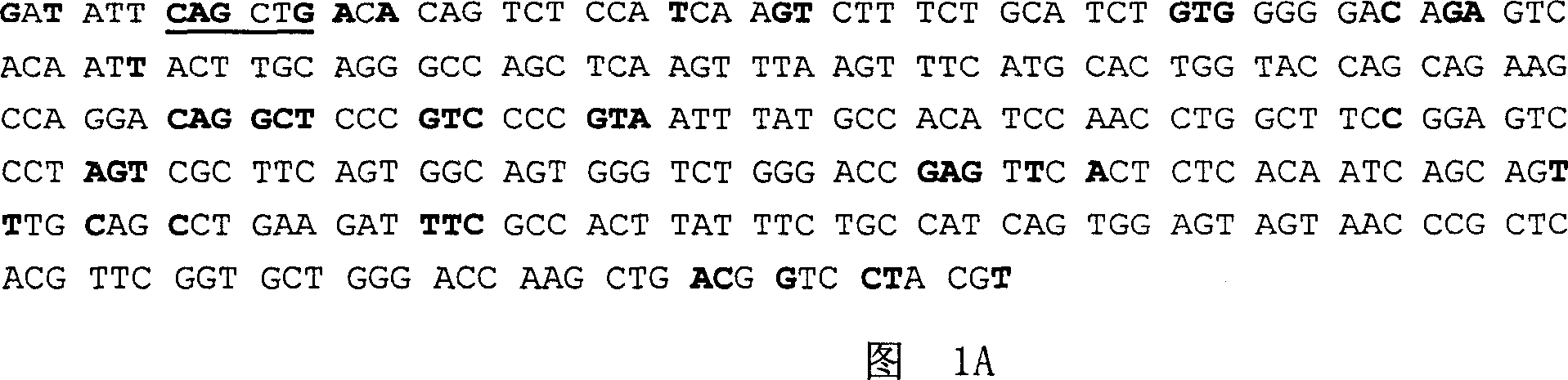
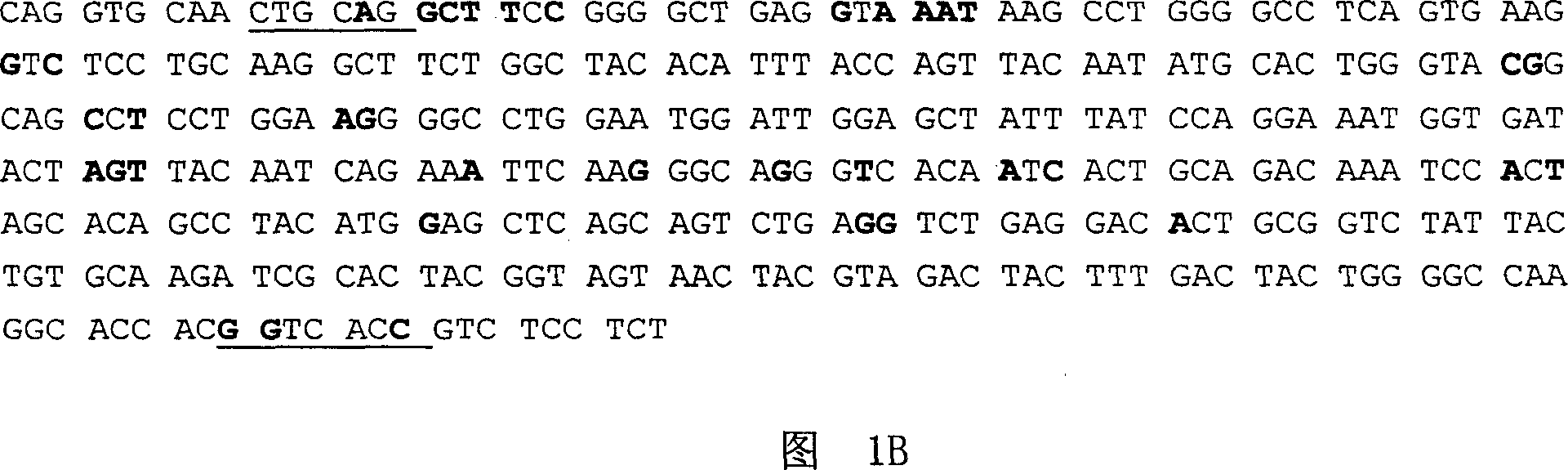
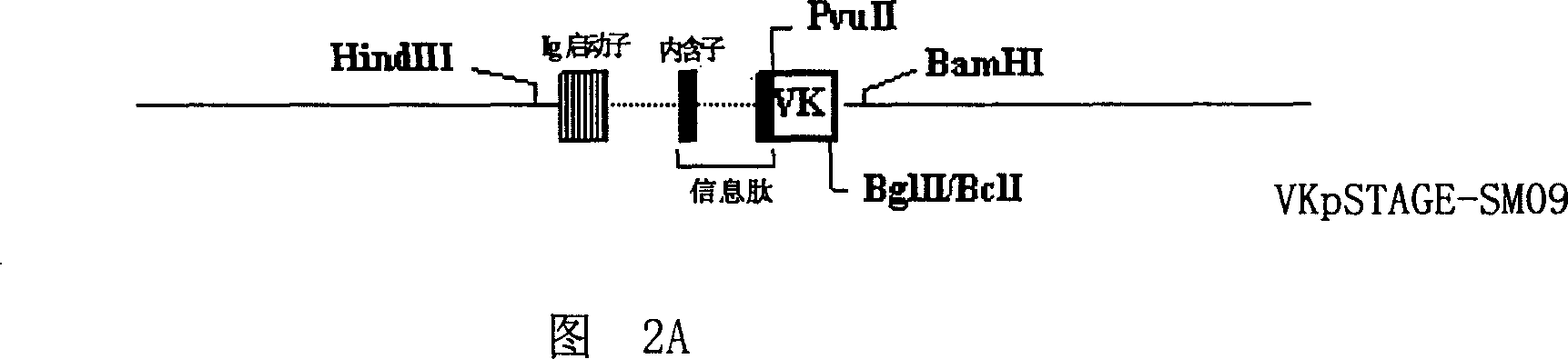
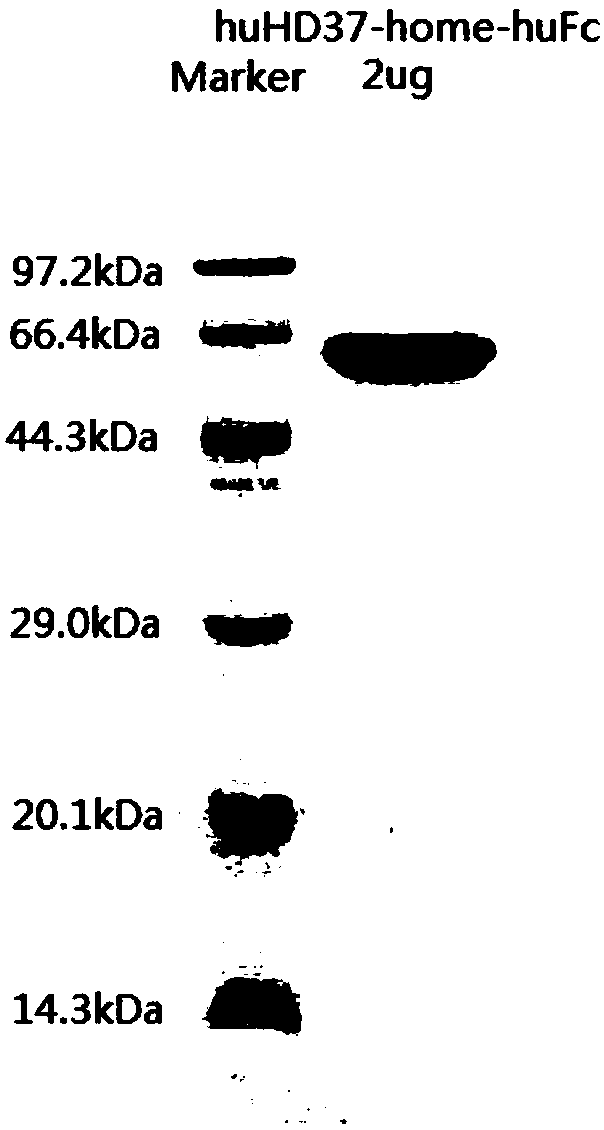
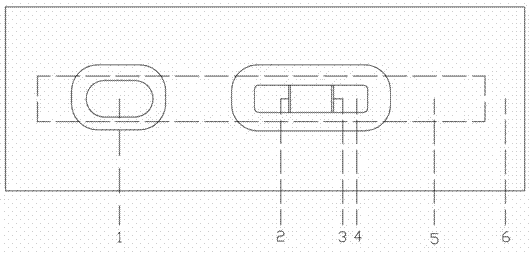
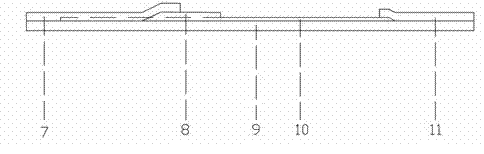
Patents
Literature
289 results about "Murine antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Definition of murine antibodies. Antibodies, also called immunoglobulins, make up the main part of the specific endogenous defence. They are made by the immune system in response to substances which are foreign to the organism (antigens = antibody generating).
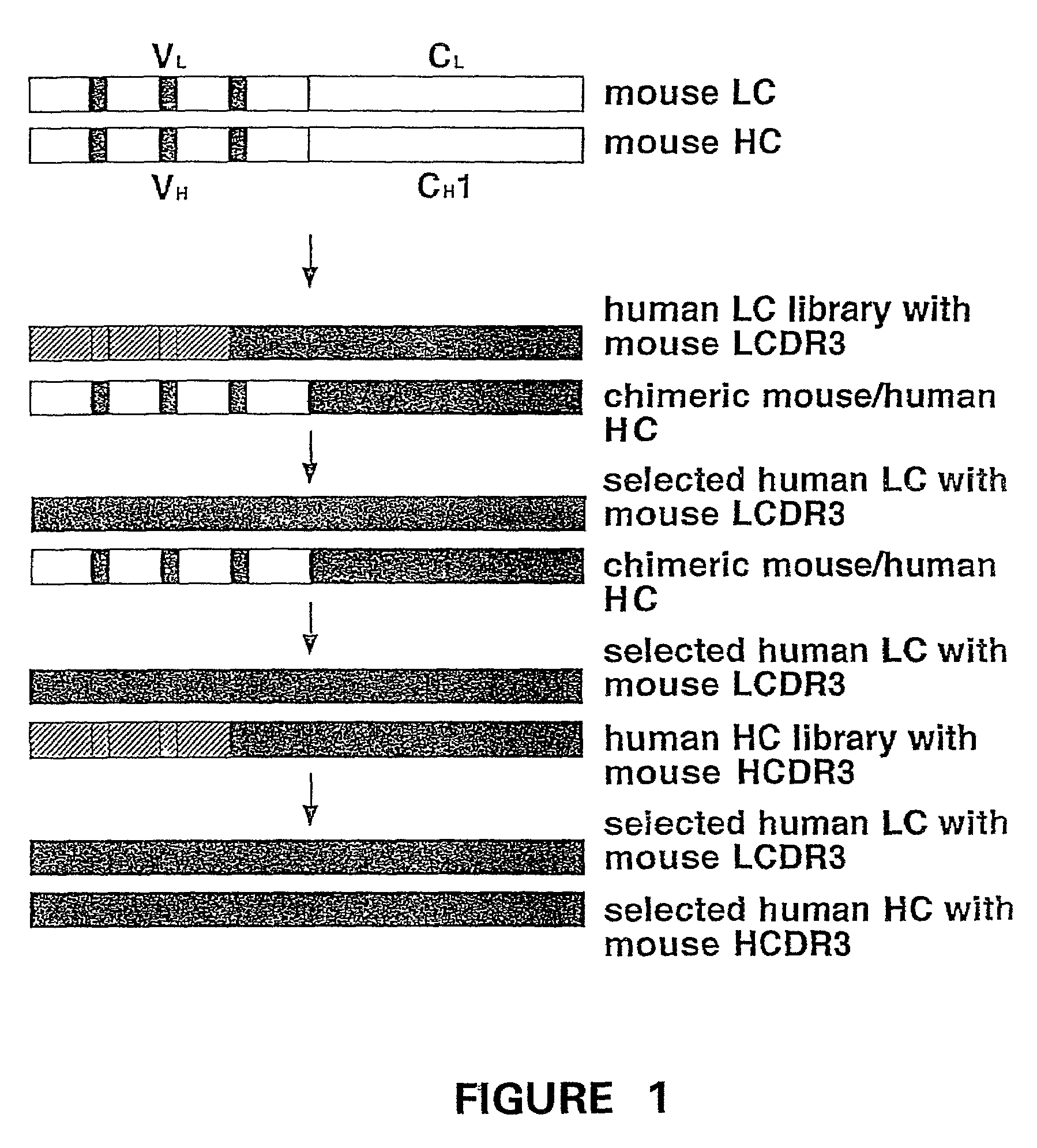
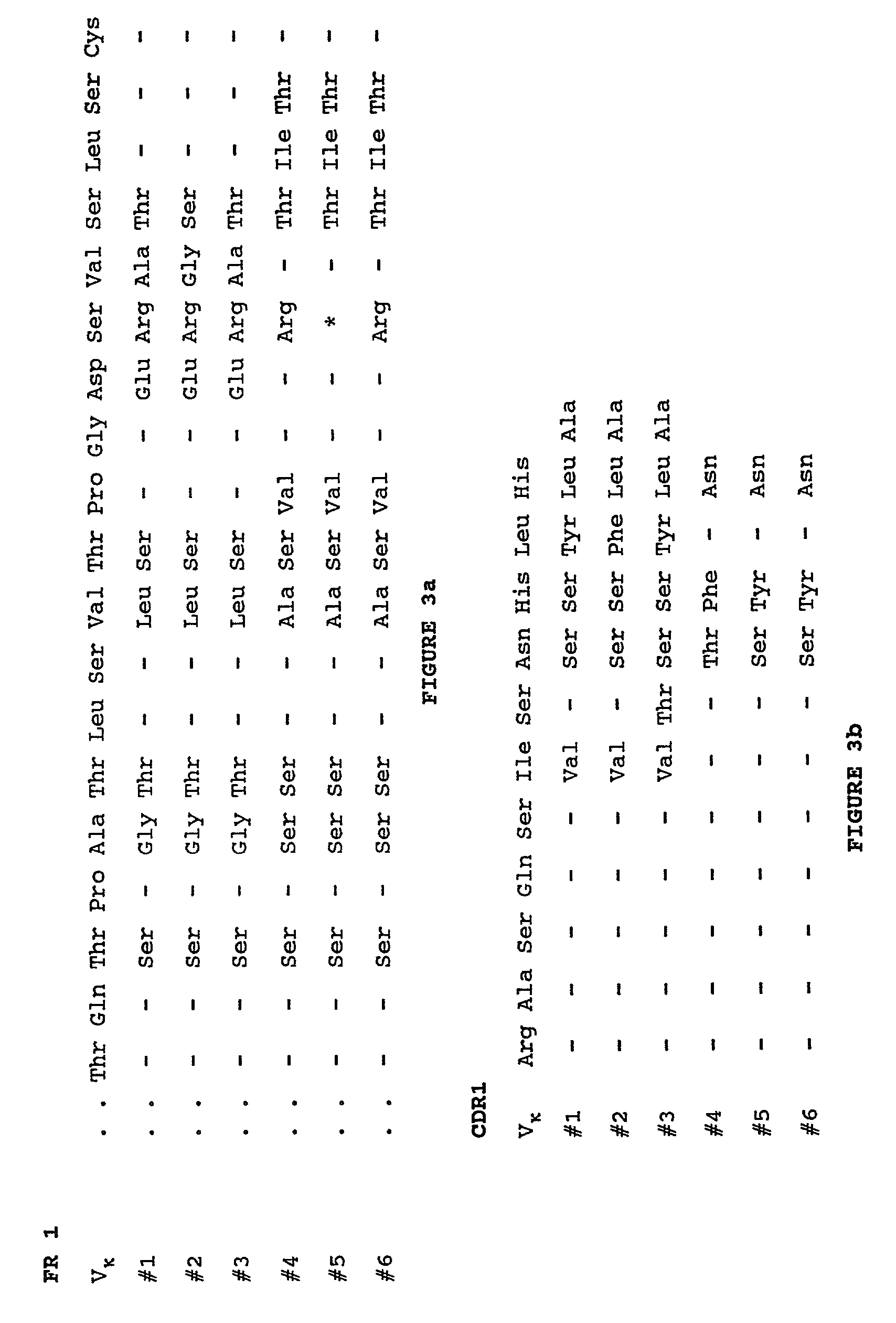
