Patents
Literature
566 results about "Wound site" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Remote augmented motor-sensory interface for surgery
InactiveUS20060087746A1Easy to controlImprove surgeon performanceDiagnosticsSurgical manipulatorsTelecommunications linkInput control
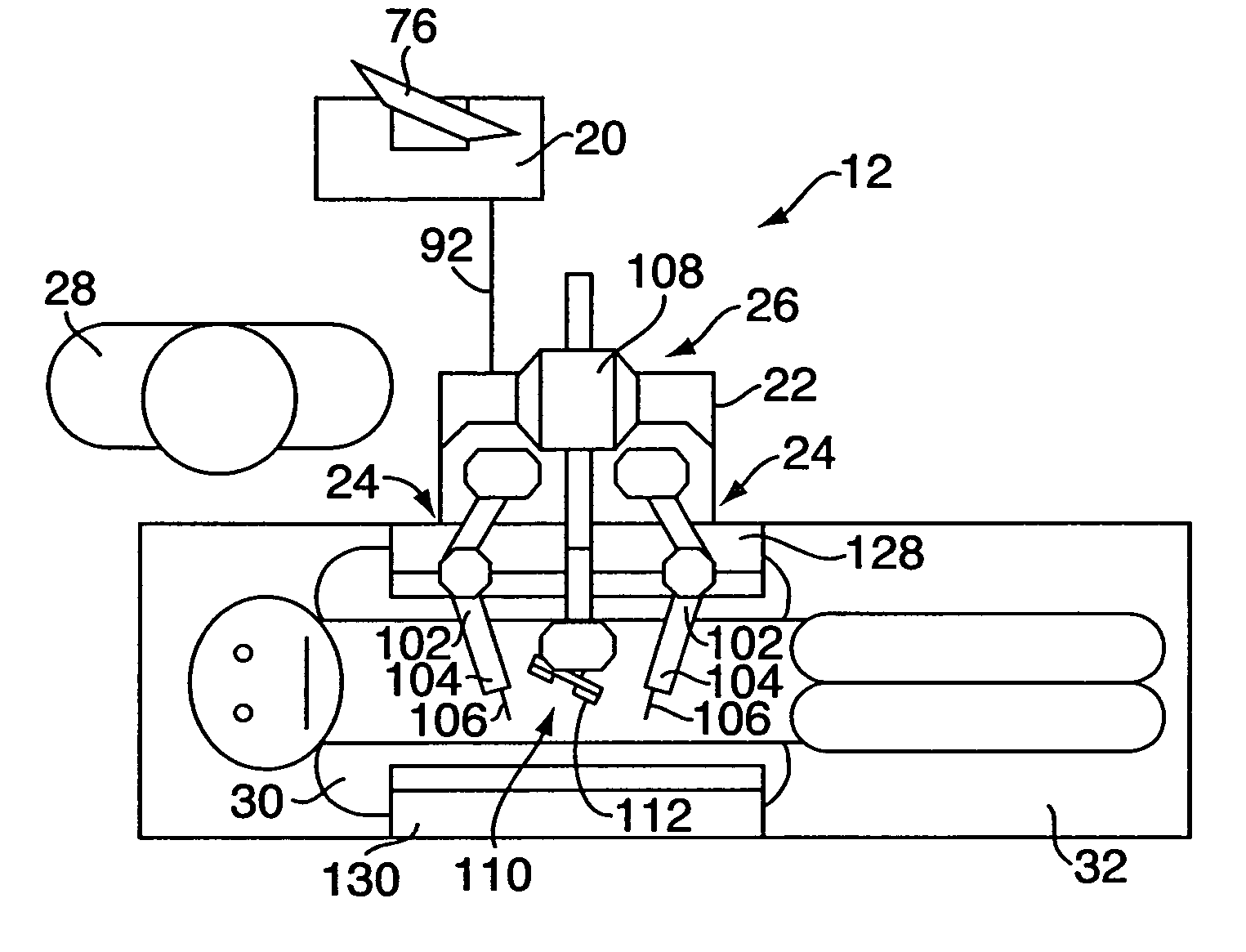
A portable augmented motor-sensory interface (“PAMI”) system for “tele-controlled” surgery includes a PAMI tele-controller and a portable PAMI medical field unit positioned at a different, distant location and configured for wireless communications with the tele-controller. (“Tele-control” refers to electronically translating and / or transmitting a physician's actions over a long distance.) The field unit has a surgical module, including robotic effectors / arms, configured for carrying out medical procedures, and a video sensor boom for electronically viewing a wound site. The tele-controller has a user interface with input controls and a display that outputs sensory information relating to the patient and field unit. For treating a patient, the field unit is deployed next to the patient and a communications link is established to the tele-controller. A physician at the tele-controller receives sensory input from the field unit through the user interface display, and manipulates the input controls for tele-controlling the surgical module.
Owner:LIPOW KENNETH
Vacuum assisted wound dressing
ActiveUS8715256B2Small and lightImprove portabilityPlastersAdhesive dressingsTopical Negative-Pressure TherapyVacuum assisted
Owner:SMITH & NEPHEW PLC
Sutures and surgical staples for anastamoses, wound closures, and surgical closures
InactiveUS8016881B2Improve featuresControlled release rateSuture equipmentsStentsSurgical stapleMicroparticle
The invention relates to sutures and surgical staples useful in anastomoses. Various aspects of the invention include wound closure devices that use amphiphilic copolymer or parylene coatings to control the release rate of an agent, such as a drug or a biological material, polymerizing a solution containing monomers and the agent to form a coating, using multiple cycles of swelling a polymer with a solvent-agent solution to increase loading, microparticles carrying the agent, biodegradable surgical articles with amphiphilic copolymer coatings, and sutures or surgical staples the deliver a drug selected from the group consisting of triazolopyrimidine, paclitaxol, sirolimus, derivatives thereof, and analogs thereof to a wound site.
Owner:MIRUS LLC
Wound dressing and method for controlling severe, life-threatening bleeding
ActiveUS7371403B2Stanching flowProhibiting flow of bloodBiocideSuspensory bandagesHydrophilic polymersWound dressing
This invention is directed to advanced hemorrhage control wound dressings, and methods of using and producing same. The subject wound dressing is constructed from a non-mammalian material for control of severe bleeding. The wound dressing for controlling severe bleeding is formed of a biomaterial comprising chitosan, a hydrophilic polymer, a polyacrylic polymer or a combination thereof. The kind of severe, life-threatening bleeding contemplated by this invention is typically of the type not capable of being stanched when a conventional gauze wound dressing is applied with conventional pressure to the subject wound. The wound dressing being capable of substantially stanching the flow of the severe life-threatening bleeding from the wound by adhering to the wound site, to seal the wound, to accelerate blood clot formation at the wound site, to reinforce clot formation at the wound site and prevent bleed out from the wound site, and to substantially prohibit the flow of blood out of the wound site.
Owner:PROVIDENCE HEALTH SYST OREGONDBA ST VINCENTMEDICAL CENT
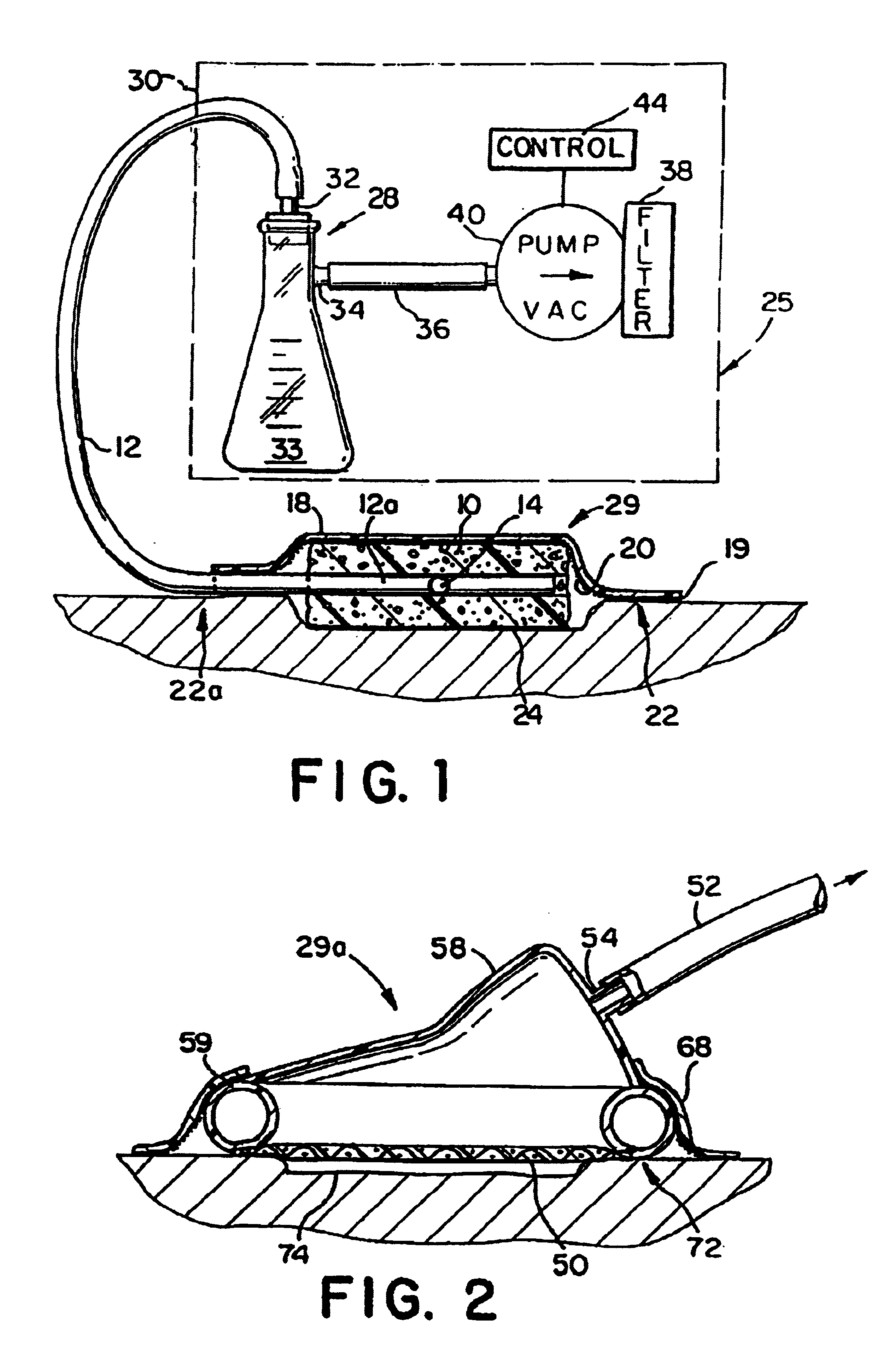
Wound treatment employing reduced pressure
InactiveUS7216651B2Reduce pressureIncreased formationDiagnosticsRespiratory masksWound siteVacuum pump
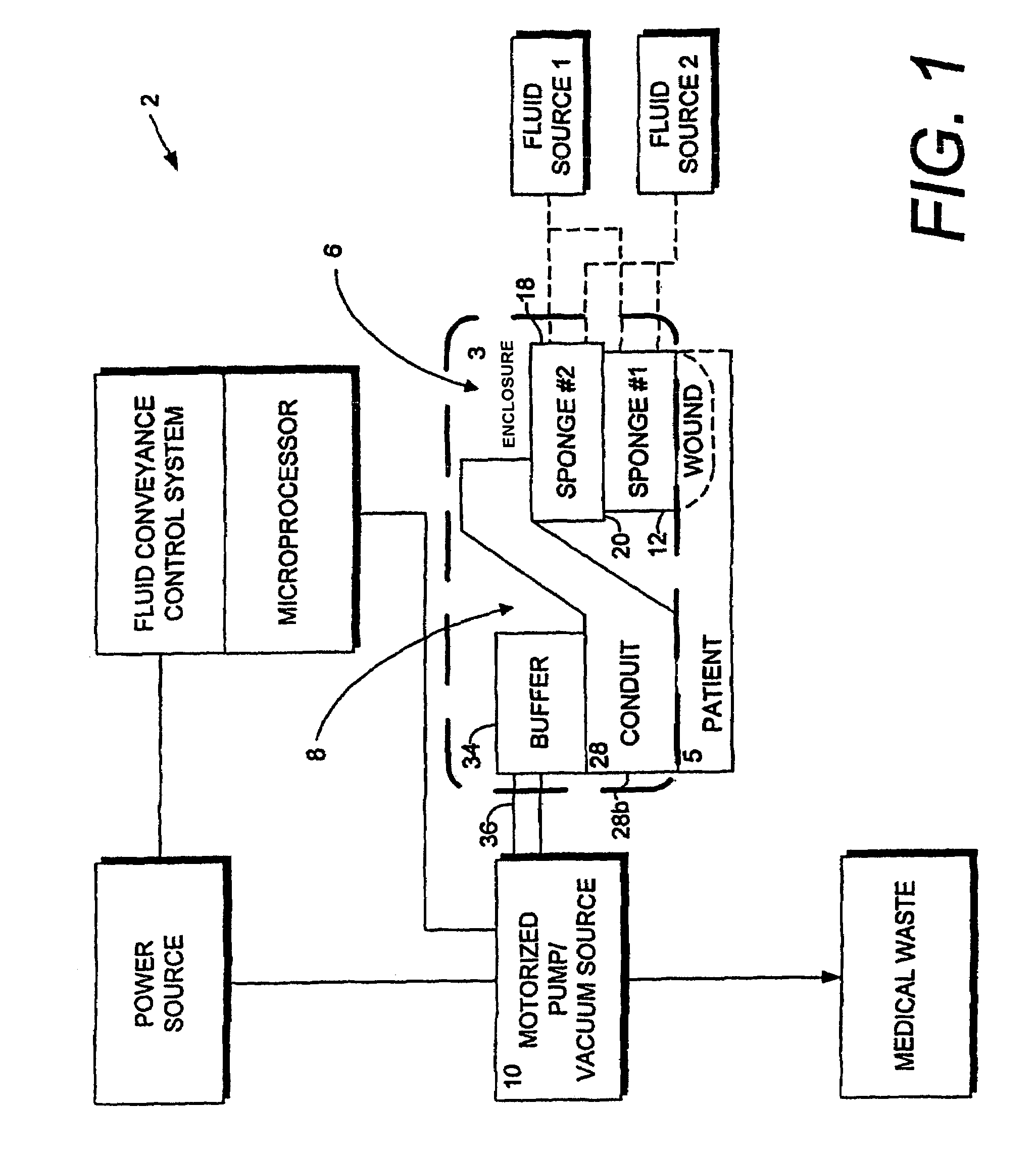
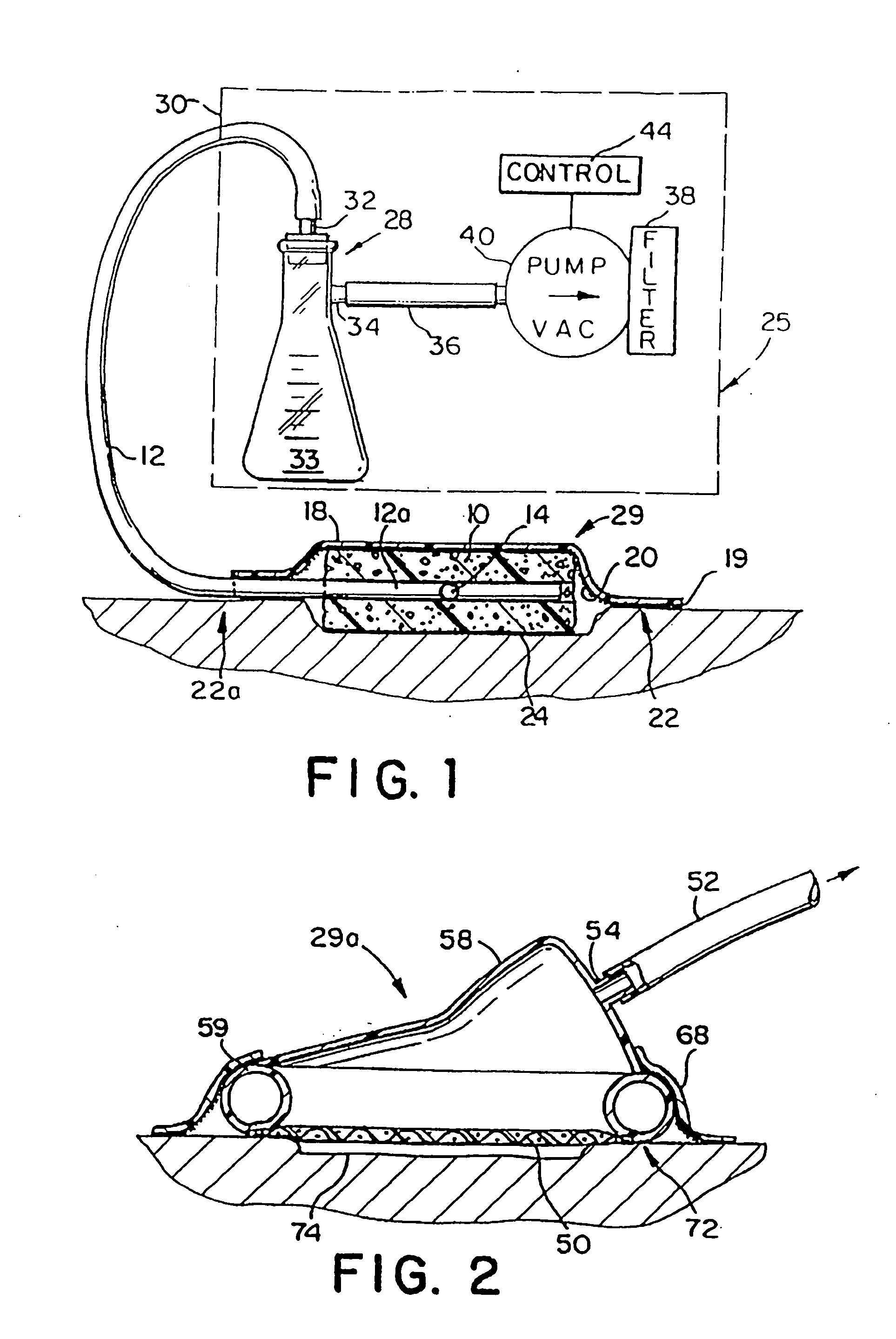
A method of treating tissue damage comprises applying a negative pressure to a wound sufficient in time and magnitude to promote tissue migration and thus facilitate closure of the wound. The method is applicable to wounds, burns, infected wounds, and live tissue attachments. A wound treatment apparatus is provided in which a fluid impermeable wound cover is sealed over a wound site. A screen in the form of an open-cell foam screen or a rigid porous screen is placed beneath the wound cover over the wound. A vacuum pump supplies suction within the wound cover over the treatment site.
Owner:WAKE FOREST UNIV HEALTH SCI INC
Biocompatible wound dressing
InactiveUS7070584B2Promote cell growthPrevent vacuum leakageWound drainsMedical applicatorsWound dressingWound site
A biocompatible wound dressing comprised of a pad for insertion substantially into a wound site and a wound drape for sealing enclosure of the foam pad at the wound site. The pad, comprised of a foam or other like material having relatively few open cells in contact with the areas upon which cell growth is to be encouraged so as to avoid unwanted adhesions, but having sufficiently numerous open cells so that drainage and negative pressure therapy may continue unimpaired, is placed in fluid communication with a vacuum source for promotion of fluid drainage, as known in the art. The pad is further comprised of an ultra-low density fused-fibrous ceramic, or a bioabsorbable branched polymer, or cell growth enhancing matrix or scaffolding.
Owner:KCI LICENSING INC
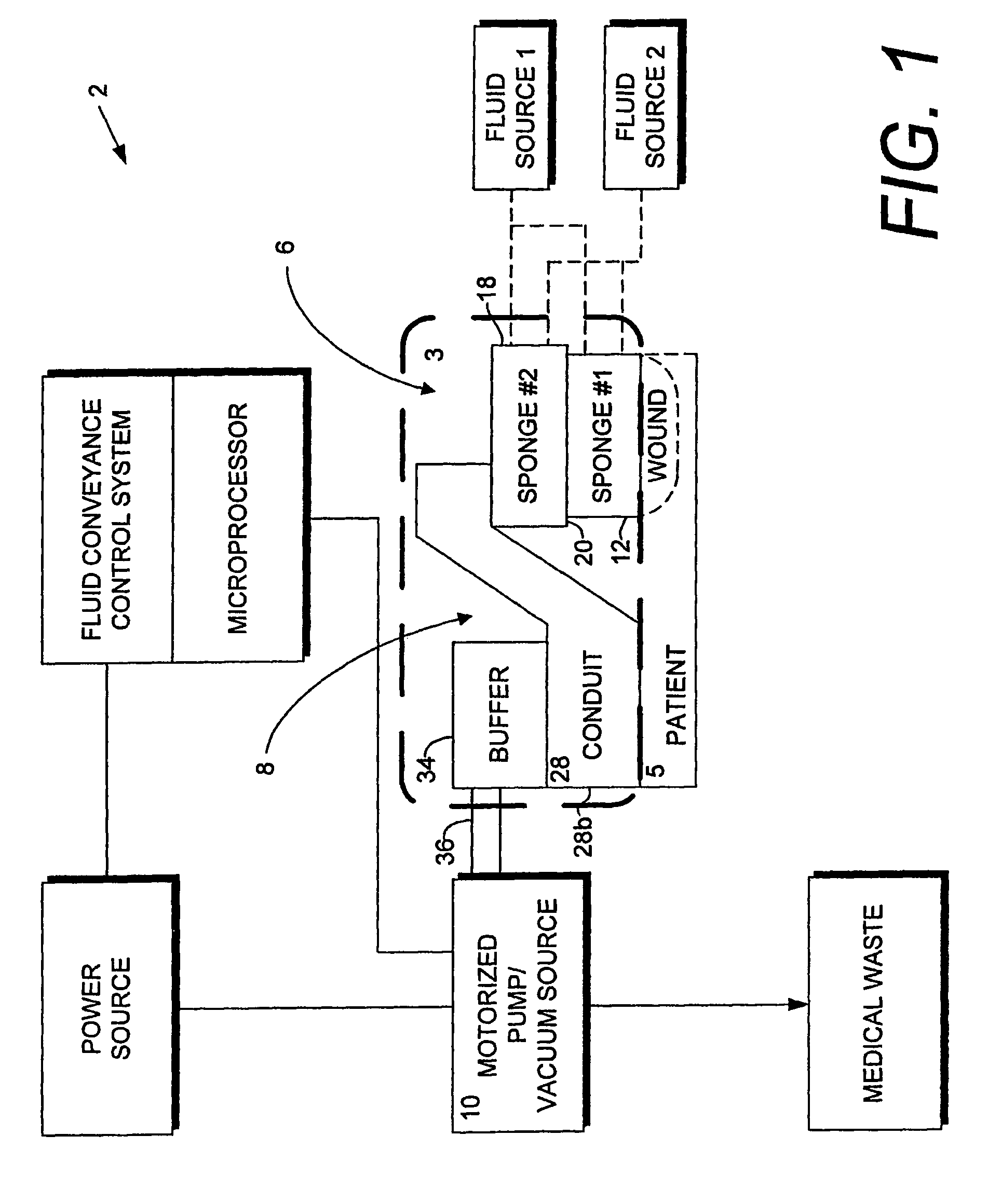
Wound therapy and tissue management system and method with fluid differentiation
A wound therapy and tissue management system utilizes fluid differentiation. Fluid is differentiated by establishing a gradient within the system. A gradient can be established with matter or energy. Patient interfaces for establishing, maintaining and varying one or more gradients include transfer elements with first and second zones having different flow coefficients. The transfer elements exchange fluid with a patient, generally through a wound site, and with external components of the system. Osmotic solution gradients are controlled by a methodology involving the present invention for extracting solutions, which can include toxins, from patients and for introducing fluids and sumping air to wound sites.
