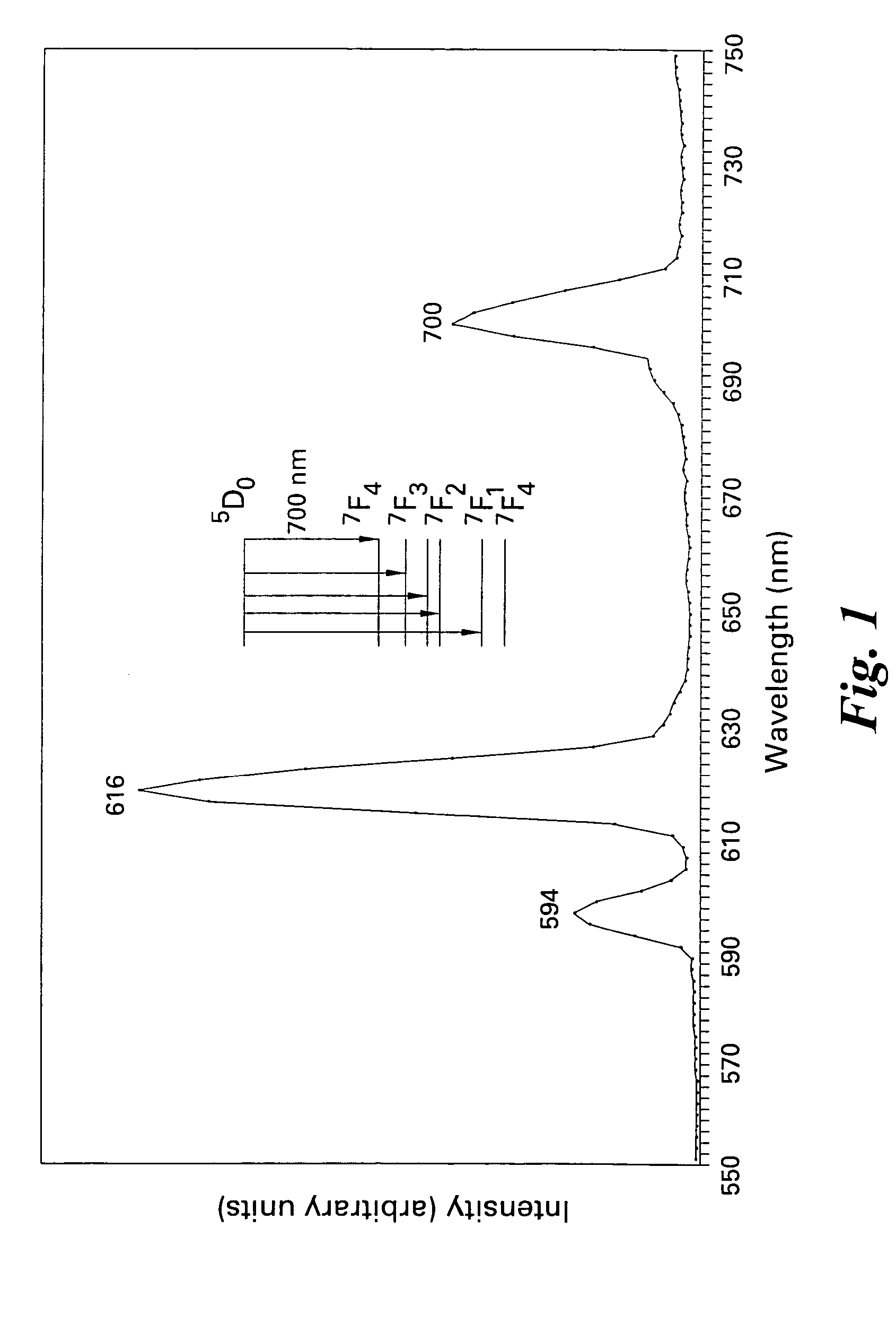
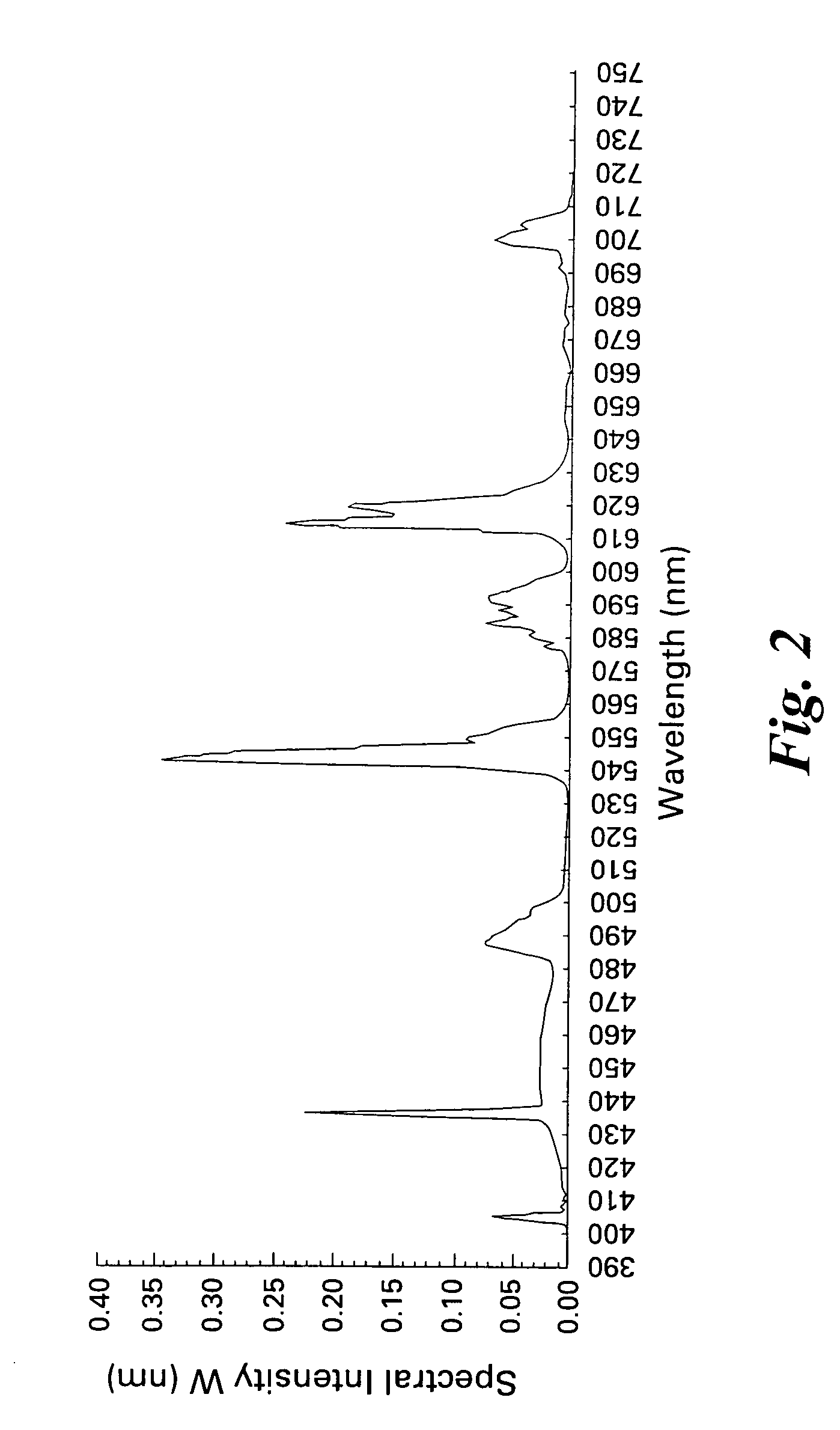
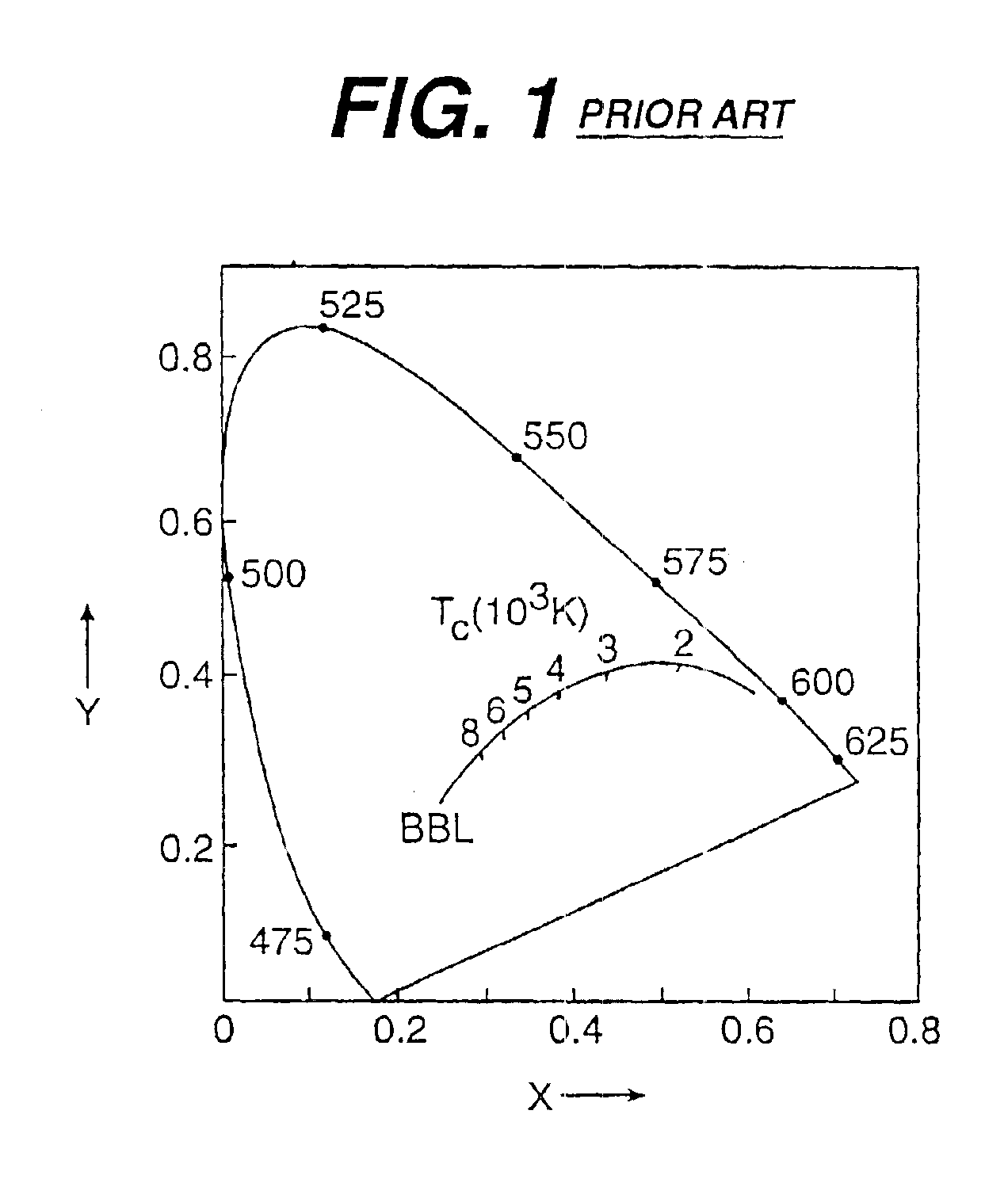
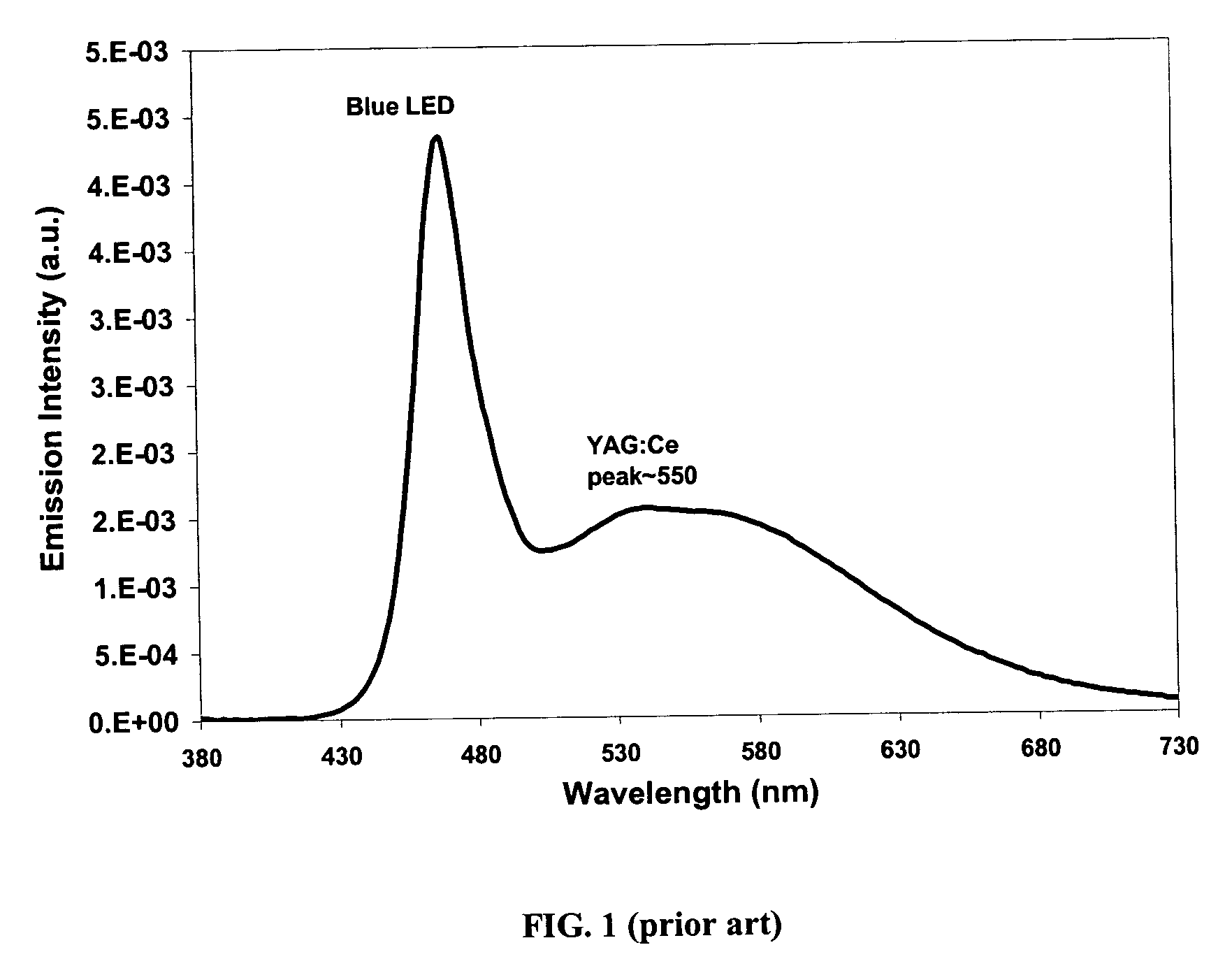
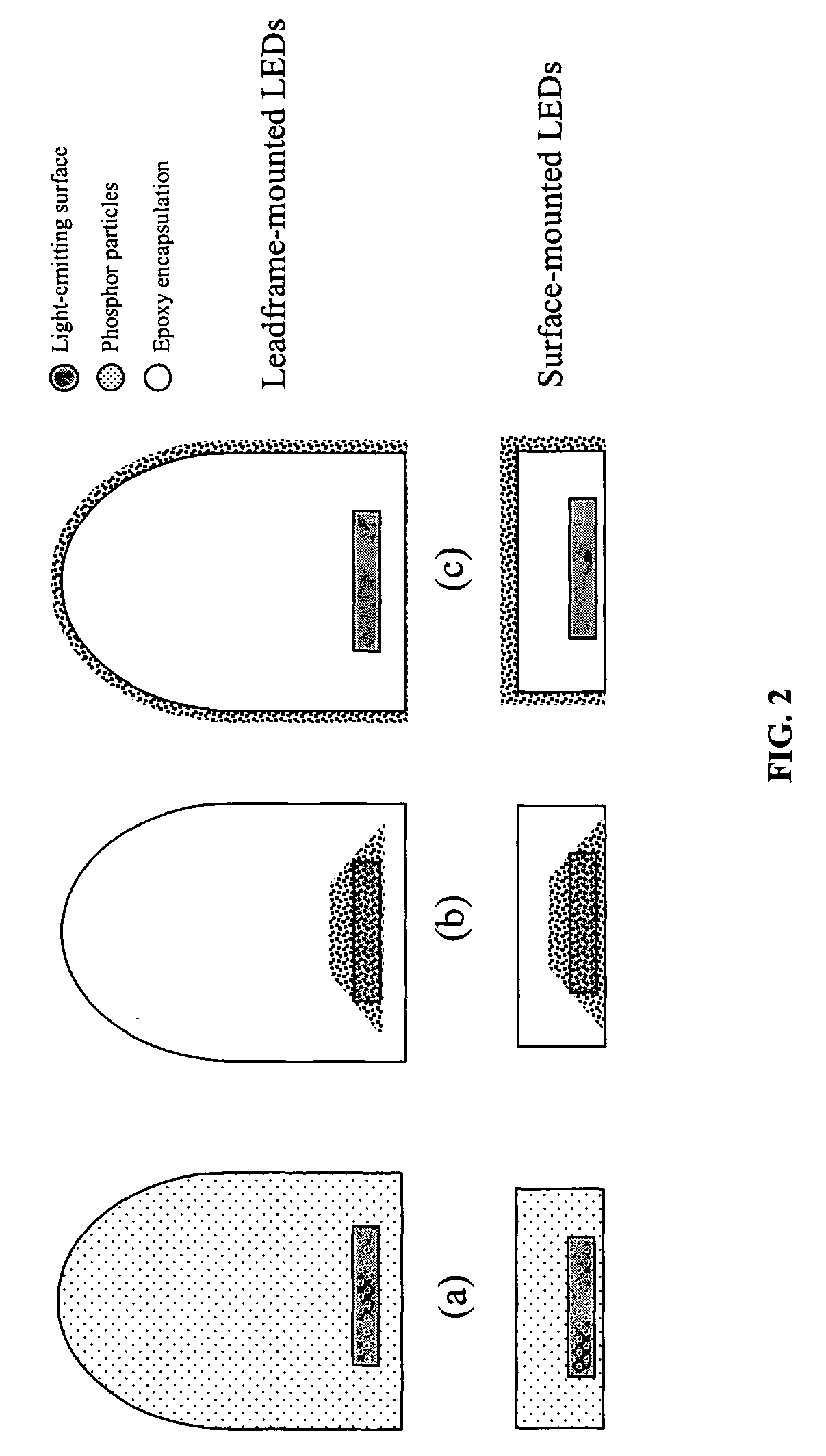
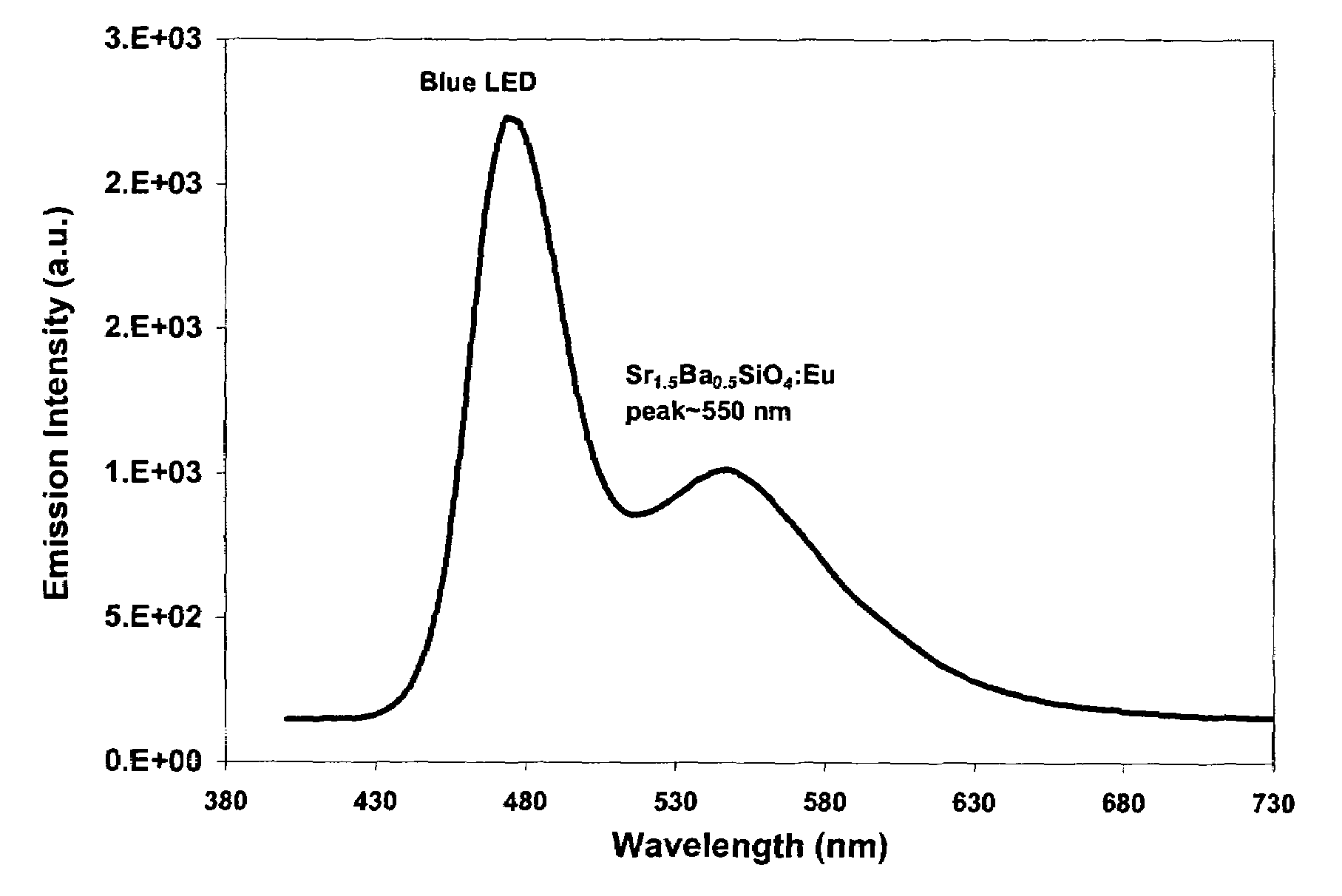
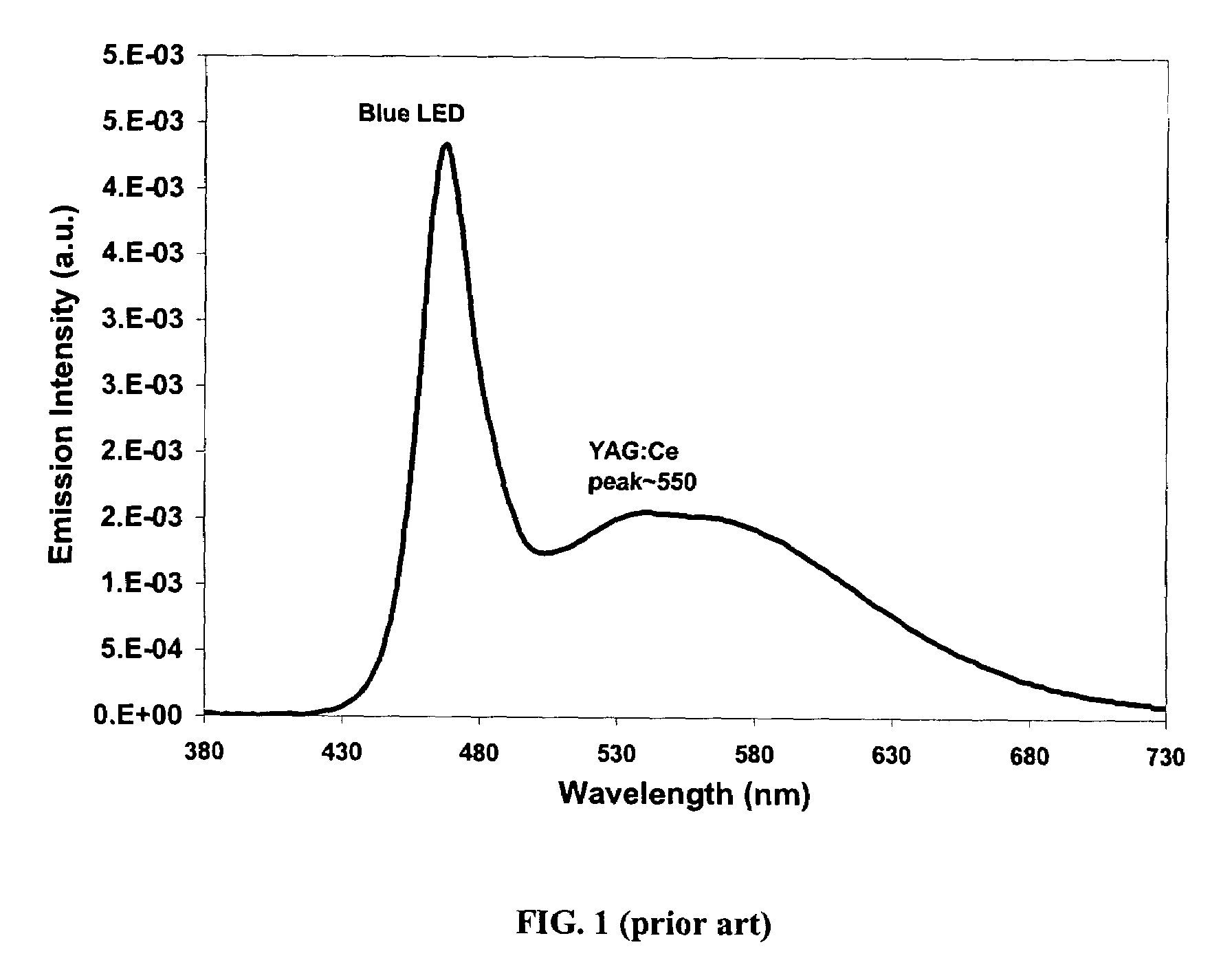
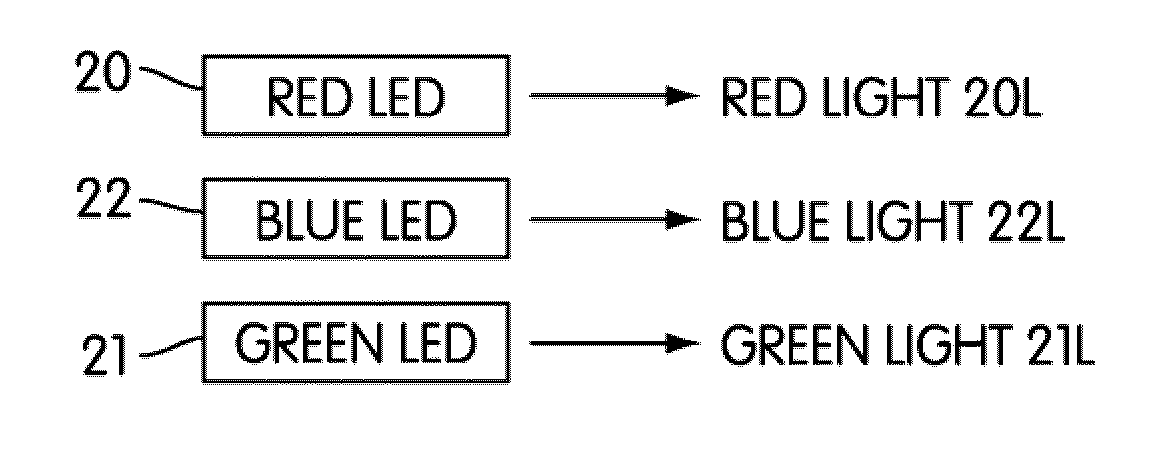
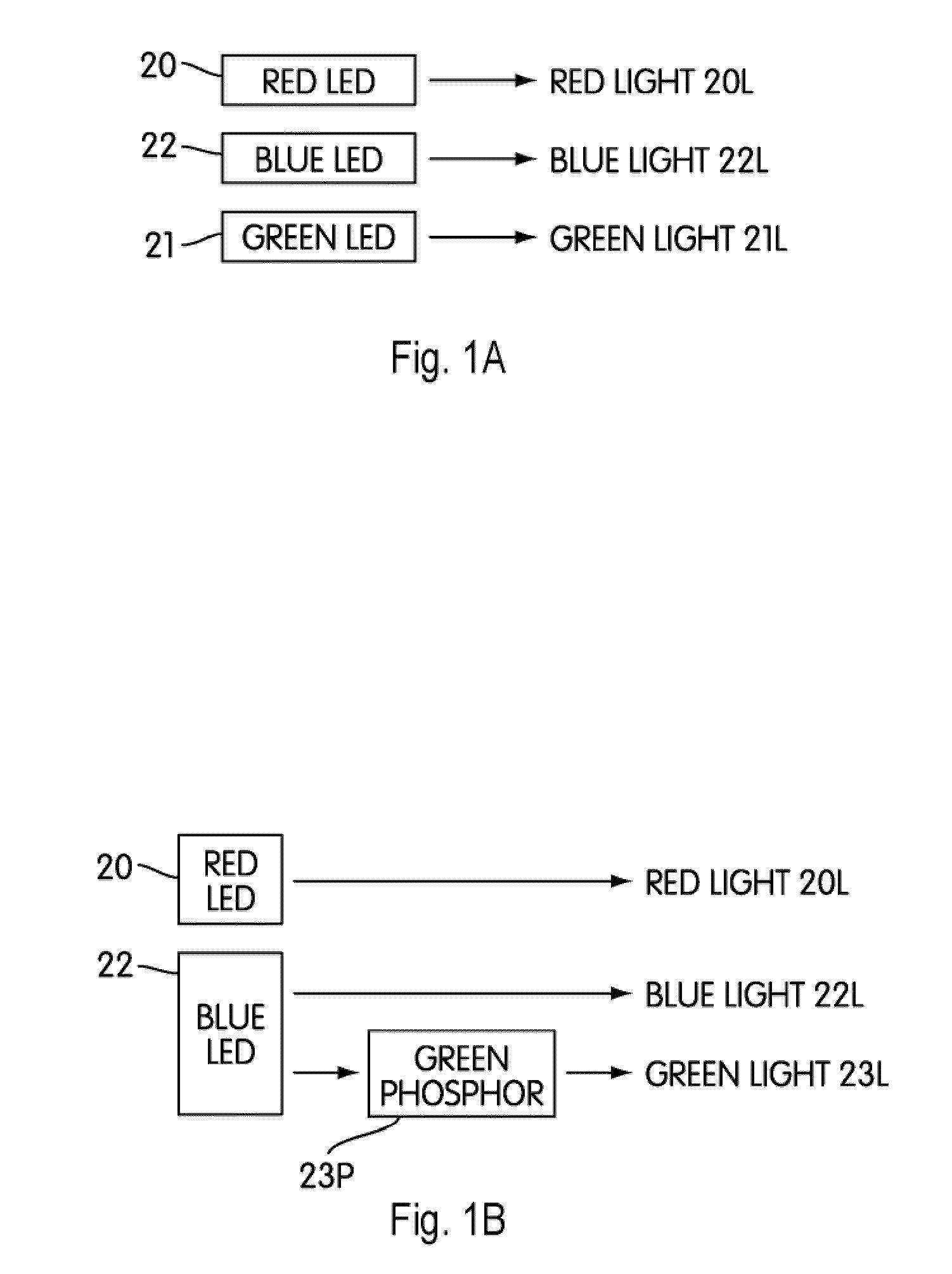
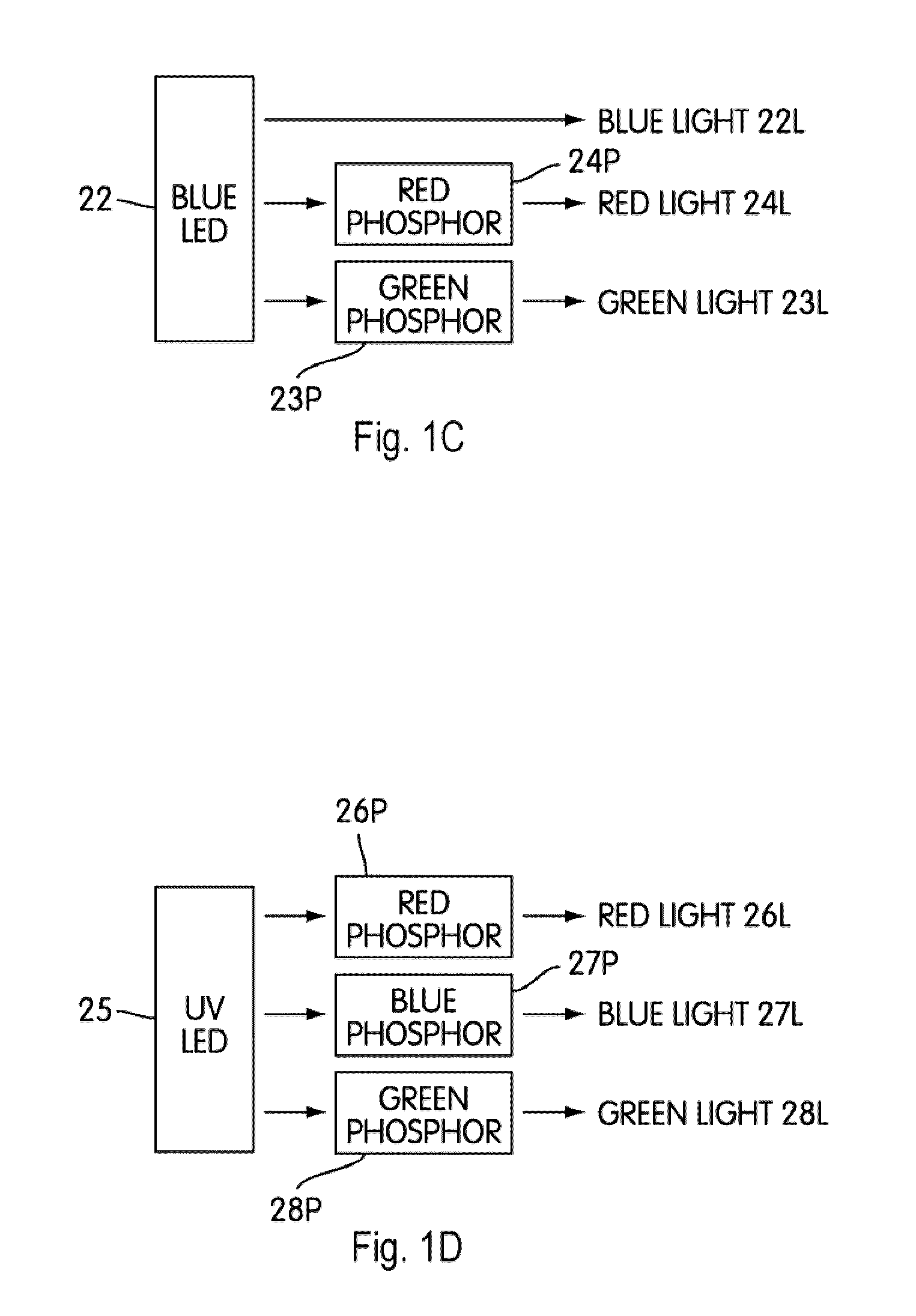
Patents
Literature
2684 results about "Europium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Europium is a chemical element with the symbol Eu and atomic number 63. Europium is the most reactive lanthanide by far, having to be stored under an inert fluid to protect it from atmospheric oxygen or moisture. Europium is also the softest lanthanide, as it can be dented with a finger nail and easily cut with a knife. When oxidation is removed a shiny-white metal is visible. Europium was isolated in 1901 and is named after the continent of Europe. Being a typical member of the lanthanide series, europium usually assumes the oxidation state +3, but the oxidation state +2 is also common. All europium compounds with oxidation state +2 are slightly reducing. Europium has no significant biological role and is relatively non-toxic compared to other heavy metals. Most applications of europium exploit the phosphorescence of europium compounds. Europium is one of the rarest of the rare earth elements on Earth.
