Patents
Literature
178 results about "Ionic radius" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
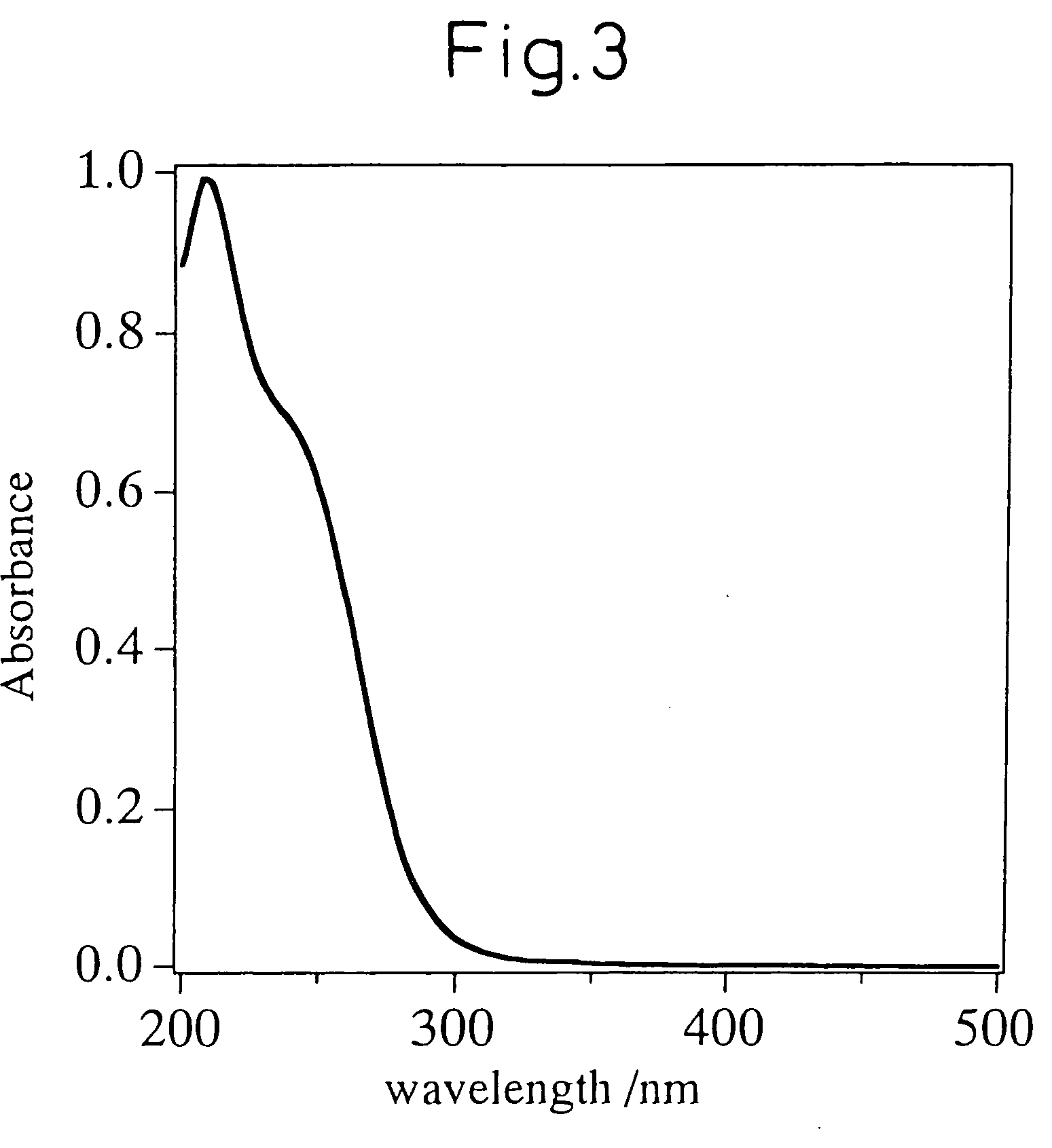
Ionic radius, rᵢₒₙ, is the radius of an atom's ion in ionic crystals structure. Although neither atoms nor ions have sharp boundaries, they are sometimes treated as if they were hard spheres with radii such that the sum of ionic radii of the cation and anion gives the distance between the ions in a crystal lattice. Ionic radii are typically given in units of either picometers (pm) or angstroms (Å), with 1 Å = 100 pm. Typical values range from 30 pm (0.3 Å) to over 200 pm (2 Å).
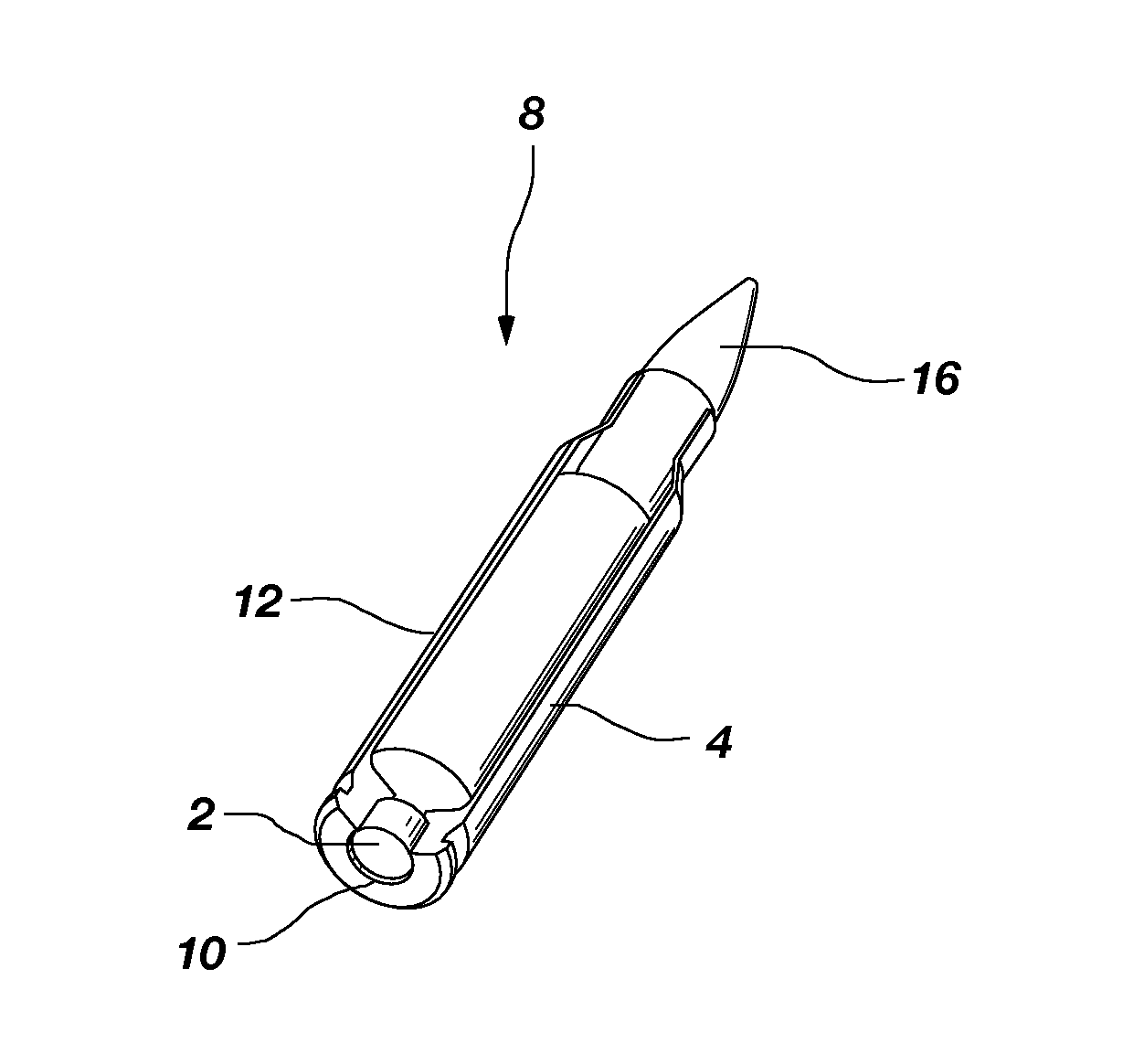
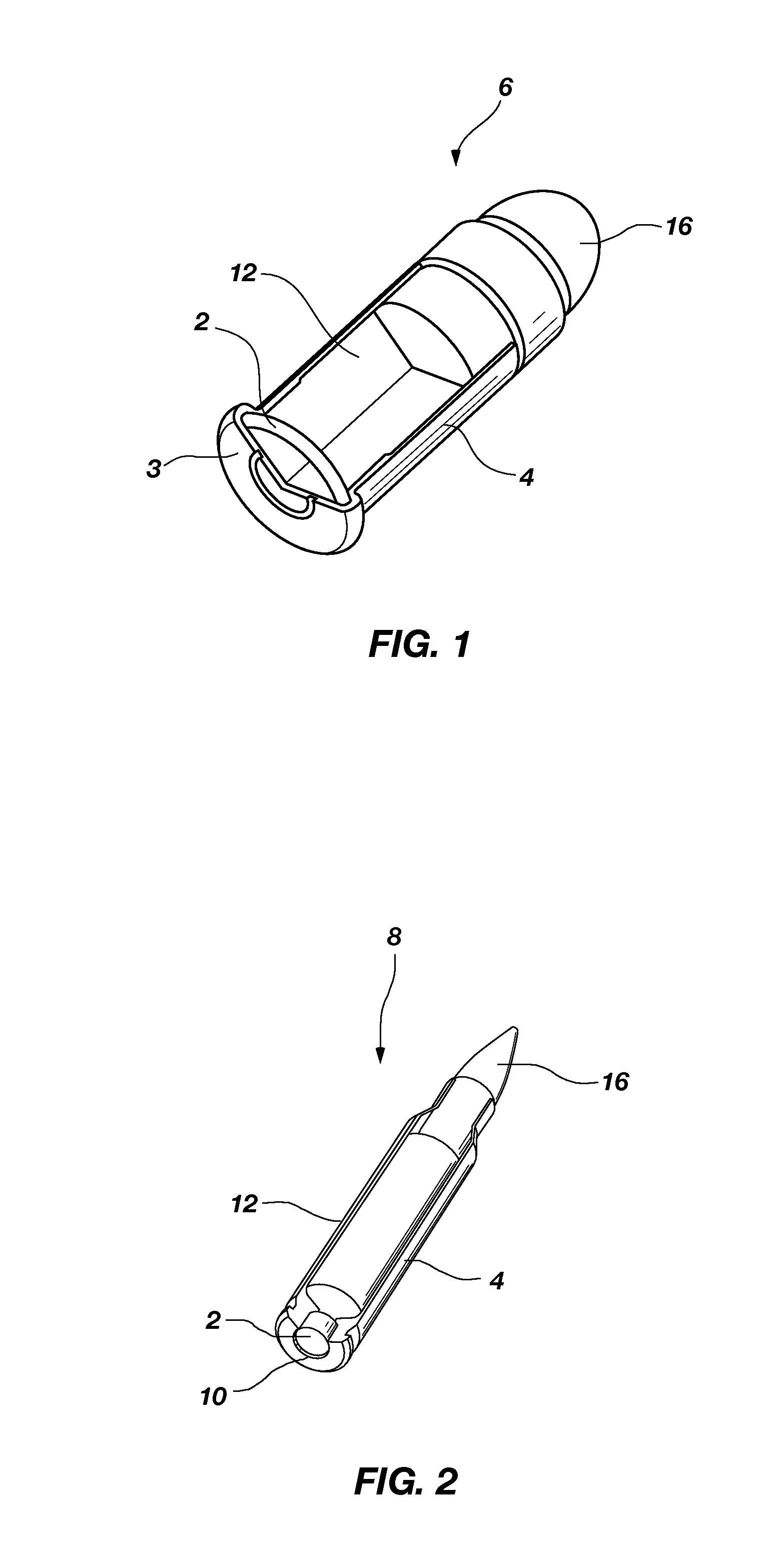
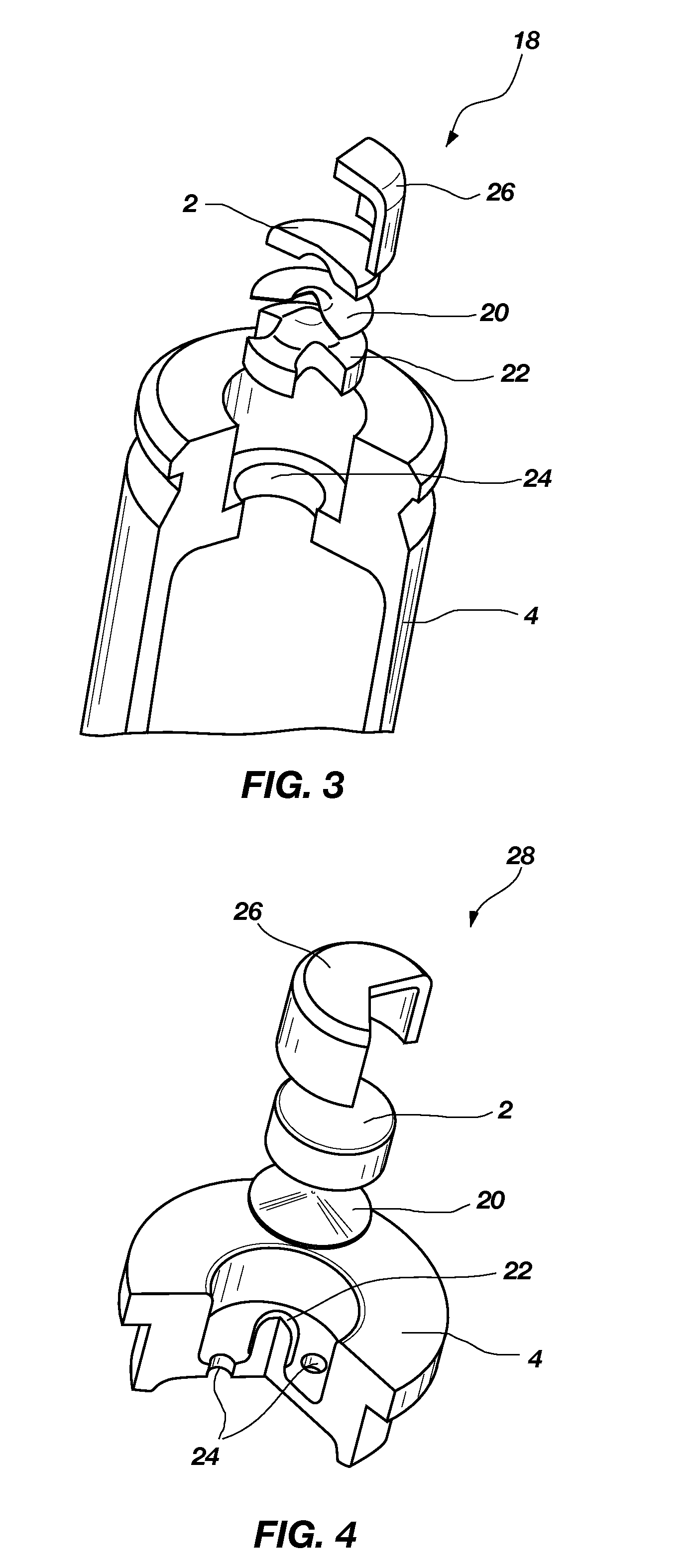
Nontoxic, noncorrosive phosphorus-based primer compositions and an ordnance element including the same
A primer composition that includes red phosphorus having an acid scavenger and a polymer thereon. The primer composition includes at least one other component that is substantially free of lead. The other component is at least one oxidizer, or at least one oxidizer and at least one of at least one secondary explosive composition and at least one energetic binder. The primer composition optionally includes at least one element having an ionic charge to ionic radius ratio of 4 or of 8, such as magnesium, zirconium, aluminum, silicon, titanium, tungsten, alloys thereof, and combinations thereof. The red phosphorus and the at least one oxidizer are present in the primer composition at approximately stoichiometric amounts. An ordnance element including the primer composition is also disclosed.
