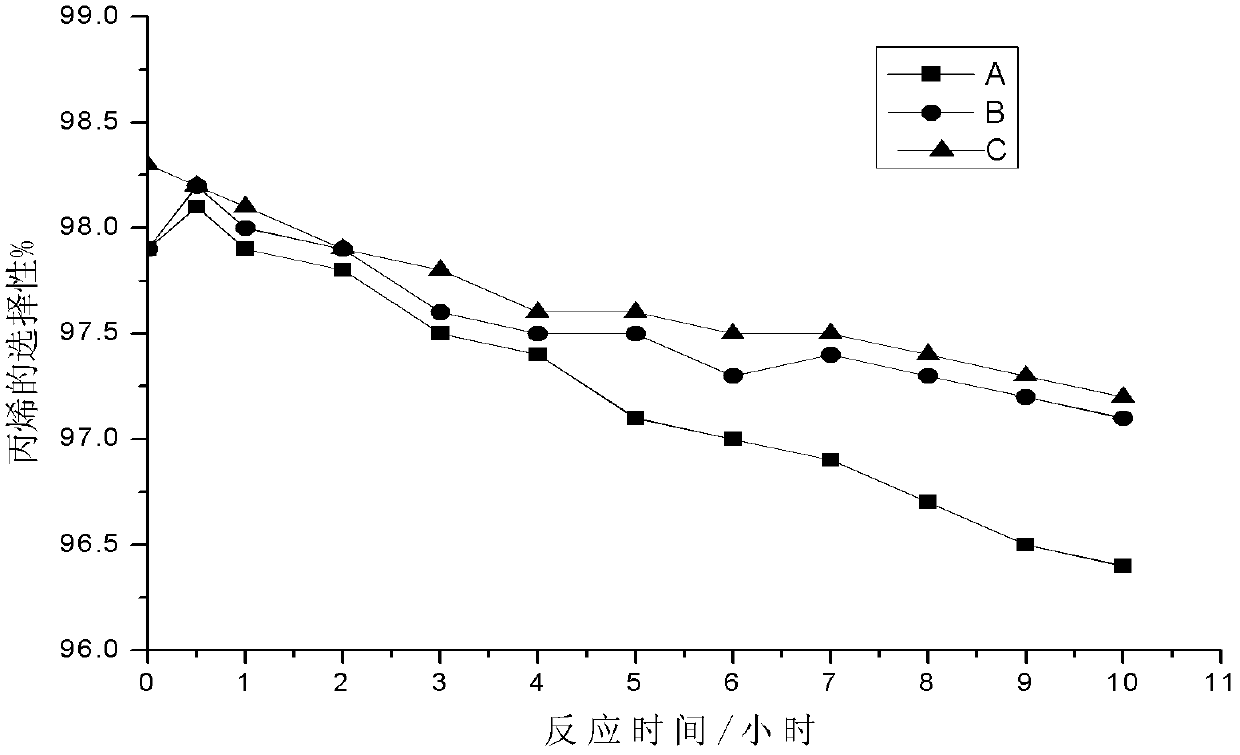
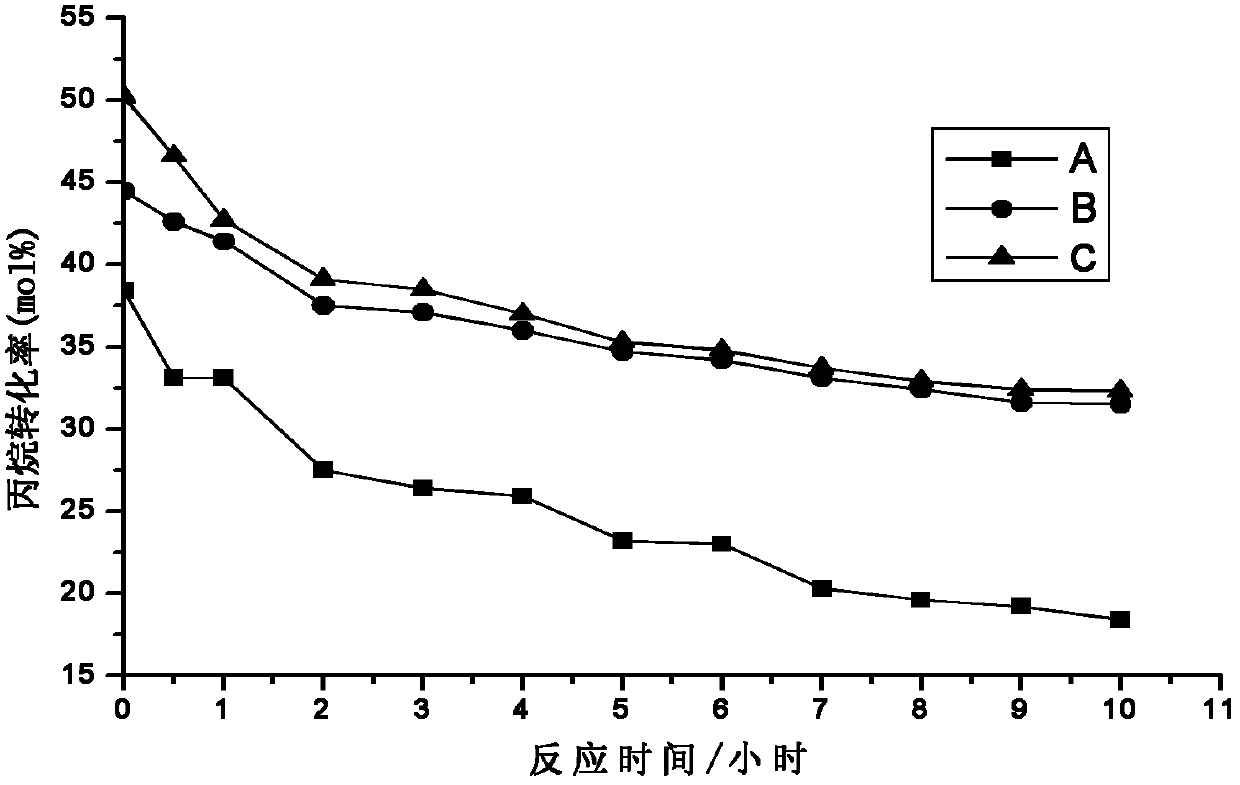
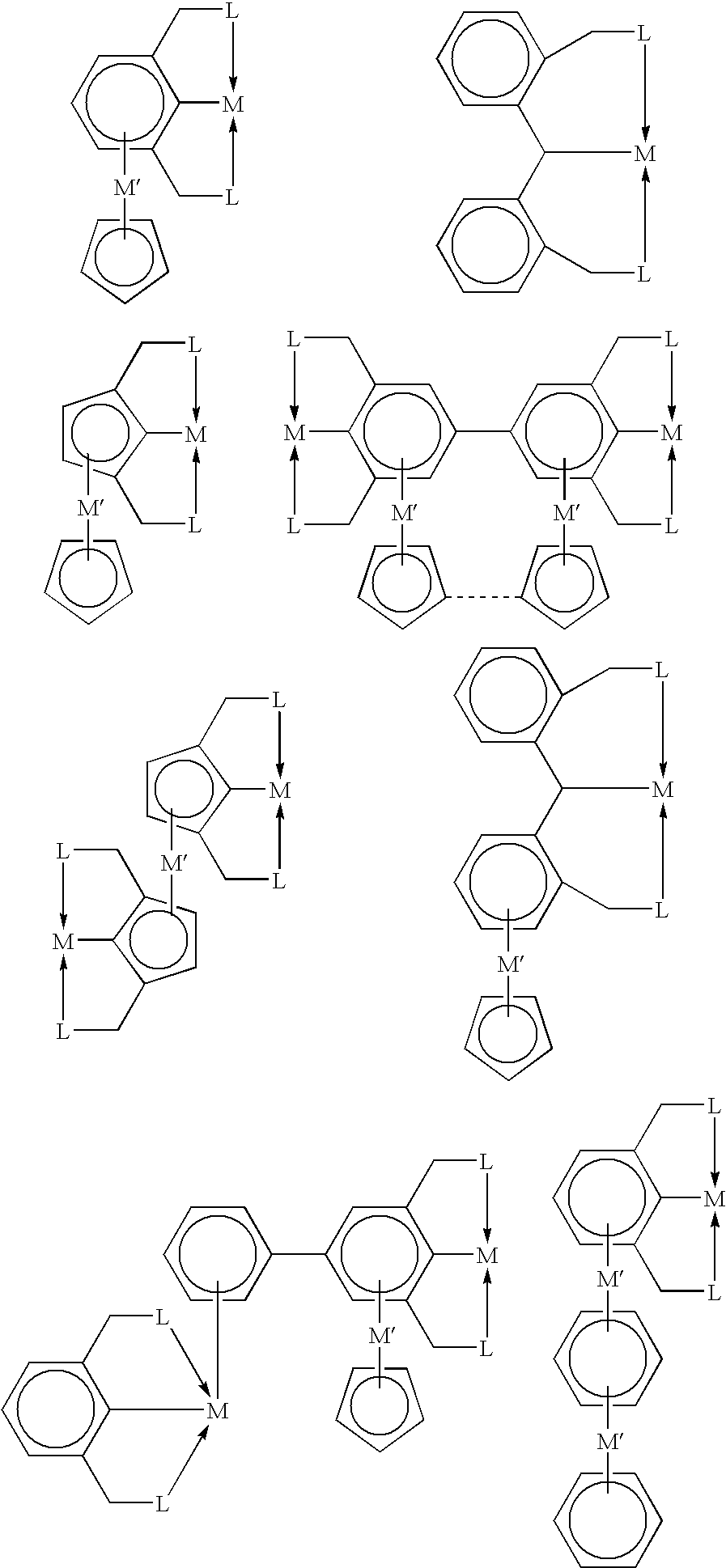
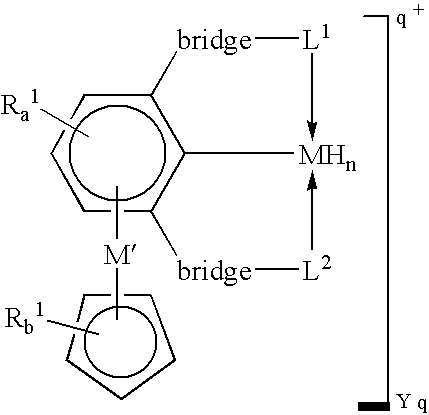
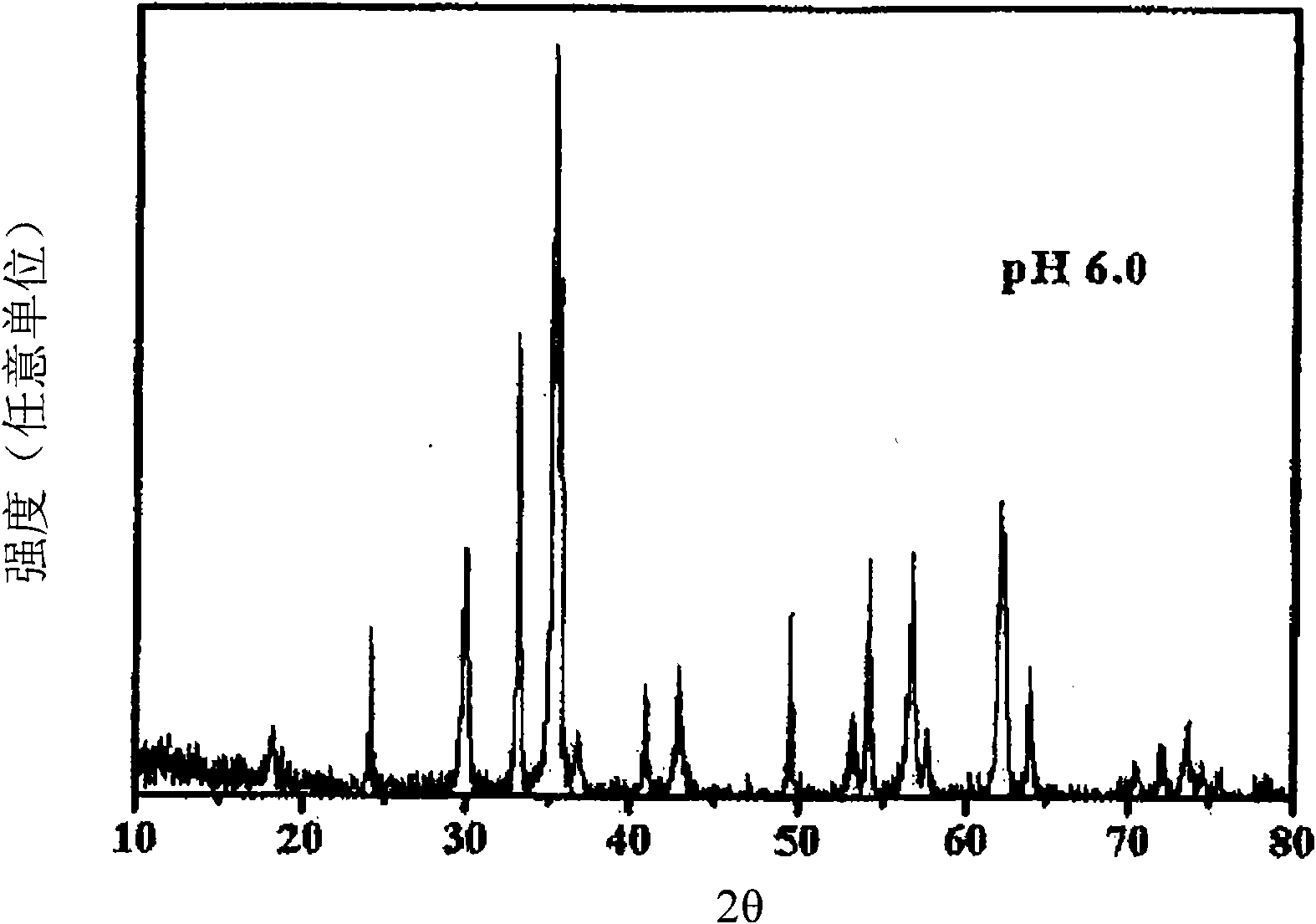
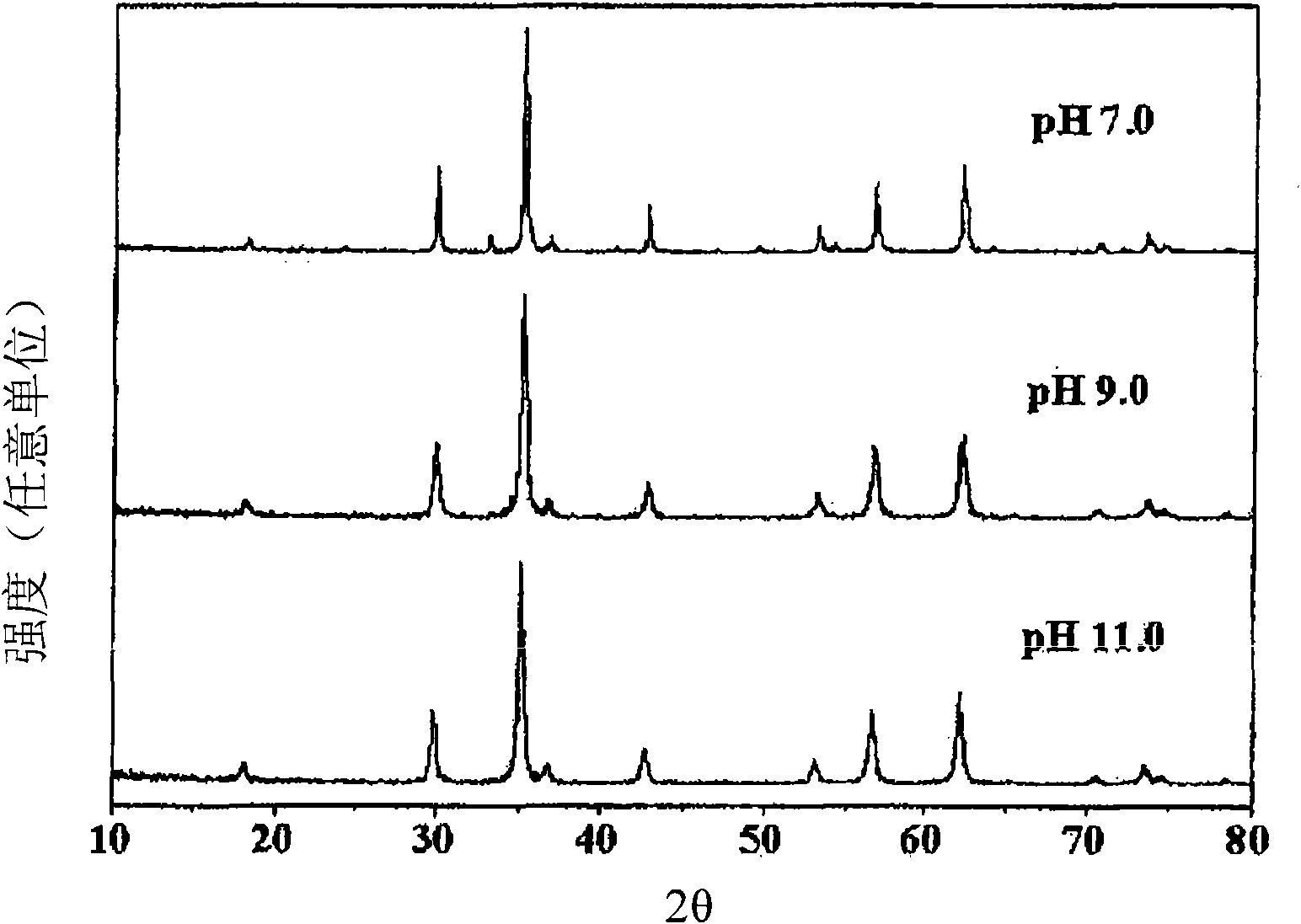
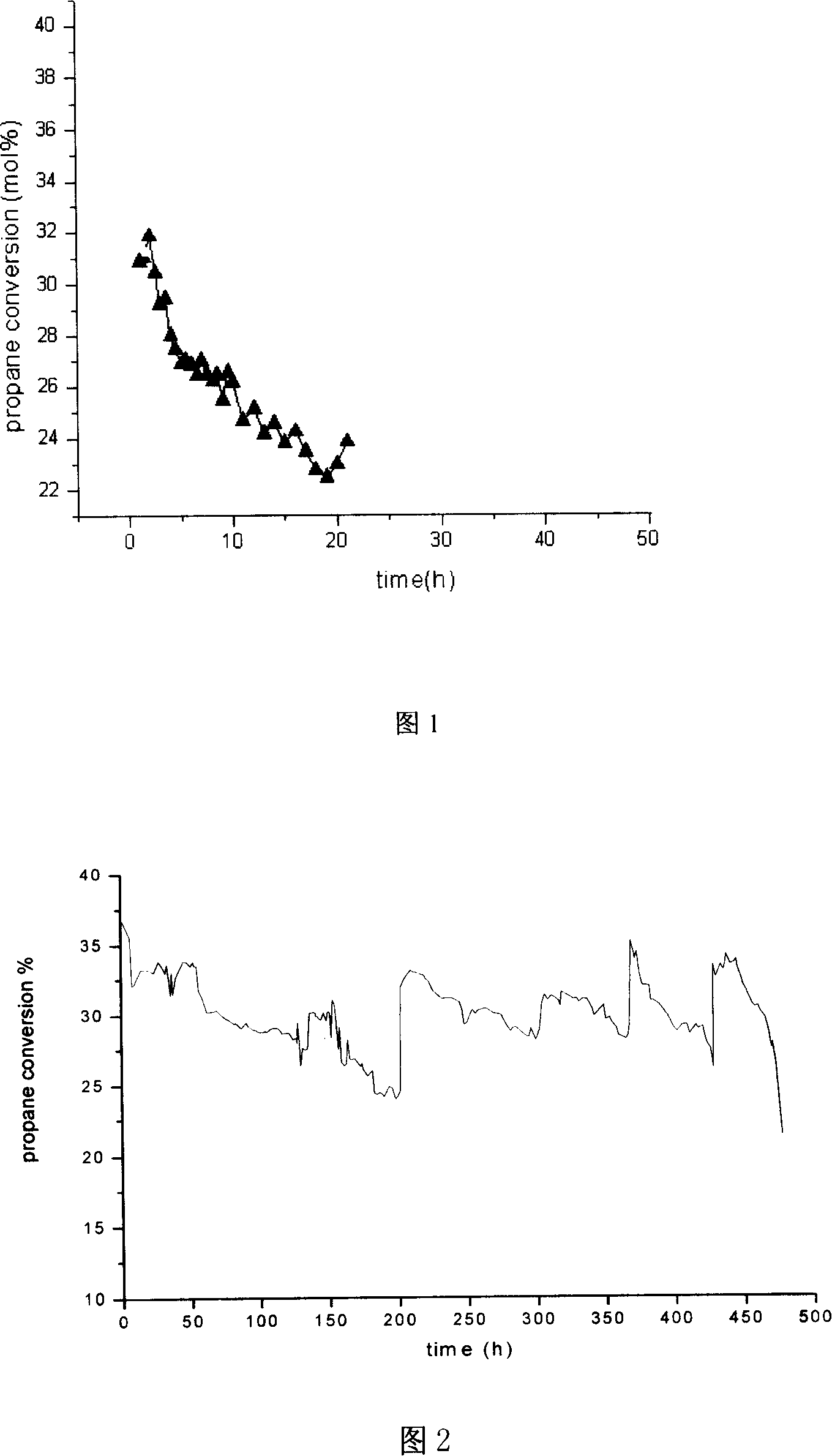
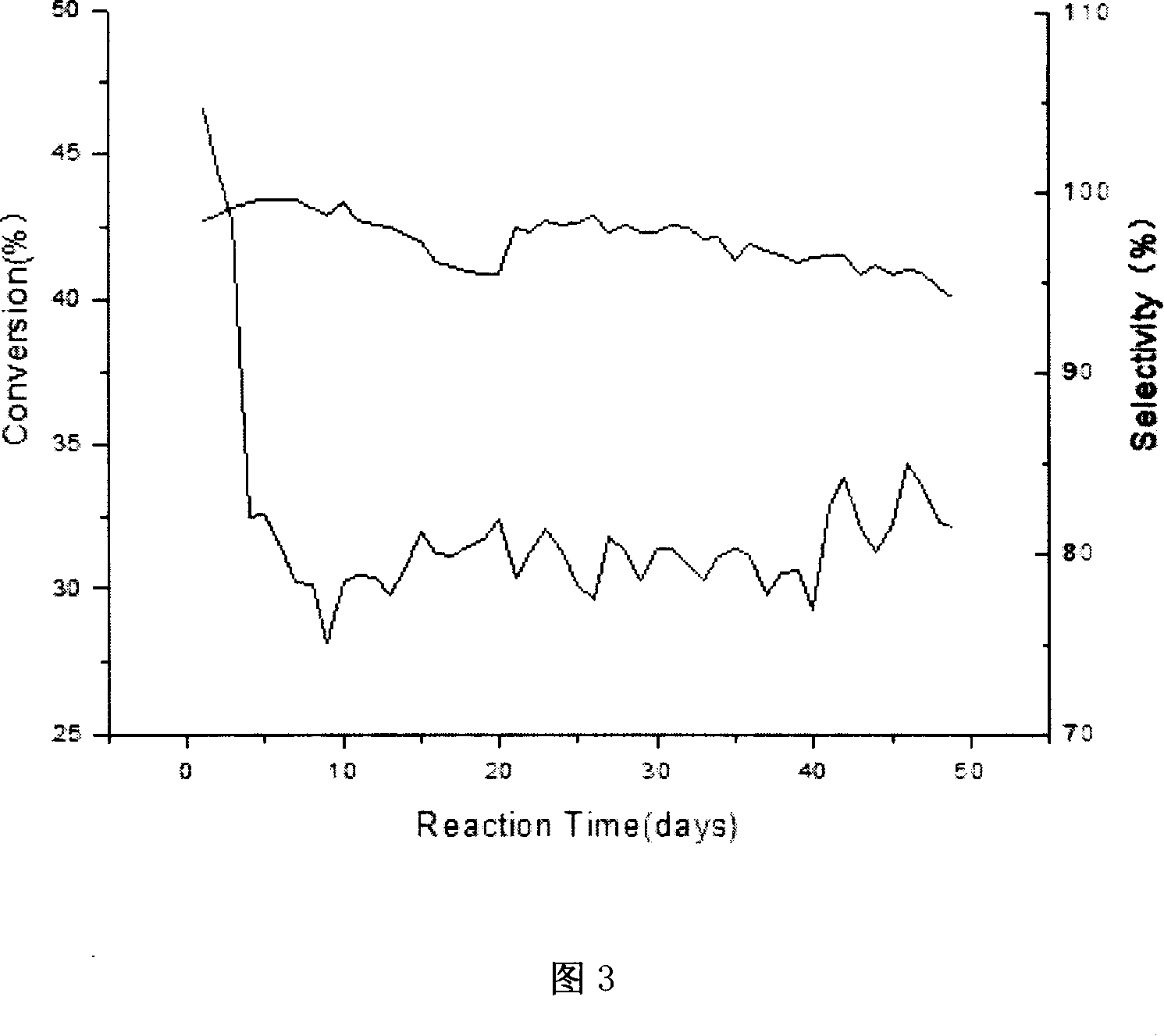
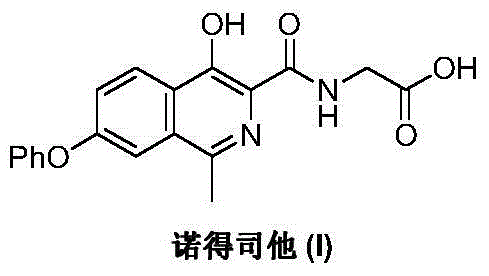
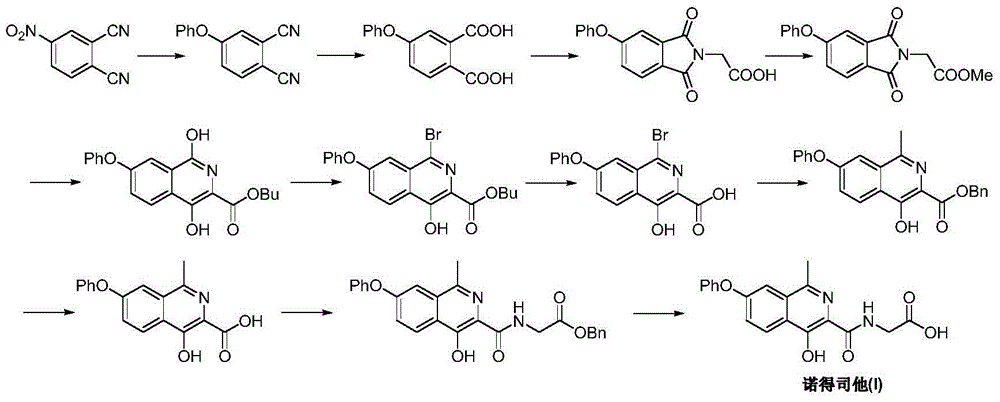
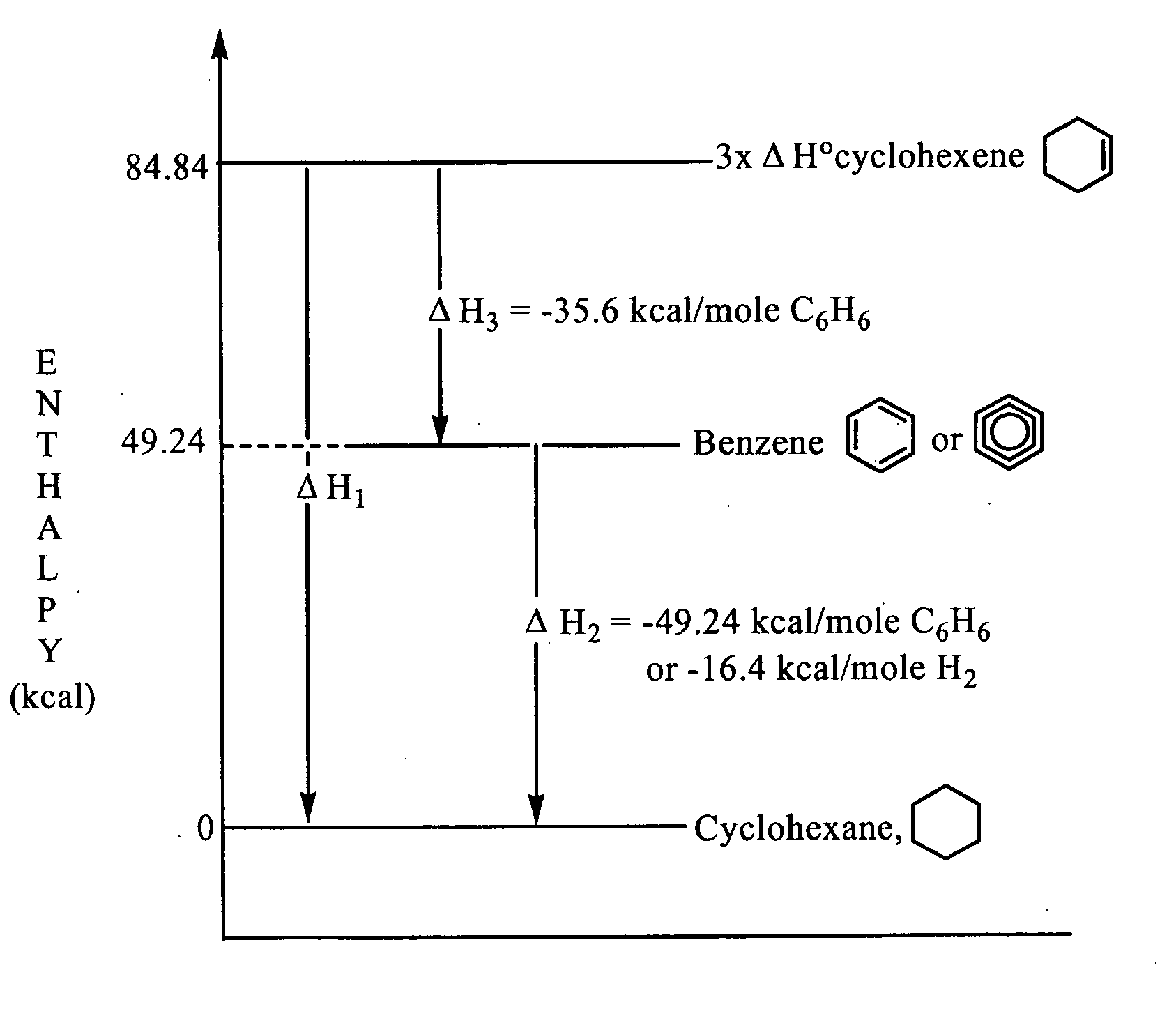
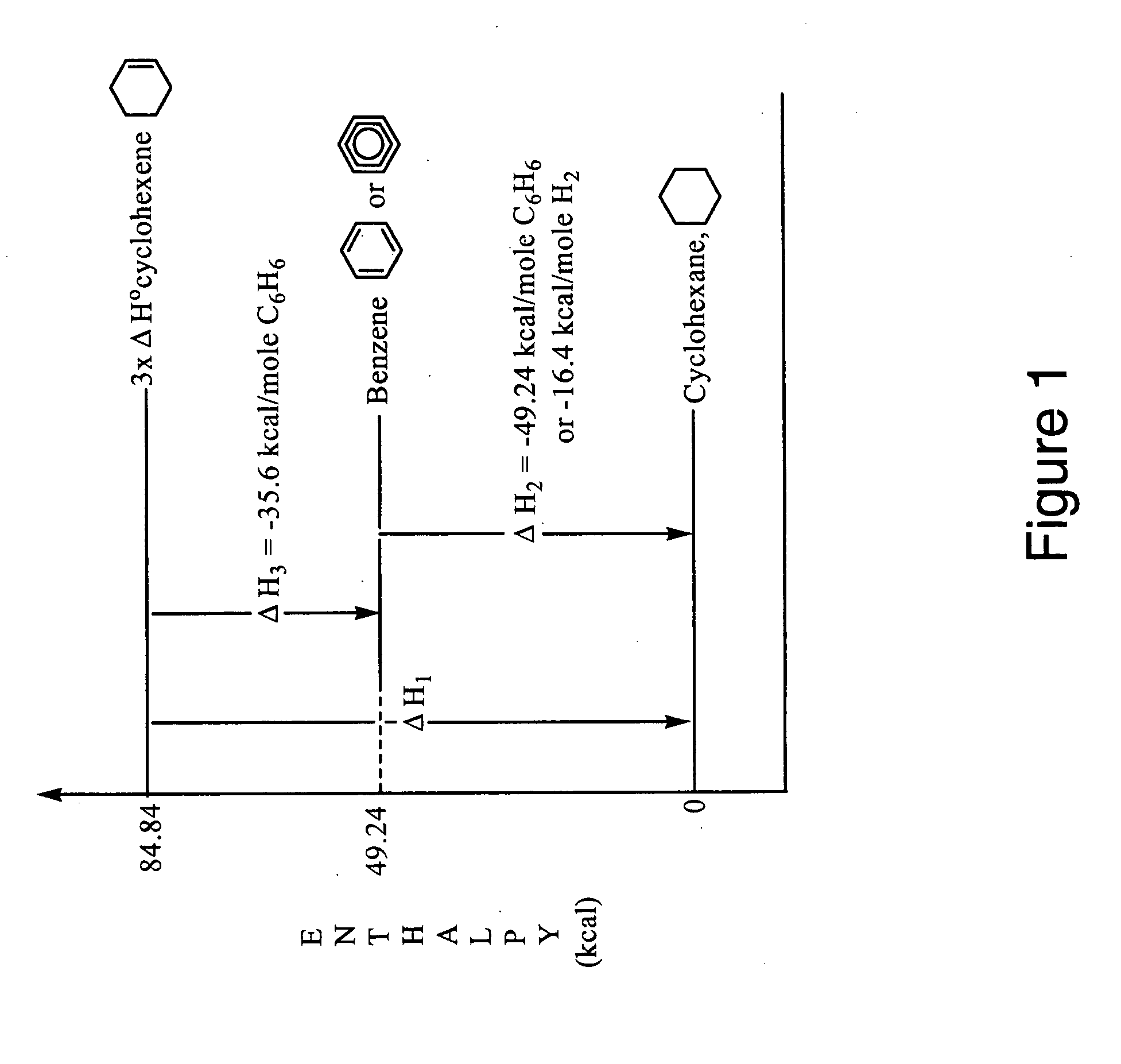
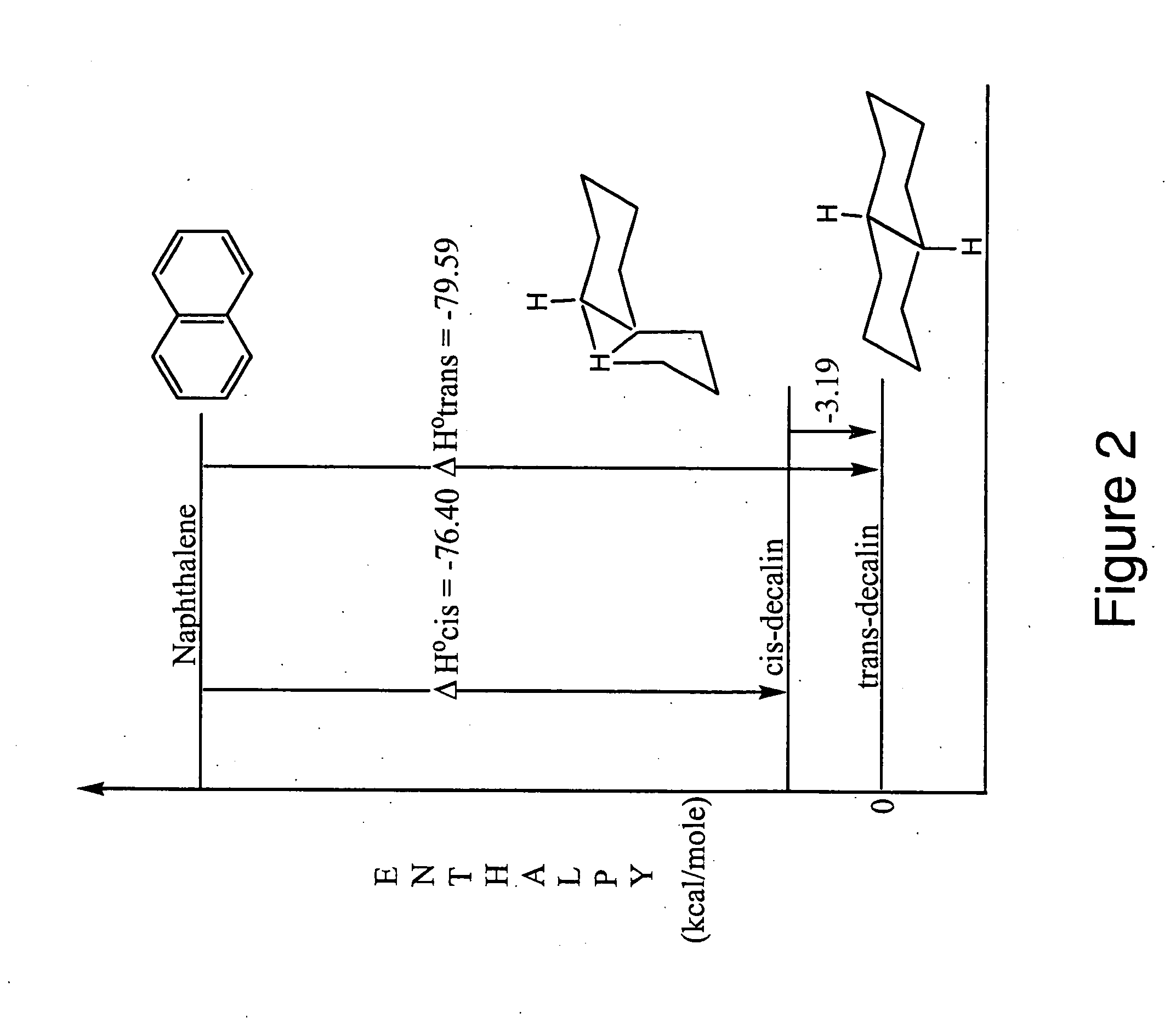
