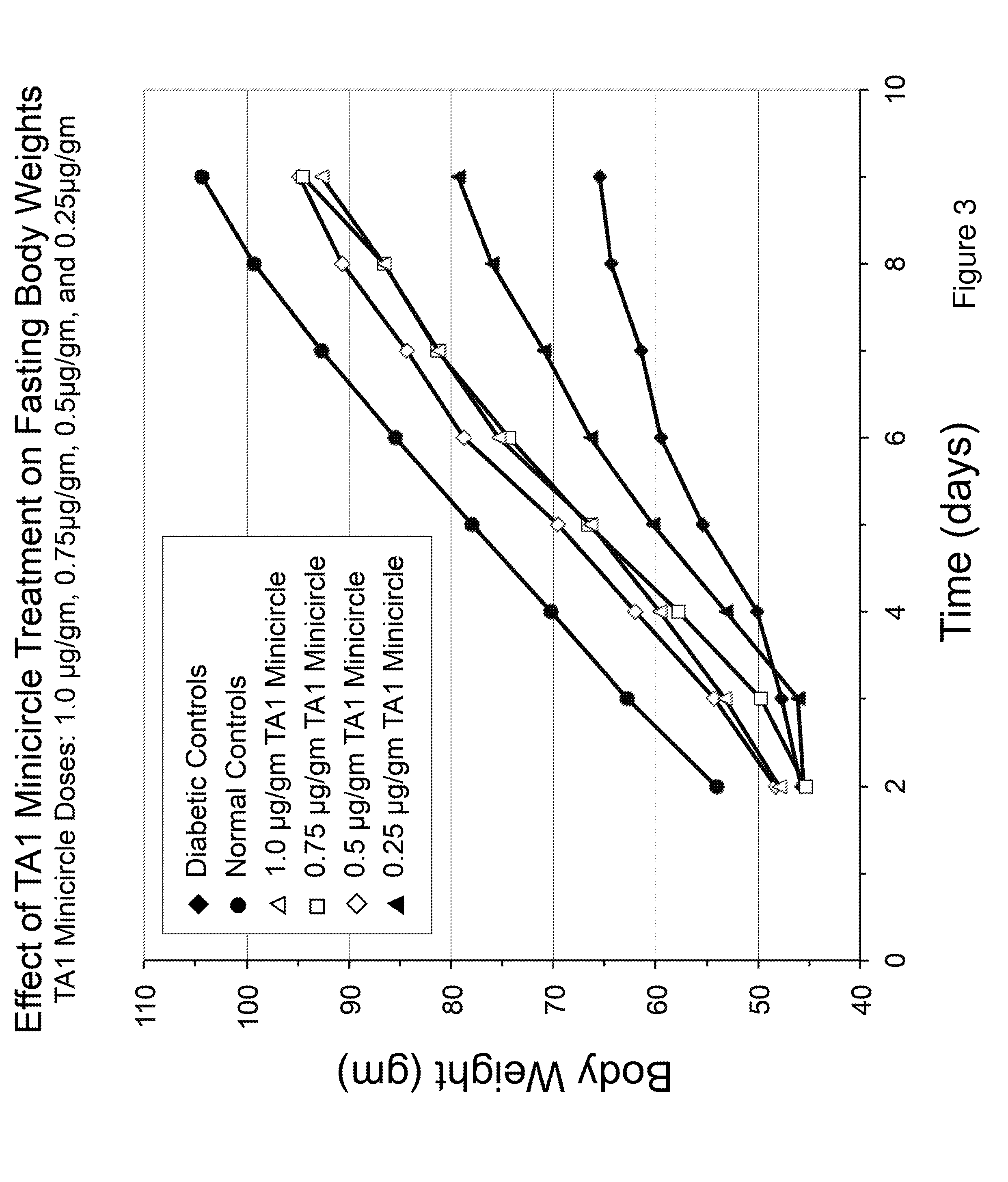
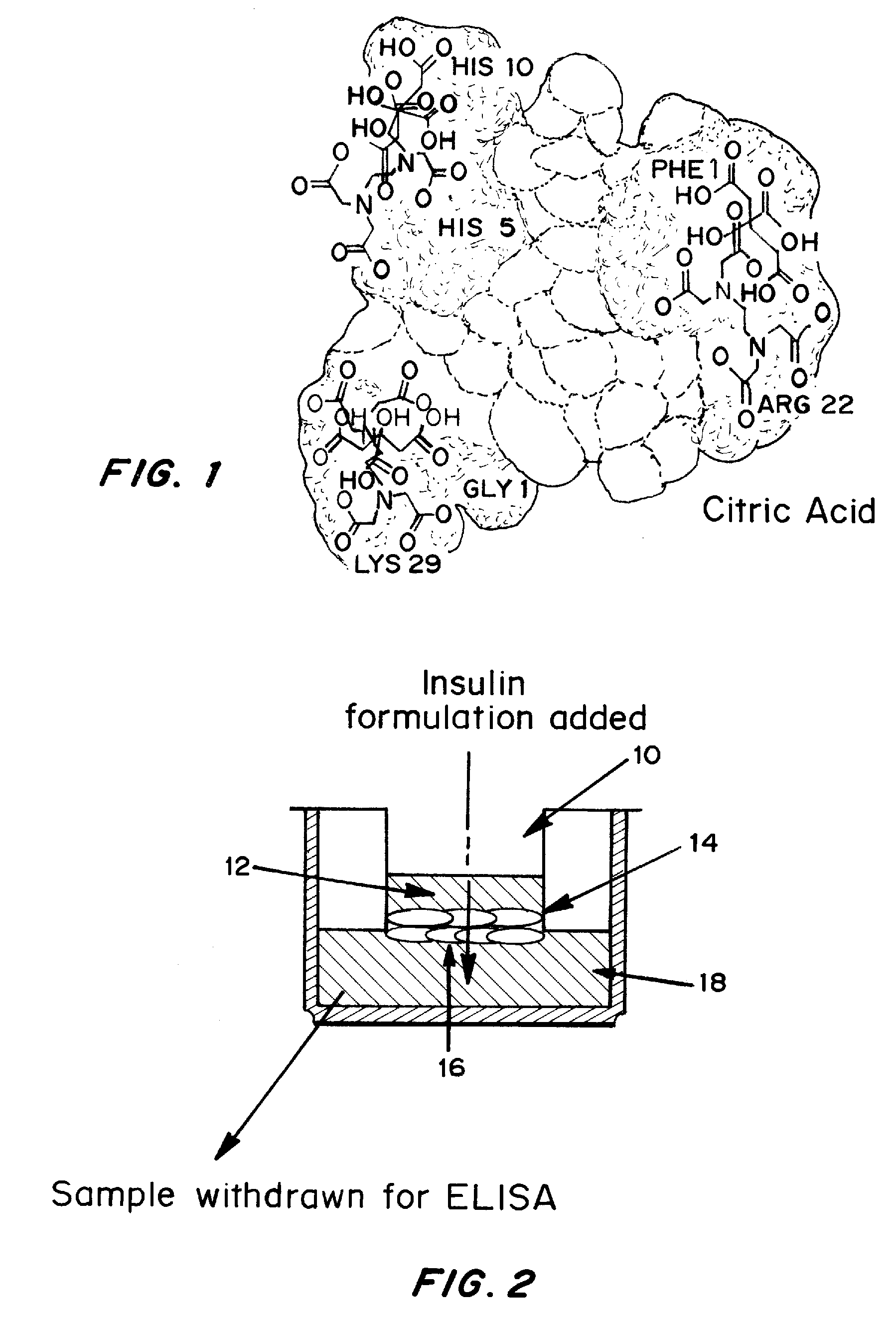
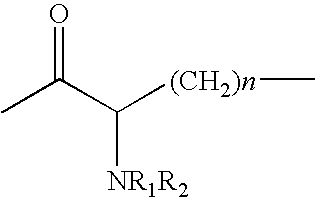
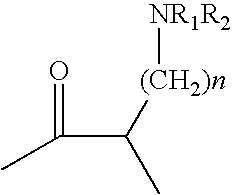
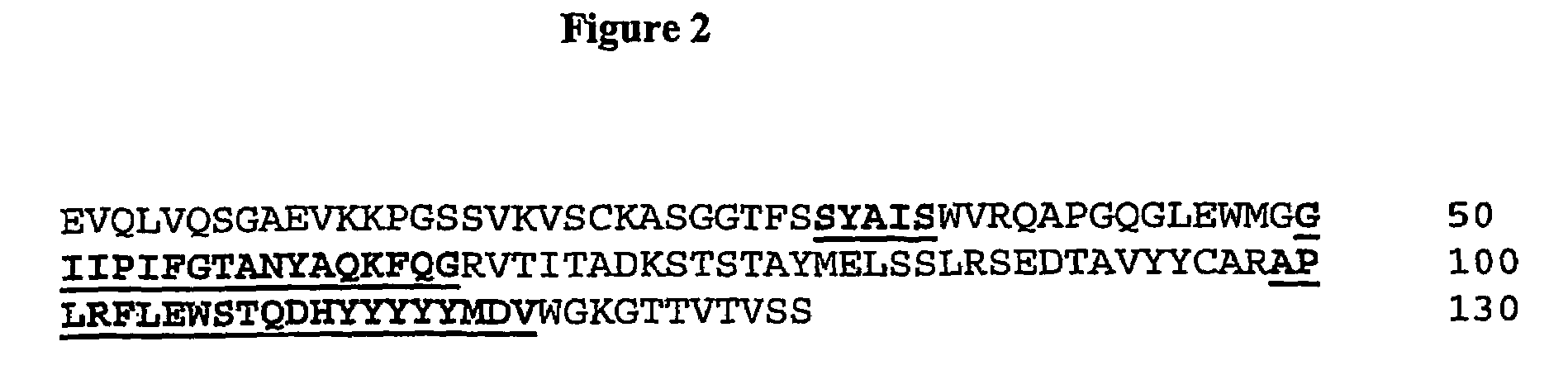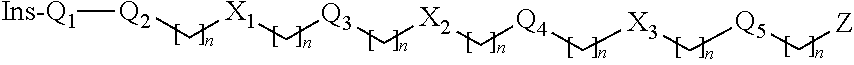Patents
Literature
396 results about "Human insulin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
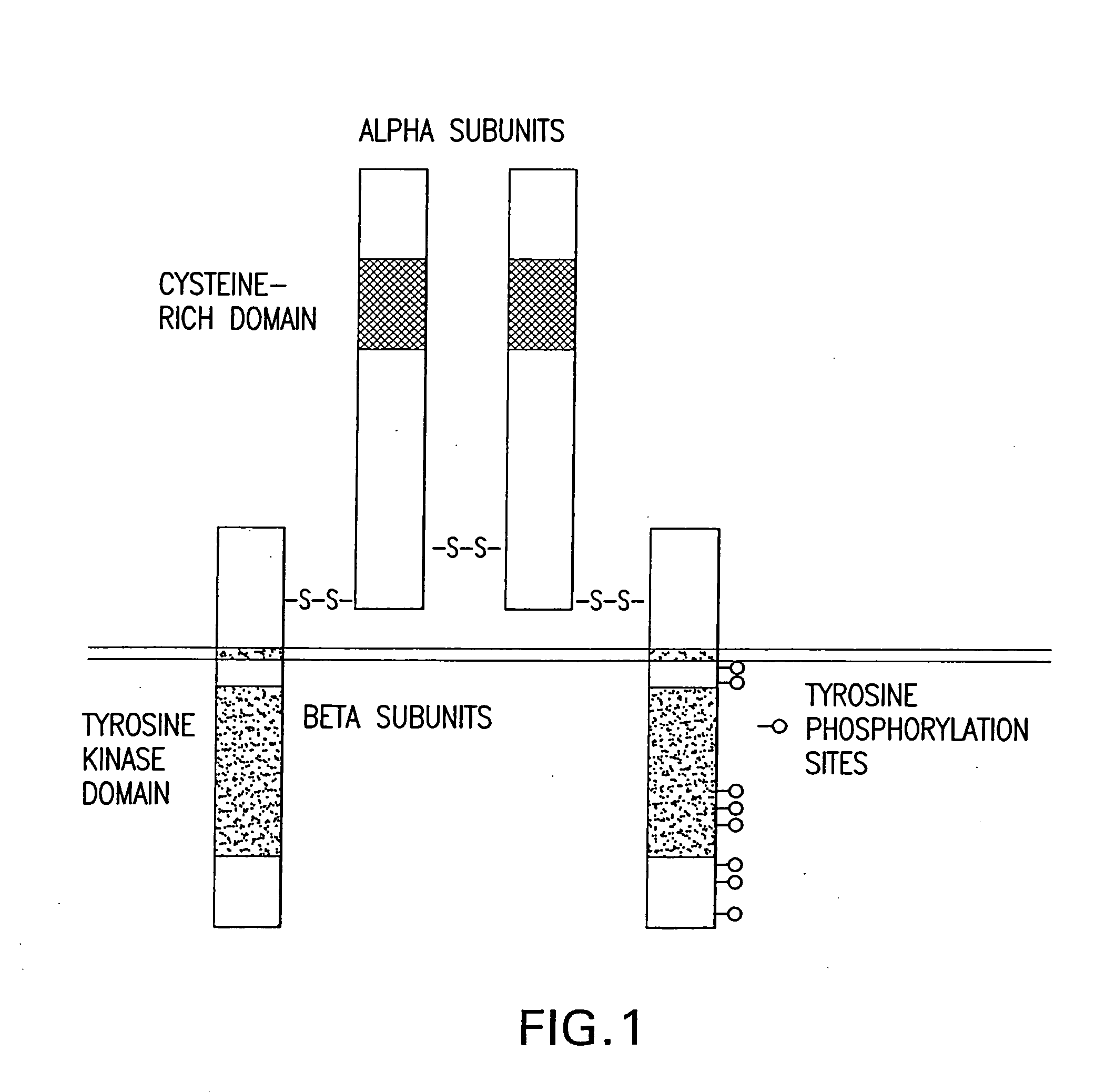
Insulin human is an Insulin. The chemical classification of insulin human is Insulin. A 51-amino acid pancreatic hormone that plays a major role in the regulation of glucose metabolism, directly by suppressing endogenous glucose production (GLYCOGENOLYSIS; GLUCONEOGENESIS) and indirectly by suppressing GLUCAGON secretion...
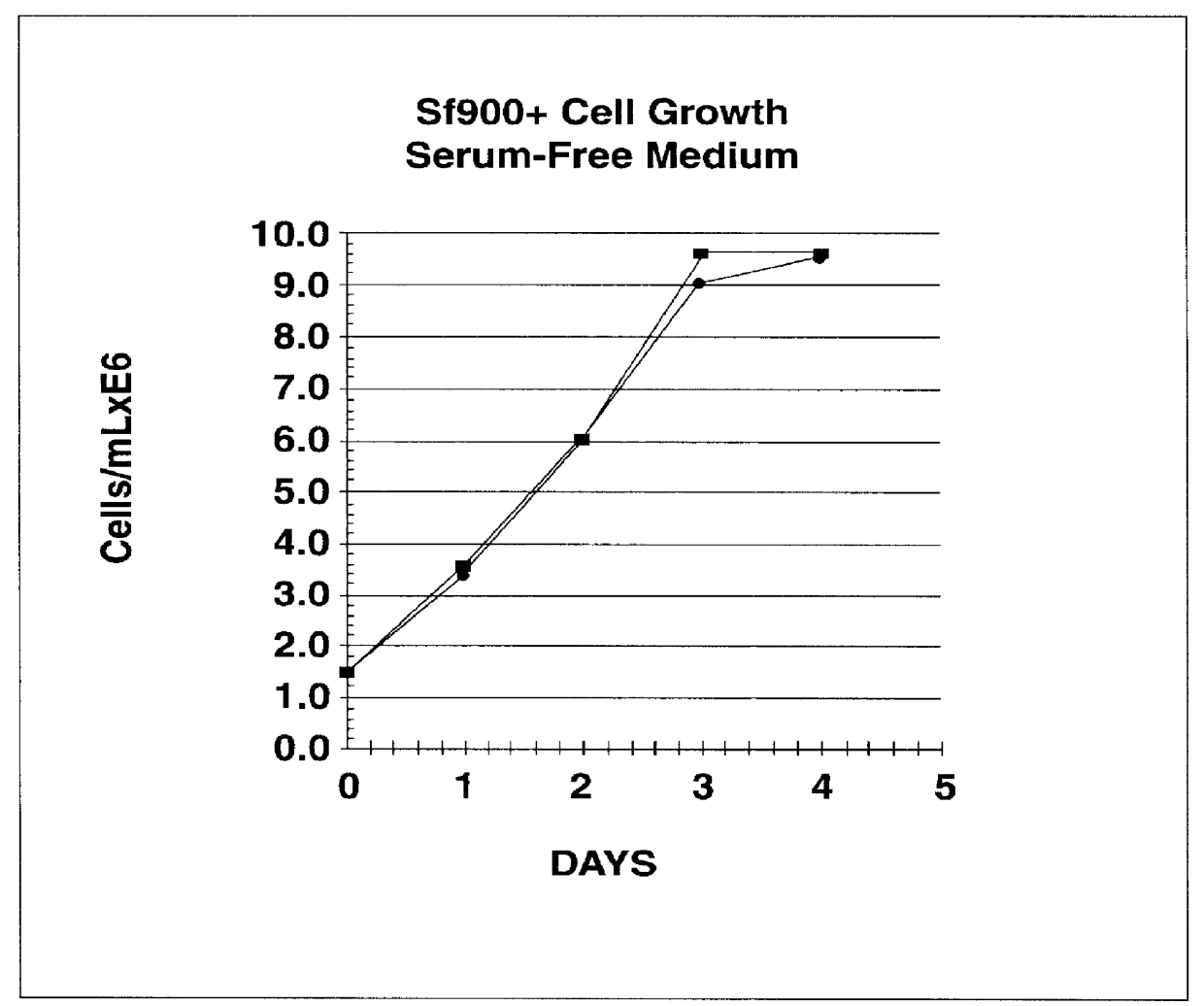
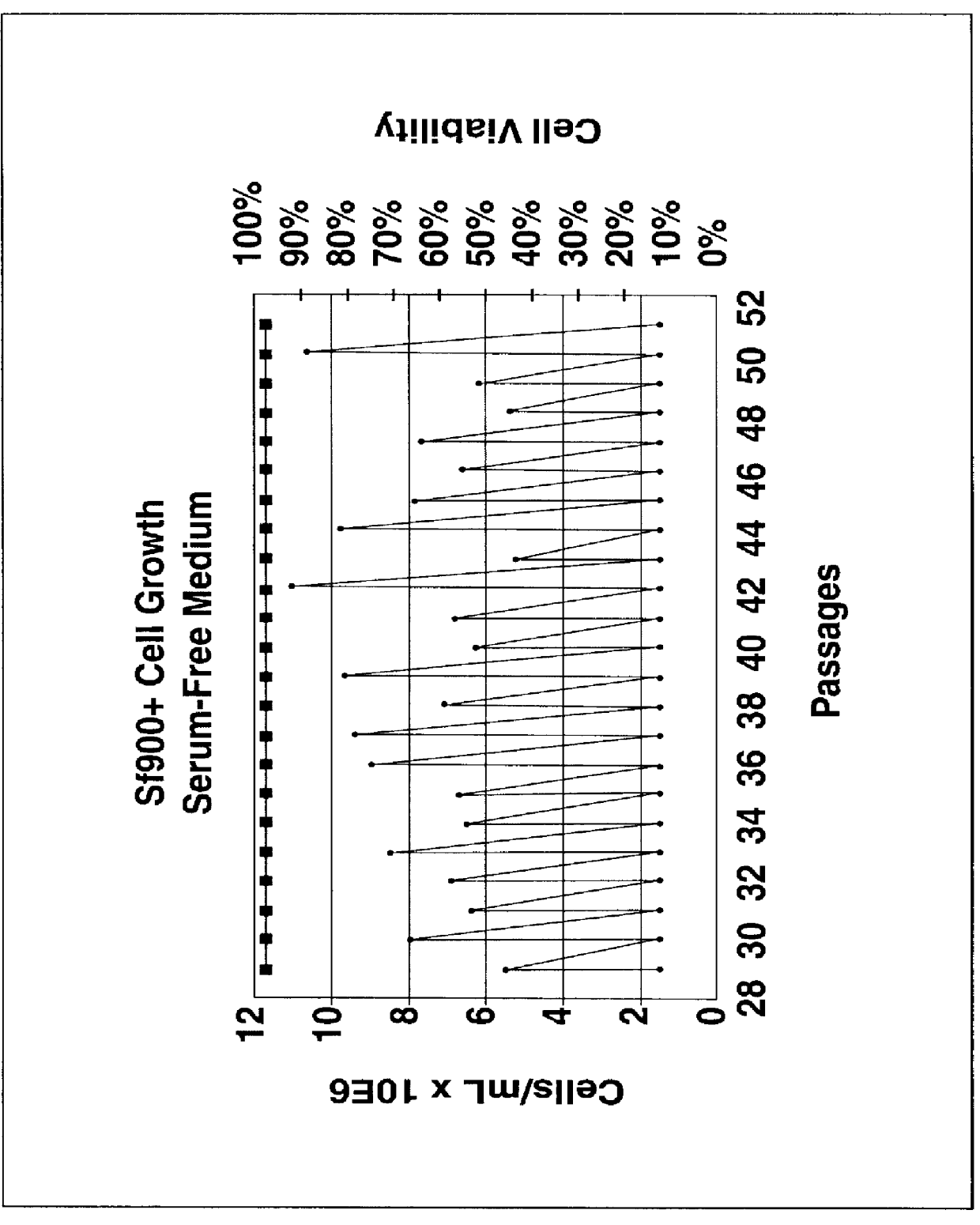
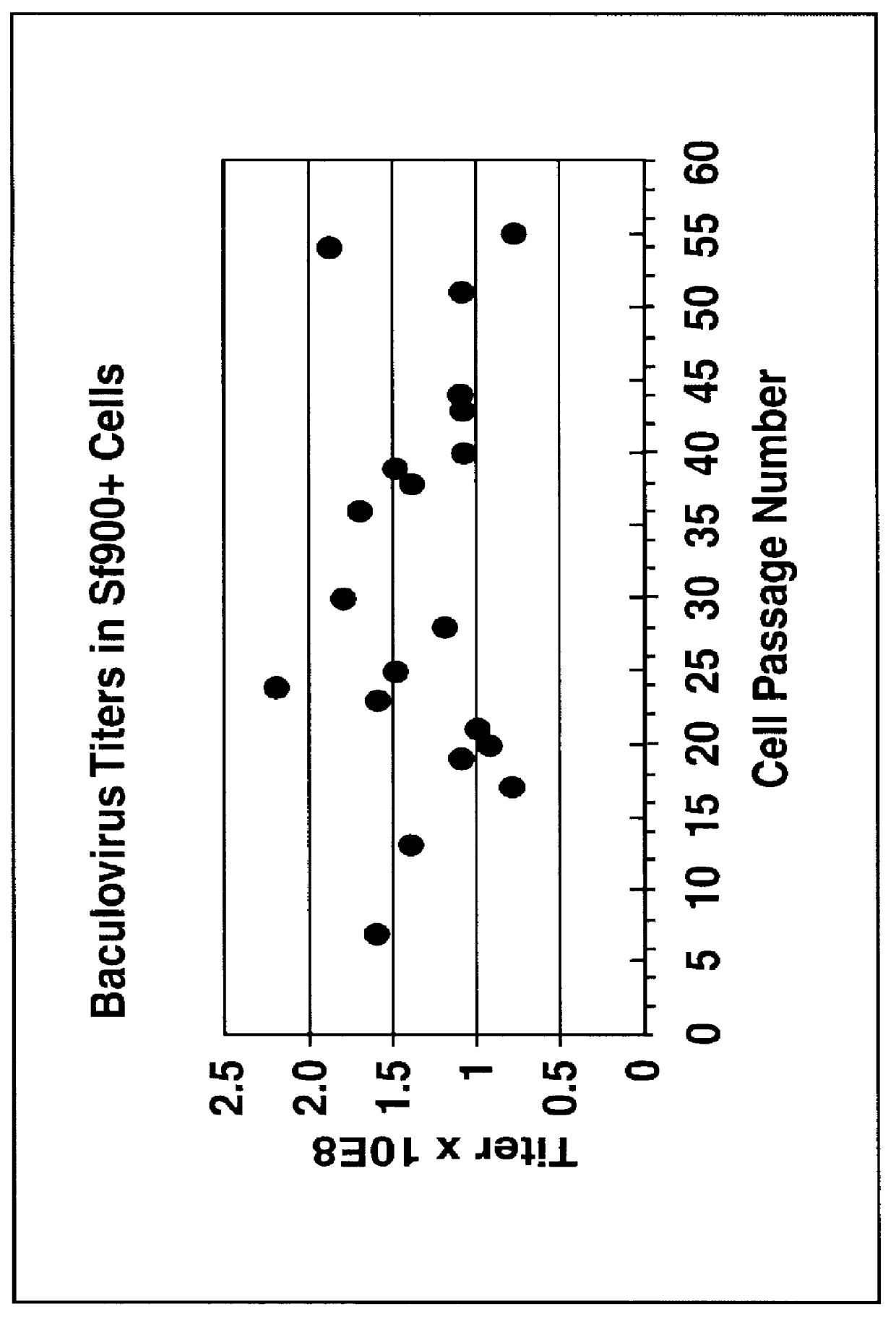
Spodoptera frugiperda single cell suspension cell line in serum-free media, methods of producing and using
InactiveUS6103526AAvoid infectionHigh densityConnective tissue peptidesInvertebrate cellsSerum free mediaAdjuvant
Disclosed and claimed is a new insect cell line, Sf900+, ATCC CRL-12579. The insect cell line was established from Lepidoptera, Noctuidae, Spodoptera frugiperda Sf-9 (ATCC CRL-1711) through multiple rounds of limiting dilution and selection in a serum-free insect medium supplemented with added human insulin. The insect cell line is useful in BEVS or as an adjuvant and has many characteristics and advantages. Also disclosed and claimed are recombinant proteins from recombinant baculovirus expression in insect cells such as Sf900+ cells, for instance, HA, NA, EPO, CD4, CEA, and thrombospondin.
