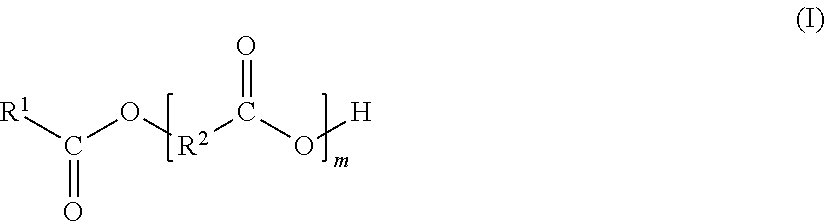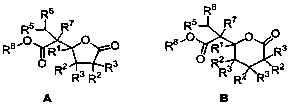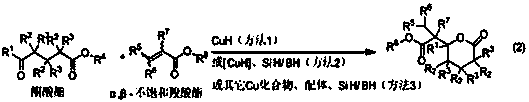Patents
Literature
286 results about "Valerolactone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
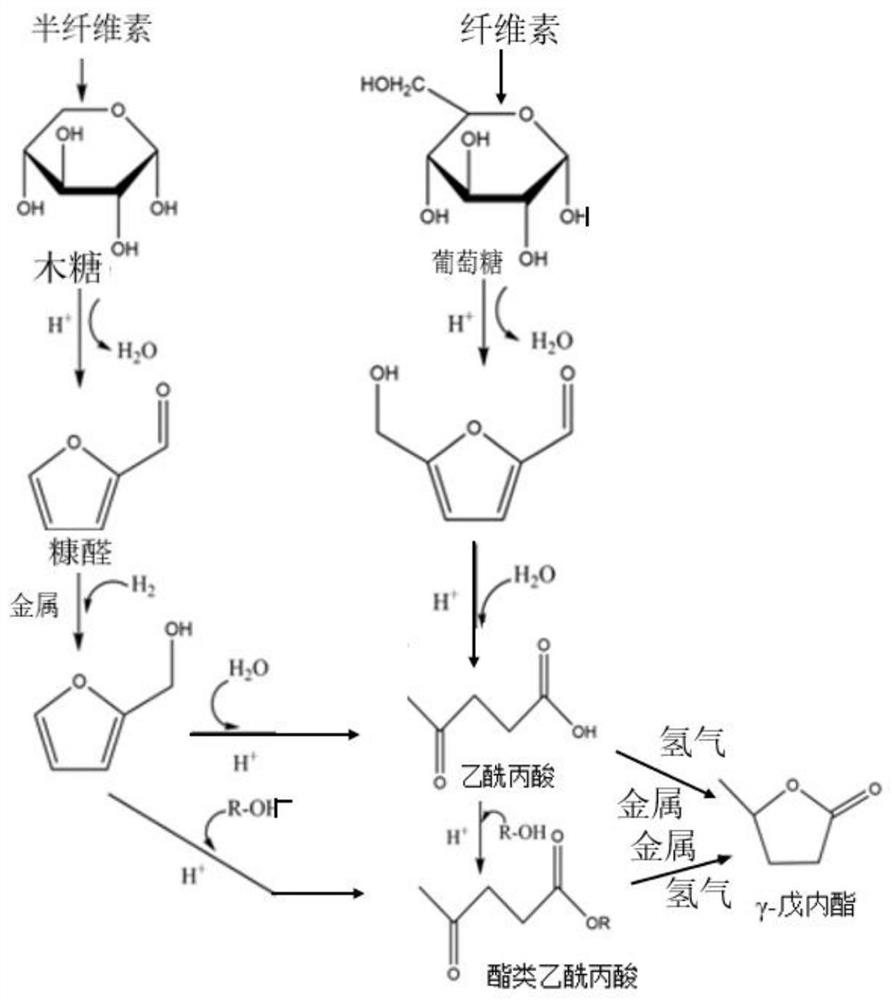
Valerolactone may refer to: delta-Valerolactone gamma-Valerolactone
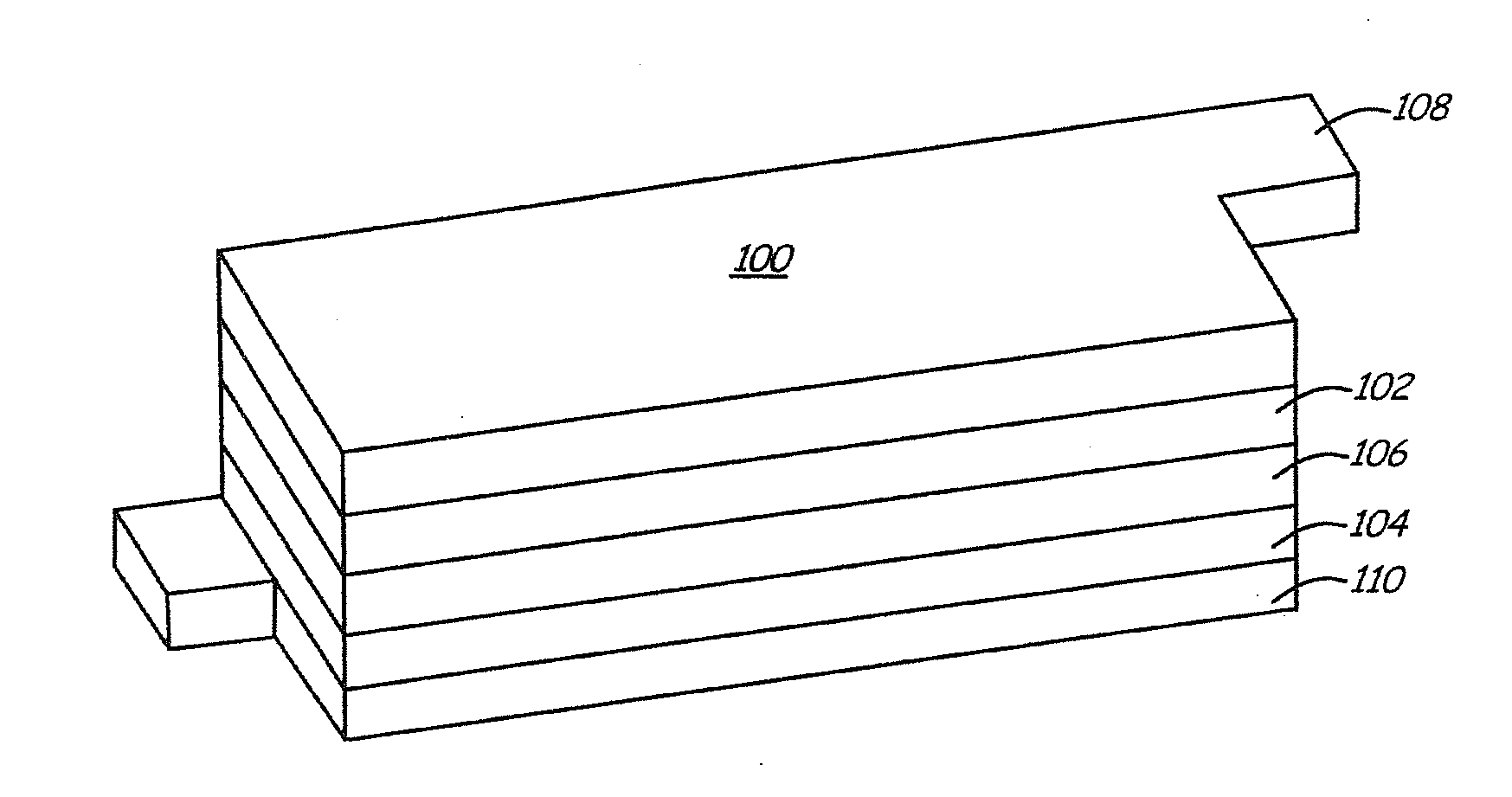
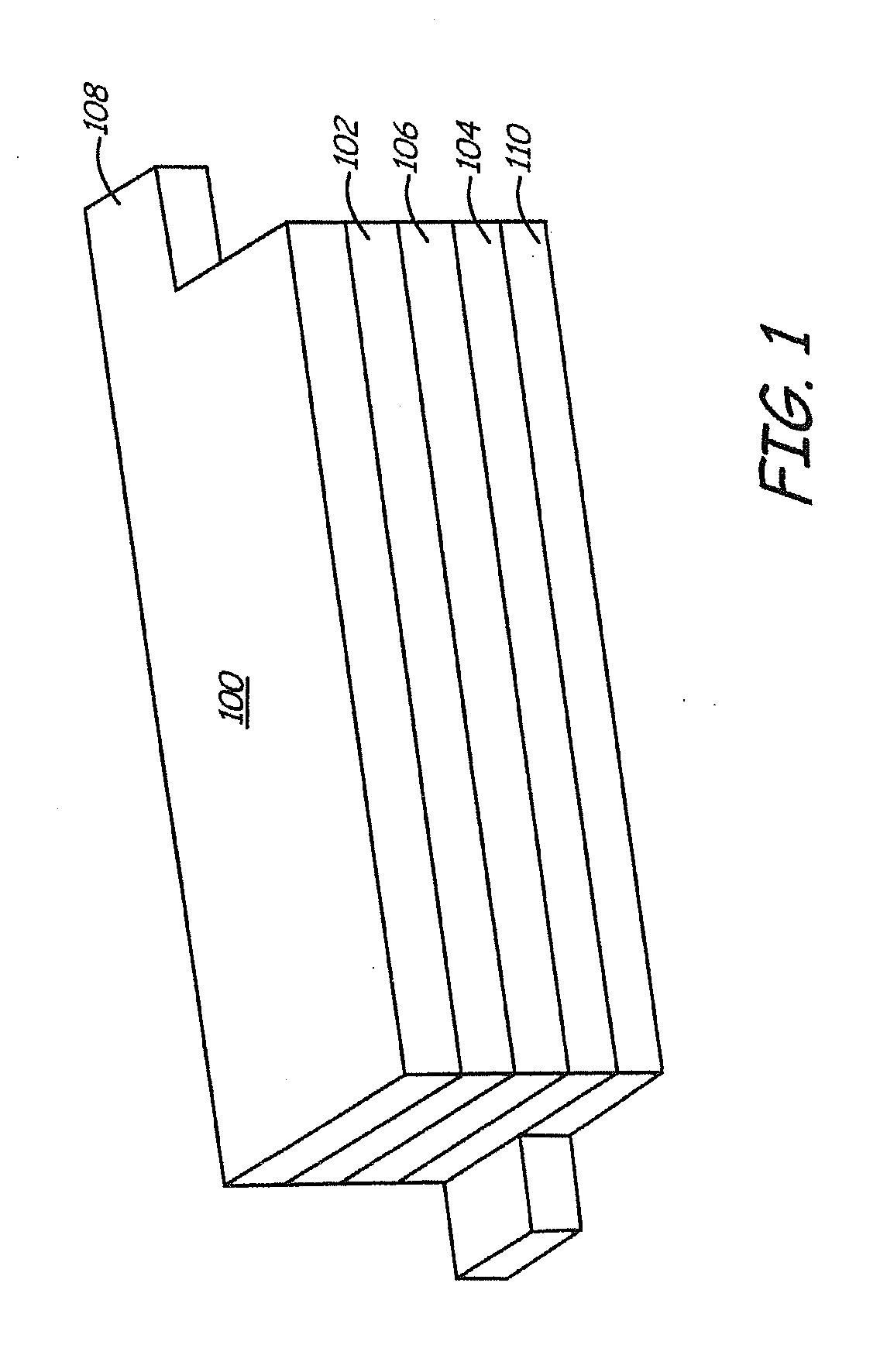
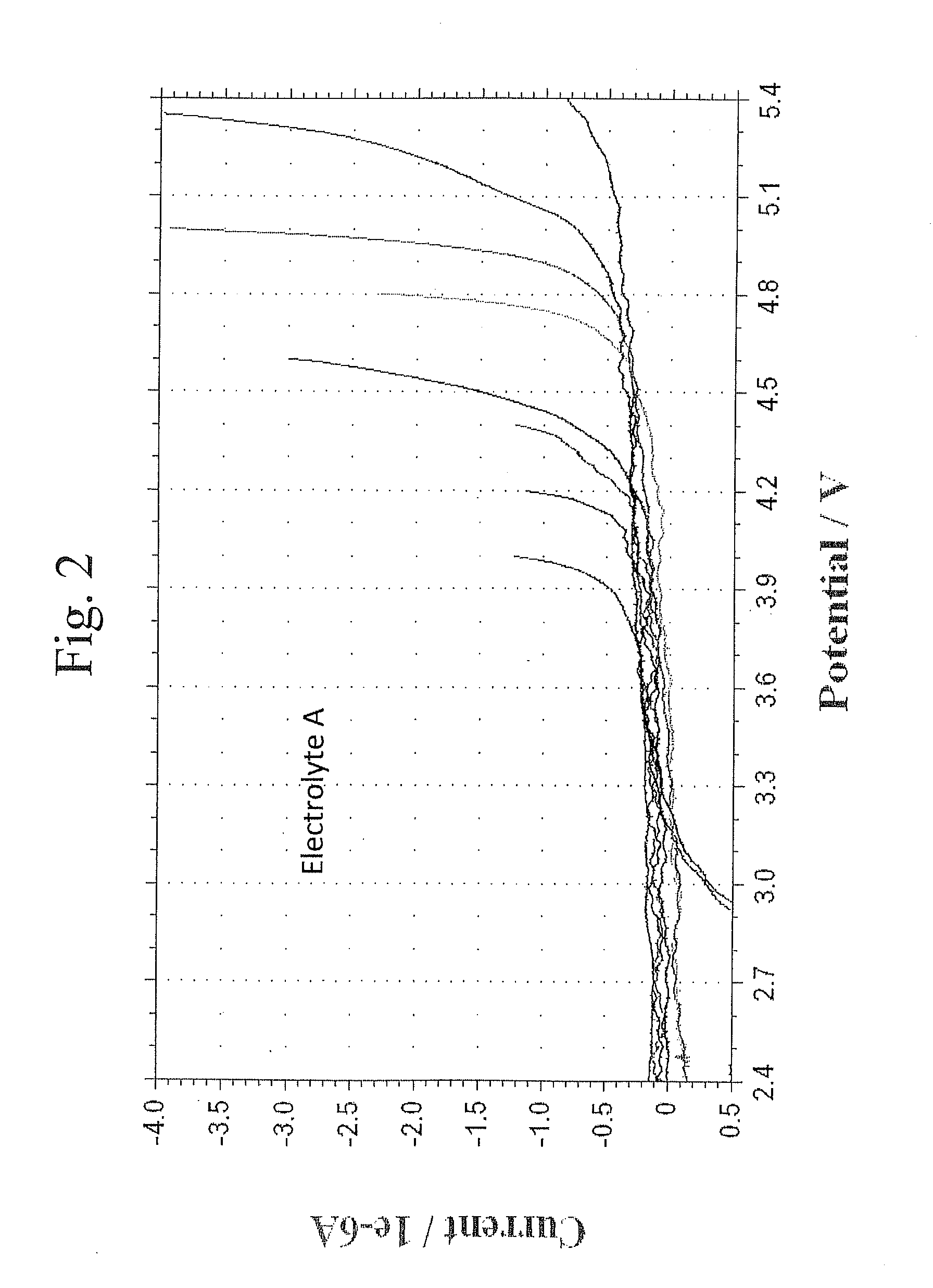
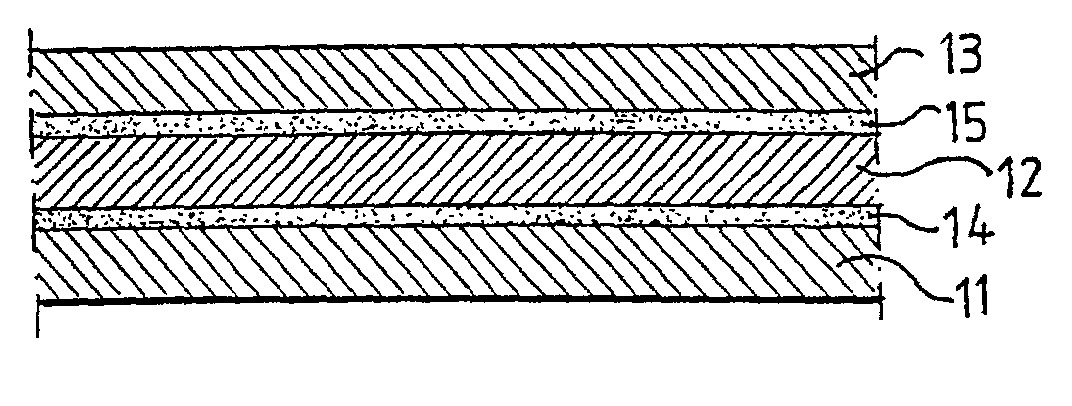
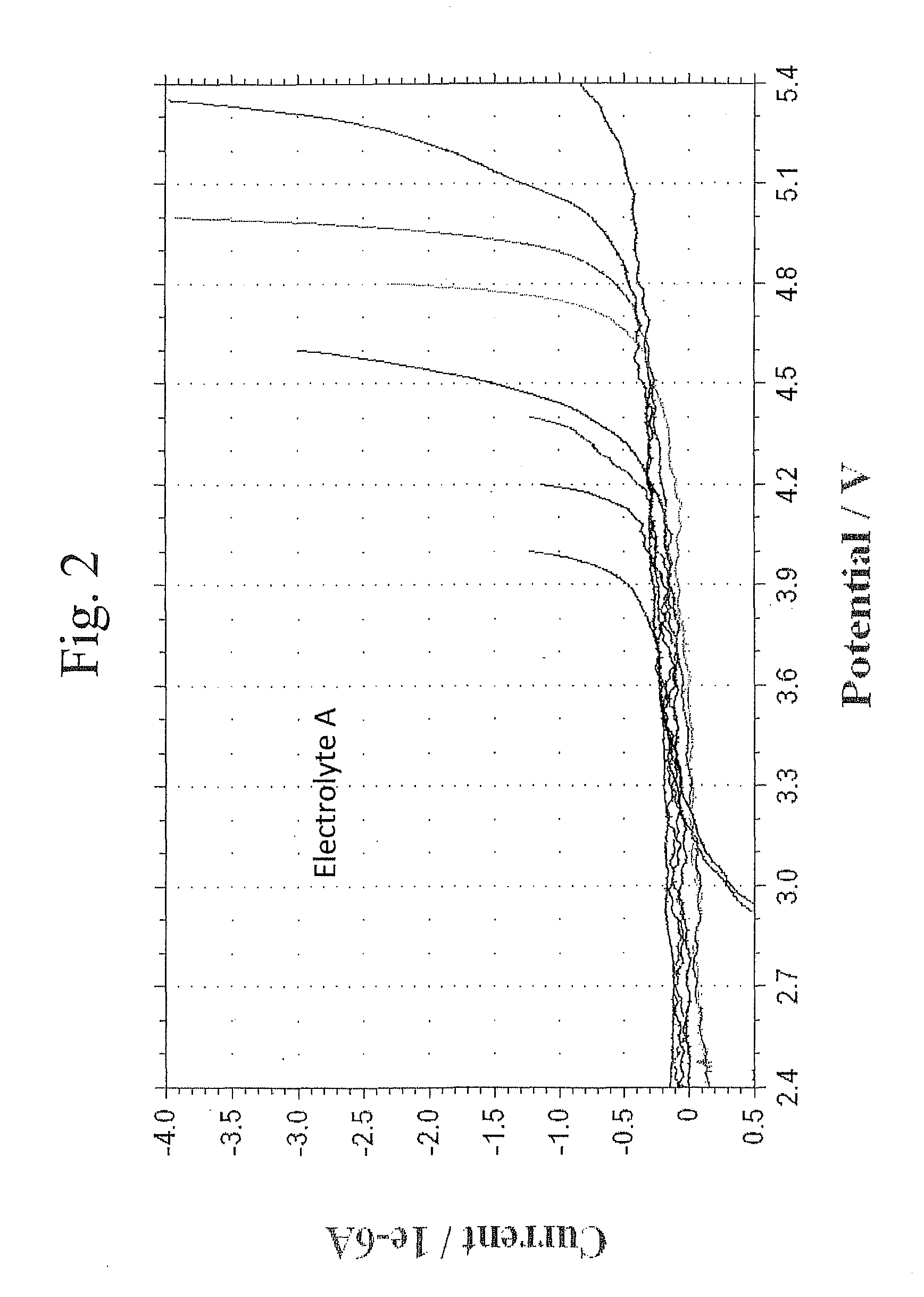
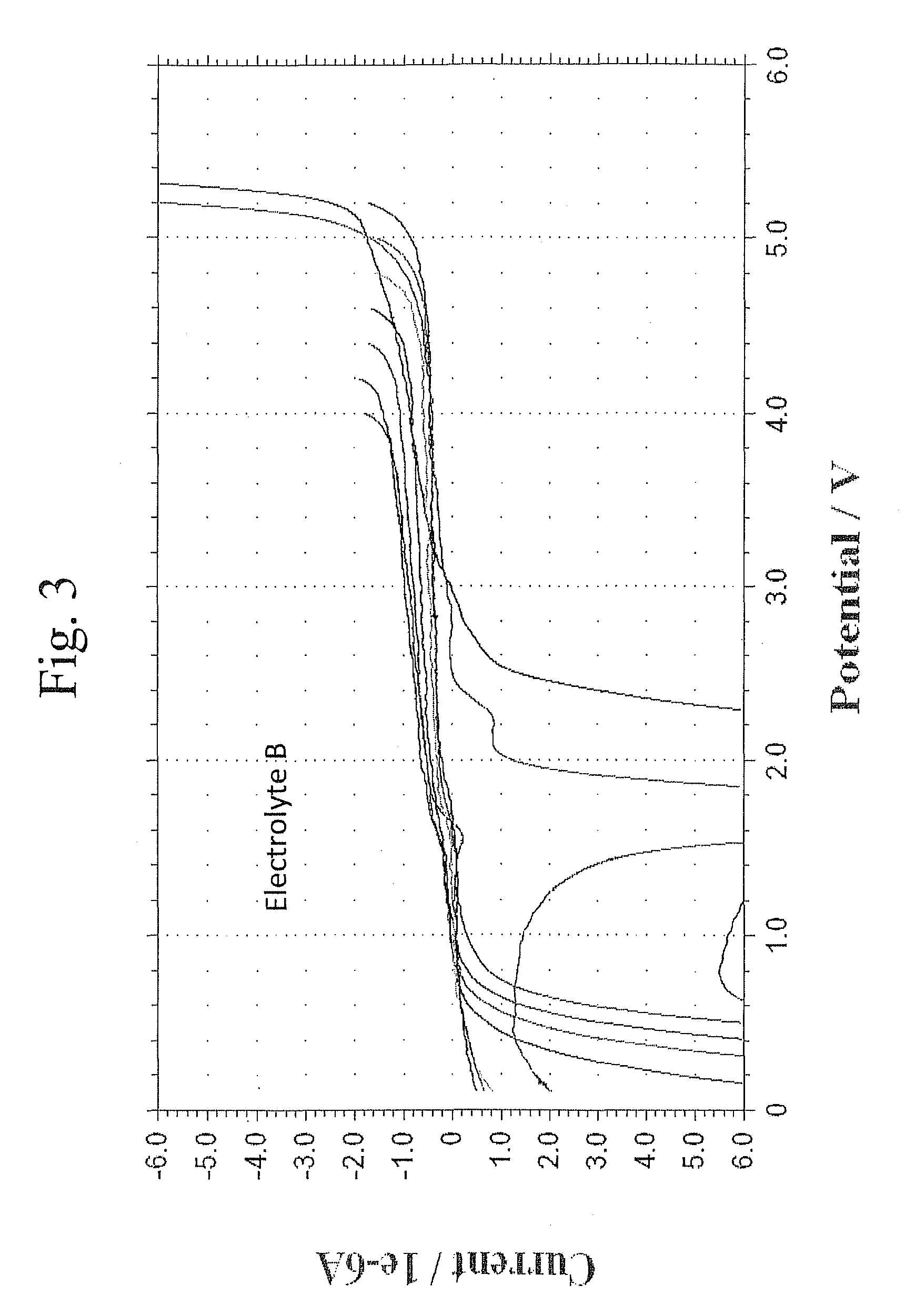
Lithium ion battery with high voltage electrolytes and additives
ActiveUS20110136019A1Final product manufactureElectrode carriers/collectorsMethyl carbonateHigh pressure
Desirable electrolyte compositions are described that are suitable for high voltage lithium ion batteries with a rated charge voltage at least about 4.45 volts. The electrolyte compositions can comprise ethylene carbonate and solvent composition selected from the group consisting of dimethyl carbonate, methyl ethyl carbonate, γ-butyrolactone, γ-valerolactone or a combination thereof. The electrolyte can further comprise a stabilization additive. The electrolytes can be effectively used with lithium rich positive electrode active materials.
