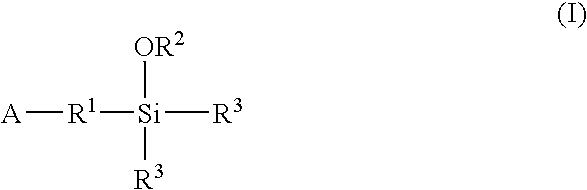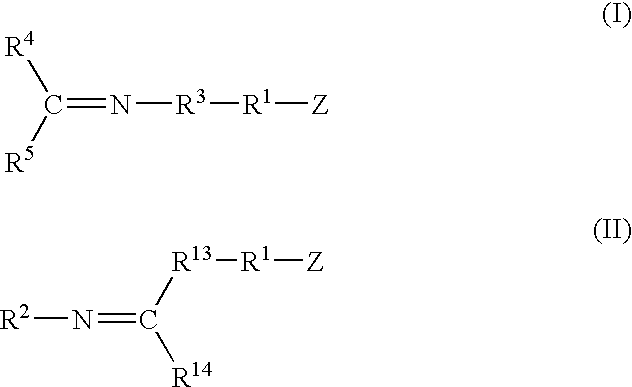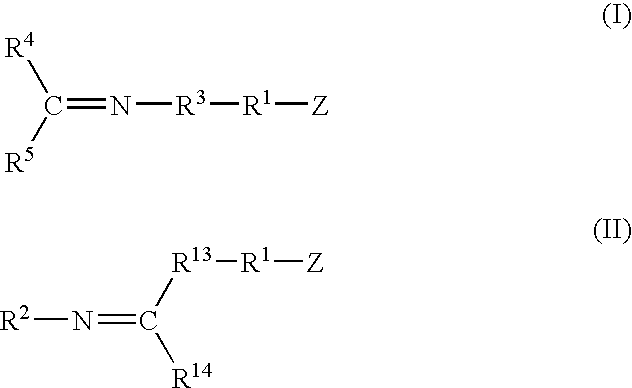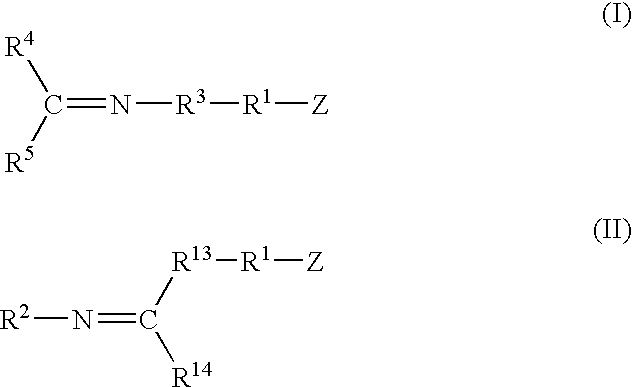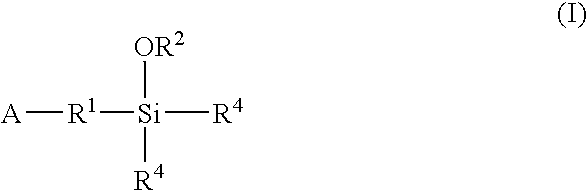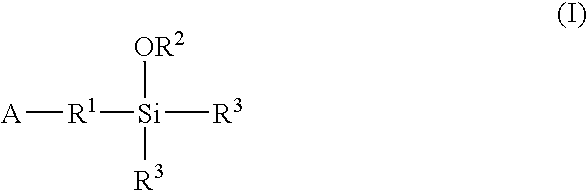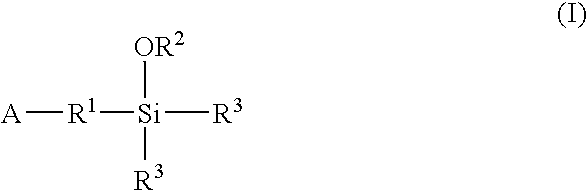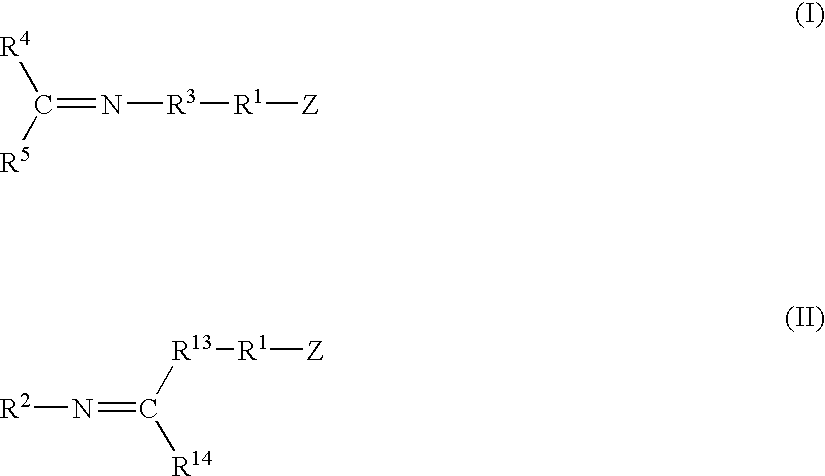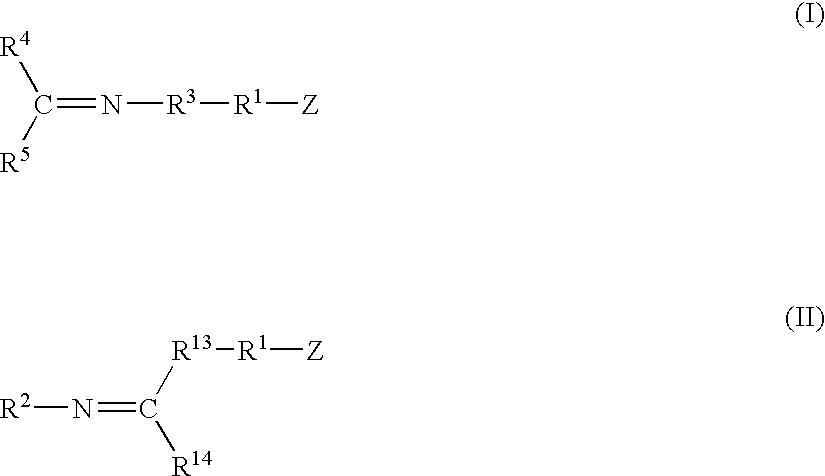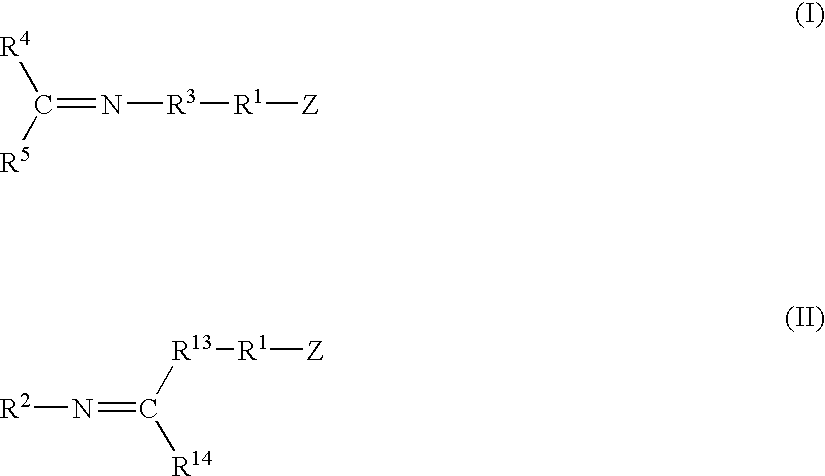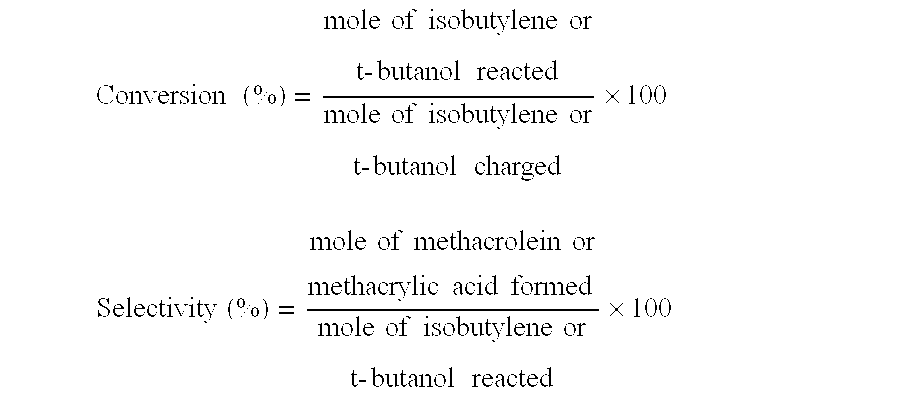Patents
Literature
2848 results about "Lanthanide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The lanthanide (/ˈlænθənaɪd/) or lanthanoid (/ˈlænθənɔɪd/) series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yttrium, are often collectively known as the rare earth elements.
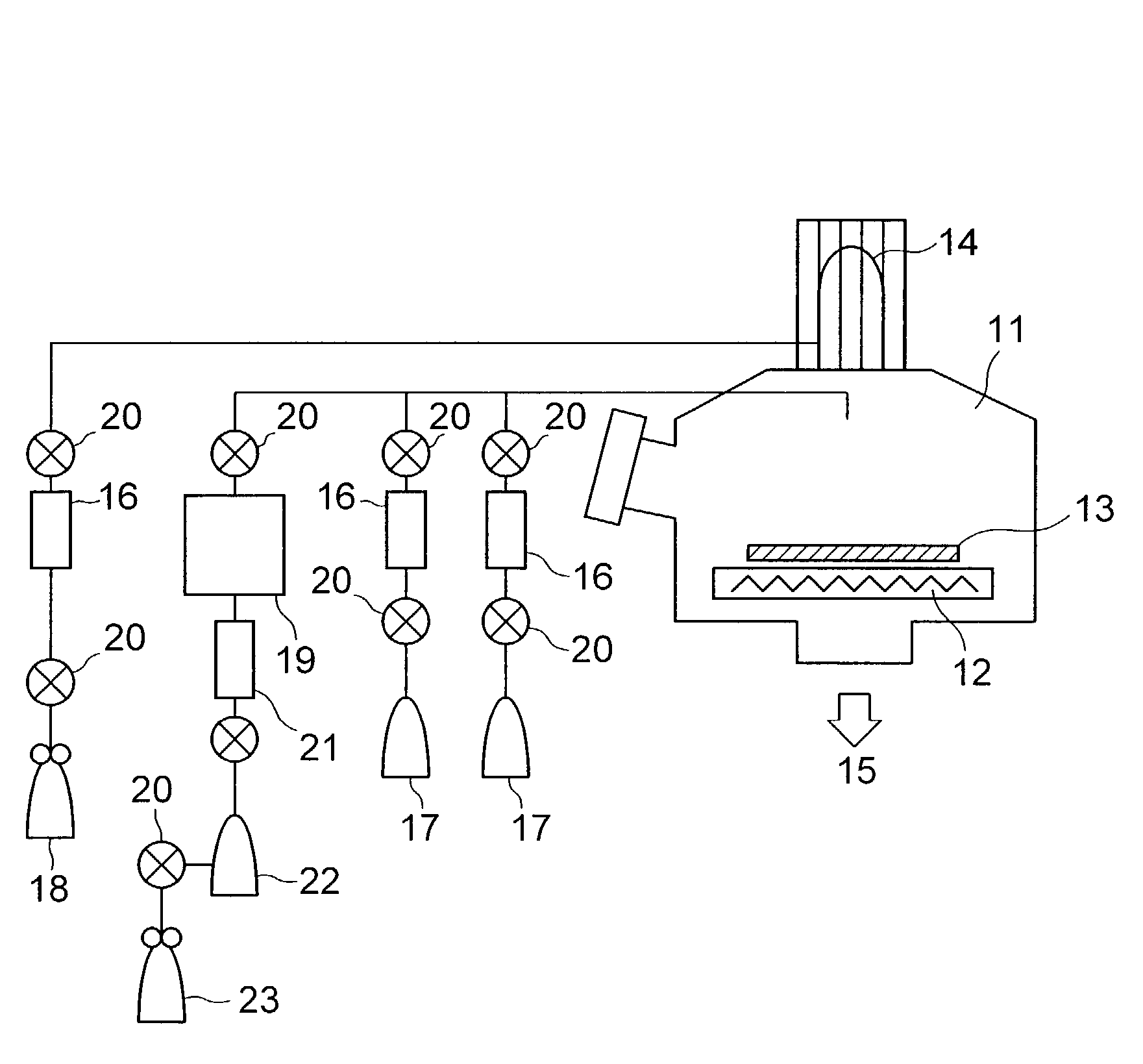
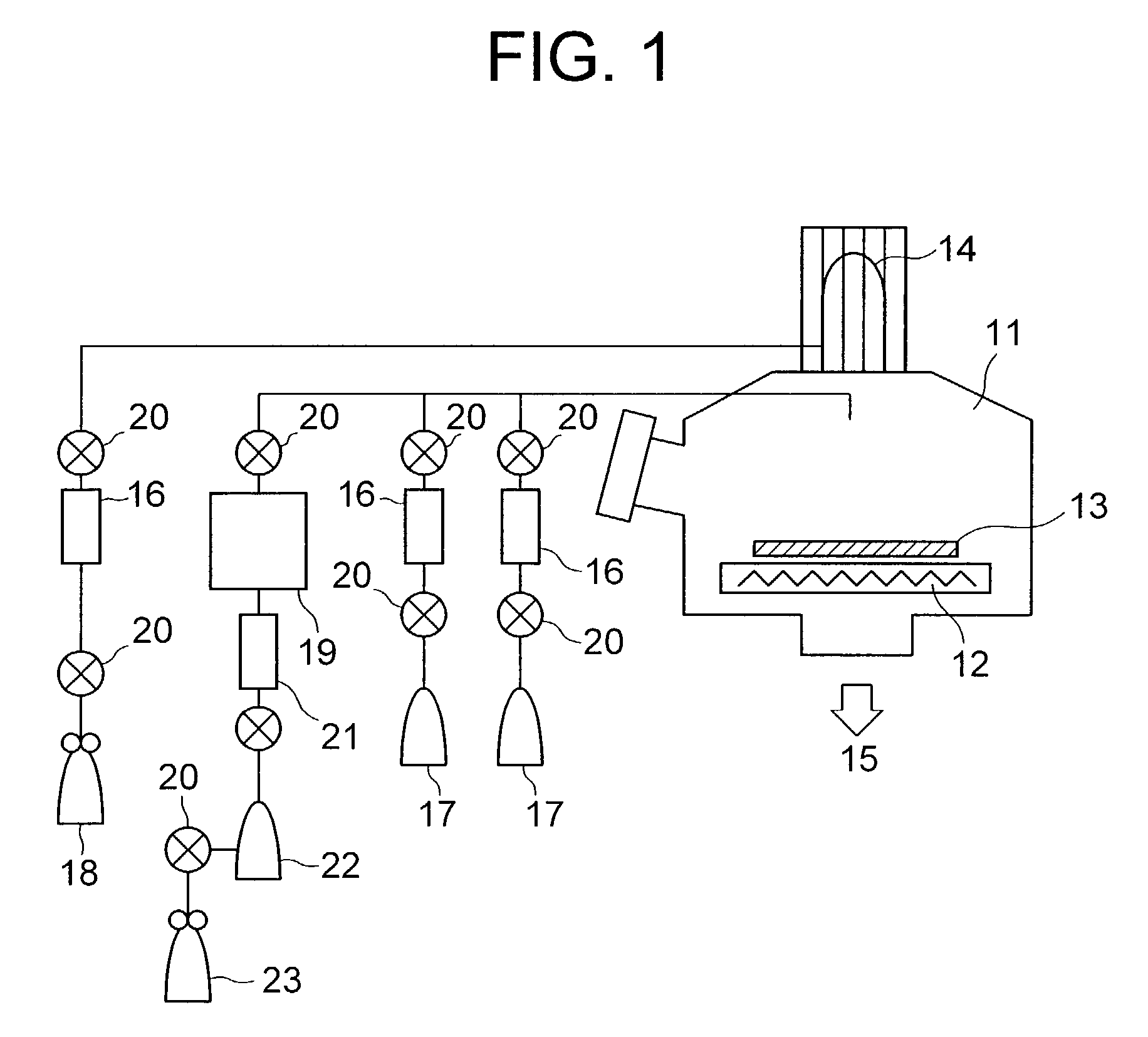
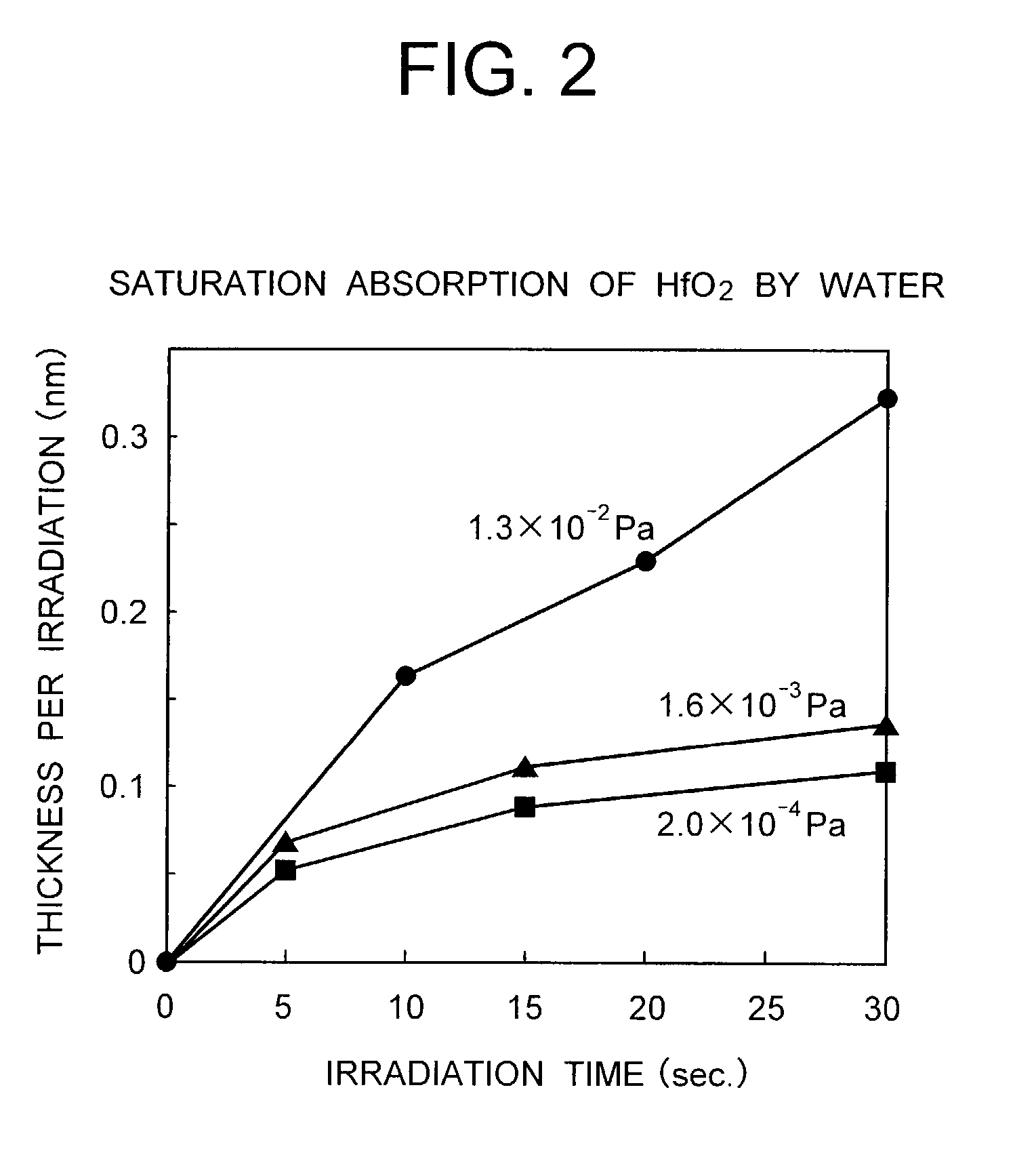
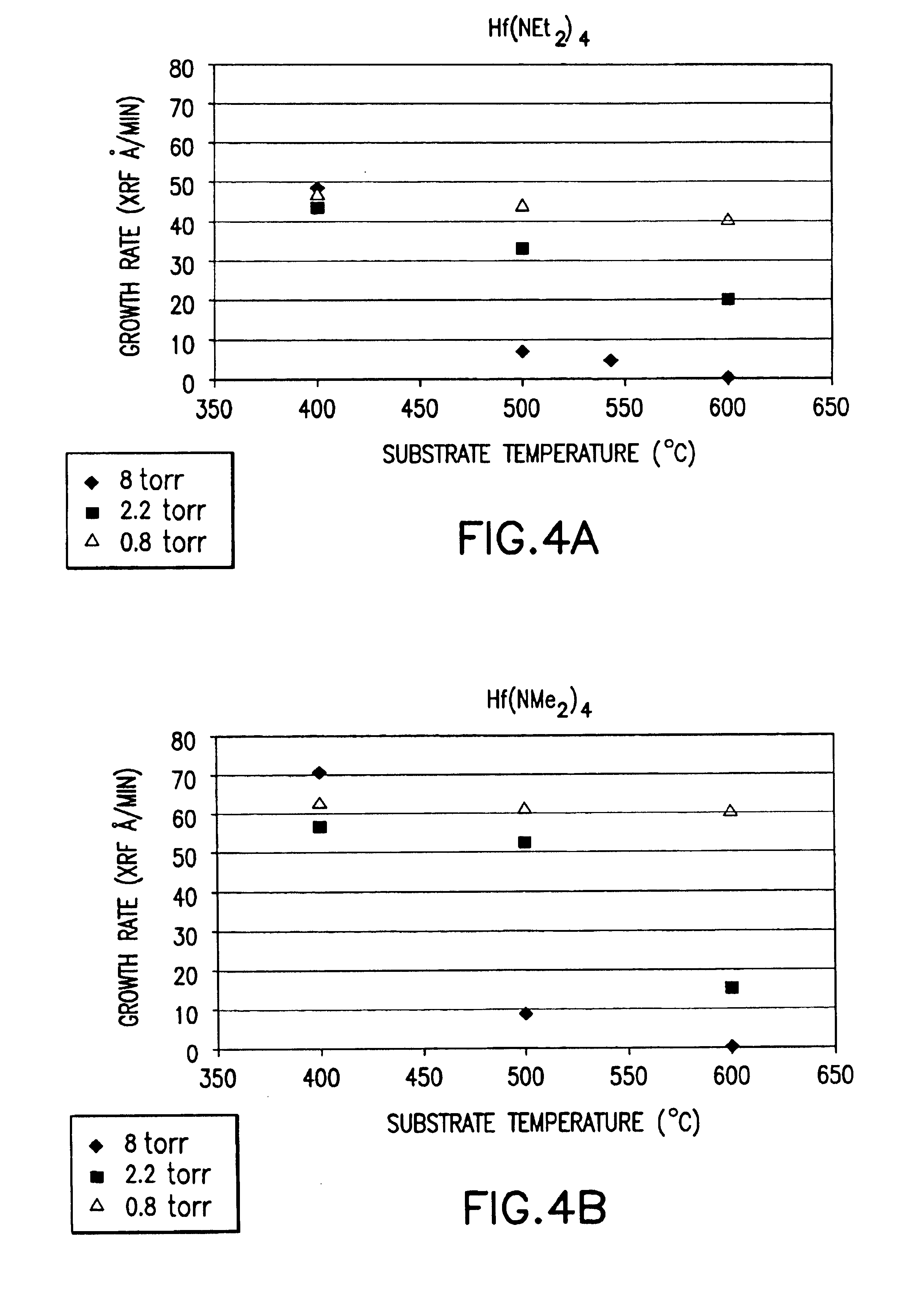
Method for vapor deposition of a metal compound film
InactiveUS20020172768A1Improve film propertiesEfficiently stackedTransistorSemiconductor/solid-state device detailsLanthanideNitrogen
A method for forming a metal compound film includes alternate irradiation of an organometal compound and oxygen or nitrogen radicals to deposit monoatomic layers of the metal compound. The organometal compound includes zirconium, hafnium, lanthanide compounds. The resultant film includes little residual carbon and has excellent film characteristic with respect to leakage current.
