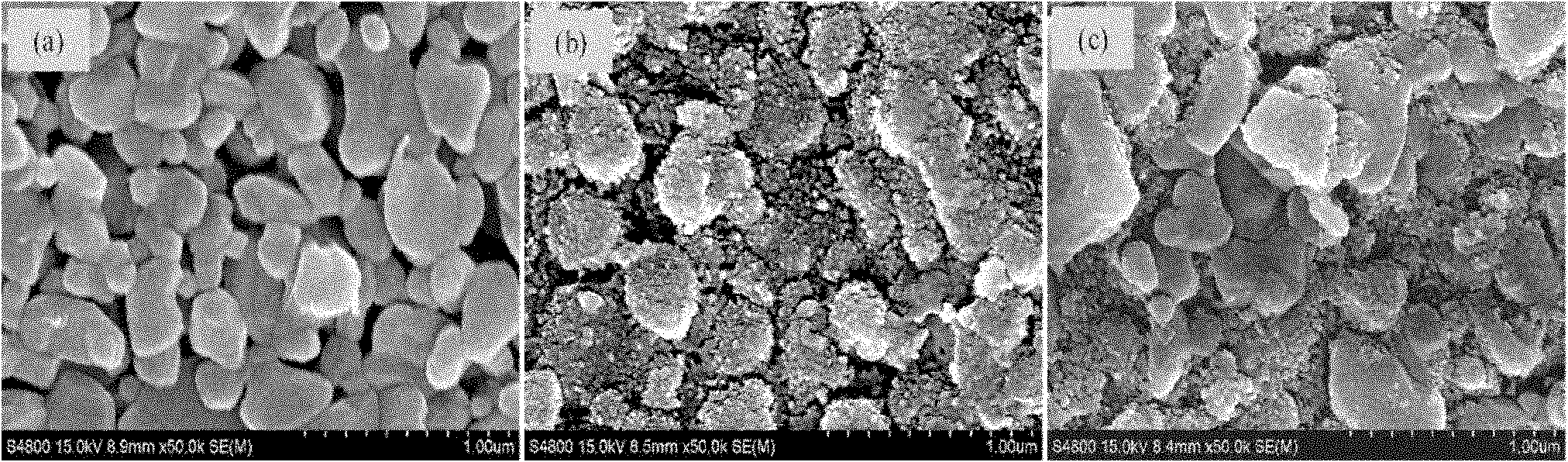
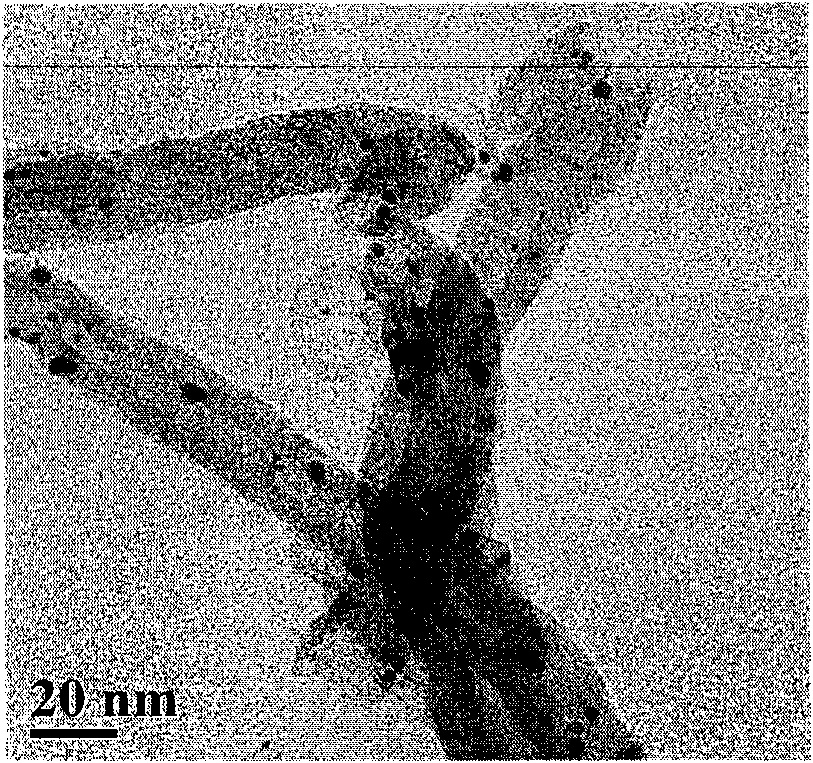
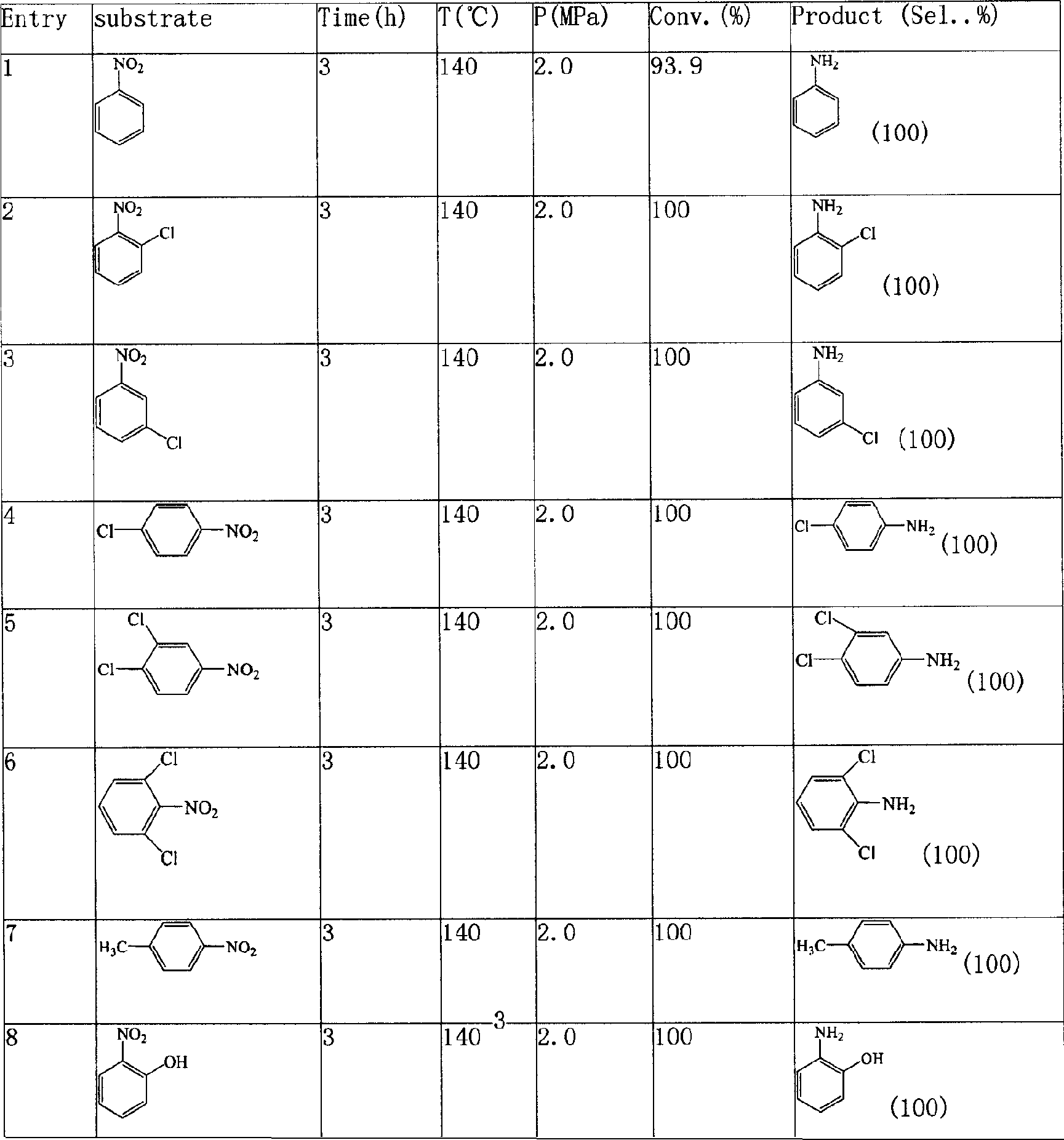
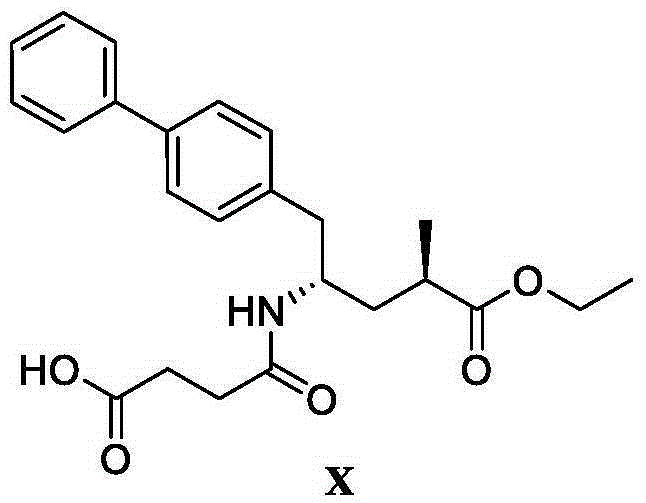
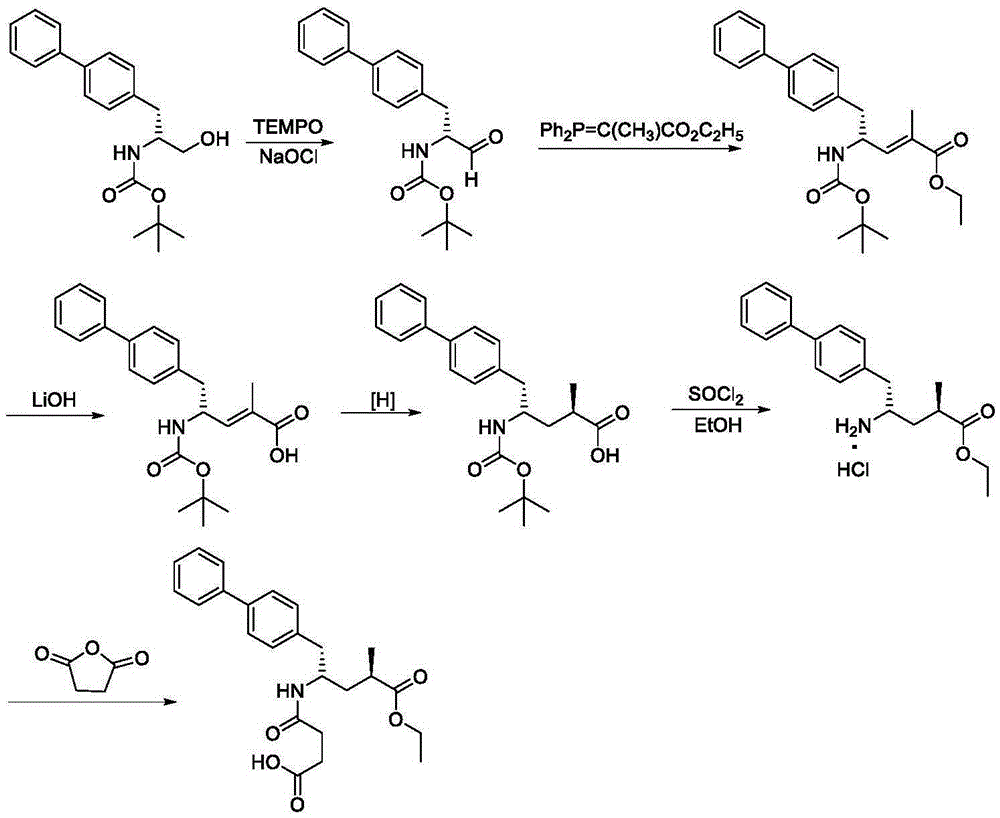
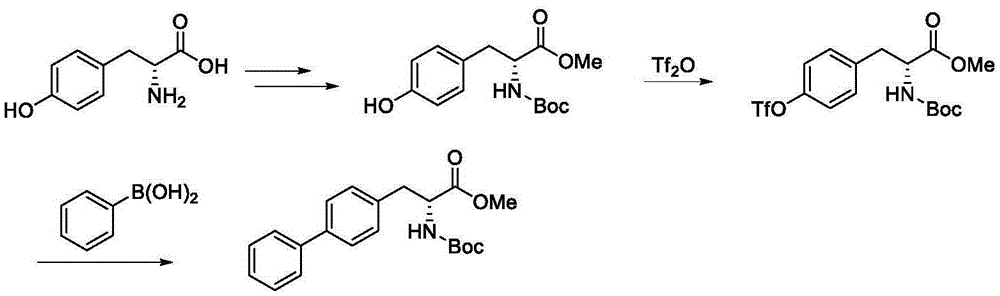
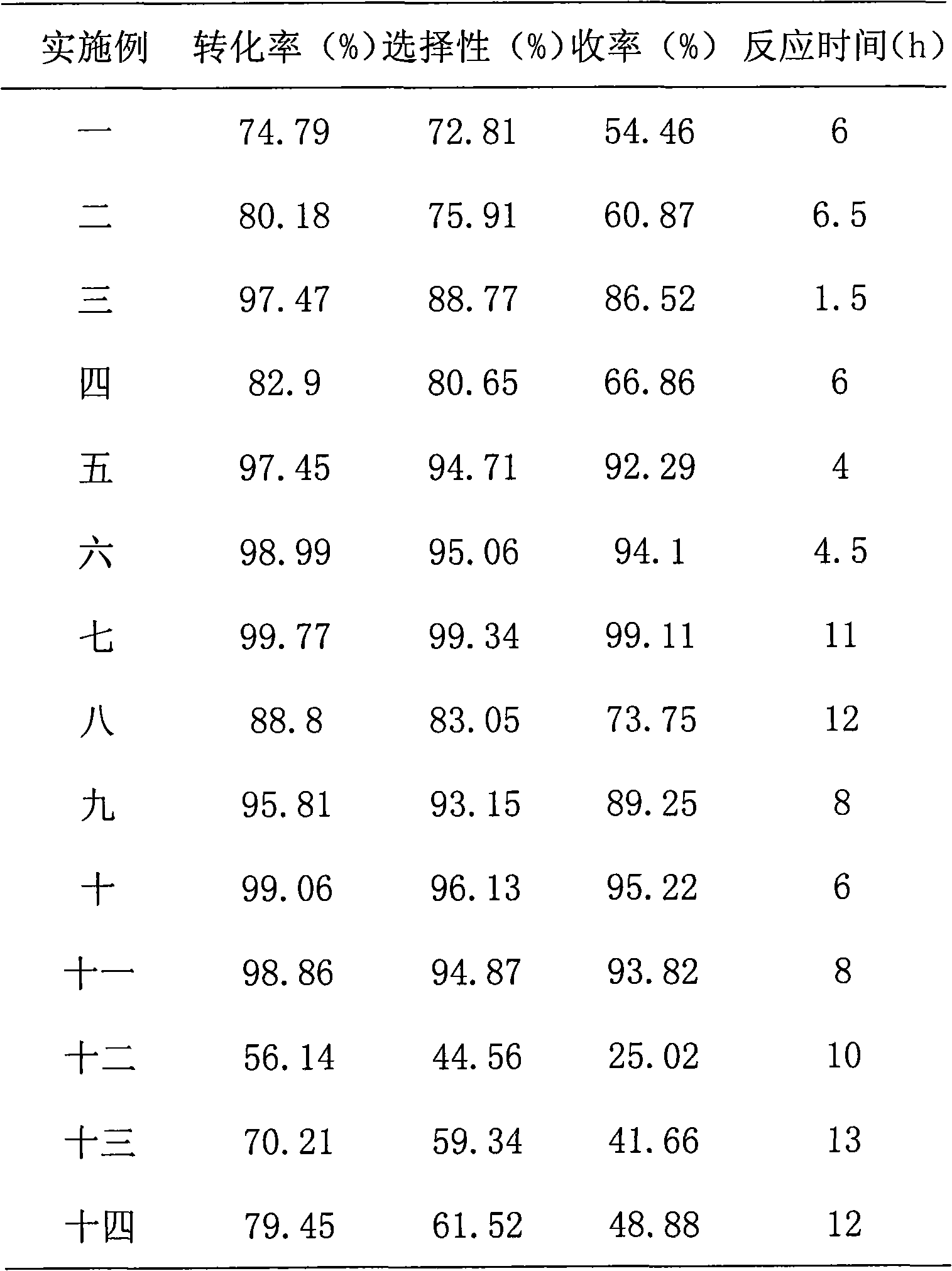
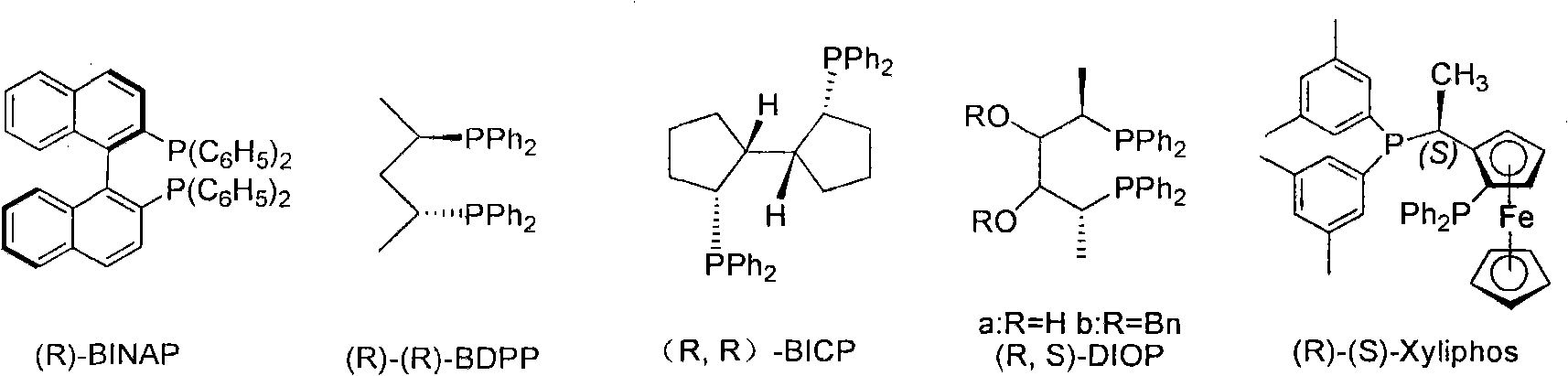
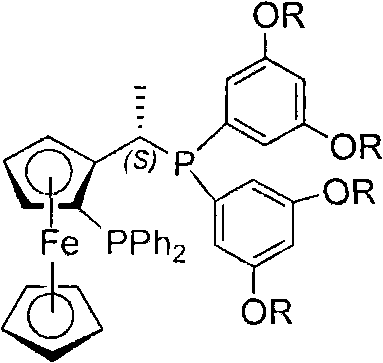
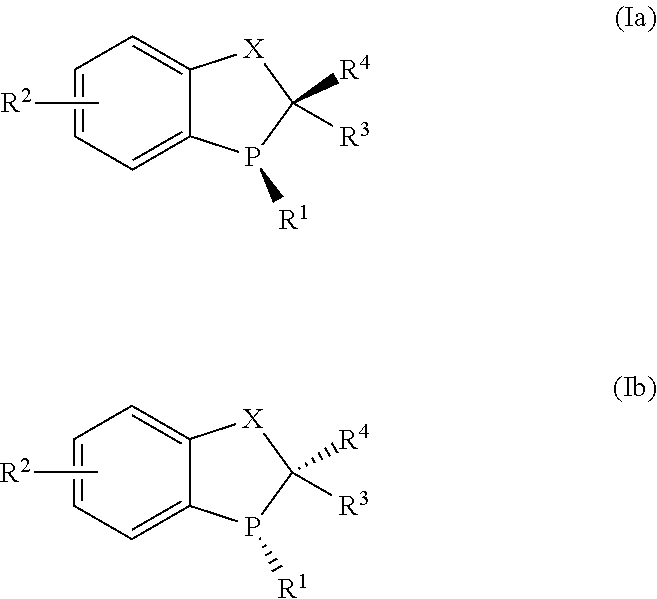
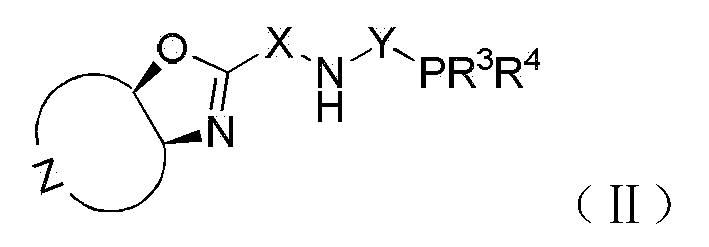
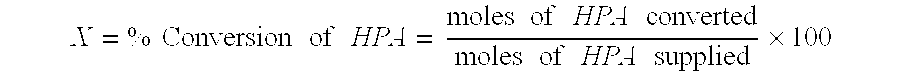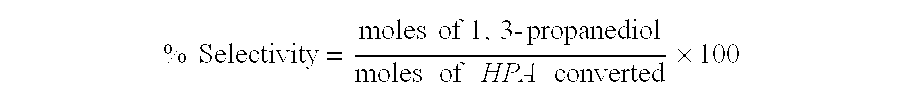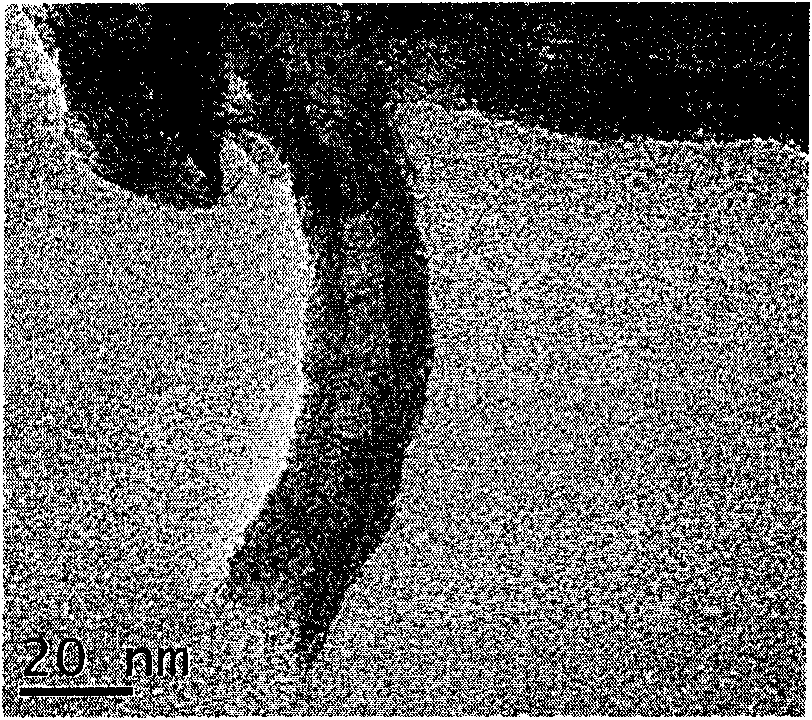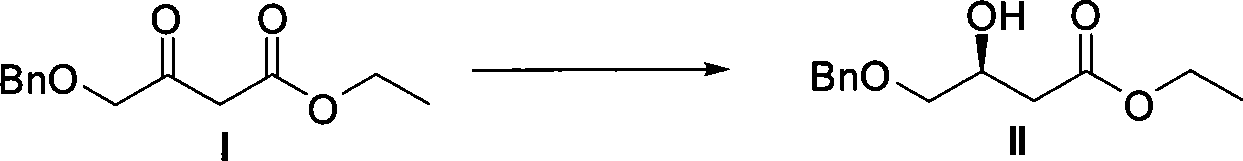Patents
Literature
886 results about "Asymmetric hydrogenation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Asymmetric hydrogenation is a chemical reaction that adds two atoms of hydrogen preferentially to one of two faces of an unsaturated substrate molecule, such as an alkene or ketone. The selectivity derives from the manner that the substrate binds to the chiral catalysts. In jargon, this binding transmits spatial information (what chemists refer to as chirality) from the catalyst to the target, favoring the product as a single enantiomer. This enzyme-like selectivity is particularly applied to bioactive products such as pharmaceutical agents and agrochemicals.
