Method of cloning reproductive and respiratory syndrome resisting pig
A blue-ear disease and pig fiber-forming technology, which is applied in the fields of animal genetic engineering and genetic modification to achieve the effect of low cost and shortened time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The construction of embodiment 1 porcine CD163 gene modification carrier
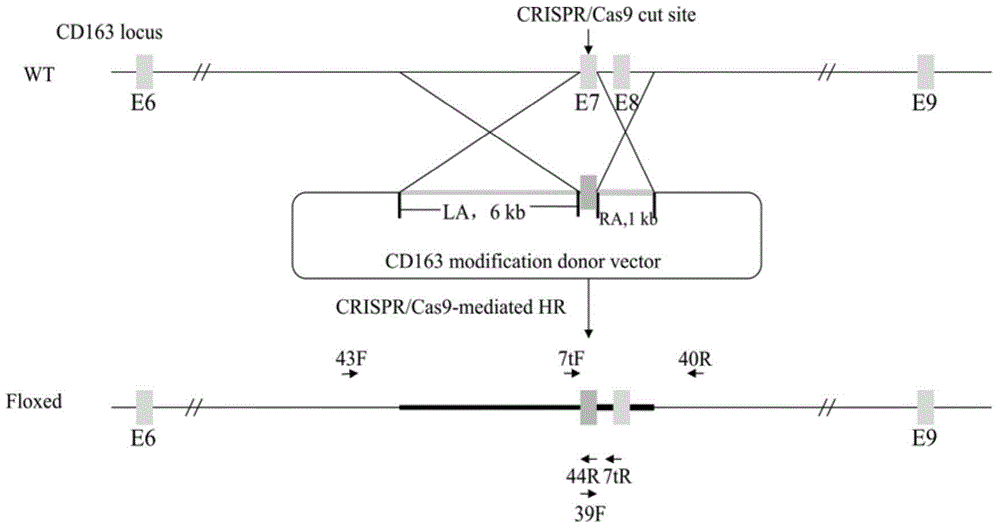
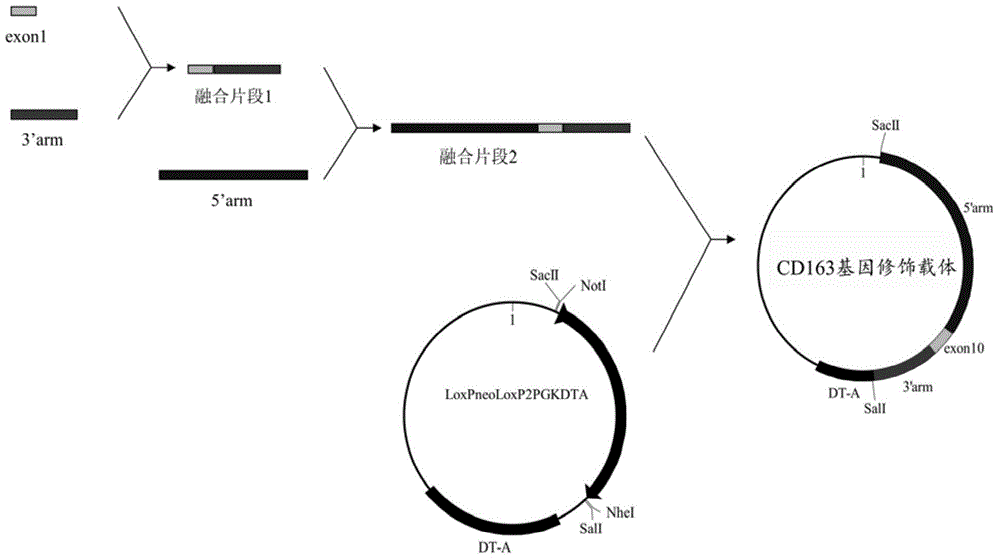
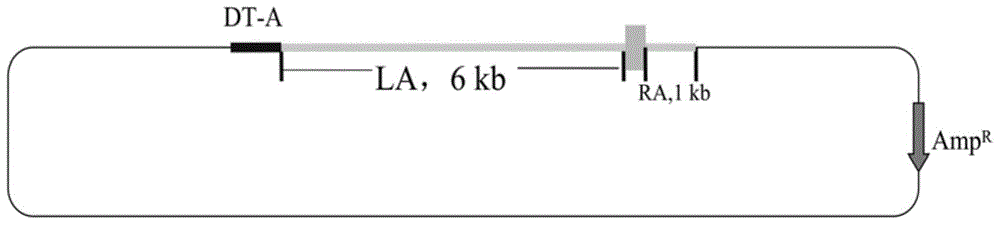
[0035] The vector construction process is divided into three steps, the process see figure 2 :
[0036] In the first step, exon 10 of human CD163-L1 (nucleotide sequence shown in SEQ ID NO.2) is fused with the homologous right arm. Using the genomic DNA of human cells as a template, exon 10 of human CD163L1 was amplified, and the primers used for the amplification were CD163L1-F5'-AAATGCTATTTTTCAGCCACAGGCAGCCCAGGCT-3' and CD163L1-R5'-CACATTCCCTGGGTCTCACGGGAACAGACAACTCCAACTT-3'. The PCR products were purified and sequenced to verify correctness. Using pig cell genomic DNA as a template, a 999bp homologous right arm (nucleotide sequence shown in SEQ ID NO.3) was amplified, and the primers were RIGHT-F5'-AAGTTGGAGTTGTCTGTTCCCGTGAGACCCAGGGAATGTG-3' and RIGHT-R 5'- TAT GTC GAC AGTGTTAGATAGATGTGCTC-3', the underline is the SalⅠ restriction site, and the sequence verification is correct. The exon...
Embodiment 2
[0039] Example 2 Construction of CRISPR-Cas9 Targeting Vector
