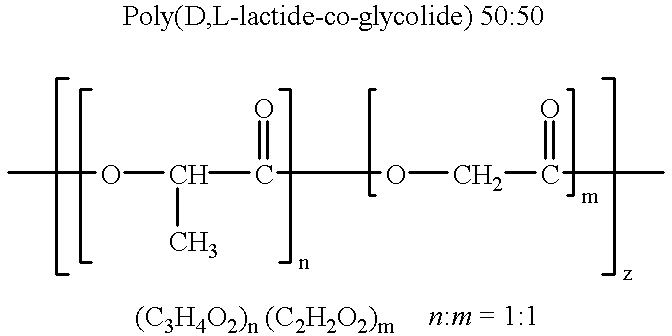Patents
Literature
1970 results about "Lactide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lactide is the lactone cyclic di-ester derived from lactic acid (2-hydroxypropionic acid). With the formula (OCHCO₂)₂, it exists in three different stereoisomeric forms. All are colorless or white solids. Lactide has attracted great interest because it is derived from abundant renewable resources and is the precursor to a polymer similar to polystyrene, but biodegradable.
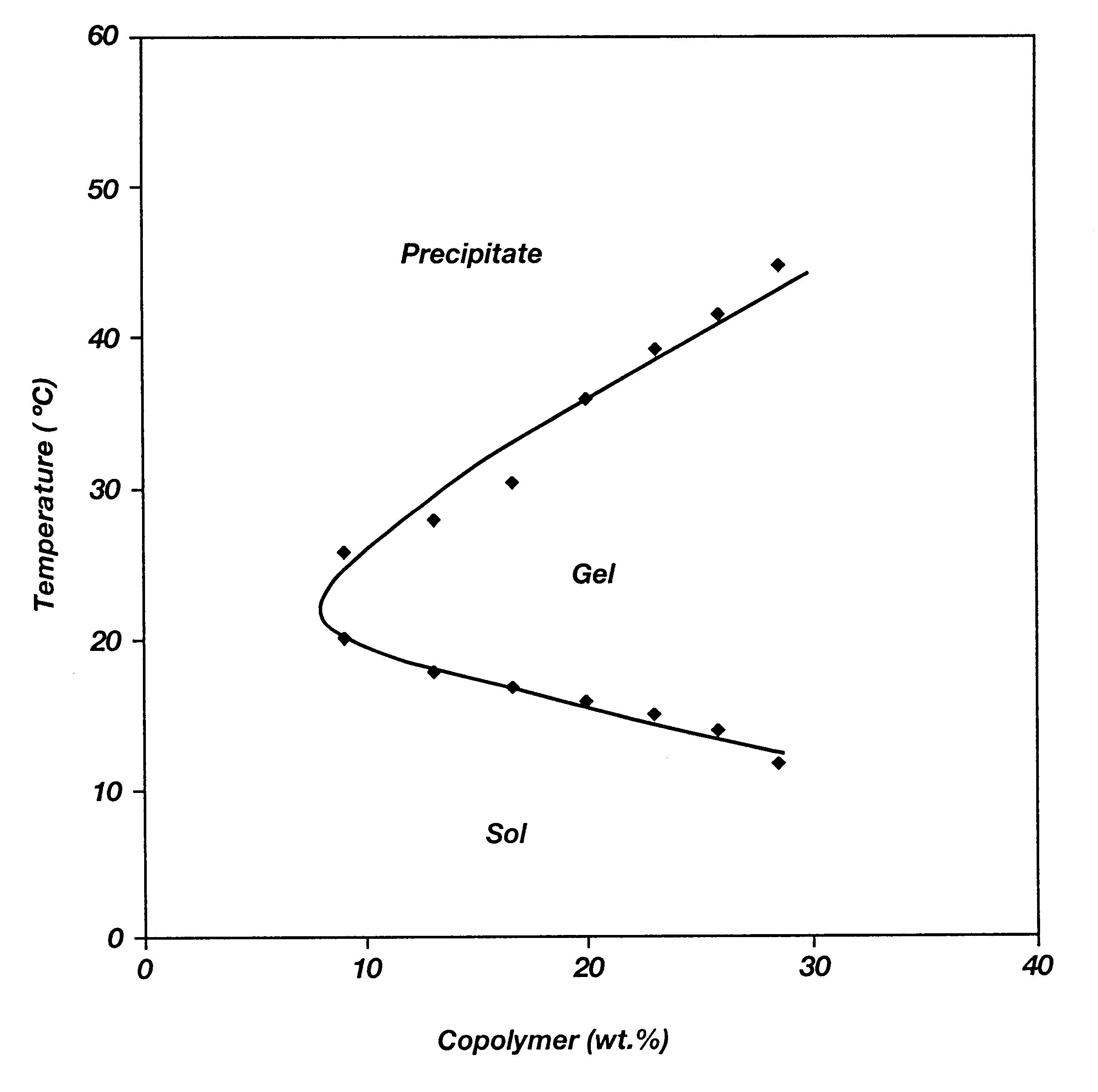
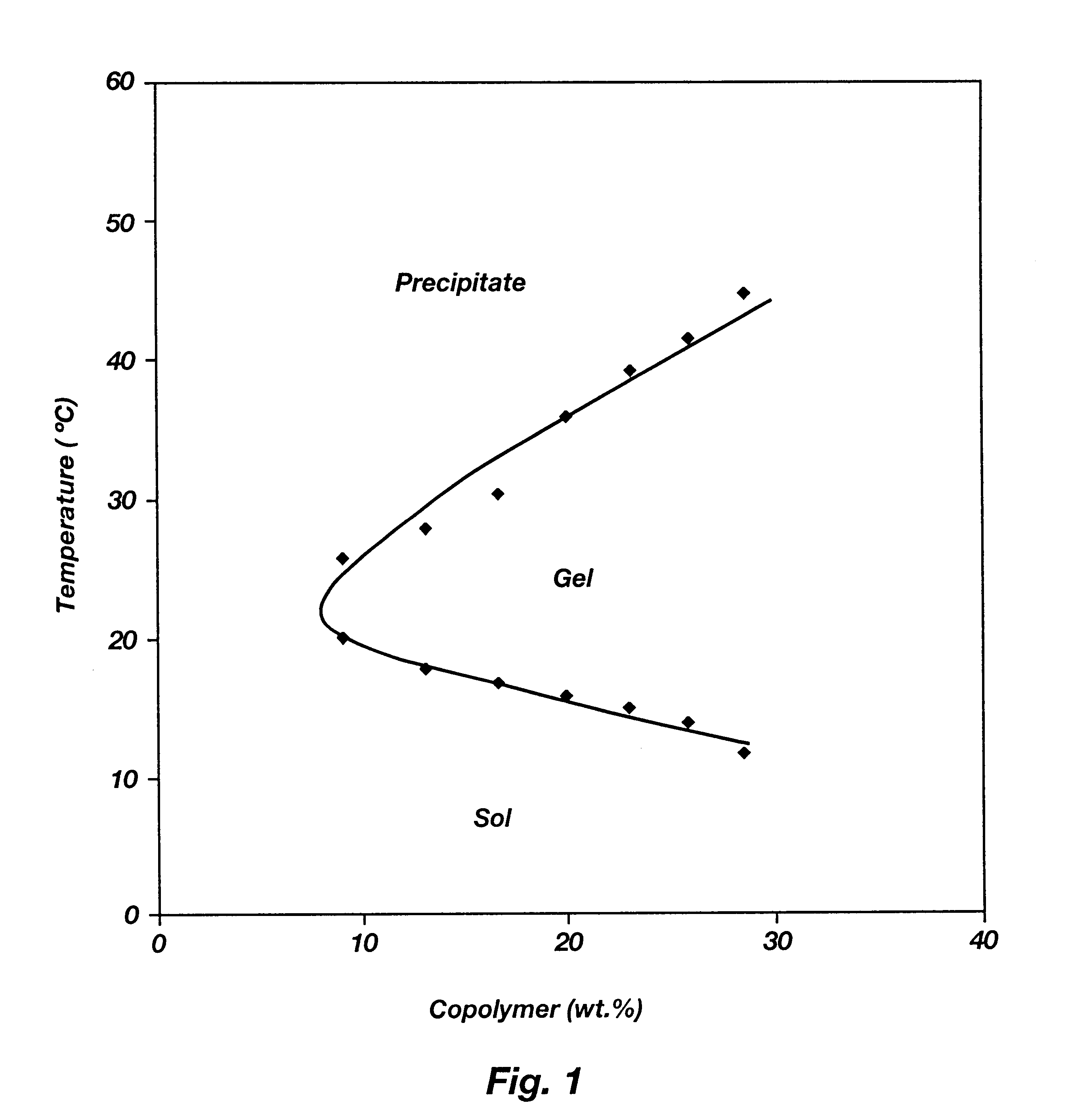
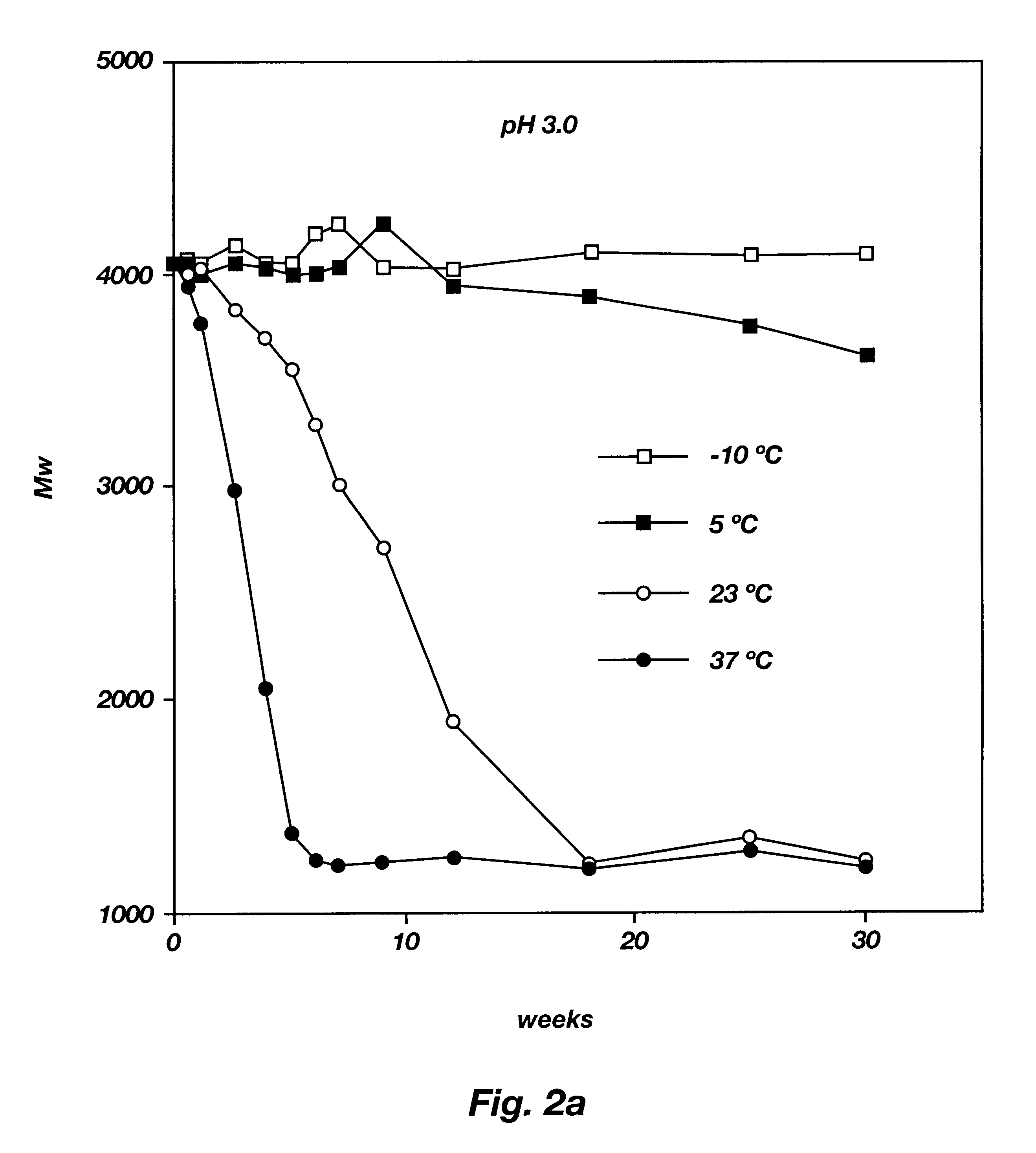
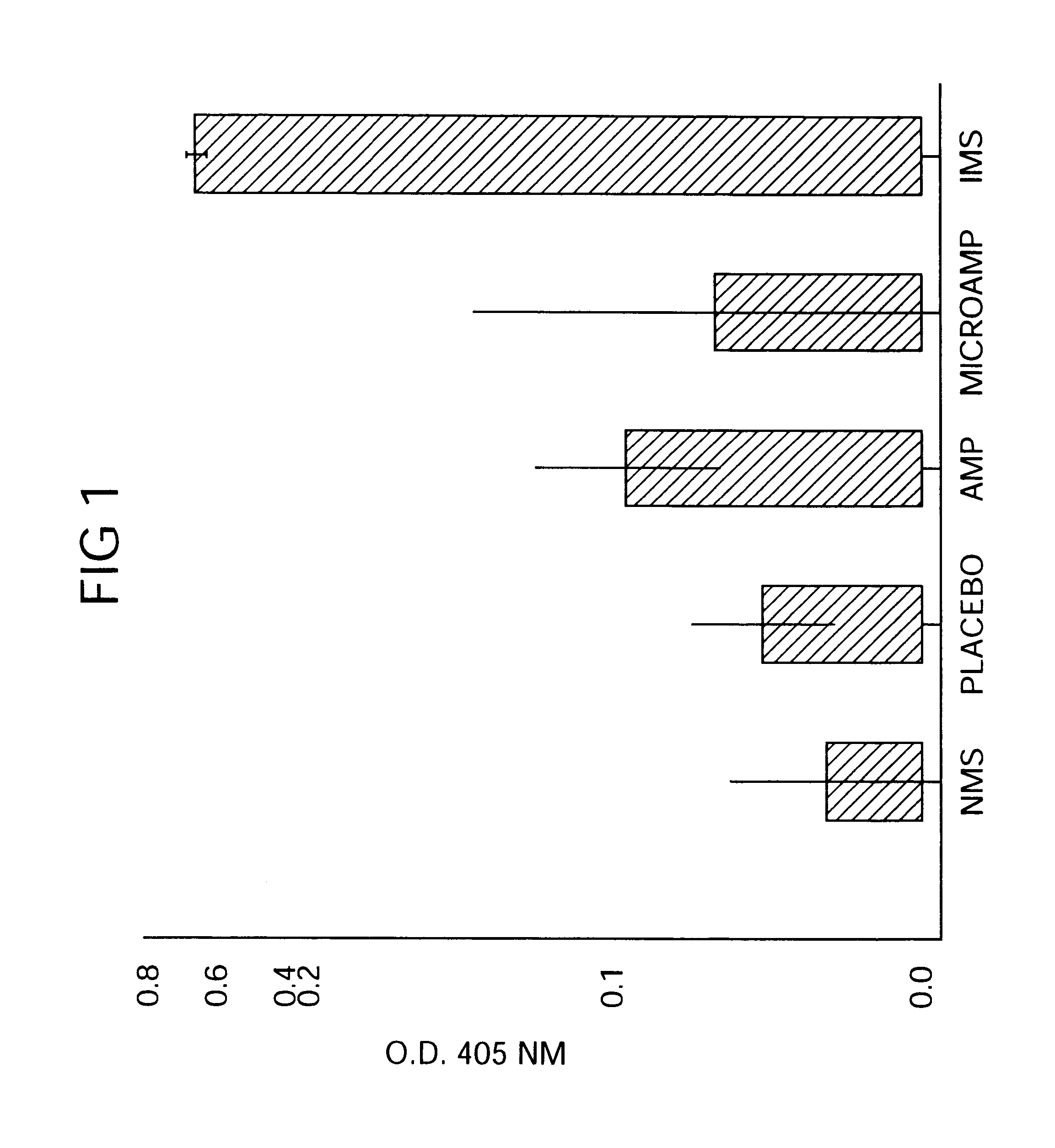
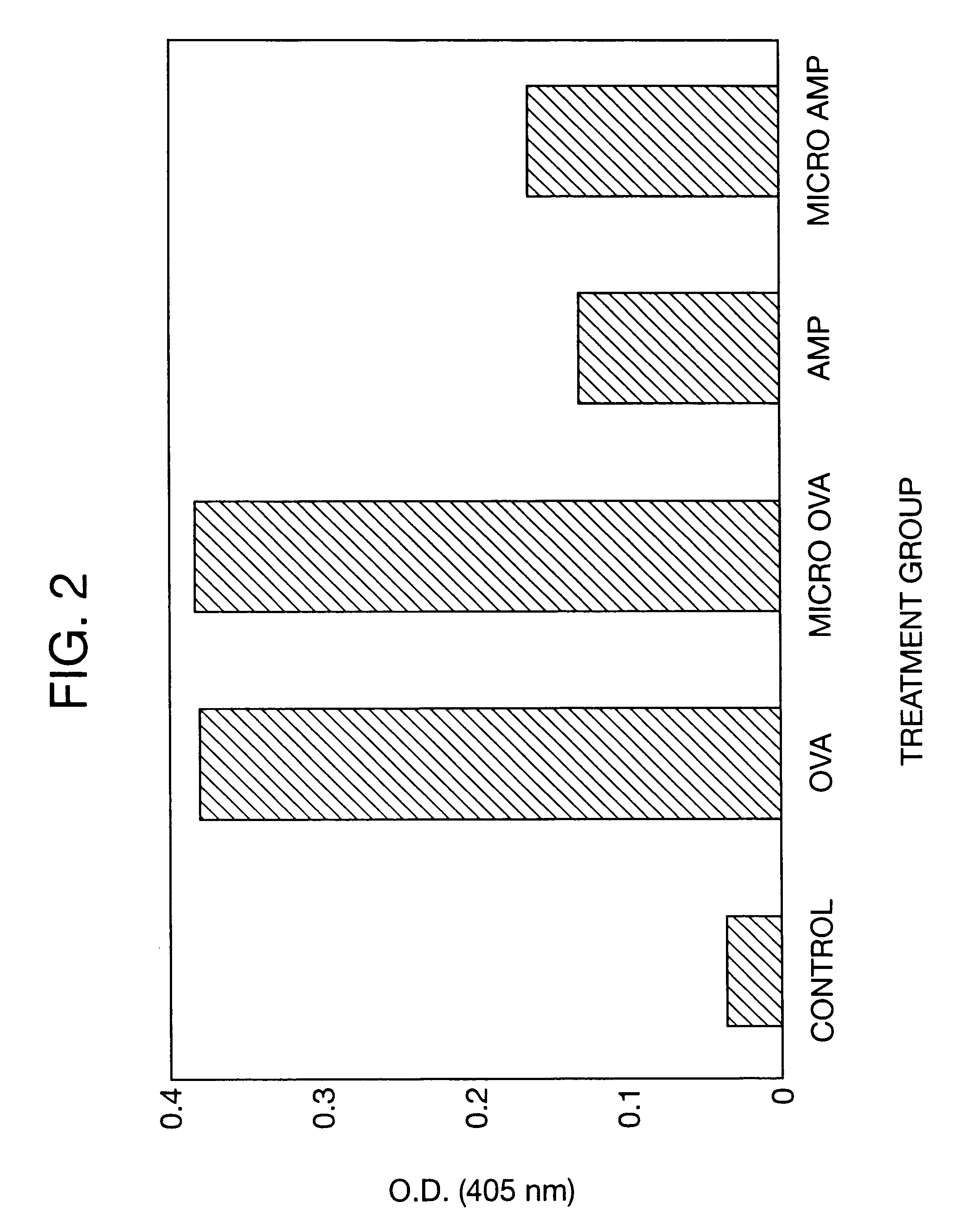
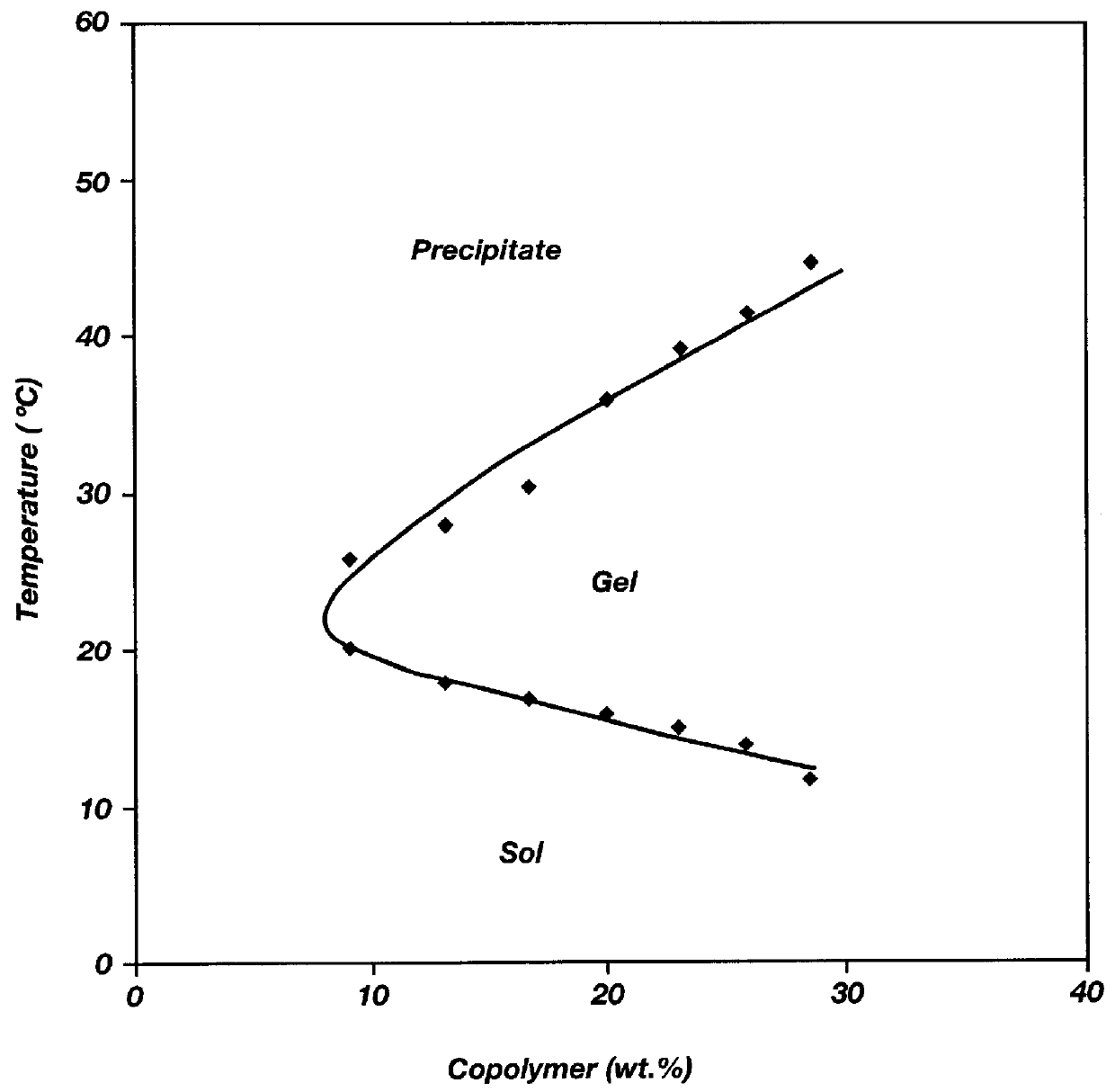
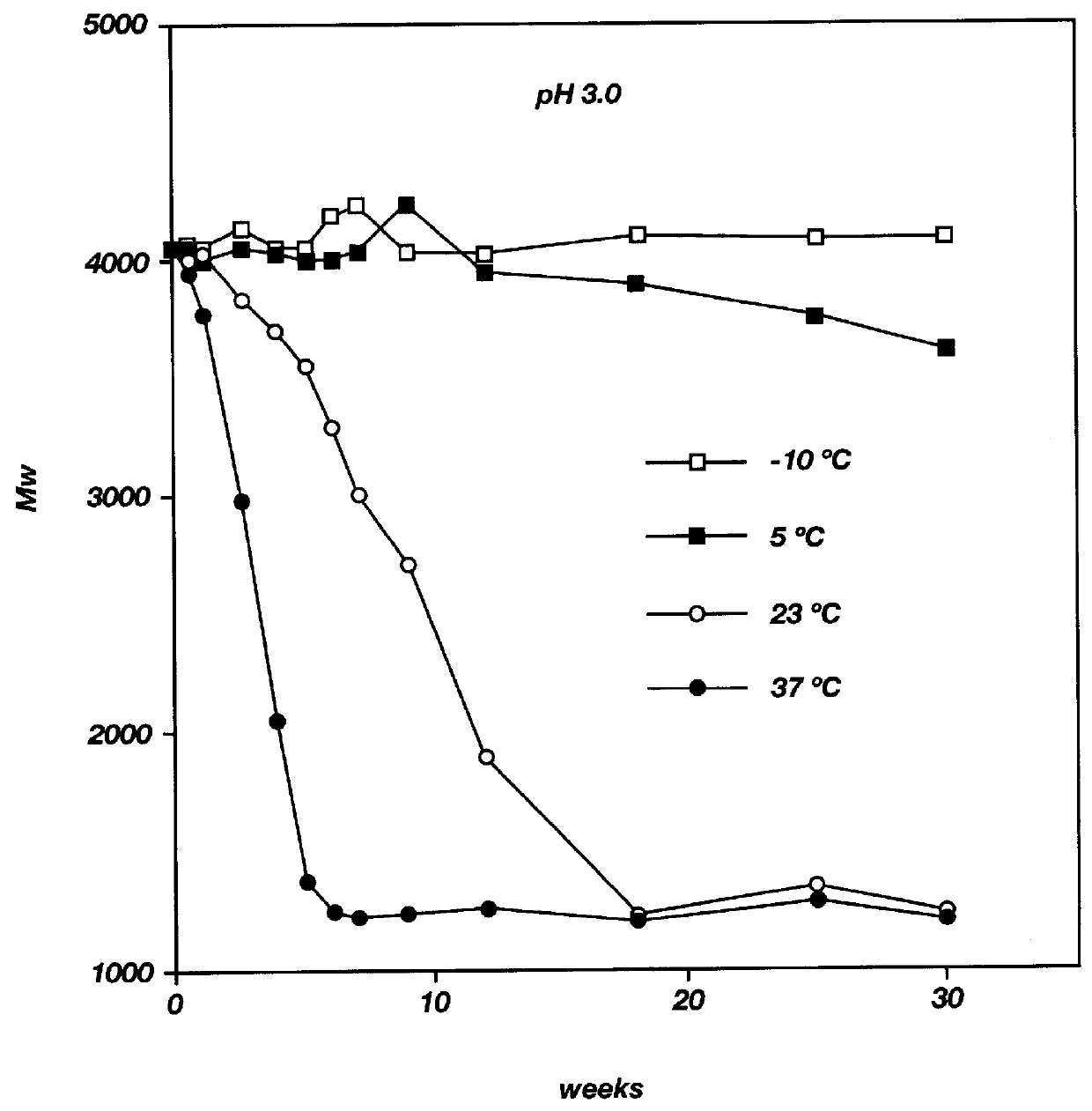
Biodegradable low molecular weight triblock poly(lactide-co- glycolide) polyethylene glycol copolymers having reverse thermal gelation properties
InactiveUS6201072B1Difficult to formulateDifficult to administerOrganic active ingredientsPowder deliverySolubilityPolymer science
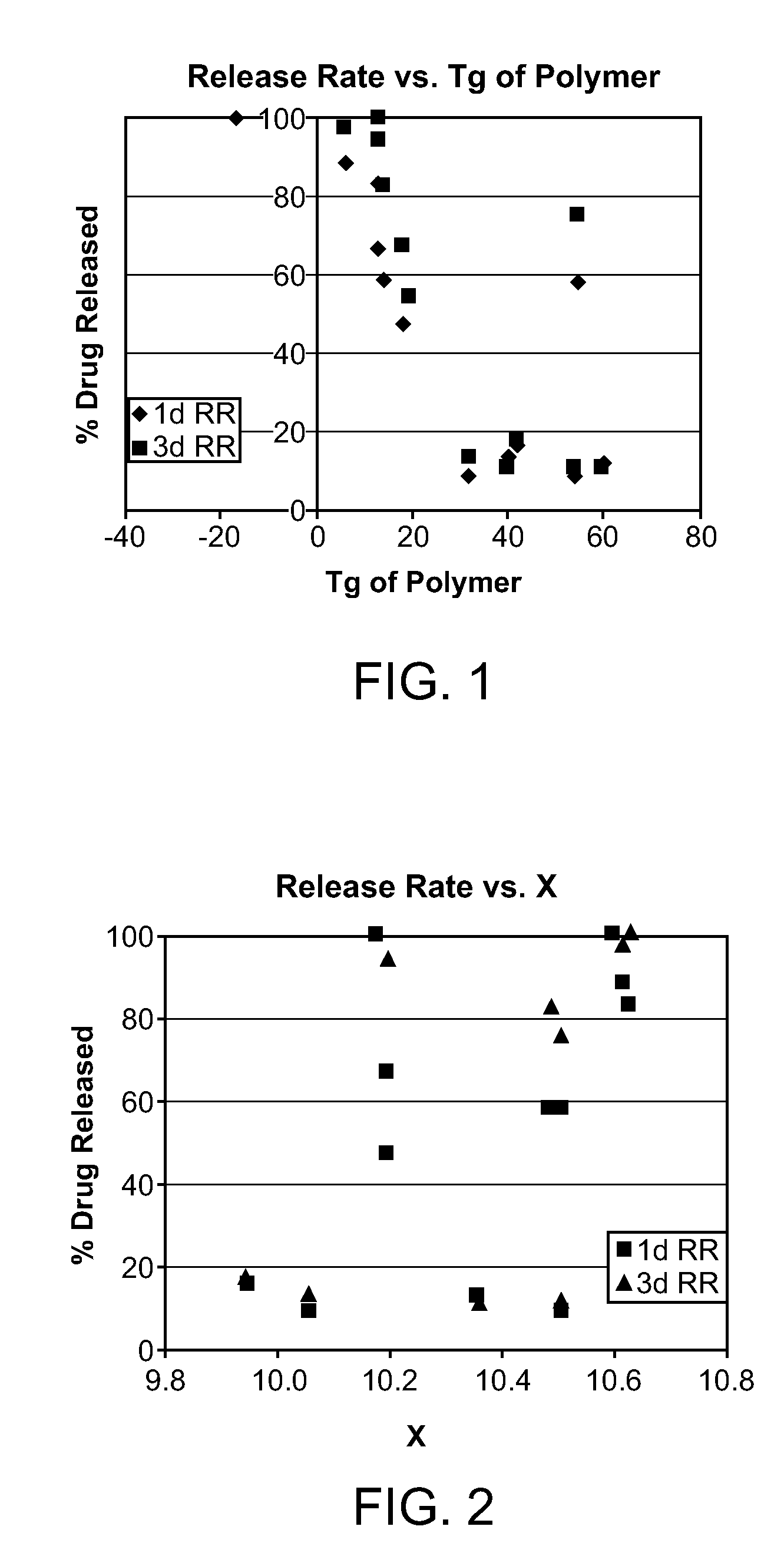
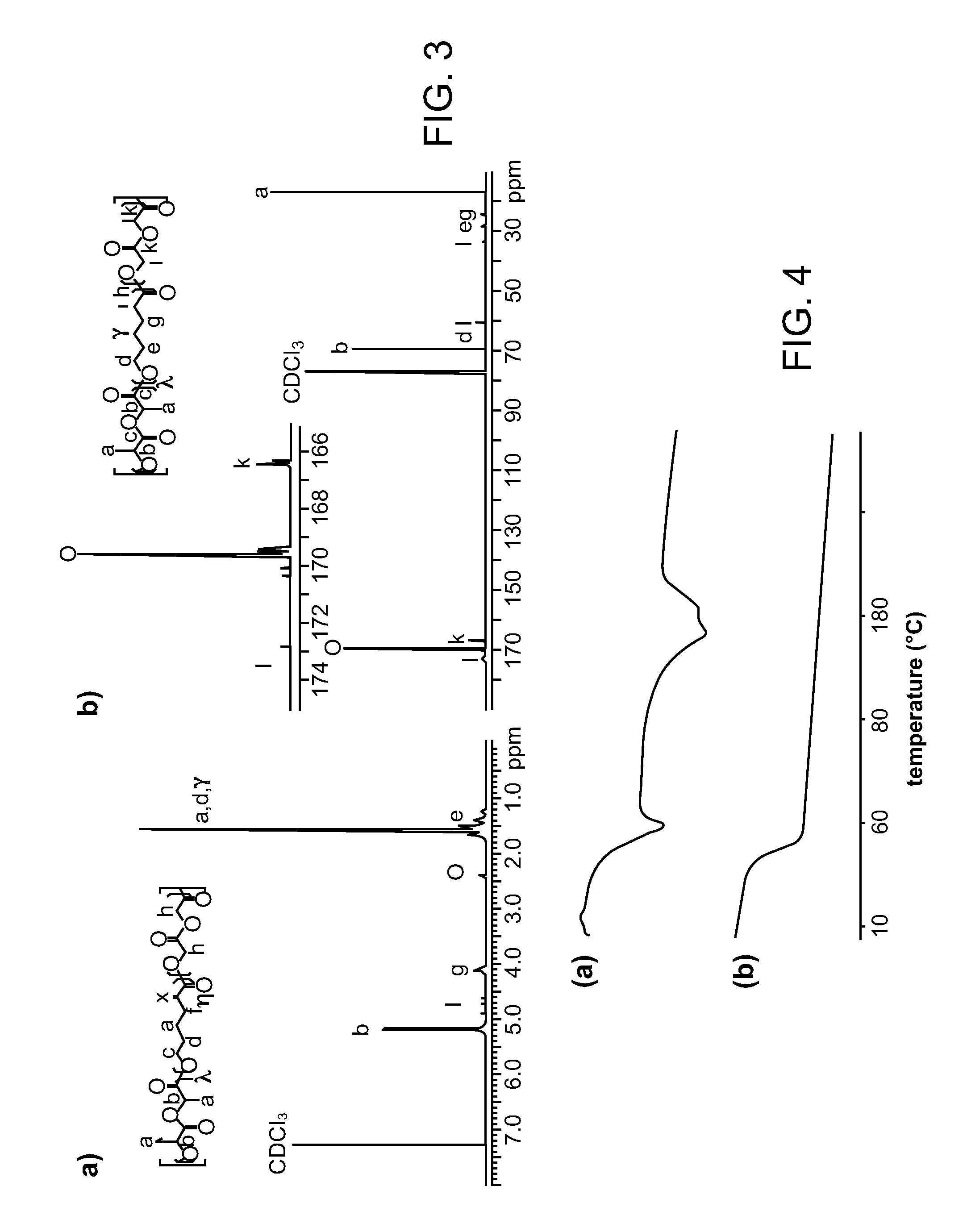
A water soluble, biodegradable ABA- or BAB-type tri-block polymer is disclosed that is made up of a major amount of a hydrophobic A polymer block made of a biodegradable polyester and a minor amount of a hydrophilic polyethylene glycol(PEG) B polymer block, having an overall average molecular weight of between about 2000 and 4990, and that possesses reverse thermal gelation properties. Effective concentrations of the tri-block polymer and a drug may be uniformly contained in an aqueous phase to form a drug delivery composition. At temperatures below the gelation temperature of the tri-block polymer the composition is a liquid and at temperatures at or above the gelation temperature the composition is a gel or semi-solid. The composition may be administered to a warm-blooded animal as a liquid by parenteral, ocular, topical, inhalation, transdermal, vaginal, transurethral, rectal, nasal, oral, pulmonary or aural delivery means and is a gel at body temperature. The composition may also be administered as a gel. The drug is released at a controlled rate from the gel which biodegrades into non-toxic products. The release rate of the drug may be adjusted by changing various parameters such as hydrophobic / hydrophilic component content, polymer concentration, molecular weight and polydispersity of the tri-block polymer. Because the tri-block polymer is amphiphilic, it functions to increase the solubility and / or stability of drugs in the composition.
Owner:KIM PH D SUNG WAN +2
