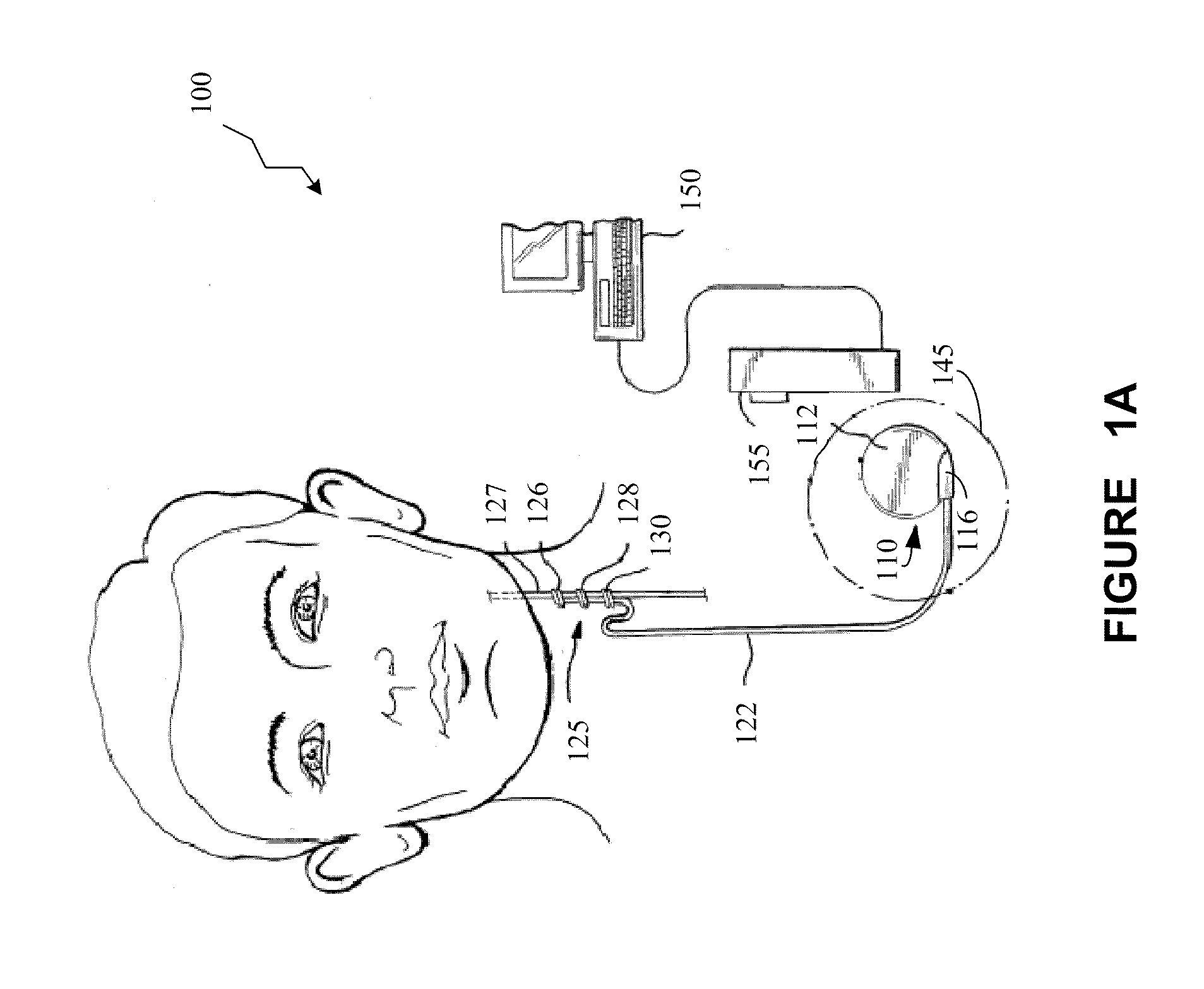
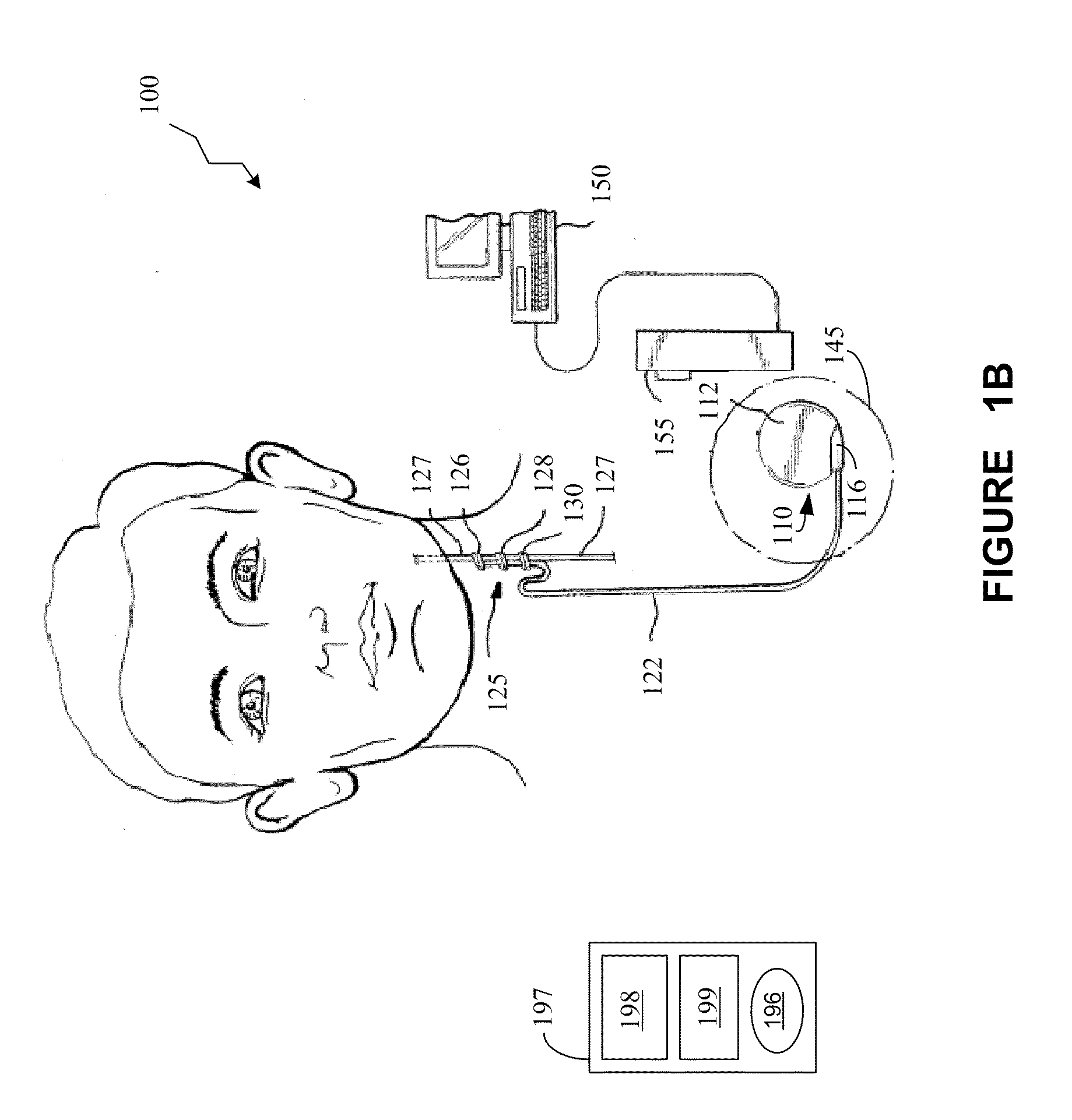
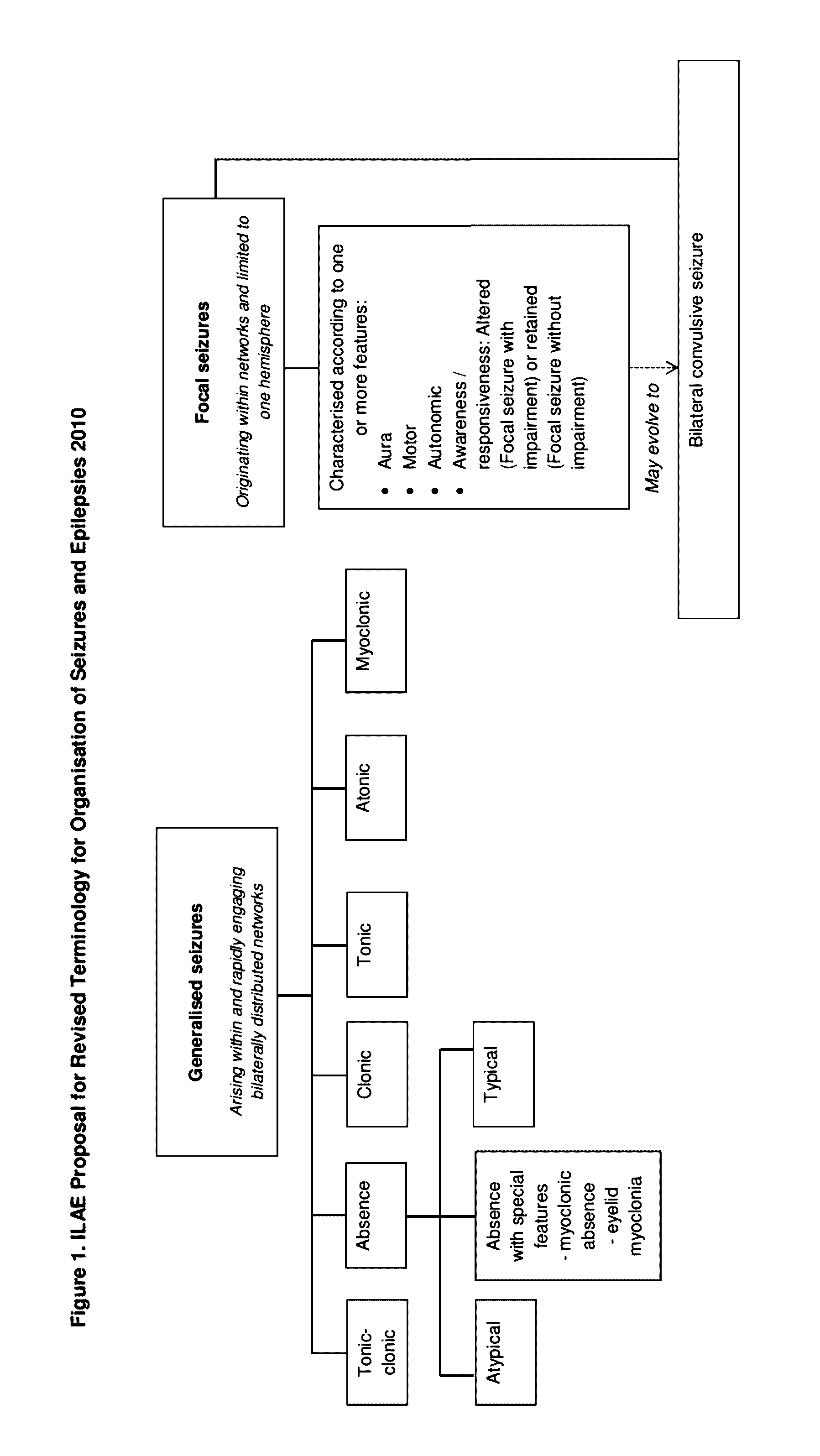
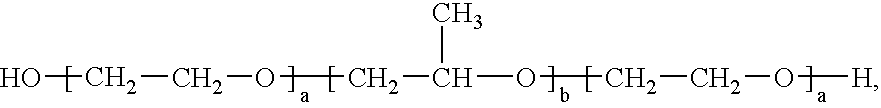Patents
Literature
97 results about "Seizure Disorders" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
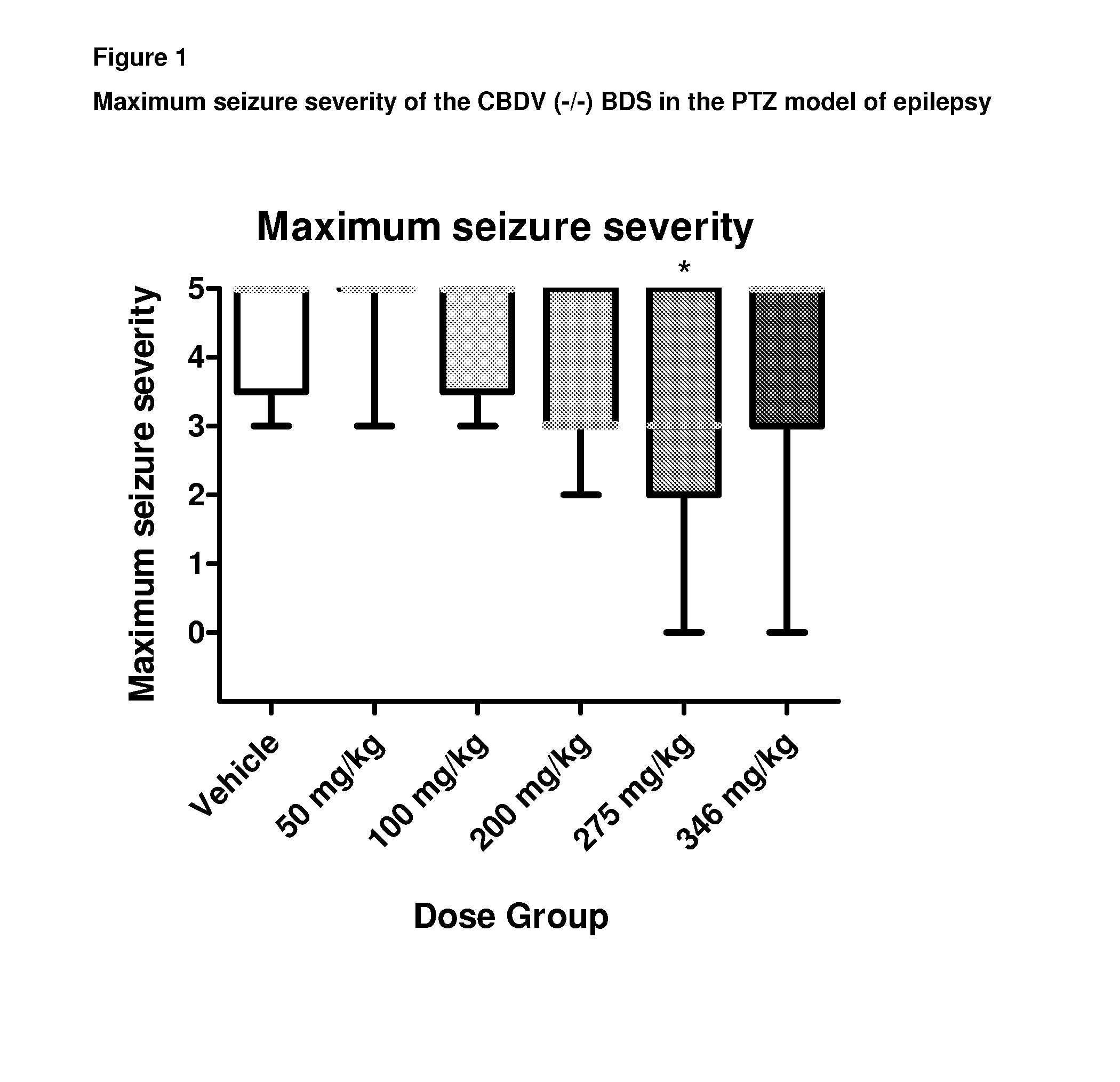
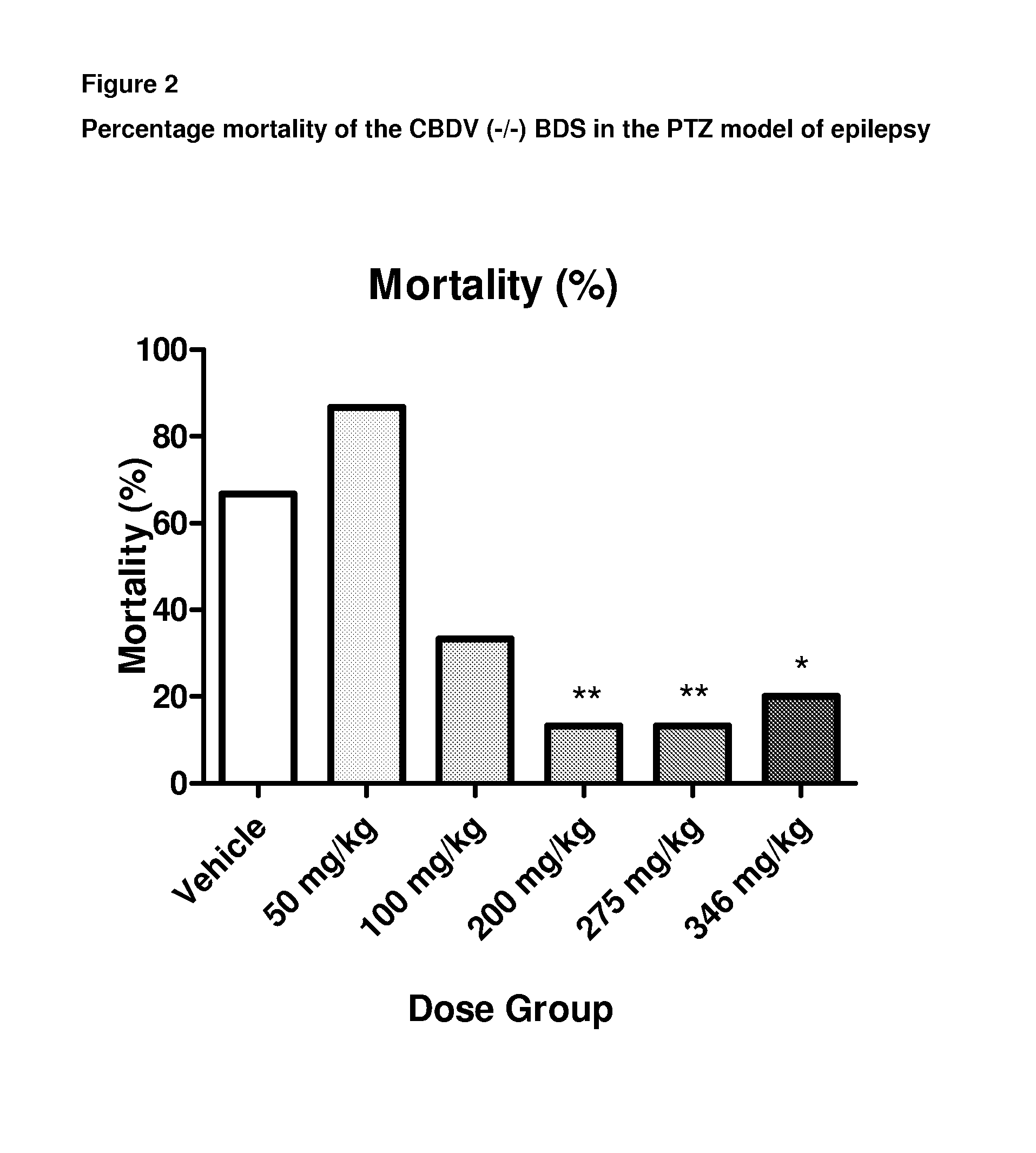
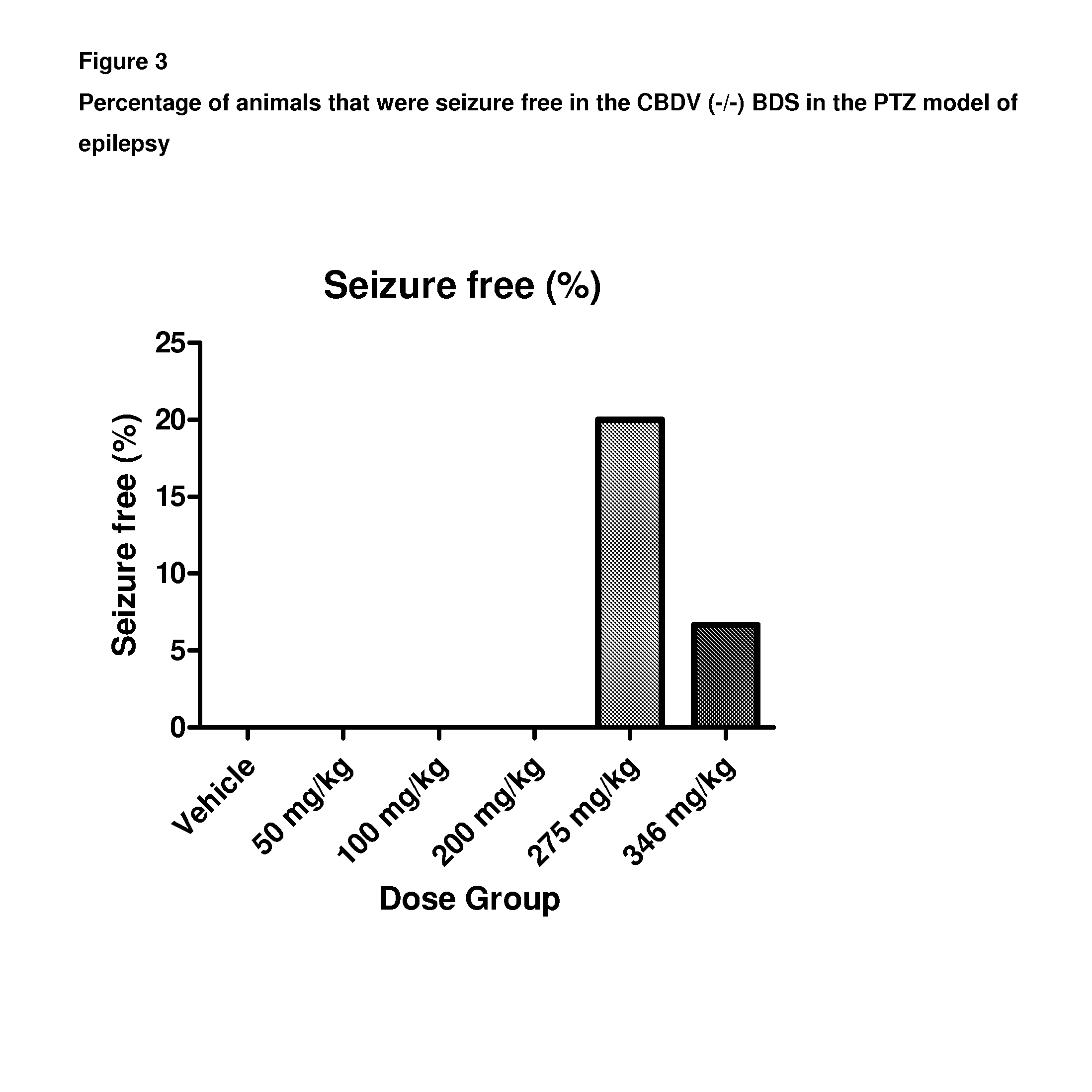
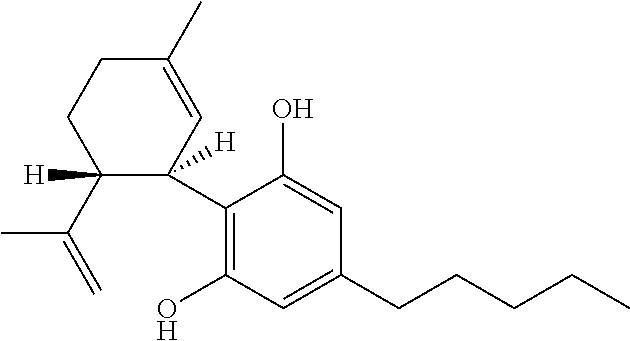
A pharmaceutical composition comprising the phytocannabinoids cannabidivarin (CBDV) and cannabidiol (CBD)
This invention relates to a pharmaceutical composition comprising or consisting essentially of the phytocannabinoids cannabidivarin (CBDV) and cannabidiol (CBD). The composition is particularly safe and efficacious for use in the treatment of neurological conditions, characterized by hyper-excitability of the central nervous system, convulsions or seizures such as occur in epilepsy. Preferably the CBDV and the CBD are present with at least one non-cannabinoid component of cannabis such as one or more terpenes or a terpene fraction. More particularly the composition further comprises one or more cannabichromene type compounds. Particularly cannabichromene propyl variant (CBCV) and / or cannabichromene (CBC). More particularly still the composition is absent or substantially absent of other cannabinoids, including in particular tetrahydrocannabinol (THC) and tetrahydrocannabivarin (THCV), which would normally be present in significant amounts in cannabis chemotypes bred to contain a significant amount of CBDV and / or CBD.
