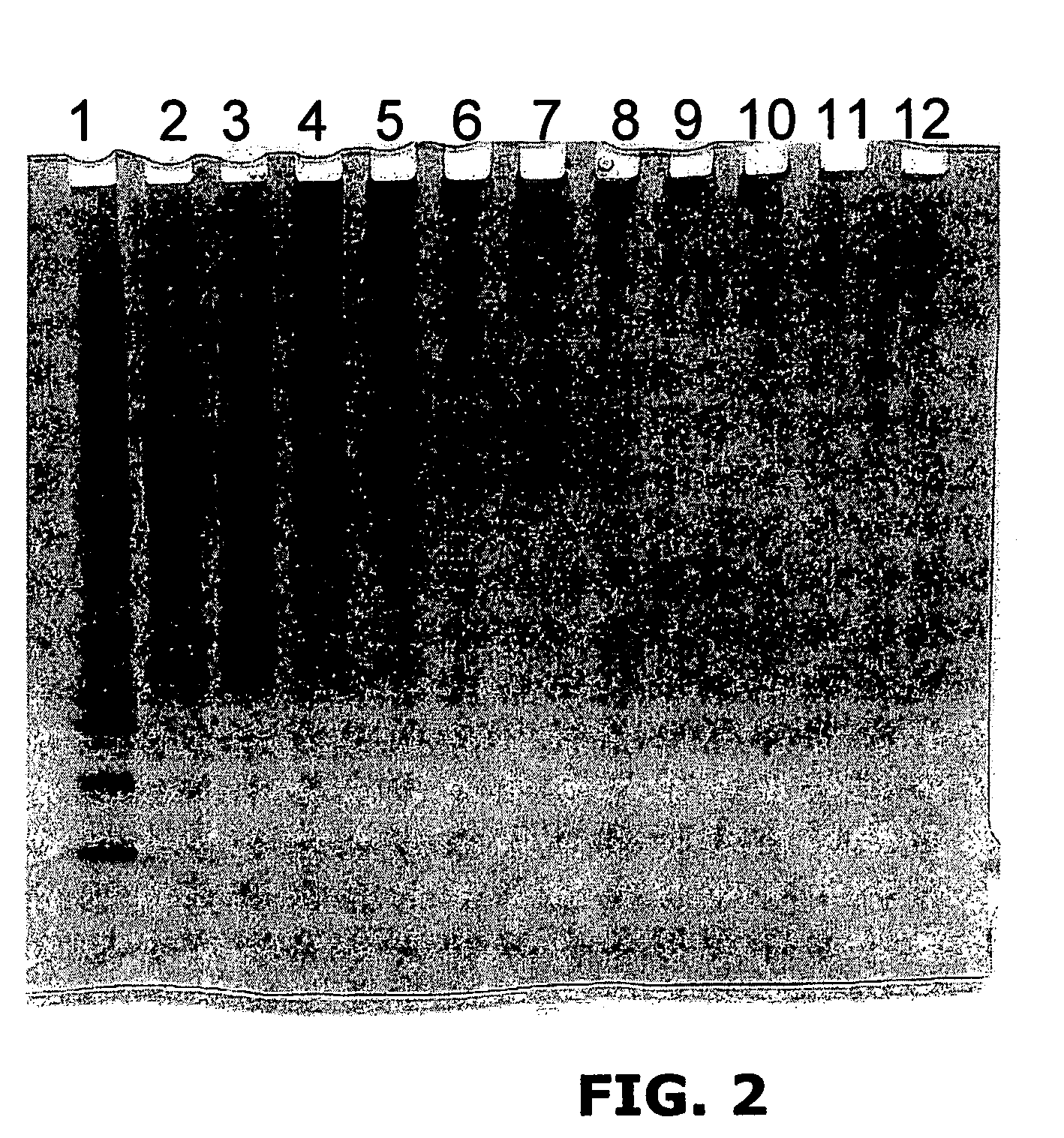
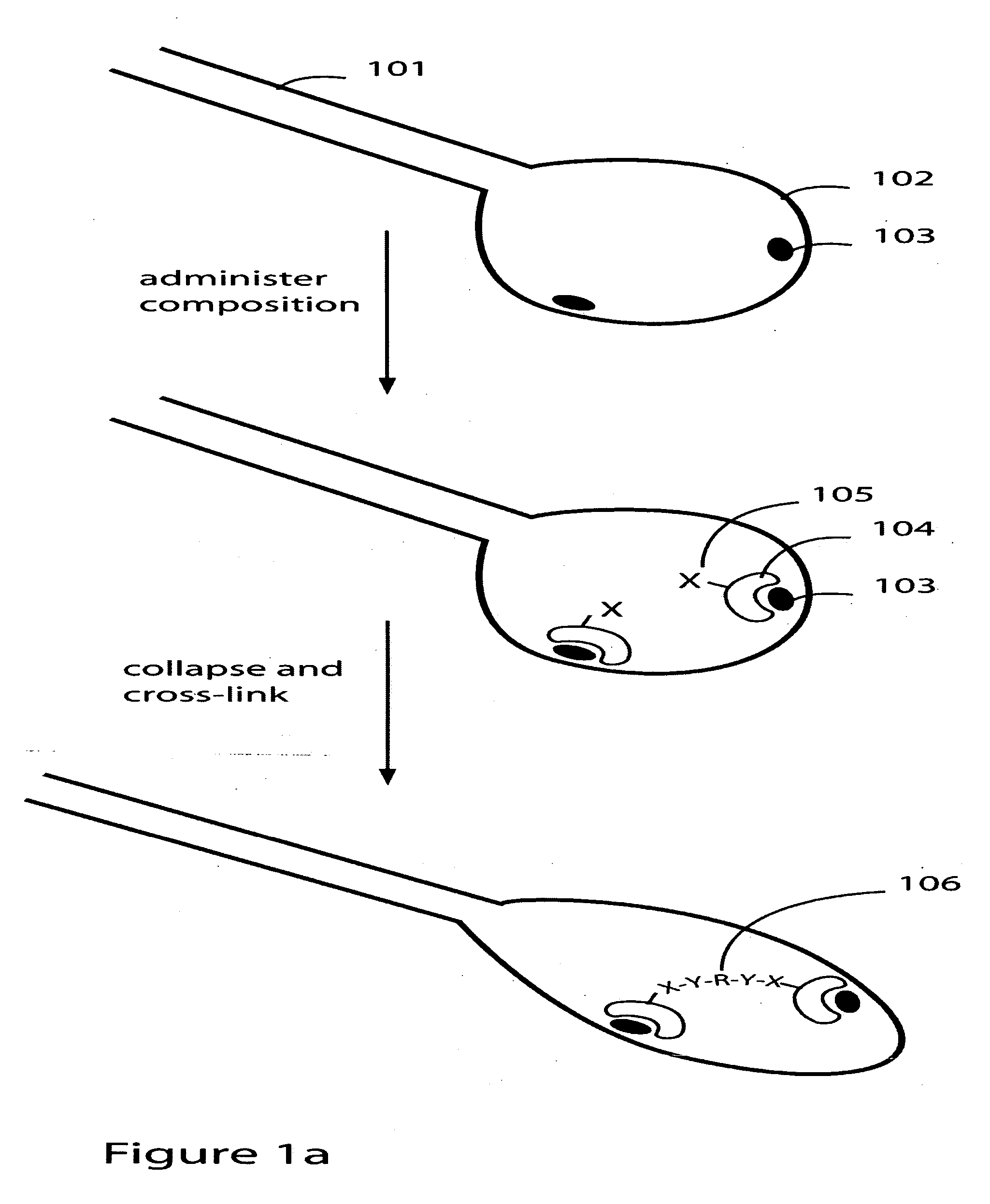
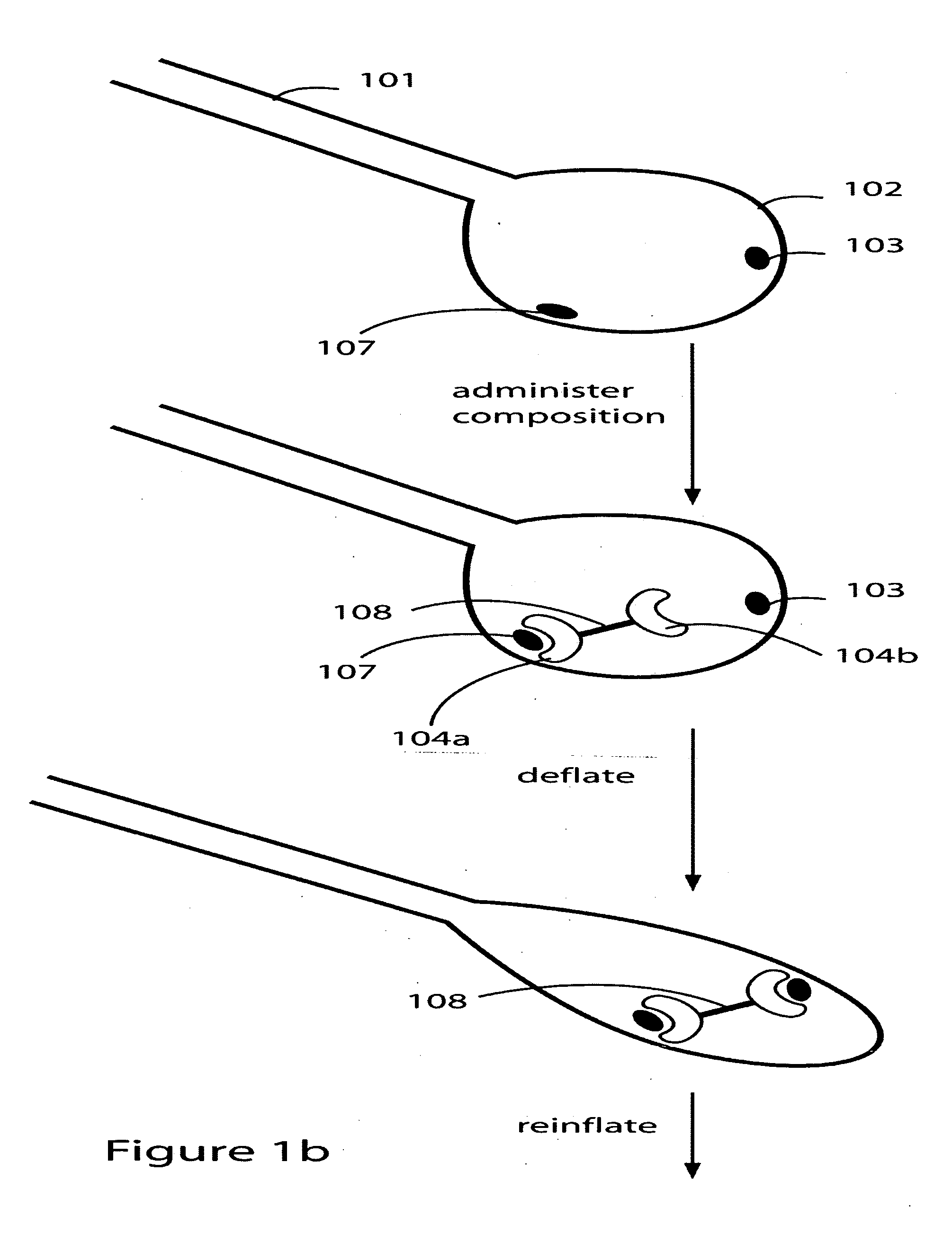
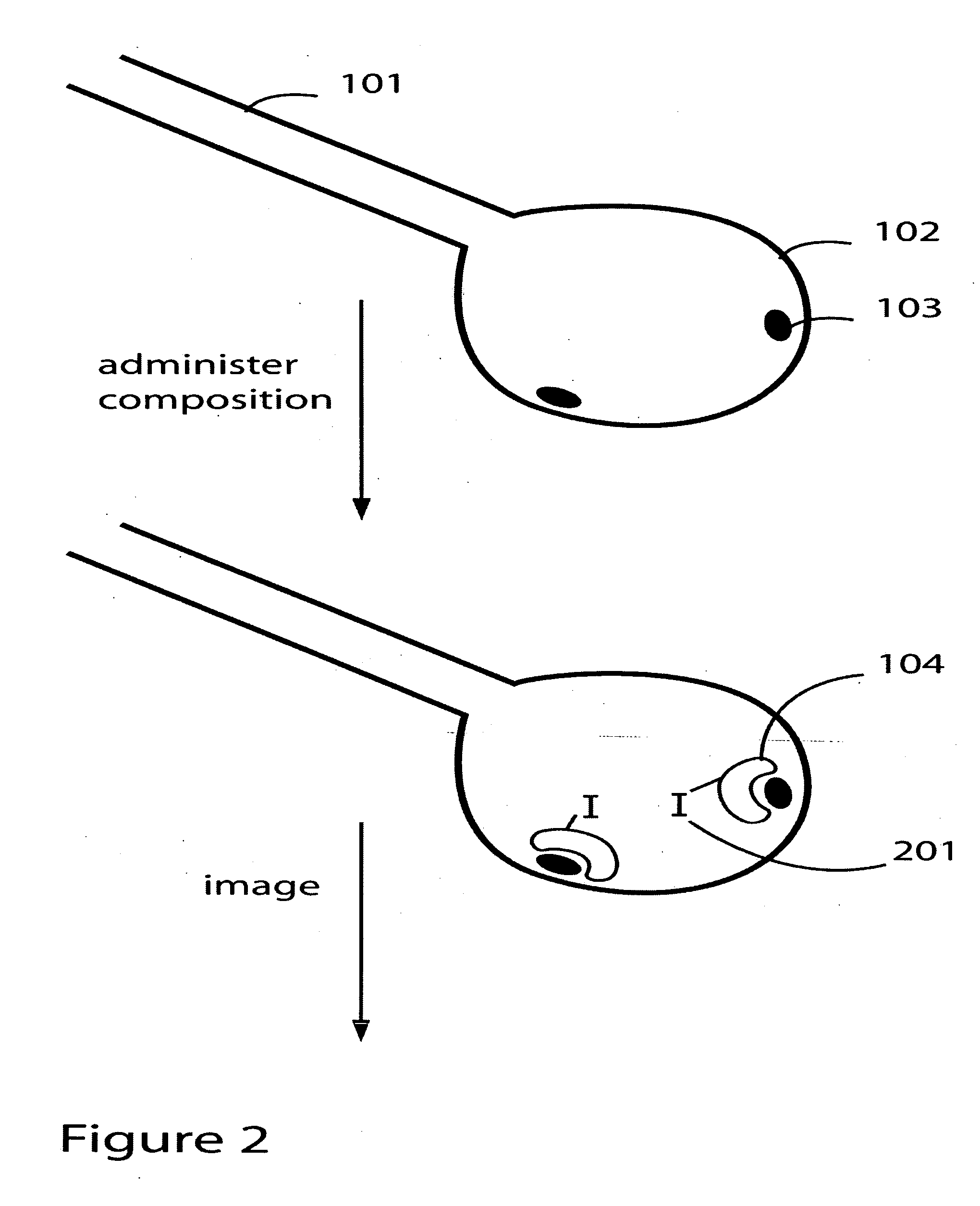
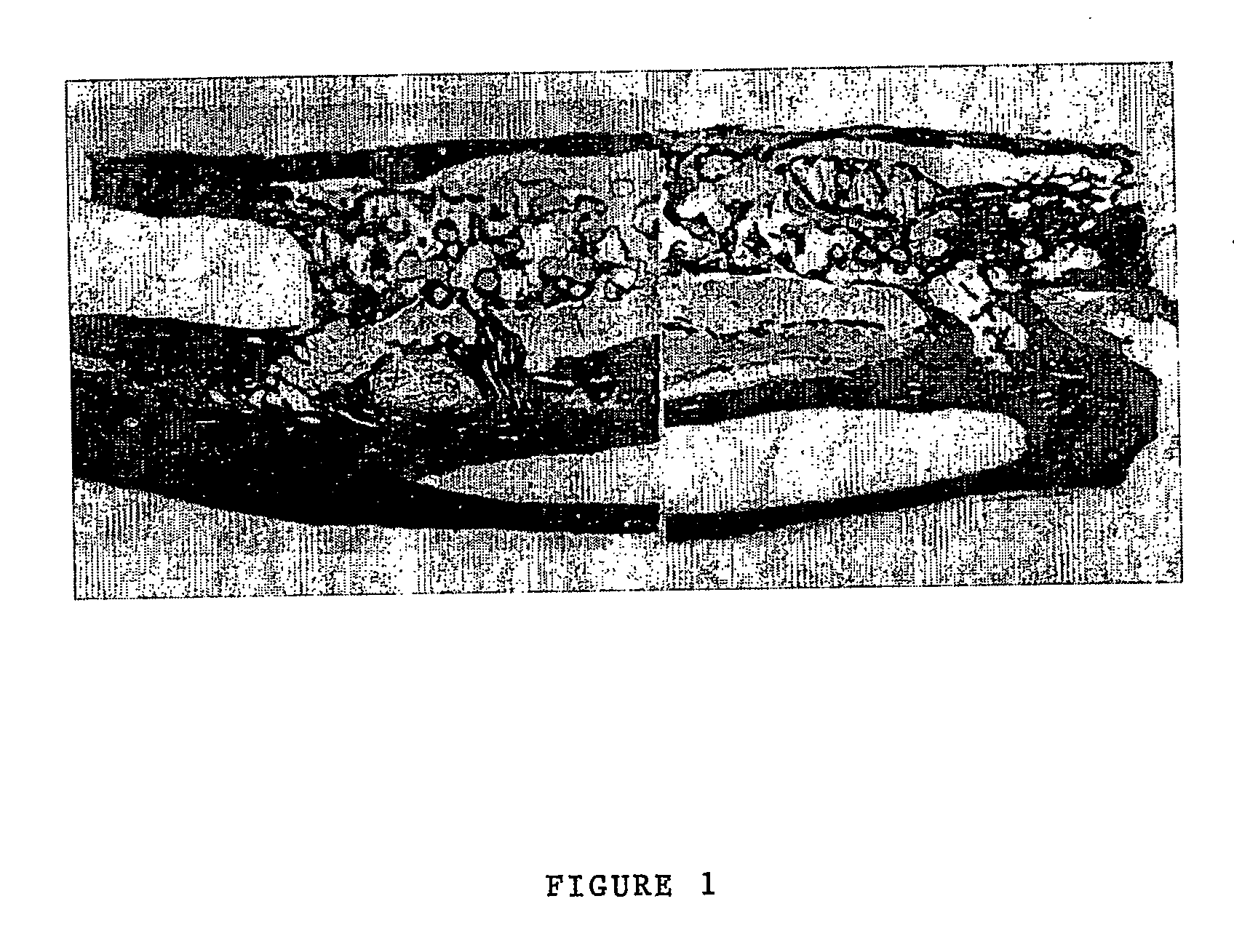
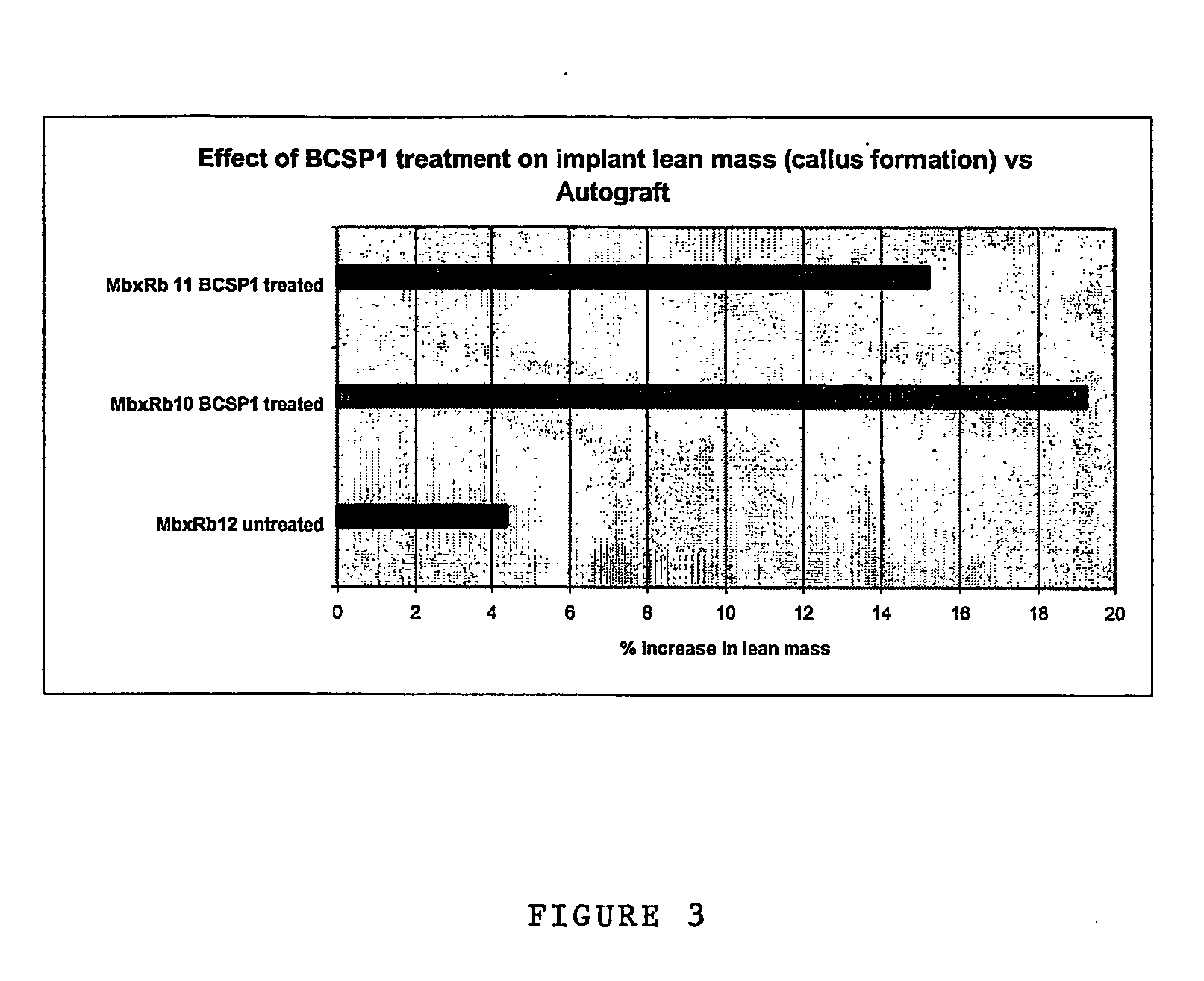
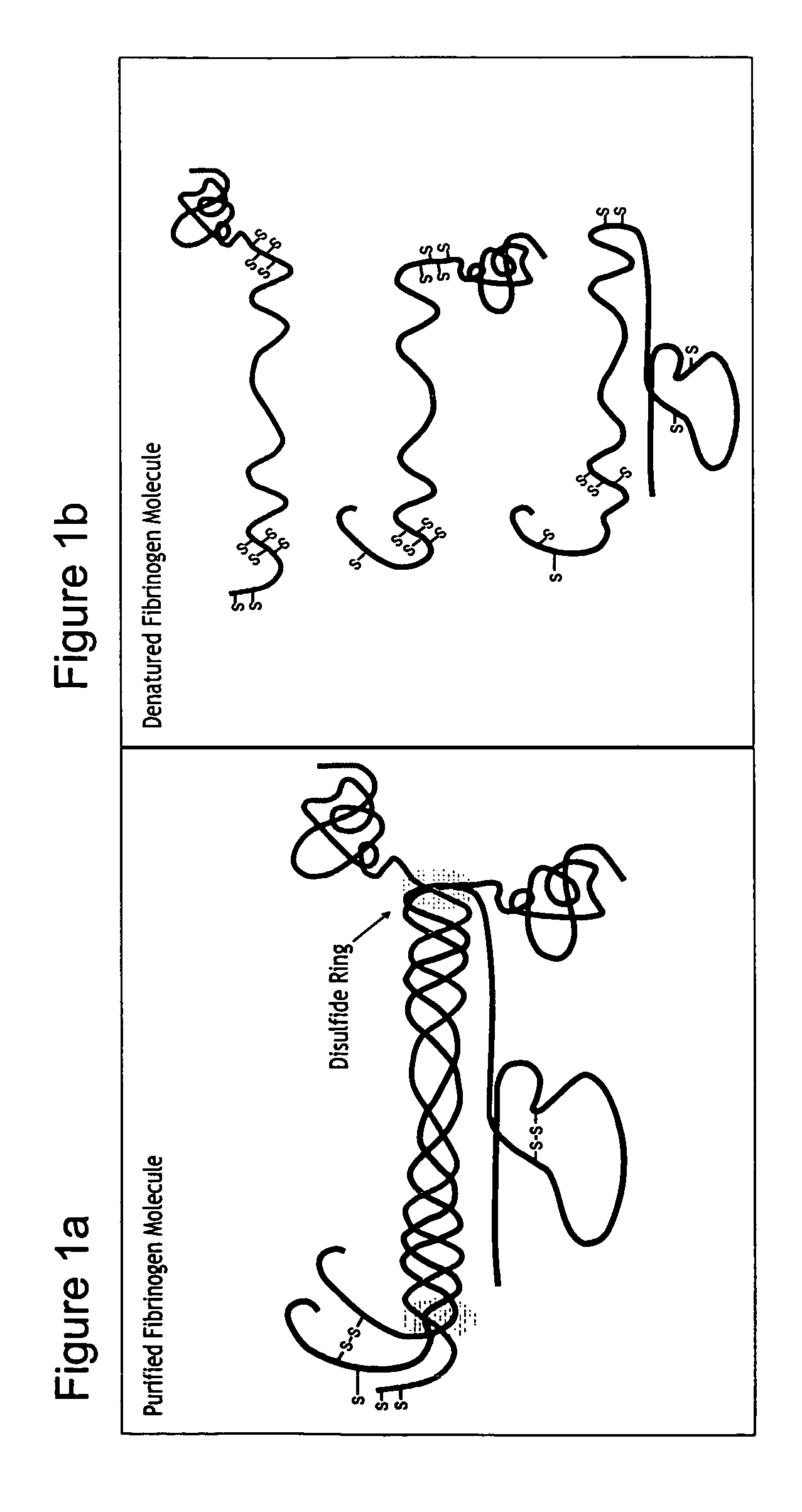
Patents
Literature
475results about "Fibrinogen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Delivery of highly lipophilic agents via medical devices
InactiveUS20060240070A1Easy to transportIncrease drug retentionBiocideFibrinogenMedicineMedical device
An apparatus and system for delivering a lipophilic agent associated with a medical device including: a medical