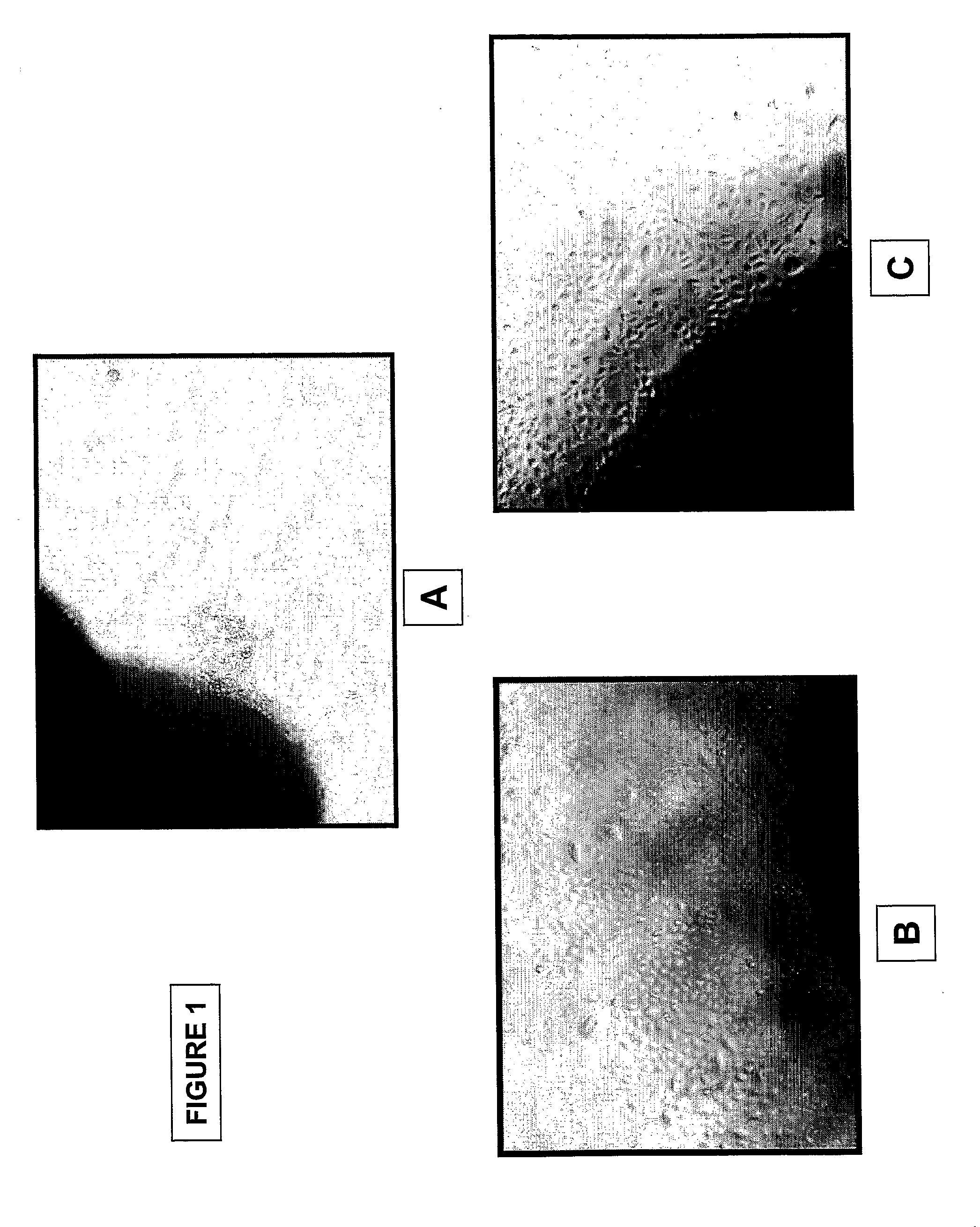
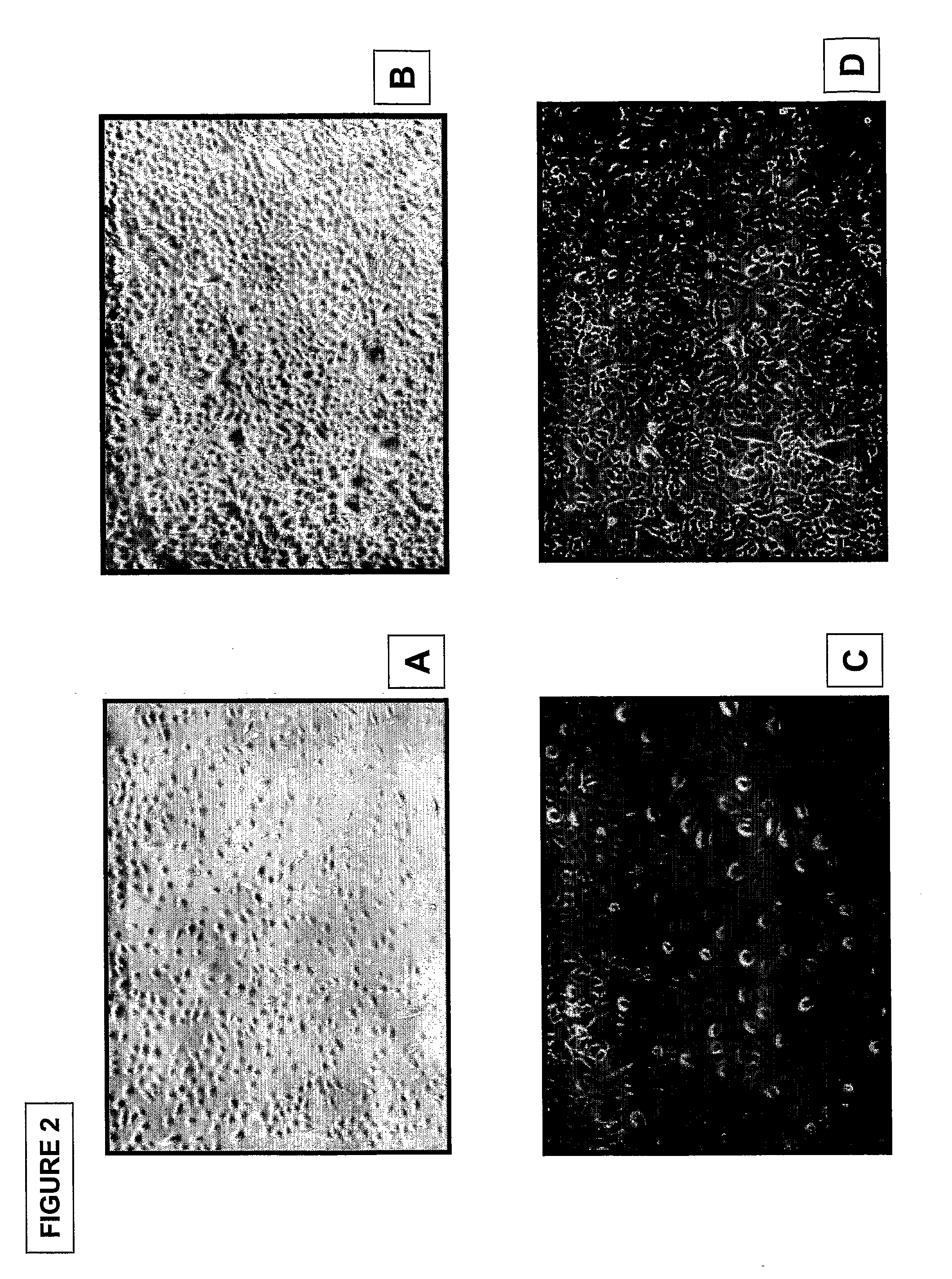
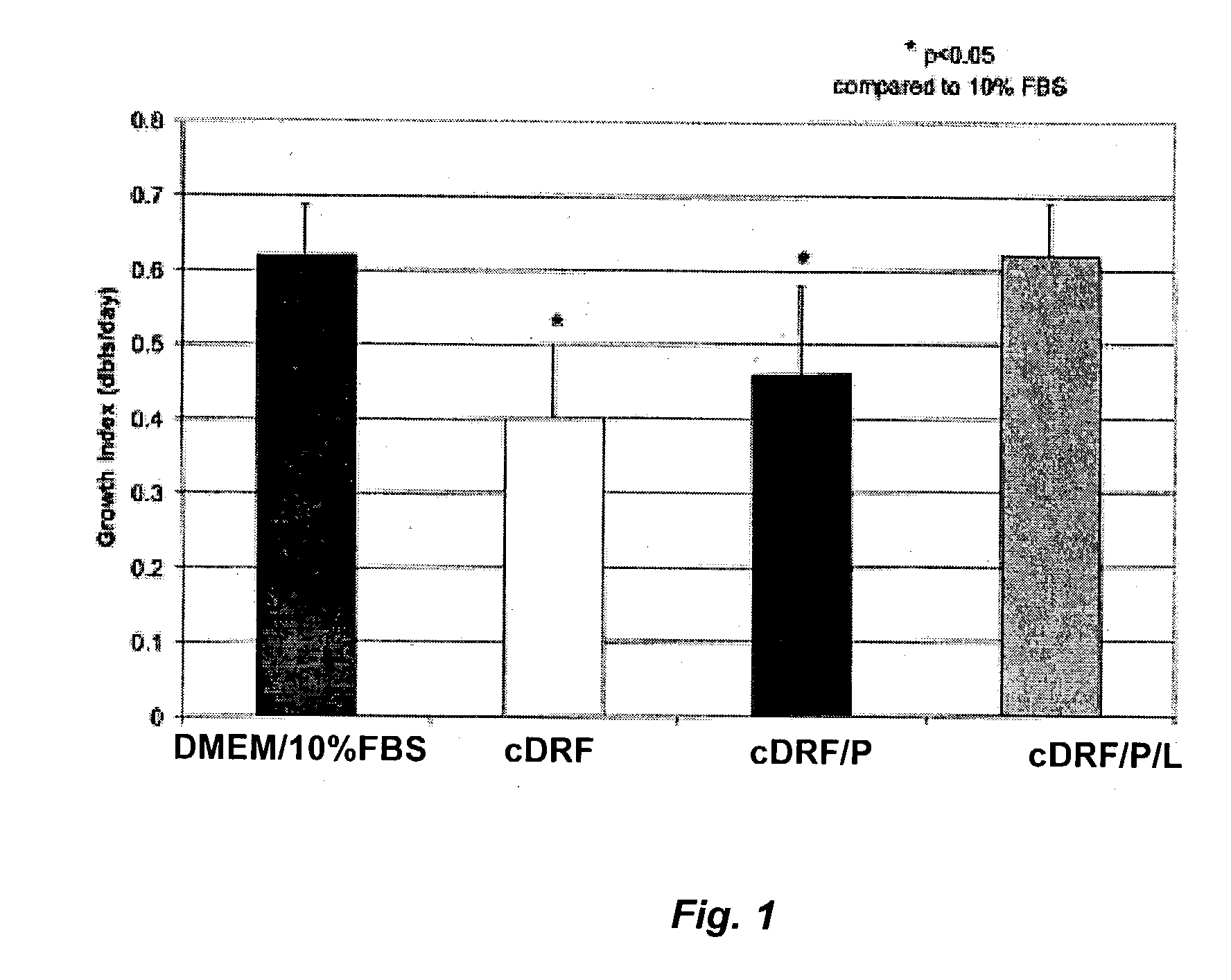
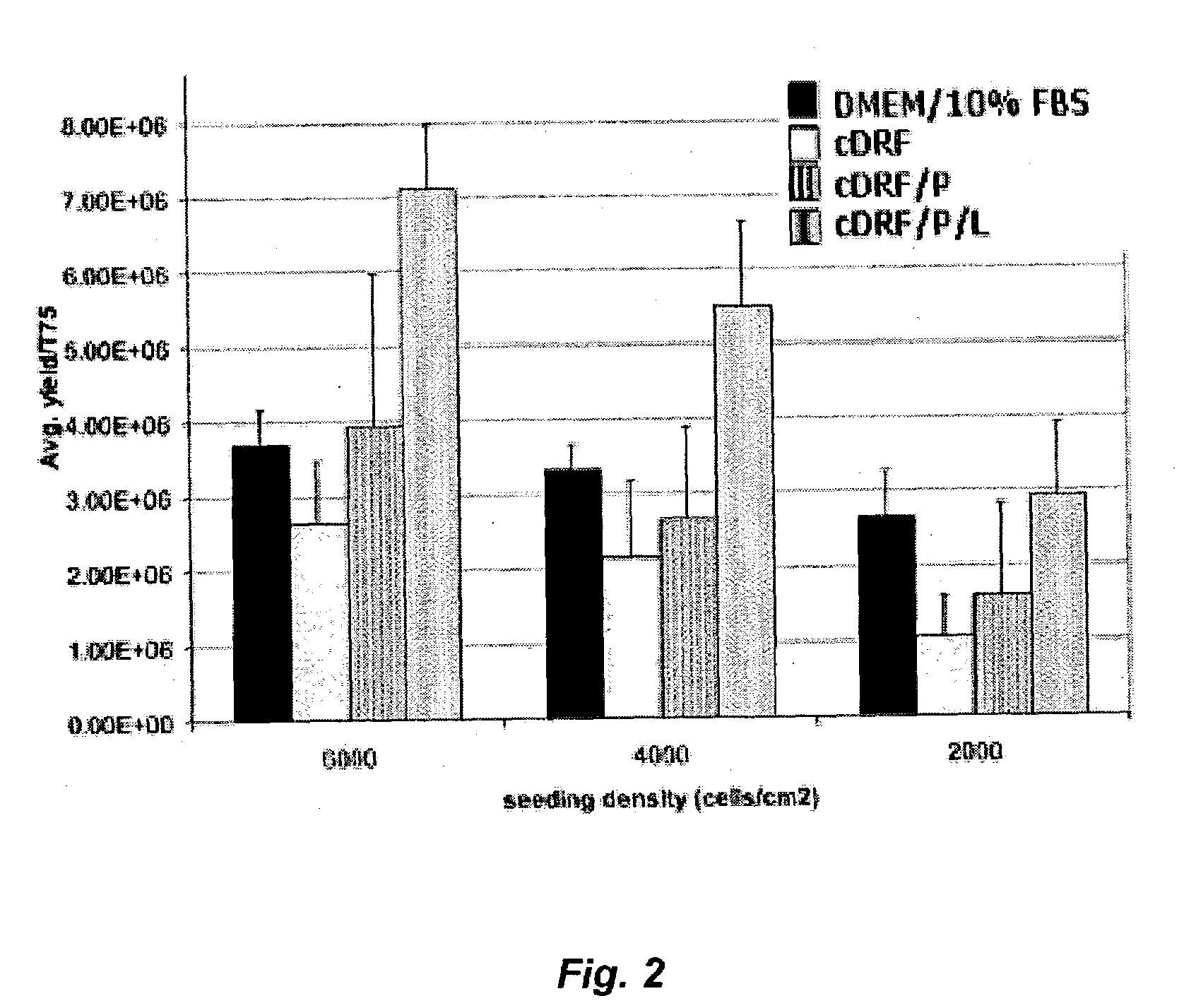
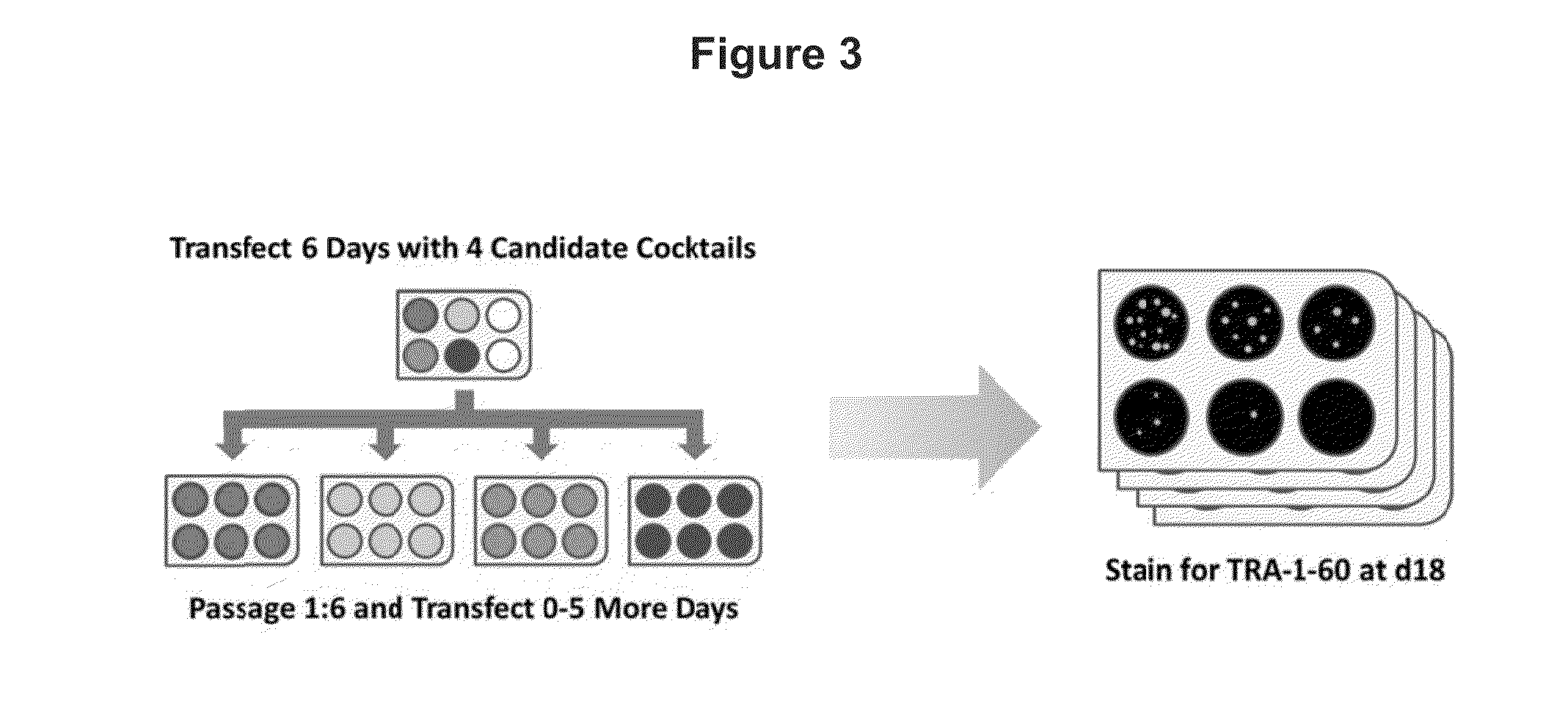
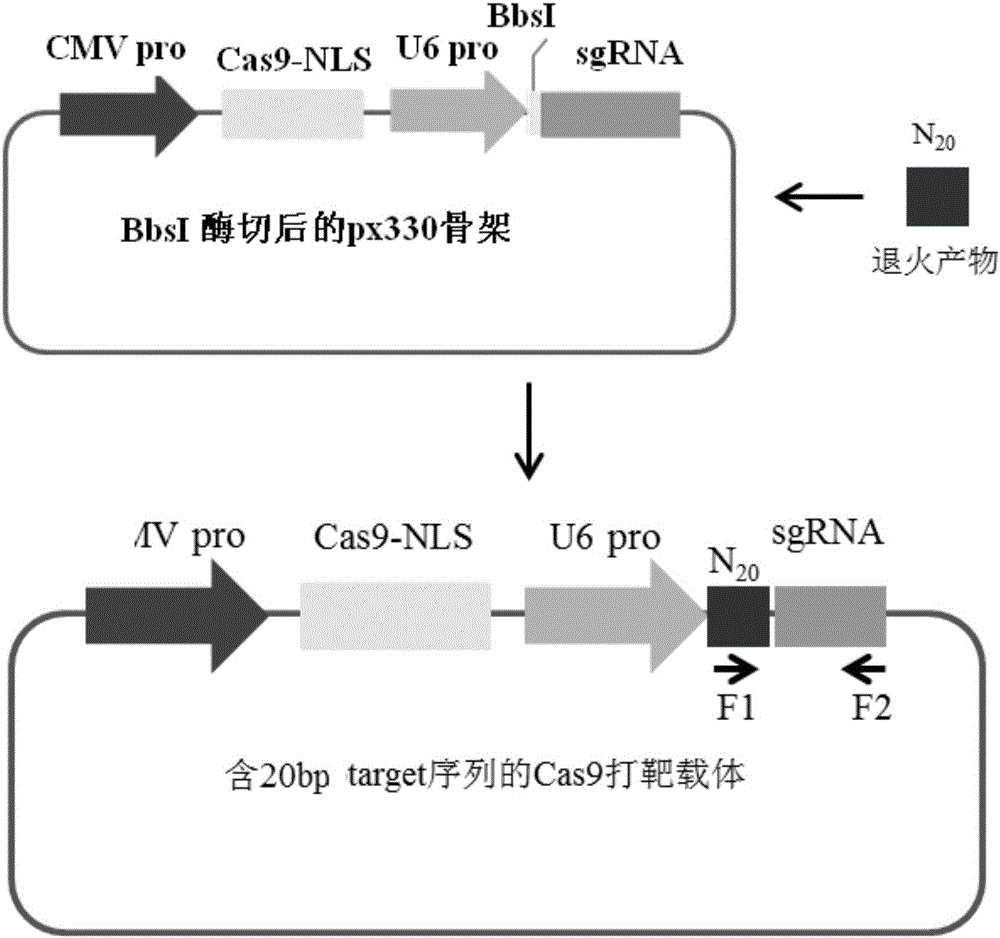
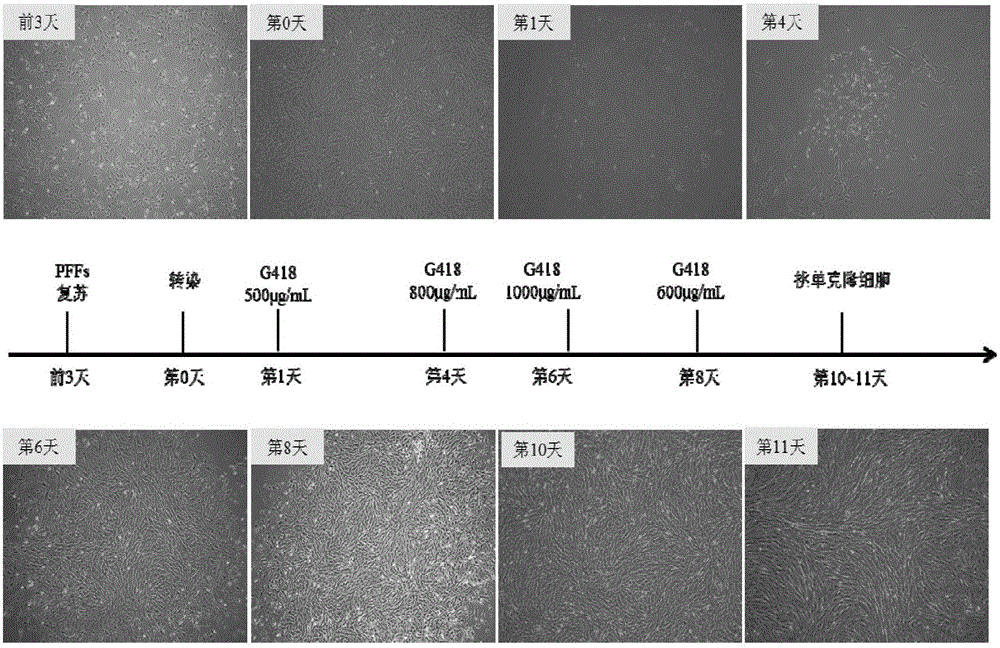
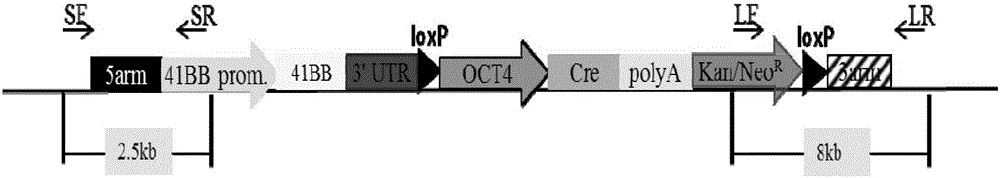
Patents
Literature
2145 results about "Fibroblast" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
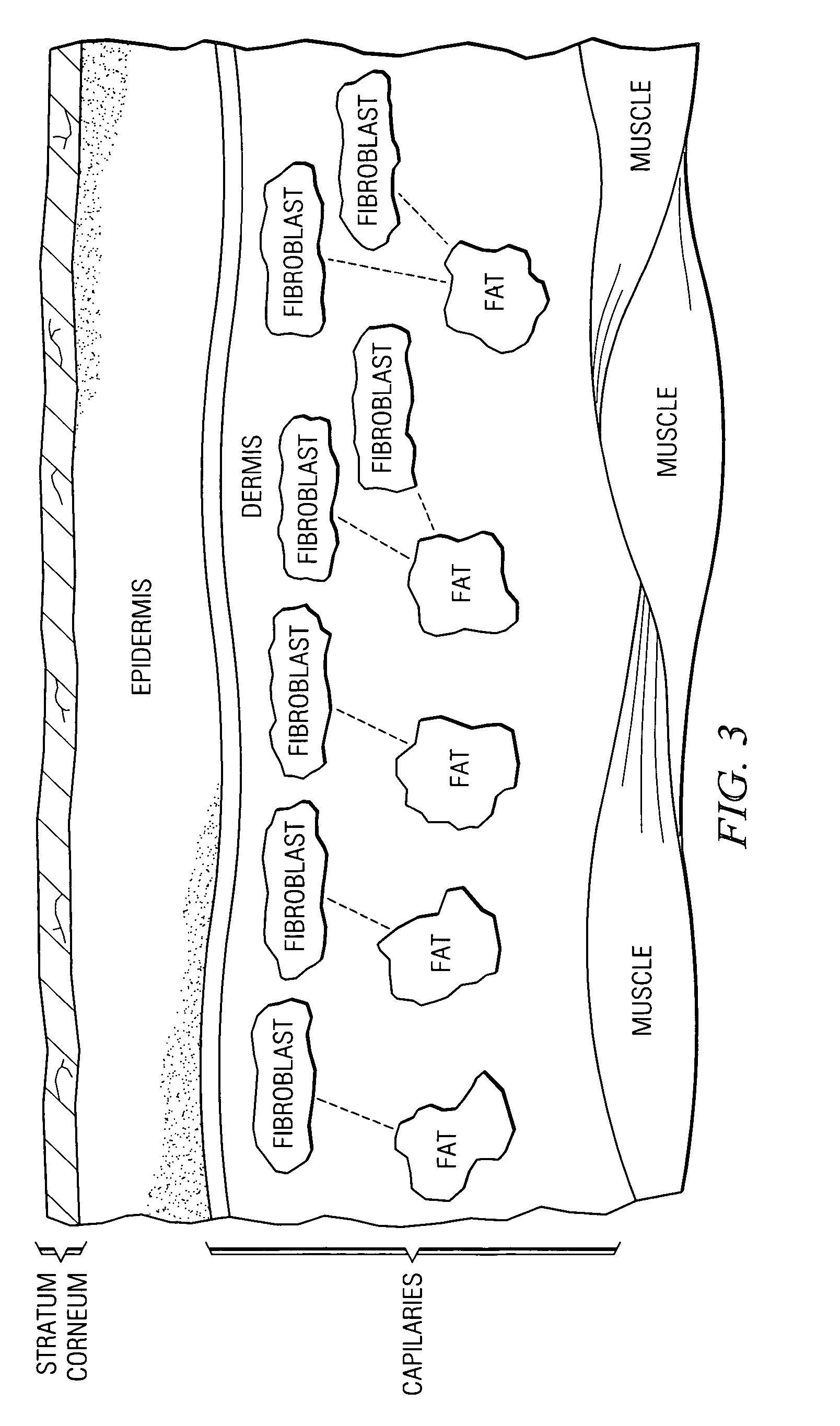
A fibroblast is a type of biological cell that synthesizes the extracellular matrix and collagen, produces the structural framework (stroma) for animal tissues, and plays a critical role in wound healing. Fibroblasts are the most common cells of connective tissue in animals.
