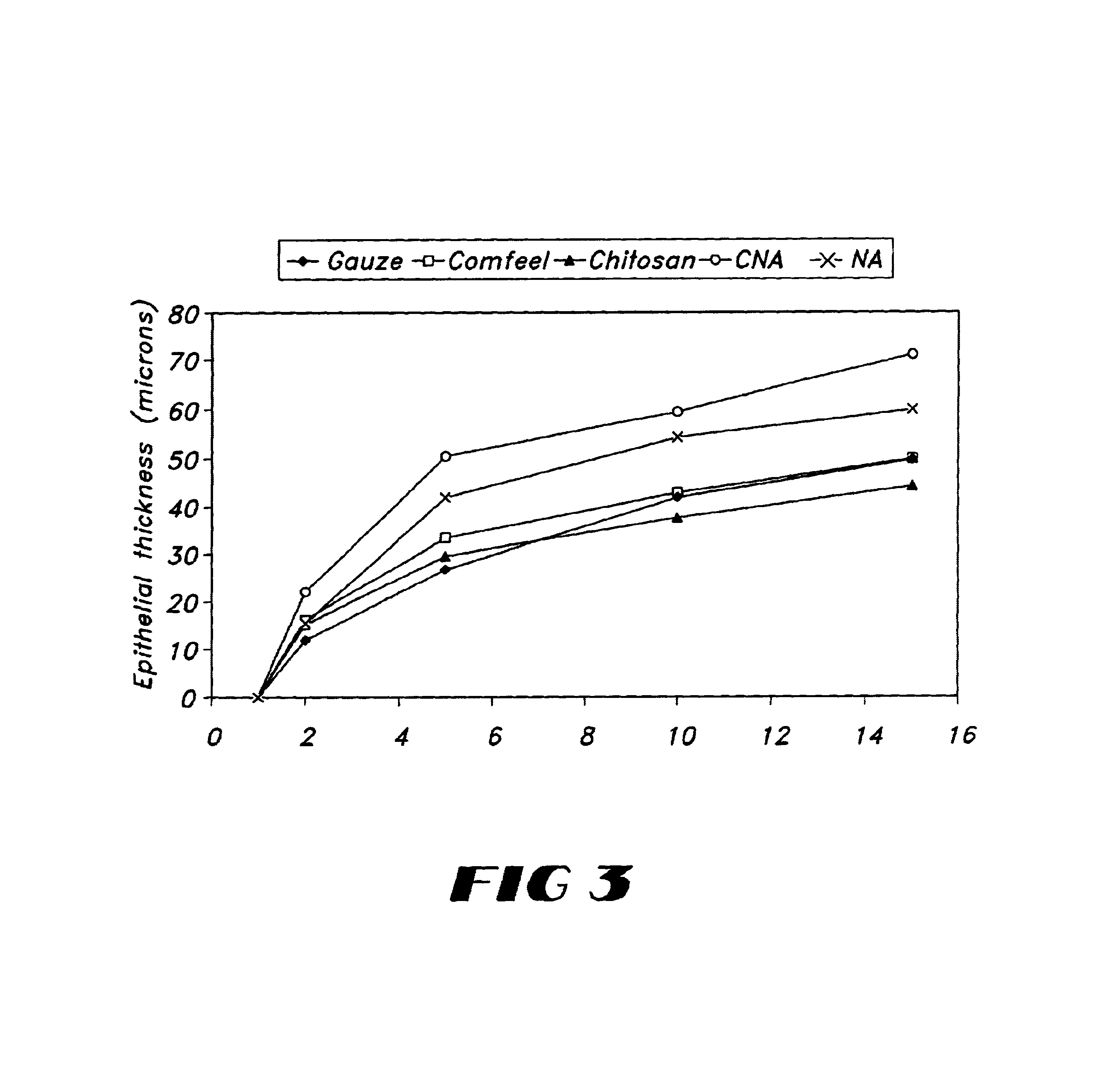
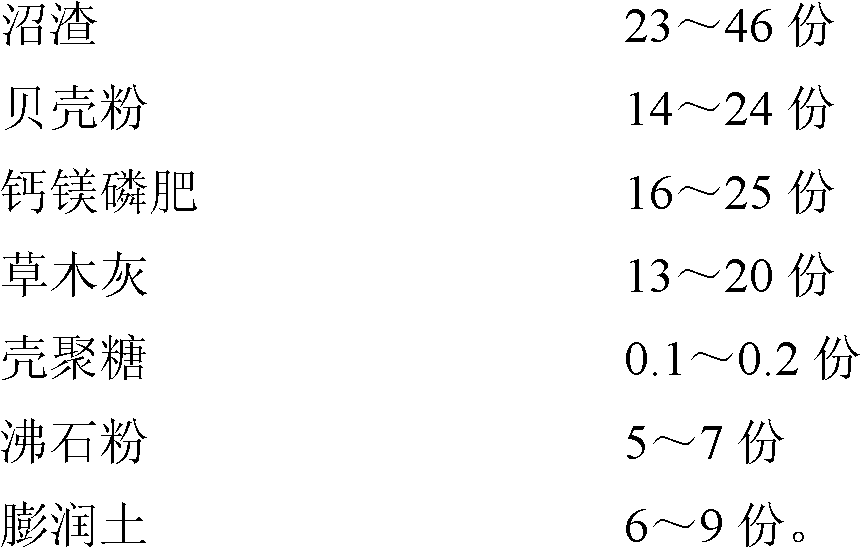Patents
Literature
25877 results about "Chitosan" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
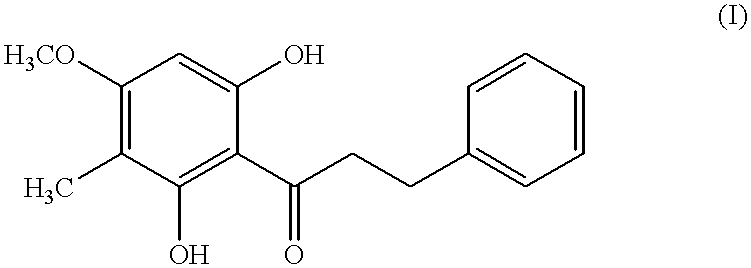
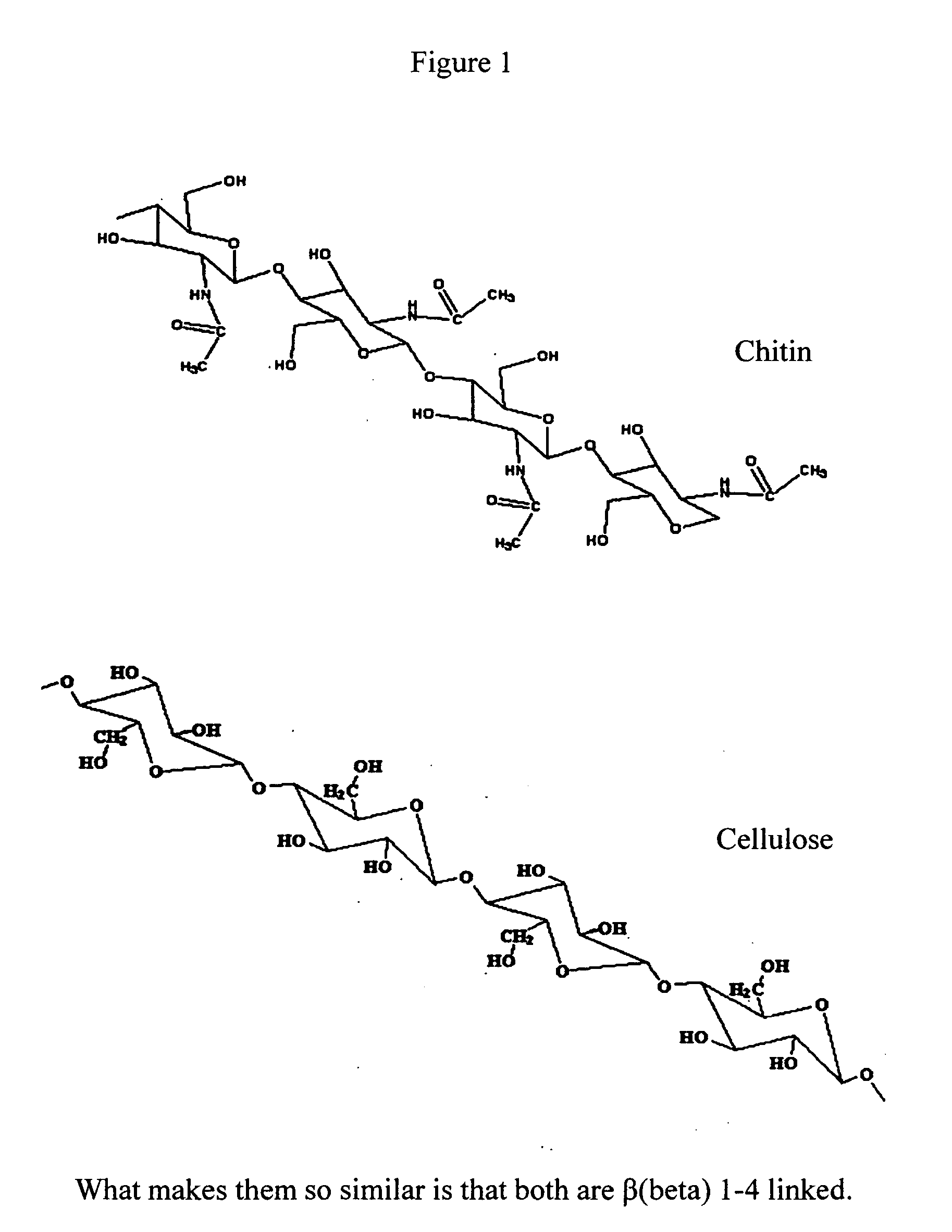
Chitosan /ˈkaɪtəsæn/ is a linear polysaccharide composed of randomly distributed β-(1→4)-linked D-glucosamine (deacetylated unit) and N-acetyl-D-glucosamine (acetylated unit). It is made by treating the chitin shells of shrimp and other crustaceans with an alkaline substance, like sodium hydroxide.
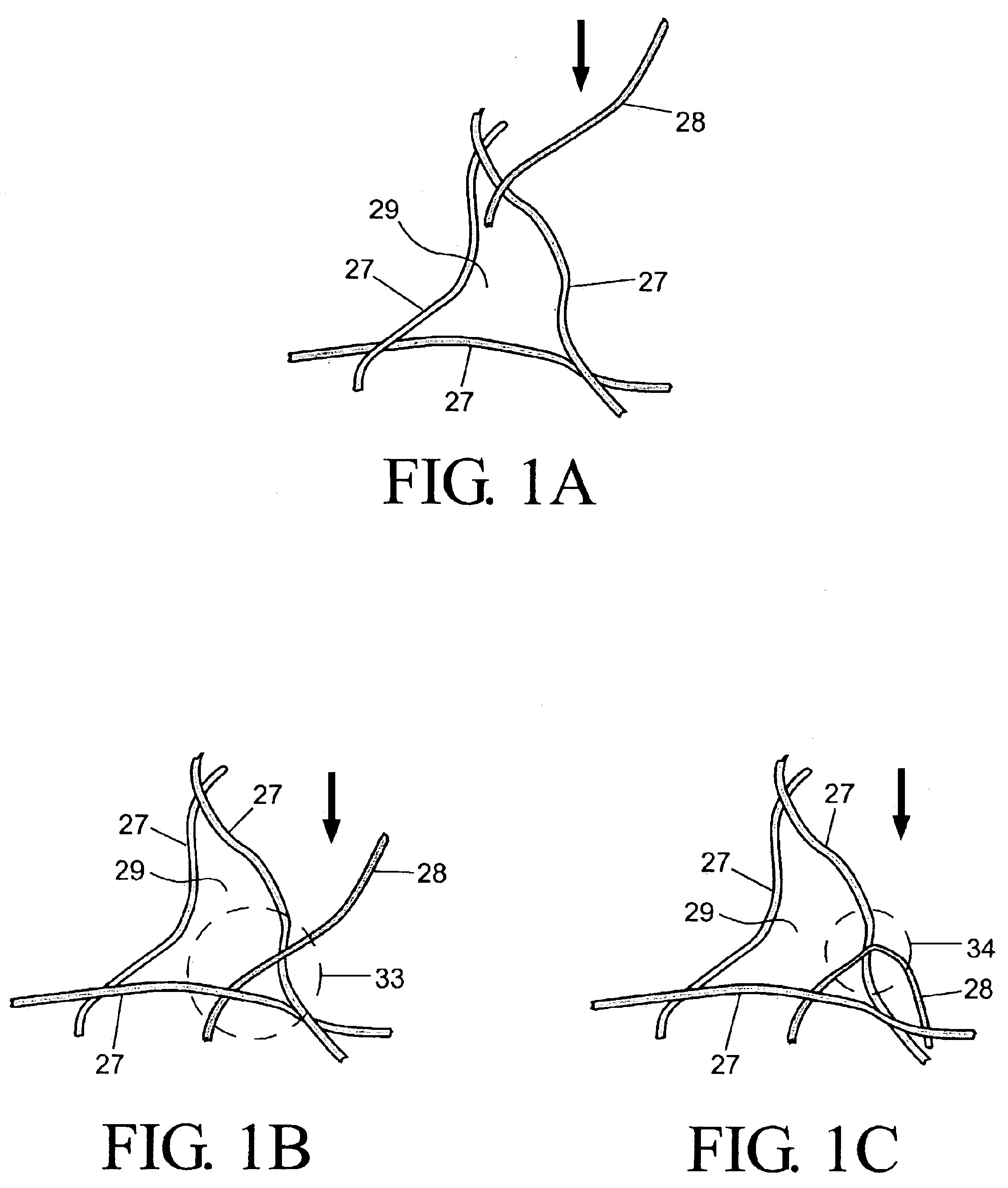
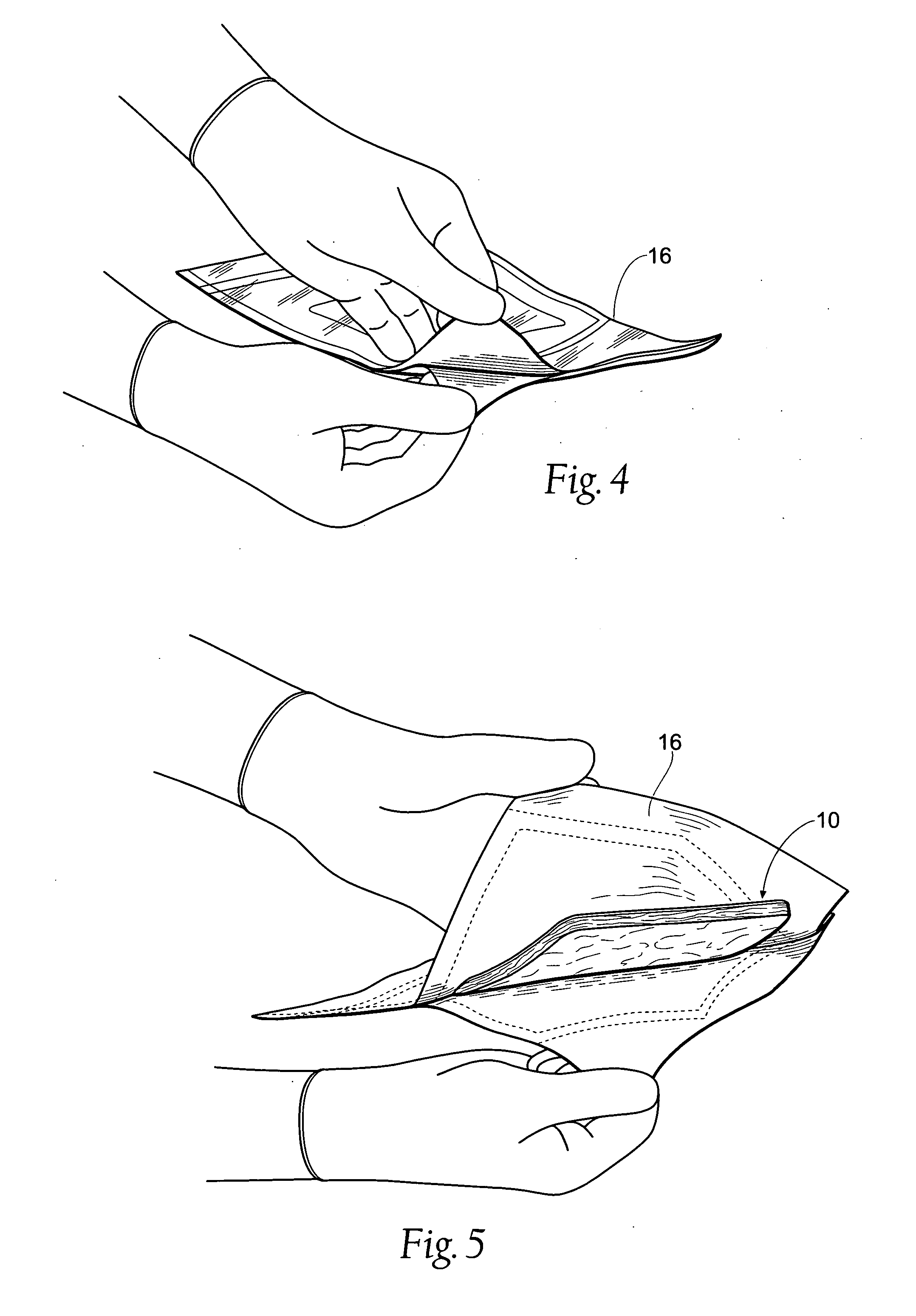
Wound dressing and method for controlling severe, life-threatening bleeding
ActiveUS7371403B2Stanching flowProhibiting flow of bloodBiocideSuspensory bandagesHydrophilic polymersWound dressing
This invention is directed to advanced hemorrhage control wound dressings, and methods of using and producing same. The subject wound dressing is constructed from a non-mammalian material for control of severe bleeding. The wound dressing for controlling severe bleeding is formed of a biomaterial comprising chitosan, a hydrophilic polymer, a polyacrylic polymer or a combination thereof. The kind of severe, life-threatening bleeding contemplated by this invention is typically of the type not capable of being stanched when a conventional gauze wound dressing is applied with conventional pressure to the subject wound. The wound dressing being capable of substantially stanching the flow of the severe life-threatening bleeding from the wound by adhering to the wound site, to seal the wound, to accelerate blood clot formation at the wound site, to reinforce clot formation at the wound site and prevent bleed out from the wound site, and to substantially prohibit the flow of blood out of the wound site.
Owner:PROVIDENCE HEALTH SYST OREGONDBA ST VINCENTMEDICAL CENT
Wound dressing and method for controlling severe, life-threatening bleeding
InactiveUS20050137512A1Reduce the temperatureStanching flowPlastersAdhesive dressingsWound dressingClot formation
This invention is directed to advanced hemorrhage control wound dressings, and methods of using and producing same. The subject wound dressing is constructed from a non-mammalian material for control of severe bleeding. The wound dressing for controlling severe bleeding is formed of a biomaterial comprising chitosan, a hydrophilic polymer, a polyacrylic polymer or a combination thereof. The kind of severe, life-threatening bleeding contemplated by this invention is typically of the type not capable of being stanched when a conventional gauze wound dressing is applied with conventional pressure to the subject wound. The wound dressing being capable of substantially stanching the flow of the severe life-threatening bleeding from the wound by adhering to the wound site, to seal the wound, to accelerate blood clot formation at the wound site, to reinforce clot formation at the wound site and prevent bleed out from the wound site, and to substantially prohibit the flow of blood out of the wound site.
