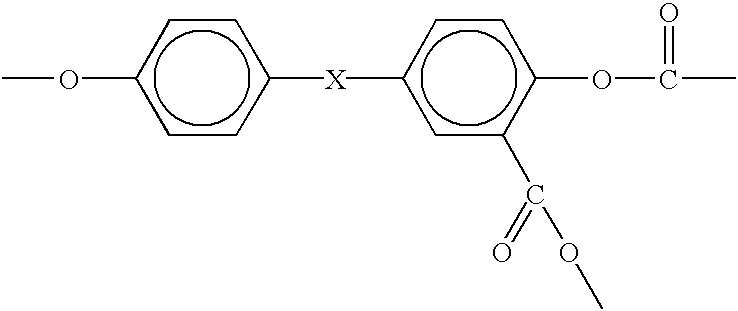
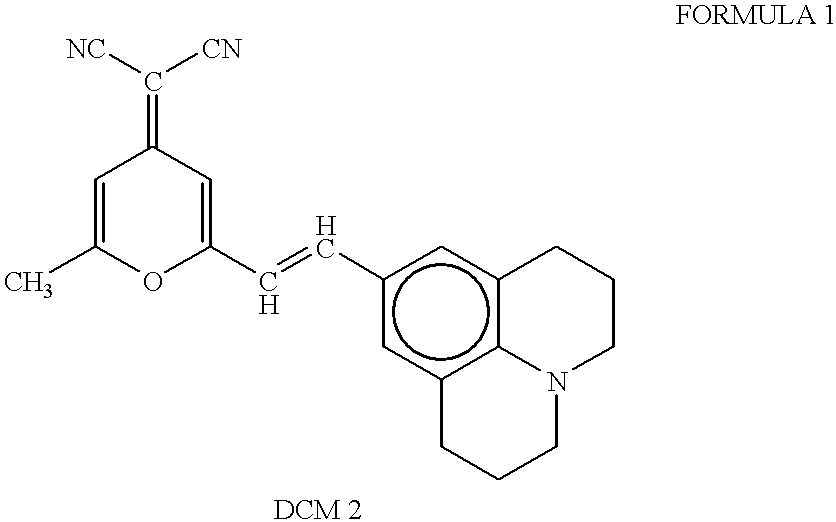
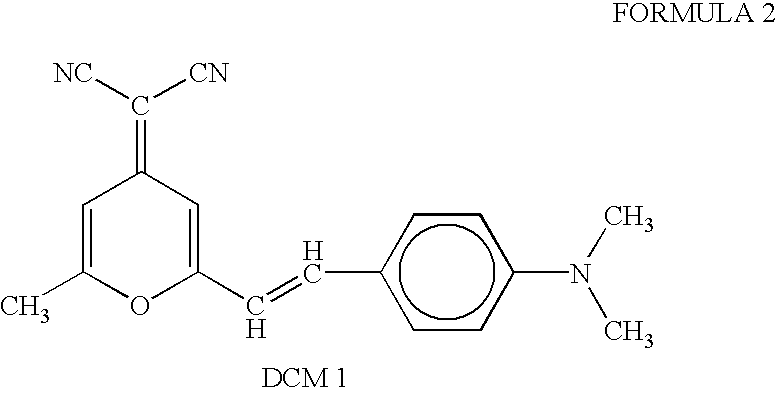
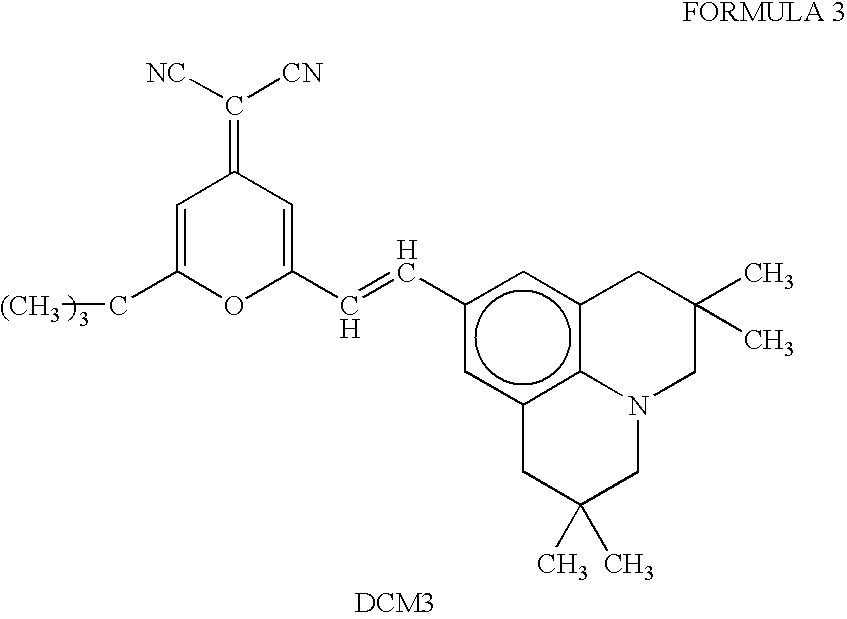
Patents
Literature
8484 results about "Alkaline earth metal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). The elements have very similar properties: they are all shiny, silvery-white, somewhat reactive metals at standard temperature and pressure.
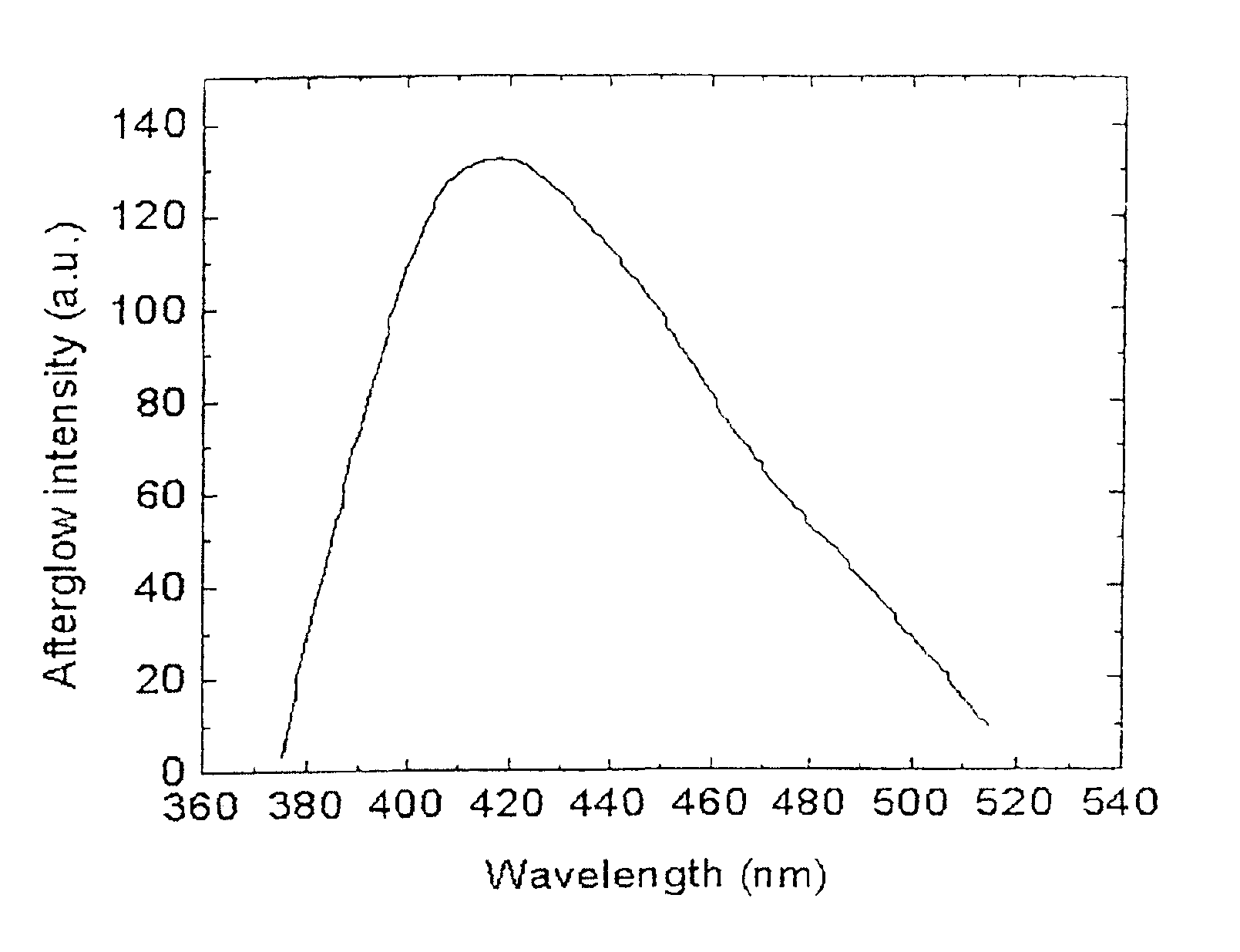
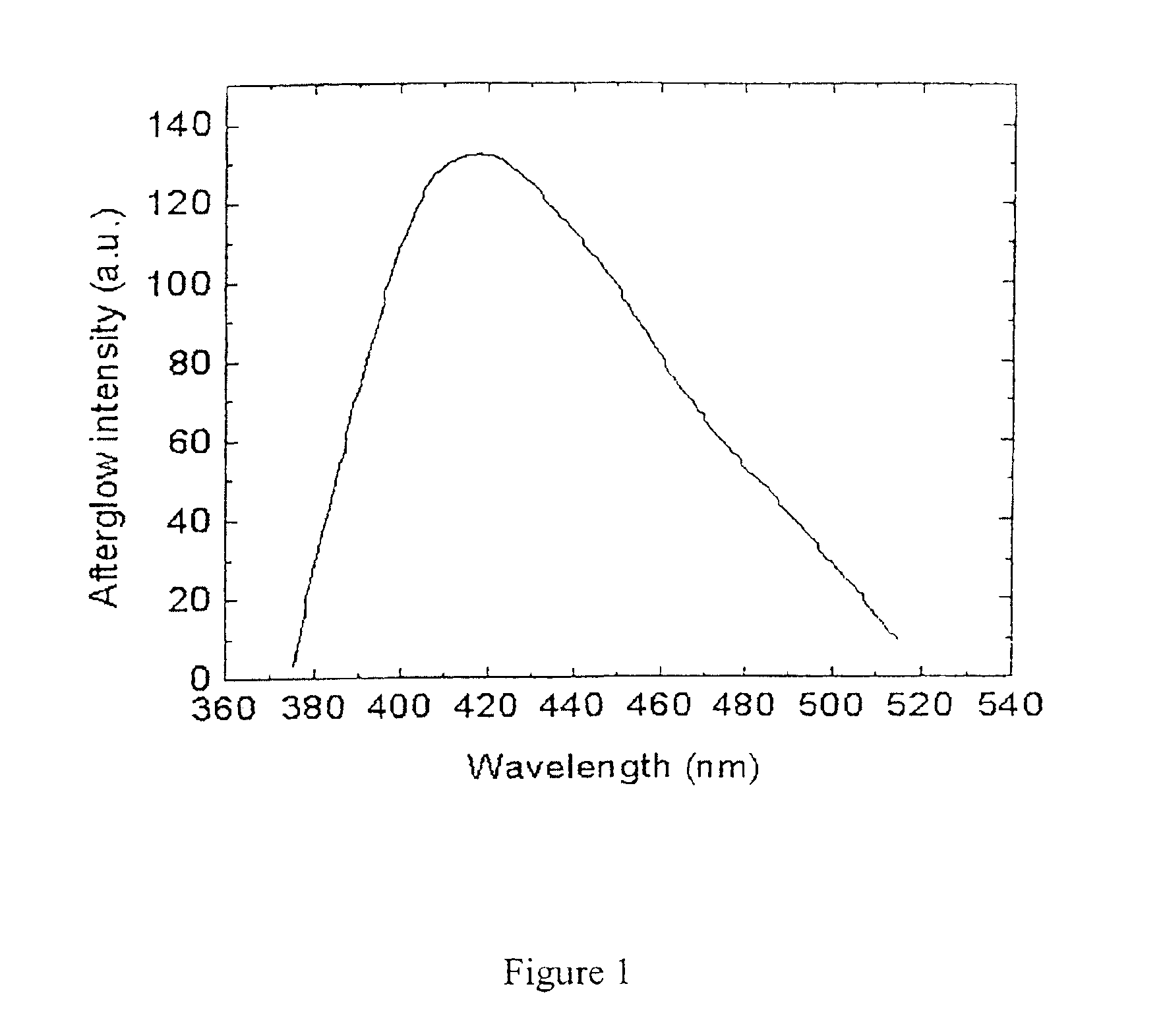
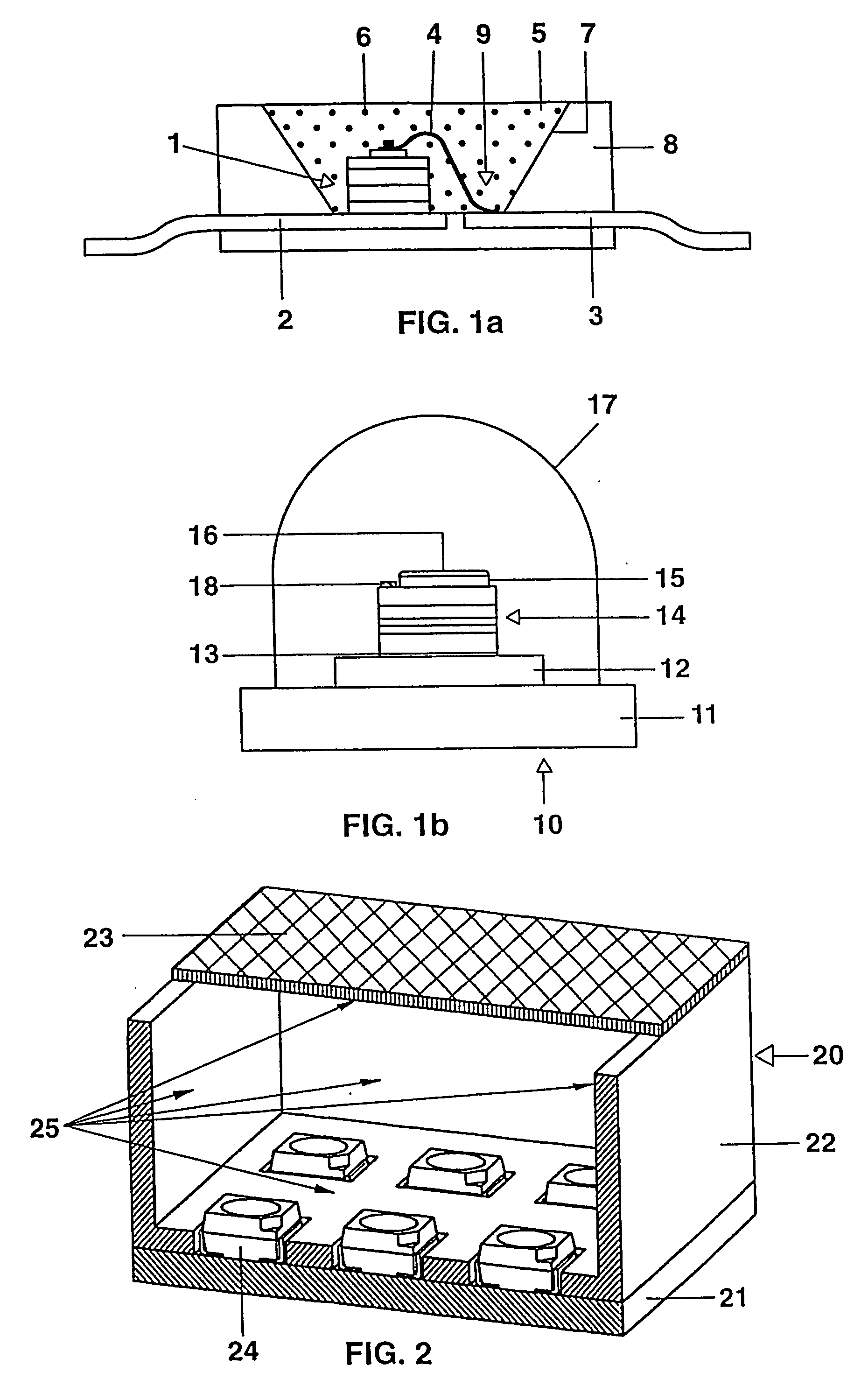
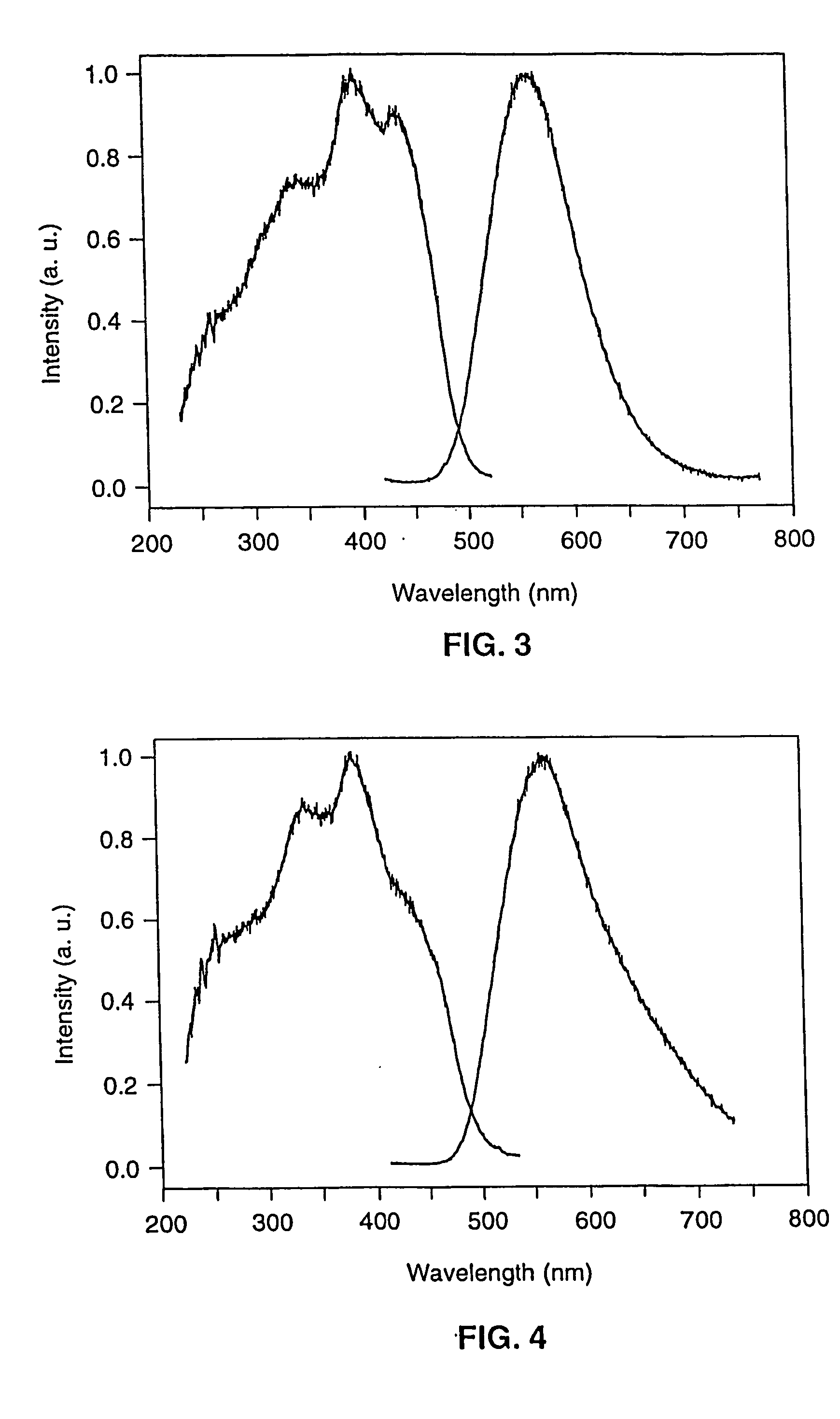
Long persistent phosphors and persistent energy transfer technique
The invention provides long-persistent phosphors, methods for their manufacture and phosphorescent articles. The invention also provides a method for generating a long-persistent phosphorescence at a selected color. The phosphors of the invention may be alkaline earth aluminates, alkaline earth silicates, and alkaline earth aluminosilicates. The phosphors include those activated by cerium. The phosphors also include those in which persistent energy transfer occurs from a donor ion to an acceptor ion, producing persistent emission largely characteristic of the acceptor ion.