Owner:GW PHARMA LTD
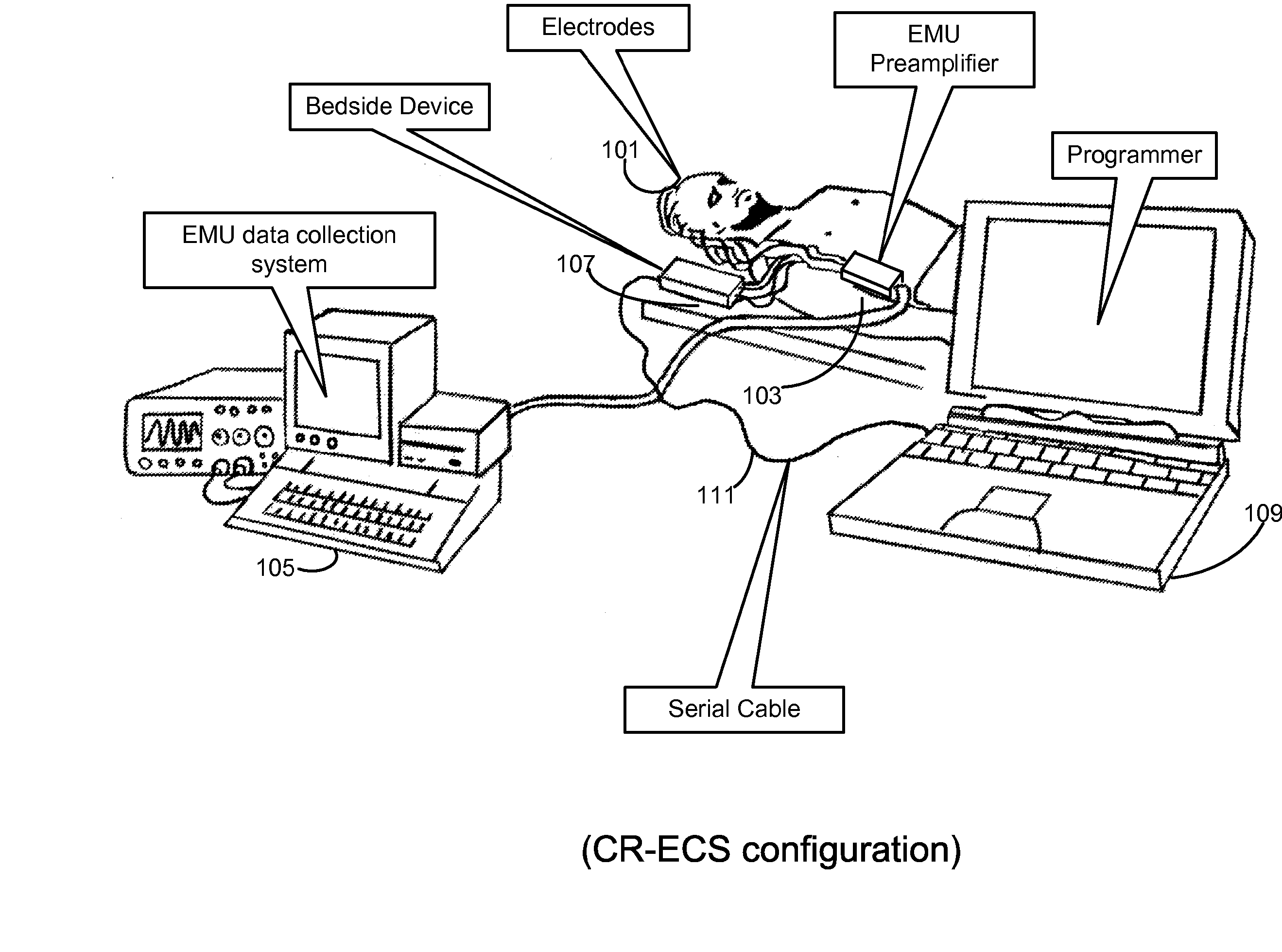
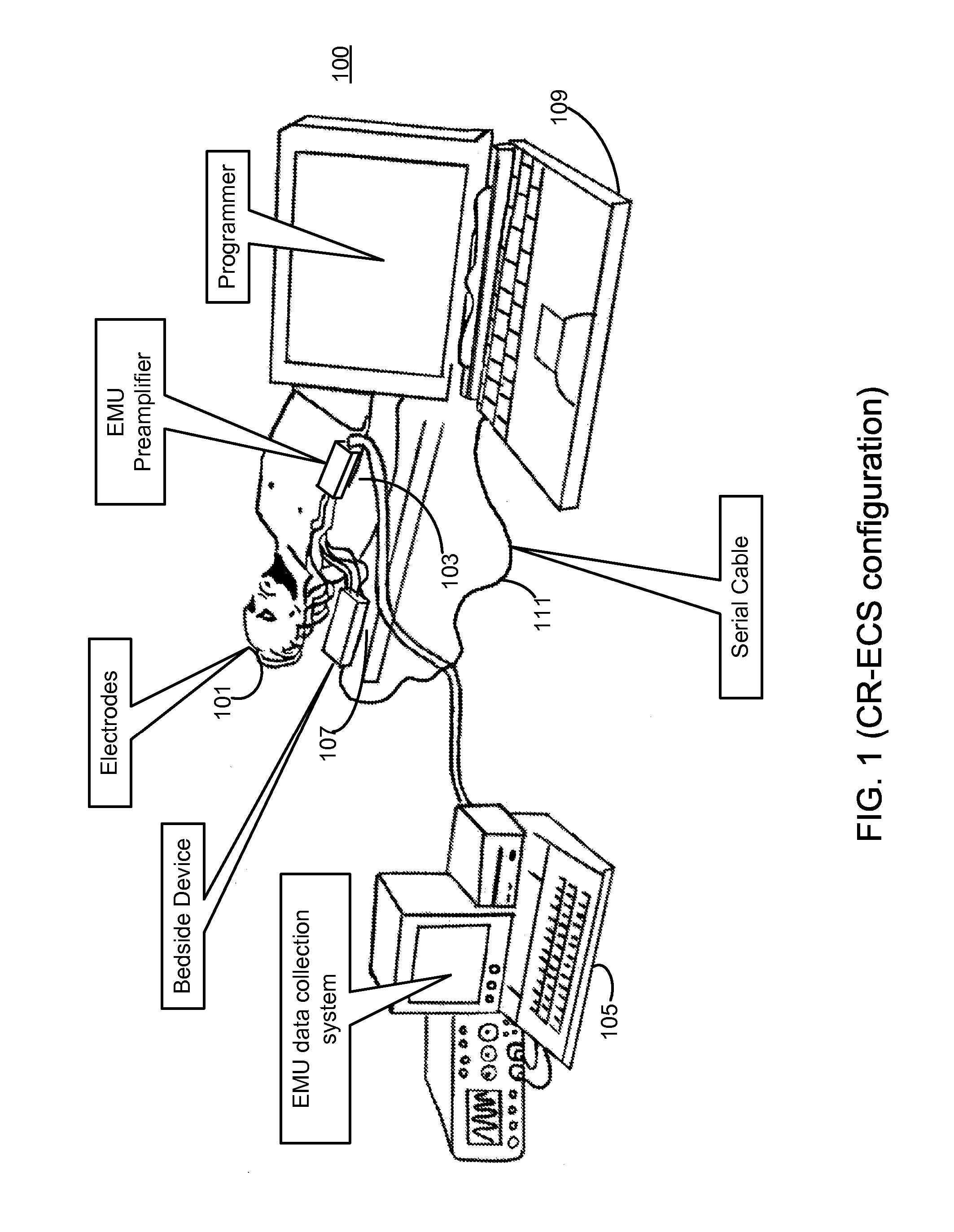
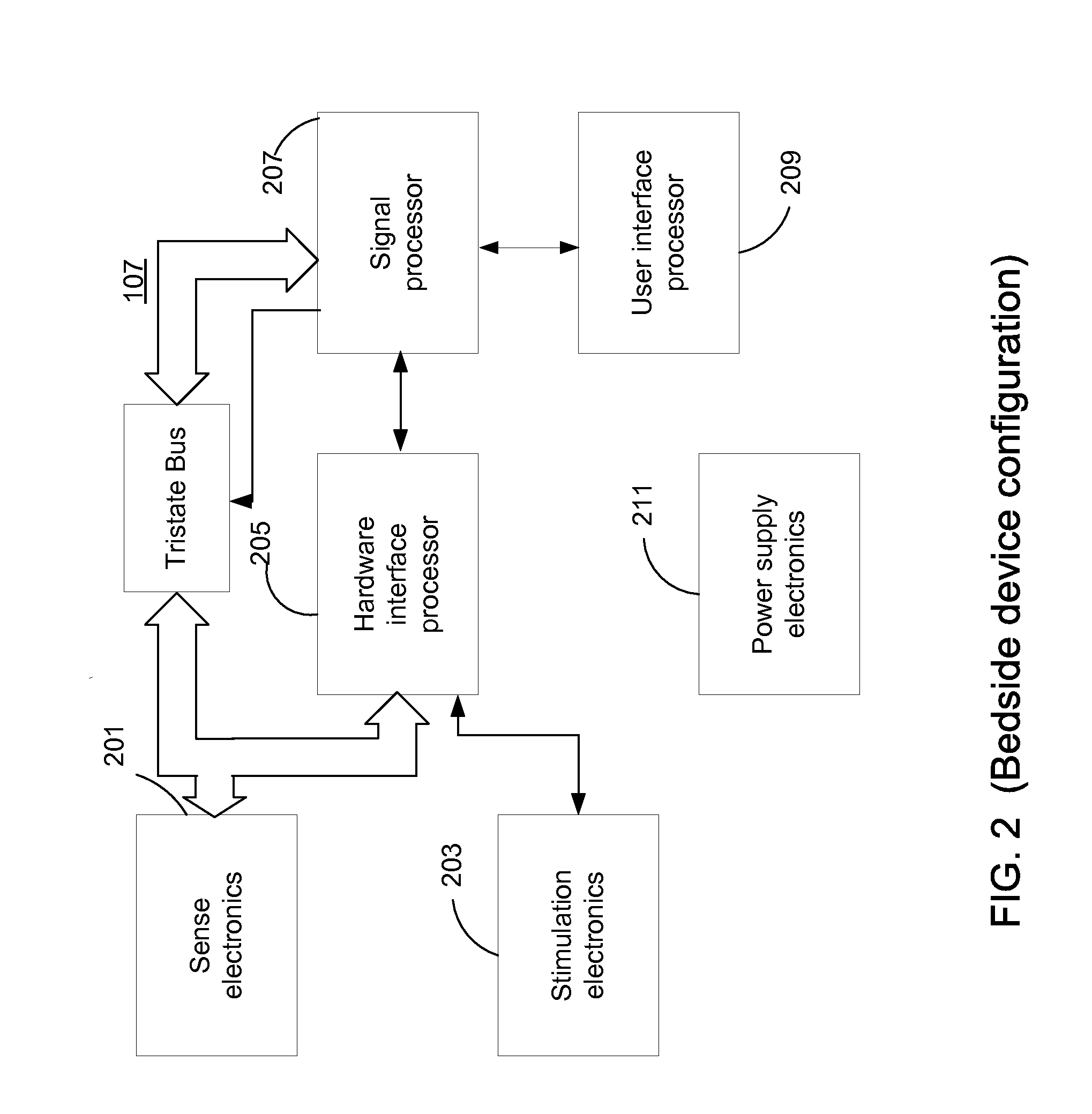
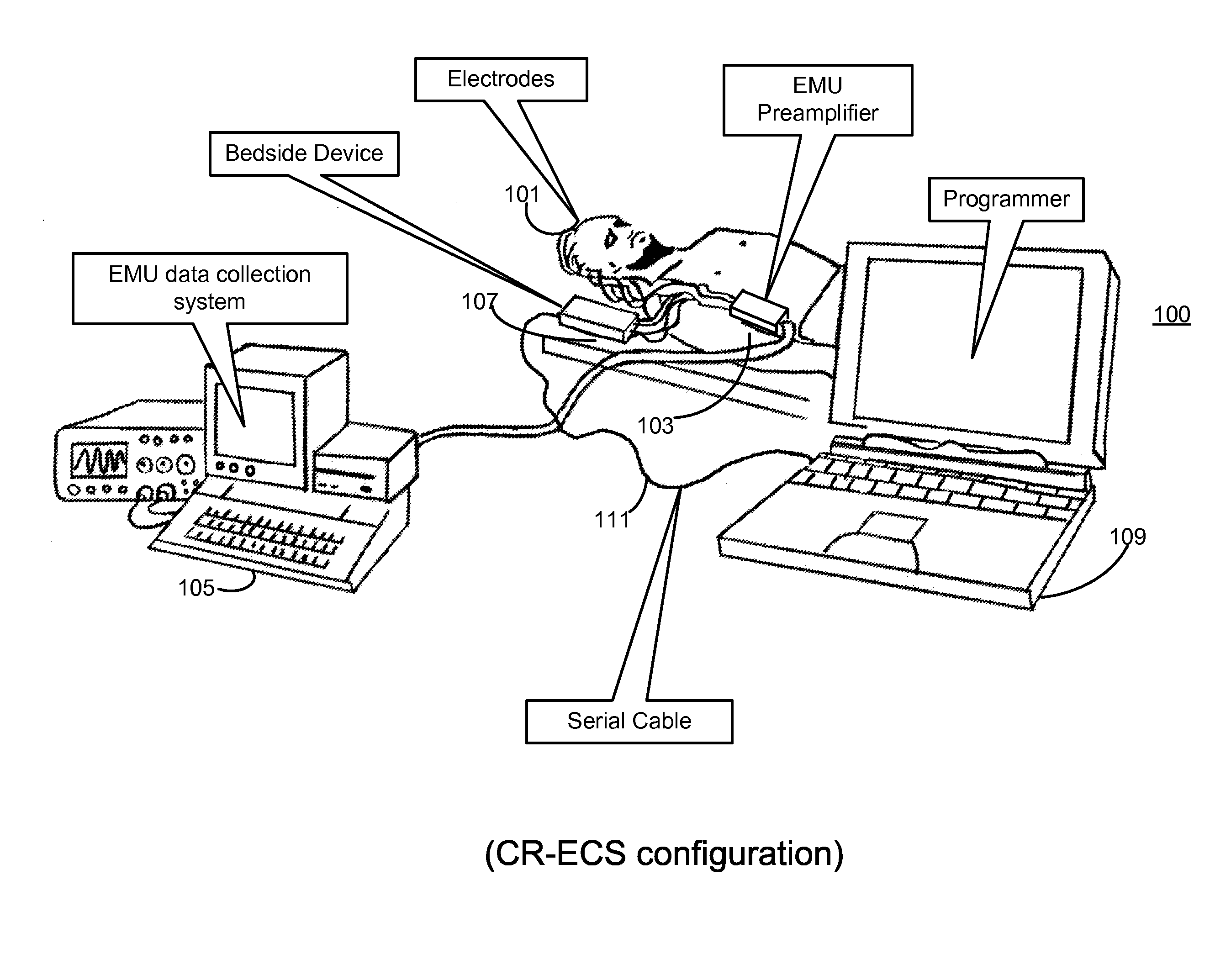
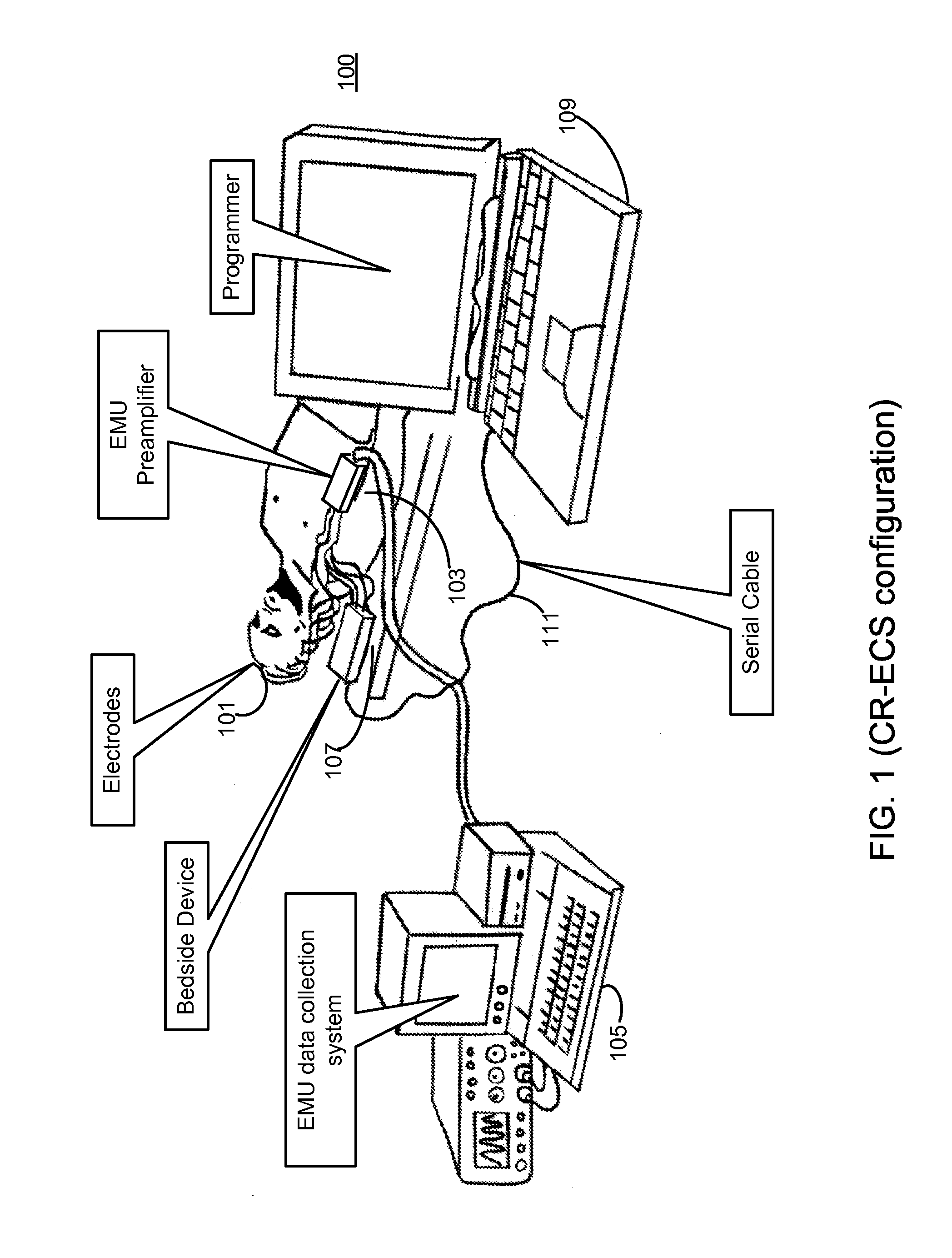
Clustering of recorded patient neurological activity to determine length of a neurological event
ActiveUS20080033508A1Good curative effectElectroencephalographyImplantable neurostimulatorsDiseaseNervous system
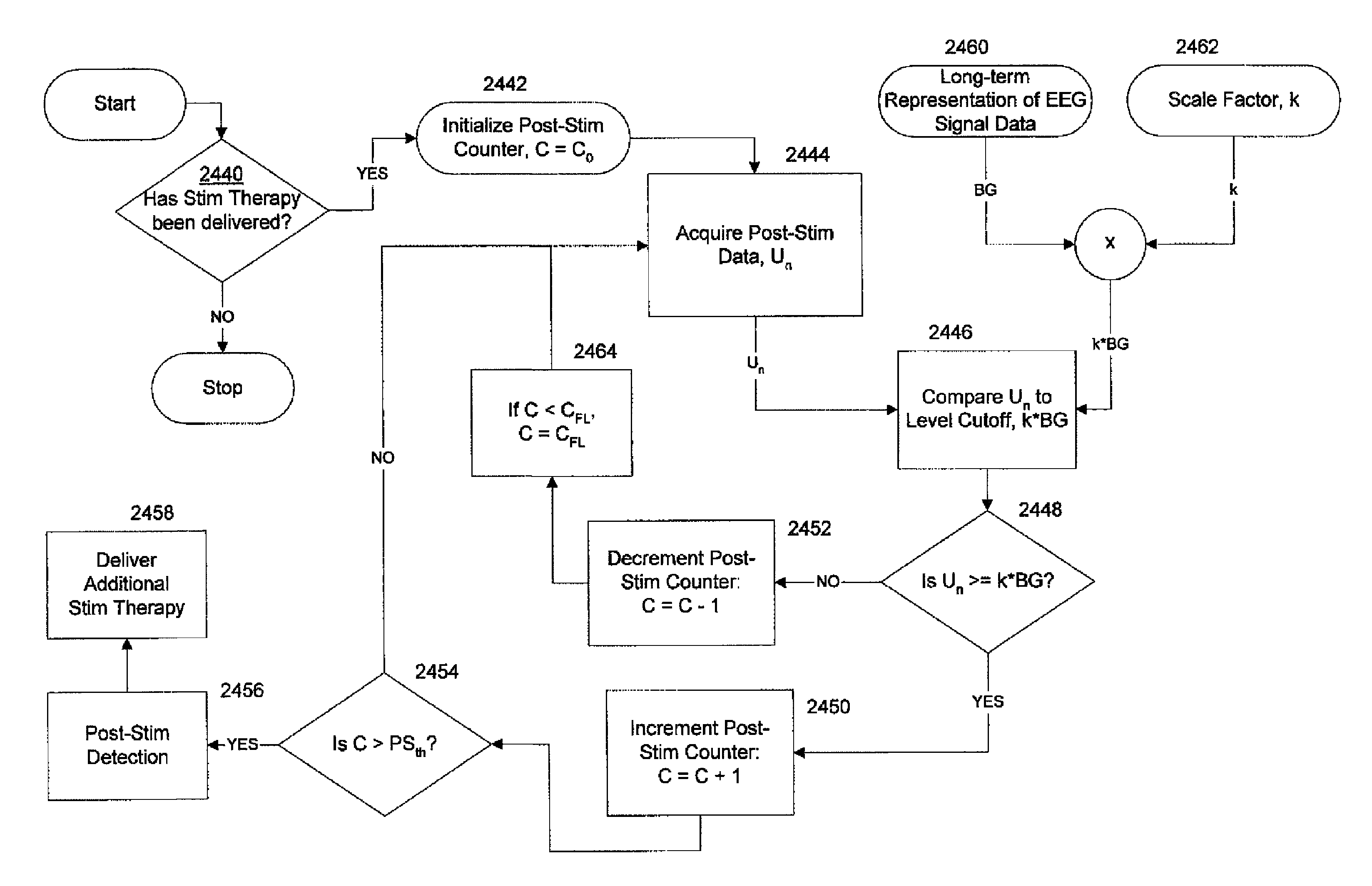
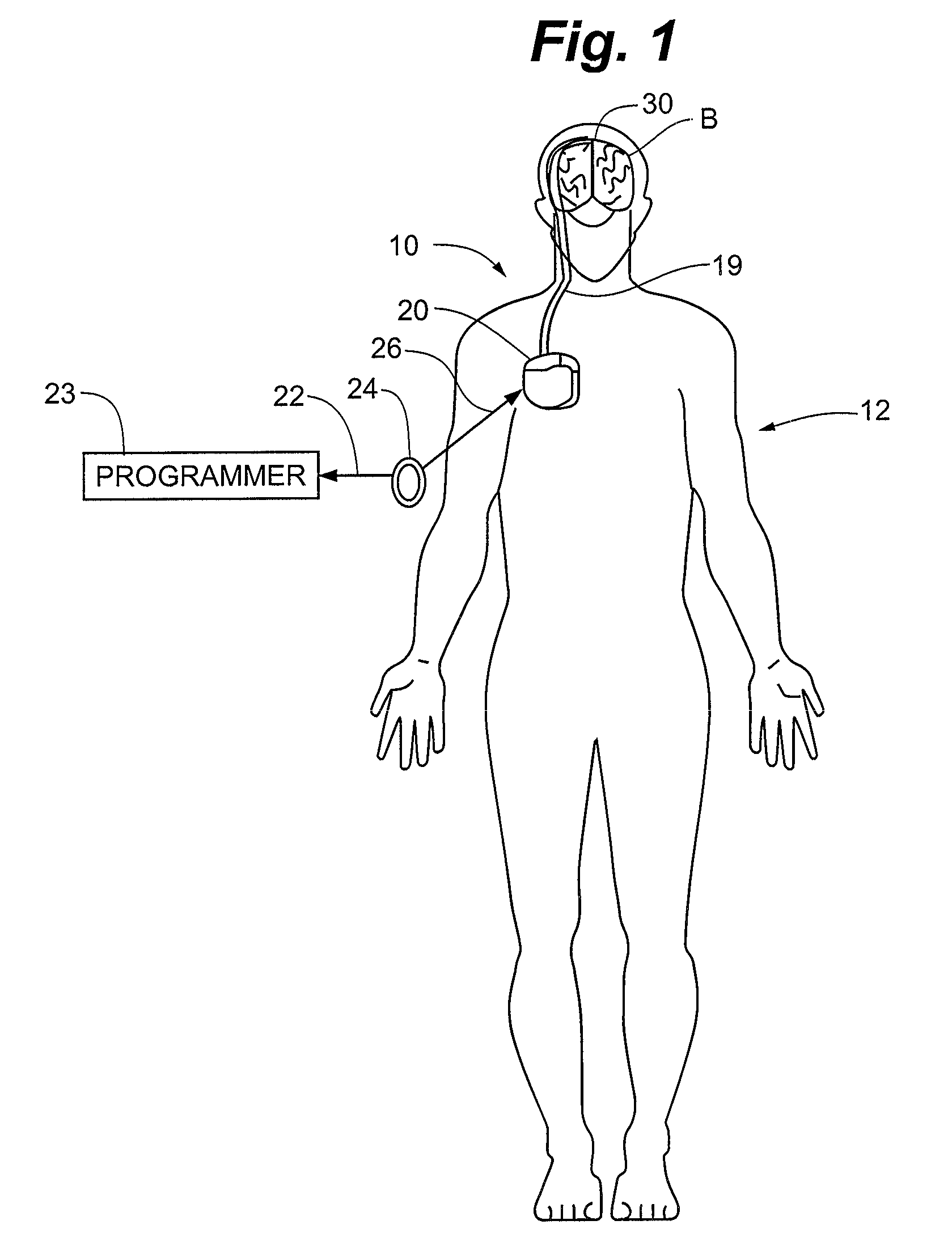
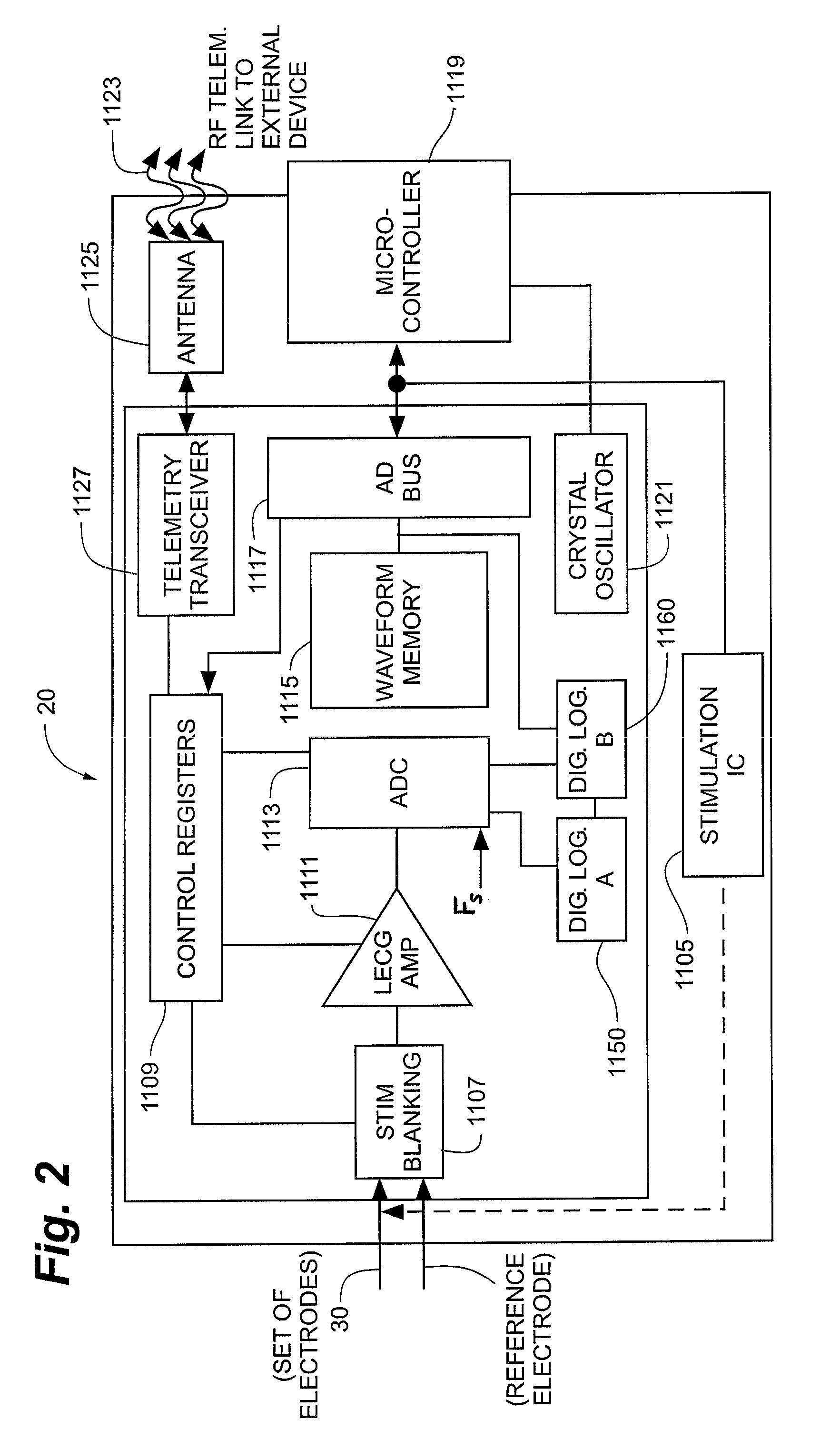
Apparatus and method detect a detection cluster that is associated with a neurological event, such as a seizure, of a nervous system disorder and update therapy parameters that are associated with a treatment therapy. The occurrence of the detection cluster is detected when the maximal ratio exceeds an intensity threshold. If the maximal ratio drops below the intensity threshold for a time interval that is less than a time threshold and subsequently rises above the intensity threshold, the subsequent time duration is considered as being associated with the detection cluster rather than being associated with a different detection cluster. Consequently, treatment of the nervous system disorder during the corresponding time period is in accordance with one detection cluster. Treatment therapy may be provided by providing electrical stimulation, drug infusion or a combination. Therapy parameters may be updated for each mth successive group of applications of the treatment therapy or for each nth detection cluster.
Owner:MEDTRONIC INC
Clustering of recorded patient neurological activity to determine length of a neurological event
Apparatus and method detect a detection cluster that is associated with a neurological event, such as a seizure, of a nervous system disorder and update therapy parameters that are associated with a treatment therapy. The occurrence of the detection cluster is detected when the maximal ratio exceeds an intensity threshold. If the maximal ratio drops below the intensity threshold for a time interval that is less than a time threshold and subsequently rises above the intensity threshold, the subsequent time duration is considered as being associated with the detection cluster rather than being associated with a different detection cluster. Consequently, treatment of the nervous system disorder during the corresponding time period is in accordance with one detection cluster. Treatment therapy may be provided by providing electrical stimulation, drug infusion or a combination. Therapy parameters may be updated for each mth successive group of applications of the treatment therapy or for each nth detection cluster.
