Patents
Literature
1404 results about "Fibrin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
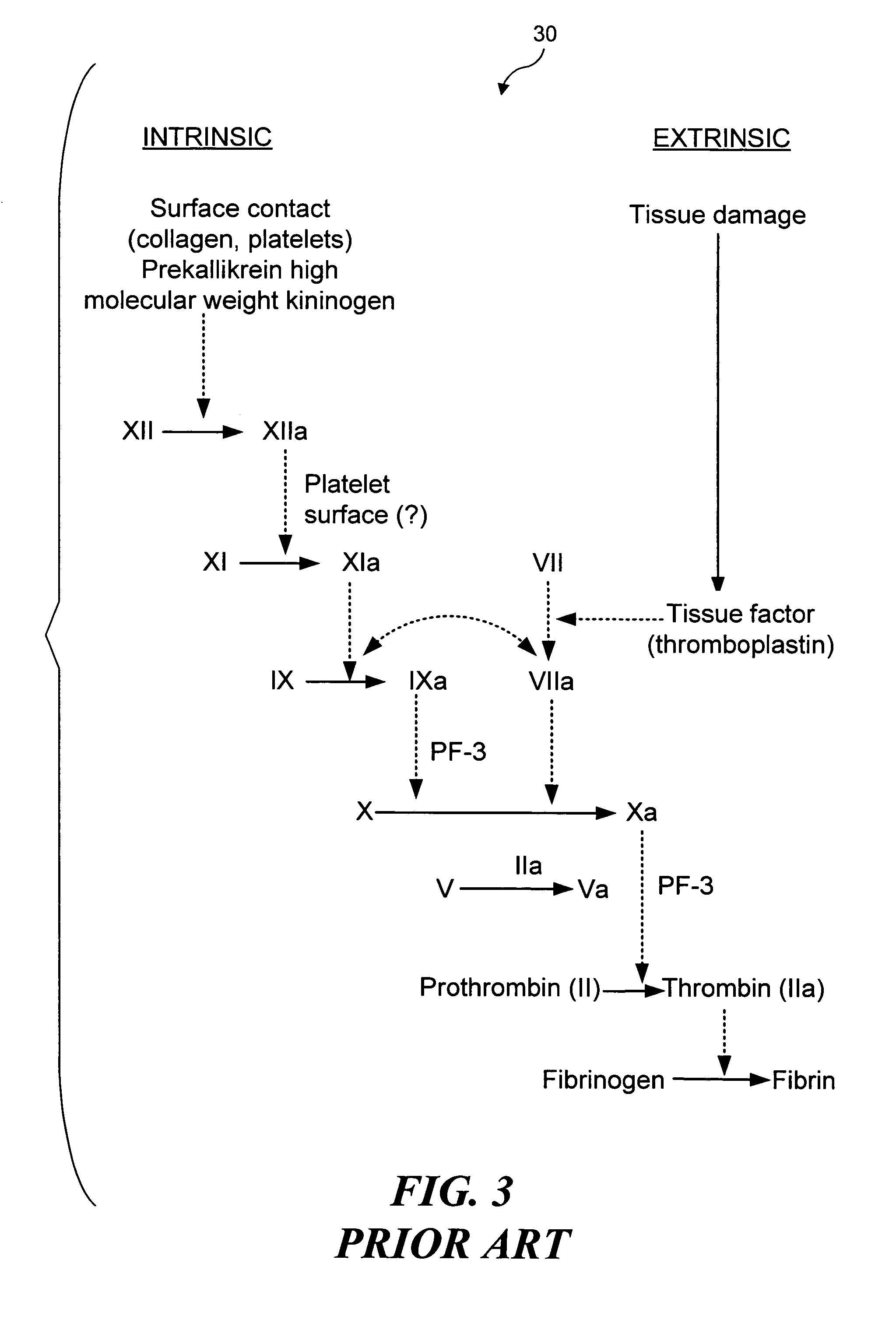
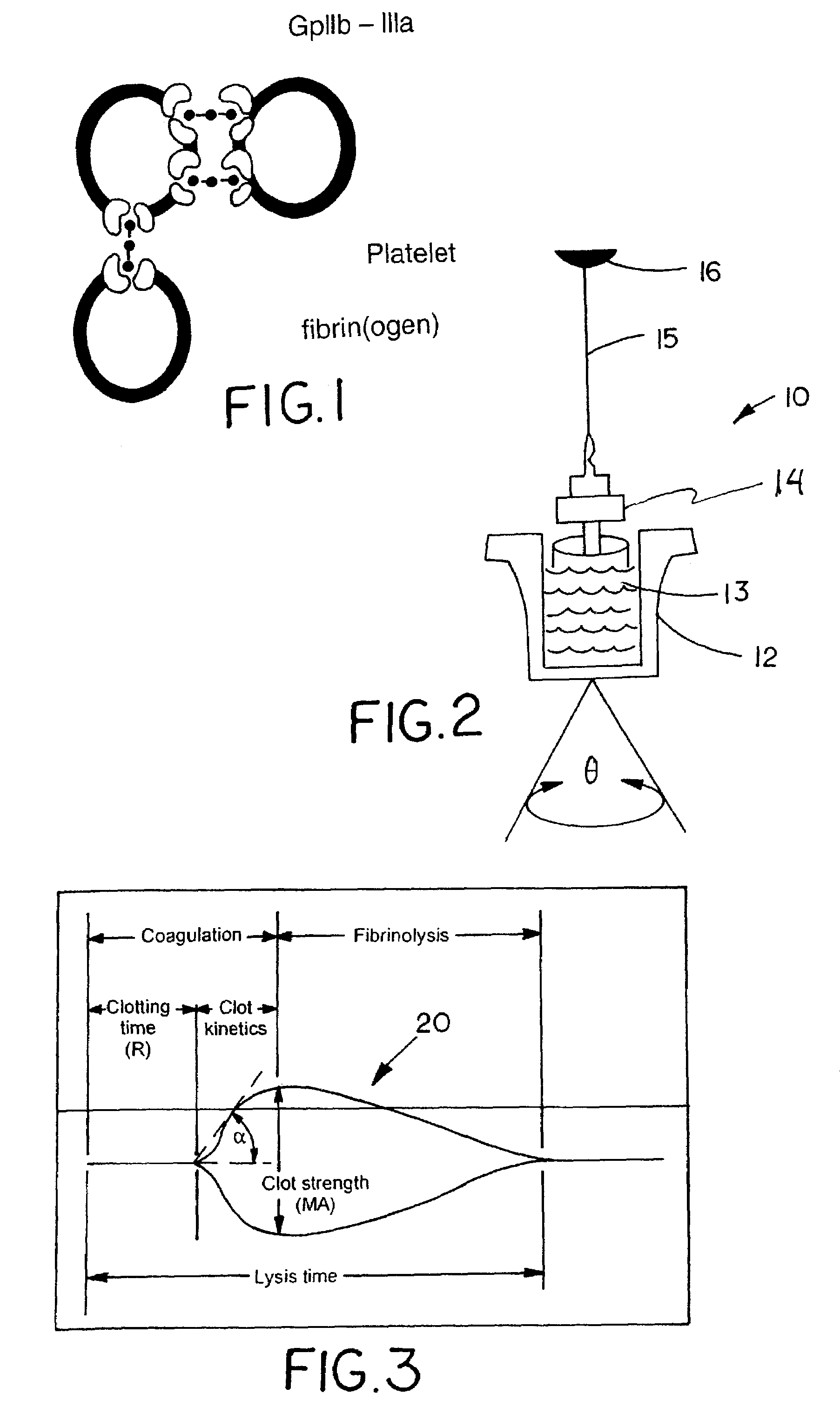
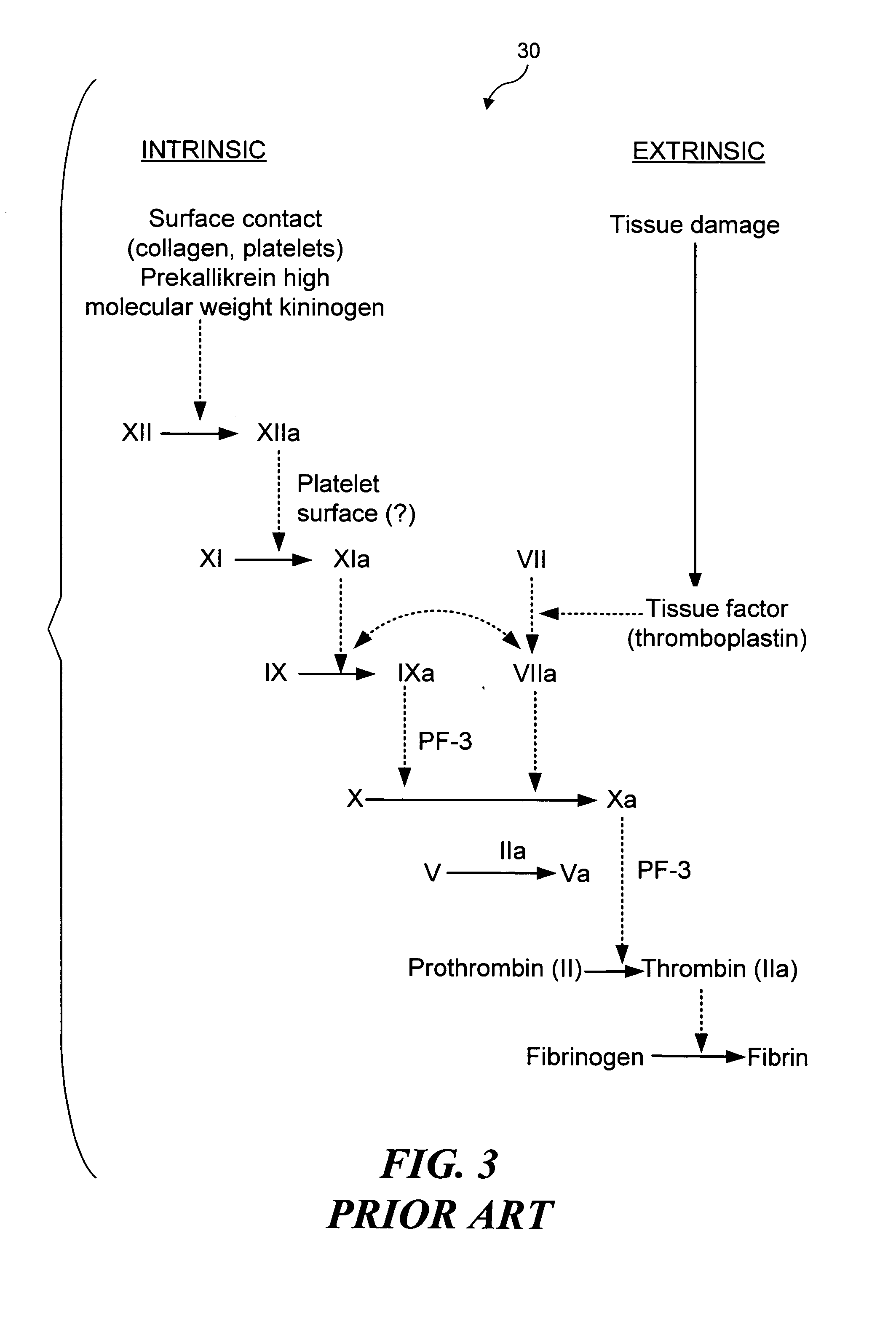
Fibrin (also called Factor Ia) is a fibrous, non-globular protein involved in the clotting of blood. It is formed by the action of the protease thrombin on fibrinogen which causes it to polymerize. The polymerized fibrin together with platelets forms a hemostatic plug or clot over a wound site.
Device for removal of thrombus through physiological adhesion
InactiveUS7004954B1Raise the level of performanceRestoring native blood flowBalloon catheterSurgeryMedicineThrombus
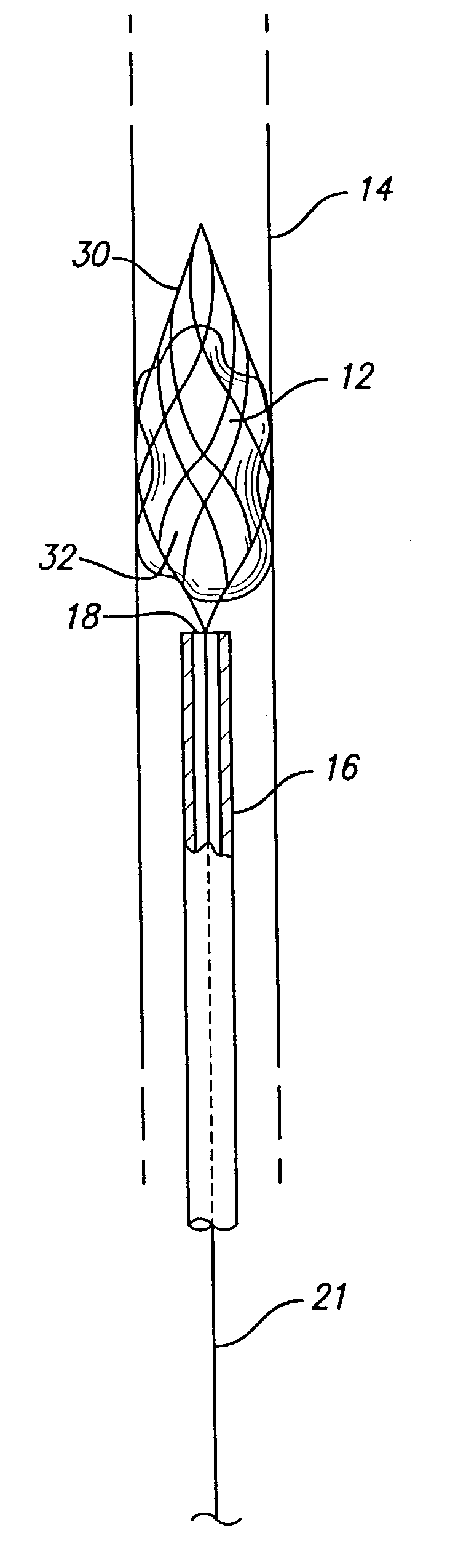
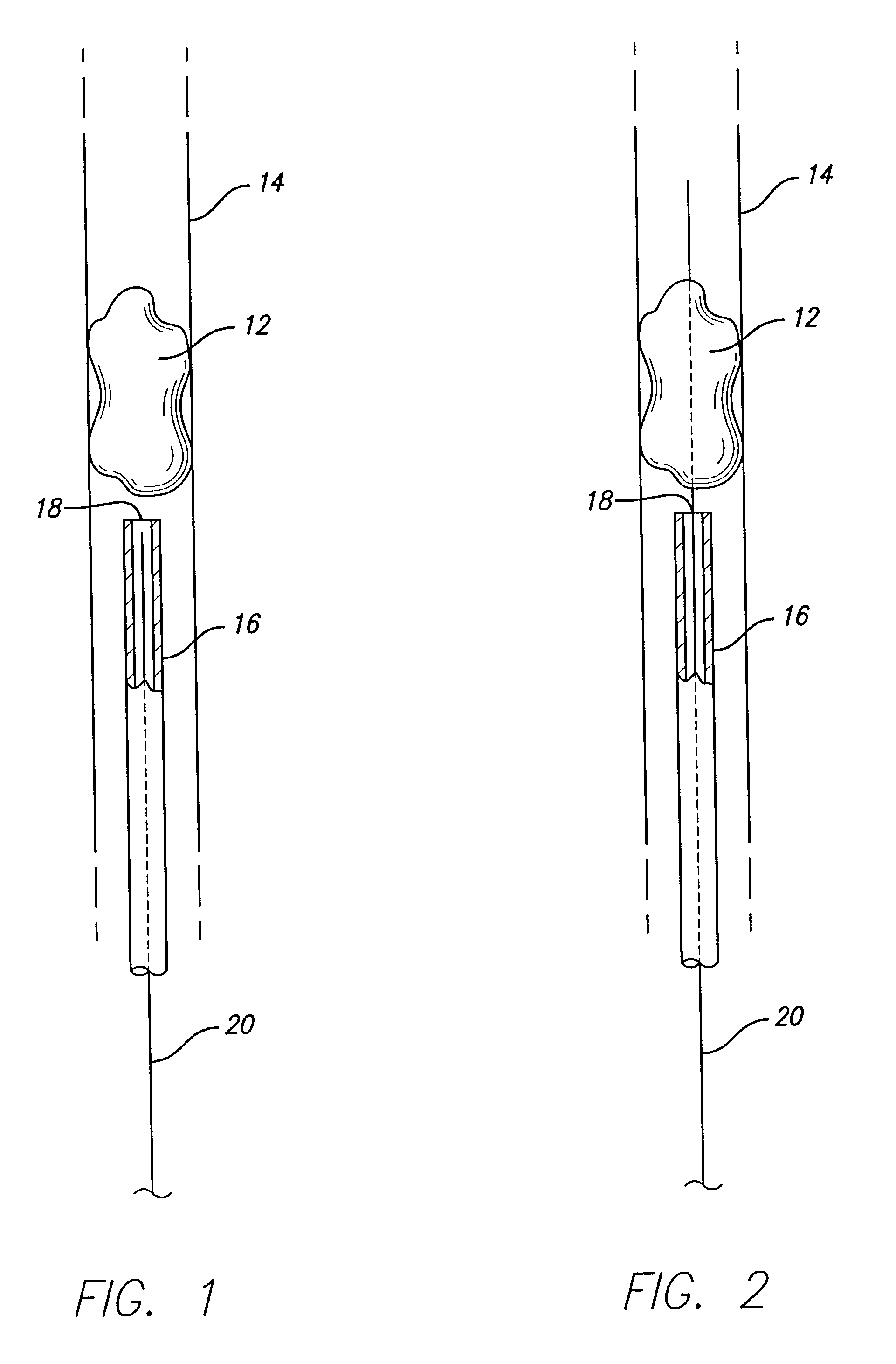
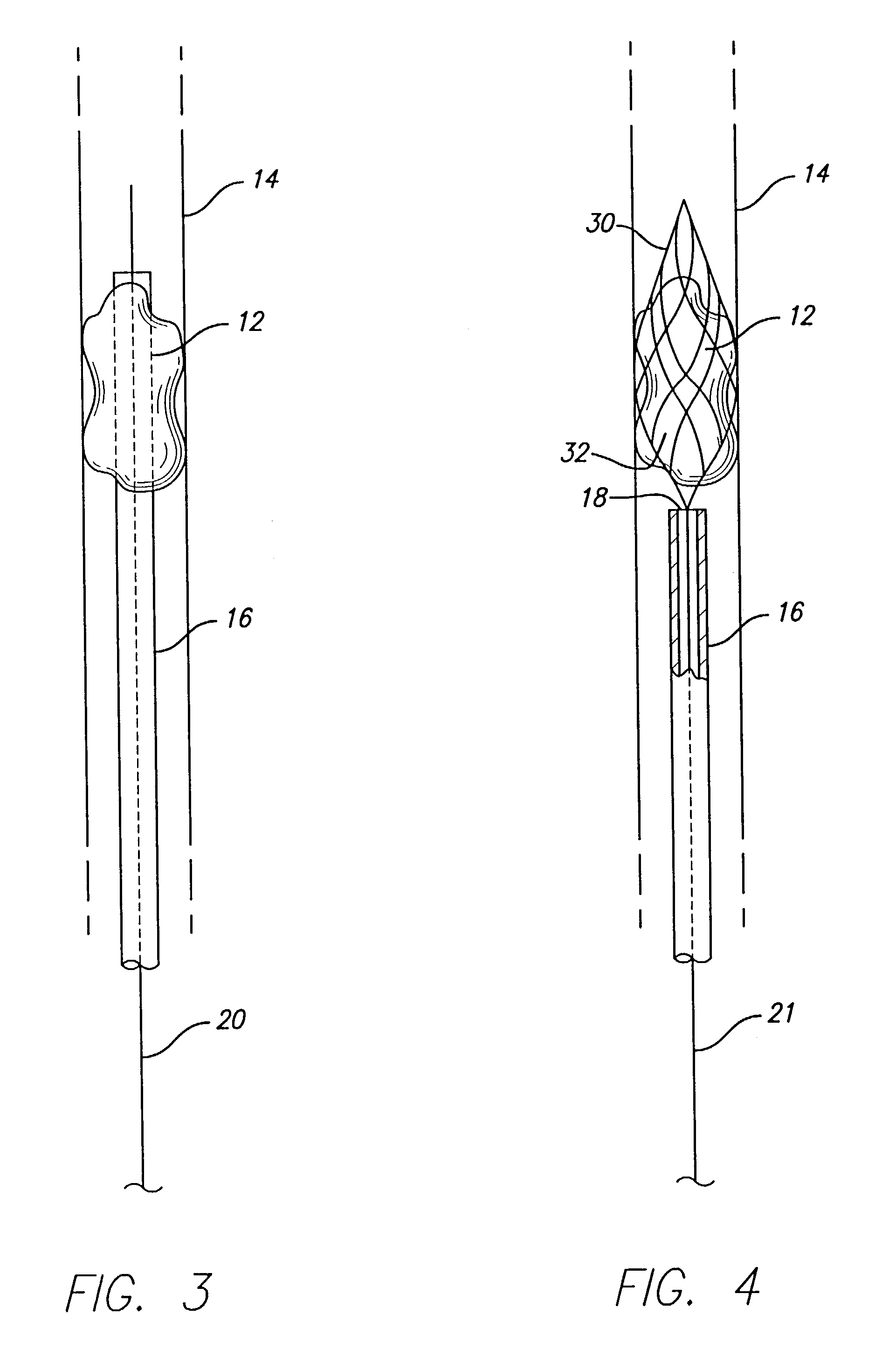
A device that is useful for removing obstructions from vessels. Various embodiments and methods of use are contemplated for the effective removal of obstructions. The disclosed devices utilize a thrombogenic material to promote the formation of fibrin bonds, thus enhancing adhesion. It is further contemplated that the disclosed devices may be used in all vasculature including the cerebral vasculature and the neurovasculature.
Owner:ENDOVASCULAR TECH
Hemostatic sandwich bandage
The present invention relates to a haemostatic multilayer bandage that comprises preferably a thrombin layer between two fibrinogen layers. The dressing may contain other resorbable materials such as glycolic acid or lactic acid based polymers or copolymers. The inventive haemostatic bandage is useful for the treatment of wounded tissue.