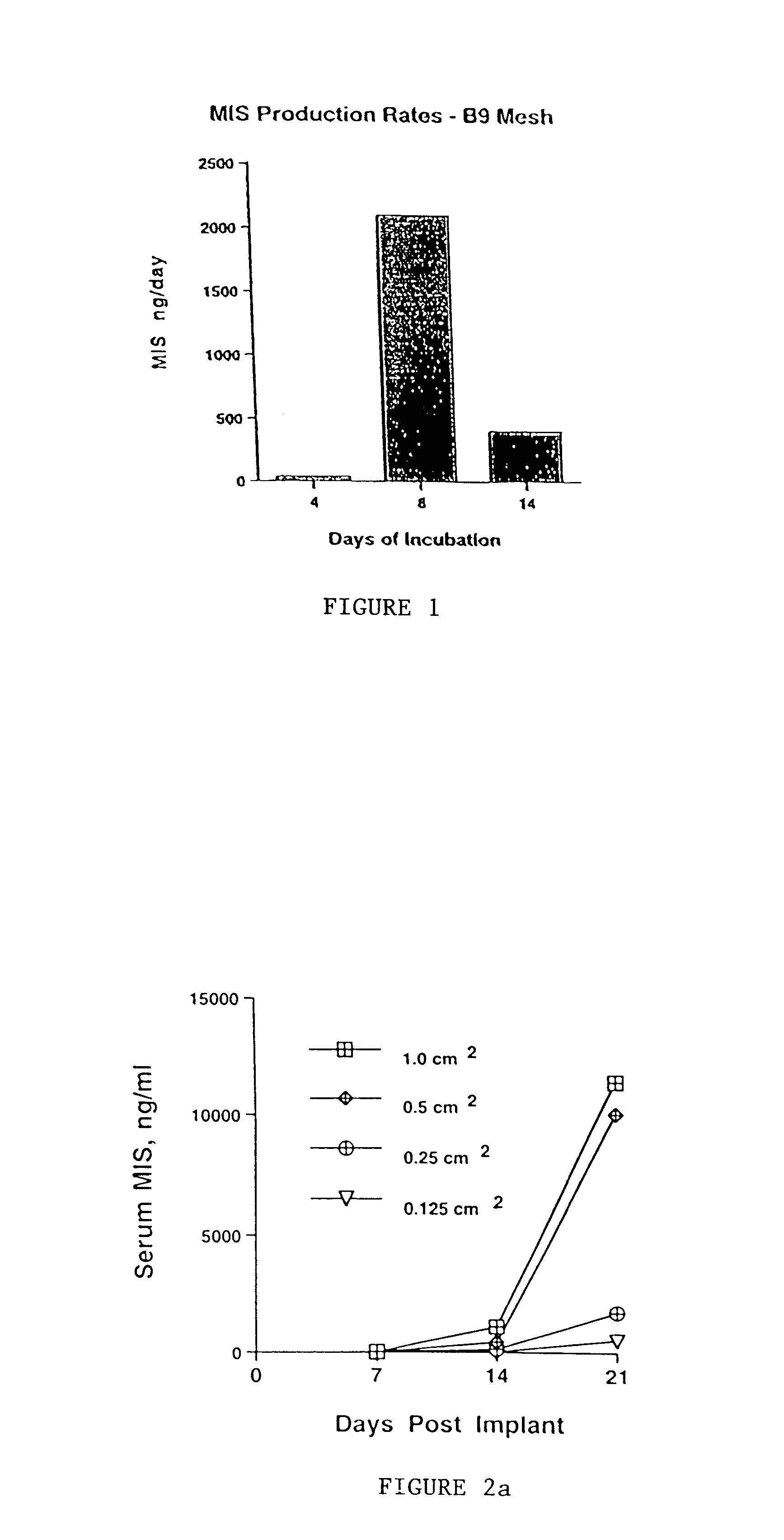
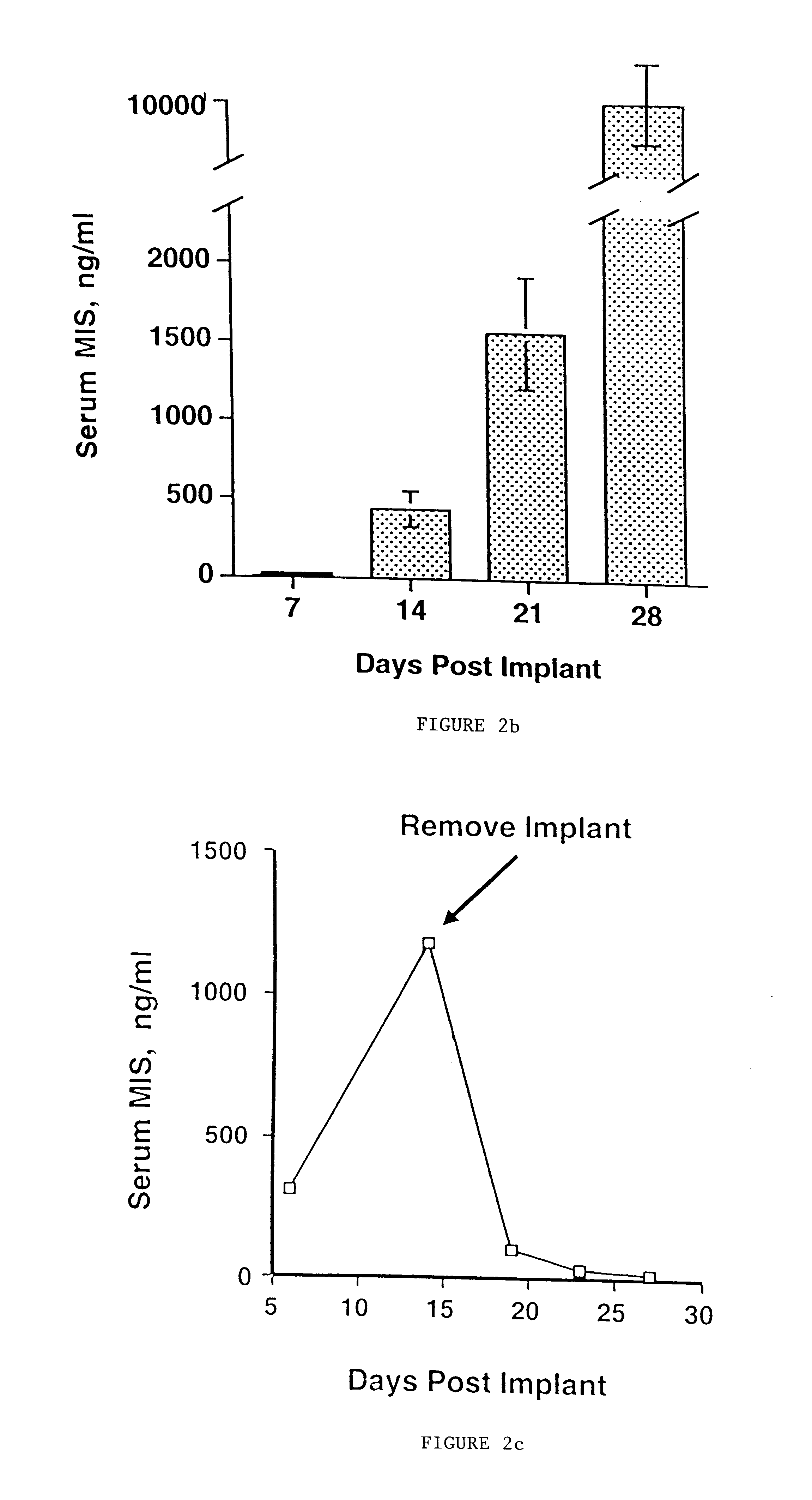
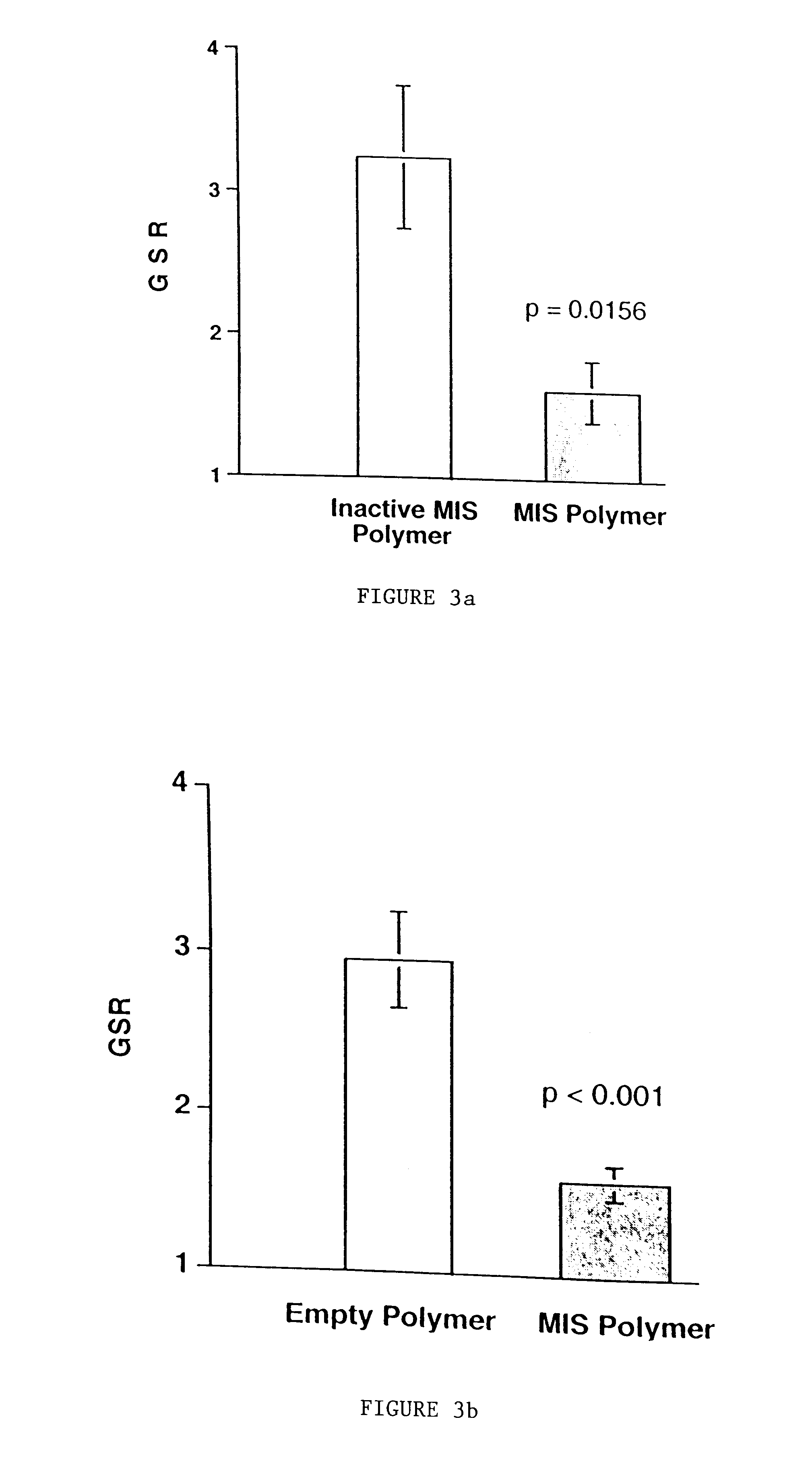
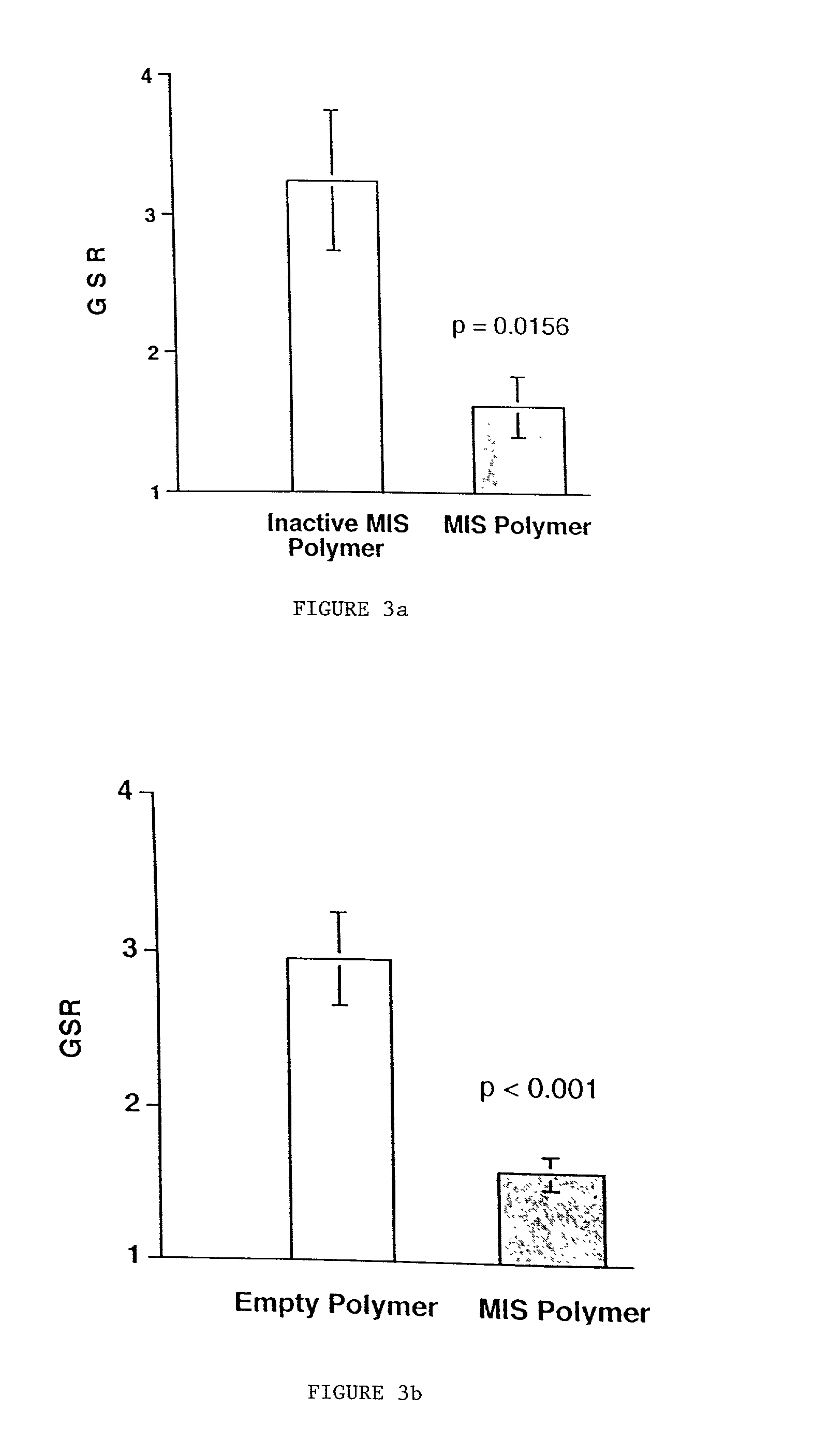
Delivery of therapeutic biologicals from implantable tissue matrices
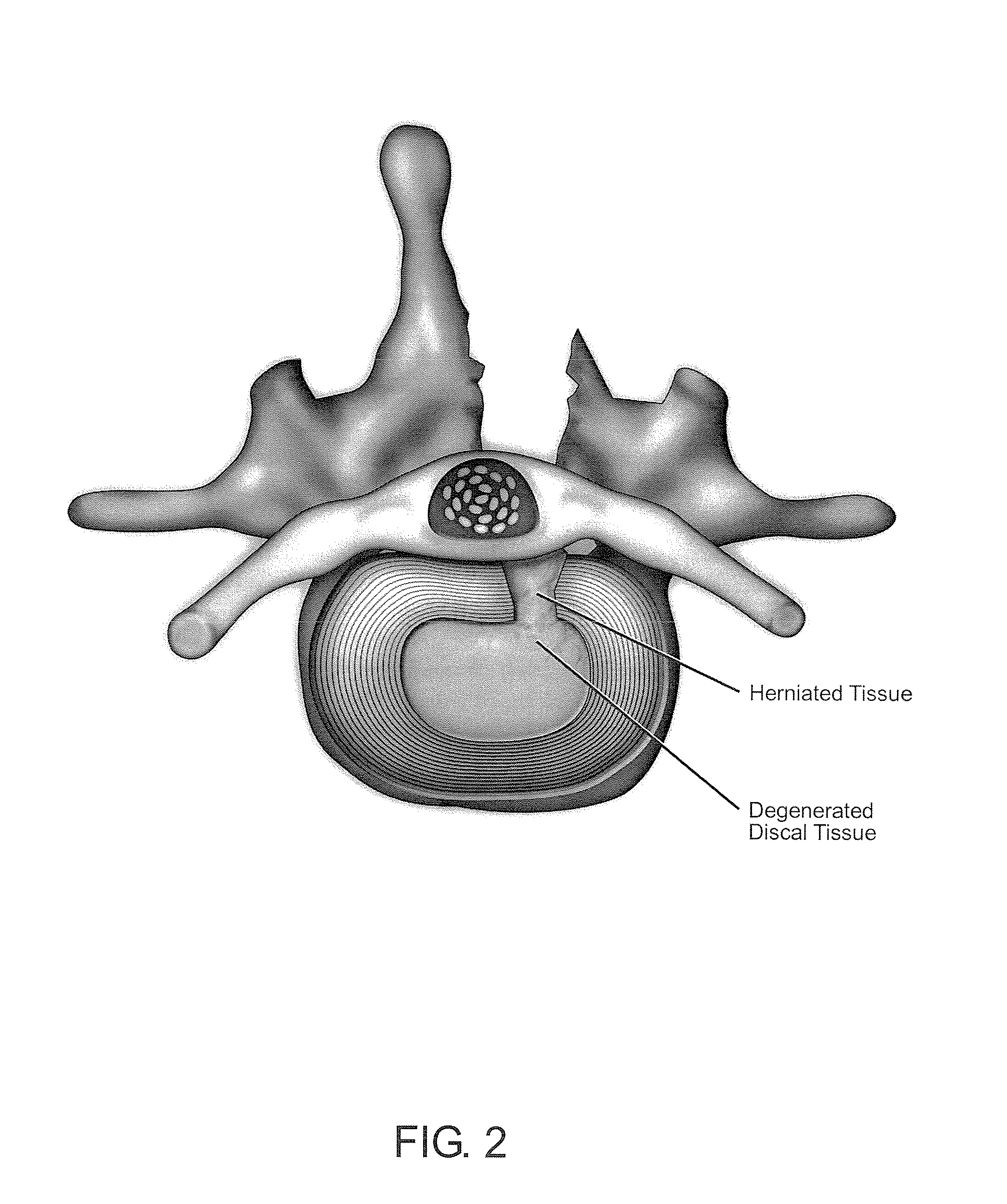
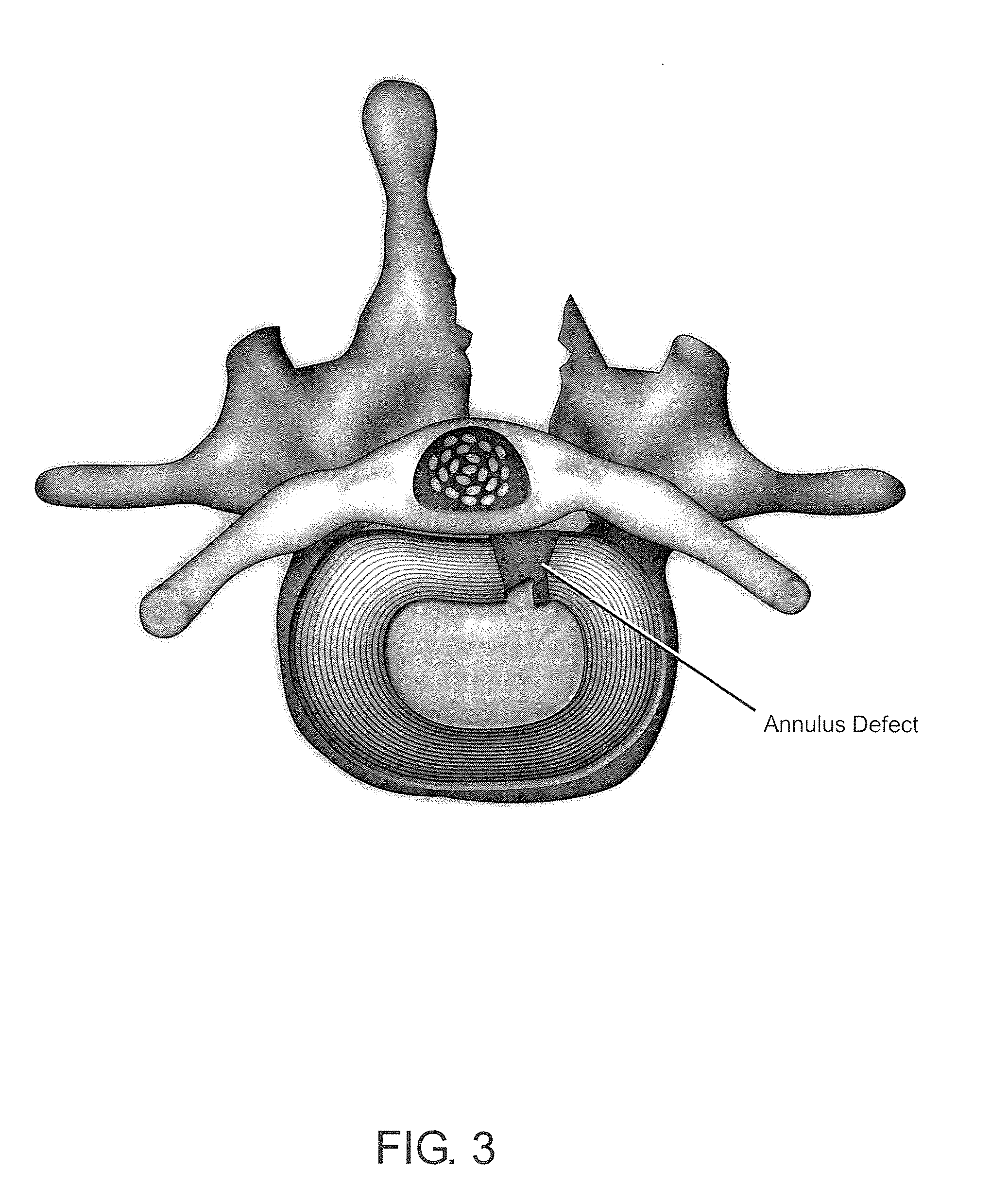
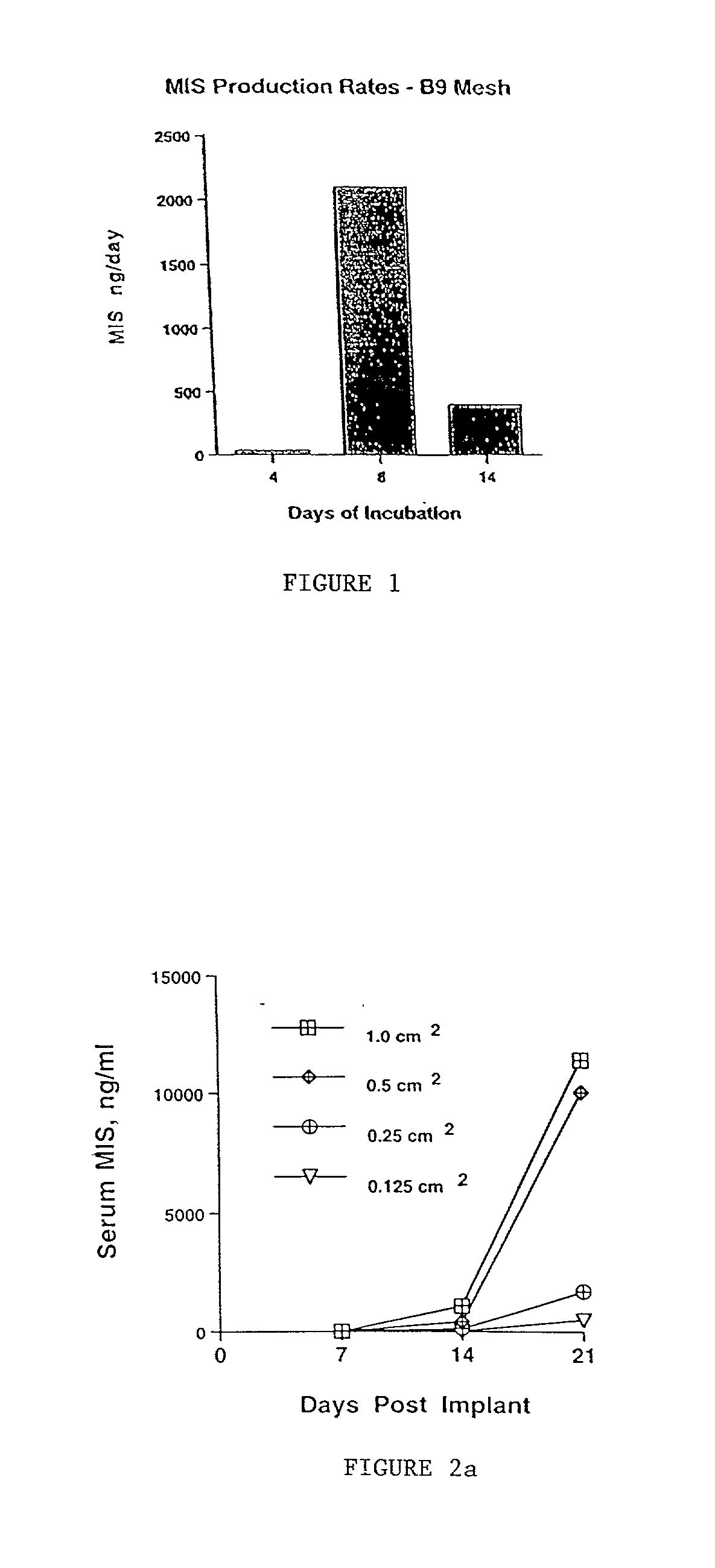
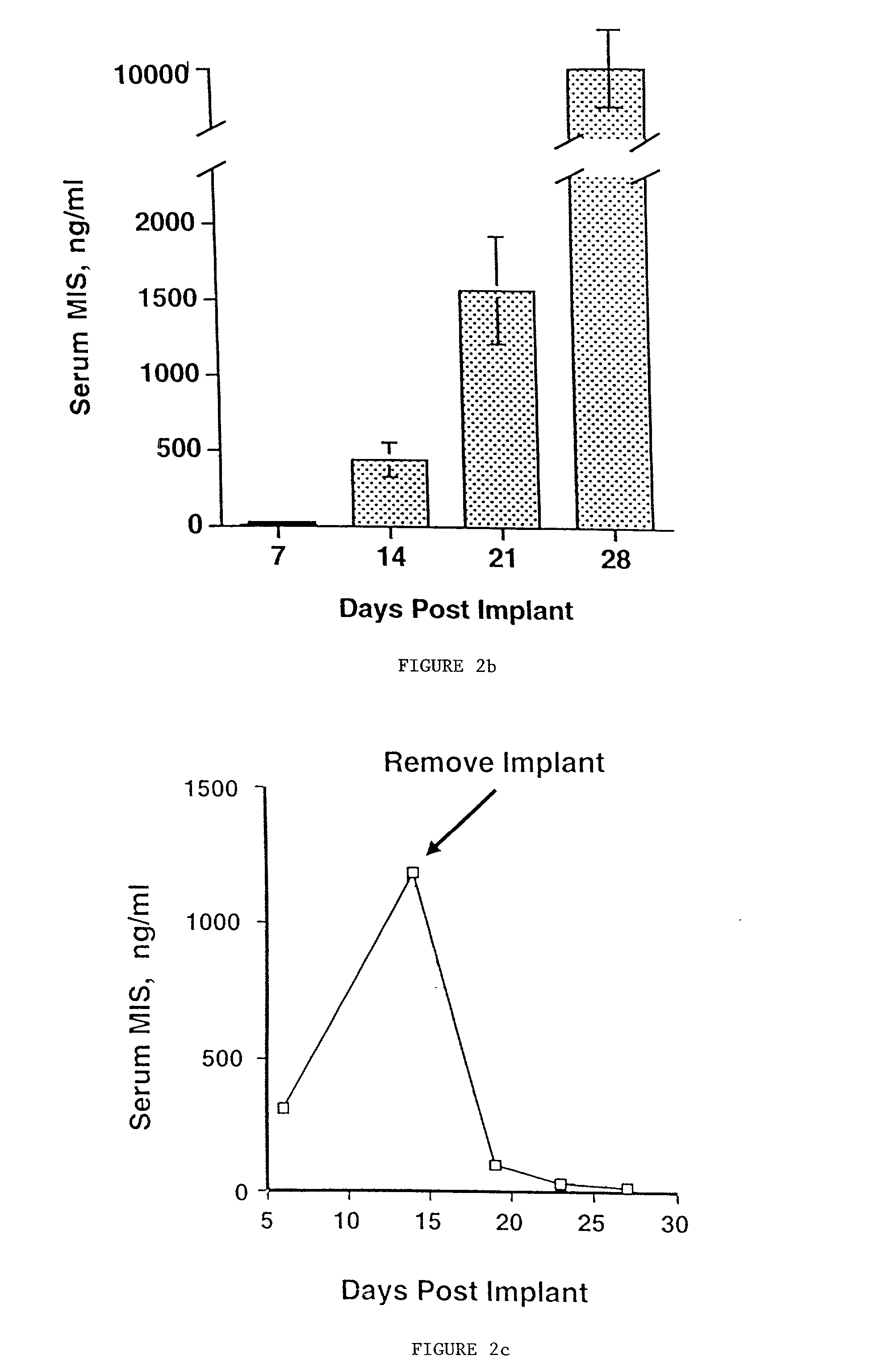
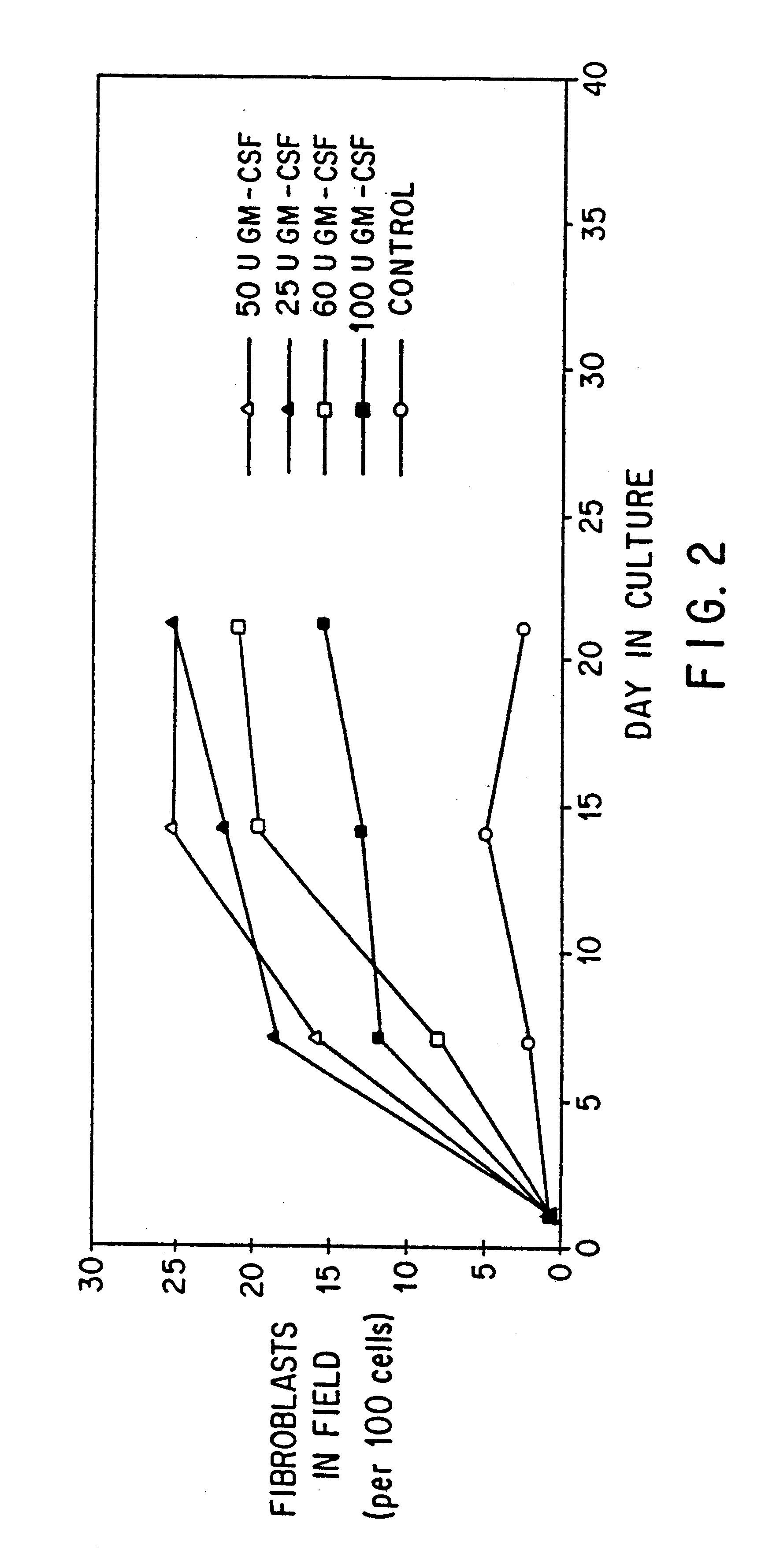
Normal cells, such as fibroblasts or other tissue or organ cell types, are genetically engineered to express biologically active, therapeutic agents, such as proteins that are normally produced in small amounts, for example, MIS, or other members of the TGF-beta family Herceptin(TM), interferons, andanti-angiogenic factors. These cells are seeded into a matrix for implantation into the patient to be treated. Cells may also be engineered to include a lethal gene, so that implanted cells can be destroyed once treatment is completed. Cells can be implanted in a variety of different matrices. In a preferred embodiment, these matrices are implantable and biodegradable over a period of time equal to or less than the expected period of treatment, when cells engraft to form a functional tissue producing the desired biologically active agent. Implantation may be ectopic or in some cases orthotopic. Representative cell types include tissue specific cells, progenitor cells, and stem cells. Matrices can be formed of synthetic or natural materials, by chemical coupling at the time of implantation, using standard techniques for formation of fibrous matrices from polymeric fibers, and using micromachining or microfabrication techniques. These devices and strategies are used as delivery systems via standard or minimally invasive implantation techniques for any number of parenterally deliverable recombinant proteins, particularly those that are difficult to produce in large amounts and / or active forms using conventional methods of purification, for the treatment of a variety of conditions that produce abnormal growth, including treatment of malignant and benign neoplasias, vascular malformations (hemangiomas), inflammatory conditions, keloid formation, abdominal or plural adhesions, endometriosis, congenital or endocrine abnormalities, and other conditions that can produce abnormal growth such as infection. Efficacy of treatment with the therapeutic biologicals is detected by determining specific criteria, for example, cessation of cell proliferation, regression of abnormal tissue, or cell death, or expression of genes or proteins reflecting the above.