Owner:PROTEIN SCI
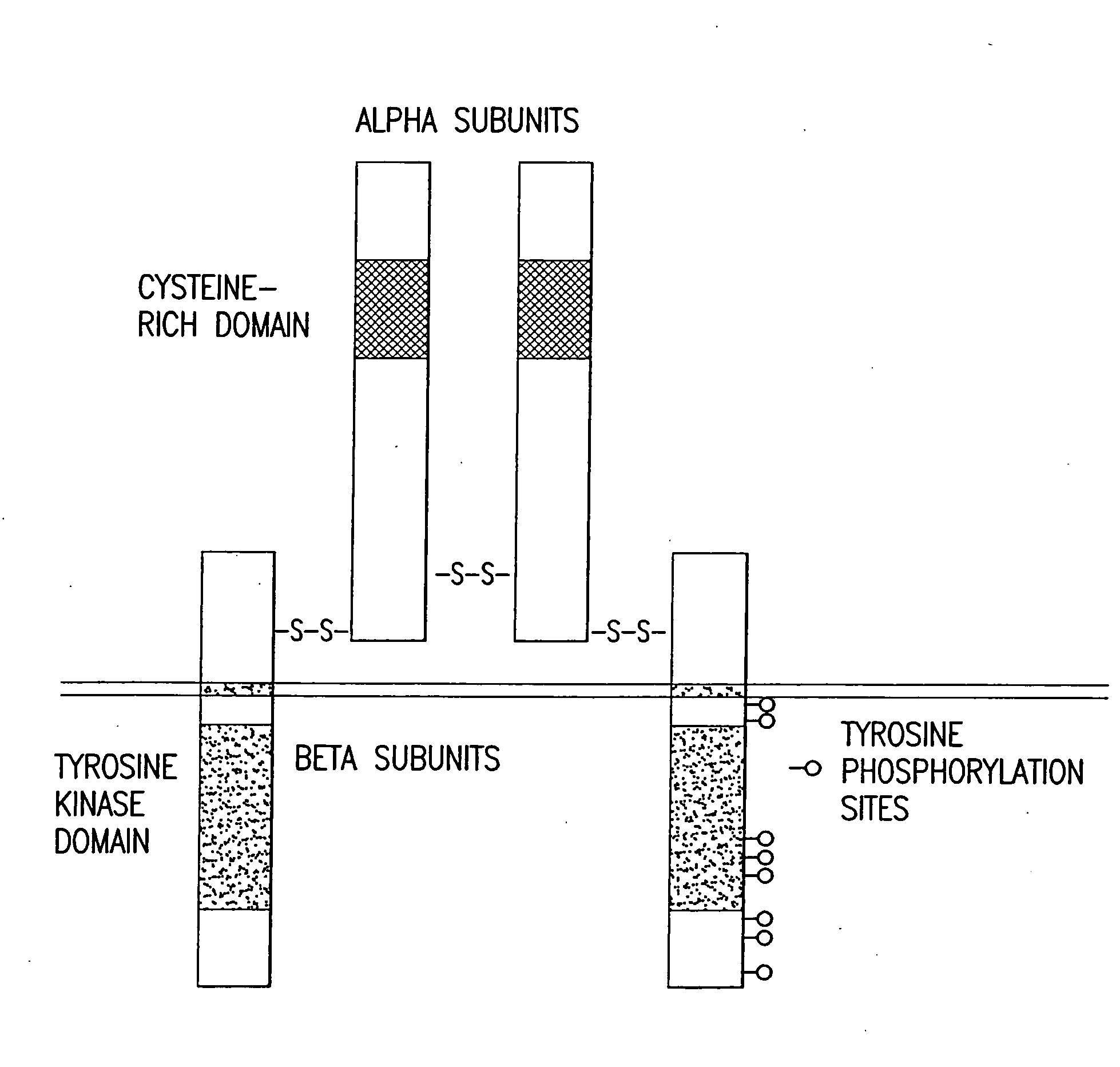
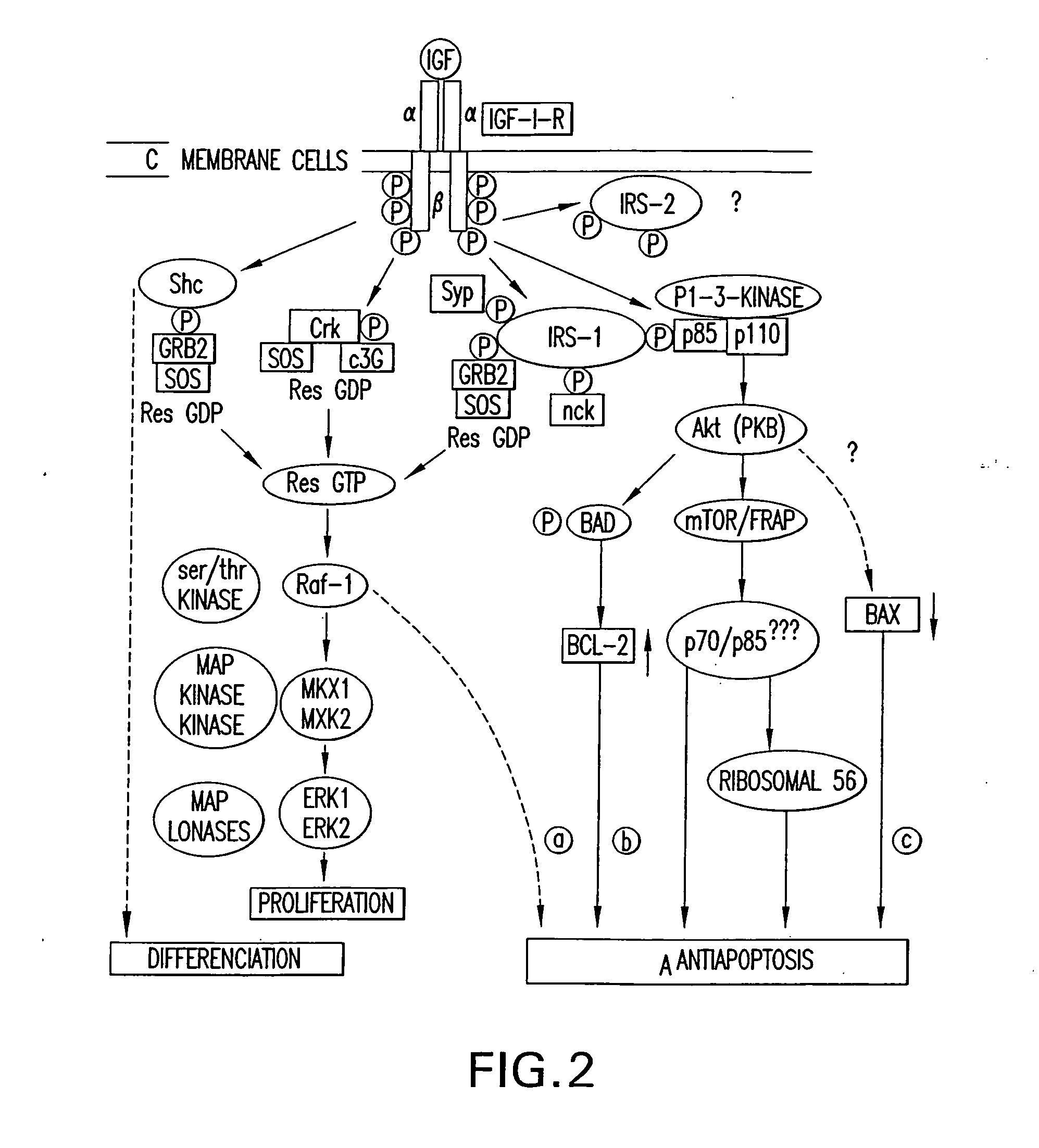
Novel anti-IGF-IR antibodies and uses thereof
InactiveUS20080193445A1Stimulate interestPromote secretionMicrobiological testing/measurementImmunoglobulins against growth factorsDiseaseAnticarcinogen
The present invention relates to novel antibodies capable of binding specifically to the human insulin-like growth factor I receptor IGF-IR and / or capable of specifically inhibiting the tyrosine kinase activity of said IGF-IR receptor, especially monoclonal antibodies of murine, chimeric and humanized origin, as well as the amino acid and nucleic acid sequences coding for these antibodies. The invention likewise comprises the use of these antibodies as a medicament for the prophylactic and / or therapeutic treatment of cancers overexpressing IGF-IR or any pathology connected with the overexpression of said receptor as well as in processes or kits for diagnosis of illnesses connected with the overexpression of the IGF-IR receptor. The invention finally comprises products and / or compositions comprising such antibodies in combination with anti-EGFR antibodies and / or compounds and / or anti-cancer agents or agents conjugated with toxins and their use for the prevention and / or the treatment of certain cancers.
Owner:INST DE RECH PIERRE FABRE +1
Serum-free culture medium for mesenchymal stem cells
ActiveCN102827807AAvoid instabilityClear chemical compositionSkeletal/connective tissue cellsCell phenotypeSodium bicarbonate
The invention relates to the field of biology, and discloses a serum-free culture medium which essentially comprises an IMDM (Iscove Modified Dulbecco Medium), L-glutamine, sodium bicarbonate, Hepes, recombinant human insulin, recombinant human transferrin, recombinant human albumin, 2-mercaptoethanol, protocatechuic acid, lipid, amino acid, vitamins, trace elements, Pluronic F-68, hydrocortisone, vitamin C, bonding amine or recombinant human fibronectin, progesterone, putrescine, heparin, serotonin, epidermal growth factors (EGFs), b-fibroblast growth factors (FGF), platelet derive growth factor (PDGF)-BB and insulin-like growth factor (IGF)-I. The serum-free culture medium is clear in chemical components, free from animal sources and serum and safe and ideal in cell cultivation and avoids the doped animal components and unstable batches, and the results of the cultured mesenchymal stem cells show that the total cellular score, the cell phenotype and the secretory cell factors are normal, so that the serum-free culture medium has good industrial application prospect.
Owner:内蒙古干细胞医学工程技术研究中心
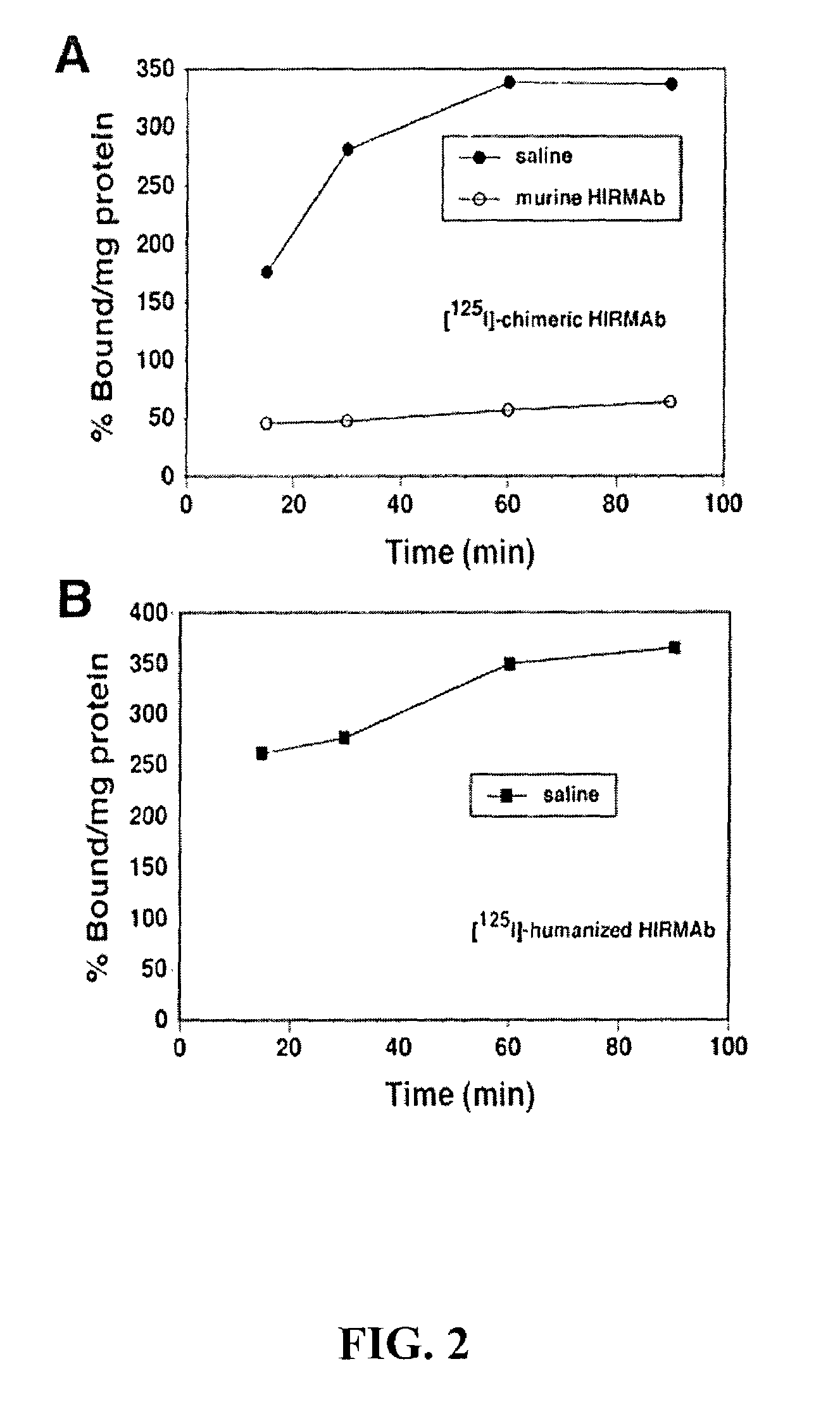
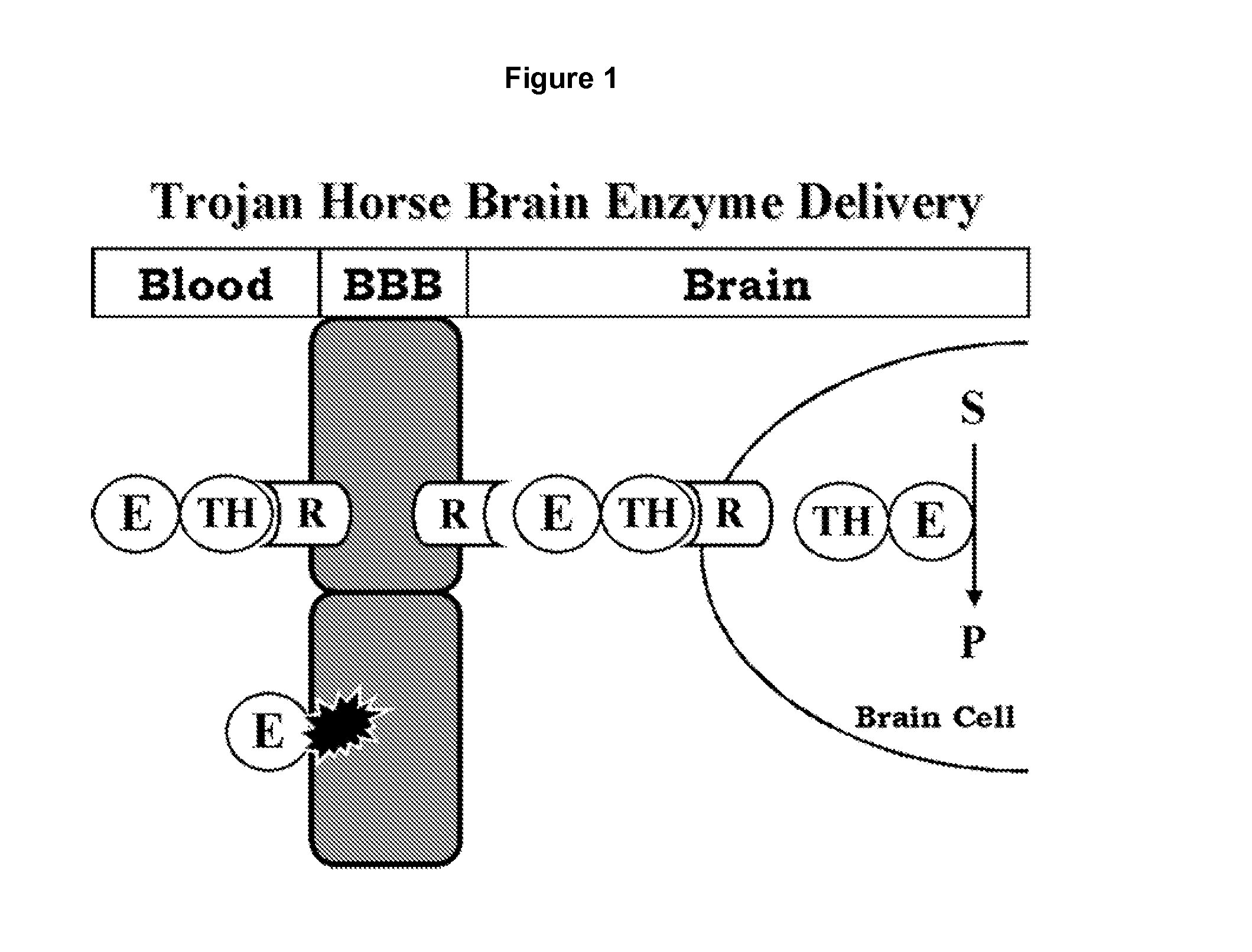
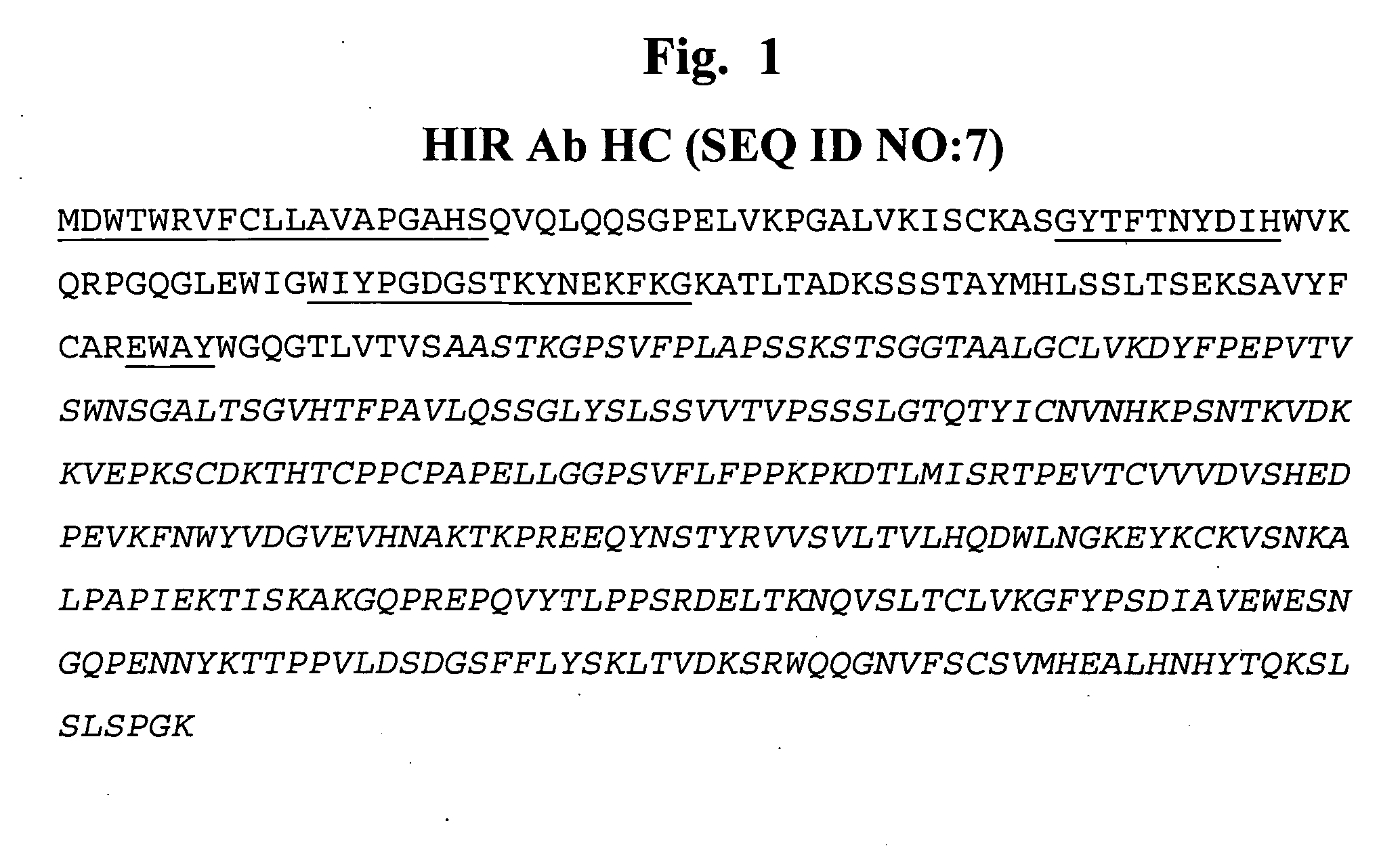
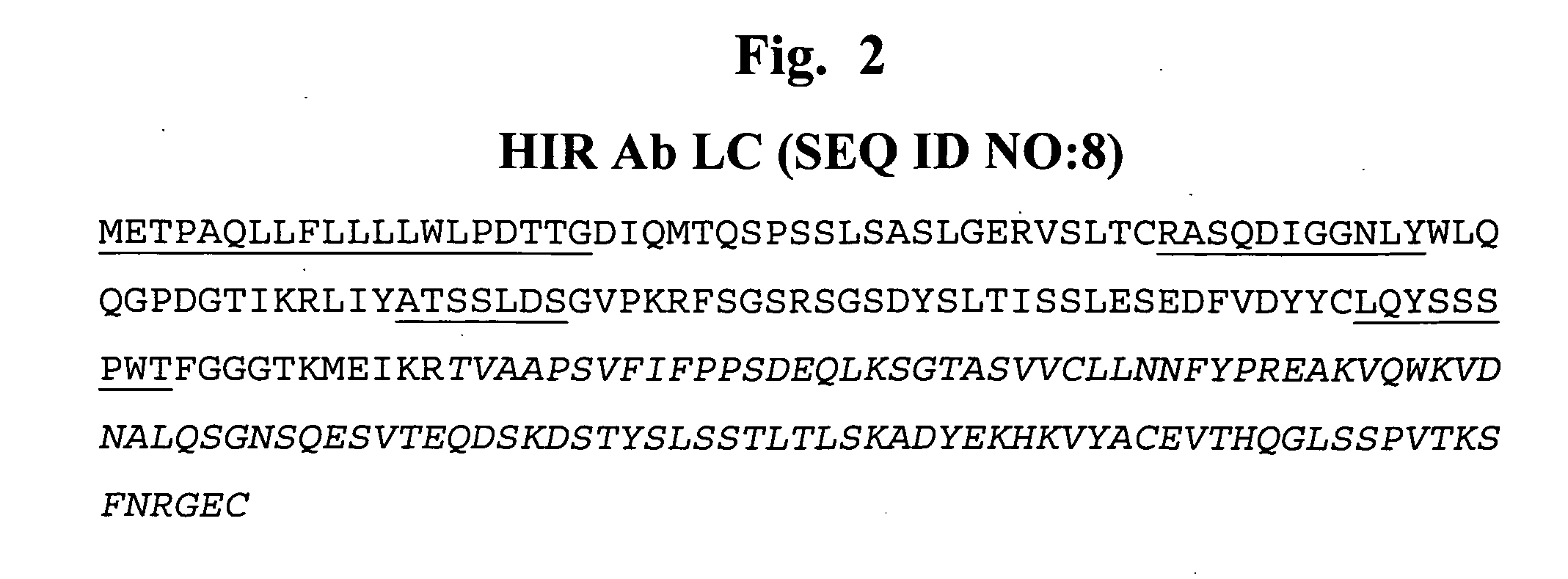
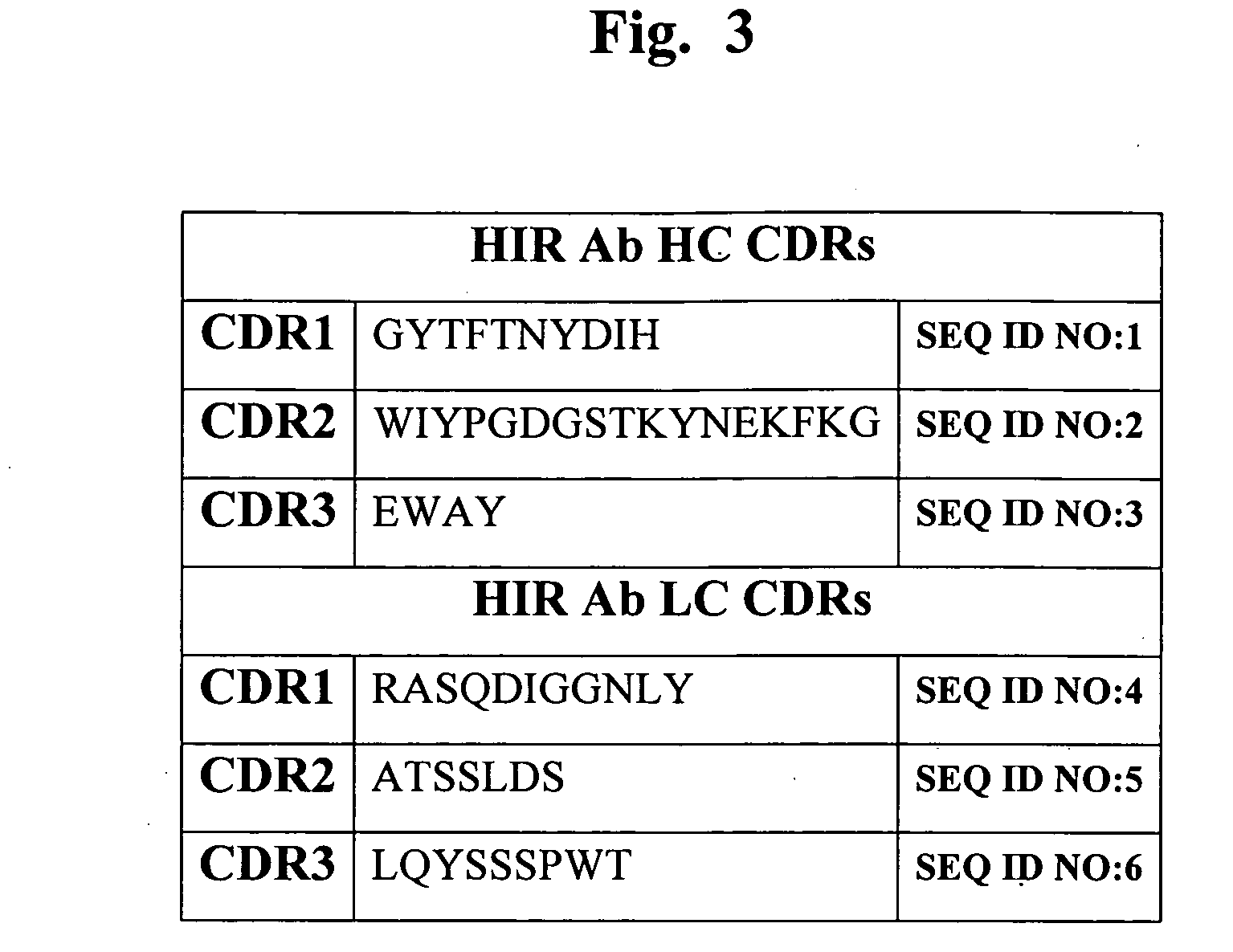