Owner:UNIV OF GEORGIA RES FOUND INC +1
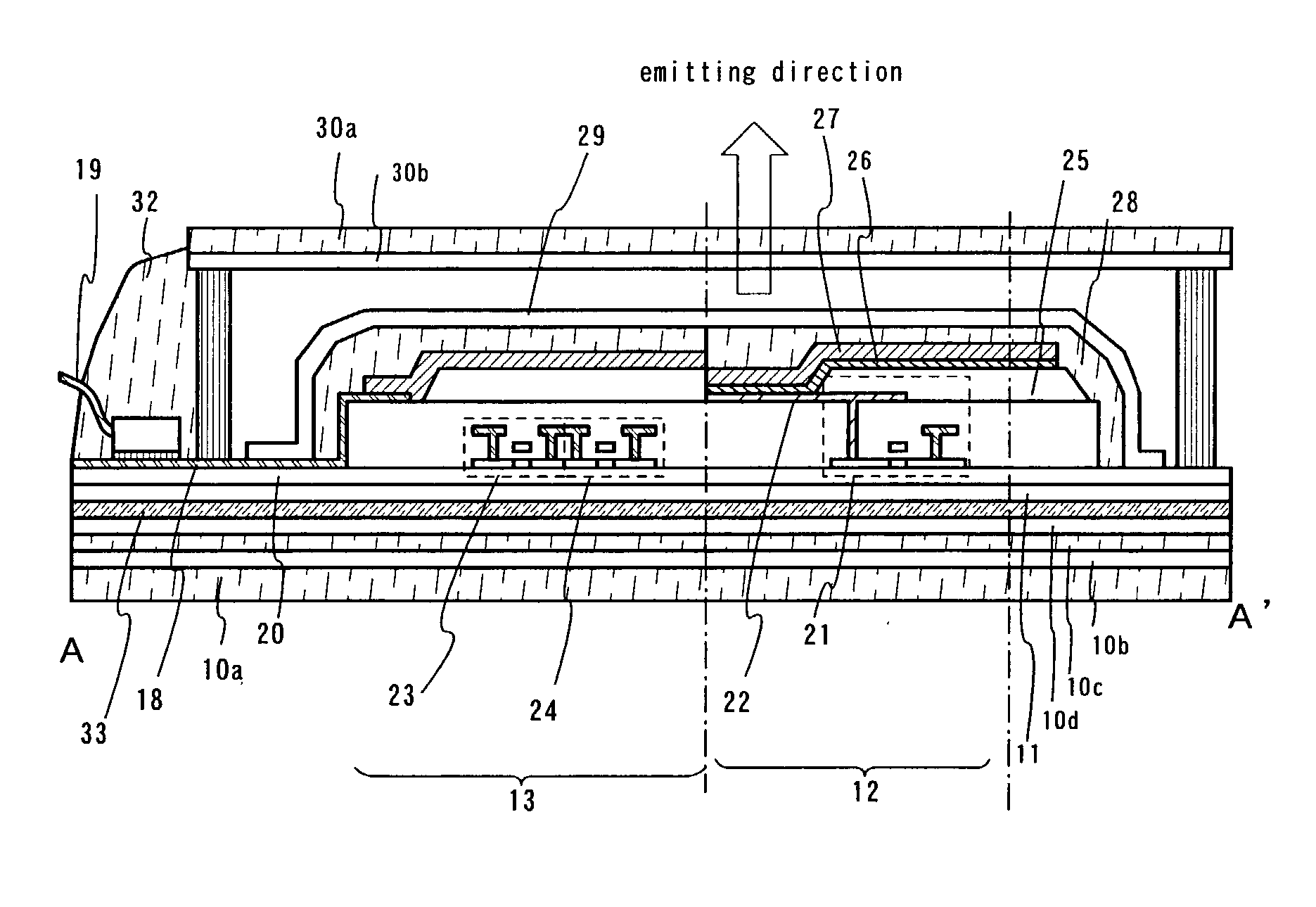
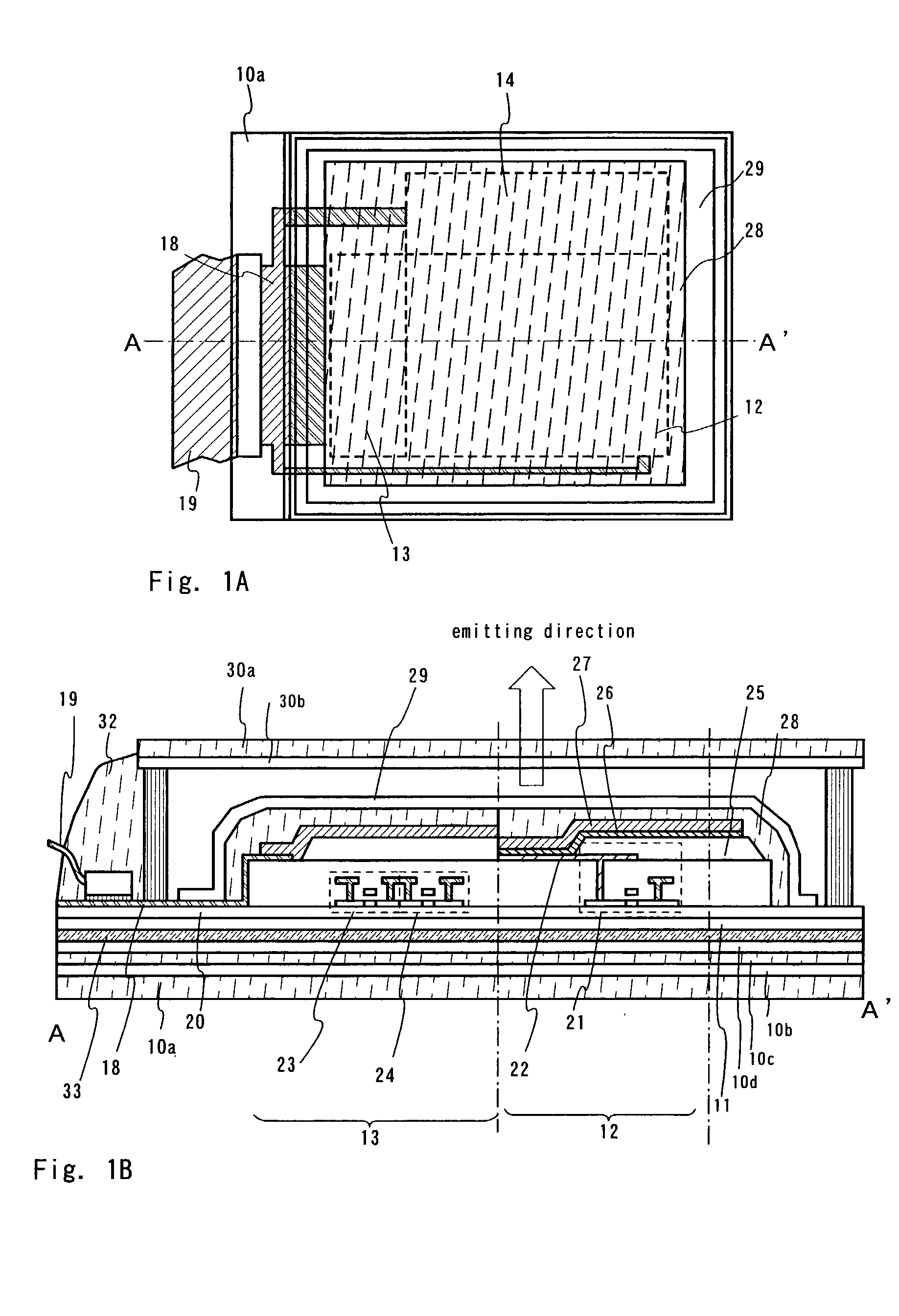
Light emitting element
InactiveUS20130105787A1Improve light emission efficiencySufficient durability lifeOrganic chemistrySolid-state devicesSilyleneAlkaline earth metal
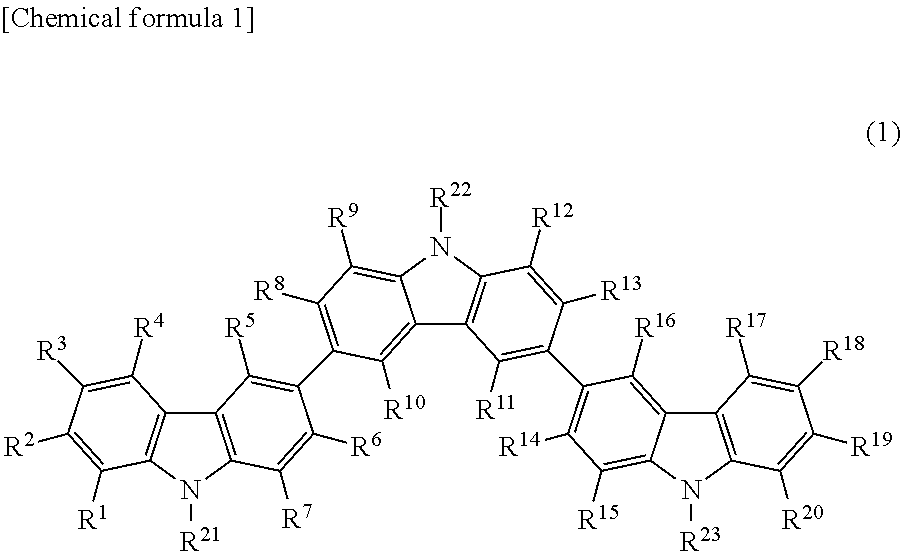
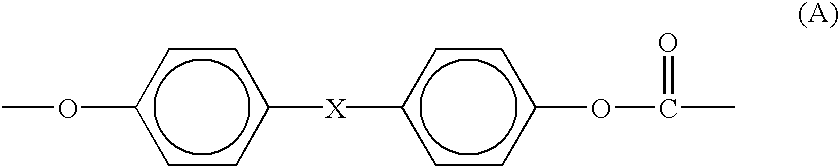
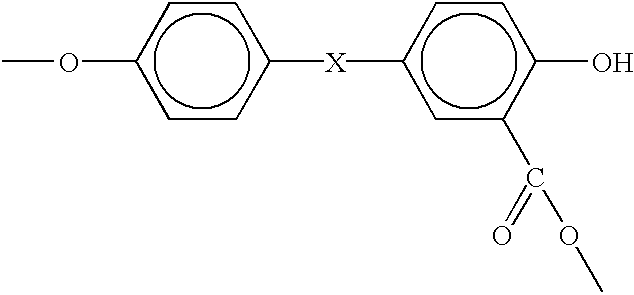
Provided is an organic thin film light emitting element which has achieved all of improved luminous efficiency, improved driving voltage and improved durability life. Specifically provided is a light emitting element which comprises a hole transport layer and an electron transport layer between a positive electrode and a negative electrode and emits light by means of electrical energy. The light emitting element is characterized in that: the hole transport layer of the light emitting element contains a compound represented by general formula (1); the electron transport layer contains a donor compound; and the donor compound is an alkali metal, an inorganic salt containing an alkali metal, a complex of an alkali metal and an organic substance, an alkaline earth metal, an inorganic salt containing an alkaline earth metal, or a complex of an alkaline earth metal and an organic substance. (In the formula, R1-R20 each represents one group selected from the group consisting of hydrogen, deuterium, an alkyl group, a cycloalkyl group, an amino group, an aryl group, a heterocyclic group, a heteroaryl group, an alkenyl group, a cycloalkenyl group, an alkynyl group, analkoxy group, an alkylthio group, an arylether group, an arylthioether group, a halogen, a cyano group, a —P(═O)R24R25 group and a silyl group; R24 and R25 each represents an aryl group or a heteroaryl group; and these substituents may be further substituted, or adjacent two substituents may combine together to form a ring. Meanwhile, R21-R23 may be the same or different and each represents one group selected from the group consisting of an alkyl group, a cycloalkyl group, an aryl group and a heteroaryl group; and these substituents maybe further substituted.)
Owner:TORAY IND INC
Light emitting apparatus and method of manufacturing the same
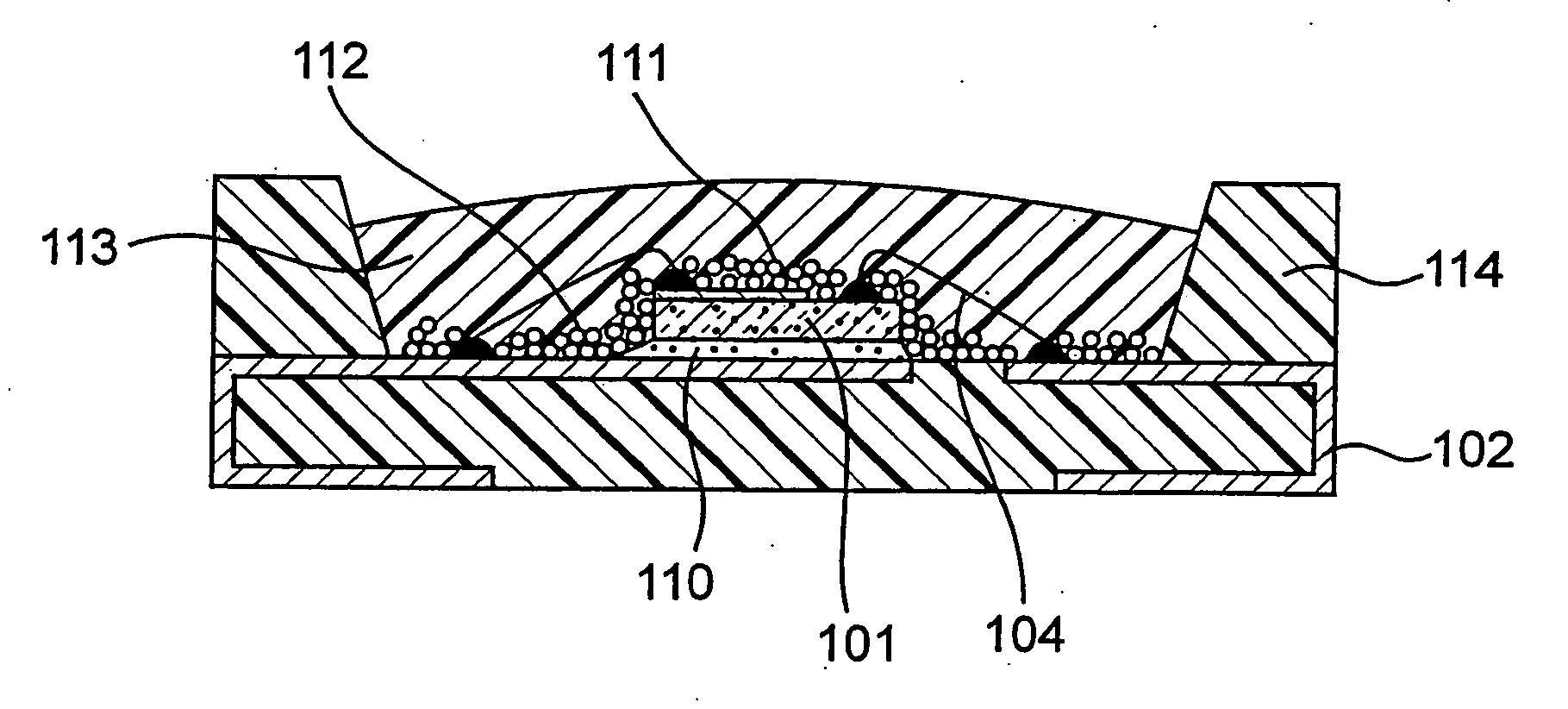
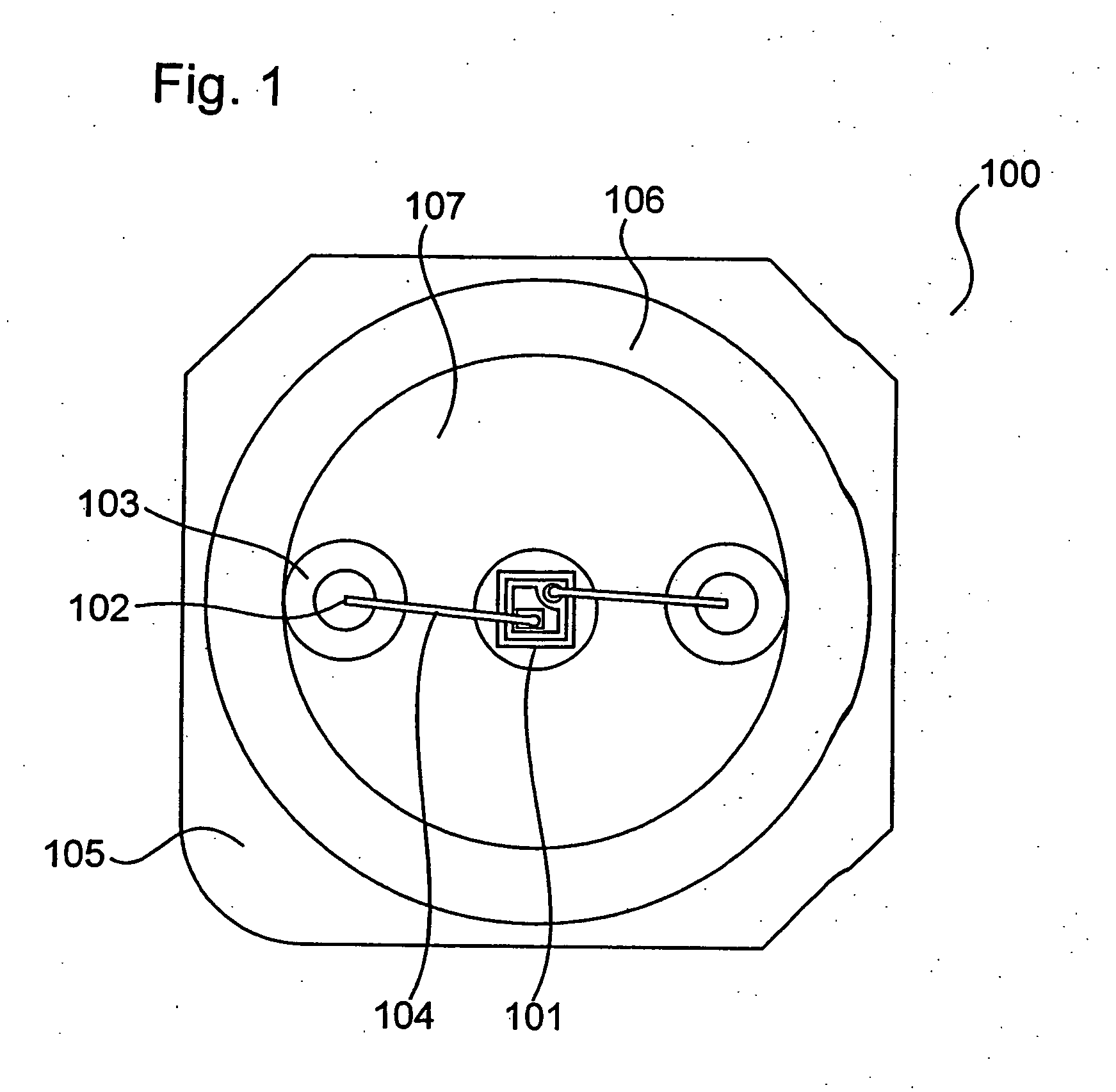
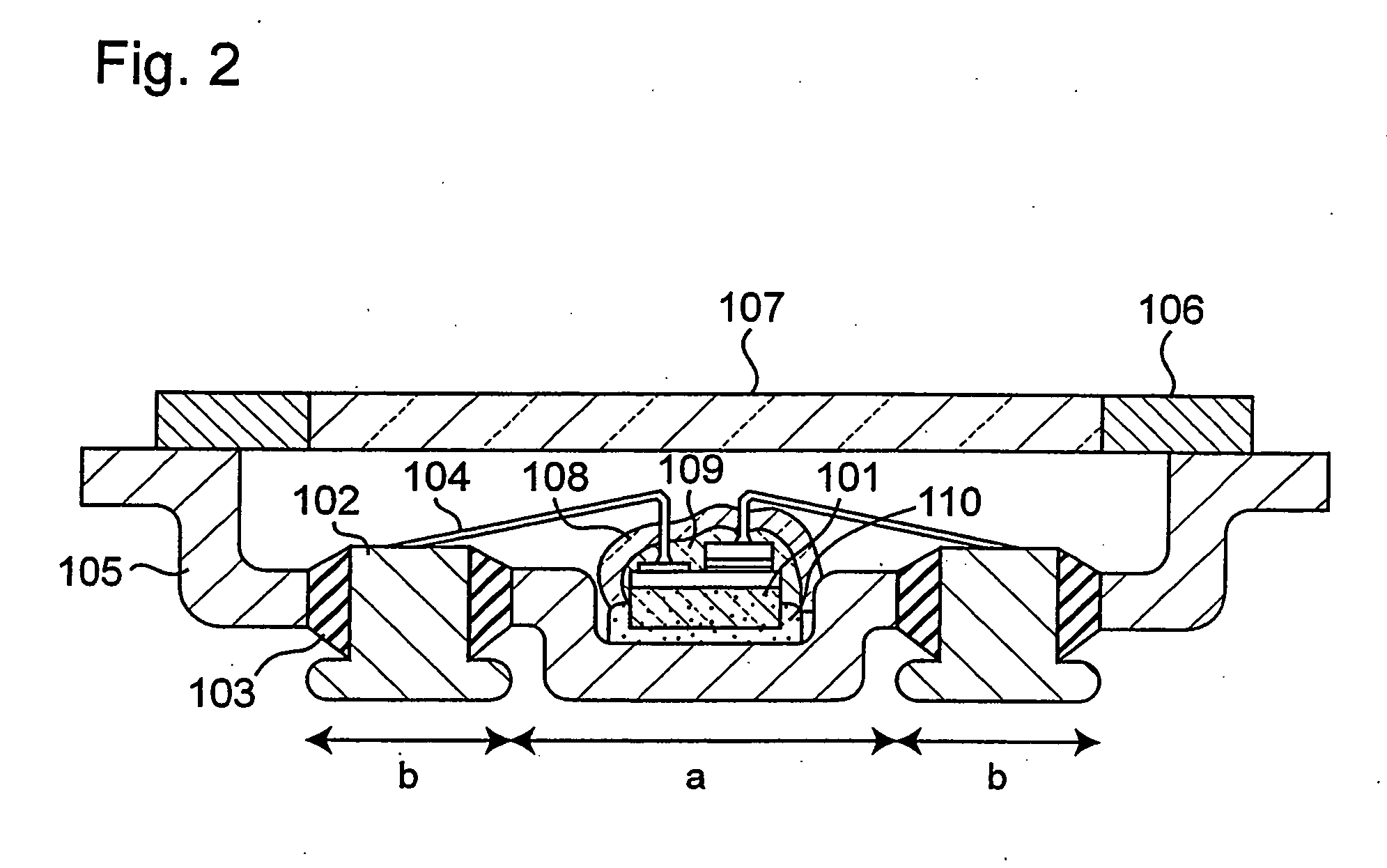
ActiveUS20040061433A1Facilitated DiffusionLarge particle sizeDischarge tube luminescnet screensLamp detailsAlkaline earth metalFluorescence
A light emitting apparatus comprising a light emitting device (101) disposed on a supporting body (105), and coating layers ((108, 109) that bind a fluorescent substance that absorbs light emitted by the light emitting device (101) and emits light of a different wavelength and secures the fluorescent substance onto the surface of the light emitting device (101). The coating layers (108, 109) are made of an inorganic material including an oxide and a hydroxide, each containing at least one element selected from the group consisting of Si, Al, Ga, Ti, Ge, P, B, Zr, Y, Sn, Pb and alkali earth metals. Also an adhesive layer (110) is made of the same inorganic material as that of the coating layers (108, 109).
