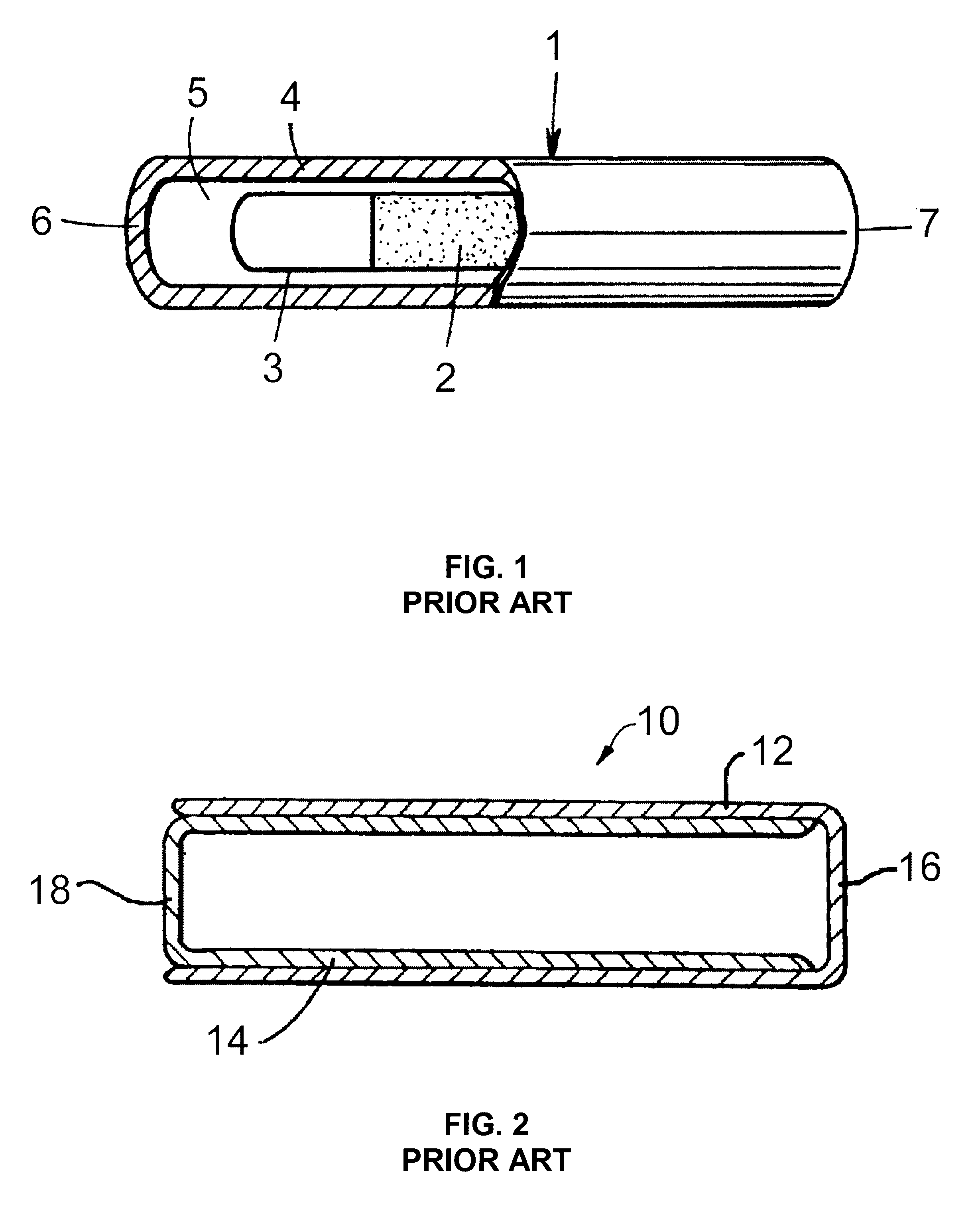
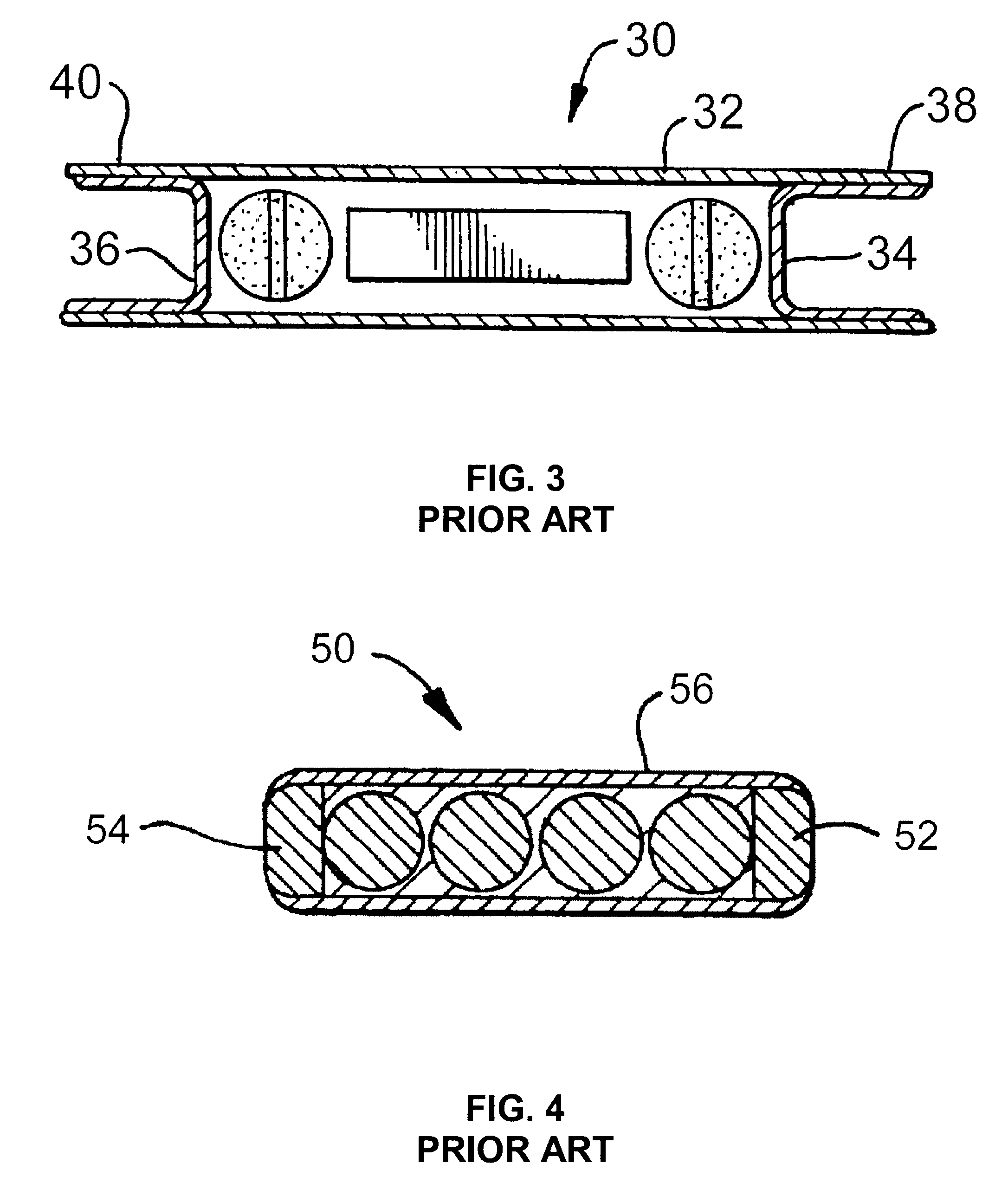
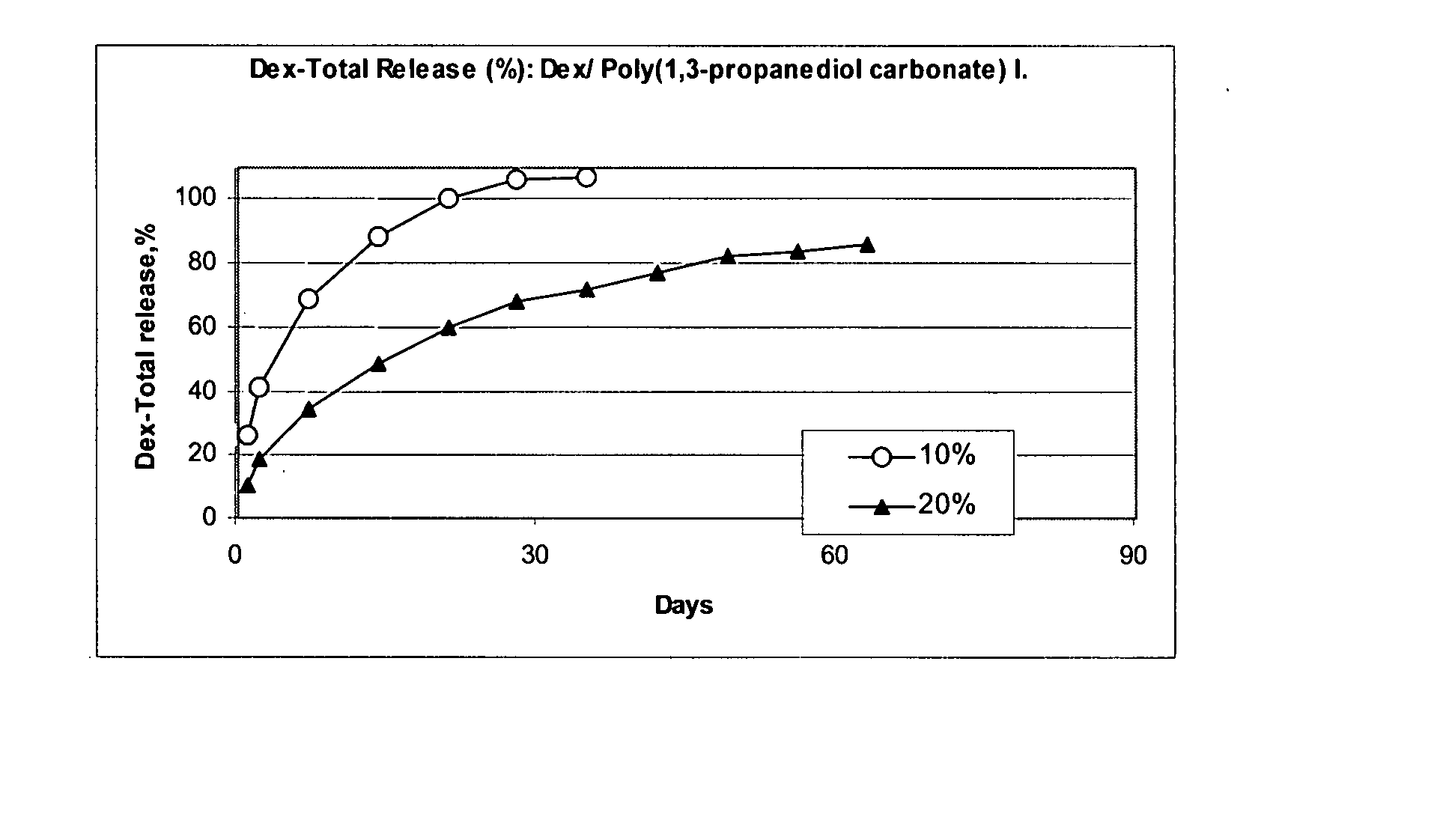
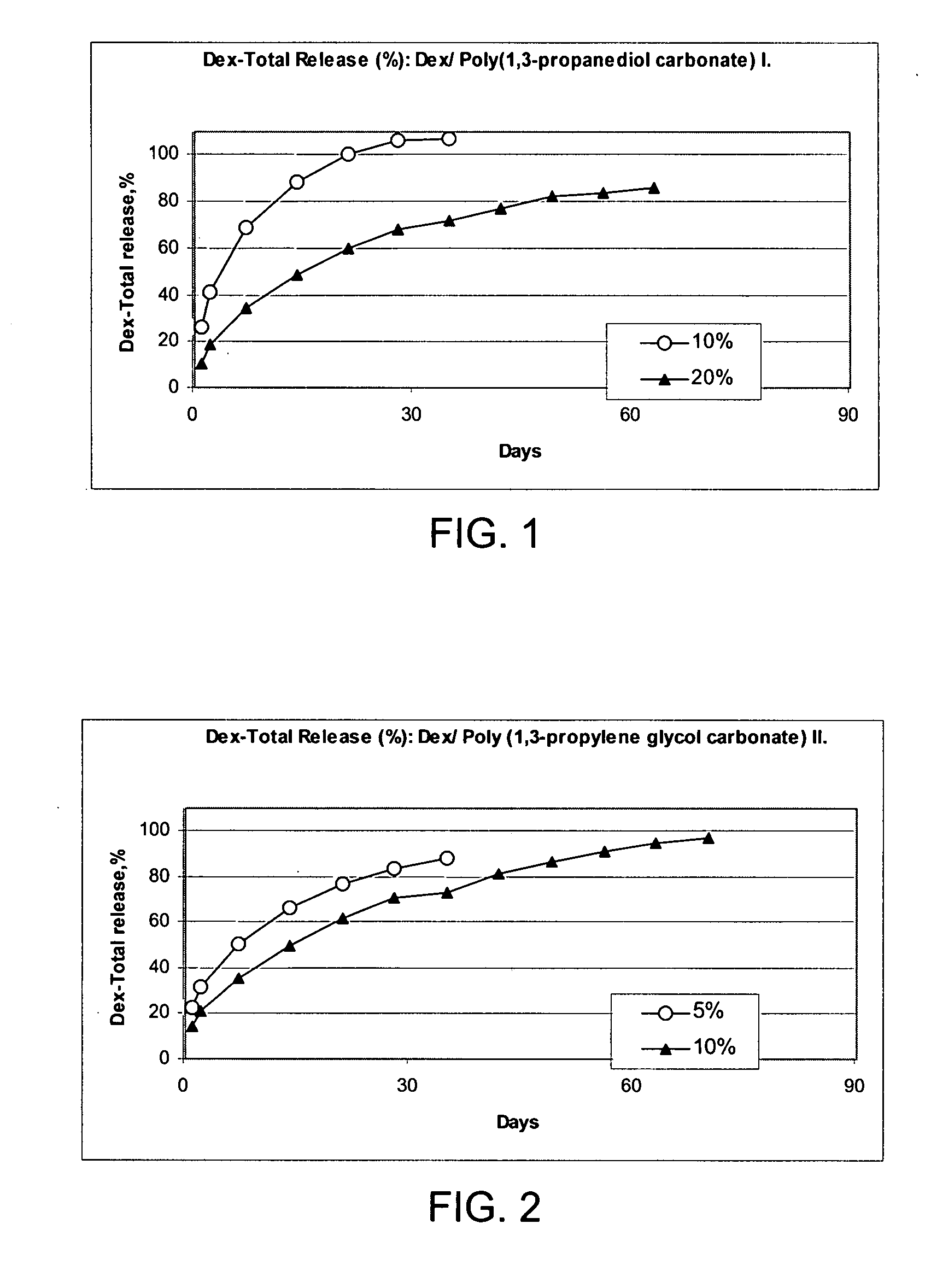
Patents
Literature
7523 results about "Biocompatibility" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Biocompatibility is related to the behavior of biomaterials in various contexts. The term refers to the ability of a material to perform with an appropriate host response in a specific situation. The ambiguity of the term reflects the ongoing development of insights into how biomaterials interact with the human body and eventually how those interactions determine the clinical success of a medical device (such as pacemaker, hip replacement or stent). Modern medical devices and prostheses are often made of more than one material so it might not always be sufficient to talk about the biocompatibility of a specific material.