Owner:NORTHROP GRUMMAN SYST CORP
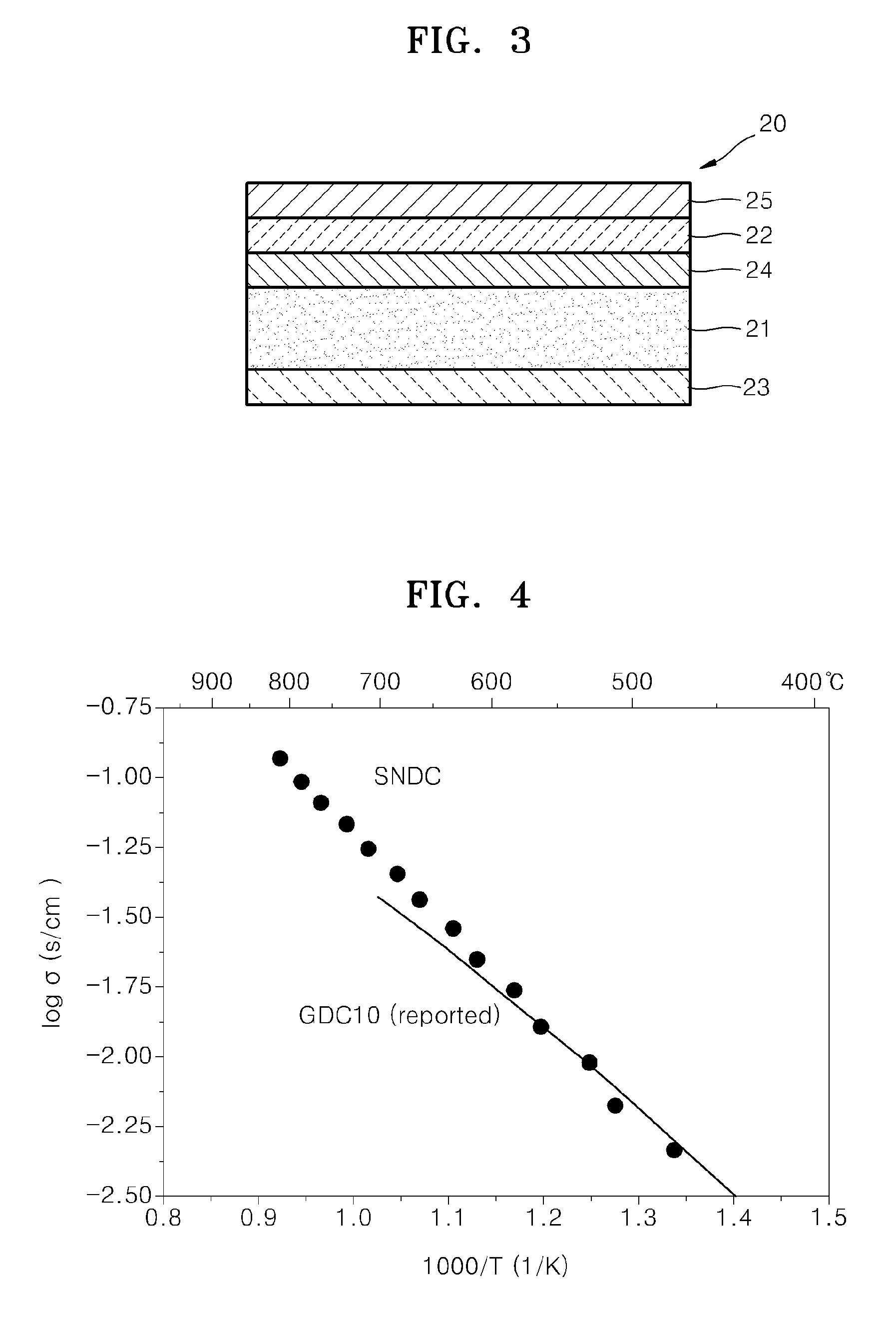
Garnet-type lithium ion-conducting oxide and all-solid-state lithium ion secondary battery containing the same
ActiveUS20110244337A1Improve lithium ion conductivitySmall rate of changeSolid electrolyte cellsElectrolytesAll solid stateIntensity normalization
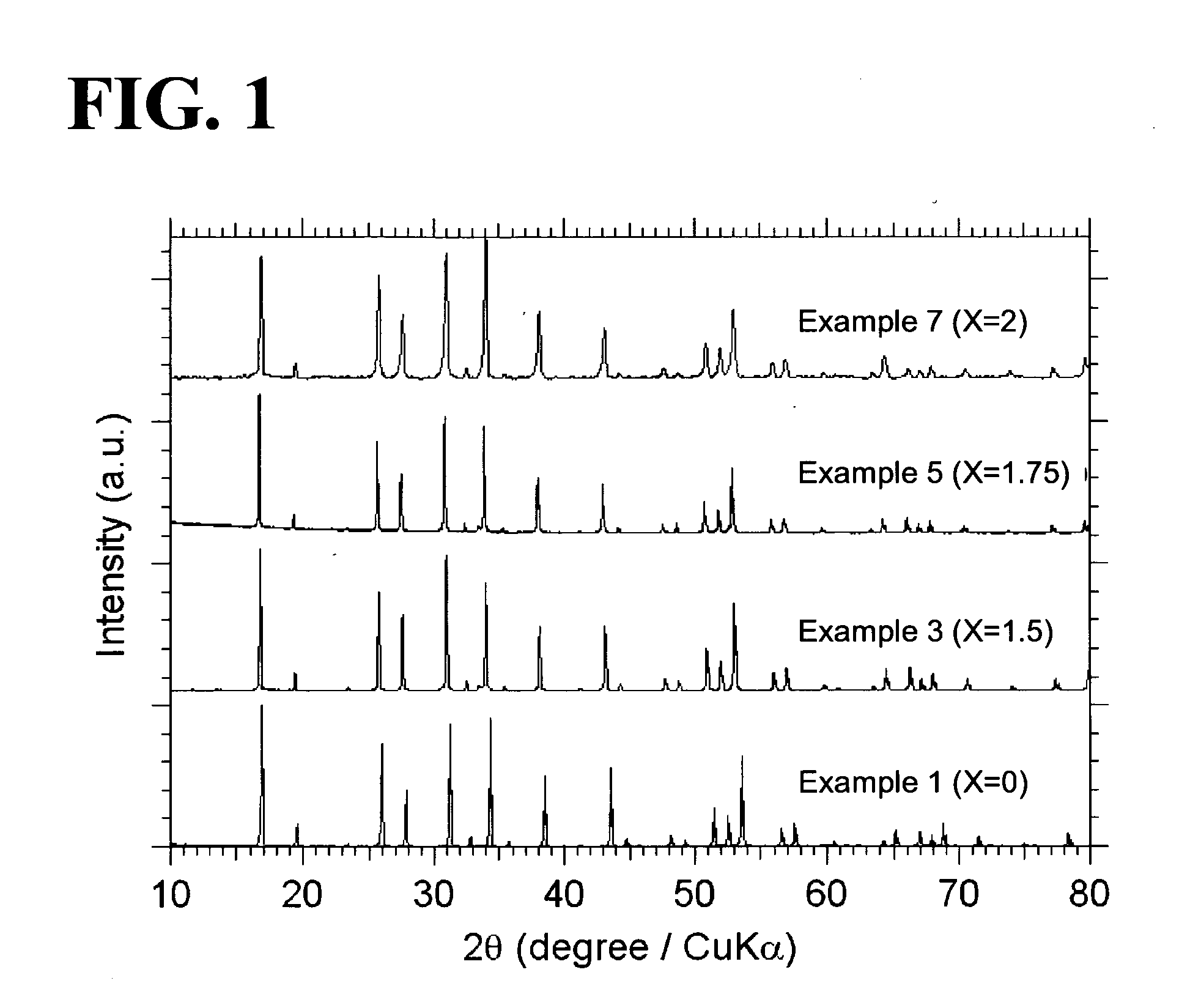
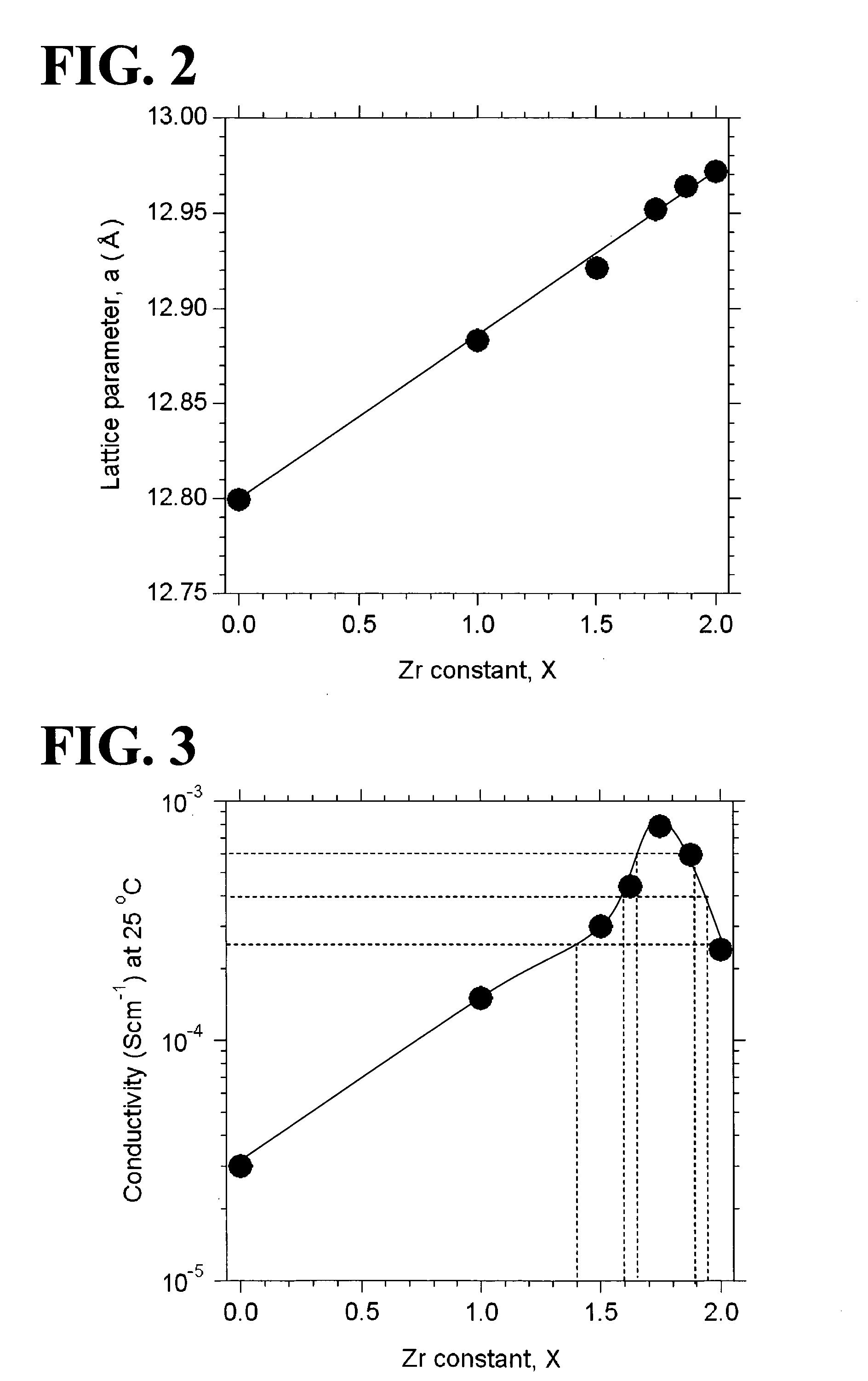
An all-solid-state lithium ion secondary battery containing a novel garnet-type oxide serving as a solid electrolyte. The garnet-type lithium ion-conducting oxide is one represented by the formula Li5+XLa3(ZrX, A2-X)O12, wherein A is at least one selected from the group consisting of Sc, Ti, V, Y, Nb, Hf, Ta, Al, Si, Ga, Ge, and Sn and X satisfies the inequality 1.4≦X<2, or is one obtained by substituting an element having an ionic radius different from that of Zr for Zr sites in an garnet-type lithium ion-conducting oxide represented by the formula Li7La3Zr2O12, wherein the normalized intensity of an X-ray diffraction (XRD) pattern with a diffraction peak, as normalized on the basis of the intensity of a diffraction peak, is 9.2 or more.
Owner:TOYOTA CENT RES & DEV LAB INC
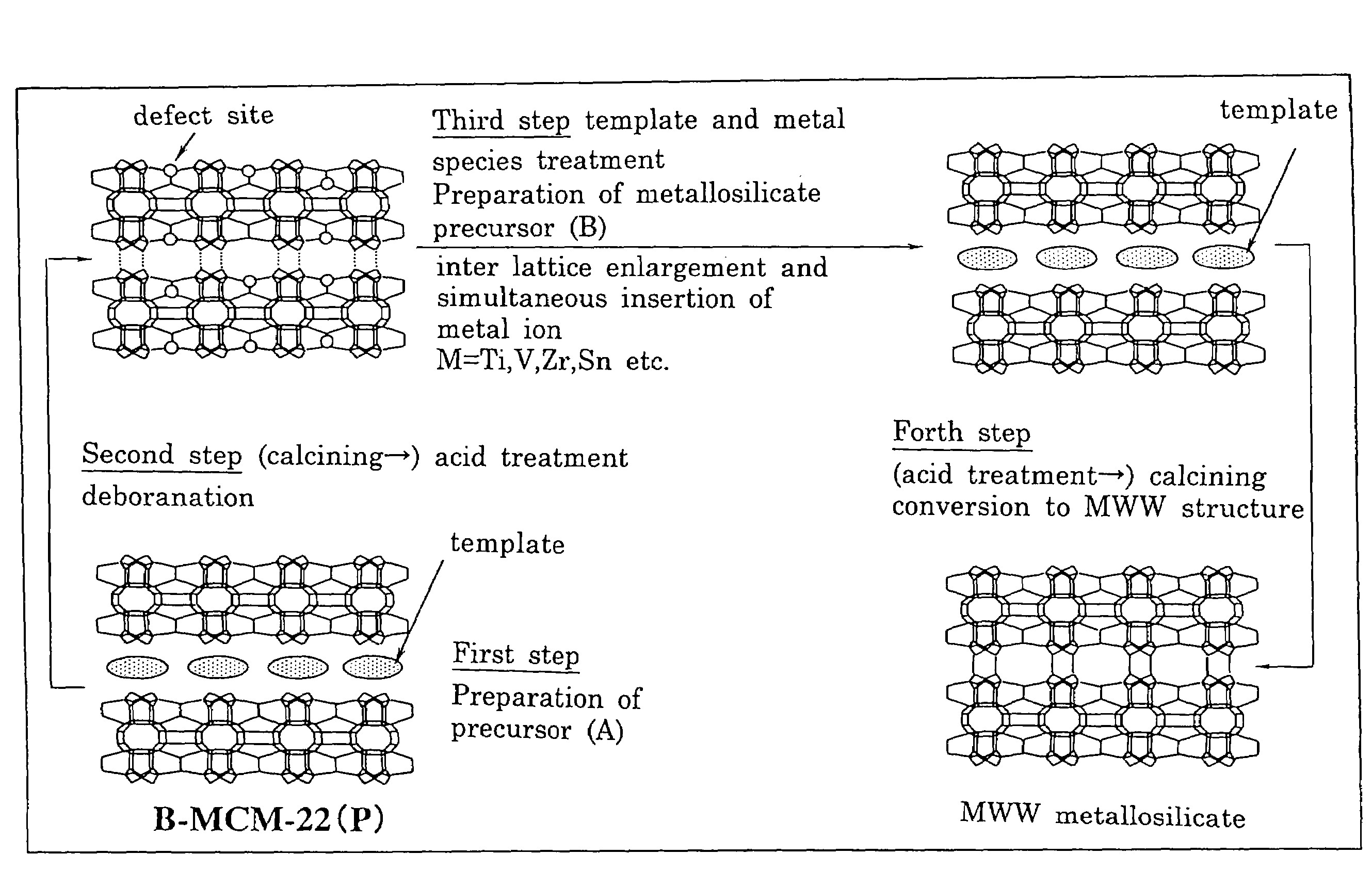
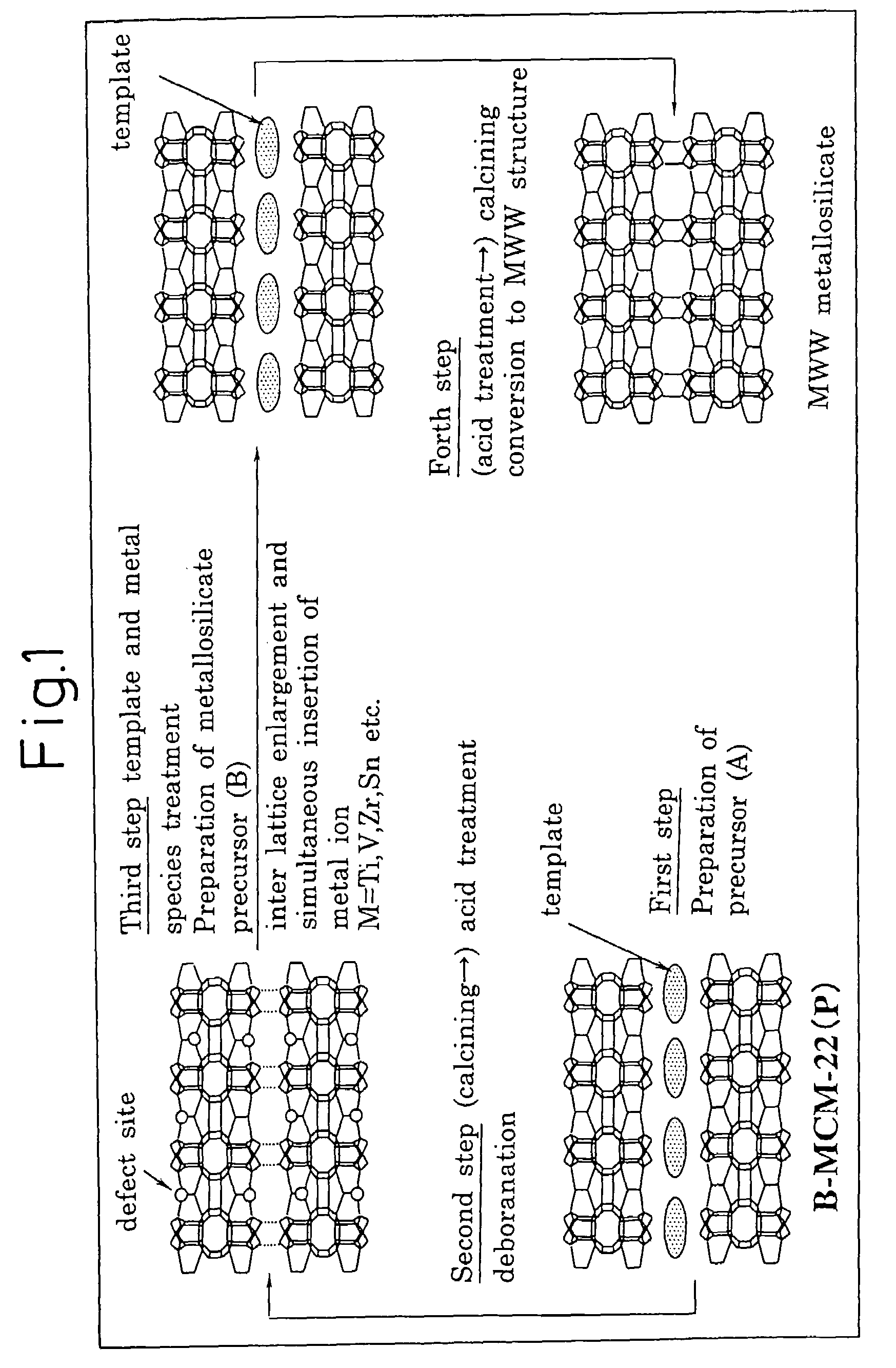
Mww type zeolite substance, precursor substance therefor, and process for producing these substances
ActiveUS20050158238A1Easy to synthesizeRaise the ratioAluminium compoundsMolecular sieve catalystsCompound (substance)Silicon
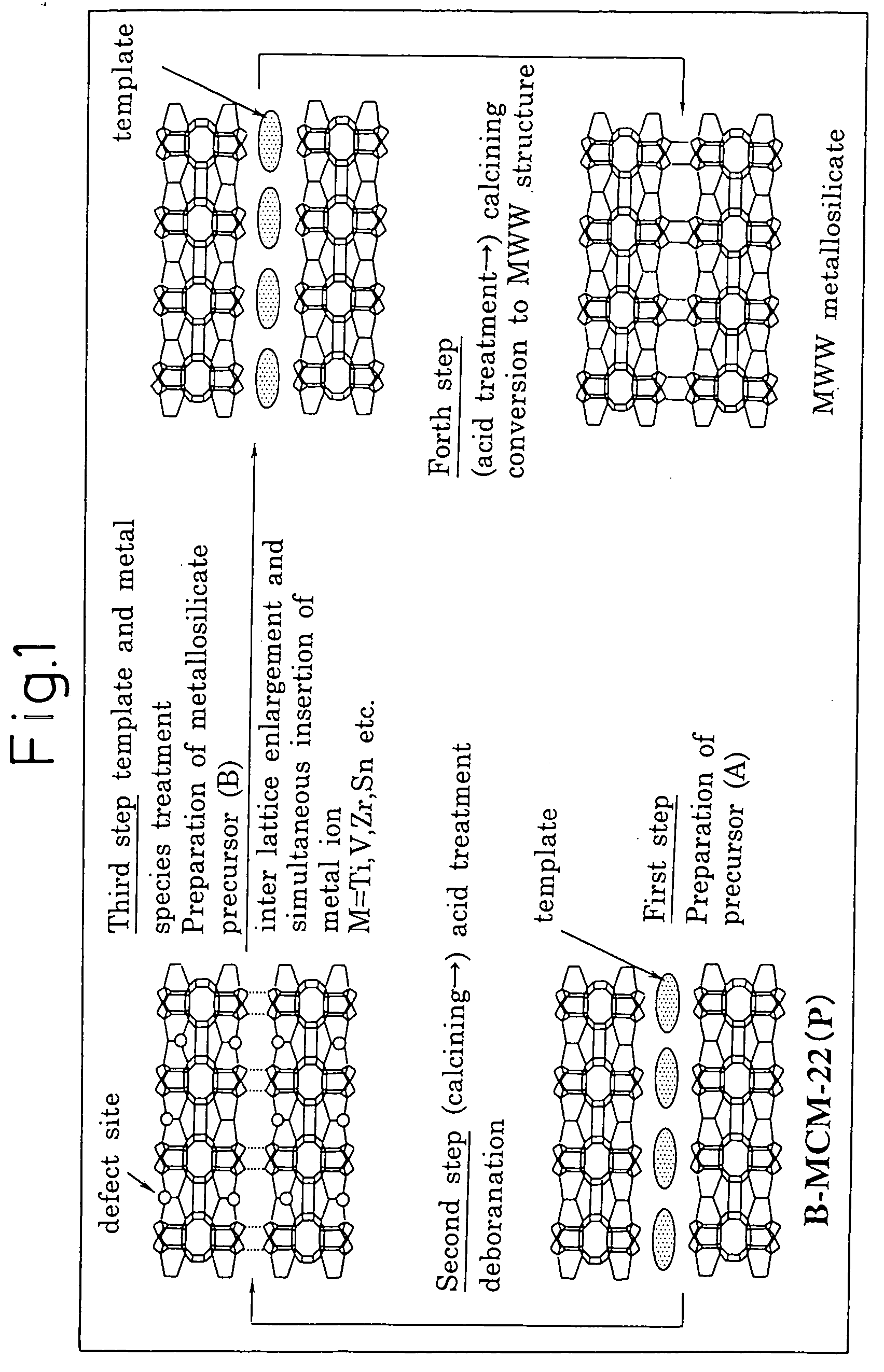
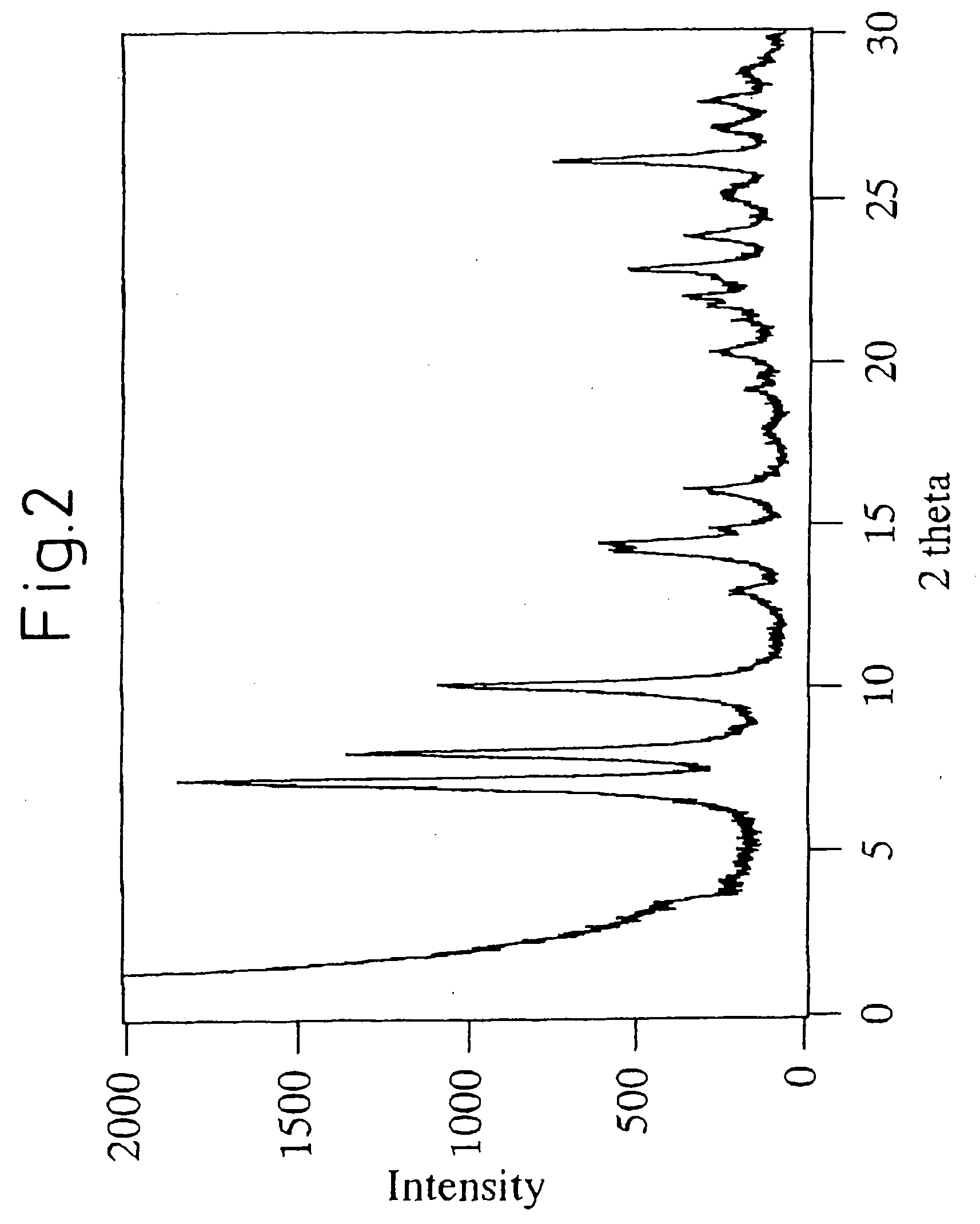
A process for easily synthesizing a zeolite substance containing an element having a large ionic radius in the framework at a high ratio. This process comprises the following first to fourth steps: First Step: a step of heating a mixture containing a template compound, a compound containing a Group 13 element of the periodic table, a silicon-containing compound and water to obtain a precursor (A); Second Step: a step of acid-treating the precursor (A) obtained in the first step; Third Step: a step of heating the acid-treated precursor (A) obtained in the second step together with a mixture containing a template compound and water to obtain a precursor (B); and Fourth Step: a step of calcining the precursor (B) obtained in the third step to obtain a zeolite substance.
Owner:SHOWA DENKO KK
Display device and method of manufacturing the same
InactiveUS6050870AVessels or leading-in conductors manufactureNon-linear opticsMolten stateDisplay device
