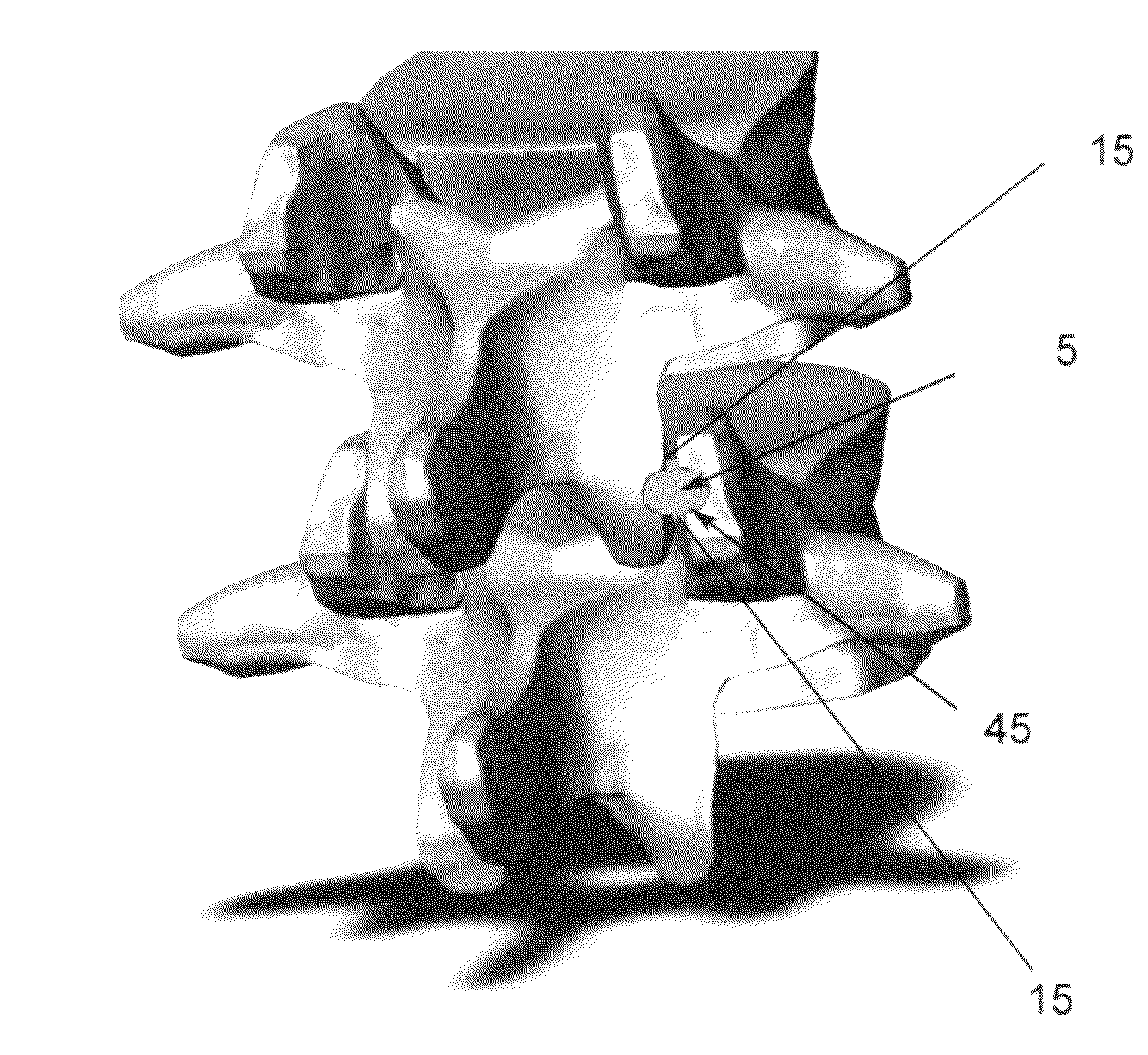Patents
Literature
161 results about "Spinal fusion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
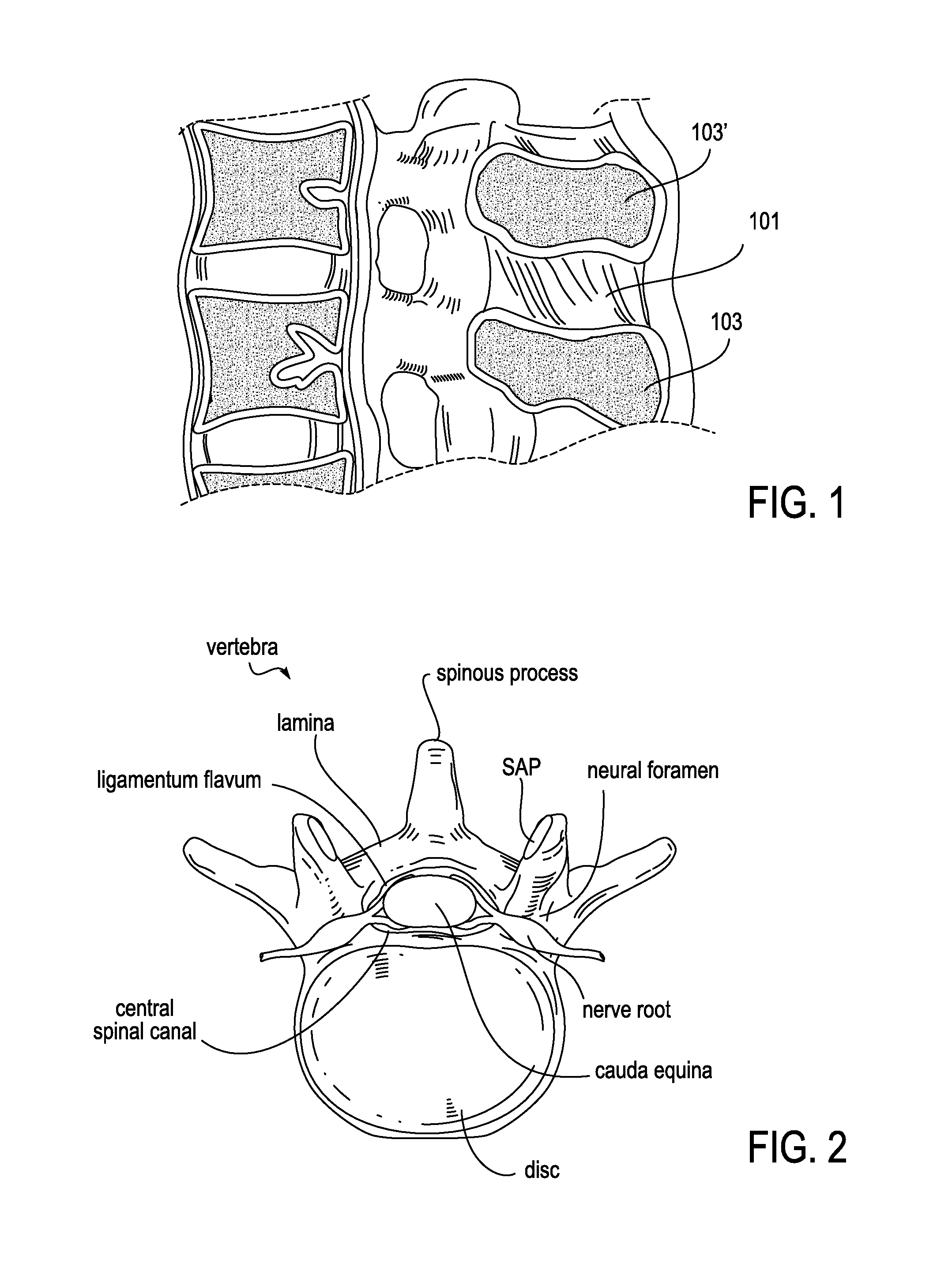
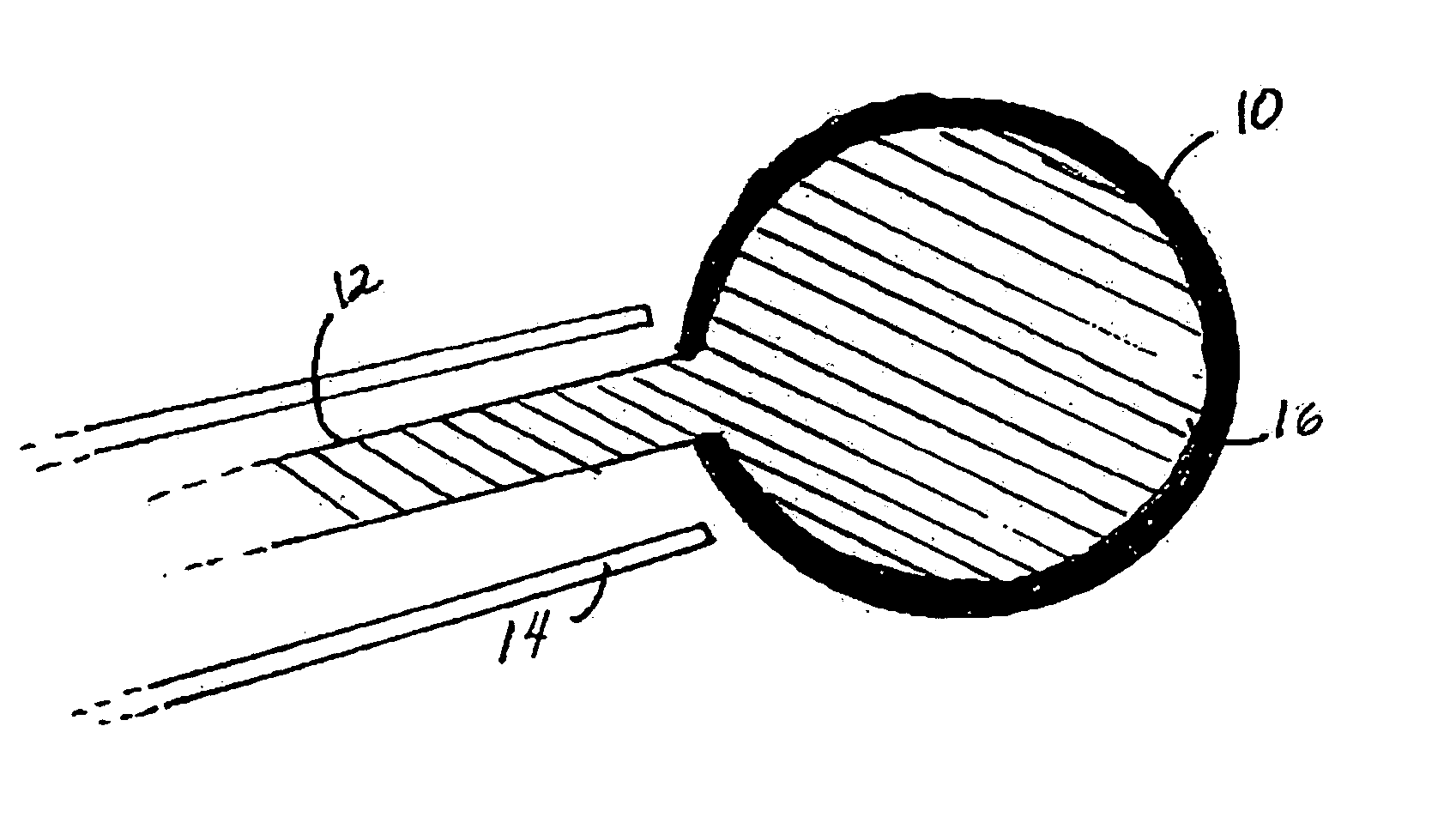
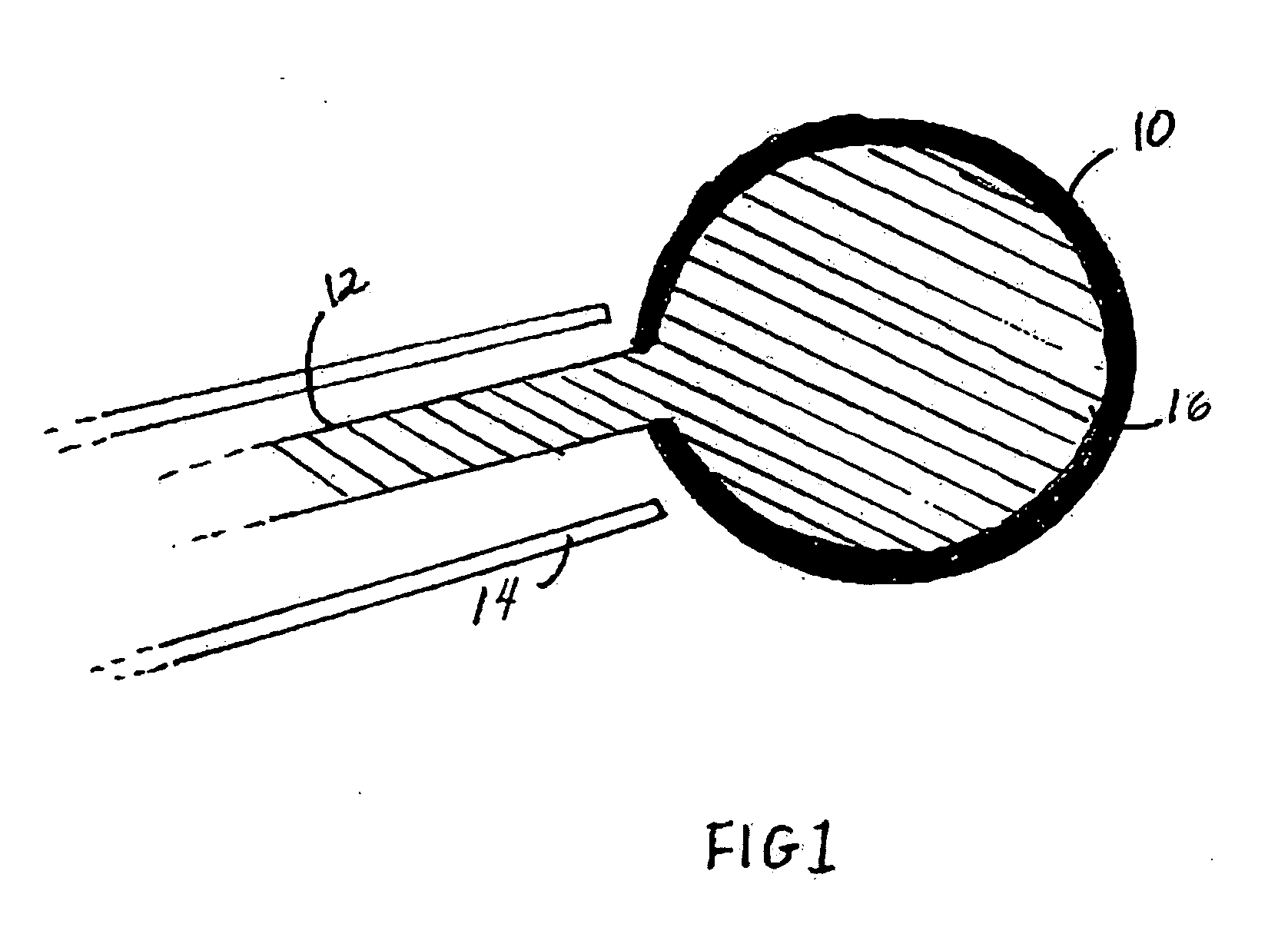
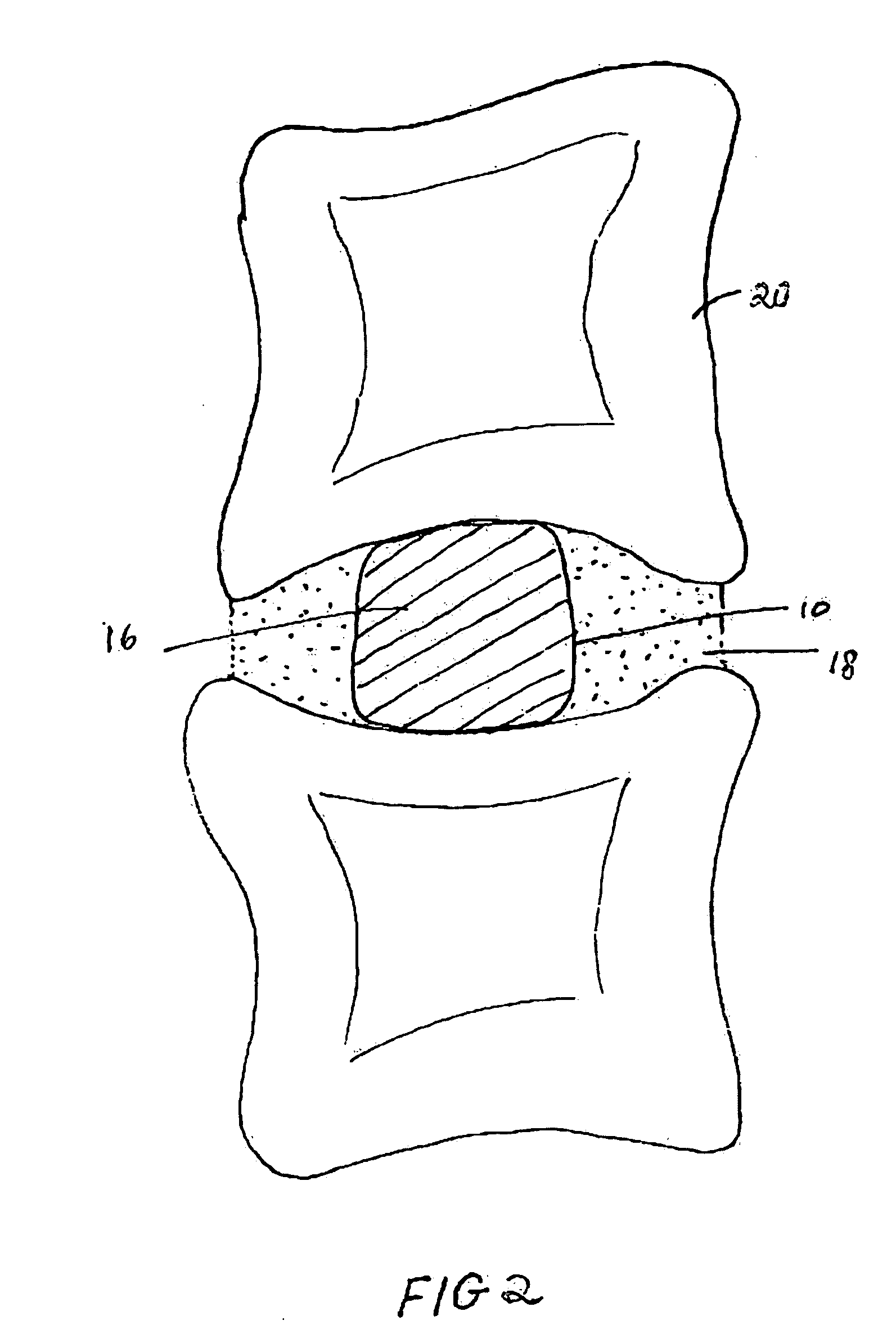
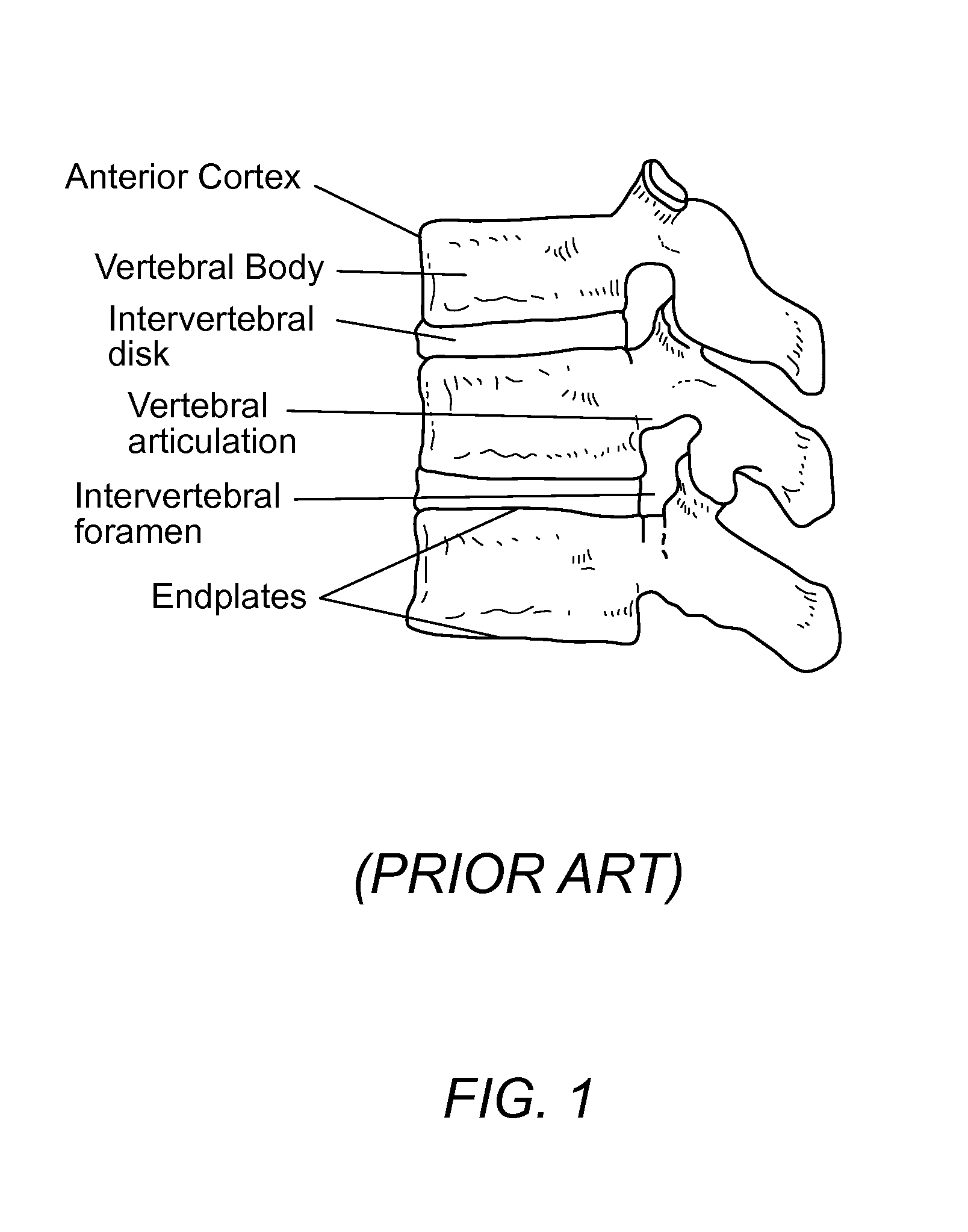
Spinal fusion, also called spondylodesis or spondylosyndesis, is a neurosurgical or orthopedic surgical technique that joins two or more vertebrae. This procedure can be performed at any level in the spine (cervical, thoracic, or lumbar) and prevents any movement between the fused vertebrae. There are many types of spinal fusion and each technique involves using bone grafting—either from the patient (autograft), donor (allograft), or artificial bone substitutes—to help the bones heal together. Additional hardware (screws, plates, or cages) is often used to hold the bones in place while the graft fuses the two vertebrae together.