Catalytic hydrogenation of 3-hydroxypropanal to 1,3-propanediol
InactiveUS6342646B1Improves performance and lifetimeImprove hydrogenation activityOrganic compound preparationOxygen compounds preparation by reductionAsymmetric hydrogenation1,3-Propanediol
The present invention provides an improved process for the hydrogenation of 3-hydroxypropanal to 1,3-propanediol which comprises purifying an aqueous solution of 3-hydroxypropanal by contacting said aqueous solution with a purifying agent prior to hydrogenation.
Owner:EI DU PONT DE NEMOURS & CO
Method for preparation of (substituted radical containted) aminophenol by catalytic hydrogenation of (substituted radical containted) nitrophenol
ActiveCN101041623AOvercoming pollutionSolve problems such as recycling difficultiesMolecular sieve catalystsOrganic compound preparationMolecular sieveNitrophenol
The invention discloses a making method of aminophenol with substituted group through hydrogenating nitrophenol with substituted group, which adopts Ni-A / X typed loaded catalyst, wherein A is one of Co, Mn, Cu, Cr, Mo, Ca, Zn, Fe and W; the carrier X is one of their composition of zeolite molecular sieve, Al2O3, SiO2, TiO2, MgO, active charcoal or amorphic aluminium silicate; the mass percentage of Ni in the catalyst is 2-45% and the mass percentage of component A is 1-40%.
Owner:SHANGHAI HUAYI NEW MATERIAL
Process for the preparation of chiral beta amino acid derivatives by asymmetric hydrogenation
ActiveUS7468459B2Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsMetalloleEnantiomer
The present invention relates to a process for the efficient preparation of enantiomerically enriched beta amino acid derivatives which are useful in the asymmetric synthesis of biologically active molecules. The process comprises an enantioselective hydrogenation of a prochiral beta amino acrylic acid derivative substrate in the presence of a transition metal precursor complexed with a chiral ferrocenyl diphosphine ligand.
Owner:MERCK SHARP & DOHME LLC
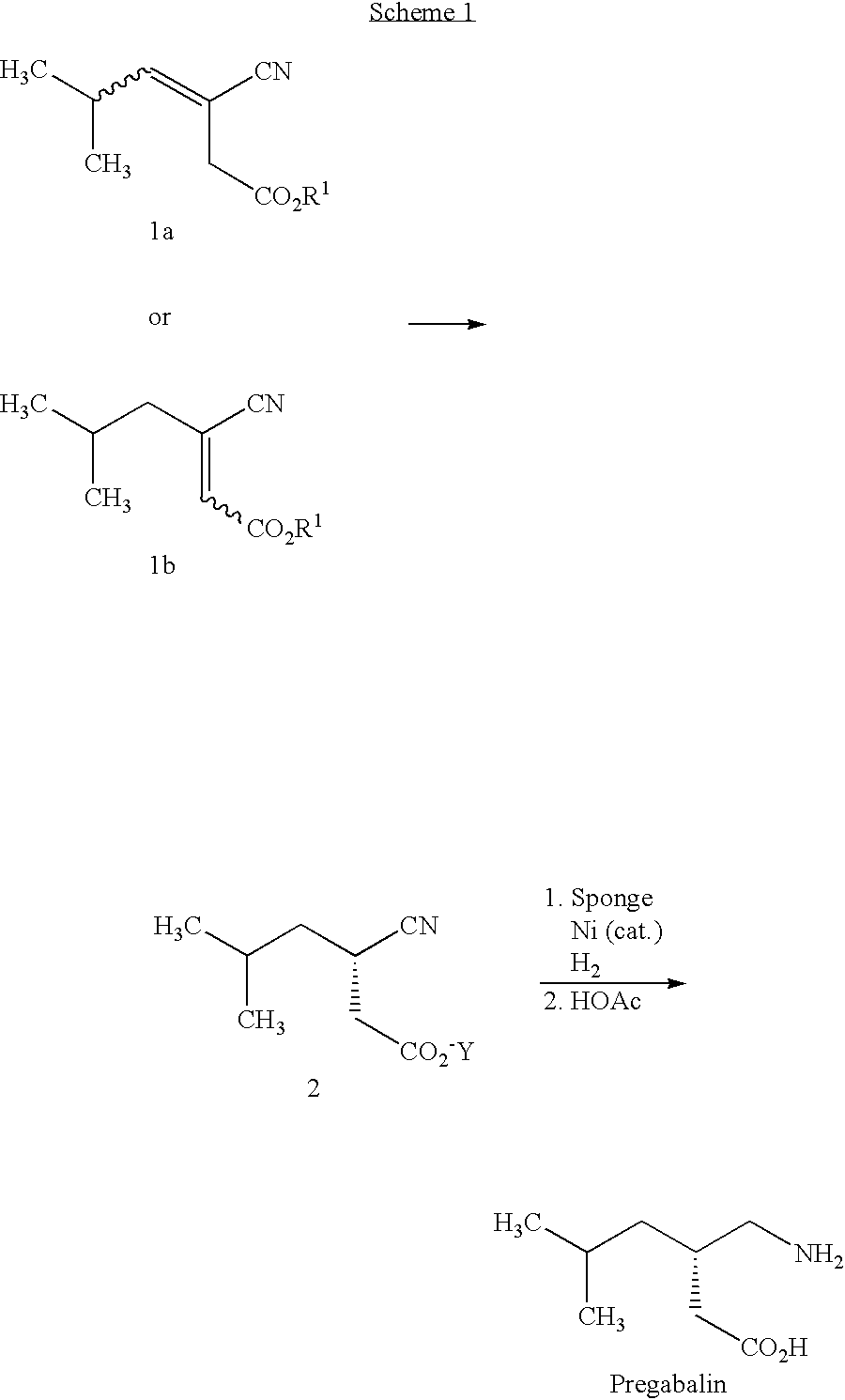
Asymmetric synthesis of pregabalin
InactiveUS6891059B2Effective approachDisposal of minimizedNervous disorderCarboxylic acid nitrile preparationPregabalinAsymmetric hydrogenation
This invention provides a method of making (S)-(+)-3-(aminomethyl)-5-methylhexanoic acid (pregabalin) or a salt thereof via an asymmetric hydrogenation synthesis. Pregabalin is useful for the treatment and prevention of seizure disorders, pain, and psychotic disorders. The invention also provides intermediates useful in the production of pregabalin.
Owner:WARNER-LAMBERT CO
Catalyst for catalytic hydrogenation of p-nitrophenol and preparation method thereof
ActiveCN102091626AEasy to separateSimple production processCatalyst carriersOrganic compound preparationSilanesP-Aminophenol
The invention relates to a catalyst for preparing p-aminophenol through catalytic hydrogenation of p-nitrophenol, and a preparation method thereof, and belongs to the technical field of catalysis. The carrier of the catalyst is a ceramic membrane, and the preparation method comprises the following steps of: modifying the membrane surface by using aminosilane; immersing in salt solution of an active ingredient; and performing hydrazine hydrate reduction to prepare a catalytic membrane. The invention has the advantages that: nano-scale catalyst particles are supported on the surface of the ceramic membrane modified by silane, and the activity and stability of the catalyst are improved; moreover, a problem that the catalyst is hardly separated from the product subsequently is avoided. The catalyst is simple in preparation process and high in activity and stability and can be widely applied to preparing the p-aminophenol through catalytic hydrogenation of the p-nitrophenol.
Owner:NANJING UNIV OF TECH
Process for the catalytic hydrogenation of organic compounds and supported catalyst therefor
InactiveUS20020087036A1Extended shelf lifeConstant compositionOrganic compound preparationOrganic chemistry methodsRutheniumAsymmetric hydrogenation
A process for the catalytic hydrogenation of an organic compound, in particular a labile organic compound, in the presence of a support catalyst with a coating containing ruthenium as active metal and a total of 1.01 to 30 wt. % of active metals. Higher stereoselectivity and a greater catalyst shelf life may be obtained by using a support catalyst of which the oxide, carbide, nitride or siliceous support material has a BET (N2) surface area smaller than 10 m2 / g, particularly preferably 0.1 to 5 m2 / g, prior to loading with at least one active metal diatomaceous earth with a BET (N2) surface area greater than 2 m2 / g is excluded and the ruthenium content thereof makes up at least 50 wt. %, preferably at least 99 wt. % of the active metals. The process and the catalysts are particularly suitable for the hydrogenation of polyfunctional compounds such as hydroxycarbonyl compounds and aromatic amines.