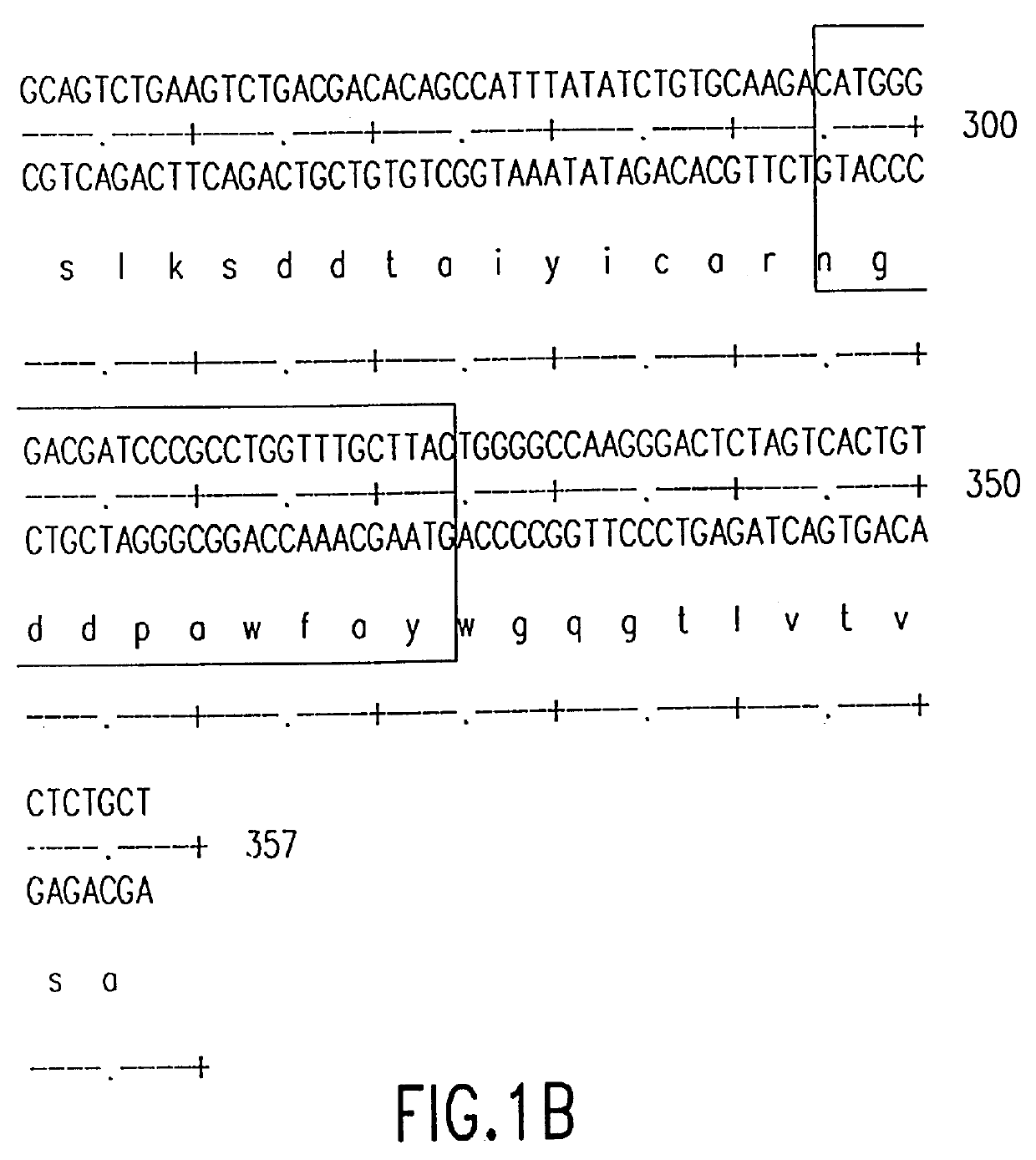
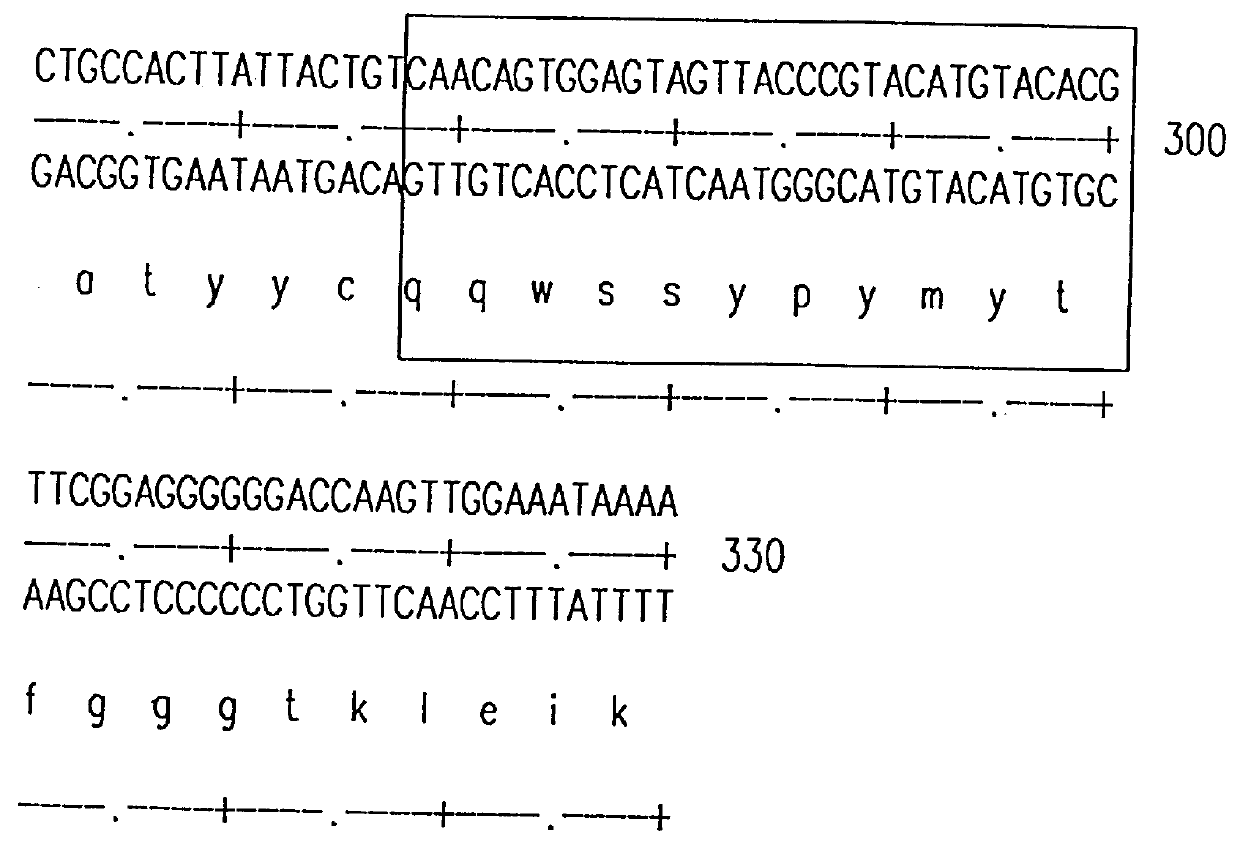
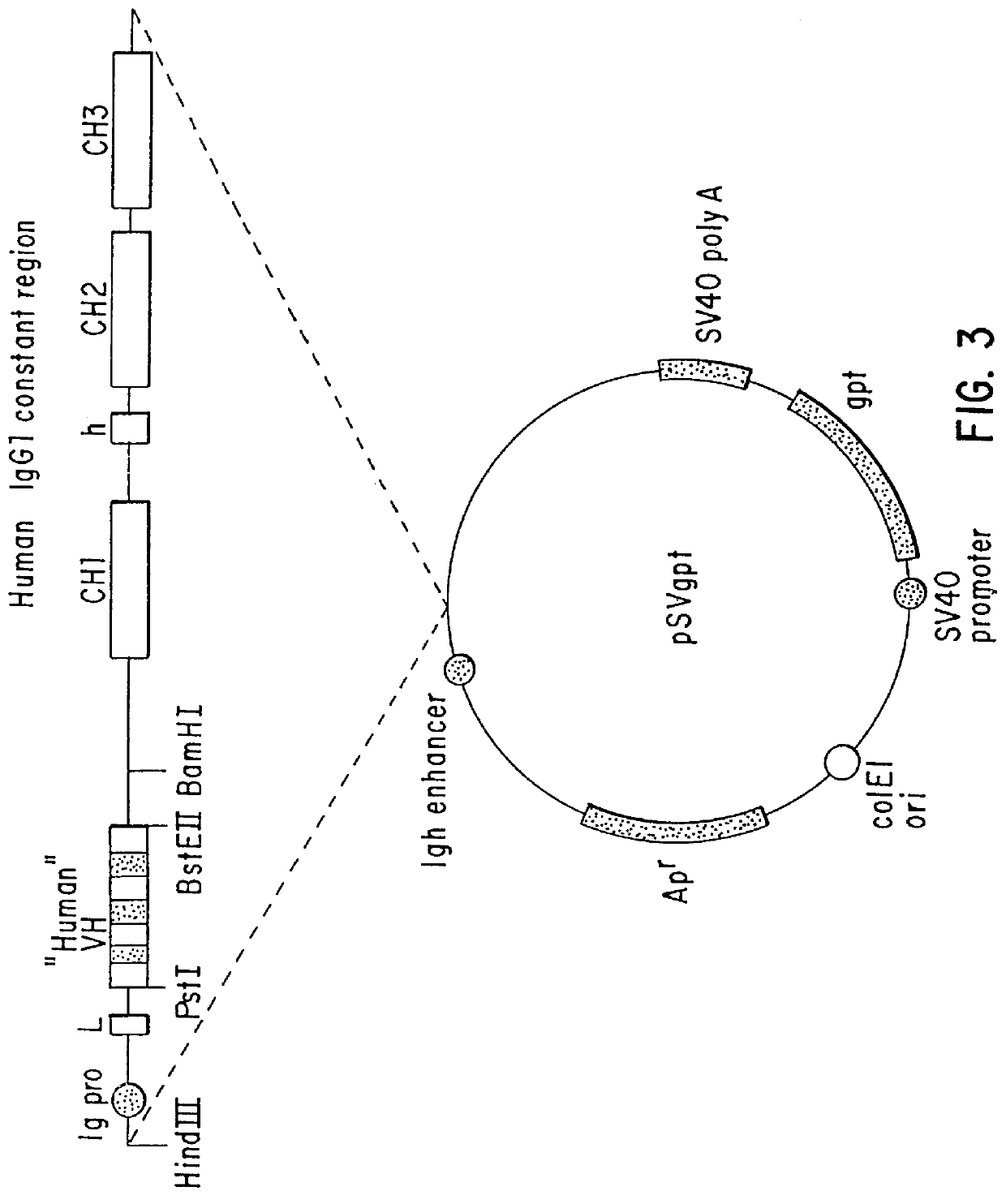
Humanization of murine antibody
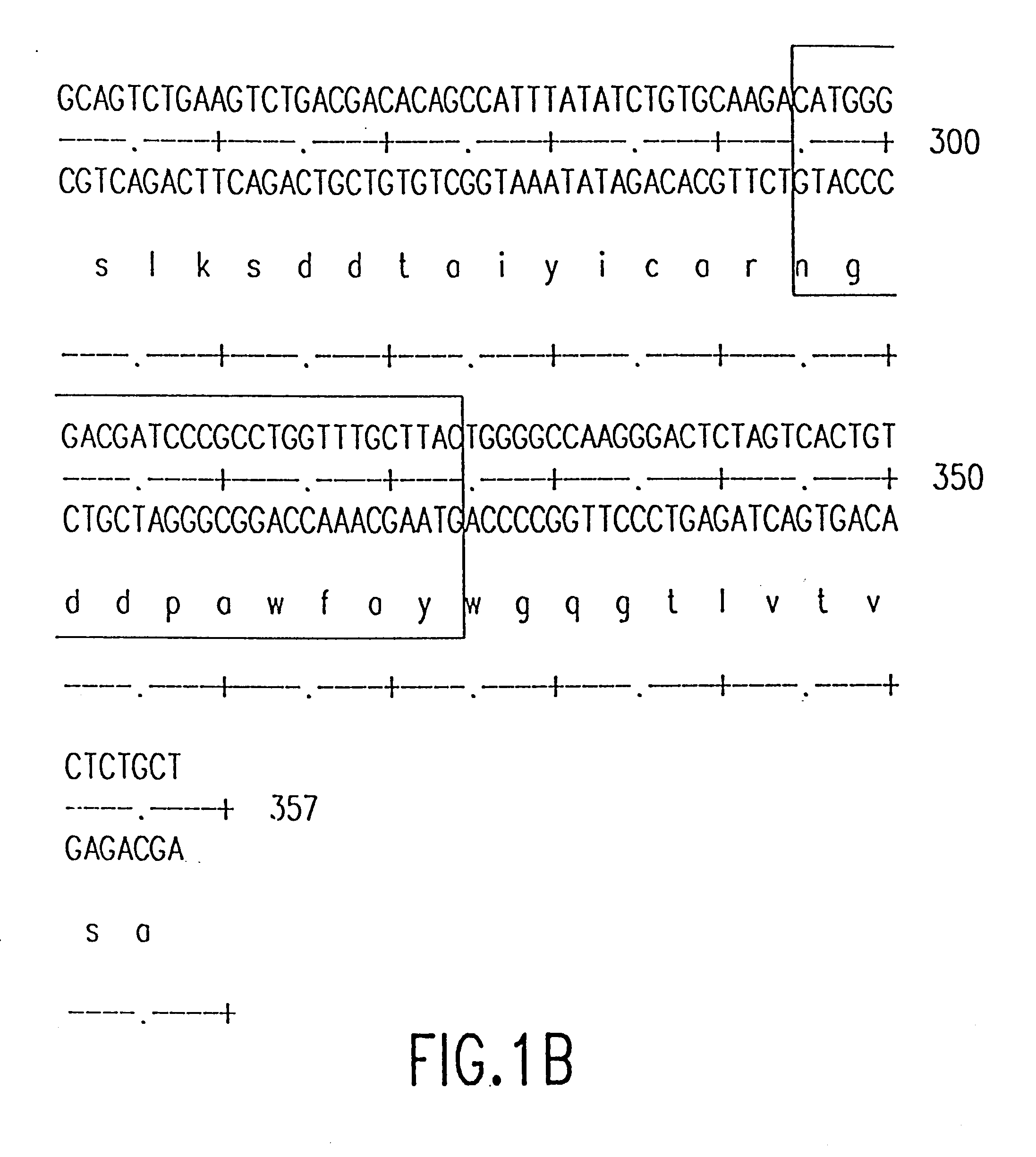
InactiveUS7087409B2Hybrid immunoglobulinsSugar derivativesComplementarity determining regionHeavy chain
A humanized murine antibody is provided. The amino acid sequences of a light chain complementarity determining region from a mouse antibody are grafted onto a human light chain, and a heavy chain complementarity determining region from a mouse antibody are grafted onto a human antibody heavy chain to produce libraries from which a humanized murine antibody having the desired specificity is selected.