Owner:RENESAS ELECTRONICS CORP
Red emitting phosphor materials for use in LED and LCD applications
ActiveUS7358542B2Discharge tube luminescnet screensElectroluminescent light sourcesPhosphorPhosphate
Phosphor compositions including those having the formulas A2-xEuxW1-yMoyO6, where A is selected from Y, Gd, Lu, La, and combinations thereof; and where 0.5≦x≦1.0, 0.01≦y≦1.0; MmOnX, wherein M is selected from the group of Sc, Y, a lanthanide, an alkali earth metal and mixtures thereof; X is a halogen; 1≦m≦3; and 1≦n≦4, and wherein the lanthanide doping level can range from 0.1 to 40% spectral weight; and Eu3+ activated phosphate or borate phosphors. Also disclosed are light emitting devices including a light source and at least one of the above phosphor compositions.
Owner:GENERAL ELECTRIC CO
Modified polymers prepared with lanthanide-based catalysts
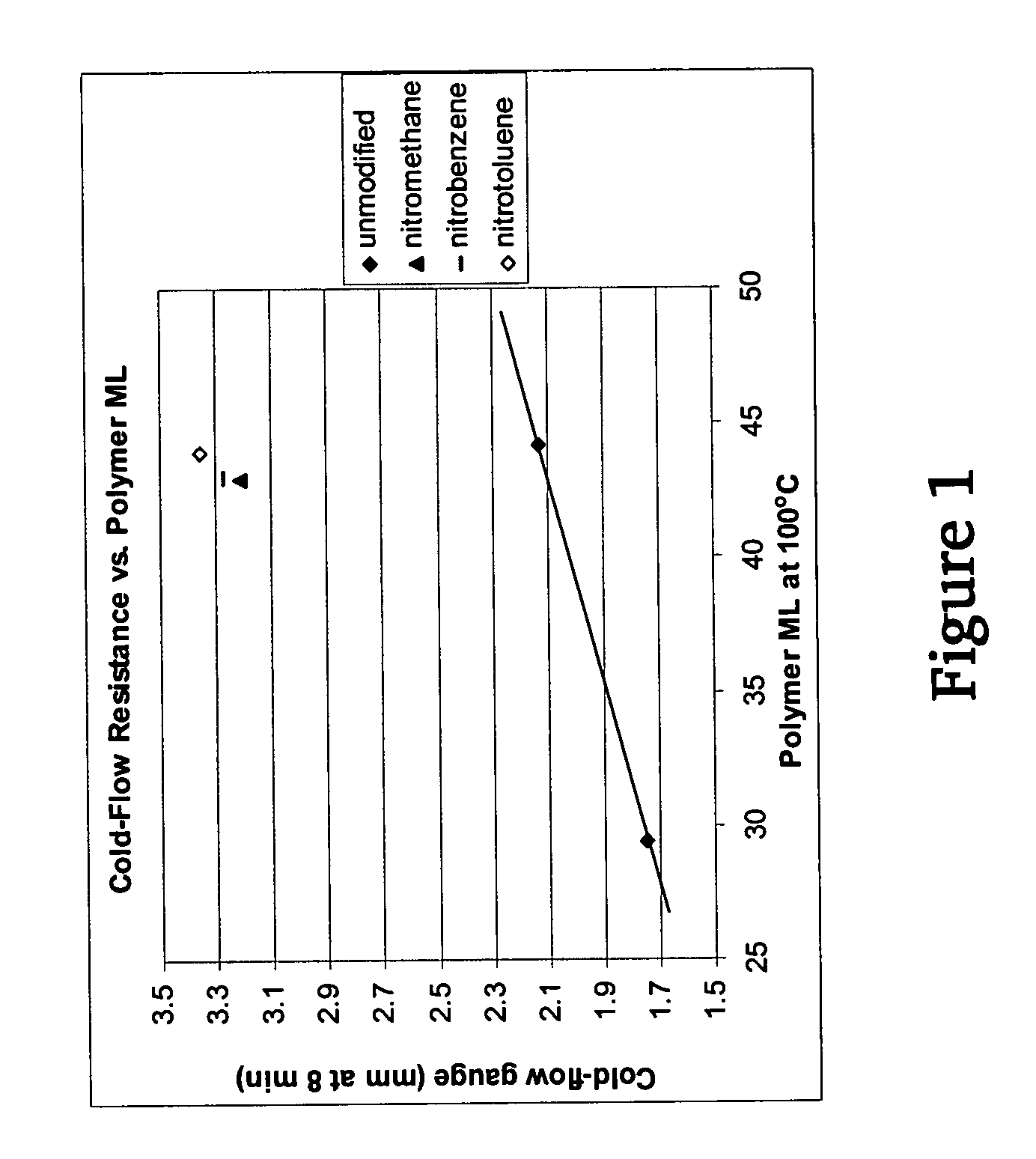
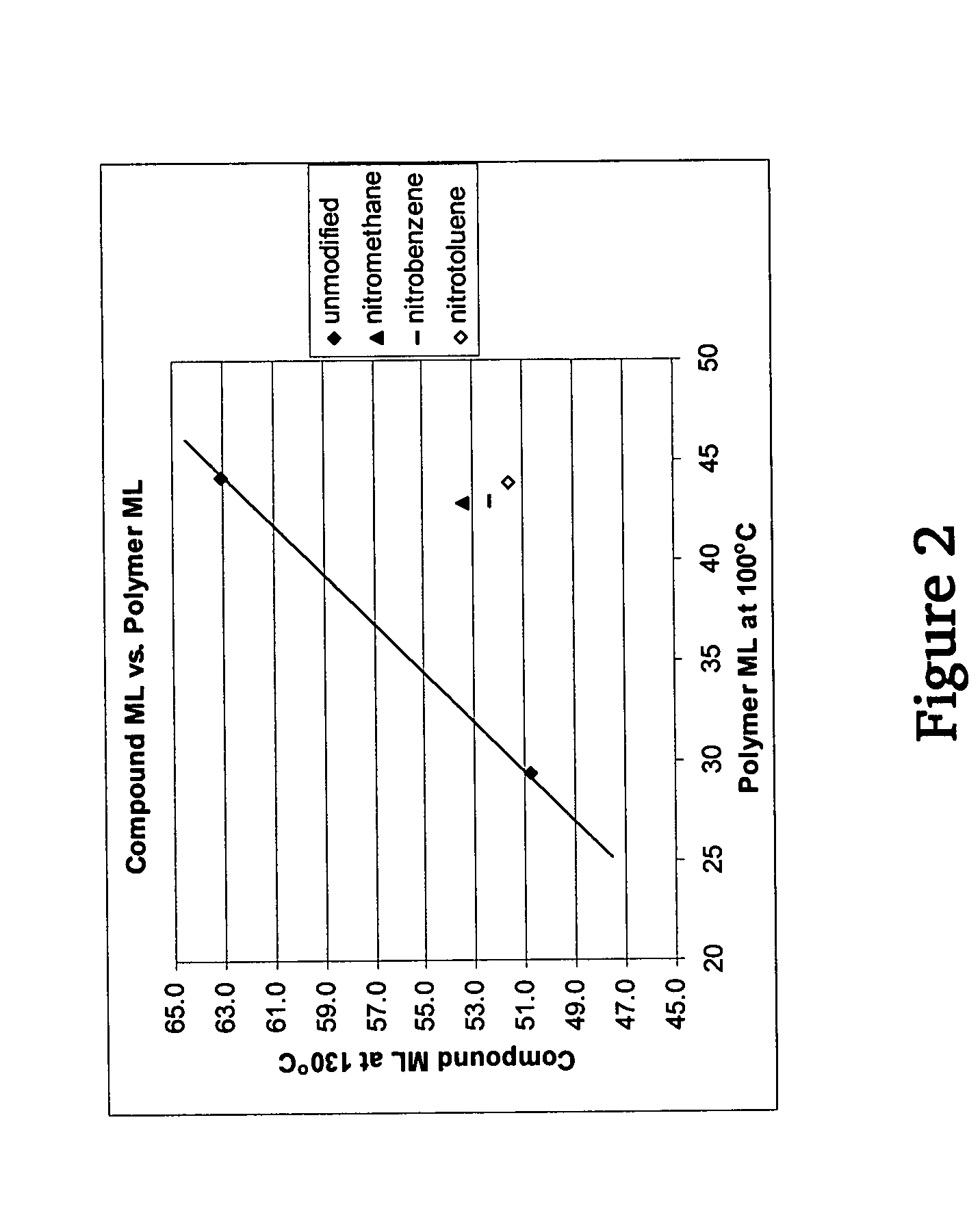
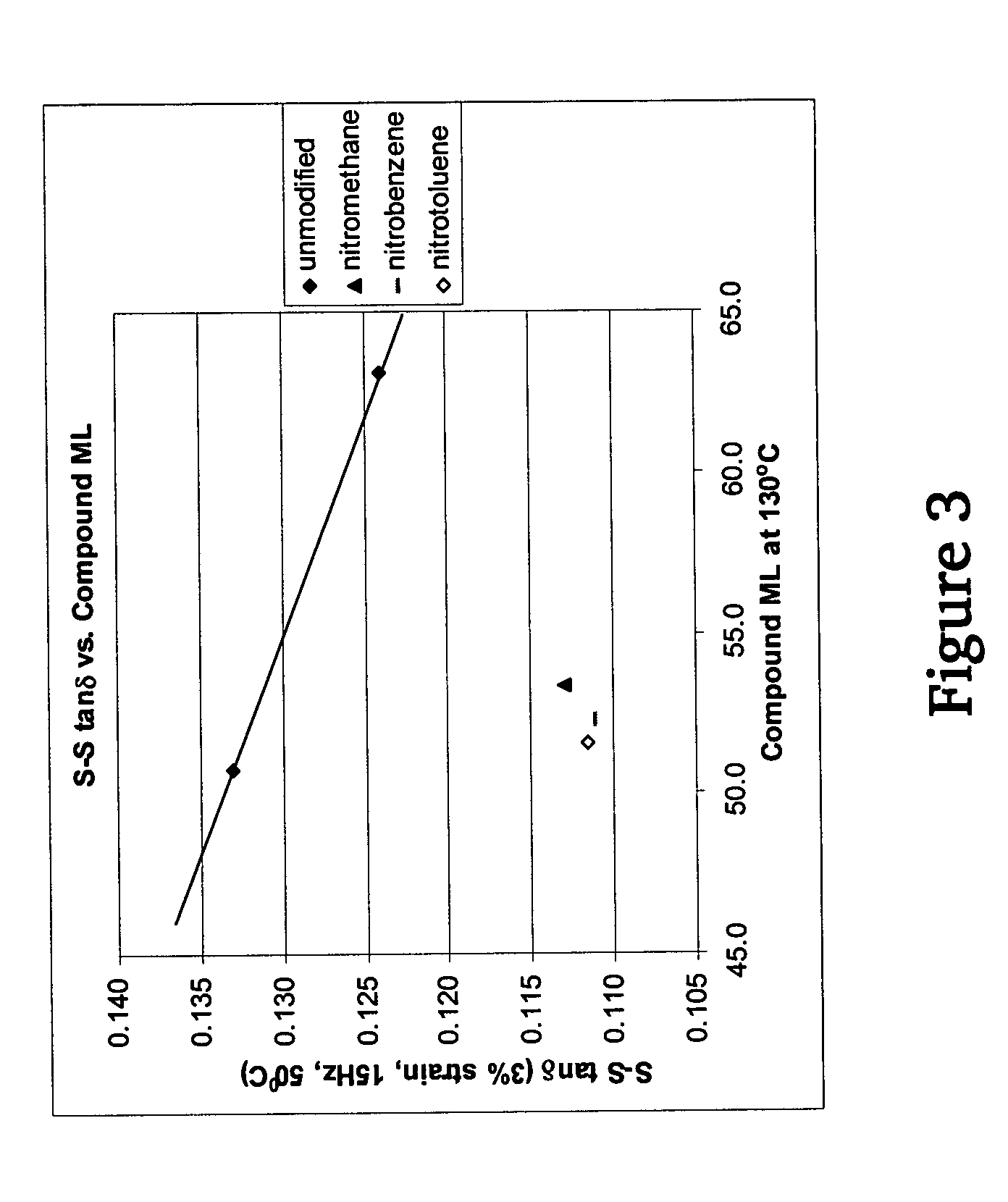
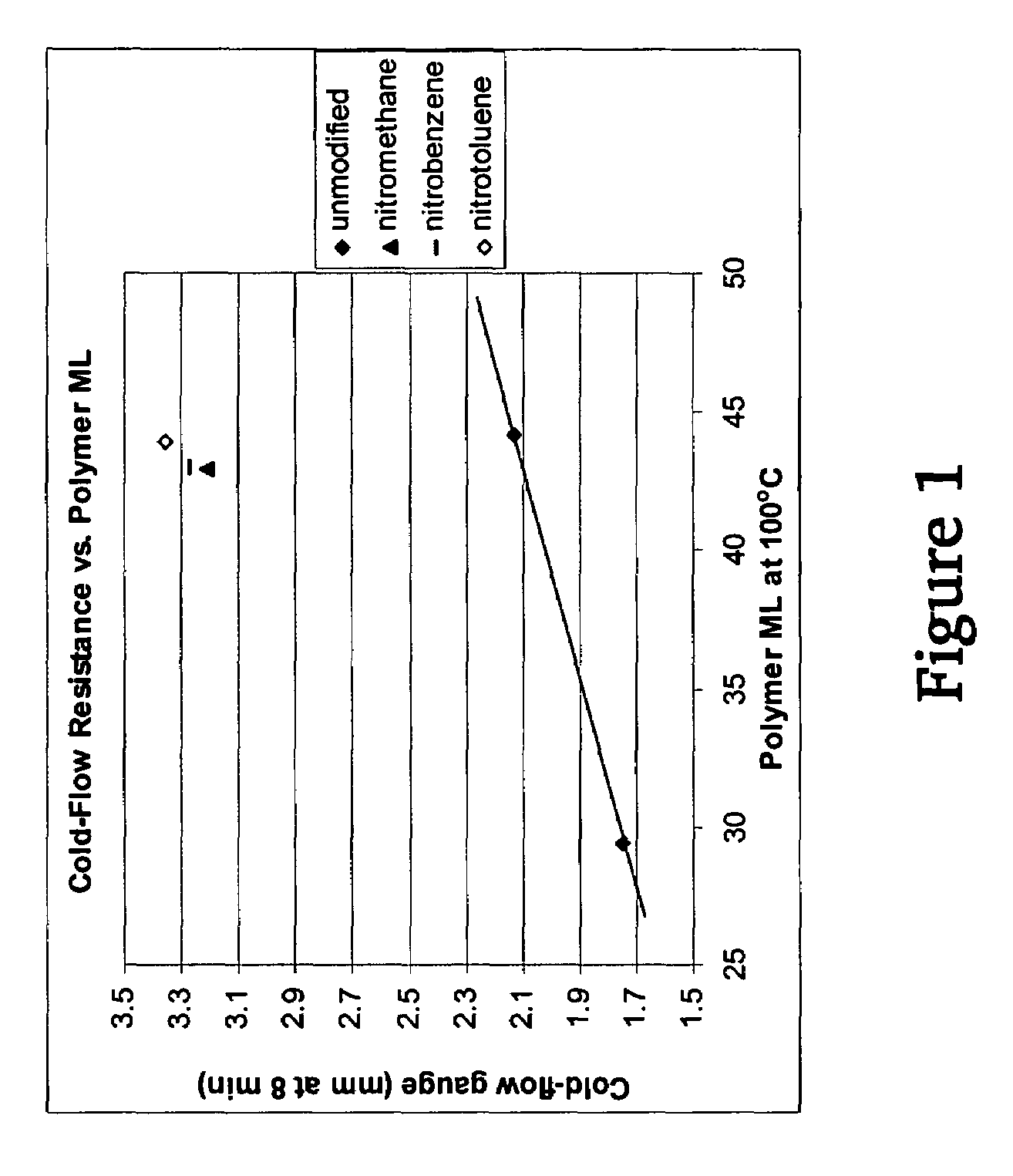
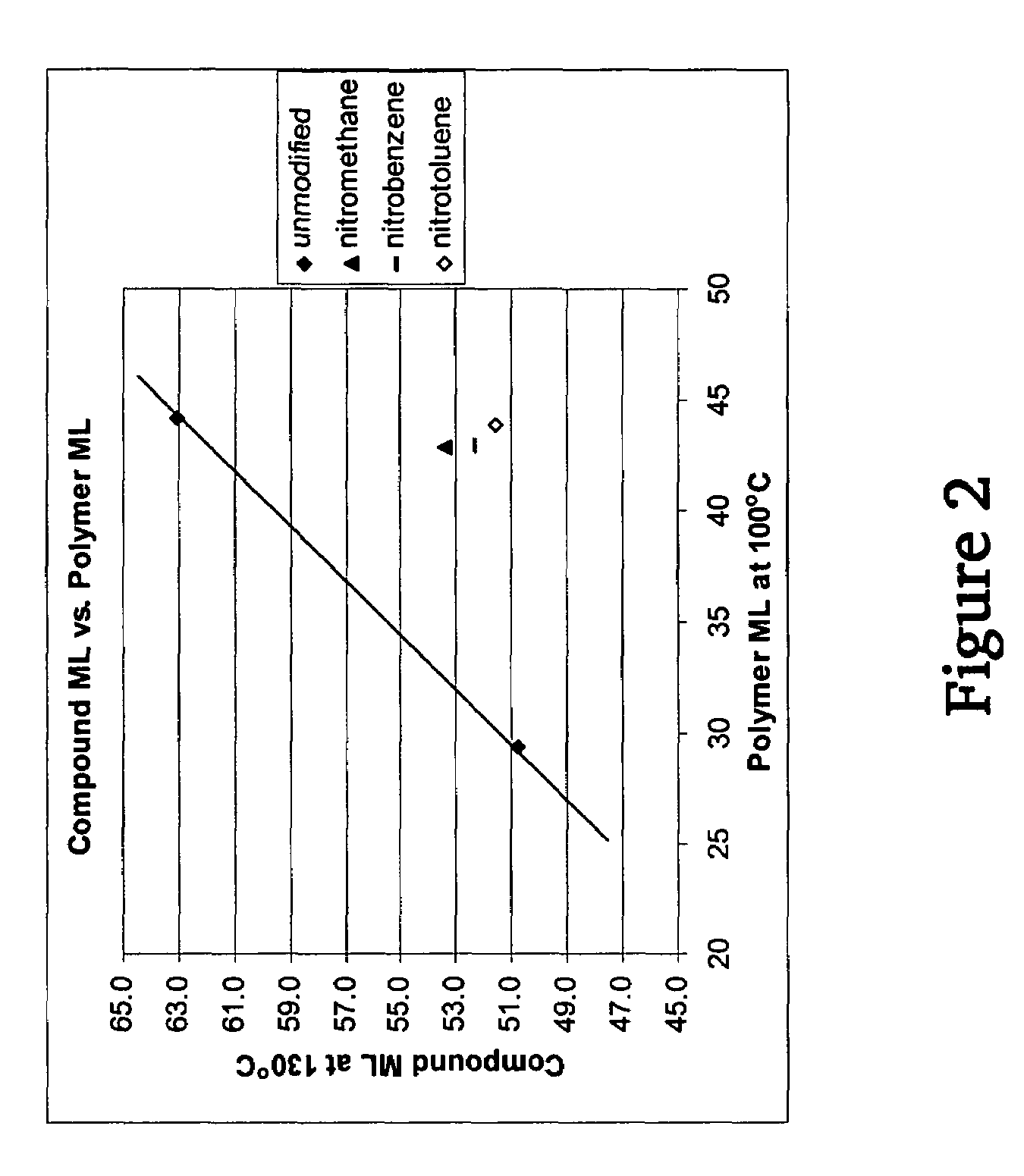
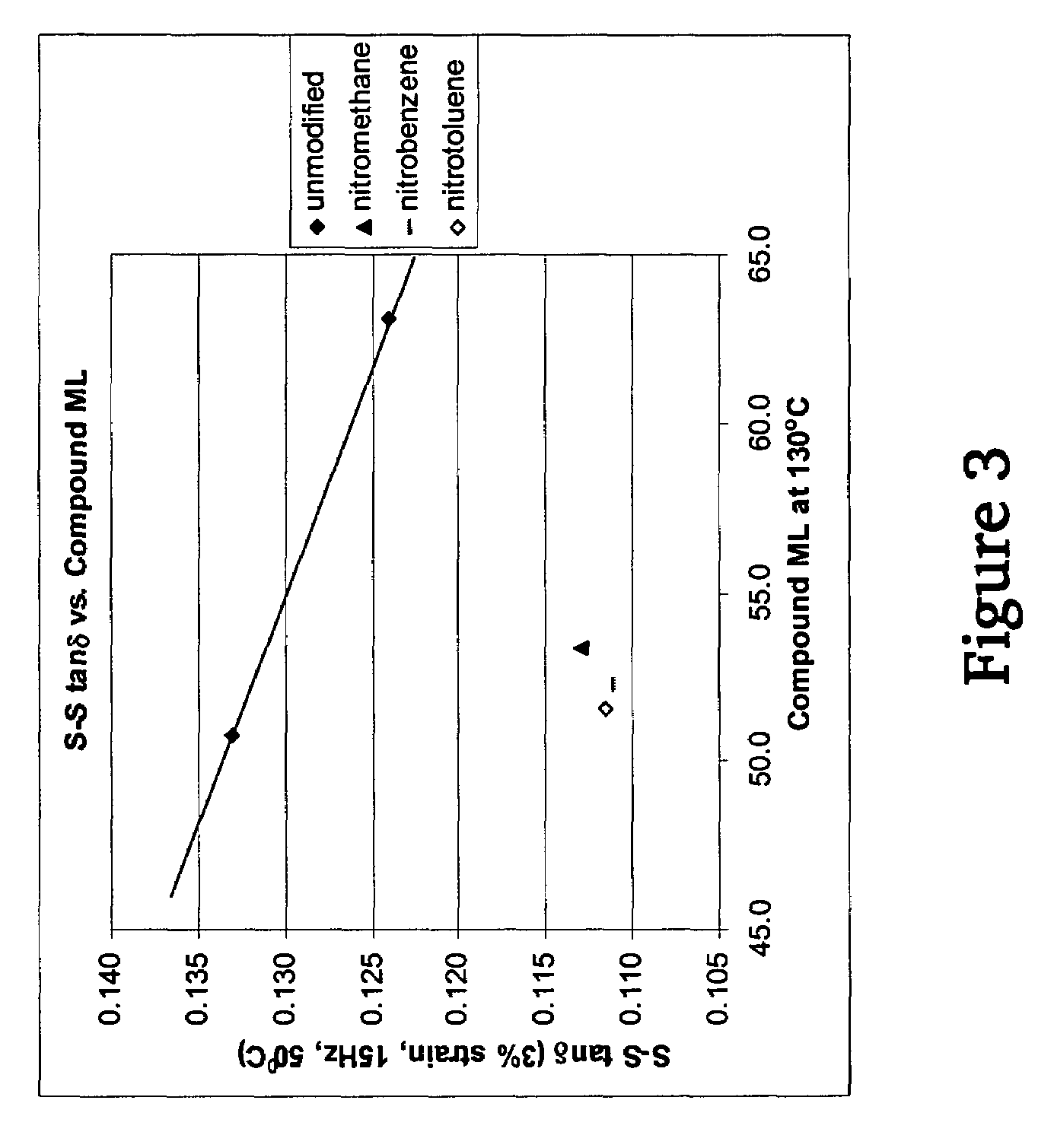
A method for preparing a functionalized polymer comprising the steps of preparing a pseudo-living polymer by polymerizing conjugated diene monomer with a lanthanide-based catalyst, and reacting the pseudo-living polymer with at least one functionalizing agent defined by the formula (I) where A is a substituent that will undergo an addition reaction with a pseudo-living polymer, R1 is a divalent organic group, R2 is a monovalent organic group, and each R4, which may be the same or different, is a monovalent organic group or a substituent defined by —OR5 where R5 is a monovalent organic group, with the proviso that A, R1, R2, R4, and R5 are substituents that will not protonate a pseudo-living polymer. Also, the functionalized polymer and a vulcanizable composition containing the polymer.
Owner:BRIDGESTONE CORP
Modified polymers prepared with lanthanide-based catalysts
A method for preparing a functionalized polymer comprising the steps of preparing a pseudo-living polymer by polymerizing conjugated diene monomer with a lanthanide-based catalyst, where said pseudo-living polymer is characterized by having greater than about 85 percent of the polymer in the cis microstructure and less than about 3 percent of the polymer is in the 1,2- or 3,4-microstructure, and reacting the pseudo-living polymer with at least one functionalizing agent defined by the formula (I) or (II) where Z is a substituent that will react or interact with organic or inorganic fillers; R1 is a single bond or a divalent organic group; R2 is a monovalent organic group or a divalent organic group that forms a cyclic organic group with R13 or R14; R3 is a single bond, a divalent organic group, or a trivalent