Owner:HEMCON
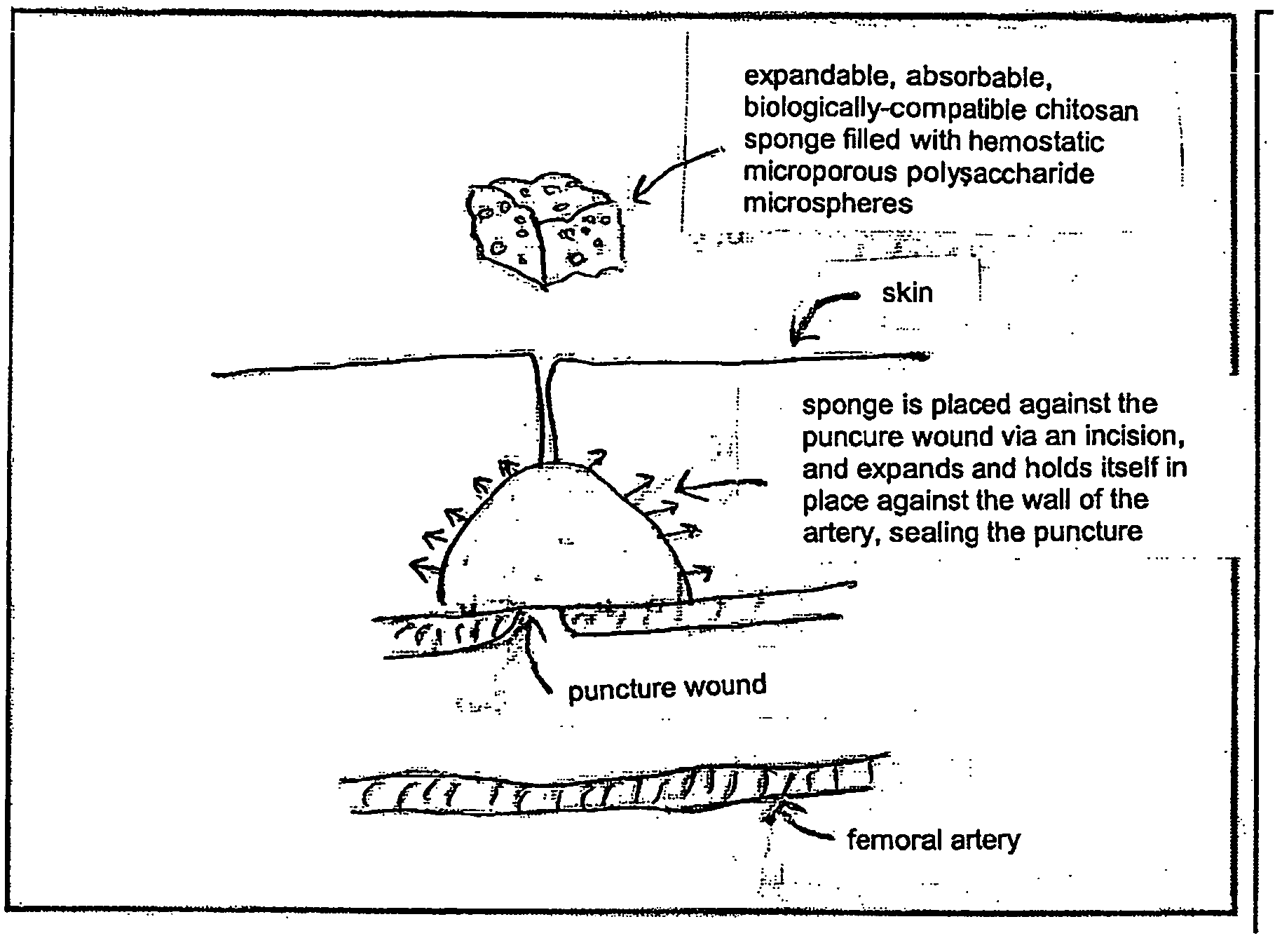
Deployable multifunctional hemostatic agent
InactiveUS20050123588A1Good hemostasisRapid and effective hemostasisSuture equipmentsPharmaceutical non-active ingredientsVeinMicrosphere
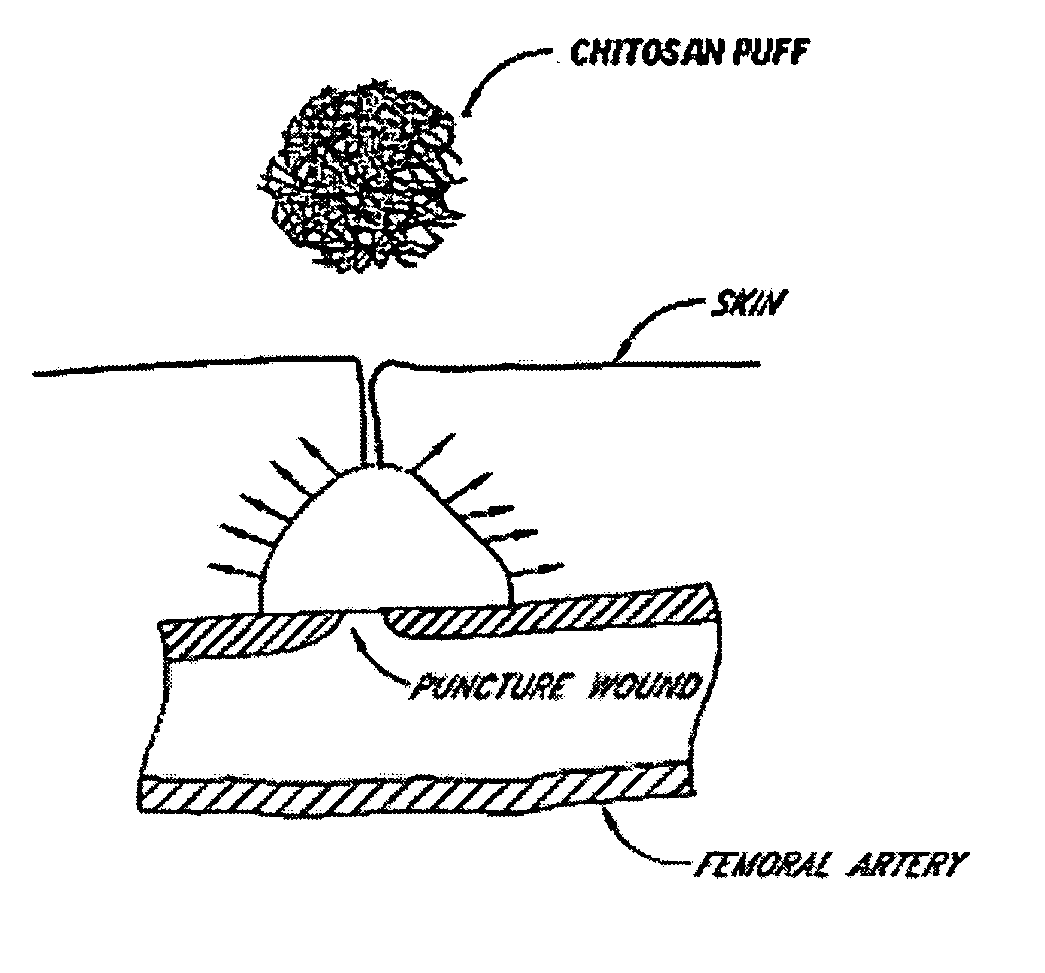
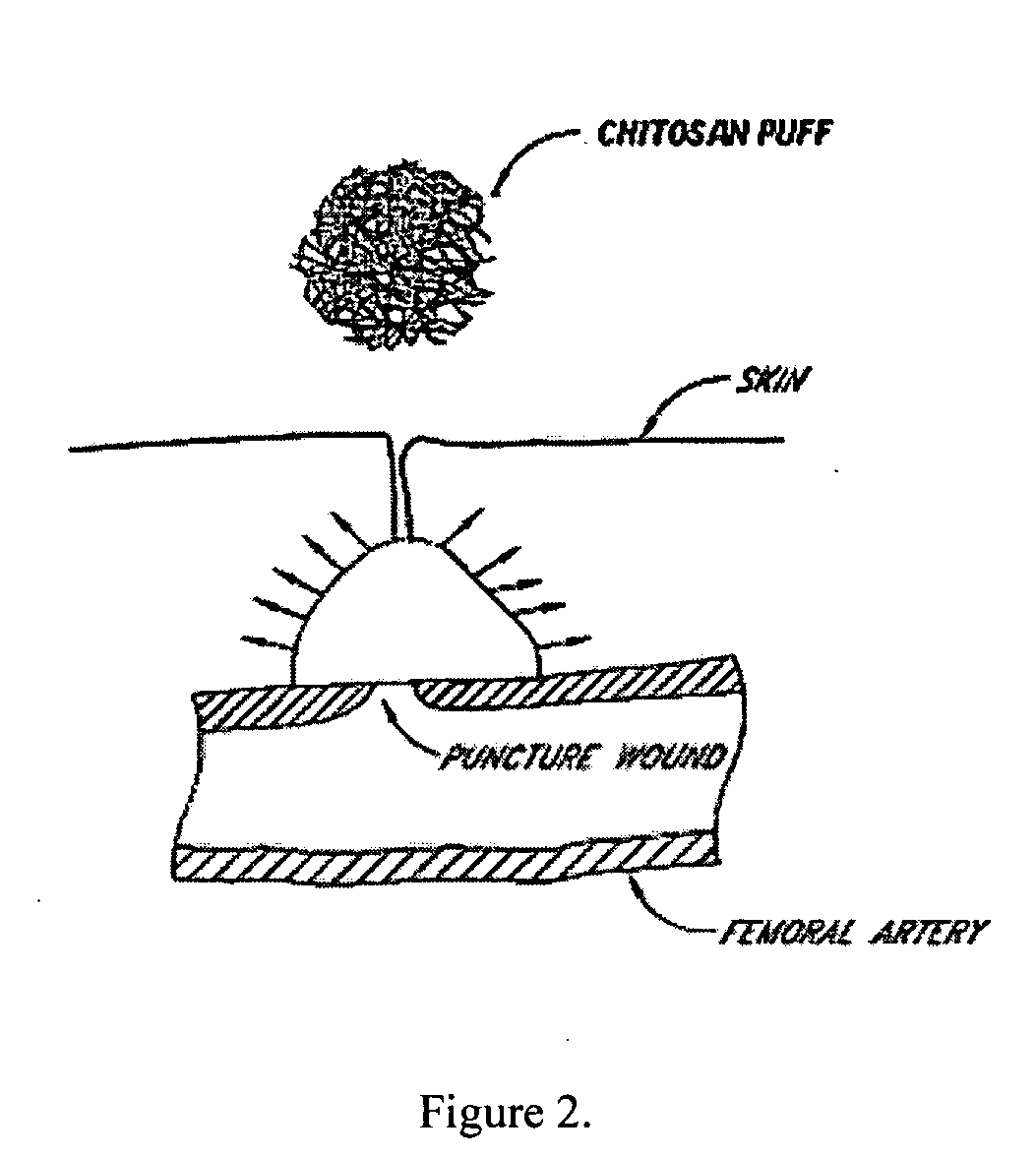
This invention relates to deployable hemostatic materials comprising chitosan fibers upon which hemostatic microporous polysaccharide microspheres and a medicament or biologically active substance are deposited. The hemostatic materials are suitable for use in controlling active bleeding from artery and vein lacerations, sealing femoral artery punctures, and controlling oozing from tissue.
Owner:LOMA LINDA UNIV MEDICAL CENT
Method of treating neurological diseases and etiologically related symptomology using carbonyl trapping agents in combination with medicaments
This invention defines a novel method for treatment of several neurological diseases and pathophysiologically related symptomology, said diseases including peripheral neuropathies, secondary symptomology of diabetes, Alzheimer's disease, Parkinson's disease, alcoholic polyneuropathy and age-onset symptomology, as well as analogous veterinary disease states. An opportunity exists for pharmacological intervention in some neurological diseases by use of water soluble, small molecular weight primary amine agents and chemical derivatives thereof. Examples of such primary pharmacological agents include 4-aminobenzoic acid and derivatives thereof. The present invention also includes: (1) oral use of optional non-absorbable polyamine polymeric co-agents such as chitosan, (2) oral use of optional known antioxidant co-agents and nutritional factors related thereto, and (3) use of the primary agents and co-agents noted above in optional combination with medicaments recognized as effective for treatment of the diseases addressed herein or symptoms thereof.
Owner:SECANT PHARMA
Pharmaceutical composition of nanoparticles
InactiveUS8257740B1Enhances intestinal epithelial barrier functionReduce intestinal permeabilityPowder deliveryPharmaceutical non-active ingredientsNanoparticleMedicine
The invention discloses a composition of chitosan-shelled nanoparticles and methods of manufacturing. The chitosan-shelled nanoparticles are characterized with a positive surface charge and enhanced epithelial ermeability for oral drug delivery.
Owner:NANOMEGA MEDICAL CORP
High density fibrous polymers suitable for implant
InactiveUS6974862B2Easy to shapeEvenly dispersedFinger jointsInternal osteosythesisFiberHigh density
This invention includes malleable, biodegradable, fibrous compositions for application to a tissue site in order to promote or facilitate new tissue growth. One aspect of this invention is a fibrous component (e.g., collagen, chitosan, alginate, hyaluronic acid, poly-lactic acid, poly-capralactone, and polyurethane) that provides unique mechanical and physical properties. The invention may be created by providing a vessel containing a slurry, said slurry comprising a plurality of natural or synthetic polymer fibers and at least one suspension fluid, wherein the polymer fibers are substantially evenly dispersed and randomly oriented throughout the volume of the suspension fluid; applying a force, e.g., centrifugal, to said vessel containing said slurry, whereupon said force serves to cause said polymer fibers to migrate through the suspension fluid and amass at a furthest extent of the vessel, forming a polymer material, with said polymer material comprising polymer fibers of sufficient length and sufficiently viscous, interlaced, or interlocked to retard dissociation of said polymer fibers.
Owner:DSM IP ASSETS BV
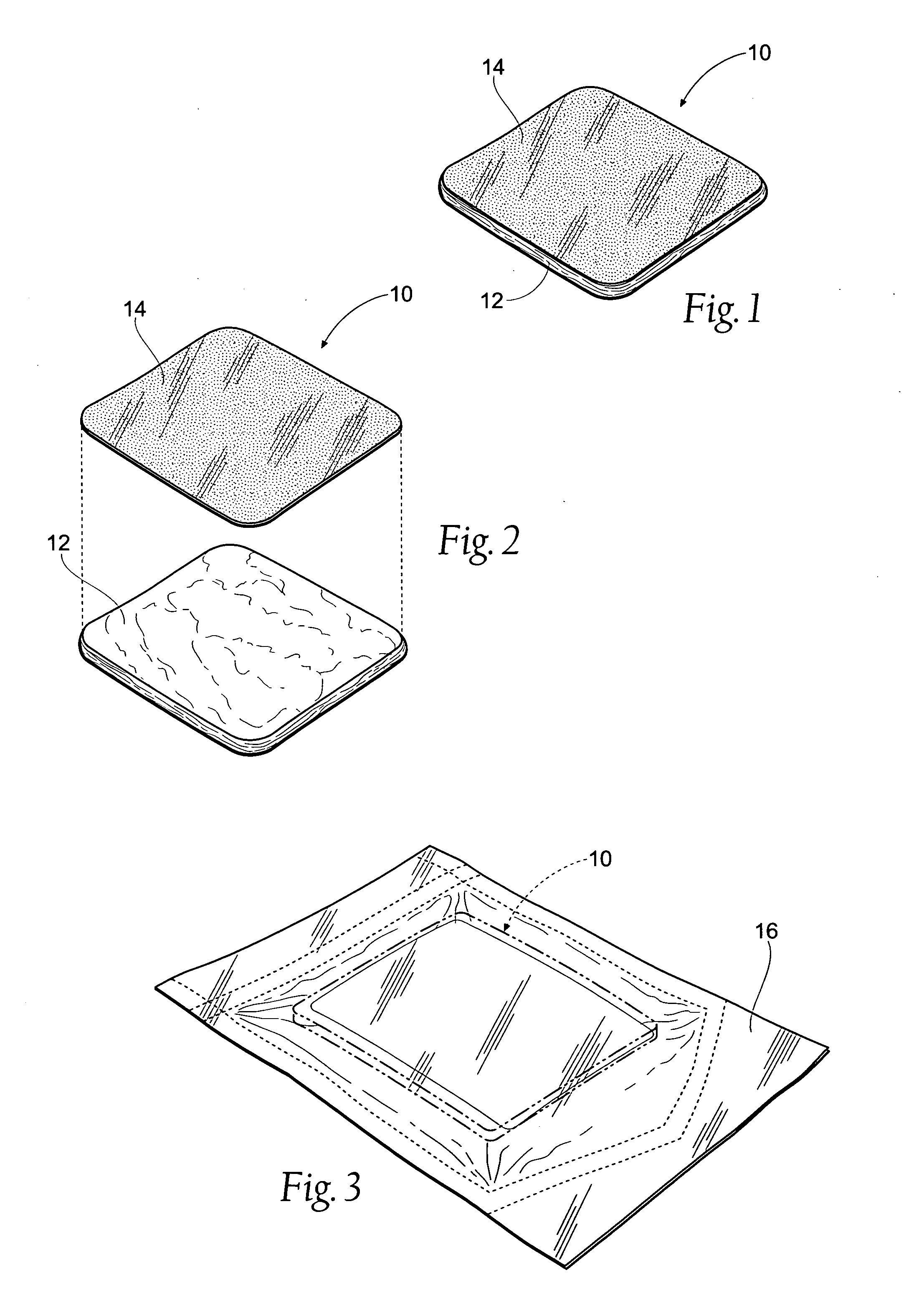
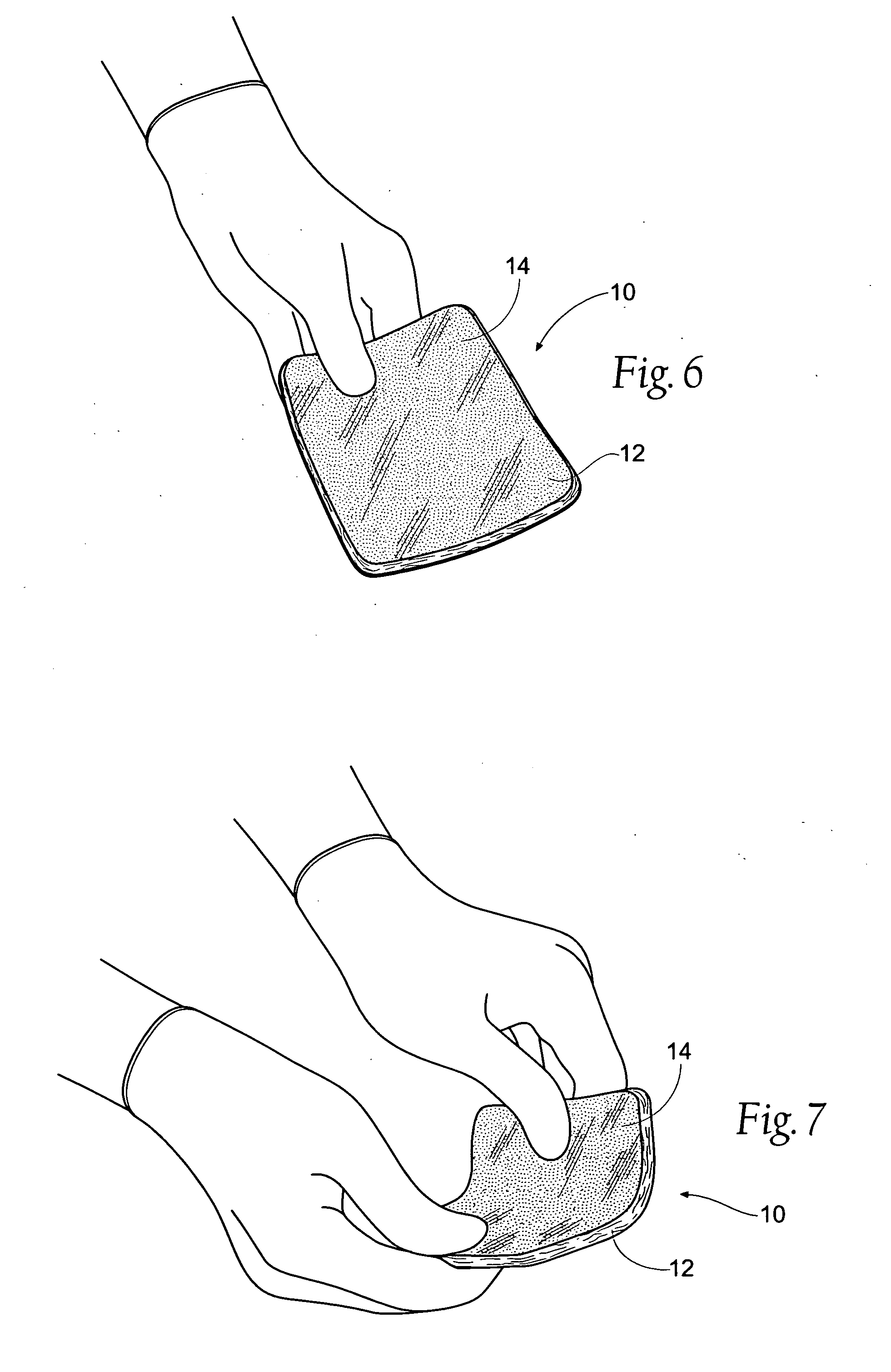
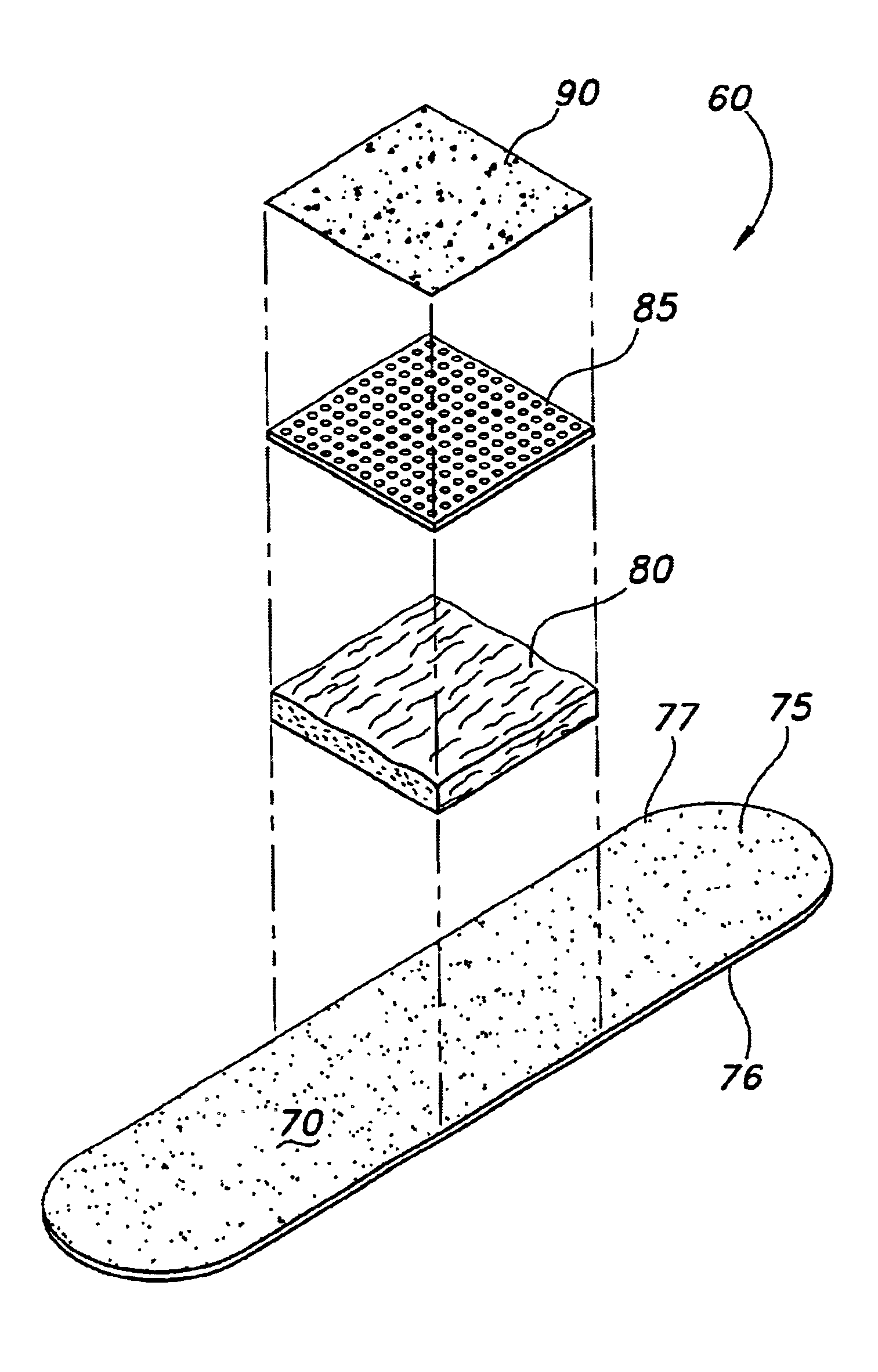
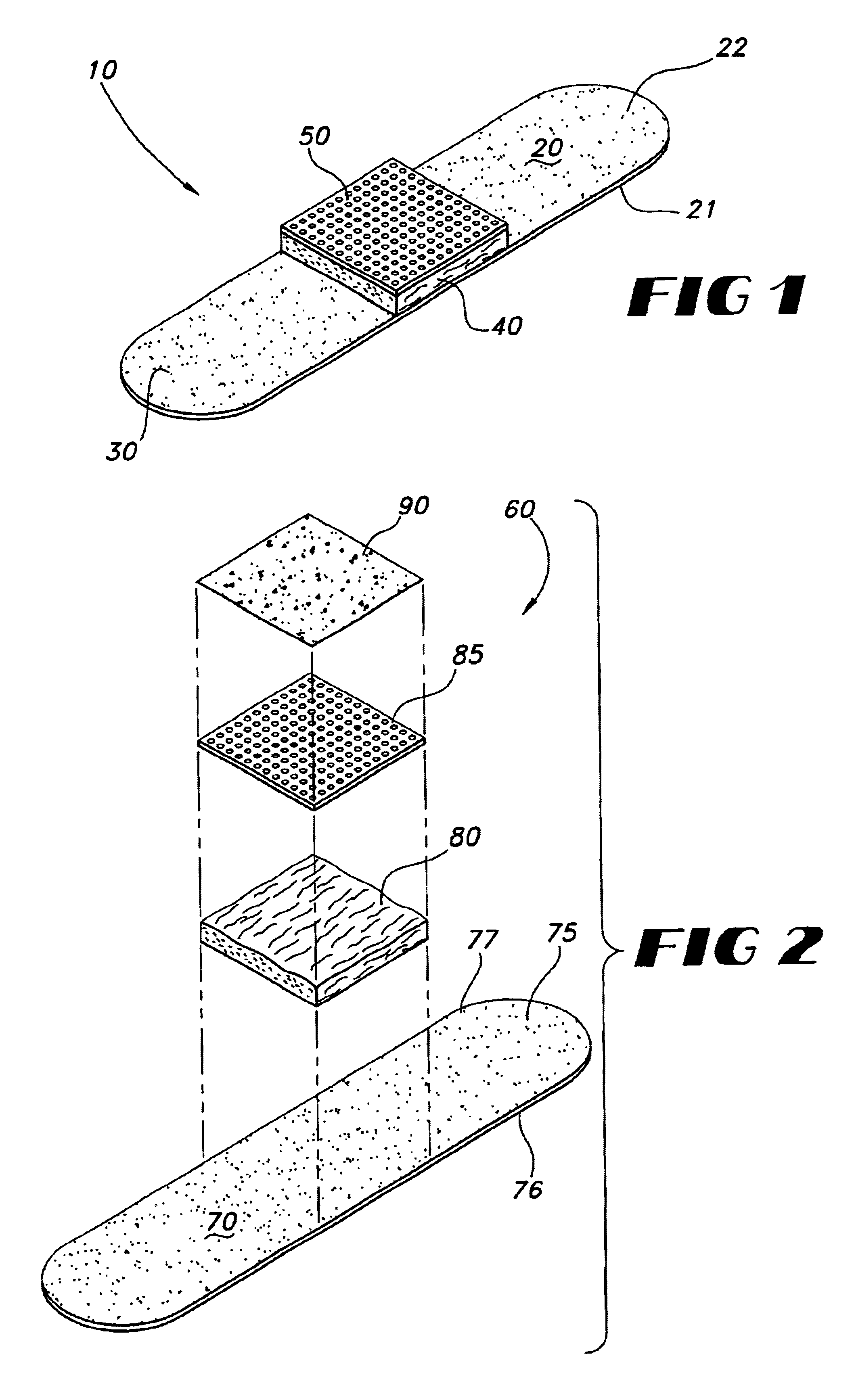
Tissue dressing assemblies, systems, and methods formed from hydrophilic polymer sponge structures such as chitosan
InactiveUS20050147656A1Imparts complianceImparts flexibilityOrganic active ingredientsBiocideInlet channelHydrophilic polymers
Tissue dressing assemblies are formed from hydrophilic polymer sponge structures. The tissue dressing assemblies can be used, e.g., (i) stanch, seal, or stabilize a site of tissue injury, tissue trauma, or tissue access; or (ii) form an anti-microbial barrier; or (iii) form an antiviral patch; or (iv) intervene in a bleeding disorder; or (v) release a therapeutic agent; or (vi) treat a mucosal surface; or (vii) combinations thereof. The tissue dressing structures are made compliant, e.g., by (i) micro-fracturing of a substantial portion of the sponge structure by mechanical manipulation prior to use, or (ii) a surface relief pattern formed on a substantial portion of the sponge structure prior to use, or (iii) a pattern of fluid inlet channels formed in a substantial portion of the sponge structure prior to use, or (iv) the impregnation of a sheet material within the sponge structure.