In a display device in which glass substrates are pasted together and a display material is filled therebetween and a method of manufacturing the same, provided is a display device composed of soda glass chemically strengthened by substituting atoms having an ionic radius larger than that of sodium atoms for sodium atoms on the surfaces of the glass substrates, and a method of manufacturing a display device wherein a chemical strengthening is carried out by dipping a soda glass substrate or a cell for the display device manufactured by using the substrate in a solution or a molten solution of a salt including cations having an ionic radius larger than that of sodium atoms.
Owner:BEIJING METIS TECH SERVICE CENT LLP
Method of manufacturing an ion-exchanged glass article
ActiveUS20130061636A1Maintain stable propertiesLittle change in intensityBase layer manufactureMolten saltIon exchange
An ion-exchanged glass article manufacturing method includes an ion-exchange step of bringing a glass article with a composition containing Li into contact with a molten salt dissolved solution containing an alkali metal element having an ionic radius larger than an ionic radius of the Li contained in the glass article, thereby ion-exchanging the Li in the glass article with the alkali metal element in the molten salt dissolved solution. At least one kind of additive selected from the group consisting of NaF, KF, K3AlF6, Na2CO3, NaHCO3, K2CO3, KHCO3, Na2SO4, K2SO4, KAl(SO4)2, Na3PO4, and K3PO4 is added to the molten salt dissolved solution so that the ion-exchange step is carried out while the additive is in a solid state.
Owner:HOYA CORP
Light emissive ceramic laminate and method of making same
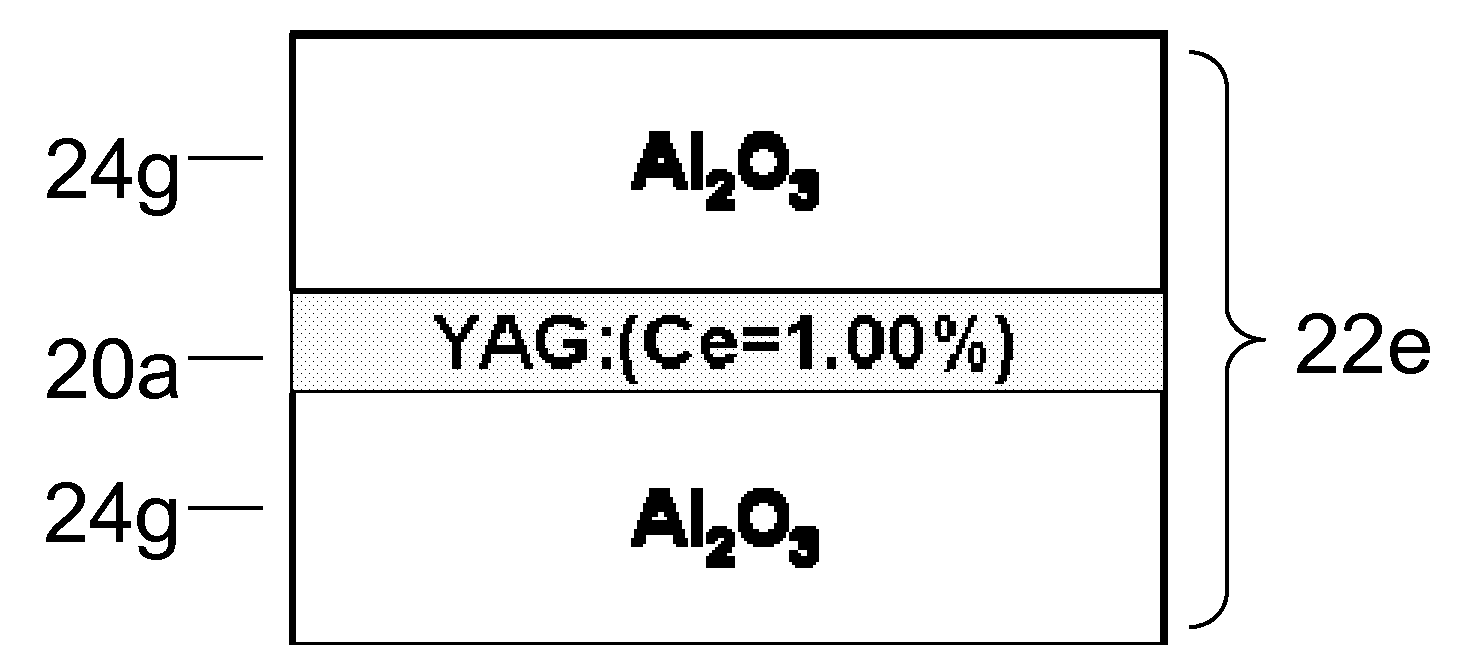
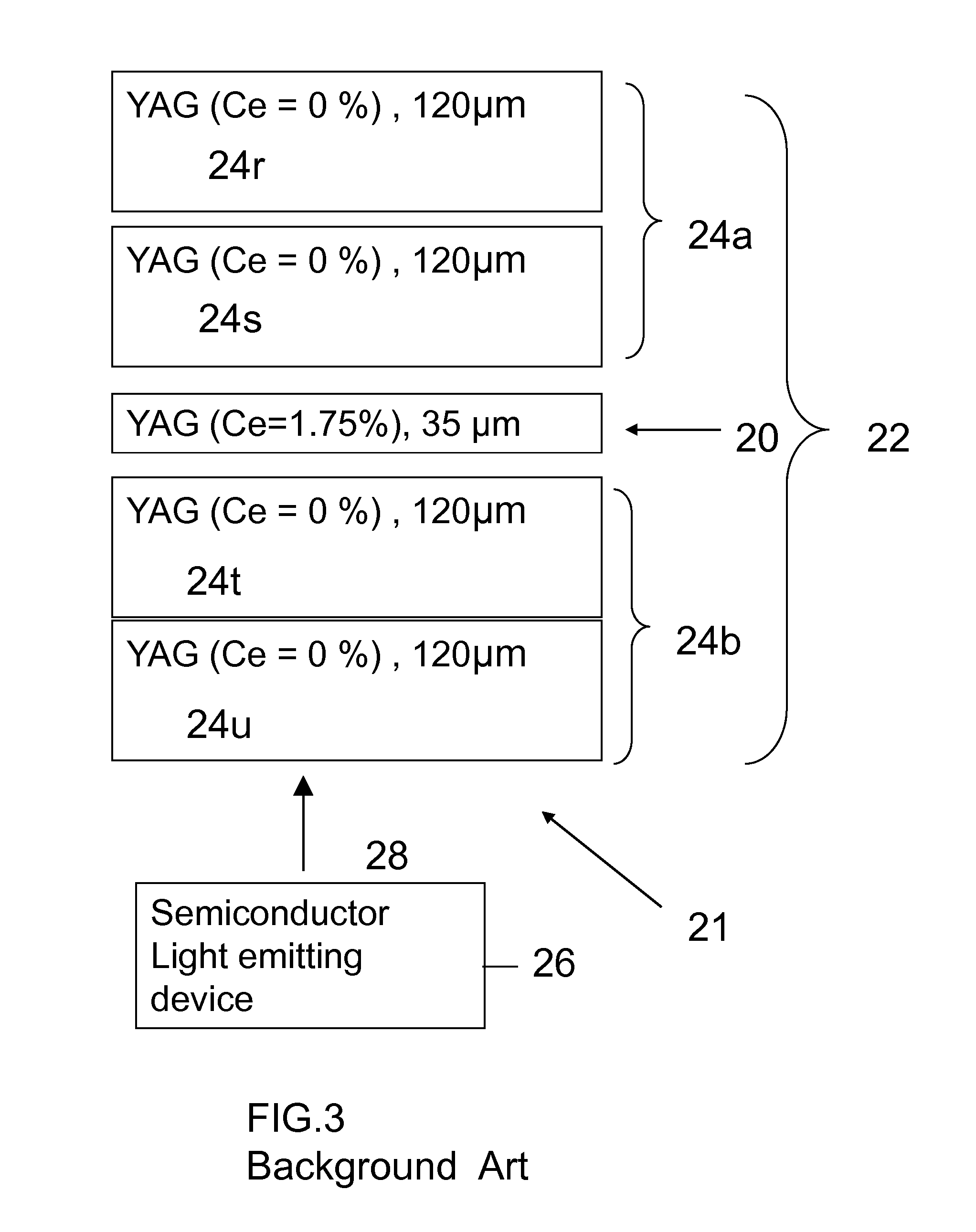
A laminated composite includes a wavelength-converting layer and a non-emissive blocking layer, wherein the emissive layer includes a garnet host material and an emissive guest material, and the non-emissive blocking layer includes a non-emissive blocking material. The metallic element constituting the non-emissive blocking material has an ionic radius which is less than about 80% of an ionic radius of an A cation element when the garnet or garnet-like host material is expressed as A3B5O12 and / or an element constituting the emissive guest material, and the non-emissive blocking layer is substantially free of the emissive guest material migrated through an interface between the emissive layer and the non-emissive blocking layer.