organic group that forms a cyclic organic group with R4 or R5; R13 is a single bond, a divalent organic group, or a trivalent organic group that forms a cyclic organic group with R2 or R14; R4 is a monovalent organic group or a divalent organic group that forms a cyclic organic group with R3 or R5; R14 is a monovalent organic group or a divalent organic group that forms a cyclic organic group with R2 or R13; and R5 is a monovalent organic group or a divalent organic group that forms a cyclic organic group with R3 or R4; with the proviso that each group attached to the imino carbon is attached via a carbon atom and R1, R2, R3, R4, R5, R13, R14 and Z are substituents that will not protonate a pseudo-living polymer.
Owner:BRIDGESTONE CORP
Phosphors containing boron and metals of Group IIIA and IIIB
A phosphor comprises: (a) at least a first metal selected from the group consisting of yttrium and elements of lanthanide series other than europium; (b) at least a second metal selected from the group consisting of aluminum, gallium, indium, and scandium; (c) boron; and (d) europium. The phosphor is used in light source that comprises a UV radiation source to convert UV radiation to visible light.
Owner:GENERAL ELECTRIC CO
Novel lanthanide beta-diketonate precursors for lanthanide thin film deposition
InactiveUS20100034719A1Group 3/13 organic compounds without C-metal linkagesSemiconductor/solid-state device manufacturingLanthanideInorganic chemistry
Methods and compositions for depositing a film on one or more substrates include providing a reactor and at least one substrate disposed in the reactor. At least one lanthanide precursor is provided in vapor form and a lanthanide thin film layer is deposited onto the substrate.
Owner:AIR LIQUIDE AMERICA INC
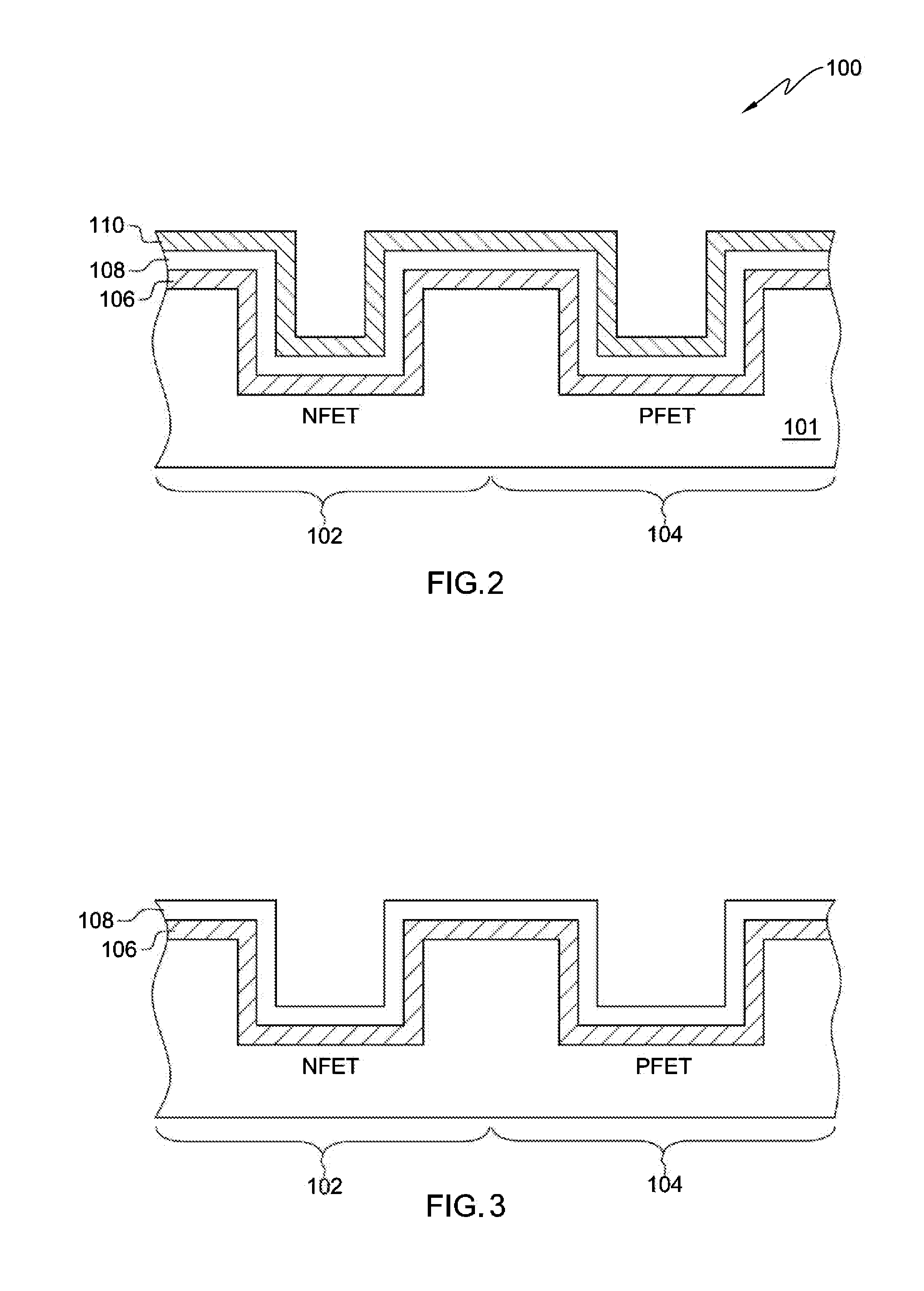
Semiconductor gate structure for threshold voltage modulation and method of making same
InactiveUS20140231922A1Lower work functionSemiconductor/solid-state device manufacturingSemiconductor devicesDielectricTitanium nitride
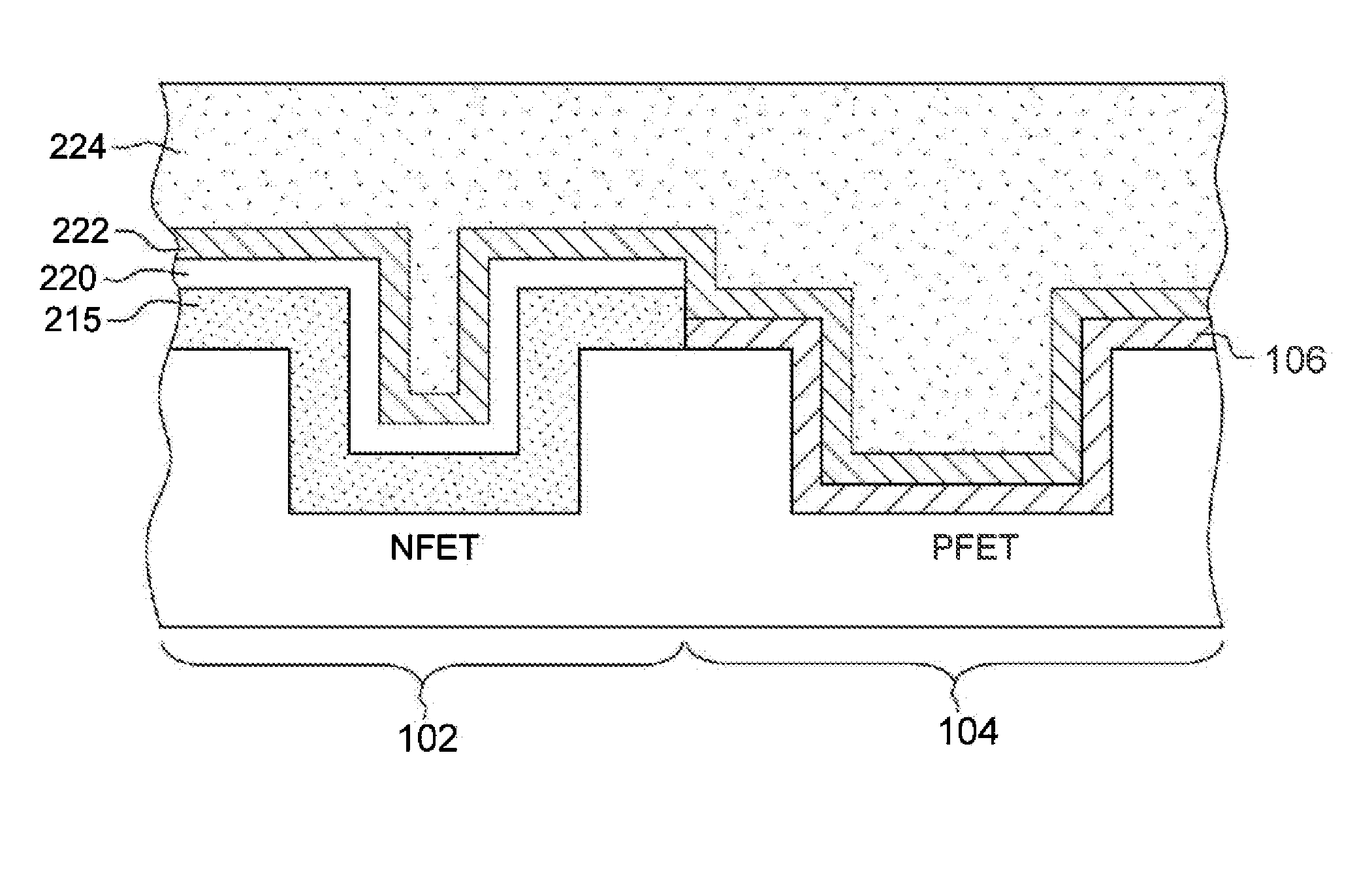
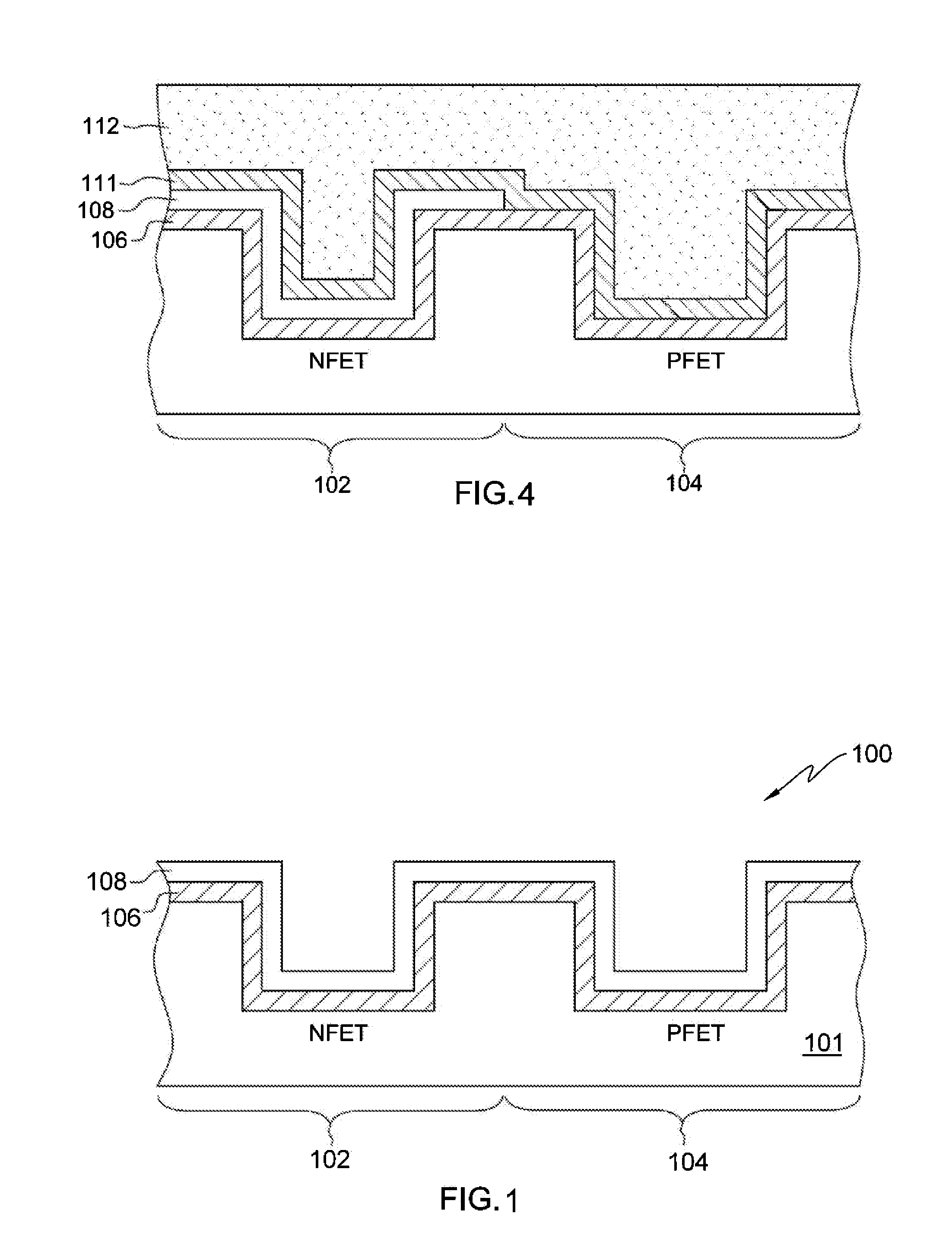
A gate structure of a semiconductor device having a NFET and a PFET, includes a lower layer of a hafnium-based dielectric over the gates of the NFET and PFET, and an upper layer of a lanthanide dielectric. The dielectrics are annealed to mix them above the NFET resulting in a lowered work function, and corresponding threshold voltage reduction. An annealed, relatively thick titanium nitride cap over the mixed dielectric above the NFET gate also lowers the work function and threshold voltage. Above the TiN cap and the hafnium-based dielectric over the PFET gate, is another layer of titanium nitride that has not been annealed. A conducting layer of tungsten covers the structure.