device, a first lipophilic agent capable of penetrating a body lumen, wherein the transfer coefficients of the first lipophilic agent is by an amount that is statistically significant of at least approximately 5,000, wherein the first lipophilic agent is associated with the medical device, wherein the first lipophilic agent / medical device is placed adjacent to said body lumen, and wherein a therapeutically effective amount of the first lipophilic agent is delivered to a desired area within a subject. Furthermore, the invention relates to a method for improving patency in a subject involving placement of a medical device in a body lumen for treating and / or preventing adjacent diseases or maintaining patency of the body lumen.
Owner:ABBOTT LAB INC
Method of providing hemostasis to a wound
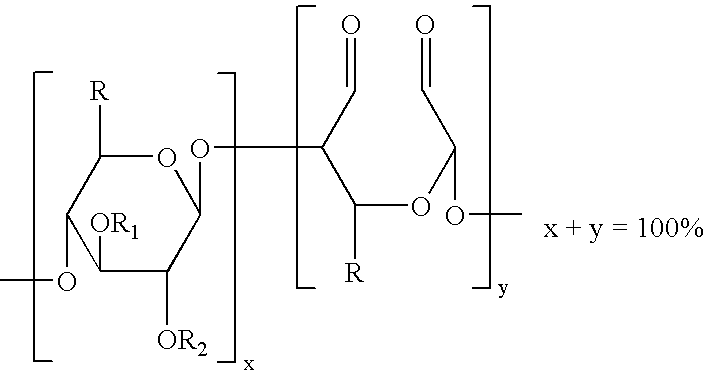
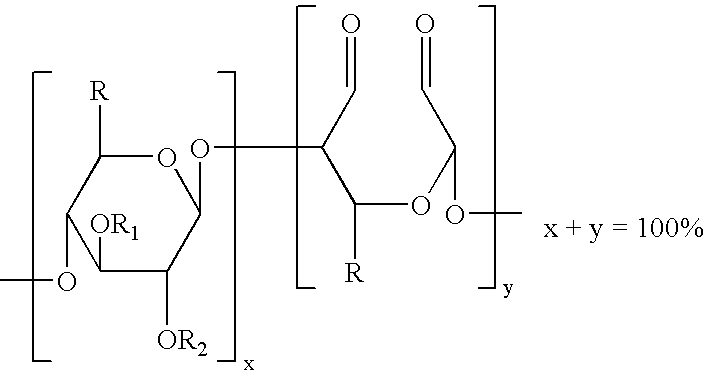
The present invention is directed to hemostatic wound dressings that contain a substrate for contacting a wound, wherein the substrate includes a wound-contacting surface and is fabricated at least in part from a biocompatible aldehyde-modified polysaccharide having covalently conjugated there with a hemostatic agent and to methods of providing hemostasis to a wound that include applying the wound dressing described herein to a wound.