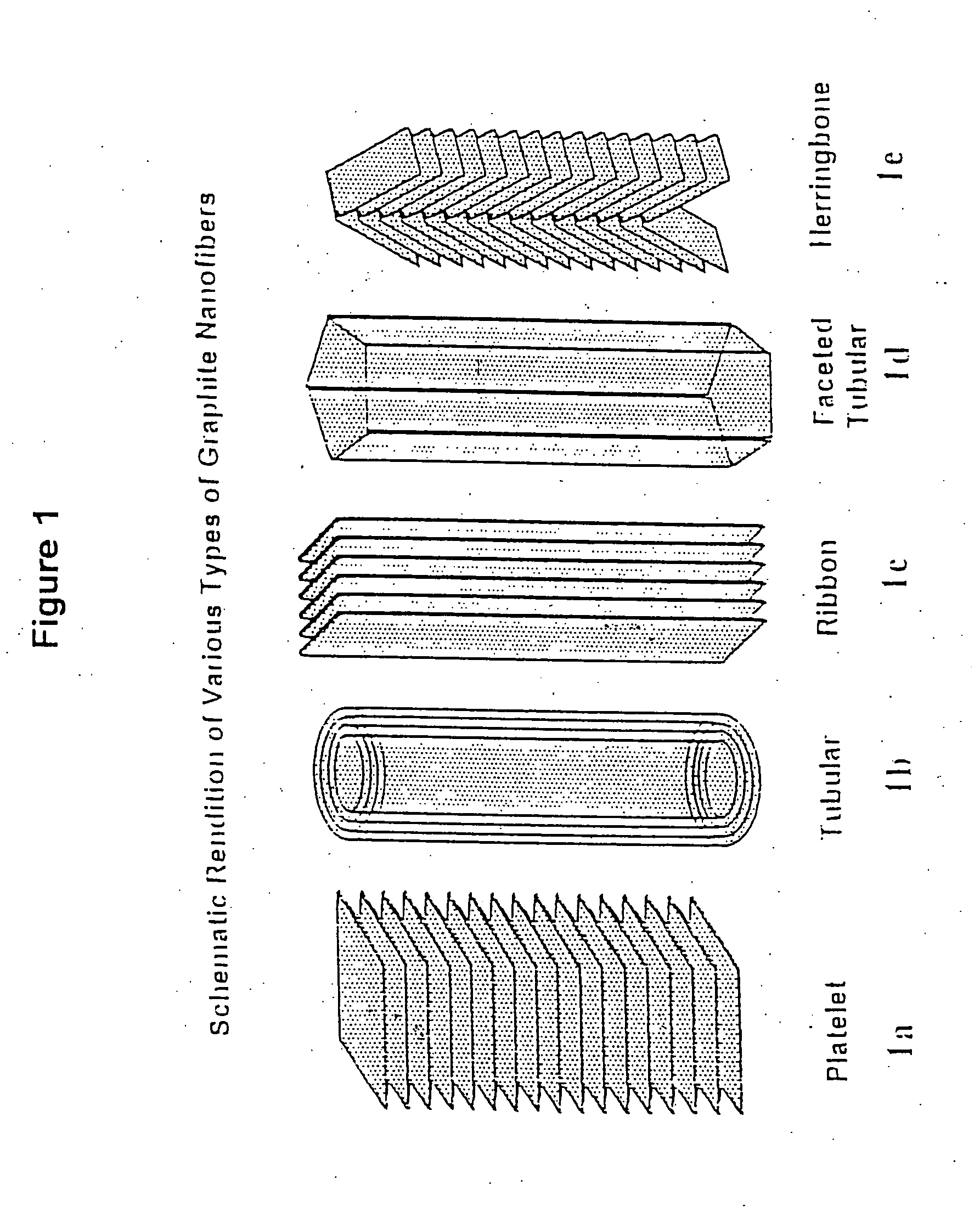
Patents
Literature
6656 results about "Dehydrogenation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dehydrogenation is a chemical reaction that involves the removal of hydrogen from an organic molecule.It is the reverse of hydrogenation. Dehydrogenation is an important reaction because it converts alkanes, which are relatively inert and thus low-valued, to olefins (including alkenes), which are reactive and thus more valuable. Alkenes are precursors to aldehydes, alcohols, polymers, and