Therapeutic treatment and prevention of infections with a bioactive materials encapsulated within a biodegradable-biocompatible polymeric matrix
InactiveUS6309669B1Sustained release of active agent over timeEfficient and effective usePowder deliveryPeptide/protein ingredientsAdjuvantEnd-group
Novel burst-free, sustained release biocompatible and biodegrable microcapsules which can be programmed to release their active core for variable durations ranging from 1-100 days in an aqueous physiological environment. The microcapsules are comprised of a core of polypeptide or other biologically active agent encapsulated in a matrix of poly(lactide / glycolide) copolymer, which may contain a pharmaceutically-acceptable adjuvant, as a blend of upcapped free carboxyl end group and end-capped forms ranging in ratios from 100 / 0 to 1 / 99.
Owner:ARMY GOVERNMENT OF THE UNITED STATES AS REPRESENTED BY THE SEC OF THE
Biodegradable low molecular weight triblock poly (lactide-co-glycolide) polyethylene glycol copolymers having reverse thermal gelation properties
InactiveUS6117949AReduce solubilityReduced stabilityPowder deliveryPeptide/protein ingredientsSolubilityPolymer science
A water soluble biodegradable ABA- or BAB-type triblock polymer is disclosed that is made up of a major amount of a hydrophobic polymer made of a poly(lactide-co-glycolide) copolymer or poly(lactide) polymer as the A-blocks and a minor amount of a hydrophilic polyethylene glycol polymer B-block, having an overall weight average molecular weight of between about 2000 and 4990, and that possesses reverse thermal gelation properties. Effective concentrations of the triblock polymer and a drug may be uniformly contained in an aqueous phase to form a drug delivery composition. At temperatures below the gelation temperature of the triblock polymer the composition is a liquid and at temperatures at or above the gelation temperature the composition is a gel or semi-solid. The composition may be administered to a warm-blooded animal as a liquid by parenteral, ocular, topical, inhalation, transdermal, vaginal, transurethral, rectal, nasal, oral, pulmonary or aural delivery means and is a gel at body temperature. The composition may also be administered as a gel. The drug is released at a controlled rate from the gel which biodegrades into non-toxic products. The release rate of the drug may be adjusted by changing various parameters such as hydrophobic / hydrophilic componenet content, polymer concentration, molecular weight and polydispersity of the triblock polymer. Because the triblock polymer is amphiphilic, it functions to increase the solubility and / or stability of drugs in the composition.