Owner:MEDTRONIC INC
Methods and compositions for the treatment of epilepsy, seizure disorders, and other CNS disorders
InactiveUS20050245460A1Benefit maximizationReducing unwanted side effectBiocideNervous disorderDiseaseEpileptic disorder
Owner:NEUROMOLECULAR INC
Topiramate pharmaceutical composition
InactiveUS20060121112A1Improve bioavailabilityDifferential bioavailabilityBiocideCarbohydrate active ingredientsDiseaseGeneralized seizure
A once daily controlled-release pharmaceutical formulation which contains therapeutic amounts of topiramate and which is capable of being administered to specific regions along the gastrointestinal tract used to treat various types of conditions, for example, partial seizures with or without secondarily generalized seizures, primary generalized tonic-clonic seizures, seizures associated with Lennox Gastaut Syndrome, migraines, and obesity.
Owner:ALKERMES PHARMA IRELAND LTD
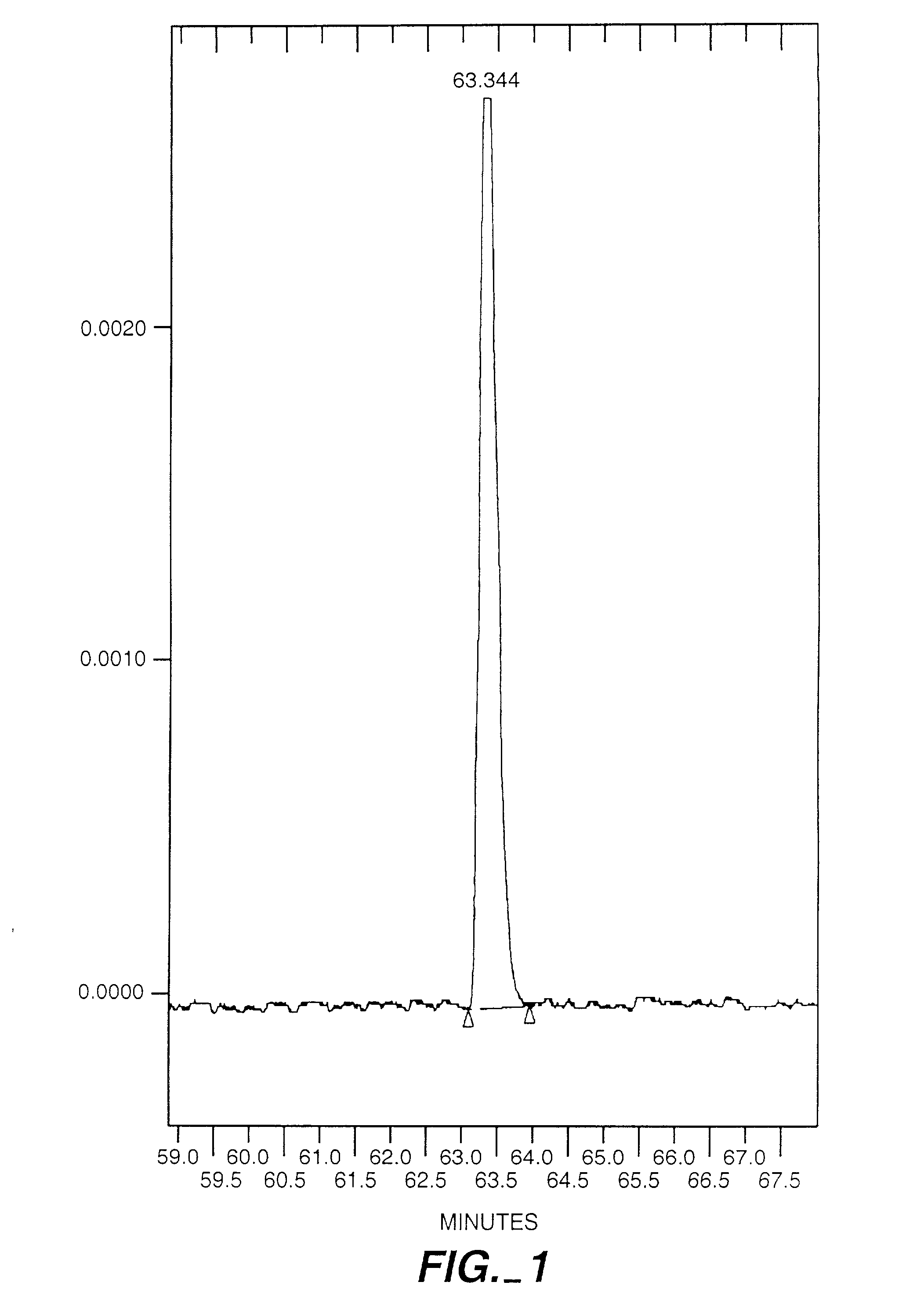
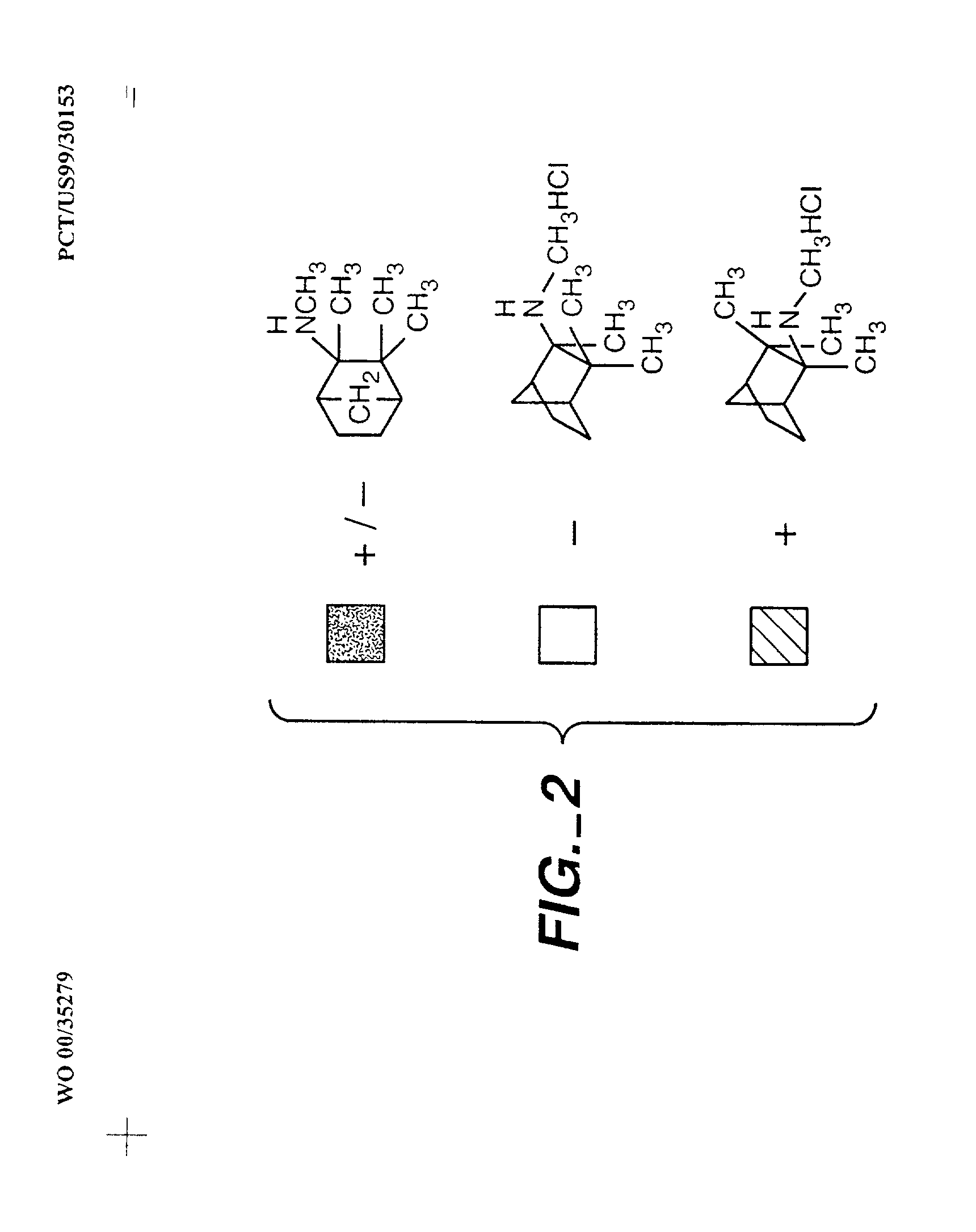
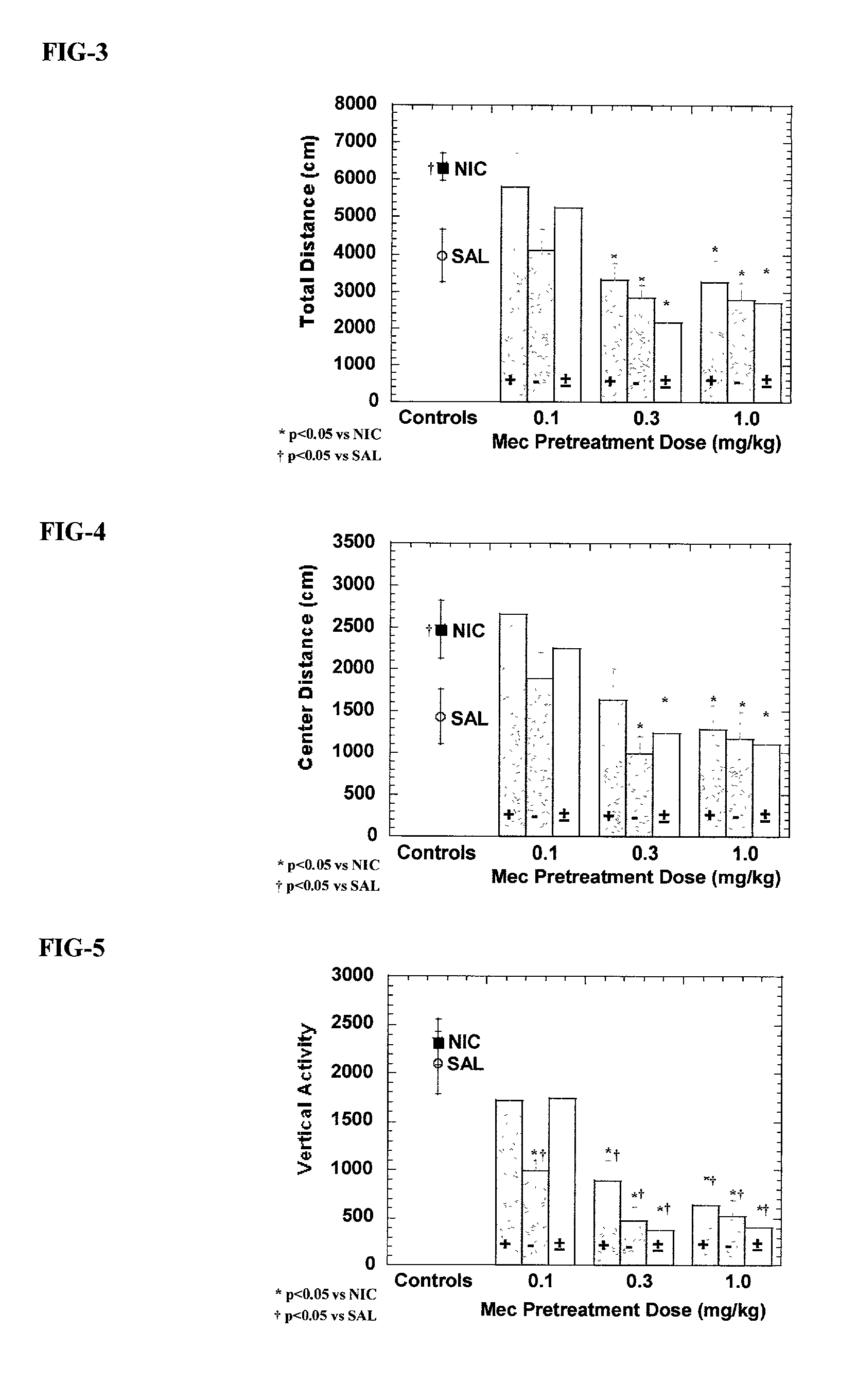
Exo-R-mecamylamine formulation and use in treatment
InactiveUS20020016370A1Convenient treatmentImprove Medication AdherenceBiocideUrea derivatives preparationStimulantS syndrome
A pharmaceutical composition includes a therapeutically effective amount of exo-R-mecamylamine or a pharmaceutically acceptable salt thereof, substantially free of exo-S-mecamylamine in combination with a pharmaceutically acceptable carrier. Preferably the amount is about 0.5 mg to about 20 mg. Medical conditions are treated by administering a therapeutically effective amount of exo-R-mecamylamine or a pharmaceutically acceptable salt thereof, substantially free of its exo-S-mecamylamine, said amount being sufficient to ameliorate the medical condition. The medical conditions include but are not limited to substance addiction (involving nicotine, cocaine, alcohol, amphetamine, opiate, other psychostimulant and a combination thereof), aiding smoking cessation, treating weight gain associated with smoking cessation, hypertension, hypertensive crisis, Tourette's Syndrome and other tremors, cancer (such as small cell lung cancer), atherogenic profile, neuropsychiatric disorders (such as bipolar disorder, depression, an anxiety disorder, schizophrenia, a seizure disorder, Parkinson's disease and attention deficit hyperactivity disorder), chronic fatigue syndrome, Crohn's disease, autonomic dysreflexia, and spasmogenic intestinal disorders.