Medical devices and applications of polyhydroxyalkanoate polymers
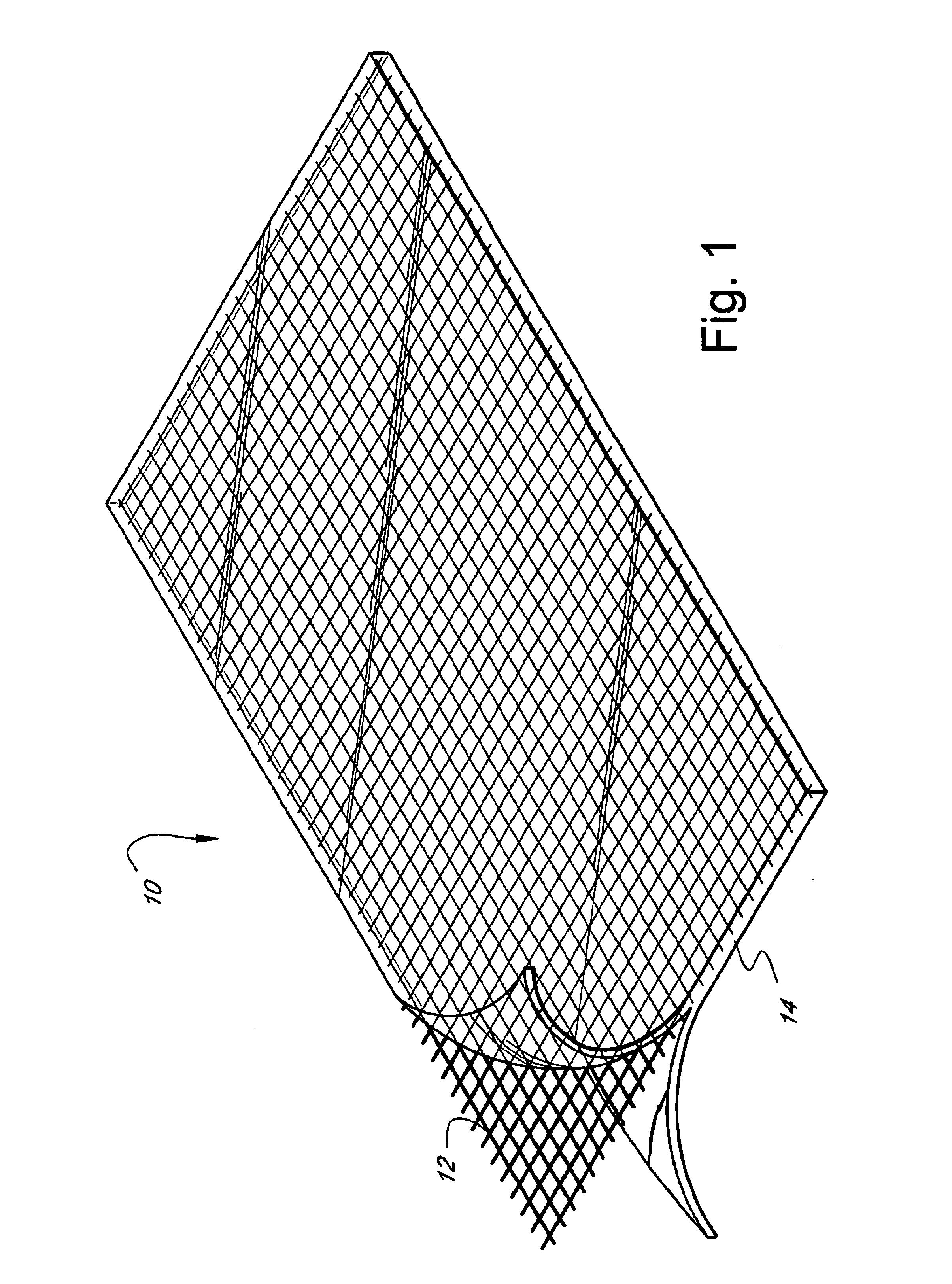
InactiveUS6838493B2High porosityReduce probabilitySuture equipmentsOrganic active ingredientsTissue repairBiocompatibility Testing
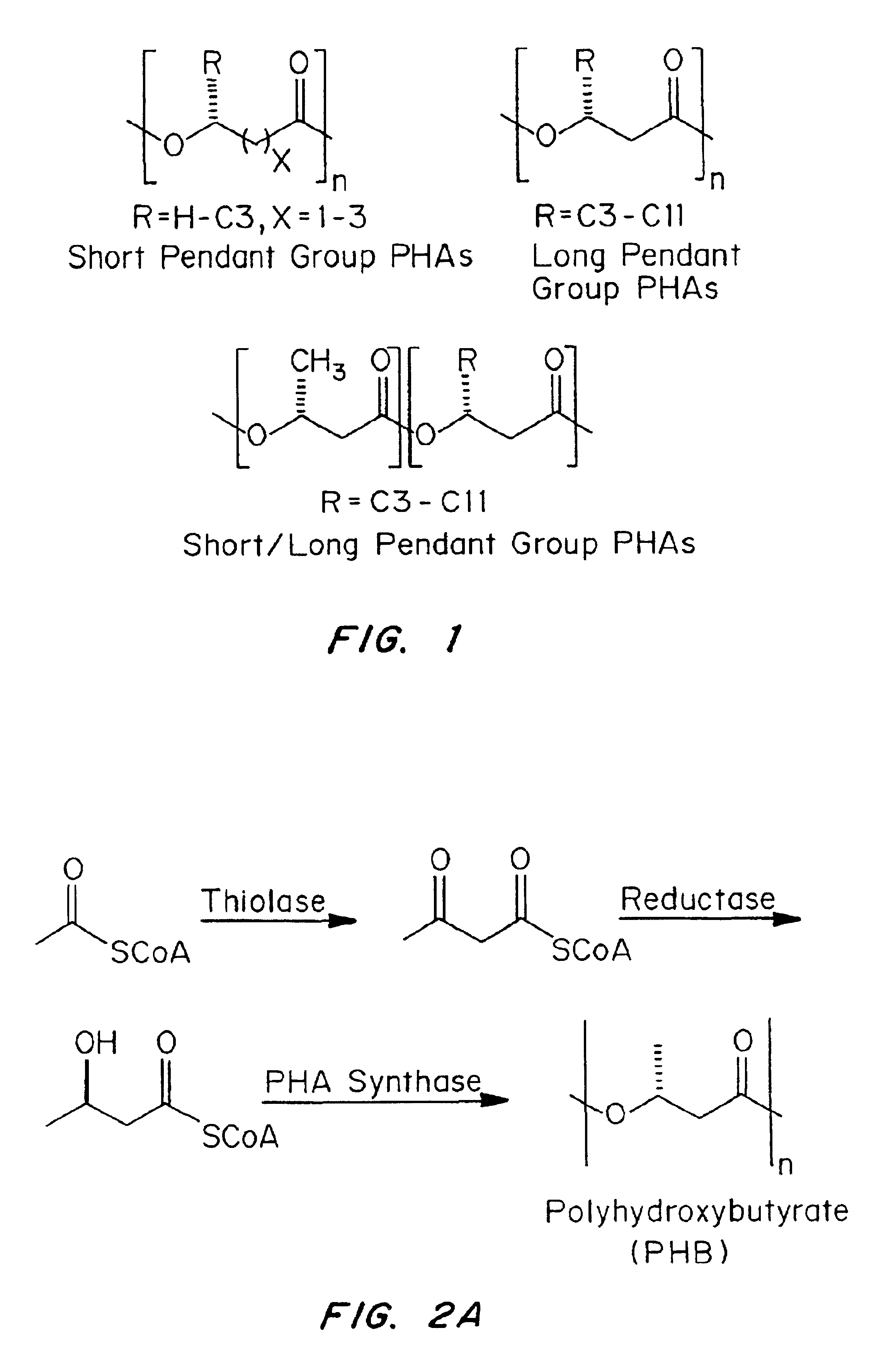
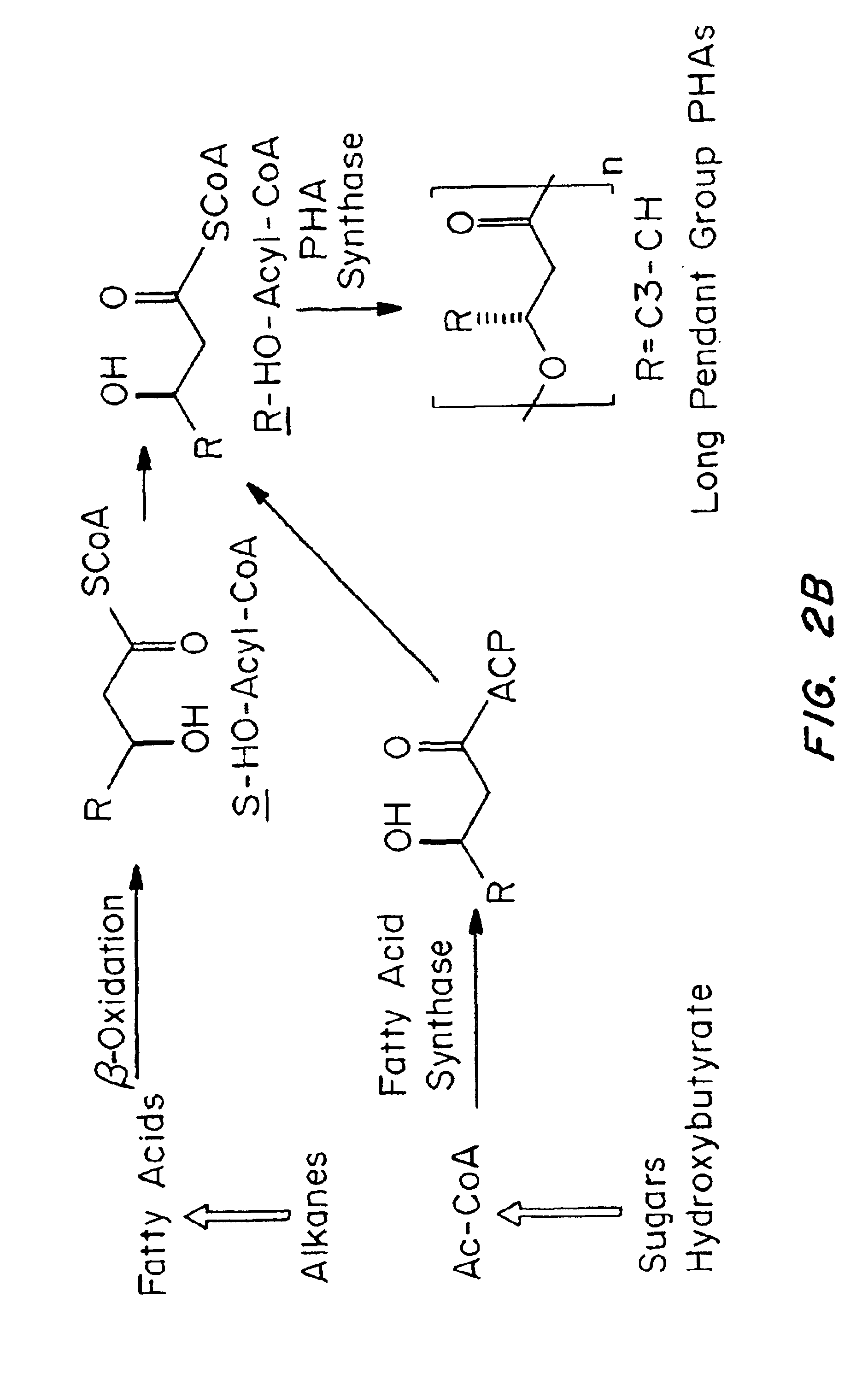
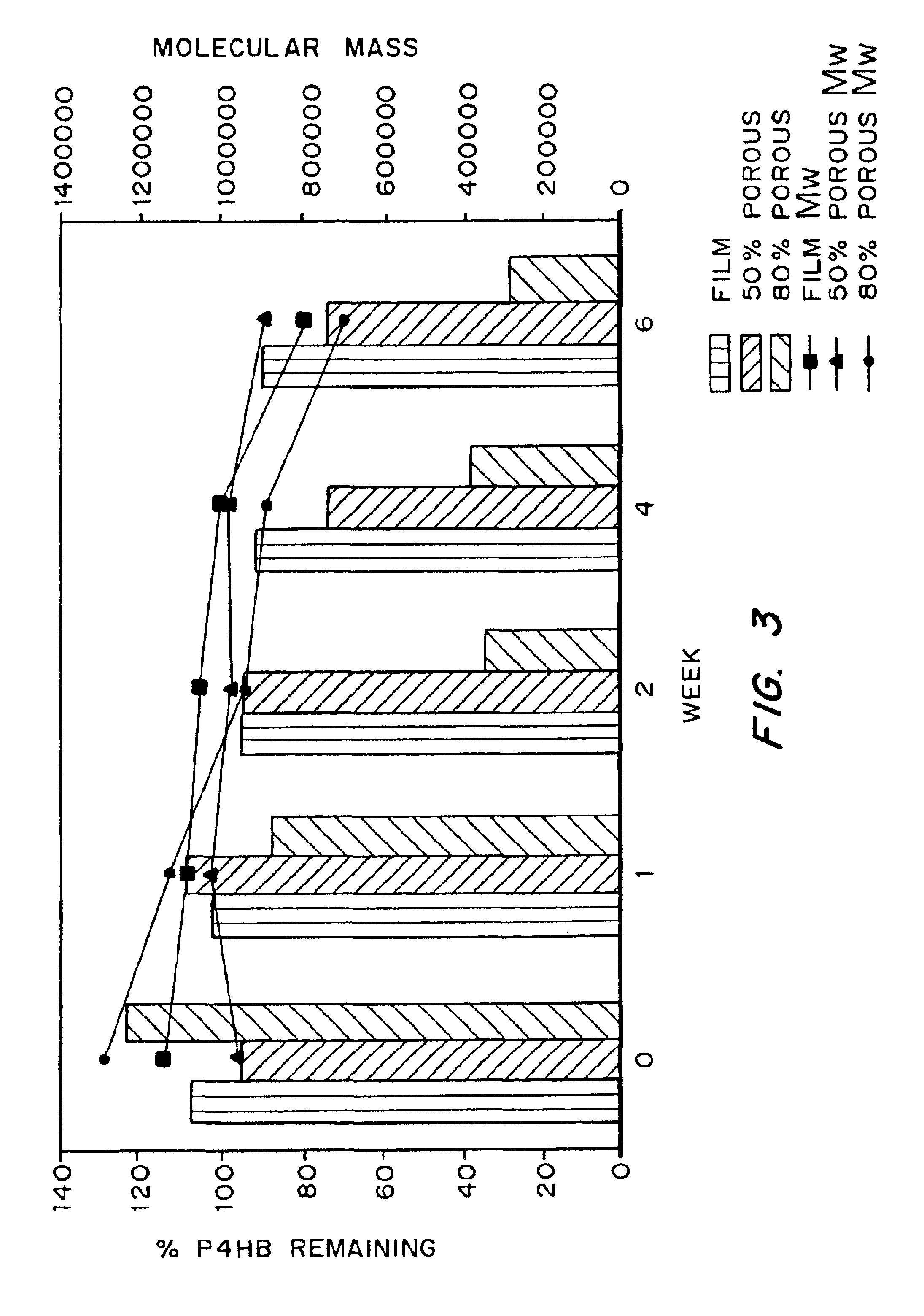
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
Self-supporting, shaped, three-dimensional biopolymeric materials and methods
Self-supporting, shaped, three-dimensional cross-linked proteinaceous biopolymeric materials that may be implanted in vivo, and methods of making such materials are disclosed. The biopolymeric materials most preferably include reinforcing media, such as biocompatible fibrous or particulate materials. In use, the preformed, shaped biopolymeric materials may be applied to tissue in need of repair and then sealed around its edges with a liquid bioadhesive. In such a manner, repaired tissue which is capable of withstanding physiological pressures may be provided.
Owner:CRYOLIFE
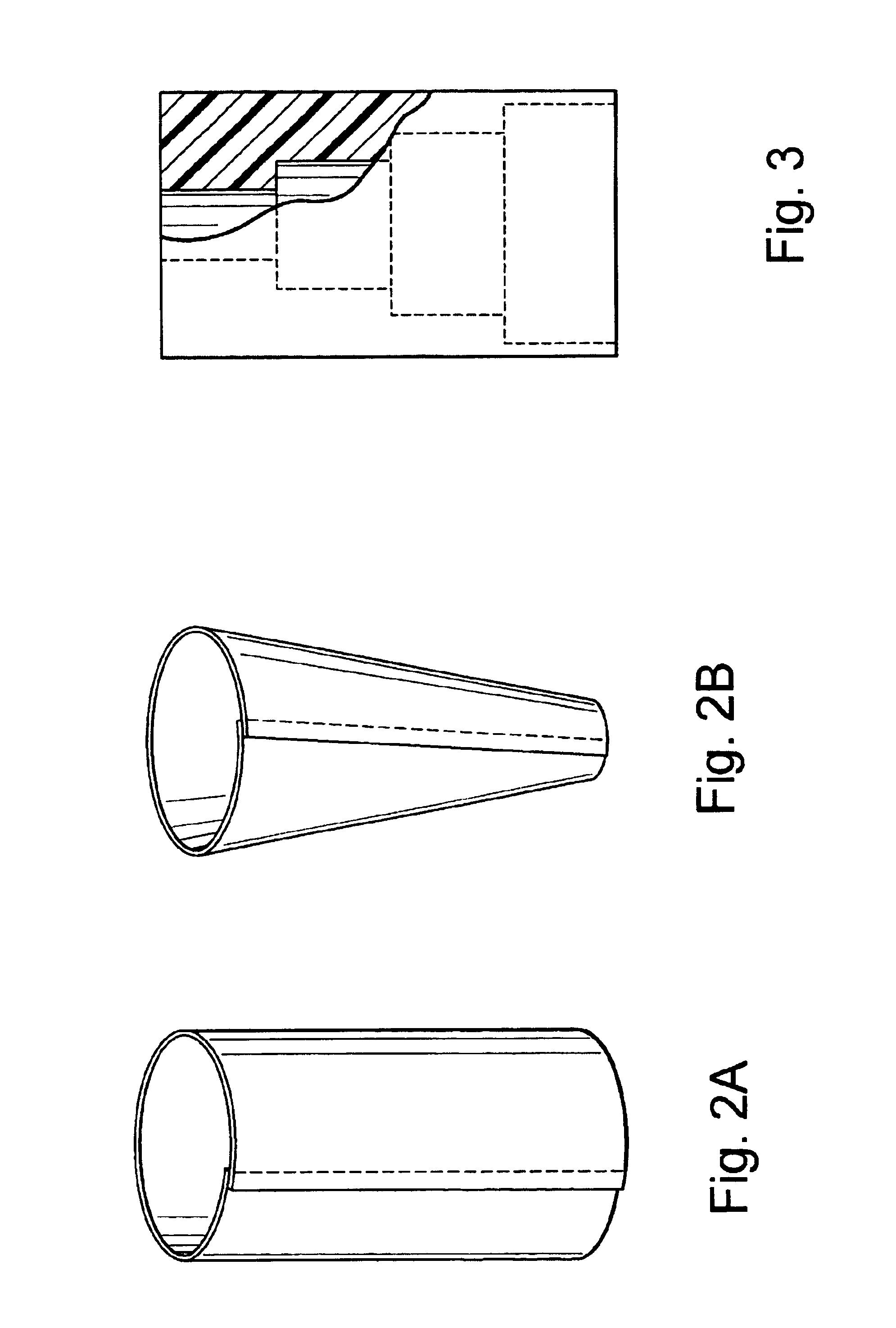
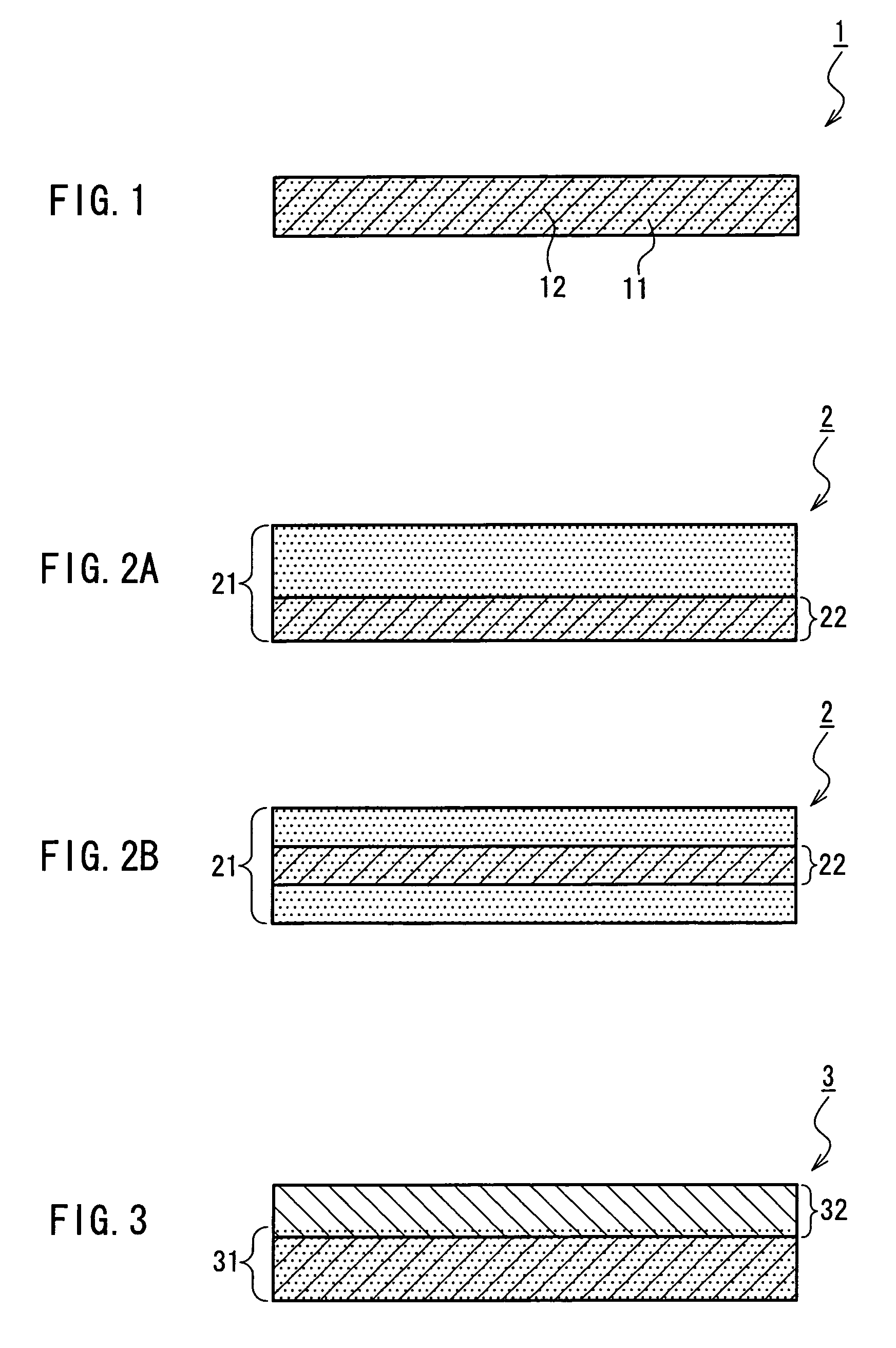
Medical film
InactiveUS7718556B2Good biocompatibilityHigh strengthSuture equipmentsBiocideGelatin filmThin membrane
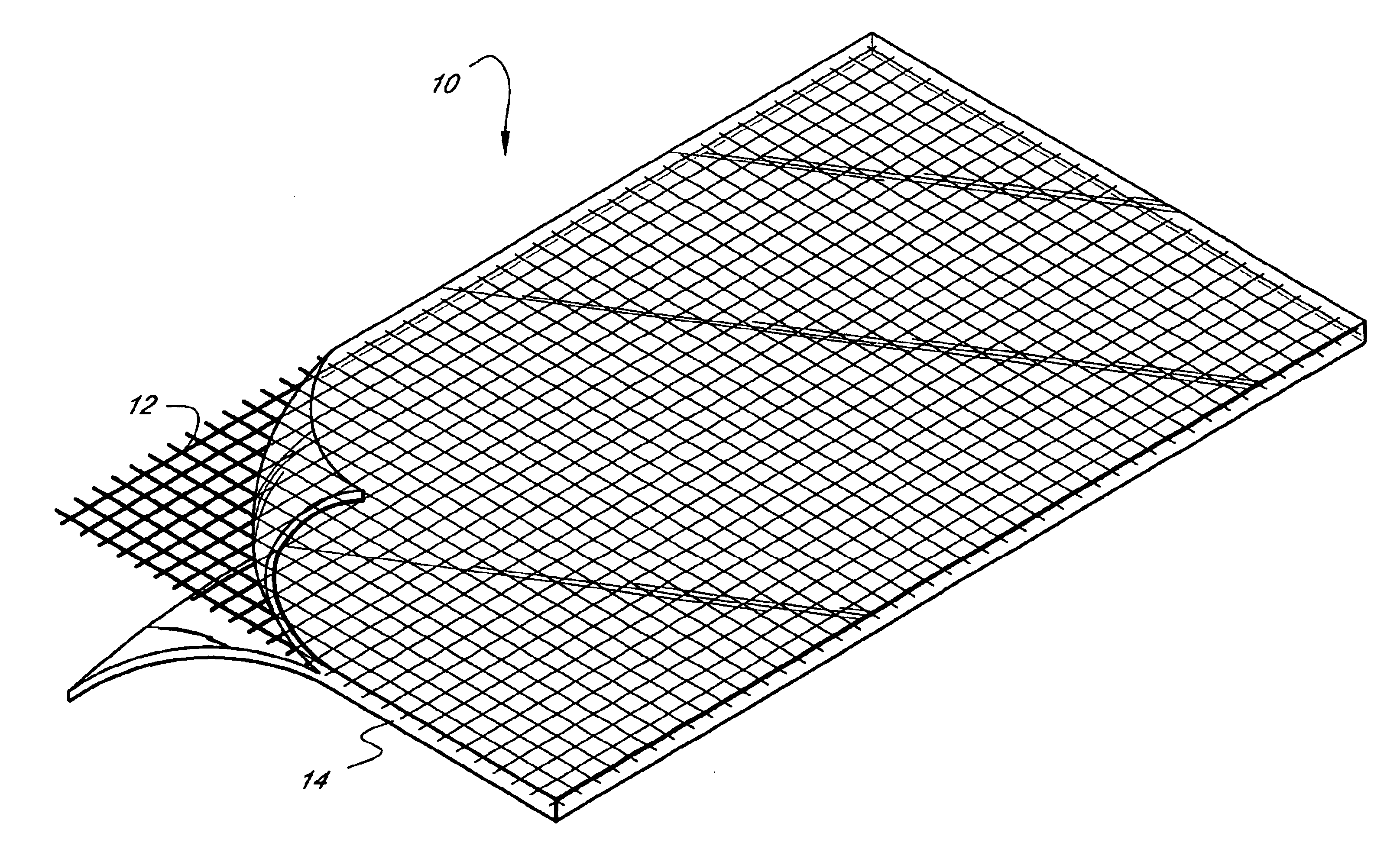
A medical film that is excellent in biocompatibility and bioabsorbability and has an excellent strength in suturing and bonding is provided. A reinforcing material 12 made of a biodegradable polymer is placed in a gelatin solution so as to allow the solution to infiltrate in the reinforcing material 12 and then the gelatin is dried. This allows the gelatin that has infiltrated entirely in an internal part of the reinforcing material 12 to gel, thereby forming a gelatin film 11. Thus, a medical film 1 in which the reinforcing material 12 and the gelatin film 11 are integrated is obtained. The gelatin film 11 preferably is a cross-linked gelatin film.