Owner:HEMCON MEDICAL TECH
Bandage, methods of producing and using same
ActiveUS6967261B1Improve health environmentPromote rapid healingAbsorbent padsCoatingsAcute woundIrritation
A bandage of the type used on acute wounds, minor wounds, burn wounds and irritations, includes a first layer for covering the wound site and an area around the wound site, with the first layer including a top surface and bottom surface; a second layer over the first layer bottom surface, for absorbing exudates from the wound site; the second layer including a poly(ethyleneoxide)-based compound and a chitosan-based compound. A third layer is situated over the second layer, the third layer being of a perforated film, and wherein, at least one antimicrobial agent is associated with the bandage in a position where the antimicrobial agent will come in contact with the wound site, and which is transferable from the bandage to the wound site, upon contact with the wound site.
Owner:O&M HALYARD INC
Organic environmentally-friendly soil conditioner for improving acidified or acid soil
ActiveCN102321484ARaw material safetyEfficient use ofOrganic fertilisersSoil conditioning compositionsSodium BentoniteHeavy metal poisons
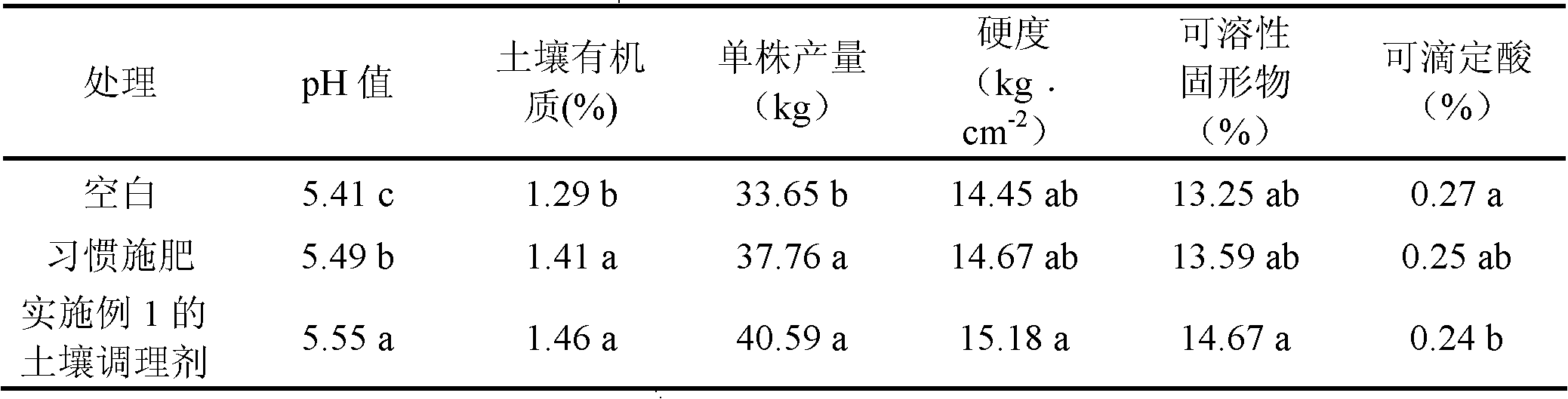
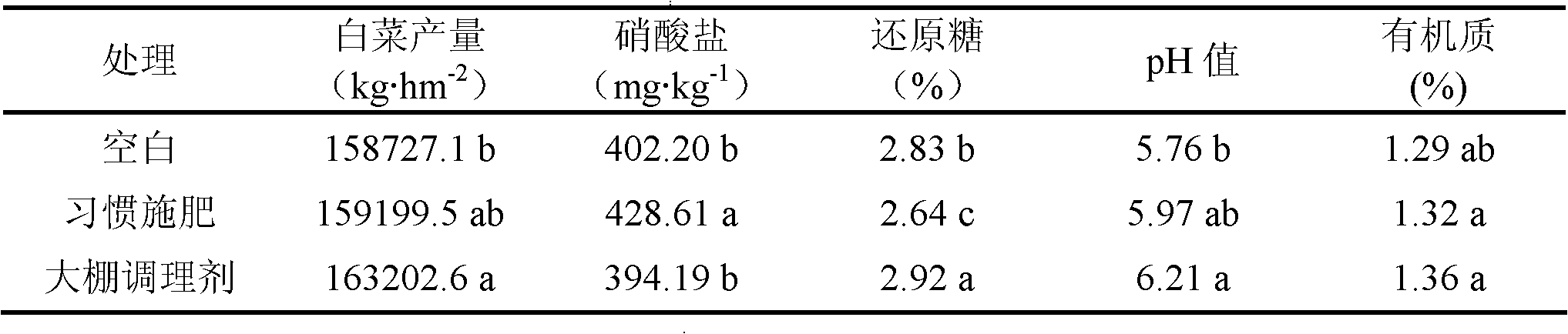
The invention relates to an organic environmentally-friendly soil conditioner for improving acidified or acid soil and a preparation method thereof. The soil conditioner is prepared from the following raw materials in parts by weight: 23-46 parts of biogas residue, 14-24 parts of shell powder, 16-25 parts of calcium magnesium phosphate fertilizer, 13-20 parts of plant ash, 0.1-0.2 part of chitosan, 5-7 parts of zeolite powder and 6-9 parts of bentonite. The soil conditioner is prepared through pretreatment of the biogas residue, pretreatment of the shell powder and the like; through the produced soil conditioner, the pH value of soil can be increased; nutritive elements such as calcium, magnesium, phosphorus, potassium and the like in the soil are increased; the organic matter content in the soil is increased; the water retention and fertilizer retention capacity of the soil is improved; the physicochemical property of the soil is improved; the antiretroviral (diseases, pests, drought and the like) capacity of crops is improved; heavy metal poison is reduced; and the soil conditioner is convenient to apply, is not influenced by wind power and is suitable for mechanical working.
Owner:INST OF AGRI RESOURCES & ENVIRONMENT SHANDONG ACADEMY OF AGRI SCI
Methods of treating chronic inflammatory diseases using carbonyl trapping agents
InactiveUS6444221B1Improved therapeutic propertyImprove propertiesBiocidePeptide/protein ingredientsEtiologyBenzoic acid
Owner:SECANT PHARMA
Edible biological preservative film and preparation method thereof
ActiveCN104194354AImprove mechanical propertiesImprove barrier propertiesFlexible coversWrappersAntioxidantPlasticizer
The invention discloses an edible biological preservative film and a preparation method thereof. The edible biological preservative film comprises the following components in parts by weight: 30-60 parts of a film former, 1-10 parts of a natural antioxidant, 1-10 parts of a natural bacterial inhibitor, 1-4 parts of a plasticizer and 0.5-4 parts of an emulsifier. The preparation method comprises the following steps: mixing the components in proportion to form preservative film liquid, and preparing the preservative film by adopting a tape-casting process. According to the preservative film, excellent film forming performances of materials such as marine polysaccharides, chitosan, gelatin and the like are utilized, the natural antioxidant and the natural bacterial inhibitor are selected and used, and the modification process is carried out through the plasticizer, the emulsifier and the like, so that the prepared preservative film is good in mechanical property, delays the spoilage of an aquatic product and can effectively prolong the shelf life of the aquatic product.
Owner:MARINE BIOLOGY INST OF SHANDONG PROVINCE
Bioactive Complexes Compositions and Methods of Use Thereof
InactiveUS20070085059A1Enhanced soluble complexesGood fluidityCosmetic preparationsNervous disorderDiseaseCholesterol blood
A bioactive complex composition having enhanced oxidative stability, emulsion stability, mineral rich transparent beverages and a wide range of functional health benefits. The composition may include as a base composition individual ingredients or a synergistic blend of mineral salts, Omega-3 rich oils, phospholipids, chitosan, and alpha-casein, beta-casein, kappa-casein or protein fragments, glycopeptides, phosphopeptides. The composition may optionally be further utilized for the prevention of hypercholesterolemia, bone (and teeth) mineral loss, treatment of mental health diseases, heart health, additional nutritional supplementation, and treatment of additional medical conditions.
