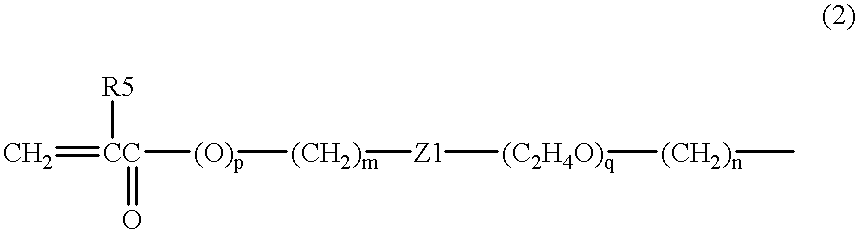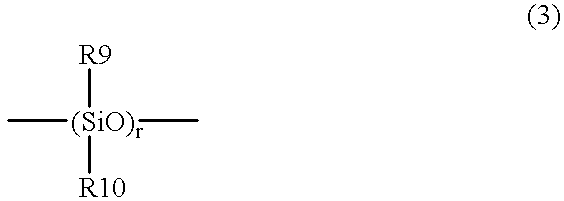Patents
Literature
40747 results about "Monomer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
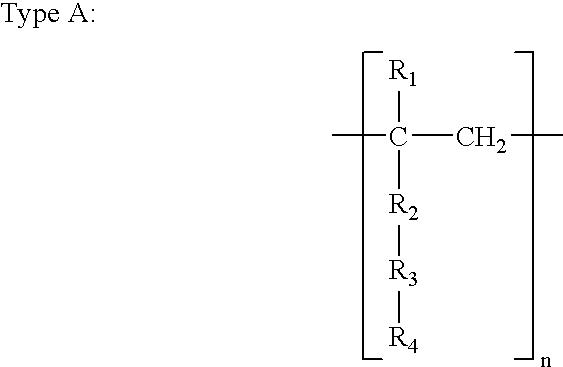
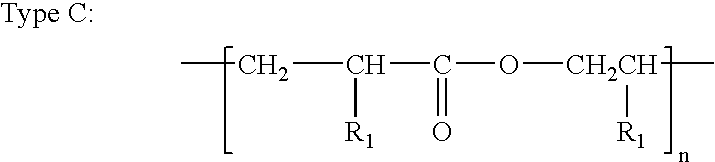
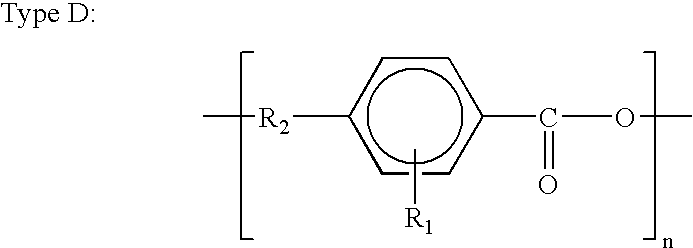
A monomer (/ˈmɒnəmər/ MON-ə-mər; mono-, "one" + -mer, "part") is a molecule that can be reacted together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Medical devices and applications of polyhydroxyalkanoate polymers
InactiveUS6838493B2High porosityReduce probabilitySuture equipmentsOrganic active ingredientsTissue repairBiocompatibility Testing
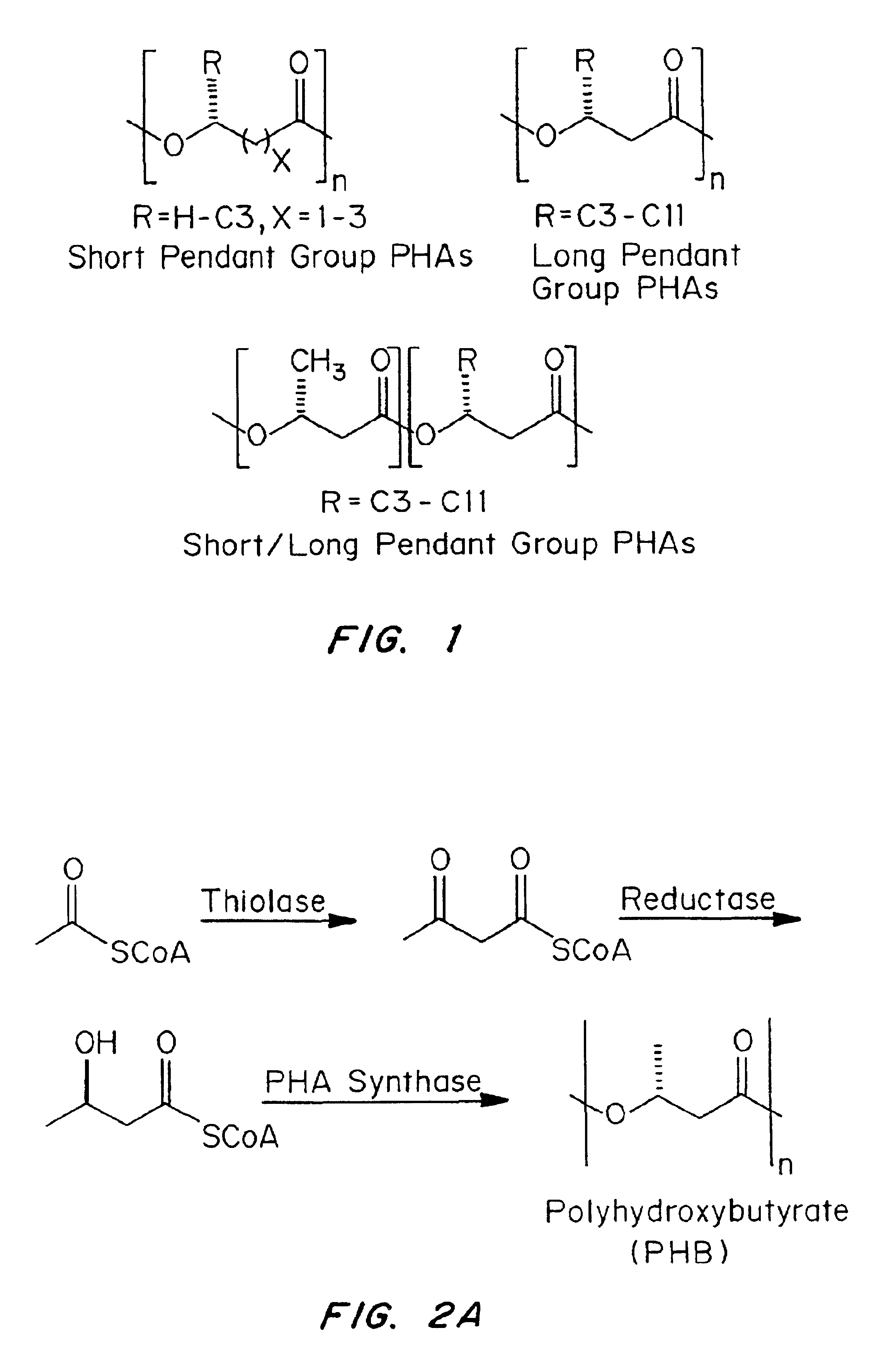
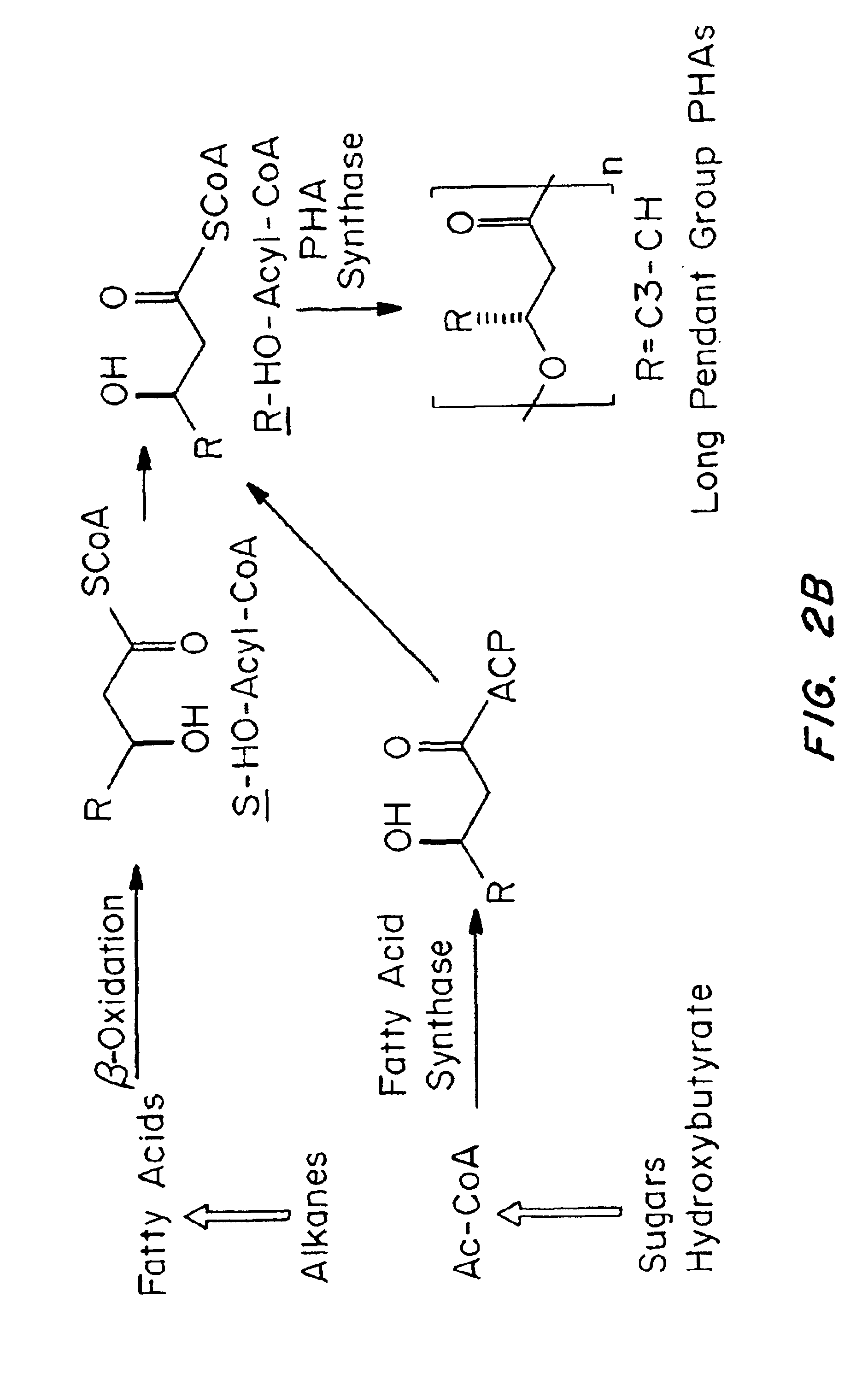
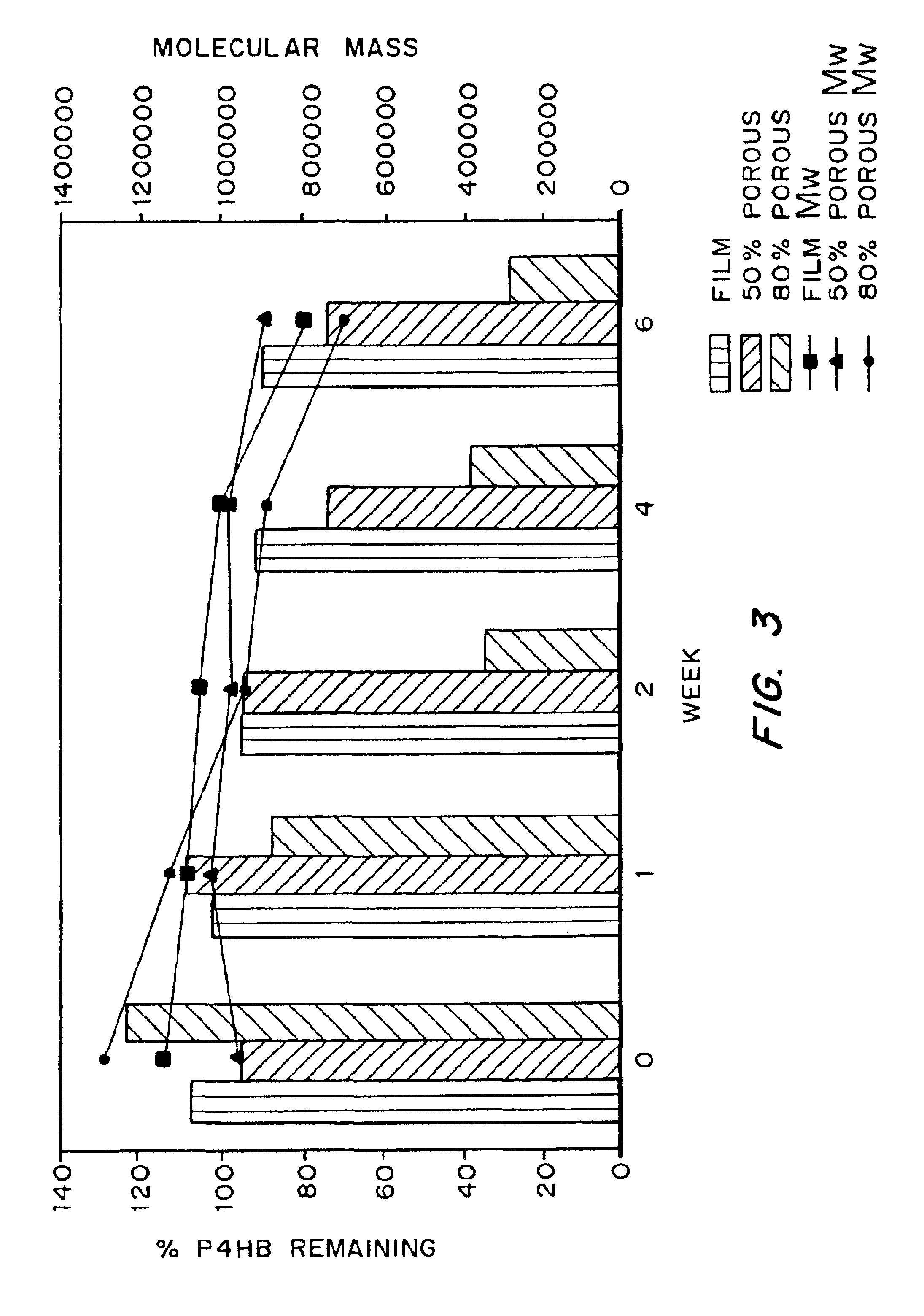
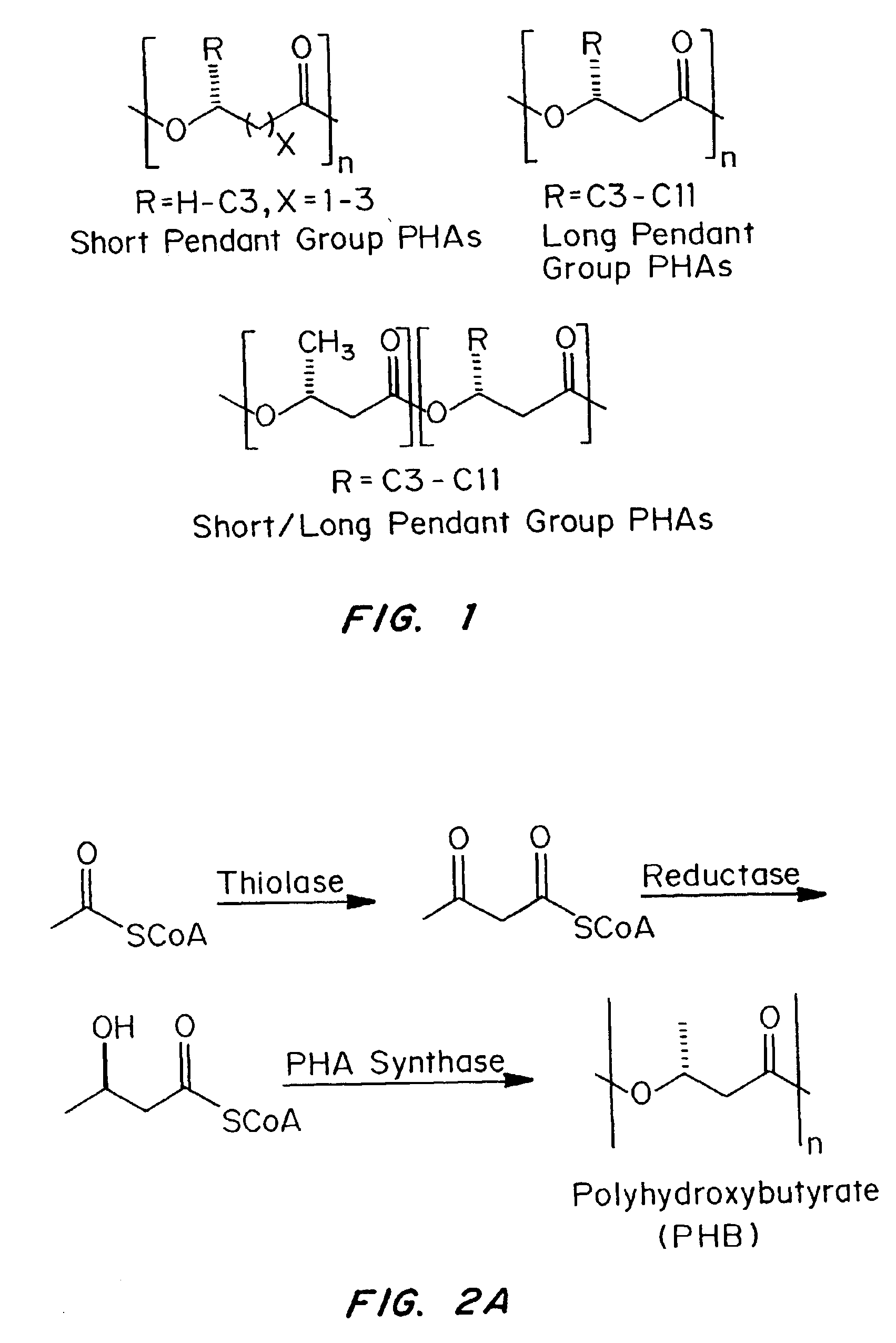