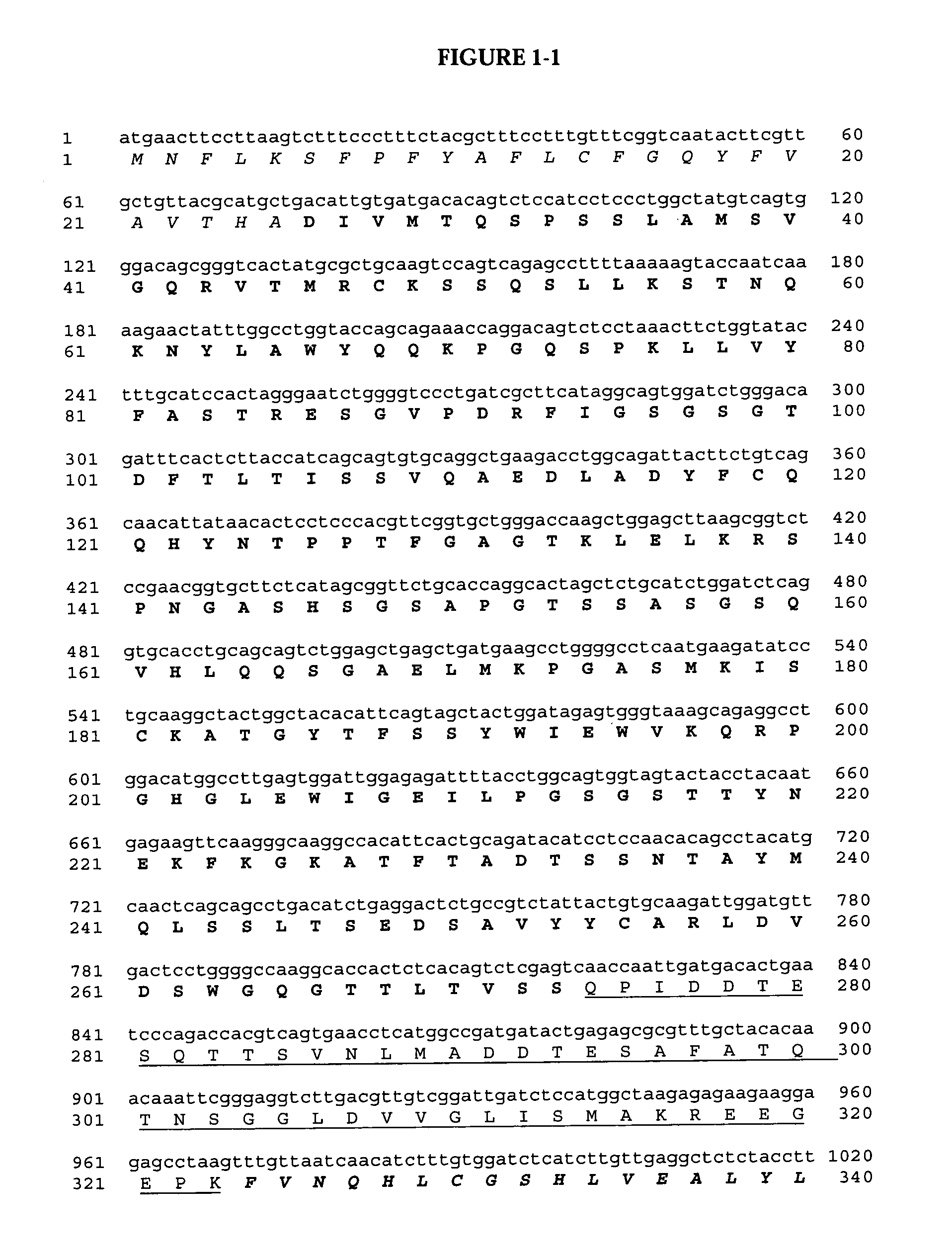
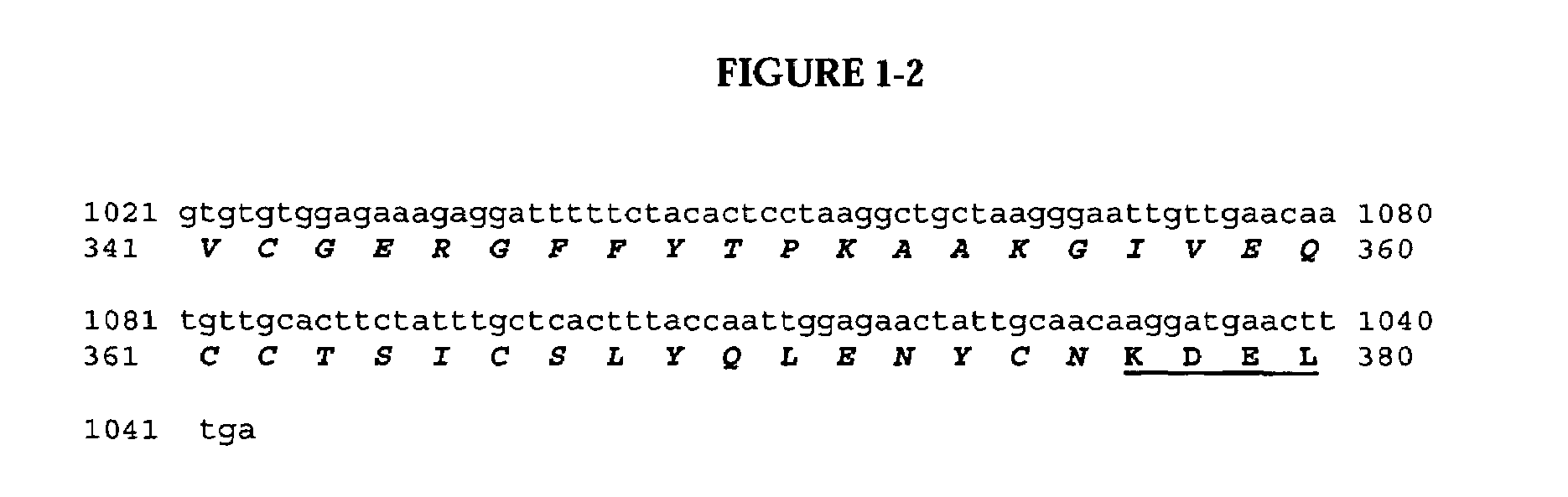
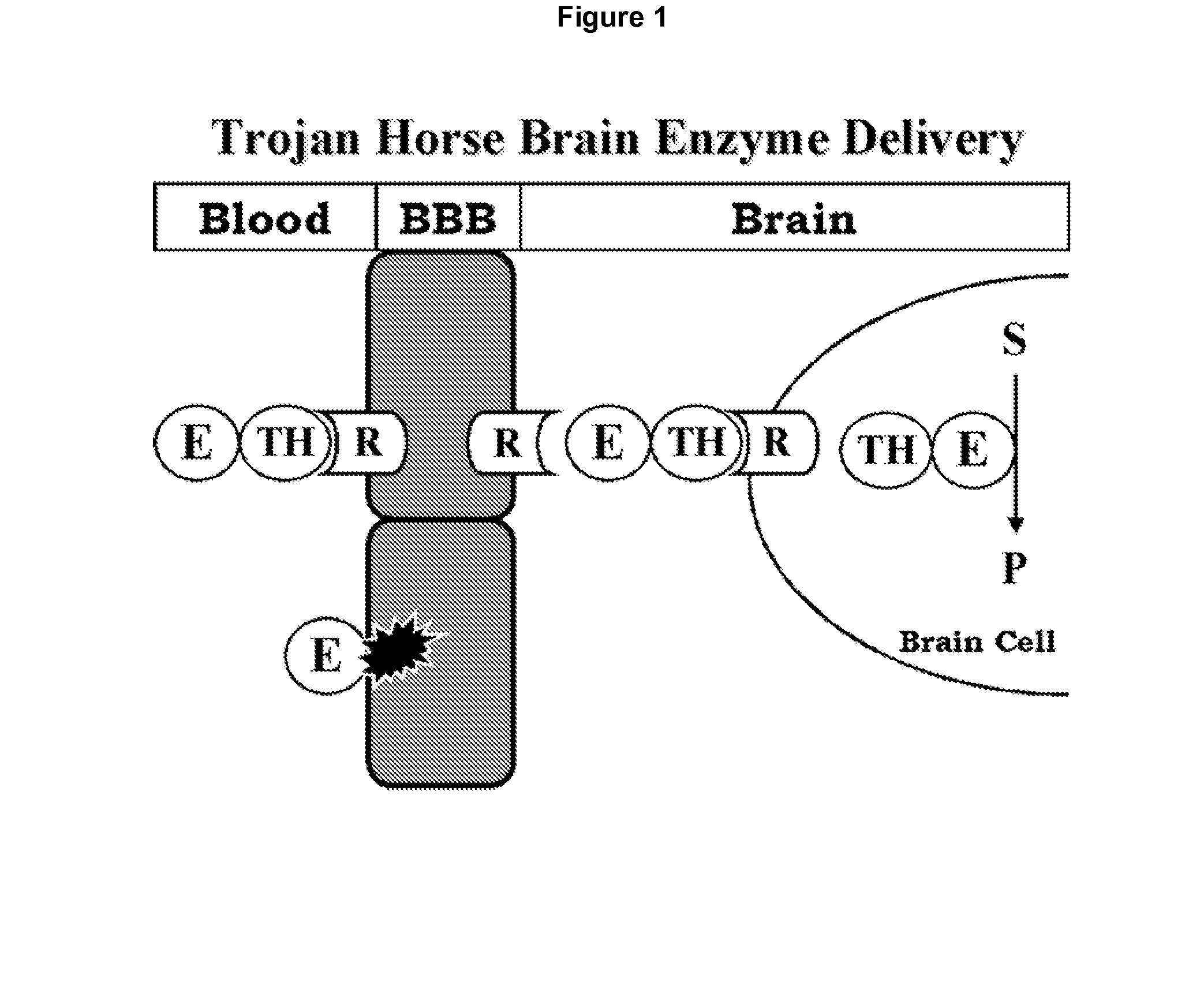
Delivery of pharmaceutical agents via the human insulin receptor
ActiveUS7388079B2Reduces immunogenic reactionNervous disorderImmunoglobulins against cell receptors/antigens/surface-determinantsMurine antibodyInsulin receptor
A humanized murine antibody is provided that binds to the human insulin receptor (HIR). The humanized murine antibody is suitable for use as a Trojan horse to deliver pharmaceutical agents to human organs and tissue that express the HIR. The humanized munne antibody is especially well suited for delivering neuropharmaceutical agents from the blood stream to the brain across the blood brain barrier (BBB). The humanized murine antibody may be genetically fused to the pharmaceutical agent or it may be linked to the pharmaceutical agent using an avidin-biotin conjugation system.
Owner:RGT UNIV OF CALIFORNIA
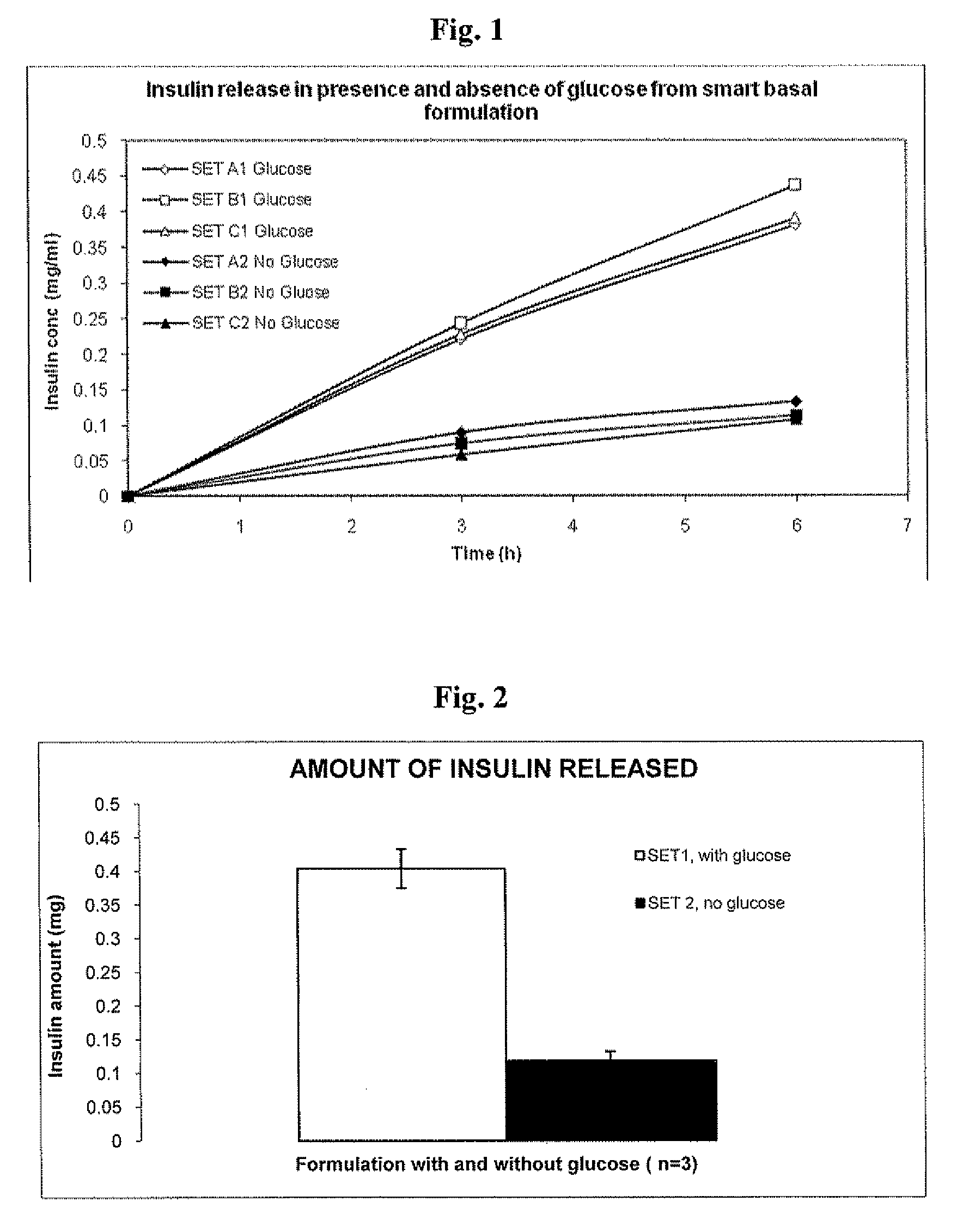
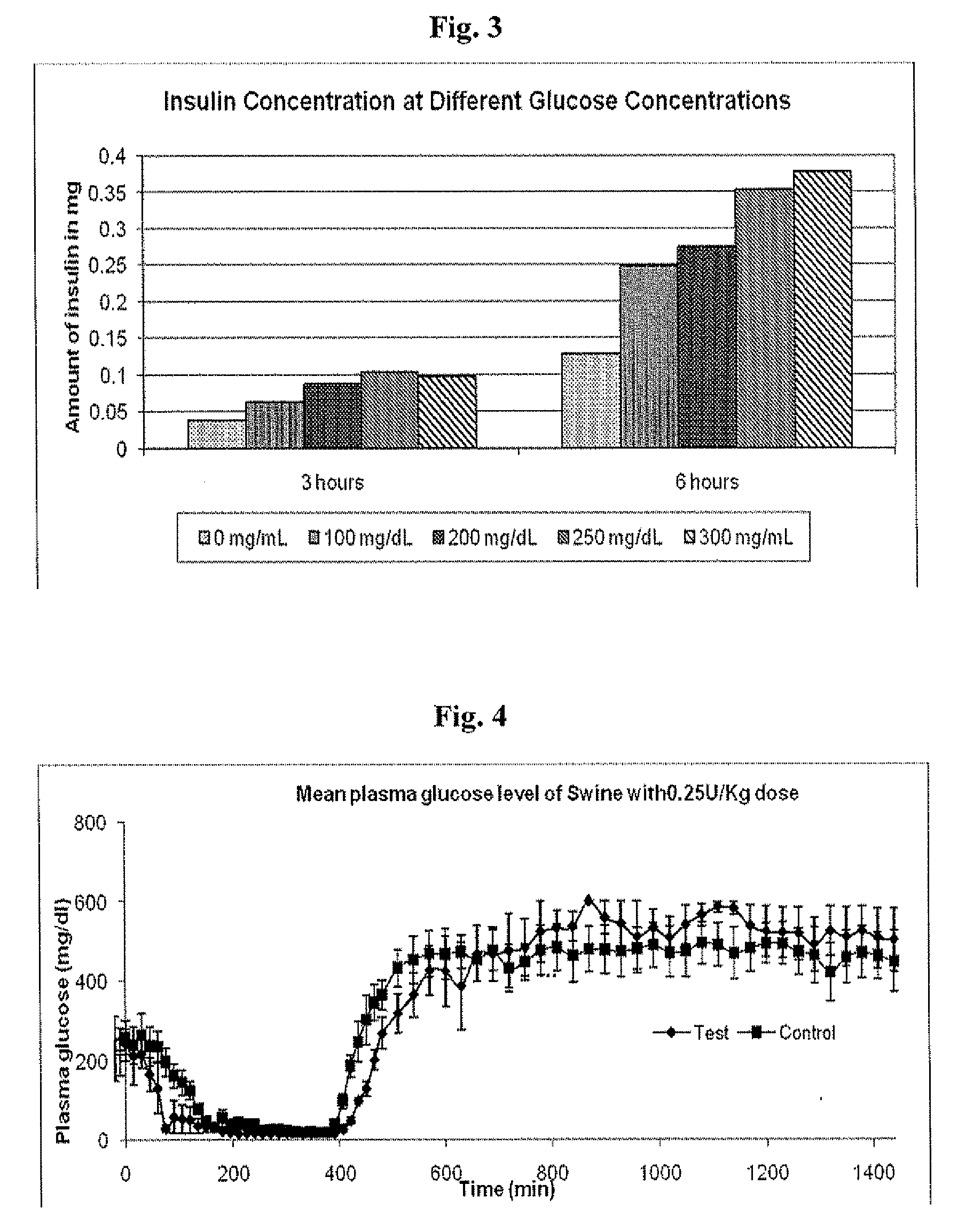
Insulin formulations for insulin release as a function of tissue glucose levels
InactiveUS20090175840A1Reduce productionRaise the pHPeptide/protein ingredientsMetabolism disorderInjections insulinReducing agent
Injectable insulin formulations that are capable of modifying the amount of insulin released based on the patient's tissue glucose levels, methods for making and using these formulations are described herein. The formulation may be administered via subcutaneous, intradermal or intramuscular administration. In one preferred embodiment, the formulations are administered via subcutaneous injection. The formulations contain insulin, an oxidizing agent or enzyme and a reducing agent or enzyme, a diluent and optionally one or more thickening agents. If a thickening agent is present in the formulation, the thickening agent increases the viscosity of the formulation following administration. Preferably the formulation contains an insulin, a diluent, glucose oxidase and peroxidase. Following administration to a patient, the insulin is released from the formulations as a function of the patient's tissue glucose level, which in turn maintains the patient's blood glucose level within an optimum range. The formulation is often referred to as a “smart” formulation since it modifies its release rate of insulin according to the patient's needs at a particular time. In a preferred embodiment, the formulation is designed to release insulin into the systemic circulation over time with a basal release profile following injection in a patient. In another embodiment, the formulation is designed to release insulin into the systemic circulation over time with a non-basal release profile following injection in a patient, such as a regular human insulin release profile or a prandial release profile.