Owner:DEGUSSA AG
Method for synthesizing gamma-valerolactone based on catalytic hydrogenation
ActiveCN103193736AHigh activityImprove stabilityOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsHydrogenAsymmetric hydrogenation
The invention discloses a method for synthesizing gamma-valerolactone based on catalytic hydrogenation. According to the method, levulinic acid or levulinate derivatives in a reaction solvent is / are subject to catalytic hydrogenation for 1-10 hours by adding hydrogen gas and keeping the hydrogen gas pressure at 0.5-5.0 MPa under the action of a supported cobalt catalyst and the temperature of 50-240 DEG C, and the reaction liquid is treated to obtain the gamma-valerolactone. The cobalt catalyst prepared according to the method has excellent catalytic activity and selectivity, the conversion rate of levulinic acid or levulinate is 100 percent, the yield of the gamma-valerolactone reaches up to 99 percent above; and the method has a simple operation technology, and is low in cost, safe and environment-friendly.
Owner:ZHEJIANG UNIV OF TECH
Platinum/carbon nanotube catalyst and preparation method and application thereof
InactiveCN102039121AMild conditionsEasy to manufactureOrganic reductionMaterial nanotechnologyCarbon nanotubeRoom temperature
The invention relates to a platinum / carbon nanotube catalyst suitable for multiphase asymmetric hydrogenation reaction. Platinum is loaded on a carbon nanotube carrier. A preparation method comprises the following steps of: heating a purified carbon nanotube in nitric acid, washing, filtering, washing by using water until the pH value of filtrate is neutral, drying to obtain the carbon nanotube carrier, soaking in aqueous solution of chloroplatinic acid, and performing ultrasonic treatment at room temperature; and stirring and impregnating a mixture of the carbon nanotube and the aqueous solution of chloroplatinic acid, raising the temperature to 110 DEG C from room temperature, drying at the temperature of 110 DEG C, grinding into fine powder, reducing by using aqueous solution of sodiumformate with heating, filtering, washing by using deionized water and drying. The invention also provides the preparation method of the catalyst and application of the catalyst to the multiphase asymmetric hydrogenation reaction.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Catalyst for preparing aromatic amine by catalytic hydrogenation of nitrobenzene compounds and its use method
InactiveCN1775351ALow costEasy to manufactureCatalyst carriersOrganic compound preparationPorous carbonCarbon nanotube
The present invention relates to a catalyst for preparing arylamine by utilizing catalytic hydrogenation of nitrobenzene compound and its application method, belonging to the field of catalytic synthesis technology in application chemistry. Said catalyst is formed from carrier and active component, its carrier can be metal oxide; carbon materials of active carbon, carbon nano tube and porous carbon, etc.; micropore molecular sieve with different compositions or silica-base material into which one or several kinds of hetero-atoms are added, also can be organic high-molecular polymer. The active component can be double-component (multicomponent) metal formed from metal silver and gold or silver gold and palladium, platinum and copper, etc. in which the weight percentage of active metal component and carrie.r is 0.5-30%.
Owner:DALIAN UNIV OF TECH
Preparation method of (R)-2-(N-tertbutyloxycarbonylamino)biphenylpropanol
InactiveCN105330569AMild reaction conditionsImprove conversion rateCarbamic acid derivatives preparationOrganic compound preparationAlcoholTert-Butyloxycarbonyl protecting group
The invention discloses a preparation method of (R)-2-(N-tertbutyloxycarbonylamino)biphenylpropanol represented by formula (I). The method comprises the following steps: carrying out an Erlenmeyer-Plochl cyclization reaction on biphenylcarboxaldehyde and N-acylglycine, hydrolyzing or alcoholyzing, and carrying out asymmetric hydrogenation to obtain (R)-N-acylbiphenylalanine or an ester thereof; and carrying out acid hydrolysis, reduction and amino protection on the (R)-N-acylbiphenylalanine or the ester thereof, or carrying out reduction, acid hydrolysis and amino protection on the (R)-N-acylbiphenylalanine or the ester thereof, or directly reducing the (R)-N-acylbiphenylalanine or the ester thereof in order to obtain the product (R)-2-(N-tertbutyloxycarbonylamino)biphenylpropanol. The product is a key intermediate of Sacubitril (AHU-377) which is one of a novel blood pressure reducing medicine LCZ696. The method has the advantages of easily available raw materials, and suitableness for industrial production.
Owner:天台宜生生化科技有限公司
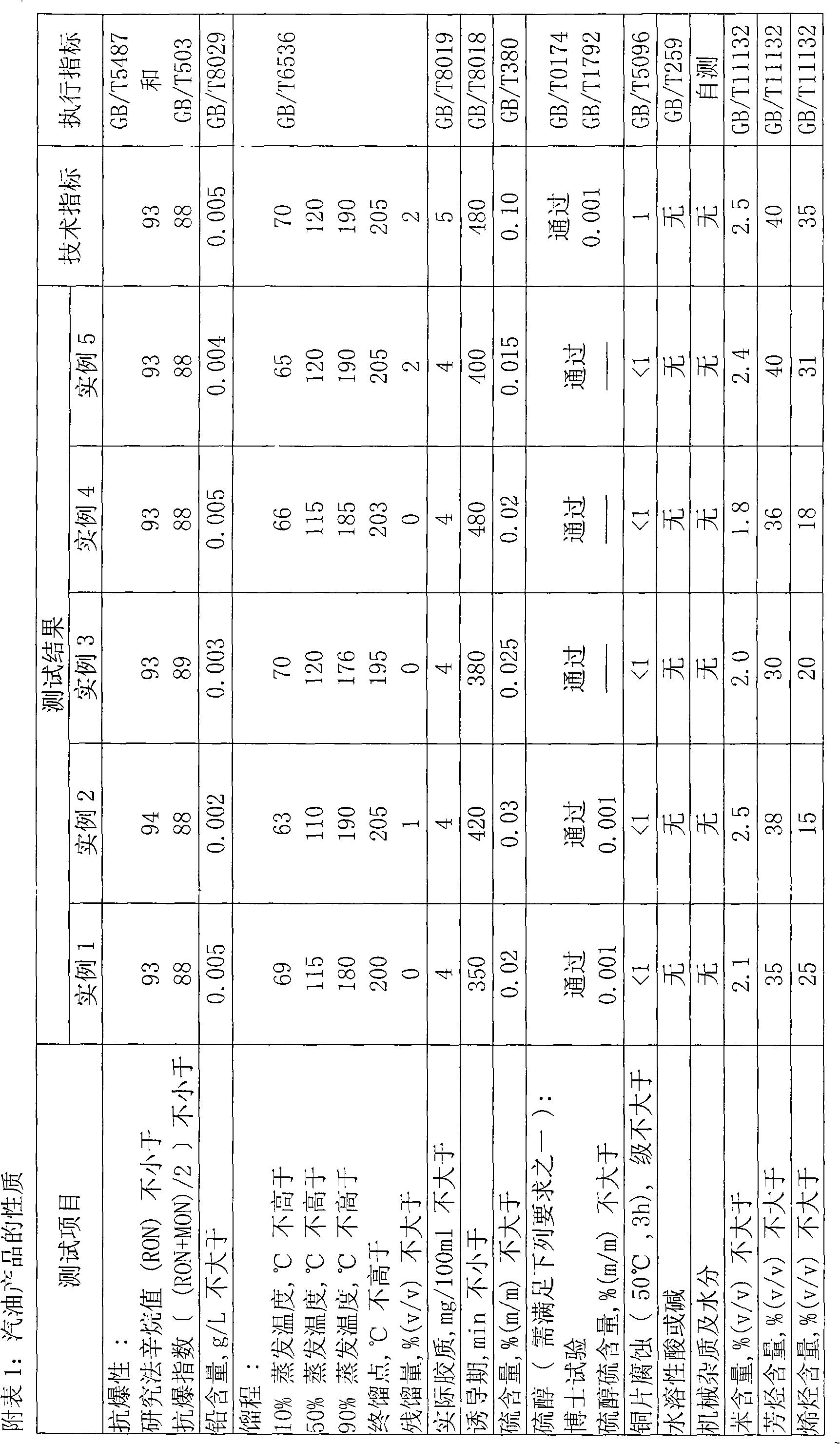
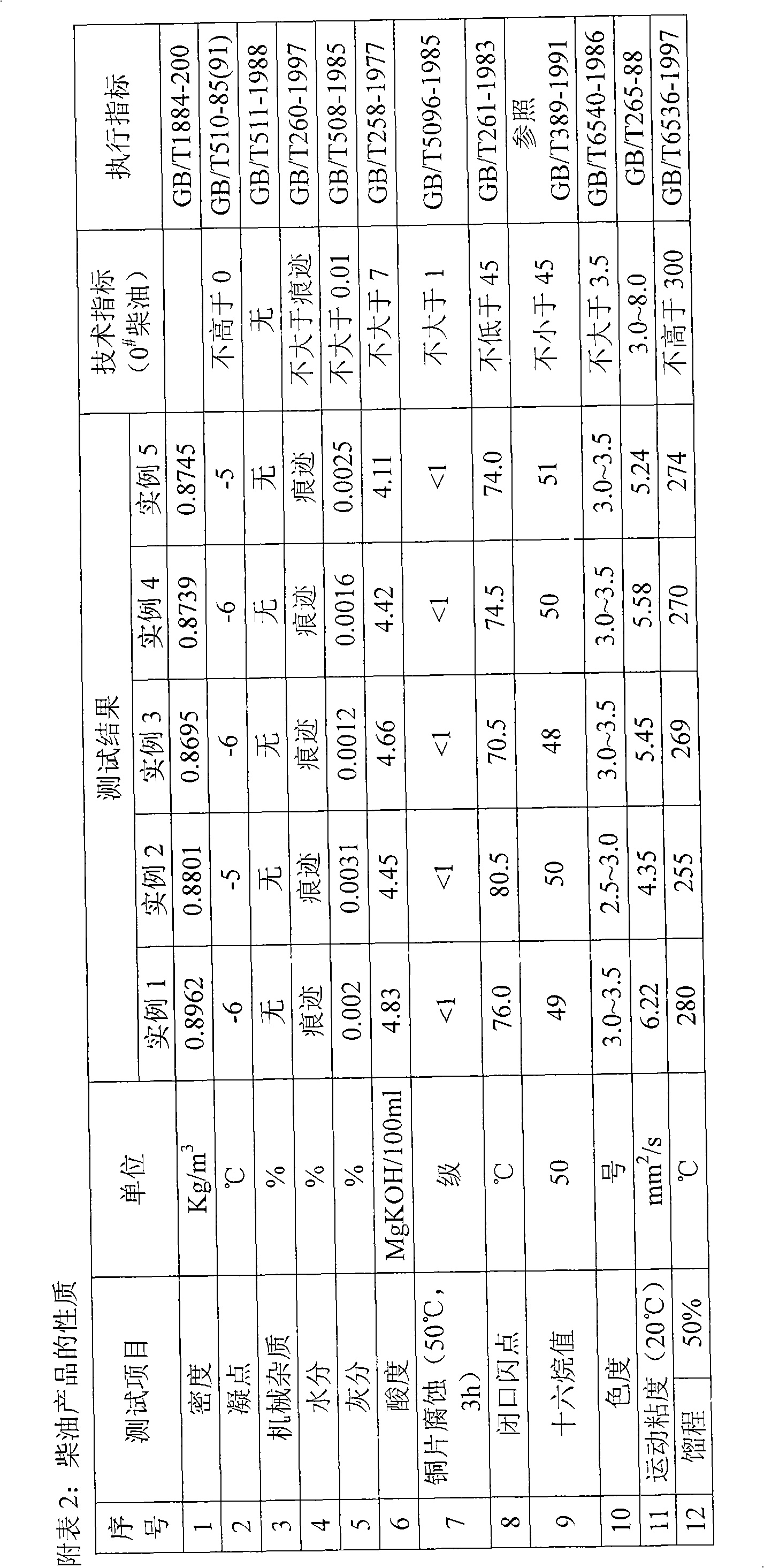
Inferior diesel oil quality change method
ActiveCN1769392AIncrease cetane numberReduce contentTreatment with hydrotreatment processesLiquid productSulfur
The invention provides a method of improving poor diesel. Said method is made up of two technique: quanlity-improving through hydrogenation and solvent extraction. Poor diesel raw material is treated through quality-improving hydrogenation catalysis bed to strip sulfur, nitrogen and aromatic hydrocarbons, then the final liquid product is extracted through solvent oil to make sure that most of the aromatic hydrocarbons are extracted and get clean diesel products which is low in sulfur, nitrogen and aromatic hydrocarbons, but high in cetane ratio, and the extracted aromatic hydrocarbons are circulated to catalysis bed. Group techniques in said invention could deeply decrease the content of sulfur, nitrogen and aromatic hydrogenation in diesel, increase the cetane ratio at the same time through making full use of their own features, therefore, making the quality of the production meet the need of fuel specification and environmental legislation, also maintain a higher diesel rate.