aromatics. Dehydrogenation processes are used extensively to produce aromatics and styrene in the petrochemical industry. Such processes are highly endothermic and require temperatures of 500 °C and above. Dehydrogenation also converts saturated fats to unsaturated fats. Enzymes that catalyze dehydrogenation are called dehydrogenases.
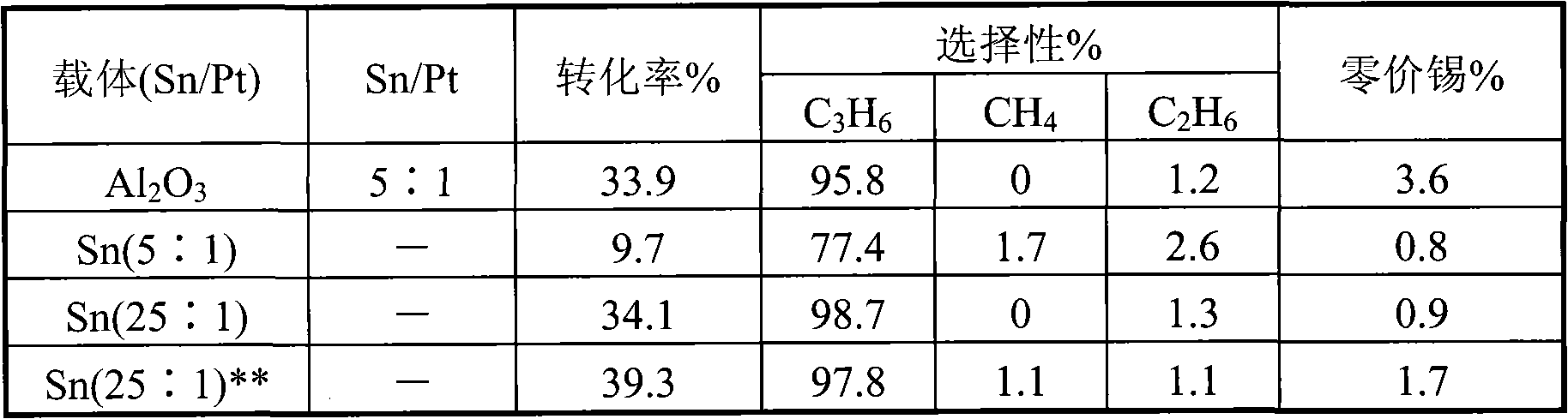
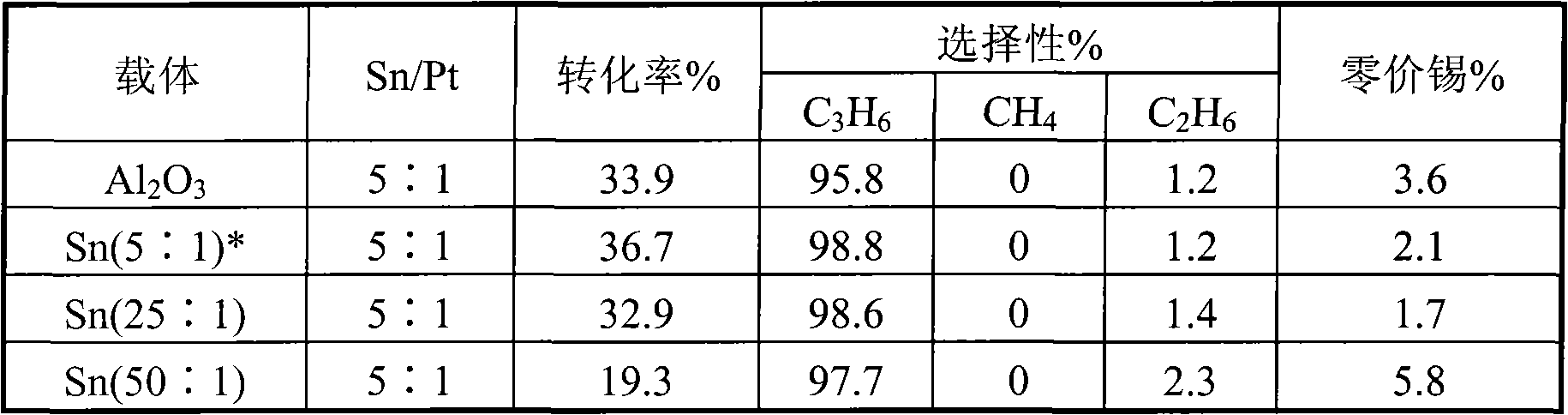
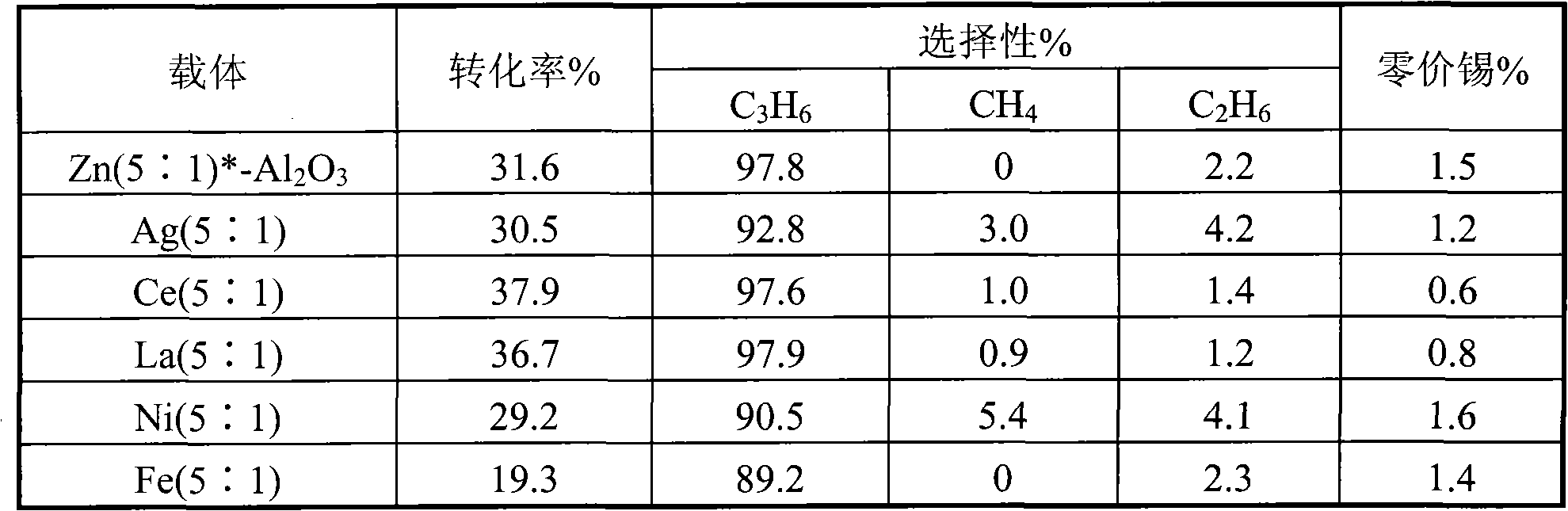
Catalyst for preparing propylene by propane dehydrogenation and its prepn.