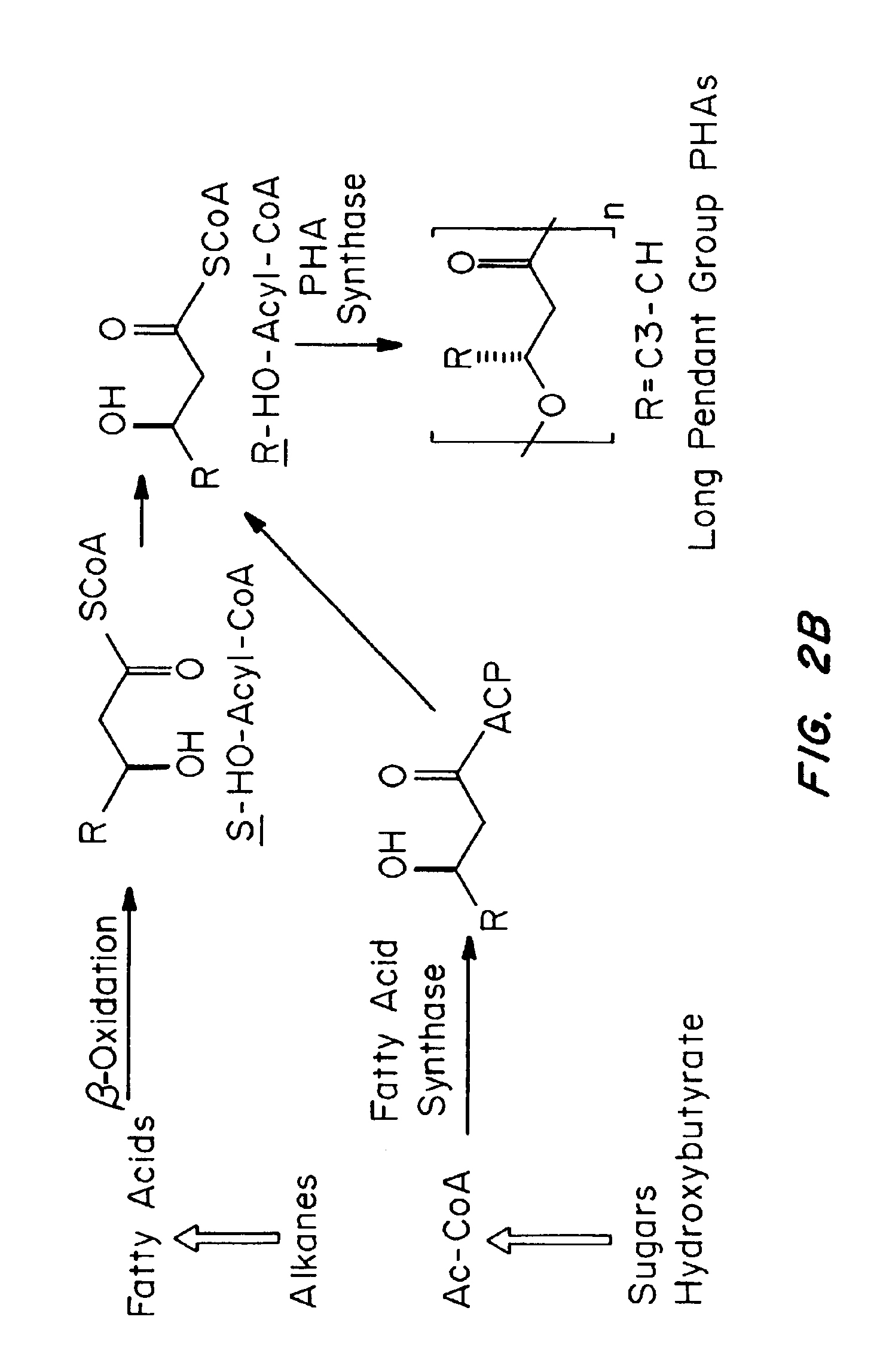
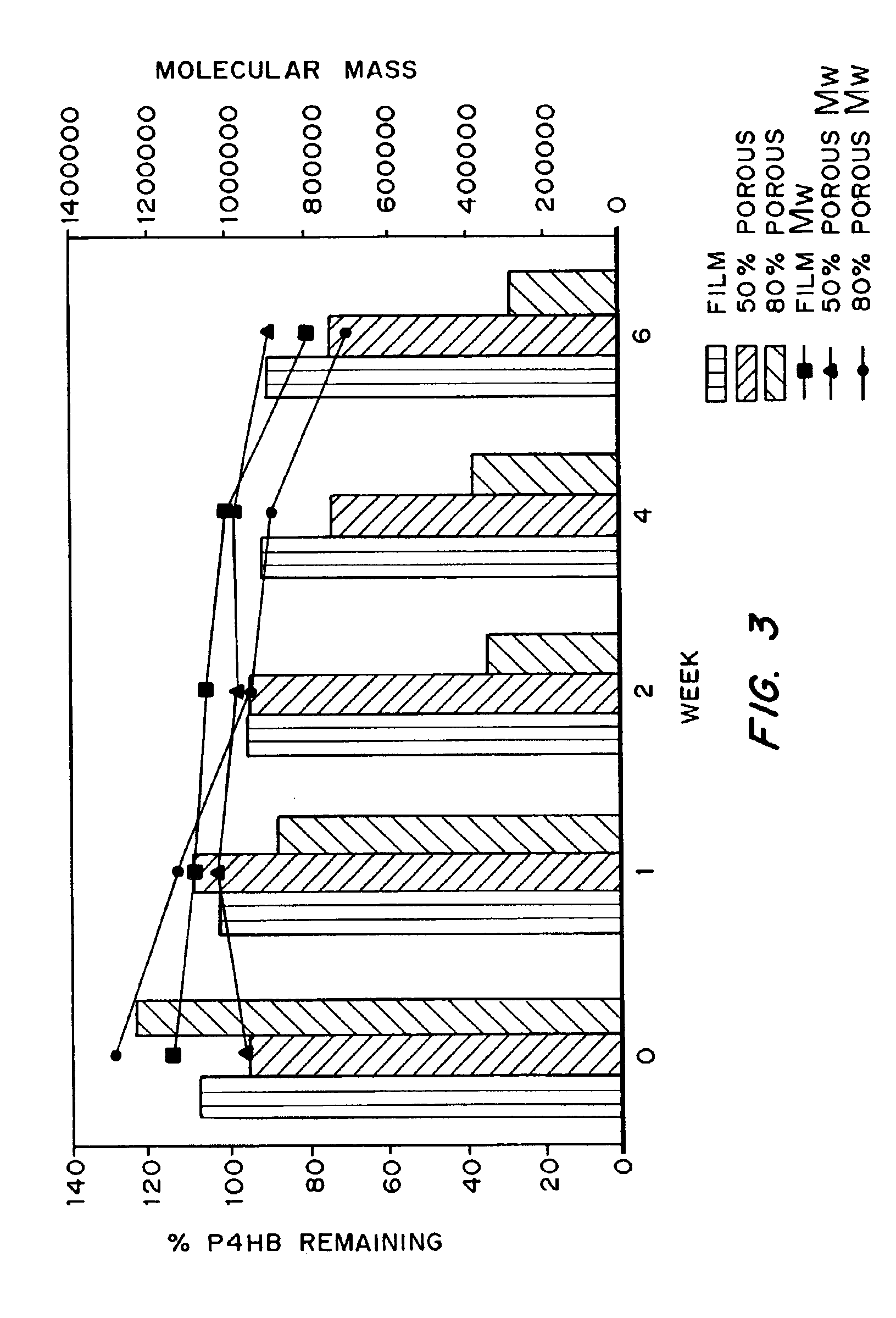
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
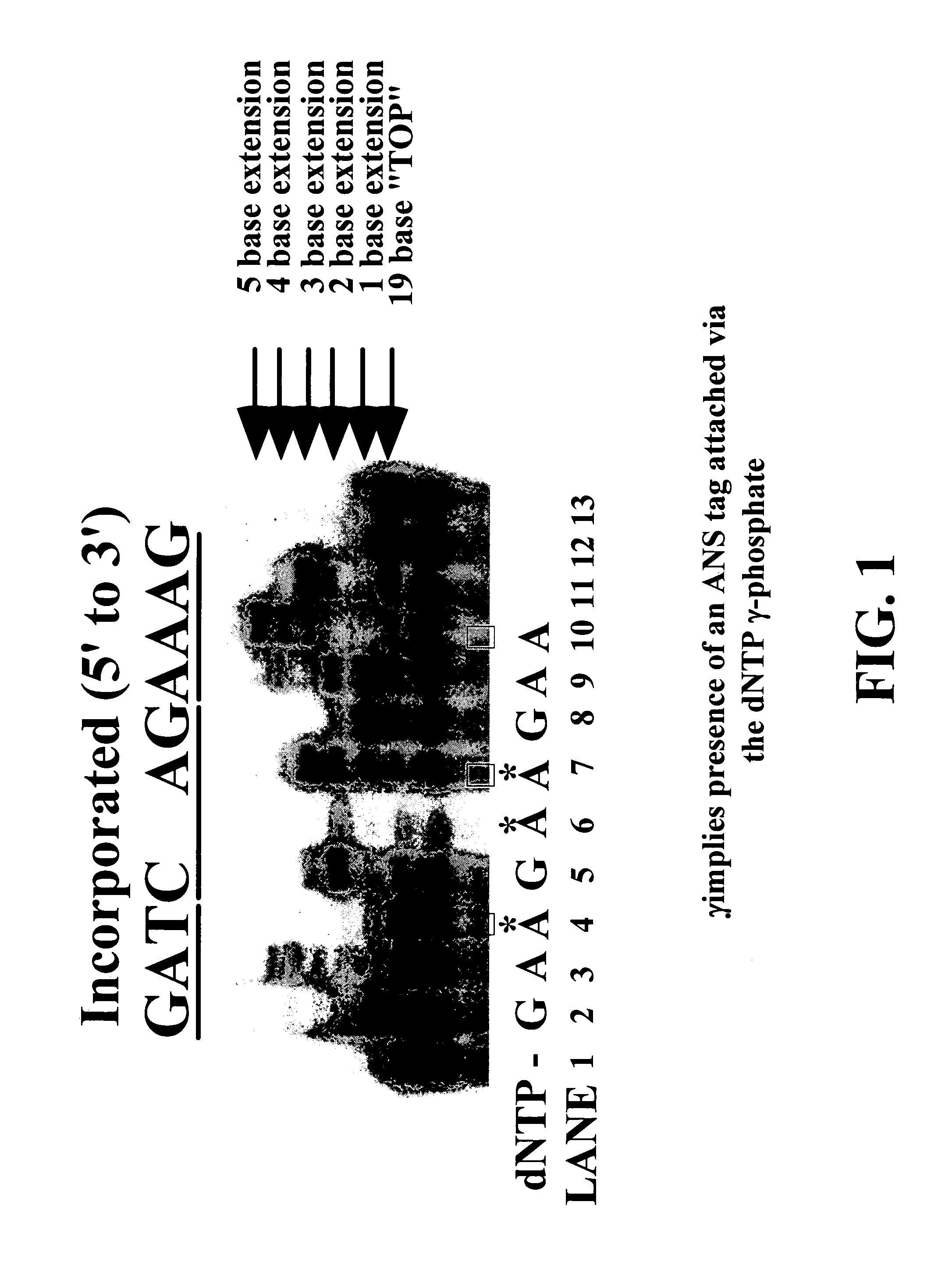
Enzymatic nucleic acid synthesis: compositions and methods for altering monomer incorporation fidelity
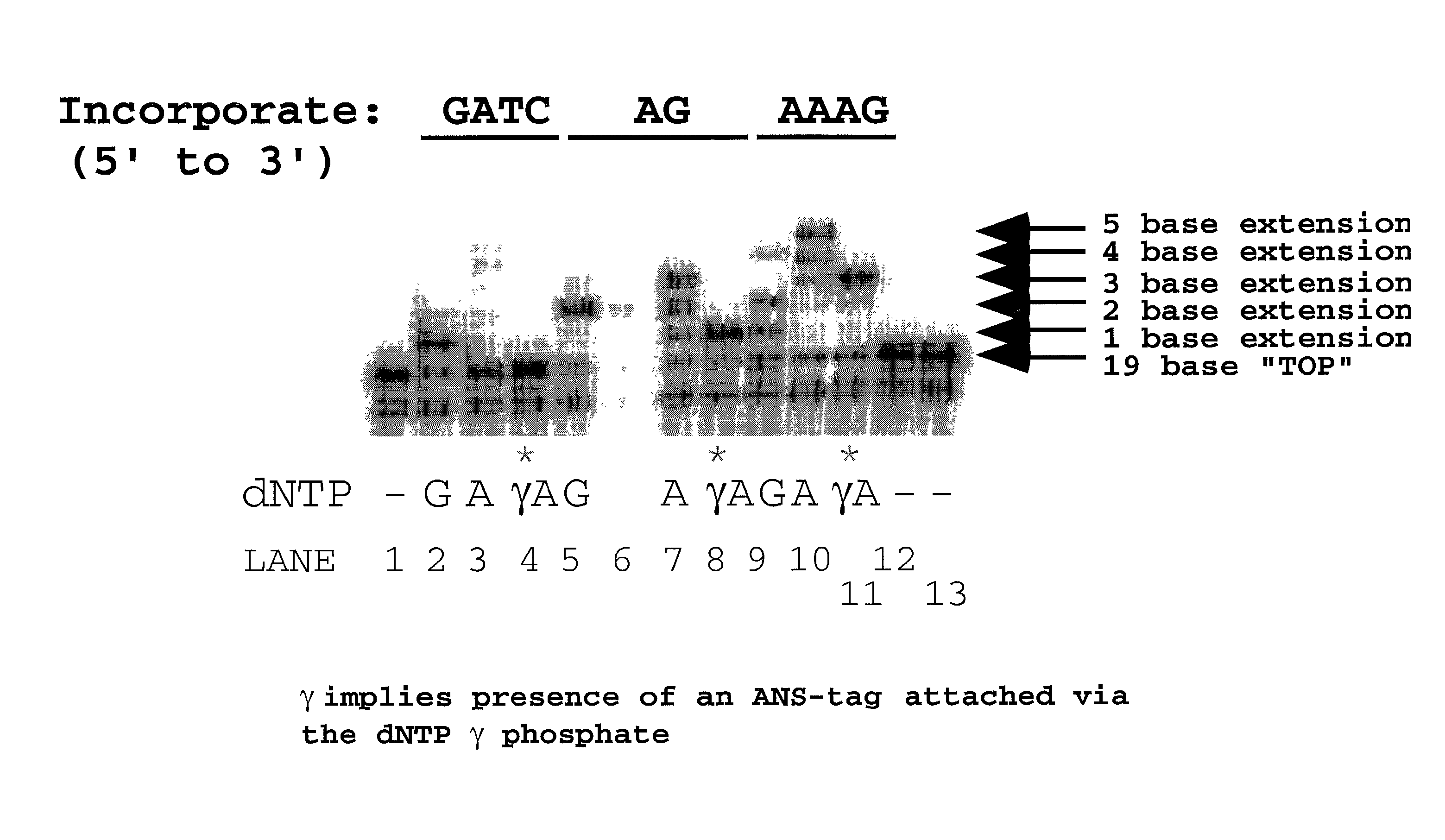
InactiveUS7211414B2Extent of pyrophosphorolysis of a primer extension product is reducedImprove fidelityBiocideSugar derivativesPhosphatePhosphoric acid
Nucleotide triphosphate probes containing a molecular and / or atomic tag on a a γ and / or β phosphate group and / or a base moiety having a detectable property are disclosed, and kits and method for using the tagged nucleotides in sequencing reactions and various assay. Also, phosphate and polyphosphate molecular fidelity altering agents are disclosed.
