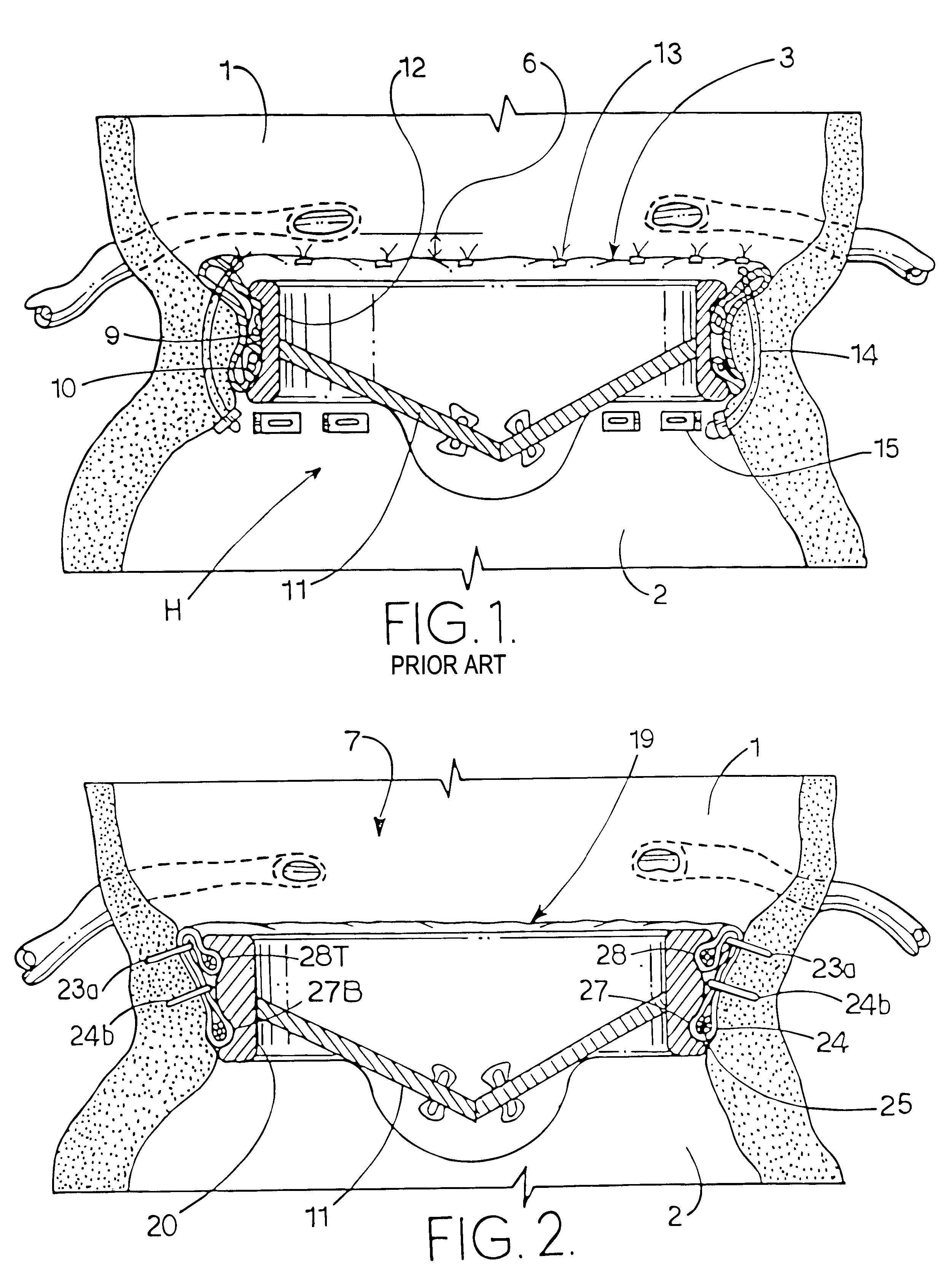
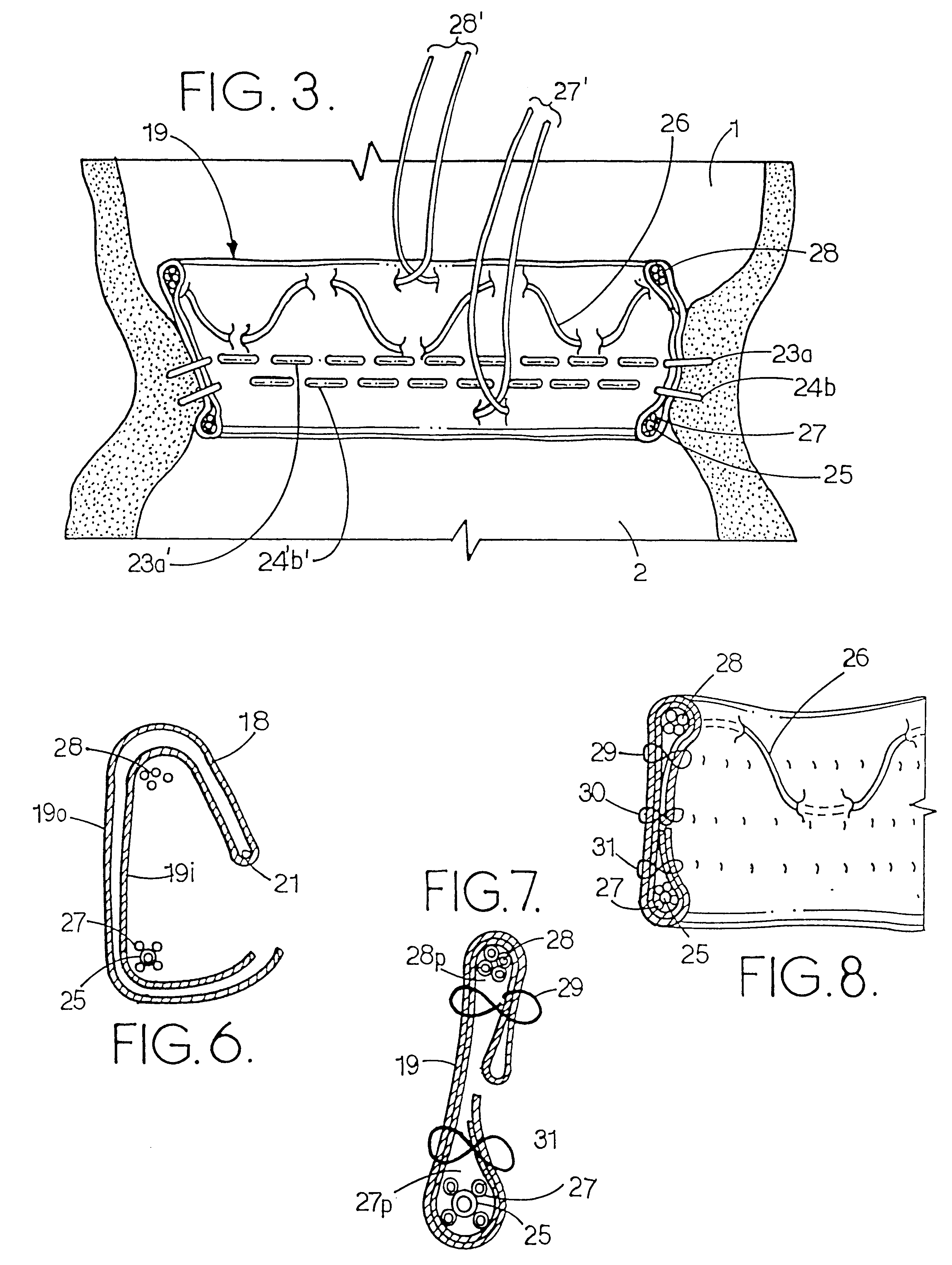
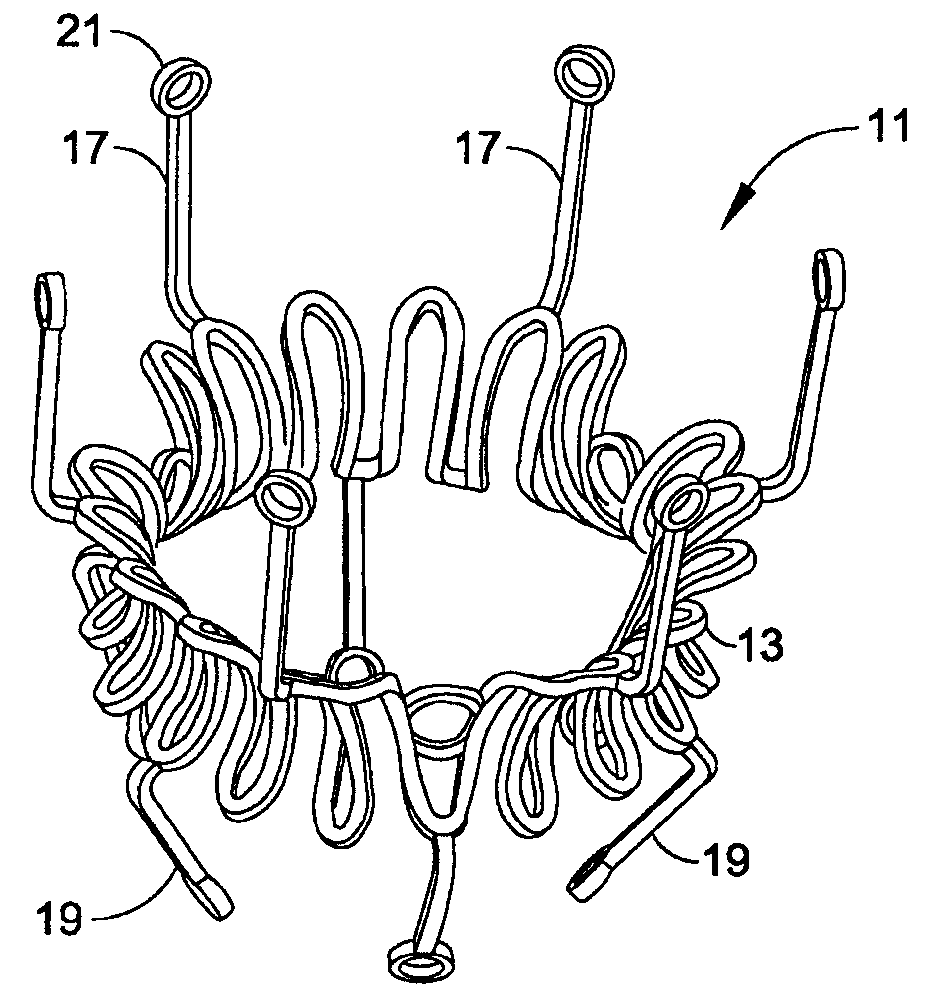
Patents
Literature
12004results about "Blood vessels" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
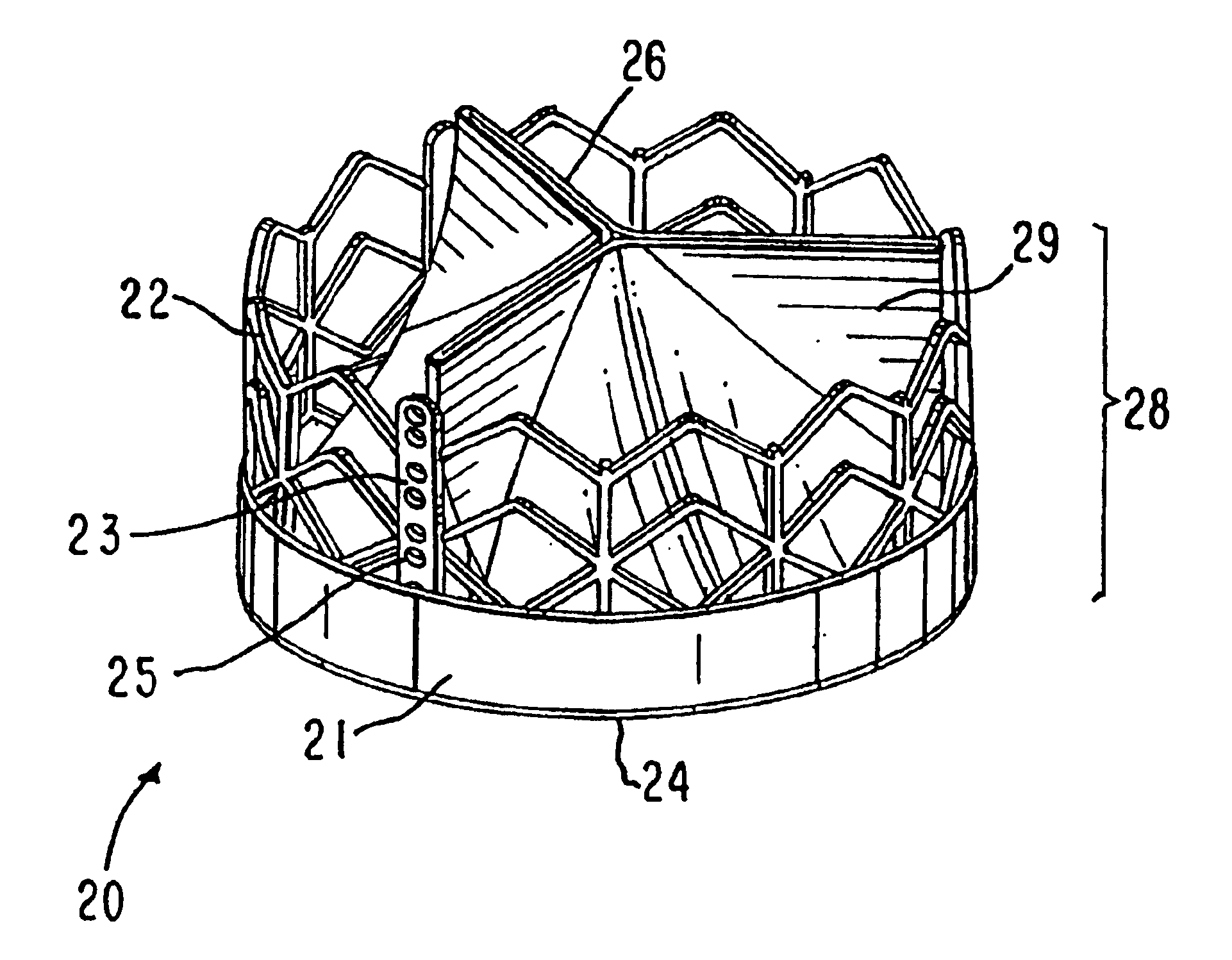
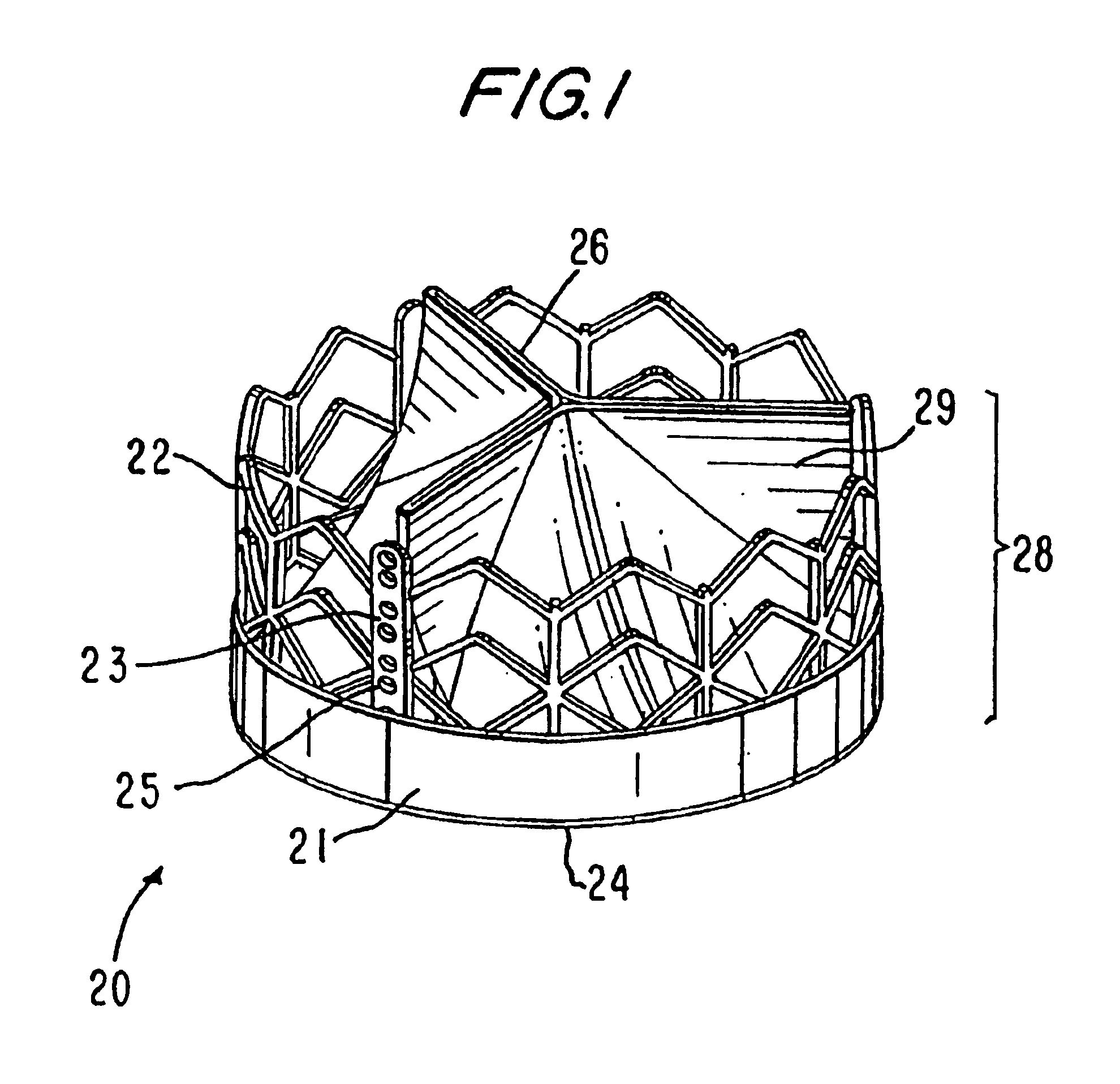
Porous tissue scaffoldings for the repair of regeneration of tissue
InactiveUS6333029B1Promote growthPromote regenerationPeptide/protein ingredientsAntipyreticRepair tissueOpen cell
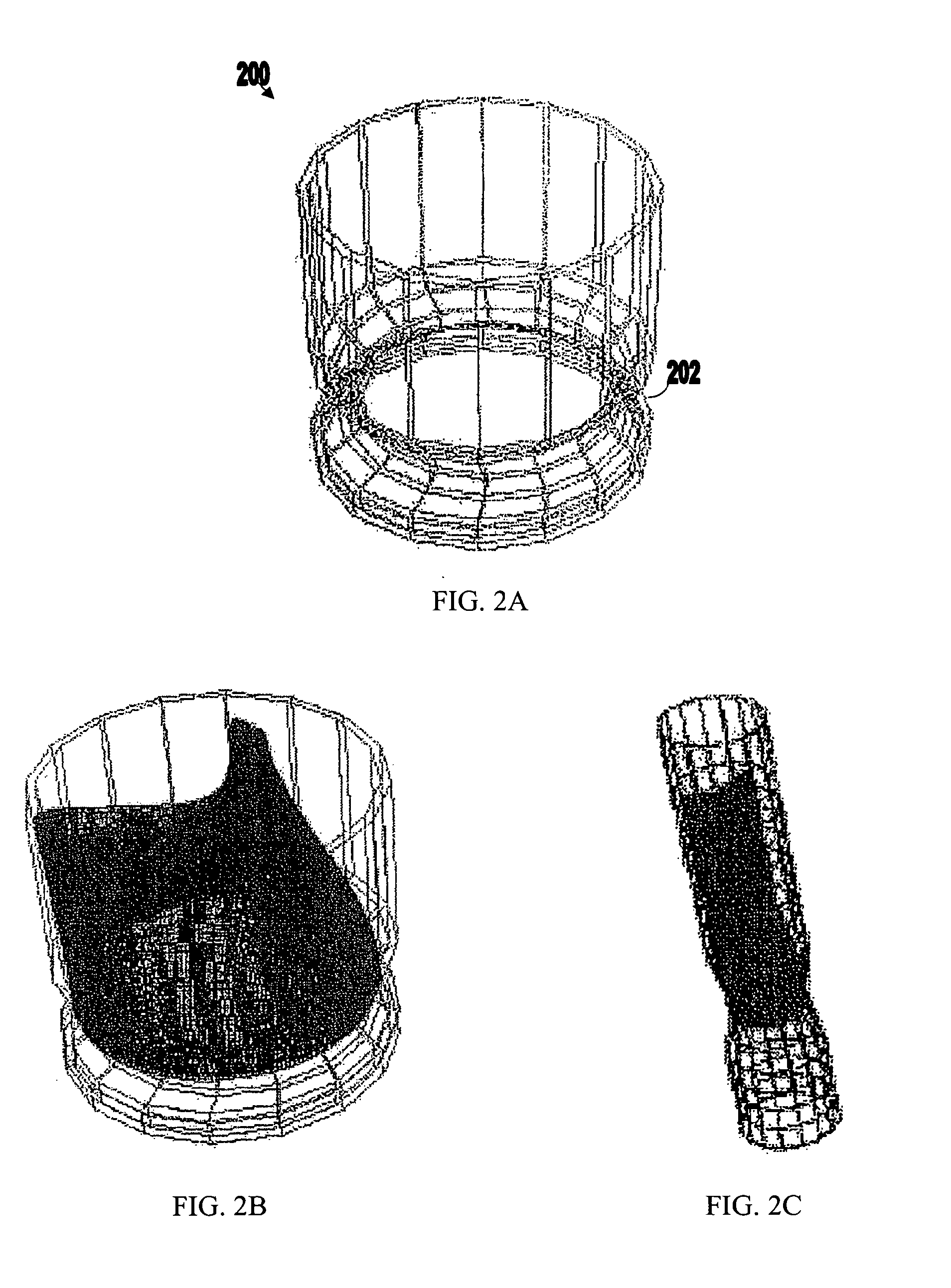
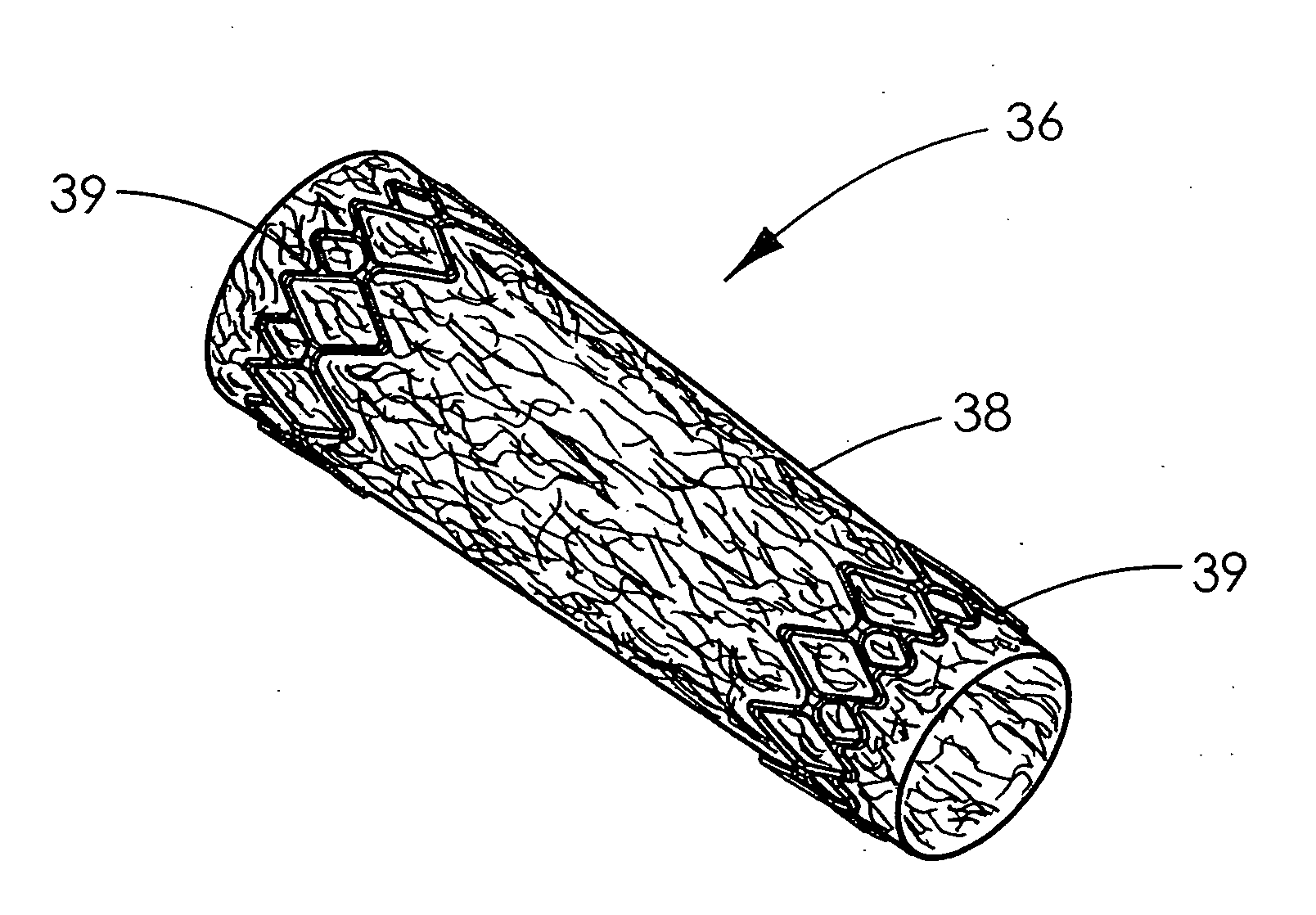
The present patent describes a three-dimensional inter-connected open cell porous foams that have a gradient in composition and / or microstructure through one or more directions. These foams can be made from a blend of absorbable and biocompatible polymers that are formed into foams having a compositional gradient transitioning from predominately one polymeric material to predominately a second polymeric material. These gradient foams are particularly well suited to tissue engineering applications and can be designed to mimic tissue transition or interface zones.
Owner:ETHICON ENDO SURGERY INC
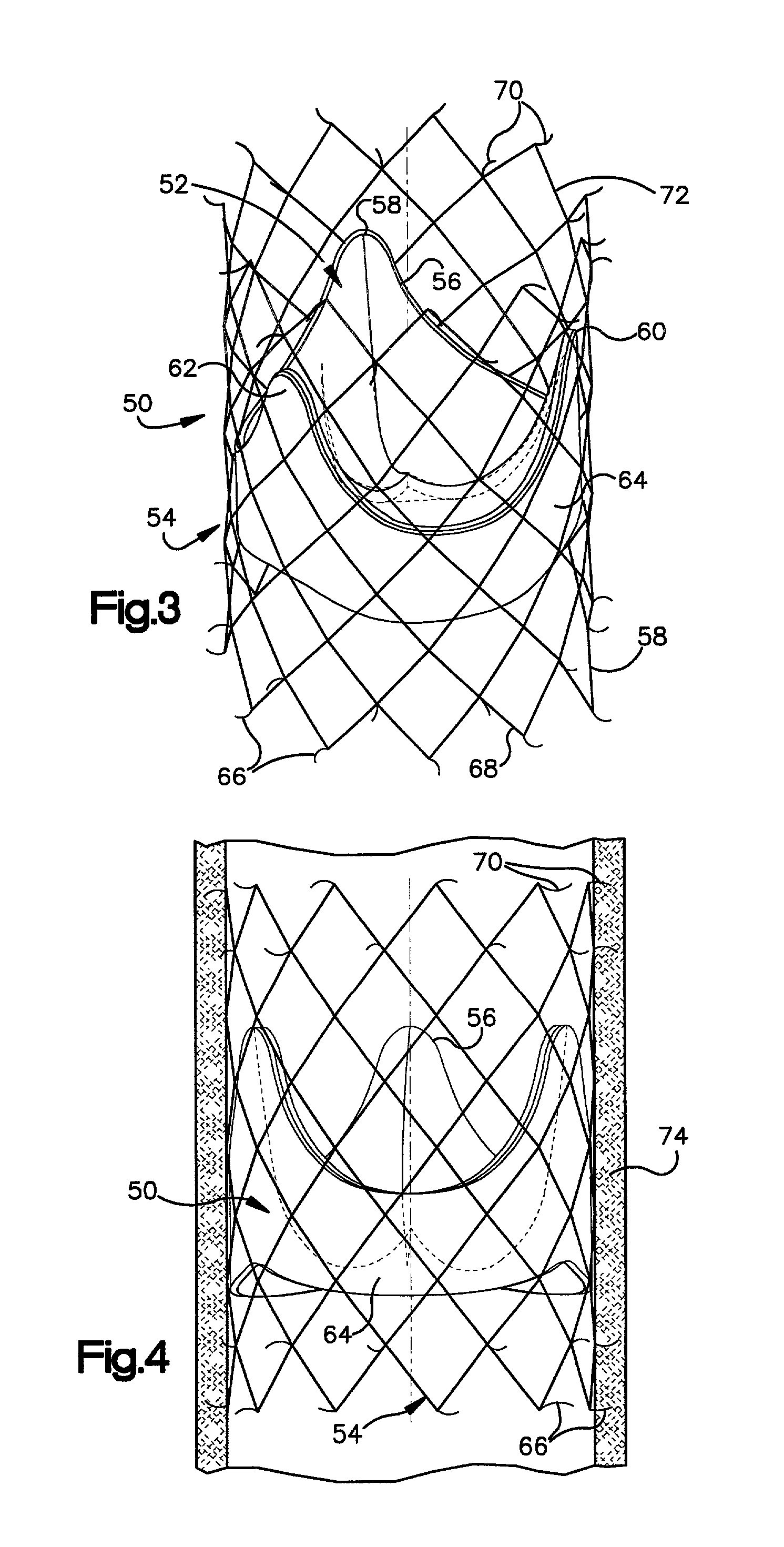
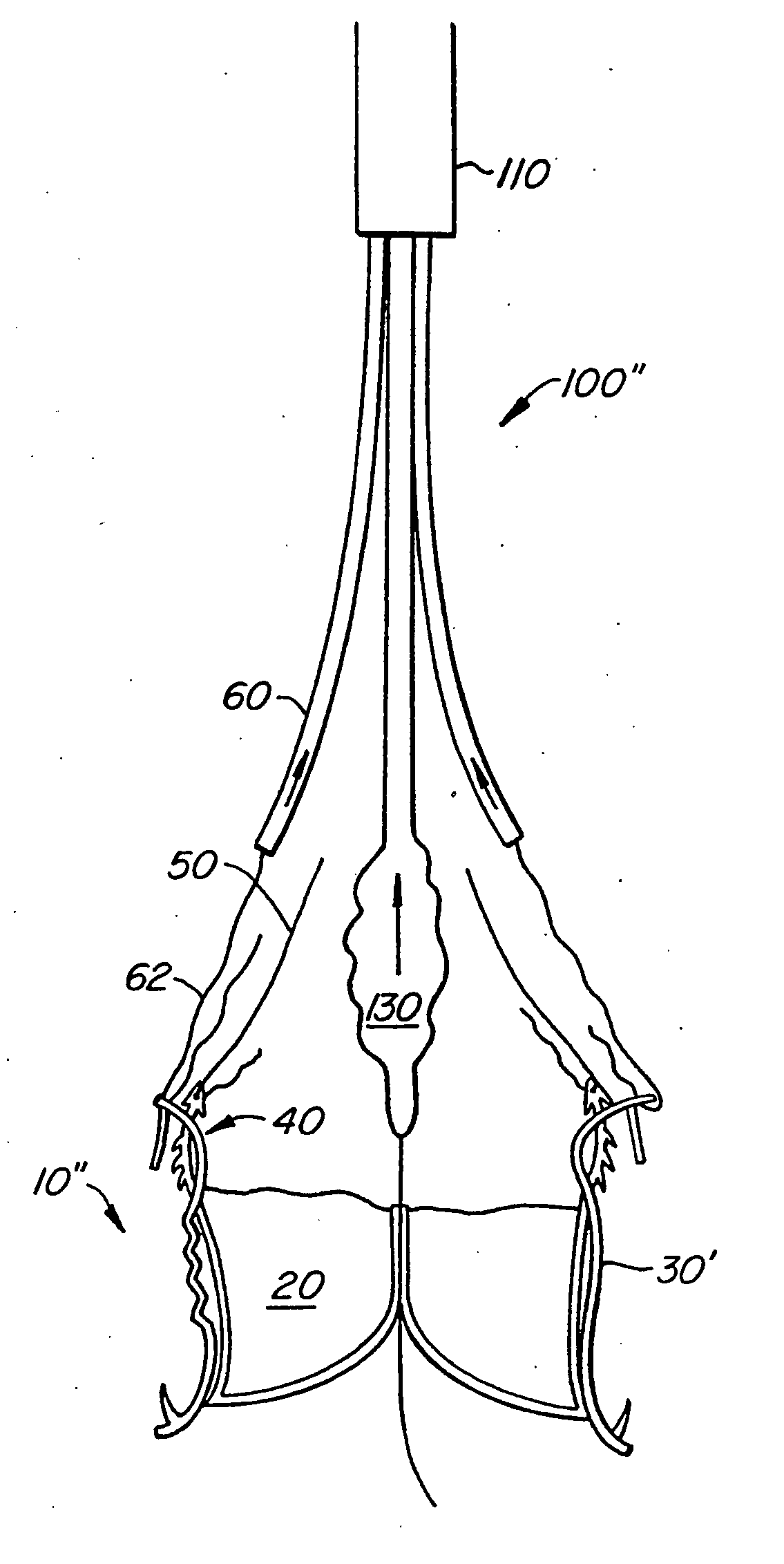
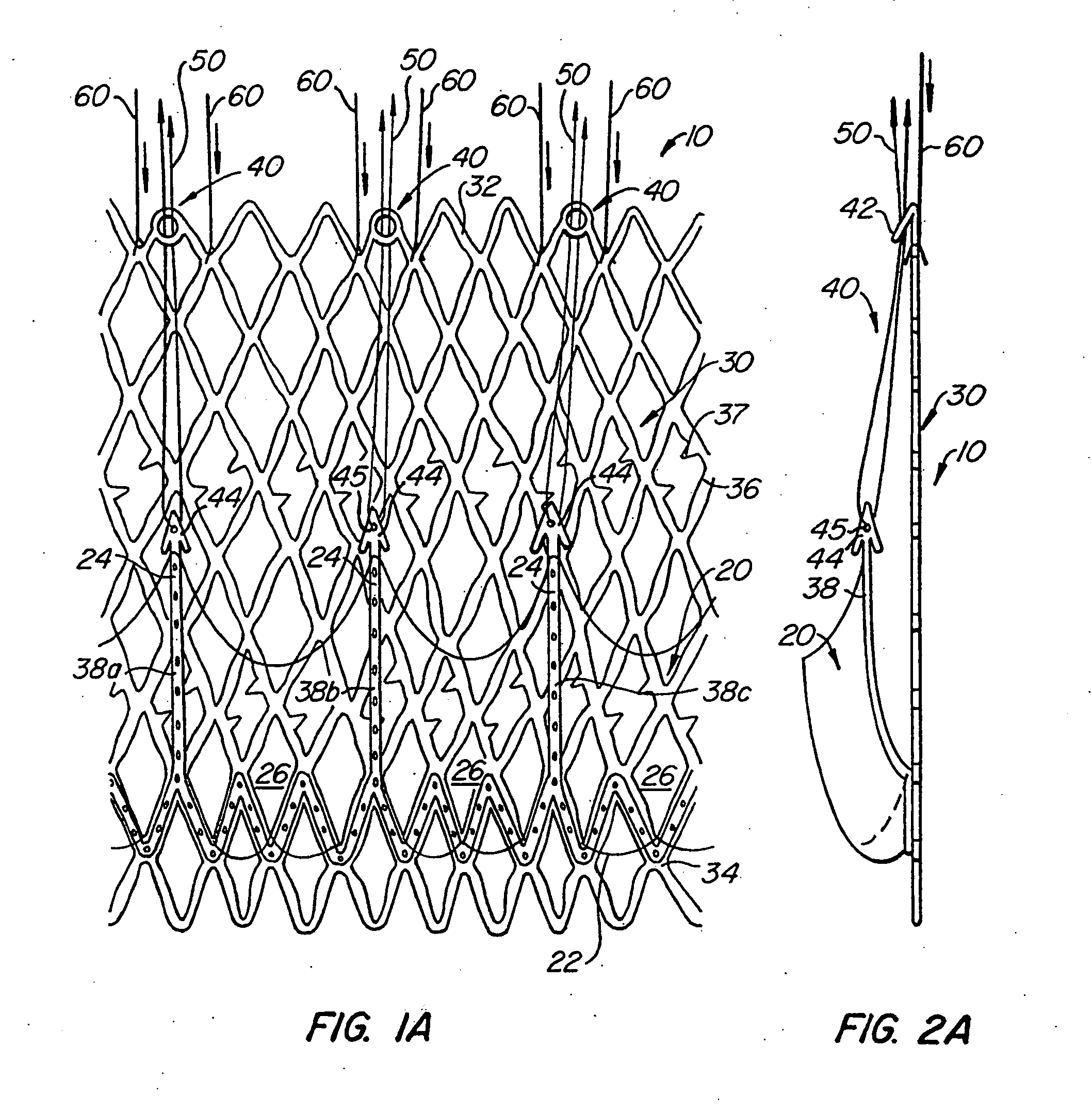
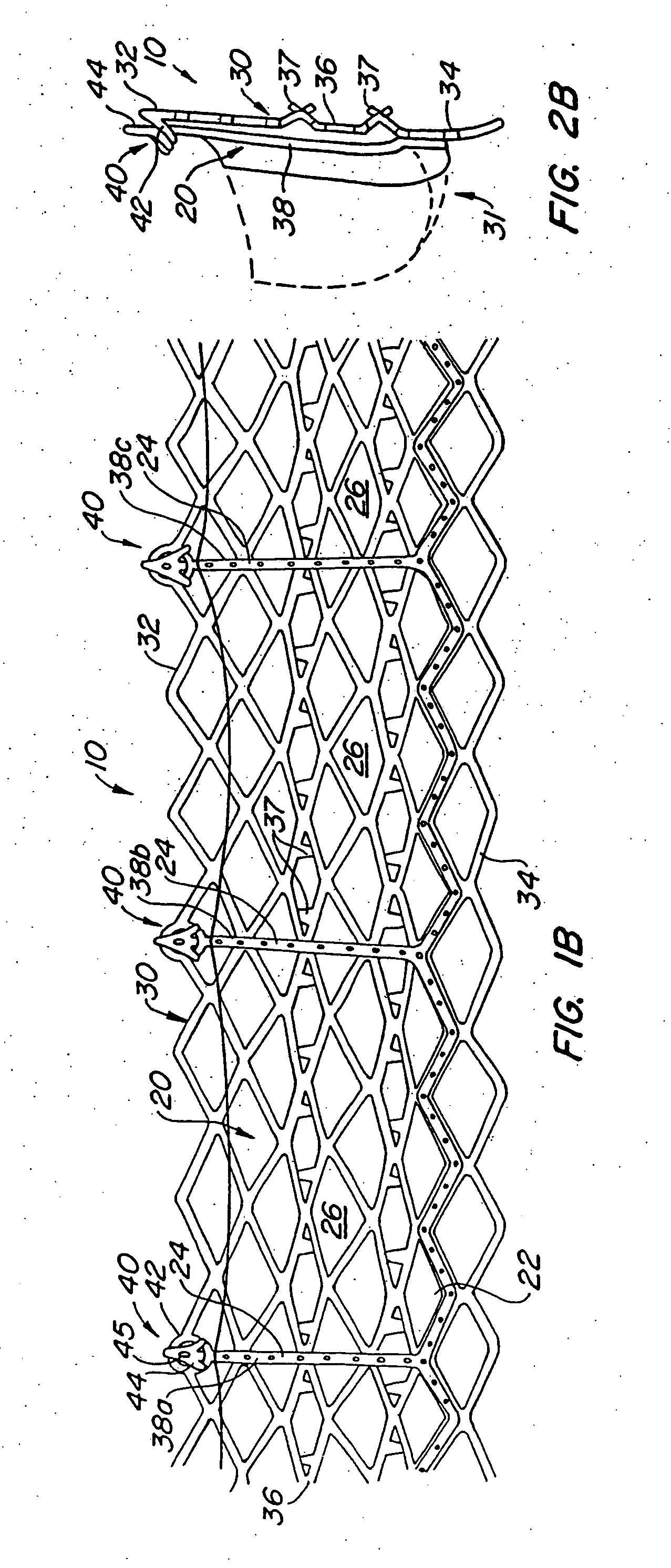
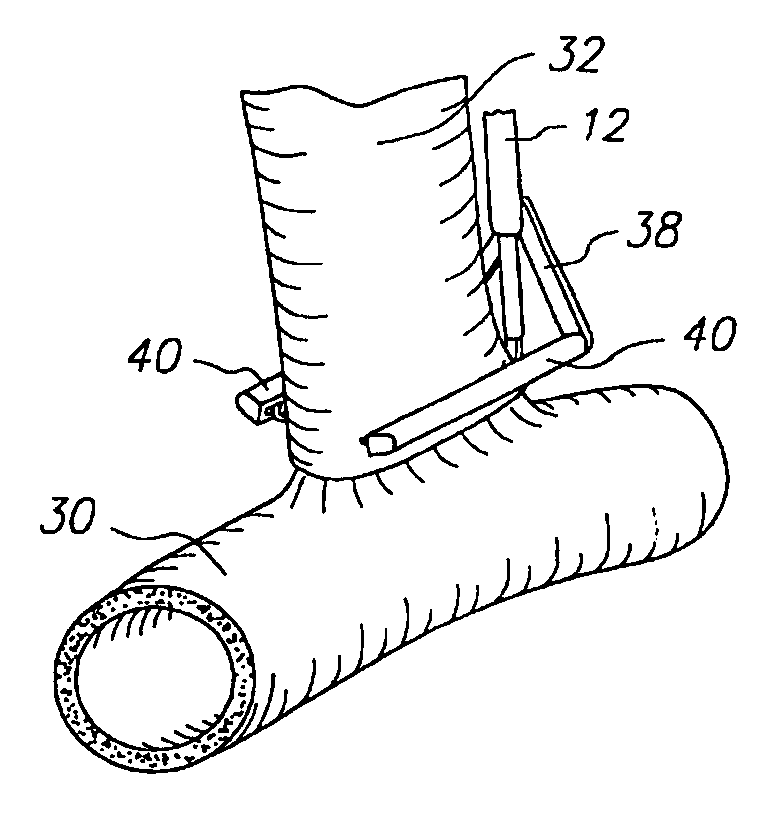
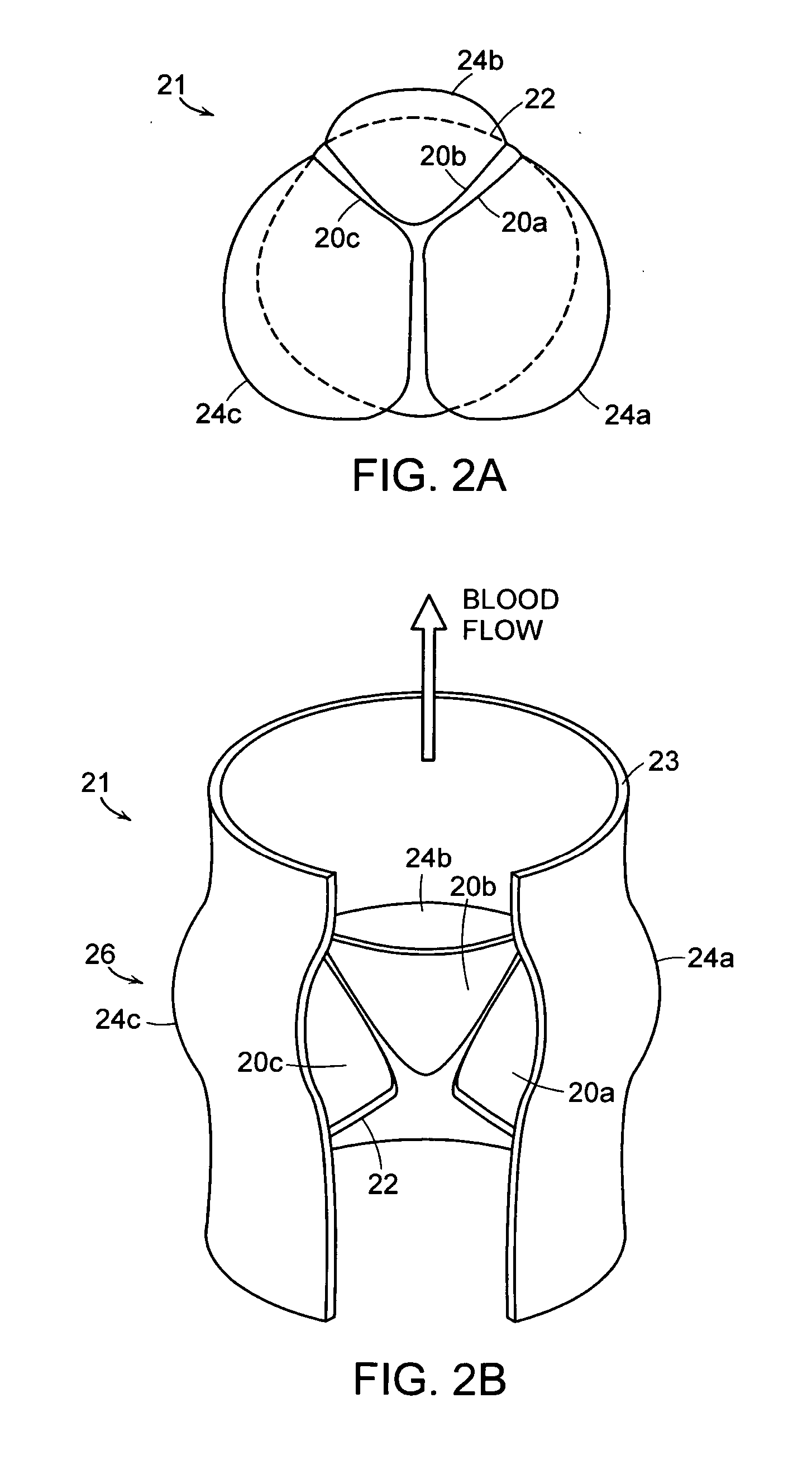