Owner:TEXAS A&M UNIVERSITY
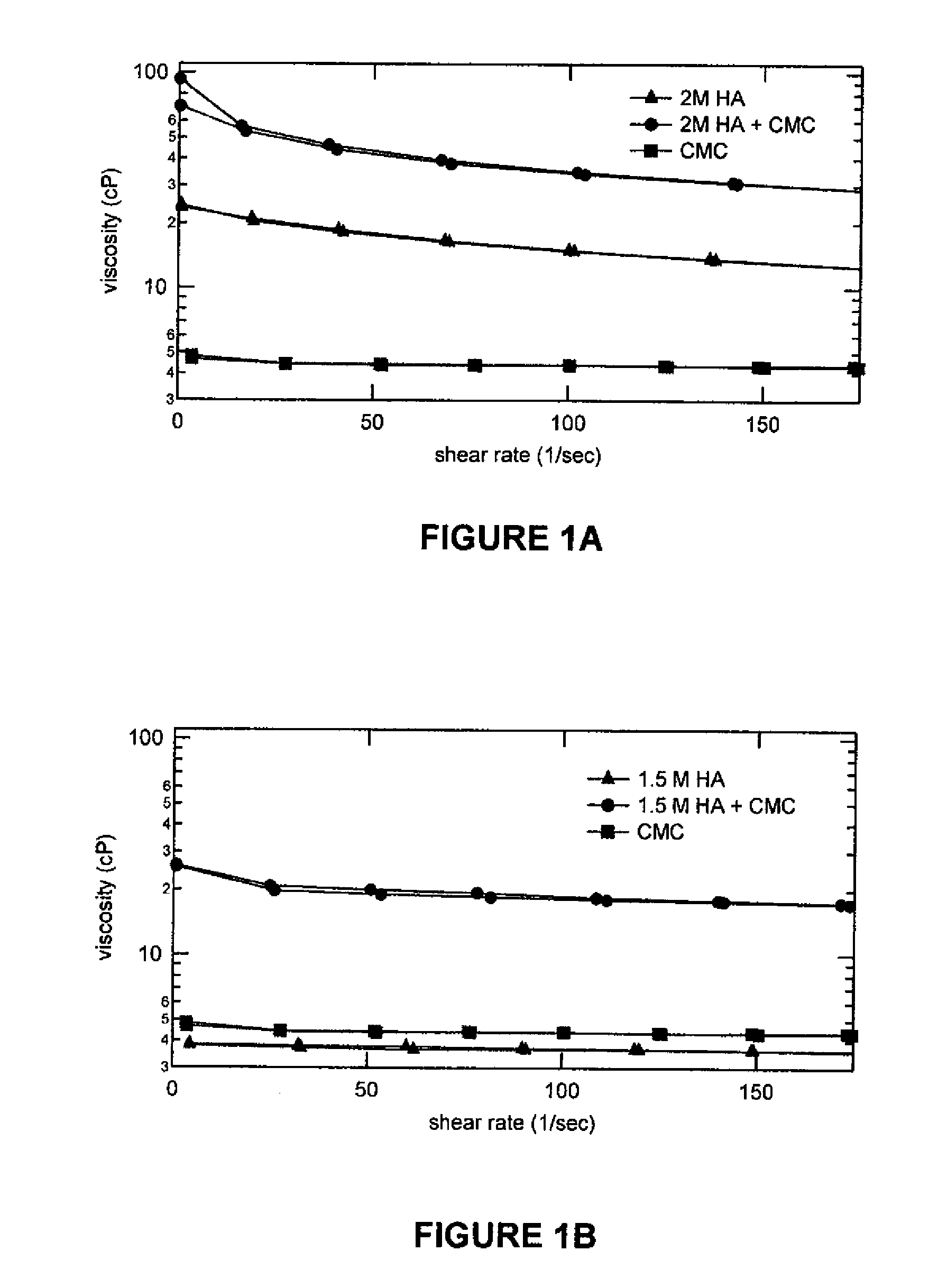
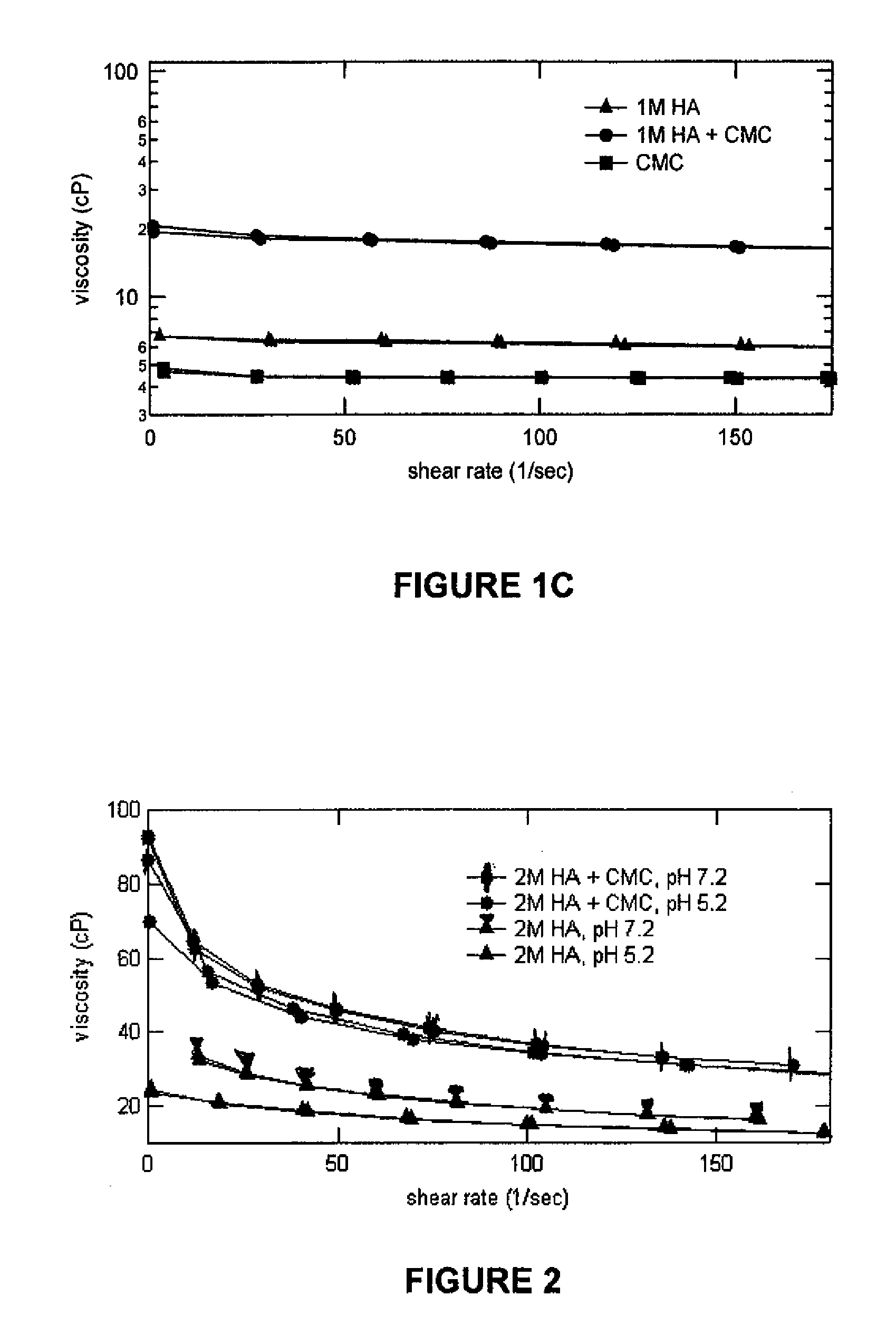
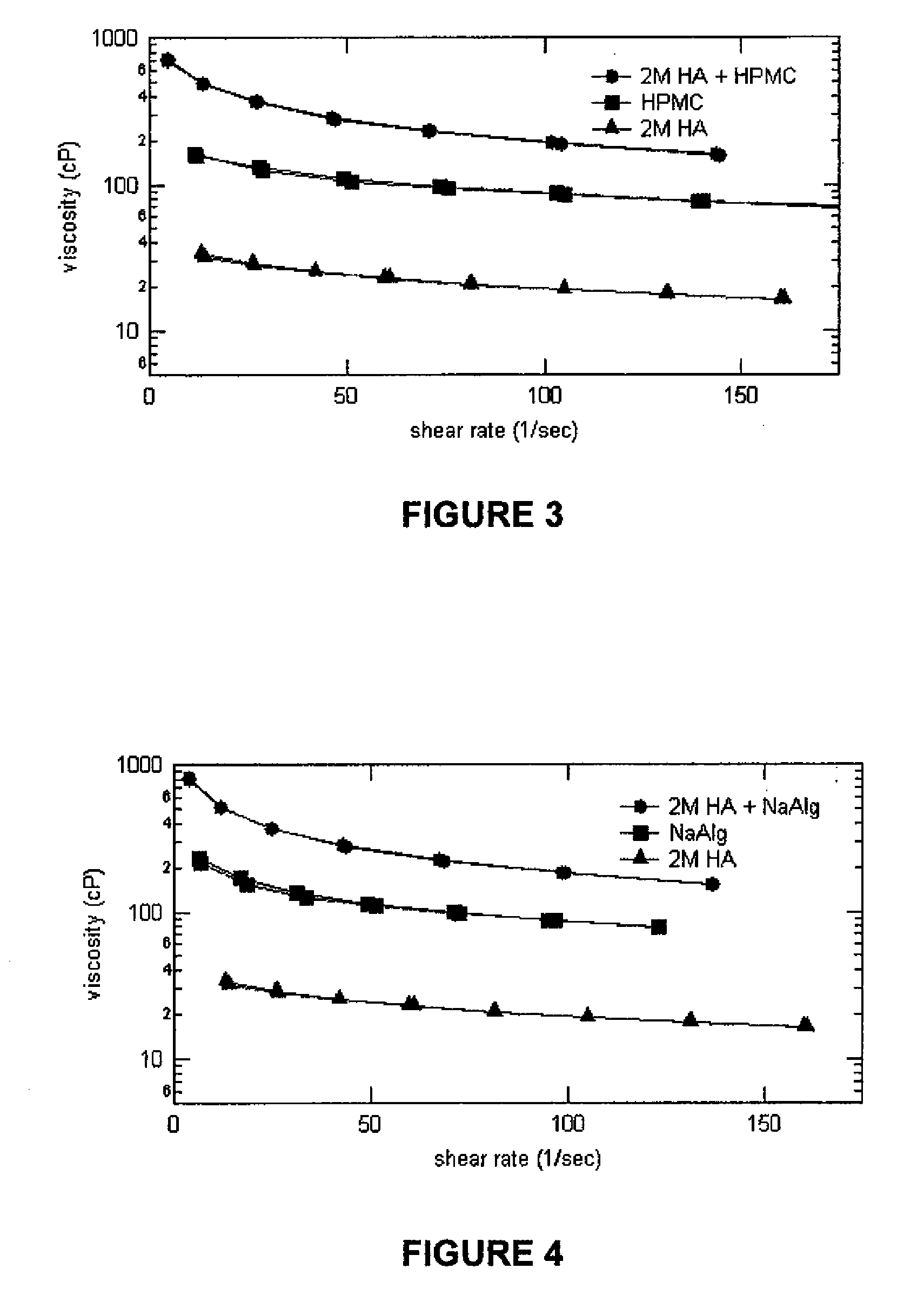
Stabilized Glycosaminoglycan Preparations and Related Methods
Compositions comprising a glycosaminoglycan (e.g., a hyaluronan, hyaluronic acid, hyaluronate, sodium hyaluronate, dermatan sulfate, karatan sulfate, chondroitin 6-sulfate, heparin, etc.) in combination with at least one component selected from; i) polyglycols (e.g., polyethylene glycol), ii) long chain hydroxy polyanionic polysaccharides (e.g., dextran, sodium alginate, alginic acid, propylene glycol alginate, carboxymethyl cellulose and carboxyethyl cellulose, hydroxyl ethyl starch, hydroxyl propyl methyl cellulose, hydroxy propyl ethyl cellulose, hydroxy propyl cellulose, methyl cellulose, polylysine, polyhistidine, polyhydroxy proline, poly ornithine, polyvinyl pyrolidone, polyvinyl alcohol, chitosan, etc.) and iii) long chain Nitrogen containing polymers (e.g., Polylysine, Polyvinylpyrrolidone, and polyvinyl alcohol). The invention also includes methods for using such compositions (e.g., as substance delivery materials, tissue fillers or bulking agents, as moistening or hydrating agents, etc.)
Owner:S K PHARMA INC
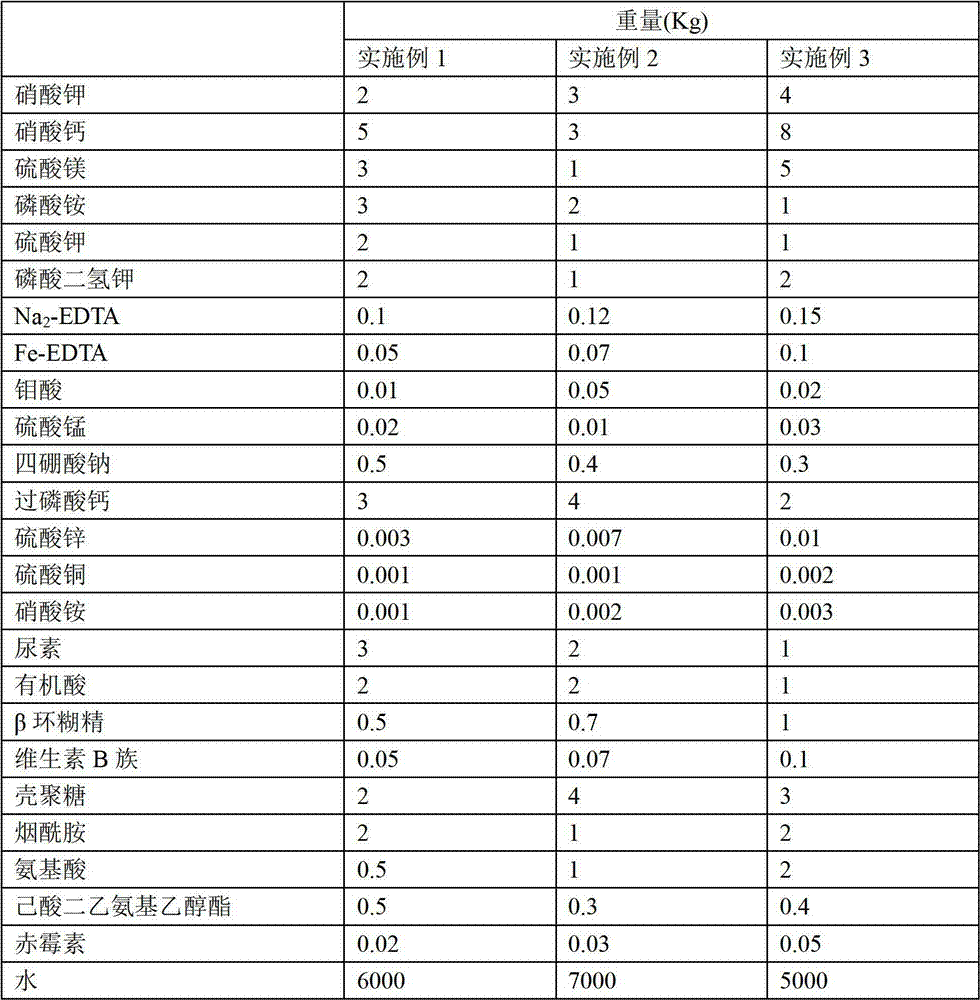
Plant nutrient solution for soilless culture of tomato
The invention provides a plant nutrient solution for soilless culture of tomato. The plant nutrient solution is prepared by the following raw materials in part by weight: 2 to 4 parts of potassium nitrate, 3 to 8 parts of calcium nitrate, 1 to 5 parts of magnesium sulfate, 1 to 3 parts of potassium phosphate, 1 to 2 parts of potassium sulphate, 1 to 2 parts of monopotassium phosphate, 0.1 to 0.15 part of Na2-EDTA, 0.05 to 0.1 part of Fe-EDTA, 0.01 to 0.05 part of molybdic acid, 0.01 to 0.03 part of manganese sulfate, 0.3 to 0.5 part of sodium tetraborate, 2 to 4 parts of superphosphate, 0.003 to 0.01 part of zinc sulfate, 0.001 to 0.002 part of copper sulfate, 0.001 to 0.003 part of ammonium nitrate, 1 to 3 parts of urea, 1 to 2 parts of organic acid, 0.5 to 1 part of beta cyclodextrin, 0.05 to 0.1 part of vitamin B, 2 to 4 parts of chitosan, 1 to 2 parts of nicotinamide, 0.5 to 2 parts of amino acid, 0.3 to 0.5 part of diethyl aminoethyl hexanoate, 0.02 to 0.5 part of gibberellin, and 5,000 to 7,000 parts of water. According to experiment, the plant nutrient solution provided by the invention can be used for carrying out soilless culture of tomato, and the tomato has high plant height, thick stem, high cluster and high output.
Owner:山西田森杜氏番茄科技有限公司
Bactericides
An object of the present invention is to provide a bactericide (and a fungicide) that is highly safe and has strong bactericidal power even when used in low concentration, and that can be used repeatedly in a sterilizing process.The object can be achieved by the bactericide (or the fungicide) comprising a polar solvent extract of leaves of eucalyptus plants and chitosan. The polar solvent is preferably chosen from the group consisting of lower alcohols and glycols.
Owner:OJI PAPER CO LTD
Hemostatic agent for topical and internal use
ActiveUS20050240137A1Good hemostasisRapid and effective hemostasisSuture equipmentsSurgical adhesivesVeinDermatology
Owner:LOMA LINDA UNIV MEDICAL CENT
Hemostatic compositions, assemblies, systems, and methods employing particulate hemostatic agents formed from hydrophilic polymer foam such as chitosan
ActiveUS20070021703A1Safe and effective deliveryIncrease ratingsPharmaceutical delivery mechanismAbsorbent padsParticulatesPolymer
Improved hemostatic agents take the form of granules or particles that can be used to stanch, seal, or stabilize a site of hemorrhage, including a noncompressible hemorrhage.
Owner:TRICOL BIOMEDICAL INC
Enteric-coated multilayer encapsulated probiotic microcapsule and preparation method thereof
InactiveCN1969889AGrowth inhibitionPromote formationMetabolism disorderBacteria material medical ingredientsAcid-fastSolubility
The invention discloses an enteric-solubility multilayer encysted bacterium microcapsule and making method in the biological agent technical domain, which is characterized by the following: adopting sodium alginate, calcium chloride and chitose as clad material of microcapsule; making lactic acid bacteria or bifidobacteria as core-clad material; proceeding ionic exchange for sodium alginate and calcium chloride; forming second clad on the surface of calcium alginate through different isoelectric points of calcium alginate and chitose; freezing at low temperature to obtain the product.