Owner:BIODEL
Insulin production methods and pro-insulin constructs
InactiveUS20080146492A1Reduce eliminateImprove efficiencyBacteriaSugar derivativesInsulin activityImproved method
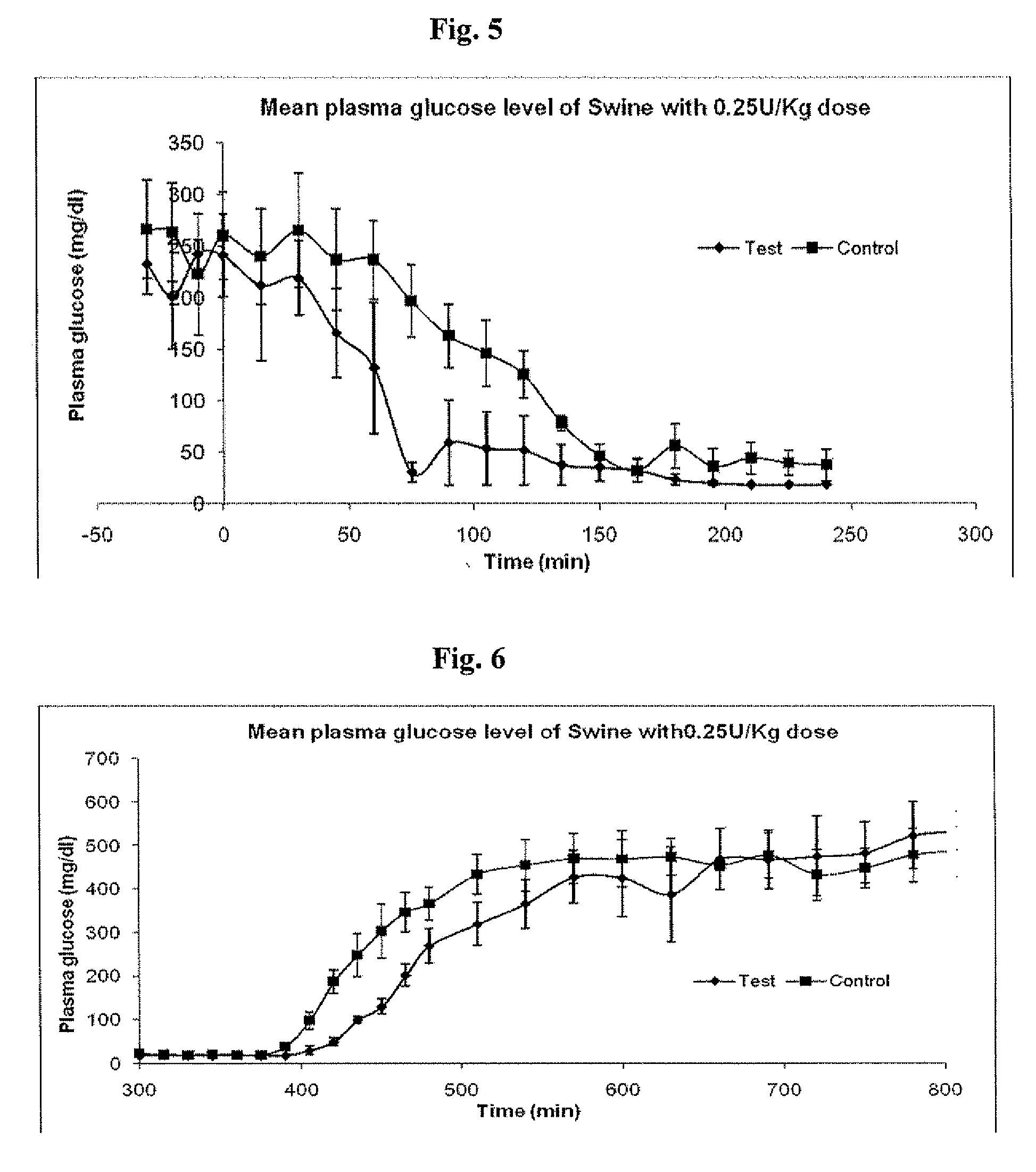
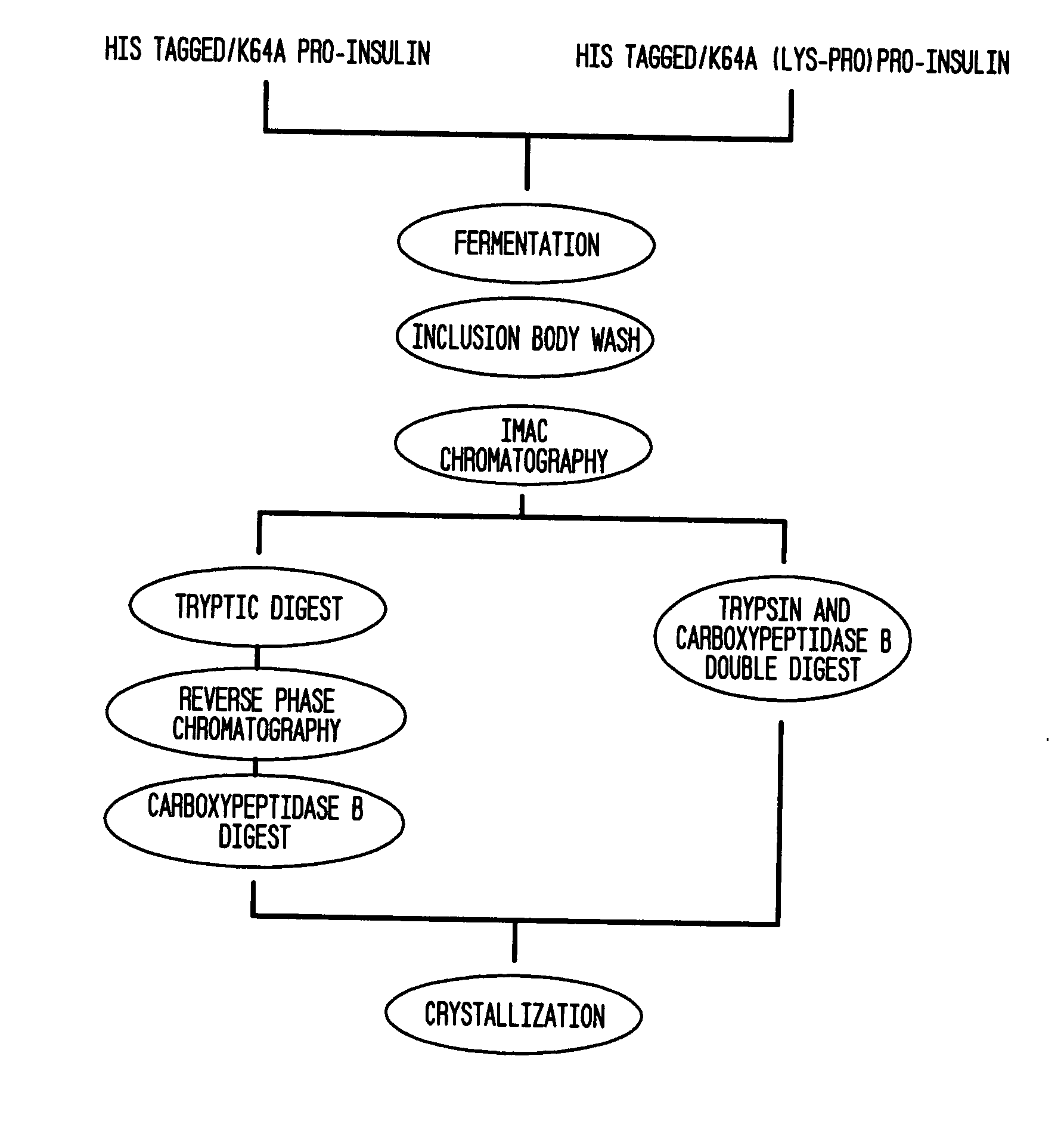
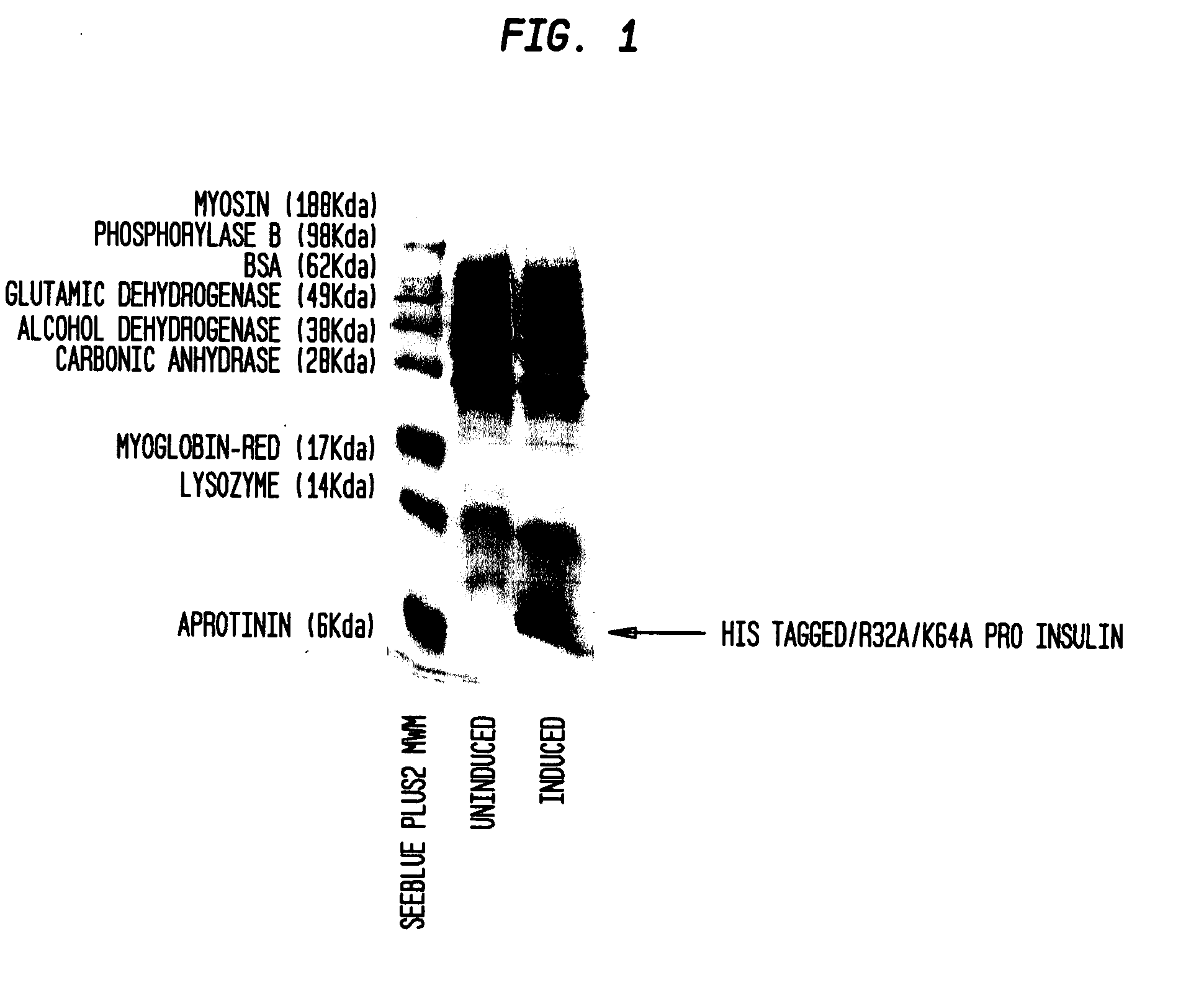
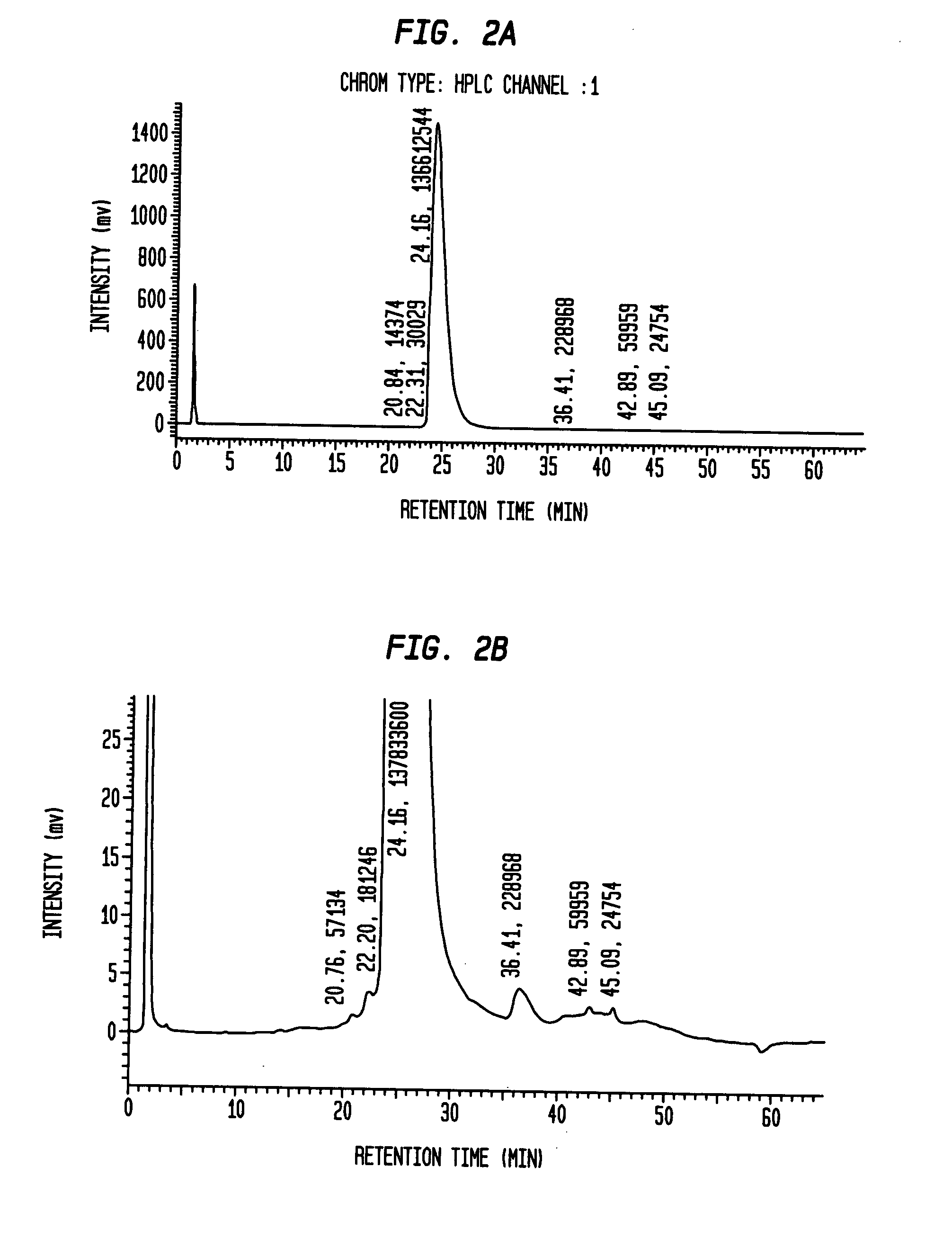
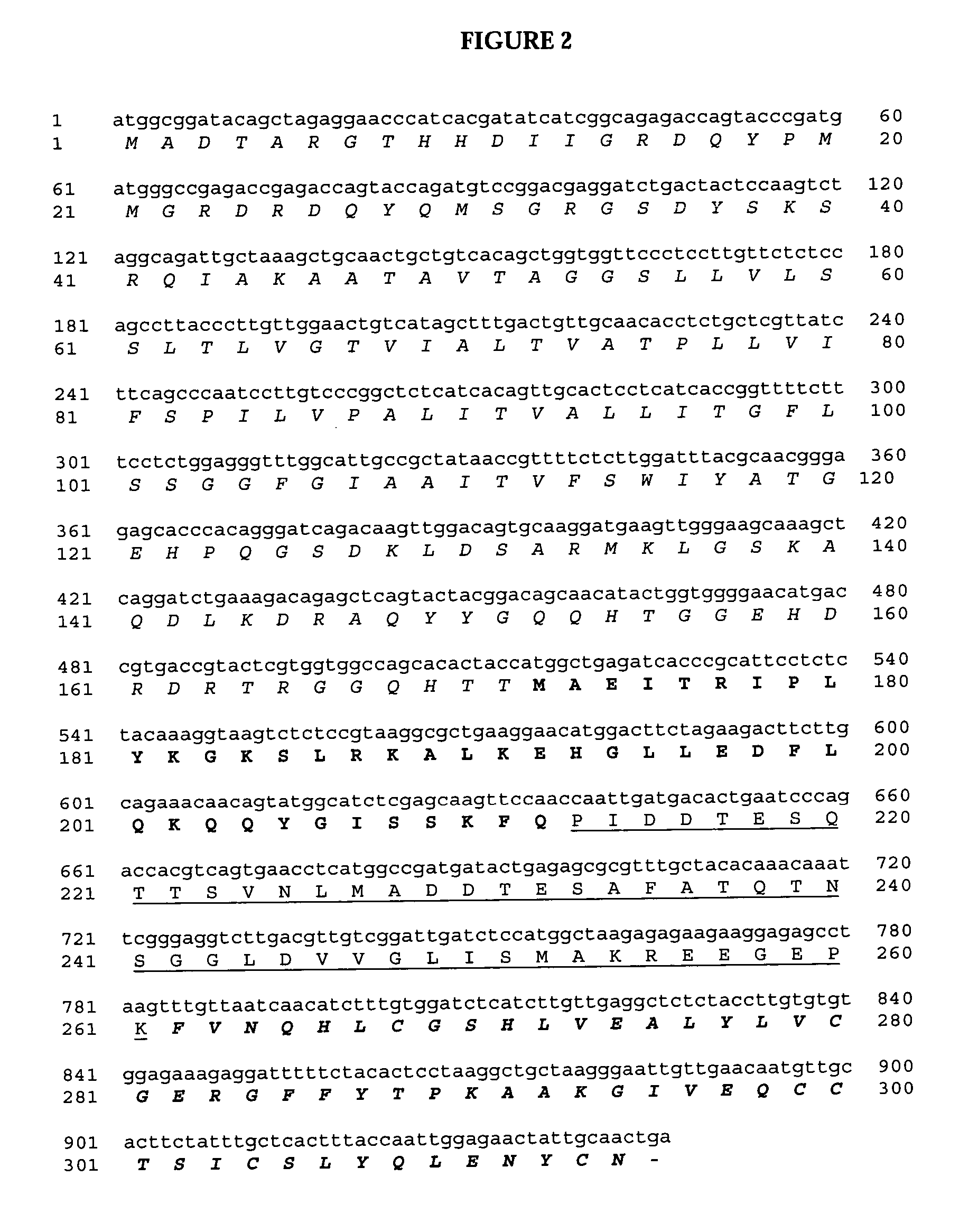
Novel pro-insulin having specific amino acid and / or nucleic acid modifications suitable for improved methods of insulin production are provided. Novel and highly efficient processes for preparing the pro-insulin preparations and preparations containing them are also disclosed. The novel pro-insulin preparations may be converted into human insulin useful in therapeutic preparations. Novel peptides of the C-peptide, and N terminus, including RREAEALQVGQVELGGGPGAGSLQPLALEGSLQAR (SEQ ID NO: 32), and MHHHHHHGGR (SEQ ID NO: 2) respectively are provided, as well as the unique nucleic acid molecules encoding them.
Owner:AGILA BIOTECH PVT LTD
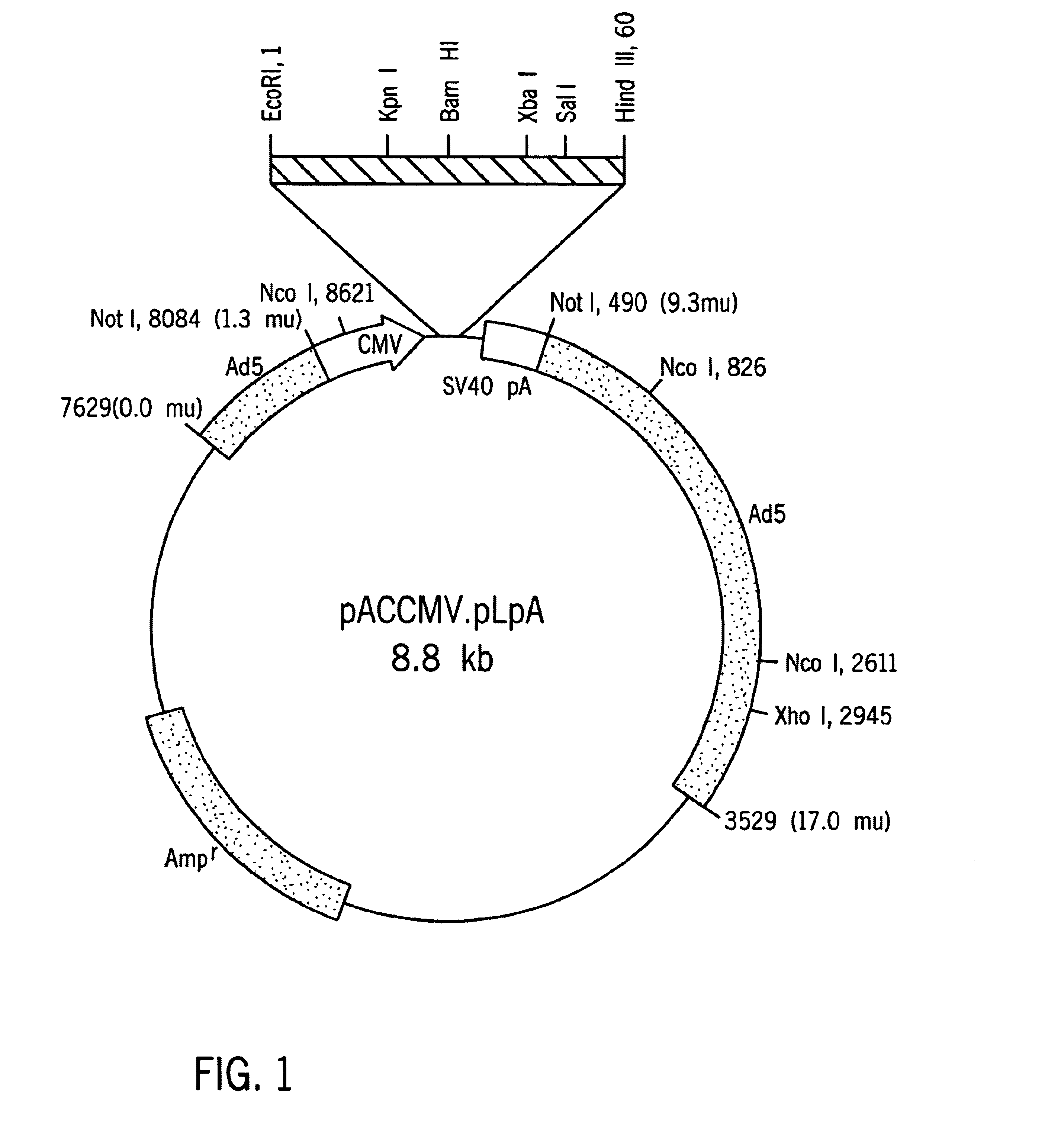
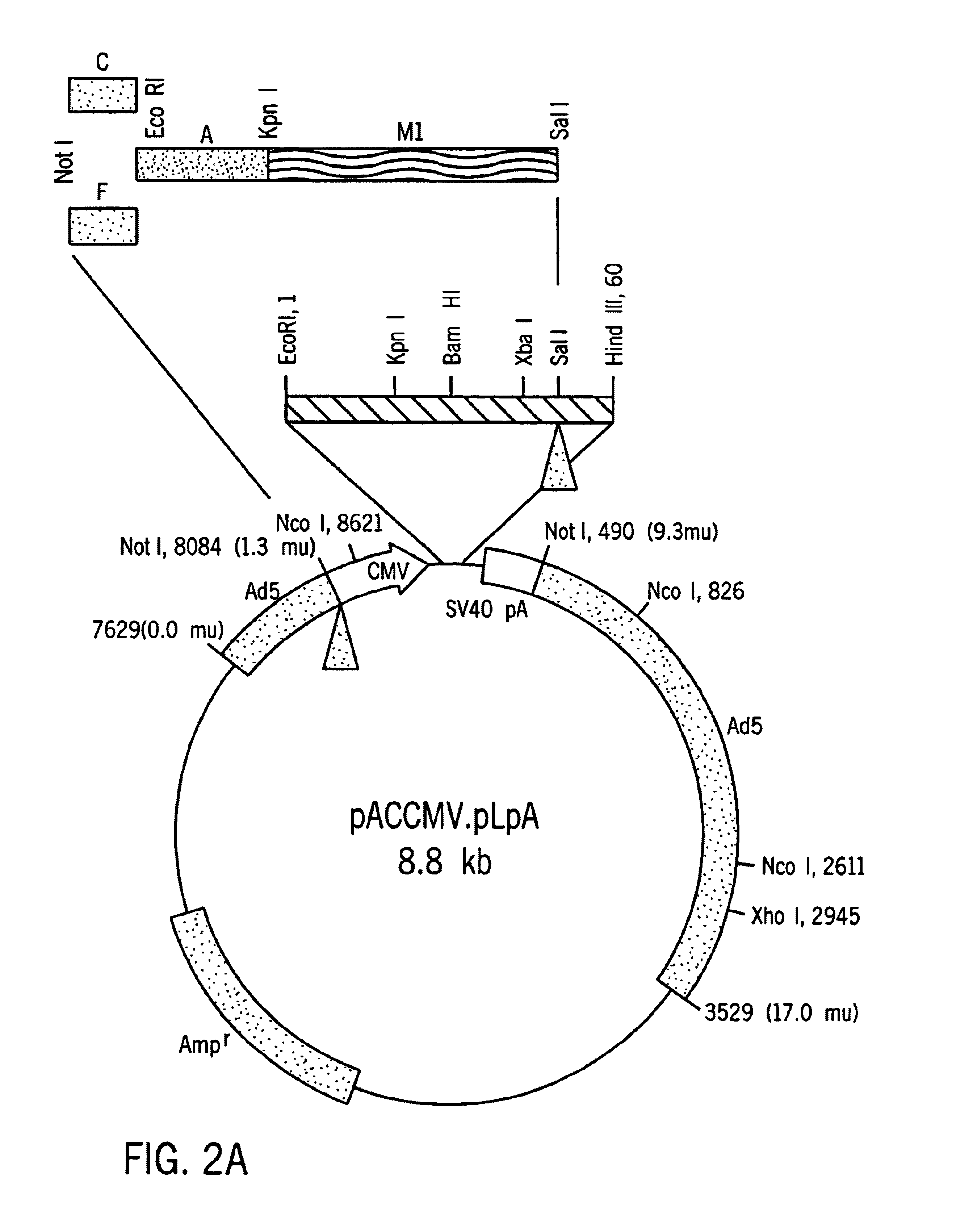
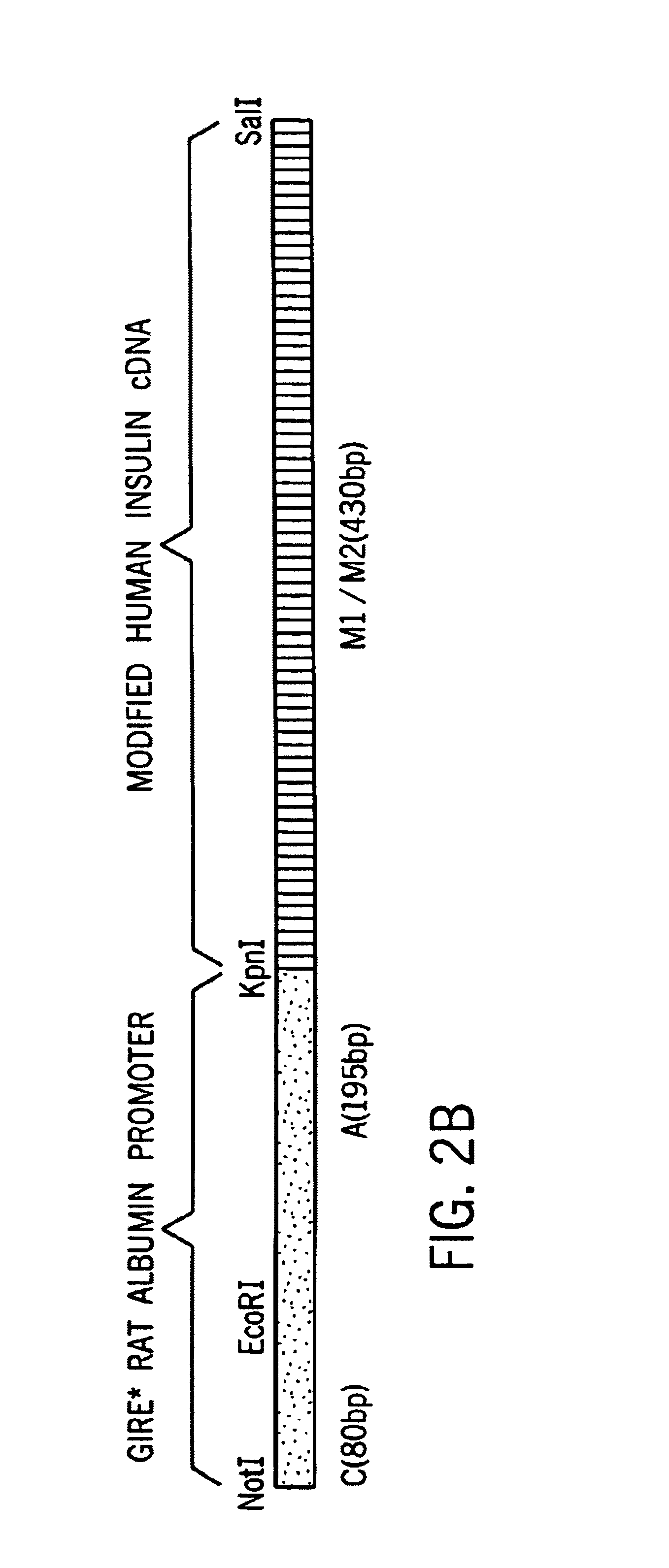
Hepatocyte Based Insulin Gene Therapy For Diabetes
A method and vectors for controlling blood glucose levels in a mammal are disclosed. In one embodiment, the method comprises the steps of: treating the hepatocyte cells of a patient with a first, second or third vector, wherein the first vector comprises a promoter enhancer, glucose inducible regulatory elements, a liver-specific promoter, a gene encoding human insulin with modified peptidase and an albumin 3′UTR and lacks an HGH intron, wherein the second vector comprises an HGH intron, glucose inducible regulatory elements, a liver-specific promoter, a gene encoding human insulin with modified peptidase site and an albumin 3′UTR and lacks a promoter enhancer, wherein the third vector comprises an HGH intron, glucose inducible regulatory elements, a liver-specific promoter, a gene encoding human insulin with modified peptidase site, an albumin 3′UTR and a promoter enhancer and observing the patient's insulin levels, wherein the patient's insulin levels are controlled.
