Patents
Literature
79 results about "Chronic fatigue" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
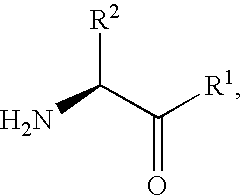
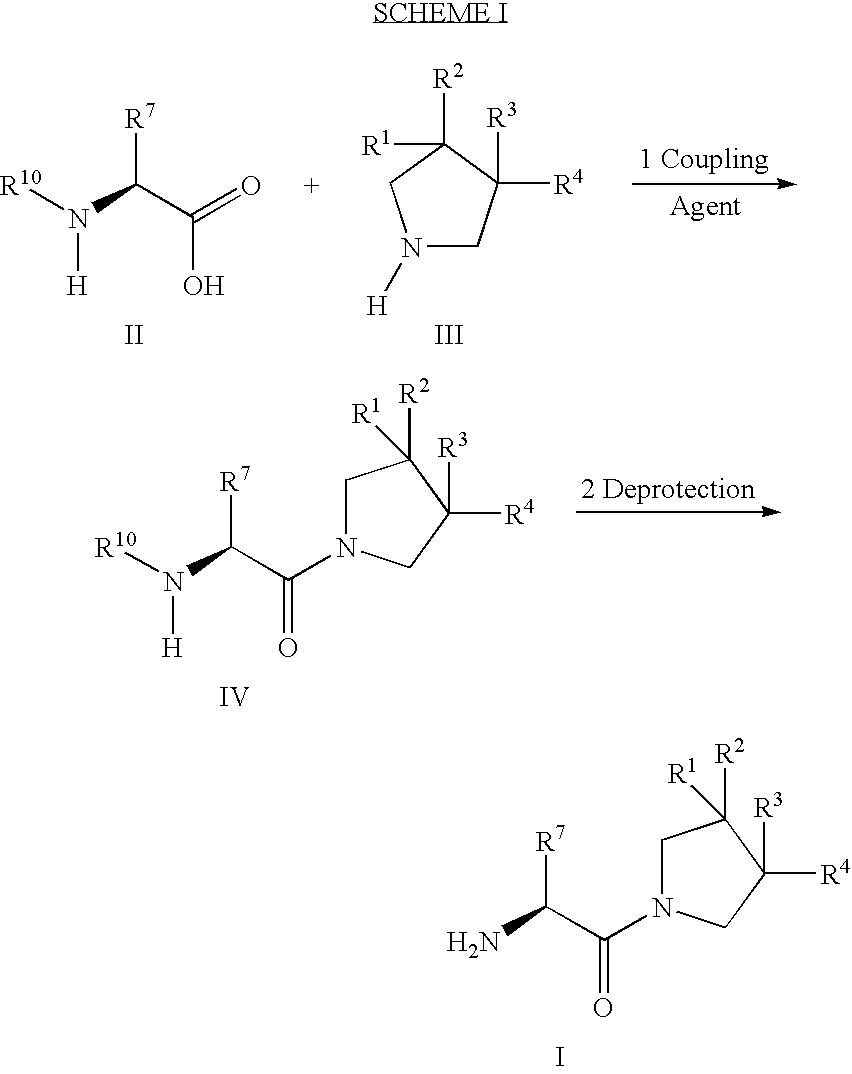
Fluorinated cyclic amides as dipeptidyl peptidase IV inhibitors
InactiveUS6710040B1Easy to prepareEase of detectabilityBiocideOrganic chemistryAcute coronary syndromeDisease progression
The invention relates to new therapeutically active and selective inhibitors of the enzyme dipeptidyl peptidase-IV, pharmaceutical compositions comprising the compounds and the use of such compounds for treating diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, hyperglycemia, impaired glucose tolerance, metabolic syndrome (Syndrome X or insulin resistance syndrome), glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of identifying an insulin secretagogue agent for diabetes.