Owner:GLOBALFOUNDRIES US INC
Lanthanide-based catalyst composition for producing cis-1,4-polydienes
InactiveUS7008899B2Easy to processHigh viscosityOrganic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationPtru catalystLanthanide
A catalyst composition that is the combination of or the reaction product of ingredients comprising (a) a lanthanide compound, (b) an organoaluminum hydride, and (c) a tin halide compound.
Owner:BRIDGESTONE CORP
Modified polymers prepared with lanthanide-based catalysts
A method for preparing a functionalized polymer comprising the steps of preparing a pseudo-living polymer by polymerizing conjugated diene monomer with a lanthanide-based catalyst, and reacting the pseudo-living polymer with at least one functionalizing agent defined by the formula (I) where A is a substituent that will undergo an addition reaction with a pseudo-living polymer, R1 is a divalent organic group, R2 is a monovalent organic group, and each R4, which may be the same or different, is a monovalent organic group or a substituent defined by —OR5 where R5 is a monovalent organic group, with the proviso that A, R1, R2, R4, and R5 are substituents that will not protonate a pseudo-living polymer. Also, the functionalized polymer and a vulcanizable composition containing the polymer.
Owner:ENEOS MATERIALS CORP
Modified polymers prepared with lanthanide-based catalysts
A method for preparing a functionalized polymer comprising the steps of preparing a pseudo-living polymer by polymerizing conjugated diene monomer with a lanthanide-based catalyst, where said pseudo-living polymer is characterized by having greater than about 85 percent of the polymer in the cis microstructure and less than about 3 percent of the polymer is in the 1,2- or 3,4-microstructure, and reacting the pseudo-living polymer with at least one functionalizing agent defined by the formula (I) or (II) where Z is a substituent that will react or interact with organic or inorganic fillers; R1 is a single bond or a divalent organic group; R2 is a monovalent organic group or a divalent organic group that forms a cyclic organic group with R13 or R14; R3 is a single bond, a divalent organic group, or a trivalent organic group that forms a cyclic organic group with R4 or R5; R13 is a single bond, a divalent organic group, or a trivalent organic group that forms a cyclic organic group with R2 or R14; R4 is a monovalent organic group or a divalent organic group that forms a cyclic organic group with R3 or R5; R14 is a monovalent organic group or a divalent organic group that forms a cyclic organic group with R2 or R13; and R5 is a monovalent organic group or a divalent organic group that forms a cyclic organic group with R3 or R4; with the proviso that each group attached to the imino carbon is attached via a carbon atom and R1, R2 R3, R4, R5, R13, R14 and Z are substituents that will not protonate a pseudo-living polymer.
Owner:BRIDGESTONE CORP
Method for producing functionalized cis-1,4-polydienes having high cis-1,4-linkage content and high functionality
This invention relates to a method for producing functionalized cis-1,4-polydienes having a combination of a high cis-1,4-linkage content and a high functionality, the resulting polymers and the vulcanized products containing the polymers. The functionalized cis-1,4-polydienes of the present invention are produced by a method comprising the steps of: (1) preparing a reactive polymer by polymerizing conjugated diene monomer with a lanthanide-based catalyst in the presence of less than 20% by weight of organic solvent based on the total weight of monomer, organic solvent, and resulting polymer, where the lanthanide-based catalyst is the combination of or reaction product of (a) a lanthanide compound, (b) an aluminoxane, (c) an organoaluminum compound other than an aluminoxane, and (d) a halogen-containing compound; and (2) contacting the reactive polymer with a functionalizing agent.
Owner:BRIDGESTONE CORP
Lanthanide oxide/zirconium oxide atomic layer deposited nanolaminate gate dielectrics
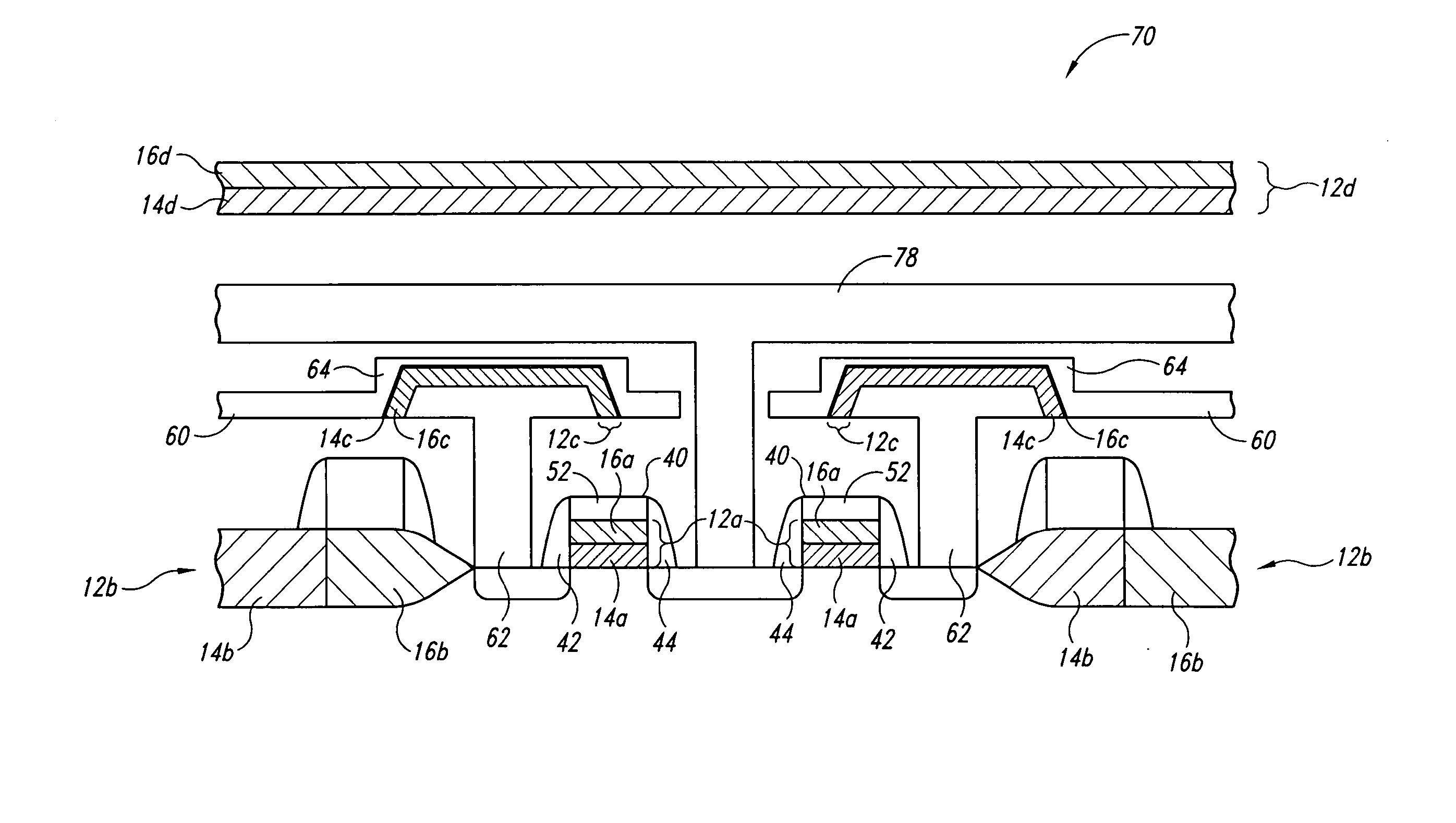
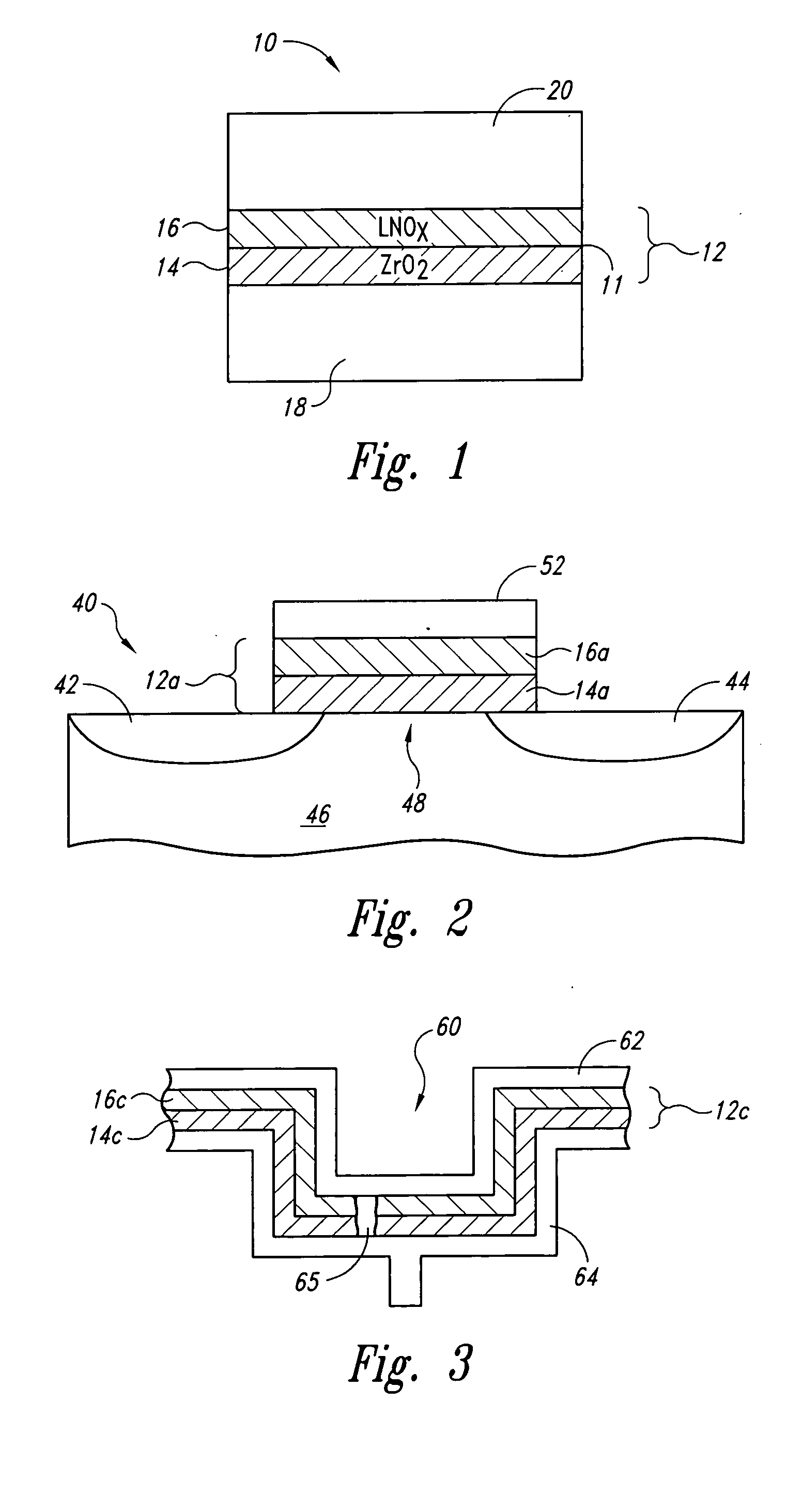
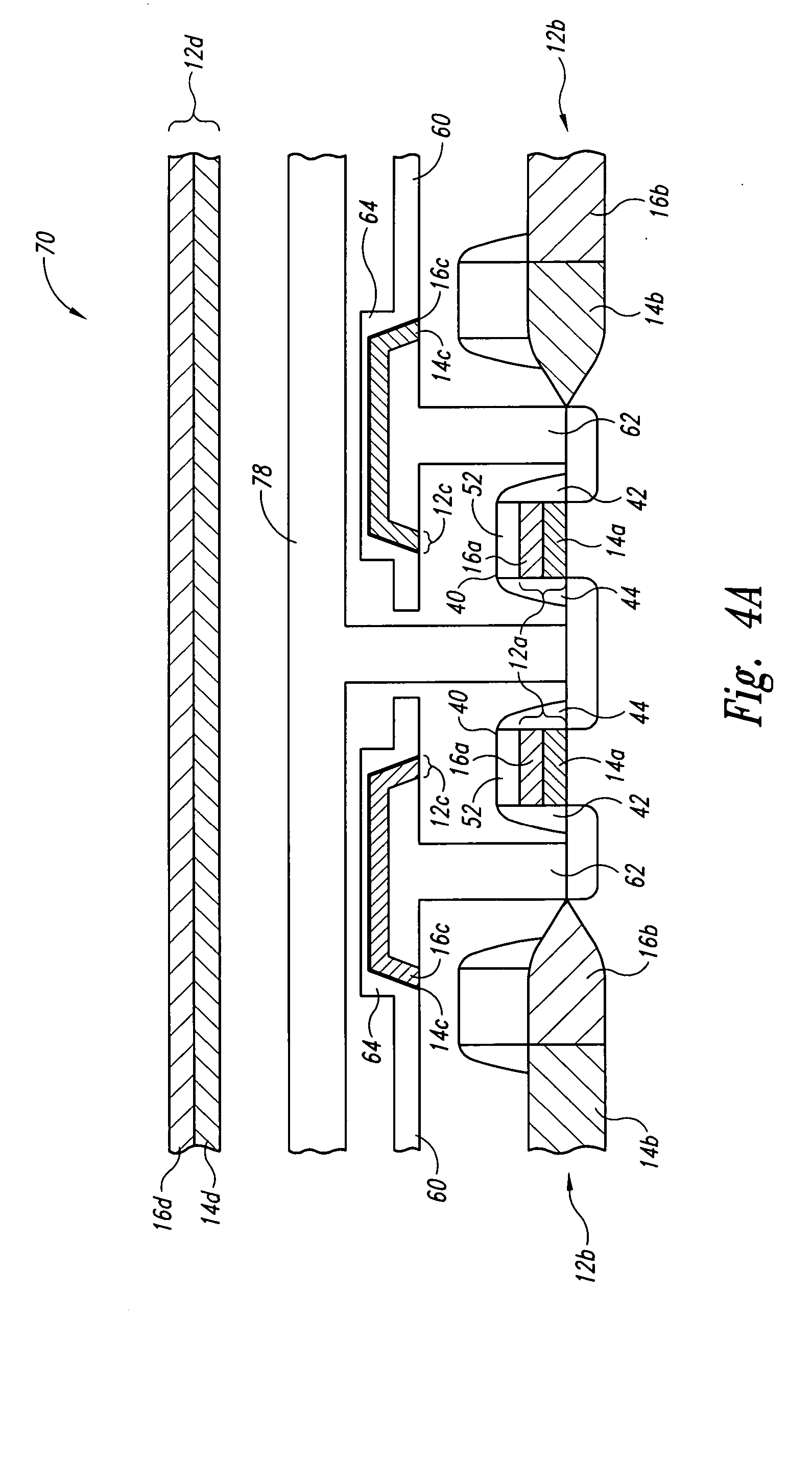
The invention provides a laminated dielectric layer for semiconductor devices formed by a combination of ZrO2 and a lanthanide oxide on a semiconductor substrate and methods of making the same. In certain methods, the ZrO2 is deposited by multiple cycles of reaction sequence atomic layer deposition (RS-ALD) that includes depositing a ZrI4 precursor onto the surface of the substrate in a first pulse followed by exposure to H2O / H2O2 in a second pulse, thereby forming a thin ZrO2 layer on the surface. After depositing the ZrO2 layer, the lanthanide oxide layer is deposited by electron beam evaporation. The composite laminate zirconium oxide / lanthanide oxide dielectric layer has a relatively high dielectric constant and can be formed in layers of nanometer dimensions. It is useful for a variety of semiconductor applications, particularly for DRAM gate dielectric layers and DRAM capacitors.
Owner:MICRON TECH INC
Polymers functionalized with nitro compounds
A method for preparing a functionalized polymer, the method comprising the steps of (i) polymerizing conjugated diene monomer by employing a lanthanide-based catalyst to form a reactive polymer, and (ii) reacting the reactive polymer with a nitro compound.
Owner:BRIDGESTONE CORP
Continuous process for the production of conjugated diene polymers having narrow molecular weight distribution and products therefrom
InactiveUS6897270B2Narrow molecular weight distributionImprove responseHydrocarbon solventsContinuous reactor
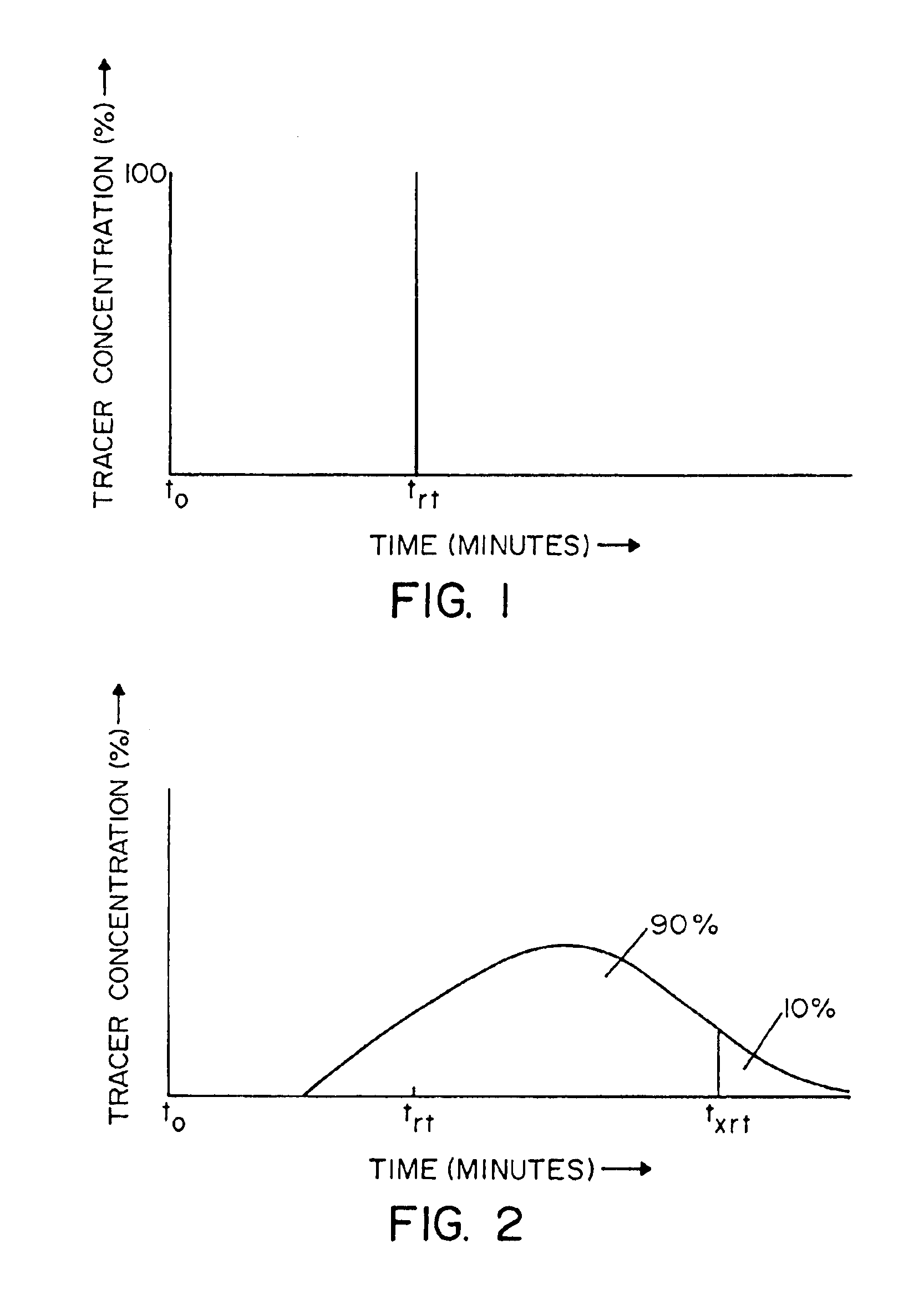
A continuous process for producing conjugated diene polymers comprising the steps of contacting, within an hydrocarbon solvent and within a continuous reactor, conjugated diene monomer and a catalyst composition prepared by combining: (a) a lanthanide compound, (b) an alkylating agent, and (c) a halogen-containing compound, and maintaining a non-ideal flow pattern within the continuous reactor so that 10% of the reagents entering the reactor at a reference time t0 are still present within the continuous reactor at a time t0+xtrt, where trt is the residence time corresponding to ideal flow within the continuous reactor and x is a numeral greater than 1.5.