Owner:JINAN SYNBIOTICS BIOENG
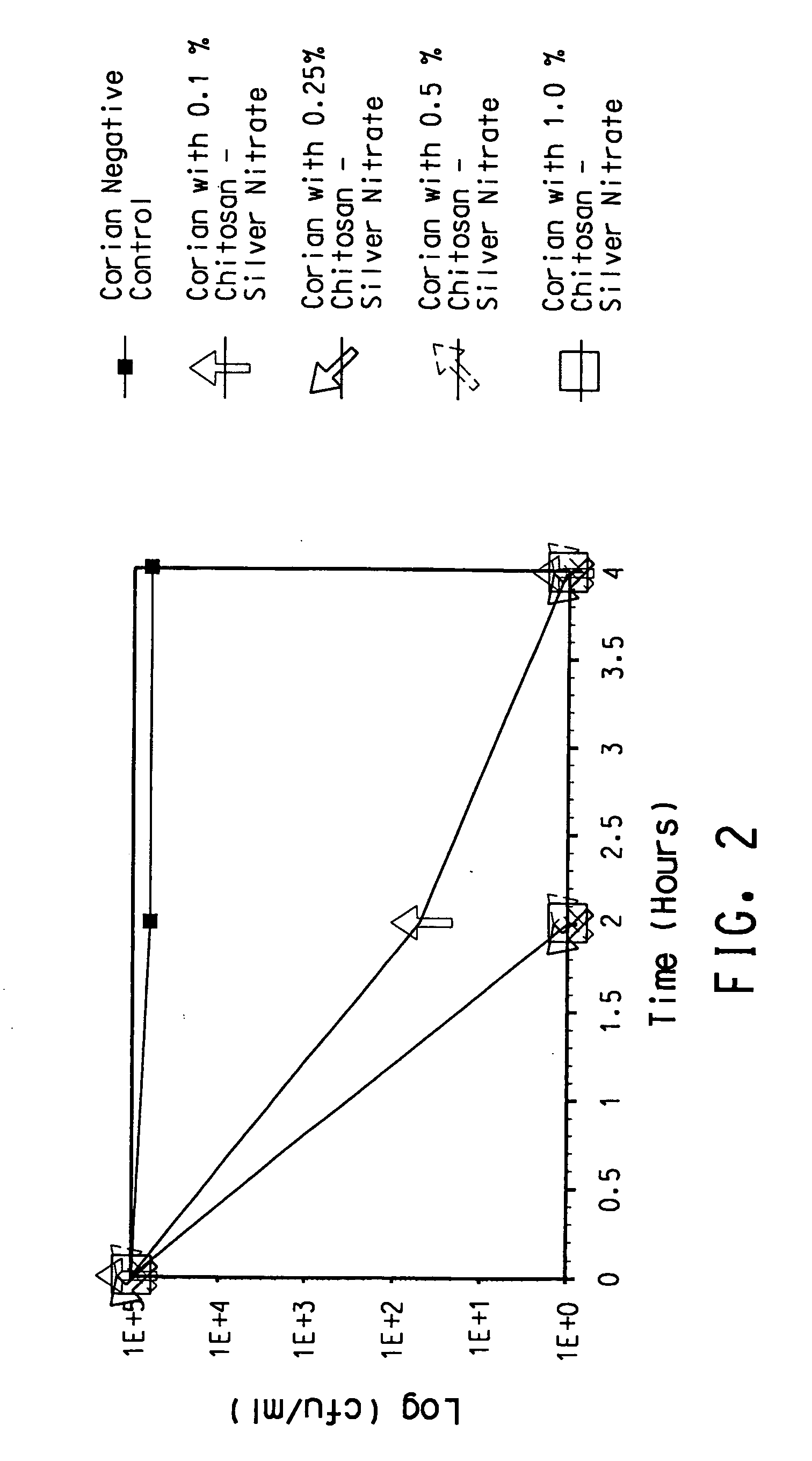
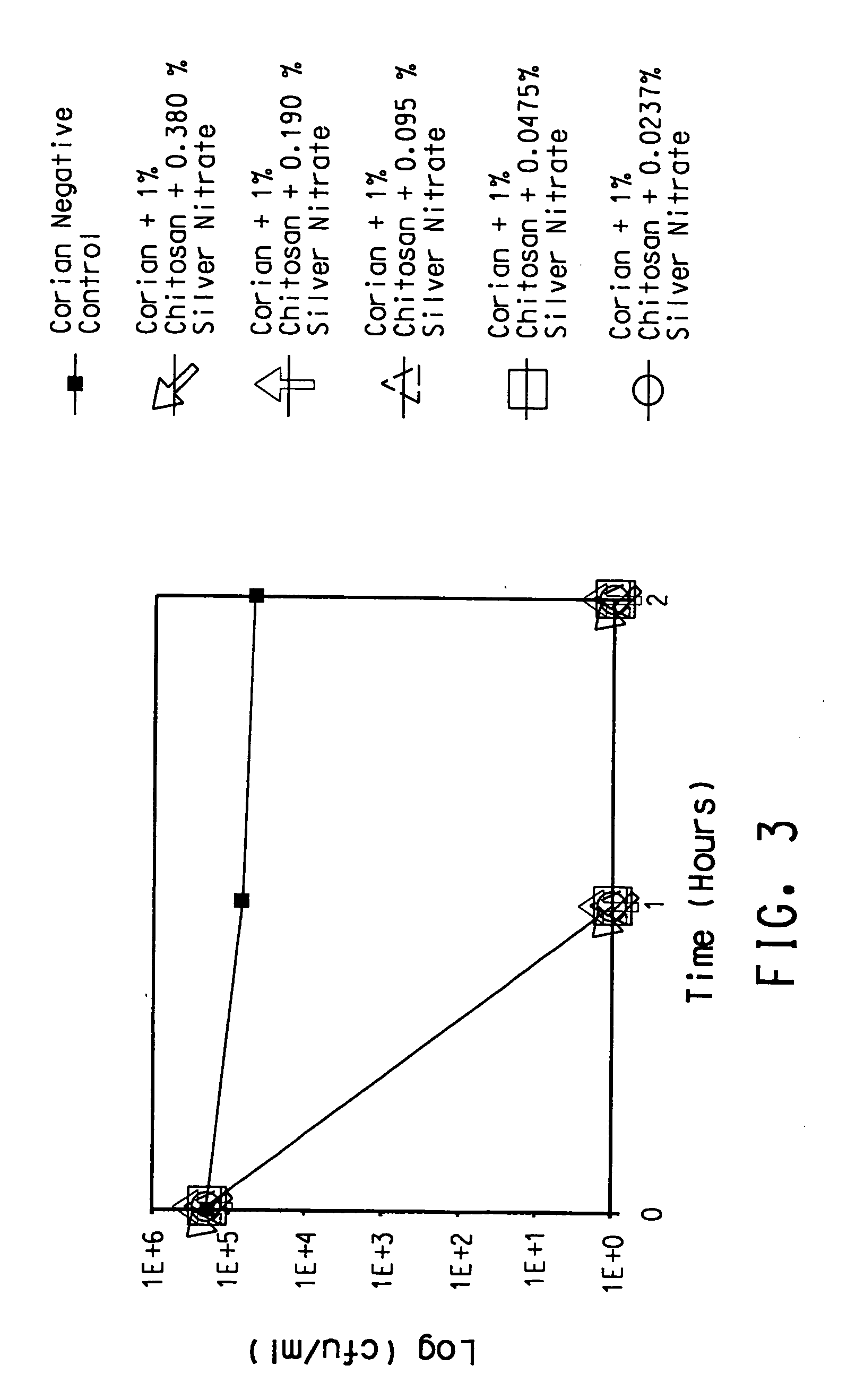
Antimicrobial solid surface materials containing chitosan-metal complexes
A solid surface material with an antimicrobial agent in a thermoset and / or thermoplastic resin matrix where the antimicrobial agent comprises a chitosan-metal complex.
Owner:EI DU PONT DE NEMOURS & CO
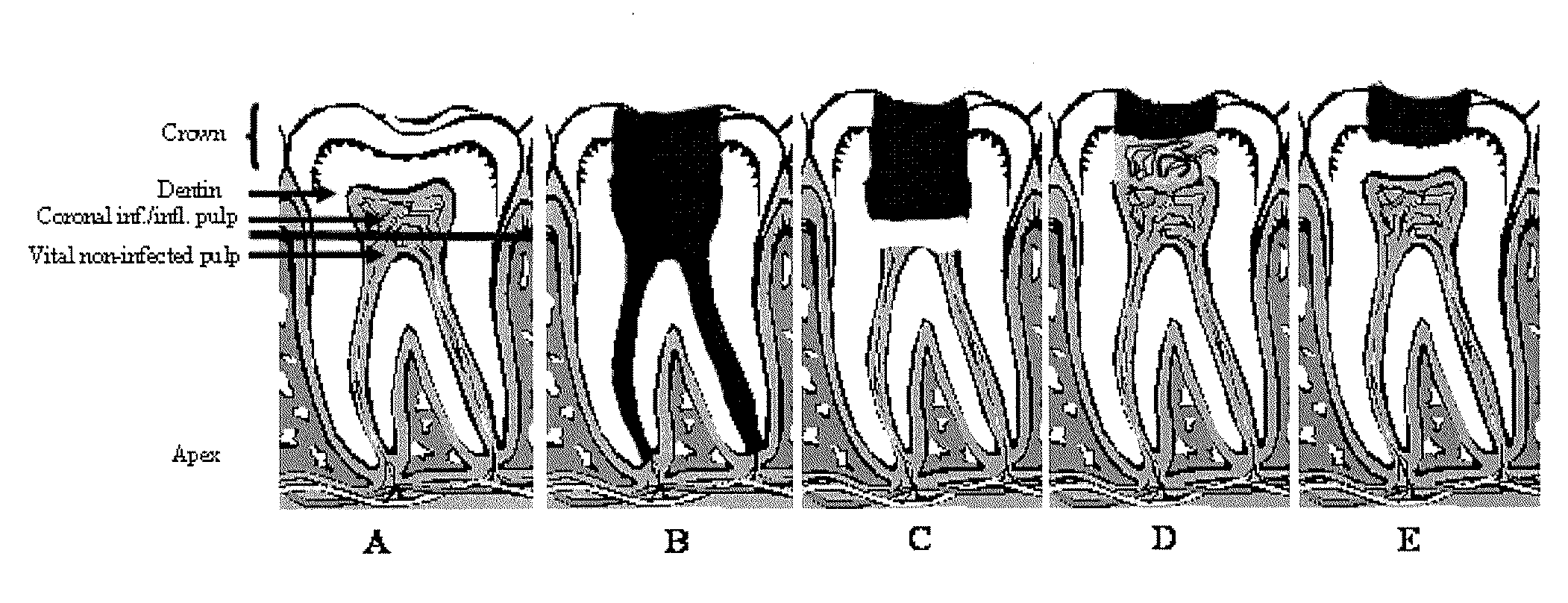
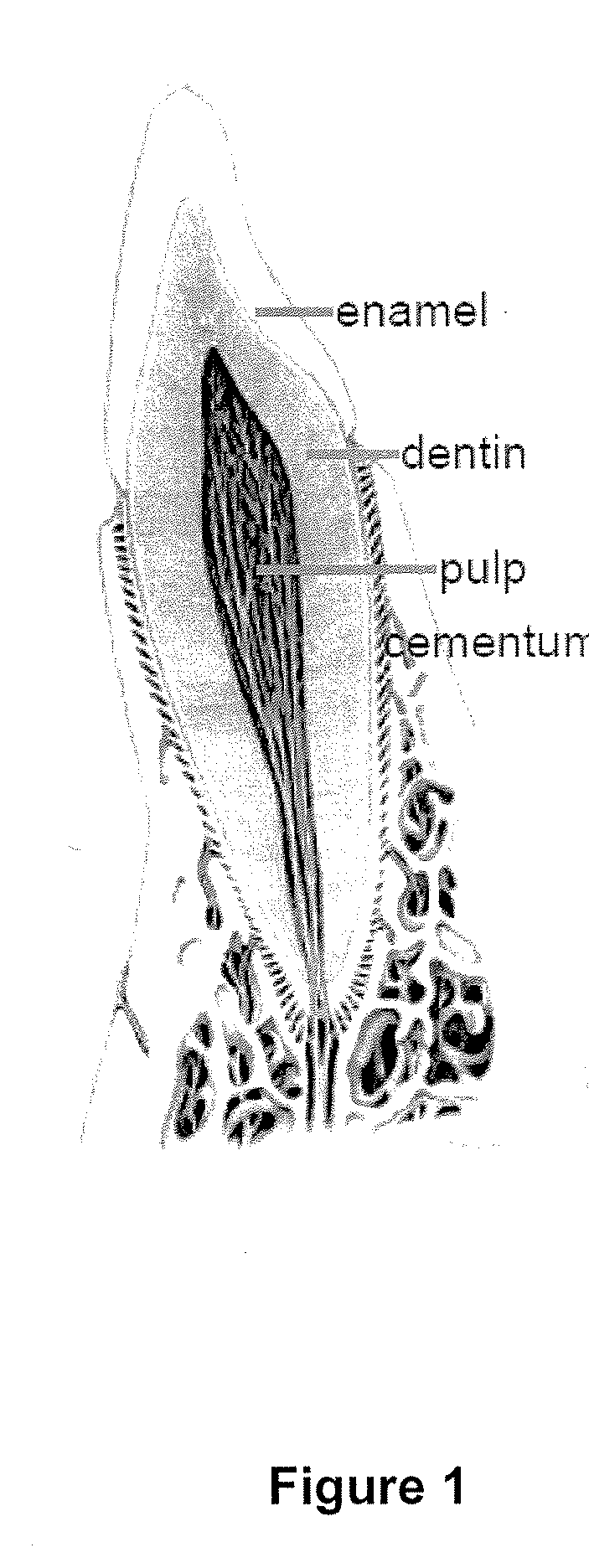
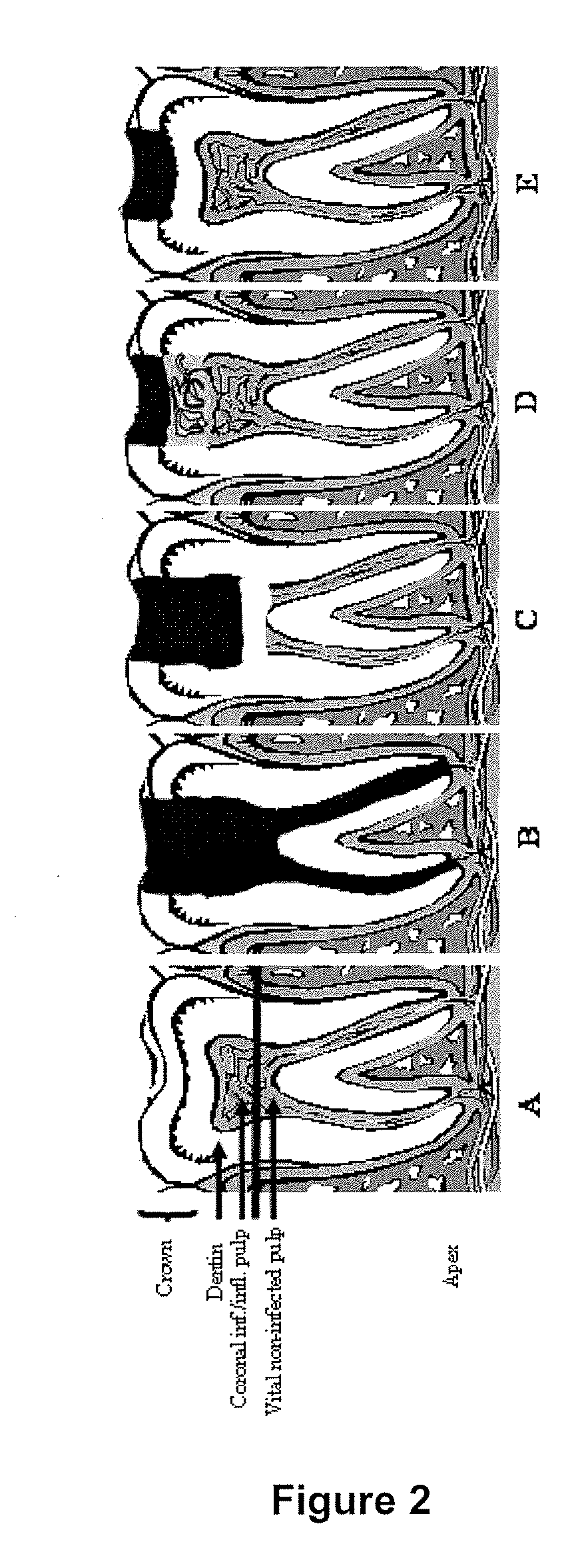
Compositions and methods for treating pulp inflammations caused by infection or trauma
The present disclosed subject matter relates to methods and compositions for restoring a diseased or damaged tooth such that infection is inhibited or eliminated and pulp regeneration is facilitated. The disclosed subject matter also includes a composition comprising a physiologically acceptable matrix seeded with pulp cells. The matrix can be capable of being injected into the pulp chamber of a tooth. In some embodiments, the matrix of a composition includes a hydrogel (e.g., collagen, chitosan, alginate, MATRIGEL™, gelatin, JELL-O®, fibrin), a mesh (e.g., polylactide-coglycolide (PLGA) mesh, polylactide (PLA) mesh, or polyglycolide (PGA) mesh, a cross-linked fiber mesh, a nanofiber mesh, a mesh fabric, biodegradable polymer mesh), a microsphere (biodegradable polymer microsphere, a hydrogel microsphere), or a combination of any of the foregoing. In yet other embodiments, the matrix includes a nanofiber, an artificial three-dimensional scaffold material, or a synthetic three-dimensional scaffold material.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Dressing material containing medicine chitoholosida and its preparation method
InactiveCN1579559AHas therapeutic effectSustained releaseAbsorbent padsBandagesSolid componentPhosphate
The invention produces polyethylene alcohol hydrogel dressing containing medicine and chitosan with 60Co gamma-radial or high energy electron beam radial cross linking. Additional, adds in some humectant, plasticizer, medicine, the solvent is the secondary distilled water, physiological saline or phosphate neutral buffer liquid. The product can release medicine slowly and has natural amylose