Owner:THE SCRIPPS RES INST
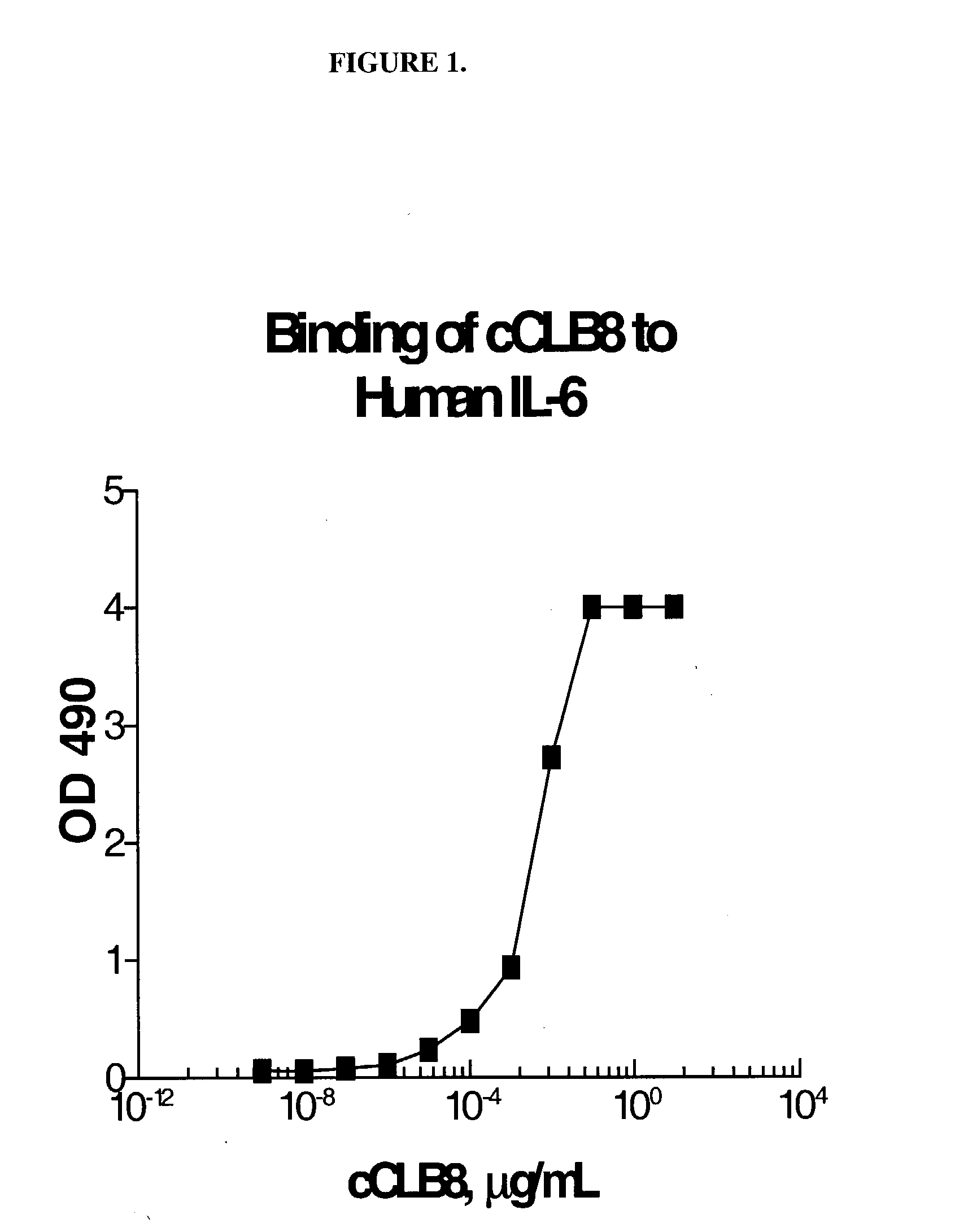
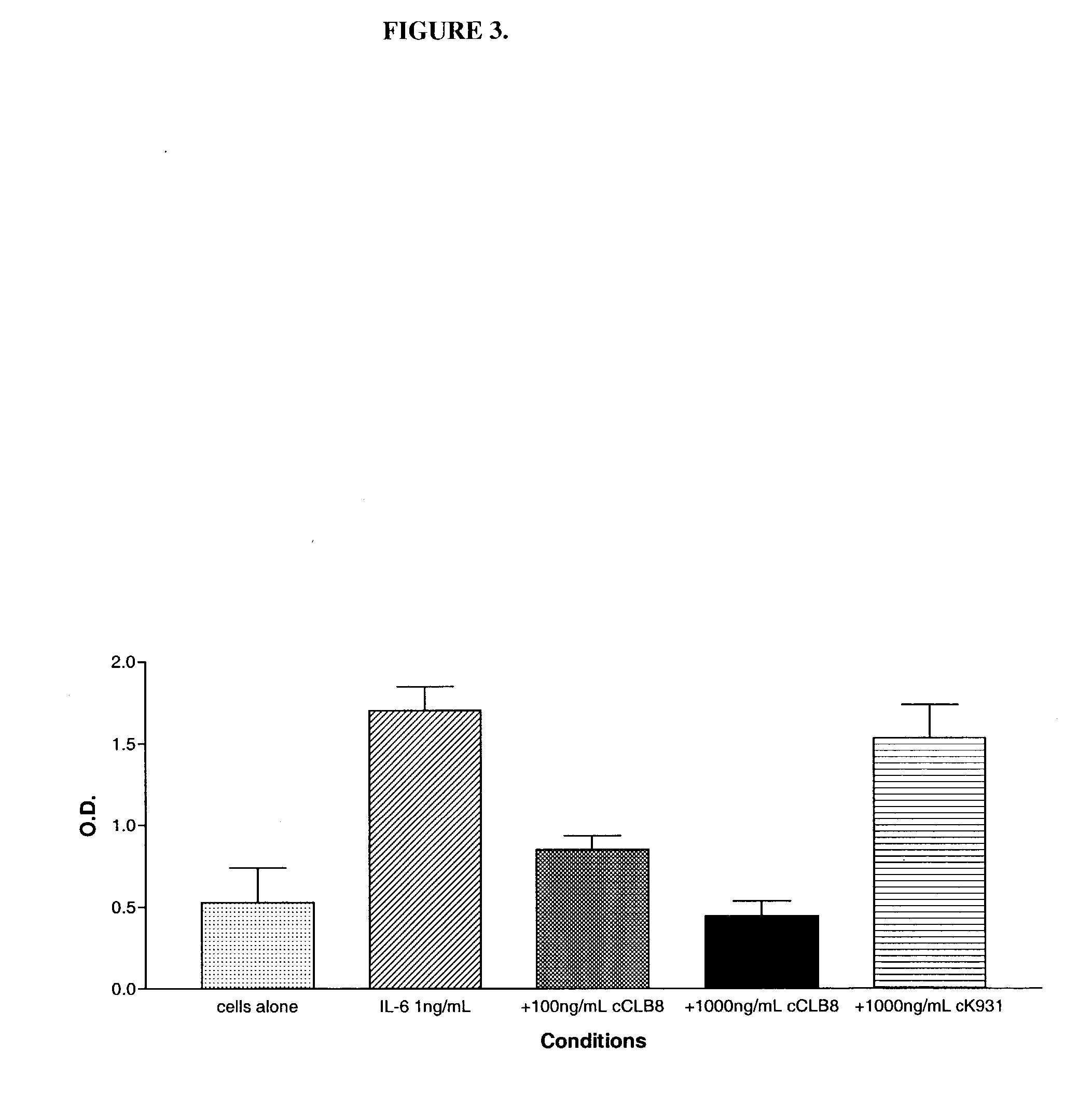
Anti-IL-6 antibodies, compositions, methods and uses
The present invention relates to at least one novel chimeric, humanized or CDR-grafted anti-IL-6 antibodies derived from the murine CLB-8 antibody, including isolated nucleic acids that encode at least one such anti-IL-6 antibody, vectors, host cells, transgenic animals or plants, and methods of making and using thereof, including therapeutic compositions, methods and devices.
Owner:CENTOCOR
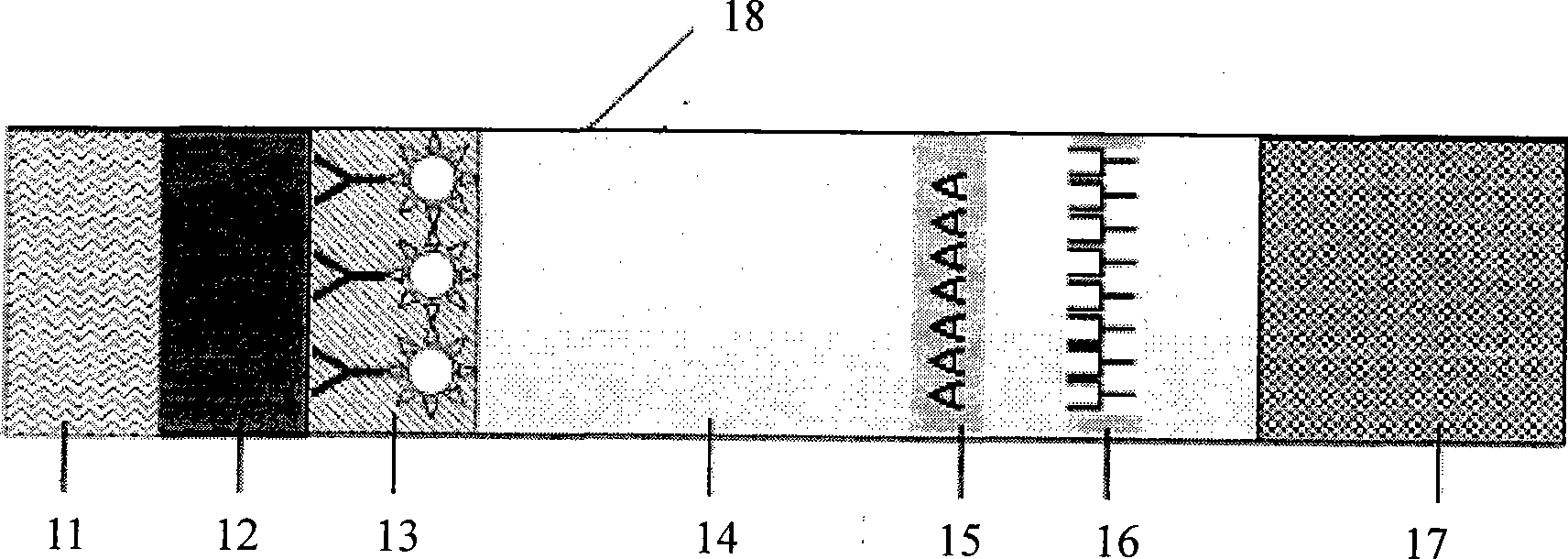
Fluorescent micro-ball immune chromatography test paper strip for detecting residual animal medicine and preparing method thereof
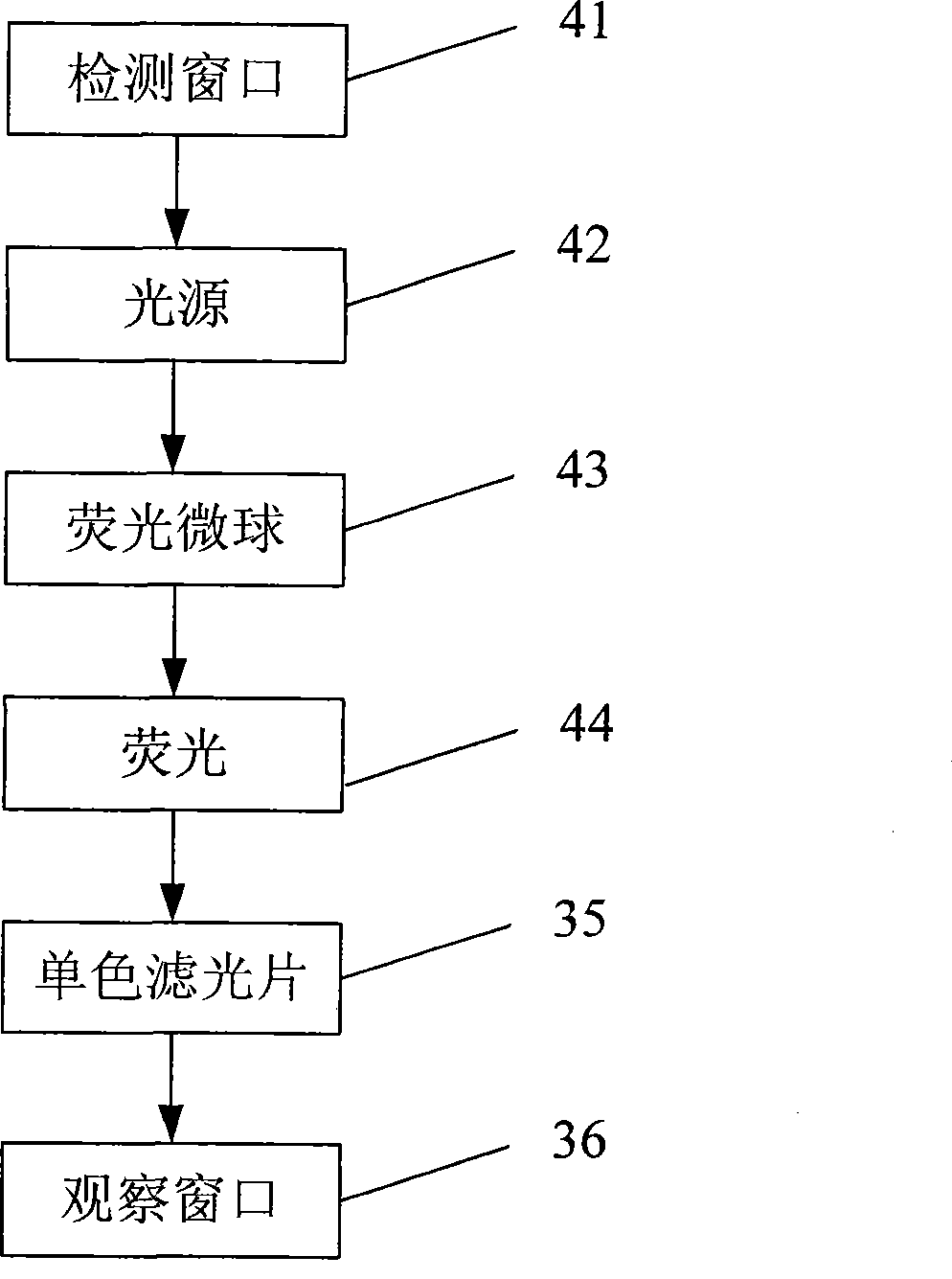
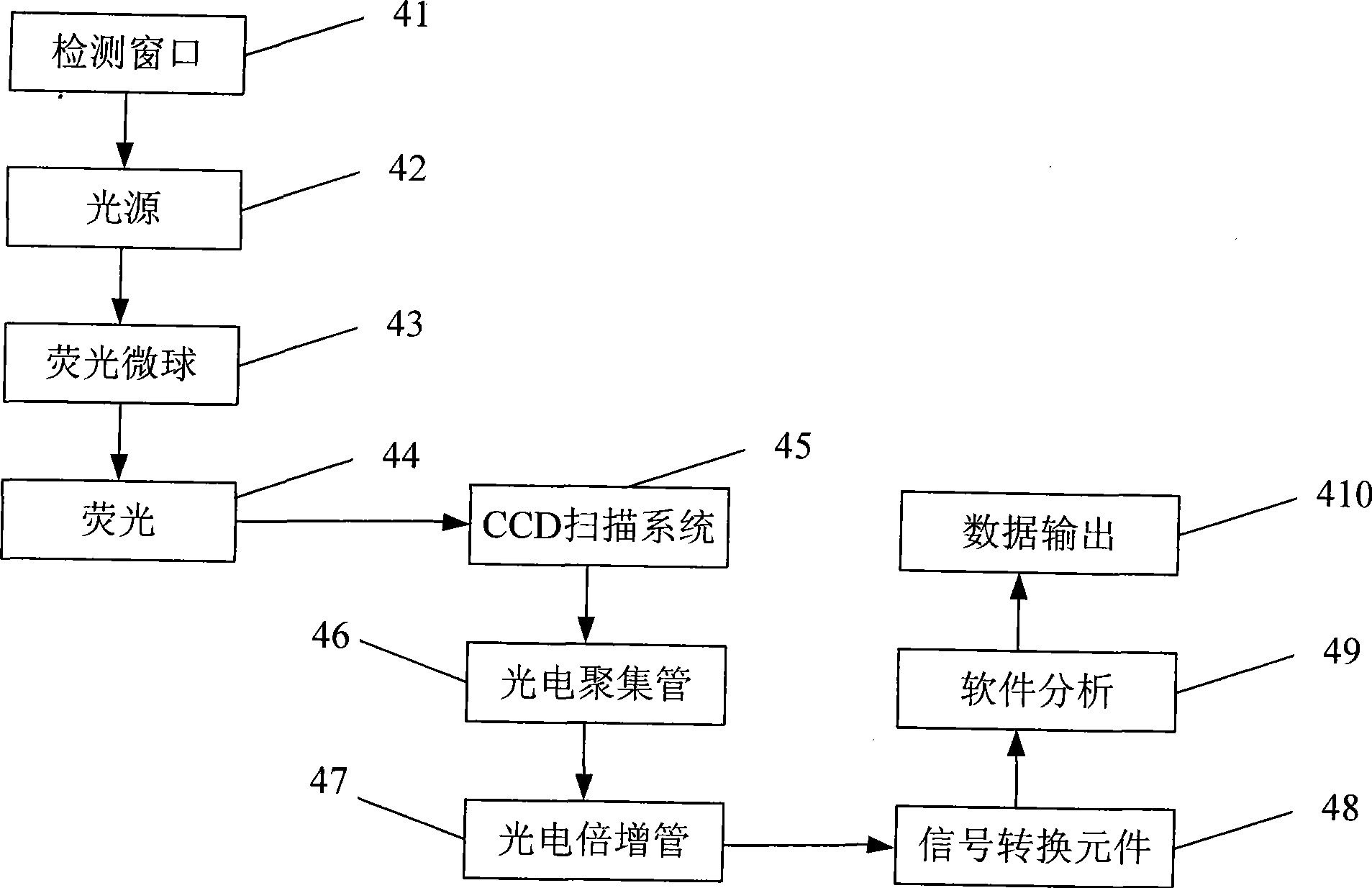
The invention discloses a test paper strip for the fluorescent bead immunochromatography of veterinary medicine remained in a detected sample and a preparation method thereof. A filter paper, a sample pad, a fiberglass membrane, a pyroxylin membrane and water sucking paper are sequentially stuck on a base plate in related joint; fluorescent beads are sprayed on the pyroxylin membrane for marking a veterinary medicine resistant molecule monoantibody; the pyroxylin membrane coated with a veterinary medicine molecule holoantigen is used as a detection region; the pyroxylin membrane coated with an anti-mouse antibody is used as a quality control region; and the test paper strip is prepared through the following steps: (1) the preparation of the pyroxylin membrane; (2) the preparation of a fluorescent bead pad; and (3) the assembling of the test paper strip. In the detecting process, emitted spectrums pass through a proper optical filter device; and all the emitted spectrums are collected through CCD scanning technology, are congregated, are multiplied and are analyzed through a fluorescence analyzer and relevant software to obtain a quantized fluorescence signal, thereby realizing quantitative detection. The test paper strip is mainly used for the qualitative detection and the quantitative detection of all the veterinary medicine classes in food safety detection.