Owner:WISCONSIN ALUMNI RES FOUND
Rapid mucosal gel or film insulin compositions
InactiveUS20080096800A1Improve stabilityRapid onsetPowder deliveryPeptide/protein ingredientsWhole bodyDissolution
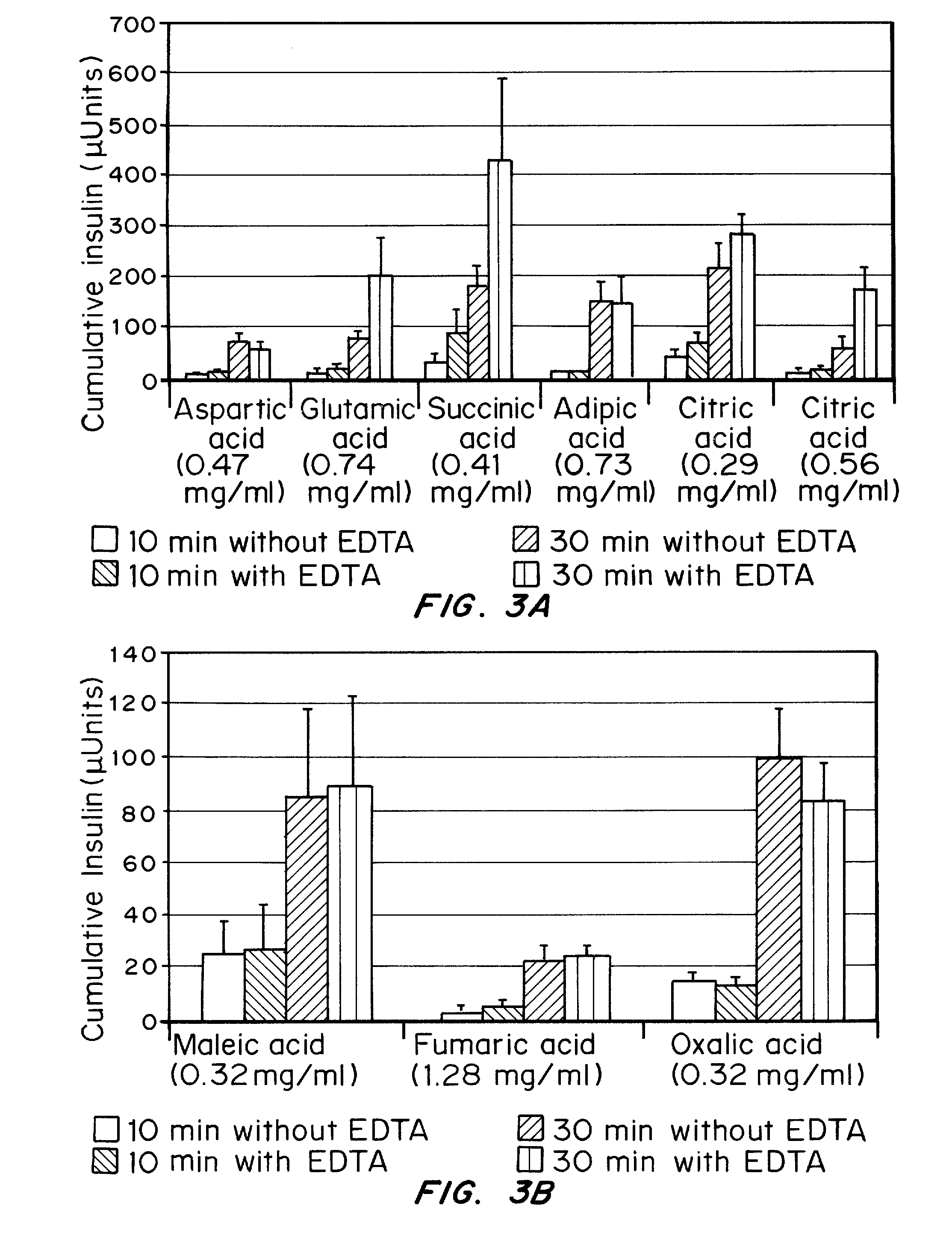
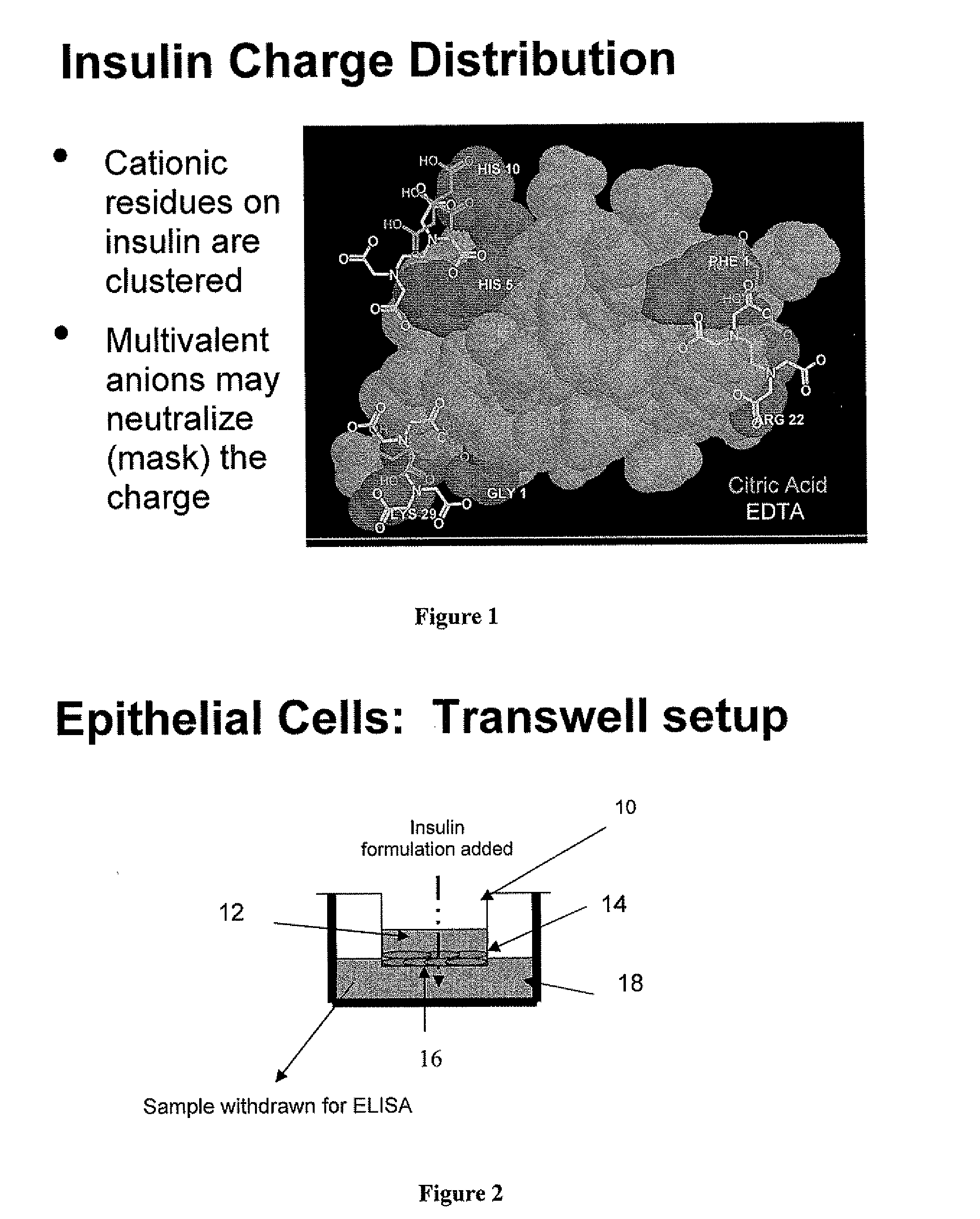
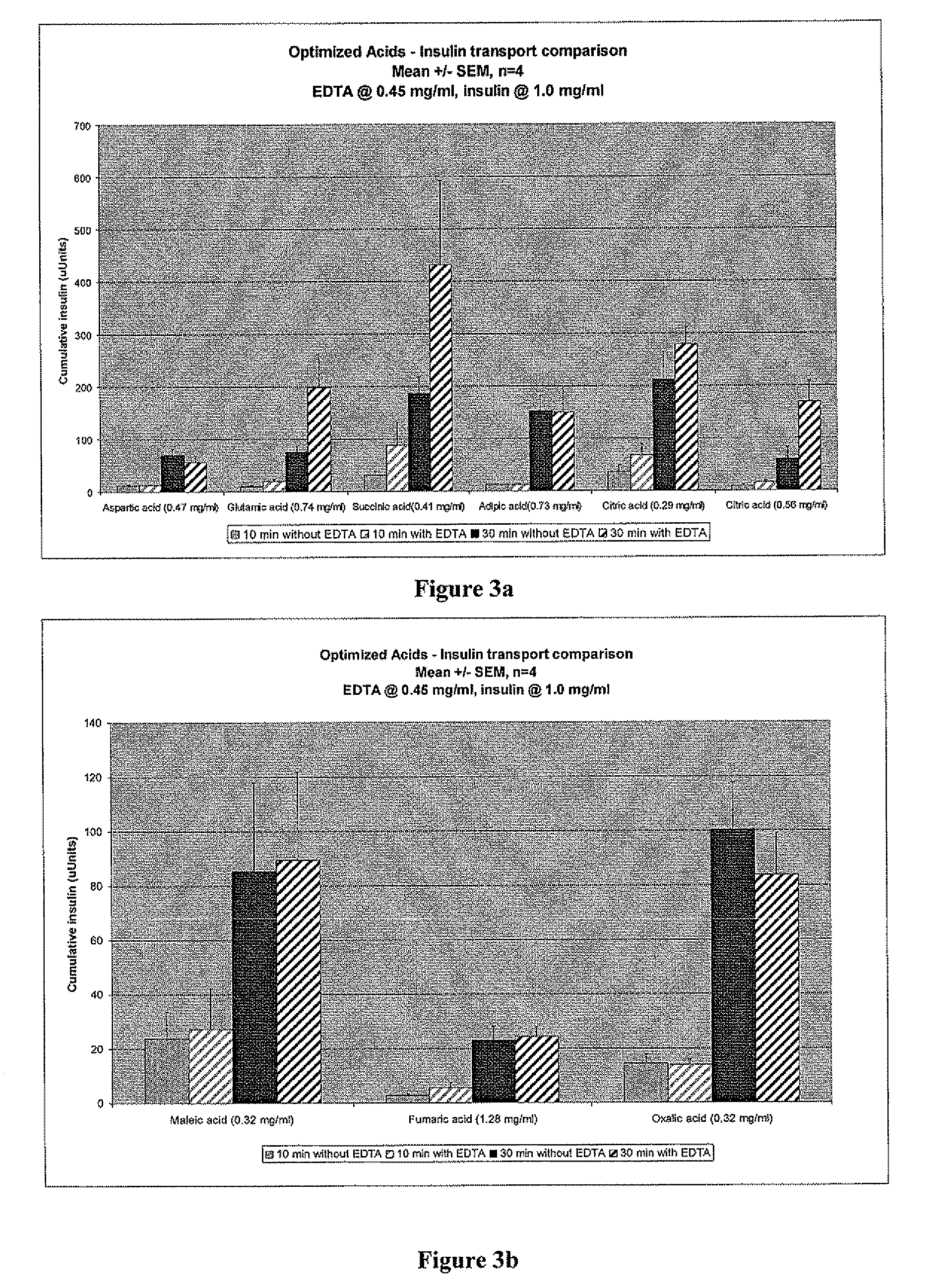
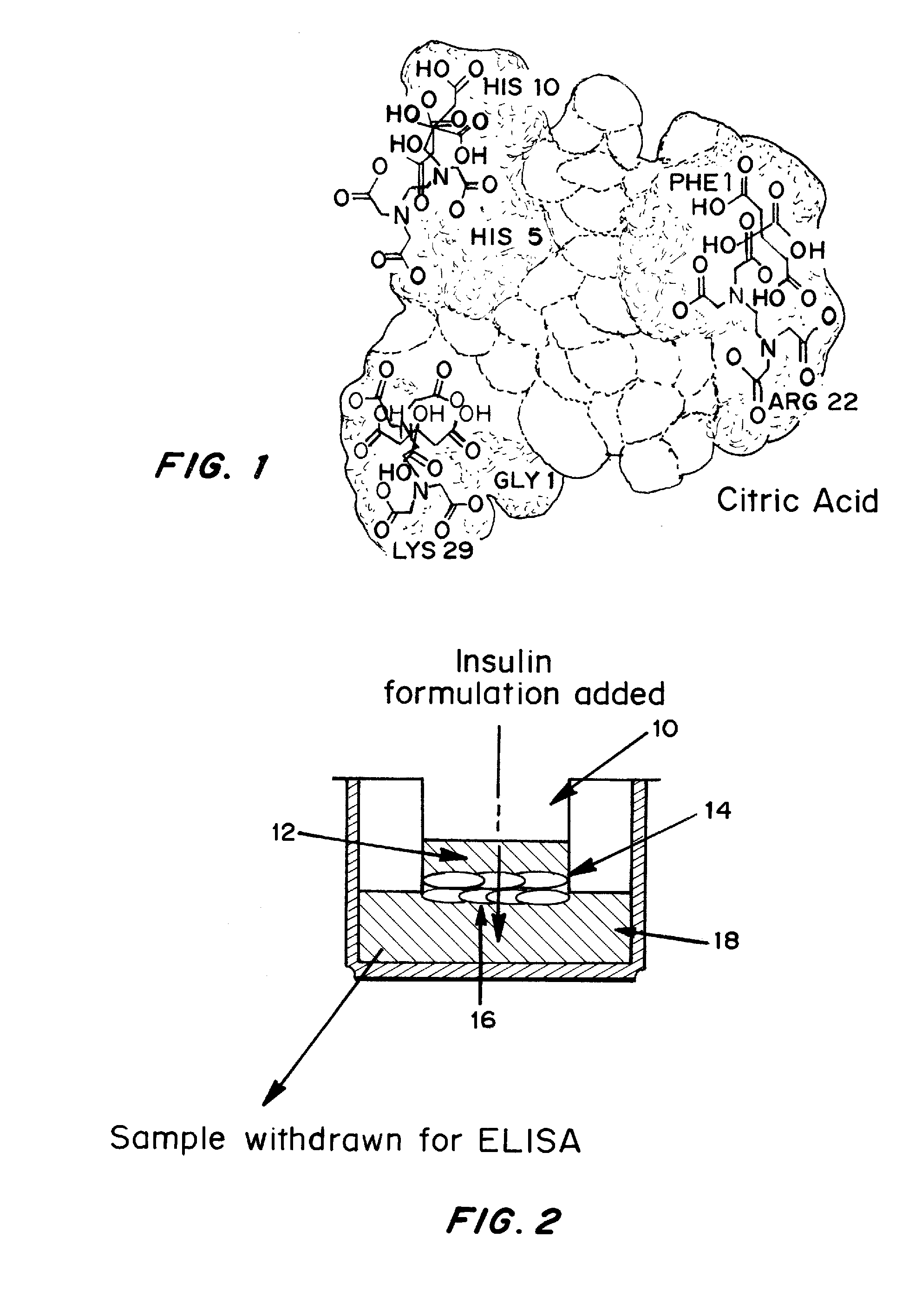
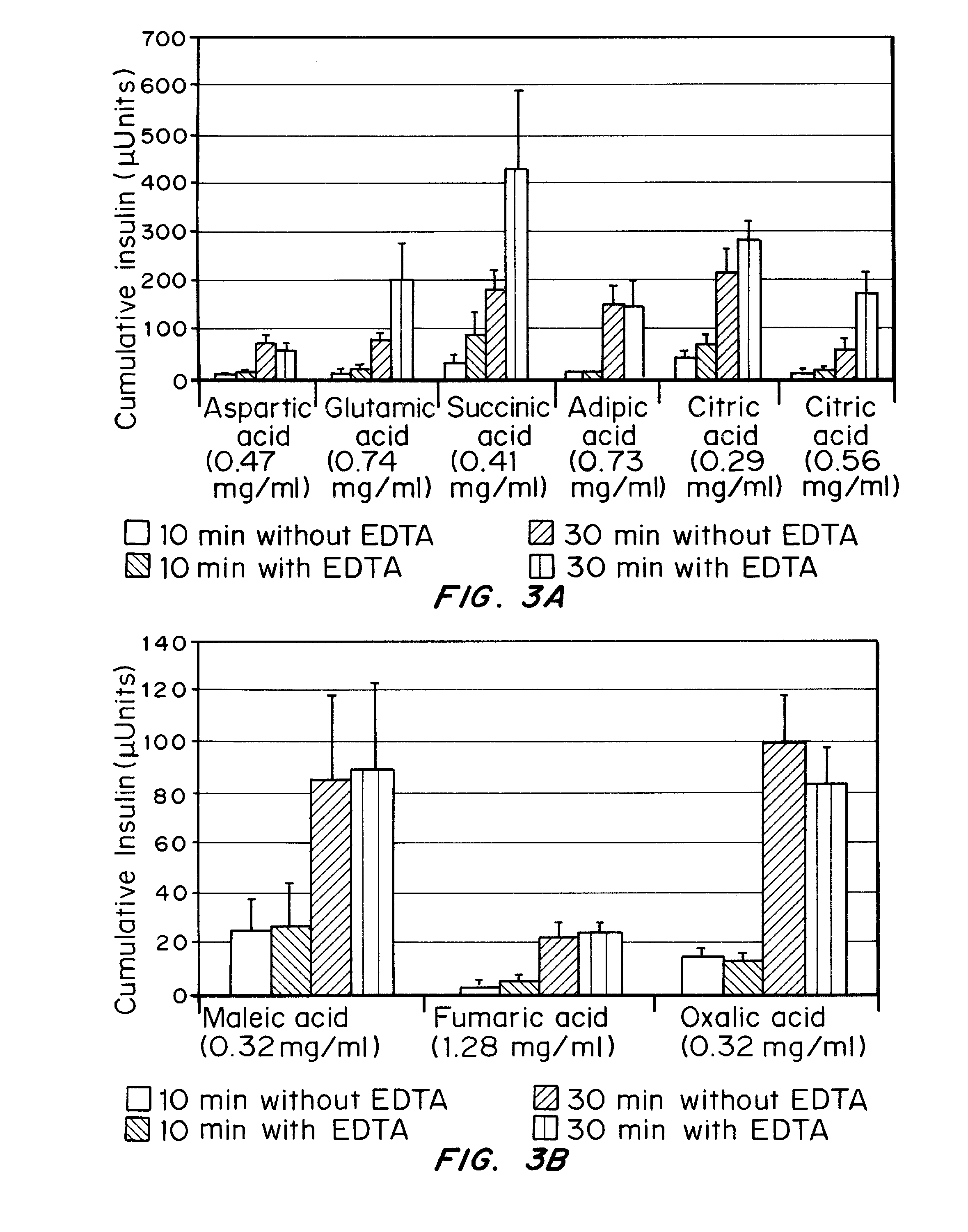
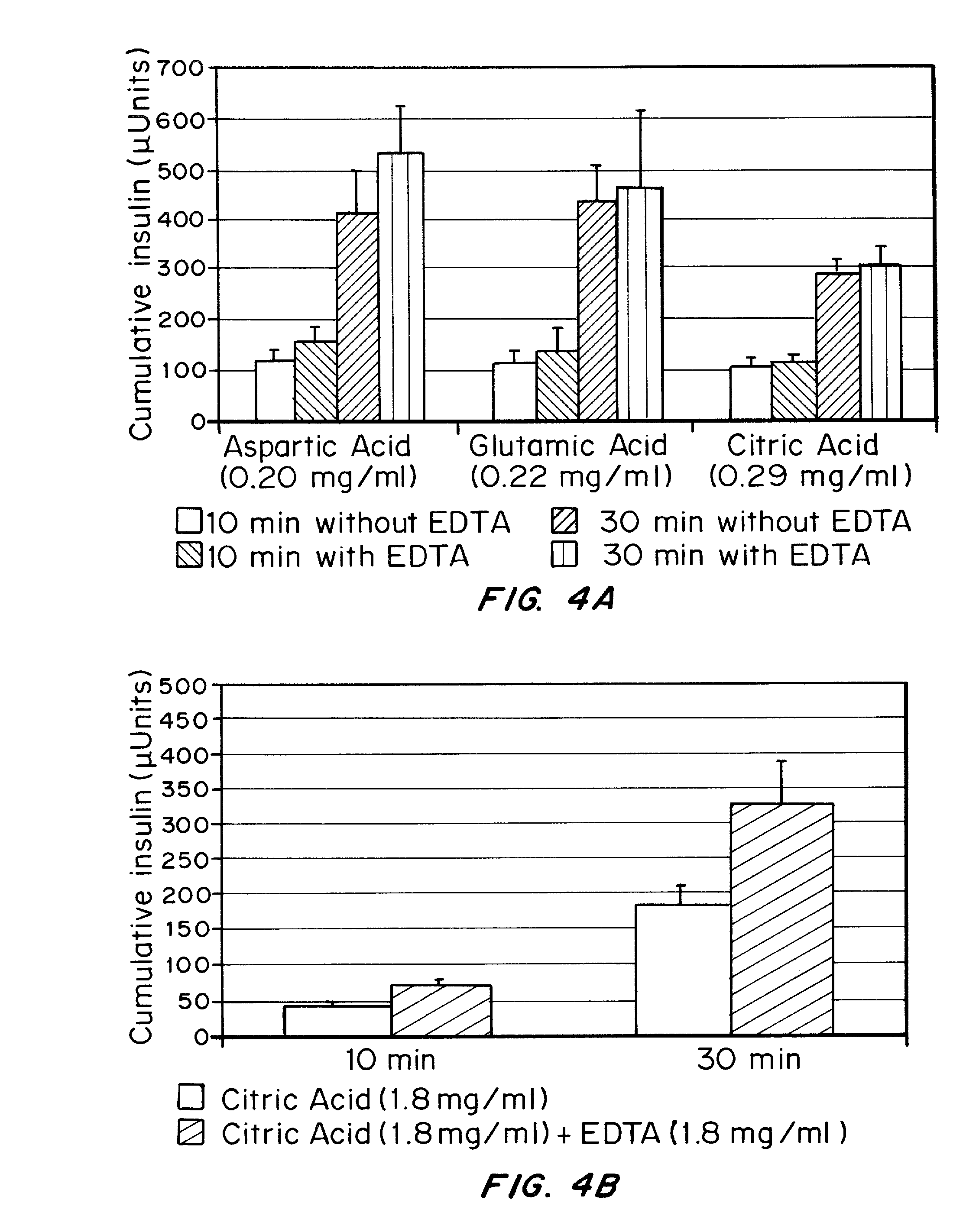
Gel, powder, suspension, emulsions or film formulations for systemic delivery of insulin with improved stability and rapid onset of action are described herein. The formulations are preferably absorbed to a mucosal surface, most preferably via buccal or sublingual administration, although rectal, vaginal, nasal or ocular administration is possible. The formulations contain insulin in combination with a chelator and dissolution agent, and optionally additional excipients. In the preferred embodiment, the formulation contains human insulin, a zinc chelator such as EDTA and a dissolution agent such as citric acid. Following administration, these formulations are rapidly absorbed into the blood stream. The formulation is preferably a polymeric gel, powder or film which adheres to the mucosal surface, thereby enhancing uptake of the incorporated drug. In the preferred embodiment, this formulation is administered sublingually, most preferably before a meal or after a meal.