Owner:KCI LICENSING INC
Removable wound closure
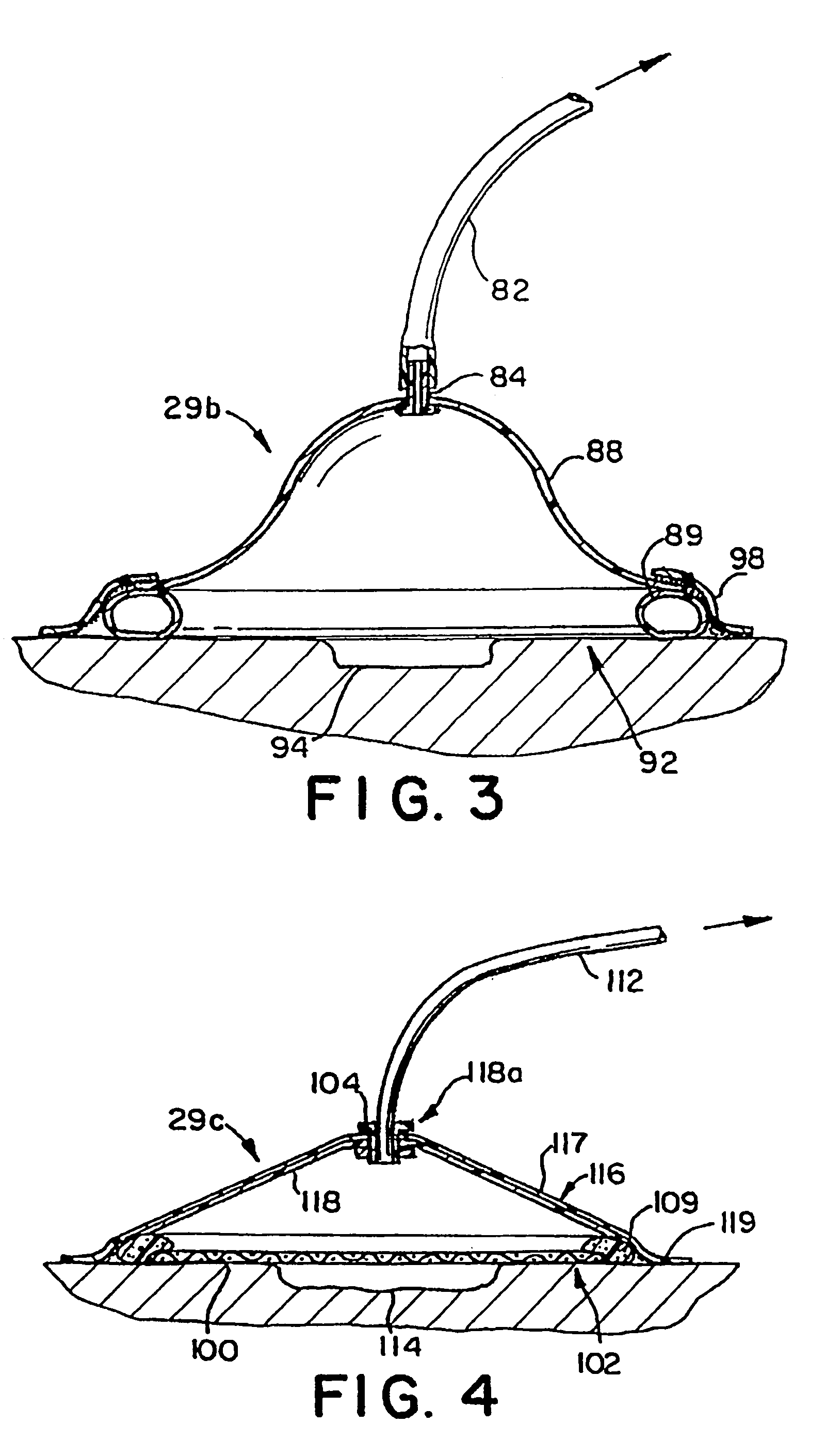
InactiveUS7381859B2Promote wound healingMinimizes adhesion formationNon-adhesive dressingsWound drainsElastomerPorosity
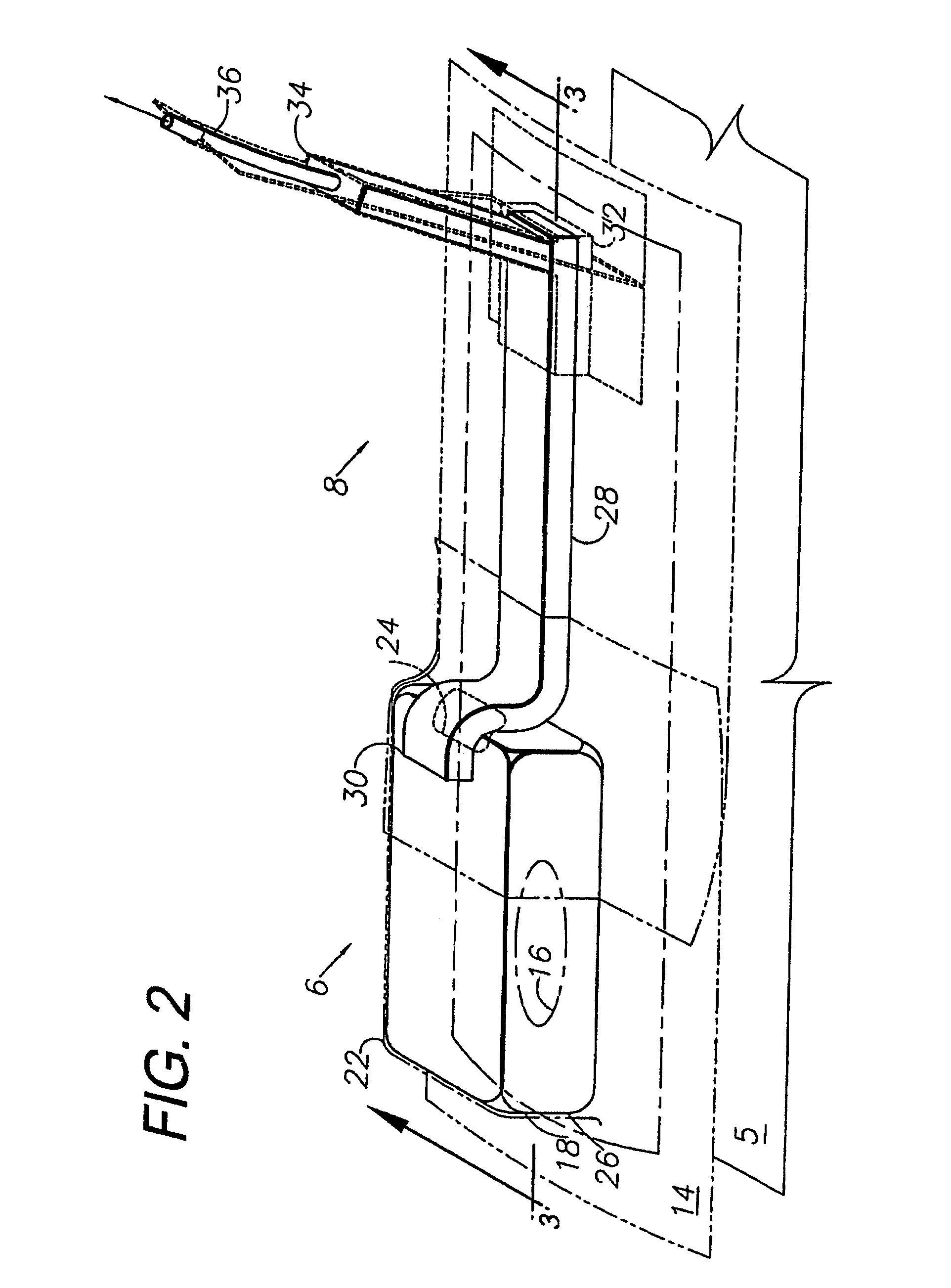
A system and method for the temporary closure of a wound, especially an abdominal wound, to facilitate re-entry, final closure, and long term healing of the wound. An abdominal wound dressing and methods of use are described that enable the application of negative pressure to the wound site in a site healing promoting manner while also limiting the formation of adhesions that would prevent the removal of the dressing. The dressing comprises a layer of porous foam material (36) enclosed by sheets of elastomeric material (38) punctuated by a number of appropriately placed holes (34). Multiple layers of porous foam may also be used. A suction tube connector (16) is provided on an upper surface of a layer of foam (12) for connection to a negative pressure source. At least one layer of foam is enclosed in elastomeric material and is placed in direct contact with the tissue within the open wound. Fluids are drawn by negative pressure through the holes positioned in the elastomeric envelope, and through the foam. If multiple foam layers are employed, the lower layer(s) of foam are of a finer porosity while the upper layer of foam is coarse. An adhesive elastomeric sheet (14) covers the entire wound dressing and seals the edges to the skin surrounding the wound. An appropriate vacuum device is attached to the suction tube connector.
Owner:KCI LICENSING INC
Multi-fastener surgical apparatus and method
A fastener preferably made from a shape memory alloy is provided which can access internal tissue or other synthetic material by catheter delivery through an endovascular pathway. After the fastener is deployed through layers of tissue or other material, it assumes a shape that automatically applies to the layers of tissue or other material an appropriate hemostatic compression which is relatively independent of tissue or material thickness. The fastener is a suitable replacement for conventional non bio-absorbable sutures and staples in certain clinical applications. The shape, method of deployment, and low force requirements make the disclosed apparatus suitable for endosurgical procedures where access to the wound site is limited. A method for deploying the fastener is also provided.
Owner:ONUX MEDICAL
Wound treatment employing reduced pressure
A method of treating tissue damage comprises applying a negative pressure to a wound sufficient in time and magnitude to promote tissue migration and thus facilitate closure of the wound. The method is applicable to wounds, burns, infected wounds, and live tissue attachments. A wound treatment apparatus is provided in which a fluid impermeable wound cover is sealed over a wound site. A screen in the form of an open-cell foam screen or a rigid porous screen is placed beneath the wound cover over the wound. A vacuum pump supplies suction within the wound cover over the treatment site.
Owner:WAKE FOREST UNIV HEALTH SCI INC
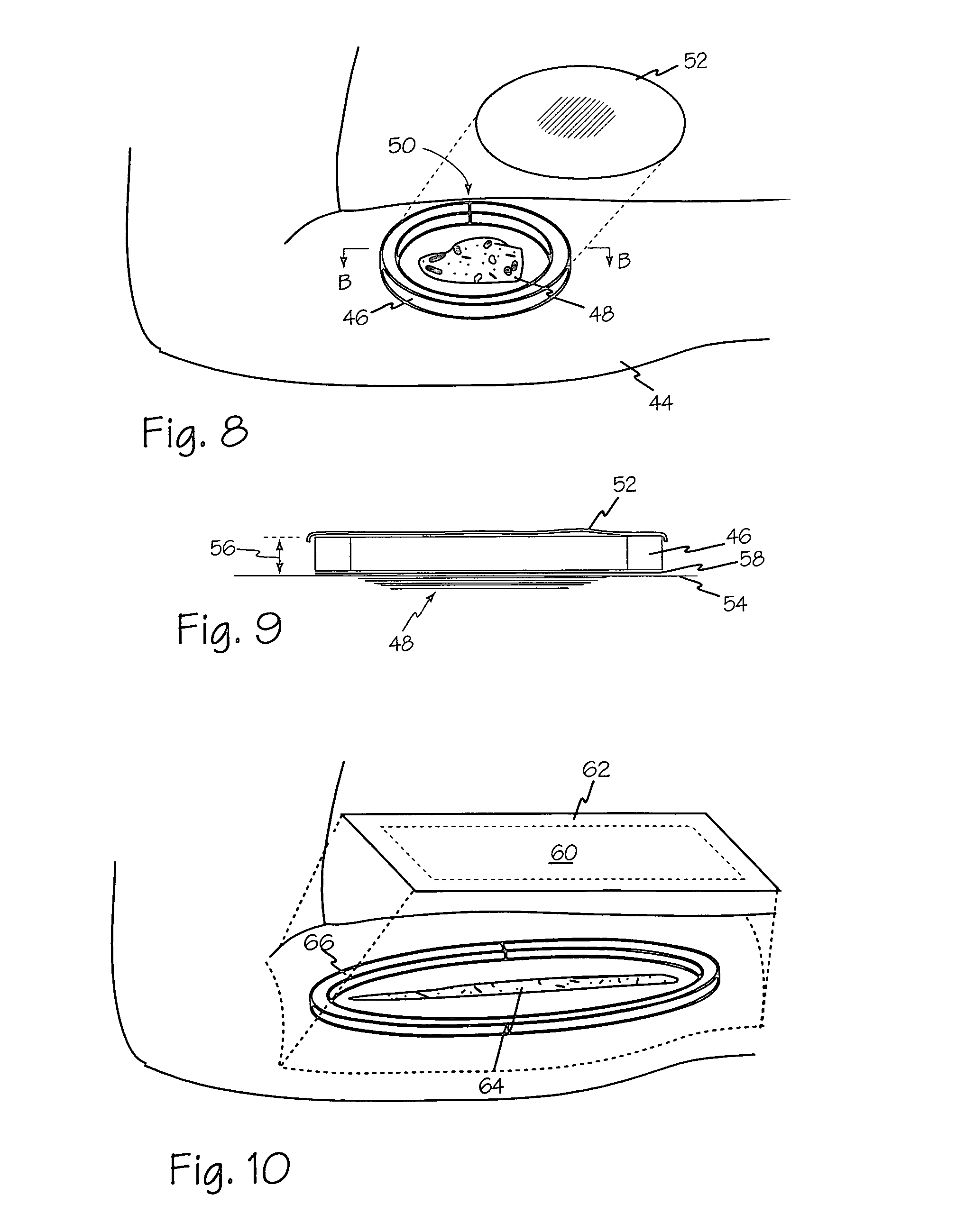
Vacuum assisted closure pad with adaptation for phototherapy
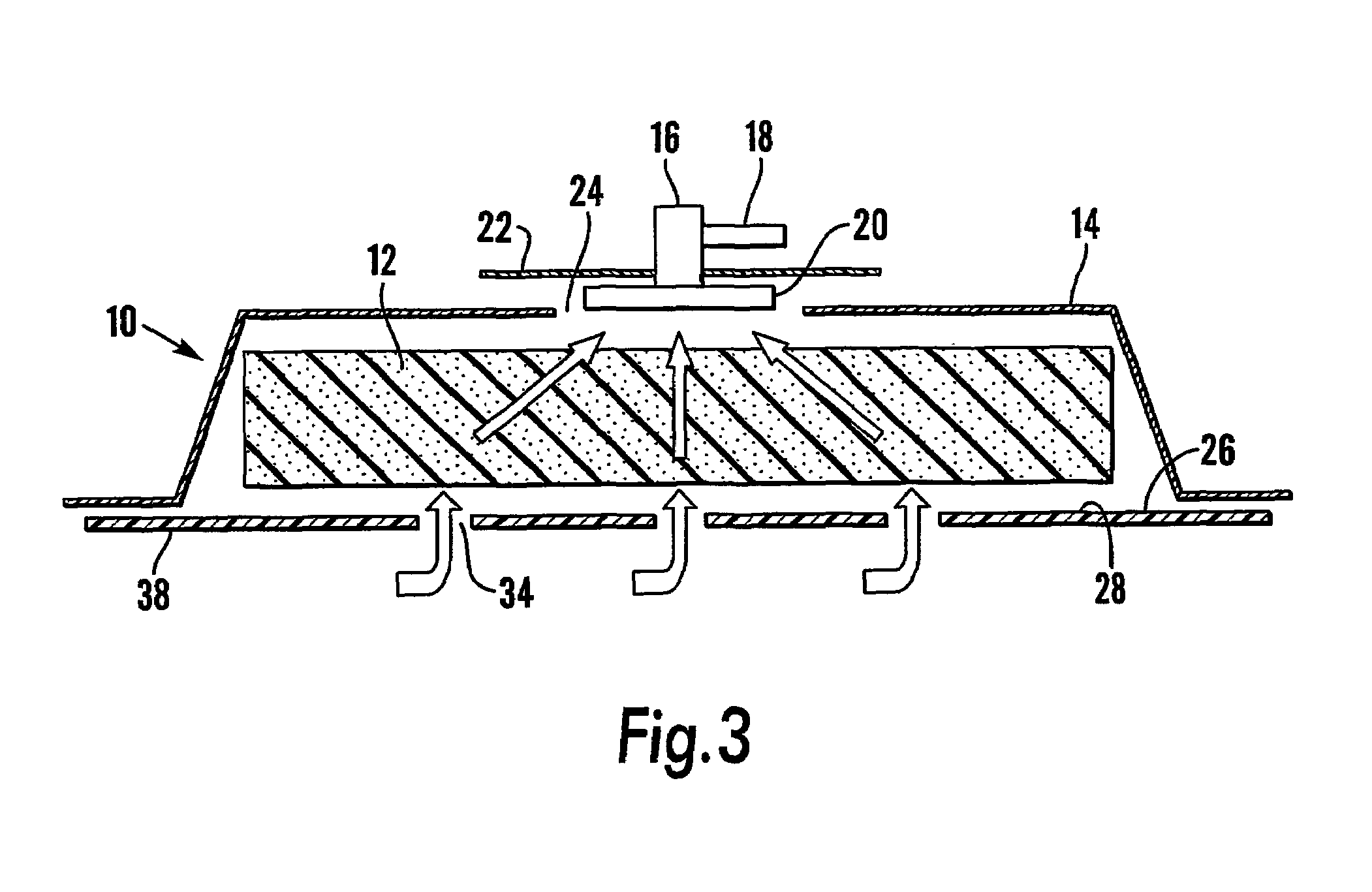
InactiveUS6994702B1Prevent vacuum leakageIncrease costPlastersSurgical instrument detailsFiberVacuum assisted
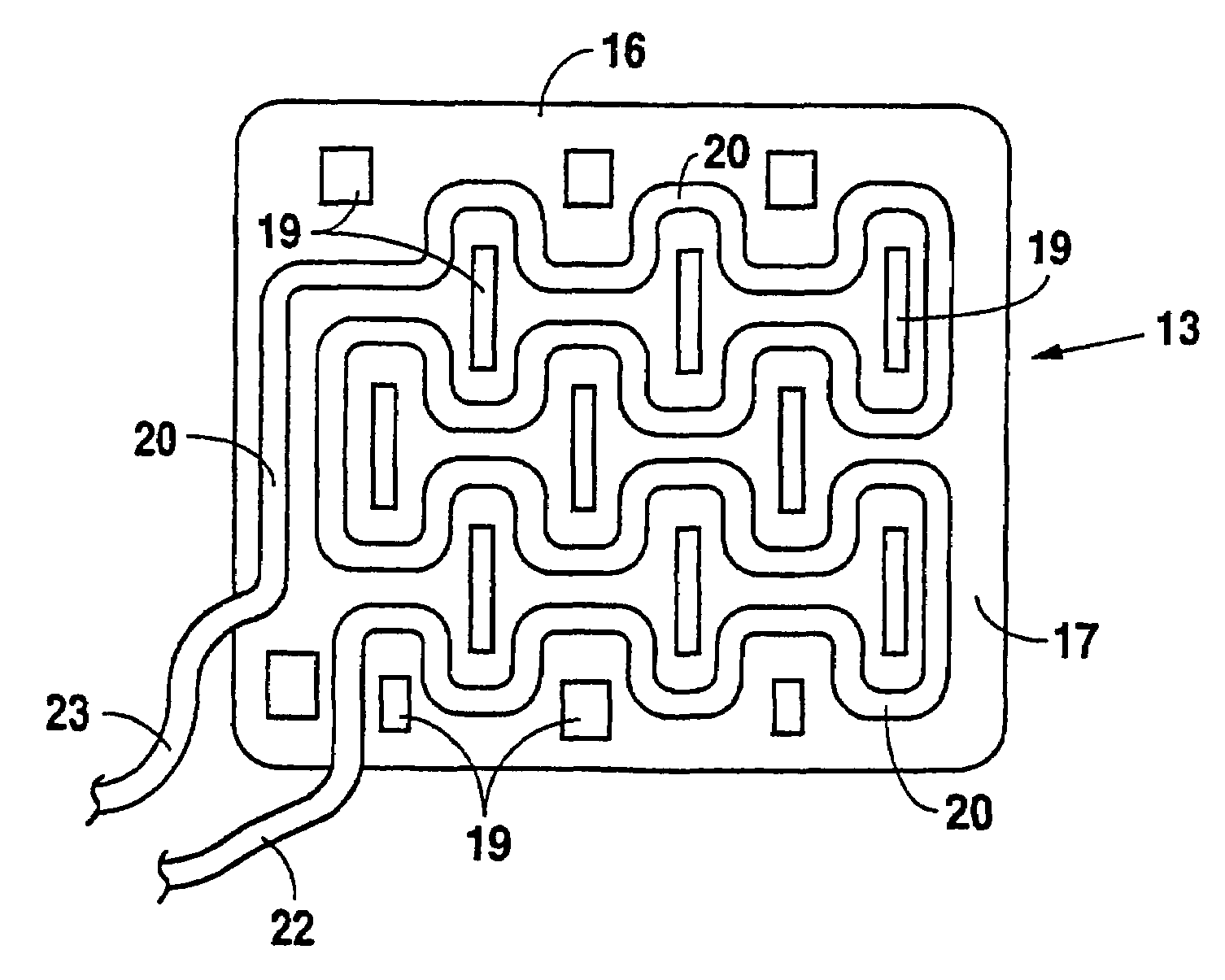
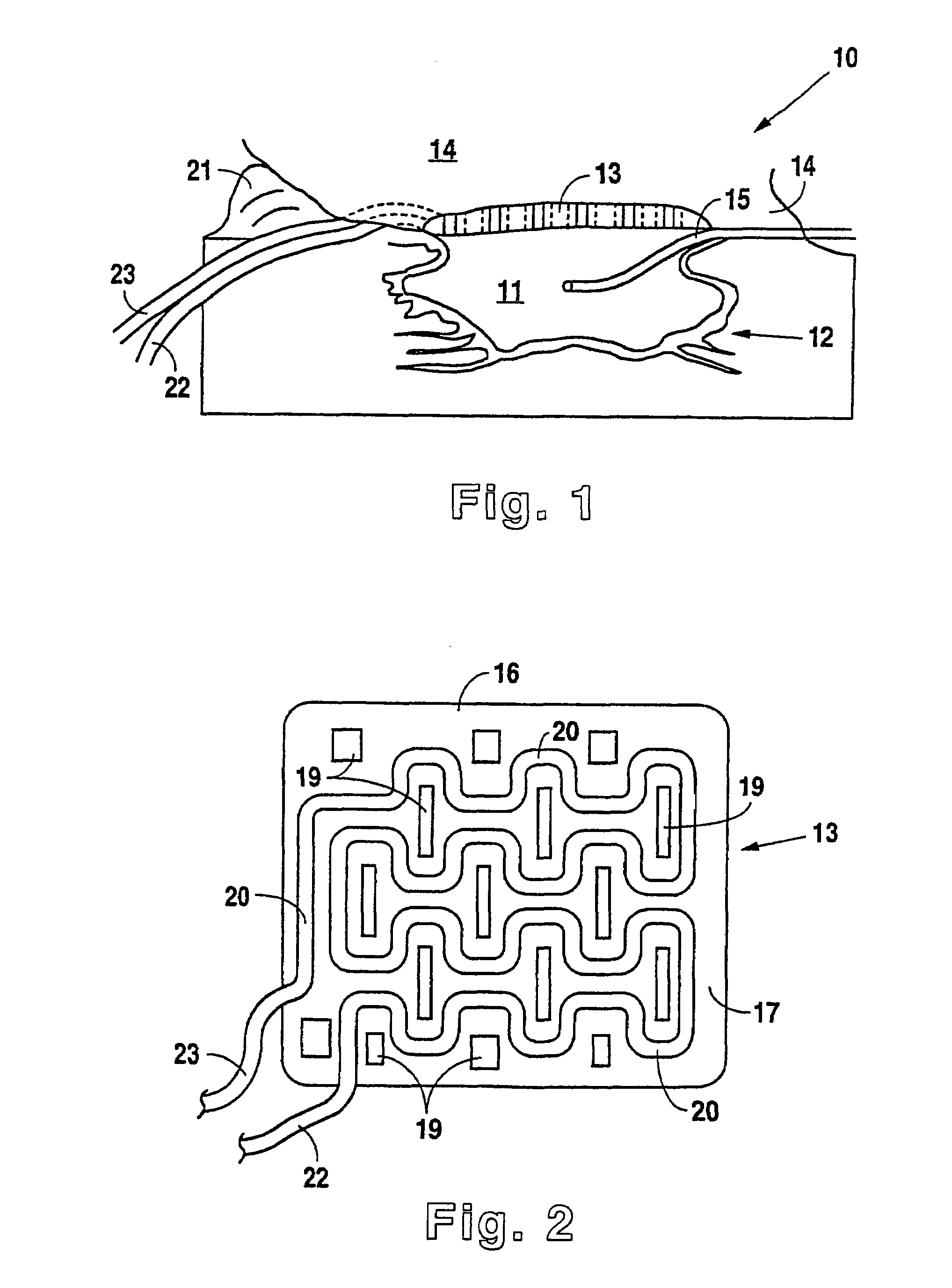
A modified vacuum assisted wound closure system adapted for concurrent applications of phototherapy having a foam pad for insertion substantially into the wound site and a wound drape for sealing enclosure of the foam pad at the wound site. The foam pad includes an optical pigtail, whereby desired wavelength of light may be directed into and about the wound site. The foam pad is placed in fluid communication with a vacuum source for promotion of fluid drainage. The foam pad is made of a highly reticulated, open-cell polyurethane or polyether foam for good permeability of wound fluids while under suction and is also embedded with an optical pigtail. The optical pigtail comprises an optical fiber that has been formed to fan into a plurality of sections. The fibers of the most distal fanned sections, which are implanted in the foam pad at its base, are provided with tiny optical slots, oriented away from the foam pad and toward the wound site. Each optical slot is made by stripping the cladding from the optical fiber in the desired areas of the fanned sections to form slot radiators. Because it is necessary to trim the foam pad in preparation for therapy, the optical fibers comprise plastics, such as acrylic or styrene. Upon placement of the pad, having the optical pigtail embedded therein, the wound drape is firmly adhered about the VAC therapy suction hose as well as the extending optical fiber to prevent vacuum leakage.