Owner:WUXI ZODOLABS BIOTECH
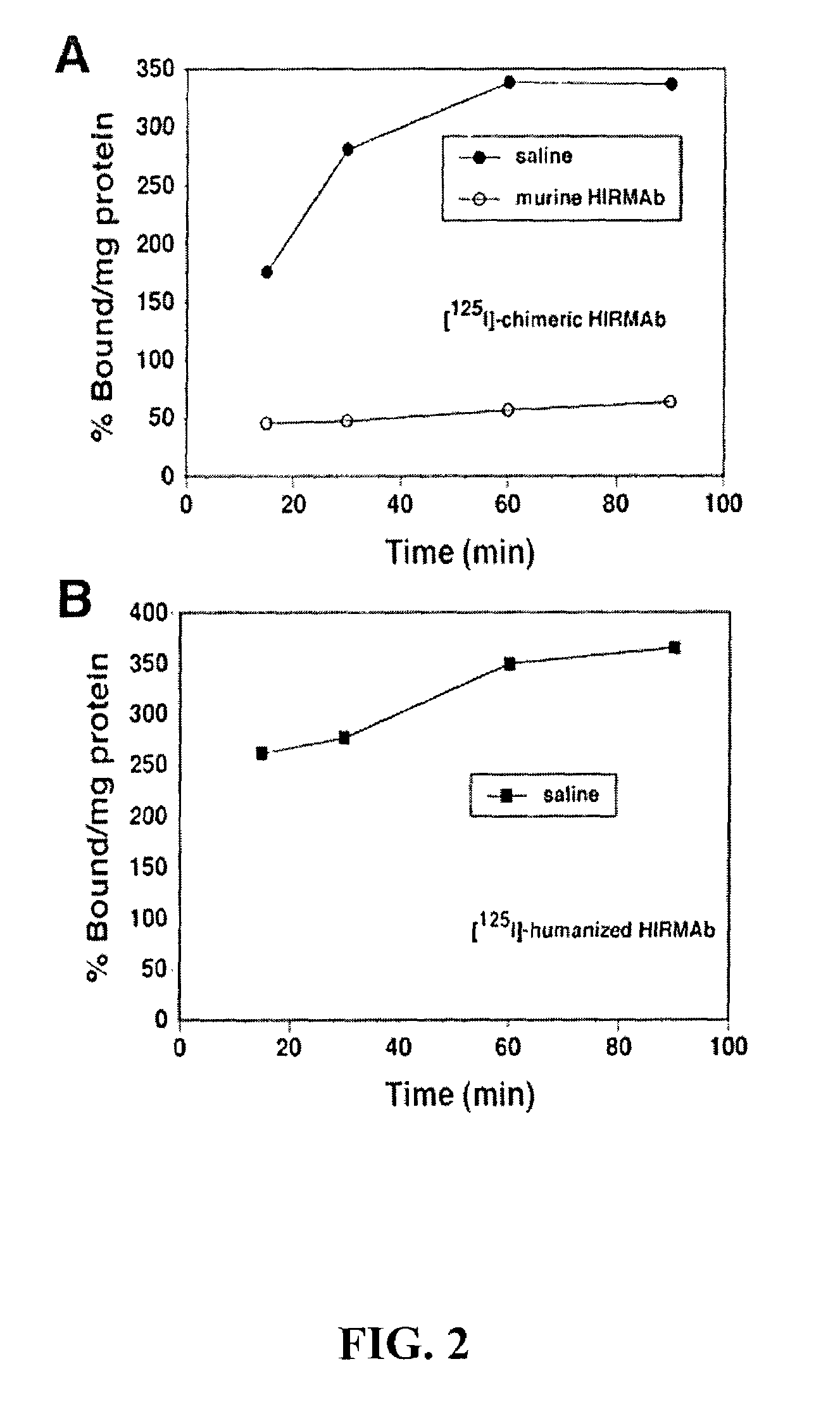
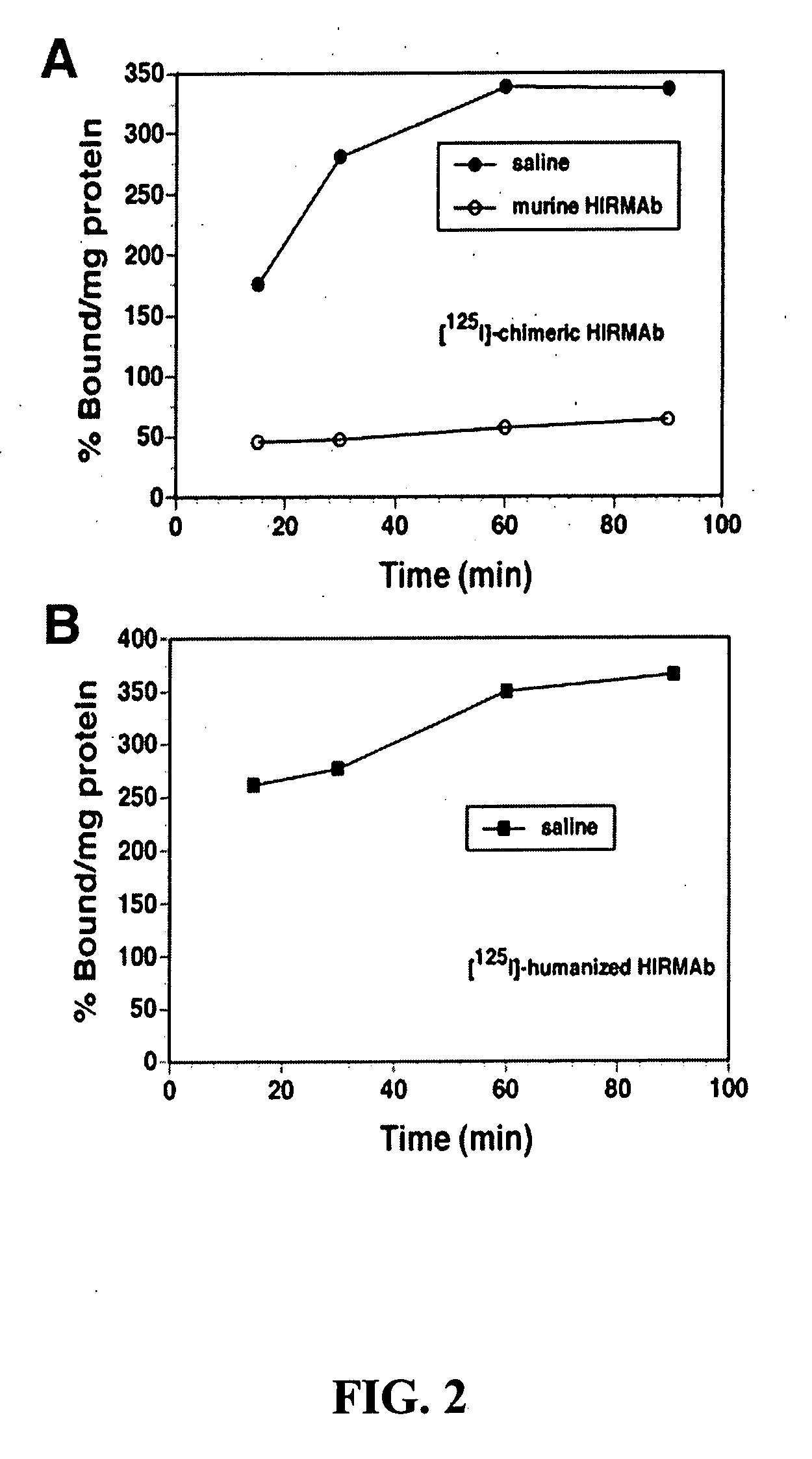
Delivery of pharmaceutical agents via the human insulin receptor
ActiveUS7388079B2Reduces immunogenic reactionNervous disorderImmunoglobulins against cell receptors/antigens/surface-determinantsMurine antibodyInsulin receptor
A humanized murine antibody is provided that binds to the human insulin receptor (HIR). The humanized murine antibody is suitable for use as a Trojan horse to deliver pharmaceutical agents to human organs and tissue that express the HIR. The humanized munne antibody is especially well suited for delivering neuropharmaceutical agents from the blood stream to the brain across the blood brain barrier (BBB). The humanized murine antibody may be genetically fused to the pharmaceutical agent or it may be linked to the pharmaceutical agent using an avidin-biotin conjugation system.
Owner:RGT UNIV OF CALIFORNIA
Novel antibody molecule aiming at human CLDN18.2, antigen binding fragment and medical application thereof
ActiveCN110606891AHigh binding activityInanimate material medical ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsComplement-dependent cytotoxicityAntigen Binding Fragment
The invention discloses an anti-human CLDN18.2 antibody as well as an antigen binding fragment and a medical application thereof. Specifically, the present invention relates to a murine antibody comprising a CDR region of the anti-human CLDN18.2 antibody, a chimeric antibody and a humanized antibody, a fully humanized antibody, an antibody-dependent cytotoxicity (ADCC) comprising the antibody andthe antigen binding fragment thereof, complement dependent cytotoxicity (CDC), the killing effect on CLDN18.2 positive cells, the effect of inhibiting the growth of CLDN18.2 positive tumors, the in-vivo drug effect, a medicine containing the anti-human CLDN18.2 antibody and the antigen binding fragment thereof, a composition of the medicine thereof, and an application of the medicine in tumor treatment, in particular to treatment of CLDN18.2 positive tumor patients..
Owner:L&L BIOPHARMA CO LTD
Delivery of pharmaceutical agents via the human insulin receptor
InactiveUS20080051564A1Reduces immunogenic reactionNervous disorderImmunoglobulins against cell receptors/antigens/surface-determinantsMurine antibodyInsulin receptor
A humanized murine antibody is provided that binds to the human insulin receptor (HIR). The humanized murine antibody is suitable for use as a Trojan horse to deliver pharmaceutical agents to human organs and tissue that express the HIR. The humanized murine antibody is especially well suited for delivering neuropharmaceutical agents from the blood stream to the brain across the blood brain barrier (BBB). The humanized murine antibody may be genetically fused to the pharmaceutical agent or it may be linked to the pharmaceutical agent using an avidin-biotin conjugation system.
Owner:RGT UNIV OF CALIFORNIA
Method for detecting cancers
The invention provides for the production of several humanized murine antibodies specific for the antigen LK26, which is recognized by the murine antibody LK26. This antigen is expressed in all choriocarcinoma, teratocarcinoma and renal cancer cell lines whereas it is not expressed on cell lines of leukaemias, lymphomas, neuroectodermally-derived and epithelial tumour cell lines (excepting a small subset of epithelial cell lines). Furthermore, whereas renal cancer cell lines express the LK26 antigen, normal renal epithelial cells do not. Similarly, with the exception of the trophoblast, all normal adult and fetal tissues tested are negative for the LK26 phenotype. The invention also provides for numerous polynucleotide encoding humanized LK26 specific antibodies, expression vectors for producing humanized LK26 specific antibodies, and host cells for the recombinant production of the humanized antibodies. The invention also provides methods for detecting cancerous cells (in vitro and in vivo) using humanized LK26 specific antibodies. Additionally, the invention provides methods of treating cancer using LK26 specific antibodies.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Test paper for rapidly detecting immunochromatography of cadmium ion colloidal gold and preparation method and application thereof
The invention discloses test paper for rapidly detecting the immunochromatography of cadmium ion colloidal gold and a preparation method and application thereof. The test paper consists of a sample pad, a colloidal gold combination pad, a nitrocellulose membrane, absorbent paper and a plastic base plate; The sample pad, the colloidal gold combination pad, the nitrocellulose membrane and the absorbent paper are sequentially stuck on the plastic base plate; an anti-cadmium ion monoclonal colloidal gold label is coated on the colloidal gold combination pad; a goat anti-rat IgG antibody and a complete antigen Cd-iEDTA-BSA of heavy metal cadmium are sequentially coated on the nitrocellulose membrane; the goat anti-rat IgG antibody is used as a quality control line; and the complete antigen Cd-iEDTA-BSA of the heavy metal cadmium is used as a test line. The test paper detects residual metal cadmium in ambient soil, a water body and an aquatic product based on the immunology theory of antigen antibody; and compared with the prior detection system, the test paper has the advantages of rapidness, cheapness, convenience, sensitivity and speciality, can give a result within five minutes and is convenient to carry along to carry out field monitoring.
Owner:JINAN UNIVERSITY
Clenobuterol hydrochloride, salbutamol and paylean three joint inspection card and method for processing detecting sample
The invention relates to a clenobuterol hydrochloride, salbutamol and paylean three joint inspection card and a method for processing a detecting sample, and belongs to the technical field of detection of a beta-receptor stimulating agent, wherein a test strip is arranged inside a shell of the three joint inspection card, and the clenobuterol hydrochloride, salbutamol and paylean three joint inspection card is formed by the adhesion of a sample gasket, a colloidal gold membrane, a cellulose nitrate membrane and a water absorbing membrane to a bearing backboard in turn; the colloidal gold membrane is a glass fiber membrane of a colloidal gold marker containing a clenobuterol hydrochloride antibody, a salbutamol antibody and a paylean antibody; three detection strips are arranged on the cellulose nitrate membrane and contain clenobuterol hydrochloride protein conjugate, salbutamol protein conjugate and paylean protein conjugate respectively; and a quality control strip containing an anti-rabbit antibody or an anti-mouse antibody is arranged. The clenobuterol hydrochloride, salbutamol and paylean three joint inspection card has the advantages of simultaneously detecting the clenobuterol hydrochloride, the salbutamol and the paylean in urine or feed, animal tissue, meat and liver. The inspection card is easy to prepare and quick and convenient to use, saves the detection cost, and has accurate result.
Owner:无锡安迪生物工程有限公司
Method for treating cancers
The invention provides for the production of several humanized murine antibodies specific for the antigen LK26, which is recognized by the murine antibody LK26. This antigen is expressed on all choriocarcinoma, teratocarcinoma and renal cancer cell lines whereas it is not expressed on cell lines of leukaemias, lymphomas, neuroectodermally-derived and epithelial tumor cell lines (excepting a small subset of epithelial cell lines). Furthermore, whereas renal cancer cell lines express the LK26 antigen, normal renal epithelial cells do not. Similarly, with the exception of the trophoblast, all normal adult and fetal tissues tested are negative for the LK26 phenotype. The invention also provides for numerous polynucleotide encoding humanized LK26 specific antibodies, expression vectors for producing humanized LK26 specific antibodies, and host cells for the recombinant production of the humanized antibodies. The invention also provides methods for detecting cancerous cells (in vitro and in vivo) using humanized LK26 specific antibodies. Additionally, the invention provides methods of treating cancer using LK26 specific antibodies.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Anti-H7N9 full-human-derived monoclonal antibody 5J13 and preparation method and application thereof
ActiveCN106519027AHigh affinityImprove featuresImmunoglobulins against virusesAntiviralsHemagglutininSide effect
The invention relates to an anti-H7N9 full-human-derived monoclonal antibody 5J13 and a preparation method and application thereof. Amino acid sequences of heavy and light chain CDR1, CDR2 and CDR3 of the antibody are GFSFSNYG in the heavy chain CDR1 area, ISYDGTNK in the heavy chain CDR2 area, AKGRGPYCSSSICYHGMDV in the heavy chain CDR3 area, QSVLSGSINMNY in the light chain CDR1 area, WAS in the light chain CDR2 area and QQYYSTPLT in the light chain CDR3 area correspondingly. The antibody can be combined with hemagglutinin A of the H7N9virus in a targeted mode and has remarkable neutralization activity in resisting H7N9 virus infection. Compared with a murine antibody, genes of the full-human-derived antibody are completely from human genes and have no components of other species, toxic and side effects such as the anti-mouse anti-antibody are avoided, the biocompatibility is better, and the anti-H7N9 full-human-derived monoclonal antibody 5J13 is more suitable and has more potential in becoming a macromolecular drug for treating influenza virus.