Owner:UNIV OF SOUTH FLORIDA
Responsiveness testing of a patient having brain state changes
PendingUS20110251468A1Risk minimizationHigh riskPhysical therapies and activitiesElectroencephalographyDiseaseGeneralized seizure
A method of determining a responsiveness of a patient having brain state changes, comprising receiving an indication of a triggering event; administering to the patient, in response to the indication, a test of responsiveness; determining, based upon a result of the test, at least one responsiveness parameter selected from the group consisting of (i) a time of occurrence of a change in the patient's responsiveness, (ii) a duration of a change in the patient's responsiveness; (iii) a magnitude of a change in the patient's responsiveness, (iv) a time interval from the indication of event occurrence to a change in the patient's responsiveness, (v) a type of change in the patient's responsiveness, (vi) an estimation of a seizure severity; (vii) a classification of a seizure into clinical or subclinical; (viii) a classification of a clinical seizure into simple partial, complex partial, or generalized; (ix) an assessment of efficacy of a therapy for the patient's medical condition; (x) an assessment of the state of the disease and formulation of a prognosis for the patient; (xi) an estimation of a risk of injury or death for the patient; and (xii) two or more thereof A medical device system capable of implementing the method.
Owner:FLINT HILLS SCI L L C
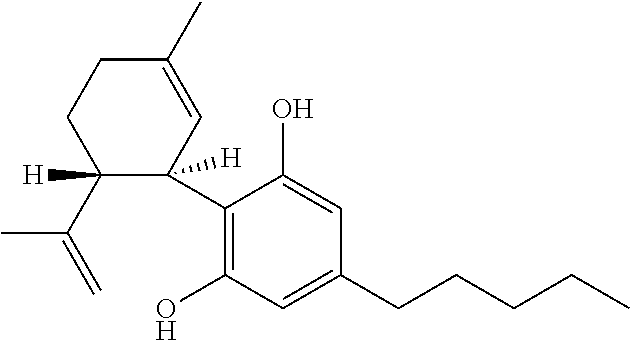
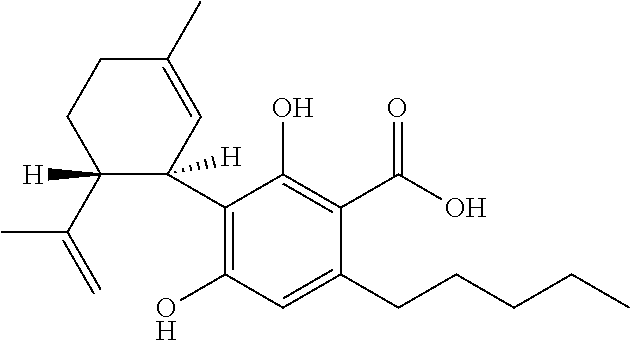
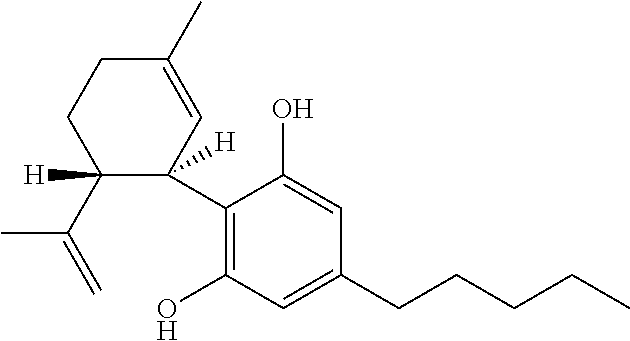
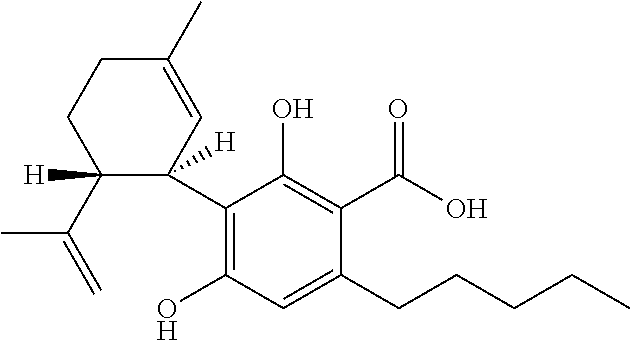
Use of cannabinoids in the treatment of epilepsy
ActiveUS20170007551A1Reduce in quantityReduce doseNervous disorderHydroxy compound active ingredientsAicardi's syndromeFocal Epilepsies
The present invention relates to the use of cannabidiol (CBD) in the treatment of focal seizures. In one embodiment the patients suffering from focal seizures are children and young adults. CBD appears particularly effective in reducing focal seizures in patients suffering with etiologies that include: Lennox-Gastaut Syndrome; Tuberous Sclerosis Complex; Dravet Syndrome; CDKL5; Neuronal ceroid lipofuscinoses (NCL); febrile infection related epilepsy syndrome (FIRES); Aicardi syndrome and brain abnormalities in comparison to other seizure types. Significantly CBD additionally is very effective in the reduction of a sub-type of focal seizures, focal seizures with impairment.
Owner:GW RES LTD
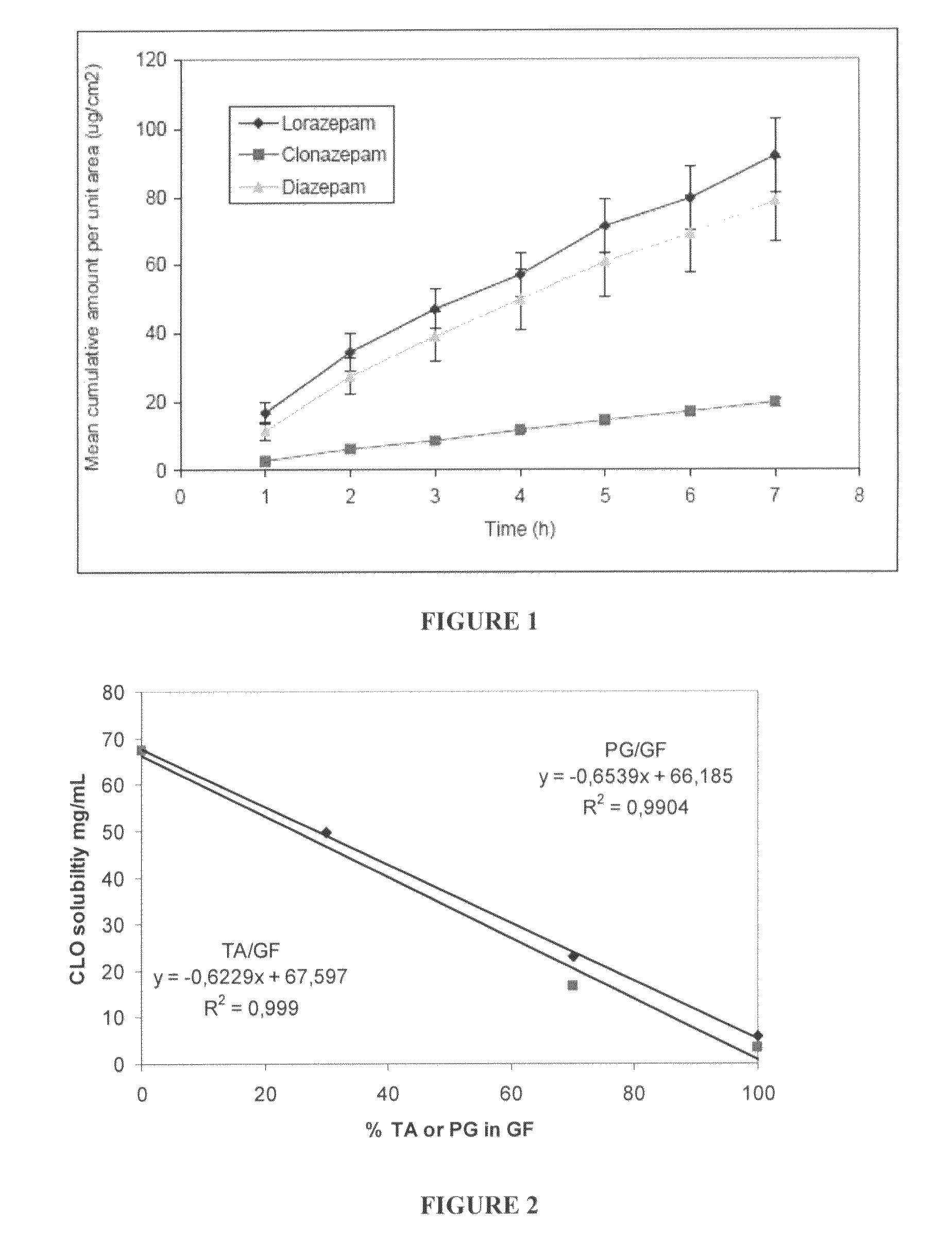
Pharmaceutical compositions of benzodiazepines and method of use thereof
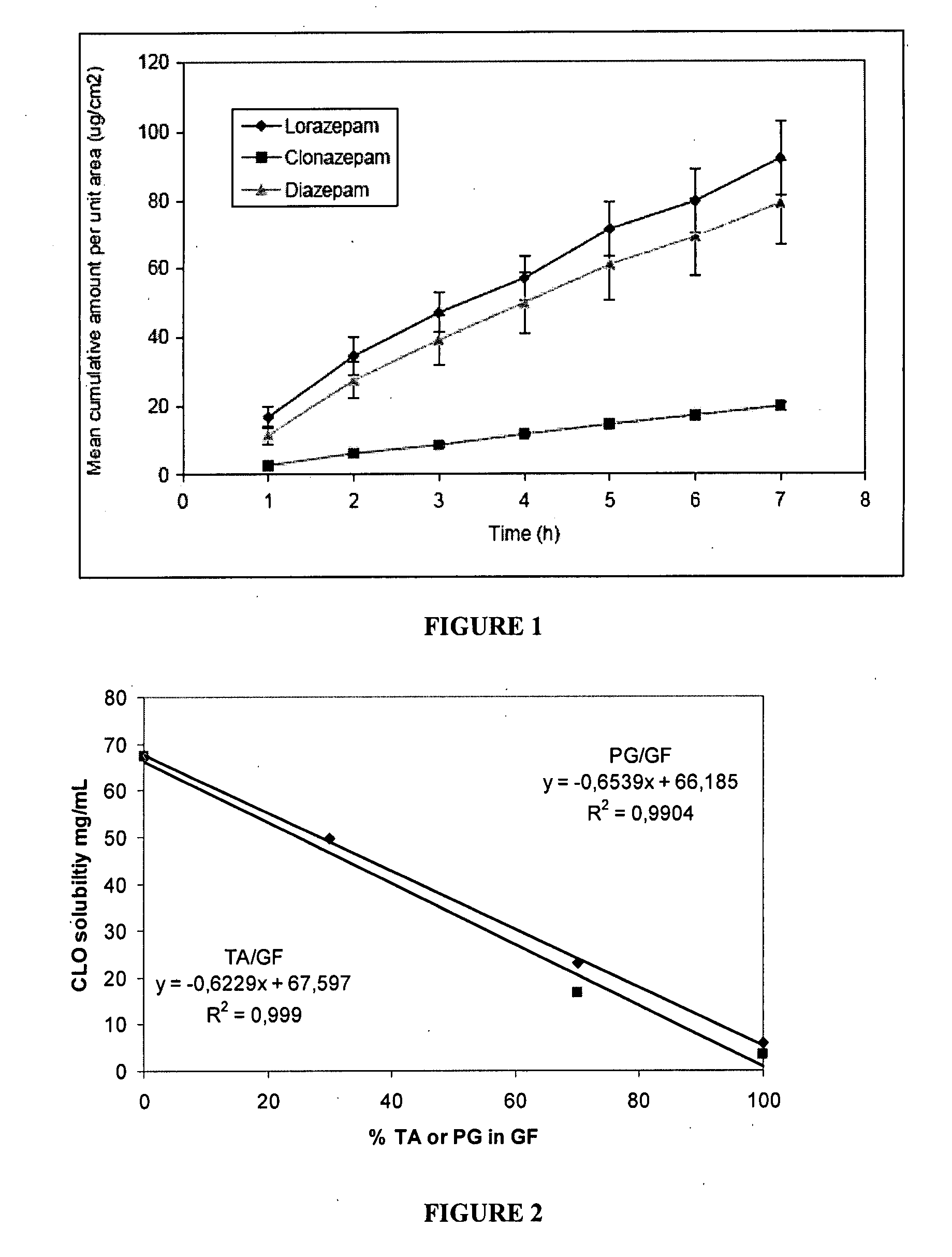
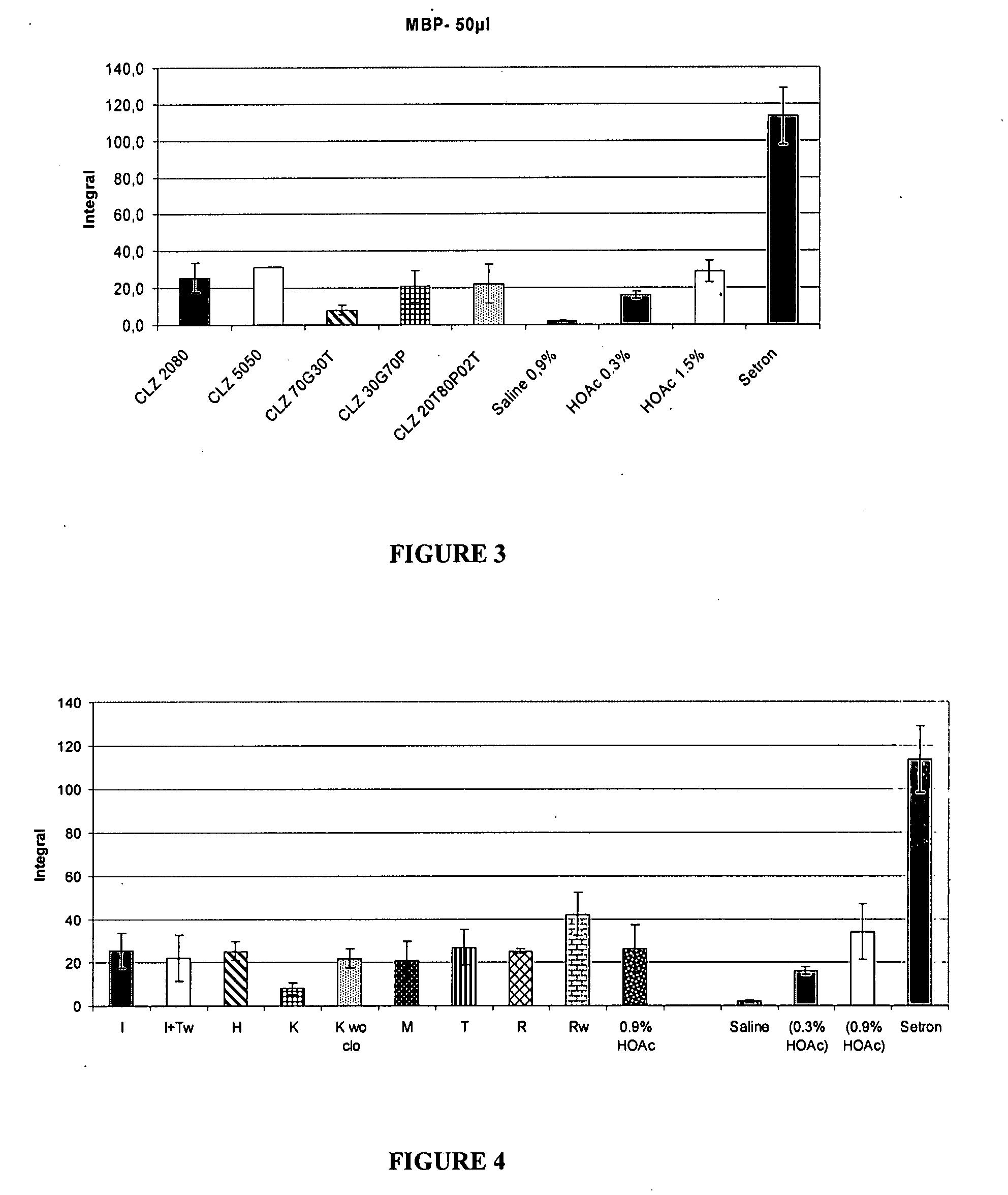
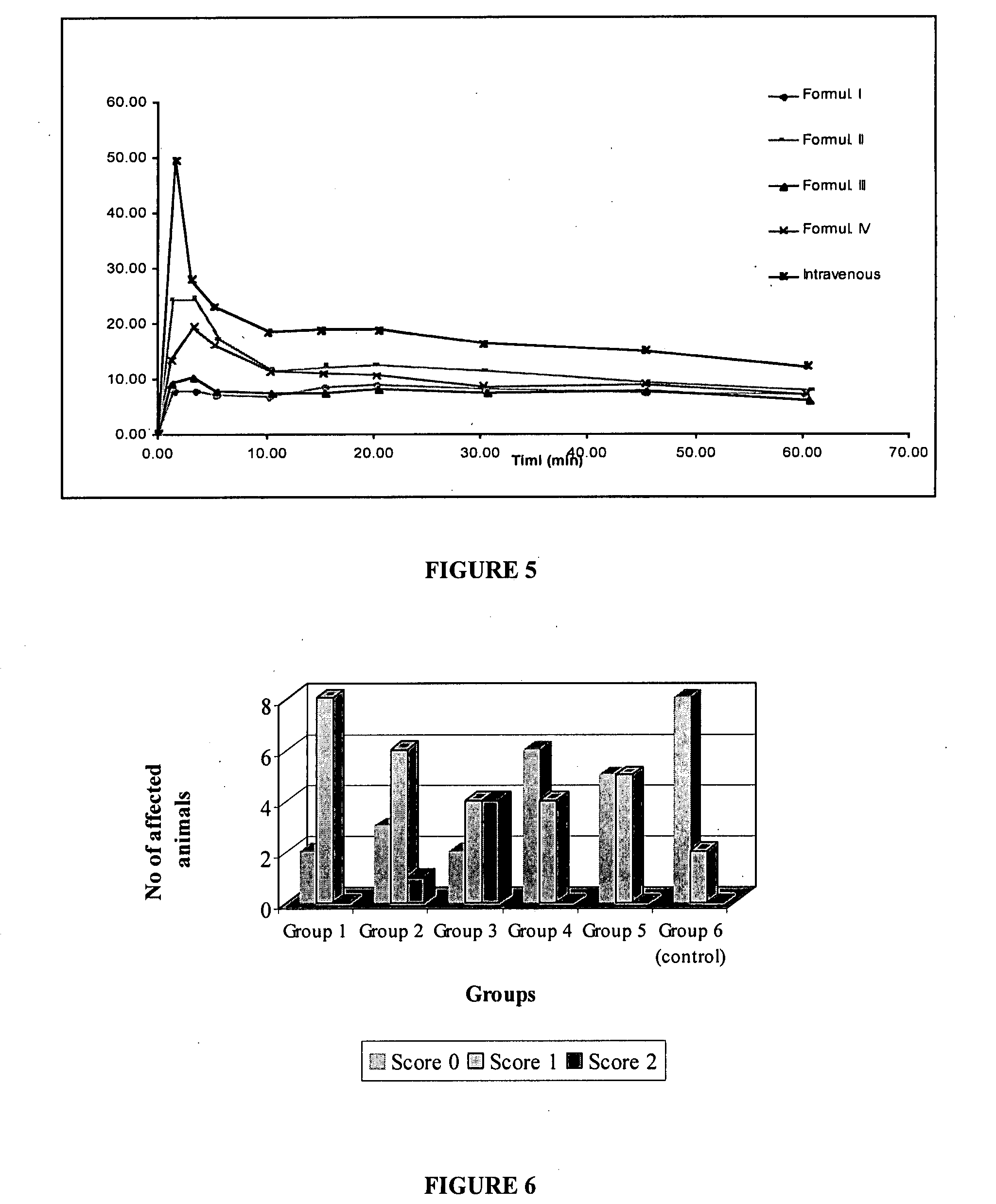
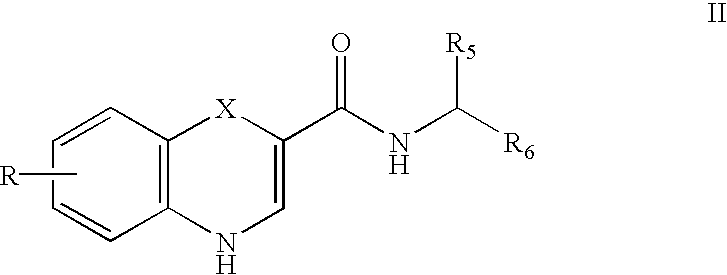
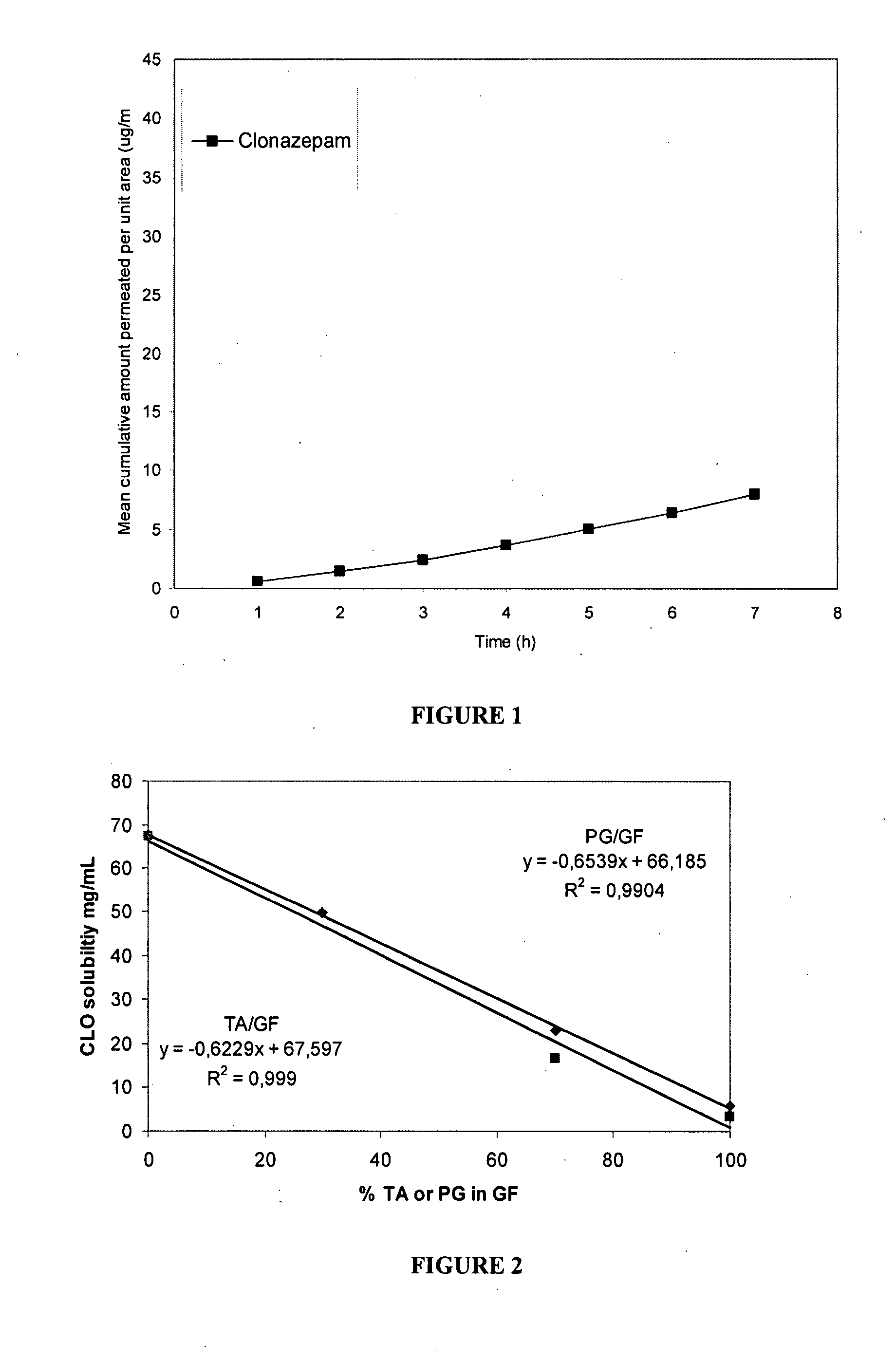
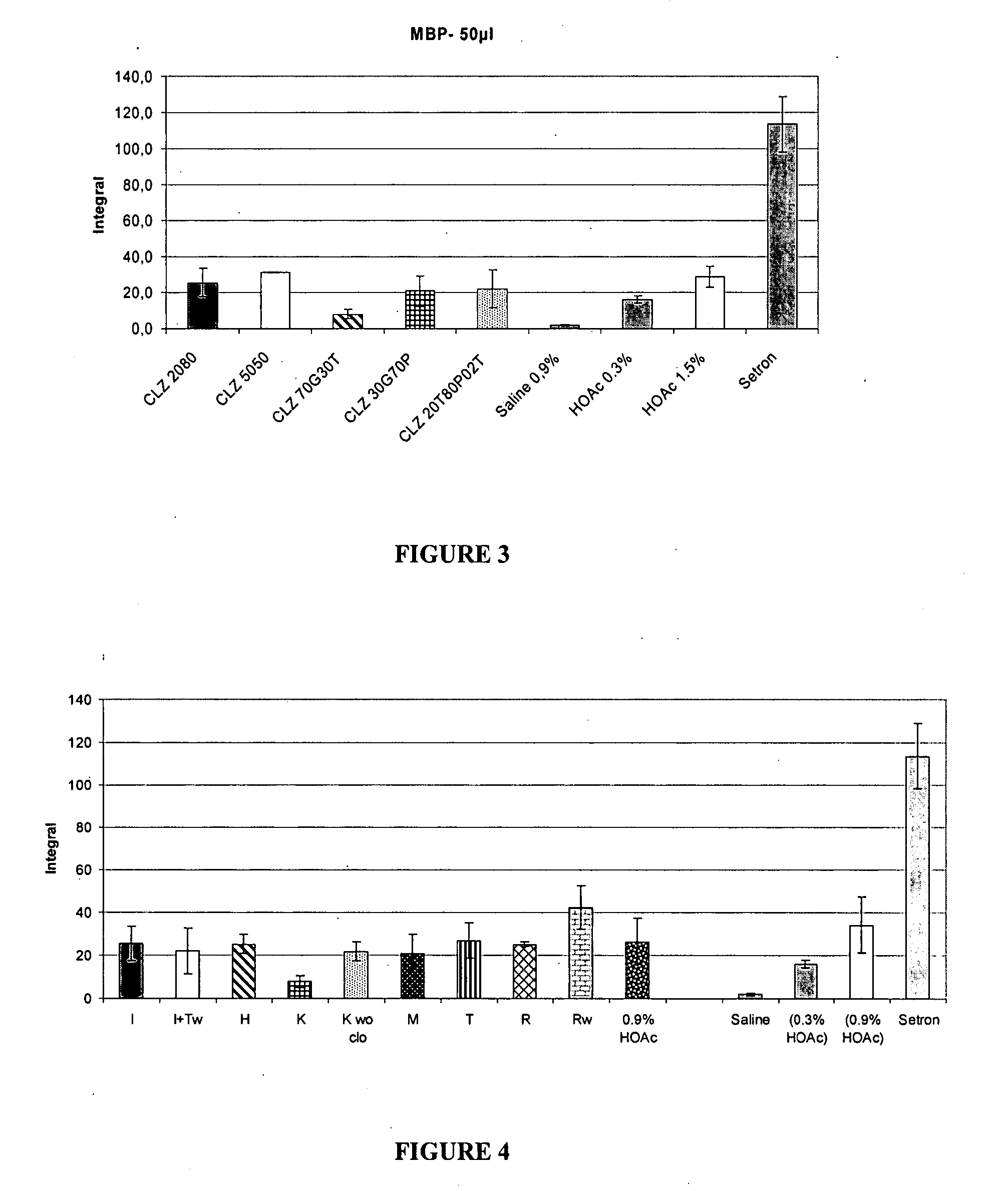
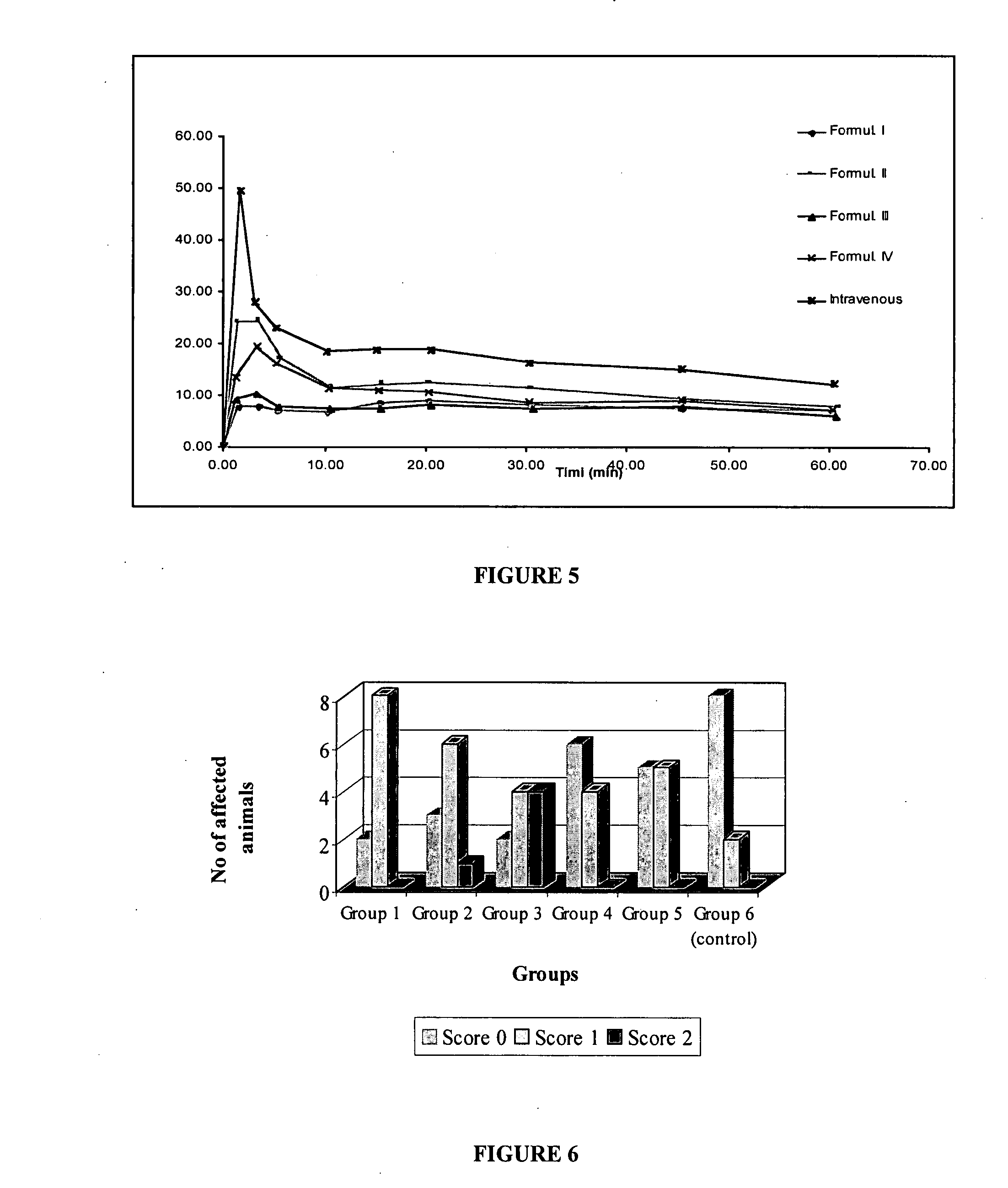