Medical devices and applications of polyhydroxyalkanoate polymers
InactiveUS6838493B2High porosityReduce probabilitySuture equipmentsOrganic active ingredientsTissue repairBiocompatibility Testing
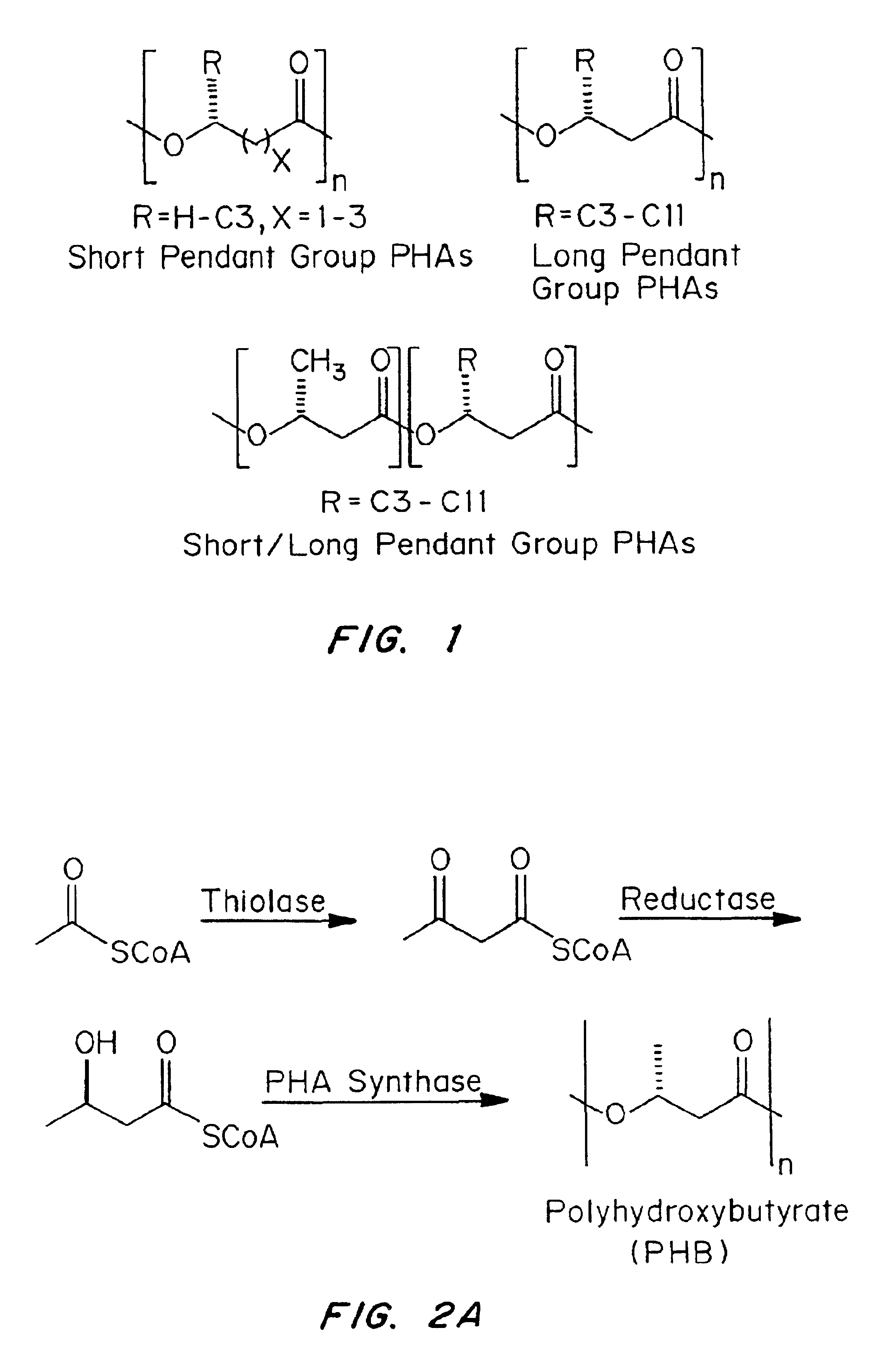
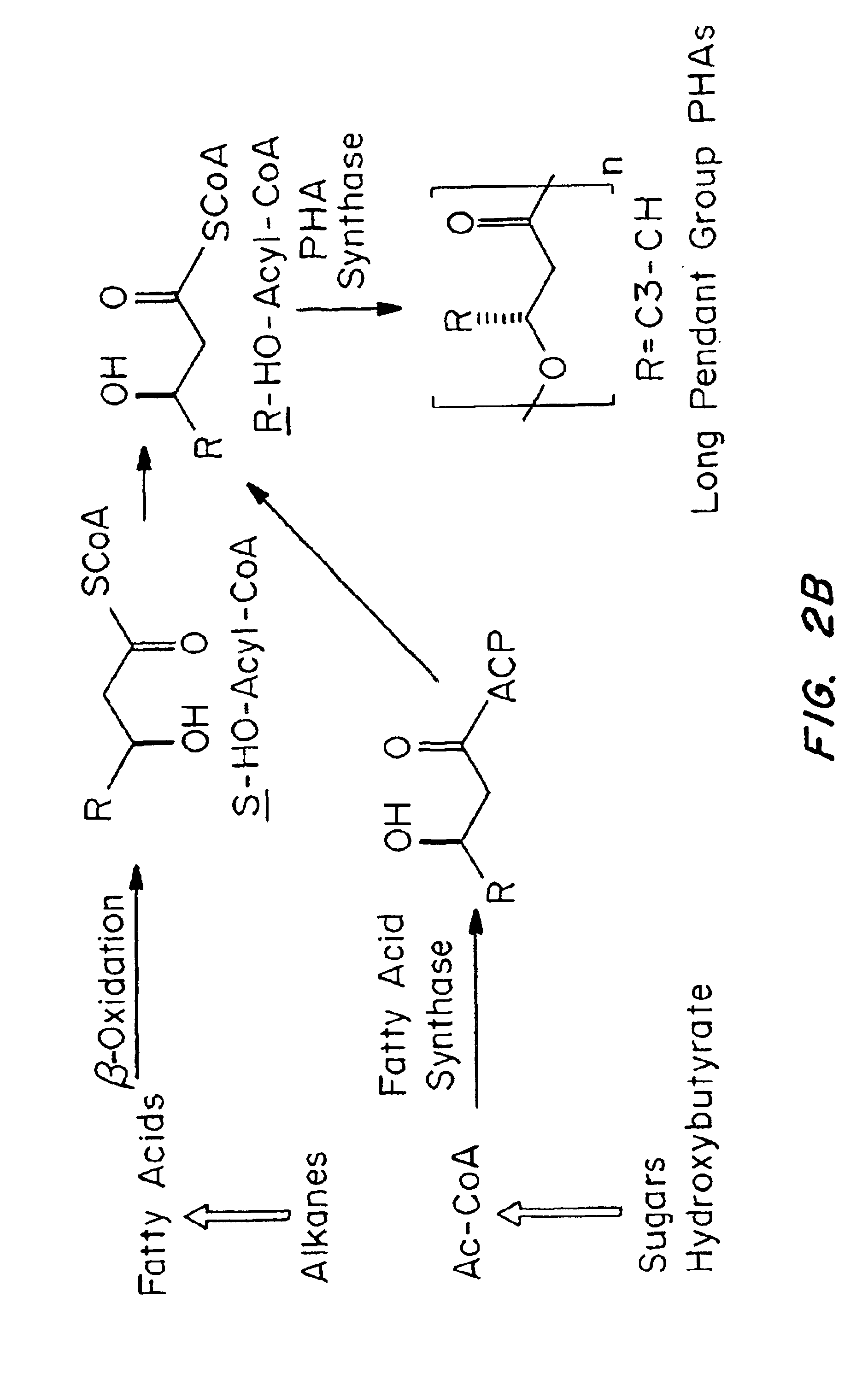
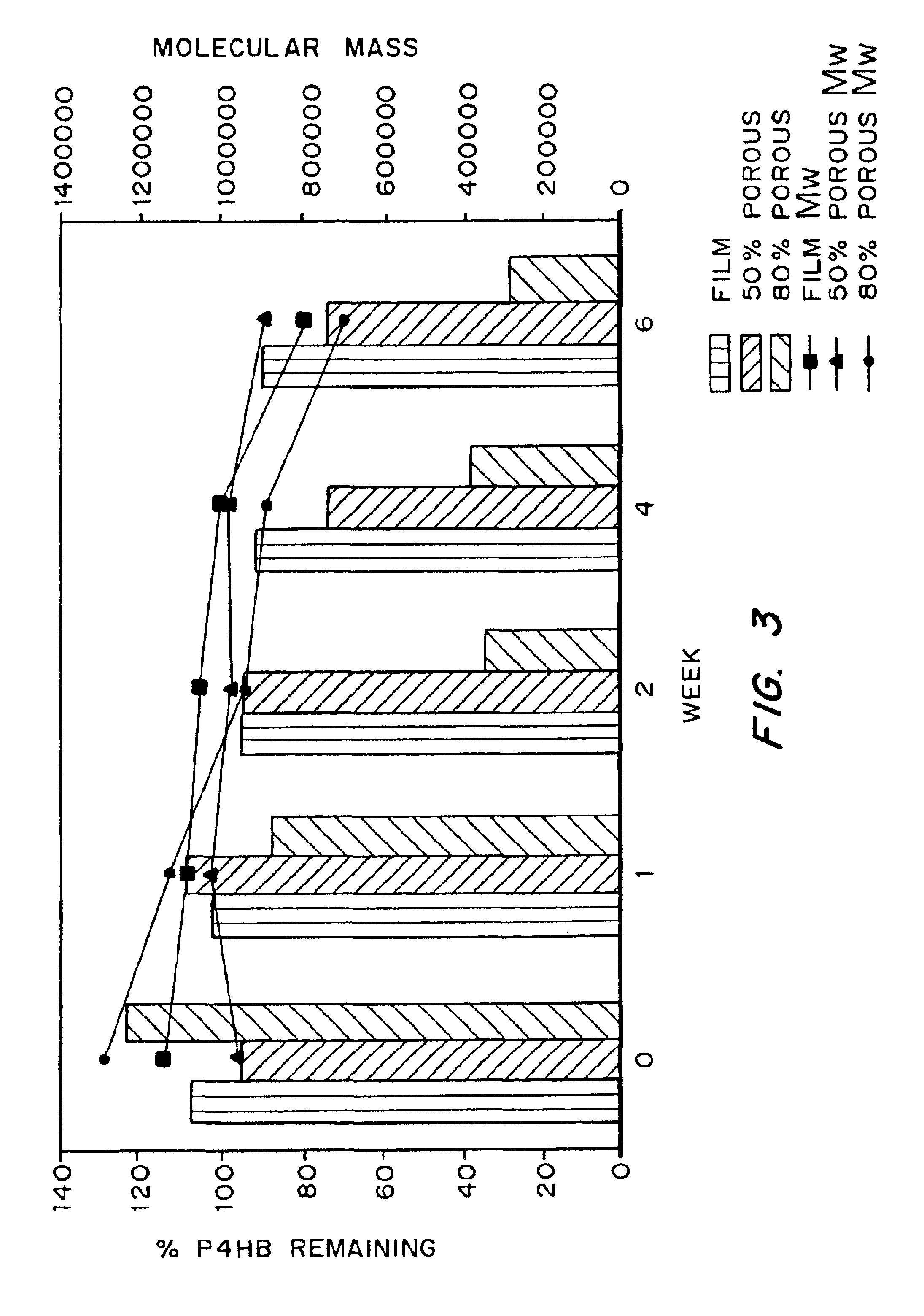
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
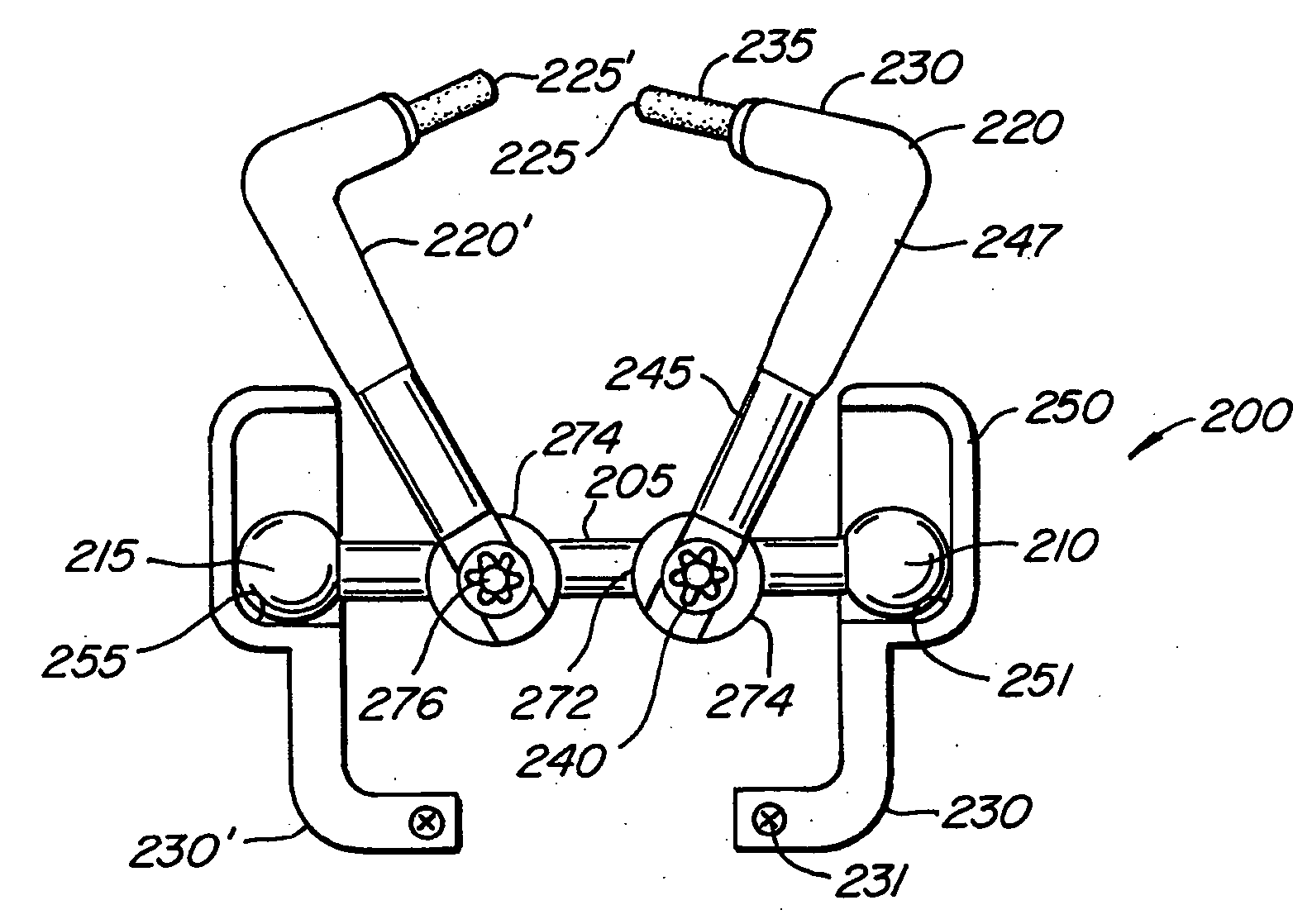
Articulating spinal fixation rod and system
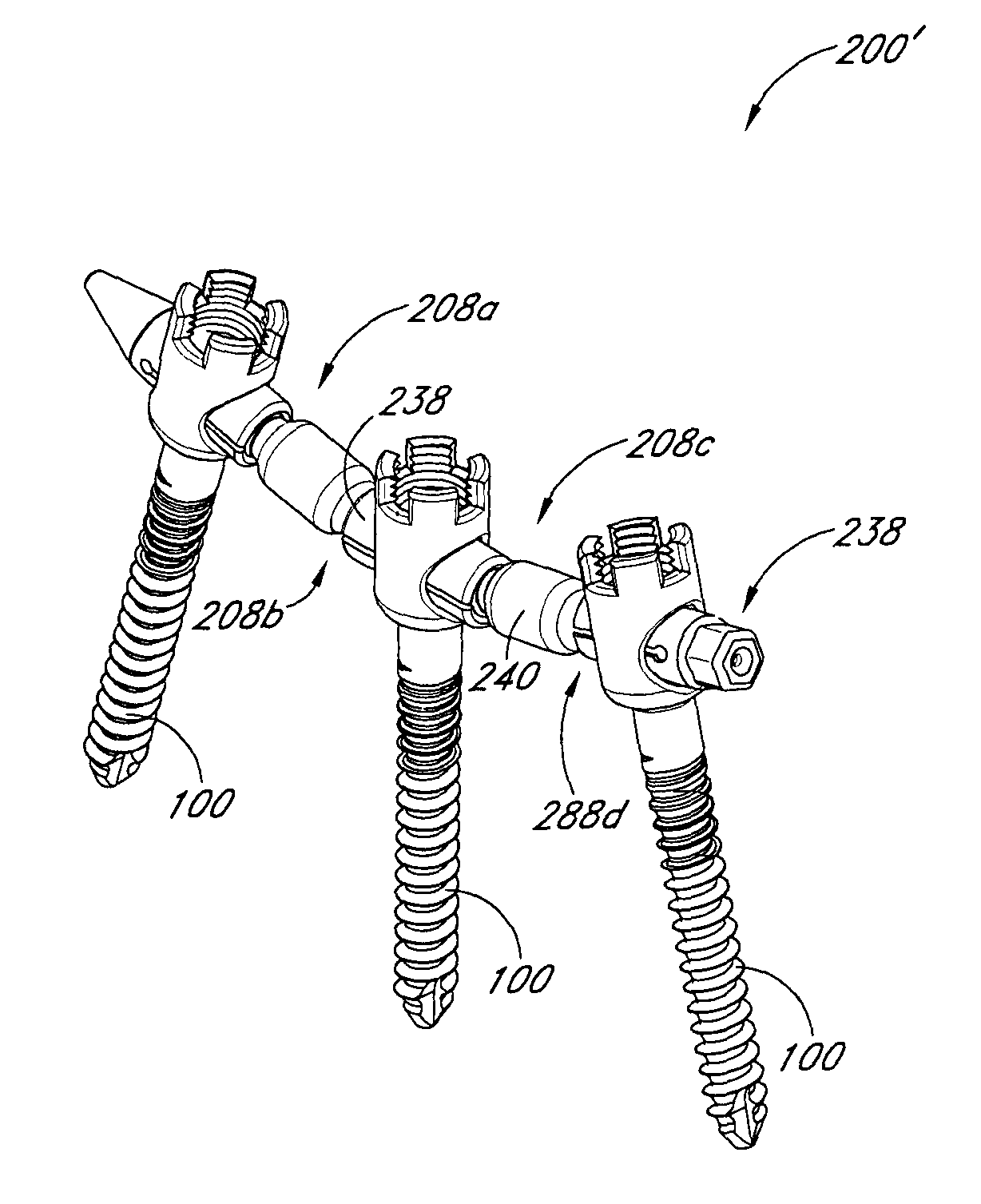
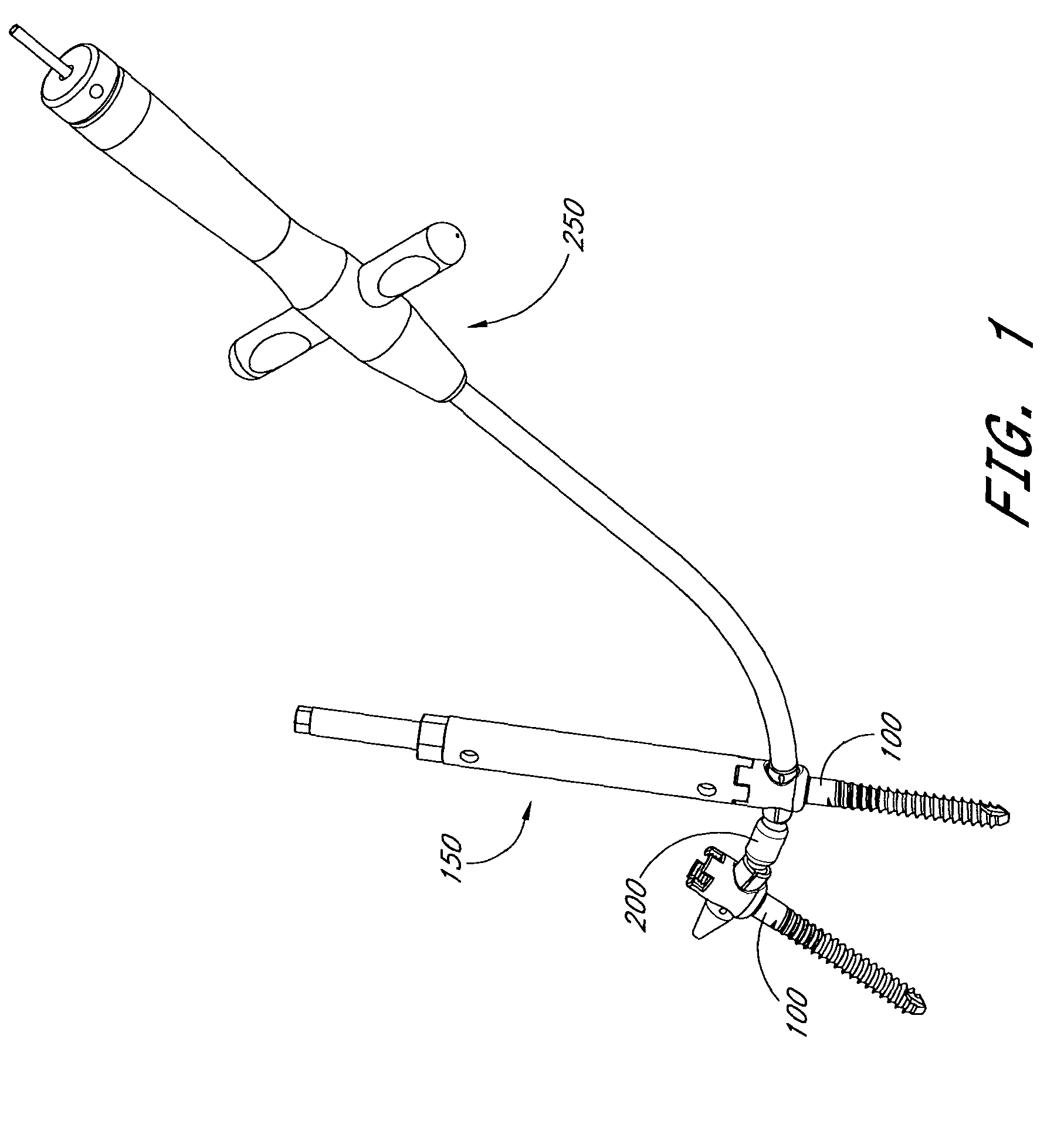
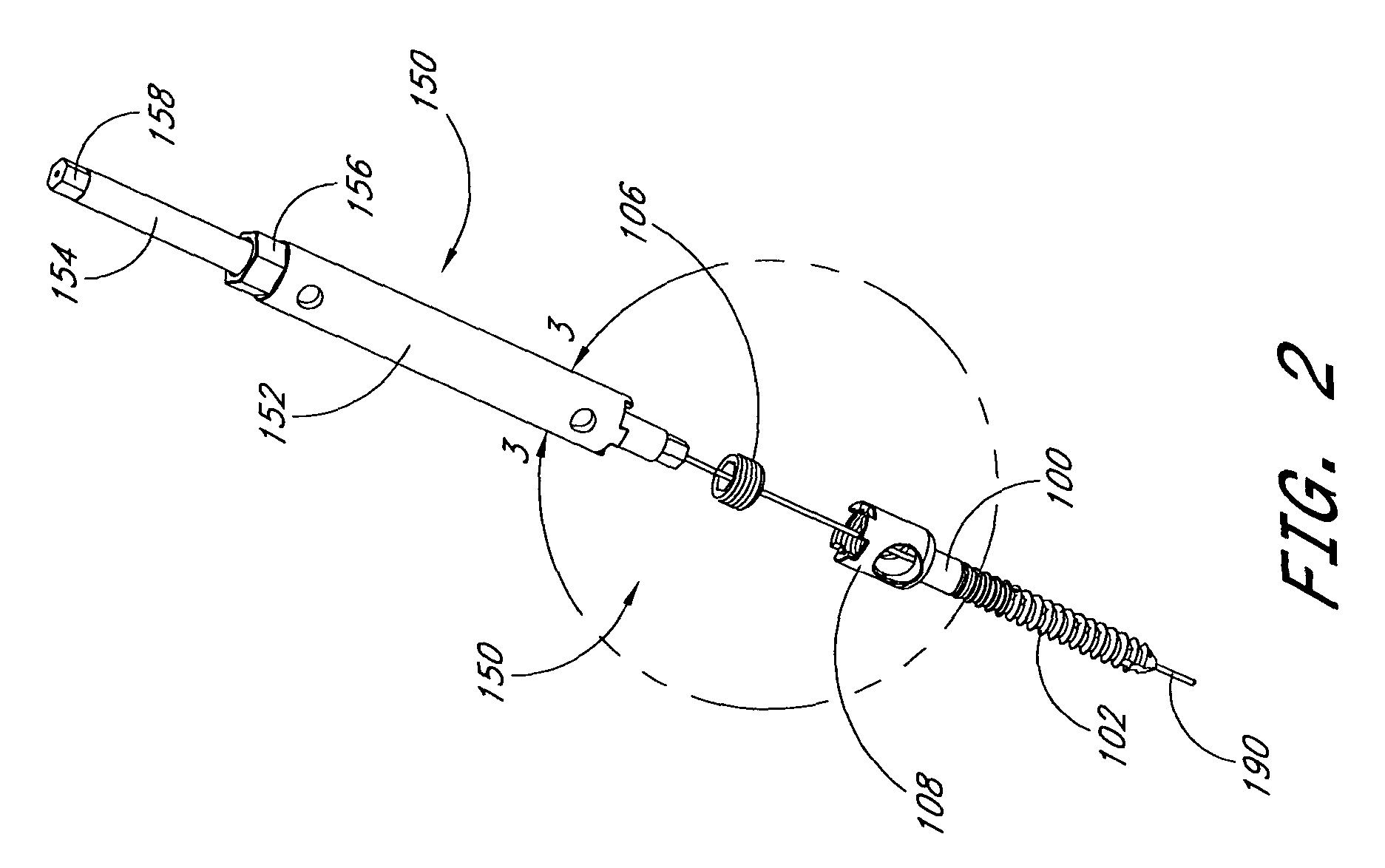
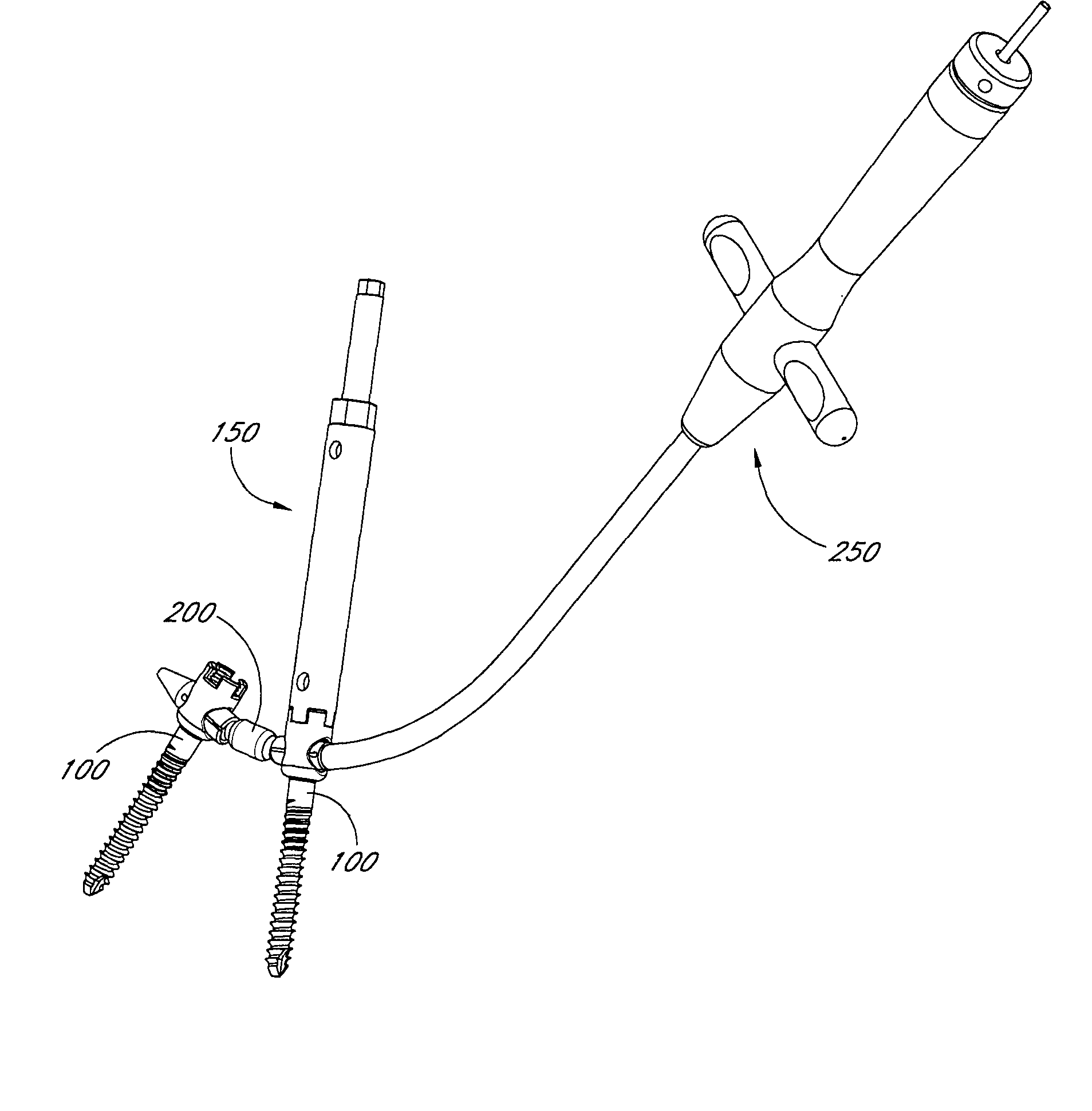
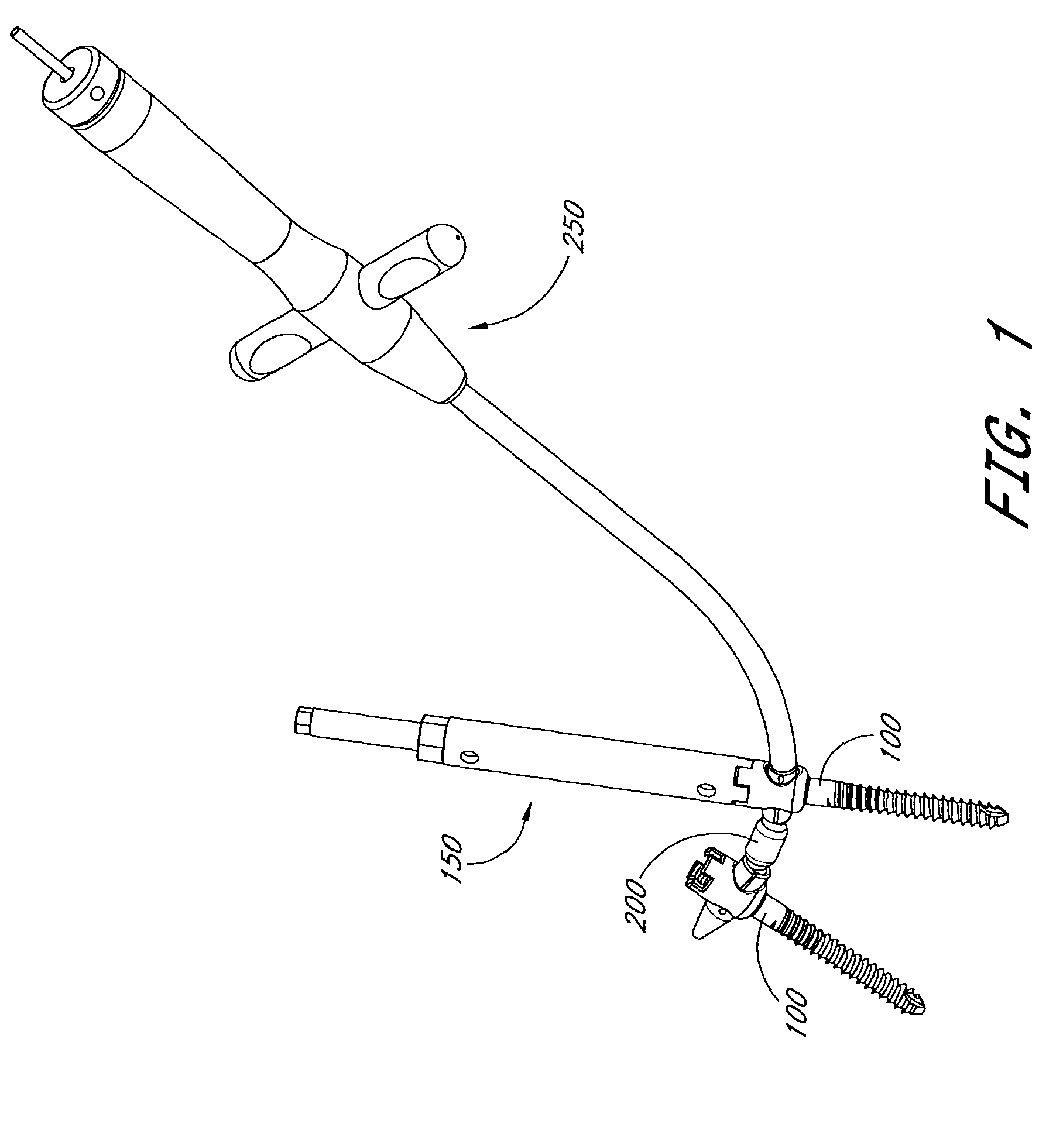
The present invention relates generally to systems and methods for aligning and implanting orthopedic fixation or stabilization implants within the body. In one embodiment, the system includes at least two bone anchors, at least one of which is provided with a transverse portal and a locking member. In one aspect, the system also includes at least one linkage rod, for linking two or more bone anchors through their respective locking members. The linking rod may include at least one angularly adjustable joint, which may be fixed by actuating the locking member. The bone anchors and the linkage rod may be locked into place to form a spinal fusion or fixation prosthesis.
