Patents
Literature
540 results about "Human plasma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Human Plasma. Processed human plasma is a cost-effective, industry-proven, human-based diluent. SeraCon™ and Basematrix are suitable human blood-based matrices for use as negative diluents in serology and molecular-based assays. Choose from multiple custom processing options to fit your needs.
Modified Human Plasma Polypeptide or Fc Scaffolds and Their Uses
InactiveUS20080125574A1Improve stabilityGood water solubilityPeptide/protein ingredientsAntibody mimetics/scaffoldsBlood plasmaChemistry
Owner:AMBRX
Use of human plasma hyaluronidase in cancer treatment
InactiveUS7148201B2Control of level of activityQuick filterBiocidePeptide/protein ingredientsPurification methodsScreening method
Owner:RGT UNIV OF CALIFORNIA
Human plasma hyaluronidase
InactiveUS7105330B2Less likely to induceControl of level of activityPeptide/protein ingredientsMicroorganism based processesPurification methodsScreening method
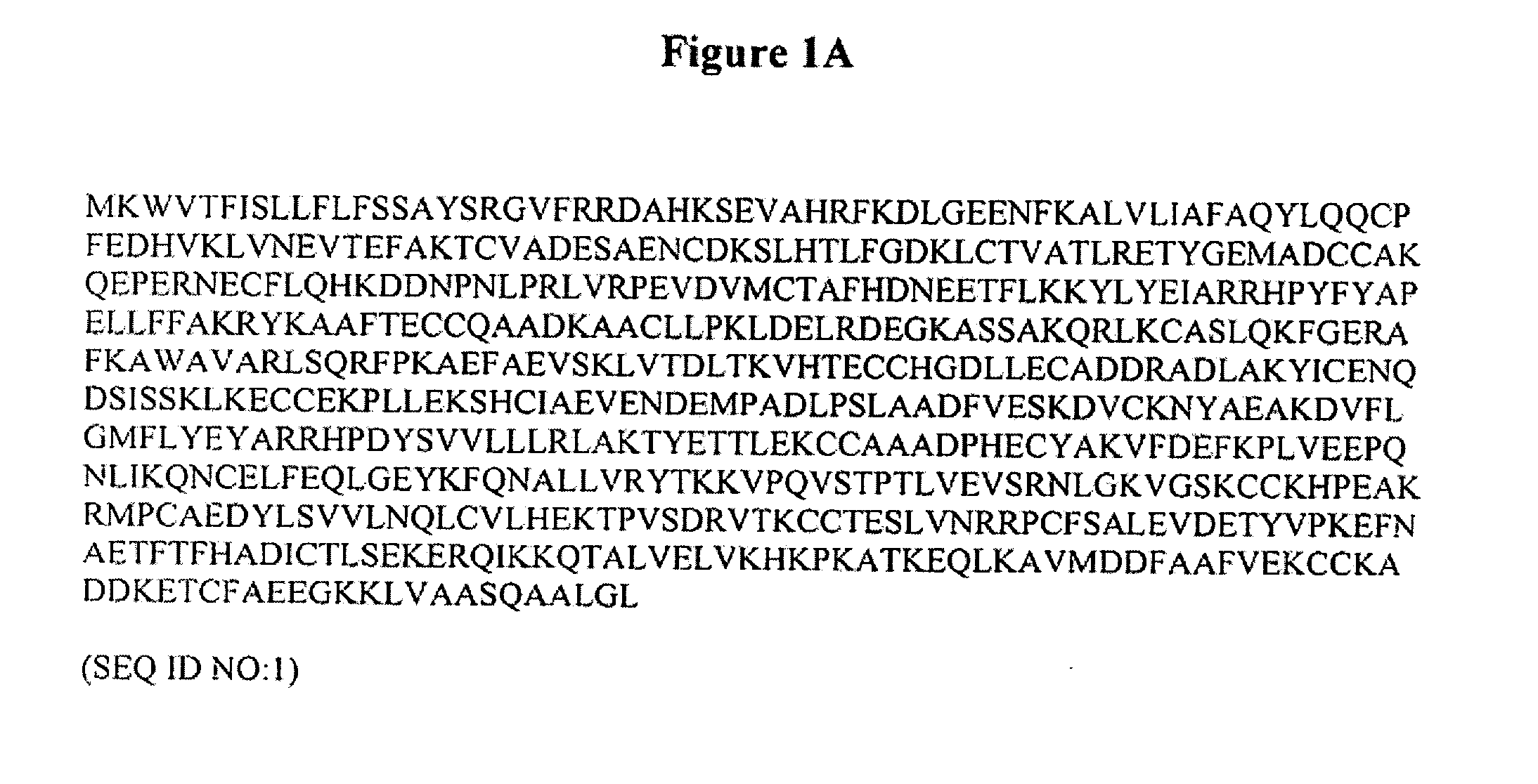
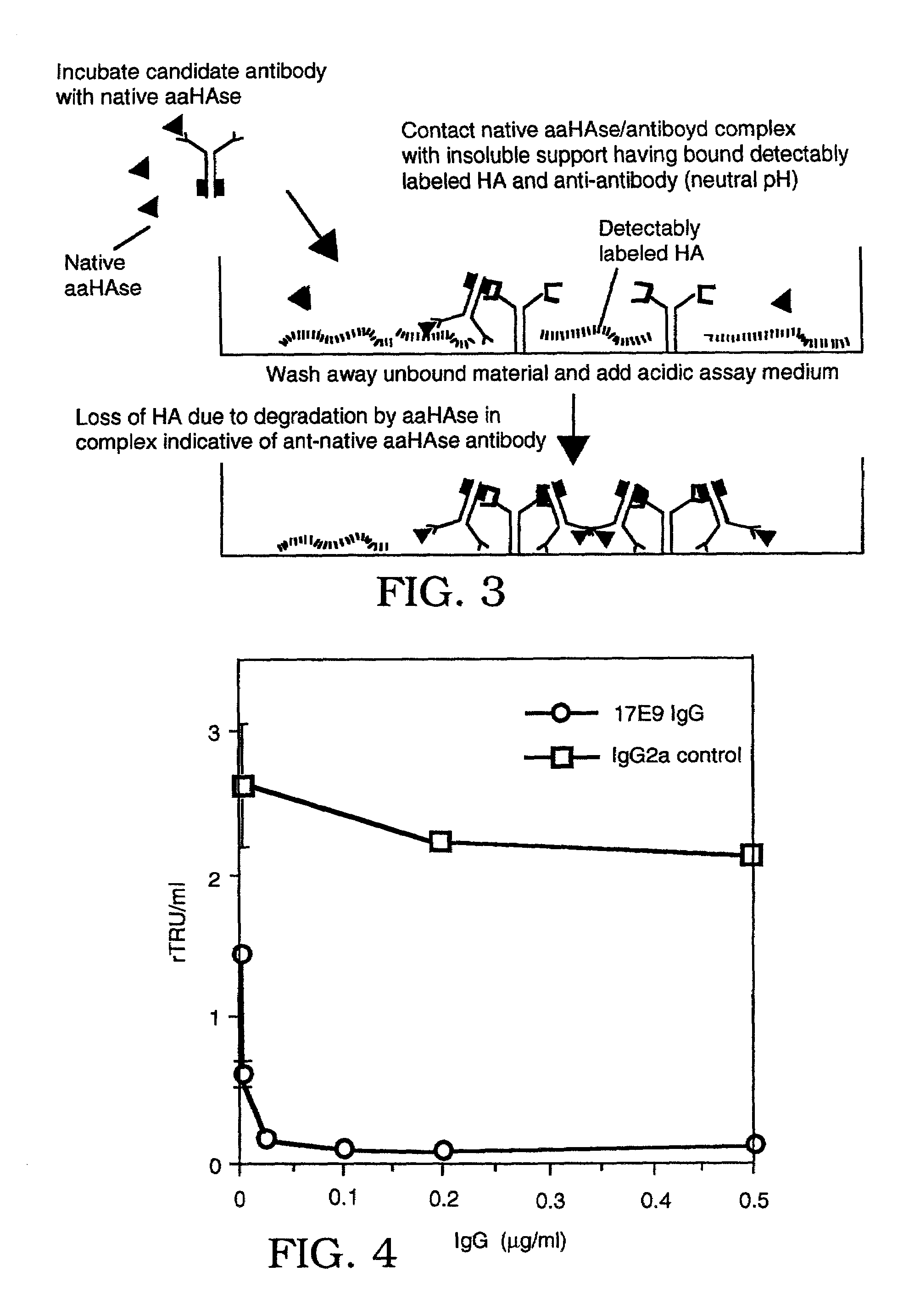
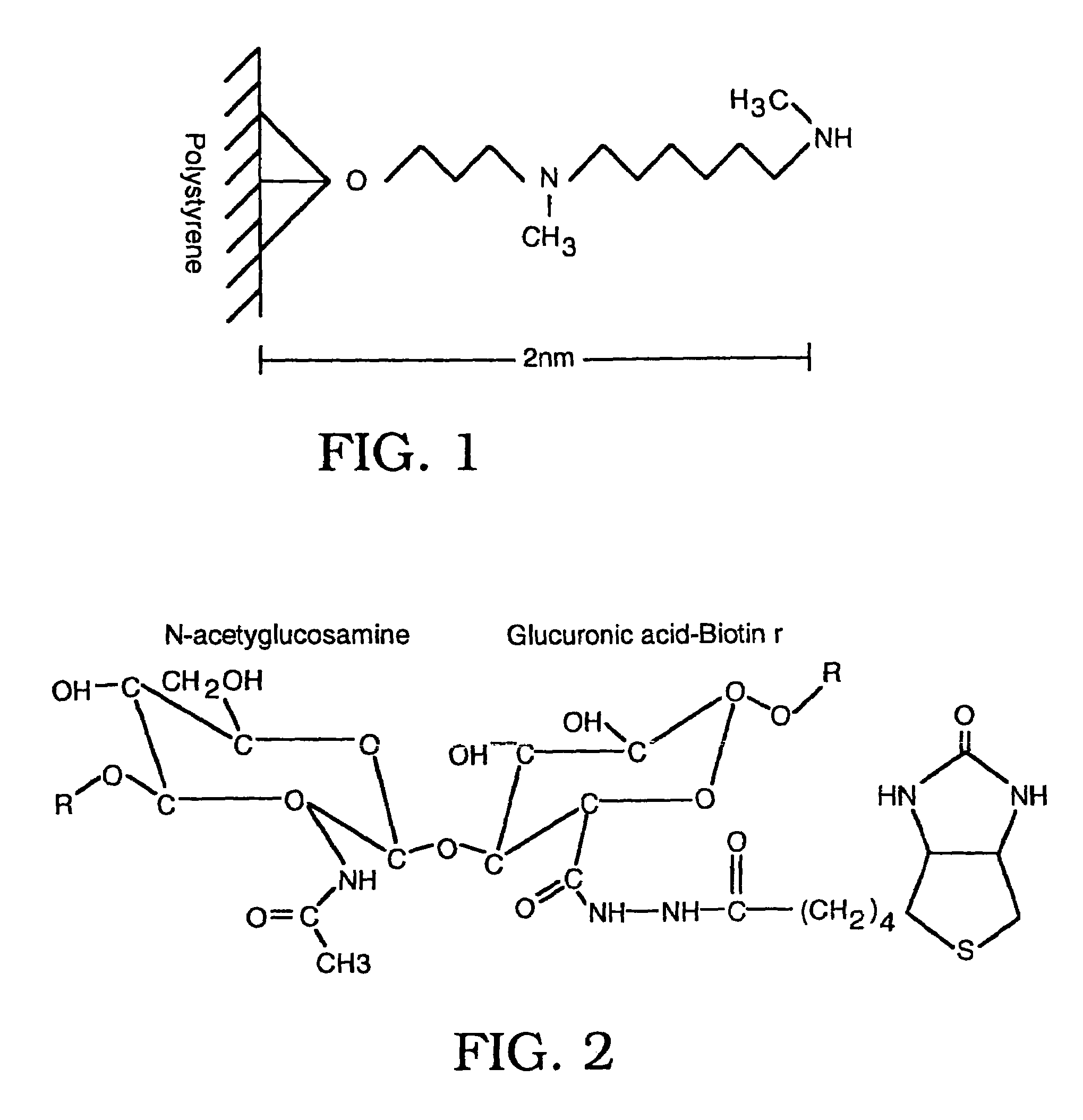
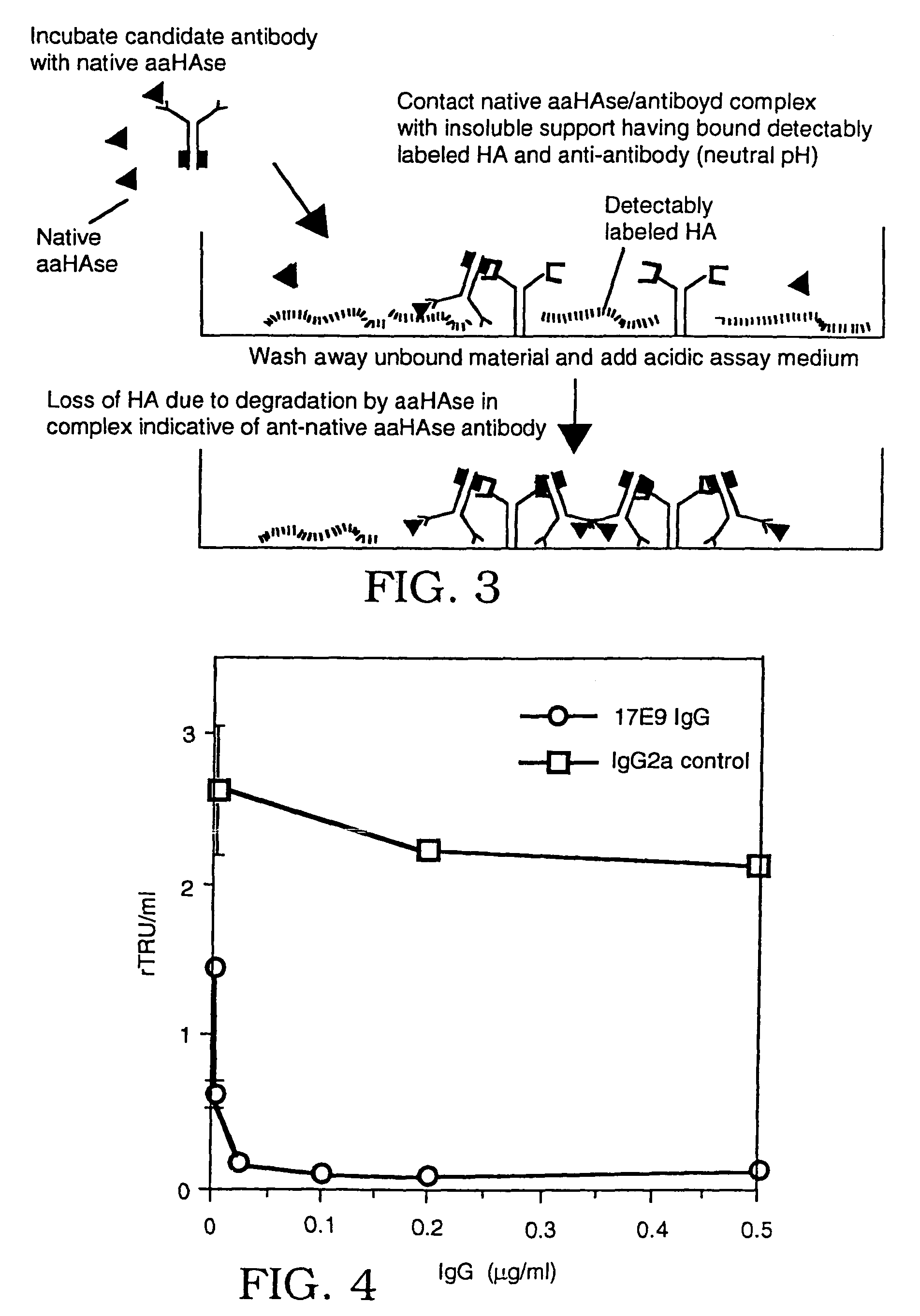
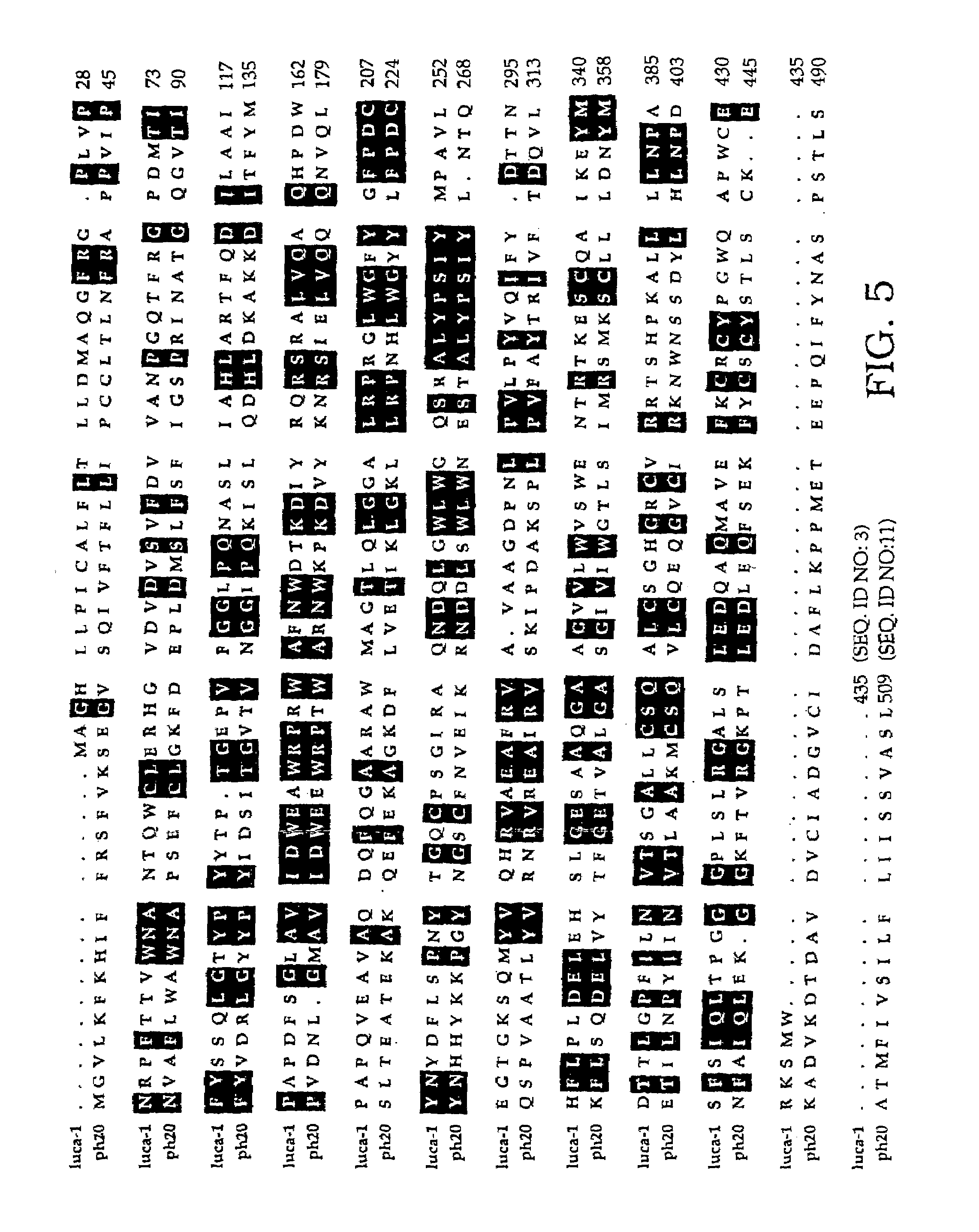
The invention is based on the discovery of methods for purification of an acid active hyaluronidase found in human plasma (hpHAse), including both biochemical and immunoaffinity purification methods. The method of immunoaffinity purification of the invention is based on the discovery of a method for identifying antibodies that specifically bind native hpHAse (anti-native hpHAse antibodies), and anti-native hpHAse antibodies identified by this screening method. The invention also features an assay for sensitive detection of HAse activity using biotinylated hyaluronic acid (bHA). Purification and characterization of hpHAse lead to the inventors' additional discovery that hpHAse is encoded by the LuCa-1 gene, which gene is present in the human chromosome at 3p21.3, a region associated with tumor suppression. The invention additionally features methods of treating tumor-bearing patients by administration of hpHAse and / or transformation of cells with hpHAse-encoding DNA.