Owner:PFIZER INC
Prolonged administration of NMDA antagonist drug and safener drug to create improved stable neural homeostasis
InactiveUS20050222270A1Stable and lasting improved neural homeostasisBiocideOrganic active ingredientsDiseaseNervous system
An NMDA antagonist (such as ketamine) is administered with a safener (such as clonidine) in patients suffering from neurologic disorders other than pain. The ketamine is adminsitered at a dosage that causes slurred speech, for a span of several days. This treatment enables a patient's nervous system to return to a healthy “set point”, also called an improved stable neural homeostasis, in a manner similar to a broken bone healing itself if protected from jostling and reinjury by a cast. In at least some patients, this treatment can ease problems such as addictions to illegal or pain-killing drugs, nicotine, or alcohol, compulsive or criminal behavioral problems, severe depression, obsessive-compulsive disorders, phobias, etc. It may also provide some relief in some patients for problems such as chronic fatigue, chemical sensitivities, allergies, autoimmune disorders, and diabetes.
Owner:OLNEY JOHN W +3
Therapy for enteric infections
There is disclosed herein a composition for treating gastrointestinal or neurological disorders, constipation, functional constipation, irritable bowel syndrome, diverticulitis, travelers diarrhea, chronic idiopathic nausea, IBD-associated constipation and diarrhea, pseudo-obstruction, diabetic gastroparesis, cyclic vomiting, reflux oesophagitis, autism enteropathy, flatulence, halitosis, chronic fatigue, bloating, proctalgia fugax, Parkinsons disease, MS, Alzheimers Disease, Motor Neurone Disease or autism, the composition comprising: (i) at least two anti-clostridial agents selected from the group consisting of: vancomycin, vancomycin derivatives, a multi-valent polymer of vancomycin, aminoglycosides, nitroimidazoles, ansamysins, nifuroxazide, colchicine, prucalopride, prokinetic agent and 5-aminosalicylic acid; or (ii) at least one anti-clostridial agent selected from the above combined with an opioid blocking agent. There is also disclosed herein a method of treating various gastrointestinal or neurological disorders, constipation, functional constipation, irritable bowel syndrome, diverticulitis, travelers diarrhea, chronic idiopathic nausea, IBD-associated constipation and diarrhea, pseudo-obstruction, diabetic gastroparesis, cyclic vomiting, reflux oesophagitis, autism enteropathy, flatulence, halitosis, chronic fatigue, bloating, proctalgia fugax, Parkinsons disease, MS, Alzheimers Disease, Motor Neurone Disease or autism, the method comprising administering orally, via enema or by suppository: (i) a composition of the invention; (ii) at least two anti-clostridial agents selected from the group consisting of: vancomycin, vancomycin derivatives, a multi-valent polymer of vancomycin, aminoglycosides, nitroimidazoles, ansamysins, nifuroxazide, colchicine, prucalopride, prokinetic agent and 5-aminosalicylic acid; or (iii) at least one anti-clostridial agent selected from the above and an opioid blocking agent to a patient in need of such treatment.
Owner:BORODY THOMAS JULIUS
Fluorinated cyclic amides as dipeptidyl peptidase IV inhibitors
InactiveUS20040132713A1Ease of preparation and detectabilityGood metabolic stabilityBiocideOrganic chemistryDisease progressionDiabetic nephropathy
The invention relates to new therapeutically active and selective inhibitors of the enzyme dipeptidyl peptidase-IV, pharmaceutical compositions comprising the compounds and the use of such compounds for treating diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, hyperglycemia, impaired glucose tolerance, metabolic syndrome (Syndrome X or insulin resistance syndrome), glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of identifying an insulin secretagogue agent for diabetes.
Owner:PFIZER INC
Diagnosis and treatment of human dormancy-related sequellae
Owner:POWELL CO LTD
Nutritive powder and its making process
ActiveCN101036519ATo promote metabolismPromote absorptionVegetable proteins working-upFood preparationDiseaseVitamin b6
The invention relates to a nutrient powder and its preparation method. The nutrient powder is made of soybean protein isolate, soybean oligopeptide, vegetable fat powder, milk powder, complex vitamine, mineral substance components, etc, viz. soybean protein isolate, soybean oligopeptide, vegetable fat powder, milk powder, soybean lecithin, vitamine A. vitamine D, vitamine E, vitamine C, vitamine B1, vitamine B2, vitamine B6, nicotinic acid, choline, folic acid, Ca, Fe, Zn, Se, Mg, and taurine. The invention is provided with unique formulation, and can rapidly supplying energy and nutrient, especially protein, for dissipating fatigue, promoting restoration of skeletal muscle, strengthening immunologic function, improving motion ability, rapidly restoring physical strength, etc, thereby it can contribute to health, disease-resistance improvement, and motion score improvement, accordingly, the health-care food can be used for soldiers, sportsmen, moderate or hard physical labors in training or working, and be suitable for chronic fatigue patients to use for a long time.