The present invention includes benzodiazepine compositions formulated for intranasal administration, comprising a binary solvent system comprising a first solvent in which the benzodiazepine is soluble, the first solvent capable of penetrating nasal mucosal tissue, and a second solvent in which the benzodiazepine in less soluble. The compositions of the present invention may be used to treat a variety of disorders including, but not limited to, panic attacks, muscle spasms, anxiety, and seizures. In one aspect, the present invention relates to a fast-acting, clonazepam composition for transnasal administration that can be used for the treatment of seizure clusters.
Owner:JAZZ PHARMA
Immunosorbent blood tests for assessing paroxysmal cerebral discharges
InactiveUS20050181466A1Effective therapeutic interventionRapid inexpensiveNervous disorderDisease diagnosisDiseaseParoxysmal AF
Immunosorbents, kits and compositions for diagnosing a central nervous system disorder, particularly paroxysmal cerebral discharges and epilepsy, comprising measuring the concentration of GluR1 or fragment thereof and / or GluR1 antibodies in a biological sample from a human subject. The method is particularly useful for identifying individuals that are at risk for brain related seizures and epilepsy, for distinguishing epilepsy from pseudo-epilepsy and epilepsy-like disorders, for following up after anticonvulsive treatment, and for the adjustment of adequate therapy and doses.
Owner:GRACE LAB
Compositions of a cyclooxygenase-2 selective inhibitor and an anticonvulsant agent for the treatment of central nervous system disorders
InactiveUS20050070524A1Good effectShorten the durationBiocideCarbohydrate active ingredientsDiseaseDepressant
The present invention provides compositions and methods for the treatment of central nervous system disorders or related conditions in a subject. More particularly, the invention provides a combination therapy for the treatment of seizures, or seizure disorders comprising the administration to a subject of an anticonvulsant agent in combination with a cyclooxygenase-2 selective inhibitor.
Owner:PHARMACIA CORP
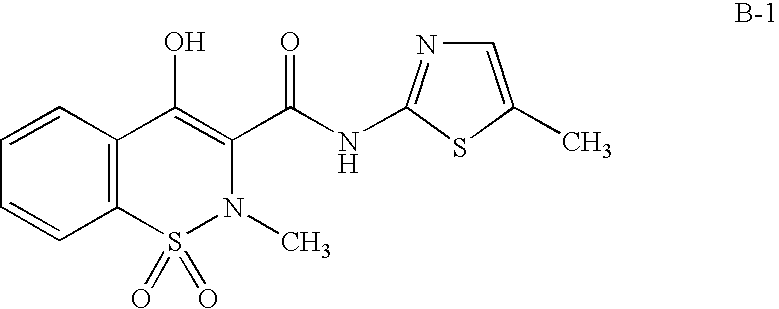
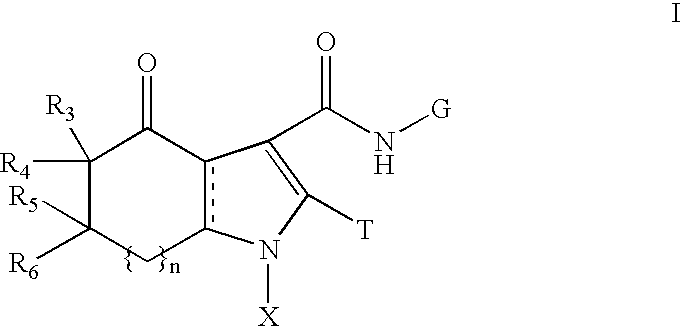
Fused pyrrolecarboxamides: GABA brain receptor ligands
Substituted pyrrolecarboxamide compounds are disclosed. These compounds are highly selective agonists, antagonists or inverse agonists for GABAA brain receptors or prodrugs of agonists, antagonists or inverse agonists for GABAA brain receptors and are therefore useful in the diagnosis and treatment of anxiety, depression, Alzheimer's dementia, sleep and seizure disorders, overdose with benzodiazepine drugs and for enhancement of memory. Pharmaceutical compositions, including packaged pharmaceutical compositions, are further provided. Compounds of the invention are also useful as probes for the localization of GABAA receptors in tissue samples.