Owner:RGT UNIV OF CALIFORNIA
Fibrin-containing composition
InactiveUS20060128016A1Improve performanceRapidly and simply producingSenses disorderFibrinogenFiberPurification methods
It is intended to provide a scaffold material having favorable properties and being appropriate for cell proliferation and differentiation in regeneration therapy. Namely, a fibrin-containing biological scaffold material to be used in the case of employing a fibrin composition for the regeneration of a human tissue and cell proliferation, characterized by containing a mixture of a fibrinogen concentrate, which is obtained from human plasma by a quick and rough purification method, with a fibrinogen activator.
Owner:ASAHI KASEI MEDICAL CO LTD
Sustained release ranolazine formulations
InactiveUS6852724B2Without fluctuationBiocidePharmaceutical non-active ingredientsRanolazineDissolution
A sustained release ranolazine formulation contains an intimate mixture of ranolazine and a partially neutralized pH-dependent binder to form a film that is mostly insoluble in aqueous media below pH 4.5 and soluble in aqueous media above pH 4.5. The formulation is suitable for twice daily administration of ranolazine and is useful for controlling the rate of dissolution of ranolazine, and to maintain human plasma ranolazine levels at between 850 and 4000 ng base / mL.
Owner:GILEAD SCI INC
Chromatographic method for high yield purification and viral inactivation of antibodies
InactiveUS6955917B2Minimizes post virus treatment manipulationYield maximizationPeptide/protein ingredientsSerum immunoglobulinsLipid formationLow ionic strength
An improved process for the purification of antibodies from human plasma or other sources is disclosed. The process involves suspension of the antibodies at pH 3.8 to 4.5 followed by addition of caprylic acid and a pH shift to pH 5.0 to 5.2. A precipitate of contaminating proteins, lipids and caprylate forms and is removed, while the majority of the antibodies remain in solution. Sodium caprylate is again added to a final concentration of not less than about 15 mM. This solution is incubated for 1 hour at 25° C. to effect viral inactivation. A precipitate (mainly caprylate) is removed and the clear solution is diluted with purified water to reduce ionic strength. Anion exchange chromatography using two different resins is utilized to obtain an exceptionally pure IgG with subclass distribution similar to the starting distribution. The method maximizes yield and produces a gamma globulin with greater than 99% purity. The resin columns used to obtain a high yield of IgG retain IgM and IgA. IgA and IgM may be eluted from these resins in high yield and purity.
Owner:BAYER HEALTHCARE LLC
Apparatus and methods for making, storing, and administering freeze-dried materials such as freeze-dried plasma
InactiveUS20090107001A1Prevent deterioration of materialAvoid contactSurgical furnitureDrying solid materials without heatBlood componentMedicine
Single unit human plasma is dispensed from a source transfer bag into a receptacle in which the plasma under goes freeze-drying. During the dispensing of the plasma from the source transfer container into the freeze-drying receptacle, an inline treatment device treats the plasma prior to freeze-drying, e.g., by the removal of cellular blood components, or by the removal of pathogens or viral or bacterial agents, or by the removal or neutralization of blood group specific antibodies.
Owner:HEMCON LIFE SCI
Modified Human Plasma Polypeptide or Fc Scaffolds and Their Uses
ActiveUS20100160212A1Improve stabilityGood water solubilityAntibacterial agentsFungiBlood plasmaChemistry
Owner:AMBRX
Kit using nanometer magnetic beads for purifying nucleic acid
The invention provides a kit using nanometer magnetic beads for purifying nucleic acid, particularly can purify nucleic acid of virus and Gram negative bacteria from human plasma or serum or urine andcan quickly and agilely purify nucleic acid on a large scale. The kit is composed of lysis solution, magnetic bead buffer solution 1, washing buffer solution 2, washing buffer solution 3, elution buffer solution and equilibrium liquid. The invention has high nucleic acid extracting speed and high sensitivity, has the potential of automatic upgrading compared with the traditional nucleic acid purifying method and makes large scale integration and homogeneity for extracting nucleic acid feasible.
Owner:上海裕隆生物科技有限公司
Method of preparing alpha-1 proteinase inhibitor
InactiveUS20110237781A1High yieldHigh purityDepsipeptidesPeptide preparation methodsAnion-exchange chromatographyBlood plasma
Purification of α-1 proteinase inhibitor (α-1 PI) from solutions comprising α-1 PI is accomplished using hydrophobic interaction chromatography (HIC). In some embodiments, purification of α-1 PI is accomplished by precipitation of contaminating proteins from a starting solution comprising α-1 PI, such as human plasma, followed by anion exchange resin chromatography prior to HIC. Further purification may be accomplished by an optional cation exchange chromatography subsequent to anion exchange chromatography but prior to HIC. Some embodiments of the invention also include virus removal and / or inactivation by methods such as nano filtration and such as contact with a non-ionic detergent. The methods of the present invention result in greater yield, purity, and pathogenic clearance of plasma fractions than known methods.
Owner:GRIFOLS THERAPEUTICS INC
Preparation and composition of inter-alpha inhibitor proteins from human plasma for therapeutic use
ActiveUS7932365B2Reduce riskAntibacterial agentsPeptide/protein ingredientsBlood plasmaRheumatoid arthritis
The invention relates to Inter-alpha inhibitor proteins (IαIp). The invention further relates to processes for purification of IαIp compositions and their use for treatment of human diseases such as sepsis and septic shock, rheumatoid arthritis, cancer and infectious diseases.
Owner:POROTHERA BIOLOGY
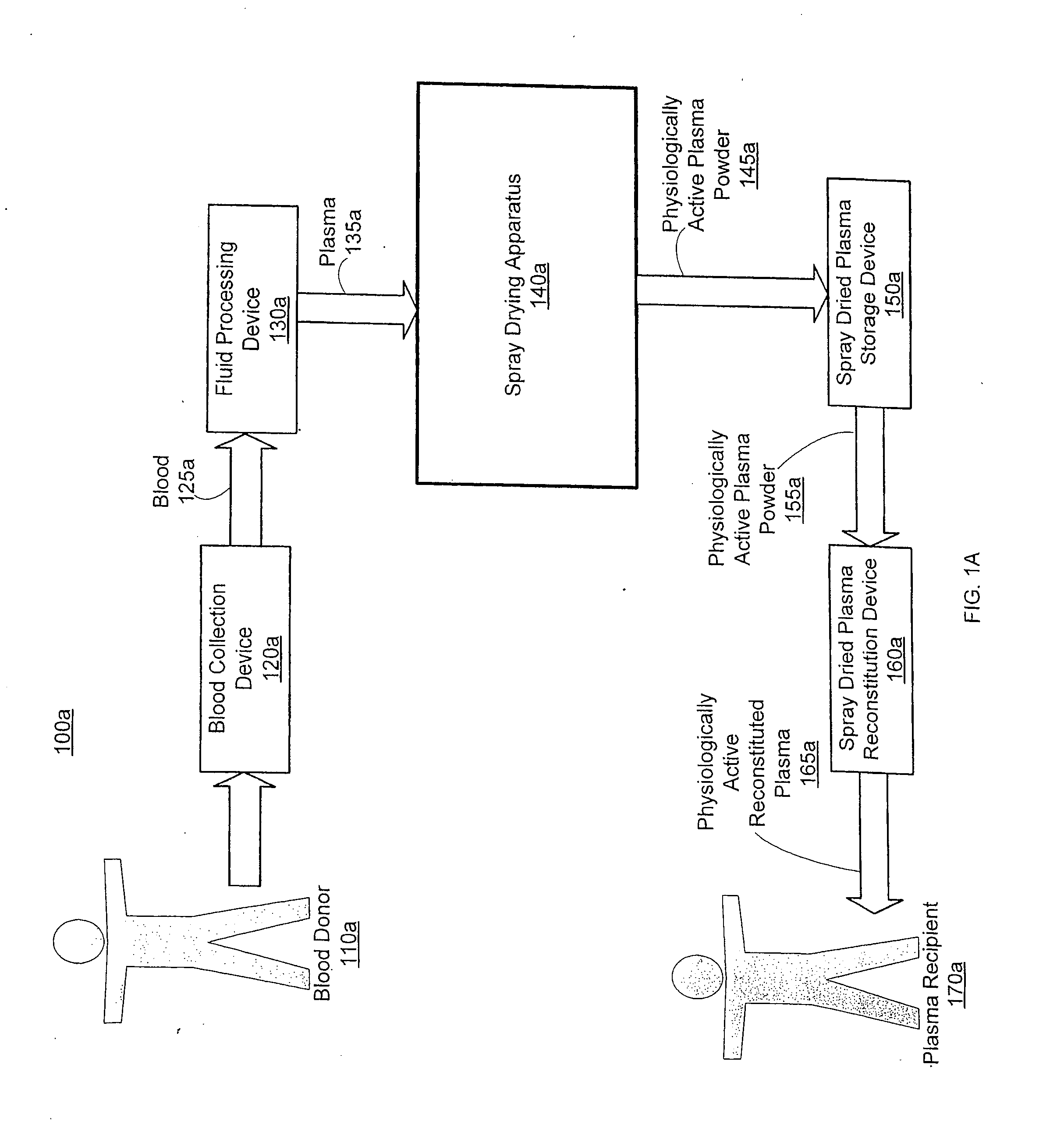
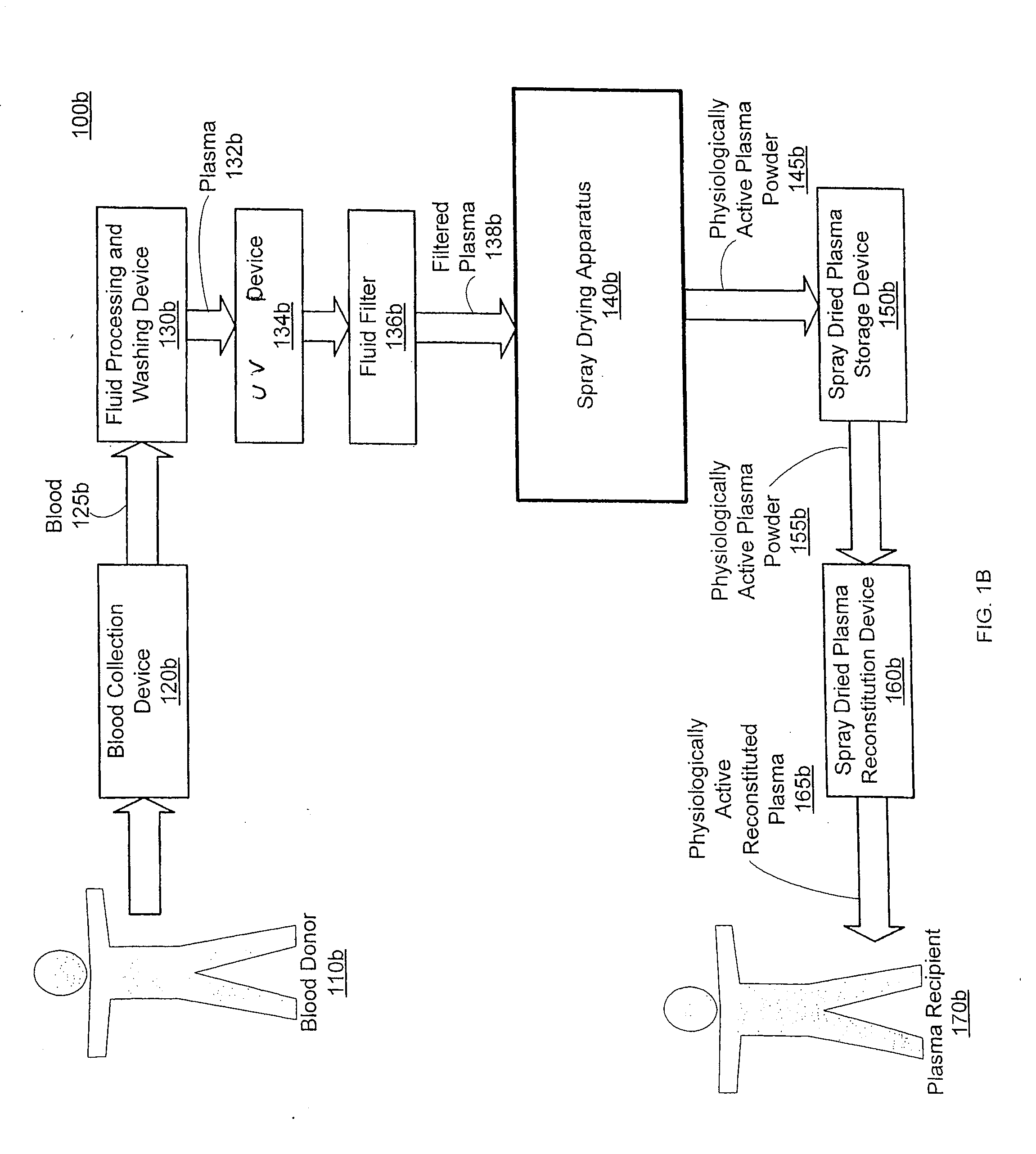
Spray dried human plasma
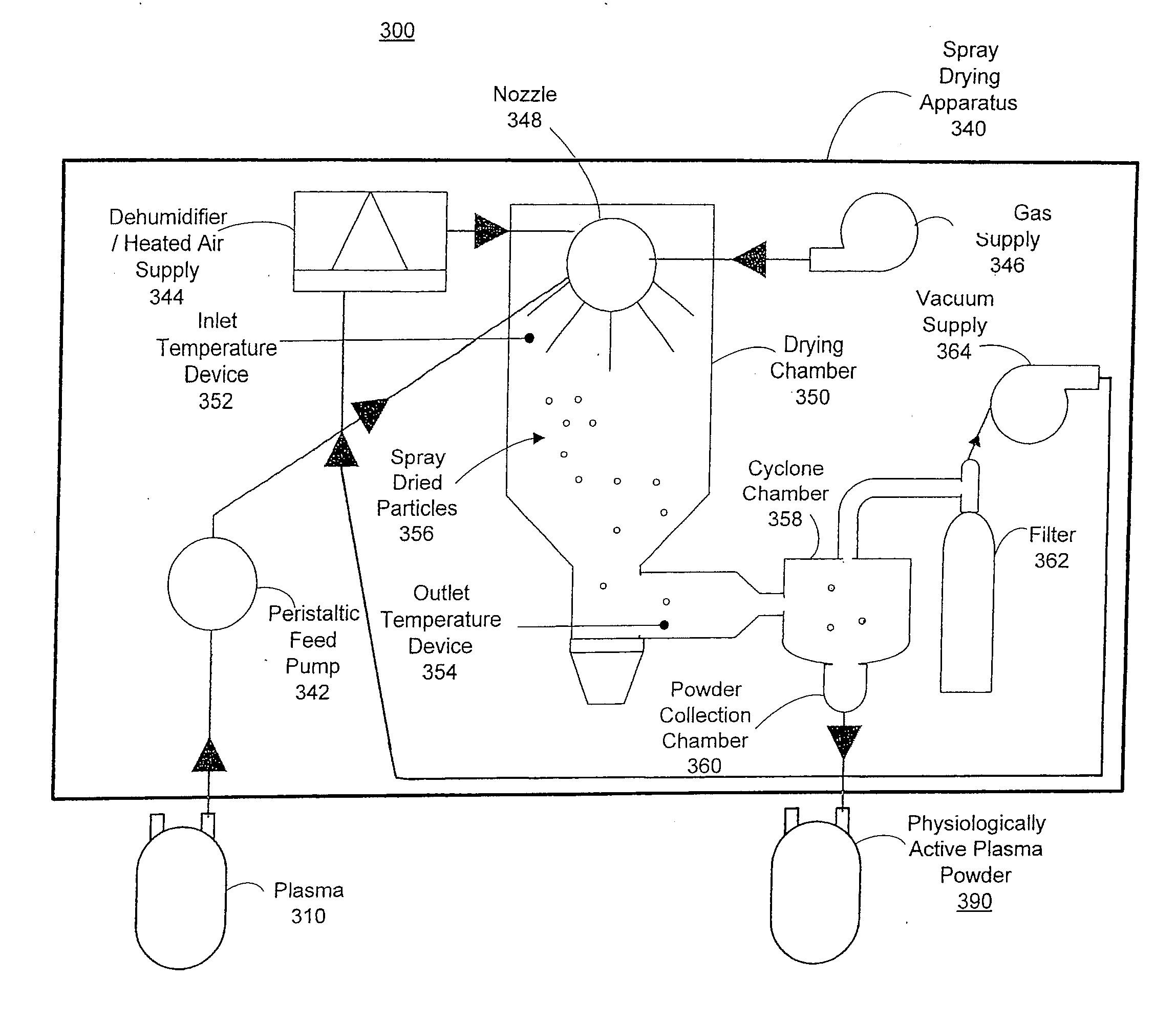
ActiveUS20120167405A1Easy to shipEasy to storeDrying solid materials with heatDrying gas arrangementsSpray dried plasmaEngineering
The technology relates to spray dried plasma and methods of making the same. The method includes providing plasma to a spray drying apparatus, spray drying the plasma, at the spray drying apparatus, to form physiologically active plasma powder, the spray drying apparatus configured utilizing one or more parameters, and storing the physiologically active plasma powder.