Owner:IONBLOX INC
Biodegradable packaging laminate, a method of producing the packaging laminate, and packaging containers produced from the packaging laminate
InactiveUS20020127358A1Improve adhesionExcellent gas barrier performanceAdhesive processesLiquid surface applicatorsLactideBiopolymer
Packaging laminate for packages for liquid foods having excellent liquid and oxygen gas barrier properties in which all included layers are biodegradable. The packaging laminate includes at least one liquidtight layer (11, 13) of homo or copolymers of monomers selected from a group consisting of lactic acid, glycol acid, lactide, glycolide, hydroxy butyric acid, hydroxy valeric acid, hydroxy caproic acid, valerolactone, butyrolactone and caprolactone, as well as an oxygen gas barrier layer (12) of ethylene vinyl alcohol, polyvinyl alcohol, starch or starch derivatives. The oxygen gas barrier layer is preferably applied by a dispersion coating process. The layers may be laminated directly to one another or indirectly by means of interjacent adhesive layers. The packaging laminate may also include a core layer of, for example, paper or paperboard, or a biopolymer. The invention also realises a method of producing the biodegradable packaging laminate according to the invention.
Owner:TETRA LAVAL HLDG & FINANCE SA
Coating Employing an Anti-Thrombotic Conjugate
InactiveUS20090018646A1Few stepsImprove versatilityStentsOrganic active ingredientsActive agentSide chain
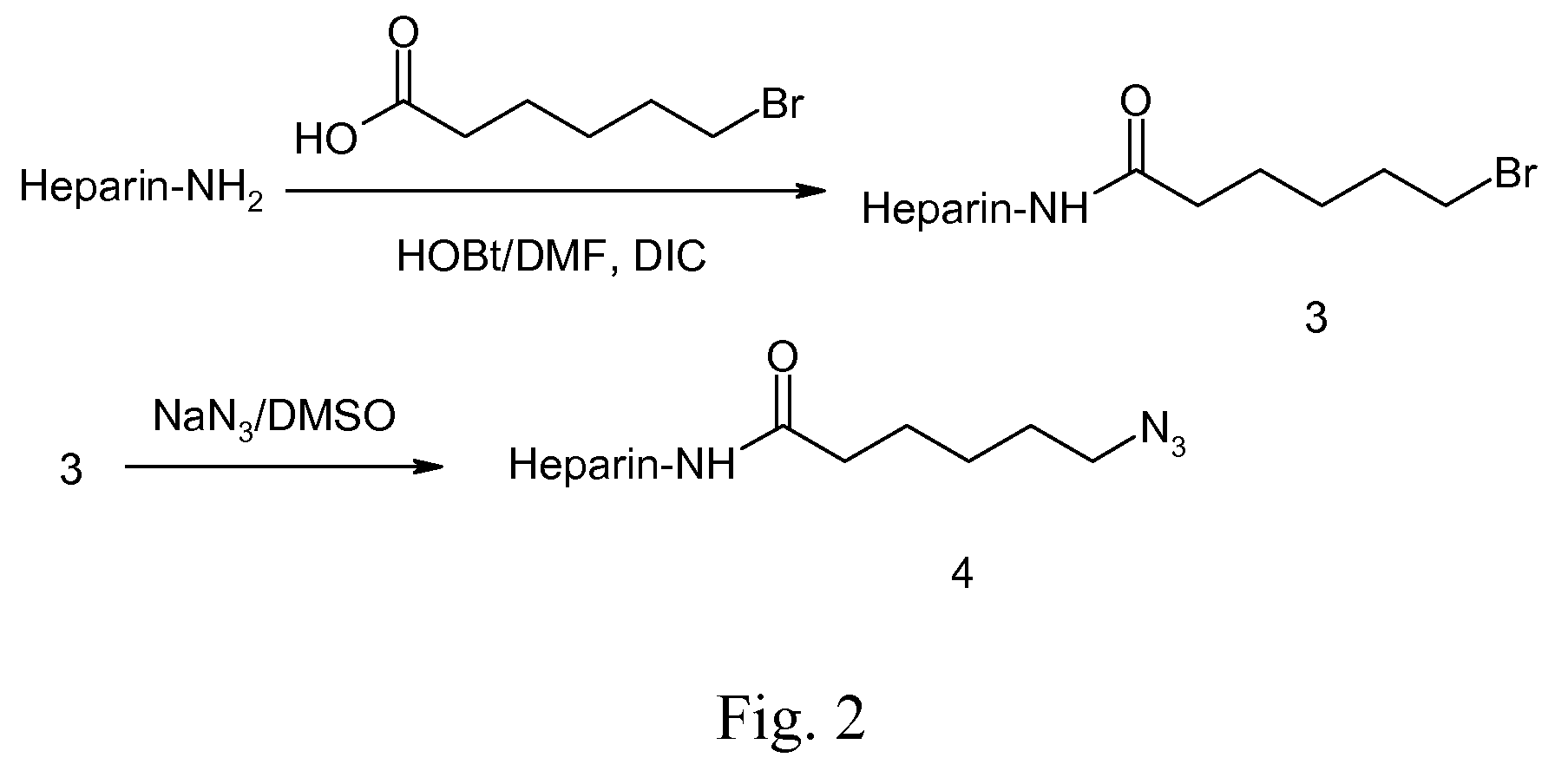
A biodegradable antithrombotic conjugate having heparin and other anti-thrombotic moieties are introduced as side chains to the polymer backbone modified by click chemistry. Various bioabsorbable monomers and dimers such as valerolactone may be used in the monomer derivation, homo- and co-polymerization, and the conjugation with a biologically active molecule by click chemistry. A coating comprising a biocompatible and bioabsorbable polymer anti-thrombotic conjugate is applied to at least a portion of an implantable device to prevent or reduce the formation of thrombosis on the surface of the implantable device. A first or sub-layer of the coating is prepared by mixing a polymeric material and a biologically active agent with a solvent, thereby forming a homogeneous solution. A second or outer layer comprising the present anti-thrombotic conjugate may be applied over the inner drug-containing layers using, for example, a dip coating or spray coating process.
Owner:CORDIS CORP
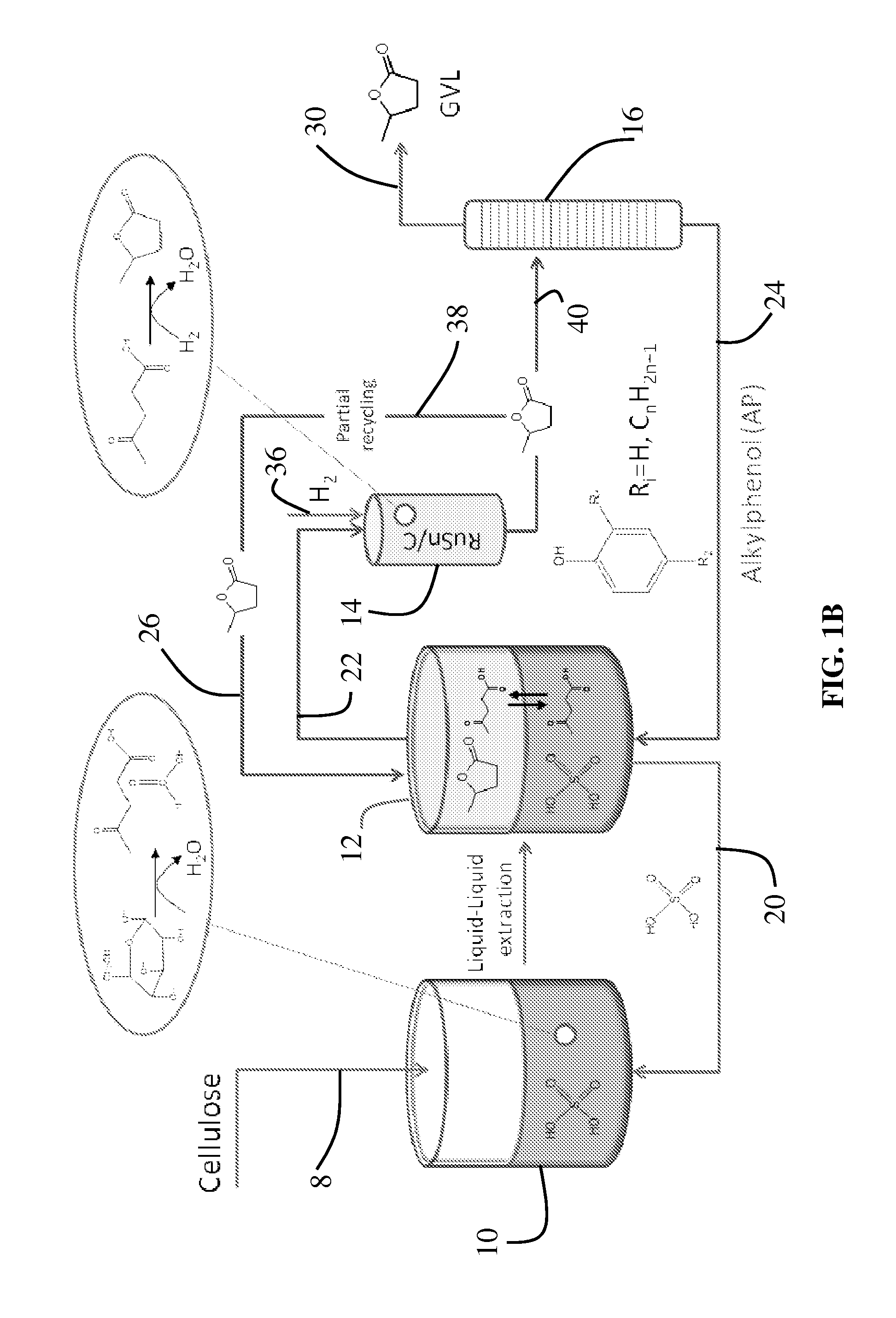
Method to produce, recover and convert furan derivatives from aqueous solutions using alkylphenol extraction
ActiveUS20120302765A1Cost-effectiveLow production costOrganic compound preparationCarboxylic acid esters preparationFuranAlkylphenol
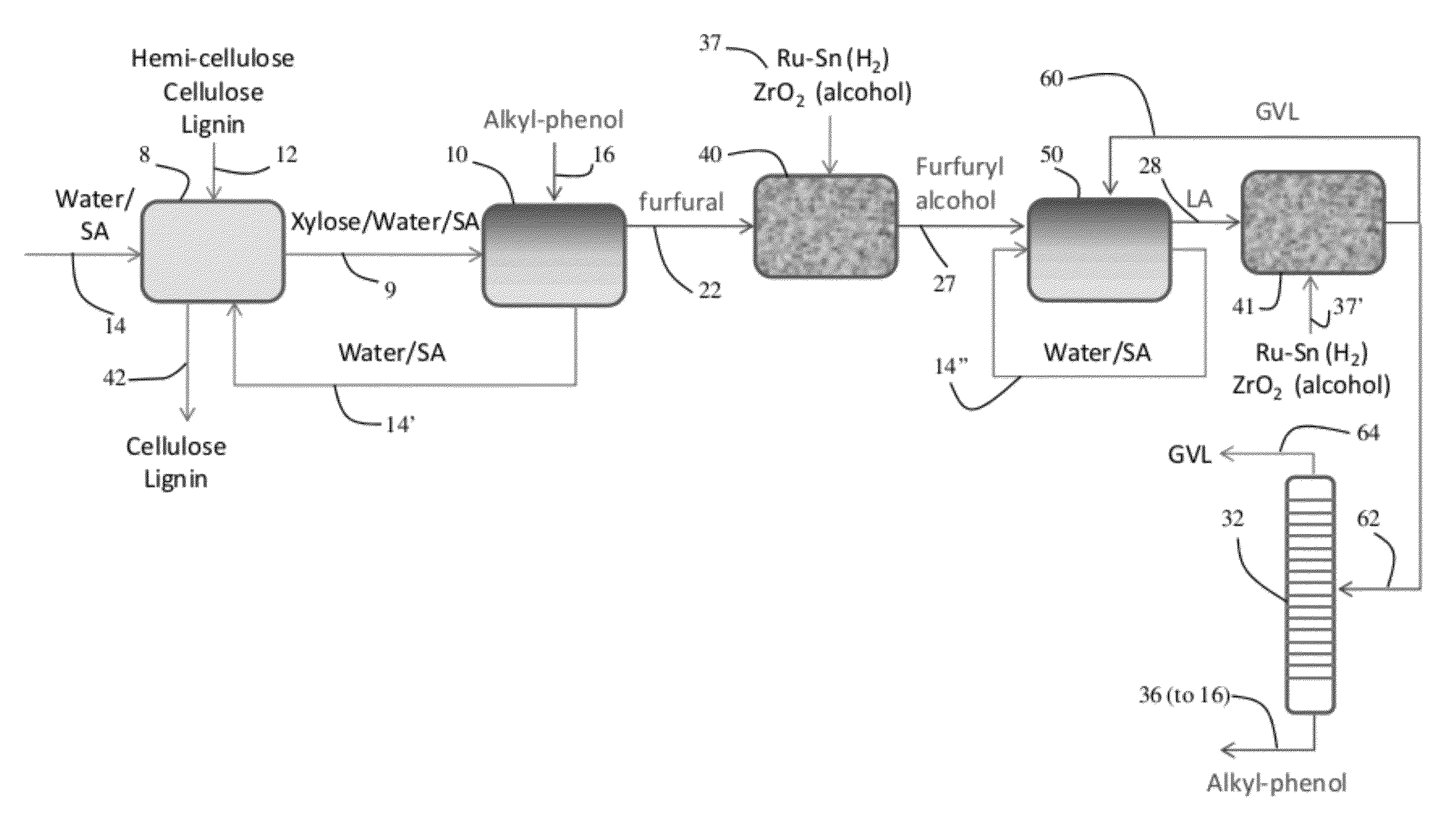
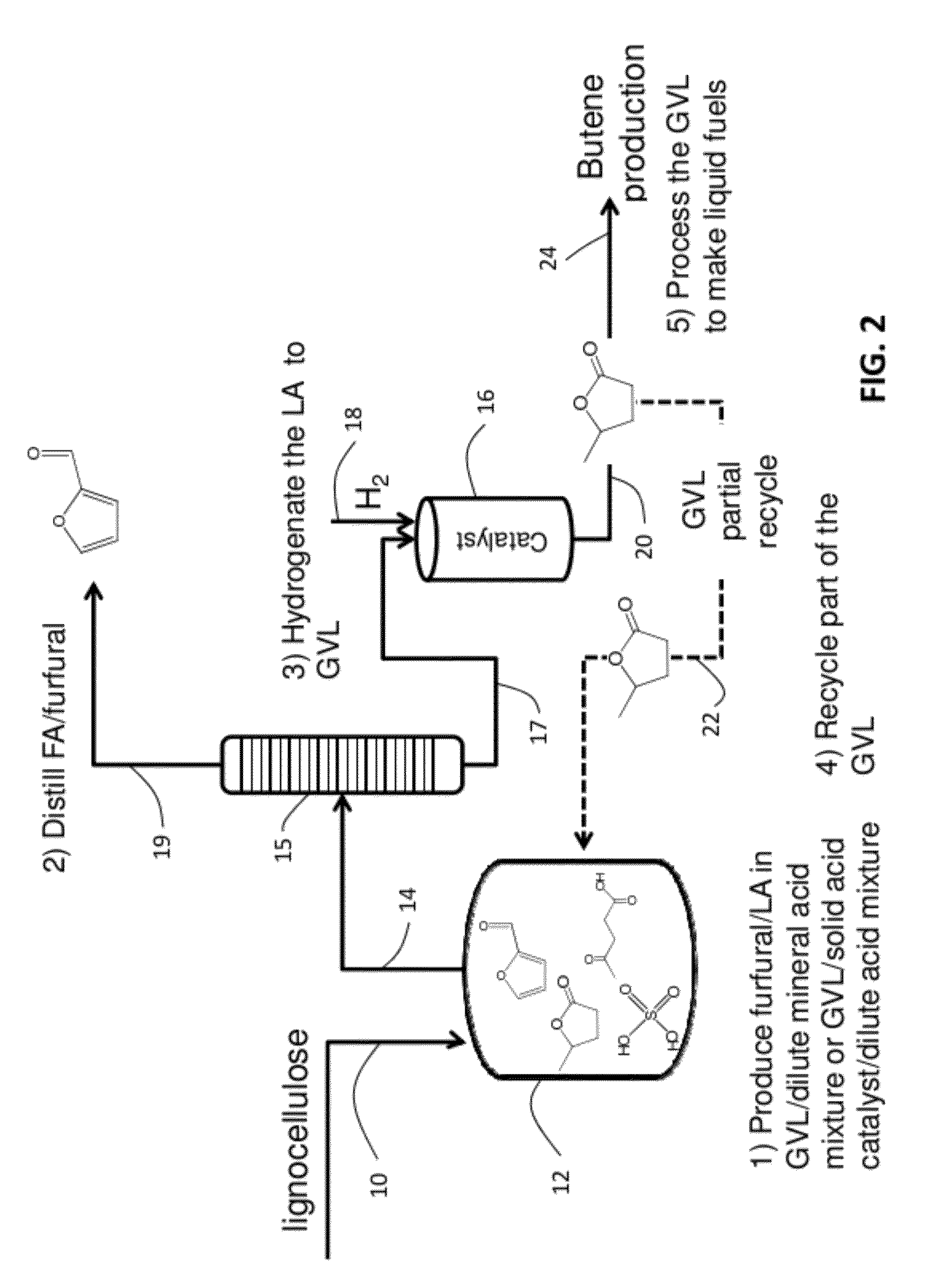
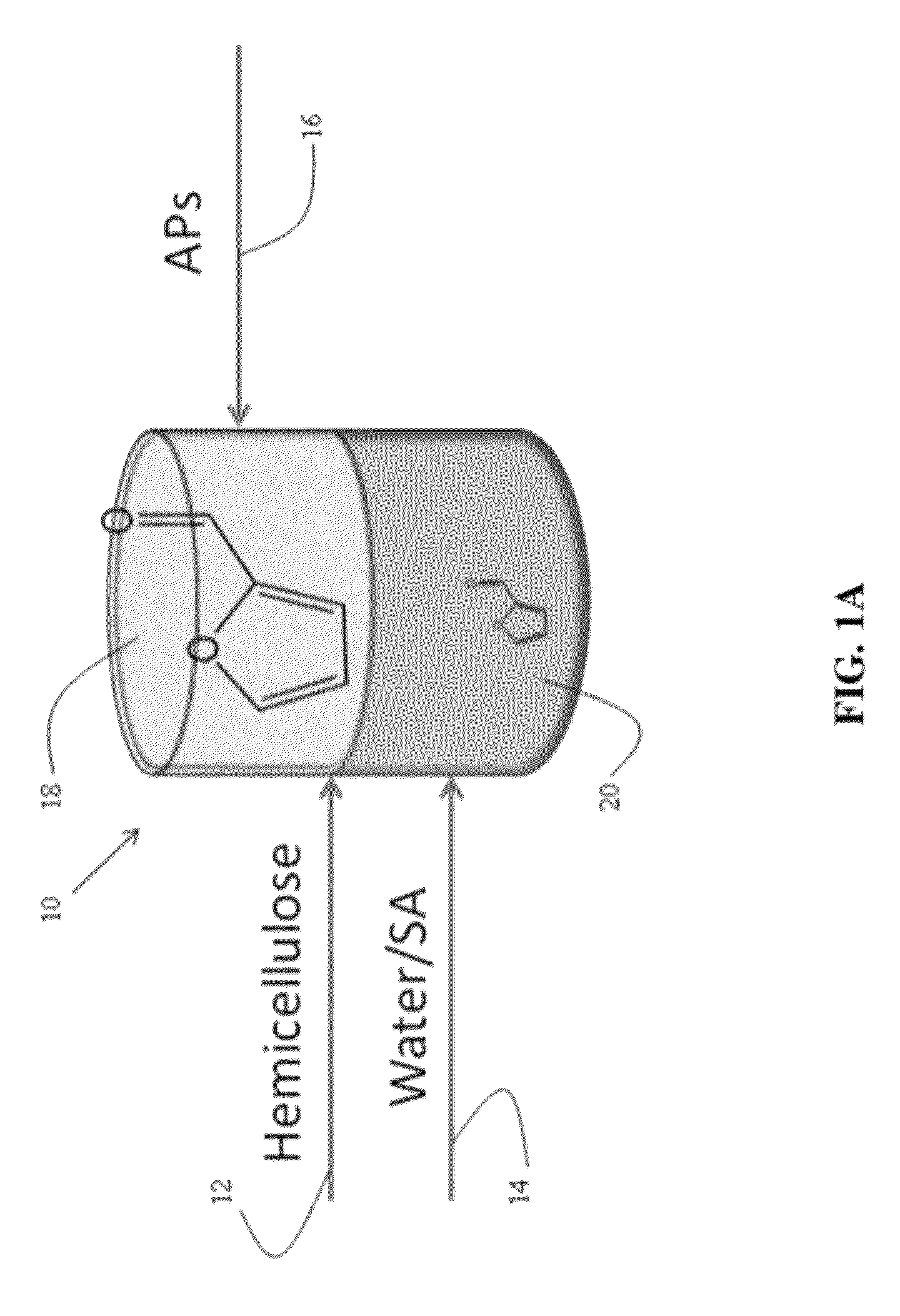
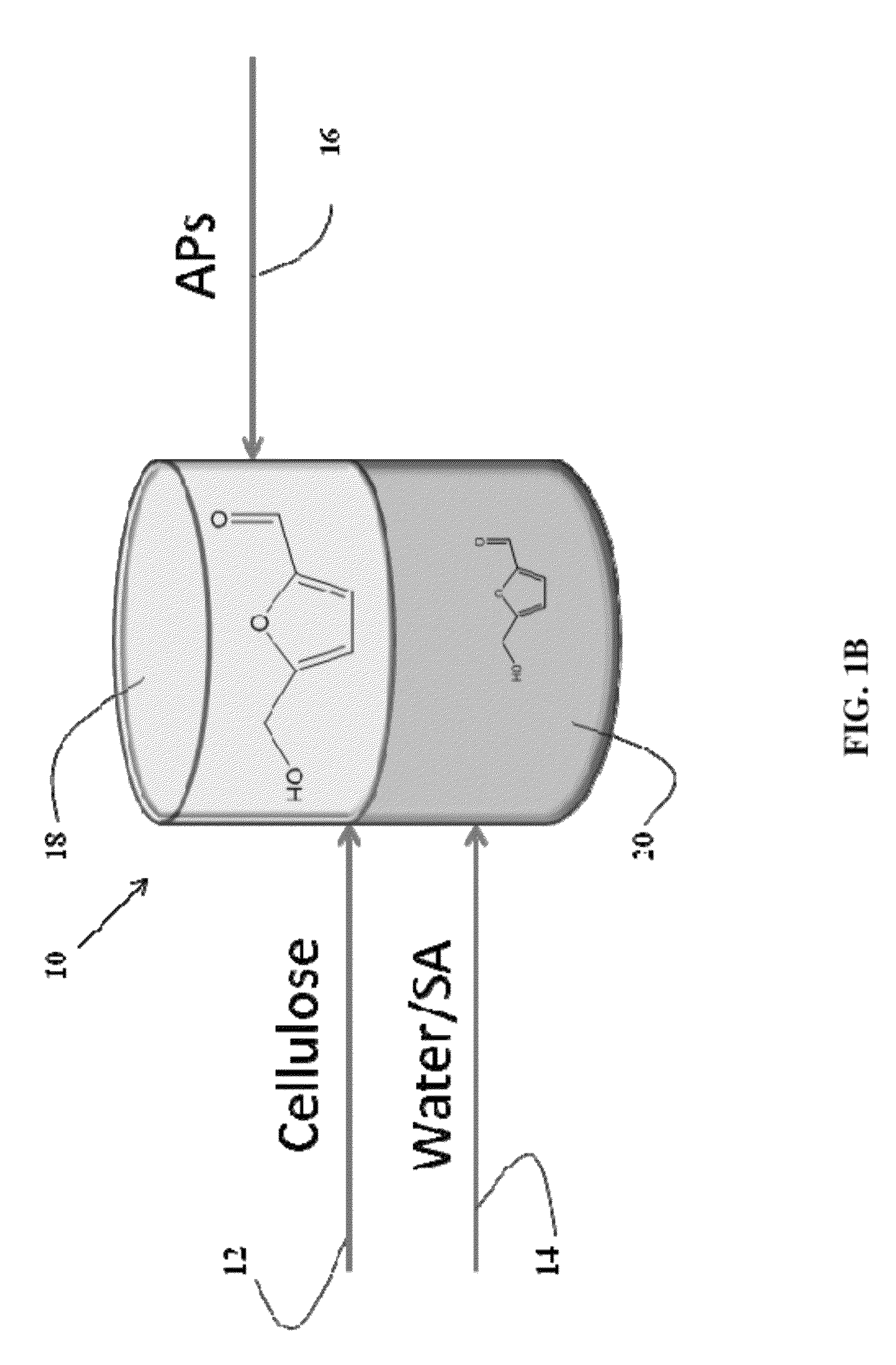
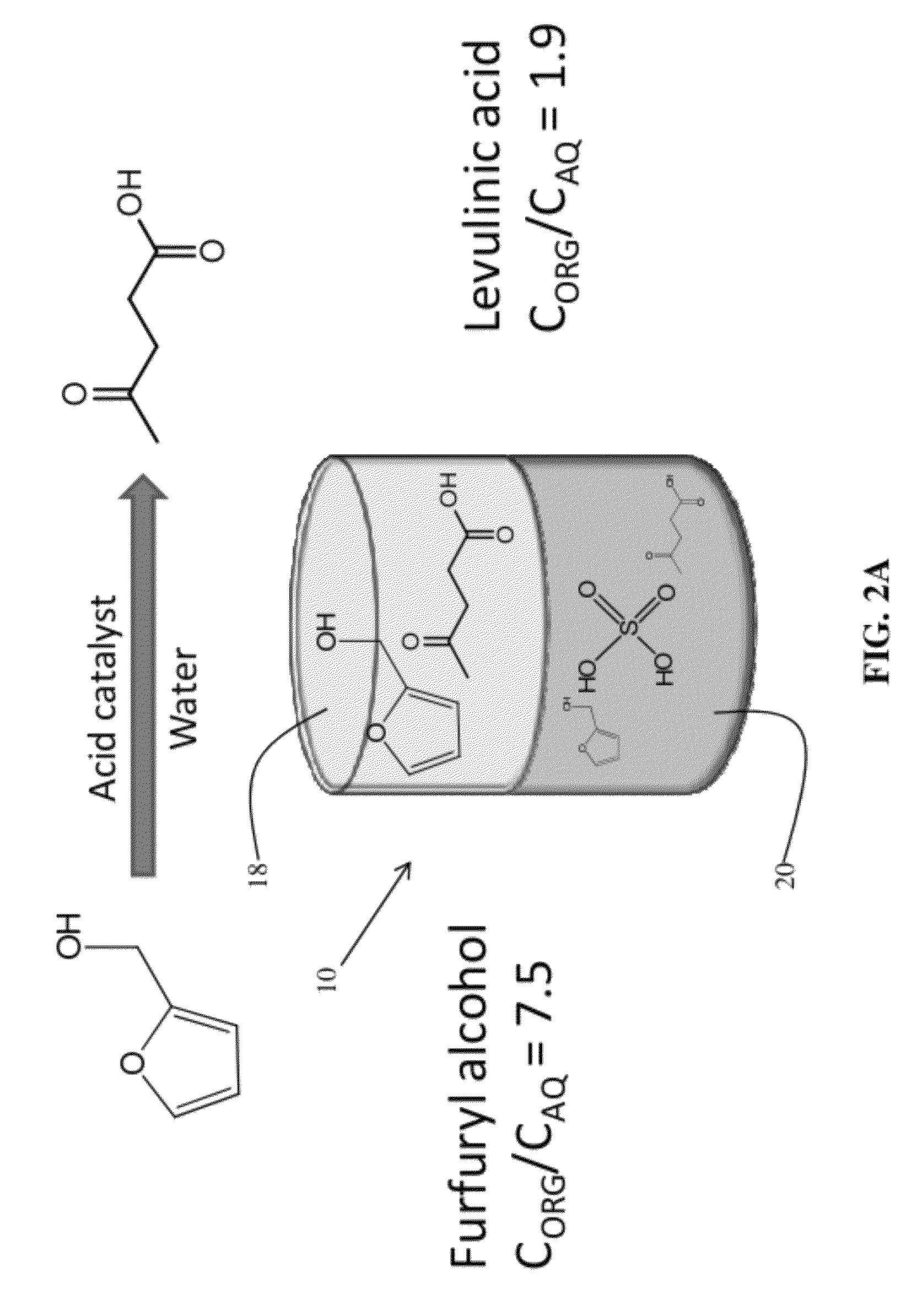
Described is a catalytic process for converting biomass to furan derivatives (e.g., furfural, furfuryl alcohol, etc.) using a biphasic reactor containing a reactive aqueous phase and an organic extracting phase containing an alkylphenol. The process provides a cost-effective route for producing furfural, furfuryl alcohol, levulinic acid hydroxymethylfurfural, γ-valerolactone, and the like. The products formed are useful as value-added intermediates to produce polymers, as precursors to diesel fuel, and as fuel additives.