Owner:CHINA PETROLEUM & CHEM CORP +1
Process for the preparation of chiral beta amino acid derivatives by asymmetric hydrogenation
InactiveCN1761642AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsMetalloleAsymmetric hydrogenation
The present invention relates to a process for the efficient preparation of enantiomerically enriched beta amino acid derivatives which are useful in the asymmetric synthesis of biologically active molecules. The process comprises an enantioselective hydrogenation of a prochiral beta amino acrylic acid derivative substrate in the presence of a transition metal precursor complexed with a chiral ferrocenyl diphosphine ligand.
Owner:MERCK SHARP & DOHME BV
Method for enantioselective hydrogenation of chromenes
A method for preparing an enantiomeric chromane, by asymmetrically hydrogenating a chromene compound in the presence of an Ir catalyst having a chiral ligand. The method includes the enantioselective preparation of enantiomeric equol. A preferred Ir catalyst has a chiral phosphineoxazoline ligand. Enantiomeric chromanes of high stereoselective purity can be obtained.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI +1
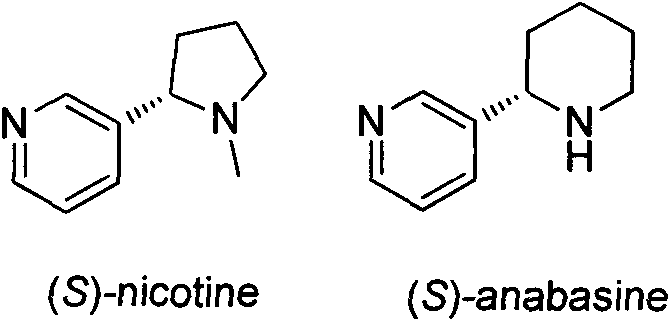
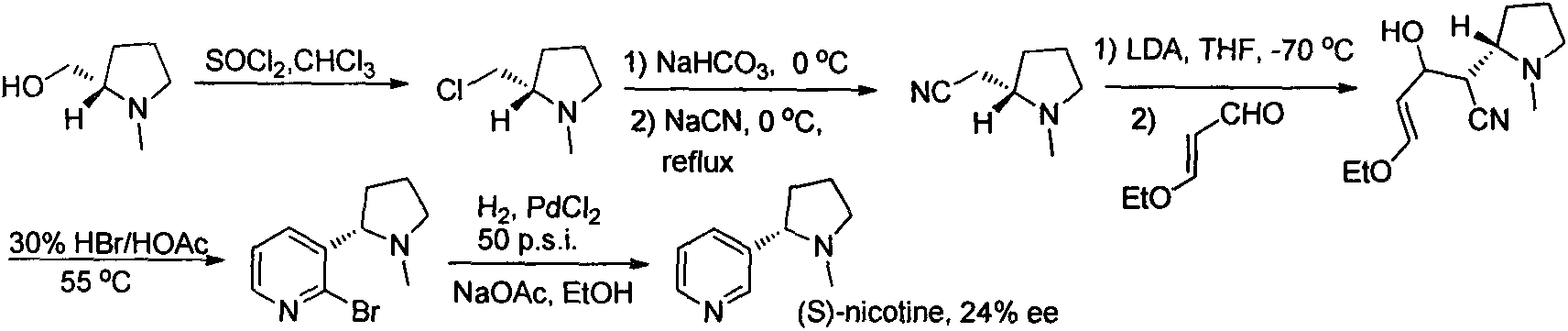
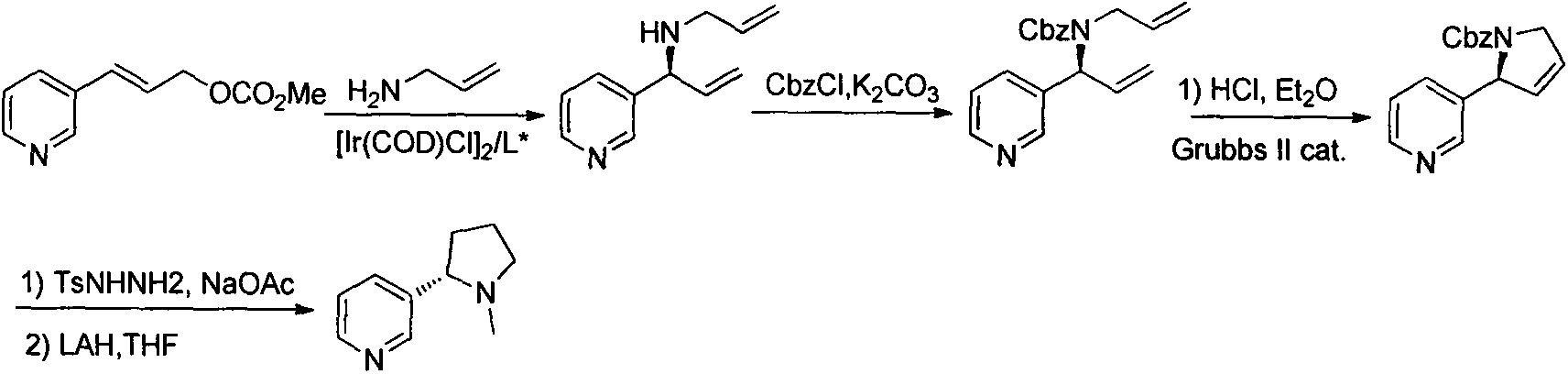
Asymmetric synthesis method for botanical pesticide nicotine and anabasine
ActiveCN104341390AHigh purityEasy to operateOrganic chemistryBulk chemical productionIridiumSynthesis methods
The invention relates to an asymmetric synthesis method for botanical pesticide nicotine and anabasine. The low-cost and easily acquired 2,5-dibromopyridine is taken as an initial raw material and is processed in two steps, so that the hydrogenation precursor annular imine is acquired; under the induction of the chiral catalyst, iridium-phosphine oxazoline, an important hydrogenated product intermediate is acquired through high enantioselectivity; the intermediate is processed in two steps, so that L-nicotine is acquired; the intermediate is converted into L-anabasine in one step. The asymmetric hydrogenation of the annular imine containing pyridine gene is taken as the key step of the method. According to the invention, the chiral catalyst, iridium-phosphine oxazoline, is used for catalyzing the asymmetric hydrogenation and the key intermediate with ultrahigh ee value is acquired, and then the methylation and reduction bromine-removing two-step reaction is performed for converting, so that the target products, natural nicotine and anabasine, are acquired. According to the invention, the operation is stable, the purity is high and the cost is low.
Owner:NANKAI UNIV
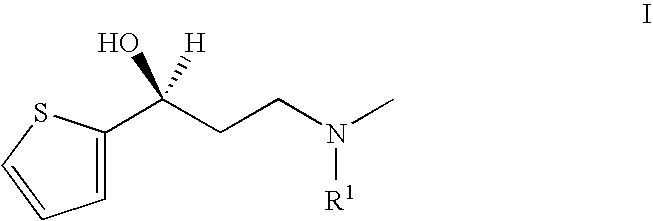
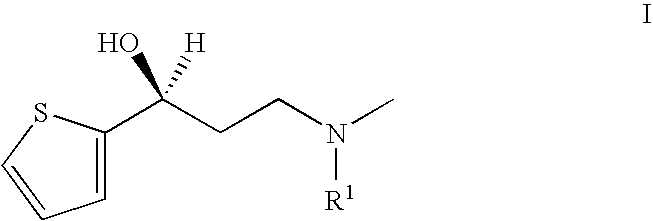
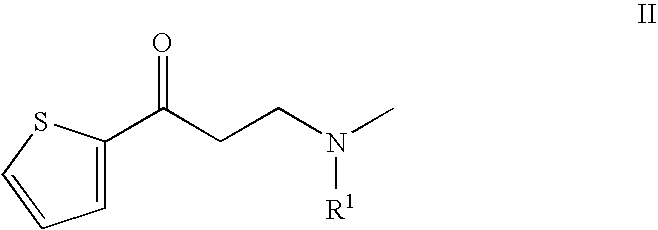
Process for the preparation of N-alkyl-N-methyl-3-hydroxy-3-(2-thienyl)-propylamines
InactiveUS20050197503A1High chemical purityHigh optical purityOrganic chemistryPropylamineAsymmetric hydrogenation
The present invention relates to an improved process for preparing chiral N-substituted N-methyl-3-hydroxy-3-(2-thienyl)-propylamine on an industrial scale using an asymmetric hydrogenation as a key step and optionally a special sequence of subsequent steps, using a catalyst system consisting of rhodium and (2R, 4R)-4-(dicyclohexylphosphino)-2-(diphenyl-phosphino-methyl)-N-methyl-aminocarbonyl-pyrrolidine.