Owner:NEUROGEN
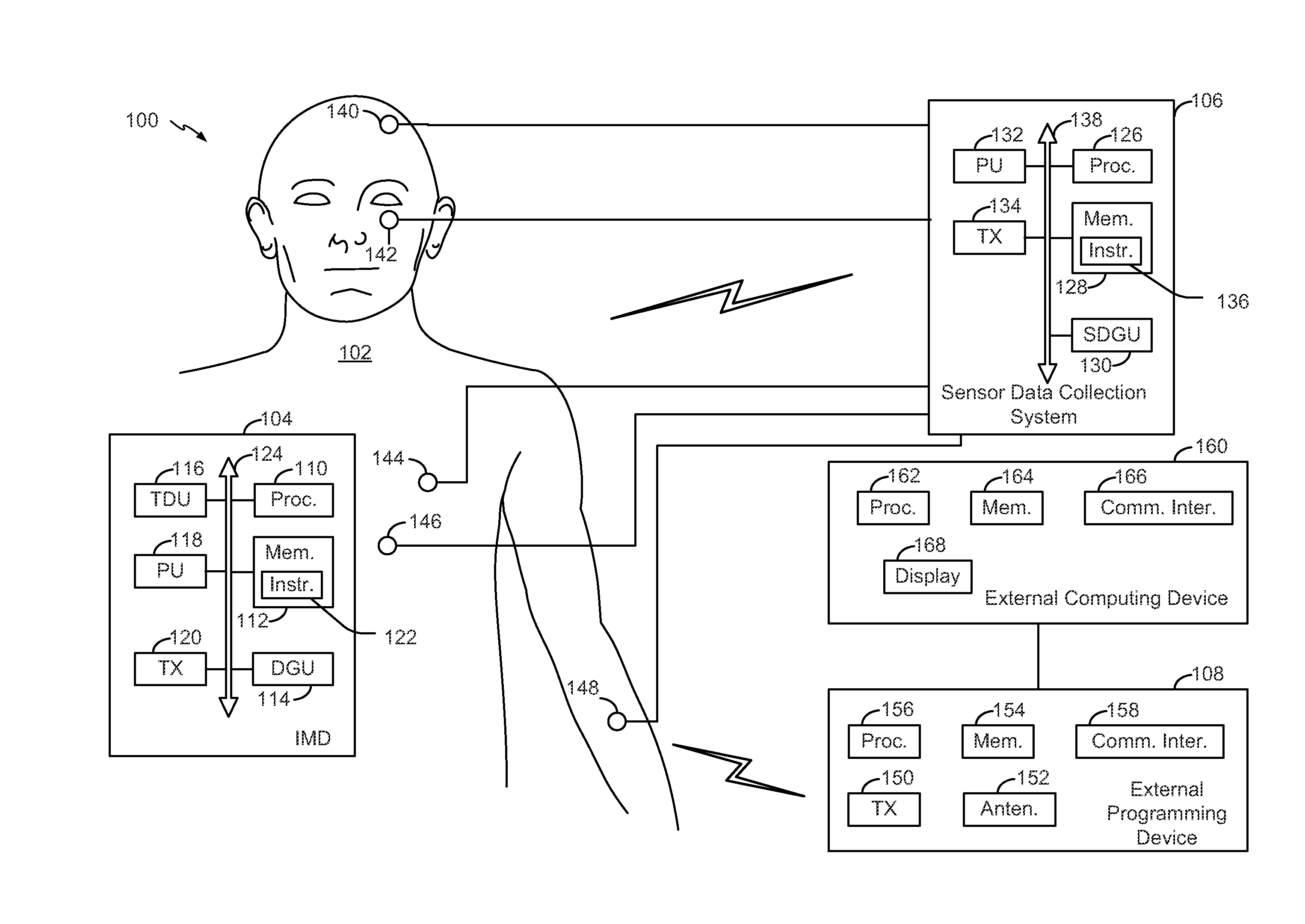
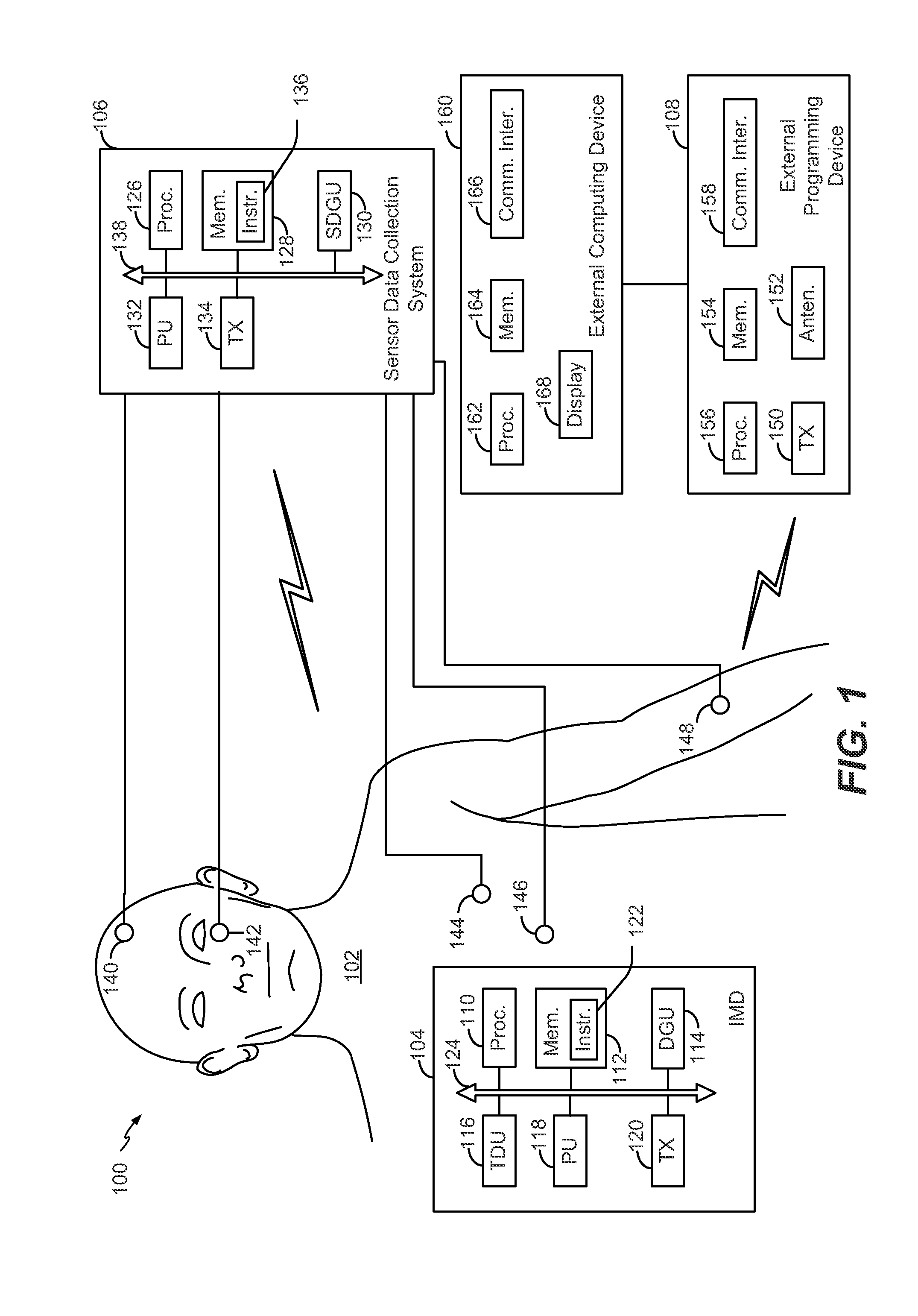
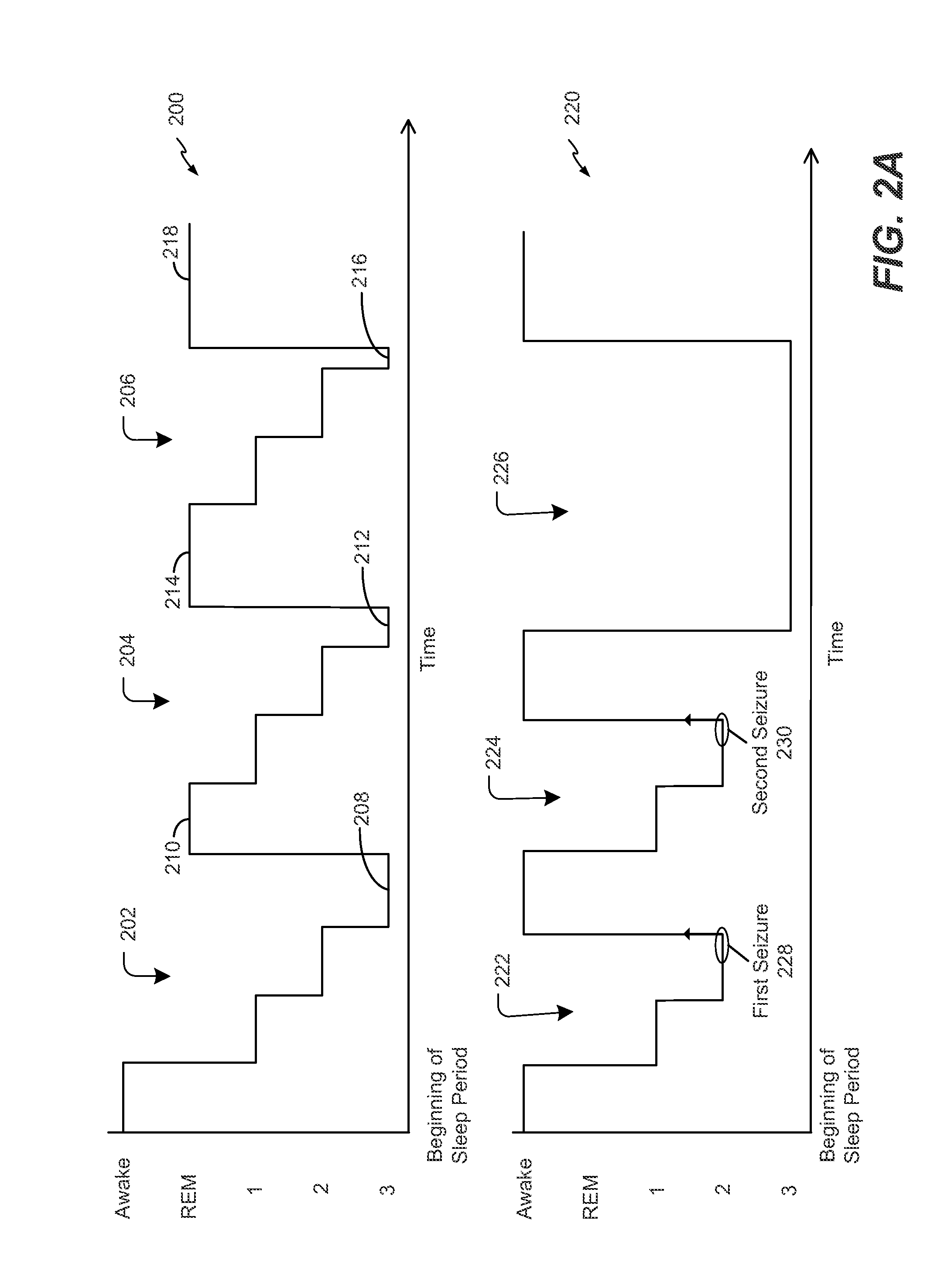
Optimization of cranial nerve stimulation to treat seizure disorders during sleep
ActiveUS20140277255A1Improve sleep qualityReduce the amount of solutionHead electrodesImplantable neurostimulatorsMedicineCranial nerve I
A method includes determining sleep cycle information related to a sleep cycle of a patient based on body parameter data. The method also includes adjusting a cranial nerve stimulation parameter based on the sleep cycle information.
Owner:LIVANOVA USA INC
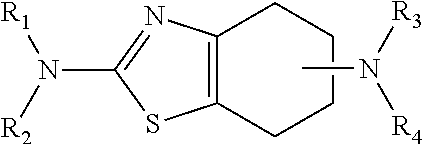
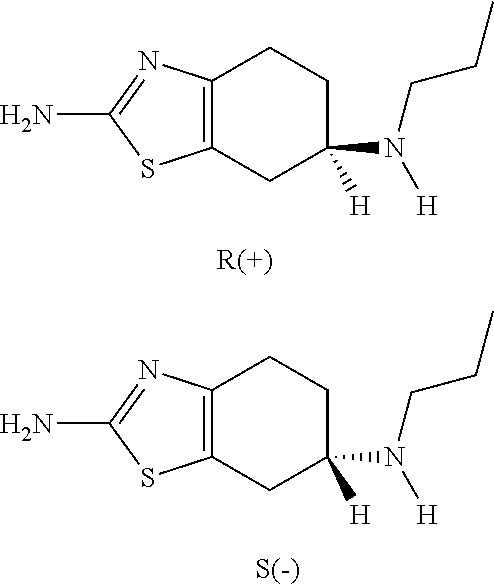
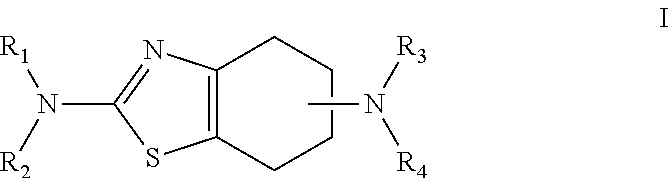
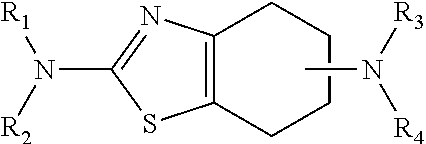
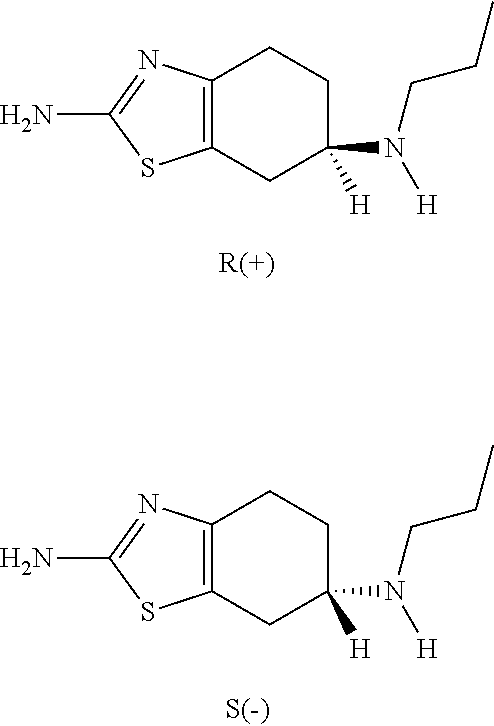
Neurorestoration with r(+) pramipexole
Formulations and methods of use thereof for restoring neuronal, muscular (cardiac and striated) and / or retinal tissue function in children and adults afflicted with chronic neurodegenerative diseases, such as neurodegenerative movement disorders and ataxias, seizure disorders, motor neuron diseases, and inflammatory demyelinating disorders, are described herein. Examples of disorders include Alzheimer's disease (AD), Parkinson's disease (PD), and amyotrophic lateral sclerosis (ALS). The method involves administering a pharmaceutical composition containing an effective amount of a tetrahydrobenzathiazole, preferably a formulation consisting substantially of the R(+) enantiomer of pramipexole. R(+) pramipexole is generally administered in doses ranging from 0.1-300 mg / kg / daily, preferably 0.5-50 mg / kg / daily, and most preferably 1-10 mg / kg / daily for oral administration. Daily total doses administered orally are typically between 10 mg and 500 mg. Alternatively, R(+) pramipexole can be administered parenterally to humans with acute brain injury in single doses between 10 mg and 100 mg, and / or by continuous intravenous infusions between 10 mg / day and 500 mg / day.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Neurorestoration with r(+) pramipexole
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
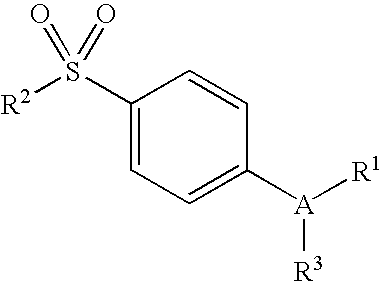
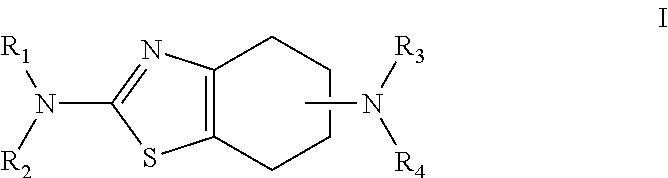
Derivatives of 4-(n-azacycloalkyl) anilides as potassium channel modulators
Owner:XENON PHARMACEUTICALS INC +1
Diagnostic polymorphisms for the ecnos promoter
InactiveUS20050084849A1Eliminate the effects ofDigital data processing detailsMicrobiological testing/measurementValvular diseaseProstate cancer
Disclosed are single nucleotide polymorphisms (SNIps) associated with breast cancer, lung cancer, prostate cancer, non-insulin dependent diabetes, end stage renal disease due to non-insulin dependent diabetes, hypertension, end stage renal disease F due to hypertension, myocardial infarction, colon cancer, hypertension, atherosclerotic peripheral vascular disease due to hypertension, cerebrovascular accident due to hypertension, cataracts due to hypertension, cardiomyopathy with hypertension, myocardial infarction due to hypertension, non-insulin dependent diabetes mellitus, atherosclerotic peripheral vascular disease due to non-insulin dependent diabetes mellitus, cerebrovascular accident due to non-insulin dependent diabetes mellitus, ischemic cardiomyopathy, ischemic cardiomyopathy with non-insulin dependent diabetes mellitus, myocardial infarction due to non-insulin dependent diabetes mellitus, atrial fibrillations without valvular disease, alcohol abuse, anxiety, asthma, chronic obstructive pulmonary disease. cholecystectomy, degenerative joint disease, end stage renal disease and frequent de-clots, end stage renal disease due to focal segmental glomerular sclerosis, end stage renal disease due to insulin dependent diabetes mellitus, or seizure disorder. Also disclosed are methods for using SNPs to determine susceptibility to these diseases; nucleotide sequences containing SNPs; kits for determining the presence of SNPs; and methods of treatment or prophylaxis based on the presence of SNPs.