Owner:THE GENERAL HOSPITAL CORP
Three-dimensional filamentous tissue having tendon or ligament function
A stromal cell-based three-dimensional cell culture system is provided which can be used to culture a variety of different cells and tissues in vitro for prolonged periods of time. The stromal cells along with connective tissue proteins naturally secreted by the stromal cells attach to and substantially envelope a framework composed of a biocompatible non-living material formed into a three-dimensional structure having interstitial spaces bridged by the stromal cells. Living stromal tissue so formed provides support, growth factors, and regulatory factors necessary to sustain long-term active proliferation of cells in culture and / or cultures implanted in vivo. When grown in this three-dimensional system, the proliferating cells mature and segregate properly to form components of adult tissues analogous to counterparts in vivo, which can be utilized in the body as a corrective tissue. The three-dimensional cultures can be used to form tubular tissue structures, like those of the gastrointestinal and genitourinary tracts, as well as blood vessels; tissues for hernia repair and / or tendons and ligaments. A three-dimensional filamentous tissue having tendon or ligament function is prepared containing fibroblasts and collagen naturally secreted by the fibroblasts attached to and substantially enveloping a three-dimensional filamentous framework.
Owner:SMITH & NEPHEW WOUND MANAGEMENT LA JOLLA
Cultured skin and method of manufacturing the same
InactiveUS6916655B2High successful grafting rateSkin implantsEpidermal cells/skin cellsEpitheliumFibroblast
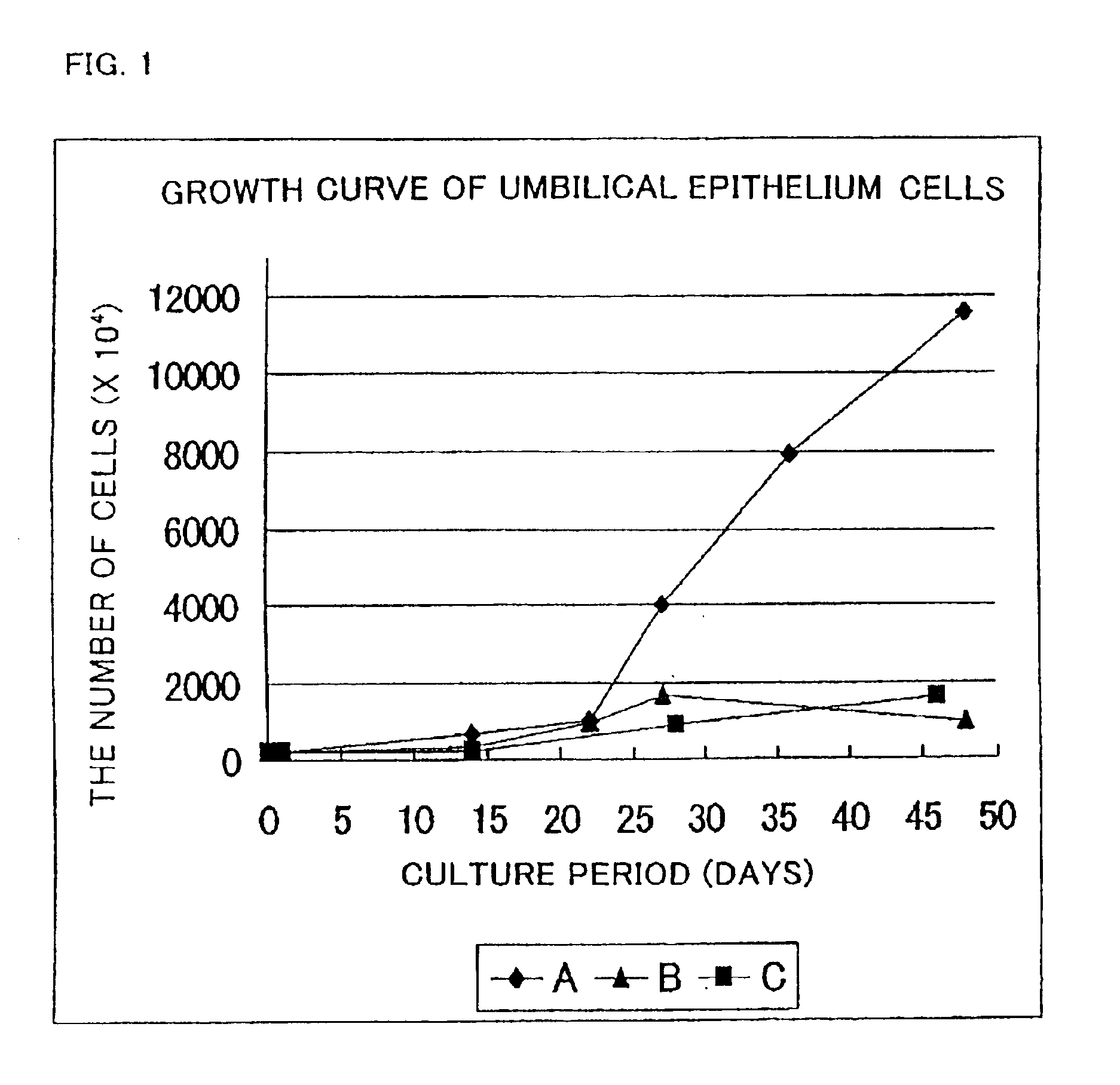
A cultured skin and a grafting cultured skin sheet are provided, each of which is a cultured reconstructive skin with a high take rate using cells collectable from cells originated from tissue included in an umbilical cord such as tissue included in an umbilical cord originated from a human fetus. The grafting cultured skin stratified sheet is prepared by placing an epithelium sheet on the top surface of a cultured dermis. The cultured dermis includes as components a cultured skin containing cells originated from a tissue included in an umbilical cord, such as umbilical cells, more concretely, umbilical fibroblast cells, being separated and cultured, preferably in a collagen nonwoven fabric. On the other hand, the epithelium sheet is prepared by culturing and stratifying the umbilical cord epithelium cells.