Owner:NICHIA CORP
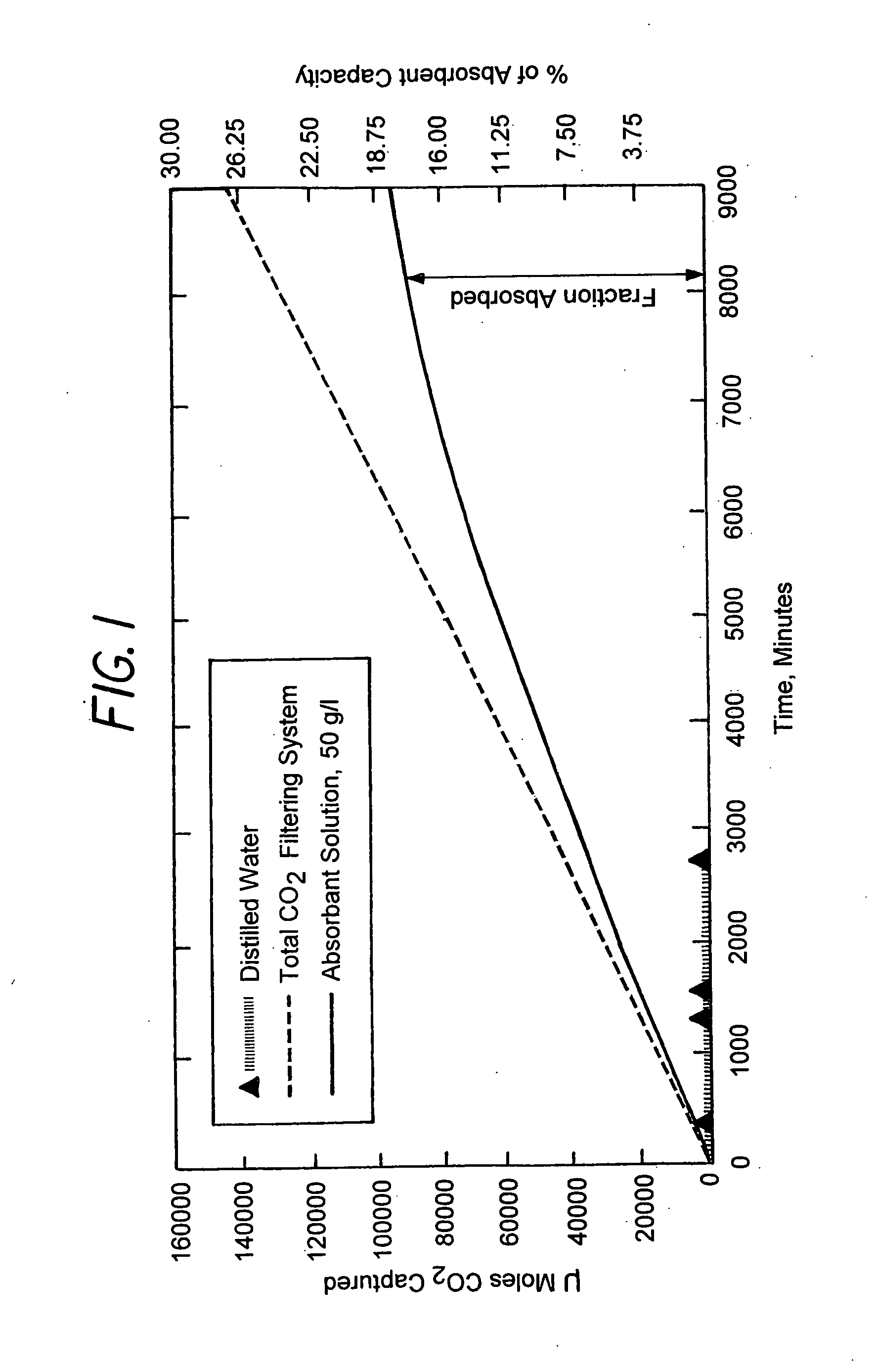
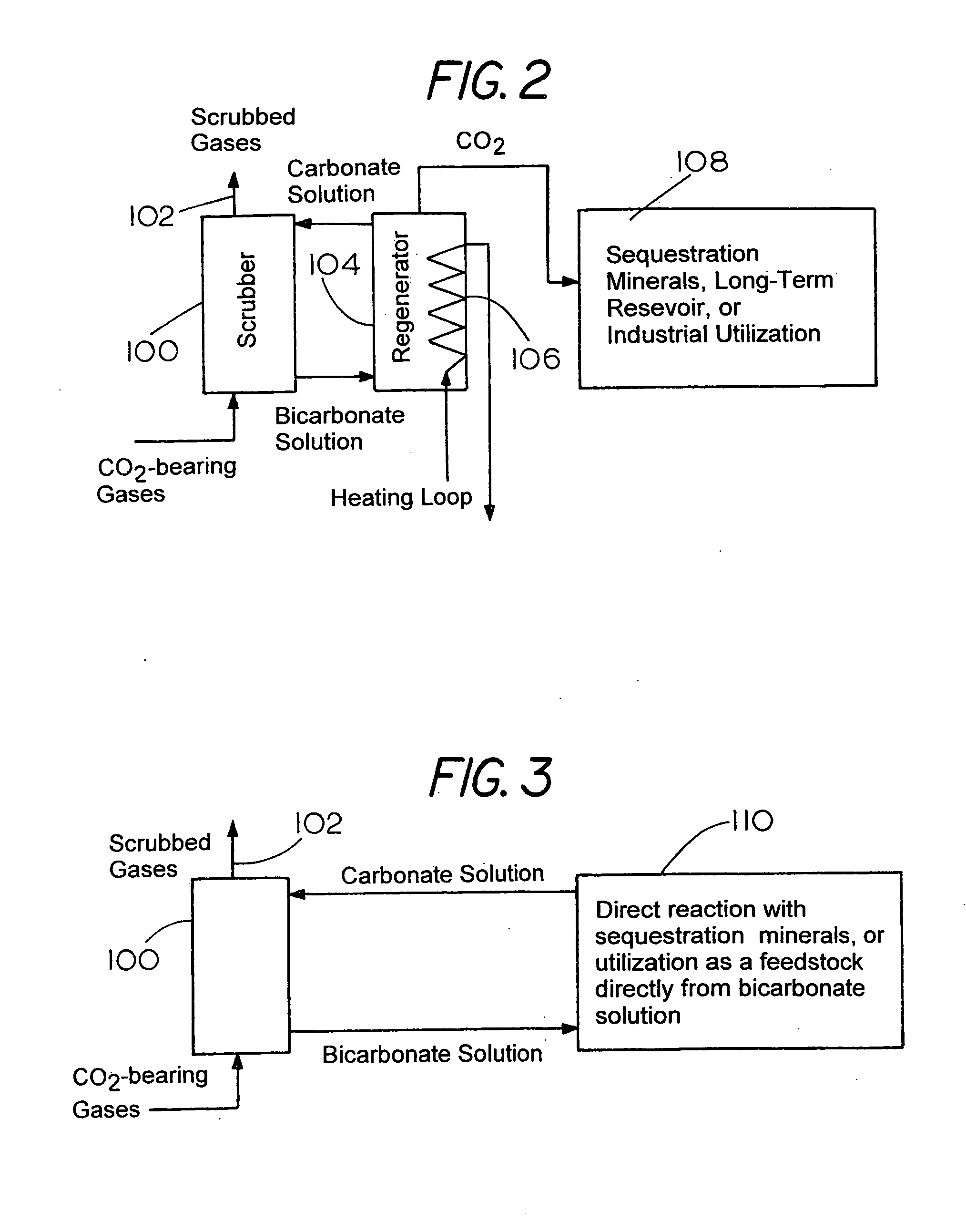
Method for extracting and sequestering carbon dioxide
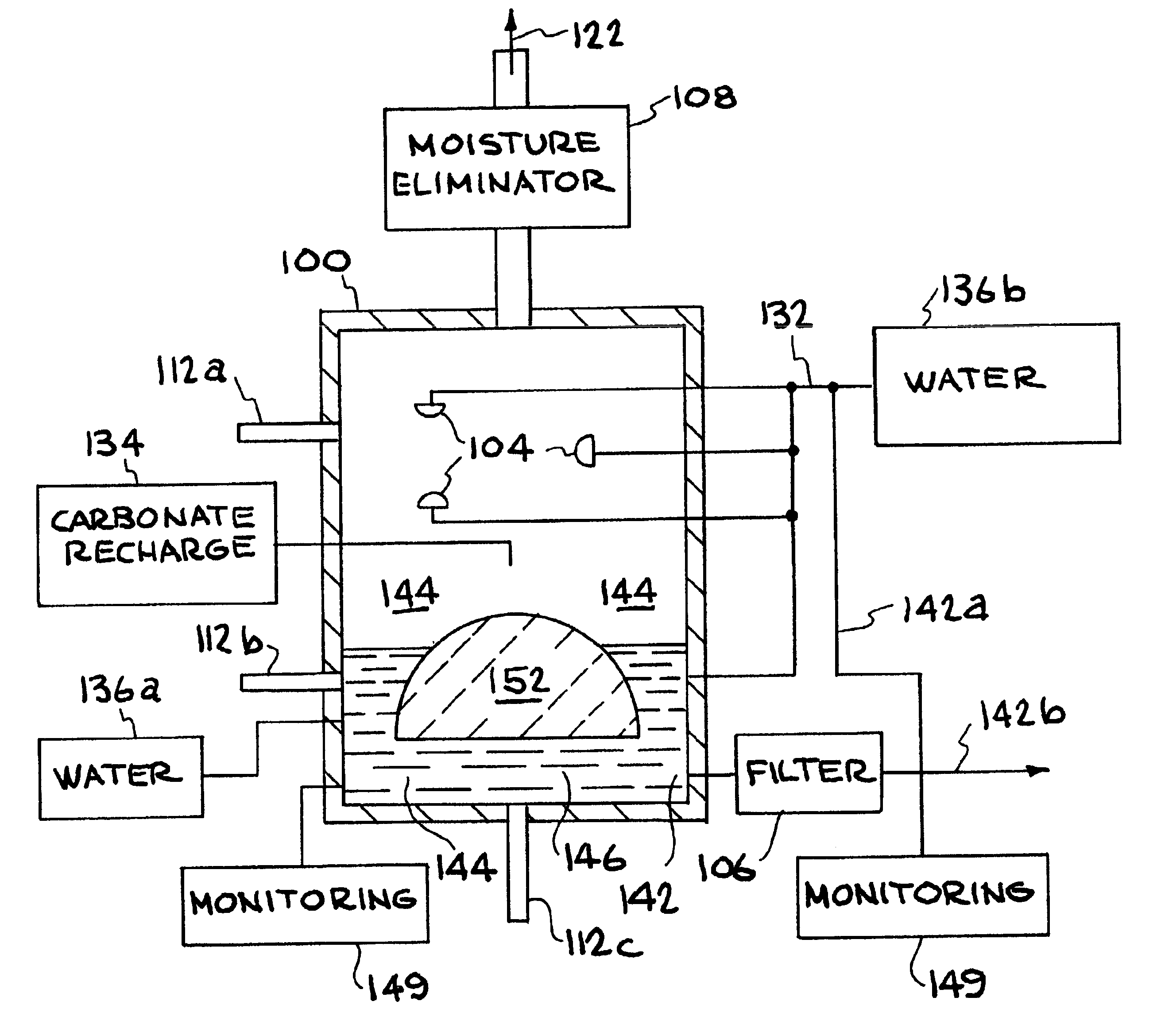
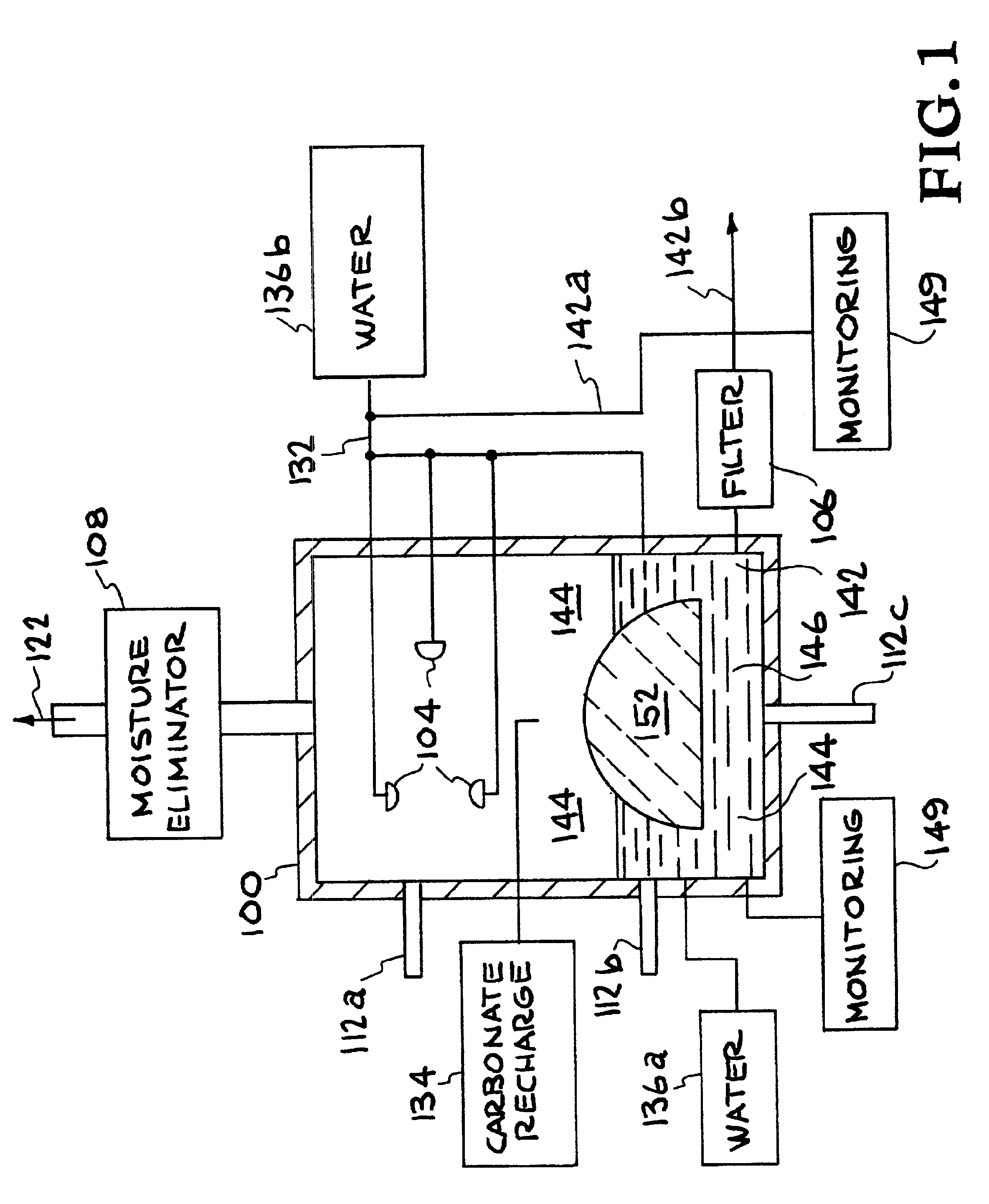
InactiveUS6890497B2Reduce CO burdenWithout significant expenditureCalcium/strontium/barium carbonatesCombination devicesDicarbonateAlkaline earth metal
A method and apparatus to extract and sequester carbon dioxide (CO2) from a stream or volume of gas wherein said method and apparatus hydrates CO2, and reacts the resulting carbonic acid with carbonate. Suitable carbonates include, but are not limited to, carbonates of alkali metals and alkaline earth metals, preferably carbonates of calcium and magnesium. Waste products are metal cations and bicarbonate in solution or dehydrated metal salts, which when disposed of in a large body of water provide an effective way of sequestering CO2 from a gaseous environment.
Owner:LAWRENCE LIVERMORE NAT SECURITY LLC
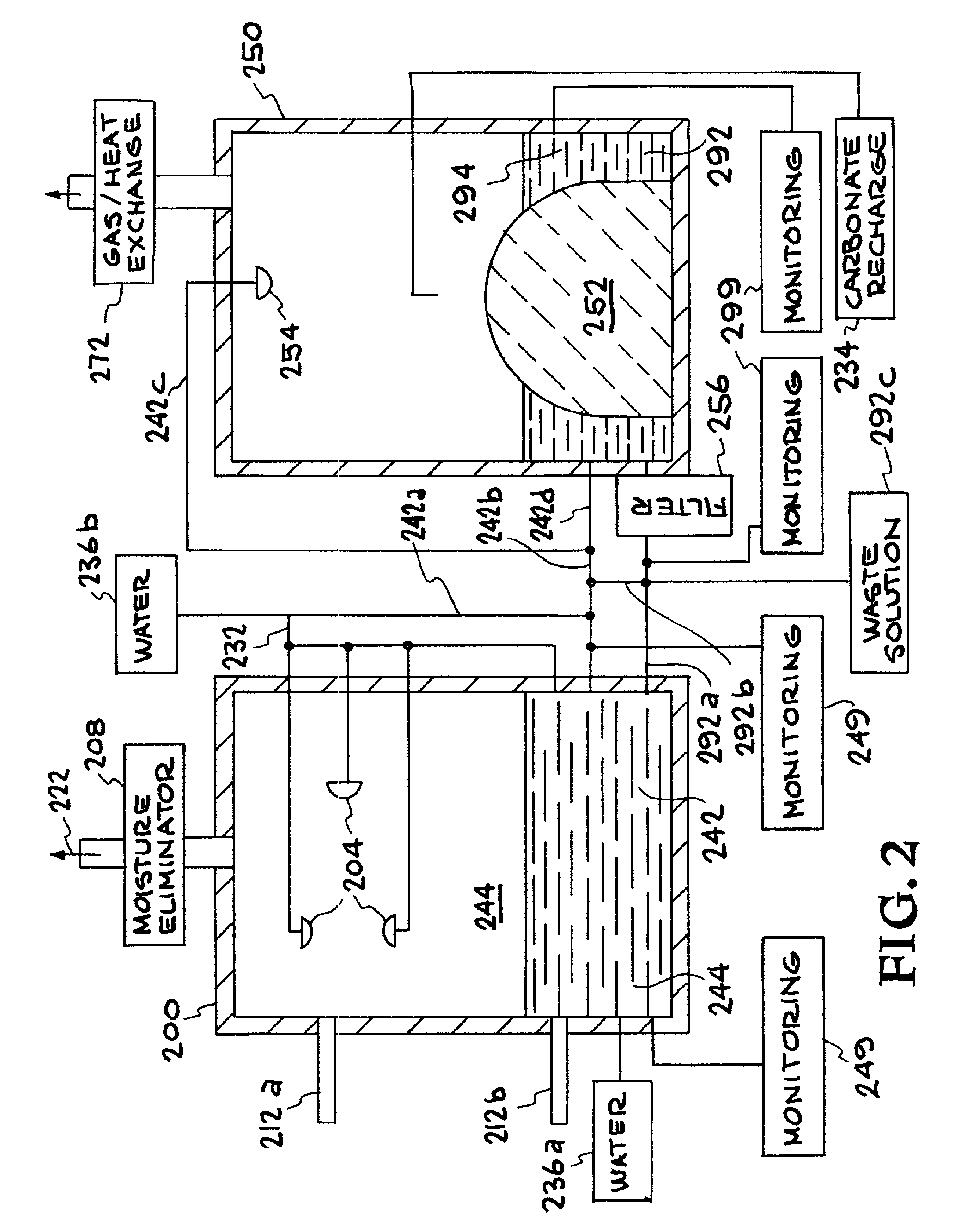
Methods of sequestering co2
Methods of sequestering carbon dioxide (CO2) are provided. Aspects of the methods include precipitating a storage stable carbon dioxide sequestering product from an alkaline-earth-metal-containing water and then disposing of the product, e.g., by placing the product in a disposal location or using the product as a component of a manufactured composition. Also provided are systems for practicing methods of the invention.
Owner:ARELAC INC
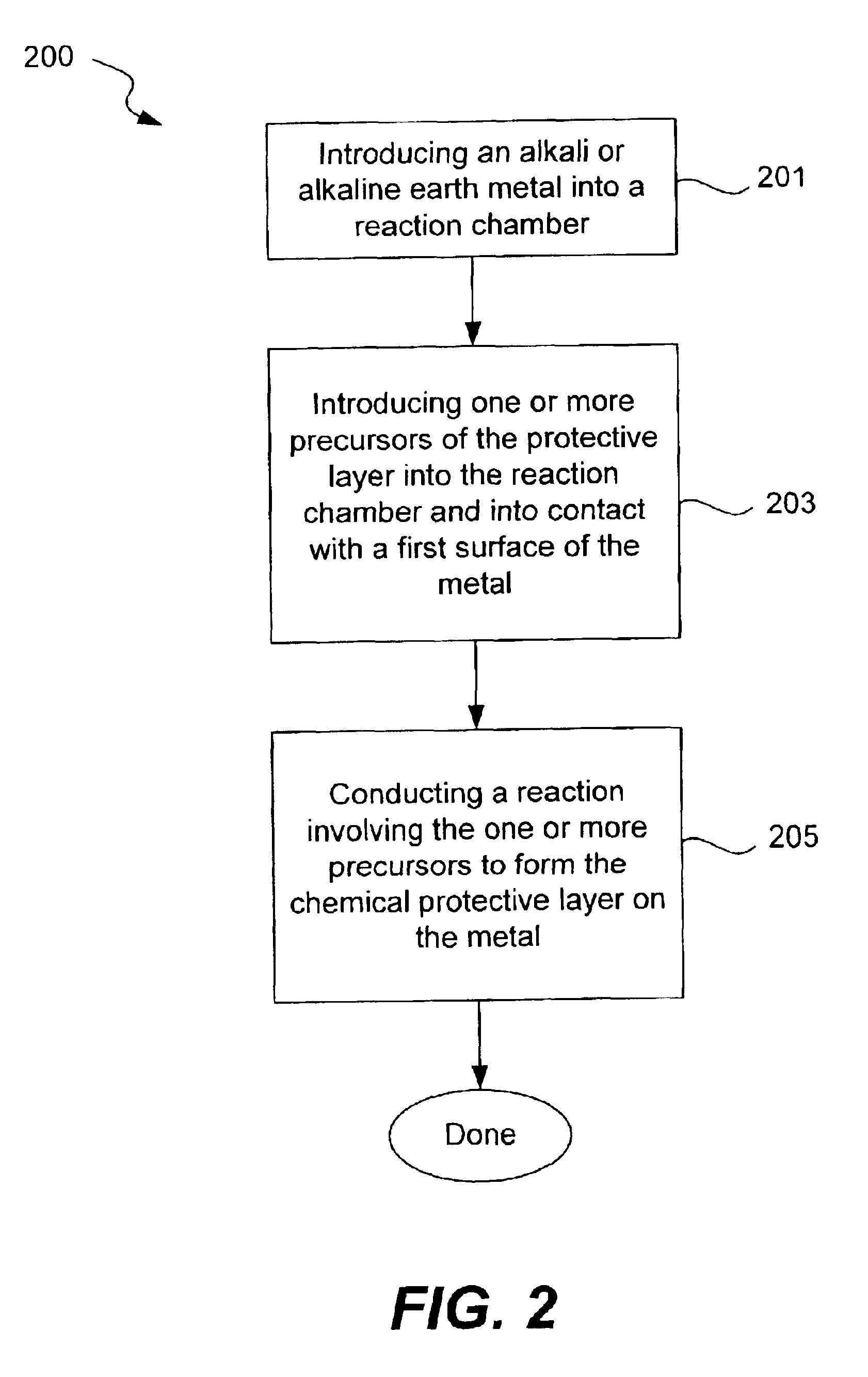
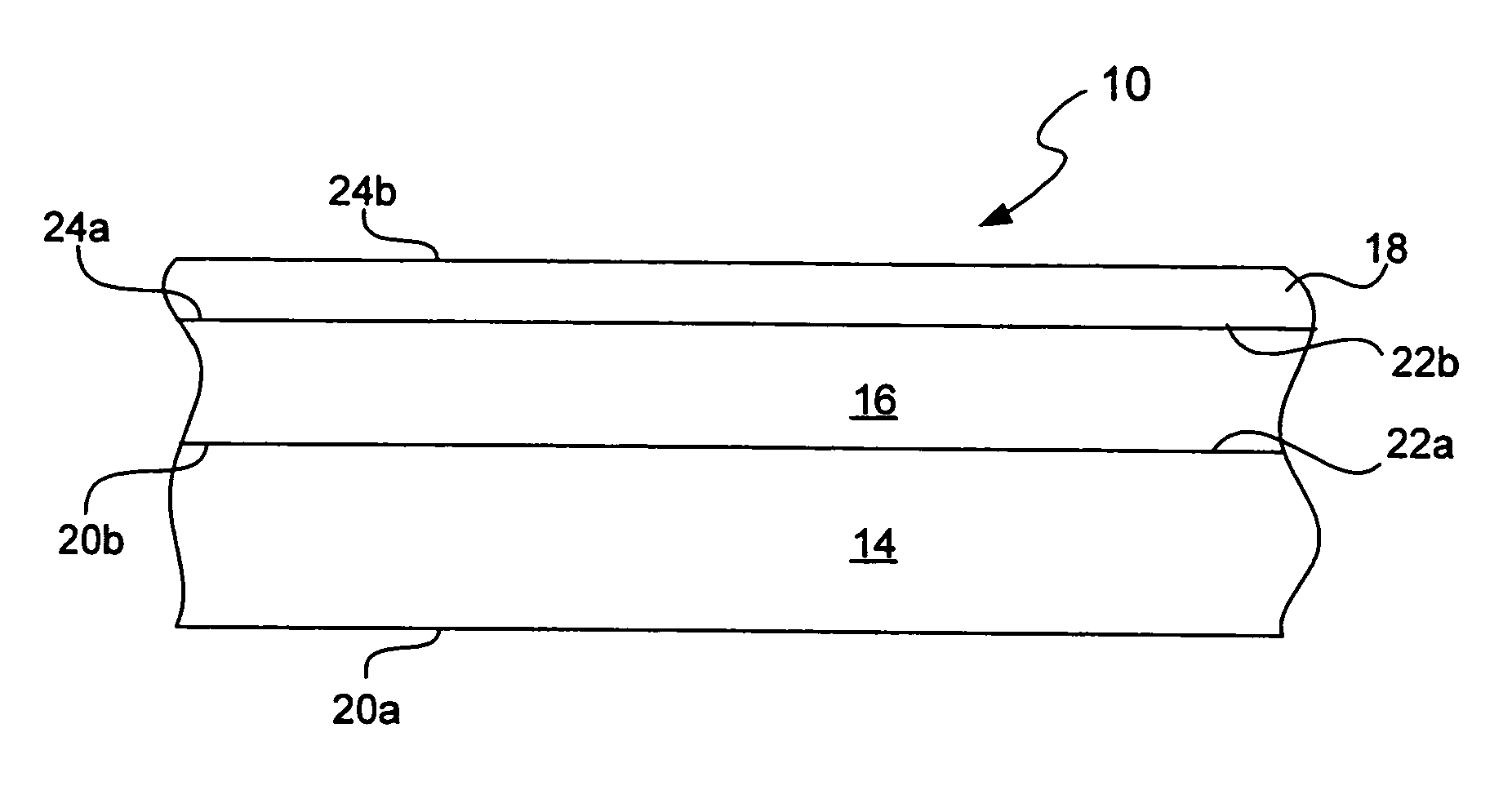
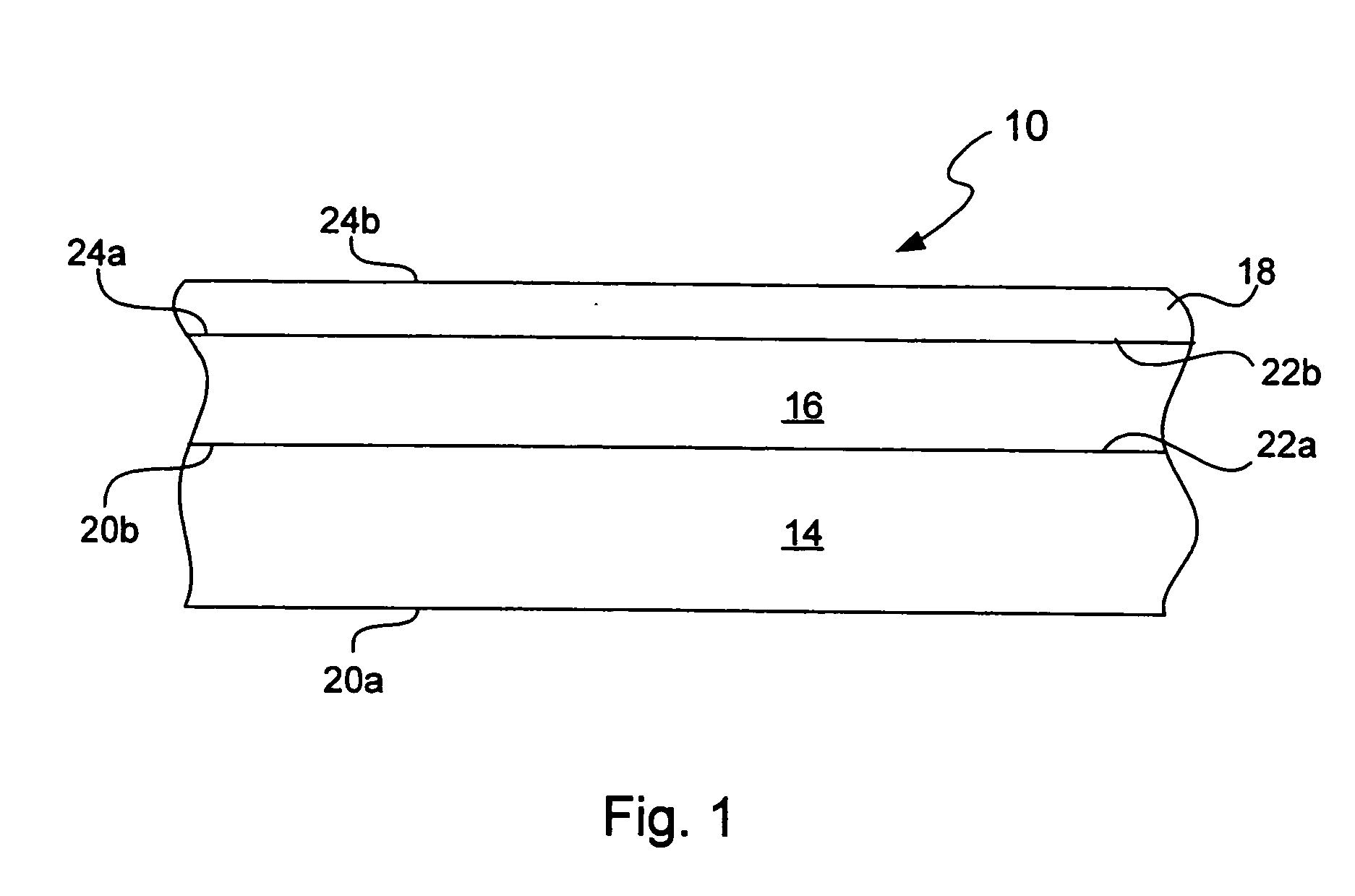
Chemical protection of a lithium surface
InactiveUS6911280B1Easy to produceSimple processElectrochemical processing of electrodesFinal product manufactureAlkaline earth metalLithium metal
Disclosed are compositions and methods for alleviating the problem of reaction of lithium or other alkali or alkaline earth metals with incompatible processing and operating environments by creating a ionically conductive chemical protective layer on the lithium or other reactive metal surface. Such a chemically produced surface layer can protect lithium metal from reacting with oxygen, nitrogen or moisture in ambient atmosphere thereby allowing the lithium material to be handled outside of a controlled atmosphere, such as a dry room. Production processes involving lithium are thereby very considerably simplified. One example of such a process in the processing of lithium to form negative electrodes for lithium metal batteries.