Owner:BIODEL INC
Human amnion mesenchymal stem cell serum-free culture medium and culture method thereof
The invention relates to a human amnion mesenchymal stem cell serum-free culture medium and a culture method thereof. The culture medium is formed by adding human serum albumin, human transferrin, human insulin and sodium selenite into a DMEM / F12 basic culture medium. The culture method for the culture medium comprises the following steps of: digesting human amnion by using trypsin, then digesting the human amnion by using collagenase IV and deoxyribonuclease I, and filtering the mixture to obtain single cell suspension; and adding the human serum albumin, the transferrin, the insulin and the sodium selenite into the DMEM / F12 basic culture medium in a ratio of VDMEM to VF12 of 1:1, and putting human amnion mesenchymal stem cells in a 37 DEG C CO2 incubator with saturated humidity and volume fraction of 5 percent under the serum-free condition, wherein culture in vitro and amplification are realized by solution change and transfer of culture, potentiality of multi-direction differentiation is maintained, and the amplified cells can be induced in vitro to form cartilage cells, osteoblasts and adipocytes. The culture medium and the culture method have the characteristics of no other animal sources, wide source and no limitation of ethics.
Owner:辽宁艾米奥干细胞与再生医学研究院有限公司
Delivery of pharmaceutical agents via the human insulin receptor
InactiveUS20080051564A1Reduces immunogenic reactionNervous disorderImmunoglobulins against cell receptors/antigens/surface-determinantsMurine antibodyInsulin receptor
A humanized murine antibody is provided that binds to the human insulin receptor (HIR). The humanized murine antibody is suitable for use as a Trojan horse to deliver pharmaceutical agents to human organs and tissue that express the HIR. The humanized murine antibody is especially well suited for delivering neuropharmaceutical agents from the blood stream to the brain across the blood brain barrier (BBB). The humanized murine antibody may be genetically fused to the pharmaceutical agent or it may be linked to the pharmaceutical agent using an avidin-biotin conjugation system.
Owner:RGT UNIV OF CALIFORNIA
Rapid Acting Injectable Insulin Compositions
InactiveUS20080090753A1Improve stabilityQuick effectPowder deliveryPeptide/protein ingredientsDissolutionExcipient
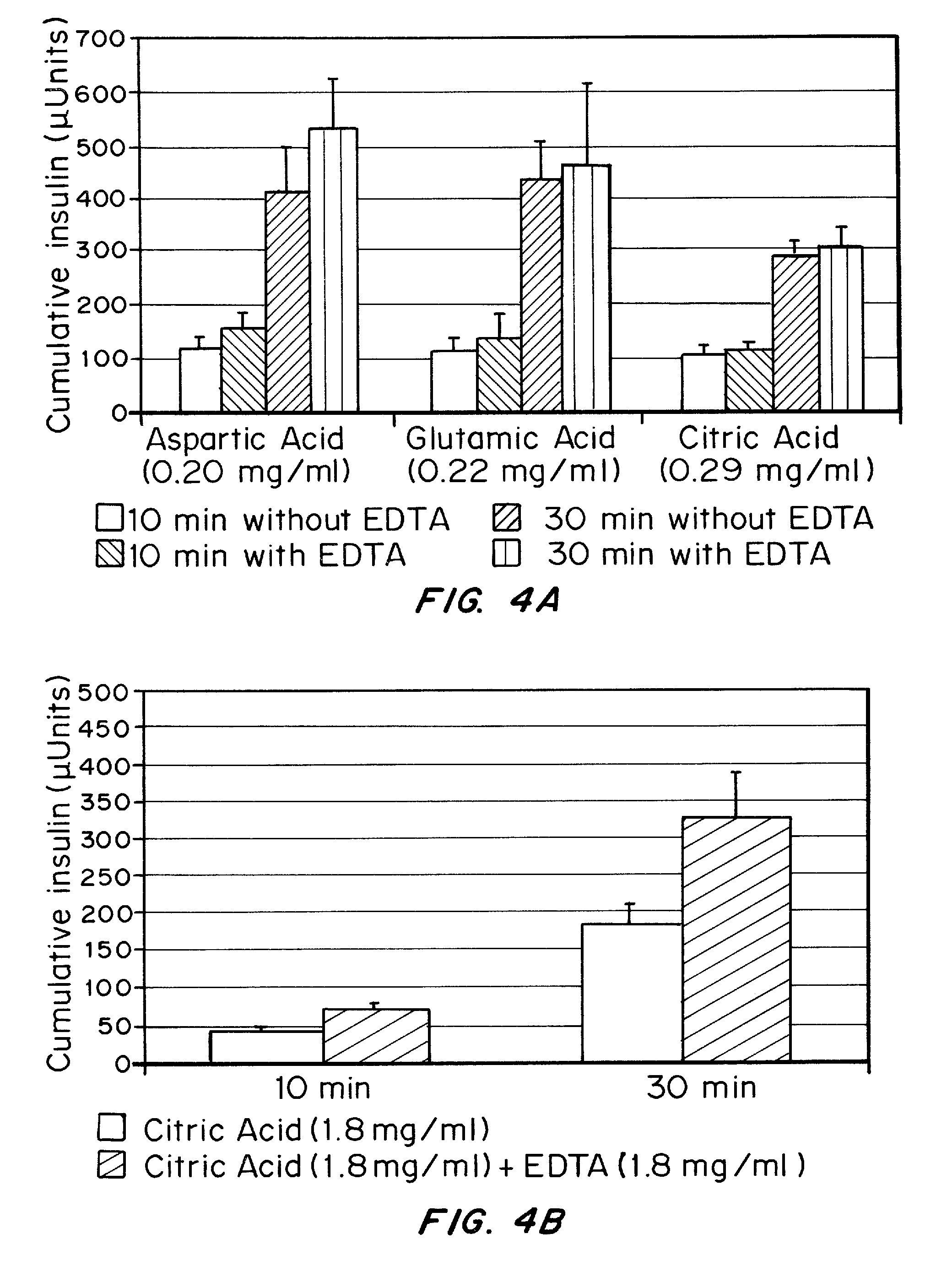
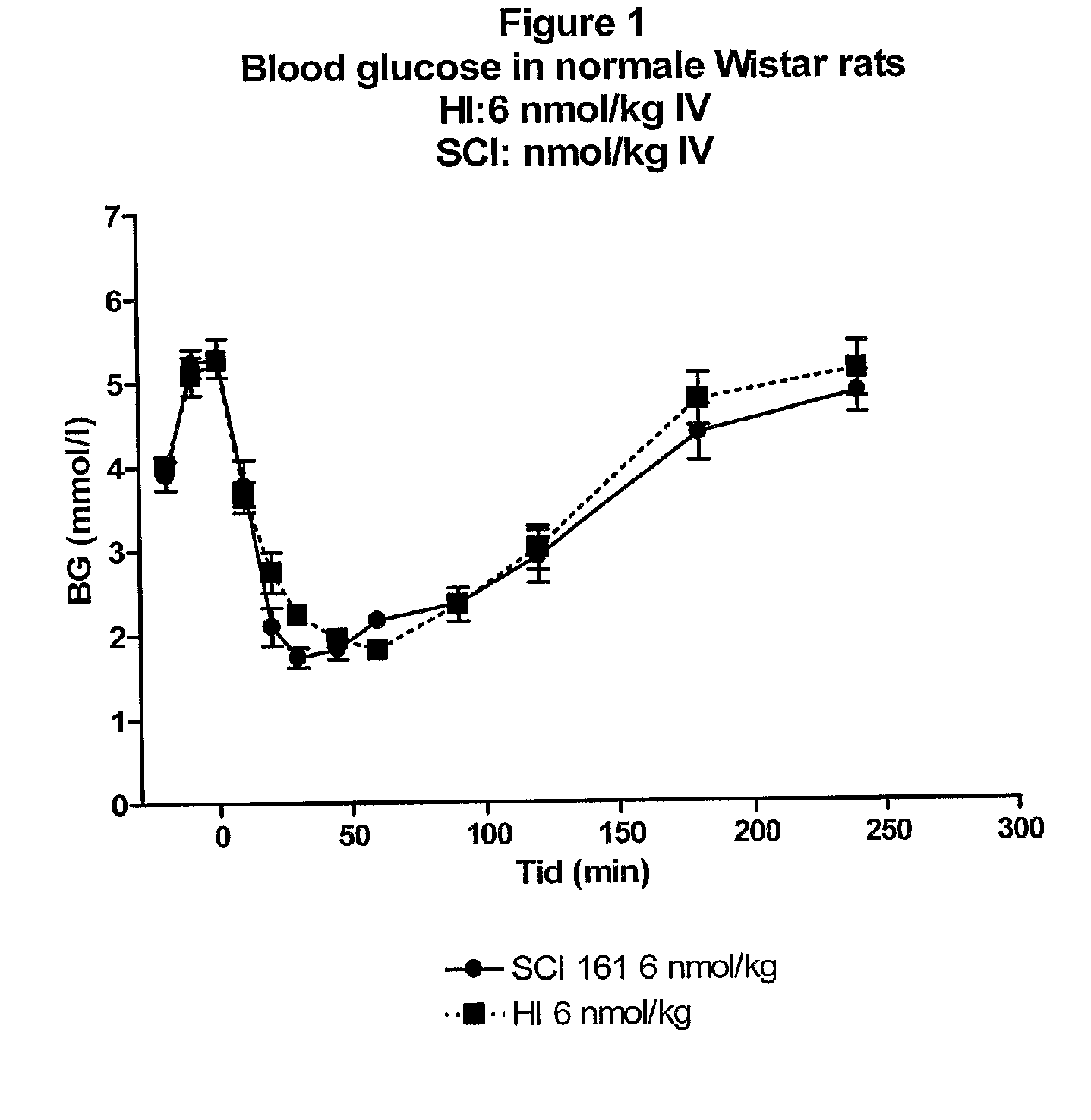
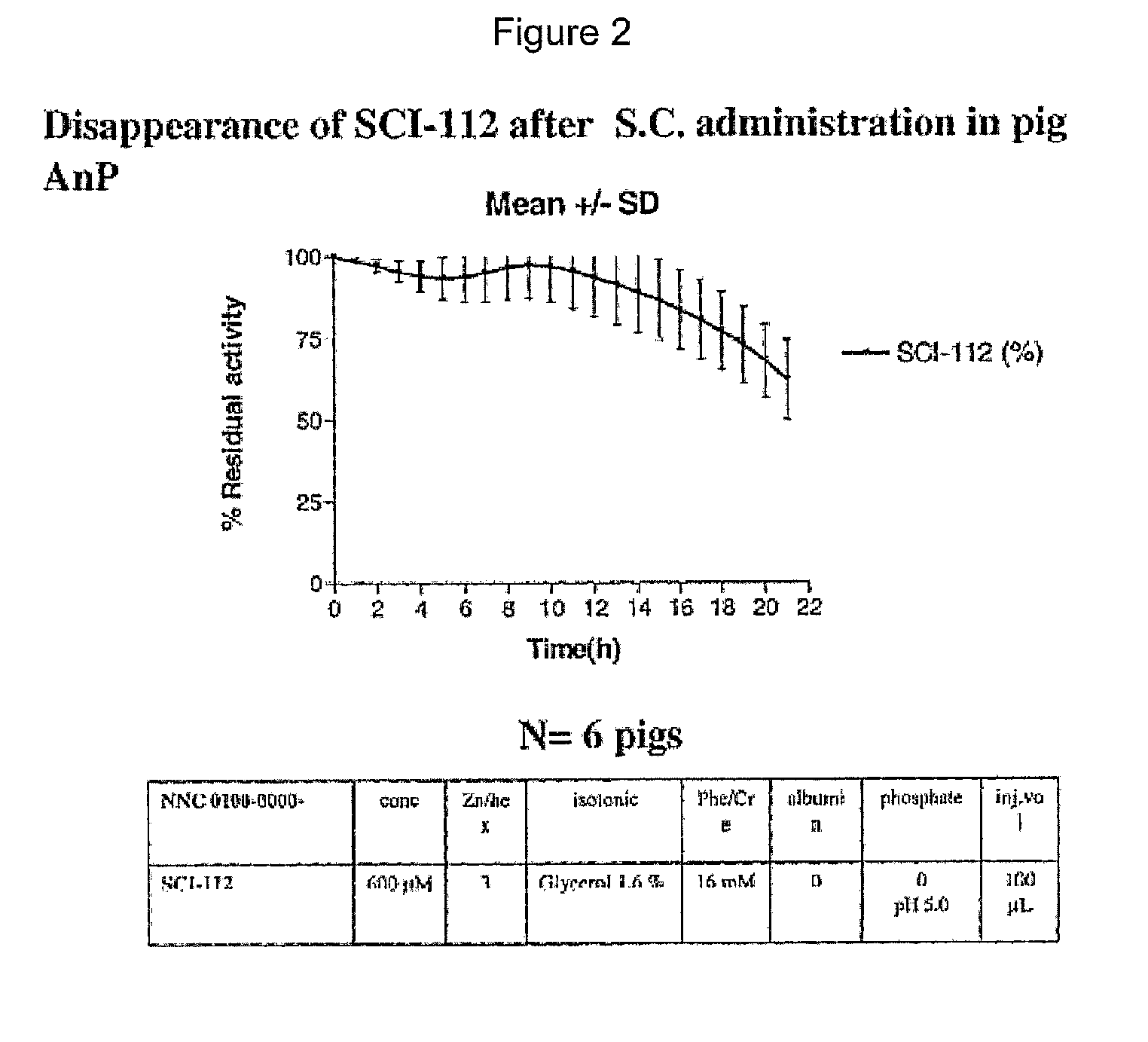
Injectable insulin formulations with improved stability and rapid onset of action are described herein. The formulations may be for subcutaneous, intradermal or intramuscular administration, In the preferred embodiment, the formulations are administered via subcutaneous injection. The formulations contain insulin in combination with a chelator and dissolution agent, and optionally additional excipients. In the preferred embodiment, the formulation contains human insulin, a zinc chelator such as EDTA and a dissolution agent such as citric acid. These formulations are rapidly absorbed into the blood stream when administered by subcutaneous injection. In the preferred embodiment, the insulin is provided as a dry powder in a sterile vial. This is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water, a zinc chelator such as EDTA and a dissolution agent such as citric acid shortly before or at the time of administration. In another embodiment, the insulin is stored as a frozen mixture, ready for use upon thawing.
Owner:BIODEL
Single-Chain Insulin
InactiveUS20090170750A1Lower blood sugar levelsSugar derivativesPeptide/protein ingredientsInsulin activityPhysical stability
The present invention is related to single-chain insulin having insulin activity comprising a B- and an A-chain or a modified B- and A-chain connected by a connecting peptide of from 6-11 amino acids. The single-chain insulins will have biological insulin activity and an IGF-1 receptor affinity similar to or lower than that of human insulin and a high physical stability. The single-chain insulin may contain at least one basic amino acid residues in the connecting peptide. The single-chain insulins may also be acylated in one or more Lys residues.
Owner:NOVO NORDISK AS
Method of designing agonists and antagonists to IGF receptor
InactiveUS7020563B1Inhibit bindingReduced activityOrganic chemistry methodsBiological material analysisAgonistInsulin receptor
Identification of the human insulin receptor binding site sequence of certain spatial molecular structures conforming to such binding site and of certain insulinomimetic sequences including the binding site sequence are disclosed.
Owner:WALTER & ELIZA HALL INST OF MEDICAL RES
Fully Human Antibodies Directed Against the Human Insulin-Like Growth Factor-1 Receptor
InactiveUS20080025990A1Reduces IGF-IRCut surfaceSenses disorderFungiInsulin-like growth factorSingle-Chain Antibodies
This invention relates to human antibodies that bind to human insulin-like growth factor-1 receptor (IGF-IR), to derivatives of these antibodies (Fabs, single chain antibodies, bi-specific antibodes, or fusion proteins), and to uses of the antibodies and derivatives in therapeutic, and diagnostic methods. The invention relates to nucleic acids encoding the anti-IGF-IR, methods of generating the antibodies and expression. The invention further relates to combination therapies using ant-IGF-IR antibodies with anti-neoplastic drugs.
Owner:IMCLONE SYSTEMS
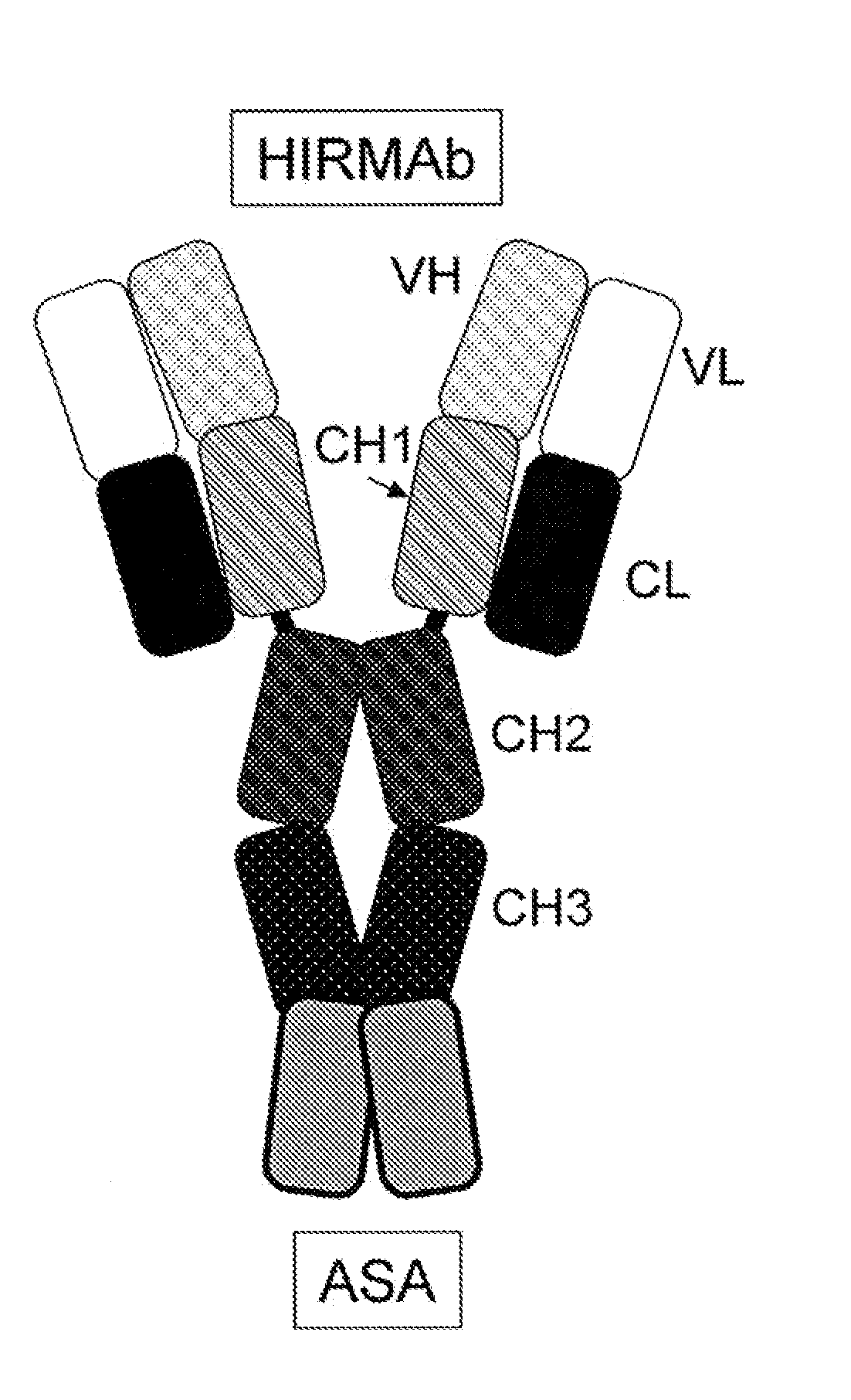
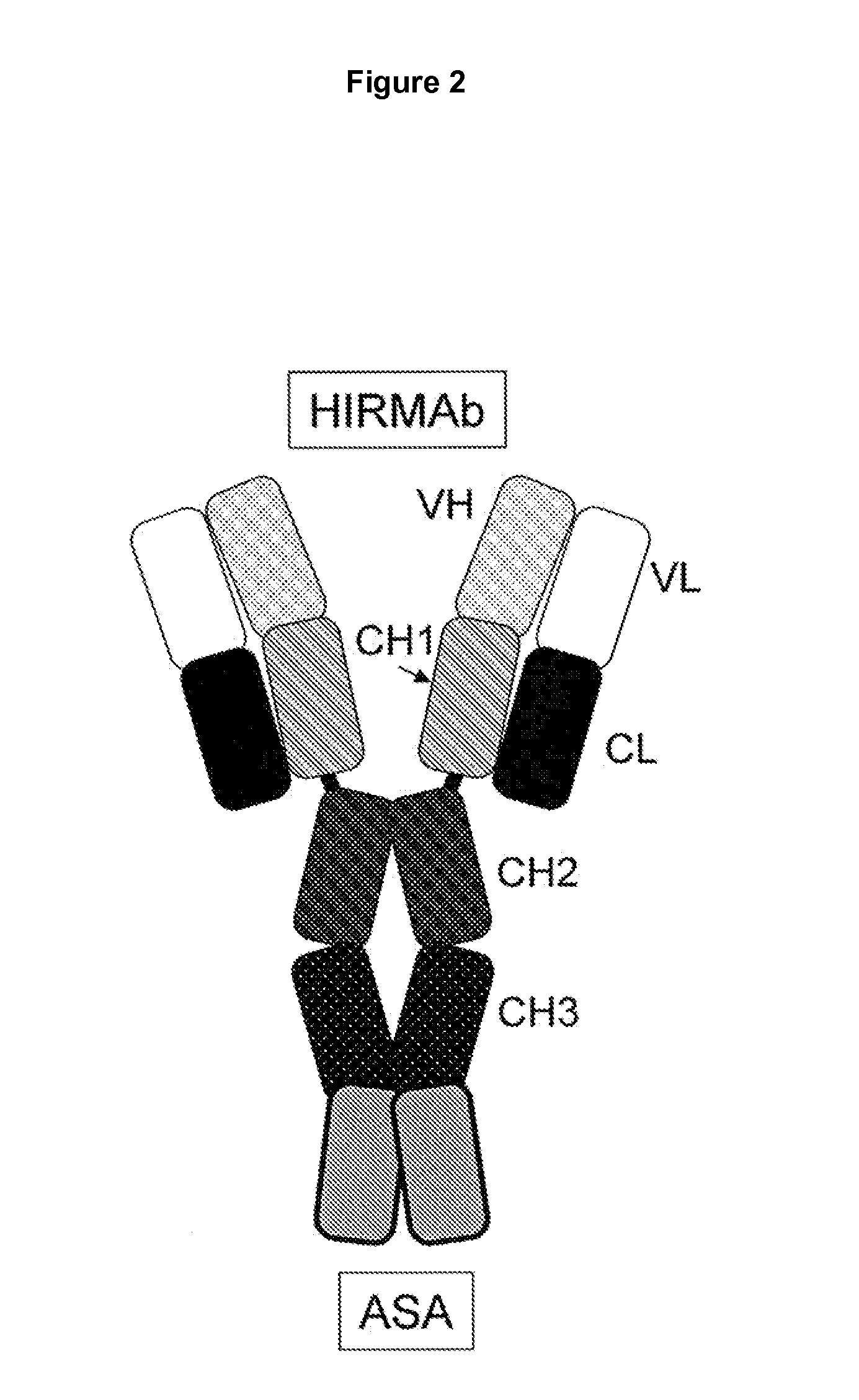
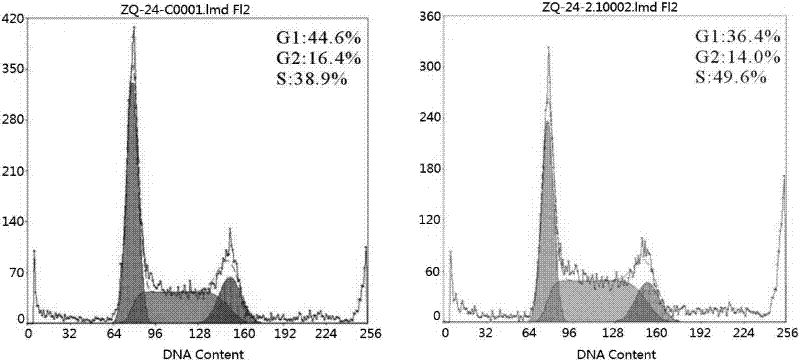
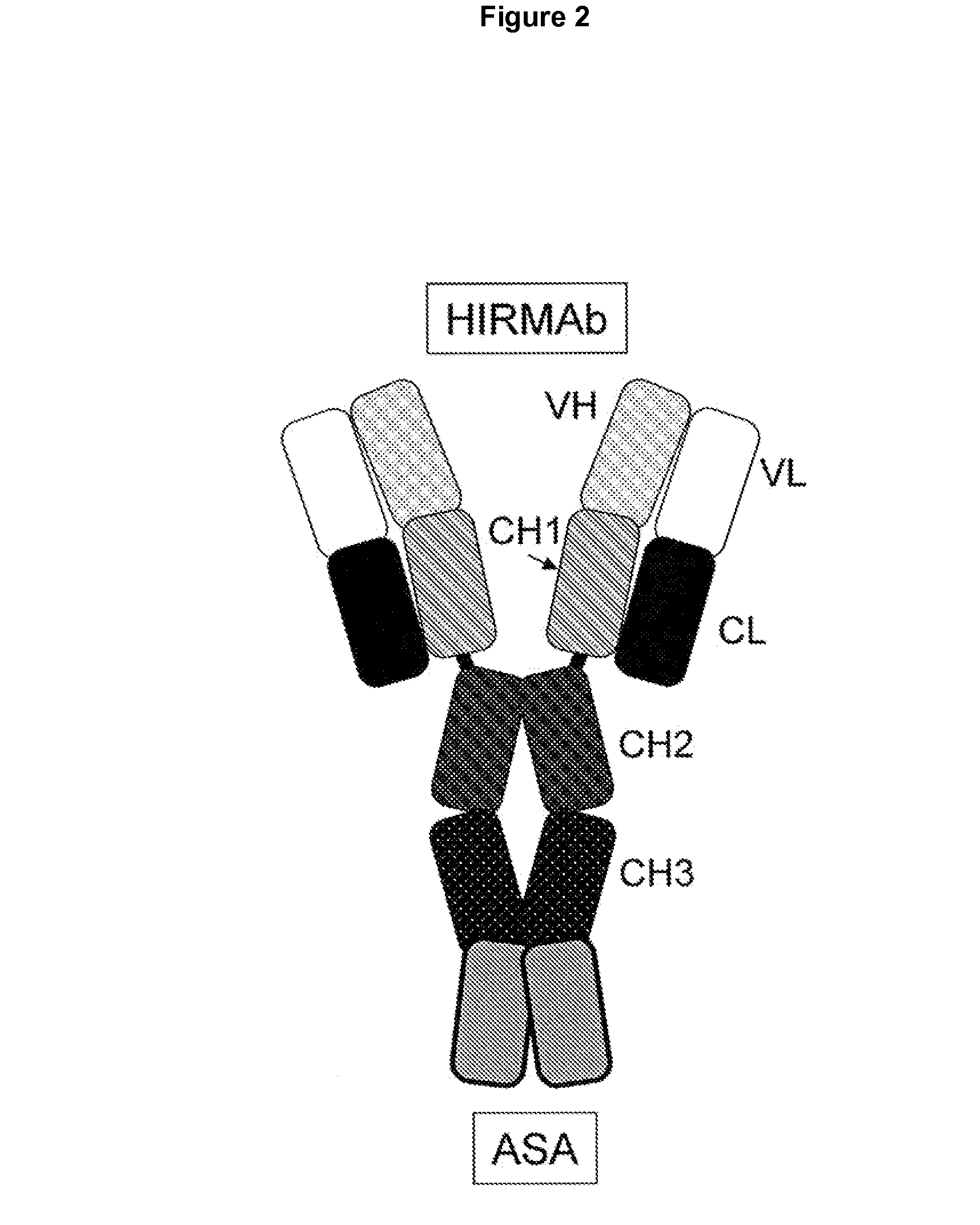
Methods and Compositions for Increasing Arylsulfatase A Activity in the CNS
ActiveUS20130142794A1Polypeptide with localisation/targeting motifNervous disorderArylsulfatase A activityInsulin receptor
Provided herein are methods and compositions for treating a subject suffering from a deficiency in arylsulfatase A in the CNS. The methods include systemic administration of a bifunctional fusion antibody comprising an antibody to a human insulin receptor and an arylsulfatase A.