Owner:NIPRO CORP
Apparatus, system, and method to deliver optimal elements in order to enhance the aesthetic appearance of the skin
ActiveUS20070073217A1Eliminate and greatly reduce disadvantageEliminate and greatly reduce and problemMicroneedlesSurgeryFiberGrowth Agents
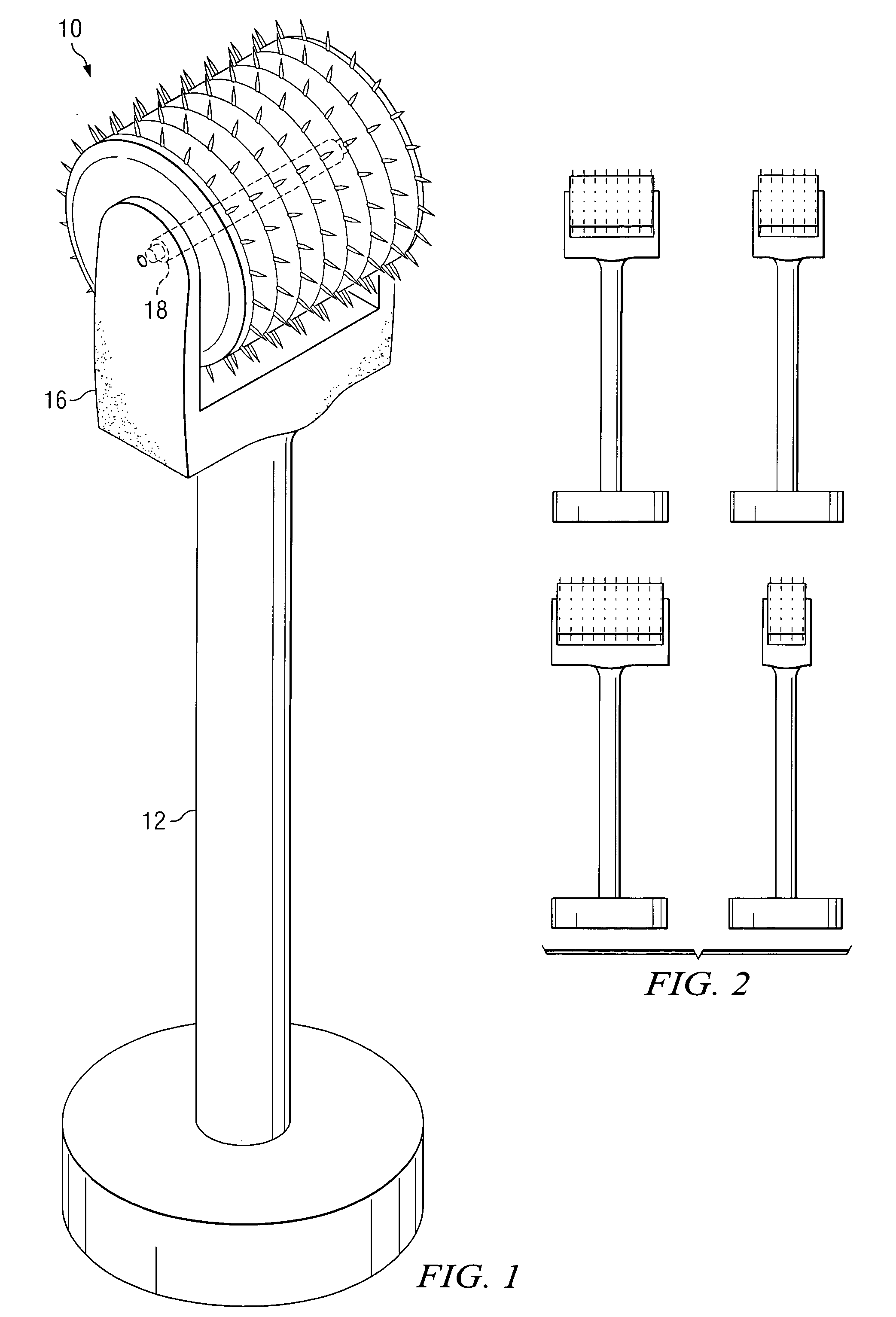
An apparatus for delivering a bioactive material to a subterranean layer of a skin architecture is provided that includes a head including one or more needles that are operable to penetrate a stratum corneum of a skin. A bioactive material is disposed on one or more of the needles, whereby movement of the head operates to pick up the bioactive material and to deliver a portion of the bioactive material to a selected location, the selected location being a dermis, or an epidermis, or both the dermis and the epidermis. In more particular embodiments, the bioactive material is a macromolecule substance that is part of a group of substances, the group consisting of a protein, a vitamin, a gene, a growth agent, a drug, and a peptide. The needles create an injury that triggers collagen production from one or more fibroblasts in the skin.
Owner:BEAUTY BIOSCI
Methods For Treating Wounds
InactiveUS20030125264A1Increasing cell proliferationPromote cell growthBiocideCarbohydrate active ingredientsFibroblastPharmaceutical drug
Pharmaceutical compositions, and methods of using the same, are provided utilizing effective amounts of one or more flavones, flavonols, flavanones, isoflavanones and isoflavones to increase cell proliferation in various tissues and cell lines. As examples, the composition and methods of the present invention can be used to increase proliferation of fibroblast cells and, more particularly, in the treatment of wounds as well as strengthening of the skin.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Bioactive, resorbable scaffolds for tissue engineering
InactiveUS20050118236A1High porosityImprove manufacturabilityBiocideSynthetic resin layered productsPorosityFiber
Flexible, bioactive glass meshes and scaffolds made therefrom are provided. The meshes comprise interwoven bioactive glass fibers that can be coated with resorbable polymers. Meshes can also be woven from glass fibers and resorbable polymers. Scaffolds can be constructed by a plurality of meshes, which can have varying porosities to create porosity gradients in the scaffold. Methods of making scaffolds are provided which can comprise pulling bioactive glass fibers, winding the fibers, forming the fibers into bundles, coating the fibers with a resorbable polymer, and creating a biaxial weave with the bundles. Soft tissue engineering methods are also provided for creating scaffolds for incubating cells such as fibroblasts and chondroblasts. Meshes and scaffolds are suitable for tissue engineering, such as bone tissue engineering and cartilage tissue engineering.
Owner:GENTIS
Isolation and Cultivation of Stem/Progenitor Cells From the Amniotic Membrane of Umbilical Cord and Uses of Cells Differentiated Therefrom
The present invention relates to a skin equivalent and a method for producing the same, wherein the skin equivalent comprises a scaffold and stem / progenitor cells isolated from the amniotic membrane of umbilical cord. These stem / progenitor cells may be mesenchymal (UCMC) and / or epithelial (UCEC) stem cells, which may then be further differentiated to fibroblast and keratinocytes. Further described is a method for isolating stem / progenitor cells from the amniotic membrane of umbilical cord, wherein the method comprises separating the amniotic membrane from the other components of the umbilical cord in vitro, culturing the amniotic membrane tissue under conditions allowing cell proliferation, and isolating the stem / progenitor cells from the tissue cultures. The invention also refers to therapeutic uses of these skin equivalents. Another aspect of the invention relates to the generation of a mucin-producing cell using stem / progenitor cells obtained from the amniotic membrane of umbilical cord and therapeutic uses of such mucin-producing cells. In yet another aspect, the invention relates to a method for generating an insulin-producing cell using stem / progenitor cells isolated from the amniotic membrane of umbilical cord and therapeutic uses thereof. The invention further refers to a method of treating a bone or cartilage disorder using UCMC. Furthermore, the invention refers to a method of generating a dopamin and tyrosin hydroxylase as well as a HLA-G and hepatocytes using UCMC and / or UCEC. The present invention also refers to a method of inducing proliferation of aged keratinocytes using UCMC.
Owner:CELLRESEARCH CORP PTE LTD
Serum-free media for chondrocytes and methods of use thereof
InactiveUS7169610B2Safe effective inexpensiveSafe and effective and inexpensiveCulture processArtificial cell constructsLipid formationSerum free media
The present invention provides defined serum-free cell culture media useful in culturing fibroblasts, especially articular chondrocytes, that avoids problems inherent in the use of serum-containing media. The defined media comprise platelet-derived growth factor (PDGF), and chemically defined lipids, or combinations of these compounds. In another aspect, the present invention also provides tissue culture methods that comprise incubating chondrocytes in the defined serum free media. The methods enhance attachment and proliferative expansion of chondrocytes seeded at low density while maintaining their redifferentiation potential.
Owner:GENZYME CORP
Graft collar and scaffold apparatuses for musculoskeletal tissue engineering and related methods
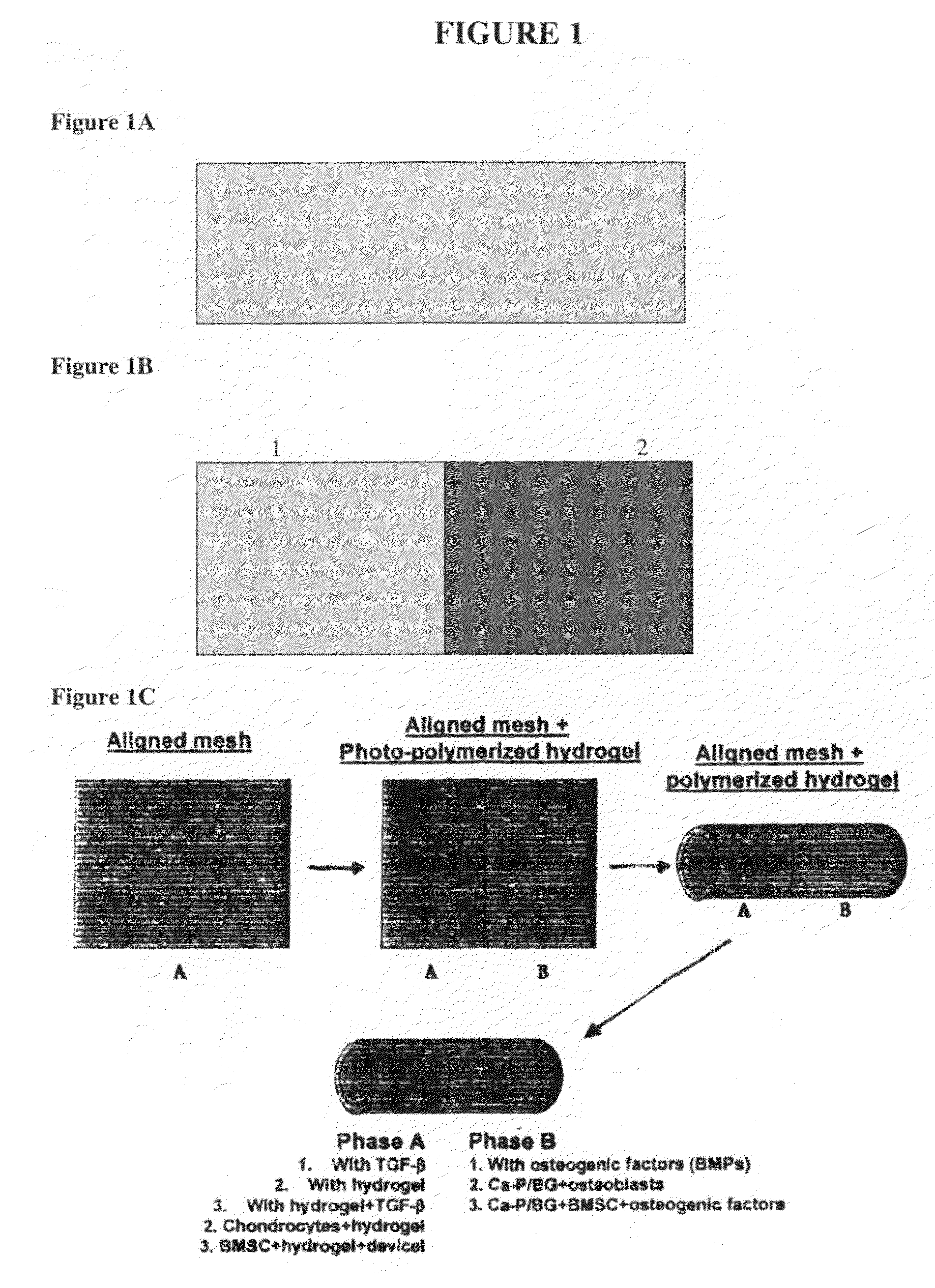
This application describes apparatuses and methods for musculoskeletal tissue engineering. Specifically, graft collar and scaffold apparatuses are provided for promoting fixation of musculoskeletal soft tissue to bone.This application provides for graft collars comprising biopolymer mesh and / or polymer-fiber mesh for fixing tendon to bone. In one aspect, the graft collar comprises more than one region, wherein the regions can comprise different materials configured to promote integration of and the regeneration of the interfacial region between tendon and bone.This application also provides for scaffold apparatuses and methods for fixing musculoskeletal soft tissue to bone. The scaffold apparatus is multiphasic, preferably triphasic, and each phase is configured promote growth and proliferation of a different cell and its associated tissue. In one aspect, the scaffold apparatus is triphasic, with phases comprising materials to promote growth and proliferation of fibroblasts, chondroblasts, and osteoblasts. In addition, an apparatus comprising two portions, each of said portion being the scaffold apparatus described above is provided, wherein each of said portion encases one end of a soft tissue graft. Further, a triphasic interference screw is provided.This application further provides apparatuses and methods for inducing formation of fibrocartilage comprising wrapping a graft collar with polymer-fiber mesh configured to apply compression to the graft collar. In another aspect, the polymer-fiber is applied directly to the graft to apply compression to the graft.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
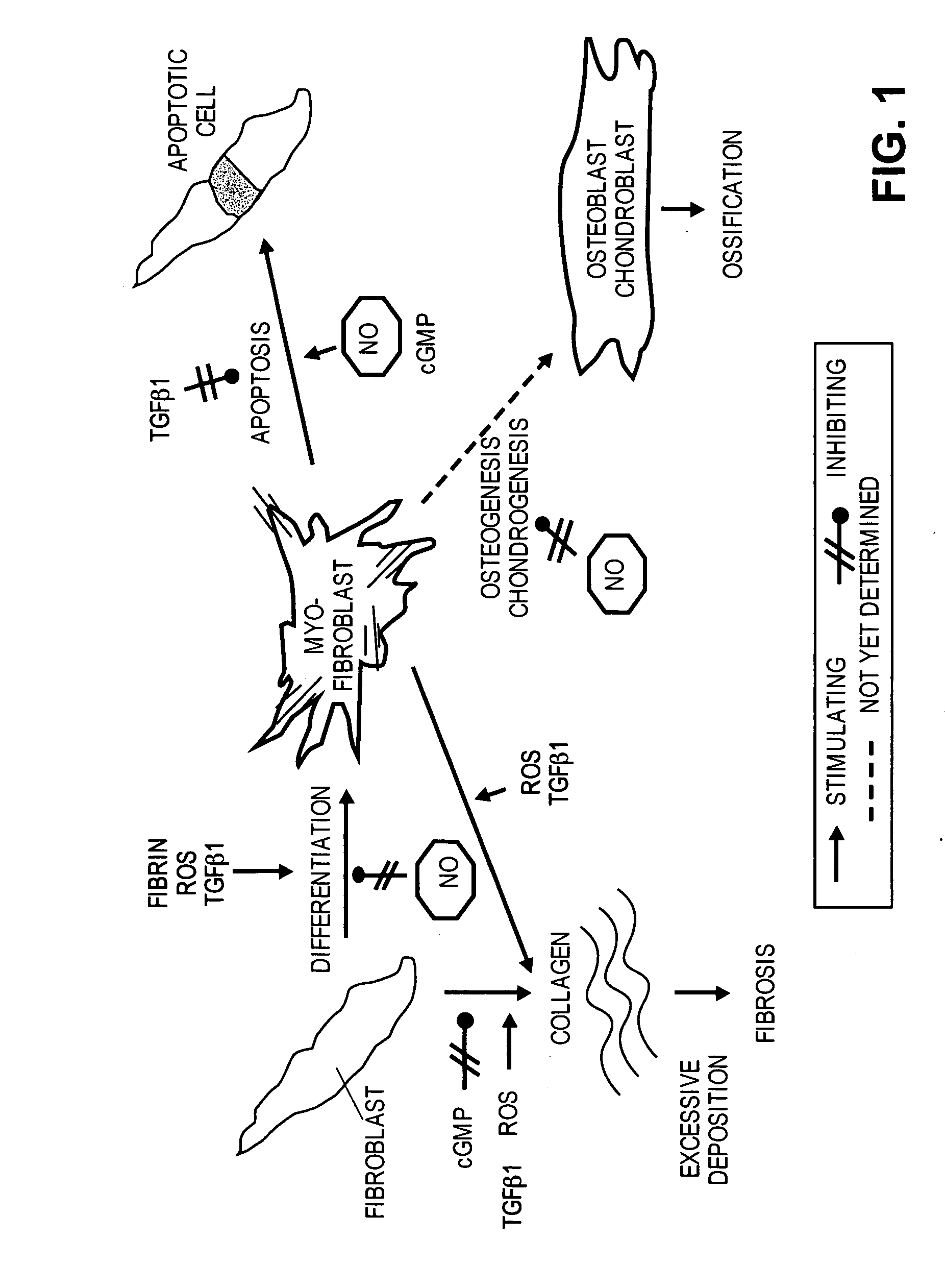
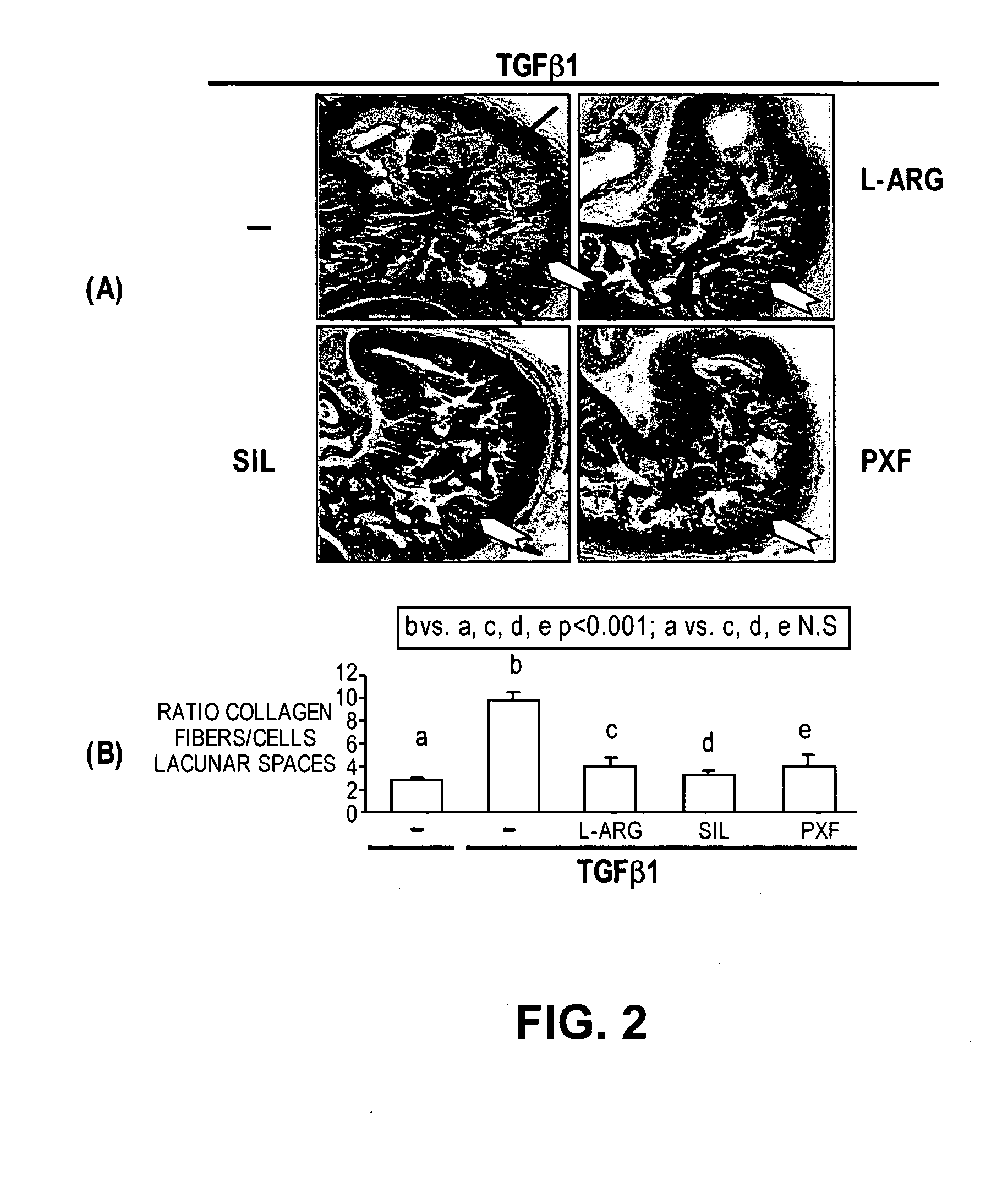
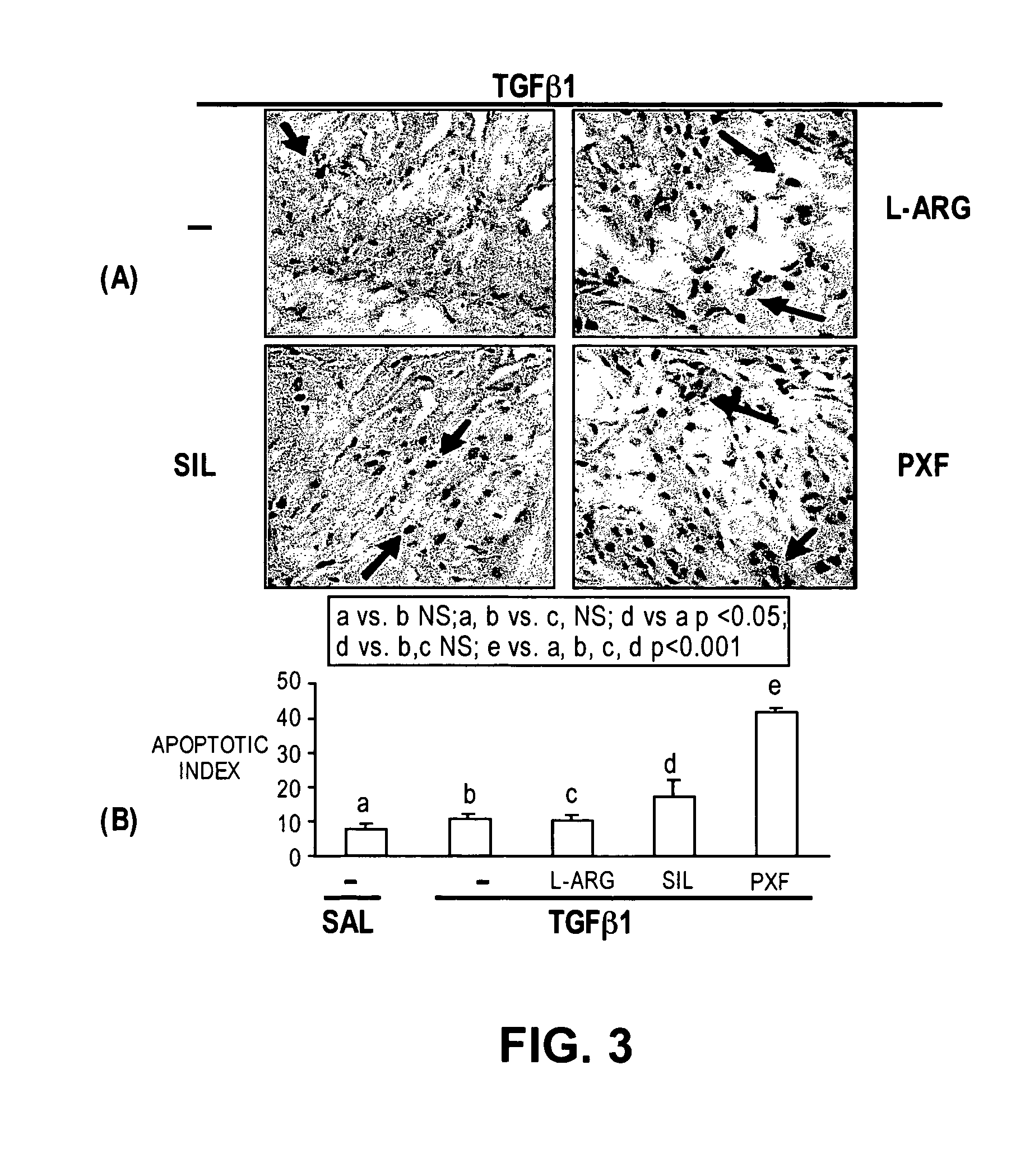
Methods of use of inhibitors of phosphodiesterases and modulators of nitric oxide, reactive oxygen species, and metalloproteinases in the treatment of peyronie's disease, arteriosclerosis and other fibrotic diseases
ActiveUS20050085486A1Increasing NO levelReduce expressionBiocidePharmaceutical delivery mechanismFemale Sexual Arousal DisorderCyclase
The present methods and compositions are of use for treatment of conditions involving fibrosis, such as Peyronie's disease plaque, penile corporal fibrosis, penile veno-occlusive dysfunction, Dupuytren's disease nodules, vaginal fibrosis, clitoral fibrosis, female sexual arousal disorder, abnormal wound healing, keloid formation, general fibrosis of the kidney, bladder, prostate, skin, liver, lung, heart, intestines or any other localized or generalized fibrotic condition, vascular fibrosis, arterial intima hyperplasia, atherosclerosis, arteriosclerosis, restenosis, cardiac hypertrophy, hypertension or any condition characterized by excessive fibroblast or smooth muscle cell proliferation or deposition of collagen and extracellular matrix in the blood vessels and / or heart. In certain embodiments, the compositions may comprise a PDE-4 inhibitor, a PDE-5 inhibitor, a compound that elevates cGMP and / or PKG, a stimulator of guanylyl cyclase and / or PKG, a combination of a compound that elevates cGMP, PKG or NO with an antioxidant that decreases ROS, or a compound that increases MMP activity.