Owner:VELICO MEDICAL
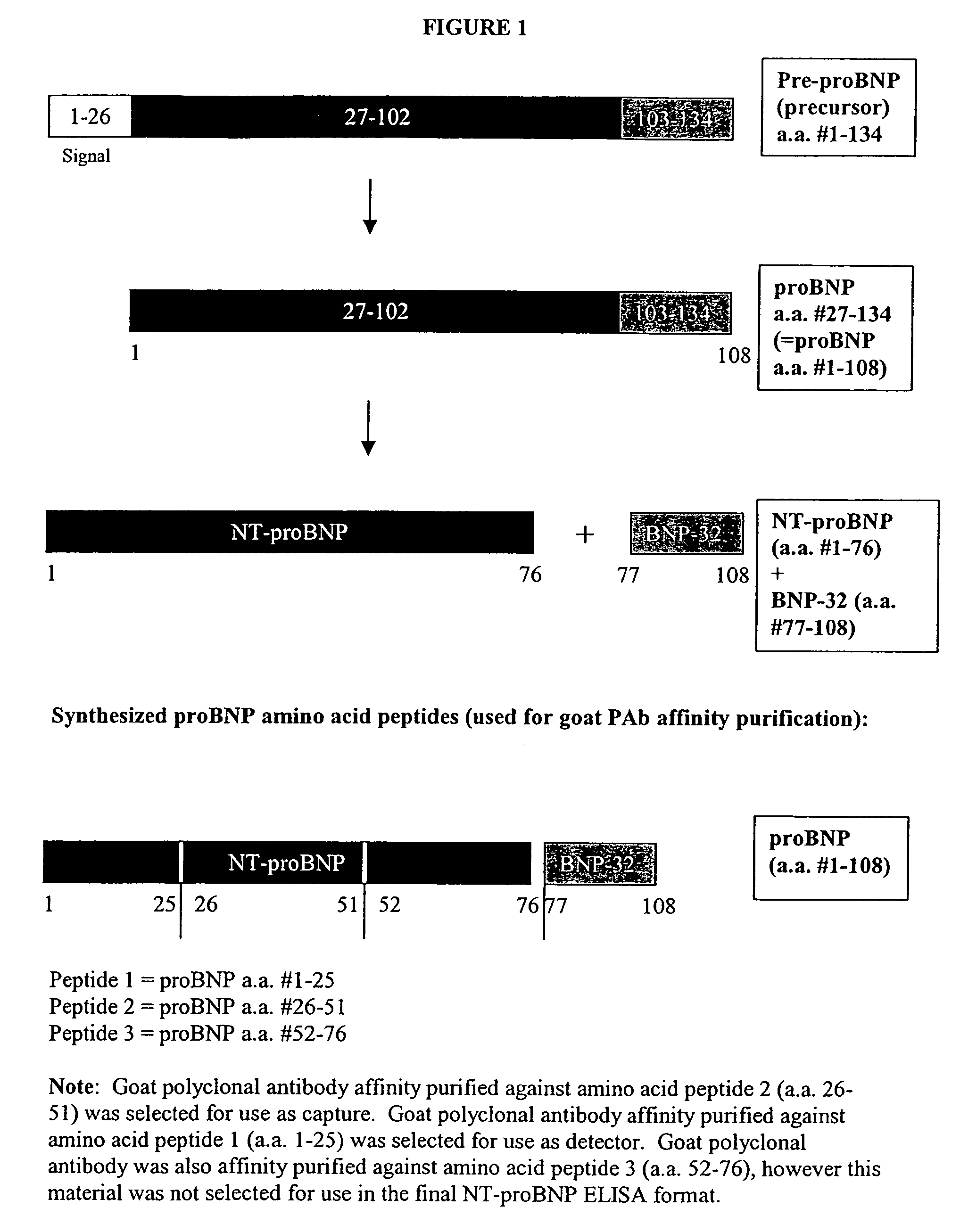
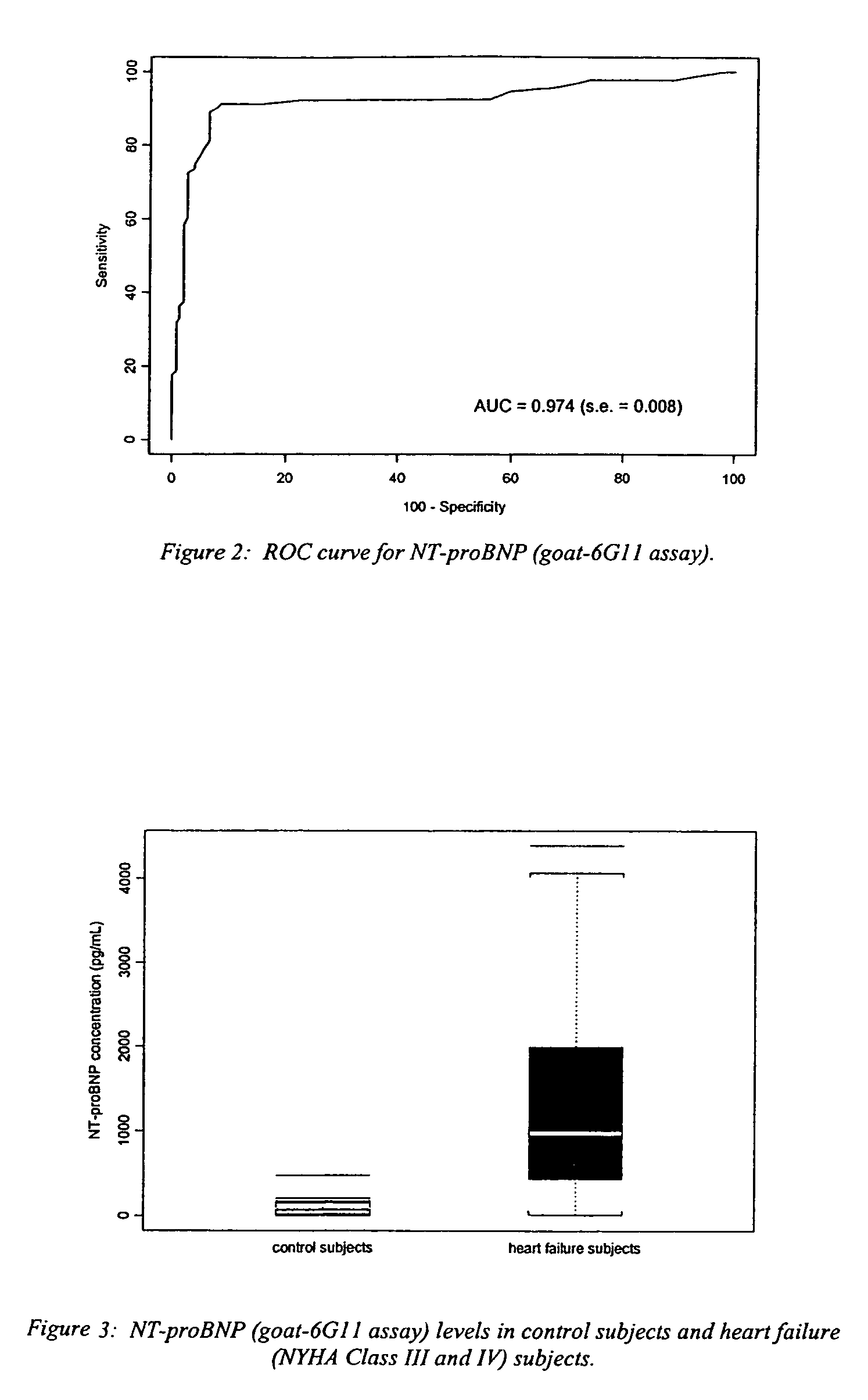
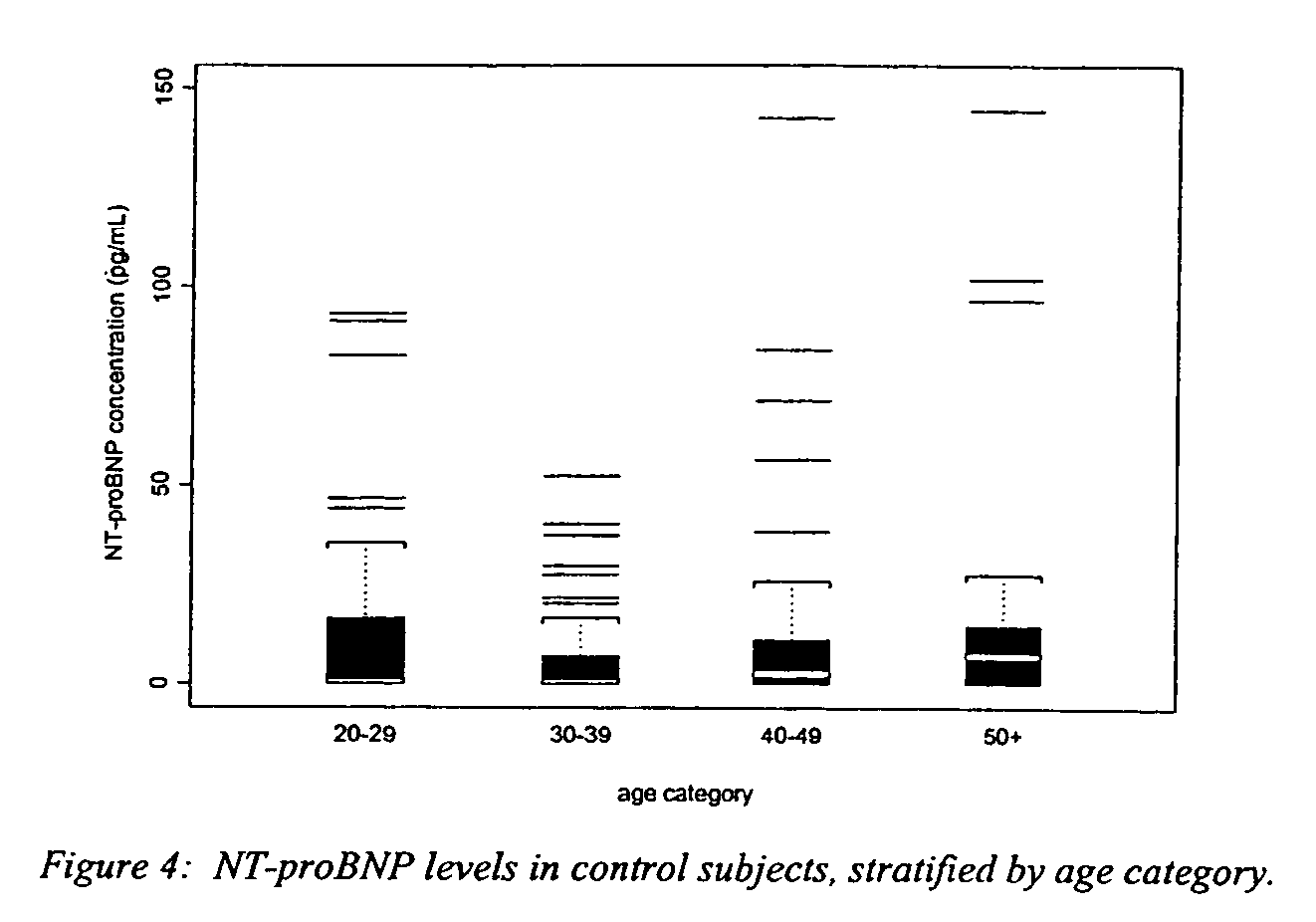
Polyclonal-monoclonal ELISA assay for detecting N-terminus proBNP
InactiveUS7527939B2Accurately predicting mortalityDisease diagnosisImmunoglobulins against hormonesBlood plasmaAmino acid
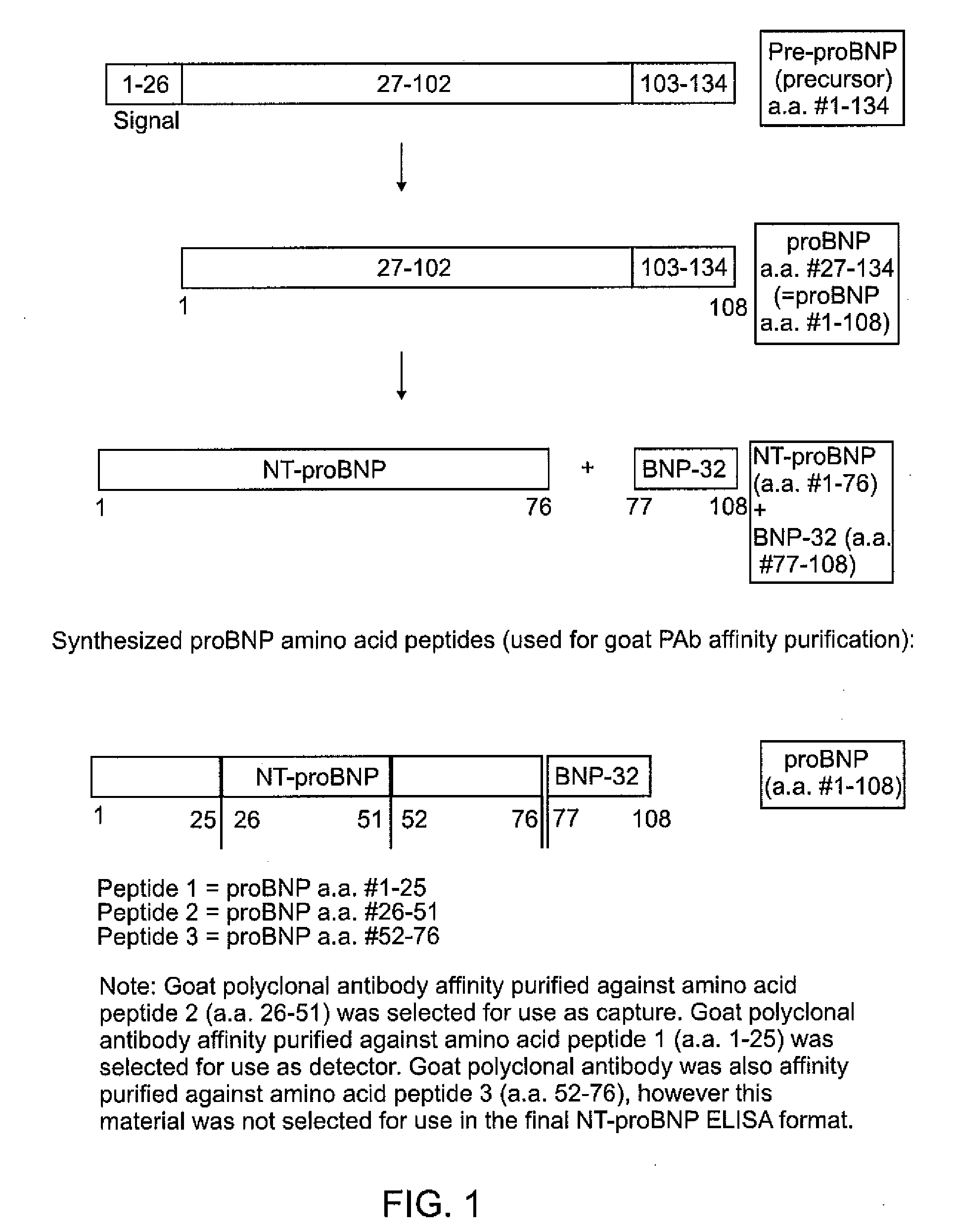
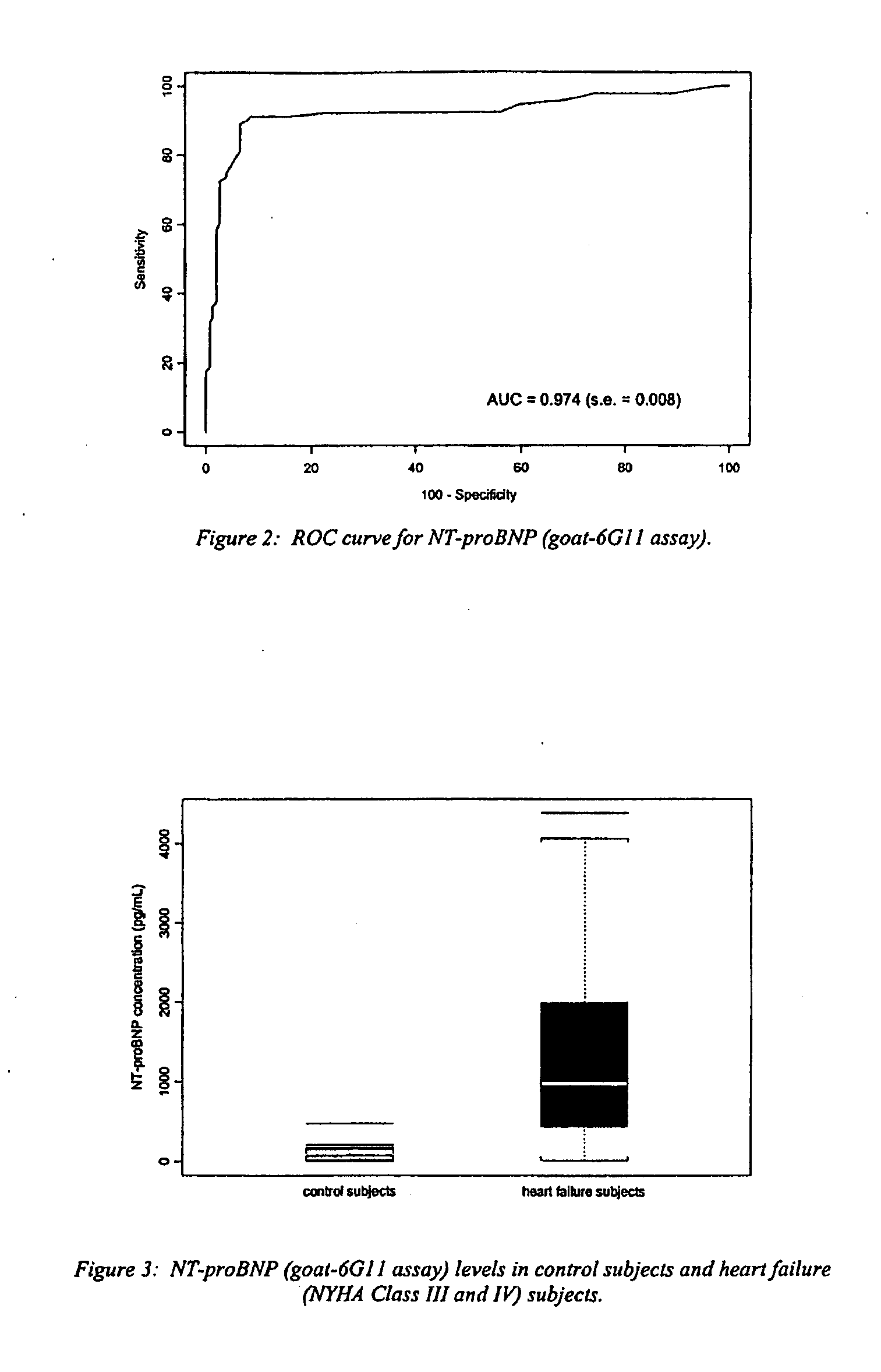
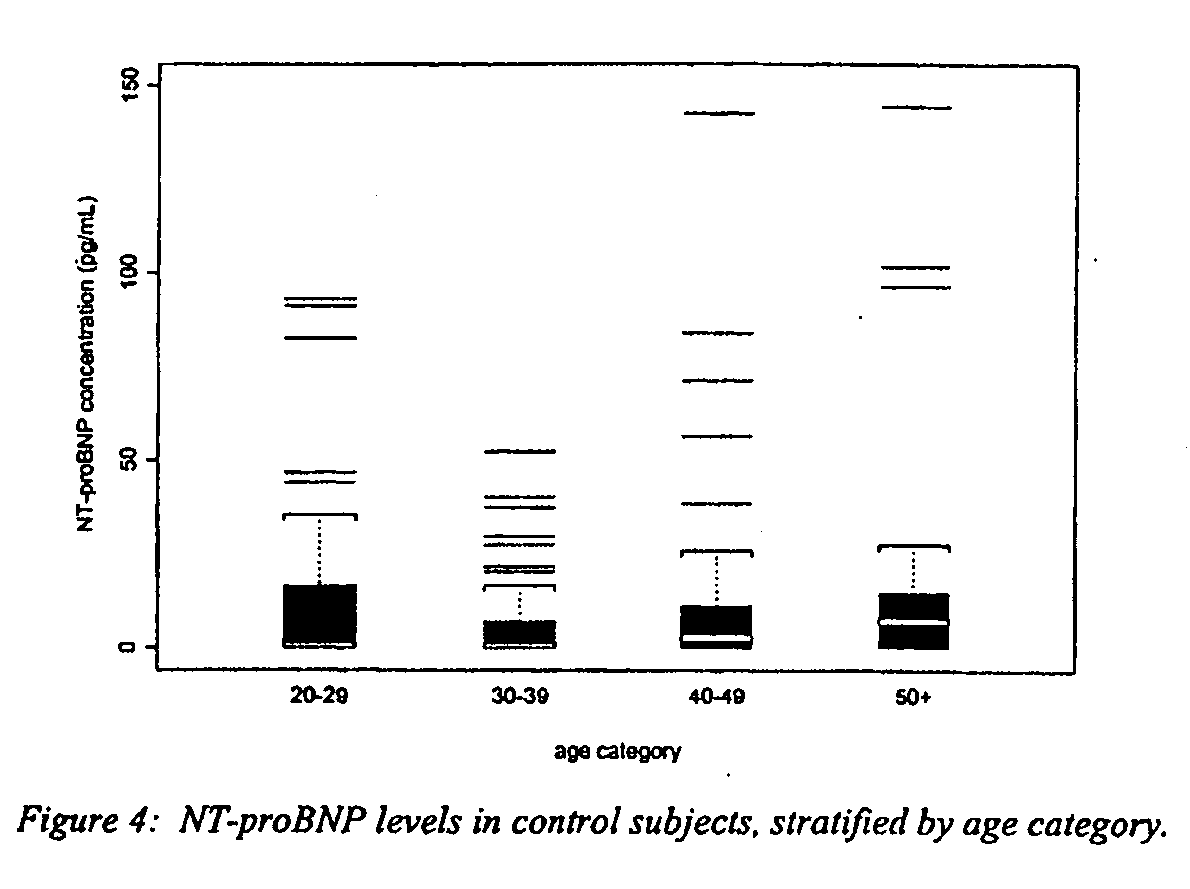
A specific and sensitive in vitro ELISA assay and diagnostic test kit is disclosed for determining levels of NT-proBNP protein in a variety of bodily fluids, non-limiting examples of which are blood, serum, plasma, urine and the like. The NT-proBNP ELISA assay test employs the sandwich ELISA technique to measure circulating NT-proBNP in human plasma. In order to obtain antibodies with specific binding properties for targeted amino acid sequences within human proBNP, recombinant human proBNP (or rhproBNP) was expressed and purified for use as an immunogen. Polyclonal antibodies (PAb) to specific amino acid sequences were subsequently purified from goat serum by sequential affinity purification. Monoclonal antibodies were raised against specific polypeptides. Recombinant human NT-proBNP (or rhNT-proBNP) was expressed and purified in order to obtain material for use in calibration of a quantitative method for measurement of human NT-proBNP.
Owner:NANOGEN POINT OF CARE
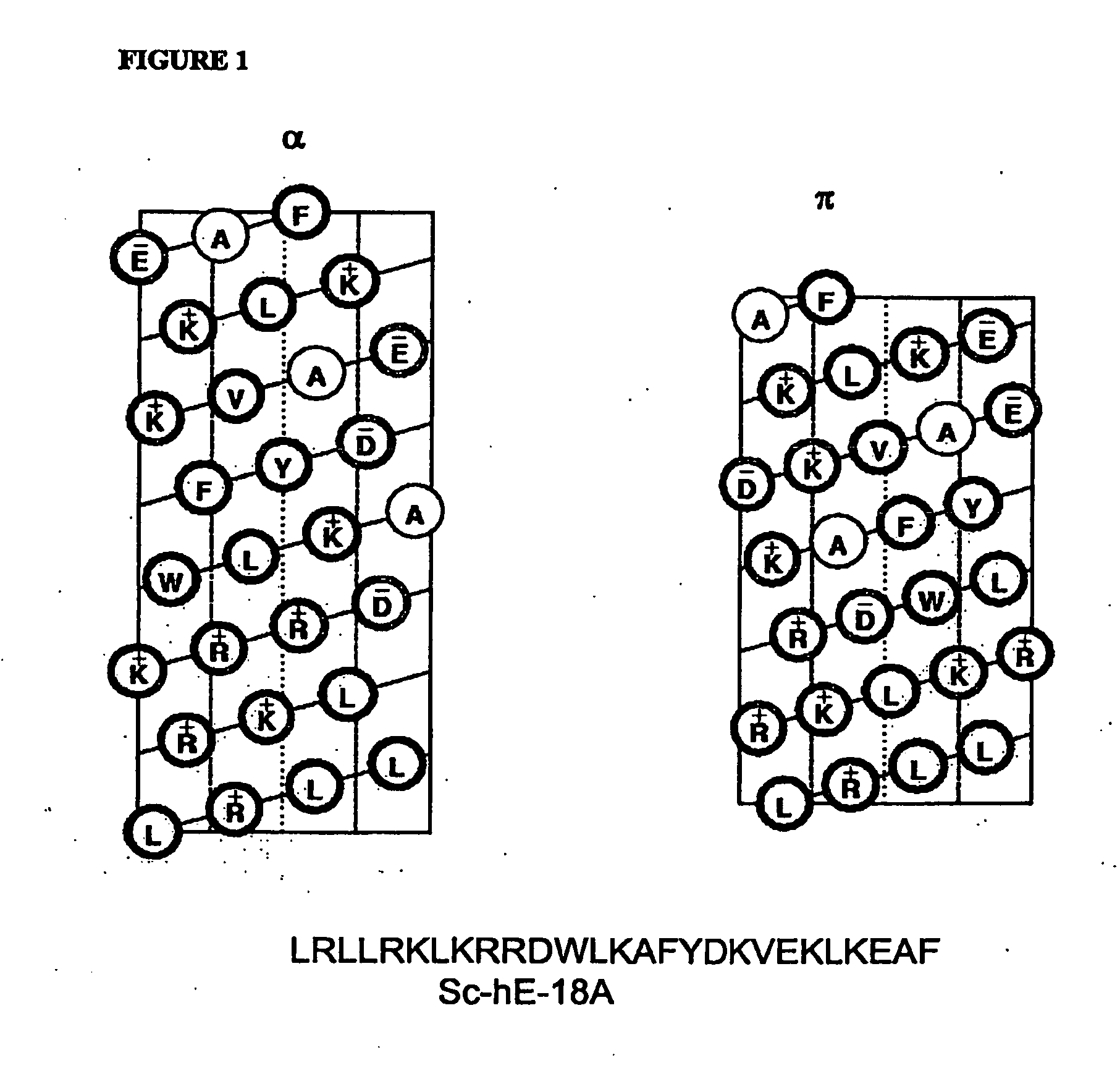
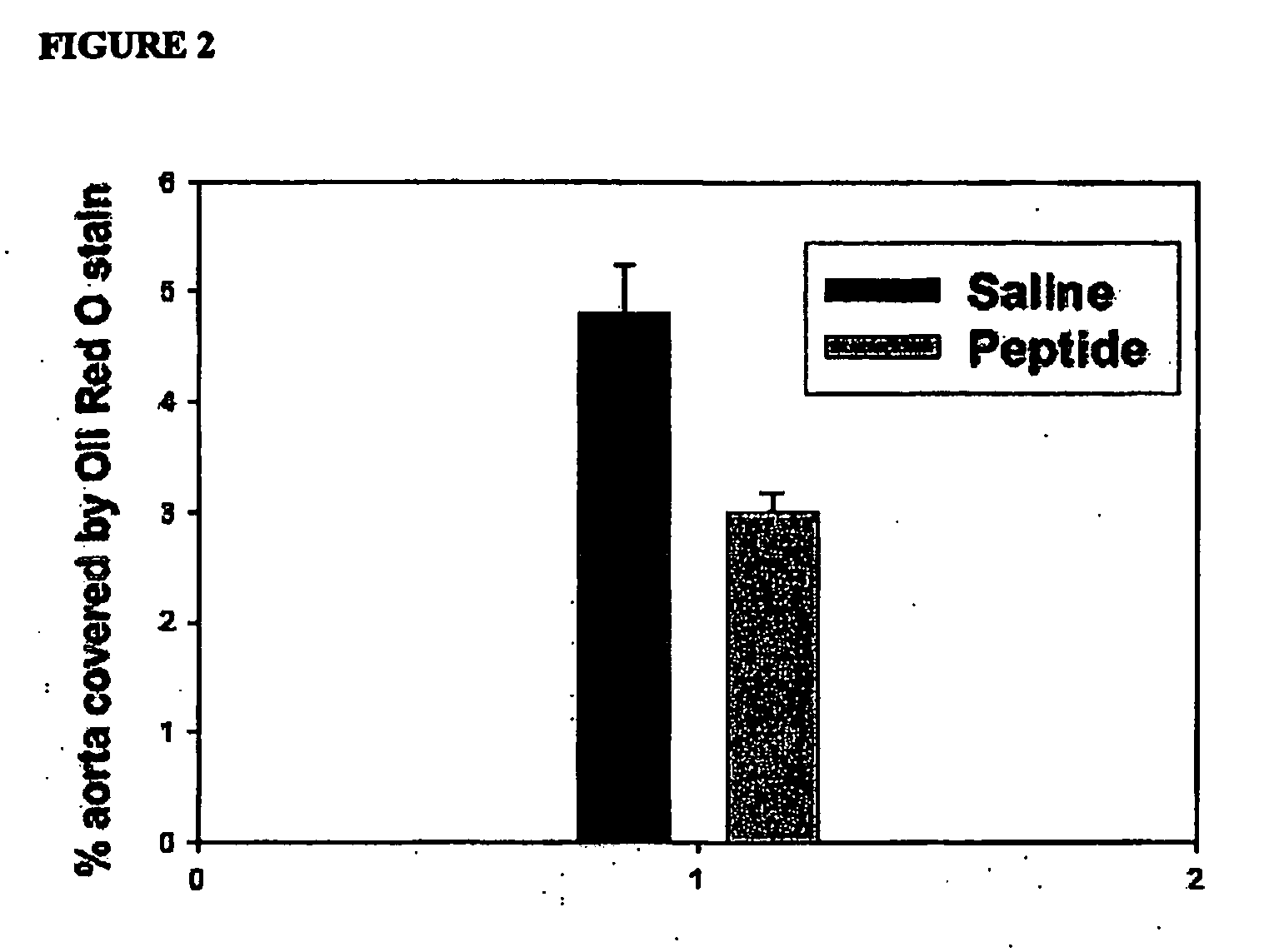
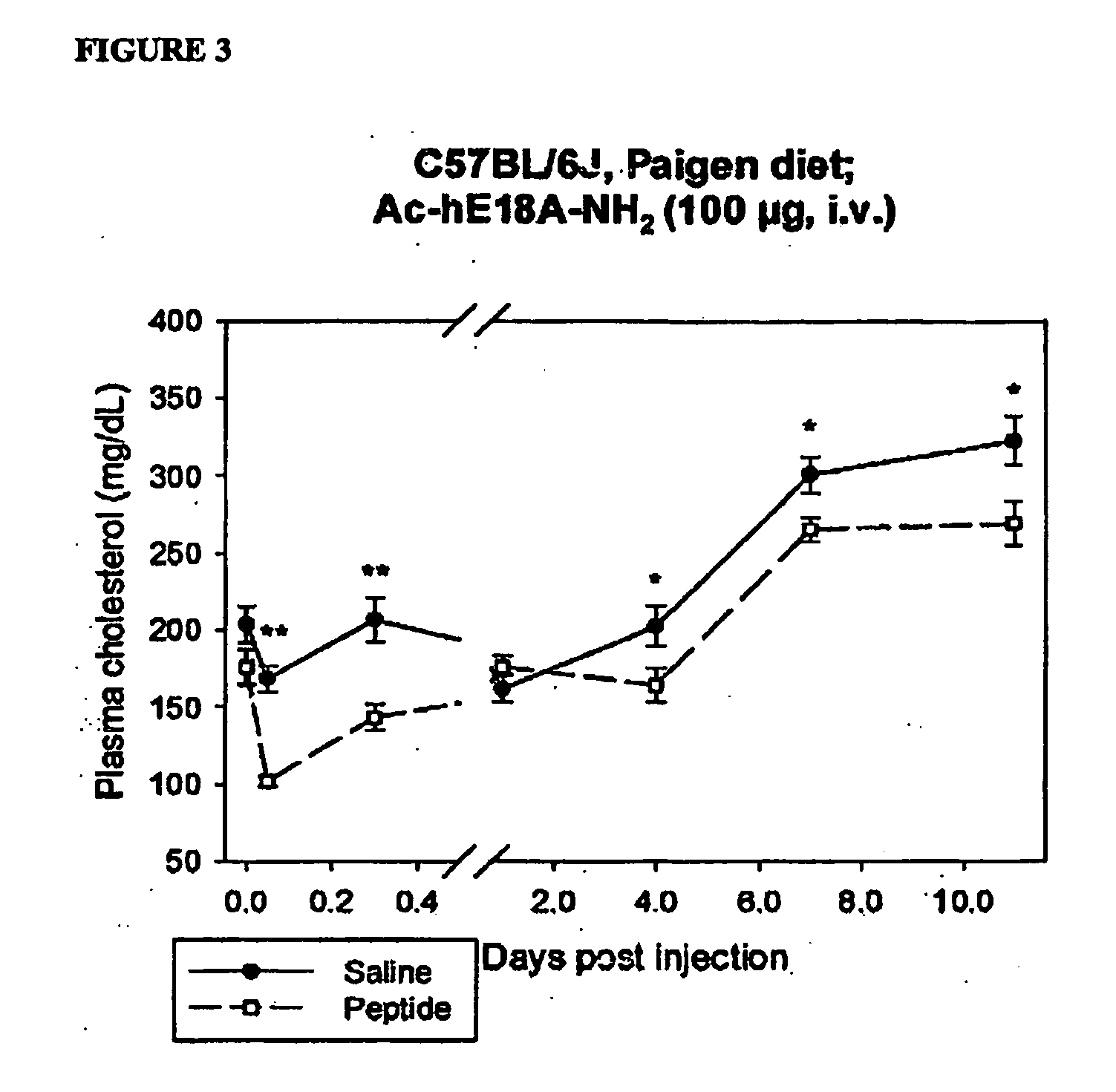