InactiveCN101015802AHigh selectivityImprove reaction stabilityCatalyst carriersHydrocarbonsAdhesiveDehydrogenation
The invention relates to a catalyst for dehydrogenating propane to prepare propone, which uses thermal-resistant oxide as carrier, uses palladium-group metal as main catalyst, uses IV A group metal and rare-earth metal as agents, uses halogen as modifier, and uses inorganic oxide with high temperature resistance as adhesive. The inventive catalyst under high temperature and low pressure has higher propane transfer rate, propone selectivity and reaction stability. And the preparation comprises that at 60-100Deg. C, immerging thermal-resistant oxide with rare-earth metal water solution for 2-10h, at 60-180Deg. C baking for 2-10h, at 400-600Deg. C baking for 3-10h, immerging said carrier and the water solution which contains palladium metal and IVA metal at 60-100Deg. C for 2-10h, and baking for 2-10h at 60-180Deg. C, adding adhesive, protruding agent and acid gel solvent, protruding and shaping, baking for 2-10h at 60-180Deg. C, activating for 3-10h at 400-600Deg. C, and reducing for 2-10h in hydrogen flow at 400-600Deg. C.
Owner:SOUTHEAST UNIV
Low-carbon alkane dehydrogenation catalyst and preparation method thereof
ActiveCN101940922AEvenly distributedEnhanced charcoal capacityCatalyst activation/preparationHydrocarbonsAlkaneDehydrogenation
The invention discloses a low-carbon alkane dehydrogenation catalyst and a preparation method thereof. The catalyst is prepared from chromium serving as an active metal ingredient, alkali metal serving as an auxiliary catalytic ingredient and chromium-containing aluminum oxide serving as a carrier, wherein the weight content of the chromium oxide in the carrier is 2.0 to 15.0 percent. In the method, the active metal ingredient, namely chromium, is introduced into the aluminum oxide carrier by partially using a kneading method and partially using an immersion method; pseudo-boehmite mixed with chromium is processed by adopting a three-step roasting method and a hydrothermal method; and thus, the porous structure and the surface character of the carrier can be improved, the content and the distribution of the active metal chromium in the carrier and the mutual effect between the active metal and the aluminum oxide are further modulated, the activity and the stability of the catalyst are improved, the carbon depositing resistance of the catalyst is enhanced, and the service life of the catalyst is prolonged.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for improving catalyst reacting activity in the propylene producing through propane dehydrogenation
InactiveCN101138734AHigh reactivityHigh mechanical strengthMolecular sieve catalystsHydrocarbonsRare earthDehydrogenation
A method to promote the activity of a catalyst for dehydrogenation of propane to propylene is as follows: (1) an inorganic oxide bonding agent, a promoter and an acid solvent are added into a heat-resistant oxide; then after the oxide bonding agent, the promoter, the acid solvent and the oxide are kneaded evenly, the oxide is molded by rolling or band-extruding; (2) the catalyst carrier prepared is dried for 2 to 10 hours under the temperature of 60 centigrade degrees, and calcined under the temperature of 400 to 800 degrees; (3) the calcined carrier is immersed in a rare earth metal water solution under the temperature of 60 to 100 centigrade degrees for 2 to 10 hours; (4) the catalyst carrier modified by the rare earth is immersed in a water solution comprising platinum metal elementsand the fourteenth metal elements under the temperature of 400 to 600 centigrade degrees for 2 to 10 hours, and then the carrier is filtered, washed with distilled water, dried under the temperature of 60 to 180 centigrade degrees for 2 to 10 hours, and calcined under the temperature of 400 to 600 centigrade degrees for 2 to 10 hours; (5) the catalyst prepared is activated in the air under the temperature of 400 to 600 centigrade degrees for 3 to 10 hours, and reduced in a hydrogen flow under the temperature of 400 to 600 centigrade degrees for 2 to 10 hours; the reduced catalyst is used for catalytic reaction for dehydrogenation of propane to propylene.
Owner:SOUTHEAST UNIV
Complex comprising oxidative dehydrogenation unit
ActiveUS20140249339A1Consumes lotThermal non-catalytic crackingSequential/parallel process reactionsAlkaneDehydrogenation
Oxidative dehydrogenation of paraffins to olefins provides a lower energy route to produce olefins. Oxidative dehydrogenation processes may be integrated with a number of processes in a chemical plant such as polymerization processes, manufacture of glycols, and carboxylic acids and esters. Additionally, oxidative dehydrogenation processes can be integrated with the back end separation process of a conventional steam cracker to increase capacity at reduced cost.
Owner:NOVA CHEM (INT) SA
Apparatus and process for production of high purity hydrogen
InactiveUS20060248800A1High purityEnhanced overall recoveryCarbon compoundsIndirect carbon-dioxide mitigationSteam reformingCombustion chamber
The invention relates to a new and improved process and apparatus for the production of high purity hydrogen by steam reforming. The apparatus is an integrated flameless distributed combustion-membrane steam reforming (FDC-MSR) or reactor for steam reforming of a vaporizable hydrocarbon to produce H2 and CO2, with minimal CO, and minimal CO in the H2 stream. The flameless distributed combustion drives the steam reforming reaction which pro-vides great improvements in heat exchange efficiency and load following capabilities. The reactor may contain multiple flameless distributed combustion chambers and multiple hydrogen-selective, hydrogen-permeable, membrane tubes. The feed and reaction gases may flow through the reactor either radially or axially. A further embodiment of the invention involves producing high purity hydrogen by dehydrogenation using an integrated FDC-membrane de-hydrogenation reactor. A still further embodiment of the invention involves a zero emission hybrid power system wherein the produced hydrogen is used to power a high-pressure internally manifolded molten carbonate fuel cell. In addition, the design of the FDC-SMR powered fuel cell makes it possible to capture good concentrations of CO2 for sequestration or use in other processes.
Owner:SHELL OIL CO
Catalyst propane using aluminium oxide modified mesonore molecular sieve as carrier for dehydrogenation producing propylene
InactiveCN101125298AHigh propane conversionGood dehydrogenation stabilityMolecular sieve catalystsHydrocarbonsMolecular sievePlatinum
The invention relates to a catalyst to make the propene by dehydrogenation propane which takes the modifying alumina medium molecular sieve as the carrier. The catalyst takes the modifying medium molecular sieve by the Al2O3 as the carrier; the platinum and tin double metal catalyst has an alumina weight proportion of 5.0 to 30.0 percent. The active component of the catalyst is the platinum; the weight proportion of the platinum is 0.4 percent; and the weight proportion of the accessory ingredient Sn is 0.1 to 2.0 percent.
Owner:NANJING UNIV
Catalyst for preparing propylene through propane catalytic dehydrogenation and preparation method thereof
InactiveCN102389831ALarge specific surface areaHigh catalytic activityMolecular sieve catalystsHydrocarbonsDehydrogenationEconomic benefits
The invention relates to a catalyst for preparing propylene through propane catalytic dehydrogenation, which is a load type platinum-based catalyst and is characterized in that home-made mesoporous molecular sieves MCM-41 are used as carriers, metal Pt is used as an active ingredient, and metals Sn, Sn-Ce or Sn-Ce-Ca are used as auxiliary agents. In a preparation method, an isovolumetric continuous immersion method is adopted for preparation. The catalyst and the preparation method have the advantages that the isovolumetric continuous immersion method is adopted for preparing multi-component catalysts, wherein the content of any one component is easy to control, the prepared catalyst has higher specific surface area (700 to 900m<2> / g) and has high catalytic activity, the propane conversion rate is high, the propylene selectivity is good, the one-path stability is good, in addition, the service life is longer, the preparation method of the catalyst carriers is simple and feasible, the economic benefit is obvious, and good industrial development and application prospects are realized.
Owner:NANKAI UNIV
Alkane and alkane group dehydrogenation with organometallic catalysts
Novel polynuclear organometallic complexes useful as catalysts for the reversible deshydrogenation of alkanes and alkane group are disclosed. The novel compounds comprise a first transition, a second transition metal p-bonded to an ?5-aromatic ligand, and a pincer ligand. The pincer ligand comprises a 6p-electron aromatic ring having at least 2 ring atoms in an 1, 3 relationship bonded each to a neutral Lewis base through a bridge, the bridge being a diradical. The pincer ligand binds the first transition metal through each of the Lewis bases and through the ring atom adjacent to both Lewis bases and p-coordinates the second transition metal through all aromatic ring atoms. The first transition metal may also bond to 2 or 4 hydrogen atoms.
Owner:POWERNOVA TECH
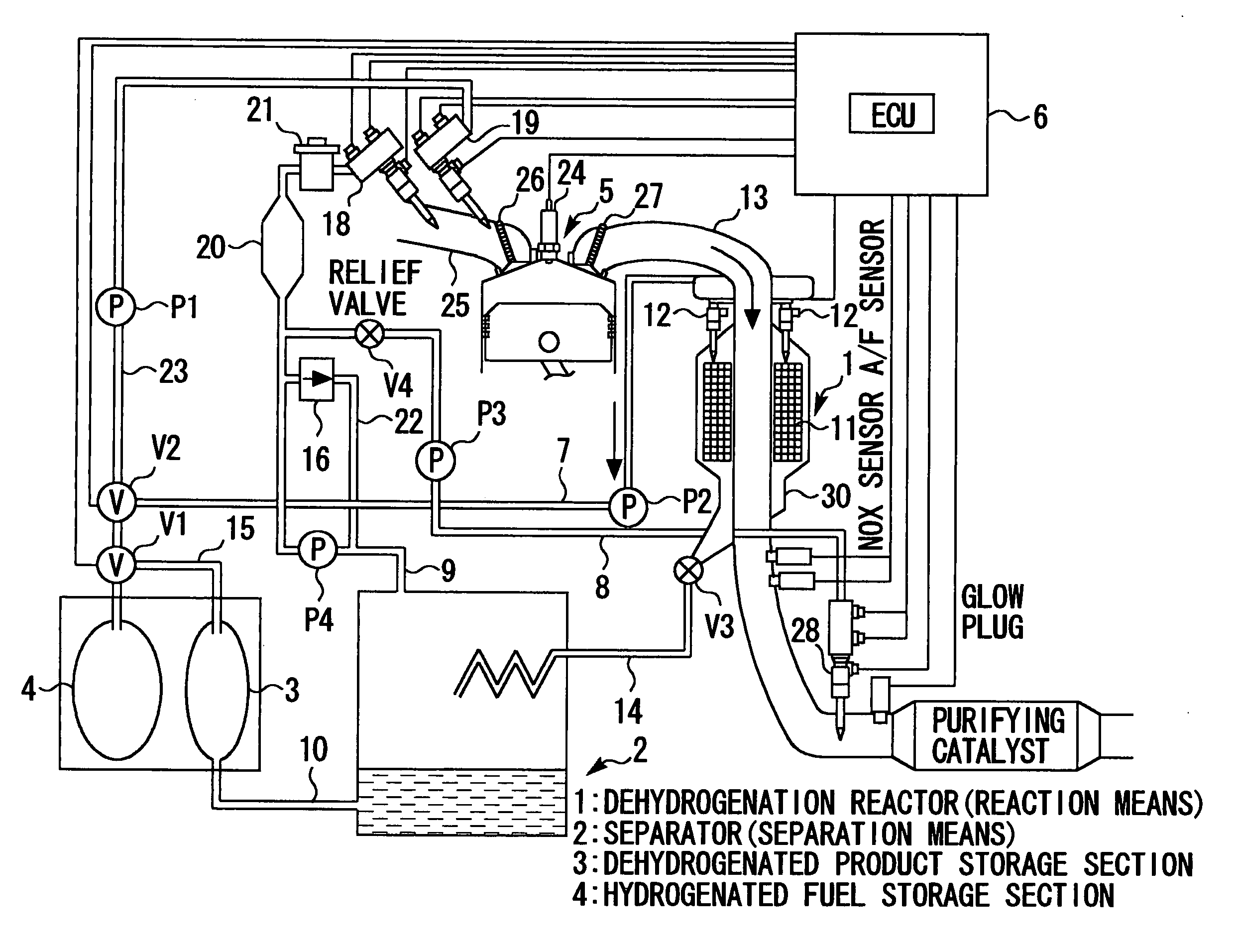
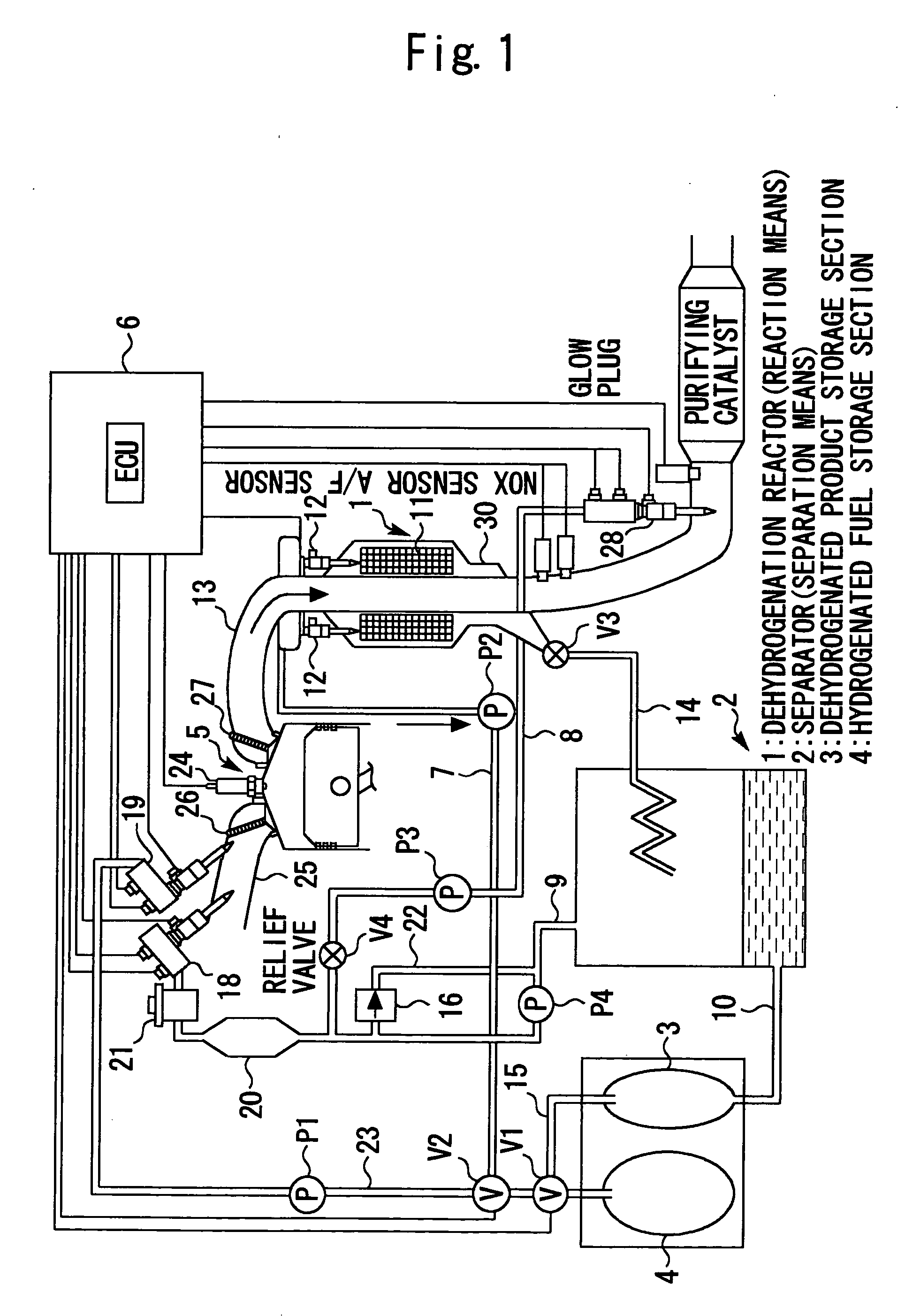
Internal combustion engine utilizing hydrogen
The present invention relates to a system that is capable of freely selecting one or two or more types of fuel and supplying the selected types of fuel to an internal combustion engine. Disclosed is a hydrogen-fueled internal combustion engine that operates upon receipt of one or more types of fuel that are selected from hydrogenated fuel and a dehydrogenated product and hydrogen, which are obtained by dehydrogenating the hydrogenated fuel. The hydrogen-fueled internal combustion engine comprises: a hydrogenated fuel storage section; reaction means for invoking a dehydrogenation reaction; separation means for separating hydrogen-rich gas and dehydrogenated product; and a dehydrogenated product storage section for storing the separated dehydrogenated product.