Owner:LOS ANGELES BIOMEDICAL RES INST AT HARBOR UCLA MEDICAL CENT
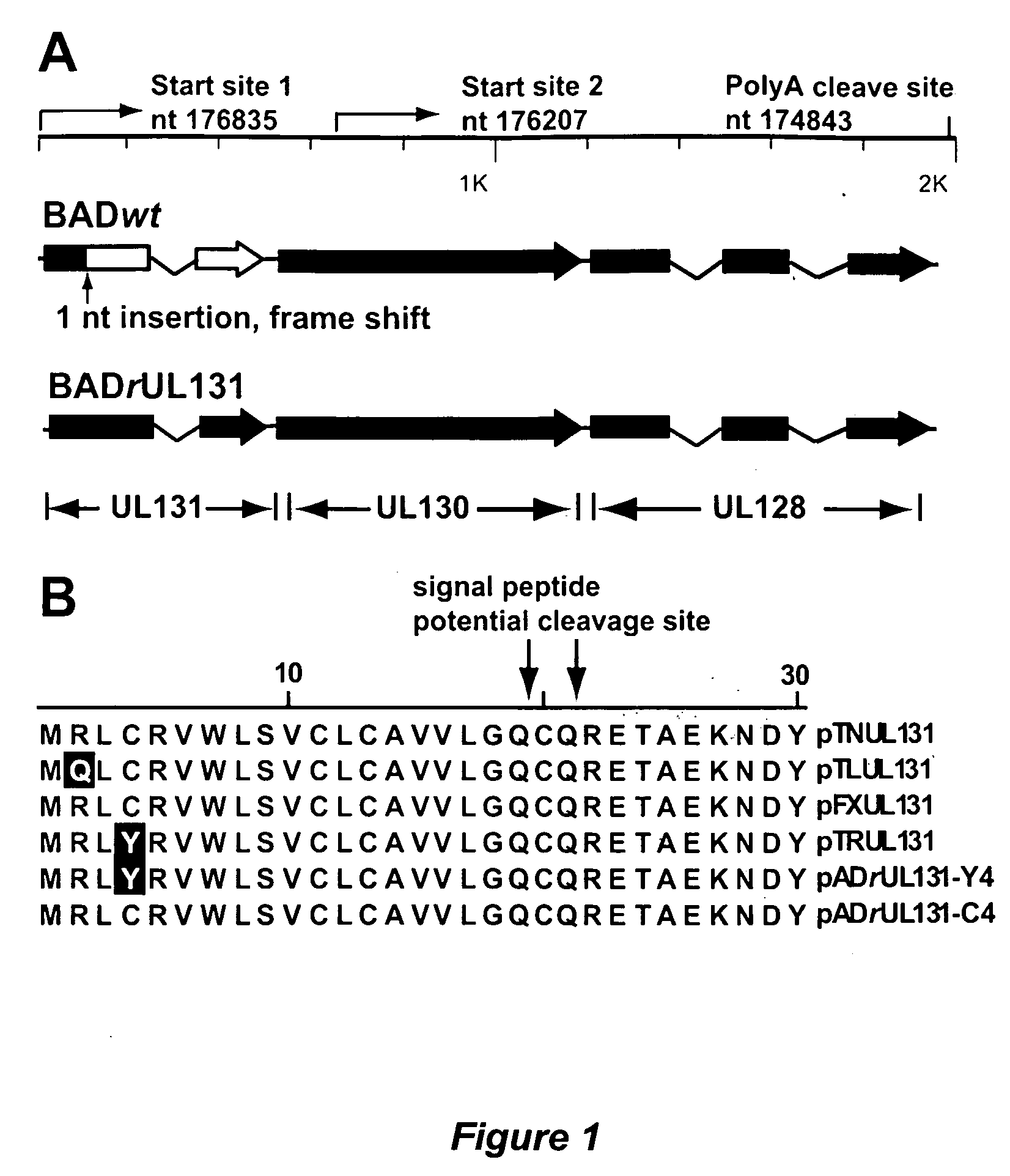
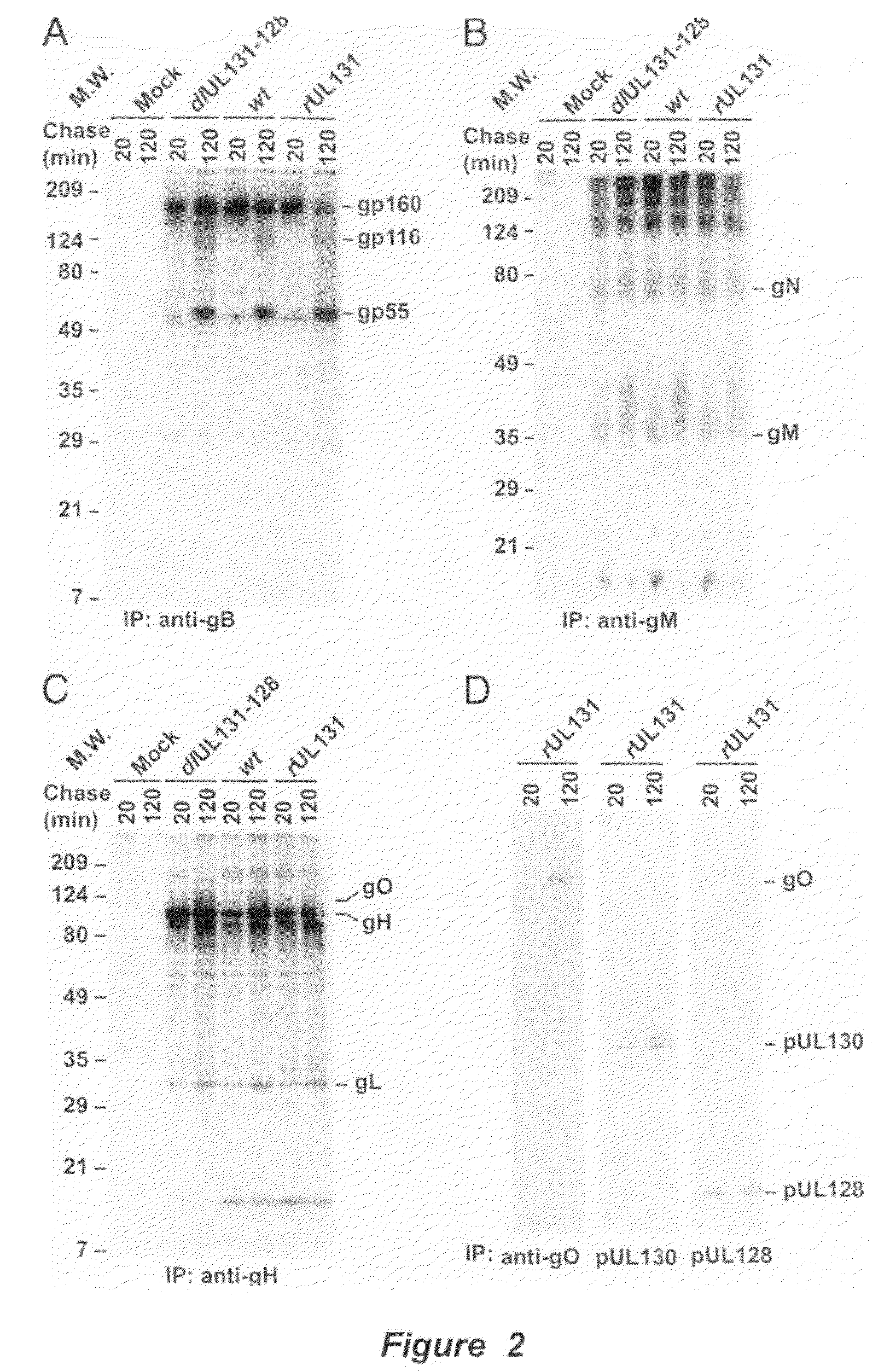
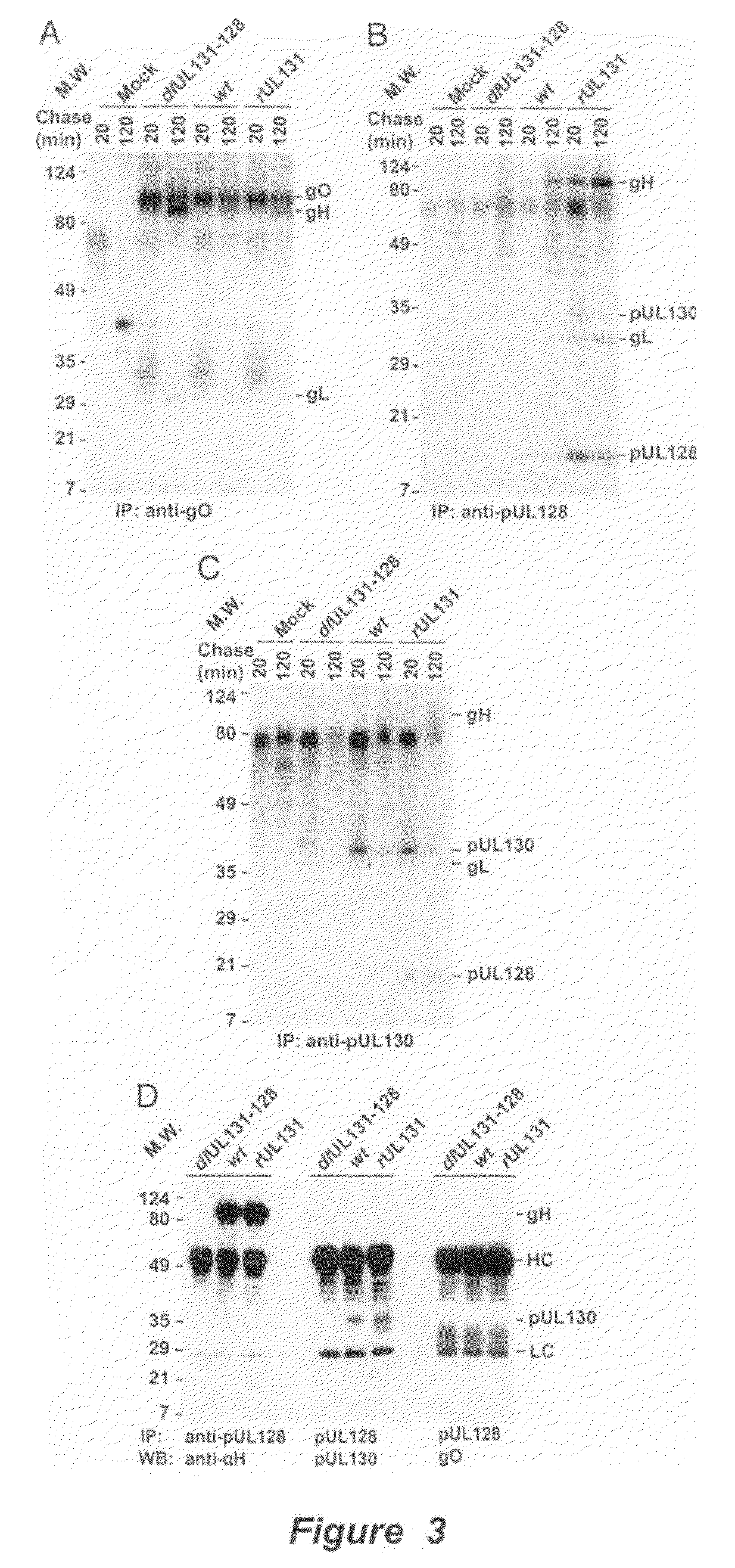
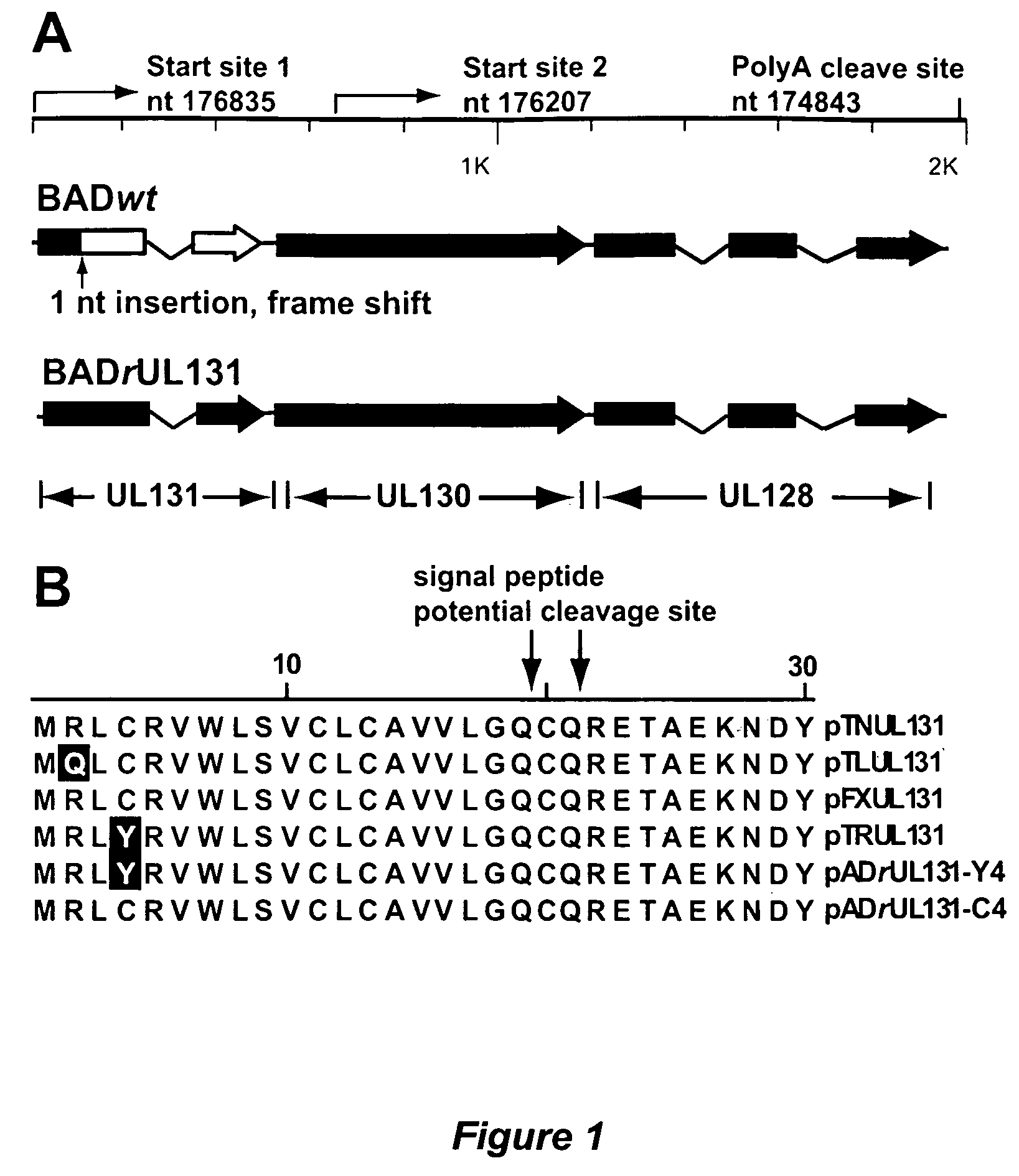
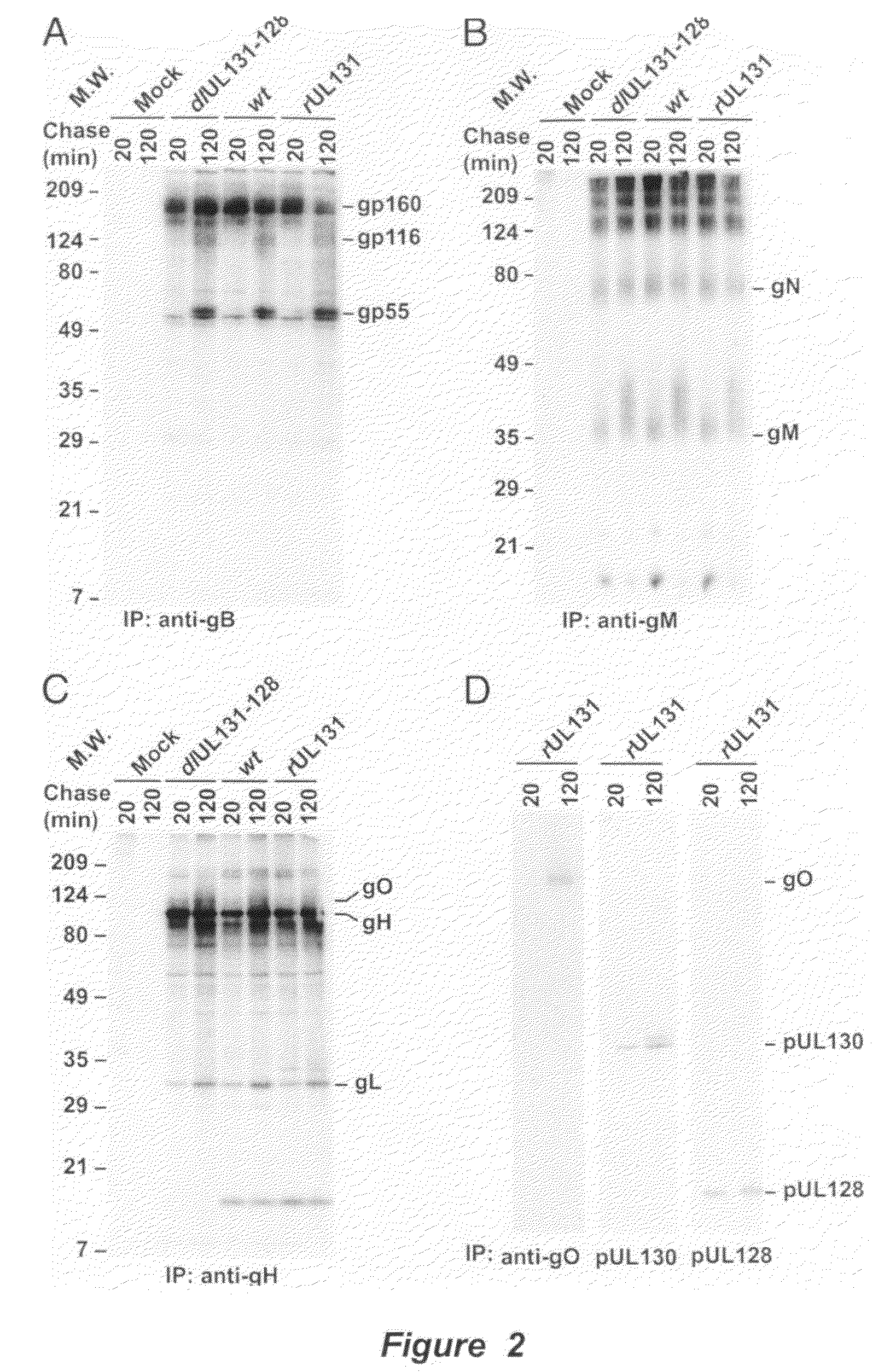
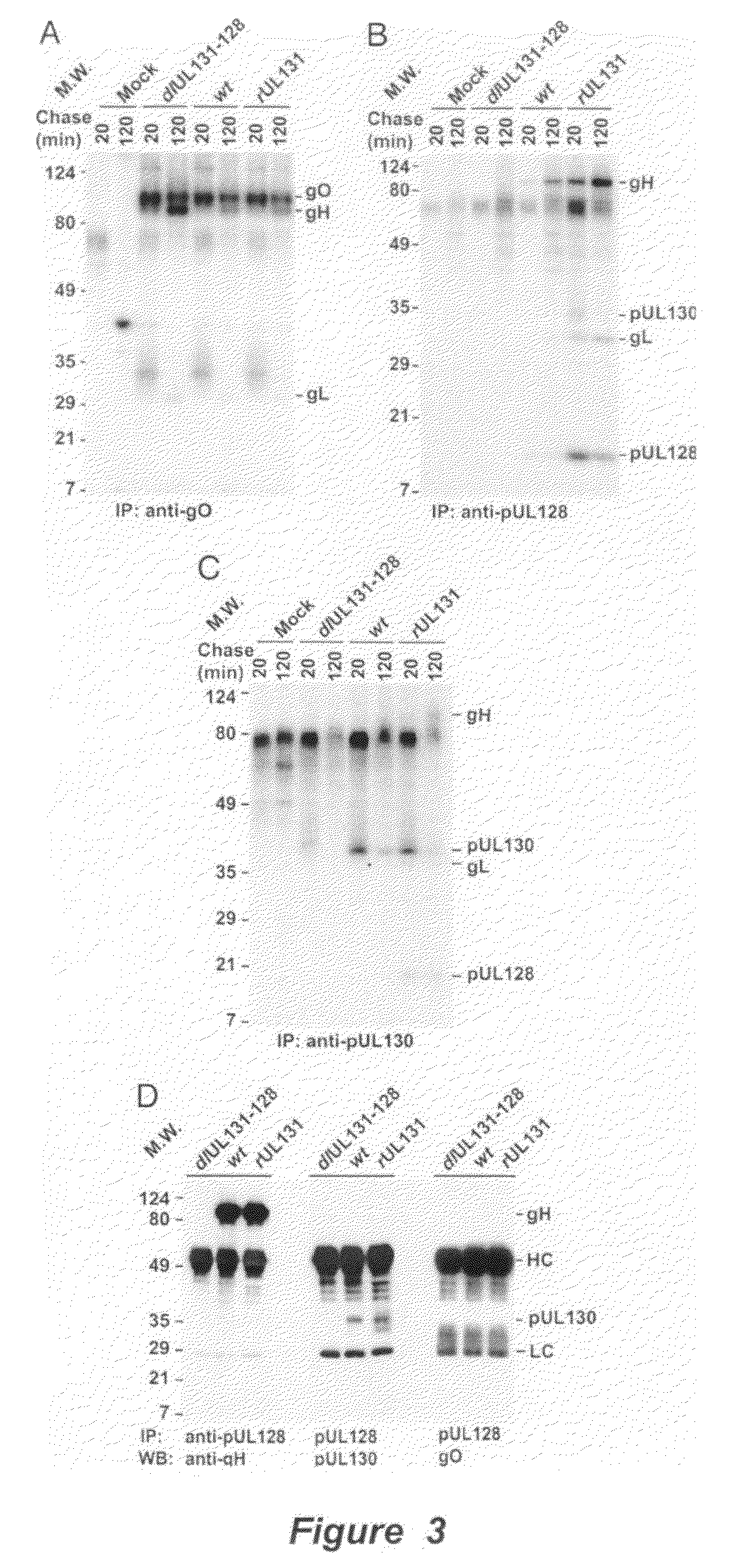
Cytomegalovirus surface protein complex for use in vaccines and as a drug target
Immunogenic compositions and prophylactic or therapeutic vaccines for use in protecting and treating against human cytomegalovirus (CMV) are disclosed. Subunit vaccines comprising a human CMV protein complex comprising pUL128 or pUL130, and nucleic acid vaccines comprising at least one nucleic acid encoding a CMV protein complex comprising pUL128 or pUL130 are described. Also disclosed are therapeutic antibodies reactive against a CMV protein complex comprising pUL128 or pUL130, as well as methods for screening compounds that inhibit CMV infection of epithelial and endothelial cells, methods for immunizing a subject against CMV infection, methods for determining the capability of neutralizing antibodies to inhibit human CMV infection of cell types other than fibroblasts, and methods of diminishing an CMV infection.