Synthetic apolipoprotein e mimicking polypeptides and methods of use
InactiveUS20100286025A1Antibacterial agentsSenses disorderAmphipathic helixVery low-density lipoprotein
The present invention provides novel synthetic apolipoprotein E (ApoE)-mimicking peptides wherein the receptor binding domain of apolipoprotein E is covalently linked to 18A, the well characterized lipid-associating model class A amphipathic helical peptide, or a modified version thereof. Such peptides enhance low density lipoprotein (LDL) and very low density lipoprotein (VLDL) binding to and degradation by fibroblast or HepG2 cells. Also provided are possible applications of the synthetic peptides in lowering human plasma LDL / VLDL cholesterol levels, thus inhibiting atherosclerosis. The present invention also relates to synthetic peptides that can improve HDL function and / or exert anti-inflammatory properties.
Owner:UAB RES FOUND
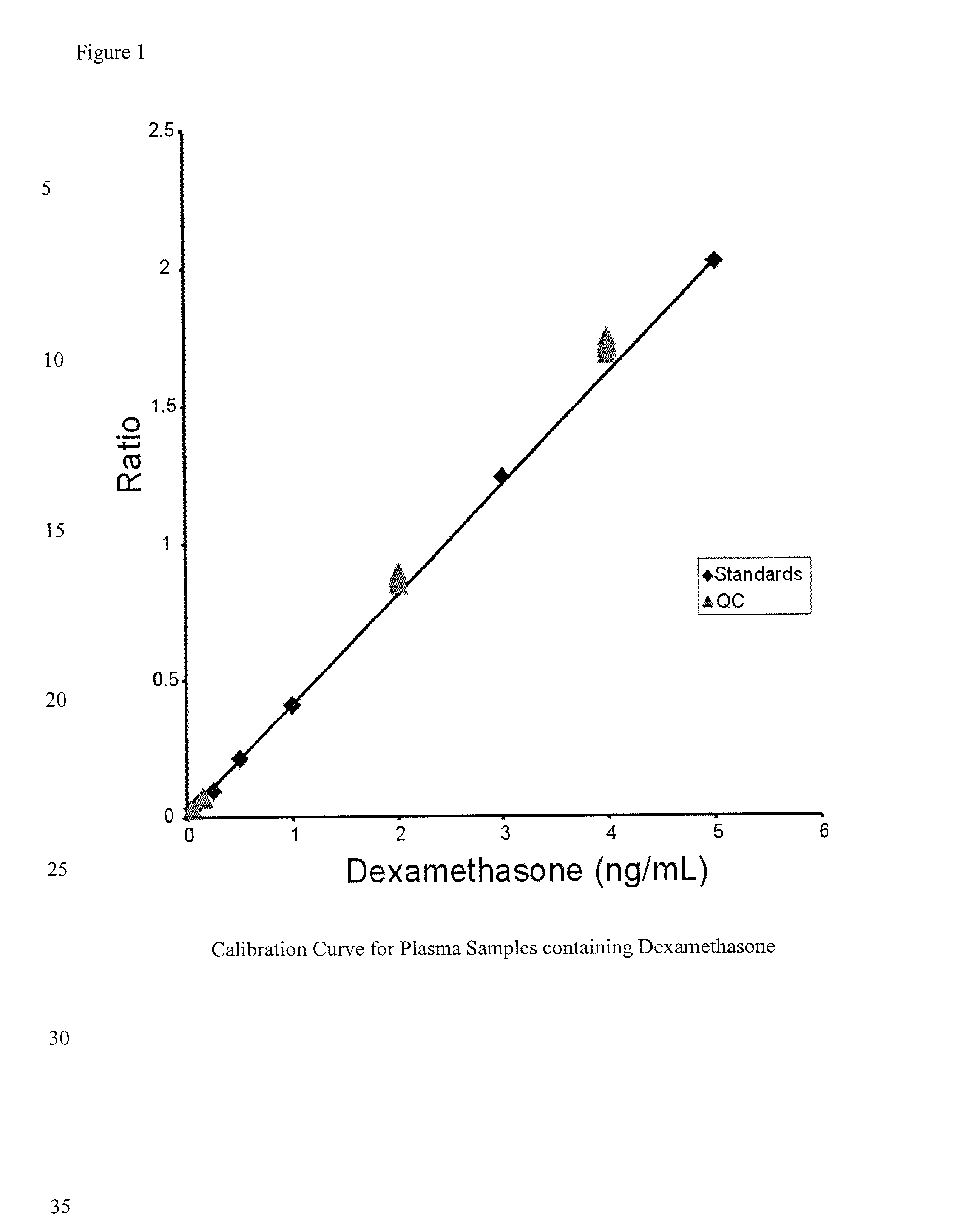
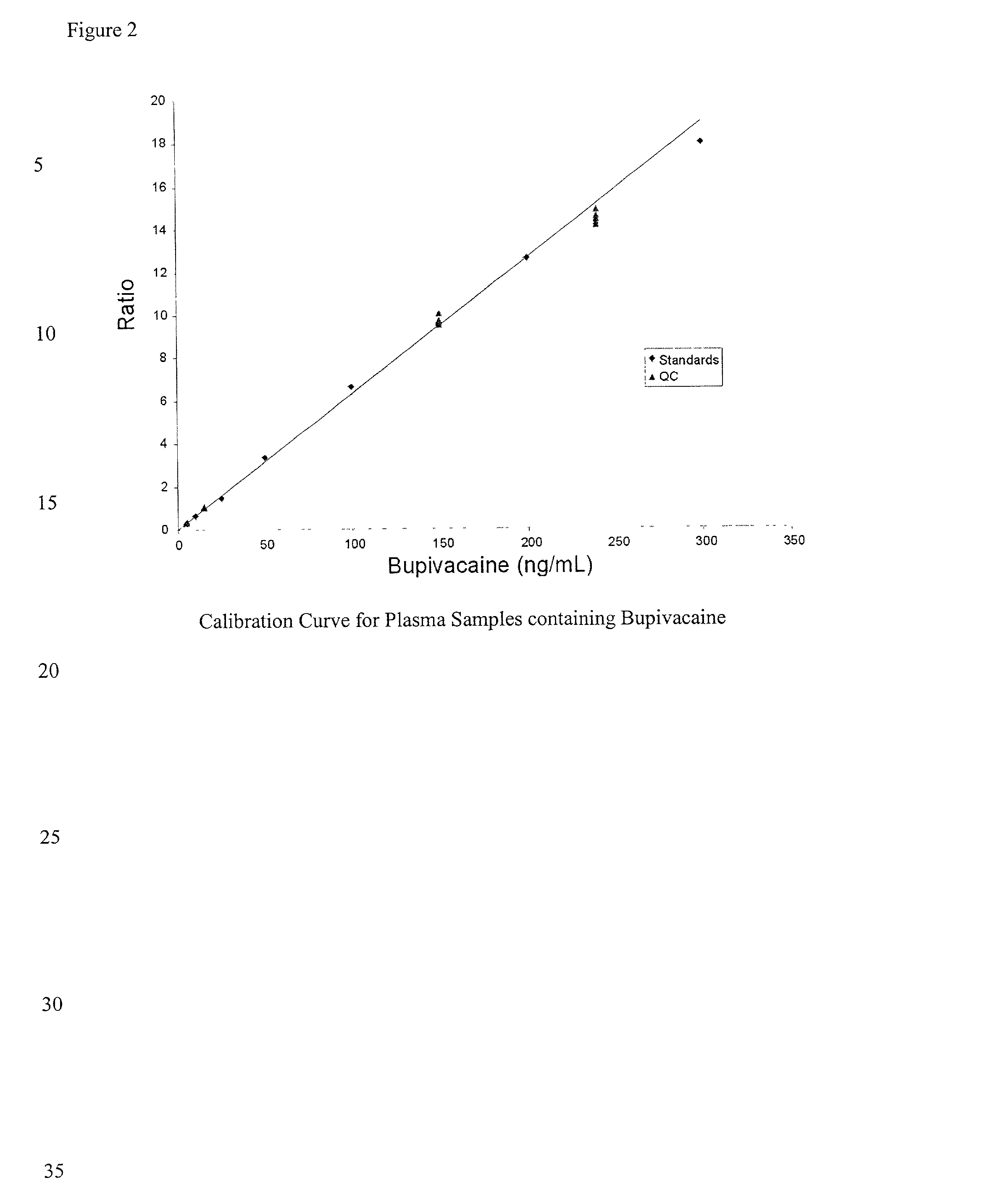
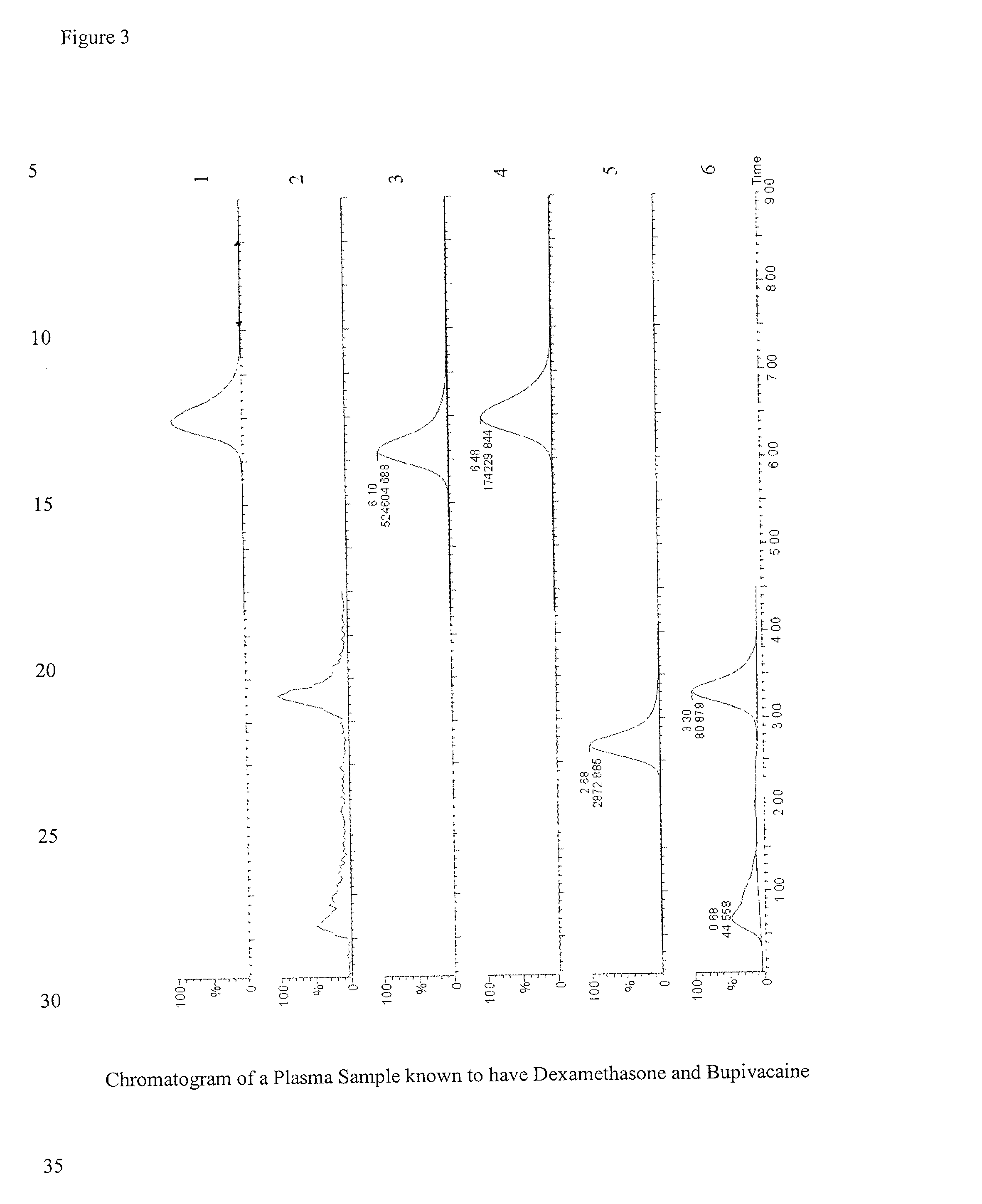
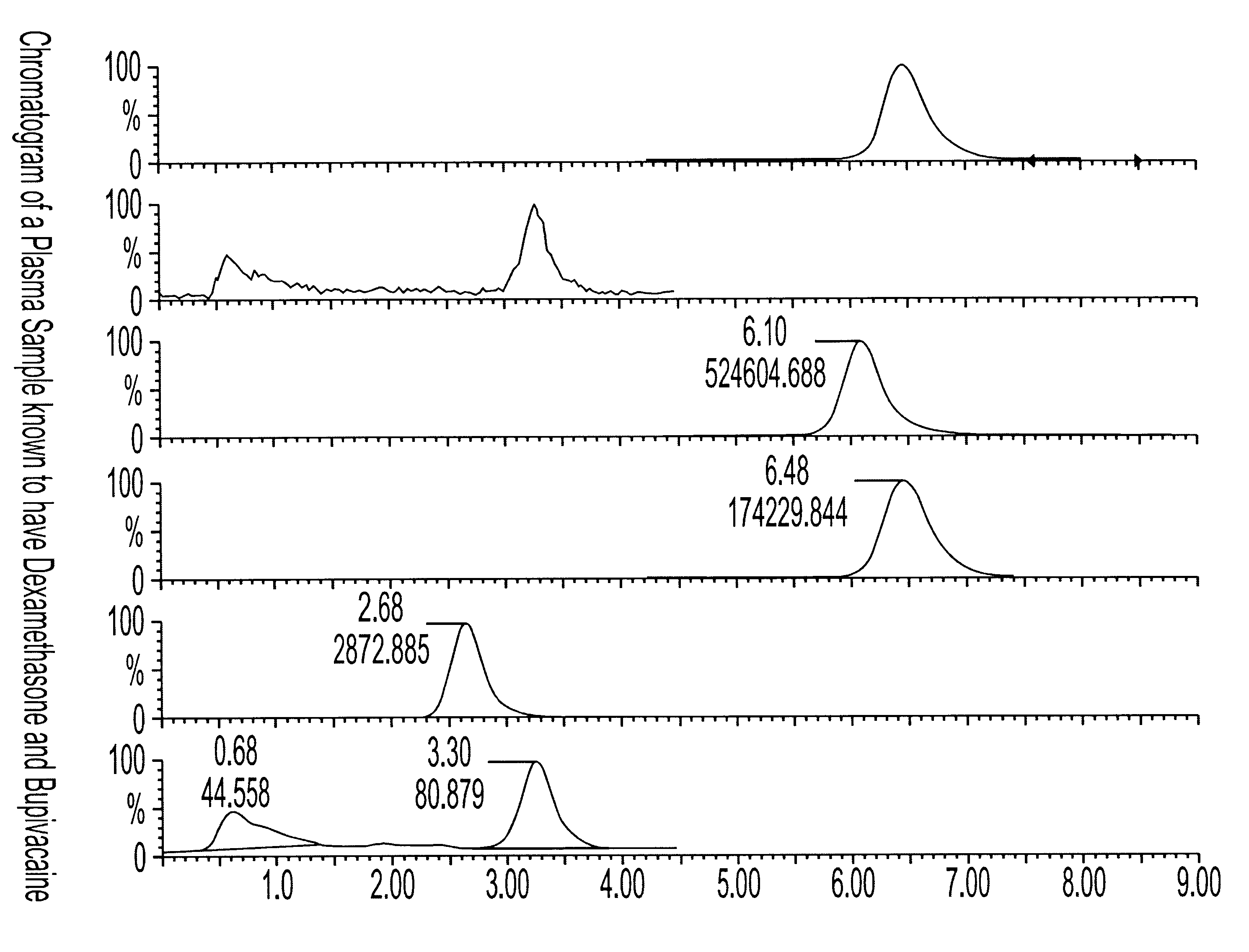
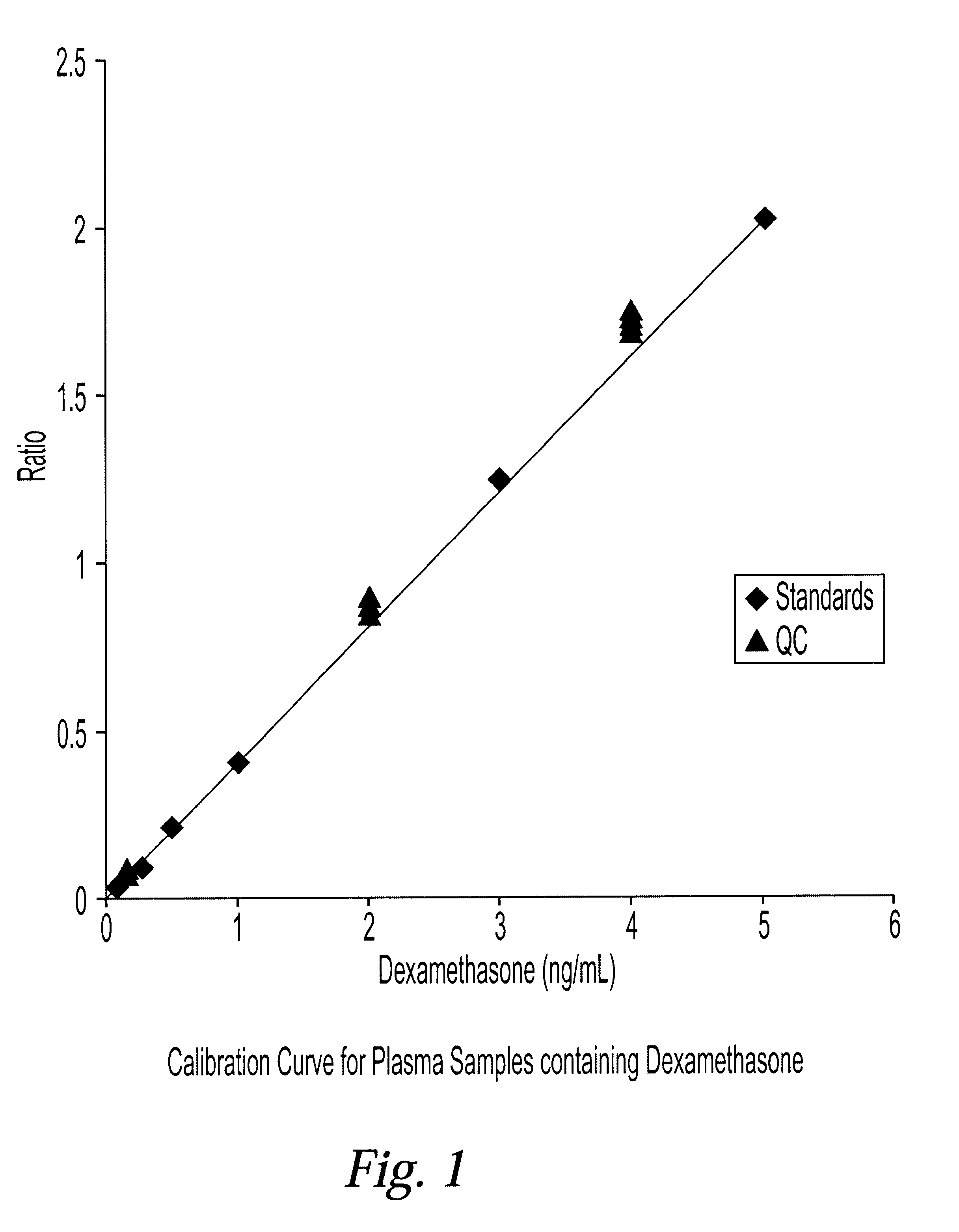
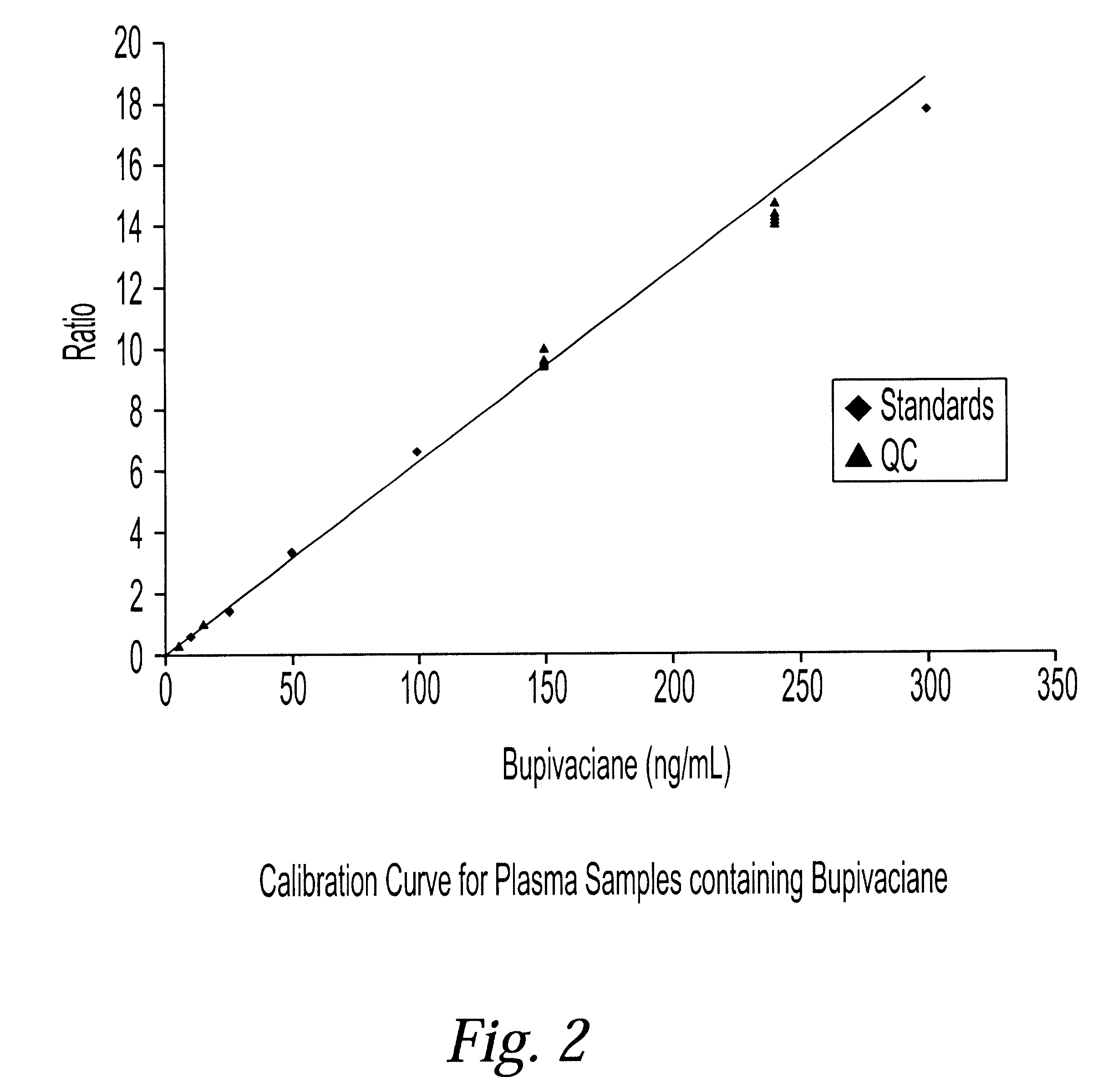
Determination of corticosteroids in human plasma using micromass LC/MS/MS/
InactiveUS20020168775A1Component separationBiological testingLiquid chromatography mass spectroscopyInternal standard