Light source comprising a light-emitting element
InactiveUS6809347B2High luminous efficiencyHigh degreePlanar light sourcesPoint-like light sourceLuminophoreManganese
Owner:TOYODA GOSEI CO LTD
Phosphors containing boron and metals of Group IIIA and IIIB
A phosphor comprises: (a) at least a first metal selected from the group consisting of yttrium and elements of lanthanide series other than europium; (b) at least a second metal selected from the group consisting of aluminum, gallium, indium, and scandium; (c) boron; and (d) europium. The phosphor is used in light source that comprises a UV radiation source to convert UV radiation to visible light.
Owner:GENERAL ELECTRIC CO
Single phosphor for creating white light with high luminosity and high CRI in a UV LED device
InactiveUS6853131B2Avoids and reduces problemGas-filled discharge tubesDischarge tube luminescnet screensX-rayUltraviolet
There is provided a white light illumination system. The illumination system includes a radiation source which emits either ultra-violet (UV) or x-ray radiation. The illumination system also includes a luminescent material which absorbs the UV or x-ray radiation and emits the white light. The luminescent material has composition A2−2xNa1+xExD2V3O12. A may be calcium, barium, strontium, or combinations of these three elements. E may be europium, dysprosium, samarium, thulium, or erbium, or combinations thereof. D may be magnesium or zinc, or combinations thereof. The value of x ranges from 0.01 to 0.3, inclusive.
Owner:GENERAL ELECTRIC CO
Polymer nanocomposite implants with enhanced transparency and mechanical properties for administration within humans or animals
Polymer nanocomposite implants with nanofillers and additives are described. The nanofillers described can be any composition with the preferred composition being those composing barium, bismuth, cerium, dysprosium, europium, gadolinium, hafnium, indium, lanthanum, neodymium, niobium, praseodymium, strontium, tantalum, tin, tungsten, ytterbium, yttrium, zinc, and zirconium. The additives can be of any composition with the preferred form being inorganic nanopowders comprising aluminum, calcium, gallium, iron, lithium, magnesium, silicon, sodium, strontium, titanium. Such nanocomposites are particularly useful as materials for biological use in applications such as drug delivery, biomed devices, bone or dental implants.
Owner:PPG IND OHIO INC
Light emitting device having silicate fluorescent phosphor
InactiveUS20040227465A1Alternating current plasma display panelsGas discharge lamp usagePhysical chemistryLight emitting device
Provided herein are novel phosphors useful in the manufacture of white light emitting diodes. The phosphors provided by the invention are described by the formula: SrxBayCazSiO4:Eu in which x, y, and z are each independently variable to be any value between about 0 and about 2, including without limitation 0.001 and 2, and every thousandth therebetween, subject to the proviso that the sum of x, y, or z is equal to at least 1, and in which Eu is present in any amount between about 0.0001% and about 5% by weight based upon the phosphor's total weight, wherein substantially all of the europium present is present in the divalent state. A phosphor according to the invention may optionally further comprise an element selected from the group consisting of: Ce, Mn, Ti, Pb, and Sn and is present in any amount between about 0.0001% and about 5% by weight based on the phosphor's total weight. The silicate phosphor materials provided by the present invention do not require the addition of dissimilar blue and red phosphor compounds, and do not contain zinc and / or magnesium. In addition, the present invention provides materials which emit a broad yellowish color containing both green and red emissions. Standard techniques used in phosphor deposition for the manufacture of light emitting diodes which comprise phosphors may be employed to produce LED's having a white light output when the phosphors of the invention are utilized.
Owner:PHOSPHORTECH
Light emitting device having silicate fluorescent phosphor
InactiveUS6982045B2Improve efficiencyDischarge tube luminescnet screensLamp detailsFluorescenceProviding material
Provided herein are novel phosphors useful in the manufacture of white light emitting diodes. The phosphors provided by the invention are described by the formula:SrxBayCazSiO4:Euin which x, y, and z are each independently variable to be any value between about 0 and about 2, including without limitation 0.001 and 2, and every thousandth therebetween, subject to the proviso that the sum of x, y, or z is equal to at least 1, and in which Eu is present in any amount between about 0.0001% and about 5% by weight based upon the phosphor's total weight, wherein substantially all of the europium present is present in the divalent state. A phosphor according to the invention may optionally further comprise an element selected from the group consisting of: Ce, Mn, Ti, Pb, and Sn and is present in any amount between about 0.0001% and about 5% by weight based on the phosphor's total weight. The silicate phosphor materials provided by the present invention do not require the addition of dissimilar blue and red phosphor compounds, and do not contain zinc and / or magnesium. In addition, the present invention provides materials which emit a broad yellowish color containing both green and red emissions.Standard techniques used in phosphor deposition for the manufacture of light emitting diodes which comprise phosphors may be employed to produce LED's having a white light output when the phosphors of the invention are utilized.
Owner:PHOSPHORTECH
Light emitting device for generating specific colored light, including white light
InactiveUS6850002B2Improve efficiencyDischarge tube luminescnet screensElectric discharge tubesCeriumMetallic sulfide
A device for the generation of specific colored light including white light by luminescent down conversion and additive color mixing based on a light-emitting diode (LED) comprising a semiconductor light-emitting layer emitting near UV light about 370-420 nm or blue light about 420-480 nm and phosphors which absorb completely or partly the light emitted by the light-emitting component and emit light of wavelengths longer than that of the absorbed primary light, wherein the light emitting layer of the light emitting component is preferably a Ga(In)N-based semiconductor; and at least one of the phosphors contains a metal sulfide fluorescent material activated with europium containing at least one element selected from the group consisting of Ba, Sr, Ca, Mg and Zn; and / or at least another phosphor which contains a complex thiometalate fluorescent material activated with either europium, cerium or both europium and cerium containing 1) at least one element selected from the group consisting of Ba, Sr, Ca, Mg and Zn and 2) at least one element selected from the group consisting of Al, Ga, In, Y, La and Gd.
Owner:OSRAM OPTO SEMICONDUCTORS GMBH +1
Nitride-based, red-emitting phosphors
ActiveUS8274215B2Discharge tube luminescnet screensElectroluminescent light sourcesDisplay deviceEuropium
Embodiments of the present invention are directed to nitride-based, red-emitting phosphors in red, green, and blue (RGB) lighting systems, which in turn may be used in backlighting displays and warm white-light applications. In particular embodiments, the red-emitting phosphor is based on CaAlSiN3 type compounds activated with divalent europium. In one embodiment, the nitride-based, red emitting compound contains a solid solution of calcium and strontium compounds (Ca,Sr)AlSiN3:Eu2+, wherein the impurity oxygen content is less than about 2 percent by weight. In another embodiment, the (Ca,Sr)AlSiN3:Eu2+ compounds further contains a halogen in an amount ranging from about zero to about 2 atomic percent, where the halogen may be fluorine (F), chlorine (Cl), or any combination thereof. In one embodiment at least half of the halogen is distributed on 2-fold coordinated nitrogen (N2) sites relative to 3-fold coordinated nitrogen (N3) sites.
Owner:INTEMATIX
Inorganic material that has metal nanoparticles that are trapped in a mesostructured matrix
An inorganic material that consists of at least two elementary spherical particles, each of said spherical particles comprising metal nanoparticles that are between 1 and 300 nm in size and a mesostructured matrix with an oxide base of at least one element X that is selected from the group that consists of aluminum, titanium, tungsten, zirconium, gallium, germanium, tin, antimony, lead, vanadium, iron, manganese, hafnium, niobium, tantalum, yttrium, cerium, gadolinium, europium and neodymium is described, whereby said matrix has a pore size of between 1.5 and 30 nm and has amorphous walls with a thickness of between 1 and 30 nm, said elementary spherical particles having a maximum diameter of 10 μm. Said material can also contain zeolitic nanocrystals that are trapped within said mesostructured matrix.
Owner:INST FR DU PETROLE
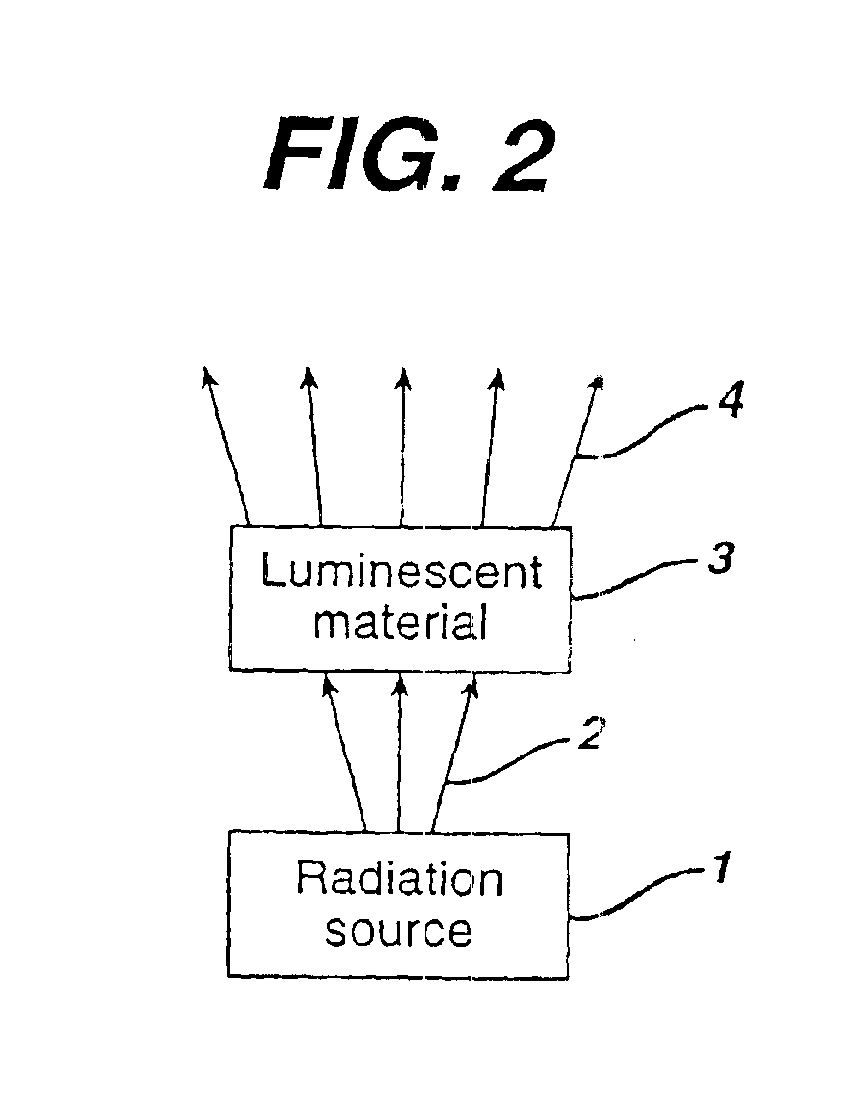
Illumination system comprising a radiation source and a fluorescent material
ActiveUS20060255710A1High temperature resistancePointing stableDischarge tube luminescnet screensElectroluminescent light sourcesCeriumEuropium
The invention concerns an illumination system for generation of colored, especially amber or red light, comprising a radiation source and a fluorescent material comprising at least one phosphor capable of absorbing a part of light emitted by the radiation source and emitting light of wavelength different from that of the absorbed light; wherein said at least one phosphor is a amber to red emitting a rare earth metal-activated oxonitridoalumosilicate of general formula (Ca1−x−y−zSrxBayMgz)1−n(Al1−a+bBa)Si1−bN3−bOb:REn, wherein 0≦x≦1, 0≦y≦1, 0≦z≦1, 0≦a≦1, 0<b≦1 and 0.002≦n≦0.2 and RE is selected from europium(II) and cerium(III).
Owner:PHILIPS LUMILEDS LIGHTING CO LLC +1
Red light emitting long afterglow photoluminescence phosphor and afterglow lamp thereof
InactiveUS20010043042A1Simple structureLow costDischarge tube luminescnet screensLamp detailsPhotoluminescenceRare earth
A red light emitting afterglow photoluminescence phosphor is a rare earth oxysulfide phosphor activated by Europium, which chemical formula is follows: Ln2O2S:Eux,My 0.00001<=x<=0.5 0.00001<=y<=0.3 wherein Ln is at least one selected from the group consisting of Y, La, Gd and Lu; M is a coactivator which is at least one selected from the group consisting of Mg, Ti, Nb, Ta and Gain the chemical formula.
Owner:NICHIA CORP
Inorganic material that has metal nanoparticles that are trapped in a mesostructured matrix
An inorganic material that consists of at least two elementary spherical particles, each of said spherical particles comprising metal nanoparticles that are between 1 and 300 nm in size and a mesostructured matrix with an oxide base of at least one element X that is selected from the group that consists of aluminum, titanium, tungsten, zirconium, gallium, germanium, tin, antimony, lead, vanadium, iron, manganese, hafnium, niobium, tantalum, yttrium, cerium, gadolinium, europium and neodymium is described, whereby said matrix has a pore size of between 1.5 and 30 nm and has amorphous walls with a thickness of between 1 and 30 nm, said elementary spherical particles having a maximum diameter of 10 μm. Said material can also contain zeolitic nanocrystals that are trapped within said mesostructured matrix.
Owner:INST FR DU PETROLE
Light source comprising a light-emitting element
InactiveUS20040090174A1High luminous efficiencyHigh degreePlanar light sourcesPoint-like light sourceAlkaline earth metalLuminophore
The invention relates to a light source comprising a light-emitting element, which emits light in a first spectral region, and comprising a luminophore, which comes from the group of alkaline-earth orthosilicates and which absorbs a portion of the light emitted by the light source and emits light in another spectral region. According to the invention, the luminophore is an alkaline-earth orthosilicate, which is activated with bivalent europium and whose composition consists of: (2-x-y)SrOx(Ba, Ca)O(1-a-b-c-d)SiO2aP2O5bAl2O3cB2O3dGeO2:yEu<2+> and / or (2-x-y)BaOx((Sr, Ca)O(1-a-b-c-d)SiO2aP2O5bAl2O3cB2O3dGeO2:yEu<2+>. The desired color (color temperature) can be easily adjusted by using a luminophore of the aforementioned type. The light source can contain an additional luminophore selected from the group of alkaline-earth aluminates, activated with bivalent europium and / or manganese, and / or can contain an additional red-emitting luminophore selected from the group Y(V, P, Si)O4:Eu or can contain alkaline-earth magnesium disilicate.