Owner:GUNZE LTD
Adhesive formulations
ActiveUS8349987B2Organic non-macromolecular adhesiveSynthetic polymeric active ingredientsArylPolymer science
The disclosure relates to biocompatible components useful for forming compositions for use as medical / surgical synthetic adhesives and sealants. Biocompatible components of the present disclosure may include a polymeric polyol core, which may be treated with a nitroaryl compound to form a nitro ester. The resulting nitro ester groups may be reduced to form amino groups which, in turn, may be treated to form isocyanate groups. The resulting isocyanate may then be reacted with a second component to form adhesive and / or sealant compositions.
Owner:COVIDIEN LP
Biocompatible wound dressing
InactiveUS7070584B2Promote cell growthPrevent vacuum leakageWound drainsMedical applicatorsWound dressingWound site
A biocompatible wound dressing comprised of a pad for insertion substantially into a wound site and a wound drape for sealing enclosure of the foam pad at the wound site. The pad, comprised of a foam or other like material having relatively few open cells in contact with the areas upon which cell growth is to be encouraged so as to avoid unwanted adhesions, but having sufficiently numerous open cells so that drainage and negative pressure therapy may continue unimpaired, is placed in fluid communication with a vacuum source for promotion of fluid drainage, as known in the art. The pad is further comprised of an ultra-low density fused-fibrous ceramic, or a bioabsorbable branched polymer, or cell growth enhancing matrix or scaffolding.
Owner:KCI LICENSING INC
Personal fit medical implants and orthopedic surgical instruments and methods for making
InactiveUS20070118243A1Minimizing Ni toxicityImprove visualizationElectrotherapyMechanical/radiation/invasive therapiesPersonalizationManufacturing technology
The present invention provides methods, techniques, materials and devices and uses thereof for custom-fitting biocompatible implants, prosthetics and interventional tools for use on medical and veterinary applications. The devices produced according to the invention are created using additive manufacturing techniques based on a computer generated model such that every prosthesis or interventional device is personalized for the user having the appropriate metallic alloy composition and virtual validation of functional design for each use.
Owner:VANTUS TECH CORP
Therapeutic treatment and prevention of infections with a bioactive materials encapsulated within a biodegradable-biocompatible polymeric matrix
InactiveUS6309669B1Sustained release of active agent over timeEfficient and effective usePowder deliveryPeptide/protein ingredientsAdjuvantEnd-group
Novel burst-free, sustained release biocompatible and biodegrable microcapsules which can be programmed to release their active core for variable durations ranging from 1-100 days in an aqueous physiological environment. The microcapsules are comprised of a core of polypeptide or other biologically active agent encapsulated in a matrix of poly(lactide / glycolide) copolymer, which may contain a pharmaceutically-acceptable adjuvant, as a blend of upcapped free carboxyl end group and end-capped forms ranging in ratios from 100 / 0 to 1 / 99.
Owner:ARMY GOVERNMENT OF THE UNITED STATES AS REPRESENTED BY THE SEC OF THE
Methods for treating a patient using a bioengineered flat sheet graft prostheses
InactiveUS20020103542A1Easy to assembleEasy to cleanSuture equipmentsAnimal materialTissue architectureProsthesis
This invention is directed to tissue engineered prostheses made from processed tissue matrices derived from native tissues that are biocompatible with the patient or host in which they are implanted. When implanted into a mammalian host, these prostheses can serve as a functioning repair, augmentation, or replacement body part or tissue structure.
Owner:ORGANOGENESIS
Implantable or insertable medical device resistant to microbial growth and biofilm formation
InactiveUS6887270B2Prevent preferential partitioningPrevent chemical modificationAntipyreticAnalgesicsActive agentMicrobial adhesion
Disclosed are implantable or insertable medical devices that provide resistance to microbial growth on and in the environment of the device and resistance to microbial adhesion and biofilm formation on the device. In particular, the invention discloses implantable or insertable medical devices that comprise at least one biocompatible matrix polymer region, an antimicrobial agent for providing resistance to microbial growth and a microbial adhesion / biofilm synthesis inhibitor for inhibiting the attachment of microbes and the synthesis and accumulation of biofilm on the surface of the medical device. Also disclosed are methods of manufacturing such devices under conditions that substantially prevent preferential partitioning of any of said bioactive agents to a surface of the biocompatible matrix polymer and substantially prevent chemical modification of said bioactive agents.