Owner:MORGAN STANLEY
Analyte test device
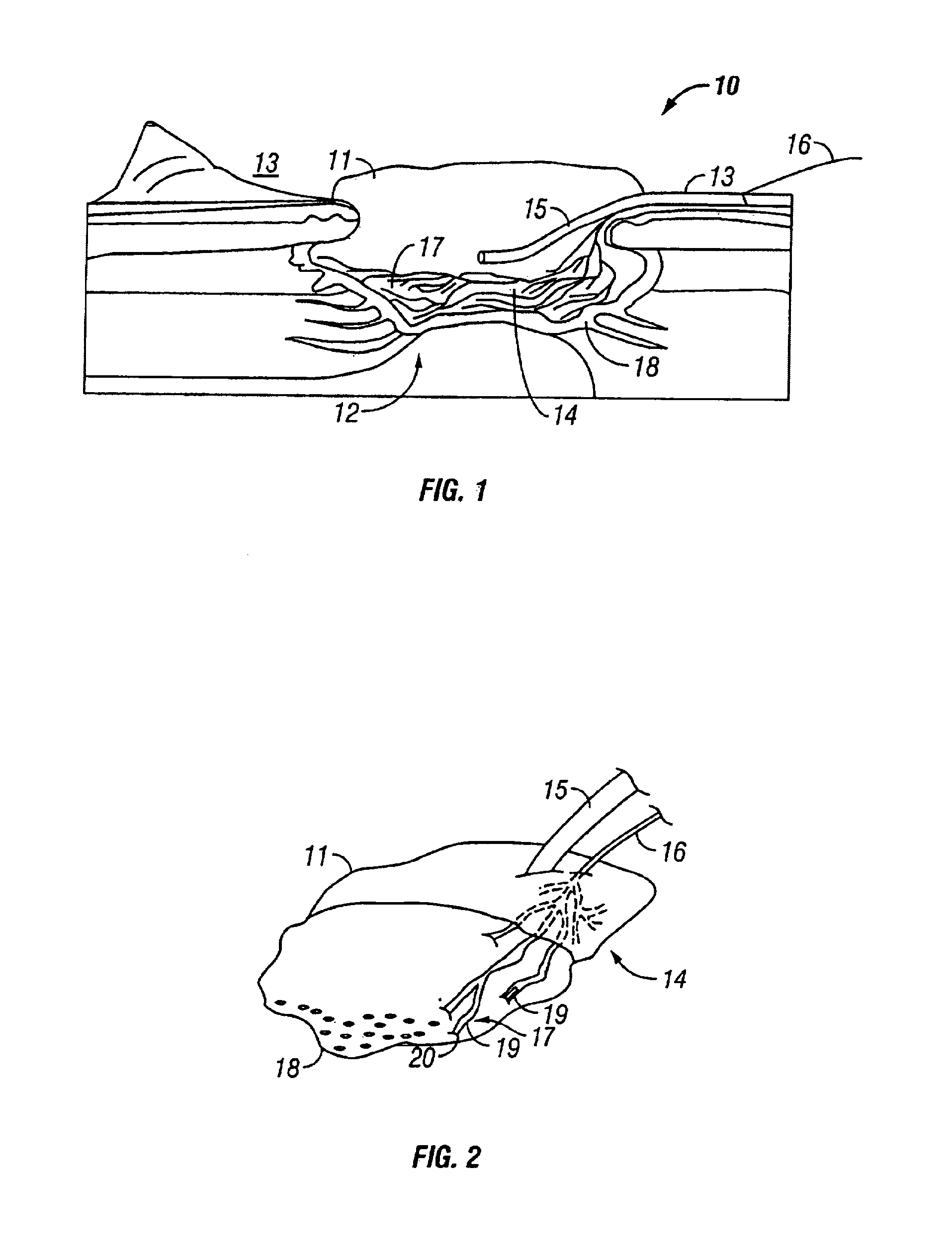
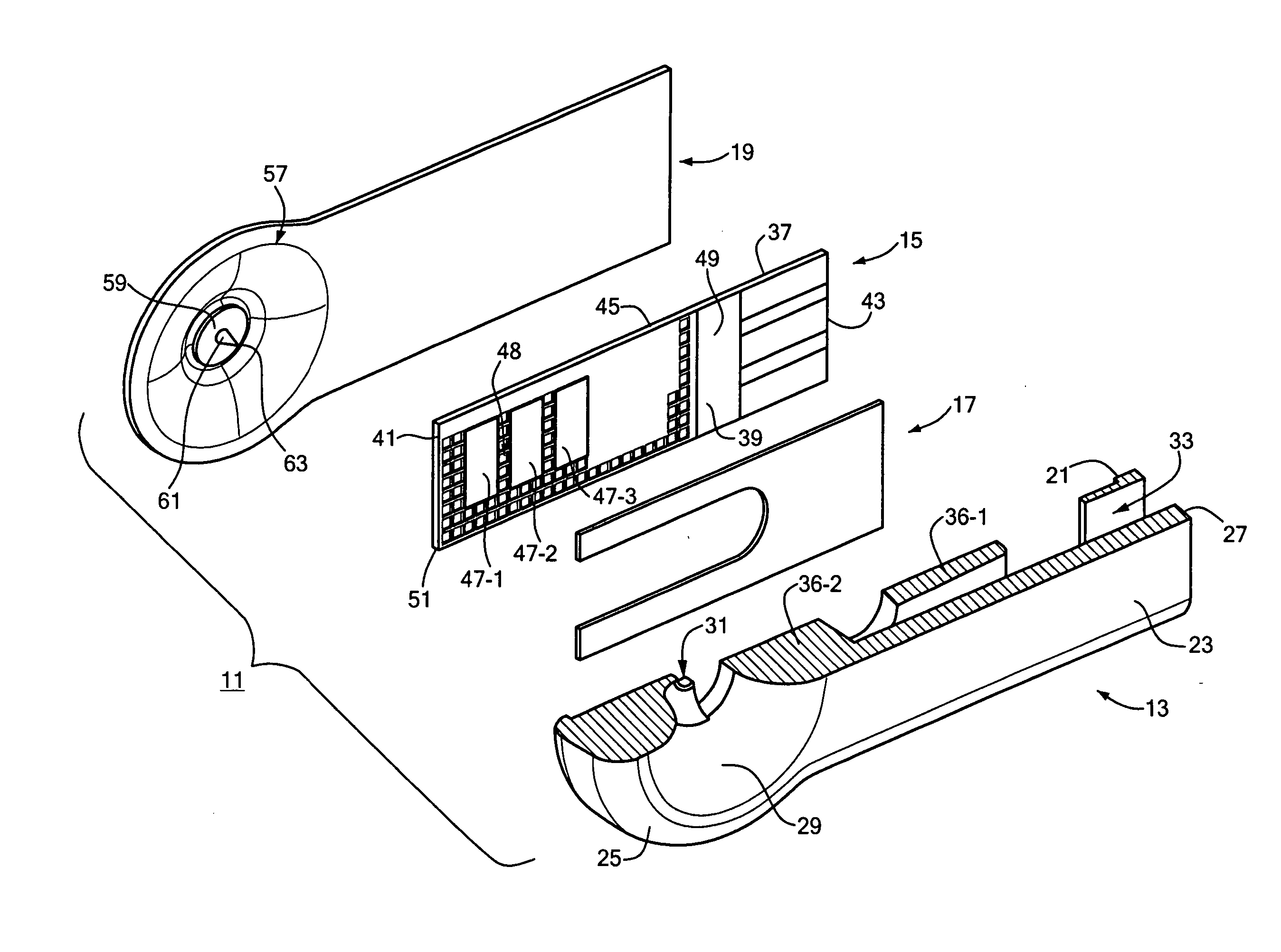
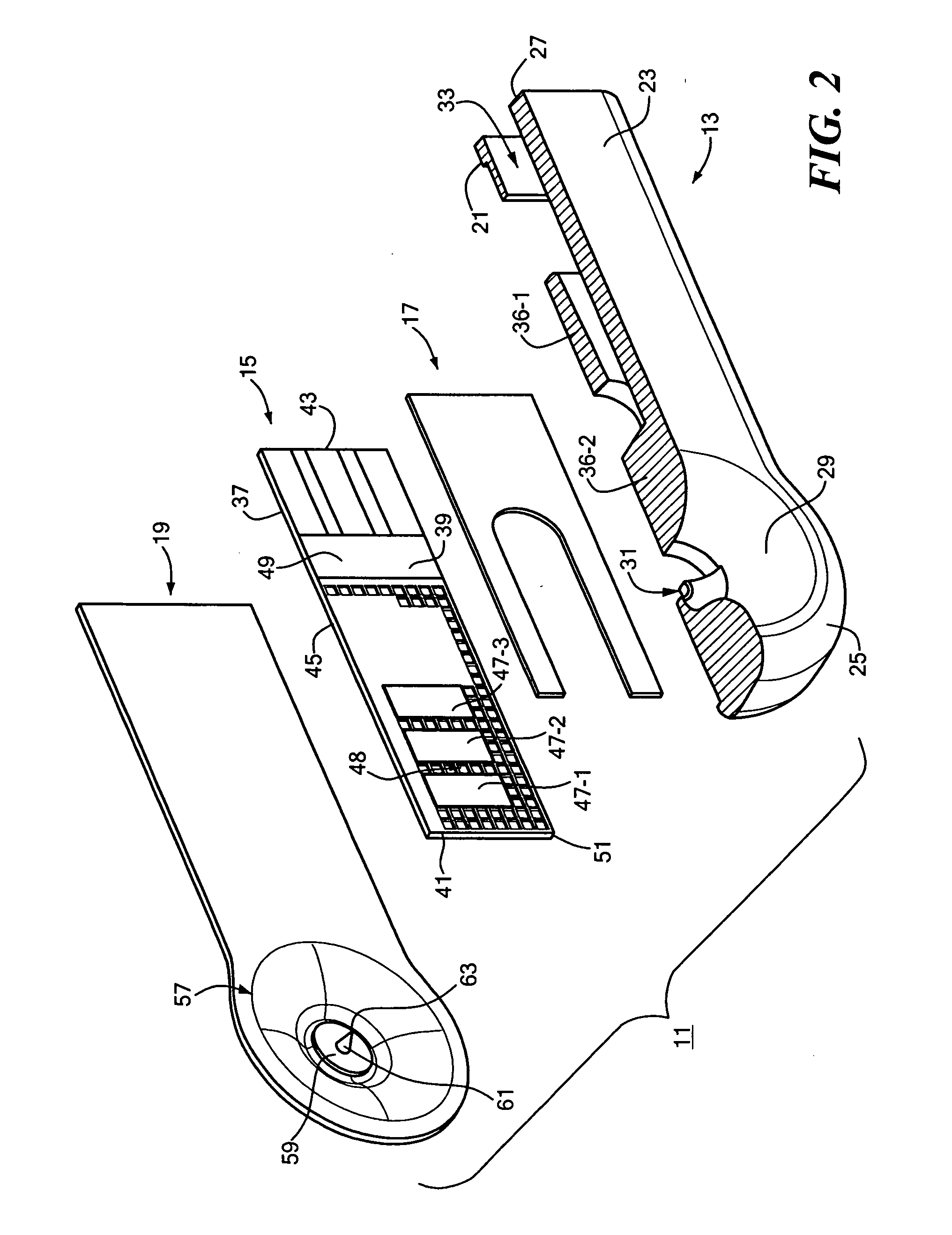
ActiveUS20050245844A1Easy to useMinimal discomfortImmobilised enzymesBioreactor/fermenter combinationsAnalyteWound site
An analyte test device is constructed as an integrated, single-use, disposable cartridge which can be releasably installed into a compatible analyte test monitor. In use, the device can be used in conjunction with the monitor to lance the skin of a patient to create a blood sample, express the blood sample from the wound site using vacuum forces and calculate the concentration of a particular analyte in the expressed blood sample. In one embodiment, the device includes a base which includes a top surface and a bottom surface. The base is also shaped to define an aperture which extends transversely through its top and bottom surfaces. An electrochemical test sensor is affixed to the base in such a manner so that a vacuum path is at least partially defined between the base and the test sensor, the vacuum path being in fluid communication with the aperture. A cover is affixed to the top surface of the base over the aperture, the cover comprising a flexible dome-shaped member and a lancet coupled to the member, the lancet being orientated such that its longitudinal axis extends at an approximate right angle relative to the longitudinal axis of the test sensor. The bottom surface of the base is shaped to include a skin receiving surface which at least partially defines the aperture in the base, the skin receiving surface having a steep inward contour to distend the skin of the patient when pressed thereagainst.
Owner:ABBOTT DIABETES CARE INC
Gradient wound treatment system and method
A wound therapy and tissue management system utilizes fluid differentiation. Fluid is differentiated by establishing a gradient within the system. A gradient can be established with matter or energy. Patient interfaces for establishing, maintaining and varying one or more gradients include transfer elements with first and second zones having different flow coefficients. The transfer elements exchange fluid with a patient, generally through a wound site, and with external components of the system. Osmotic solution gradients are controlled by a methodology involving the present invention for extracting solutions, which can include toxins, from patients and for introducing fluids and sumping air to wound sites.