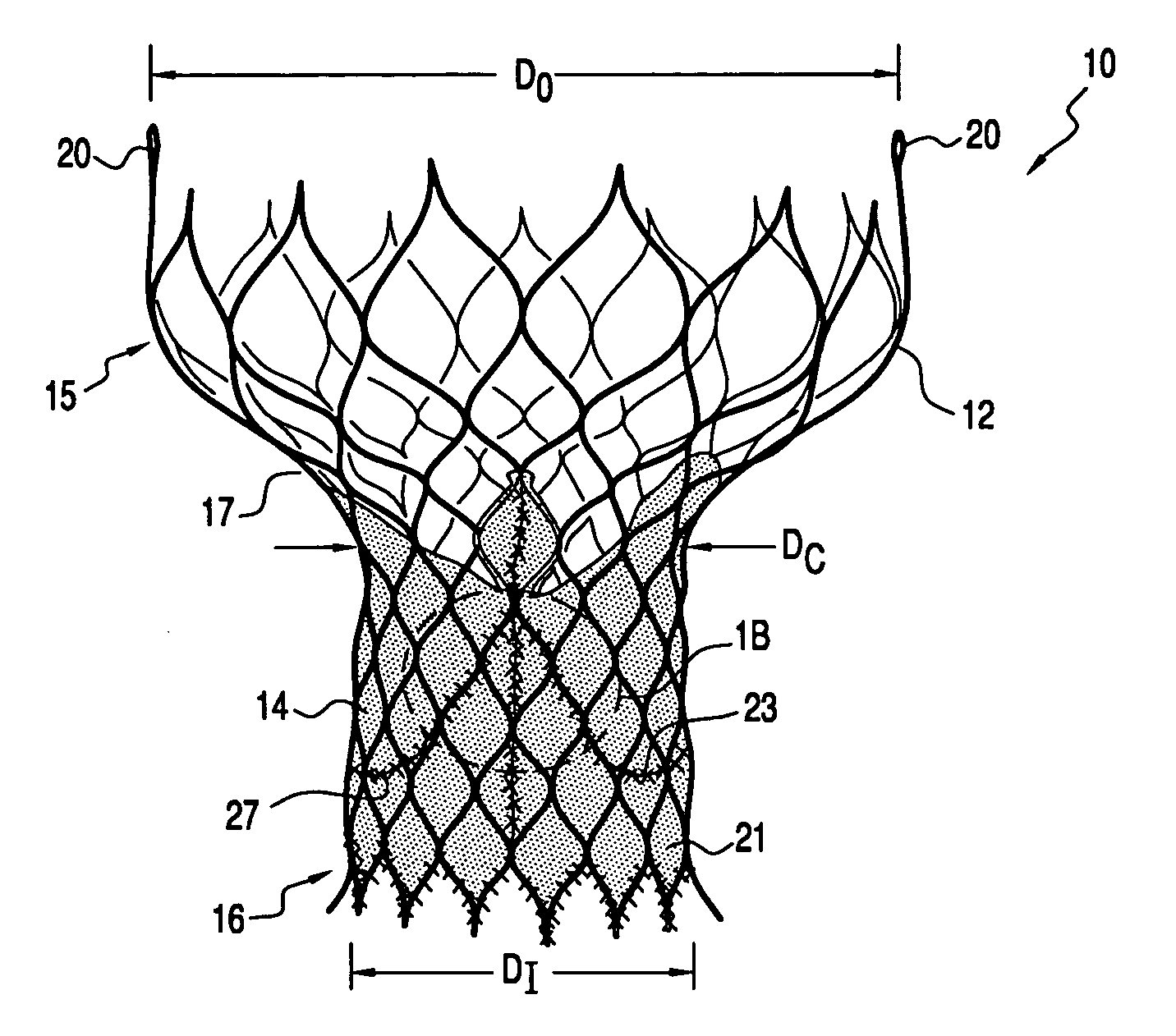
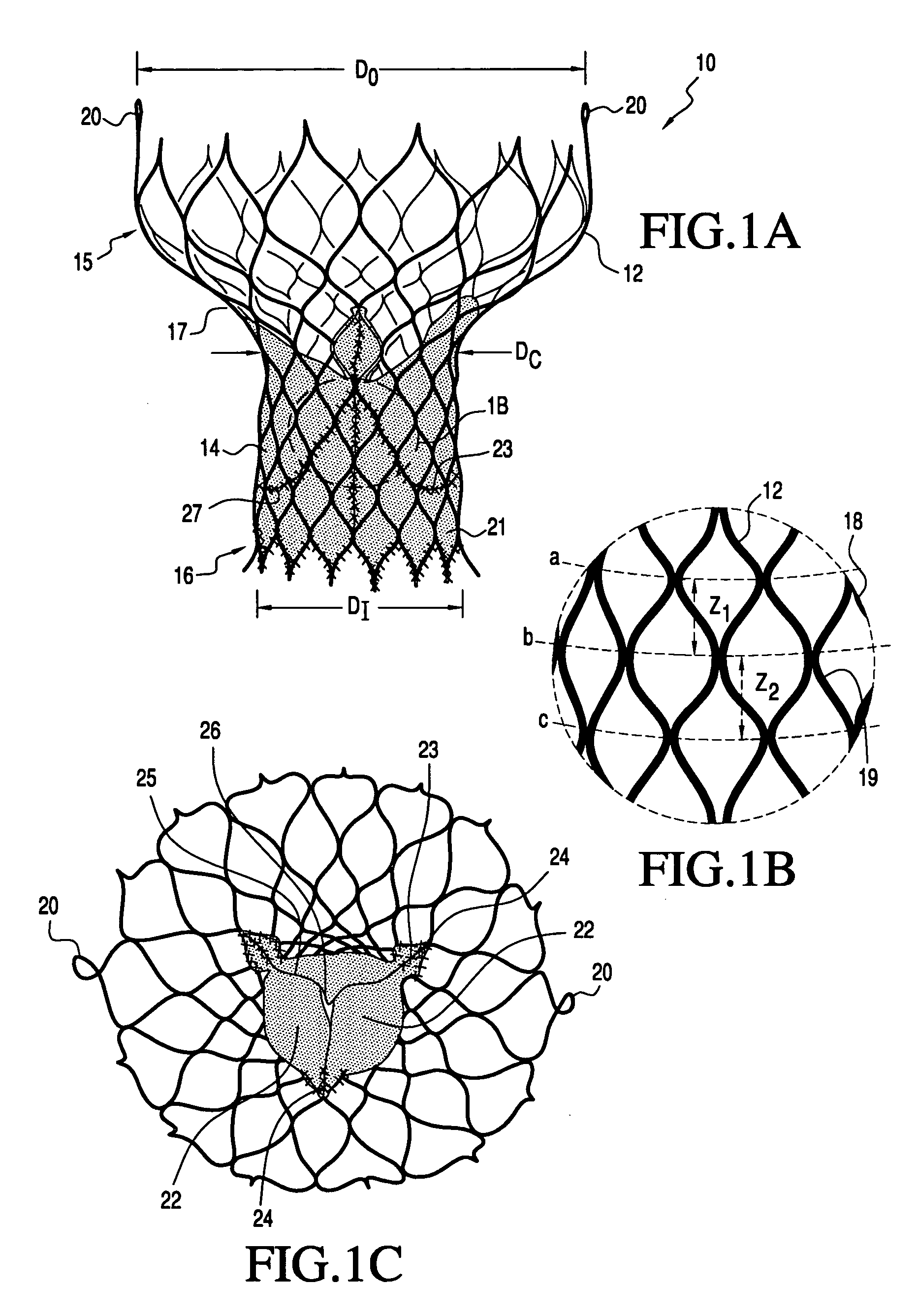
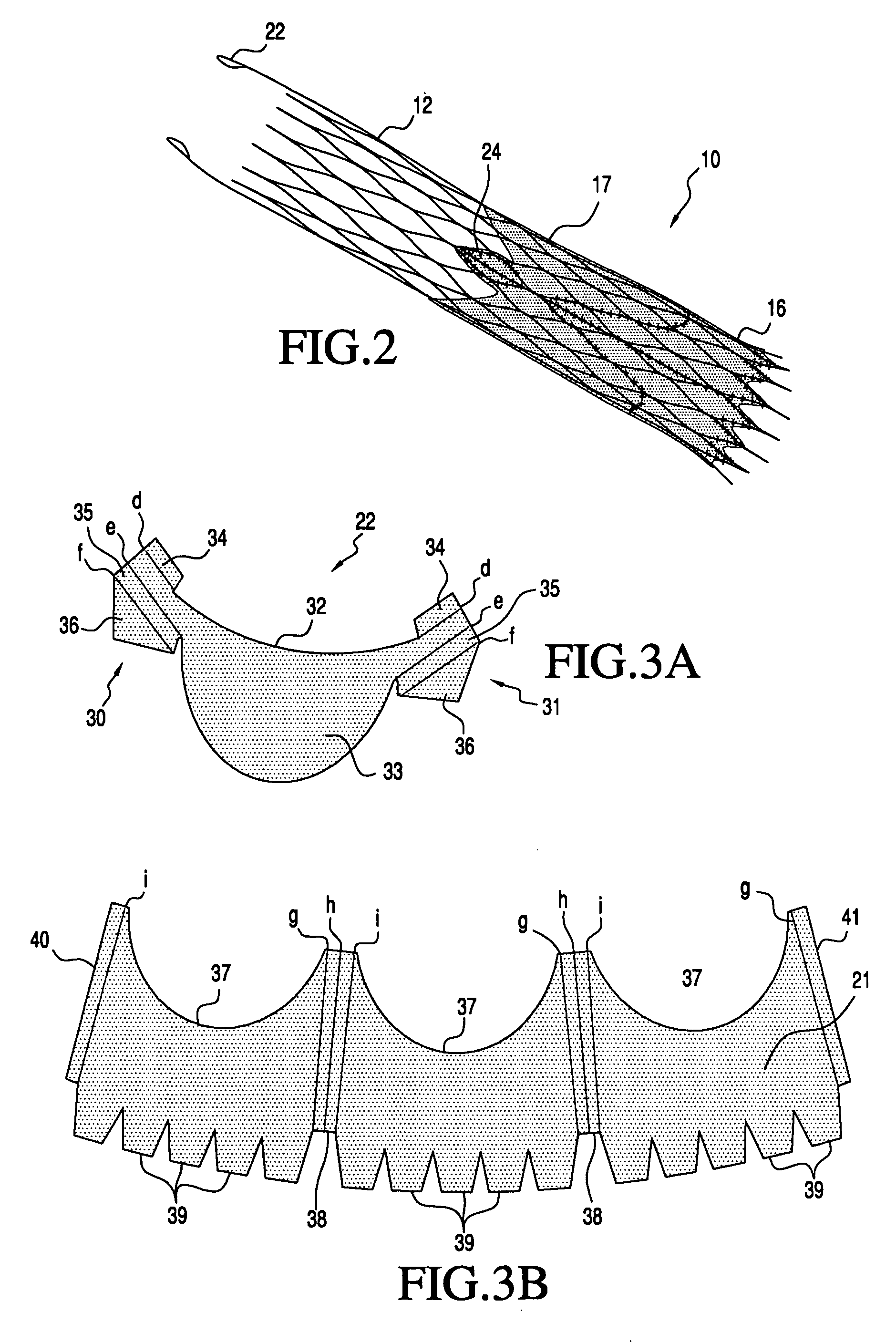
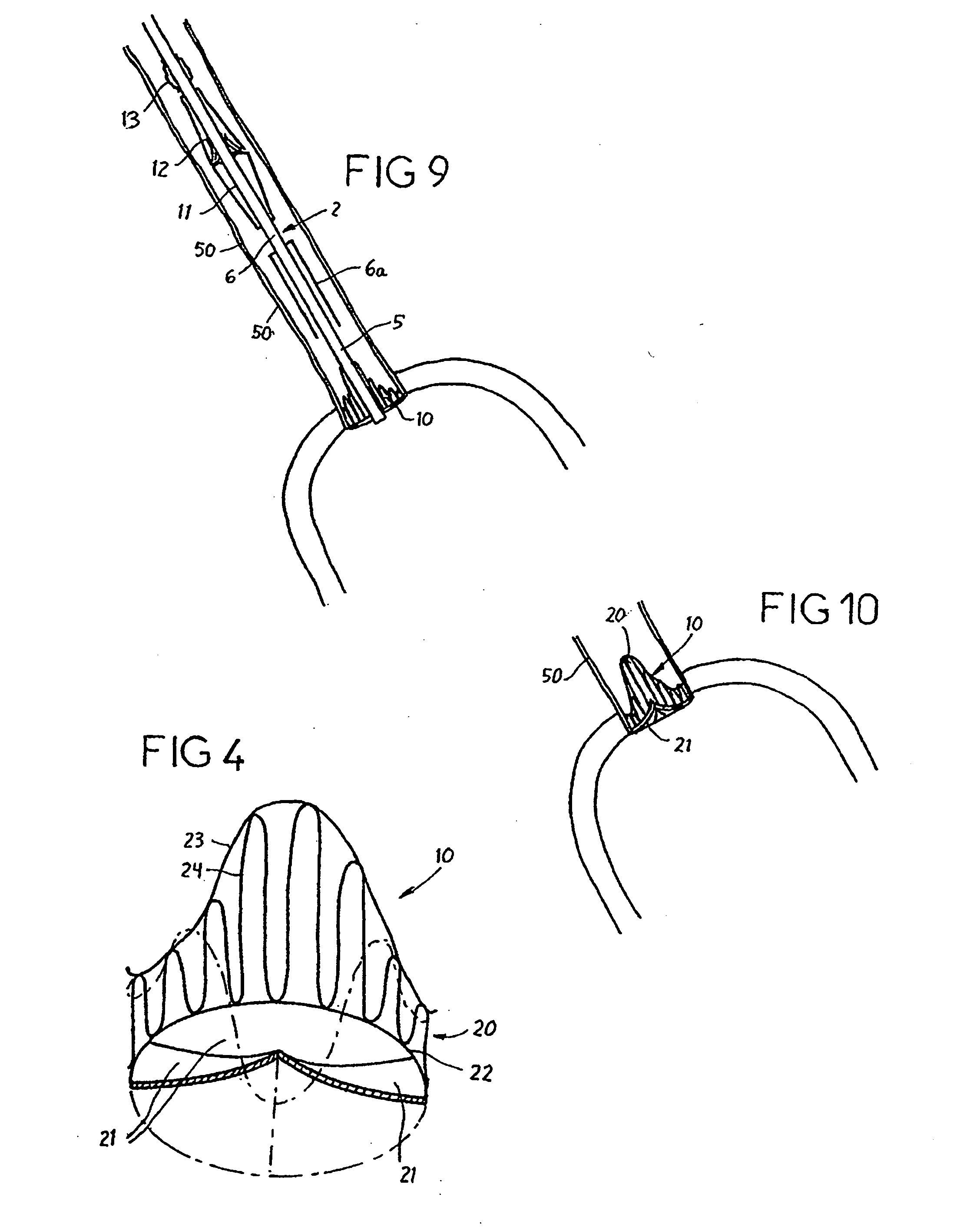
Prosthetic valve for transluminal delivery
InactiveUS7018406B2Preventing substantial migrationEliminate the problemBalloon catheterHeart valvesProsthesisCommissure
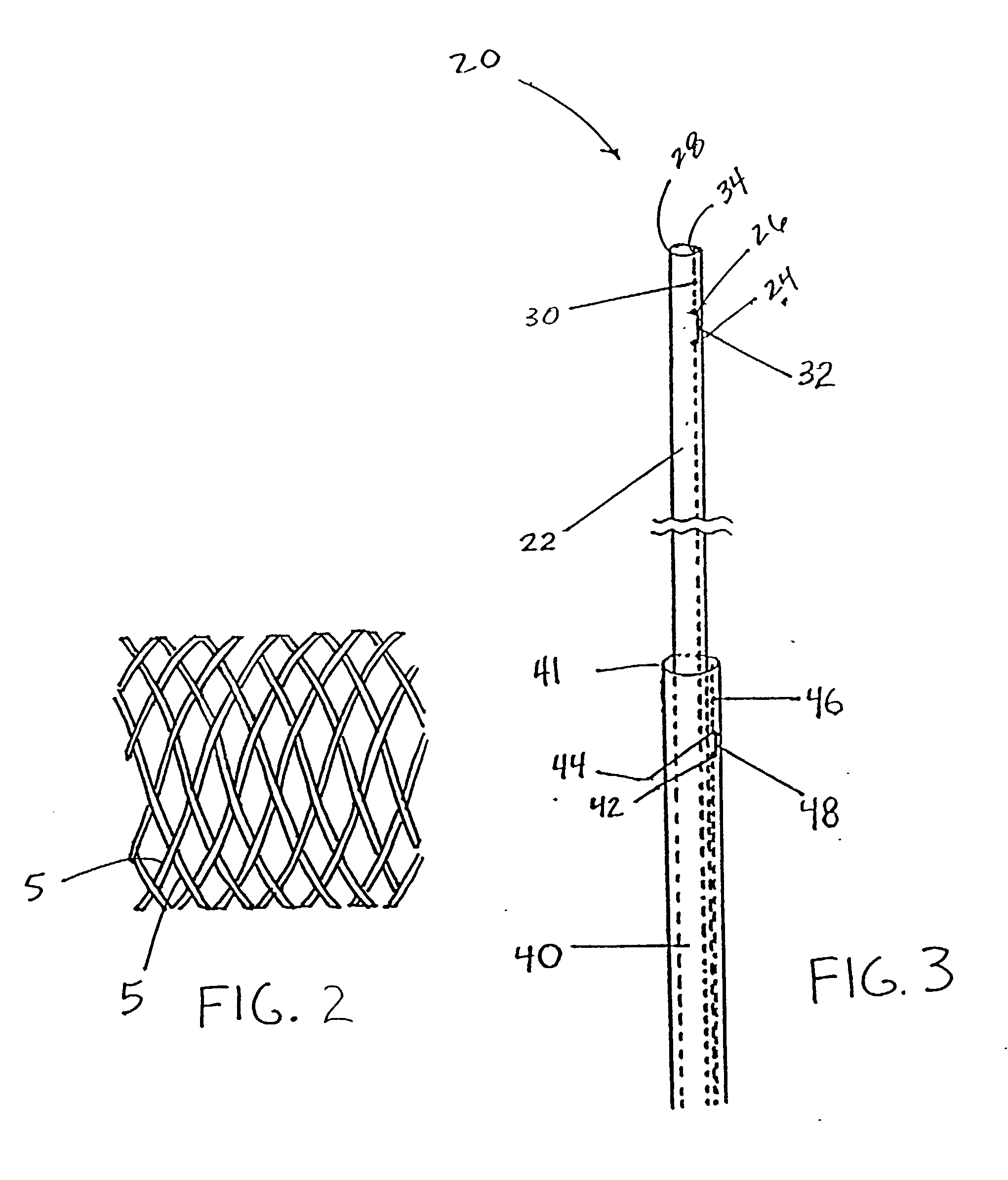
A prosthetic valve assembly for use in replacing a deficient native valve comprises a replacement valve supported on an expandable valve support. If desired, one or more anchor may be used. The valve support, which entirely supports the valve annulus, valve leaflets, and valve commissure points, is configured to be collapsible for transluminal delivery and expandable to contact the anatomical annulus of the native valve when the assembly is properly positioned. The anchor engages the lumen wall when expanded and prevents substantial migration of the valve assembly when positioned in place. The prosthetic valve assembly is compressible about a catheter, and restrained from expanding by an outer sheath. The catheter may be inserted inside a lumen within the body, such as the femoral artery, and delivered to a desired location, such as the heart. When the outer sheath is retracted, the prosthetic valve assembly expands to an expanded position such that the valve and valve support expand within the deficient native valve, and the anchor engages the lumen wall.