Owner:NITTO DENKO CORP
MWW type zeolite substance, precursor substance therefor, and process for producing these substances
ActiveUS7326401B2Easy to synthesizeRaise the ratioAluminium compoundsMolecular sieve catalystsSiliconPrecursor substance
A process for easily synthesizing a zeolite substance containing an element having a large ionic radius in the framework at a high ratio. This process comprises the following first to fourth steps:First Step:a step of heating a mixture containing a template compound, a compound containing a Group 13 element of the periodic table, a silicon-containing compound and water to obtain a precursor (A);Second Step:a step of acid-treating the precursor (A) obtained in the first step;Third Step:a step of heating the acid-treated precursor (A) obtained in the second step together with a mixture containing a template compound and water to obtain a precursor (B); andFourth Step:a step of calcining the precursor (B) obtained in the third step to obtain a zeolite substance.
Owner:SHOWA DENKO KK
Cathode material for a fuel cell, cathode including the cathode material, and a solid oxide fuel cell including the cathode material
A cathode material for a fuel cell, the cathode material including a first metal oxide having a perovskite crystal structure, and a second metal oxide including cerium and at least two lanthanide elements, the lanthanide elements having an average ionic radius of about 0.90 to about 1.02 Å.
Owner:SAMSUNG ELECTRO MECHANICS CO LTD
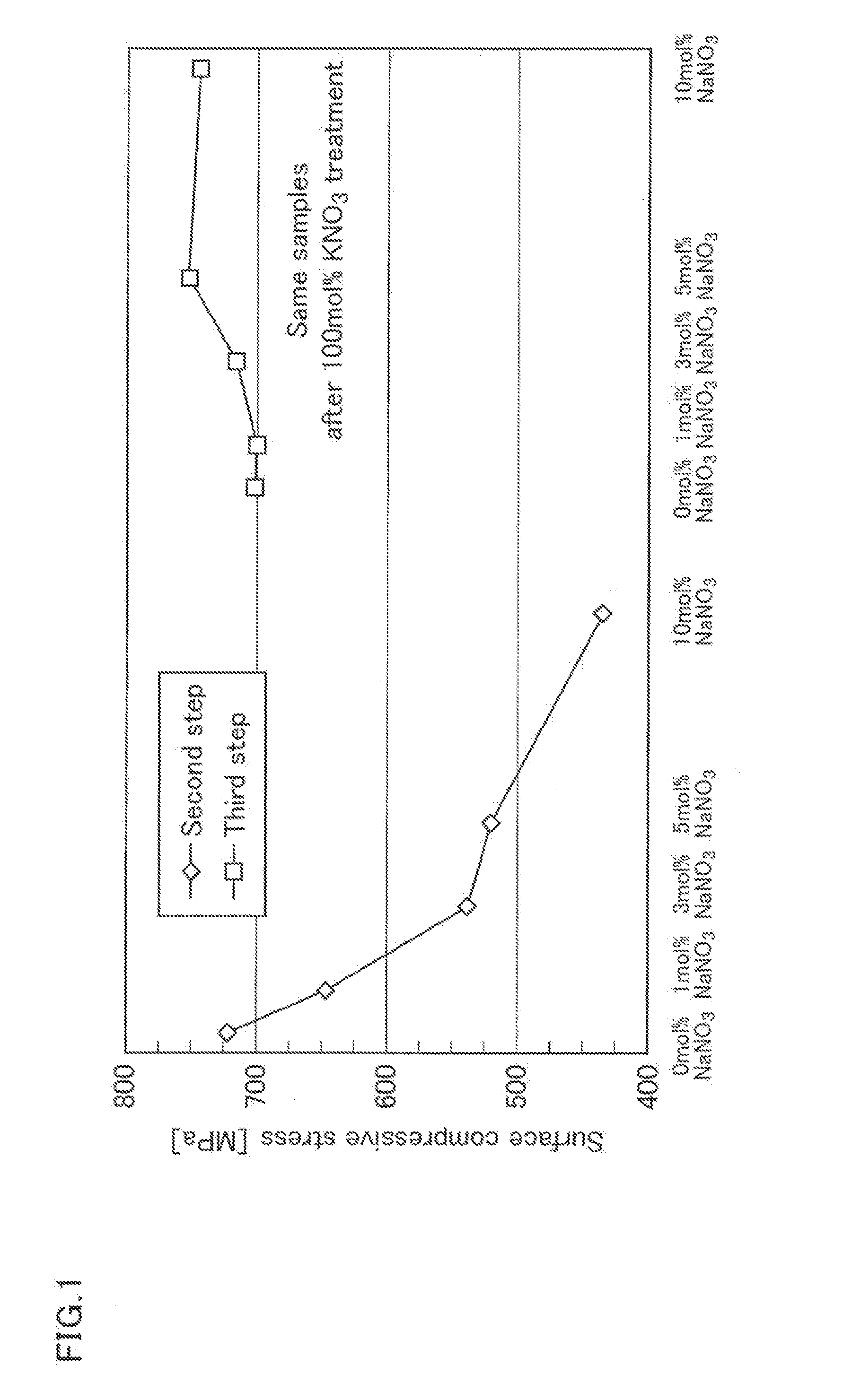
Method of manufacturing chemically strengthened glass plate
InactiveUS20130219966A1Increase the surface compressive stressEfficient productionIon exchangeGlass sheet
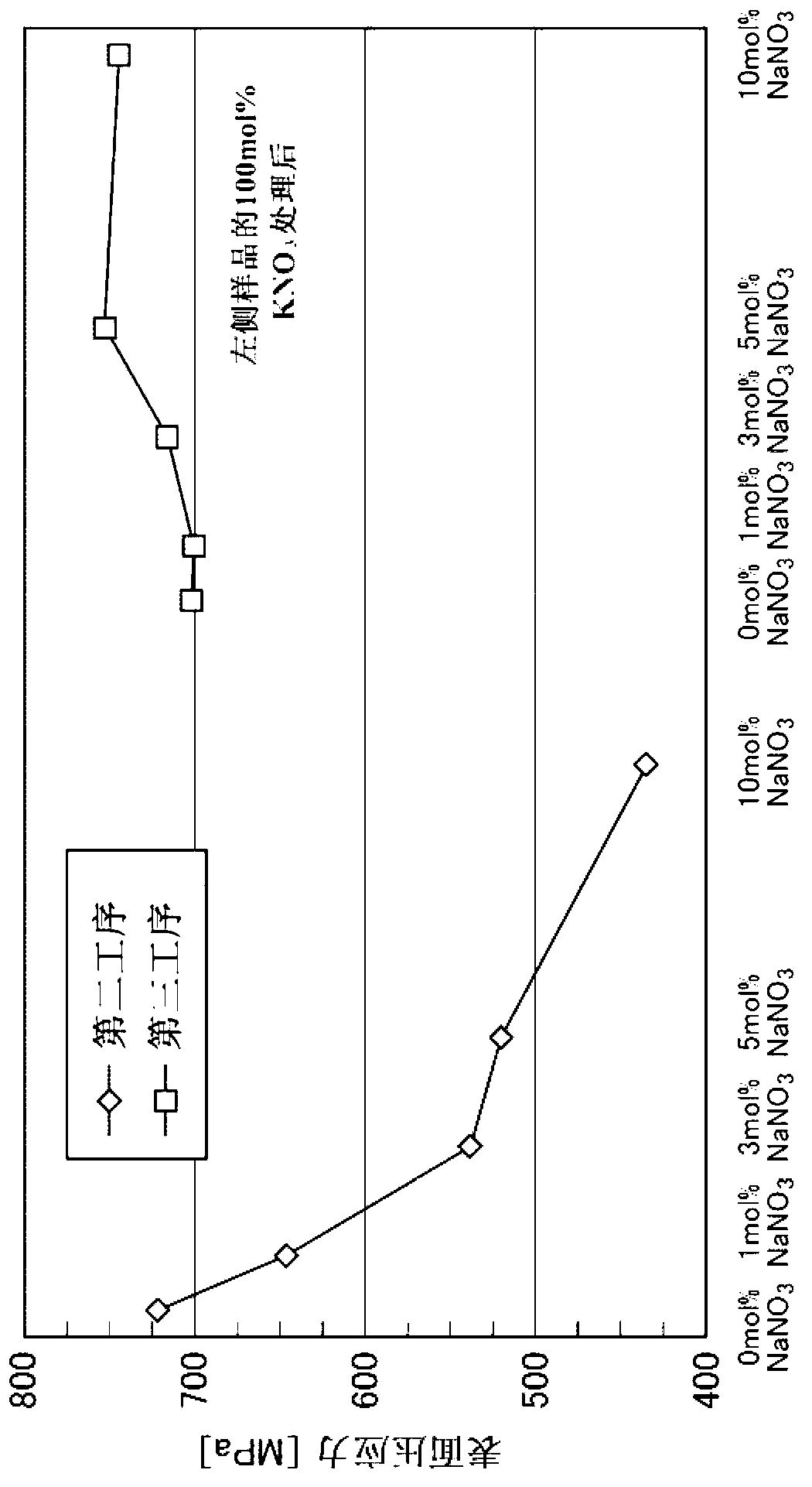
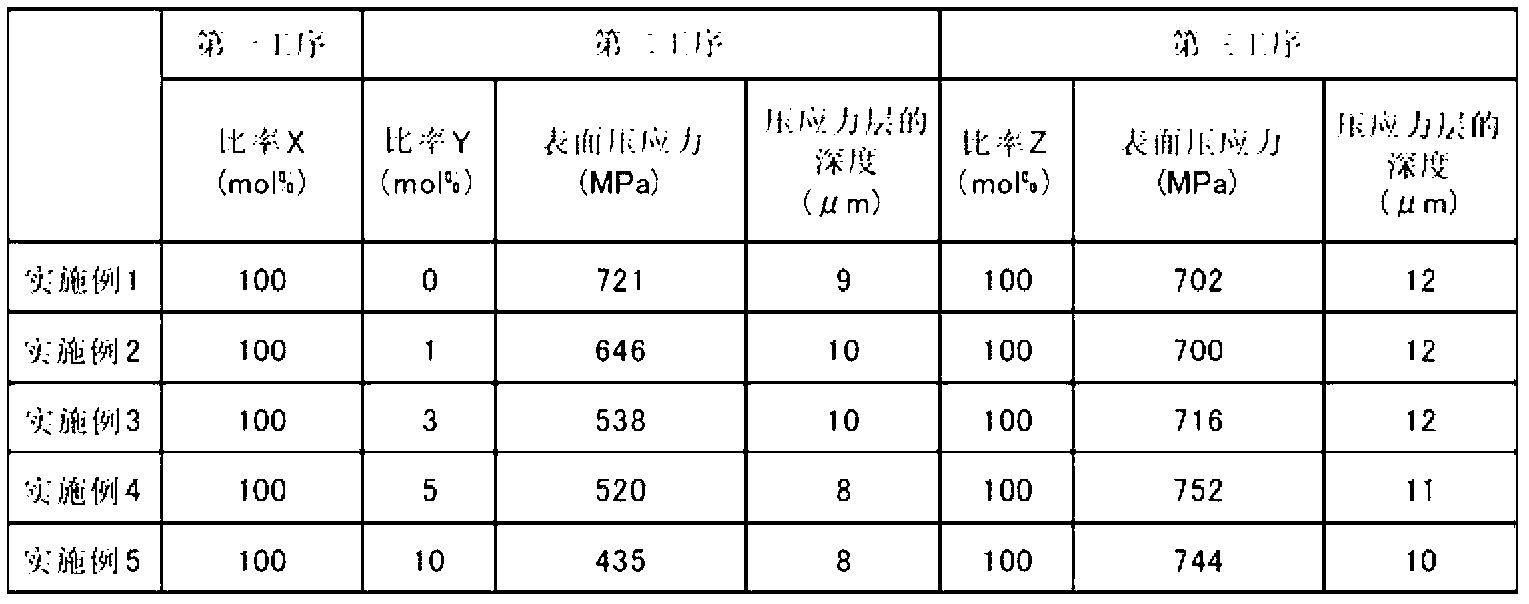
[Subject]An object of the present invention is to provide a method for manufacturing a chemically strengthened glass plate having a high surface compressive stress with high efficiency using a soda-lime glass, the composition of which is not particularly suited for chemical strengthening.[Solution]The present invention provides a method of manufacturing a chemically strengthened glass plate by ion-exchanging a glass base plate to replace alkali metal ions A that are the main alkali metal ion component of the glass base plate with alkali metal ions B having a larger ionic radius than the alkali metal ions A at a surface of the glass base plate,the unexchanged glass base plate made of a soda-lime glass,the method including:a first step of contacting the glass base plate with a first salt containing the alkali metal ions A, the first salt containing the alkali metal ions A at a ratio X, as expressed as a molar percentage of total alkali metal ions, of 90 to 100 mol %;a second step of contacting the glass plate with a second salt containing the alkali metal ions B after the first step, the second salt containing the alkali metal ions A at a ratio Y, as expressed as a molar percentage of the total alkali metal ions, of 0 to 10 mol %; anda third step of contacting the glass plate with a third salt containing the alkali metal ions B after the second step, the third salt containing the alkali metal ions B at a ratio Z, as expressed as a molar percentage of the total alkali metal ions, of 98 to 100 mol %.
Owner:CENT GLASS CO LTD
Bismuth ferrite-based leadless piezoelectric ceramic with high Curie temperature and preparation method thereof
The invention discloses a bismuth ferrite-based leadless piezoelectric ceramic with high Curie temperature and a preparation method thereof. The general formula for the piezoelectric ceramic is (1-x-y)(BizM1-z)t(FeuM'1-u)O3-xBaTiO3-yBiMnO3, wherein, M is a trivalent metallic element with large ionic radius, M' is a trivalent metallic element with small ionic radius, and x, y, u, t and z represent mole content in a ceramic system and satisfy the following relations: 0<=x<=1.0, 0<=y<=0.1, 0<z<1, 0.85<t<1.2, 0<u<1 and x+y<1. The piezoelectric ceramic is prepared by a conventional ceramic preparation method through selection of proper technological parameters. The piezoelectric constant d33 of the ceramic can reach 140 pC / N, Curie temperature can reach 490 DEG C, and Kt is more than 0.50. The preparation method has the advantages of a simple and stable process, and obtained leadless piezoelectric ceramic has excellent performance and is suitable for being used under high temperature.