Owner:TOYODA GOSEI CO LTD
Light-emitting device
InactiveUS20070052342A1Improve efficiencyGood colorDischarge tube luminescnet screensElectroluminescent light sourcesCeriumLength wave
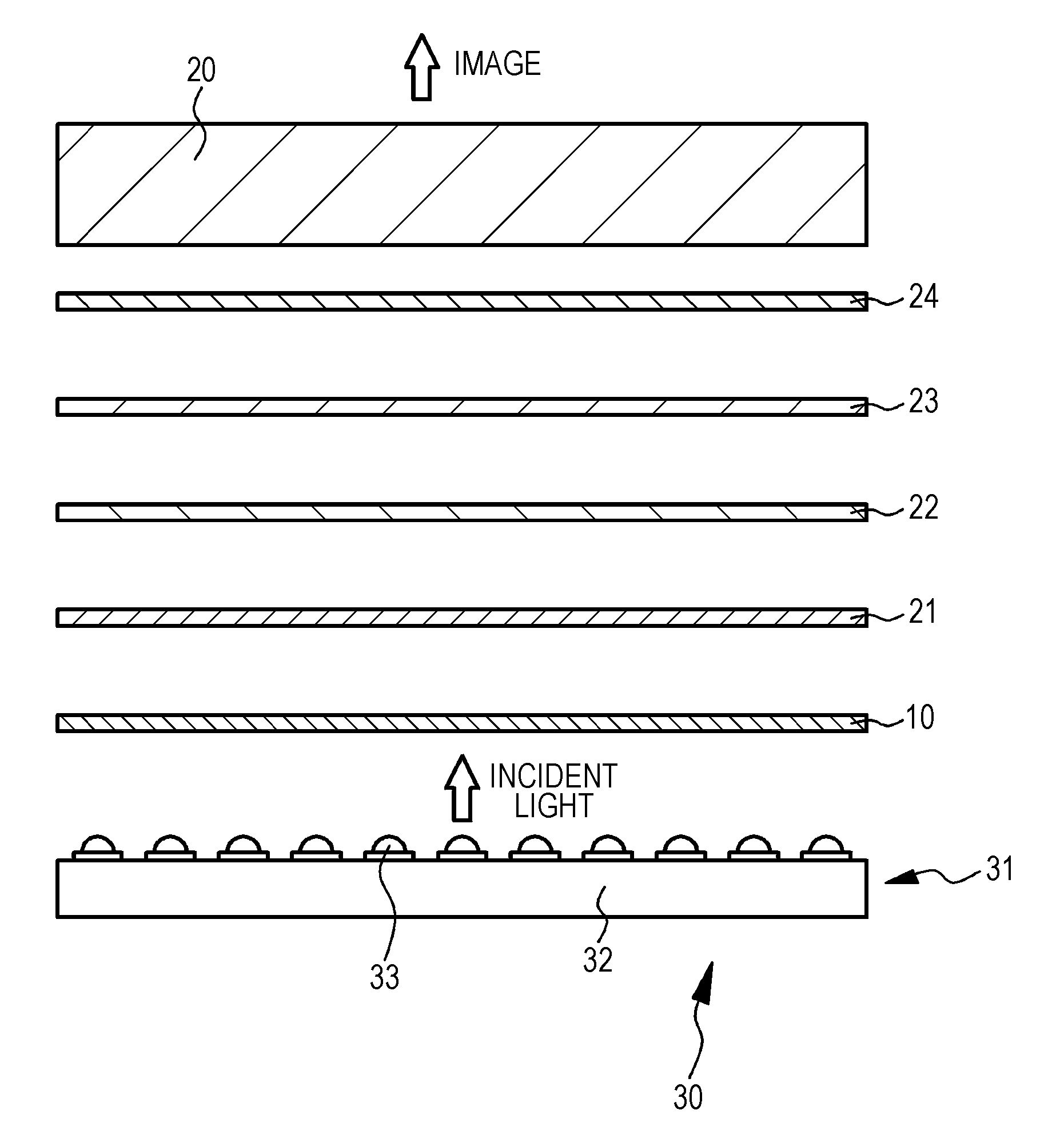
A light-emitting device includes a light-emitting element emitting primary light and a wavelength conversion portion absorbing a part of the primary light and emitting secondary light having a wavelength equal to or longer than the wavelength of the primary light. The wavelength conversion portion includes a plurality of green or yellow light-emitting phosphors and a plurality of red light-emitting phosphors. The green or yellow light-emitting phosphor is implemented by at least one selected from a specific europium (II)-activated silicate phosphor (A-1) and a specific cerium (III)-activated silicate phosphor (A-2). The red light-emitting phosphor is implemented by a specific europium (II)-activated nitride phosphor (B). The light-emitting device emitting white light at efficiency and color rendering property higher than in a conventional example can thus be provided.
Owner:SHARP KK
Phosphor, method of producing the same, and light emitting apparatus
ActiveUS20080258602A1Improve efficiencyStable characteristicsMaterial nanotechnologyDischarge tube luminescnet screensLength waveNitride phosphor
There are provided a phosphor which is a divalent europium-activated oxynitride phosphor substantially represented by General formula (A): EuaSibAlcOdNe, a divalent europium-activated oxynitride phosphor substantially represented by General formula (B): MIfEugSihAlkOmNn or a divalent europium-activated nitride phosphor substantially represented by General formula (C): (MIIl-pEup)MIIISiN3, having a reflectance of light emission in a longer wavelength region of visible light than a peak wavelength of 95% or larger, and a method of producing such phosphor; a nitride phosphor and an oxynitride phosphor which emit light efficiently and stably by the light having a wavelength ranging from 430 to 480 nm from a semiconductor light emitting device by means of a light emitting apparatus using such phosphor, and a producing method of such phosphor; and a light emitting apparatus having stable characteristics and realizing high efficiency.
Owner:DENKA CO LTD +1
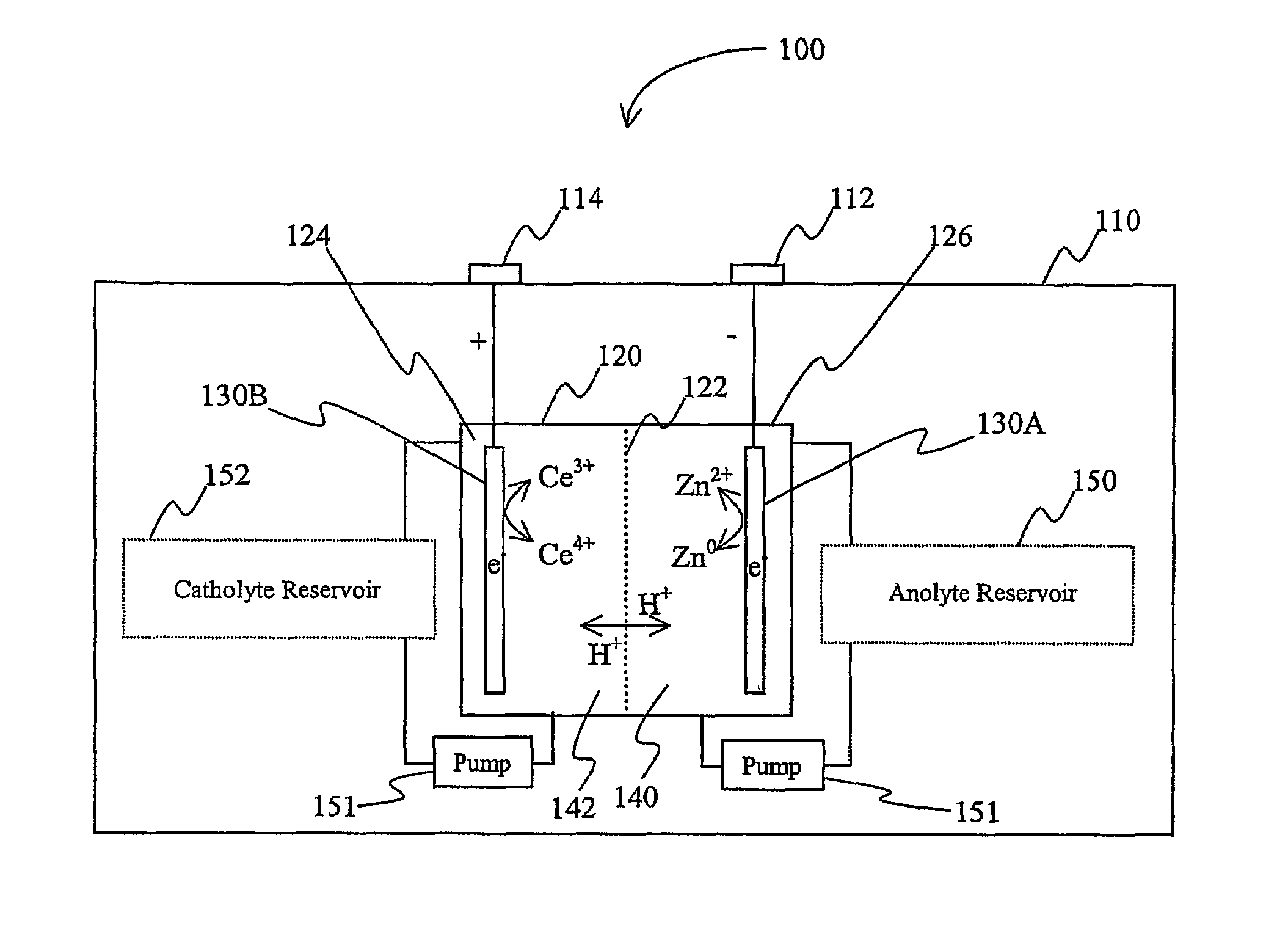
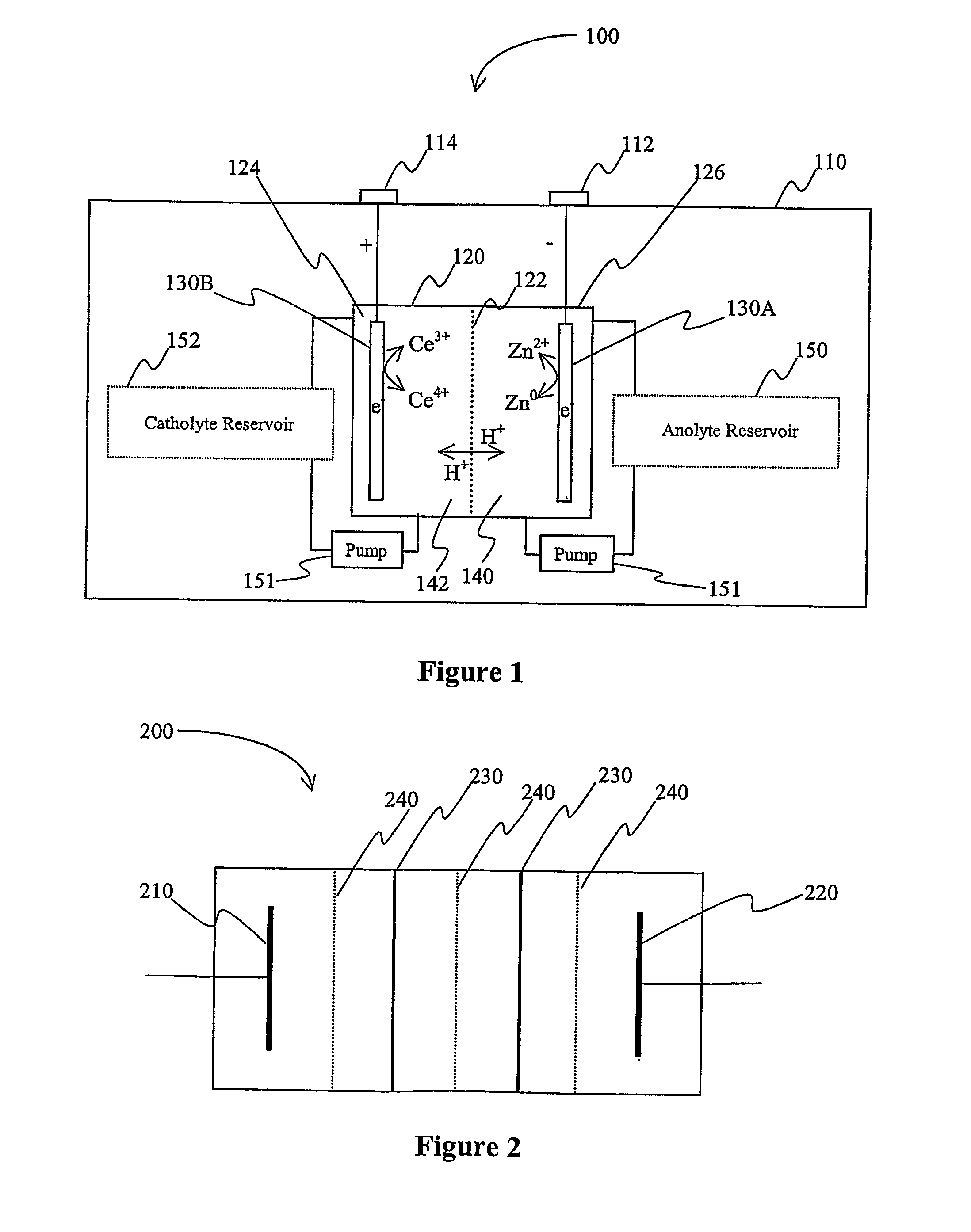
Lanthanide batteries
InactiveUS7252905B2Wide capacity rangeLarge capacityAlkaline accumulatorsSolid electrolyte cellsLanthanideCerium
A battery (100) comprises an electrolyte in which a lanthanide and zinc form a redox pair. Preferred electrolytes are acid electrolytes, and most preferably comprise methane sulfonic acid, and it is further contemplated that suitable electrolytes may include at least two lanthanides. Contemplated lanthanides include cerium, praseodymium, neodymium, terbium, and dysprosium, and further contemplate lanthanides are samarium, europium, thulium and ytterbium.