Owner:LIFE TECH CORP
Sutures and surgical staples for anastamoses, wound closures, and surgical closures
InactiveUS8016881B2Improve featuresControlled release rateSuture equipmentsStentsSurgical stapleMicroparticle
The invention relates to sutures and surgical staples useful in anastomoses. Various aspects of the invention include wound closure devices that use amphiphilic copolymer or parylene coatings to control the release rate of an agent, such as a drug or a biological material, polymerizing a solution containing monomers and the agent to form a coating, using multiple cycles of swelling a polymer with a solvent-agent solution to increase loading, microparticles carrying the agent, biodegradable surgical articles with amphiphilic copolymer coatings, and sutures or surgical staples the deliver a drug selected from the group consisting of triazolopyrimidine, paclitaxol, sirolimus, derivatives thereof, and analogs thereof to a wound site.
Owner:MIRUS LLC
Long wearable soft contact lens
The present invention relates to a soft contact lens, and provides a contact lens which shows small and stable contact angle to water at its surface in water as well as in air, little deposition in wearing, high oxygen permeability, no adhesion of lens to a cornea and superior extended-wearing characteristics. The present invention provides a hydrogel soft contact lens which has contact angle at a lens surface in a range of 10-50° by the captive bubble method in water and 30-90° by the sessile drop method in air, oxygen permeability of not less than 30 and water content of not less than 5%, and also a hydrogel soft contact lens consisting of a polymer comprising a hydrophilic siloxanyl monomer shown by a specified general formula.
Owner:COOPERVISION INT LTD
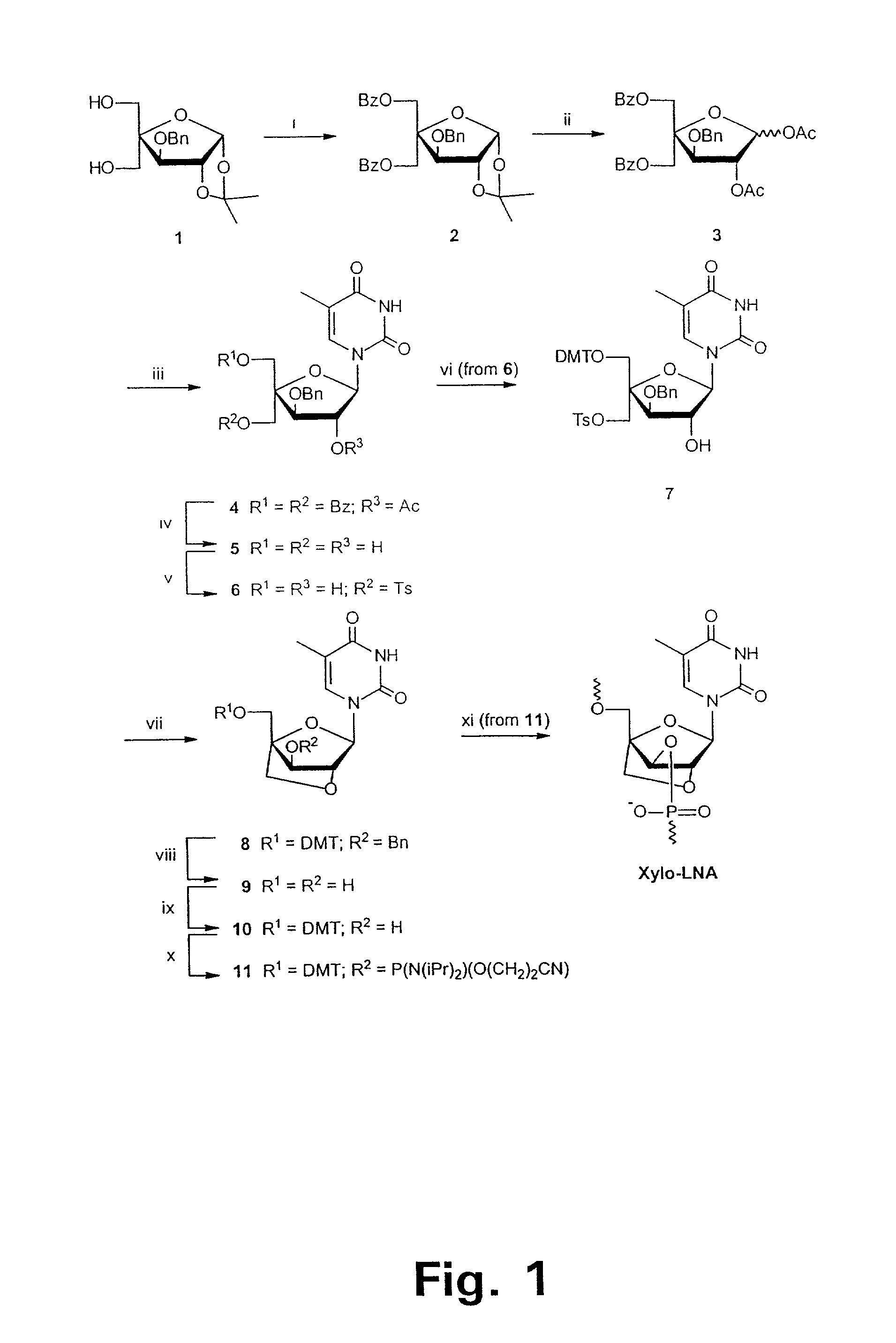
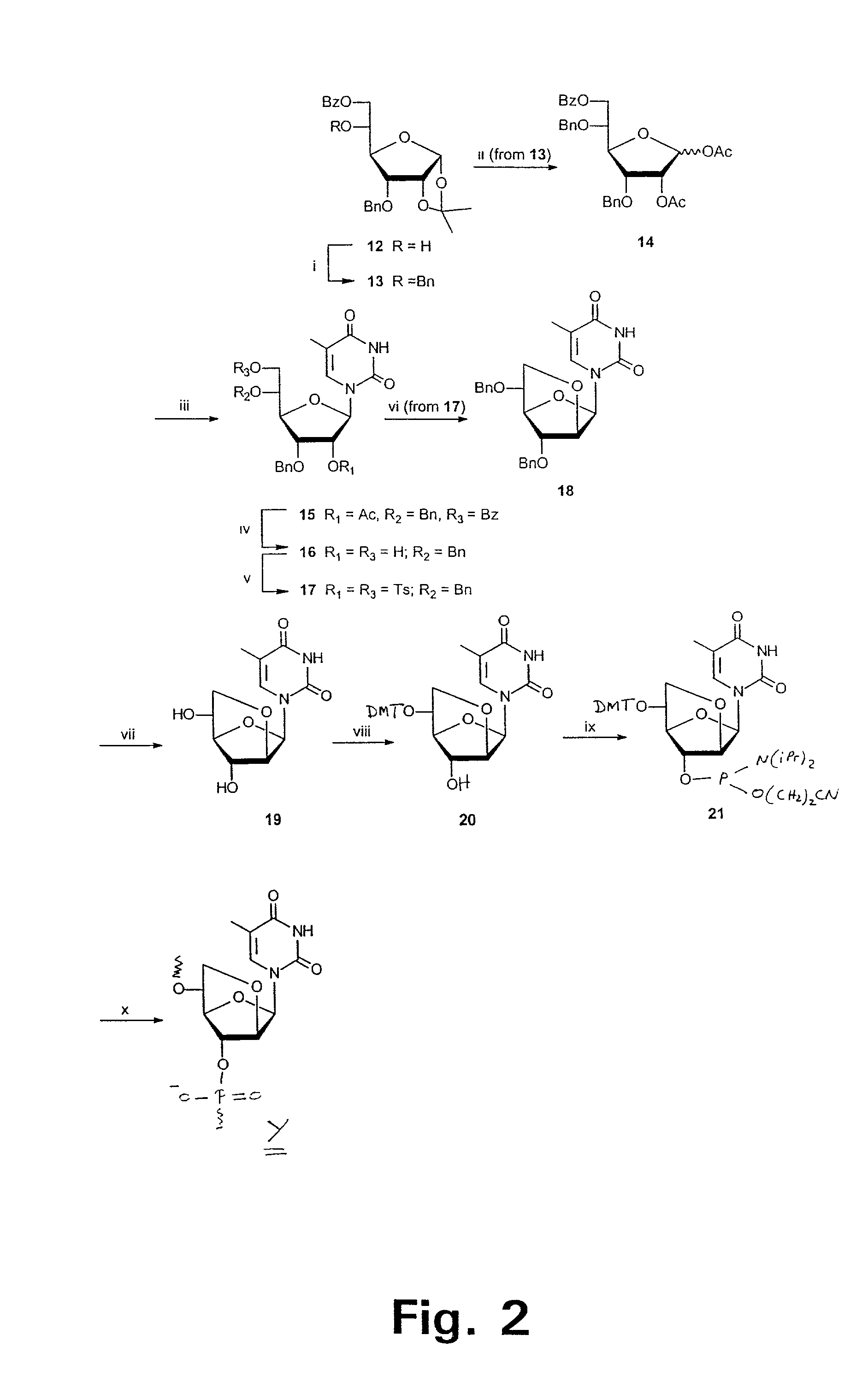
Xylo-LNA analogues
Based on the above and on the remarkable properties of the 2′-O,4′-C-methylene bridged LNA monomers it was decided to synthesise oligonucleotides comprising one or more 2′-O,4′-C-methylene-β-D-xylofuranosyl nucleotide monomer(s) as the first stereoisomer of LNA modified oligonucleotides. Modelling clearly indicated the xylo-LNA monomers to be locked in an N-type furanose conformation. Whereas the parent 2′-deoxy-β-D-xylofuranosyl nucleosides were shown to adopt mainly an N-type furanose conformation, the furanose ring of the 2′-deoxy-β-D-xylofuranosyl monomers present in xylo-DNA were shown by conformational analysis and computer modelling to prefer an S-type conformation thereby minimising steric repulsion between the nucleobase and the 3′-O-phopshate group (Seela, F.; Wömer, Rosemeyer, H. Helv. Chem. Acta 1994, 77, 883). As no report on the hybridisation properties and binding mode of xylo-configurated oligonucleotides in an RNA context was believed to exist, it was the aim to synthesise 2′-O,4′-C-methylene-β-D-xylofuranosyl nucleotide monomer and to study the thermal stability of oligonucleotides comprising this monomer. The results showed that fully modified or almost fully modified Xylo-LNA is useful for high-affinity targeting of complementary nucleic acids. When taking into consideration the inverted stereochemistry at C-3′ this is a surprising fact. It is likely that Xylo-LNA monomers, in a sequence context of Xylo-DNA monomers, should have an affinity-increasing effect.
Owner:QIAGEN GMBH
Electrophoretic particles and processes for the production thereof
InactiveUS6822782B2Improve stabilityEasy to adaptMaterial nanotechnologyStatic indicating devicesCross-linkOligomer
In electrophoretic media, it is advantageous to use pigment particles having about 1 to 15 percent by weight of a polymer chemically bonded to, or cross-linked around, the pigment particles. The polymer desirably has a branched chain structure with side chains extending from a main chain. Charged or chargeable groups can be incorporated into the polymer or can be bonded to the particles separately from the polymer. The polymer-coated particles can be prepared by first attaching a polymerizable or polymerization-initiating group to the particle and then reacting the particle with one or more polymerizable monomers or oligomers.