Owner:BTG INT LTD +2
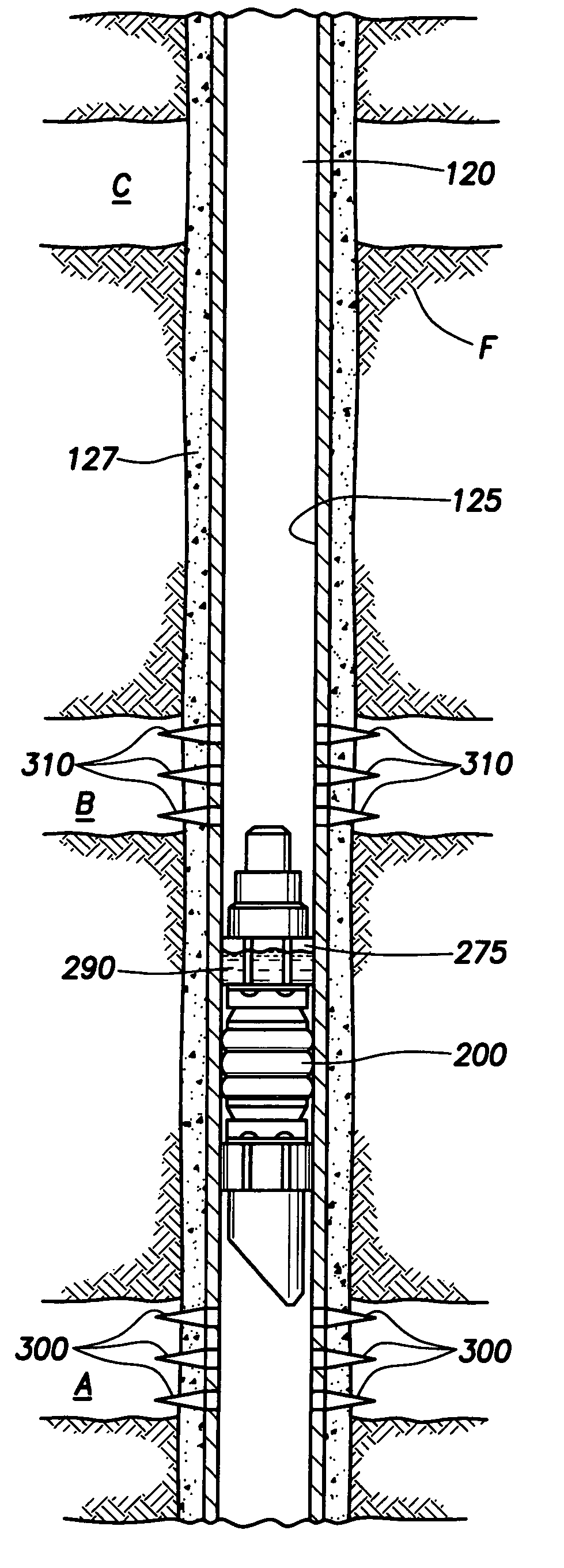
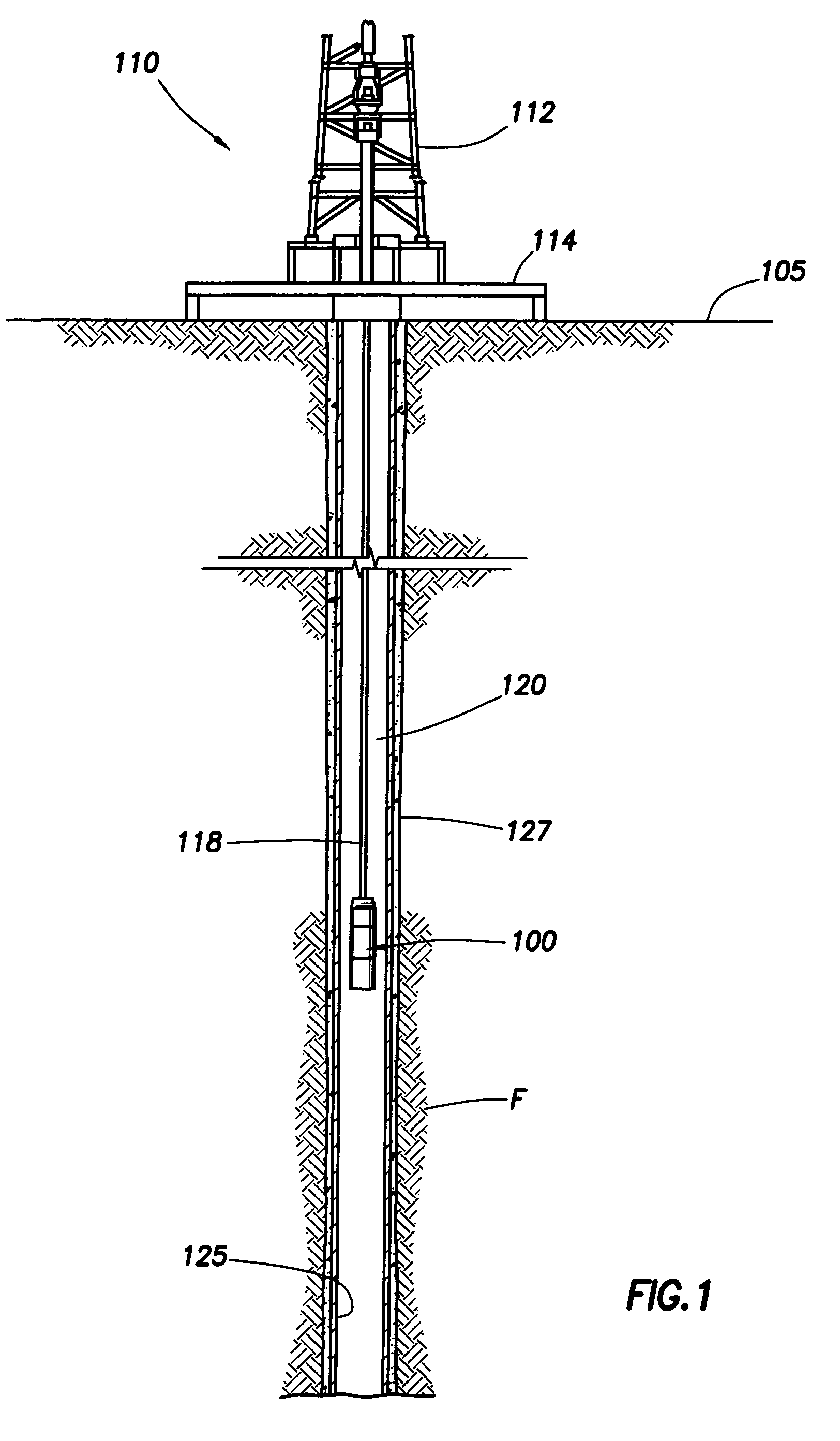
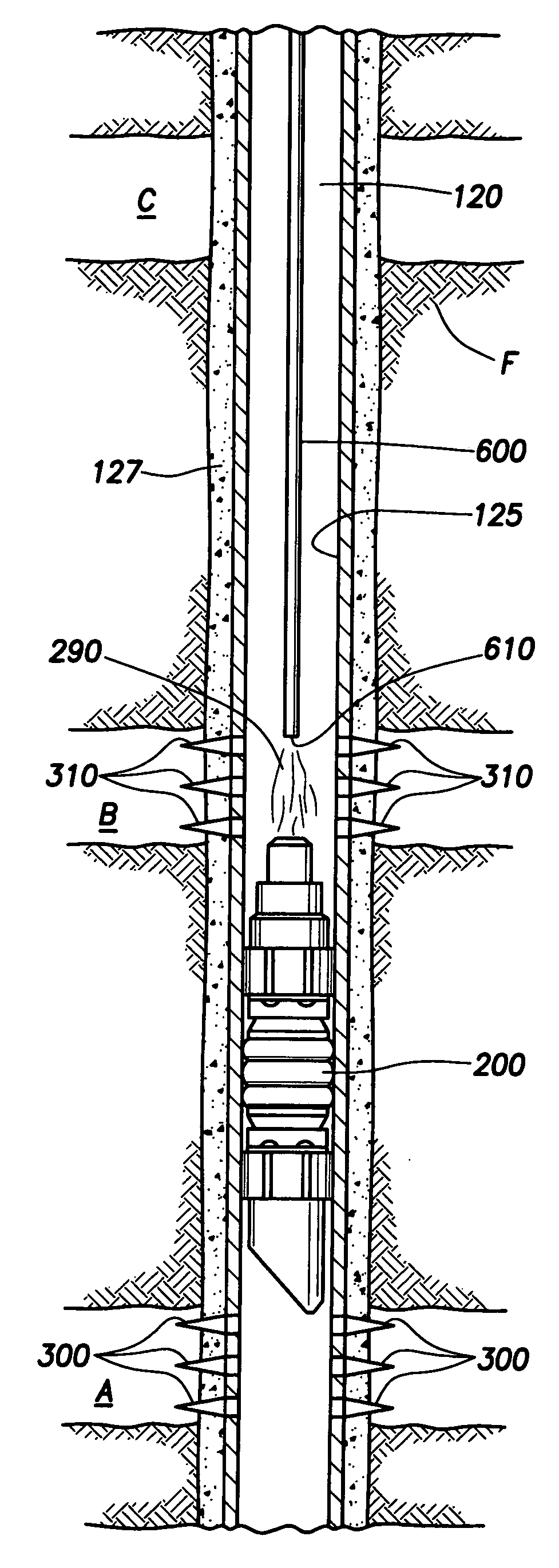
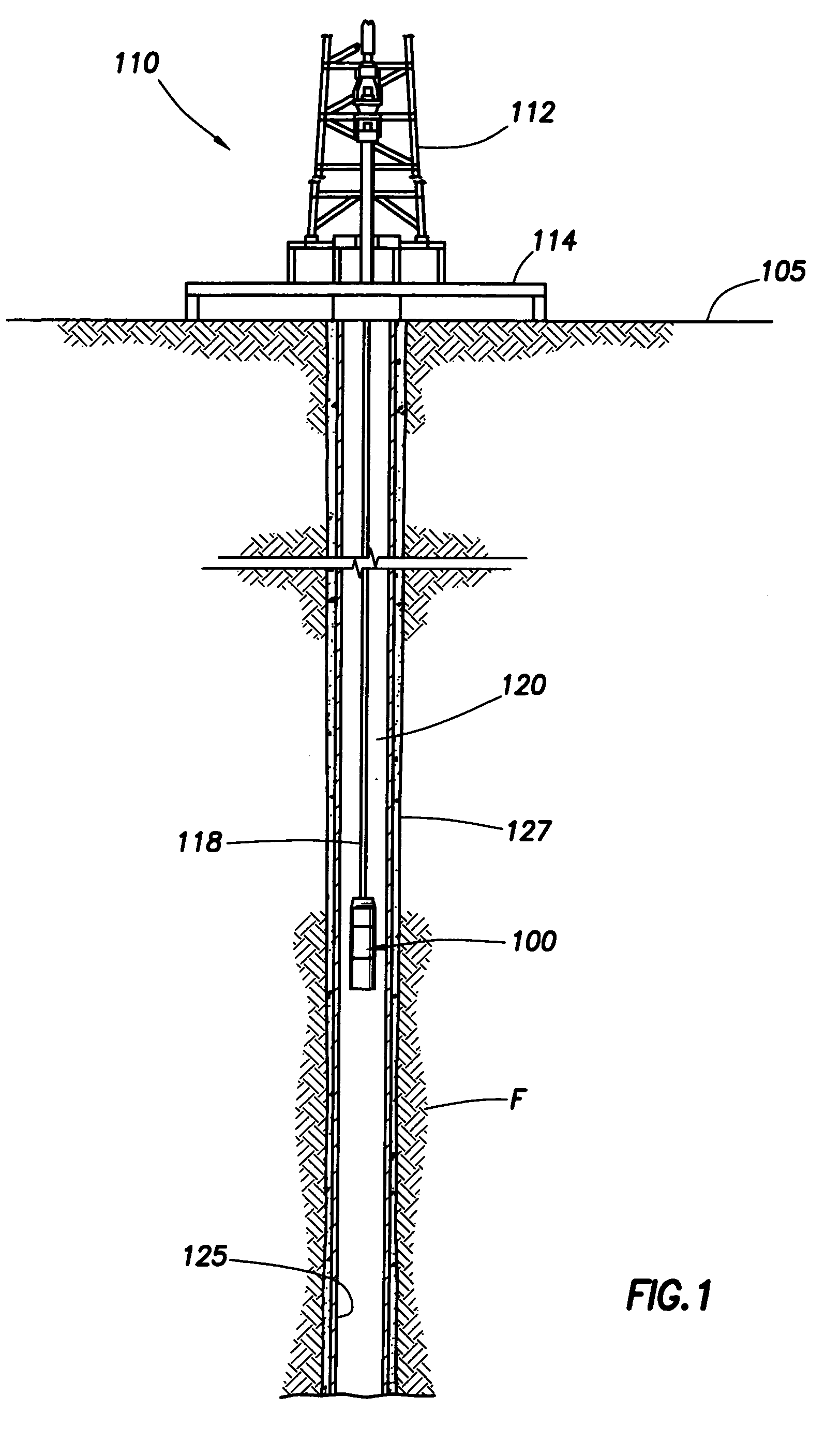
Generating Acid Downhole in Acid Fracturing
An acid fracturing method is provided in which the acid is generated in the fracture by hydrolysis of a solid acid-precursor selected from one or more than one of lactide, glycolide, polylactic acid, polyglycolic acid, a copolymer of polylactic acid and polyglycolic acid, a copolymer of glycolic acid with other hydroxy-, carboxylic acid-, or hydroxycarboxylic acid-containing moieties, and a copolymer of lactic acid with other hydroxy-, carboxylic acid or hydroxycarboxylic acid-containing moieties. The solid acid-precursor may be mixed with a solid acid-reactive material to accelerate the hydrolysis and / or coated to slow the hydrolysis. Water-soluble liquid compounds are also given that accelerate the hydrolysis. The method ensures that the acid contacts fracture faces far from the wellbore.
Owner:SCHLUMBERGER TECH CORP
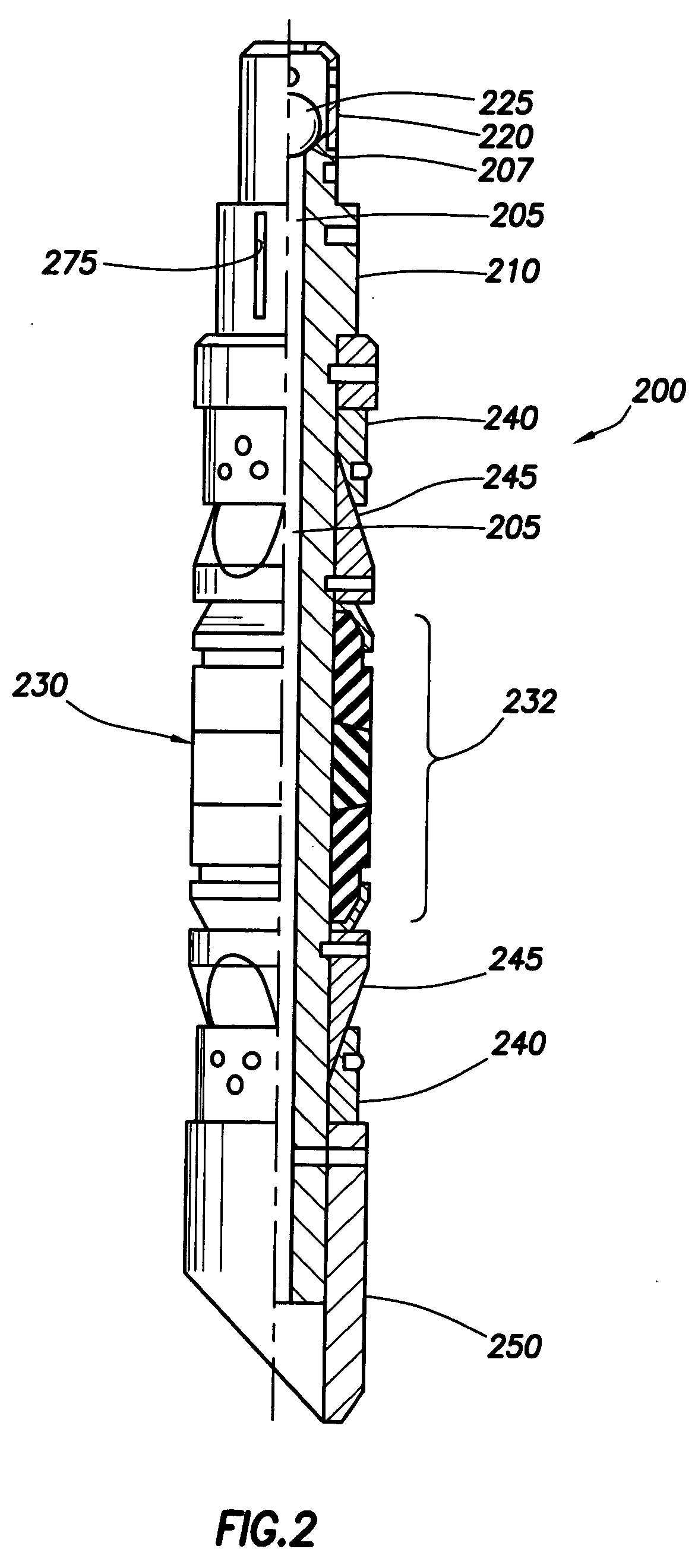
One-time use composite tool formed of fibers and a biodegradable resin
ActiveUS7093664B2Eliminates and at least minimizes drawbackFluid removalWell/borehole valve arrangementsFiberChemical Linkage
The present invention is directed to disposable composite downhole tool formed of a resin-coated fiber. The fiber is formed of a degradable polymer, such as a poly(lactide) or polyanhydride. The resin is formed of the same degradable polymer as the fiber. It chemically bonds to the fiber, thereby making a strong rigid structure once cured. The fiber may be formed into a fabric before being coated with the resin. Alternatively, the fiber is formed of a non-biodegradable material.
Owner:HALLIBURTON ENERGY SERVICES INC
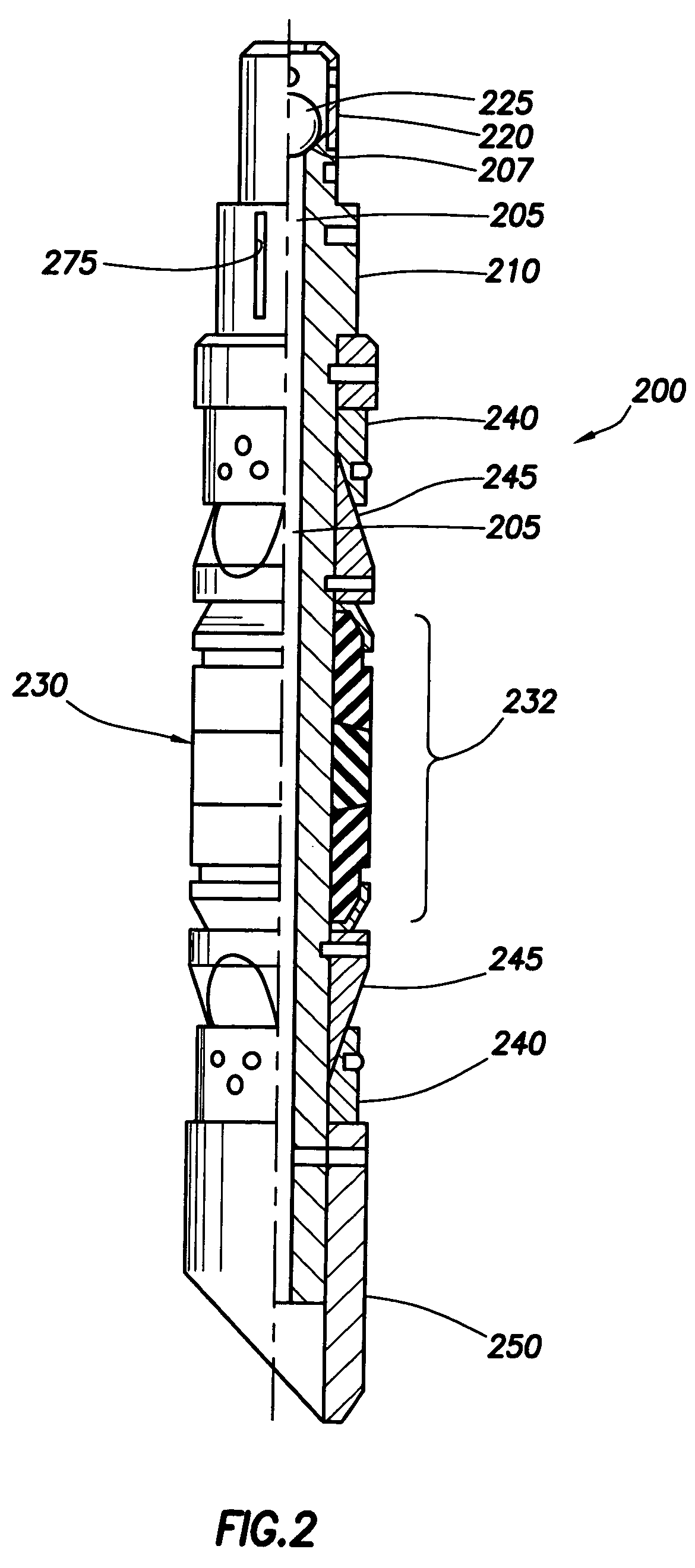
Drug eluting implantable medical device
A drug eluting medical device is provided for implanting into vessels or luminal structures within the body of a patient. The coated medical device, such as a stent, vascular, or synthetic graft comprises a coating consisting of a controlled-release matrix of a bioabsorbable, biocompatible, bioerodible, biodegradable, nontoxic material, such as a Poly(DL-Lactide-co-Glycolide) polymer, and at least one pharmaceutical substance, or bioactive agent incorporated within the matrix or layered within layers of matrix. In particular, the drug eluting medical device when implanted into a patient, delivers the drugs or bioactive agents within the matrix to adjacent tissues in a controlled and desired rate depending on the drug and site of implantation.
Owner:ORBUSNEICH MEDICAL PTE LTD
One-time use composite tool formed of fibers and a biodegradable resin
ActiveUS20050205265A1Eliminates and at least minimizes drawbackFluid removalWell/borehole valve arrangementsLactideFiber Chemistry
The present invention is directed to disposable composite downhole tool formed of a resin-coated fiber. The fiber is formed of a degradable polymer, such as a poly(lactide) or polyanhydride. The resin is formed of the same degradable polymer as the fiber. It chemically bonds to the fiber, thereby making a strong rigid structure once cured. The fiber may be formed into a fabric before being coated with the resin. Alternatively, the fiber is formed of a non-biodegradable material.
Owner:HALLIBURTON ENERGY SERVICES INC
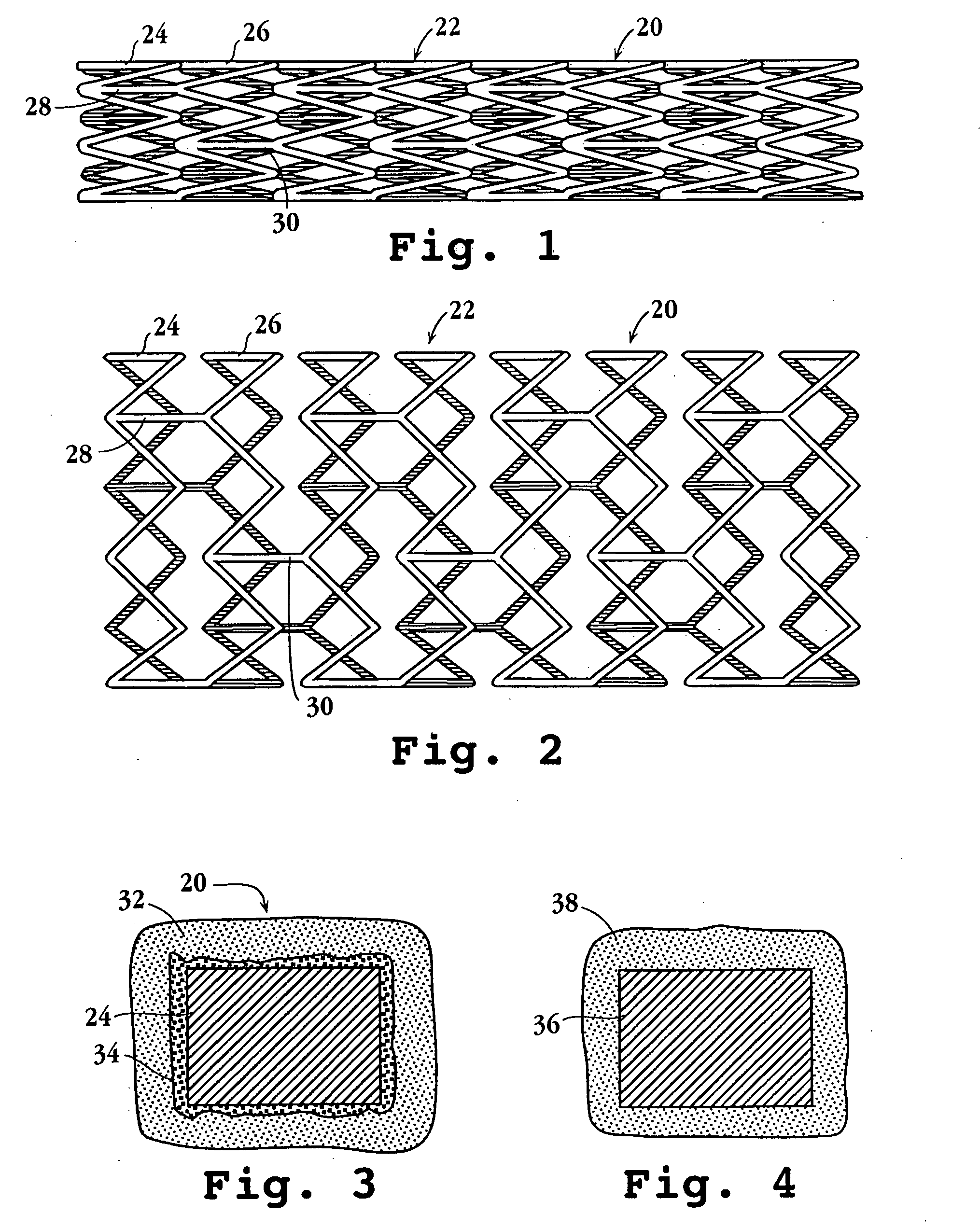
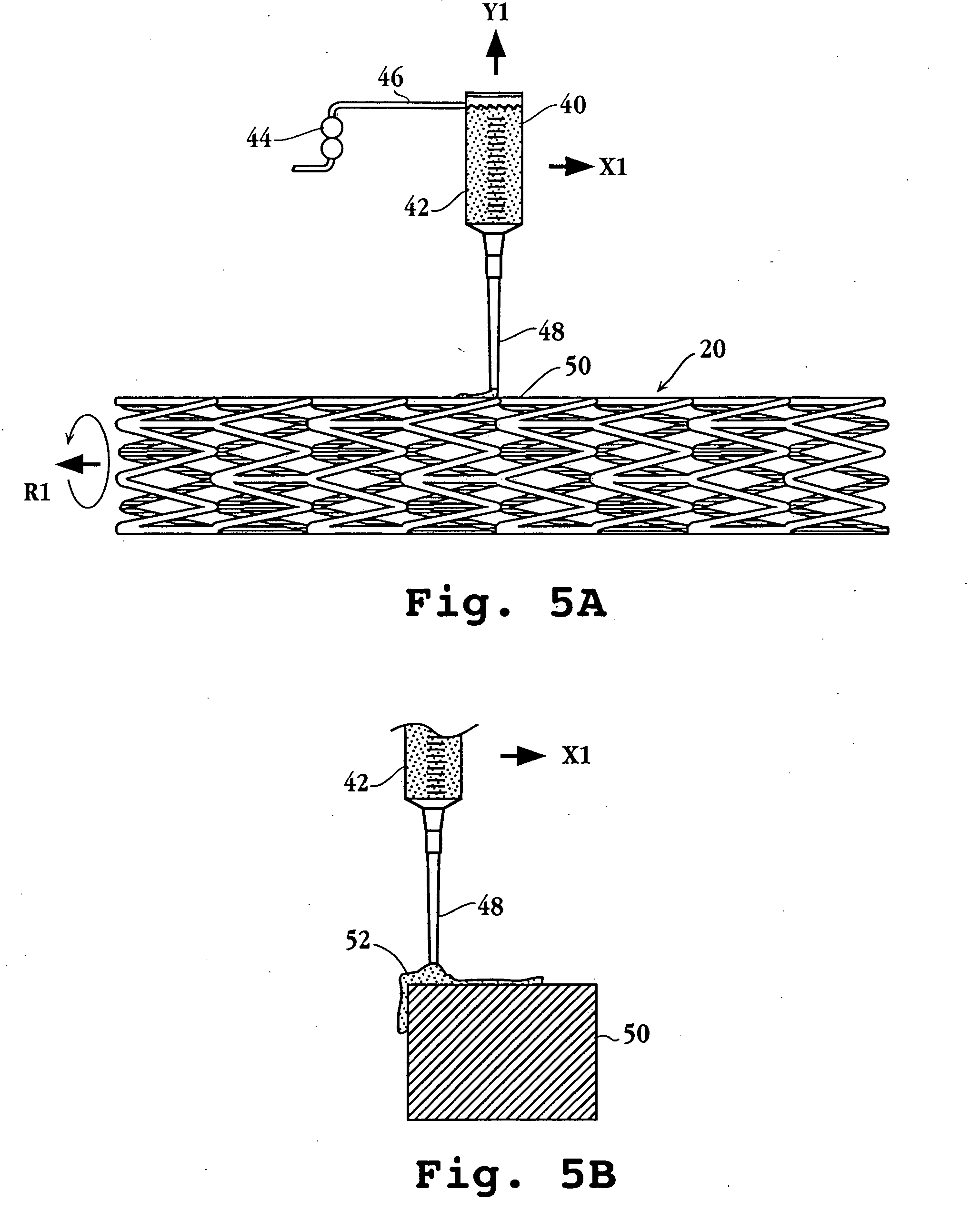
Drug-delivery endovascular stent and method of forming the same
ActiveUS20050038505A1Prevent restenosisEfficient releaseOrganic active ingredientsOrganic chemistryPolymer substrateInsertion stent
An intravascular stent and method for inhibiting restenosis, following vascular injury, is disclosed. The stent has an expandable, linked-filament body and a drug-release coating formed on the stent-body filaments, for contacting the vessel injury site when the stent is placed in-situ in an expanded condition. The coating releases, for a period of at least 4 weeks, a restenosis-inhibiting amount of a monocyclic triene immunosuppressive compound having an alkyl group substituent at carbon position 40 in the compound. The stent, when used to treat a vascular injury, gives good protection against clinical restenosis, even when the extent of vascular injury involves vessel overstretching by more than 30% diameter. Also disclosed is a stent having a drug-release coating composed of (i) 10 and 60 weight percent poly-d / -lactide polymer substrate and (ii) 40-90 weight percent of an anti-restenosis compound, and a polymer undercoat having a thickness of between 1-5 microns.