Owner:PLURION LTD
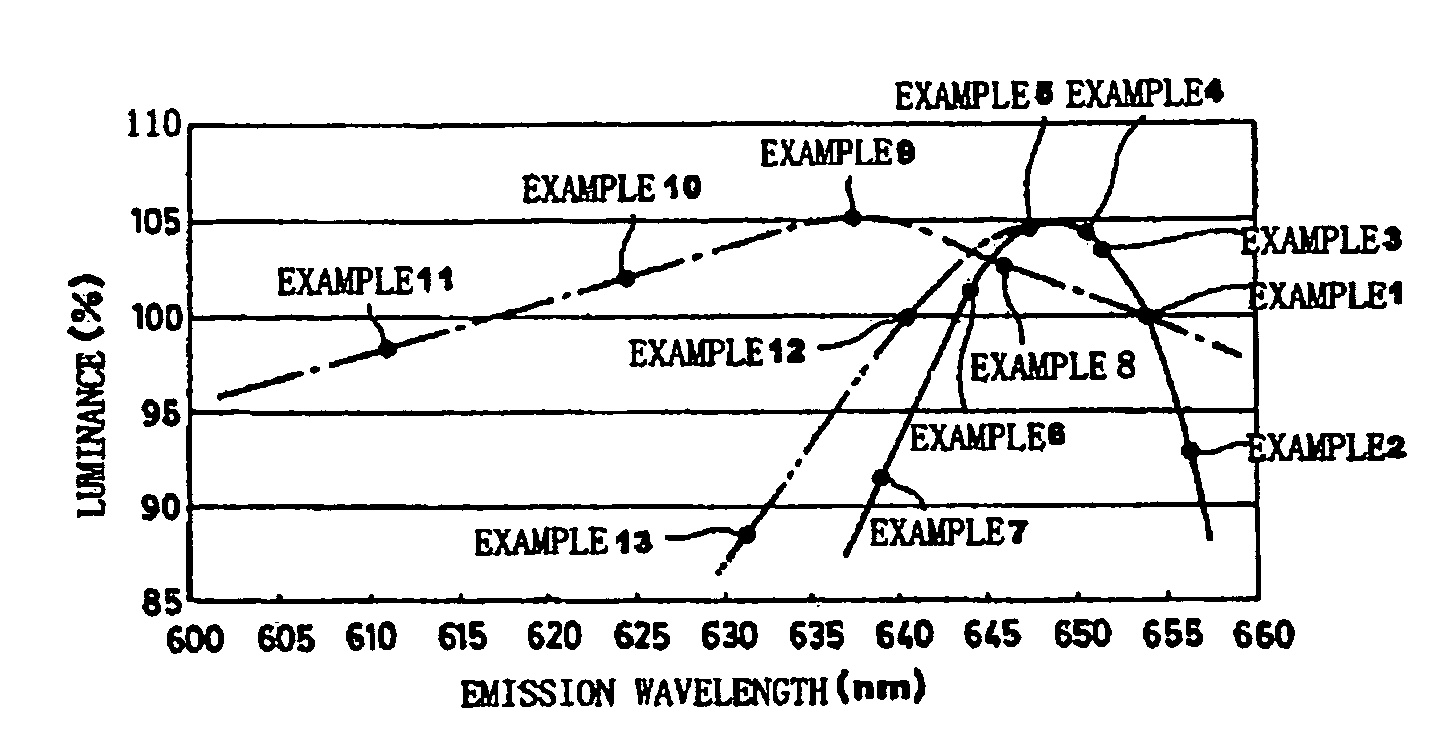
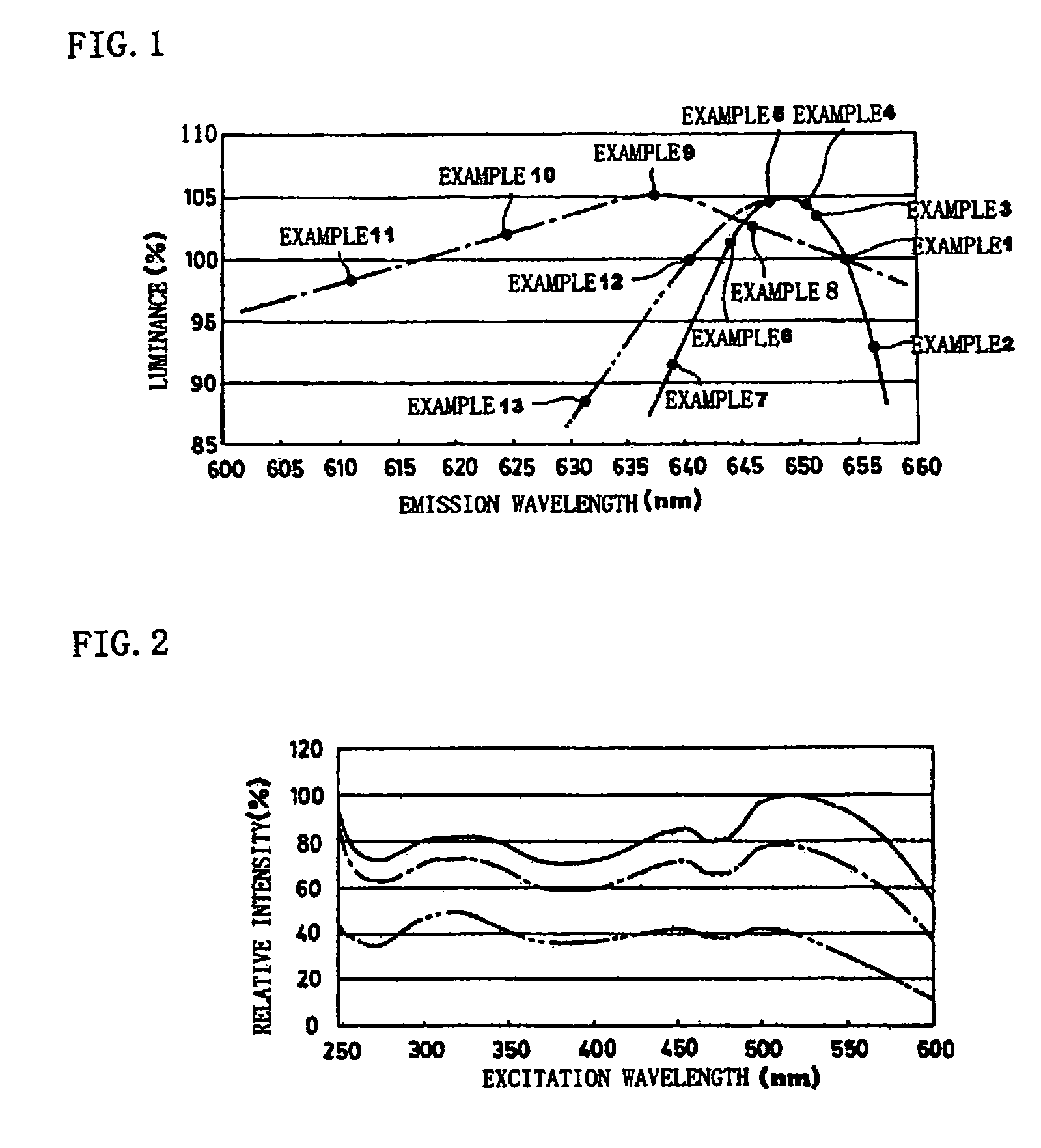
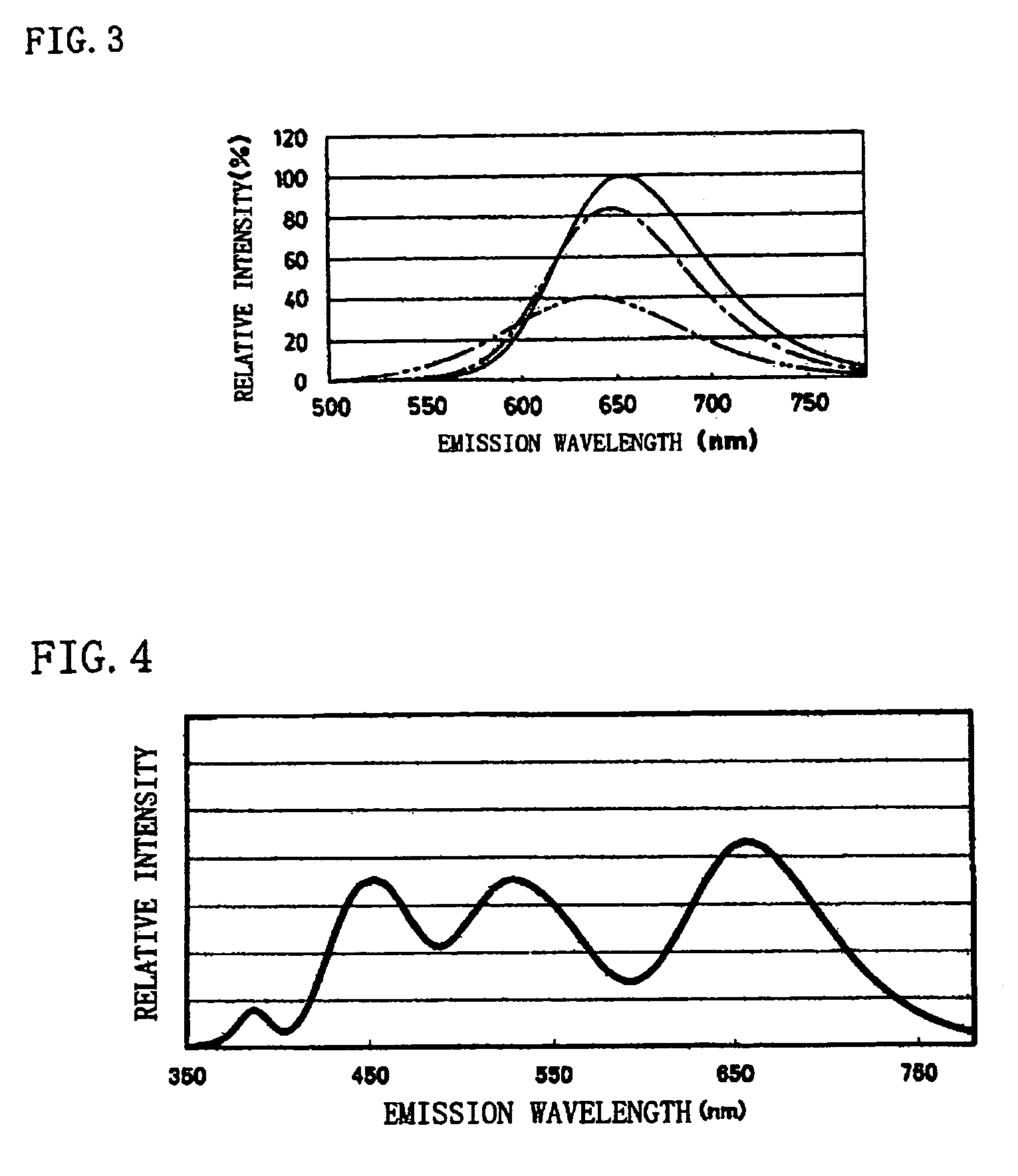
Phosphor and production method of the same, method of shifting emission wavelength of phosphor, and light source and LED
ActiveUS7273568B2High sensitivityImprove luminous performanceDischarge tube luminescnet screensLamp detailsOxygenEuropium
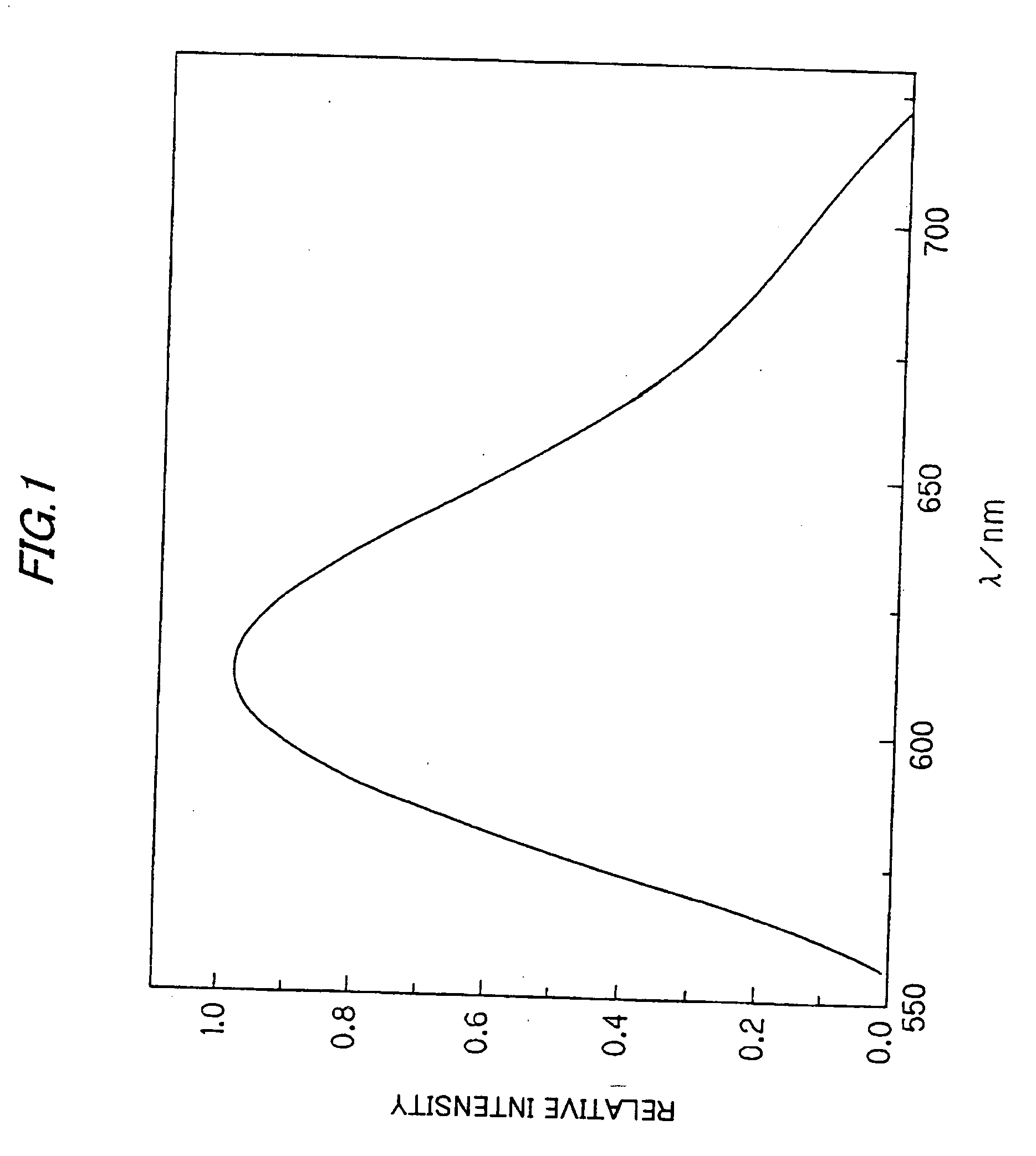
A phosphor including a main production phase of a phosphor expressed by a composition formula of MmAaBbOoNn:Zz (where an element M is one or more bivalent elements, an element A is one or more trivalent elements, an element B is one or more tetravalent elements, O is oxygen, N is nitrogen, an element Z is an activator, n=2 / 3m+a+4 / 3b−2 / 3o, m / (a+b)≧1 / 2, (o+n) / (a+b)>4 / 3, wherein m=a=b=1 and o and n is not 0). A phosphor including 24 wt % to 30 wt % of Ca (calcium), 17 wt % to 21 wt % of Al (aluminum), 18 wt % to 22 wt % of Si (silicon), 1 wt % to 15 wt % of oxygen, 15 wt % to 33 wt % of nitrogen and 0.01 wt % to 10 wt % of Eu (europium), wherein an emission maximum in an emission spectrum is in a range of 600 nm to 660 nm; and wherein color chromaticity x of light emission is in a range of 0.5 to 0.7, and color chromaticity y of the light emission is in a range of 0.3 to 0.5.
Owner:NICHIA CORP
Ceramic bonding composition, method of making, and article of manufacture incorporating the same
A ceramic bonding composition comprises a first oxide and at least a second oxide having a formula of Me2O3; wherein the first oxide is selected from the group consisting of aluminum oxide, scandium oxide, and combinations thereof; Me is selected from the group consisting of yttrium, lanthanum, cerium, praseodymium, neodymium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, lutetium, and combinations thereof. The ceramic bonding composition can further comprise silica. An article of manufacture comprising at least two members attached together with the ceramic bonding composition.
Owner:GENERAL ELECTRIC CO
Nitride-based red-emitting phosphors in RGB red-green-blue lighting systems
ActiveUS20100308712A1Low levelDischarge tube luminescnet screensElectroluminescent light sourcesDisplay deviceEffect light
Embodiments of the present invention are directed to nitride-based, red-emitting phosphors in red, green, and blue (RGB) lighting systems, which in turn may be used in backlighting displays and warm white-light applications. In particular embodiments, the red-emitting phosphor is based on CaAlSiN3 type compounds activated with divalent europium. In one embodiment, the nitride-based, red emitting compound contains a solid solution of calcium and strontium compounds (Ca,Sr)AlSiN3:Eu2+, wherein the impurity oxygen content is less than about 2 percent by weight. In another embodiment, the (Ca,Sr)AlSiN3:Eu2+ compounds further contains a halogen in an amount ranging from about zero to about 2 atomic percent, where the halogen may be fluorine (F), chlorine (Cl), or any combination thereof. In one embodiment at least half of the halogen is distributed on 2-fold coordinated nitrogen (N2) sites relative to 3-fold coordinated nitrogen (N3) sites.
Owner:INTEMATIX
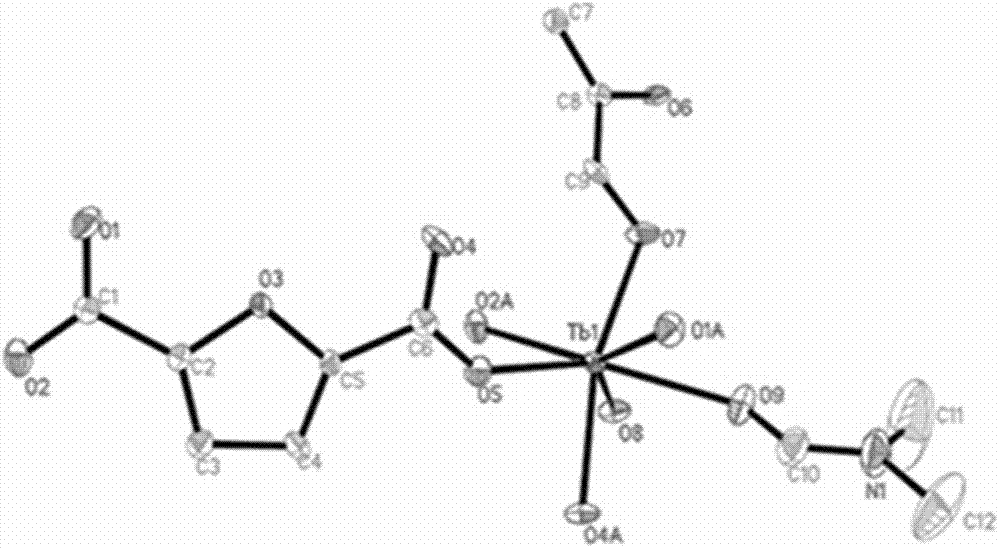
Multifunctional rare earth metal-organic framework and preparation method thereof
InactiveCN102757453ANovel structureSensitive recognitionCarbon compoundsOther chemical processesCooking & bakingN dimethylformamide
The invention relates to a multifunctional rare earth metal-organic framework of which the chemical formula is {[Ln(FDA)1.5(DMF)].DMF}n, wherein Ln is Eu or Tb, the FDA is furyl-2,5-dicarboxylic acid, and the DMF is N,N-dimethylformamide; and the rare earth metal framework structure has an Eu<3+> / Tb<3+> ion in a coordination environment, and forms a three-dimensional framework structure containing one-dimensional honeycomb channels. The preparation method comprises the following steps: by using europium nitrate hexahydrate or terbium nitrate hexahydrate as a metal salt, H2FDA as a ligand and DMF as a solvent, stirring the mixed solution, baking, separating and washing the solid to obtain the multifunctional rare earth metal-organic framework. The multifunctional rare earth metal-organic framework provided by the invention has an fluorescent identification function on cancerigenic organic solvents, such as methylbenzene, benzene and acetone, has excellent selective adsorptivity for gas and high acting force with H2, contains high adsorptive enthalpy, and thus, is a multifunctional novel-structure microporous MOF (metal-organic framework) material.