Owner:VIRAL THERAPEUTICS
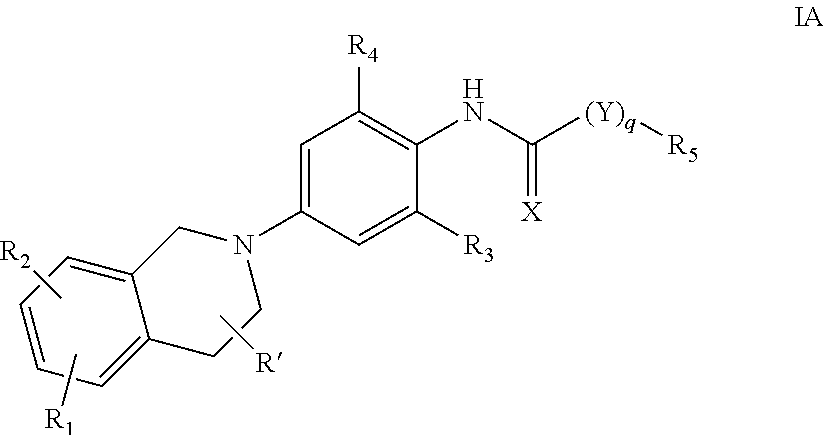
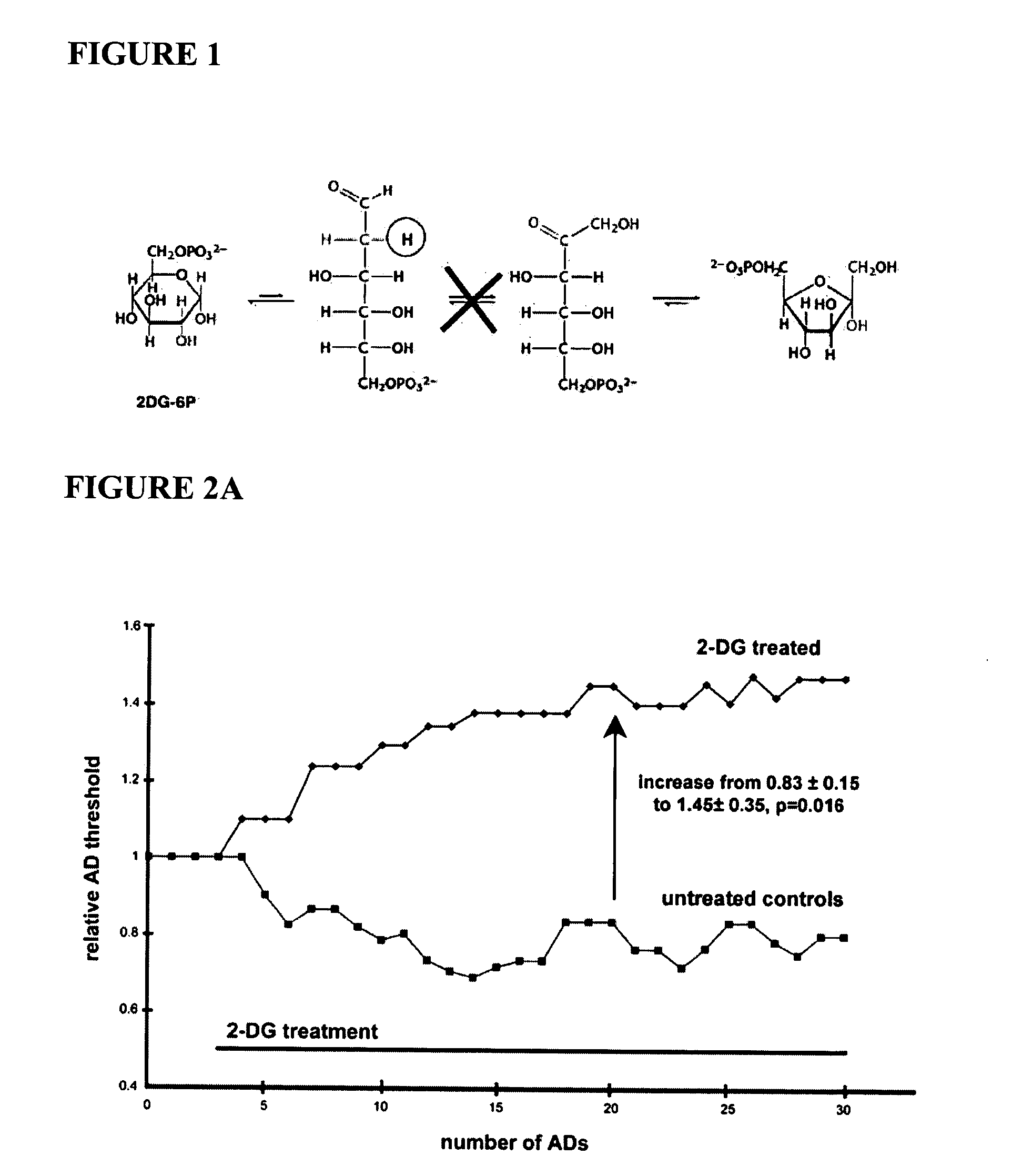
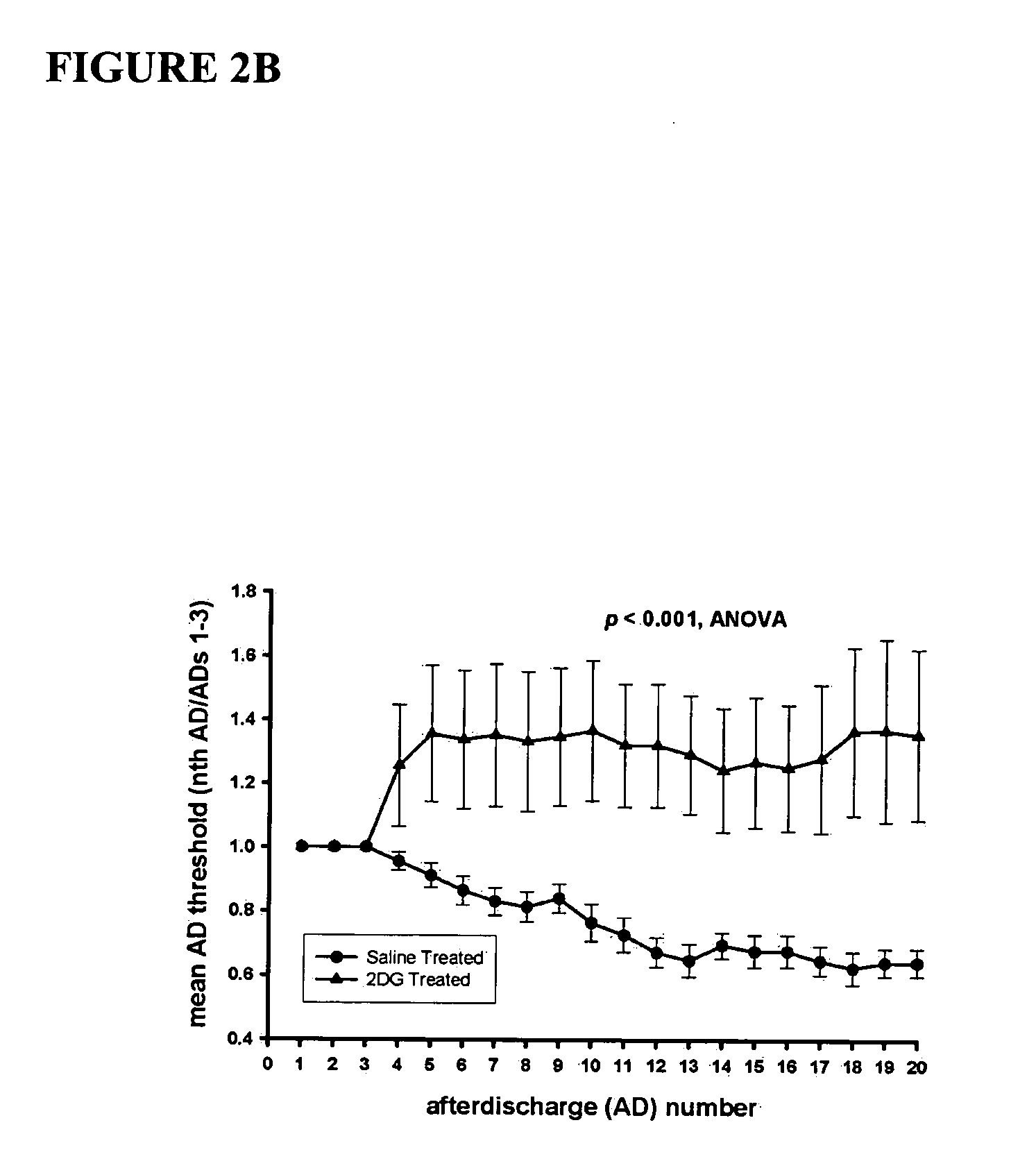
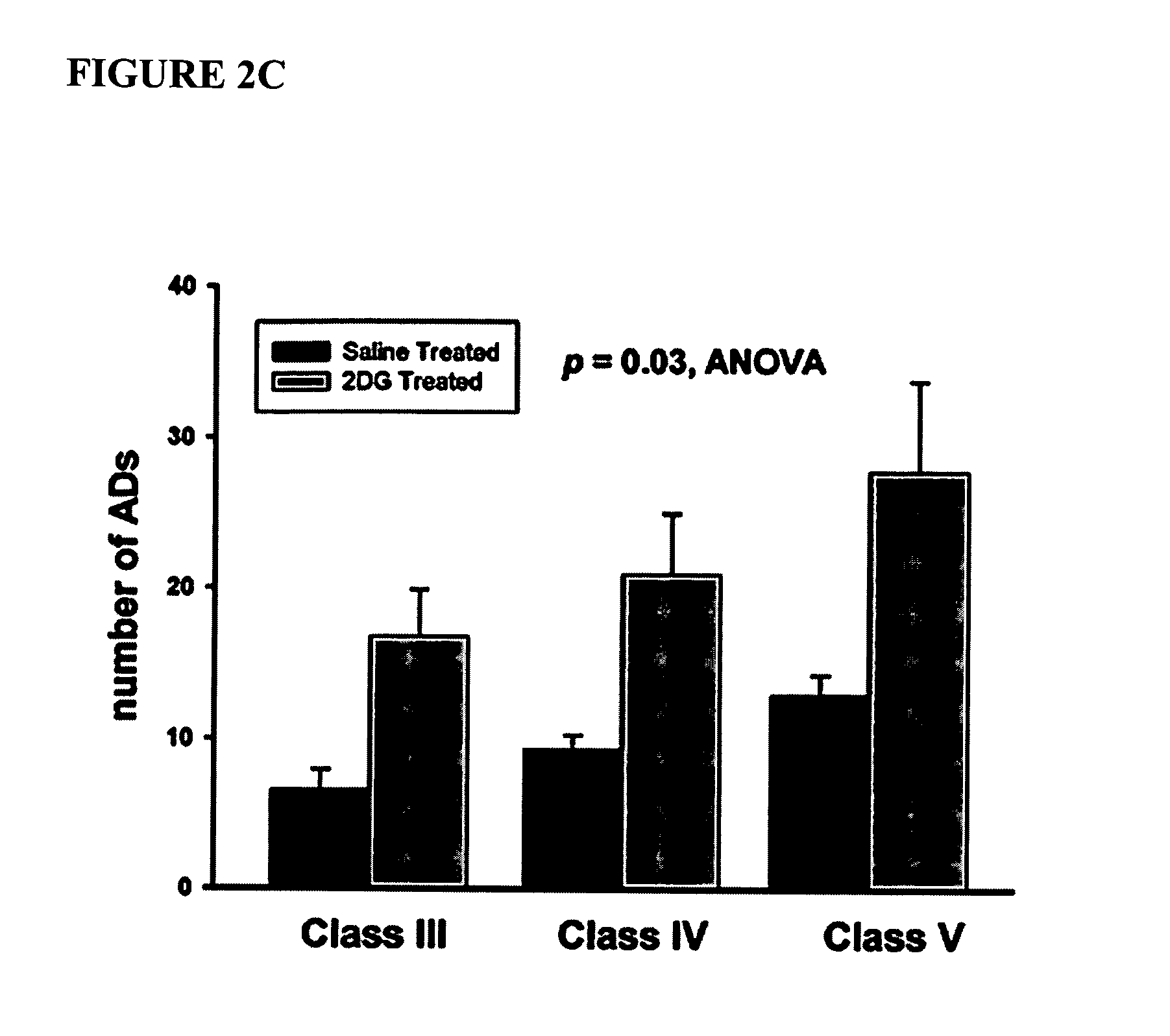
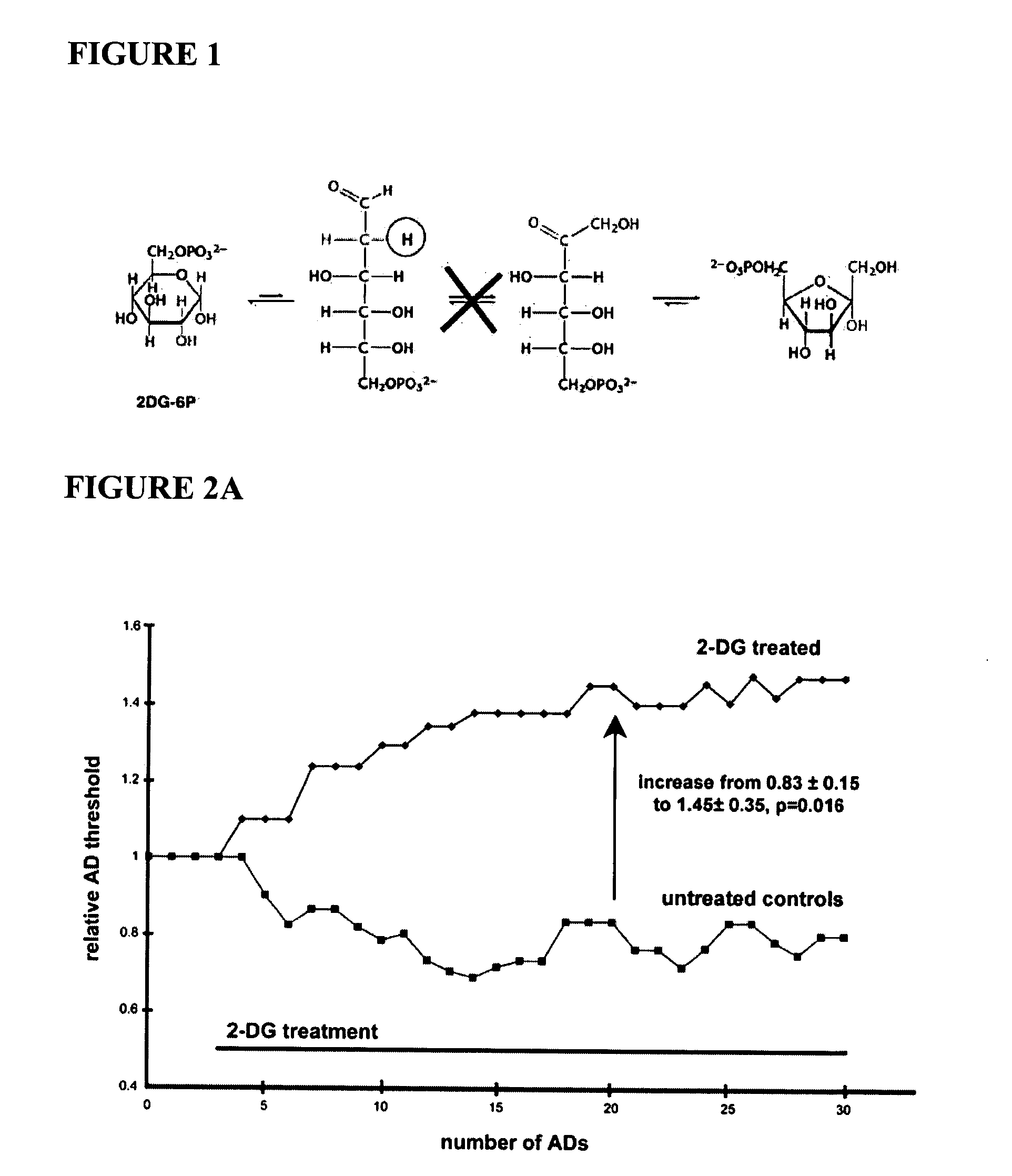
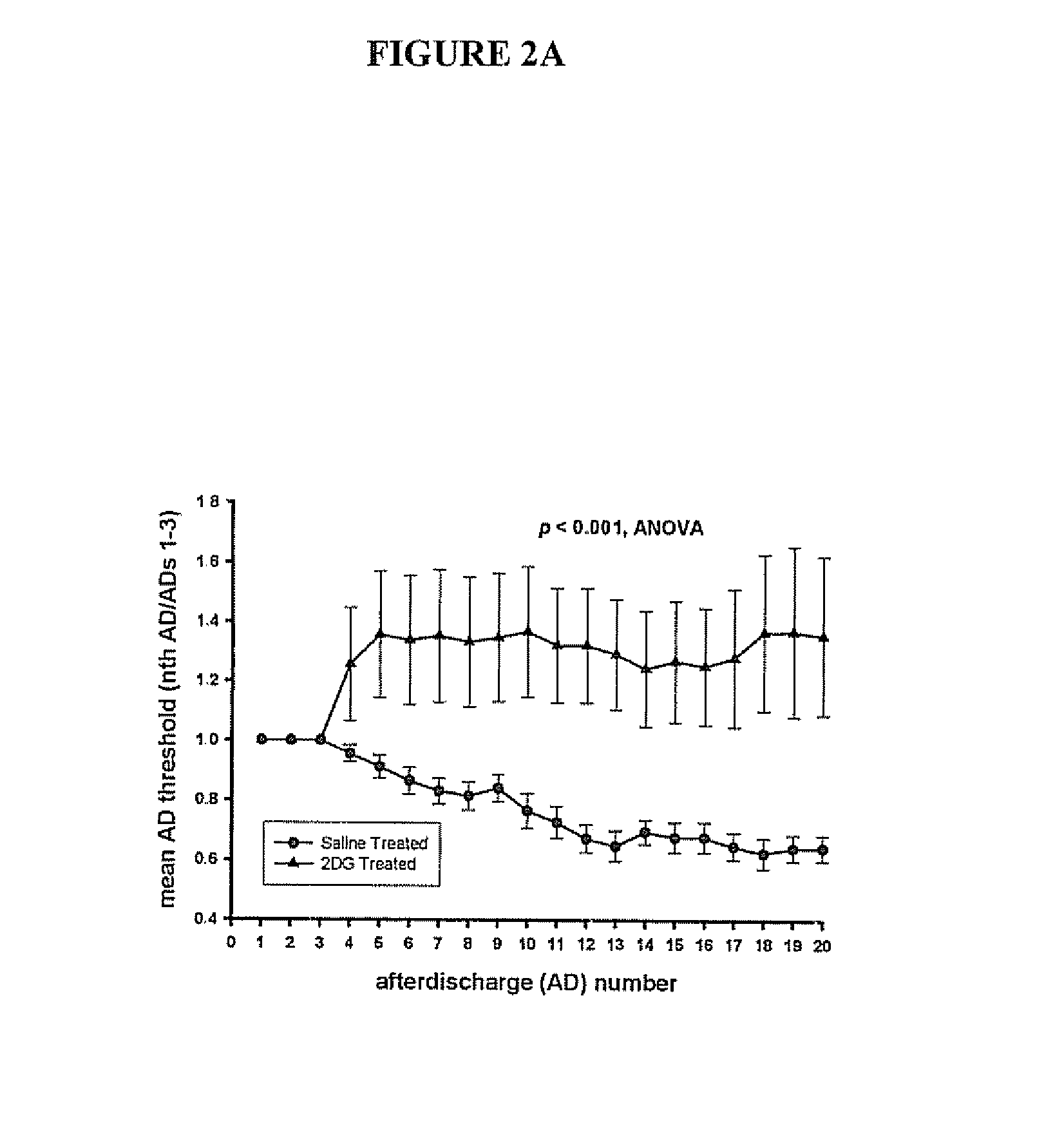
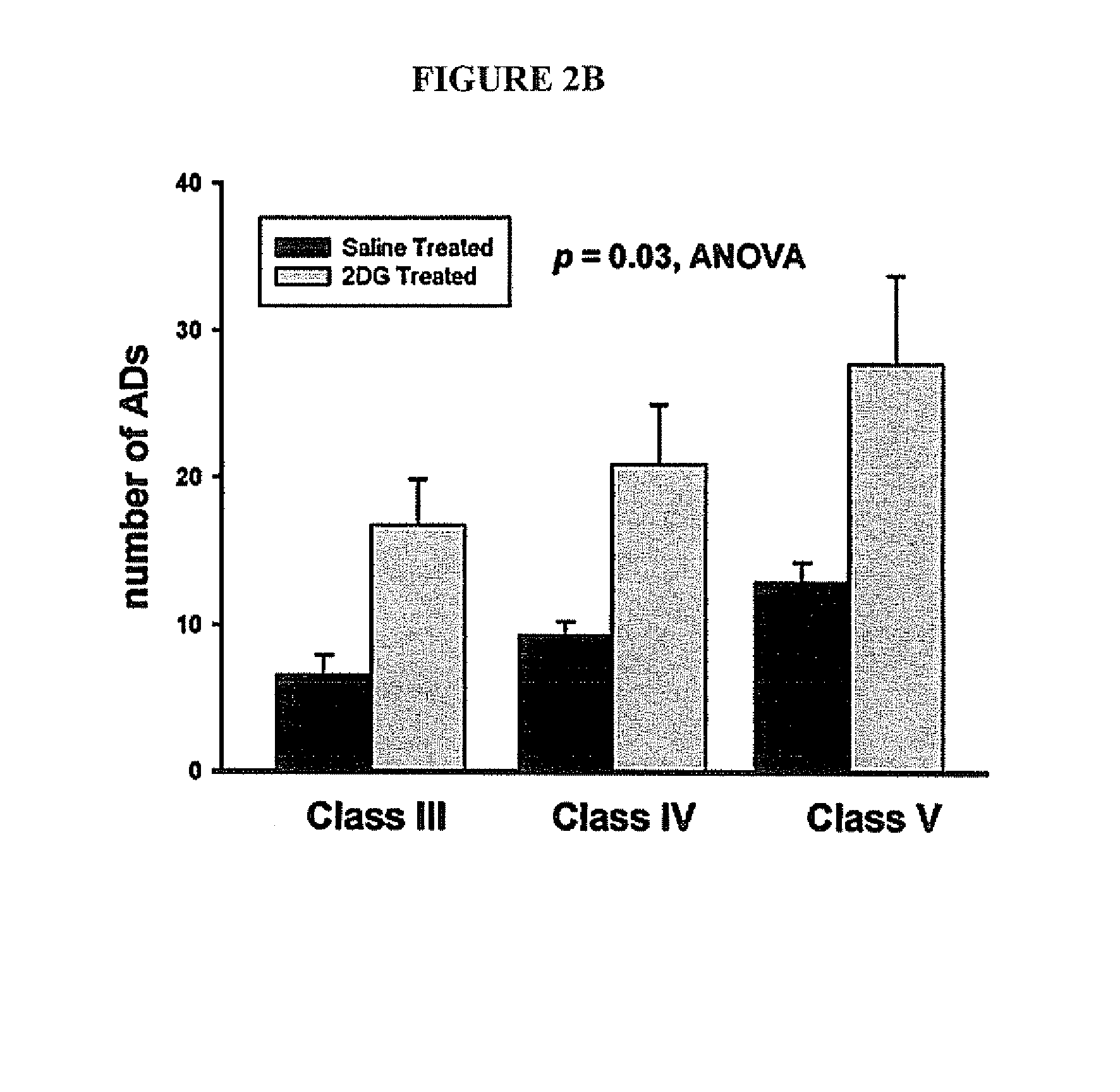
Compounds and methods for treating seizure disorders
ActiveUS20060088517A1Restore responseIncreased Von Frye scoreBiocideNervous disorderParoxysmal AFMedicine
This invention provides methods for alleviating paroxysmal disorders in an animal, particularly epilepsy, by modulating glycolysis in brain cells.
Owner:WISCONSIN ALUMNI RES FOUND
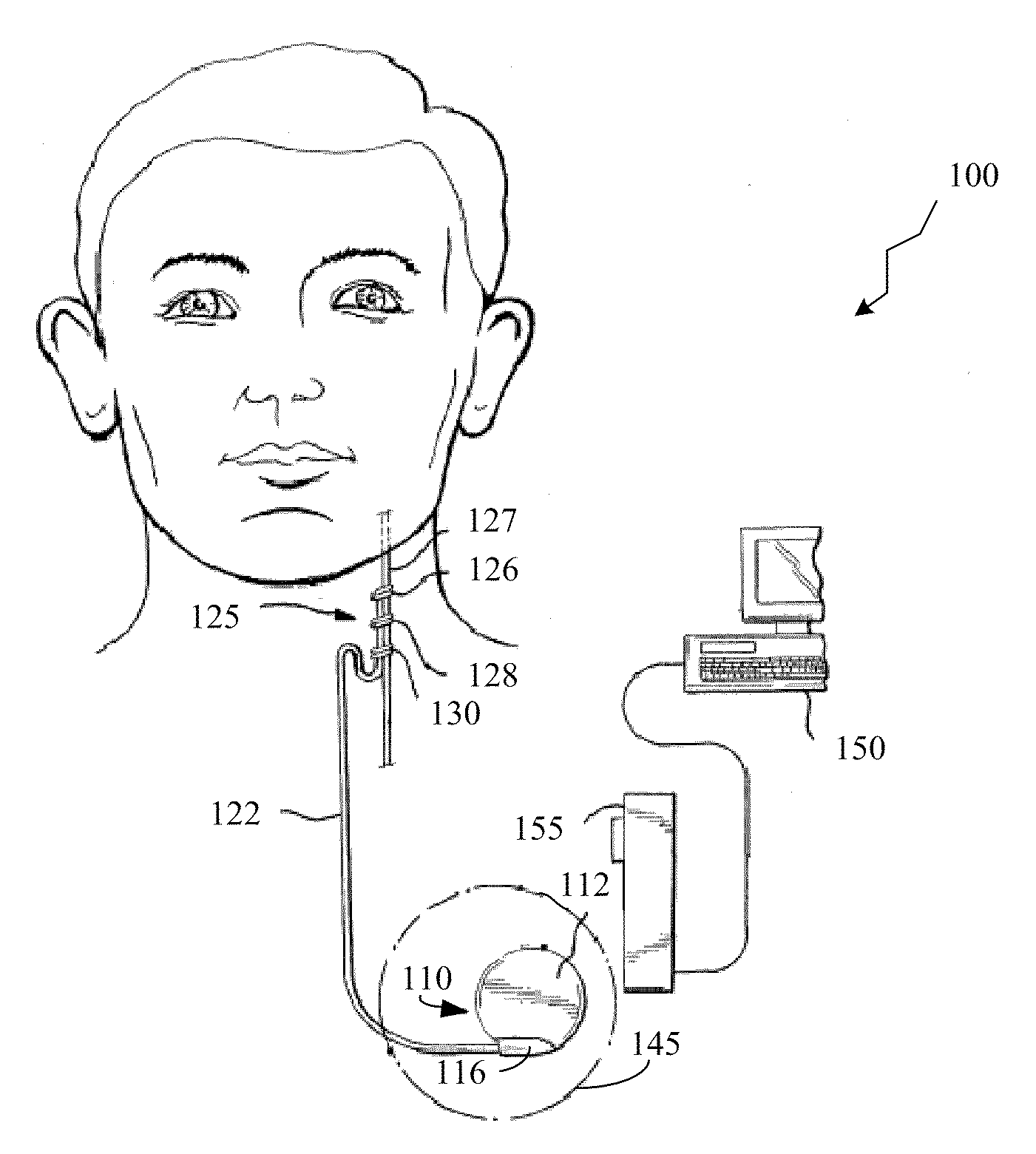
Method and apparatus for detection of nervous system disorders
ActiveUS7761146B2ElectroencephalographyPhysical therapies and activitiesNeurological problemsPhysical therapy
Owner:MEDTRONIC INC
Use of cannabinoids in the treatment of epilepsy
ActiveUS20200297656A1Reduce in quantityReduce doseNervous disorderHydroxy compound active ingredientsAicardi's syndromeYoung adult
The present invention relates to the use of cannabidiol (CBD) in the treatment of focal seizures. In one embodiment the patients suffering from focal seizures are children and young adults. CBD appears particularly effective in reducing focal seizures in patients suffering with etiologies that include: Lennox-Gastaut Syndrome; Tuberous Sclerosis Complex; Dravet Syndrome; CDKLS; Neuronal ceroid lipofuscinoses (NCL); febrile infection related epilepsy syndrome (FIRES); Aicardi syndrome and brain abnormalities in comparison to other seizure types. Significantly CBD additionally is very effective in the reduction of a sub-type of focal seizures, focal seizures with impairment.
