Patents
Literature
12703 results about "Tungsten" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. The name tungsten comes from the former Swedish name for the tungstate mineral scheelite, tung sten or "heavy stone". Tungsten is a rare metal found naturally on Earth almost exclusively combined with other elements in chemical compounds rather than alone. It was identified as a new element in 1781 and first isolated as a metal in 1783. Its important ores include wolframite and scheelite.
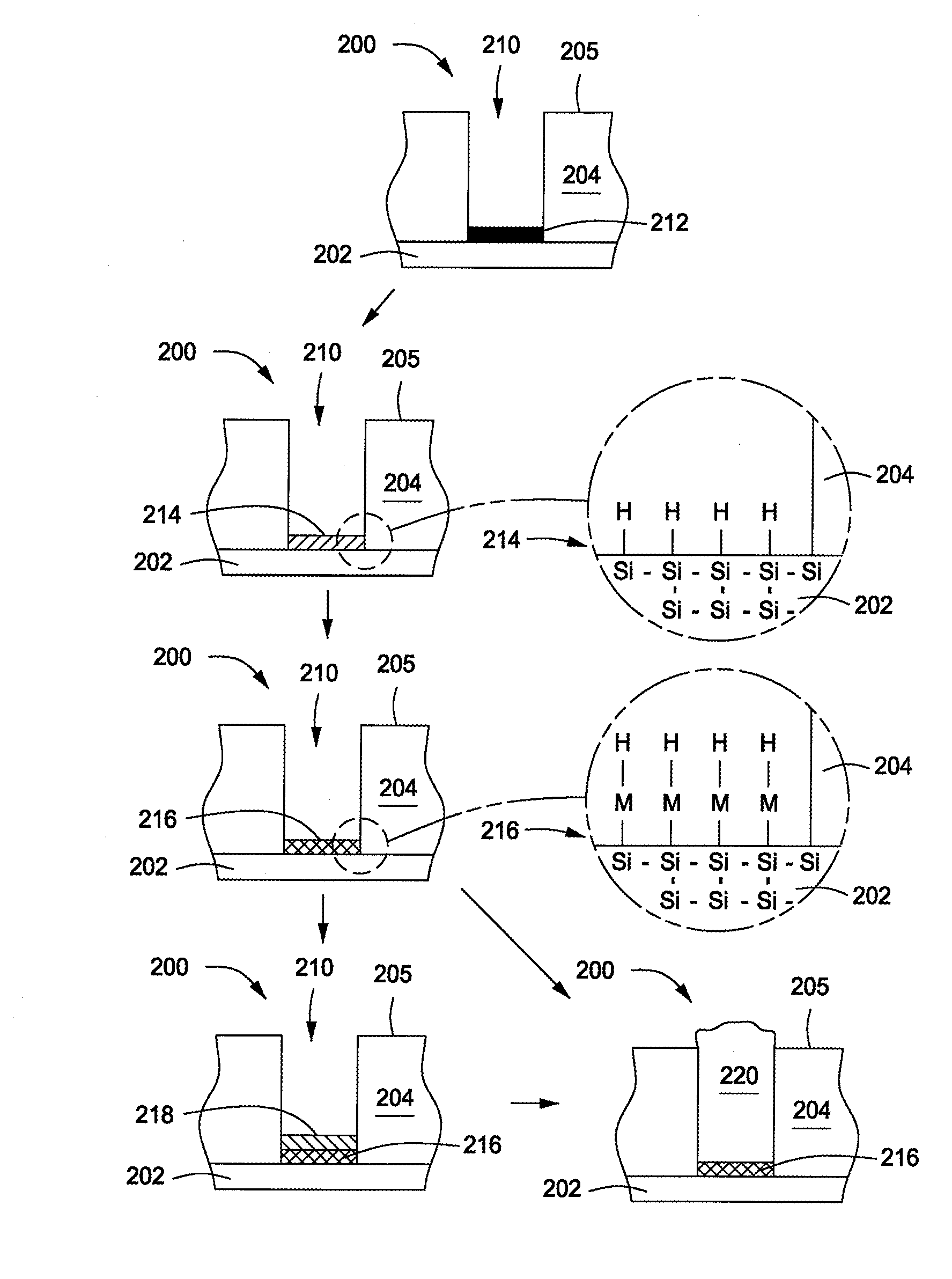
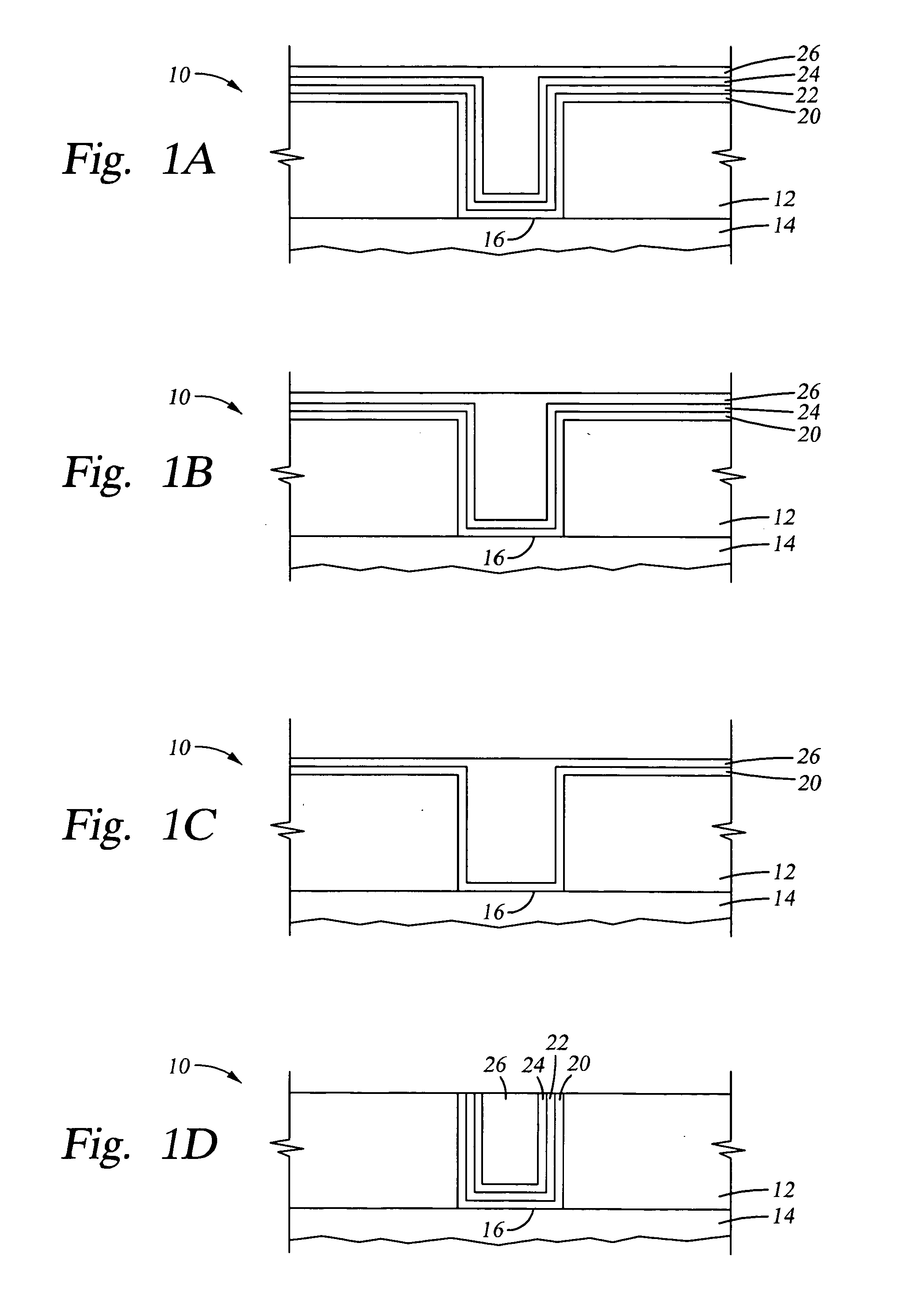
Method of forming metal layer using atomic layer deposition and semiconductor device having the metal layer as barrier metal layer or upper or lower electrode of capacitor
InactiveUS6287965B1High thermal resistantEasy to adjustSemiconductor/solid-state device manufacturingCapacitorsNiobiumDevice material
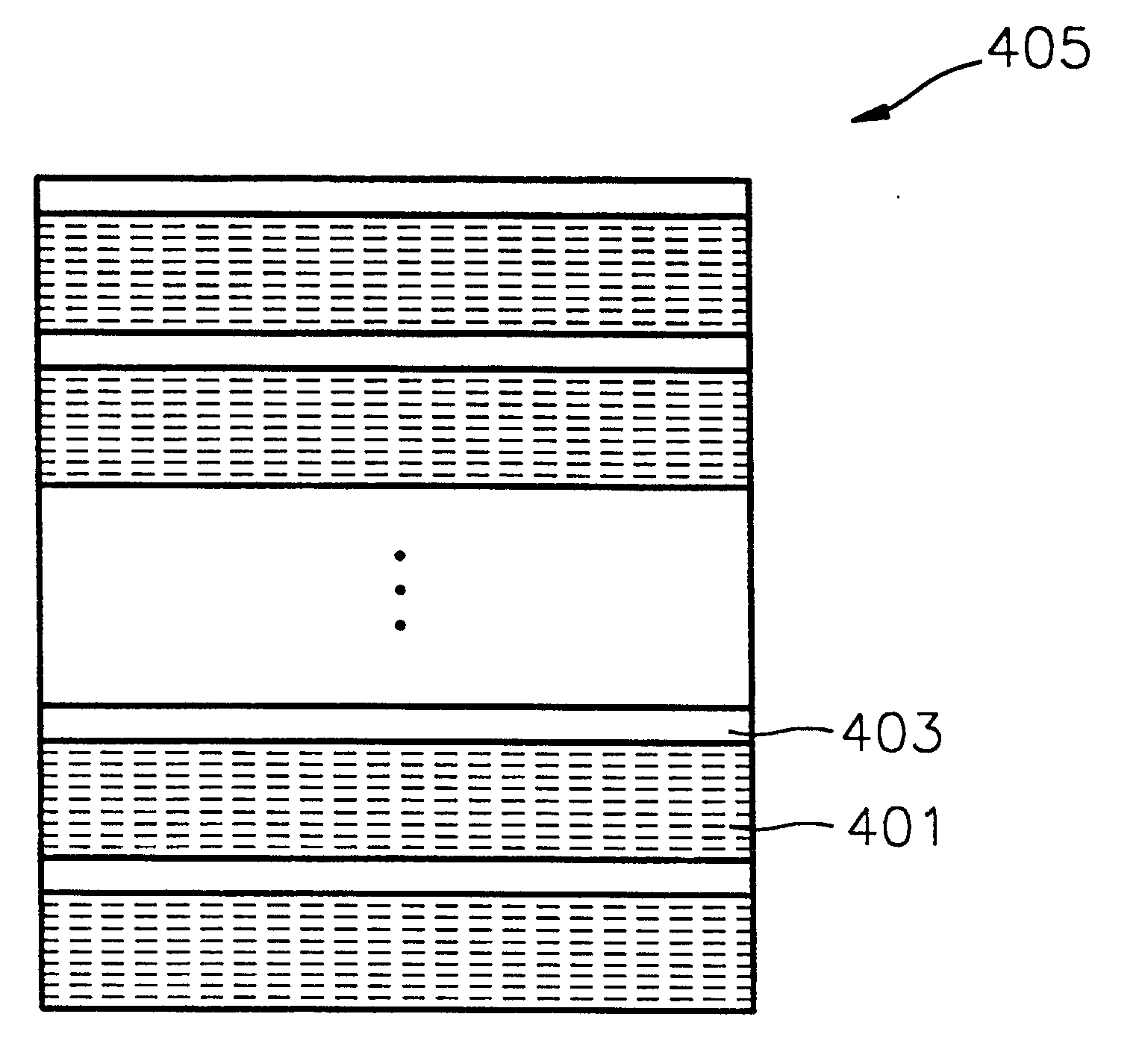
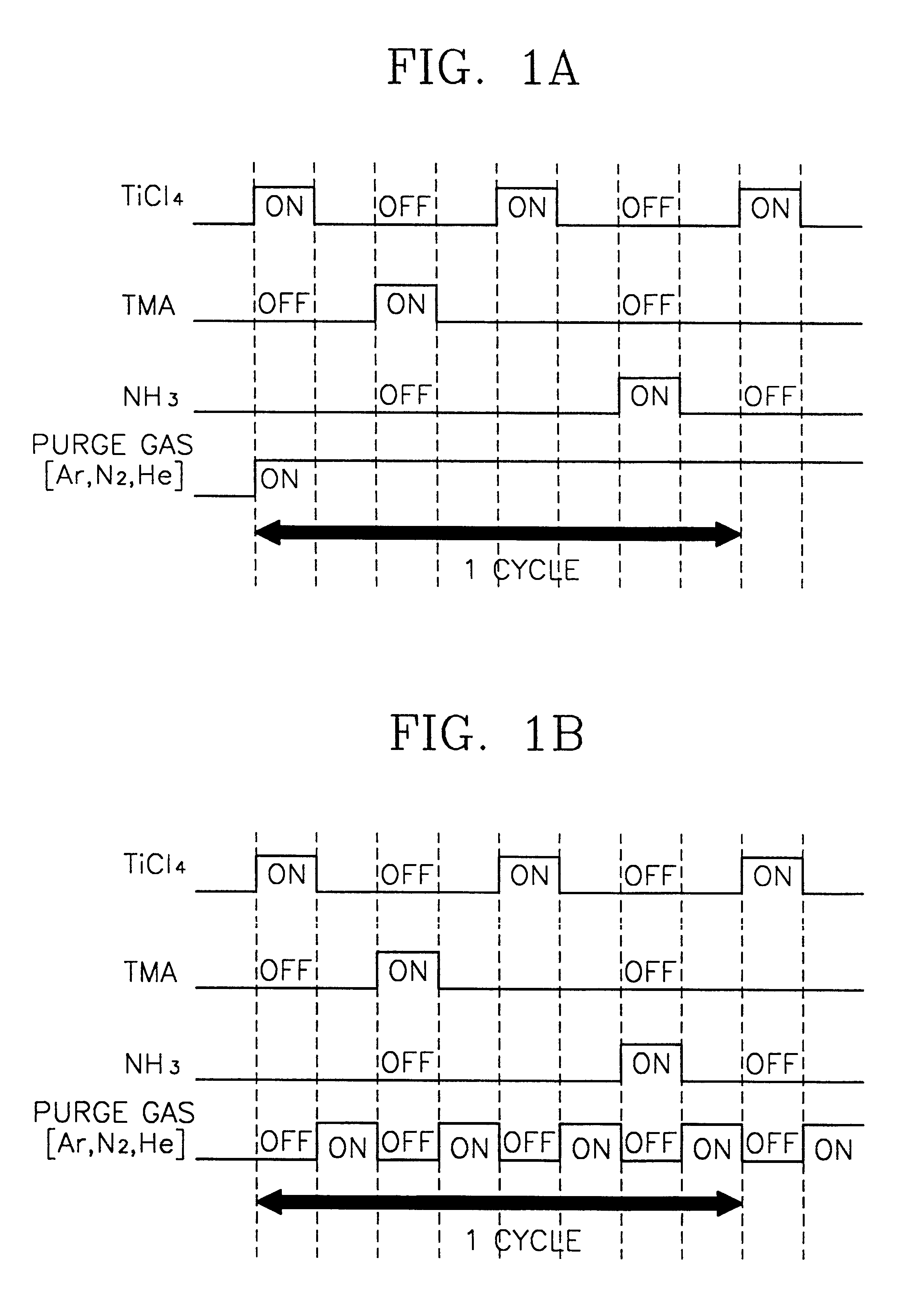
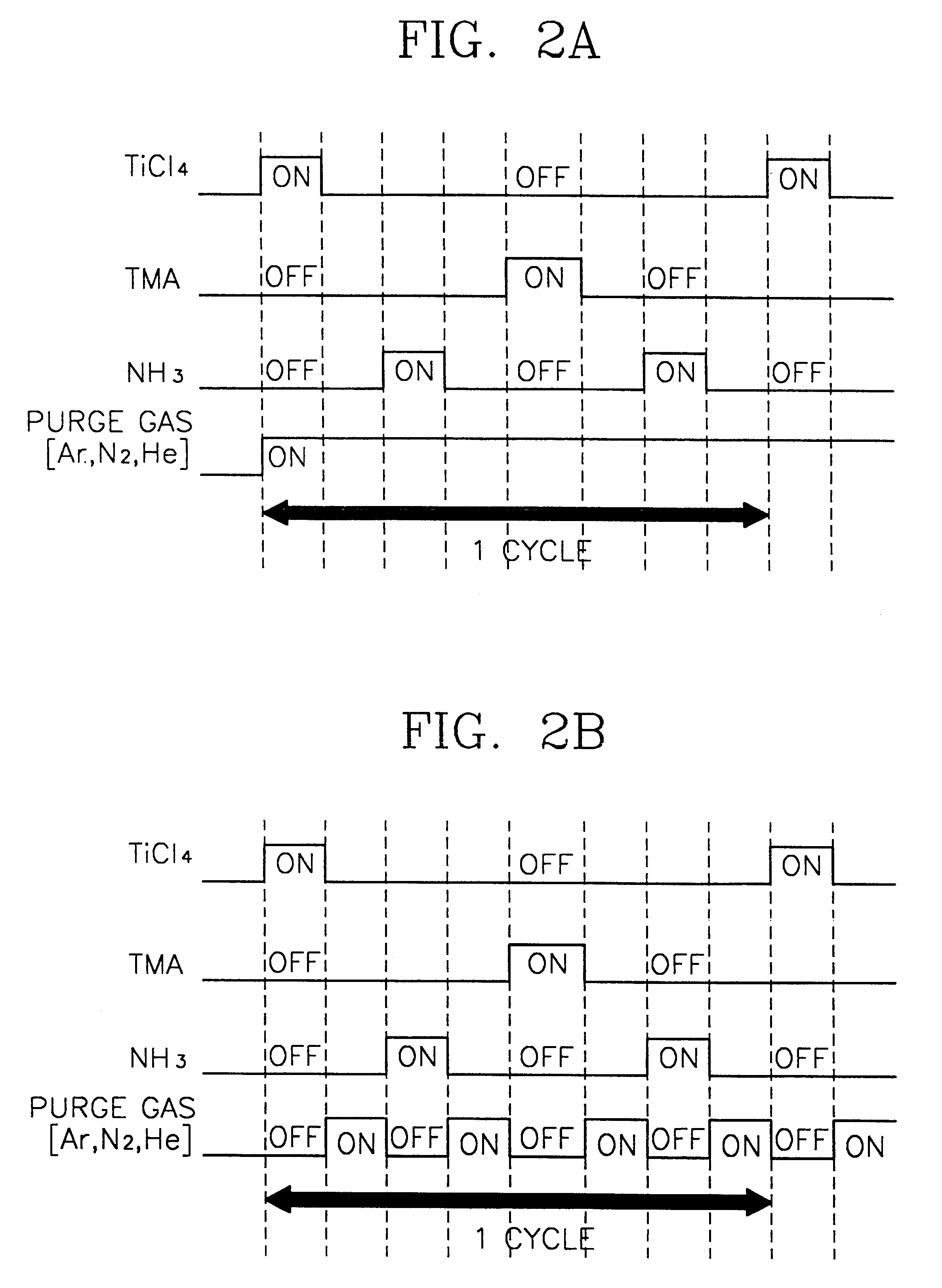
A method of forming a metal layer having excellent thermal and oxidation resistant characteristics using atomic layer deposition is provided. The metal layer includes a reactive metal (A), an element (B) for the amorphous combination between the reactive metal (A) and nitrogen (N), and nitrogen (N). The reactive metal (A) may be titanium (Ti), tantalum (Ta), tungsten (W), zirconium (Zr), hafnium (Hf), molybdenum (Mo) or niobium (Nb). The amorphous combination element (B) may be aluminum (Al), silicon (Si) or boron (B). The metal layer is formed by alternately injecting pulsed source gases for the elements (A, B and N) into a chamber according to atomic layer deposition to thereby alternately stack atomic layers. Accordingly, the composition ratio of a nitrogen compound (A-B-N) of the metal layer can be desirably adjusted just by appropriately determining the number of injection pulses of each source gas. According to the composition ratio, a desirable electrical conductivity and resistance of the metal layer can be accurately obtained. The atomic layers are individually deposited, thereby realizing excellent step coverage even in a complex and compact region. A metal layer formed by atomic layer deposition can be employed as a barrier metal layer, a lower electrode or an upper electrode in a semiconductor device.
Owner:SAMSUNG ELECTRONICS CO LTD
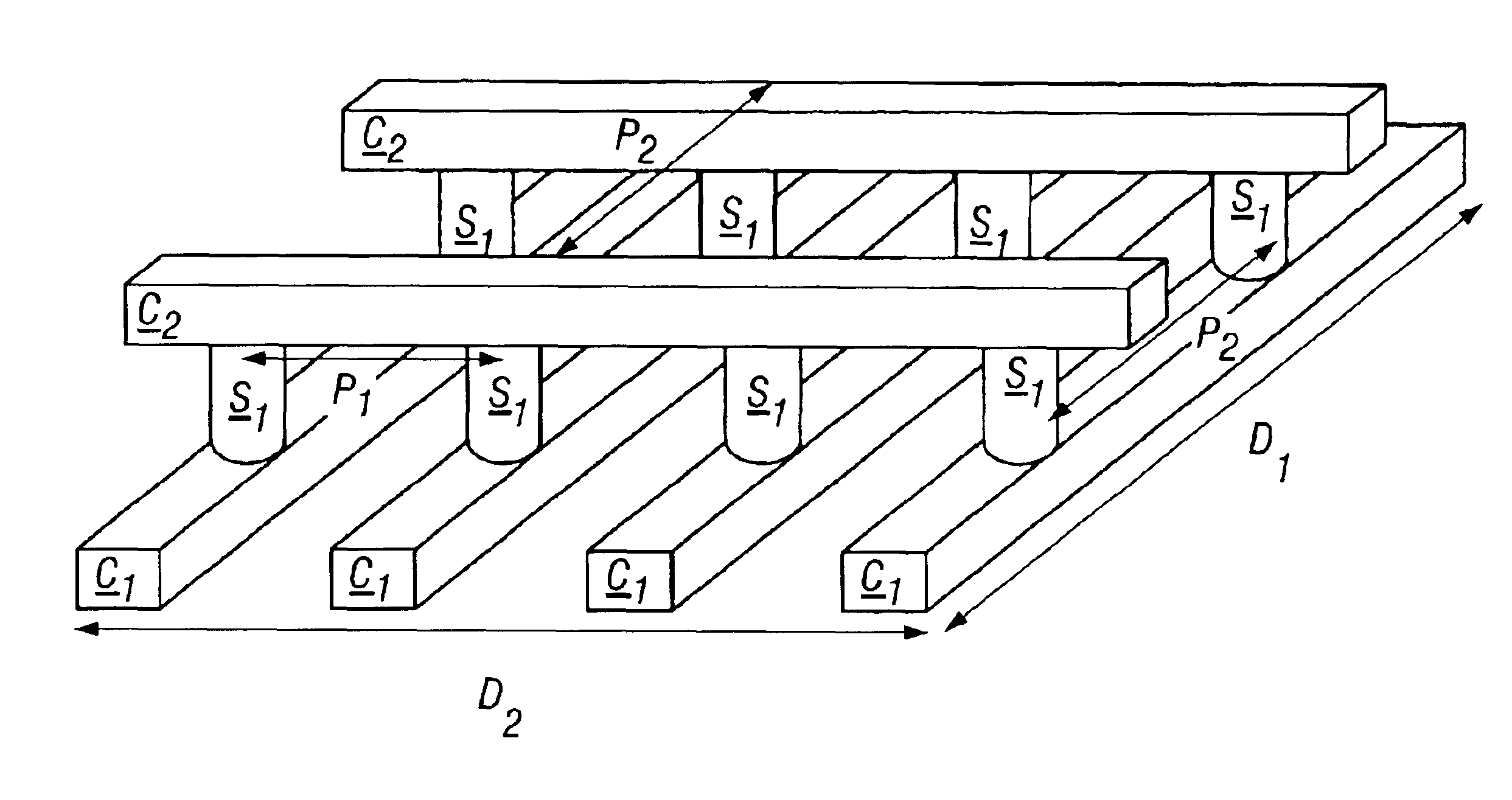
High-density three-dimensional memory cell
InactiveUS6952030B2High densityReduce the overall heightTransistorSemiconductor/solid-state device detailsElectrical conductorHigh density
A three dimensional monolithic memory comprising a memory cell allowing for increased density is disclosed. In the memory cell of the present invention, a bottom conductor preferably comprising tungsten is formed. Above the bottom conductor a semiconductor element preferably comprises two diode portions and an antifuse. Above the semiconductor element are additional conductors and semiconductor elements in multiple stones of memories. The arrangement of processing steps and the choice of materials decreases aspect ratio of each memory cell, improving the reliability of gap fill and preventing etch undercut.
Owner:SANDISK TECH LLC
Methods for the reduction and elimination of particulate contamination with CVD of amorphous carbon
InactiveUS20060014397A1Minimal defect formationReduce particle pollutionSemiconductor/solid-state device manufacturingSpecial surfacesVariable thicknessMicroparticle
A method is provided for forming an amorphous carbon layer, deposited on a dielectric material such as oxide, nitride, silicon carbide, carbon doped oxide, etc., or a metal layer such as tungsten, aluminum or poly-silicon. The method includes the use of chamber seasoning, variable thickness of seasoning film, wider spacing, variable process gas flows, post-deposition purge with inert gas, and post-deposition plasma purge, among others, to make the deposition of an amorphous carbon film at low deposition temperatures possible without any defects or particle contamination.
Owner:APPLIED MATERIALS INC +1
LED substrate processing
ActiveUS8404499B2Improve efficiencyFast response timeSemiconductor/solid-state device testing/measurementMuffle furnacesLong pulseLight-emitting diode
Embodiments of the present invention pertain to substrate processing equipment and methods incorporating light emitting diodes (LEDs) for thermally processing substrates. Such light sources offer a variety of advantages including higher efficiency and more rapid response times. Pulse widths are selectable down to under a millisecond but can be for long pulses up to and exceeding a second. LEDs are preferable to tungsten-halogen lamps even in circumstances that allow longer processing times, since LEDs produce light with greater than 50% efficiency and tungsten-halogen lamps operate with less than 5% efficiency.