Owner:WARSAW ORTHOPEDIC INC
Articulating spinal fixation rod and system
The present invention relates generally to systems and methods for aligning and implanting orthopedic fixation or stabilization implants within the body. In one embodiment, the system includes at least two bone anchors, at least one of which is provided with a transverse portal and a locking member. In one aspect, the system also includes at least one linkage rod, for linking two or more bone anchors through their respective locking members. The linking rod may include at least one angularly adjustable joint, which may be fixed by actuating the locking member. The bone anchors and the linkage rod may be locked into place to form a spinal fusion or fixation prosthesis.
Owner:WARSAW ORTHOPEDIC INC
Medical devices and applications of polyhydroxyalkanoate polymers
InactiveUS6867247B2Reduce probabilityHigh porositySuture equipmentsStentsTissue repairBiocompatibility Testing
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
Adjacent level facet arthroplasty devices, spine stabilization systems, and methods
ActiveUS20060052785A1Relieve pressureAvoid levelingInternal osteosythesisJoint implantsAdjacent segment diseaseFacet arthroplasty
The invention discloses an implantable facet arthroplasty device suitable for treating adjacent level disease. The device is designed for implantation between a first vertebra and a second vertebra. Components of the device include: a crossbar; a first component having a first attachment mechanism adapted to attach to a first location of a spinal fusion device attached to a first vertebra and a second attachment mechanism adapted to attach to the crossbar; and a second component having a second attachment mechanism adapted to attach to a second location of a spinal fusion device attached to the first vertebra and a second attachment mechanism adapted to attach to the crossbar. The first component articulates relative to the second component and the first vertebra articulates relative to the device itself.
Owner:GLOBUS MEDICAL INC
Systems and methods for performing spinal fusion
InactiveUS20110160772A1Increase success rateInternal osteosythesisJoint implantsFistSacroiliac joint
Described herein are methods, devices and systems for performing an interspinous fusion, in particular for performing an interspinous fusion unilaterally. In general an interspinous fusion system may include a first fixation plate configured to couple to a first lateral side of a spinous process, a rod extending from the first fixation plate at a joint such that the rod is pivotable with respect to the first fixation plate, and a second fixation plate configured to couple to a second lateral side of a spinous process opposite from the first fixation plate. In general, a method of performing an interspinous fusion unilaterally may include the steps of placing a