Owner:BIOSENSORS INT GROUP
Wound healing polymer compositions and methods for use thereof
The present invention provides bioactive polymer compositions that can be formulated to release a wound healing agent at a controlled rate by adjusting the various components of the composition. The composition can be used in an external wound dressing, as a polymer implant for delivery of the wound healing agent to an internal body site, or as a coating on the surface of an implantable surgical device to deliver wound healing agents that are covalently attached to a biocompatible, biodegradable polymer and / or embedded within a hydrogel. Methods of using the invention bioactive polymer compositions to promote natural healing of wounds, especially chronic wounds, are also provided. Examples of biodegradable copolymer polyesters useful in forming the blood-compatible, hydrophilic layer or coating include copolyester amides, copolyester urethanes, glycolide-lactide copolymers, glycolide-caprolactone copolymers, poly-3-hydroxy butyrate-valerate copolymers, and copolymers of the cyclic diester monomer, 3-(S)[(alkyloxycarbonyl)methyl]-1,4-dioxane-2,5-dione, with L-lactide. The glycolide-lactide copolymers include poly(glycolide-L-lactide) copolymers formed utilizing a monomer mole ratio of glycolic acid to L-lactic acid ranging from 5:95 to 95:5 and preferably a monomer mole ratio of glycolic acid to L-lactic acid ranging from 45:65 to 95:5. The glycolide-caprolactone copolymers include glycolide and ε-caprolactone block copolymer, e.g., Monocryl or Poliglecaprone.
Owner:MEDIVAS LLC
Polymer coating for medical devices
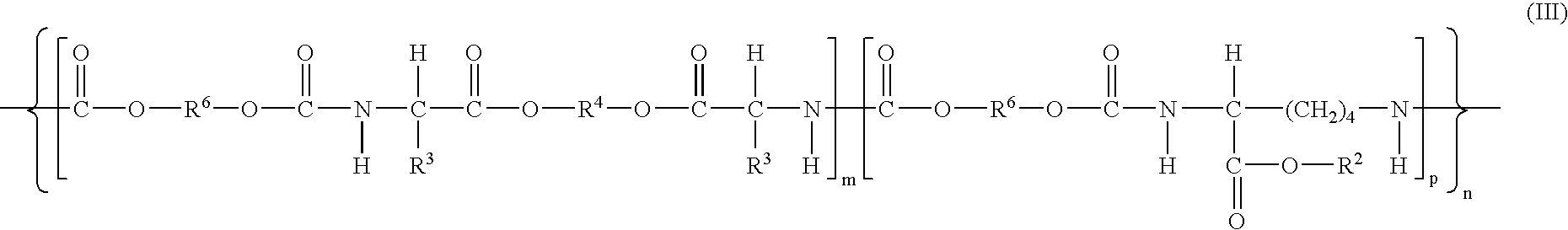
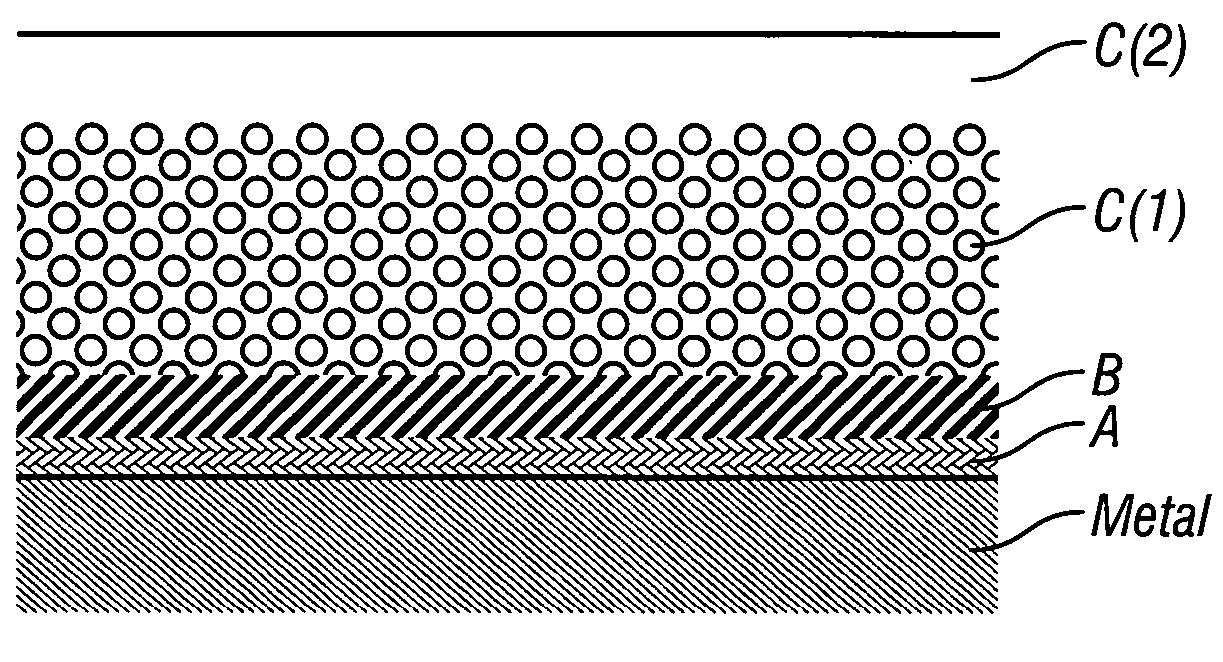
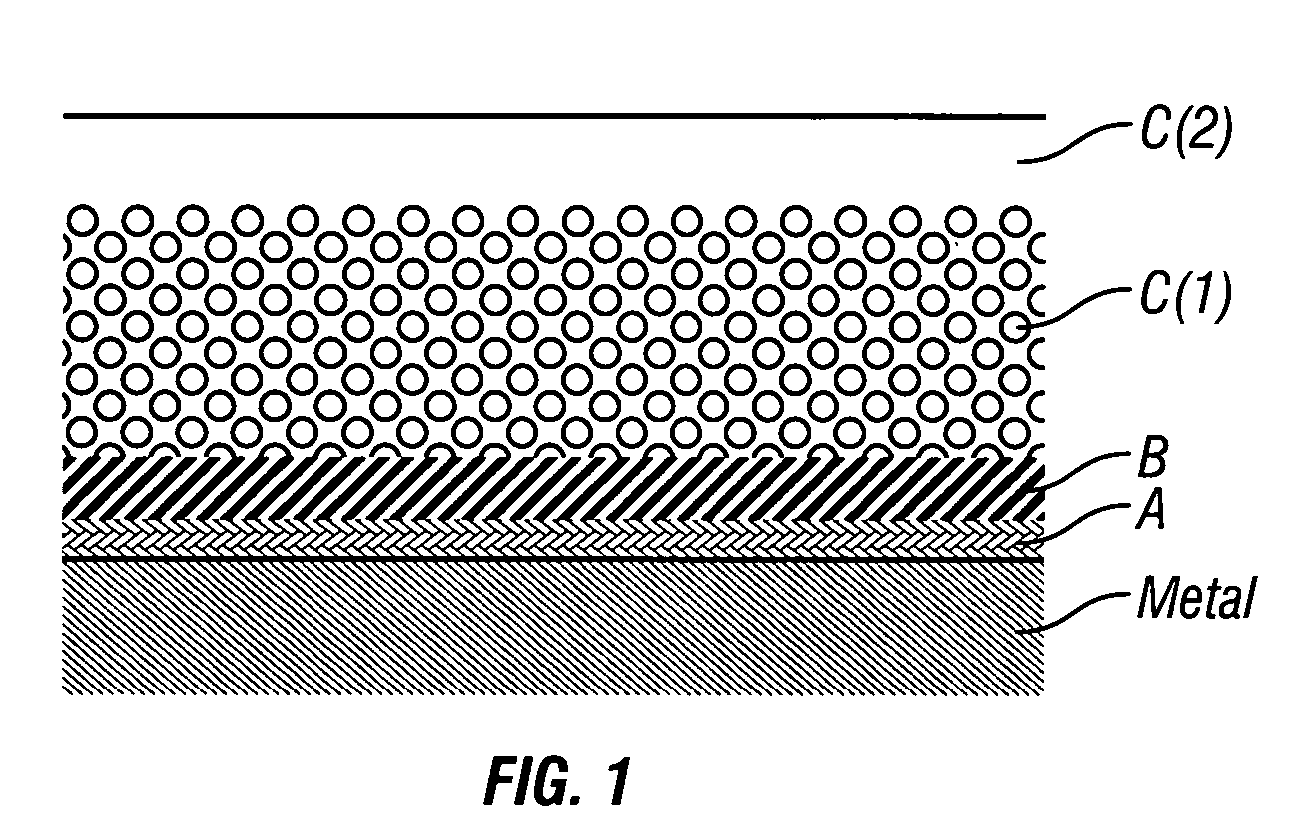
Coatings are provided in which surfaces may be activated by covalently bonding a silane derivative to the metal surface, covalently bonding a lactone polymer to the silane derivative by in situ ring opening polymerization, and depositing at least one layer of poly(lactide-co-caprolactone) copolymer on the bonded lactone. Biologically active agents may be disposed with the poly(lactide-co-caprolactone) copolymer layers. Such coated surfaces may be useful in medical devices, in particular stents.