Owner:GUILIN UNIV OF ELECTRONIC TECH
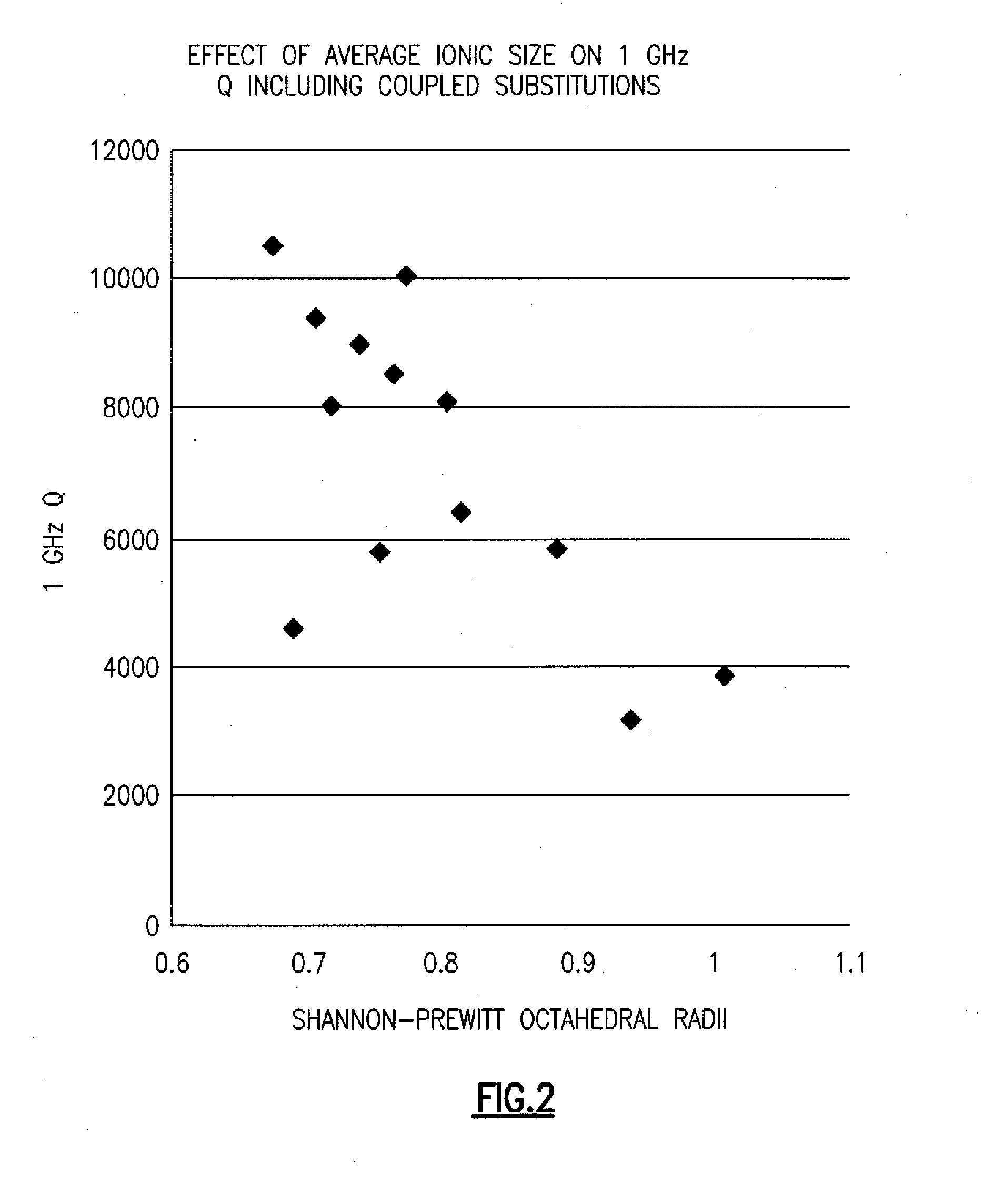
Novel enhanced high q material compositions and methods of preparing same
A framework for developing high quality factor (Q) material for electronic applications in the radio frequency range is provided. In one implementation, ceramic materials having a tungsten bronze crystal structure is modified by substituting one or more elements at one or more lattice sites on the crystal structure. The substitute elements are selected based on the ionic radius and other factors. In other implementations, the modified ceramic material is prepared in combination with compositions such as rutile or a perovskite to form a orthorhombic hybrid of perovskite and tetragonal tungsten bronze.
Owner:ALLUMAX TTI LLC
Cathode material for a fuel cell, cathode including the cathode material, and a solid oxide fuel cell including the cathode material
The invention relates to a cathode material for a fuel cell, a cathode including the cathode material, and a solid oxide fuel cell including the cathode material. The cathode material for the fuel cell includes a metal oxide having a perovskite crystal structure at a metal defected position, and a metal oxide including at least two mixed lanthanide elements based on a cerium dioxide, wherein the lanthanide elements have an average ionic radius of about 0.90 to about 1.02.
Owner:SAMSUNG ELECTRO MECHANICS CO LTD
Multi-element in-situ co-doped ternary material precursor as well as preparation method and application thereof
The invention discloses a multi-element in-situ co-doped ternary material precursor as well as a preparation method and an application thereof. The chemical formula of the precursor is (NixCoyMnz)(1-a-c)MaNc(OH)(2+k), wherein x is larger than or equal to 1 / 3 and smaller than or equal to 0.9, y is larger than 0 and smaller than or equal to1 / 3, z is larger than 0 and smaller than or equal to 0.4, the sum of x, y and z is 1, a is larger than or equal to 0.0001 and smaller than or equal to 0.01, and c is larger than or equal to 0.0001 and smaller than or equal to 0.01; radius of a doped ion M is close to that of the lithium ion, and M is selected from one or more of Mg<2+>, Zn<2+>, Zr<4+>, Nb<5+>, Ta<4+>, In<3+>, Sc<3+>, Y<3+>, Ce<4+> and Gd<3+>; radius of a doped ion N is close to that of metal ions Mn and Co in the ternary material, and N is selected from one or more of Al<3+>, Ti<4+>, Ge<4+>, W<6+> and V<5+>. In the preparation process of the ternary material precursor, two kinds metalions with different radii are introduced in situ, so that the doped metal ions are uniformly distributed in a precursor phase, and uniform mixing on the atomic grade is realized. The two kinds of metal ions with different radii are doped in different positions, cell parameters have coordinated variation, so that not only can a lithium ion transmission channel be expanded, but also good lattice structure of the ternary material can be kept, and the ternary material with excellent electrochemical performance is obtained.
Owner:圣戈莱(北京)科技有限公司
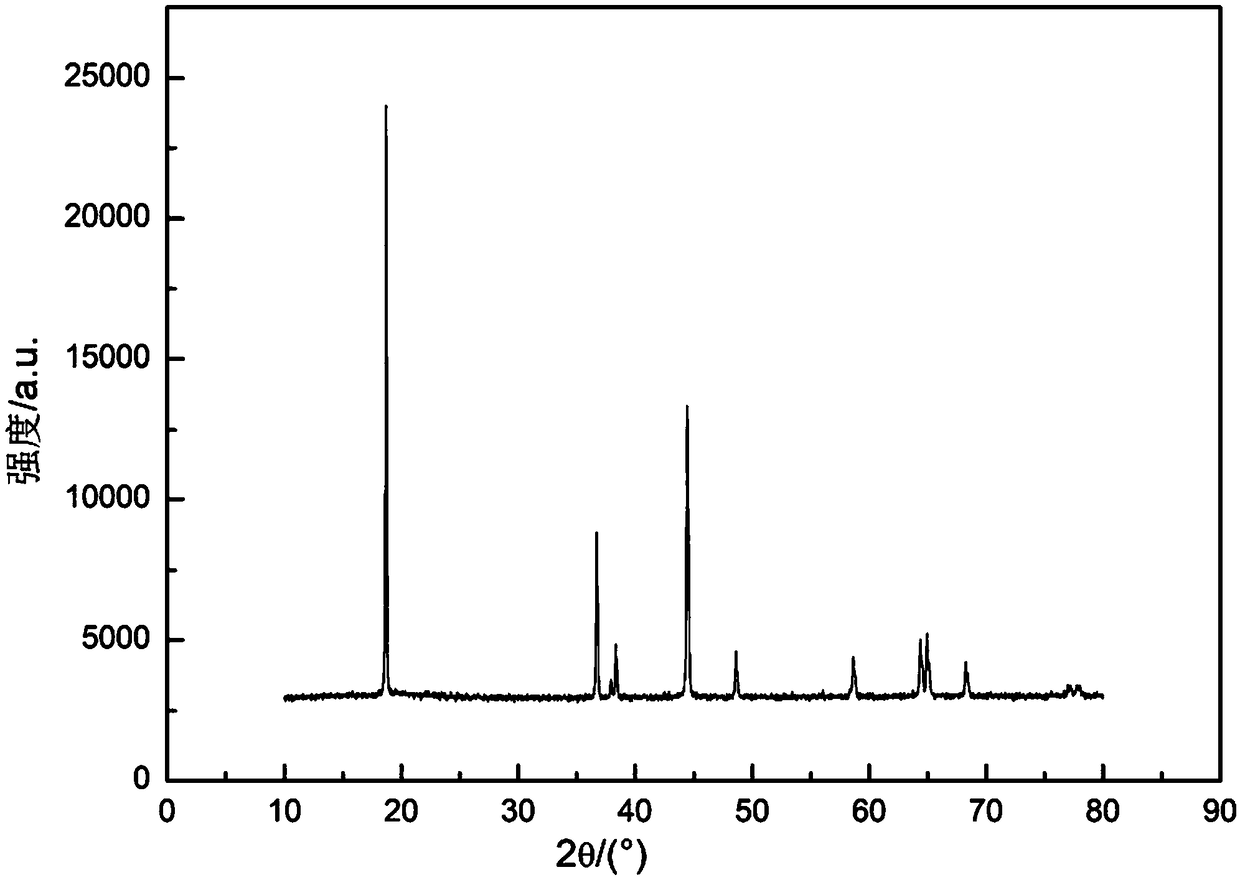
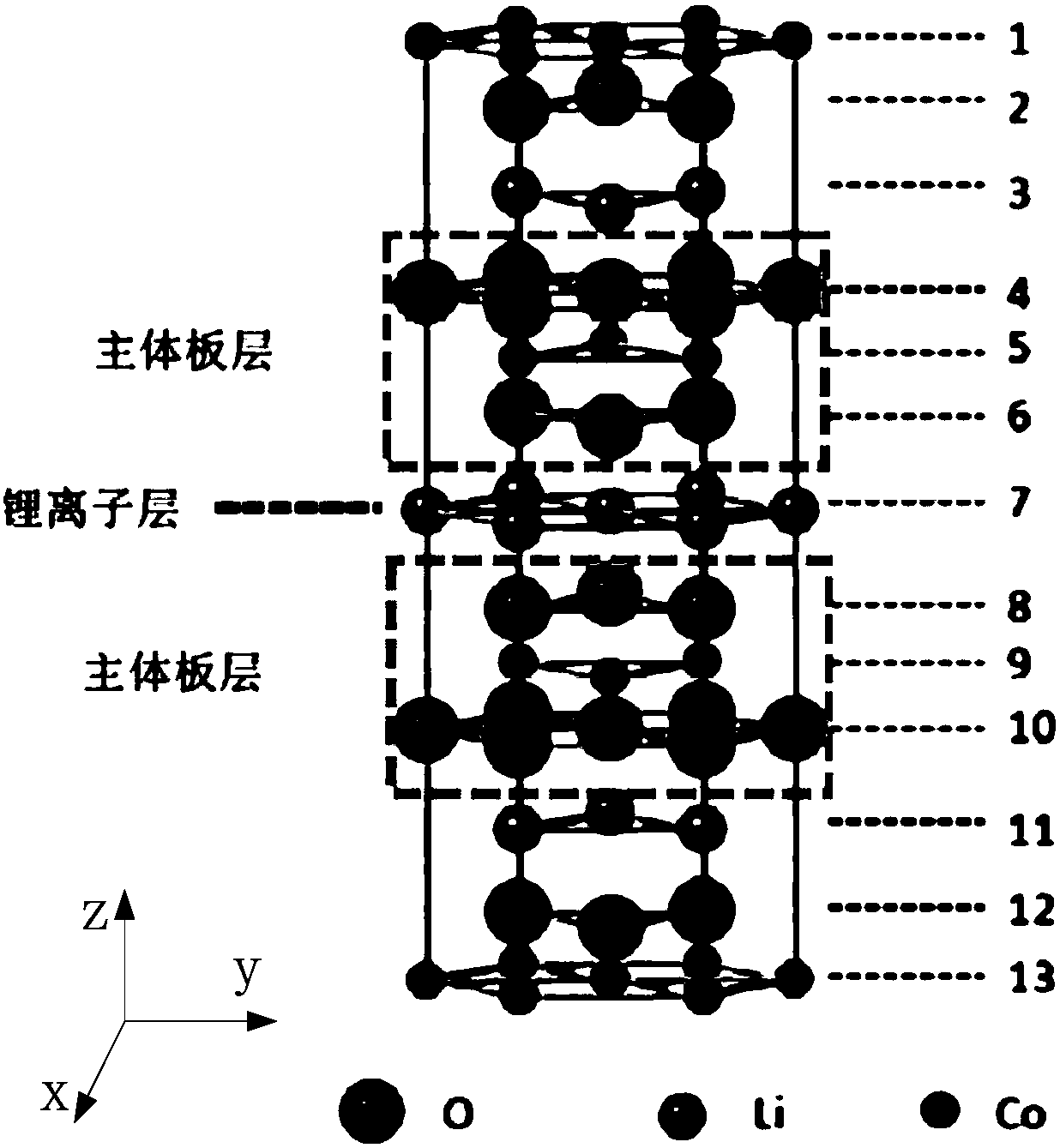
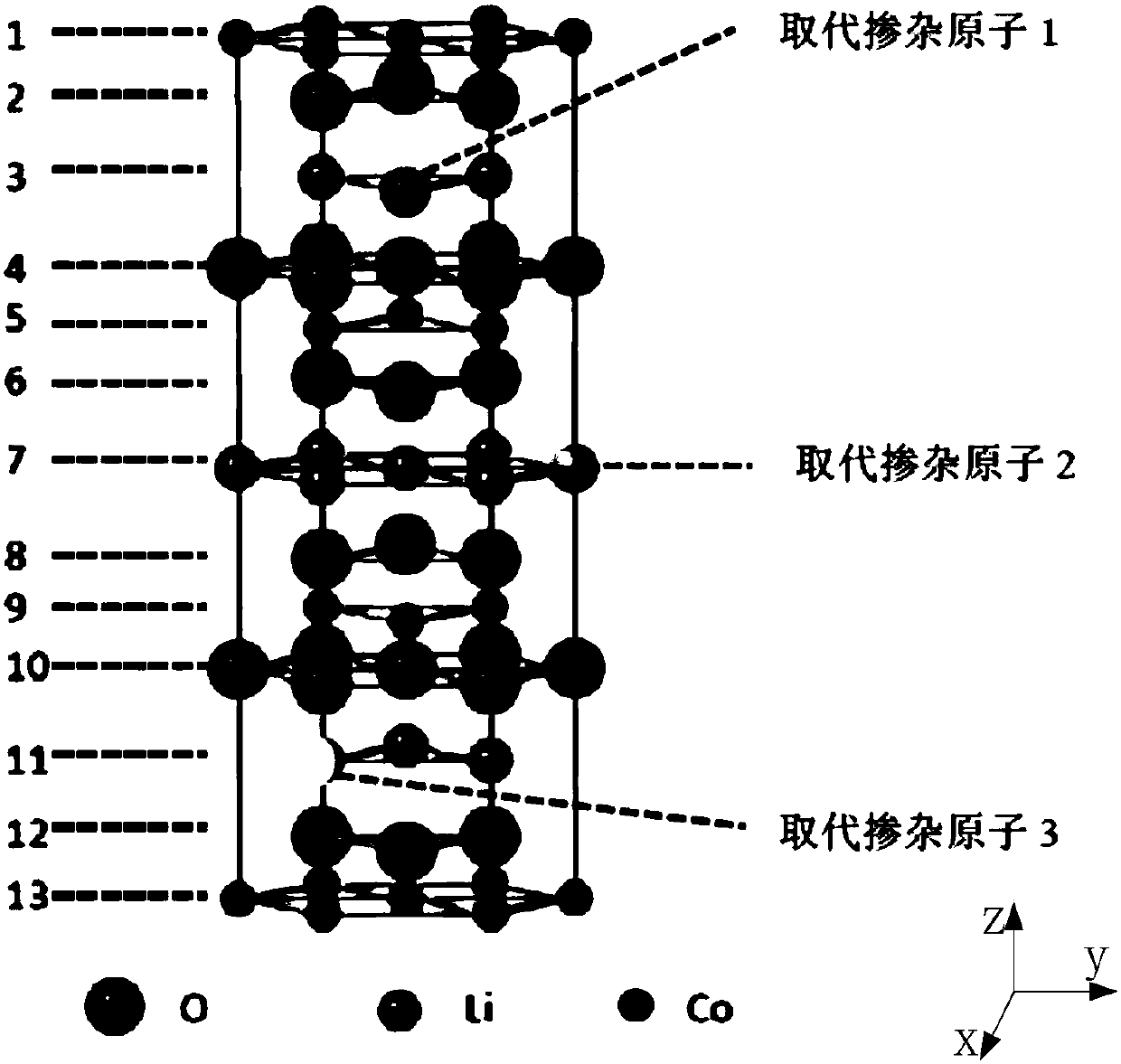
High-voltage lithium cobaltate positive electrode material and preparation method thereof, and lithium ion battery
ActiveCN109786738AImprove cycle stabilityAlleviate volume changesCell electrodesSecondary cellsDissolutionLithium-ion battery
Embodiments of the present invention provide a high voltage lithium cobaltate positive cathode material, including a lithium cobaltate substituted and doped at lithium positions. The lithium cobaltatesubstituted and doped at lithium positions has a general formula of Li1-xMaxCoO2, where x is greater than 0 and less than or equal to 0.05, Ma is a doping element, Ma is selected from one or more ofelements having an ionic radius ranging from 68 pm to 90 pm and an ion valence state greater than or equal to 1. The high-voltage lithium cobaltate positive electrode material is formed by substituting and doping lithium cobaltate at lithium positions, and the electrostatic interaction and cobalt dissolution of lithium cobaltate caused by lithium de-intercalation at a high voltage is alleviated, so that the structure and cycle stability of the material is improved, and the material has a high capacity and good cycle stability at the high voltage. Embodiments of the present invention further provide a high-voltage lithium cobaltate positive electrode material preparation method and a lithium ion battery.
Owner:HUAWEI TECH CO LTD
Spinel substrate and heteroepitaxial growth of III-V materials thereon
InactiveUS6844084B2Quality improvementImprove the situationPolycrystalline material growthSemiconductor/solid-state device manufacturingSpinelSingle crystal
A spinel composition of the invention includes a monocrystalline lattice having a formula Mg1-wαwAlx-yβyOz, where w is greater than 0 and less than 1, x is greater than 2 and less than about 8, y is less than x, z is equal to or greater than about 4 and equal to or less than about 13, α is a divalent cationic element having an ionic radius greater than divalent magnesium, and β is a trivalent cationic element having an ionic radius greater than trivalent aluminum. The monocrystalline lattice has tetrahedral and octahedral positions, and most of the magnesium and α occupy tetrahedral positions. In one embodiment, the molar ratio of aluminum to the amount of magnesium, α and β can be controlled during growth of the monocrystalline lattice thereby forming a spinel substrate suitable for heteroepitaxial growth of III-V materials. A method of the invention, includes forming a monocrystalline lattice of a spinel composition. A composite includes the spinel composition layer and a III-V layer at the surface of the spinel layer. A method of forming a composite includes depositing the III-V layer onto the surface of the spinel composition using heteroepitaxial techniques.