first fixation plate, having a rod extending from the fixation plate, between two adjacent spinous processes from a fist lateral side of the spinous processes, pivoting the rod with respect to the first fixation plate such that the plate abuts the second, opposite, lateral side of at least one of the spinous processes, and placing a second fixation plate such that it abuts the fist lateral side of at least one of the spinous processes.
Owner:BAXANO
Allograft implant
ActiveUS7491237B2Inhibit migrationMaximize sizeBone implantJoint implantsBone CortexIntervertebral fusion
Owner:SYNTHES USA
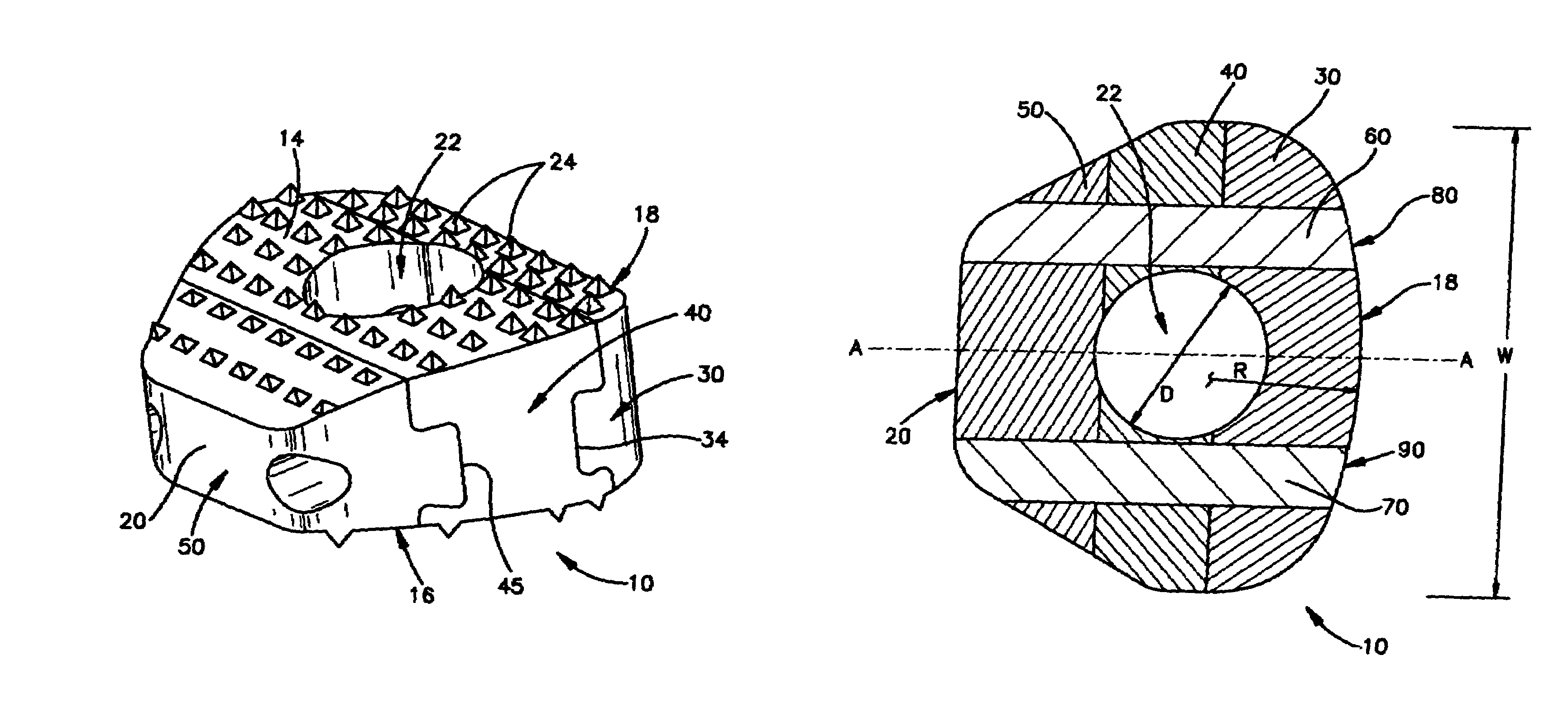
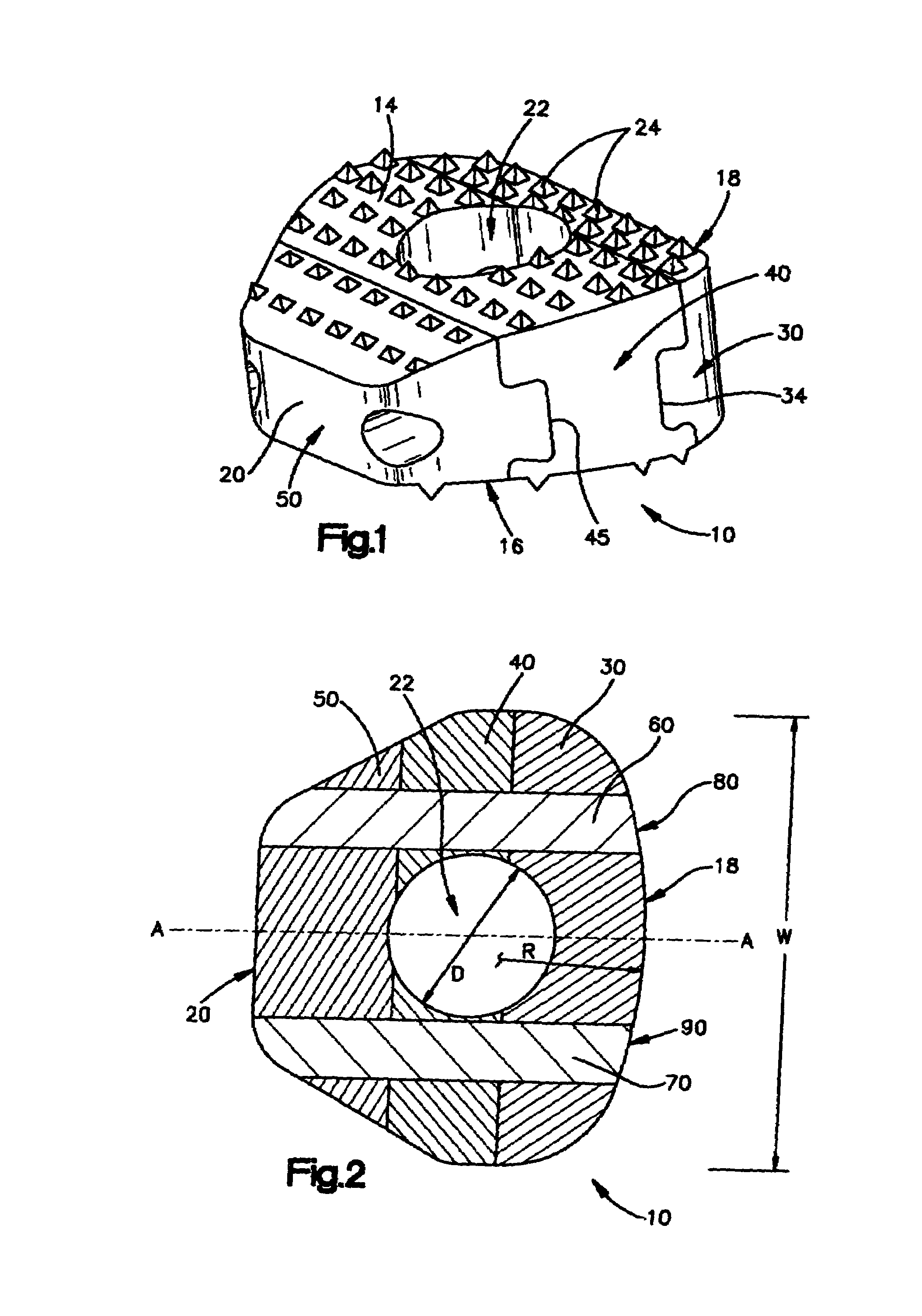
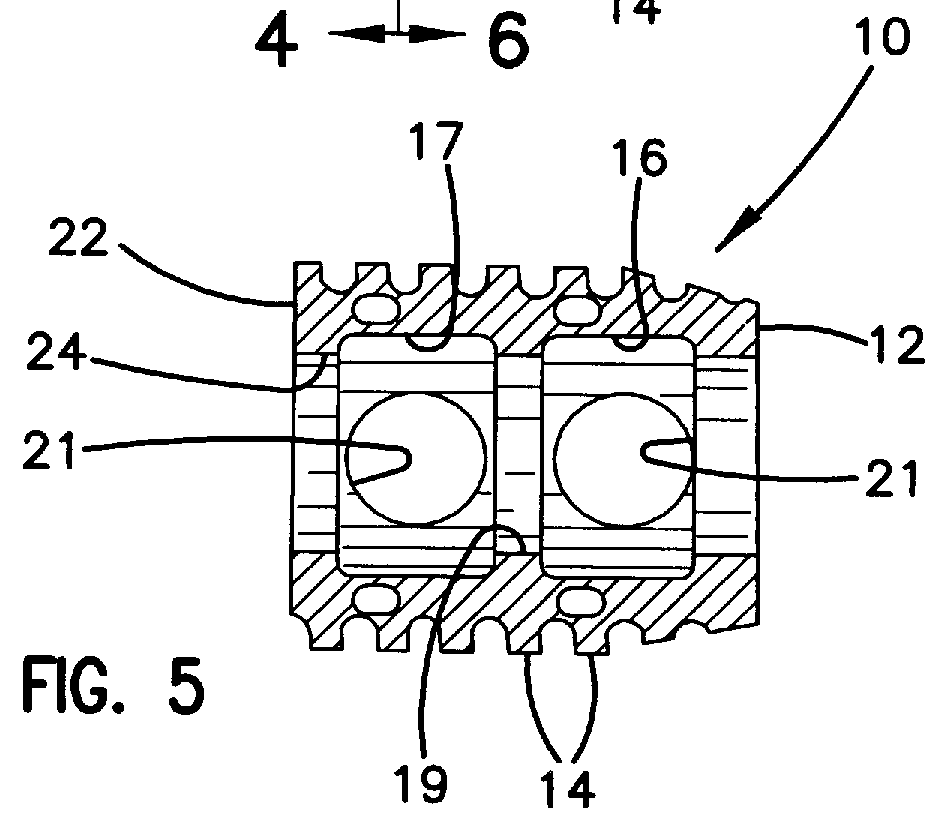
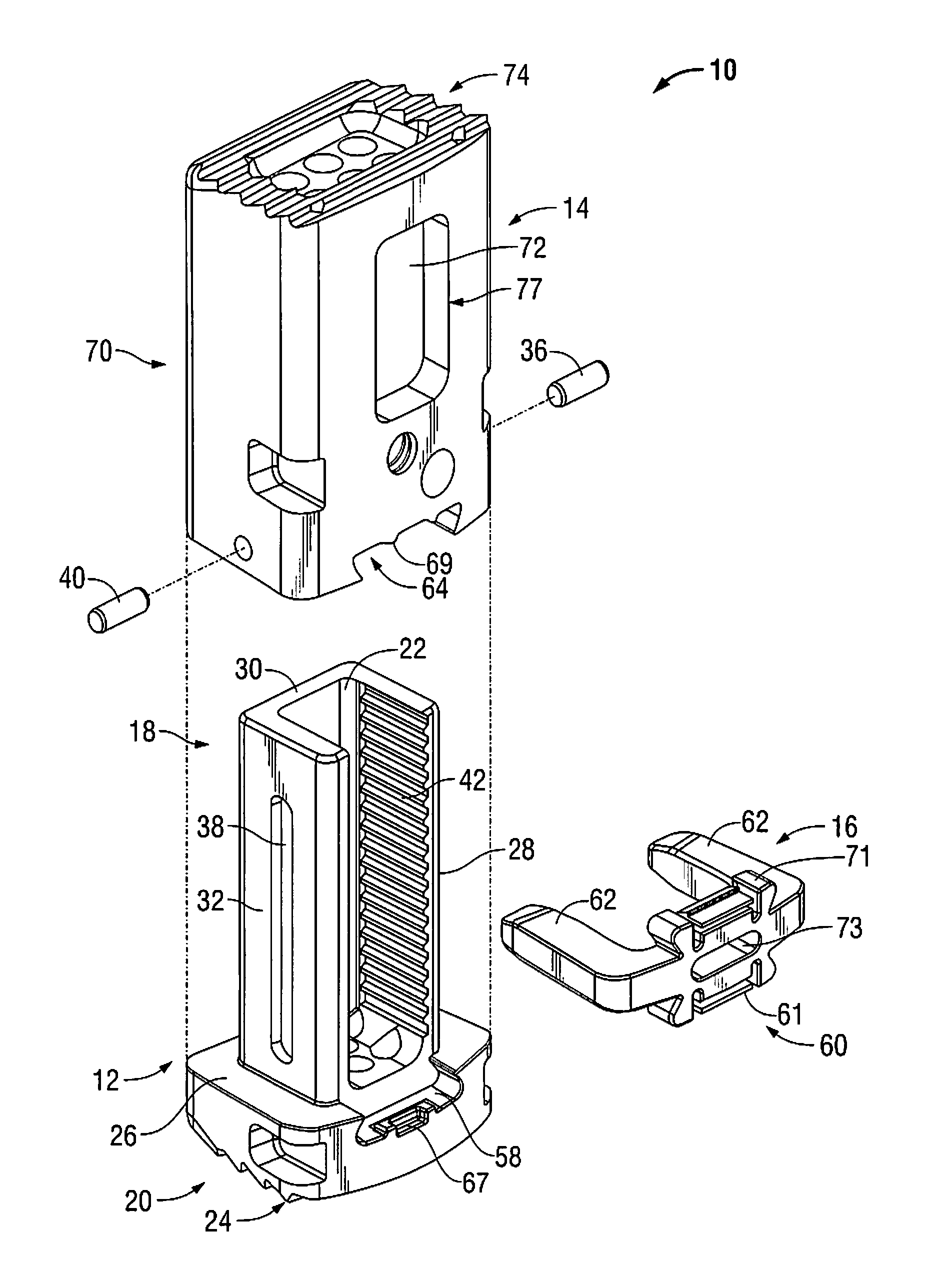
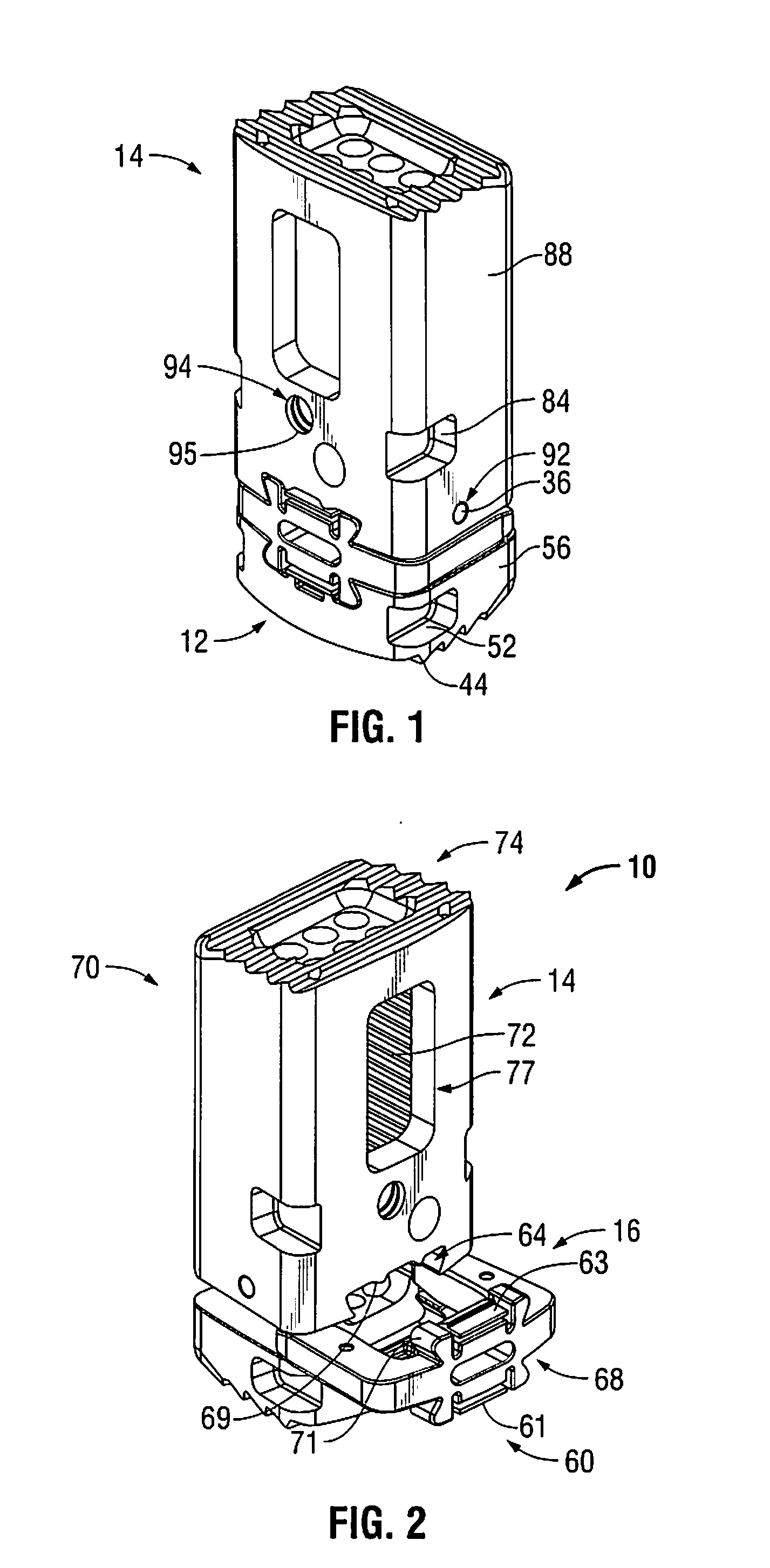
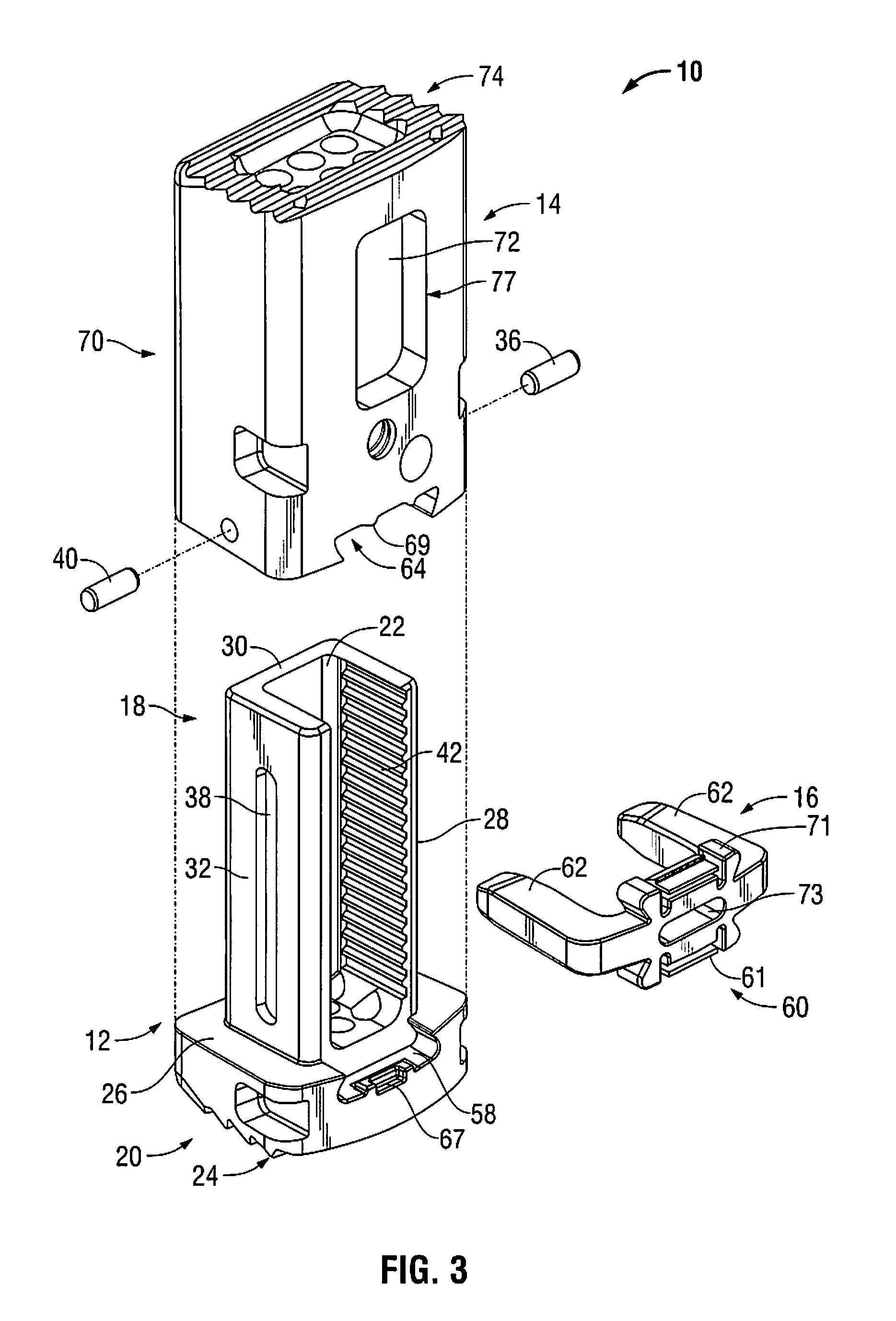
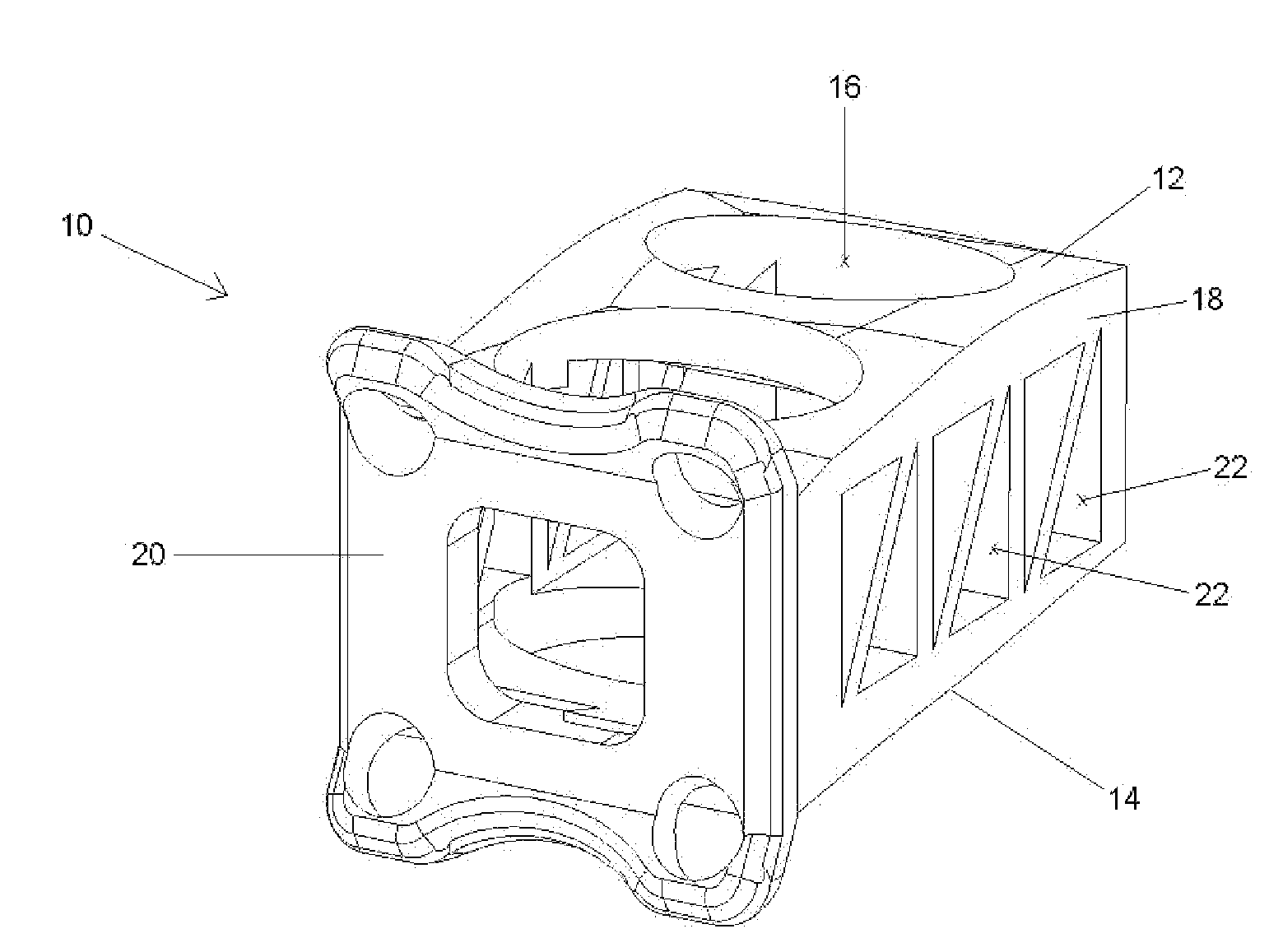
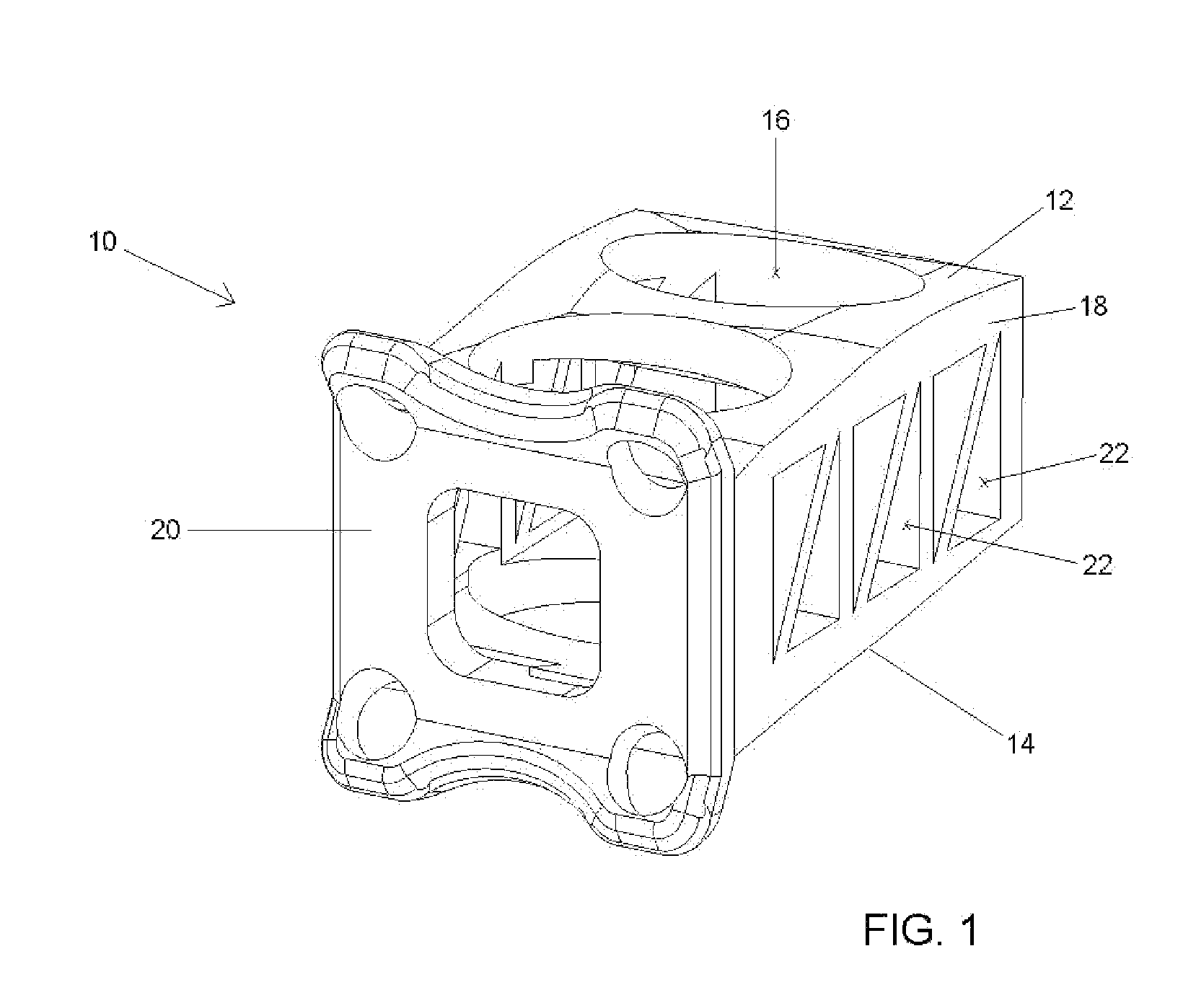
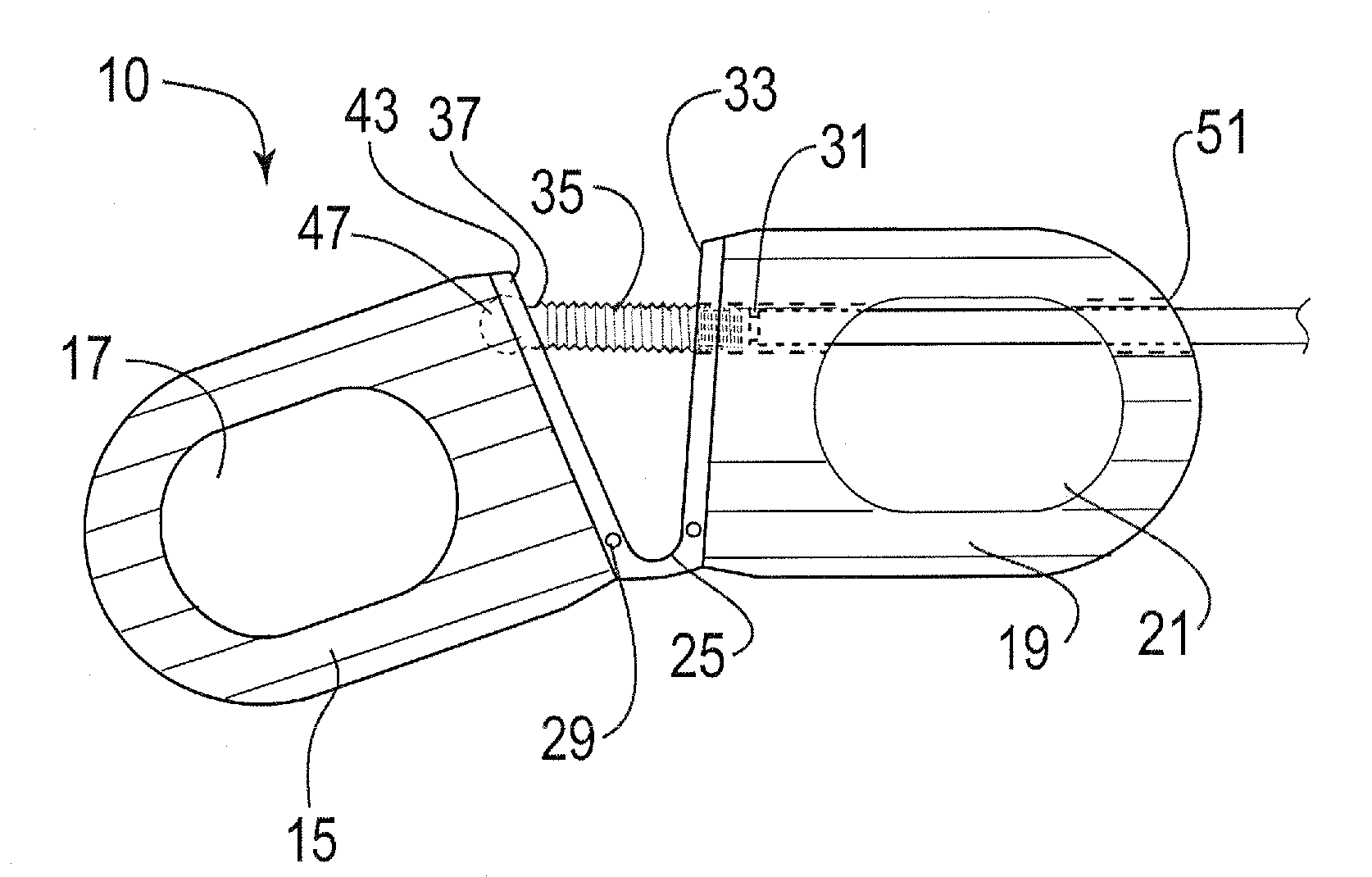
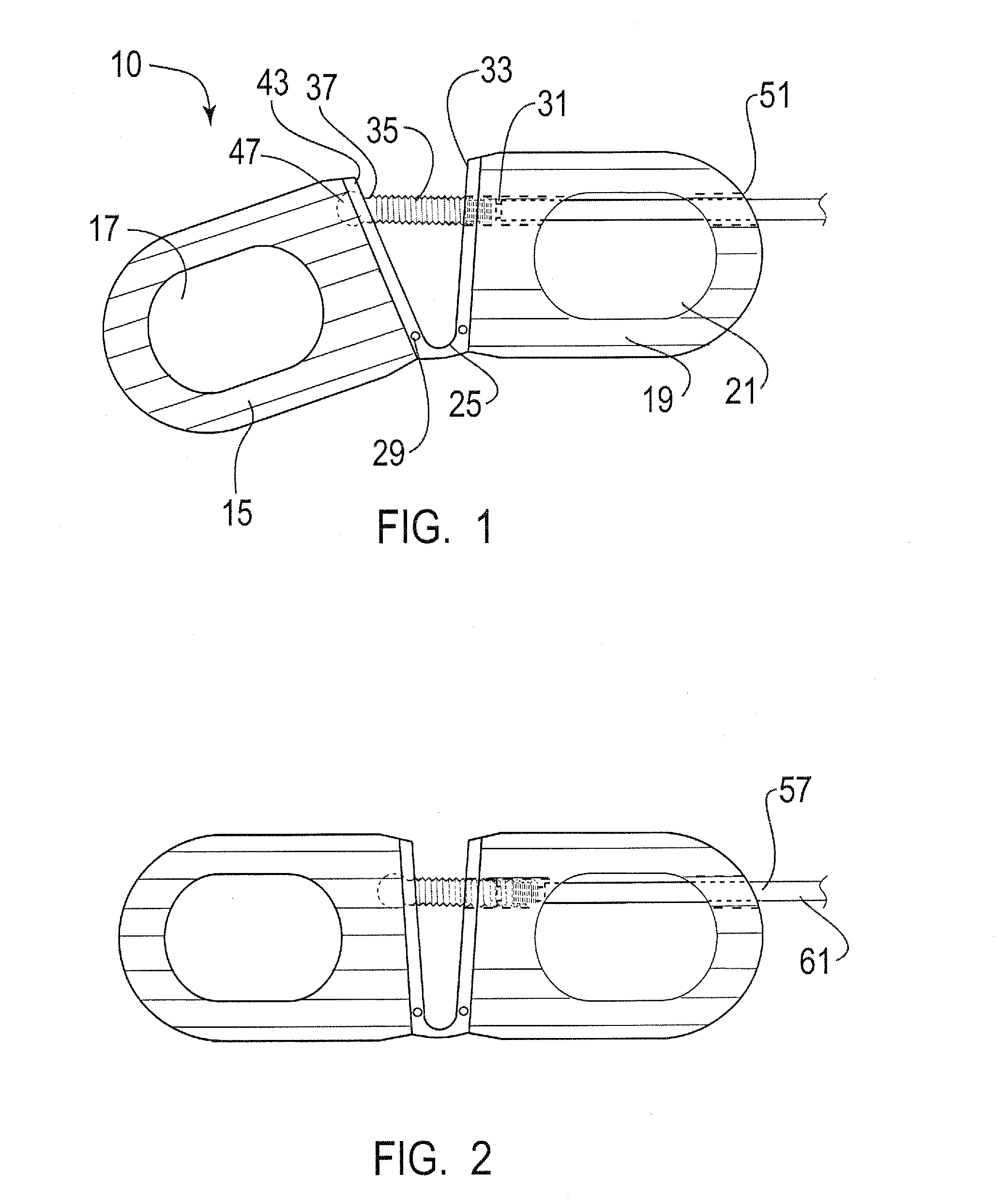
Modular spinal fusion device
An implant is disclosed for use in stabilization of one or more bones, for example, adjacent vertebrae. The implant includes two or more coupled structural elements. Each structural element includes a first end spaced apart by a side surface from a second end. The side surfaces of each structural element include at least one recess and the structural elements are coupled by matingly engaging one or more recesses.
Owner:ZIMMER BIOMET SPINE INC
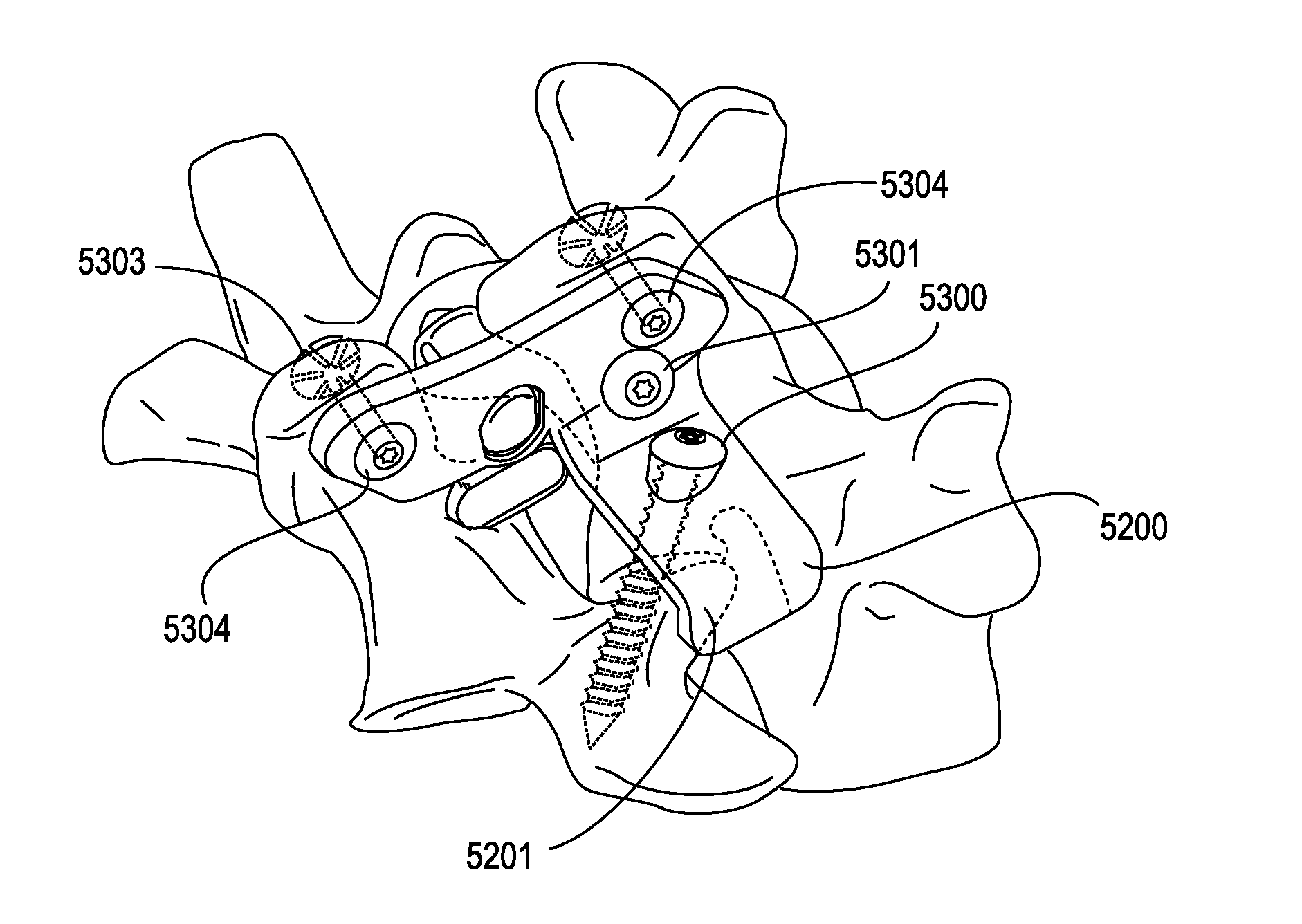
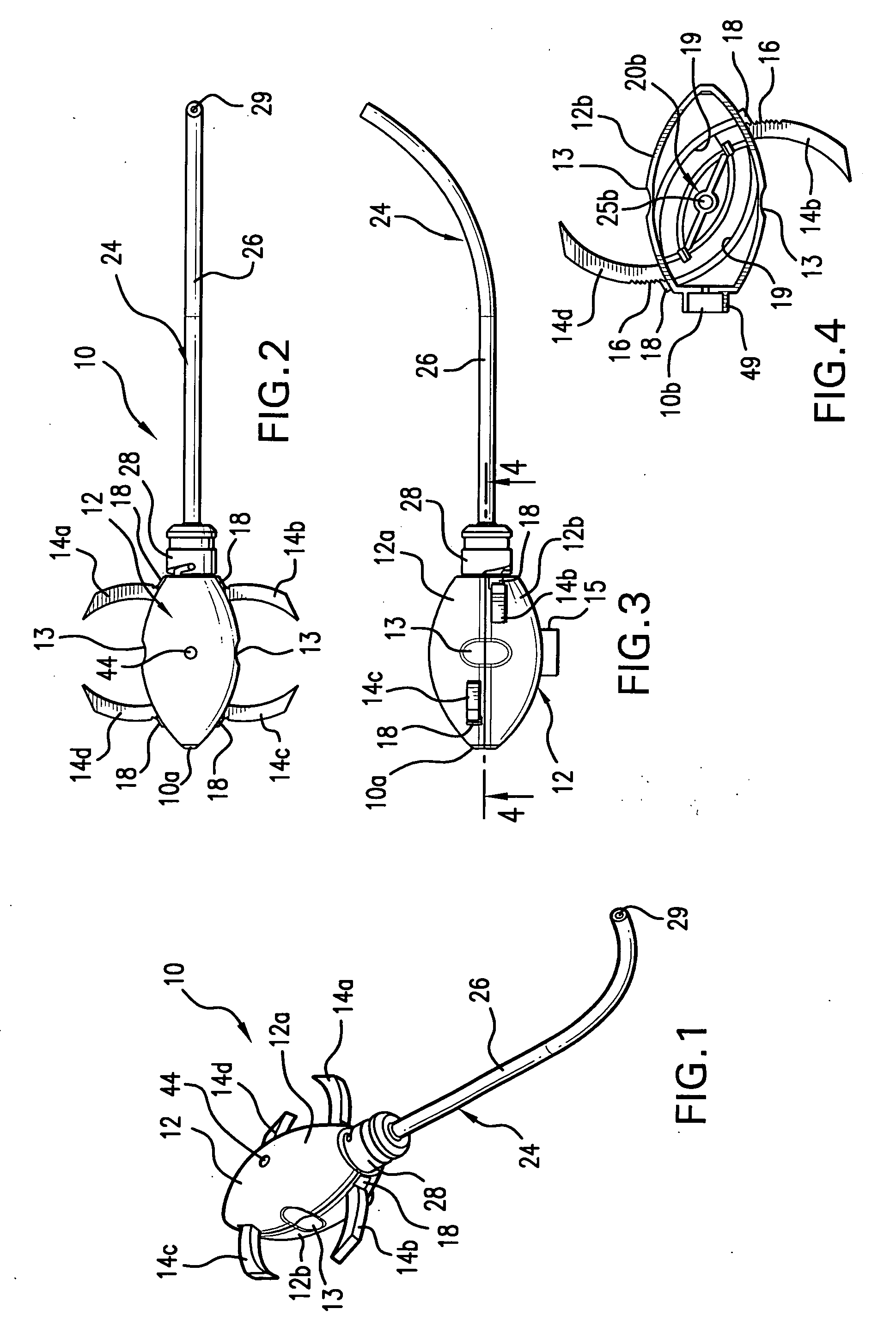
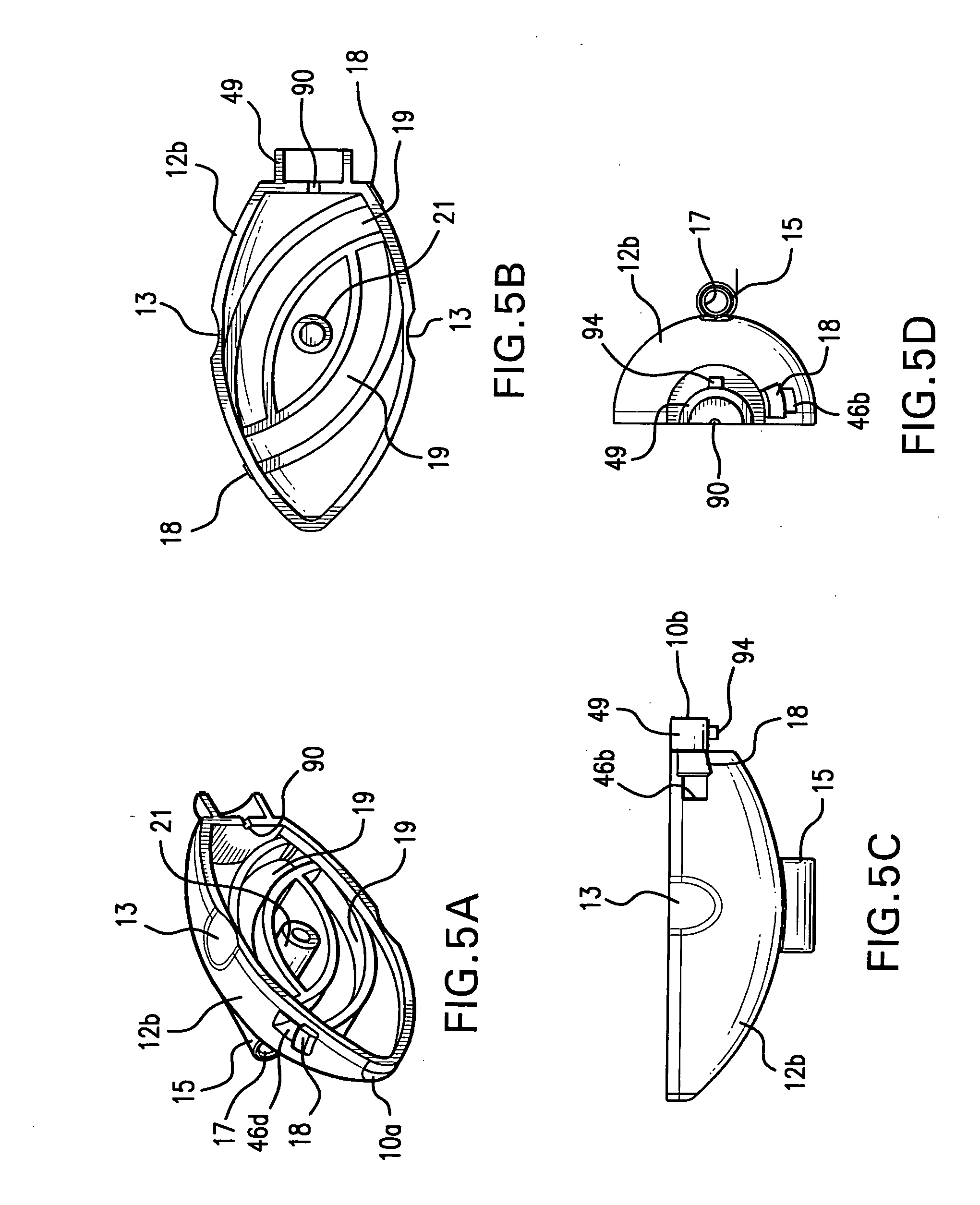
Method and apparatus for spinal facet fusion
A spinal facet fusion implant comprising:an elongated body having a distal end, a proximal end and a longitudinal axis extending between the distal end and the proximal end, the elongated body having a cross-sectional profile characterized by a primary axis and a secondary axis; andat least one stabilizer extending radially outwardly from the elongated body in the secondary axis;wherein the elongated body has a length along the primary axis which is less than the combined width of the spinal facets making up a facet joint;and further wherein the at least one stabilizer has a width which is sized to make a press fit into the gap between the spinal facets making up a facet joint.
Owner:VG INNOVATIONS
Method for processing and using adipose-derived stem cells
The present invention relates to a device comprising a cell carrier portion containing regenerative cells, e.g., stem and progenitor cells, and a cell carrier containment portion. The device is useful for the treatment of bone related disorders, including spinal fusion related disorders and long bone or flat bone related defects. The device may be used in conjunction with disclosed automated systems and methods for separating and concentrating regenerative cells.