Owner:WISCONSIN ALUMNI RES FOUND
PRODUCTION OF LEVULINIC ACID, FURFURAL, AND GAMMA VALEROLACTONE FROM C5 and C6 CARBOHYDRATES IN MONO- AND BIPHASIC SYSTEMS USING GAMMA- VALEROLACTONE AS A SOLVENT
ActiveUS20120302767A1Organic compound preparationCarbonyl compound preparationCellulosePropanoic acid
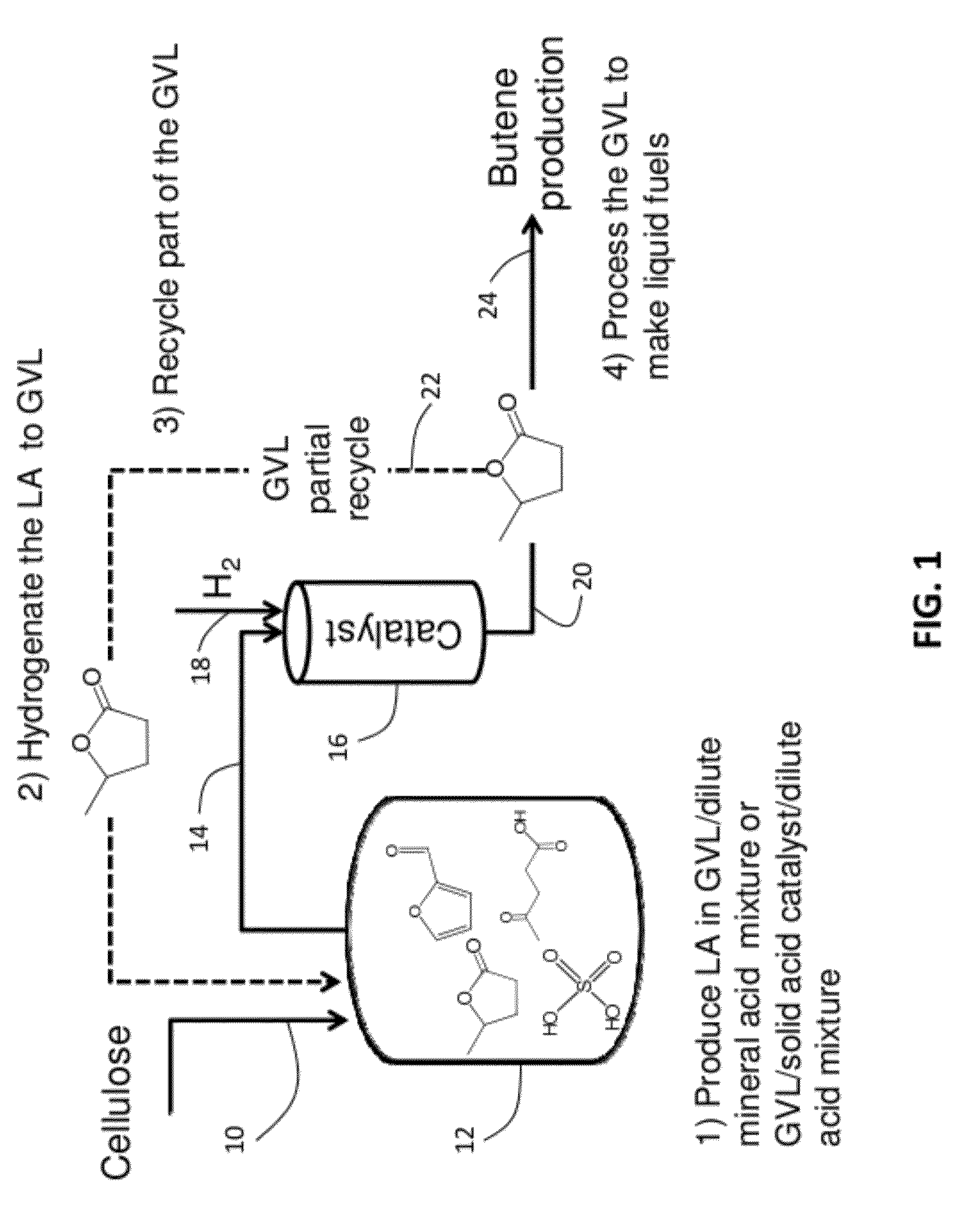
A method to make levulinic acid (LA), furfural, or gamma-valerolactone (GVL). React cellulose (and / or other C6 carbohydrates) or xylose (and / or other C5 carbohydrates) or combinations thereof in a monophasic reaction medium comprising GVL and an acid; or (ii) a biphasic reaction system comprising an organic layer comprising GVL, and a substantially immiscible aqueous layer. At least a portion of the cellulose (and / or other C6 carbohydrates), if present, is converted to LA and at least a portion of the xylose (and / or other C5 carbohydrates), if present, is converted into furfural.
Owner:WISCONSIN ALUMNI RES FOUND
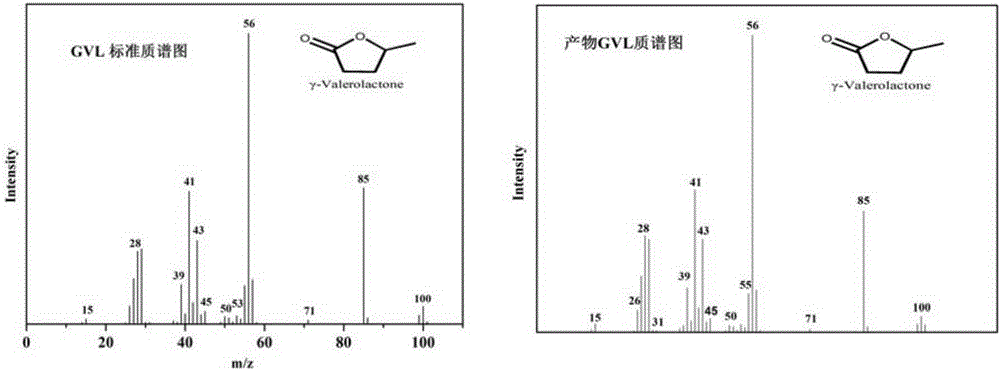
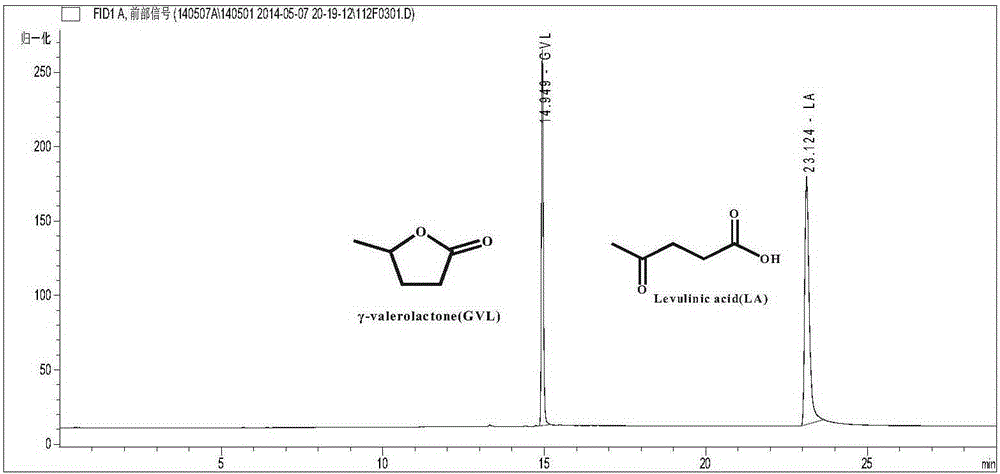
Method for preparing gamma-valerolactone by acetylpropionic acid catalytic hydrogenation
ActiveCN105289592AHigh activityGood reaction selectivityOrganic chemistryMolecular sieve catalystsOrganic acidPtru catalyst
The invention discloses a method for preparing gamma-valerolactone by acetylpropionic acid catalytic hydrogenation. Through high efficiency catalysis of levulinic acid hydrogenation based on a loaded ruthenium catalyst under mild conditions, gamma-valerolactone is prepared. The method has a levulinic acid conversion rate of 100% and gamma-valerolactone selectivity of 99.9%. The loaded ruthenium catalyst has a low active metal load capacity (less than 1.5w.t.%) and high activity (TOF, 7676h<1>), has good water and acid resistance and is suitable for an intermittent reactor and a continuous fixed bed reactor. The method solves the problem that the existing gamma-valerolactone preparation method needs a high temperature and high pressure and utilizes organic acids and bases and an organic solvent, improves preparation method economy and safety, utilizes a small amount of a catalyst, realizes catalyst recycle, has a high product yield and product separation easiness, and has a latent industrial application value.
Owner:SYNFUELS CHINA TECH CO LTD
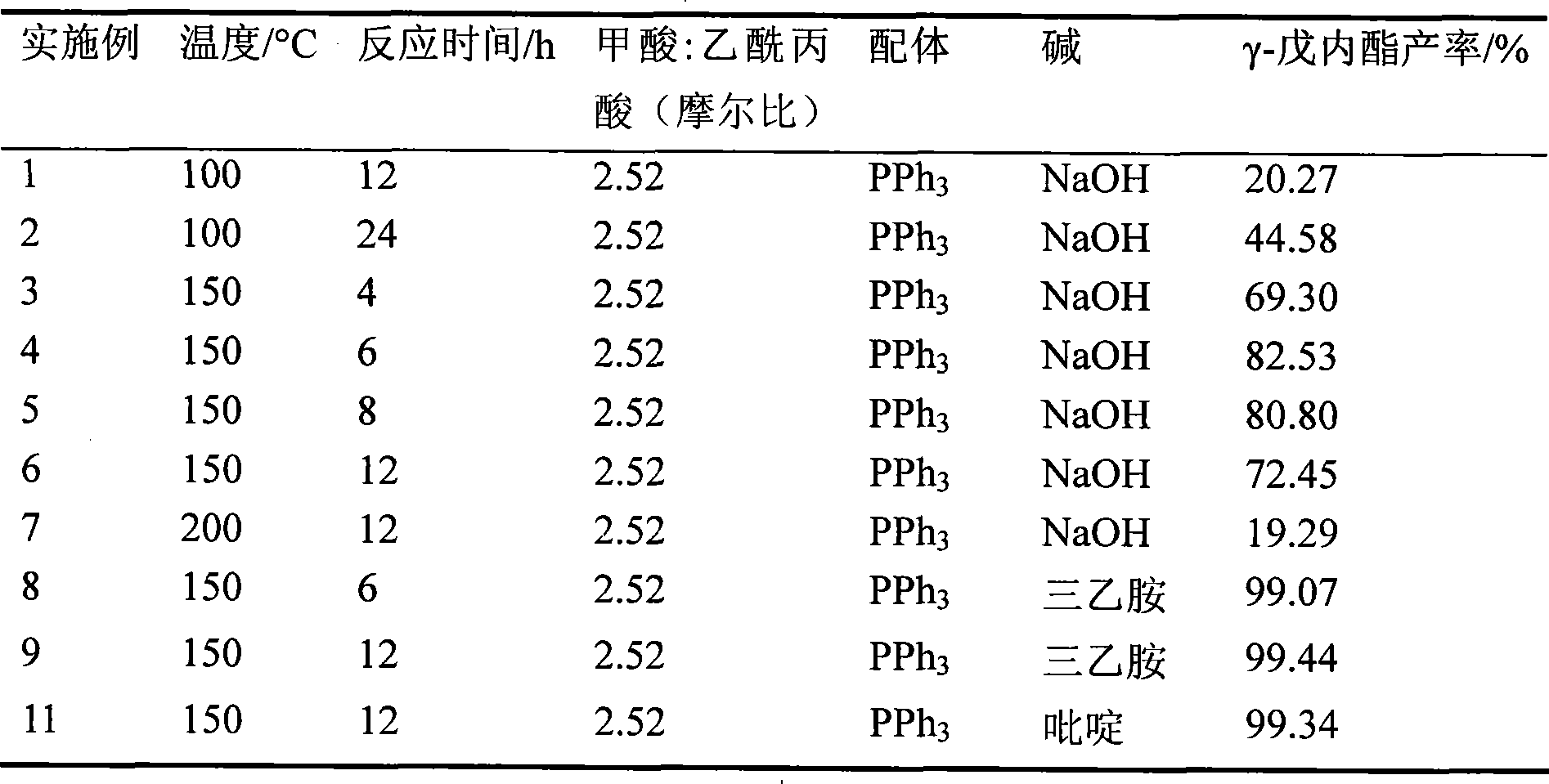
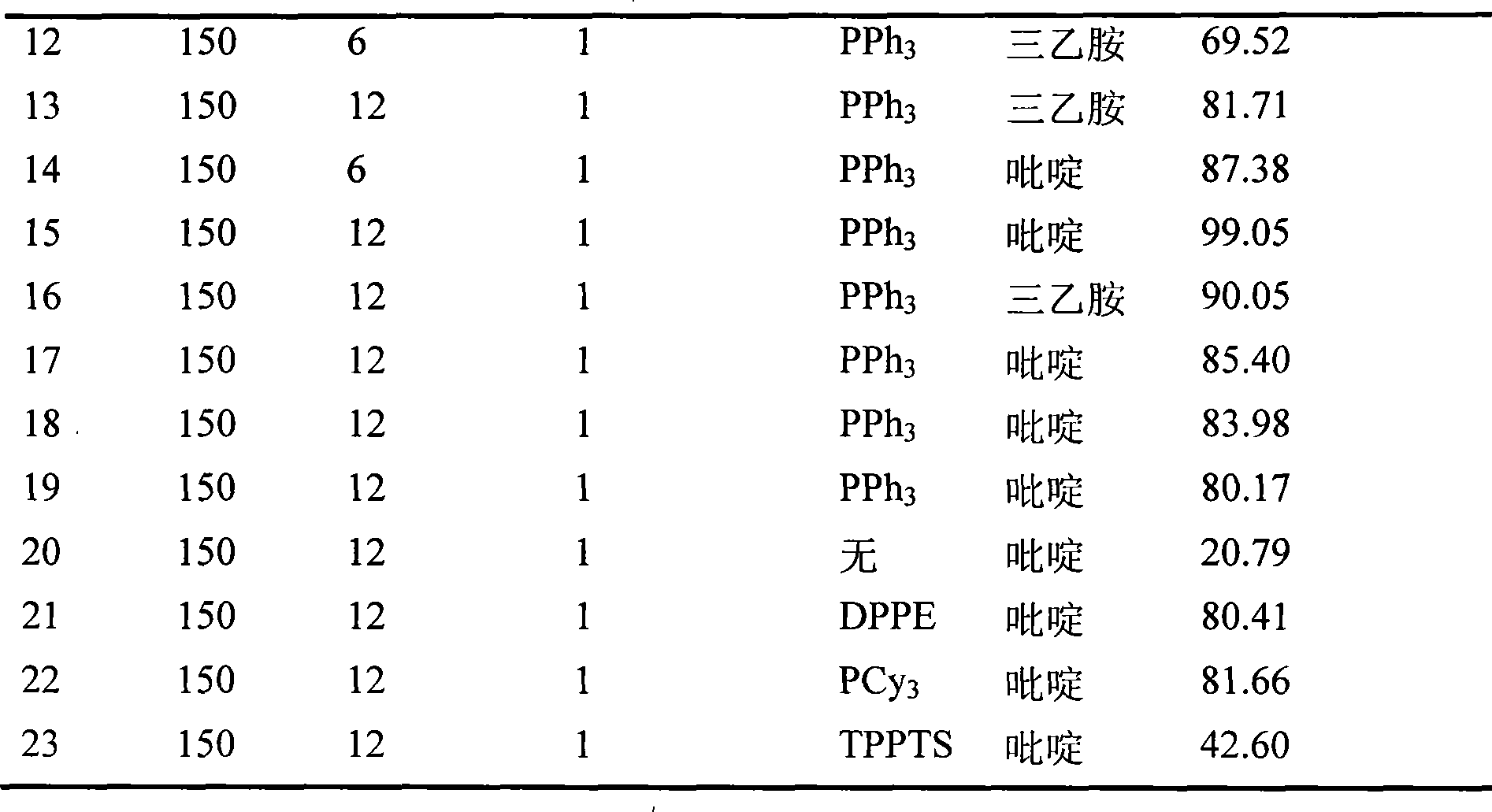
Method for directly preparing gamma-valerolactone from acetylpropionic acid and aminic acid
The invention discloses a method for preparing GAMMA-valerolactone from levulinic acid and formic acid, which is characterized in that the levulinic acid is in situ reduced at 100-200 DEG C in the presence of ruthenium catalyst and formic acid contained in a system as reducer, and distillation is carried out to obtain mixture containing the product GAMMA valerolactone and used catalyst; and mother liquor containing the used catalyst and raw material are mixed to realize circulation of the catalyst, thereby greatly improving environmental friendliness. The method avoids the energy source consumption during valerolactone purification process, avoids hydrogen source acquired from the system exterior, and improves the economy and security of a production system, with the advantages of simple operation process. The formic acid is decomposed to generate large amount of carbon dioxide besides hydrogen, which is convenient for collection and utilization.