Owner:BOEHRINGER INGELHEIM INT GMBH
Method for preparing diaminonaphthalene by catalytic hydrogenation of dinitronaphthalene
InactiveCN101575295AImprove conversion rateHigh selectivityOrganic compound preparationAmino compound preparationPalladium catalystReaction temperature
A method for preparing diaminonaphthalene by catalytic hydrogenation of dinitronaphthalene relates to a preparation technology of the diaminonaphthalene, which comprises the following steps: adding a palladium catalyst comprising active components and a carrier and adding the dinitronaphthalene and solvent to a stainless steel high-pressure reactor with an agitator, closing the reactor, replacing air in the reactor with nitrogen for at least three times and then replacing the nitrogen in the reactor with hydrogen for at least three times, and then filling the hydrogen in the reactor so that the reaction pressure in the reactor reaches 0.4-4.0MPa, heating the reactor so that the reaction temperature reaches 30-150 DEG C so as to carry out catalytic hydrogenation to prepare the diaminonaphthalene. The method adopts the hydrogenation reaction technology in the stainless steel high-pressure reactor with the agitator; a catalyst carrier is pretreated to improve the activity of the catalyst and reduce the consumption of the catalyst. By optimizing the technological conditions, the rate of conversion from the dinitronaphthalene to the diaminonaphthalene is effectively improved and high selectivity of the product diaminonaphthalene is maintained.
Owner:JIANGSU POLYTECHNIC UNIVERSITY +1
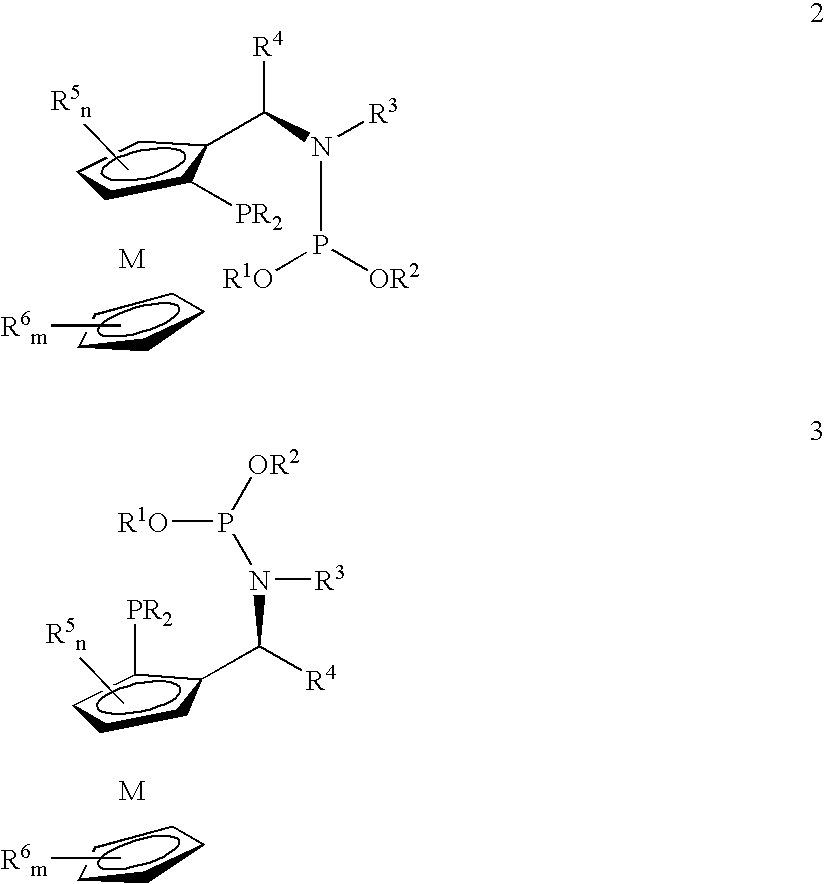
Phosphine-phosphoramidite compounds
ActiveUS6906212B1Hydrocarbon by hydrogenationGroup 8/9/10/18 element organic compoundsIsomerizationSilica hydride
Disclosed are novel phosphine-phosphoramidite compounds which may be employed in combination with a catalytically-active metal to effect a wide variety of reactions such as asymmetric hydrogenations, asymmetric reductions, asymmetric hydroborations, asymmetric olefin isomerizations, asymmetric hydrosilations, asymmetric allylations, asymmetric conjugate additions, and asymmetric organometallic additions. Also disclosed are a process for the preparation of the phosphine-phosphoramidite compounds, metal complex compounds comprising at least one of the phosphine-phosphoramidite compounds and a catalytically-active metal and hydrogenation processes utilizing the metal complex compounds.
Owner:JOHNSON MATTHEY PLC
Catalyst for preparing halogenated aniline through catalytic hydrogenation of halogenated nitrobenzene and application thereof
ActiveCN103349983AImprove blockageImprove adsorption capacityOrganic compound preparationAmino compound preparationNitrobenzeneAniline
The invention discloses a catalyst for preparing halogenated aniline through catalytic hydrogenation of halogenated nitrobenzene. The catalyst comprises an activated carbon carrier and active component platinum adhered to the activated carbon carrier, and the mass percent of platinum in the catalyst is 0.1%-5%. The preparation method of the catalyst comprises the following steps: 1, pretreating activated carbon with inorganic acid, then washing to be neutral, and reserving after drying; 2, placing the dried activated carbon in carbonate aqueous solution for impregnating; 3, dissolving a platinum compound in water to obtain active component solution; 4, dripping the active component solution into the carbonate aqueous solution with impregnated activated carbon, carrying out insulated impregnating, adjusting the pH value of the system, and filtering after cooling to obtain a filter cake; 5, reducing the filter cake with a reducing agent, washing to be neutral, and drying to obtain the catalyst. The catalyst is simple in preparation process, can be repeatedly used, is high in reactivity, high in selectivity and good in stability, and can inhibit the side reactions of dehalogenation effectively.
Owner:XIAN CATALYST NEW MATERIALS CO LTD
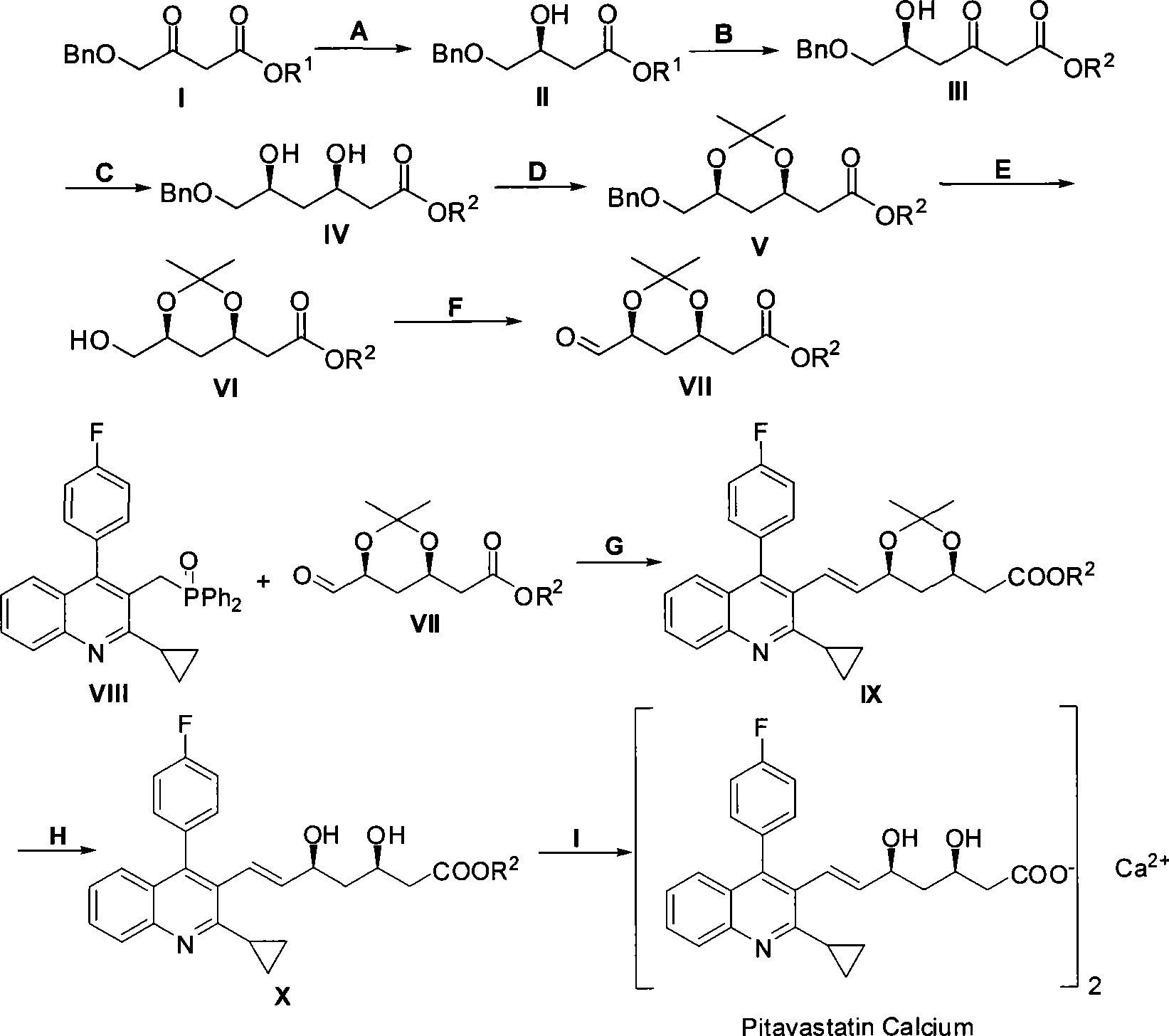
Method for preparing pitavastatin calcium raw material medicine using asymmetric hydrogenation
ActiveCN101386592ASolve the problem of expensive chiral side chainsMetabolism disorderAsymmetric synthesesClaisen condensationEthyl butyrate
The invention relates to a method for preparing a pitavastatin calcium raw material by asymmetric hydrogenation in the technical field of medicinal chemistry. The method comprises the following steps: performing catalytic hydrogenation on 4-benzyloxy-ethyl-acetoacetate I by using a chiral catalyst to prepare (S)-4-benzyloxy-3-hydroxy-ethyl-butyrate, and then obtaining the pitavastatin calcium through a series of reactions such as Claisen condensation, reduction, hydroxyl protection, removal of benzyl, oxidation and the like. In the invention, the highly-efficient chirality added value is realized by the use of catalytic amount of the chiral catalyst (wherein the ratio of substrate to the catalyst can reach 3, 000 to 1), and by performing the asymmetric hydrogenation and the reduction on non-chiral ketones to generate chiral alcohols compounds directly (wherein the enantioselectivity can be up to 94.3 percent).