Owner:ETHICON INC
Removal of plasmin(ogen) from protein solutions
A method for specifically removing or isolating plasmin(ogen) or plasmin in presence of fibrinogen from a mixture containing plasmin(ogen) or plasmin by contacting the mixture with a rigid amino acid wherein the amino group of the amino acid and the carboxylic group of the amino acid are about 6–8 Angstroms, preferably about 7 Angstroms apart and the rigid amino acid is covalently bound to the support via the amino group of the amino acid.
Owner:OMRIX BIOPHARM
Crosslinking agents and methods of use
Methods and compositions are provided for preparing protein concentrates from protein comprising aqueous compositions. In the subject methods, an initial protein comprising aqueous compositions, such as whole blood or a derivative thereof, is contacted with a non-protein denaturant hydrogel under conditions sufficient for a substantial amount of water present in the composition to be absorbed by the hydrogel, resulting in the production of a protein concentrate, such as a fibrinogen rich composition. Of particularl interest is the use of the subject methods to prepare fibrinogen rich compositions, where such compositions produced according to the subject invention are useful in fibrin sealants, drug delivery vehicles and in a number of other diverse applications.
Owner:INCEPT LLC
Biocompatible crosslinked polymers with visualization agents
InactiveUS7332566B2Improve performanceEasy to usePowder deliveryFibrinogenWound dressingBlood vessel
Owner:INCEPT LLC
Enzyme-mediated modification of fibrin for tissue engineering
The invention provides fibrin-based, biocompatible materials useful in promoting cell growth, wound healing, and tissue regeneration. These materials are provided as part of several cell and tissue scaffolding structures that provide particular application for use in wound-healing and tissue regenerating. Methods for preparing these compositions and using them are also disclosed as part of the invention. A variety of peptides may be used in conjunction with the practice of the invention, in particular, the peptide IKVAV, and variants thereof. Generally, the compositions may be described as comprising a protein network (e.g., fibrin) and a peptide having an amino acid sequence that comprises a transglutaminase substrate domain (e.g., a factor XIIIa substrate domain) and a bioactive factor (e.g., a peptide or protein, such as a polypeptide growth factor), the peptide being covalently bound to the protein network. Other applications of the technology include their use on implantable devices (e.g., vascular graphs), tissue and cell scaffolding. Other applications include use in surgical adhesive or sealant, as well as in peripheral nerve regeneration and angiogenesis.
Owner:CALIFORNIA INST OF TECH
Collagen type I and type III compositions for use as an adhesive and sealant
InactiveUS20030032143A1Reduce riskInherently hemostatic propertiesFibrinogenSurgical adhesivesWound healingCollagen i
Polymerized type I and / or III collagen based compositions for medical use as adhesives and sealants and preparation thereof are described. Prior to polymerization, the collagen monomers are prepared recombinantly whereby chemical modifications of the collagen are not needed to form such monomers. The type I and / or III collagen compositions are useful as medical adhesives for bonding soft tissues or in a sealant film for a variety of medical uses. In a further aspect of the present invention, the polymerized type I and / or III collagen composition includes agents which induce wound healing or provide for additional beneficial characteristics desired in a tissue adhesive and sealant.
Owner:NEFF THOMAS B +1
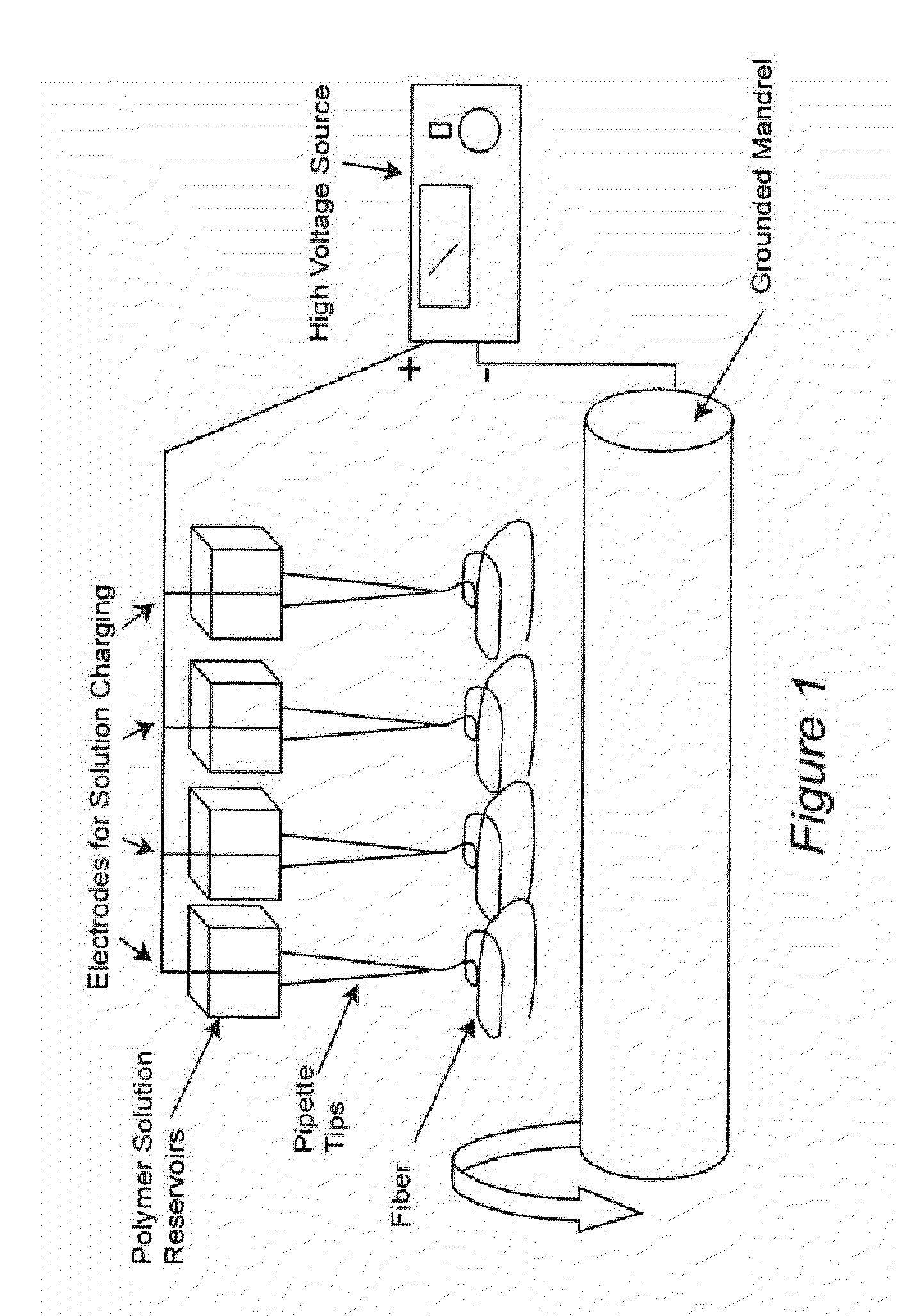
Electrospun dextran fibers and devices formed therefrom
The invention generally relates to dextran fibers which are preferably electrospun and devices formed from such fibers. In particular, such devices may include substances of interest (such as therapeutic substances) associated with the electrospun fibers. Upon exposure to a liquid the electrospun fibers dissolve immediately and the substances of interest are released into the liquid. Exemplary devices include bandages formed from electrospun dextran fibers and associated agents that promote hemostasis, such as thrombin and fibrinogen.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC +1
Methods for treating cardiovascular disease using a soluble CTLA4 molecule
The present invention relates to compositions and methods for treating cardiovascular system diseases by administering to a subject soluble CTLA4 molecules that block endogenous B7 molecules from binding their ligands.
Owner:BRISTOL MYERS SQUIBB CO
Biocompatible phase invertable proteinaceous compositions and methods for making and using the same
InactiveUS20040081676A1Improve adhesionImprove satisfactionBiocideCosmetic preparationsPolymer scienceCross linker
Biocompatible phase invertable proteinaceous compositions and methods for making and using the same are provided. The subject phase invertable compositions are prepared by combining a proteinaceous substrate and a cross-linker. The proteinaceous substrate includes one or more proteins and an adhesion modifier, and may also include one or more of: a pasticizer, a carbohydrate, or other modification agent. In certain embodiments, the cross-linker is a heat-treated dialdehyde, e.g., heat-treated glutaraldehyde. Also provided are kits for use in preparing the subject compositions. The subject compositions, kits and systems find use in a variety of different applications.
Owner:BAXTER INT INC
Biocompatible phase invertable proteinaceous compositions and methods for making and using the same
Biocompatible phase invertable proteinaceous compositions and methods for making and using the same are provided. The subject phase invertable compositions are prepared by combining a proteinaceous substrate and a cross-linker. The proteinaceous substrate includes one or more proteins and an adhesion modifier, and may also include one or more of: a pasticizer, a carbohydrate, or other modification agent. In certain embodiments, the cross-linker is a heat-treated dialdehyde, e.g., heat-treated glutaraldehyde. Also provided are kits for use in preparing the subject compositions. The subject compositions, kits and systems find use in a variety of different applications.
Owner:BAXTER INT INC
Fibrin material and method for producing and using the same
This invention describes a bioerodible fibrin material which is obtained by mixing fibrinogen and thrombin reconstituted or diluted with a particular high tonic strength medium, free of calcium. Such a fibrin-based biomaterial develops a tight structure with thin fibers and small pore size suitable for use as an anti-adhesion barrier. In this invention, thrombin is no longer the variable which governs the tightness and the porosity of the fibrin material obtained, but still controls the clotting time. The mechanical behavior, high-water capacity, and releasable retention properties for therapeutic agents of this fibrin structure causes the fibrin material to be ideally suited for use as a drug delivery device, capable of delivering proteins, hormones, enzymes, antibiotics, antineoplastic agents and even cells for local and systemic treatment of human and non-human patients.