Owner:LOREM VASCULAR PTE LTD
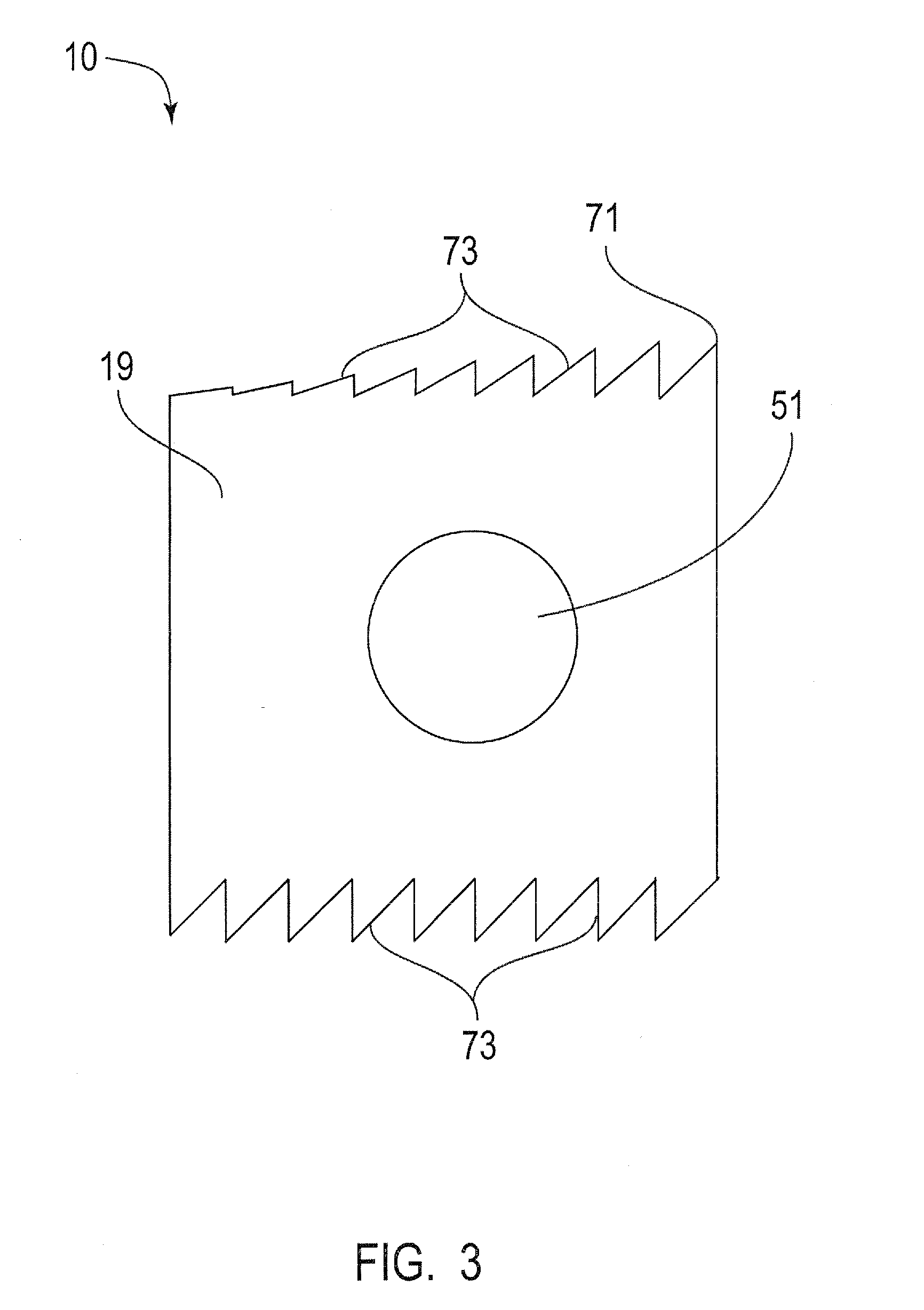
Apparatus and method for spinal stabilization
A surgical method and apparatus for implanting a spinal fusion implant includes a rigid centering guide having a distal end sized to be inserted into the disc space with the guide extending along a longitudinal axis from a distal end to a proximal end. The guide includes a first external guide surface which extends at least partially between the distal end and the proximal end. The external guide surface is shaped complimentary to an external guided surface of a drill guide. The external guide surface and the guided surface are nested such that the guided surface slides against the external guide surface along a path of travel parallel to the longitudinal axis.
Owner:ZIMMER SPINE INC
Expandable cage
An apparatus supports the spine between vertebrae and promotes spinal fusion. The apparatus generally includes a first supporting member, a second support member, and an expansion member. The first support member has a first longitudinal passage extending therethrough, a first supporting end configured to engage tissue, and a rack configured to engage a tool. The second supporting member contains a second longitudinal passage extending therethrough and a second supporting end configured to engage tissue. The second longitudinal passage is dimensioned to receive at least a portion of the first supporting member. The first and second supporting members are configured to move with respect to each other. The expansion member is removably positioned between the first and second supporting members and is adapted to maintain the first and second supporting members in a fixed relative position.
Owner:K2M
Anterior Spinal Fusion and Fixation Cage with Integrated Plate and Method of Use
The present invention provides a fixation and fusion cage with interlocking plate comprising a central cage portion and interlocking plate that rigidly attaches to the front of the cage. The cage can be varied in shape to account for various clinical necessities such as lordosis. The invention may be installed in an offset configuration so as to avoid damage to blood vessels. The device may be constructed from radiolucent material to allow for visualization of bone fusion within the cage.
Owner:ABERNATHIE DENNIS LEE
Interspinous implants and methods for implanting same
A spinal implant for treating lumbar spinal stenosis or as an adjunct to spinal fusion. The implant includes a body portion having an interior cavity. A plurality of locking wings are adapted and configured to move between a stowed position retracted within the interior cavity of the body portion and a deployed position extended from the interior cavity of the body portion. In the deployed position, the wings fix the implant in a selected interspinous space. A cable and wheel arrangement moves the plurality of locking wings from the stowed position to the deployed position and a ratchet / pawl assembly prevents backward movement of the wings.
Owner:SPINAL SIMPLICITY
Transforaminal lumbar interbody fusion cage
InactiveUS20070260314A1Easy to insertShrink the necessary spaceSpinal implantsSpinal cordEngineering
A cage to separate and support adjacent vertebrae in the spine that have undergone orthopedic spinal fusion procedures. The cage has first and second spacer members for insertion between adjacent vertebrae with a hinge located between the spacers. An advancing mechanism is located between the first and second spacer members that pivotally moves the first and second spacer members relative to each other at an angle which facilitates the insertion of the cage around the spinal cord. After insertion, the advancing mechanism is operable to position the first and second spacer members in the desired position between the two adjacent vertebrae.
Owner:BIYANI ASHOK
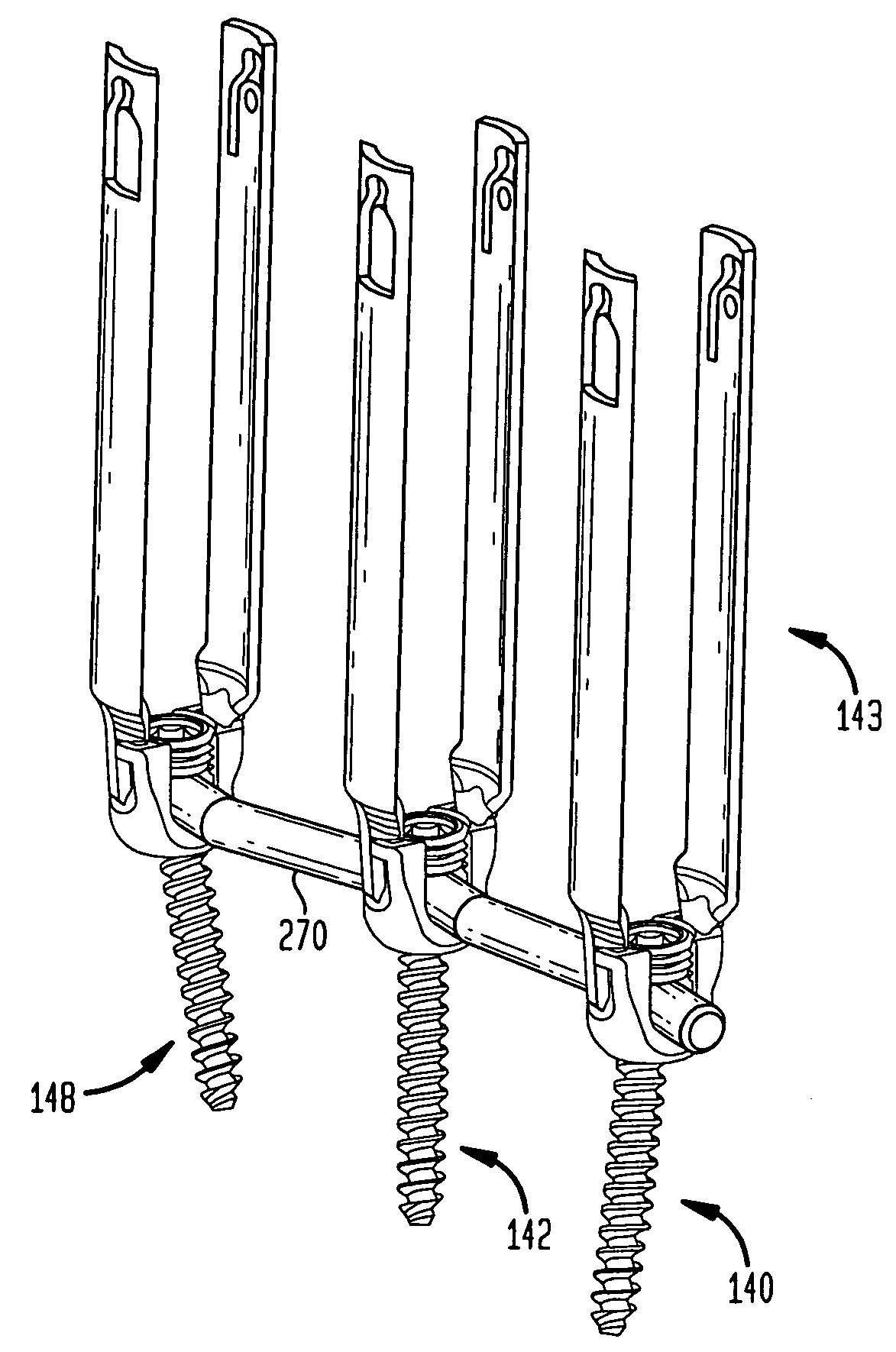
Rod contouring apparatus for percutaneous pedicle screw extension
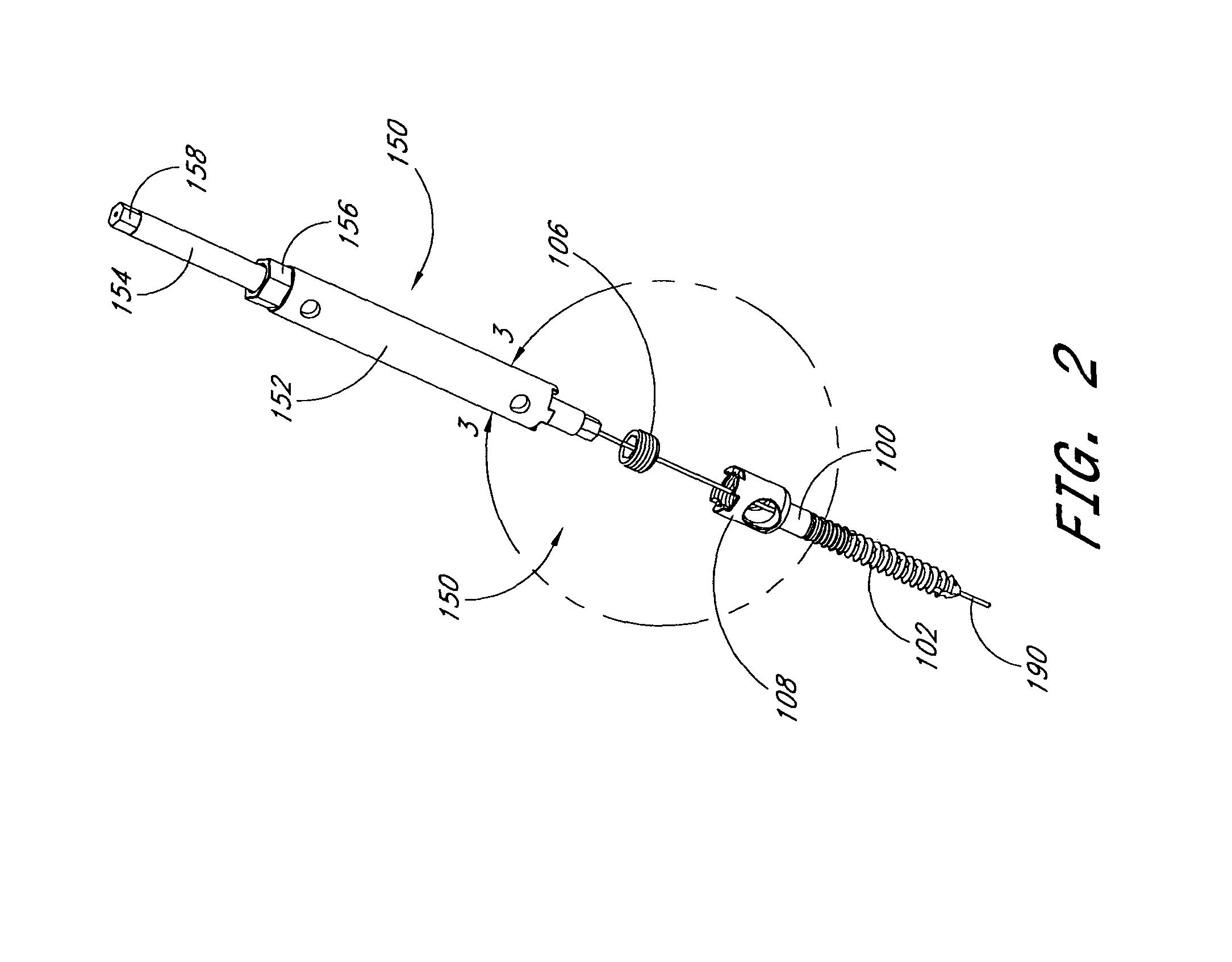
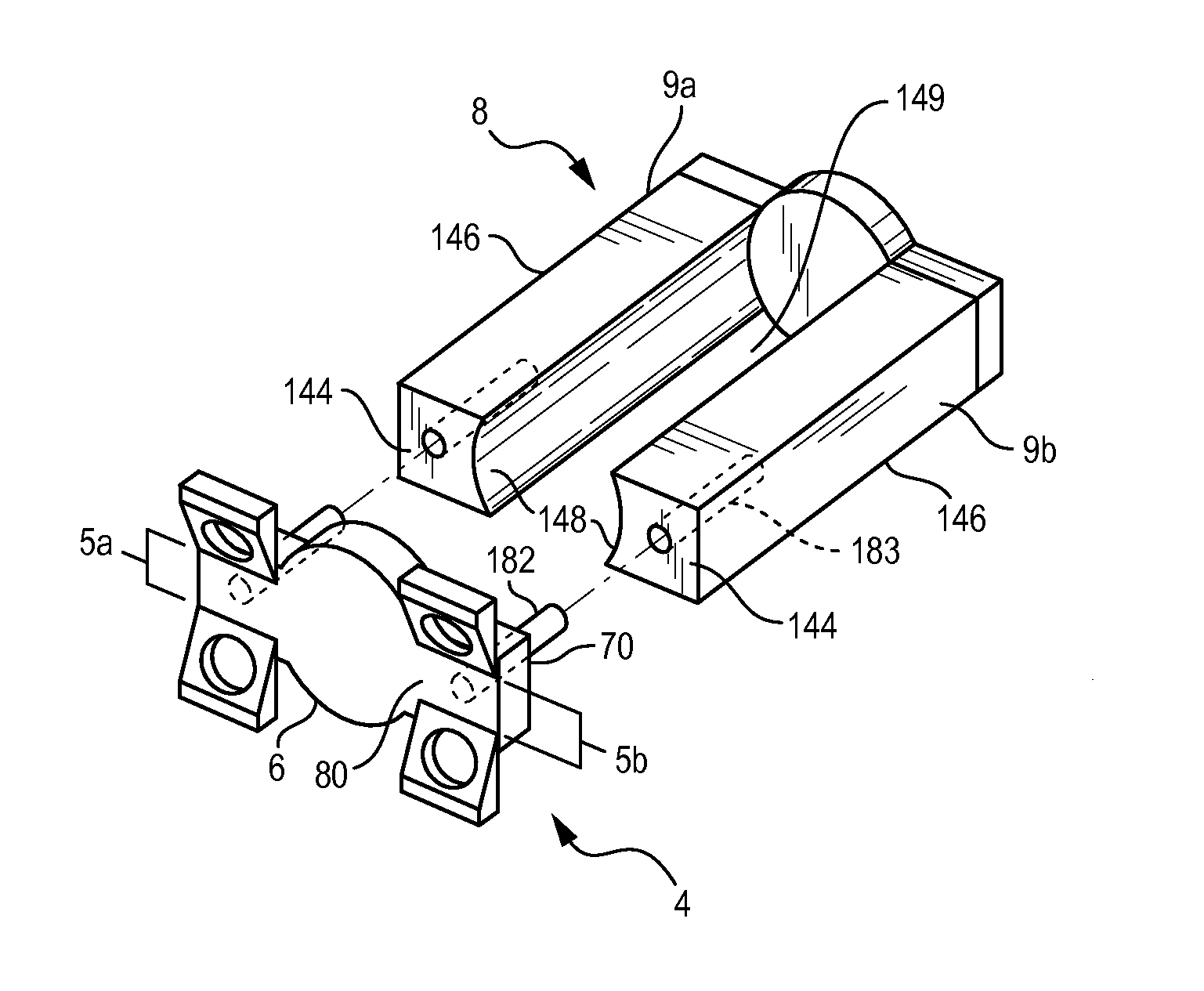