Owner:JCR PHARMA +1
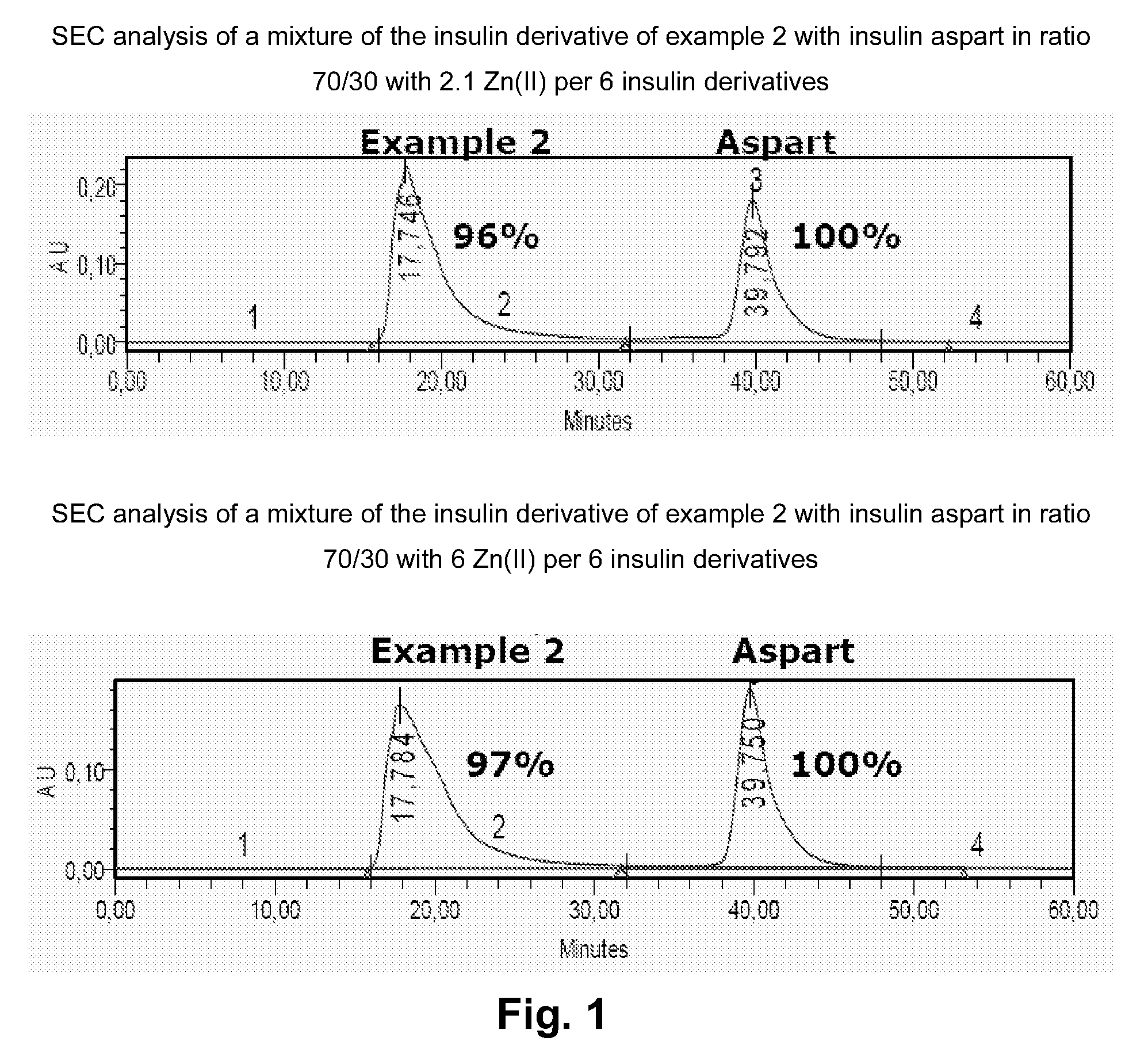
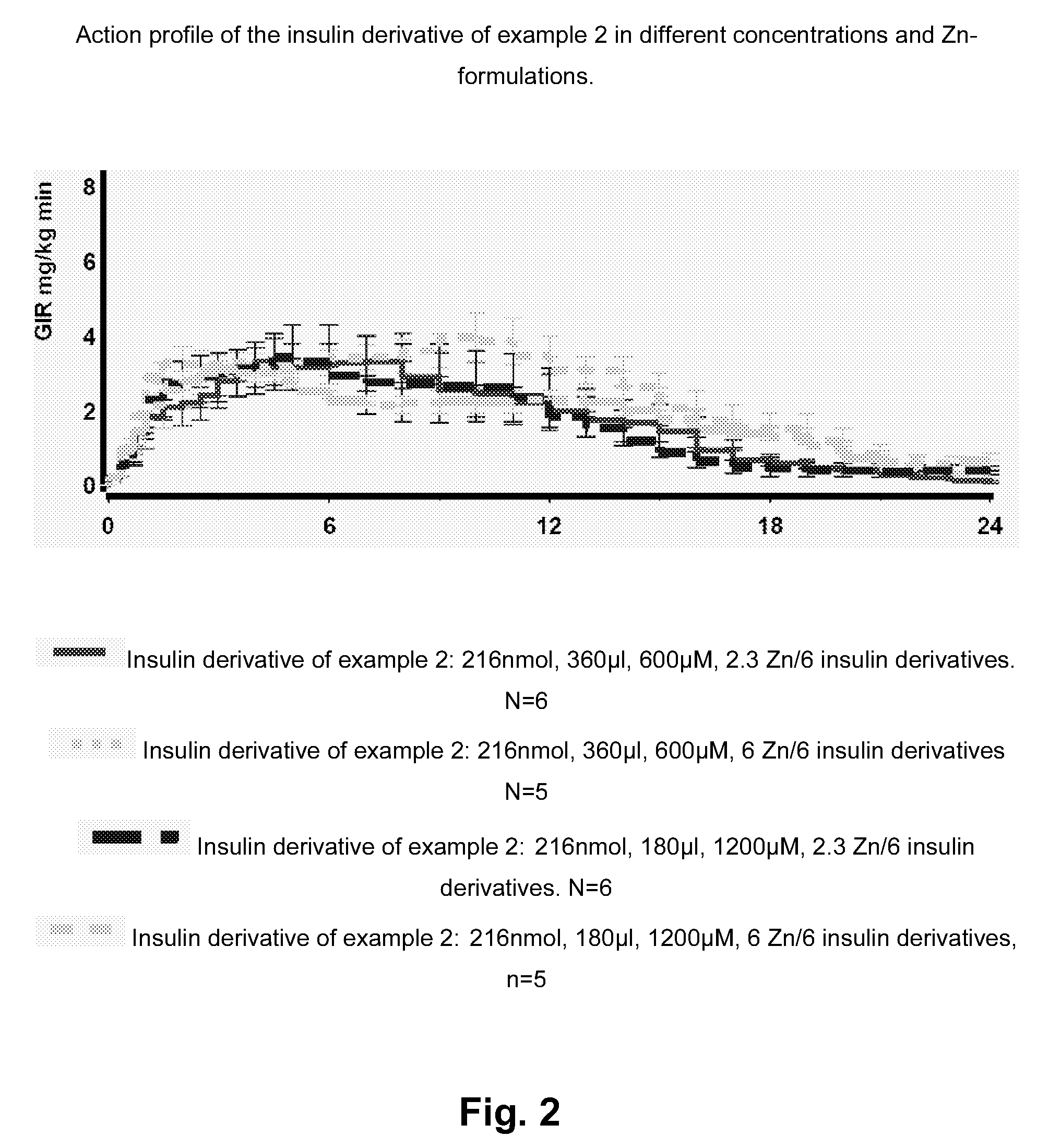
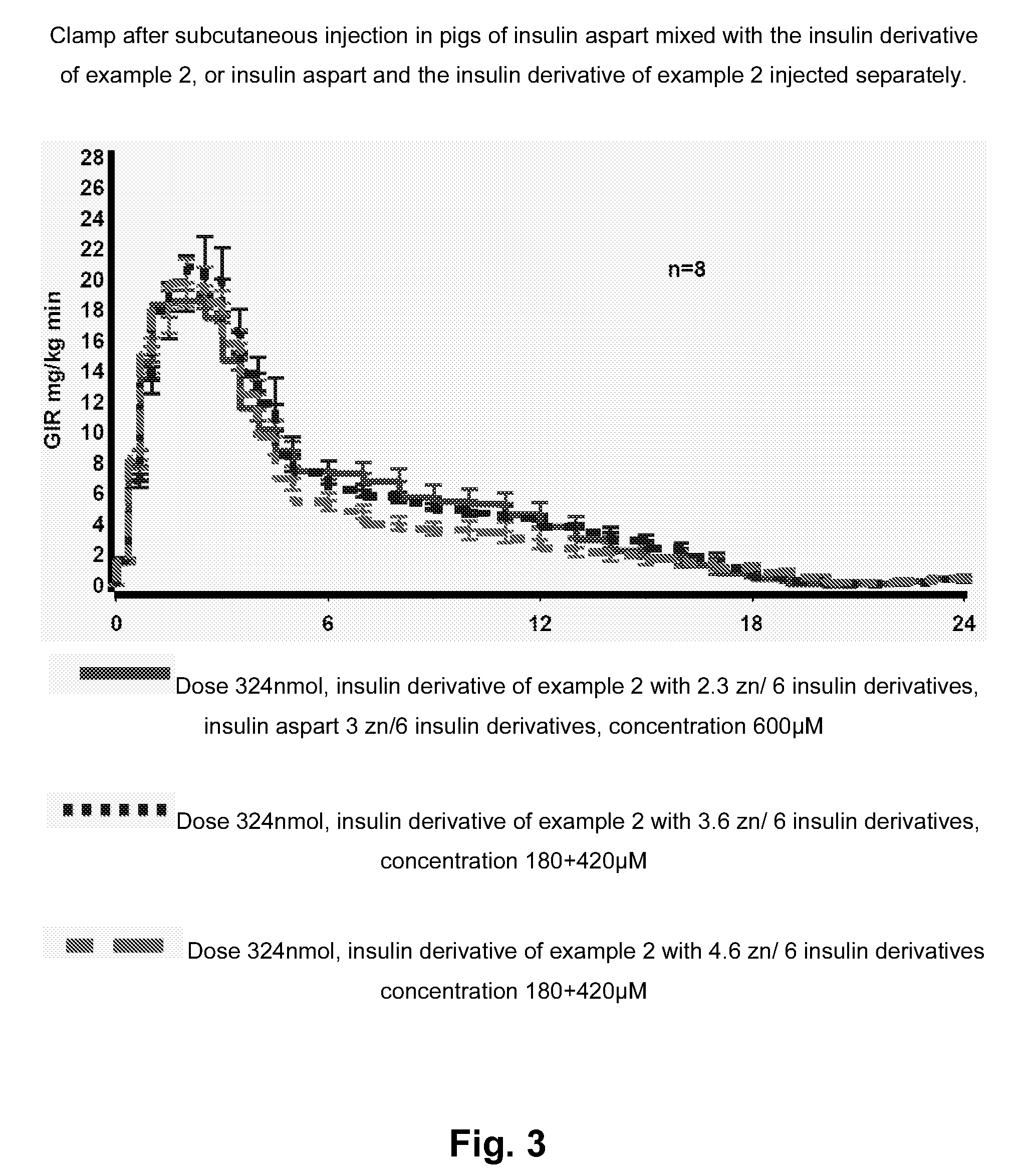
Insulin Derivative
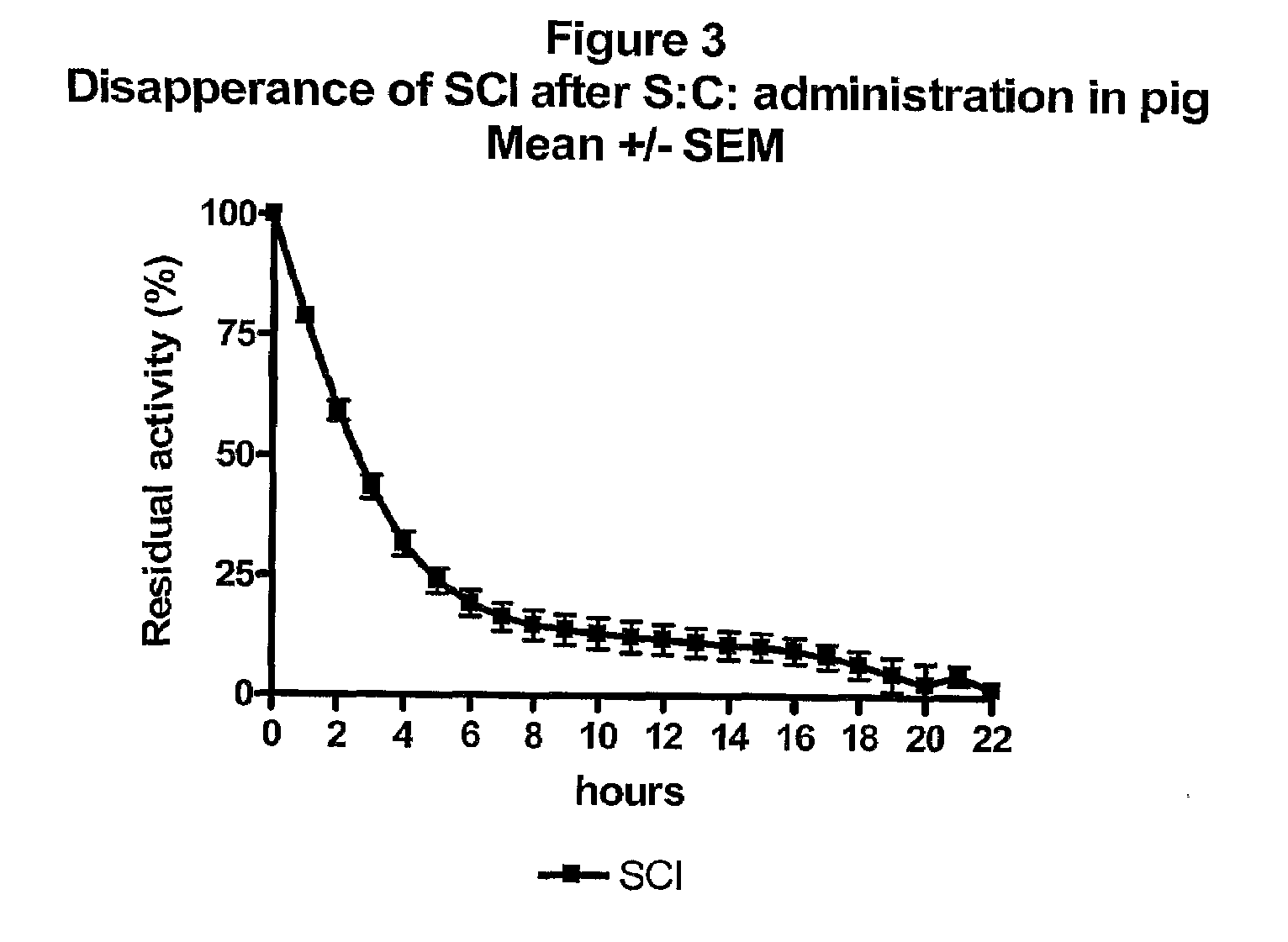
InactiveUS20100279931A1Prolonged profile of actionPeptide/protein ingredientsMetabolism disorderDrugHuman insulin
Owner:NOVO NORDISK AS
Antibody against human insulin-like growth factor
InactiveUS7438911B2Improve stabilitySuppression problemBacteriaAntipyreticInsulin-like growth factorDisease
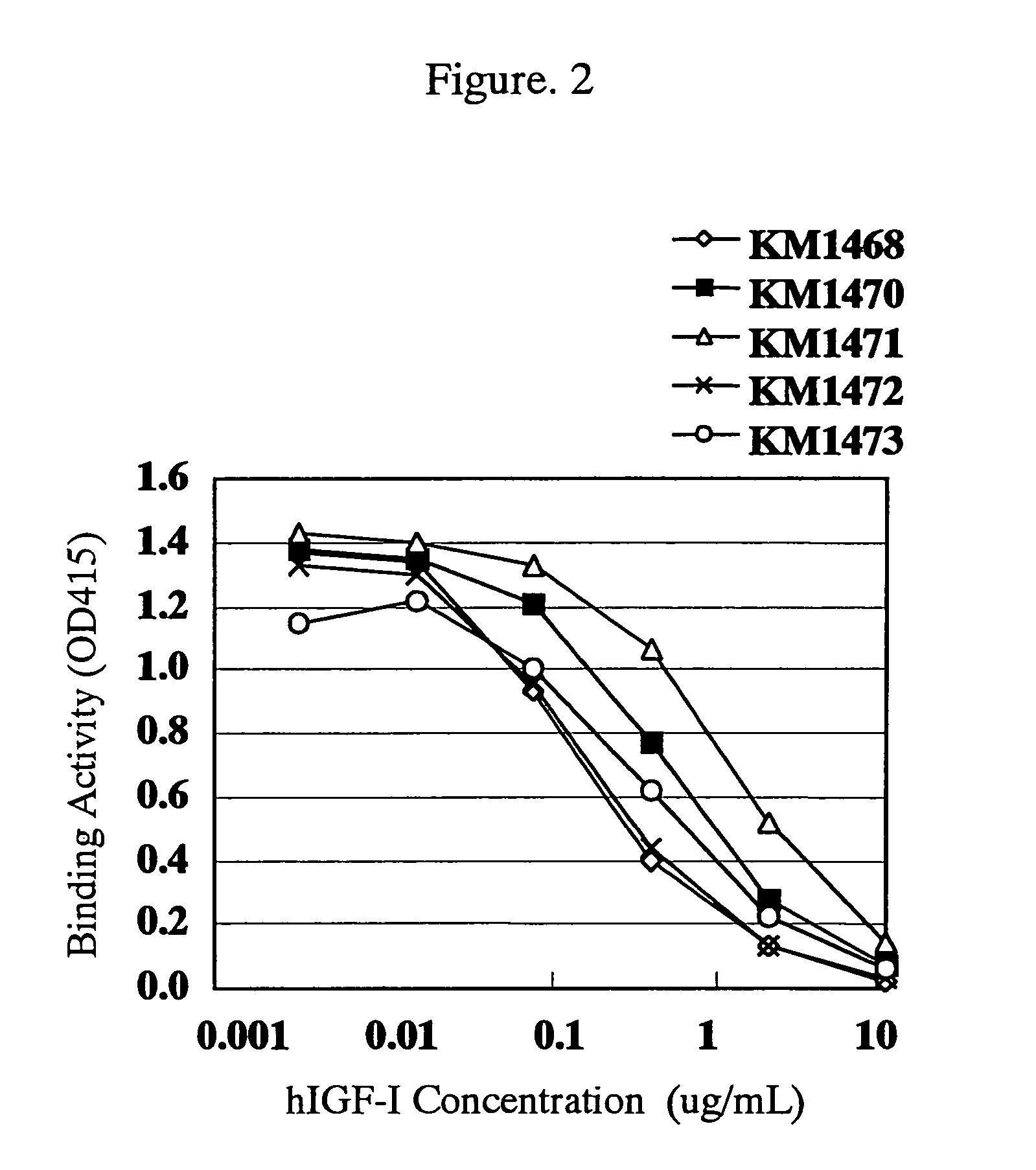
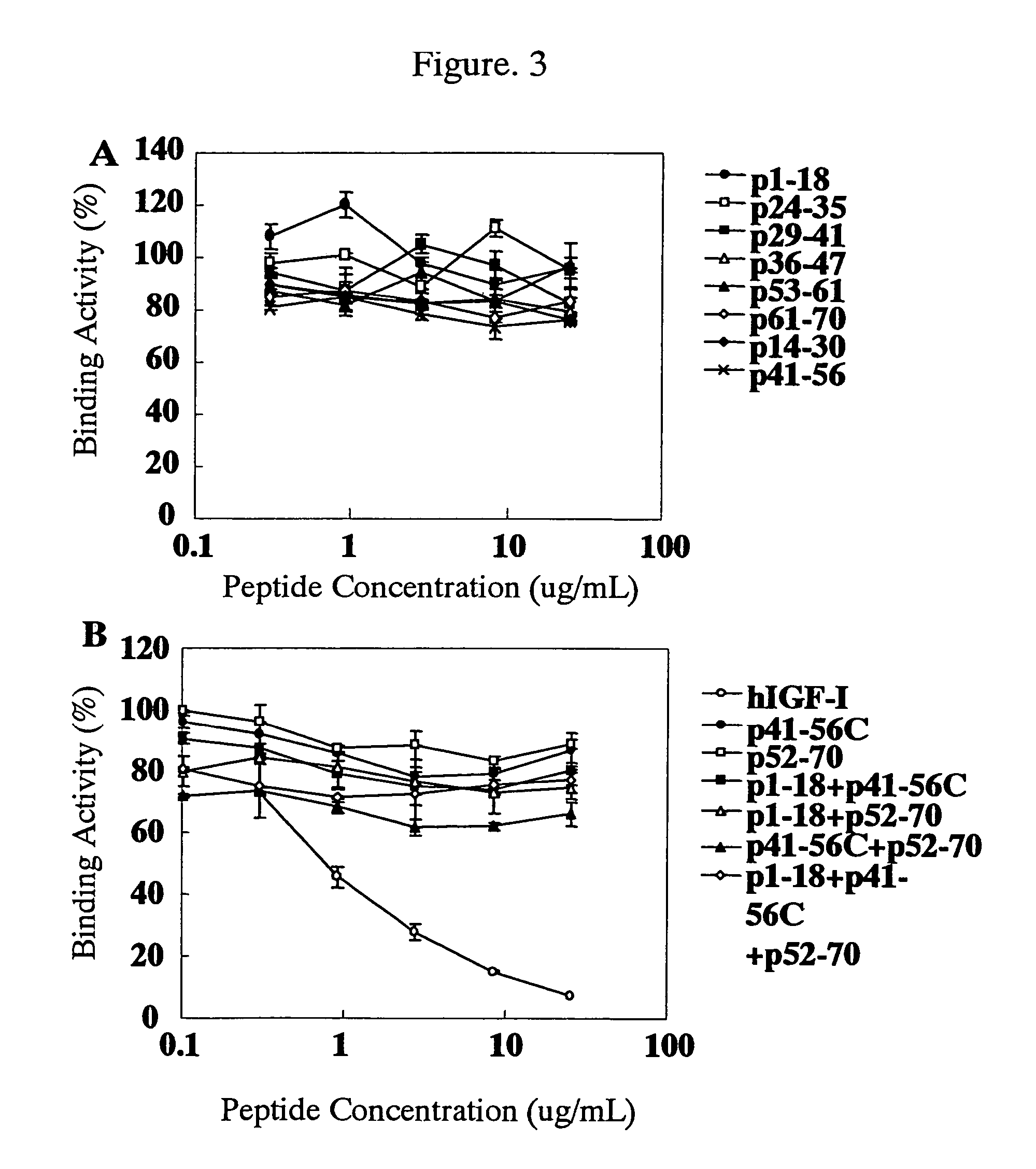
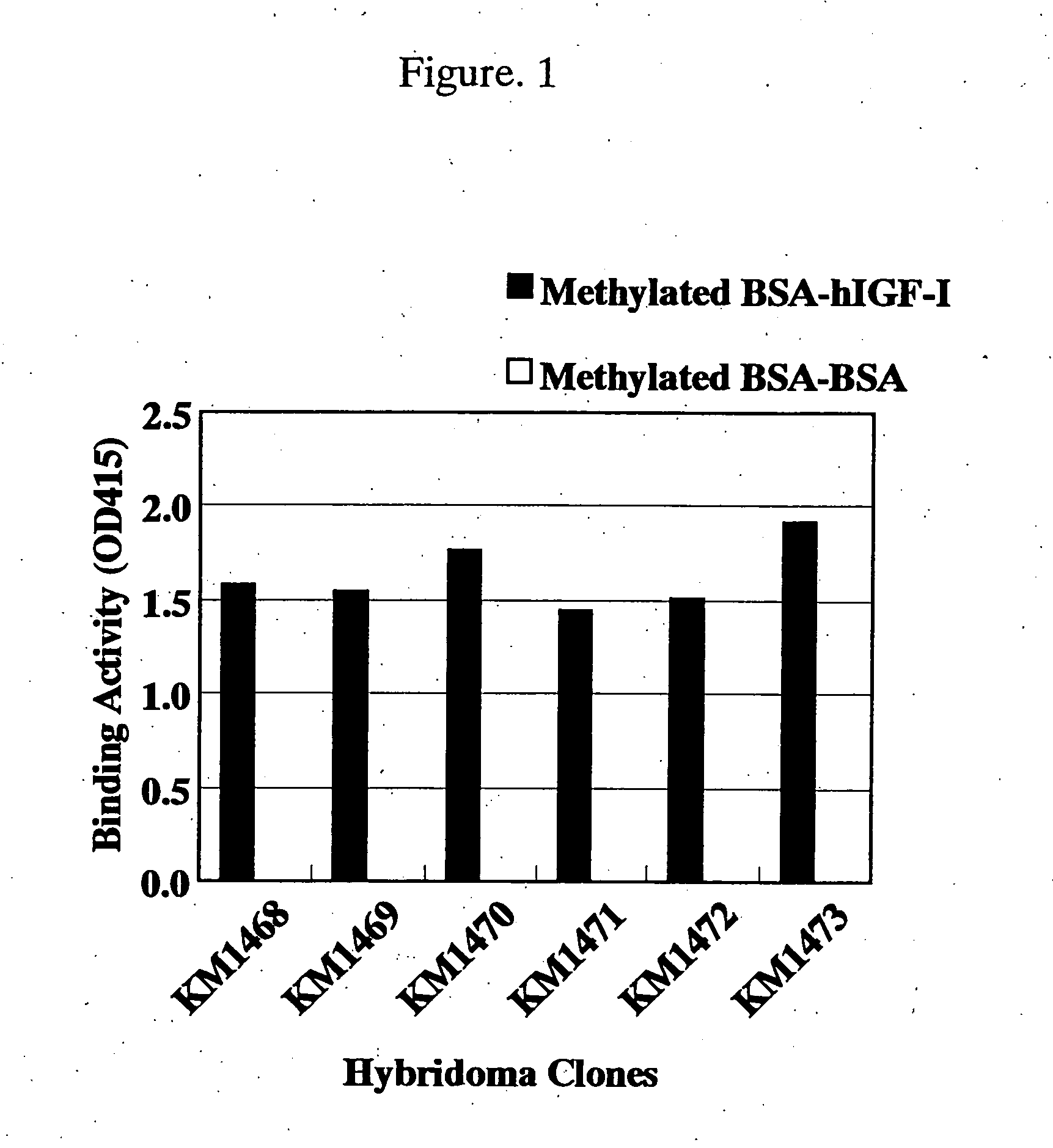
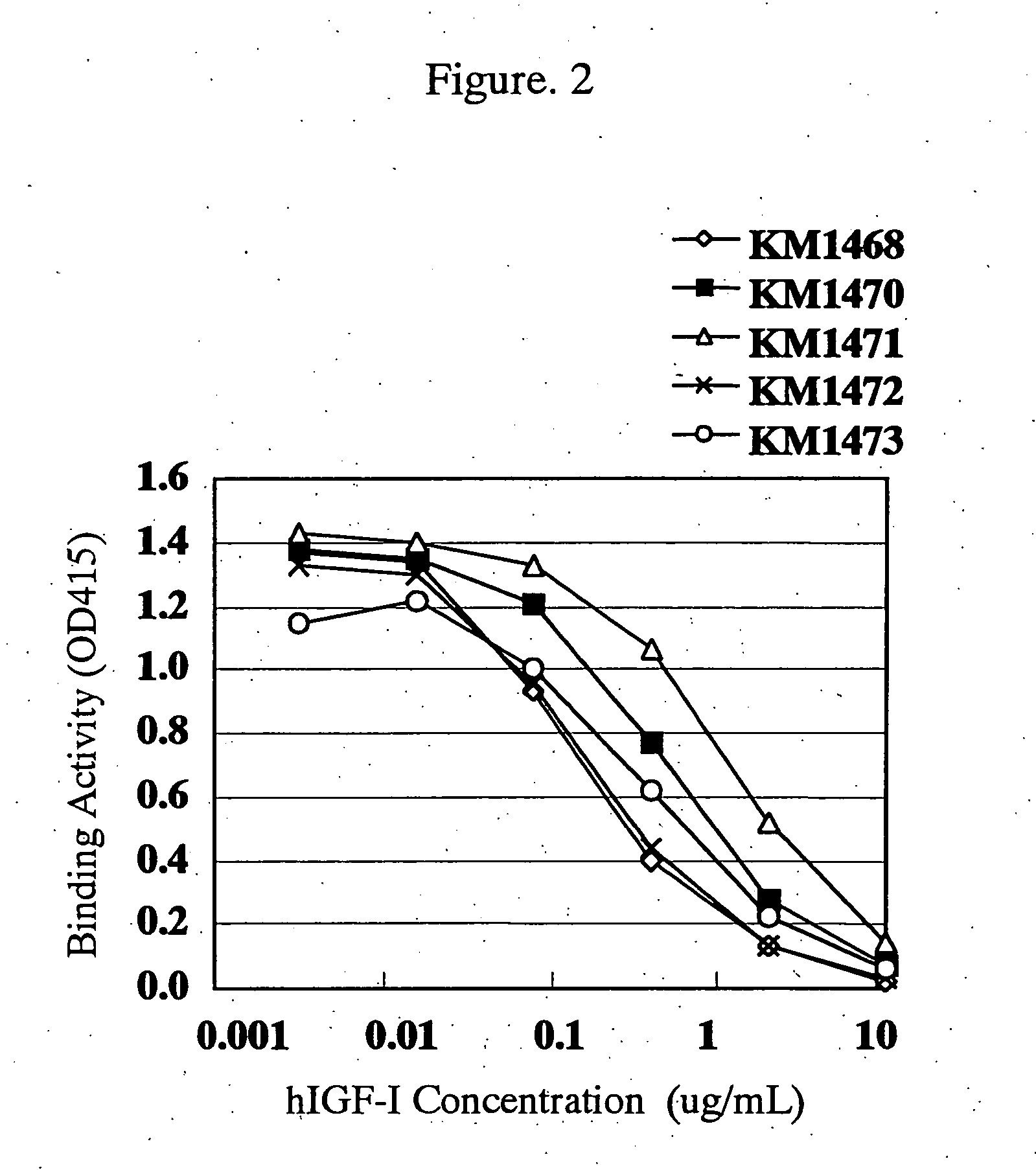
For the effective treatment of diseases such as cancer in which hIGF participates, there have been desired to be developed antibodies which strongly bind to both factors hIGF-I and hIGF-II and inhibit their functions and fragments of these antibodies. The present invention provides antibodies which have the ability to specifically bind to human IGF-I and IGF-II to thereby inhibit the functions of human IGF-I and IGF-II and have binding activity with a binding constant of 5×109 M−1 or more measured with a biosensor BIACORE. In addition, the present invention provides diagnostics, preventives and remedies for an hIGF-mediated disease and a disease showing pathological progressing due to abnormally promoted hIGF production, which use said antibodies.
Owner:KYOWA HAKKO KIRIN CO LTD
Antibody against human insulin-like growth factor
InactiveUS20060165695A1Improve stabilitySuppression problemBacteriaAntipyreticInsulin-like growth factorDisease
For the effective treatment of diseases such as cancer in which hIGF participates, there have been desired to be developed antibodies which strongly bind to both factors hIGF-I and hIGF-II and inhibit their functions and fragments of these antibodies. The present invention provides antibodies which have the ability to specifically bind to human IGF-I and IGF-II to thereby inhibit the functions of human IGF-I and IGF-II and have binding activity with a binding constant of 5×109 M−1 or more measured with a biosensor BIACORE. In addition, the present invention provides diagnostics, preventives and remedies for an hIGF-mediated disease and a disease showing pathological progressing due to abnormally promoted hIGF production, which use said antibodies.
Owner:KYOWA HAKKO KIRIN CO LTD
Rapid Mucosal Gel or Film Insulin Compositions
InactiveUS20080085298A1Improve stabilityRapid onsetPowder deliveryPeptide/protein ingredientsNasal cavityWhole body
Gel, powder, suspension, emulsions or film formulations for systemic delivery of insulin with improved stability and rapid onset of action are described herein. The formulations are preferably absorbed to a mucosal surface, most preferably via buccal or sublingual administration, although rectal, vaginal, nasal or ocular administration is possible. The formulations contain insulin in combination with a chelator and dissolution agent, and optionally additional excipients. In the preferred embodiment, the formulation contains human insulin, a zinc chelator such as EDTA and a dissolution agent such as citric acid. Following administration, these formulations are rapidly absorbed into the blood stream. The formulation is preferably a polymeric gel, powder or film which adheres to the mucosal surface, thereby enhancing uptake of the incorporated drug. In the preferred embodiment, this formulation is administered sublingually, most preferably before a meal or after a meal.
Owner:BIODEL
Insulin Derivative
InactiveUS20090239785A1Prolonged profile of actionNo bluntingPeptide/protein ingredientsMetabolism disorderDrugHuman insulin
The present invention relates to novel human insulin derivatives which are soluble at physiological pH values and have a prolonged profile of action. The invention also relates to methods of providing such derivatives, to pharmaceutical compositions containing them, to methods of treating diabetes and hyperglycaemia using the insulin derivatives of the invention and to the use of such insulin derivatives in the treatment of diabetes and hyperglycaemia.
Owner:NOVO NORDISK AS
Methods and compositions for increasing alpha-l-iduronidase activity in the CNS
Provided herein are methods and compositions for treating a subject suffering from a deficiency in α-L-Iduronidase in the CNS. The methods include systemic administration of a bifunctional fusion antibody comprising an antibody to a human insulin receptor and an α-L-Iduronidase. A therapeutically effective systemic dose is based on the specific CNS uptake characteristics of human insulin receptor antibody-α-L-Iduronidase fusion antibodies as described herein.
Owner:ARMAGEN INC +1
Autoantigen composition
ActiveUS20060183670A1Reduce incidenceHigh incidencePeptide/protein ingredientsMetabolism disorderAntigenDisease
The invention features methods for the prevention or treatment of autoimmune disorders in humans. The methods include administering an autoantigen in combination with an oil-based carrier. Included are methods for the prevention and treatment of diabetes mellitus which include treating a patient with a diabetes type 1 autoantigen, e.g., human insulin B-chain or GAD65, and an oil-based carrier approved for human use. Also included are vaccines and kits for the treatment of diabetes mellitus.
Owner:JOSLIN D ABETES CENTER INC
Methods for the production of insulin in plants
InactiveUS7547821B2Simple methodReadily apparentSugar derivativesOther foreign material introduction processesSingle-Chain AntibodiesPlanting seed
Commercial production of human insulin can be affected via transgenic expression in plant seeds. Thus, levels of insulin accumulation exceeding 0.1% of total cellular protein can be achieved recombinantly, through expression of the insulin with a single-chain antibody as a fusion partner. Production in seeds offers flexibility in storage and shipment of insulin as a raw material, and insulin retains its activity upon extraction from stored seed. Further, the amount of biomass subjected to extraction is limited, due to the relatively low water content of plant seeds.
Owner:SEMBIOSYS GENETICS INC
SFM (serum-free medium) for culturing MSCs (mesenchymal stem cells)
ActiveCN103555665AClear ingredientsAvoid heterogeneous contaminationSkeletal/connective tissue cellsSodium bicarbonateSerum free media