Owner:TOYOTA JIDOSHA KK
Method for the oxidative dehydrogenation of ethane
InactiveUS7319179B2Organic compound preparationHeterogenous catalyst chemical elementsAlkaline earth metalDehydrogenation
Owner:CONSEJO SUPERIOR DE INVESTIGACIONES CIENTIFICAS (CSIC) +1
Catalytically inactive heat generator and improved dehydrogenation process
An improved dehydrogenation catalyst bed system for olefin production utilizing classical processing techniques is disclosed. The catalyst bed system comprises a dehydrogenation catalyst comprising an active component selected from an oxide of a metal of Group 4 or Group 5 or Group 6 and combinations thereof and a support selected from aluminum oxide, aluminas, alumina monohydrate, alumina trihydrate, alumina-silica, transition aluminas, alpha-alumina, silica, silicate, aluminates, calcined hydrotalcites, zeolites and combinations thereof mixed with a first inert material selected from any material that is catalytically inactive when subjected to reaction conditions that can effect dehydrogenation of olefins and that has a high density and high heat capacity and that is not capable of producing heat during any stage of the dehydrogenation process, and the dehydrogenation catalyst plus the first inert material then being physically mixed with a secondary component comprising a heat-generating inert material and a carrier capable of supporting the heat-generating inert material, wherein the secondary component is catalytically inert with respect to dehydrogenation reactions or to cracking or to coking and generates heat after being exposed to reducing and / or to oxidizing reaction conditions.
Owner:CLARIANT INT LTD
Zinc ferrite catalysts, method of preparing thereof and method of preparing 1,3-butadiene using thereof
ActiveCN101674883ASimple structureEasy to synthesizeHeterogenous catalyst chemical elementsCatalystsButeneDehydrogenation
Owner:SK INNOVATION CO LTD +1
Catalyzer used for low carbon alkane catalytic dehydrogenation and method of manufacturing propylene by paraffin hydrocarbons catalytic dehydrogenation with the same as catalyzer
InactiveCN101108362AReactiveMitigation of heightened demandMolecular sieve catalystsHydrocarbonsAlkaneReaction temperature
The invention relates to a catalyst used for catalyzing and dehydrogenation of low-carbon alkane and the method of producing propylene by alkane catalyzing and dehydrogenation with the catalyst. The former catalyst used for catalyzing and dehydrogenation of low-carbon alkane, each molecule of the hydrocarbon has about 2 to 8 carbon atom, which is characterized in that: the catalyst makes a molecular sieve the carrier, the Pt family metal is loaded on the carrier as the active component, makes the IVA family metal element and alkalinity metal element as additional agent and high temperature standing inorganic oxide as connection agent; when the catalyst is used in producing propylene by alkane catalyzing and dehydrogenation, the reaction temperature is 500 to 700 DEG C., the pressure is 0 to 0.2Mpa, the quality air speed is 2 to 5h to 1, the regenerating temperature of the catalyst is 500 to 700 DEG C., the air speed is 100 to 1000h to 1, the pressure is 0 to 1.0MPa. With adopting the invention, the reaction of producing propylene by alkane catalyzing and dehydrogenation is good, the average transforming rate is 30 per cent, the selectivity above 95 per cent can keep for 50 days.
Owner:SINOPEC JINLING PETROCHEMICAL CO LTD
Lithium aluminate layered catalyst and a selective oxidation process using the catalyst
InactiveUS6858769B2Thermal non-catalytic crackingHydrocarbon by isomerisationHydrogenDehydrogenation
A catalyst for the selective oxidation of hydrogen has been developed. It comprises an inert core such as cordierite and an outer layer comprising a lithium aluminate support. The support has dispersed thereon a platinum group metal and a promoter metal, e.g. platinum and tin respectively. This catalyst is particularly effective in the selective oxidation of hydrogen in a dehydrogenation process.
Owner:UOP LLC
Process for the simultaneous conversion of methane and organic oxygenate to C2 to C10 hydrocarbons
A process for the non-oxidative conversion of methane simultaneously with the conversion of an organic oxygenate, represented by a general formula: CnH2n+1OCmH2m+1, wherein C, H and O are carbon, hydrogen and oxygen elements, respectively; n is an integer having a value between 1 and 4; and m is an integer having a value between zero and 4, to C2+ hydrocarbons, particularly to gasoline range C6–C10 hydrocarbons and hydrogen, using a bifunctional pentasil zeolite catalyst, having strong acid and dehydrogenation functions, at a temperature below 700° C. is disclosed. In this process the moles of methane converted per mole of oxygenate converted is above 1.0, depending upon the process conditions.
Owner:COUNCIL OF SCI & IND RES
Dehydrogenation catalyst for feed gas containing carbon monoxide, preparation method and application method thereof
ActiveCN101543776AWide range of CO content requirementsGood choiceCarbon monoxideMetal/metal-oxides/metal-hydroxide catalystsPlatinum saltsCerium
The invention discloses a dehydrogenation catalyst for feed gas containing carbon monoxide, a preparation method and an application method thereof. The catalyst takes alumina as a carrier, palladium and / or platinum as an active component and 2 to 4 MOxes as an additive, M is sodium, potassium, magnesium, titanium, zirconium, vanadium, manganese, iron, nickel, cobalt, copper, molybdenum, tungsten or cerium, and components of the catalyst (calculated by carrier mass) are: 0.01 to 2 percent of the palladium and / or 0.01 to 1 percent of the platinum, and 1 to 2 percent of MOxes. The preparation method for the catalyst comprises the following steps that: firstly, an additive metal salt solution is used to impregnate the carrier; a palladium and / or platinum salt solution is used to impregnate the carrier after the carrier is dried and roasted; and the impregnated carrier is roasted at a temperature of between 450 and 850 DEG C to obtain the catalyst. Before the use, H2-N2 mixed gas containing more than 10 percent of hydrogen or pure hydrogen is activated by the catalyst at a temperature of between 450 and 650 DEG C. The catalyst can perform deep removal on less than 5 percent of hydrogen in the feed gas with the CO content of between 10 and 99 percent, the using temperature is between 100 and 300 DEG C, the space velocity is between 500 and 9,000h<-1>, the dehydrogenation rate is more than 99 percent, the content of outlet hydrogen is less than 100 ppm, and the loss of the carbon monoxide is less than 0.5 percent.
Owner:HAISO TECH
Biofuels via hydrogenolysis and dehydrogenation-condensation
ActiveUS20110282115A1Easy to oxidizePromote hydrolysis reactionBiofuelsEfficient propulsion technologiesHydrogenDehydrogenation
A method comprising providing a carbohydrate feed; contacting at least a portion of the carbohydrate feed directly with hydrogen in the presence of a hydrogenolysis catalyst to produce a first reaction product comprising a stable hydroxyl intermediate; contacting at least a portion of the first reaction product comprising the stably hydroxyl intermediates with a dehydrogenation catalyst to form a second reaction product; and contacting at least a portion of the second reaction product with a condensation catalyst comprising a base functionality to form a fuel blend.
Owner:SHELL USA INC
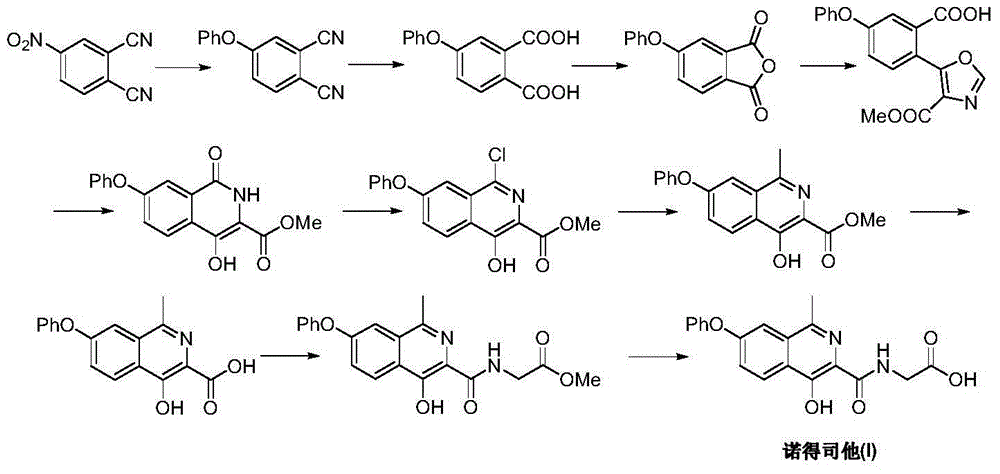
Preparation method of Roxadustat
ActiveCN104892509AEase of industrial productionRaw materials are easy to getOrganic chemistryDehydrogenationTyrosine
The invention discloses a preparation method of Roxadustat. The preparation steps are as follows: the Roxadustat is prepared from tyrosine through esterification, etherification, cyclization, dehydrogenation, oxidative rearrangement and acylation reaction. The preparation method has the advantages that the raw materials are easily obtained; the process is simple; and the preparation method is economic and environmental-friendly, and is suitable for industrialized production.
Owner:南通华宇化工科技有限公司
Process for Preparing Butadiene by Oxidative Dehydrogenation of N-Butenes with Monitoring of the Peroxide Content During Work-Up of the Product
InactiveUS20140200381A1Analysis using chemical indicatorsHydrocarbon by hydrogenationWater vaporDesorption
The invention relates to a process for preparing butadiene from n-butenes, which comprises the following steps:A) provision of a feed gas stream a comprising n-butenes;B) introduction of the feed gas stream a comprising n-butenes and an oxygen-comprising gas into at least one dehydrogenation zone and oxidative dehydrogenation of n-butenes to butadiene, giving a product gas stream b comprising butadiene, unreacted n-butenes, water vapor, oxygen, low-boiling hydrocarbons, possibly carbon oxides and possibly inert gases;C) cooling and compression of the product gas stream b in at least one cooling stage and at least one compression stage, with the product gas stream b being brought into contact with a circulated coolant to give at least one condensate stream c1 comprising water and a gas stream c2 comprising butadiene, n-butenes, water vapor, oxygen, low-boiling hydrocarbons, possibly carbon oxides and possibly inert gases;D) separation of incondensable and low-boiling gas constituents comprising oxygen, low-boiling hydrocarbons, possibly carbon oxides and possibly inert gases as gas stream d2 from the gas stream c2 by absorption of the C4-hydrocarbons comprising butadiene and n-butenes in a circulated absorption medium, giving an absorption medium stream loaded with C4-hydrocarbons and the gas stream d2, and subsequent desorption of the C4-hydrocarbons from the loaded absorption medium stream to give a C4 product gas stream d1;E) separation of the C4 product stream d1 by extractive distillation using a solvent which is selective for butadiene into a stream e1 comprising butadiene and the selective solvent and a stream e2 comprising n-butenes;F) distillation of the stream e1 comprising butadiene and the selective solvent to give a stream f1 consisting essentially of the selective solvent and a stream f2 comprising butadiene;where samples are taken from the circulated coolant in step C) and / or the circulated absorption medium in step D) and the peroxide content of the samples taken is determined by means of iodometry, differential scanning calorimetry (DSC) or microcalorimetry.
Owner:BASF AG
Nanometer grade low carbon paraffin dehydrogen catalyst
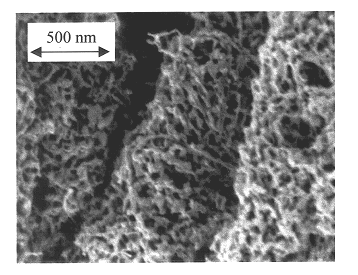
InactiveCN1911502ALarge specific surface areaHigh catalytic activityMetal/metal-oxides/metal-hydroxide catalystsAlkaneCarbon nanotube
The present invention relates to one kind of nanometer level low carbon alkane dehydrogenating catalyst, and features that the C3-C5 low carbon alkane dehydrogenating catalyst comprises carrier of single wall or multiple wall carbon nanotube and two active components selected from chromic oxide in 2-30 wt%, alumina in 2-25 weight and nickel oxide in 2-30 wt%. The catalyst has high catalysis activity, increased active structures, long service life, low catalytic dehydrogenation reaction temperature, high conversion rate, high selectivity and other advantages.
Owner:DAQING PETROLEUM ADMINISTRATION
Hydrogen storage by reversible hydrogenation of pi-conjugated substrates
ActiveUS20050002857A1Less energy expenditureEasy to separateCatalytic naphtha reformingVariable capacity gas holdersPartial hydrogenationDehydrogenation
Processes are provided for the storage and release of hydrogen by means of a substantially reversible catalytic hydrogenation of extended pi-conjugated substrates which include large polycyclic aromatic hydrocarbons, polycyclic aromatic hydrocarbons with nitrogen heteroatoms, polycyclic aromatic hydrocarbons with oxygen heteroatoms, polycyclic aromatic hydrocarbons with alkyl, alkoxy, nitrile, ketone, ether or polyether substituents, pi-conjugated molecules comprising 5 membered rings, pi-conjugated molecules comprising six and five membered rings with nitrogen or oxygen hetero atoms, and extended pi-conjugated organic polymers. The hydrogen, contained in the at least partially hydrogenated form of the extended pi-conjugated system, can be facilely released for use by a catalytic dehydrogenation of the latter in the presence of a dehydrogenation catalyst which can be effected by lowering the hydrogen gas pressure, generally to pressures greater than 0.1 bar or raising the temperature to less than 250° C. or less, or by a combination of these two process parameters.