Owner:CV THERAPEUTICS INC
Hemostatic textile
ActiveUS20070160653A1Quick activationNon-adhesive dressingsPeptide/protein ingredientsLactideSisal fiber
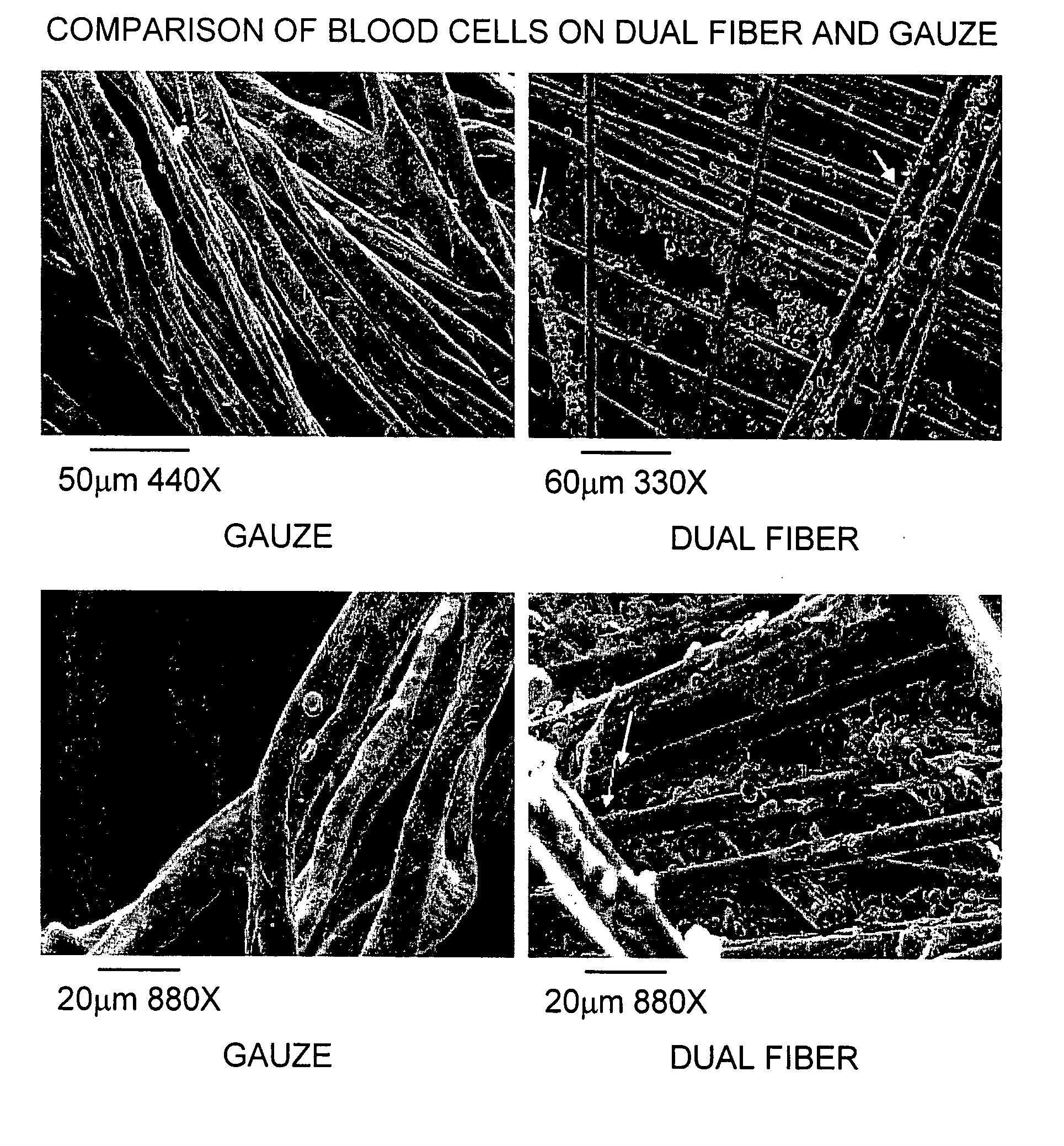
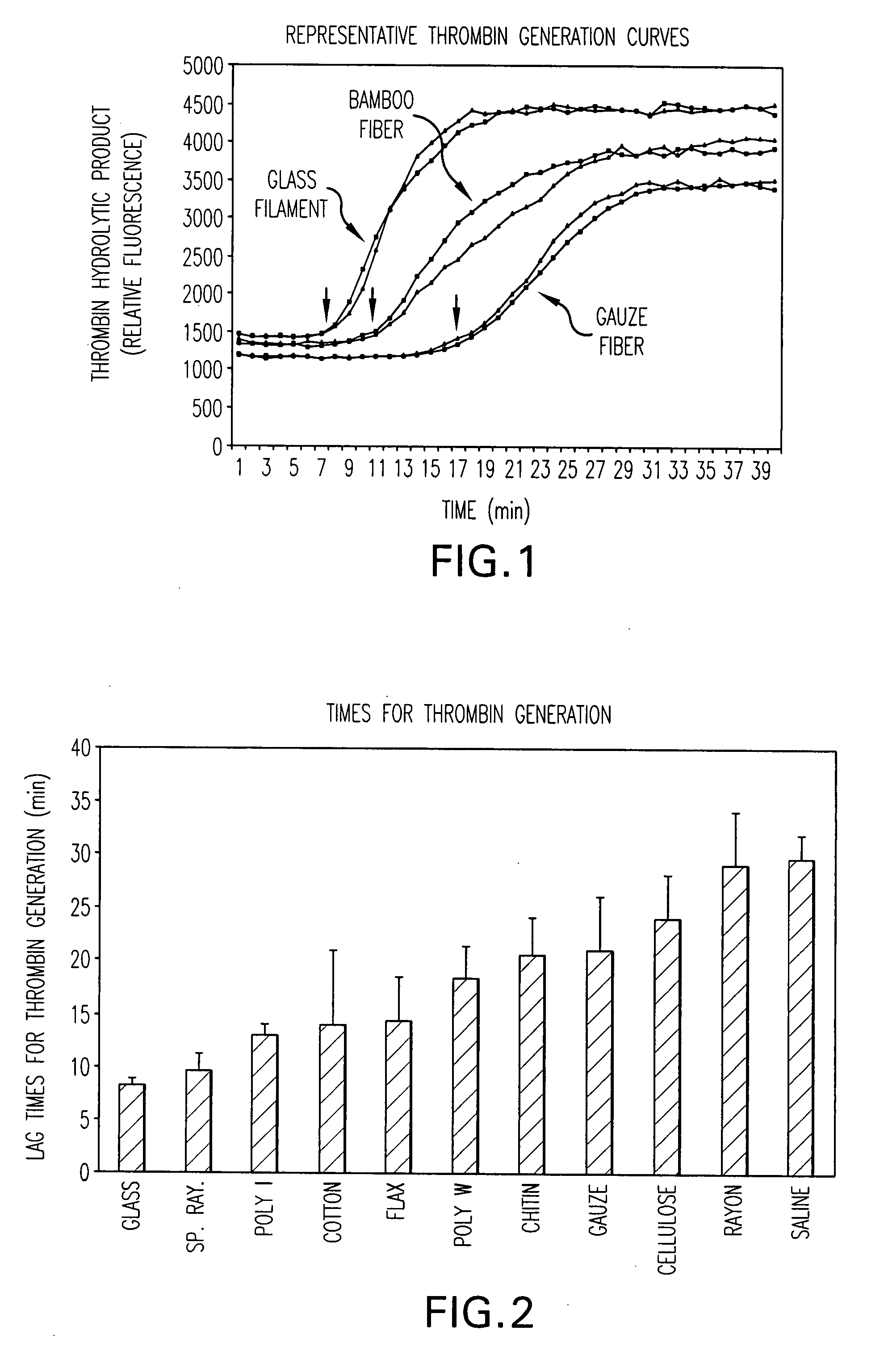
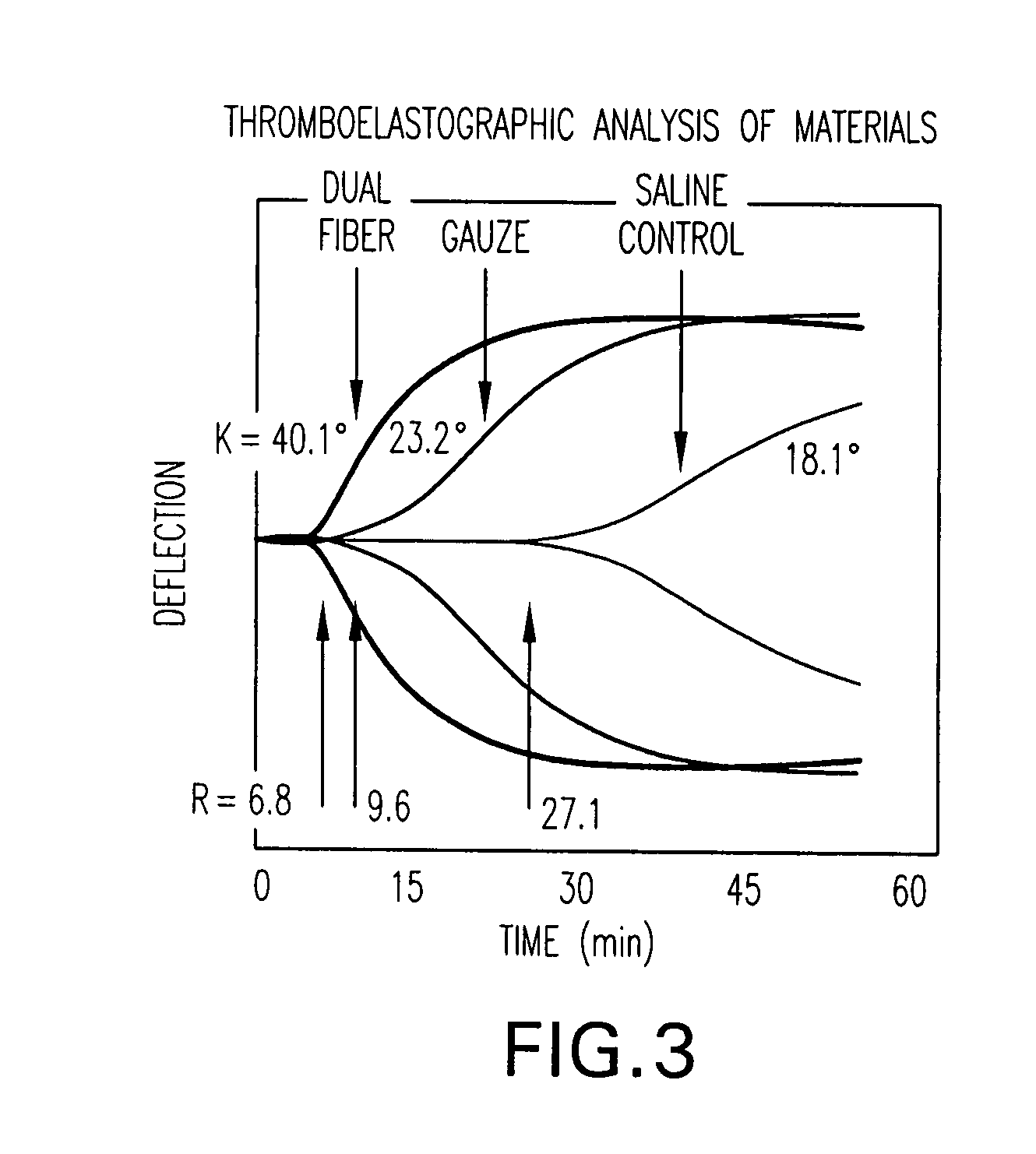
The present invention is directed to a hemostatic textile, comprising: a material comprising a combination of glass fibers and one or more secondary fibers selected from the group consisting of silk fibers; ceramic fibers; raw or regenerated bamboo fibers; cotton fibers; rayon fibers; linen fibers; ramie fibers; jute fibers; sisal fibers; flax fibers; soybean fibers; corn fibers; hemp fibers; lyocel fibers; wool; lactide and / or glycolide polymers; lactide / glycolide copolymers; silicate fibers; polyamide fibers; feldspar fibers; zeolite fibers, zeolite-containing fibers, acetate fibers; and combinations thereof; the hemostatic textile capable of activating hemostatic systems in the body when applied to a wound. Additional cofactors such as thrombin and hemostatic agents such as RL platelets, RL blood cells; fibrin, fibrinogen, and combinations thereof may also be incorporated into the textile. The invention is also directed to methods of producing the textile, and methods of using the textile to stop bleeding.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL +1
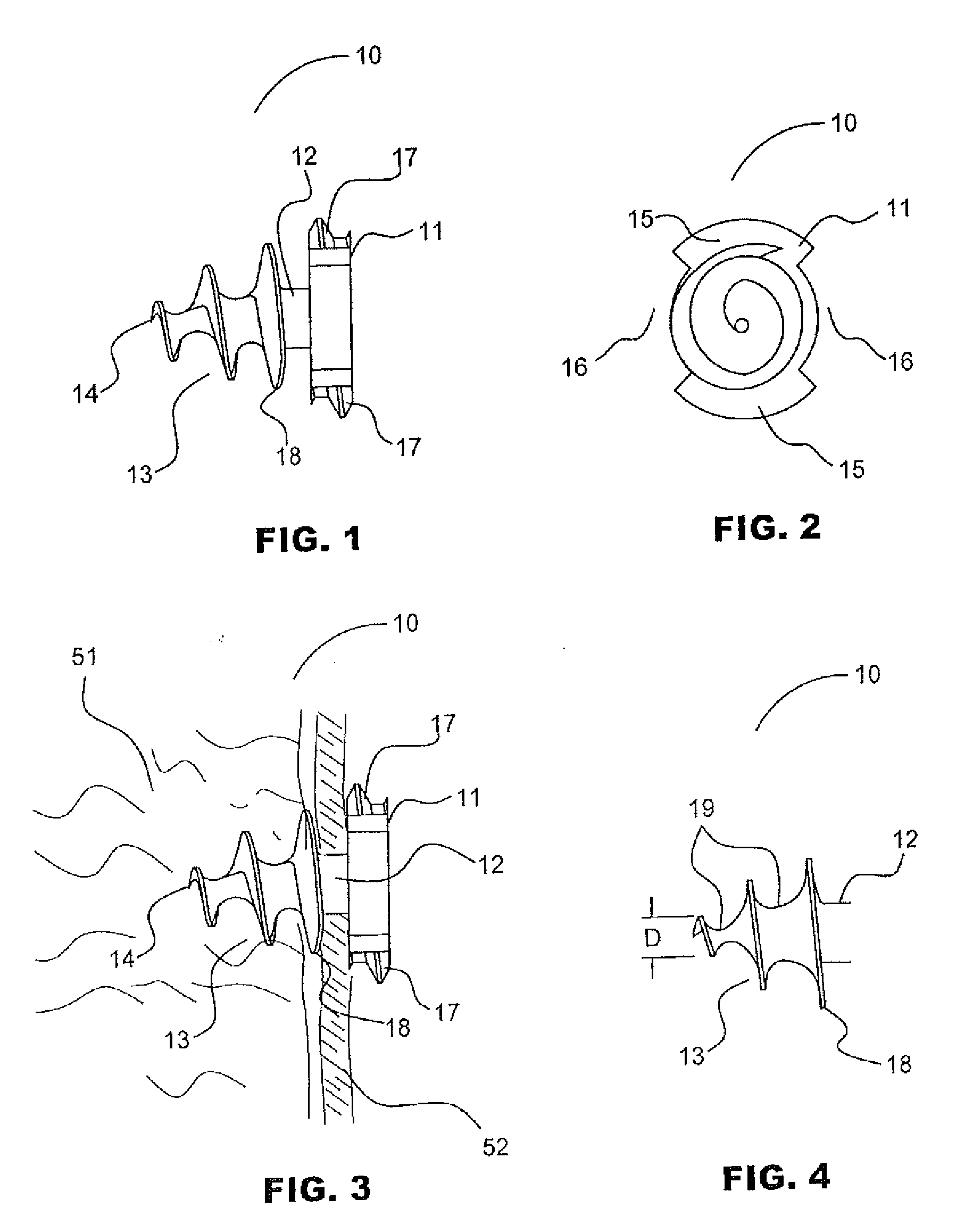
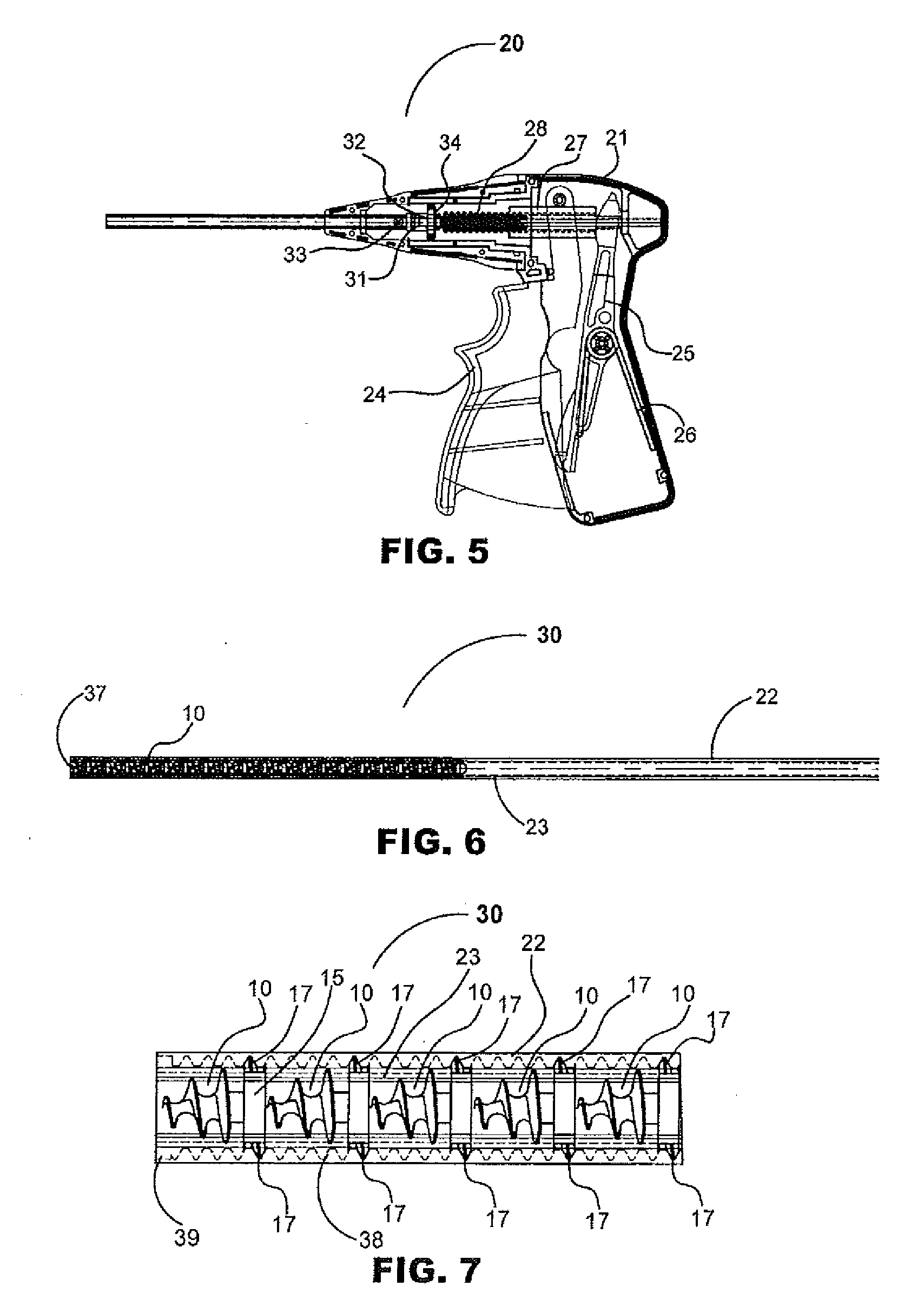
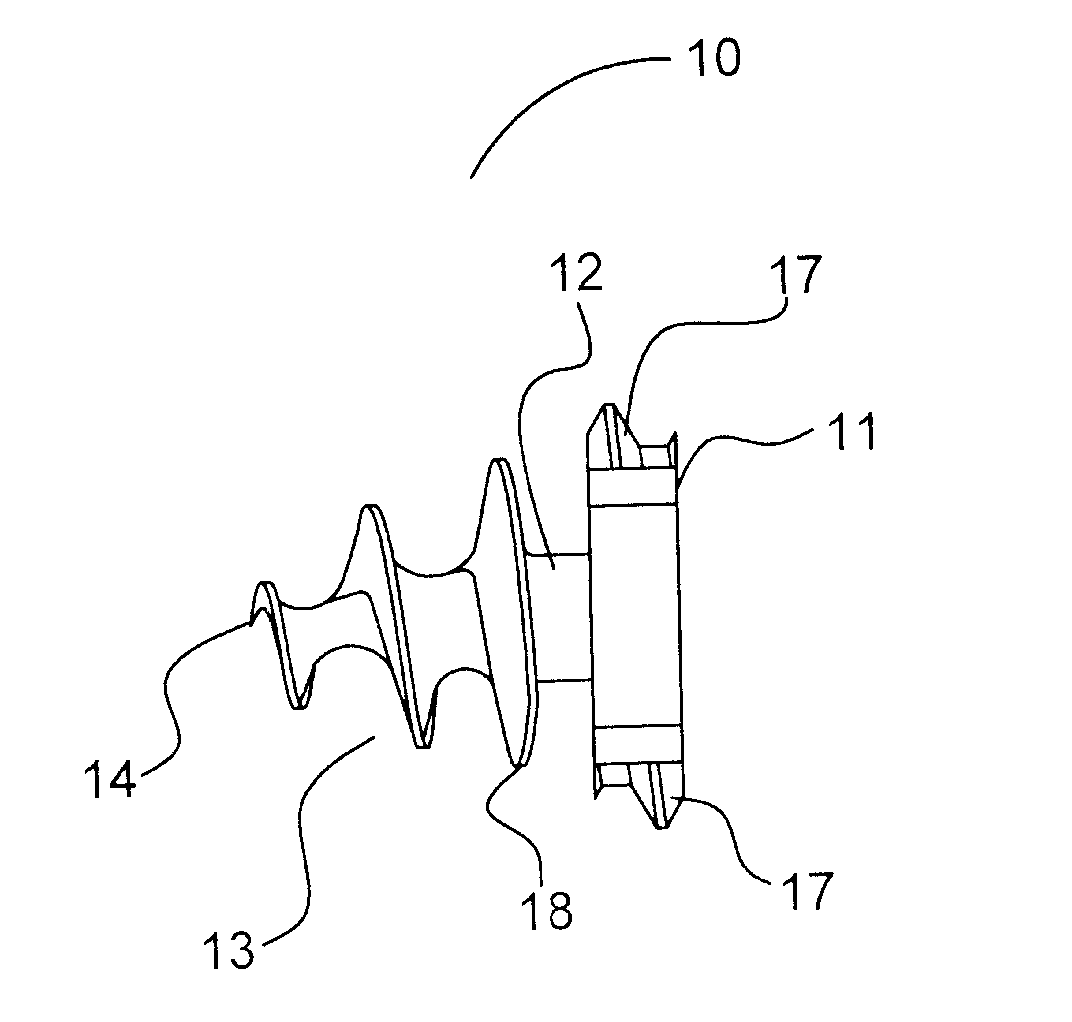
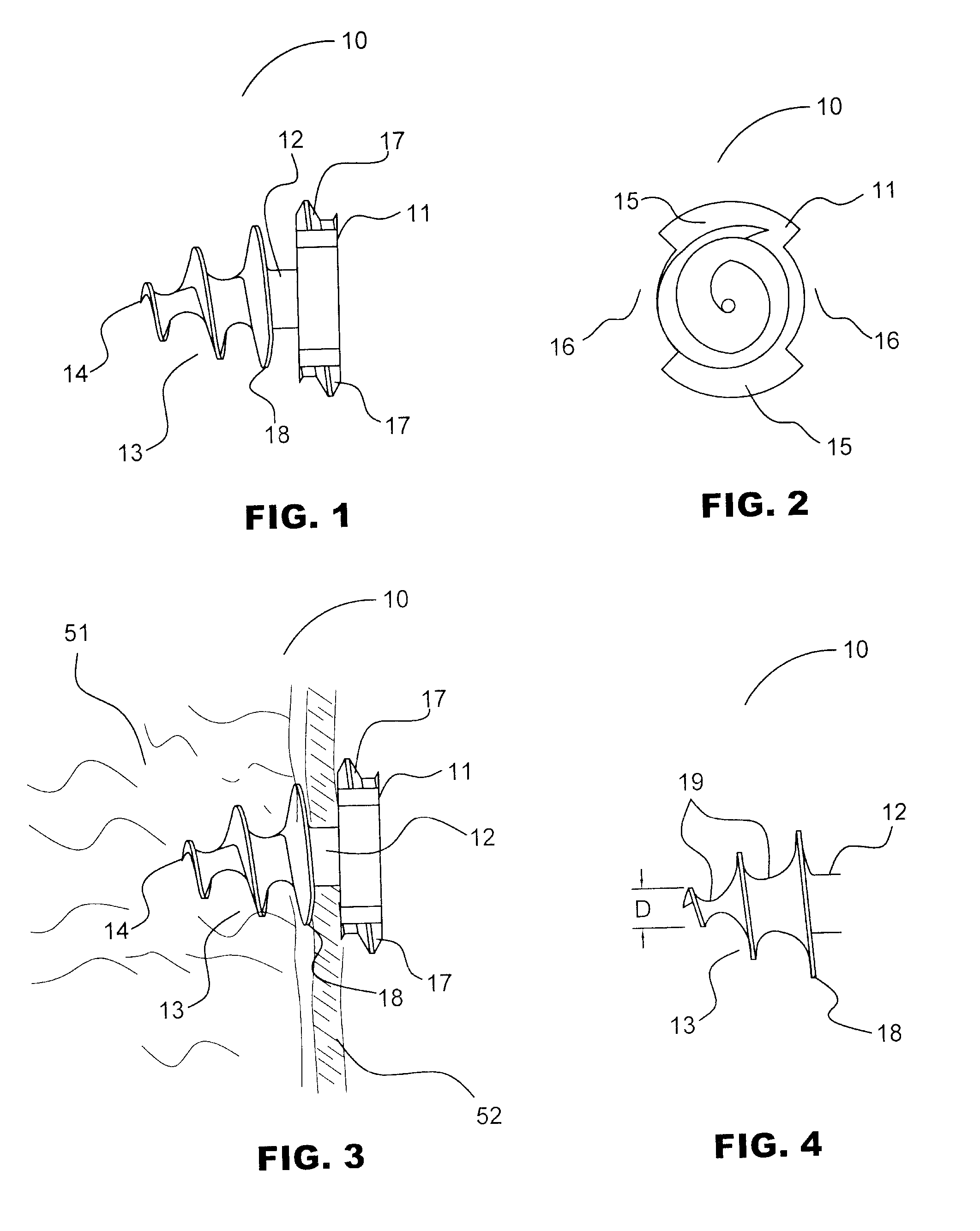
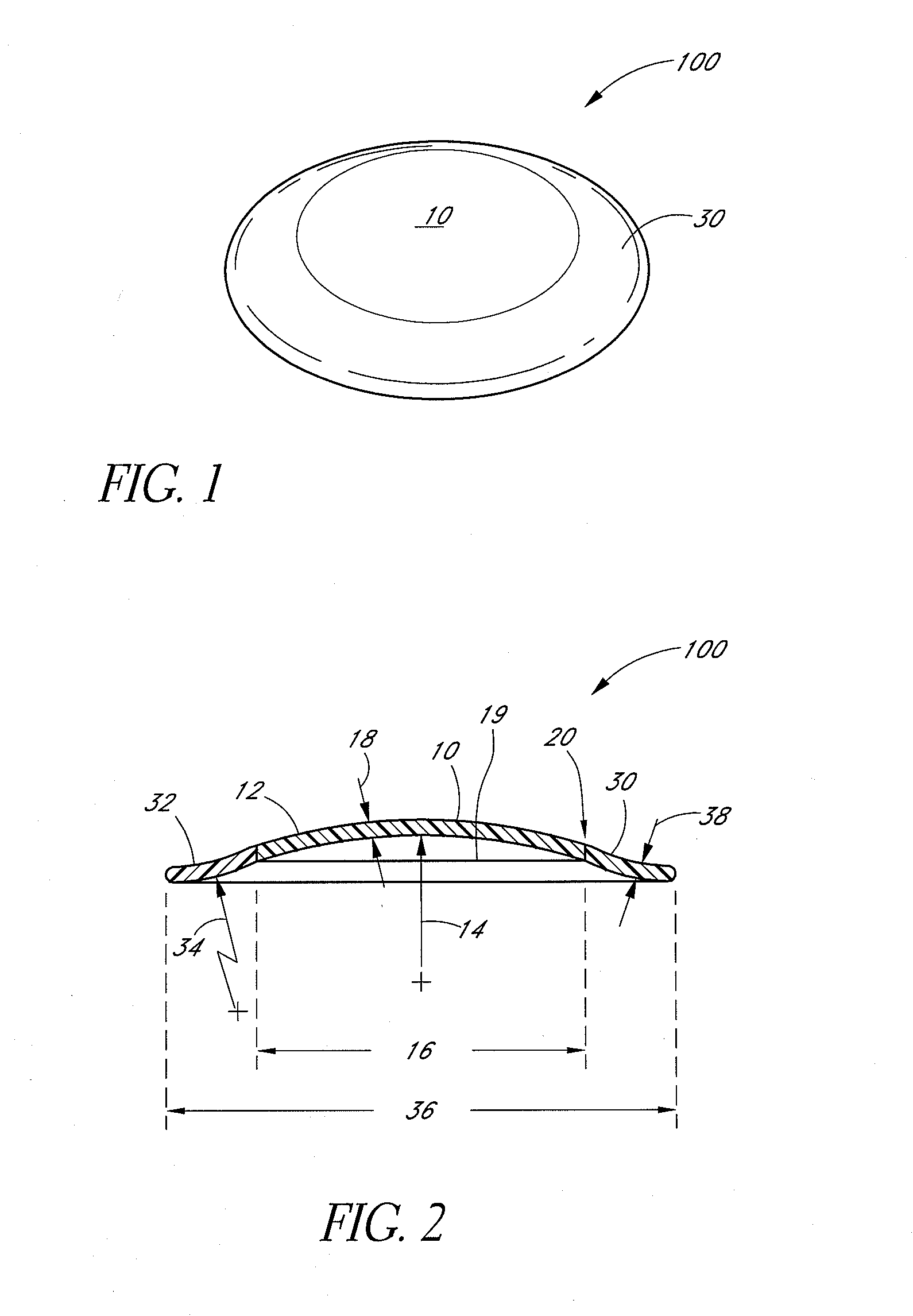
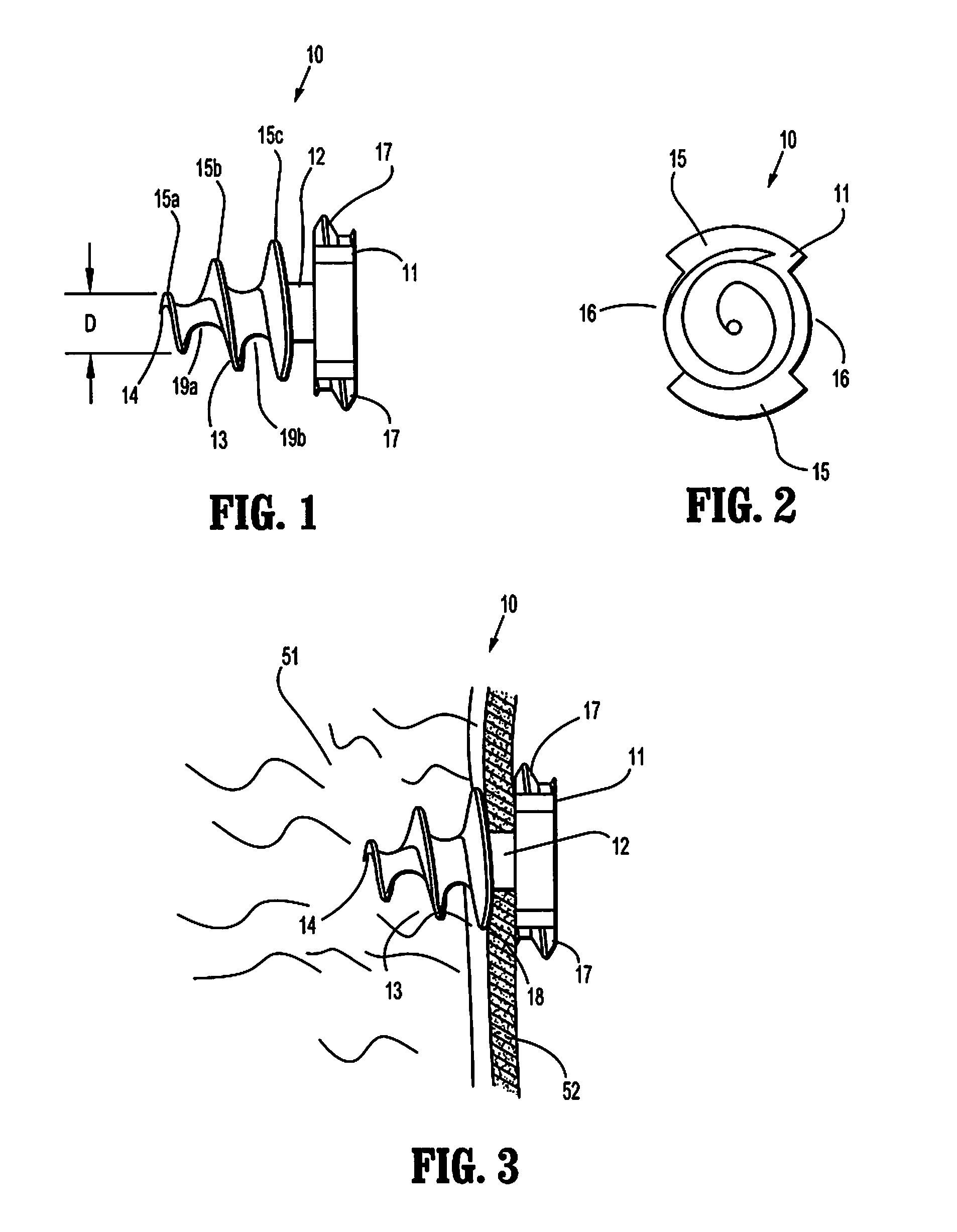
Absorbable anchor for hernia mesh fixation
A method of forming and deploying an improved absorbable anchor for hernia mesh fixation is disclosed. The absorbable anchor of the present invention functions to securely fasten tough, non macro-porous, and relative inelastic mesh to soft tissue. The anchor is formed from co-polymers of lactide and glycolide.