Owner:POLYPLUS BATTERY CO INC
Chemical protection of a lithium surface
InactiveUS20050186469A1Easy to produceEasy to processElectrochemical processing of electrodesFinal product manufactureAlkaline earth metalLithium metal
Disclosed are compositions and methods for alleviating the problem of reaction of lithium or other alkali or alkaline earth metals with incompatible processing and operating environments by creating a ionically conductive chemical protective layer on the lithium or other reactive metal surface. Such a chemically produced surface layer can protect lithium metal from reacting with oxygen, nitrogen or moisture in ambient atmosphere thereby allowing the lithium material to be handled outside of a controlled atmosphere, such as a dry room. Production processes involving lithium are thereby very considerably simplified. One example of such a process is the processing of lithium to form negative electrodes for lithium metal batteries.
Owner:POLYPLUS BATTERY CO INC
Light emitting apparatus and method of manufacturing the same
ActiveUS20060267042A1Improve reliabilityLess variabilitySolid-state devicesSemiconductor/solid-state device manufacturingAlkaline earth metalFluorescence
A light emitting apparatus comprising a light emitting device (101) disposed on a supporting body (105), and coating layers ((108, 109) that bind a fluorescent substance that absorbs light emitted by the light emitting device (101) and emits light of a different wavelength and secures the fluorescent substance onto the surface of the light emitting device (101). The coating layers (108, 109) are made of an inorganic material including an oxide and a hydroxide, each containing at least one element selected from the group consisting of Si, Al, Ga, Ti, Ge, P, B, Zr, Y, Sn, Pb and alkali earth metals. Also an adhesive layer (110) is made of the same inorganic material as that of the coating layers (108, 109).
Owner:NICHIA CORP
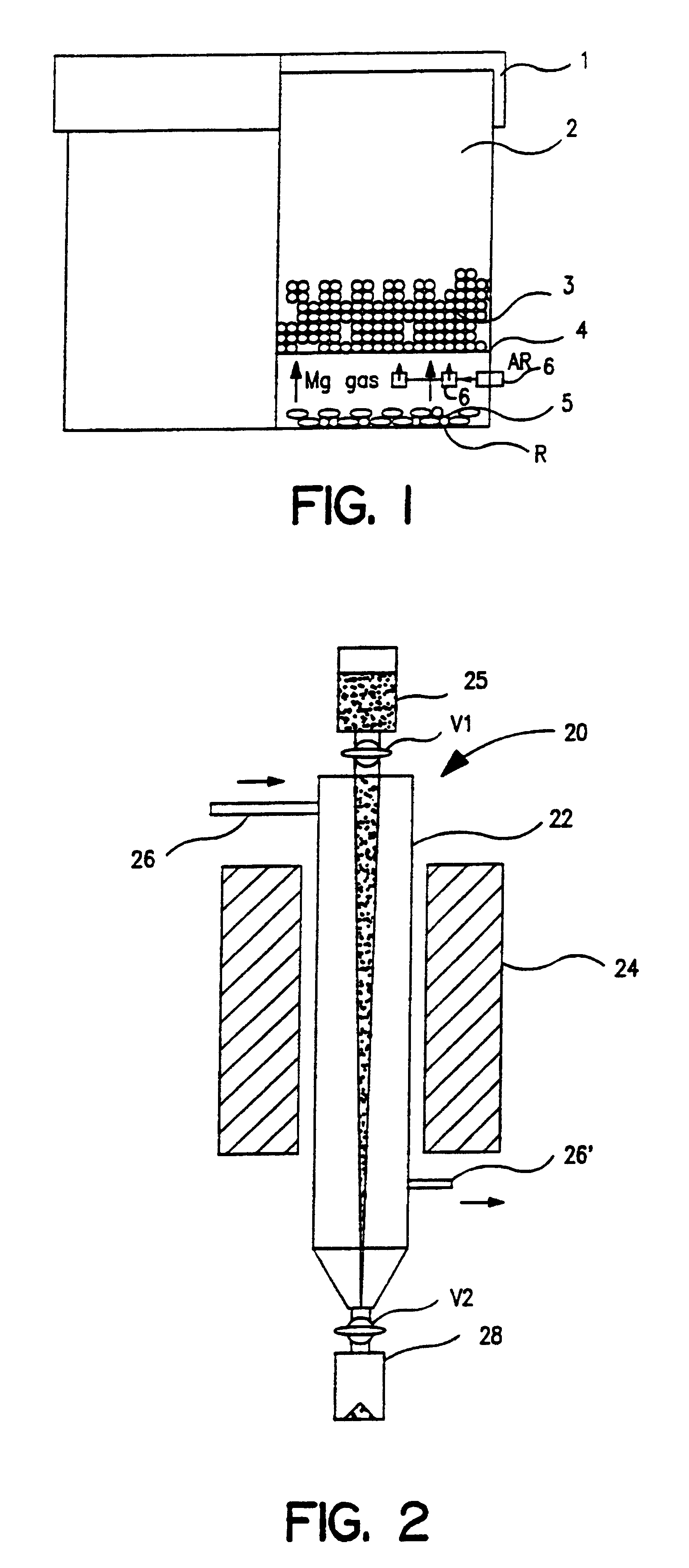
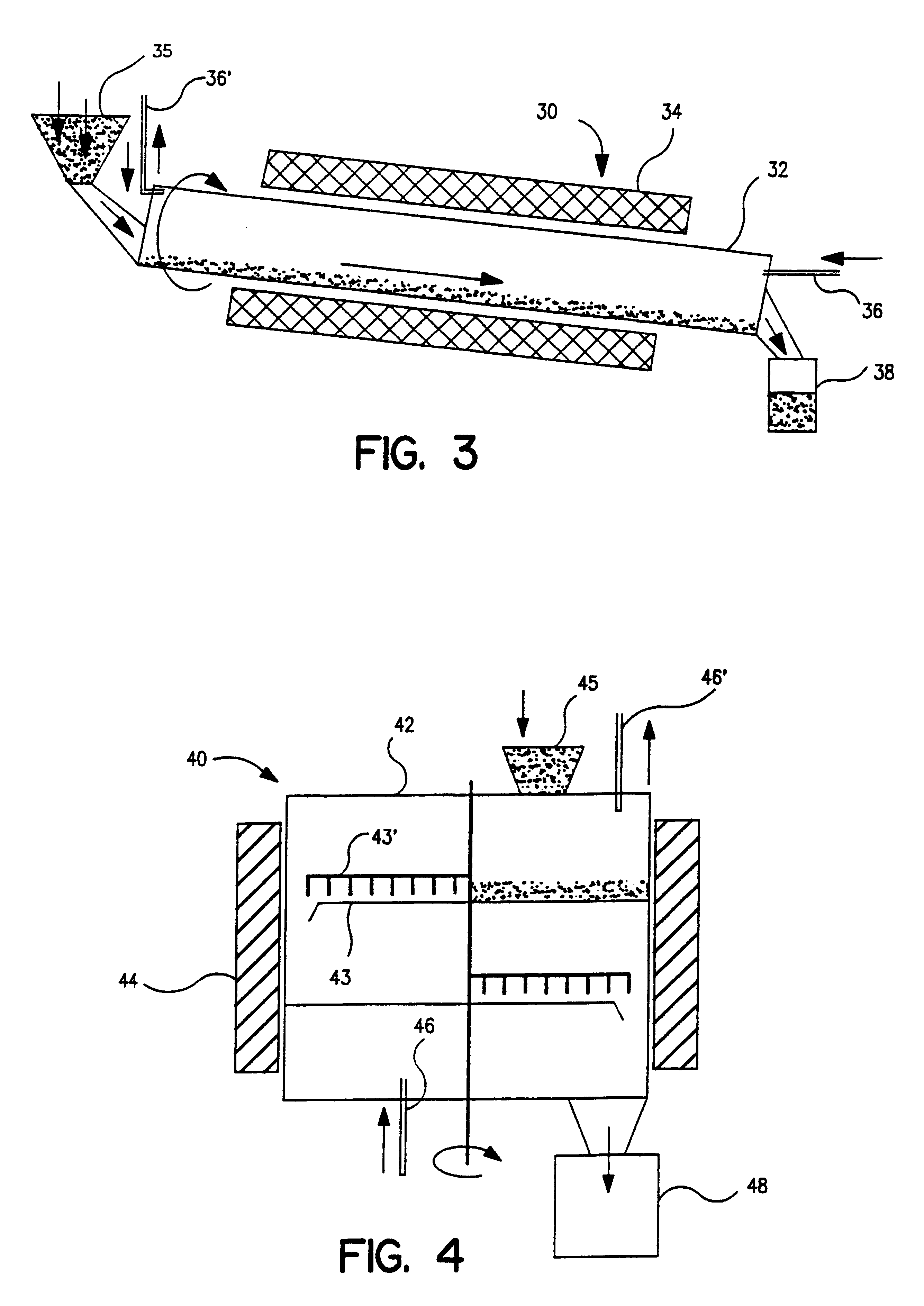
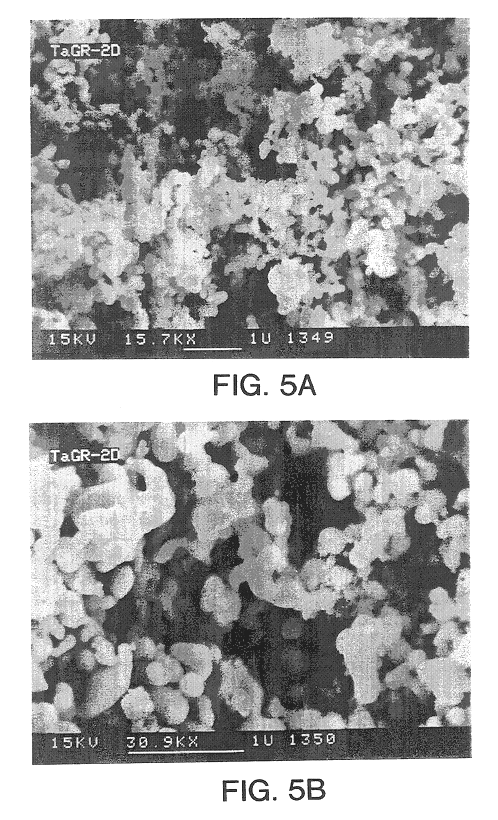
Metal powders produced by the reduction of the oxides with gaseous magnesium
InactiveUS6558447B1Improve performanceEliminate the problemElectrolytic capacitorsTransportation and packagingAlkaline earth metalMetal powder
Metal powder Ta and / or Nb, with or without one or metals from the group Ta, Nb, Ti, Mo, W, V, Zr and Hf, is made in a fine powder form by reduction of metal oxide by contact with a gaseous reducing agent, preferably an alkaline earth metal, to near complete reduction, leaching, further deoxidation and agglomeration, the powder so produced being sinterable to capacitor anode form and processable to other usages.
Owner:H C STARCK TANTALUM & NIOBIUM GMBH
Process for the production of branched melt polycarbonate by late addition of fries-inducing catalyst
The invention relates to a method for the production of a branched polycarbonate composition, having increased melt strength, by late addition of branch-inducing catalysts to the polycarbonate oligomer in a melt polycondensation process. Surprisingly, it has been found that by adding branch-inducing catalysts, such as alkali metal compounds and / or alkaline earth metal compounds, to the melt polycarbonate oligomer at a later stage of the melt polycondensation process, preferably after the oligomer has reached an average molecular weight of between about 3,000 and 30,000 g / mole, a unique branched polycarbonate composition is formed that has improved properties. It is believed that the addition of the branch-inducing catalysts at the later stages of the process produces a branched polycarbonate composition having longer chains between the branching points, and thus a new composition is produced. The invention also relates to various applications of the branched polycarbonate composition.
Owner:SABIC GLOBAL TECH BV
Organic electroluminescent display element, finder screen display device, finder and optical device
InactiveUS6468676B1Discharge tube luminescnet screensElectroluminescent light sourcesNitrosoAlkaline earth metal
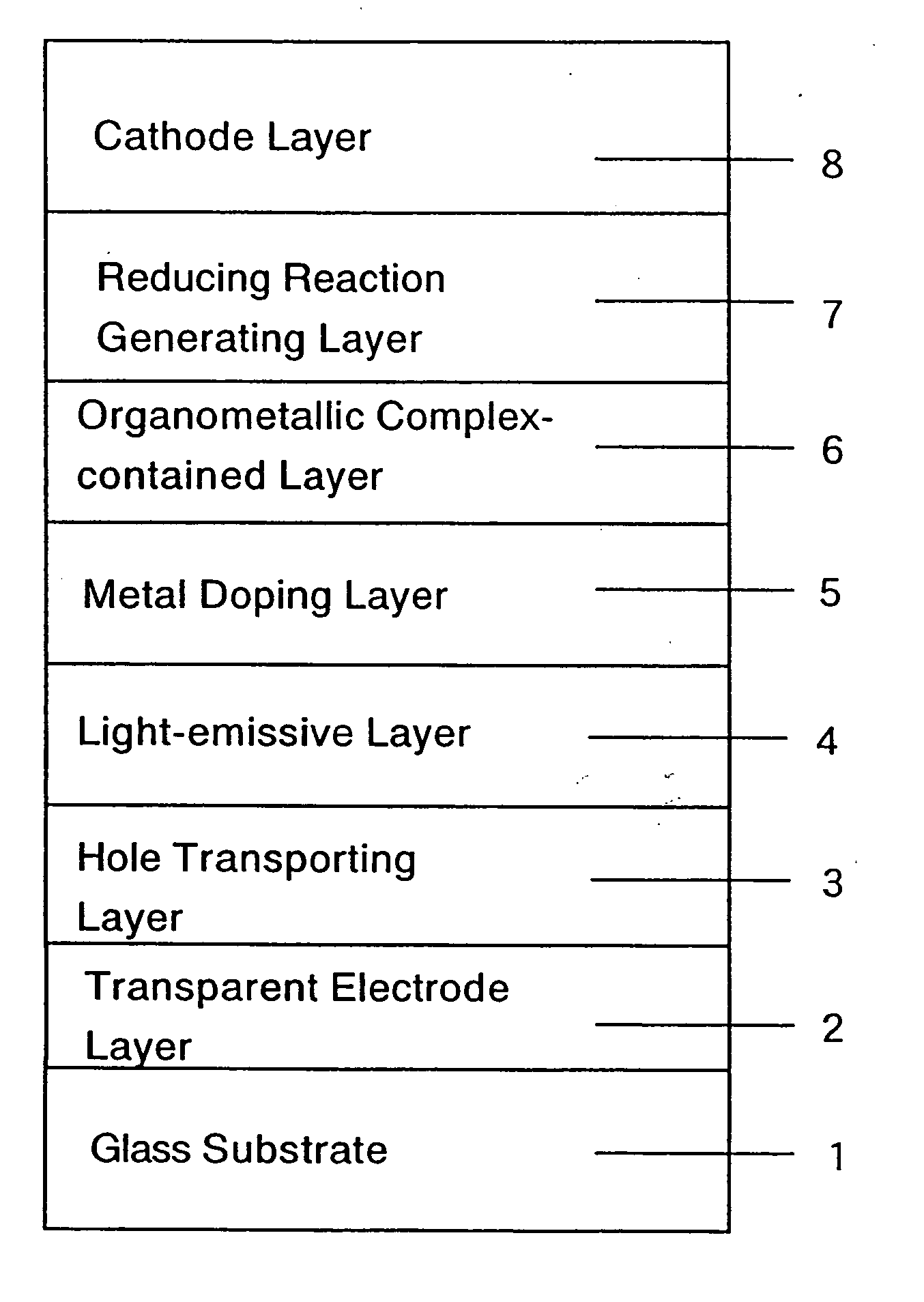
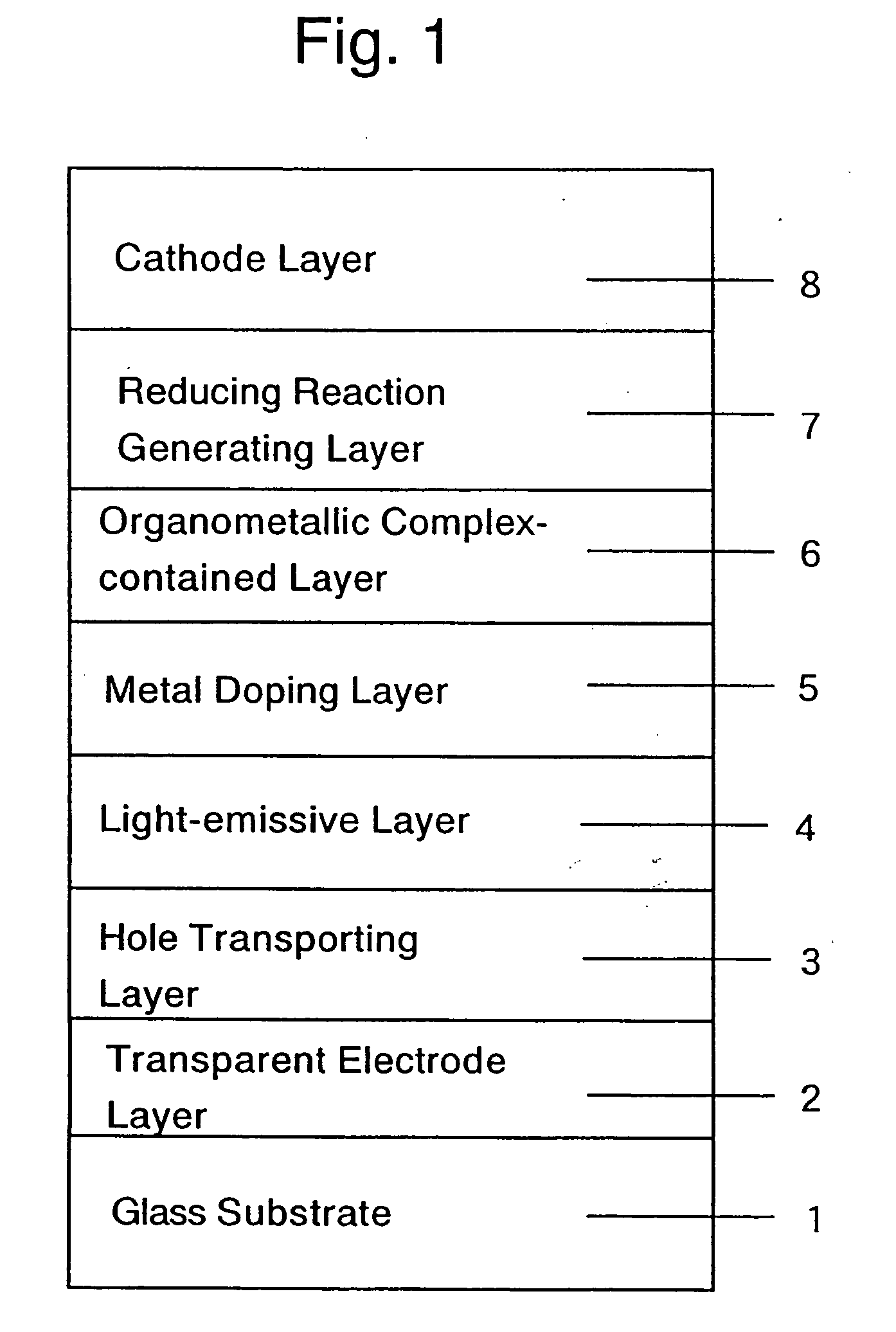
An organic electroluminescent display element has at least a positive electrode, an organic luminescent film, an electron injection layer and a negative electrode. Each of the positive and negative electrodes is formed of a transparent conductive film, the electron injection layer is formed of a thin transparent film made of a halogenide of an alkali metal or an alkaline earth metal, or an organic metal complex containing an alkali metal or an alkaline earth metal as a metal, and the organic metal complex is at least one complex selected from the group consisting of acetylacetonate complexes, alpha-nitroso-beta-naphthol complexes, salicylaldoxime complexes, cupferron complexes, benzoinoxime complexes, bipyridine complexes, phenanthroline complexes, crown complexes, proline complexes and benzoylacetone complexes.