Owner:APPLIED MATERIALS INC
Niobium suboxide powder
ActiveUS20050013765A1High currentReduce residual currentOxide/hydroxide preparationLiquid electrolytic capacitorsCapacitorTungsten
A niobium suboxide powder comprising 100 to 600 ppm of magnesium is described. The niobium suboxide powder may (alternatively or in addition to 100 to 600 ppm of magnesium) further include 50 to 400 ppm of molybdenum and / or tungsten. The niobium suboxide powder is suitable for the production of: capacitors having an insulator layer of niobium pentoxide; capacitor anodes produced from the niobium suboxide powder; and corresponding capacitors.
Owner:TANIOBIS GMBH
Method of selectively depositing a thin film material at a semiconductor interface
InactiveUS20070108404A1Detergent mixture composition preparationSemiconductor/solid-state device manufacturingDevice formMetal silicide
Embodiments of the invention provide processes to form a high quality contact level connection to devices formed on a substrate. In one embodiment, a method for depositing a material on a substrate is provided which includes exposing the substrate to a buffered oxide etch solution to form a silicon hydride layer during a pretreatment process, depositing a metal silicide layer on the substrate, and depositing a first metal layer (e.g., tungsten) on the metal silicide layer. The buffered oxide etch solution may contain hydrogen fluoride and an alkanolamine compound, such as ethanolamine diethanolamine, or triethanolamine. The metal silicide layer may contain cobalt, nickel, or tungsten and may be deposited by an electroless deposition process. In one example, the substrate is exposed to an electroless deposition solution containing a solvent and a complexed metal compound.
Owner:APPLIED MATERIALS INC
Conductive lithium storage electrode
A compound comprising a composition Ax(M′1-aM″a)y(XD4)z, Ax(M′1-aM″a)y(DXD4)z, or Ax(M′1-aM″a)y(X2D7)z, and have values such that x, plus y(1-a) times a formal valence or valences of M′, plus ya times a formal valence or valence of M″, is equal to z times a formal valence of the XD4, X2D7, or DXD4 group; or a compound comprising a composition (A1-aM″a)xM′y(XD4)z, (A1-aM″a)xM′y(DXD4)z(A1-aM″a)xM′y(X2D7)z and have values such that (1-a)x plus the quantity ax times the formal valence or valences of M″ plus y times the formal valence or valences of M′ is equal to z times the formal valence of the XD4, X2D7 or DXD4 group. In the compound, A is at least one of an alkali metal and hydrogen, M′ is a first-row transition metal, X is at least one of phosphorus, sulfur, arsenic, molybdenum, and tungsten, M″ any of a Group IIA, IIIA, IVA, VA, VIA, VIIA, VIIIA, IB, IIB, IIIB, IVB, VB, and VIB metal, D is at least one of oxygen, nitrogen, carbon, or a halogen, 0.0001<a≦0.1, and x, y, and z are greater than zero. The compound can have a conductivity at 27° C. of at least about 10−8 S / cm. The compound can be a doped lithium phosphate that can intercalate lithium or hydrogen. The compound can be used in an electrochemical device including electrodes and storage batteries and can have a gravimetric capacity of at least about 80 mAh / g while being charged / discharged at greater than about C rate of the compound.
Owner:MASSACHUSETTS INST OF TECH
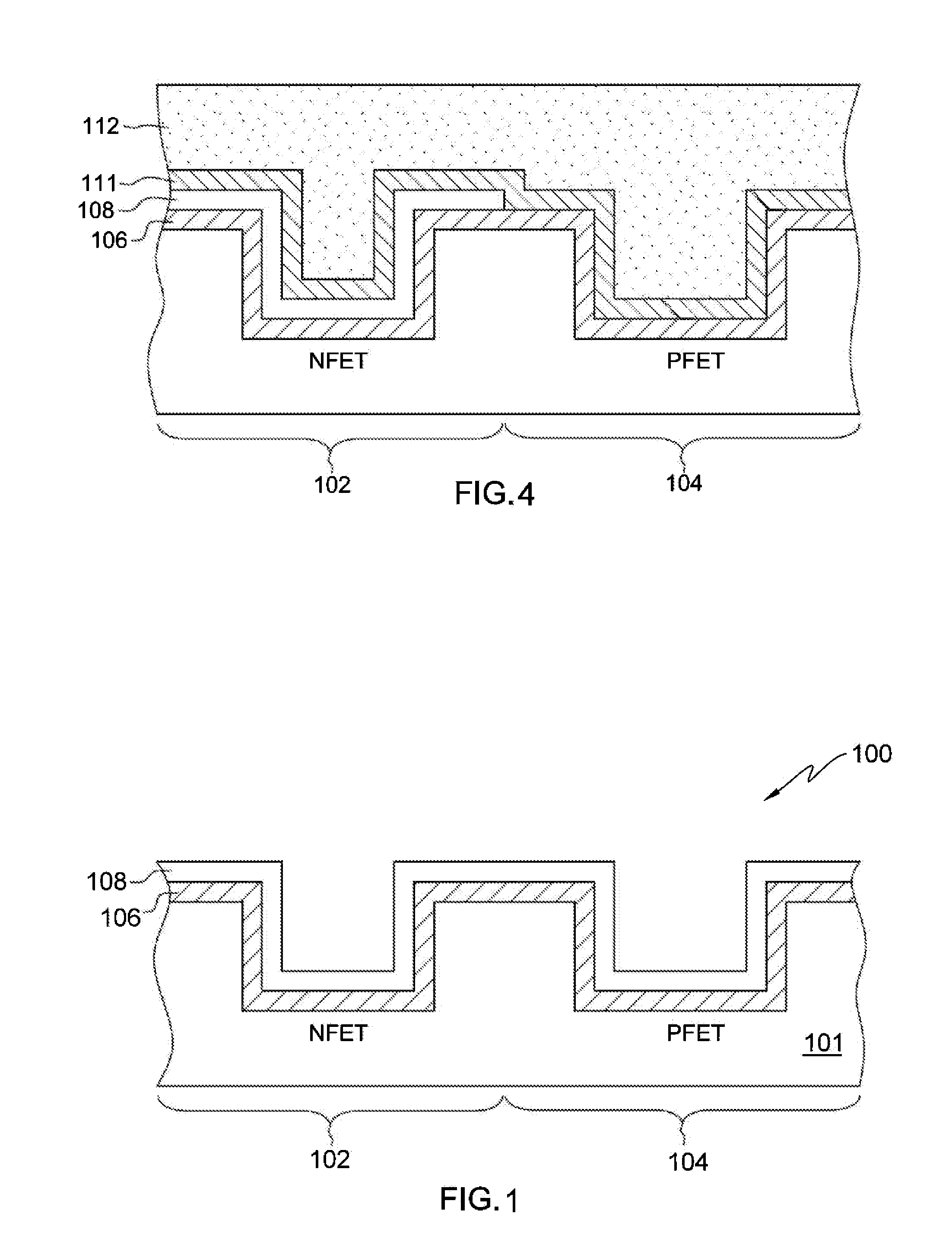
Structure and method for metal replacement gate of high performance
ActiveUS20050051854A1Improve performanceSemiconductor/solid-state device manufacturingSemiconductor devicesTitanium nitrideDiffusion barrier
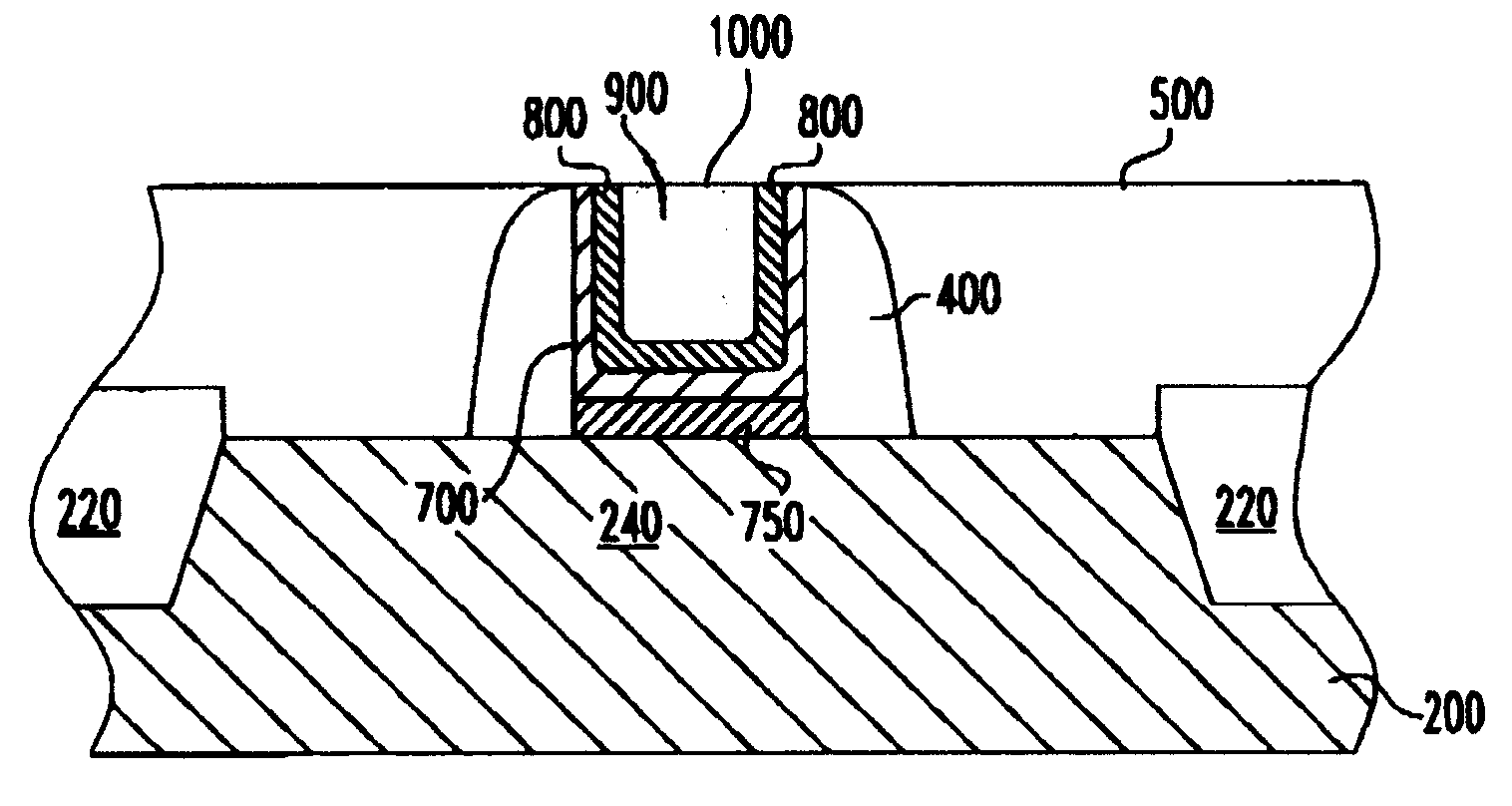
A structure and method for a metal replacement gate of a high performance device is provided. A sacrificial gate structure is first formed on an etch stop layer provided on a semiconductor substrate. A pair of spacers is provided on sidewalls of the sacrificial gate structure. The sacrificial gate structure is then removed, forming an opening. Subsequently, a metal gate including an first layer of metal such as tungsten, a diffusion barrier such as titanium nitride, and a second layer of metal such as tungsten is formed in the opening between the spacers.
Owner:GLOBALFOUNDRIES US INC
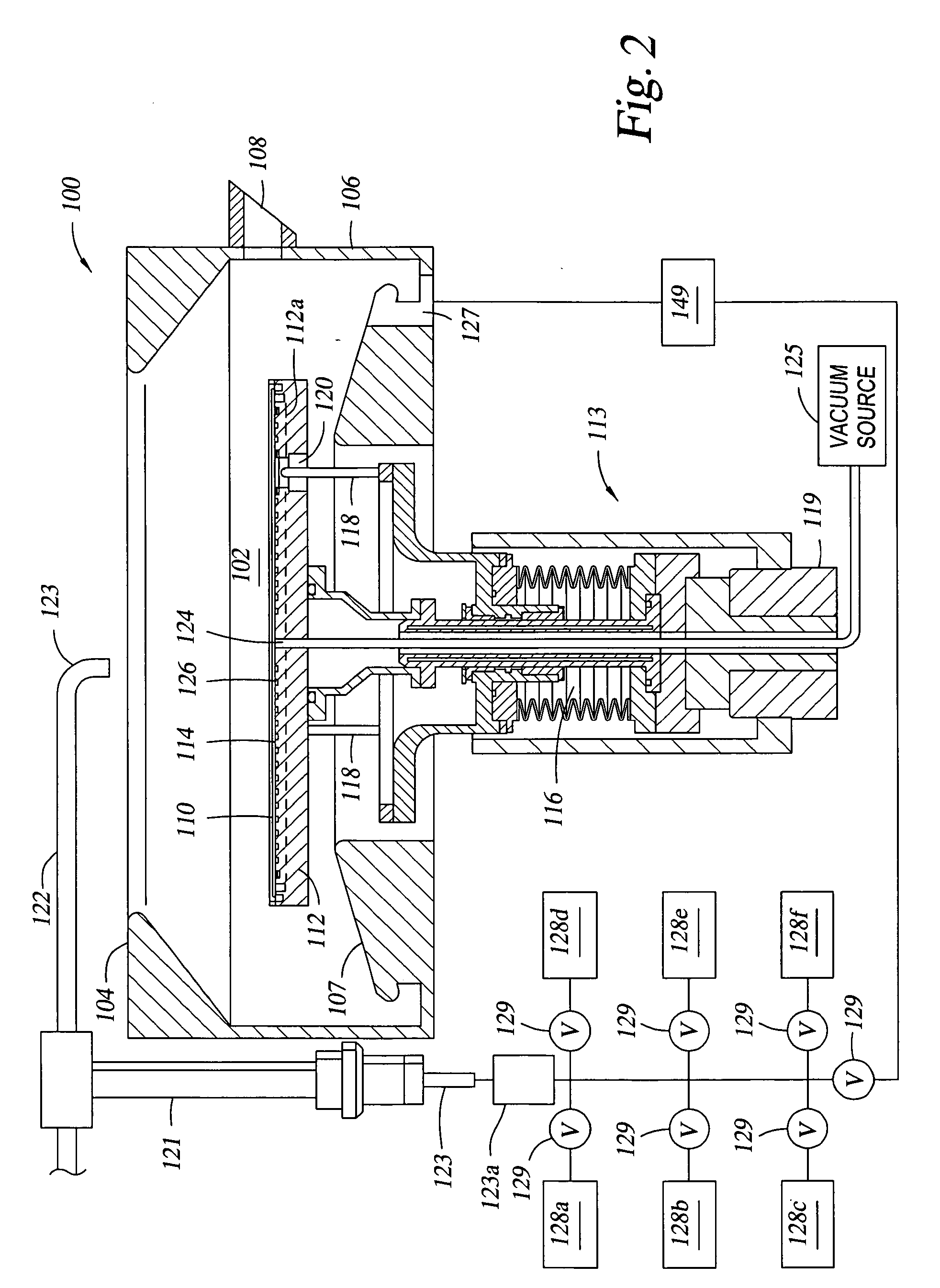
Electroless deposition apparatus
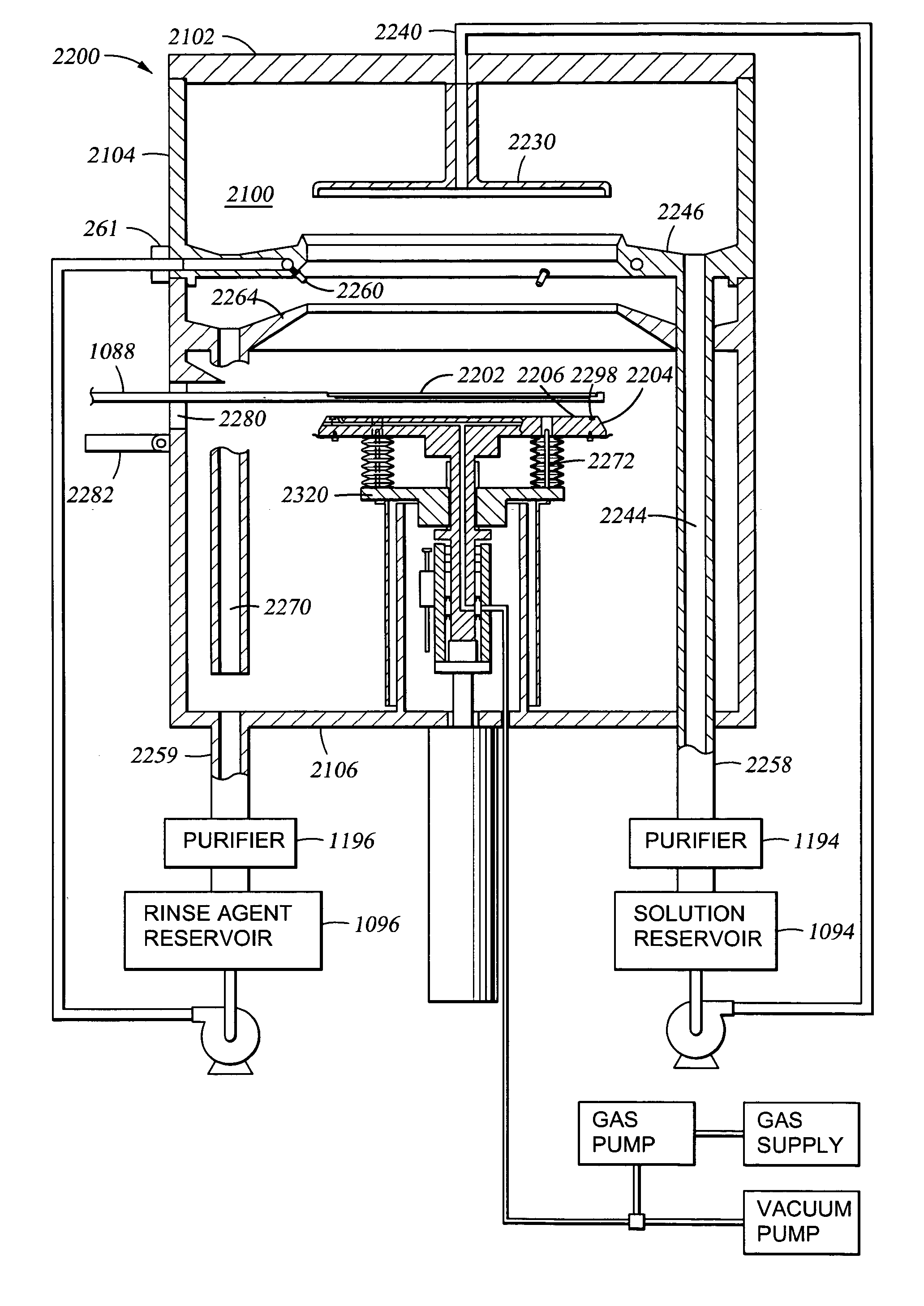
An apparatus and a method of depositing a catalytic layer comprising at least one metal selected from the group consisting of noble metals, semi-noble metals, alloys thereof, and combinations thereof in sub-micron features formed on a substrate. Examples of noble metals include palladium and platinum. Examples of semi-noble metals include cobalt, nickel, and tungsten. The catalytic layer may be deposited by electroless deposition, electroplating, or chemical vapor deposition. In one embodiment, the catalytic layer may be deposited in the feature to act as a barrier layer to a subsequently deposited conductive material. In another embodiment, the catalytic layer may be deposited over a barrier layer. In yet another embodiment, the catalytic layer may be deposited over a seed layer deposited over the barrier layer to act as a “patch” of any discontinuities in the seed layer. Once the catalytic layer has been deposited, a conductive material, such as copper, may be deposited over the catalytic layer. In one embodiment, the conductive material is deposited over the catalytic layer by electroless deposition. In another embodiment, the conductive material is deposited over the catalytic layer by electroless deposition followed by electroplating or followed by chemical vapor deposition. In still another embodiment, the conductive material is deposited over the catalytic layer by electroplating or by chemical vapor deposition.
Owner:APPLIED MATERIALS INC
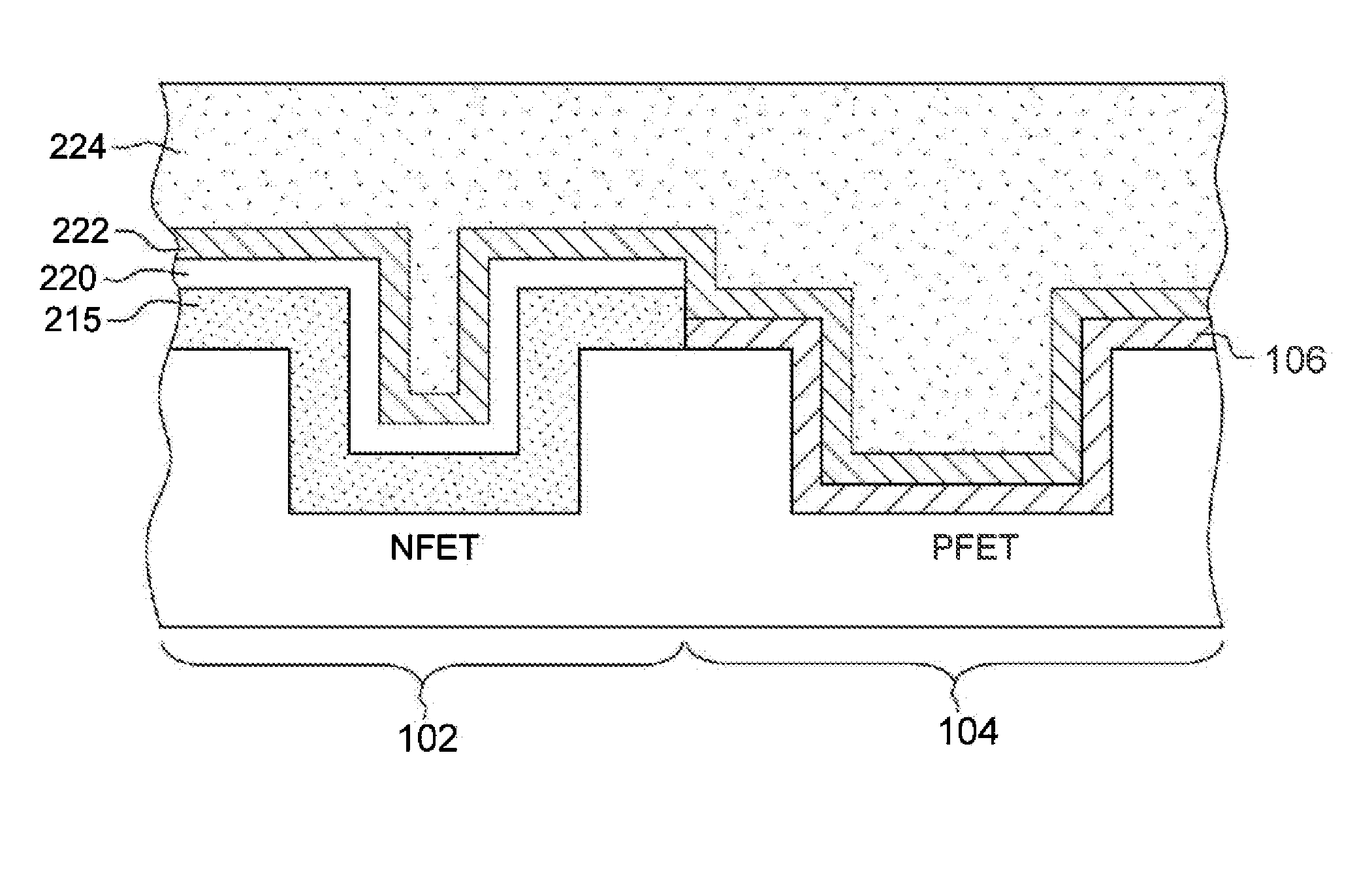
Semiconductor gate structure for threshold voltage modulation and method of making same
InactiveUS20140231922A1Lower work functionSemiconductor/solid-state device manufacturingSemiconductor devicesDielectricTitanium nitride
A gate structure of a semiconductor device having a NFET and a PFET, includes a lower layer of a hafnium-based dielectric over the gates of the NFET and PFET, and an upper layer of a lanthanide dielectric. The dielectrics are annealed to mix them above the NFET resulting in a lowered work function, and corresponding threshold voltage reduction. An annealed, relatively thick titanium nitride cap over the mixed dielectric above the NFET gate also lowers the work function and threshold voltage. Above the TiN cap and the hafnium-based dielectric over the PFET gate, is another layer of titanium nitride that has not been annealed. A conducting layer of tungsten covers the structure.