Owner:AMERICAN NAT RED CROSS
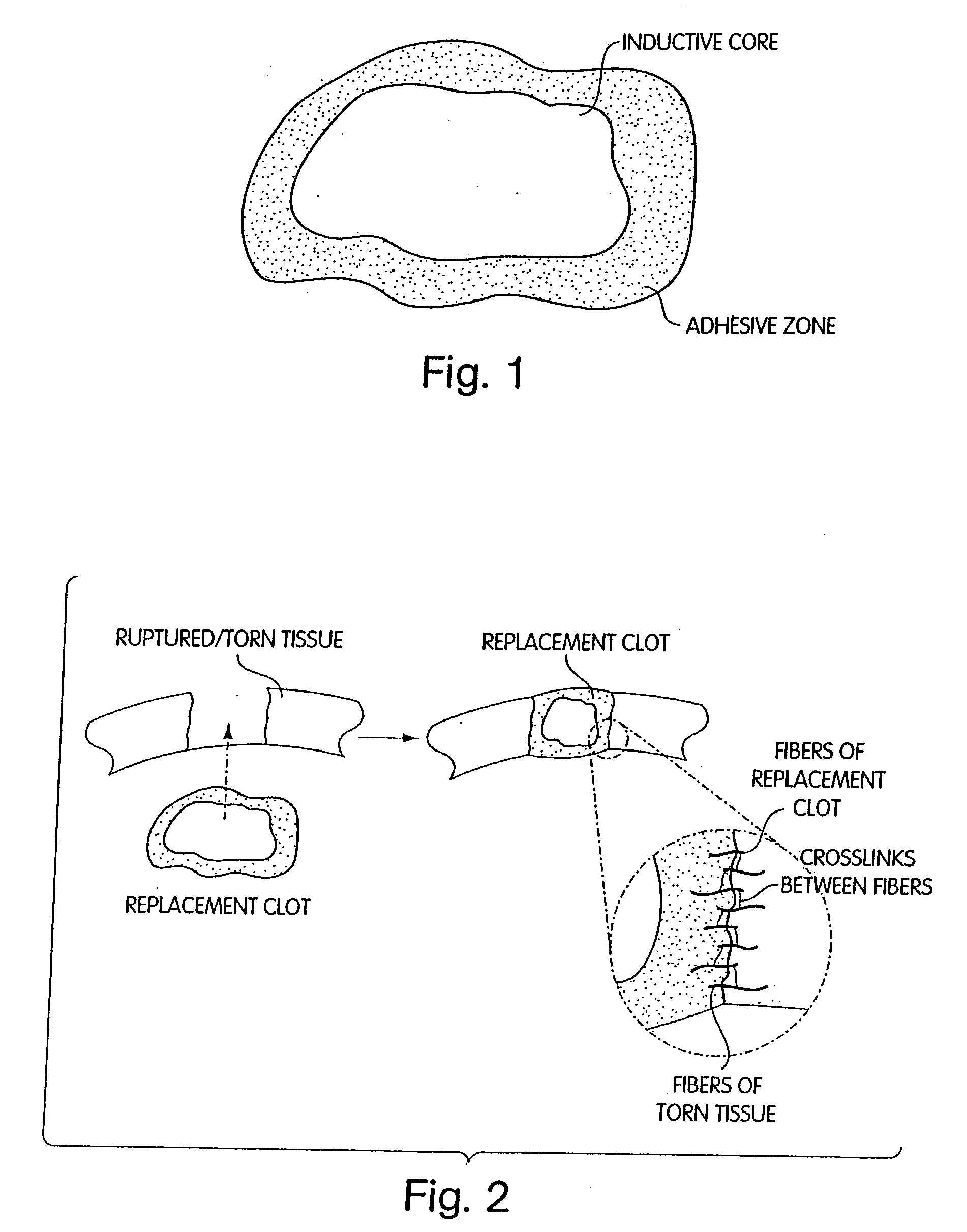
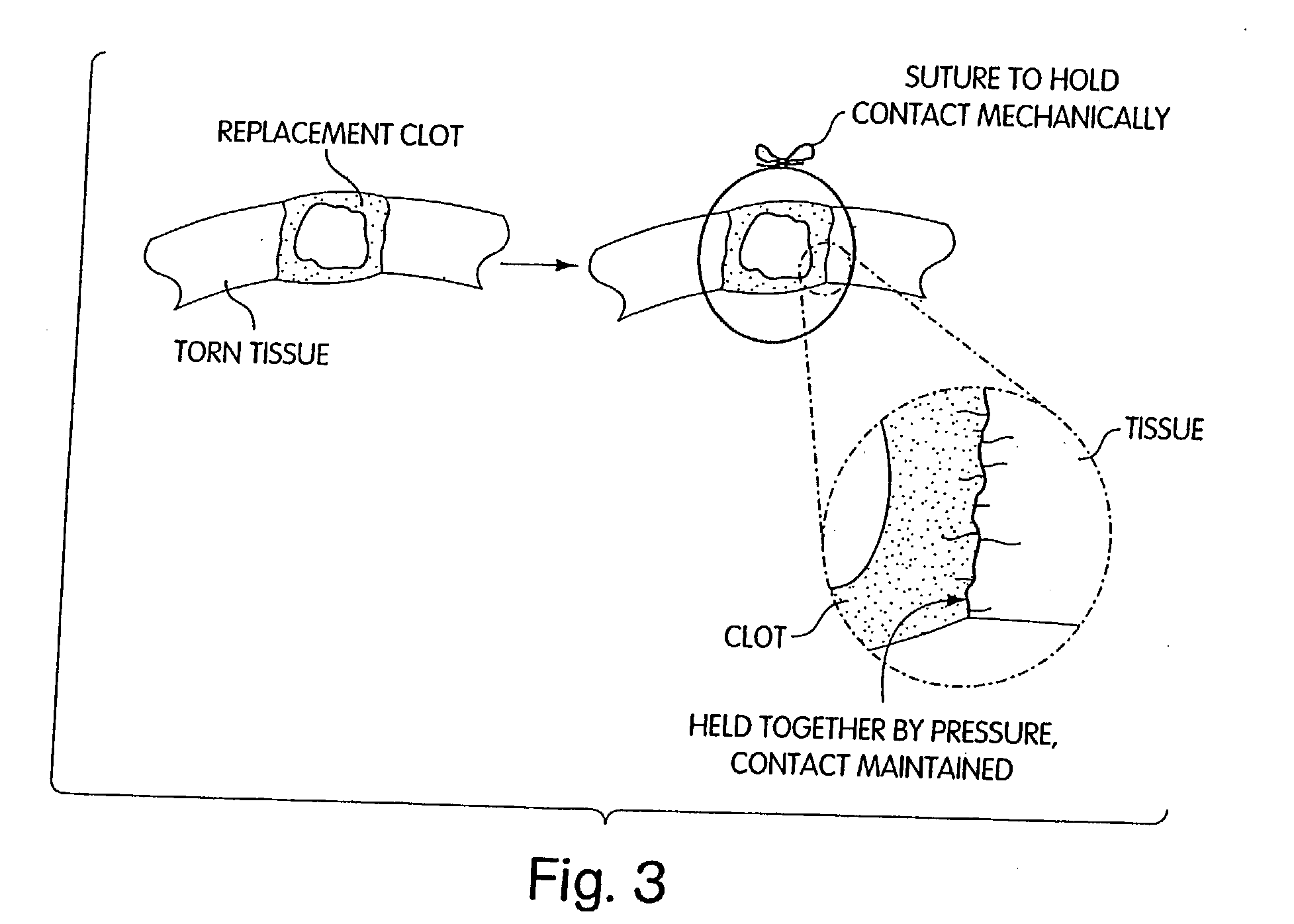
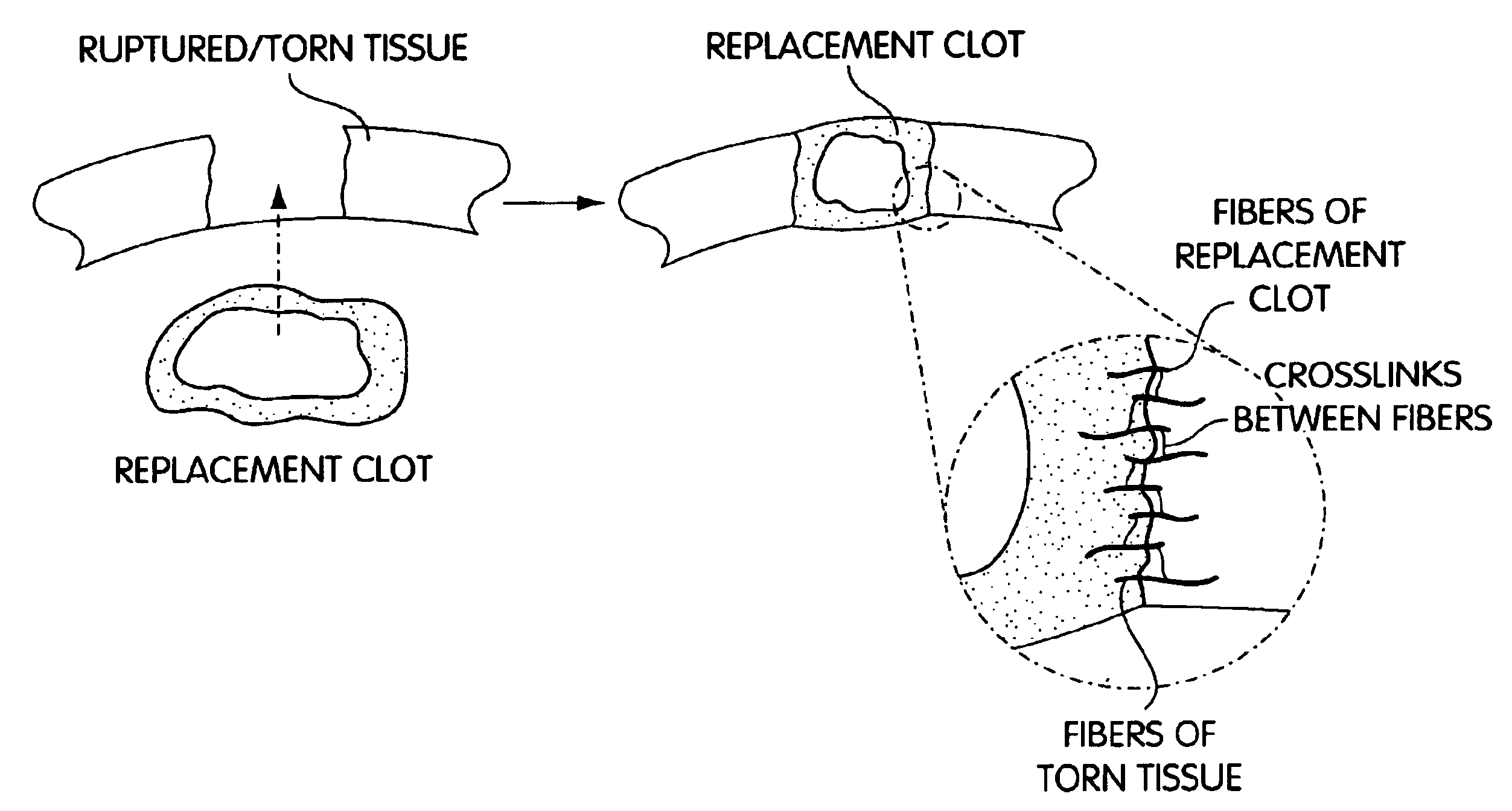
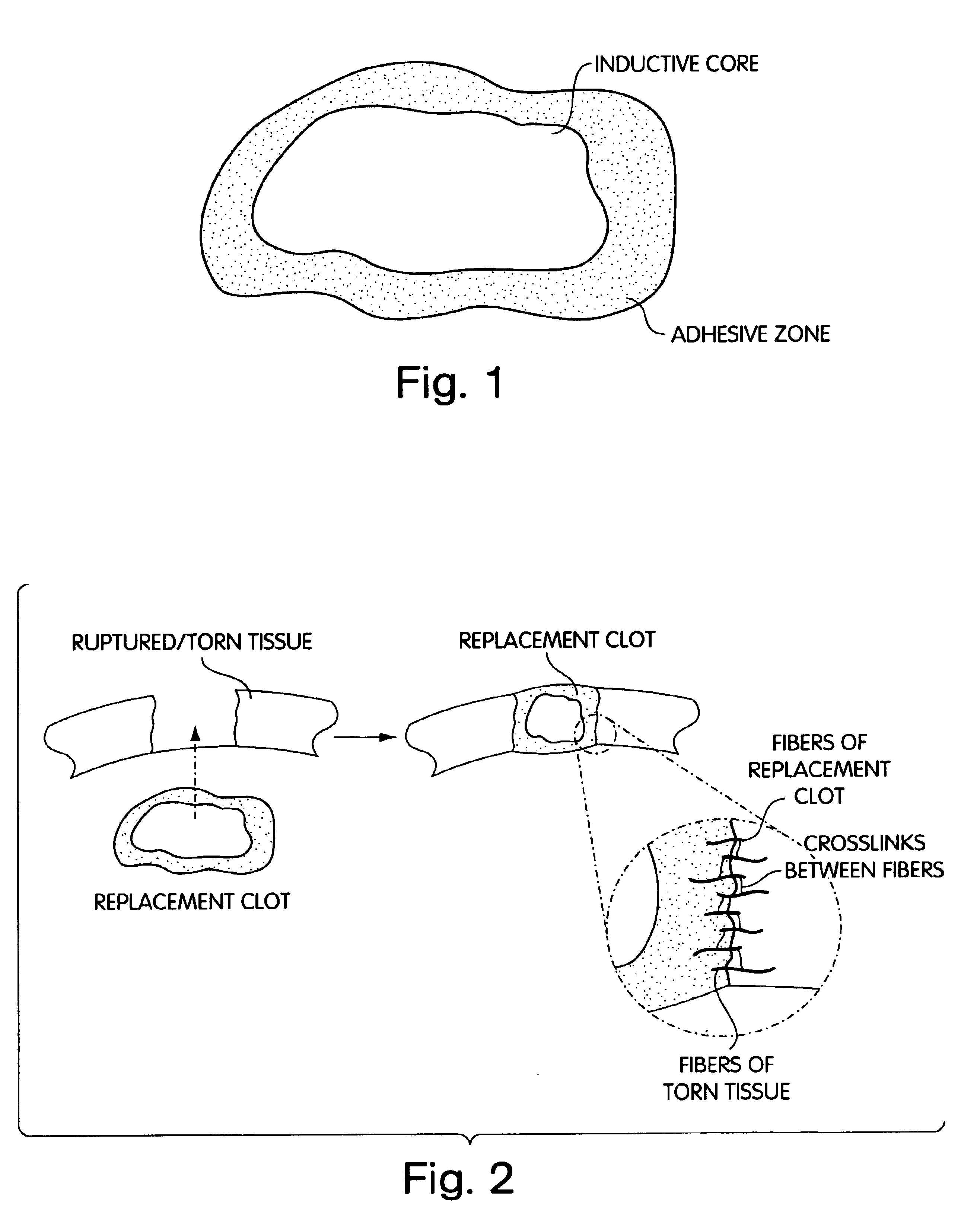
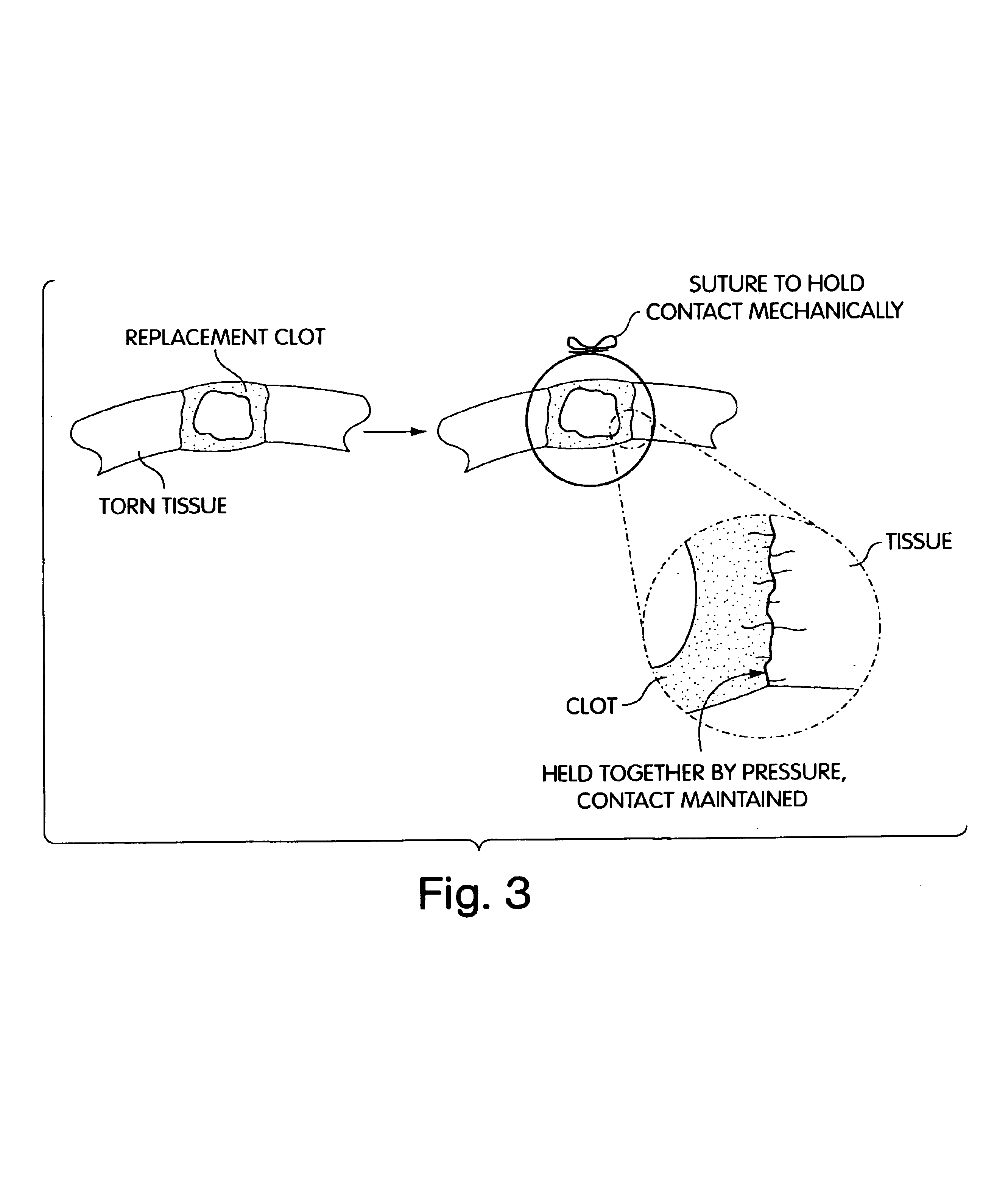
Biologic replacement for fibrin clot
InactiveUS20040059416A1Promote regenerationWound closure is subsequentlySuture equipmentsVirusesLigament structureBiomedical engineering
Owner:CHILDRENS MEDICAL CENT CORP +1
Fibrin delivery device and method for forming fibrin on a surface
InactiveUS6461325B1High mechanical strengthEasy to handleSurgeryMedical devicesMechanical energyMedical device
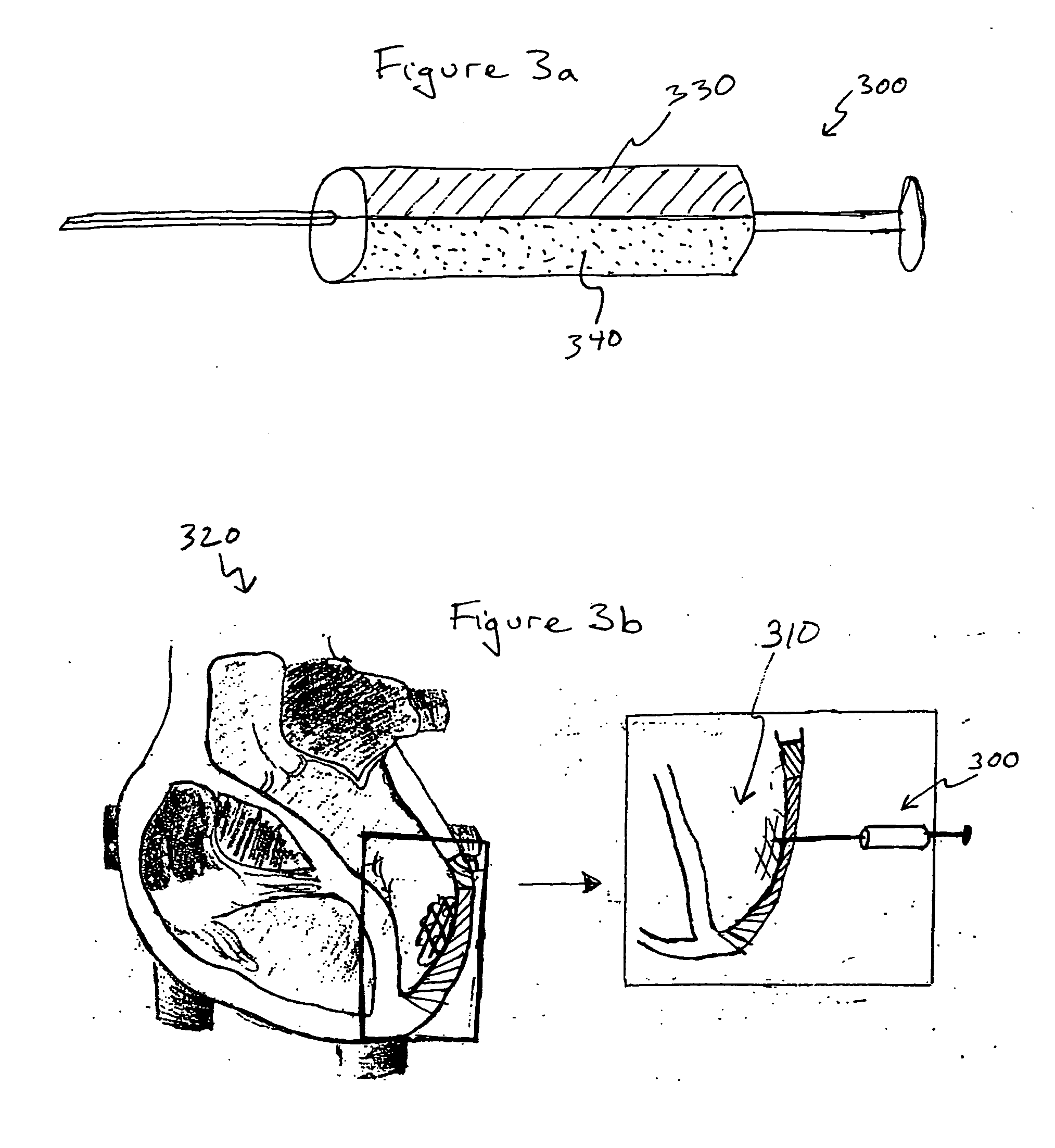
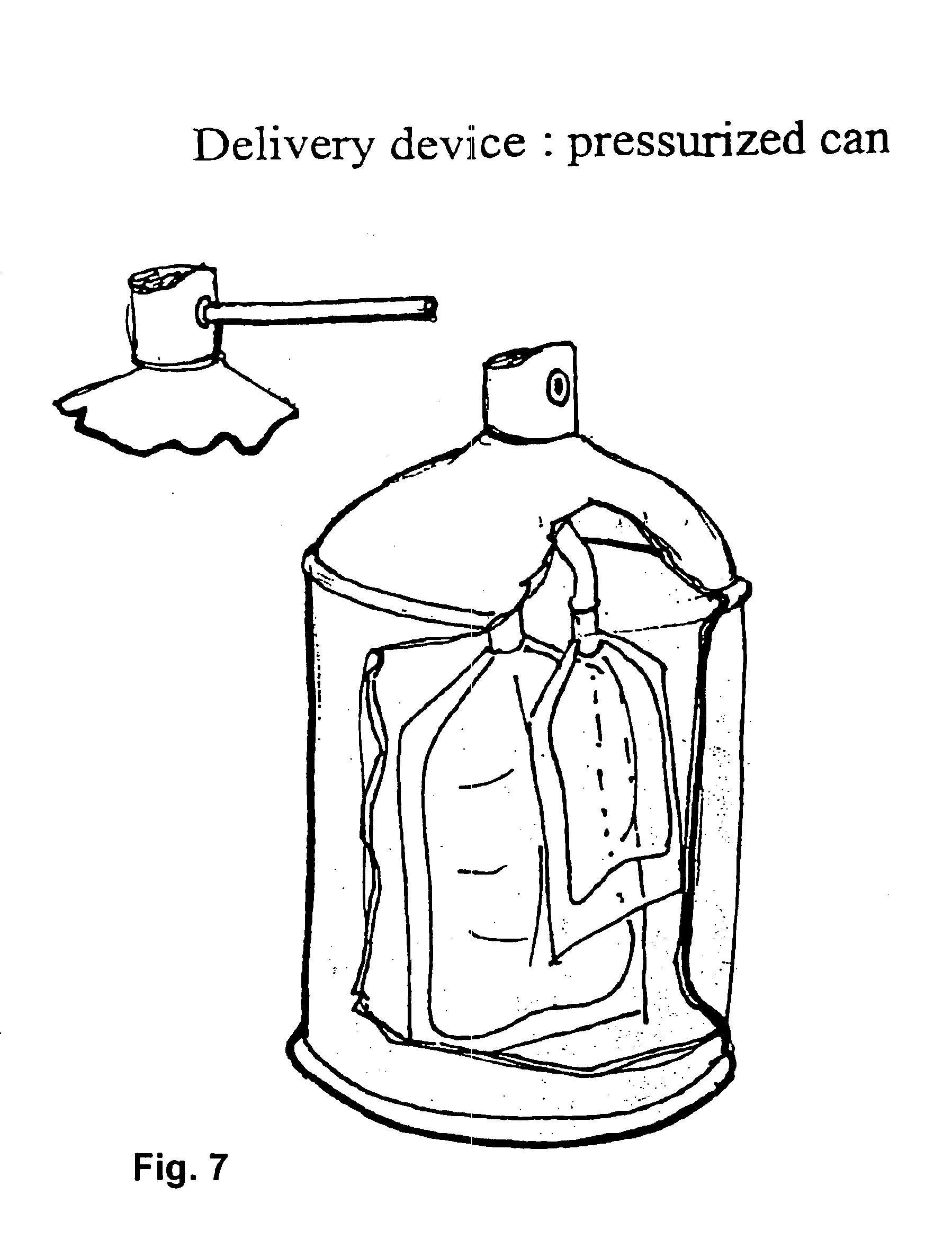
This invention provides a medical device for delivering volumetric quantities of a first and a second biochemically reactive fluid comprising a first container having an opening, the first container being adapted to contain the first biochemically reactive fluid; a second container having a second fluid opening adjacent the first fluid opening, the second container being adapted to contain the second biochemically reactive fluid; a spray unit for separately atomizing the first and second biochemically reactive fluids into an aerosol with at least one energy source of a liquid energy, a mechanical energy, a vibration energy, and an electric energy; a fluid pressurizer for pressurizing the first and the second biochemically reactive fluids for delivery under pressure through the spray unit onto a surface; and wherein the first and second biochemically reactive fluids first mix on the surface.