Owner:BOSTON SCI SCIMED INC
Biocompatible crosslinked coating and crosslinkable coating polymer composition for forming such a coating
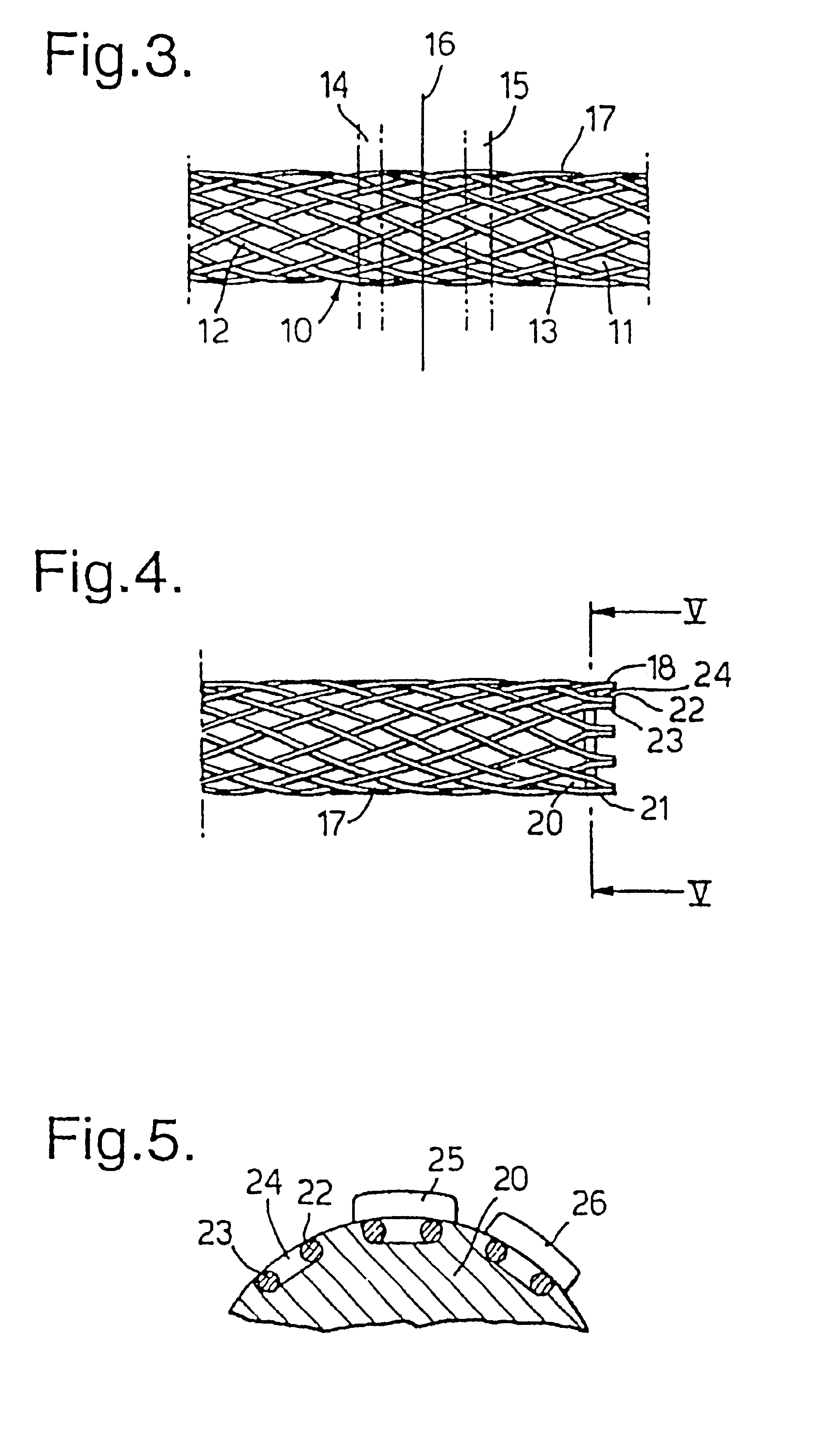
A braided stent (1) for transluminal implantation in body lumens is self-expanding and has a radial expanded configuration in which the angle α between filaments is acute. Some or all of filaments (6,7) are welded together in pairs at each end (4,5) of the stent to provide beads (8), thereby strengthening the stent and assisting its deployment from a delivery device. The stent is preferably completely coated using a biocompatible polymeric coating, said polymer preferably having pendant phosphoryl choline groups. A method of making the stent by braiding and welding is described as well as a delivery device for deploying the device.The present invention provides a biocompatible crosslinked coating and a crosslinkable coating polymer composition for forming such a coating. The biocompatible crosslinked coating may be formed by curing a polymer of 23 mole % (methacryloyloxy ethyl)-2-(trimethylammonium ethyl) phosphate inner salt, 47 mole % lauryl methacrylate, 5 mole % γtrimethoxysilyl propyl methacrylate and 25 mole % of hydroxy propyl methacrylate. The crosslinkable coating polymer may include 23 mole % (methacryloyloxy ethyl)-2-(trimethylammonium ethyl) phosphate inner salt, 47 mole % lauryl methacrylate, 5 mole % γtrimethoxysilyl propyl methacrylate and 25 mole % of hydroxy propyl methacrylate.<?insert-end id="INS-S-00001" ?>
Owner:BIOCOMPATIBLES UK LTD
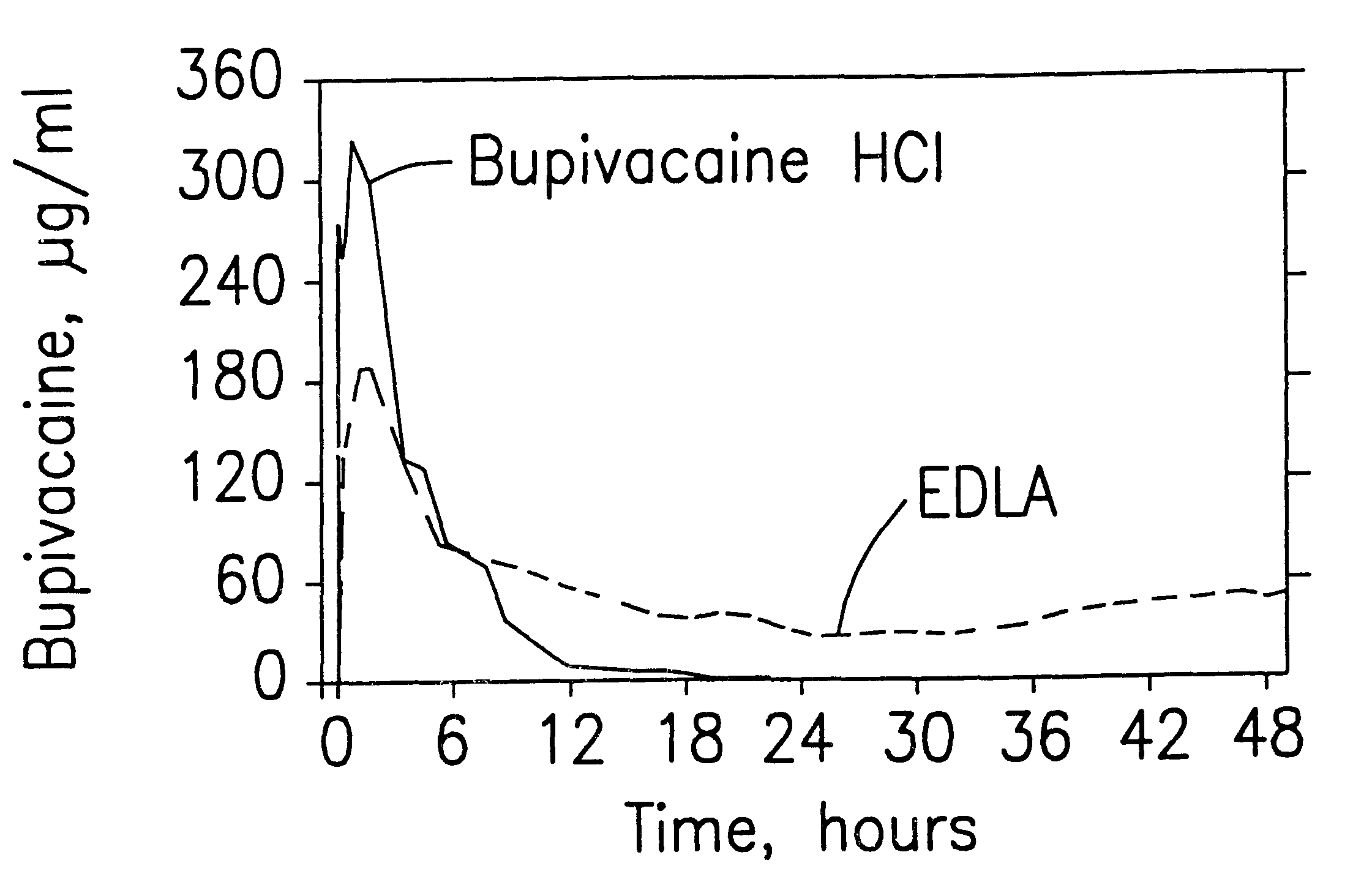
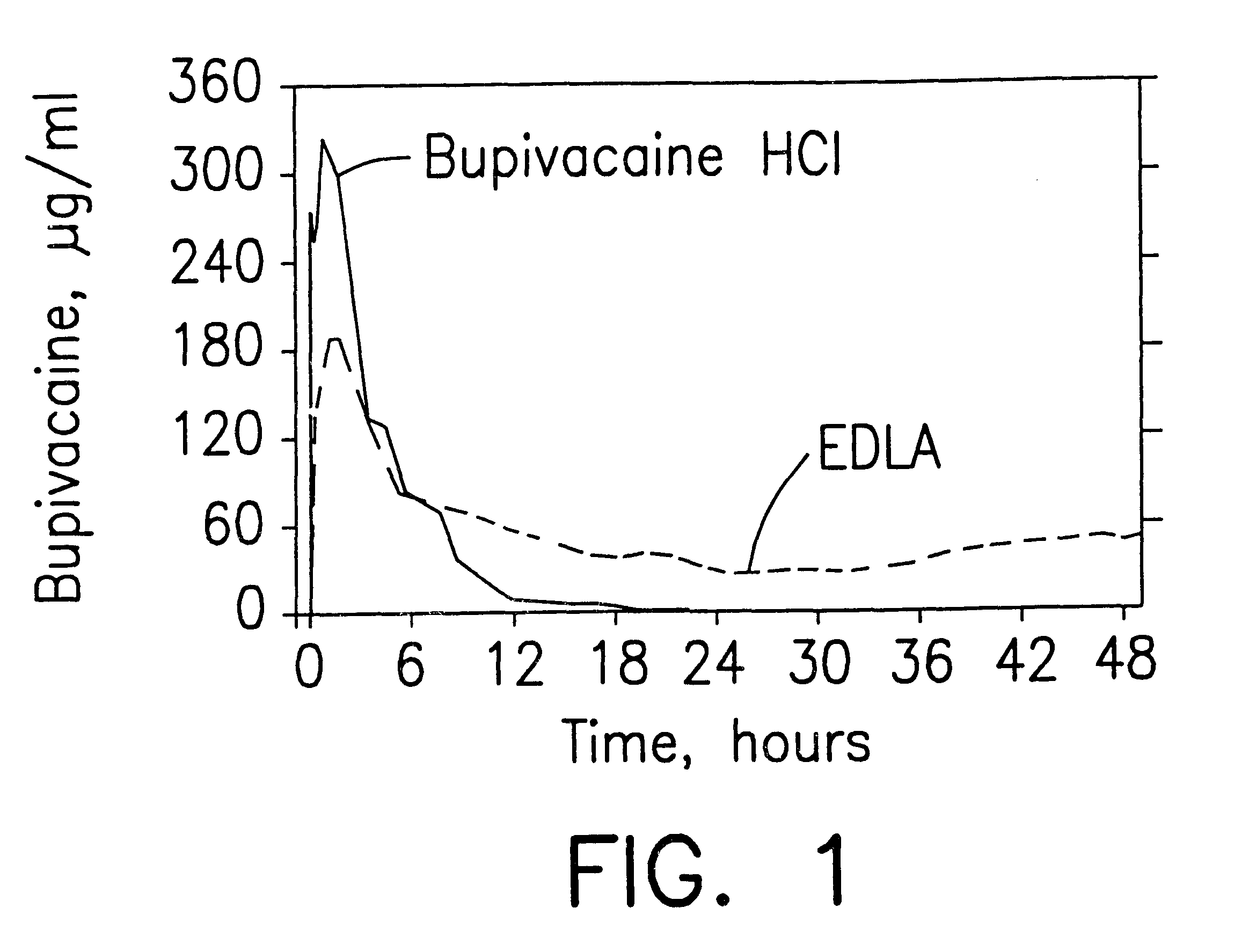
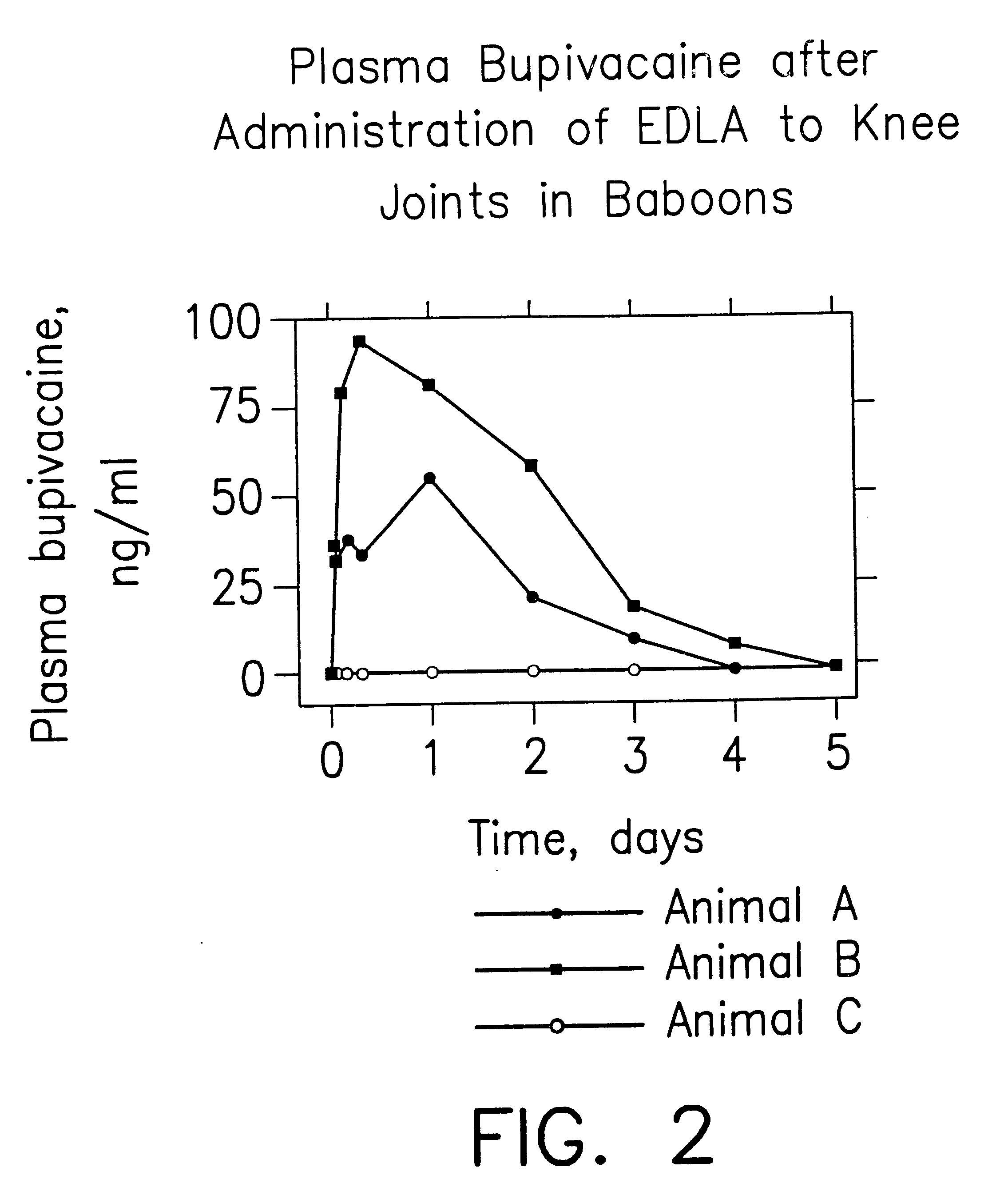
Prolonged anesthesia in joints and body spaces
InactiveUS6248345B1Enhance and prolong local anesthesiaImprovement in administrationInorganic non-active ingredientsAnaestheticsAnesthetic AgentPharmaceutical medicine
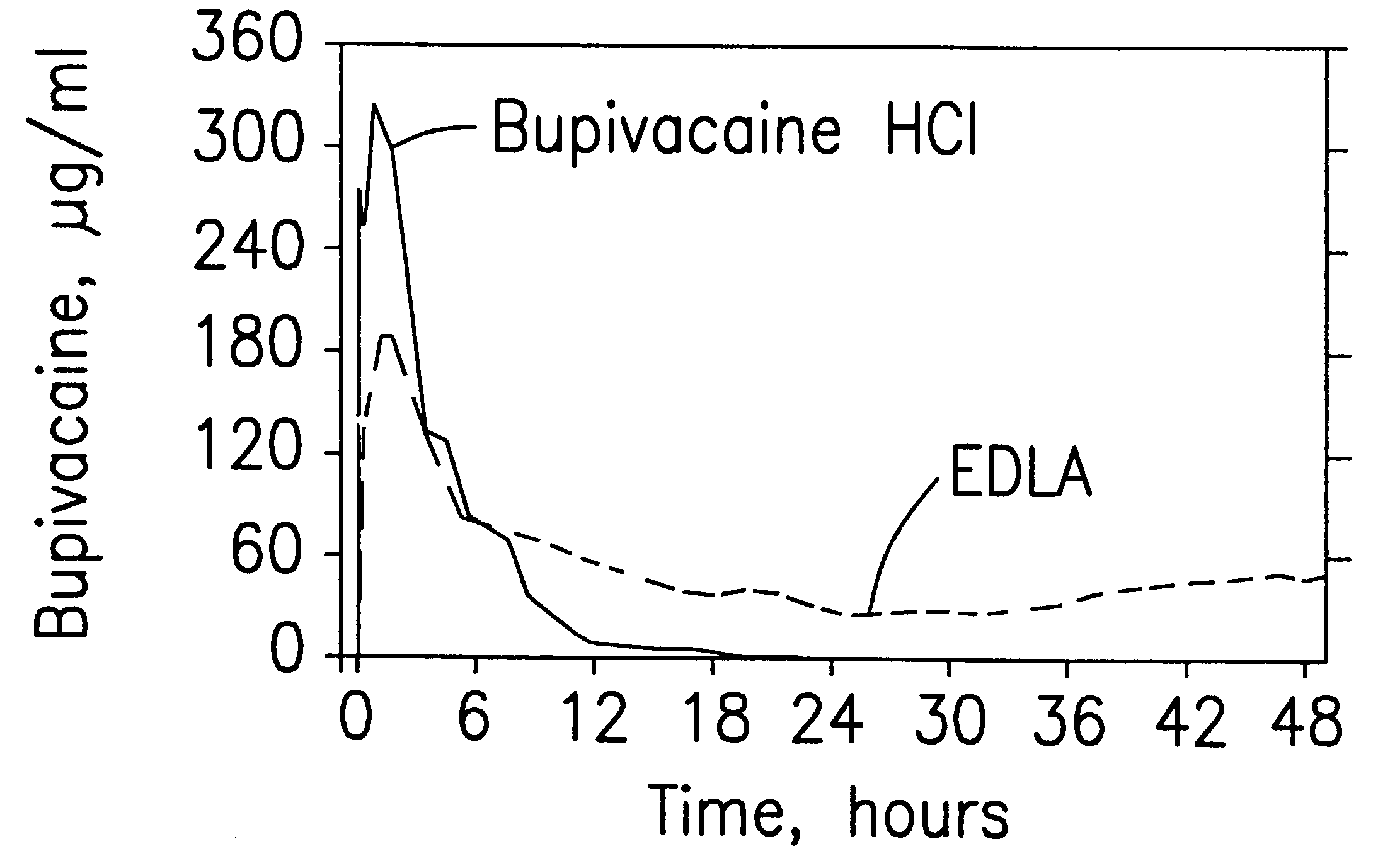
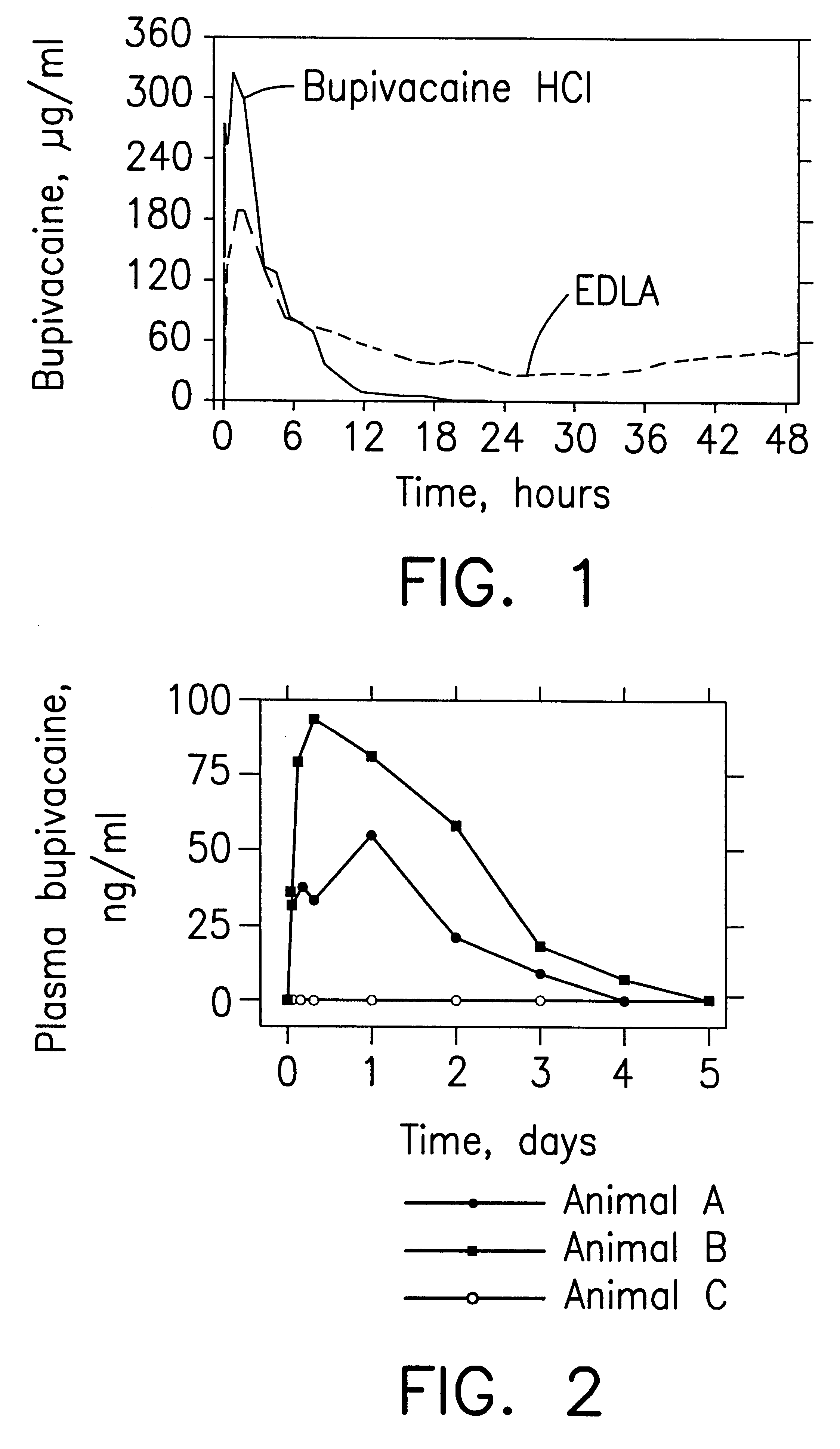
Sustained release local anesthetic formulations are administered intra articularly and / or into body spaces / cavities. The formulation is preferably a plurality of injectable microparticles including a local anesthetic and an effective amount of a biocompatible, biodegradable, sustained release material prolonging the release of the local anesthetic and optionally and a pharmaceutically acceptable, i.e., non-toxic, augmenting agent effective to prolong the duration of the local anesthesia for a time period longer than that obtainable without the augmenting agent.
Owner:PURDUE PHARMA LP
Implant with composite coating
InactiveUS6261322B1High strengthCost effectiveImpression capsBone implantBiocompatible coatingBiocompatibility Testing
Systems and methods are described for implants with composite coatings to promote tissue in-growth and / or on-growth. An implant includes: a substrate; a structured surface formed on at least a portion of the substrate; and a biocompatible coating deposited on at least a fraction of the structured surface. The systems and methods provide advantages in that the implant has good biocompatibility while the biocompatible coating has good strength.
Owner:SHALBY ADVANCED TECH INC
Bioabsorbable brachytherapy device
InactiveUS6575888B2Controlled release rateMinimally shieldsRadioactive preparation formsX-ray/gamma-ray/particle-irradiation therapyBrachytherapy deviceRadiopaque medium
A bioabsorbable brachytherapy device includes a tubular housing with sealed ends and an enclosed radioactive material. The radioactive material includes a radioisotope, such as palladium-103 or iodine-125. The tubular housing is made from a biocompatible and bioabsorbable polymeric material, and is sealed by means such as heat welding or solvent fixing. The device may further include a radiopaque medium and one or more therapeutic drugs.
Owner:FERRING BV
Conveniently implantable sustained release drug compositions
InactiveUS20080038316A1Economical and practical and efficientEasy to produceBiocideOrganic active ingredientsDiseaseSustained release drug
This invention provides for biocompatible and biodegradable syringeable liquid, implantable solid, and injectable gel pharmaceutical formulations useful for the treatment of systemic and local disease states.