Owner:KCI LICENSING INC
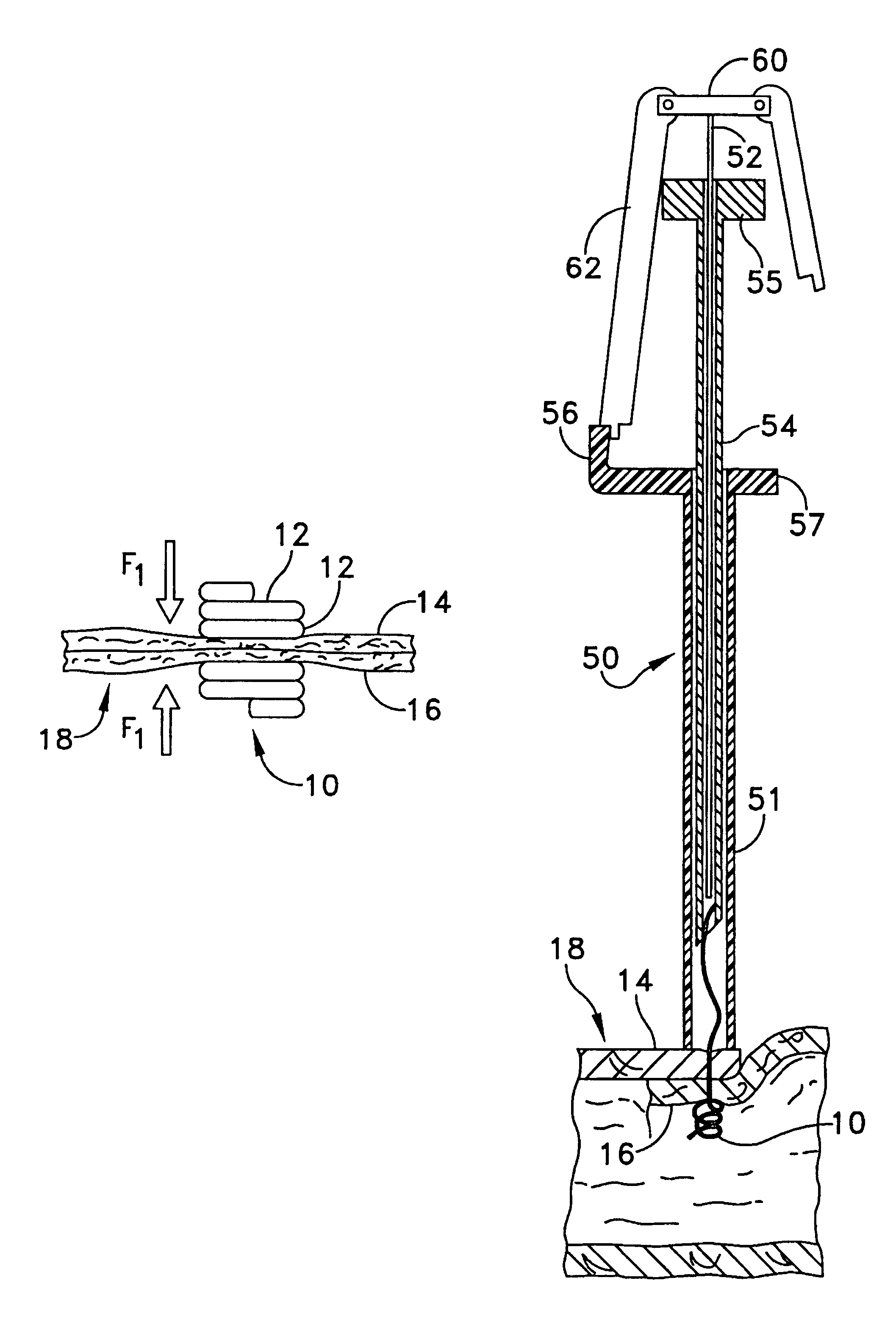
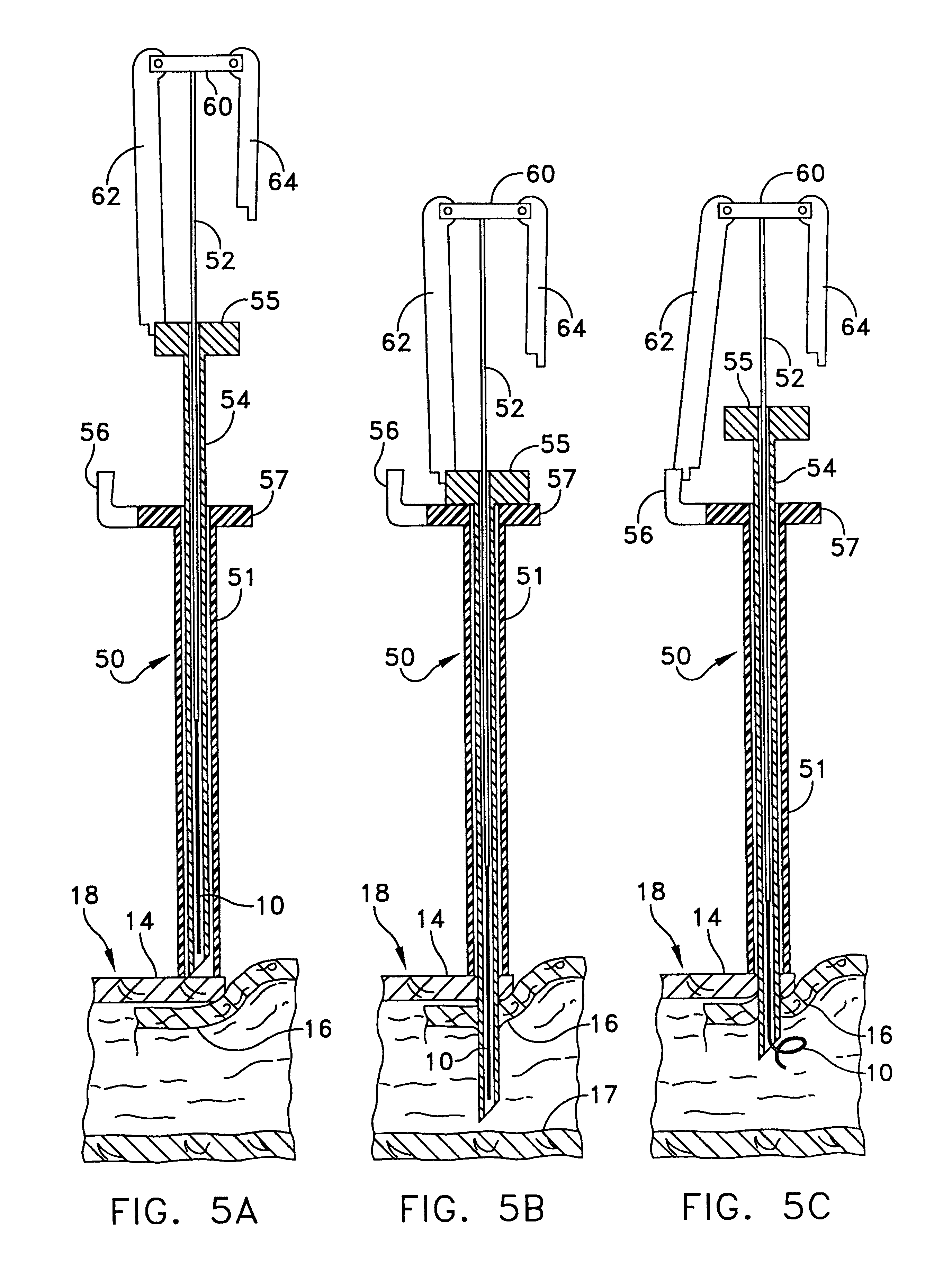
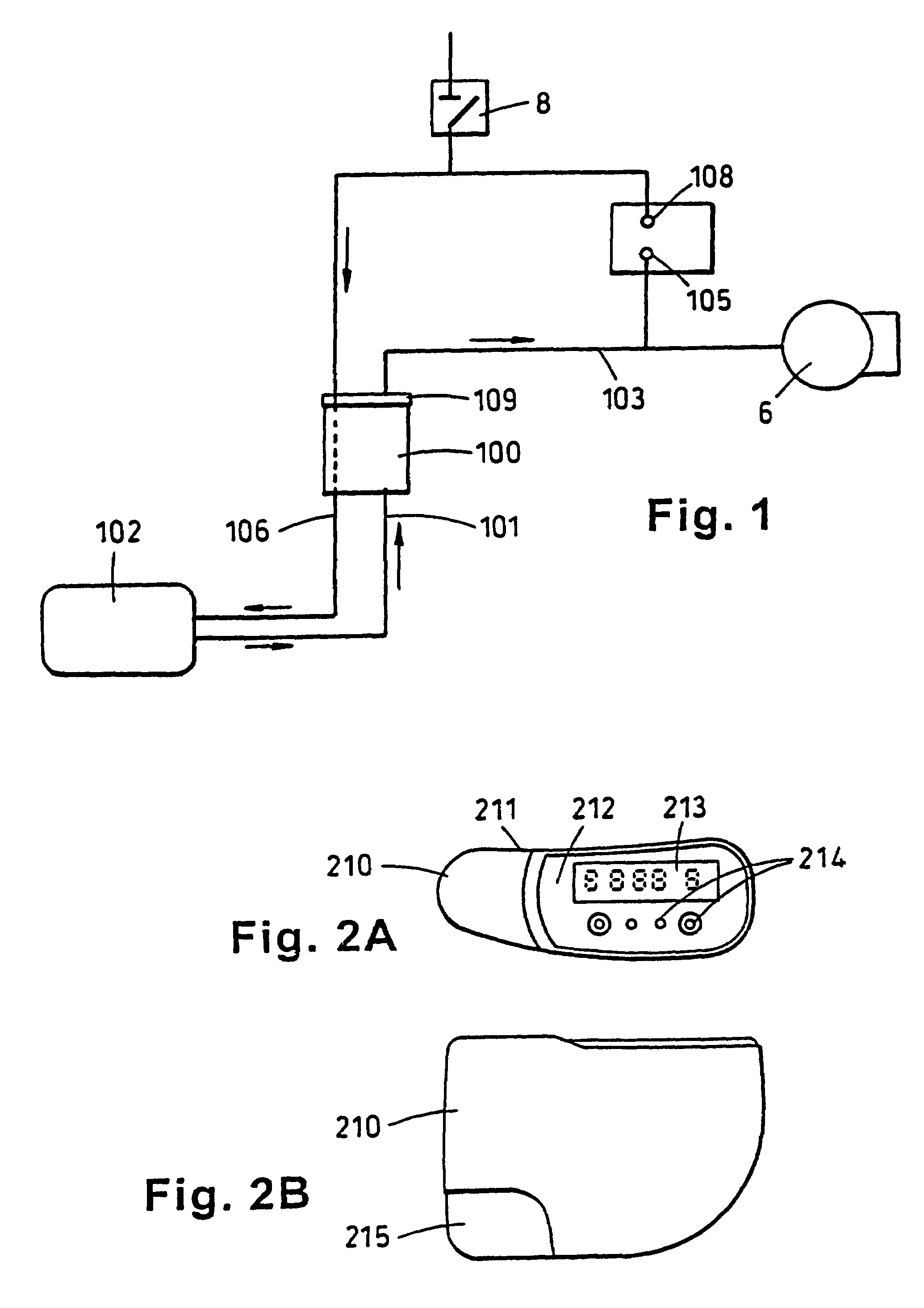
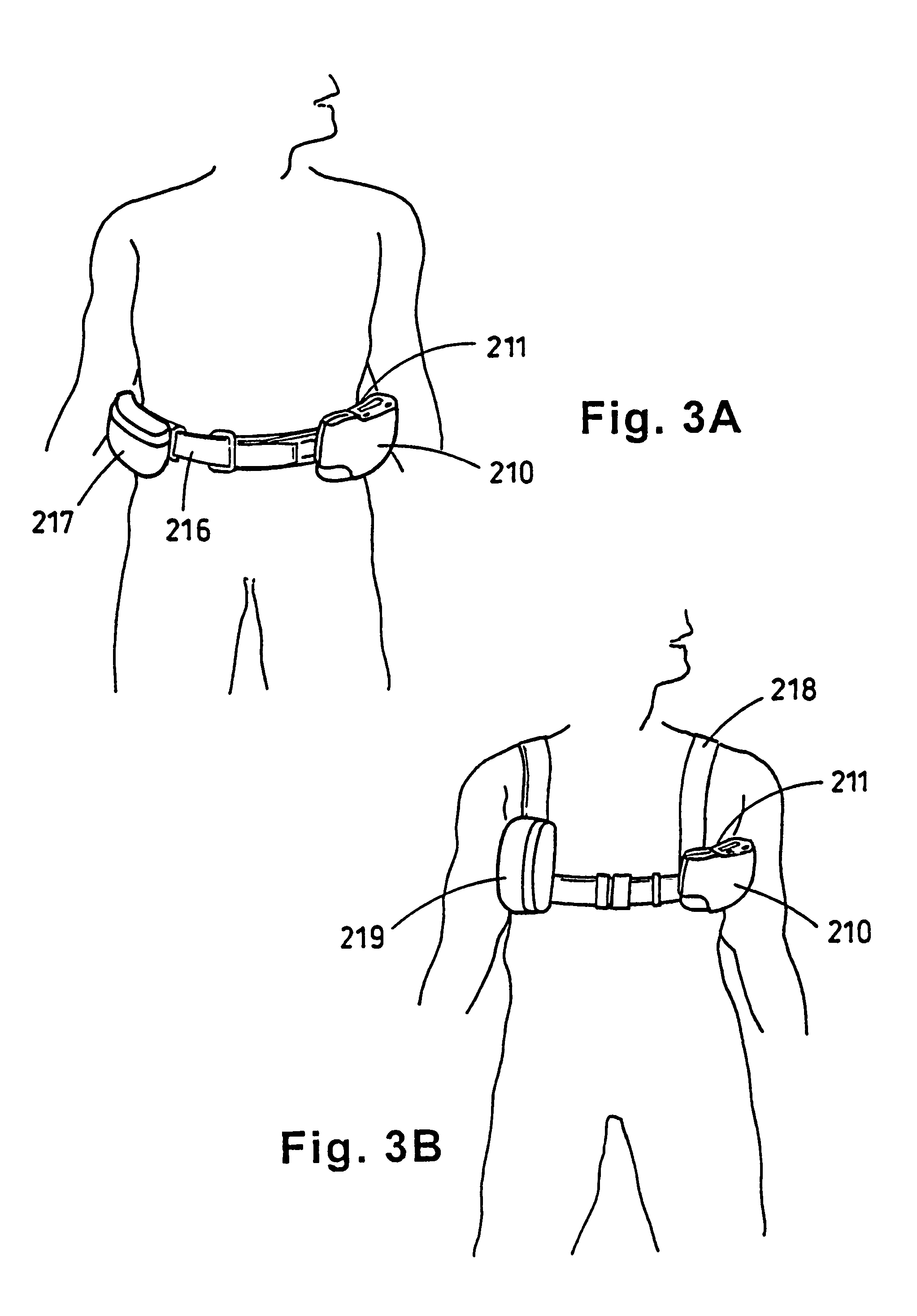
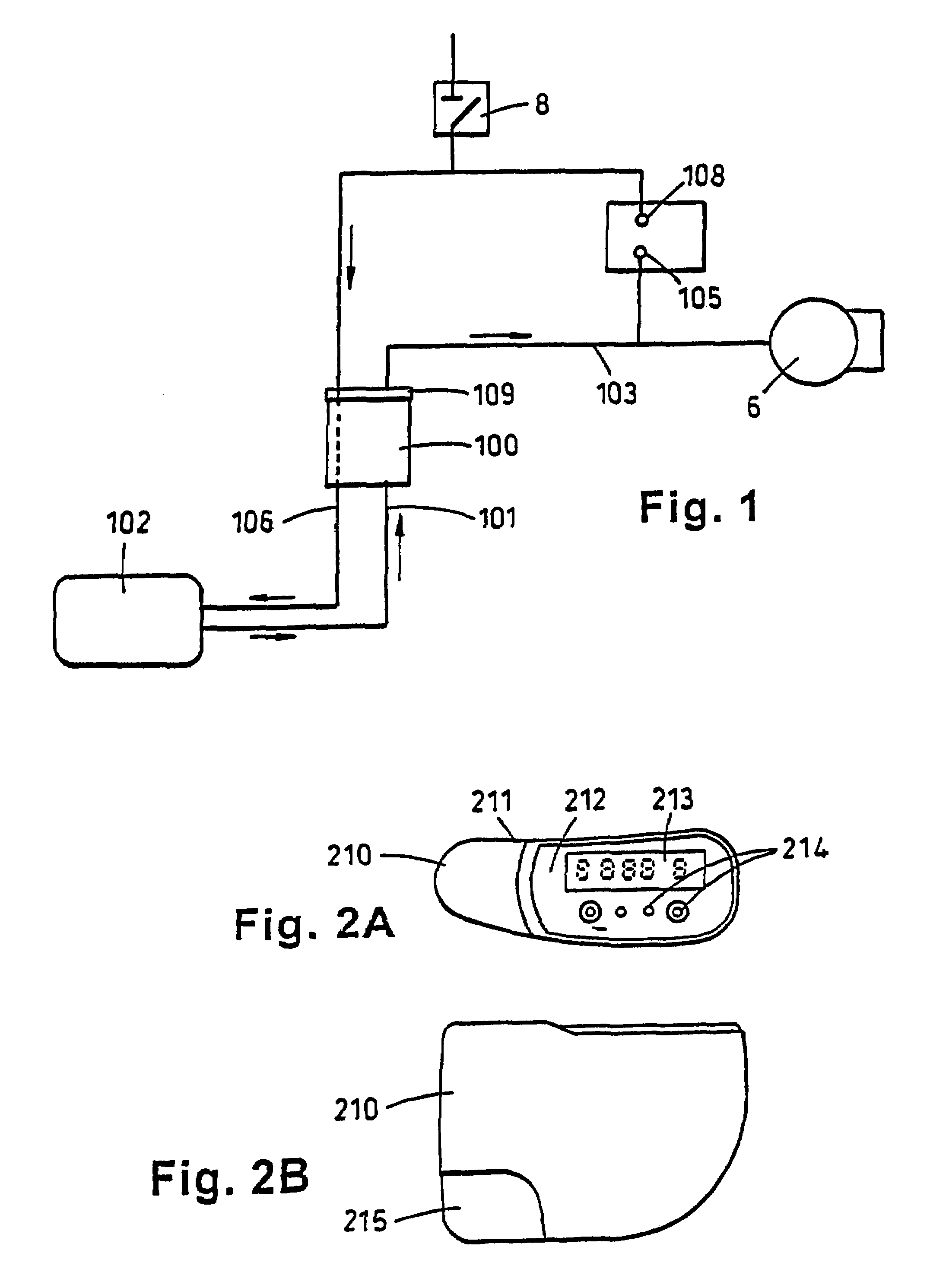
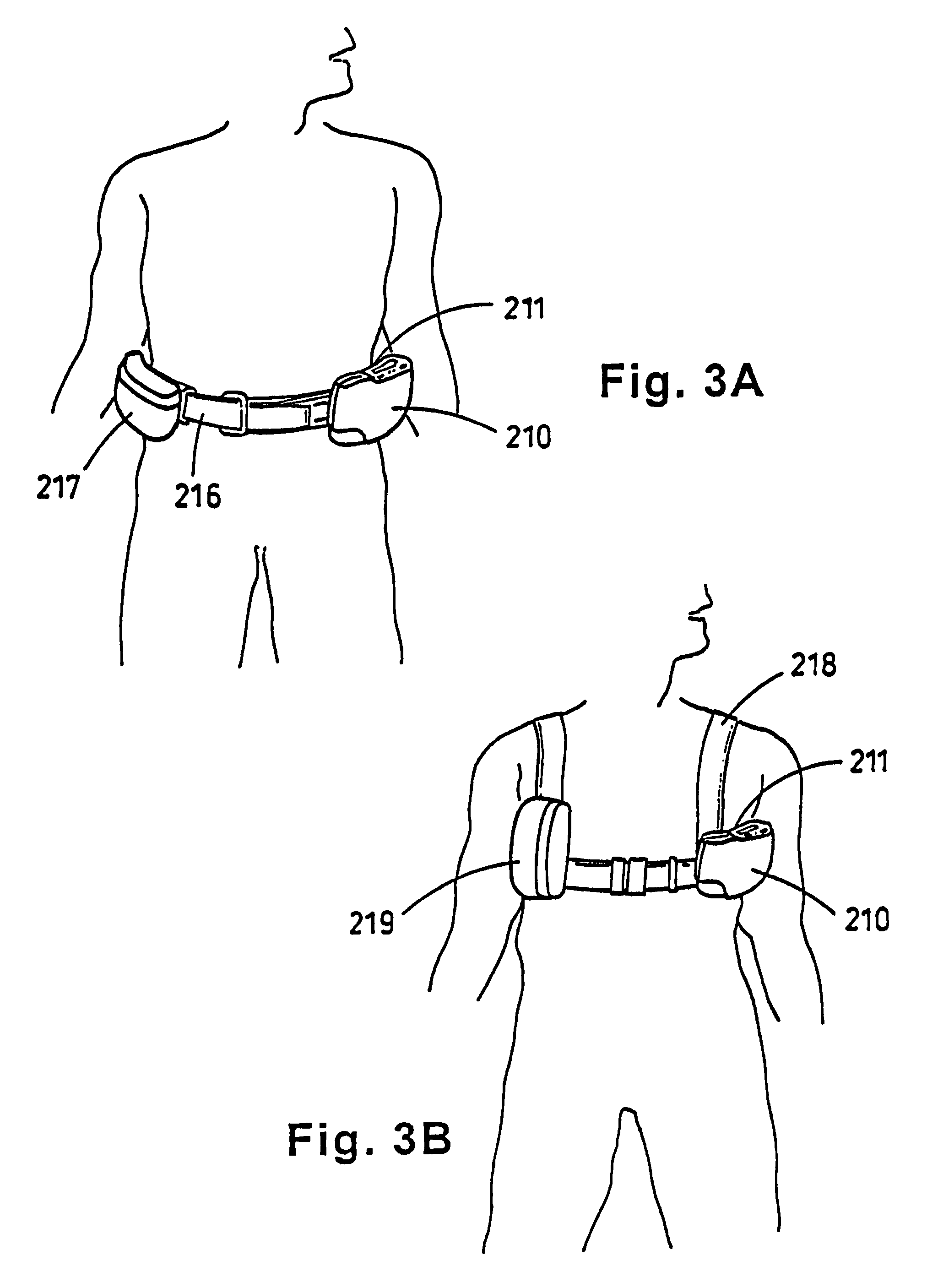
Portable wound treatment apparatus
A portable wound treatment apparatus, for stimulating the healing of superficial wounds, comprises a housing containing a suction pump and a canister for containing fluids drawn from the wound. The housing is supported on a harness or belt worn by the patient. The canister is connected to a porous wound dressing at the wound site via a plurality of tubes, a multi-lumen tube or a combination thereof. A rechargeable battery pack may be incorporated within the housing or externally thereto. The external battery pack may be shaped to balance the housing on the harness or belt. Pressure transducers are provided to monitor and report pressures at the wound site or internal to the canister. Monitored pressures may also be utilized to determine the filled state of the canister and, thereafter, either report this state to the operator or automatically discontinue suction from the wound, or both.
Owner:KCI LICENSING INC
Gradient wound treatment system and method
A wound therapy and tissue management system utilizes fluid differentiation. Fluid is differentiated by establishing a gradient within the system. A gradient can be established with matter or energy. Patient interfaces for establishing, maintaining and varying one or more gradients include transfer elements with first and second zones having different flow coefficients. The transfer elements exchange fluid with a patient, generally through a wound site, and with external components of the system. Osmotic solution gradients are controlled by a methodology involving the present invention for extracting solutions, which can include toxins, from patients and for introducing fluids and sumping air to wound sites.