Owner:THE TRUSTEES FOR PRINCETON UNIV
Methods and compositions based on inhibition of cell invasion and fibrosis by anionic polymers
Owner:TRIAD
Method of cloning reproductive and respiratory syndrome resisting pig
Owner:CHINA AGRI UNIV
Compositions for regenerating tissue that has deteriorated, and methods for using such compositions
InactiveUS6878383B2Promote tissue regenerationPrevent emergenceBiocideImpression capsDiseaseFibroblast
The invention provides a composition for promoting regeneration of tissue which has degenerated in a subject as a result of a disease or disorder and a method of using the composition is provided. The composition comprises a biodegradable acellular matrix, and passaged autologous fibroblasts substantially free of immunogenic proteins, e.g., culture medium serum-derived proteins, integrated within the matrix. Also provided is an injectable composition comprising an acellular filler material (e.g., any type of collagen) and passaged autologous fibroblasts substantially free of immunogenic proteins, e.g., culture medium serum-derived proteins, for correcting defects in skin, such as wrinkles or scars, and for augmenting tissue in the subject, particularly facial tissue.
Owner:CASTLE CREEK BIOSCIENCES LLC
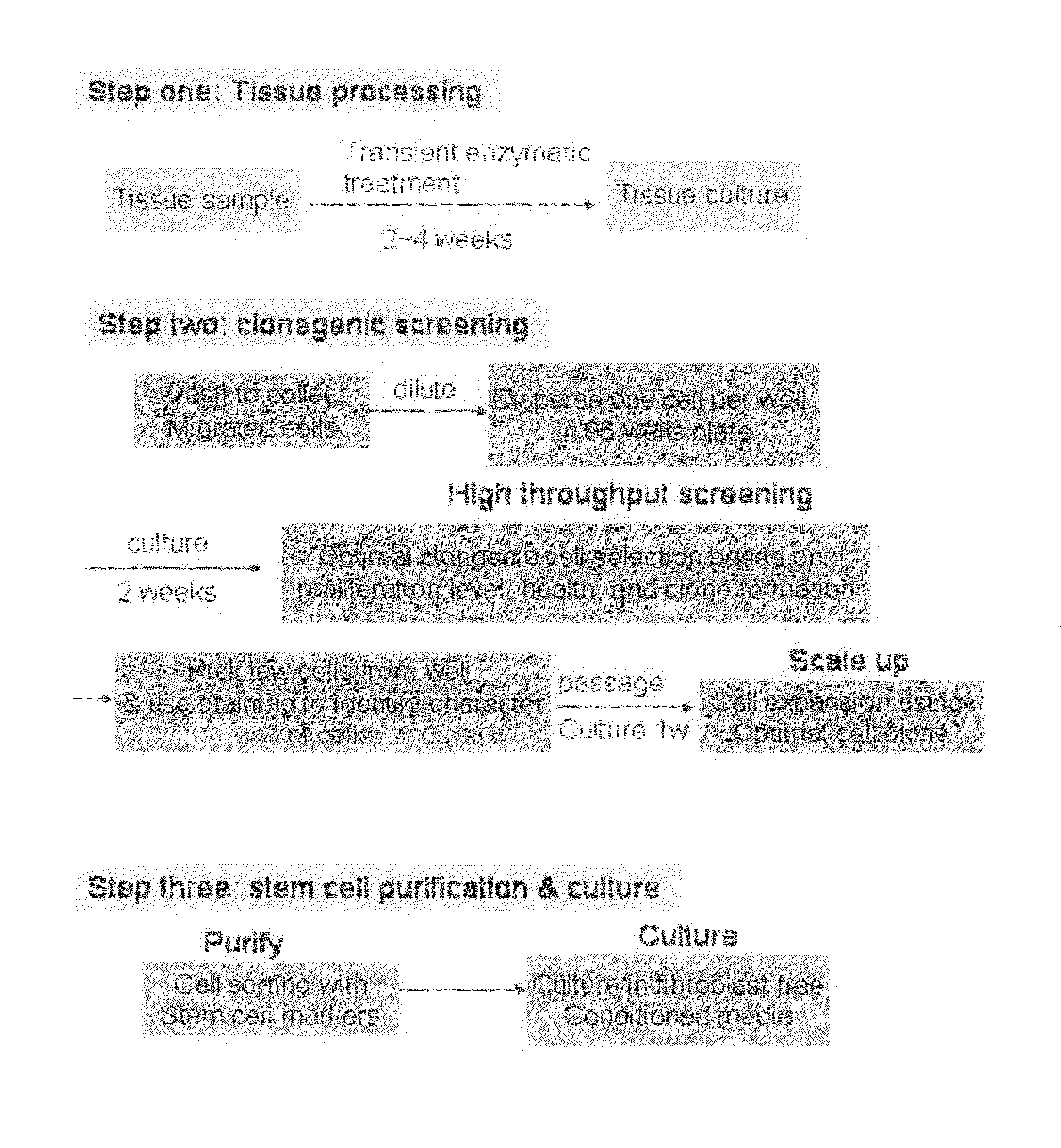
Method for preparing cell cultures from biological specimens for chemotherapeutic and other assays
InactiveUS6887680B2Promote growthMicrobiological testing/measurementMicroorganism separationParticulatesAnticarcinogen
An improved system for screening a multiple of candidate therapeutic or chemotherapeutic agents for efficacy as to a specific patient, in which a tissue sample from the patient is harvested, cultured and separately exposed to a plurality of treatments and / or therapeutic agents for the purpose of objectively identifying the best treatment or agent for the particular patient. Specific method innovations such as tissue sample preparation techniques render this method practically as well as theoretically useful. One particularly important tissue sample preparation technique is the initial preparation of cohesive multicellular particulates of the tissue sample, rather than enzymatically dissociated cell suspensions or preparations, for initial tissue culture monolayer preparation. With respect to the culturing of malignant cells, for example, it is believed (without any intention of being bound by the theory) that by maintaining the malignant cells within a multicellular particulate of the originating tissue, growth of the malignant cells themselves is facilitated versus the overgrowth of fibroblasts or other cells which tends to occur when suspended tumor cells are grown in culture. Practical monolayers of cells may thus be formed to enable meaningful screening of a plurality of treatments and / or agents. Growth of cells is monitored to ascertain the time to initiate the assay and to determine the growth rate of the cultured cells; sequence and timing of drug addition is also monitored and optimized. By subjecting uniform samples of cells to a wide variety of active agents (and concentrations thereof), the most promising agent and concentration for treatment of a particular patient can be determined. For assays concerning cancer treatment, a two-stage evaluation is contemplated in which both acute cytotoxic and longer term inhibitory effect of a given anti-cancer agent are investigated.
Owner:PRECISION THERAPEUTICS
FAP-activated anti-tumor compounds
The invention relates to a prodrug that is capable of being converted into a drug by the catalytic action of human fibroblast activation protein (FAPalpha), said prodrug having a cleavage site which is recognised by FAPalpha, and said drug being cytotoxic or cytostatic under physiological conditions.
Owner:BOEHRINGER INGELHEIM PHARM KG
Double membrane tissue patching material and preparation method thereof
The invention discloses a double-layer membranous tissue repair material and a preparation method thereof, wherein, a cell-free membranous biological derivative material is used as a surface layer, and a fibroblast is compounded in the interior of a biological support material to form a substrate, and then the surface layer and the substrate are combined in a chimeric way to form the double-layer membranous tissue repair material; a compact surface layer structure can effectively reduce the loss of water, electrolytes and protein from surface of wound, avoid the invading and the reproduction of bacteria to the impaired surface of wound as well as prevent the infection of the surface of wound, thus being beneficial to epitheliosis and epithelial growth; the substrate can directly repair the surface of wound, promote the ingrowth of cells around the surface of wound and the angiogenesis, induce the differentiation from stem cells to skin cells and quicken wound healing; compared with the existing products, the tissue repair material has the advantages of being capable of promoting the regeneration of skin, improving the elasticity, the flexibility and the mechanical abrasion resistance of skin after the surface of wound is healed, reducing hyperplasia of scar tissues, controlling the contracture, having excellent biocompatibility, increasing the success rate of transplant and improving the quality of healing; the invention has wide material resources and simple production method; the double-layer membranous tissue repair material prepared is applicable to the clinical treatment of skin defect caused by inflammation, ulcer, thermal burns, iatrogenicity and the like.
Owner:SHAANXI RUISHENG BIOTECH
Feeder-free derivation of human-induced pluripotent stem cells with synthetic messenger RNA
ActiveUS20130302295A1Improve efficiencyShorten the timeBiocideNervous disorderReprogrammingFibroblast
The present disclosure relates generally to novel methods and compositions for using engineered reprogramming factor(s) for the creation of induced pluripotent stem cells (iPSCs) through a kinetically controlled process. Specifically, this disclosure relates to establishing combinations of reprogramming factors, including fusions between conventional reprogramming factors with transactivation domains, optimized for reprogramming various types of cells. More specifically, the exemplary methods disclosed herein can be used for creating induced pluripotent stem cells from various mammalian cell types, including human fibroblasts. Exemplary methods of feeder-free derivation of human induced pluripotent stem cells using synthetic messenger RNA are also disclosed.
Owner:ALLELE BIOTECH & PHARMA
Overexpression porcine co-stimulatory 4-1BB vector and application thereof
InactiveCN105087620AHigh copy numberLower activation thresholdVector-based foreign material introductionAnimal husbandryInteinEmbryo
The invention provides an overexpression porcine co-stimulatory 4-1BB vector and application thereof. PCR (polymerase chain reaction) amplification is performed on a left homologous arm and a right homologous arm of an intron 1 of a rosa26 gene, a 4-1BB regulatory sequence and an OCT4 specific promoter; the left homologous arm, a 4-1BB expression cassette, LoxP locus-contained Cre and Neo expression cassettes, the right homologous arm and negative selection DTA diphtheria toxin are connected in sequence to obtain a 4-1BB homologous recombinant vector p4BOCNDR; the vector and a CRISPR / Cas9 (clustered regularly interspaced short palindromic repeats / CRISPR-associated) targeting vector of sgRNA (small guide ribonucleic acid) containing the intron 1 of the specific targeting porcine rosa26 gene are transferred together into a porcine fetus fibroblast; by taking a positive cell as a donor cell and an oocyte as a recipient cell, a cloned embryo is obtained through a somatic cell nuclear transfer technique; the cloned embryo is transplanted into a porcine uterus for fetation to obtain a transgenic pig integrating a 4-1BB gene at the fixed point of a first intron of the rosa26 gene and automatically deleting a marker gene.
Owner:CHINA AGRI UNIV
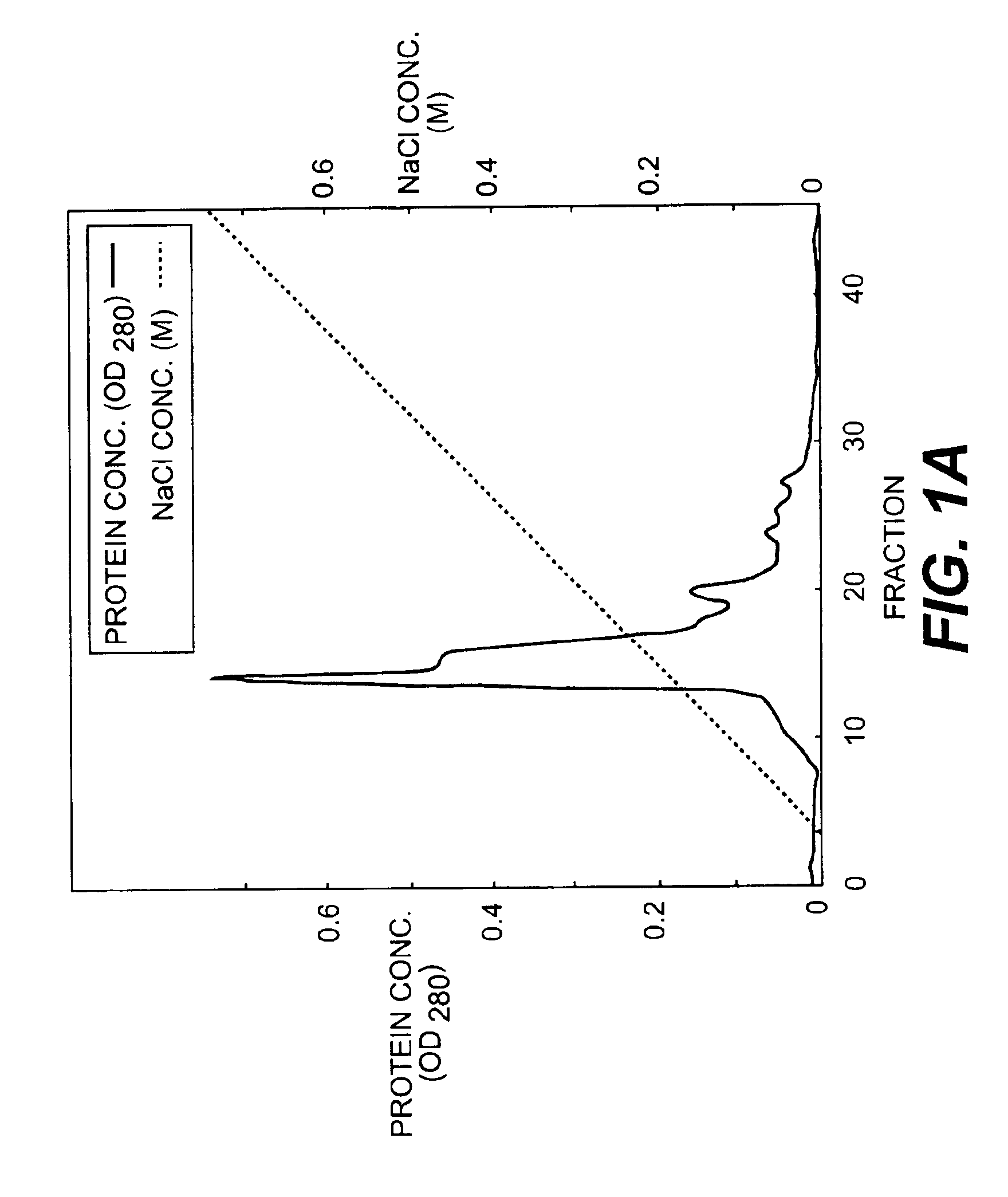
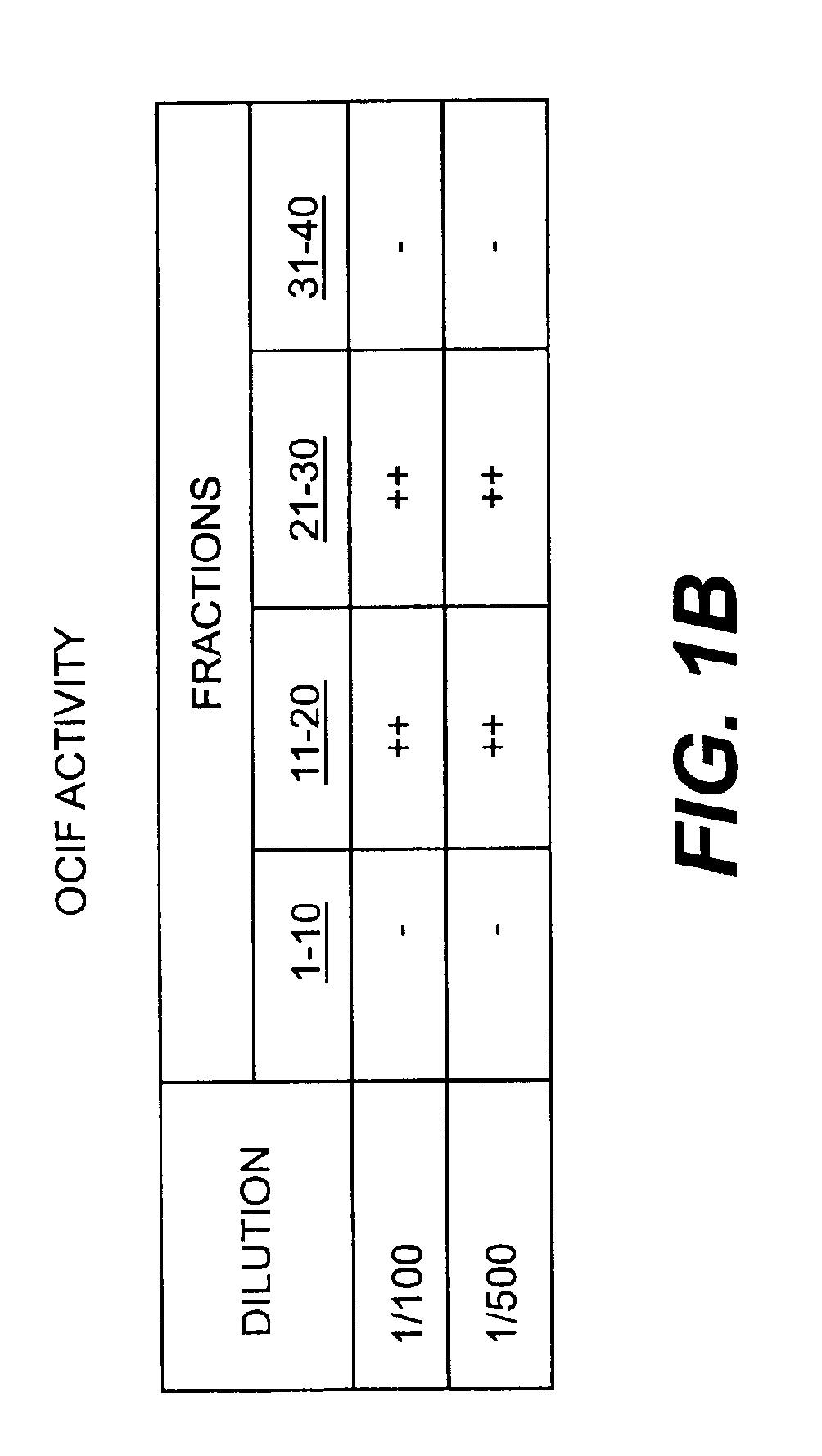
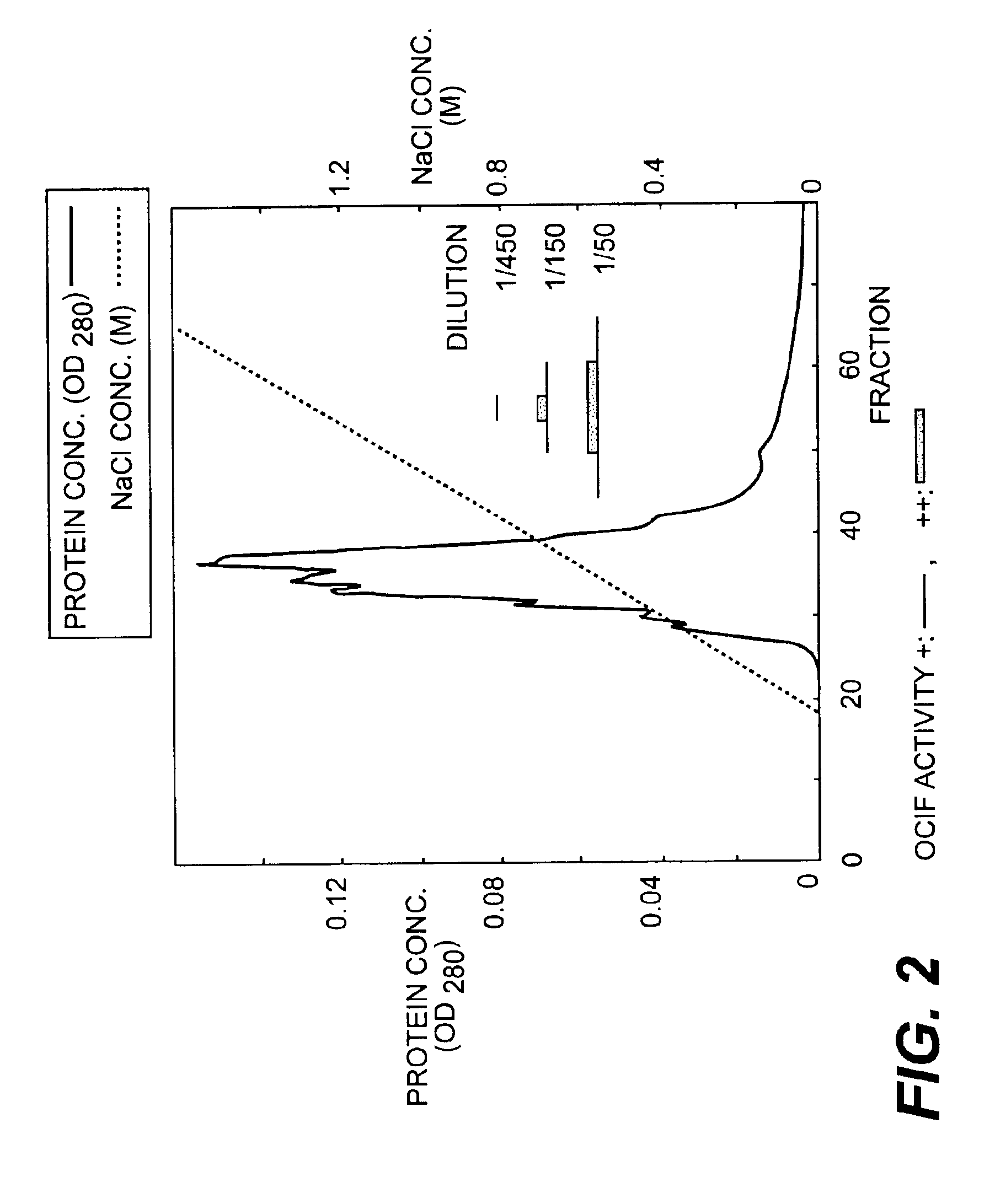
Monoclonal antibodies that bind OCIF
InactiveUS6919434B1Efficient productionMicrobiological testing/measurementEnzymologyADAMTS ProteinsEmbryo
A protein which inhibits osteoclast diffraction and / or maturation and a method for producing the protein. The protein is produced by human embryonic lung fibroblasts and has a molecular weight of about 60 kD and about 120 kD under non-reducing conditions and about 60 kD under reducing conditions an SDS-polyacrylamide gel electrophoresis. The protein can be isolated and purified from the culture medium of fibroblasts. Furthermore, the protein can be produced by gene engineering. The present invention includes cDNA for producing the protein by gene engineering, antibodies having specific affinity for the protein or a method for determining protein concentration using these antibodies.