Owner:GLOBALFOUNDRIES US INC
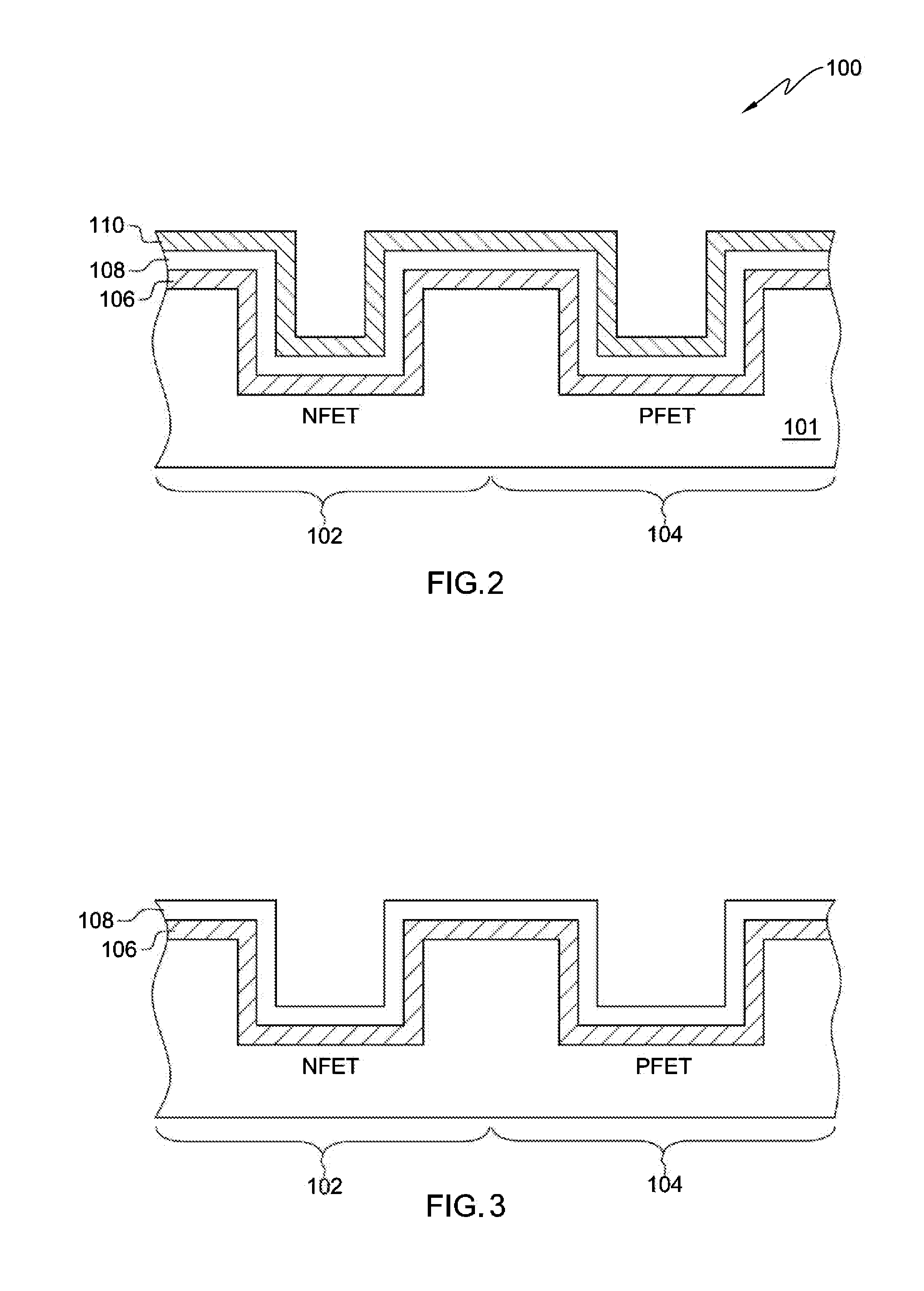
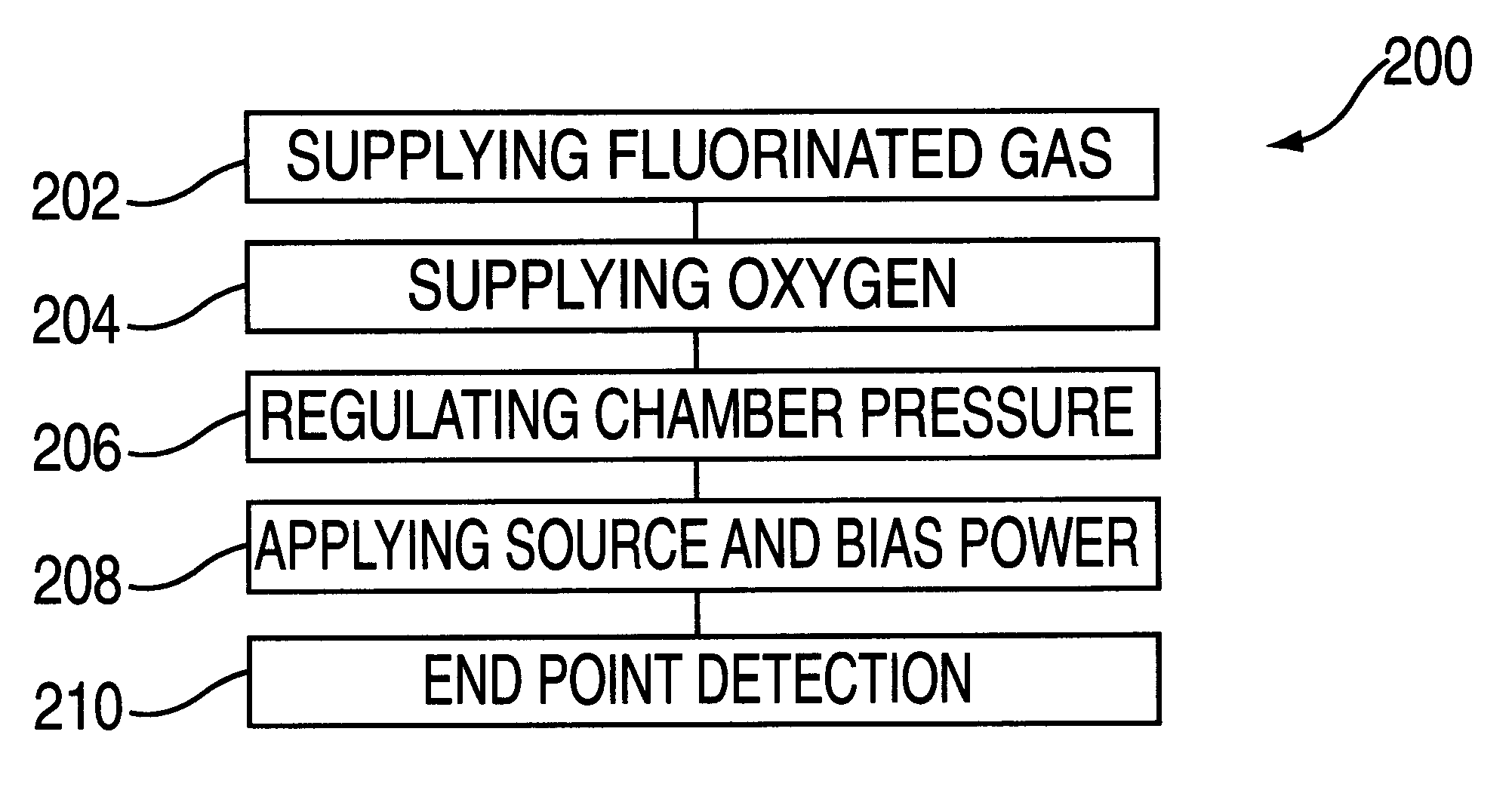
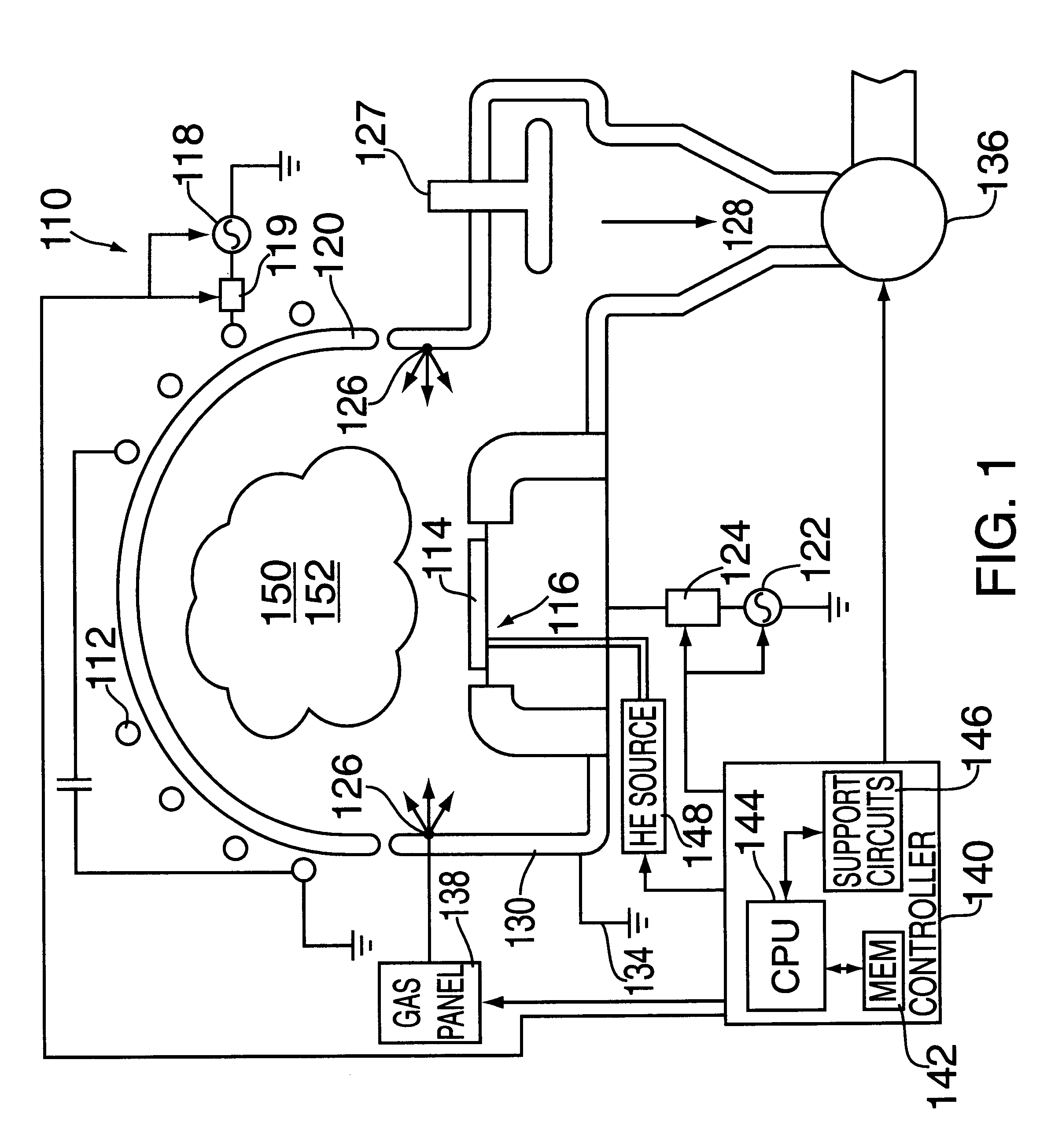
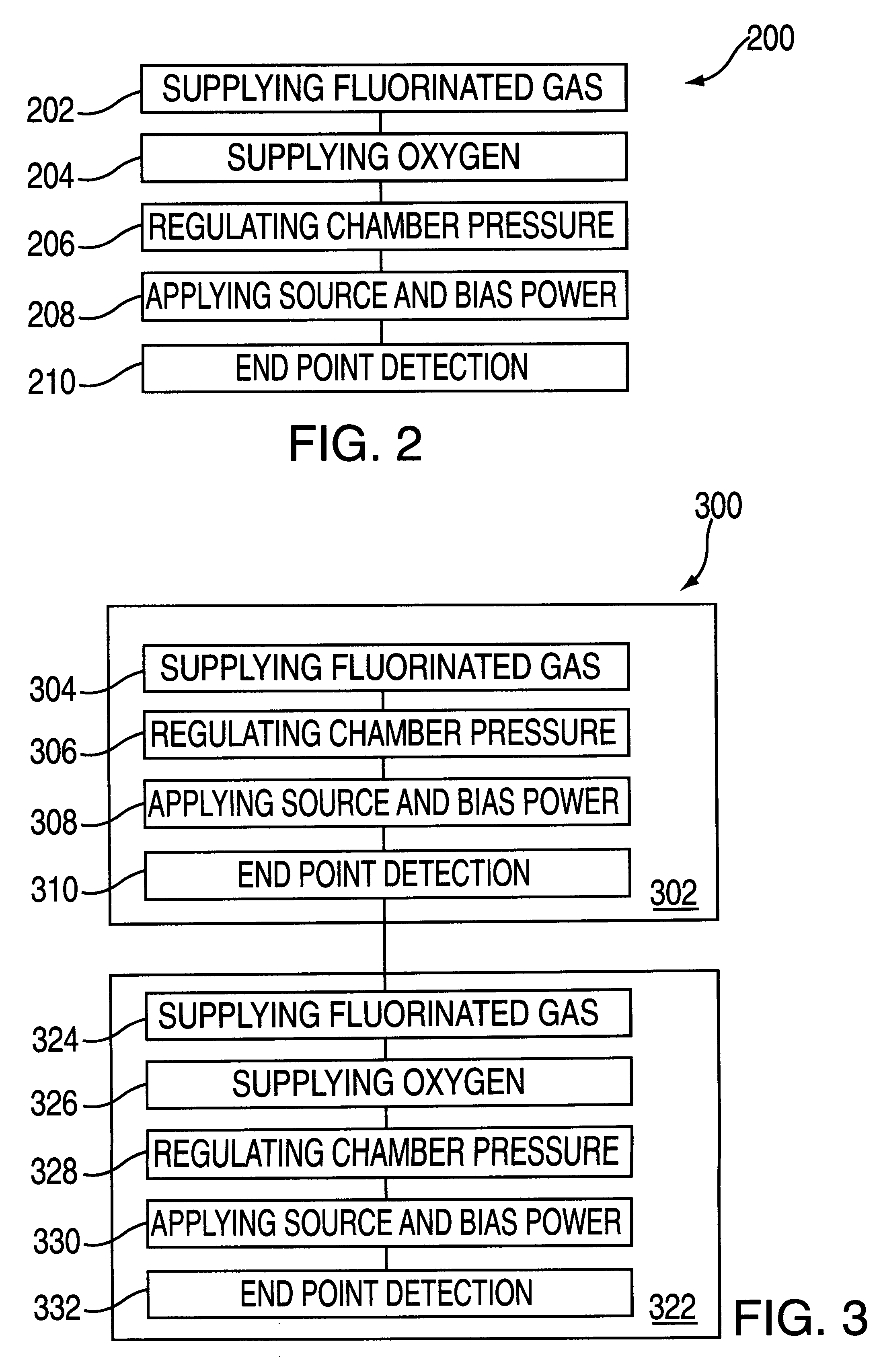
Plasma processing of tungsten using a gas mixture comprising a fluorinated gas and oxygen
InactiveUS6277763B1Good choiceElectric discharge tubesVacuum gauge using ionisation effectsFluorinated gasesProduct gas
Owner:APPLIED MATERIALS INC
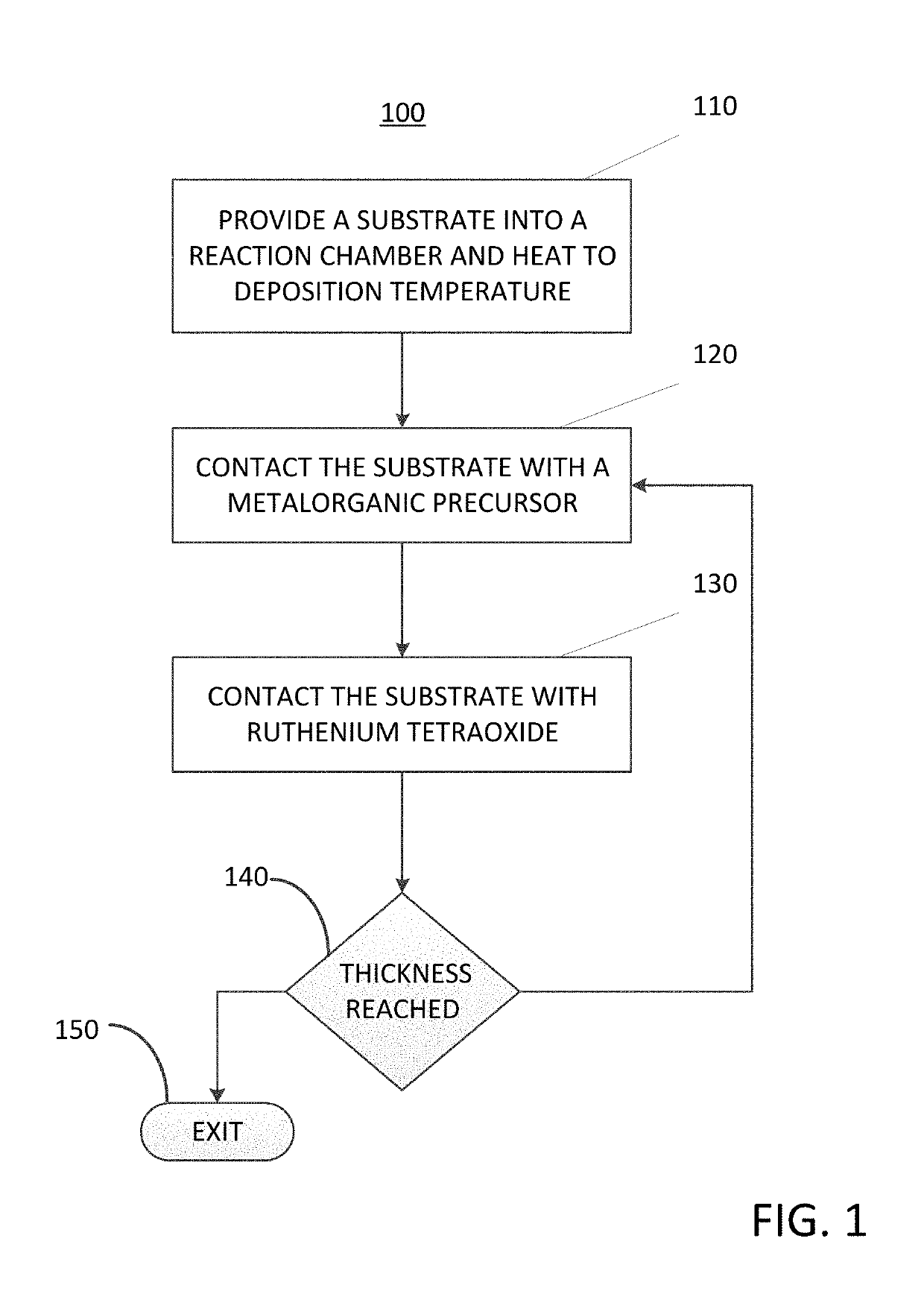
Method for depositing a ruthenium-containing film on a substrate by a cyclical deposition process
A method for depositing a ruthenium-containing film on a substrate by a cyclical deposition process is disclosed. The method may include: contacting the substrate with a first vapor phase reactant comprising a metalorganic precursor, the metalorganic precursor comprising a metal selected from the group consisting of a cobalt, nickel, tungsten, molybdenum, manganese, iron, and combinations thereof. The method may also include; contacting the substrate with a second vapor phase reactant comprising ruthenium tetroxide (RuO4); wherein the ruthenium-containing film comprises a ruthenium-metal alloy. Semiconductor device structures including ruthenium-metal alloys deposited by the methods of the disclosure are also disclosed.
Owner:ASM IP HLDG BV
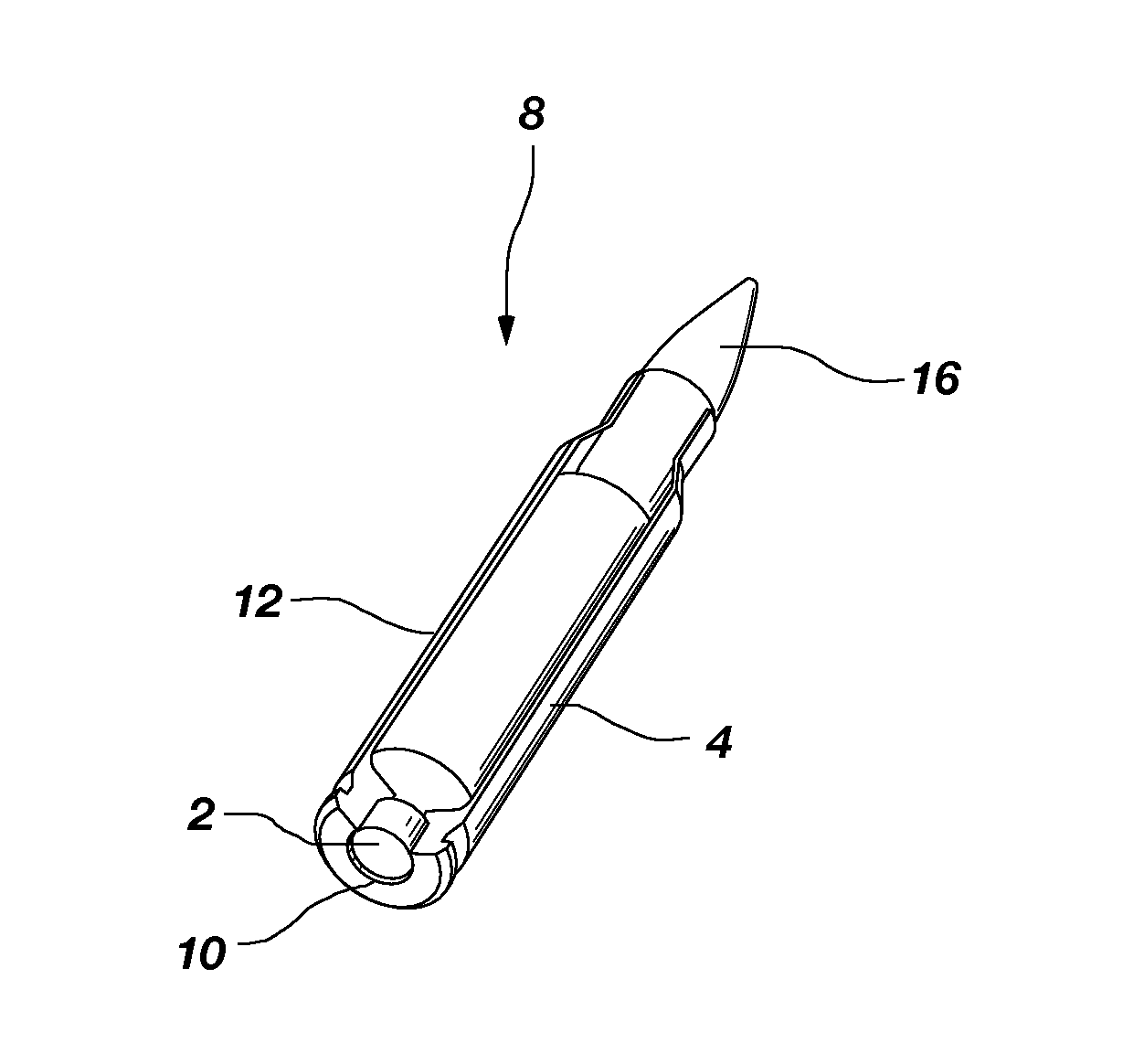
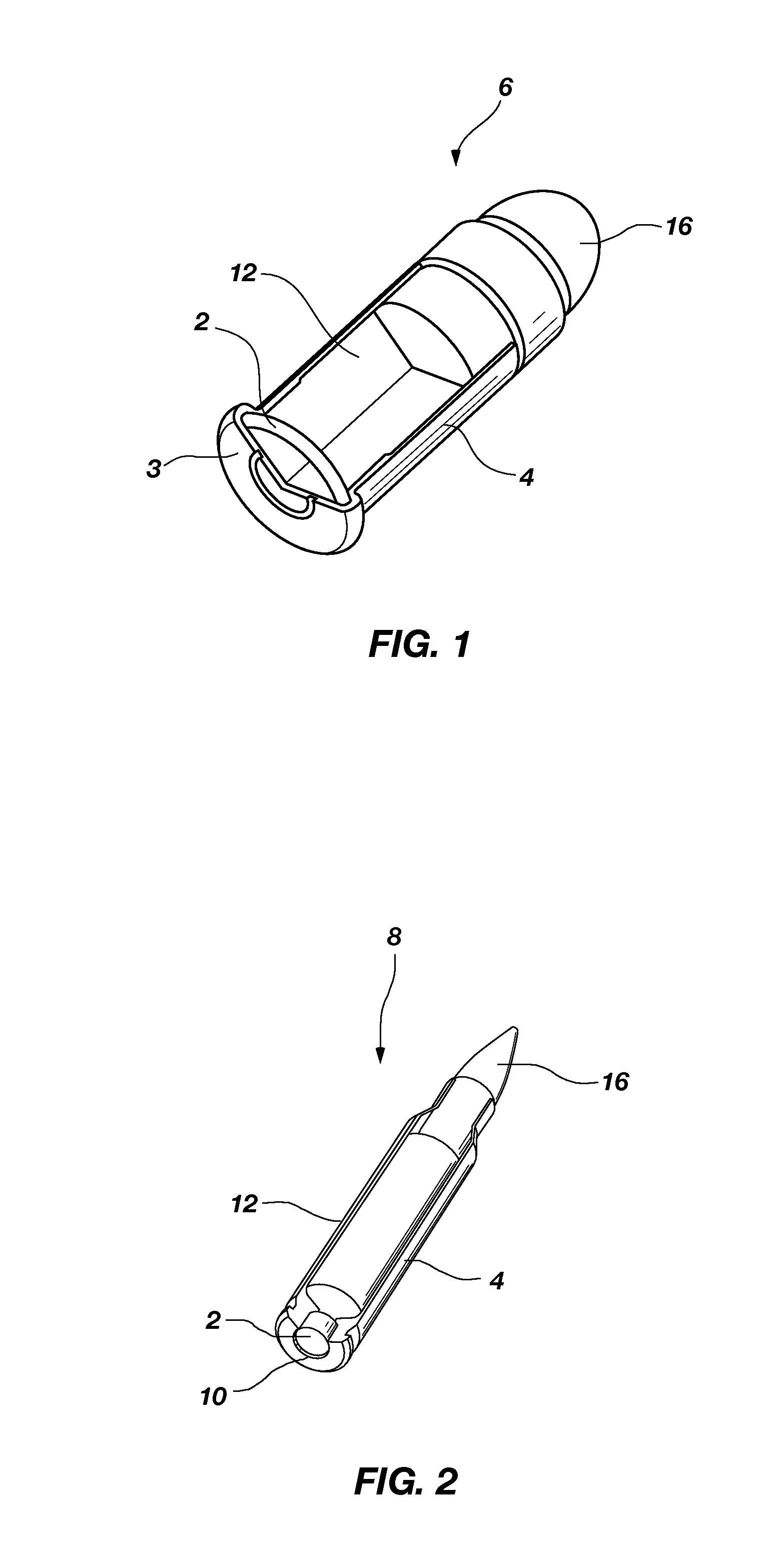
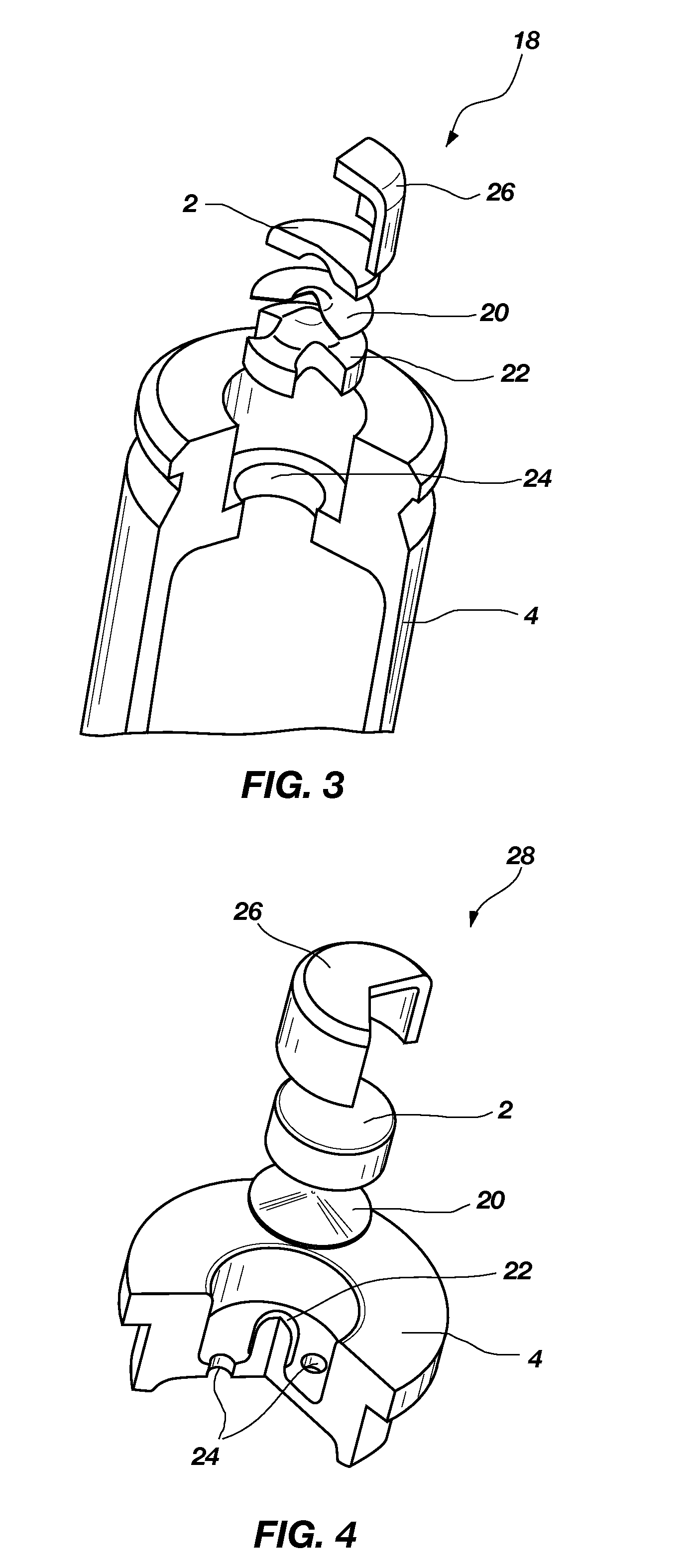
Composite material containing tungsten and bronze
High-density composite materials comprising tungsten and bronze are useful as lead replacements in the production of ammunition, weights and other high density articles. The composition of the composite, articles manufactured using the composite, and a process for making the composite are disclosed.