Owner:KONICA MINOLTA INC
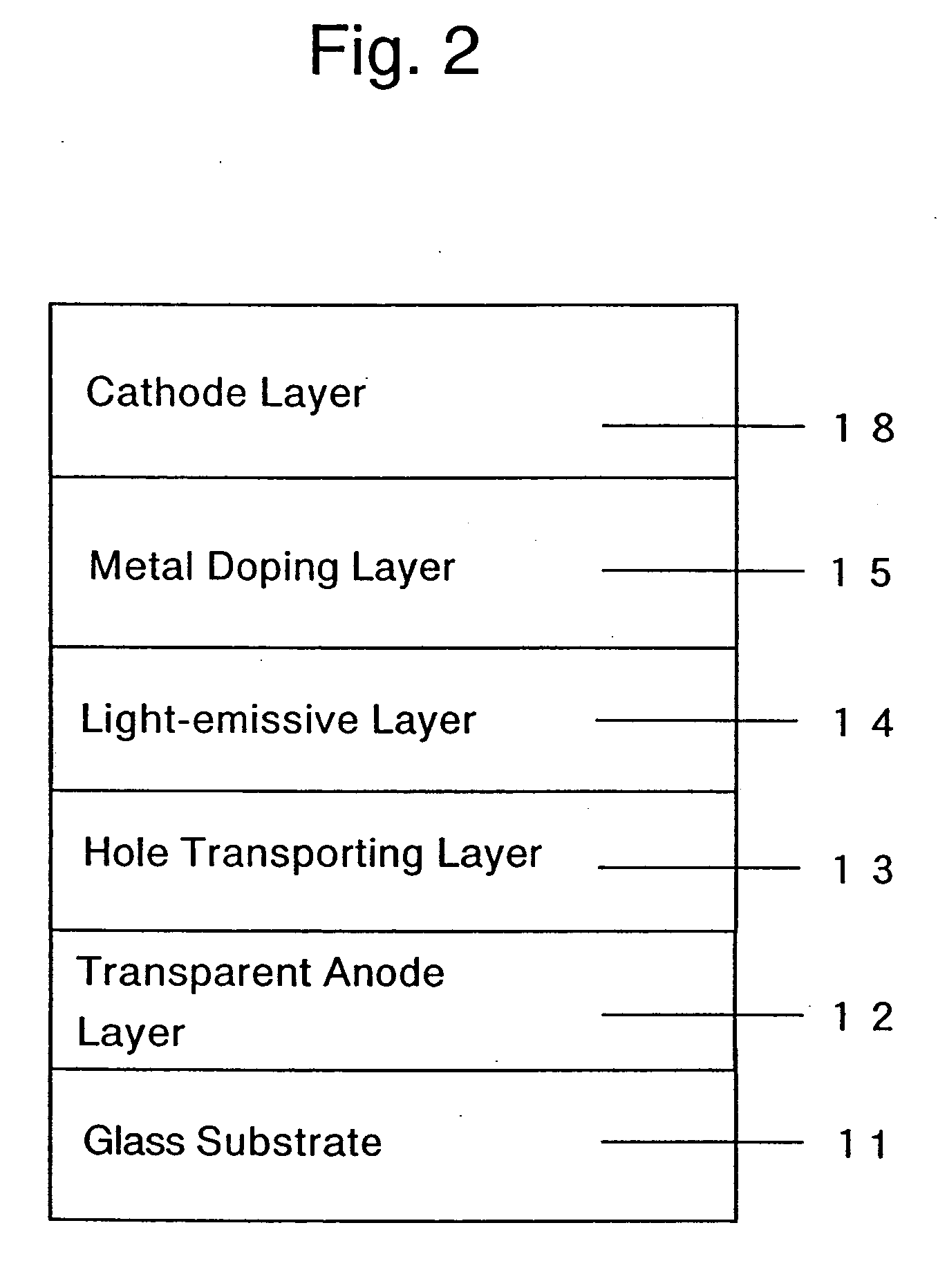
Organic electroluminescent device and production process thereof
ActiveUS20050084713A1Stable device propertyIncrease the driving voltageDischarge tube luminescnet screensElectroluminescent light sourcesOrganic structureAlkaline earth metal
Owner:ROHM CO LTD +1
Light-emitting element, lighting device, and electronic appliance
ActiveUS20170012232A1Element characteristicIncrease the driving voltageMechanical apparatusDomestic lightingAlkaline earth metalEngineering
A tandem light-emitting element employing an inverted-structure is provided. The light-emitting element includes a cathode, a first EL layer over the cathode, a second EL layer over the first EL layer, an anode over the second EL layer, and an intermediate layer. The intermediate layer is between the first EL layer and the second EL layer. The intermediate layer includes a first layer, a second layer over the first layer, and a third layer over the second layer. The first layer includes a hole-transport material and an electron acceptor. The third layer includes an alkali metal or an alkaline earth metal. The second layer includes an electron-transport material.
Owner:SEMICON ENERGY LAB CO LTD
Well treatment fluid compositions and methods for their use
InactiveUS6983801B2High viscosityIncrease volumeFluid removalFlushingAlkaline earth metalCarboxylic acid
Owner:COMBINED SYST +1
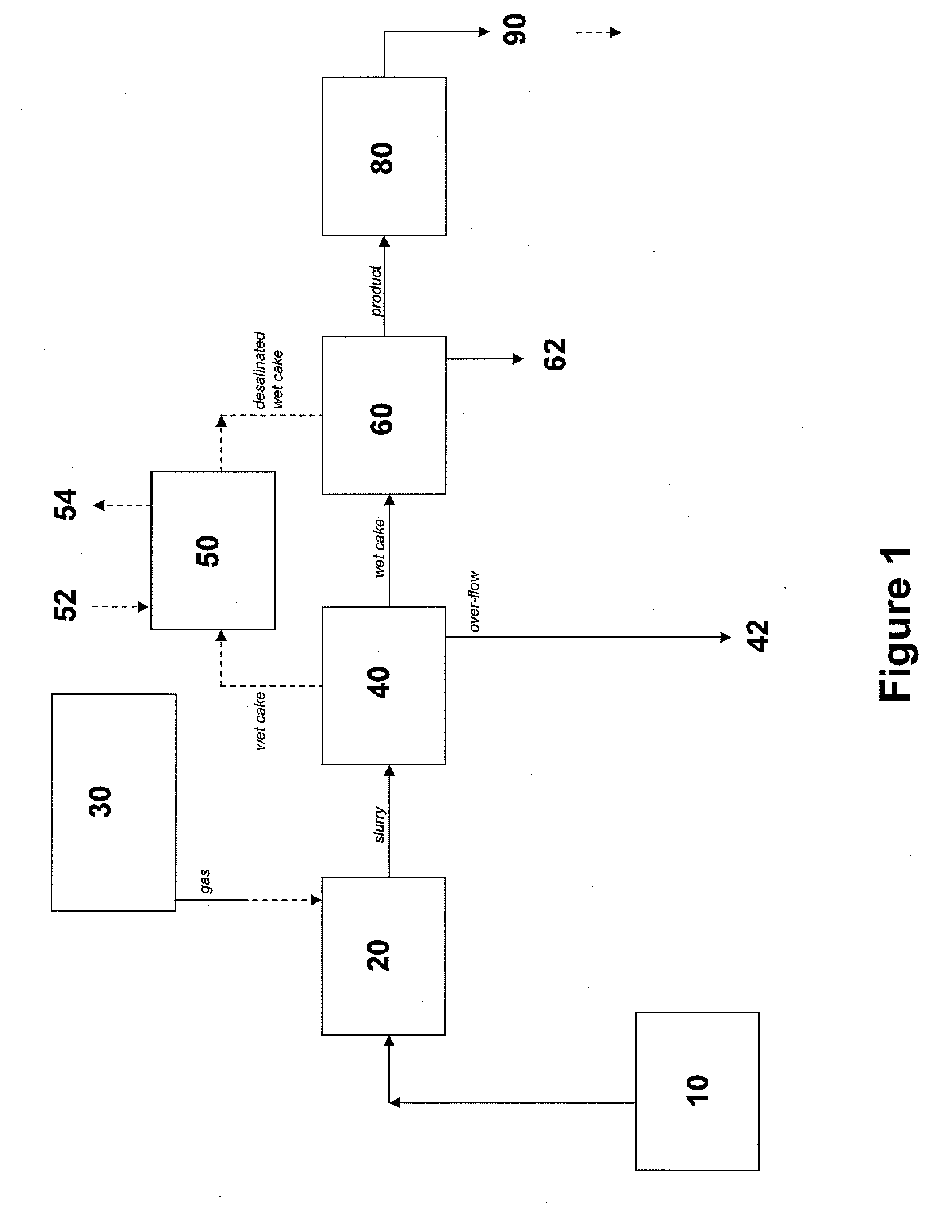
Carbon dioxide sequestration using alkaline earth metal-bearing minerals
ActiveUS20050180910A1High dissolution rateEfficient removalCalcium/strontium/barium carbonatesProductsParticulatesAlkaline earth metal
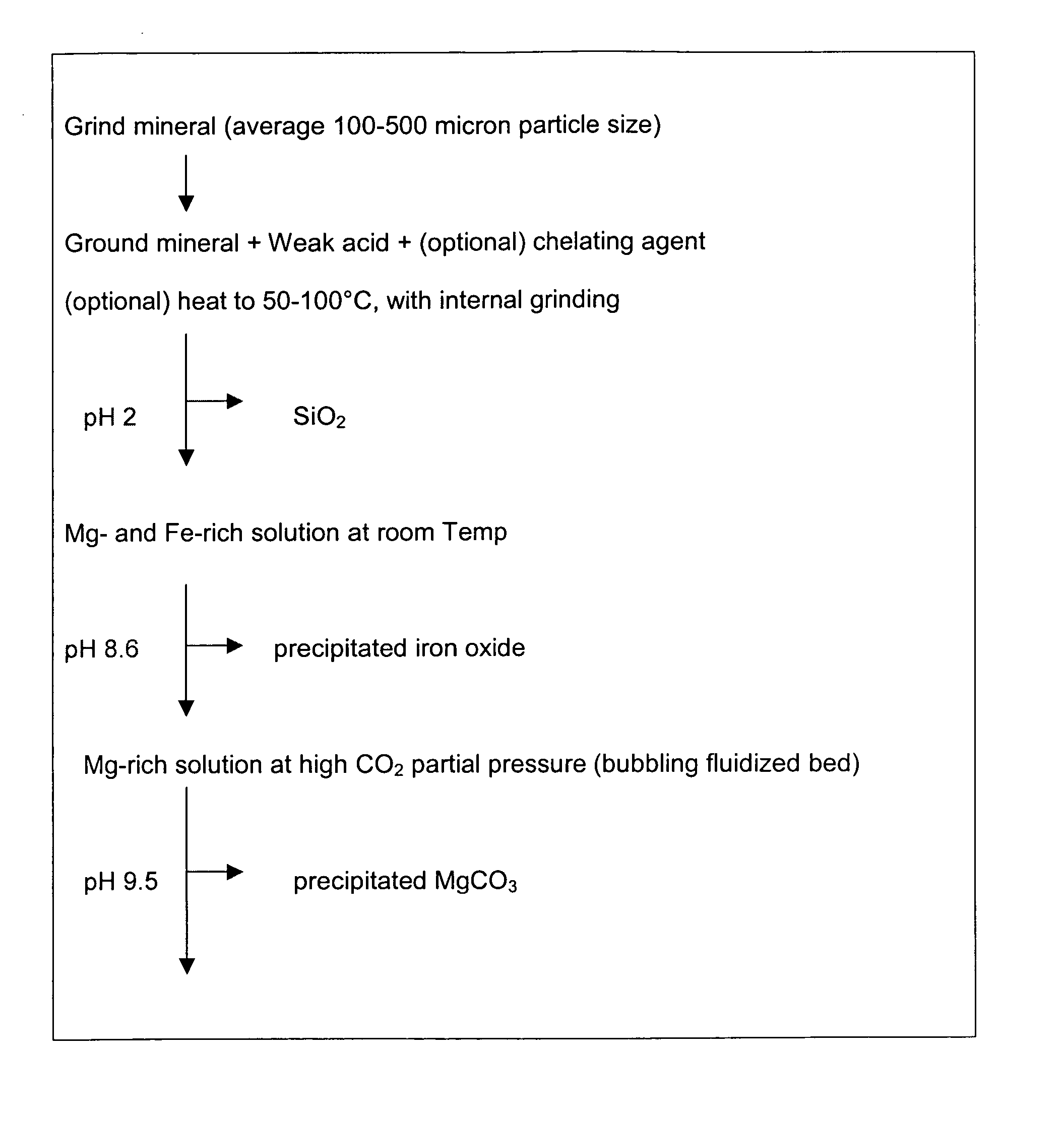
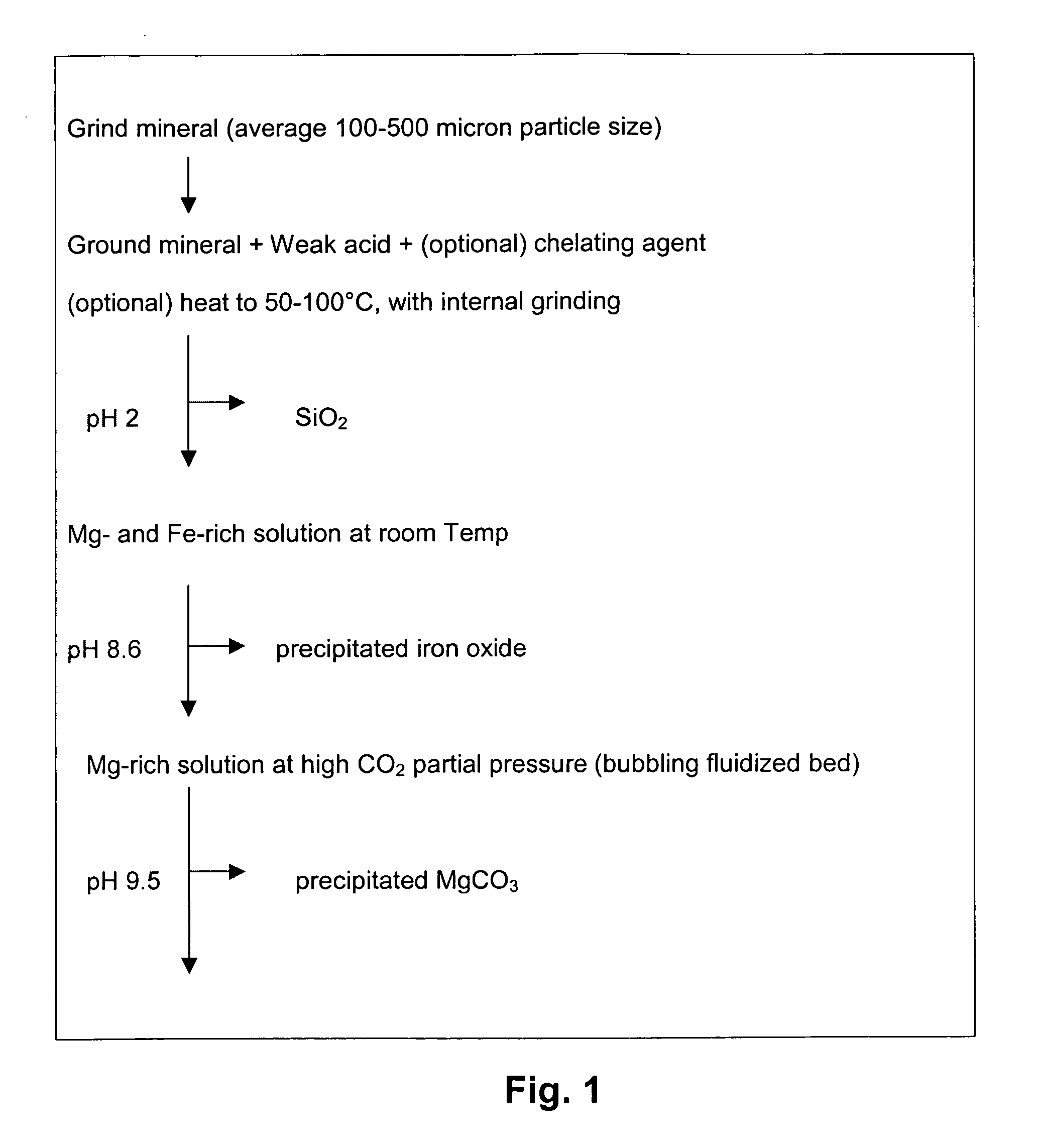
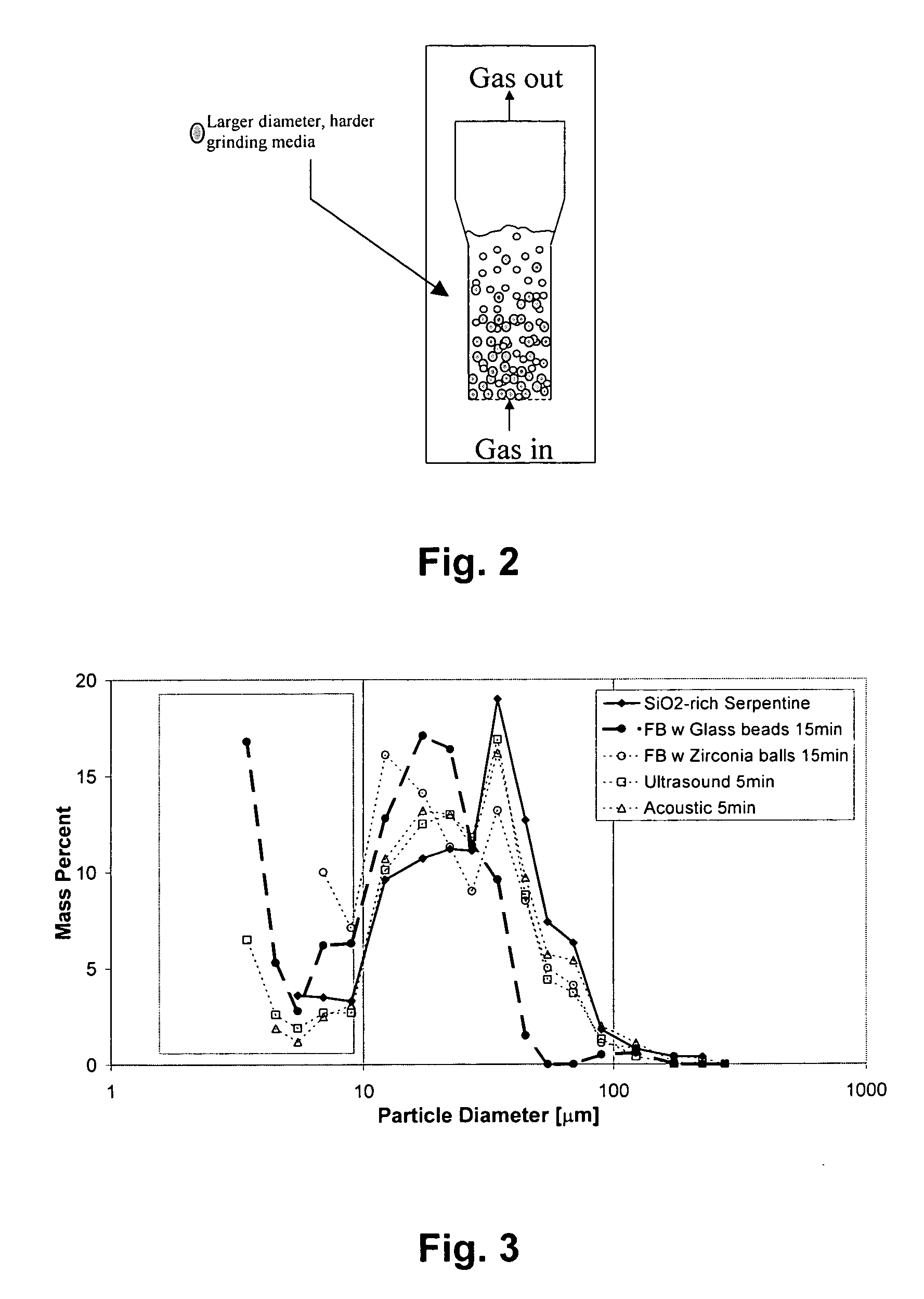
A method for mineral sequestration of pollutant gases resulting from the combustion of carbon-based fuels such as carbon and sulfur dioxides is provided and includes, providing a particulate magnesium-containing mineral and exposing the magnesium-containing mineral to a weak acid to dissolve magnesium from the mineral and form a magnesium-containing solution. The surface of the particulate magnesium-containing mineral is physically activated to expose and dissolve additional magnesium into the solution. Pollutant gases such as carbon dioxide are mixed with the magnesium-containing solution. When the pH of the magnesium-containing solution is increased, solid magnesium carbonate is formed.