ActiveUS20090099605A1Suitable level of positional controlEasy to shapeSuture equipmentsInternal osteosythesisDilatorIliac screw
Anatomic points within the body are projected outside the body through the use of extenders (180, 182, 188). The projected points may then be used for measurement, or to facilitate the selection or configuration of an implant that is positioned proximate the anatomic points using a slotted cannula (143). Such an implant may be a rod (270) for a posterior spinal fusion system. Pedicle screws (140, 142, 148) may be implanted into pedicles of the spine, and may then serve as anchors for the extenders. The extenders (180, 182, 188) may have rod interfaces (214, 216, 218) that receive the rod (270) in a manner that mimics the geometry of the pedicle screws (140, 142, 148) so that the selected or configured contoured rod (270) will properly fit into engagement with the pedicle screws (140, 142, 148).
Owner:STRYKER EURO OPERATIONS HLDG LLC
Balloon technologies for tissue repair
A medical device containing an inflatable balloon structure for use in minimally invasive surgery and minimally invasive diagnostic and therapeutic procedures are described herein. The device is delivered by a catheter and expanded using gases, liquids or liquids that solidify in situ. The inflatable balloon may be constructed from a wide variety of materials and may be reinforced by supporting structures, when necessary. The device may form an endoprosthesis in a patient. In the preferred embodiment, the device is used in spinal fusion. Optionally, the device may also be used in combination with bone graft materials and bioactive factors.
Owner:KUROS BIOSURGERY AG
Method and apparatus for spinal facet fusion
A spinal facet fusion implant comprising:an elongated body having a distal end, a proximal end and a longitudinal axis extending between the distal end and the proximal end, the elongated body having a cross-sectional profile characterized by a primary axis and a secondary axis; andat least one stabilizer extending radially outwardly from the elongated body in the secondary axis;wherein the elongated body has a length along the primary axis which is less than the combined width of the spinal facets making up a facet joint;and further wherein the at least one stabilizer has a width which is sized to make a press fit into the gap between the spinal facets making up a facet joint.
Owner:VG INNOVATIONS
Interspinous implants and methods for implanting same
A spinal implant for treating lumbar spinal stenosis or as an adjunct to spinal fusion. The implant includes a body portion having an interior cavity. A plurality of locking wings are adapted and configured to move between a stowed position retracted within the interior cavity of the body portion and a deployed position extended from the interior cavity of the body portion. In the deployed position, the wings fix the implant in a selected interspinous space. A cable and wheel arrangement moves the plurality of locking wings from the stowed position to the deployed position and a ratchet / pawl assembly prevents backward movement of the wings.
Owner:SPINAL SIMPLICITY
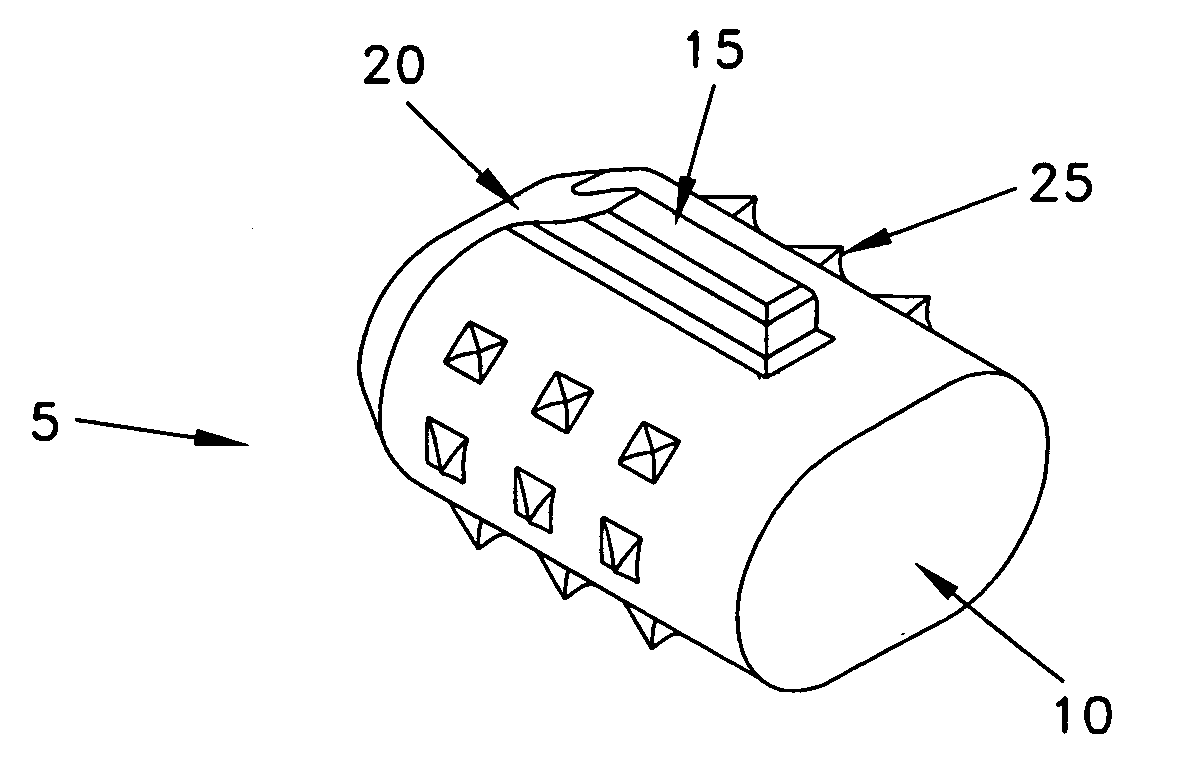
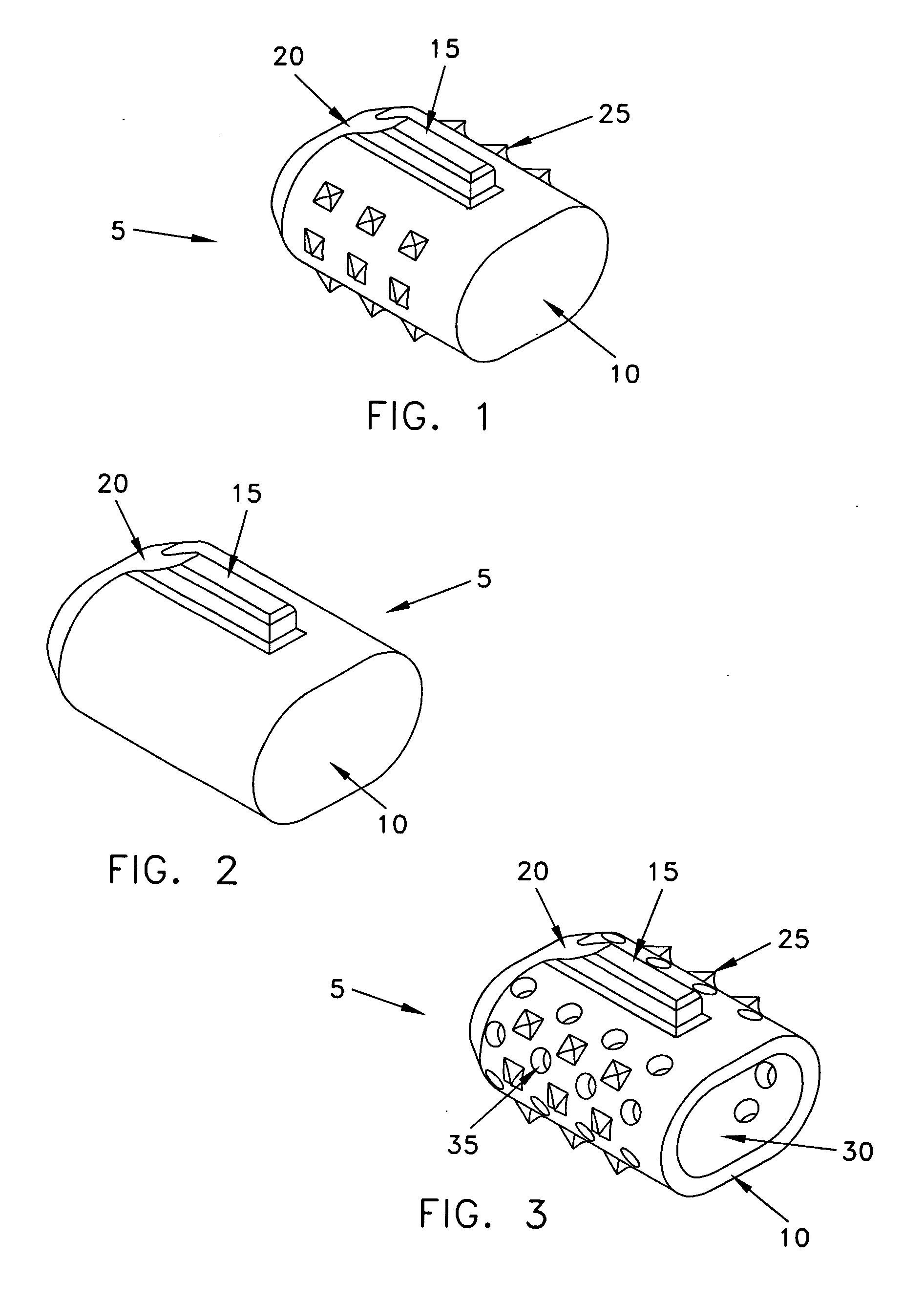
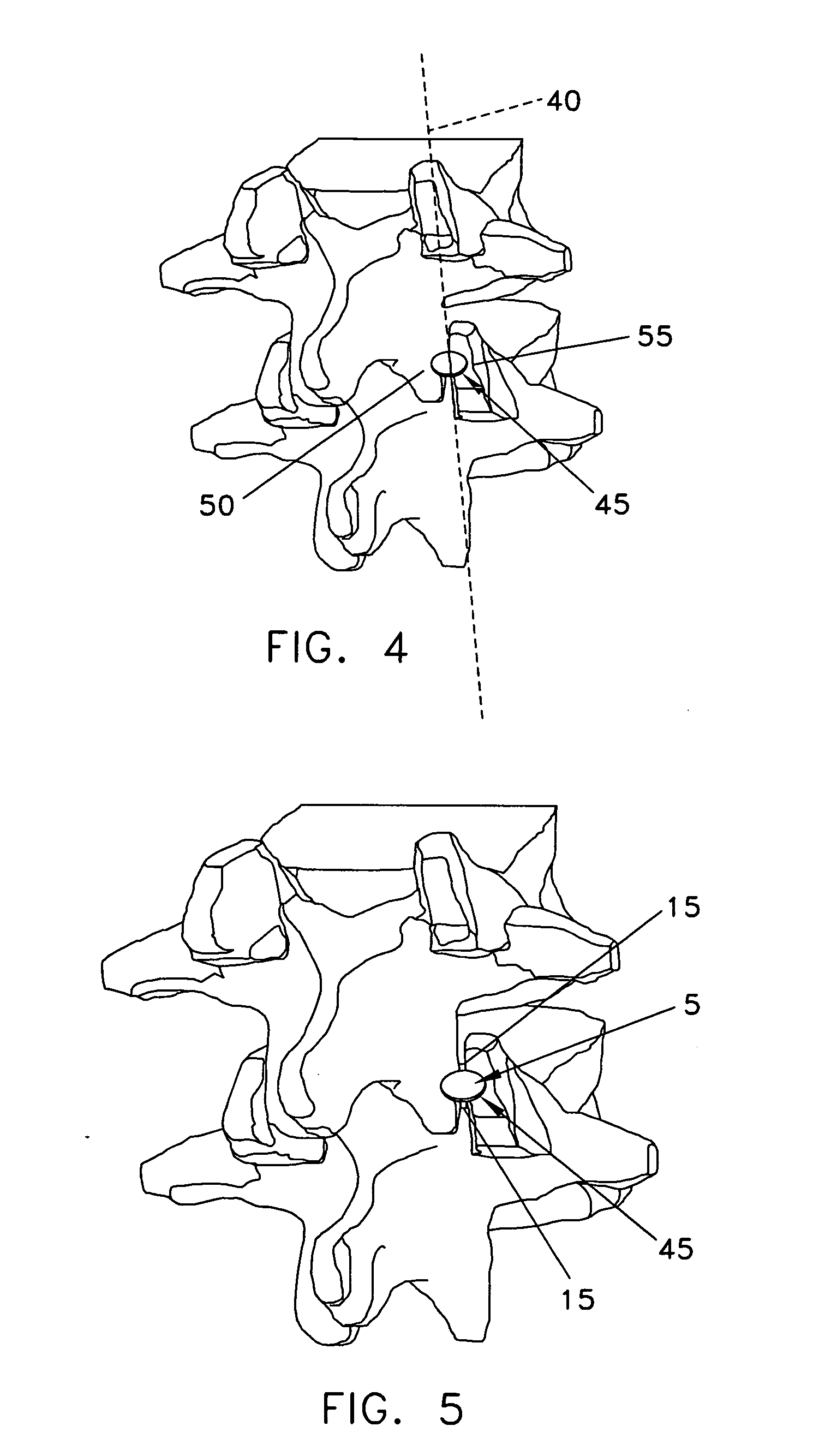
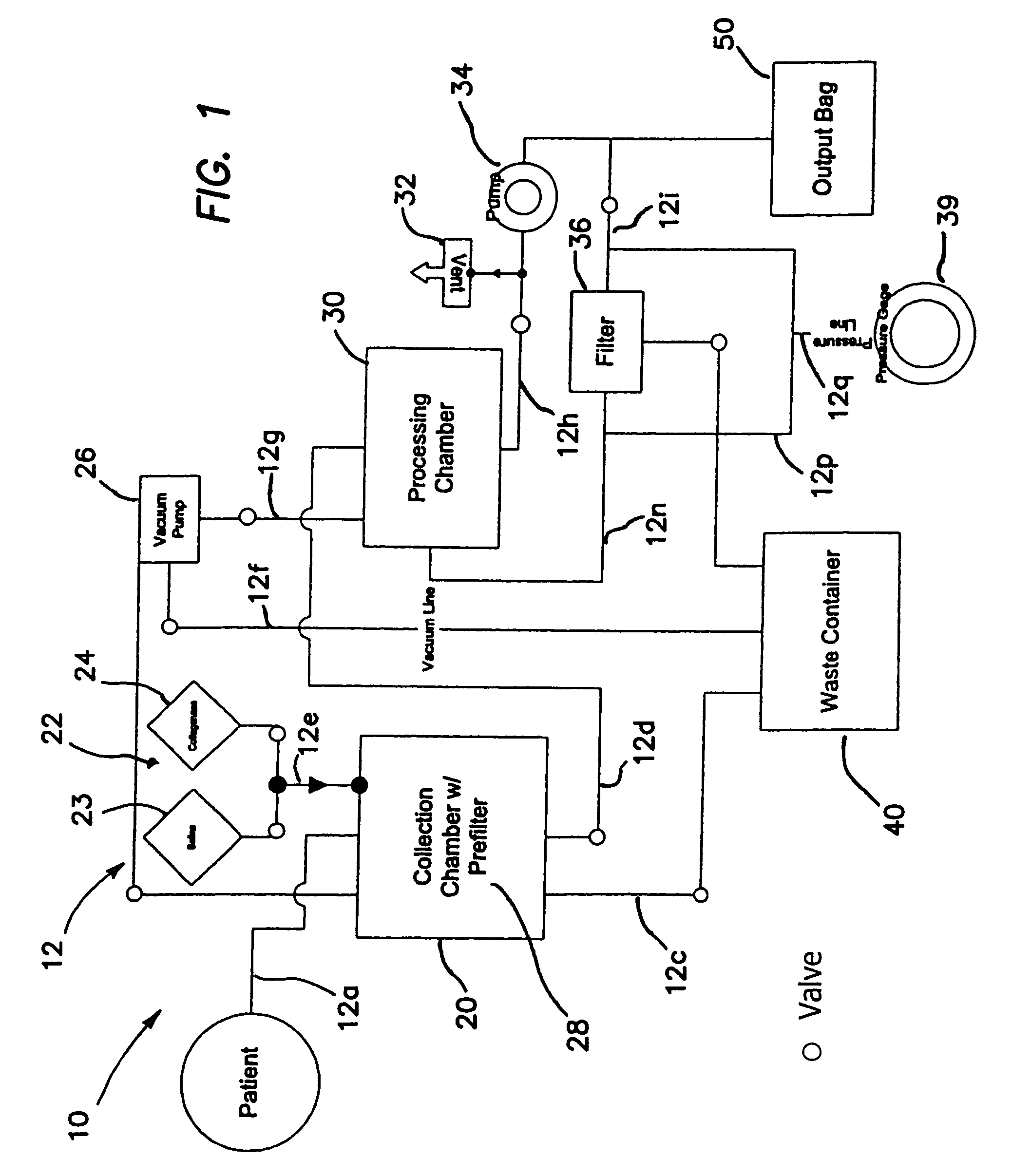
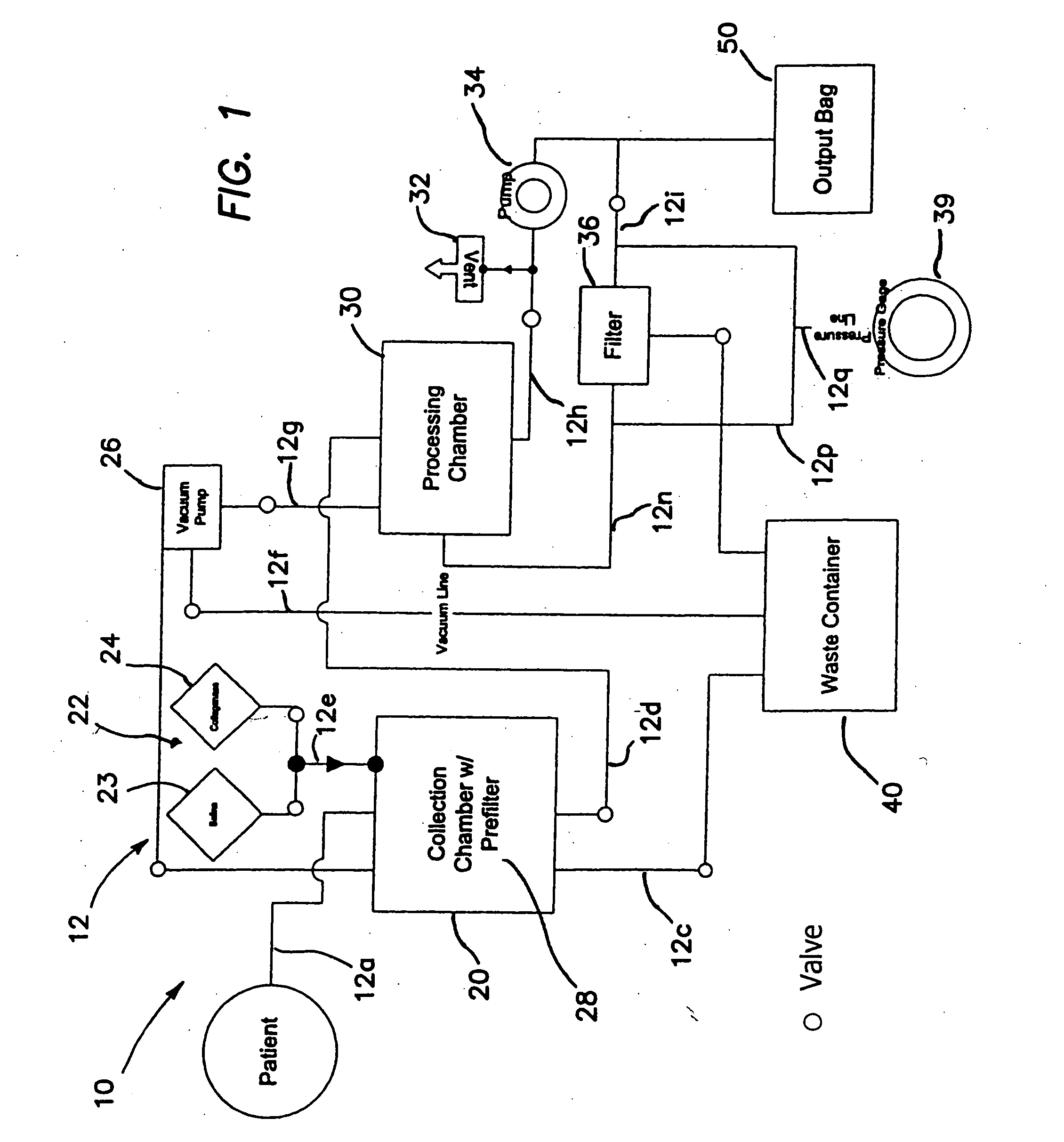
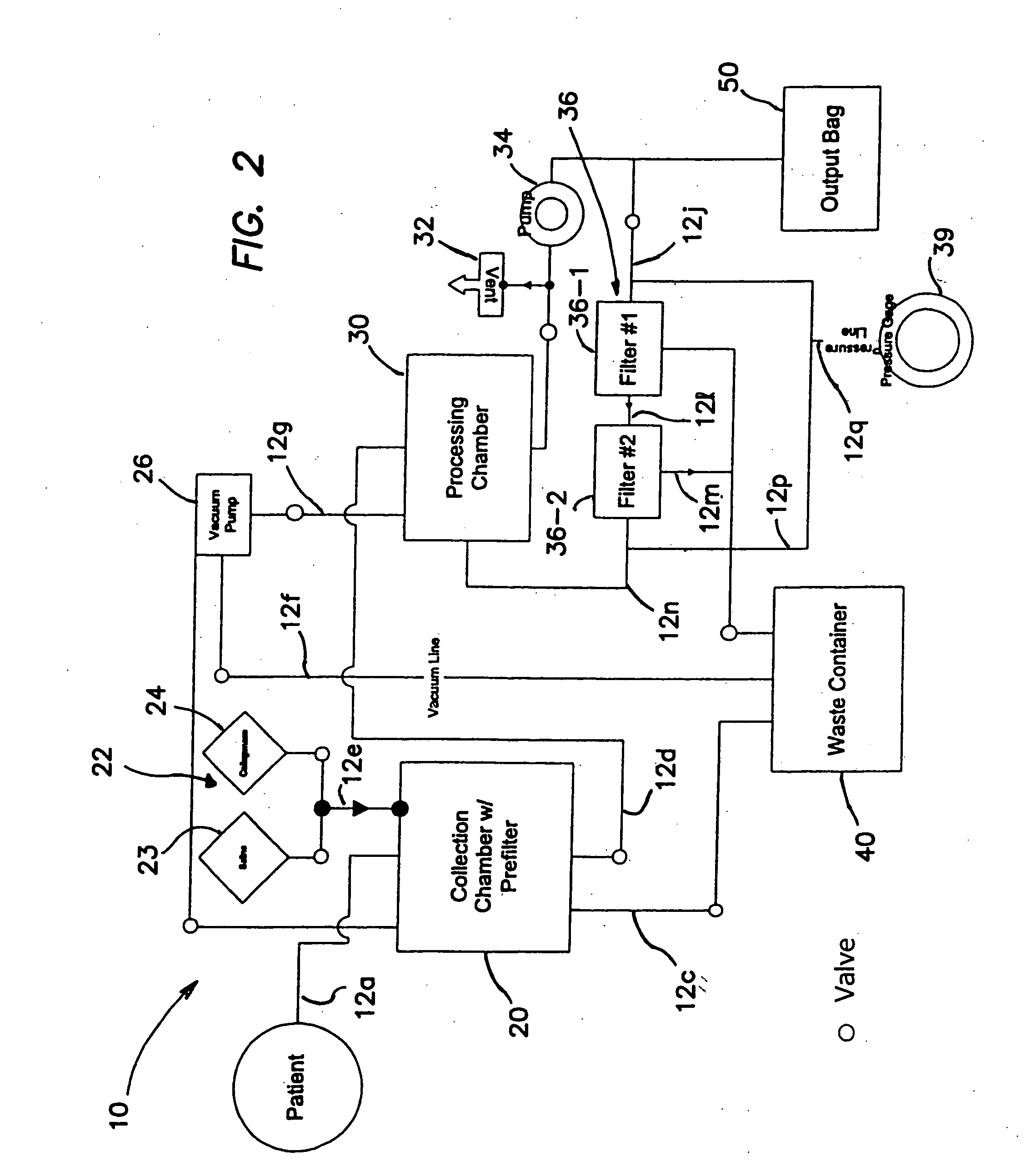
Device for collecting bone material during a surgical procedure
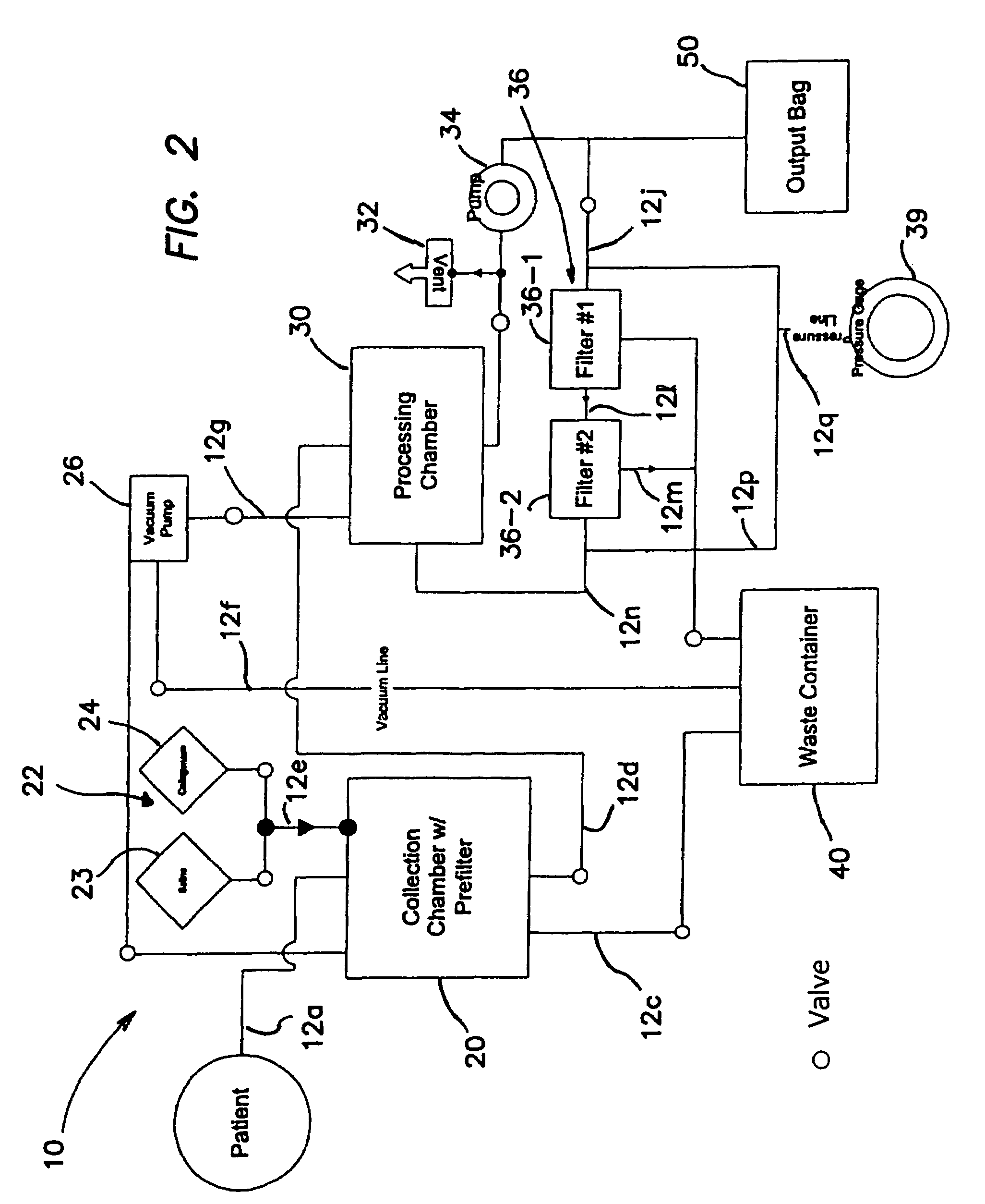
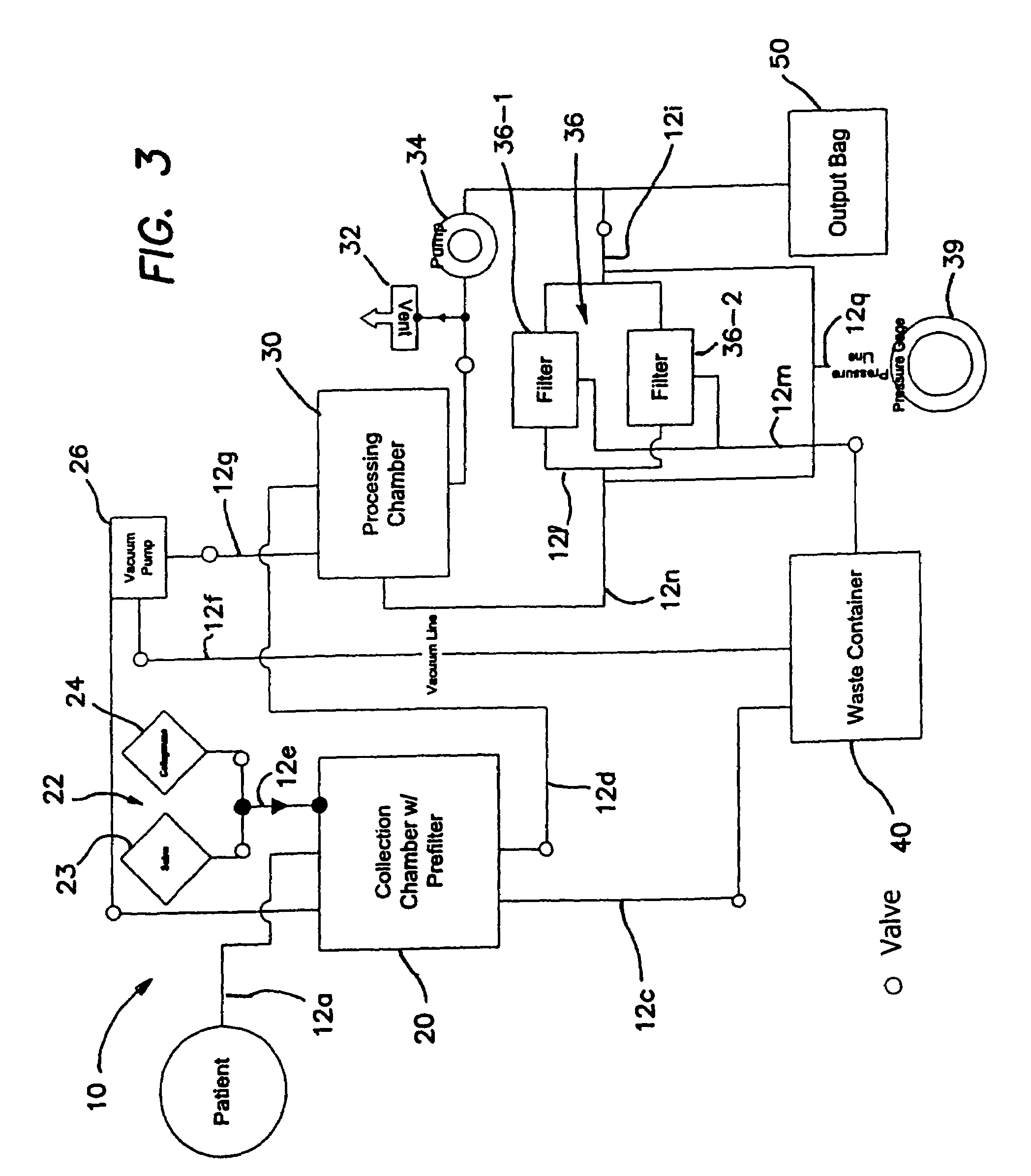
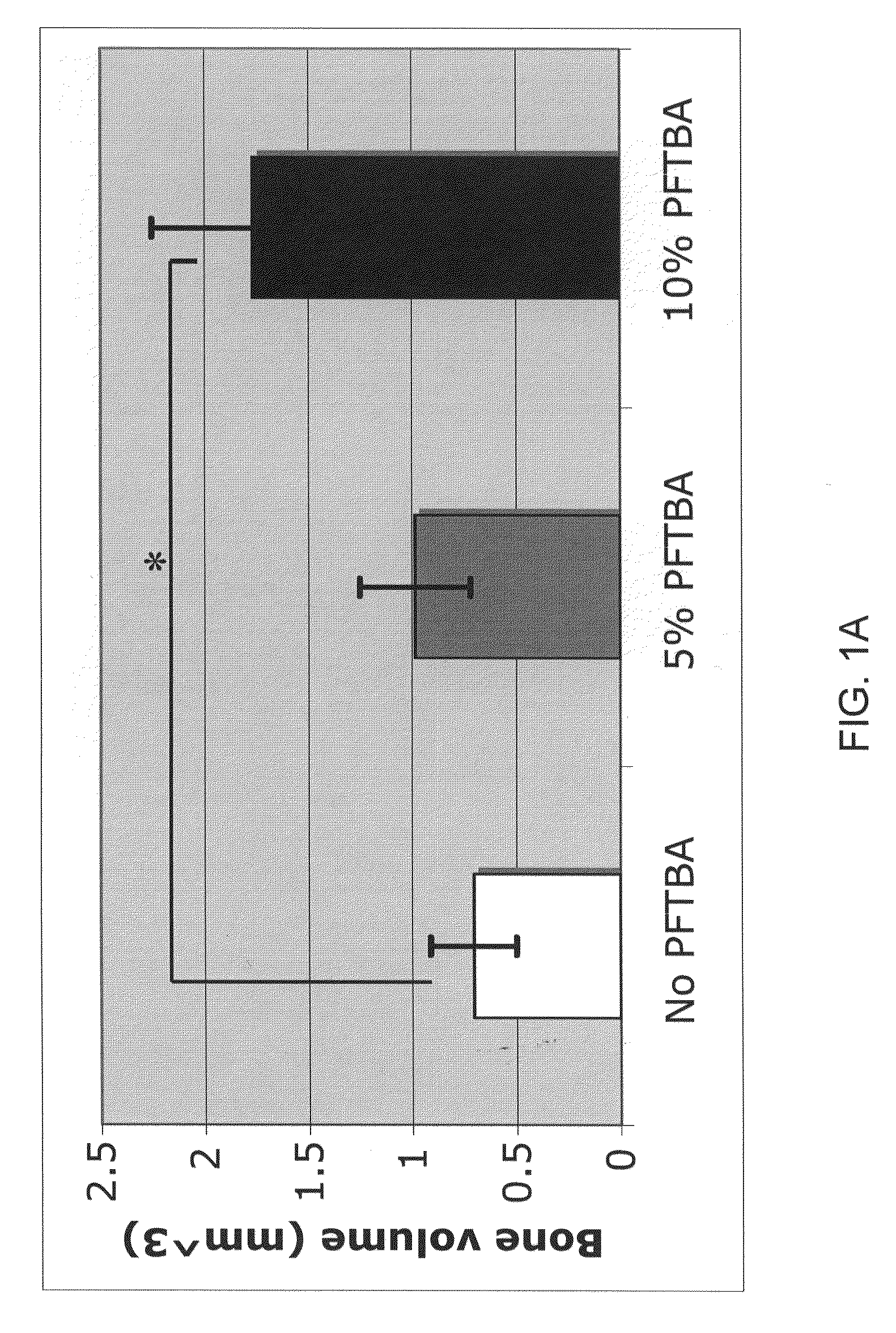
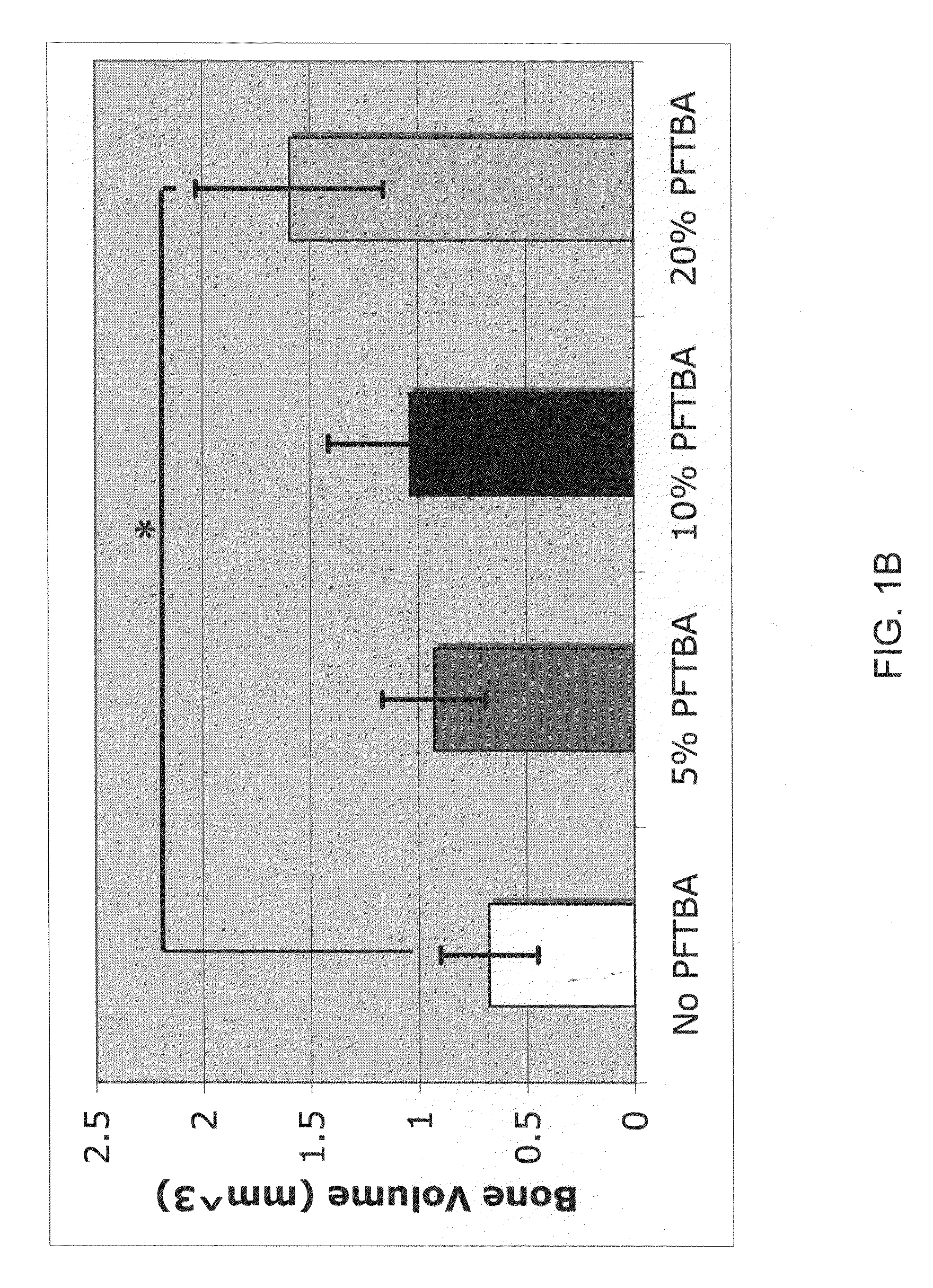
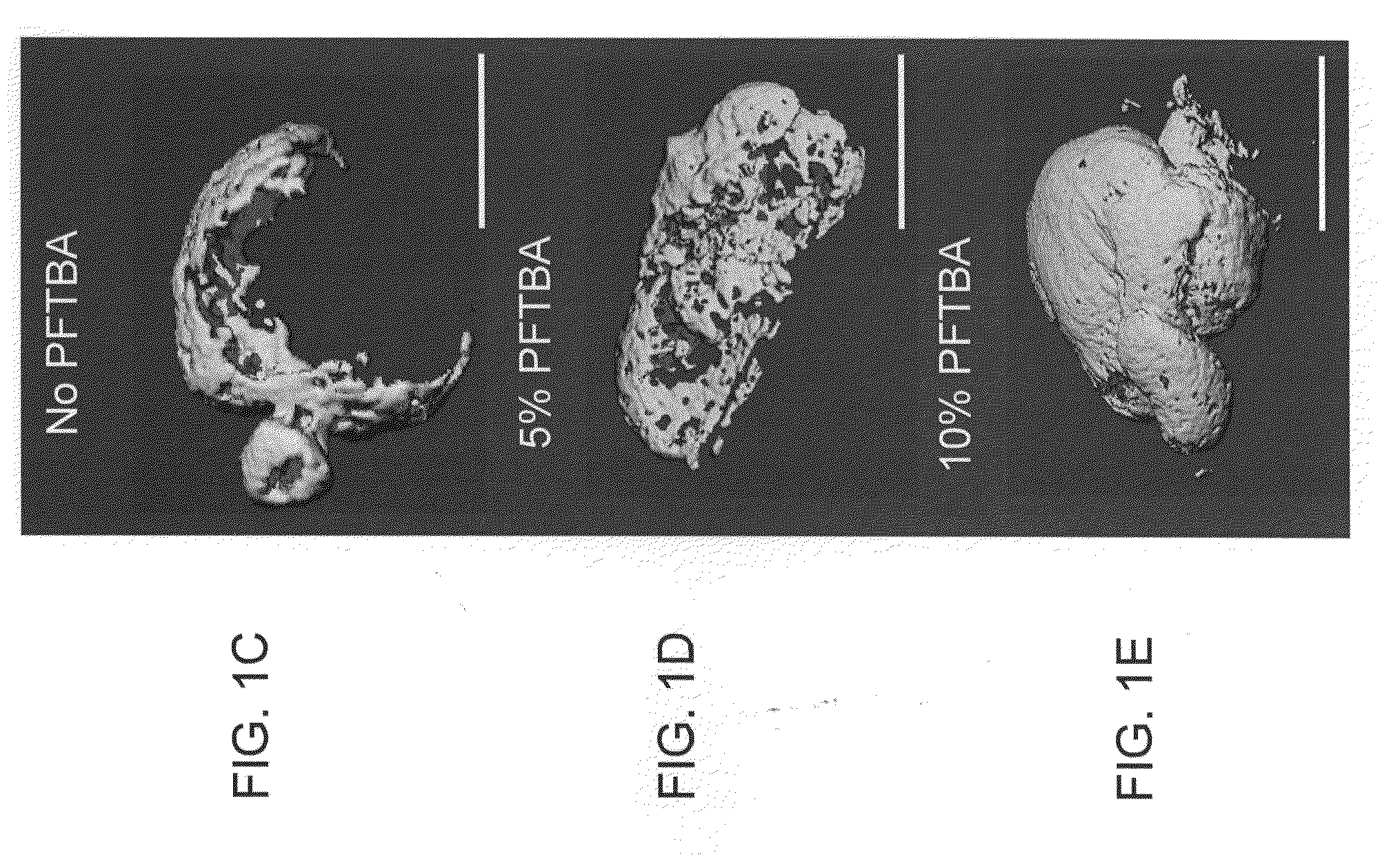
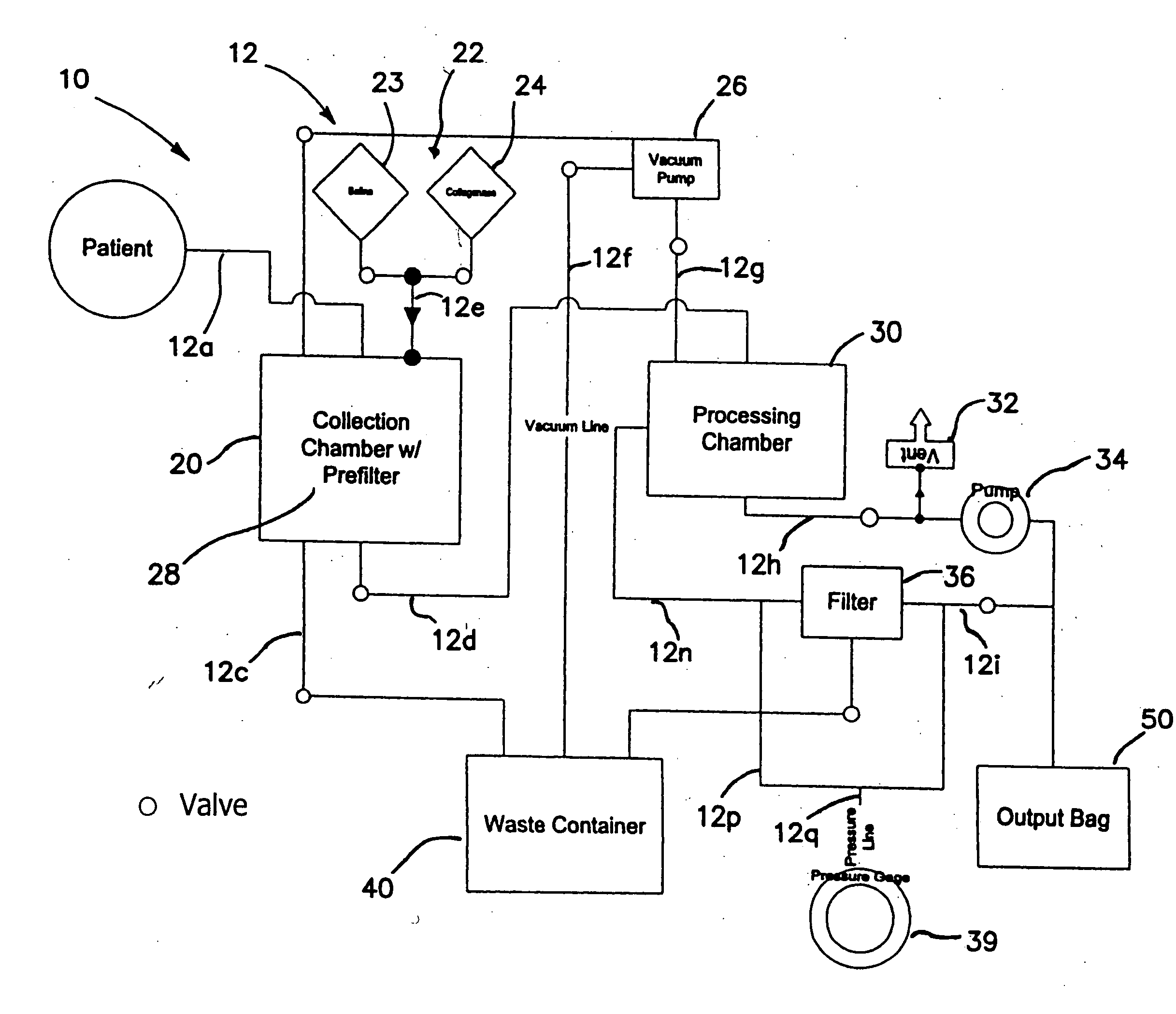
A collection device for collecting bone material during a spinal fusion surgical procedure so that the bone material can later be used as a graft material for the fusion procedure. The device includes a container that collects the bone and other material that is removed from the patient during the surgical procedure through a suction hose. The device includes a plunger having a filter plate sealed to the inside of the container. The plunger is pushed down into the collected material in the container so that blood and other liquids are forced through the plate to be separated from the bone material. A cover at the bottom of the container can be removed to remove the collected bone material.