The present invention is directed to a method of detecting a corticosteroid in a sample by adding an internal standard to a sample suspected of containing a corticosteroid; removing interfering compounds from the sample; placing the sample on an HPLC column equilibrated with a NH4OAc:MeOH solution and collecting an eluent; and analyzing the eluent of the HPLC column with a MS, wherein if contained in the sample, the corticosteroid forms an adduct that is detected by the MS.
Owner:PURDUE PHARMA LP
Natural killer cell culture medium and natural killer cell amplification culture method
ActiveCN104593324AImprove efficiencyHigh purityBlood/immune system cellsMicrobiologyNatural killer cell
The invention relates to the cell culture technical field, and particularly relates to a natural killer cell culture medium and a natural killer cell amplification culture method. The invention provides the culture medium used for amplification culture of natural killer cells and containing a serum-free culture medium, human plasma, IL-2, IL-21, IL-15 and OKT-3. The culture medium provided by the invention is used for amplification culture of the natural killer cells, can avoid risks caused by exogenous serum, has high amplification efficiency and allows the obtained natural killer cells to have high purity. With adopting the method for amplification of the natural killer cells, amplification of the natural killer cells can be maintained at a logarithmic phase for a longer period of time. Moreover, the cultured natural killer cells have good killing activity on tumor cells.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Process for preparing human serum albumin
ActiveCN103394076ANon-deterministicHigh yieldPeptide/protein ingredientsSerum albuminUltrafiltrationFiltration
The invention discloses a process for preparing human serum albumin. According to the process, a low-temperature ethanol separation method is adopted, and the human serum albumin is prepared from human plasma. The process comprises the steps of dissolving plasma; preparing an ingredient I; preparing an ingredient II and an ingredient III; preparing an ingredient IV; preparing an ingredient V; refining the ingredient V; carrying out ultrafiltration; diluting; carrying out pasteurization; sterilizing and packaging albumen; incubating products; and packaging finished products. The process has the advantages that solid-liquid separation is carried out by adopting a pressure filtration technology, so that the albumin yield which is higher than 29 g / L plasma is increased remarkably, the purity is higher than 98%, and the stability of the products is improved remarkably; Zetaplus deep filter-core filtration is combined with the prolongation of pasteurization time, so that the PKA (Protein Kinase A) level of the products is effectively controlled to be lower than 20IU / ml, and the risks of excessive heat source and virus infection in the products are reduced; and during the process, sodium chloride solutions of two gradient concentrations are used for carrying out ultrafiltration, so that not only can the ethanol residual quantity of the products be controlled to be lower than 0.025%, but also the aluminum residual quantity can be effectively minimized to be lower than 50 micrograms / L.