Owner:AIR PROD & CHEM INC
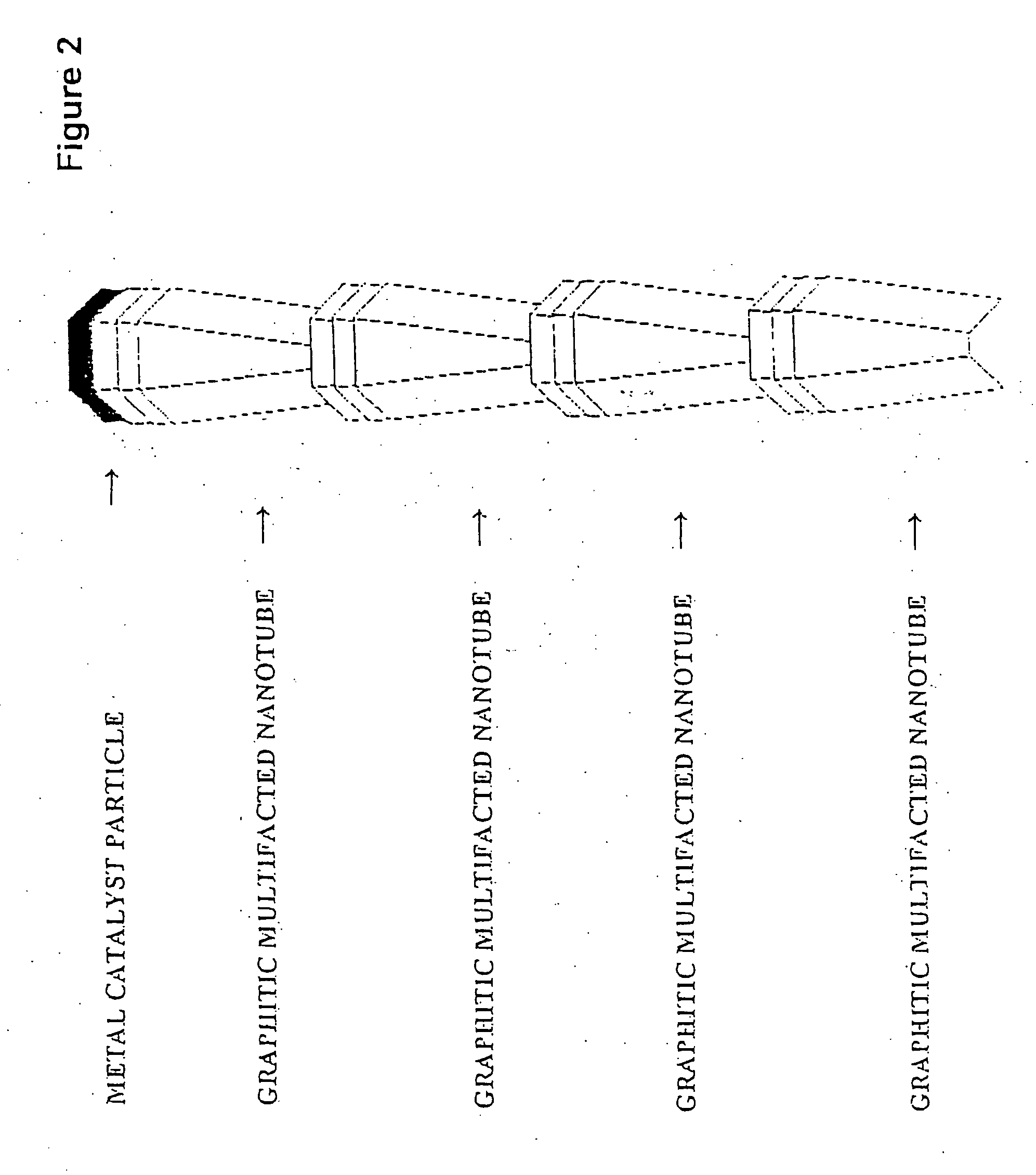
Novel graphite nanocatalysts
Novel catalysts comprised of graphitic nanostructures. The graphitic nanostructure catalysts are suitable for catalyzing reactions such as oxidation, hydrogenation, oxidative-hydrogenation, and dehydrogenation.
Owner:CATALYTIC MATERIALS
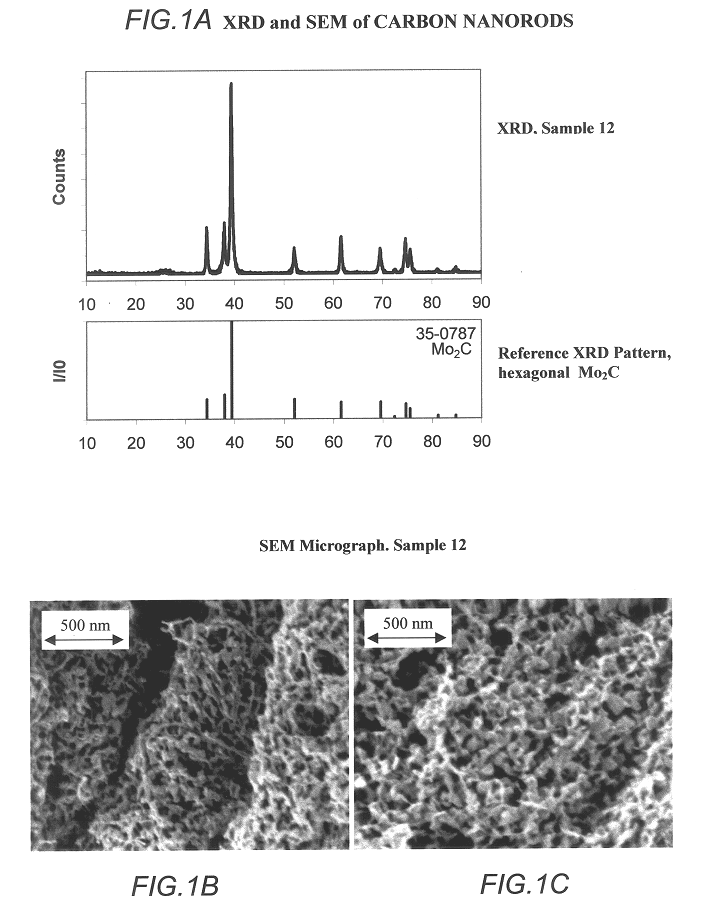
Carbide and oxycarbide based compositions, rigid porous structures including the same, methods of making and using the same
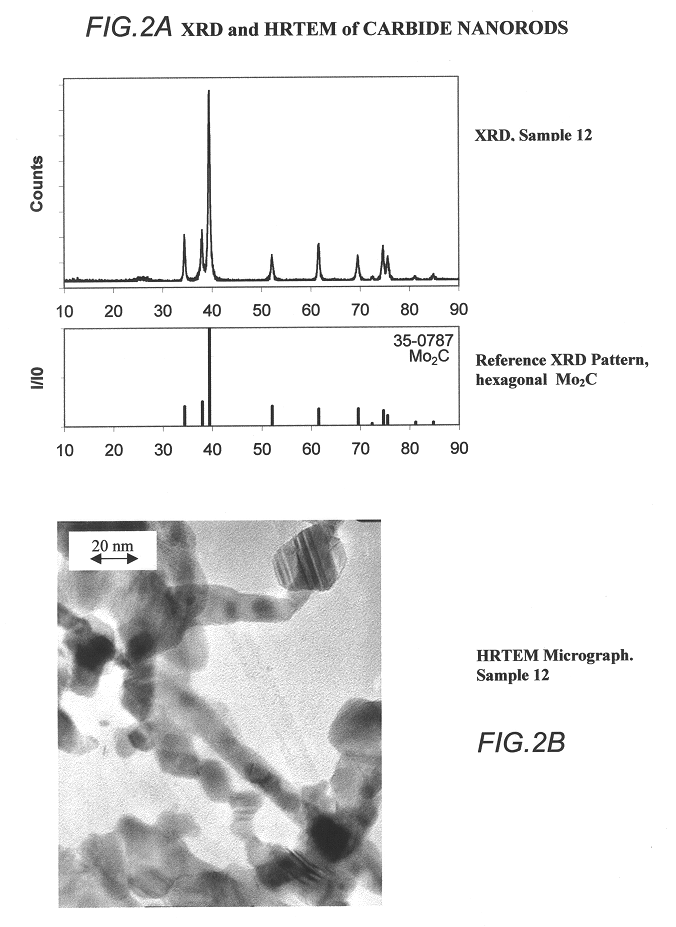
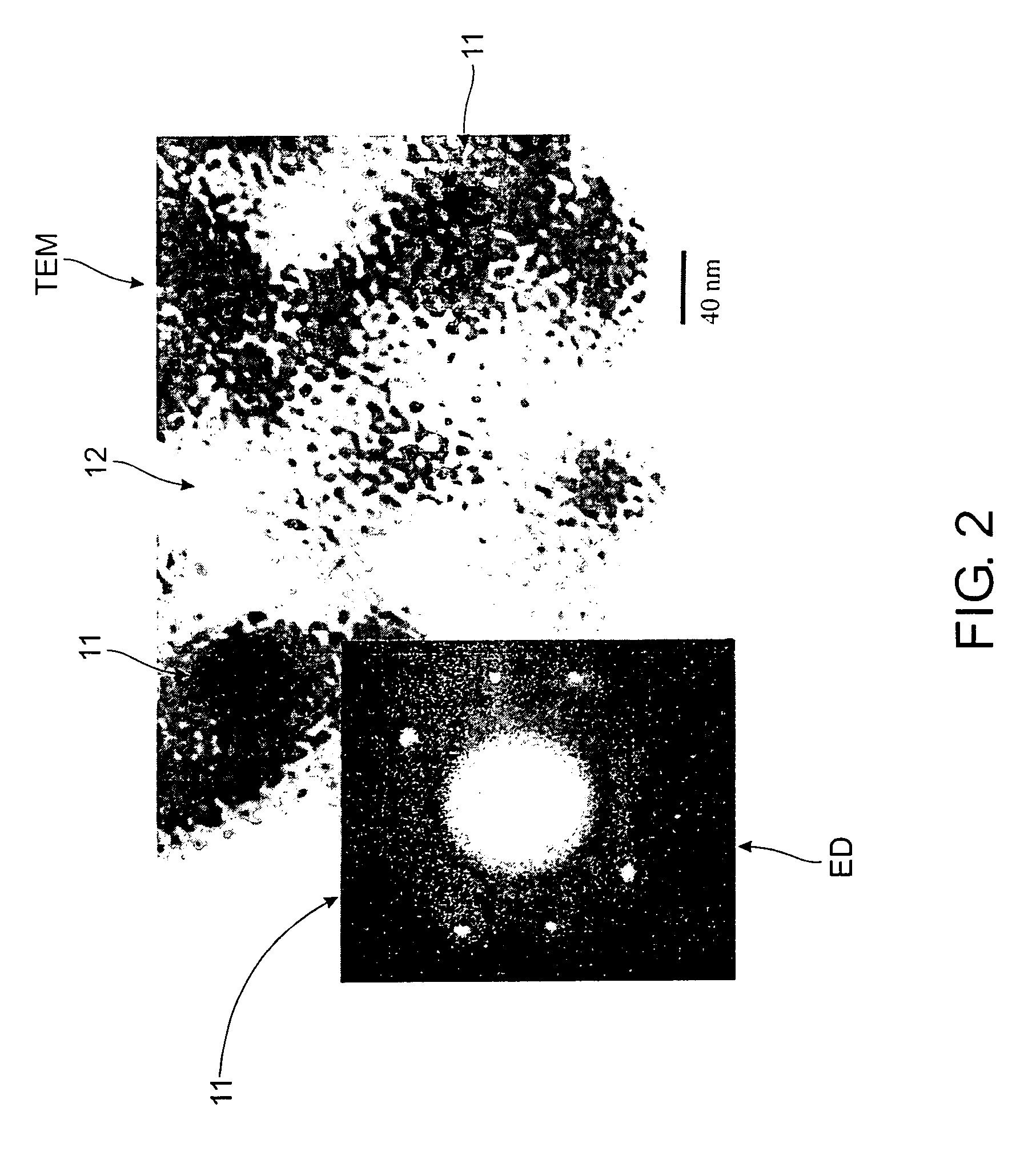
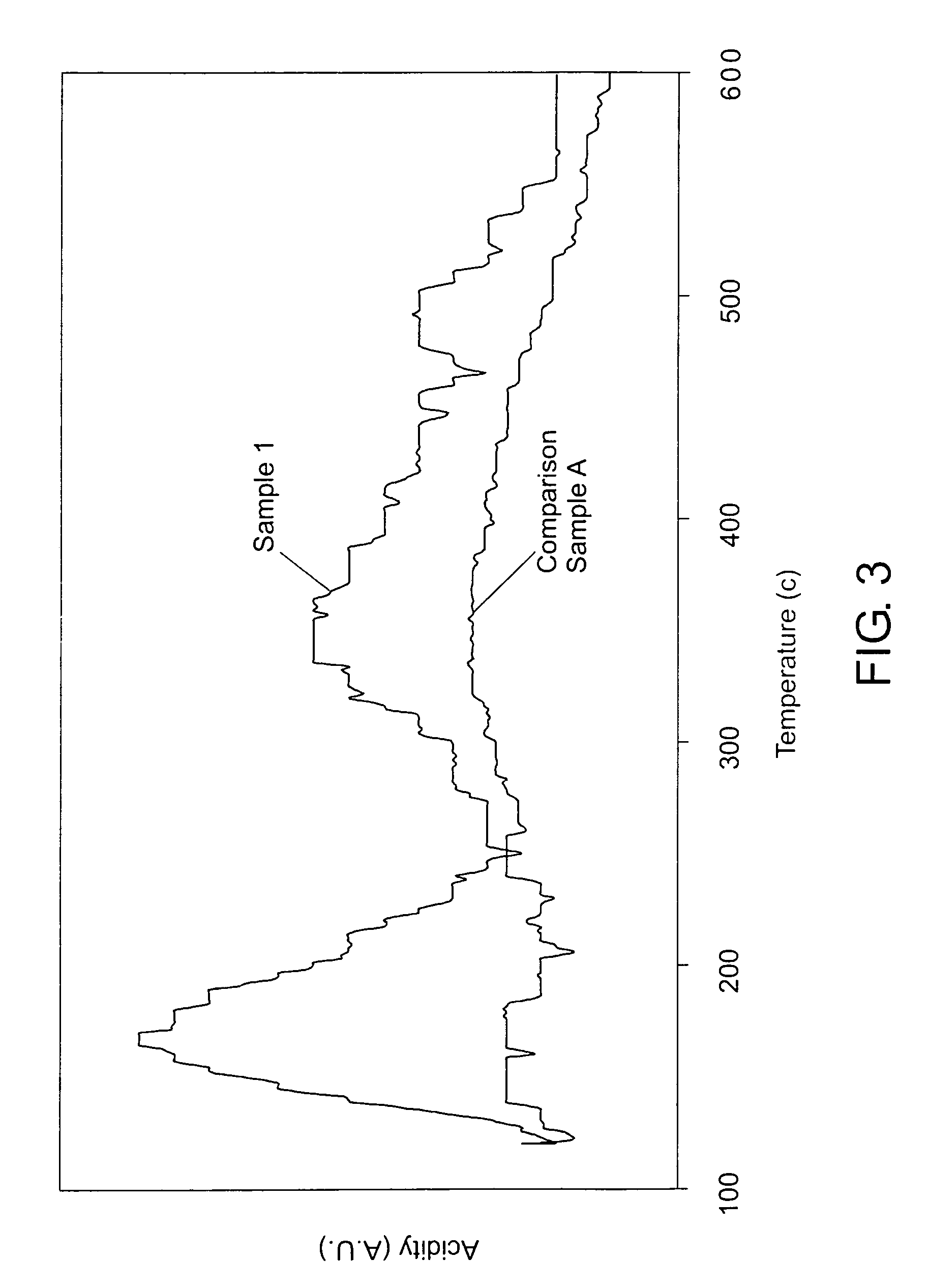
InactiveUS6514897B1Easy to diffuseSpeed up the flowMaterial nanotechnologyHydrocarbon by isomerisationIsomerizationChemical reaction
The present invention relates to compositions and rigid porous structures that contain nanorods having carbides and / or oxycarbides and methods of making and using such compositions and such rigid porous structures. The compositions and rigid porous structures can be used either as catalysts and / or catalyst supports in fluid phase catalytic chemical reactions. Processes for making supported catalyst for selected fluid phase catalytic reactions are also provided. The fluid phase catalytic reactions catalyzed include hydrogenation hydrodesulfuriaation, hydrodenitrogenation, hydrodemetallization, hydrodeoxygenation, hydrodearomatization, dehydrogenation, hydrogenolyis, isomerization, alkylation, dealkylation, oxidation and transalkylation.
Owner:HYPERION CATALYSIS INT
Propane dehydrogenation catalyst with hetero atom molecule sieve as carrier and preparation method thereof
InactiveCN101513613AEnhanced interactionPromote migrationMolecular sieve catalystsCatalyst activation/preparationMolecular sievePtru catalyst
The invention relates to a propane dehydrogenation catalyst with a hetero atom molecule sieve as a carrier. The hetero atom molecule sieve carrier for preparing the catalyst is a ZSM-5 molecule sieve with the skeleton containing metallic elements of the fourth A group and rare earth elements. The catalyst is shaped by taking metallic elements of platinum group as a main catalyst, taking metallic elements of the fourth A group or the metallic elements of the forth A group or the second A group as an assistant, taking halogen elements as a modifying agent, and taking inorganic oxides as a bonder. The preparation of the catalyst adopts a step impregnation method, that is, the method comprises the steps of firstly impregnating the alkaline metal assistant so as to effectively adjust the acidity and alkaline of the catalyst and the variety of positive ions in the pore path of the molecule sieve by cation interchange technology; and then impregnating the metallic elements of platinum group. The catalyst has excellent anti-carbon performance, and higher propane utilization rate, propane selectivity and reaction reliability at the reaction conditions of high temperature and low pressure.