Owner:KCI LICENSING INC
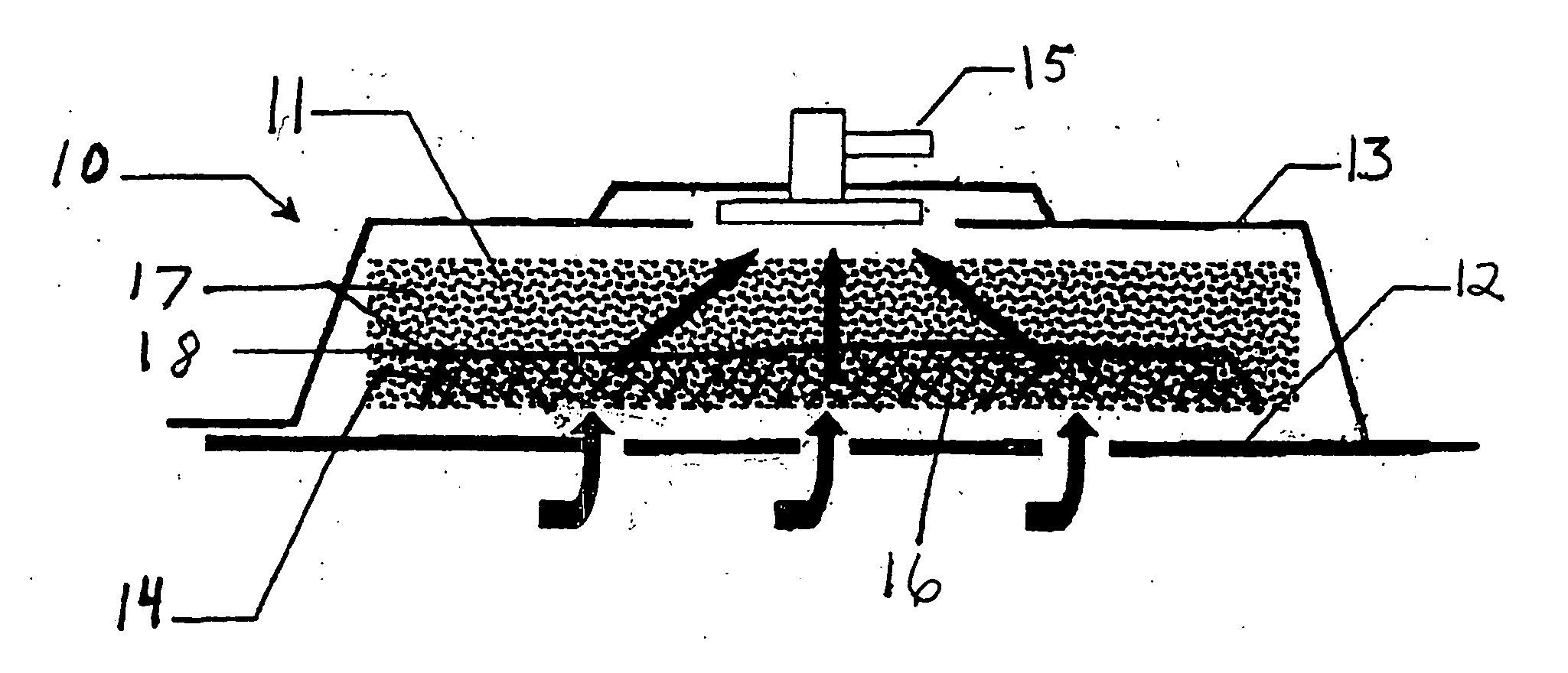
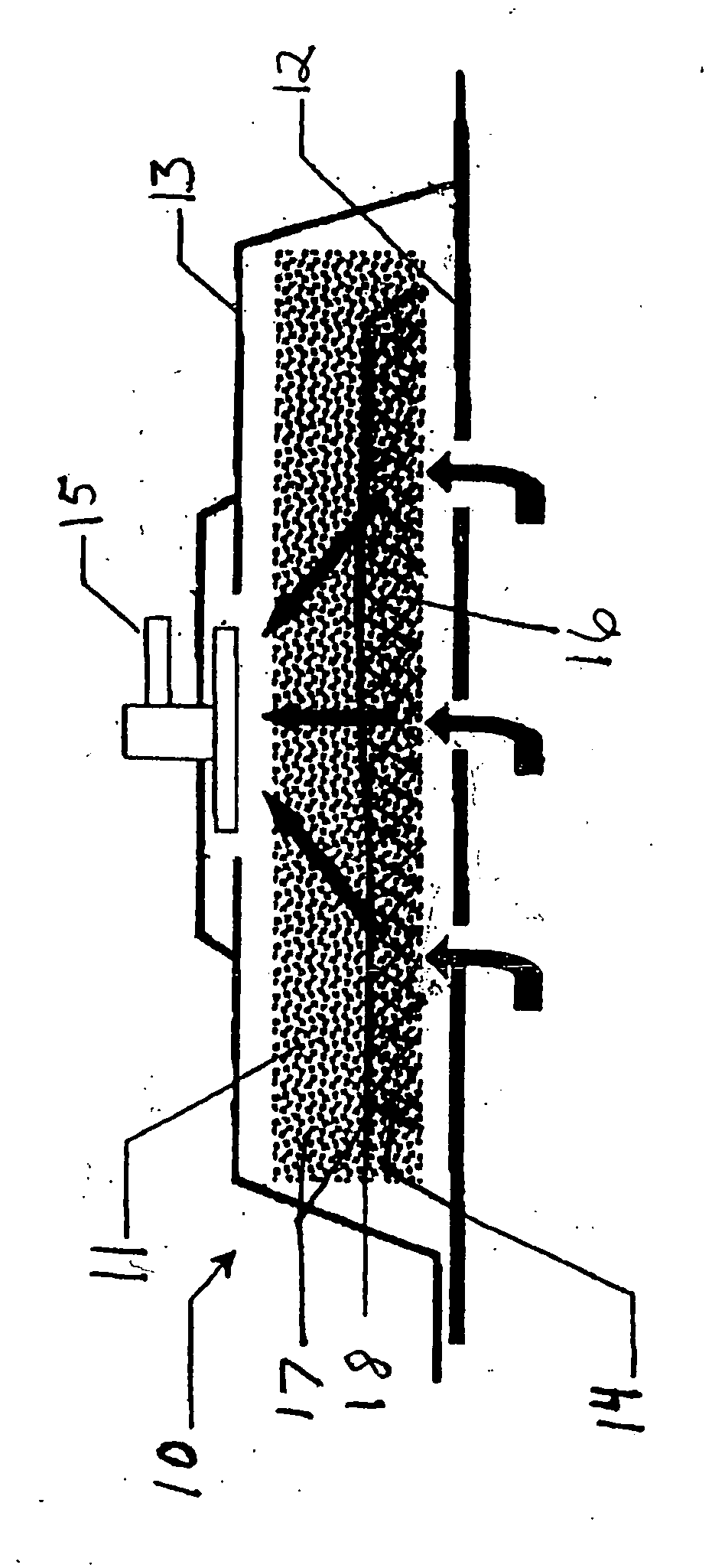
Negative pressure treatment system with heating and cooling provision
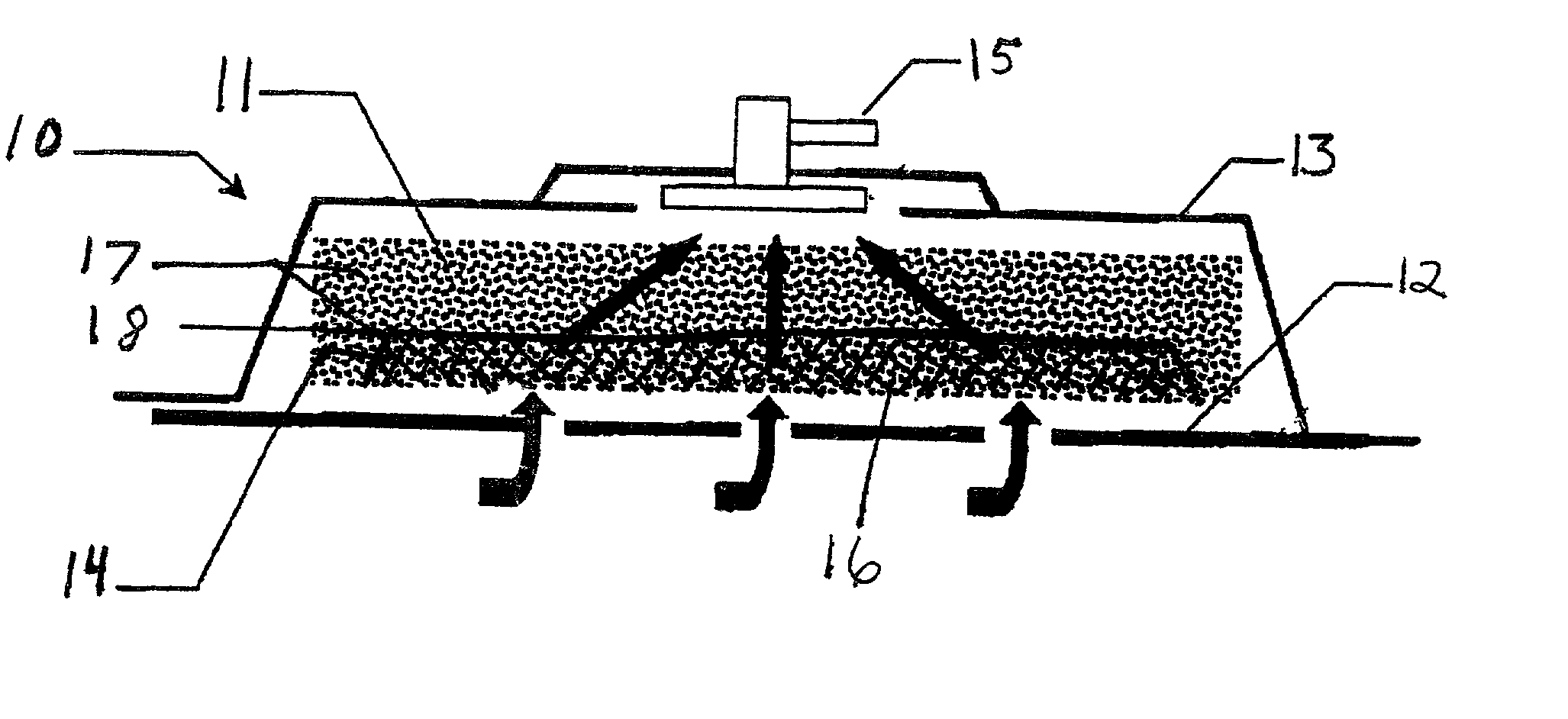
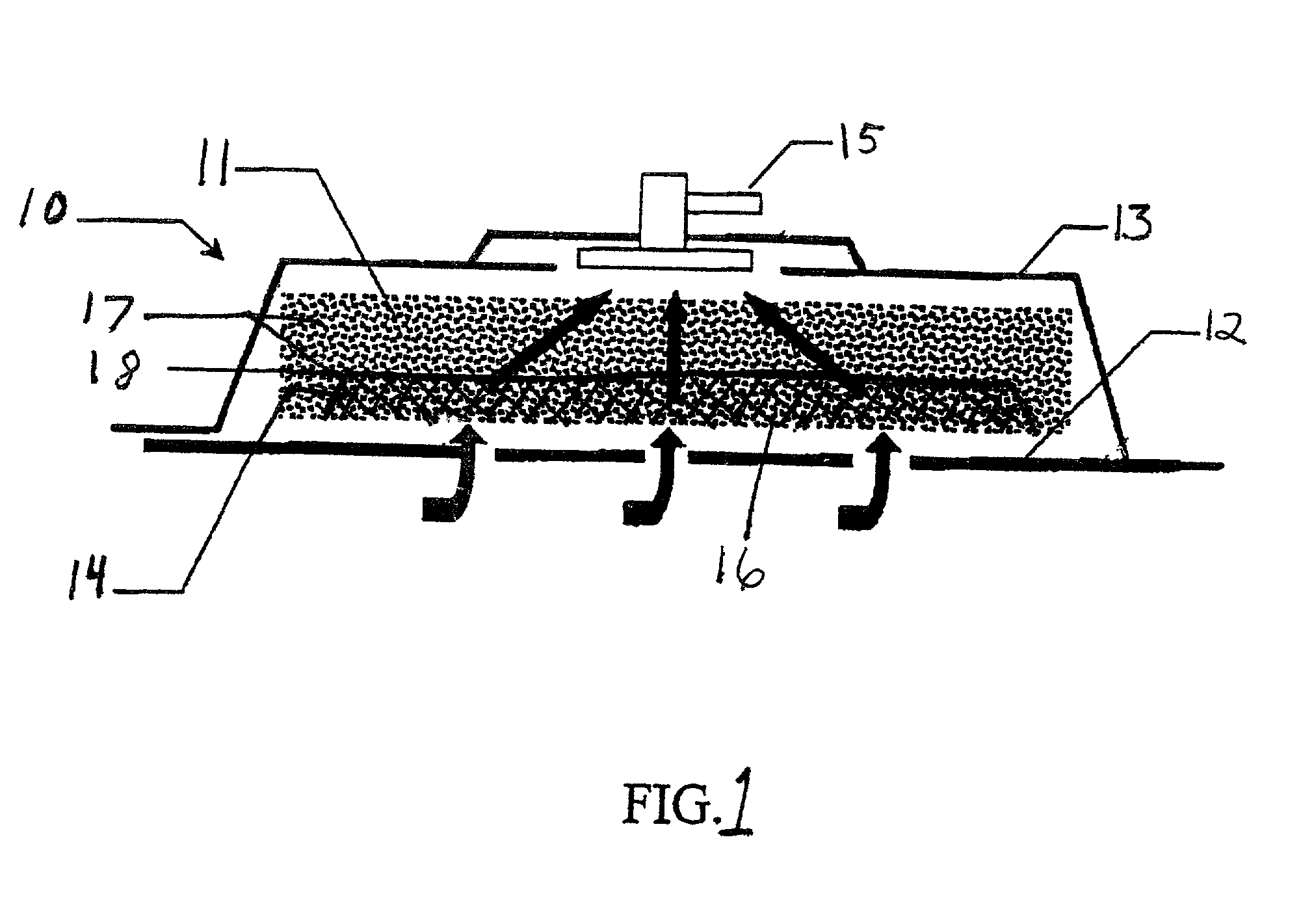
InactiveUS7144390B1Prevent vacuum leakageReduce inflammationSurgical needlesPlastersWound siteSurgery
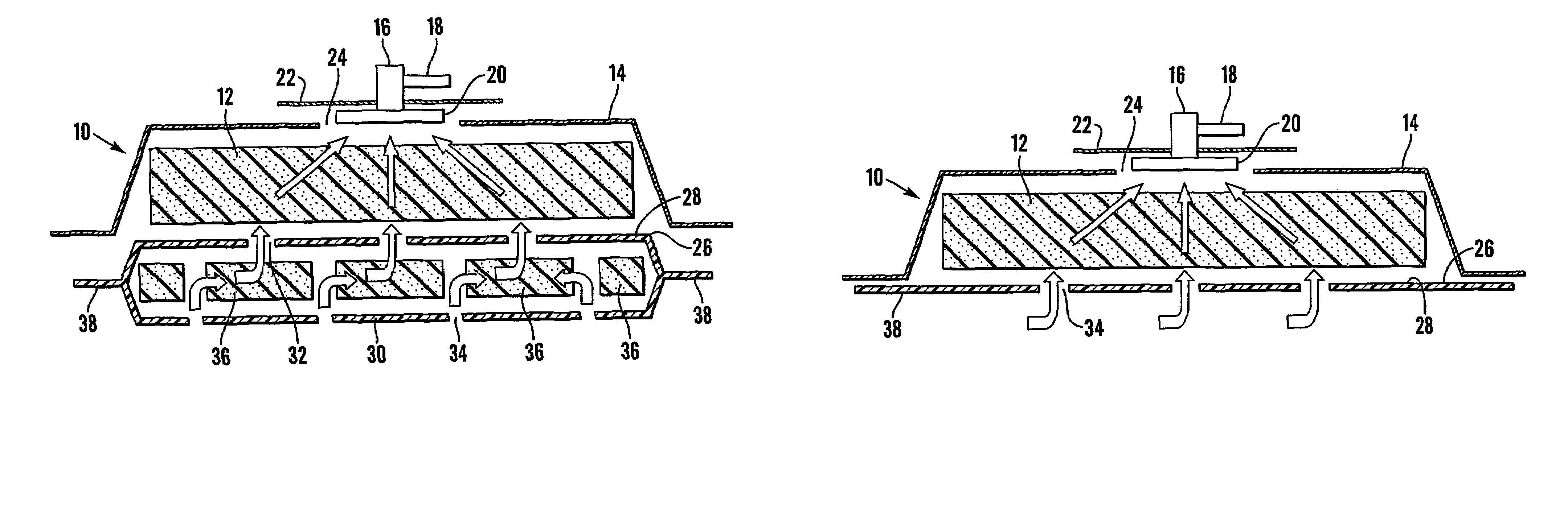
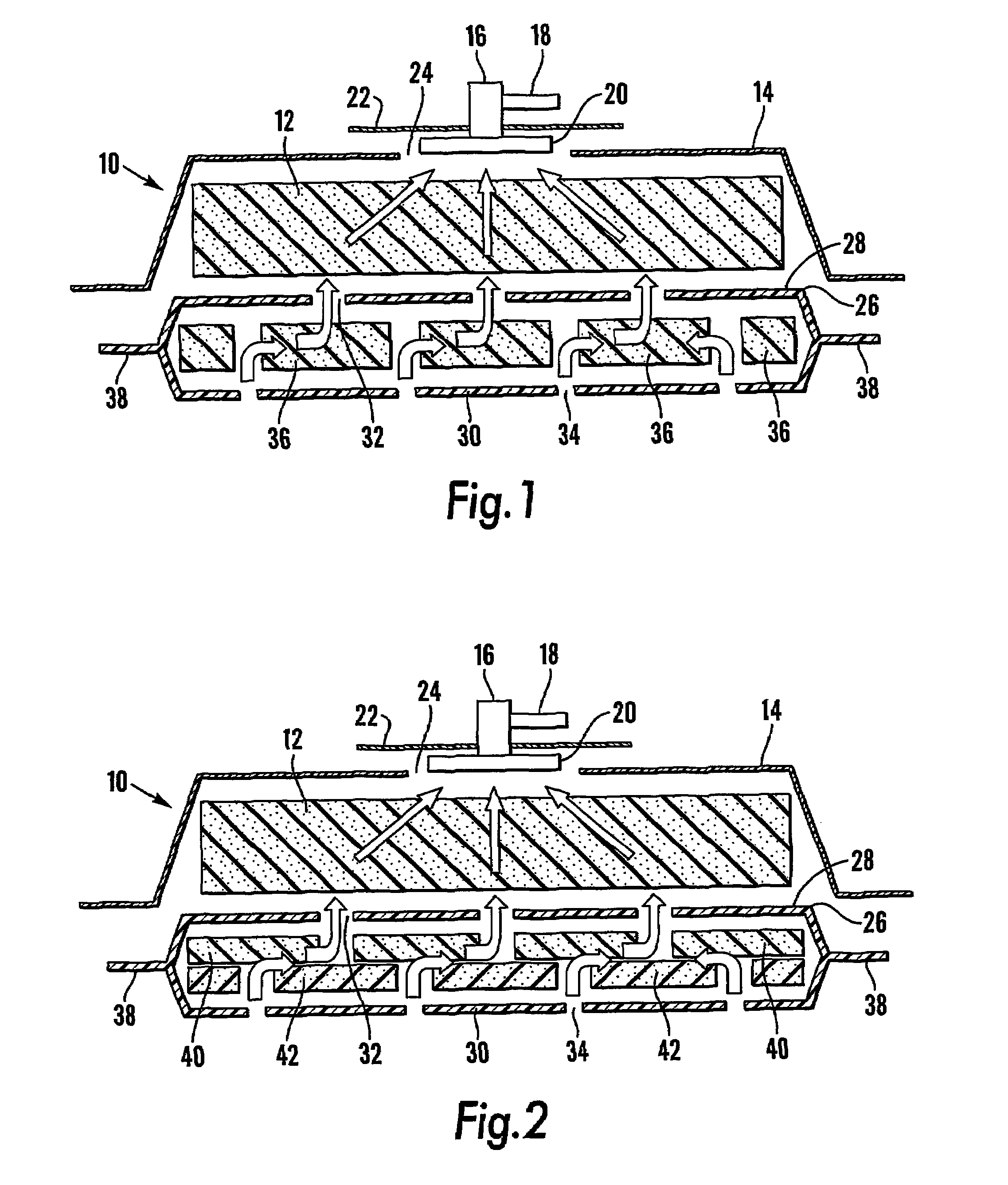
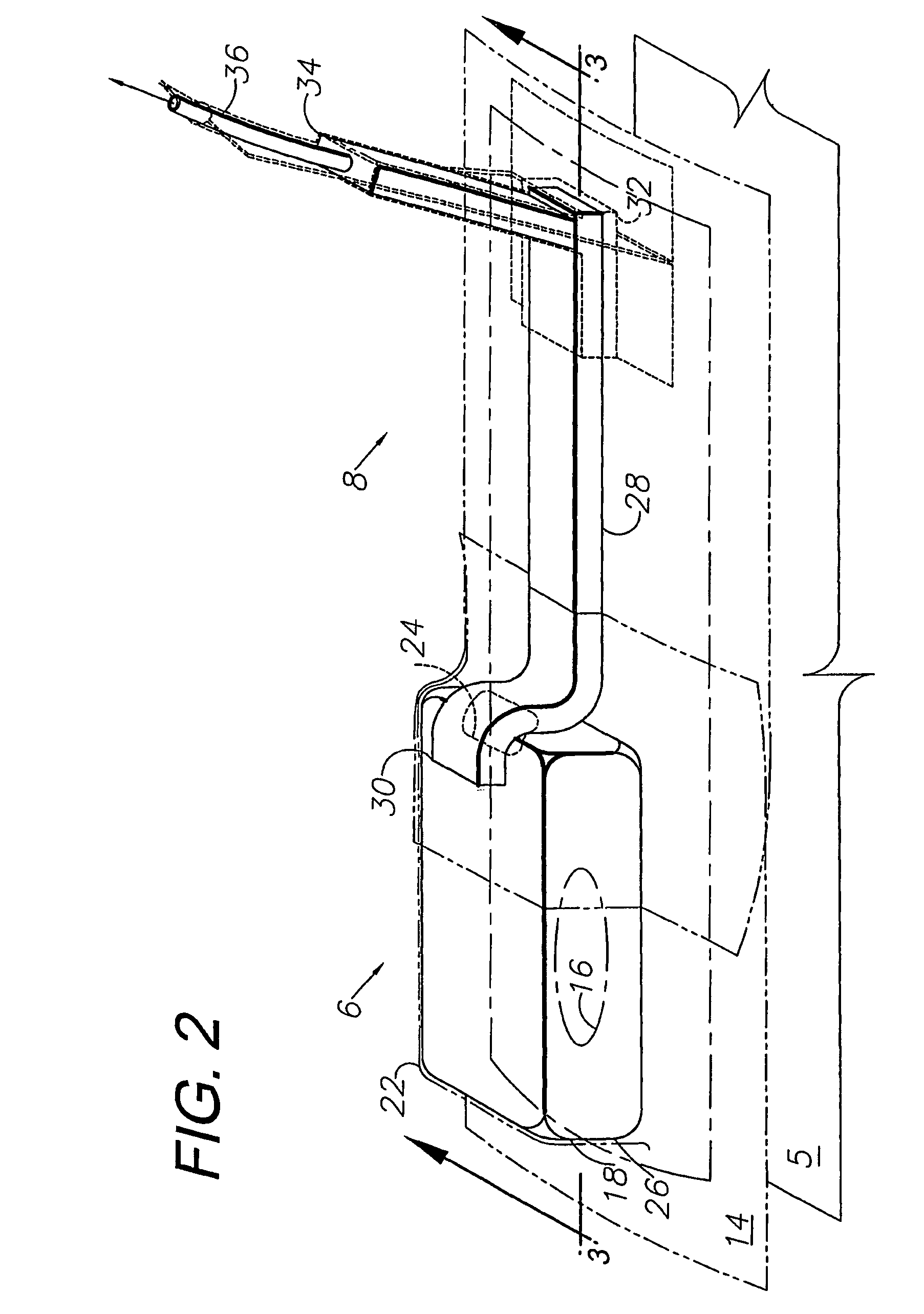
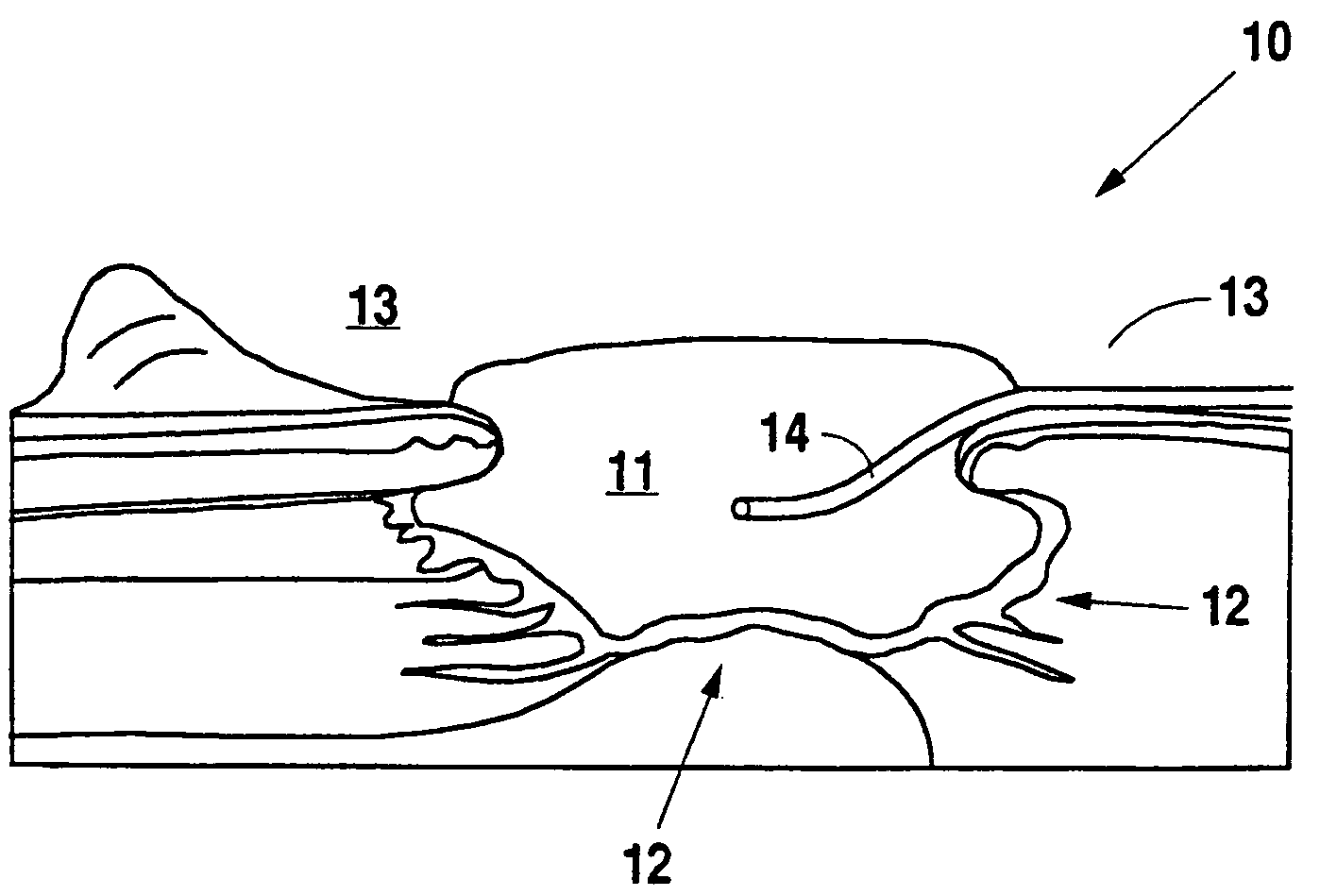
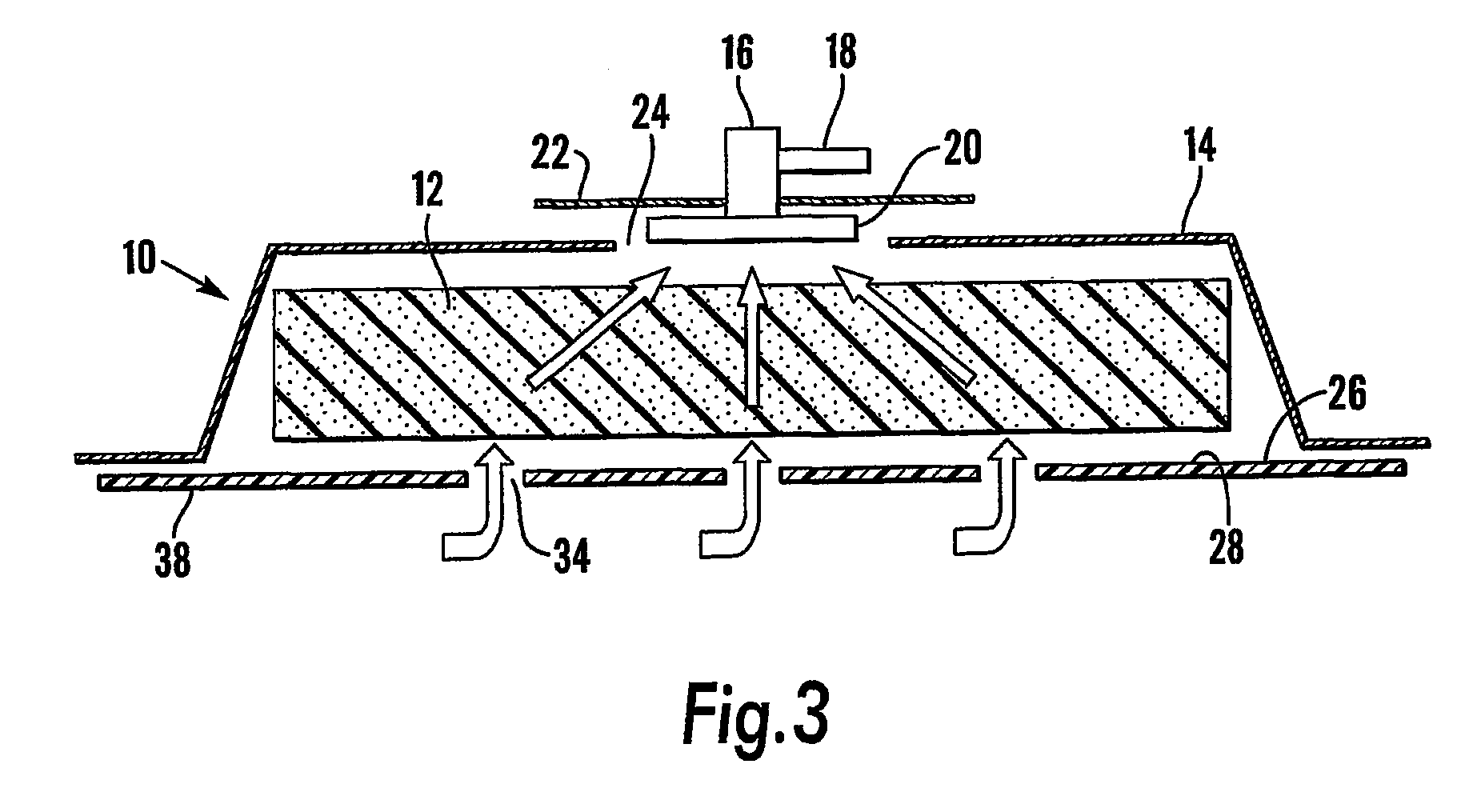
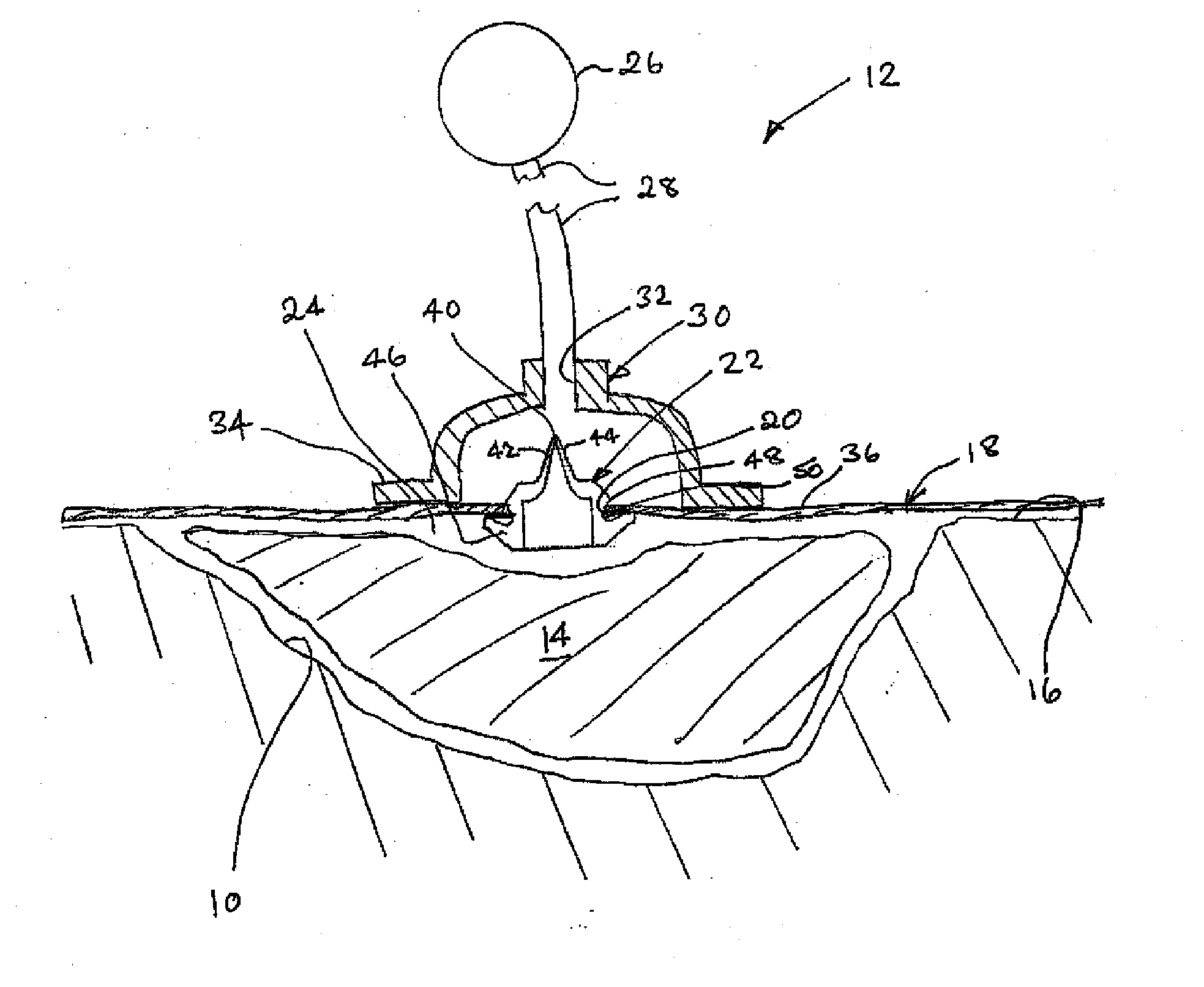
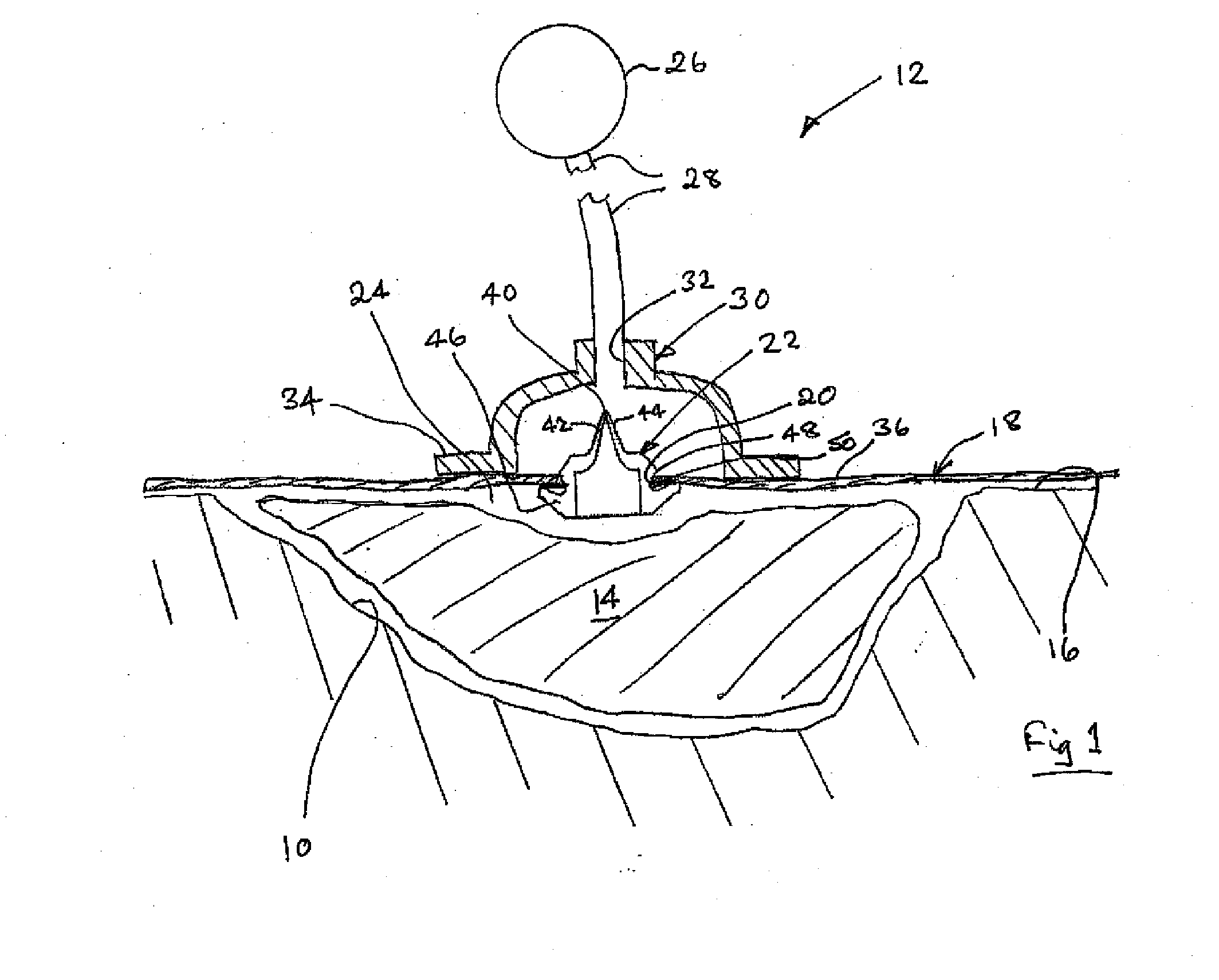
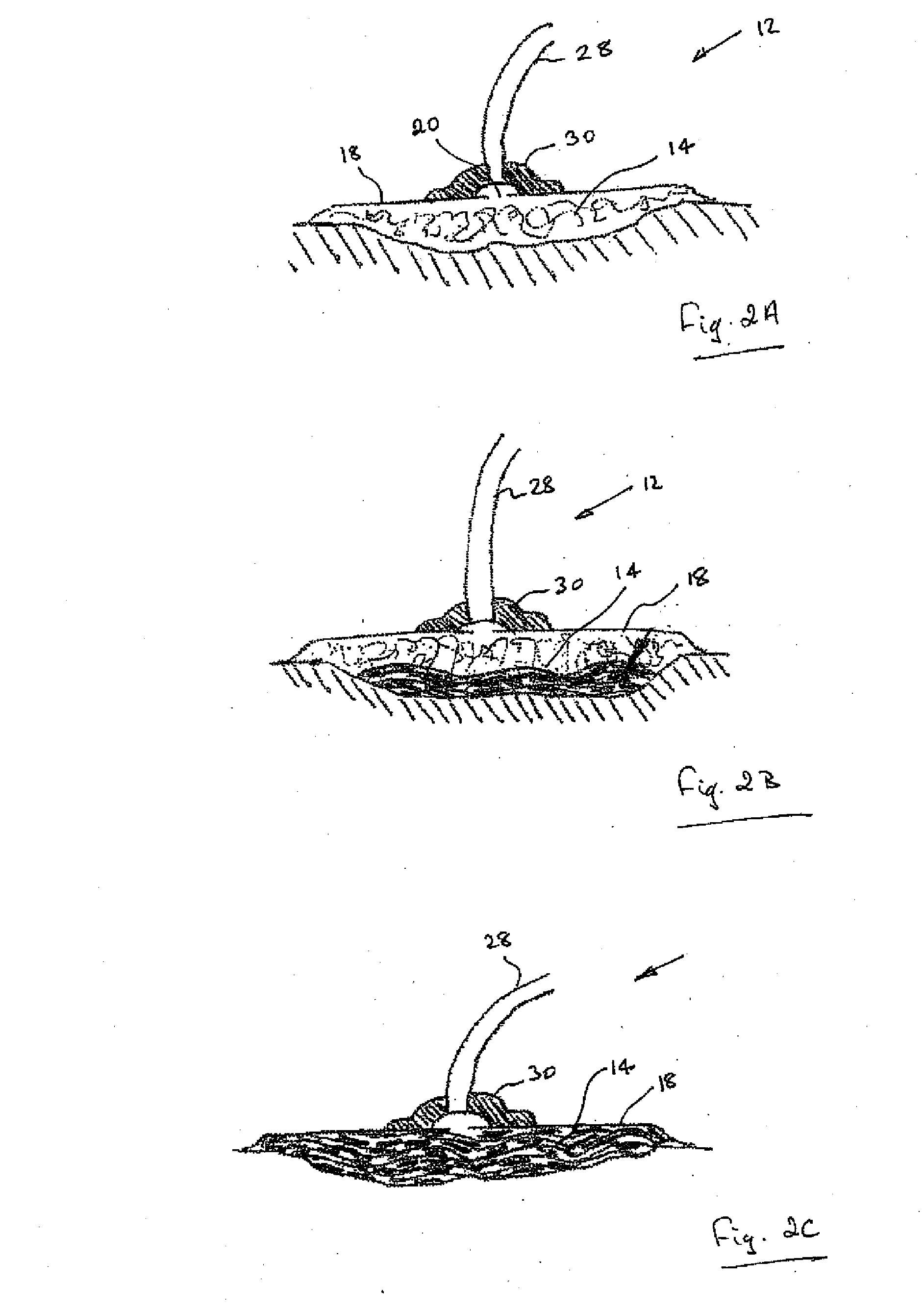
A method, and apparatus (10) for the controlled acceleration, and / or retardation of the body's inflammatory response generally comprises a foam pad (11) for insertion substantially into a wound site, a heating, a cooling pad (13) for application over the wound site (12), a wound drape (14) or sealing enclosure of the foam pad (11), the heating, and cooling pad (13) at wound site (12). The foam pad (11) is placed in fluid communication with a vacuum source for promotion of the controlled acceleration or retardation of the body's inflammatory response. The heating, and cooling provision controls the local metabolic function as part of the inflammatory response.
Owner:KCI LICENSING INC
Negative pressure wound therapy system with provision for introduction of an agent
InactiveUS7534240B1Promote wound healingRapid reepithelializationPeptide/protein ingredientsSurgical needlesWound siteGrafting