Owner:RAMSCOR
Articles comprising large-surface-area bio-compatible materials and methods for making and using them
ActiveUS20100303722A1Improve cell adhesionAccelerated cell growth characteristicImmobilised enzymesBioreactor/fermenter combinationsCell culture mediaBone growth
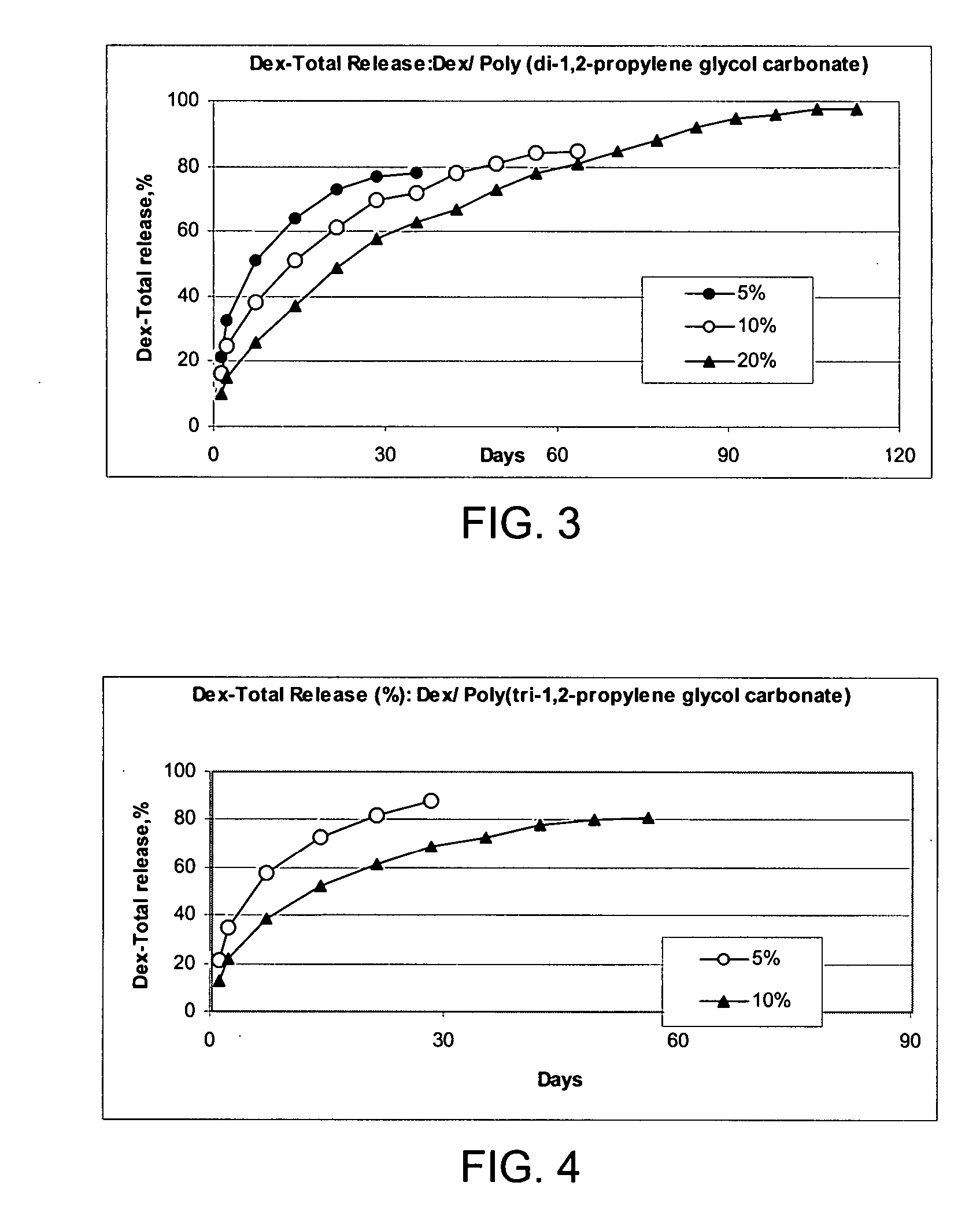
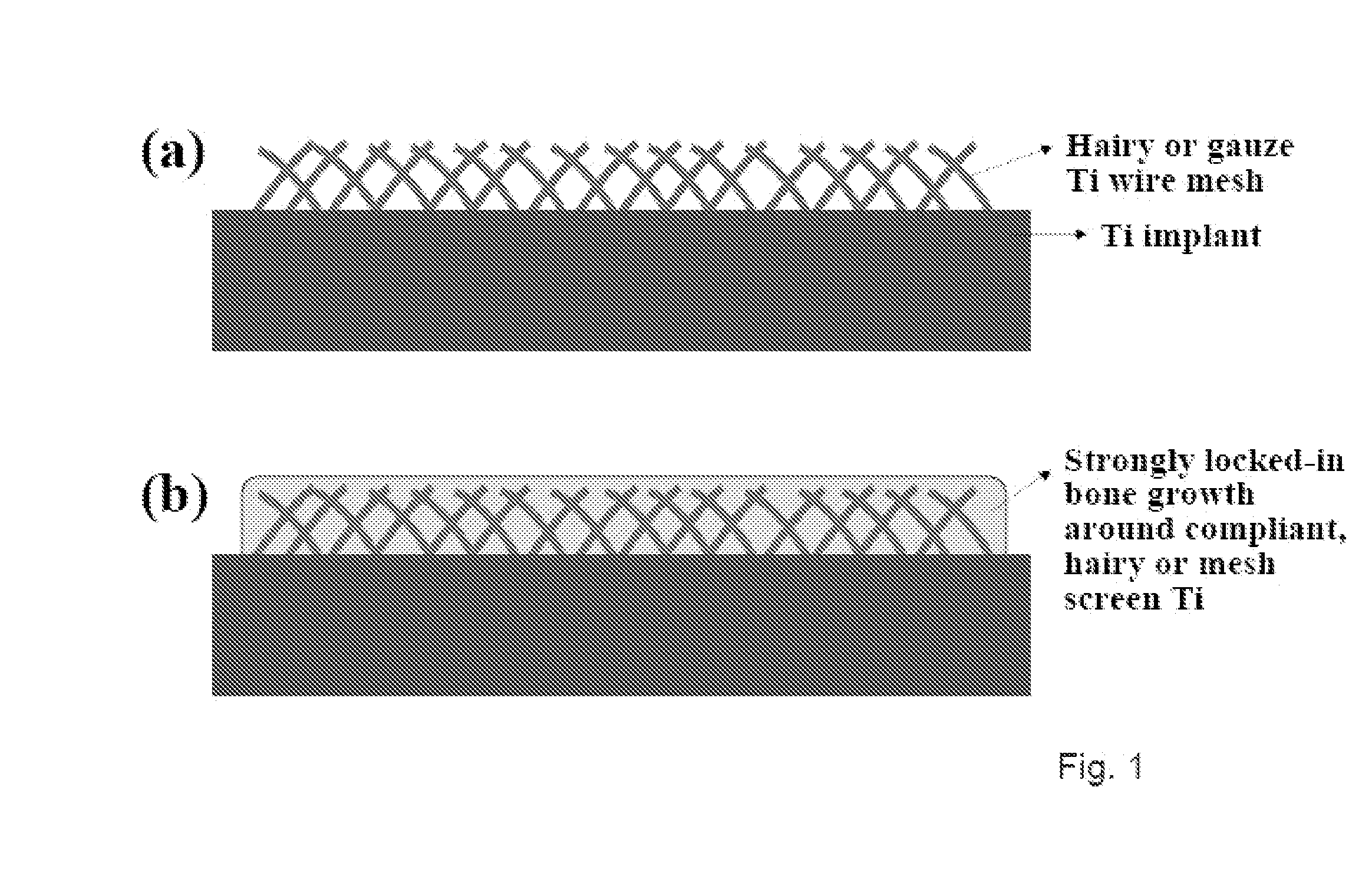
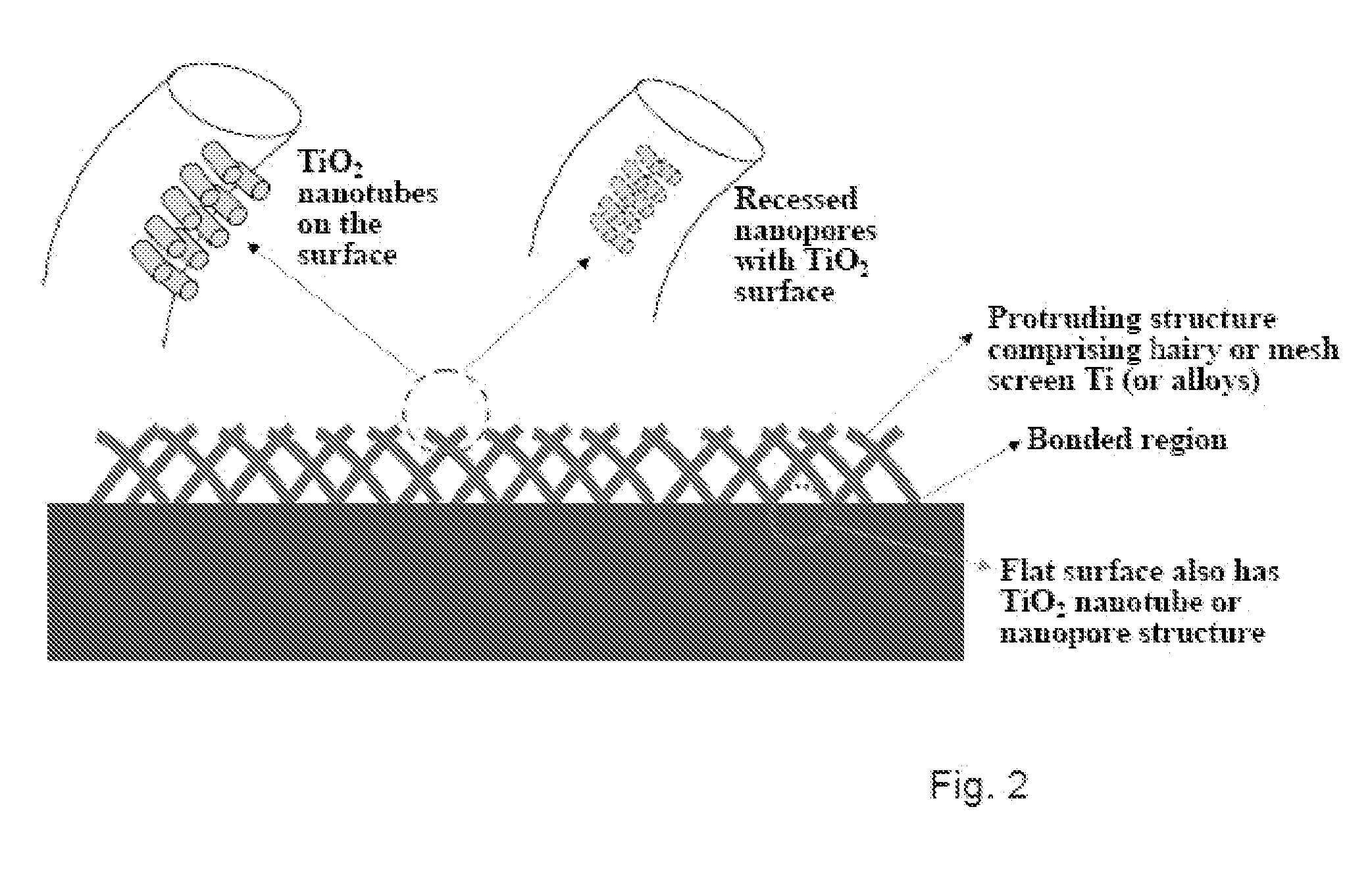
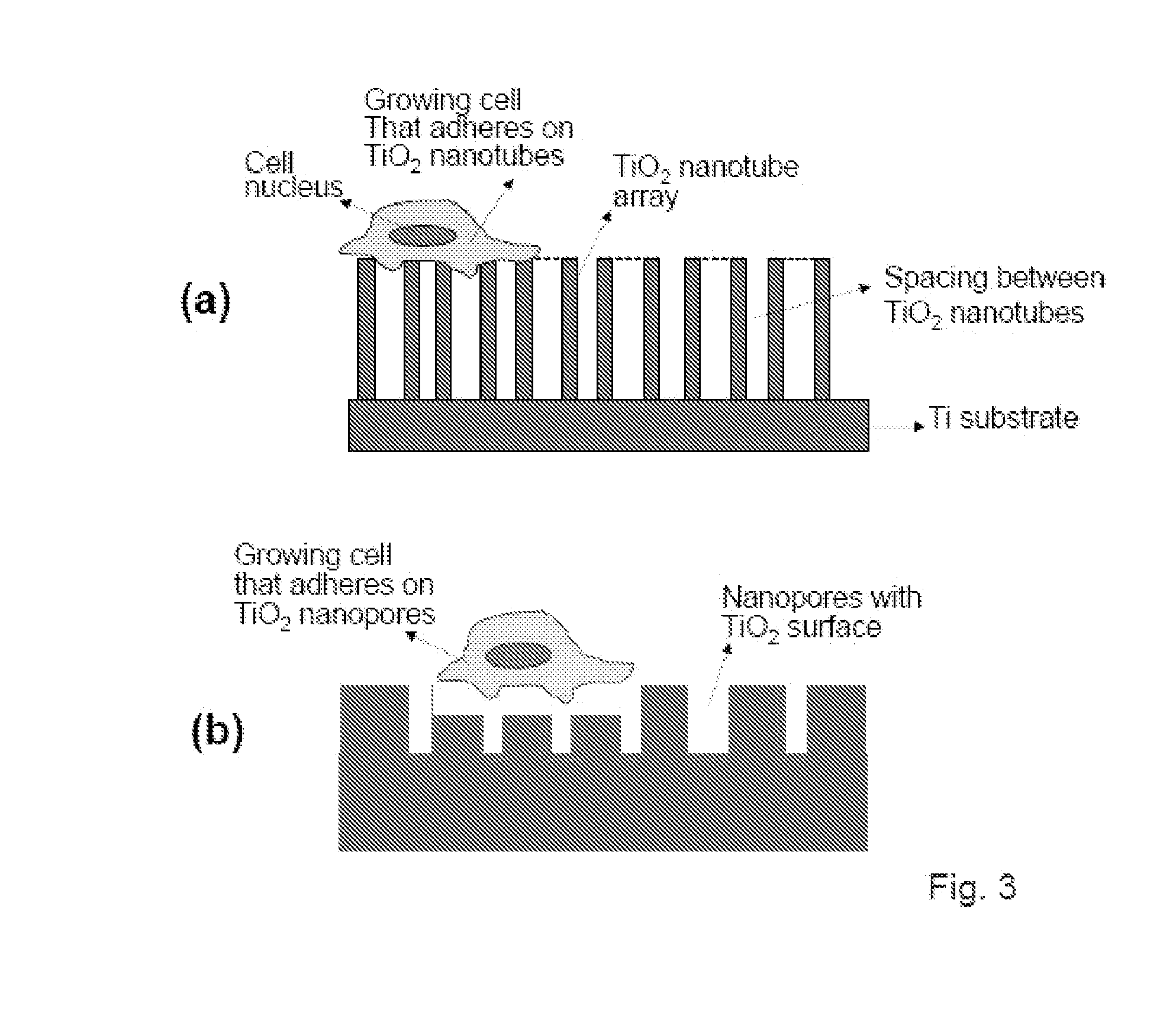
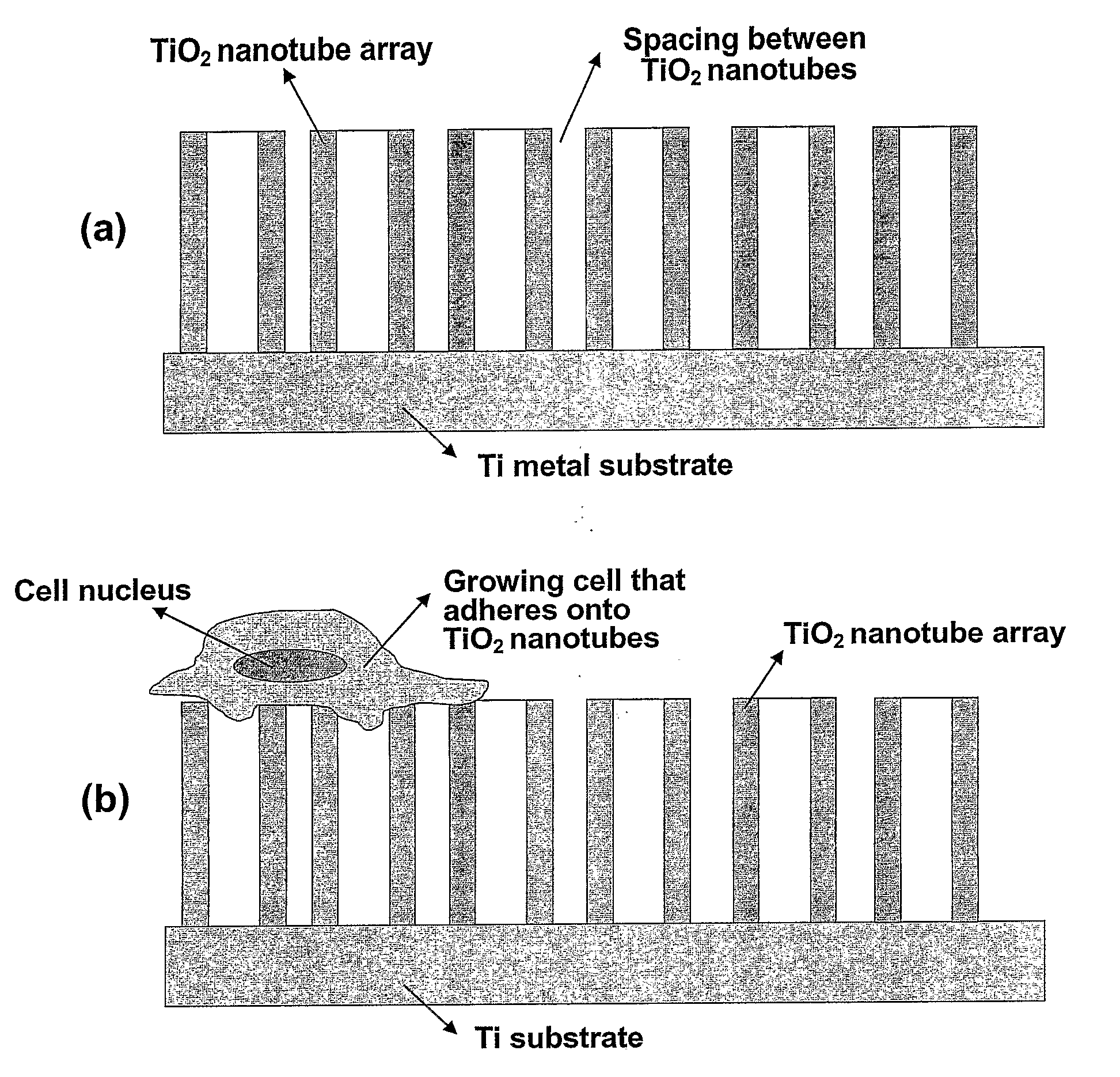
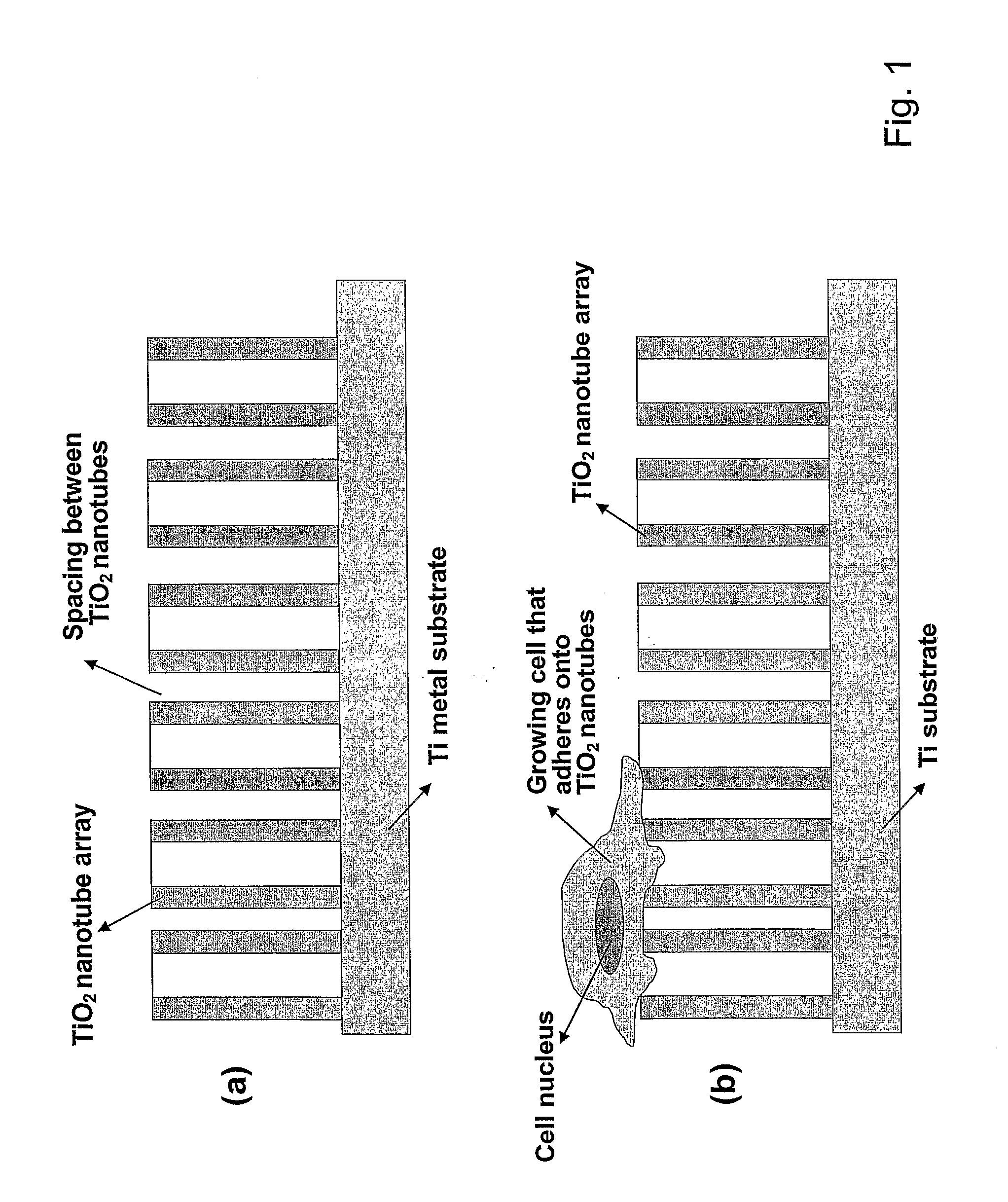
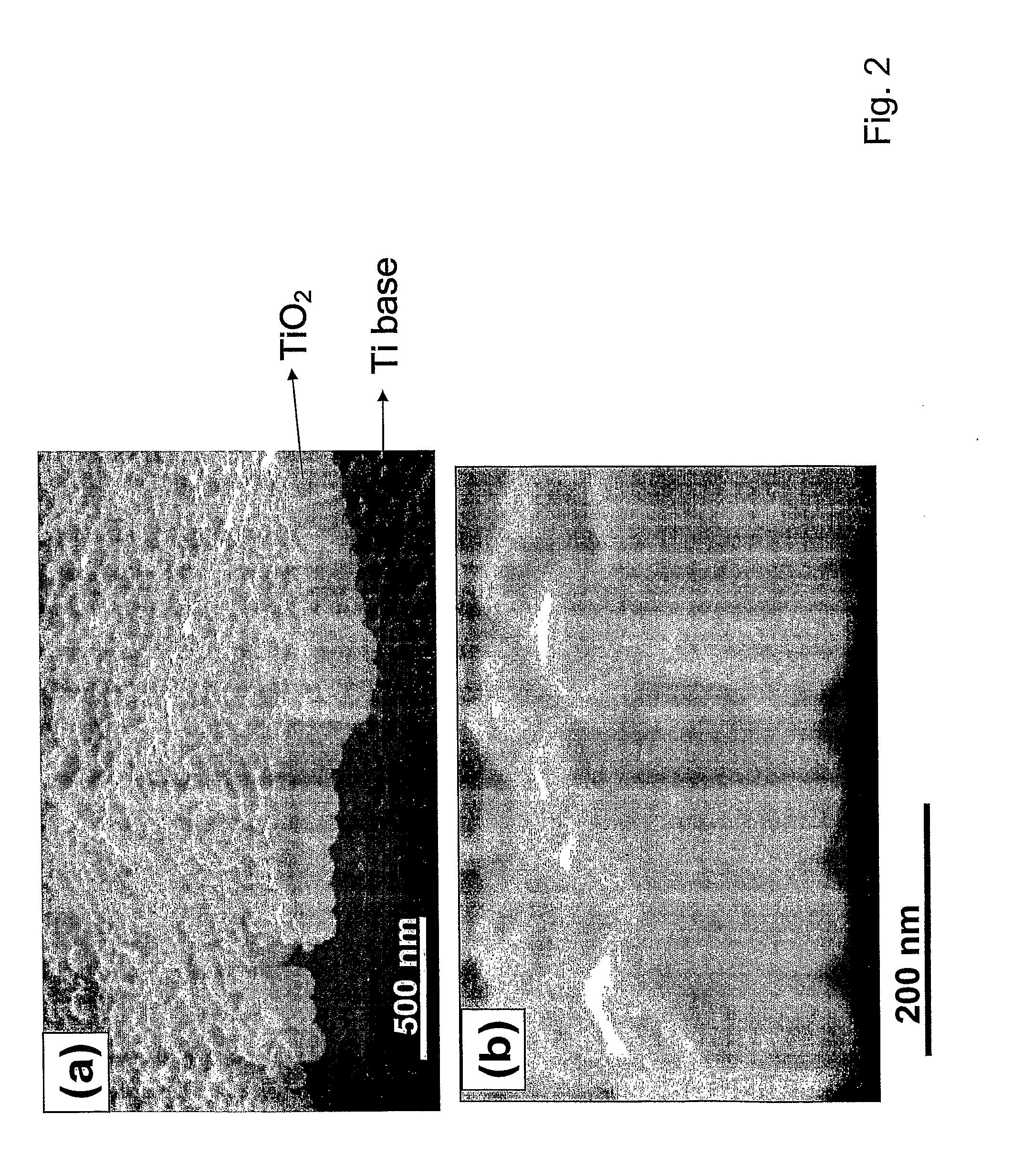
The present invention provides articles of manufacture comprising biocompatible nanostructures comprising significantly increased surface area for, e.g., organ, tissue and / or cell growth, e.g., for bone, tooth, kidney or liver growth, and uses thereof, e.g., for in vitro testing of drugs, chemicals or toxins, or as in vivo implants, including their use in making and using artificial tissues and organs, and related, diagnostic, screening, research and development and therapeutic uses, e.g., as drug delivery devices. The present invention provides biocompatible nanostructures with significantly increased surface area, such as with nanotube and nanopore array on the surface of metallic, ceramic, or polymer materials for enhanced cell and bone growth, for in vitro and in vivo testing, cleansing reaction, implants and therapeutics. The present invention provides optically transparent or translucent cell-culturing substrates. The present invention provides biocompatible and cell-growth-enhancing culture substrates comprising elastically compliant protruding nanostructure substrates coated with Ti, TiO2 or related metal and metal oxide films.
Owner:RGT UNIV OF CALIFORNIA
Compositions comprising nanostructures for cell, tissue and artificial organ growth, and methods for making and using same
ActiveUS20090220561A1Improve bone formationIncreased durabilityBioreactor/fermenter combinationsElectrolysis componentsIn vivoNanostructure
The invention provides articles of manufacture comprising biocompatible nanostructures comprising nanotubes and nanopores for, e.g., organ, tissue and / or cell growth, e.g., for bone, kidney or liver growth, and uses thereof, e.g., for in vitro testing, in vivo implants, including their use in making and using artificial organs, and related therapeutics. The invention provides lock-in nanostructures comprising a plurality of nanopores or nanotubes, wherein the nanopore or nanotube entrance has a smaller diameter or size than the rest (the interior) of the nanopore or nanotube. The invention also provides dual structured biomaterial comprising micro- or macro-pores and nanopores. The invention provides biomaterials having a surface comprising a plurality of enlarged diameter nanopores and / or nanotubes.
Owner:RGT UNIV OF CALIFORNIA
Composite bone graft, method of making and using same
InactiveUS6902578B1Avoid significant donor site morbidityHigh mechanical strengthBone implantJoint implantsDiseaseOssicular Prosthesis Implantation
The invention is directed to a composite bone graft for implantation in a patient, and methods of making and using the composite bone graft, along with methods for treating patients by implanting the composite bone graft at a site in a patient. The composite bone graft includes two or more connected, discrete, bone portions, and includes one or more biocompatible connectors which hold together the discrete bone portions to form the composite bone graft. The composite bone graft may include one or more textured bone surfaces. The textured surface preferably includes a plurality of closely spaced protrusions, preferably closely spaced continuous protrusions. The composite bone graft is useful for repairing bone defects caused by congenital anomaly, disease, or trauma, in a patient, for example, for restoring vertical support of the anterior and / or posterior column. Implantation of the composite bone graft results in improved graft stability and osteoinductivity, without a decrease in mechanical strength. The composite bone graft does not shift, extrude or rotate, after implantation. The present composite bone graft can be appropriately sized for any application and can be used to replace traditional non-bone prosthetic implants.
Owner:LIFENET HEALTH
Soft tissue implants with improved interfaces
InactiveUS20060293760A1Facilitates dual functionInduce new tissue formationAnkle jointsBone implantHost tissueDual function