Owner:BAXTER INT INC
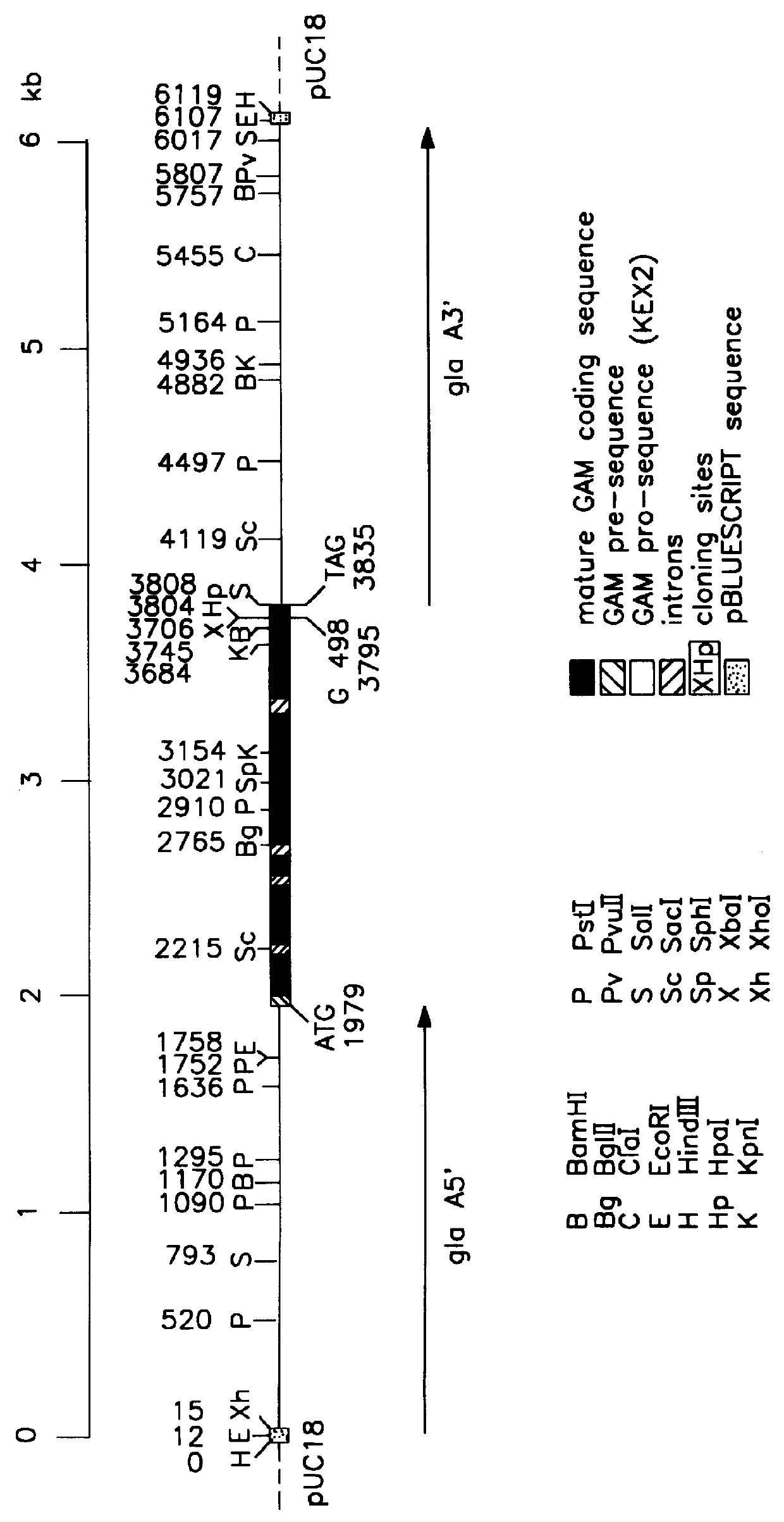
Recombinant fibrin chains, fibrin and fibrin-homologs
The invention is directed to fibrin materials for use in fibrin compositions and methods that avoid the need to use thrombin as an activating agent for fibrin monomer-based sealants. The invention provides for substantially pure fibrin chains, fibrin chain precursors, fibrin chains with other N-terminal extensions, fibrin monomer, fibrin-homolog and fibrin-analog. The invention further provides for variant fibrin .gamma.-chains. The variant gamma-chain contains one or more mutations and / or deletions in the C-terminal region following the coiled-coil forming region such that, when incorporated into fibrin-homolog, the homolog lacks the ability to self-polymerize but has the ability to form non-covalent bonds, and thereby form mixed polymers useful as sealants, with fibrinogen. The invention also provides nucleotide sequences encoding fibrin chains or fibrin chain variants and cells expressing fibrin chains, fibrin chain variants, fibrin monomer, fibrin precursor or fibrinogen-analog. The invention further provides a method of forming fibrin-related proteins in vitro from their component fibrin chains. The invention additionally provides a method for forming a fibrin sealant by a reacting a first fibrin-related protein that is incapable of self-polymerizing with a second fibrin-related protein that is incapable of self-polymerizing. Fibrin chains produced by methods of the present invention may be used as sources of substantially pure starting material for the production of important fibrin-derived factors that regulate angiogenesis, platelet aggregation, and other physiological processes.
Owner:BRISTOL MYERS SQUIBB CO
Method for treatment and repair of meniscal injuries
A method for repair of meniscal injuries comprising induction of meniscal regeneration by introducing a strongly adhesive collagen-polyethylene glycol (PEG) hydrogel to a site of injury.
Owner:OCUGEN INC +1
Matrix composed of a naturally-occurring protein backbone cross linked by a synthetic polymer and methods of generating and using same
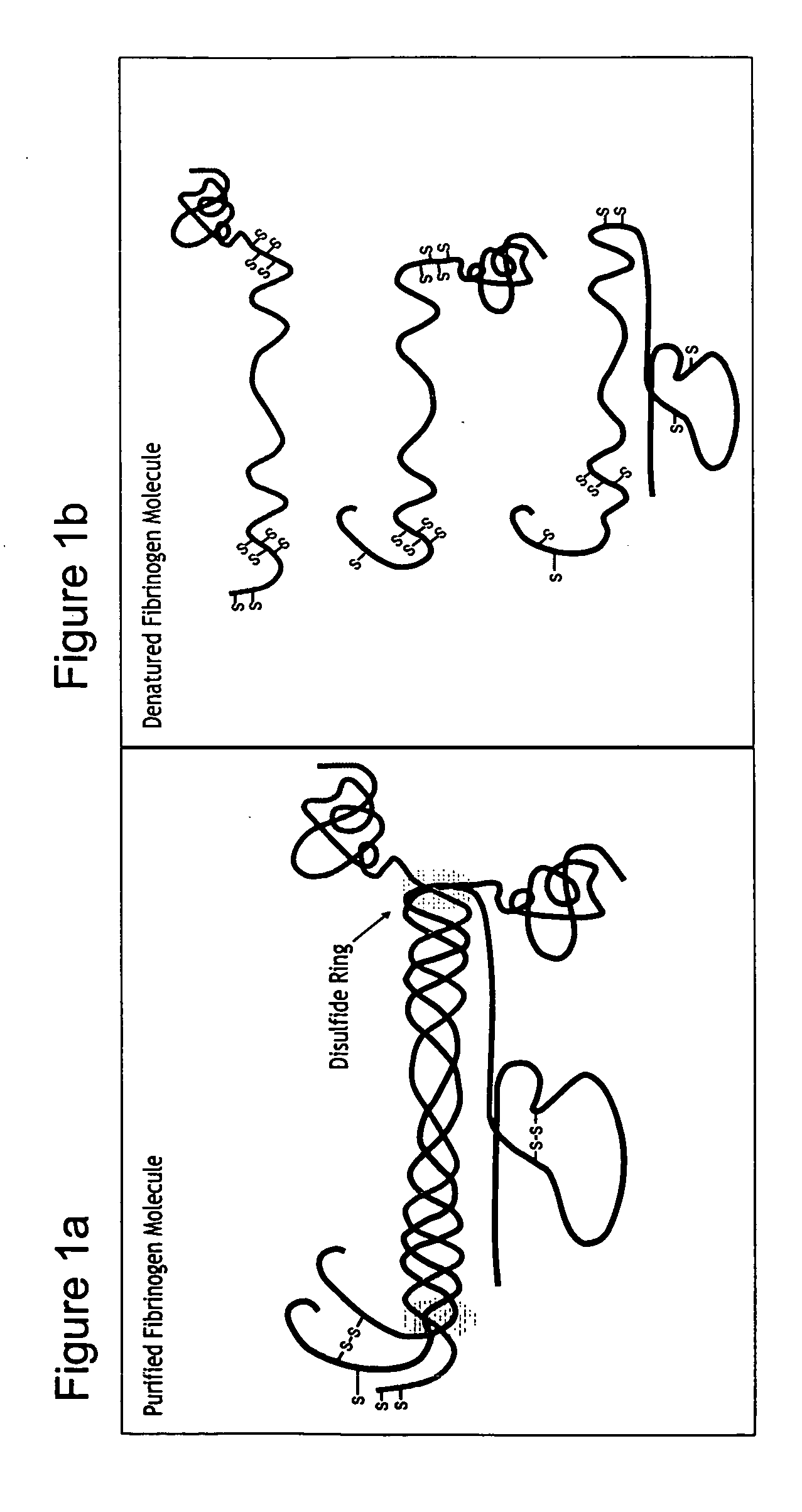
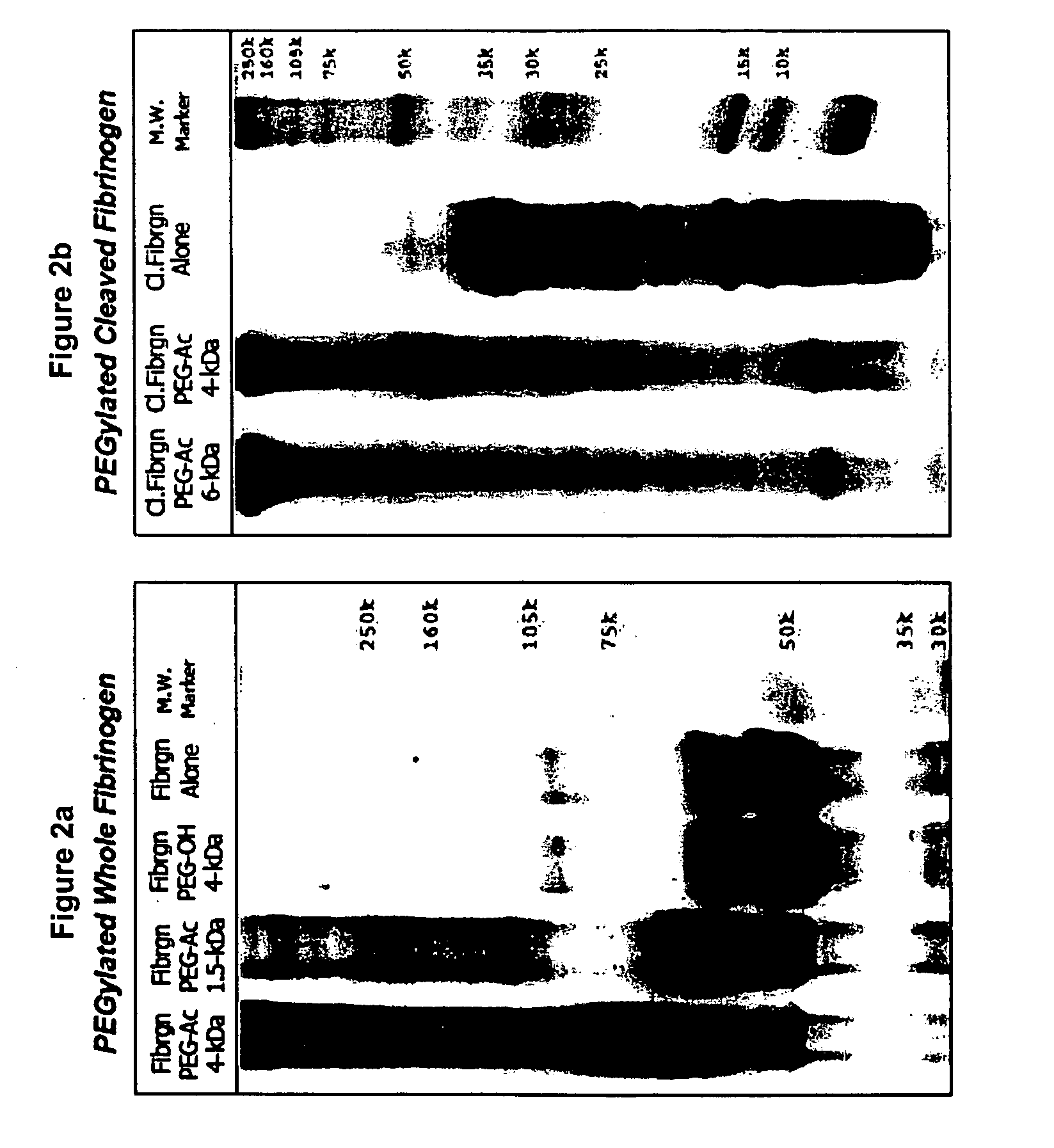
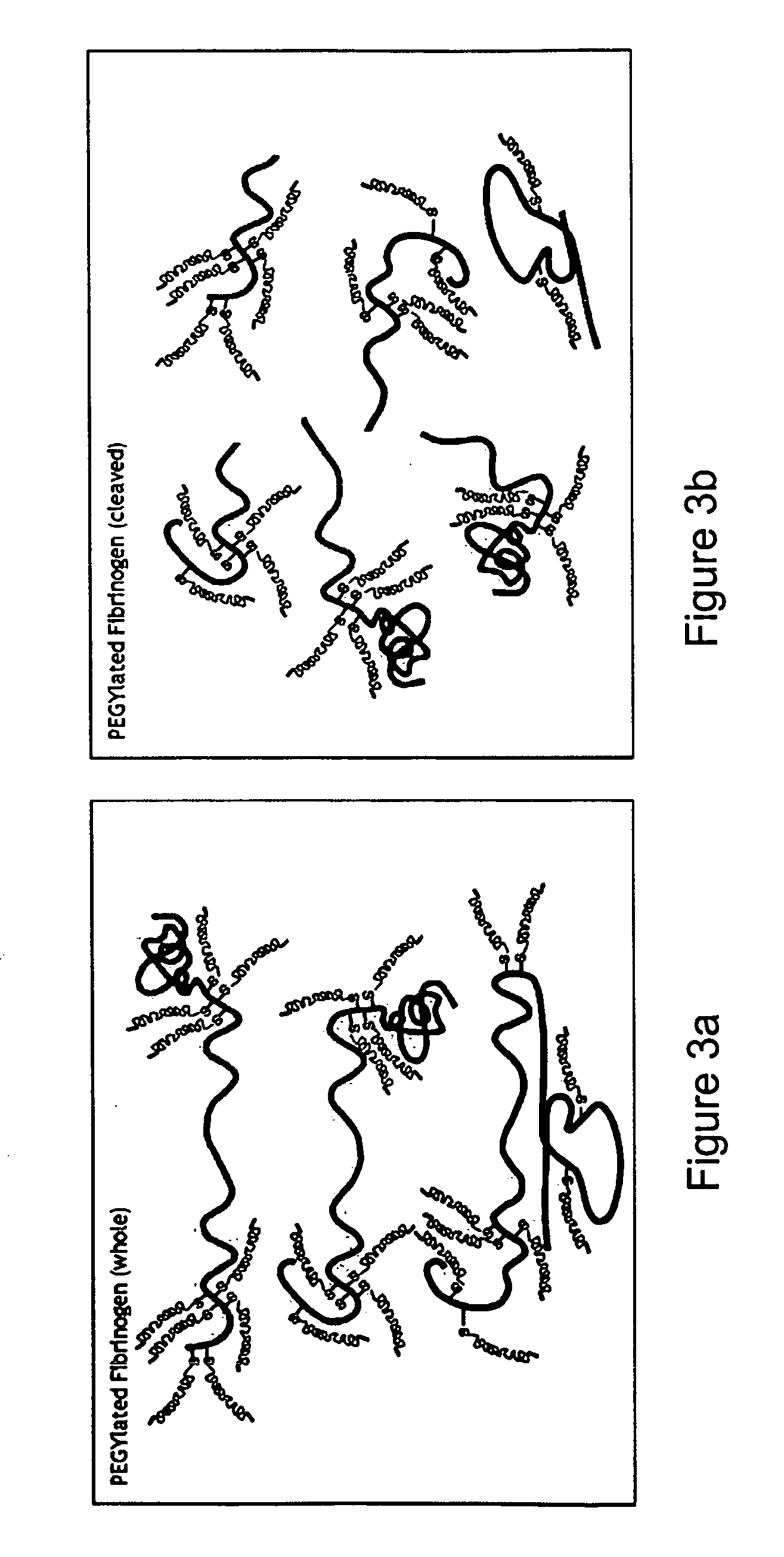
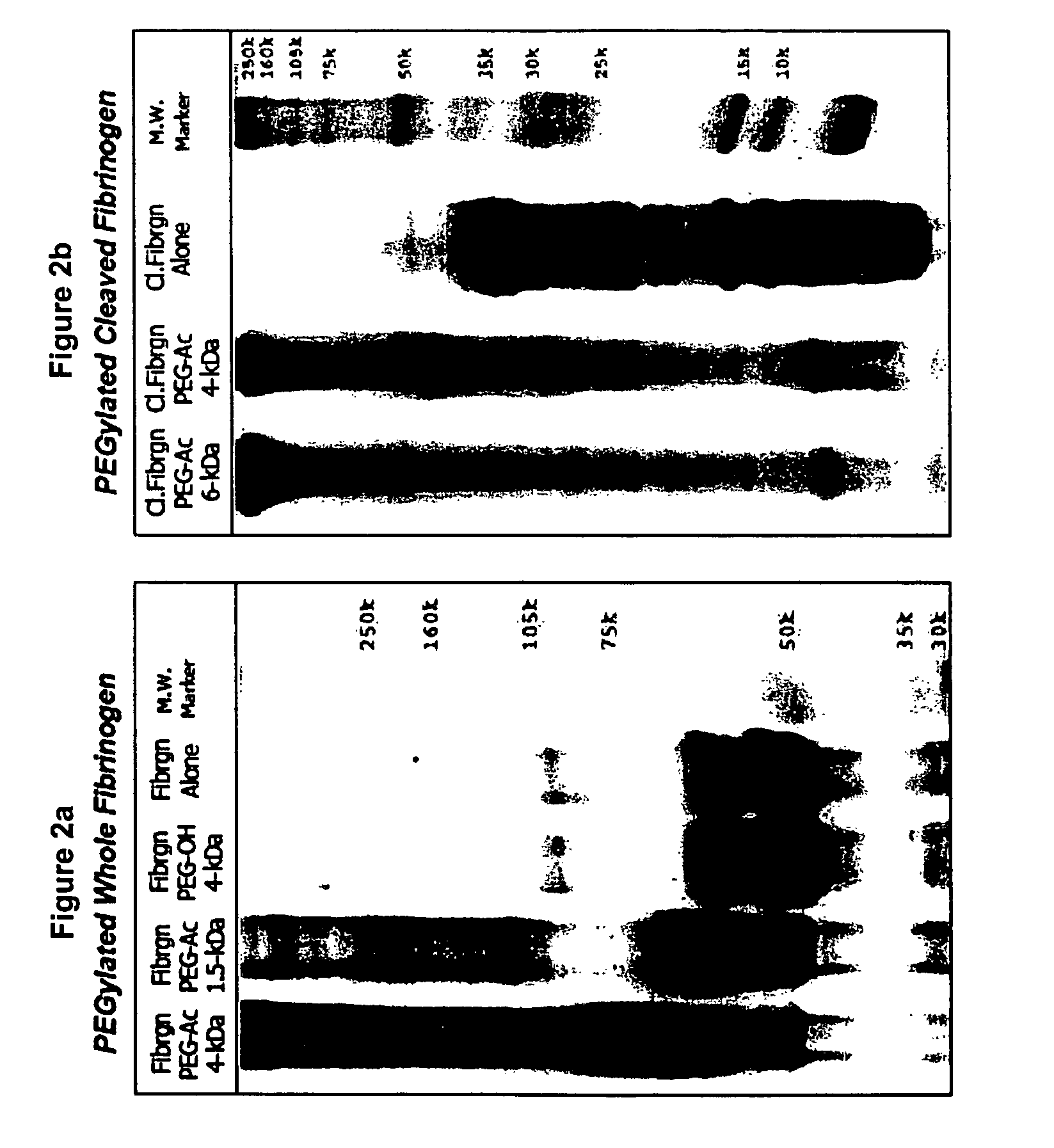
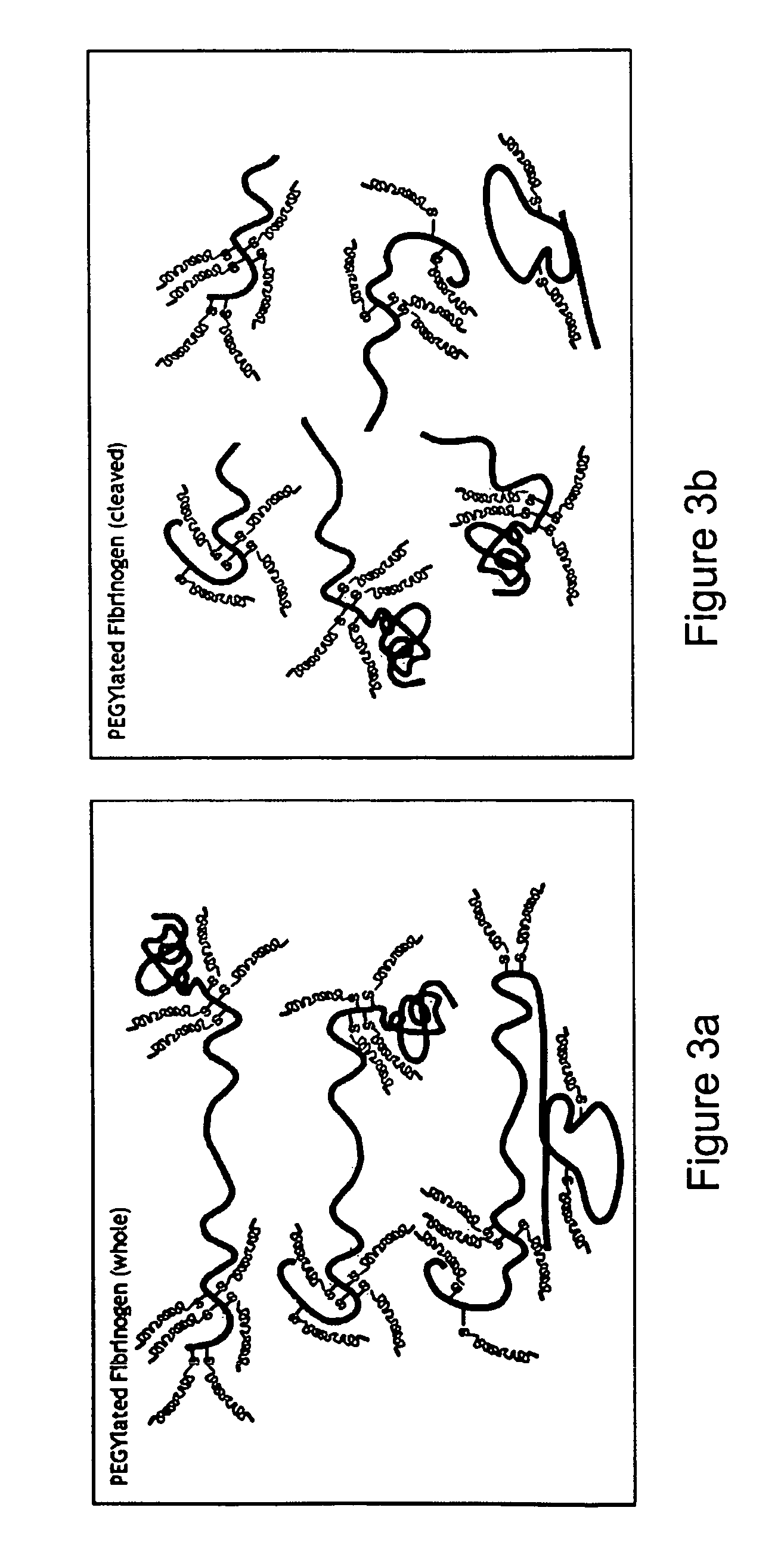
A method of treating a disorder characterized by tissue damage is provided. The method comprising providing to a subject in need-thereof a composition which comprises a synthetic polymer attached to denatured fibrinogen or a therapeutic portion of the fibrinogen, the composition being formulated for releasing the therapeutic portion of the fibrinogen in a pharmacokinetically regulated manner, thereby treating the disorder characterized by tissue damage or malformation.