A method and apparatus for the introduction to a wound under negative pressure therapy of a wound healing agent, generally comprises a foam pad (11) for insertion substantially into a wound site (12), and a wound drape (13) for sealing enclosure of the foam pad at the wound site. The foam pad is placed in fluid communication with a vacuum source for promotion of fluid drainage. Additionally, the foam pad is predisposed, through grafting or other techniques known to those of ordinary skill in the art, with basic fibroblast growth factor (bFGF), anti-microbial or other factors, also known to those of ordinary skill in the art, for the promotion of increased wound healing.
Owner:KCI LICENSING INC
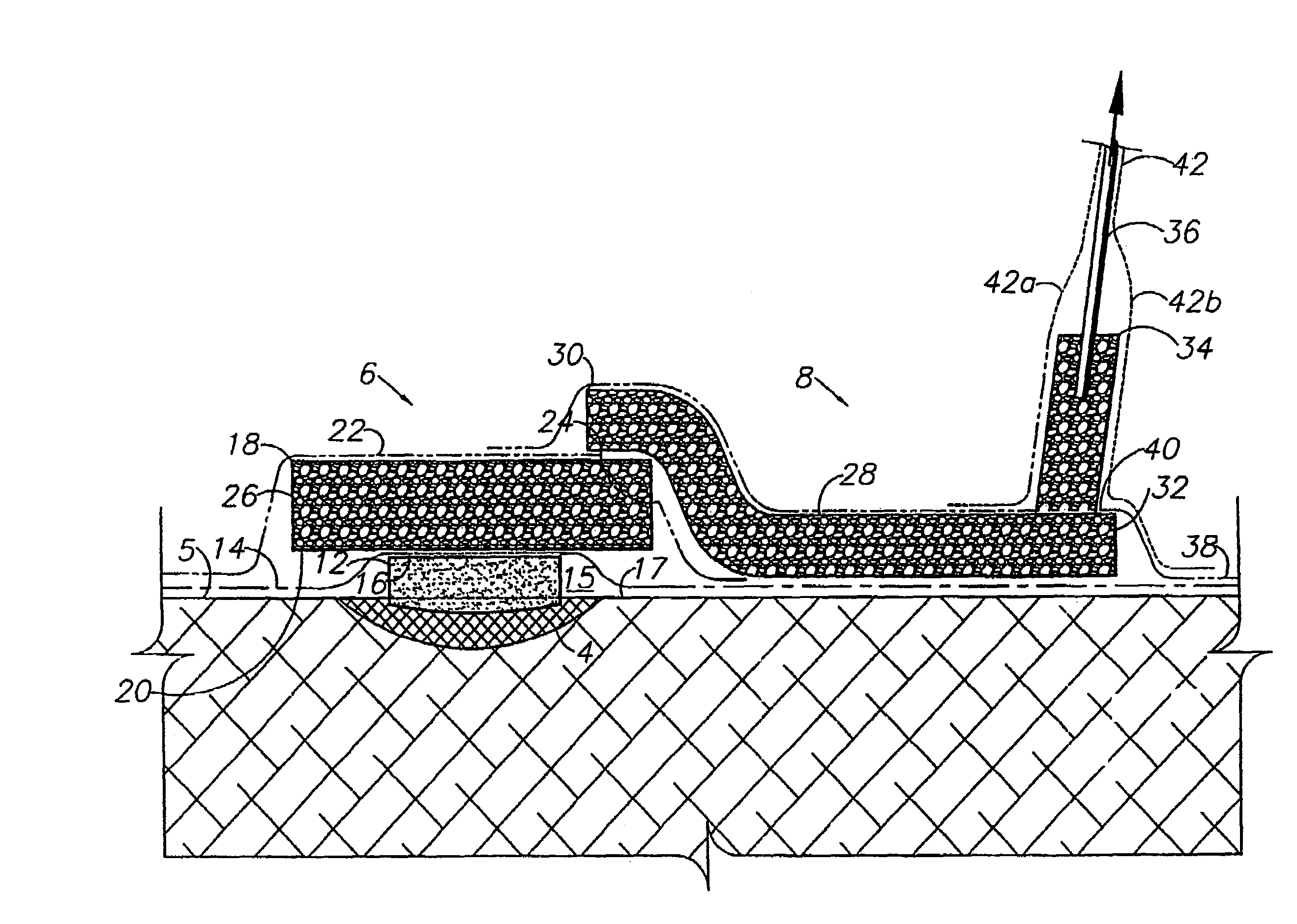
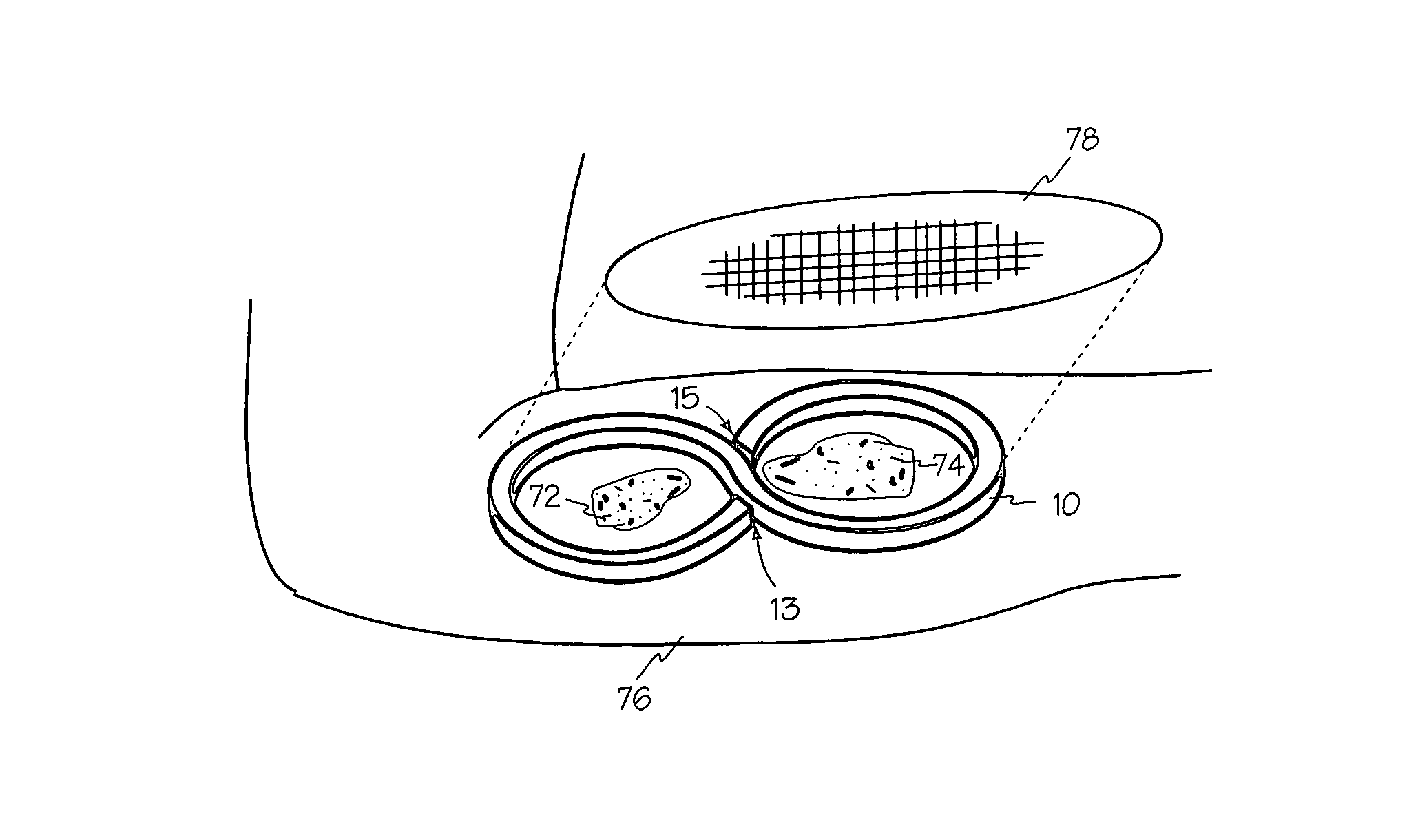
Negative pressure wound treatment dressing
A wound treatment dressing for applying negative pressure wound therapy to a wound of an extremity, such as on the heel of a foot. The dressing comprises a fluid manifold 14 positioned within a fenestrated drape 16 to form a contoured occlusive wrapping 10 for placement over a wound on an extremity. A contoured porous pad 26 is placed adjacent or within the wound, such that the contoured pad 26 is enveloped by the occlusive wrapping 10. Flexible tubing 42 is attached to or through a fluid communication port 18, which forms an opening to the outer layer 16b of the occlusive wrapping 10, so as to allow for fluid communication of negative pressure to the contoured pad 26 from a source of negative pressure 44 connected to an opposite end of the flexible tubing 42. The negative gauge pressure is communicated from the source 44, through the tube 42, through the fenestrations 19 of the occlusive wrapping 10, such that negative gauge pressure is applied to the wound on the extremity. The fluid manifold 14 serves to direct the negative pressure from a position away from the wound site to the contoured pad 26 at the wound site.