Owner:MEDTRONIC COREVALVE
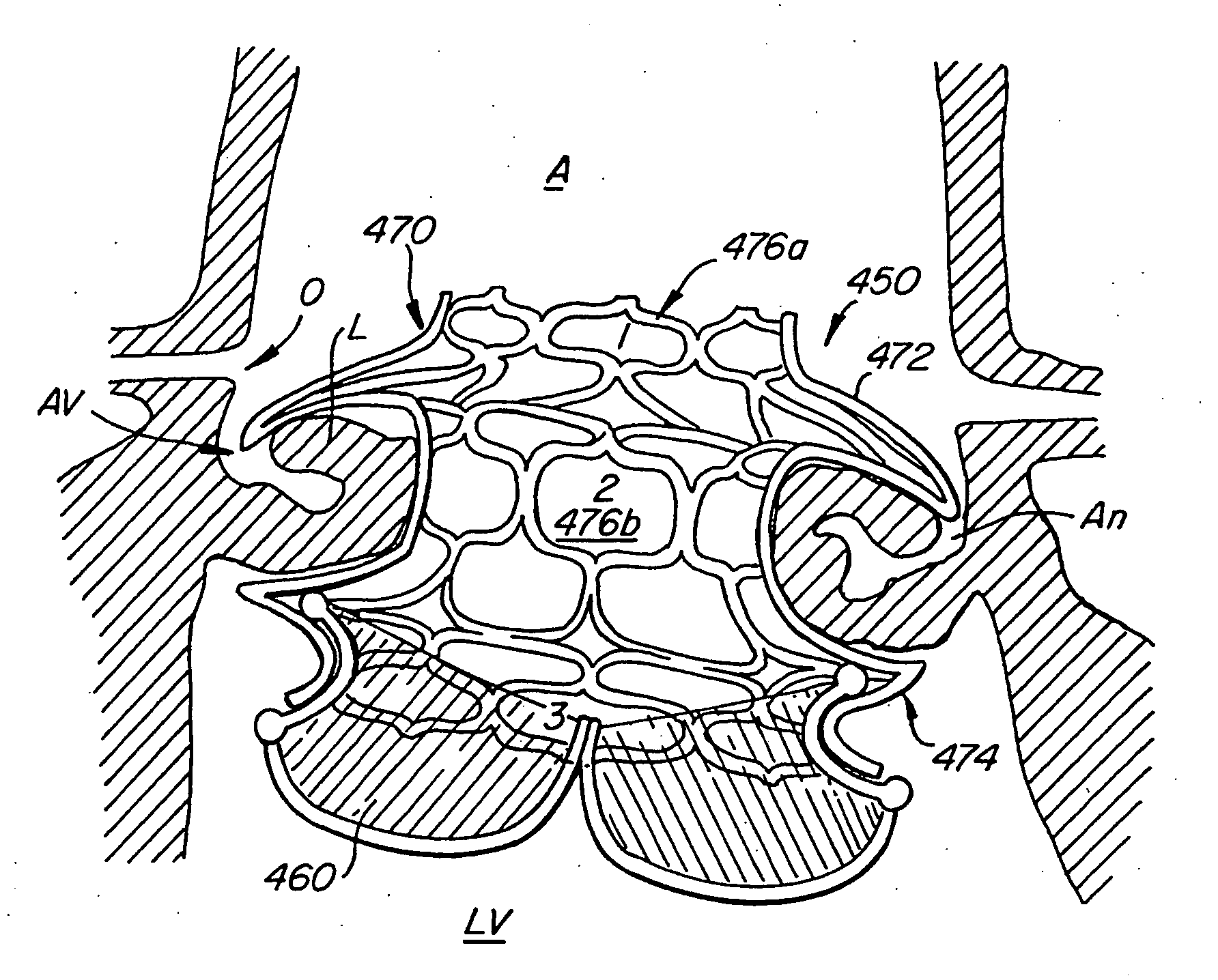
Heart valve prosthesis and sutureless implantation of a heart valve prosthesis
A heart valve prosthesis and method of implanting the prosthesis are disclosed. A valve is mounted within a support apparatus that is deformable between a first condition and a second condition. The prosthesis has a cross-sectional dimension in the second condition that is less than a cross-sectional dimension of the supported valve when in first condition. The prosthesis can be implanted into a patient's heart, such as during a direct vision procedure through a tubular implantation apparatus that maintains the prosthesis in its second condition until discharged from the tubular apparatus.
Owner:EDWARDS LIFESCI CARDIAQ
Implantable prosthetic valve
InactiveUS6893460B2Prevent blood flowImprove sealingStentsBalloon catheterGuide tubeBiomedical engineering
A valve prosthesis device is disclosed suitable for implantation in body ducts. The device comprises support stent, comprised of a deployable construction adapted to be initially crimped in a narrow configuration suitable for catheterization through the body duct to a target location and adapted to be deployed by exerting substantially radial forces from within by means of a deployment device to a deployed state in the target location, the support stent provided with a plurality of longitudinally rigid support beams of fixed length; valve assembly comprising a flexible conduit having an inlet end and an outlet, made of pliant material attached to the support beams providing collapsible slack portions of the conduit at the outlet. When flow is allowed to pass through the valve prosthesis device from the inlet to the outlet the valve assembly is kept in an open position, whereas a reverse flow is prevented as the collapsible slack portions of the valve assembly collapse inwardly providing blockage to the reverse flow.
Owner:EDWARDS LIFESCI PVT
Value prosthesis for implantation in body channels
A valve prosthesis which is especially useful in the case of aortic stenosis and capable of resisting the powerful recoil force and to stand the forceful balloon inflation performed to deploy the valve and to embed it in the aortic annulus, comprises a collapsible valvular structure and an expandable frame on which said valvular structure is mounted. The valvular structure is composed of physiologically compatible valvular tissue that is sufficiently supple and resistant to allow the valvular structure to be deformed from a closed state to an opened state. The valvular tissue forms a continuous surface and is provided with strut members that create stiffened zones which induce the valvular structure to follow a patterned movement in its expansion to its opened state and in its turning back to its closed state.