Owner:REGENTIS BIOMATERIALS
Implantable preparations
Implantable preparation comprising a material which can be obtained from globin that has been modified, especially chemically, to be, at least partially, soluble at physiological pH, the material being biocompatible, and biodegradable in the organism. The material may be soluble at physiological pH, or insoluble at that pH. The preparation may be in the form of a solution, suspension, paste, gel, film, sponge, powder or granules, or a solid implant. Application in particular to the healing, protection or filling of external skin wounds, the filling of wrinkles and skin flaws, the filling of tissue, as means for fixing prostheses or biomaterials, or means for preventing adhesion.
Owner:KHORIONYX
Fibrin-containing composition
InactiveUS20060128016A1Improve performanceRapidly and simply producingSenses disorderFibrinogenFiberPurification methods
It is intended to provide a scaffold material having favorable properties and being appropriate for cell proliferation and differentiation in regeneration therapy. Namely, a fibrin-containing biological scaffold material to be used in the case of employing a fibrin composition for the regeneration of a human tissue and cell proliferation, characterized by containing a mixture of a fibrinogen concentrate, which is obtained from human plasma by a quick and rough purification method, with a fibrinogen activator.
Owner:ASAHI KASEI MEDICAL CO LTD
Methods, compositions and devices for treating lesioned sites using bioabsorbable carriers
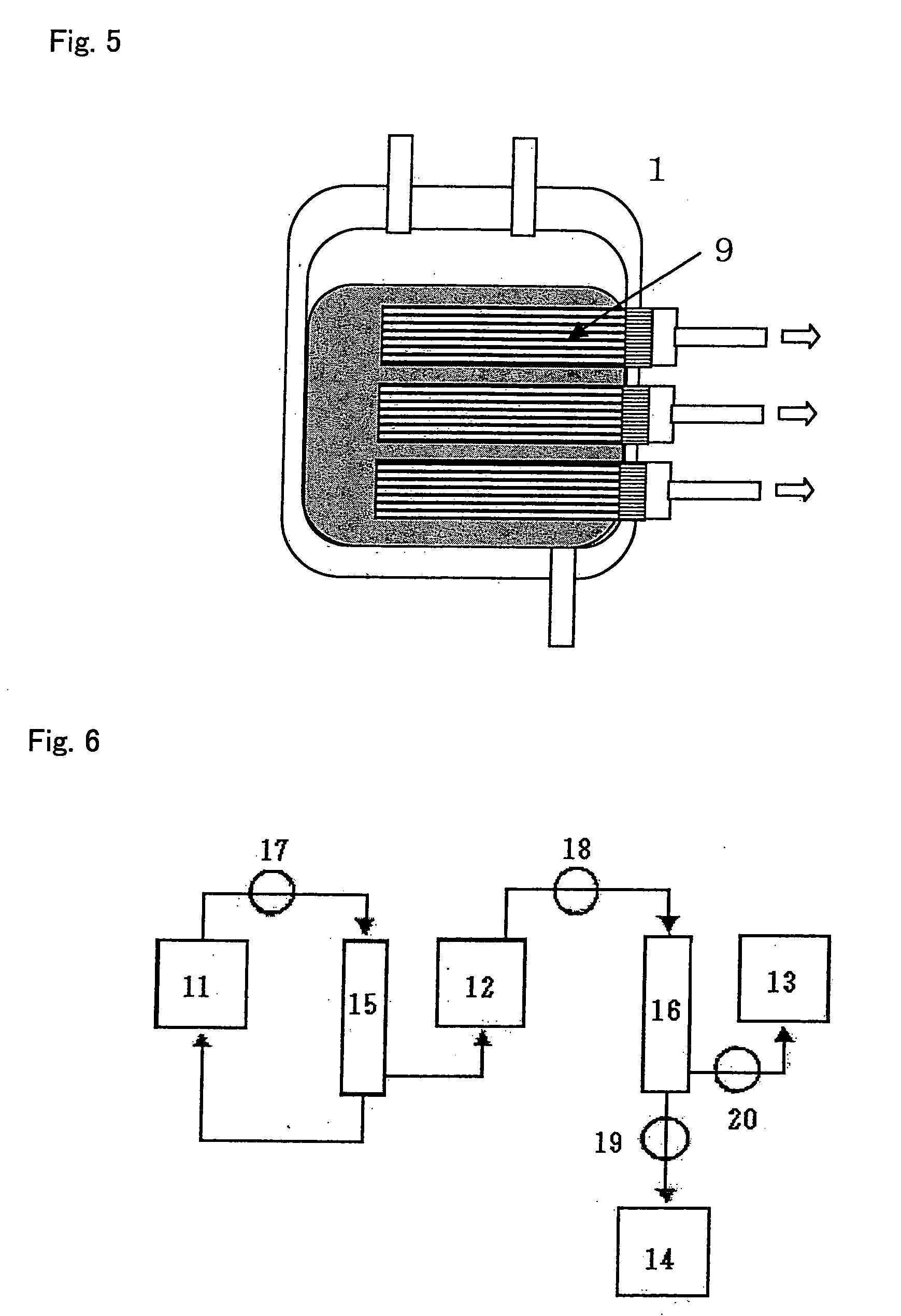
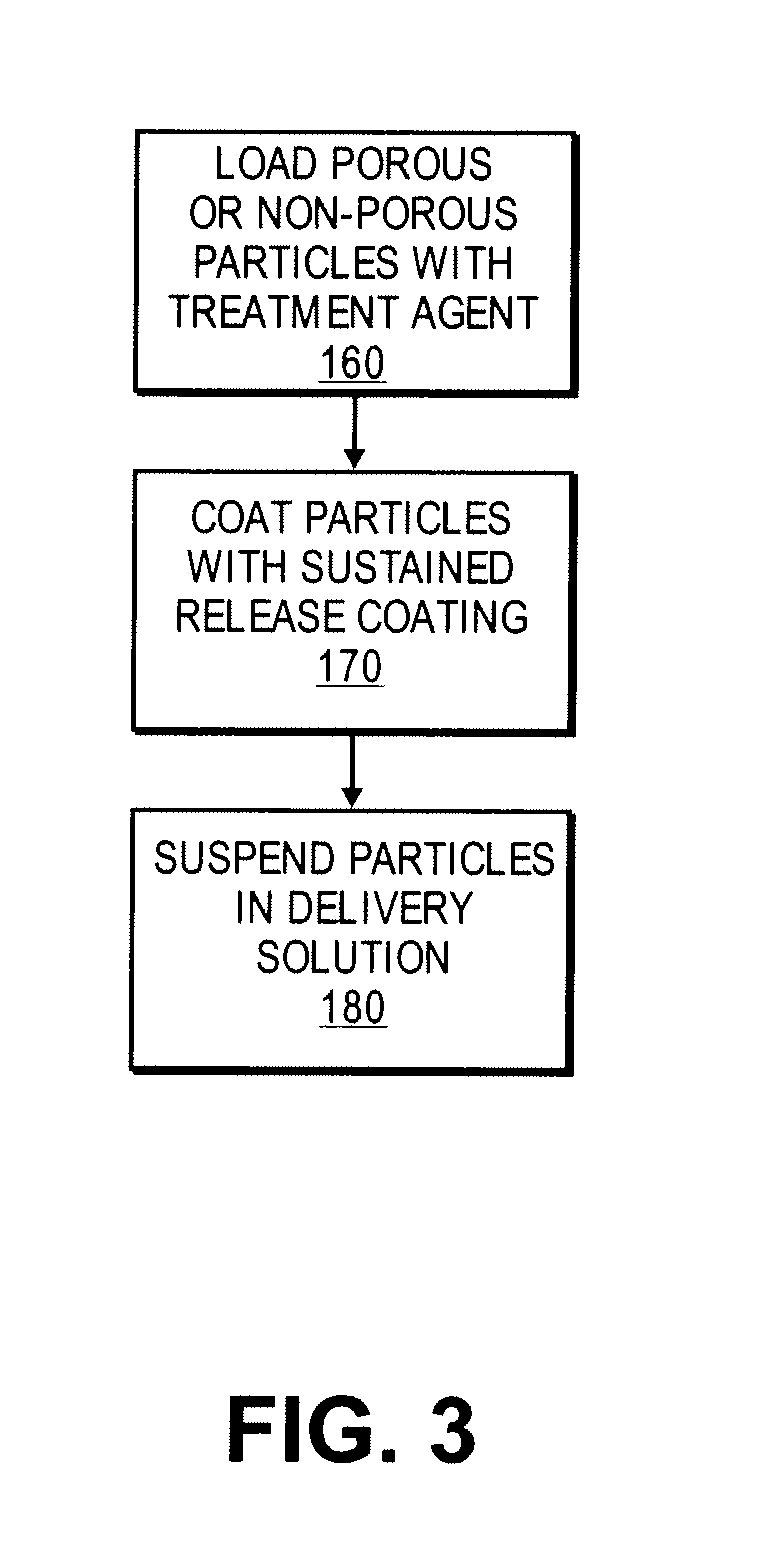
Methods and compositions for the sustained release of treatment agents to treat an occluded blood vessel and affected tissue and / or organs are disclosed. Porous or non-porous bioabsorbable glass, metal or ceramic bead, rod or fiber particles can be loaded with a treatment agent, and optionally an image-enhancing agent, and coated with a sustained-release coating for delivery to an occluded blood vessel and affected tissue and / or organs by a delivery device. Implantable medical devices manufactured with coatings including the particles or embedded within the medical device are additionally disclosed.
Owner:ABBOTT CARDIOVASCULAR
Composite materials for controlled release of water soluble products
InactiveUS7211275B2Inhibits inflammatory and immune responsePowder deliveryFibrinogenControlled releaseWater soluble
Composite materials comprising a water-soluble compound adsorbed onto a basic inorganic material and a bio-degradable polymer which yields acidic degradation products, methods of producing same, and methods of use thereof are described, wherein the composite materials are designed so as to provide controlled release of the water soluble molecule.