Owner:BRIDGESTONE CORP
Bulk polymerization process for producing polydienes
ActiveUS7094849B2Organic-compounds/hydrides/coordination-complexes catalystsHalogenBulk polymerization
A method of producing cis-1,4-polydienes, the method comprising the step of contacting conjugated diene monomer with a lanthanide-based catalyst system in the presence of less than 20% by weight of organic solvent based on the total weight of monomer, organic solvent, and resulting polymer, where the lanthanide-based catalyst system is the combination of or reaction product of (a) a lanthanide compound, (b) an organoaluminum hydride, (c) a trihydrocarbylaluminum, and (d) a halogen-containing compound.
Owner:BRIDGESTONE CORP
Source reagent compositions for CVD formation of gate dielectric thin films using amide precursors and method of using same
InactiveUS6869638B2Group 4/14 organic compounds without C-metal linkagesGroup 8/9/10/18 element organic compoundsGate dielectricHydrogen
A CVD Method of forming gate dielectric thin films on a substrate using metalloamide compounds of the formula M(NR1R2)x, or wherein M is Zr, Hf, Y, La, Lanthanide series elements, Ta, Ti, or Al; N is nitrogen; each of R1 and R2 is same or different and is independently selected from H, aryl, perfluoroaryl, C1-C8 alkyl, C1-C8 perfluoroalkyl, alkylsilyl; and x is the oxidation state on metal M; and an aminosilane compound of the formula HxSiAy(NR1R2)4-x-y or wherein H is hydrogen; x is from 0 to 3; Si is silicon; A is a halogen; Y is from 0 to 3; N is nitrogen; each of R1 and R2 is same or different and is independently selected from the group consisting of H, aryl, perfluoroaryl, C1-C8 alkyl, and C1-C8 perfluoroalkyl; and n is from 1-6. By comparison with the standard SiO2 gate dielectric materials, these gate dielectric materials provide low levels of carbon and halide impurity.
Owner:ENTEGRIS INC
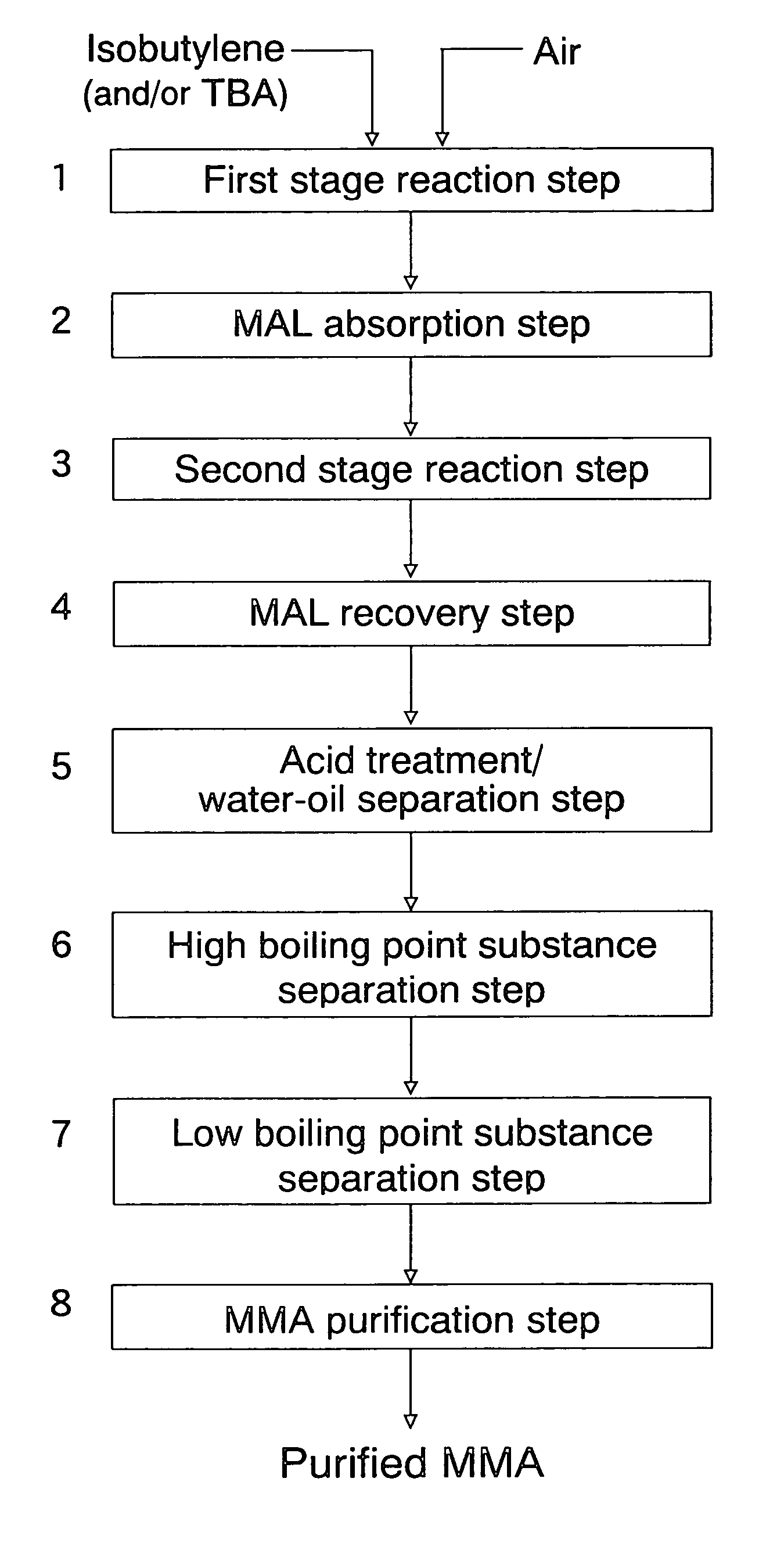
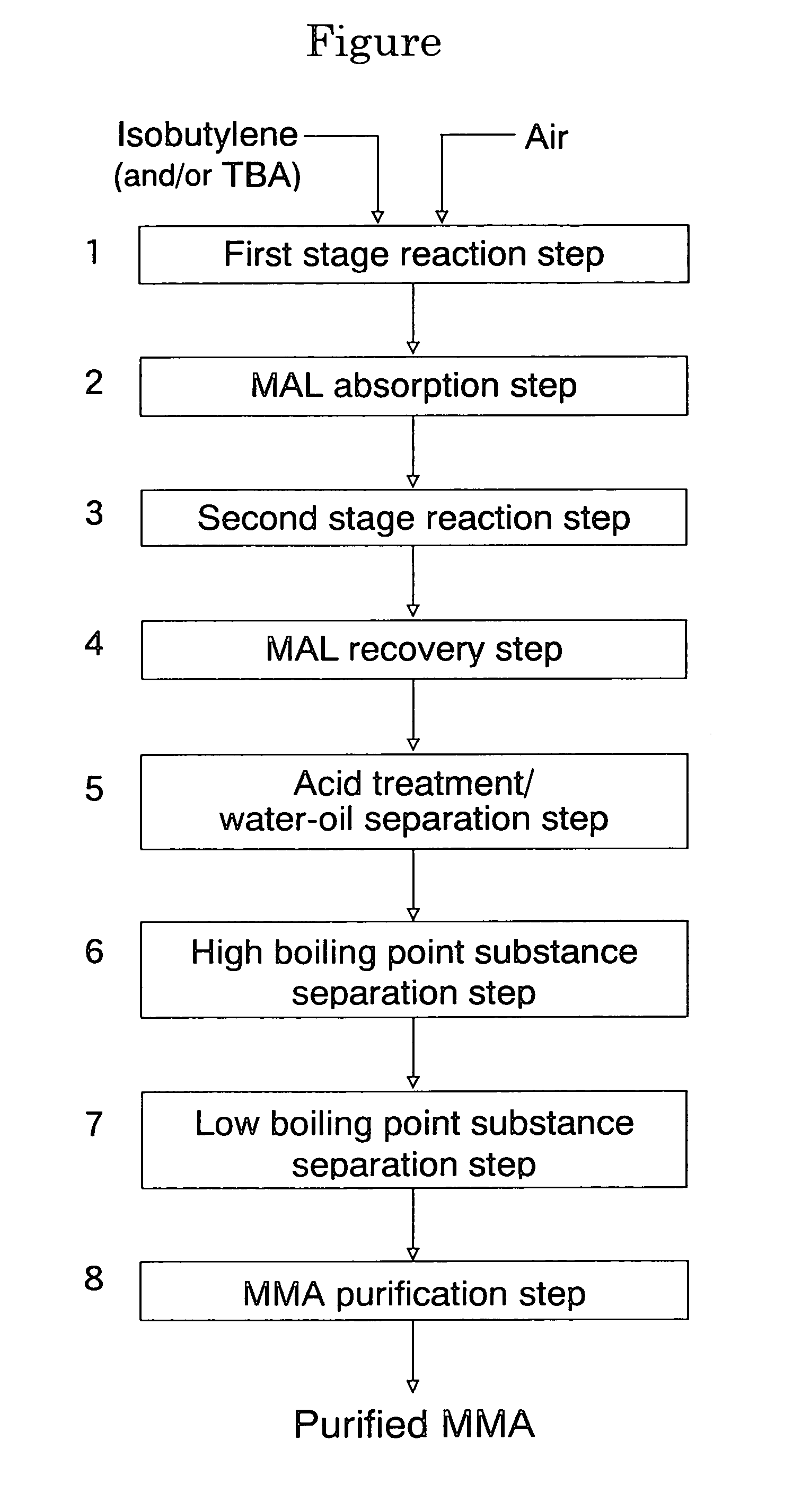
Oxide catalyst composition
InactiveUS7012039B2High selectivityLow selectivityOrganic compound preparationCarboxylic acid esters preparationLanthanideMethacrolein
An oxide catalyst composition for use in producing methacrolein or a mixture of methacrolein and methacrylic acid, wherein the oxide catalyst composition is represented by the formula (Mo+W)l2BiaAbBcFedXeSbfOg, wherein: A is at least one member selected from the group consisting of Y and the elements of the lanthanoid series exclusive of Pm; B is at least one member selected from the group consisting of K, Rb and Cs; X is Co solely, or a mixture of Co and at least one member selected from the group consisting of Mg and Ni; and a, b, c, d, e, f and g are, respectively, the atomic ratios of Bi, A, B, Fe, X, Sb and O, relative to twelve atoms of the total of Mo and W, wherein the atomic ratios (a to f) of the elements and the relationship between the amounts of the elements are chosen so as to satisfy specific requirements.
Owner:ASAHI KASEI CHEM CORP
Ammonothermal process for bulk synthesis and growth of cubic GaN
InactiveUS20030209191A1Easy to transportQuality improvementPolycrystalline material growthFrom chemically reactive gasesLanthanideSingle crystal
A method of growing single-crystals of a cubic (zinc blende) form of gallium nitride, the method comprising the steps of: placing into a reaction tube or acid resistant vessel a gallium source, anhydrous ammonia, an acid mineralizer and a metal halide salt selected from the group consisting of alkali metal halides, copper halides, tin halides, lanthanide halides and combinations thereof; closing said reaction tube or vessel; heating said reaction tube; cooling said reaction tube or vessel; and collecting single-crystals of cubic (zinc blende) form of GaN; wherein said reaction tube or vessel has a temperature gradient with a hot zone of at least 250° C., wherein said reaction tube or vessel has a temperature gradient with a cool zone of at least 150° C., and wherein said acid mineralizer has a sufficient concentration to permit chemical transport of GaN in said reaction tube or vessel from said hot zone to said cool zone due to said temperature gradient within said reaction tube or vessel.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
Metallocene compounds, process for their preparation and their use in catalytic systems for the polymerization of olefins
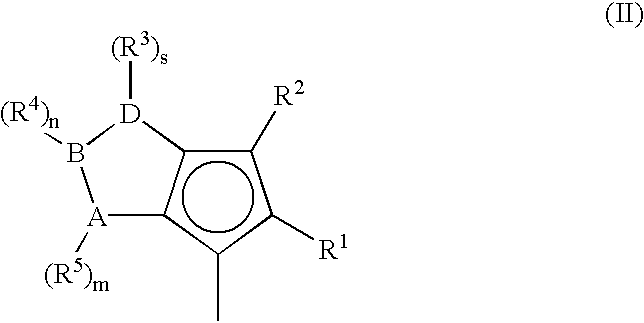
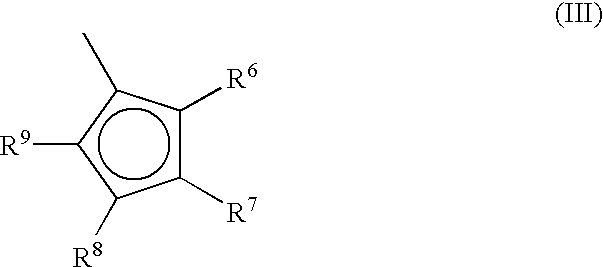
A class of metallocene compounds is disclosed having general formula (I) wherein Y is a moiety of formula (II) wherein A, B, and D, same or different from each other, are selected from an element of the groups 14 to 16 of the Periodic Table of the Elements (new IUPAC version), with the exclusion of nitrogen and oxygen; R<1>, R<2>, R<3>, R<4 >and R<5 >are hydrogen or hydrocarbon groups, Z is selected from a moiety of formula (II) as described above and from a moiety of formula (III) wherein R<6>, R<7>, R<8 >and R<9>, are hydrogen or hydrocarbon groups; L is a divalent bridging group; M is an atom of a transition metal selected from those belonging to group 3, 4, 5, 6 or to the lanthanide or actinide groups in the Periodic Table of the Elements (new IUPAC version), X, same or different, is hydrogen, a halogen, a R<10>, OR<10>, OSO2CF3, OCOR<10>, SR<10>, NR<10>2 or PR<10>2 group, wherein the substituents R<10 >are hydrogen or alkyl groups; p is an integer of from 1 to 3, being equal to the oxidation state of the metal M minus 2. The above metallocenes are particularly useful in the polymerization of propylene.