Owner:UNIV OF SCI & TECH OF CHINA
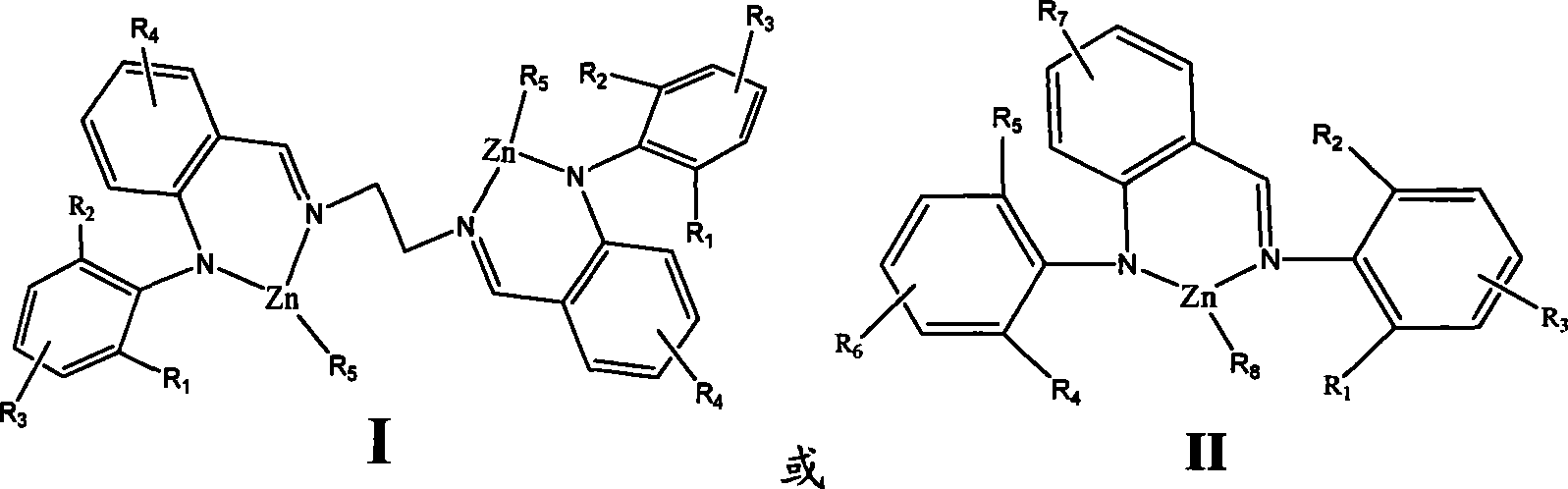
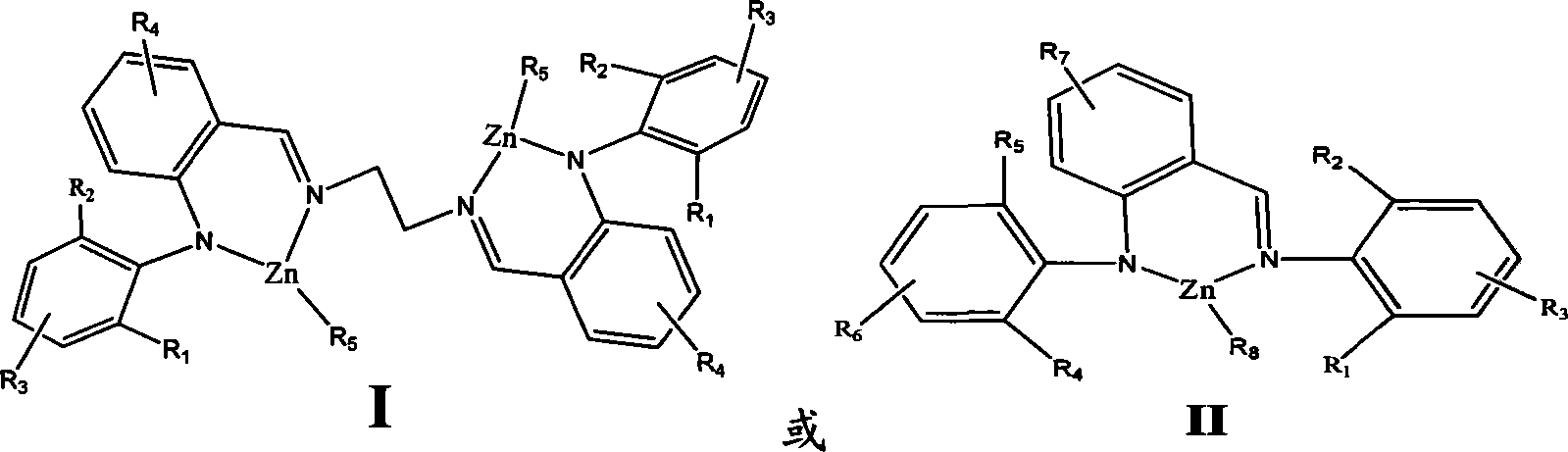
Amine imines zinc catalyst and preparation method and use thereof
The present invention relates to the of amine imine zinc catalyst and the preparation method as well as the application of the amine imine zinc catalyst, which belongs to the macromolecule technology field. The amine imine zinc catalyst is (2-2, 6-isopropyl benzeneimino methyl phenyl)-2. 6-diisopropyl amino ethyl benzene Zinc, (2-2,6-diisopropyl pheny benzeneimino methyl phenyl) ethylenediamine-diethyl zinc and so on. The amine imine zinc catalyst is used for catalysis lactones and virgule or lactide for homopolymerization and random as well as block polymerization; the cyclolactone compound is glycolide, lactide, beta-butyrolactone, beta- valerolactone or Epsilon -caprolactone. The catalyst of the present invention is of varied structure. Through the change of the substituent on the ligand and the central metal, the performance of the catalyst can be substantially controlled. The catalyst of the present invention is of good stability, high catalytic activity. The polymerization reaction can occur at a lower temperature to gat macromolecule polymer; through the control over the polymerization condition, the molecular weight of the polymer can be controlled.
Owner:JILIN UNIV
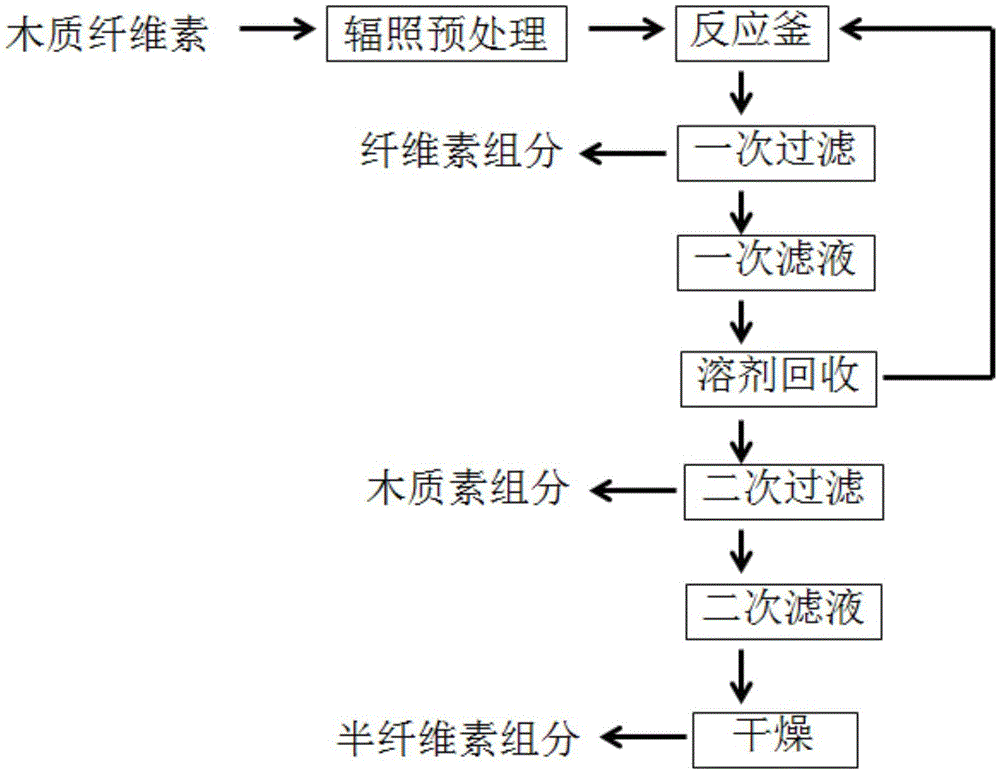
Green separating process for components of lignocellulose
ActiveCN105484083AEasy to operateReduce energy consumptionPaper material treatmentAlcoholOrganic solvent
The invention provides a green separating process for components of lignocellulose, which belongs to the fields of high-value utilization of biomass resources and biochemical industry. According to the process, biomass is pretreated with irradiation and then coupled with low-boiling-point tetrahydrofuran or high-boiling-point gamma-valerolactone for a reaction, and primary filtering is carried out so as to obtain a cellulose component; tetrahydrofuran is recovered from obtained filtrate or a saturated NaCl solution is added into the filtrate for phase-split precipitation of lignin; and secondary filtering is carried out, the obtained residue is a lignin component, and secondary filtrate is dried so as to obtain a hemicellulose component. The process has the advantages that no organic solvent like acid, alkali and alcohol is needed for separation of the components of lignocellulose and the process is simple, highly efficient and clean.
Owner:BEIJING UNIV OF CHEM TECH
Production of levulinic acid, furfural, and gamma valerolactone from C5 and C6 carbohydrates in mono- and biphasic systems using gamma-valerolactone as a solvent
A method to make levulinic acid (LA), furfural, or gamma-valerolactone (GVL). React cellulose (and / or other C6 carbohydrates) or xylose (and / or other C5 carbohydrates) or combinations thereof in a monophasic reaction medium comprising GVL and an acid; or (ii) a biphasic reaction system comprising an organic layer comprising GVL, and a substantially immiscible aqueous layer. At least a portion of the cellulose (and / or other C6 carbohydrates), if present, is converted to LA and at least a portion of the xylose (and / or other C5 carbohydrates), if present, is converted into furfural.
Owner:WISCONSIN ALUMNI RES FOUND
Method to produce, recover and convert furan derivatives from aqueous solutions using alkylphenol extraction
ActiveUS8389749B2Cost-effectiveLow production costOrganic chemistryChemical recyclingFuranAlkylphenol
Described is a catalytic process for converting biomass to furan derivatives (e.g., furfural, furfuryl alcohol, etc.) using a biphasic reactor containing a reactive aqueous phase and an organic extracting phase containing an alkylphenol. The process provides a cost-effective route for producing furfural, furfuryl alcohol, levulinic acid hydroxymethylfurfural, γ-valerolactone, and the like. The products formed are useful as value-added intermediates to produce polymers, as precursors to diesel fuel, and as fuel additives.
Owner:WISCONSIN ALUMNI RES FOUND
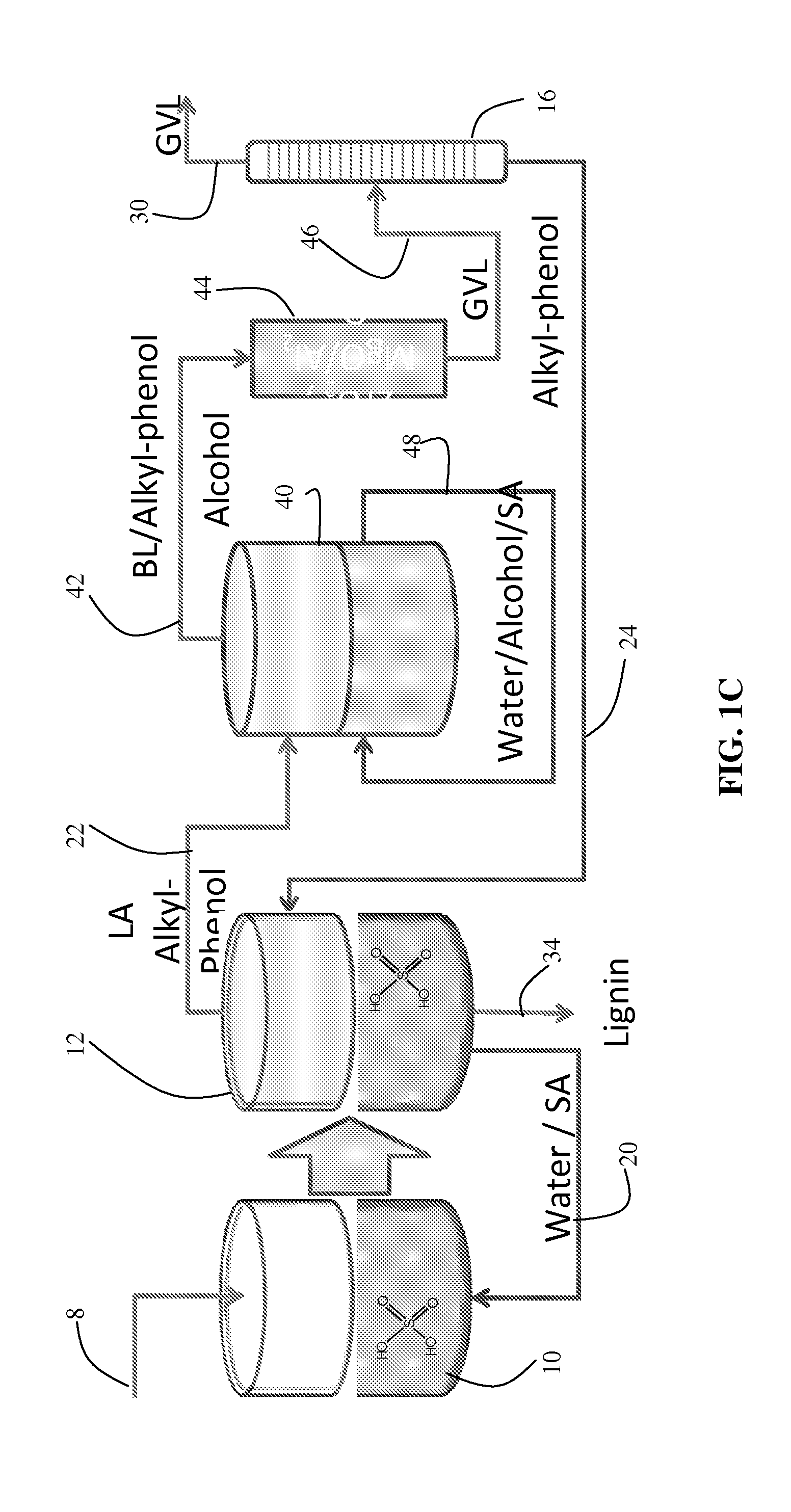
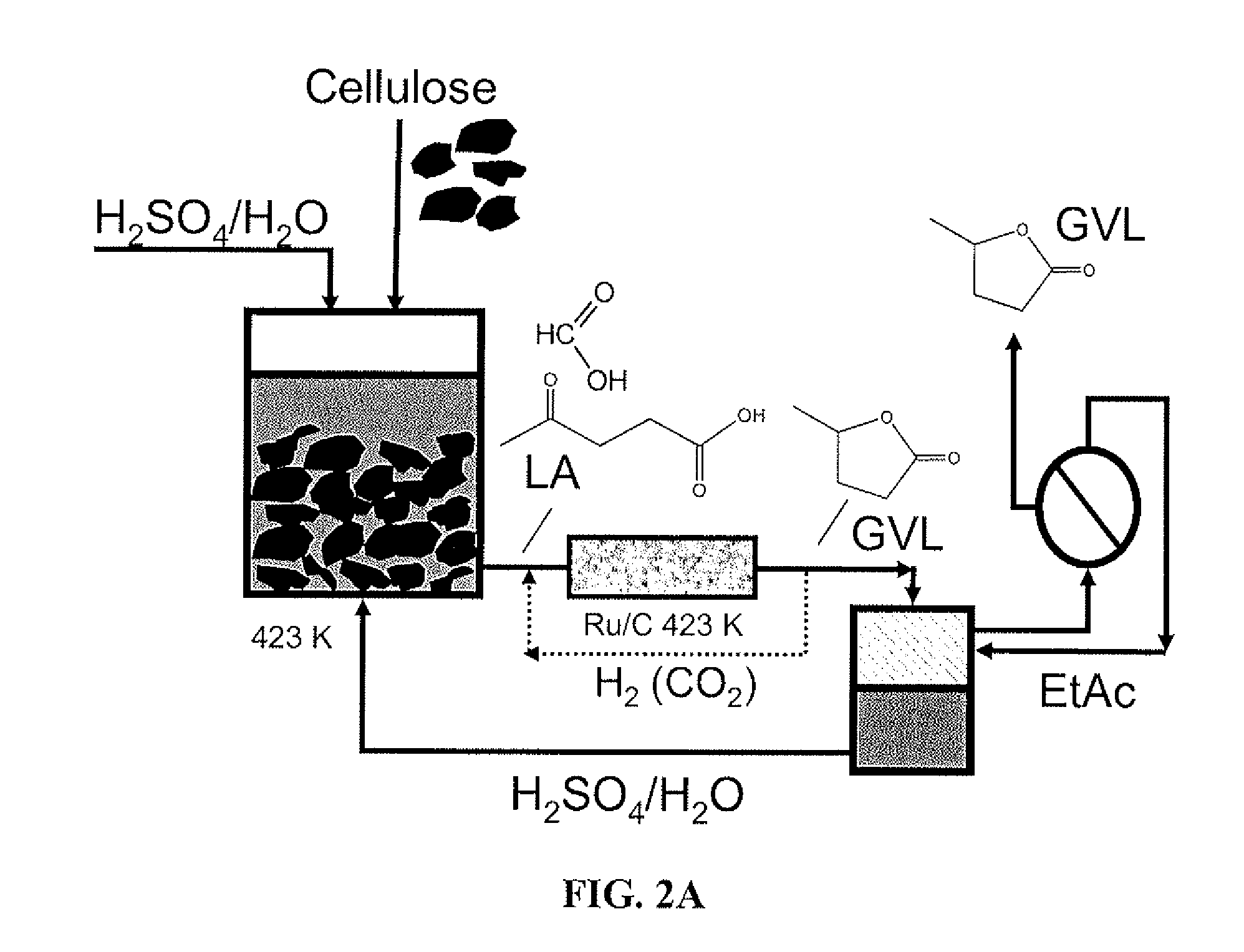
Method to produce and recover levulinic acid and/or gamma-valerolactone from aqueous solutions using alkylphenols
ActiveUS20120302764A1Enhancing GVL concentrationEasy and cost-efficient recoveryOrganic compound preparationCarboxylic acid esters preparationCellulosePropanoic acid
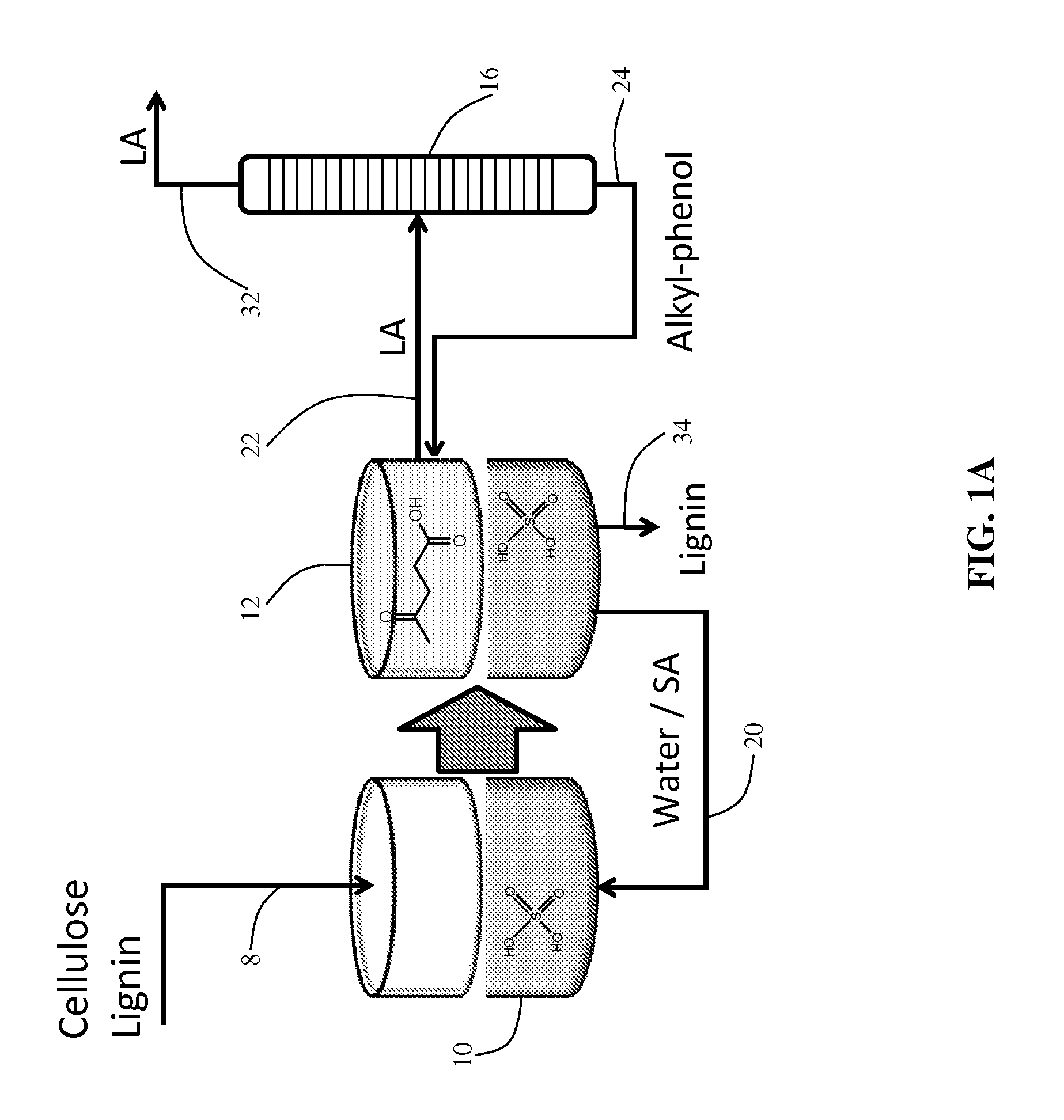
A method to produce levulinic acid (LA) and γ-valerolactone (GVL) from biomass-derived cellulose by selective extraction of LA by alkylphenol (AP) and hydrogenation of LA, in which mineral acid used in the method is recycled and the final concentration of GVL is increased by successive extraction / hydrogenation steps to allow for effective separation by distillation.
Owner:WISCONSIN ALUMNI RES FOUND
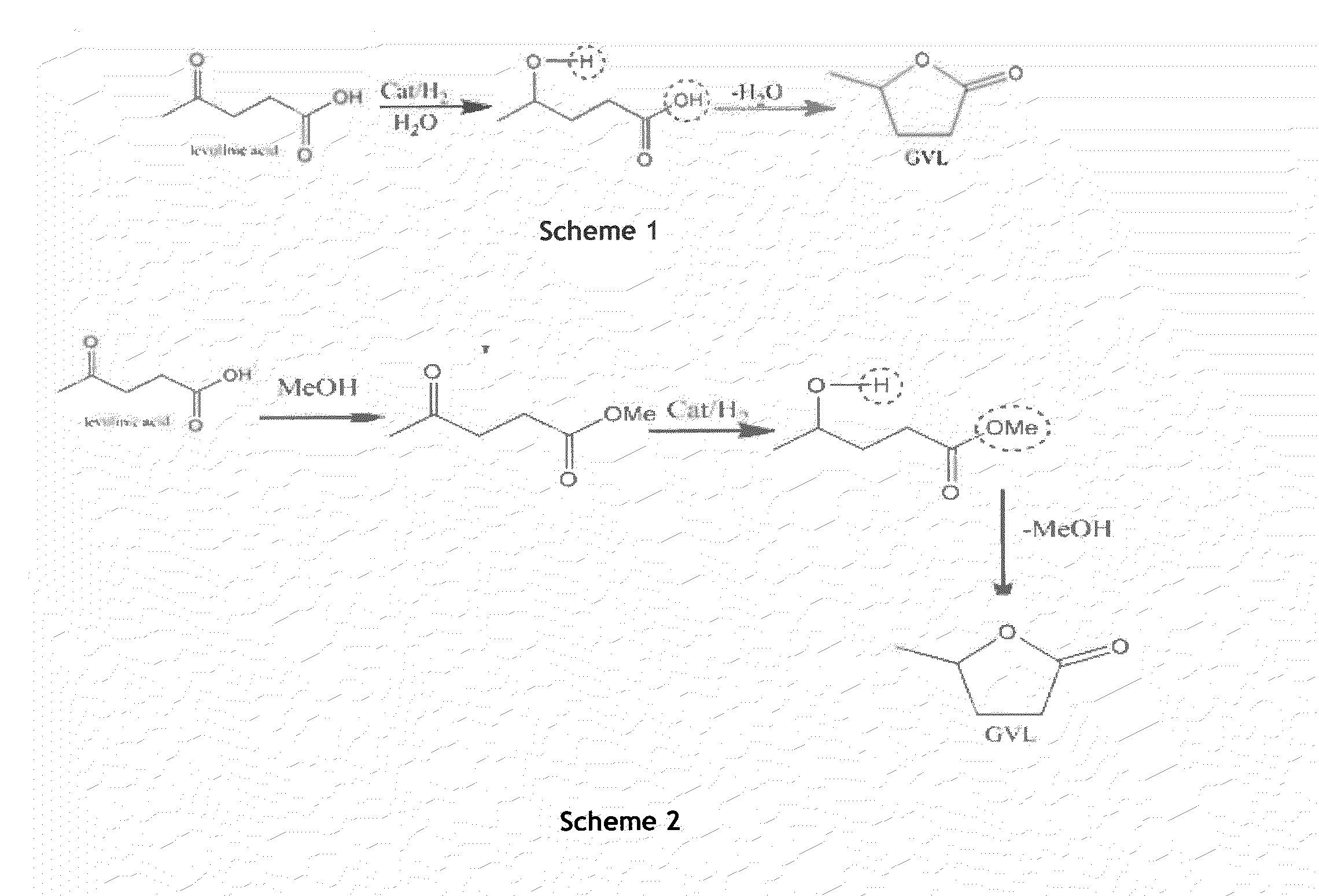
PROCESS FOR PREPARATION OF gamma-VALEROLACTONE VIA CATALYTIC HYDROGENATION OF LEVULINIC ACID
ActiveUS20130296579A1Avoid deactivationOrganic chemistryChemical recyclingGamma-ValerolactoneNon noble metal
An industrially viable process for selective preparation of γ-valerolactone using recyclable non noble metal catalyst is provided. This process provides 80-100% conversion to γ-valerolactone, with selectivity in the range of 80-100%.