Owner:E INK CORPORATION
Water-soluble copolymer film packet
Owner:MONOSOL LLC +1
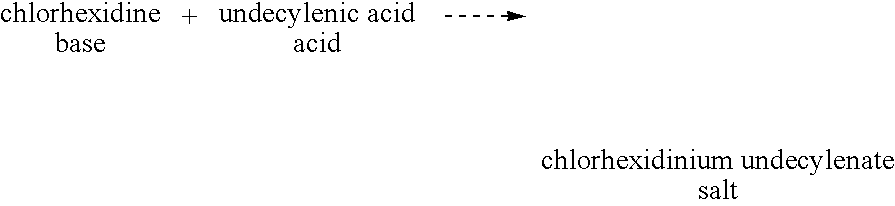
Dermatological compositions
InactiveUS20090318557A1Decrease of bioactivityReduced effectivenessCosmetic preparationsBiocideCarboxylic acidAcid–base reaction
A dermatological composition comprising a salt of a cation and an anion. The cation is derived from a monomeric or polymeric molecule that will generate an amidine moieity, a guanidine moieity or a biguanide moieity. The anion is derived from a monomeric or polymeric molecule that will generate a carboxylic acid moieity. The composition may be prepared by a metathesis or acid-base reaction.
Owner:STOCKEL RICHARD F
Novel proteins with targeted binding
InactiveUS20050089932A1Stimulate and inhibit activityEasy screeningPeptide librariesPeptide/protein ingredientsMonomerComputational biology
Methods for identifying discrete monomer domains and immuno-domains with a desired property are provided. Methods for generating multimers from two or more selected discrete monomer domains are also provided, along with methods for identifying multimers possessing a desired property. Presentation systems are also provided which present the discrete monomer and / or immuno-domains, selected monomer and / or immuno-domains, multimers and / or selected multimers to allow their selection. Compositions, libraries and cells that express one or more library member, along with kits and integrated systems, are also included in the present invention.
Owner:AMGEN MOUNTAIN VIEW
Coupled electrochromic compounds with photostable dication oxidation states
InactiveUS6249369B1High photochemical stabilityTenebresent compositionsNon-linear opticsAction spectrumElectronic communication
Coupling of anodic electrochromic compounds by a covalent bond or a bridge link which provides for electronic communication between the coupled electrochromic compounds results in coupled electrochromic compounds which exhibit greater stability as well as electrochromic activity that differs from the monomeric electrochromic compounds. Extension of the absorption spectrum into the near-infrared region of the spectrum is frequently observed. The coupled electrochromic compounds are highly suitable for use in electrochromic media used to produce electrochromic devices.
Owner:GENTEX CORP
Acidic activator-supports and catalysts for olefin polymerization
ActiveUS7294599B2Increase acidityImprove productivityOrganic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationAlkyneOlefin polymerization
This invention relates to the field of olefin polymerization catalyst compositions, and methods for the polymerization and copolymerization of olefins, typically using a supported catalyst composition. In one aspect, this invention encompasses precontacting a metallocene with an olefin or alkyne monomer and an organoaluminum compound, prior to contacting this mixture with the acidic activator-support.
Owner:CHEVRON PHILLIPS CHEMICAL CO LP
Method for continuous production of water-absorbent resin
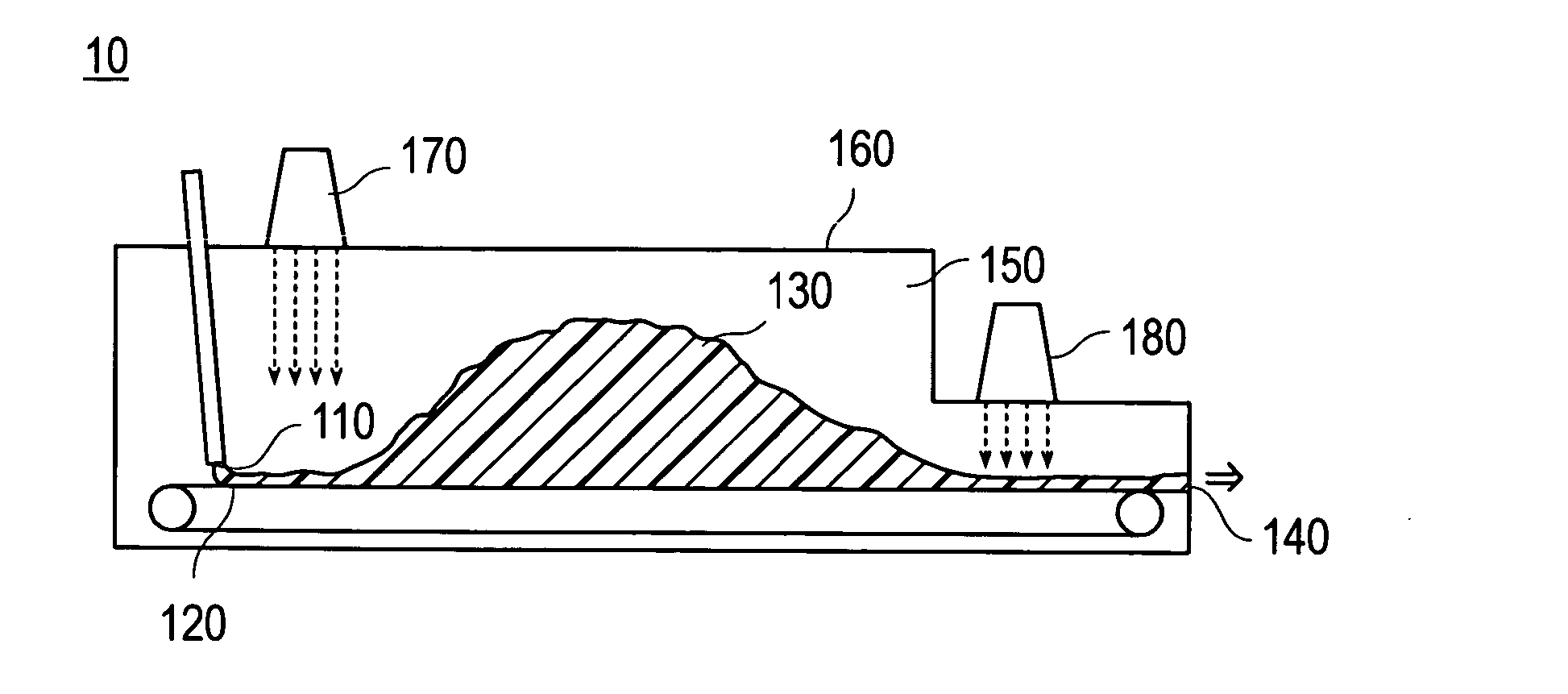
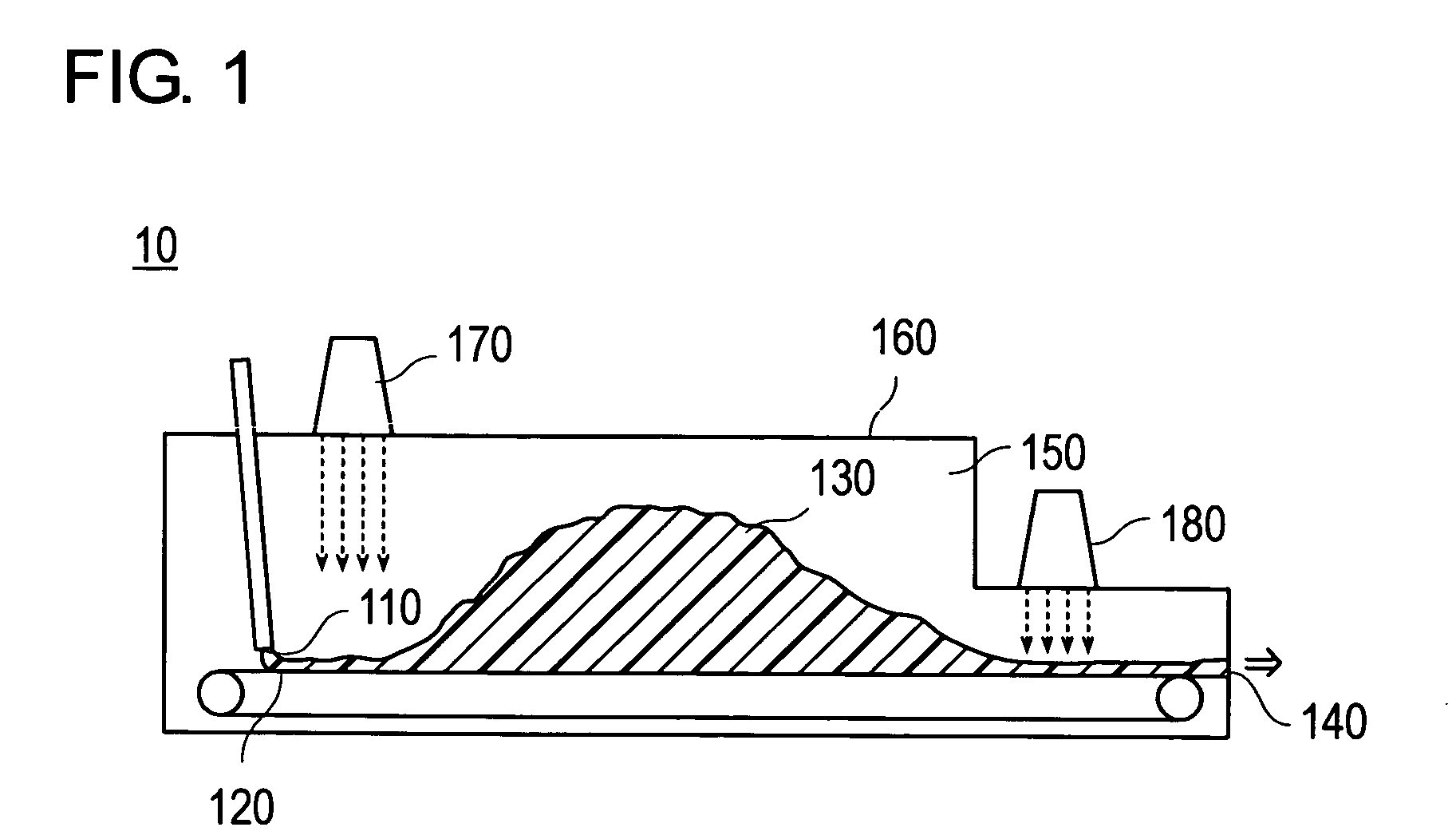
The present invention relates to a method for continuous production of a water-absorbent resin by use of an continuous polymerization device having a charge part of a monomer aqueous solution; an endless belt on which the monomer and a hydropolymer formed are conveyed; and a discharge part of the hydropolymer, wherein the continuous polymerization device has side walls and a ceiling, and the space ratio in the device represented by the equation, “space ratio in the device=B / A”, is in the range of 1.2 to 20. In the equation, A is a maximum cross-sectional area (cm2) of the hydropolymer during the polymerization in the width direction of the endless belt, and B is a maximum cross-sectional area (cm2) of the space between the endless belt of the continuous polymerization device and the ceiling of the continuous polymerization device in the width direction of the endless belt.
Owner:NIPPON SHOKUBAI CO LTD
Compositions that can produce polymers
InactiveUS7601665B2Organic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationMonomer
Owner:CHEVRON PHILLIPS CHEMICAL CO LP
Polymer solution for nanoimprint lithography to reduce imprint temperature and pressure
InactiveUS20040110856A1Reduce pressureReduce the temperatureNanostructure manufactureDecorative surface effectsCross-linkVitrification
A method of forming features on substrates by imprinting is provided. The method comprises: (a) forming a polymer solution comprising at least one polymer dissolved in at least one polymerizable monomer; and (b) depositing the polymer solution on a substrate to form a liquid film thereon; and then either: (c) curing the liquid film by causing the monomer(s) to polymerize and optionally cross-linking the polymer(s) to thereby form a polymer film, the polymer film having a glass transition temperature (Tg); and imprinting the polymer film with a mold having a desired pattern to form a corresponding negative pattern in the polymer film, or (d) imprinting the liquid film with the mold and curing it to form the polymer film. The temperature of imprinting is as little as 10° C. above the Tg, or even less if the film is in the liquid state. The pressure of the imprinting can be within the range of 100 to 500 psi.
Owner:HEWLETT PACKARD DEV CO LP
Combinatorial libraries of monomer domains
InactiveUS20050221384A1Specificity of bindingEasy screeningPeptide librariesMicrobiological testing/measurementMonomerChemistry
Methods for identifying discrete monomer domains and immuno-domains with a desired property are provided. Methods for generating multimers from two or more selected discrete monomer domains are also provided, along with methods for identifying multimers possessing a desired property. Presentation systems are also provided which present the discrete monomer and / or immuno-domains, selected monomer and / or immuno-domains, multimers and / or selected multimers to allow their selection. Compositions, libraries and cells that express one or more library member, along with kits and integrated systems, are also included in the present invention.
Owner:AMGEN MOUNTAIN VIEW
c-Met kinase binding proteins
InactiveUS20060008844A1Stimulate and inhibit activityCompound screeningPeptide librariesKinase bindingC-Met
Owner:AMGEN MOUNTAIN VIEW
Isosorbide containing polyesters and methods for making same
InactiveUS6063464AHigher inherent viscosityInherent viscosityBottlesSynthetic resin layered productsPolyesterDiol
A polyester polymer and method for making the polyester, wherein the polyester is prepared by (1) combining in a reactor a monomer containing a diacid moiety; a monomer comprising a diol moiety; and a monomer containing an isosorbide moiety; with a condensation catalyst suitable for condensing aromatic diacids and diols; and (2) heating the monomers and catalyst to polymerize the monomers to yield a polyester having an inherent viscosity of at least about 0.15 dL / g.
Owner:EI DU PONT DE NEMOURS & CO
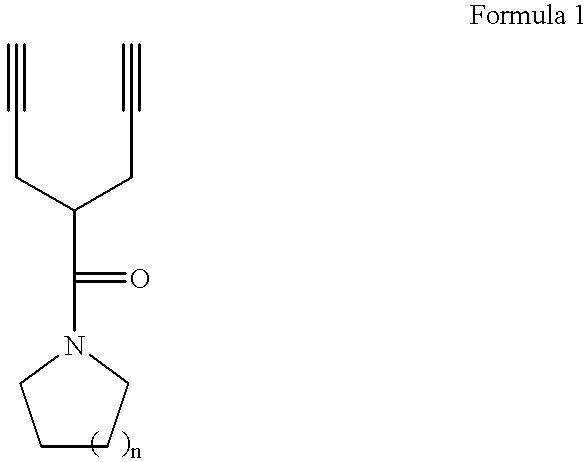
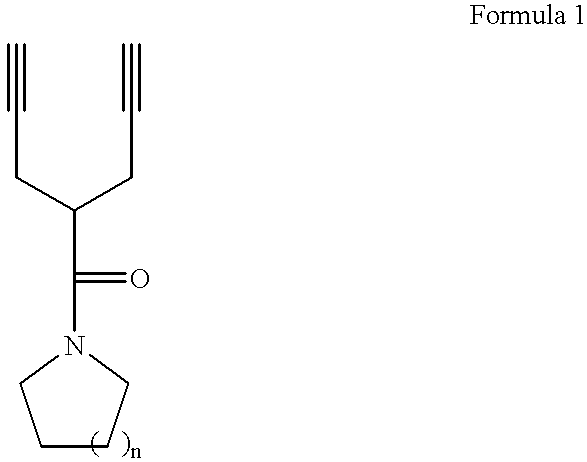
Photoresist monomers, polymers thereof, and photoresist compositions containing the same
Dipropargyl acetamide derivatives of following Formula 1 which are photoresist monomers, polymers thereof, and photoresist compositions containing the same. The photoresist polymer has high etching resistance, adhesiveness and post-exposure delay stability. As a result, the photoresist composition is suitable to form a fine pattern in a deep ultraviolet region.wherein, n is an integer from 0 to 5.