Owner:BAXTER INT INC
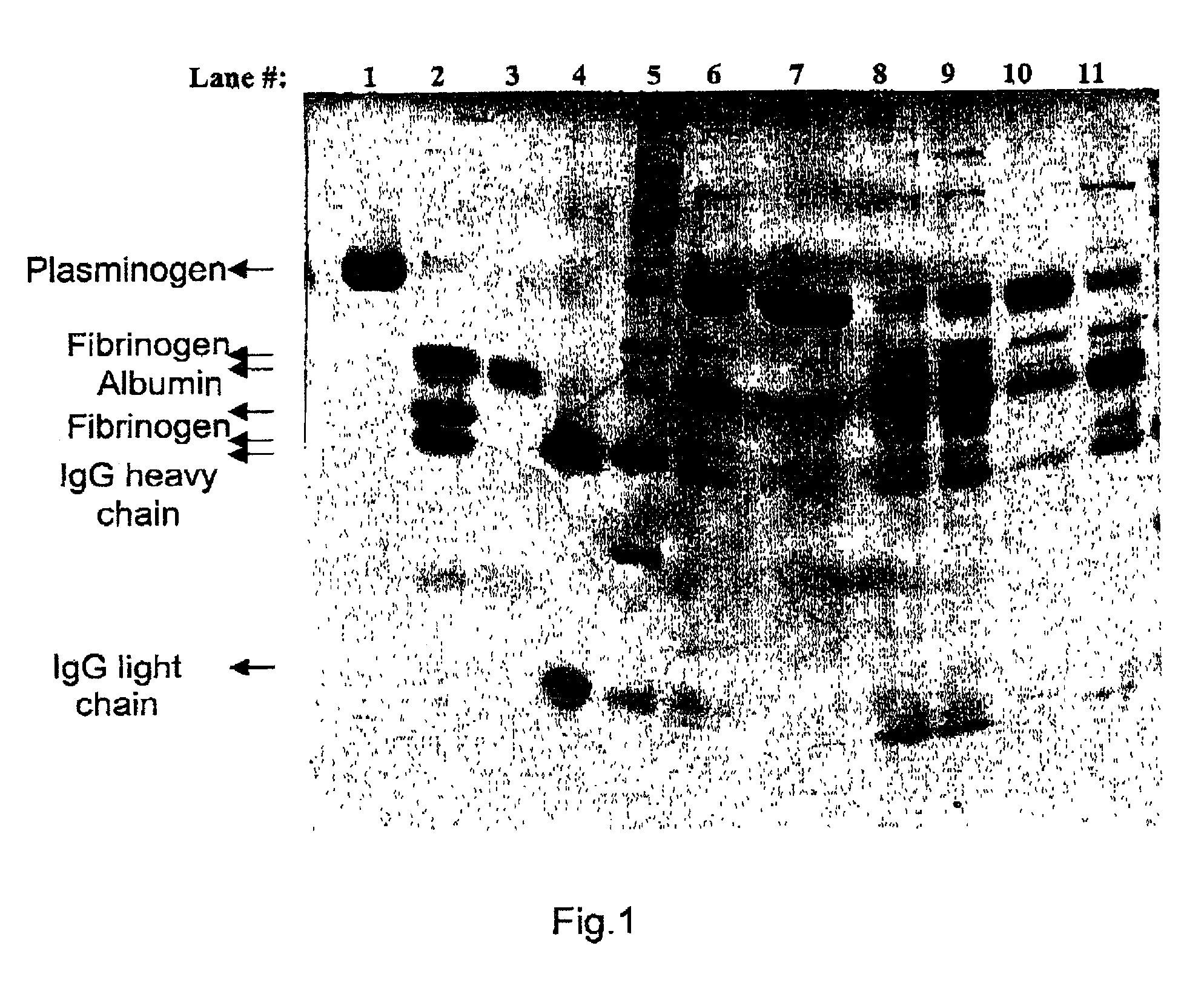
Removal of plasmin(ogen) from protein solutions
A method for specifically removing or isolating plasmin(ogen) or plasmin in presence of fibrinogen from a mixture containing plasmin(ogen) or plasmin by contacting the mixture with a rigid amino acid wherein the amino group of the amino acid and the carboxylic group of the amino acid are about 6–8 Angstroms, preferably about 7 Angstroms apart and the rigid amino acid is covalently bound to the support via the amino group of the amino acid.
Owner:OMRIX BIOPHARM
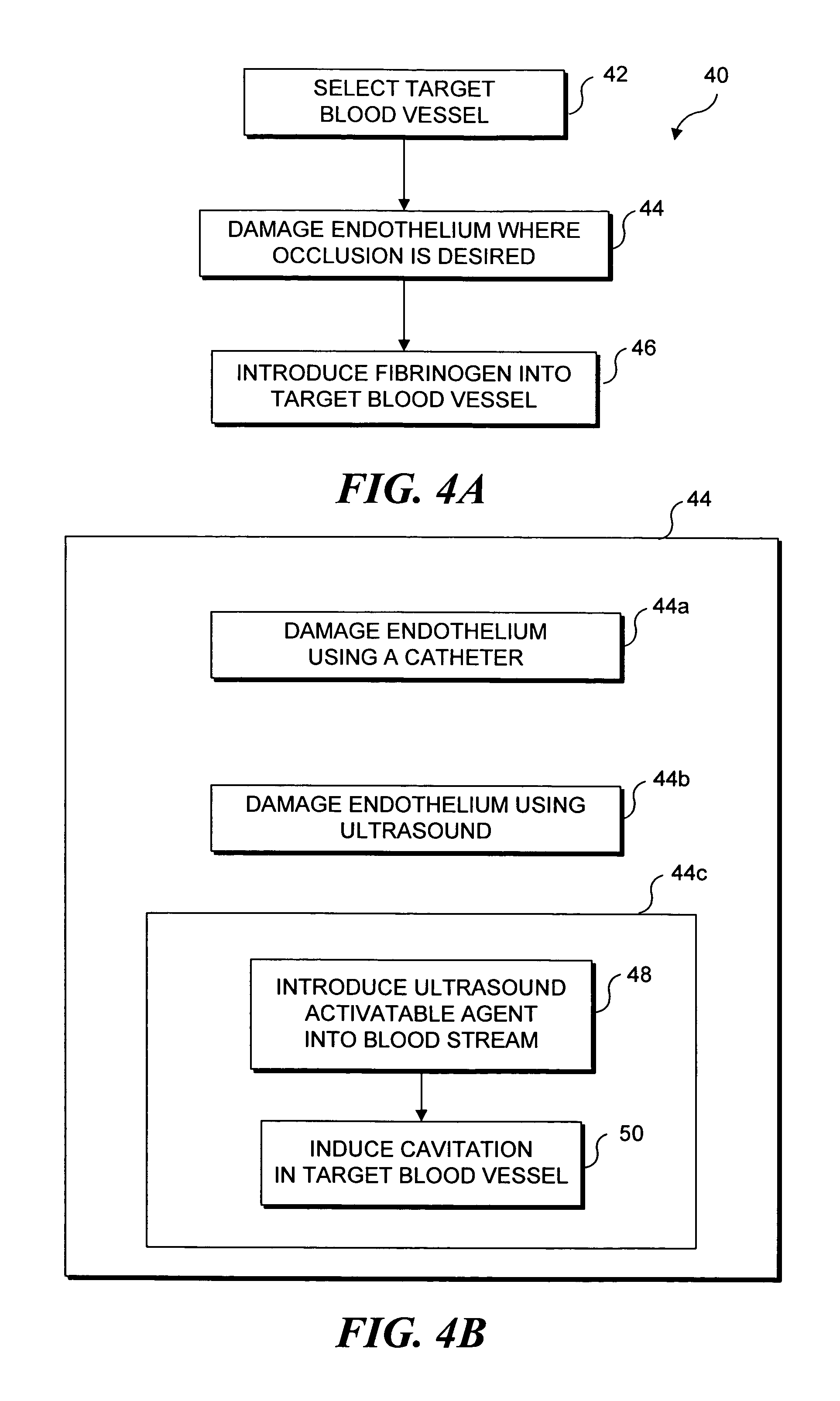
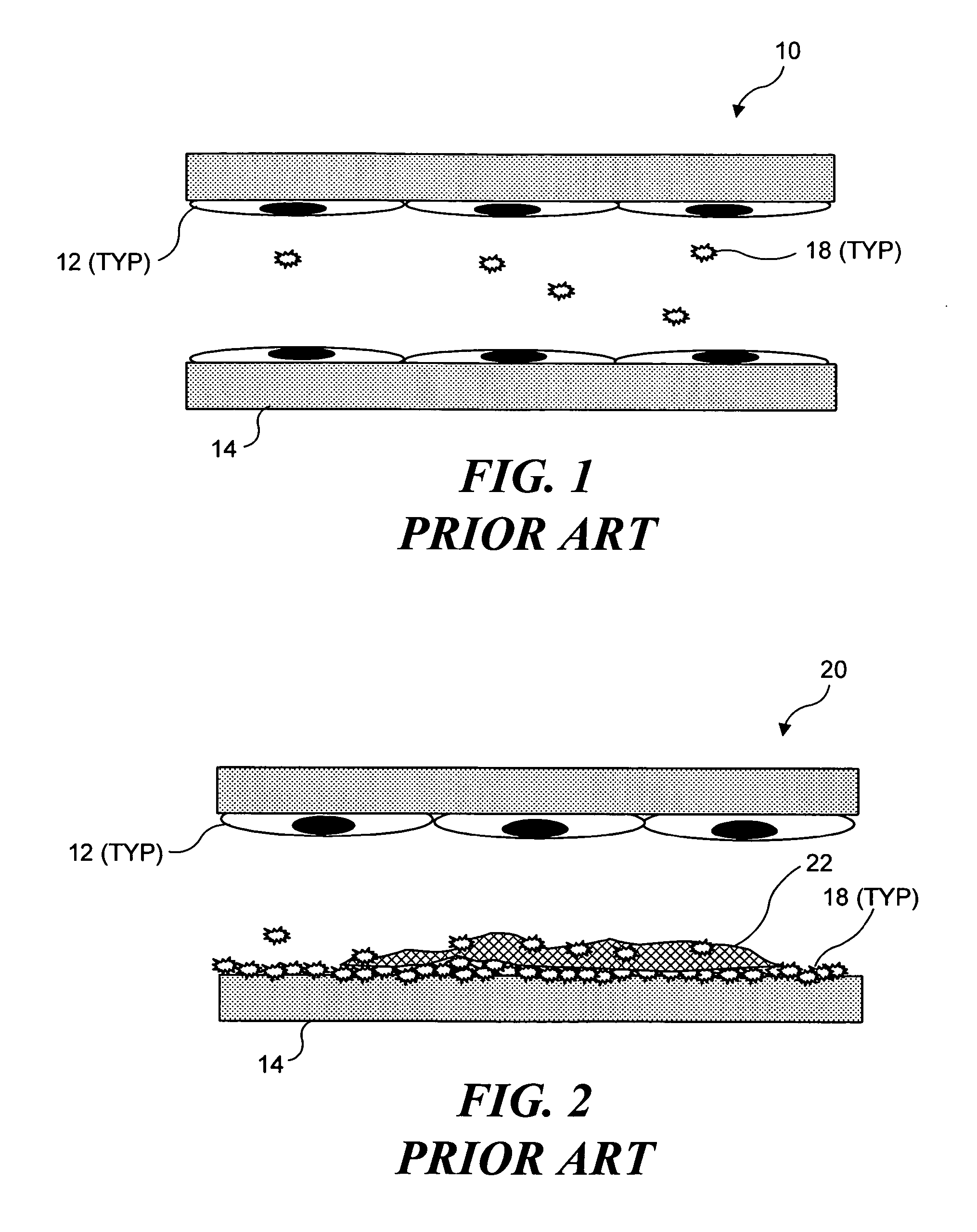
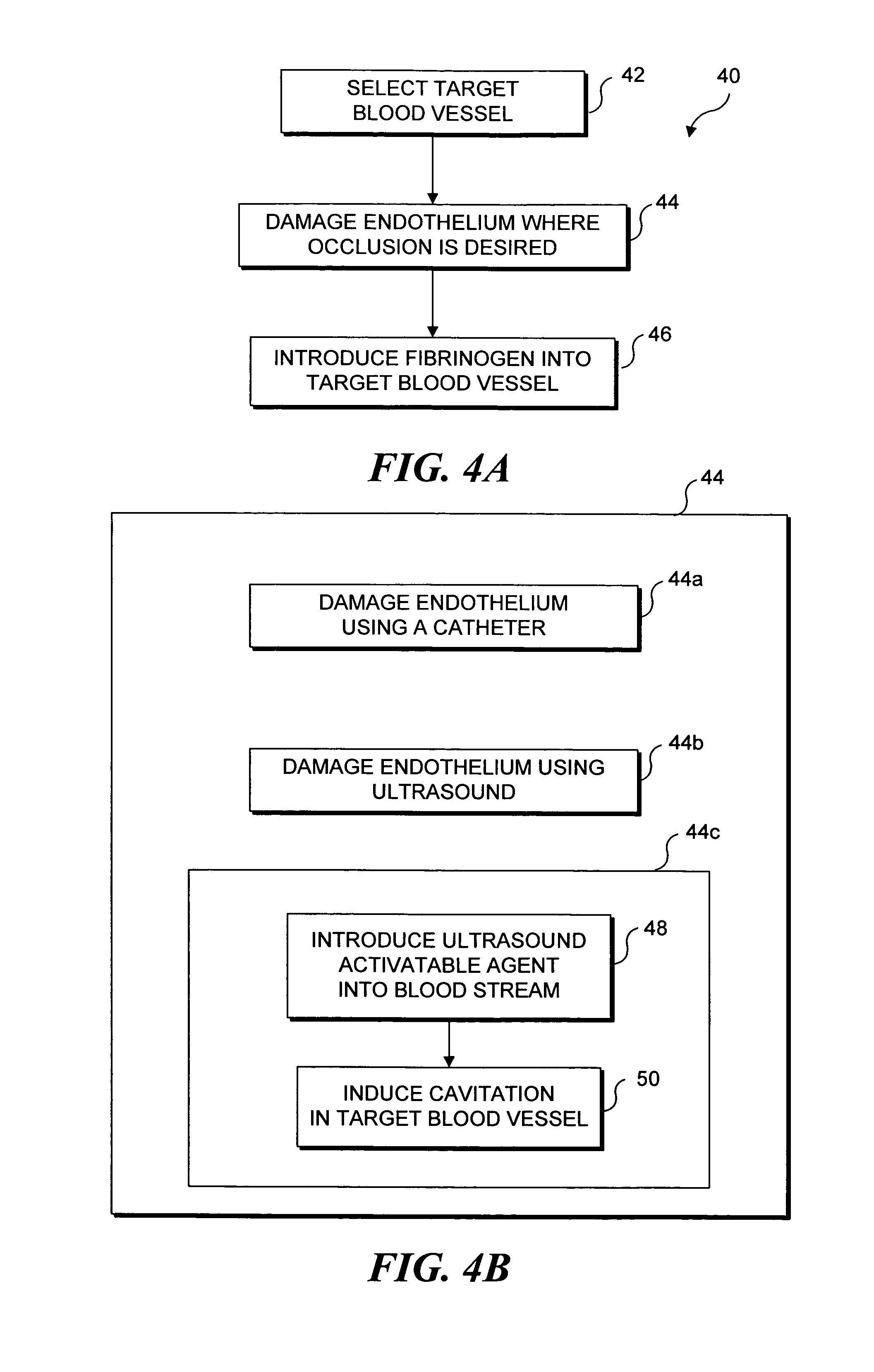
Ultrasound target vessel occlusion using microbubbles
InactiveUS7591996B2Eliminate heat damageFew techniqueUltrasonic/sonic/infrasonic diagnosticsUltrasound therapyCavitationThrombus
Owner:UNIV OF WASHINGTON
Biologic replacement for fibrin clot
InactiveUS6964685B2Increase contactResists premature degradationSurgical adhesivesPeptide/protein ingredientsExtra-ArticularExtracellular proteins
The invention provides compositions and methods for repairing intra-articular and extra-articular tissue including ligament, meniscus, cartilage, tendon, and bone. The method includes contacting the ends of an injured tissue from a patient with a composition. The repair composition includes soluble type 1 collagen, a platelet, and at least one of an extracellular protein and a neutralizing agent.
Owner:CHILDRENS MEDICAL CENT CORP
Hemostatic textile
ActiveUS20070160653A1Quick activationNon-adhesive dressingsPeptide/protein ingredientsLactideSisal fiber
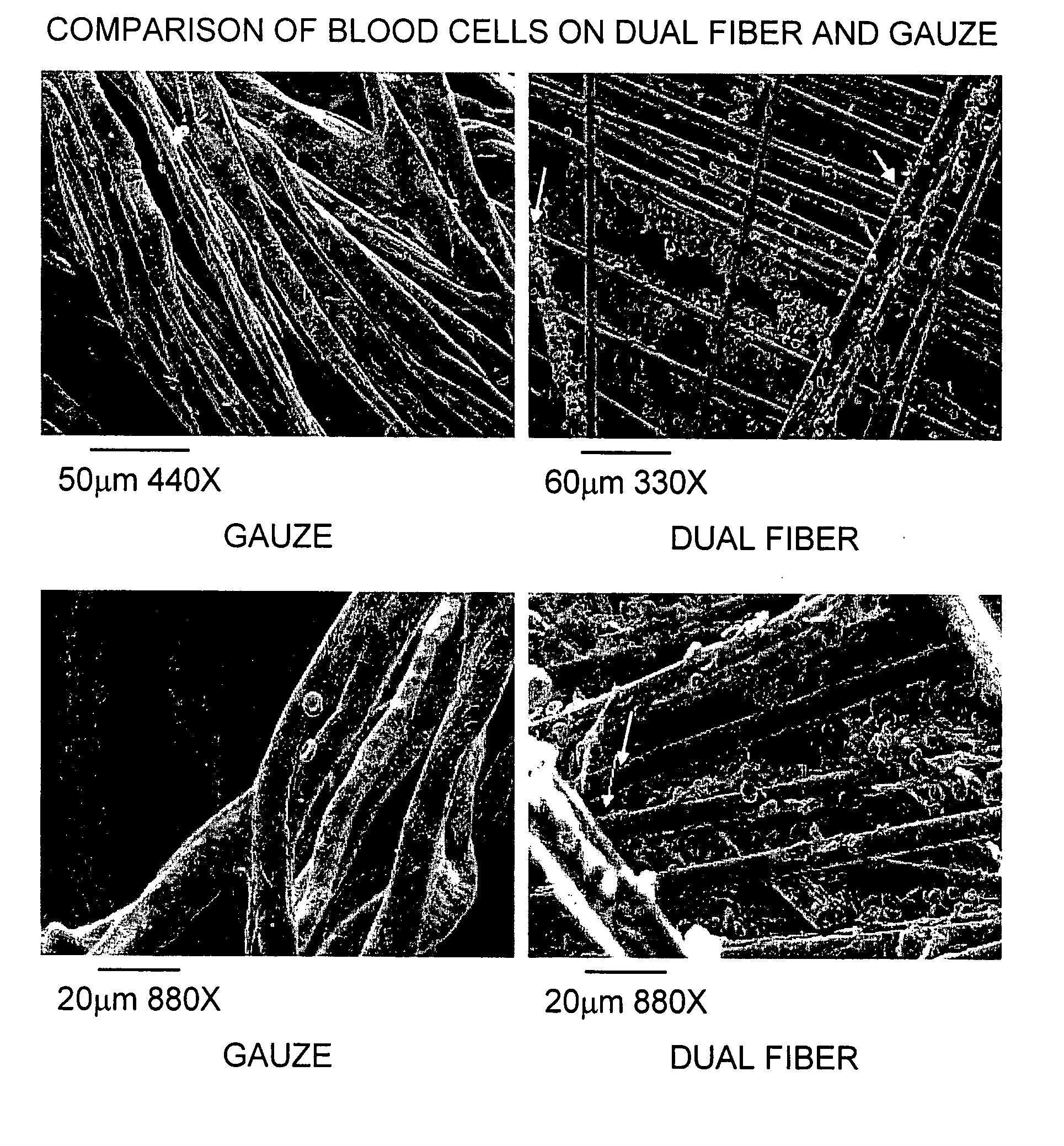
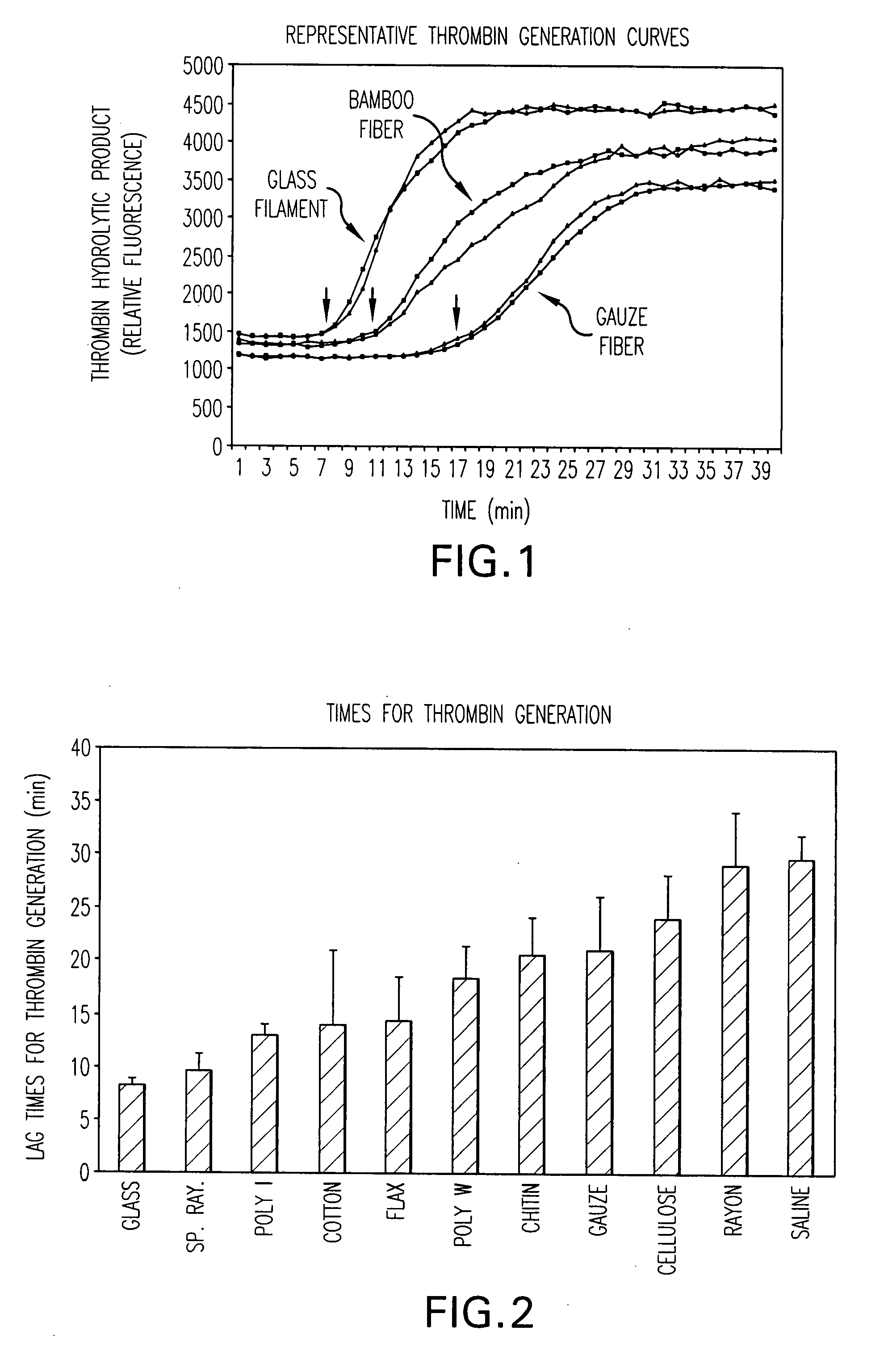
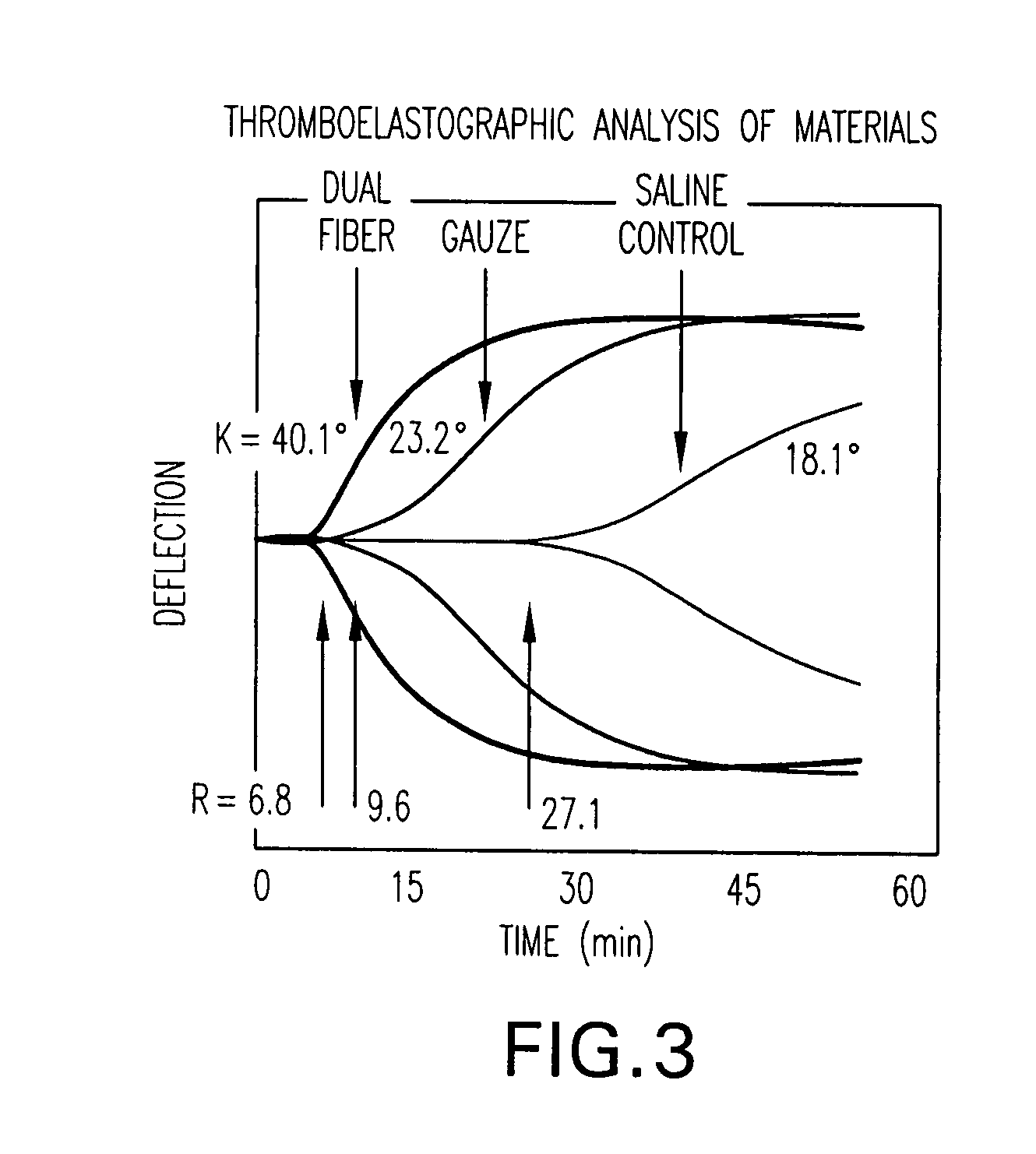
The present invention is directed to a hemostatic textile, comprising: a material comprising a combination of glass fibers and one or more secondary fibers selected from the group consisting of silk fibers; ceramic fibers; raw or regenerated bamboo fibers; cotton fibers; rayon fibers; linen fibers; ramie fibers; jute fibers; sisal fibers; flax fibers; soybean fibers; corn fibers; hemp fibers; lyocel fibers; wool; lactide and / or glycolide polymers; lactide / glycolide copolymers; silicate fibers; polyamide fibers; feldspar fibers; zeolite fibers, zeolite-containing fibers, acetate fibers; and combinations thereof; the hemostatic textile capable of activating hemostatic systems in the body when applied to a wound. Additional cofactors such as thrombin and hemostatic agents such as RL platelets, RL blood cells; fibrin, fibrinogen, and combinations thereof may also be incorporated into the textile. The invention is also directed to methods of producing the textile, and methods of using the textile to stop bleeding.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL +1
Hydrogel bioscaffoldings and biomedical device coatings
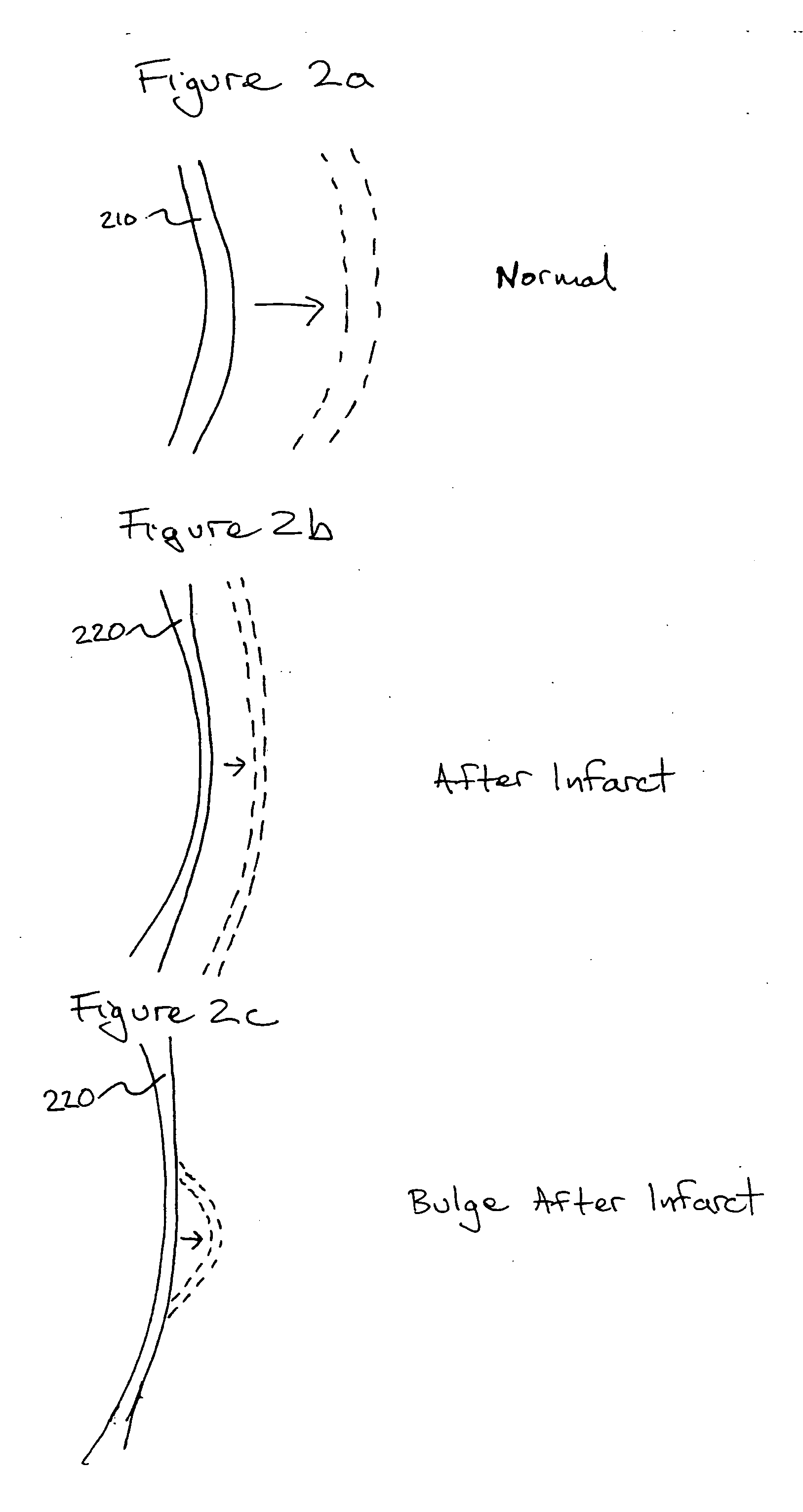
Bioscaffoldings formed of hydrogels that are crosslinked in situ in an infarcted region of the heart (myocardium) by a Michael's addition reaction or by a disulfide bond formed by an oxidative process are described. Each of the bioscaffoldings described includes hyaluronan as one of the hydrogel components and the other component is selected from collagen, collagen-laminin, poly-l-lysine, and fibrin. The bioscaffolding may further include an alginate component. The bioscaffoldings may have biofunctional groups such as angiogenic factors and stem cell homing factors bound to the collagen, collagen-laminin, poly-l-lysine, or fibrinogen hydrogel component. In particular, the biofunctional groups may be PR11, PR39, VEGF, bFGF, a polyarginine / DNA plasmid complex, or a DNA / polyethyleneimine (PEI) complex. Additionally, the hydrogel components may be injected into the infarct region along with stem cells and microspheres containing stem cell homing factors. The bioscaffolding may be formed on a stent or a cardiac medical device.