Owner:THE OHIO STATES UNIV
Nitride-based red phosphors
InactiveUS20090283721A1Gas discharge lamp usageLuminescent compositionsAlkaline earth metalFluorescence
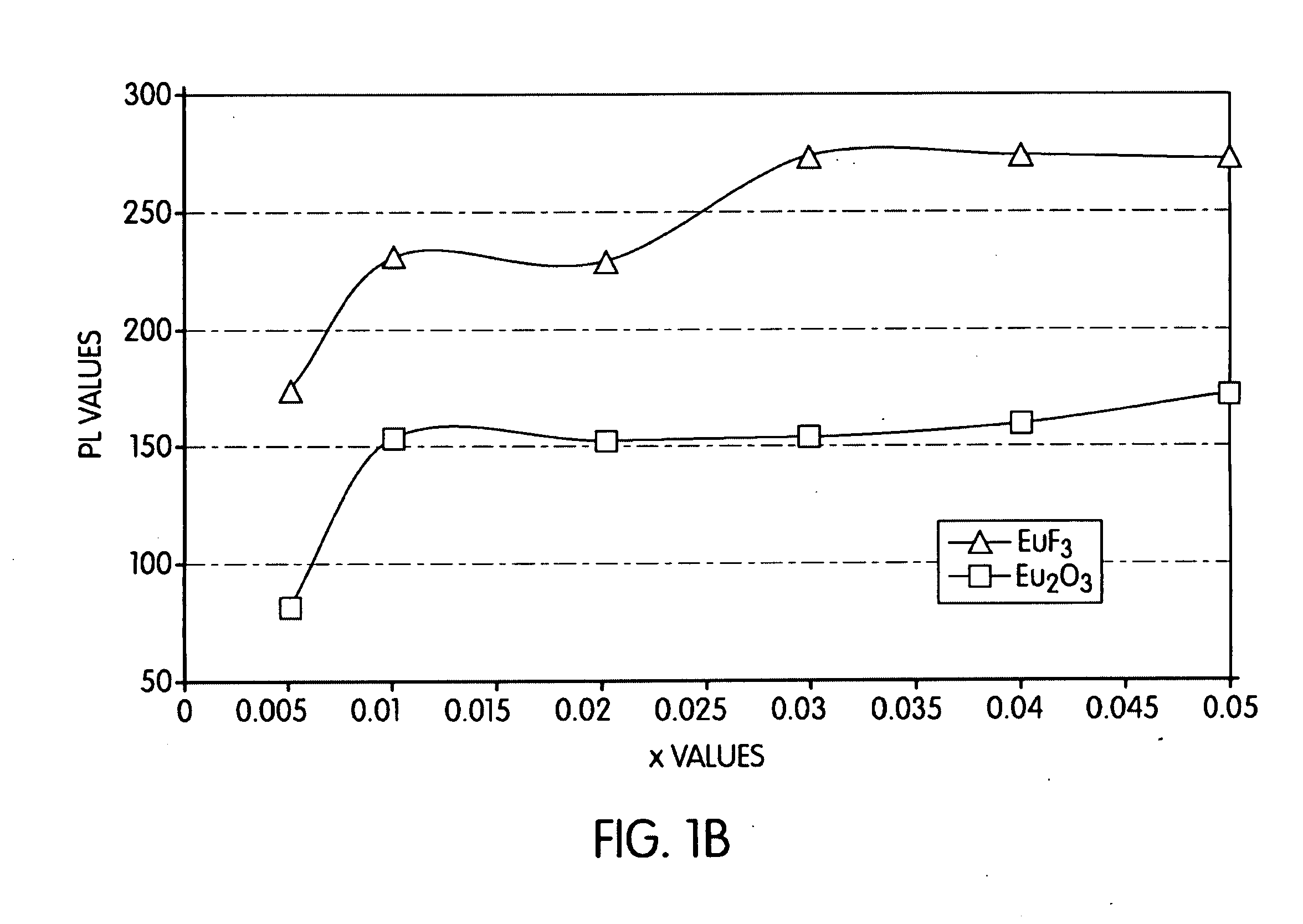
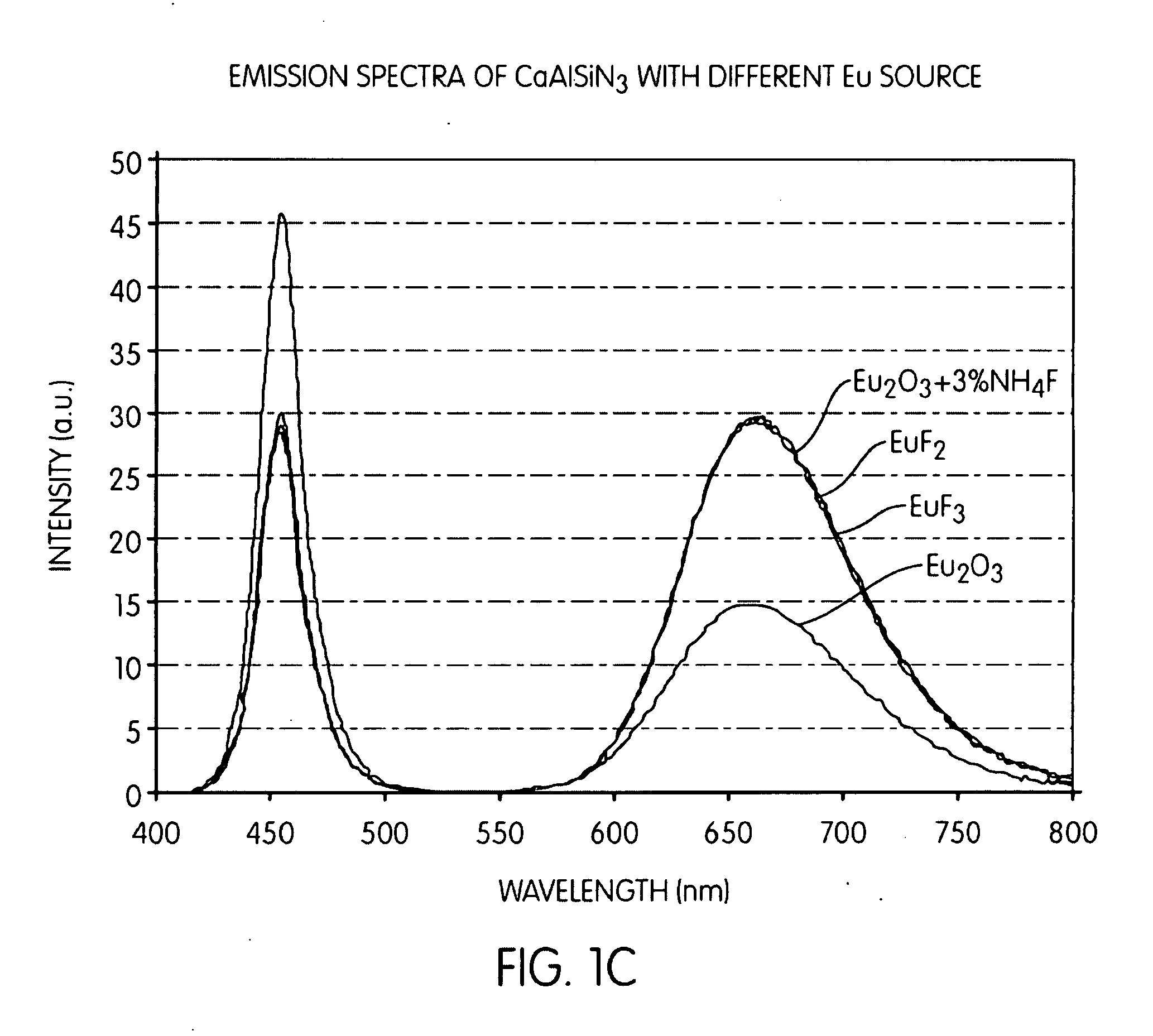
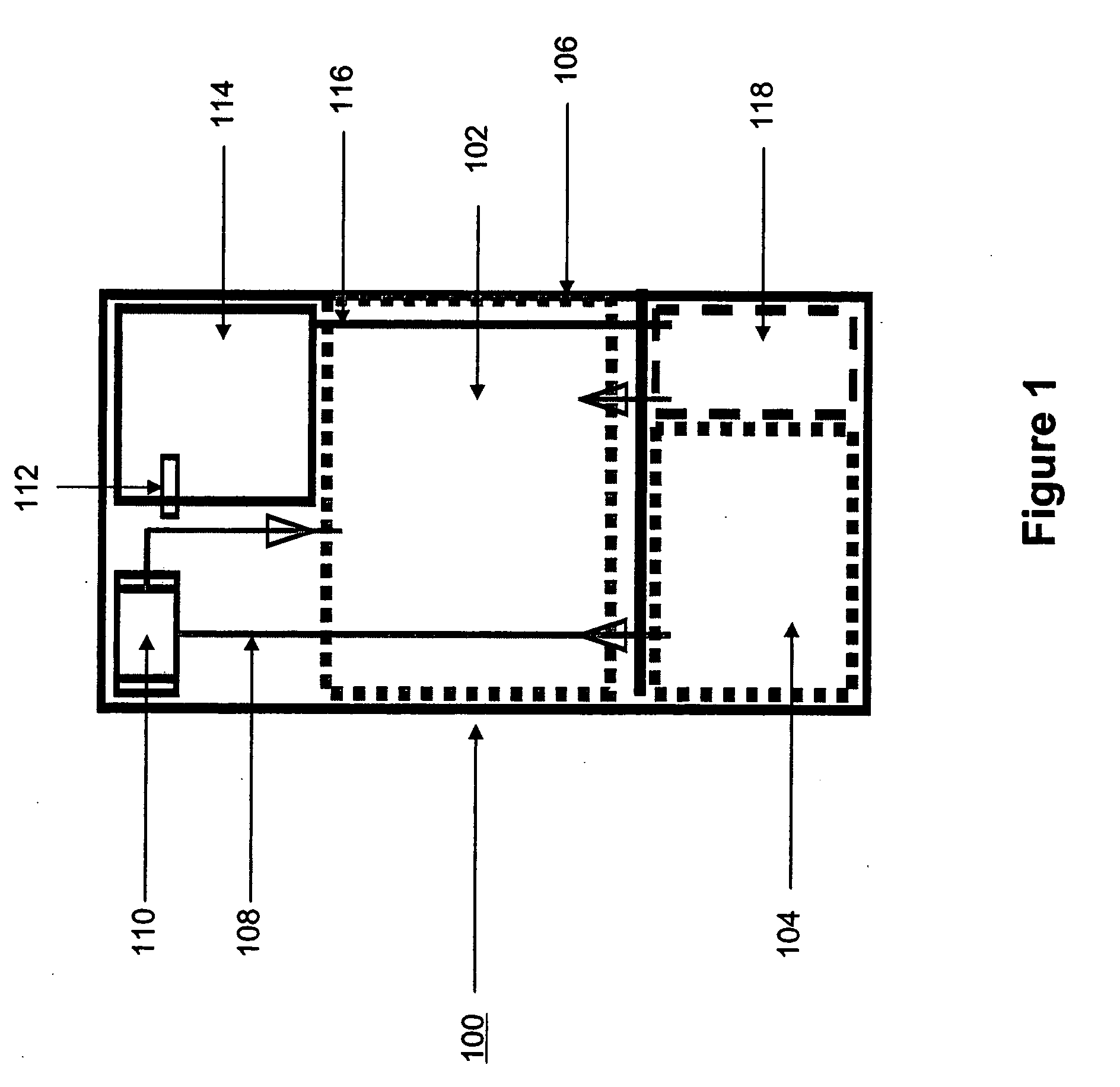
Embodiments of the present invention are directed to the fluorescence of a nitride-based deep red phosphor having at least one of the following novel features: 1) an oxygen content less than about 2 percent by weight, and 2) a halogen content. Such phosphors are particularly useful in the white light illumination industry, which utilizes the so-called “white LED.” The selection and use of a rare earth halide as a raw material source of not only the activator for the phosphor, but also the halogen, is a key feature of the present embodiments. The present phosphors have the general formula MaMbBC(N,D):Eu2+, where Ma is a divalent alkaline earth metal such as Mg, Ca, Sr, Ba; Mb is a trivalent metal such as Al, Ga, Bi, Y, La, and Sm; and Mc is a tetravalent element such as Si, Ge, P, and B; N is nitrogen, and D is a halogen such as F, Cl, or Br. An exemplary compound is CaAlSi(N1-xFx): Eu2+.
Owner:INTEMATIX
Composite metal oxide for unsaturated aldehyde selective oxidation and preparing method thereof
A composite metallic oxide as catalyst for selective oxidization of unsaturated aldehyde, especially the acrylaldehyde or methyl acrylaldehyde to obtain acrylic acid or methyl acrylic acid, is composed of active components (Mo, V and Cu), stabilizer (at least Sb and Ti) and the composite oxide of Ni, Fe, Si, Al, alkali metal and alkali-earth metal. Its preparing process is also disclosed.
Owner:兰州金润宏成新材料科技有限公司
Systems and methods for hydrogen generation from solid hydrides
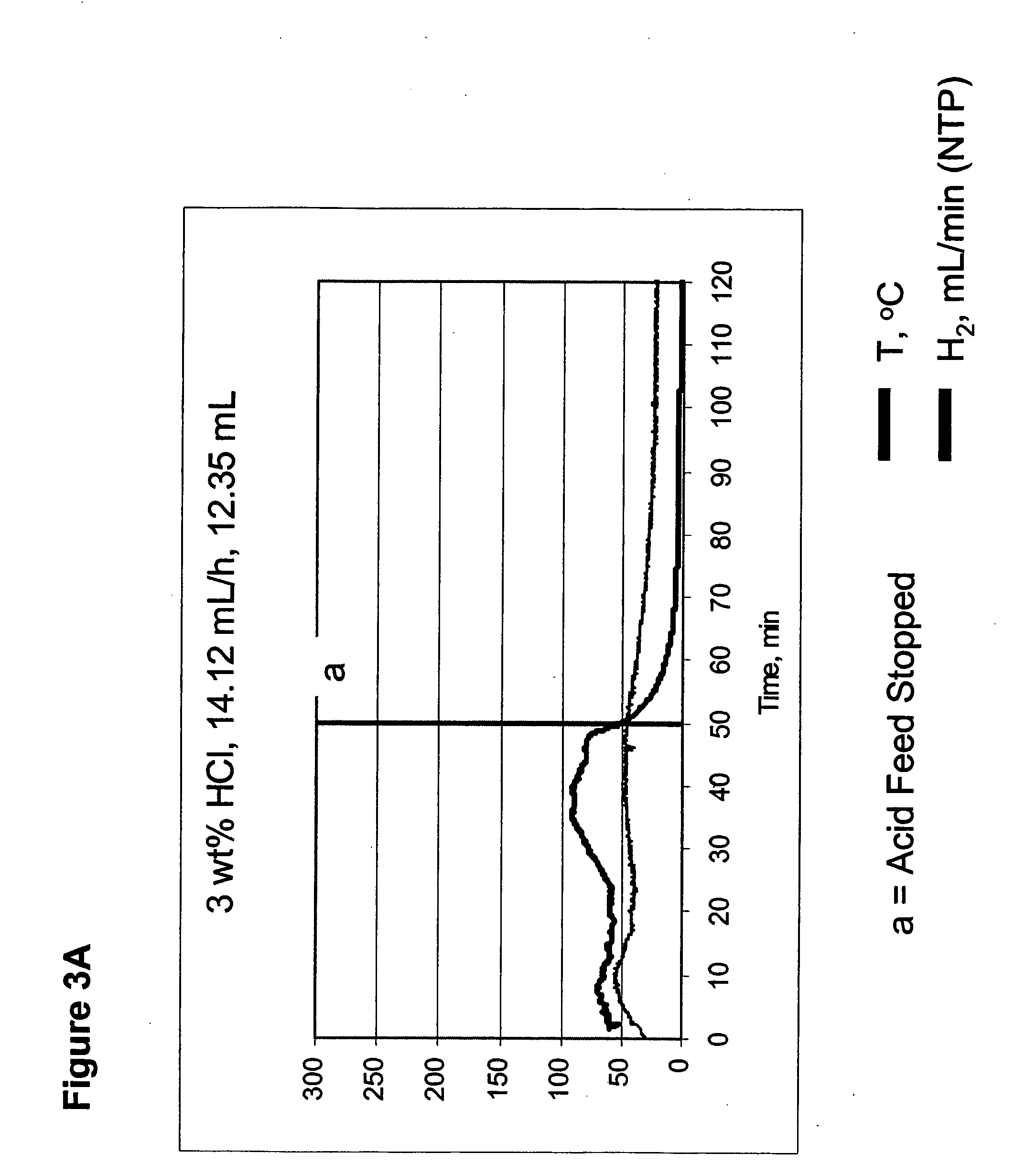
InactiveUS20050238573A1Regulate rateReactant parameters controlHydrogen productionO-Phosphoric AcidAlkaline earth metal
A system is disclosed for hydrogen generation based on hydrolysis of solid chemical hydrides with the capability of controlled startup and stop characteristics wherein regulation of acid concentration, acid feed rate, or a combination of both control the rate of hydrogen generation. The system comprises a first chamber for storing a solid chemical hydride and a second chamber for storing an acidic reagent. The solid chemical hydride is a solid metal borohydride having the general formula MBH4, where M is selected from the group consisting of alkali metal cations, alkaline earth metal cations, aluminum cation, zinc cation, and ammonium cation. The acidic reagent may comprise inorganic acids such as the mineral acids hydrochloric acid, sulfuric acid, and phosphoric acid, and organic acids such as acetic acid, formic acid, maleic acid, citric acid, and tartaric acid, or mixtures thereof.
Owner:MILLENNIUM CELL
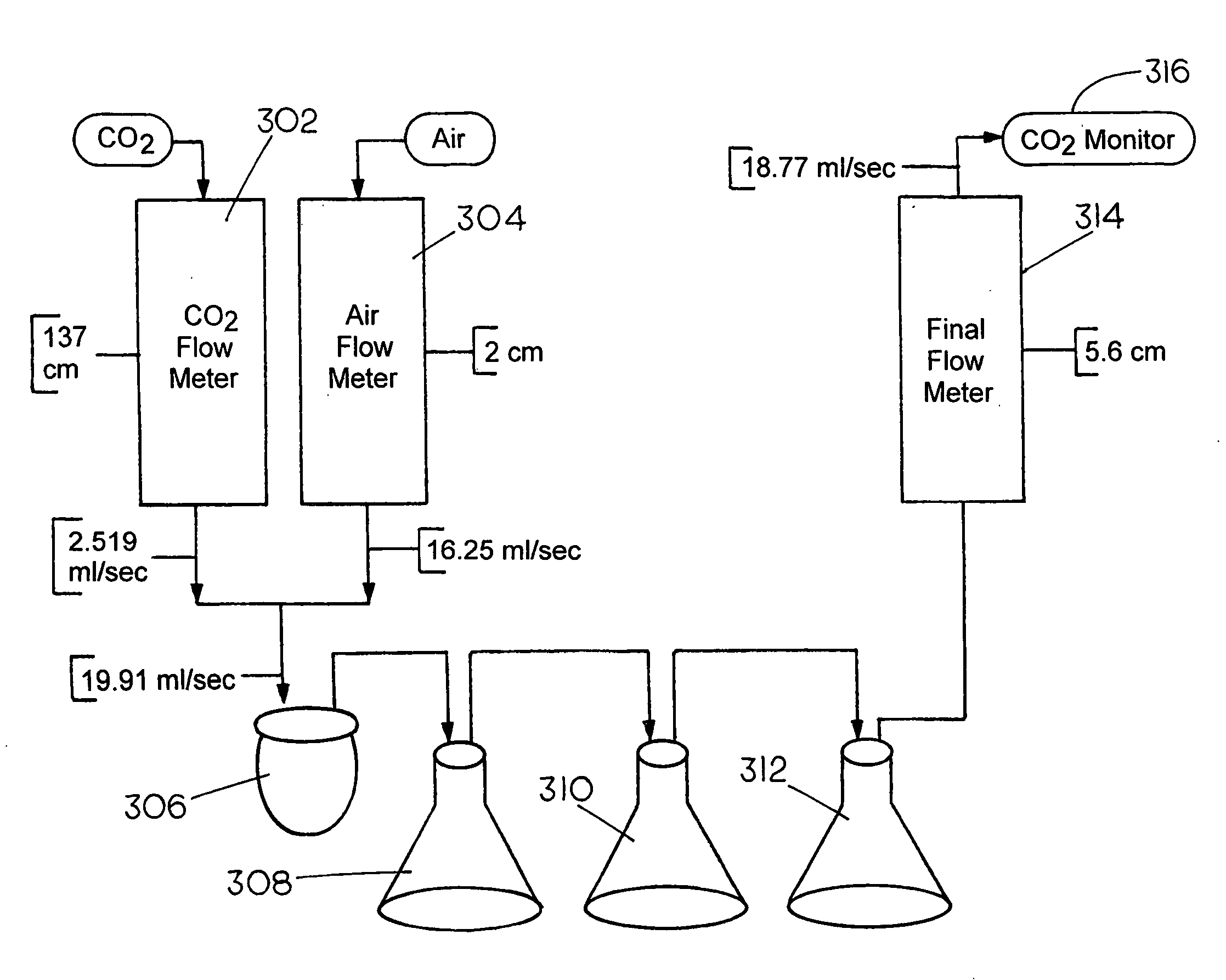
Capture and Sequestration of Carbon Dioxide in Flue Gases
ActiveUS20090202410A1Calcium/strontium/barium carbonatesPigmenting treatmentAlkaline earth metalOxidation state
There is provided a process for the capture and sequestration of carbon dioxide that would otherwise enter the atmosphere and contribute to global warming and other problems. CO2 capture is accomplished by reacting carbon dioxide in flue gas with an alkali metal carbonate, or a metal oxide, particularly containing an alkaline earth metal or iron, to form a carbonate salt. A preferred carbonate for CO2 capture is a dilute aqueous solution of additive-free (Na2CO3). Other carbonates include (K2CO3) or other metal ion that can produce both a carbonate and a bicarbonate salt. Examples of suitable metal oxides include several alkaline earths including CaO and MgO. The captured CO2 is preferably sequestered using any available mineral or industrial waste that contains calcium magnesium or iron in non-carbonate forms, or iron in the Fe+2 oxidation state.
Owner:MICHIGAN TECHNOLOGICAL UNIVERSITY
Alkaline, post plasma etch/ash residue removers and photoresist stripping compositions containing metal-halide corrosion inhibitors
InactiveUS20070060490A1Avoiding consequenceAvoid expensesDetergent mixture composition preparationElectrostatic cleaningAlkaline earth metalMetal halides
The invention provides alkaline compositions useful in the microelectronics industry for stripping or cleaning semiconductor wafer substrates by removing photoresist residues and other unwanted contaminants. The compositions contain (a) one or more bases and (b) one or more metal corrosion inhibiting metal halides of the formula: WzMXy where M is a metal selected from the group Si, Ge, Sn, Pt, P, B, Au, Ir, Os, Cr, Ti, Zr, Rh, Ru, and Sb; X is a halide selected from F, Cl, Br and I; W is selected from H, to an alkali or alkaline earth metal, and a metal ion-free hydroxide base moiety; y is a numeral of from 4 to 6 depending on the metal halide; and z is a numeral of 1, 2 or 3.
Owner:AVANTOR PERFORMANCE MATERIALS INC
Oxide films with nanodot flux pinning centers
InactiveUS20050159298A1Increasing critical current densitySimple and versatileMaterial nanotechnologyMolecular sieve catalystsNanodotRare-earth element
A method for producing a thin film includes disposing a precursor solution onto a substrate to form a precursor film. The precursor solution contains precursor components to a rare-earth / alkaline-earth-metal / transition-metal oxide including a salt of a rare earth element, a salt of an alkaline earth metal, and a salt of a transition metal in one or more solvents, wherein at least one of the salts is a fluoride-containing salt. The precursor solution also contains an additive component comprising one or more metal compounds capable of forming a second phase nanoparticle, either alone or in combination with one or more of the precursor components of the precursor solution or a dopant component comprising one or more metal compounds capable of substituting for an element of the rare-earth / alkaline-earth-metal / transition-metal oxide, and treating the precursor film to form an intermediate metal oxyfluoride including the rare earth, the alkaline earth metal, the transition metal and the additive metal or dopant metal of the precursor solution.
Owner:AMERICAN SUPERCONDUCTOR
Oxygen scavenging films
InactiveUS20100255231A1Metal-working apparatusGlass/slag layered productsParticulatesAlkaline earth metal
A well dispersed oxygen scavenging particulate compounded in a polymer matrix. The oxygen scavenging formulation consists of iron powder with a mean particle sizes within 1-25 um and pre-coated with at least one or more activating and acidifying powdered compounds, usually in the form of solid organic and inorganic salts of alkaline and alkaline earth metals such as sodium chloride and sodium bisulfate. The pre-coated iron particulate is dispersed into a polymer resin by using a conventional melt processing method such as twin-screw extrusion. The oxygen scavenging compound is mixed with polymer pellets in the solid state prior to melting. The polymer resin pellets and the coated iron powder are preferably treated with a surfactant in the dry state to help dispersing the iron / salt powder with the resin pellets. The melt extruded compounds are pelletized and kept in the dry state to prevent premature activation.