Owner:SK HYNIX INC
Photoresist monomers, polymers and photoresist compositions for preventing acid diffusion
Photoresist monomers, polymers thereof, photoresist compositions containing the same for preventing acid generated in the exposed area during the course of a photolithography process from being diffused to the unexposed area. The line edge roughness and slope pattern are improved when an ultrafine photoresist pattern is formed using photoresist copolymer having a multi-oxygen-containing compound as a repeating unit such as an ethyleneoxy moiety represented by Formula 1 with at least one polymerizable carbon-carbon double bond. In addition, the shape of pattern is improved by eliminating top loss and the adhesion of pattern to the substrate is improved. wherein n is an integer ranging from 1 to 5.
Owner:SK HYNIX INC +1
Medical devices and applications of polyhydroxyalkanoate polymers
InactiveUS6867247B2Reduce probabilityHigh porositySuture equipmentsStentsTissue repairBiocompatibility Testing
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
Solid electrolytic capacitor containing a conductive polymer
ActiveUS7515396B2Hybrid capacitor electrolytesSolid electrolytic capacitorsOligomerConductive polymer
A method for forming an electrolytic capacitor is disclosed. The method includes forming a conductive polymer coating by polymerizing a monomer in the presence of less than a stoichiometric amount of an oxidative polymerization catalyst. The present inventor has found that the use of less than the stoichiometric amount of the oxidative polymerization catalyst per mole of monomer can slow the polymerization of the monomer, creating oligomers that are shorter in length than if fully polymerized into a polymer. Without wishing to be bound by theory, it is believed that these shorter oligomers provide better penetration into the porous anode. Thus, the resulting conductive polymer layer can be more intimately positioned with respect to the anode. As a result, the formed capacitor can exhibit better performance.
Owner:KYOCERA AVX COMPONENTS CORP
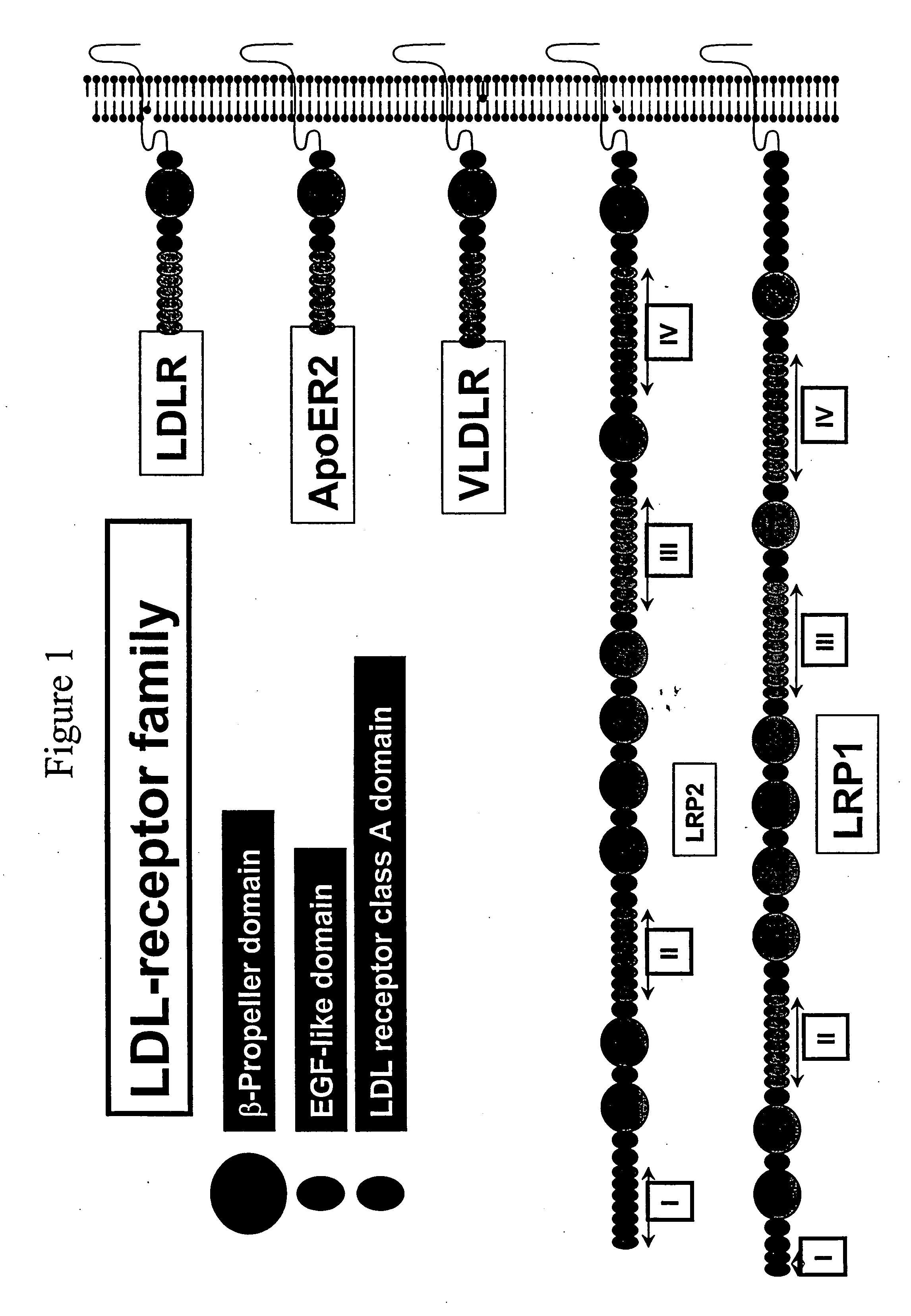
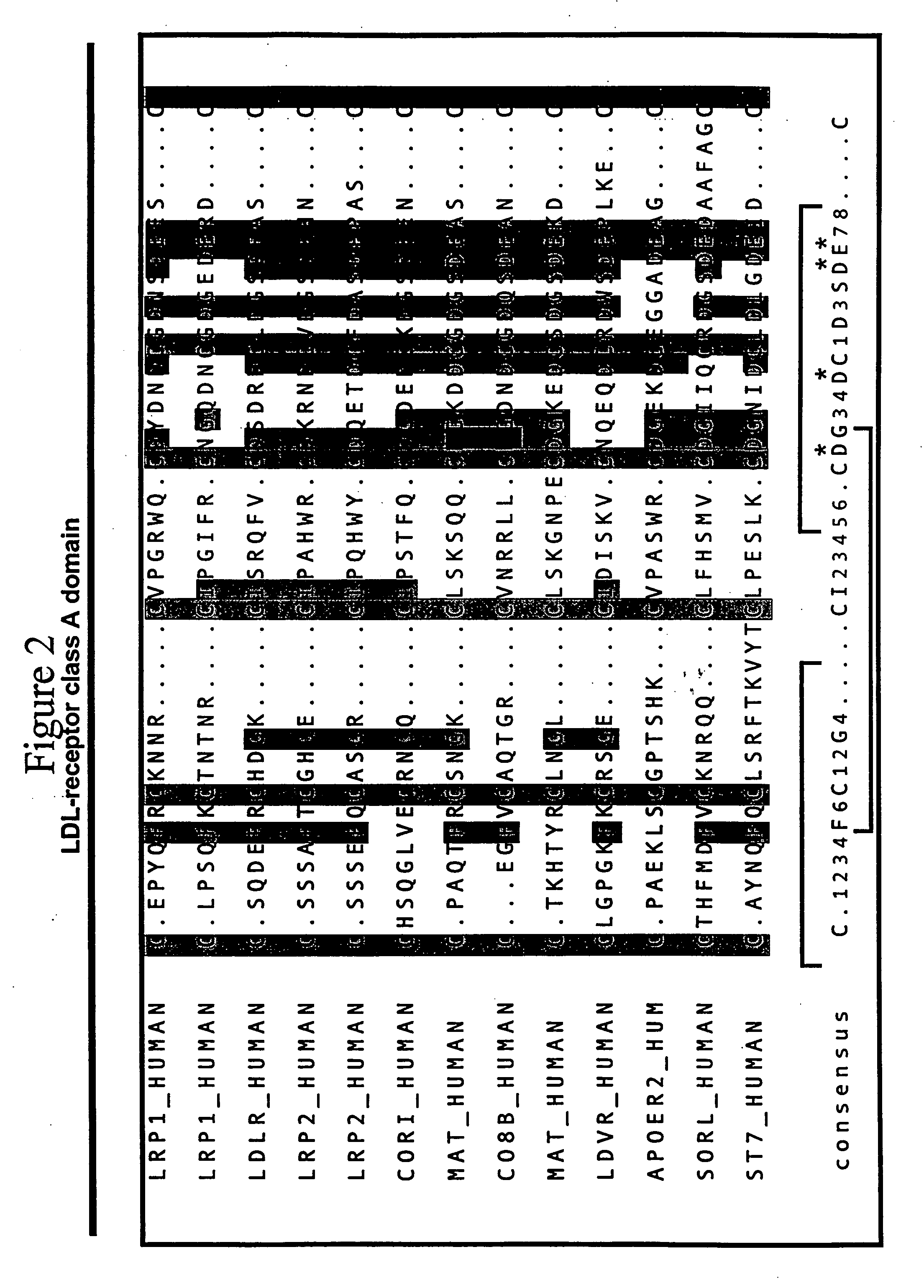
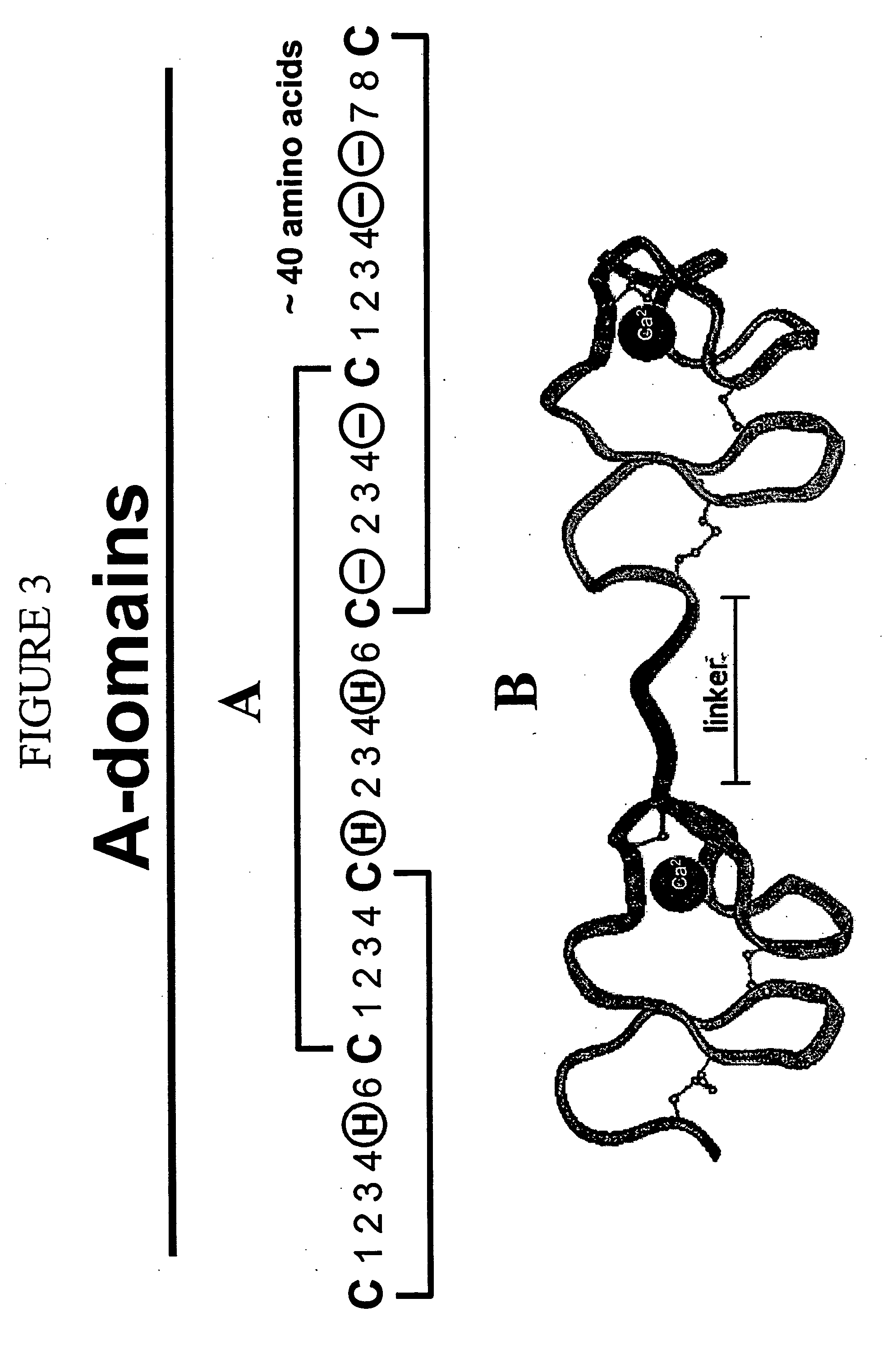
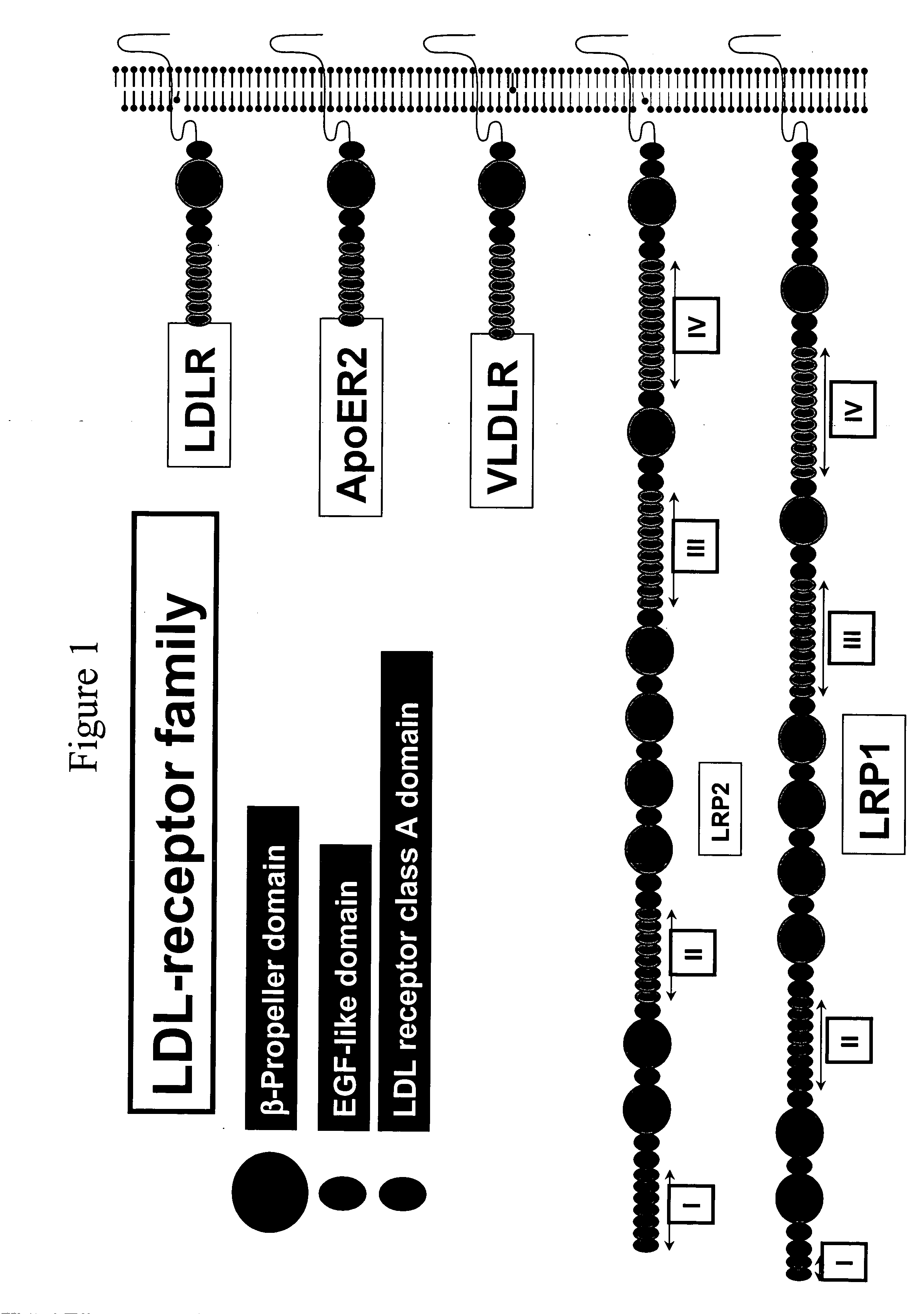
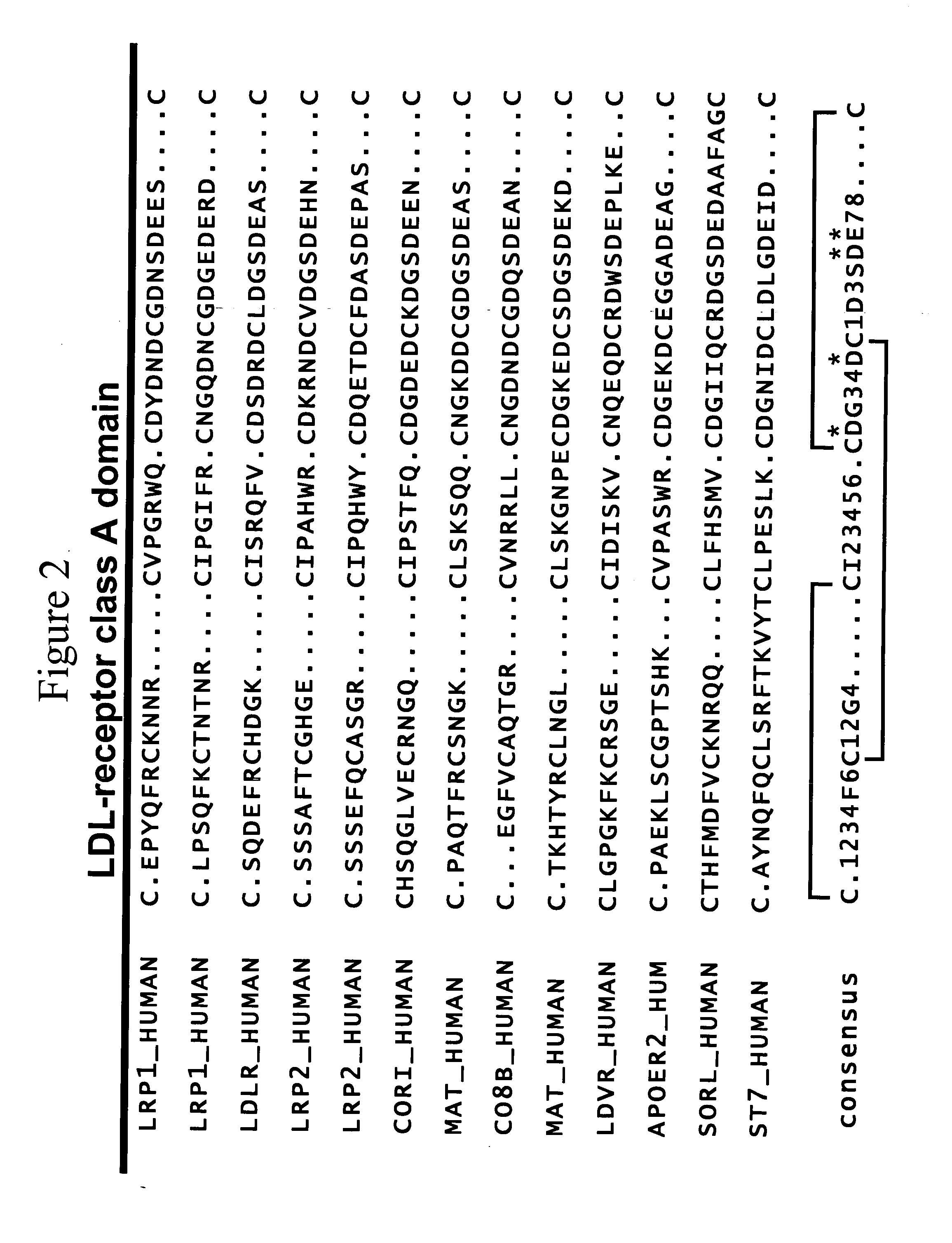
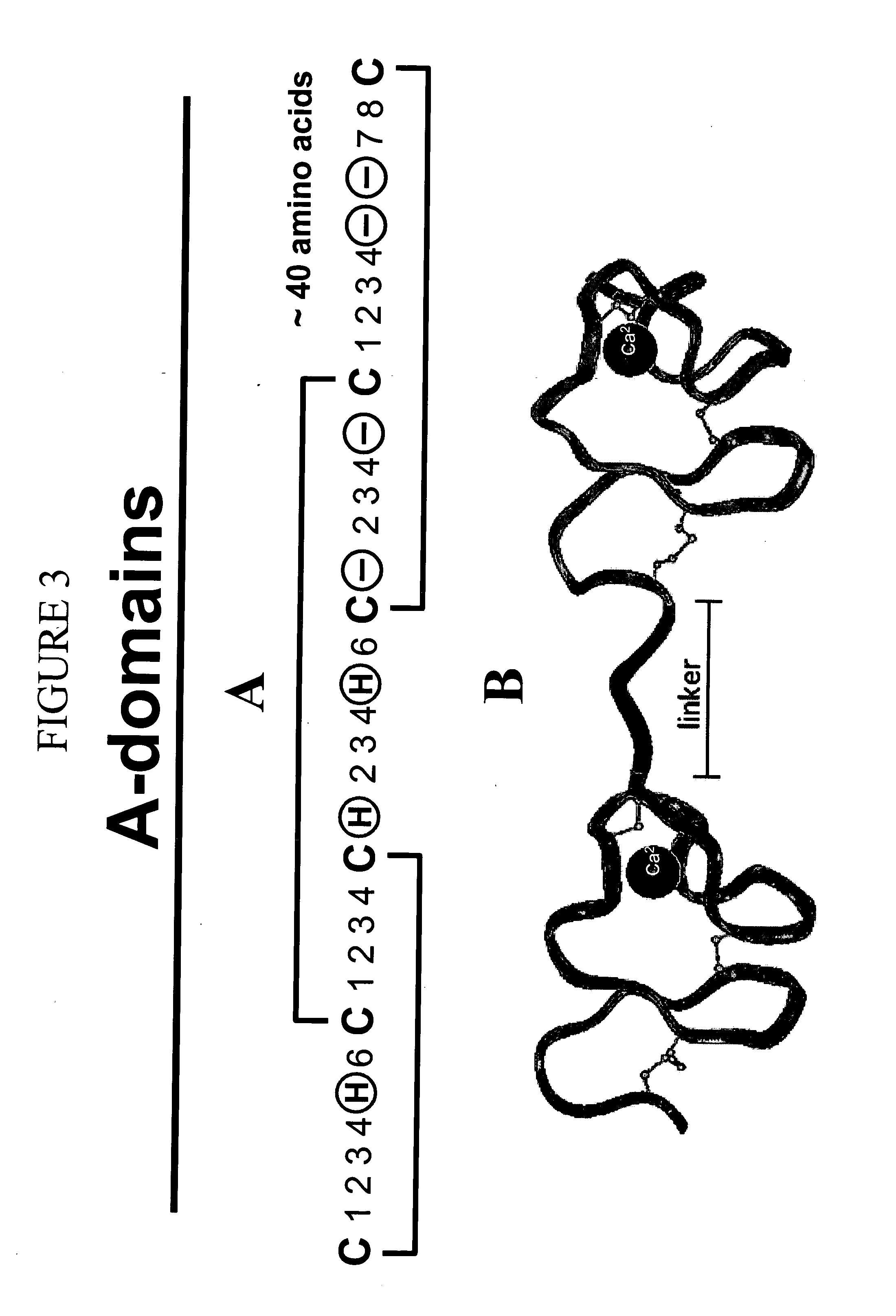
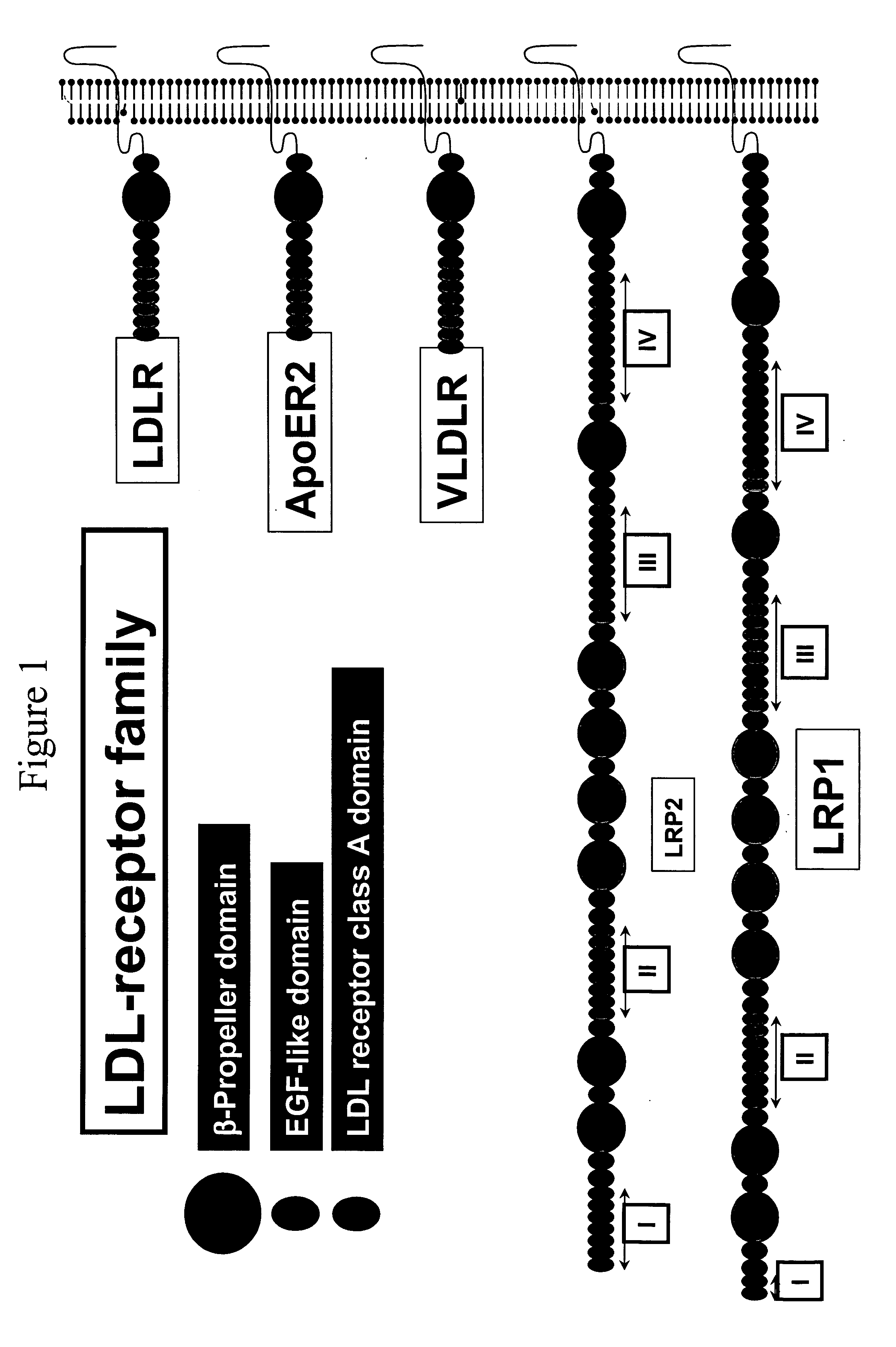
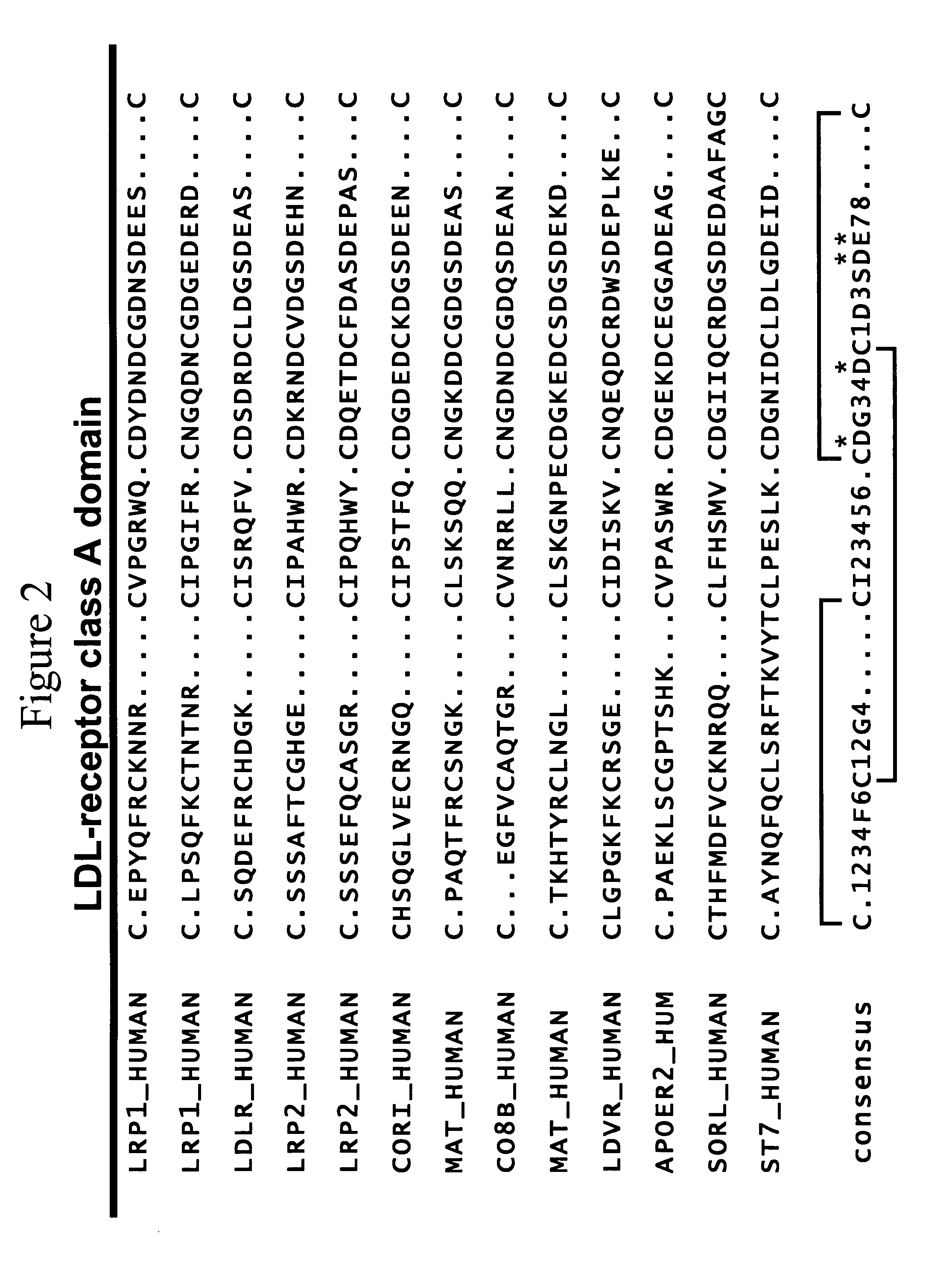
LDL receptor class A and EGF domain monomers and multimers
InactiveUS20050164301A1High affinityStimulate and inhibit activityPeptide librariesPeptide/protein ingredientsMonomerIntegrated systems
Specific monomer domains and multimers comprising the monomer domains are provided. Methods, compositions, libraries and cells that express one or more library member, along with kits and integrated systems, are also included in the present invention.