Owner:ZHONGSHI TAILING BEIJING BIO SCI & TECH +1
Diagnosis and treatment of human dormancy-related sequellae
InactiveUS20060052278A1Effective treatmentReduce doseBiocideSnake antigen ingredientsAntigenAutoimmune condition
New methods for diagnosis and treatment of human dormancy syndrome-related sequellae are provided. Human dormancy syndrome (HDS) is characterized by elevated serum ratio of rT3 / fT3 compared to a population of normal subjects. HDS includes fibromyalgia, chronic fatigue, cancer, autoimmune disease, obesity and related dormancy conditions. Dormancy and HDS-related sequellae are imposed on humans by infection with lipopolysaccharide (LPS; or endotoxin)-producing organisms, especially those that are intracellular and those that create antigens that stimulate the TLR pathways. In such instances, the elimination or neutralization of the LPS signal along with the infectious source is required to impact the sequellae of HDS. Treatment includes use of novel and non-obvious doses of antibiotics, optionally including agents that decrease the adverse effects of endotoxin.
Owner:POWELL CO LTD
Medicinal composition and use thereof
InactiveCN1451426AImprove sexual functionImprove securityUnknown materialsSexual disorderSide effectCitrulline
A medical composition for treating sexual disfunction, impotence, immunodeficiency, chronic fatigue, and hepal and renal disfunction is prepared from ornithin and citrulline or their salts, jujube, ginkgo seed and ginseng. Its advantages are high curative effect and no toxic by-effect.
Owner:ZHEJIANG ADAMERCK BIOPHARMLABS
Physical recovery healthy food and preparation process thereof
The invention relates to a strength-restoring health-care food and preparation method thereof. The health-care food is made of soybean oligopeptide as main component and polysaccharide, complex vitamines, mineral matter, etc, viz. soybean oligopeptide, high-maltose powder, white granulated sugar, vitamine B1, vitamine B2, vitamine C, vitamine E, nicotinic acid, calcium lactate, potassium chloride, sodium chloride, zinc gluconate, L-carnitine tartrate, anhydrous citric acid, acesulfame-k, and IFF concentrated vanilla. The invention is provided with unique formulation, and can rapidly supplying energy and nutrient, especially protein, for dissipating fatigue, promoting restoration of skeletal muscle, strengthening immunologic function, improving motion ability, rapidly restoring physical strength, etc, thereby it can contribute to health, disease-resistance improvement, and motion score improvement, accordingly, the health-care food can be used for soldiers, sportsmen, moderate or hard physical labors in training or working, and be suitable for acute or chronic fatigue patients to use for a long time.