Owner:ELLIOTT CARTRIDGE COMPANY (CANADA) LTD
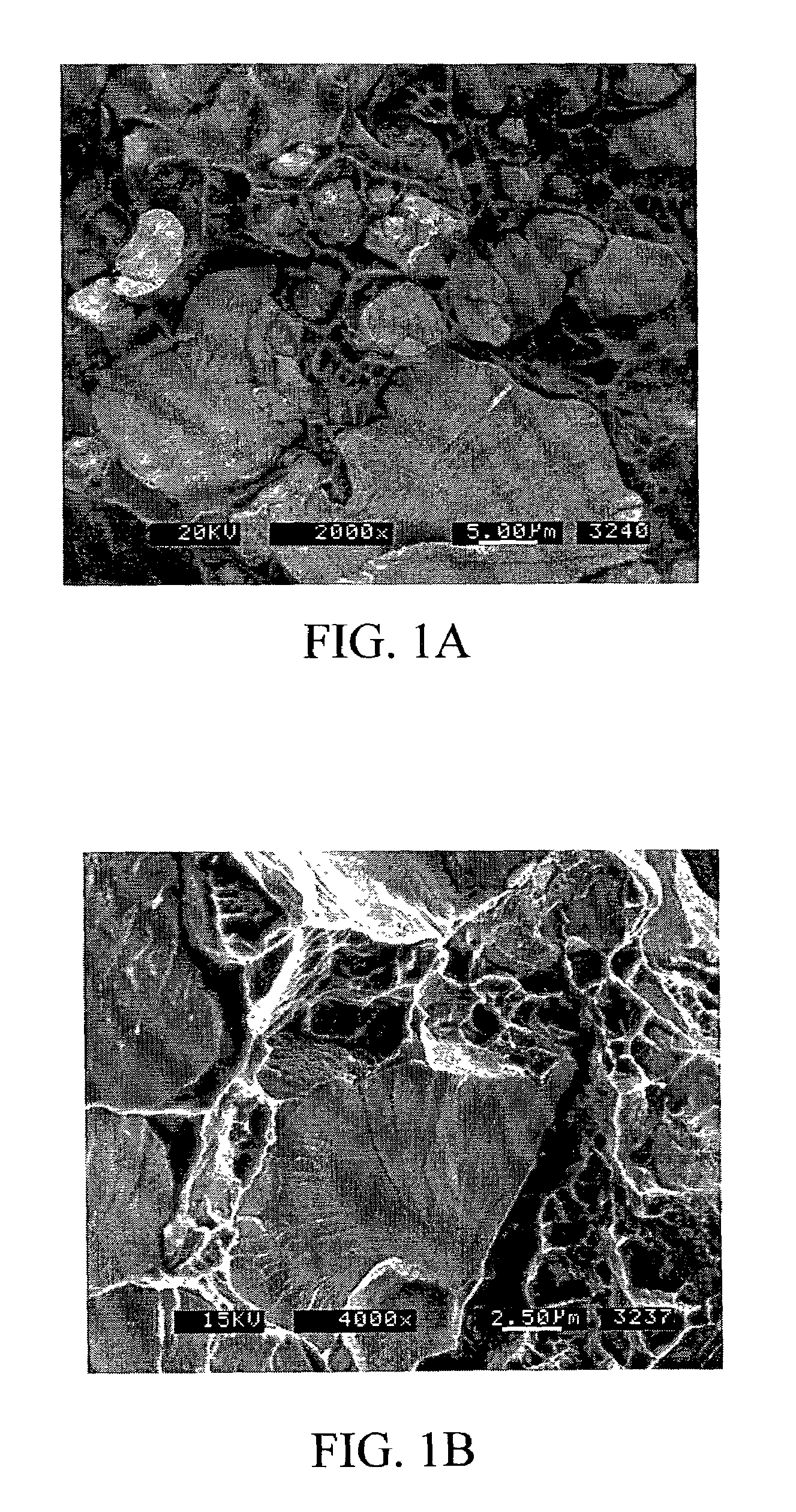
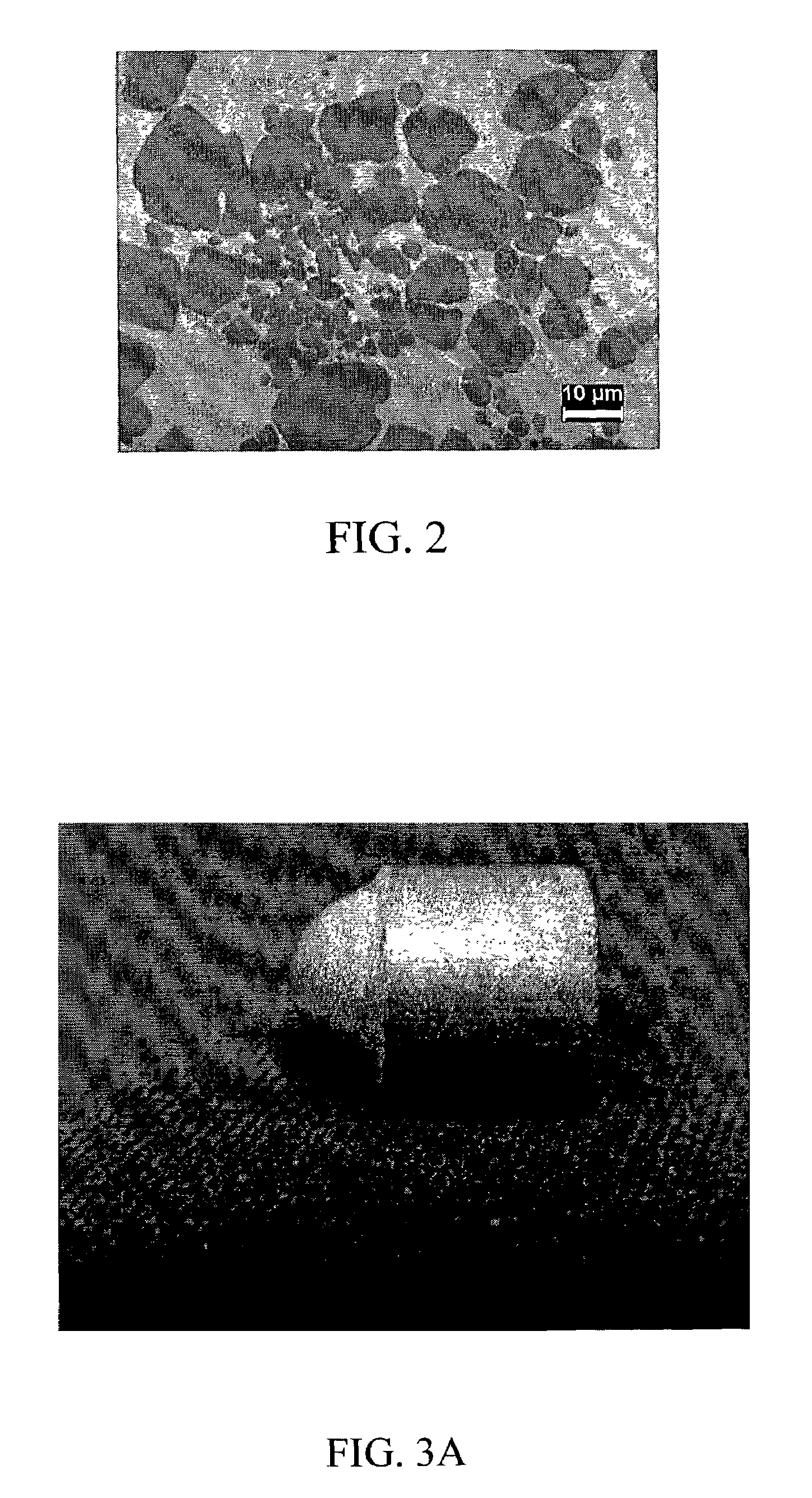
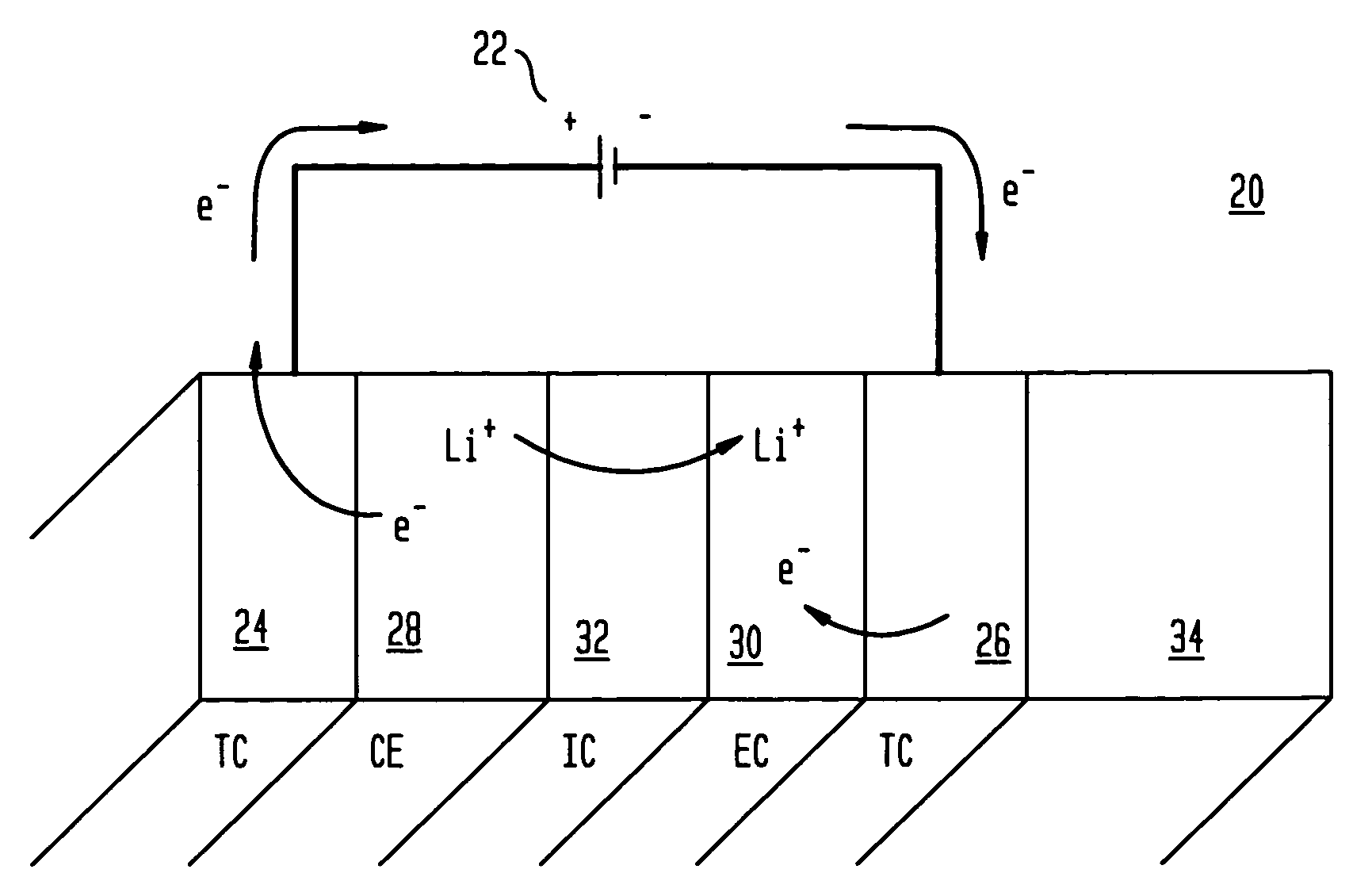
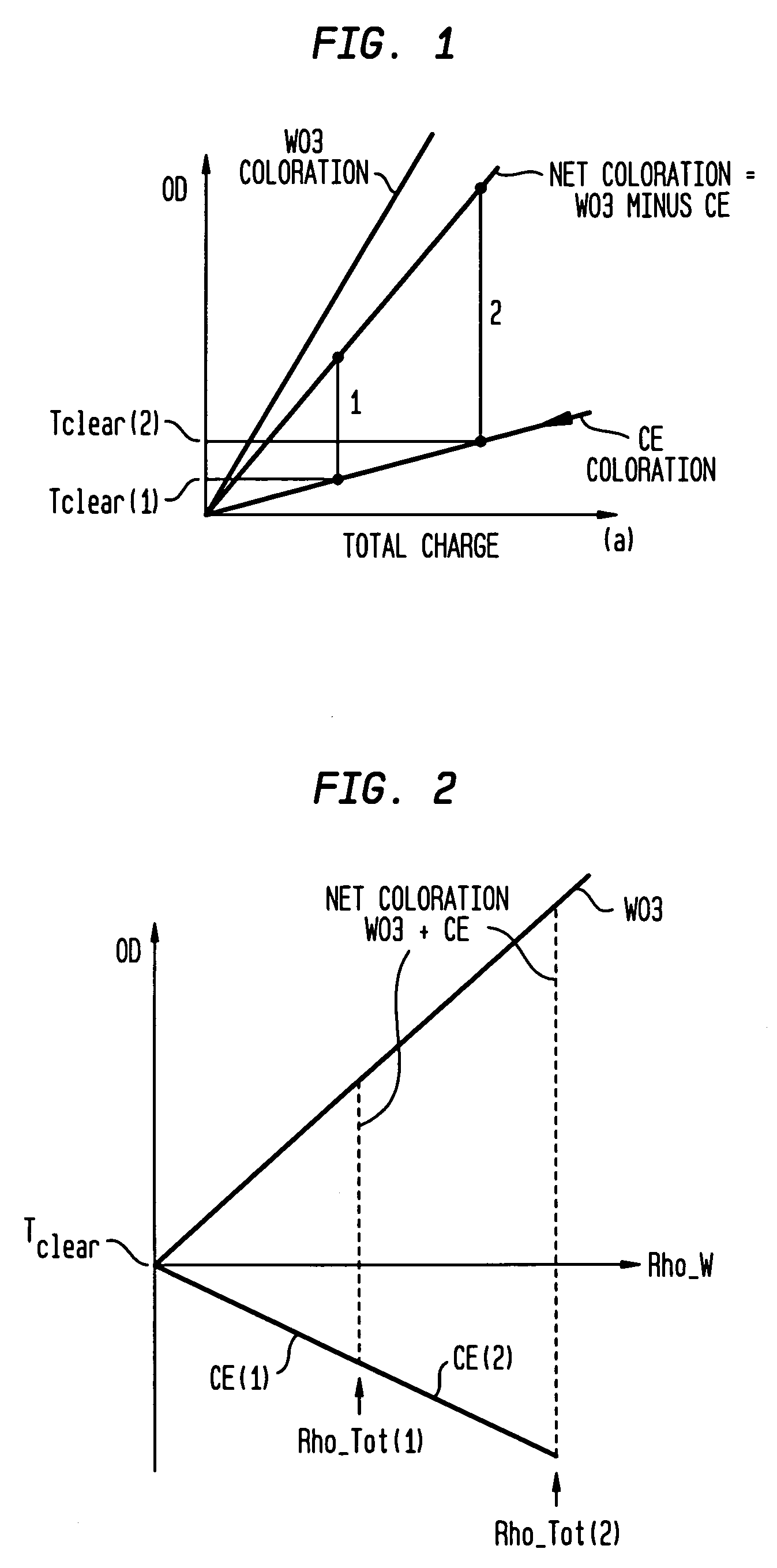
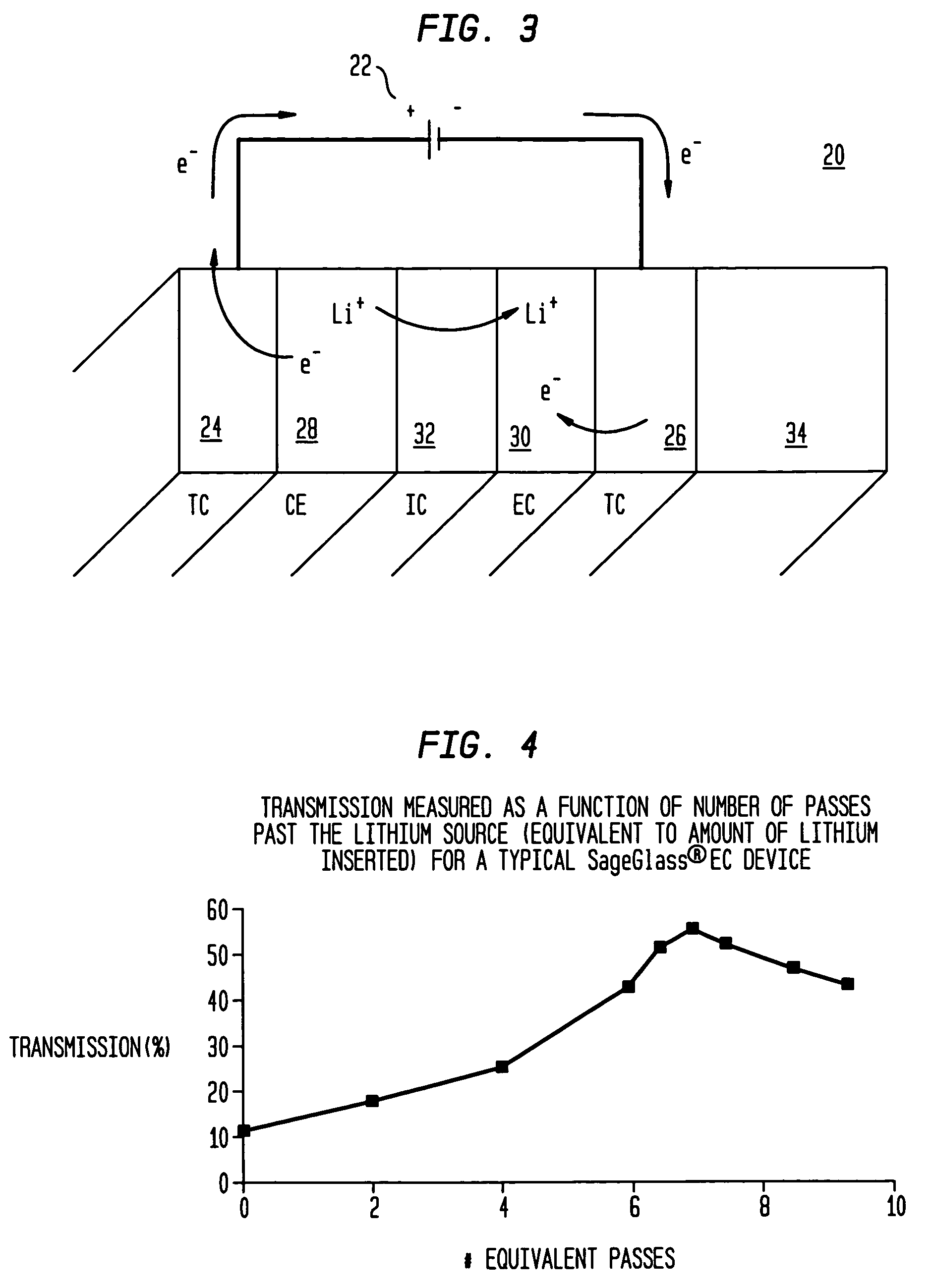
Electrochromic devices and methods
A heat treated electrochromic device comprising an anodic complementary counter electrode layer comprised of a mixed tungsten-nickel oxide and lithium, which provides a high transmission in the fully intercalated state and which is capable of long term stability, is disclosed. Methods of making an electrochromic device comprising an anodic complementary counter electrode comprised of a mixed tungsten-nickel oxide are also disclosed.