Owner:COUNCIL OF SCI & IND RES
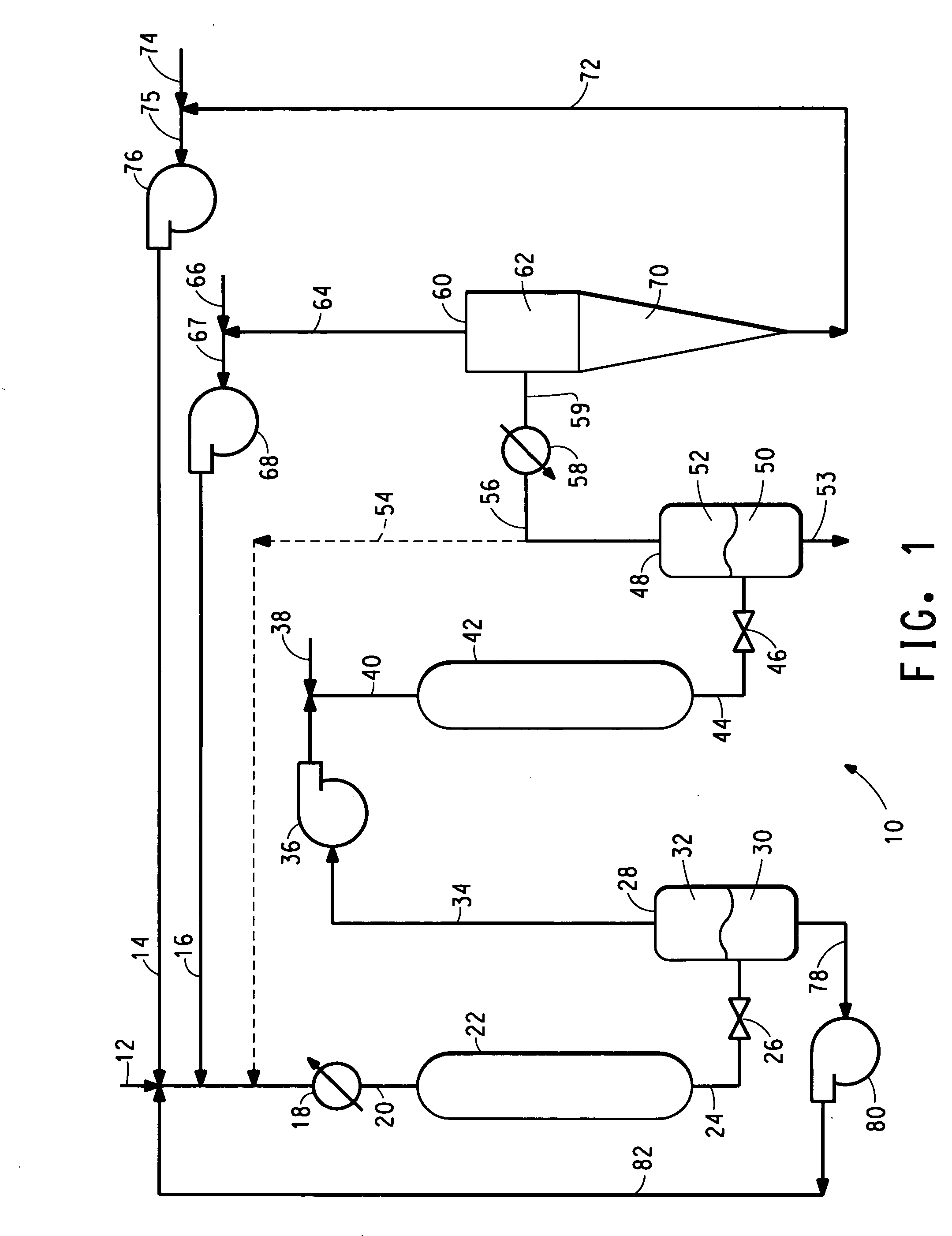
Process for the production of y-methyl-a-methylene-y-butyrolactone from reaction of levulinic acid and hydrogen with recycle of unreacted levulinic acid followed by reaction of crude y-valerolactone and formaldehyde, both reactions being carried out in the supercritical or near-critical fluid phase
InactiveUS20060100447A1Lower cost of capitalVariable costOrganic chemistryBulk chemical productionPropanoic acidHydrogen
Process for the production of γ-methyl-α-methylene-γ-butyrolactone from reaction of levulinic acid and hydrogen with recycle of unreacted levulinic acid and reaction of crude γ-valerolactone and formaldehyde, both reactions being carried out in the supercritical or near-critical fluid phase.
Owner:EI DU PONT DE NEMOURS & CO
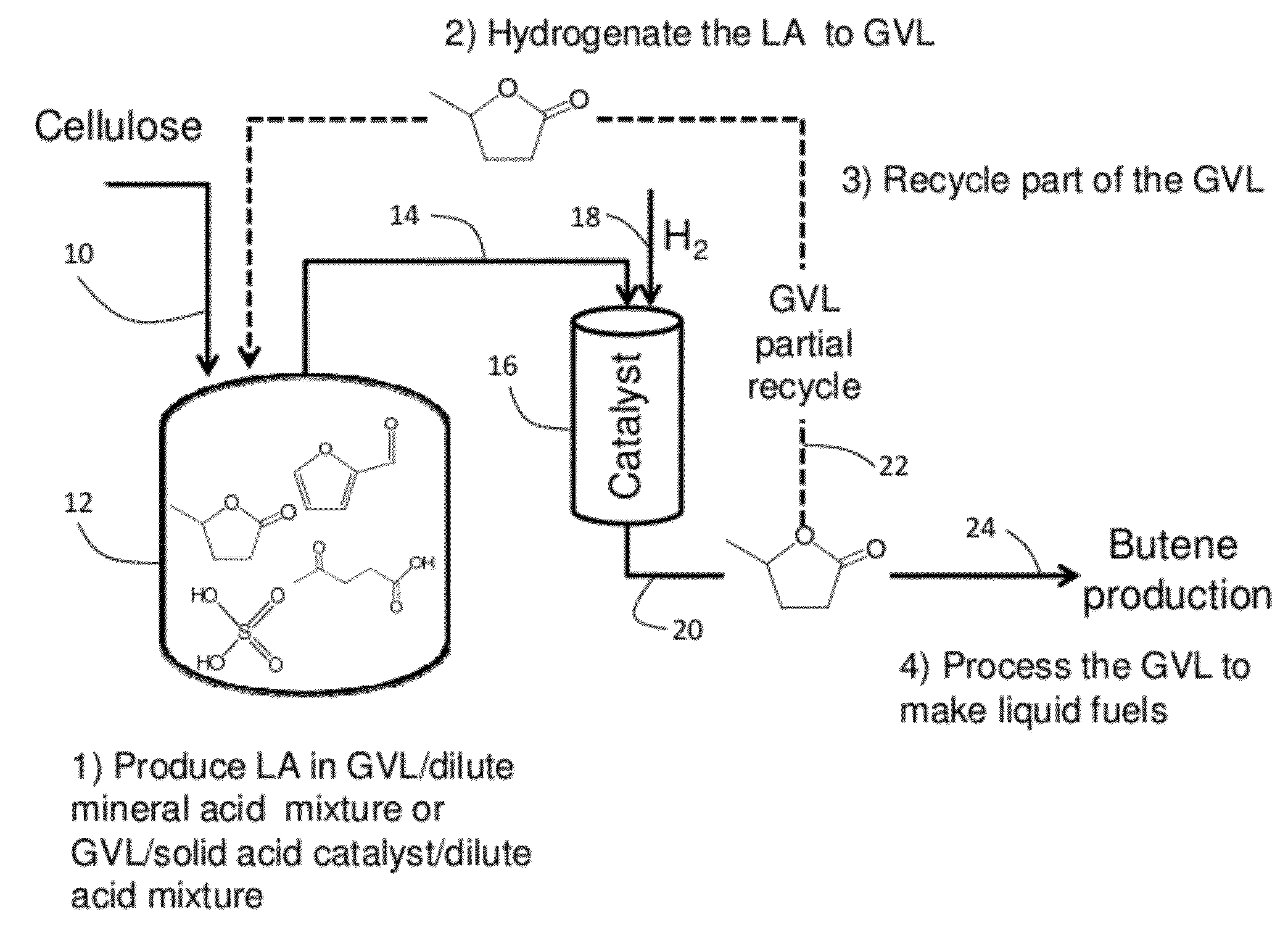
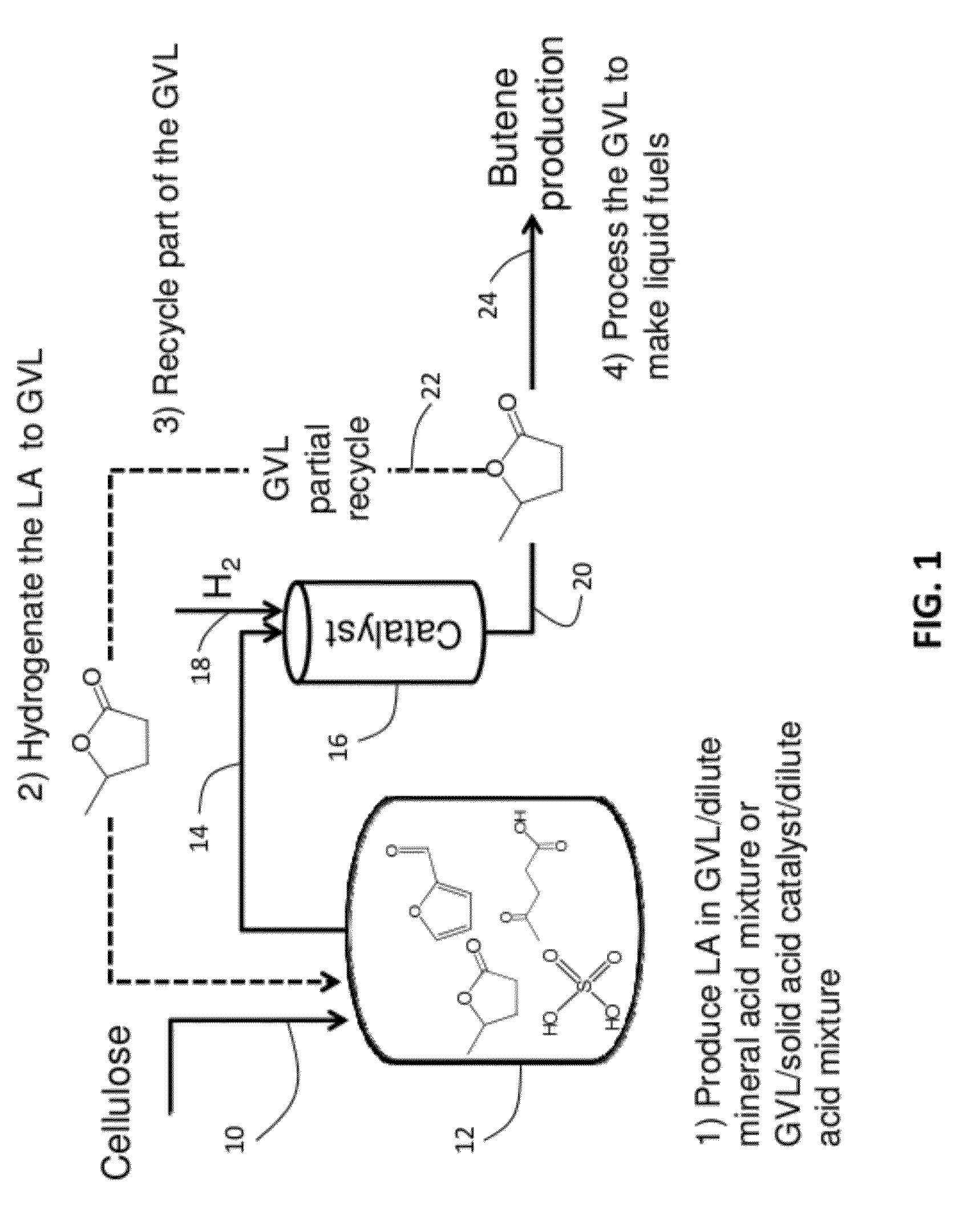
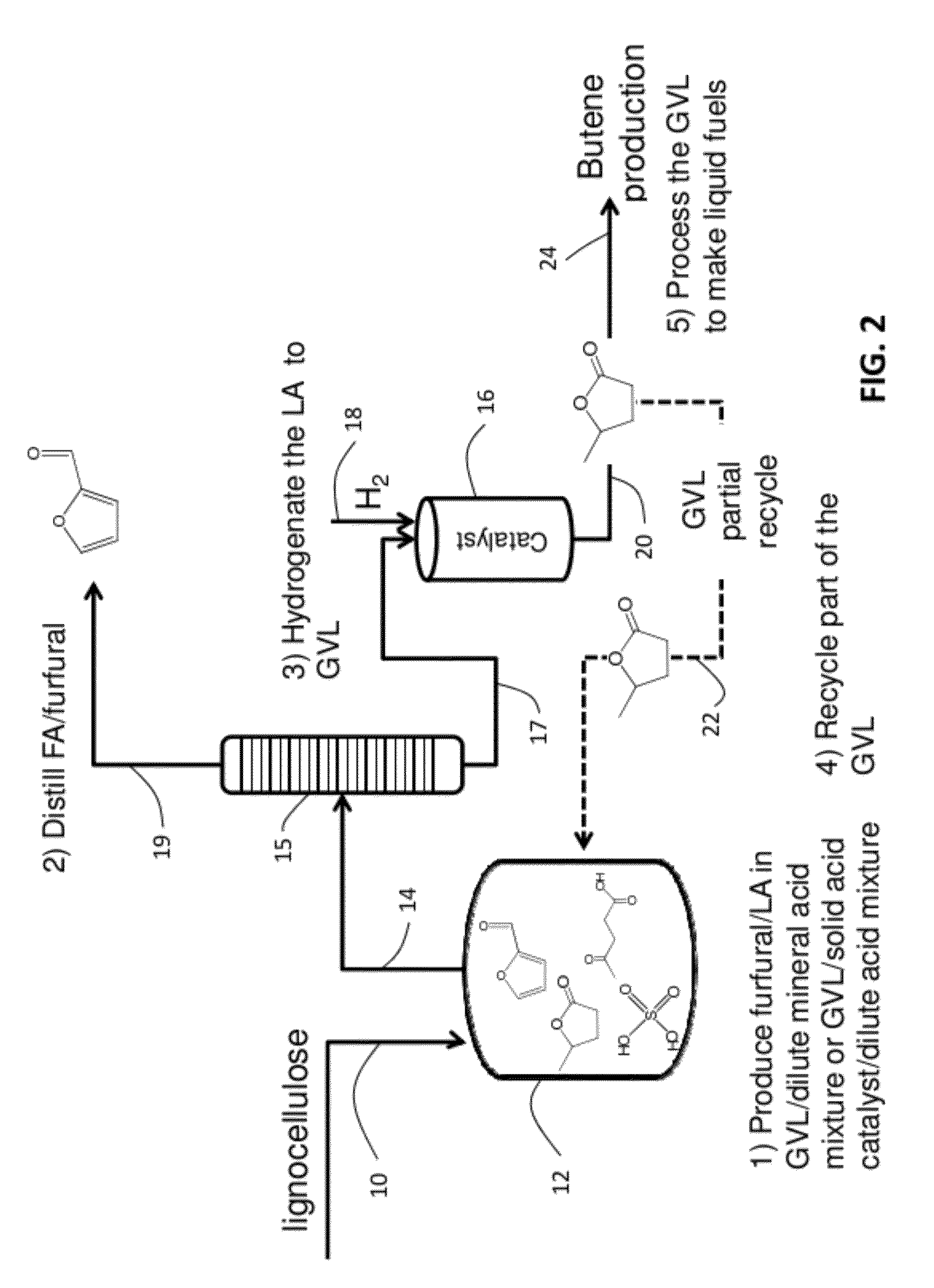
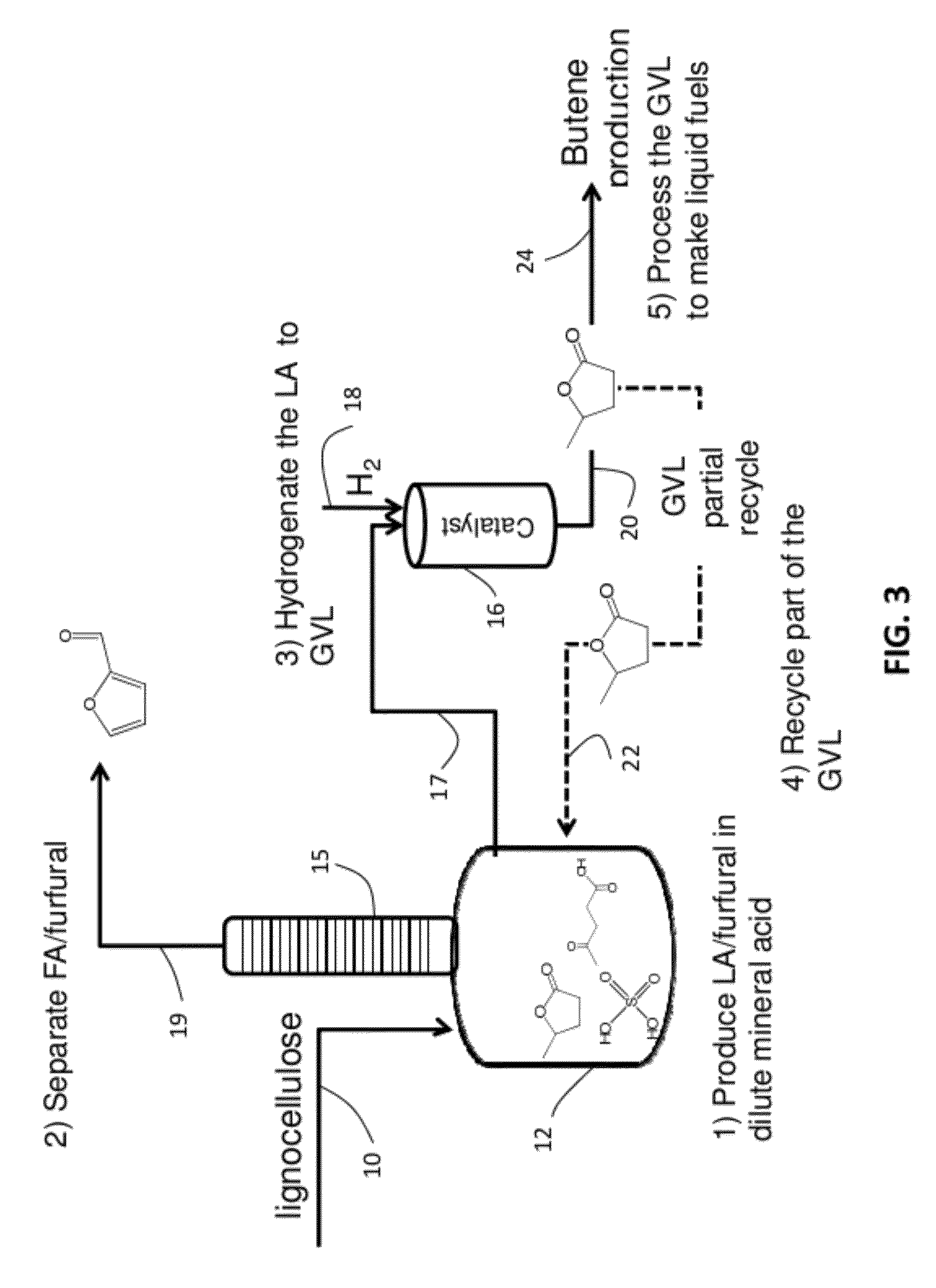
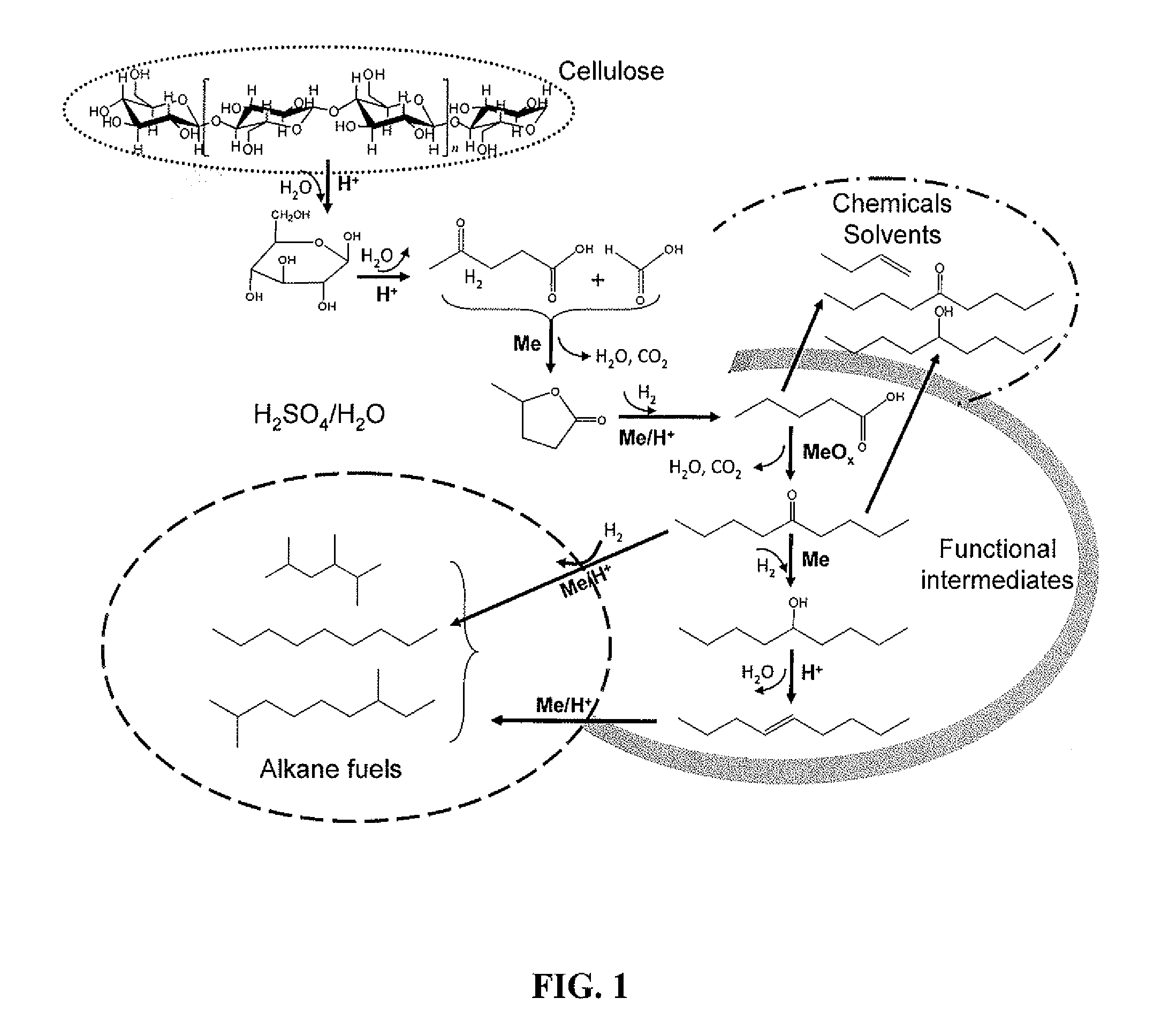
Catalytic conversion of cellulose to liquid hydrocarbon fuels by progressive removal of oxygen to facilitate separation processes and achieve high selectivities
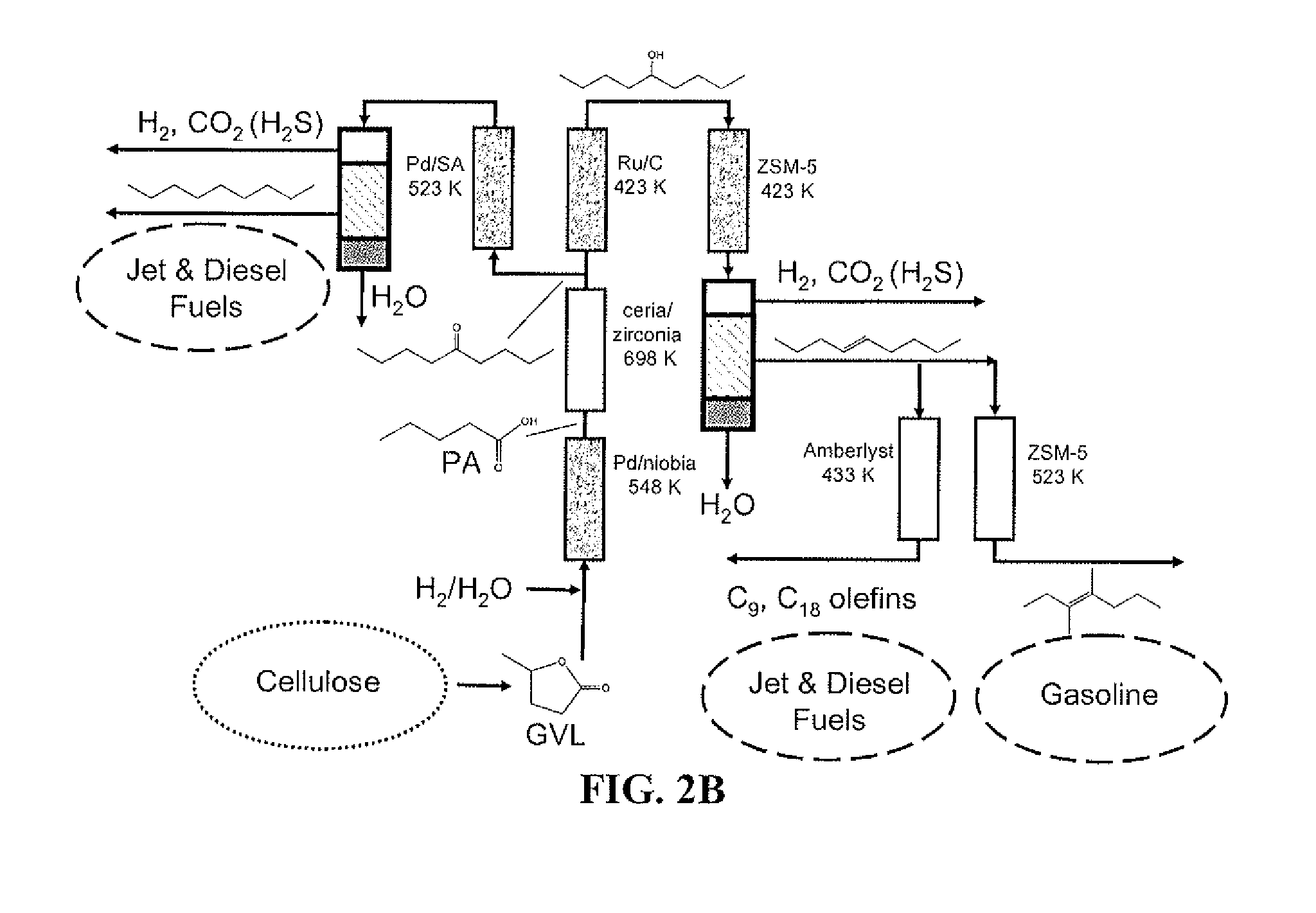
Described is a method to make liquid chemicals, such as functional intermediates, solvents, and liquid fuels from biomass-derived cellulose. The method is cascading; the product stream from an upstream reaction can be used as the feedstock in the next downstream reaction. The method includes the steps of deconstructing cellulose to yield a product mixture comprising levulinic acid and formic acid, converting the levulinic acid to γ-valerolactone, and converting the γ-valerolactone to pentanoic acid. Alternatively, the γ-valerolactone can be converted to a mixture of n-butenes. The pentanoic acid so formed can be further reacted to yield a host of valuable products. For example, the pentanoic acid can be decarboxylated yield 1-butene or ketonized to yield 5-nonanone. The 5-nonanone can be hydrodeoxygenated to yield nonane, or 5-nonanone can be reduced to yield 5-nonanol. The 5-nonanol can be dehydrated to yield nonene, which can be dimerized to yield a mixture of C9 and C18 olefins, which can be hydrogenated to yield a mixture of alkanes. Alternatively, the nonene may be isomerized to yield a mixture of branched olefins, which can be hydrogenated to yield a mixture of branched alkanes. The mixture of n-butenes formed from γ-valerolactone can also be subjected to isomerization and oligomerization to yield olefins in the gasoline, jet and Diesel fuel ranges.