Owner:EDWARDS LIFESCI PVT
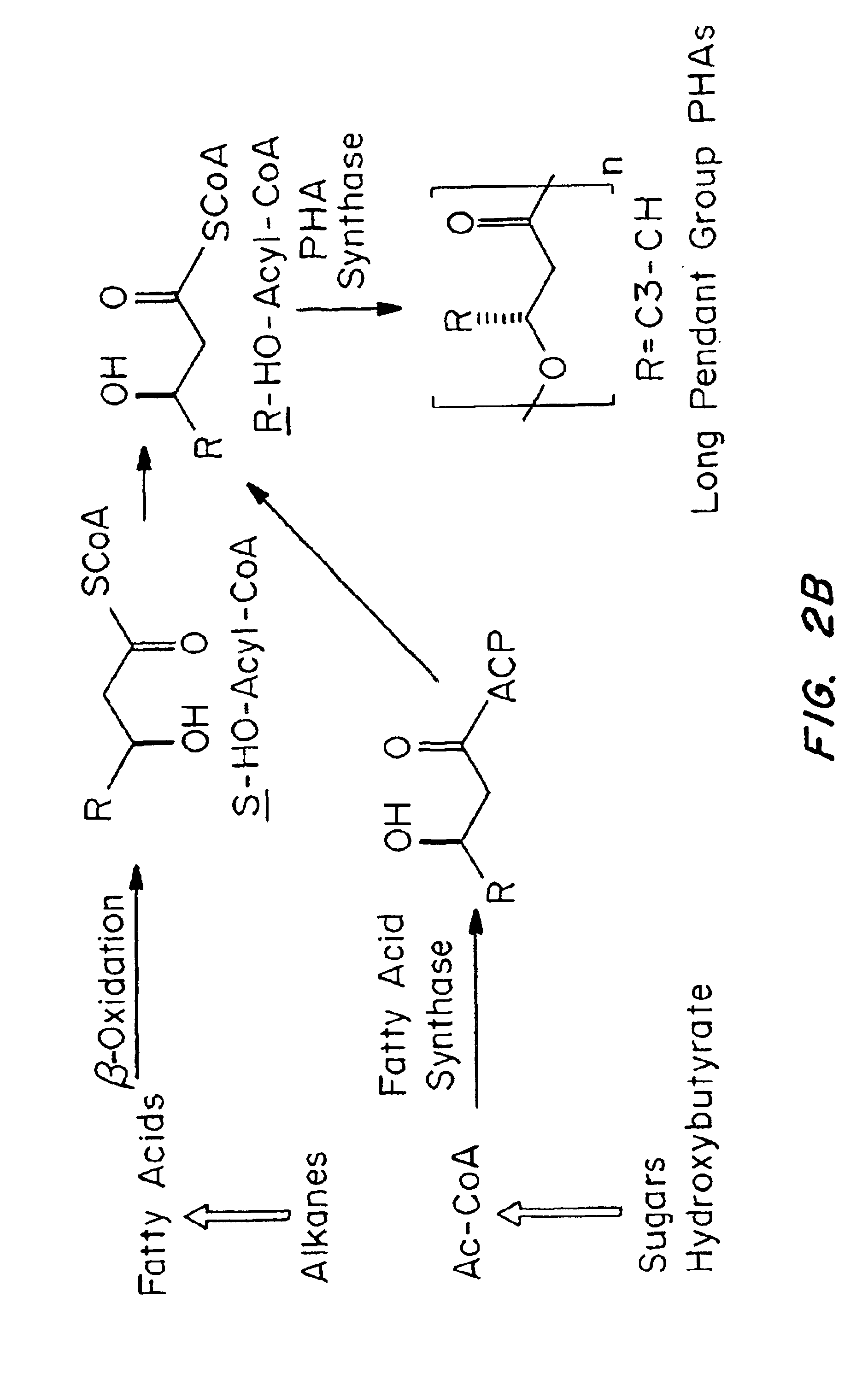
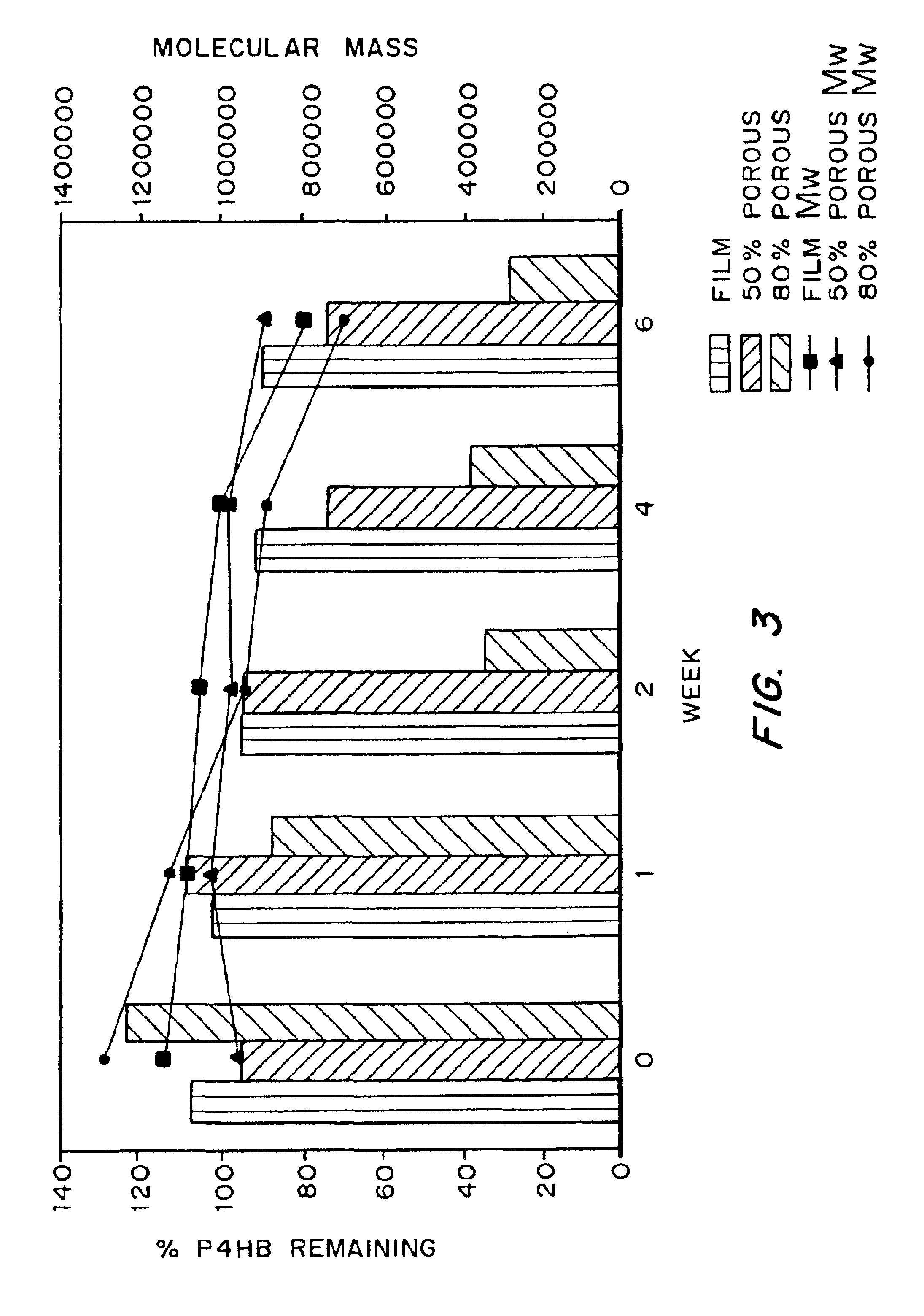
Medical devices and applications of polyhydroxyalkanoate polymers
InactiveUS6838493B2High porosityReduce probabilitySuture equipmentsOrganic active ingredientsTissue repairBiocompatibility Testing
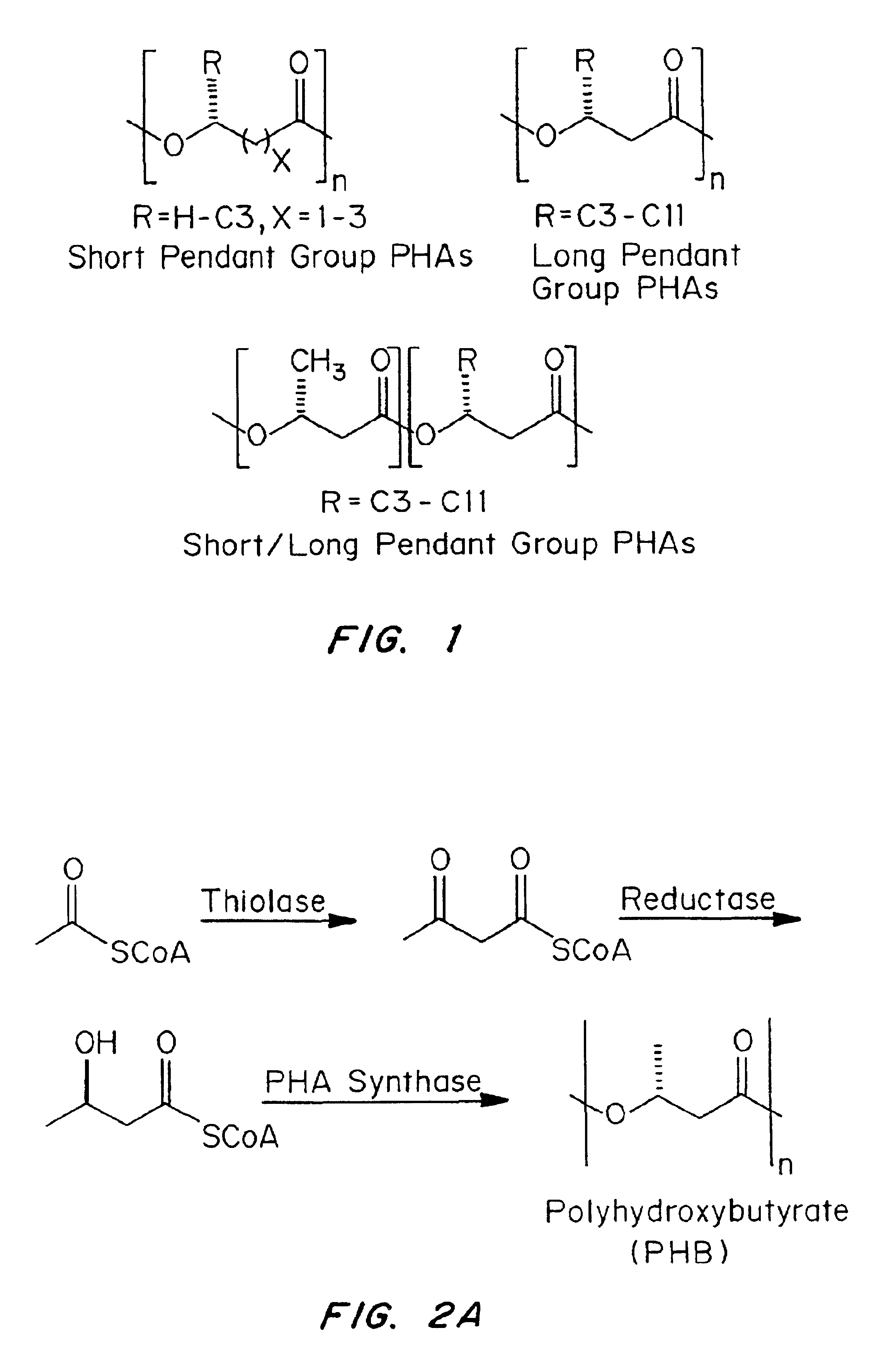
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
Drug releasing anastomosis devices and methods for treating anastomotic sites
ActiveUS7108701B2Reduce drug toxicityGood curative effectSuture equipmentsSurgical needlesBiological bodyReady to use
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:WYETH
Method of performing anastomosis
InactiveUS7699859B2Precise positioningSecurely holdSuture equipmentsStapling toolsGeneral surgeryDistal anastomosis
Owner:AESCULAP AG
Medical film
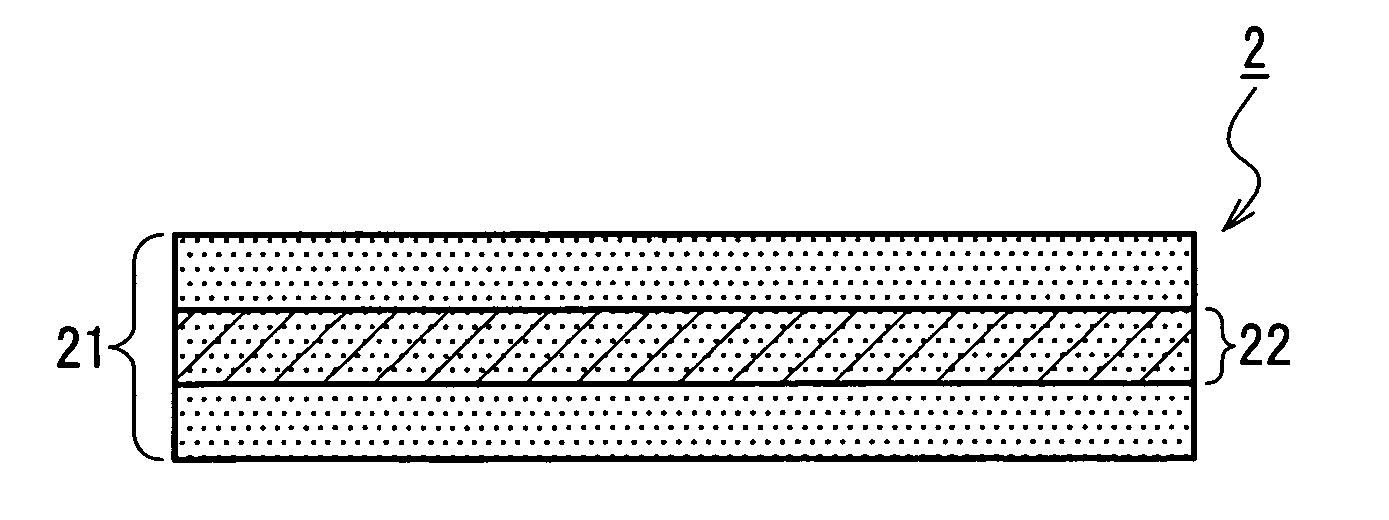
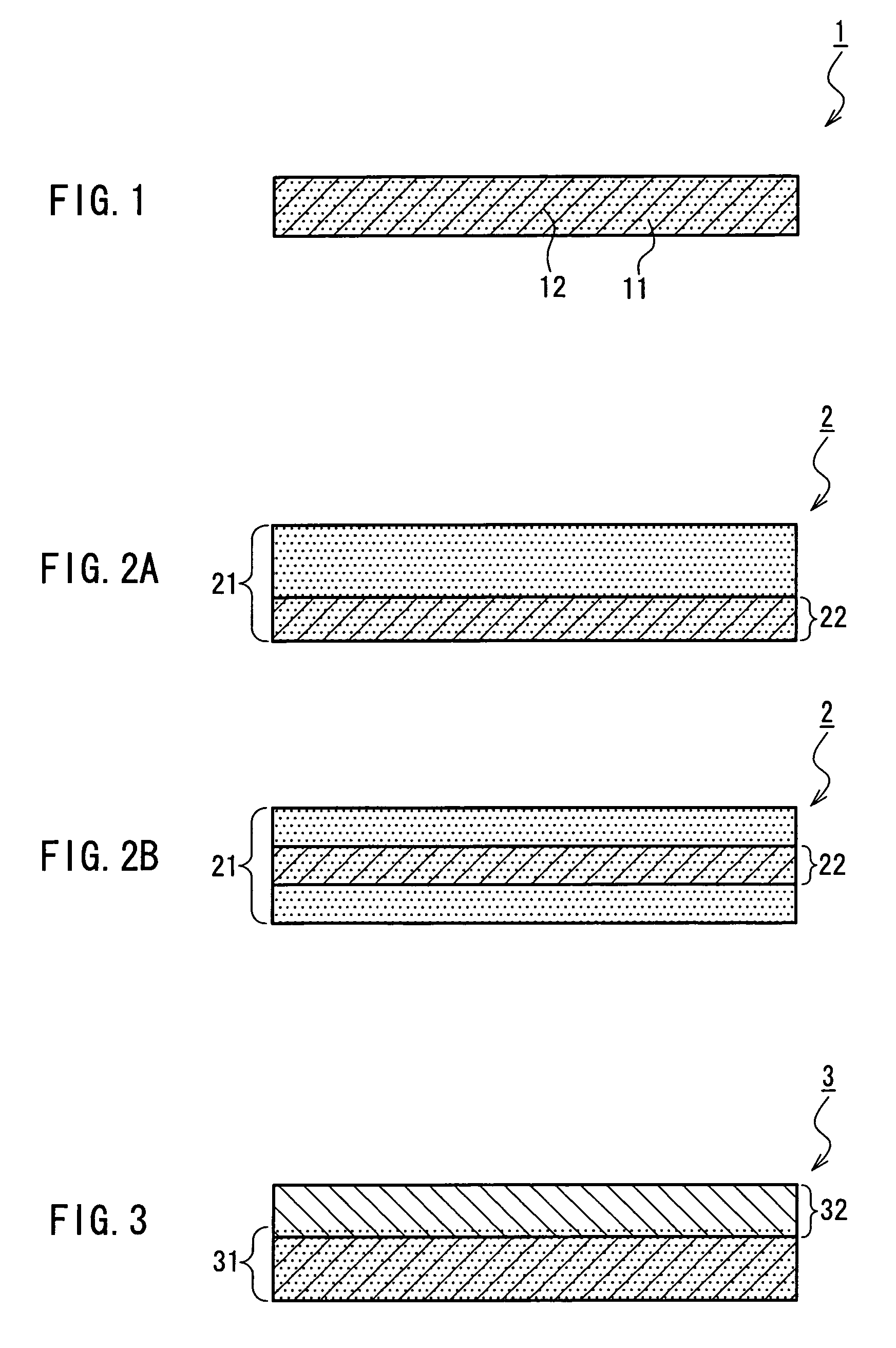
InactiveUS7718556B2Good biocompatibilityHigh strengthSuture equipmentsBiocideGelatin filmThin membrane
A medical film that is excellent in biocompatibility and bioabsorbability and has an excellent strength in suturing and bonding is provided. A reinforcing material 12 made of a biodegradable polymer is placed in a gelatin solution so as to allow the solution to infiltrate in the reinforcing material 12 and then the gelatin is dried. This allows the gelatin that has infiltrated entirely in an internal part of the reinforcing material 12 to gel, thereby forming a gelatin film 11. Thus, a medical film 1 in which the reinforcing material 12 and the gelatin film 11 are integrated is obtained. The gelatin film 11 preferably is a cross-linked gelatin film.