Owner:SHENZHEN INST OF ADVANCED TECH
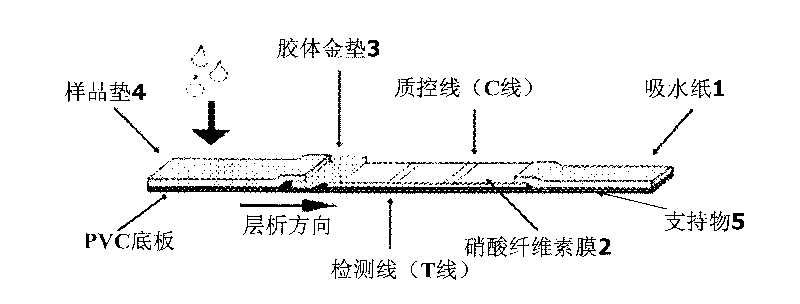
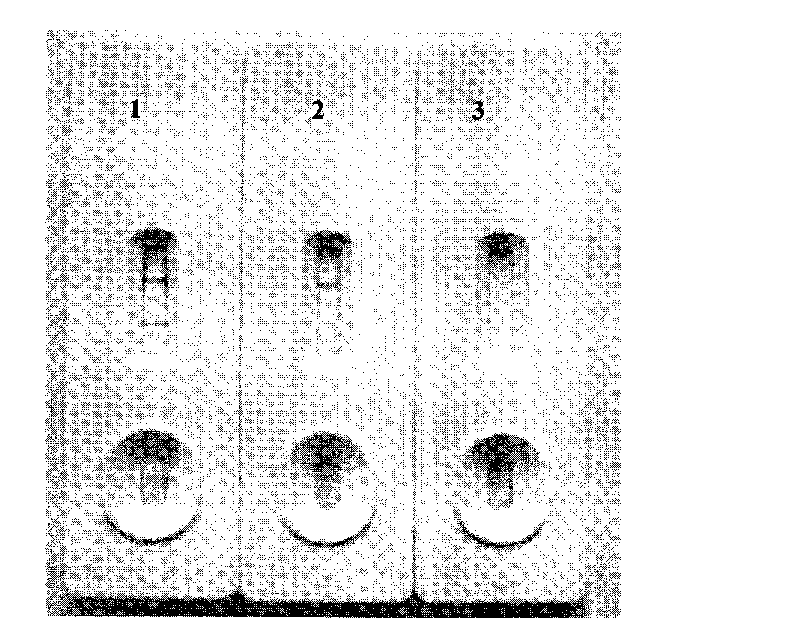
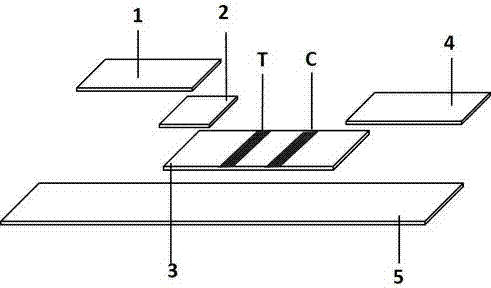
Colloidal gold immunochromatographic test strip for detecting wild-type classical swine fever virus
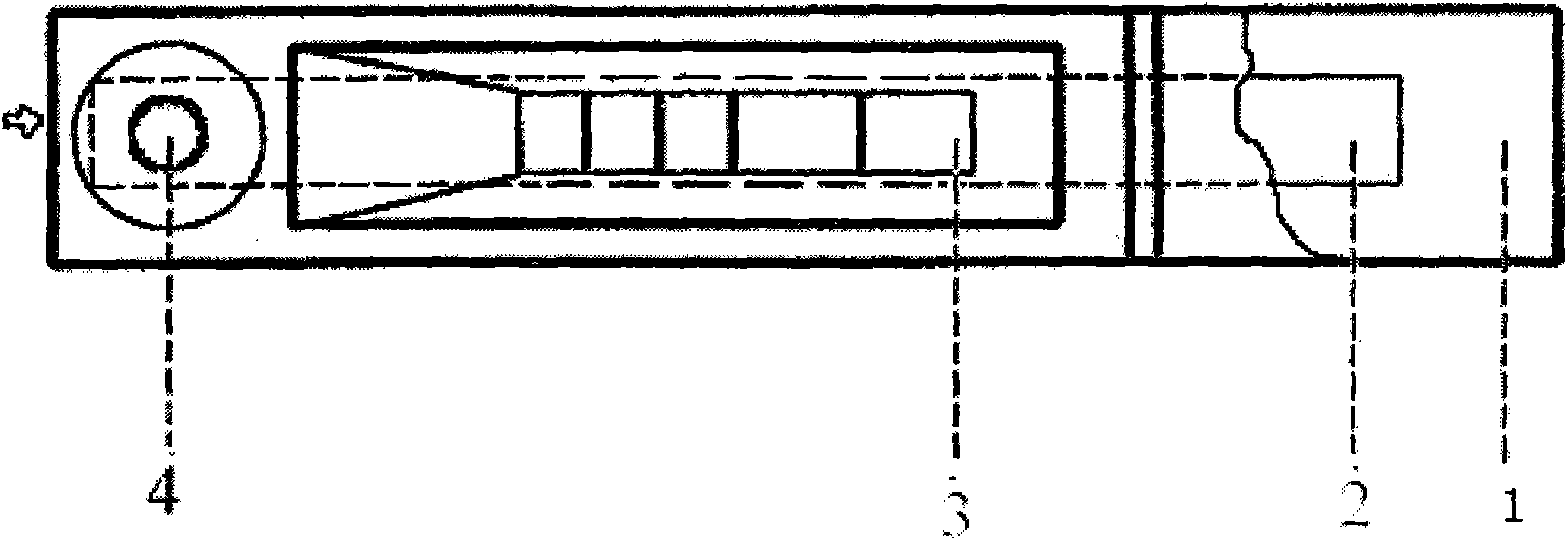
The invention discloses a colloidal gold immunochromatographic test strip for detecting wild-type classical swine fever virus, which consists of water absorbent paper (1), a cellulose nitrate membrane (2), a colloidal gold pad (3), a sample pad (4) and a support (5), wherein the cellulose nitrate membrane contains a detection line which is formed by coating monoclonal antibody HQ06 of anti-classical swine fever virus E2 protein and a quality control line which is formed by coating rabbit anti-mouse IgG antibody; and the colloidal gold pad is combined with colloidal gold-labeled monoclonal antibody 6E10 of the anti-classical swine fever virus E2 protein. The test strip does not react with C-strain of classical swine fever virus, bovine viral diarrhea virus, porcine reproductive and respiratory syndrome virus, transmissible gastroenteritis virus, porcine epidemic diarrhea virus, porcine rotavirus, pseudorabies virus, porcine parvovirus and porcine circovirus type 2, and can accurately and sensitively identify the wild-type classical swine fever virus, thereby having good specificity, sensitivity and repeatability.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
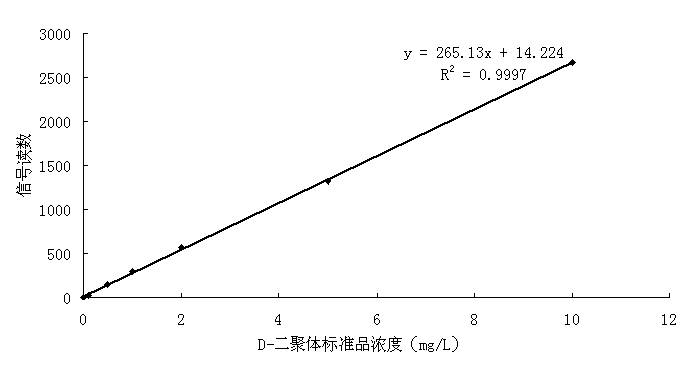
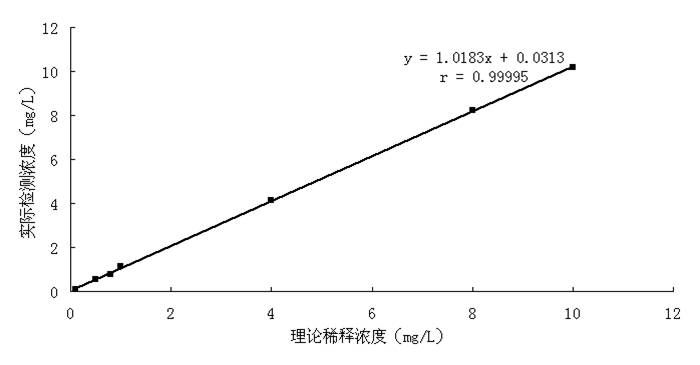
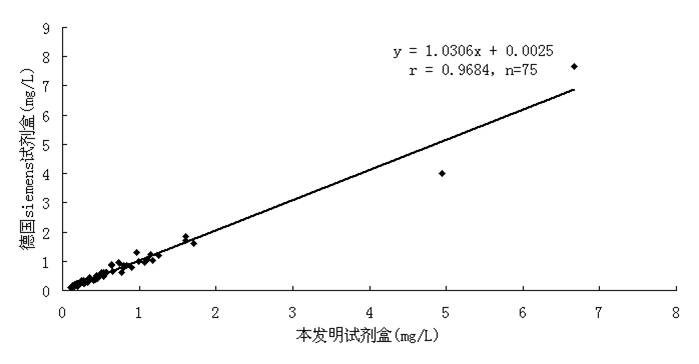
D-dimer quantitative fluorescence immunoassay test strip and preparation method thereof
InactiveCN102692504ASimple and fast operationImprove accuracyMaterial analysisDimerAntiendomysial antibodies
The invention relates to a D-dimer quantitative fluorescence immunoassay test strip. The test strip comprises a sample pad, a bind pad, a nitrocellulose film and absorbent paper, wherein the sample pad is a two-layer sample pad; the bind pad is coated with a fluorescence latex microsphere marked anti D-dimer antibody A; the nitrocellulose film is coated with an anti D-dimer antibody B serving as an assay line and a rabbit antimouse IgG antibody serving as a control line; and the fluorescence late microsphere is prepared from latex microsphere adsorptive fluorescence marked streptavidin. The test strip can be used for accurately and quantitatively assaying the content of D-dimer in human blood plasma, and has the characteristics of convenience in operation, high accuracy, high sensitivity, low cost and the like.
Owner:GETEIN BIOTECH
Antibody of anti human CD20 from human resources functionally, and application
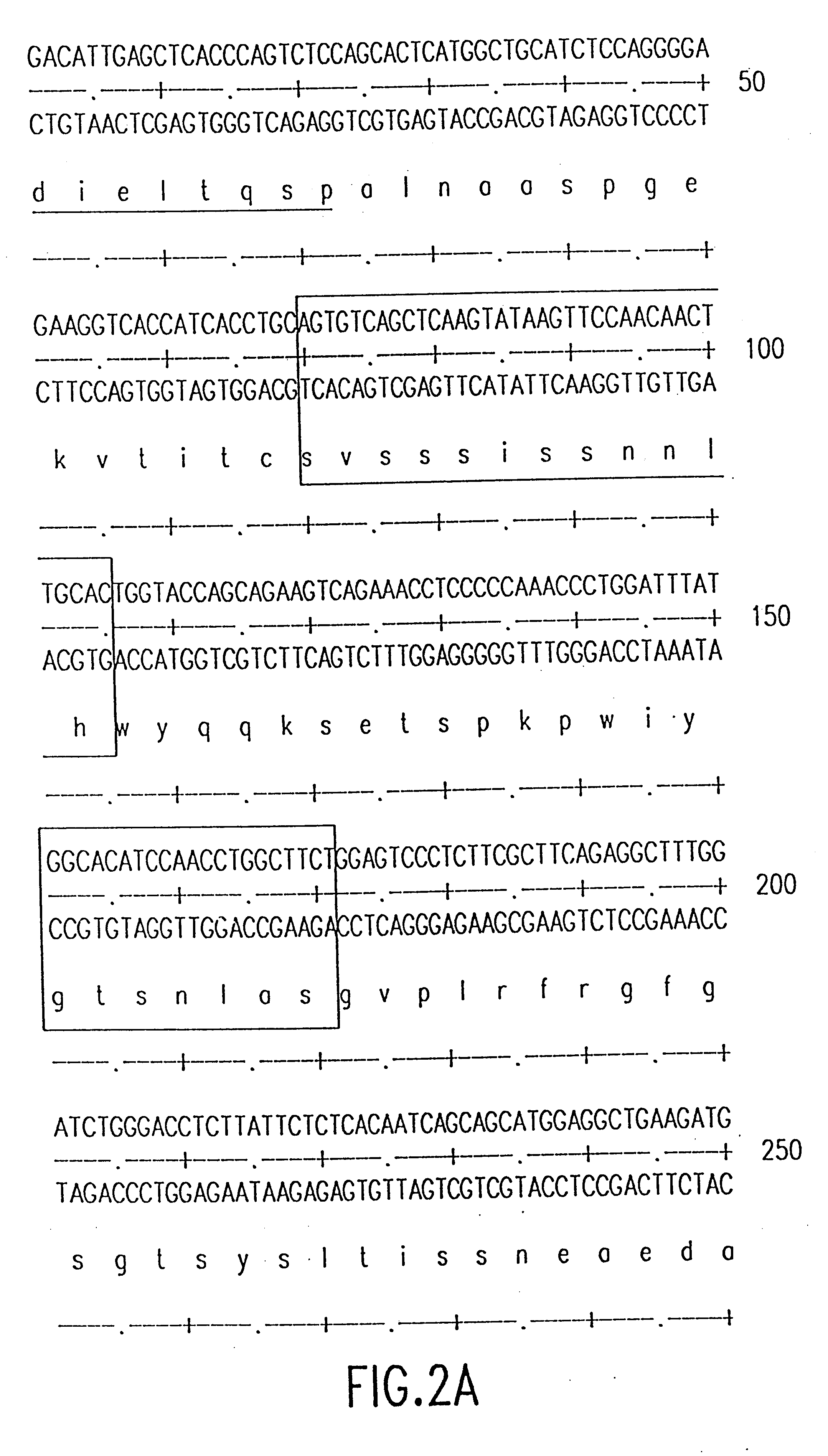
ActiveCN1958615ALow immunogenicityMaintain propertiesImmunoglobulins against cytokines/lymphokines/interferonsRadioactive preparation carriersDiseaseCD20
This invention discloses functional humanized antibody against CD20 molecules and its application. The functional humanized antibody specifies human CD20 molecules, and is composed of a light chain and a heavy chain, each of which comprises a constant region and a variable region. The constant region of the heavy chain is isotypic human IgG1 constant region, and that of the light chain is isotypic human kappa constant region. The variable region of the heavy chain is shown in SEQ ID No.2, and that of the light chain is shown in SEQ ID No.1. The functional humanized antibody has low immunogenicity. Experiments show that the functional humanized antibody has the same specificity and affinity as the original murine antibody. Drugs containing the functional humanized antibody and its derivatives can be used for treating cancers or immune system diseases with the high-expression of CD20, such as rheumatic arthritis and lupus erythematosus.