Owner:SAGE ELECTROCHROMICS
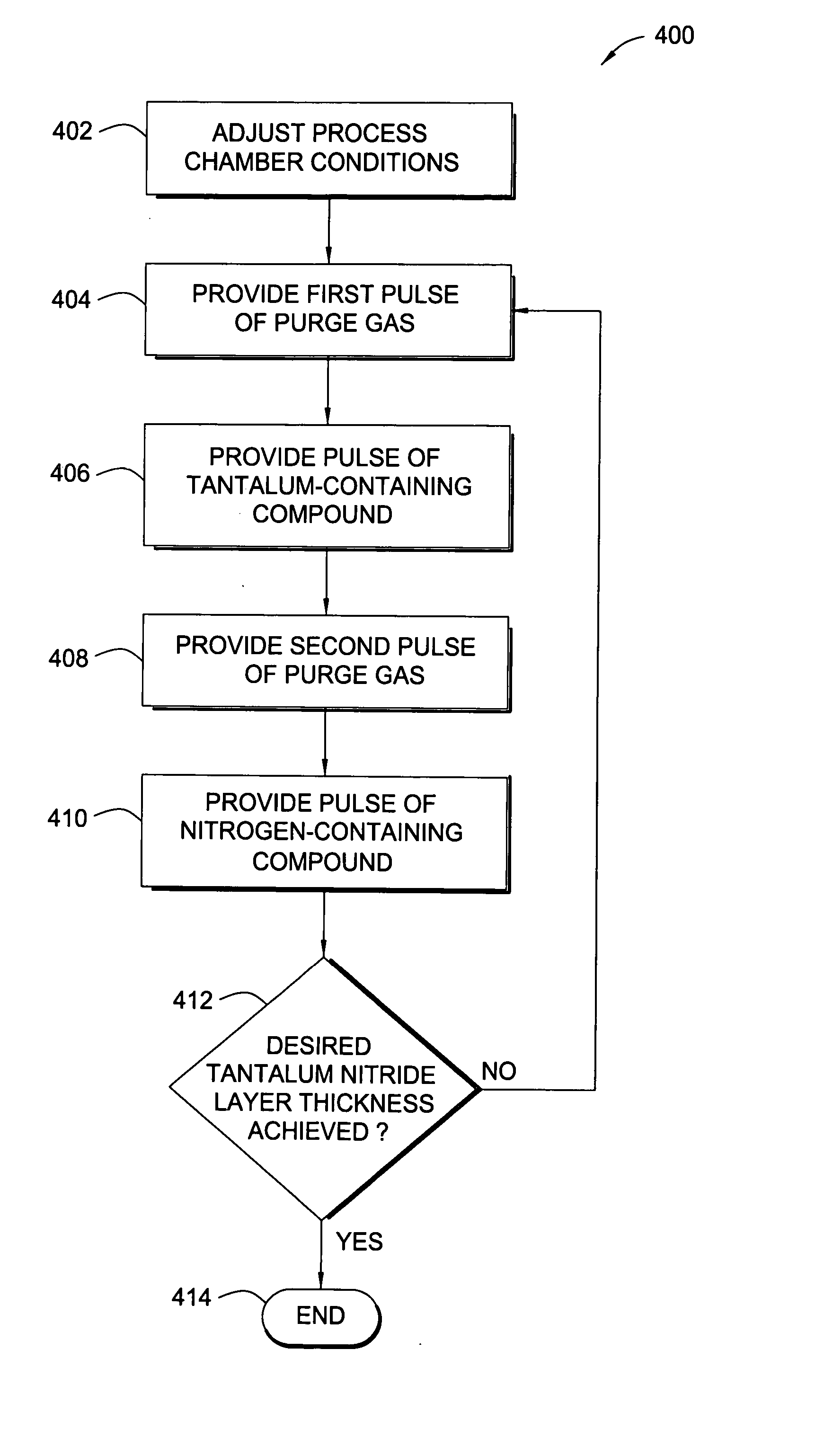
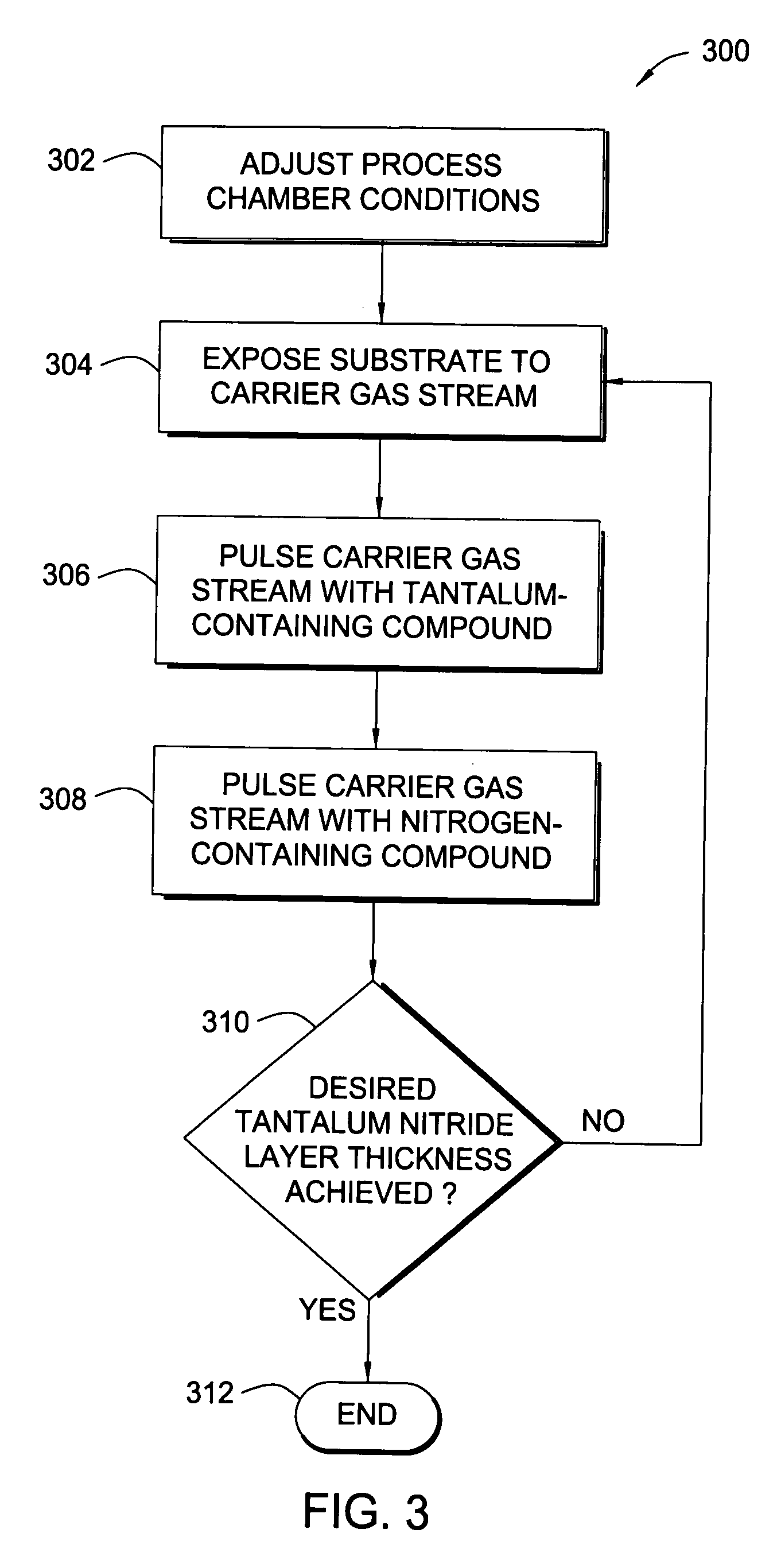
Atomic layer deposition of tantalum-containing materials using the tantalum precursor taimata
InactiveUS20060019495A1Semiconductor/solid-state device manufacturingChemical vapor deposition coatingTantalum nitrideBoron nitride
In one example of the invention, a method for depositing a tantalum-containing material on a substrate in a process chamber is provided which includes exposing the substrate to a tantalum precursor that contains TAIMATA and to at least one secondary precursor to deposit a tantalum-containing film during an atomic layer deposition (ALD) process. The ALD process is repeated until the tantalum-containing film is deposited with a predetermined thickness. Usually, the TAIMATA is preheated prior pulsing the tantalum precursor into the process chamber. A metal layer, such as tungsten or copper, may be deposited on the tantalum-containing material. The tantalum-containing material may include tantalum, tantalum nitride, tantalum silicon nitride, tantalum boron nitride, tantalum phosphorous nitride or tantalum oxynitride. The tantalum-containing material may be deposited as a barrier or adhesion layer within a via or as a gate electrode material within a source / drain device.
Owner:APPLIED MATERIALS INC
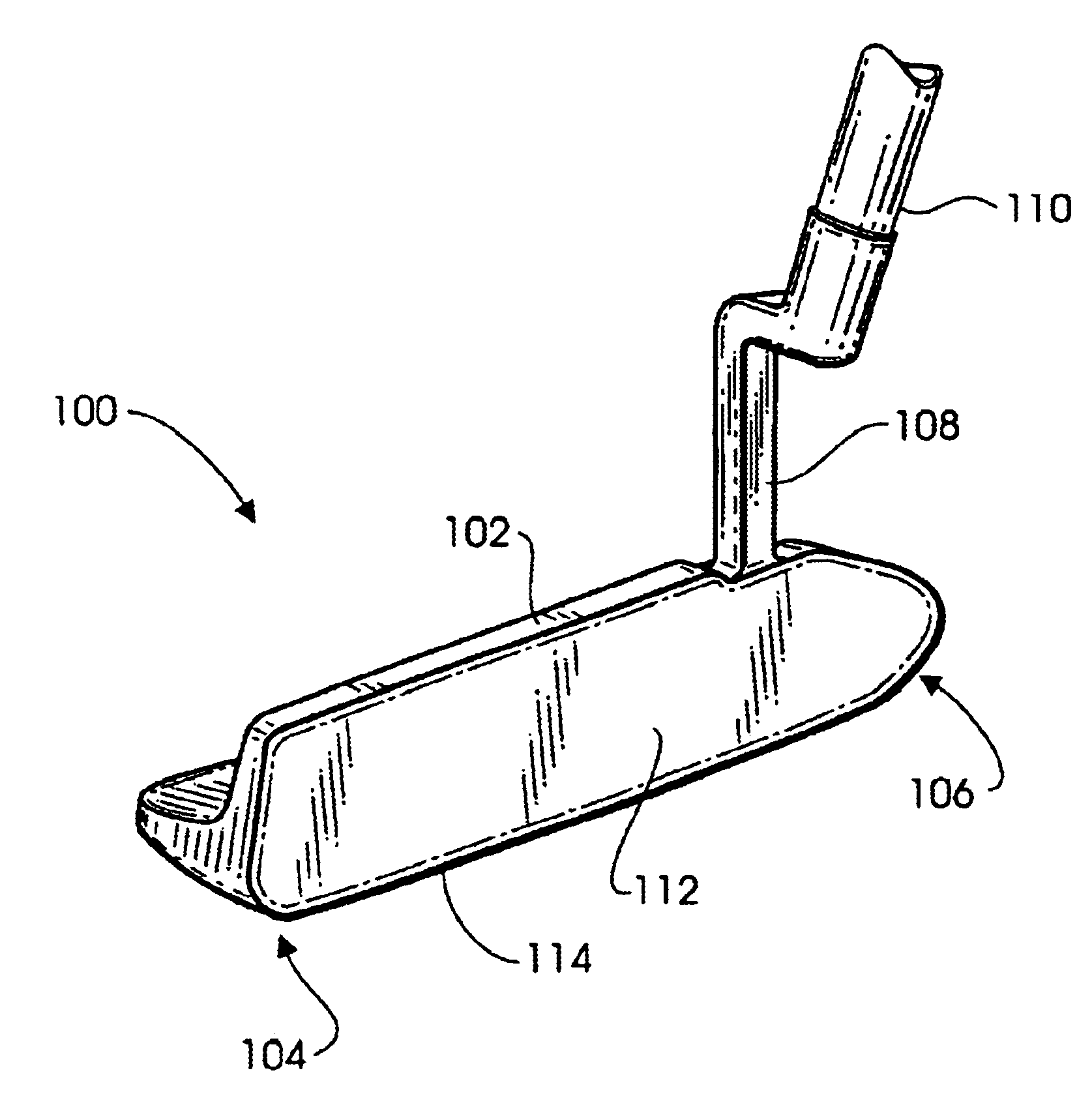
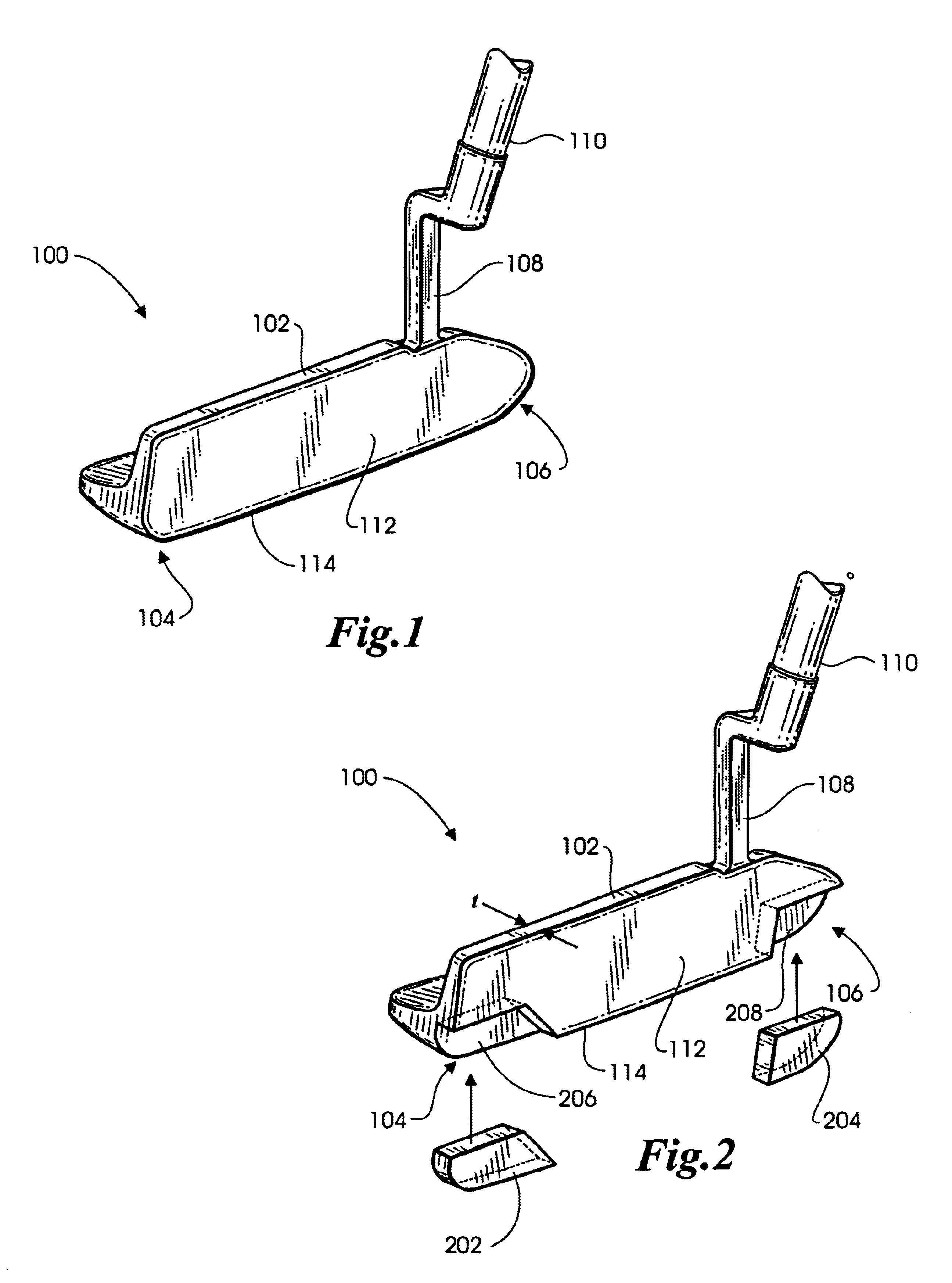
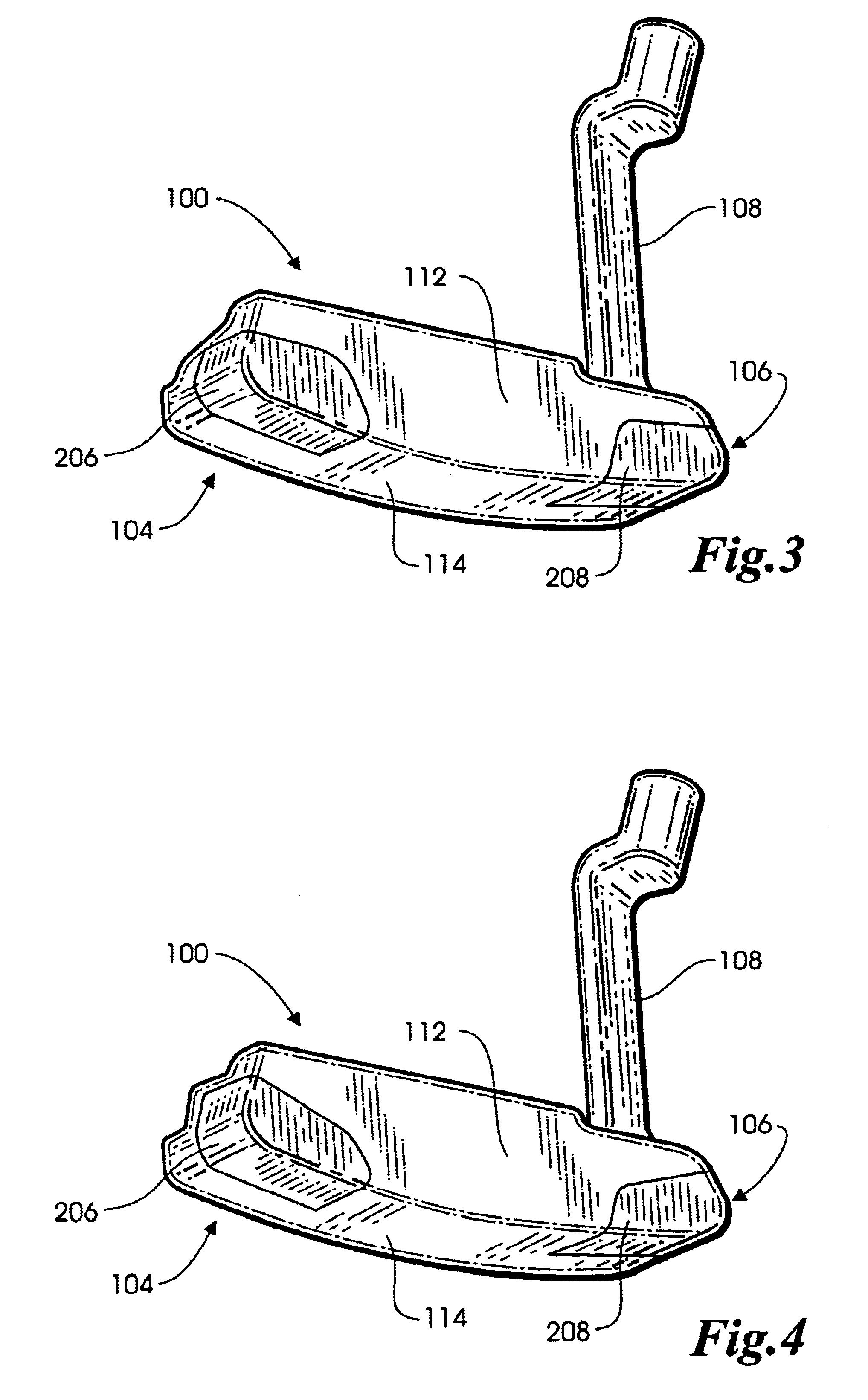
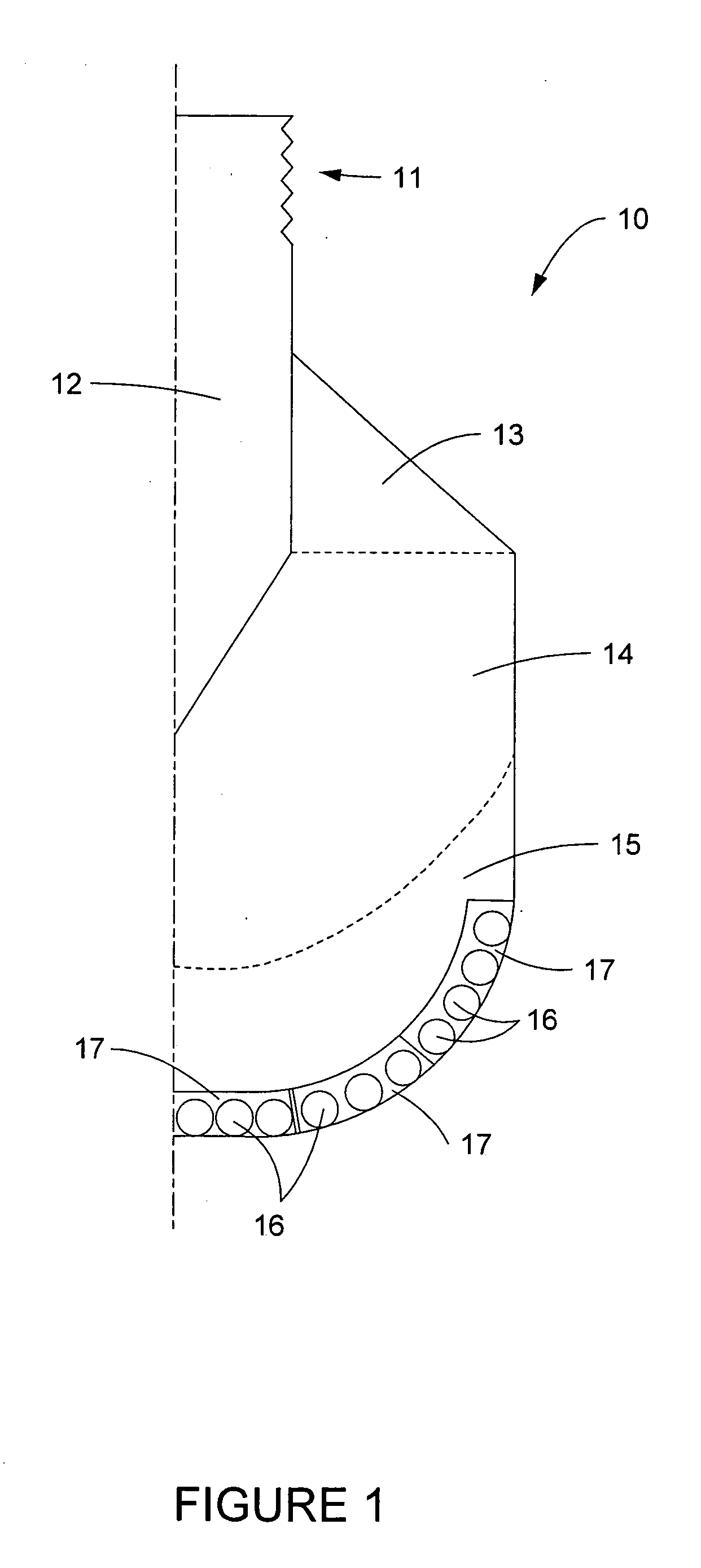
Methods and apparatus for a putter club head with high-density inserts
A putter golf club head includes a body fitted with relatively high-density heel and toe inserts. A golf putter club head includes a body formed of a first material (e.g., titanium) having a density of approximately 3.0 g / cm3 to 7.0 g / cm3. Two cavities are formed in the body, one adjacent to the heel region of the body, and another adjacent to the toe region. Inserts are provided in each of the two cavities and are formed of a second material (e.g., tungsten) having a density ranging from approximately 15.0 g / cm3 to 20.0 g / cm3. The cavities may be configured such that they extend to (or are bounded by) the bottom surface and / or the front face of the body. In one embodiment, the body of the putter club head has an elastic modulus greater than approximately 90 GPa while the density of the insert material is greater than the density of the body material by a factor of at least 3.0.
Owner:KARSTEN MFG CORP
Reactive preclean prior to metallization for sub-quarter micron application
InactiveUS6107192AReduce the amount requiredIncrease resistanceVacuum evaporation coatingSputtering coatingRemote plasmaHydrogen
The present invention generally provides a precleaning process prior to metallization for submicron features on substrates. The method includes cleaning the submicron features with radicals from a plasma of a reactive gas such as oxygen, a mixture of CF4 / O2, or a mixture of He / NF3, wherein the plasma is preferably generated by a remote plasma source and the radicals are delivered to a chamber in which the substrate is disposed. Native oxides remaining in the submicron features are preferably reduced in a second step by treatment with radicals from a plasma containing hydrogen. Following the first or both precleaning steps, the features can be filled with metal by available metallization techniques which typically include depositing a barrier / liner layer on exposed dielectric surfaces prior to deposition of aluminum, copper, or tungsten. The precleaning and metallization steps can be conducted on available integrated processing platforms.