Owner:TYCO HEALTHCARE GRP LP
Tumor targeting drug-loaded particles
A composition for delivering a tumor therapeutic agent to a patient includes a fast-release formulation of a tumor apoptosis inducing agent, a slow-release formulation of a tumor therapeutic agent, and a pharmaceutically acceptable carrier. An apoptosis-inducing agent in a pharmaceutically acceptable carrier may be administered before or concomitantly therewith. Nanoparticles or microparticles (e.g., cross-linked gelatin) of the therapeutic agent (e.g., paclitaxel) also may be used. The nanoparticles or microparticles may be coated with a bioadhesive coating. Microspheres that agglomerate to block the entrance of the lymphatic ducts of the bladder to retard clearance of the microparticles through the lymphatic system also may be employed. This invention also uses drug-loaded gelatin and poly(lactide-co-glycolide) (PLGA) nanoparticles and microparticles to target drug delivery to tumors in the peritoneal cavity, bladder tissues, and kidneys.
Owner:AU JESSIE L S +1
Absorbable Anchor for Hernia Mesh Fixation
A method of forming and deploying an improved absorbable anchor for hernia mesh fixation is disclosed. The absorbable anchor of the present invention functions to securely fasten tough, non macro-porous, and relative inelastic mesh to soft tissue. The anchor is formed from co-polymers of lactide and glycolide.
Owner:TYCO HEALTHCARE GRP LP
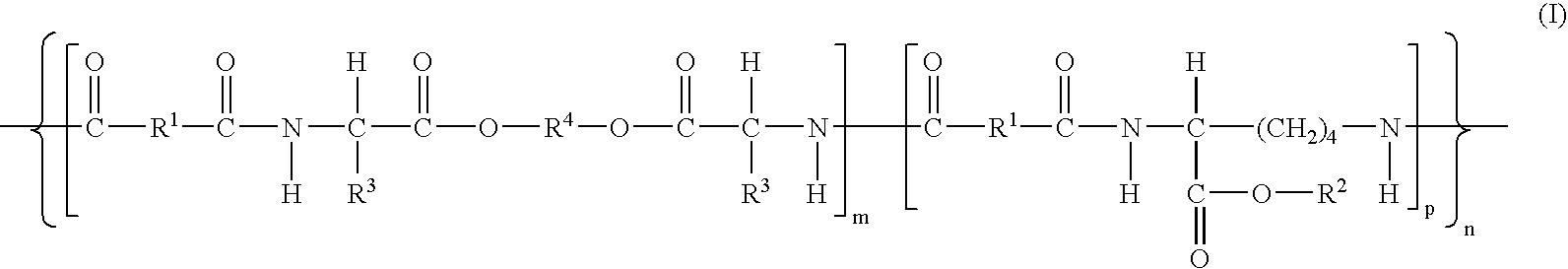
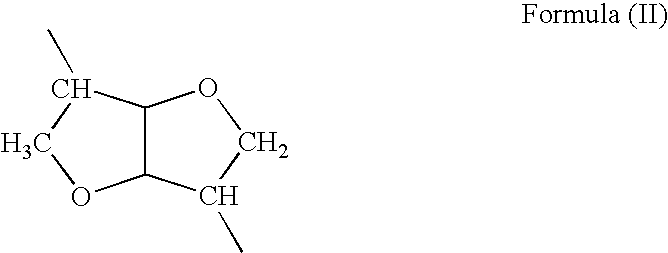
Polymeric nanoparticles with enhanced drug-loading and methods of use thereof
The invention is directed to modified polymers with increased drug-loading including compounds of formula (I): wherein Z is a poly(lactic-co-glycolic acid) (PLGA) polymer having molecular weight from 1-15 kDa and where the ratio of lactide to glycolide in the PLGA polymer is from 1:10 to 10:1; formula (II) R1 are independently H, R2, OH, O-alkyl, —O—R2, NH—R2, -linker-R2, or -and R2 are independently one or more therapeutic agents. The invention is also directed to nanoparticle drug delivery systems including a PLGA-b-PEG block copolymer; and a stabilizer and to drug delivery systems including PLGA-b-PEG block copolymer polyvinyl alcohol (PVA) nanoparticle; and the modified polymer substantially as described herein.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
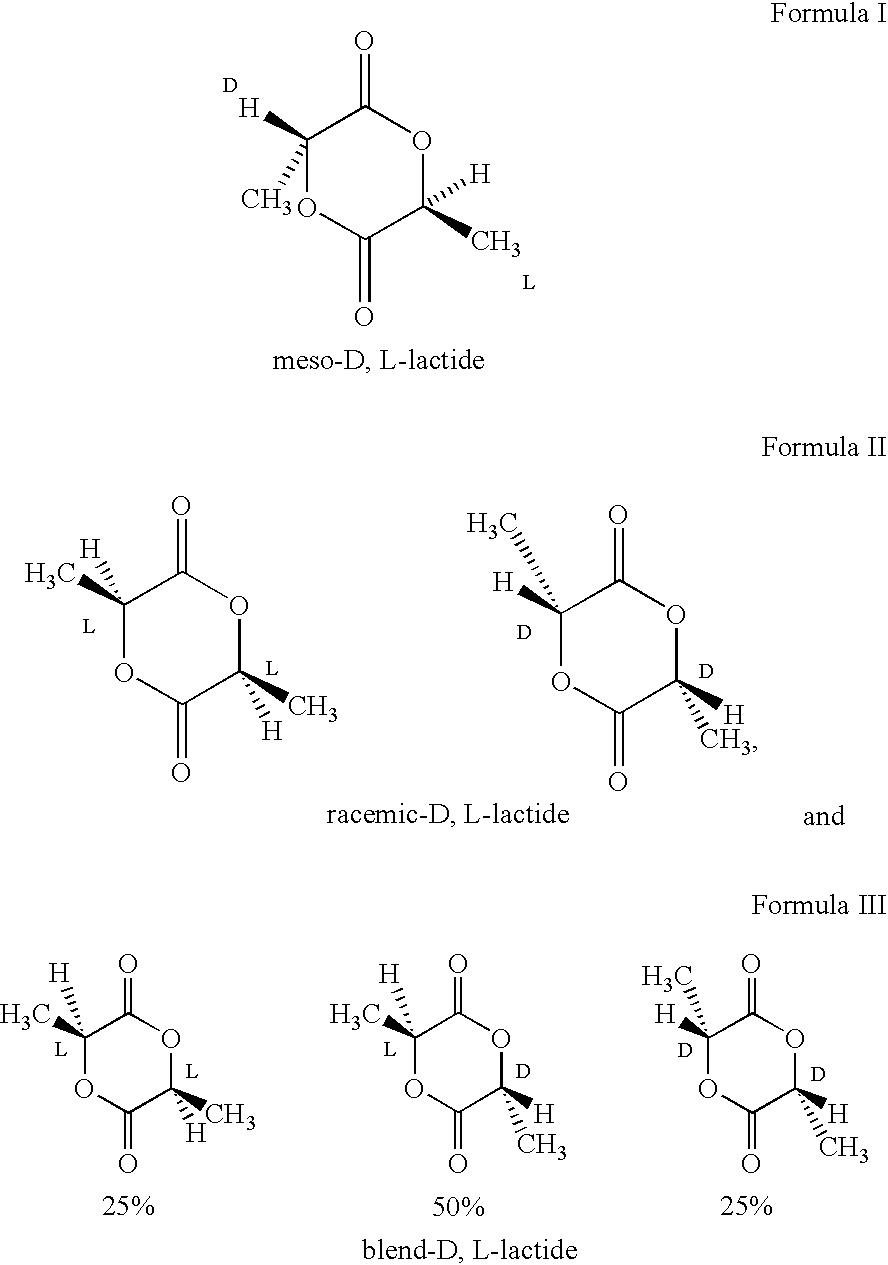
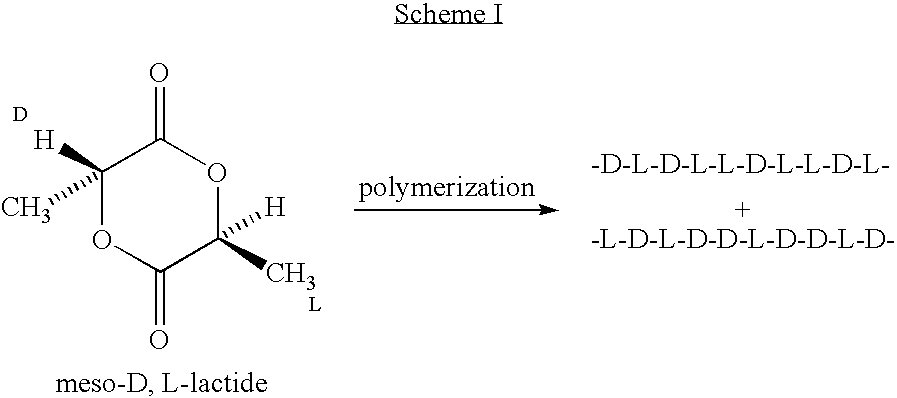
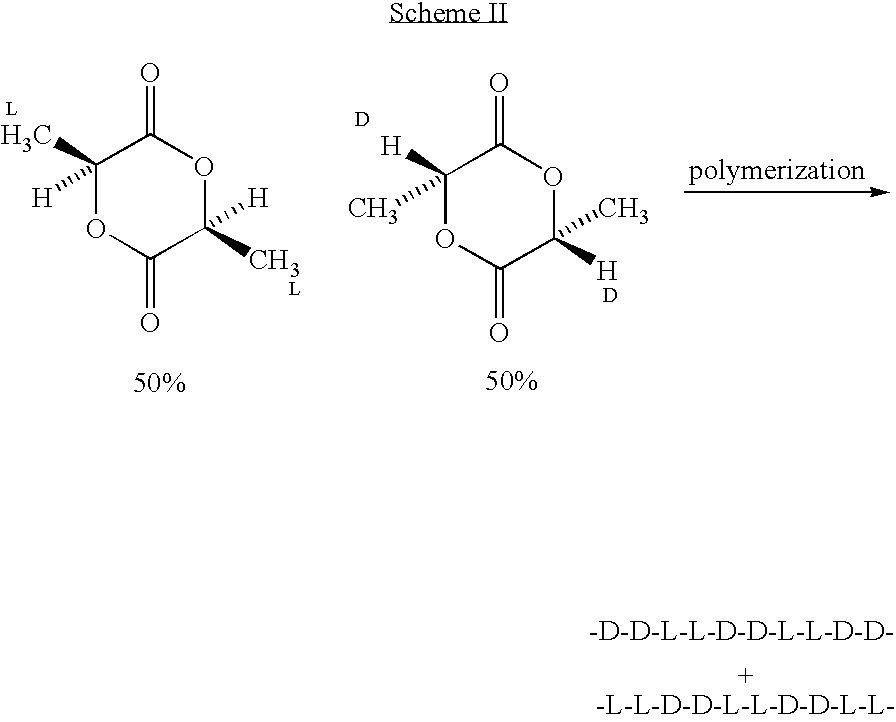
Amorphous poly(D,L-lactide) coating
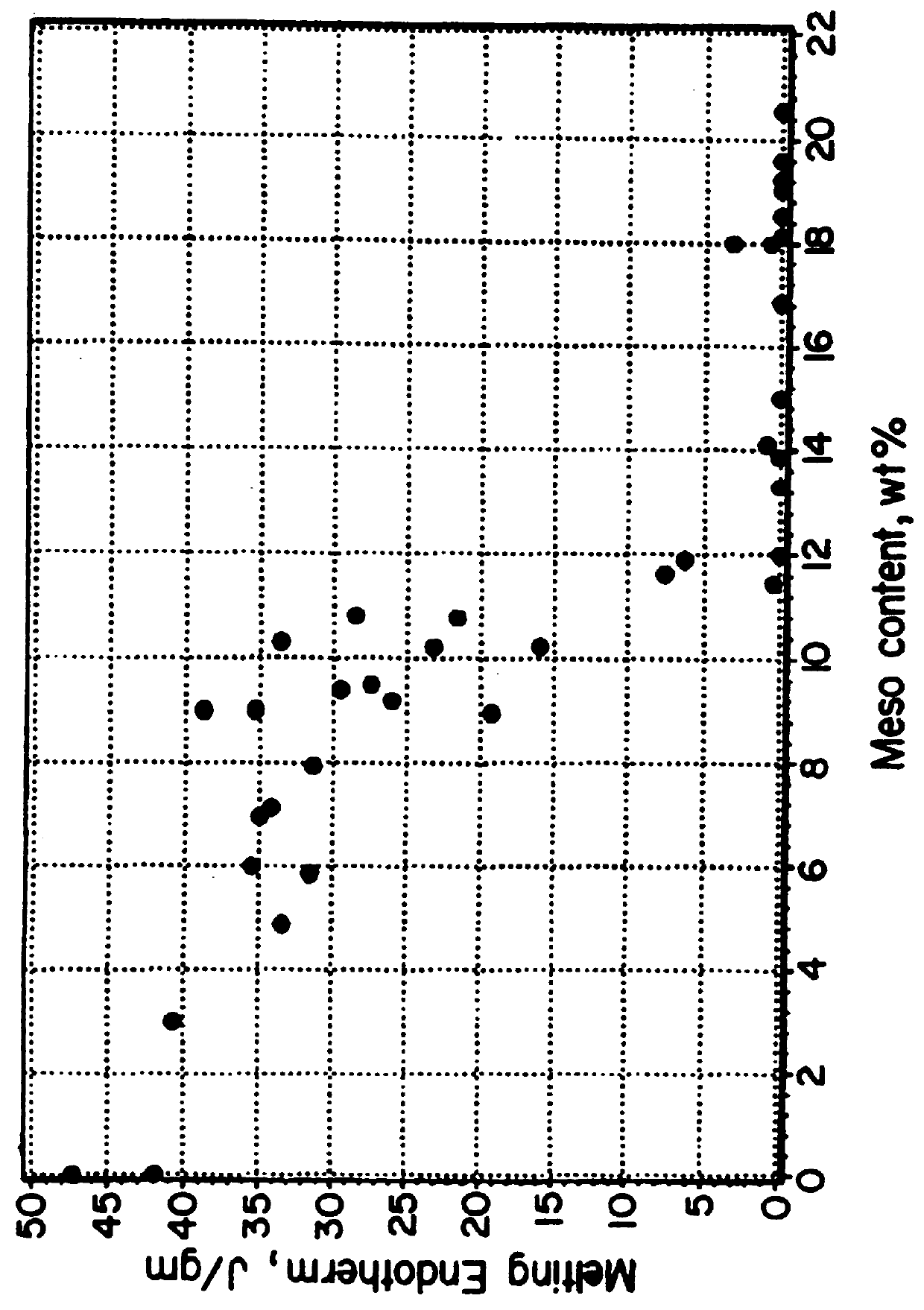
Implantable devices formed of or coated with a material that includes an amorphous poly(D,L-lactide) formed of a starting material such as meso-D,L-lactide are provided. The implantable device can be used for the treatment, mitigation, prevention, or inhibition of a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, patent foramen ovale, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
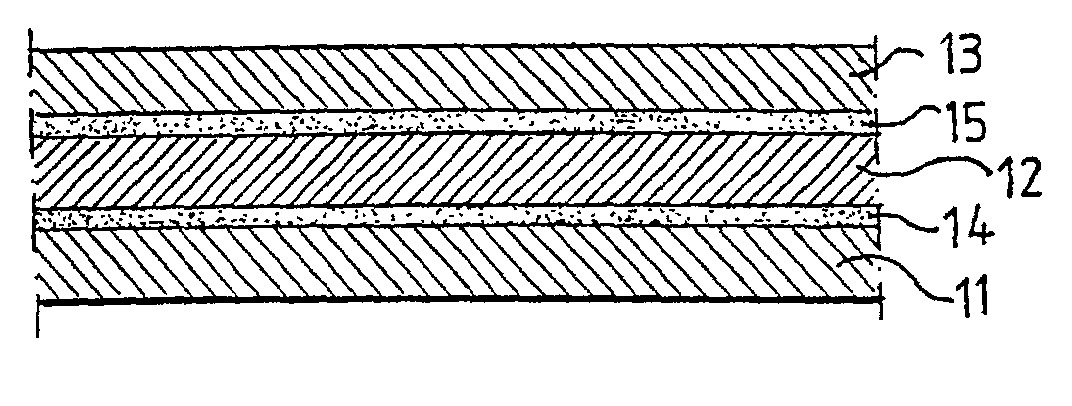
Biodegradable packaging laminate, a method of producing the packaging laminate, and packaging containers produced from the packaging laminate
InactiveUS20020127358A1Improve adhesionExcellent gas barrier performanceAdhesive processesLiquid surface applicatorsLactideBiopolymer
Packaging laminate for packages for liquid foods having excellent liquid and oxygen gas barrier properties in which all included layers are biodegradable. The packaging laminate includes at least one liquidtight layer (11, 13) of homo or copolymers of monomers selected from a group consisting of lactic acid, glycol acid, lactide, glycolide, hydroxy butyric acid, hydroxy valeric acid, hydroxy caproic acid, valerolactone, butyrolactone and caprolactone, as well as an oxygen gas barrier layer (12) of ethylene vinyl alcohol, polyvinyl alcohol, starch or starch derivatives. The oxygen gas barrier layer is preferably applied by a dispersion coating process. The layers may be laminated directly to one another or indirectly by means of interjacent adhesive layers. The packaging laminate may also include a core layer of, for example, paper or paperboard, or a biopolymer. The invention also realises a method of producing the biodegradable packaging laminate according to the invention.
Owner:TETRA LAVAL HLDG & FINANCE SA
Bioabsorbable blends and surgical articles therefrom
InactiveUS7138441B1Excellent flexibiltyExcellent Adhesive PropertiesSuture equipmentsSurgical adhesivesCyanoacrylatePolymer science
Blends made of bioabsorbable materials including glycolide, lactide, caprolactone, dioxanone, trimethylene carbonate, alkylene glycols, esteramides, etc., and polymers and copolymers thereof with cyanoacrylates are described. Processes for making the polymers and surgical articles made totally or in part from such polymers, including sutures, are also described.
Owner:UNITED STATES SURGICAL CORP
Melt-stable semi-crystalline lactide polymer film and process for manufacture thereof
InactiveUS6121410AHigh surface energyEasy to printCoatings with pigmentsSurgeryLactidePolymer chemistry
A semi-crystalline film comprised of a lactide polymer. The lactide polymer comprises a plurality of poly(lactide) polymer chains, residual lactide in concentration of less than about 5 percent and water in concentration of less than about 2000 parts-per-million. A process for manufacturing a semi-crystalline film with the lactide polymer composition is also disclosed.