Owner:SINOMAB BIOSCI
Novel humanized anti-CD22 antibody
ActiveCN103214578AMaintain biological propertiesLow immunogenicityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsMurine antibodyImmunogenicity
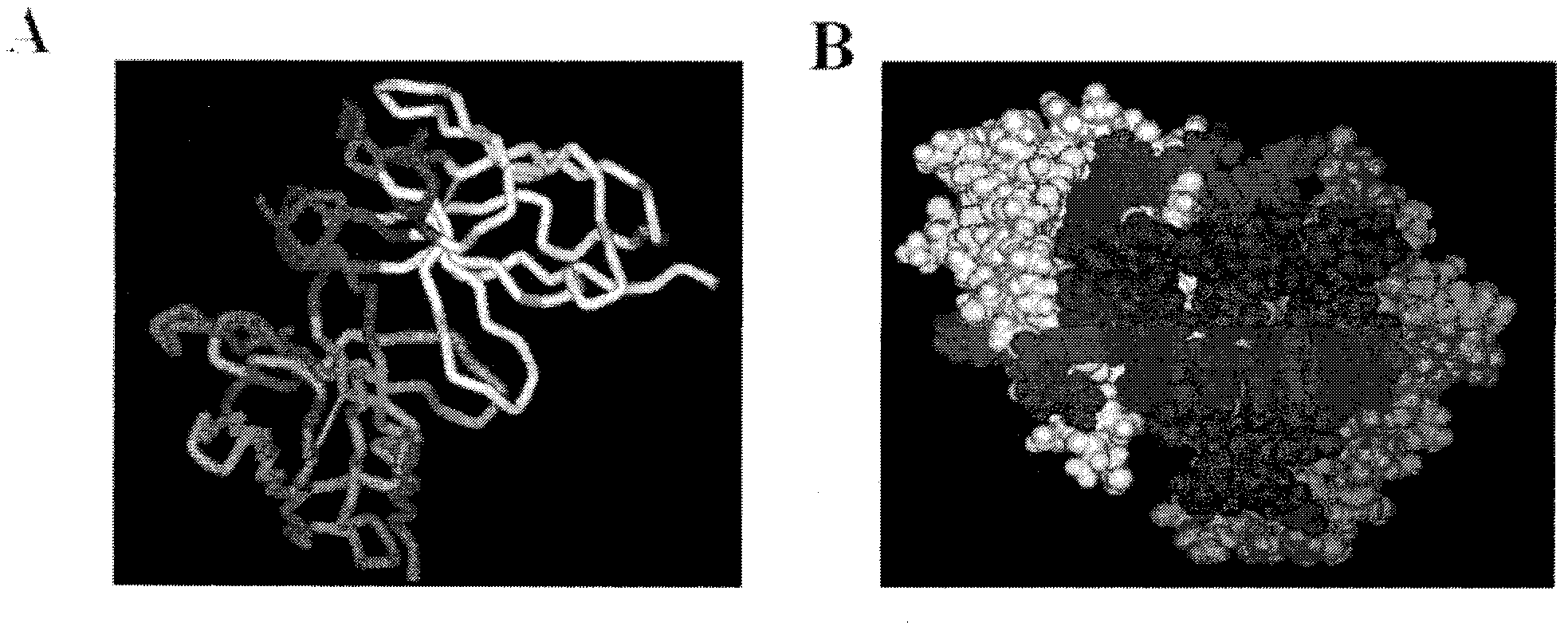
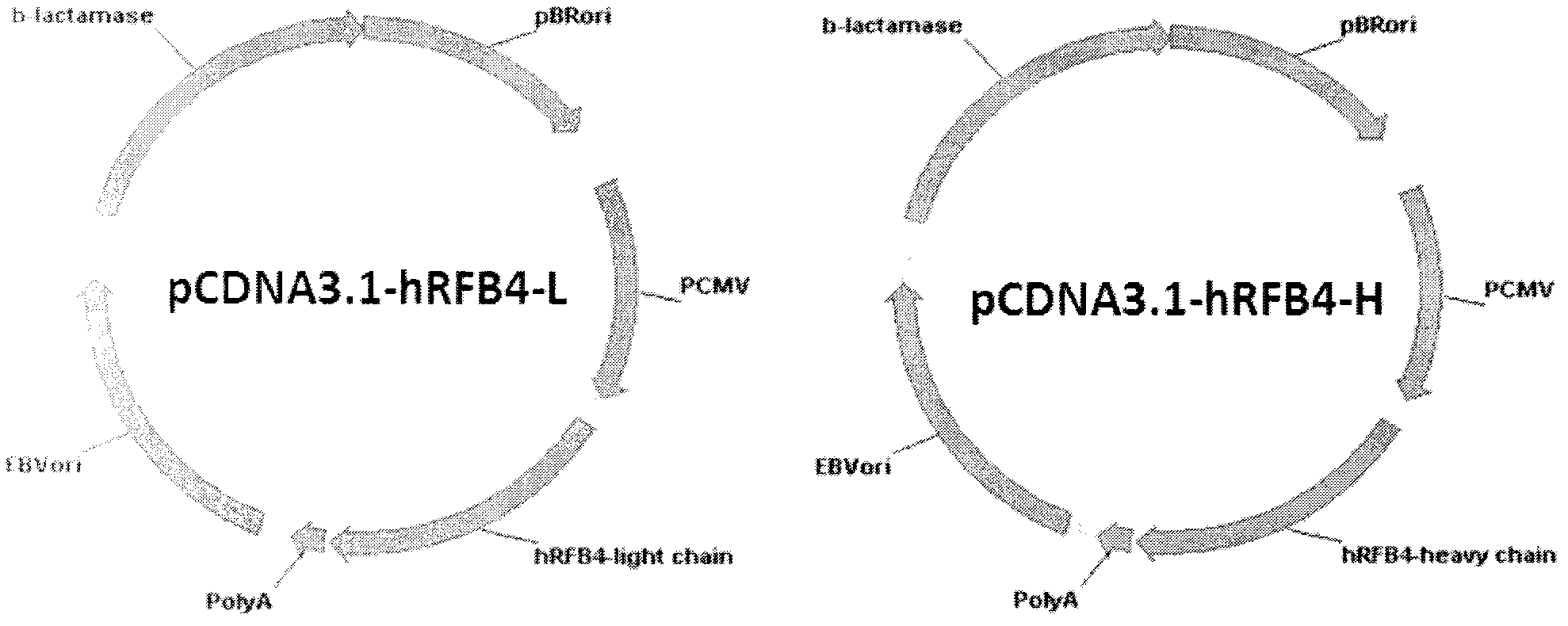
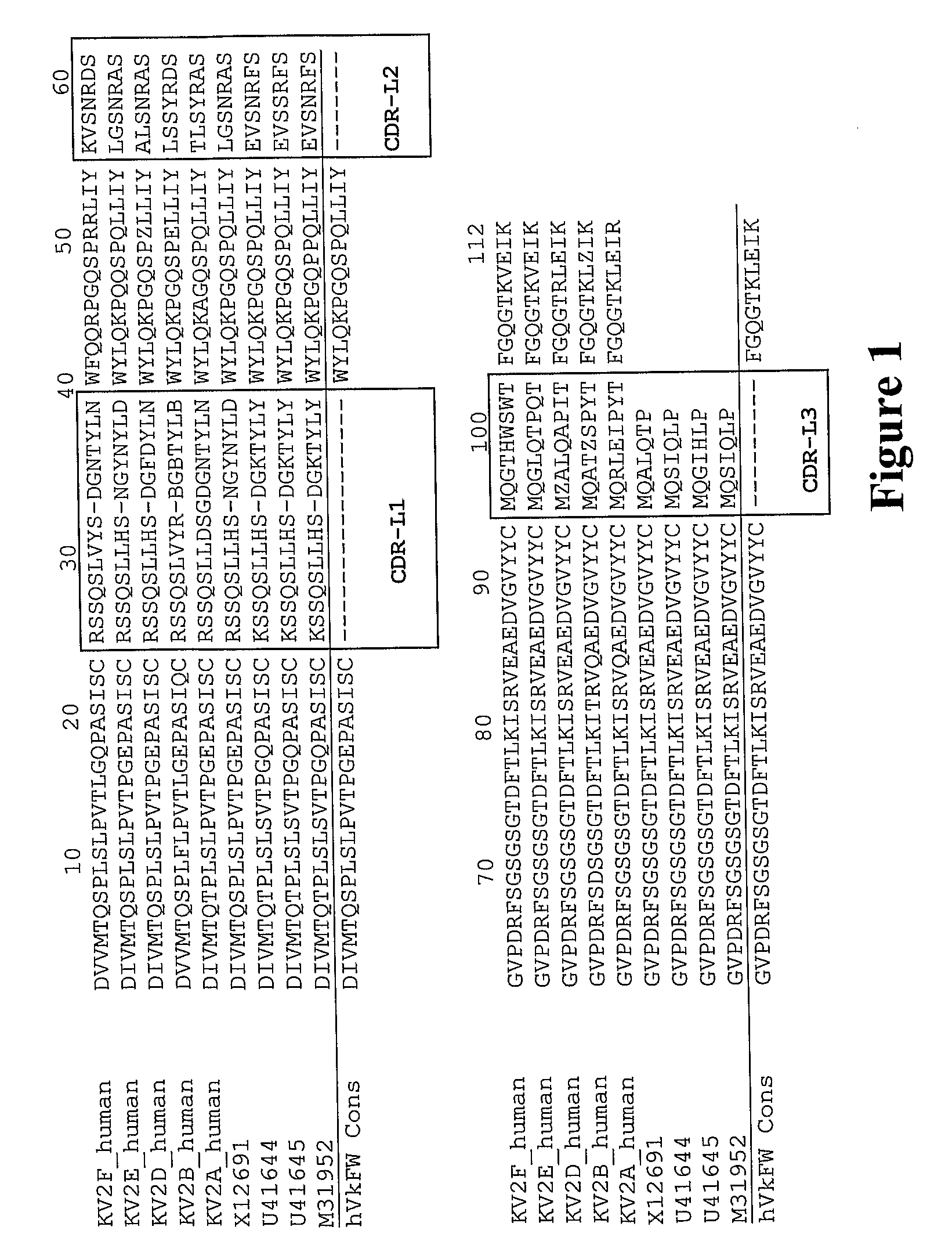
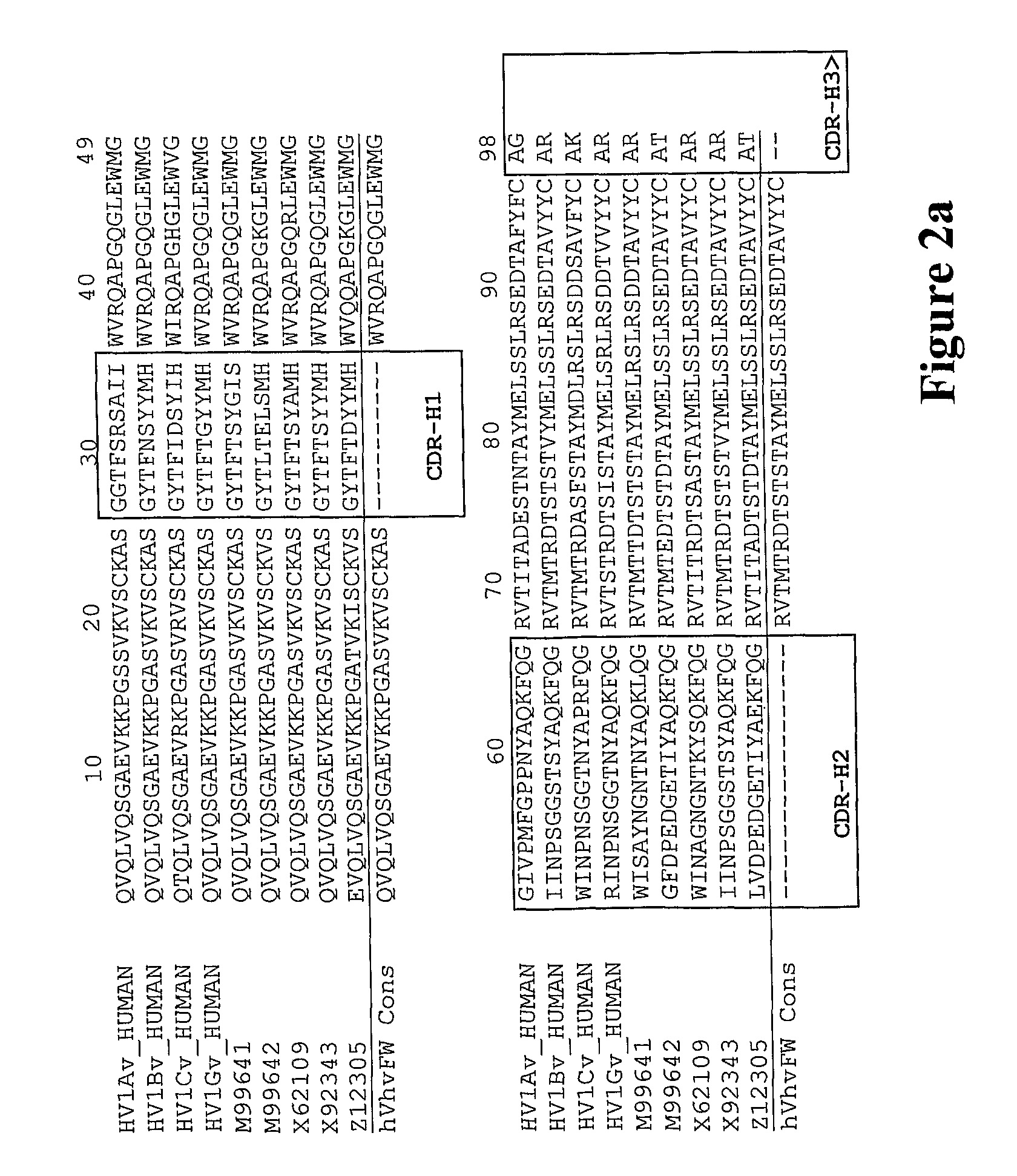
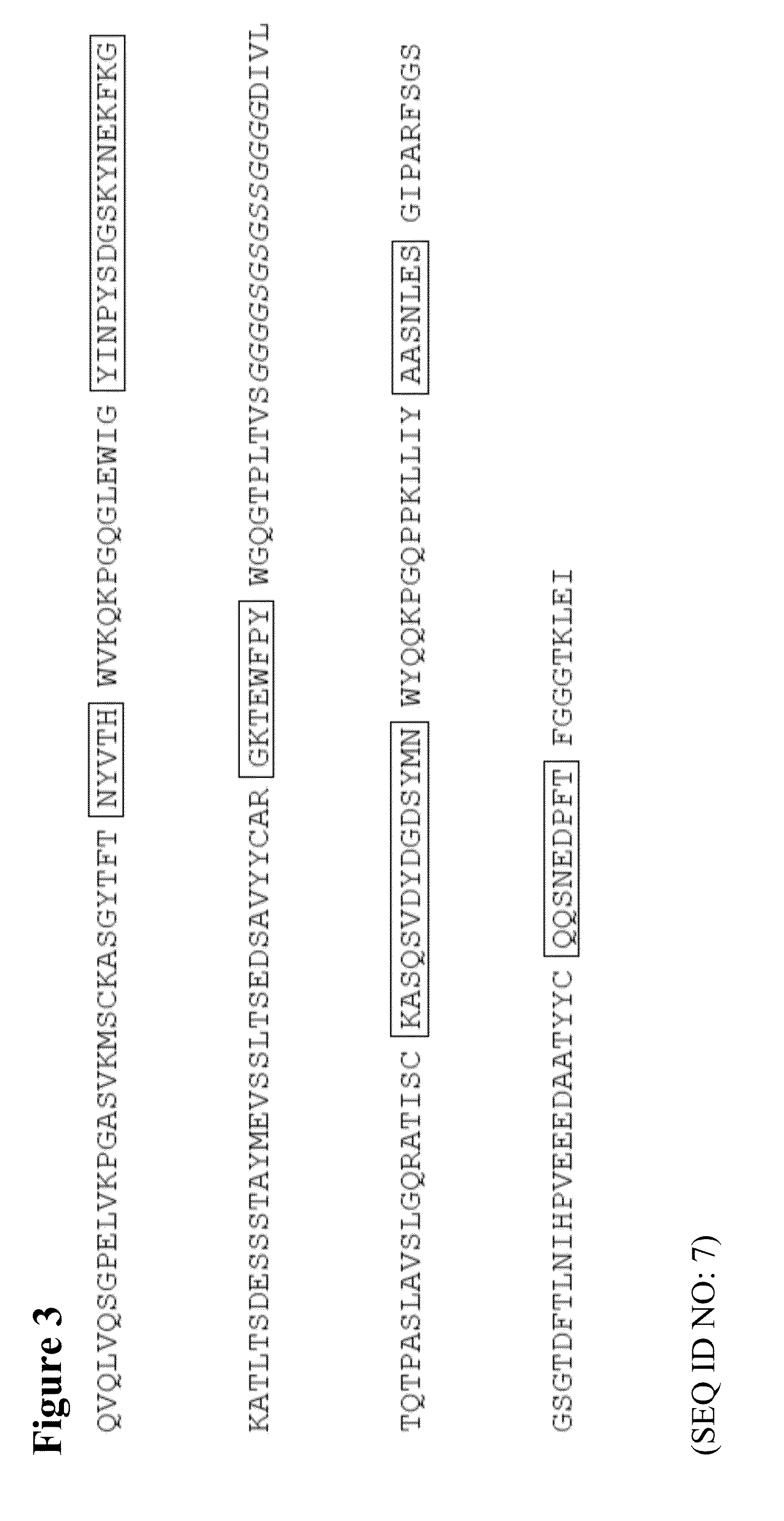
The invention relates to the field of bioengineering, and specifically relates to a novel humanized anti-CD22 antibody. The novel humanized anti-CD22 antibody takes a murine antibody RFB4 as a parental antibody and uses a CDR implantation technique to carry out humanization; and the humanized new antibody hRFB4 has affinity similar with the parental antibody, and keeps the bonding capability of the parental antibody with the CD22 antigen and the cell internalizing activity of antigen-antibody complex caused by the antibody. Compared with the parental antibody, the humanized protein sequence proportion in the humanized hRFB4 antibody reaches over 90%, so that the immunogenicity is greatly reduced and the occurrence of HAMA reaction is avoided.
Owner:BEIJING DONGFANG BIOTECH
Humanized anti-C5aR antibodies
The present invention is directed to humanized antibodies which bind the human C5a receptor and their use as therapeutic and diagnostic agents. The present invention is further directed toward nucleic acid sequences which encode said humanized antibodies, and their expression in recombinant host cells. In particular, the present invention is directed towards humanized antibodies derived from murine antibody 7F3 which specifically binds to the human C5a receptor.
Owner:NOVO NORDISK AS
Immunochromatography reagent strip for quantitative detection of 25-hydroxyvitamin D and preparation method of immunochromatography reagent strip
InactiveCN104749385ASampling is simpleSimple and fast operationBiological testingImmune profilingCellulose
The invention relates to the technical field of biology, and discloses an immunochromatography reagent strip for quantitative detection of 25-hydroxyvitamin D and a preparation method of the immunochromatography reagent strip. The immunochromatography reagent strip for quantitative detection of the 25-hydroxyvitamin D comprises a sample adding region, a conjugate release region, a reaction region and a water absorption region, which are sequentially arranged; the conjugate release region is a glass cellulose membrane enveloped with colloidal gold or a quantum dot or an up-conversion luminescent material UCP-marked mouse-anti-human 25-hydroxyvitamin D monoclonal antibody; the reaction region is a test region and a control region; the test region is enveloped with 25-hydroxyvitamin D-coupling protein; and the control region is enveloped with a nitrocellulose membrane of a goat anti-mouse IgG antibody. The immunochromatography reagent strip is capable of accurately, quantitatively, rapidly and sensitively detecting the 25-hydroxyvitamin D in human whole blood, serum and plasma; the required instrument is different from a traditional large expensive instrument for a clinical laboratory; and only one corresponding miniature immunity analyzer is required, therefore, real-time detection is realized.