Owner:GUNZE LTD
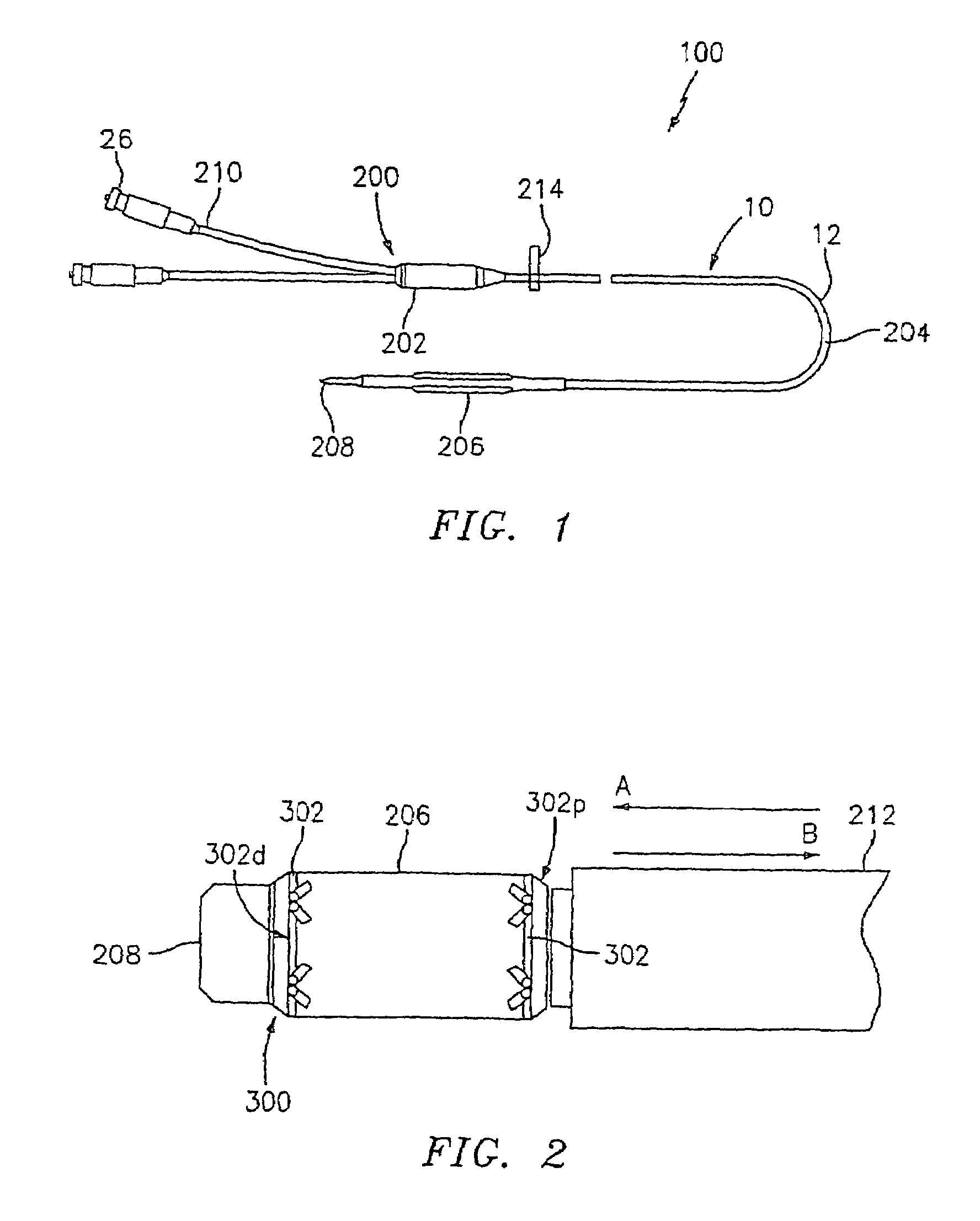
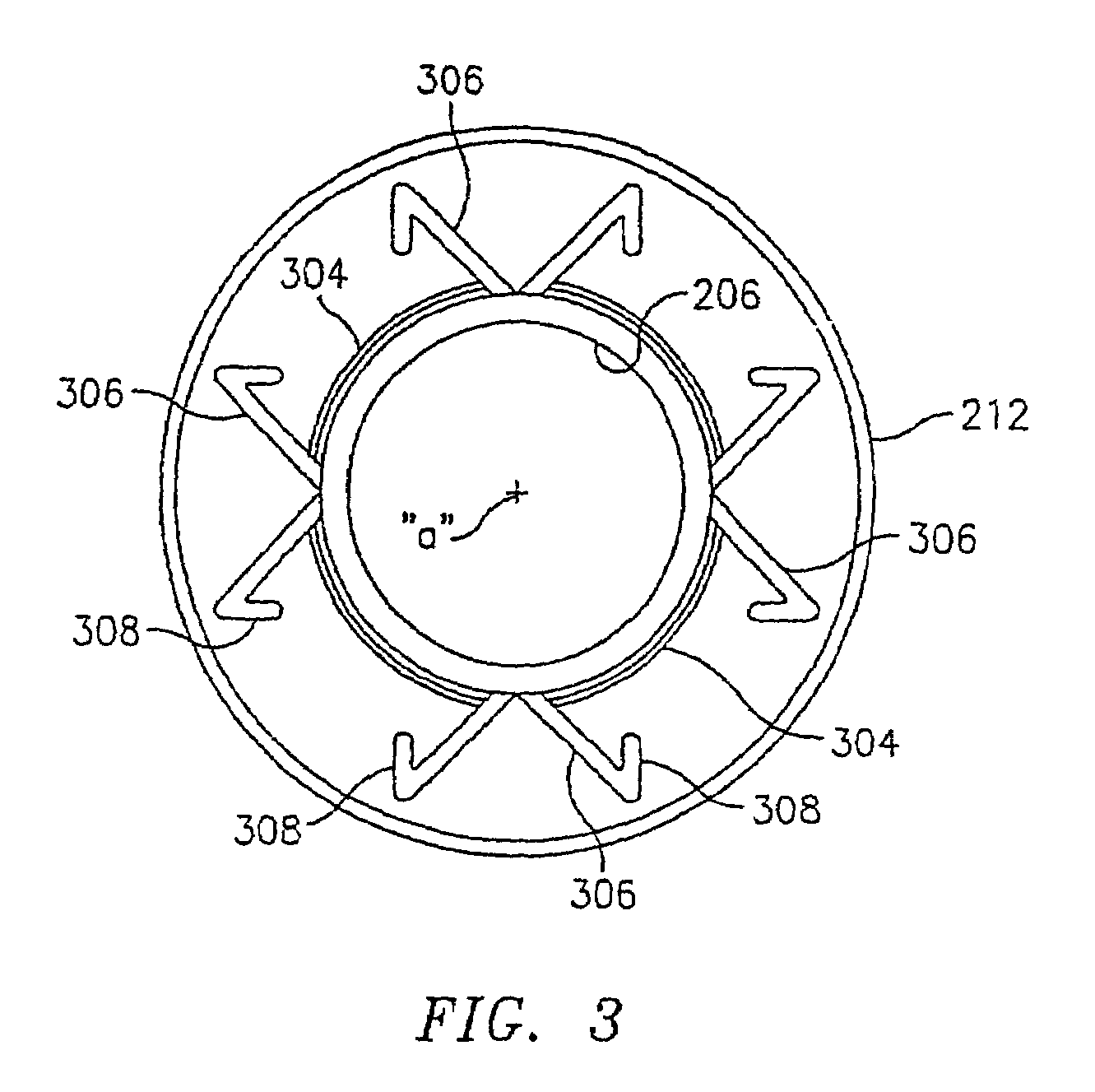
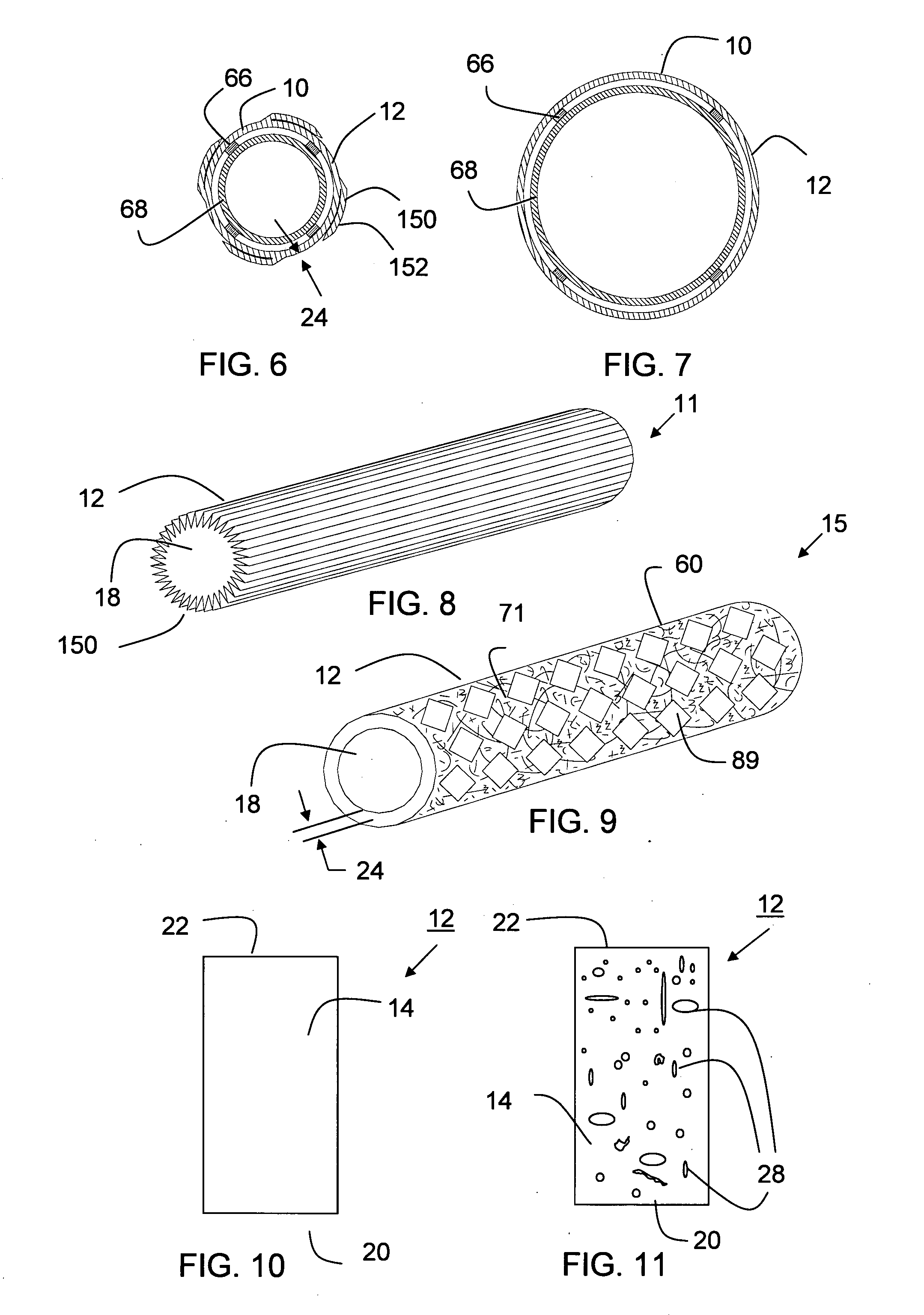
Apparatus and method for fixation of vascular grafts
InactiveUS7351258B2Reduce leakageOvercome disadvantagesStentsBlood vesselsThree vesselsVascular graft
An apparatus for facilitating securement of a vascular graft within a blood vessel, includes a shaft dimensioned for passage within a blood vessel and having an expansion member movable between a contracted condition and an expanded condition and a fastener array comprising at least one fastener disposed about a peripheral portion of the expansion member. The one fastener is deployable into a wall of the blood vessel upon movement of the expansion member to the expanded condition thereof, to thereby engage the vascular graft to secure the vascular graft to a wall of the blood vessel. The fastener array preferably includes a plurality of fasteners. The fasteners may be operatively connected to each other and releasably secured to the peripheral portion of the expansion member.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Tear and abrasion resistant expanded material and reinforcement
InactiveUS20070207186A1Increase flexibilityLess complex manufacturing processStentsSurgeryDiseaseEngineering
The present invention is a more durable expanded material that enables thinner wall thicknesses and a more flexible reinforcement suitable for stenting. The present invention is especially useful in the construction of grafts, stents, and stent-grafts which are used, for example, in repairing or replacing blood vessels that are narrowed or occluded by disease, aneurismal blood vessels, or other medical treatments. The inventive material and configurations allow expansion or contraction in size or adjustment in size in an incremental manner so that the optimum size, shape, and fit with other objects can be obtained. The present invention is also optionally capable of more accurately delivering one or more active ingredients such as drugs over longer periods of time. The present invention optionally includes surface modifications and additives that increase the surface adhesion of active ingredients, coatings, or combinations thereof. Finally, the present invention optionally includes growing cells on the inventive material so that the expanded material, reinforcement, or combinations thereof are useful, for example, in producing lab-grown blood vessels or organs.
Owner:SCANLON JOHN JAMES +1
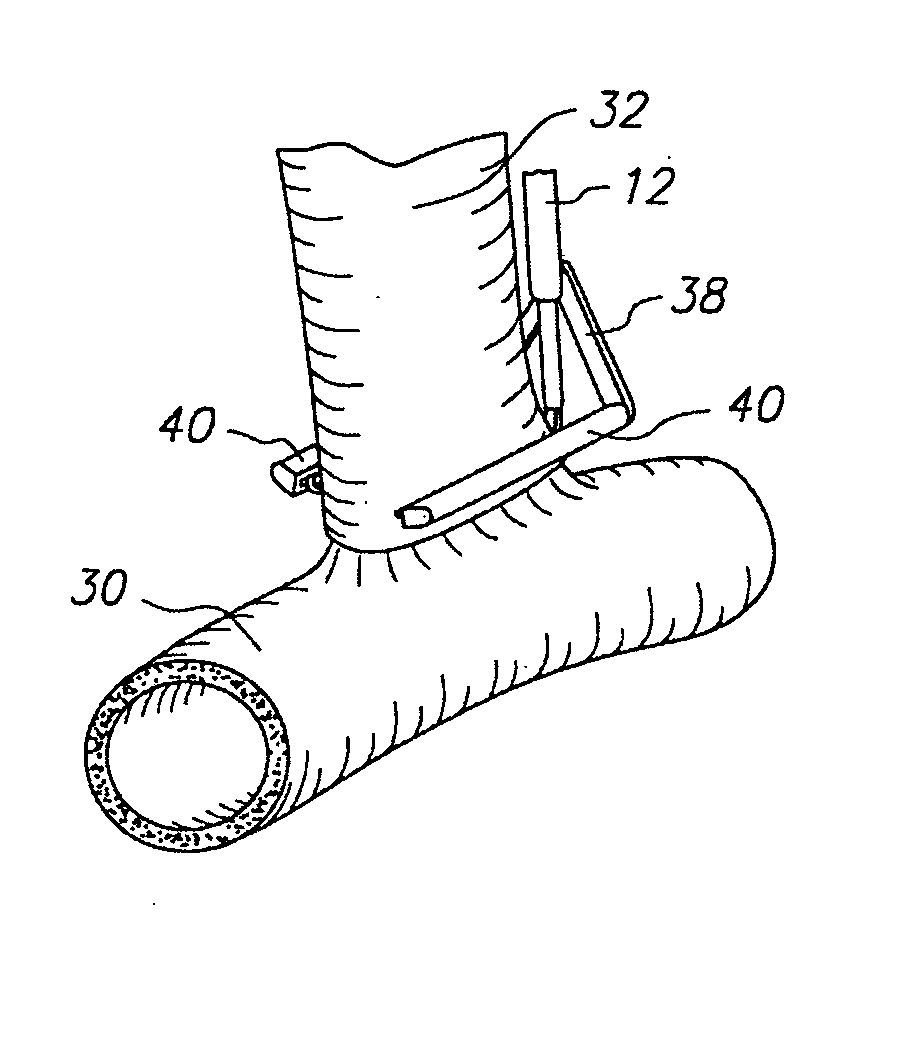
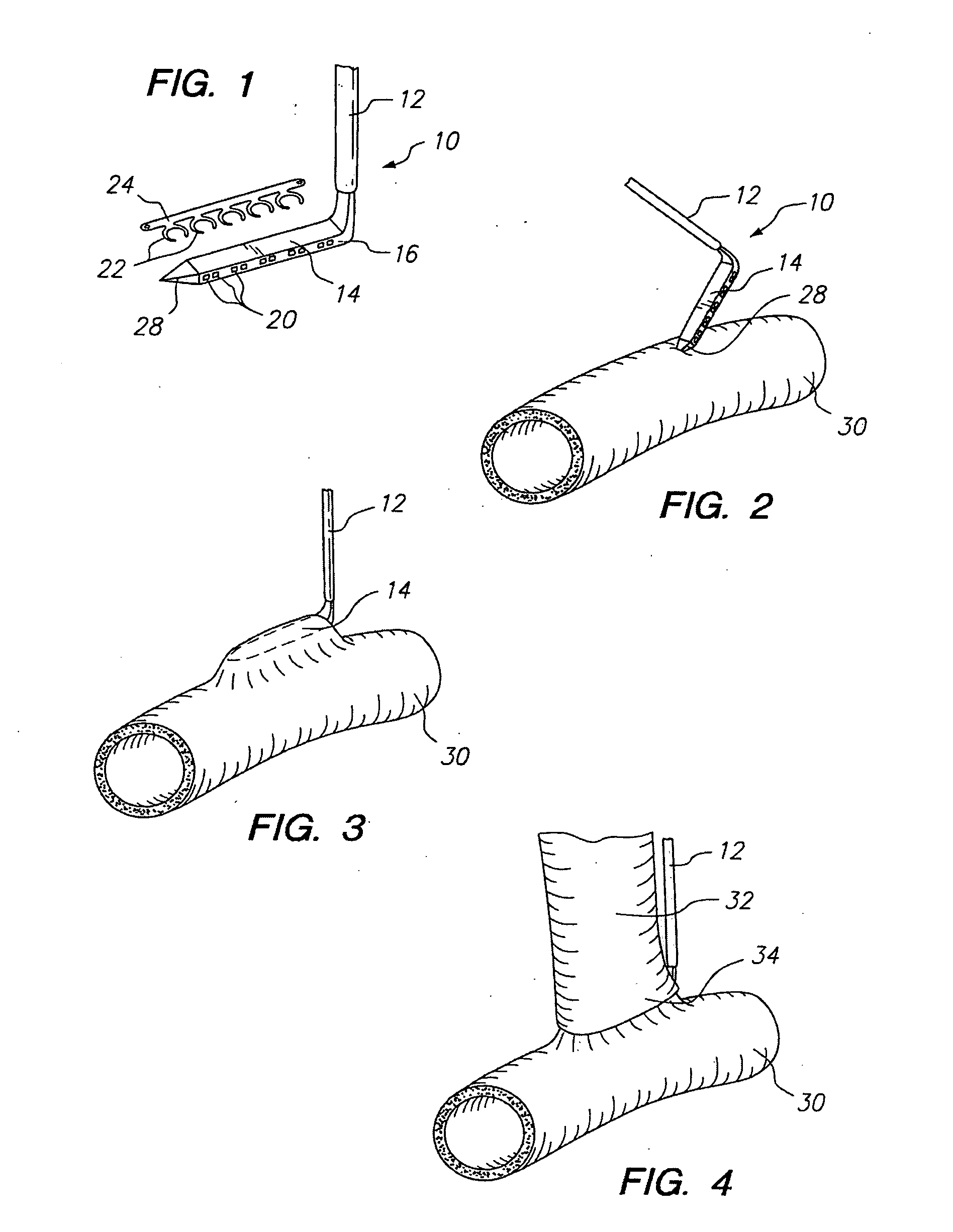
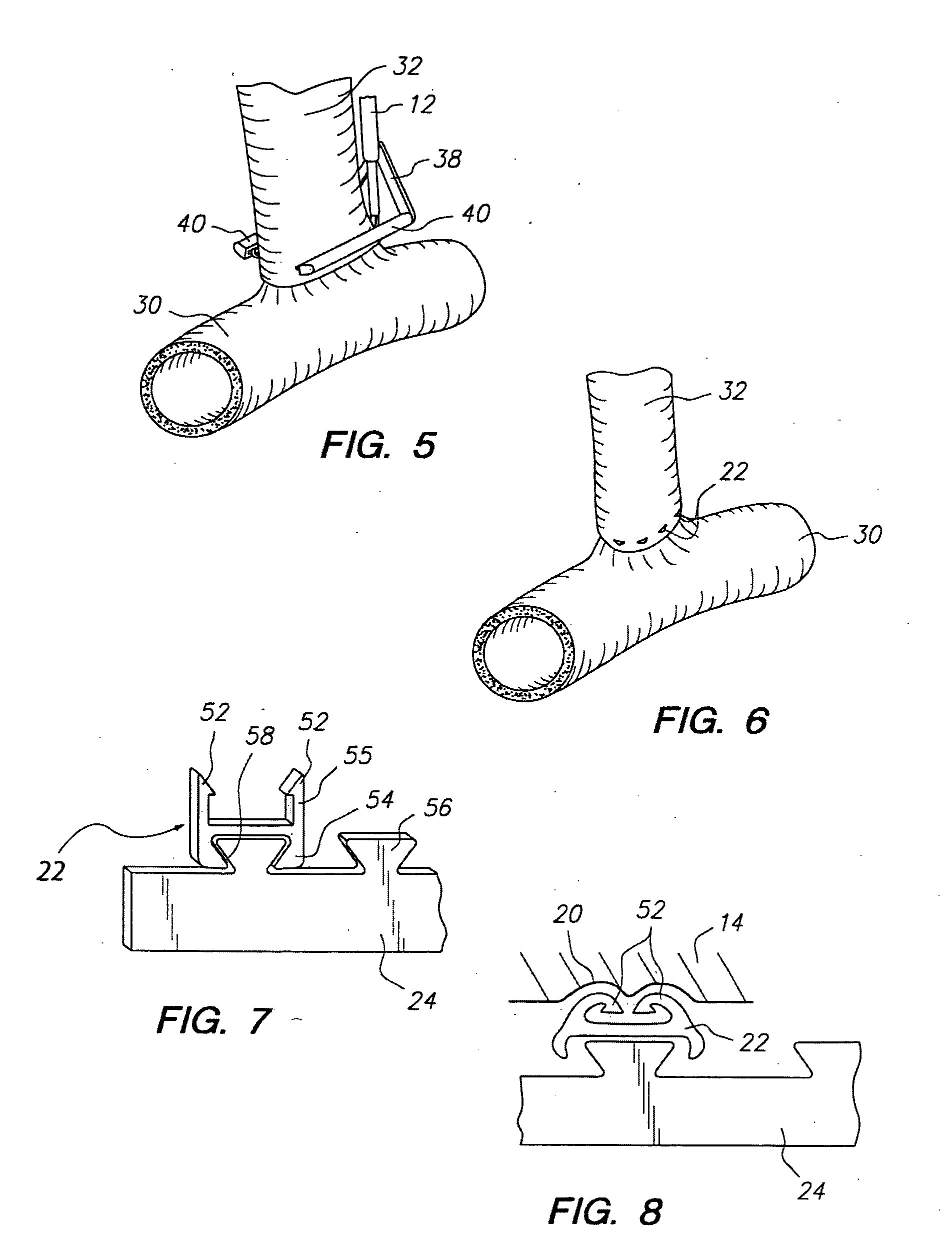
Method for anastomosing vessels
InactiveUS20050154406A1Precise positioningSecurely holdSuture equipmentsDiagnosticsSurgeryBlood vessel
A method for connecting a graft vessel to a target vessel, each vessel having a wall surrounding a lumen, may include providing a connector holder, associating an end of the graft vessel with the connector holder, positioning the connector holder outside of the lumen of the target vessel, outside the lumen of the graft vessel, and in proximity to the outer surface of the wall of the target vessel, and actuating the connector holder to secure the end of the graft vessel to the side of the target vessel.