Owner:BASELL POLYOLEFINE GMBH
Thin film solar cell
InactiveUS20080078444A1Good choiceImprovement factorPhotovoltaic energy generationSemiconductor devicesHigh energyUltraviolet
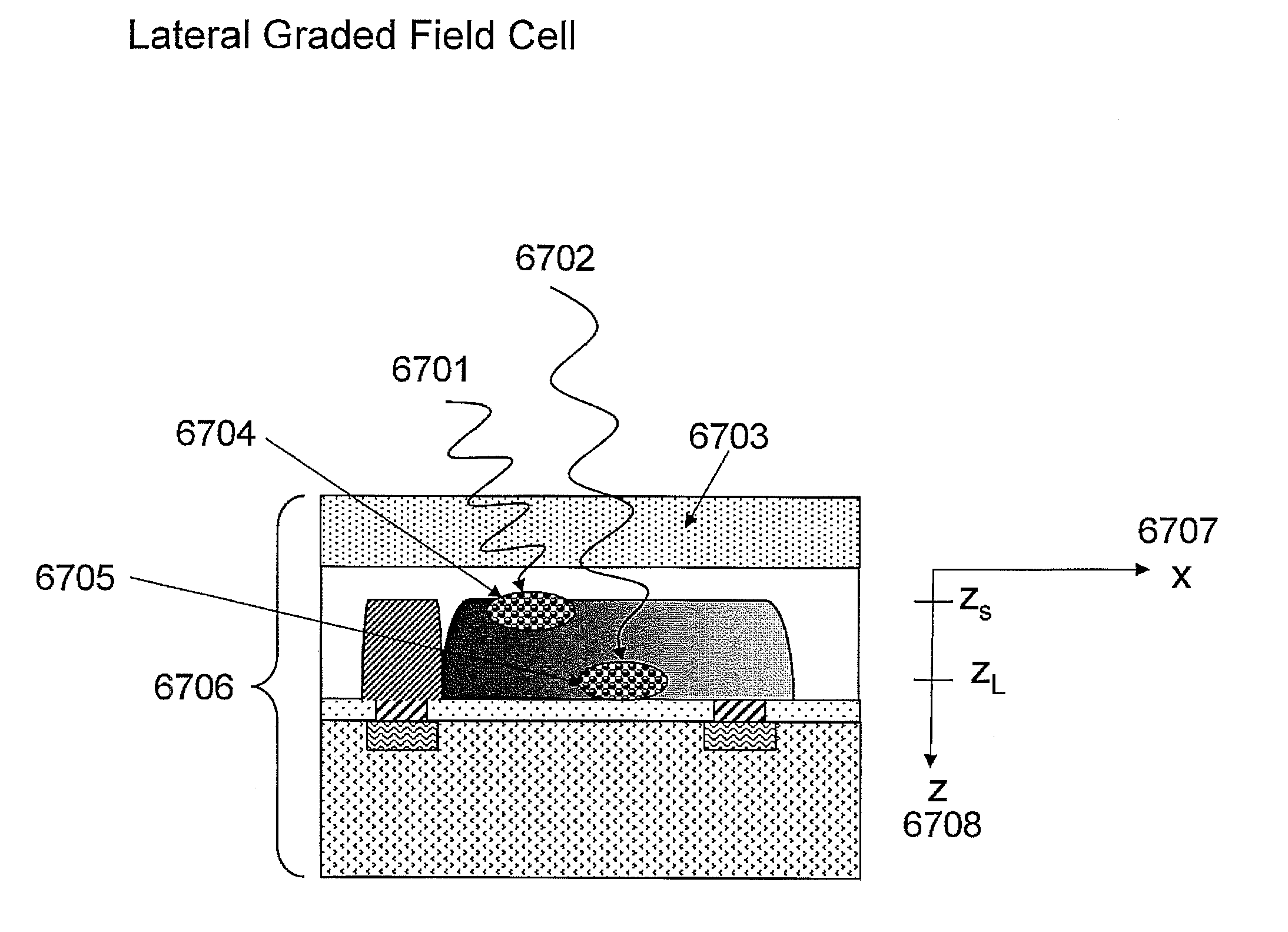
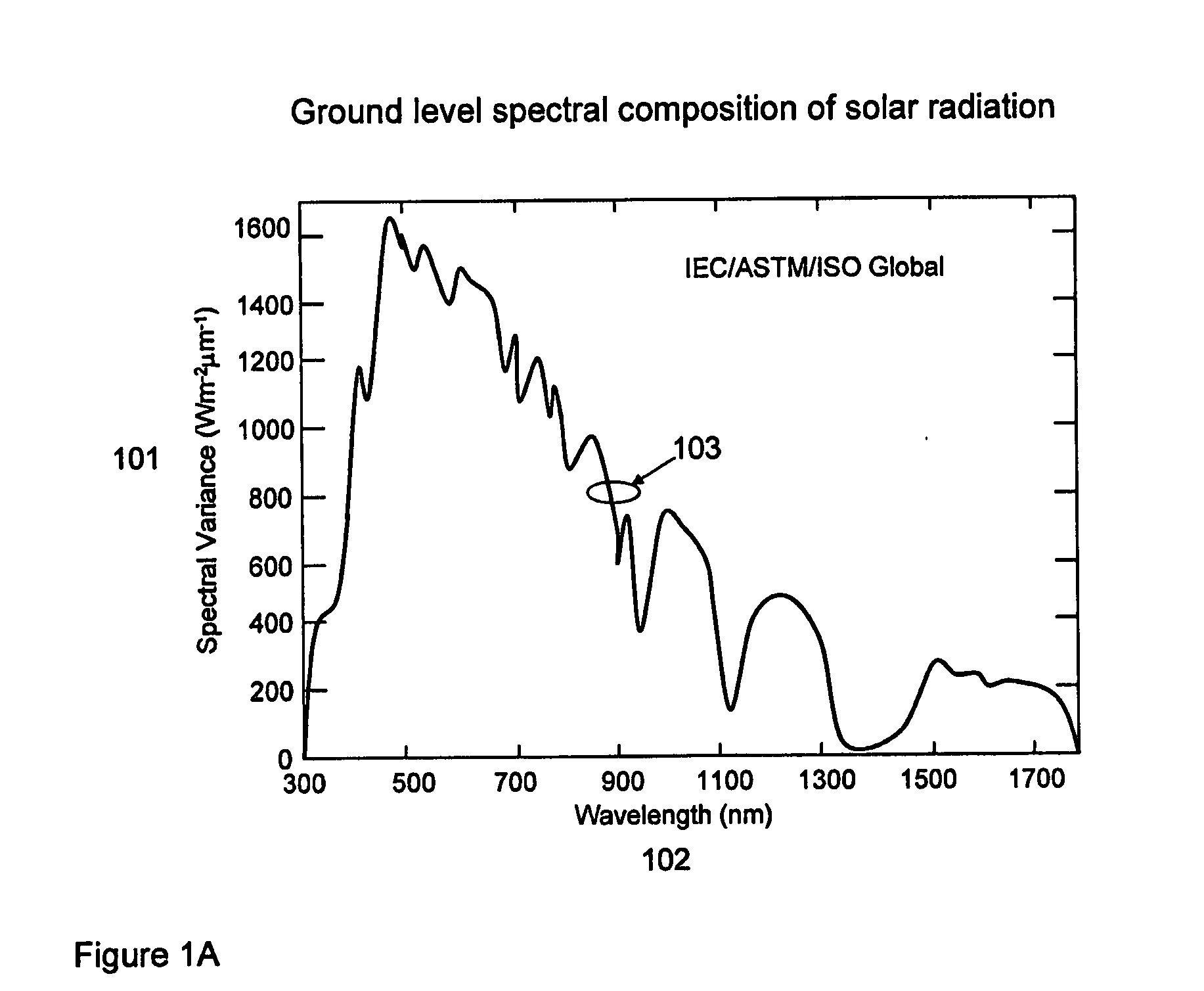
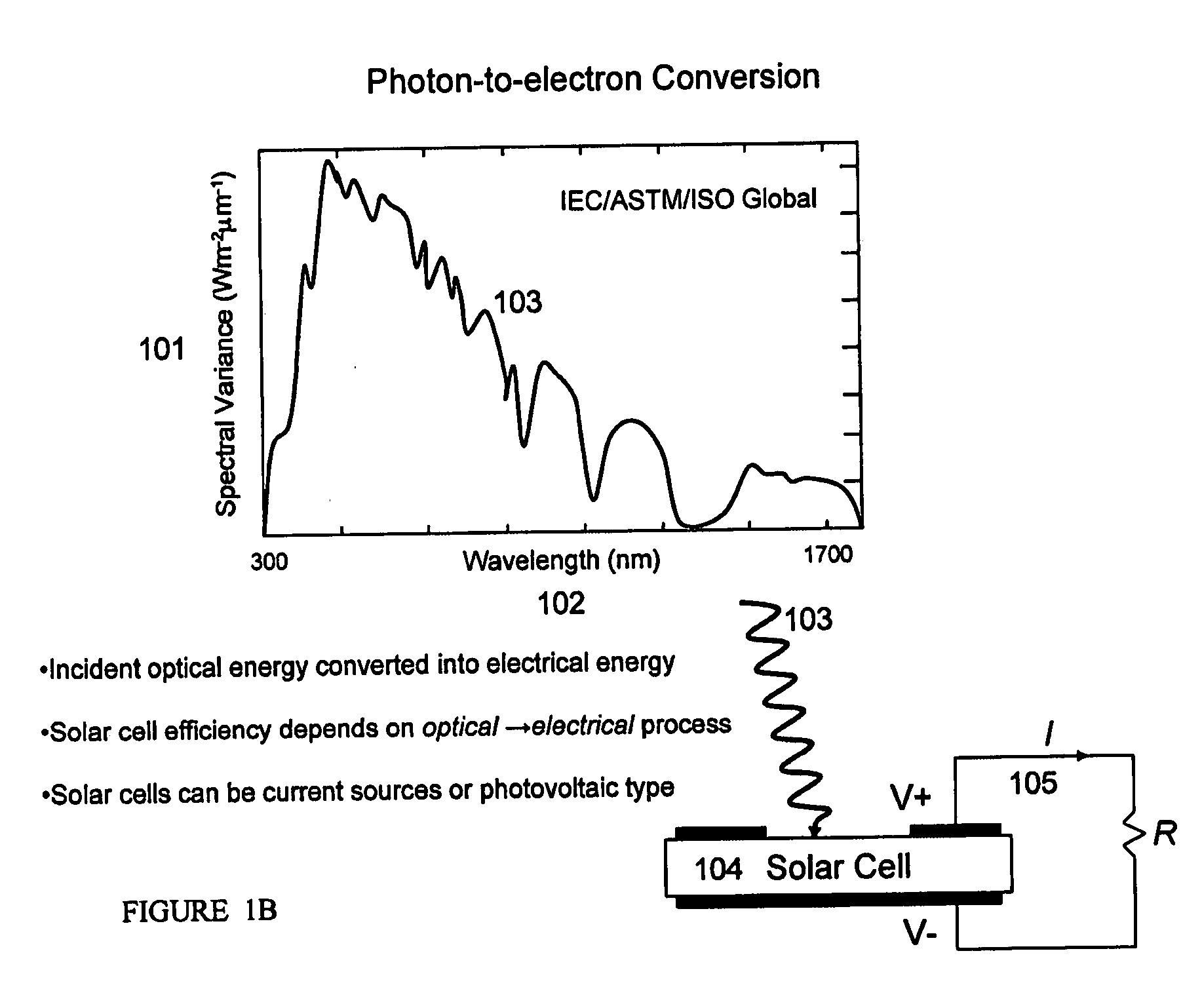
Optimal structures for high efficiency thin film silicon solar energy conversion devices and systems are disclosed. Thin film silicon active layer photoelectron conversion structures using ion implantation are disclosed. Thin film semiconductor devices optimized for exploiting the high energy and ultraviolet portion of the solar spectrum at the earths surface are also disclosed. Solar cell fabrication using high oxygen concentration single crystal silicon substrates formed using in preference the CZ method are used advantageously. Furthermore, the present invention discloses optical coatings for advantageous coupling of solar radiation into thin film solar cell devices via the use of rare-earth metal oxide (REOx), rare-earth metal oxynitride (REOxNy) and rare-earth metal oxy-phosphide (REOxPy) glasses and or crystalline material. The rare-earth metal is chosen from the group commonly known in the periodic table of elements as the lanthanide series.
Owner:IQE
Scintillator compositions, and related processes and articles of manufacture
ActiveUS20050082484A1Improve performanceExcellent and reproducible scintillation responseMaterial analysis by optical meansLuminescent compositionsIodideHigh energy
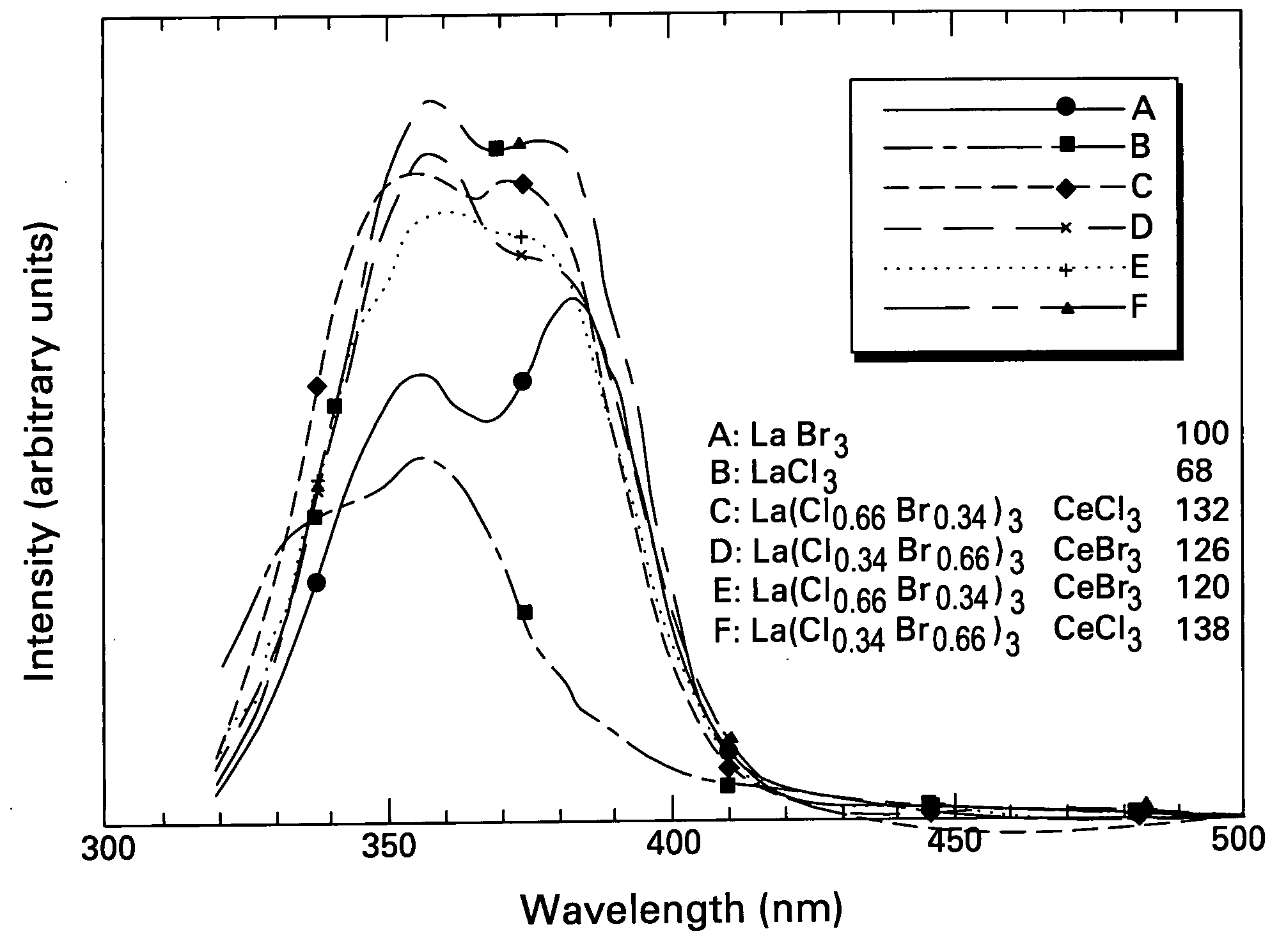
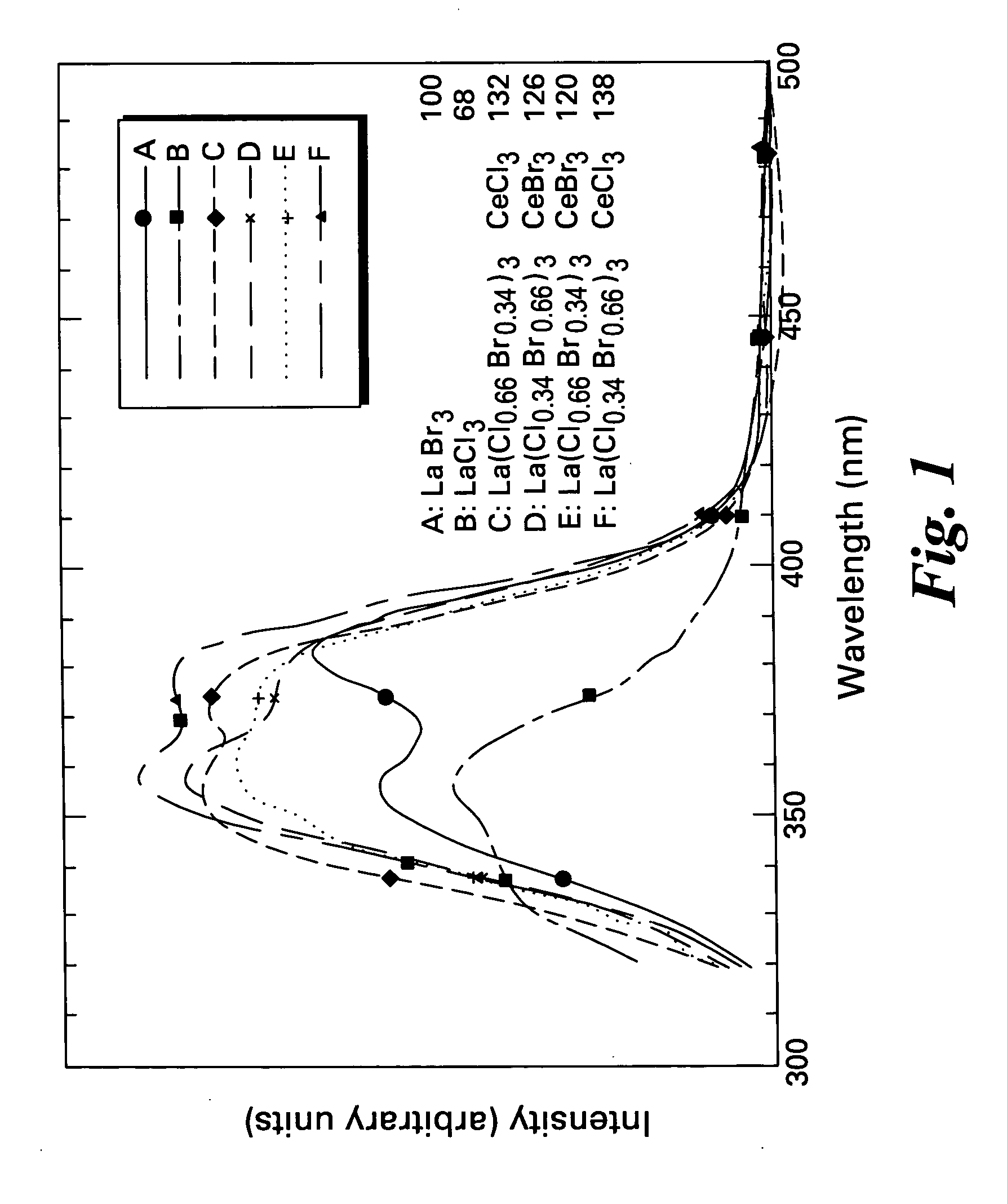
Scintillator materials based on certain types of halide-lanthanide matrix materials are described. In one embodiment, the matrix material contains a mixture of lanthanide halides, i.e., a solid solution of at least two of the halides, such as lanthanum chloride and lanthanum bromide. In another embodiment, the matrix material is based on lanthanum iodide alone, which must be substantially free of lanthanum oxyiodide. The scintillator materials, which can be in monocrystalline or polycrystalline form, also include an activator for the matrix material, e.g., cerium. Radiation detectors that use the scintillators are also described, as are related methods for detecting high-energy radiation.
Owner:BAKER HUGHES OILFIELD OPERATIONS LLC
Garnet phosphor materials having enhanced spectral characteristics
Phosphor compositions having the formulas (Tb1−x−y−z−wYxGdyLuzCew)3MrAls−rO12+δ, where M is selected from Sc, In, Ga, Zn, or Mg, and where 0<w≦0.3, 0≦x<1, 0≦y≦0.4, 0≦z<1, 0≦r≦4.5, 4.5≦s≦6, and −1.5≦δ≦1.5; (RE1−x−yScxCey)2A3−pBpSiz−qGeqO12+δ, where RE is selected from a lanthanide ion or Y3+, A is selected from Mg, Ca, Sr, or Ba, B is selected from Mg and Zn, and where 0≦p≦3, 0≦q≦3, 2.5≦z≦3.5, 0≦x≦1, 0≦y≦0.3, −1.5≦δ≦1.5; and (Ca1−x−y−zSrxBayCez)3(Sc1−a−cLuaDc)2Sin−wGewO12+δ, where D is either Mg or Zn, 0≦x<1, 0≦y<1, 0<z≦0.3, 0≦a<1, 0≦c≦1, 0≦w≦3, 2.5≦n≦3.5, and −1.5≦δ≦1.5. Also disclosed are light emitting devices including a light source and at least one of the above phosphor compositions.
Owner:GE LIGHTING SOLUTIONS LLC
Polymers functionalized with nitro compounds
A method for preparing a functionalized polymer, the method comprising the steps of (i) polymerizing conjugated diene monomer by employing a lanthanide-based catalyst to form a reactive polymer, and (ii) reacting the reactive polymer with a nitro compound.