Owner:MULTISORB TECH INC
Light emitting device and electronic apparatus
InactiveUS7211828B2Inhibit deteriorationFinal product manufactureSolid-state devicesAlkaline earth metalStress relaxation
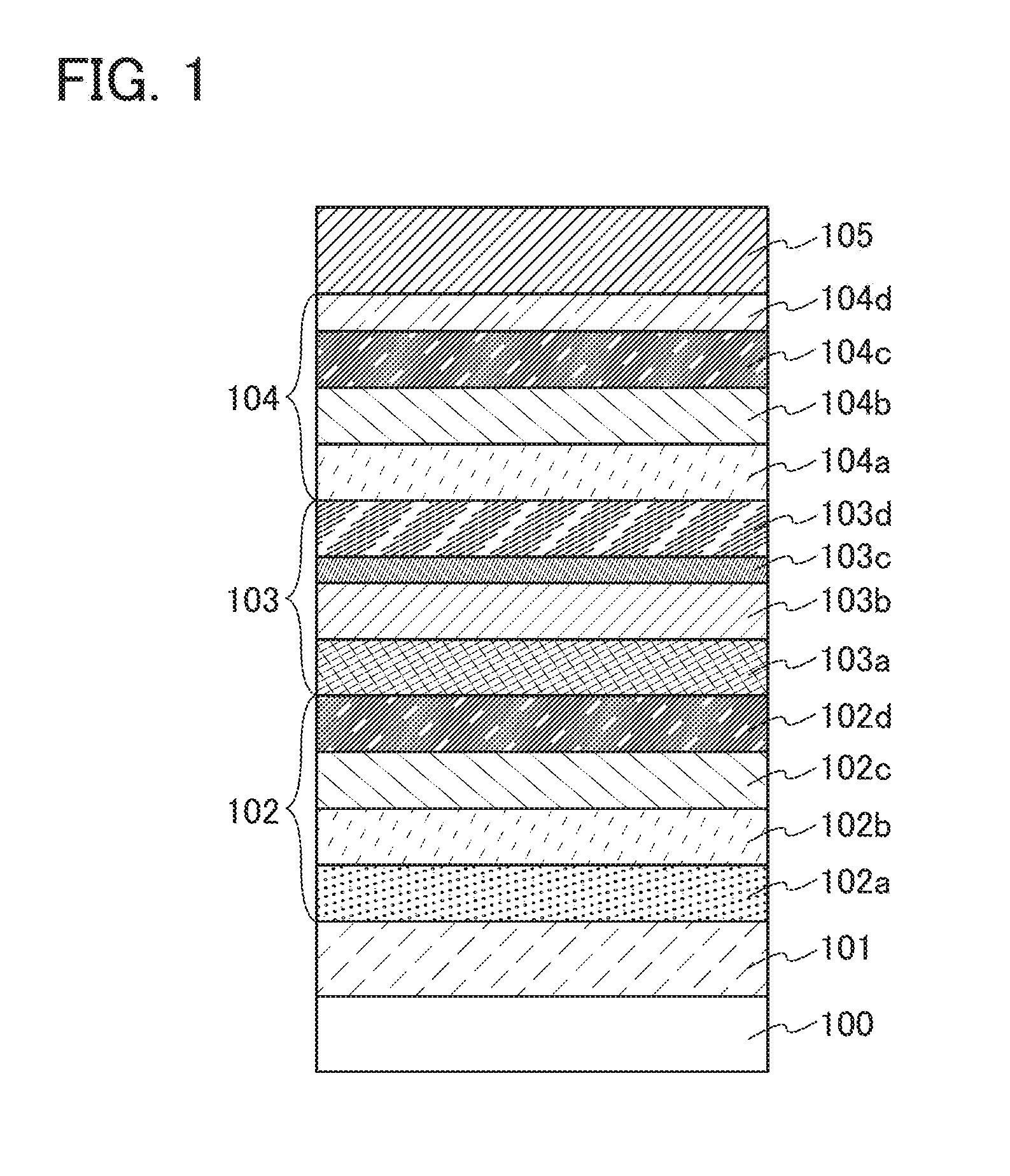
A light emitting device which is capable of suppressing deterioration by diffusion of impurities such as moisture, oxygen, alkaline metal and alkaline earth metal, and concretely, a flexible light emitting device which has light emitting element formed on a plastic substrate. On the plastic substrate, disposed are two layers and more of barrier films comprising a layer represented by AlNxOy which is capable of blocking intrusion of moisture and oxygen in a light emitting layer and blocking intrusion of impurities such as an alkaline metal and an alkaline earth metal in an active layer of TFT, and further, a stress relaxation film containing resin is disposed between two layers of barrier films.
Owner:SEMICON ENERGY LAB CO LTD
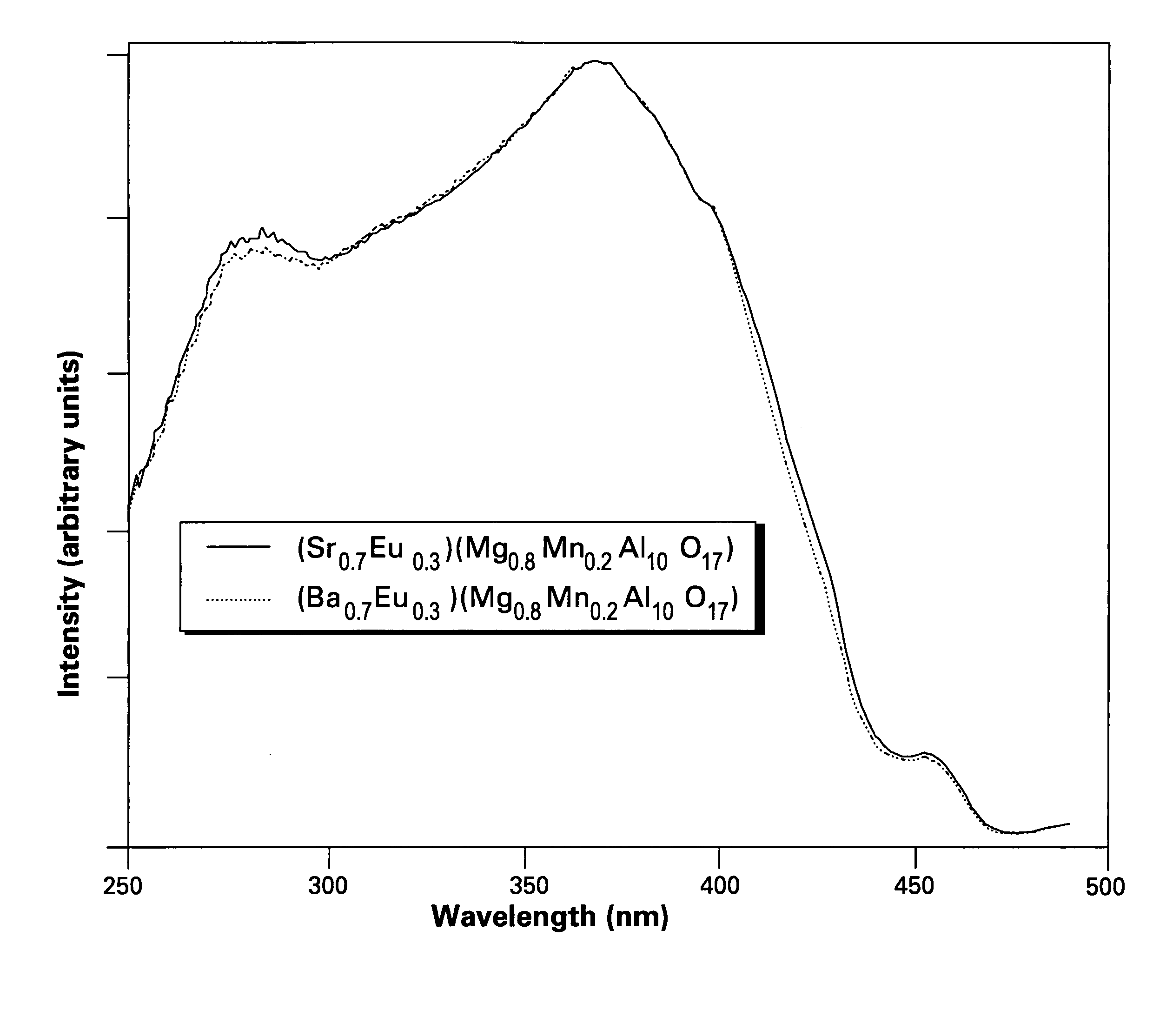
Phosphors containing oxides of alkaline-earth and group-IIIB metals and white-light sources incorporating same
InactiveUS7077978B2Discharge tube luminescnet screensCathode ray tubes/electron beam tubesAlkaline earth metalManganese
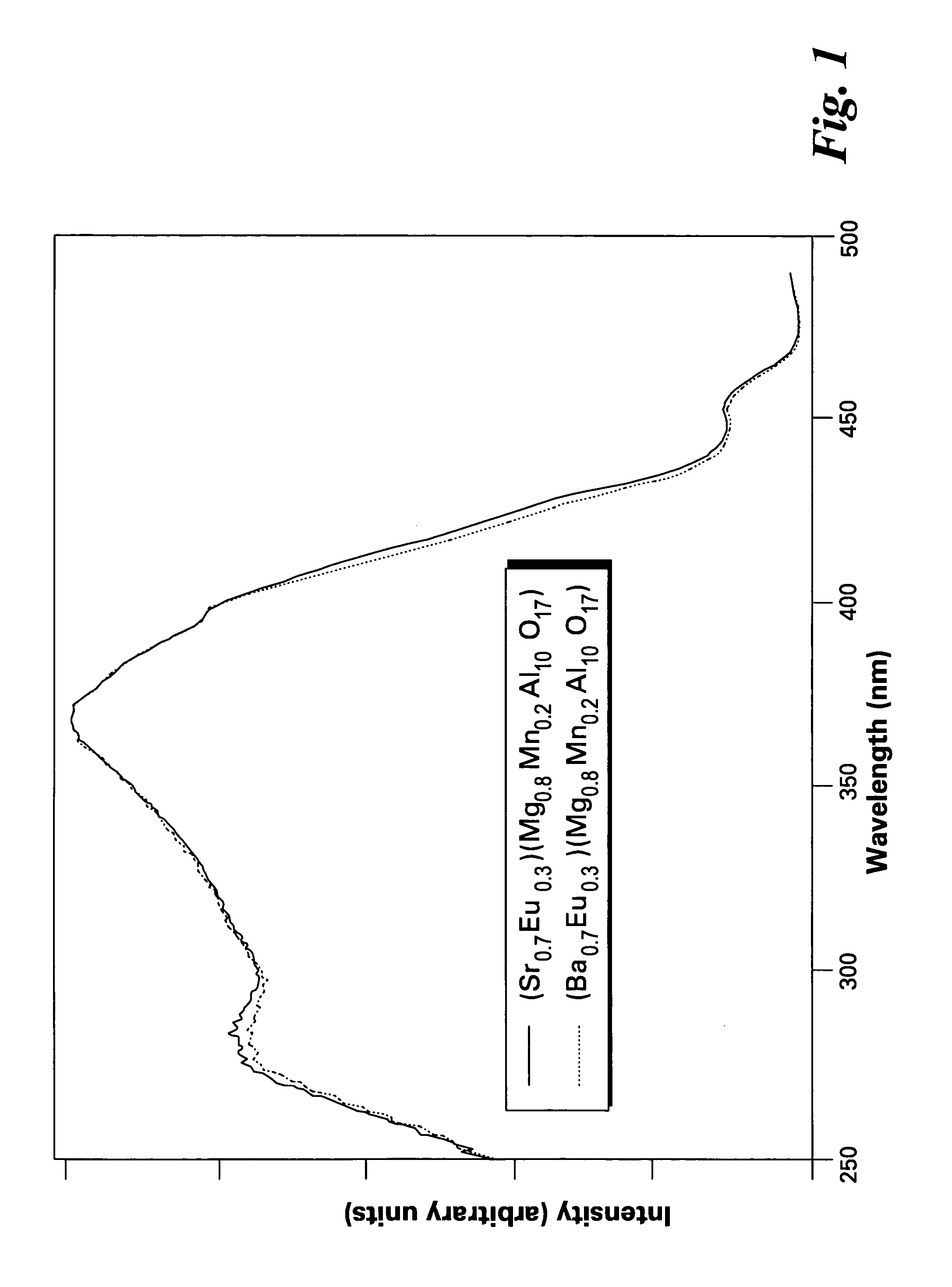
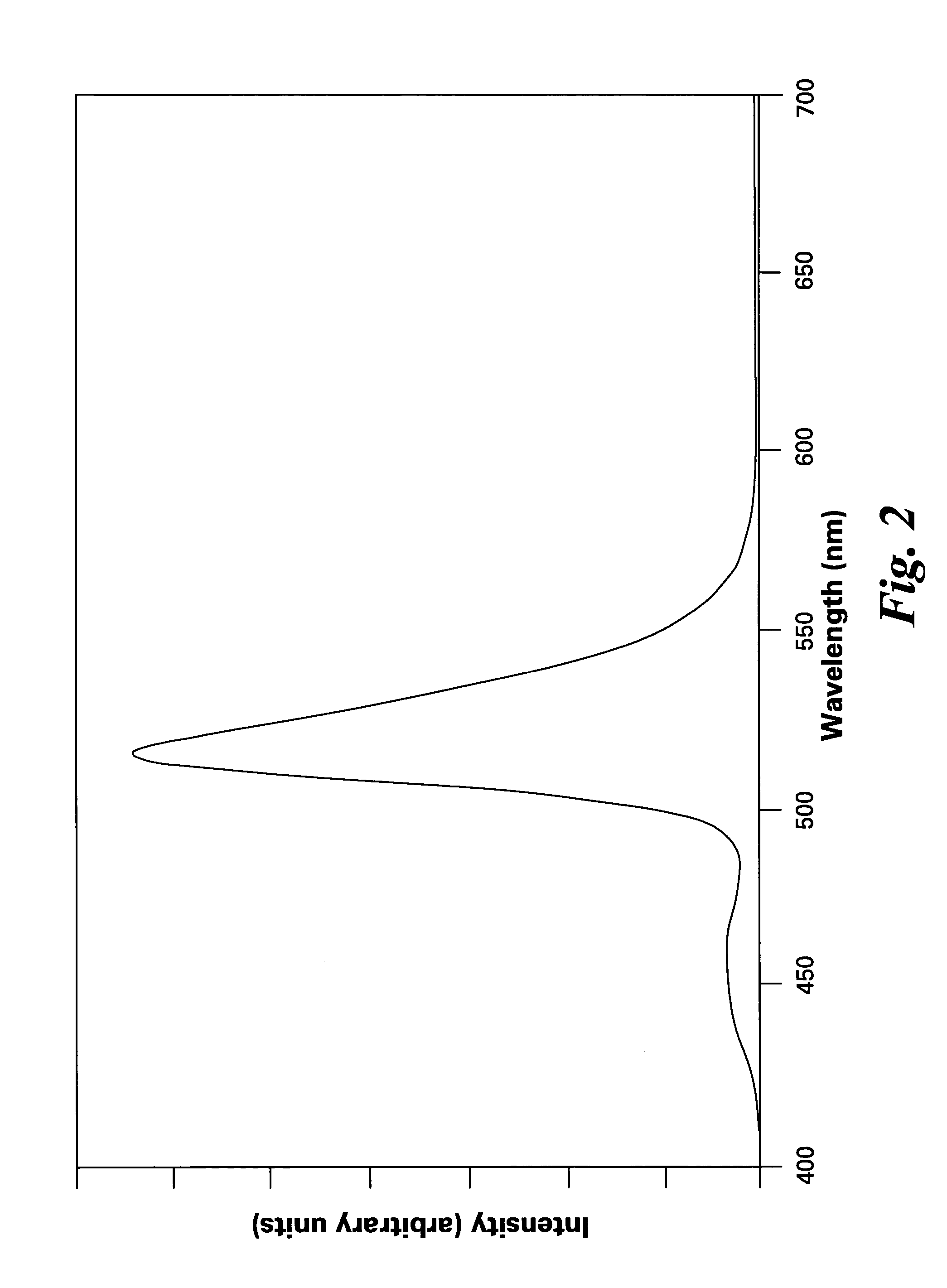
A phosphor comprises a material having a formula of AMgD10O17:Eu2+,Mn2+, wherein A is at least an alkaline-earth metal selected from the group consisting of Ba, Sr, Ca, and combinations thereof; and D is at least a metal selected from the group consisting of Al, Ga, In, and combinations thereof; wherein Eu2+ ions are present in an amount from about 10 to about 50 atom percent of a combined quantity of A ions and europium ions, and Mn2+ ions are present in an amount from about 5 to about 30 atom percent of a combined quantity of magnesium ions and manganese ions. The phosphor is used alone or in conjunction with other phosphors to convert UV / blue radiation emitted by a source, such as an LED or a gas discharge device, to visible light.
Owner:GENERAL ELECTRIC CO
Inorganic and organic nitrate additives for nonaqueous electrolyte in alkali metal electrochemical cells
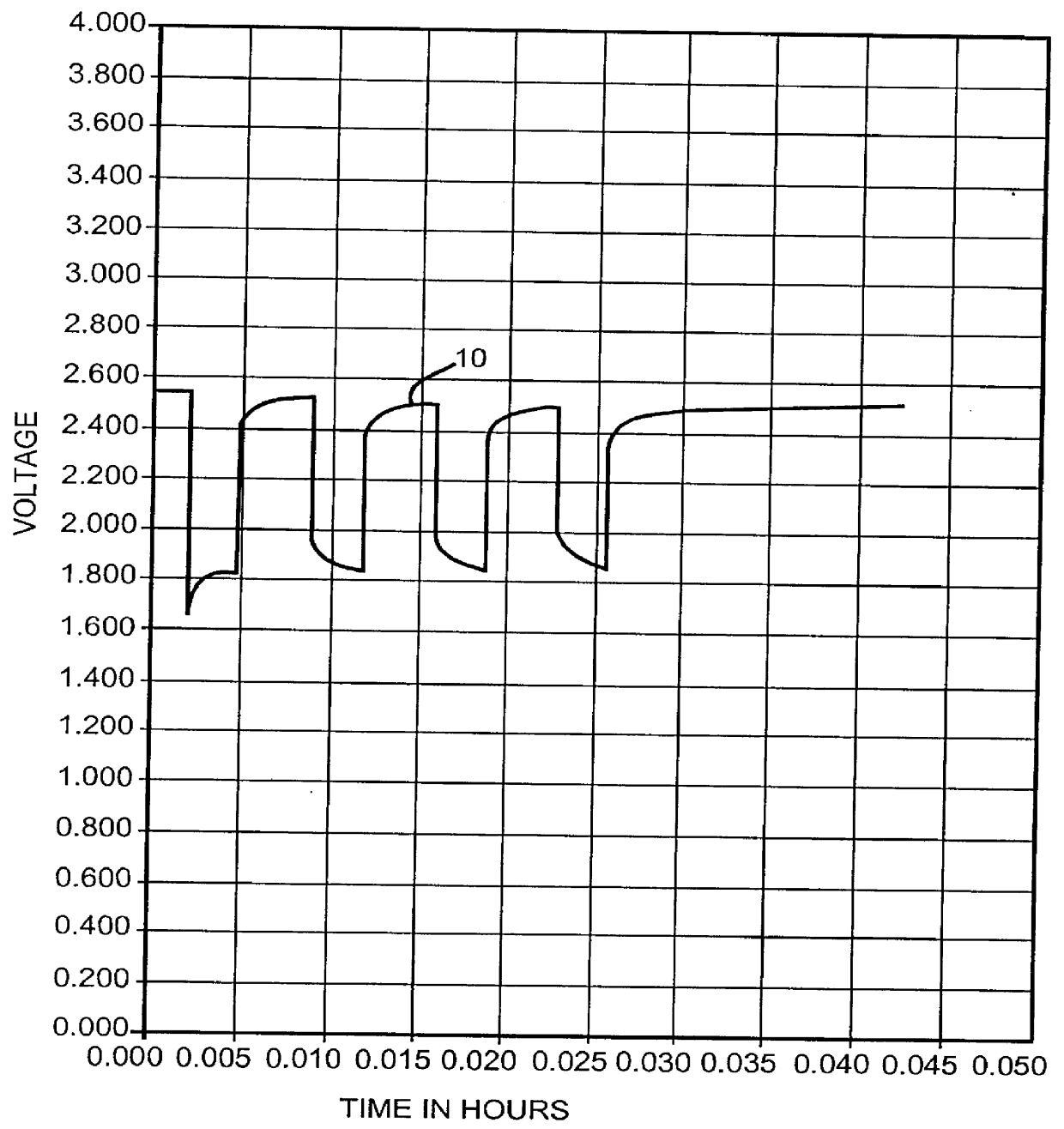
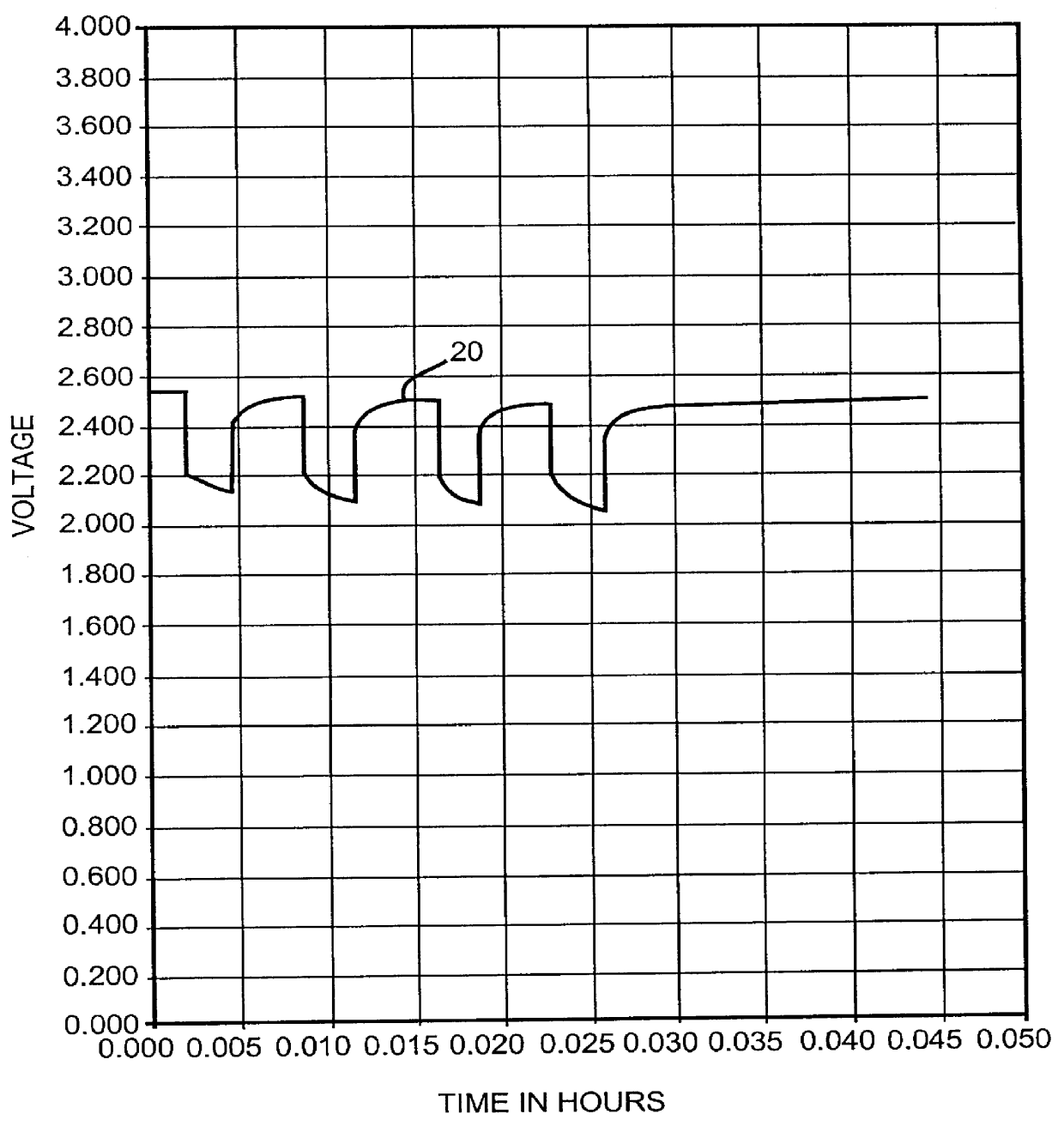
InactiveUS6060184AGood charge and discharge cycleImprove efficiencyOrganic electrolyte cellsSecondary cells charging/dischargingAlkaline earth metalOrganic nitrates
An alkali metal, solid cathode, nonaqueous electrochemical cell capable of delivering high current pulses, rapidly recovering its open circuit voltage and having high current capacity, is described. The stated benefits are realized by the addition of at least one nitrate additive to an electrolyte comprising an alkali metal salt dissolved in a mixture of a low viscosity solvent and a high permittivity solvent. A preferred solvent mixture includes propylene carbonate, dimethoxyethane and an alkali metal nitrate, alkaline earth metal nitrate and / or an organic alkyl nitrate additive.
Owner:WILSON GREATBATCH LTD
Methods of sequestering co2
Methods of sequestering carbon dioxide (CO2) are provided. Aspects of the methods include precipitating a storage stable carbon dioxide sequestering product from an alkaline-earth-metal-containing water and then disposing of the product, e.g., by placing the product in a disposal location or using the product as a component of a manufactured composition. Also provided are systems for practicing methods of the invention.