Owner:APPLIED MATERIALS INC
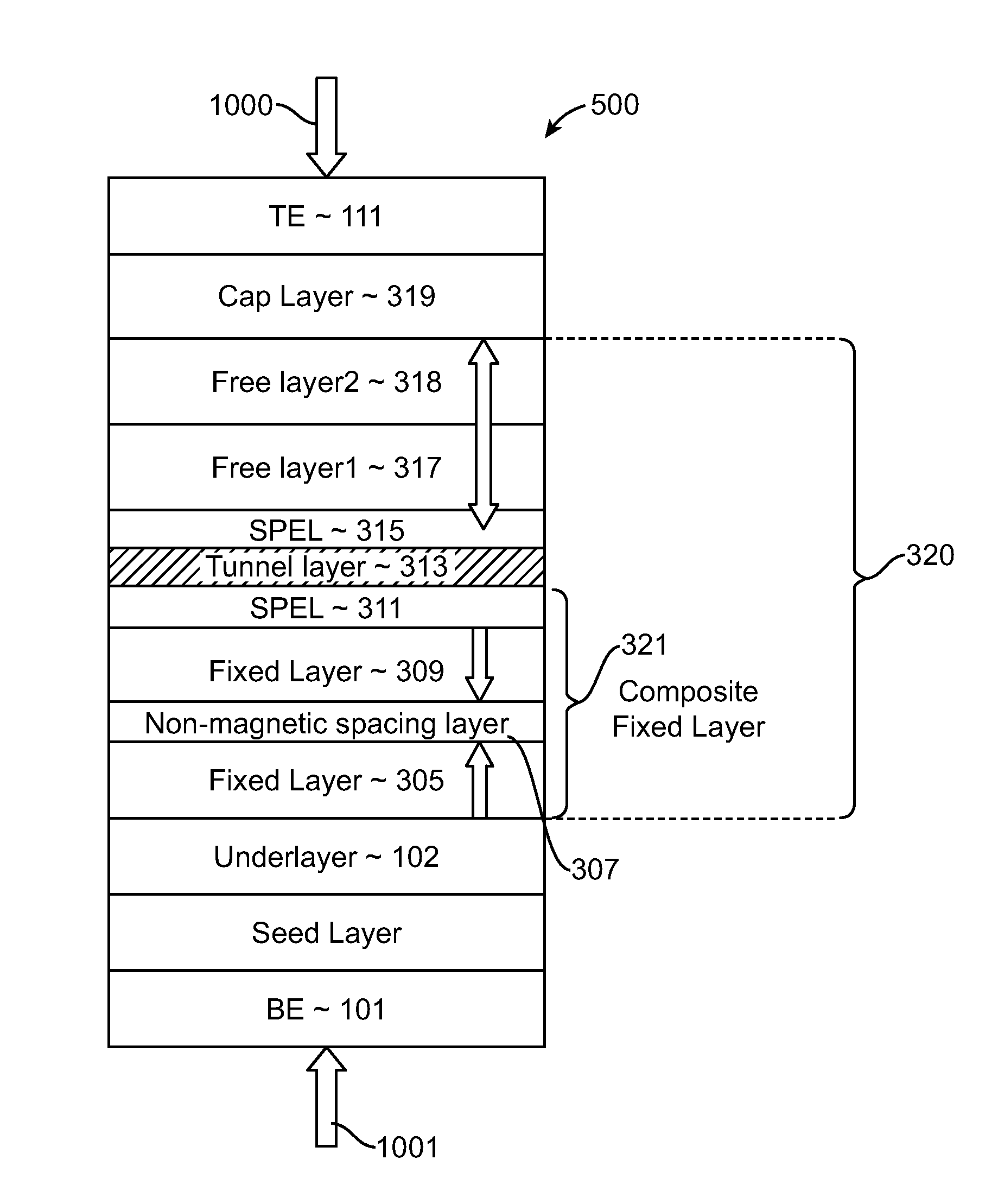
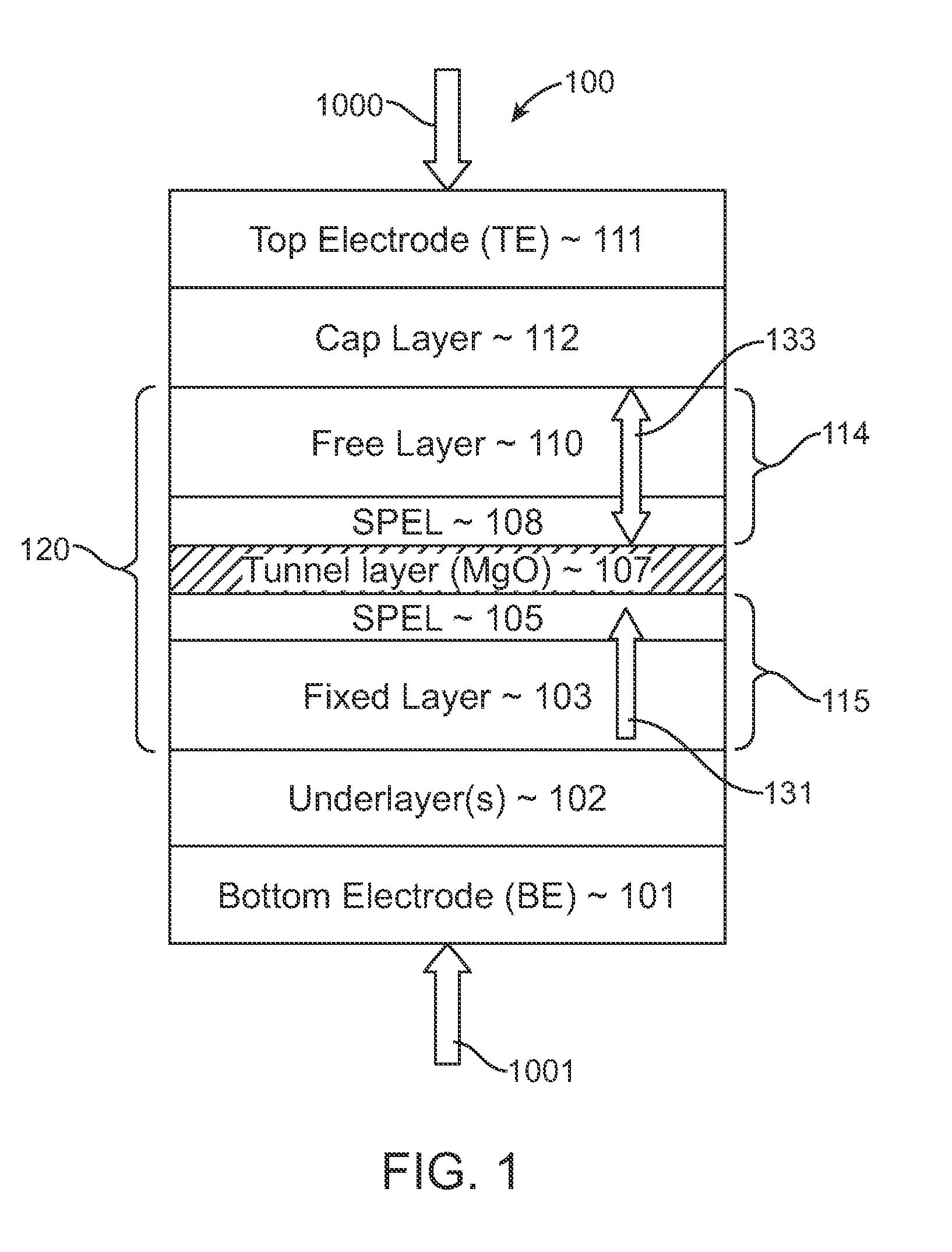
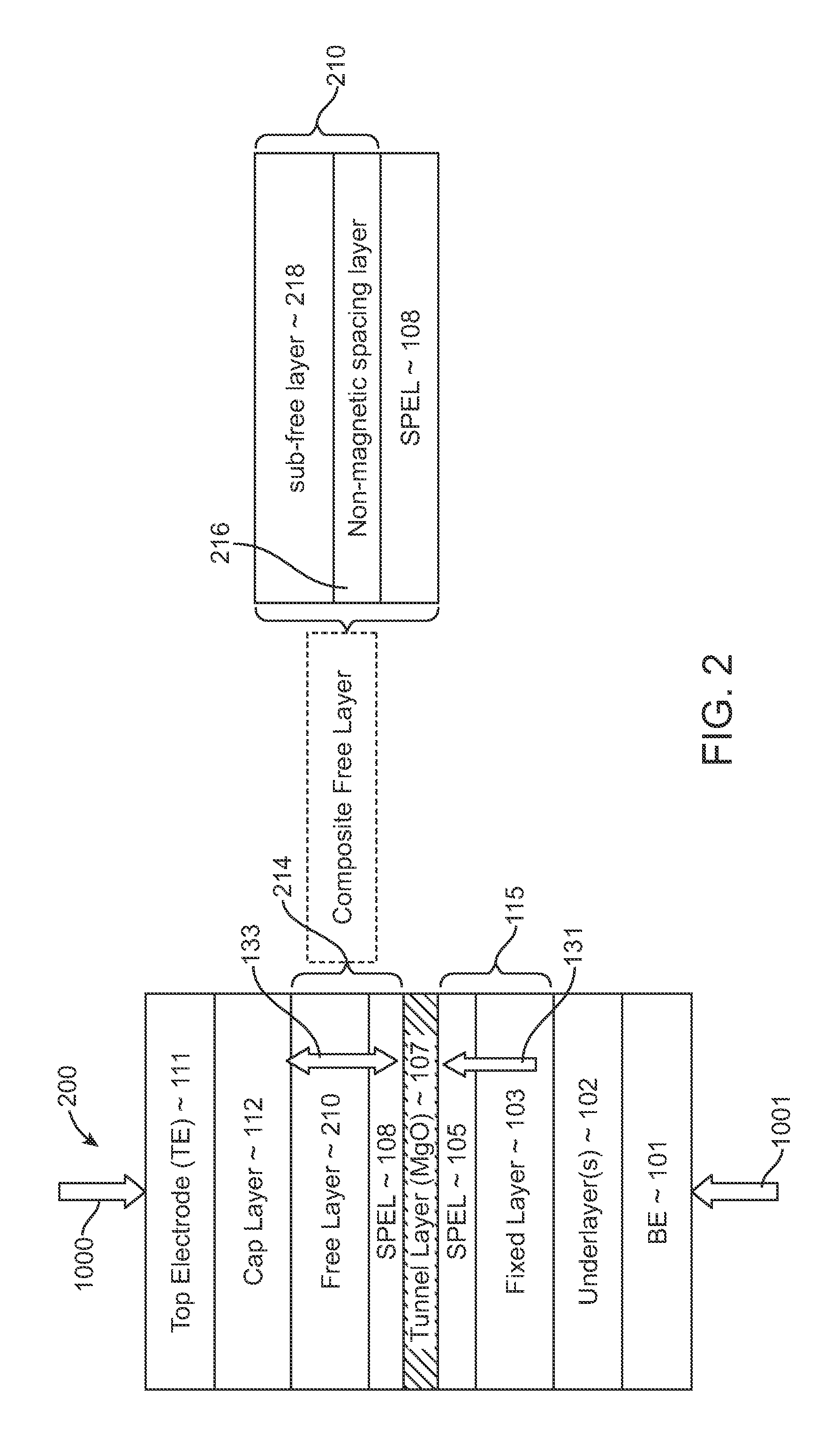
Spin-transfer torque magnetic random access memory having magnetic tunnel junction with perpendicular magnetic anisotropy
A spin-torque transfer memory random access memory (STTMRAM) element includes a fixed layer formed on top of a substrate and a a tunnel layer formed upon the fixed layer and a composite free layer formed upon the tunnel barrier layer and made of an iron platinum alloy with at least one of X or Y material, X being from a group consisting of: boron (B), phosphorous (P), carbon (C), and nitride (N) and Y being from a group consisting of: tantalum (Ta), titanium (Ti), niobium (Nb), zirconium (Zr), tungsten (W), silicon (Si), copper (Cu), silver (Ag), aluminum (Al), chromium (Cr), tin (Sn), lead (Pb), antimony (Sb), hafnium (Hf) and bismuth (Bi), molybdenum (Mo) or rhodium (Ru), the magnetization direction of each of the composite free layer and fixed layer being substantially perpendicular to the plane of the substrate.
Owner:AVALANCHE TECH
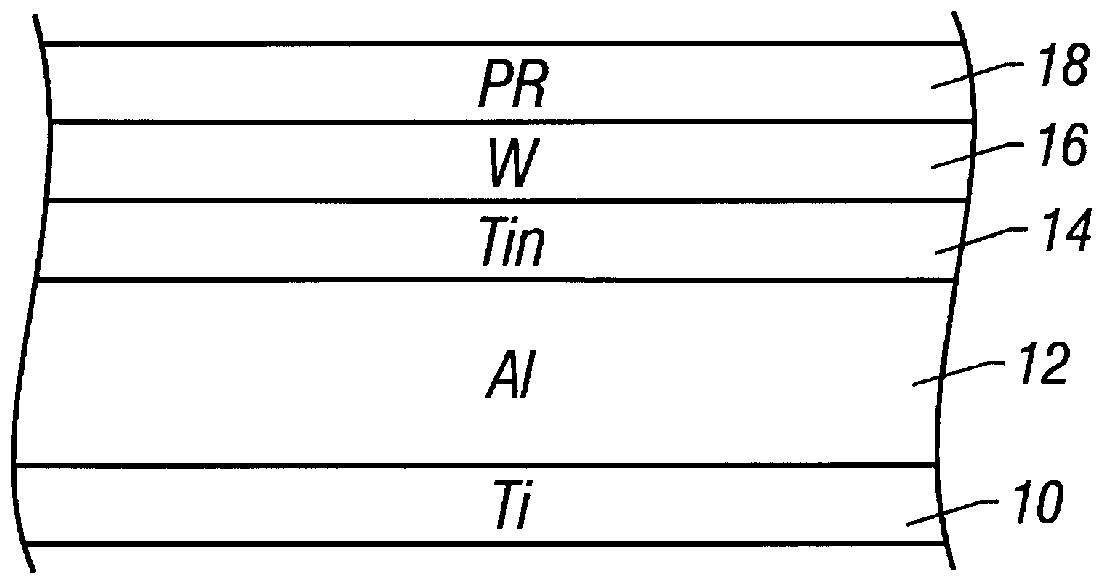
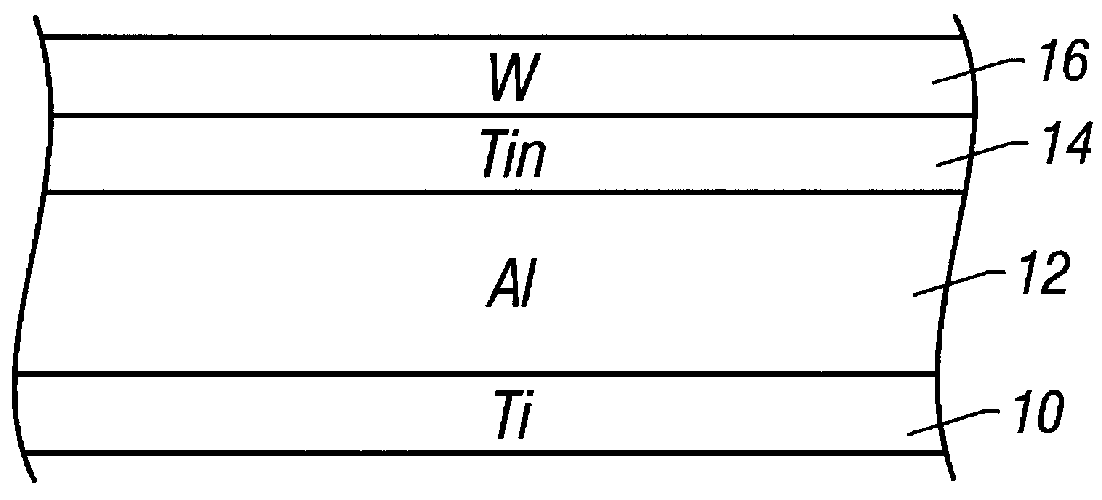
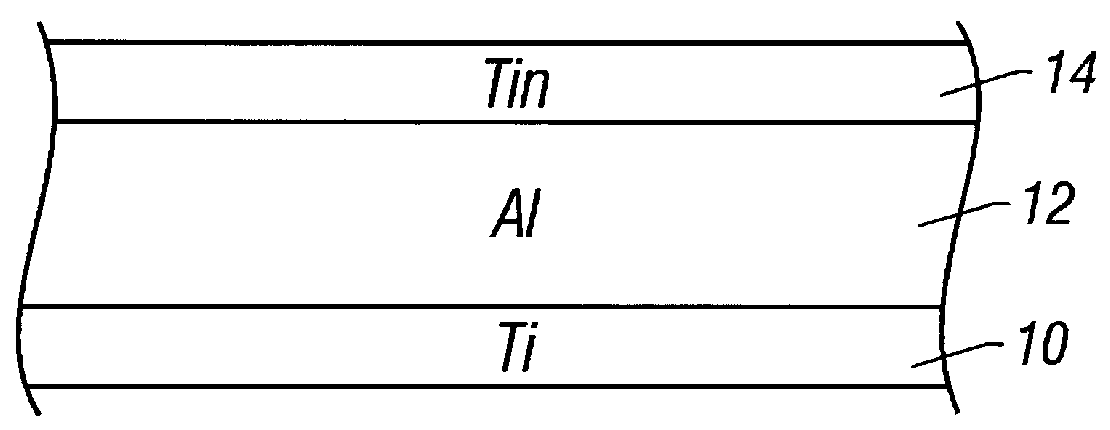
Method of making an interconnect using a tungsten hard mask
InactiveUS6087269AReduce thicknessImproved metal pitchSemiconductor/solid-state device manufacturingPhotoresistTungsten
An interconnect layer is fabricated using a tungsten hard mask by forming a tungsten based layer over an aluminum based layer. A photoresist layer is deposited over the tungsten based layer and patterned. The tungsten based layer is patterned by applying a fluorine-based etchant using the photoresist layer as an etch mask. Then the aluminum based layer is patterned by applying a chlorine based etchant using the tungsten based layer as an etch mask.
Owner:ADVANCED MICRO DEVICES INC
Lead free reduced ricochet limited penetration projectile
A frangible projectile with a specific gravity similar to a lead projectile. The projectile comprises 34-94%, by weight, binder. The binder comprises poly ether block amide resin. The projectile further comprises 6-66%, by weight, ballast. The ballast comprises at least one member selected from a group consisting of tungsten, tungsten carbide, molybdenum, tantalum, ferro-tungsten, copper, bismuth, iron, steel, brass, aluminum bronze, beryllium copper, tin, aluminum, titanium, zinc, nickel silver alloy, cupronickel and nickel. The projectile can be prepared with a particularly preferred specific gravity of 5-14 and more preferably 11-11.5.
Owner:ACCUTEC USA
Nontoxic, noncorrosive phosphorus-based primer compositions and an ordnance element including the same
A primer composition that includes red phosphorus having an acid scavenger and a polymer thereon. The primer composition includes at least one other component that is substantially free of lead. The other component is at least one oxidizer, or at least one oxidizer and at least one of at least one secondary explosive composition and at least one energetic binder. The primer composition optionally includes at least one element having an ionic charge to ionic radius ratio of 4 or of 8, such as magnesium, zirconium, aluminum, silicon, titanium, tungsten, alloys thereof, and combinations thereof. The red phosphorus and the at least one oxidizer are present in the primer composition at approximately stoichiometric amounts. An ordnance element including the primer composition is also disclosed.
Owner:NORTHROP GRUMMAN SYST CORP
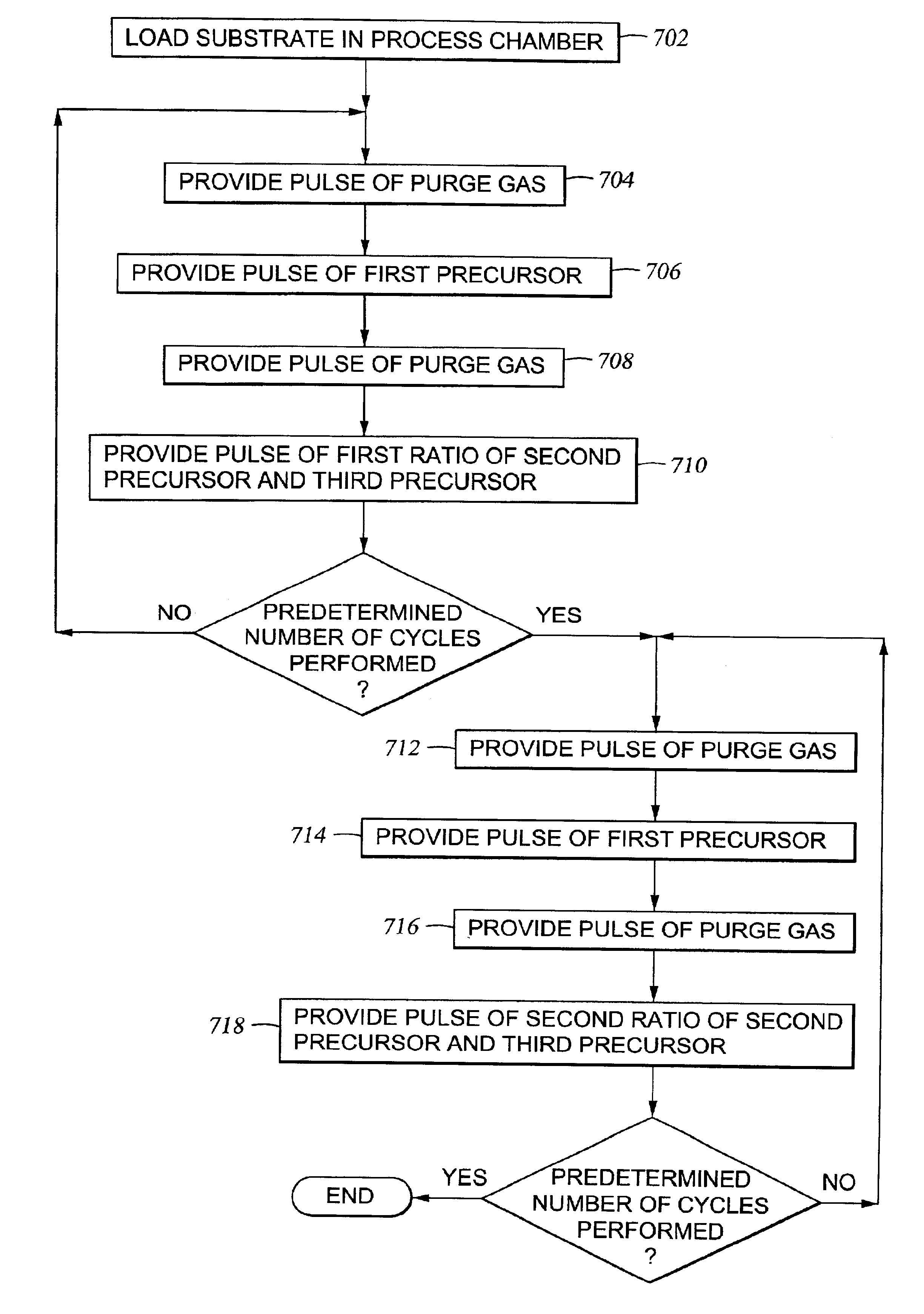
Multiple precursor cyclical deposition system
Embodiments of the present invention relate to an apparatus and method of cyclical deposition utilizing three or more precursors in which delivery of at least two of the precursors to a substrate structure at least partially overlap. One embodiment of depositing a ternary material layer over a substrate structure comprises providing at least one cycle of gases to deposit a ternary material layer. One cycle comprises introducing a pulse of a first precursor, introducing a pulse of a second precursor, and introducing a pulse of a third precursor in which the pulse of the second precursor and the pulse of the third precursor at least partially overlap. In one aspect, the ternary material layer includes, but is not limited to, tungsten boron silicon (WBxSiy), titanium silicon nitride (TiSixNy), tantalum silicon nitride (TaSixNy), silicon oxynitride (SiOxNy), and hafnium silicon oxide (HfSixOy). In one aspect, the composition of the ternary material layer may be tuned by changing the flow ratio of the second precursor to the third precursor between cycles.
Owner:APPLIED MATERIALS INC
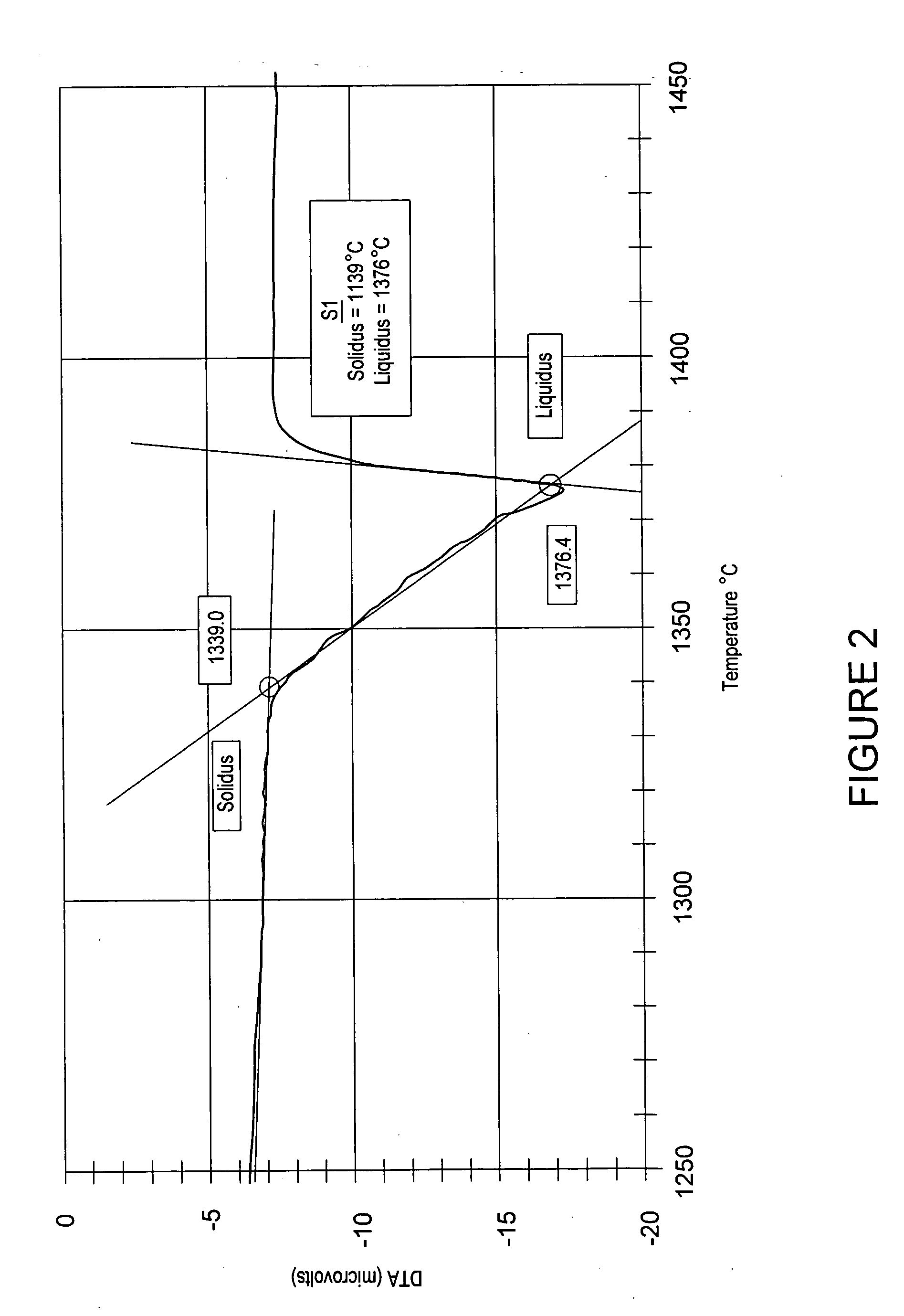
Earth-boring bits
ActiveUS20050247491A1Low melting pointLowered melting point of the binder facilitates proper infiltration of the massDrill bitsCutting machinesBorideNiobium
The present invention relates to compositions and methods for forming a bit body for an earth-boring bit. The bit body may comprise hard particles, wherein the hard particles comprise at least one carbide, nitride, boride, and oxide and solid solutions thereof, and a binder binding together the hard particles. The binder may comprise at least one metal selected from cobalt, nickel, and iron, and, optionally, at least one melting point reducing constituent selected from a transition metal carbide in the range of 30 to 60 weight percent, boron up to 10 weight percent, silicon up to 20 weight percent, chromium up to 20 weight percent, and manganese up to 25 weight percent, wherein the weight percentages are based on the total weight of the binder. In addition, the hard particles may comprise at least one of (i) cast carbide (WC+W2C) particles, (ii) transition metal carbide particles selected from the carbides of titanium, chromium, vanadium, zirconium, hafnium, tantalum, molybdenum, niobium, and tungsten, and (iii) sintered cemented carbide particles.