Owner:华润博雅生物制药集团股份有限公司
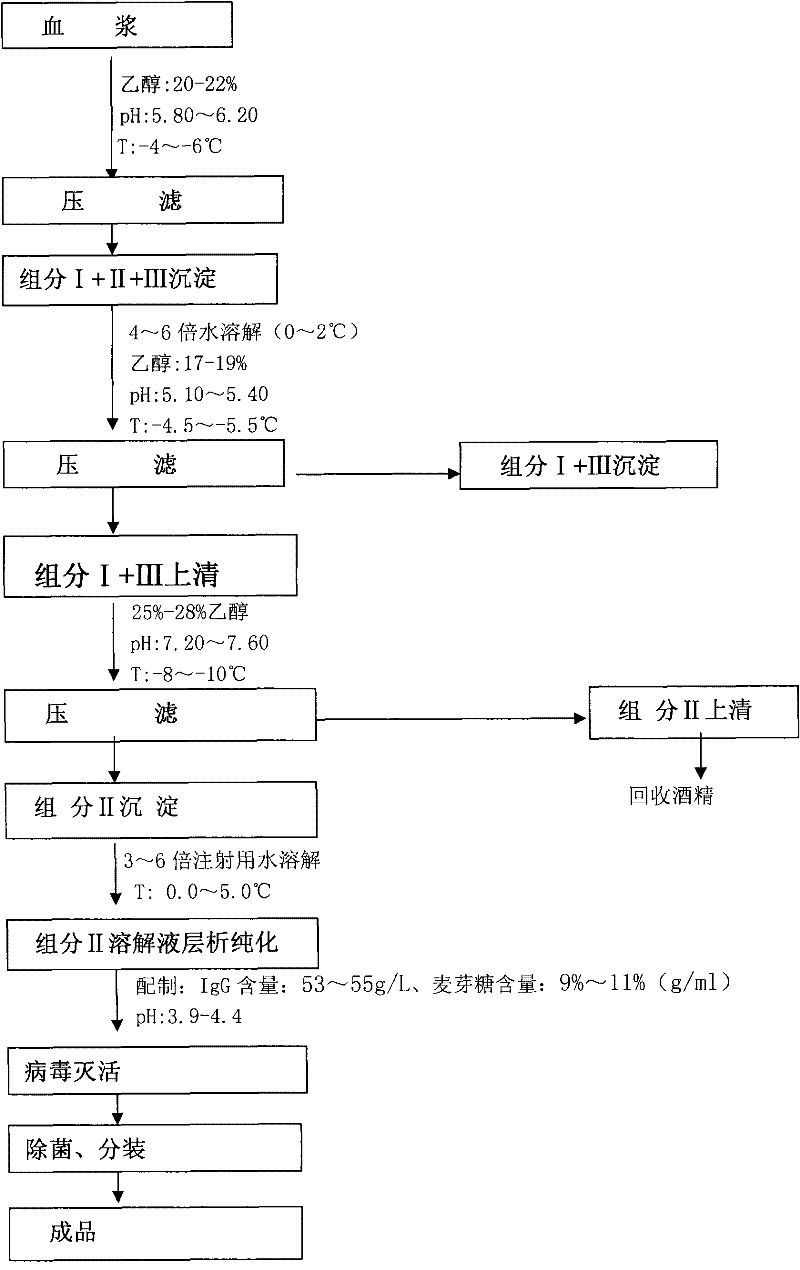
Method for producing intravenous injection human immune globulin
ActiveCN102178951AEasy temperature controlFast separationSerum immunoglobulinsAntibody ingredientsIon exchangeEngineering
The invention relates to a preparation method of human plasma protein, in particular a method for producing intravenous injection human immune globulin by using filter press technique for separation in combination with chromatograph for purification. The method is characterized in that a filter press is adopted as a main separation device to replace a centrifugal machine for producing intravenous injection human immune globulin, so that the temperature and other conditions for separation are easy to control, the separation speed is quick, and the safety is high without a high-speed operating device. Meanwhile, ion-exchange column chromatography is adopted to further purify the product and acquire the intravenous injection human immune globulin with the purity as high as 98.5-100%. The sum of monomer and dipolymer reaches 99-99.5%. PKA (proteinkinase A) is no more than 3IU / ml and ACA (Anti Cardiolipin Antibodies) is no more than 9%. Besides, the process is high in recovery rate, and for each ton of blood plasma, 5600-6300g of intravenous injection human immune globulin can be harvested.
Owner:哈尔滨派斯菲科生物制药有限公司
Determination of corticosteroids in human plasma using micromass LC/MS/MS
InactiveUS6541263B2Component separationBiological testingInternal standardLiquid chromatography mass spectroscopy
The present invention is directed to a method of detecting a corticosteroid in a sample by adding an internal standard to a sample suspected of containing a corticosteroid; removing interfering compounds from the sample; placing the sample on an HPLC column equilibrated with a NH4OAc:MeOH solution and collecting an eluent; and analyzing the eluent of the HPLC column with a MS, wherein if contained in the sample, the corticosteroid forms an adduct that is detected by the MS.
Owner:PURDUE PHARMA LP
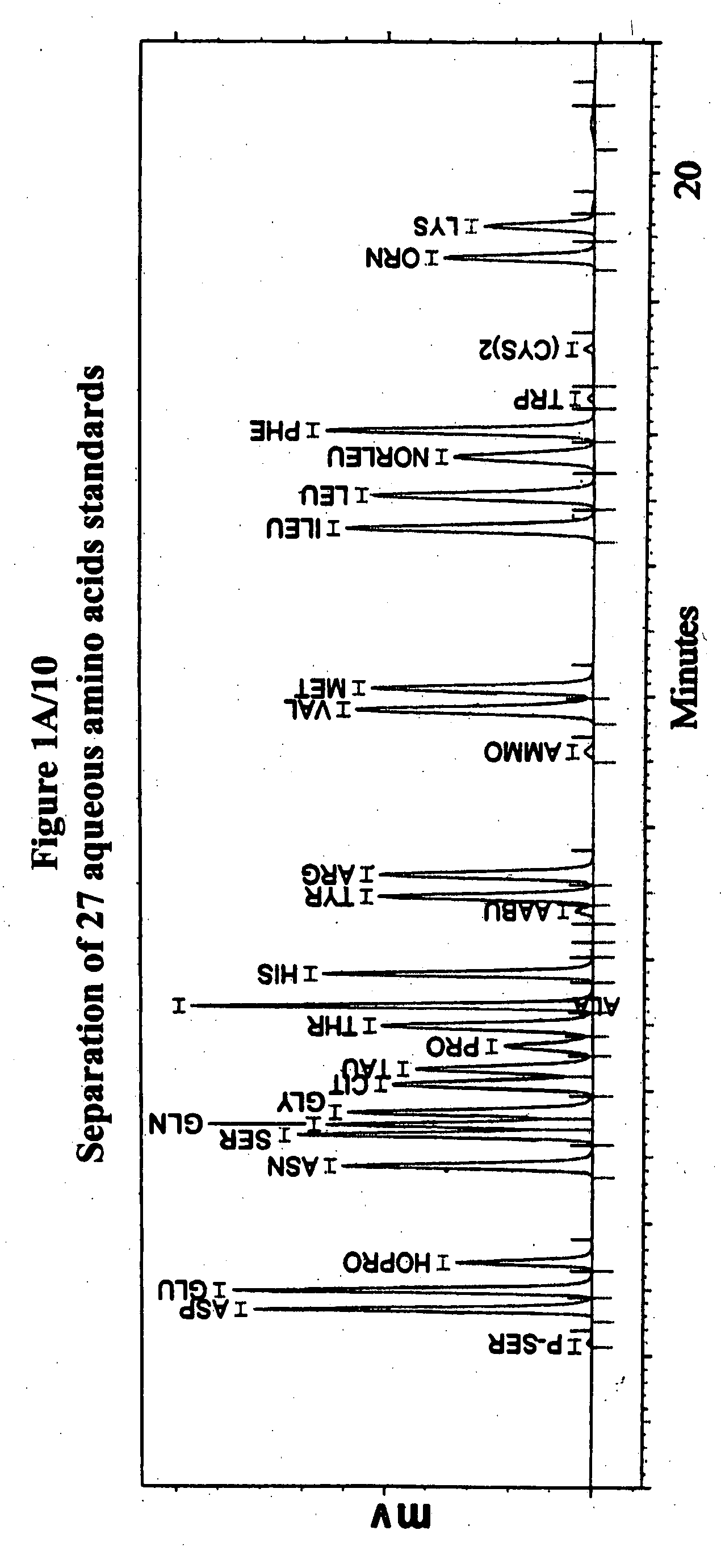
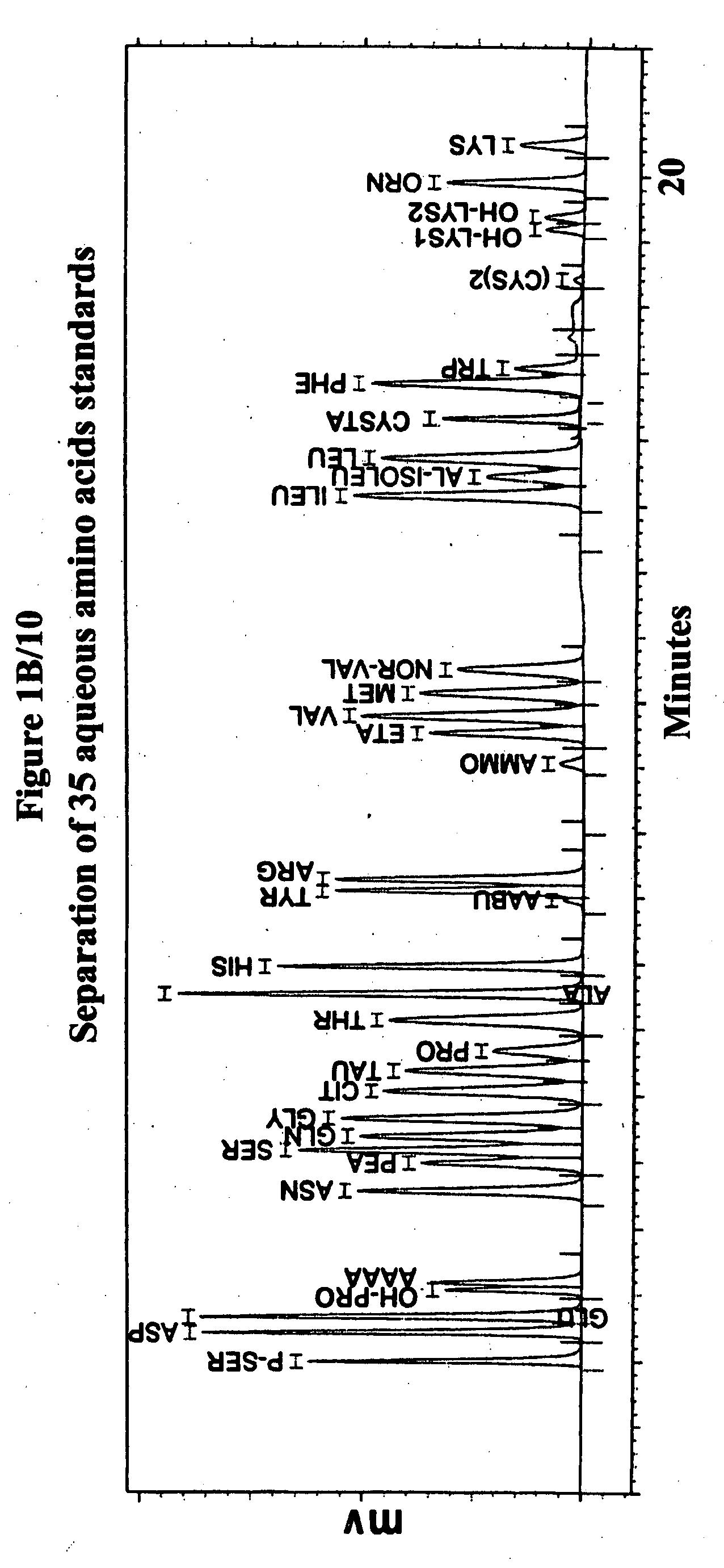
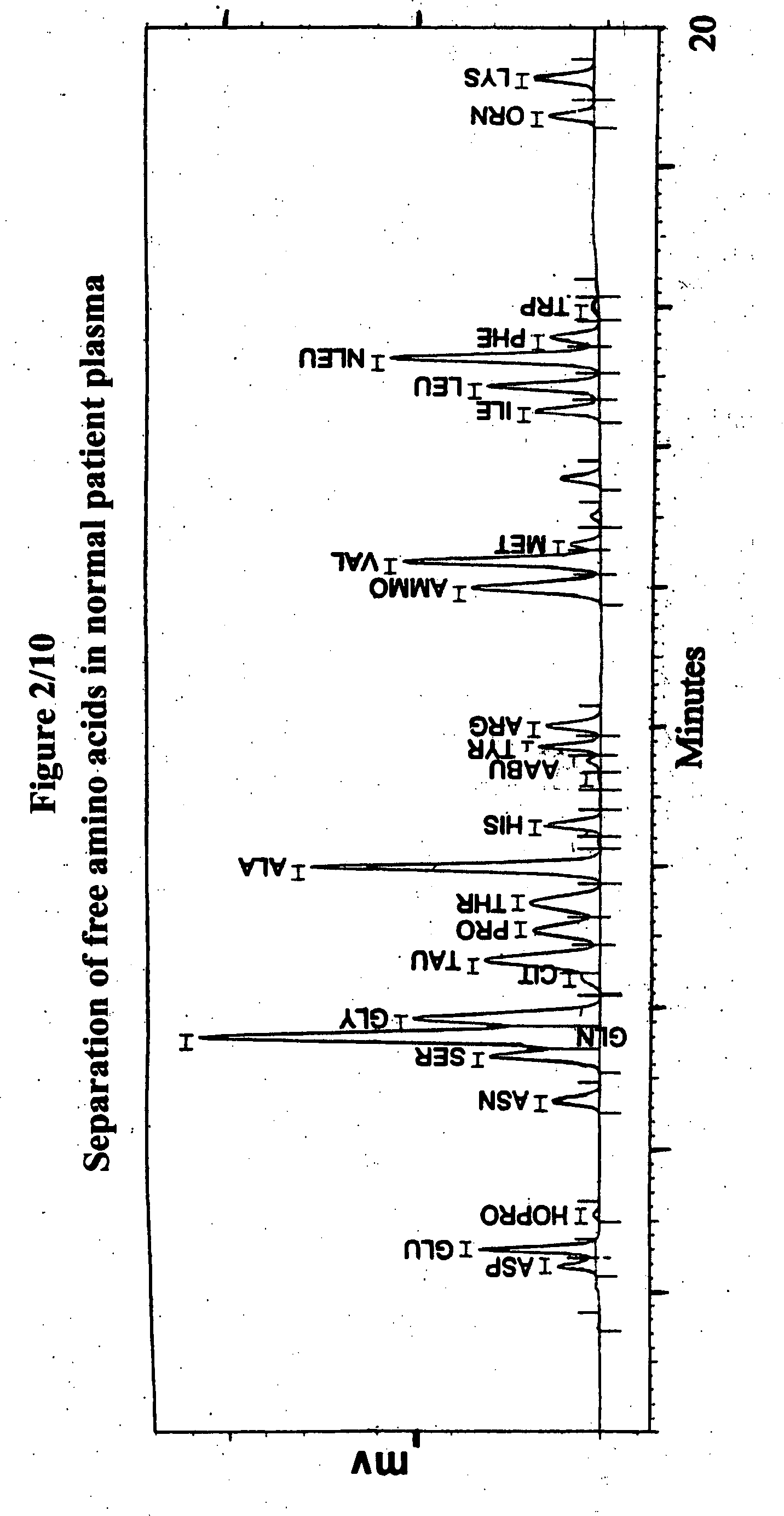
Human plasma free amino acids profile using pre-column derivatizing reagent- 1-naphthylisocyanate and high performance liquid chromatographic method
InactiveUS20070281361A1Component separationSolid sorbent liquid separationCysteine thiolateHplc method
After many decades, 1-Naphthylisocyanate (NIC) has been identified as the most ideal pre column derivatization fluorescent tag for reversed phase Liquid Chromatographic (HPLC) analysis of all free amino acids (AA) in biological samples. NIC forms very stable derivatives with all AAs in one minute. Using NIC, the first, most simple, robust, sensitive (femto mole), and economical high pressure binary gradient, HPLC method, has been developed. It estimates 35 (and 2 internal standards) AAs in human plasma in record shortest time of 20 minutes and has been validated for precision (n=16, <6%), accuracy 95 %, linearity (0 to 1200 μM / L), and analyzing normal and abnormal patients. It can provide with in 20 minutes the first and best plasma free AAs profile that includes Homocysteine, Cysteine, Alloisoleucine, and Cystathionine and a 27 AAs profile using a blood spot (3 μl plasma). A sample can be analyzed with in one hour of its arrival in the laboratory.