Owner:AESCULAP AG
Externally expandable heart valve anchor and method
InactiveUS20050137686A1Reduce paravalvular leakageReduce regurgitationStentsBalloon catheterBlood vesselVALVE PORT
Owner:BOSTON SCI SCIMED INC
Apparatus for performing anastomosis
An apparatus for performing anastomosis between a graft vessel and a target vessel may include a connector holder having spaced-apart arms, and a member connected to the connector holder, where the member is insertable through an opening in a wall of the target vessel at least partially into the lumen of the target vessel. One or more connectors, such as staples, may be deployed from each arm to connect the graft vessel to the target vessel. One or more connectors may be deformable against the member.
Owner:AESCULAP AG
Stent-valves for valve replacement and associated methods and systems for surgery
InactiveUS20070213813A1Simple methodReduce riskStentsBalloon catheterLess invasive surgeryInsertion stent
Stent-valves (e.g., single-stent-valves and double-stent-valves), associated methods and systems for their delivery via minimally-invasive surgery, and guide-wire compatible closure devices for sealing access orifices are provided.
Owner:SYMETIS
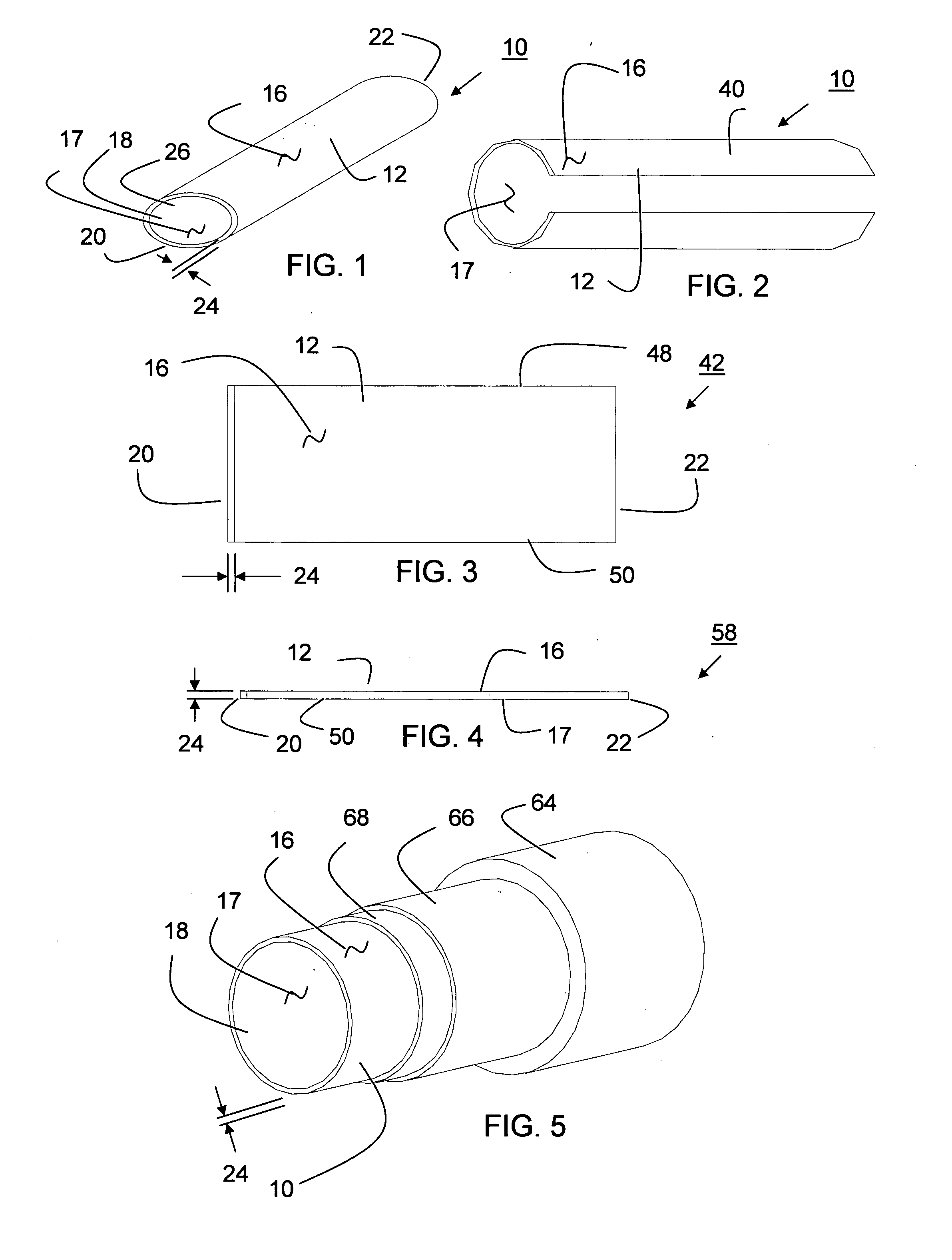
Sutures and surgical staples for anastamoses, wound closures, and surgical closures
InactiveUS8016881B2Improve featuresControlled release rateSuture equipmentsStentsSurgical stapleMicroparticle
The invention relates to sutures and surgical staples useful in anastomoses. Various aspects of the invention include wound closure devices that use amphiphilic copolymer or parylene coatings to control the release rate of an agent, such as a drug or a biological material, polymerizing a solution containing monomers and the agent to form a coating, using multiple cycles of swelling a polymer with a solvent-agent solution to increase loading, microparticles carrying the agent, biodegradable surgical articles with amphiphilic copolymer coatings, and sutures or surgical staples the deliver a drug selected from the group consisting of triazolopyrimidine, paclitaxol, sirolimus, derivatives thereof, and analogs thereof to a wound site.
Owner:MIRUS LLC
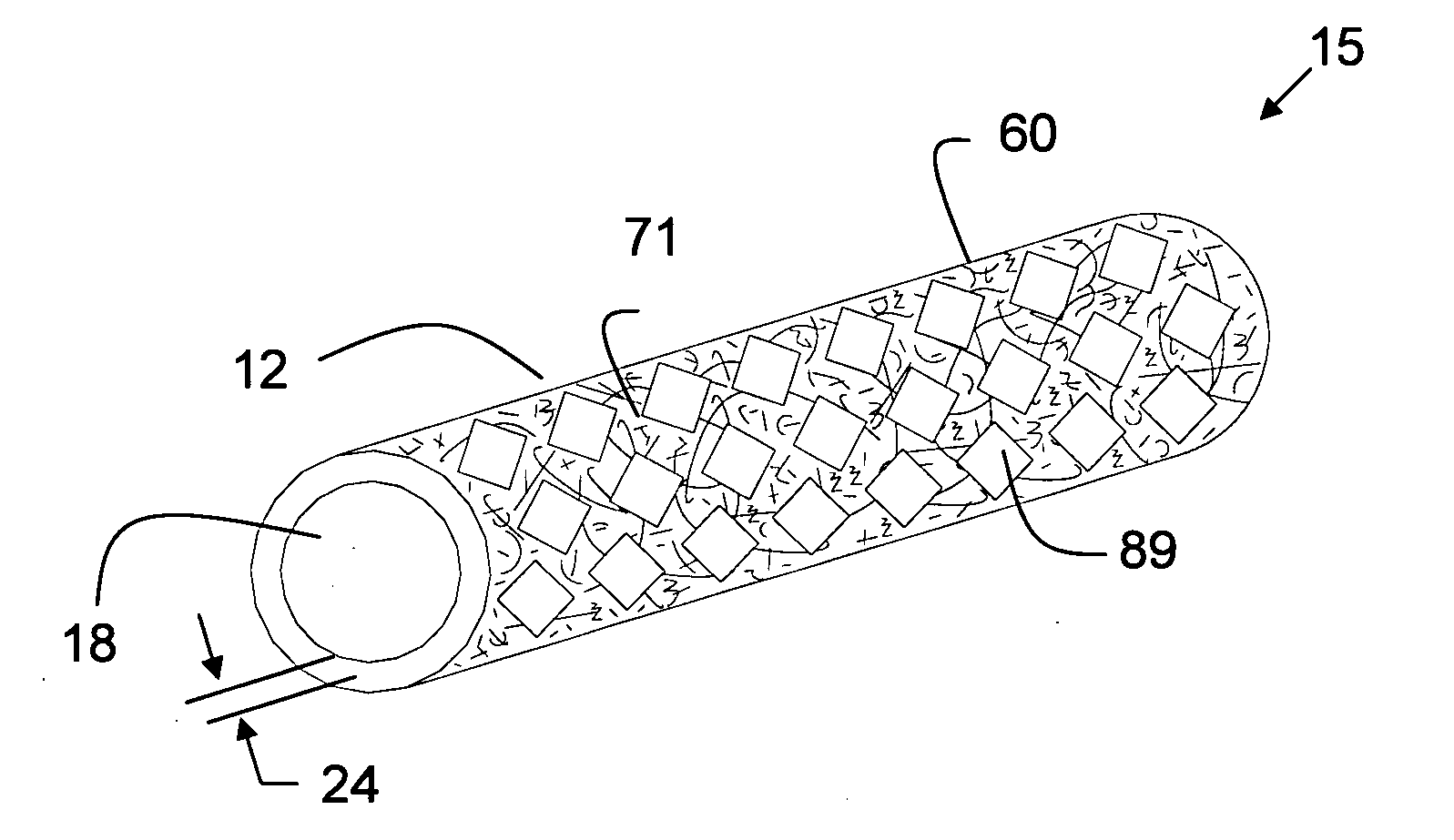
Composite self-cohered web materials
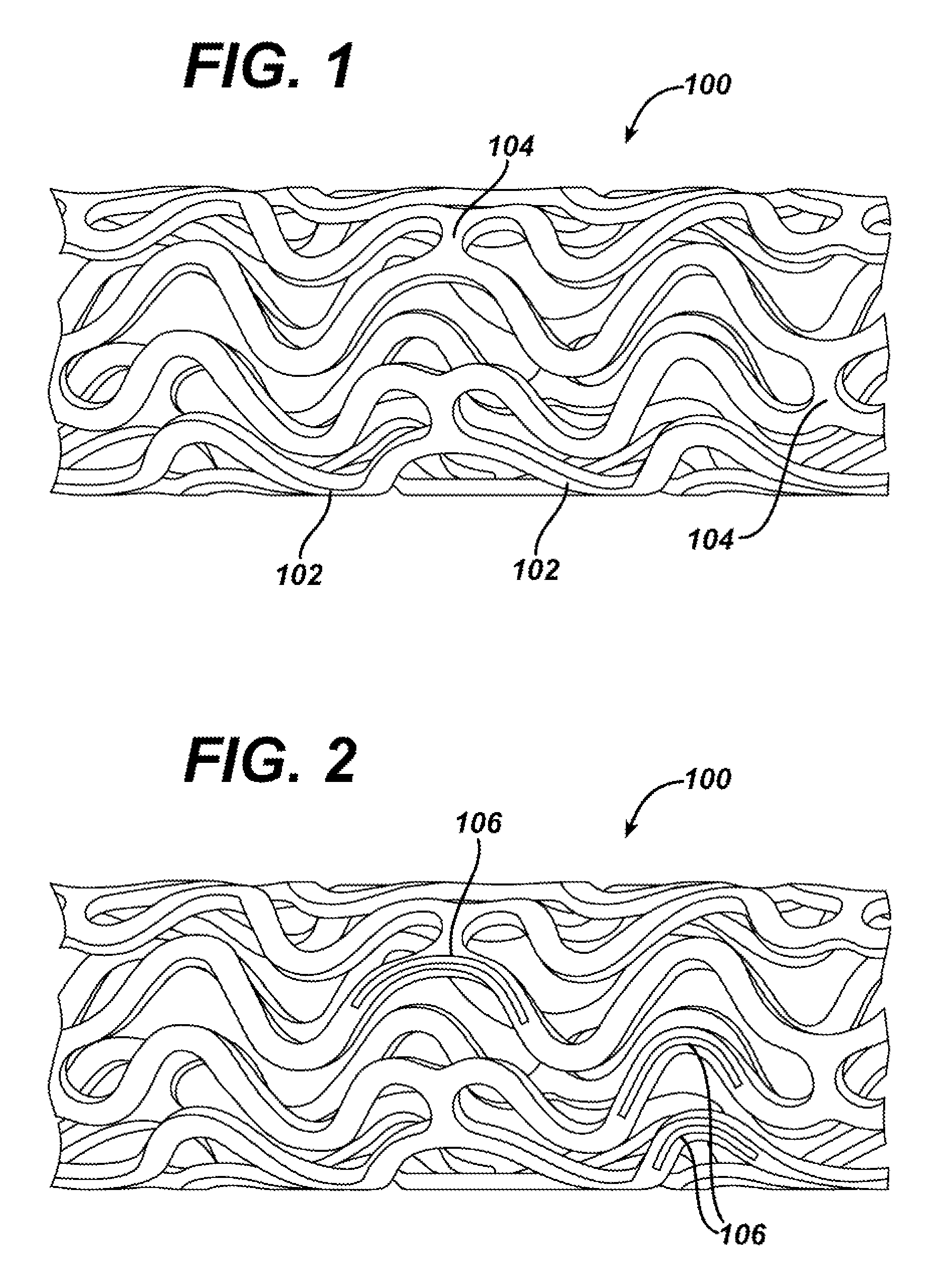
The present invention is directed to implantable bioabsorbable non-woven self-cohered web materials having a high degree of porosity. The web materials are very supple and soft, while exhibiting proportionally increased mechanical strength in one or more directions. The web materials often possess a high degree of loft. The web materials can be formed into a variety of shapes and forms suitable for use as implantable medical devices or components thereof.
Owner:WL GORE & ASSOC INC
Delivery devices
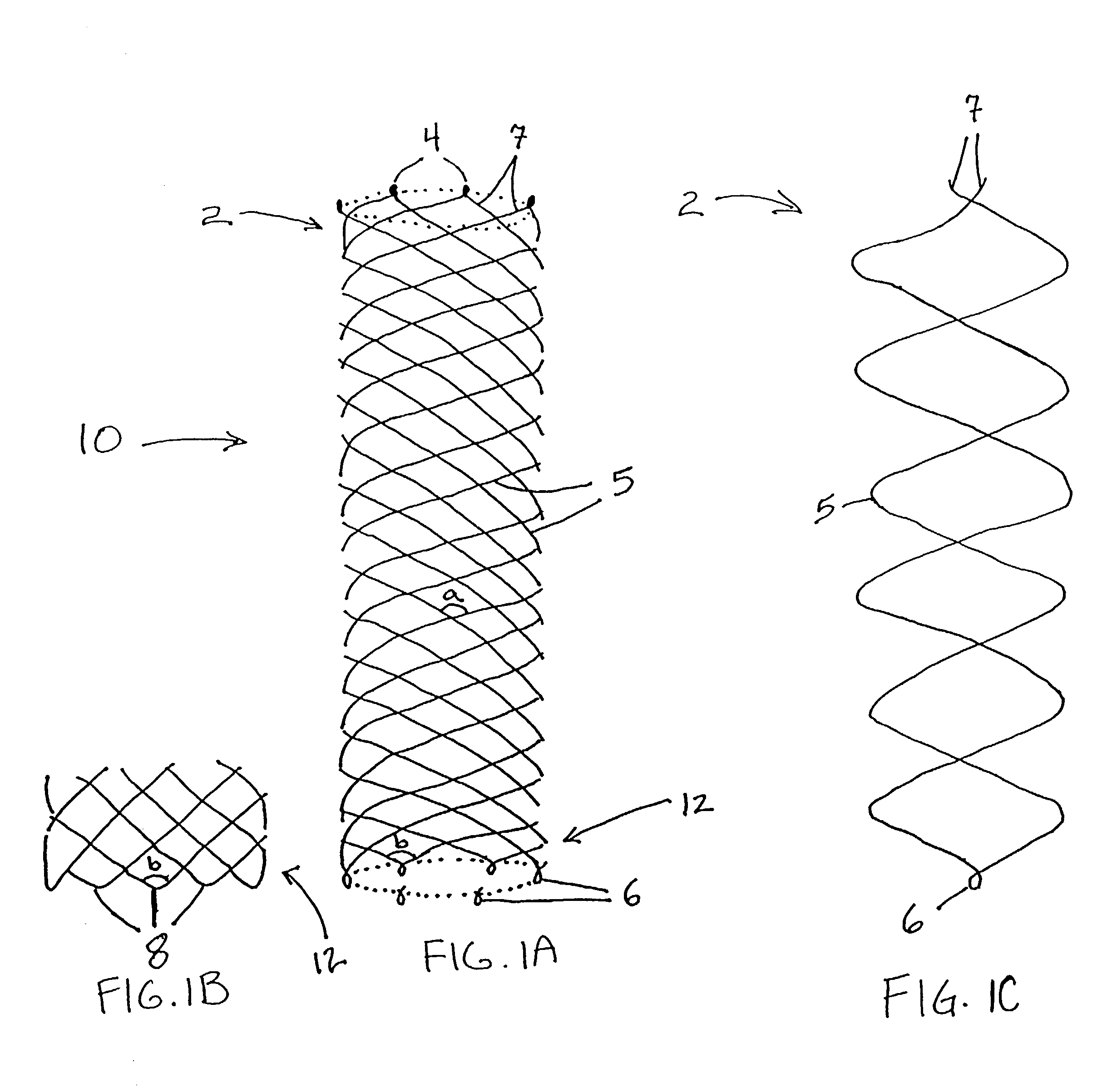
Self-expandable, woven intravascular devices for use as stents (both straight and tapered), filters (both temporary and permanent) and occluders for insertion and implantation into a variety of anatomical structures. The devices may be formed from shape memory metals such as nitinol. The devices may also be formed from biodegradable materials. Delivery systems for the devices include two hollow tubes that operate coaxially. A device is secured to the tubes prior to the implantation and delivery of the device by securing one end of the device to the outside of the inner tube and by securing the other end of the device to the outside of the outer tube. The stents may be partially or completely covered by graft materials, but may also be bare. The devices may be formed from a single wire. The devices may be formed by either hand or machine weaving. The devices may be created by bending shape memory wires around tabs projecting from a template, and weaving the ends of the wires to create the body of the device such that the wires cross each other to form a plurality of angles, at least one of the angles being obtuse. The value of the obtuse angle may be increased by axially compressing the body.