The invention relates to an SFM (serum-free medium) for culturing MSCs (mesenchymal stem cells). Based on volume, the SFM comprises the following components: 10.2 grams per liter of alpha-MEM (alpha-minimum essential medium), 2.4 grams per liter of sodium bicarbonate, 1 to 5 millimoles of L-glutamine, 50 to 300 milligrams per liter of poloxamer 188, 2 to 8 grams per liter of recombinant human albumin, 10 to 20 milligrams per liter of recombinant human transferrin, 2 to 10 milligrams per liter of recombinant human insulin, 1 to 5 millimoles per liter of Hepes, 50 nanomoles of beta-mercaptoethanol, 0.1 to 1 milligram per liter of lipid, 1 to 5 milligrams per liter of trace element, 0.1 to 5 milligrams per liter of glutathione, 0.5 to 5 milligrams per liter of para-aminobenzoic acid, 1 to 50 nanograms per milliliter of hydrocortisone, 20 to 50 milligrams per liter of vitamin PP, 5 to 50 milligrams per liter of vitamin C, 2 to 10mu M of compound shown in a formula I, 5 to 20mu M of compound shown in a formula II, 10 to 20 nanograms per milliliter of progestin, 1 to 10 milligrams per liter of putrescine, 1 to 10 international units per liter of heparin, 1 to 10 nanograms per milliliter of EGF (epidermal growth factor), 1 to 10 nanograms per milliliter of b-FGF (b-fibroblast growth factor), 1 to 10 nanograms per milliliter of HGF (hepatocyte growth factor) and 1 to 10 nanograms per milliliter of VEGF (vascular endothelial growth factor). The SFM for culturing the MSCs is a BPS-SFM which has determinate chemical components and is free of animal-derived substances.
Owner:BEIJING DONGFANG HUAHUI BIOMEDICAL TECH
Treatment of diabetes with synthetic beta cells
InactiveUS6933133B2Reduce formationReduce expressionBiocidePeptide/protein ingredientsGlucose polymersActrapid insulin
Disclosed is a method for obtaining glucose-regulated expression of active insulin in the cells of a mammalian subject. The method involves delivering into the subject a genetic construct comprising a coding sequence for a human proinsulin operably connected a promoter functional in the host cells. The construct includes a glucose responsive regulatory module having at least one glucose inducible regulatory element comprising a pair of CACGTG motifs linked by a five base nucleotide sequence, which confers glucose inducible expression of the proinsulin coding sequence. To ensure proper processing of the proinsulin to active insulin, the coding sequence was modified to direct the synthesis of a mutant proinsulin polypeptide having amino acid sequences that can be cleaved to mature insulin in suitable host cells, such as hepatocytes.
Owner:WISCONSIN ALUMNI RES FOUND
Transgenic tilapia comprising a humanized insulin gene
InactiveUS6476290B1Stable integrationDevelopmental stability and uniformityNew breed animal cellsMammal material medical ingredientsTilapiaIslet cells
In accordance with the present invention, there are provided humanized fish insulin genes. Humanized insulin the present invention encode human insulin alpha and / or beta chains while using fish-preferred codons and regulatory sequences. These humanized genes are thus expressible in fish islet cells. Also provided are transgenic fish having islet cells containing and capable of expressing humanized insulin genes. These islet cells (Brockmann Bodies) can be xenotransplanted into subjects having diabetes. In this manner normoglycemia can be achieved in the recipient of the islets.
Owner:DALHOUSIE UNIV
Serum-free complete medium for mesenchymal stem cell
InactiveCN102634482AAvoid stabilityAvoid cytotoxicitySkeletal/connective tissue cellsCholesterolCytotoxicity
The invention discloses a serum-free complete medium for mesenchymal stem cell, consisting of a basal medium and an additive component, wherein the basal medium is DMEM / F12 (dulbecco modified eagle medium), and the additive component consists of recombinant human insulin, human serum albumin, human transferrin, cholesterol, sodium selenite, ferric nitrate and glucan. The complete medium is free from serum, so that the defects that the serum-containing medium is unstable in serum batches, high in cell toxicity, abundant in heterologous protein and the like can be greatly avoided, and a good tool can be provided for the basic research and the clinic treatment of the mesenchymal stem cell; and the invention further achieves the effect which is approximate or equal to the serum-containing complete medium and the other serum-free complete mediums in the aspect of cell proliferation, so that the long-term subcultring of the cell can be supported.
Owner:四川全组生命科技有限公司
Methods and Compositions for Increasing Arylsulfatase A Activity in the CNS
Provided herein are methods and compositions for treating a subject suffering from a deficiency in arylsulfatase A in the CNS. The methods include systemic administration of a bifunctional fusion antibody comprising an antibody to a human insulin receptor and an arylsulfatase A.
Owner:JCR PHARMA +1
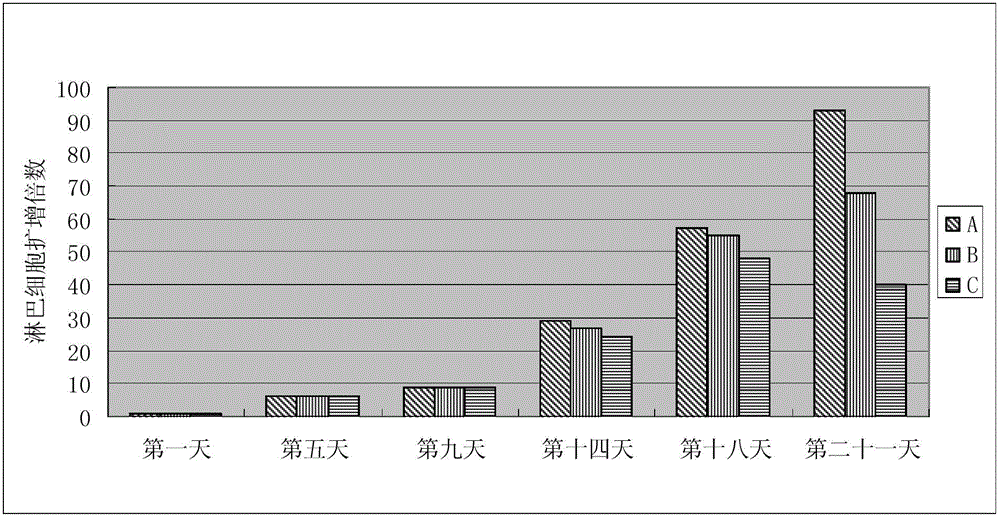
Animal source-free and serum-free culture medium of lymphocyte
InactiveCN103146648AGood amplification factorEnhance cell viabilityBlood/immune system cellsCell phenotypeSodium bicarbonate
The invention relates to the biological field and discloses an animal source-free and serum-free culture medium of lymphocyte. The culture medium disclosed by the invention essentially consists of IMDM (Iscove Modified Dulbecco Medium), L-glutamine, sodium bicarbonate, recombinant human insulin, human transferrin, human serum albumin, 2-mercaptoethanol, N-acetyl-cysteine, lipid, amino acid, vitamin, microelement, ferric citrate, hydrocortisone, cholamine and non-essential amino acid. The serum-free culture medium disclosed by the invention has the advantages of clear chemical components, no animal source, no serum, safe and ideal culture cells; the instability caused by the doping of animal components and batches is avoided; the result of culturing lymphocyte shows that the total number of the cells and the cell phenotypes are normal; and the serum-free culture medium disclosed by the invention has a good industrial application prospect.
Owner:BEIJING JING MENG STEM CELL TECH
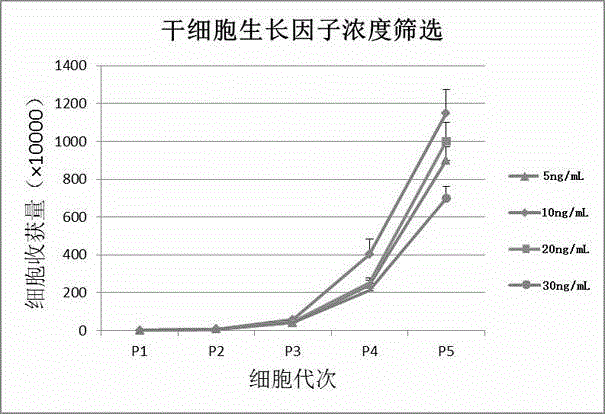
Serum-free adipose-derived stem cell culture medium and preparation method thereof
InactiveCN104877962AStem cells are in good shapeGood cell stemnessSkeletal/connective tissue cellsPhenolic content in teaCell culture media
The invention provides a serum-free adipose-derived stem cell culture medium, belonging to the technical field of stem cells. The serum-free adipose-derived stem cell culture medium comprises an IMDM (Iscove's modified Dulbecco's medium) basal culture medium, recombinant human insulin, recombinant transferrin, vitamin C, human serum albumin, fibroblast growth factor, platelet-derived growth factor, epidermal growth factor, insuline-like growth factor, transforming growth factor, fiber connexin, fetuin, tea polyphenol and stem cell growth factor. The serum-free adipose-derived stem cell culture medium provided by the invention does not need to perform culture bottle coating, and has the advantages of cell adherent property, favorable morphology and high cell proliferation rate.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com