Owner:JAZZ PHARM RES UK LTD
Substituted 4H-1,4-benzothiazine-2-carboxamide: GABA brain receptor ligands
Disclosed are 4H-1,4-Benzothiazine-2-carboxamides. These compounds are highly selective agonists, antagonists or inverse agonists for GABAa brain receptors or prodrugs of agonists, antagonists or inverse agonists for GABAa brain receptors. These compounds are useful in the diagnosis and treatment of anxiety, depression, sleep, cognitive and seizure disorders, and overdose with benzodiazepine drugs and for enhancement of alertness. Pharmaceutical compositions, including packaged pharmaceutical compositions, are further provided. Compounds of the invention are also useful as probes for the localization of GABAA receptors in tissue samples.
Owner:NEUROGEN
Pharmaceutical compositions of clonazepam and method of use thereof
The present invention includes benzodiazepine compositions formulated for intranasal administration, comprising a binary solvent system comprising a first solvent in which the benzodiazepine is soluble, the first solvent capable of penetrating nasal mucosal tissue, and a second solvent in which the benzodiazepine in less soluble. The compositions of the present invention may be used to treat a variety of disorders including, but not limited to, panic attacks, muscle spasms, anxiety, and seizures. In one aspect, the present invention relates to a fast-acting, clonazepam composition for transnasal administration that can be used for the treatment of seizure clusters.
Owner:JAZZ PHARMA INC
Methods and compositions for the treatment of epilepsy, seizure disorders, and other CNS disorders
InactiveUS20090306051A1Benefit maximizationReducing unwanted side effectBiocideNervous disorderPharmacologyRelated disorder
Owner:MEYERSON LAURENCE R +2
Compositions and methods for treating seizures
Owner:DRAGTEK CORP
Use of cannabinoids in the treatment of epilepsy
ActiveUS10709671B2Nervous disorderHydroxy compound active ingredientsAicardi's syndromeFocal Epilepsies
Owner:GW RES LTD
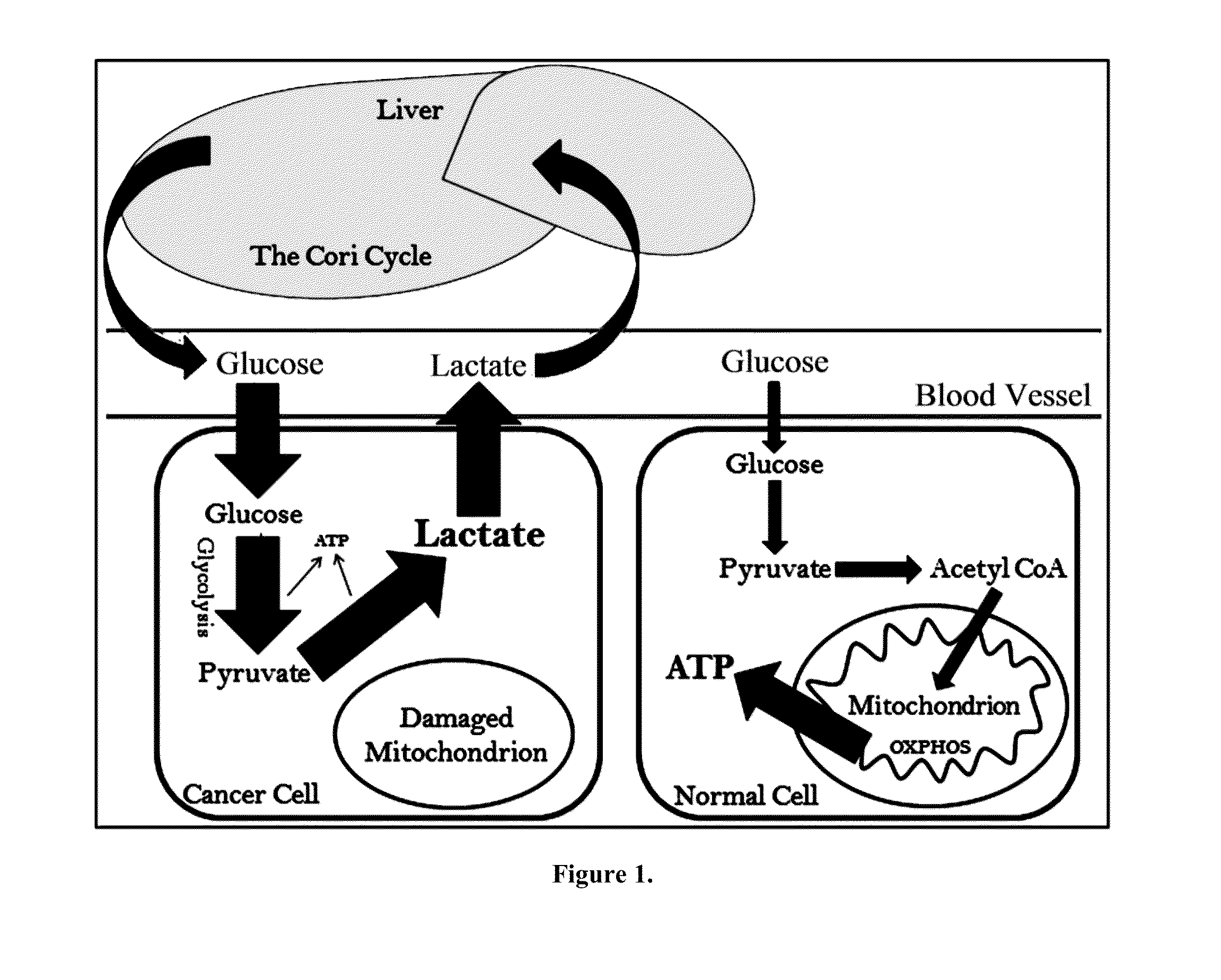
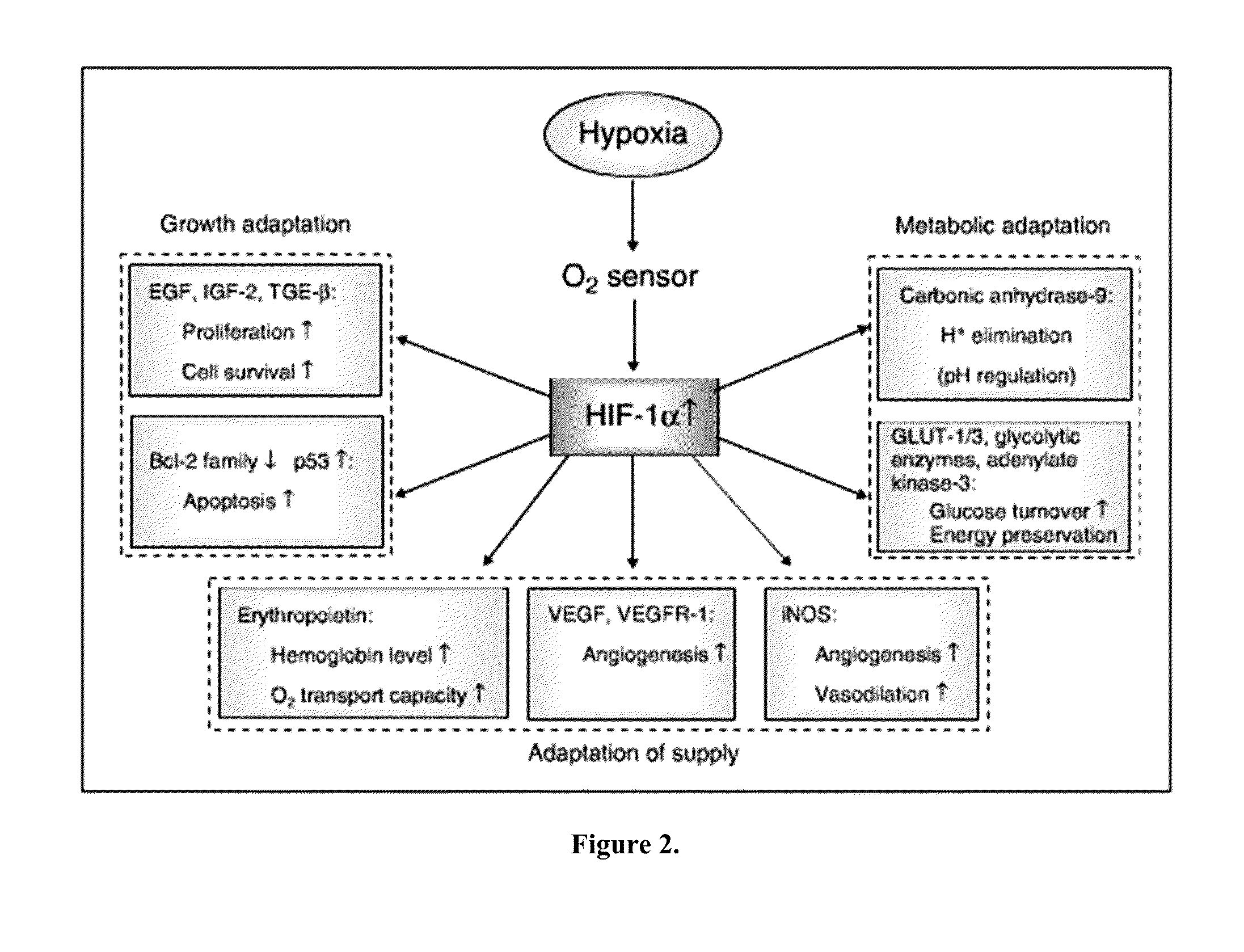
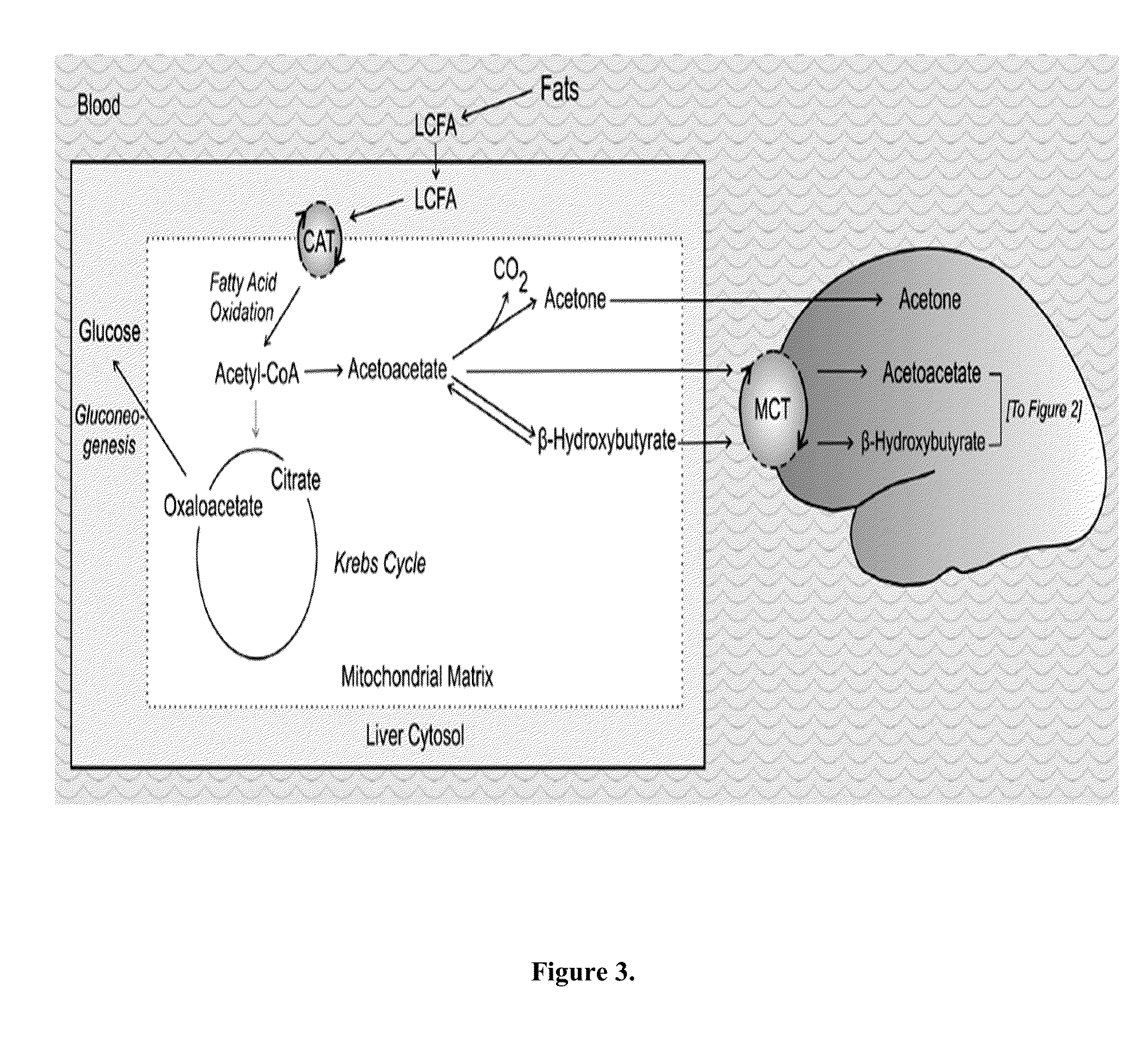
Cancer with metabolic therapy and hyperbaric oxygen
InactiveUS20140072654A1Raise the possibilityIncrease productionBiocidePeroxide active ingredientsDiseaseAcetoacetates
The present invention demonstrates the therapeutic use of ketone esters for seizure disorders, Alzheimer's disease malignant brain cancer, and other cancers, which are associated with metabolic dysregulation. The administration of a ketogenic diet, such as ketone esters, while concurrently subjecting the patient to a hyperbaric, oxygen-enriched environment resulted in therapeutic ketosis. Optionally, the hyperbaric, oxygen-enriched environment is 100% oxygen at 2.5 ATA absolute. The ketone esters may be derived from acetoacetate and can include R,S-1,3-butanediol acetoacetate monoester, R,S-1,3-butanediol acetoacetate diester, or a combination of the two. The treatment may further include administering at least 10% ketone supplementation, such as acetoacetate, adenosine monophosphate kinase, 1,3-butanediol, or ketone ester, to the patient.
Owner:UNIV OF SOUTH FLORIDA
Pharmaceutical compositions of benzodiazepines and method of use thereof
The present invention includes benzodiazepine compositions formulated for intranasal administration, comprising a binary solvent system comprising a first solvent in which the benzodiazepine is soluble, the first solvent capable of penetrating nasal mucosal tissue, and a second solvent in which the benzodiazepine in less soluble. The compositions of the present invention may be used to treat a variety of disorders including, but not limited to, panic attacks, muscle spasms, anxiety, and seizures. In one aspect, the present invention relates to a fast-acting, clonazepam composition for transnasal administration that can be used for the treatment of seizure clusters.
Owner:JAZZ PHARMA INC
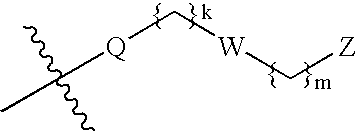
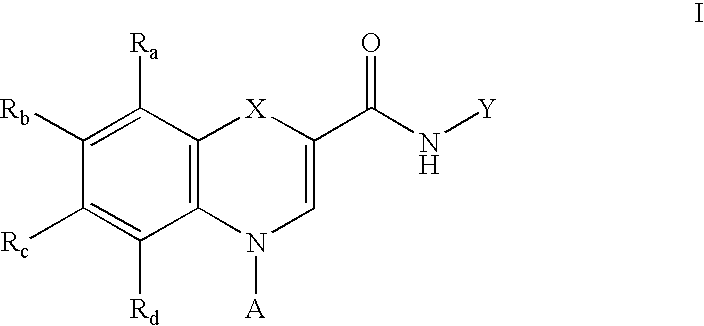
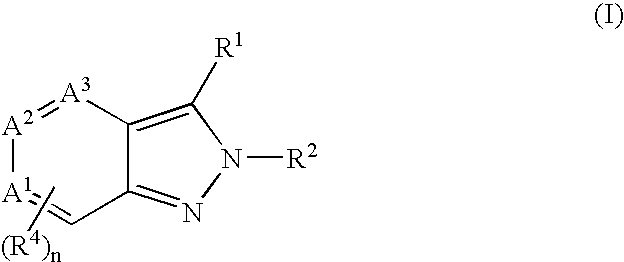
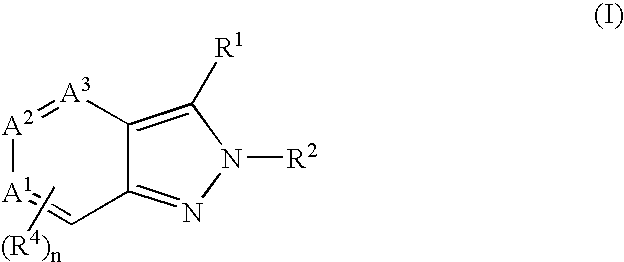
Heterocyclic GABAA subtype selective receptor modulators
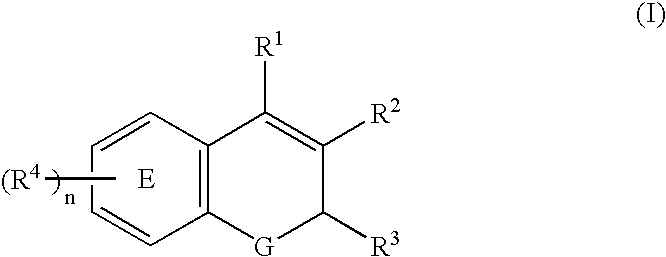
This invention relates to a method for modulating α2 subtype GABAA receptors with heterocyclic compounds of formula I, and salts, solvates and prodrugs thereof. The invention further relates to novel heterocyclic compounds and pharmaceutical compositions containing said compounds. In addition the invention relates to the treatment of depression, an anxiety disorder, a psychiatric disorder, a learning or cognitive disorder, a sleep disorder, a convulsive or seizure disorder or pain
Owner:ROCHE PALO ALTO LLC
Compounds and methods for treating seizure disorders
This invention provides methods for alleviating paroxysmal disorders in an animal, particularly epilepsy, by modulating glycolysis in brain cells.
Owner:WISCONSIN ALUMNI RES FOUND
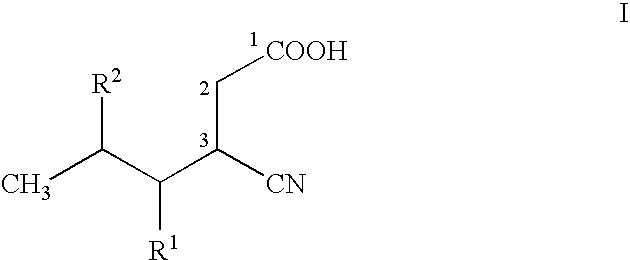
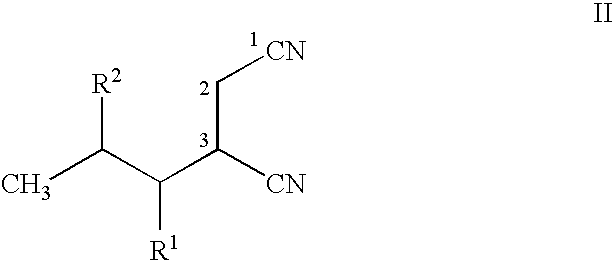
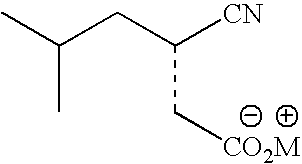
Stereoselective bioconversion of aliphatic dinitriles into cyano carboxylic acids
The present invention is directed to a regio- and stereoselective bioconversion of selected aliphatic dinitriles into corresponding cyanocarboxylic acids. More particularly, the present invention provides methods for the conversion of 2-isobutyl-succinonitrile into (S)-3 cyano-5-methylhexanoic acid, which is a useful intermediate in the synthesis of (S)-3(aminomethyl)-5-methylhexanoic acid (pregabalin). Pregabalin can be used for treating certain cerebral diseases, for example, in the treatment and prevention of seizure disorders, pain, and psychotic disorders.
Owner:PFIZER INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com