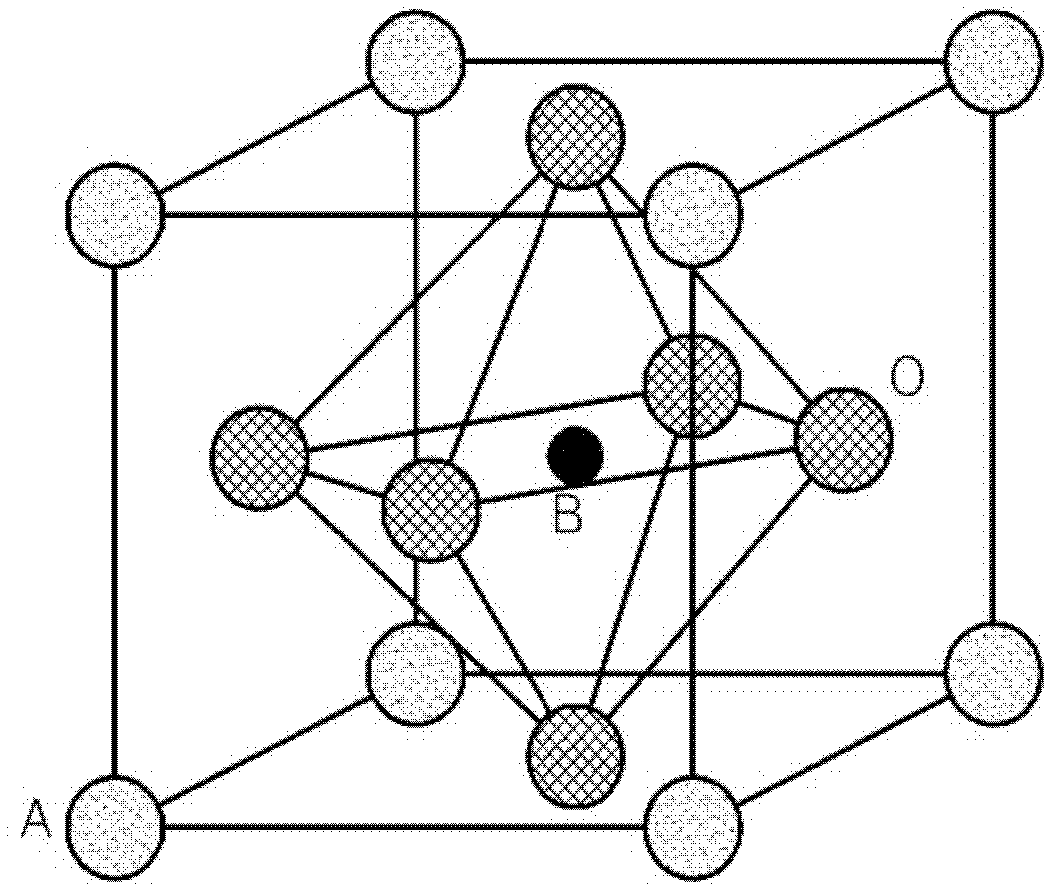
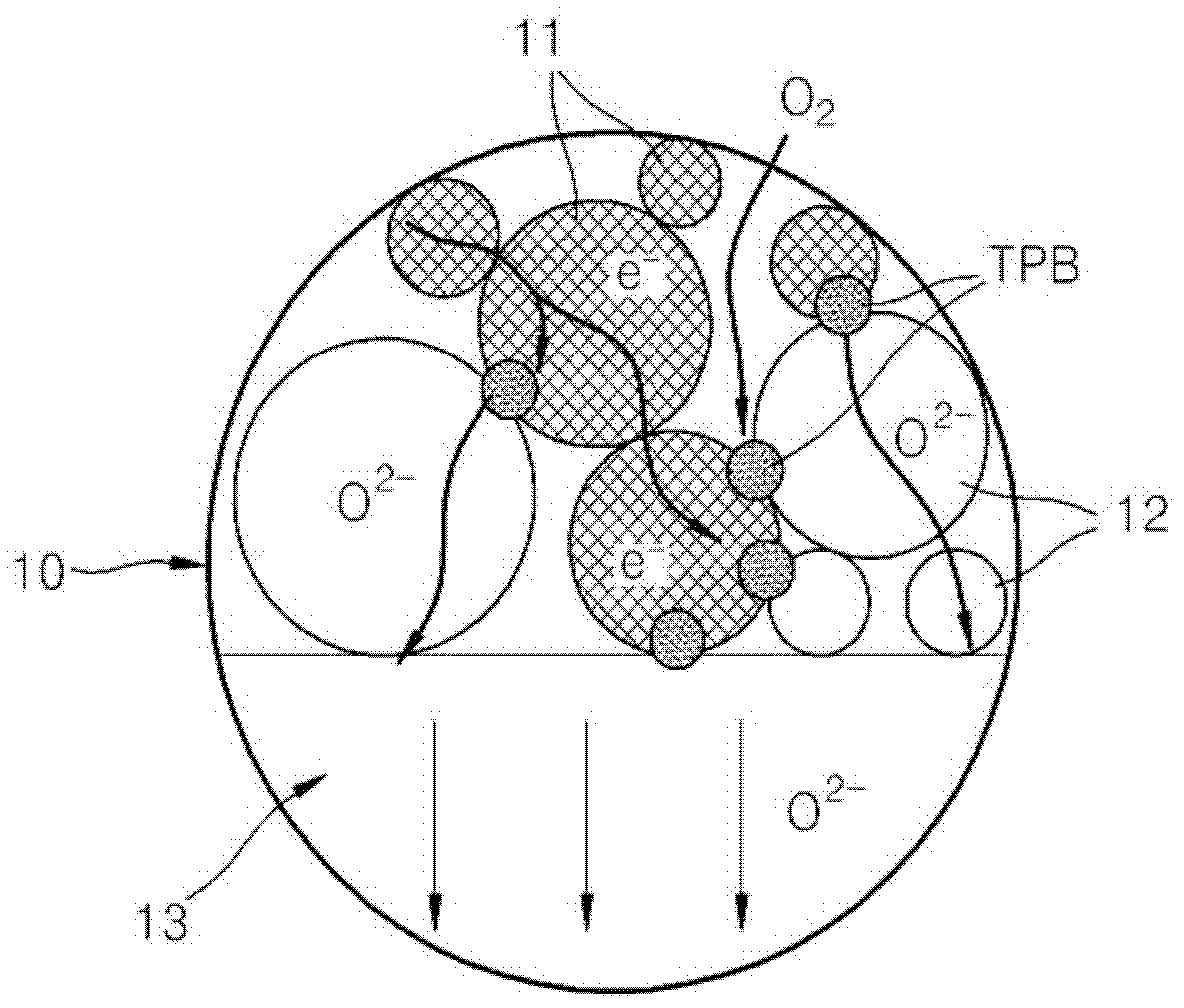
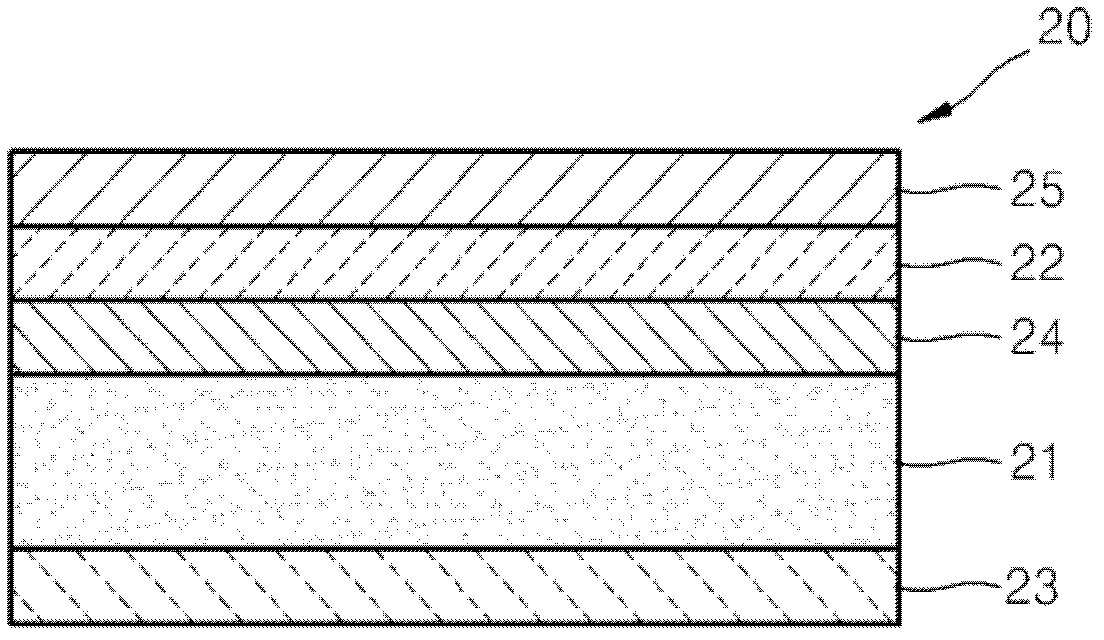
Cathode material for a fuel cell, cathode including the cathode material, and a solid oxide fuel cell including the cathode material
A fuel cell and cathode material technology, which is applied in the field of solid oxide fuel cells, and can solve problems such as the increase in the resistance of SOFC cathode materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0119] Preparation Example 1: Manufacture of Cathode (1)
[0120] To fabricate Ce as Sm, Nd-doped ceria SNDC 0.8 SM0.15 Nd 0.05 o 2 Ion-conducting powder, firstly 19.920g of Ce(NO 3 ) 3 ·6H 2 O, 3.823g of Sm(NO 3 ) 3 ·6H 2 O, 1.257g Nd(NO 3 ) 3 ·6H 2 O and 6.816g of urea were placed in 100ml of distilled water and stirred with a magnetic bar until they were completely dissolved. Using a hot plate, the resulting solution was heated at about 150°C for 12 hours to obtain a dry powder. Ce with a fluorite structure was obtained by heating the resulting dry powder at about 800 °C for two hours 0.80 SM 0.15 Nd 0.05 o 2 (referred to as SNDC).