Owner:BAKER HUGHES INC +1
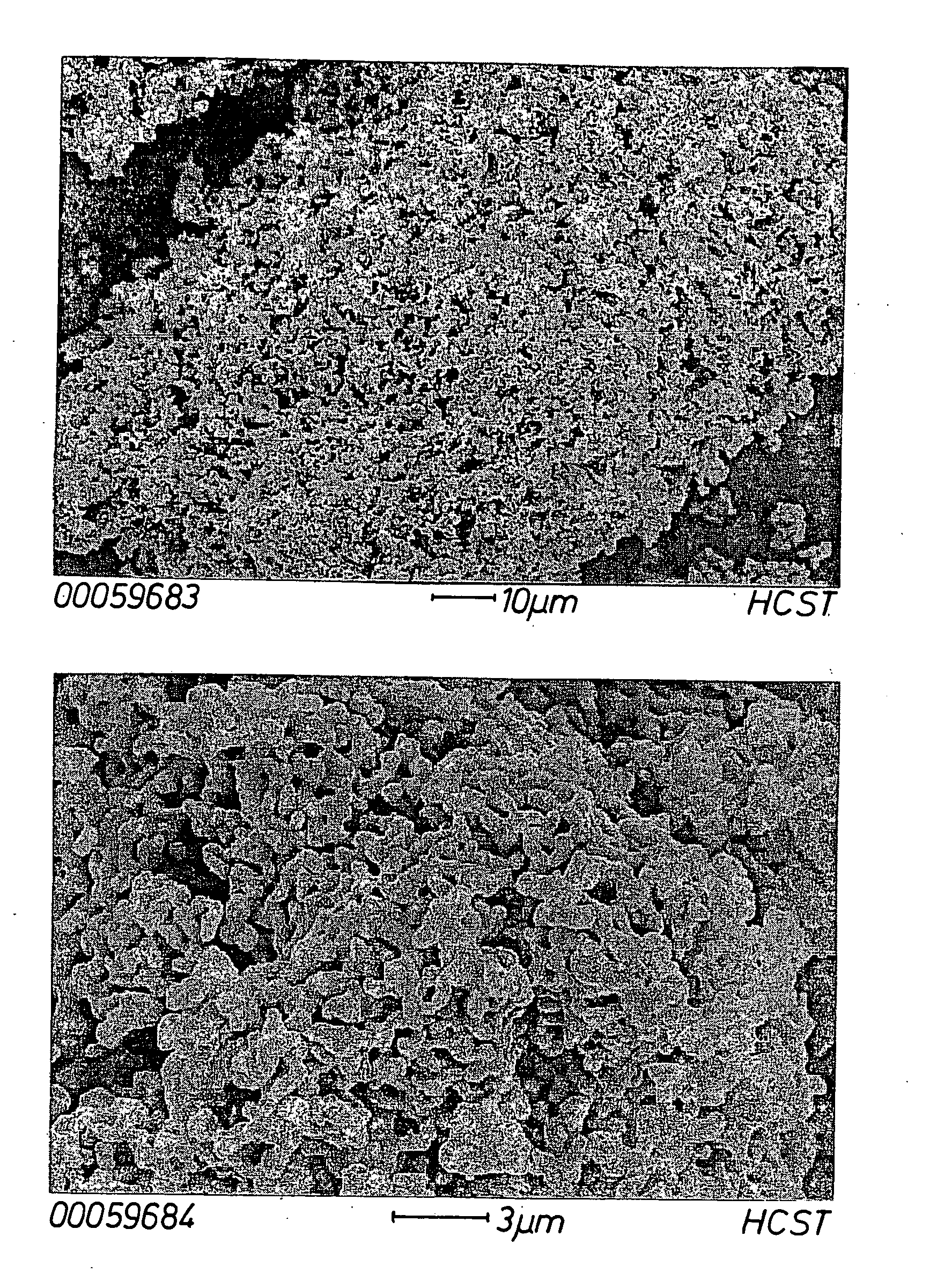
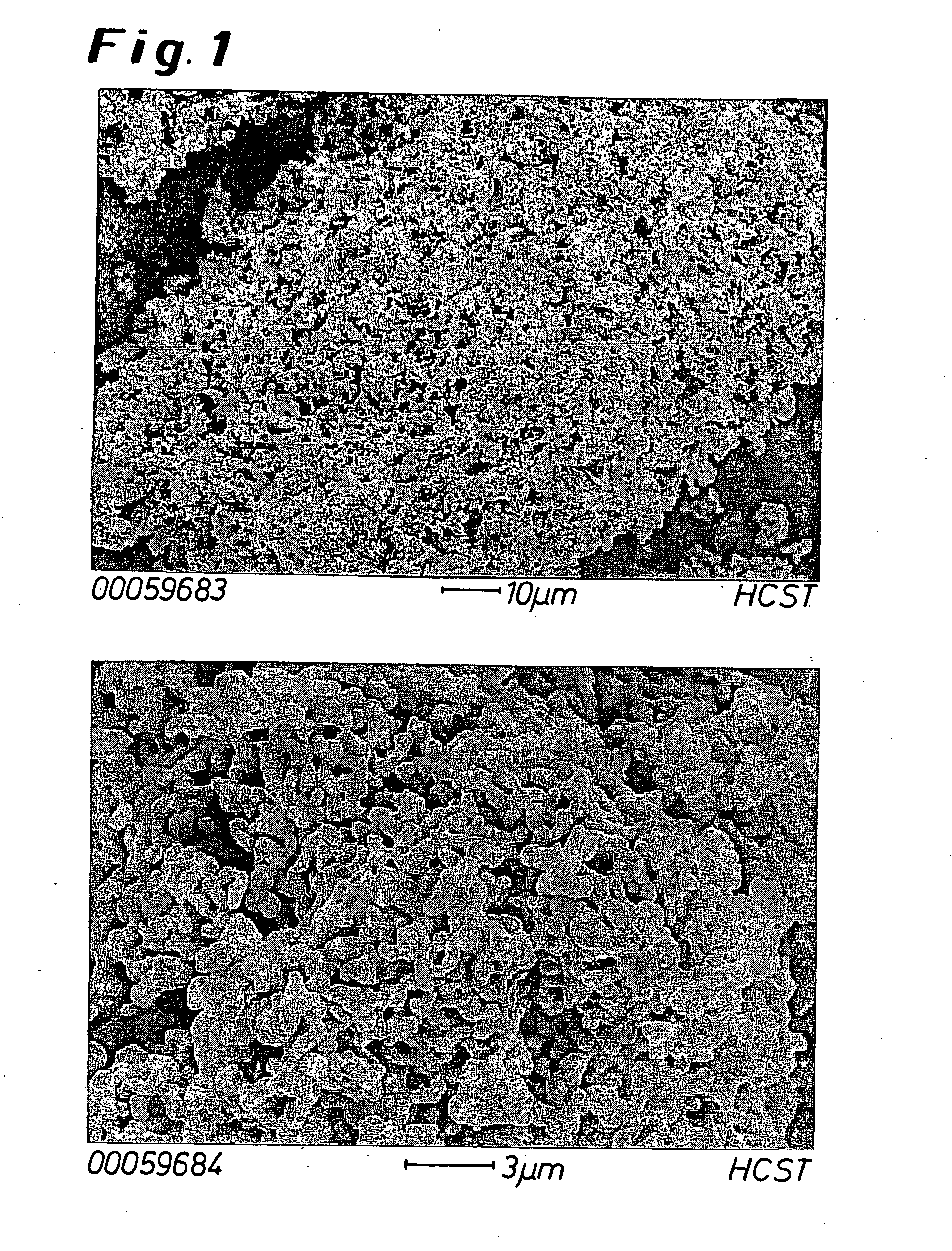
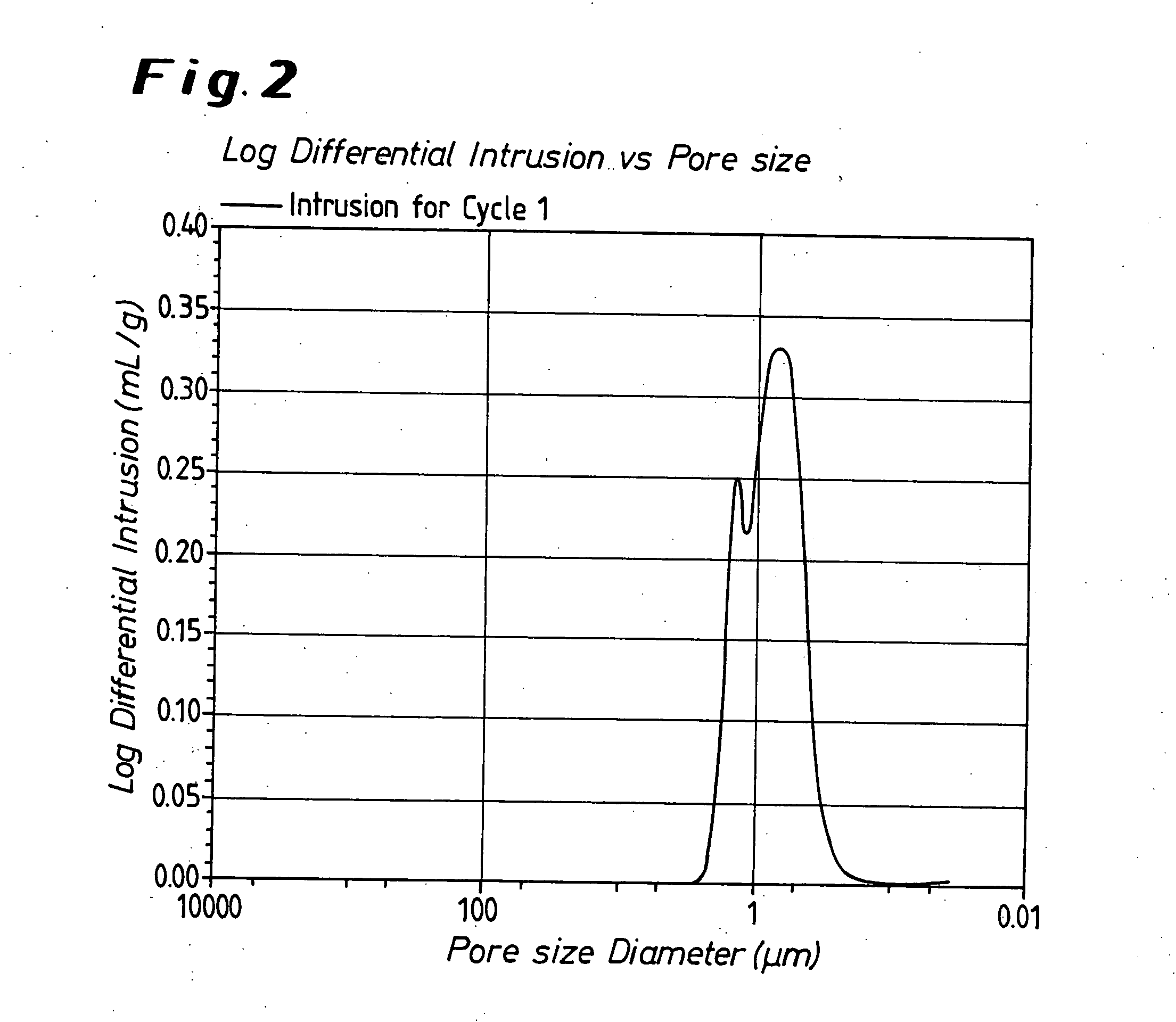
Tungsten deposition sequence
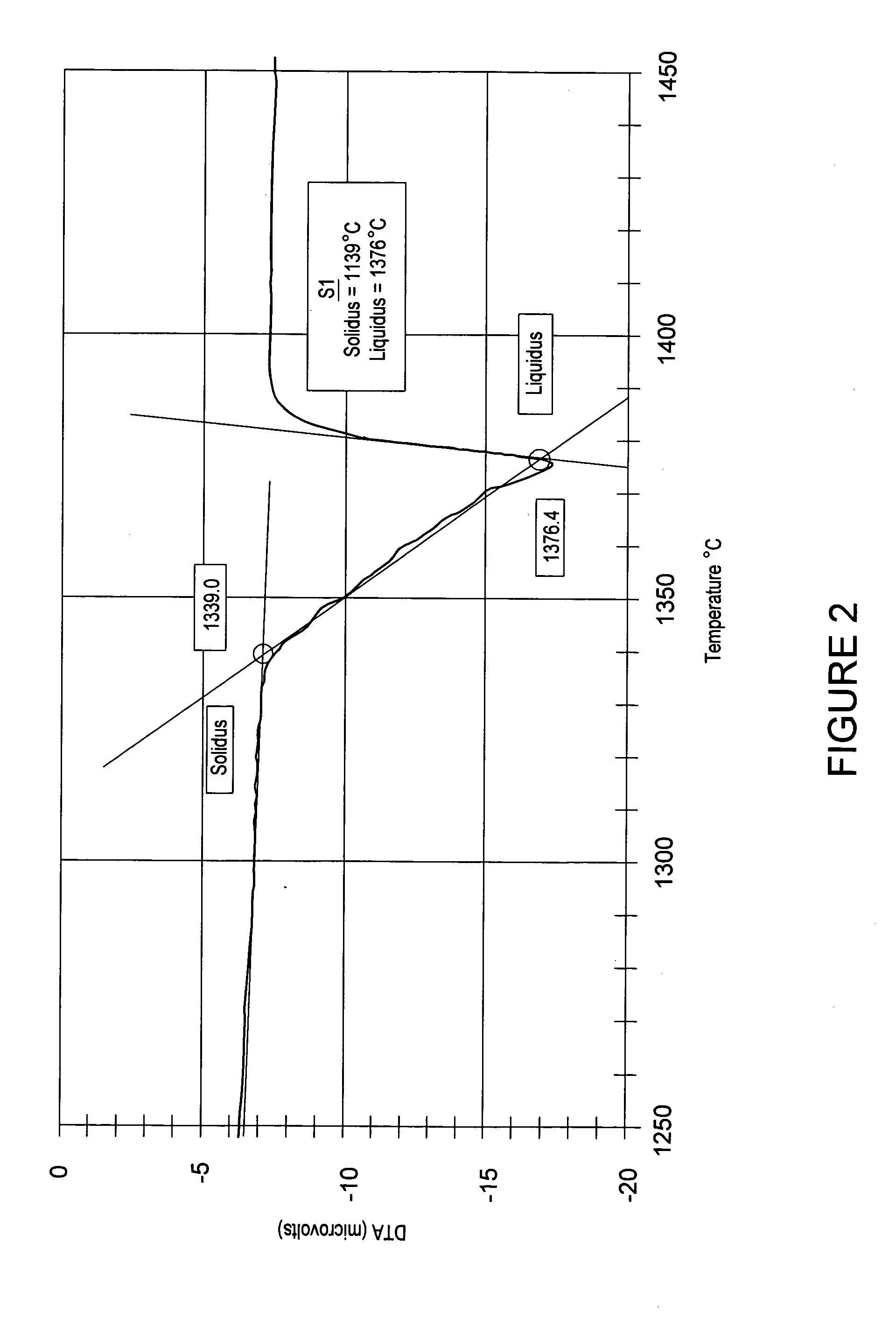
InactiveUS20140273451A1Enhance gapfillingAvoid difficultySemiconductor/solid-state device manufacturingChemical vapor deposition coatingHalogenRoom temperature
Methods of filling gaps with tungsten are described. The methods include a tungsten dep-etch-dep sequence to enhance gapfilling yet avoid difficulty in restarting deposition after the intervening etch. The first tungsten deposition may have a nucleation layer or seeding layer to assist growth of the first tungsten deposition. Restarting deposition with a less-than-conductive nucleation layer would impact function of an integrated circuit, and therefore avoiding tungsten “poisoning” during the etch is desirable. The etching step may be performed using a plasma to excite a halogen-containing precursor while the substrate at relatively low temperature (near room temperature or less). The plasma may be local or remote. Another method may be used in combination or separately and involves the introduction of a source of oxygen into the plasma in combination with the halogen-containing precursor.
Owner:APPLIED MATERIALS INC
Radiopaque nitinol alloys for medical devices
Owner:ABBOTT CARDIOVASCULAR
Earth-boring bits
InactiveUS20050211475A1Low melting pointLowered melting point of the binder facilitates proper infiltration of the massDrill bitsMetal-working drilling toolsBorideNiobium
The present invention relates to compositions and methods for forming a bit body for an earth-boring bit. The bit body may comprise hard particles, wherein the hard particles comprise at least one carbide, nitride, boride, and oxide and solid solutions thereof, and a binder binding together the hard particles. The binder may comprise at least one metal selected from cobalt, nickel, and iron, and at least one melting point reducing constituent selected from a transition metal carbide in the range of 30 to 60 weight percent, boron up to 10 weight percent, silicon up to 20 weight percent, chromium up to 20 weight percent, and manganese up to 25 weight percent, wherein the weight percentages are based on the total weight of the binder. In addition, the hard particles may comprise at least one of (i) cast carbide (WC+W2C) particles, (ii) transition metal carbide particles selected from the carbides of titanium, chromium, vanadium, zirconium, hafnium, tantalum, molybdenum, niobium, and tungsten, and (iii) sintered cemented carbide particles.
Owner:ATI PROPERTIES +1
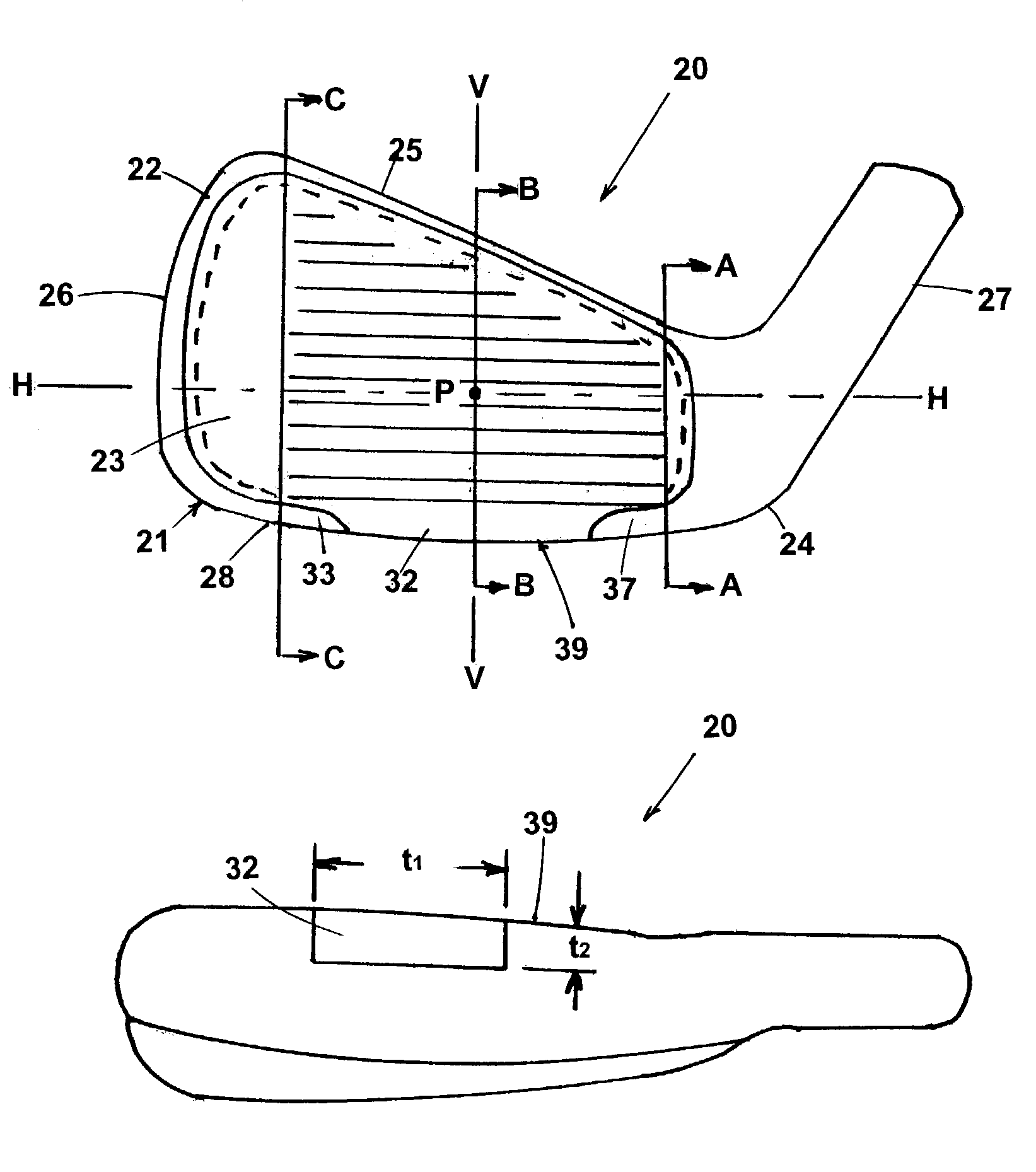
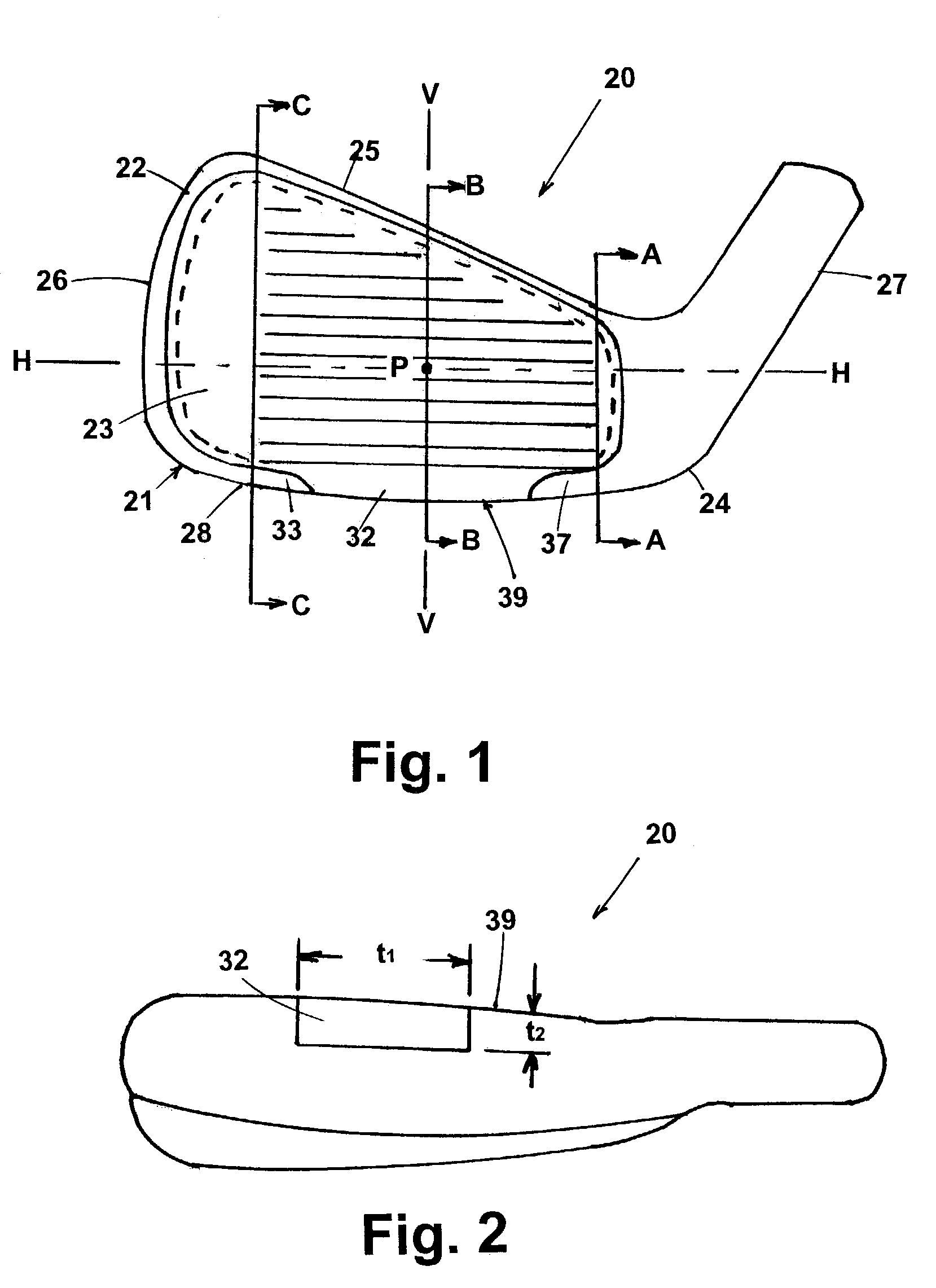
Golf club iron
An iron golf club head having a thin (less than 0.12 inches) first section that has an expanded unsupported front face region. The first section including a central portion forming part of a leading edge and wrapping around a sole section of the club, to create an increase the coefficient of restitution of the club head to greater than 0.8. The club head utilizes a rear insert that in addition to providing support for the front face, also allows for the fine tuning of swing weights with no change in geometry or size of the club head. This is accomplished this by the utilization of weight adjustment inserts that impregnate tungsten loaded plastic into sheets of carbon graphite and epoxy. The percentage of tungsten creating a weight range without any size change in the sheets.
Owner:ACUSHNET CO
Semiconductor device comprising a contact structure based on copper and tungsten
ActiveUS20070099414A1Reduce resistanceLower resistanceTransistorSemiconductor/solid-state device detailsInter layerDevice material
By providing contact plugs having a lower plug portion, formed on the basis of well-established tungsten-based technologies, and an upper plug portion, which may comprise a highly conductive material such as copper or a copper alloy, a significant increase in conductivity of the contact structure may be achieved. For this purpose, after the deposition of a first dielectric layer of the inter-layer stack, a planarization process may be performed so as to allow the formation of the lower plug portions on the basis of tungsten, while, after the deposition of the second dielectric layer, a corresponding copper-based technology may be used for forming the upper plug portions of significantly enhanced conductivity.