Owner:AMGEN MOUNTAIN VIEW
Characterization of individual polymer molecules based on monomer-interface interactions
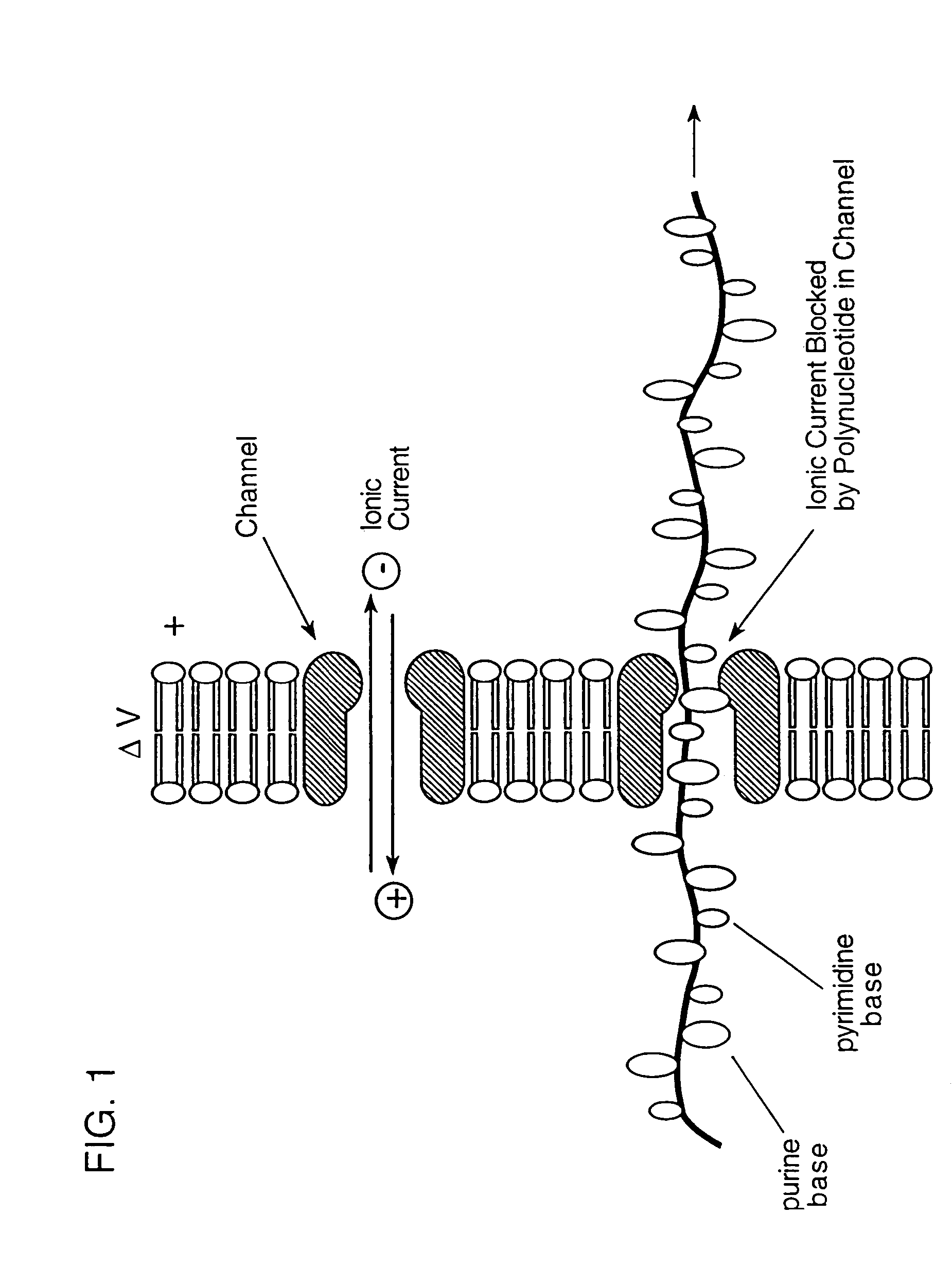
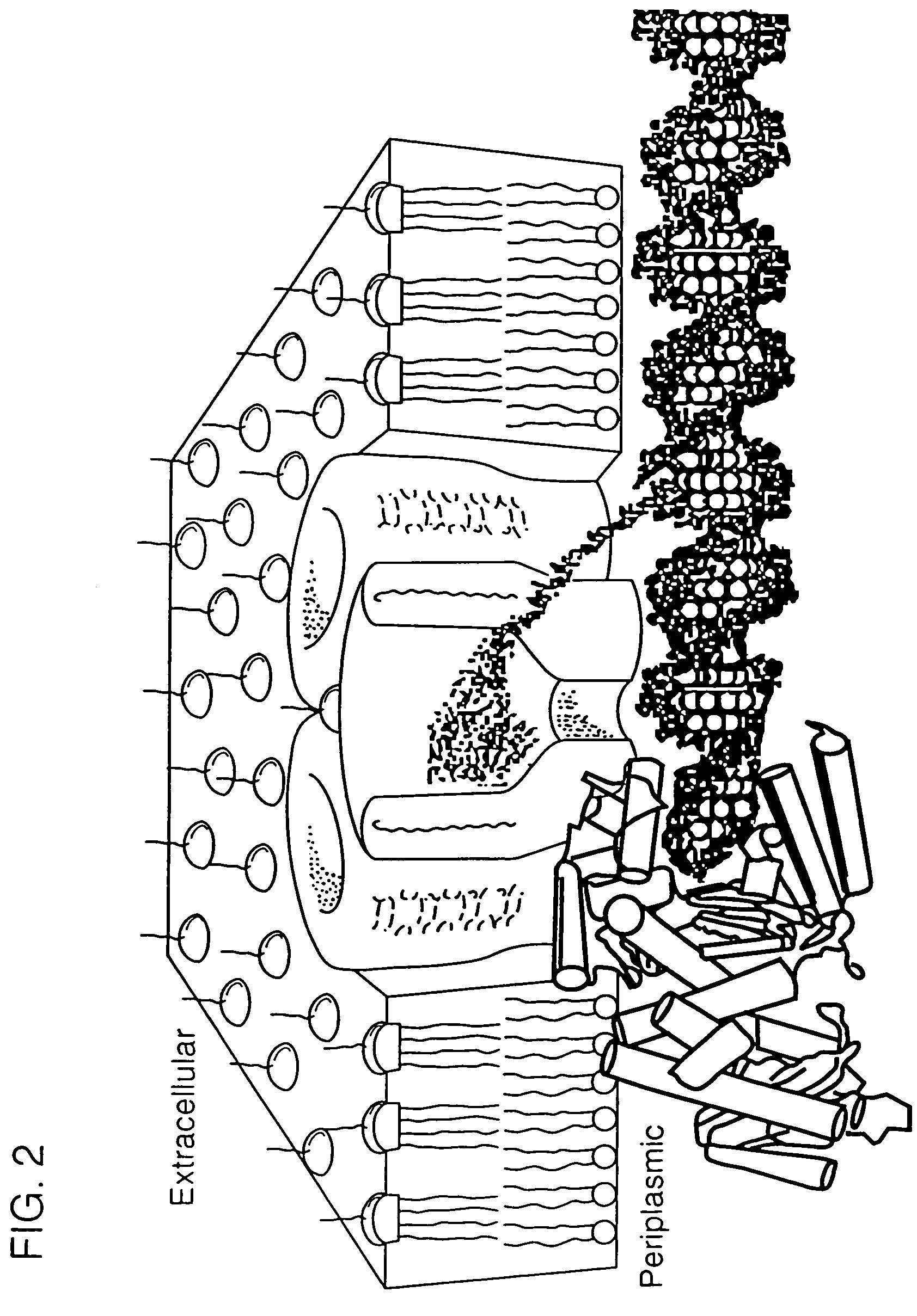
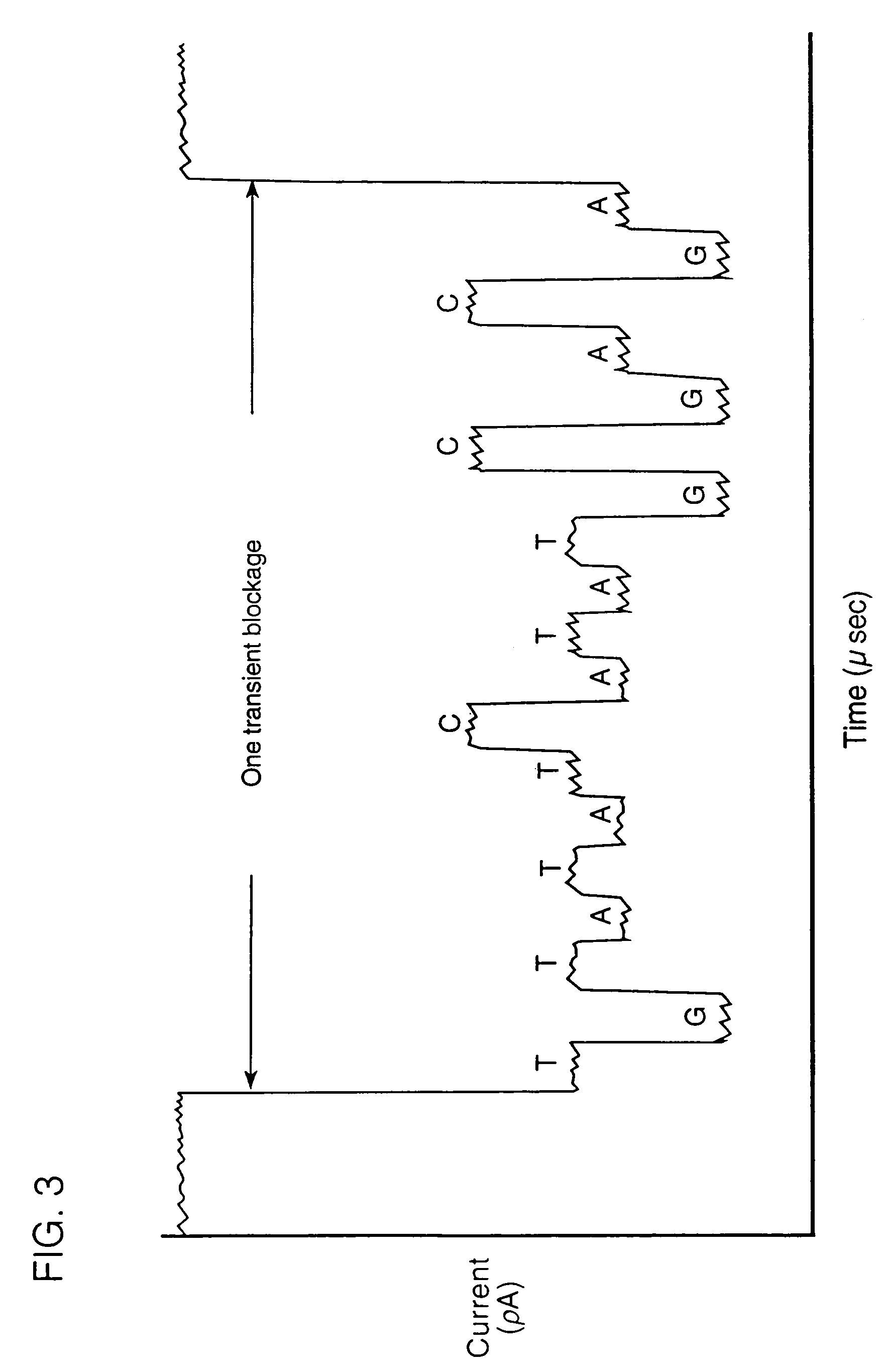
InactiveUS7189503B2Bioreactor/fermenter combinationsMaterial nanotechnologySingle strandDouble strand
The invention relates to a method for detecting a double-stranded region in a nucleic acid by (1) providing two separate, adjacent pools of a medium and a interface between the two pools, the interface having a channel so dimensioned as to allow sequential monomer-by-monomer passage of a single-stranded nucleic acid, but not of a double-stranded nucleic acid, from one pool to the other pool; (2) placing a nucleic acid polymer in one of the two pools; and (3) taking measurements as each of the nucleotide monomers of the single-stranded nucleic acid polymer passes through the channel so as to differentiate between nucleotide monomers that are hybridized to another nucleotide monomer before entering the channel and nucleotide monomers that are not hybridized to another nucleotide monomer before entering the channel.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +1
Articles having bioactive surfaces and solvent-free methods of preparation thereof
Methods for preparing articles having a bioactive surface comprising treating a substrate to form free reactive groups, depositing a monomer onto the treated substrate, and covalently immobilizing a biologically functional molecule onto the deposited monomer. Additional embodiments include methods for the deposition of the monomer onto the treated substrate in a solvent-free environment. Further embodiments include articles having surfaces prepared using the methods described herein. Additional embodiments include articles prepared using the methods described herein.