Owner:CARGILL INC
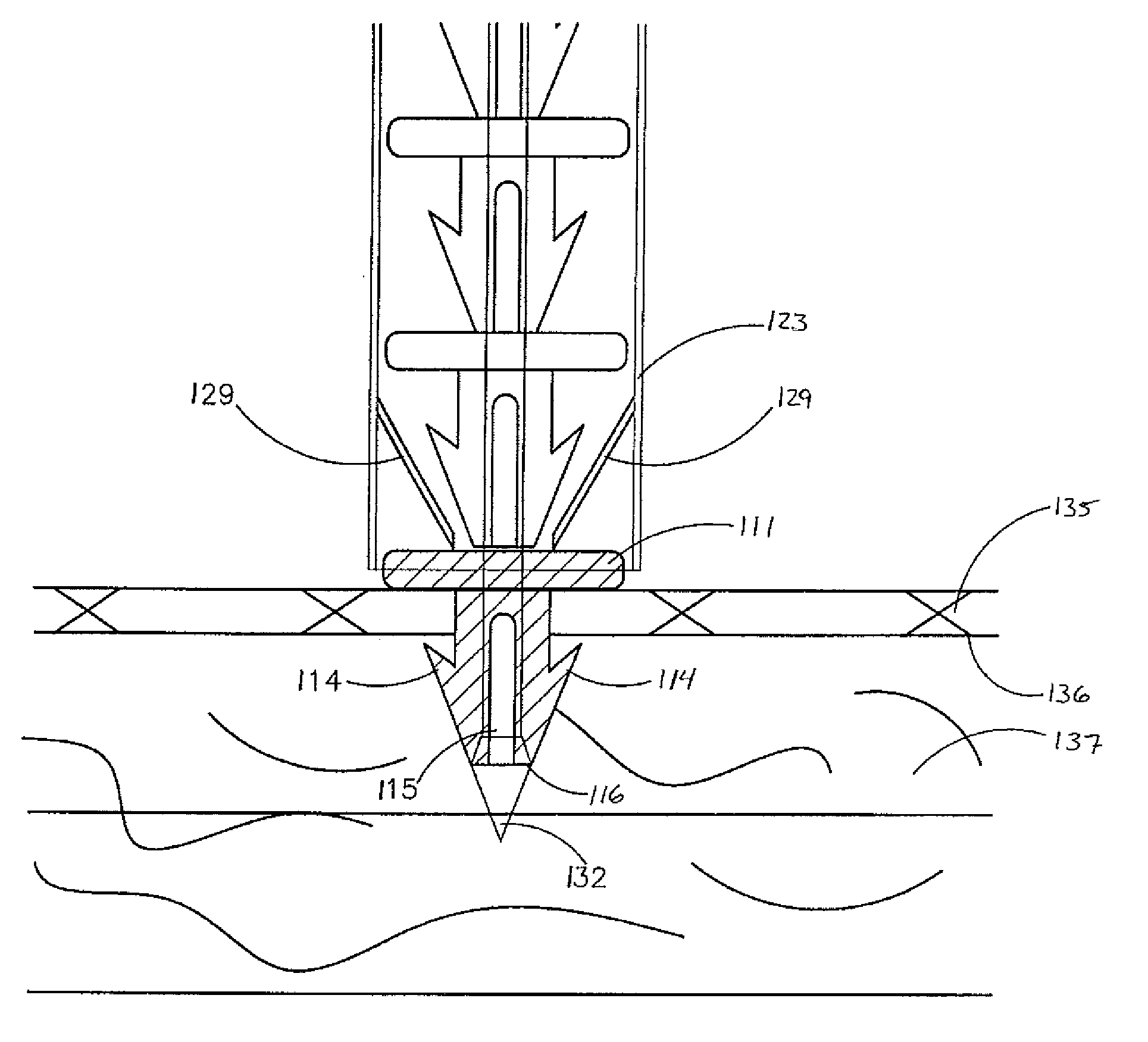
Methods and devices for delivering injections
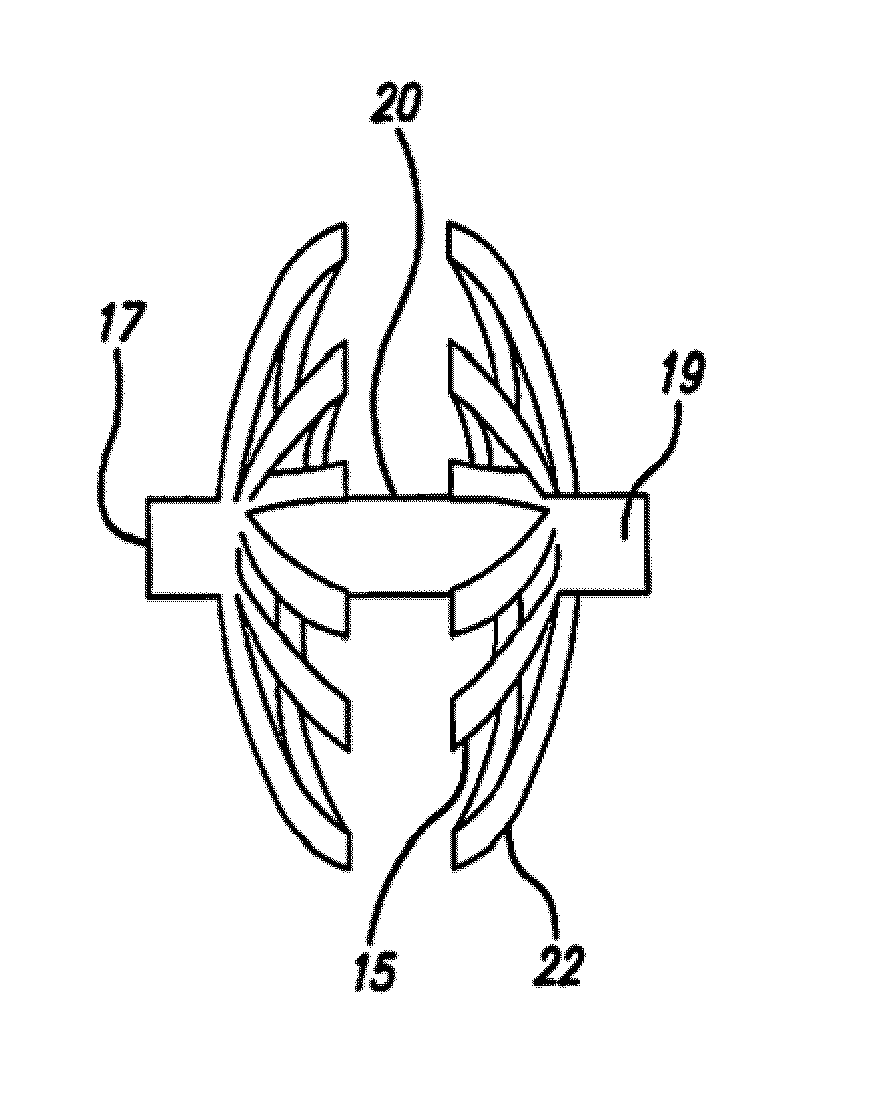
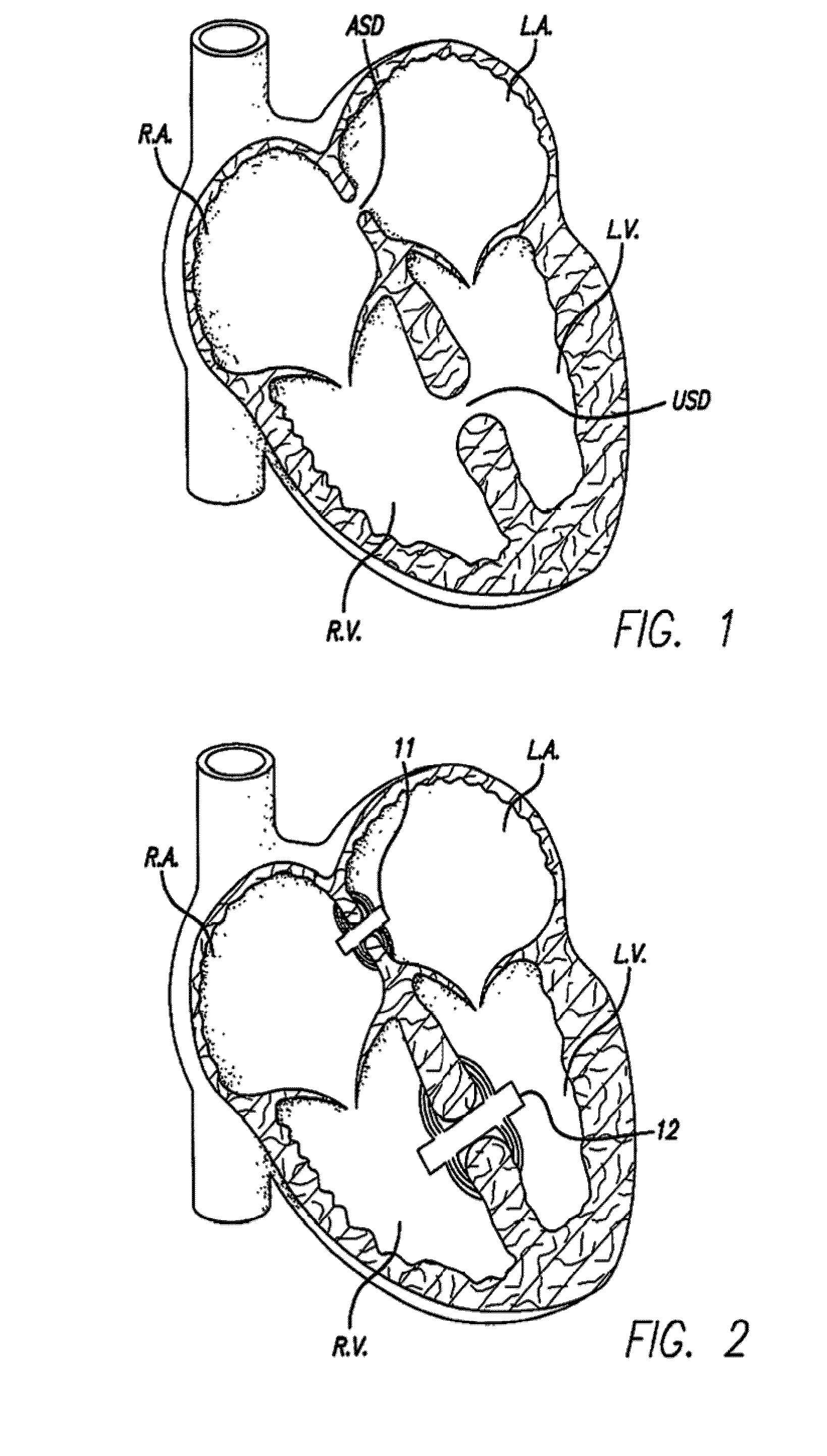
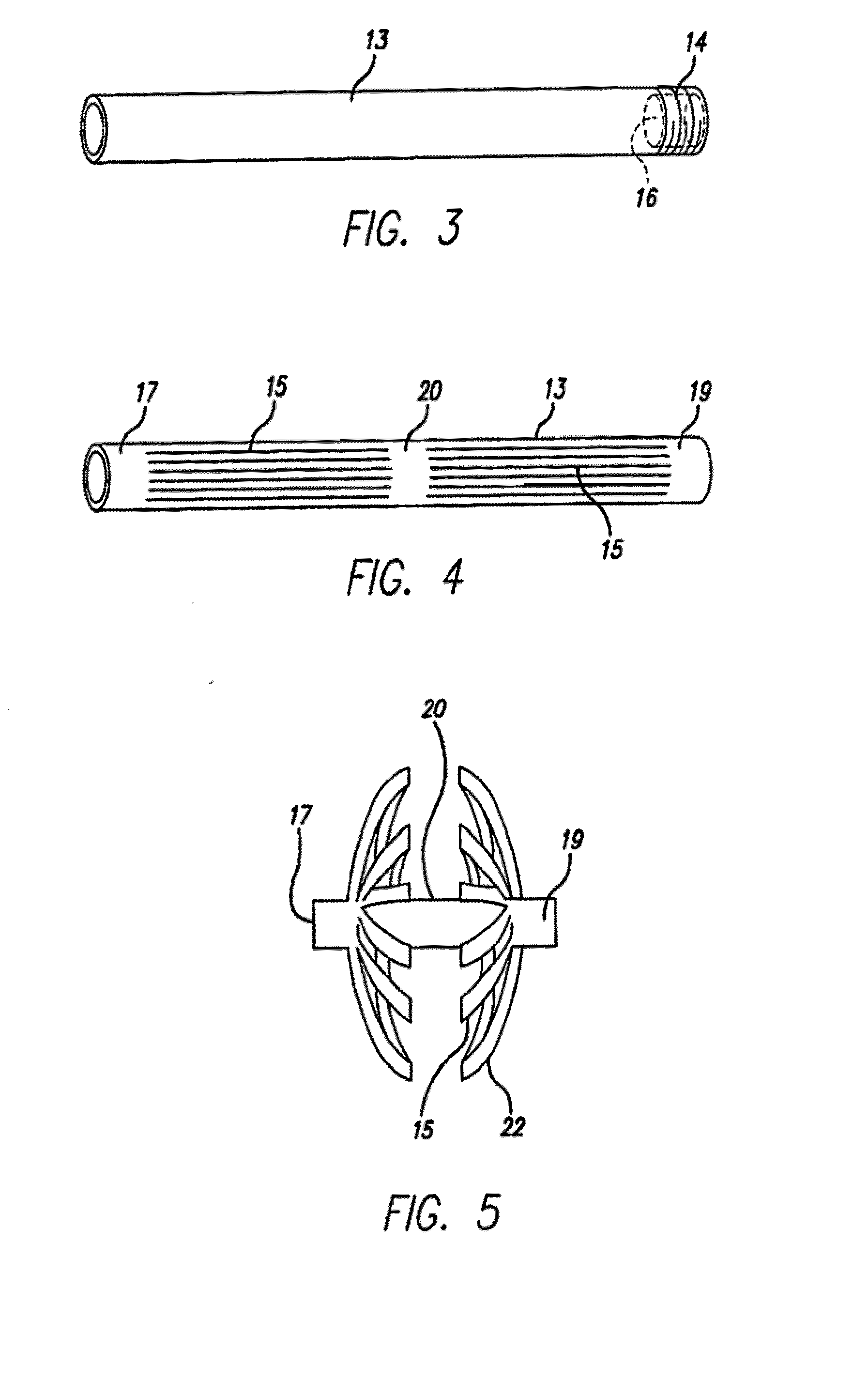
Septal defect occluders are disclosed which can be used with a catheter deployment system to occlude a septal defect. The septal defect occluders of the present invention comprise a metallic frame structure that supports a biodegradable member. The frame structure is made from a shape memory metal such as Nitinol. The frame forms two opposing umbrella or disc shaped halves that are connected via a central region. The biodegradable member is attached to the umbrella or disc shaped halves and can be any of numerous biodegradable materials and is preferably a co-polymer of glycolide and lactide. This material initially forms a barrier to blood flow that occludes the defect. Over time, this material is replaced by the body with scar tissue formation and endothelial cells. The metal frame is left coated with the body's own material that blocks the defect.
Owner:DEBEER NICHOLAS +1
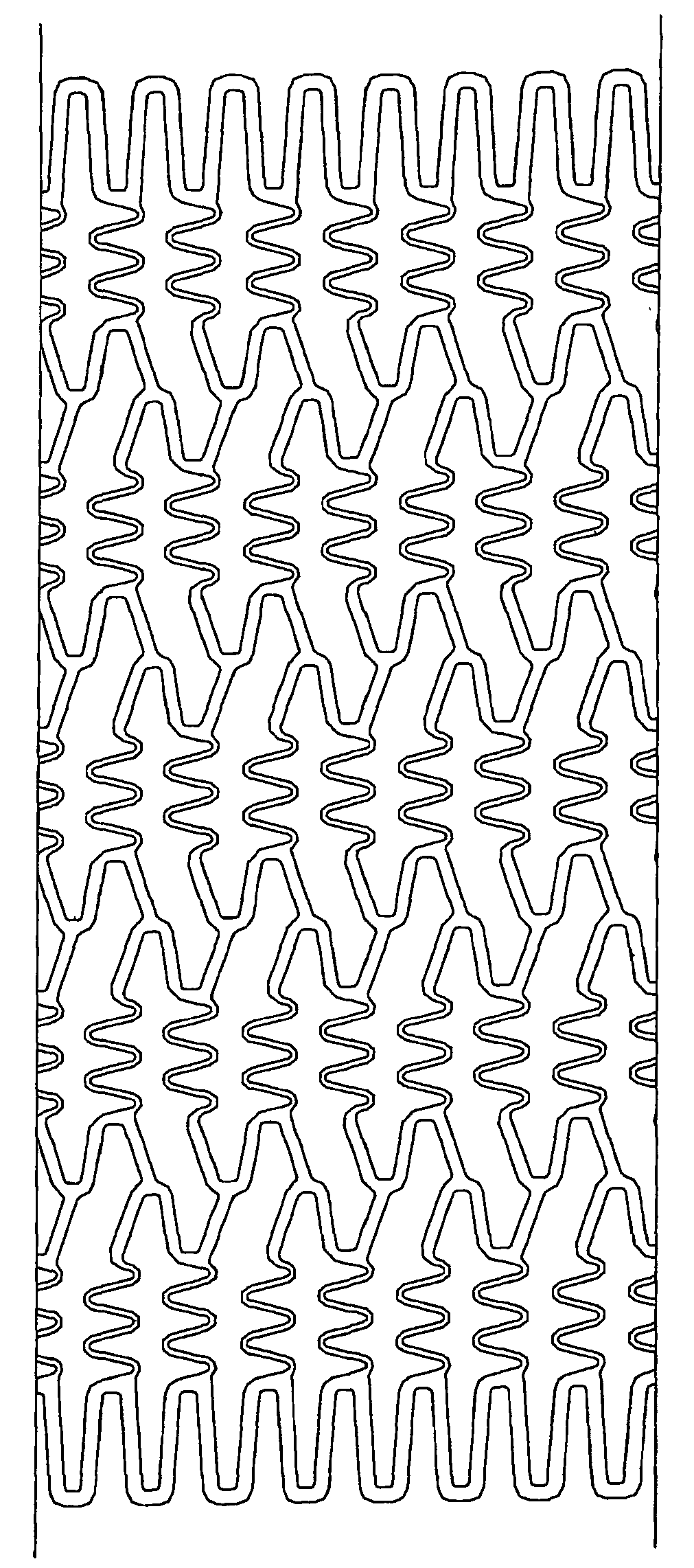
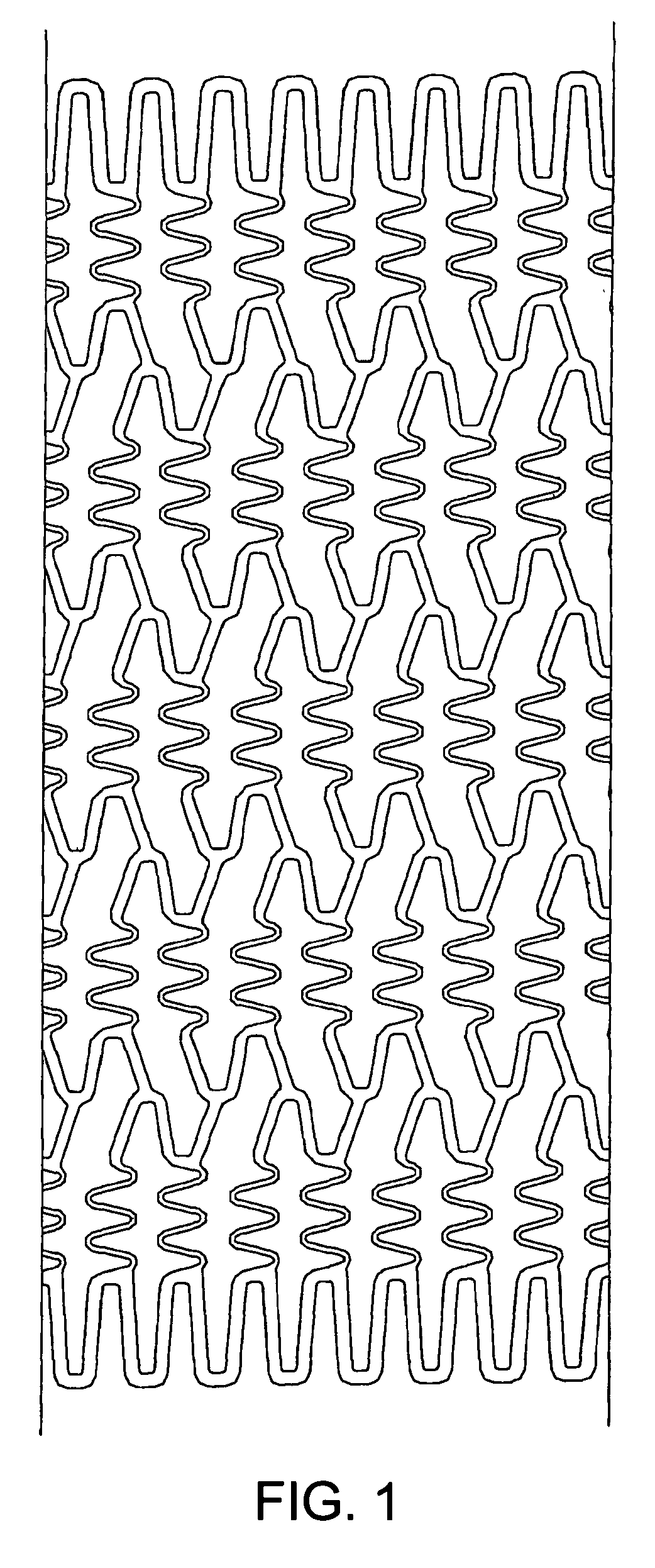
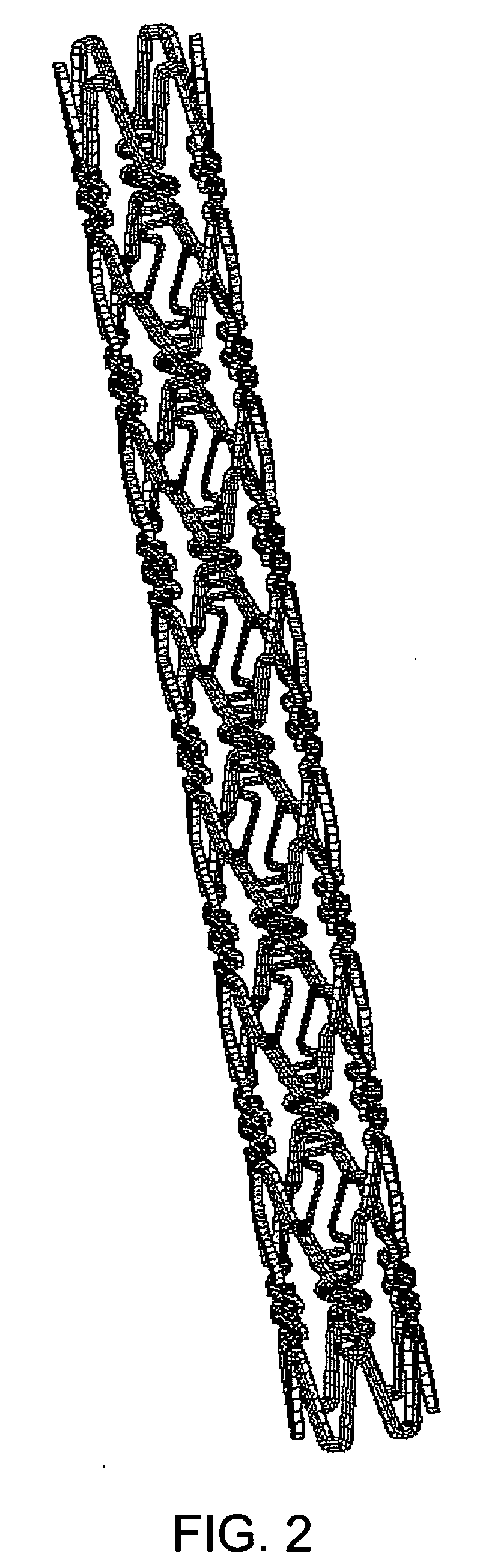
Stent to be placed in vivo
InactiveUS20070038289A1Low rateHighly preventive effectStentsSurgeryLactidePercent Diameter Stenosis
As a treatment for angiostenosis, angioplasty (PTA or PTCA) of expanding a small-sized balloon in a vessel has been commonly conducted. However, this treatment easily causes repeated stenosis (restenosis) after the treatment. Placement of a stent in a vessel is also effective in decreasing restenosis, but this treatment may also cause restenosis. The present invention provides a stent containing a poly (lactide-co-glycolide) or both a poly (lactide-co-glycolide) and an immunosuppressive agent in at least a portion of a surface of the stent, and further containing a material nondegradable in vivo.