Owner:NANKAI UNIV
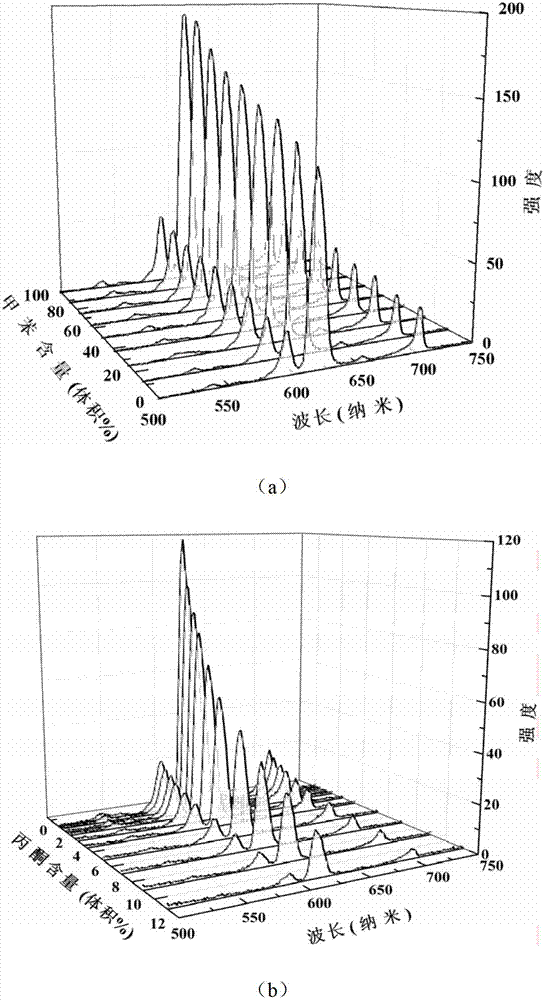
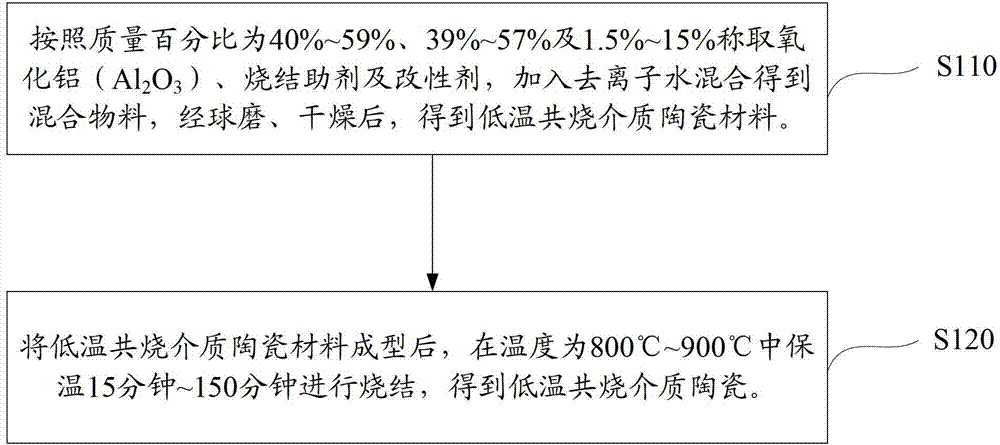
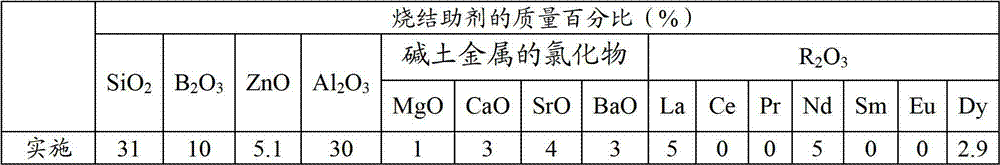
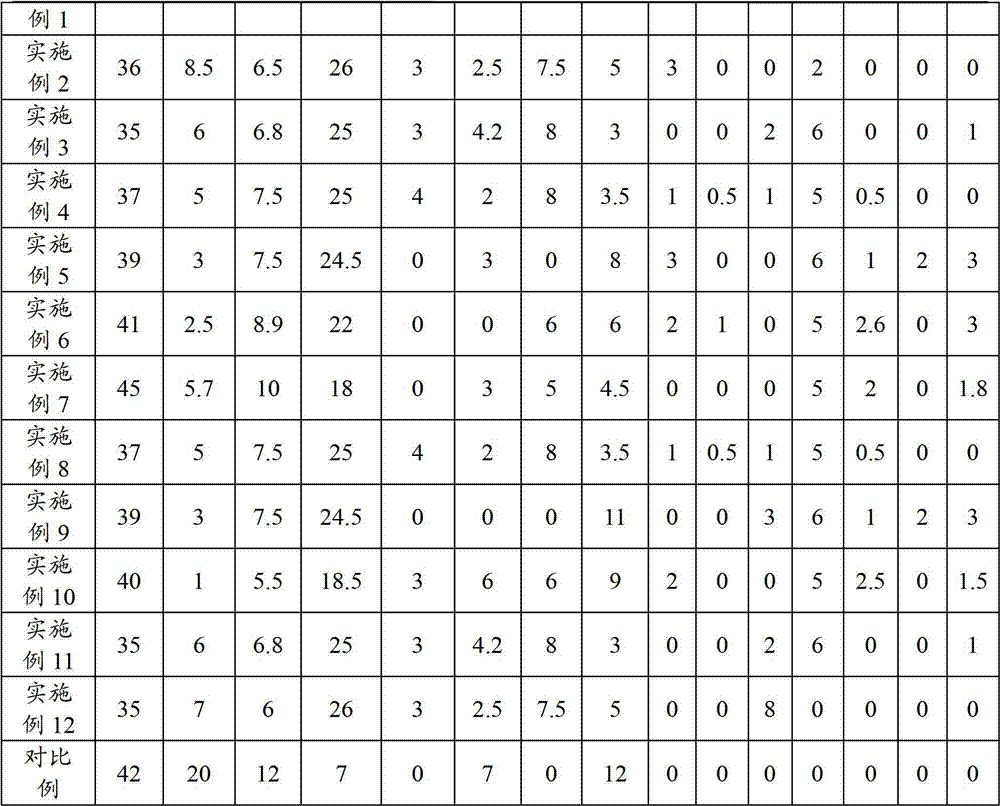
Method, sintering aid and materials for preparation of low-temperature cofired medium ceramic and application
A sintering aid for a low-temperature cofired medium ceramic material is composed of, by weight, 31%-45% of silicon dioxide, 1%-10% of boron oxide, 5.1%-10% of zinc oxide, 18%-30% of aluminum oxide, 11%-24% of alkaline earth metallic oxide and 5%-15% of oxide with the general formula of R2O3, wherein R refers to at least one of lanthanum, cerium, praseodymium, neodymium, samarium, europium and dysprosium, and the alkaline earth metallic oxide refers to one of magnesium oxide, calcium oxide, barium oxide and strontium oxide. Adding the sintering aid into the low-temperature cofired medium ceramic material enables the prepared low-temperature cofired medium ceramic to have excellent thermal mechanical performance and dielectric performance. In addition, the invention provides the low-temperature cofired medium ceramic material and application thereof and a method for preparing the low-temperature cofired medium ceramic.
Owner:GUANGDONG FENGHUA ADVANCED TECH HLDG
Light-emitting device
ActiveUS20110043101A1Improve color gamutEffective absorptionDischarge tube luminescnet screensElectroluminescent light sourcesManganeseGreen-light
Disclosed is a light-emitting device including a light-emitting element emitting primary light, and a light converter absorbing a part of the primary light emitted from the light-emitting element and emitting secondary light having a longer wavelength than the primary light. The light converter contains a green light-emitting phosphor and a red light-emitting phosphor. The green light-emitting phosphor is composed of at least one phosphor selected from a divalent europium-activated oxynitride phosphor substantially represented by the following formula: EuaSibAlcOdNe and a divalent europium-activated silicate phosphor substantially represented by the following formula: 2(Ba1-f-gMIfEug)O.SiO2, while the red light-emitting phosphor is composed of at least one phosphor selected from tetravalent manganese-activated fluoro-tetravalent metalate phosphors substantially represented by the following formulae: MII2(MIII1-hMnh)F6 and / or MIV(MIII1-hMnh)F6. Consequently, the light-emitting device has excellent color gamut (NTSC ratio).
Owner:GE PHOSPHORS TECH LLC
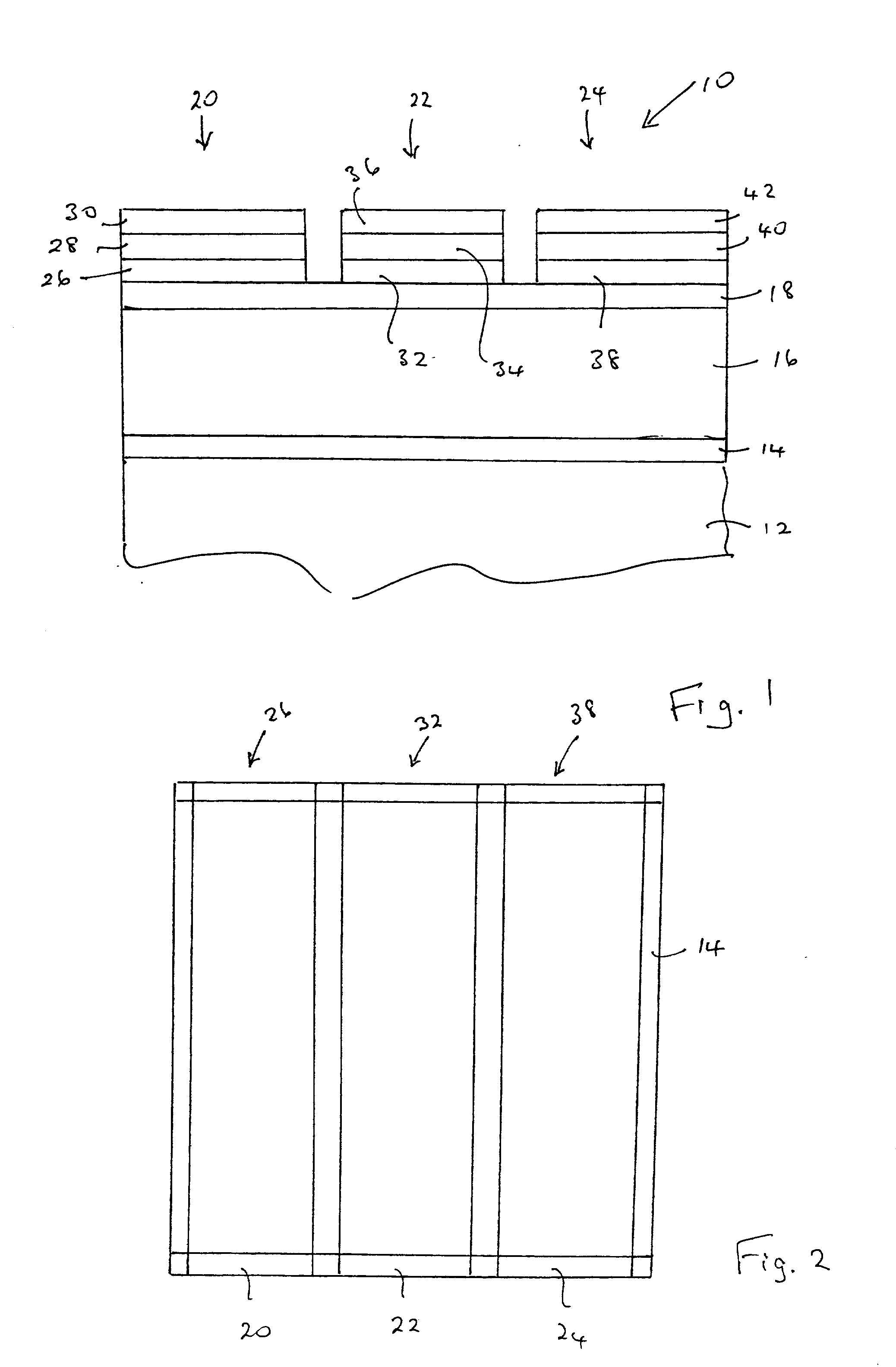
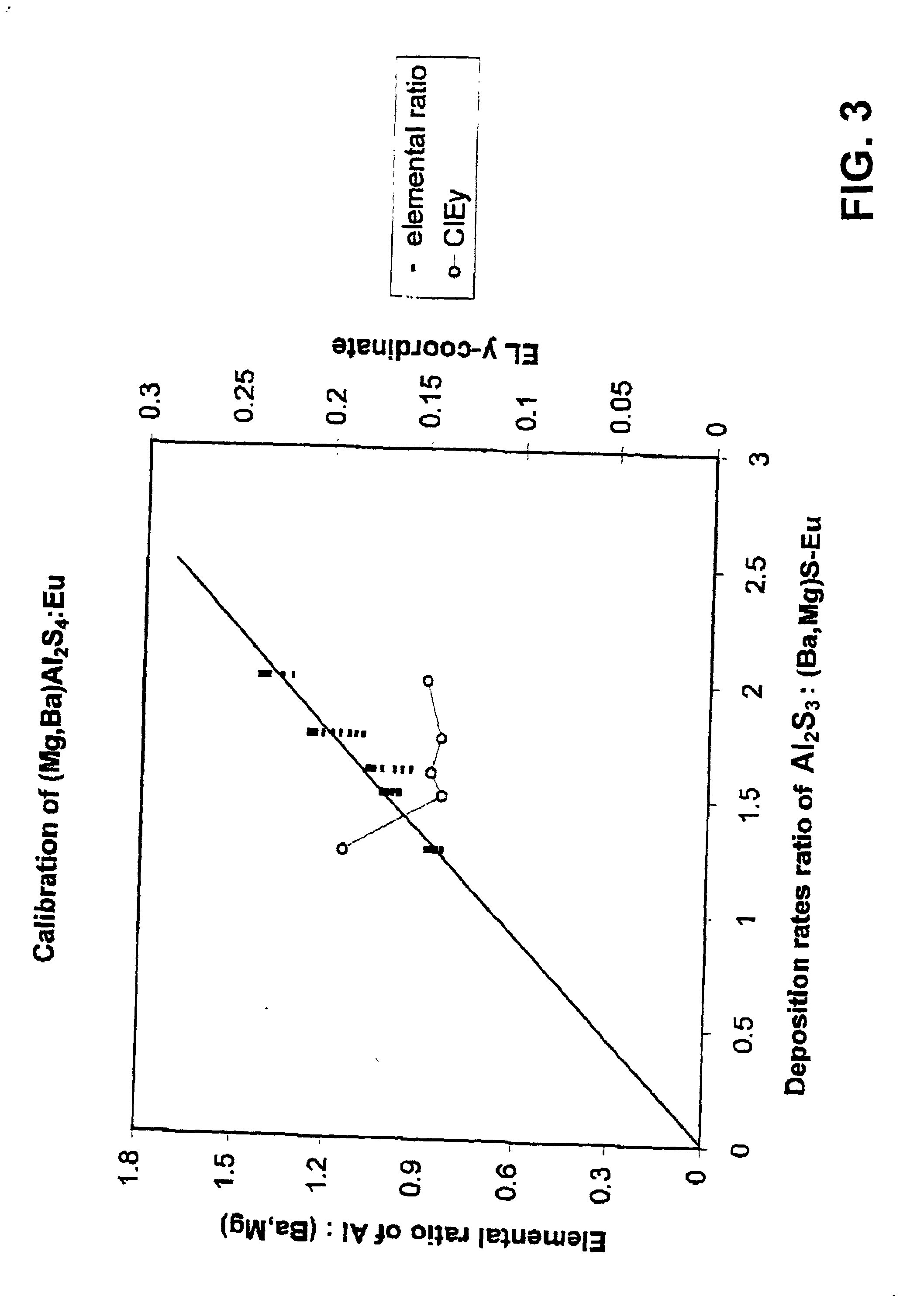
Magnesium barium thioaluminate and related phosphor materials
A phosphor and a method of deposition. The phosphor comprises a composition of the formula M'aBa1-aM''2M'''4':RE, where M' is at least one element selected from magnesium and calcium, M'' is at least one element selected from aluminum, gallium and indium, M''' is at least one element selected from sulphur, selenium and tellurium, RE is at least one rare earth element, especially europium or cerium, and 0<a<1. Deposition is preferably by dual source electron beam deposition. The phosphor may be annealed. The phosphor provides a high luminosity blue emission that does not require an optical filter to achieve acceptable colour coordinates for the blue sub-pixel element for a full colour thin film or thick film electroluminescent display. The blue sub-pixel pixel performance meets the luminosity and colour temperature specifications for current generation cathode ray tube displays.