[0040] 1. Use the website of Zhang Feng Laboratory (http: / / crispr.genome-engineering.org / ) to predict the targeting site of exon 7 of the porcine CD163 gene. According to the scores in the self-assessment and prediction results, two candidate target sites were selected, named 501 and 502, and their sgRNA sequences were GGAACTACAGTGCGGCACTG and ACTTCAACACGACCAGAGCA, respectively. Complementary paired oligonucleotides were synthesized according to the sgRNA sequence, as shown in Table 1, where the lowercase letters are restriction sites.
[0041] Table 1 Oligonucleotide sequence
[0042] name
Sequence (5'-3')
px330-501F
caccgGGAACTACAGTGCGGCACTG (SEQ ID NO. 5)
px330-501R
aaacCAGTGCCGCACTGTAGTTCCc (SEQ ID NO. 6)
px330-502F
caccgACTTCAACACGACCAGAGCA (SEQ ID NO. 7)
px330-502R
aaacTGCTCTGGTCGTGTTGAAGTc (SEQ ID NO. 8)
[0043] 2. A total of two targeting v...
Embodiment 3
[0052] Example 3 Screening and Identification of Positive Cell Monoclonal
[0053] 1. Screening of positive monoclonal cells
[0054] Digest and collect porcine fibroblasts in one well of a 6-well cell culture plate (approximately 1×10 6 1), the targeting vector px330-501 constructed in Example 2 and the porcine CD163 gene homologous recombination modification vector constructed in Example 1 were mixed according to the ratio of the amount of substances 1:1, and the total mass was 4 μg, according to Example 2 After transfection by the method in step 5, put into CO 2 In the incubator, cultivate at 37.5°C. After 48 hours, the confluence of the cells reached 80-90%. At this time, the cells in one well were evenly divided into eight 10cm culture dishes. After 24 hours, the cells adhered to the wall, and the medium was replaced with fibroblast medium (10% FBS+DMEM) containing G418 (600 μg / mL), and the medium was changed every 3 to 4 days. ) fibroblast culture medium. After the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com