Owner:WISCONSIN ALUMNI RES FOUND
Delta-valerolactone compounds, preparation method and application
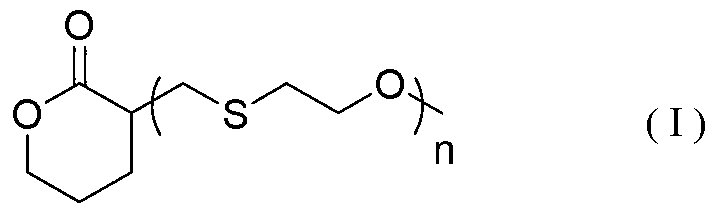
ActiveCN103288788AWide variety of sourcesBiocompatibleOrganic chemistryPharmaceutical non-active ingredientsDrug deliveryPolyester
The invention discloses delta-valerolactone compounds having structures expressed by a general formula (I); the invention further discloses a preparation method of the compounds, polyester prepared from the compounds and a preparation method thereof; the raw materials of the delta-valerolactone compounds prepared by the invention are extensive and renewable; and the polyester prepared from the delta-valerolactone compounds has biocompatibility and degradable hydrophily and can be utilized in a plurality of purposes, for example, medicine conveyance, protein protection, and the like.
Owner:YINGU PHARMA
Metal microparticle dispersion, process for production of electrically conductive substrate, and electrically conductive substrate
ActiveUS20120267151A1Good dispersionStable maintenanceMaterial nanotechnologyTransportation and packagingPolyesterPolymer science
Provided are a metal microparticle dispersion which is excellent in a dispersibility, an electrically conductive substrate which is obtained by using the above metal microparticle dispersion and which is excellent in an electrical conductivity and a production process for the same.Provided is a metal microparticle dispersion comprising metal microparticles, a polymeric dispersant and a dispersion medium, wherein an average primary particle diameter of the metal microparticles is 0.001 to 0.5 μm; the polymeric dispersant has a polyester skeleton in at least one of a principal chain and a side chain thereof; the above polyester skeleton has at least one of a constitutional unit derived from valerolactone and a constitutional unit derived from caprolactone, and a sum of the numbers of the above constitutional units is 10 or more in terms of an average value, or the polymeric dispersant has a polyether skeleton in at least one of a principal chain and a side chain thereof; and a content of the above polymeric dispersant is 0.1 to 100 parts by mass based on a content of 100 parts by mass of the metal microparticles. Further, provided is a production process for an electrically conductive substrate, comprising printing a coating solution containing the above metal microparticle dispersion in a pattern-like form to form a printed layer and subjecting the above printed layer to burning treatment to form a pattern-like metal microparticle sintered film, and an electrically conductive substrate produced by the above production process is provided as well.
Owner:DAI NIPPON PRINTING CO LTD
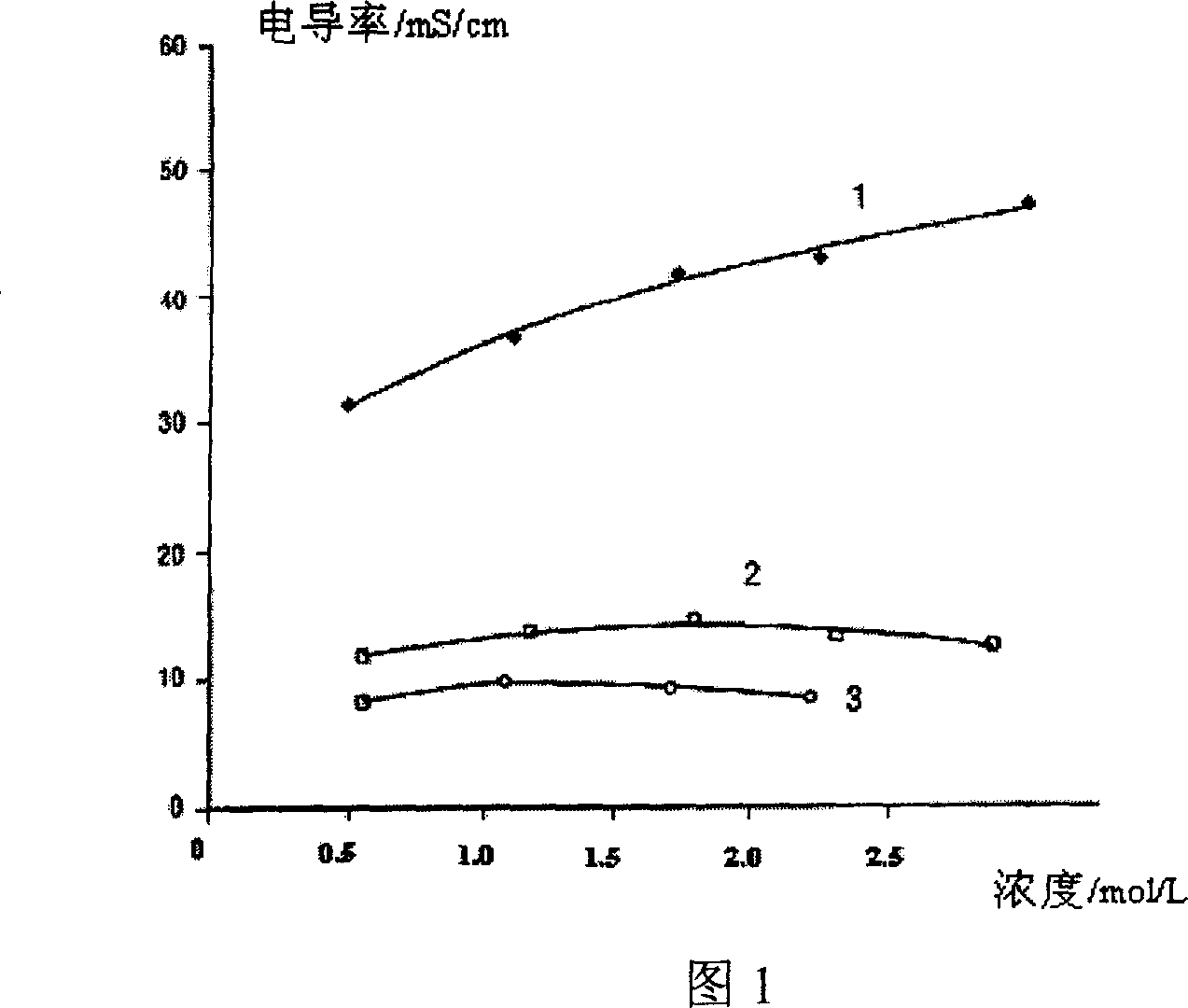
High temperature electrolyte for super capacitor
The invention relates to a super-capacitor high-temperature electrolyte. Wherein, its solute is N-trialkyl-N-alkoxy acyl fluoroboric acid or phosphorofluoric acid or Fraude's reagent, or trifluoromethyl sulfonic acid; and the solvent is aprotic solvents; its density in room temperature is 0.8-2.0mol / l; the aprotic solvents are acetonitrile, ethyl cyanide, methacrylonitrile, gamma- butyrolactone, gamma- valerolactone, vinylene carbonate, propylene carbonate, N, N- dimethyl formamide, 1- dimethyl formamide-2- pyrrolidone, methylenedioxy ethane, 2-methoxy ether, tetrahydrofuran, dioxolane, dimethyl carbonate, diethyl carbonate, diethyl carbonate, or dimethyl sulfoxide. And the inventive capacitor has high capacity and high service life at 85Deg. C.
Owner:锦州凯美能源有限公司
Process for preparation of γ-valerolactone via catalytic hydrogenation of levulinic acid
ActiveUS8975421B2Avoid deactivationOrganic chemistryChemical recyclingPropanoic acidAsymmetric hydrogenation
An industrially viable process for selective preparation of γ- valerolactone using recyclable non noble metal catalyst is provided. This process provides 80-100% conversion to γ-valerolactone, with selectivity in the range of 80-100%.
Owner:COUNCIL OF SCI & IND RES
Preparation method of bio-based copolyester containing modifiable functional group
The invention discloses a preparation method of a bio-based copolyester containing a modifiable functional group, and a random copolyester of alpha-methylene-gamma-butyrolactone, epsilon-caprolactone,delta-valerolactone, and gamma-butyrolactone prepared thereby. A binary catalysis system, which is composed of a strong alkali and a co-catalyst (urea or thiourea), is used to catalyze selective ring-opening co-polymerization of alpha-methylene-gamma-butyrolactone and other cyclic monomers to prepare the bio-based copolyester containing a modifiable functional group. The prepared bio-based copolyester can meet the application requirements of the biomedicine field, and the physical property of the bio-based copolyester can be modulated in a wider range.
Owner:QINGDAO UNIV OF SCI & TECH
Synthetic method of gamma-alkyl oxyacyl methyl-gamma-butyrolactone and delta- alkyl oxyacyl methyl-delta-valerolactone
The invention belongs to the field of chemical technology, and in particular to a synthetic method of gamma-alkyl oxyacyl methyl-gamma-butyrolactone and delta- alkyl oxyacyl methyl-delta-valerolactone. CuH compound as a reducing agent reacts with keto ester, alpha, beta-unsaturated carboxylic ester; keto ester, alpha,beta-unsaturated carboxylic ester, a silicon hydride or boron hydride react with each other with CuH compound as the catalyst; and other copper compound, phosphine ligand, silicon hydride or boron hydride react with each other to generate a catalyst, which directly catalyses silicon hydride or boron hydrogen to react with keto ester, alpha,beta-unsaturated carboxylic ester. The synthesis reaction is carried out through the above three methods. reaction of this three method of The method of the invention uses cascade reaction for synthesis, three steps of reaction are carried out continuously in the same reaction vessel; separation of intermediates is not required, so as to avoid the separation process and the resulting loss; and the method has simple operation, improved reaction efficiency, and good application value.
Owner:DALIAN UNIV
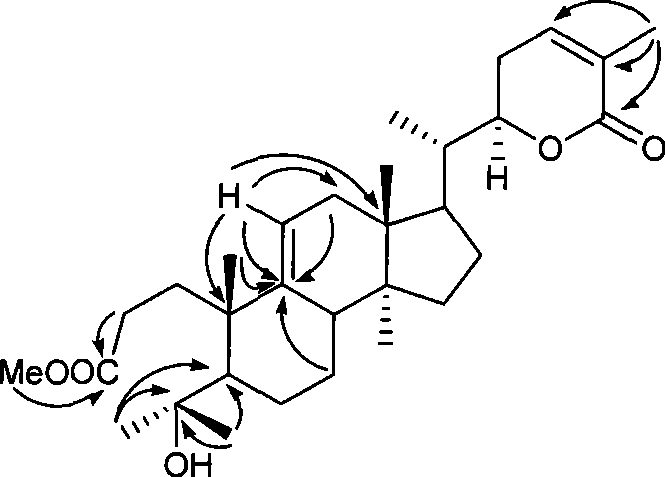
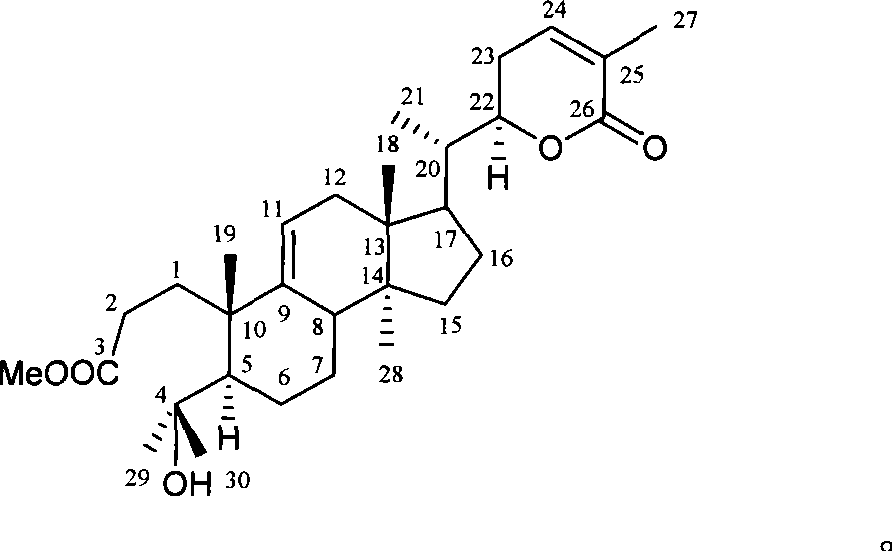
Novel triterpenoid schisanlactone H and extracting and separating method thereof
InactiveCN101376652ANovel structureSimple extraction and separation methodOrganic chemistryPlant ingredientsTherapeutic effectStructural formula
Owner:KUNMING UNIV OF SCI & TECH
Flexible absorbable composite interface screw sheath and preparation method thereof
InactiveCN110051888AImprove ductilityImprove impact toughnessSurgeryBreaking strengthPolymer science
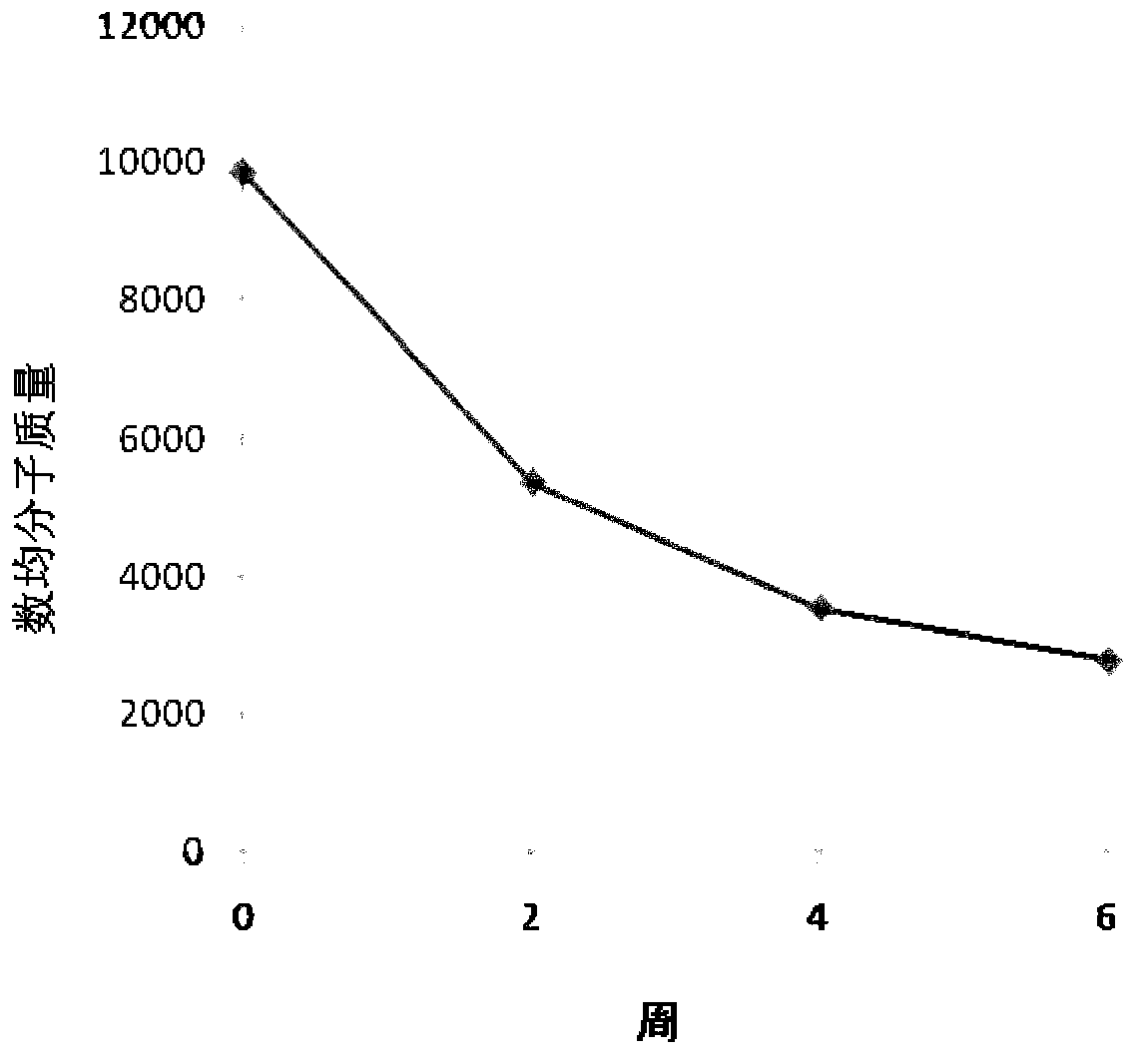
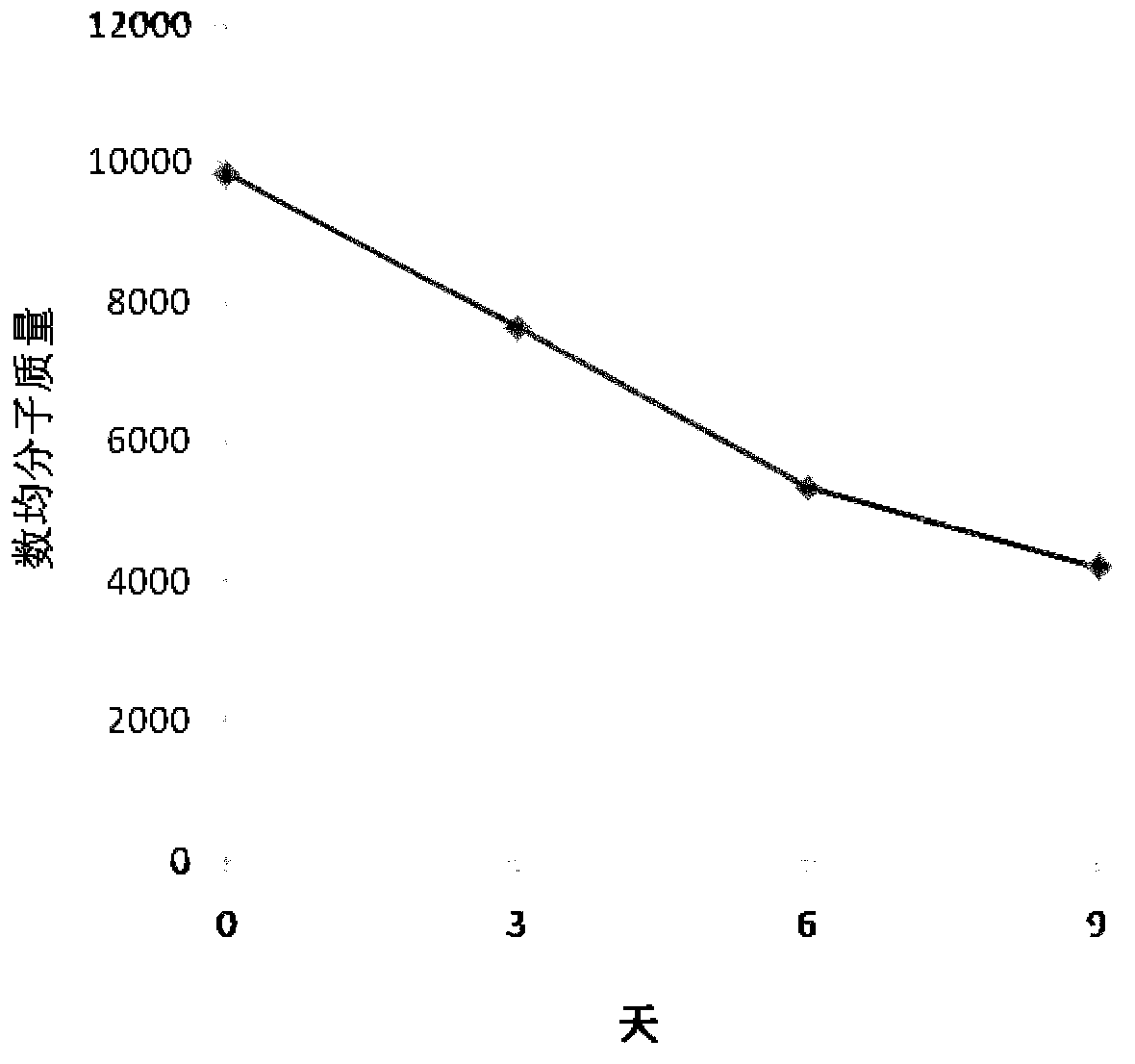
The invention relates to a flexible absorbable composite interface screw sheath and a preparation method thereof. The flexible absorbable composite interface screw sheath is prepared by melt blendingand extrusion granulation of the mixture and injection molding, wherein the mixture mainly comprises a base material and polyethylene glycol, or mainly consists of a matrix material, a polyethylene glycol-flexible polylactone copolymer and beta-TCP, wherein the matrix material is polylactic acid and / or a copolymer thereof, and the flexible polylactone is a polymer of caprolactone and / or valerolactone; the flexible absorbable composite interface screw sheath has elongation at break of not less than 103%, the impact strength of not less than 10 kJ / m<2> and the breaking strength of not less than25 MPa. By adopting the method, the ductility, strength and impact toughness of the absorbable composite interface screw sheath are improved, and the prepared absorbable composite interface screw sheath has high elongation at break, high impact strength and high enough breaking strength, and has good application prospect.