Owner:SAINT GOBAIN CERAMICS & PLASTICS INC
Functional layer material for solid oxide fuel cell, functional layer manufactured using functional layer material, and solid oxide fuel cell including functional layer
A functional layer material for a solid oxide fuel cell (SOFC) including a ceria ceramic oxide and a metal oxide including a metal, except for zirconium, having a Vegard's slope X represented by Equation 1 and having an absolute value |X| of the Vegard's slope X, wherein 27×105≦|X|≦45×105:X=(0.0220ri+0.00015zi) (1),wherein ri is an ionic radius difference between the metal and Ce4+, and zi is a charge difference between the metal and Ce4+.
Owner:SAMSUNG ELECTRO MECHANICS CO LTD
Single crystal heat treatment method
The present invention provides a single crystal heat treatment method, having a step of heating a single crystal of a cerium-doped silicate compound represented by any of general formulas (1) to (4) below in an oxygen-containing atmosphere Y2-(x+y)LnxCeySiO5 (1) (wherein Ln represents at least one elemental species selected from a group consisting of elements belonging to the rare earth elements, x represents a numerical value from 0 to 2, and y represents a numerical value greater than 0 but less than or equal to 0.2) Gd2-(z+w)LnzCewSiO5 (2) (wherein Ln represents at least one elemental species selected from a group consisting of elements belonging to the rare earth elements, z represents a numerical value greater than 0 but less than or equal to 2, and w represents a numerical value greater than 0 but less than or equal to 0.2) Gd2-(p+q)LnpCeqSiO5 (3) (wherein Ln represents at least one elemental species selected from a group consisting of Dy, Ho, Er, Tm, Yb, Lu, Y and Sc, which are rare earth elements having an ionic radius smaller than Tb, p represents a numerical value greater than 0 but less than or equal to 2, and q represents a numerical value greater than 0 but less than or equal to 0.2) Gd2-(r+s)LurCesSiO5 (4) (wherein r represents a numerical value greater than 0 but less than or equal to 2, and s represents a numerical value greater than 0 but less than or equal to 0.2).
Owner:OXIDE
Dielectric ceramic composition and electronic device
ActiveUS7262146B2High specific permittivityReducing atmosphereLayered productsFixed capacitor dielectricBarium titanateMaterials science
A dielectric ceramic composition, comprising a main component including barium titanate; a first subcomponent including at least one kind selected from MgO, CaO, BaO and SrO; a second subcomponent functioning as a sintering auxiliary agent; a third subcomponent including at least one kind selected from V2O5, MoO3 and WO3; a fourth subcomponent including an oxide of R1 (note that R1 is at least one kind selected from Sc, Er, Tm Yb and Lu); a fifth subcomponent including CaZrO3 or CaO+ZrO2; and an eighth subcomponent including an oxide of A (note that A is at least one kind selected from a cation element group having an effective ionic radius at the time of 6 coordination of 0.065 nm to 0.085 nm); wherein a ratio of the eighth subcomponent with respect to 100 moles of the main component is 0 to 4 moles (note that 0 mole and 4 moles are excluded, and the value is in terms of an A oxide).
Owner:TDK CORPARATION
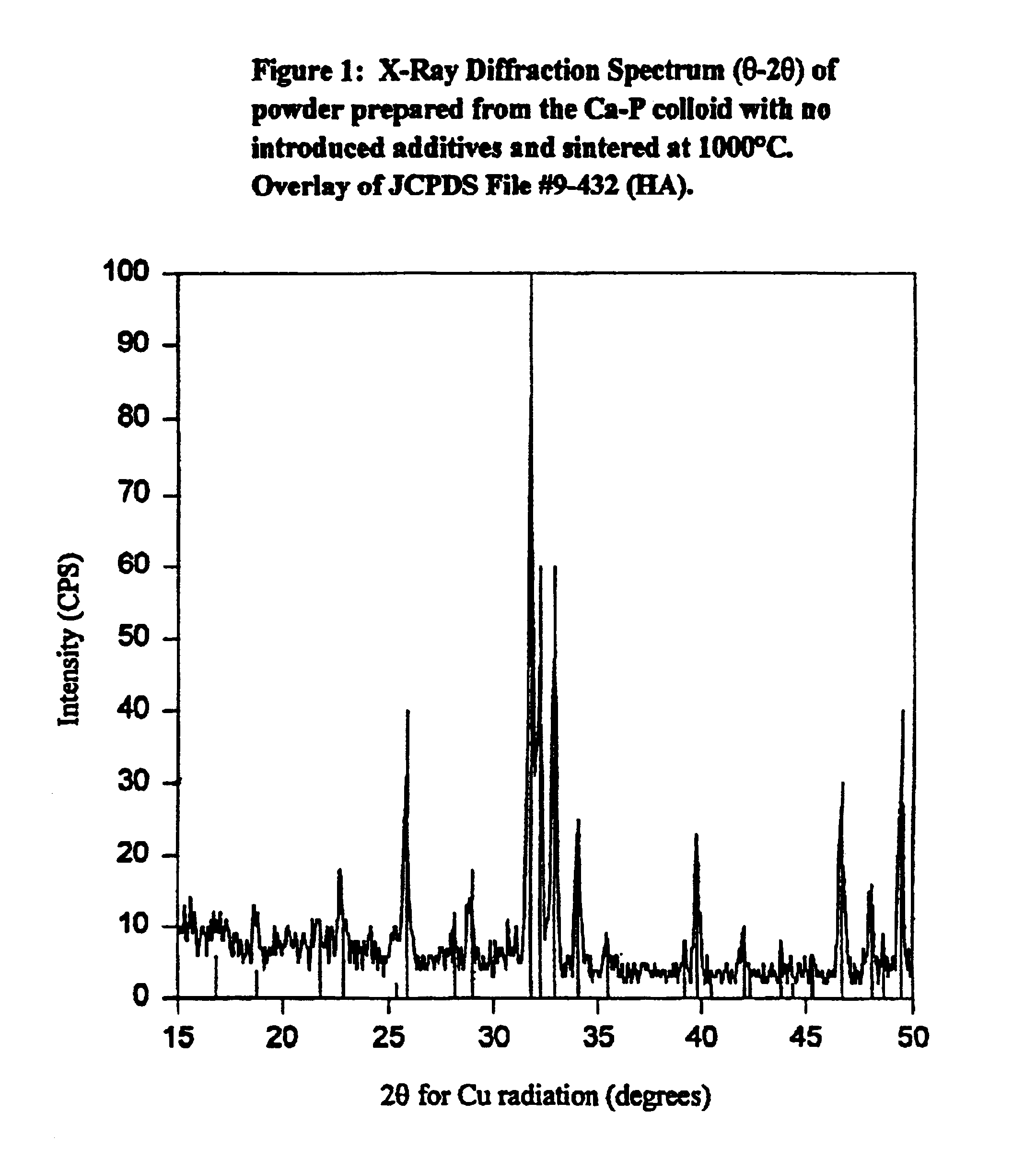
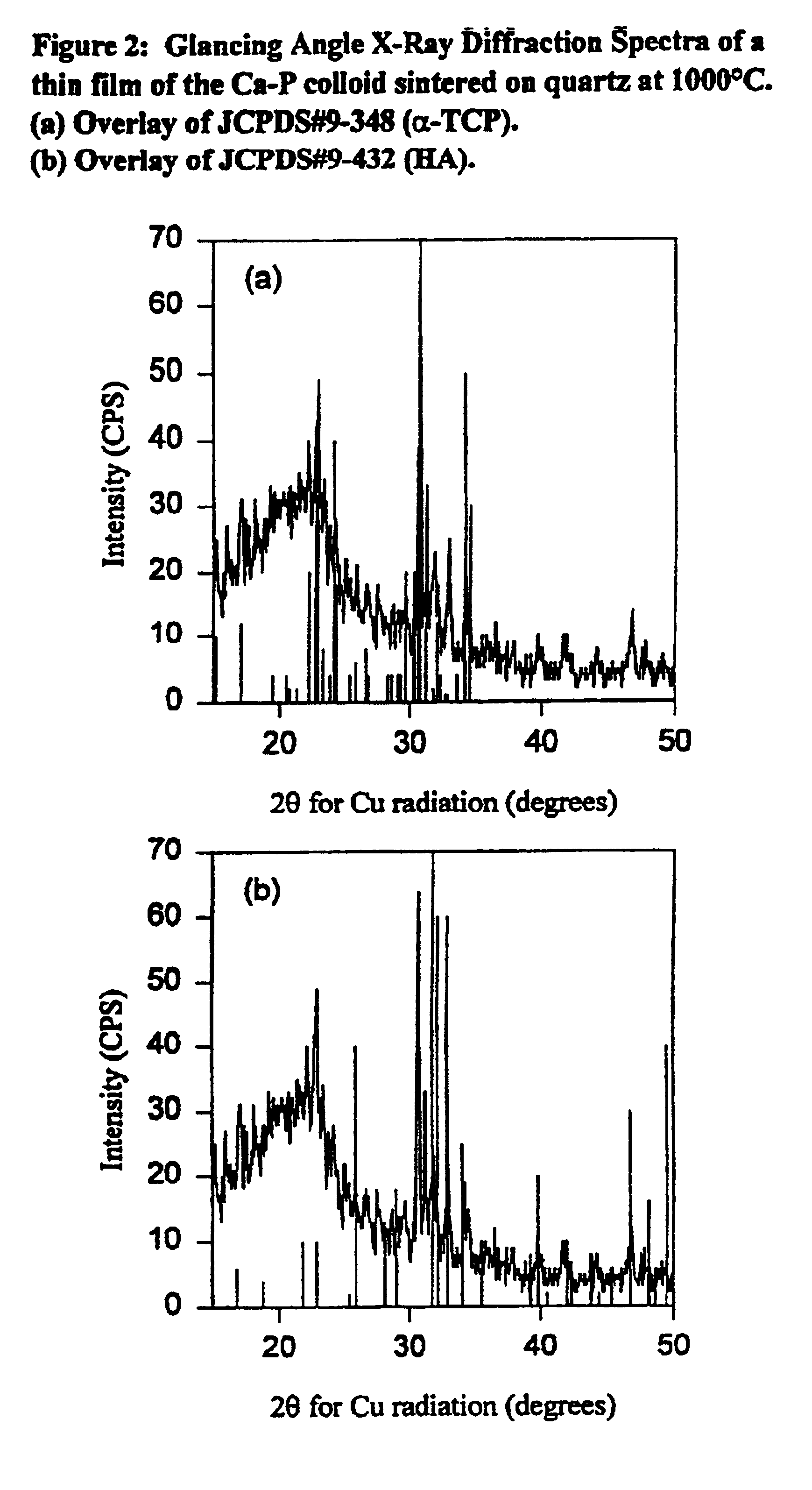
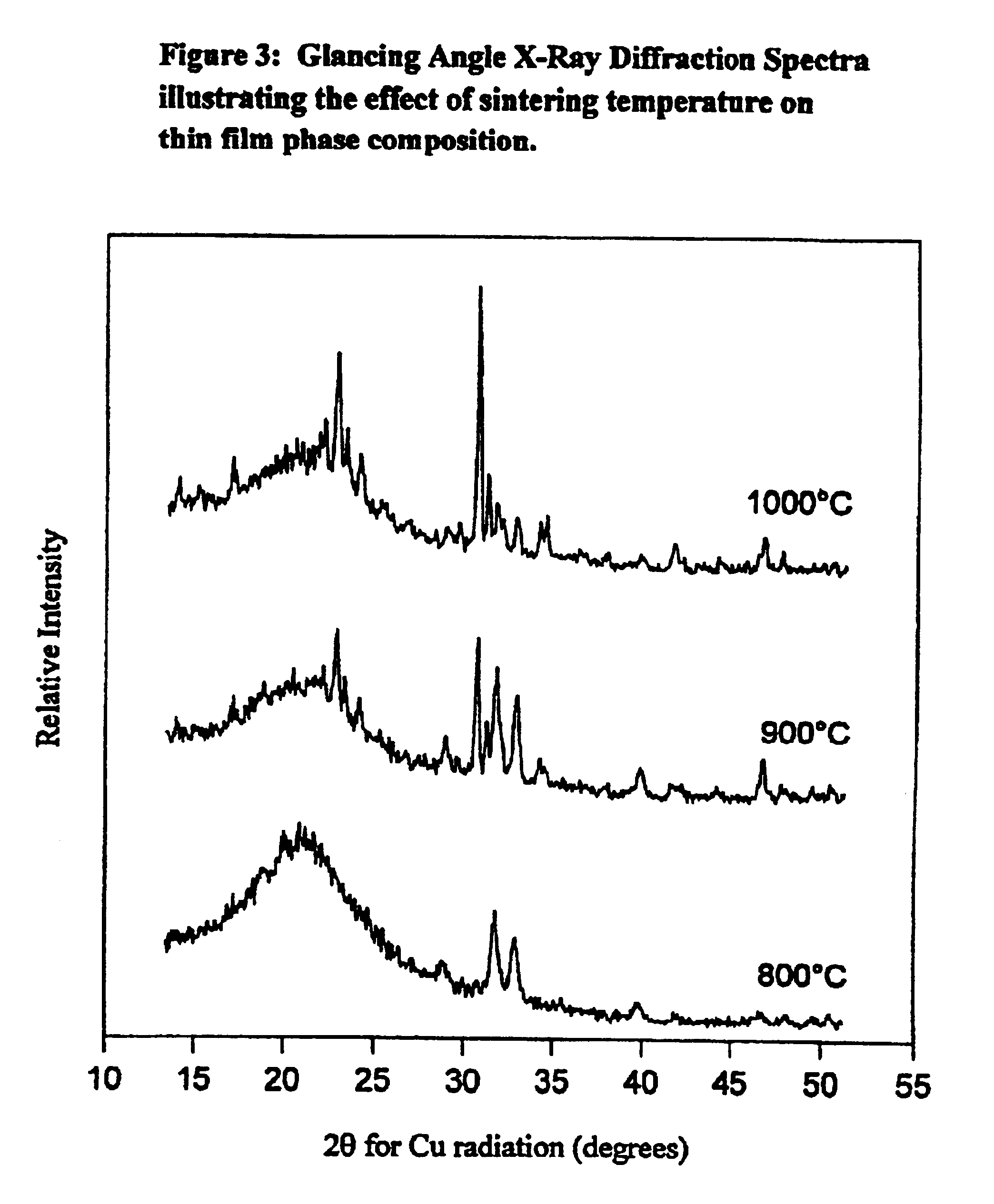
Synthetic biomaterial compound of calcium phosphate phases particularly adapted for supporting bone cell activity
InactiveUS6846493B2Maintain long-term stabilityPromote formationBiocidePhosphatesCalcium biphosphateOxygen
The present invention is directed to a synthetic biomaterial compound based on stabilized calcium phosphates and more particularly to the molecular, structural and physical characterization of this compound. The compound comprises calcium, oxygen and phosphorous, wherein at least one of the elements is substituted with an element having an ionic radius of approximately 0.1 to 1.1 Å. The knowledge of the specific molecular and chemical properties of the compound allows for the development of several uses of the compound in various bone-related clinical conditions.
Owner:WARSAW ORTHOPEDIC INC
Dielectric ceramic composition and electronic device
ActiveUS20060046922A1High specific permittivityImproved IR temperature dependencyLayered productsFixed capacitor dielectricBarium titanateMaterials science
A dielectric ceramic composition, comprising a main component including barium titanate; a first subcomponent including at least one kind selected from MgO, CaO, BaO and SrO; a second subcomponent functioning as a sintering auxiliary agent; a third subcomponent including at least one kind selected from V2O5, MoO3 and WO3; a fourth subcomponent including an oxide of R1 (note that R1 is at least one kind selected from Sc, Er, Tm Yb and Lu); a fifth subcomponent including CaZrO3 or CaO+ZrO2; and an eighth subcomponent including an oxide of A (note that A is at least one kind selected from a cation element group having an effective ionic radius at the time of 6 coordination of 0.065 nm to 0.085 nm); wherein a ratio of the eighth subcomponent with respect to 100 moles of the main component is 0 to 4 moles (note that 0 mole and 4 moles are excluded, and the value is in terms of an A oxide).