Owner:KANEKA CORP
Adhesion-Preventive Film
InactiveUS20080090936A1Good flexibilityProvide effectSuture equipmentsCosmetic preparationsLactideCaprolactone
An adhesion-preventive film is provided that is excellent in flexibility and can prevent cracks from occurring. The adhesion-preventive film contains a copolymer of lactide and caprolactone. The lactide and the caprolactone of the copolymer has a mole ratio in the range of 65:35 to 80:20. Even when this adhesion-preventive film is used in a curved state in vivo or is wound around an affected part such as a tendon, for example, it can provide an adhesion-preventive function for a sufficiently long period without cracking.
Owner:JMS CO LTD
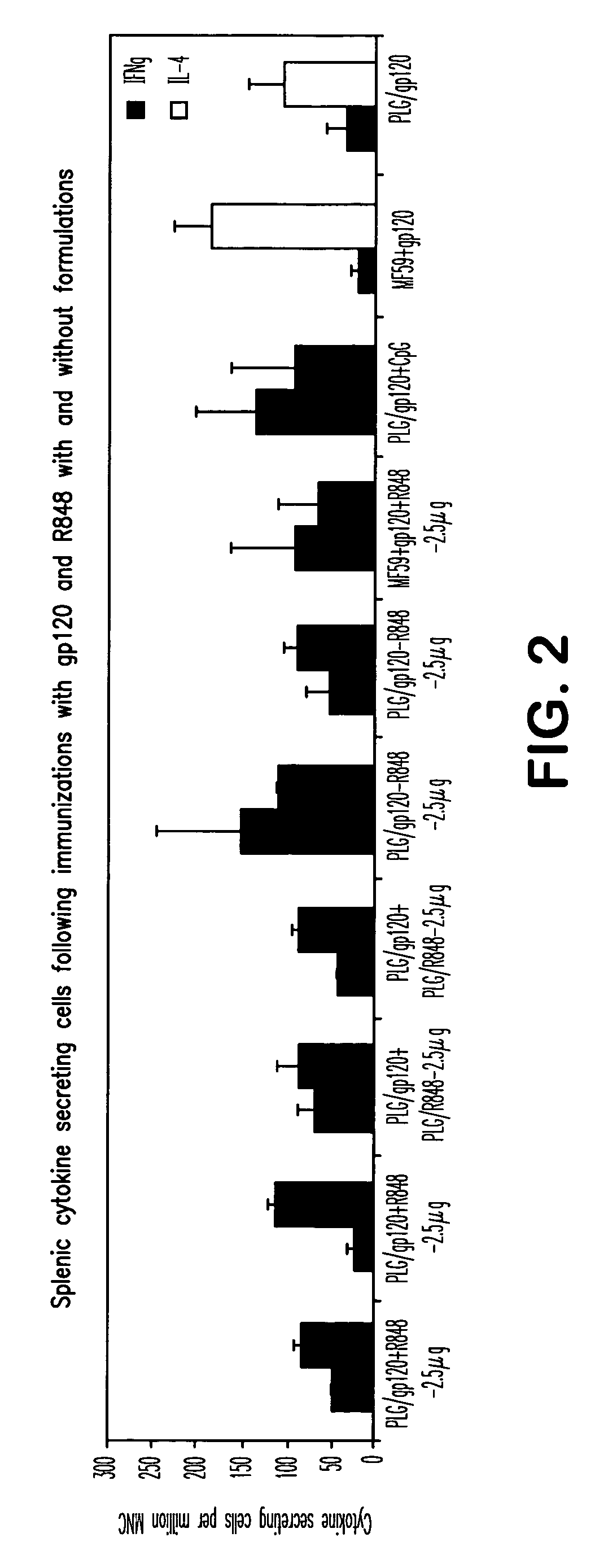
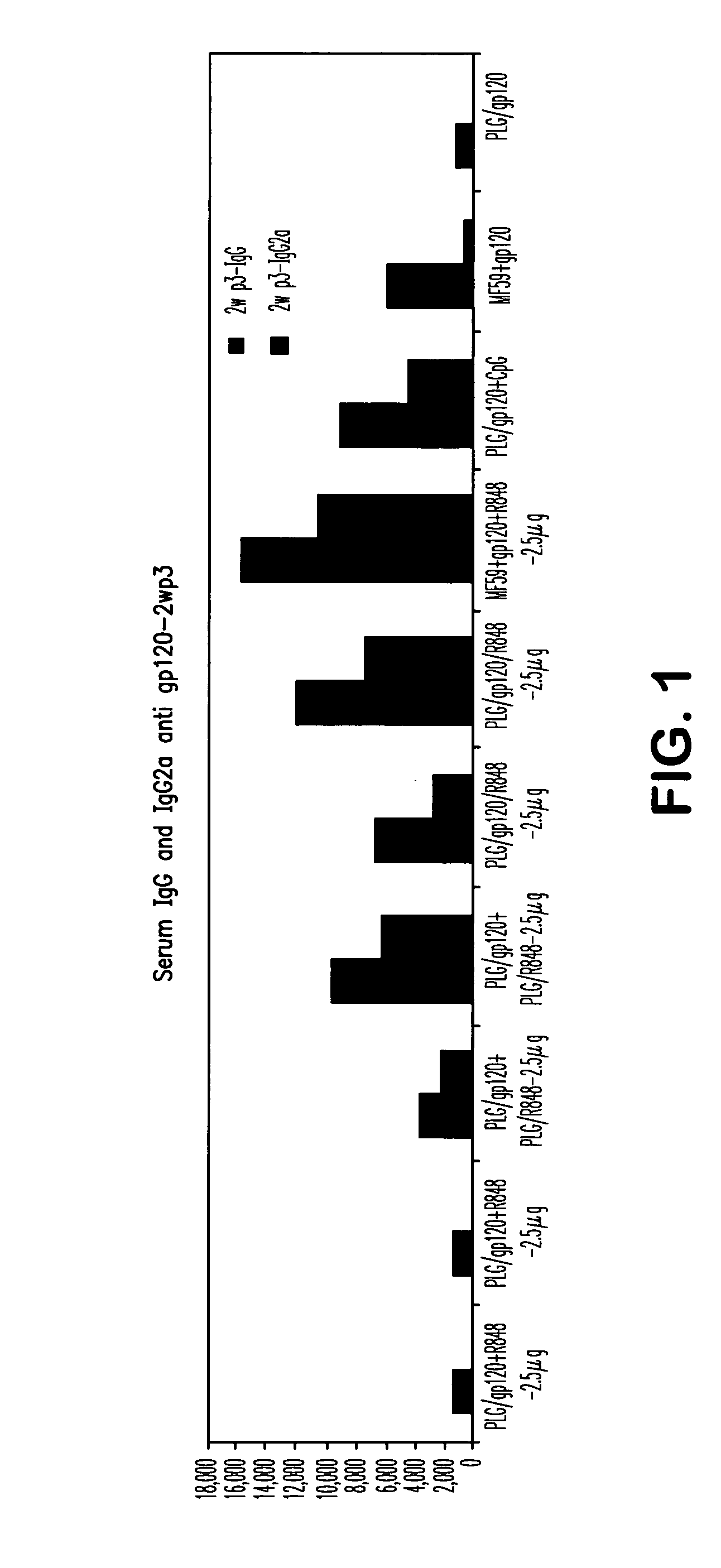
Compositions for inducing immune responses
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Liquid polymeric compositions for controlled release of bioactive substances
Controlled release of hydrophobic bioactive substances in vivo over an extended time period and without "bursts" of drug release is achieved using a liquid polymeric composition including a polymer such as poly(lactide-co-glycolide) copolymer in a mixture of hydrophilic and lipophilic solvents.
Owner:MERCK SHARP & DOHME CORP
Compositions for inducing immune responses
ActiveUS20050107322A1Avoid inductionBacterial antigen ingredientsViral antigen ingredientsAdjuvantLactide
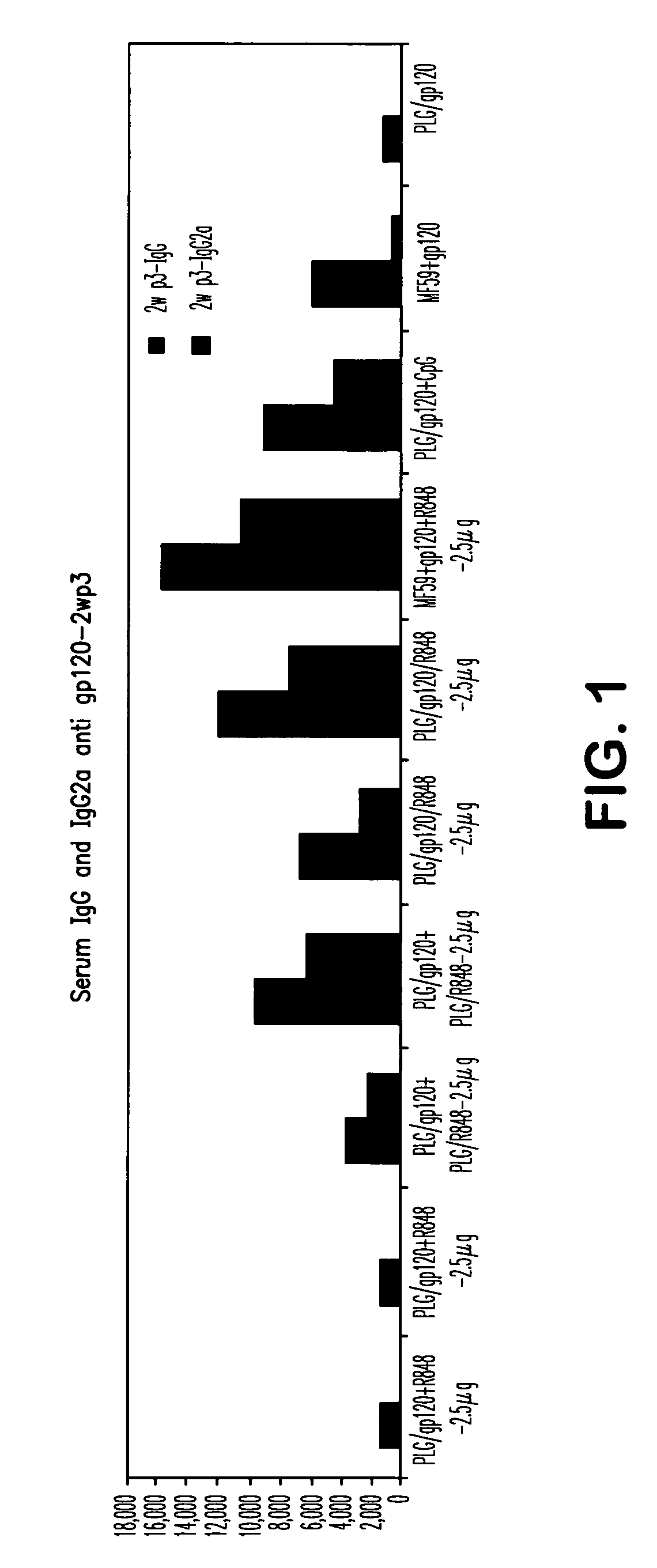
The invention provides, inter alia, immunogenic compositions comprising a first antigen, at least two adjuvants, wherein a first adjuvant comprises a polymer derived from poly(lactides) and / or poly(lactide-co-glycolides), and wherein a second adjuvant comprises an imidazoquinoline, wherein said first antigen is encapsulated within, adsorbed or conjugated to, co-lyophilized or mixed with said first adjuvant, and a pharmaceutically acceptable excipient, wherein said composition elicits a cellular immune response when administered to a vertebrate subject. The invention also provides methods of producing immunogenic compositions, methods for producing a cytotoxic-T lymphocyte (CTL) response in a vertebrate subject, and methods of immunization.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Bioabsorbable Polymeric Compositions, Processing Methods, and Medical Devices Therefrom
ActiveUS20120071566A1Improve mechanical propertiesHigh column strengthSuture equipmentsStentsP-dioxanoneLactide
Novel bioabsorbable polymeric blends are disclosed. The blends have a first component that is a polylactide polymers or a copolymer of lactide and glycolide and a second component that is poly(p-dioxanone) polymer. The novel polymeric blends provide medical devices having dimensional stability. Also disclosed are novel bioabsorbable medical devices made from these novel polymer blends, as well as novel methods of manufacture.
Owner:ETHICON INC
Hybrid contact lenses prepared with expansion controlled polymeric materials
A hybrid contact lens includes a substantially rigid center portion and a substantially flexible skirt portion connected to the center portion. The skirt portion is formed using xerogels compatible with diluents comprising at least one selected from polylactic acid, polyglycolic acid, lactide, glycolide, a polyacetal, a cyclic acetal, a polyketal, a cyclic ketal, a polyorthoester, a cyclic orthoester, di-t-butyl-dicarbonate, tris(trimethylsilyl)amine, and 2,2,2-trifluoroacetamide. These diluents are formulated to function as a stand-in for water in the xerogel polymer, allowing the xerogel to form and bond to the rigid center portion in its fully expanded state. Upon hydration of the hybrid lens, substantially little dimensional change, and thus substantially little distortion, is obtained in the skirt portion. The diluents of the present invention further preserve the mechanical integrity of the xerogel, allowing the xerogel to be machined to final shape, improving the dimensional tolerances which can be achieved in the hybrid lens.
Owner:SYNERGEYES
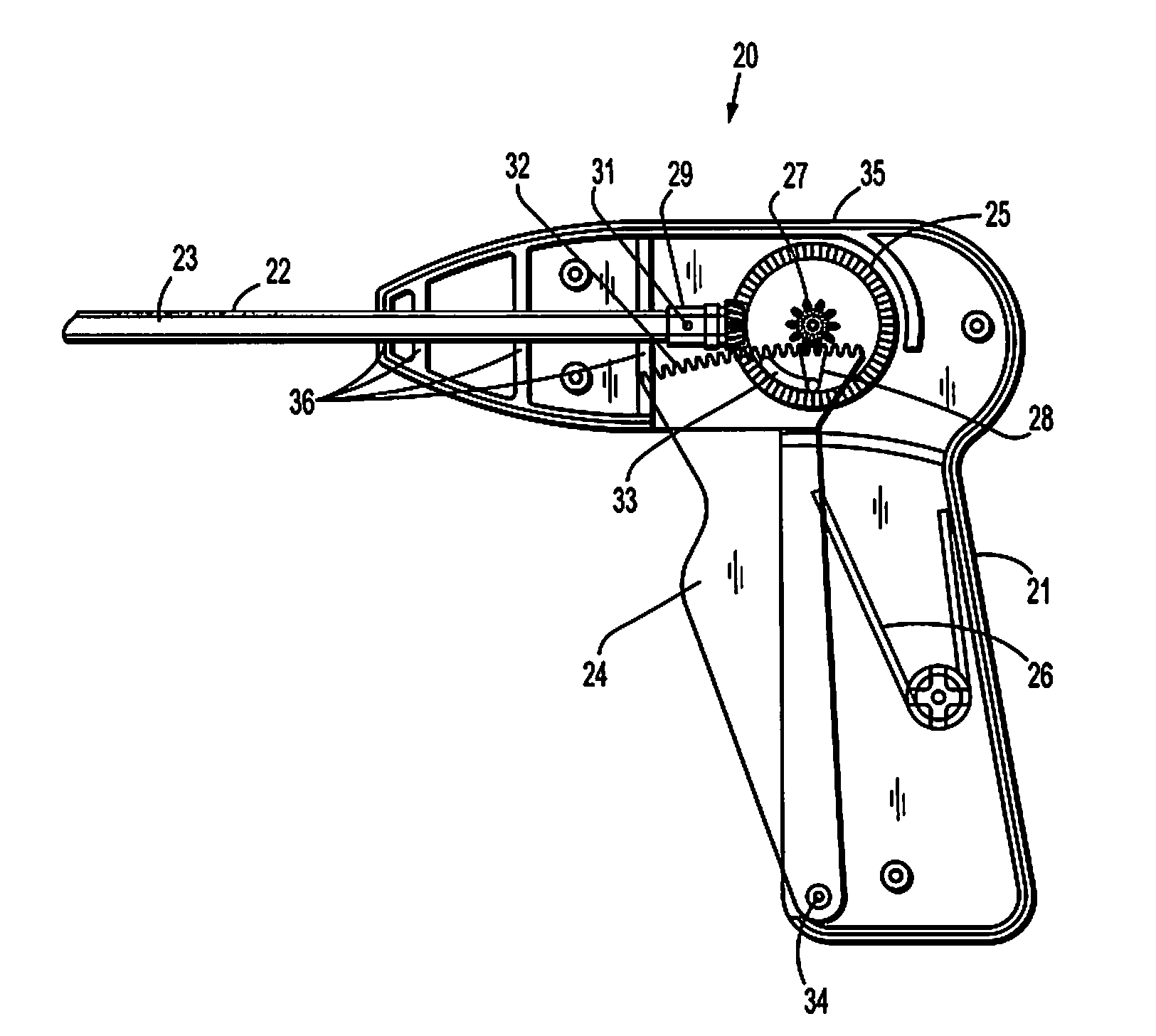
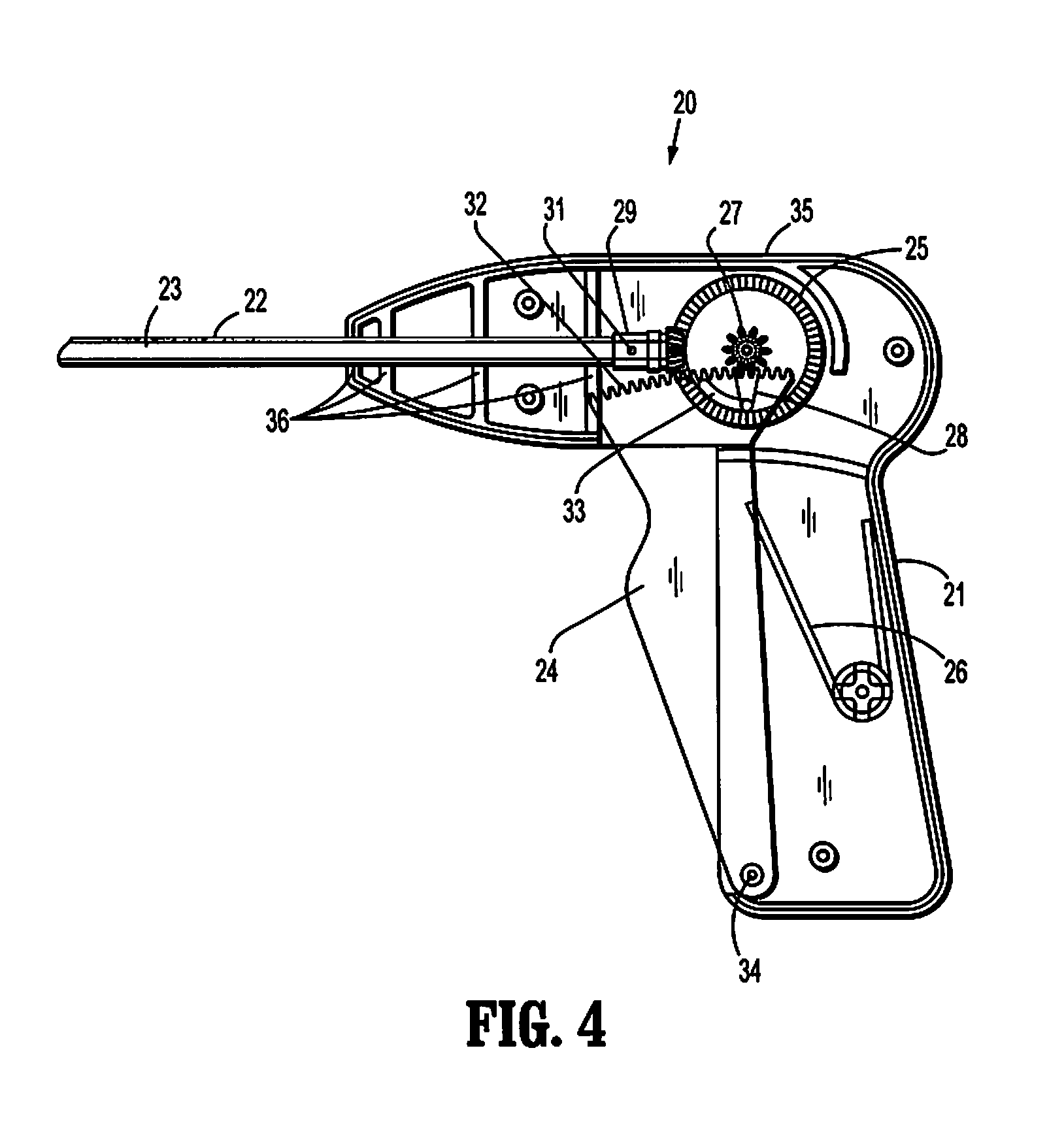
Absorbable fastener for hernia mesh fixation
A method of forming and deploying an improved absorbable fastener for hernia mesh fixation is disclosed. The absorbable fastener of the present invention functions to securely fasten tough, non macro-porous, and relative inelastic mesh to soft tissue. The fastener is formed from co-polymers of lactide and glycolide.
Owner:COVIDIEN LP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com