Owner:NANJING JINLIAN TECH +1
Zeolite composite, method for making and catalytic application thereof
A catalytic material includes microporous zeolites supported on a mesoporous inorganic oxide support. The microporous zeolite can include zeolite Beta, zeolite Y (including “ultra stable Y”—USY), mordenite, Zeolite L, ZSM-5, ZSM-11, ZSM-12, ZSM-20, Theta-1, ZSM-23, ZSM-34, ZSM-35, ZSM-48, SSZ-32, PSH-3, MCM-22, MCM-49, MCM-56, ITQ-1, ITQ-2, ITQ-4, ITQ-21, SAPO-5, SAPO-11, SAPO-37, Breck-6, ALPO4-5, etc. The mesoporous inorganic oxide can be e.g., silica or silicate. The catalytic material can be further modified by introducing some metals e.g. aluminum, titanium, molybdenum, nickel, cobalt, iron, tungsten, palladium and platinum. It can be used as catalysts for acylation, alkylation, dimerization, oligomerization, polymerization, hydrogenation, dehydrogenation, aromatization, isomerization, hydrotreating, catalytic cracking and hydrocracking reactions.
Owner:ABB LUMMUS GLOBAL INC
Catalyst, its preparation and use
A process for preparing a catalyst which process comprises preparing a mixture comprising iron oxide and at least one Column 1 metal or compound thereof, wherein the iron oxide is obtained by heating a mixture comprising an iron halide and at least 0.05 millimoles of a Column 6 metal per mole of iron; a catalyst made by the above described process; an iron oxide composition; a process for the dehydrogenation of an alkylaromatic compound which process comprises contacting the alkylaromatic compound with the catalyst; and a method of using an alkenylaromatic compound for making polymers or copolymers, in which the alkenylaromatic compound has been produced by the dehydrogenation process.
Owner:SHELL OIL CO
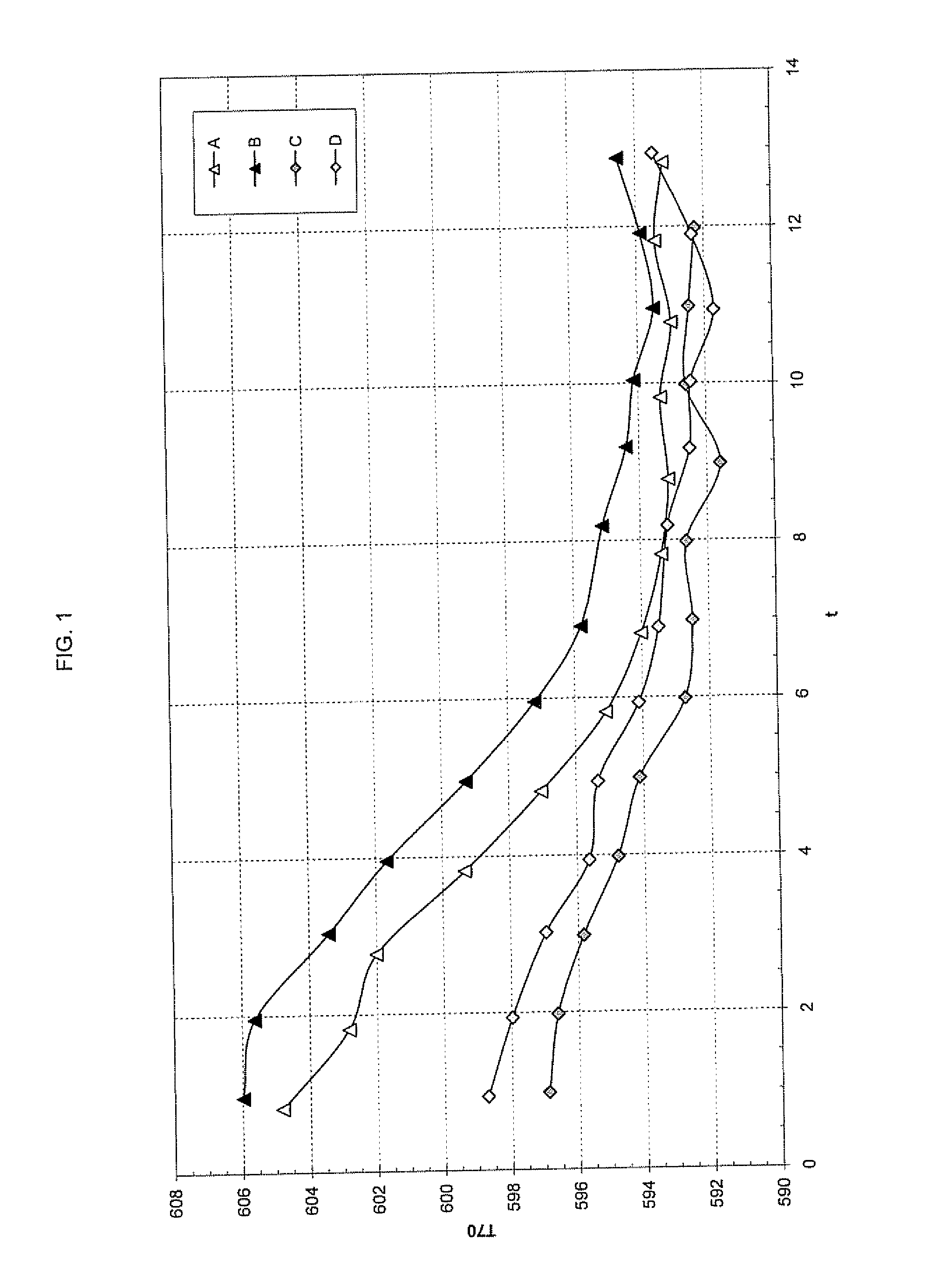
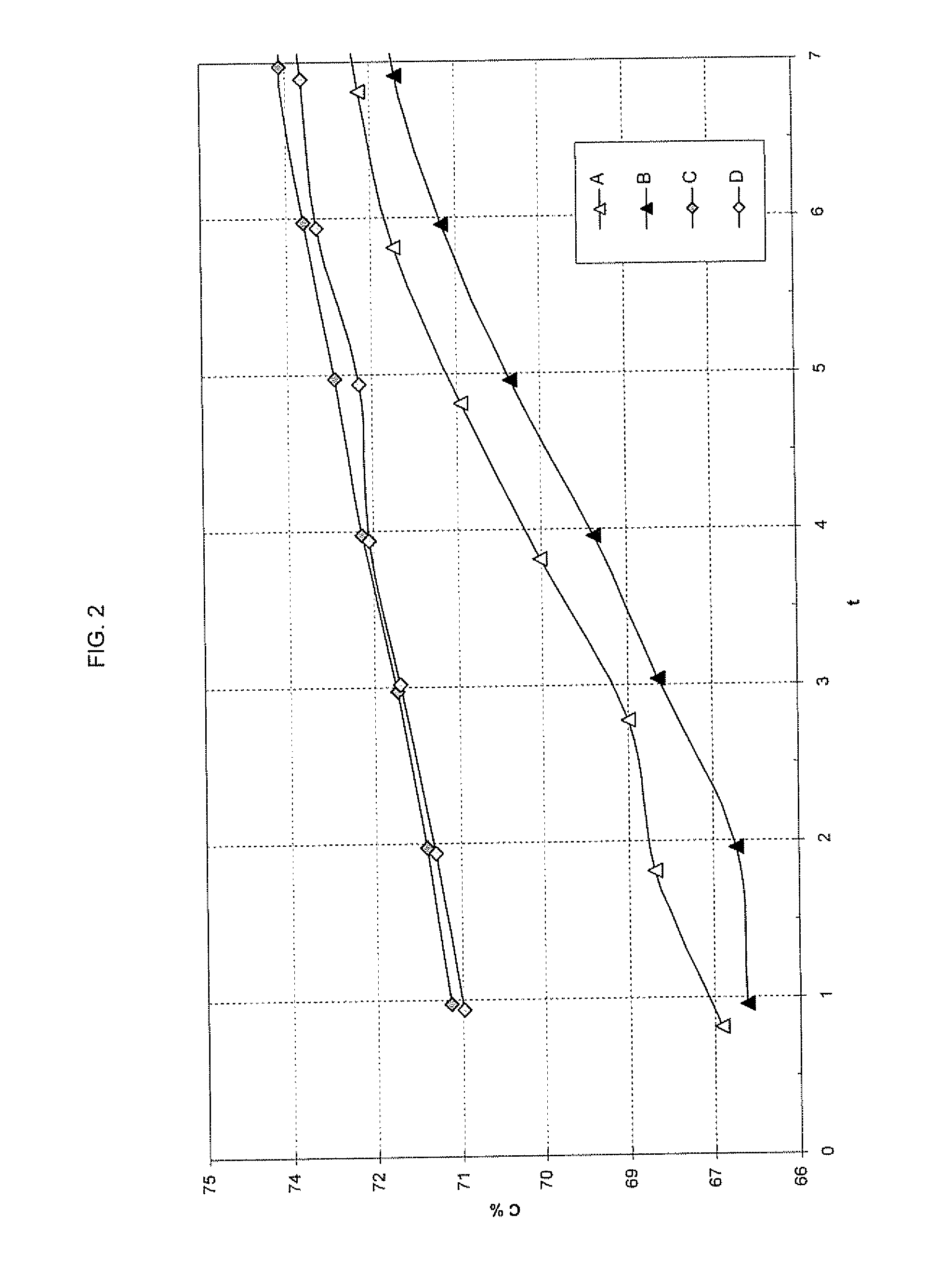
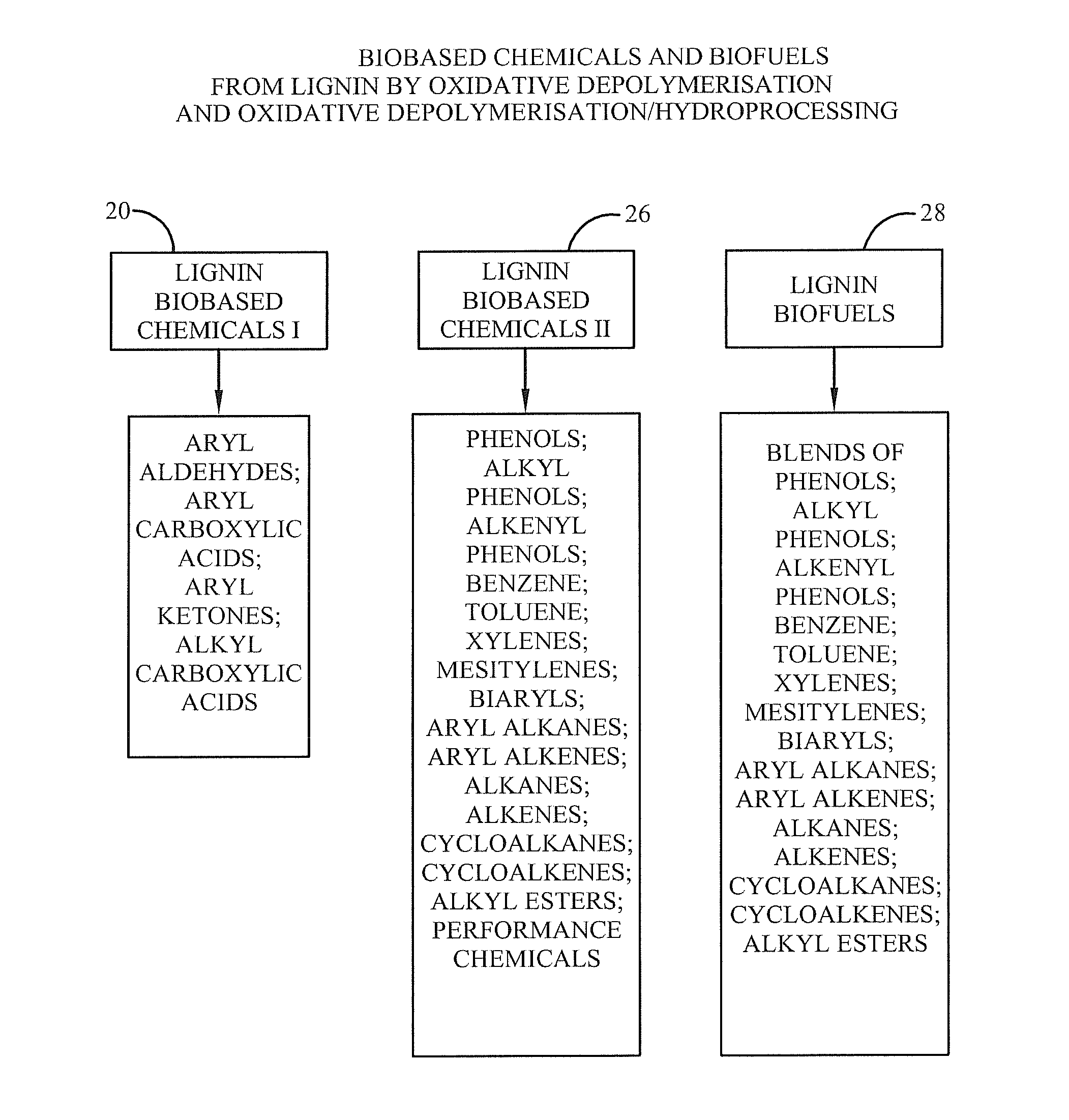
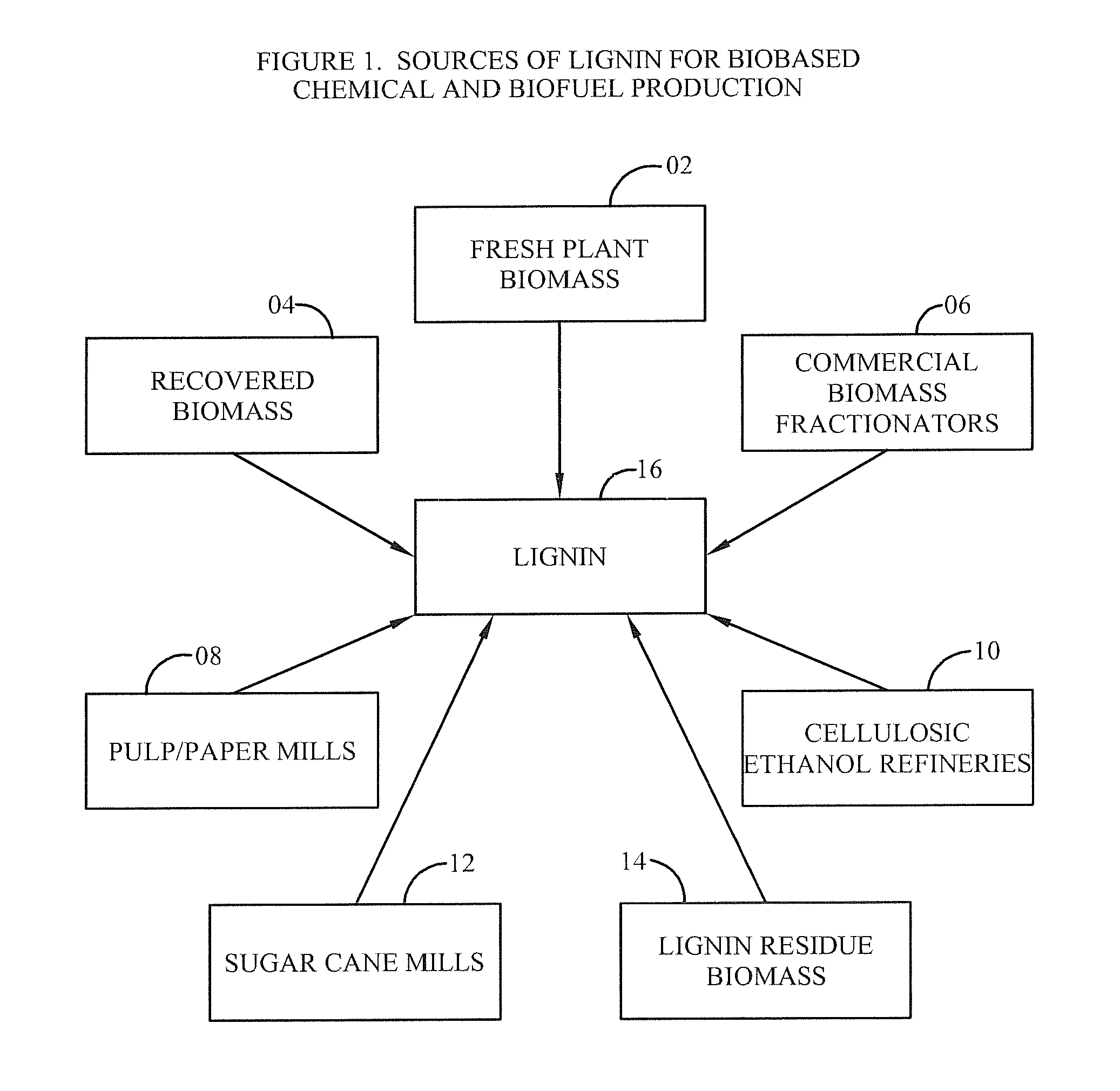
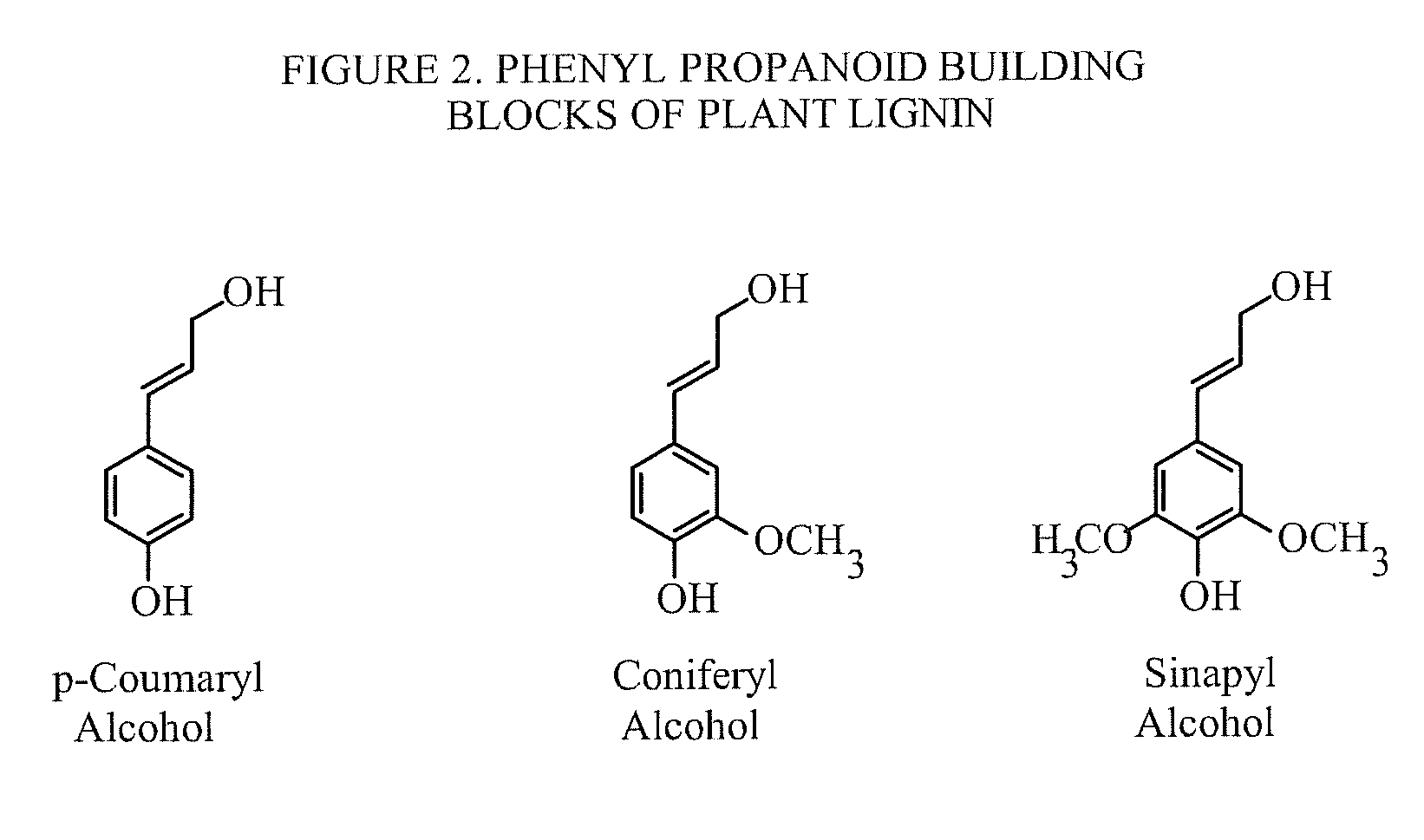
Method for selective production of biobased chemicals and biofuels from plant lignin
InactiveUS20130232853A1Easy to implementEasy to useCarboxylic acid nitrile preparationCarboxylic acid esters preparationBiofuelHydrodeoxygenation
The present invention is directed generally to a method of production of biobased chemicals, biofuels, and lignin residues from lignin sources, including waste lignin. This method may allow for selectively producing biobased chemicals, biofuels, and lignin residues from lignin sources using certain processing methods. The methods for production of these biobased chemicals, biofuels, and lignin residues may be provided by chemical-induced processing, catalytic oxidative lignin depolymerisation processing, and catalytic hydroprocessing. Further, the catalytic hydroprocessing from processes including catalytic reduction processing, catalytic hydrodeoxygenation processing, and / or catalytic / dehydrogenation processing may also be used. The method described herein also provides a means in which waste from the process(es) may be reduced and / or recycled.
Owner:VERTICHEM CORP
Method for preparing propylene catalyst by propane dehydrogenation process