Owner:MASSACHUSETTS INST OF TECH
Hemostatic compositions and devices
The present invention includes both sterilized and unsterilized hemostatic compositions that contain a biocompatible liquid having particles of a biocompatible polymer suitable for use in hemostasis and which is substantially insoluble in the liquid, up to about 20 percent by weight of glycerol and about 1 percent by weight of benzalkonium chloride, each based on the weight of the liquid, all of which are substantially homogenously dispersed throughout the liquid to form a substantially homogenous composition, methods for making such compositions, medical devices that contain such hemostatic compositions disposed therein and methods of making such devices.
Owner:ETHICON INC
Compositions and methods for selective dissolution of nascent intravascular blood clots
InactiveUS7041287B2Uncontrolled formationFibrinogenPeptide/protein ingredientsRed blood cellDissolution
Compositions and methods for prevention and treatment of uncontrolled formation of intravascular fibrin clots, which are capable of selective dissolution of pathological nascent clots formed intravascularly, with minimal risk of unwanted dissolution of pre-existing hemostatic clots, are provided wherein fibrinolytic or anticoagulant drugs are biocompatibly coupled to red blood cell carriers.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Implantable preparations
An implantable preparation comprises a material which can be obtained from globin that has been modified, especially chemically, to be, at least partially, soluble at physiological pH. The material is biocompatible, and biodegradable in the organism. The material may be soluble at physiological pH, or insoluble at that pH. The preparation may be in the form of a solution, suspension, paste, gel, film, sponge, powder or granules, or a solid implant. The preparation can be used for the healing, protection or filling of external skin wounds, the filling of wrinkles and skin flaws, the filling of tissue, as a device for fixing prostheses or biomaterials, or as a device for preventing adhesion.
Owner:KHORIONYX
Hydrogel compositions comprising vasoconstricting and Anti-hemorrhagic agents for dermatological use
InactiveUS20110171310A1Improve stabilityProlonging dermal filler durationBiocideOrganic active ingredientsAnti-Hemorrhagic AgentVasoconstrictor Agents
The present specification generally relates to hydrogel compositions and methods of treating a soft tissue condition using such hydrogel compositions.
Owner:ALLERGAN IND
Reinforced absorbable synthetic matrix for hemostatic applications
The present invention is directed to a reinforced absorbable hemostat comprising at least one hemostatic agent in a single layer of nonwoven synthetic fabric having a mixture of compressed fiber staples of a polyglycolide / polylactide copolymer and a polydioxanone.
