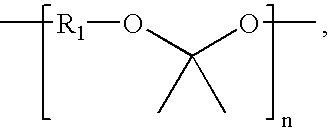
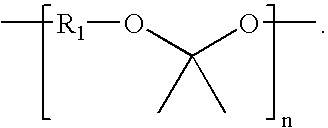
Biodegradable triblock copolymers for implantable devices
a bioabsorbable, triblock copolymer technology, applied in the direction of prosthesis, catheter, antibody medical ingredients, etc., can solve the problems of occlusion of the conduit, late thrombosis with drug-delivery stents, and collapse of the inner flap or torn arterial lining, so as to avoid adverse effects, improve mechanical properties, and perform the effect of functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
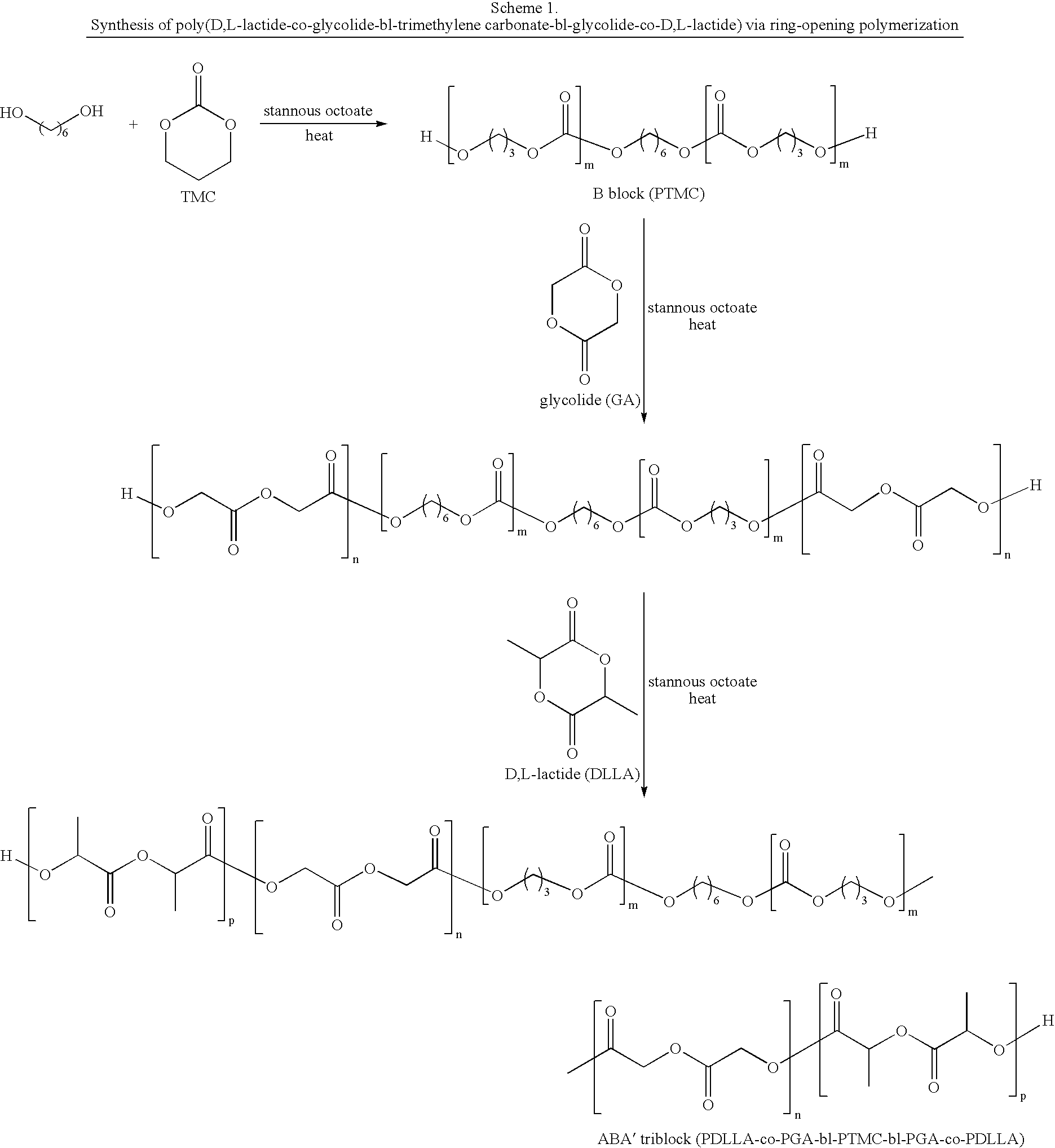
Synthesis of Poly(glycolide-ran-D,L-lactide)-block-poly(TMC)-block-poly(glycolide-ran-D,L-lactide), 51.8 mole % glycolide, 43.6 mole % trimethylene carbonate, and 4.6 mole % D,L-lactide
[0229]A flame-dried, three-neck 250 ml round-bottom flask is charged with 46.41 g (0.455 mole) trimethylene carbonate, 0.123 g (1.16 mmol) distilled diethylene glycol, and 0.053 ml of stannous octoate (0.33 M in toluene) (60,000:1 molar ratio monomer:catalyst). The flask is equipped with a flame-dried mechanical stirrer and adapter for argon purge and vacuum. The reaction vessel is purged by evacuating the flask, followed by venting with argon; this is repeated three times. The reaction flask, under an argon pressure of one atmosphere, is heated to 190° C. and maintained at this temperature for about 16 hours with slow stirring.
[0230]In the second stage of polymerization, 6.96 g (48.3 mmol) of D,L-lactide, and 62.64 g (0.54 mole) of molten glycolide, are added to the prepolymer in the reaction flask a...
example 2
Synthesis of Poly(D,L-lactide)-block-poly(caprolactone-ran-glycolide)-block-poly(D,L-lactide), 39.5 mole % caprolactone, 34.7 mole % D,L-lactide, and 25.8 mole % glycolide