Owner:BRIDGESTONE CORP
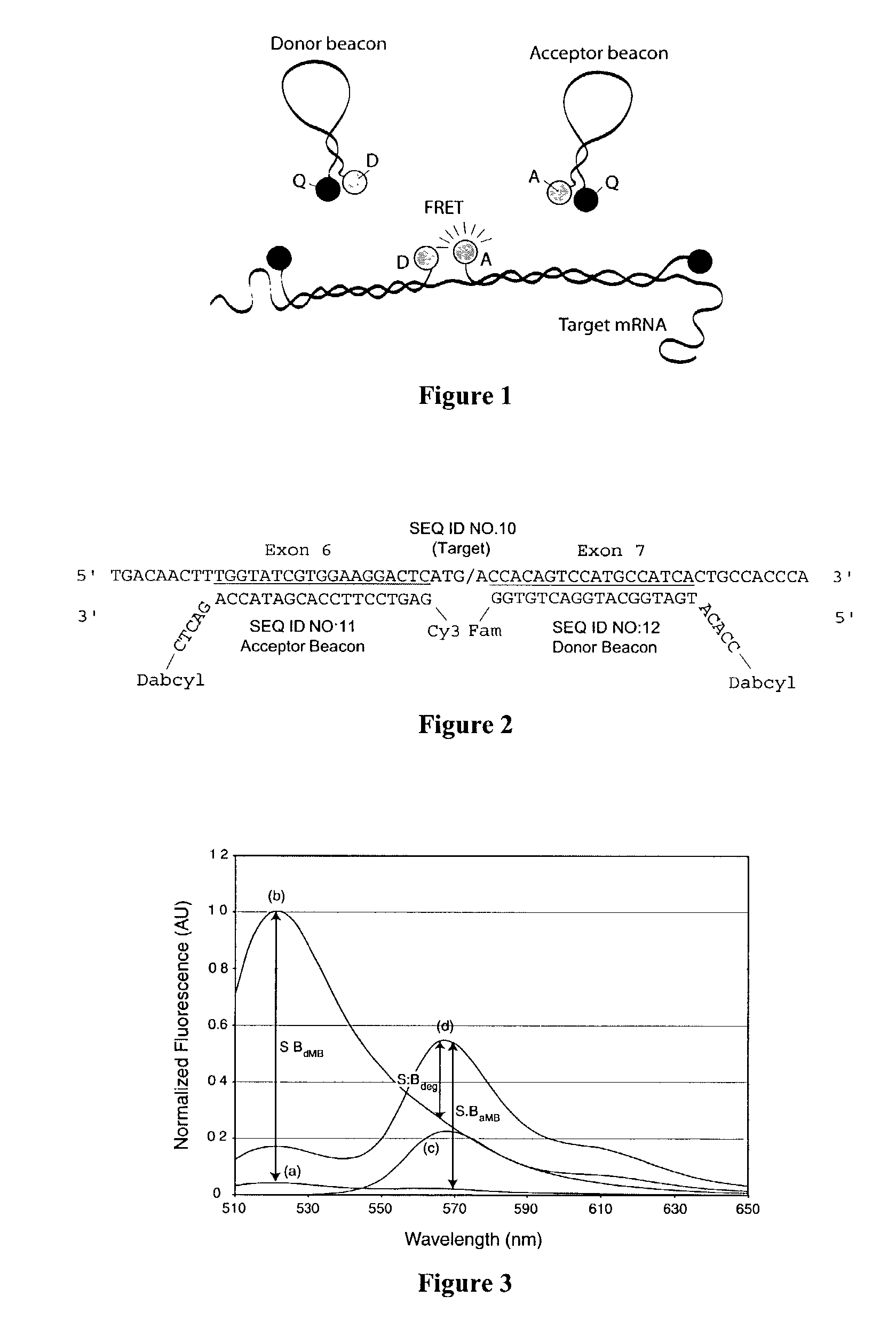
Dual resonance energy transfer nucleic acid probes
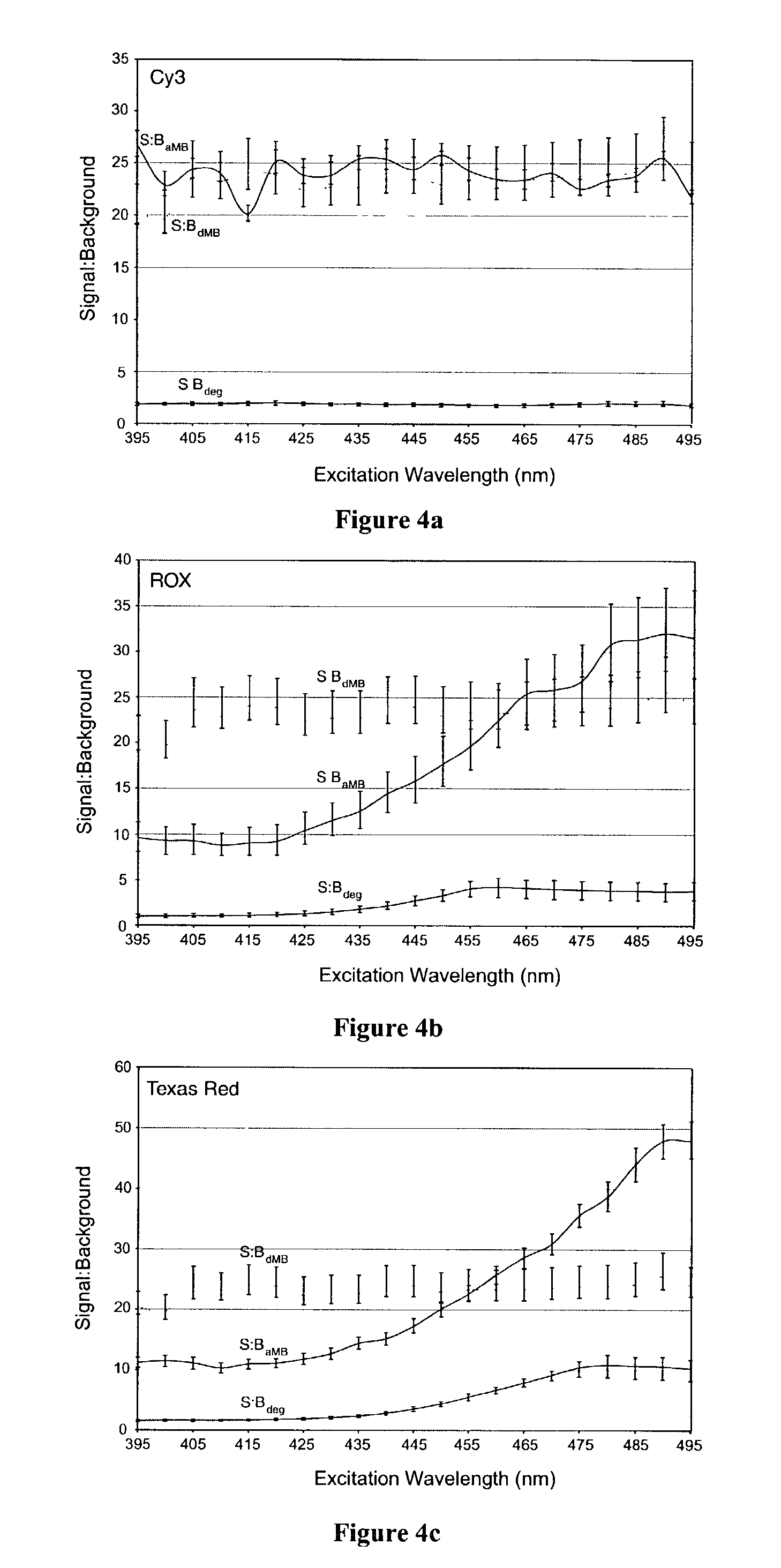
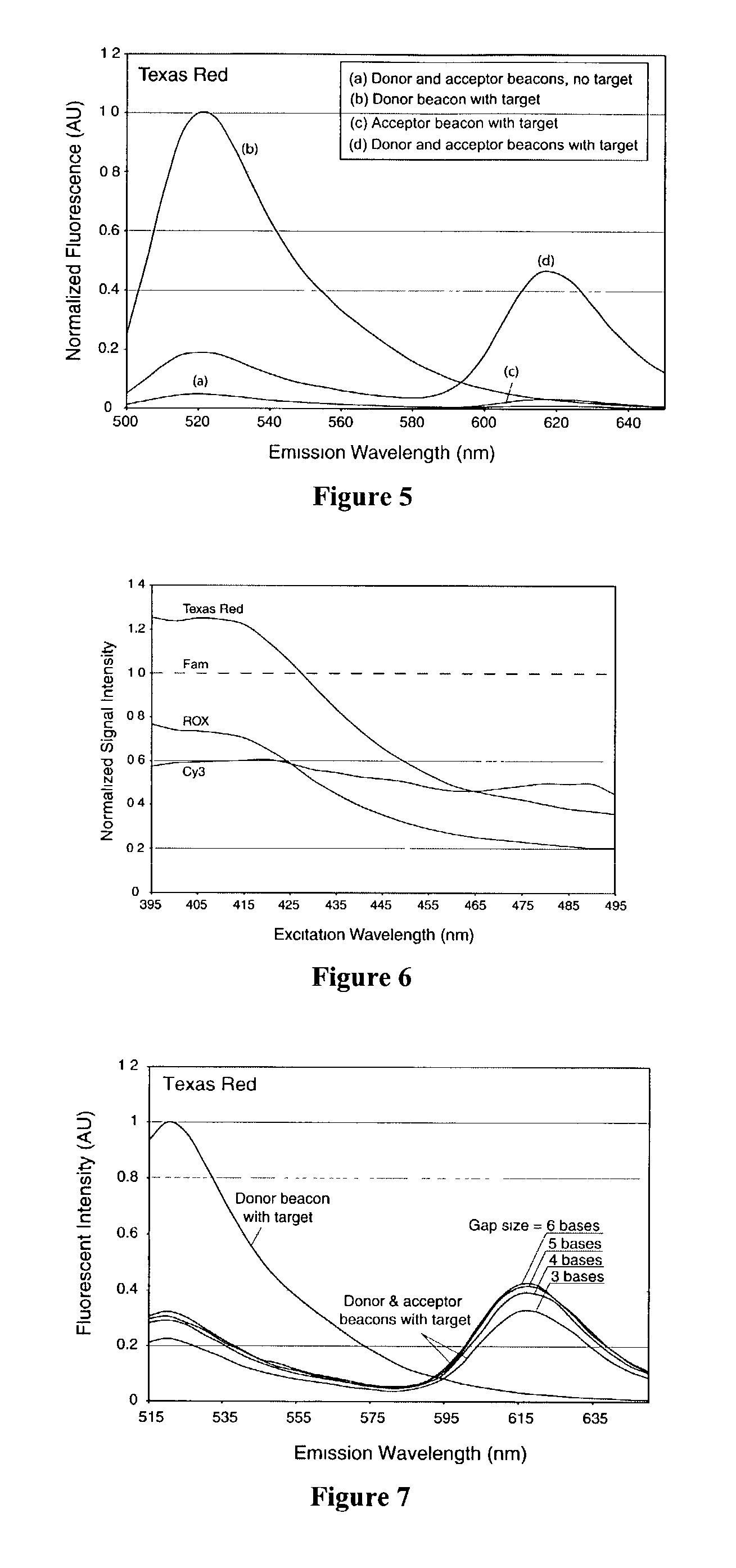
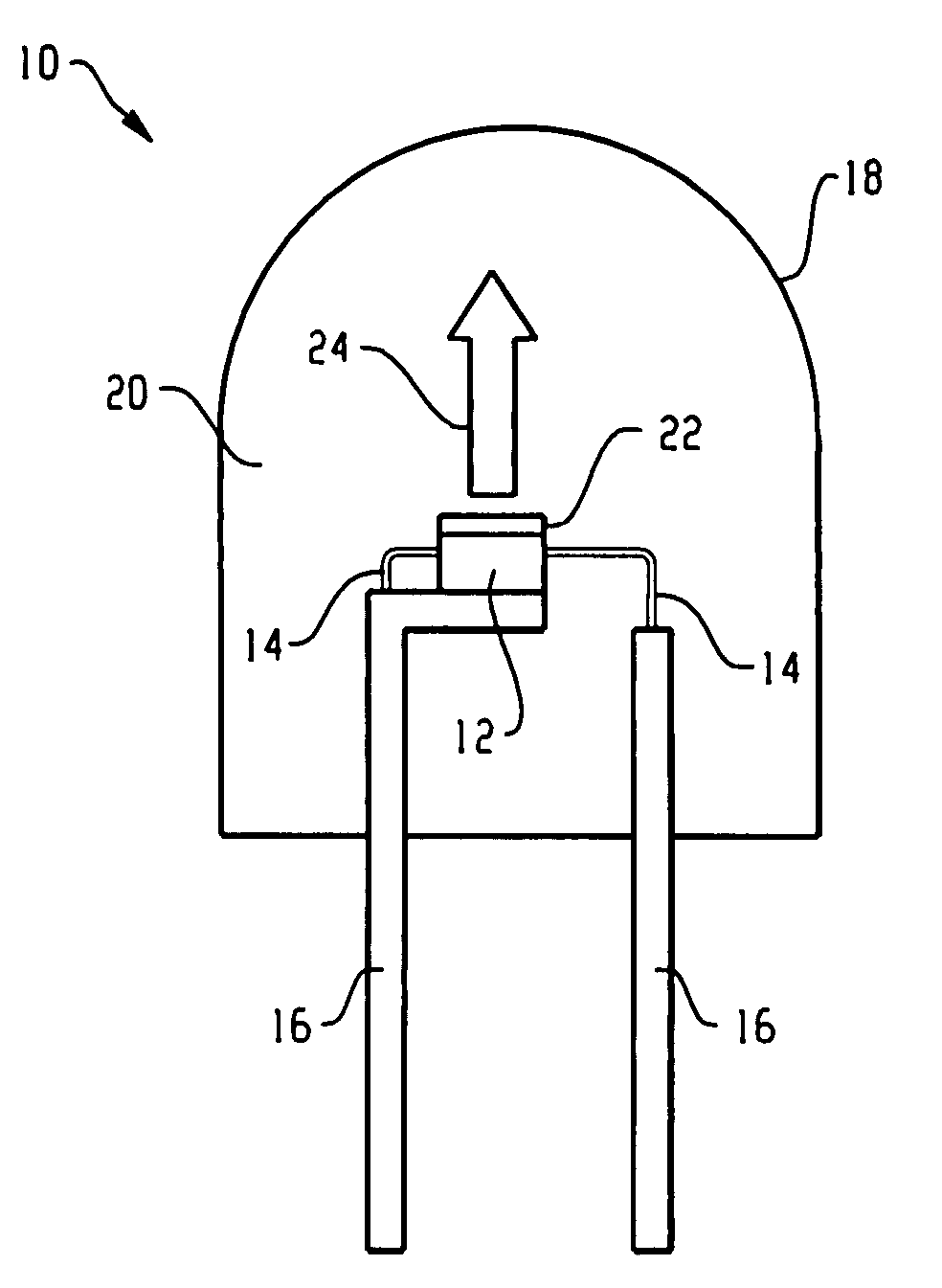
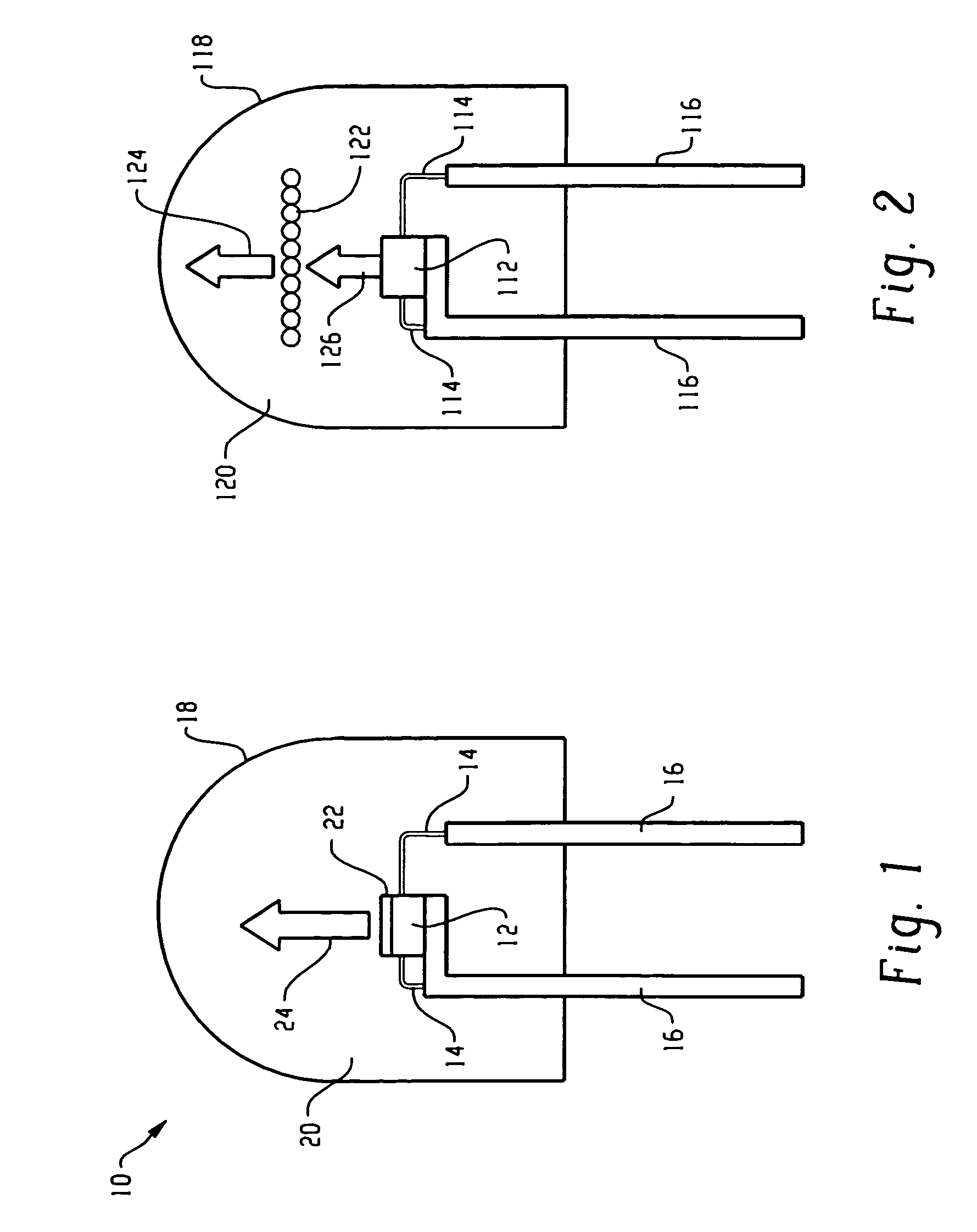
InactiveUS7081336B2Quick checkEasy to useSugar derivativesMaterial analysis by observing effect on chemical indicatorDiseaseCell specific
Owner:GEORGIA TECH RES CORP
Garnet phosphor materials having enhanced spectral characteristics
Phosphor compositions having the formulas (Tb1-x-y-z-wYxGdyLuzCew)3MrAls-rO12+δ, where M is selected from Sc, In, Ga, Zn, or Mg, and where 0<w≦0.3, 0≦x≦1, 0≦y≦0.4, 0≦z<1, 0≦r≦4.5, 4.5≦s≦6, and −1.5≦δ≦1.5; (RE1-xScxCey)2A3-pBpSiz-qGeqO12+δ, where RE is selected from a lanthanide ion or Y3+, A is selected from Mg, Ca, Sr, or Ba, B is selected from Mg and Zn, and where 0≦p≦3, 0≦q≦3, 2.5≦z≦3.5, 0≦x≦1, 0≦y≦0.3, −1.5≦δ≦1.5; and (Ca1-x-y-zSrxBayCez)3(Sc1-a-bLuaDc)2Sin-wGewO12+δ, where D is either Mg or Zn, 0≦x≦1, 0≦y<1, 0<z≦0.3, 0≦a<1, 0≦c≦1, 0≦w≦3, 2.5≦n≦3.5, and −1.5≦δ≦1.5. Also disclosed are light emitting devices including a light source and at least one of the above phosphor compositions.
Owner:GE LIGHTING SOLUTIONS LLC
Perovskite-type metal oxide compounds
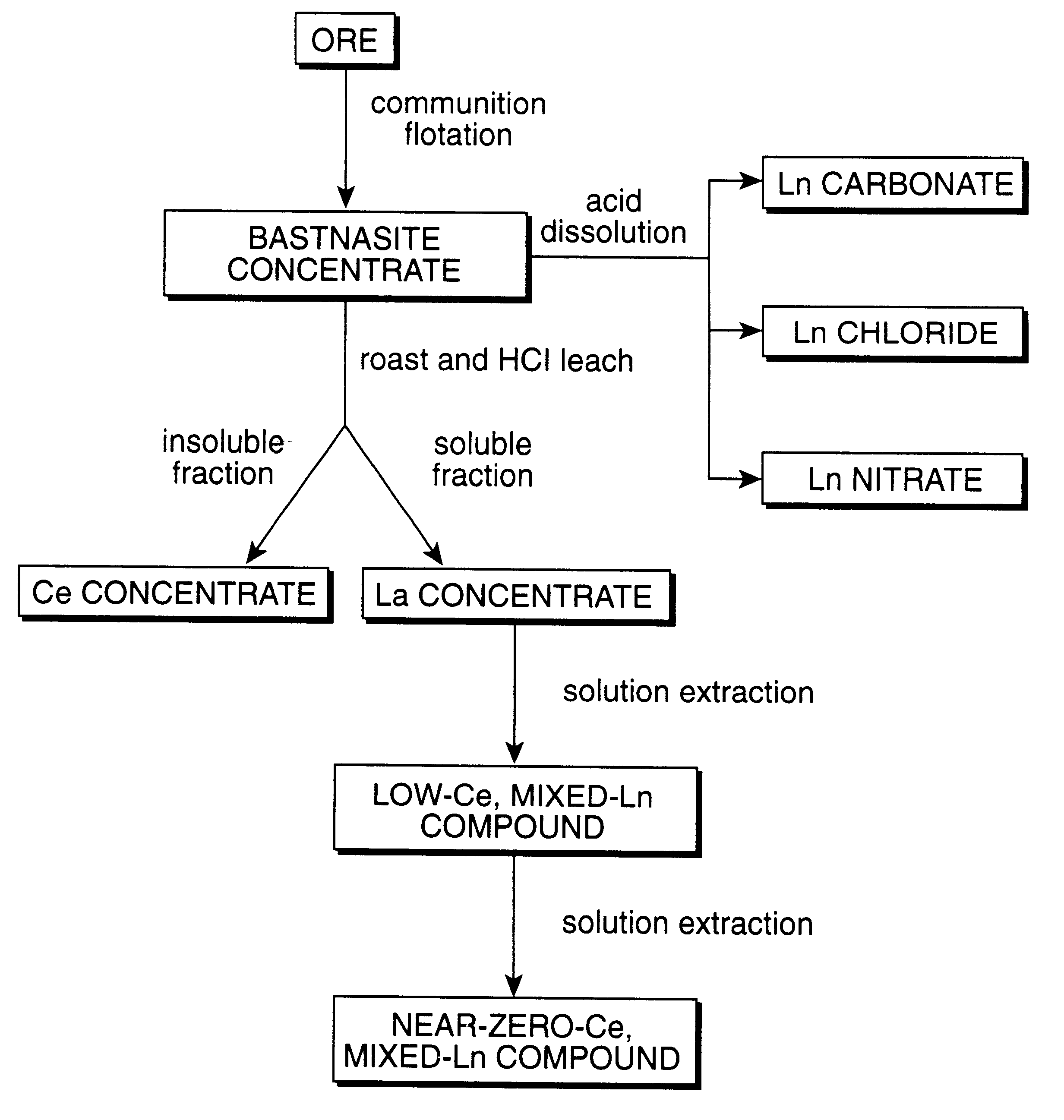
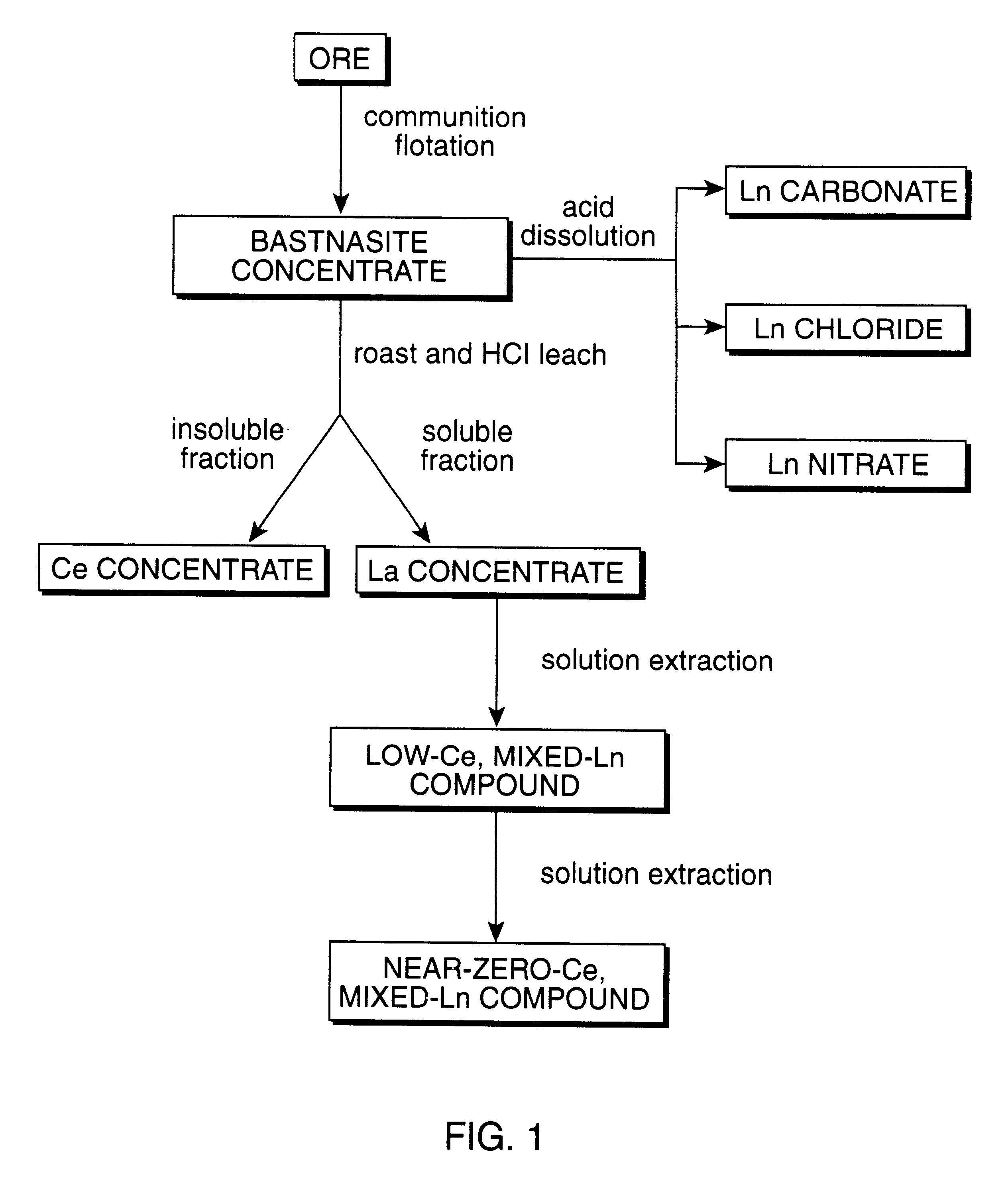
Perovskite-type catalyst consists essentially of a metal oxide composition is provided. The metal oxide composition is represented by the general formula A.sub.1-x B.sub.x MO.sub.3, in which A is a mixture of elements originally in the form of single phase mixed lanthanides collected from bastnasite; B is a divalent or monovalent cation; M is at least one element selected from the group consisting of elements of an atomic number of from 22 to 30, 40 to 51, and 73 to 80; and x is a number defined by 0.ltoreq.x<0.5.