Specialization of the end(s) or host tissue contact points of biocompatible scaffolds brings a functionality to the scaffold that facilitates the dual function of inducing new tissue formation and facilitating attachment and site-specific tissue formation at the scaffolds' functionalized points of fixation.
Owner:DEPUY SPINE INC (US) +1
Intervertebral disc prosthesis and methods of implantation
InactiveUS6936070B1Reduce harmEasy to insertJoint implantsSpinal implantsSpinal columnIntervertebral space
The present invention provides biocompatible intervertebral disc prostheses that are resilient to compressive forces, that may be adapted to an intervertebral space. When implanted in the spinal column of a patient, the intervertebral disc prostheses according to the present invention is intended to maintain the separation between adjacent vertebrae and provide shock absorbent protection. Flexibility of the spinal column may also be maintained. The present invention further provides methods for the implantation of the intervertebral disc prosthesis of the present invention and an optional intervertebral spacer into the spinal column of a human or animal patient.
Owner:MUHANNA NABIL L
Biocompatible wound dressing
InactiveUS20060189910A1Prevent vacuum leakagePromote cell growthAdhesive dressingsMedical applicatorsWound dressingWound site
A biocompatible wound dressing comprised of a pad for insertion substantially into a wound site and a wound drape for sealing enclosure of the foam pad at the wound site. The pad, comprised of a foam or other like material having relatively few open cells in contact with the areas upon which cell growth is to be encouraged so as to avoid unwanted adhesions, but having sufficiently numerous open cells so that drainage and negative pressure therapy may continue unimpaired, is placed in fluid communication with a vacuum source for promotion of fluid drainage, as known in the art. The pad is further comprised of an ultra-low density fused-fibrous ceramic, or a bioabsorbable branched polymer, or cell growth enhancing matrix or scaffolding.
Owner:KCI LICENSING INC
Intraocular Camera for Retinal Prostheses
ActiveUS20080086206A1Enhanced patient acceptabilityAdd depthHead electrodesEye treatmentControl signalRetinal Prosthesis
An intraocular camera for retinal prostheses may include an optical imaging system comprising a set of optical elements for forming an image of the external world on an image sensor array, wherein the optical elements and the image sensor array may be enclosed in an implantable biocompatible housing that may employ haptic elements for stabilization within the eye. The set of optical elements may be designed to have a short focal length and to provide adequate resolution images that can be transformed into a set of stimulation signals applied to a pixellated microstimulator array. Transmission of the signals from the intraocular camera to a microstimulator driver circuit may be accomplished either by a wired or wireless communication device. Power and control signals may be provided to the intraocular camera by a wired or wireless communication device, or optically by means of ambient illumination or an optical beam.
Owner:UNIV OF SOUTHERN CALIFORNIA
Intra-cavitary ultrasound medical system and method
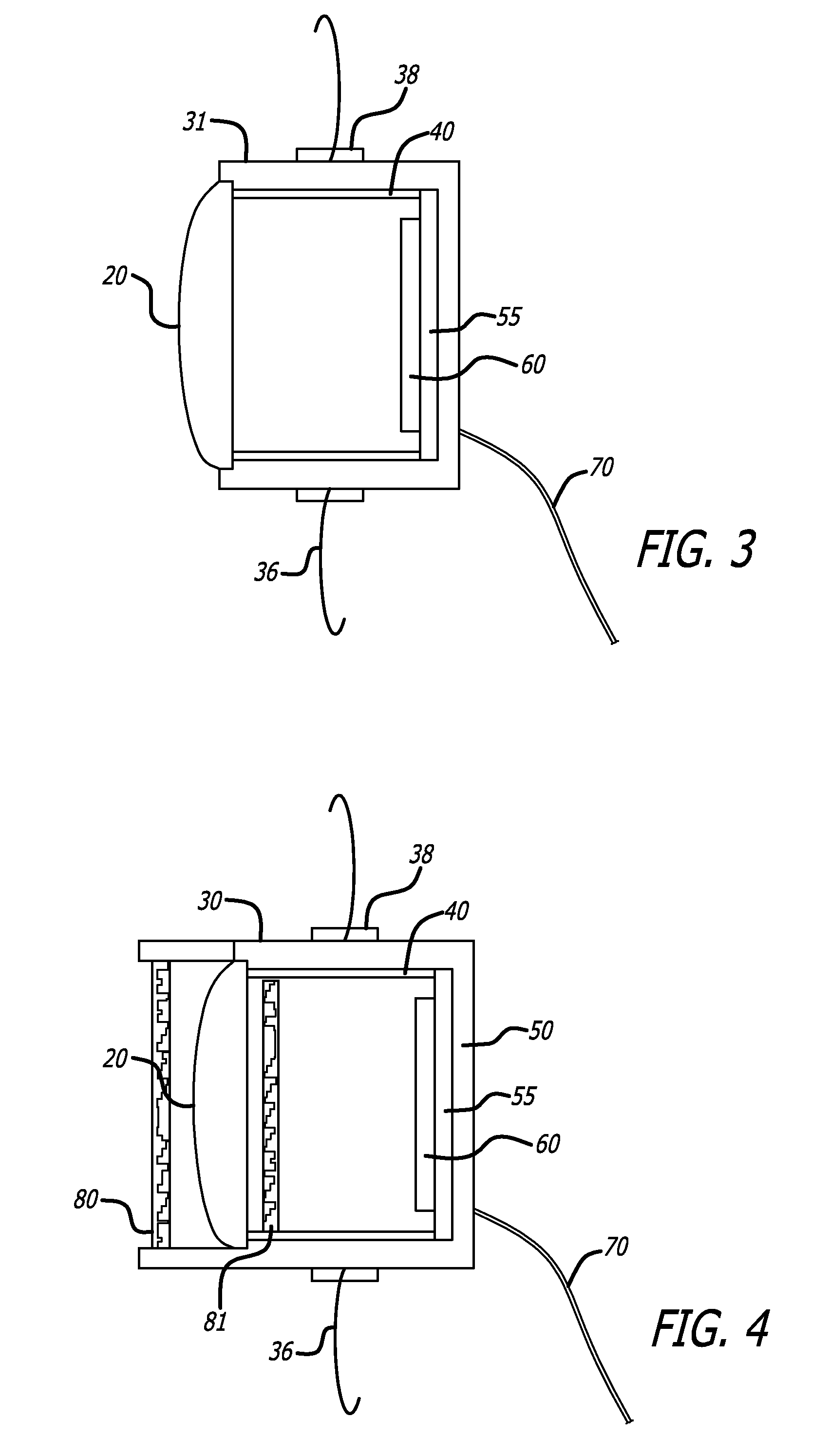
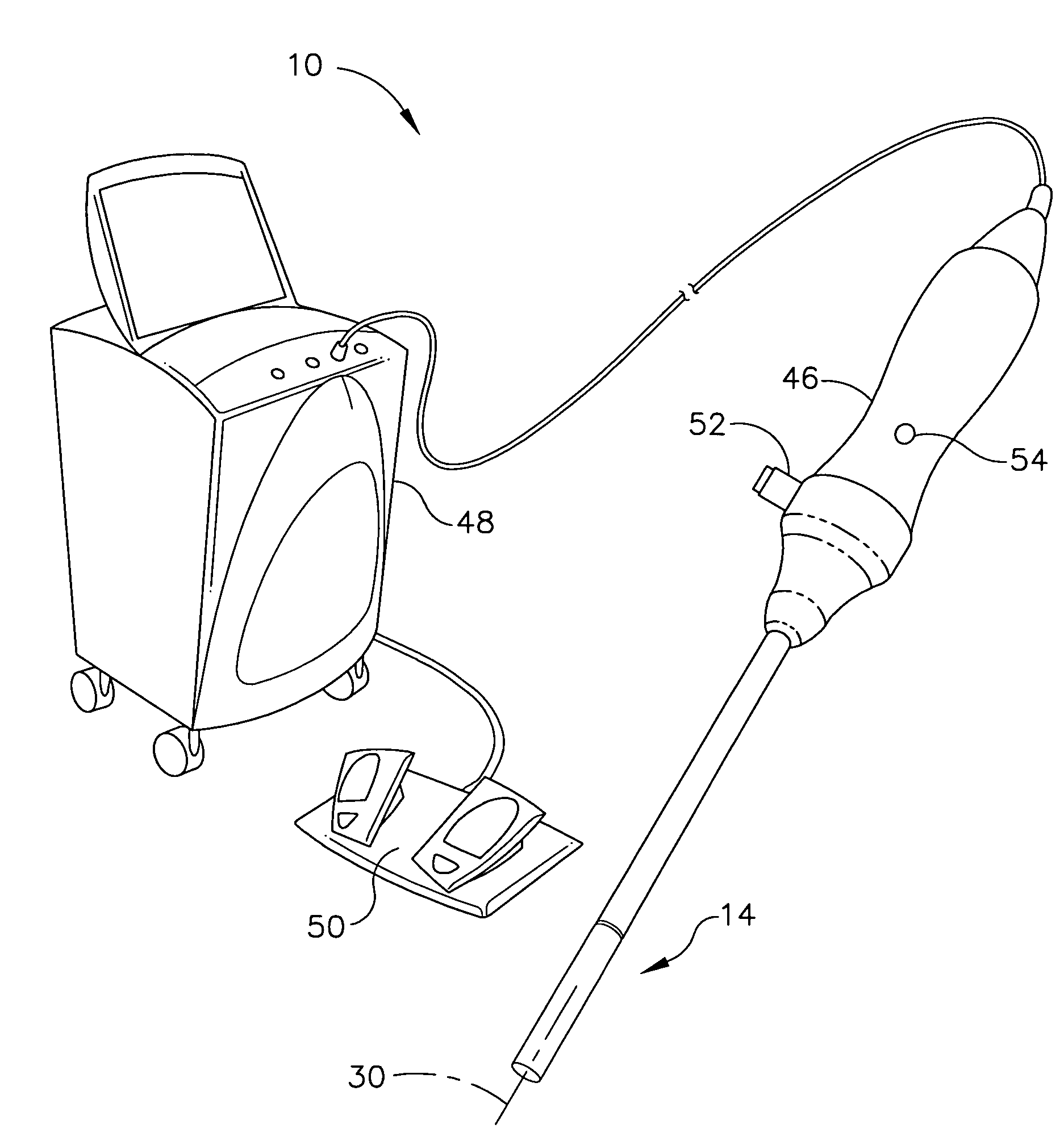
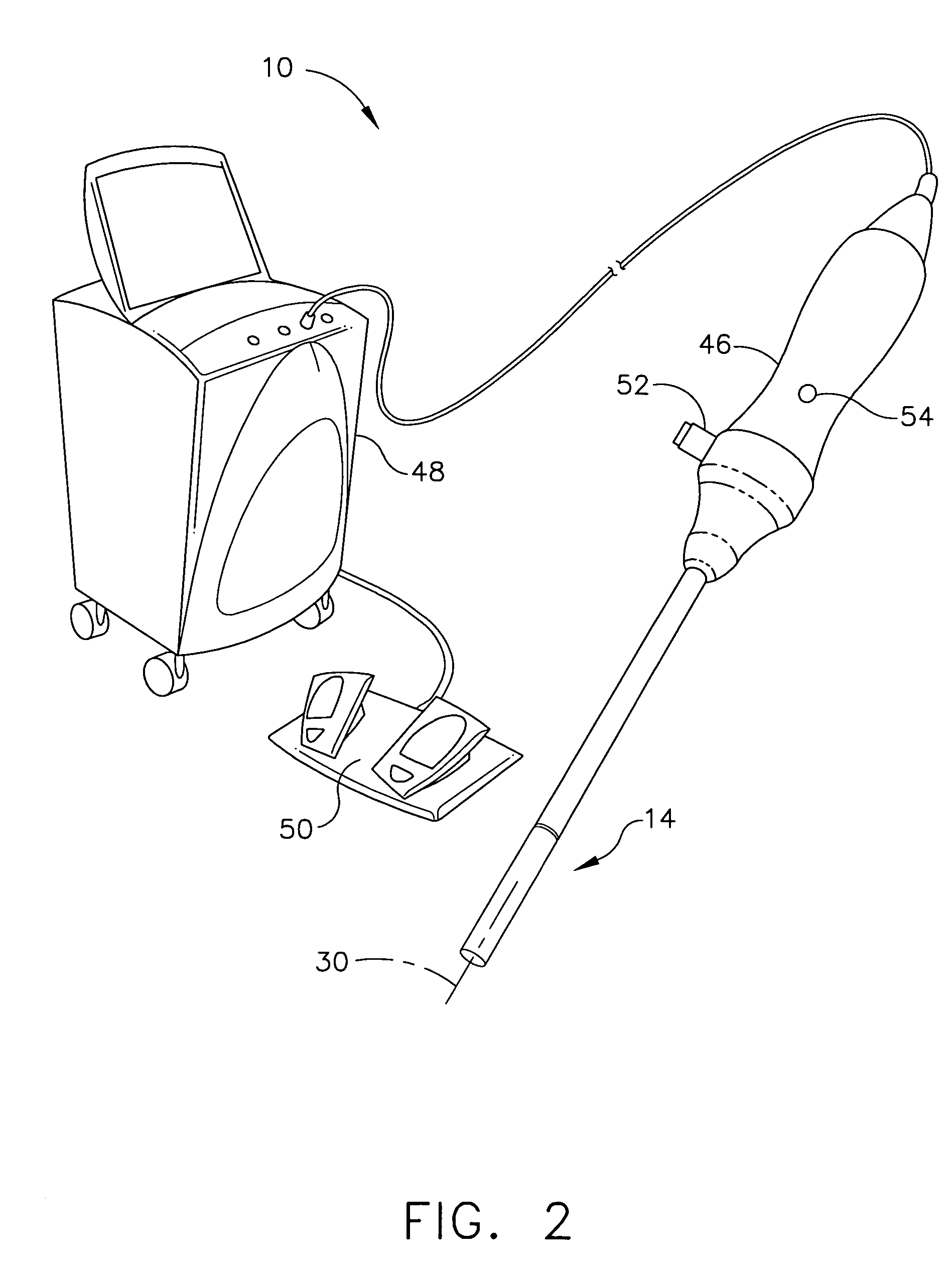
A method for medically employing ultrasound within a body cavity of a patient. An end effector is obtained having a medical ultrasound transducer assembly. A biocompatible hygroscopic substance is obtained having a non-expanded anhydrous state and having an expanded and fluidly-loculated hydrated state. The end effector, including the transducer assembly, and the substance in substantially its anhydrous state are inserted into a body cavity (such as endoscopically inserted into a uterus) of a patient. The transducer assembly is used to medically image and / or medically treat patient tissue (such as stopping blood flow to, and / or ablating, a uterine fibroid). A system for medically employing ultrasound includes the end effector and the substance. In another system, the end effector includes the substance. The substance in its hydrated state expands inside the body cavity providing acoustic coupling between the wall of the body cavity and the transducer assembly.