Owner:ABBOTT CARDIOVASCULAR
Dialysis catheter system
InactiveUS20040210180A1Reduce and prevent likelihoodIncrease flow rateMulti-lumen catheterOther blood circulation devicesArterial canalSuperior vena cava
The present invention provides a dialysis catheter that is designed to function in reverse-flow, having a dual lumen configuration. An embodiment of the present invention includes two lumen cooperatively configured in a co-axial design. The arterial lumen is circular or oval and extends beyond the termination of the venous lumen. The arterial lumen extracts the blood from the blood vessel for hemodialysis treatment. The venous lumen is also circular or oval. Terminating at a proximal point to the distal end of the arterial lumen, this configuration of the venous lumen aids in preventing recirculation. The venous lumen returns dialyzed blood back into the patient. The venous lumen can further include a plurality of apertures to aid in reducing the risk of fibrin sheath growth. In a method of use, the arterial lumen of the invention preferably resides within the right atrium with the venous lumen positioned within the superior vena cava.
Owner:ALTMAN SANFORD D
Biologic replacement for fibrin clot
InactiveUS20020123805A1Promote regenerationLess invasive treatmentSurgical adhesivesPeptide/protein ingredientsMedicineLigament
Owner:CHILDRENS MEDICAL CENT CORP
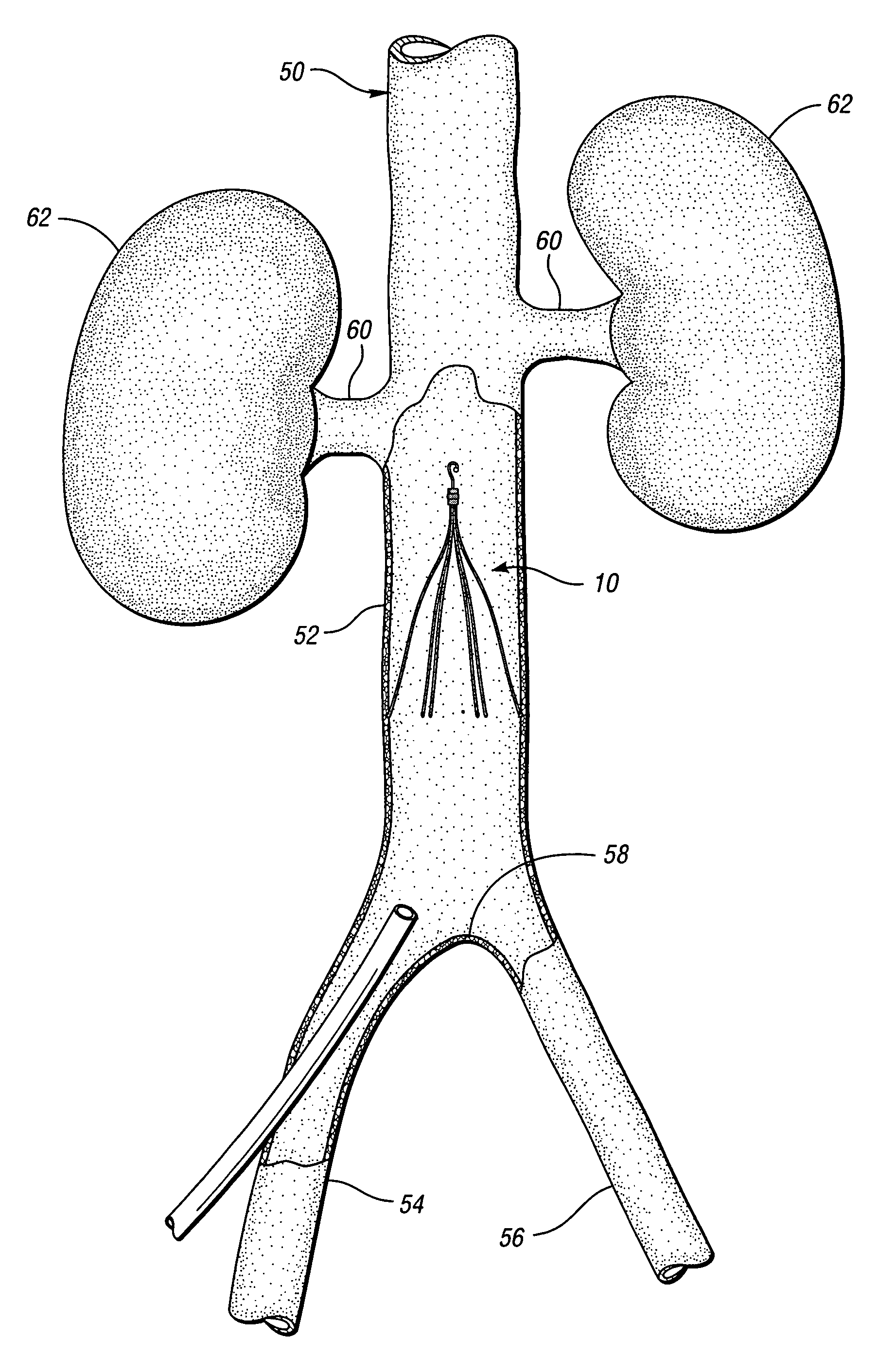
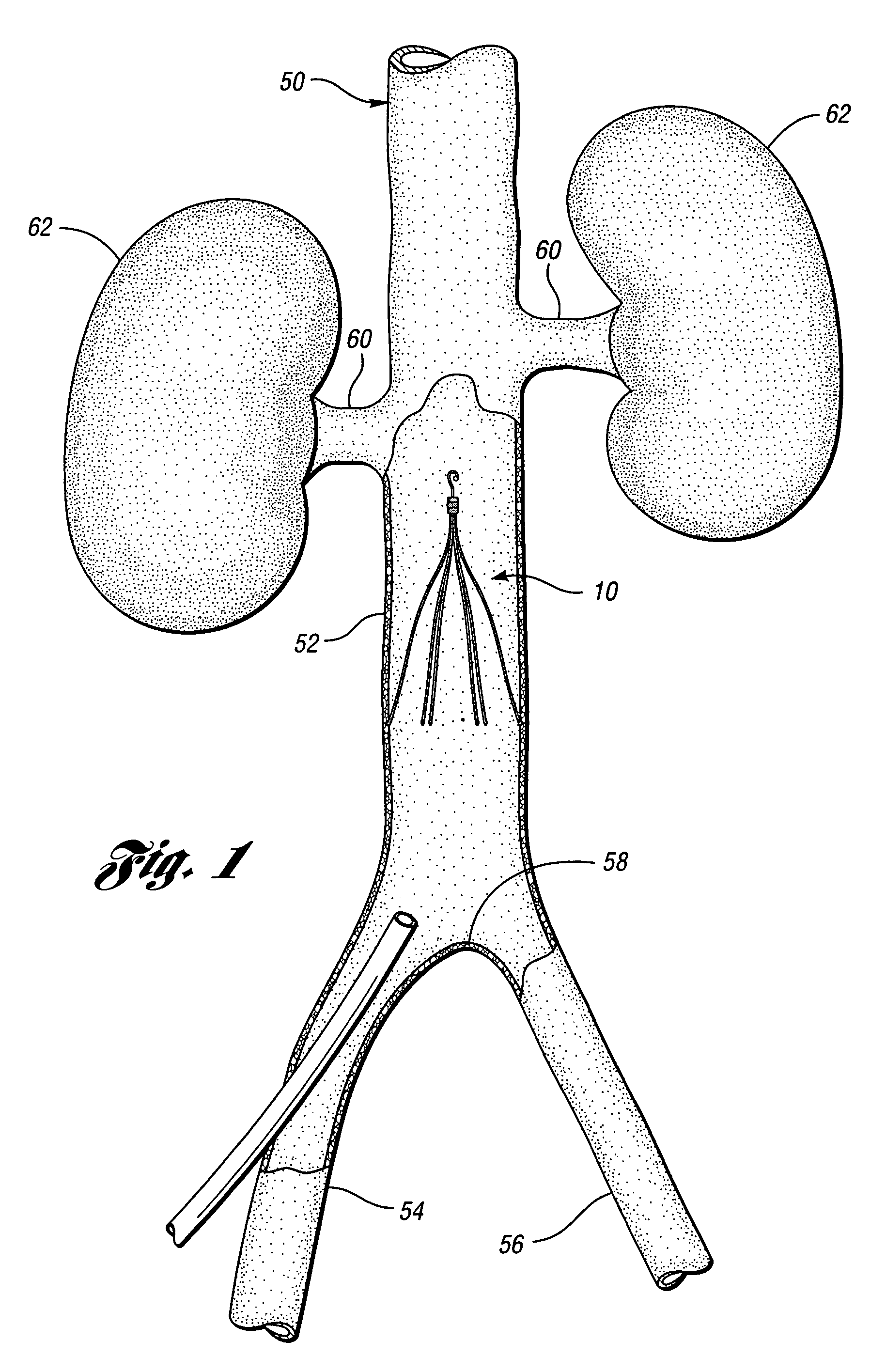
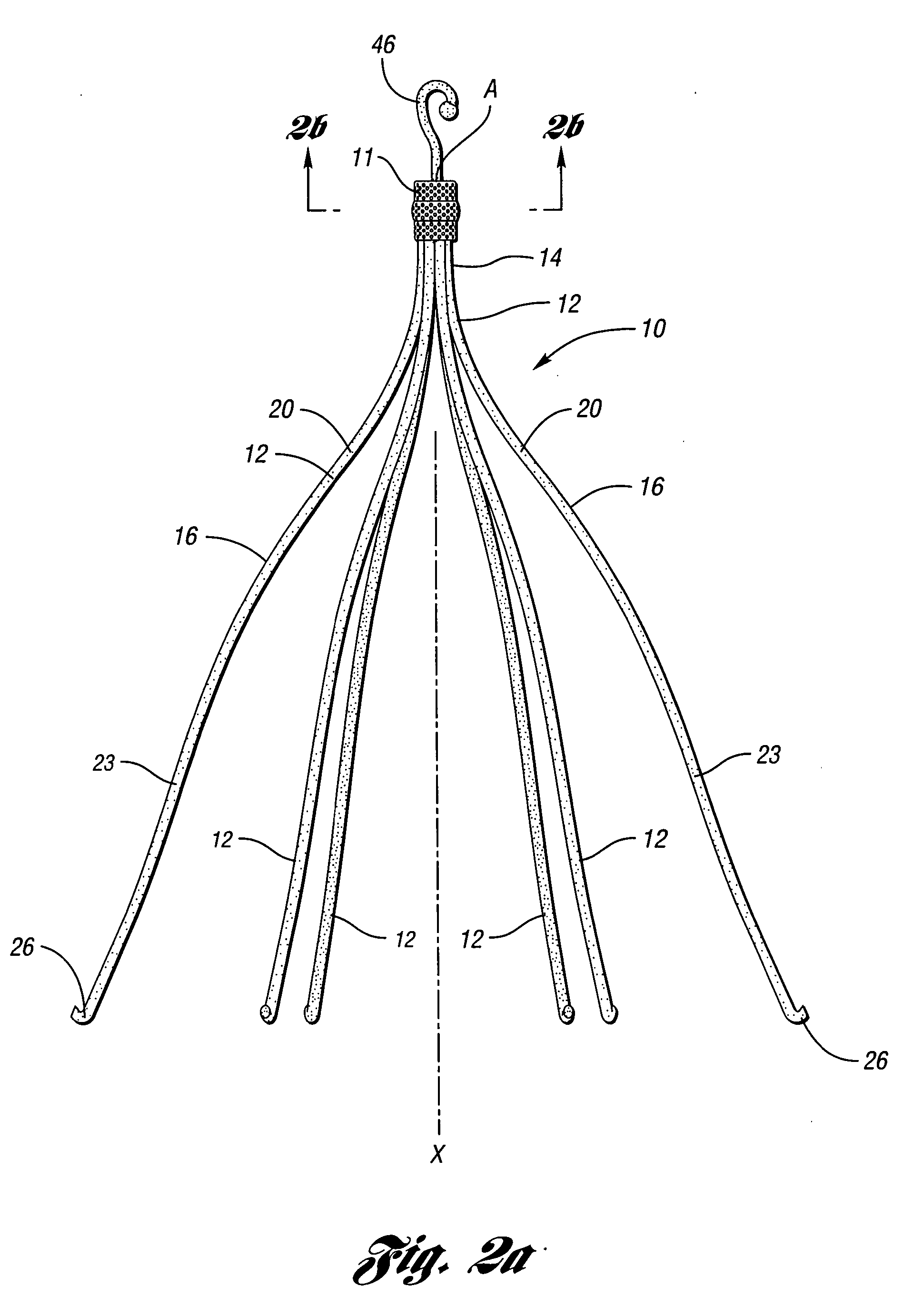
Anti-thrombus filter having enhanced identifying features
InactiveUS20060069405A1Easy to identifyReduced endotheliosis featureDiagnosticsSurgeryCeramic coatingThrombus
A removable filter for capturing thrombi in a body vessel. The filter has anti-thrombogenic, echogenic, and radiopaque features. The features of the filter provide for enhanced identifying and reduced endotheliosis in a body vessel of a patient. Generally, the anti-thrombogenic feature is preferably a fibrinolytic coating disposed on the filter to decrease the accumulation of fibrin thereon. The echogenic feature preferably is comprised of marks formed on the filter that give rise to reflections of ultrasound waves during ultrasonography. The radiopaque feature is preferably a polymeric coating, ceramic coating, or noble metal coating applied on the filter for enhanced fluoroscopy.