Owner:HARIHARAN MEENAKSHISUNDARAM
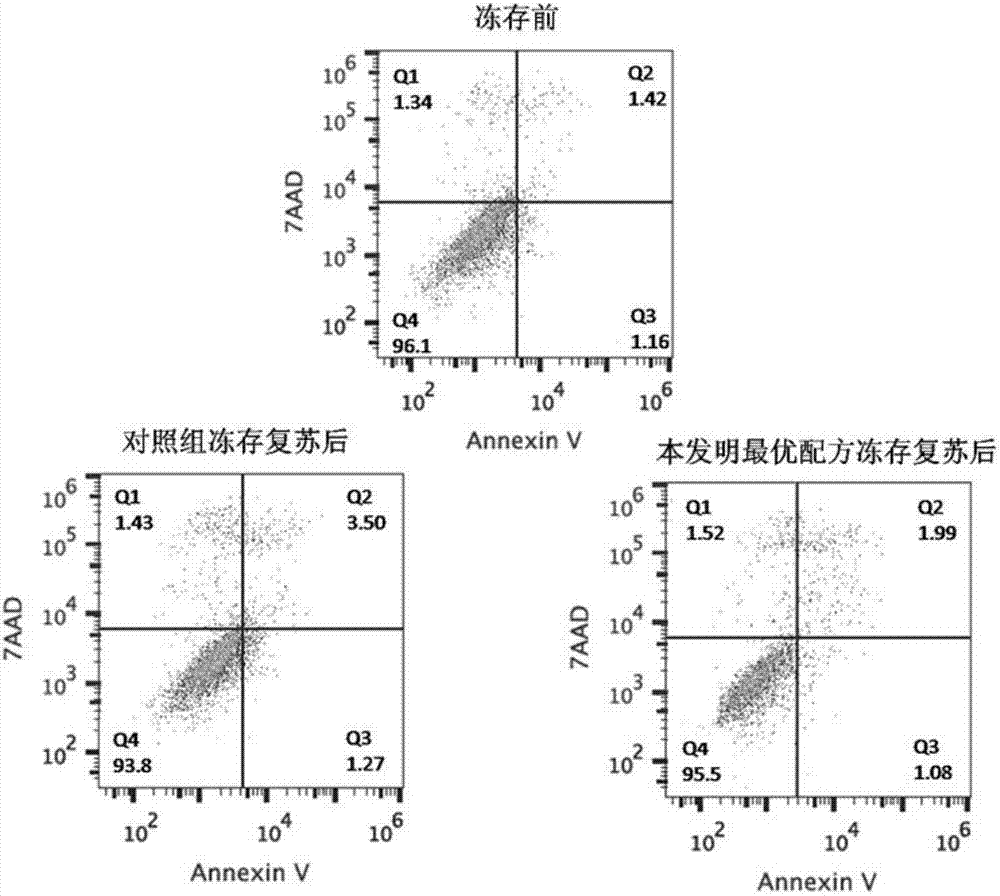
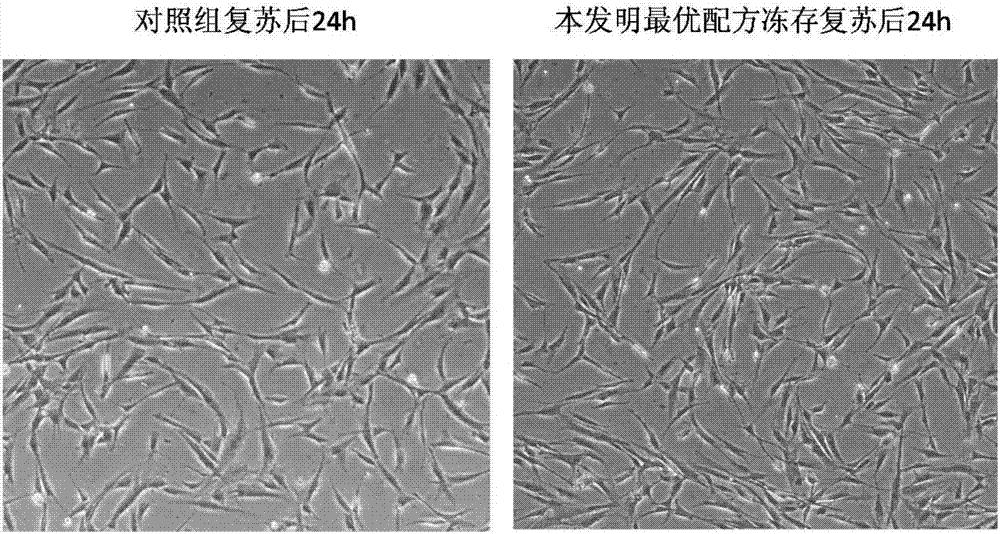
Clinic freeze-preservation protective solution composition for umbilical cord mesenchymal stem cells and application thereof
InactiveCN106982821AMaintain activityMaintain biological activityDead animal preservationAdditive ingredientCell culture media
The invention provides a clinic freeze-preservation protective solution composition for umbilical cord mesenchymal stem cells and application thereof. The protective solution composition comprises the following components: compound dextranum 40 injection, human serum albumin and DMSO. Compared with the prior art, the composition has the beneficial effects that the clinic freeze-preservation protective solution for umbilical cord mesenchymal stem cells can main the activity and biology activity of the mesenchymal stem cell for a long time, and does not contain unsafe components such as cell culture mediums, fetal calf serum and human plasma. All ingredients comprise clinical drug compound dextranum 40 injection, 20% human serum albumin or clinically used (dimethyl sulfoxide (UPS clinical level)), and the stability of the clinically standardized mesenchymal stem cell freeze-preservation system can be guaranteed while the umbilical cord mesenchymal stem cells for cryopreservation resuscitation meet the clinic using requirement. The application is safe, effective and simple in preparation method, and has good clinical application prospect.
Owner:安徽瑞杰赛尔生物科技有限公司
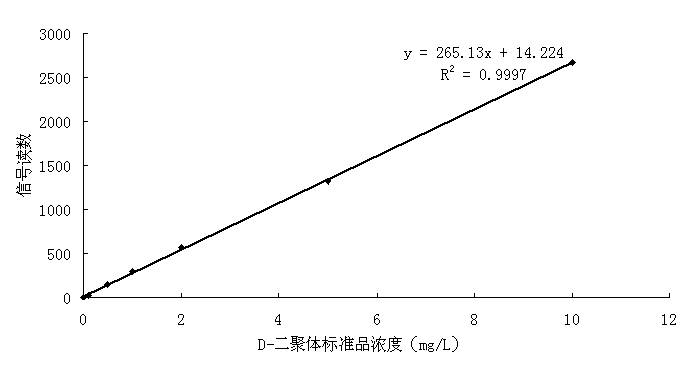
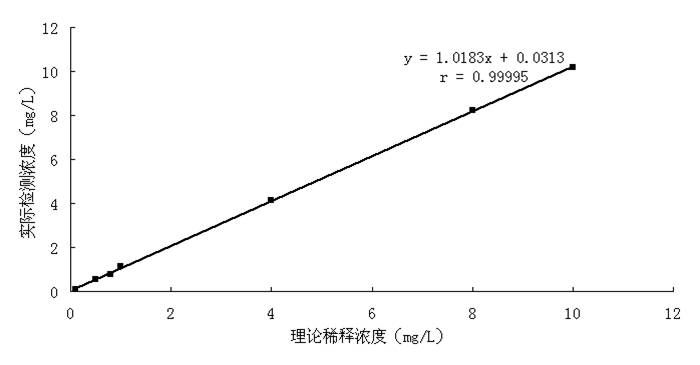
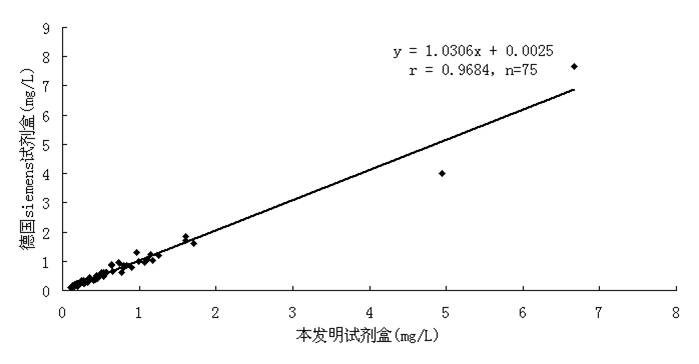
D-dimer quantitative fluorescence immunoassay test strip and preparation method thereof
InactiveCN102692504ASimple and fast operationImprove accuracyMaterial analysisDimerAntiendomysial antibodies
The invention relates to a D-dimer quantitative fluorescence immunoassay test strip. The test strip comprises a sample pad, a bind pad, a nitrocellulose film and absorbent paper, wherein the sample pad is a two-layer sample pad; the bind pad is coated with a fluorescence latex microsphere marked anti D-dimer antibody A; the nitrocellulose film is coated with an anti D-dimer antibody B serving as an assay line and a rabbit antimouse IgG antibody serving as a control line; and the fluorescence late microsphere is prepared from latex microsphere adsorptive fluorescence marked streptavidin. The test strip can be used for accurately and quantitatively assaying the content of D-dimer in human blood plasma, and has the characteristics of convenience in operation, high accuracy, high sensitivity, low cost and the like.
Owner:GETEIN BIOTECH
Method and apparatus for manufacturing plasma based plastics and bioplastics produced therefrom
A method of making a bioplastic, and a bioplastic produced thereby, by using human plasma in which human plasma is clotted, either dried through its gel phase or dried and powdered, and processed into a bioplastic with the addition of at least one plasticizer followed by forming and heating to form a final bioplastic construct.
Owner:CARNEGIE MELLON UNIV +1
Preparation method for human immunoglobulin for intravenous injection
ActiveCN103554253AShorten the production cycleHigh yieldSerum immunoglobulinsPeptide preparation methodsUltrafiltrationIon exchange
The invention discloses a preparation method for human immunoglobulin for intravenous injection. The preparation method comprises the following steps: (1) two-step filtering pressing: separating II+III precipitate from human plasma as a starting raw material, removing component III precipitate through low-temperature ethanol filter pressing, collecting supernatant, performing ultrafiltration and dialysis to the supernatant, and regulating the protein concentration; (2) two-step chromatography: performing upper column chromatography purification through a DEAF-Sepharose-FF ion exchange column, and collecting a first-step flow penetration liquid; performing ultrafiltration and dialysis to the collected first-step flow penetration liquid, regulating and performing chromatographic purification by using a Fractogel-EMD-TMA ion exchange column, and collecting a second-step flow penetration liquid; (3) performing ultrafiltration, dialysis and liquid preparation to the collected second-step flow penetration liquid; (4) inactivating virus, disinfecting, filtering and subpackaging to obtain a product. The product prepared by using the method is higher in bioactivity, yield and purity.
Owner:TONROL BIOLOGICAL PHARM CO LTD
Human medical treatment by aerosol inhalation of immunoglobulin A
Pooled human plasma is processed by cold ethanol fractionation to produce purified immunoglobulin G antibodies for intravenous administration. Immunoglobulin A is an unwanted by-product since intravenous administration of immunoglobulin A-containing immunoglobulin G can cause life-threatening anaphylaxis in some people. The present invention is the topical application of immunoglobulin A coupled with J chain, and optionally coupled with secretory component in order to render the immunoglobulin A more physiologically active, for the prevention or treatment of ocular diseases including ocular immune deficiency and infections. Antigen-specific monoclonal immunoglobulin A may be used.
Owner:SIMON MICHAEL R
Polyclonal-monoclonal elisa assay for detecting n-terminus pro-bnp
InactiveUS20090325195A1Accurately predicting mortalityDisease diagnosisFused cellsBound propertyBlood plasma
A specific and sensitive in vitro ELISA assay and diagnostic test kit is disclosed for determining levels of NT-proBNP protein in a variety of bodily fluids, non-limiting examples of which are blood, serum, plasma, urine and the like. The NT-proBNP ELISA assay test employs the sandwich ELISA technique to measure circulating NT-proBNP in human plasma. In order to obtain antibodies with specific binding properties for targeted amino acid sequences within human proBNP, recombinant human proBNP (or rhproBNP) was expressed and purified for use as an immunogen. Polyclonal antibodies (PAb) to specific amino acid sequences were subsequently purified from goat serum by sequential affinity purification. Monoclonal antibodies were raised against specific polypeptides. Recombinant human NT-proBNP (or rhNT-proBNP) was expressed and purified in order to obtain material for use in calibration of a quantitative method for measurement of human NT-proBNP.
Owner:NEXUS DX
Tumor mark quality control product preparation method
A preparation method of tumor labeled substance quality control belongs to the biological product technical field and comprises the following steps: after being added calcium chloride solution, the finished product human plasma is hatched for 1-3 hours in 36-38 DEG C and is centrifugalized at the speed of 3500-4500 r / m; after fiber protein in the plasma is eliminated, the plasma becomes quality control serum metrix; after being added tumor labeled substance quality control specimen in a certain scope of contents, the quality control serum metrix becomes the crude product of quality control product; after being added antiseptic and mixed uniformly, the quality control crude product is fractionally packed, dried in vacuum and low temperature and determined. The preparation method of tumor labeled substance quality control adopts the finished product human plasma which has adequate source and is easy to obtained as material; after the plasma is processed, the ingredients of organic substance in the plasma is totally identical with the ingredients of serum, which not only guarantees the quantity and the quality of quality control product but also has the advantages of less additive, less modulated substance, small vial to vial difference, a full set of quality control detecting projects, low production cost and long period of validity; furthermore, a lyophilizing reagent has sound stability after being reconstituted.
Owner:上海透景诊断科技有限公司
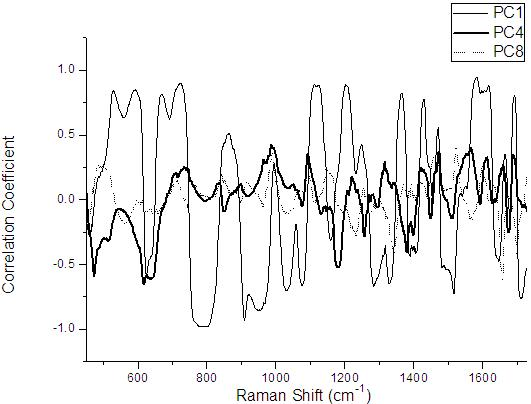
Detection method of human plasma surface enhanced raman spectroscopy by integrating main component analysis
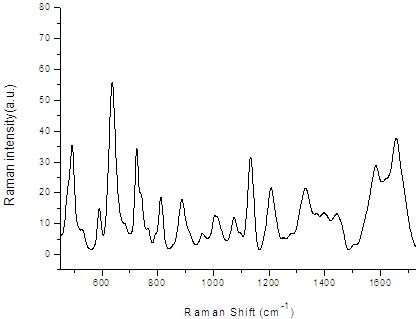
InactiveCN101806740AQuick checkNon-destructive testingRaman scatteringHigh power lasersSurface-enhanced Raman spectroscopy
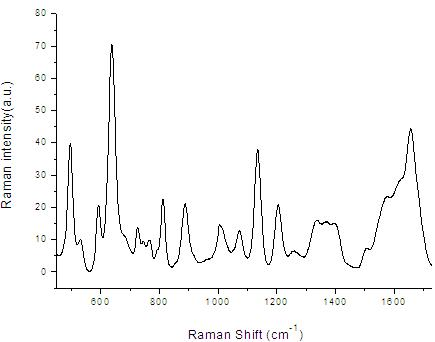
The invention provides a detection method of human plasma surface enhanced raman spectroscopy by integrating main component analysis; the method comprises the following steps that: a human plasma sample in physiological conditions is obtained, and is reduced by hydroxylamine hydrochloride to prepare silver sol; the silver sol and the human plasma sample are uniformly mixed by equal volume and incubated for 2h at 4DEG C, to measure the plasma surface enhanced raman spectroscopy; the measurement to the plasma surface enhanced raman spectroscopy can also adopt laser in different polarization states to excite the human plasma sample; and a plasma surface enhanced raman spectroscopy database is built, and the scattergraph distribution corresponding to the surface enhanced raman spectroscopy of different human plasma is obtained by integrating main component analysis. The detection method ensures the activity of biomolecules in the plasma, and has the advantages of simplicity, fastness and strong reliability. In addition, an SERS technology enables the sample to obtain very strong raman spectroscopy signals at very low laser power, so that the repeatability of the spectrum signals is good, thereby preventing carbonation and damage of high-power laser to the biological sample.
Owner:FUJIAN NORMAL UNIV +1
Compositions and methods for the treatment of immunodeficiency
ActiveUS9107906B1Antibacterial agentsSerum immunoglobulinsPassive ImmunizationsPrimary immunodeficiency
The present invention relates to compositions and methods for the treatment of immunodeficiency (e.g., primary immunodeficiency disease). In particular, the invention provides human plasma immunoglobulin compositions containing select antibody titers specific for a plurality of respiratory pathogens, methods of identifying human donors and donor samples for use in the compositions, methods of manufacturing the compositions, and methods of utilizing the compositions (e.g., for prophylactic administration and / or therapeutic treatment (e.g., passive immunization (e.g., immune-prophylaxis))).
Owner:ADMA BIOMANUFACTURING LLC
Coronary heart disease and palsy risk diagnosis reagent kit and manufacturing method thereof
The invention relates to the field of medical biotechnology, in particular to a making method for coronary heart disease and apoplexy risk diagnosis kit. The kit is used for quantitative analysis of Lp-PLA2 level in human plasma or blood serum by enzyme linked immunosorbent assay, so as to prevent heart disease and apoplexy.
Owner:SHANDONG TAIBANG BIOLOGICAL PROD CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com