Owner:CALERA CORP
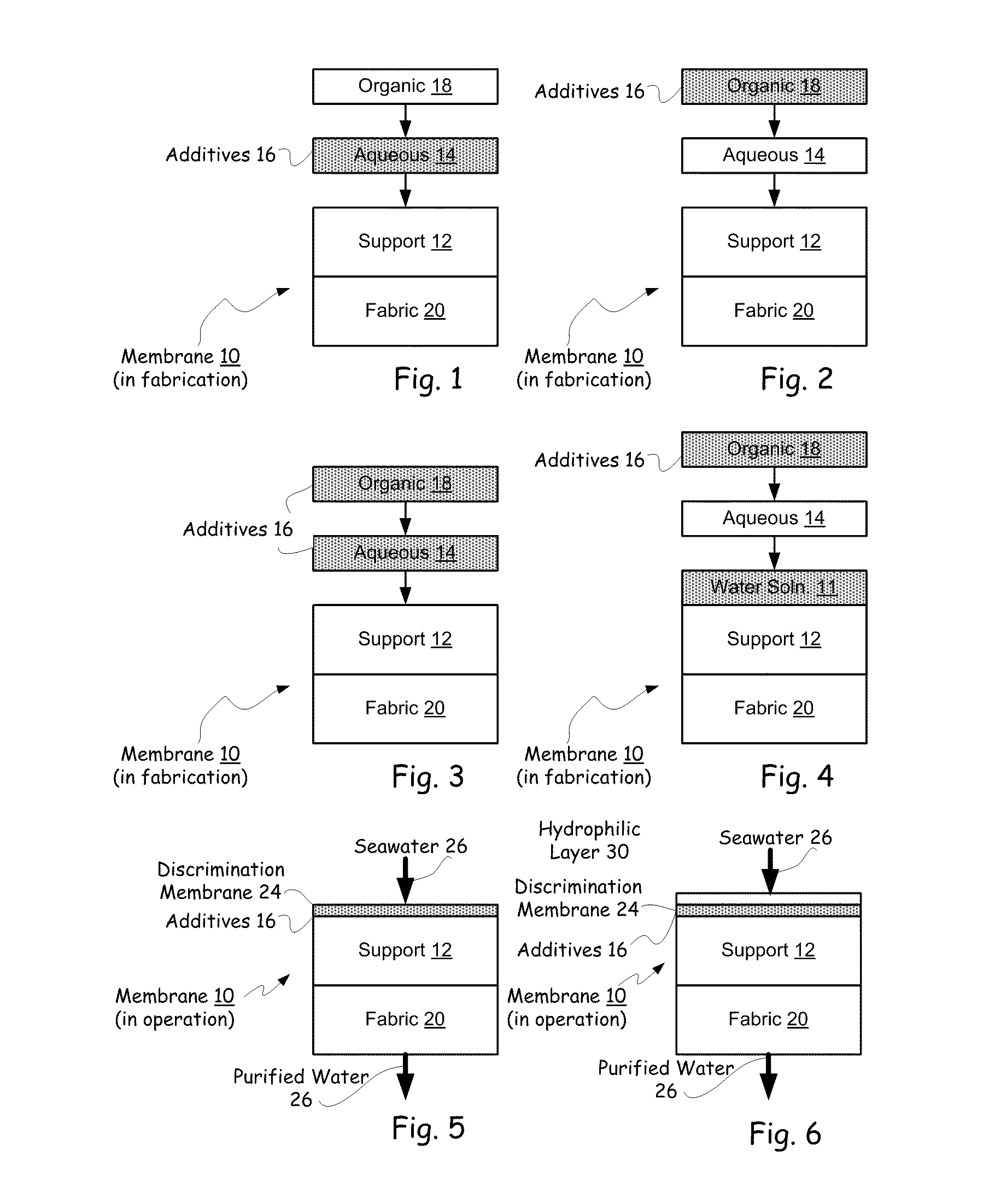
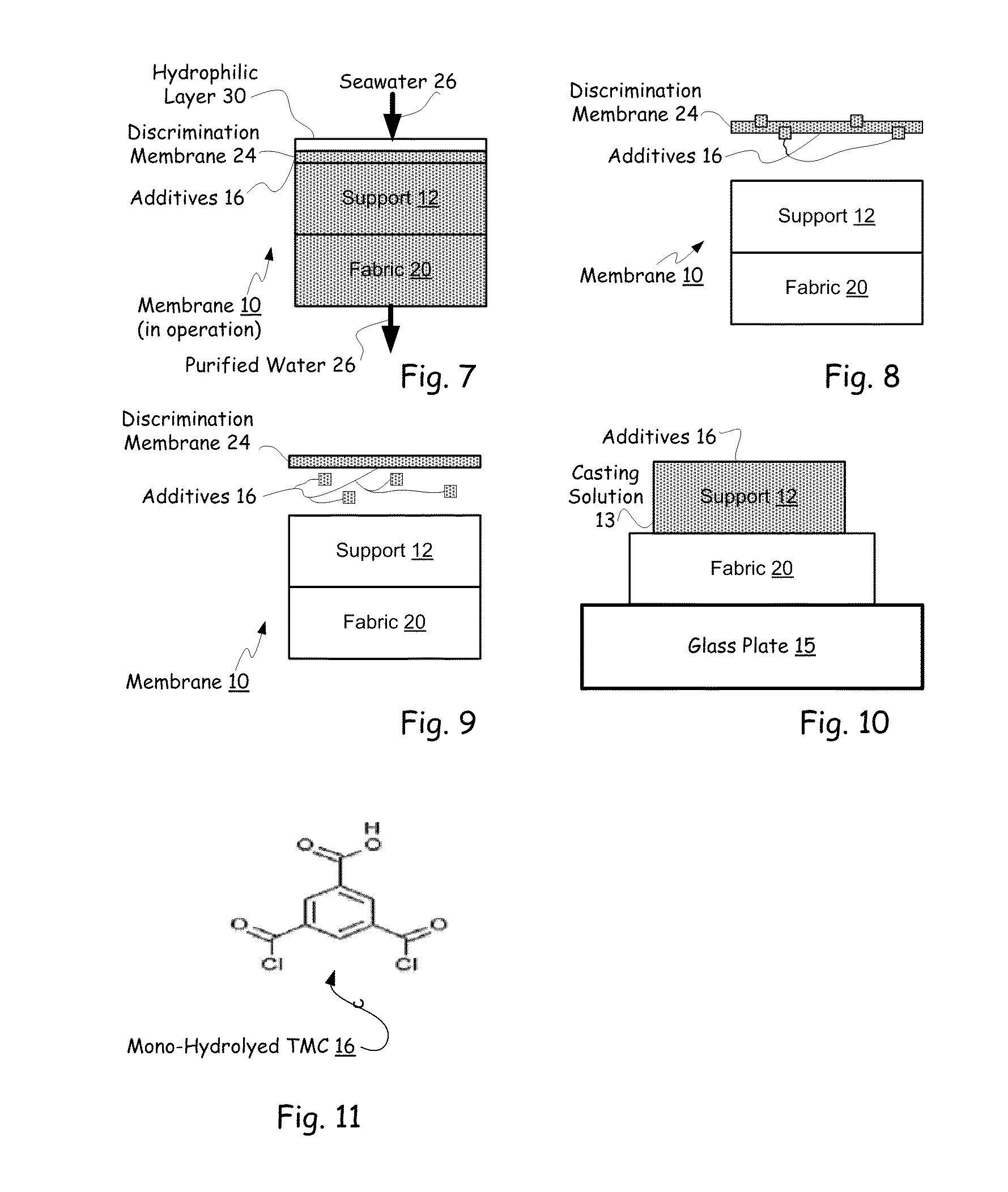
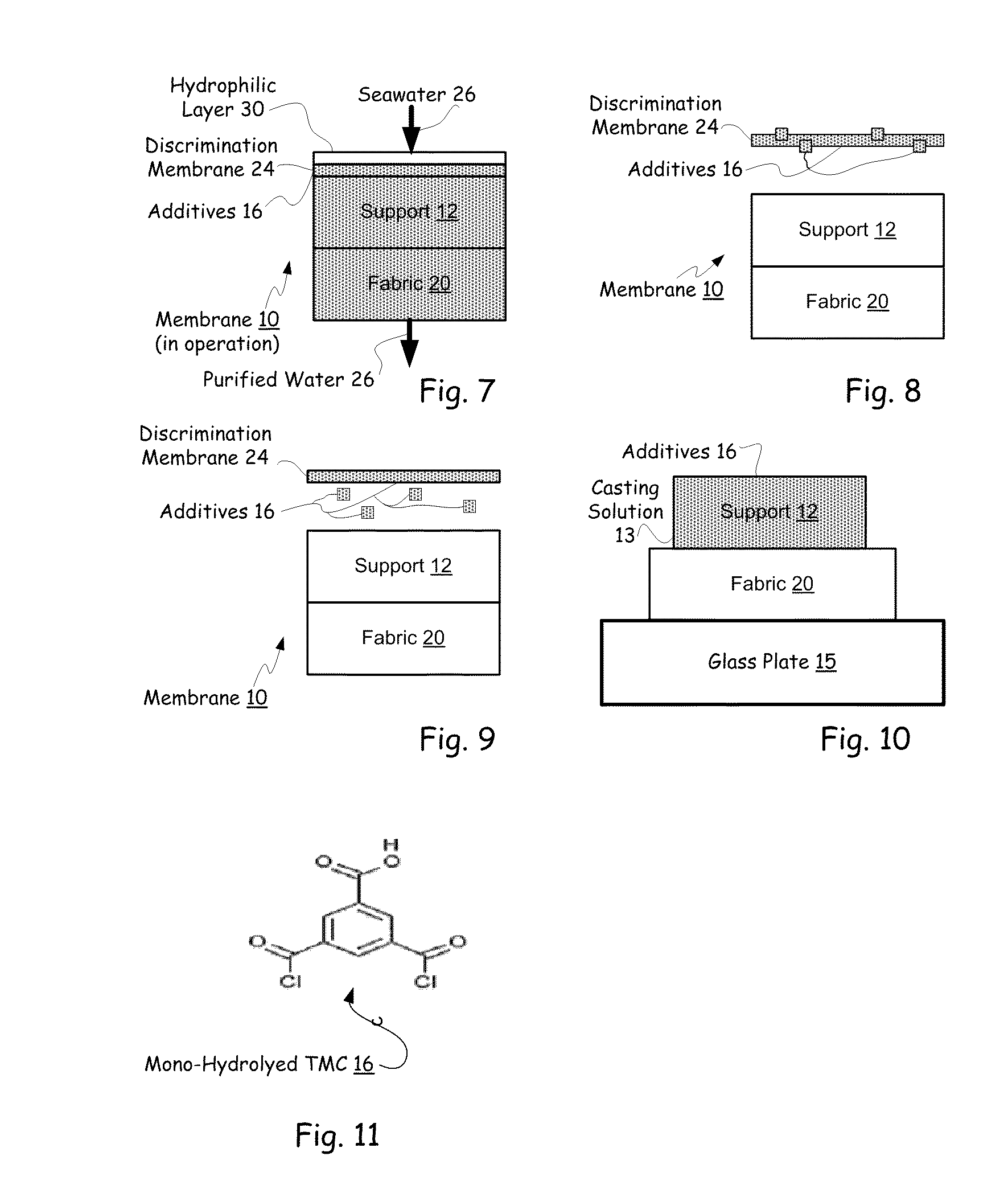
Hybrid tfc ro membranes with nitrogen additives
ActiveUS20110005997A1Increase fluxIncreased rejectionMembranesPretreated surfacesChloramine BAlkaline earth metal
Owner:NANOH2O
Luminescent material, especially for led application
ActiveUS20050205845A1Efficient excitationGood colorIncadescent screens/filtersDischarge tube luminescnet screensAlkaline earth metalOptoelectronics
UV-blue excitable green luminescent material including an Eu-doped oxynitride host lattice with general composition MSi2O2N2, wherein M is at least one of an alkaline earth metal chosen from the roup Ca, Sr, Ba.
Owner:OSRAM OLED
Method for the oxidative dehydrogenation of ethane
InactiveUS7319179B2Organic compound preparationHeterogenous catalyst chemical elementsAlkaline earth metalDehydrogenation
Owner:CONSEJO SUPERIOR DE INVESTIGACIONES CIENTIFICAS (CSIC) +1
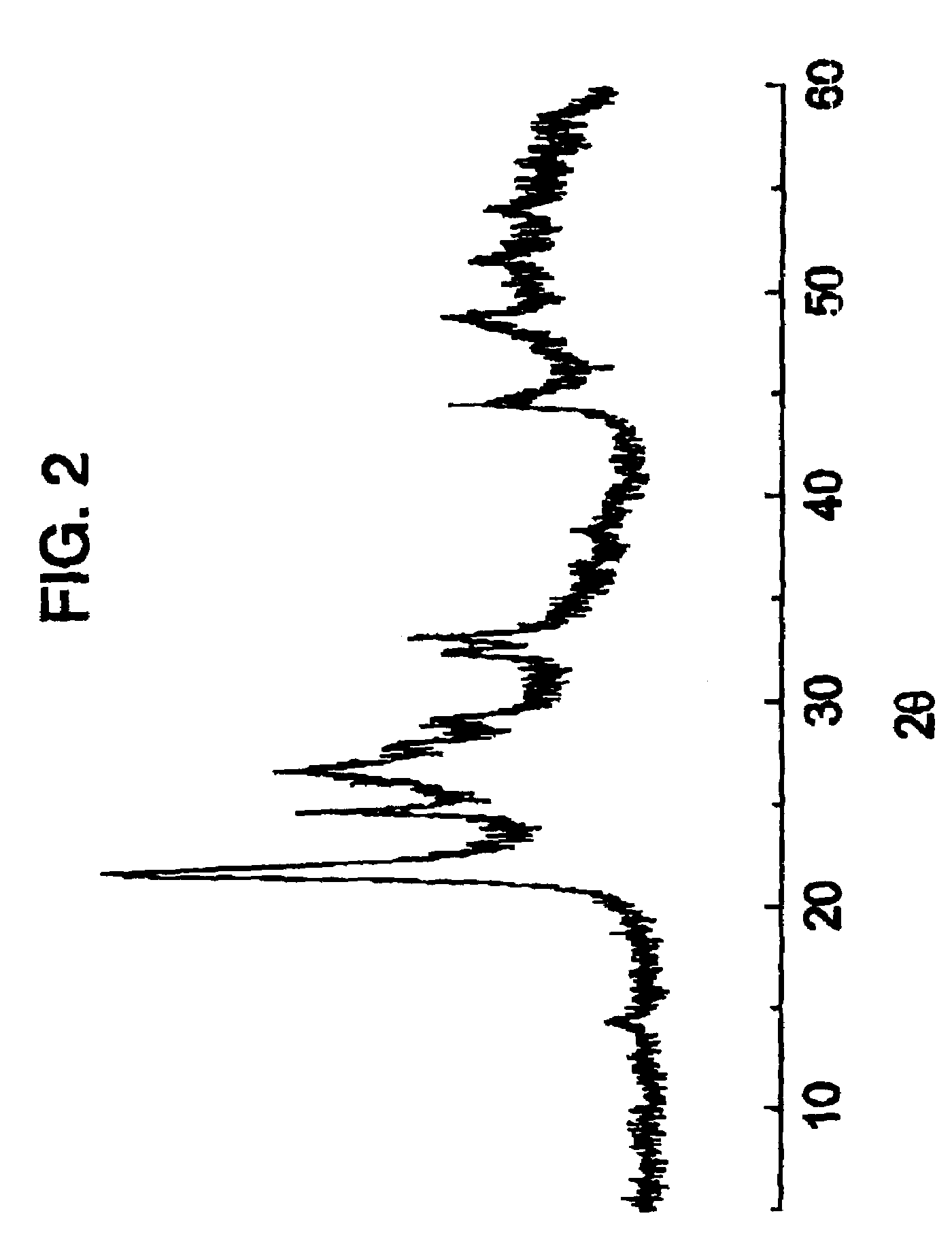
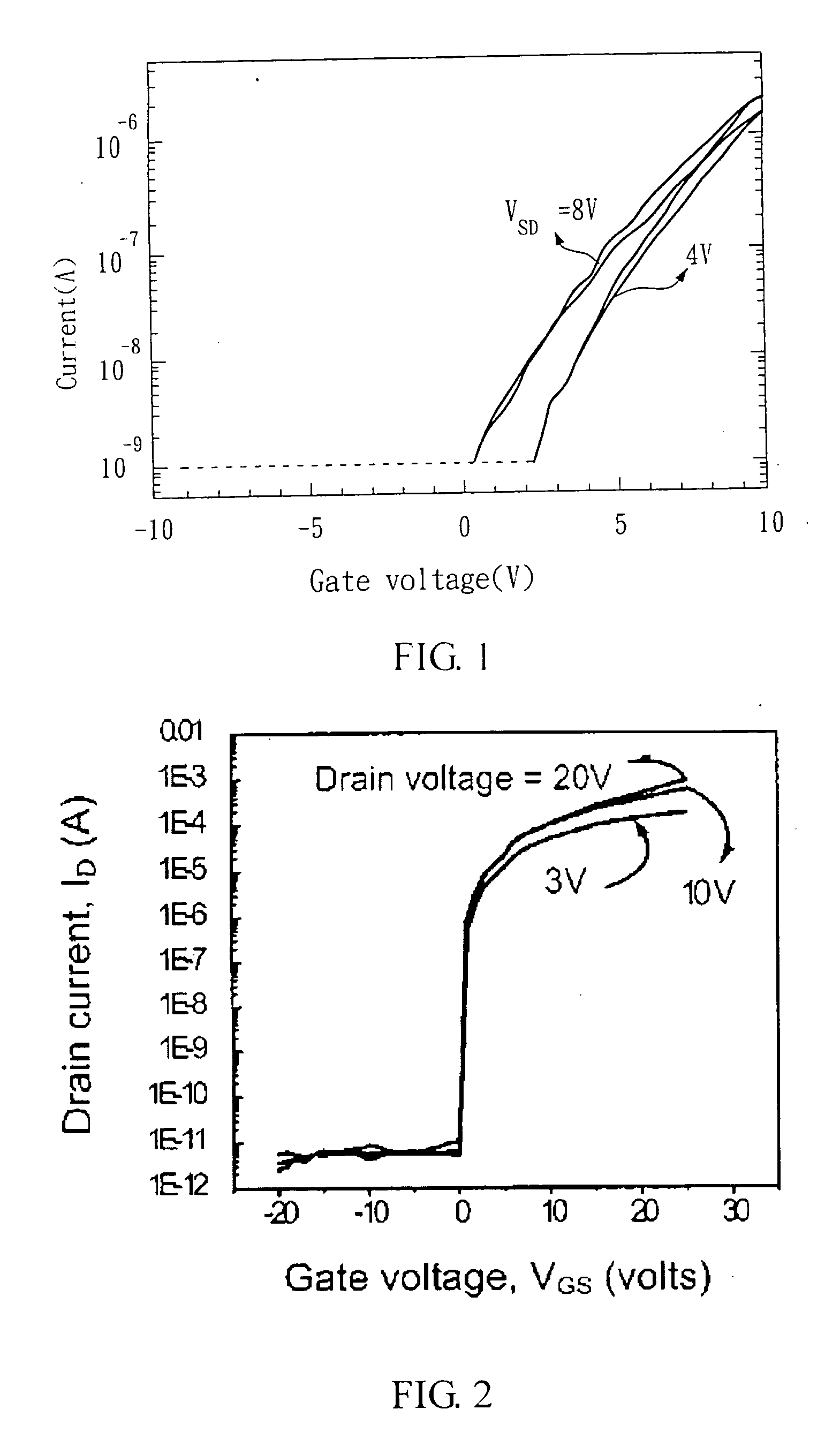
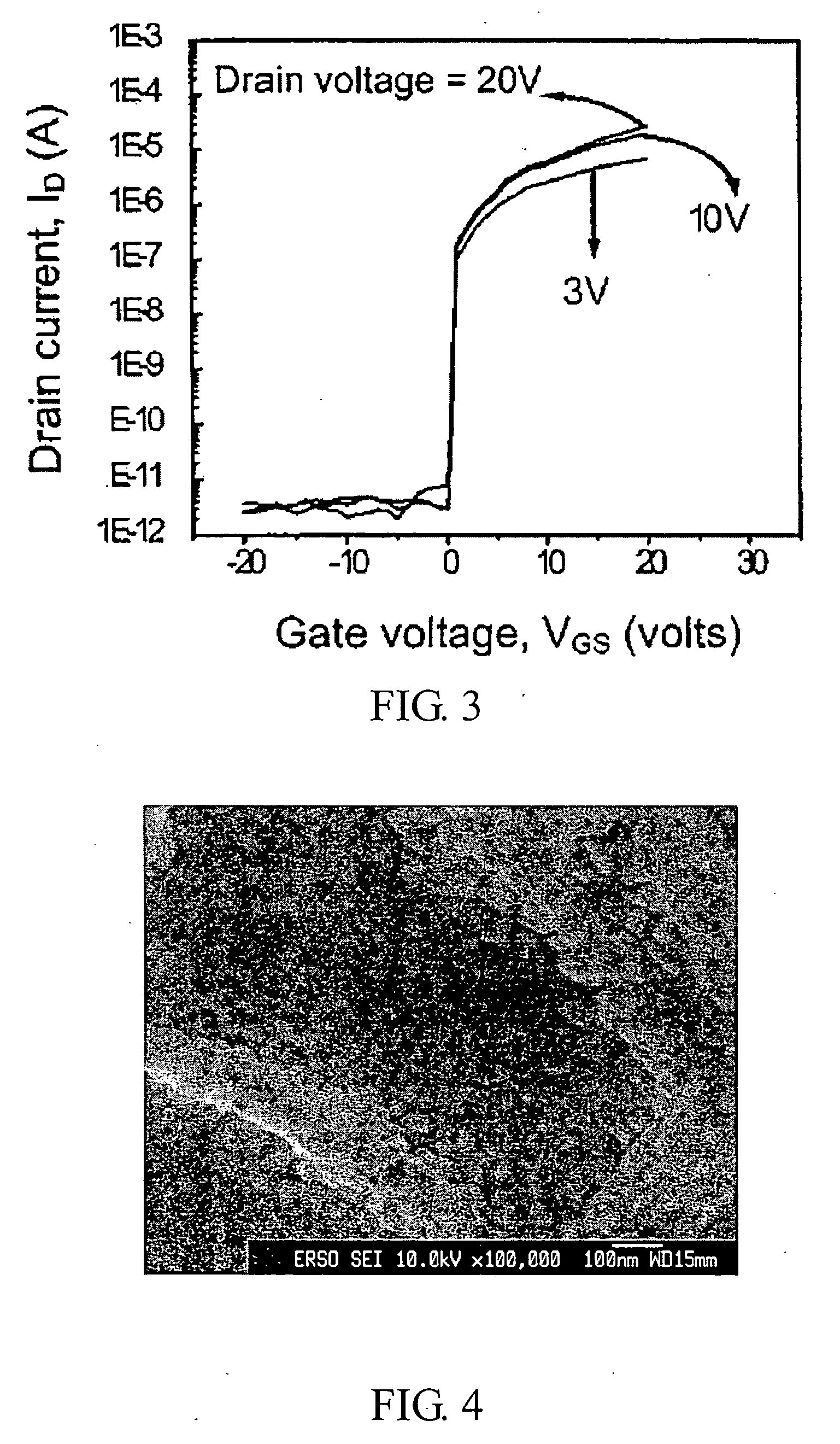
Compound semiconductor material and method for forming an active layer of a thin film transistor device
InactiveUS20050062134A1High voltage-durableImprove device characteristicsTransistorSolid-state devicesDopantAlkaline earth metal
A compound semiconductor material for forming an active layer of a thin film transistor device is disclosed, which has a group II-VI compound doped with a dopant ranging from 0.1 to 30 mol %, wherein the dopant is selected from a group consisting of alkaline-earth metals, group IIIA elements, group IVA elements, group VA elements, group VIA elements, and transitional metals. The method for forming an active layer of a thin film transistor device by using the compound semiconductor material of the present invention is disclosed therewith.
Owner:IND TECH RES INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com