Owner:GLOBALFOUNDRIES US INC
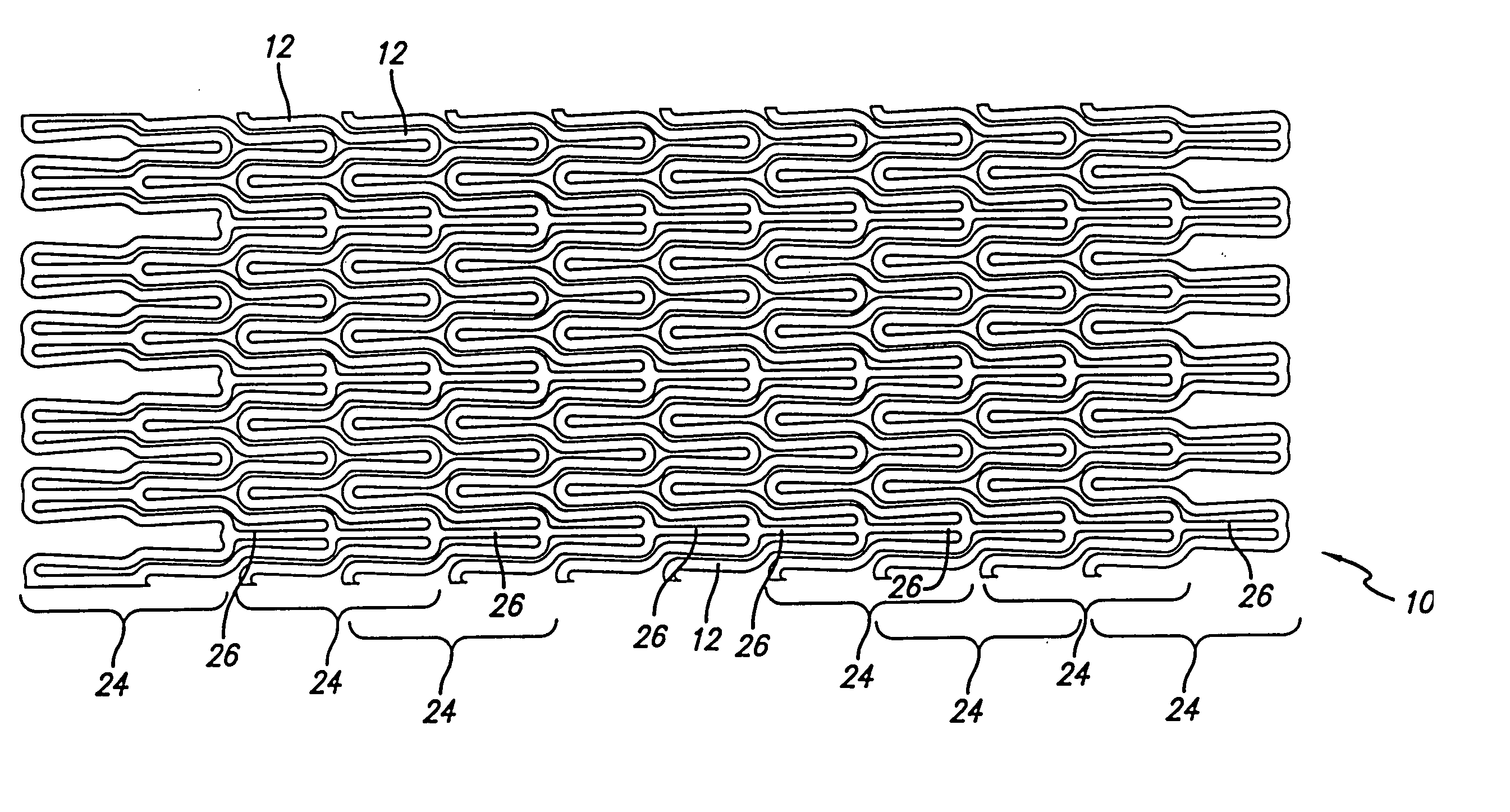
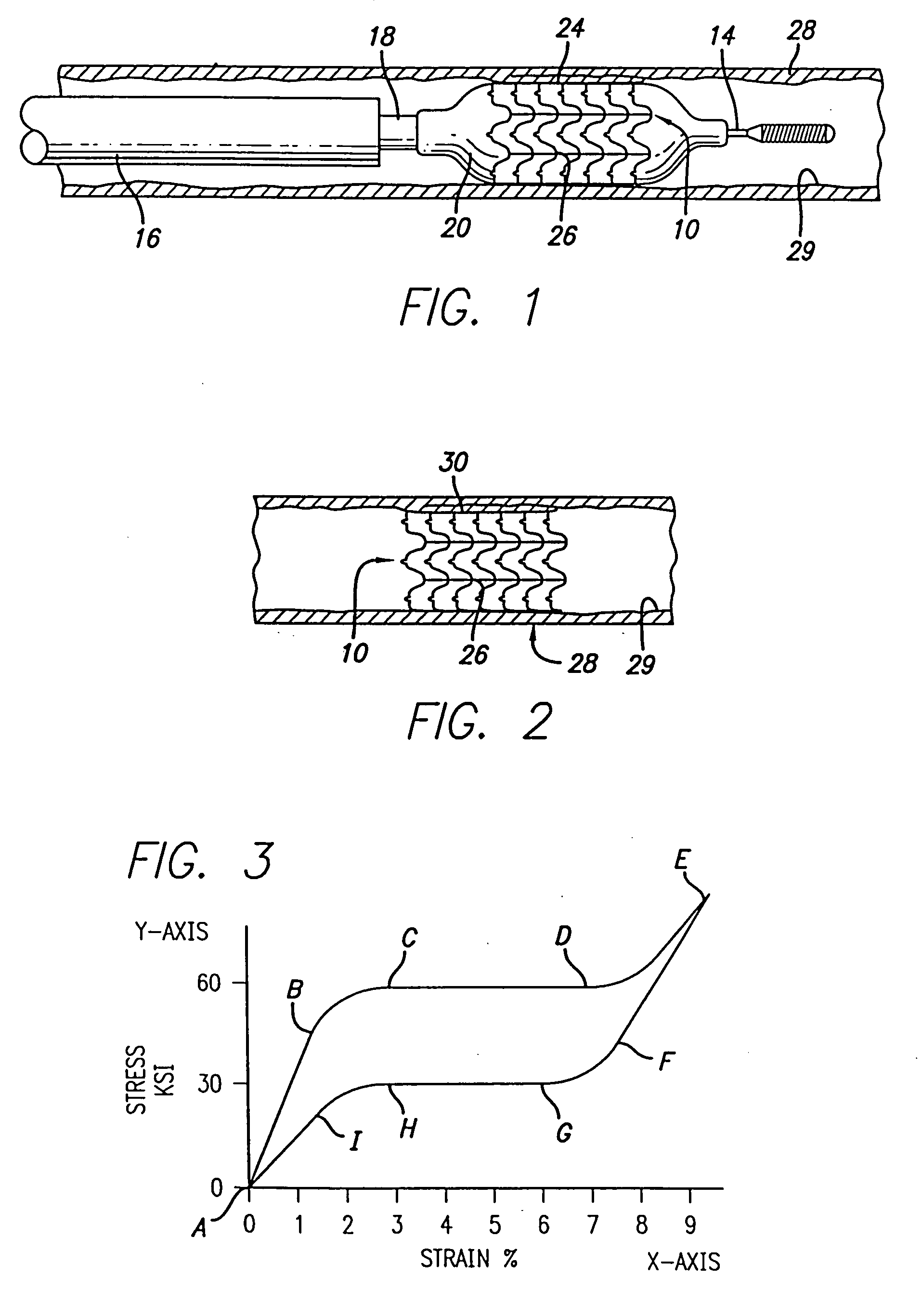
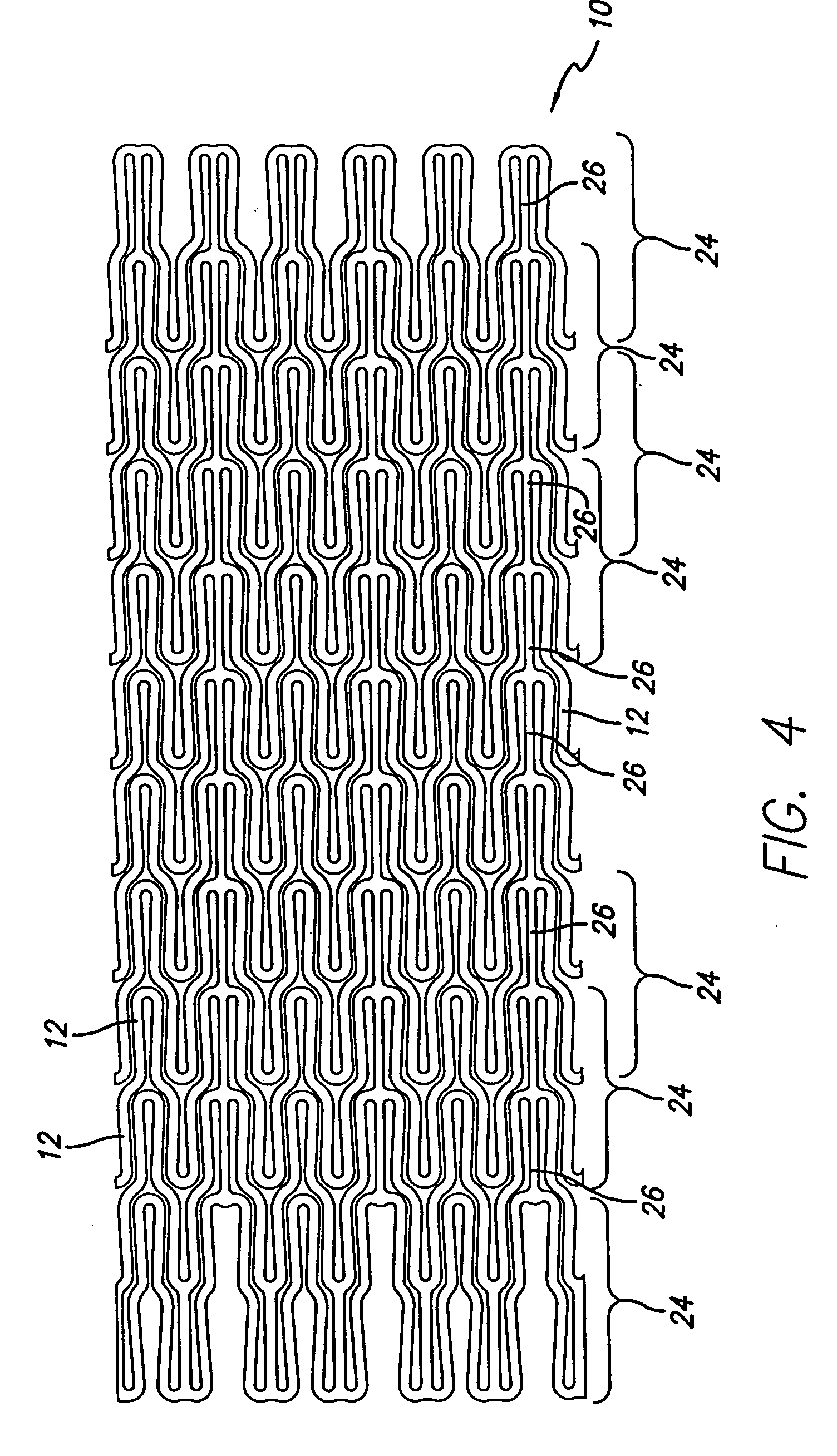
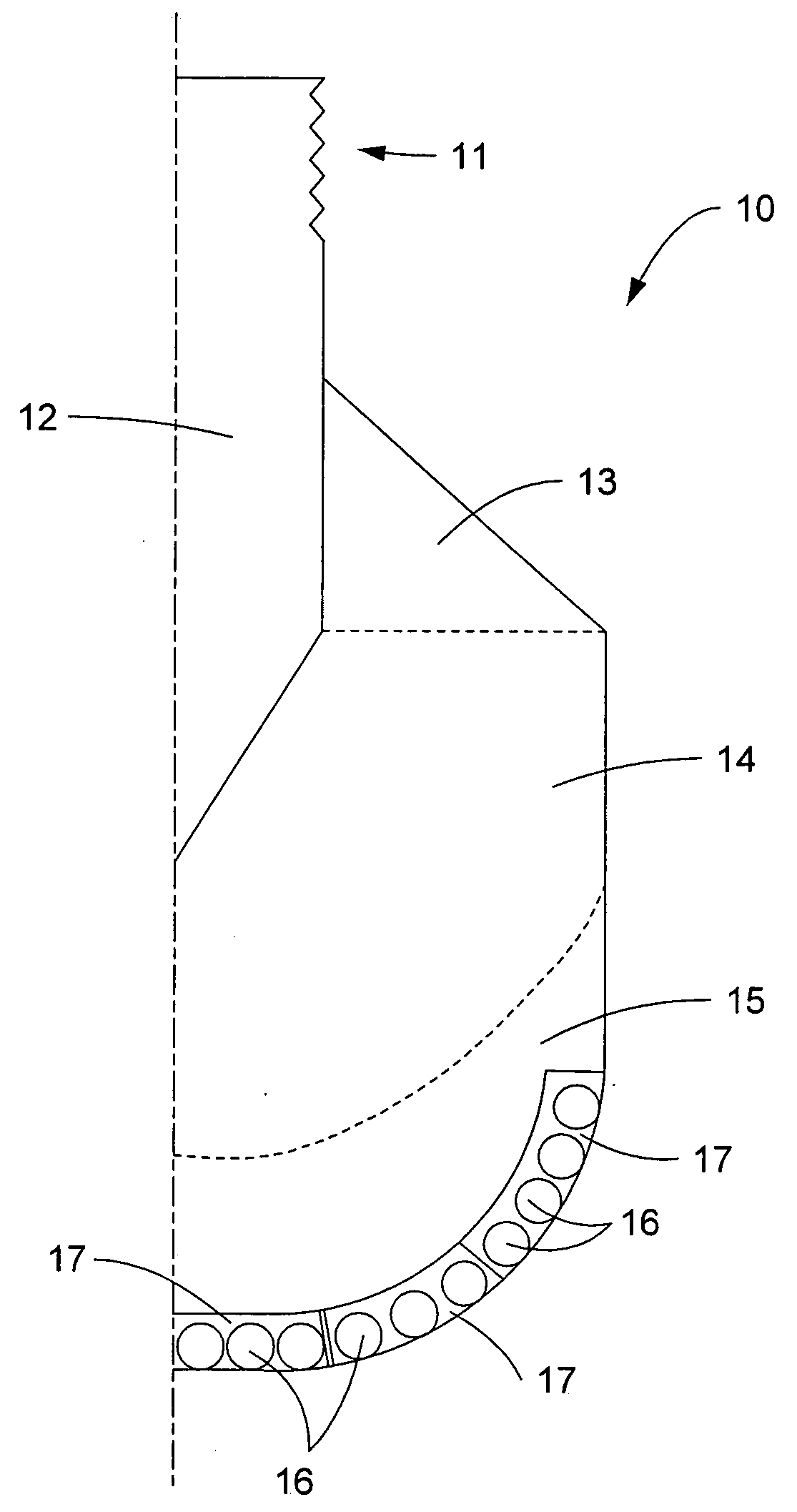
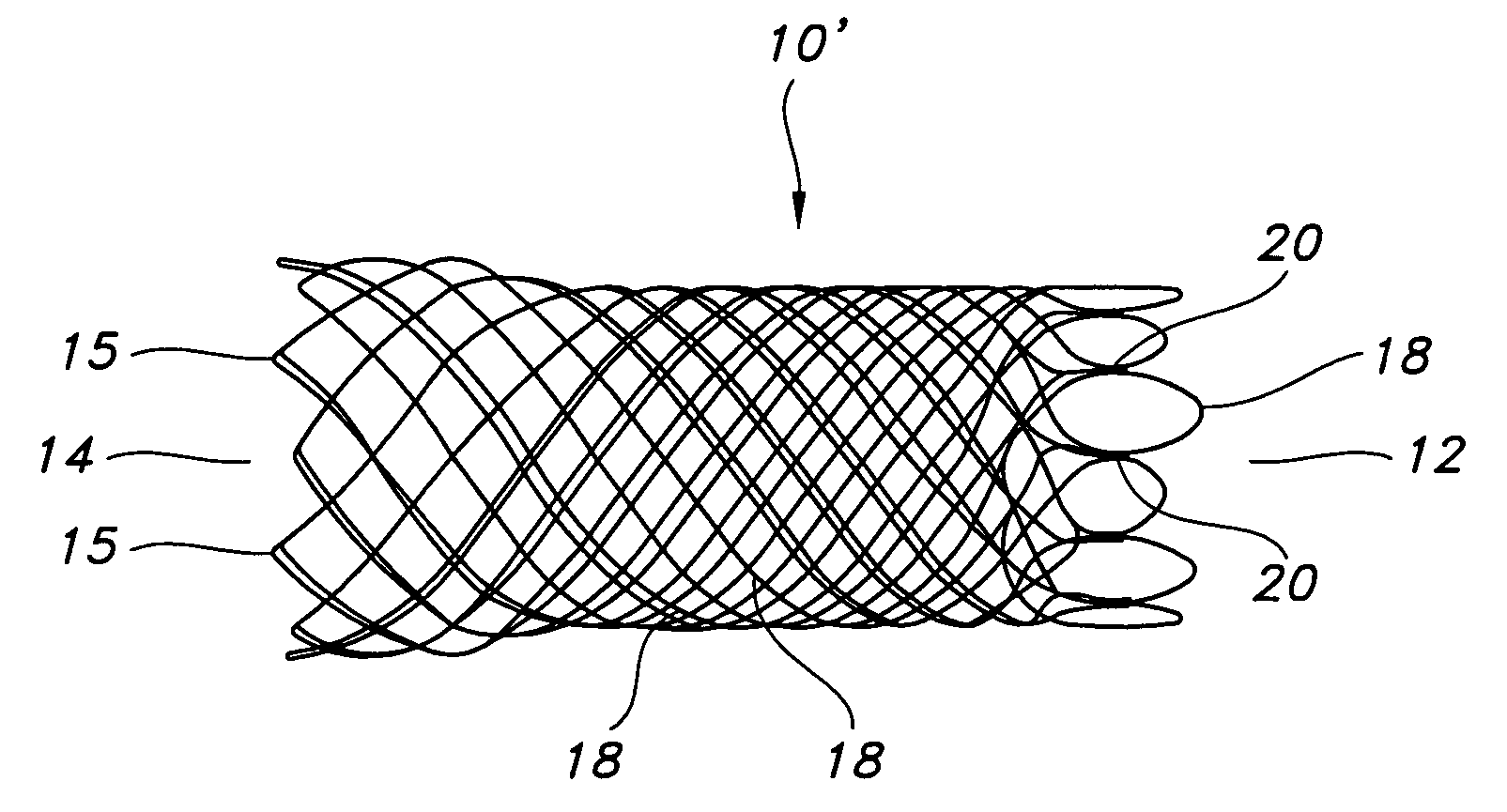
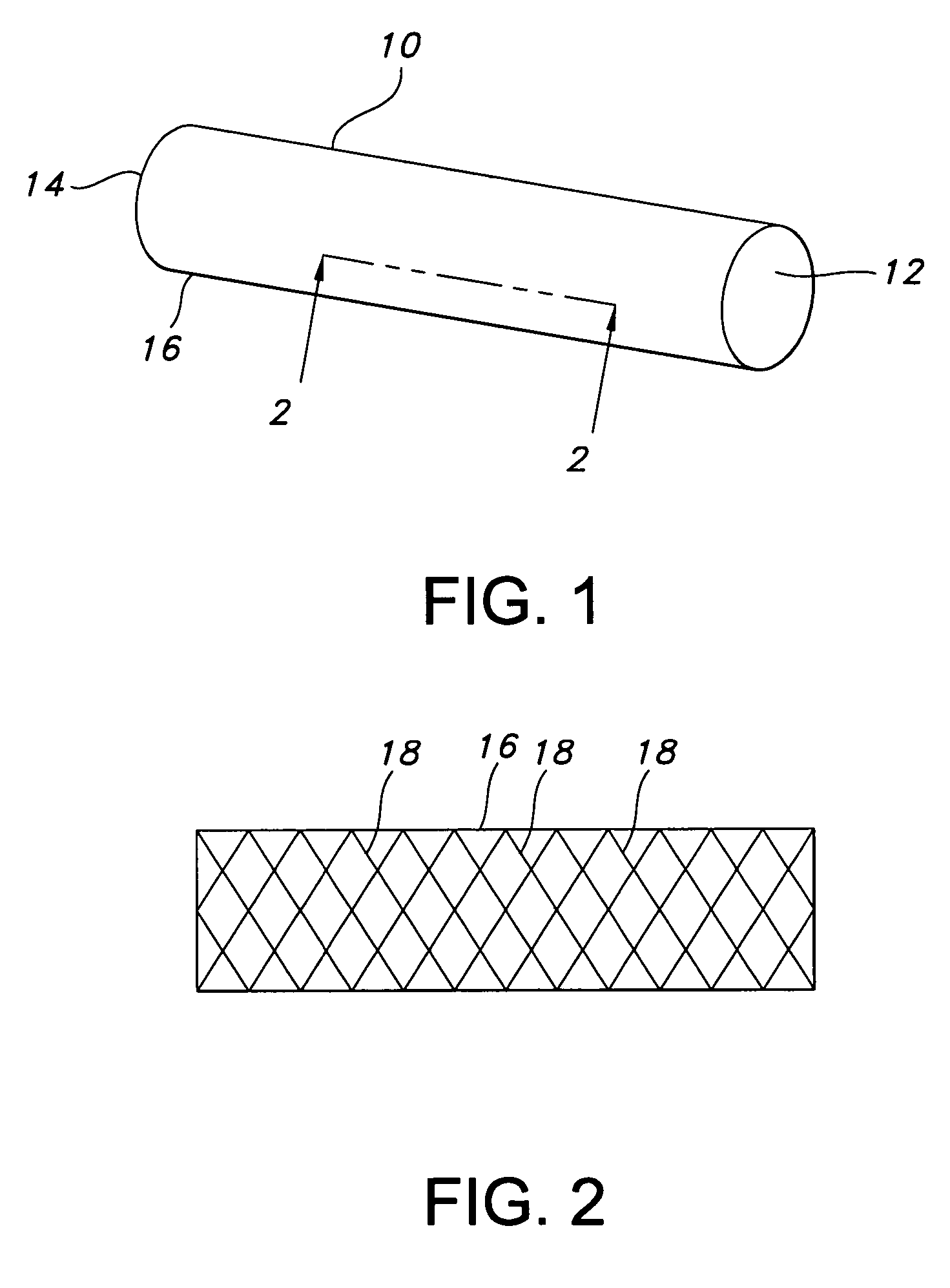
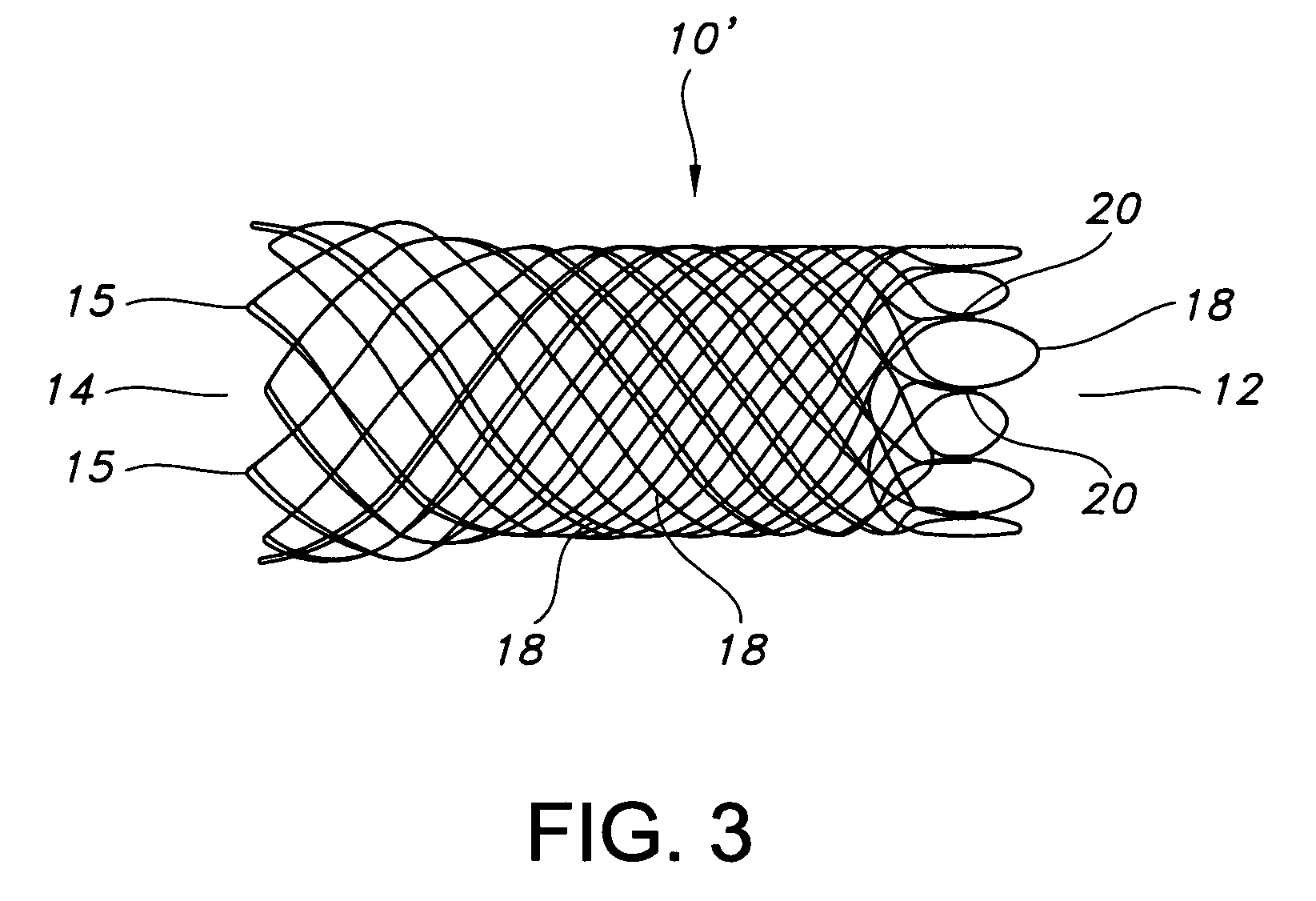
Atraumatic stent with reduced deployment force, method for making the same and method and apparatus for deploying and positioning the stent
An implantable stent includes a plurality of elongate wires braided to form a hollow tubular structure having a tubular wall to define an interior surface and an exterior surface and having opposed open first and second ends, wherein the opposed open first and second ends are atraumatic ends The atraumatic ends of the stent are desirably free of any loose wire ends. The wires include composite wires to enhance visibility of the wires to provide improved external imaging of the wires in the body. The elongate composite wires of the stent may be metallic wires having an outer metallic portion including a first metal, such as nitinol, and an inner metallic core portion including a second metal, which is a radiopaque material, such as gold, barium sulfate, ferritic particles, platinum, platinum-tungsten, palladium, platinum-iridium, rhodium, tantalum or combinations thereof.
Owner:BOSTON SCI SCIMED INC
Atomic layer deposition of tungsten material
InactiveCN101308794ASemiconductor/solid-state device manufacturingChemical vapor deposition coatingGas phaseNucleation
An implementing mode of the invention provides an improved technology for depositing materials containing tungsten. The technology utilizes an infusion technology and a gaseous phase deposition technology, such as atomic layer deposition (ALD), to provide tungsten-containing materials with obviously improved surface evenness and yield. In one implementing mode, a method for forming tungsten-containing materials on a substrate is provided. The method comprises deposing a substrate, which contains a bottom coating deposited thereon, in a technological chamber; exposing the substrate orderly in a precursor of tungsten and reducing gases so as to deposit a tungsten nucleation layer on the bottom coating, during the ALD technology; and depositing a tungsten block layer on the tungsten nucleation layer. The invention is characterized in that the reducing gases comprise a hydrogen gas / hydride flow ratio of 40:1, 100:1, 500:1, 800: 1, 1000:1 or more, and comprise hydride such as diborane, silicane or silicoethane.
Owner:APPLIED MATERIALS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com