Owner:TDK CORPARATION
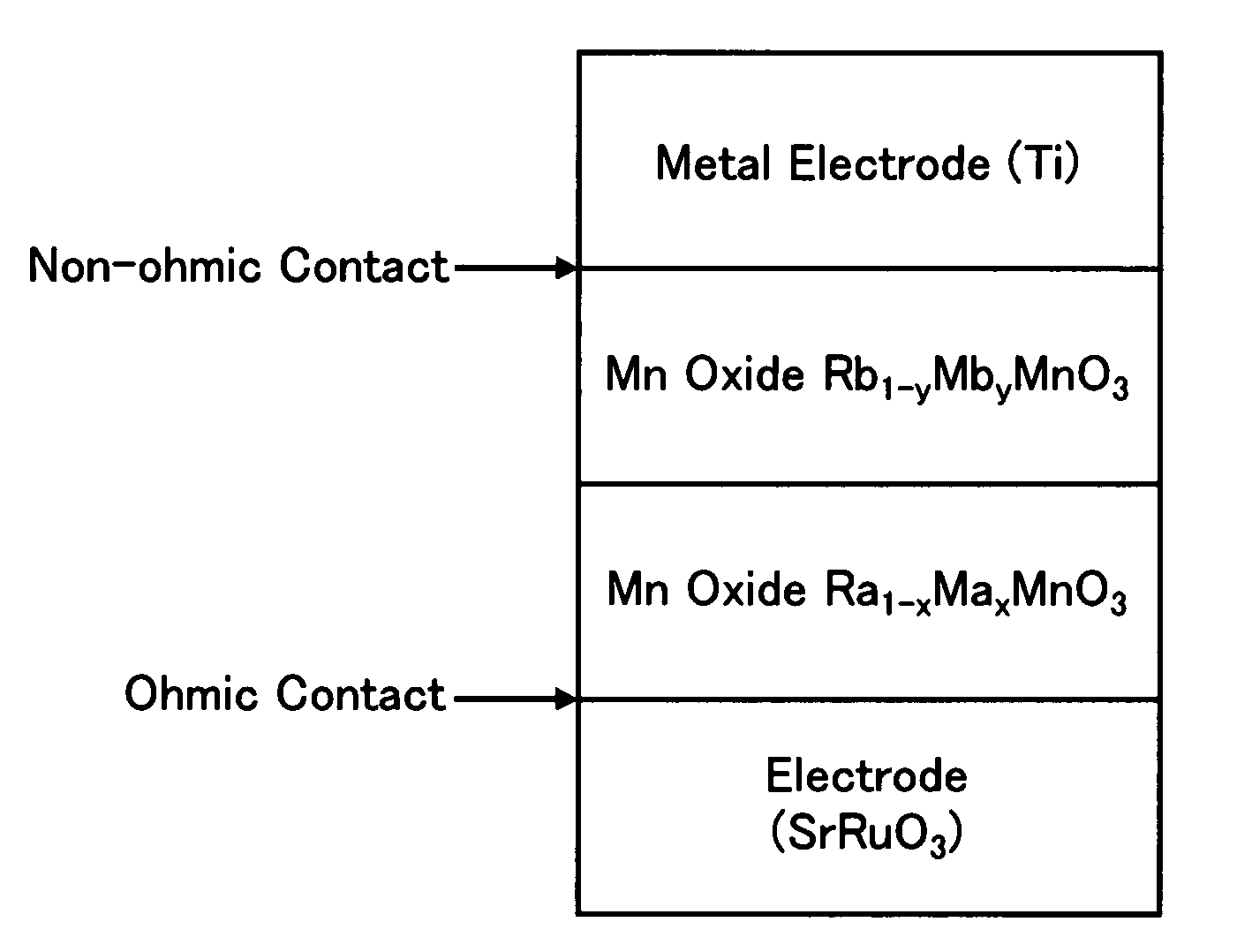
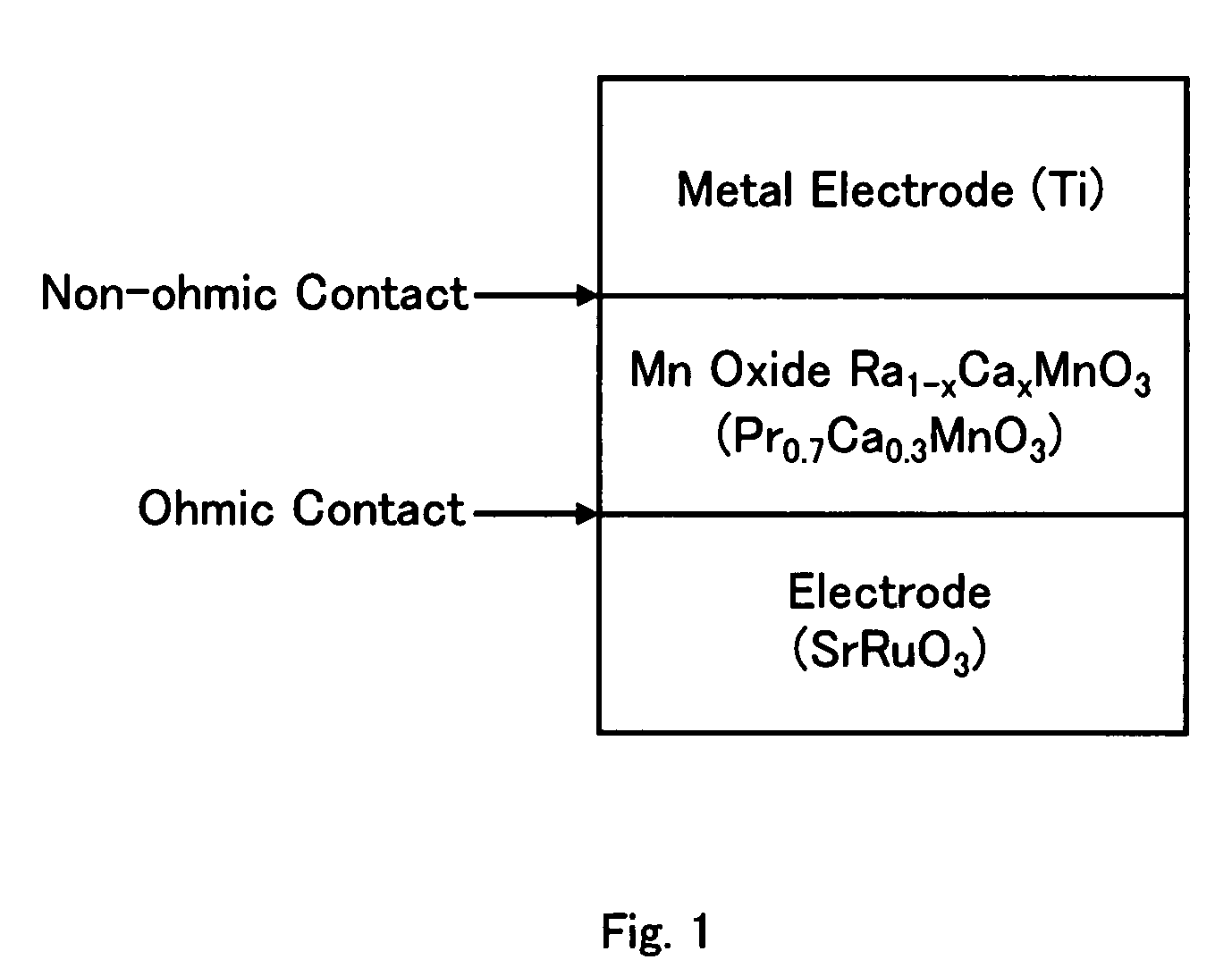
Nonvolatile memory element
InactiveUS7580276B2Property of change can be controlledImprove propertiesSolid-state devicesDigital storageRare-earth elementAlkaline earth metal
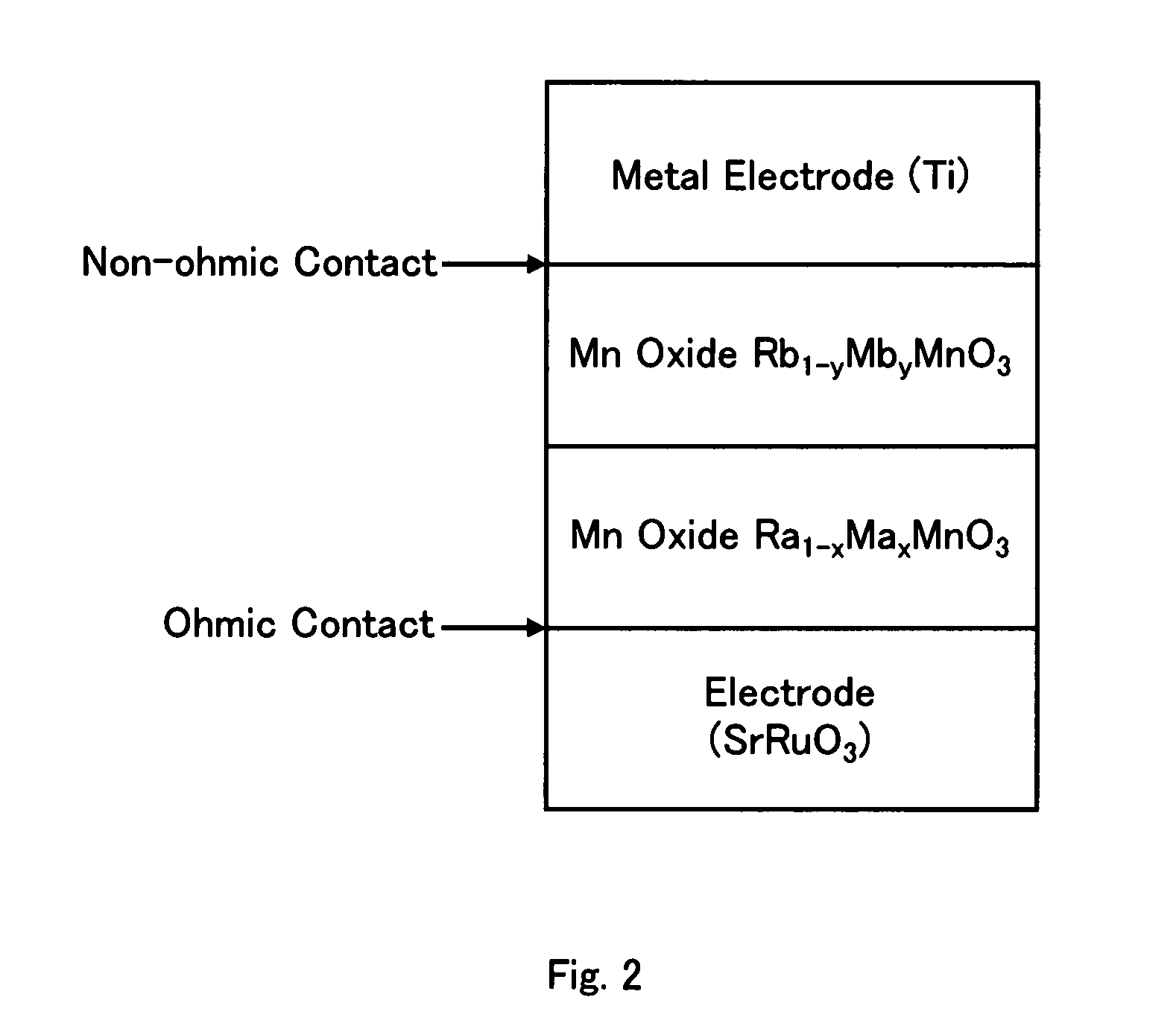
A nonvolatile memory element in which Rb1-yMbyMnO3 having higher insulation properties than Ra1-xMaxMnO3 is inserted between the Ra1-xMaxMnO3 and a metal having a shallow work function or a low electronegativity in order to improve resistance change properties and switching properties and to control the resistance change properties. (In the formulas, Ra and Rb represent rare earth elements and are solid solutions of one or more types of rare earth elements. Average ionic radius of the Rb is smaller than that of the Ra. Ma and Mb represent alkaline earth metals and are solid solutions of one or more types of alkaline earth metals. 0<x, y<1).
Owner:NAT INST OF ADVANCED IND SCI & TECH
Optical information recording medium, manufacturing method of the same and optical information recording and reproducing apparatus
InactiveUS20050213487A1Large storage capacityReduce power consumptionMechanical record carriersRecord information storageImage resolutionParticle physics
An optical information recording medium includes at least a reflecting layer, an optical information recording layer, a super-resolution layer and a protective layer. The super-resolution layer is a thin layer whose an optical constant changes nonlinearly due to a red shift of its band gap, and made of solid solution, compound or mixture of metal oxides containing two or more types of metal ions of the same valence. Ion radius unconformity ΔR=|(RMi−RMj) / RMj| between ion radii RMi and RMj of two types of metal ions Mi and Mj arbitrarily selected from the two or more types of metal ions is 8% or less. The difference ΔE=Eg−E between band gap energy Eg of the mixture of the metal oxides and energy E corresponding to a wavelength of the laser beam is a more 0.4 or less 1.4 eV.
Owner:HITACHI LTD
A-B site simultaneously substituting microwave dielectric ceramic material and preparation method thereof
The invention provides an A-B site simultaneously substituting microwave dielectric ceramic material and a preparation method thereof. The general chemical formula of the material is (Ba1-aAa)6-3x(Nd1-bBb)8+2x(Ti1-cCc)18O54, C=MN, wherein x=3 / 4, a is greater than or equal to 0.05 and smaller than or equal to 0.2, b is greater than or equal to 0.05 and smaller than or equal to 0.15, c is greater than or equal to 0.02 and smaller than or equal to 0.08, A represents divalent Ca and Sr elements substituting A1 sites, B represents trivalent Sm and Bi elements substituting A2 sites, M represents Nb with the valence state higher than tetravalency, N represents other one or more elements with a valence state lower than tetravalency and an ionic radius similar to Ti, and M and N substitute simultaneously or individually. The preparation method includes: determining the respective mass percentage content according to the general chemical formula, conducting ball mill mixing, performing presintering under 1000-1150DEG C, and then conducting sintering at 1250-1450DEG C. The prepared material has adjustable dielectric constant and frequency-temperature coefficient and at the same time maintains a high Q*f value. The formula does not contain Pb, Cd and other volatile or heavy metals, the performance is greatly enhanced, the raw materials are in abundant supply, and the price is low, so that low cost of high performance microwave ceramics becomes possible.
Owner:UNIV OF ELECTRONICS SCI & TECH OF CHINA
Hexagonal type barium titanate powder, producing method thereof, dielectric ceramic composition and electronic component
InactiveUS20110110018A1High specific permittivitySolve the lack of reliabilityAlkaline earth titanatesFixed capacitor dielectricRare-earth elementBarium titanate
Dielectric ceramic composition includes a hexagonal type barium titanate as a main component shown by a generic formula (Bai-αMα)A(Ti1-βMnβ)BO3 and having hexagonal structure wherein an effective ionic radius of 12-coordinated “M” is −20% or more to +20% or less with respect to an effective ionic radius of 12-coordinated Ba2+ and the A, B, α and β satisfy relations of 0.900≦(A / B)≦1.040, 0.003≦α≦0.05, 0.03≦β≦0.2, and as subcomponents, with respect to the main component, certain contents of alkaline earth oxide such as MgO and the like, Mn3O4 and / or Cr2O3, CuO, Al2O3, rare earth element oxide and glass component including SiO2. According to the present invention, it can be provided the hexagonal type barium titanate powder and dielectric ceramic composition which are preferable for producing electronic components such as a capacitor and the like showing high specific permittivity, having advantageous insulation property and sufficient reliability.