Owner:KCI LICENSING INC
Device for use in surgery
In one embodiment, a wound retractor includes a plurality of straps connected between an inner ring and an outer ring assembly. The inner ring is positioned internal of the wound so that it rests against the innermost tissue layer, and the outer ring is located outside of the wound adjacent the outermost tissue layer. The outer ring assembly includes an inner ring having guide holes for the passage of the straps and an outer ring to which the straps are affixed. Rotation of the outer ring of the outer ring assembly relative to the inner ring of the outer ring assembly results in a shorting of the effective length of the straps, retraction of the wound edge and exposure of the wound site.
Owner:ATROPOS LTD
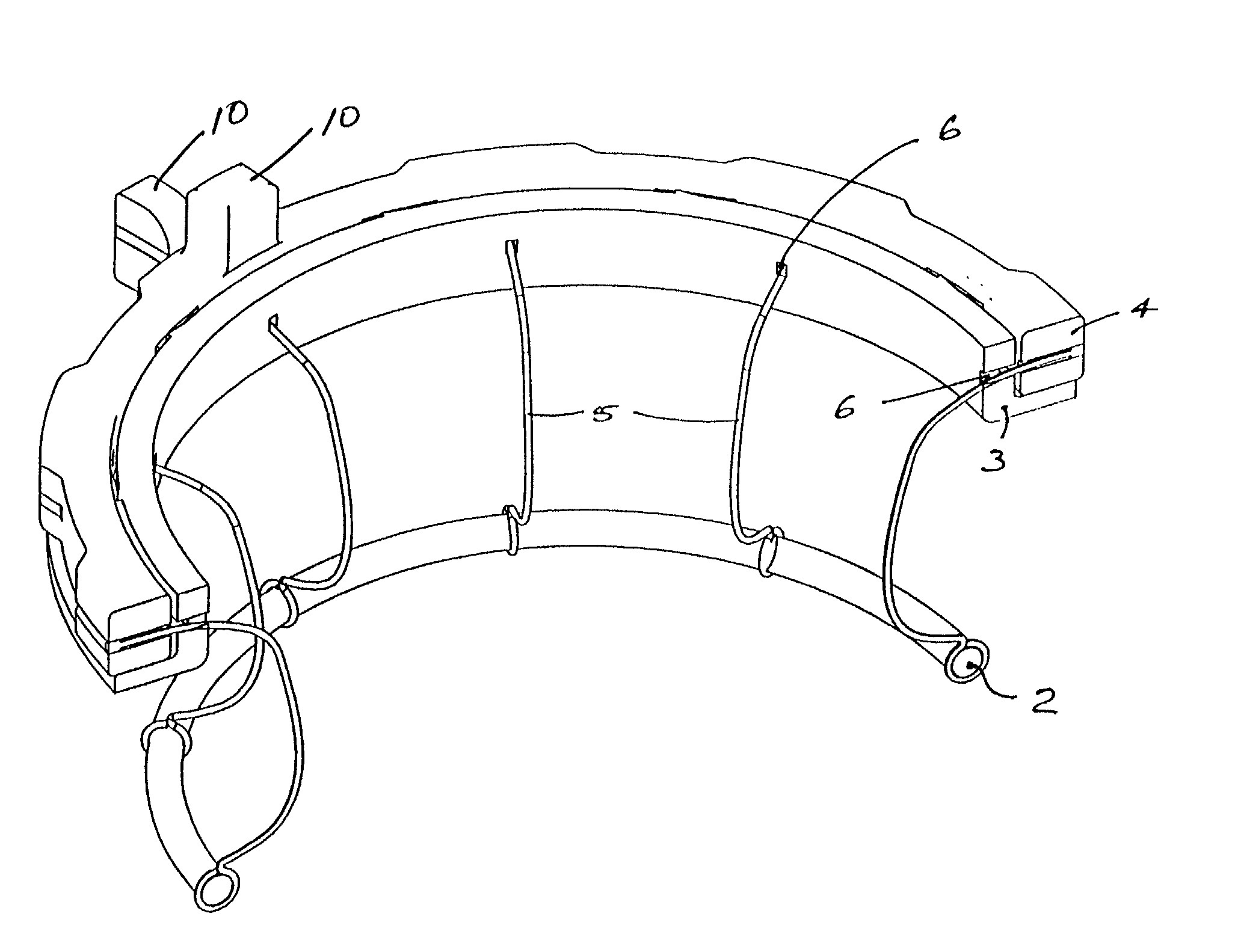
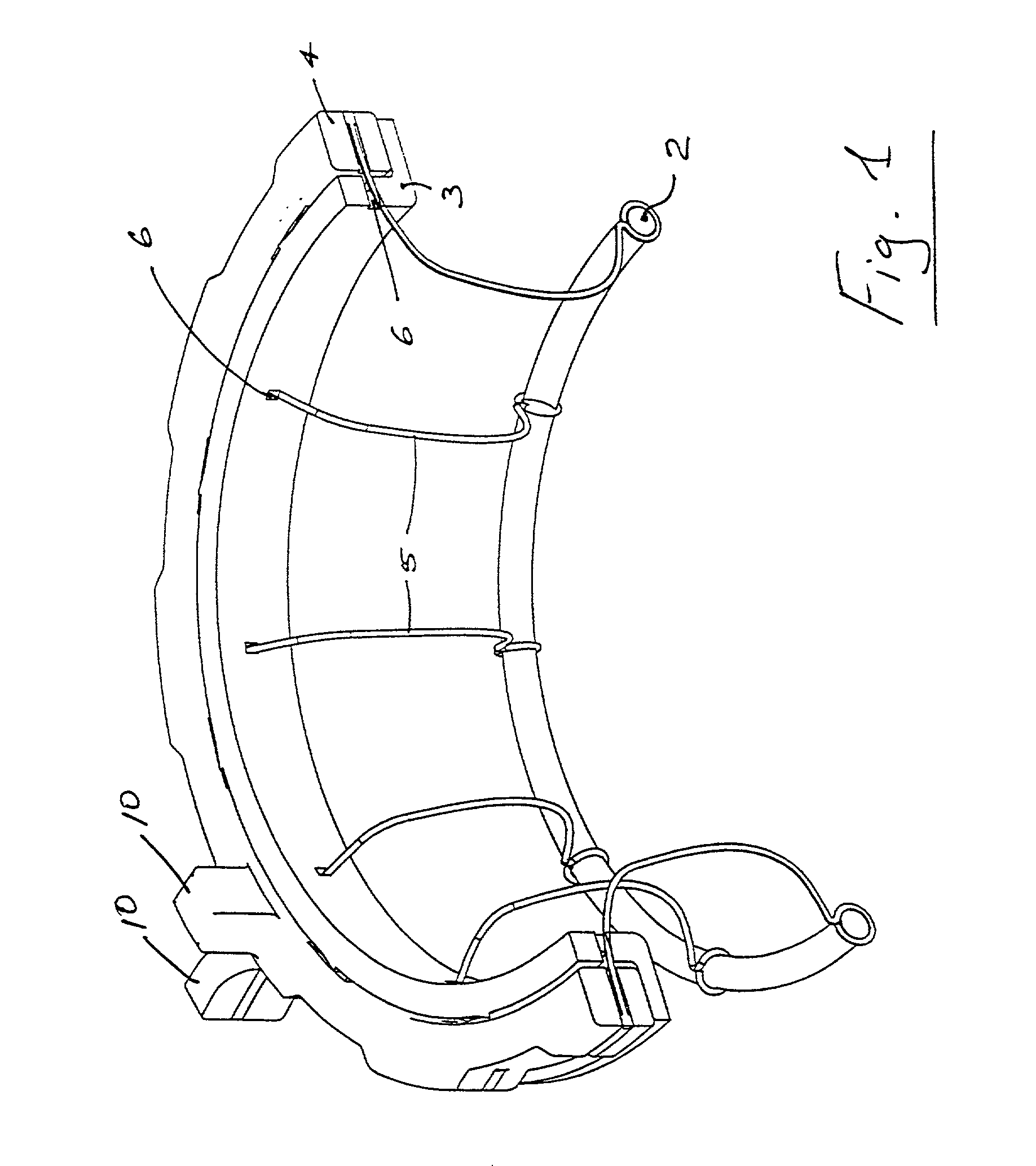
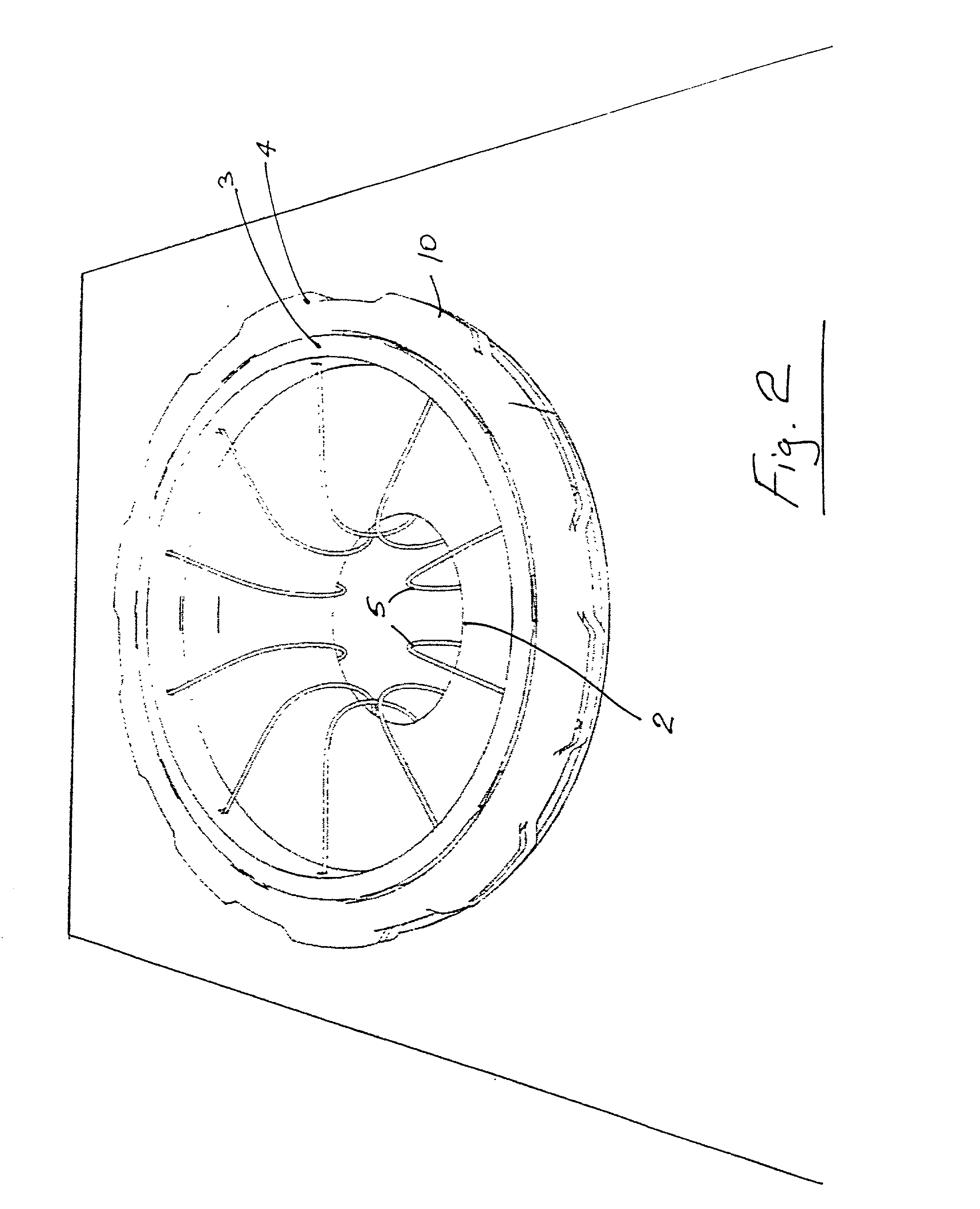
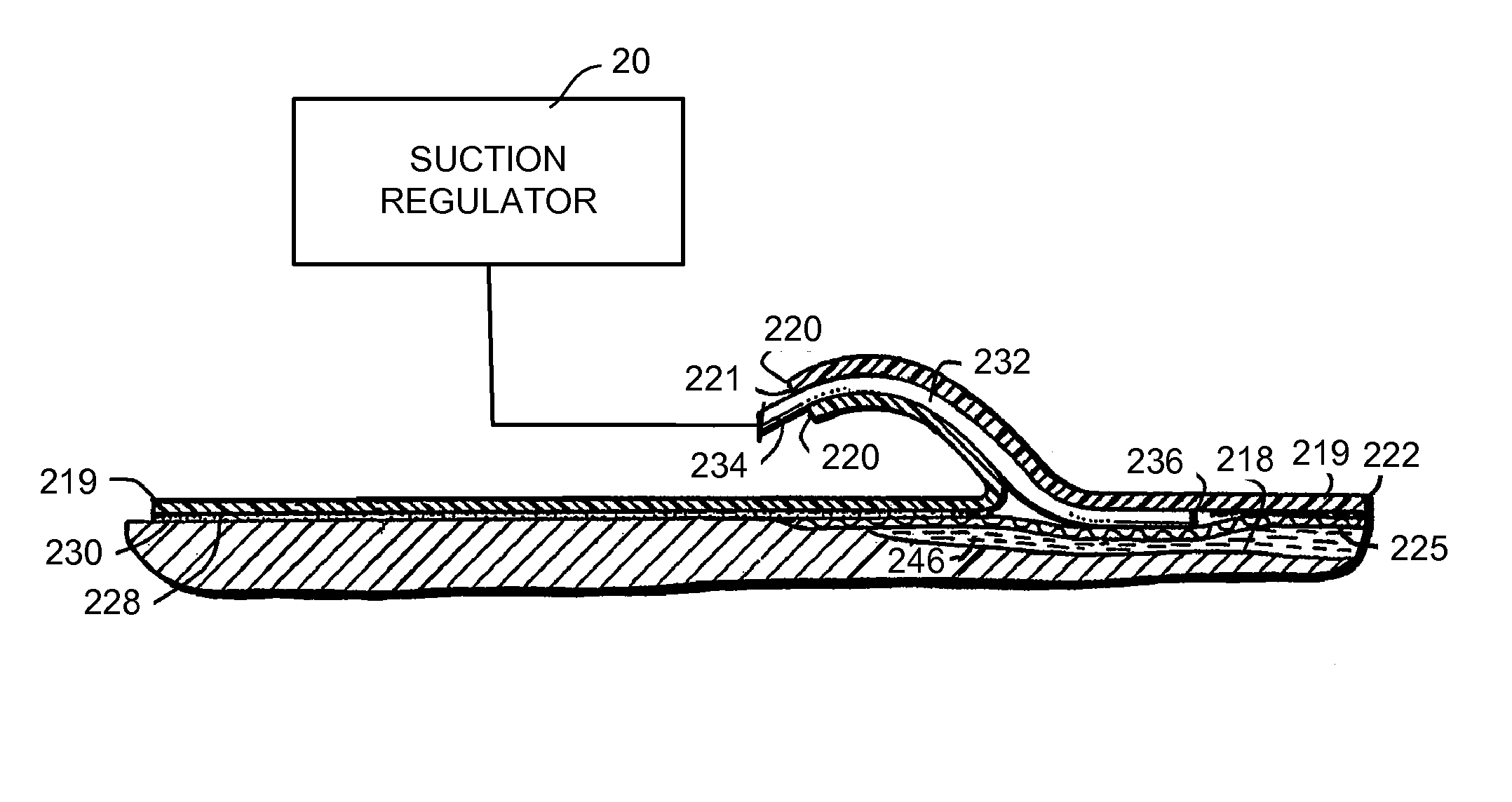
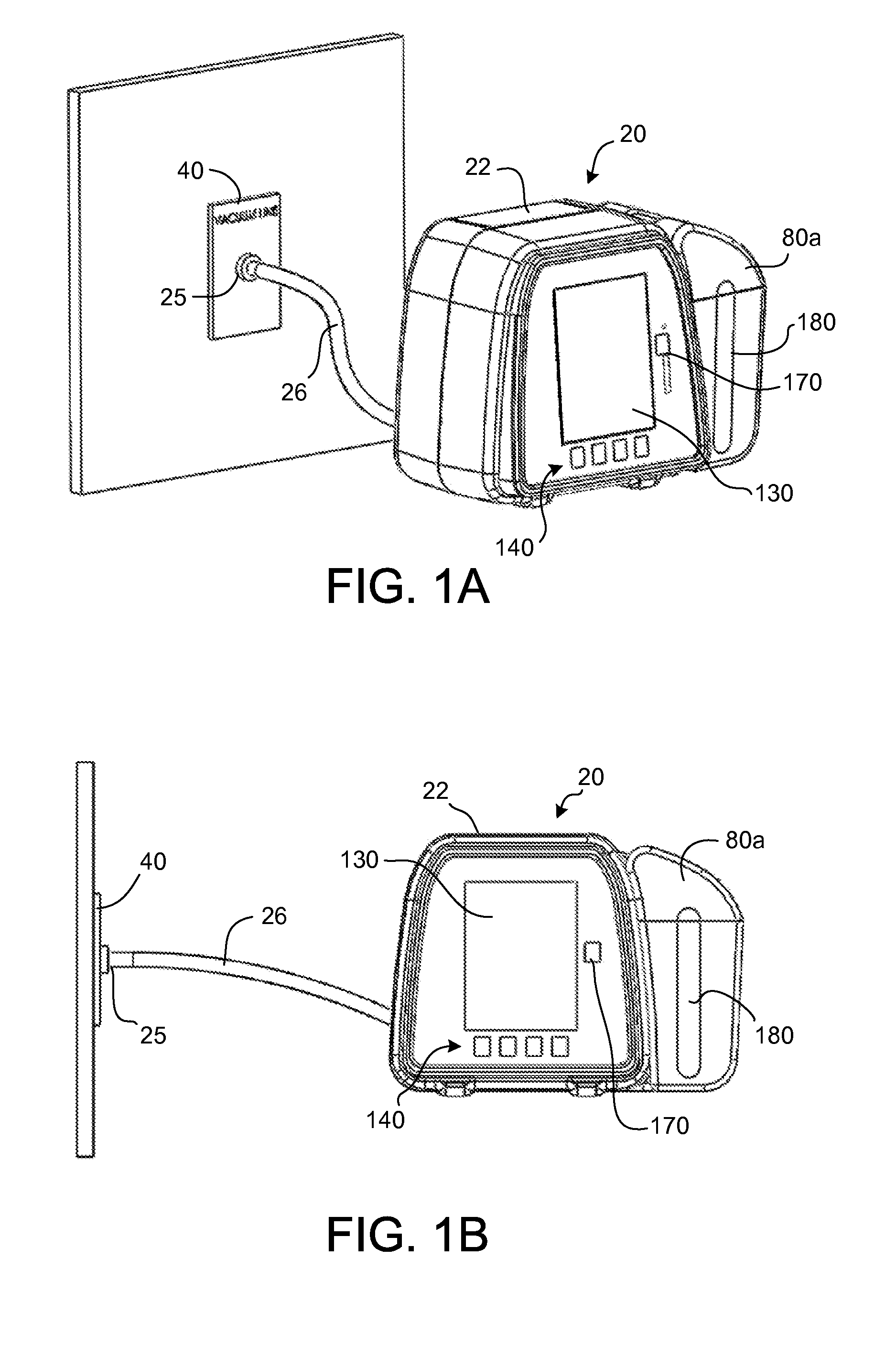
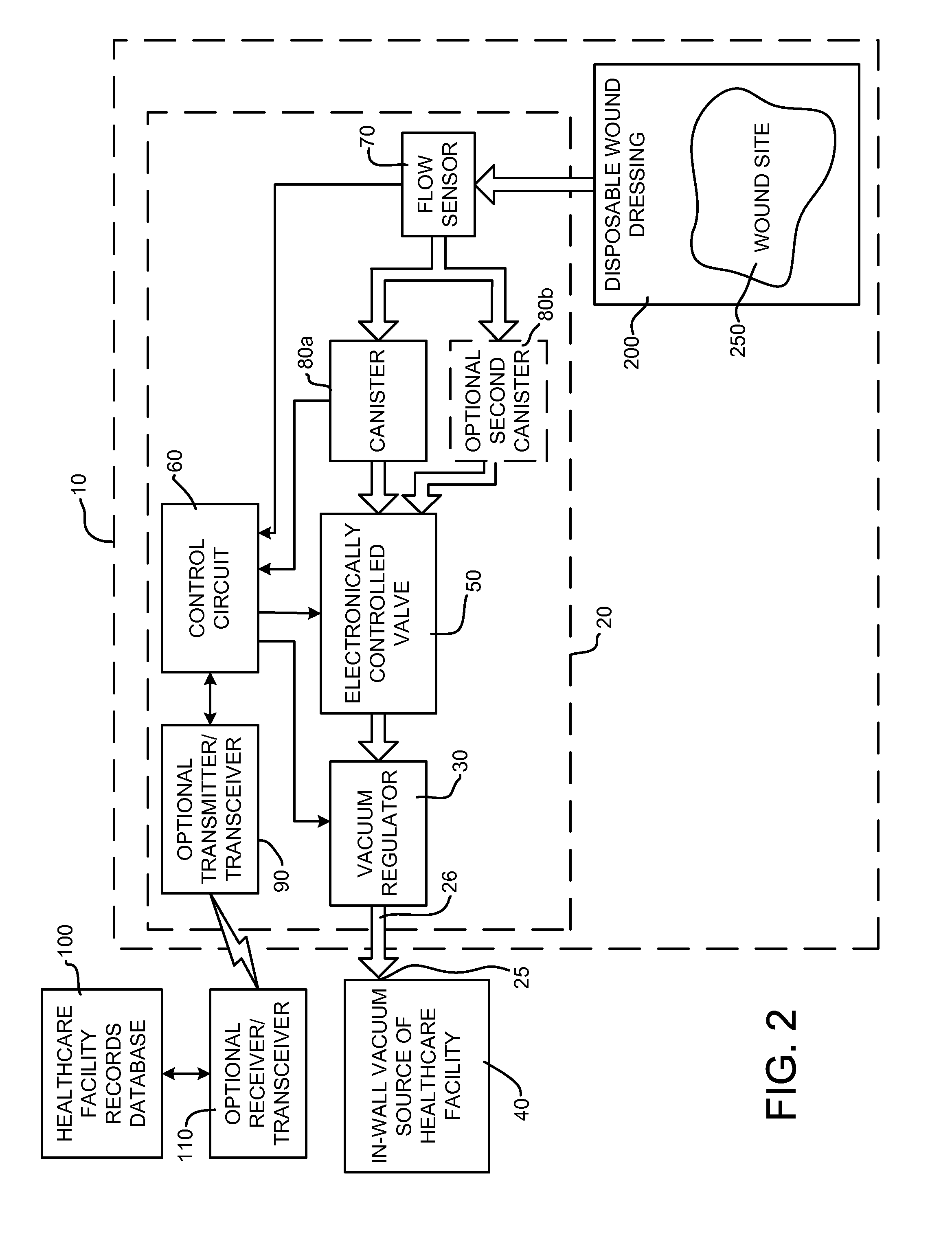
Wound treatment system and suction regulator for use therewith
A system is provided for the treatment of wounds by applying a negative pressure to a wound. The system comprises an electronically controlled suction regulator that comprises: a vacuum regulator, a coupler for coupling the vacuum regulator to an external vacuum source, a valve connected to the vacuum regulator for supplying a negative pressure to the wound, and a control circuit for generating control signals for controlling the valve so that negative pressure may be continuously or intermittently supplied to the wound. The system further comprises a wound dressing provided at the wound site and coupled to the electrically operated valve. The wound dressing comprises a wound dressing pad for placing over the wound, and a wound drape provided over the wound dressing pad and the wound site for sealing the wound site for application of the negative pressure.