chitosan with biology sterilization activity, it has active sterilization function, at the same time, it has high water quantity, and good water reserving performance, the mechanical intensity is moderate, and the light penetration and air penetration are excellent. It can accord the demands for curing each kind of wound. It can used as the permanent dressing for light skin injuries, and it also can be applied to the temporally close of severe skin organization wound or burn wound.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
Nanoparticles for protein drug delivery
InactiveUS20090004266A1Easy to transportReduce resistancePowder deliverySaccharide peptide ingredientsActive agentNanoparticle
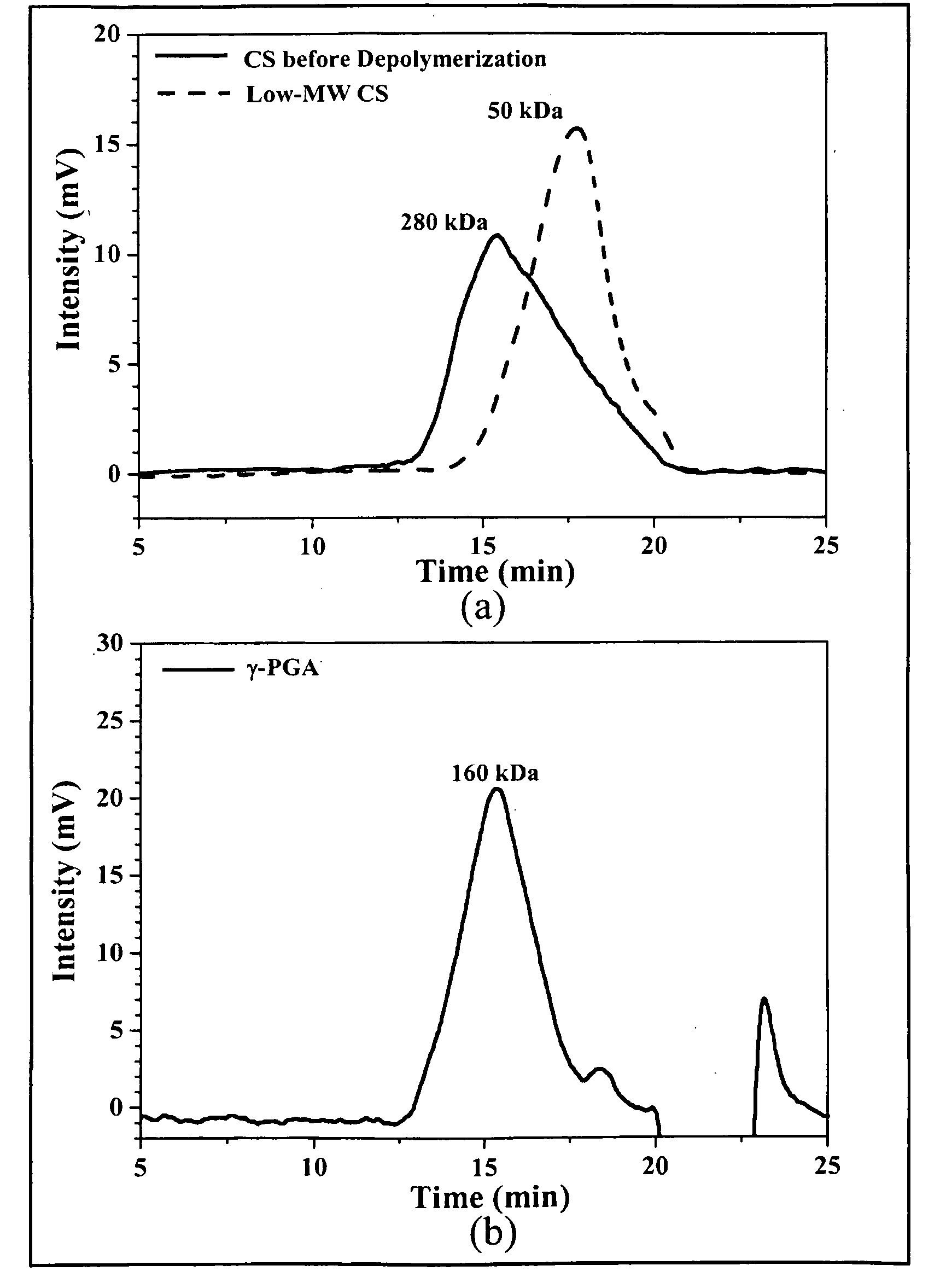
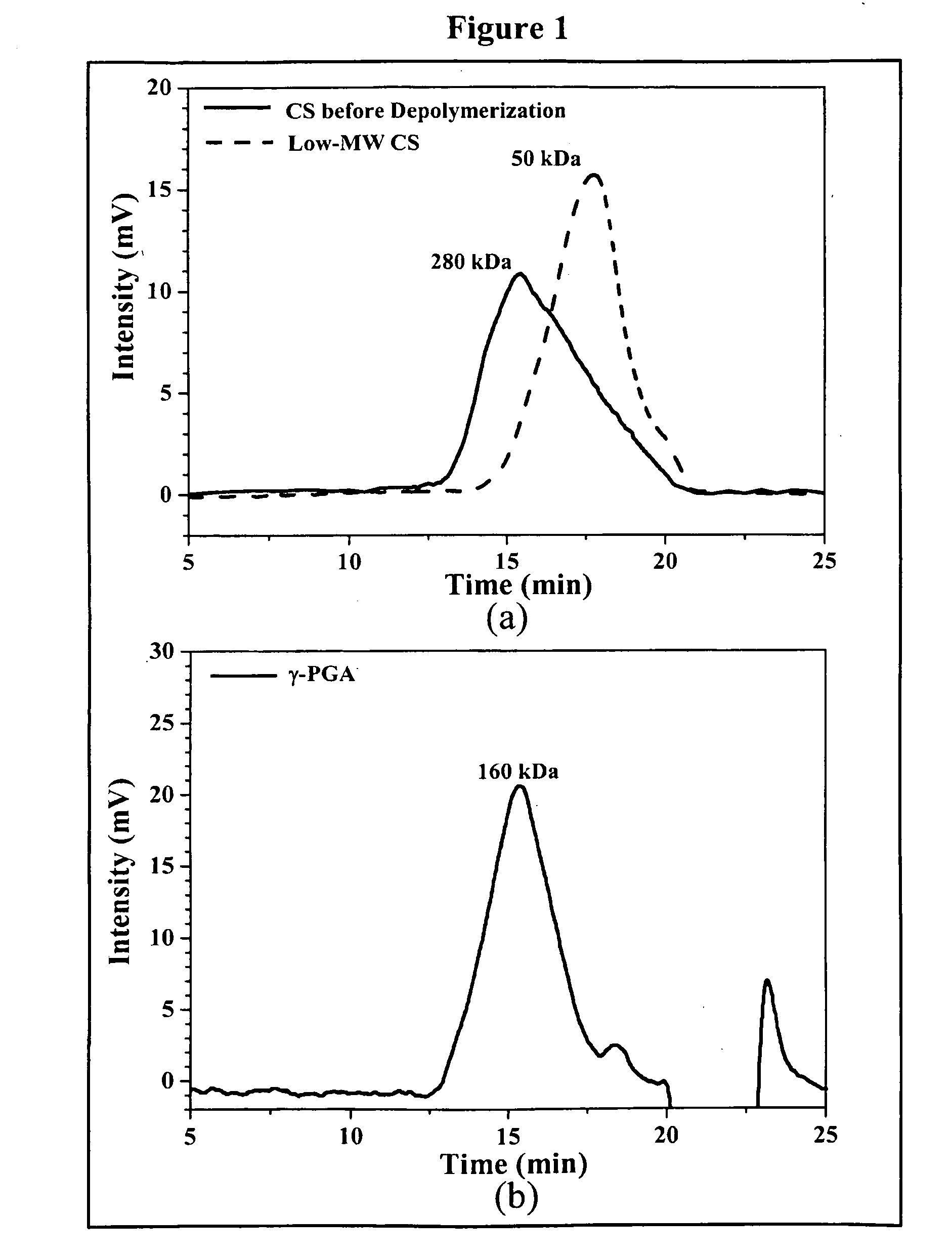
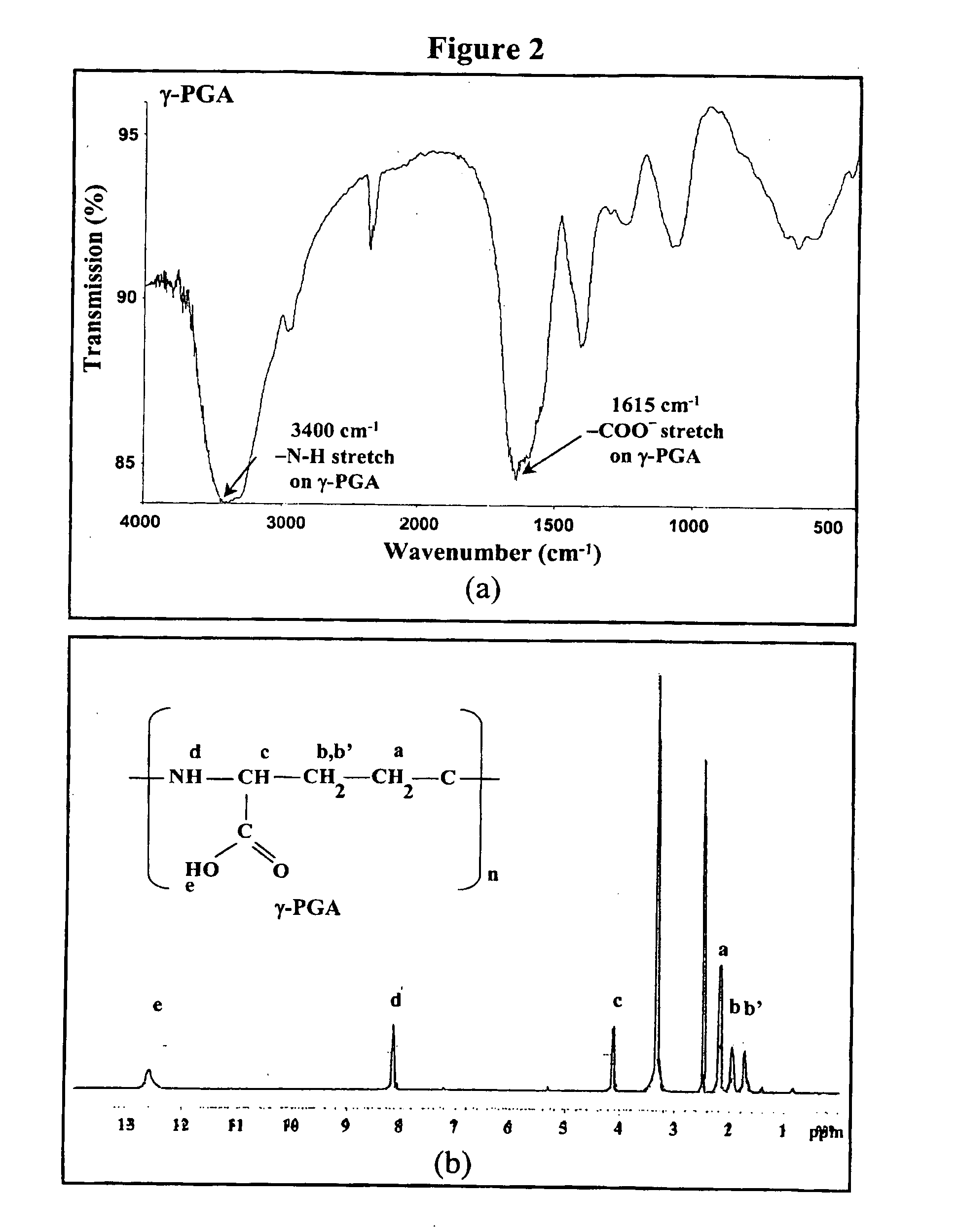
The invention discloses a chewable composition for oral delivery of bioactive agents having nanoparticles that are composed of chitosan, poly-glutamic acid, and at least one protein drug or bioactive agent characterized with a positive surface charge and their enhanced permeability for paracellular protein drug and bioactive agent delivery.
Owner:NANOMEGA MEDICAL CORP
Ionic cross-linking of ionic cotton with small molecular weight anionic or cationic molecules
InactiveUS7201778B2Improved wrinkle recovery anglePromote recoveryWrinkle resistant fibresDetergent compounding agentsCelluloseFiber
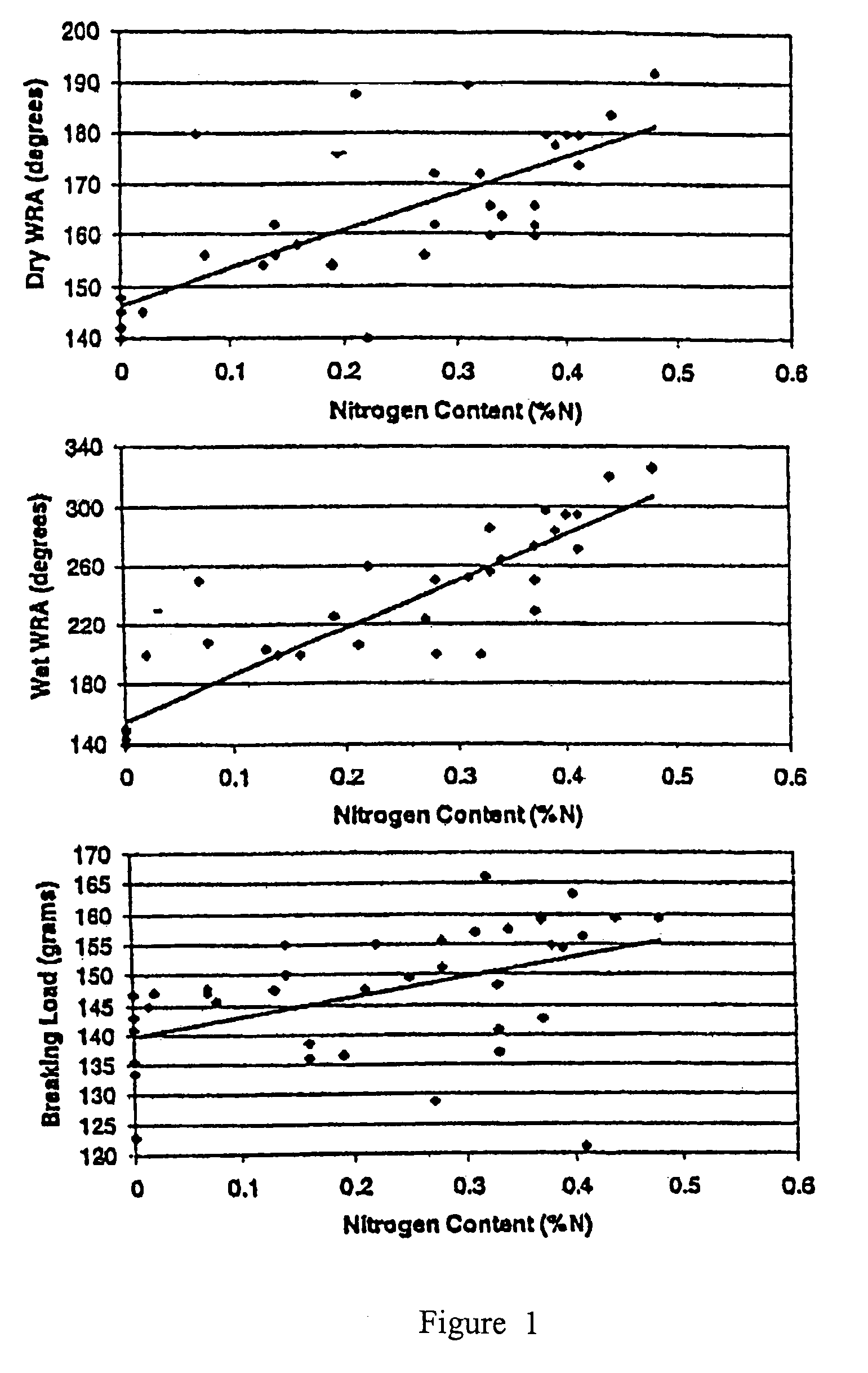
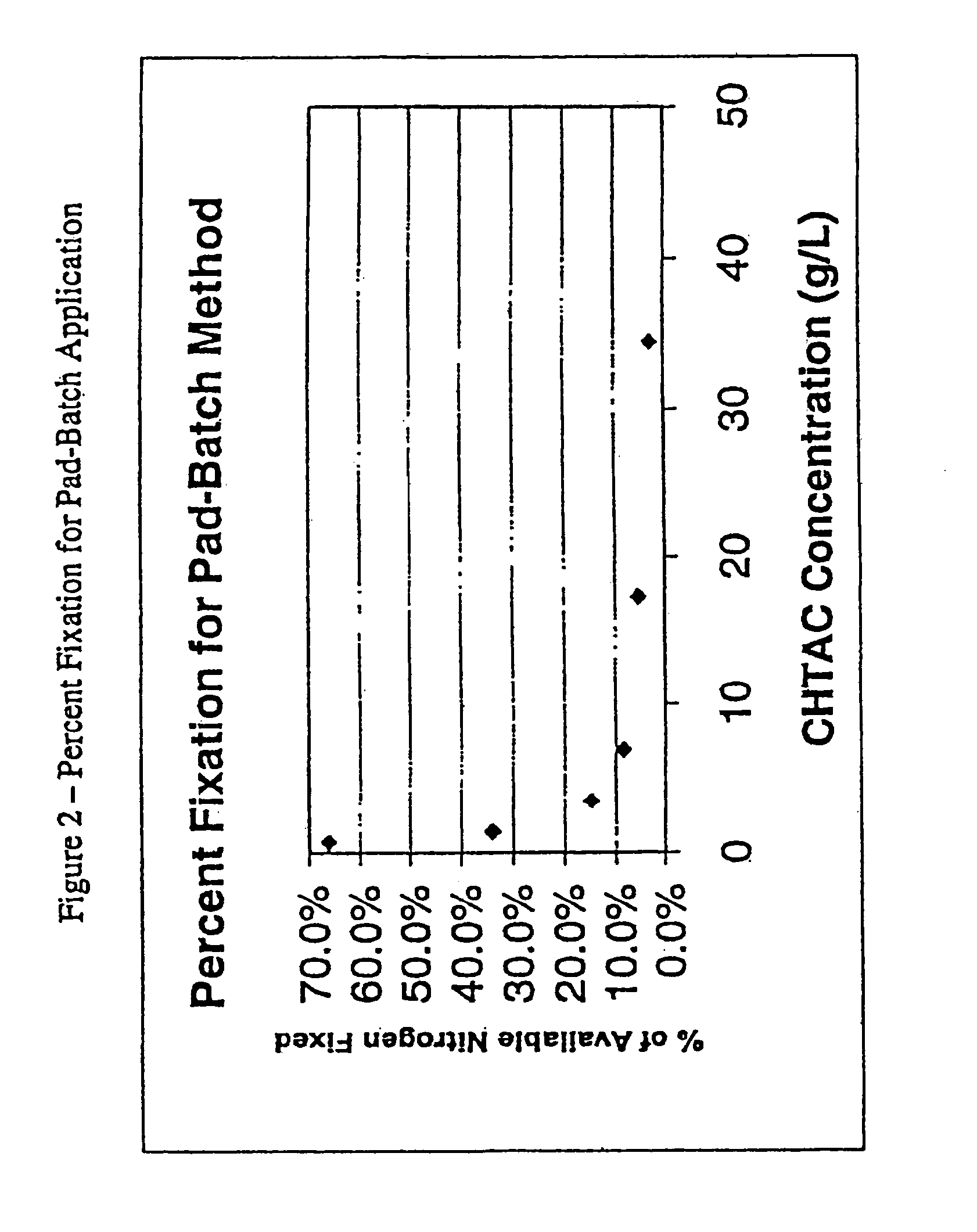
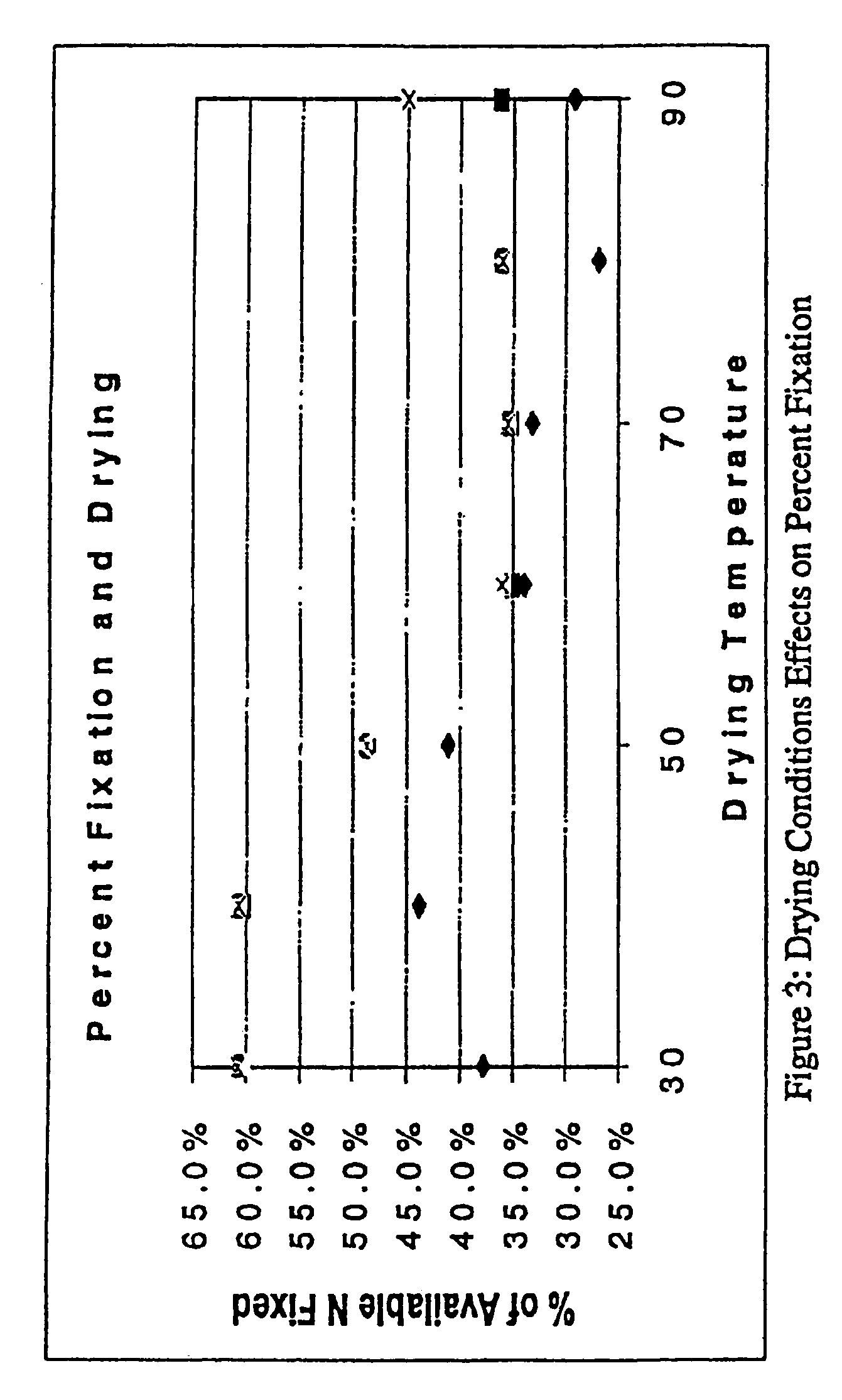
A process for producing an ionic crosslinked fibrous material, such as a cellulosic fabric, paper, or other substrate, wherein the ionic crosslinked fiber exhibits an increased wrinkle resistance angle. A process for producing a cationized chitosan, wherein the cationized chitosan exhibits cationization at the C6 and ring hydroxyl sites and the reactivity of the ring NH2 sites is preserved. A process for applying a polycation to an anionic fibrous material to form an ionic crosslinked fibrous material. A process for producing a cationized fibrous material, wherein the process is performed as a pad-batch process, an exhaust fixation process, a pad-steam process, or a pad-dry-cure process.