Owner:CATALYTIC SOLUTIONS INC
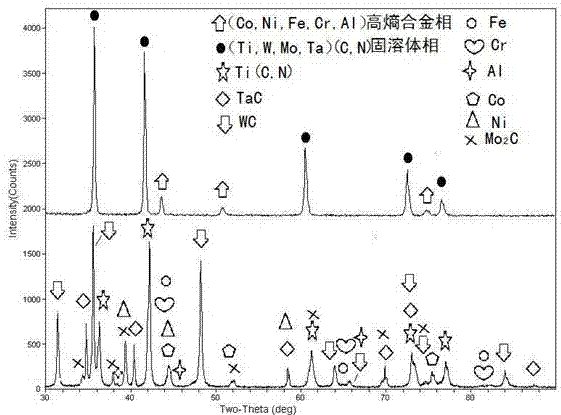
Titanium carbonitride based metal ceramic based on high-entropy alloy binder phase and preparation method of metal ceramic
InactiveCN102787266AImprove corrosion resistanceImprove wear resistanceRare-earth elementHigh entropy alloys
Disclosed is a titanium carbonitride based metal ceramic material based on a high-entropy alloy binder phase. The binder phase of the titanium carbonitride based metal ceramic material is high-entropy alloy, the hard phase of the titanium carbonitride based metal ceramic material is carbonitride solid solution, the high-entropy alloy binder phase includes at least four of ferrum, cobalt, nickel, chromium, aluminum, vanadium, titanium, copper, zirconium, molybdenum, manganese and rare earth elements, and the molar content ratio of each element ranges from 5% to 35%. In a preparation method, the titanium carbonitride based metal ceramic material based on the high-entropy alloy binder phase comprises raw materials including, in weight percent, 3-30% of the high-entropy alloy binder phase, 0-30% of second carbide powder and the balance carbonitride solid solution powder, the carbonitride solid solution powder includes at least one of Ti (Cx, N1-x), (Ti, M1...) and (Cx, N1-x), the M1 component of the (Ti, M1...) and (Cx, N1-x) includes at least one of W, Mo, Ta, Nb, V, Cr, Zr, Hf, Y and lanthanide, and 0<x<1 in the Ti (Cx, N1-x), (Ti, M1...) and (Cx, N1-x). The preparation method includes the process steps of (1) ball-milling mixing, (2) forming and (3) low-pressure sintering.
Owner:SICHUAN UNIV
Platinum group metal-free catalysts for reducing the ignition temperature of particulates on a diesel particulate filter
A catalyzed diesel particulate filter (CDPF) and a method for filtering particulates from diesel engine exhaust are provided, where the catalyzed diesel particulate filter includes a substrate and a catalyst composition, where the catalyst composition contains at least one first component, at least one second component, and at least one third component, where the first component is at least one first component selected from the group consisting of cerium and a lanthanide and mixtures thereof, the at least one second component is selected from the group consisting of cobalt, copper, manganese and mixtures thereof; and the third component comprises strontium, where the first component, the second component, and the third component are in an oxide form after calcination. The catalyst on the catalyzed diesel particulate filter lowers the temperature at which particulates are removed from the CDPF by oxidizing the particulates on the filter. The catalyzed diesel particulate filter may also include a washcoat. Washcoats prepared from colloidal aluminum oxide may have higher surface areas and pore volumes loadings than washcoats containing aluminum oxide prepared from aluminum nitrate.
Owner:CATALYTIC SOLUTIONS INC
Microspheres capable of binding radioisotopes, optionally comprising metallic microparticles, and methods of use thereof
One aspect of the present invention relates to a microsphere, comprising a hydrophilic polymer comprising a plurality of pendant anionic groups; a transition-metal, lanthanide or group 13-14 metal oxide, polyoxometalate or metal hydroxide or combination thereof; and a first radioisotope that emits a therapeutic β-particle. In certain embodiments, the microsphere further comprsies a second radioisotope that emits a diagnostic γ-ray; wherein the atomic number of the first radioisotope is not the same as the atomic number of the second radioisotope. In certain embodiments, the microsphere is composed of polymer impregnated with zirconia bound to 32p as the source of the therapeutic β-emissions and 67Ga as the source of the diagnostic γ-emissions. Another aspect of the present invention relates to the preparation of a microsphere impregnated with a radioisotope that emits therapeutic β-particles and a radioisotope that emits diagnostic β-emitting radioisotope and a γ-emitting radioistope; wherein the atomic number of the first radioisotope is not the same as the atomic number of the second radioisotope. In certain embodiments, said microspheres are administered to the patient through a catheter. In another embodiment, the microsphere is combined with the radioisotopes at the site of treatment.
Owner:BIOSPHERE MEDICAL INC
Silver-containing p-type semiconductor
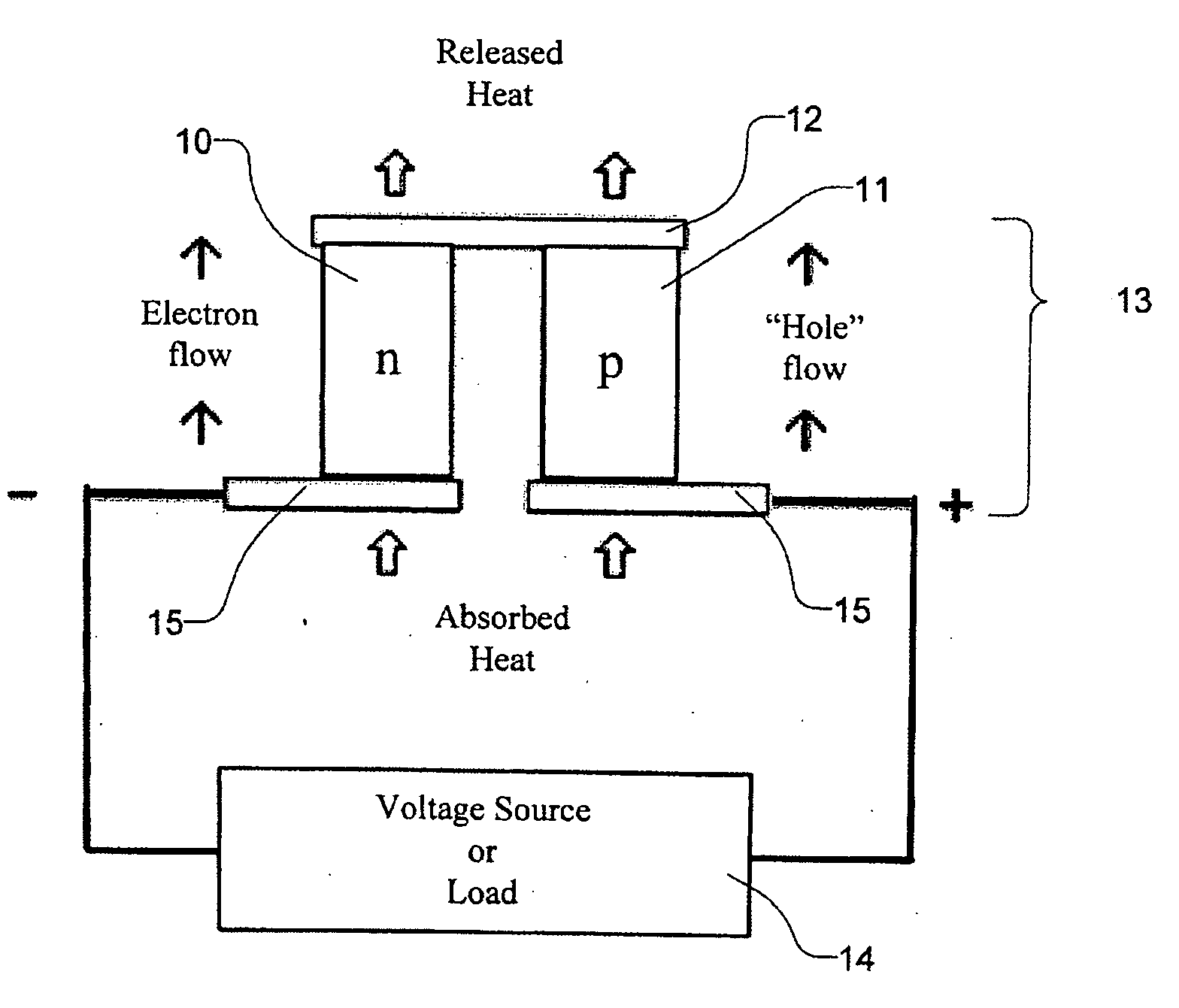
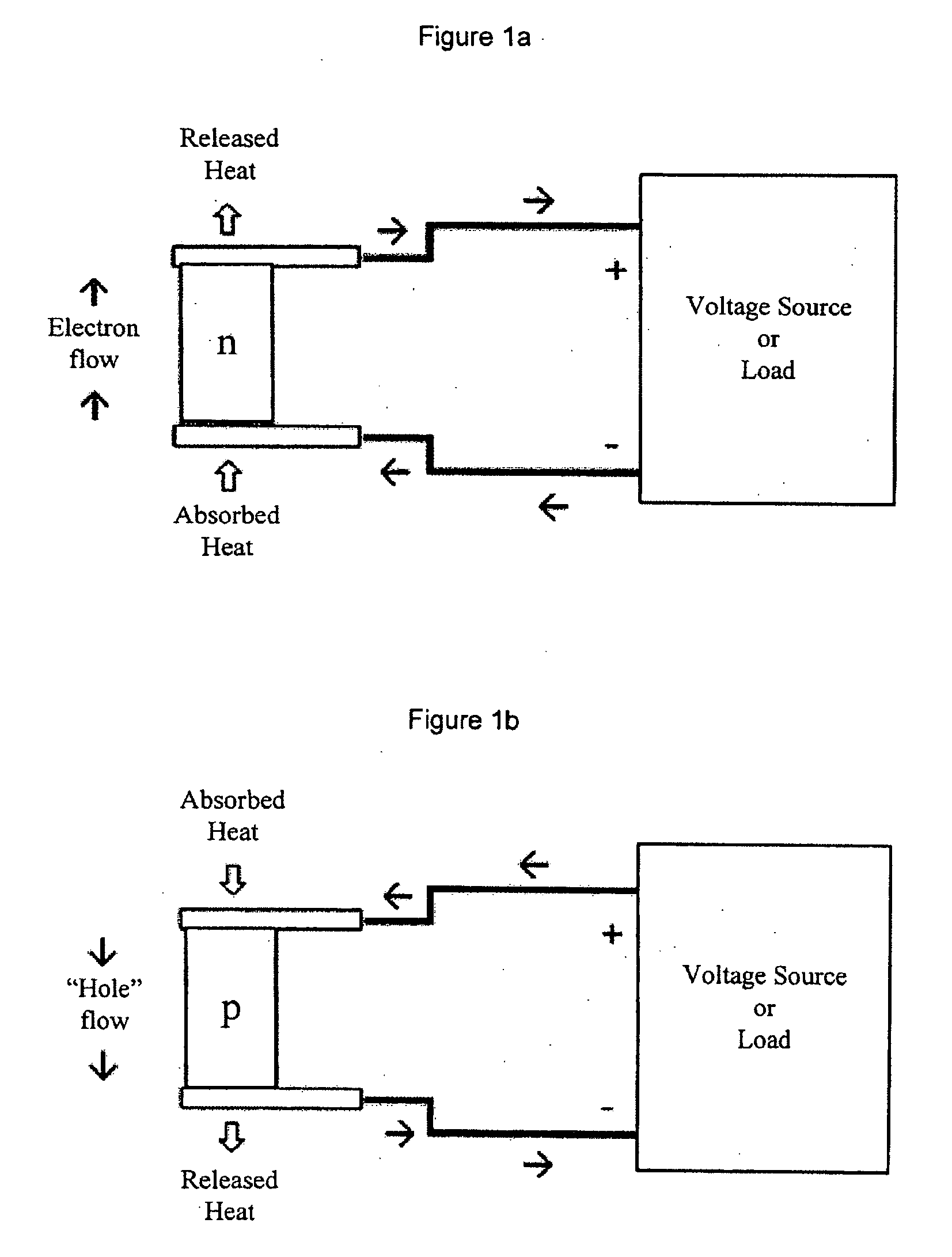
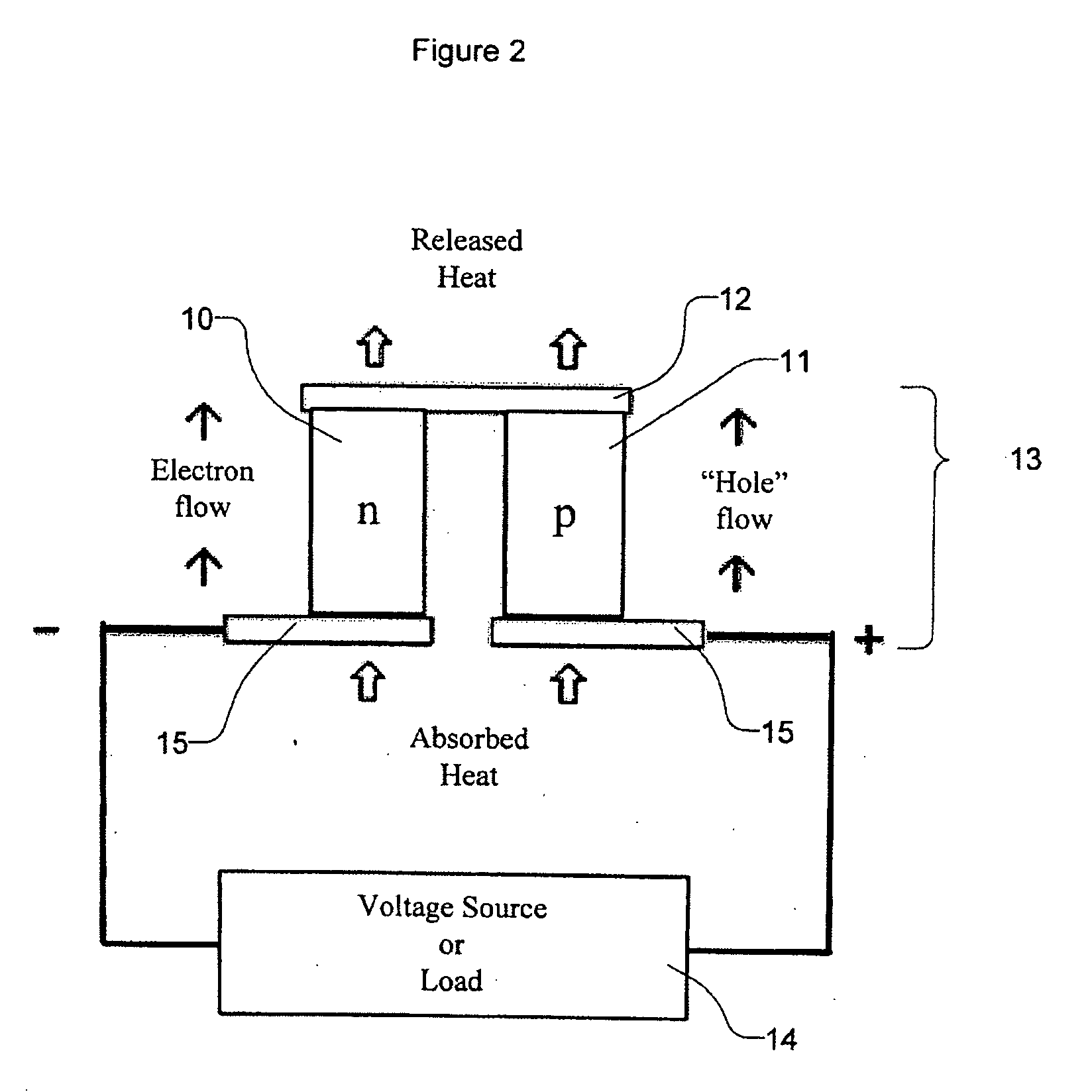
InactiveUS20050076944A1Improve efficiencyHigh thermoelectric figure of meritThermoelectric device junction materialsCrystallographyLanthanide
A thermoelectric composition comprises a material represented by the general formula (AgaX1−a)1±x(SnbPb1−b)mM′1−yQ2+m wherein X is Na, K, or a combination of Na and K in any proportion; M′ is a trivalent element selected from the group consisting of Sb, Bi, lanthanide elements, and combinations thereof; Q is a chalcogenide element selected from the group consisting of S, Te, Se, and combinations thereof; a and b are independently >0 and ≦1; x and y are independently >0 and <1; and 2≦m≦30. The compositions exhibit a figure of merit ZT of up to about 1.4 or higher, and are useful as p-type semiconductors in thermoelectric devices.
Owner:BOARD OF TRUSTEES OPERATING MICHIGAN STATE UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com