Owner:IFIRE TECH INC
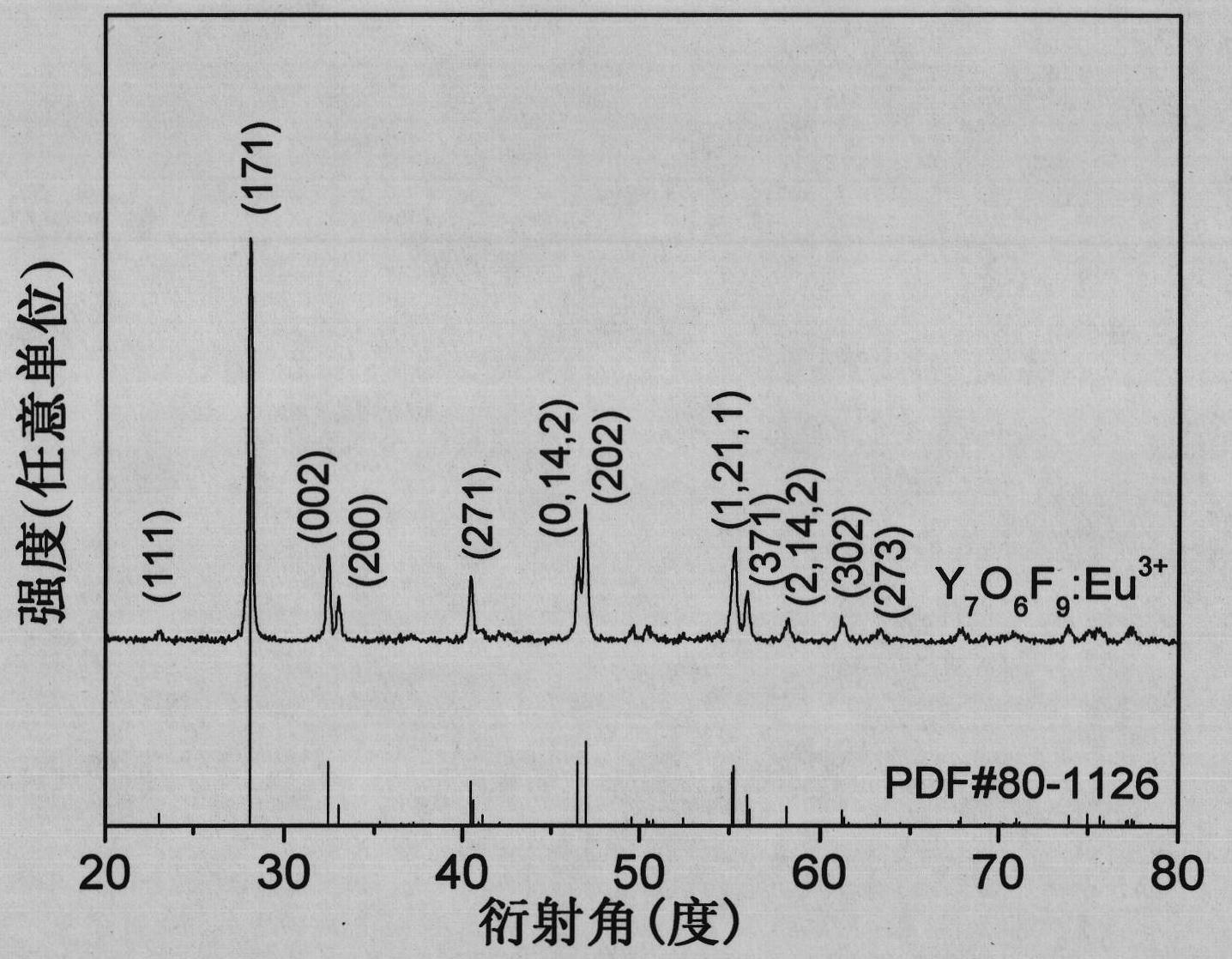
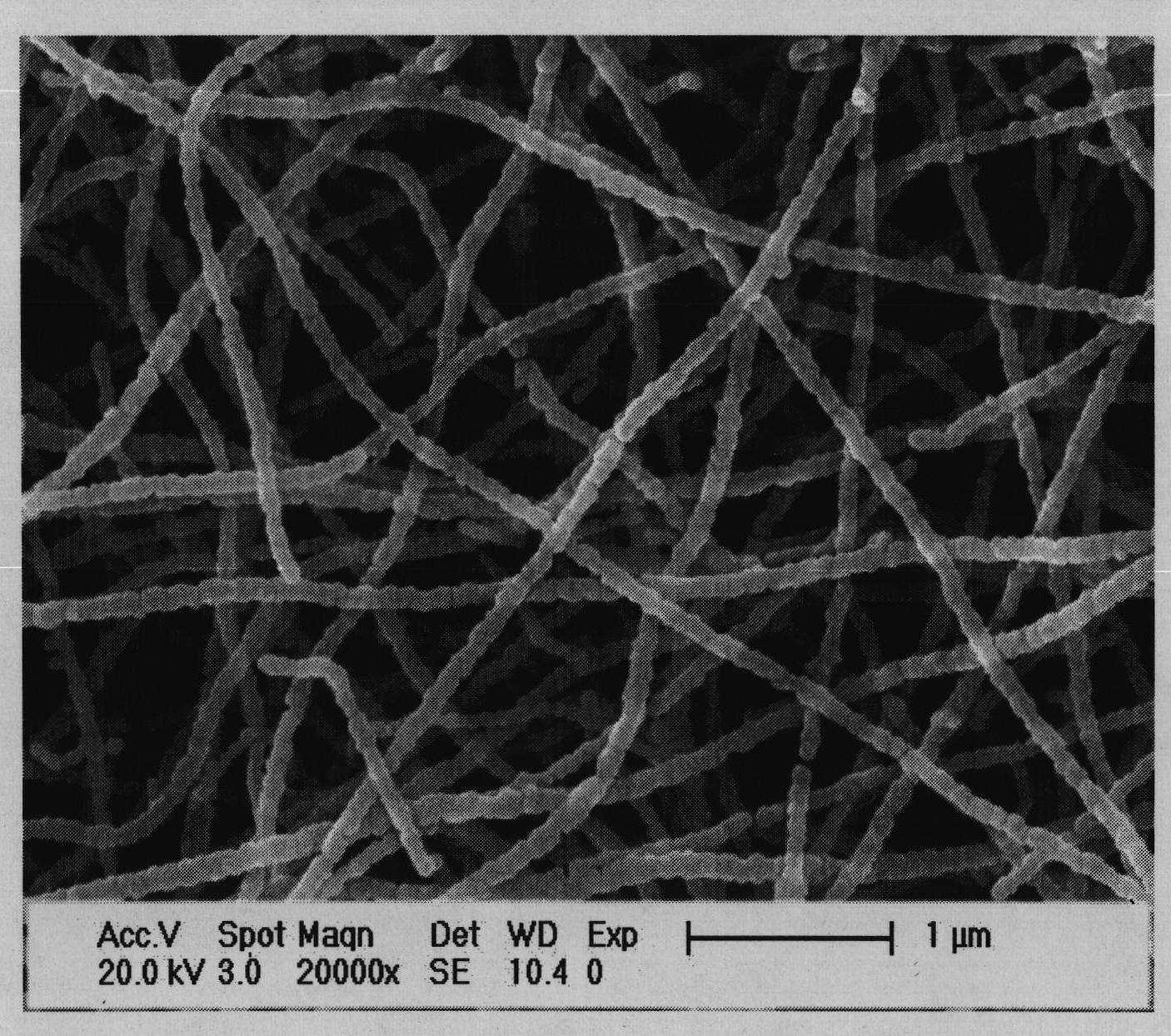
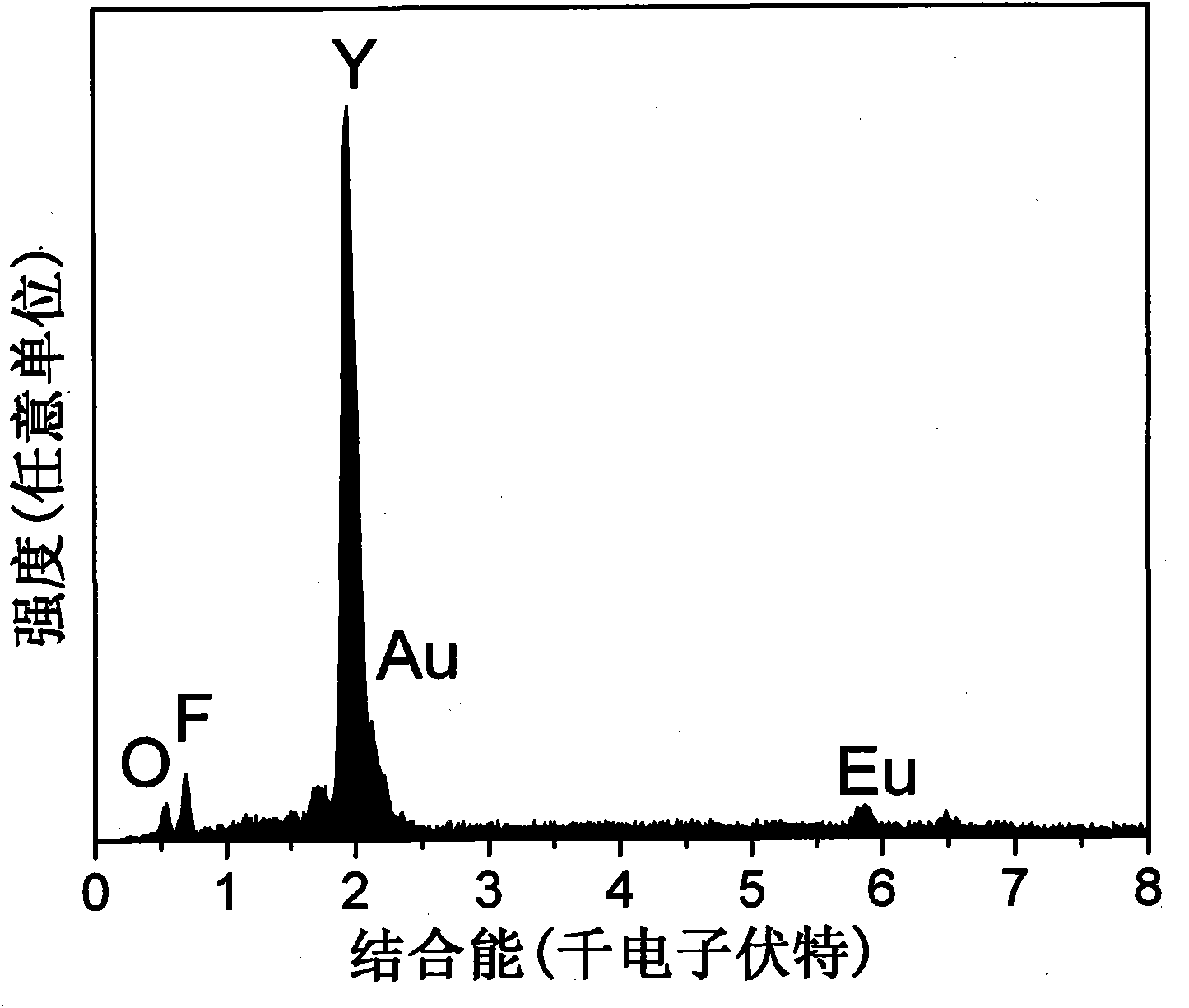
Europium-doped Y7O6F9 nano fiber and preparation method thereof
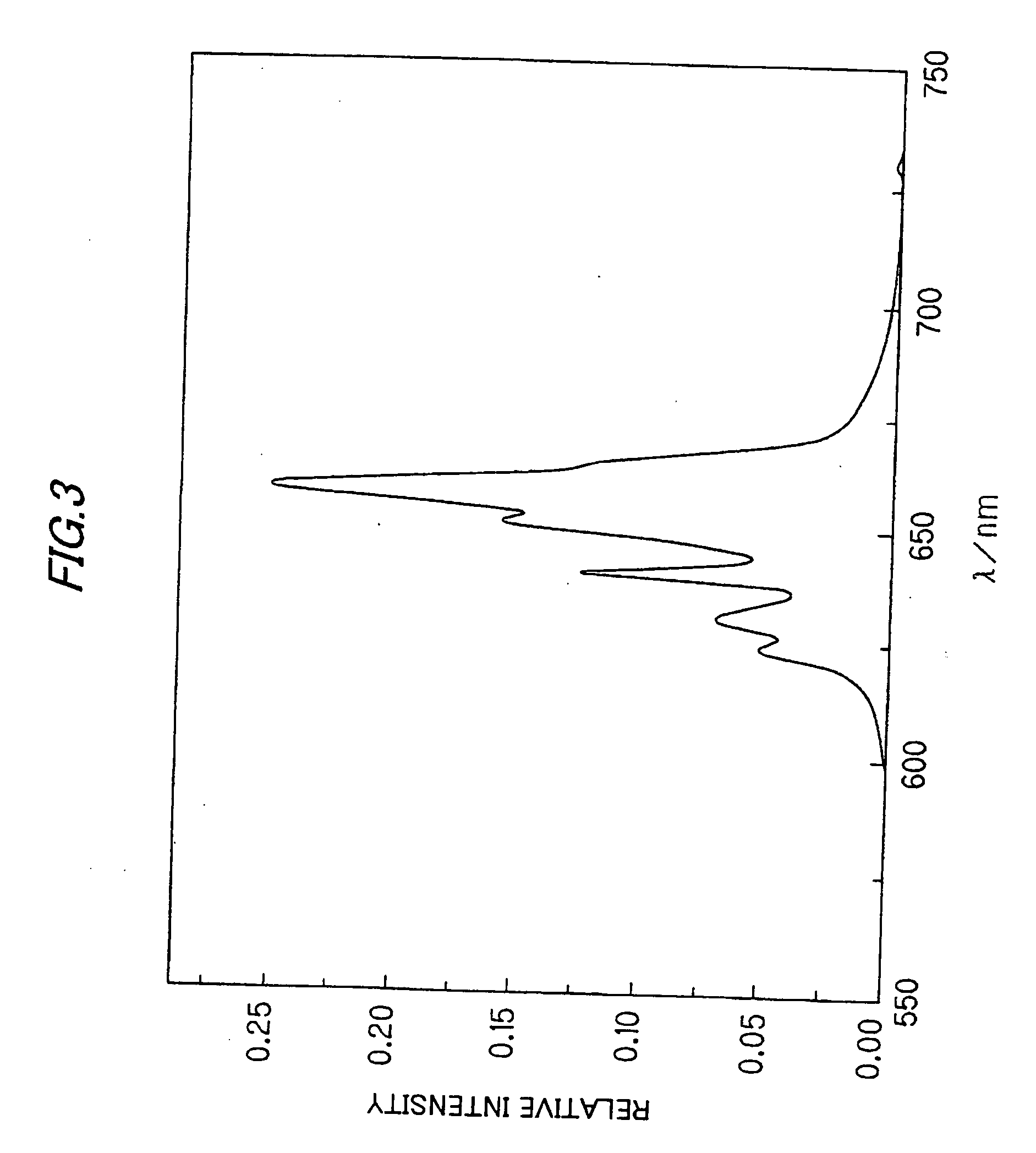
The invention relates to a europium-doped Y7O6F9 nano fiber and a preparation method thereof, belonging to the technical field of the preparation of nano materials. A rare earth fluoride / rare earth oxyfluoride composite nano fiber is prepared by the traditional electrostatic spinning technology. The preparation method provided by the invention comprises the following three steps: (1) preparation of a Y2O3:5%Eu<3+> nano fiber: preparing a PVP / [Y(NO3)3+Eu(NO3)3] composite nano fiber by adopting the electrostatic spinning technology, and then, carrying out heat treatment to obtain the Y2O3:5%Eu<3+> nano fiber; (2) preparation of a YF3:5%Eu<3+> nano fiber: fluorinating the Y2O3:5%Eu<3+> nano fiber by a double-crucible method to obtain the YF3:5%Eu<3+> nano fiber, wherein the fluorinating reagent is ammonium bifluoride; and (3) preparation of a Y7O6F9:5%Eu<3+> nano fiber: placing the YF3:5%Eu<3+> nano fiber in a muffle furnace, heating at 580 DEG C under the air atmosphere for 9 hours to obtain the Y7O6F9:5%Eu<3+> nano fiber, wherein the diameter is 181-241 nm, and the length is greater than 300 mu m. The europium-doped Y7O6F9 nano fiber is a novel important red nano fluorescent material having wide application prospects.
Owner:CHANGCHUN UNIV OF SCI & TECH
Phosphor, production method thereof, phosphor-containing composition, light emitting device, and display and illuminating device
InactiveUS20100090585A1Increase brightnessIncrease intensityDischarge tube luminescnet screensLamp detailsAluminateAlkaline earth metal
To provide a phosphor that stably shows high emission intensity and brightness as well as superior temperature characteristics, under excitation by near-ultraviolet light, the phosphor contains an alkaline-earth metal aluminate and has a crystal phase comprising an alkali metal element and, in that crystal phase, the rate of substituted Eu (europium) to the number of sites which can be substituted with Eu of the crystal phase is 25% or higher and the ratio of the alkali metal element to the number of sites which can be substituted with Eu of the crystal phase is 3% or lower.