Owner:THOMPSON MIS
Scaffolds with oxygen carriers, and their use in tissue regeneration
Provided are fibrin or silk matrices comprising an oxygen carrier, and matrices, which comprise an oxygen carrier and mesenchymal stem cells. Also provided are methods of generating and using same for ex vivo or in vivo tissue regeneration and / or repair such as for treating a non-union bone fracture and a condition requiring spinal fusion.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Cell carrier and cell carrier containment devices containing regenerative cells
ActiveUS20050058632A1Promote bone formationPromote cartilage formationBiocideElectrotherapyProgenitorBiology
The present invention relates to a device comprising a cell carrier portion containing regenerative cells, e.g., stem and progenitor cells, and a cell carrier containment portion. The device is useful for the treatment of bone related disorders, including spinal fusion related disorders and long bone or flat bone related defects. The device may be used in conjunction with disclosed automated systems and methods for separating and concentrating regenerative cells.
Owner:LOREM VASCULAR PTE LTD
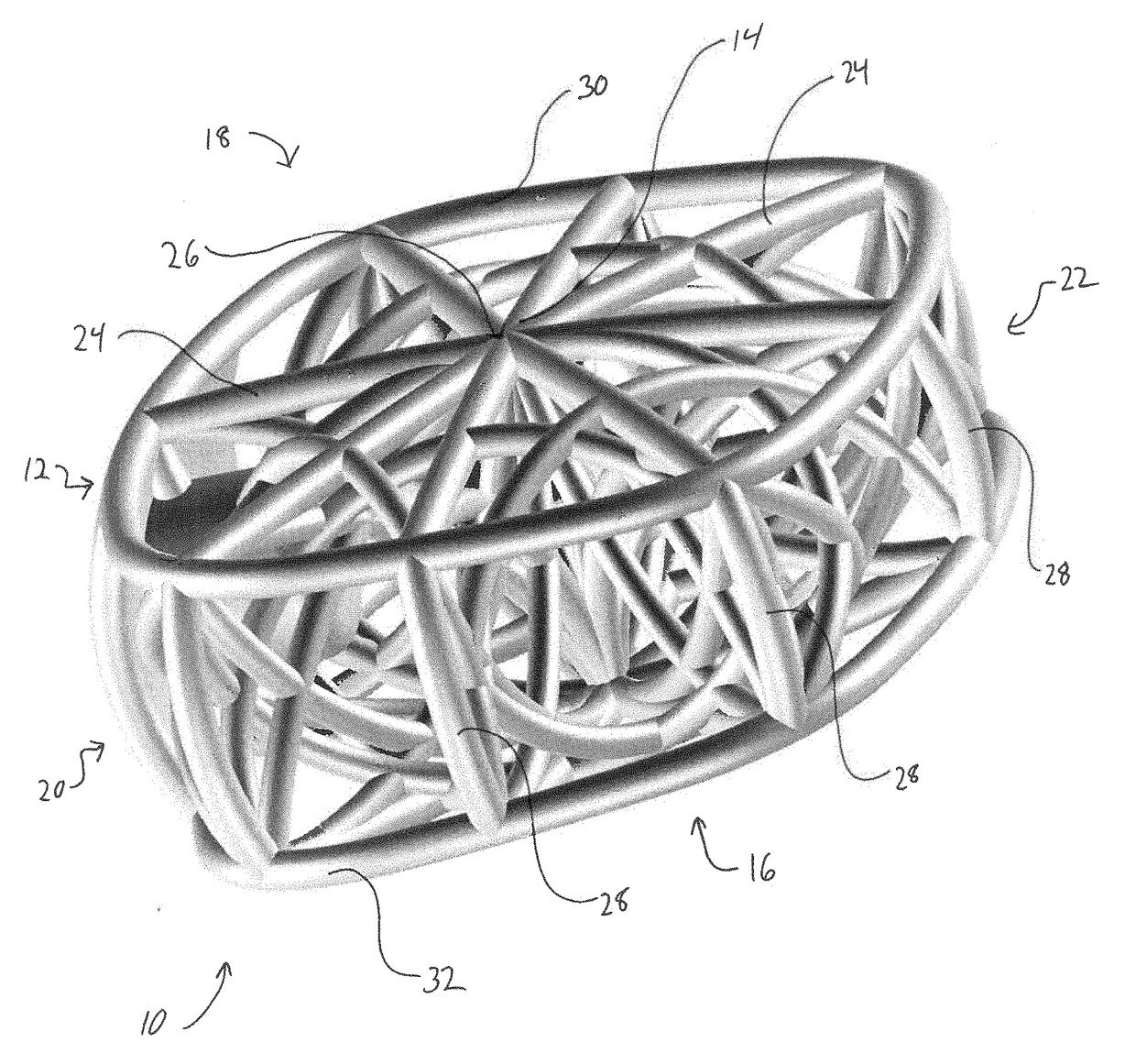
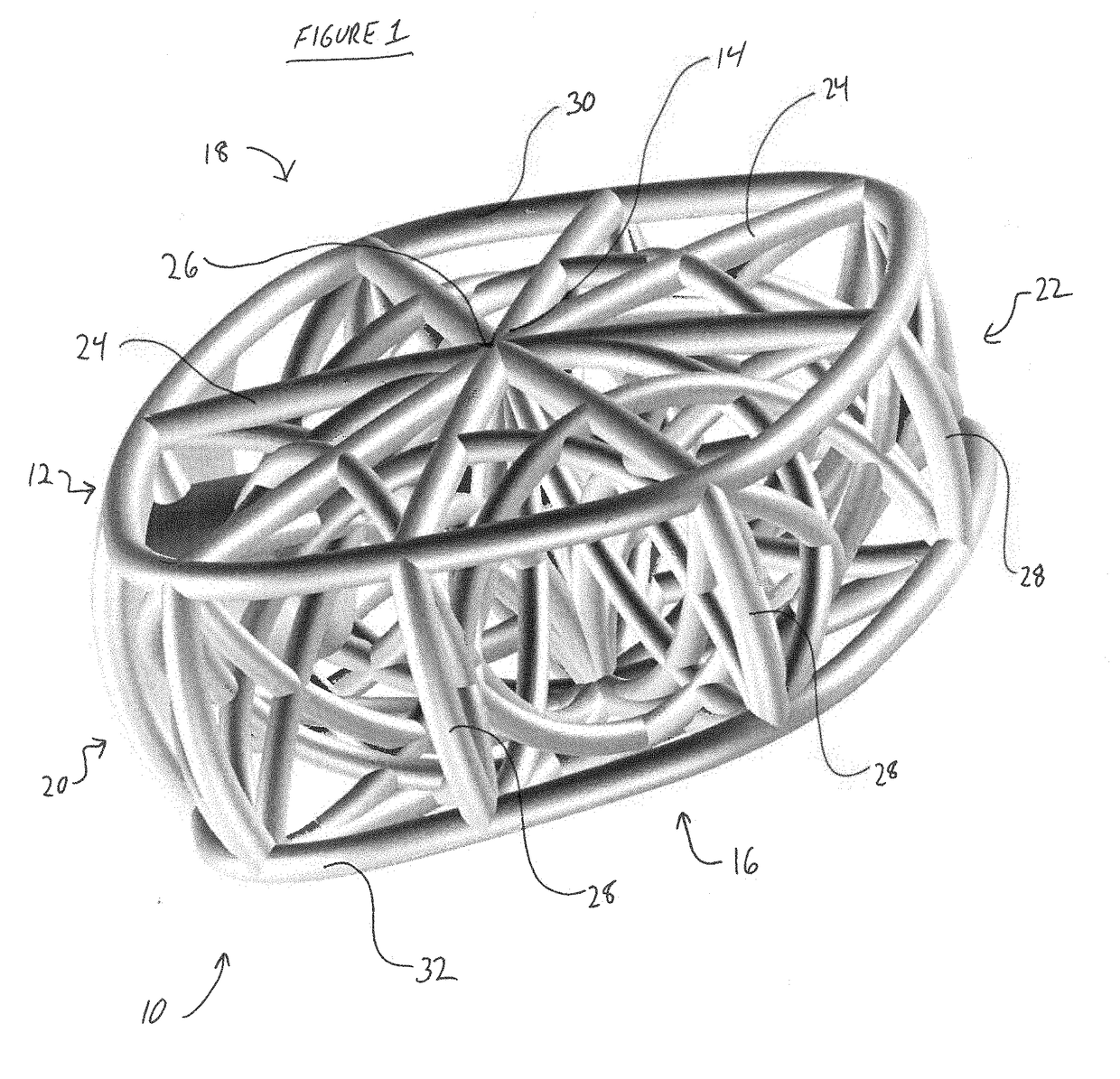
3D printed osteogenesis scaffold
Osteogenesis scaffold such as for spinal fusion or an intermedullary nail includes a number of arcuate struts. The scaffold may have a functional modulus of elasticity that is a result of the modulus of the material of the struts together with the architecture of the struts, and may be within the range of 5 GPa and 75 GPa. An anisotropy of a physical property such as stiffness, compressive strength or elastic modulus corresponds to the same physical property of native bone in the vicinity of the intended implantation site.
Owner:AFZAL THOMAS
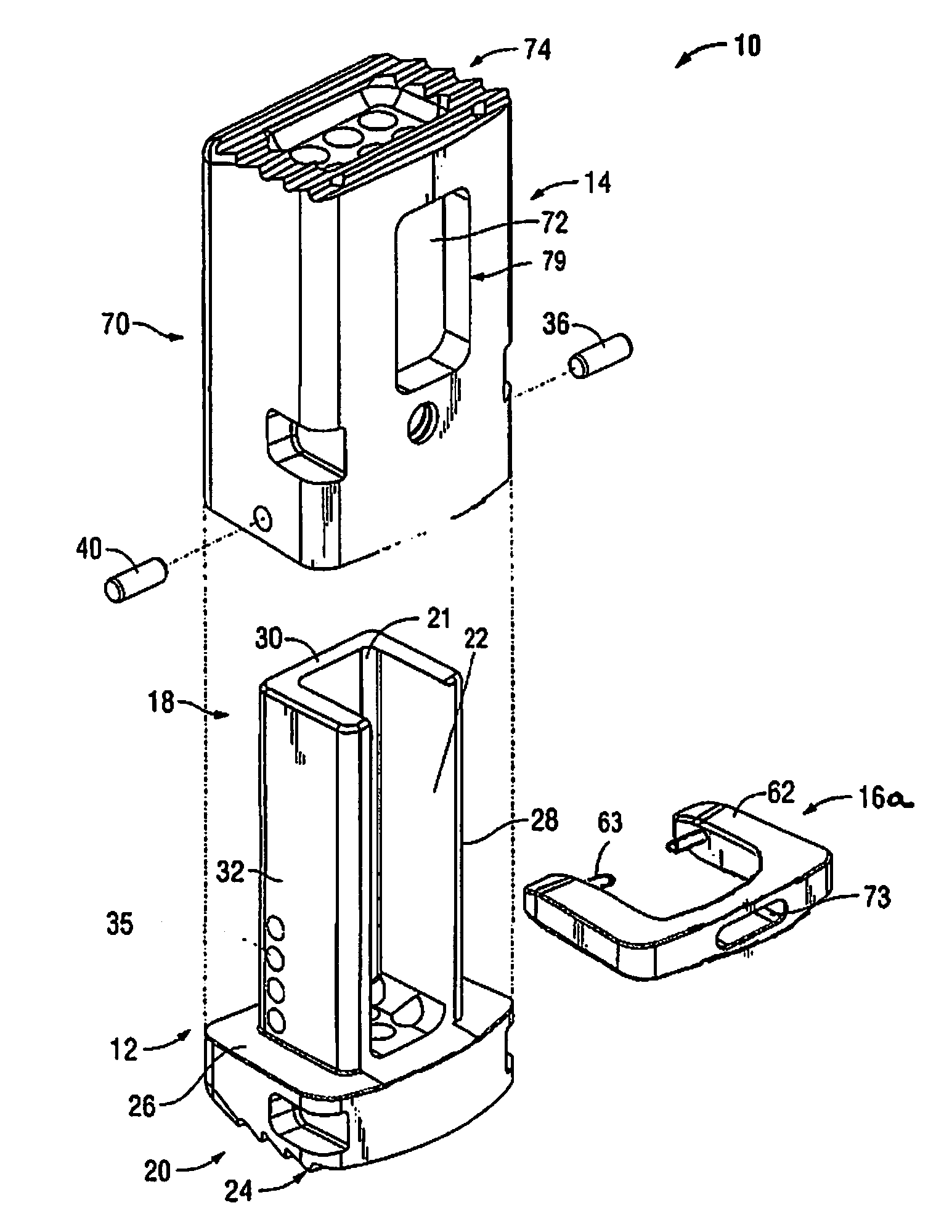
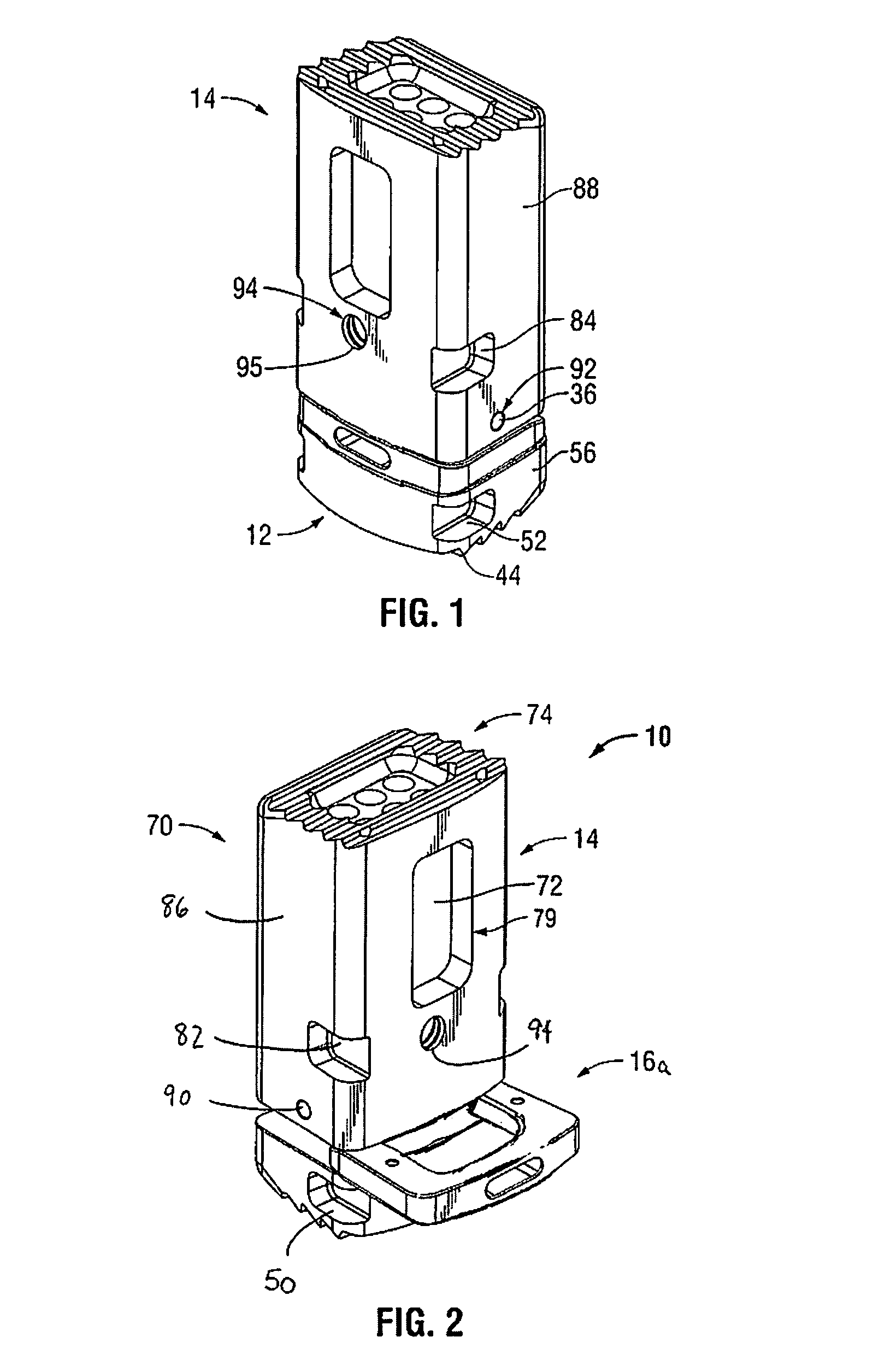
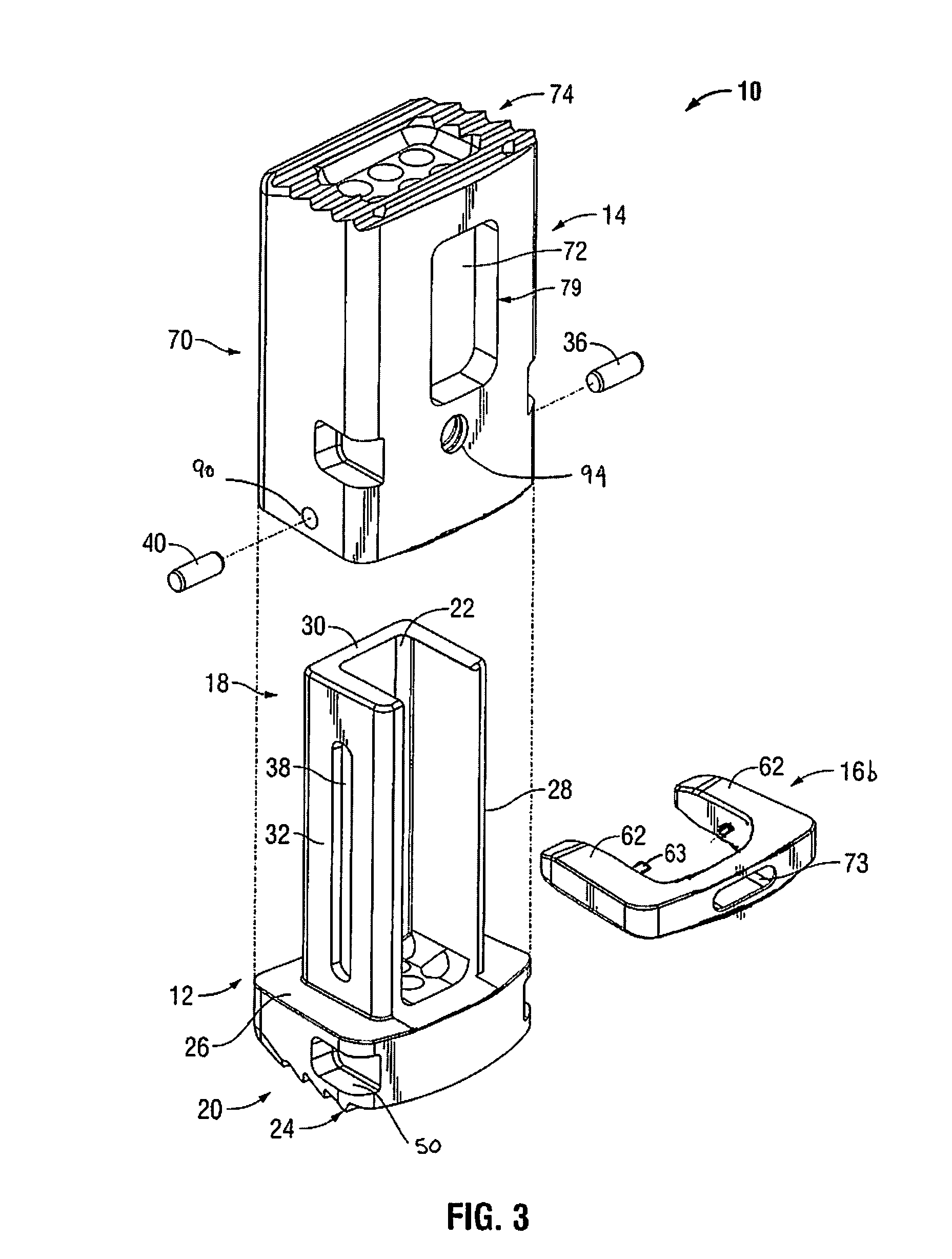
Surgical device for minimally invasive spinal fusion and surgical system comprising the same
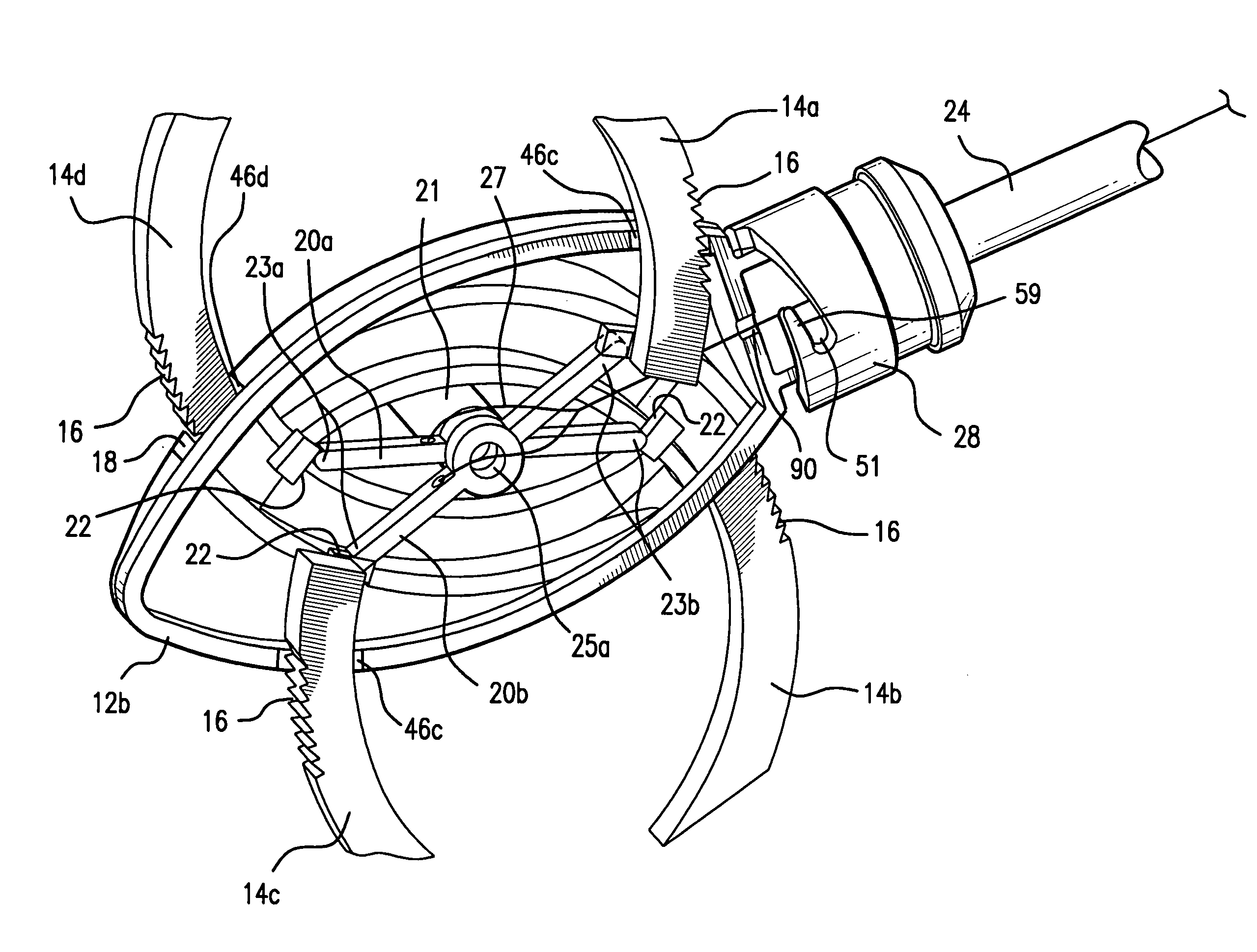
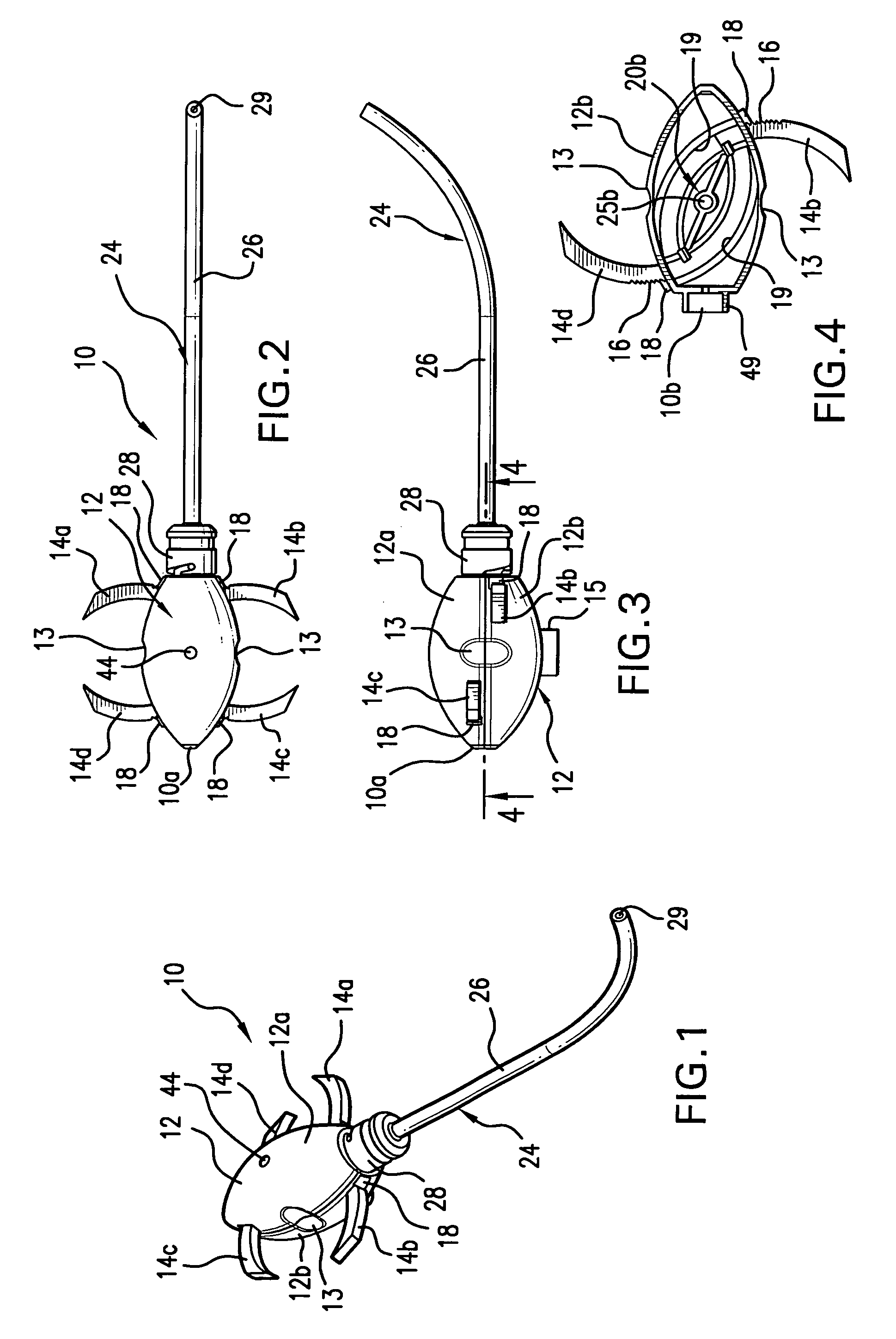
A surgical device is disclosed which may advantageously perform the functions of both a retractor and a distractor. The device has two retractor blades facing each other and held in a common frame, the retractor blades are insertable into a surgical incision in a patient and moveable away from each other along a main axis (x) so as to widen the surgical incision; and a connecting pin attached at a distal end of each retractor blade. Each connecting pin is attachable to a pedicle screw anchored to respective vertebrae of the patient, so that moving away the two retractor blades along the main axis (x) determines a distraction of the vertebrae.
Owner:MEDACTA INT SA
Monorail system
ActiveUS20070270873A1Simple methodProtective structureSpinal implantsOsteosynthesis devicesNerve structureMonorail
Systems providing improved methods of performing a spinal fusion procedure and including a monorail instrument that protects the medial neural structures, includes a sliding platform for additional instrumentation and allows controlled delivery of various instruments for disc preparation and implant insertion. The monorail instrument engages other instruments, including various instruments utilized to prepare the disc space as well as spinal fusion implants, for guided and controlled insertion into the disc space. The monorail instrument may be a distractor, and may include flutes for preparation of the disc space.
Owner:K2M
Interspinous implants and methods for implanting same