Owner:TDK CORPARATION
Method of manufacturing an ion-exchanged glass article
ActiveUS8919150B2Long lastingMaintain stable propertiesMagnetic materials for record carriersRecord information storageMolten saltIon exchange
Owner:HOYA CORP
Gas sensor
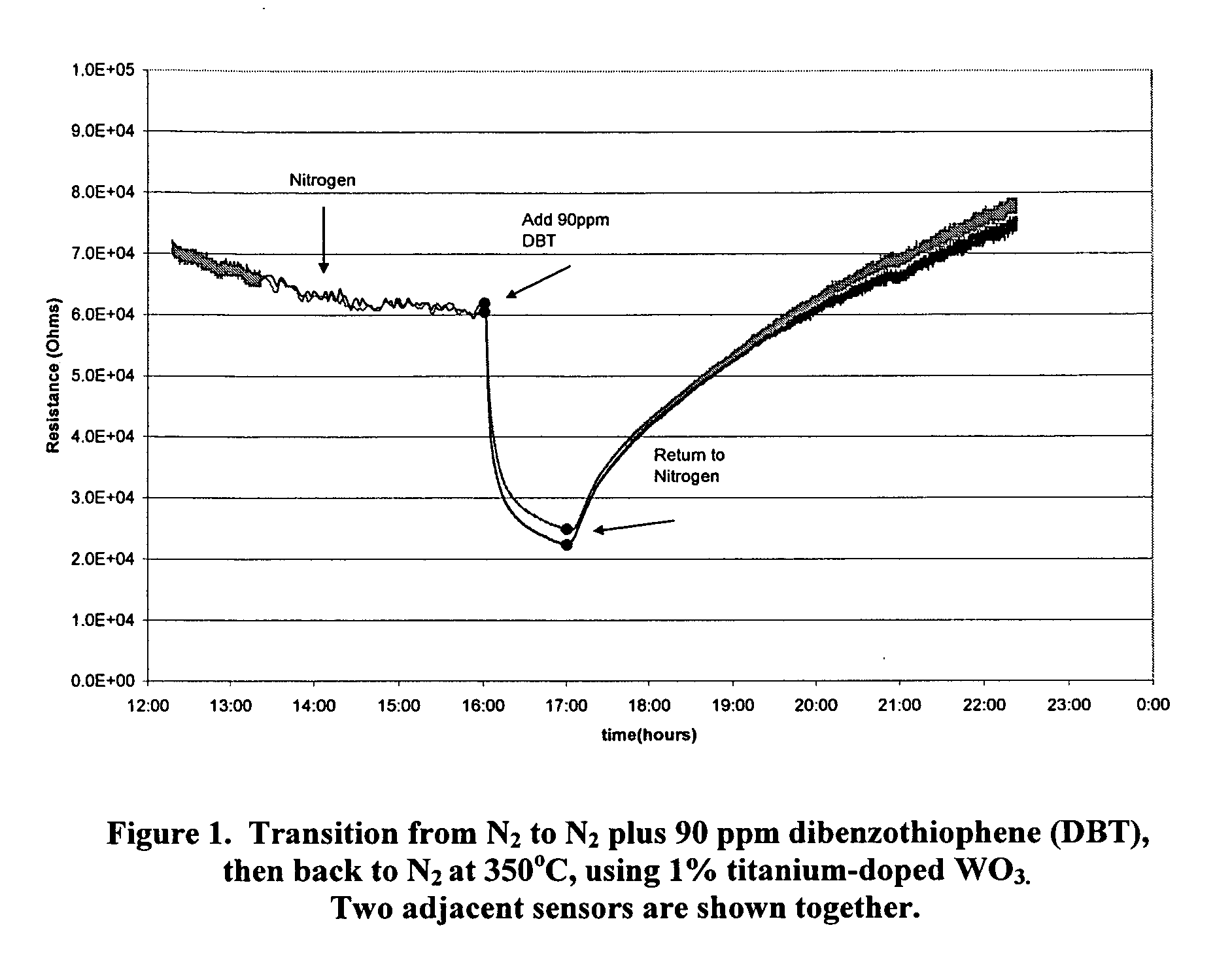
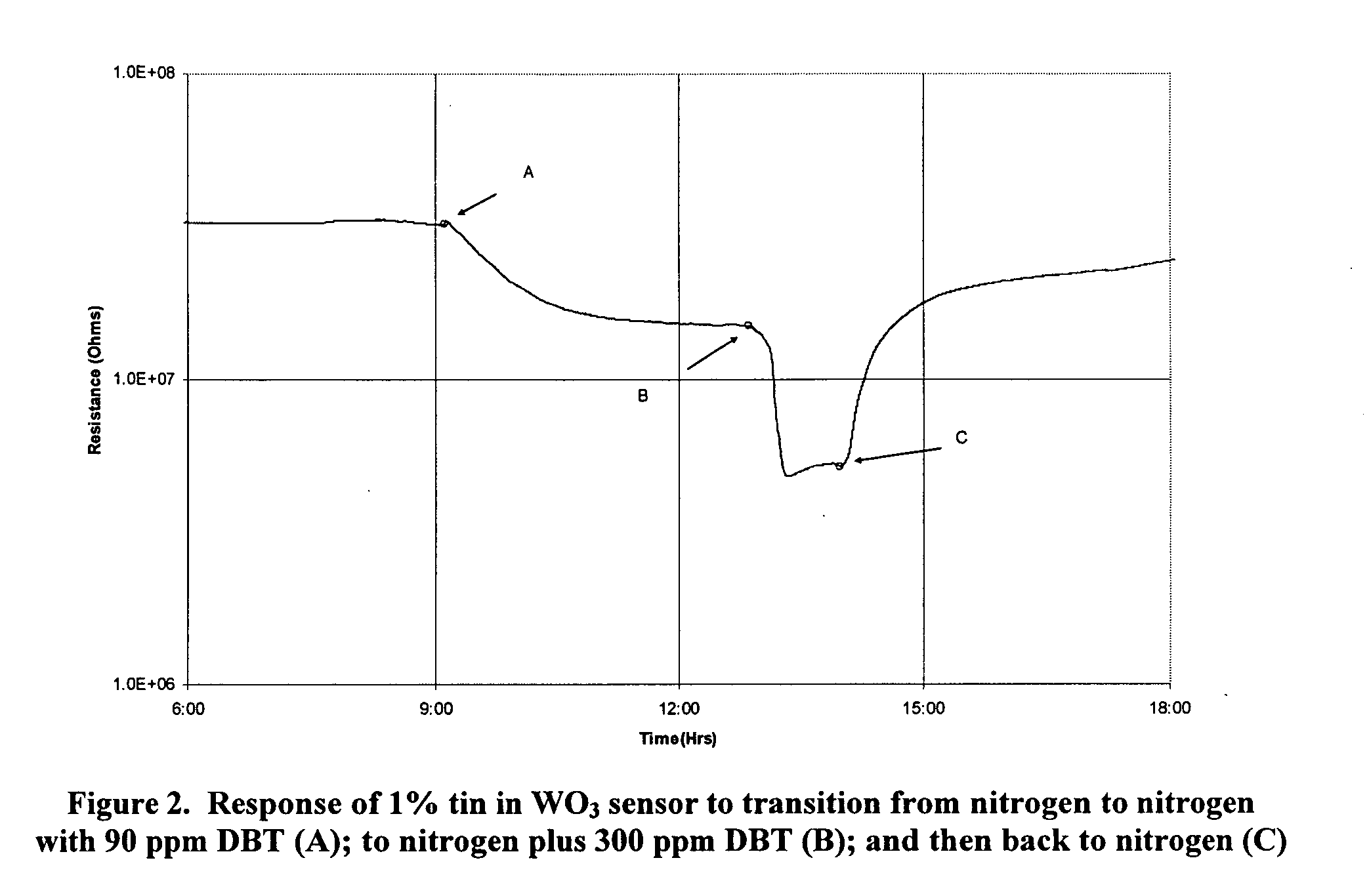
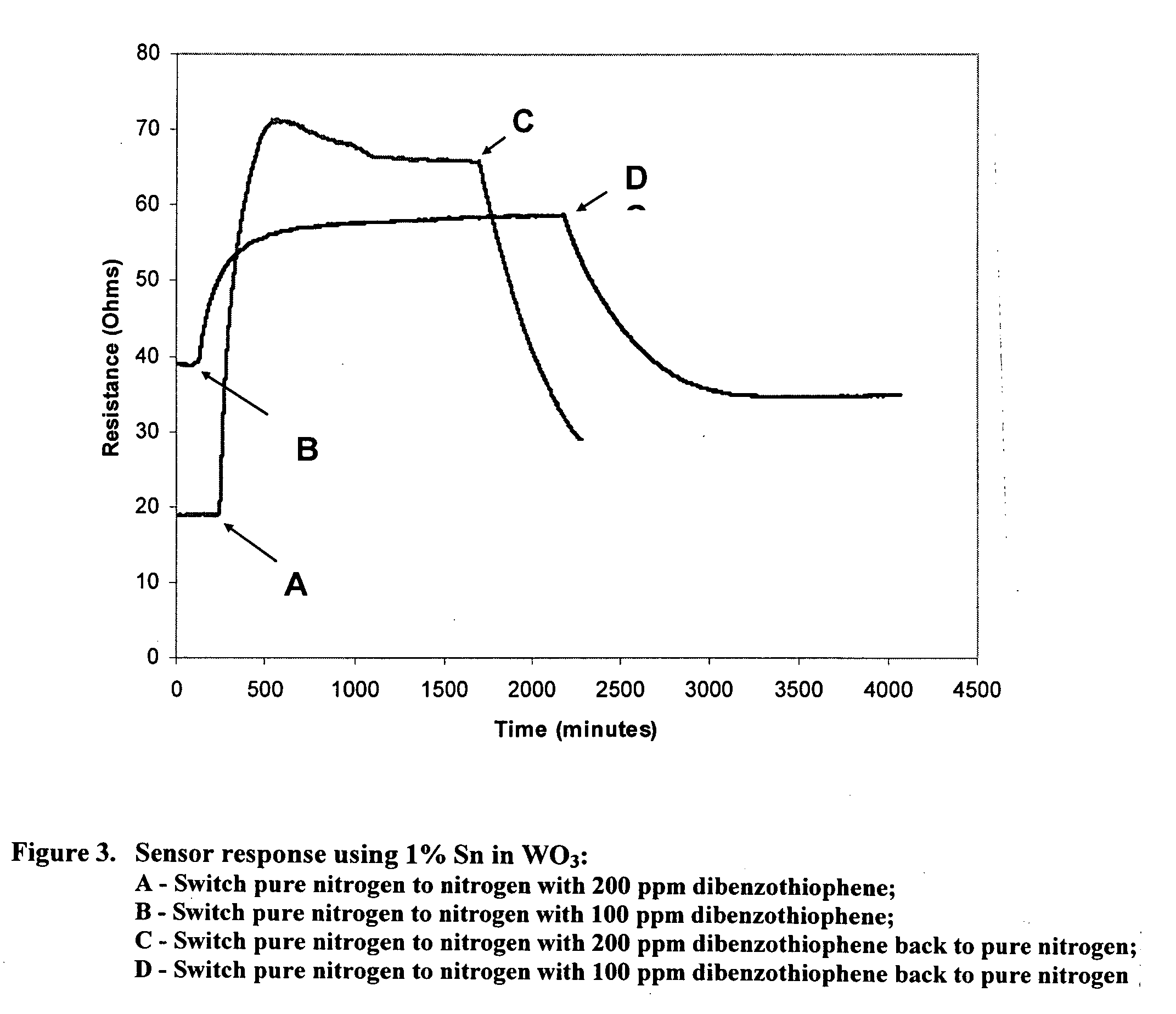
InactiveUS20090218235A1Weather/light/corrosion resistanceVolume/mass flow measurementDesorptionNanoparticle
Metal-oxide gas sensor. According to one embodiment, the sensor includes a layer or pellet of tungsten trioxide (WO3) substituted with one or more added metals. Preferably, the added metals are substituted in a concentration between about 0.005 and 10%, have an oxidation state less than +6, and possess a similar ionic radius to W6+. The substituted metal oxides are preferably formed as nanoparticles and sintered into a dense structure or coating possessing a surface-depletion layer sensitive to the surface adsorption of gas molecules and whose resistance changes in a predictable manner with gas adsorption. The extent of resistance change, rate of change and rate of desorption can be different for different gases, depending on the gas molecule's polarizability, dipole moments and electron configuration. The sensor can be used in a wide range of temperatures and corrosive conditions because of the intrinsic stability of the substituted metal oxides.
Owner:GINER INC
Method of manufacturing chemically strengthened glass plate
InactiveCN103214172AEfficient preparationIncrease the surface compressive stressGlass tempering apparatusIon exchangeGlass sheet
The present invention provides a method of manufacturing a chemically strengthened glass plate by ion-exchanging a glass base plate to replace alkali metal ions A that are the main alkali metal ion component of the glass base plate with alkali metal ions B having a larger ionic radius than the alkali metal ions A at a surface of the glass base plate, the unexchanged glass base plate made of a soda-lime glass, the method including: a first step of contacting the glass base plate with a first salt containing the alkali metal ions A, the first salt containing the alkali metal ions A at a ratio X, as expressed as a molar percentage of total alkali metal ions, of 90 to 100 mol %; a second step of contacting the glass plate with a second salt containing the alkali metal ions B after the first step, the second salt containing the alkali metal ions A at a ratio Y, as expressed as a molar percentage of the total alkali metal ions, of 0 to 10 mol %; and a third step of contacting the glass plate with a third salt containing the alkali metal ions B after the second step, the third salt containing the alkali metal ions B at a ratio Z, as expressed as a molar percentage of the total alkali metal ions, of 98 to 100 mol %.
Owner:CENT GLASS CO LTD
Method for repairing non-performing glass
The invention provides a method for repairing non-performing glass. The method comprises the following steps of: A, contacting the non-performing glass with a melting returning enhancer, thus obtaining a reduced glass product, wherein the returning enhancer is the first alkali metal salt, wherein ionic radius of the first alkali metal ion in the first alkali metal salt is less than the ionic radius of the alkali metal ion contained on the surface of the non-performing glass, and the first alkali metal ion is the same as the alkali metal ion contained in the non-performing glass; B, polishing the reduced glass product in step A, so as to obtain the pre-fabricated glass product; and C, contacting the pre-fabricated glass product in step B and the melting returning enhancer to enhance, so as to obtain the performing glass, wherein the enhancer is the second alkali metal salt, wherein the ionic radius of the second alkali metal ion in the second alkali metal salt is less than the ionic radius of the alkali metal ion contained in the non-performing glass, and the second alkali metal ion is the same as the alkali metal ion contained in the non-performing glass. The method provided by the invention brings higher yield of the non-performing glass in repair.
Owner:BYD CO LTD
Dielectric ceramic composite and electronic device
InactiveCN1432548AAccelerated life is not improvedInhibition of segregationAlkaline earth titanatesFixed capacitor dielectricRare-earth elementCapacitance
A dielectric ceramic composition, comprising a main component including barium titanate, a fourth subcomponent including an oxide of R1 (note that R1 is at least one kind selected from a first element group composed of rare-earth elements having a effective ionic radius of less than 108pm when having a coordination number of nine), and a fifth subcomponent including an oxide of R2 (note that R2 is at least one kind selected from a second element group composed of rare-earth elements having a effective ionic radius of 108pm to 113pm when having a coordination number of nine). According to the composition, a dielectric ceramic composition having excellent reducing resisting property at firing, excellent temperature. dependence of capacitance after firing, and improved accelerated lifetime of insulation resistance can be provided.
Owner:TDK CORPARATION
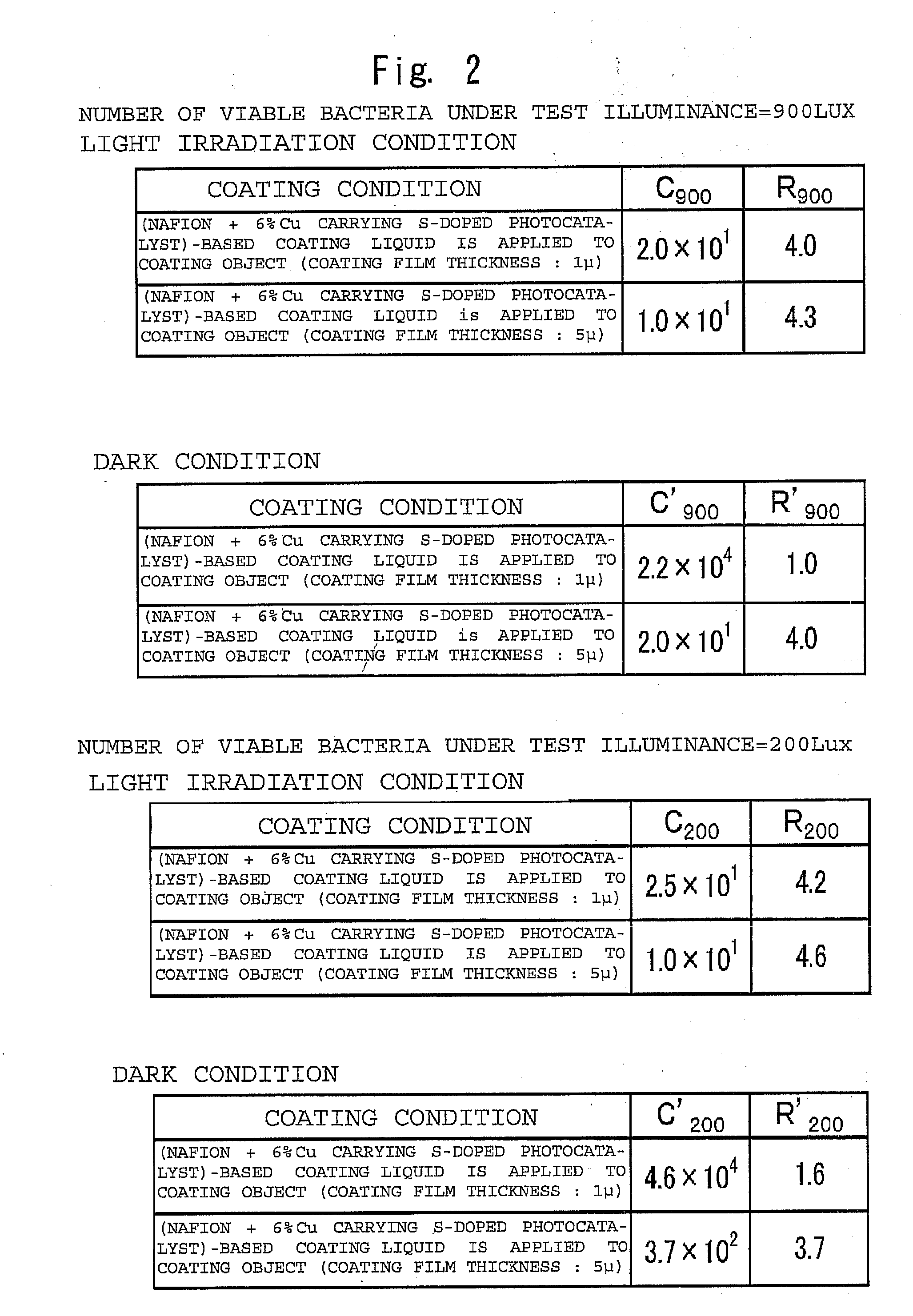
Photocatalytic coating, photocatalytic coating film and laminated coating film structure
InactiveUS20120142238A1High affinityNot easy to corrodeOrganic-compounds/hydrides/coordination-complexes catalystsSynthetic resin layered productsSolventComplex ions
Provided is a photocatalytic coating in which the coating per se is hardly eroded even when a photocatalyst is excited and a coating surface having strong hydrophobic tendency can be formed. The photocatalytic coating is prepared by dispersing or dissolving at least a photocatalyst, a tetrafluoro-ethylene-based resin obtained by graft polymerization of a sulfonic acid, a compound containing metal ion having an ionic radius not less than an ionic radius of calcium and / or complex ion having an ionic radius not less than the ionic radius of calcium into a solvent. Further, hydroxide is used as the compound containing the metal ion or an electrically neutral surfactant is added to the photocatalytic coating.
Owner:METAL TECH CO LTD +2
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com