Owner:COOK INC
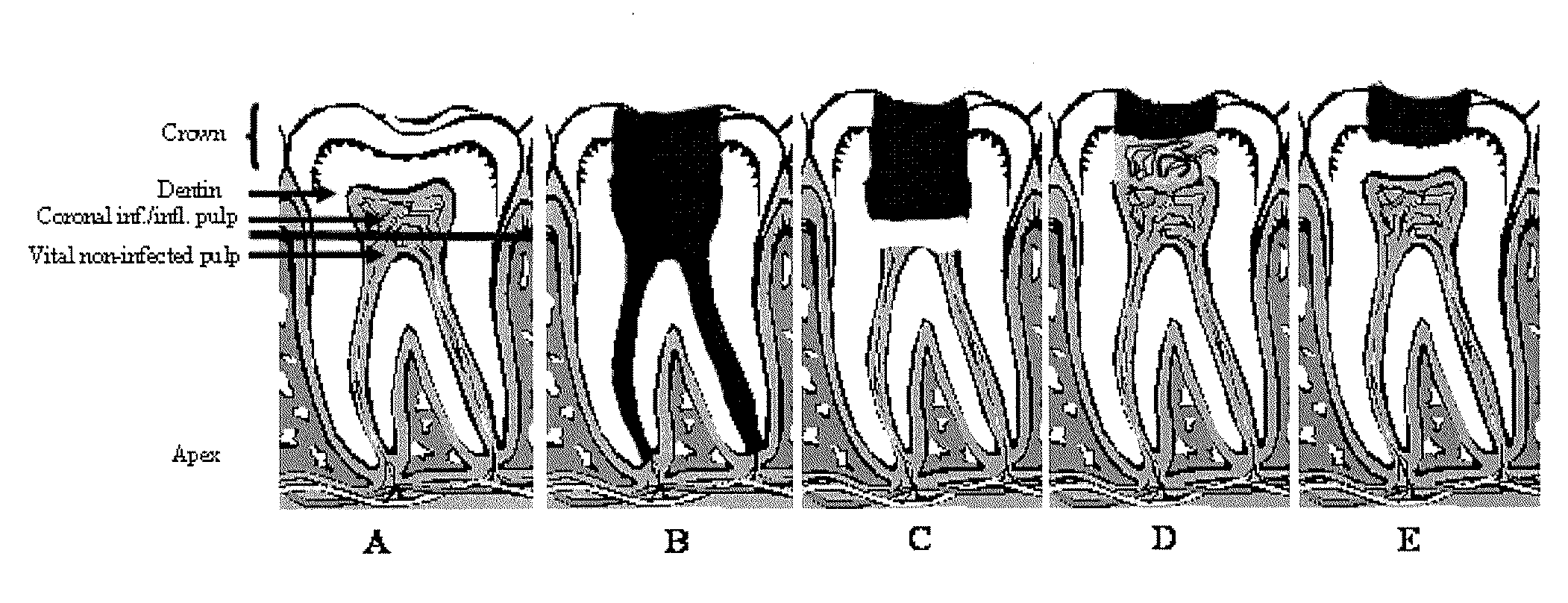
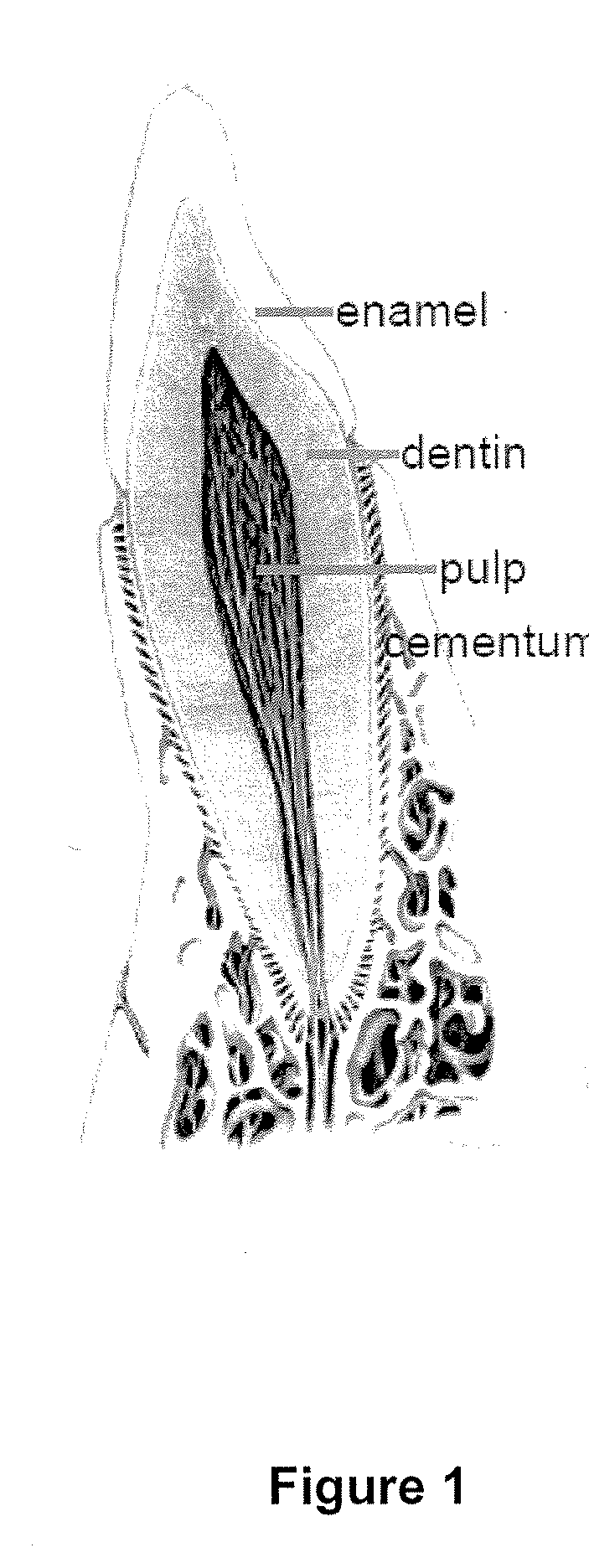
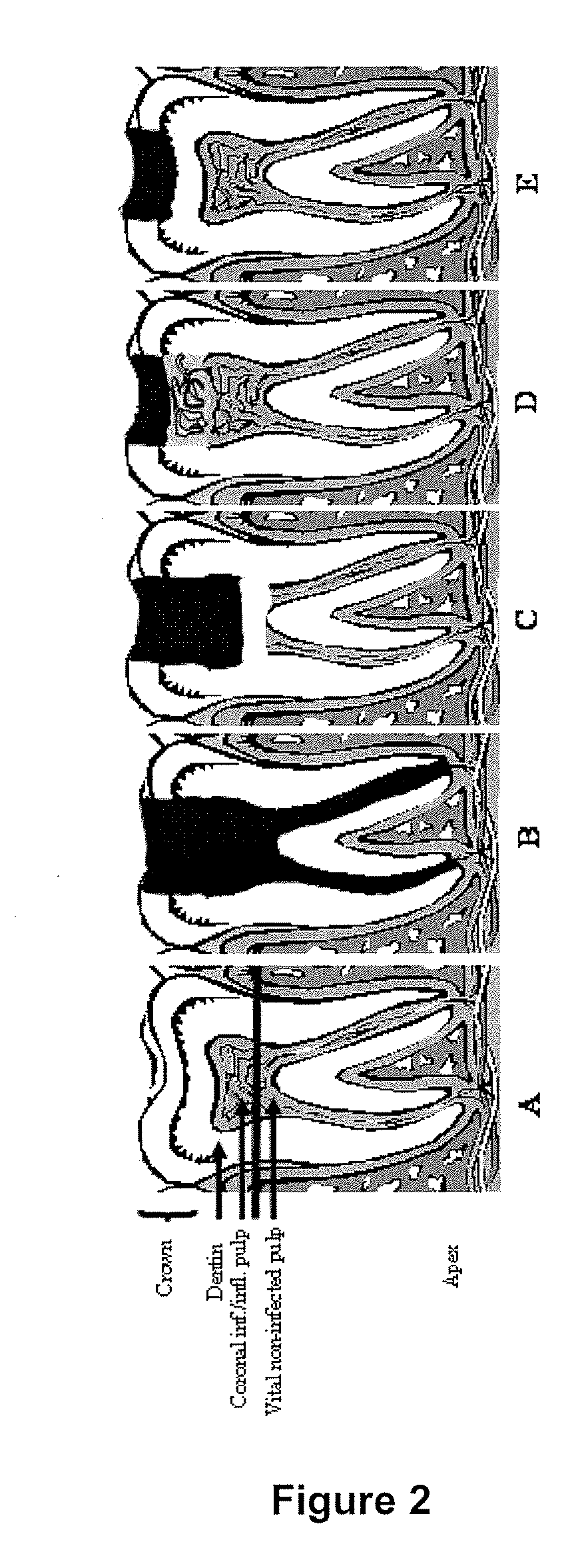
Compositions and methods for treating pulp inflammations caused by infection or trauma
The present disclosed subject matter relates to methods and compositions for restoring a diseased or damaged tooth such that infection is inhibited or eliminated and pulp regeneration is facilitated. The disclosed subject matter also includes a composition comprising a physiologically acceptable matrix seeded with pulp cells. The matrix can be capable of being injected into the pulp chamber of a tooth. In some embodiments, the matrix of a composition includes a hydrogel (e.g., collagen, chitosan, alginate, MATRIGEL™, gelatin, JELL-O®, fibrin), a mesh (e.g., polylactide-coglycolide (PLGA) mesh, polylactide (PLA) mesh, or polyglycolide (PGA) mesh, a cross-linked fiber mesh, a nanofiber mesh, a mesh fabric, biodegradable polymer mesh), a microsphere (biodegradable polymer microsphere, a hydrogel microsphere), or a combination of any of the foregoing. In yet other embodiments, the matrix includes a nanofiber, an artificial three-dimensional scaffold material, or a synthetic three-dimensional scaffold material.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
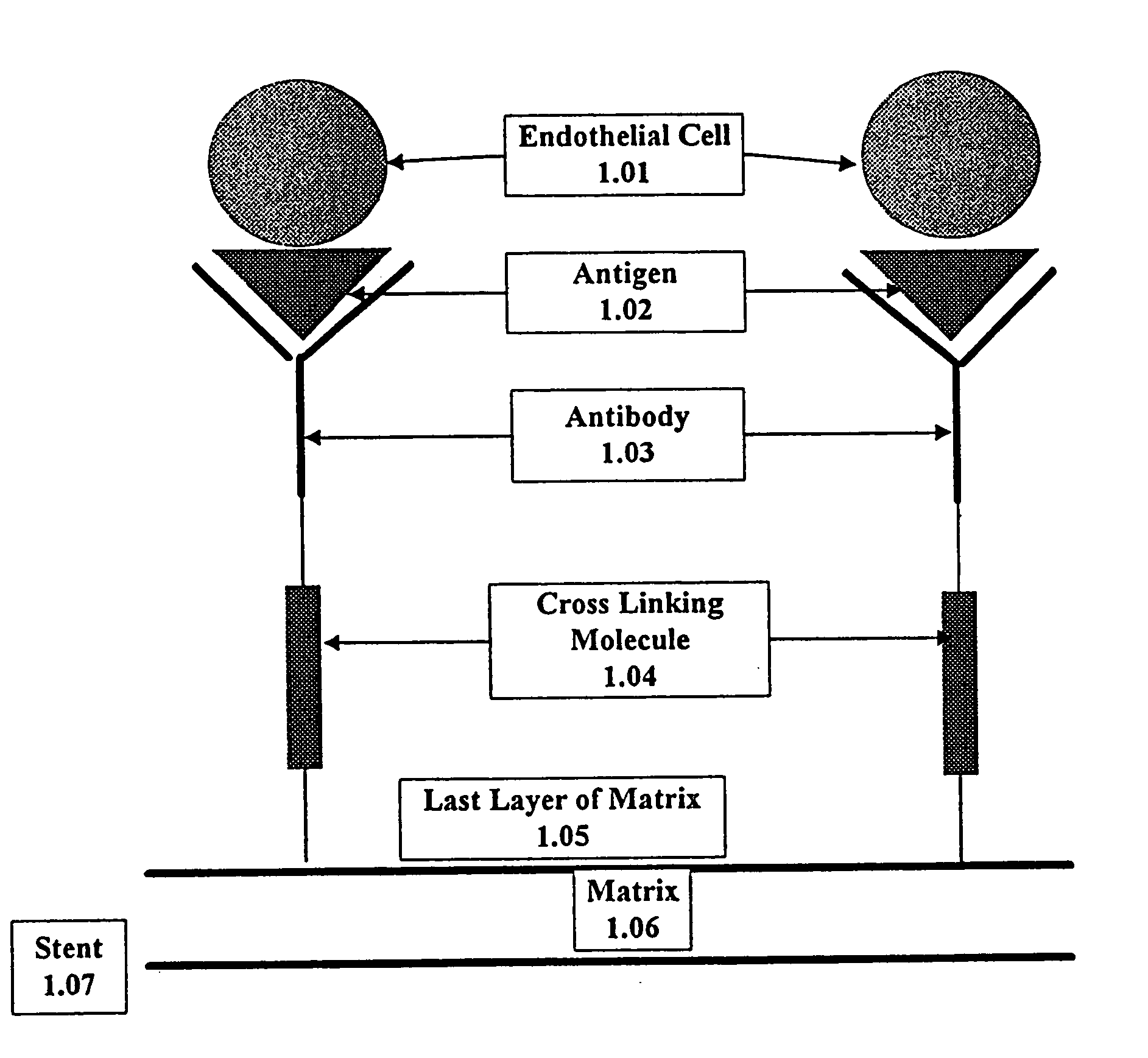
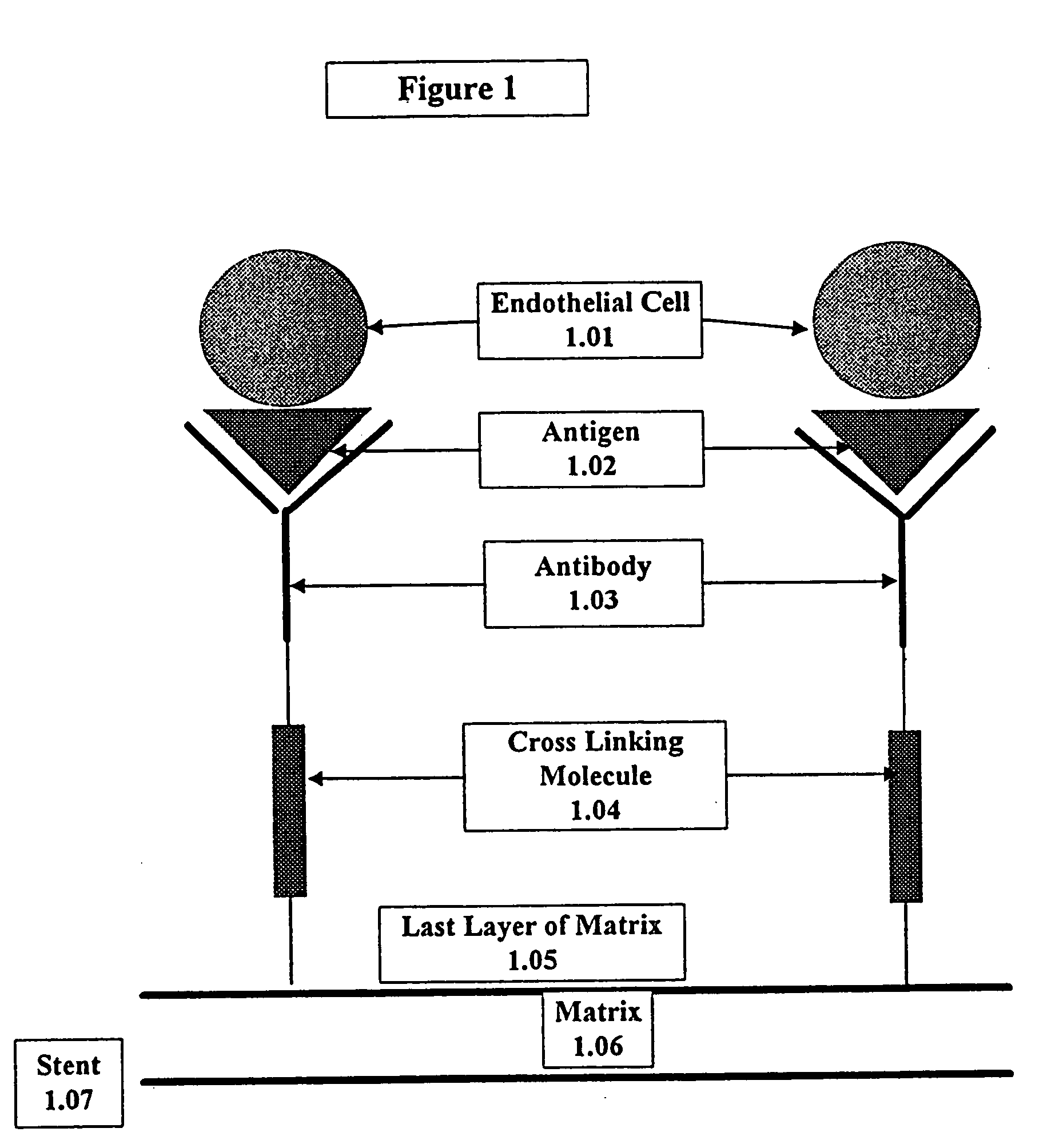
Medical device with coating that promotes endothelial cell adherence
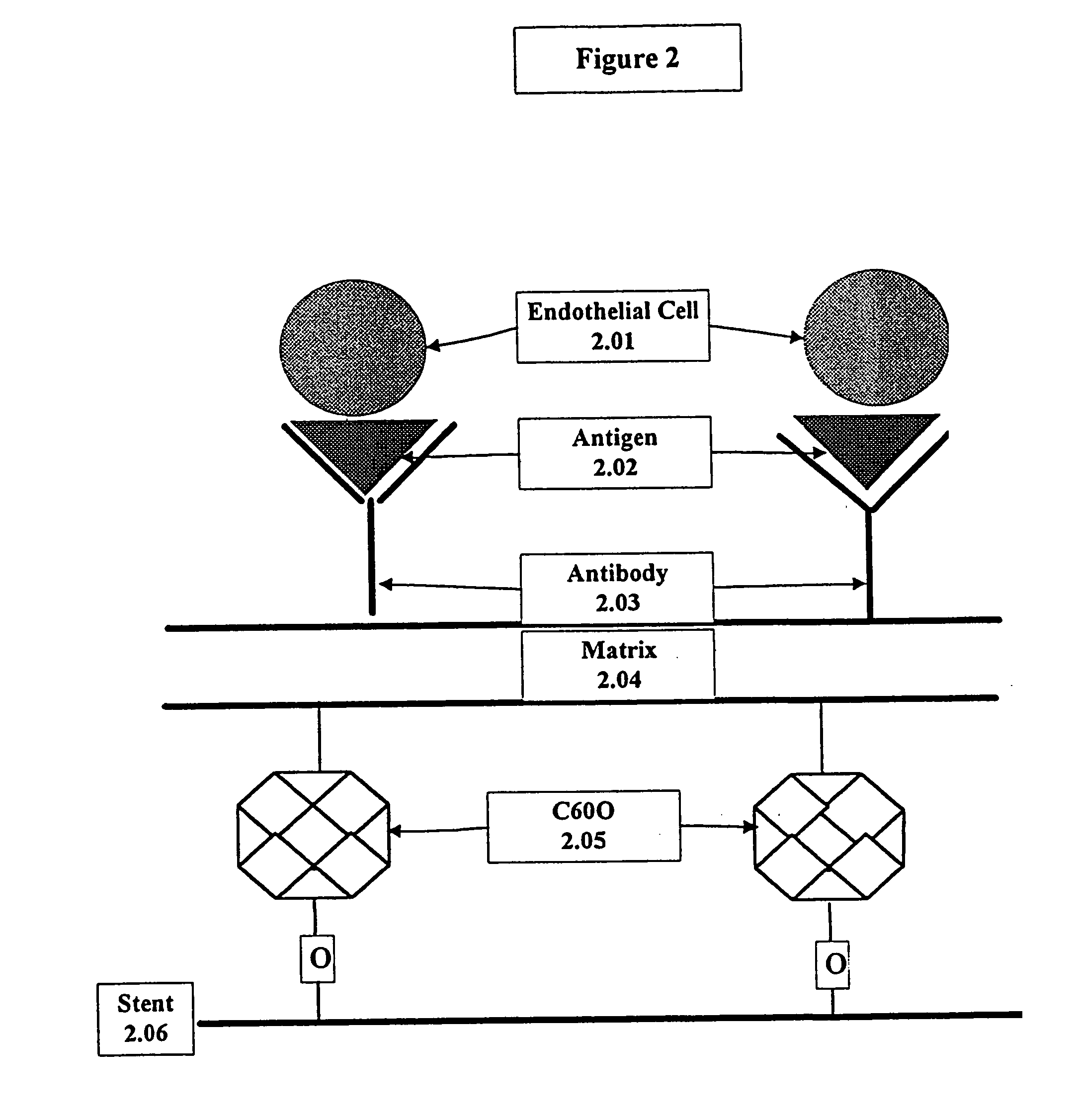
InactiveUS20050043787A1Prevent restenosisPreventing other thromboembolic complicationMaterial nanotechnologyPeptide/protein ingredientsAntigenPolyethylene glycol
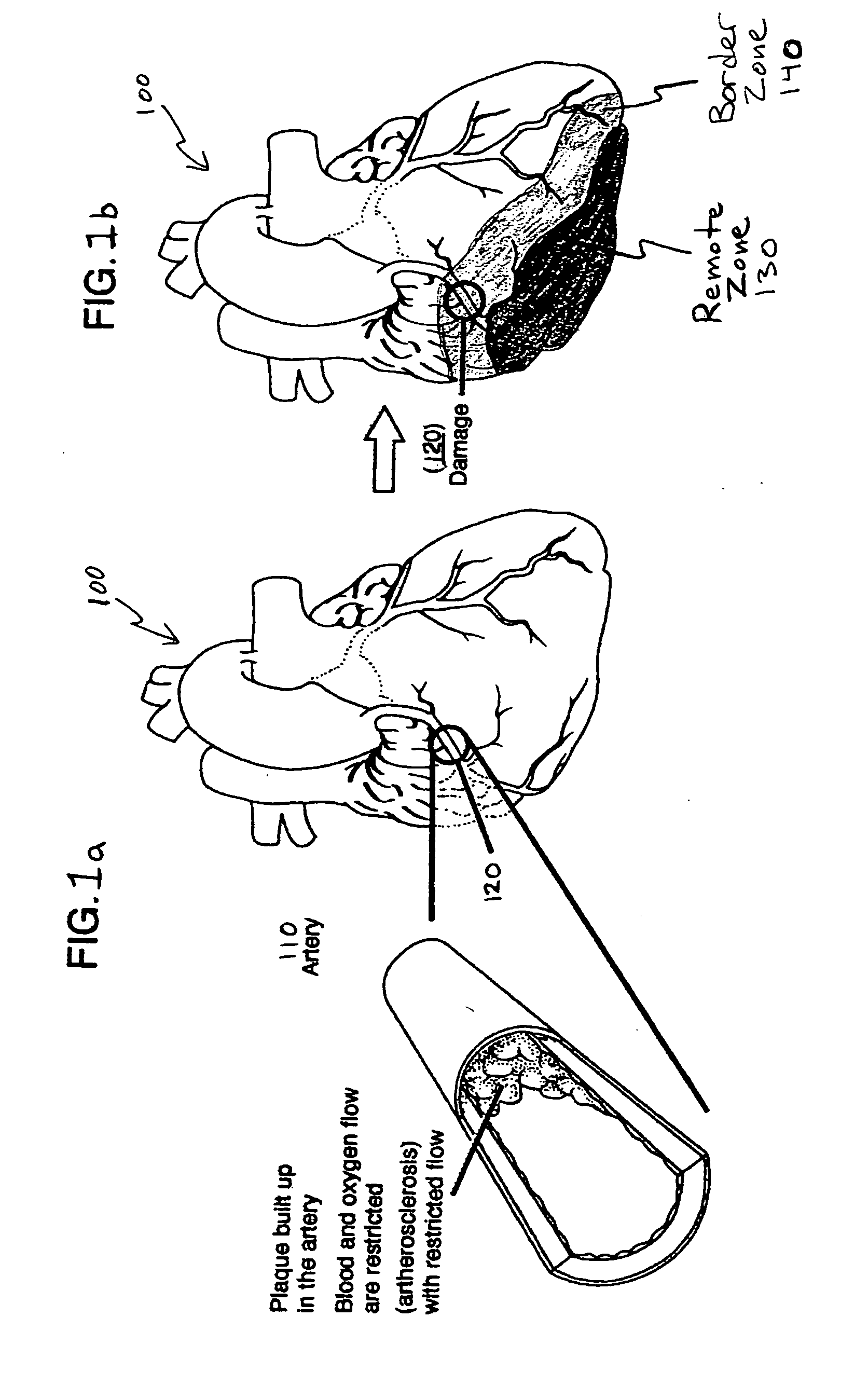
This invention provides compositions and methods for producing a medical device coated with a matrix and an antibody which reacts with an endothelial cell antigen. The matrix coating the medical device may be composed of synthetic material, such as polyurethane, poly-L-lactic acid, cellulose ester or polyethylene glycol. In another embodiment, the matrix is composed of naturally occurring materials, such as collagen, fibrin, elastin, amorphous carbon. In a third embodiment, the matrix may be composed of fullerenes. The fullerenes range from about C60 to about C100. The medical device may be a stent or a synthetic graft. The antibodies promote adherence of endothelial cells on the medical device. The antibodies may be mixed with the matrix or covalently tethered through a linker molecule to the matrix. Following adherence to the medical device, the endothelial cells differentiate and proliferate on the medical device. The antibodies may be different types of monoclonal antibodies. Methods of preparing such composition and methods of treating a mammal with atherosclerosis or other types of vessel obstruction are disclosed. By facilitating adherence of endothelial cells to the surface of the medical device, the methods and compositions of this invention will decrease the incidence of restenosis as well as other thromboembolic complications resulting from implantation of medical devices.