Owner:ETHICON INC
Photoactivated crosslinking of a protein or peptide
A method of crosslinking a protein or peptide for use as a biomaterial, the method comprising the step of irradiating a photoactivatable metal-ligand complex and an electron acceptor in the presence of the protein or peptide, thereby initiating a crosslinking reaction to form a 3-dimensional matrix of the biomaterial.
Owner:COOK MEDICAL TECH LLC
Reinforced Absorbable Synthethic Matrix for Hemostatic Applications
The present invention is directed to a reinforced absorbable hemostat comprising at least one hemostatic agent in a single layer of nonwoven synthetic fabric having a mixture of compressed fiber staples of a polyglycolide / polylactide copolymer and a polydioxanone.
Owner:ETHICON INC
Targeting damaged lung tissue
InactiveUS20050281796A1Antibacterial agentsHeavy metal active ingredientsCancer researchPneumatocele
The present invention relates to methods and compositions for targeting damaged lung tissue. Compositions provided feature a targeting moiety coupled to one or more other moieties, including, for example, a cross-linkable moiety, an imaging moiety, and / or one or more other targeting moieties. The methods and compositions of the invention find use, for example, in detecting and treating a pulmonary condition such as emphysema.
Owner:PNEUMRX
Connective Tissue Stimulating Peptides
ActiveUS20050288229A1Stimulating development maintenance repairCosmetic preparationsPeptide/protein ingredientsHydroxyprolineDrug
Novel peptides are described which comprise an amino acid motif selected from the group consisting of “PG”, “GP”, “PI” and “IG”and having up to 10 amino acids upstream and / or downstream of the amino acid motif, wherein “P” in the motif is proline or hydroxyproline and the peptide stimulates the development, maintenance and repair of bone, cartilage and associated connective tissue. The invention further relates to pharmaceutical compositions of these peptides, as well as therapeutic and prophylactic uses of such peptides.
Owner:OCTANE ORTHOBIOLOGICS INC +1
Matrix composed of a naturally-occurring protein backbone cross linked by a synthetic polymer and methods of generating and using same
A method of treating a disorder characterized by tissue damage is provided. The method comprising providing to a subject in need-thereof a composition which comprises a synthetic polymer attached to denatured fibrinogen or a therapeutic portion of the fibrinogen, the composition being formulated for releasing the therapeutic portion of the fibrinogen in a pharmacokinetically regulated manner, thereby treating the disorder characterized by tissue damage or malformation.
Owner:REGENTIS BIOMATERIALS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com