A spinal implant for treating lumbar spinal stenosis or as an adjunct to spinal fusion. The implant includes a body portion having an interior cavity. A plurality of locking wings are adapted and configured to move between a stowed position retracted within the interior cavity of the body portion and a deployed position extended from the interior cavity of the body portion. In the deployed position, the wings fix the implant in a selected interspinous space. A cable and wheel arrangement moves the plurality of locking wings from the stowed position to the deployed position and a ratchet / pawl assembly prevents backward movement of the wings.
Owner:SPINAL SIMPLICITY
Apparatus and method for spinal stabilization
A surgical method and apparatus for implanting a spinal fusion implant includes a rigid centering guide having a distal end sized to be inserted into the disc space with the guide extending along a longitudinal axis from a distal end to a proximal end. The guide includes a first external guide surface which extends at least partially between the distal end and the proximal end. The external guide surface is shaped complimentary to an external guided surface of a drill guide. The external guide surface and the guided surface are nested such that the guided surface slides against the external guide surface along a path of travel parallel to the longitudinal axis.
Owner:ZIMMER SPINE INC
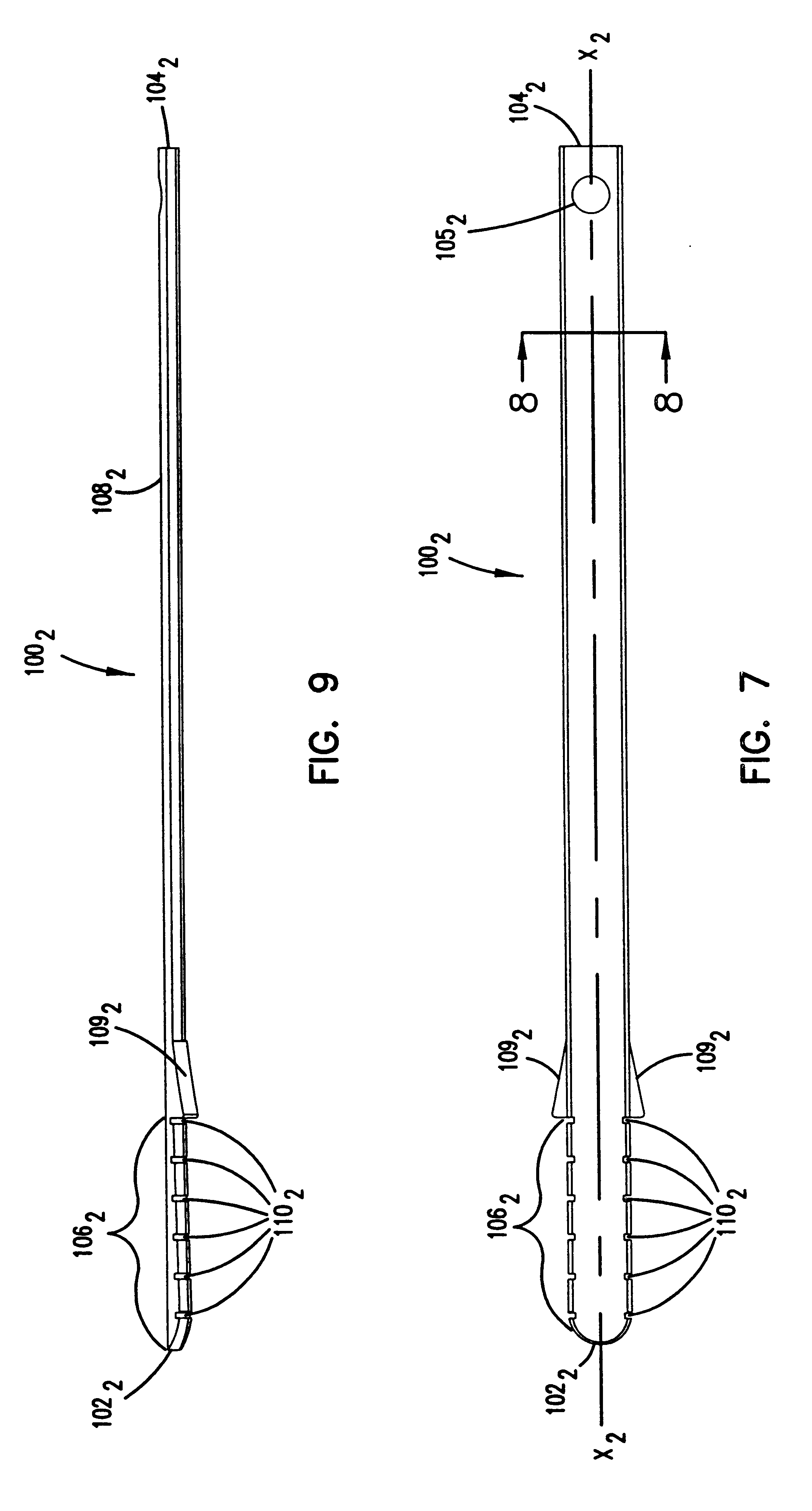
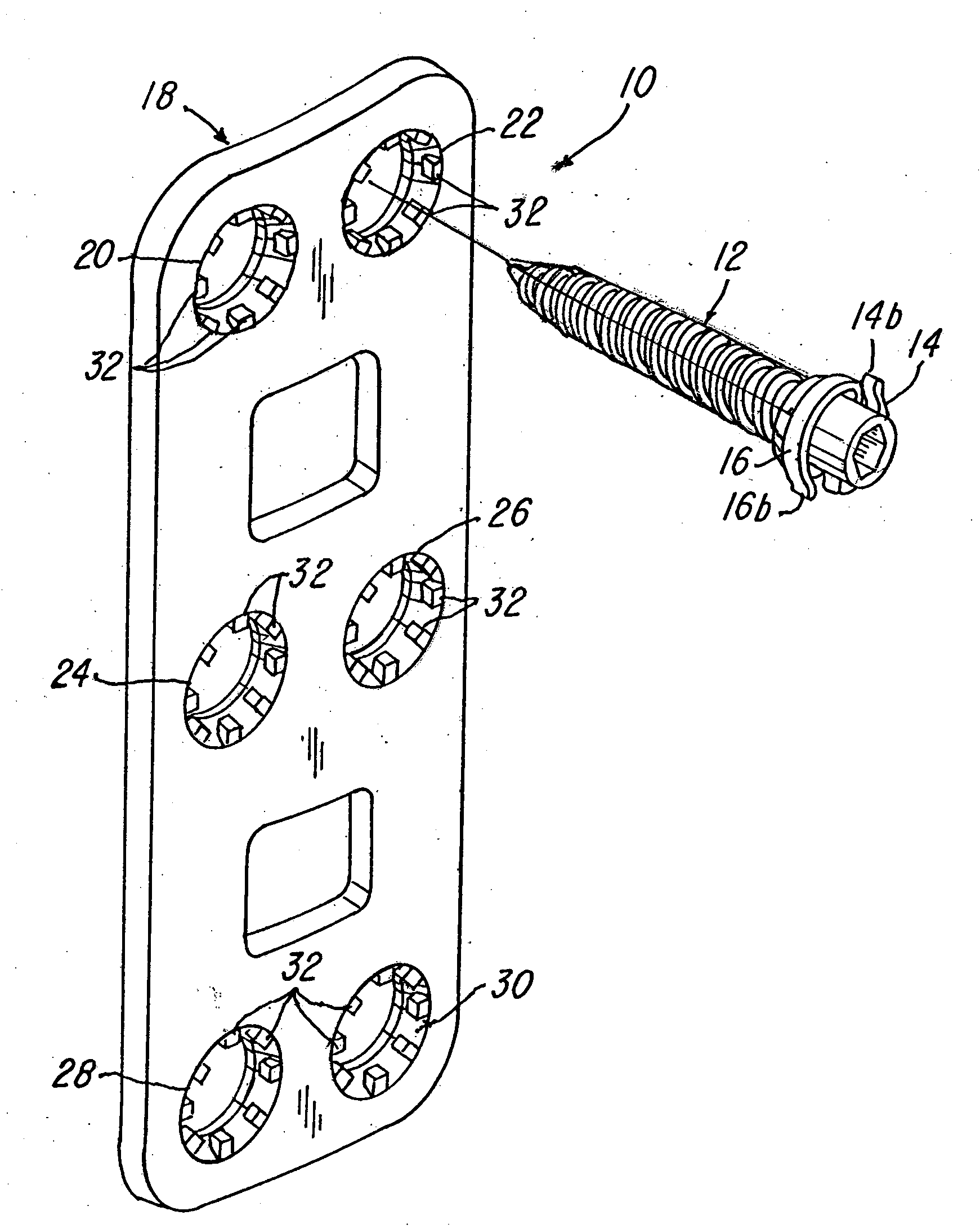
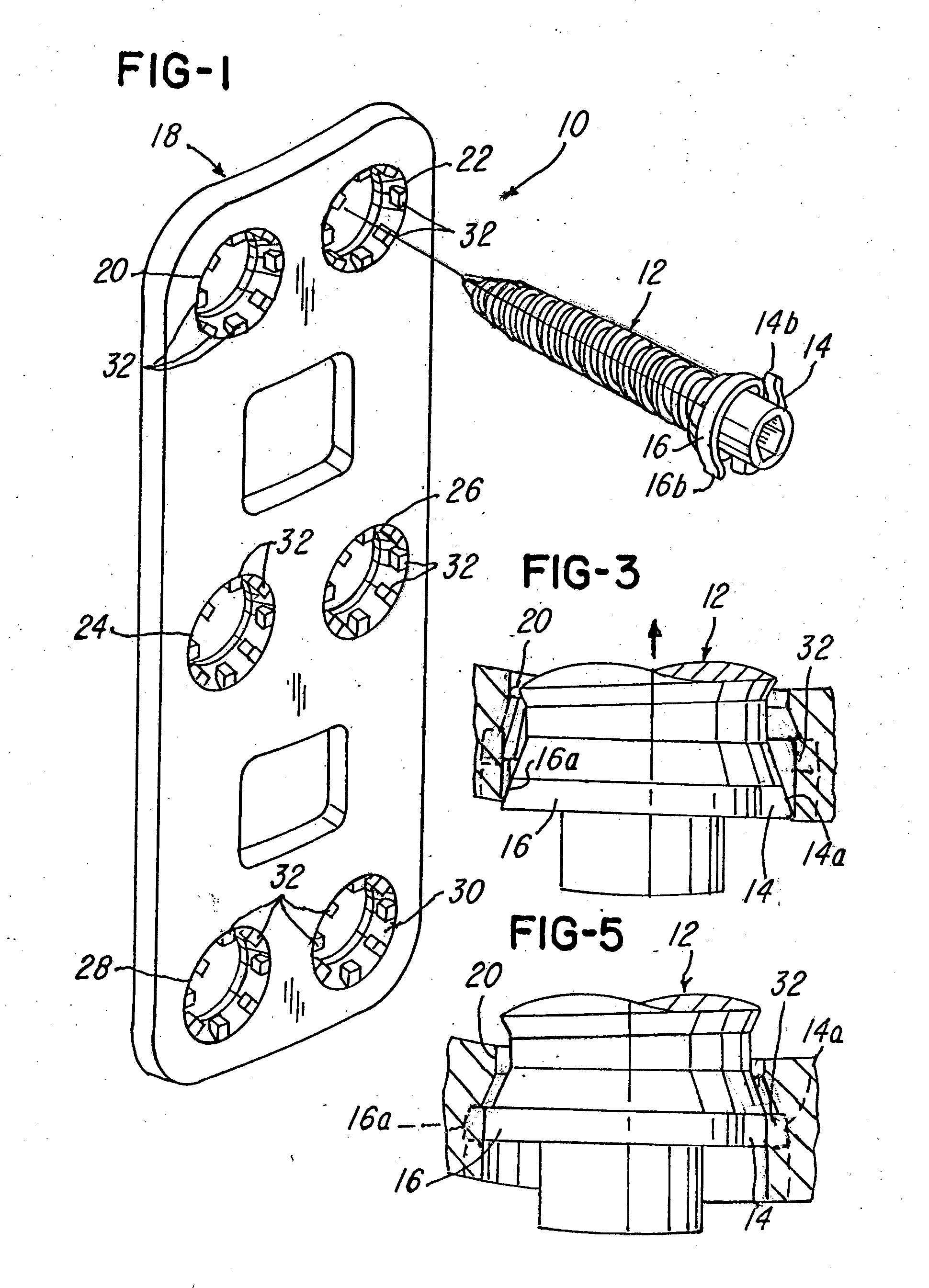
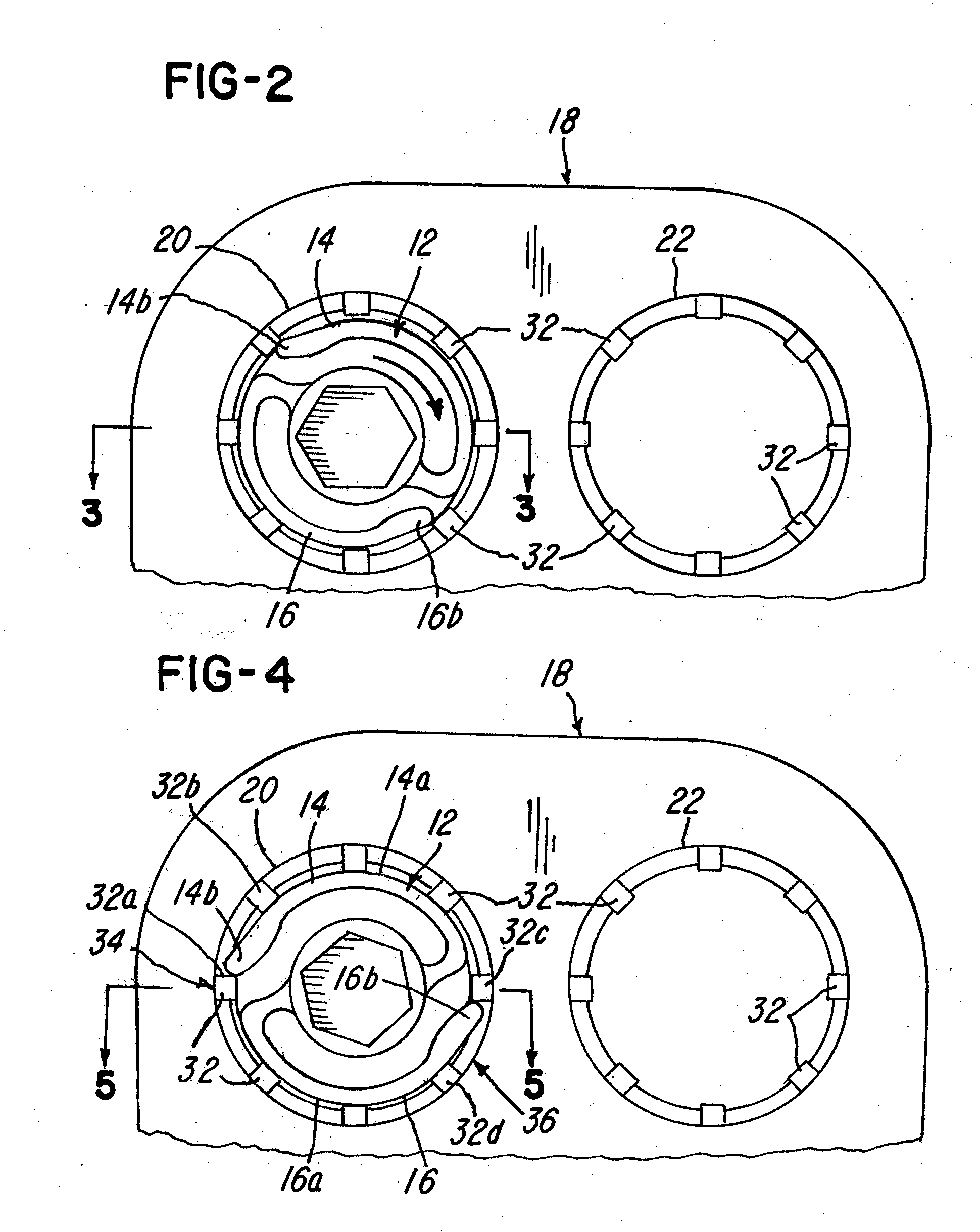
Implant plate screw locking system and screw having a locking member
ActiveUS20090024170A1Promote migrationSuture equipmentsInternal osteosythesisEngineeringSpinal fusion
Spinal fusion system and method utilizing an implant and screw, wherein at least one pawl is mounted on or integral with the screw to prevent said plate or screw from moving in at least one of an axial direction or a rotational direction.
Owner:X SPINE SYST
Expandable cage with locking device
An apparatus supports the spine between vertebrae and promotes spinal fusion. The apparatus generally includes a first supporting member, a second support member, and an expansion member. The first support member has a first longitudinal passage extending there through and a first supporting end configured to engage tissue. The second supporting member contains a second longitudinal passage extending there through and a second supporting end configured to engage tissue. The second longitudinal passage is dimensioned to receive at least a portion of the first supporting member. The first and second supporting members are configured to move with respect to each other. The expansion member is removably positioned between the first and second supporting members and is adapted to maintain the first and second supporting members in a fixed relative position.
Owner:K2M
Anterior intervertebral spacer and integrated plate assembly and methods of use
InactiveUS8932358B1Easy to fuseImprove stabilityBone implantSpinal implantsAnterior cortexIntervertebral spaces
A precisely size matched intervertebral plate and spacer assembly for ensuring a tight fit within a disc space to promote spinal fusion, comprising: a “U-shaped” spacer configured to fit within the intervertebral space; and, a matching countersunk low profile “H-shaped” anterior plate joined perpendicularly to the spacer. The plate further comprises: a plurality of anchor members configured to attach to the junctions of the anterior cortex faces and the endplates; and, channels individually traversing through the anchor members for inserting screws into the vertebral bodies' cortical bone. The spacer comprises a hollow three-sided U-shaped member, comprising two opposing parallel side walls, and a perpendicular posterior wall, while lacking a superior, inferior, and anterior wall. The exterior walls of the plate and spacer are planar, while the interior walls of the spacer are curved to house a precisely fitting cylindrical graft, or other insert such as DBM, bone dust, bone paste, bone dowel with direct contact to the endplates to promote fusion.
Owner:NEHLS DANIEL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com