Owner:HANGZHOU REJOIN MASTIN MEDICAL INSTR CO LTD
Integrated two-step process for the production of gamma-methyl-alpha-methylene-gamma-butyrolactone from levulinic acid and hydrogen
An integrated two step method for making gamma-methyl alpha-methylene-gamma-butyrolactone (meMBL) from levulinic acid, in which levulinic acid is reacted with hydrogen in the first step to form a crude reaction product containing gamma-valerolactone, and the crude reaction product, without removal of unreacted levulinic acid therefrom, is reacted with formaldehyde in the second step to produce MeMBL.
Owner:EI DU PONT DE NEMOURS & CO
Method for preparing valerolactone through catalytic hydrogenation of ethyl levulinate
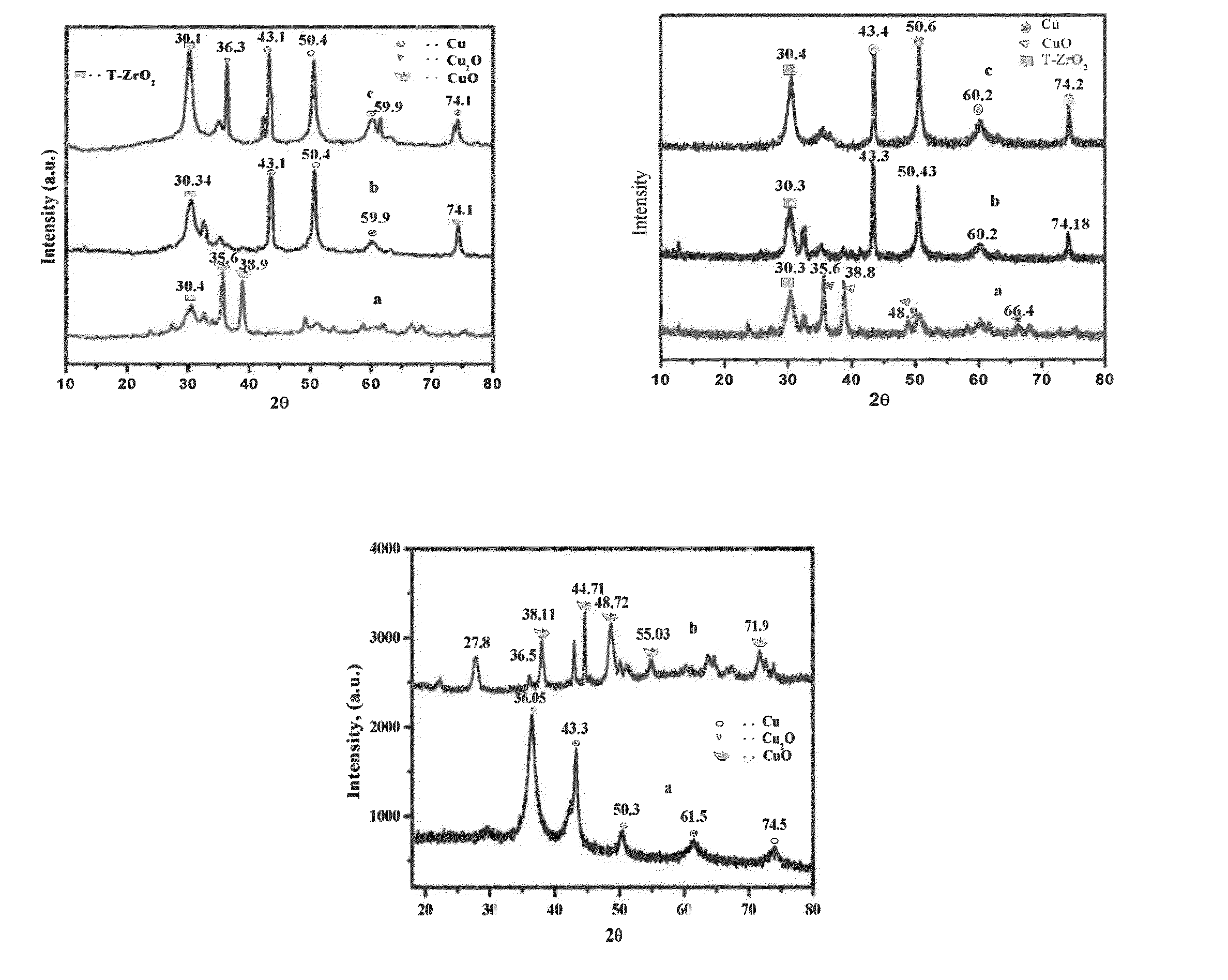
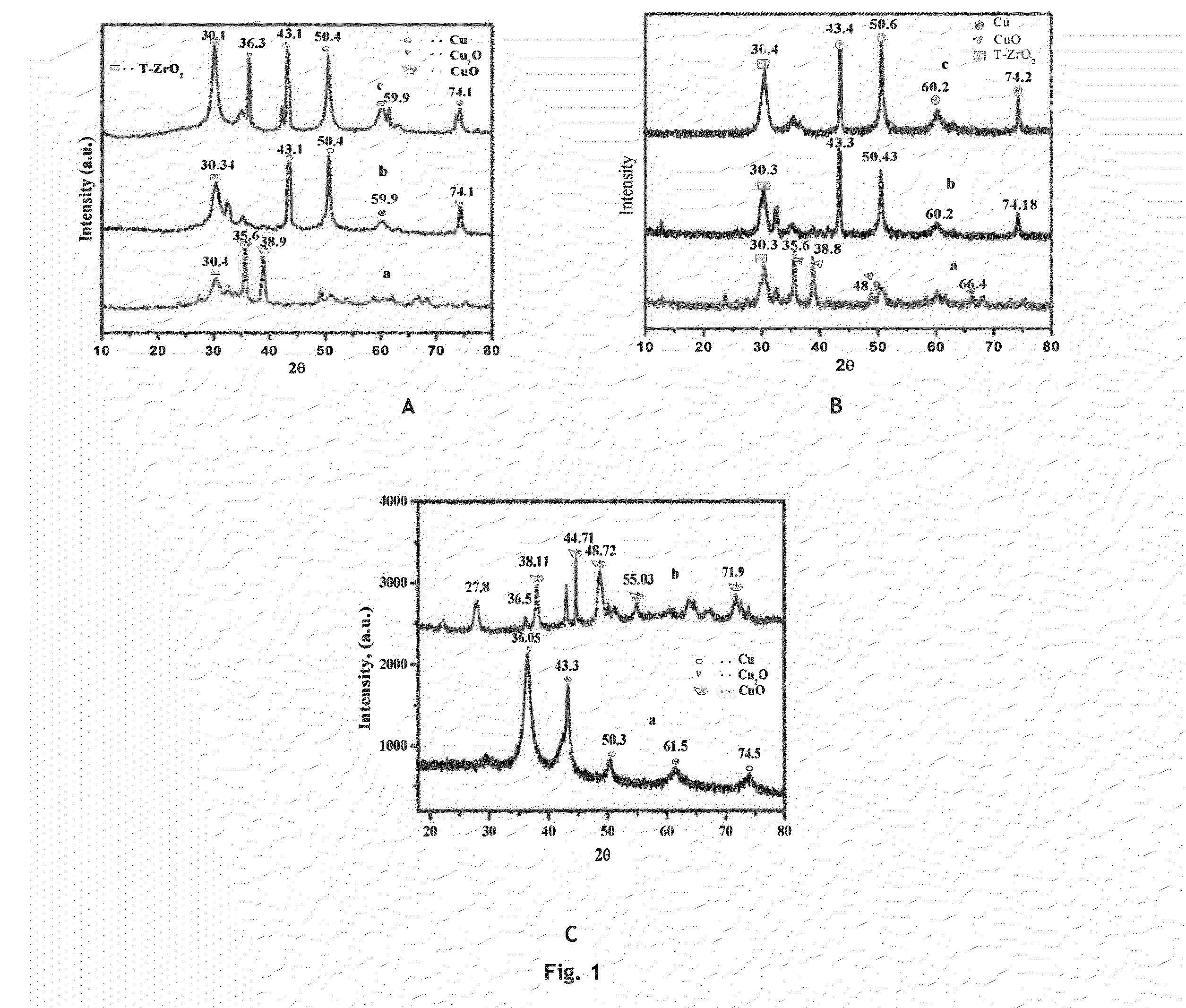
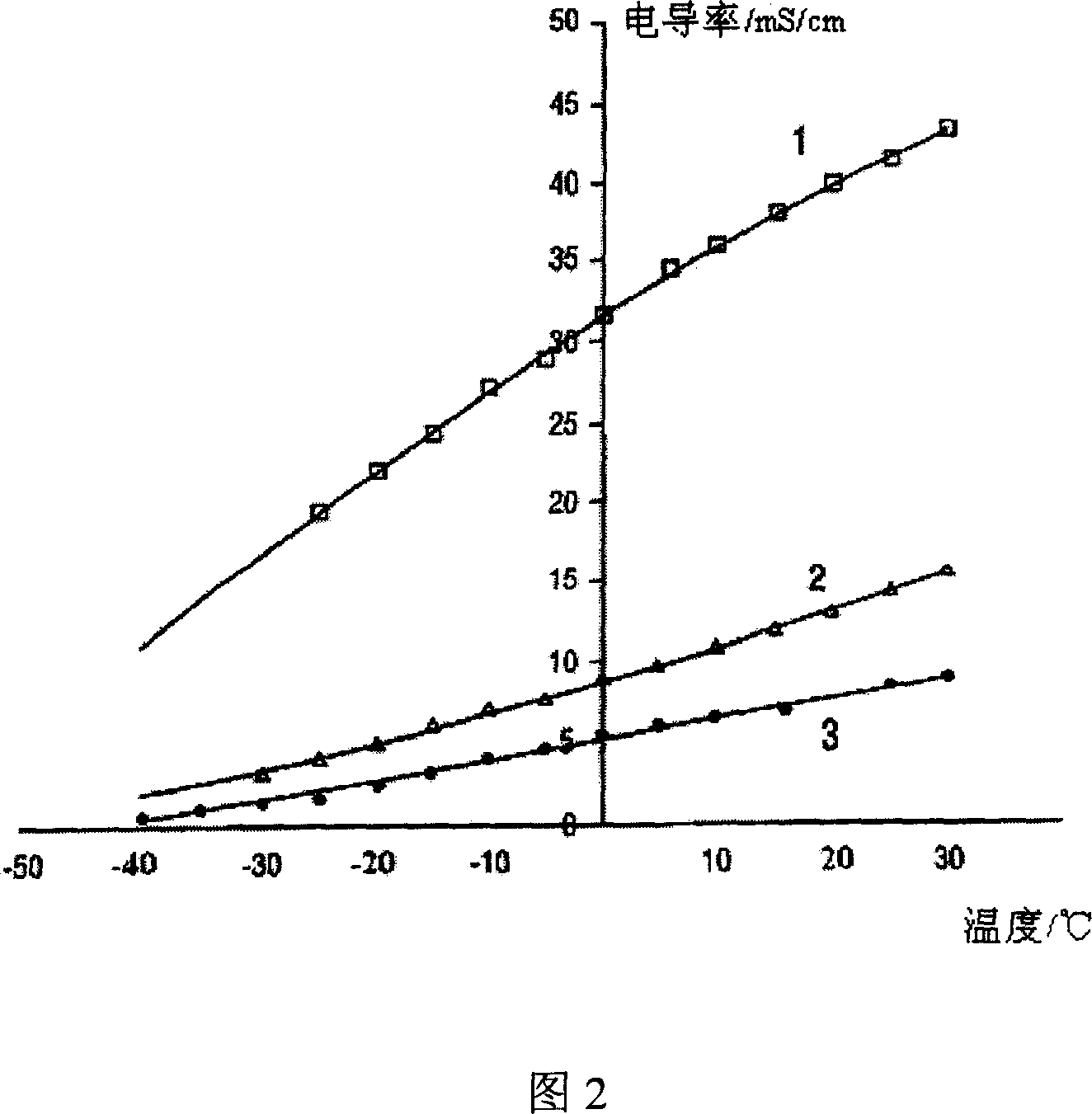
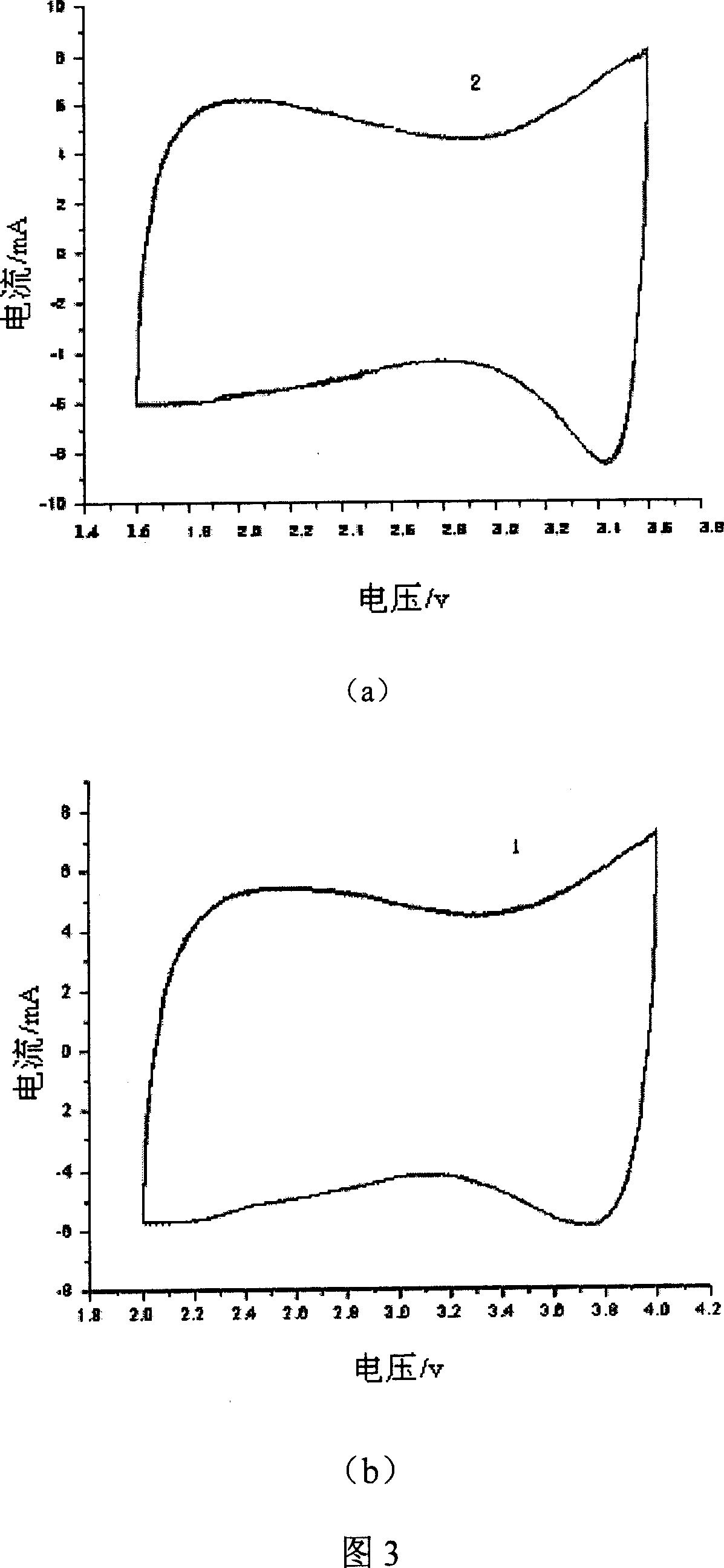
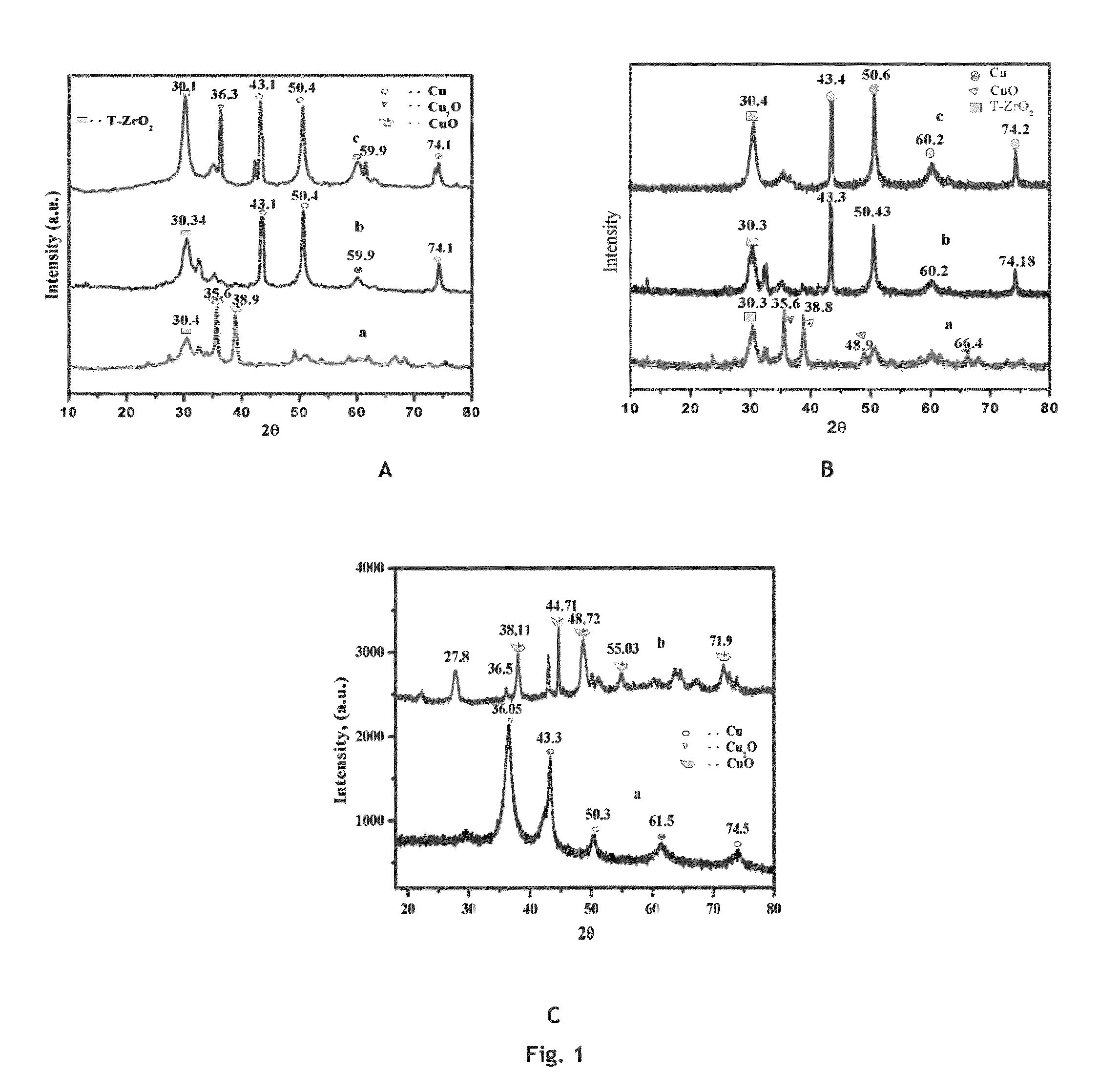
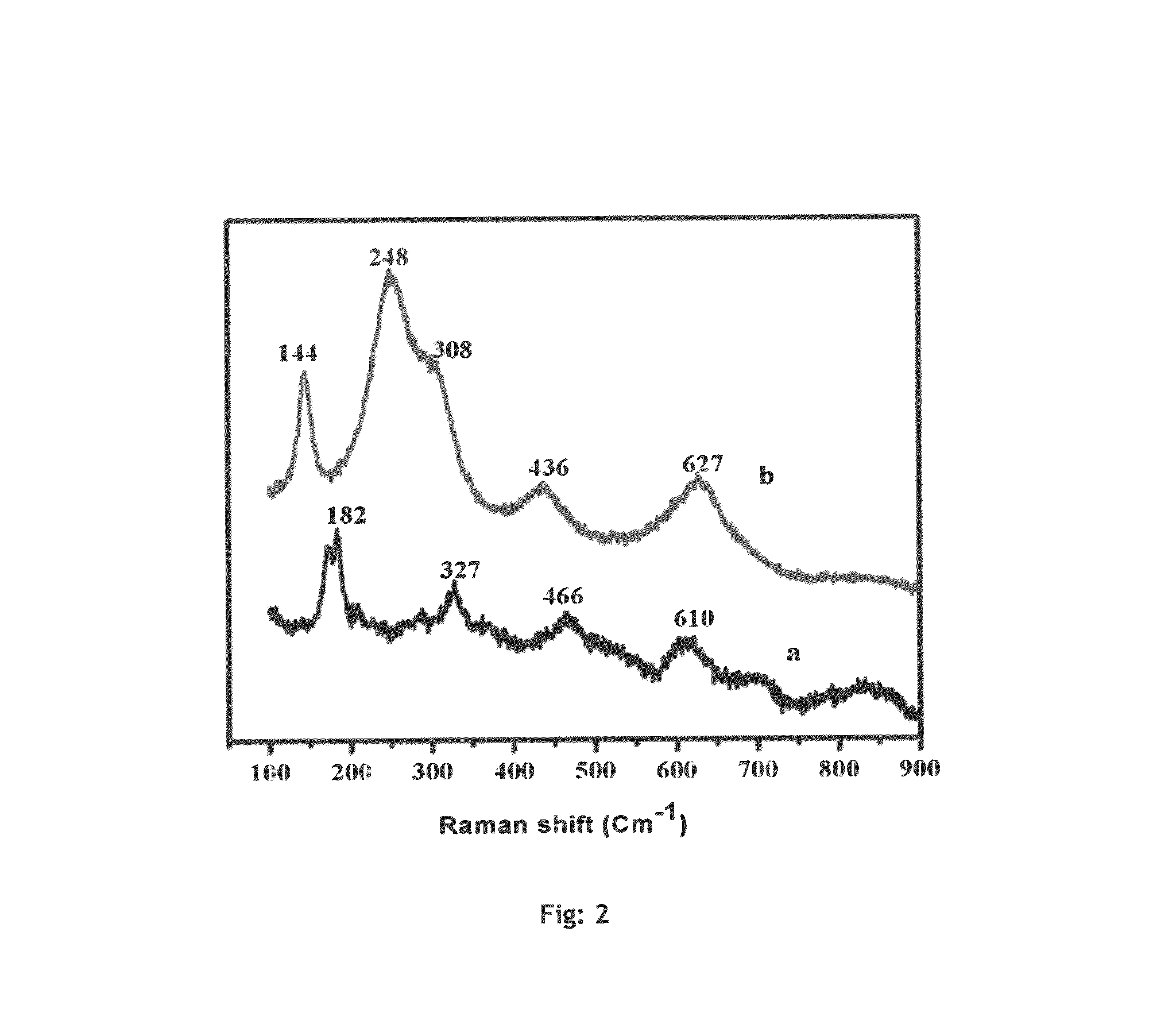
ActiveCN107245065AEasy to useReduce the hassle of re-preparationOrganic chemistryPhysical/chemical process catalystsTitanium zirconiumMicrosphere
The present invention discloses a method for preparing valerolactone through catalytic hydrogenation of ethyl levulinate. The method comprises: placing ethyl levulinate and different molar ratios of titanium zirconium microsphere catalysts in a device, adding isopropanol as a solvent and a hydrogen source, and carrying out a reaction for 2-10 h at a temperature of 160-200 DEG C, wherein a molar ratio of the titanium zirconium microsphere catalyst is Ti / Zr of 1:0, 8:2, 5:5, 2:8, and 0:1. According to the present invention, the catalytic activity of the method is good, the ethyl levulinate can be completely converted, the yield of the valerolactone can achieve 90.1%, the production cost is reduced by using the alcohol as the hydrogen source, and the catalytic system can be recycled more than 6 times while the catalytic efficiency is not significantly reduced.