Owner:ORBUSNEICH MEDICAL PTE LTD
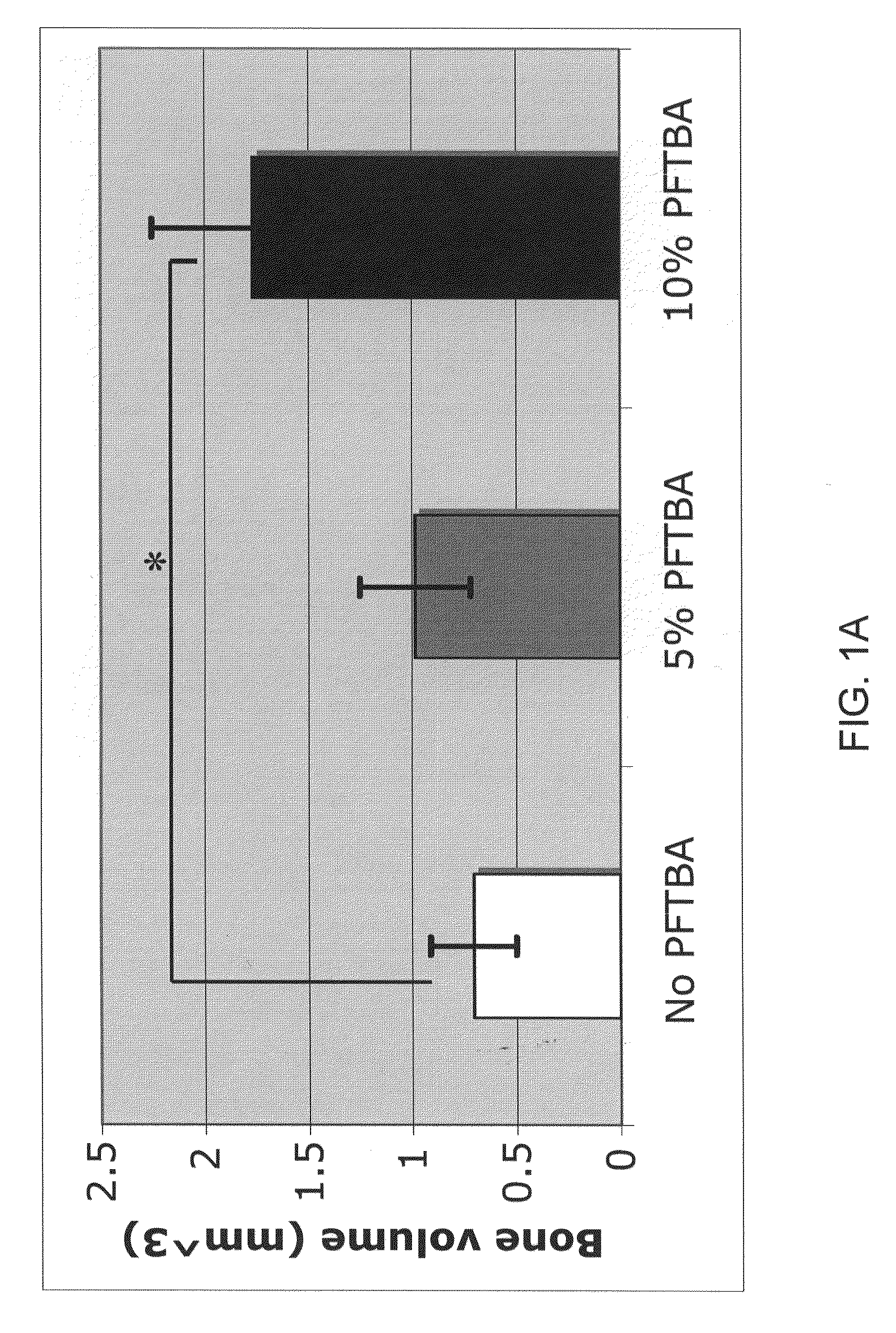
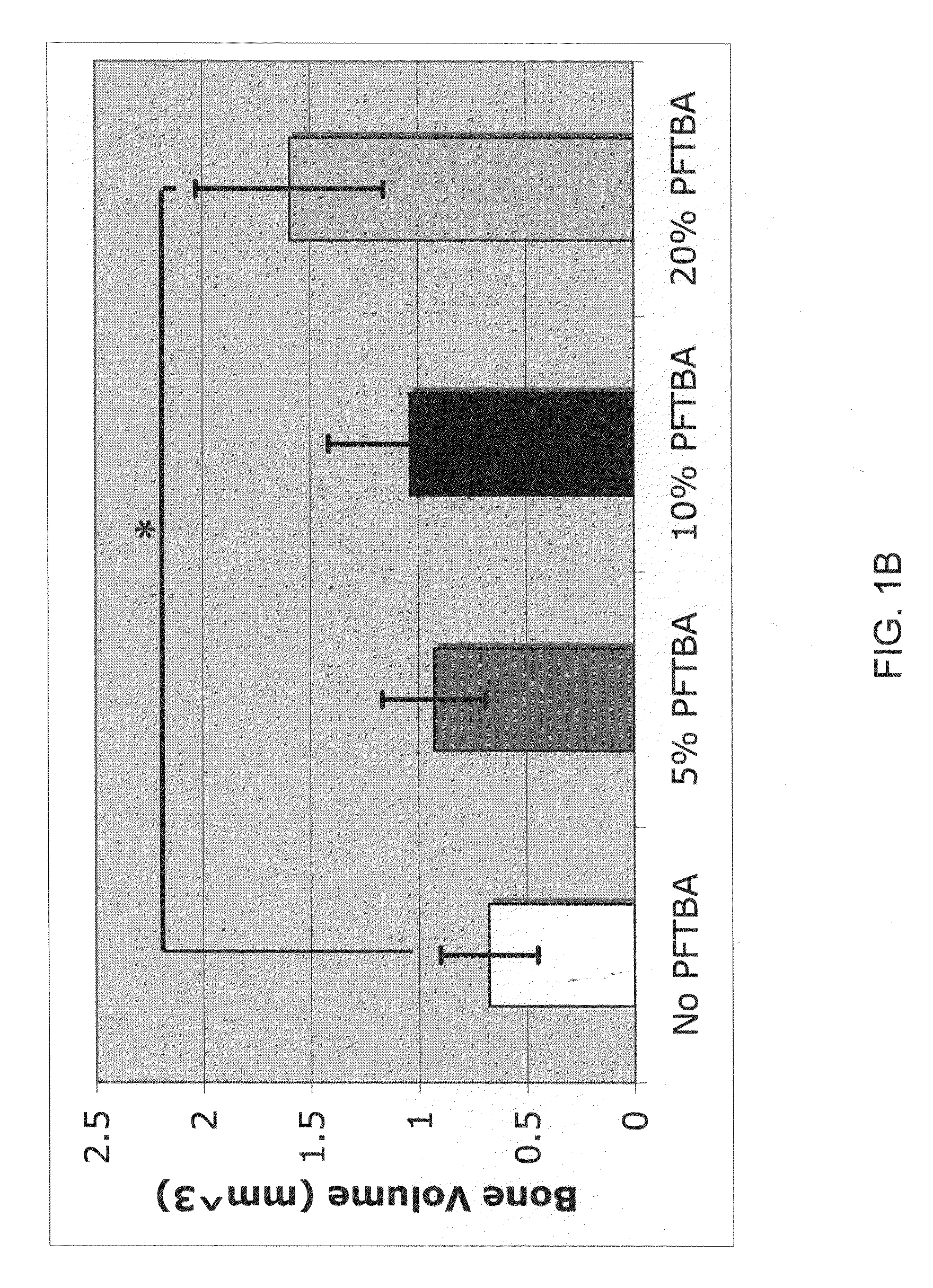
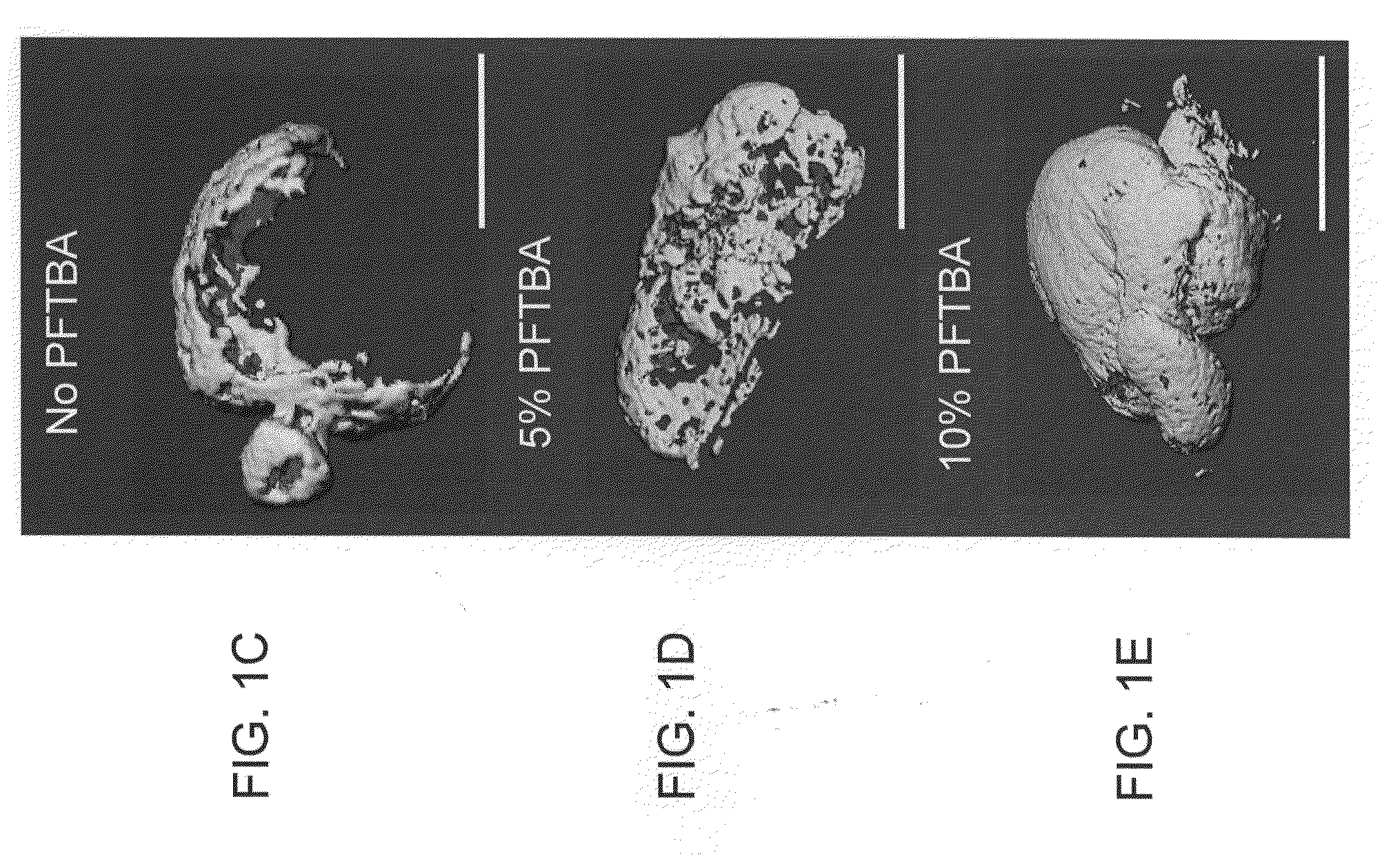
Scaffolds with oxygen carriers, and their use in tissue regeneration
Provided are fibrin or silk matrices comprising an oxygen carrier, and matrices, which comprise an oxygen carrier and mesenchymal stem cells. Also provided are methods of generating and using same for ex vivo or in vivo tissue regeneration and / or repair such as for treating a non-union bone fracture and a condition requiring spinal fusion.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
System/unit and method employing a plurality of magnetoelastic sensor elements for automatically quantifying parameters of whole blood and platelet-rich plasma
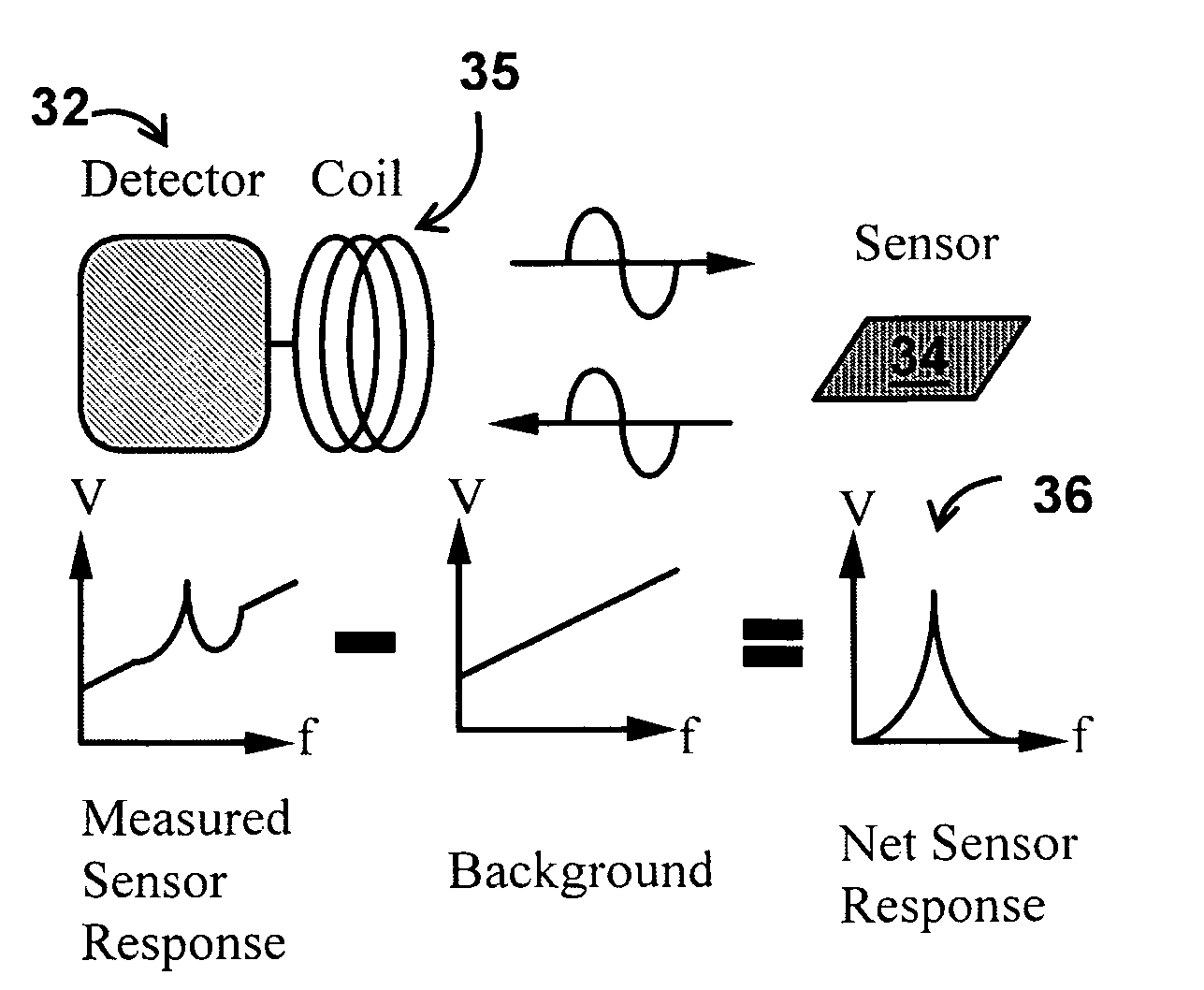
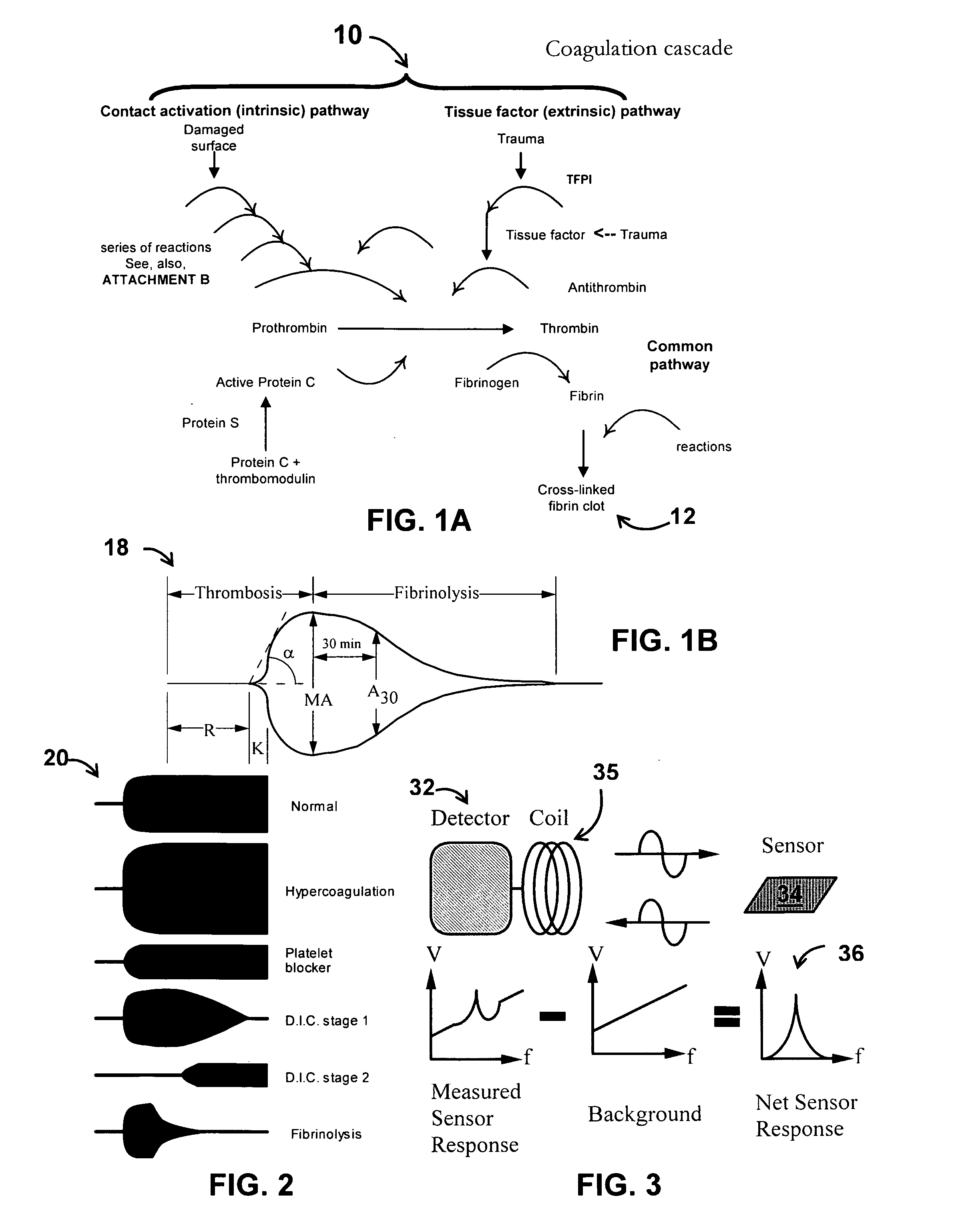
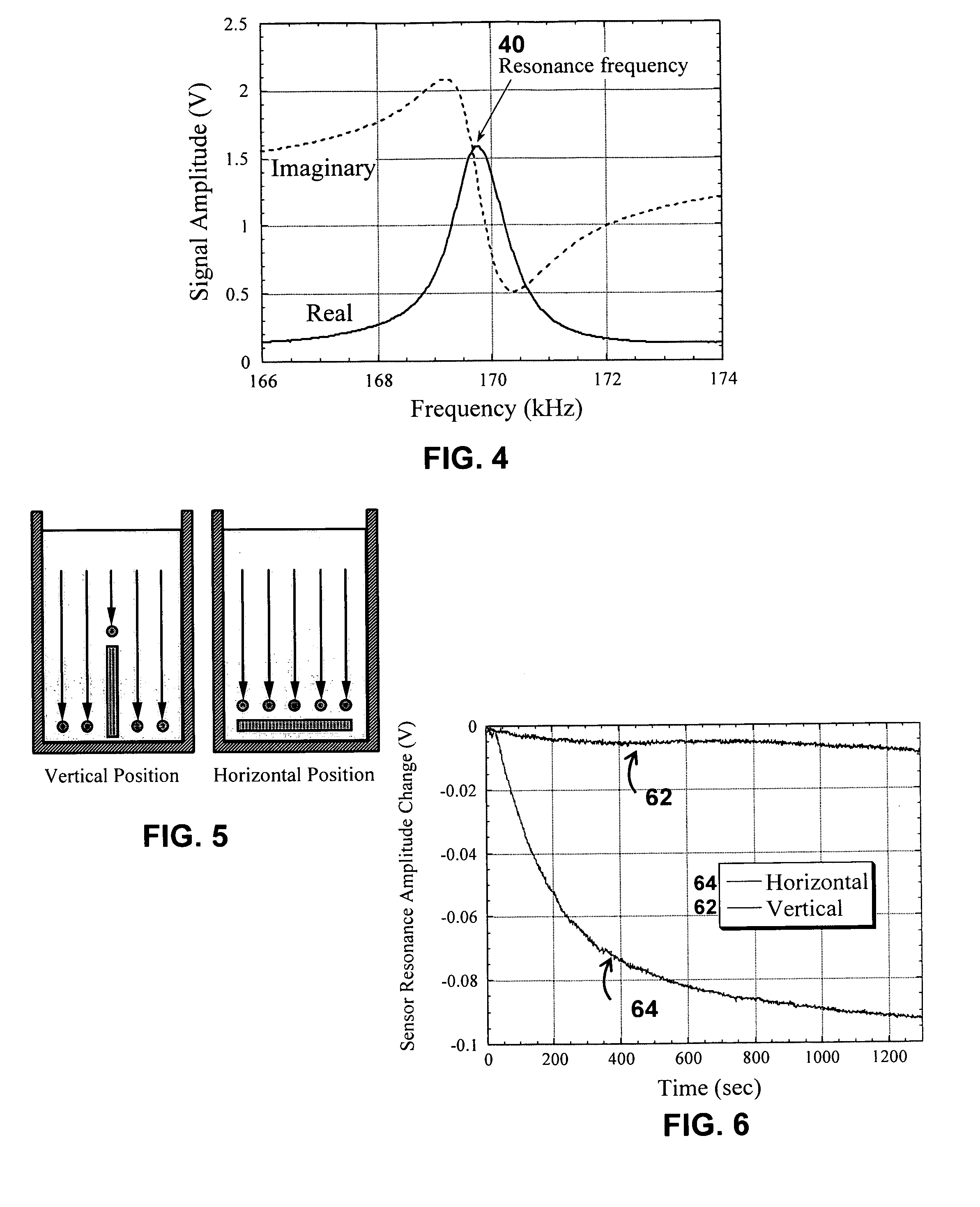
InactiveUS20080261261A1Quantifying platelet-fibrin clot strengthBioreactor/fermenter combinationsBiological substance pretreatmentsClot formationBlood plasma
A system / analyzer-unit and method / platform—using information obtained from at least one, adapted for a plurality of, magnetoelastic sensor elements in contact with one or more samples comprising blood from a patient—for automatically quantifying one or more parameters of the patient's blood. Information obtained from emissions measured from each of the sensor elements is uniquely processed to determine a quantification about the patient's blood, such as, quantifying platelet aggregation to determine platelet contribution toward clot formation; quantifying fibrin network contribution toward clot formation; quantifying platelet-fibrin clot interactions; quantifying kinetics of thrombin clot generation; quantifying platelet-fibrin clot strength; and so on. Structural aspects of the analyzer-unit include: a cartridge having at least one bay within which a sensor element is positioned; each bay in fluid communication with both (a) an entry port for injecting a first blood sample composed of blood taken from the patient (human or other mammal), and (b) a gas vent through which air displaced by injecting the first blood sample into the bay.
Owner:KMG2 SENSORS CORP
Matrix composed of a naturally-occurring protein backbone cross linked by a synthetic polymer and methods of generating and using same
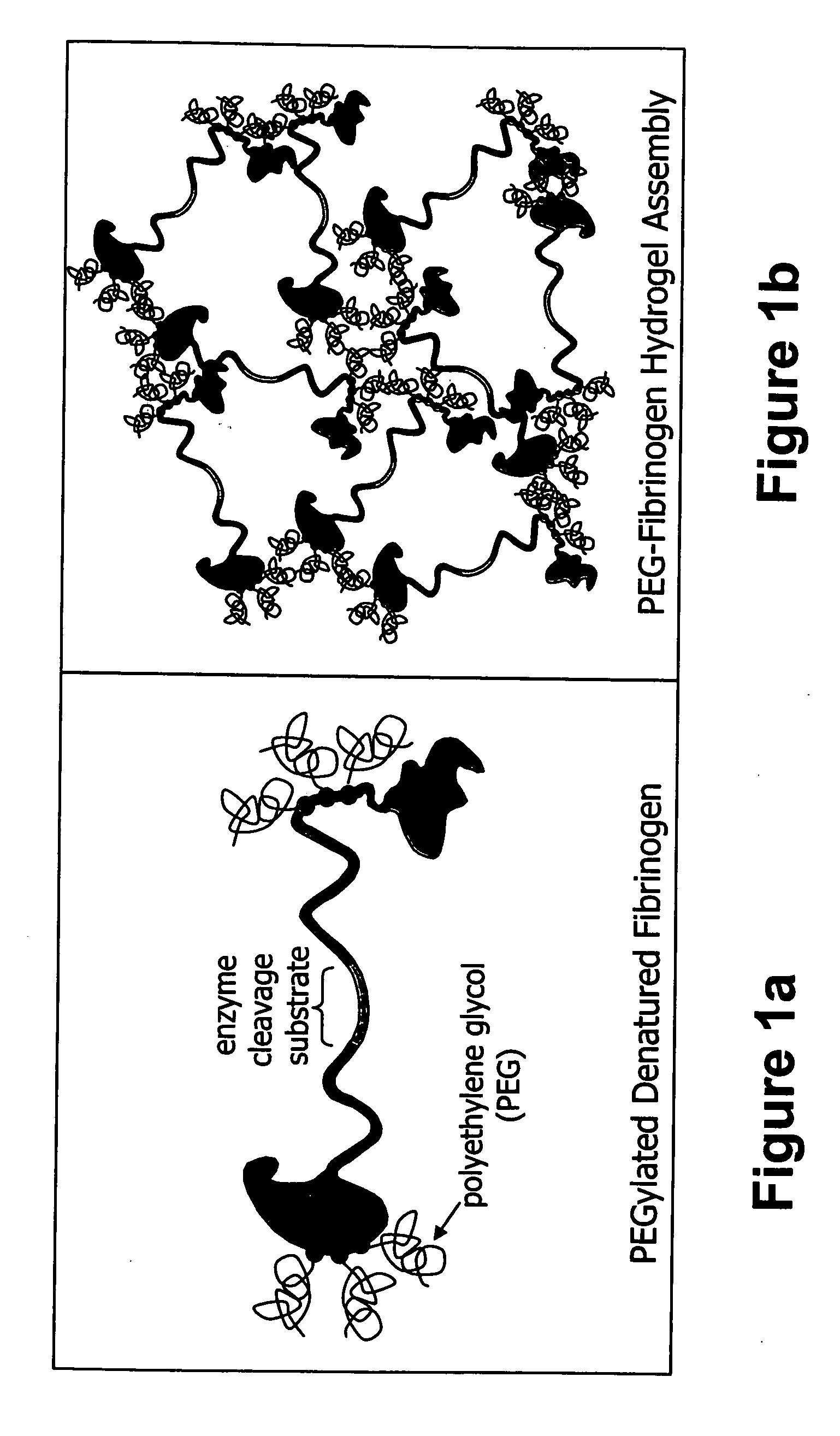
The present invention relates to biodegradable scaffolds composed of a naturally-occurring protein backbone cross-linked by a synthetic polymer. Specifically, the present invention provides PEGylated-fibrinogen scaffold and methods of generating and using same for treating disorders requiring tissue regeneration.