Owner:OHIO MEDICAL CORP
Wound dressing and method for controlling severe, life-threatening bleeding
InactiveUS20050137512A1Reduce the temperatureStanching flowPlastersAdhesive dressingsWound dressingClot formation
This invention is directed to advanced hemorrhage control wound dressings, and methods of using and producing same. The subject wound dressing is constructed from a non-mammalian material for control of severe bleeding. The wound dressing for controlling severe bleeding is formed of a biomaterial comprising chitosan, a hydrophilic polymer, a polyacrylic polymer or a combination thereof. The kind of severe, life-threatening bleeding contemplated by this invention is typically of the type not capable of being stanched when a conventional gauze wound dressing is applied with conventional pressure to the subject wound. The wound dressing being capable of substantially stanching the flow of the severe life-threatening bleeding from the wound by adhering to the wound site, to seal the wound, to accelerate blood clot formation at the wound site, to reinforce clot formation at the wound site and prevent bleed out from the wound site, and to substantially prohibit the flow of blood out of the wound site.
Owner:HEMCON
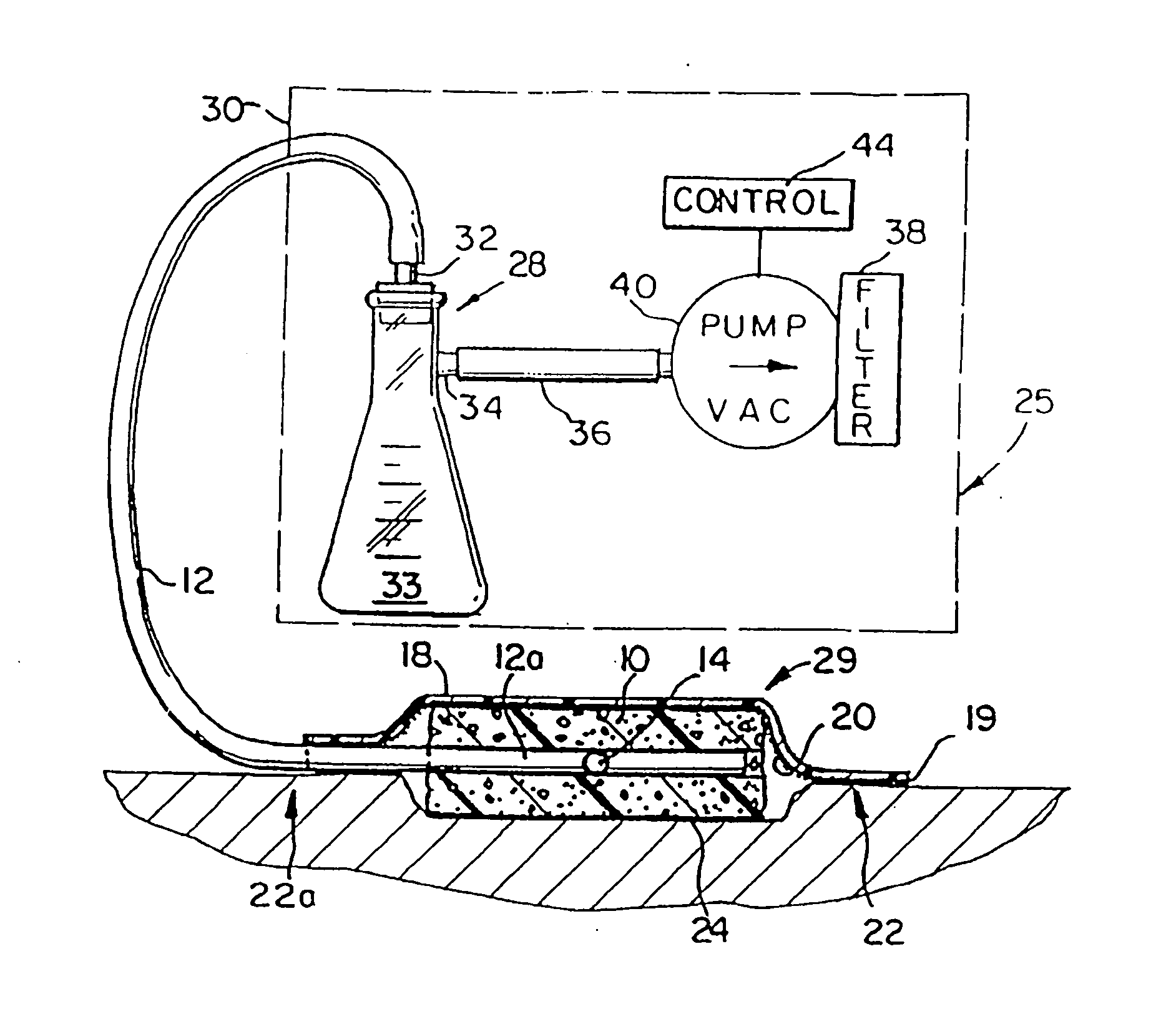
Portable wound treatment apparatus having pressure feedback capabilities
InactiveUS7670323B2Easy to remove and replaceLess-easy to determineWound drainsSurgeryWound sitePressure feedback
A reduced pressure treatment apparatus includes a drape for positioning over the wound site to create and maintain a substantially air-tight cavity between the wound site and the drape. A multi-lumen suction tube is provided to be attached to a reduced pressure source. The multi-lumen suction tube includes a center lumen and at least one outer lumen and is configured to deliver reduced pressure beneath the drape to the substantially air-tight cavity. The multi-lumen suction tube is adapted to allow fluid to be drawn from the wound site through the center lumen and pressure to be monitored at the wound site through the at least one outer lumen.
Owner:KCI LICENSING INC
Sutures and surgical staples for anastamoses, wound closures, and surgical closures
ActiveUS20050038472A1Good storage stabilityRemove moistureSuture equipmentsPowder deliverySurgical stapleMicroparticle
The invention relates to sutures and surgical staples useful in anastomoses. Various aspects of the invention include wound closure devices that use amphiphilic copolymer or parylene coatings to control the release rate of an agent, such as a drug or a biological material, polymerizing a solution containing monomers and the agent to form a coating, using multiple cycles of swelling a polymer with a solvent-agent solution to increase loading, microparticles carrying the agent, biodegradable surgical articles with amphiphilic copolymer coatings, and sutures or surgical staples the deliver a drug selected from the group consisting of triazolopyrimidine, paclitaxol, sirolimus, derivatives thereof, and analogs thereof to a wound site.
Owner:MIRUS LLC
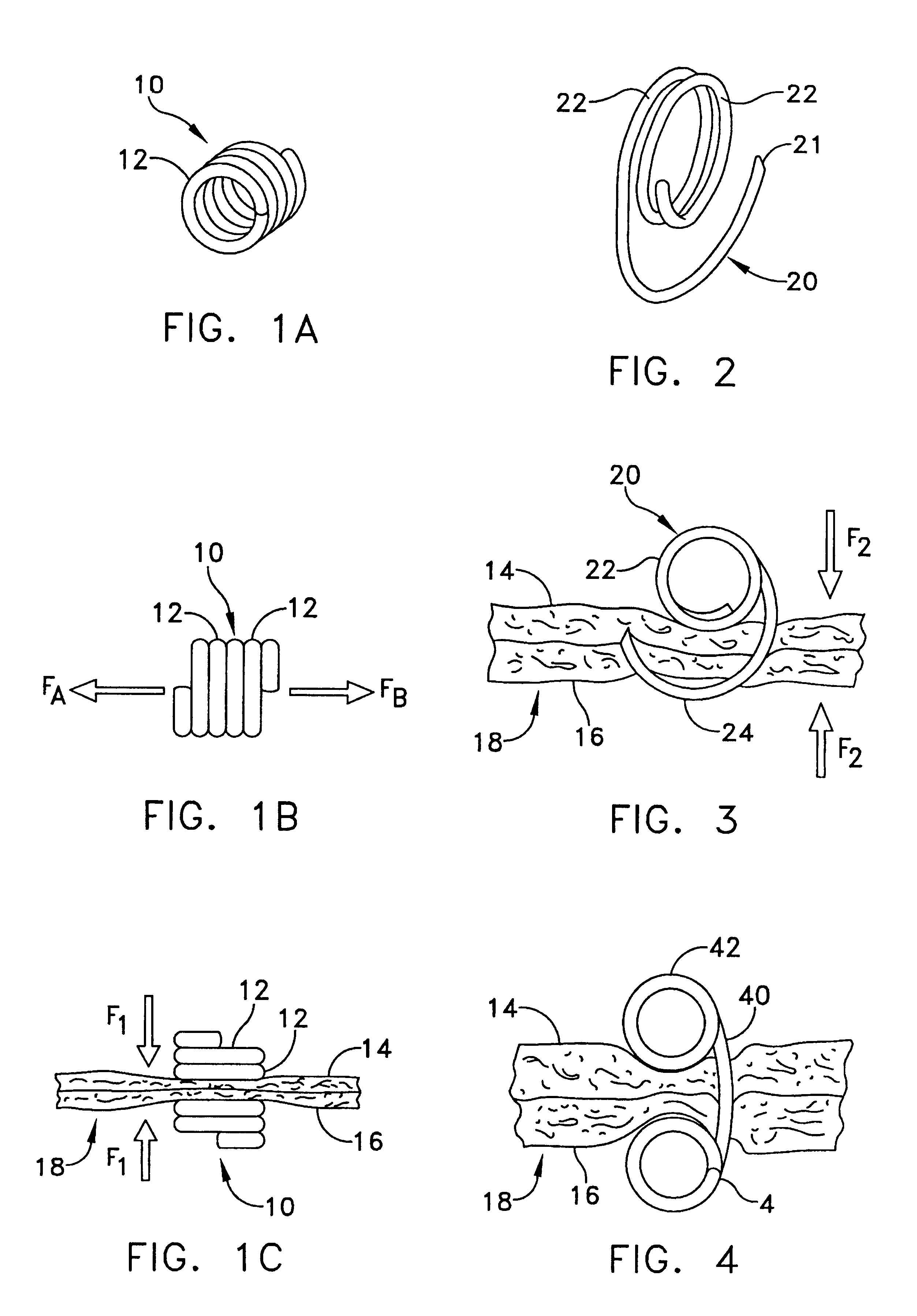
Wound shield for exudate management
A wound shield for exudate management may include a conformable frame to circumscribe a wound. The conformable frame may be formed of material for absorbing wound exudate. Exudate absorbing material may also be one of two or more layers of material forming the conformable frame. The layers may be arranged to keep the exudate absorbing layer at some selected distance from a patients skin. Any suitable dressing may be secured over the conformable frame providing separation between the wound and the dressing. The wound frame may provide pressure relief around a wound or pressure sore to permit healing. The conformable frame may be wrapped, in a spiral, around a wound providing an increasing pressure relief by increasing distance from the wound. A conformable frame may be composed of one or more layers of any suitable material and may include adhesive on one or more surfaces to secure the frame to the wound site and or to secure the dressing to the conformable frame. One layer of the two or more layers of material forming the conformable frame may be a wicking or conduit material that draws exudate from the wound and transports the exudate to any suitable media for exudate storage. The exudate storage may be one or more layers of a dressing covering the wound site, or it may be a removable reservoir.
Owner:AALNEX
Biocompatible wound dressing
InactiveUS20060189910A1Prevent vacuum leakagePromote cell growthAdhesive dressingsMedical applicatorsWound dressingWound site
A biocompatible wound dressing comprised of a pad for insertion substantially into a wound site and a wound drape for sealing enclosure of the foam pad at the wound site. The pad, comprised of a foam or other like material having relatively few open cells in contact with the areas upon which cell growth is to be encouraged so as to avoid unwanted adhesions, but having sufficiently numerous open cells so that drainage and negative pressure therapy may continue unimpaired, is placed in fluid communication with a vacuum source for promotion of fluid drainage, as known in the art. The pad is further comprised of an ultra-low density fused-fibrous ceramic, or a bioabsorbable branched polymer, or cell growth enhancing matrix or scaffolding.
Owner:KCI LICENSING INC
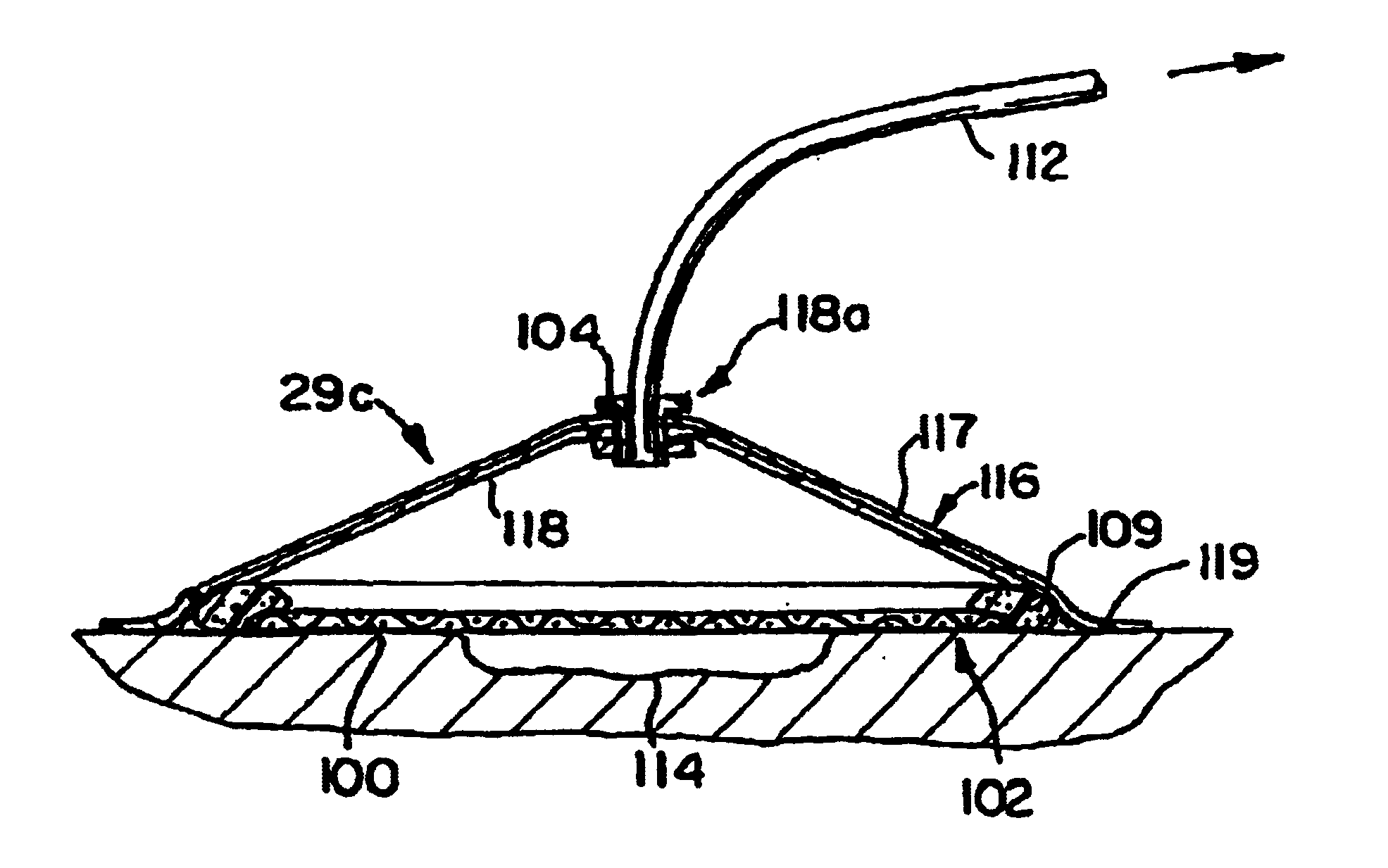
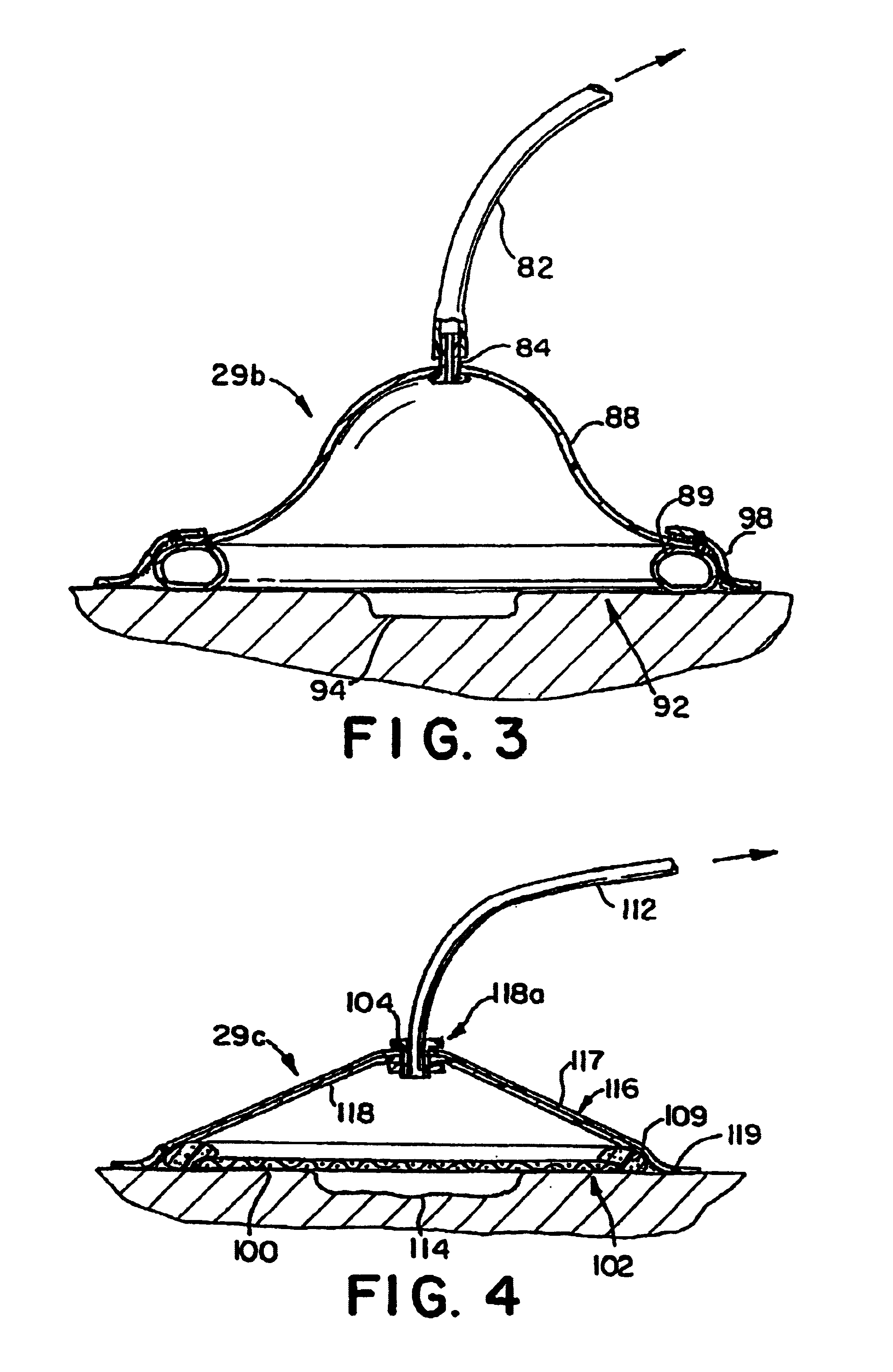
Wound treatment employing reduced pressure
InactiveUS20060213527A1Easy to attachReduce pressureRespiratory masksDiagnosticsWound siteVacuum pump
A method of treating tissue damage comprises applying a negative pressure to a wound sufficient in time and magnitude to promote tissue migration and thus facilitate closure of the wound. The method is applicable to wounds, burns, infected wounds, and live tissue attachments. A wound treatment apparatus is provided in which a fluid impermeable wound cover is sealed over a wound site. A screen in the form of an open-cell foam screen or a rigid porous screen is placed beneath the wound cover over the wound. A vacuum pump supplies suction within the wound cover over the treatment site.
Owner:ARGENTA LOUIS C +1
Abdominal wound dressing
InactiveUS20080243044A1Promote wound healingMinimizes adhesion formationNon-adhesive dressingsWound drainsElastomerWound site
Owner:KCI LICENSING INC
Vacuum assisted wound dressing
Apparatus for the application of topical negative pressure therapy to a wound site is described, the apparatus comprising: a wound contacting element for retaining wound exudate fluid therein; a wound covering element that provides a substantially airtight seal over the wound contacting element and wound site; a vacuum connection tube connecting a wound cavity to a vacuum source; and a vacuum source connected to a distal end of the vacuum connection tube.
Owner:SMITH & NEPHEW INC
Biodegradable and/or bioabsorbable member for vascular sealing
An implantable, bioresorbable and / or biodegradable sealing member, such as a staple, clip, snap or rivet, is used for clamping vessels, vessel side-branches, aneurysms, or any other tube like body-parts or for sealing vascular access sites and wound site management. The implantable, bioresorbable and / or biodegradable member comprises a combination of materials which dissolve or degrade in the human body without any harmful effects on the person that wears the member.
Owner:BIOTRONIK VI PATENT
Fluidic connector for negative pressure wound therapy
Disclosed herein are several embodiments of a wound treatment apparatus employing a fluidic connector for negative pressure wound therapy and methods of using the same. Some embodiments are directed to improved fluidic connectors for connecting to a wound site, for example a fluidic connector including a reinforcement, and methods of using the same.
Owner:SMITH & NEPHEW INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com