Owner:DAIICHI SANKYO CO LTD
Methods and compositions for repair of cartilage using an in vivo bioreactor
Methods and compositions for the biological repair of cartilage using a hybrid construct combining both an inert structure and living core are described. The inert structure is intended to act not only as a delivery system to feed and grow a living, core component, but also as an inducer of cell differentiation. The inert structure comprises concentric internal and external and inflatable / expandable balloon-like bio-polymers. The living core comprises the cell-matrix construct comprised of HDFs, for example, seeded in a scaffold. The method comprises surgically removing a damaged cartilage from a patient and inserting the hybrid construct into the cavity generated after the foregoing surgical intervention. The balloons of the inert structure are successively inflated within the target area, such as a joint, for example. Also disclosed herein are methods for growing and differentiating human fibroblasts into chondrocyte-like cells via mechanical strain.
Owner:SPINALCYTE
Delivery of therapeutic biologicals from implantable tissue matrices
InactiveUS20020031500A1Many of effectMany of inconveniencePowder deliveryBiocideProgenitorActive agent
Normal cells, such as fibroblasts or other tissue or organ cell types, are genetically engineered to express biologically active, therapeutic agents, such as proteins that are normally produced in small amounts, for example, MIS, or other members of the TGF-beta family Herceptin(TM), interferons, andanti-angiogenic factors. These cells are seeded into a matrix for implantation into the patient to be treated. Cells may also be engineered to include a lethal gene, so that implanted cells can be destroyed once treatment is completed. Cells can be implanted in a variety of different matrices. In a preferred embodiment, these matrices are implantable and biodegradable over a period of time equal to or less than the expected period of treatment, when cells engraft to form a functional tissue producing the desired biologically active agent. Implantation may be ectopic or in some cases orthotopic. Representative cell types include tissue specific cells, progenitor cells, and stem cells. Matrices can be formed of synthetic or natural materials, by chemical coupling at the time of implantation, using standard techniques for formation of fibrous matrices from polymeric fibers, and using micromachining or microfabrication techniques. These devices and strategies are used as delivery systems via standard or minimally invasive implantation techniques for any number of parenterally deliverable recombinant proteins, particularly those that are difficult to produce in large amounts and / or active forms using conventional methods of purification, for the treatment of a variety of conditions that produce abnormal growth, including treatment of malignant and benign neoplasias, vascular malformations (hemangiomas), inflammatory conditions, keloid formation, abdominal or plural adhesions, endometriosis, congenital or endocrine abnormalities, and other conditions that can produce abnormal growth such as infection. Efficacy of treatment with the therapeutic biologicals is detected by determining specific criteria, for example, cessation of cell proliferation, regression of abnormal tissue, or cell death, or expression of genes or proteins reflecting the above.
Owner:THE GENERAL HOSPITAL CORP
Single guide ribonucleic acid (sgRNA) capable of effectively editing pig ROSA26 gene, and application of sgRNA
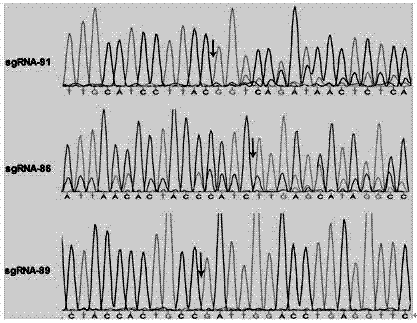
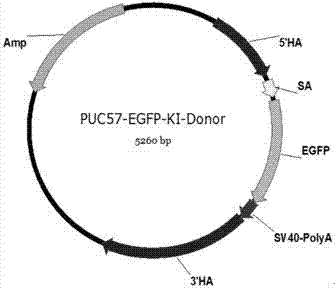
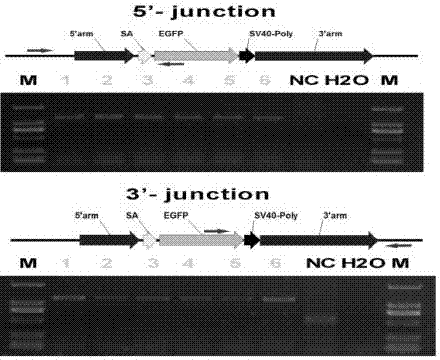
ActiveCN106916820AImprove securityEfficient and stable expressionAnimal husbandryDNA/RNA fragmentationBiotechnologyFibroblast
The invention discloses single guide ribonucleic acid (sgRNA) capable of effectively editing a pig ROSA26 gene, and application of the sgRNA. Based on the sgRNA capable of specifically identifying the pig ROSA26 gene in a pig genome, a cell line of enhanced green fluorescent protein (EGFP) site-specific integrated porcine fetal fibroblasts is successfully established by using a CRISPR / Cas9-mediated gene knock-in technique; the results show that the cell line can stably and efficiently express an EGFP gene; furthermore, in the preparation process of the cell line, exogenous promoter genes and positive and negative screening marker genes are not introduced into the cell line. Therefore, the sgRNA greatly improves the safety of transgenic pigs, and has important significance for eliminating the biological and food potential safety hazards of agricultural products of the transgenic pigs.
Owner:重庆吉渝科技有限公司
Tissue transplantation compositions and methods
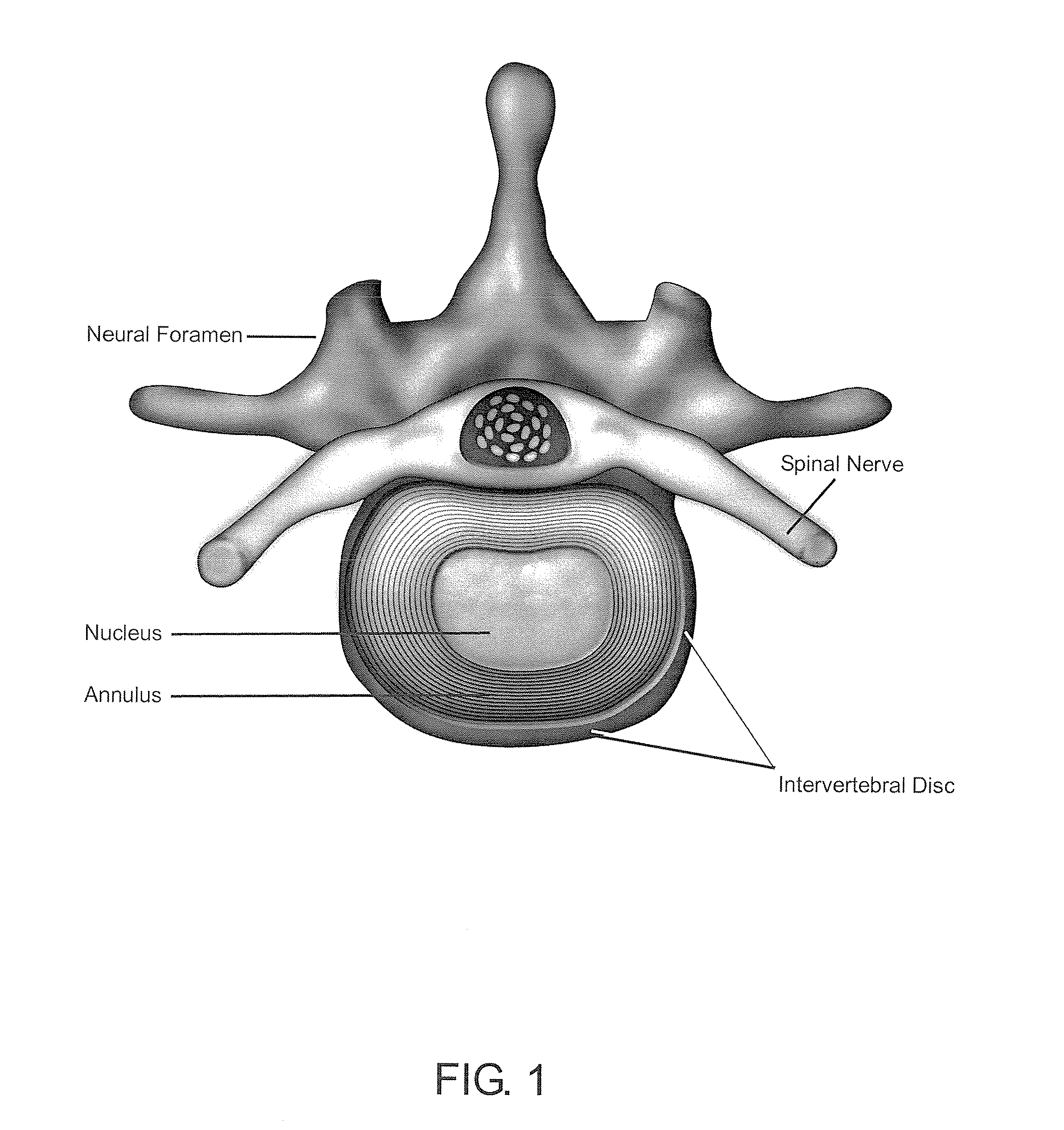
A biomedical material for transplant to a subject is provided according to embodiments of the present invention which includes an isolated donor tissue enzyme-treated to reduce the amount of proteoglycans in the donor tissue compared to untreated tissue. Isolated cells are optionally added to the enzyme-treated donor tissue, including leukocytes, particularly monocytes; macrophages; platelets; cells derived from an intervertebral disc such as chondrocyte-like nucleus pulposus cells; fibrocytes; fibroblasts; mesenchymal stem cells; mesenchymal precursor cells; chondrocytes; or a combination of any of these. The isolated donor tissue is articular cartilage or an intervertebral disc tissue such as nucleus pulposus tissue and / or annulus fibrosis tissue enzyme-treated to remove proteoglycans normally present in these tissues. A biomedical material of the present invention is administered to a subject to treat a disorder or injury, such as a disorder or injury to connective tissue.
Owner:FERREE BRET
Cytomegalovirus surface protein complex for use in vaccines and as a drug target
Owner:THE TRUSTEES FOR PRINCETON UNIV
Modulation of immune responses in blood-borne mesenchymal cells
The present invention relates to a population of blood borne mammalian cells that express a unique profile of surface markers that includes certain markers typical of connective tissue fibroblasts, and are referred to herein as "blood-borne mesenchymal cells." In particular, it relates to the isolation, characterization and uses of such blood-borne mesenchymal cells. The cells of the present invention can be distinguished from peripheral blood leukocytes by their distinct size, morphology, cell surface phenotype and biologic activities, and are likewise distinguishable from connective tissue fibroblasts by other surface phenotypic markers. These cells proliferate in culture, and in vivo, as demonstrated in animal models, are capable of migrating into wound sites from the blood. Therefore, such blood-borne mesenchymal cells may have a wide range of applications, including, but not limited to, the promotion of wound healing, tissue remodeling, and for gene therapy.
Owner:FERRING BV
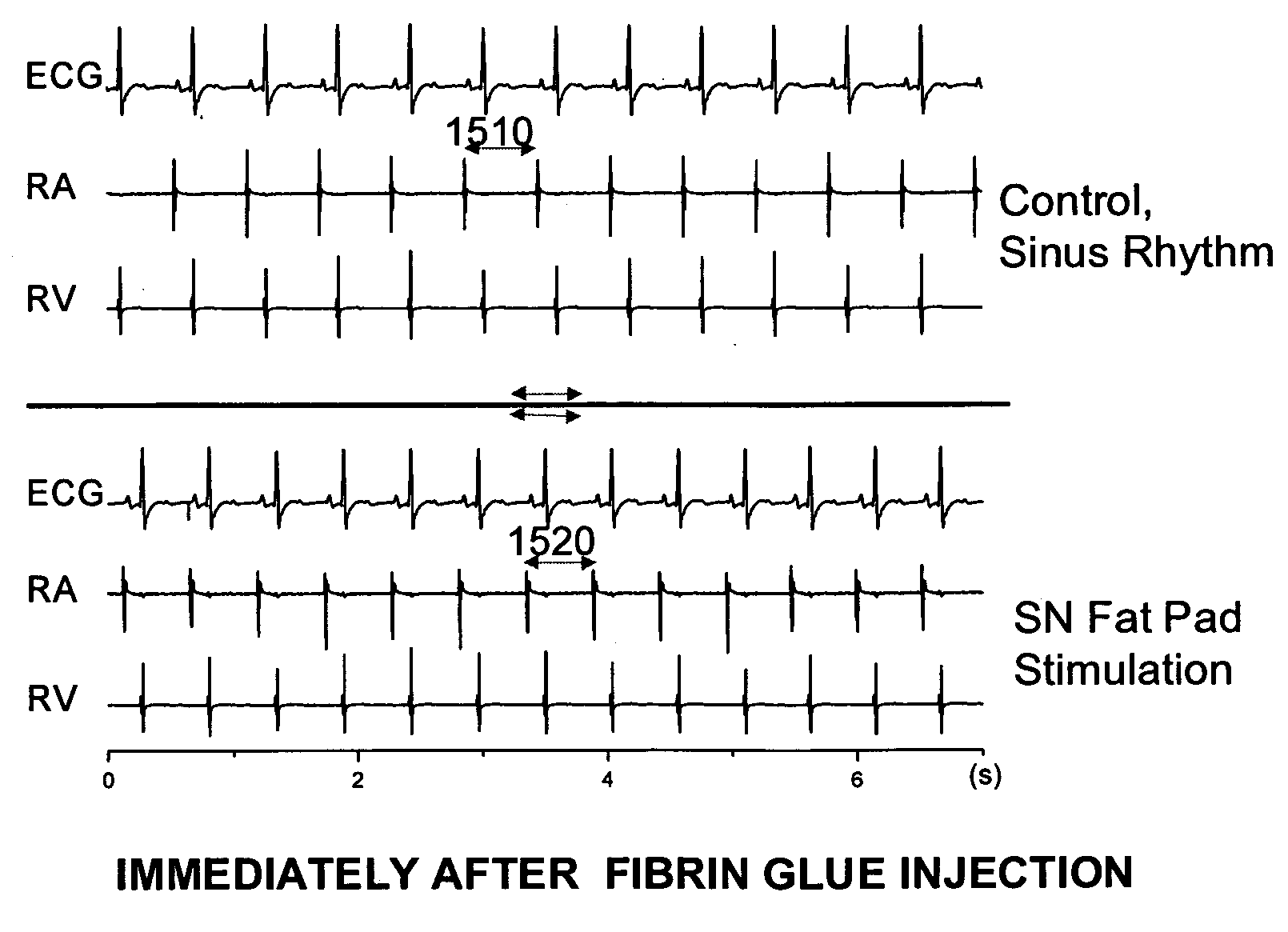
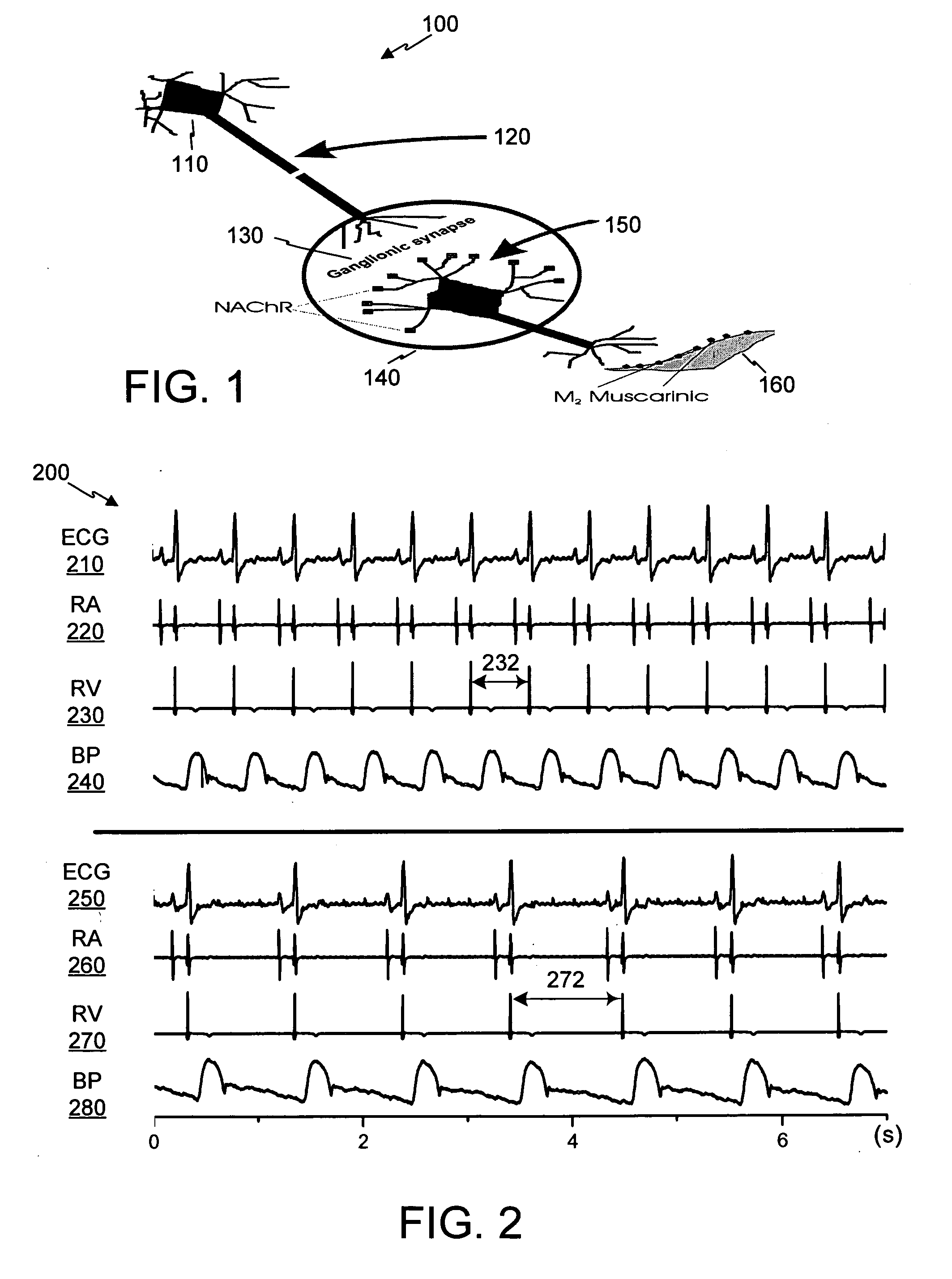
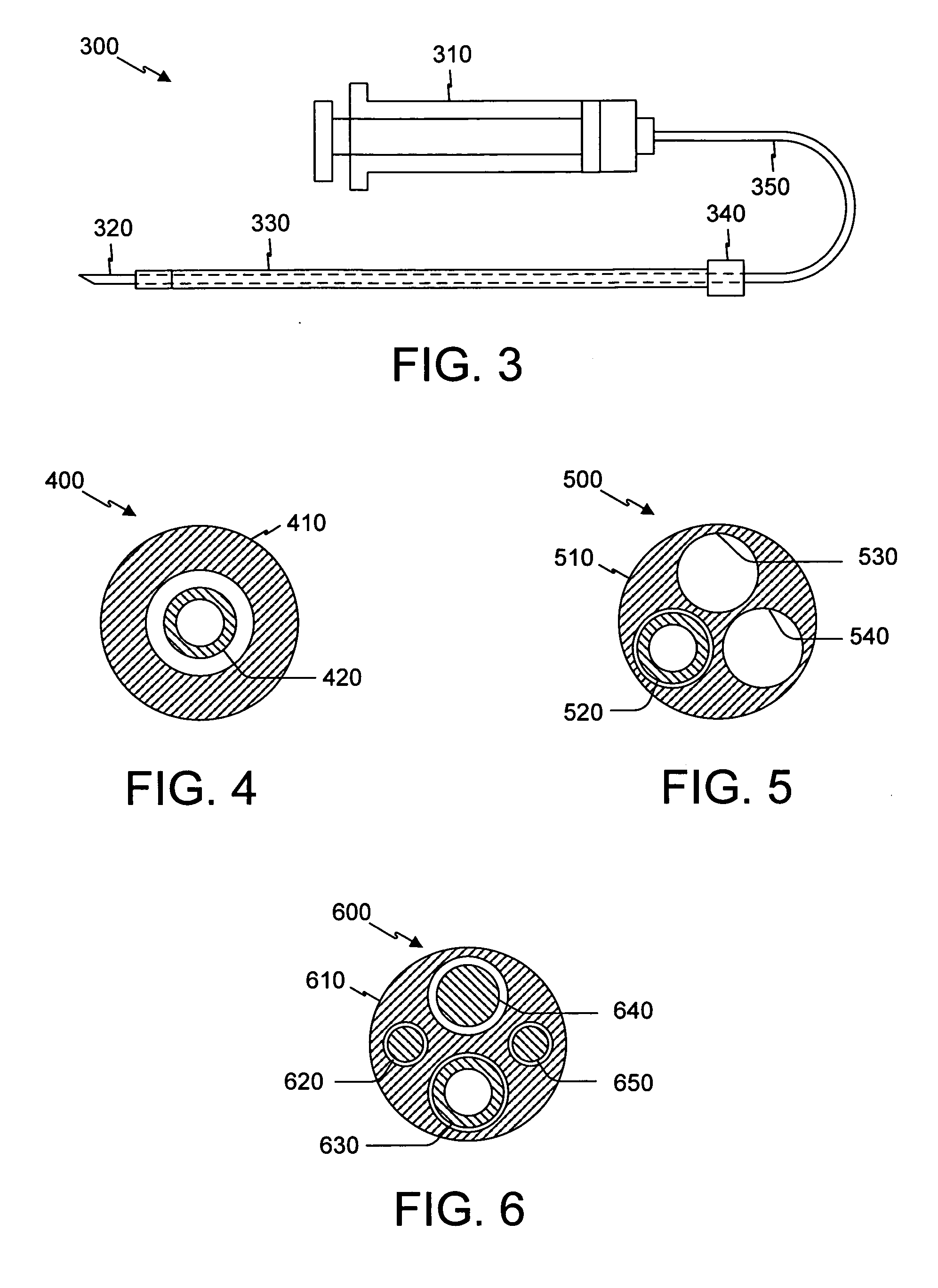
Control of cardiac arrhythmias by modification of neuronal conduction within fat pads of the heart
InactiveUS20050119704A1Efficient modificationPeptide/protein ingredientsInfusion syringesBiopolymerAdventitia
To control cardiac arrhythmias, various conduction-modifying agents include biopolymers, fibroblasts, neurotoxins, and growth factors are introduced either epicardially or endocardially to the fat pads in proximity to the ganglia therein. Any desired technique may be used for injection, including injection from a catheter inserted percutaneously, or direct injection through the epicardial during open heart surgery. Preferably the patient's heart is beating throughout the Injection.