Owner:REGENTIS BIOMATERIALS
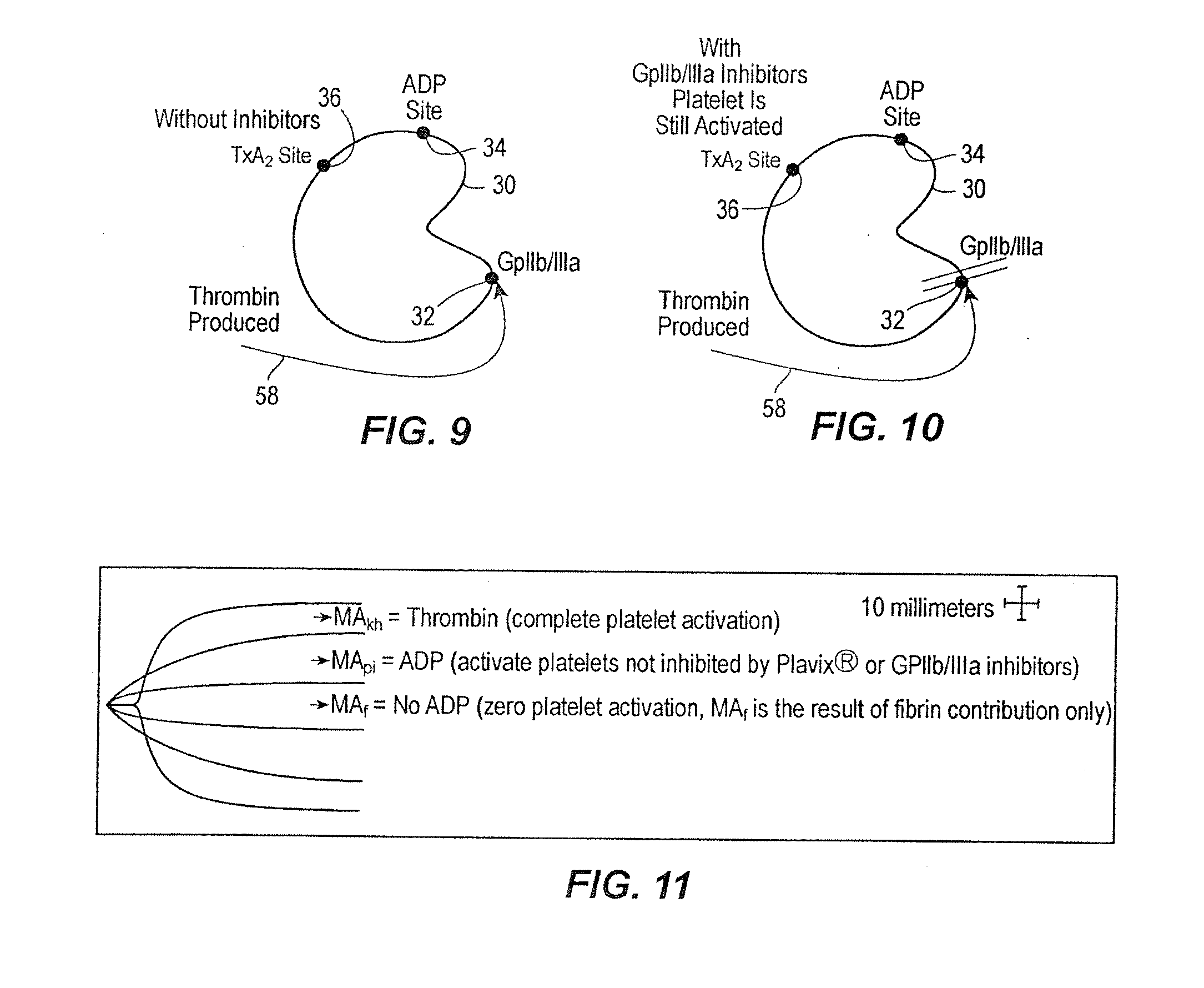
Protocol for monitoring platelet inhibition
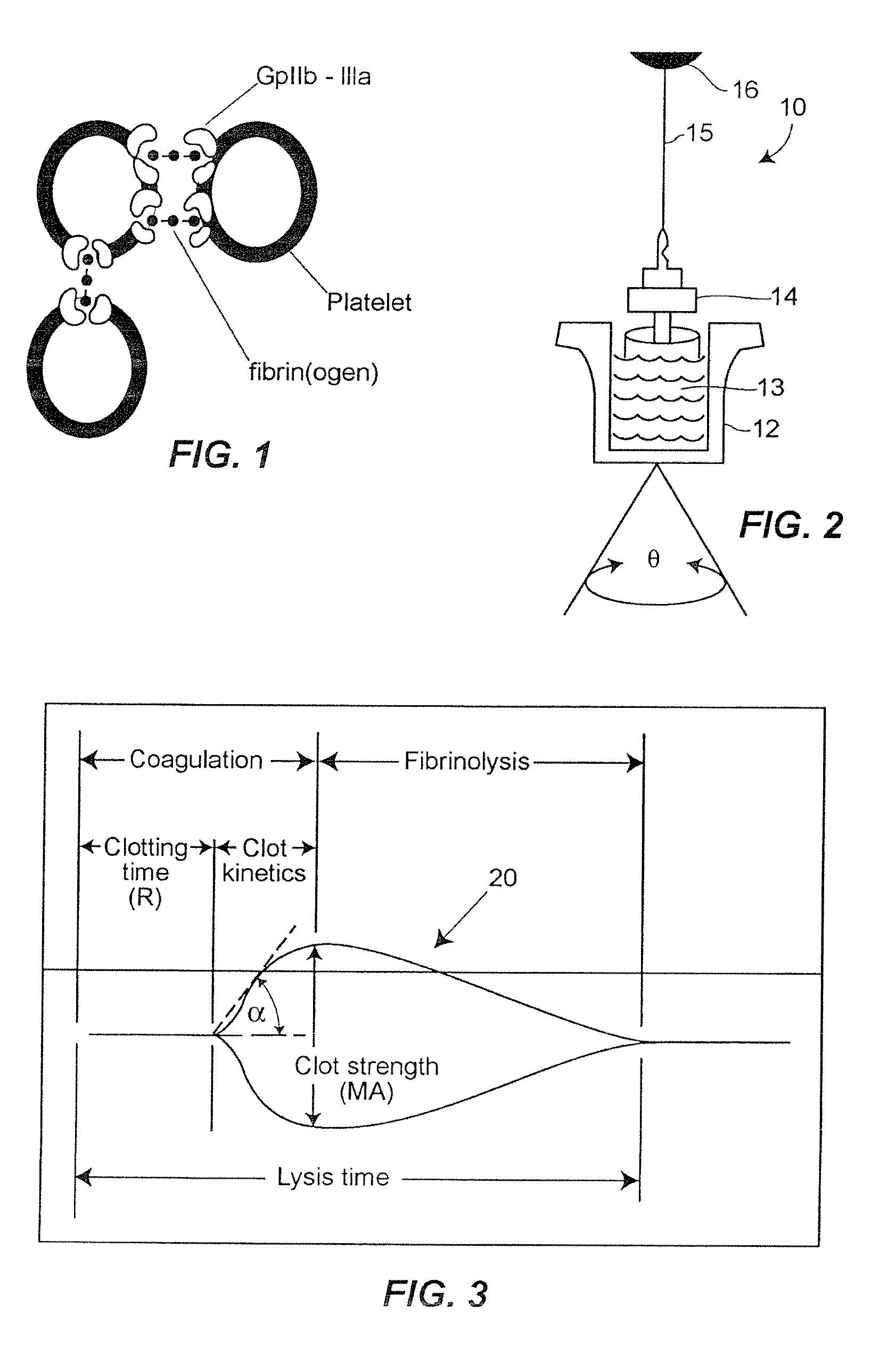
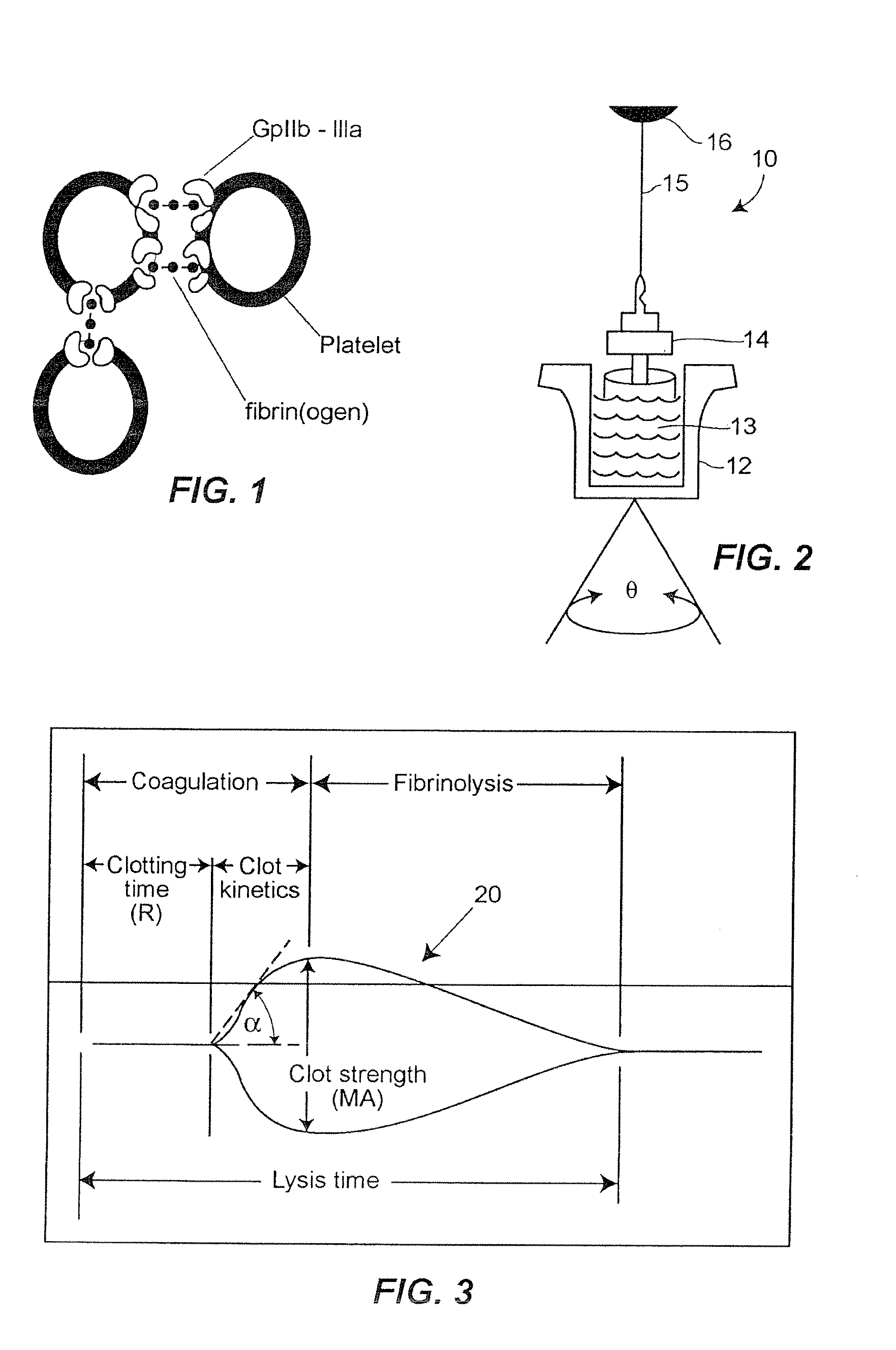
A hemostasis analyzer, such as the Thrombelastograph® (TEG®) hemostasis analyzer is utilized to measure continuously in real time, the hemostasis process from the initial fibrin formation, through platelet-fibrin interaction and lysis to generate blood hemostasis parameters. The measured blood hemostasis parameters permit evaluation of platelet inhibition therapy.
Owner:HAEMONETICS
Vascular inducing implants
Implants and associated delivery systems for promoting angiogenesis in ischemic tissue are provided. The implants may be delivered percutaneously, thoracically or surgically and are particularly well suited for implantation into the myocardium of the heart. The implants are configured to have a first configuration having a low profile and an expanded, second configuration having a large profile. The implants are delivered to the ischemic tissue location in the first configuration, implanted then expanded to the second configuration. The expanded implants maintain a stress on the surrounding tissue, irritating and slightly injuring the tissue to provoke an injury response that results in angiogenesis. The flow of blood from the surrounding tissue into the implant and pooling of the blood in and around the implant leads to thrombosis and fibrin growth. This healing process leads to angiogenesis in the tissue surrounding the implant. Additionally, the implants may contain an angiogenic substance or a thrombus of blood, preloaded or injected after implantation to aid in initiating angiogenesis.
Owner:CR BARD INC
Ultrasound target vessel occlusion using microbubbles
InactiveUS20070041961A1Less invasive techniqueSimple technologyUltrasonic/sonic/infrasonic diagnosticsUltrasound therapyCavitationThrombus
Selective occlusion of a blood vessel is achieved by selectively damaging endothelial cells at a target location in the blood vessel, resulting in the formation of a fibrin clot proximate to the damaged endothelial cells. Additional fibrinogen can then be introduced into the blood vessel if occlusion is not achieved, as the fibrinogen is converted to fibrin by enzymes released by the exposed thrombogenic tissue and activated platelets. Endothelial cells are selectively damaged using thermal effects induced by ultrasound, by mechanical effects induced by ultrasound, or by mechanical effects produced by a tool introduced into the blood vessel (such as a catheter-based tool). A particularly preferred technique for selectively damaging endothelial cells involves introducing an ultrasound activatable agent into the blood vessel, and causing cavitation in that agent using pulses of high-intensity focused ultrasound having a duration insufficient to induce thermal damage in adjacent perivascular tissue.
Owner:UNIV OF WASHINGTON
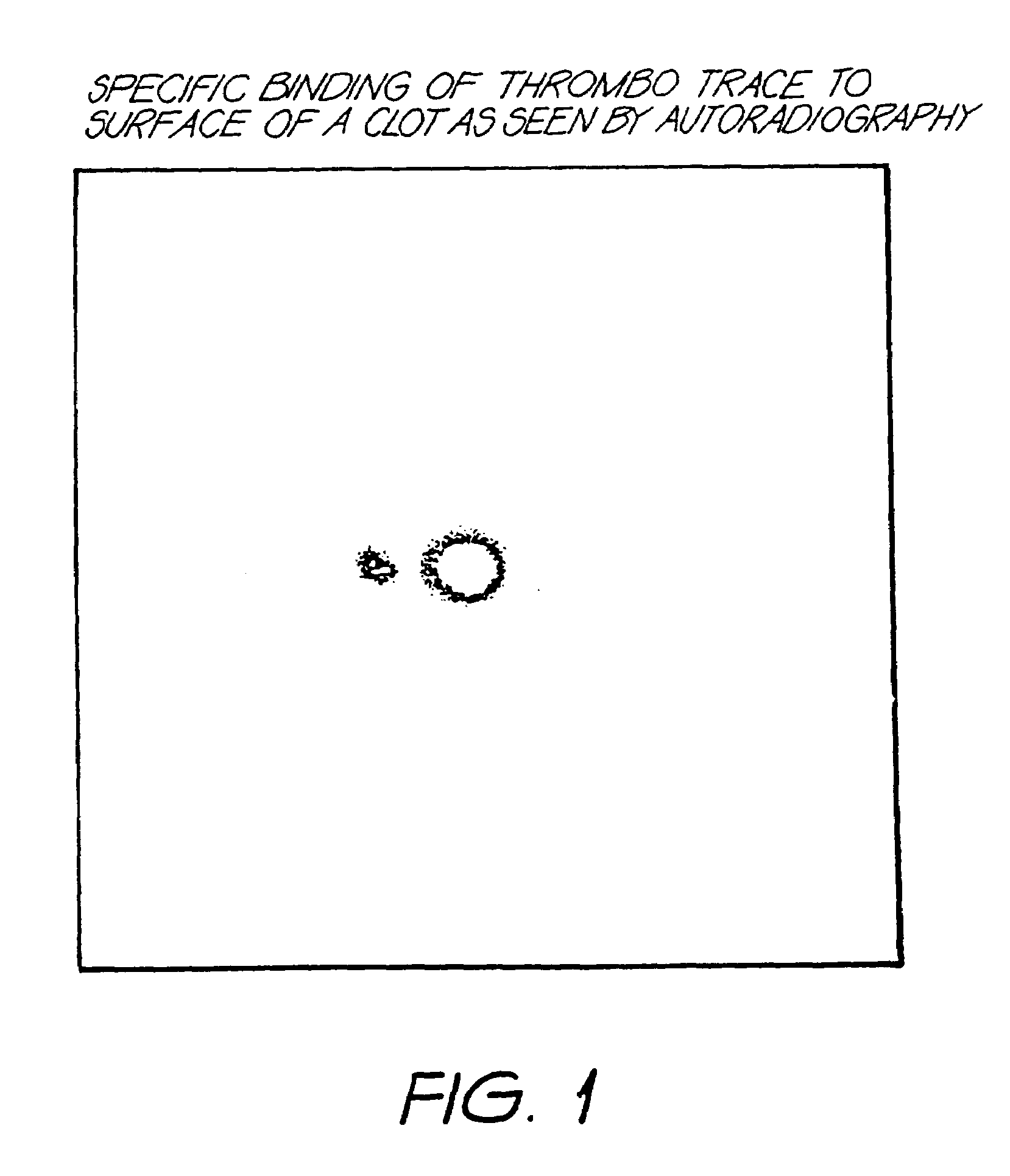
Method for detection of fibrin clots
InactiveUS6977068B1Improve binding efficiencyEasy to producePowder deliveryRadioactive preparation carriersCarbon layerIn vivo
A method for the detection of fibrin in a source, in particular the in vivo detection of a fibrin in a patient, the method comprising supplying to the source or patient an amount of a detectable reagent comprising a plurality of discrete particles, each of the particles comprising a plurality of layers of carbon and being capable of binding to fibrin; and detecting the presence of the particles in the source. The particles may also comprise a detectable marker encased in said plurality of layers of carbon, the presence of said marker being capable of detection in said source.
Owner:AUSTRALIEN NAT UNIV
Fibrin material and method for producing and using the same
This invention describes a bioerodible fibrin material which is obtained by mixing fibrinogen and thrombin reconstituted or diluted with a particular high tonic strength medium, free of calcium. Such a fibrin-based biomaterial develops a tight structure with thin fibers and small pore size suitable for use as an anti-adhesion barrier. In this invention, thrombin is no longer the variable which governs the tightness and the porosity of the fibrin material obtained, but still controls the clotting time. The mechanical behavior, high-water capacity, and releasable retention properties for therapeutic agents of this fibrin structure causes the fibrin material to be ideally suited for use as a drug delivery device, capable of delivering proteins, hormones, enzymes, antibiotics, antineoplastic agents and even cells for local and systemic treatment of human and non-human patients.
Owner:BAXTER INT INC
Method of evaluating patient hemostasis
A hemostasis analyzer, such as the Thrombelastograph® (TEG®) hemostasis analyzer is utilized to measure continuously in real time, the hemostasis process from the initial fibrin formation, through platelet-fibrin interaction and lysis to generate blood hemostasis parameters. The measured blood hemostasis parameters permit evaluation of a patient hemostasis condition.
Owner:HAEMONETICS
Plasma concentrator device
ActiveUS20060175268A1Reduce plasma polarizationSufficient amountWater/sewage treatment by centrifugal separationRotary centrifugesReciprocating motionBlood plasma
A plasma concentrator of this invention having a concentrator chamber, concentrator gel beads, a filter, and an agitator. The agitator has agitator blades extending outwardly from the lower end. The agitator end is positioned in the concentrator chamber and supported for rotation about its central axis and for reciprocal movement along its central axis. The concentrator has a top with an upper opening through which the upper end of the actuator stem extends, and a lower opening in which the filter is positioned. The concentrator chamber can have a cylindrical inner wall, and the agitator blades can have an outer edge in close proximity to the inner wall with the space between the outer edge and the inner wall being less than the diameter of the gel beads. The filter is selected to block effective flow of plasma therethrough under ambient gravity conditions and permit plasma and plasma concentrate flow therethrough under centrifugal forces of the separation gravity. The method concentrates plasma by removing water without significantly denaturing the fibrinogen in the plasma. The plasma is introduced into a concentration chamber containing a plurality of dehydrated concentrator gel beads and an agitator. Then water is removed from the plasma while stirring the beads to reduce plasma polarization and breaking up clumps of beads that form during the agitation. Then centrifugal force can be applied to the concentrated plasma in an amount sufficient to separate a substantial portion of the plasma concentrate from the beads.
Owner:HANUMAN +1
Free-standing biodegradable patch
InactiveUS20110071498A1Increase flexibilityImprove mechanical propertiesHydrolasesPeptide/protein ingredientsThrombin activityFibrinogen
Methods and apparatus for a free-standing biodegradable patch suitable for medical applications, especially intravascular, minimally-invasive and intraoperative surgical applications are provided, wherein the patch comprises a free-standing film or device having a mixture of a solid fibrinogen component and a solid thrombin component that, when exposed to an aqueous environment, undergoes polymerization to form fibrin. In alternative embodiments the patch may comprise a solid fibrinogen component, with or without an inorganic calcium salt component. The patch may take a non-adherent form during delivery to a target location within a vessel or tissue, and thereafter may be activated to adhere to vessel wall or tissue, and may include a number of additives, including materials to improve the mechanical properties of the patch, or one or more therapeutic or contrast agents.
Owner:BIOINSPIRE TECH
Method of Evaluating Patient Hemostasis
InactiveUS20070184508A1Microbiological testing/measurementDiagnostic recording/measuringLysisPlatelet
A hemostasis analyzer, such as the Thrombelastograph® (TEG®) hemostasis analyzer is utilized to measure continuously in real time, the hemostasis process from the initial fibrin formation, through platelet-fibrin interaction and lysis to generate blood hemostasis parameters. The measured blood hemostasis parameters permit evaluation of a patient hemostasis condition.
Owner:HAEMONETICS
Antagonists of IL-6 to prevent or treat thrombosis
ActiveUS8277804B2Inhibit formation and biological effectEffective treatmentImmunoglobulins against cytokines/lymphokines/interferonsDepsipeptidesDiseaseElevated C-reactive protein
The present invention is directed to therapeutic methods using IL-6 antagonists such as antibodies and fragments thereof having binding specificity for IL-6 to prevent or treat thrombosis in diseases associated with abnormal blood coagulation or fibrinolysis. In preferred embodiments these patients will comprise those exhibiting elevated D-dimer or other coagulation cascade related proteins and optionally will further exhibit elevated C reactive protein prior to treatment. The subject therapies also may include the administration of other actives such as chemotherapeutics, anti-coagulants, statins, et al.