ActiveCN101884922AHigh selectivityImprove stabilityCatalyst activation/preparationHydrocarbonsPlatinumDehydrogenation
The invention relates to a method for preparing a propylene catalyst by a propane dehydrogenation process and mainly solves the problem in the high-temperature and charcoal-burning process of the catalyst in the conventional preparation technology that a tin component is easy to reduce and separate out so as to influence the performance of the catalyst. The invention better solves the problem through the technical scheme comprising the following steps of: firstly, introducing the tin component into an aluminum sol by a sol-gel method, and drying and forming the mixture to obtain a tin-containing alumina supporter; secondly, loading a platinum component and other metallic aids by an impregnation method, namely impregnating the tin-containing alumina supporter into aqueous solution of soluble salt of platinum and other metals; and finally, drying, roasting and dechlorinating the product to obtain the platinum-tin catalyst. The method of the invention can be used for industrial preparation of the propylene catalyst by the propane dehydrogenation process.
Owner:CHINA PETROLEUM & CHEM CORP +1
Transition metal-containing catalysts and processes for their preparation and use as oxidation and dehydrogenation catalysts
ActiveUS20050176989A1Efficient oxidationMaterial nanotechnologyAmino preparation from aminesAlcoholDehydrogenation
This invention relates to the field of heterogeneous catalysis, and more particularly to catalysts including carbon supports having formed thereon compositions which comprise a transition metal in combination with nitrogen and / or carbon. The invention further relates to the fields of catalytic oxidation and dehydrogenation reactions, including the preparation of secondary amines by the catalytic oxidation of tertiary amines and the preparation of carboxylic acids by the catalytic dehydrogenation of alcohols.
Owner:MONSANTO TECH LLC
Method for manufacturing semiconductor device
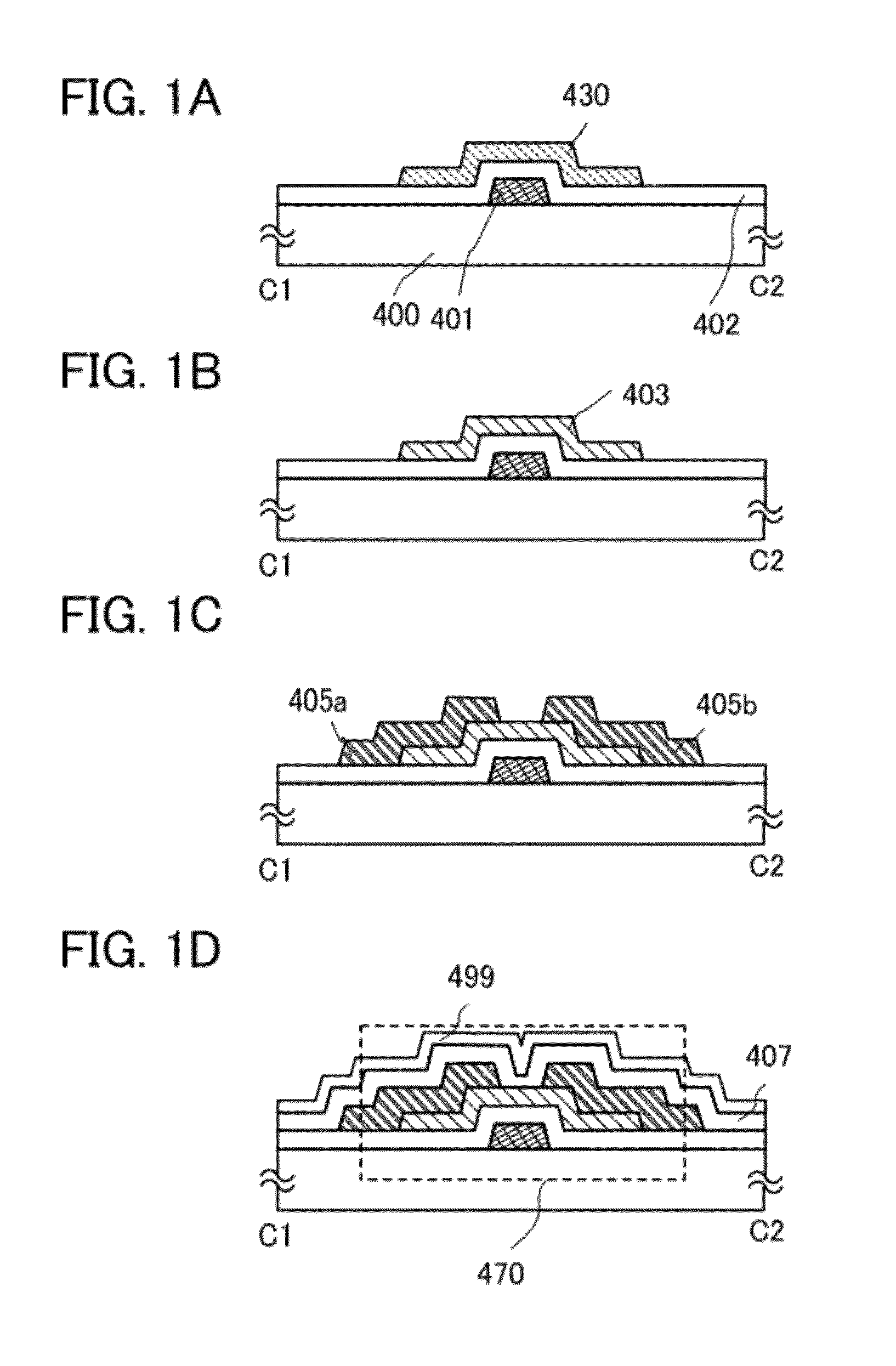
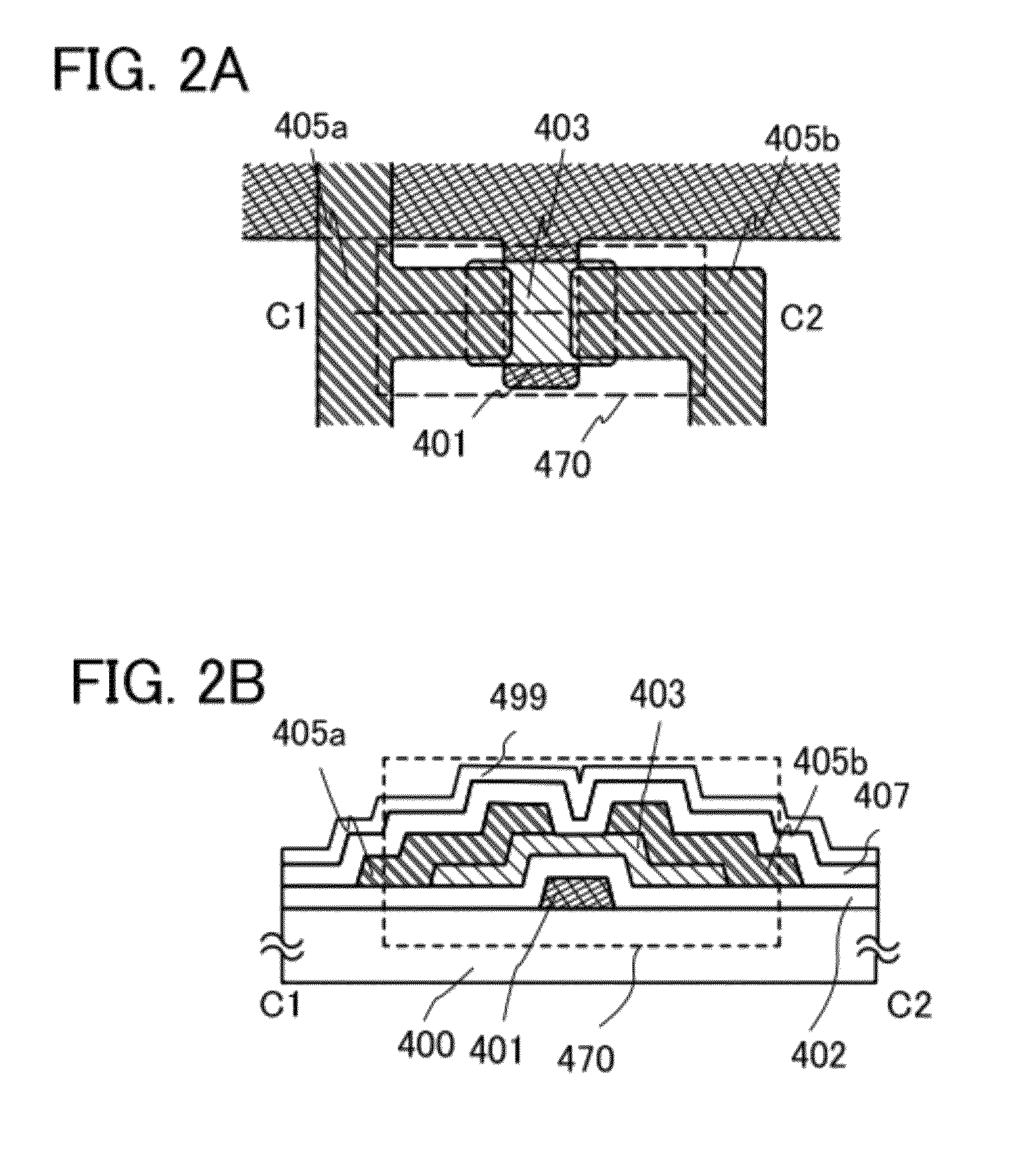
ActiveUS8193031B2Reduce the presence of impuritiesStable electrical characteristicsStatic indicating devicesSolid-state devicesNoble gasDehydrogenation
An object is to provide a semiconductor device having stable electric characteristics in which an oxide semiconductor is used. An oxide semiconductor layer is subjected to heat treatment for dehydration or dehydrogenation treatment in a nitrogen gas or an inert gas atmosphere such as a rare gas (e.g., argon or helium) or under reduced pressure and to a cooling step for treatment for supplying oxygen in an atmosphere of oxygen, an atmosphere of oxygen and nitrogen, or the air (having a dew point of preferably lower than or equal to −40° C., still preferably lower than or equal to −50° C.) atmosphere. The oxide semiconductor layer is thus highly purified, whereby an i-type oxide semiconductor layer is formed. A semiconductor device including a thin film transistor having the oxide semiconductor layer is manufactured.
Owner:SEMICON ENERGY LAB CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com