Owner:HYODOH HIDEKI +2
Extraction of solvents from drug containing polymer reservoirs
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Minimally invasive valve replacement system
InactiveUS20060259137A1Reduce and eliminate needShorten the timeBalloon catheterHeart valvesEngineeringVALVE PORT
Owner:MEDTRONIC 3F THERAPEUTICS
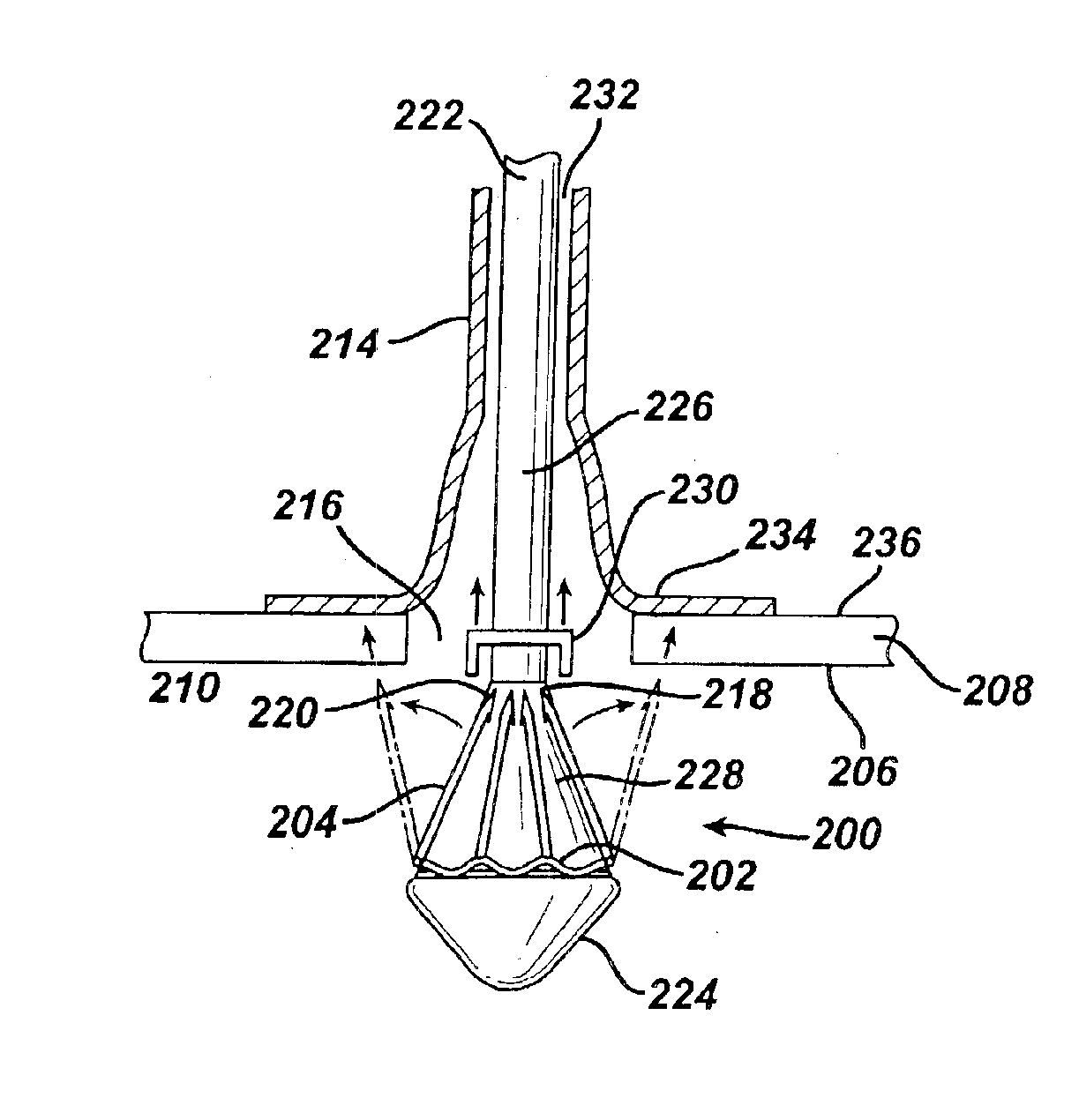
Fluid flow prosthetic device
A prosthetic device including a valve-orifice attachment member attachable to a valve in a blood vessel and including a fluid inlet, and a diverging member that extends from the fluid inlet, the diverging member including a proximal end near the fluid inlet and a distal end distanced from the proximal end, wherein a distal portion of the diverging member has a larger cross-sectional area for fluid flow therethrough than a proximal portion thereof. The diverging member may have a diverging taper that causes fluid to flow therethrough with pressure recovery at the distal end thereof.
Owner:MEDTRONIC VENTOR TECH
Means and method of replacing a heart valve in a minimally invasive manner
A heart valve can be replaced using minimally invasive methods which include a sutureless sewing cuff that and a fastener delivery tool that holds the cuff against the patient's tissue while delivering fasteners to attach the cuff to the tissue from the inside out. The tool stores a plurality of fasteners and is self-contained whereby a fastener is delivered and placed all from inside a vessel. The fasteners are self-forming whereby they do not need an anvil to be formed. Anchor elements are operated from outside the patient's body to cinch a prosthesis to an anchoring cuff of the valve body. The cuff is releasably mounted on the tool and the tool holds the cuff against tissue and drives the fastener through the cuff and the tissue before folding over the legs of the fastener whereby secure securement between the cuff and the tissue is assured. Fasteners are placed and formed whereby fasteners are located continuously throughout the entire circumference of the cuff. A minimally invasive surgical method is disclosed, and a method and tool are disclosed for repairing abdominal aortic aneurysms in a minimally invasive manner. Fasteners that are permanently deformed during the process of attaching the cuff are disclosed as are fasteners that are not permanently deformed during the attaching process.
Owner:MEDTRONIC INC +1
Minimally invasive heart valve replacement
ActiveUS20090005863A1Minimally invasiveSufficient flexibilityHeart valvesBlood vesselsHeart valve replacementThoracic cavity
A replacement valve for implantation centrally within the orifice of a malfunctioning native heart valve. The valve is designed for minimally invasive entry through an intercostal opening in the chest of a patient and an opening in the apex of the human heart. The replacement valve includes either a separate anchor (11, 87, 111) or a combined anchor (67) that folds around the malfunctioning native valve leaflets, sandwiching them in a manner so as to securely anchor the replacement valve in a precise, desired location.
Owner:VENUS MEDTECH (HANGZHOU) INC
Transcatheter delivery of a replacement heart valve
A replacement heart valve apparatus. The heart valve apparatus includes a stent and a valve frame having a substantially cylindrical body defining a lumen. The valve frame includes a plurality of curved wire pairs attached to the substantially cylindrical body. Each curved wire pair includes an inner curved wire and an outer curved wire. The wire frame further having a plurality of leaflets. Each leaflet is attached to a respective inner curved wire and extends over a respective outer curved wire, so as to position the body of the leaflet within the lumen of the valve frame.
Owner:CHILDRENS MEDICAL CENT CORP
Endovascular fastener applicator
Endovascular fastener applicator for endoluminally fastening prosthetic grafts to vessels, are provided. The endovascular fastener applicator includes a delivery assembly configured for positioning within a vessel, and a control assembly mounted to a proximal end of the outer sheath for extracorporeal control of the delivery assembly. The delivery assembly includes an expandable portion disposed adjacent a distal end of an outer sheath and being expandable to support a prosthetic in contact with an inner surface of a vessel; a yoke assembly disposed within the expandable portion; an applicator head assembly pivotably mounted to the yoke assembly and movable between a loading position longitudinally aligned with the yoke assembly, and a firing position oriented substantially perpendicular to the yoke assembly; and a fastener assembly connectable to a distal end of the expandable portion, the fastener assembly retaining at least one fastener therein.
Owner:TYCO HEALTHCARE GRP LP
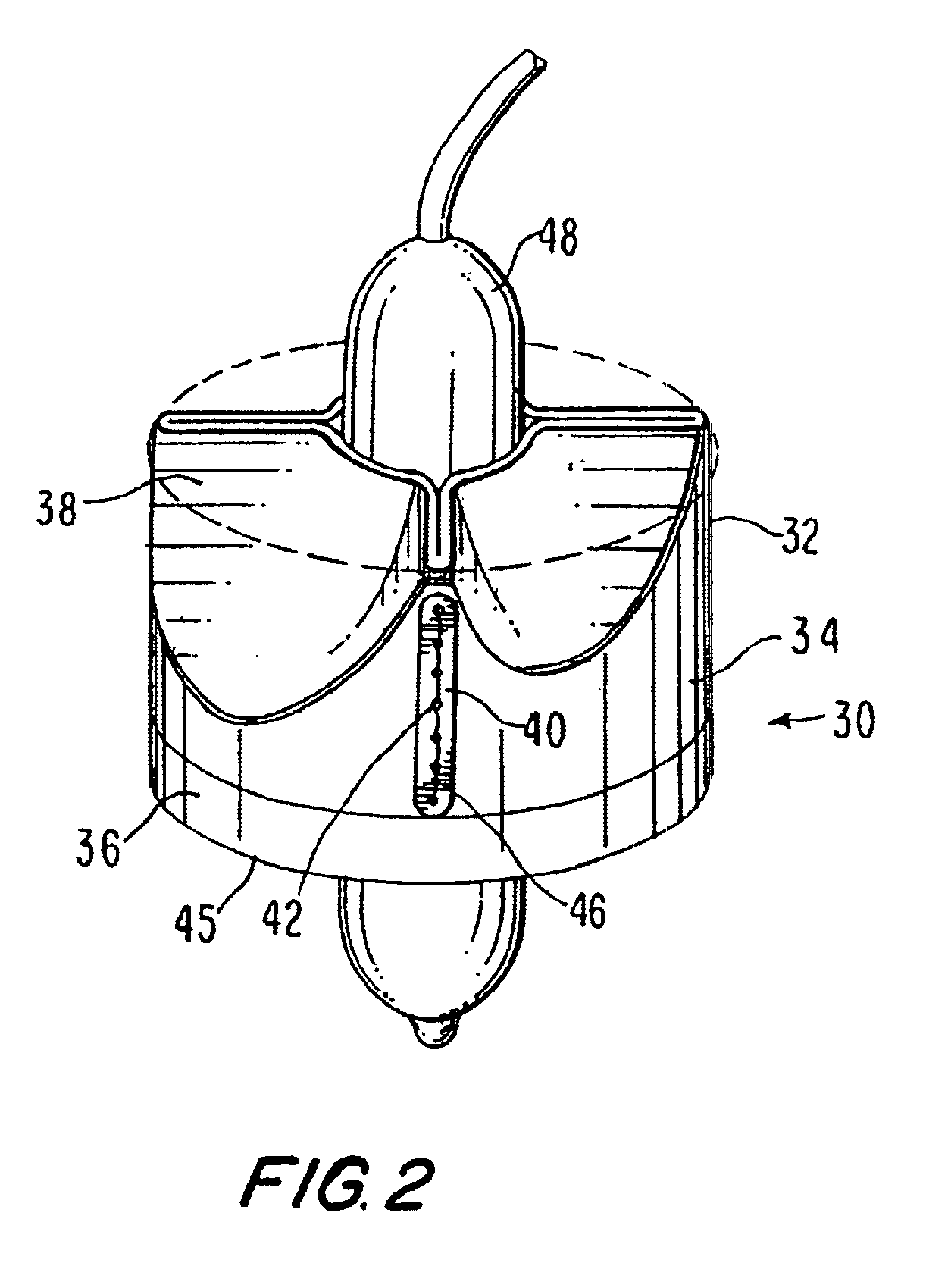
Heart valve prosthesis and methods of manufacture and use
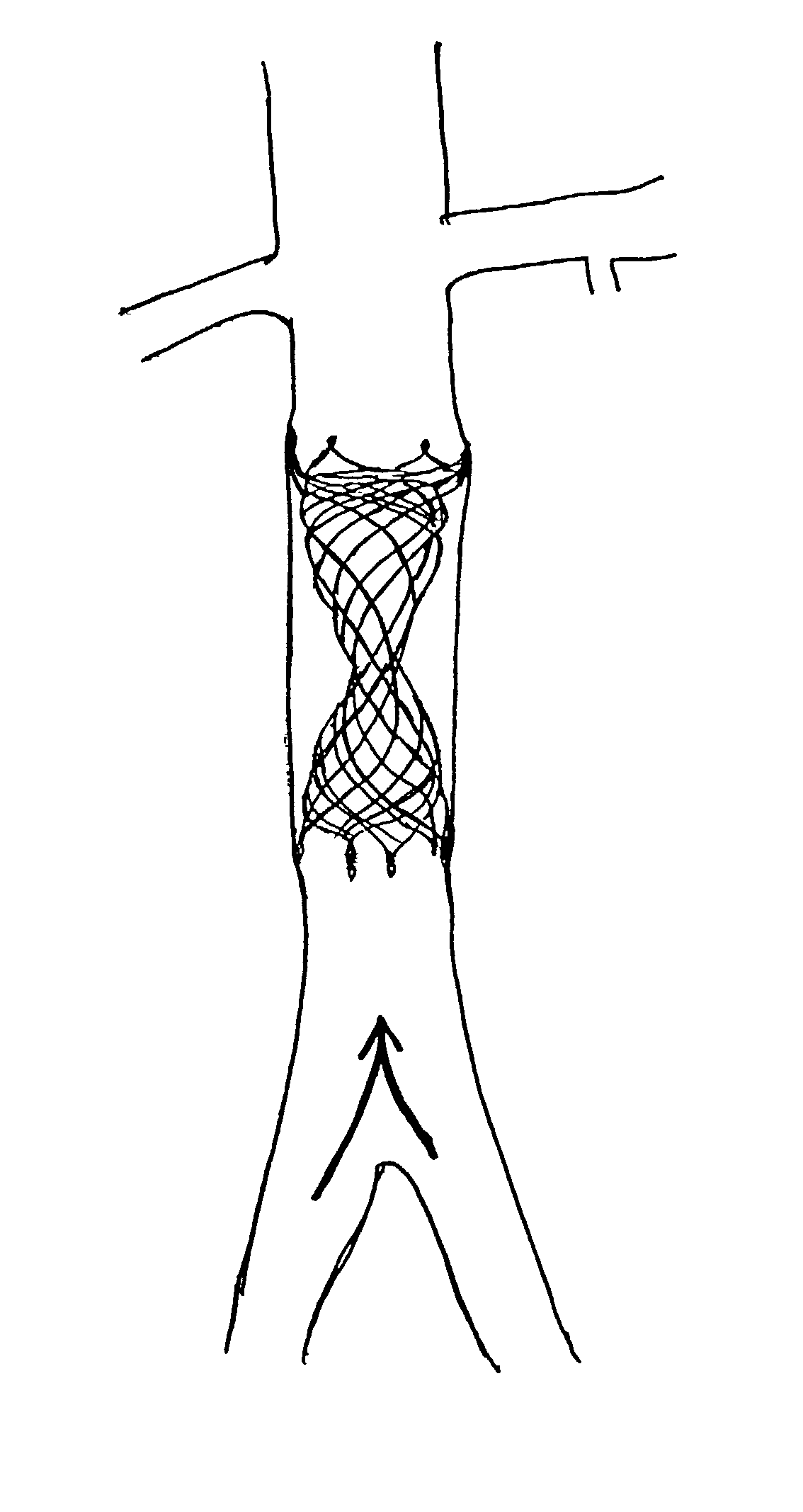
ActiveUS20060259136A1Overcomes drawbackReduce riskHeart valvesBlood vesselsHorizontal forceHourglass
A heart valve prosthesis is provided having a self-expanding multi-level frame that supports a valve body comprising a skirt and plurality of coapting leaflets. The frame transitions between a contracted delivery configuration that enables percutaneous transluminal delivery, and an expanded deployed configuration having an asymmetric hourglass shape. The valve body skirt and leaflets are constructed so that the center of coaptation may be selected to reduce horizontal forces applied to the commissures of the valve, and to efficiently distribute and transmit forces along the leaflets and to the frame. Alternatively, the valve body may be used as a surgically implantable replacement valve prosthesis.
Owner:MEDTRONIC ARDIAN LUXEMBOURG SARL
Coated endovascular AAA device
InactiveUS6852122B2Reduce drug toxicityGood curative effectSuture equipmentsStentsBiological bodyReady to use
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Non-cylindrical prosthetic valve system for transluminal delivery
InactiveUS20070043435A1Preventing substantial migrationEliminate the problemBalloon catheterHeart valvesCoronary arteriesProsthesis
A prosthetic valve assembly for use in replacing a deficient native valve comprises a replacement valve supported on an expandable prosthesis frame. If desired, one or more expandable anchors may be used. The prosthesis frame, which entirely supports the valve annulus, valve leaflets, and valve commissure points, is configured to be collapsible for transluminal delivery and expandable to contact the anatomical annulus of the native valve when the assembly is properly positioned. Portions of the prosthesis frame may expand to a preset diameter to maintain coaptivity of the replacement valve and to prevent occlusion of the coronary ostia. The prosthesis frame is compressible about a catheter, and restrained from expanding by an outer sheath. The catheter may be inserted inside a lumen within the body, such as the femoral artery, and delivered to a desired location, such as the heart. When the outer sheath is retracted, the prosthesis frame expands to an expanded position such that the valve and prosthesis frame expand at the implantation site and the anchor engages the lumen wall. The prosthesis frame has a non-cylindrical configuration with a preset maximum expansion diameter region about the valve opening to maintain the preferred valve geometry. The prosthesis frame may also have other regions having a preset maximum expansion diameter to avoid blockage of adjacent structures such as the coronary ostia.
Owner:MEDTRONIC COREVALVE
Low profile heart valve and delivery system
Apparatus for endovascularly replacing a patient's heart valve, including: a delivery catheter having a diameter of 21 french or less; an expandable anchor disposed within the delivery catheter; and a replacement valve disposed within the delivery catheter. The invention also includes a method for endovascularly replacing a heart valve of a patient. In some embodiments the method includes the steps of: inserting a catheter having a diameter no more than 21 french into the patient; endovascularly delivering a replacement valve and an expandable anchor to a vicinity of the heart valve through the catheter; and deploying the anchor and the replacement valve.
Owner:BOSTON SCI SCIMED INC
Apparatus for imparting physician-determined shapes to implantable tubular devices
A tubular sleeve is provided for enabling a physician to impart a preselected shape in an implantable tubular device, such as a cardiac lead, a catheter, or some other tubular structure. The sleeve may be deformed by the surgeon before or at the time of implantation to customize the shape of the tubular device to the particular anatomical structures to be encountered by the device. To retain the deformation imparted by the physician, the sleeve may be composed of a heat-sensitive shape-memory material or an elastomeric material provided with a plastically deformable rib.
Owner:INTERMEDICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com