Owner:GUIZHOU UNIV
Lithium ion battery with high voltage electrolytes and additives
ActiveUS8993177B2Final product manufactureElectrode carriers/collectorsHigh pressureLithium-ion battery
Desirable electrolyte compositions are described that are suitable for high voltage lithium ion batteries with a rated charge voltage at least about 4.45 volts. The electrolyte compositions can comprise ethylene carbonate and solvent composition selected from the group consisting of dimethyl carbonate, methyl ethyl carbonate, γ-butyrolactone, γ-valerolactone or a combination thereof. The electrolyte can further comprise a stabilization additive. The electrolytes can be effectively used with lithium rich positive electrode active materials.
Owner:IONBLOX INC
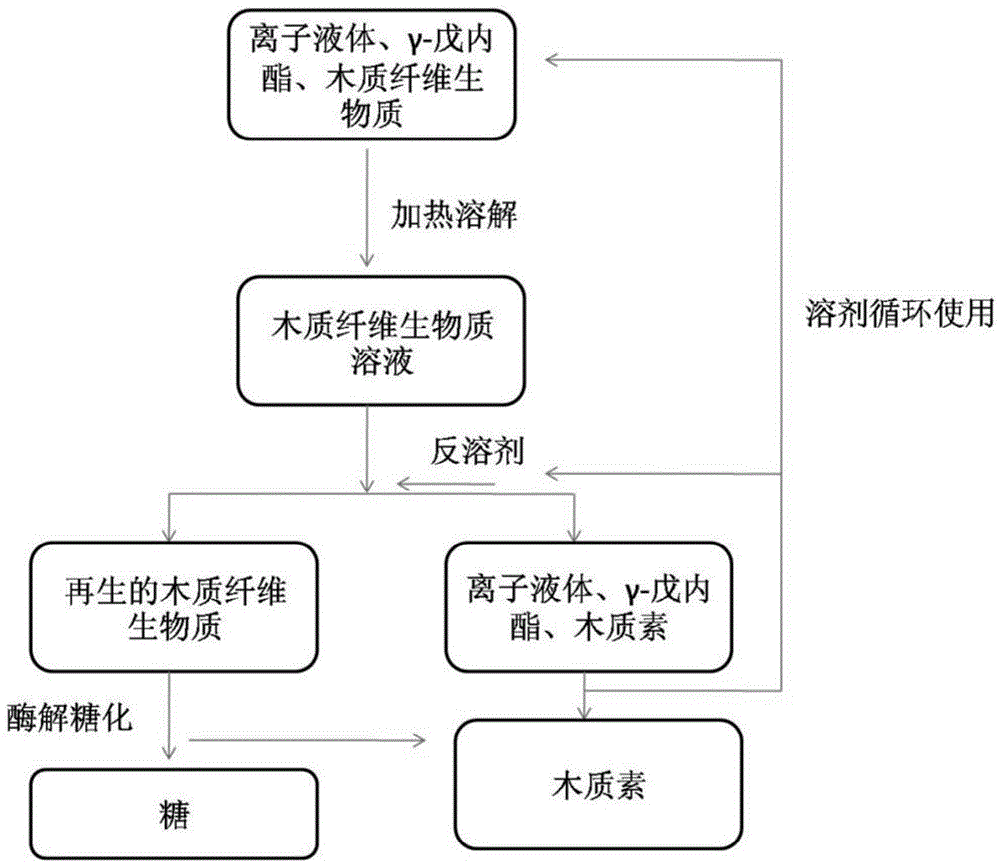
Method for pre-processing lignocellulose biomass to increase sugar field of lignocellulose biomass
The invention provides a method for dissolving and pre-processing lignocellulose biomass through a mixed solvent formed by ionic liquid and gamma-valerolactone to increase the sugar field of lignocellulose biomass. The method includes the following steps that firstly, ionic liquid, gamma-valerolactone and lignocellulose biomass are mixed to form a pre-dissolving and pre-processing system; secondly, a pre-dissolving mixture system is heated and dissolved, and a solution is formed; thirdly, an anti-solvent is added into the solution system, and the dissolved lignocellulose biomass precipitates and regenerates; fourthly, the regenerative lignocellulose biomass is taken, a buffering solution, cellulase and xylanase are added for enzymolysis, and a sugar solution is obtained. Part of the ionic liquid is replaced by biology-base gamma-valerolactone, and the mixed system is formed. The method has the advantages that the technology is simple, solvent cost is low, operation is convenient, the solvent can be recycled, and cellulose and hemicellulose in the processed lignocellulose biomass have the advantages that the enzyme hydrolysis speed is high and the sugar yield is high.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
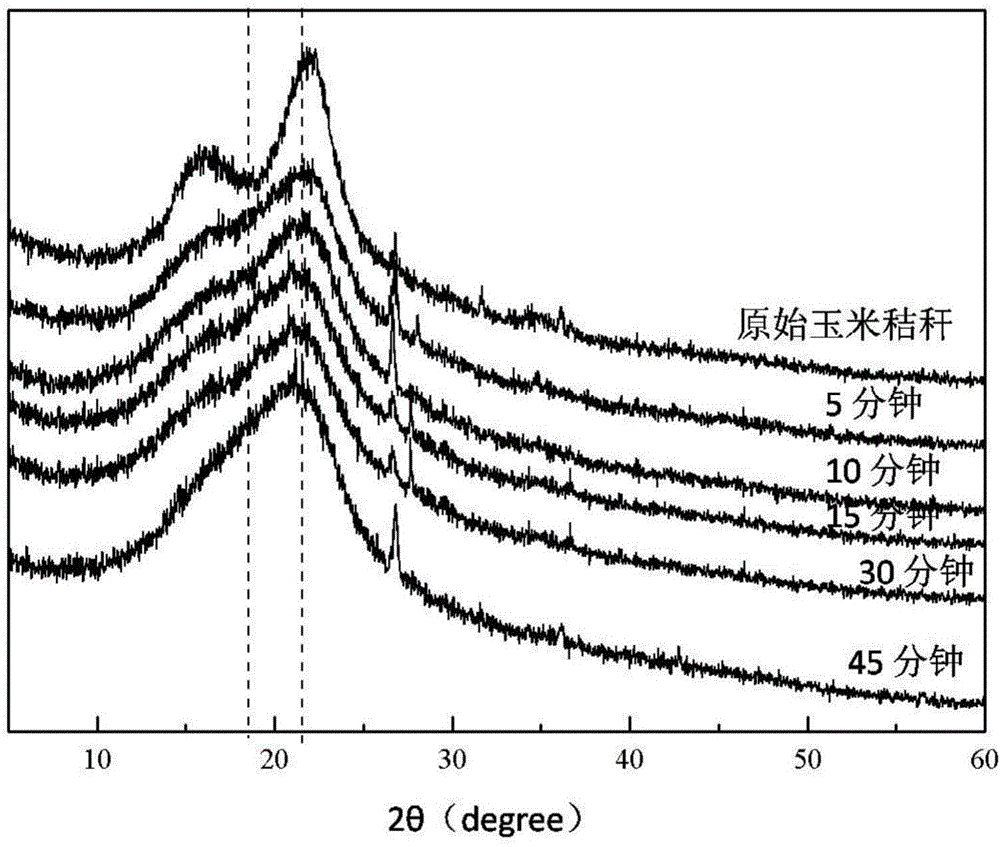
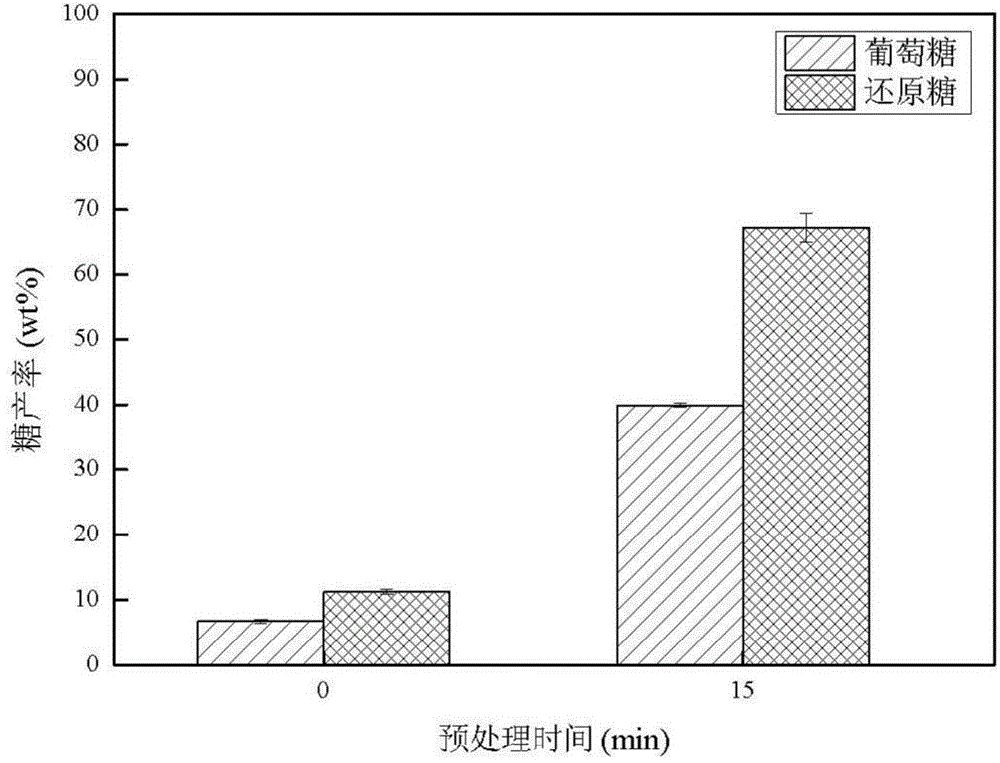
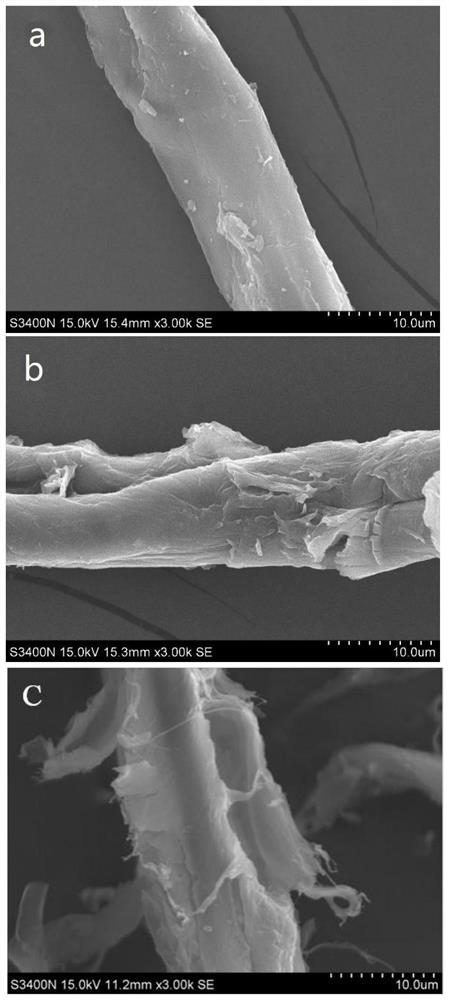
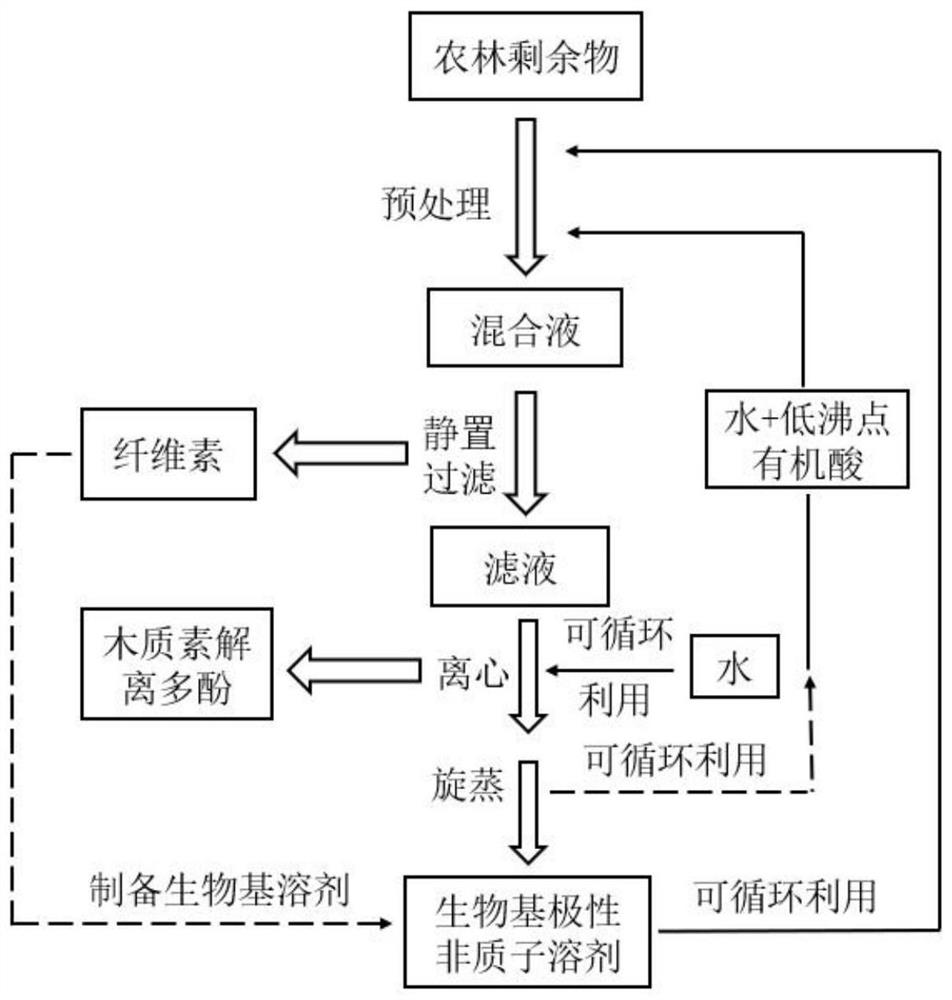
Method for pretreating wood fibers in bio-based polar aprotic solvent system
The invention provides a method for pretreating wood fibers in a bio-based polar aprotic solvent system, wherein the method comprises the steps: catalyzing and efficiently separating cellulose, hemicellulose and lignin in a bio-based polar aprotic solvent / water composite solvent by taking agricultural and forestry residues as raw materials; adding the agriculture and forestry residues, the bio-based polar aprotic solvent / water composite solvent and low-boiling-point organic acid into a pressurized reaction kettle, carrying out heating pretreatment, cooling to room temperature after pretreatment is finished, standing, filtering to obtain solid residues and filtrate, drying the solid residues, and weighing for preparing bio-based polar aprotic solvents such as gamma-valerolactone / dihydrolevoglucosenone and the like through hydrolysis and hydrogenation; and adding a certain amount of deionized water into the filtrate, filtering to obtain brown powdery lignin dissociation polyphenol, and performing reduced-pressure fractional distillation on the filtrate to recycle water, low-boiling-point organic acid and the bio-based polar aprotic solvent. The recycled water, the recycled low-boiling-point organic acid and the recycled bio-based polar aprotic solvent can be recycled.
Owner:INST OF CHEM IND OF FOREST PROD CHINESE ACAD OF FORESTRY
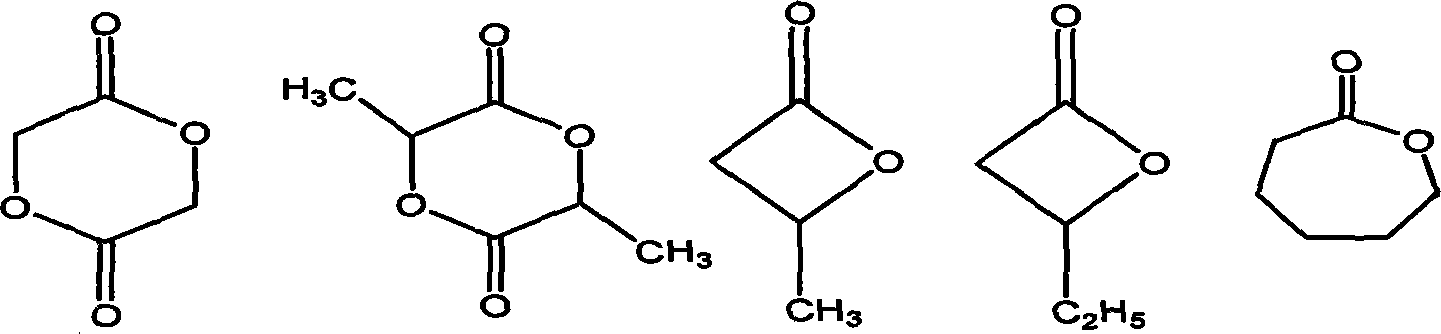
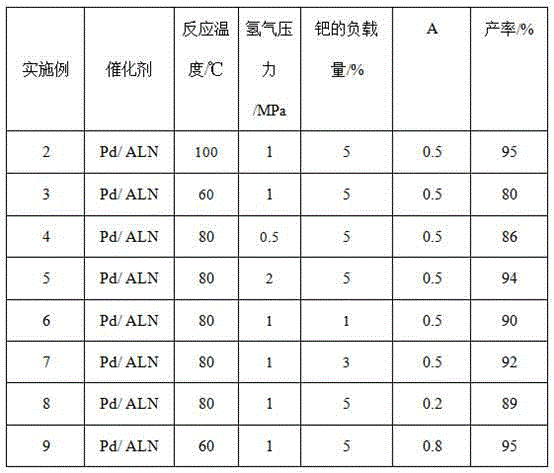
Method for preparing valerolactone
InactiveCN105348230AHigh yieldMild reaction conditionsOrganic chemistryHeterogenous catalyst chemical elementsPropanoic acidPhysical chemistry
The invention discloses a method for preparing valerolactone. The method comprises the following steps: putting acetylpropionic acid and palladium-loaded aluminum nitride into a reaction device; adding distilled water and introducing hydrogen gas to react at 60 DEG C to 100 DEG C for 1 to 6 hours, wherein the loading amount of palladium is 1 percent to 10 percent, the pressure of the hydrogen gas is 0.5MPa to 2MPa, and the mass ratio of the palladium-loaded aluminum nitride to the acetylpropionic acid is (0.2 to 0.8):1. The method has moderate reaction conditions and has a relatively high valerolactone yield.
Owner:林康艺
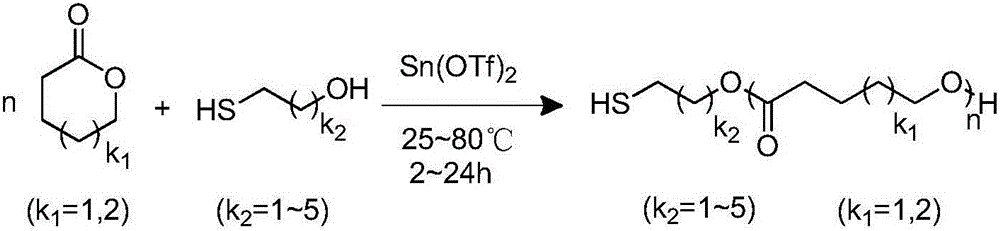
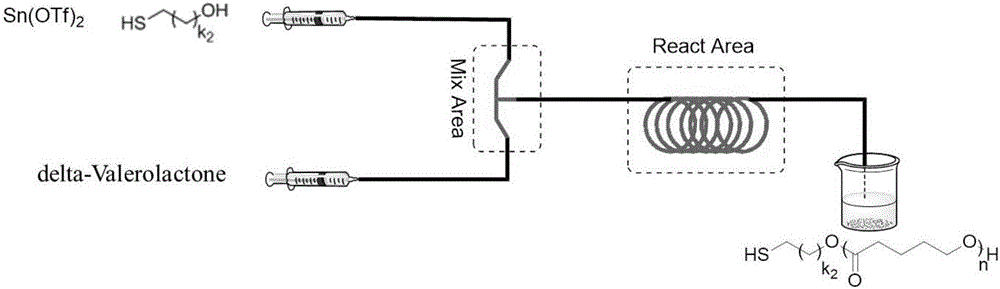
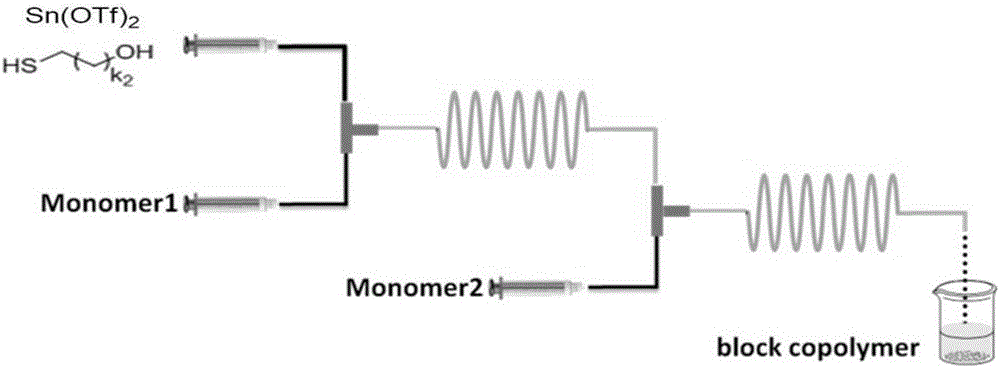
Method for preparing thiol-functionalized polyester with micro-reaction unit
The invention discloses a method for preparing thiol-functionalized polyester with a micro-reaction unit. Delta-valerolactone (VL) or epsilon-caprolactone (CL) is used as a reaction monomer, tin trifluoromethanesulfonate (Sn(OTf)2) is used as a metal complex catalyst, fatty alcohol with an end thiol group is used as a functionalization initiator, and the thiol-functionalized polyester is prepared in the micro-reaction unit. Compared with the prior art, the adopted metal complex catalyst is simple and easy to obtain and high in catalytic activity, the technology is high in operability, and cost is low. Meanwhile, the whole polymerization process is short, reaction conversion rate is high, polymer thiol introduction rate is high, and high-precision control of polymerization reaction is achieved.
Owner:NANJING UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com