Owner:CILAG GMBH INT
Methods, devices, and manufacture of the devices for musculoskeletal reconstructive surgery
ActiveUS20170014169A1Guaranteed functionAccurate analysisBone implantJoint implantsBite force quotientCombined use
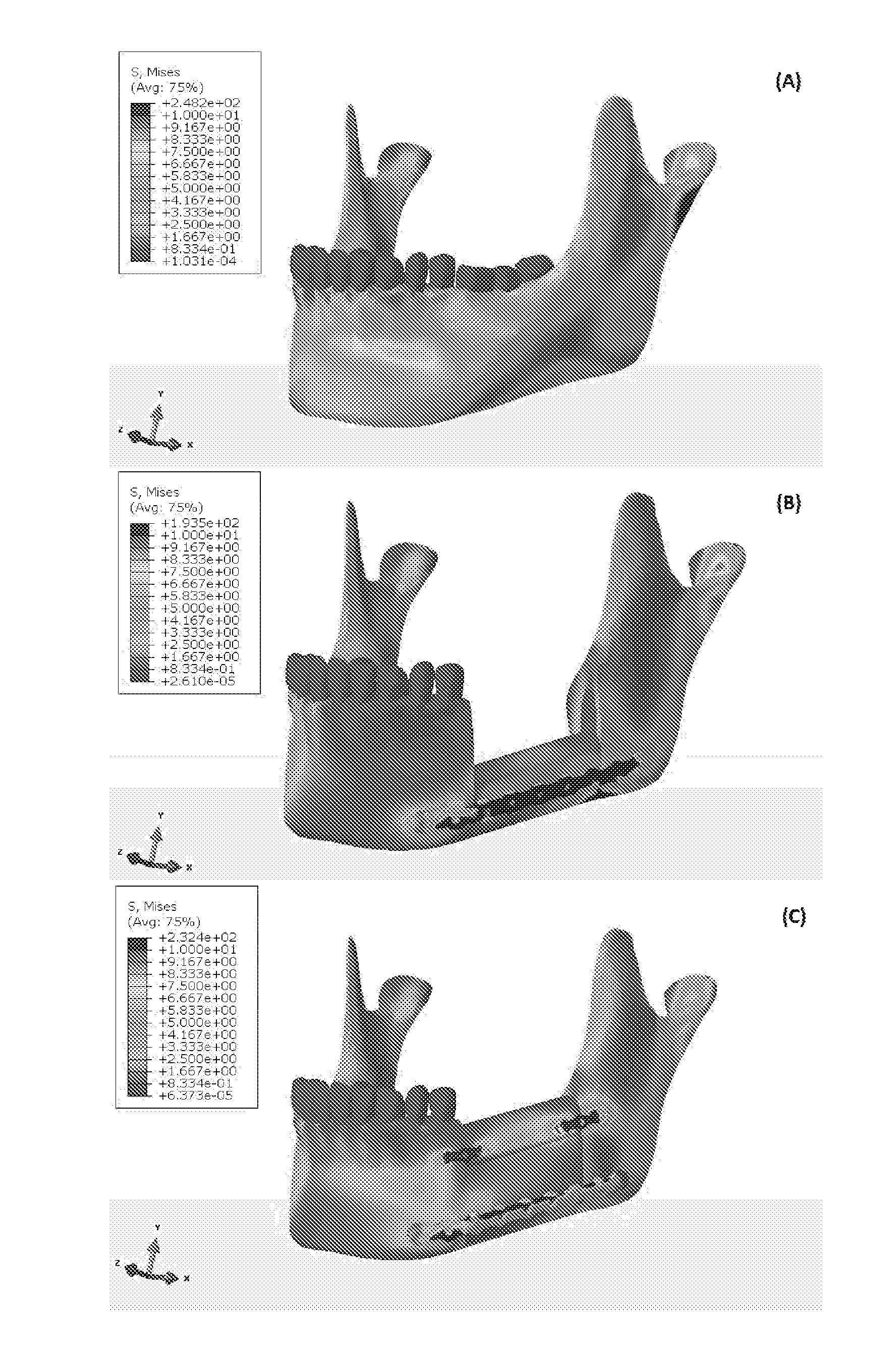
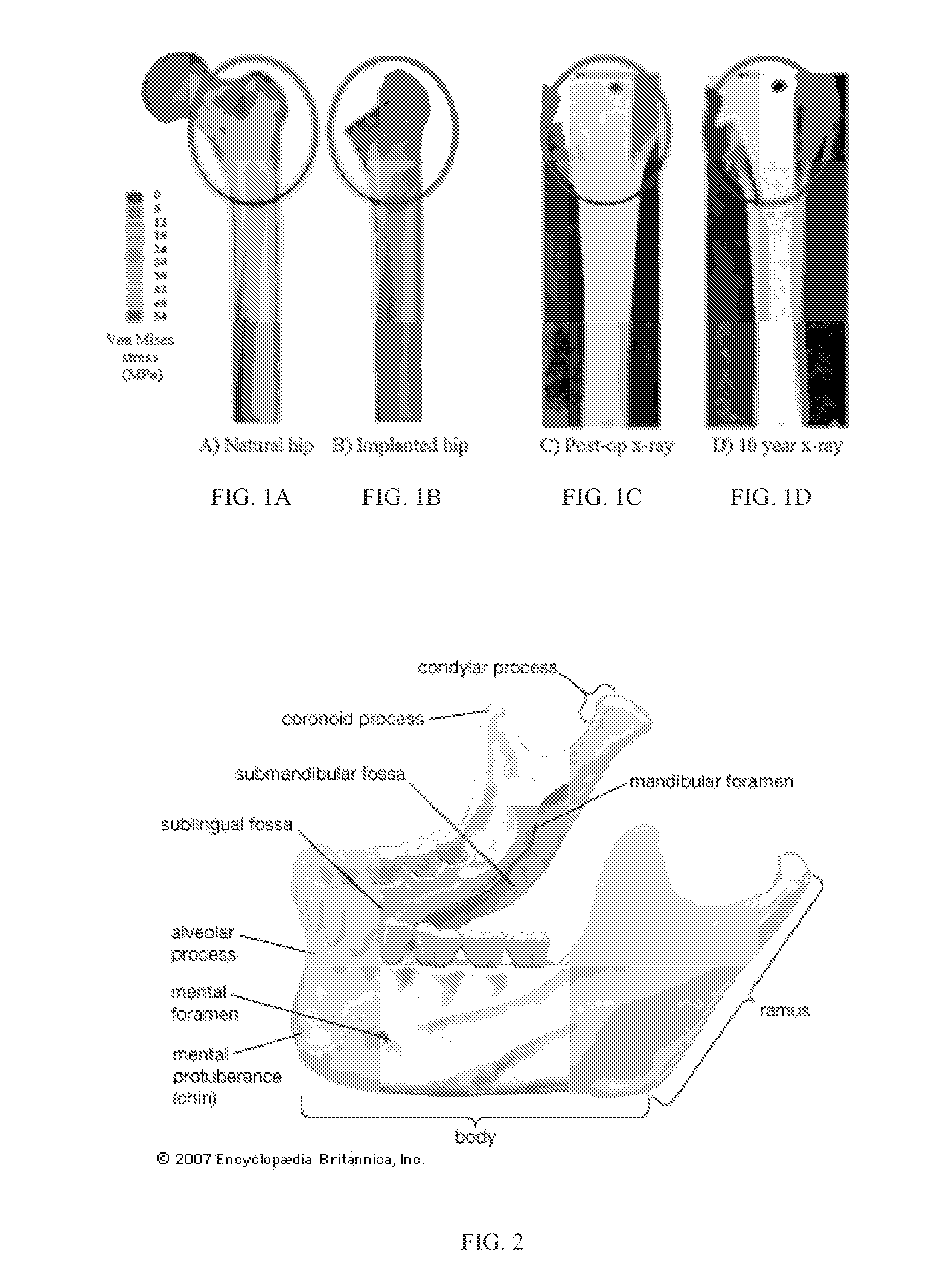
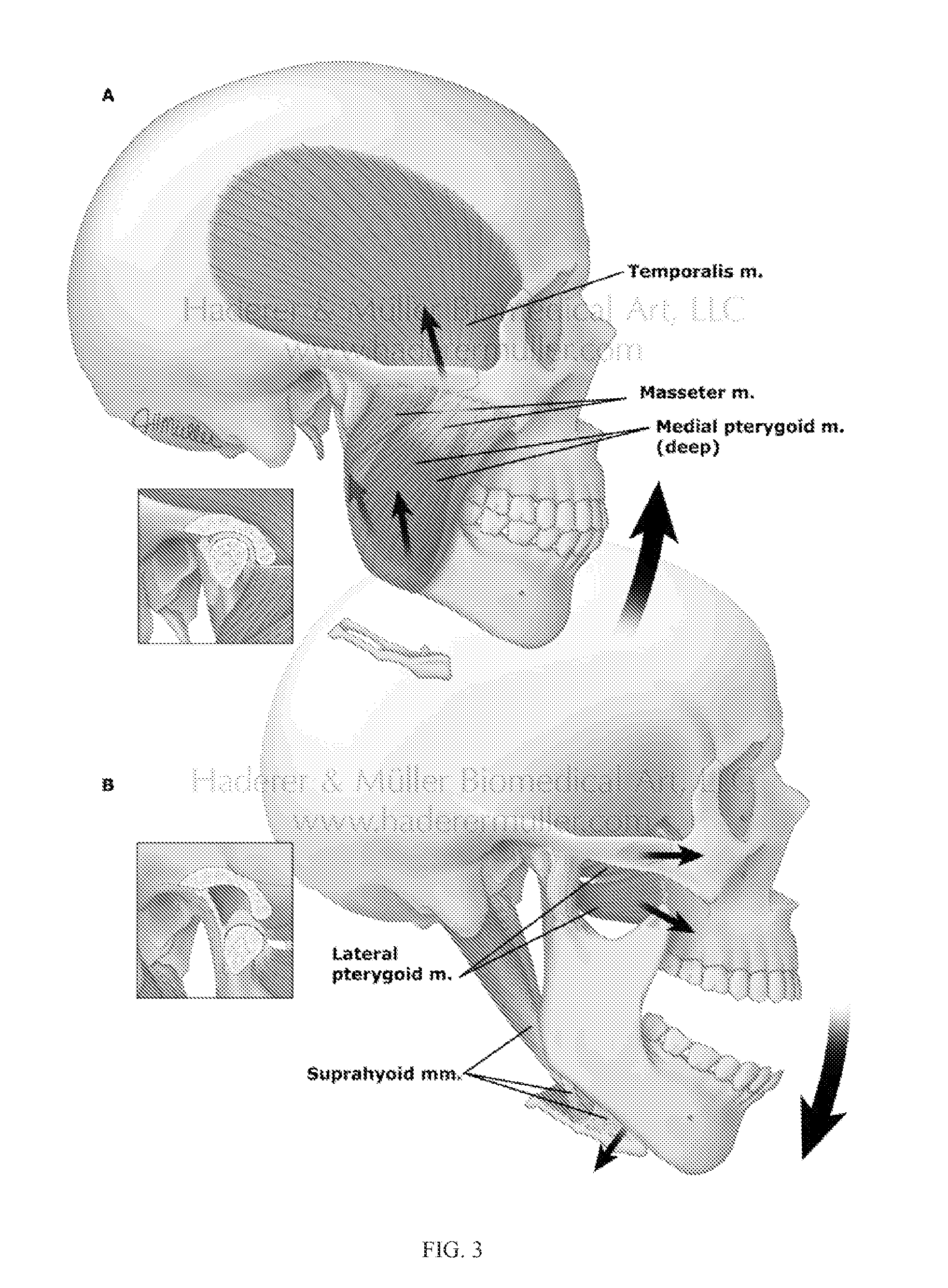
A device used in conjunction with fixation hardware to provide a two-stage process to address the competing needs of immobilization and re-establishment of normal stress-strain trajectories in grafted bone. A method of determining a patient-specific stress / strain pattern that utilizes a model based on 3D CT data of the relevant structures and cross-sectional data of the three major chewing muscles. The forces on each of the chewing muscles are determined based on the model using predetermined bite forces such that a stiffness of cortical bone in the patient's mandible is determined. Based on the stiffness data, suitable implantation hardware can be designed for the patient by adjusting external topological and internal porous geometries that reduce the stiffness of biocompatible metals to thereby restore normal bite forces of the patient.
Owner:OHIO STATE INNOVATION FOUND +1
Prosthetic bone filler and process for the production of the same
InactiveUS6203574B1Preventing good bio-compatibilityPromote resultsIon-exchange process apparatusImpression capsCeramic particleLiving body
A prosthetic bone filler including ceramic granules for use in a living body, the ceramic granules being bonded to each other with a polymeric substance, and having ventilation pores produced as a result of the presence of gaps between the adjacent granules. The prosthetic bone filler is produced by adding the polymeric substance in two portions to the ceramic granules. In addition to good flexibility, the prosthetic bone filler exhibits excellent bio-compatibility.