Owner:NORTH CAROLINA STATE UNIV
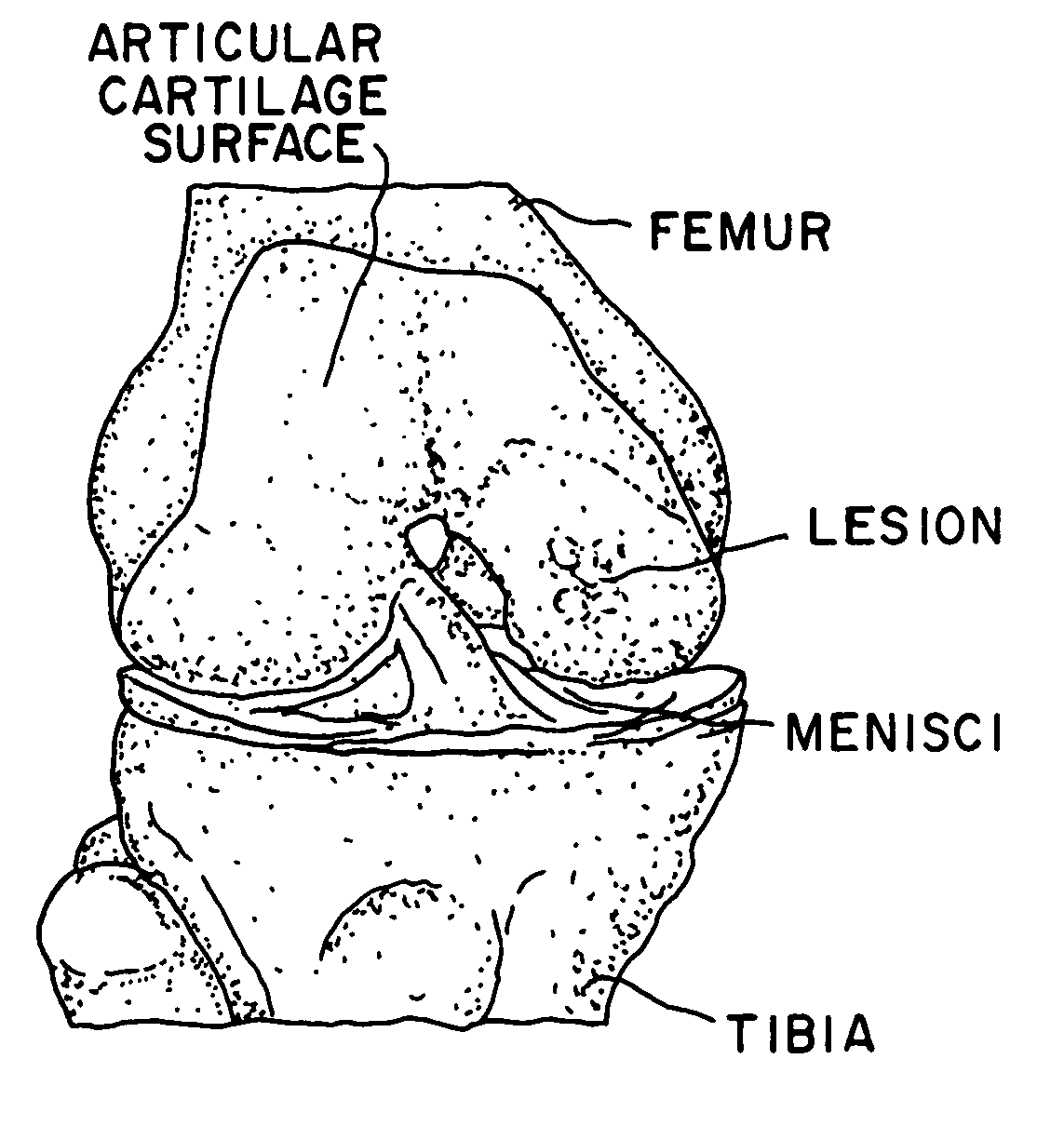
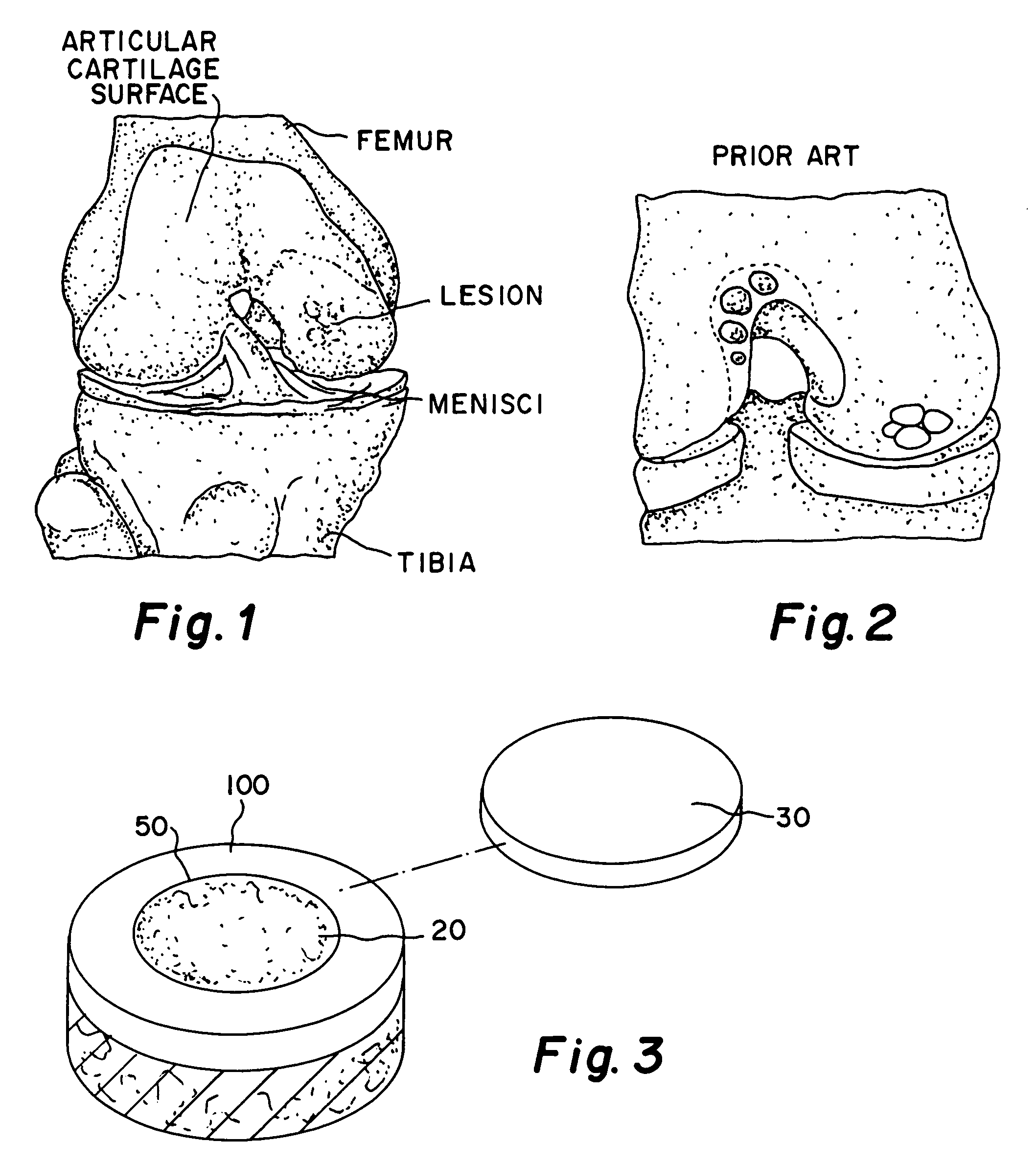
Glue for cartilage repair
ActiveUS7067123B2Promote migrationIncreased proliferationBiocidePeptide/protein ingredientsMedicineBone marrow cell
The invention is directed toward a sterile cartilage defect implant material comprising milled lyophilized allograft cartilage pieces ranging from 0.01 mm to 1.0 mm in size in a bioabsorbable carrier taken from a group consisting of sodium hyaluronate, hyaluronic acid and its derivatives, gelatin, collagen, chitosan, alginate, buffered PBS, Dextran or polymers with allogenic chondrocytes or bone marrow cells in an amount exceeding the natural occurrence of same in hyaline cartilage and adding a cell growth additive.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
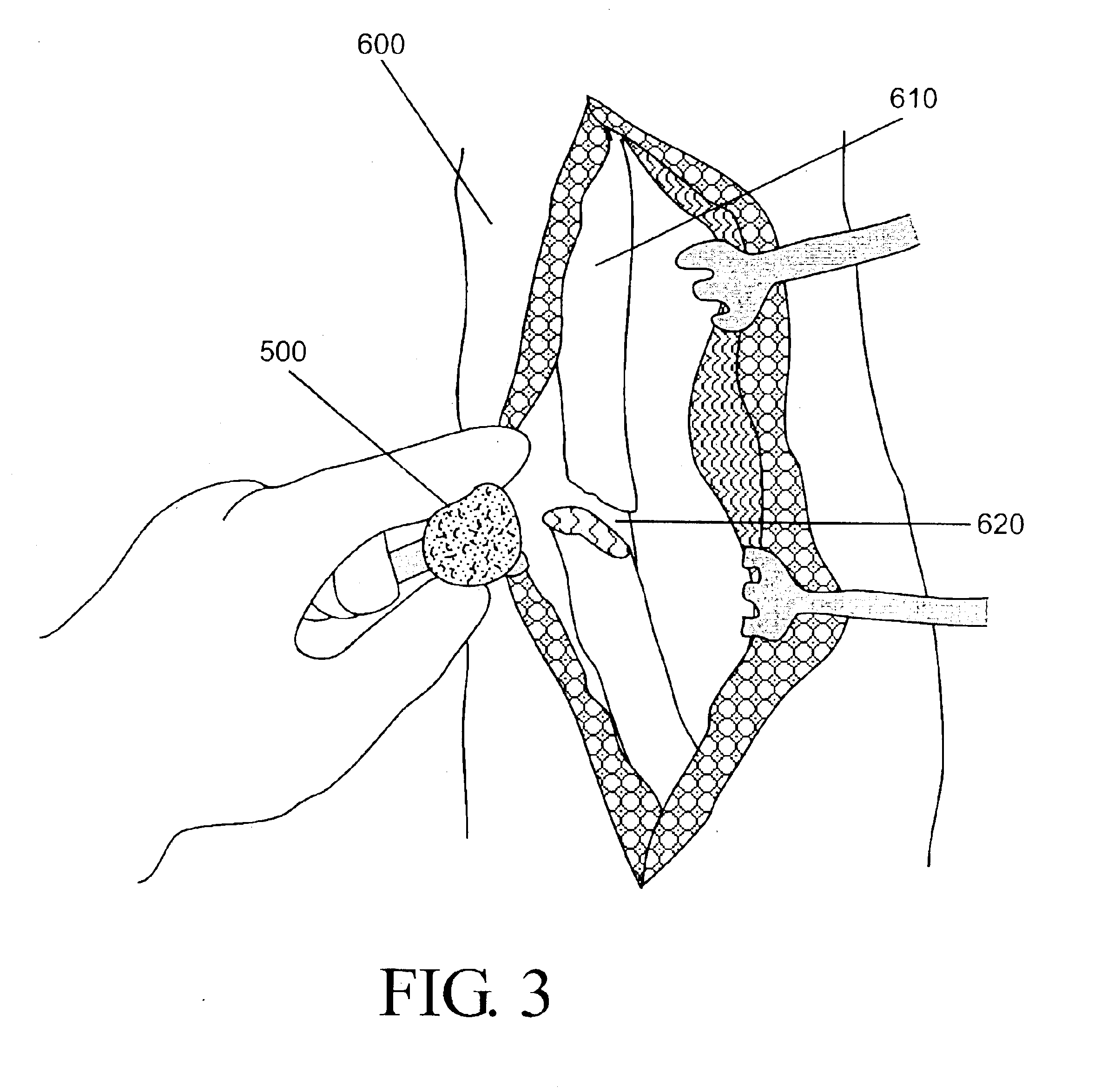
Devices and methods for promoting the formation of blood clots in esophageal varices
InactiveUS20070167971A1Inhibit injectionPromote formationBalloon catheterSurgeryThrombusSilicon dioxide
A device for promoting the clotting of blood in body cavities includes a flexible body portion; an expandable member located on the flexible body portion; and a blood clotting material attached to the expandable member. When used, insertion of at least a portion of the blood clotting material into the body cavity causes at least a portion of the blood clotting material to contact blood emanating from a bleed site. Methods of providing therapies to tube-shaped organs include the steps of providing suitable devices having expansion capabilities, positioning the devices at the appropriate bleed sites, and expanding the devices to cause blood clotting materials to contact the bleed sites. Materials that may be used as the blood clotting material include zeolites, molecular sieve materials, diatomaceous earth, clay, silica-based materials, oxidized cellulose, carboxymethyl cellulose, bioactive glass, biological hemostats, chitosan, and combinations of the foregoing.
Owner:TELEFLEX LIFE SCI LTD
Nanoformulation and methods of use of thyroid receptor beta1 agonists for liver targeting
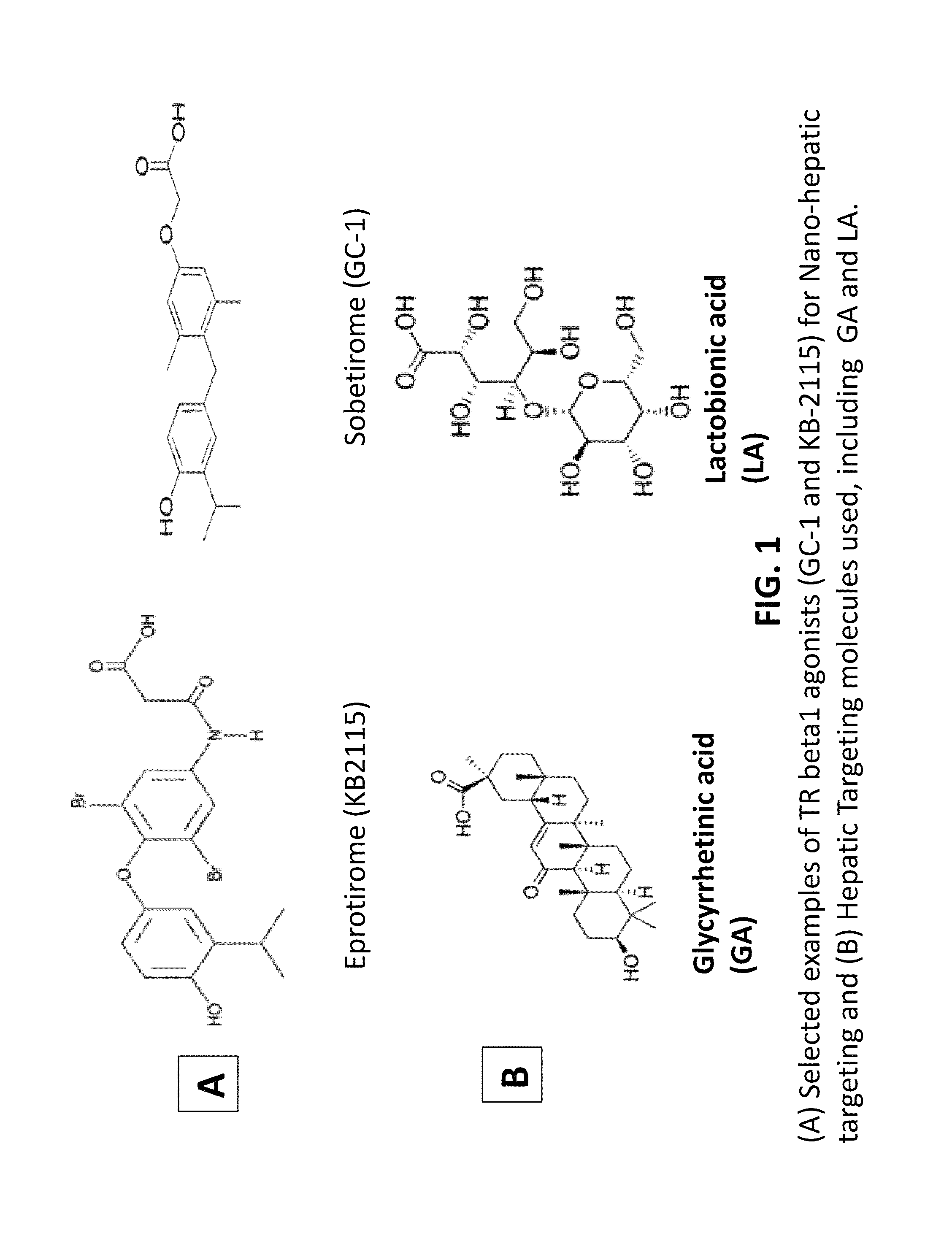
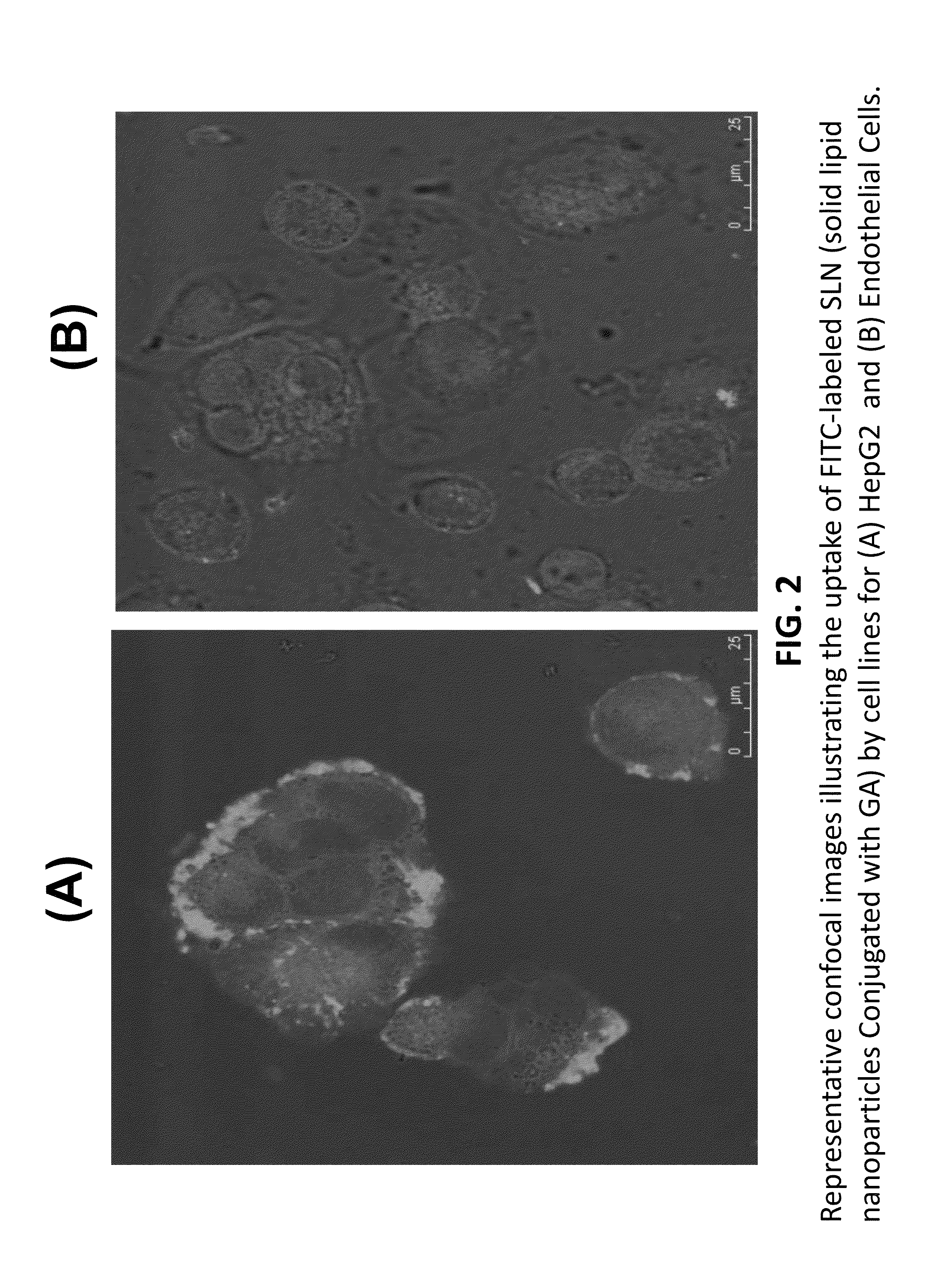
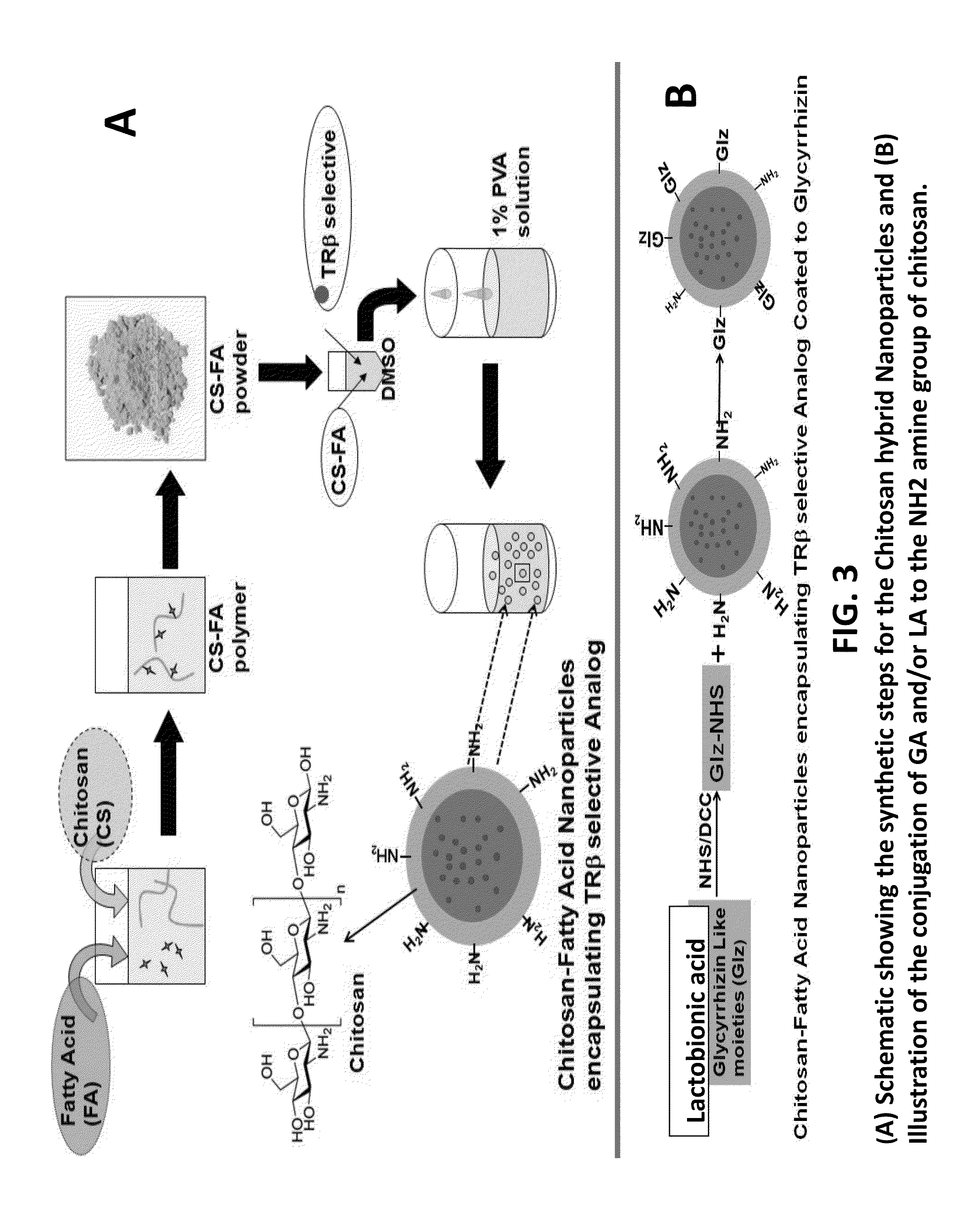
A composition and an associated method for hepatic targeted delivery of thyroid receptor beta1 (TRβ1) agonist to a liver of a subject. The composition includes hydrophobic nanoparticles, a liver targeting moiety exterior to each nanoparticle and covalently bonded to each nanoparticle, and at least one TRβ1 agonist encapsulated within each nanoparticle. The nanoparticles include chitosan hybrid nanoparticles, amine-modified PLGA nanoparticles, solid lipid nanoparticles, and combinations thereof. The liver targeting moiety includes Glycyrrhetinic acid (GA), Lactobionic acid (LA), or combinations thereof.