Owner:CARDIOPOLYMERS
Enriched stem cell and progenitor cell populations, and methods of producing and using such populations
The present invention provides a novel method to isolate and expand pure progenitor / stem cells from a primary tissue explant, which produces a population enriched in multipotent functional progenitor / stem cells free of contaminating fibroblasts and other cell types. Cardiac progenitor / stem cells isolated by this method maintain their self-renewal and clonogenic character in vitro and differentiate into normal cells in myocardium, including cardiomyocytes, endothelial cells, and smooth muscle cells, after transplantation into ischemic hearts. The present invention also includes substantially pure populations of multipotent progenitor / stem cells, e.g., cardiac progenitor / stem cells, and their use to treat and prevent diseases and injuries, including those resulting from myocardial infarction.
Owner:KECK GRADUATE INST A UNIV OF THE STATE OF CALIFORNIA
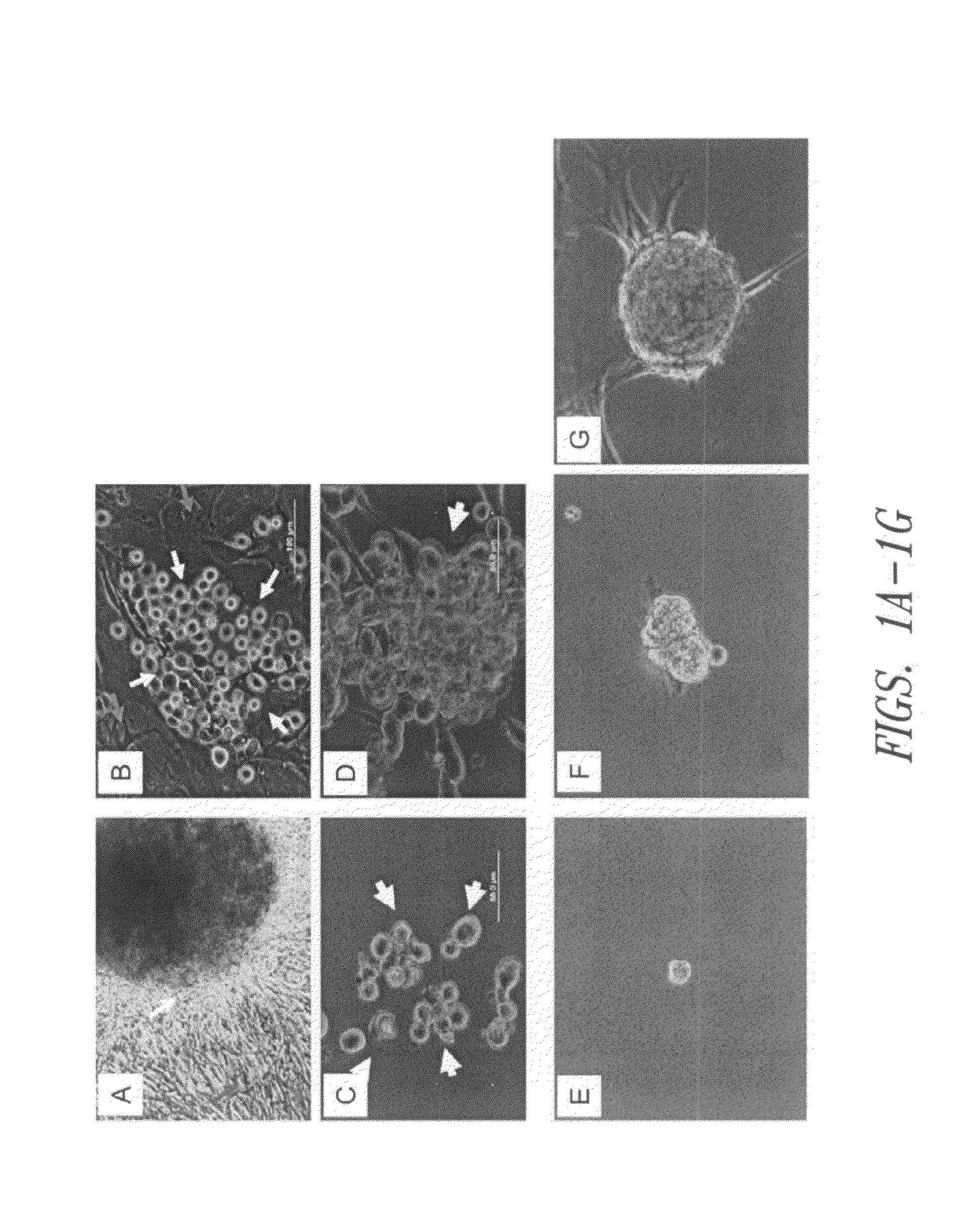
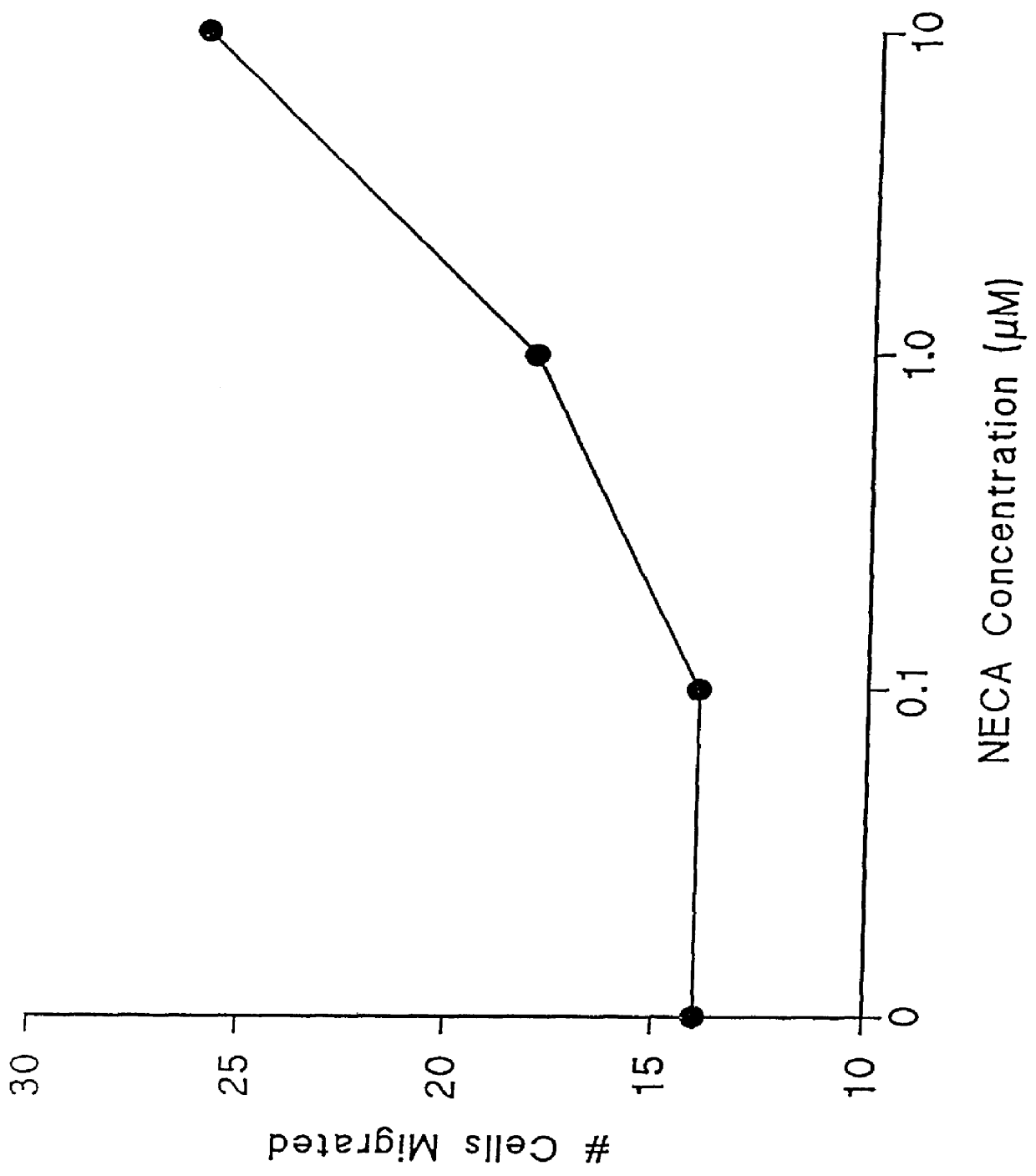
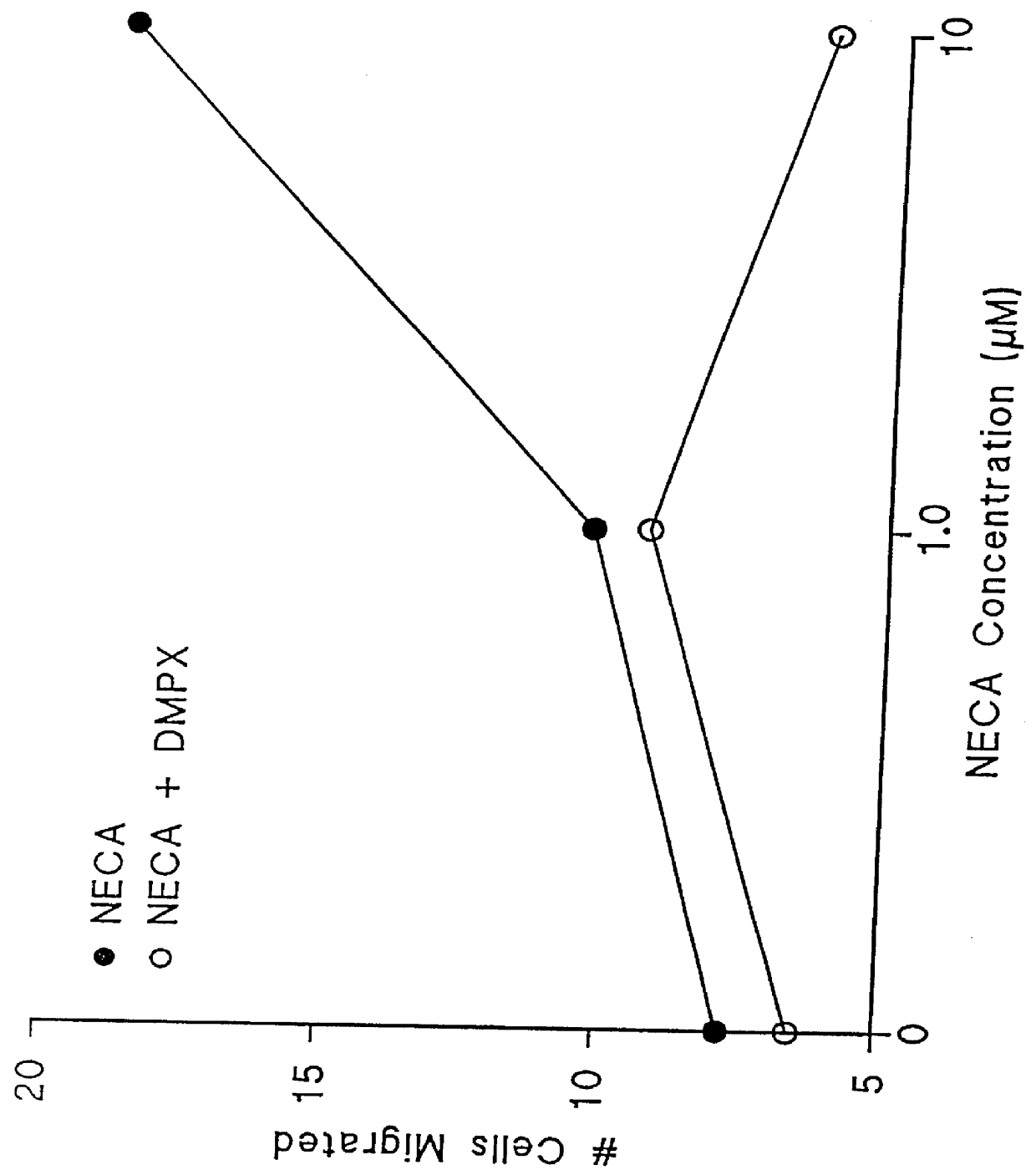
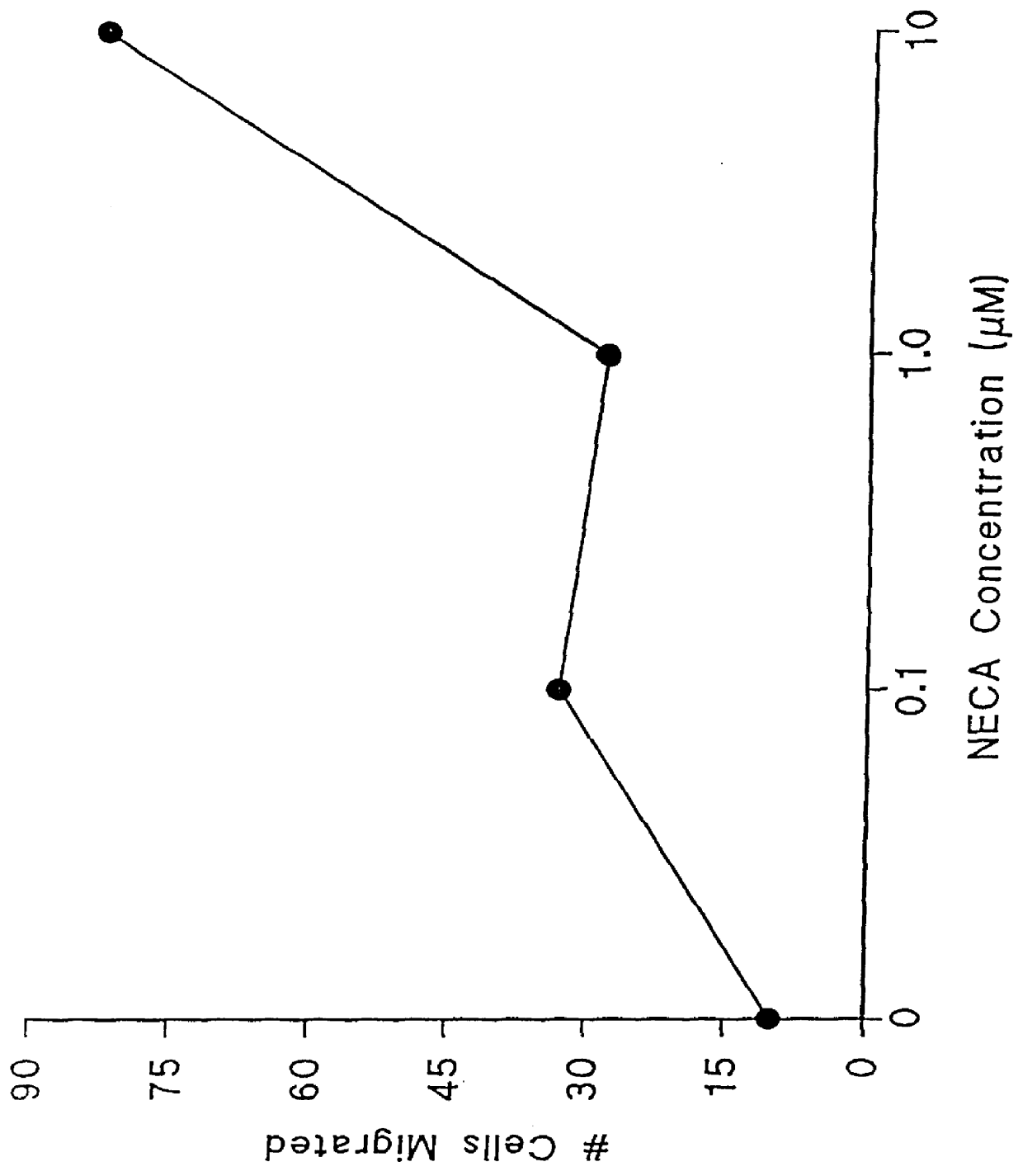
Adenosine receptor agonists for the promotion of wound healing
Agonists of the adenosine A2 receptor promote the migration of endothelial cells, fibroblasts and epithelial cells. Thus, methods and pharmaceutical compositions useful for treating wounds and promoting wound healing comprise agents which cause stimulation of the adenosine A2 receptor, preferably receptor agonists and adenosine uptake blockers. Preferred agonists include 2-phenylaminoadenosine, 2-para-2-carboxyethylphenyl-amino-5'N-ethylcarboxamidoadenosine, 5'N-ethylcarbox-amidoadenosine, 5'N-cyclo-propyladenosine, 5'N-methylcarboxamidoadenosine and PD-125944. Preferred uptake blockers include dipyridamole, nitrobenzylthio-inosine, dilazep and R75231.
Owner:NEW YORK UNIV
Blood-borne mesenchymal cells
InactiveUS6174526B1Promote wound healingBiocideMammal material medical ingredientsTissue remodelingSurface marker
The present invention relates to a population of blood borne mammalian cells that express a unique profile of surface markers that includes certain markers typical of connective tissue fibroblasts, and are referred to herein as "blood-borne mesenchymal cells." In particular, it relates to the isolation, characterization and uses of such blood-borne mesenchymal cells. The cells of the present invention can be distinguished from peripheral blood leukocytes by their distinct size, morphology, cell surface phenotype and biologic activities, and are likewise distinguishable from connective tissue fibroblasts by other surface phenotypic markers. These cells proliferate in culture, and in vivo, as demonstrated in animal models, are capable of migrating into wound sites from the blood. Therefore, such blood-borne mesenchymal cells may have a wide range of applications, including, but not limited to, the promotion of wound healing, tissue remodeling, and for gene therapy.
Owner:FERRING BV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com