Owner:BECTON DICKINSON & CO
Soft contact lenses
InactiveUS6943203B2Reduces Young 's modulusModulus is reducedOptical partsOptical elementsMonomerSilicone
A soft contact lens containing a silicone-hydrogel made by curing a reaction mixture containing a silicone-containing monomer.
Owner:JOHNSON & JOHNSON VISION CARE INC
Optically oriented three-dimensional polymer microstructures
A method and system of creating one or more waveguides and / or patterning these waveguides to form a 3D microstructure that uses mask and collimated light. In one embodiment, the system includes at least one collimated light source selected to produce a collimated light beam; a reservoir having a photo-monomer adapted to polymerize by the collimated light beam; and a mask having at least one aperture and positioned between the at least one collimated light source and the reservoir. Here, the at least one aperture is adapted to guide a portion of the collimated light beam into the photo-monomer to form the at least one polymer waveguide through a portion of a volume of the photo-monomer.
Owner:HRL LAB
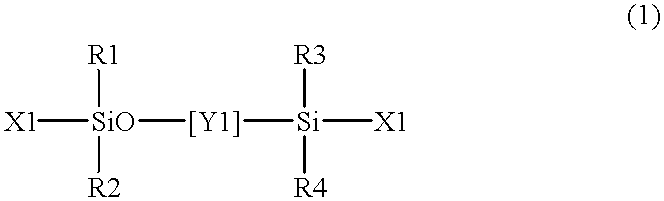
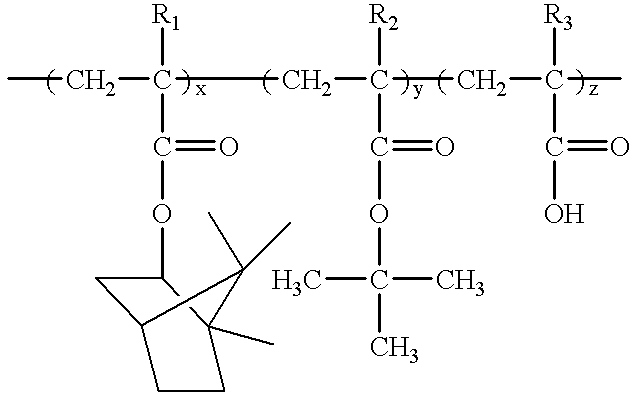
Room temperature-setting compositions
The present invention provides a room temperature-setting composition containing a polyoxyalkylene polymer (A), having a molecular weight of from 8000 to 50000 and having hydrolyzable silicon groups of the following formula (1), which comprises, as an essential component, the polyoxyalkylene polymer, having a molecular weight of from 8000 to 50000 and having hydrolyzable silicon groups of the formula (1), wherein a is 3 and a room temperature-setting composition containing a polyoxyalkylene polymer (A) having a molecular weight of from 8000 to 50000 and having hydrolyzable silicon groups of the formula (1), which comprises, as essential components, the polyoxyalkylene polymer having a molecular weight of from 8000 to 50000 and having hydrolyzable silicon groups of the formula (1) wherein a is 3, and a polymer (B) made by polymerization of a polymerizable unsaturated group-containing monomer (C):wherein R1 is a C1-20 substituted or unsubstituted monovalent organic group, X is a hydroxyl group or a hydrolyzable group, a is 1, 2 or 3, provided that when more than one R1 exist, the plurality of R1 may be the same or different, and when more than one X exist, the plurality of X may be the same or different.
Owner:ASAHI GLASS CO LTD
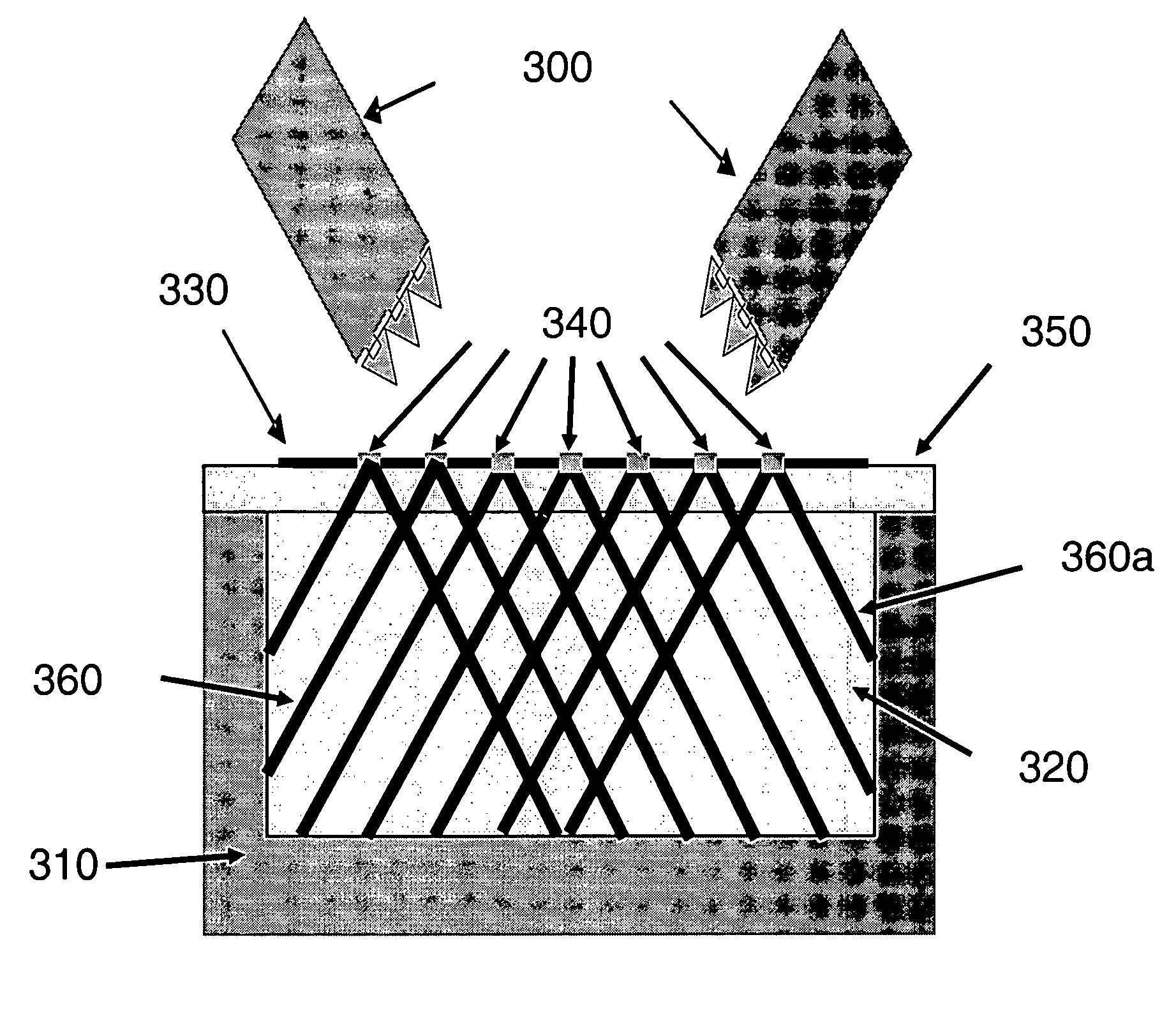
V-like domain binding molecules
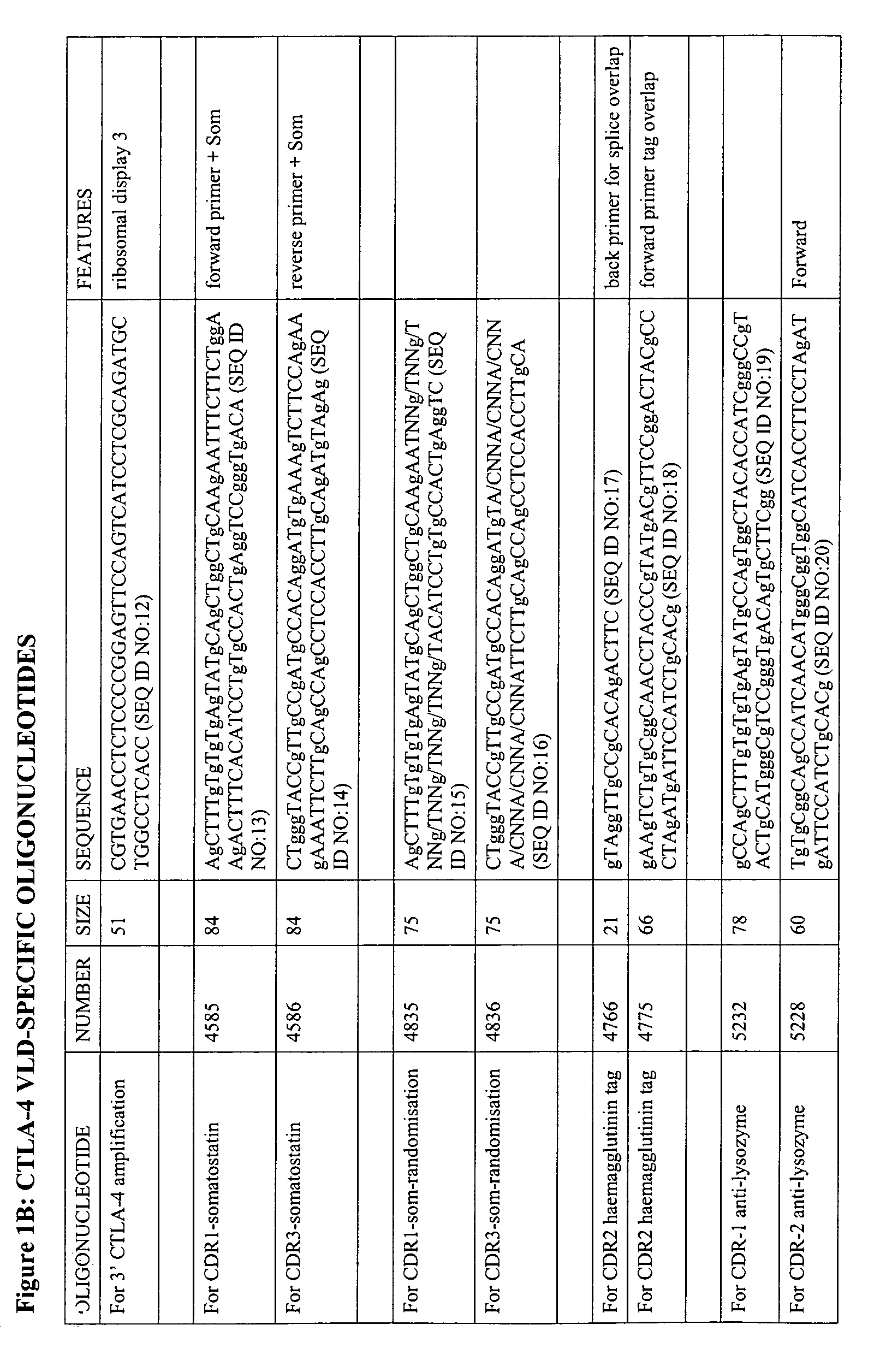
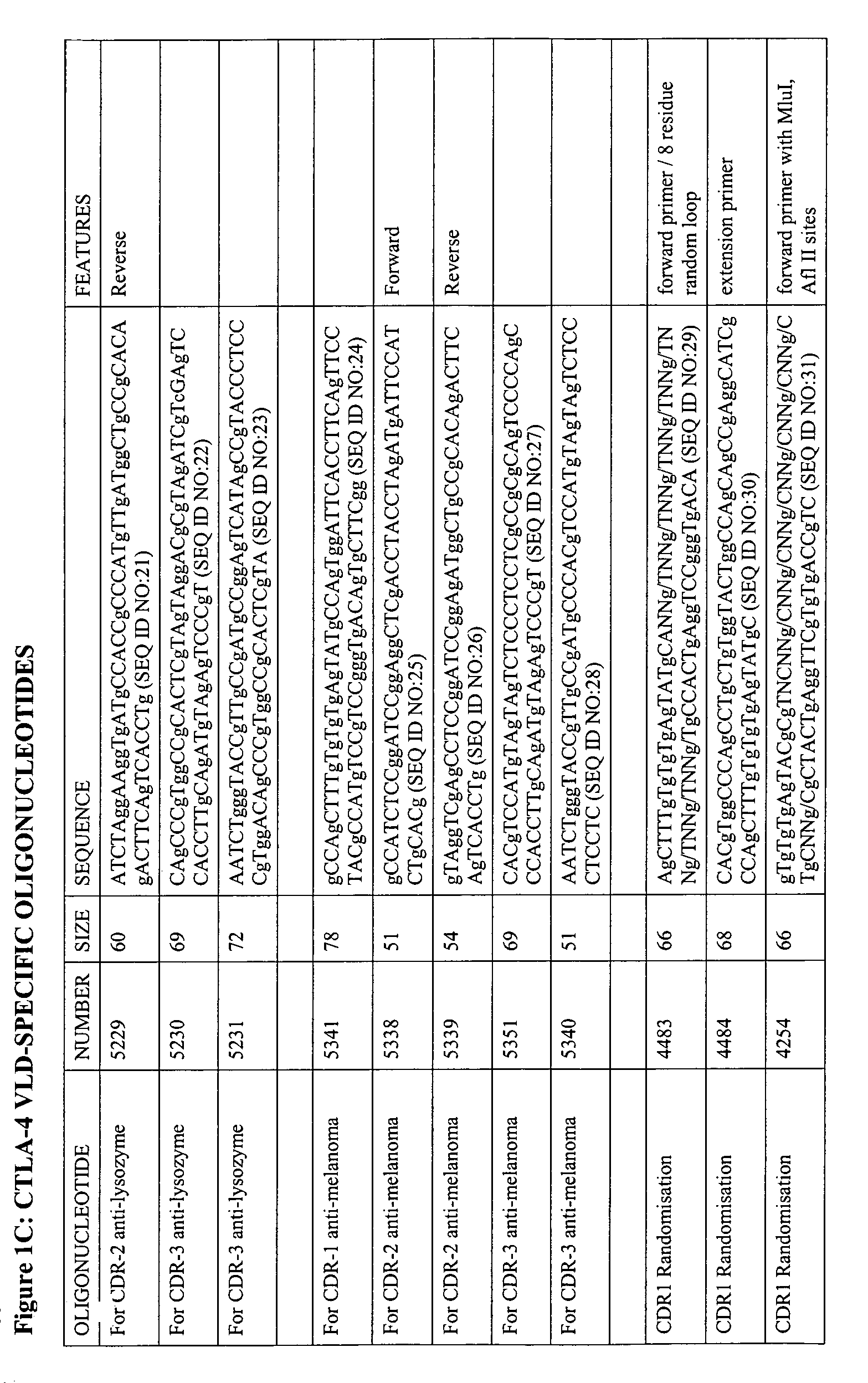
The present invention relates to binding moieties comprising at least one monomeric V-like domain (VLD) derived from a non-antibody ligand, the at least one monomeric V-like domain being characterized in that at least one CDR loop structure or part thereof is modified or replaced such that the solubility of the modified VLD is improved when compared with the unmodified VLD.
Owner:ARANA THERAPEUTICS VIC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com