Owner:MITSUBISHI CHEM CORP
Phosphor and optical device using same
InactiveUS20070035813A1Good colorSolid-state devicesEnergy efficient lightingAlkaline earth metalColored white
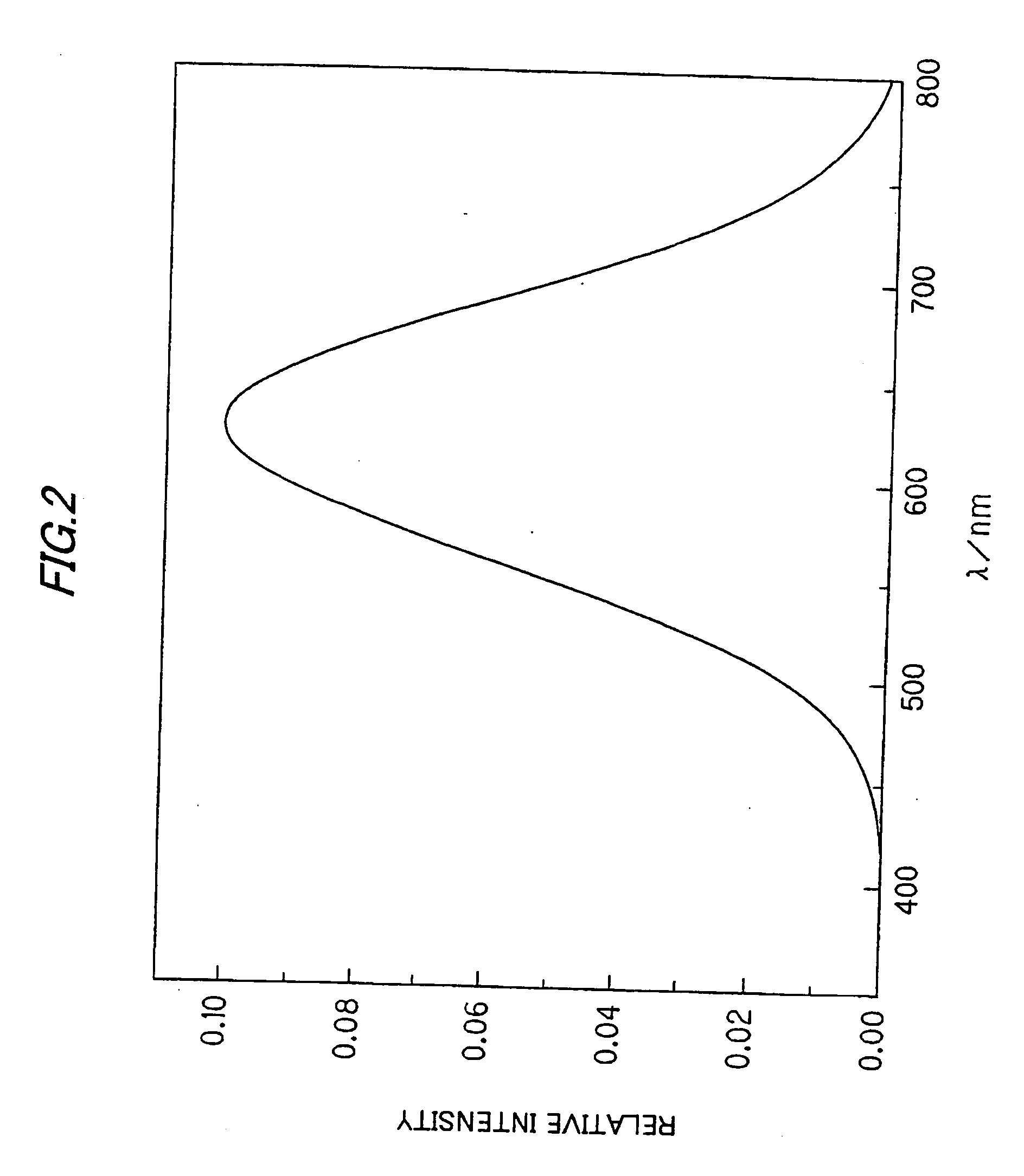
A phosphor for converting ultraviolet light or blue light emitted from a light emitting element into a visible white radiation having a high level of color rendering properties, containing a light emitting component prepared from a solid system of an alkaline earth metal antimonate and a system derived from the solid system and exhibiting intrinsic photoemission, such as a fluoroantimonate, a light emitting component prepared from a manganese(IV)-activated antimonate, a titanate, silicate-germanate, and an aluminate, a light emitting component prepared from a europium-activated silicate-germanate or from a system containing a sensitizer selected from a group consisting of europium (II) and manganese (II) as a secondary activator and having an orange color or a dark red color in the spectrum range over 600 nm, or a light emitting component composed of a mixture of eight or less light emitting components having different emission bands and brought to a state of continuous emission of about 380 to 780 nm exhibiting a color temperature of about 10,000 to 6,500 K and a color temperature of about 3,000 to 2,000 K by virtue of the superposition of the light emitting bands.
Owner:TOYODA GOSEI CO LTD +2
Green-emitting phosphor particles, method for manufacturing green-emitting phosphor particles, color conversion sheet, light-emitting device, and image display device assembly
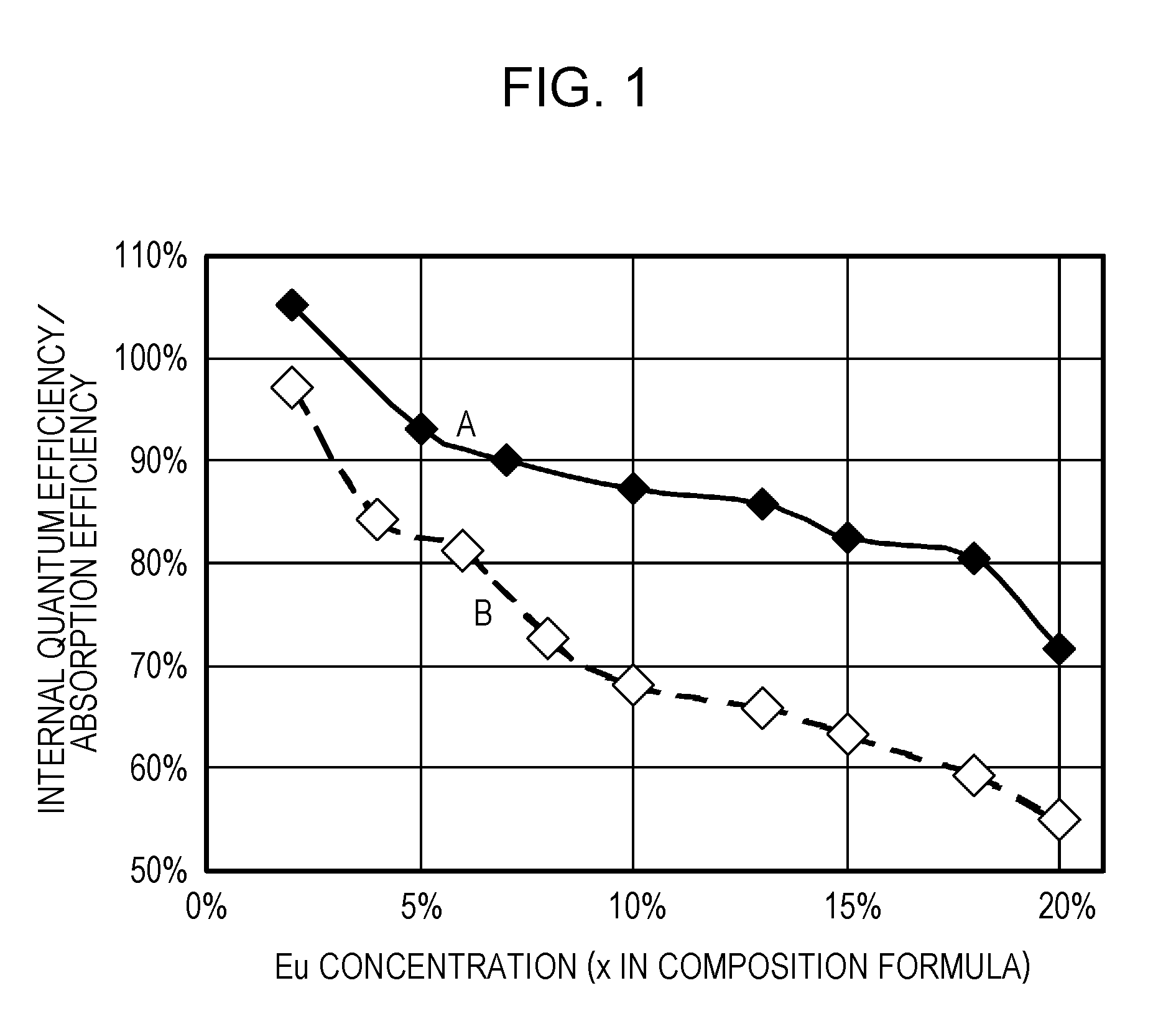
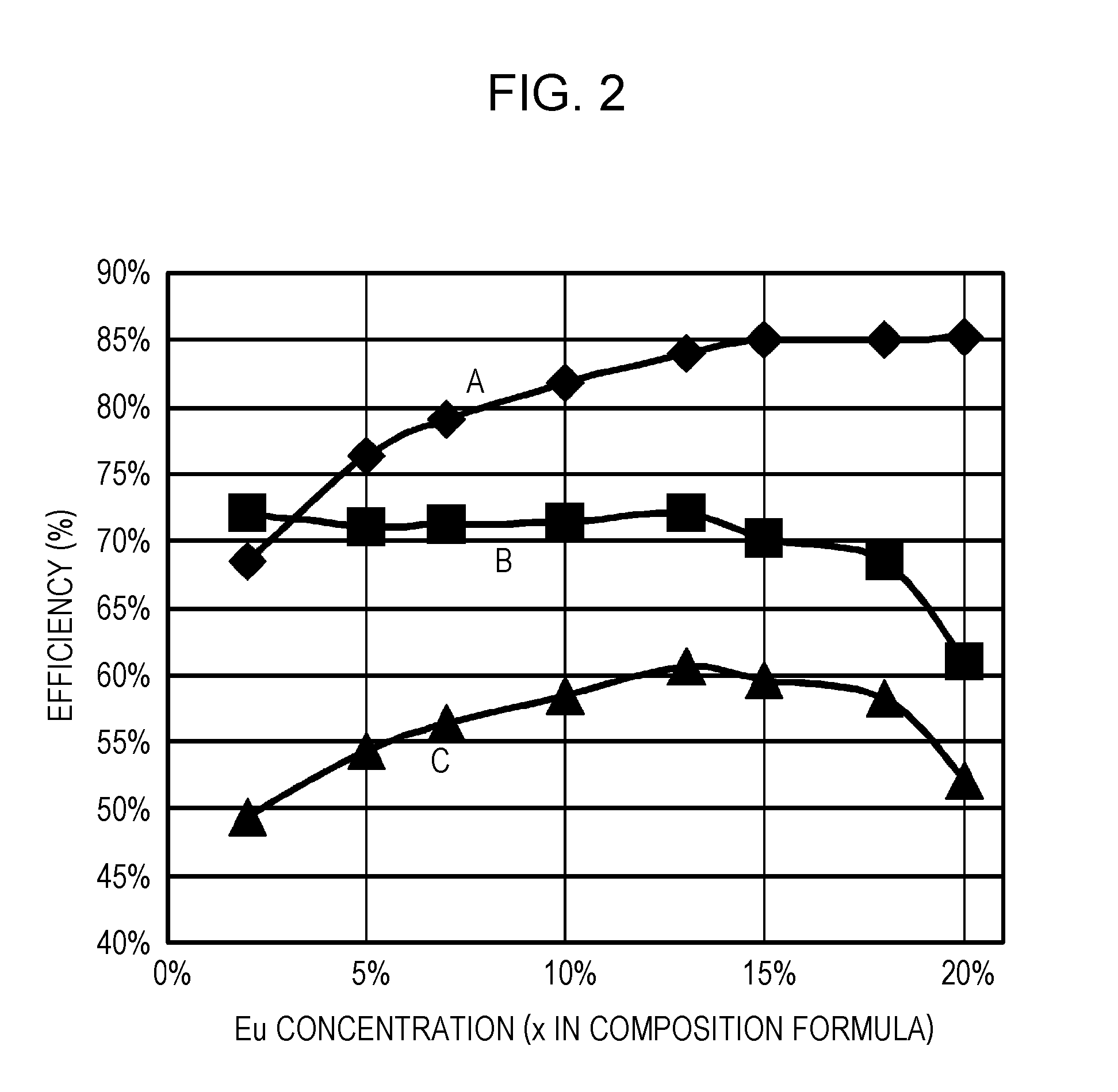
ActiveUS20110273864A1Improve internal quantum efficiencyImprove absorption efficiencySolid-state devicesLuminescent compositionsPhosphorStrontium
Owner:DEXERIALS CORP
Aflatoxin B1 flow lag immunization time distinguishing fluorescence rapid-detection kit and application thereof
The invention relates to an aflatoxin B1 flow lag immunization time distinguishing fluorescence rapid-detection kit and an application thereof. The kit comprises a fluorescent test strip and a sample reaction bottle containing an europium-labeled anti-aflatoxin B1 monoclonal antibody lyophilized product, wherein the fluorescent test strip comprises a cardboard, a water absorption pad, a detection pad and a sample pad are sequentially pasted on one surface of the cardboard from top to bottom, adjacent pads are connected at the connection in an overlapping manner, the detection pad treats a cellulose nitrate membrane as a base pad, a transverse quality control line and a detection line are arranged on the cellulose nitrate membrane from top to bottom, the quality control line is coated with a rabbit anti-mouse polyclonal antibody, and the detection line is coated with an aflatoxin B1 bovine serum albumin conjugate; and the anti-aflatoxin B1 monoclonal antibody is secreted by a hybridoma cell strain having a preservation number of CCTCC NO.C201015. The kit can be used for the quantitative determination of the content of the aflatoxin B1, and has the advantages of simple operation, rapidness and high accuracy.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
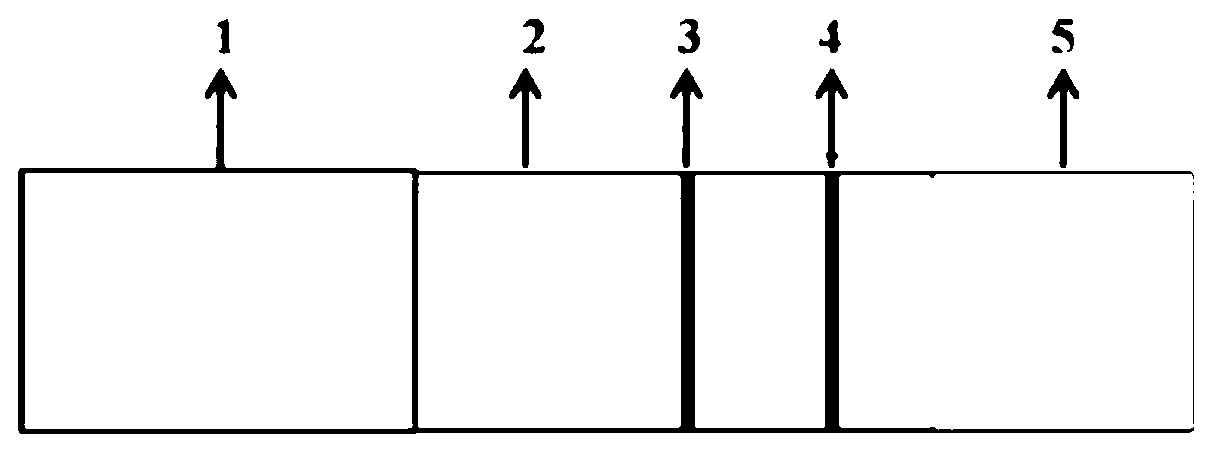
Illumination system comprising a radiation source and a luminescent material
InactiveUS20090218581A1Improve quantum efficiencyReduce lossDischarge tube luminescnet screensElectroluminescent light sourcesLithiumColor rendering index
An illumination system, comprising a radiation source (1) and a luminescent material (3,4,5) comprising at least one phosphor capable of absorbing a part of light emitted by the radiation source and emitting light of wavelength different from that of the absorbed light; wherein said at least one phosphor is a yellow red-emitting europium(II)-activated earth alkaline lithium orthosilicate of general formula (Sr1-x-y-zCaxBay)Li2SiO4:Euz wherein 0≦x≦1; 0≦y≦1; 0.001<z<0.3 can provide light sources having high luminosity and color-rendering index, especially in conjunction with a light emitting diode as a radiation source. The red to yellow-emitting europium(II)-activated earth alkaline lithium orthosilicate of general formula (Sr1-x-y-zCaxBay)Li2SiO4:Euz wherein 0<x≦1; 0≦y<1; 0.001<z<0.3 is efficiently excitable by primary radiation in the near UV-to-blue range of the electromagnetic spectrum.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
High-density scintillators for imaging system and method of making same
InactiveUS20060219927A1Material analysis using wave/particle radiationRadiation/particle handlingAlkaline earth metalCerium
A scintillator composition comprising a garnet represented by (M1-x-yNxAy)3(Al5-a-bCaDb)O12, where M comprises yttrium, or terbium, or gadolinium, or holmium, or erbium, or thulium, or ytterbium, or lutetium, or combinations thereof, where N comprises additives including a lanthanide, or an alkali metal, or an alkaline earth metal, or combinations thereof, where A comprises a suitable activator ion including cerium, or europium, or praseodymium, or terbium, or ytterbium, or combinations thereof, where C or D comprises lithium, or magnesium, or gallium, or an element from group IIIa, or IVa, or Va, or IIId transition metal, or IVd transition metal, or combinations thereof, where x ranges from about 0 to about 0.90, y ranges from about 0.0005 to about 0.30, and a sum of a and b ranges from about 0 to 2.0.
Owner:GENERAL ELECTRIC CO
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com