Owner:ASAHI KOGAKU KOGYO KK
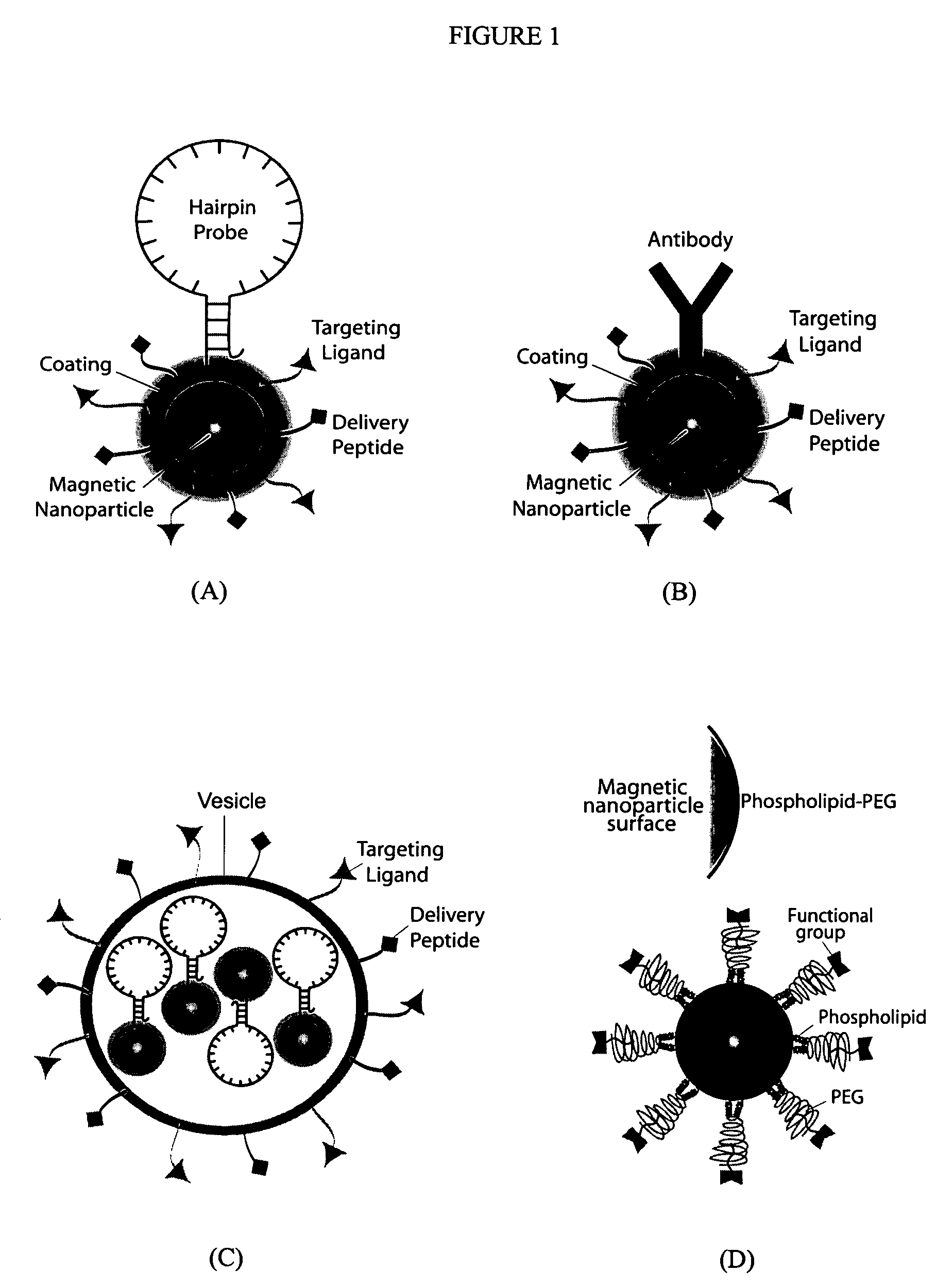
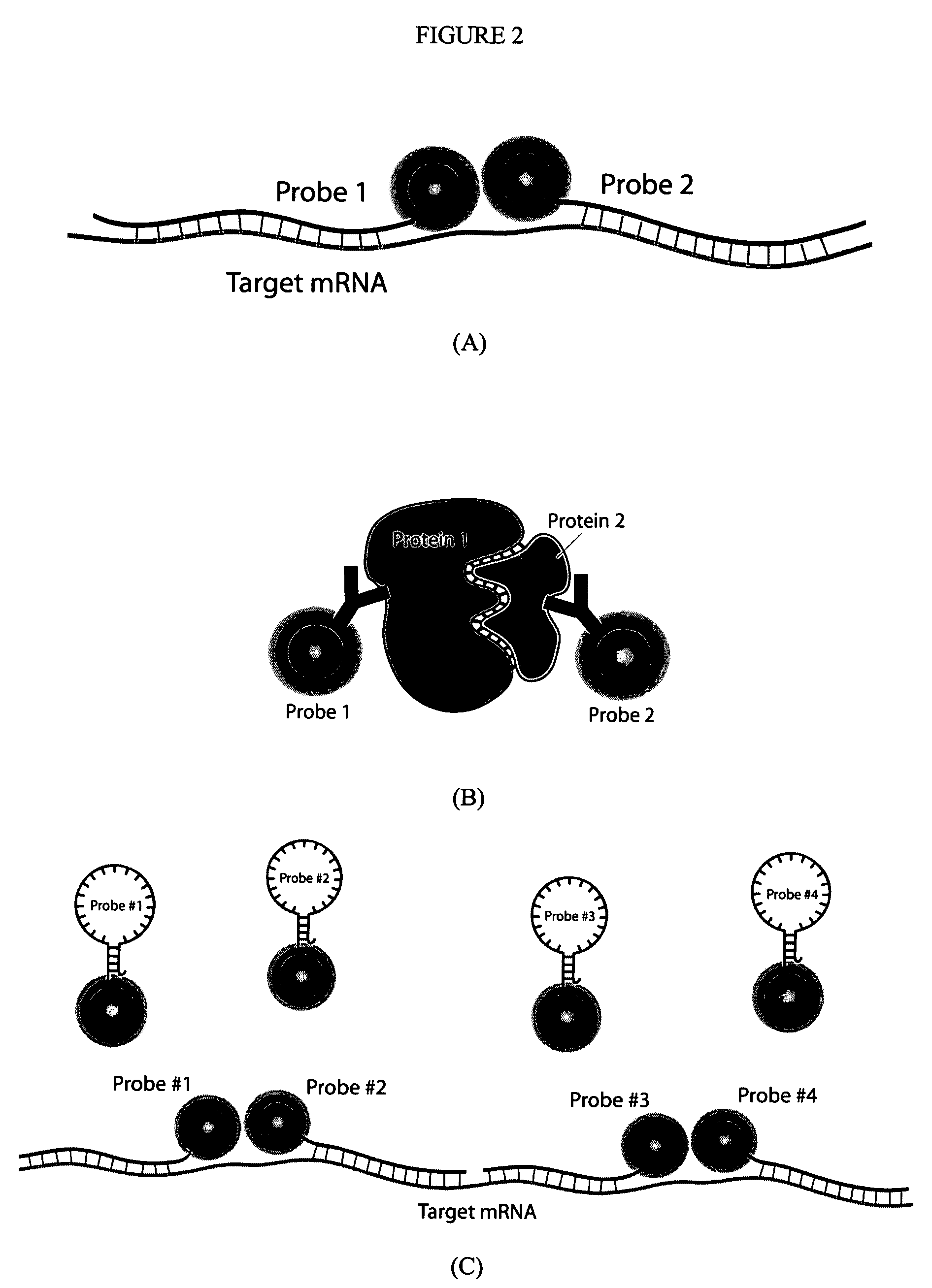
Multifunctional magnetic nanoparticle probes for intracellular molecular imaging and monitoring
InactiveUS7459145B2Efficient internalizationHigh sensitivityBiocideMaterial nanotechnologyFluorescenceBiocompatible coating
The present invention provides multifunctional magnetic nanoparticle probe compositions for molecular imaging and monitoring, comprising a nucleic acid or polypeptide probe, a delivery ligand, and a magnetic nanoparticle having a biocompatible coating thereon. The probe compositions may further comprise a fluorescent or luminescent resonance energy transfer moiety. Also provided are compositions comprising two or more such multifunctional magnetic nanoparticle probes for molecular imaging or monitoring. In particular, the nucleic acid or polypeptide probes bind to a target and generate an interaction observable with magnetic resonance imaging (MRI) or optical imaging. The invention thereby provides detectable signals for rapid, specific, and sensitive detection of nucleic acids, polypeptides, and interactions thereof in vivo.
Owner:GEORGIA TECH RES CORP +1
Formulations and methods for providing prolonged local anesthesia
InactiveUS6451335B1Slow in-vitro releaseRelease the local anestheticAnaesthesiaGranular deliveryControlled releaseAnesthetic Agent
A formulation for inducing sustained regional local anesthesia in a patient comprising a substrate comprising a local anesthetic and an effective amount of a biocompatible, biodegradable, controlled release material prolonging the release of the local anesthetic from the substrate to obtain a reversible local anesthesia when implanted or injected in a patient, and a non-toxic augmenting agent effective to prolong the duration of the local anesthesia for a time period longer than that obtainable from the substrate without the augmenting agent. In preferred embodiments, the controlled release material is a low molecular weight, acid-terminated polymer. A further aspect of the invention is directed to such formulations which release the local anesthetic in two phases, the first a rapid "bolus" to initiate anesthesia and a second, slower release to maintain anesthesia.
Owner:EURO-CELTIQUE SA
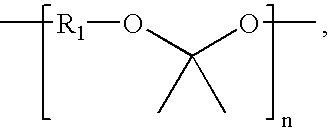
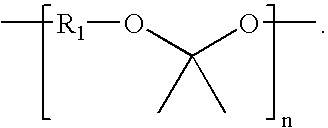
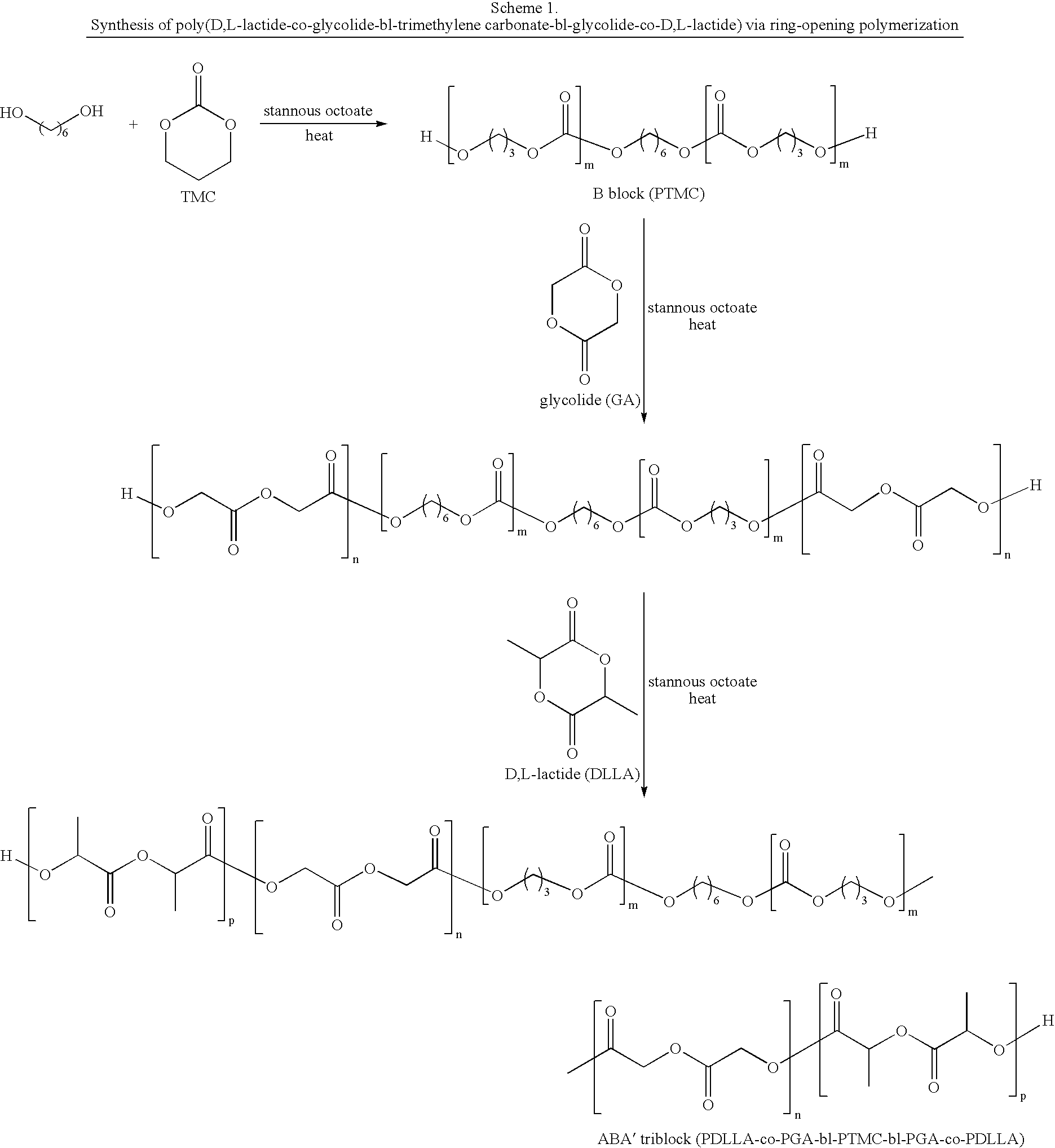
Biodegradable triblock copolymers for implantable devices
InactiveUS20090004243A1Reduce adverse effectsImprove mechanical propertiesBiocidePeptide/protein ingredientsDevice formBiocompatibility Testing
The present invention is directed to polymeric materials made of biodegradable, bioabsorbable triblock copolymers and implantable devices (e.g., drug-delivery stents) containing such polymeric materials. The polymeric materials may also contain at least one therapeutic substance. The polymeric materials are formulated so as to improve the mechanical and adhesion properties, degradation, biocompatibility and drug permeability of such materials and, thus, implantable devices formed of such materials.
Owner:ABBOTT CARDIOVASCULAR
Spray for fluent materials
ActiveUS20060189944A1Easy to measureWell mixedSurgeryIntravenous devicesSprayerBiocompatible coating
Certain embodiments relate to a sprayer or other medical apparatus for applying a biocompatible coating in situ. Such an apparatus may have a first conduit connected to a first exit opening and a second conduit connected to a second exit opening to deliver a first composition through the first conduit and a second composition through the second conduit to mix the first composition and the second composition outside both the first conduit and the second conduit. The first composition may be, e.g., a precursor to a material formed after the mixing of the first composition and the second composition. The first exit opening and the second exit opening may be approximately adjacent to each other and define an angle that is less than about 140 degrees.
Owner:CONFLUENT SURGICAL
Wound dressing material containing silk fibroin and sericin as main component and method for preparing same
There is provided a novel wound dressing material which has biocompatibility and infection controllability as essential properties required for such a material, especially excellent flexibility and water absorption properties, thereby accelerating smooth regeneration of a skin defect without stripping off the regenerating skin while removing the material from the skin. A healing agent is added to the wound dressing material which comprises an amorphous film of a crystallinity below 10% and contains fibroin and sericin as a main component.
Owner:NAT INST OF AGROBIOLOGICAL SCI +1
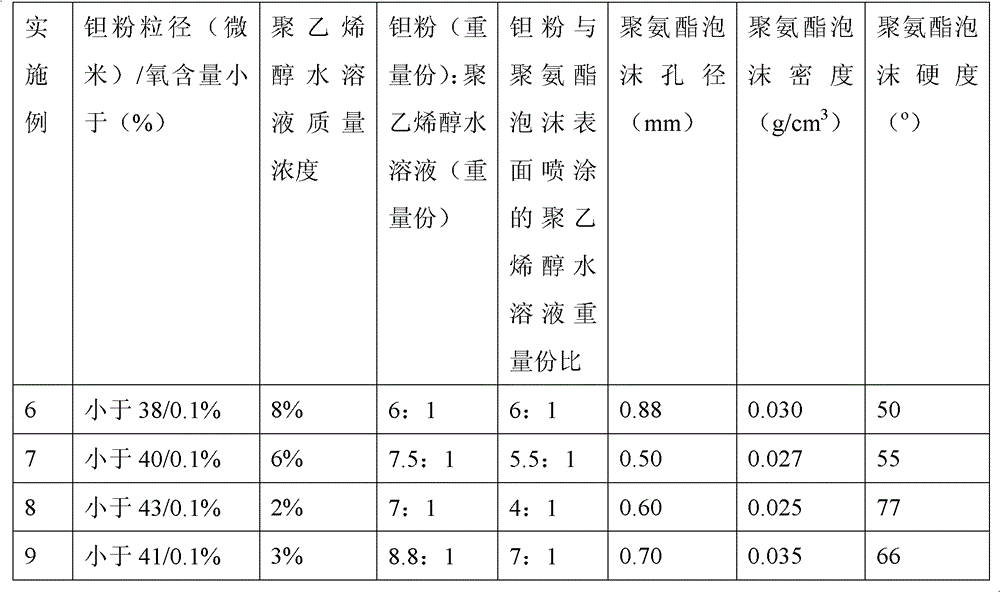
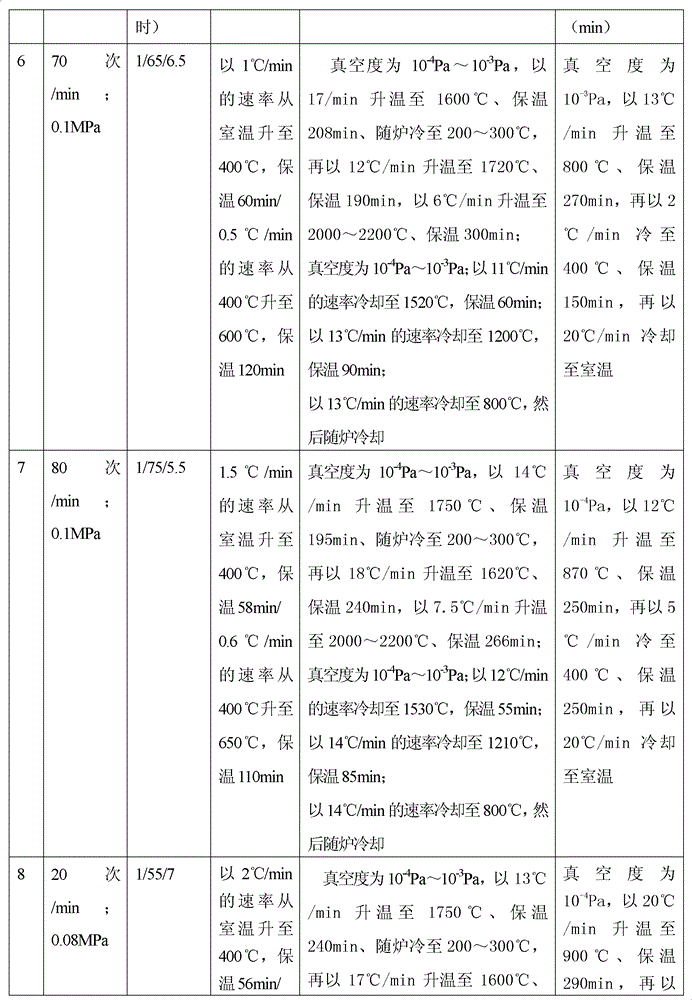
Method for preparing medical porous tantalum implant material
ActiveCN102796894AReduce contentImprove mechanical propertiesProsthesisPolyvinyl alcoholBiocompatibility Testing
The invention discloses a method for preparing a medical porous tantalum material. The method comprises the following steps of: mixing a poly ethanol aqueous solution and tantalum powder to obtain slurry, wherein the mass concentration of the poly ethanol aqueous solution is 2 to 8 percent; injecting the slurry into an organic foam by vibrating and pressurizing, wherein the vibrating frequency is 20 to 80 times / min; drying; degreasing; sintering, namely raising temperature to 1,500 to 1,800 DEG C at the speed of 10 to 20 DEG C / min under the vacuum degree of 10<-4> to 10<-3>Pa, preserving heat for 120 to 240 minutes, cooling to 200 to 300 DEG C along with a furnace, raising temperature to 1,500 to 1,800 DEG C at the speed of 10 to 20 DEG C / min again, preserving heat for 180 to 240 minutes, raising temperature to 2,000 to 2,200 DEG C at the speed of 5 to 10 DEG C / min, and preserving heat for 120 to 360 minutes; cooling; and performing thermal treatment, namely raising temperature to 800 to 900 DEG C at the speed of 10 to 20 DEG C / min under the vacuum degree of 10<-4> to 10<-3> Pa, preserving heat for 240 to 480 minutes, cooling to 400 DGE C at the speed of 2 to 5 DGE C / min, preserving heat for 120 to 300 minutes, and cooling to room temperature along with the furnace. The porous tantalum prepared by the method is very suitable to be used for the medical implant material for replacing bearing bone tissues, and biocompatibility and the mechanical property can be guaranteed simultaneously.
Owner:CHONGQING RUNZE PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com