Owner:JIANGSU WANBANG BIOPHARMLS +1
Chiral diphosphite ligand and iridium composite catalyst and preparation thereof method and application to asymmetrical hydrogenization synthesis (S)-metolachlor
ActiveCN101857612AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAlkaneDiphosphines
The invention relates to a kind of chiral diphosphite ligands, an iridium composite catalyst thereof, a preparation method and application thereof. The ligands are obtained through using chiral (R)-(S)-1-dimethylamino ethyiferroene as raw materials to react with diphenyl phosphonium chloride under the effect of butyl lithium and then to carry out displacement reaction with diaryl phosphine alkane. The chiral diphosphite ligands respectively act with homotropilidene compositions of iridous chloride, tetrabutyl ammonium iodide and glacial acetic acid, and imine asymmetrical hydrogenization catalysts can be obtained. When the iridium-diphosphine catalysts are used for catalyzing 2-methyl-6-ethyl-N-methylene aniline (EMA-imine) hydrogenization reaction, (S)-N-(1-anisyl-2-propyl)-2-methyl-6- ethylaniline ((S)-NNA) can be obtained, and the antimer excessive value (ee) can reach 86.5 percent. The (S)-NNA and chloracetyl chloride carry out acylation reaction to obtain (S)-metolachlor with the ee value of 86 percent. Thereby, the ligands provided by the invneiton can be used for synthesizing chiral herbicidal chemicals of (S)-metolachlor.
Owner:NANJING UNIV OF TECH +2
Catalyst used in the catalytic hydrogenation of p-nitrophenol and its preparation method
ActiveCN101007275AHigh activityReduce loadCatalyst carriersOrganic compound preparationFiltrationUltrasonic radiation
The invention involves a load palladium amorphous alloy catalyst used for nitrophenol hydrogenation and preparation method and belongs to catalyst technology field. Said catalyst uses NaY, MCM-41, molecular screen, Al2O3, TiO2, SiO2 or MgO as carrier, and is loaded with Pd-B amorphous alloy, the quality of Pd is 0.1-0.5% of toatl quality of catalyst. The preparation process includes ultrasonic dispersion, vacuum impregnation, KBH4 chemical reduction under vacuum conditions, filtration and washing of carriers; it is characterized by introducing vacuum and ultrasonic radiation conditions. The advantages of the invention lies in its high activity of catalyst, low preparation cost and simple preparation process, it could widely apply to the preparation of p-aminophenol through nitrophenol catalysis and hydrogenation.
Owner:NANJING UNIV OF TECH
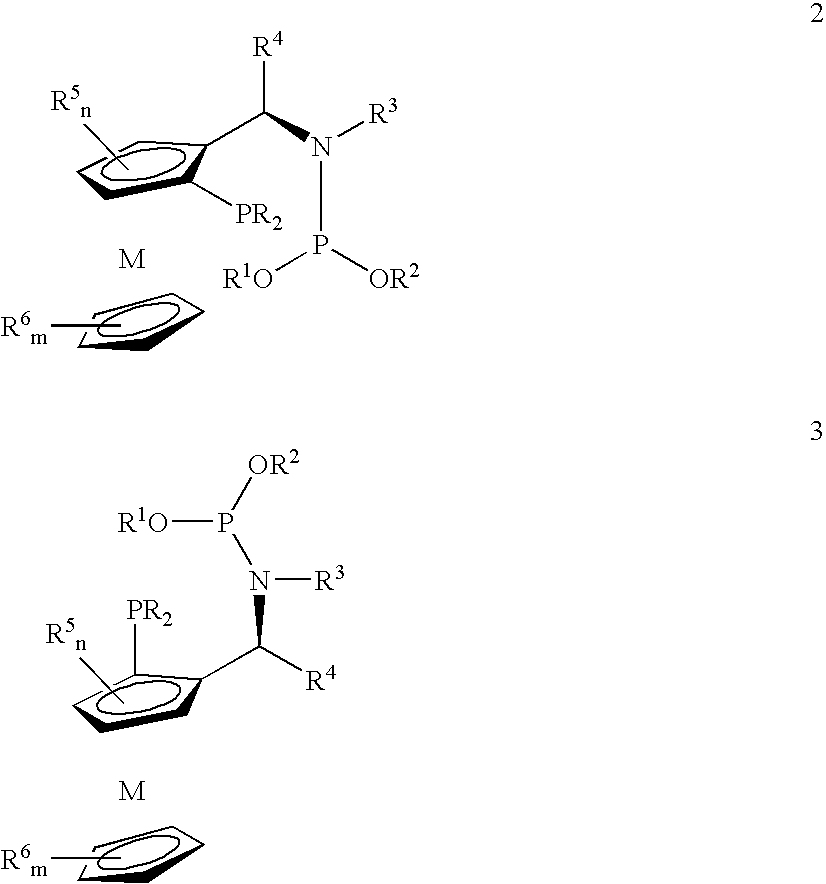
Novel chiral phosphorus ligands
InactiveUS20120277455A1High enantioselectivityHigh reactivityRhodium organic compoundsPhosphorus organic compoundsAsymmetric hydrogenationCoordination complex
Owner:BOEHRINGER INGELHEIM INT GMBH
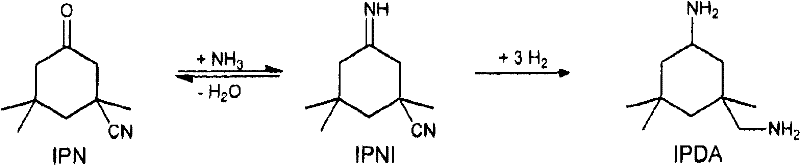
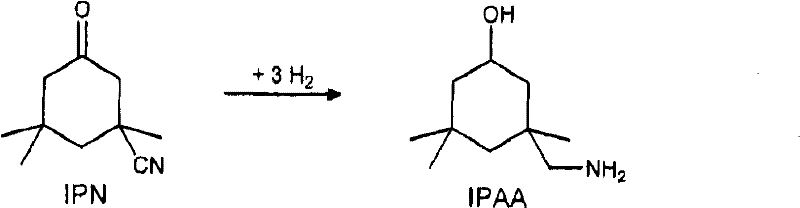
Process for preparing 3-aminomethyl-3,5,5-trimethylcyclohexylamine
InactiveCN102531916AIncrease concentrationOrganic compound preparationWater/sewage treatment by heatingIsophoroneAsymmetric hydrogenation
The invention relates to an improved process for preparing 3-aminomethyl-3,5,5-trimethylcyclohexylamine, referred to hereinafter as isophoronediamine or, in abbreviated form, IPDA, by: I. preparation of isophorone by catalyzed aldol condensations with acetone as reactant; II. Reaction of isophorone with HCN to form isophoronenitrile (IPN, 3-cyano-3,5,5-trimethylcyclohexanone); III. catalytic hydrogenation and / or catalytic reductive amination (also referred to as aminative hydrogenation) of 3-cyano-3,5,5-trimethylcyclohexanone, hereinafter called isophoronenitrile or, in abbreviated form, IPN, to give the isophoronediamine.
Owner:EVONIK DEGUSSA GMBH
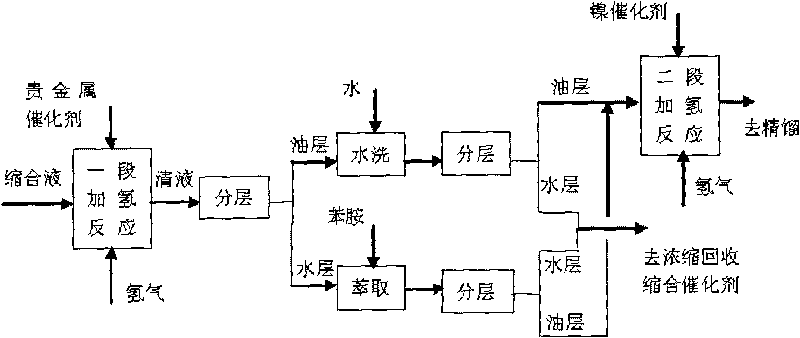
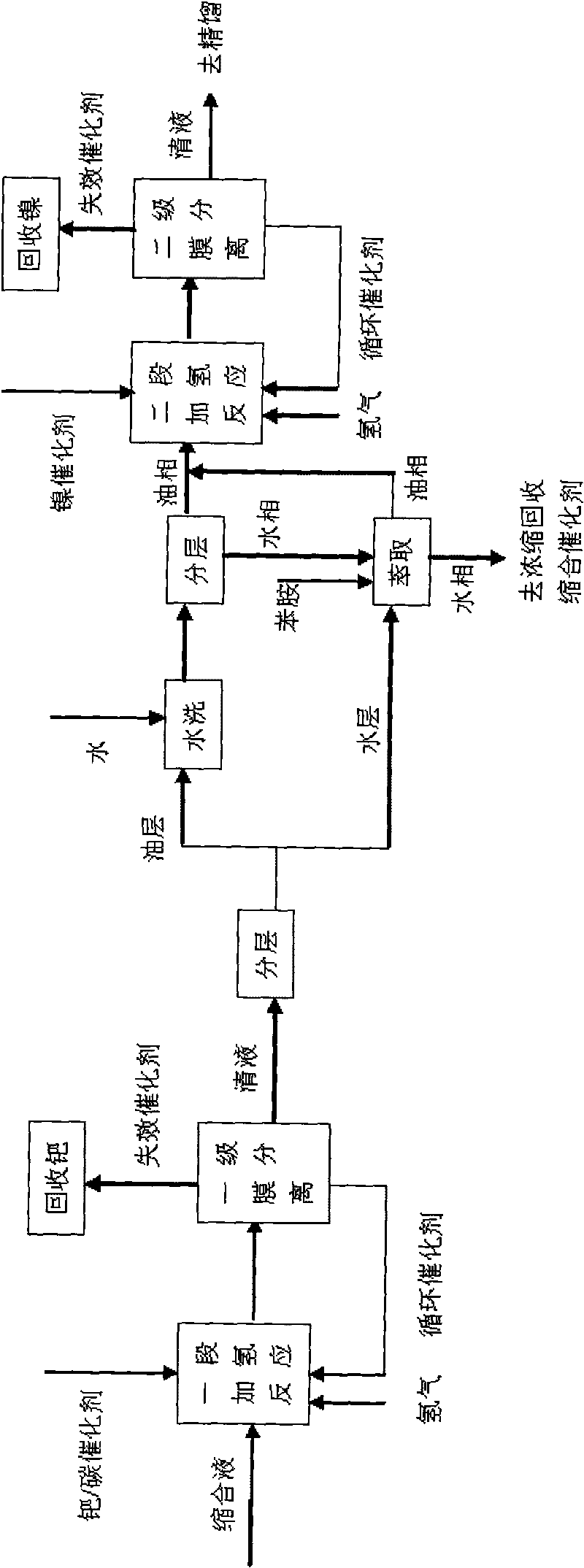
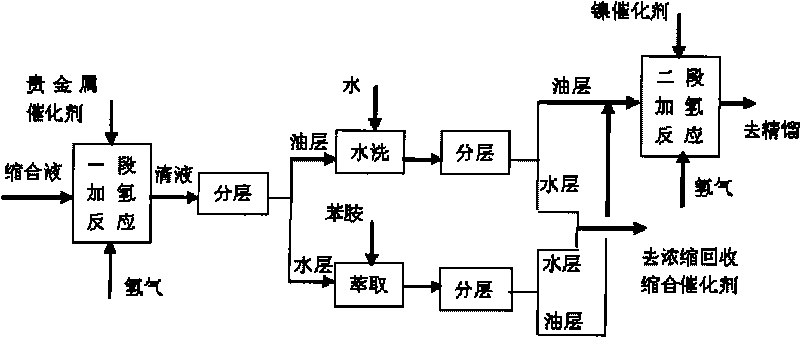
Method for preparing 4-amino diphenylamine by catalytic hydrogenation
ActiveCN101691332AEasy to cleanImprove regenerative abilitySemi-permeable membranesAmino compound purification/separationNitrosoNitrobenzene
The invention provides a method for preparing 4-amino diphenylamine by catalytic hydrogenation. The method adopts two sections of hydrogenation reaction processes, and comprises the following steps: performing the condensation reaction of condensation solution formed by the condensation reaction of nitrobenzene and aniline which serve as raw materials under an alkaline condition by using using a noble metal hydrogenation catalyst and a nickel catalyst sequentially so as to make the conversion rate of 4-nitroso diphenylamine, 4-nitro diphenylamine and azoxybenzene achieve 100 percent; and separating the noble metal hydrogenation catalyst and the nickel catalyst by using a two-stage membrane separation component system to avoid the loss of small particles of catalyst. At the same time, the method has high degree of automation and can easily realize the continuous hydrogenation process.
