Phosphor, production method thereof, phosphor-containing composition, light emitting device, and display and illuminating device
a technology of phosphor and phosphor, which is applied in the direction of solid-state devices, discharge tubes/lamp details, electrical apparatus, etc., can solve the problems of insufficient brightness of previously known green oxide phosphors, and achieve superior temperature characteristics, high emission intensity and brightness, and superior light-emitting devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0457]As phosphor materials, 0.552 g of barium carbonate (BaCO3), 0.211 g of europium oxide (Eu2O3). 0.261 g of basic magnesium carbonate (mass of 93.17 per 1 mole of Mg), 0.138 g of manganese carbonate (MnCO3), and 2.038 g of α-alumina (Al2O3) were weighed out and used. Also, as flux, 0.015 g (0.47 weight % relative to the total weight of the phosphor materials weighed out) of potassium fluoride (KF), which is a univalent metal element halide, were weighed out and used.
[0458]The phosphor materials and fluxes mentioned above were mixed in a mortar for 30 minutes and filled in an alumina crucible. In order to create a reducing atmosphere at the time of firing, bead-shaped graphites were placed in a space around the crucible. This mixture of the phosphor materials and flux was fired at 1550° C. for 2 hours under atmospheric pressure. The fired product obtained was ground, thereby to obtain a phosphor. The phosphor will be hereinafter referred to as the “phosphor of Example 1”.
[0459]Th...
examples 2 to 6
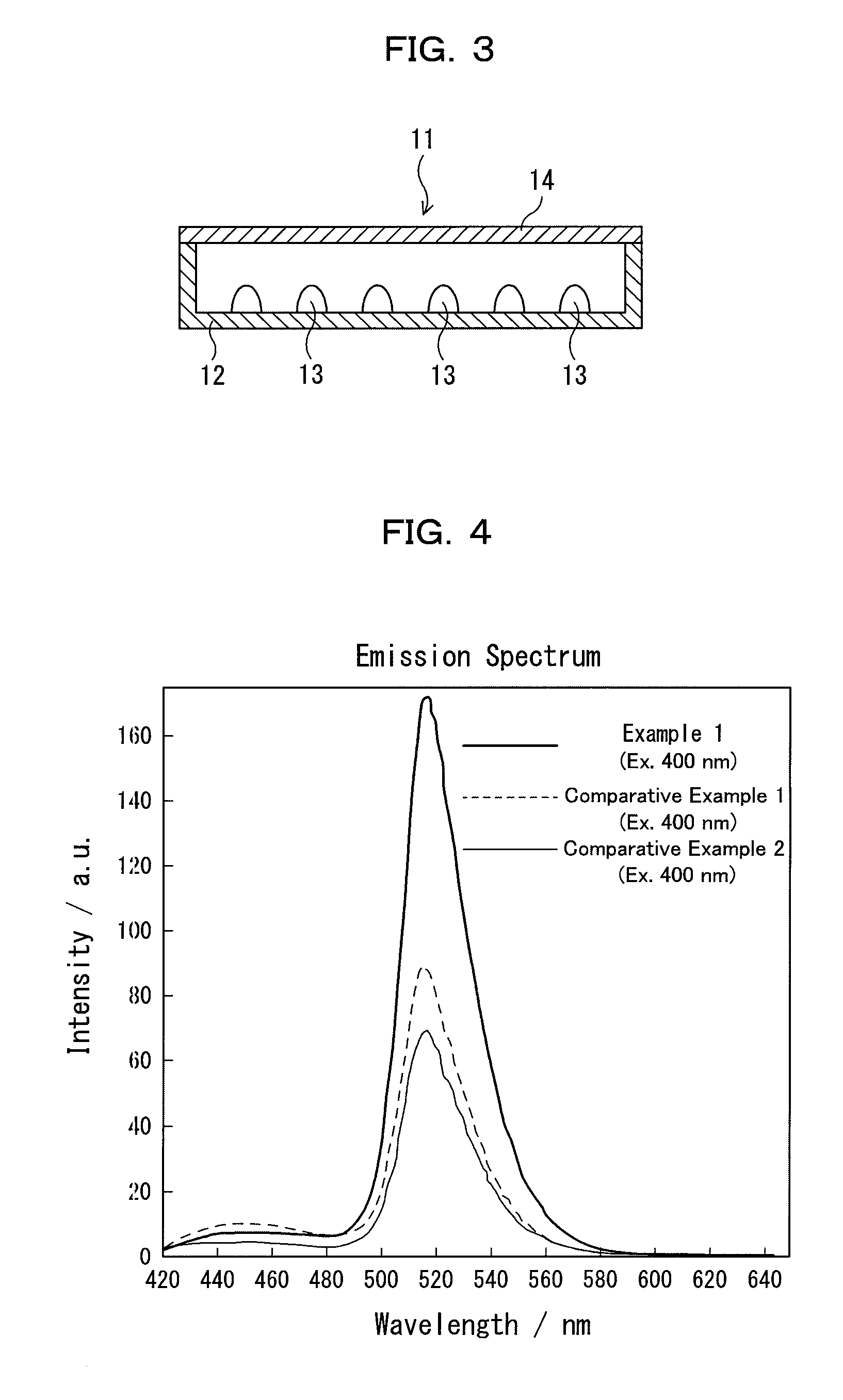
[0486]On each phosphor of Examples 2 to 6, emission spectrum and excitation spectrum were measured under excitation by light of 400 nm wavelength, and the emission spectrum characteristics and excitation spectrum characteristics were calculated, in the same procedure as Example 1.
[0487]The emission spectrum characteristics and excitation spectrum characteristics of respective phosphors of Examples 2 to 6 are shown in Table 5 below, together with the emission spectrum characteristics and excitation spectrum characteristics of the phosphor of Example 1.
TABLE 5Emission spectrum characteristics(excitation wavelength of 400 nm)Ratio of blue-emissionEmission-peakpeak intensity relative toExcitation spectrum characteristicswavelengthRelative emission-that of the greenReduction rate ofRate of change of(nm)peak intensity (%)*1emission peak (%)I(400) to I(340) (%)*2I(390) to I(382) (%)*3Example 15162024.5191.1Example 25171854.7231.5Example 35161994.5200.9Example 45162014.5191.1Example 5516196...
examples 7 to 9
[0491]On each phosphor of Examples 7 to 9, emission spectrum and excitation spectrum were measured under excitation by light of 400 nm wavelength, and the emission spectrum characteristics and excitation spectrum characteristics were calculated, in the same procedure as Example 1.
[0492]The emission spectrum characteristics and excitation spectrum characteristics of respective phosphors of Examples 7 to 9 are shown in Table 7 below, together with the emission spectrum characteristics and excitation spectrum characteristics of the phosphor of Example 1. Incidentally, the full width at half maximum of the emission peak of Example 8 was 26.5 nm.
TABLE 7Emission spectrum characteristics (excitation wavelength of400 nm)Ratio of blue-emissionpeak intensity relative toExcitation spectrum characteristicsEmission-peakRelative emission-that of the greenReduction rate ofRate of change ofwavelength (nm)peak intensity (%)*1emission peak (%)I(400) to I(340) (%)*2I(390) to I(382) (%)*3Example 151620...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength range | aaaaa | aaaaa |
| wavelength range | aaaaa | aaaaa |
| wavelength range | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com