[0121] The cathode material was obtained by dissolving 2.5 g La 0.6 Sr 0.4 co 0.2 Fe 0.8 o 3 (FCM, hereinafter referred to as LSCF) and 2.5 g of the powder of SNDC obtained above were put into a tungsten bottle, 10 cc of ethanol was added to the tungsten bottle, they were mixed with a high-energy grinder (Mixer / Mill 8000D, ...
preparation Embodiment 2
[0122] Preparation Example 2: Manufacture of Cathode (2)
[0123] Fabrication of site-defected lanthanum strontium cobalt ferrous oxide (LSCF) La using the urea method 0.55 Sr 0.4 co 0.2 Fe 0.8 o 3 powder. Specifically, 8.457g of La(NO 3 ) 3 ·6H 2 O, 3.004g of Sr(NO 3 ) 2 , 2.066gCo(NO 3 ) 3 9H 2 O, 11.472g of Fe(NO 3 ) 3 9H 2 O and 7.288g of urea (CH 4 N 2 O) Put in 100 g of distilled water and stir with a magnetic bar until they are completely dissolved. Using a hot plate, the resulting solution was heated at about 150°C for 12 hours to obtain a dry powder. By heating the resulting dry powder at about 1000 °C for two hours, La with a perovskite structure 0.55 Sr 0.4 co 0.2 Fe 0.8 o 3 (referred to as L 0.55 SCF) powder.
[0124] The cathode material was obtained by adding 2.5 g of the above obtained L 0.55 SCF and 2.5 g of the SNDC powder obtained in Preparation Example 1 were put into a tungsten bottle, 10 cc of ethanol was added to the tungsten bot...
preparation Embodiment 3
[0125] Preparation Example 3: Manufacture of Cathode (3)
[0126] The same method as in Preparation Example 2 was carried out to obtain a cathode material, except that La strontium cobalt ferrous oxide (LSCF) was La 0.55 Sr 0.35 co 0.2 Fe 0.8 o 3 (referred to as L 0.55 S 0.35 CF) was manufactured using a solution obtained by dissolving 8.586 g of La(NO 3 ) 3 ·6H 2 O, 2.669g of Sr(NO 3 ) 2 , 2.0975g of Co(NO 3 ) 3 9H 2 O, 11.674g of Fe(NO 3 ) 3 9H 2 O and 6.949g of urea (CH 4 N 2 O) Put in 100 g of distilled water, and then dissolve the mixture.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com