Owner:PRO MED BEIJING TECH
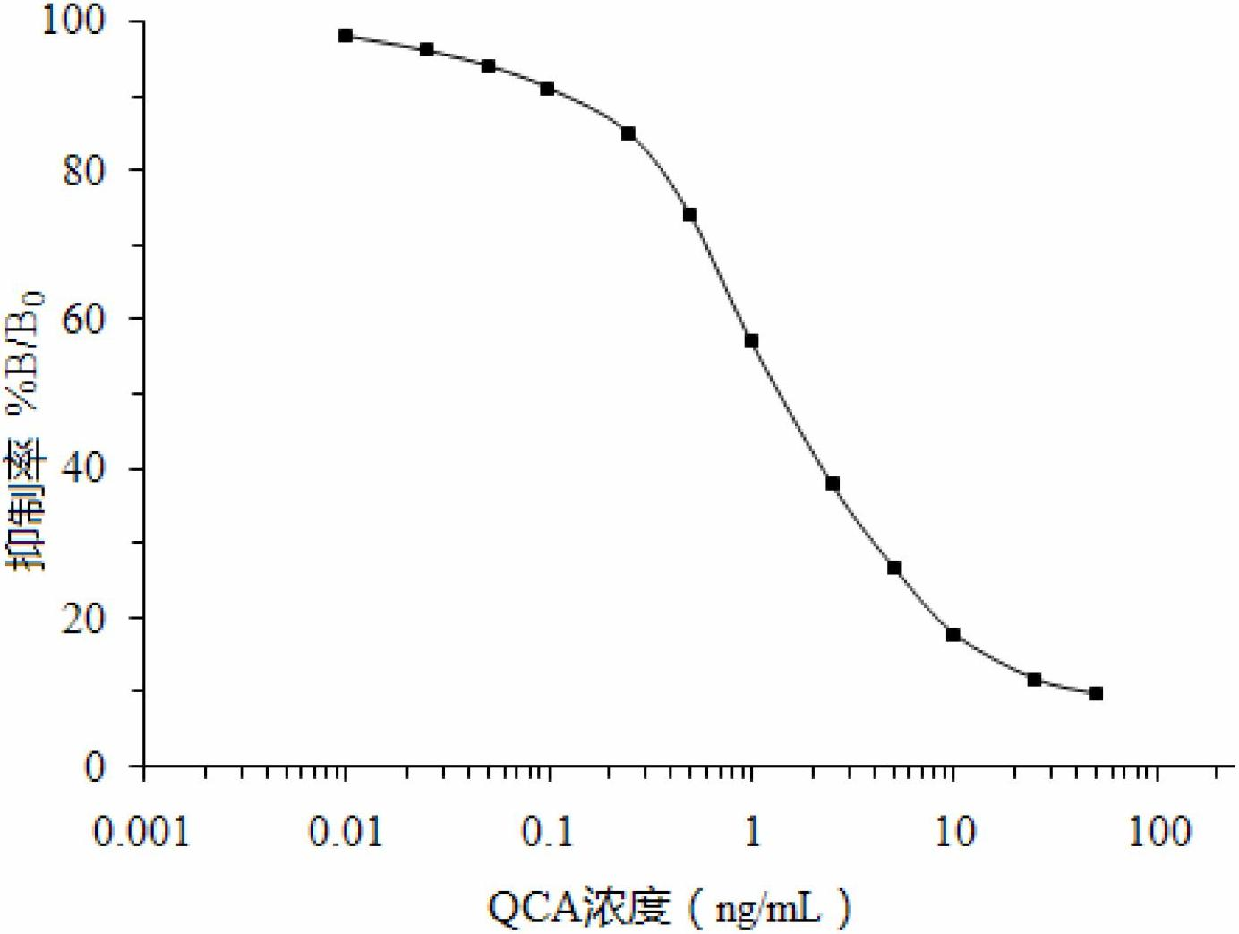
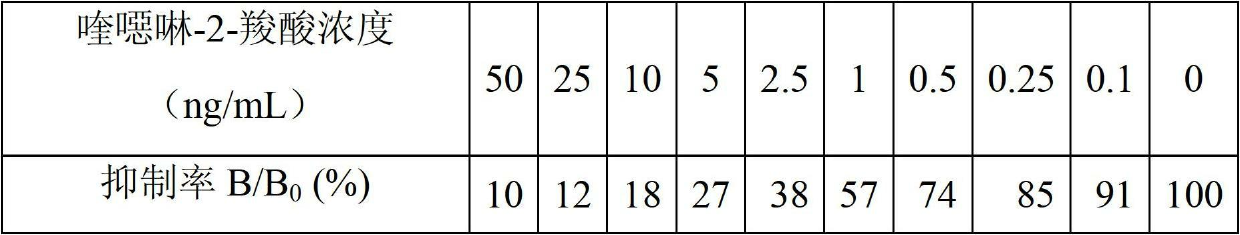
Detecting reagent kit used for detecting quinoxalinone-2-carboxylic acid and method
InactiveCN102654500AEasy to handleDetection suitable forMaterial analysisFreeze-dryingCarboxylic acid
The invention discloses a time-resolved fluorescence immunoassay reagent kit which is used for detecting quinoxalinone-2-carboxylic acid and a detection method of the time-resolved fluorescence immunoassay reagent kit. The reagent kit comprises a porous coated plate, a buffer solution, quinoxalinone-2-carboxylic acid standard, freeze-dried antibodies of quinoxalinone-2-carboxylic acid, europium-marked goat anti mouse antibodies, a cleaning solution and an enhancement solution. By measuring fluorescence intensity of counts per second (cps), the content of quinoxalinone-2-carboxylic acid in samples is calculated by a standard curve. The kit provided by the invention has the advantages of simple structure, easiness in assembly, corrosion resistance, light weight, low cost, wide application and the like. A valve core has good centering performance and can move flexibly and reliably and can play a good water-plugging role.
Owner:CHONGQING ACADEMY OF SCI & TECH
Test kit special for enzyme linked immunosorbent assay adsorption of vomitus toxin and preparing and detecting method thereof
The invention relates to a special enzyme-linked immunosorbent assay kit for emetic toxin. The detection is rapid, sensitive, accurate, quantitative, simple in operation, low in requirements on sample purity and strong in specificity, thereby being particularly applicable to the detection of large quantities of samples; and the invention also provides a preparation of the special kit and a detection method. The kit comprises washing liquid, color developing liquid A, color developing liquid B and stop solution, and the kit is characterized in that: the kit also comprises a coated plate which is coated by emetic toxin solid-phase antigen, an emetic toxin (DON) standard product, an emetic toxin (DON) monoclonal antibody freeze-dried product and an enzyme-labeled goat anti-mouse antibody free-dried product. When in detection, the coated plate is taken, 50muL-100muL of the DON standard product or a well processed sample is added into the respective micropores, 50mul-100mul of the anti-DON antibody is added, the incubation is carried out at 35 DEG C-45 DEG C for about 0.5 hour-1 hour, the washing liquid is used for washing for 3 times-5 times, 50muL-100muL of the horseradish peroxidase(HRP)-goat anti-mouse antibody is added, the incubation is carried out at about 35 DEG C-45 DEG C for about 0.5 hour-1 hour, the washing liquid is used for washing for 3 times-5 times, 50muL-100muL of the color developing liquid A and 50muL-100muL of the color developing liquid B are added, the mixture is placed still in the dark for 10 minutes-20 minutes, then the stop solution is added, the absorbance value is measured at 450nm, and the DON content in the sample is calculated from a standard curve.
Owner:BEOSON JIANGSU FOOD SAFETY TECH CO LTD
Immune colloidal gold detecting card of carbendazin and preparation method of immune colloidal gold detecting card
The invention discloses an immune colloidal gold detecting card of carbendazin and a preparation method of the immune colloidal gold detecting card, and relates to the technical field of animal source food veterinary drug residue detection. A test strip in a shell of the detecting card is composed of a PVC glue plate, a sample cushion, a colloidal gold conjugate pad, a coating film and a water absorbing cushion, wherein a colloidal gold film is a glass cellulose membrane containing carbendazin monoclonal antibodies, the coating film is a nitrocellulose film and is provided with a line T and a line C, the line T is wrapped by carbendazin protein conjugates, and the line C is wrapped by goat-anti-mouse IgG antibodies. The detecting card can be effectively used for rapidly detecting carbendazin and is convenient to use, rapid and accurate in result.
Owner:NANJING YITE BIOTECH
Humanized antibody against CD19 and immune effector cells targeting to CD19
The invention relates to an anti-CD19 humanized antibody prepared from a murine monoclonal antibody, a chimeric antigen receptor containing the humanized antibody, and immune cells expressing the humanized antibody. The humanized antibody of the invention does not produce an anti-antibody reaction (AAR) and a human anti-mouse antibody reaction (HAMA), has better affinity than a murine antibody, and presents excellent activity and safety, so a novel means is provided for treating tumors expressing CD19.
Owner:CARSGEN THERAPEUTICS
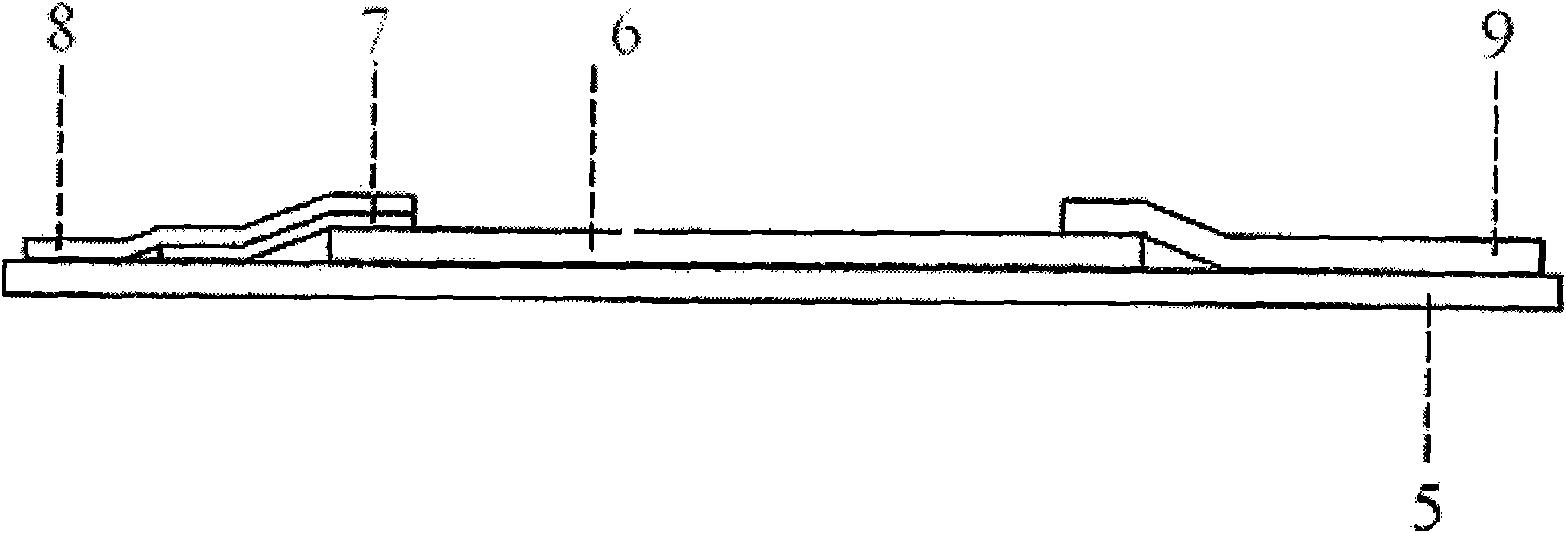
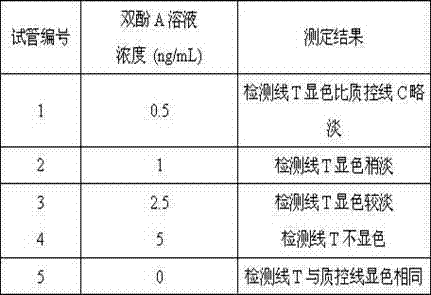
Colloidal gold immunochromatographic card for detecting bisphenol A (BPA) and preparation method thereof
ActiveCN103018467AQualitative detection is easy to operateThe detection process is fastBiological testingCelluloseAntiendomysial antibodies
The invention relates to a colloidal gold immunochromatographic card for detecting BPA and a preparation method of the colloidal gold, which belongs to the field of industrial compound environmental hormone detection technology. The test strip in a case of the colloidal gold immunochromatographic card is composed of a PVC slab, a sample cushion, a colloidal gold combination cushion, a coating film, and a water absorption cotton, wherein a colloidal gold film is a glass fiber film containing BPA monoclonal antibody; the coating film is a cellulose nitrate film and is provided with a T line and a C line; the T line is coated with a BPA protein conjugate; and the C line is coated with a rabbit antimouse IgG antibody. The colloidal gold immunochromatographic card provided by the invention is effective in detecting BPA quickly, and is simple, convenient, quick, and accurate.
Owner:JIANGSU WISE SCI & TECH DEV
Anti-cd22 Anti-idiotypic antibodies and uses thereof
ActiveUS20150175711A1Not applyBiological material analysisArtificial cell constructsComplement-dependent cytotoxicityAntibody fragments
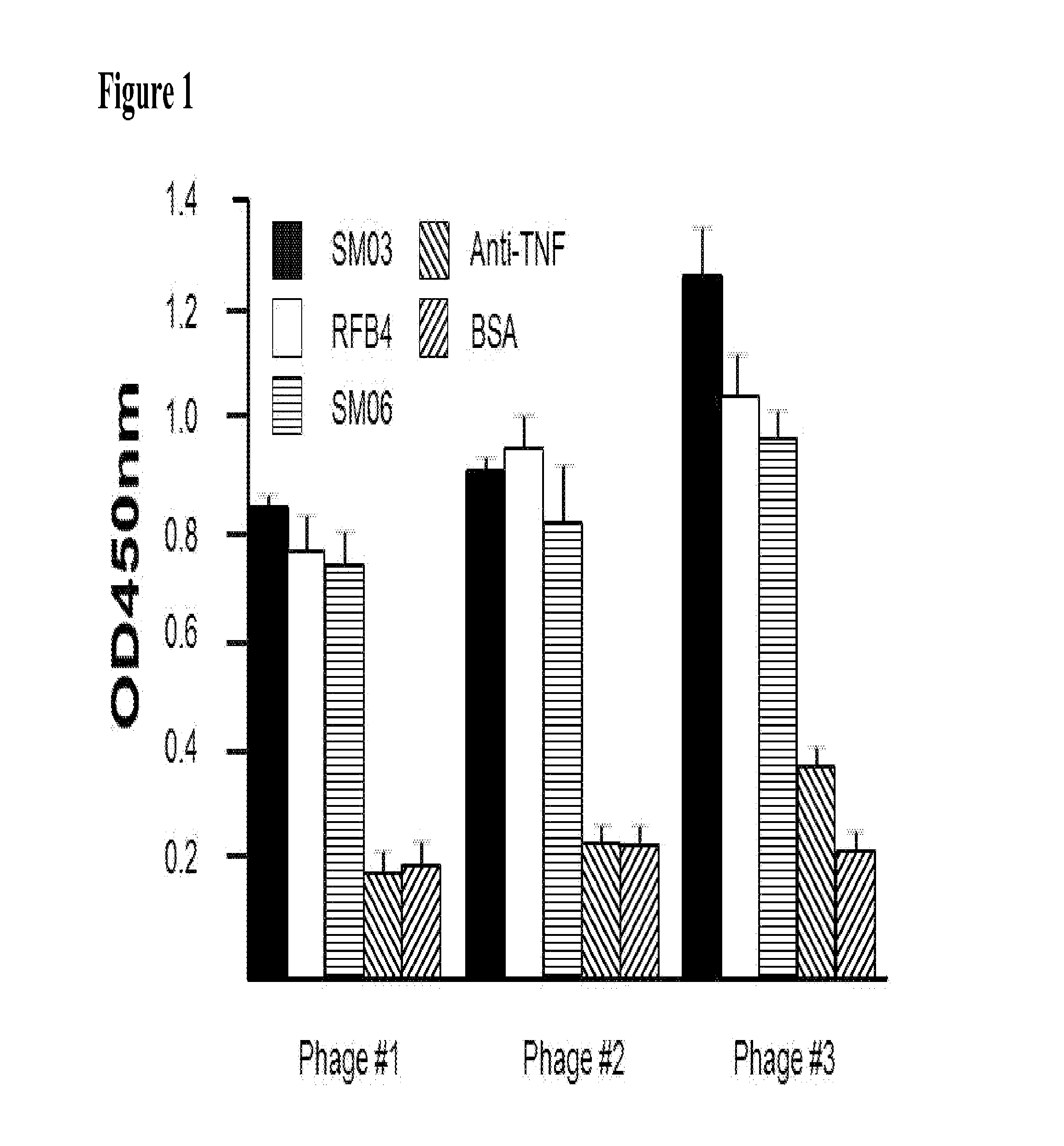
The present invention describes the generation of an anti-idiotype single-chain Fv (scFv) antibody specific for the murine (RFB4), chimeric (SM03) and humanized (SM06) versions of an anti-CD22 antibody (the anti-CD22 antibodies). The present invention further describes the construction of a murine IgG2a / kappa immunoglobulin carrying the variable region sequences of the anti-idiotype scFv sequences. Additionally, the present invention provides a cell line capable of producing an anti-idiotype murine antibody specific for the anti-CD22 antibodies. The present invention is directed against a method for identifying and evaluating the activities and concentration of the anti-CD22 antibodies. Additionally, the present invention provides a method for evaluating serum concentration of the anti-CD22 antibodies that are being used clinically. The present invention is also directed against a method to detect HAMA, HACA and HAHA responses in patients treated with the anti-CD22 antibodies. Specifically, the present invention is directed against the establishment of a cell line expressing surface concentration of the antibody of the invention; the said cell line expressing surface anti-idiotype antibodies or antibody fragments will be used as the target cell line for evaluating the functional activities of the anti-CD22 antibodies via complement dependent cytotoxicity (CDC) and / or antibody dependent cell cytotoxicity (ADCC) activities.