[0231]A flame-dried, three-neck, 250 ml round-bottom flask is charged with 36.0 g (0.316 mole) caprolactone and 24.0 g (0.207 mole) glycolide. The flask is equipped with a flame-dried mechanical stirrer and adapter for argon purge and vacuum. The contents are heated to 120° C. and stirred under vacuum for four hours. After purging with argon, and cooling to room temperature, 0.11 gm (1.4 mmol) distilled diethylene glycol, and 0.097 ml of stannous octoate (0.33 M in toluene) (25,000:1 molar ratio monomer:catalyst), are added. The reaction vessel is purged by evacuating the flask followed by venting with argon; this is repeated three times. The reaction flask, under an argon pressure of one atmosphere, is heated to 180° C. and maintained at this temperature for about 24 hours with slow stirring.
[0232]In the second st...
example 3
Method of Manufacturing a Drug-Delivery Stent Coating Using the Copolymer of Example 1 or 2
[0233]In a first step, an optional primer coating is applied to a stent. A primer solution containing between about 0.1 mass % and about 15 mass %, (e.g., about 2.0 mass %) of the copolymer of Example 1 or 2, and the balance being a solvent mixture of chloroform and 1,1,1-trichloroethane (having about 50 mass % of chloroform and about 50 mass % of 1,1,1-trichloroethane) is prepared. The solution is applied onto the stent to form a primer layer.
[0234]To apply the primer layer, a spray apparatus (e.g., Sono-Tek MicroMist spray nozzle, manufactured by Sono-Tek Corp. of Milton, N.Y.) is used. The spray apparatus is an ultrasonic atomizer with a gas entrainment stream. A syringe pump is used to supply the coating solution to the nozzle. The composition is atomized by ultrasonic energy and applied to the stent surfaces. A useful nozzle-to-stent distance is about 20 mm to about 40 mm at an ultrasonic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mn | aaaaa | aaaaa |
| weight fraction | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com