Owner:VITAERIS INC +1
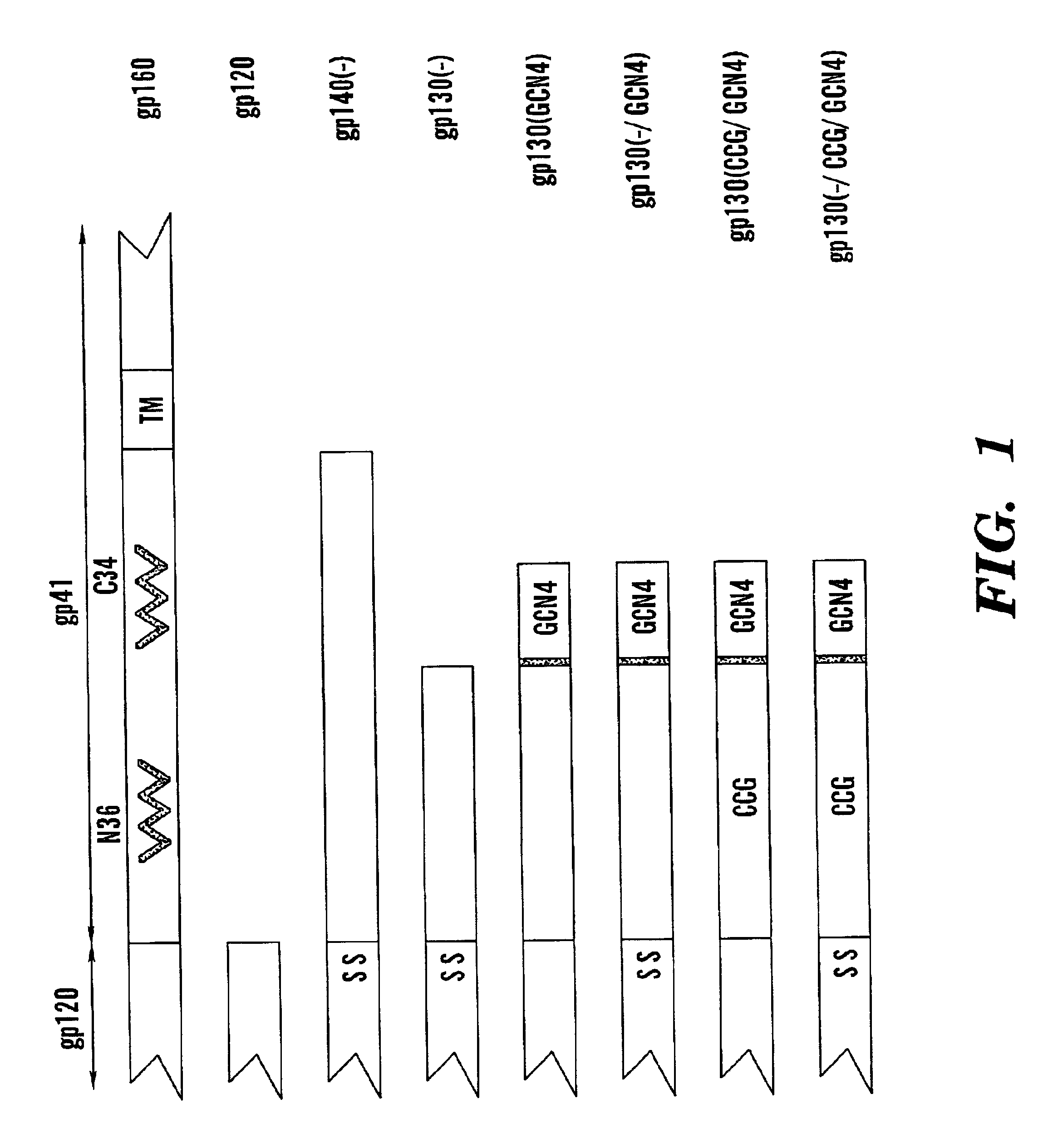
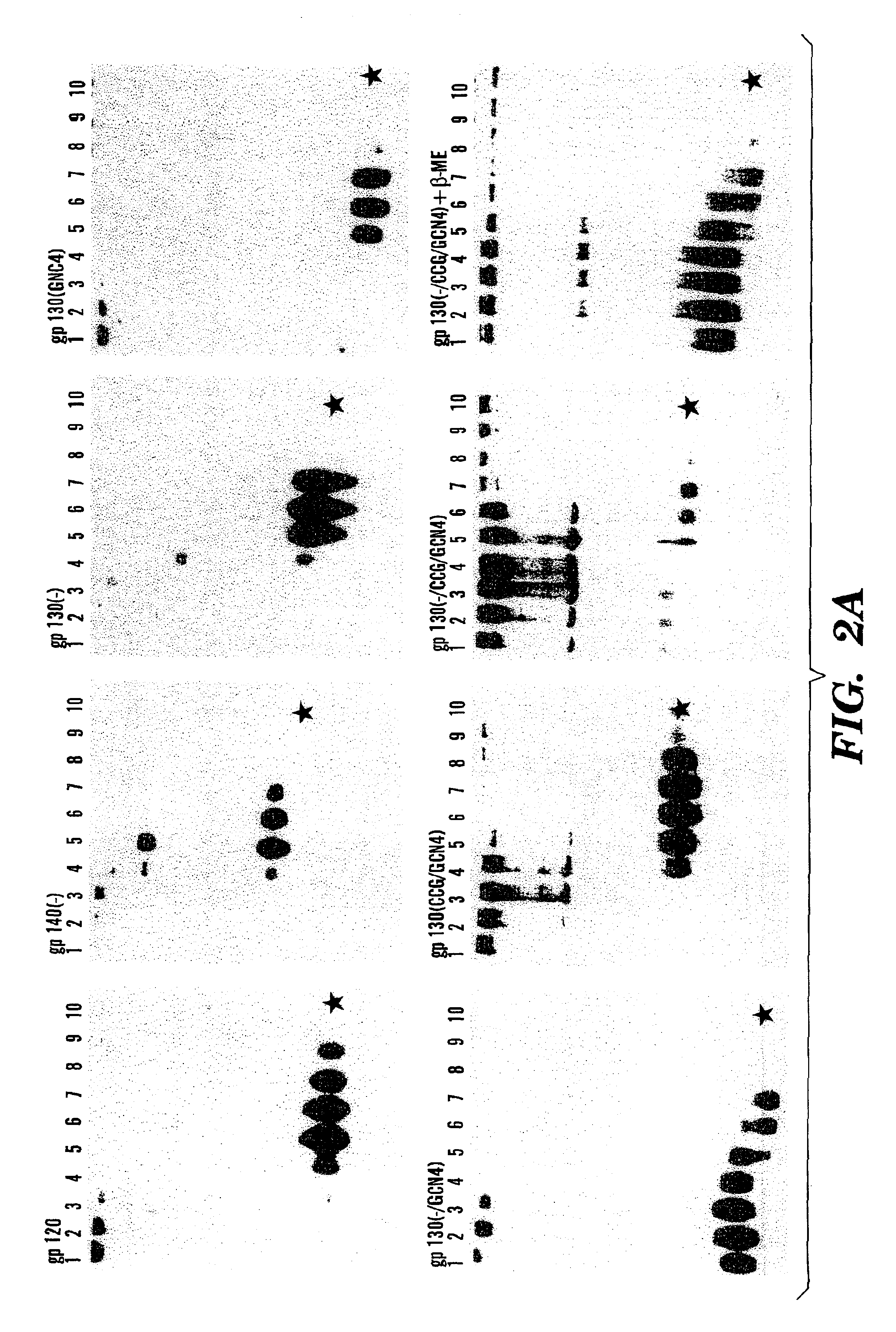
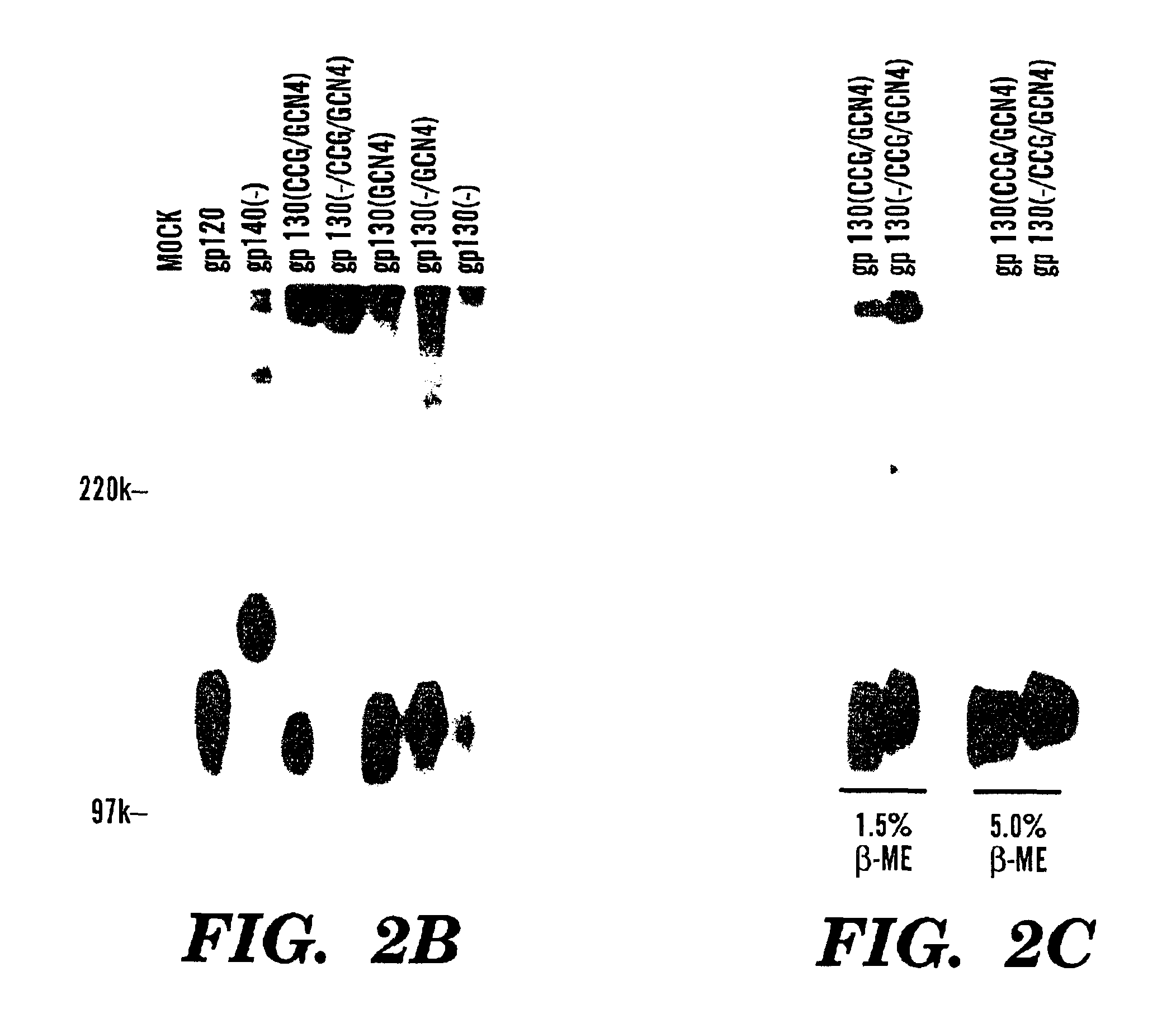
Stabilized soluble glycoprotein trimers
InactiveUS6911205B2Stabilize trimerSimplifies isolationPeptide/protein ingredientsAntibody mimetics/scaffoldsHiv envelopeGp41
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Fibrin-containing composition
InactiveUS20060128016A1Improve performanceRapidly and simply producingSenses disorderFibrinogenFiberPurification methods
It is intended to provide a scaffold material having favorable properties and being appropriate for cell proliferation and differentiation in regeneration therapy. Namely, a fibrin-containing biological scaffold material to be used in the case of employing a fibrin composition for the regeneration of a human tissue and cell proliferation, characterized by containing a mixture of a fibrinogen concentrate, which is obtained from human plasma by a quick and rough purification method, with a fibrinogen activator.
Owner:ASAHI KASEI MEDICAL CO LTD
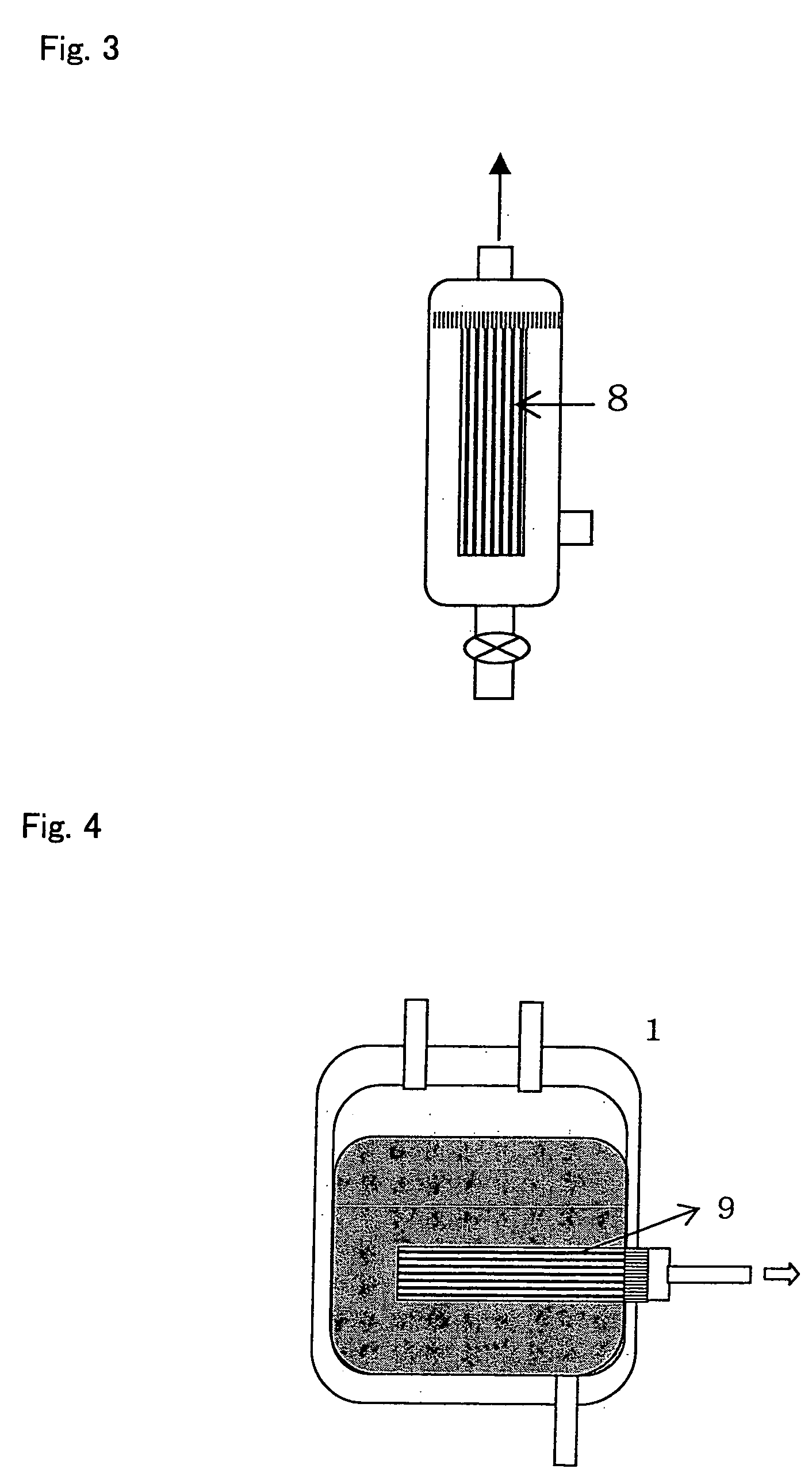
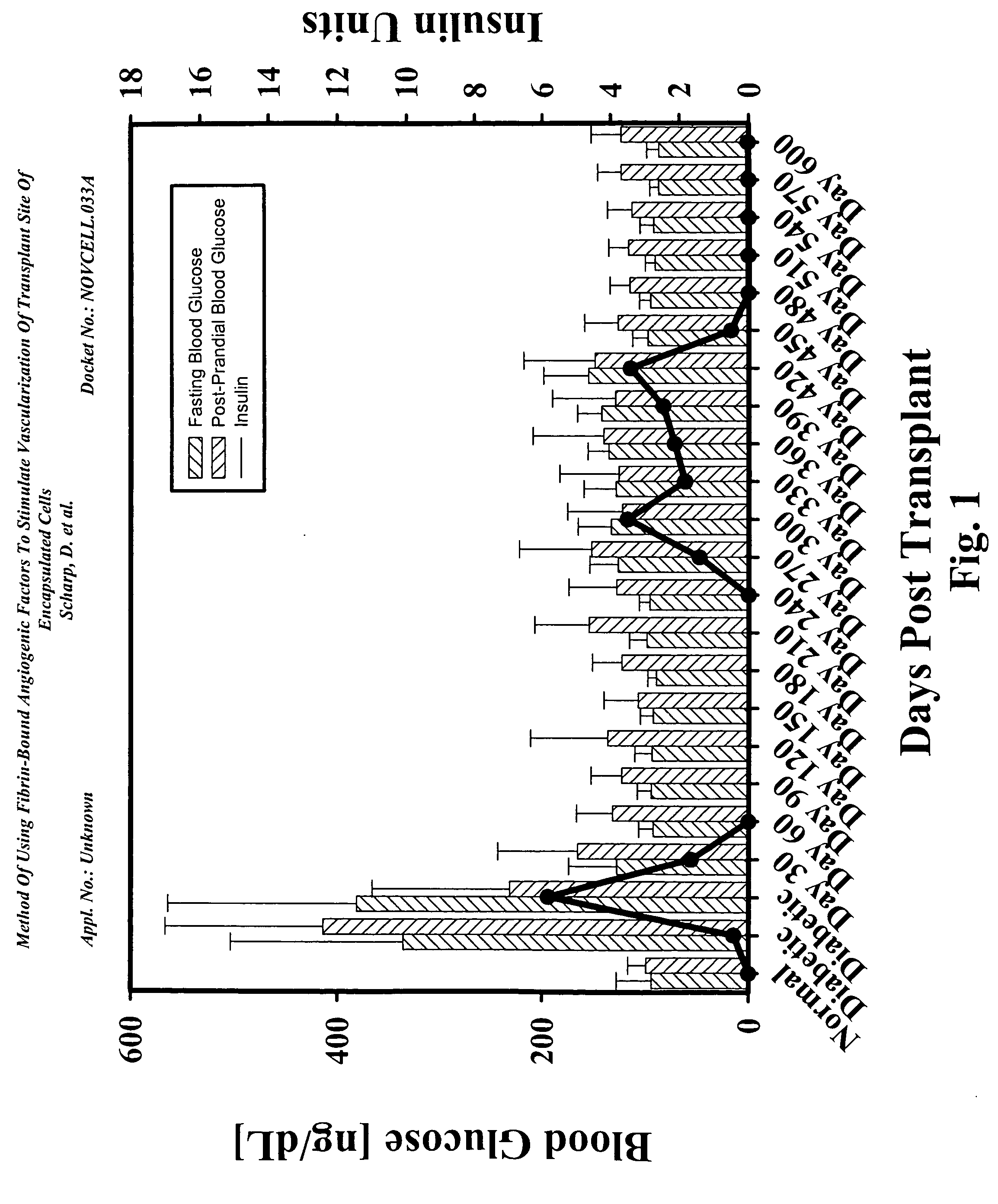
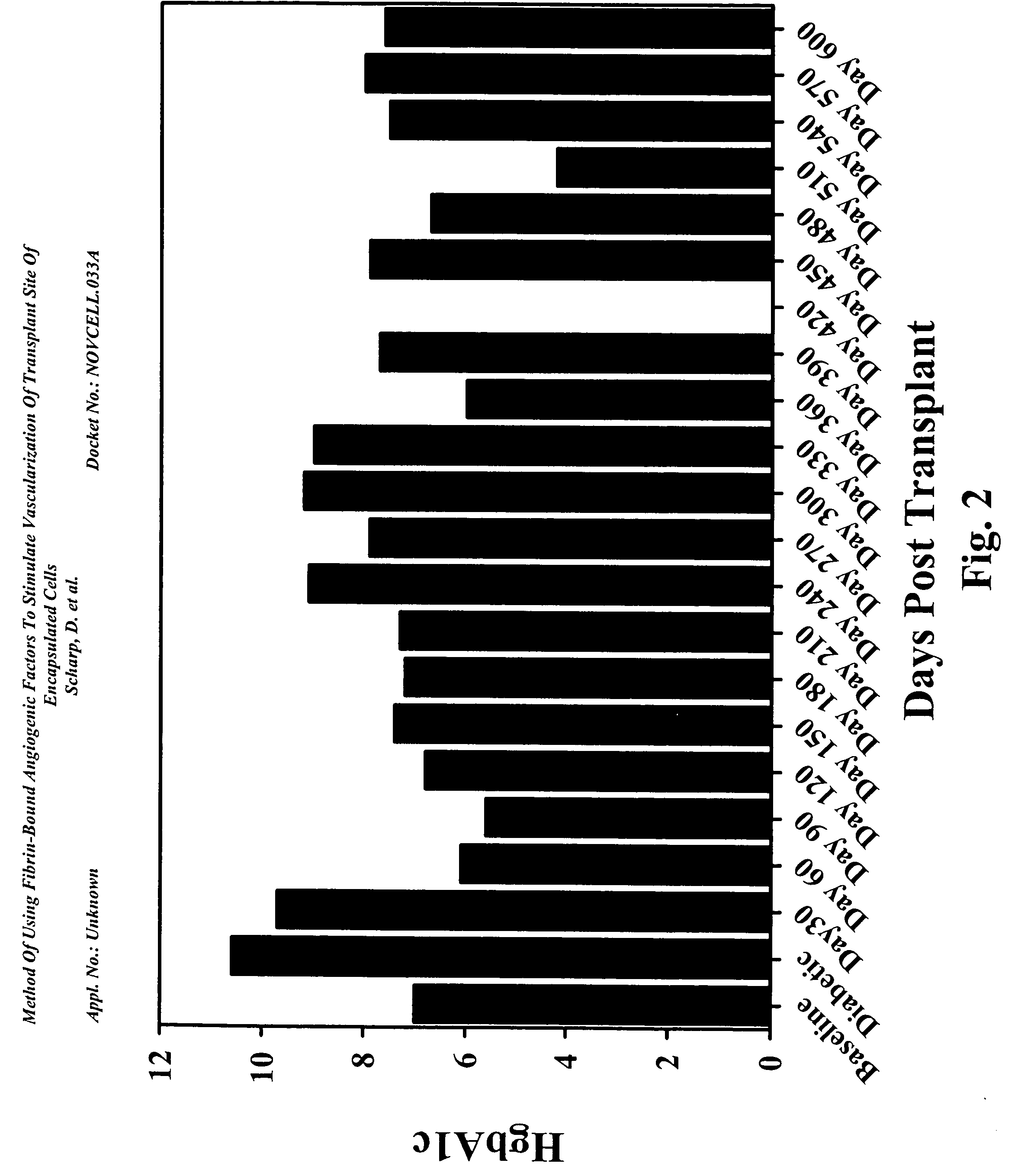
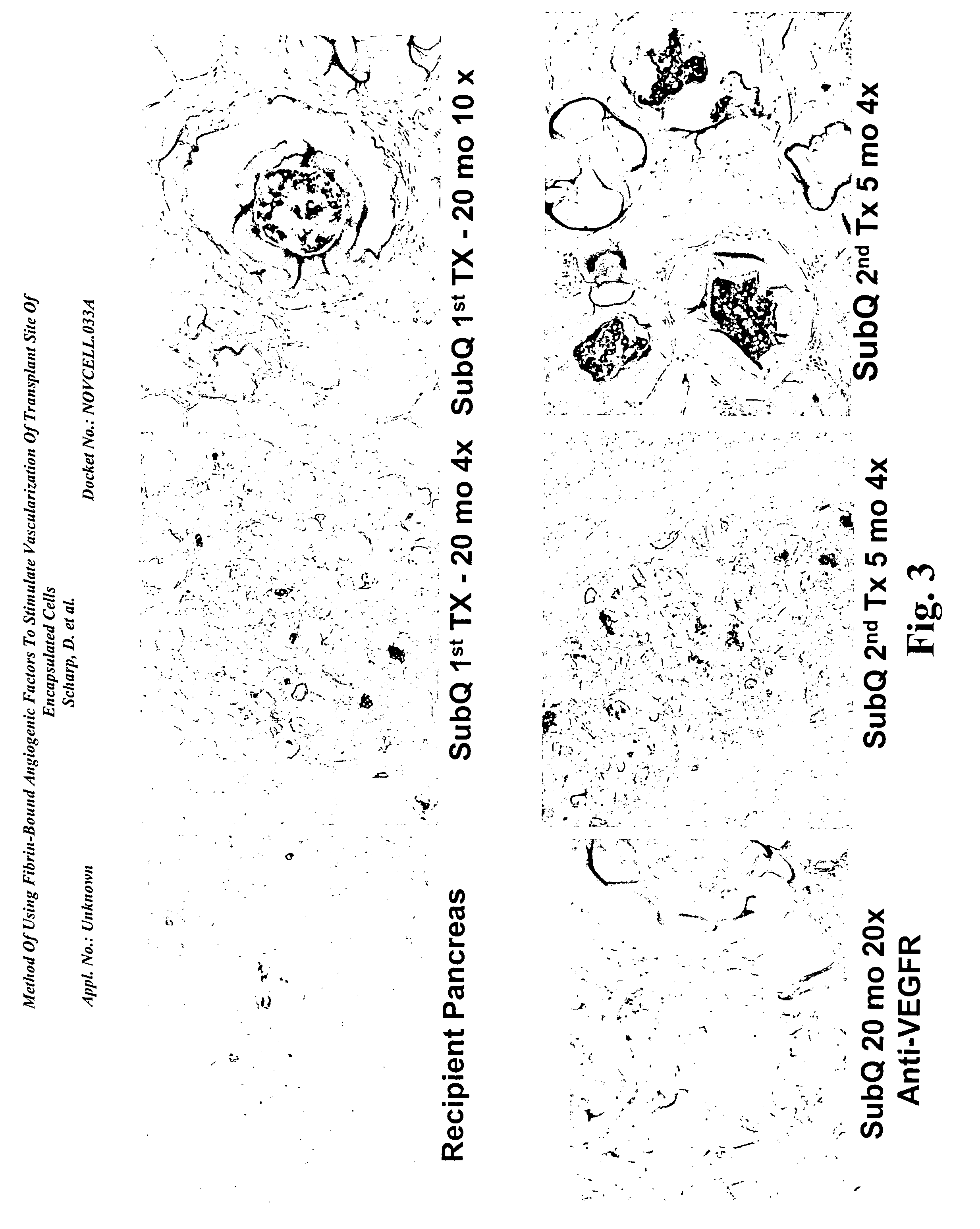
Method of using fibrin-bound angiogenic factors to stimulate vascularization of transplant site of encapsulated cells
InactiveUS20050180957A1Conducive to survivalFunction increaseBiocideSurgical adhesivesDiseaseDiabetes mellitus
The present invention relates to compositions and methods of treating a disease, such as diabetes, by implanting encapsulated biological material with a growth factor and conjugate into a patient in need of treatment. Several methods are presented to accomplish transplanting several different types of biological materials. This invention also provides methods of utilizing these encapsulated biological materials to treat different human and animal diseases or disorders by implanting them into several areas in the body including the subcutaneous site.
Owner:NOVOCELL
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com