Owner:SINOMAB BIOSCI
SARS virus antibody detecting method, rapid diagnosis kit and preparation method
InactiveCN1570638AImprove featuresIncreased sensitivityMicrobiological testing/measurementBiological testingSerum igeSARS coronavirus
This invention relates to a detection mode of SARS virus antibody and a preparing method of the box for rapid diagnosis reagent. The checking method includes that (1) putting antihuman Ig antibody marked by colloidal gold in the carrier, and coating detecting thread made of variant antigen of SARS coronavirus and controlling thread made of antipest IgG antibody in the detecting carrier connecting with the marked one. (2) putting human serum in the marked carrier. The box for rapid diagnosis reagent includes checkerboard marked by variant antibody of SARS coronavirus, which comprises water-absorbing layer of adding-kind edge, detecting layer and water-absorbing layer of water-uptake edge. There is antihuman Ig antibody layer between detecting layer and water-absorbing layer of water-uptake edge, and in the detecting layer there is detecting thread made of variant antigen of SARS coronavirus and controlling thread made of antipest IgG antibody coating.
Owner:LANZHOU YAHUA BIOTECH
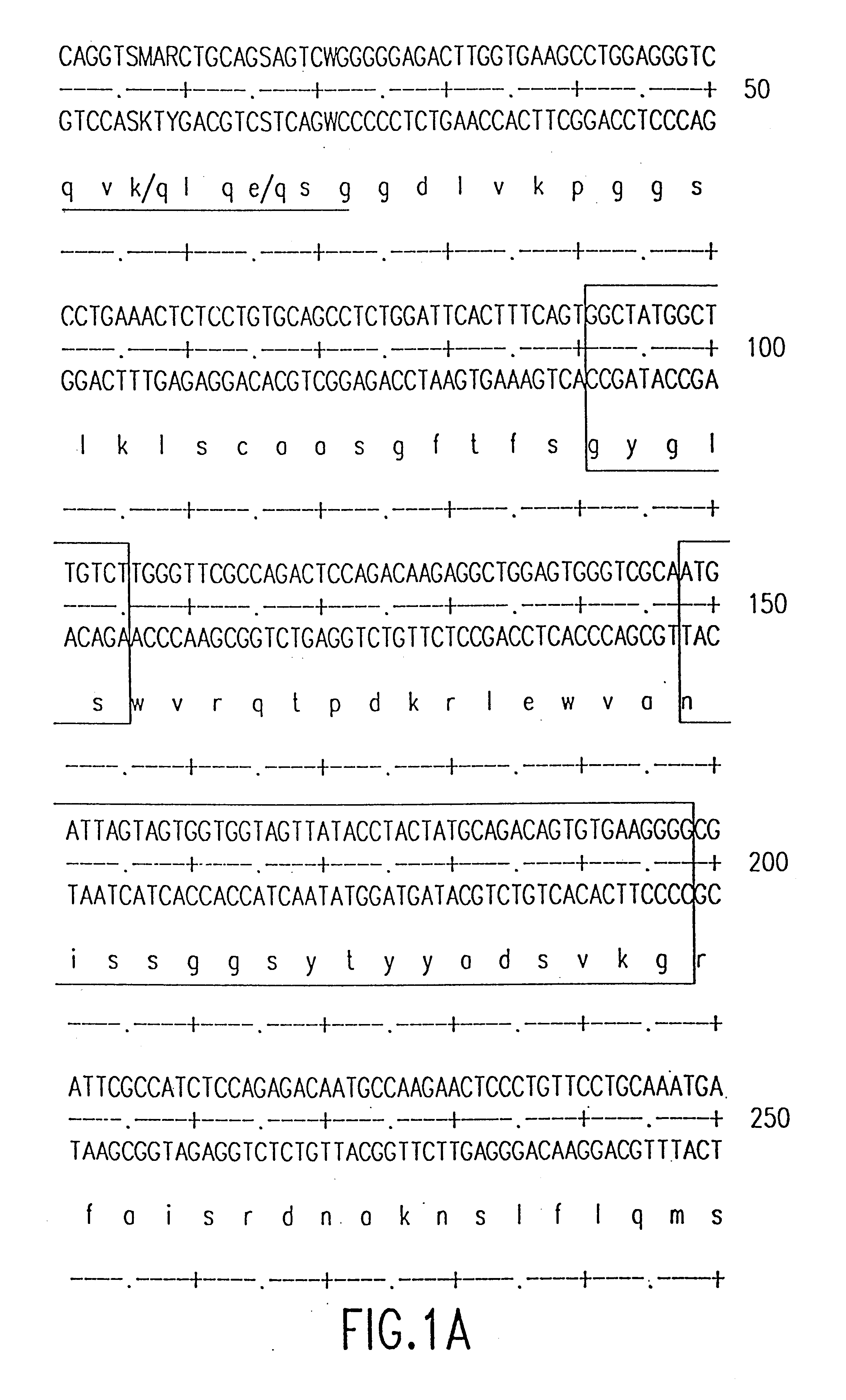
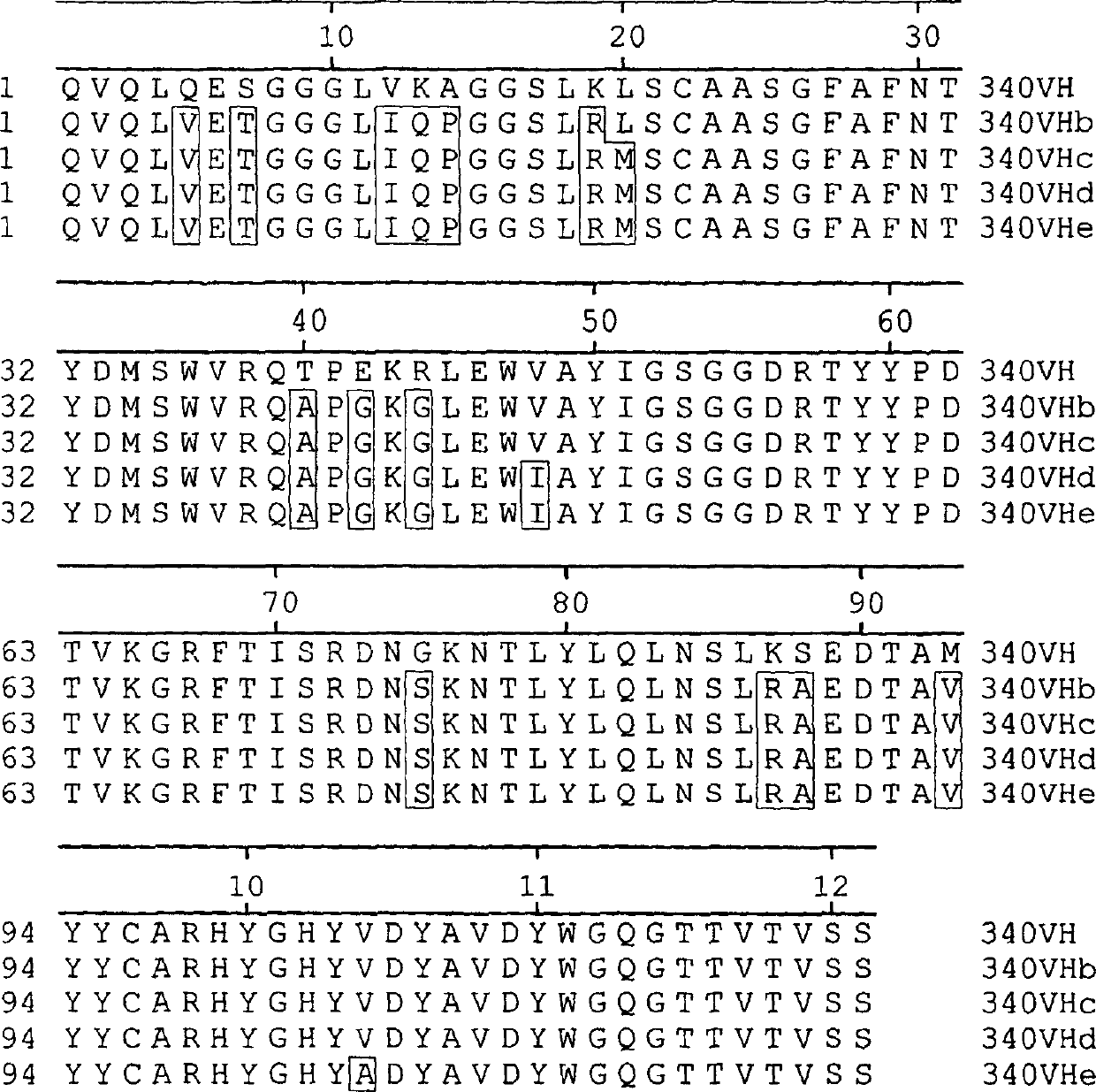
Humanised antibodies to epidermal growth factor receptor
InactiveCN1432063AOrganic active ingredientsFungiHuman epidermal growth factor receptorMurine antibody
The present invention provides a humanised form of the antibody 340 obtainable from the cell line deposited with the ECACC under accession number 97021428. Such antibodies have been found to have an increased ability to kill cells compared to the murine antibody 340. Also provided are nucleic acids encoding such antibodies, as well as the use of the antibodies in medicine, in particular in the treatment of cancer.
Owner:SCANCELL
Neutralizing Anti-ccl20 antibodies
ActiveUS20120148592A1Reduces human CCL20-induced chemotaxisShorten the progressAntibacterial agentsFungiDiseaseHeavy chain
The present invention relates to novel humanized, chimeric and murine antibodies that have binding specificity for the human CC chemokine ligand 20 (CCL20). The present invention further relates to heavy chains and light chains of said antibodies. The invention also relates to isolated nucleic acids, recombinant vectors and host cells that comprise a sequence which encodes a heavy chain and / or a light chain of said antibodies, and to a method of preparing said antibodies. The anti-CCL20 antibodies of the invention can be used in therapeutic applications to treat, for example, inflammatory and autoimmune disorders and cancer.
Owner:EISIA R&D MANAGEMENT CO LTD
Immune colloidal gold detect card of chlorpromazine, and preparation method thereof
The invention discloses an immune colloidal gold detect card of chlorpromazine, and a preparation method thereof, and belongs to the technical field of animal-derived food veterinary drug residue detection. A test strip of an outer housing of the immune colloidal gold detect card is composed of a PVC slab, a sample pad, a colloidal gold conjugate pad, a coating membrane, and an absorbent pad; a colloidal gold membrane is a glass cellulose membrane containing chlorpromazine monoclonal antibodies; the coating membrane is a nitrocellulose memebrane, and is provided with a T line and a C line; the T line contains chlorpromazine protein conjugates via coating; and the C line contains goat anti-mouse IgG antibodies via coating. The immune colloidal gold detect card is used for rapid detection of chlorpromazine effectively, and is convenient and rapid for using; and detection results are accurate.
Owner:NANJING YITE BIOTECH
Antibody of anti human CD20 from human resources functionally, and application
ActiveCN100455598CImmunoglobulins against cytokines/lymphokines/interferonsRadioactive preparation carriersCD20Disease
This invention discloses functional humanized antibody against CD20 molecules and its application. The functional humanized antibody specifies human CD20 molecules, and is composed of a light chain and a heavy chain, each of which comprises a constant region and a variable region. The constant region of the heavy chain is isotypic human IgG1 constant region, and that of the light chain is isotypic human kappa constant region. The variable region of the heavy chain is shown in SEQ ID No.2, and that of the light chain is shown in SEQ ID No.1. The functional humanized antibody has low immunogenicity. Experiments show that the functional humanized antibody has the same specificity and affinity as the original murine antibody. Drugs containing the functional humanized antibody and its derivatives can be used for treating cancers or immune system diseases with the high-expression of CD20, such as rheumatic arthritis and lupus erythematosus.
Owner:SINOMAB BIOSCI
Dual colloidal gold test strip for detecting human bone metabolic biomarker and detection device applying same
InactiveCN106370844AThe test result is accurateFast test resultsDisease diagnosisBiological testingAntigenBiotin-streptavidin complex
The invention belongs to the field of clinical laboratory techniques and in particular relates to a colloidal gold test strip for quantitatively detecting human bone metabolic biomarker and a detection device applying same. The test strip provided by the invention comprises a sample pad, a glass fiber mat coated with gold-labeled streptavidin, a glass fiber mat coated with a gold-labeled antibody, a nitrocellulose membrane and an absorbent pad which are sequentially adhered with one another on a bottom plate in a staggered manner. A quality control line pre-enveloped with a goat anti-rat IgG antibody is formed in the nitrocellulose membrane; a detection line parallel to the quality control line is formed in the nitrocellulose membrane; an antibody capable of being specifically bound with a to-be-detected antigen human bone metabolic biomarker is coated on the detection line; the gold-labeled antibody is an antibody capable of being specifically bound with the to-be-detected antigen human bone metabolic biomarker. According to the test strip provided by the invention, the human bone metabolic biomarker can be quantitatively detected, the requirement on instruments is low, the time of clinically detecting the human bone metabolic biomarker is shortened, and the detection complexity and cost can be reduced.
Owner:吕炜锋
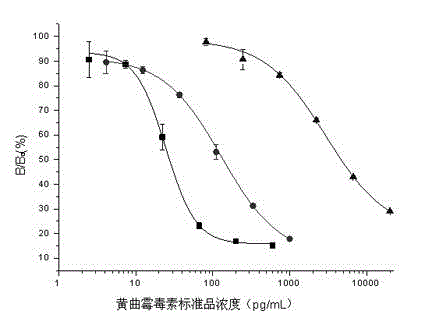
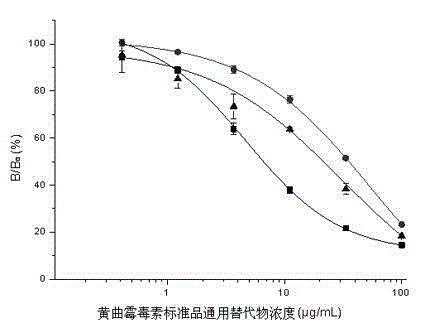
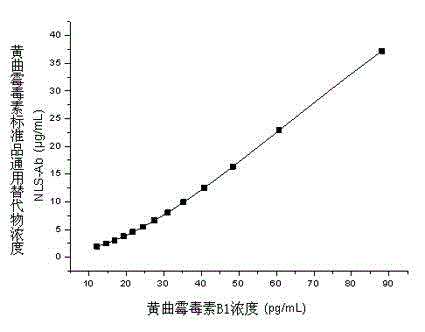
Standard substance universal alternate for aflatoxin detection by using ELISA, preparation method thereof, and ELISA detection method for aflatoxin
The present invention relates to a standard substance universal alternate for aflatoxin detection by using ELISA, a preparation method thereof, and an ELISA detection method for aflatoxin. The standard substance universal alternate is characterized in that: the standard substance universal alternate is a rabbit anti-mouse antibody capable of recognizing various mouse anti-aflatoxin monoclonal antibodies. The preparation method comprises: adopting a mouse anti-aflatoxin B1 monoclonal antibody, a mouse anti-aflatoxin M1 monoclonal antibody, and a mouse anti-aflatoxin G1 monoclonal antibody as a mixed antigen; adopting the mixed antigen to immunize New Zealand long ear white rabbits; sampling blood from carotid arteries; and carrying out affinity purification on serums to obtain the standard substance universal alternate. The standard substance universal alternate of the present invention is non-toxic and harmless, and can respectively replace various corresponding aflatoxin standard substances in ELISA detection methods for aflatoxin to quantitatively detect various corresponding aflatoxin concentrations in samples.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com