Owner:MOUSA SHAKER A
High SPF transparent or translucent, cytoprotective, biodegradable, UV radiation resistant compositions
ActiveUS20080233060A1Protect the skinEliminating possible endocrine disruption responseCosmetic preparationsToilet preparationsCarrageenanPhospholipid
A composition comprising purified water using ozonation, ionization, or distillation or any combination thereof wherein alcohol may be substituted for, or combined with water at least one emollient including but not limited to chitosan, and aloe vera gel, individually or in any combination; an oil component with spf boosting agents including but not limited to; ethyl macadamiate, non-toxic silicone oil and essential oils, butter milk, waxes impregnated with inorganic sun-block or sunscreen agent and organic / inorganic micronized particles, wood powder and bentonite clay, keratin, either individually or in any combination; at least one inorganic sun-block or sunscreen agent including any metal oxide, glass microsphere, silica and silica compound, and optionally metal oxide pigments with particles that are micronized, submicronized, nanoparticle sized, or otherwise individually or in any combination that can be homogenized in either a water phase, a water-aloe phase, an oil phase or any phase of said composition; at least one emulsifier wherein said emulsifier includes but is not limited to a phospholipid and / or liposome or an aloe vera gel or an ester of coconut oil individually or in any combination, for emulsifying the water, water-aloe, or oil phase in combination with an homogenizer; where any of components are preferably mixed with an homogenizer and where an appropriate thickening agent including but not limited to xanthan gum, carageenan, either individually or in any combination is added as required.
Owner:GRUNE GUERRY L
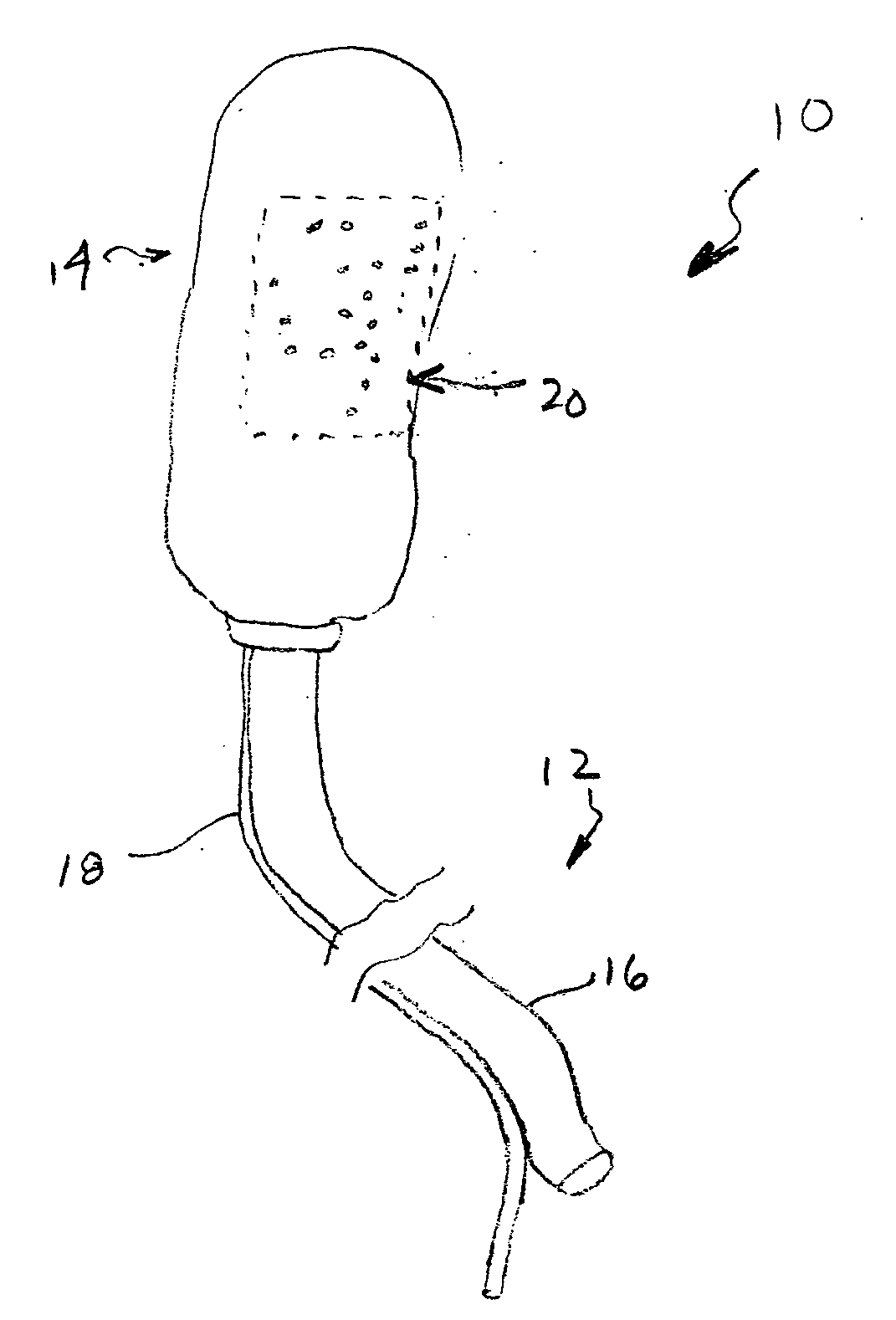
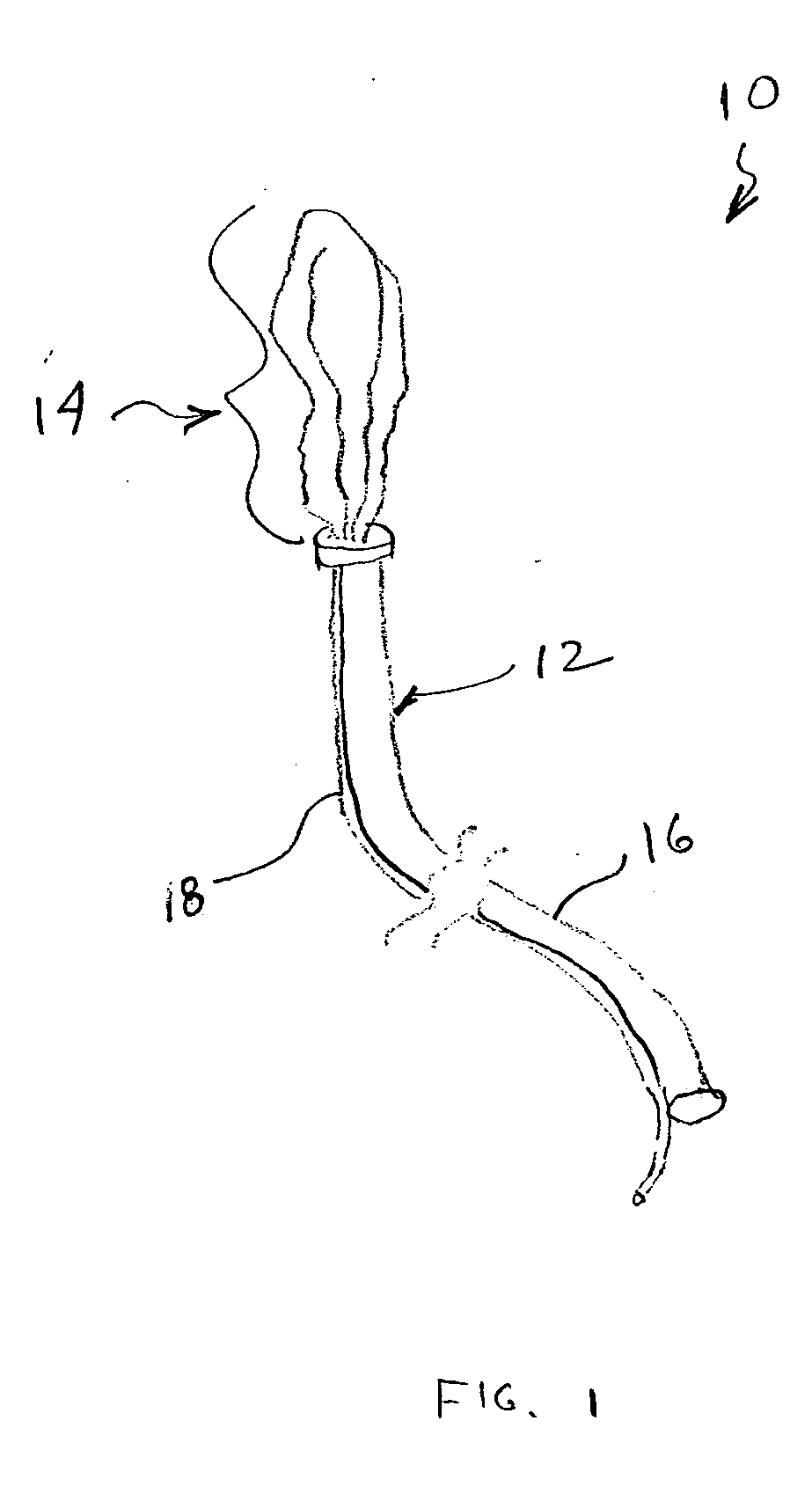
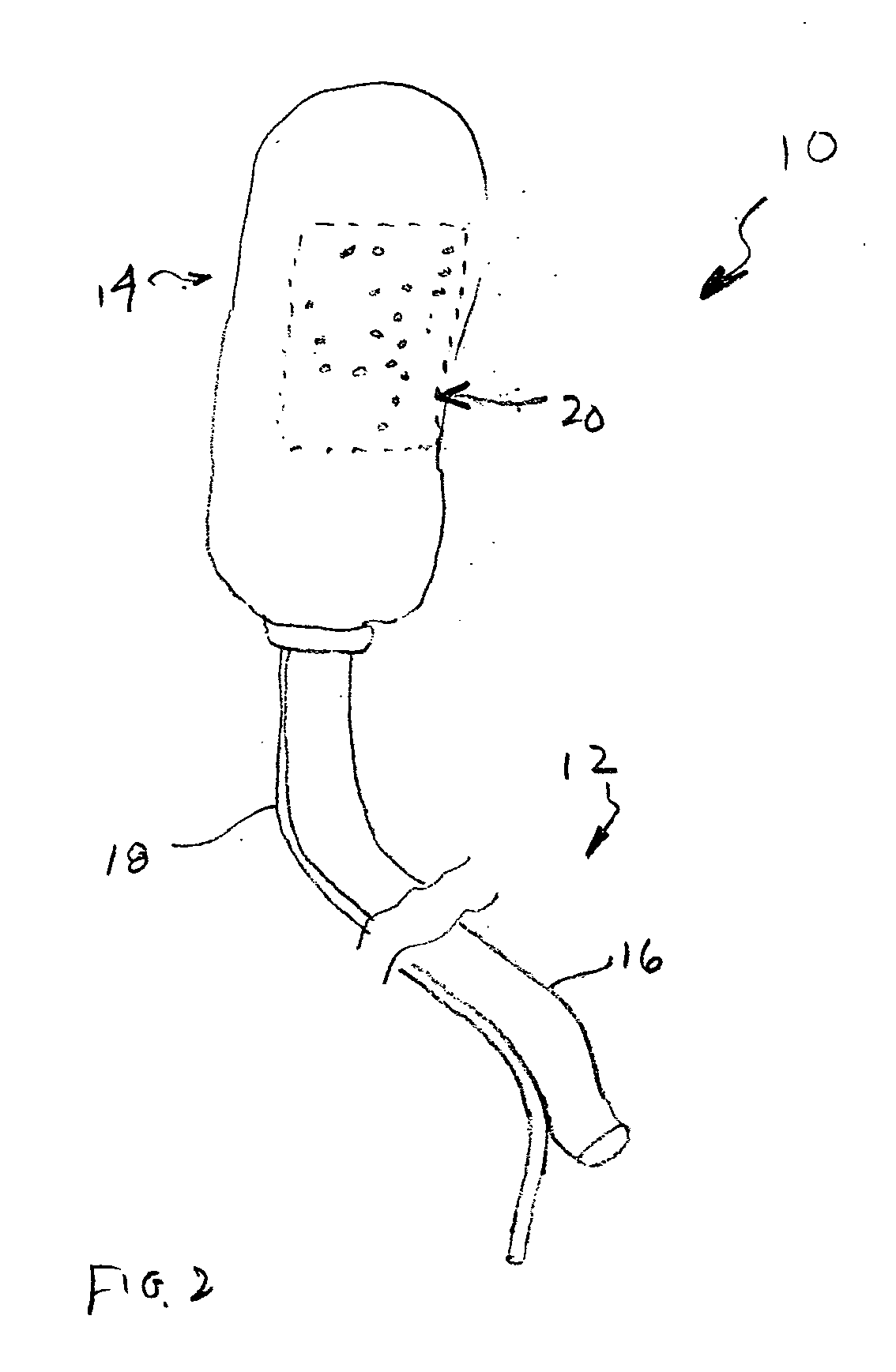
Chitosan wound dressing
InactiveUS20070237811A1Stop the bleedingBiocideOrganic active ingredientsGel preparationWound dressing
A composition is described in which chitosan is prepared in a foamed gel that may be layered onto a suitable backing for use as a wound dressing, or the gel may be directly applied to wounds to effect hemostatic activity as a result of the action of the chitosan. The composition of the foamed chitosan gel has added to it medicaments, which include antimicrobial agents, in order to reduce the risk of microbial infections in wounds where hemostatic agents including the chitosan foamed gel preparation can be applied to effect hemostasis.
Owner:SCHERR GEORGE H
Hair treatment compositions containing N-hydroxy-alkyl-O-benzyl chitosans and methods of using same
InactiveUS20050226838A1EffectiveMaintain good propertiesCosmetic preparationsHair cosmeticsMedicineAdditive ingredient
The hair treatment composition contains preferably from 0.01 to 20 percent by weight of at least one N-hydroxyalkyl-O-benzyl chitosan and from 0.01 to 20 percent by weight of at least one other hair treatment effective ingredient. The at least one N-hydroxyalkyl-O-benzyl chitosan has at least one hydroxylalkyl group, preferably a hydroxyethyl, hydroxypropyl or hydroxybutyl group, and has from 2 to 20 carbon atoms. Various methods of treating hair with hair treatment compositions containing one or more of the N-hydroxyalkyl-O-benzyl chitosans are described.
Owner:WELLA AG
Probiotic products for pet applications
An exemplary embodiment providing one or more improvements includes feeding pets with probiotic microbes encapsulated in a mixture of xanthan gum and chitosan, or in gelatin, specifically Pediococcus acidilactici and Saccharomyces boulardii. Such encapsulation protects the viability of the probiotic microbes against unfavorable temperatures. Such feeding has the benefit of reducing odors, and improving digestion in pets which have these problems.
Owner:IMAGILIN TECH LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com