Owner:JIANGSU YANGNONG CHEM GROUP +1
Chiral tridentate PNN ligand and application of same in asymmetric hydrogenation
ActiveCN105153229AHigh electronegativityGood chiral environmentOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsIridiumKetone
The invention discloses a chiral tridentate PNN ligand and application of the same in asymmetric hydrogenation and similar reactions. The tridentate nitrogen phosphine ligand disclosed in the invention has been the first reported nitrogen phosphine ligand containing chiral oxazoline so far and has been successfully applied to high-efficiency and high-selectivity hydrogenation and similar reactions of ketone and imine salts. Compared with other ligand, the ligand provided by the invention is simpler in synthetic route, higher in yield and environment-friendlier; moreover, the metal complex of the ligand shows better selectivity and a higher turn-over number in asymmetric hydrogenation. An iridium complex of the chiral tridentate PNN ligand has successfully realized asymmetric reduction of beta-ketone ester into beta-alcohol ester (which is a raw material for synthesis of molecular drugs duloxetine and atomoxetine), asymmetric hydrogenation of alpha-hydroxyacetophenone into alpha-hydroxyphenylethyl alcohol and asymmetric hydrogenation of acetophenone into phenylethyl alcohol, which is of important significance to production of the medical industry.
Owner:SHENZHEN CATALYS SCI & TECH CO LTD +2
Preparation method of Z-1,1,1,4,4,4-hexafluoro-2-butene
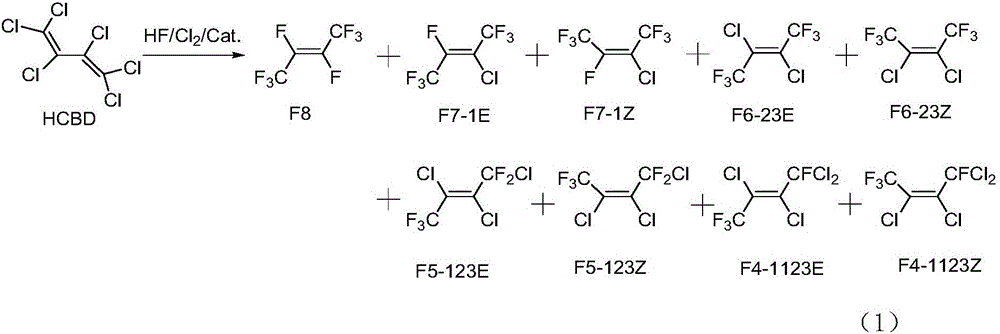
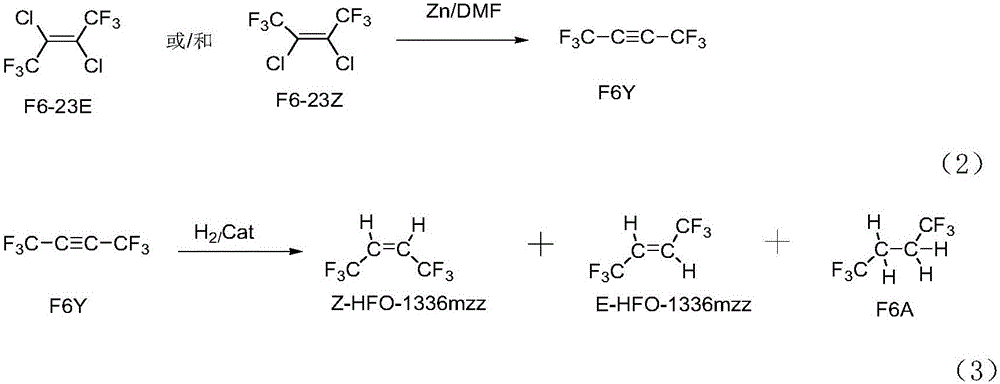
ActiveCN106008147ALow priceHigh activityPreparation by dehalogenationPreparation by halogen replacementChemical synthesisGas phase
The invention relates to a preparation method of Z-1,1,1,4,4,4-hexafluoro-2-butene, belonging to the field of chemical synthesis. The method comprises the following steps of taking hexachlorobutadiene (HCBD) as a raw material, performing gas-phase catalytic chlorination-fluoridation separation to obtain 2,3-dichlorohexafluoro-2-butene, performing liquid-phase dechlorination to obtain hexafluoro-2-butyne, and preparing the Z-1,1,1,4,4,4-hexafluoro-2-butene (Z-HFO-1336mzz) by means of performing gas-phase catalytic hydrogenation, wherein three reactions are included totally. The Z-1,1,1,4,4,4-hexafluoro-2-butene with high selectivity is obtained by means of performing catalytic hydrogenation by adopting the hexafluoro-2-butyne, and meanwhile the product is easily separated from side products and the raw material. The method disclosed by the invention has the advantages that the original raw material is easily obtained, the activity of a catalyst is high, the service life of the catalyst is long, the raw material can be recycled, and the standard of zero emissions is reached.
Owner:泉州宇极新材料科技有限公司
Catalyst for using coal tar to prepare clean fuel oil by catalytic hydrogenation, preparation method and applications
InactiveCN101574659AHigh activityHigh selectivityCatalyst activation/preparationMetal/metal-oxides/metal-hydroxide catalystsFuel oilSilicon oxide
The invention relates to a catalyst for using coal tar to prepare clean fuel oil by catalytic hydrogenation, comprising the following components in percentage by mass: 4 to 15 percent of molybdenum oxide, 3 to 9 percent of nickel oxide, 0.1 to 5 percent of cobalt oxide, 10 to 25 percent of tungsten oxide, 2.5 to 40 percent of silicon oxide and 26 to 65 percent of aluminium oxide. The preparation method of the catalyst comprises the following steps of: firstly, preparing a silicon oxide-aluminium oxide carrier, loading active components of Co, Mo, Ni and W on the carrier by an isosteric dipping method, drying, baking and forming. The catalyst can be used for the preparation of the clean fuel oil by catalytic hydrogenation of the coal tar, gasoline and diesel oil obtained from the prepared fuel oil after being separated respectively meet the national standard of 93# gasoline and 0# diesel oil.
Owner:SHANXI INST OF COAL CHEM CHINESE ACAD OF SCI
Method for preparing cis-pinane by asymmetric catalytic hydrogenation of alpha-pinene
InactiveCN104003831AImprove conversion rateHigh enantioselectivityOrganic-compounds/hydrides/coordination-complexes catalystsHydrocarbon by hydrogenationNitrogen gasHigh pressure
The invention discloses a method for preparing cis-pinane by asymmetric catalytic hydrogenation of alpha-pinene, and belongs to the chemical engineering field. The method comprises the process steps: in a nitrogen atmosphere, heating RhCl3.3H2O to dissolve in an ethanol solution, and forming a solution A; dissolving newly recrystallized PPh3 in a deoxygenated ethanol, and forming a solution B; adding the solution B into the solution A, refluxing for a certain period of time, filtering under reduced pressure while being hot, washing with deoxygenated ether, carrying out vacuum drying to obtain a rhodium phosphine complex RhCl(PPh3)3; and adding the prepared rhodium phosphine complex RhCl(PPh3)3, an ionic liquid and alpha-pinene into a high-pressure reaction kettle according to a certain proportion, putting a cover and sealing, respectively replacing with nitrogen gas and hydrogen gas, carrying out pressure maintaining and leakage detection, and carrying out a reaction under certain conditions to prepare cis-pinane. The method has the advantages of mild reaction conditions, high conversion rate of alpha-pinene and high enantioselectivity of cis-pinane; the ionic liquid catalyst system is easily separated from the product and can be recycled; and the process flow is simple, and the energy consumption is low.
Owner:KUNMING UNIV OF SCI & TECH
Catalyst for preparing halogeno anilin through catalytic hydrogenation of halogeno nitrobenzene and preparation method
InactiveCN101049562AHigh reactivityGood choiceOrganic compound preparationAmino compound preparationActive componentNitrobenzene
A catalyst with high reaction activity, selectivity and stability for preparing halophenylamine by catalytic hydrogenation of halonitrobenzene is composed of carrier (ZrO2, TiO2, SiO3, or MgO) and active component (Au) (0.5-10 Wt %). Its preparing process includes such steps as providing chloroauric acid as precursor, depositing with urea, and ageing, washing, drying, and calcining at 200-350 deg.C.
Owner:TSINGHUA UNIV
Method for manufacturing optically active menthol
InactiveUS20130253228A1Easy to operateHigh selectivityOxygen-containing compound preparationPreparation by isomerisationMentholAsymmetric hydrogenation
An object of the present invention is to provide a method for manufacturing an optically active menthol having fewer steps, which generates less environmentally polluting waste because a catalytic reaction is involved in all of the steps, and is capable of saving a production cost. The present invention relates to a method for manufacturing an optically active menthol, including the following steps: A-1) asymmetrically hydrogenating at least one of geranial and neral to thereby obtain an optically active citronellal, B-1) conducting a ring-closure reaction of the optically active citronellal in the presence of an acid catalyst to thereby obtain an optically active isopulegol, and C-1) hydrogenating the optically active isopulegol to thereby obtain an optically active menthol.
Owner:TAKASAGO INTERNATIONAL CORPORATION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com