Owner:ZHONGSHI TAILING BEIJING BIO SCI & TECH +1
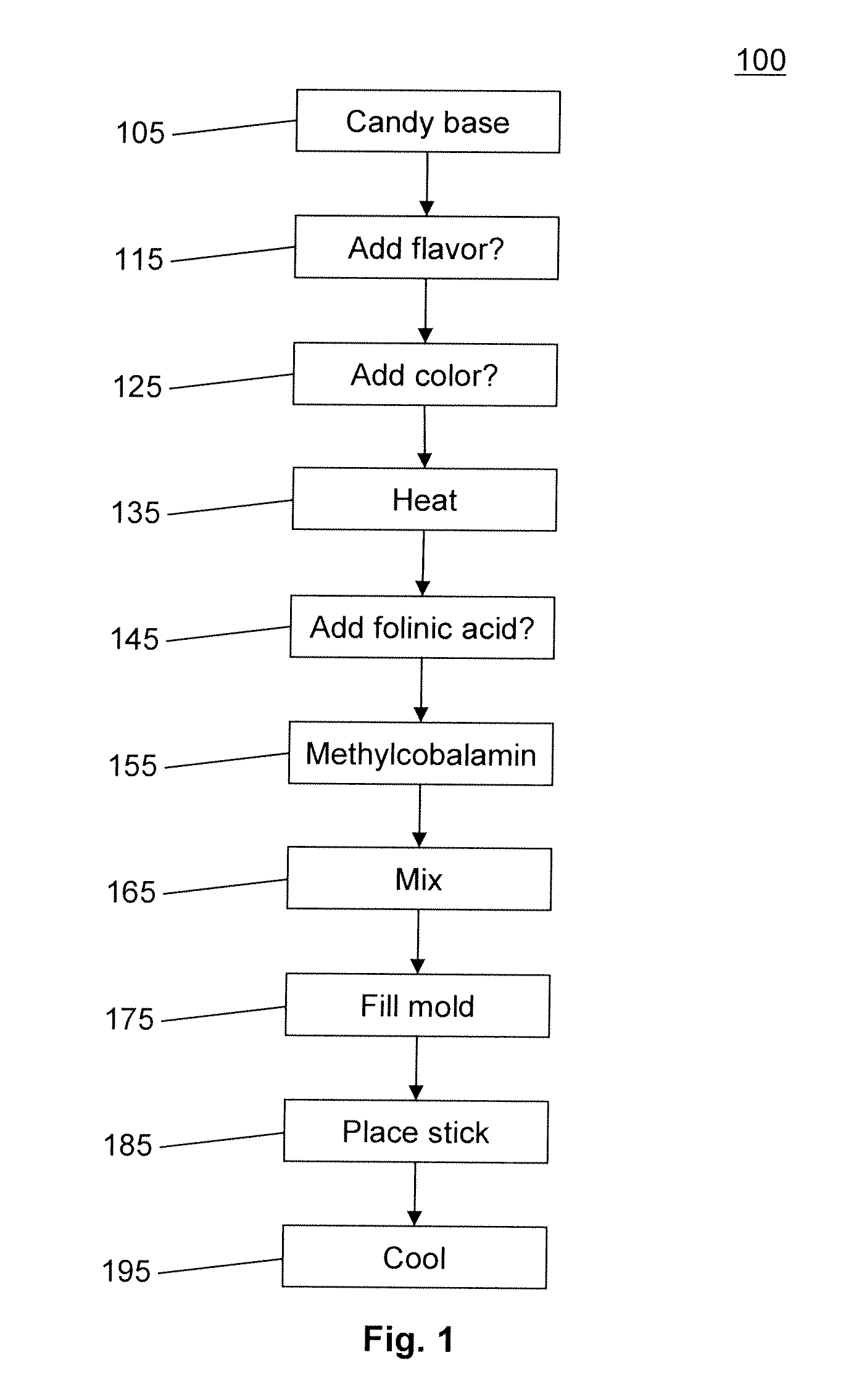
Use of methylcobalamin nasal spray to treat disorders
A method of treating a disorder by nasally administering methylcobalamin, with or without folinic acid. The disorders addressed are: a) attention deficit hyperactivity disorder (ADHD); b) anxiety, depression, stress and chronic stress; c) socialization problems, mood problems, behavior problems, memory problems; d) dislexia, depth perception problems, color viewing problems, visual and auditory processing problems, light modulation problems, night vision problems; e) speech problems such as finding words, apraxia, and articulation problems, sleep regulation problems, eye or muscle movement problems; and f) chronic fatigue problems, digestion problems, sensitivity to chemicals, viral infection, inflammatory conditions such as rheumatoid arthritis, sciatica, and fibromyalgia, asthma, irritable bowel, colitis, tinnitus, migraines, nail biting, autoimmune problems. In some embodiments, the disorders that are particularly addressed are ADHD, anxiety, stress and chronic stress, and irritable bowel.
Owner:KURTZ STAN
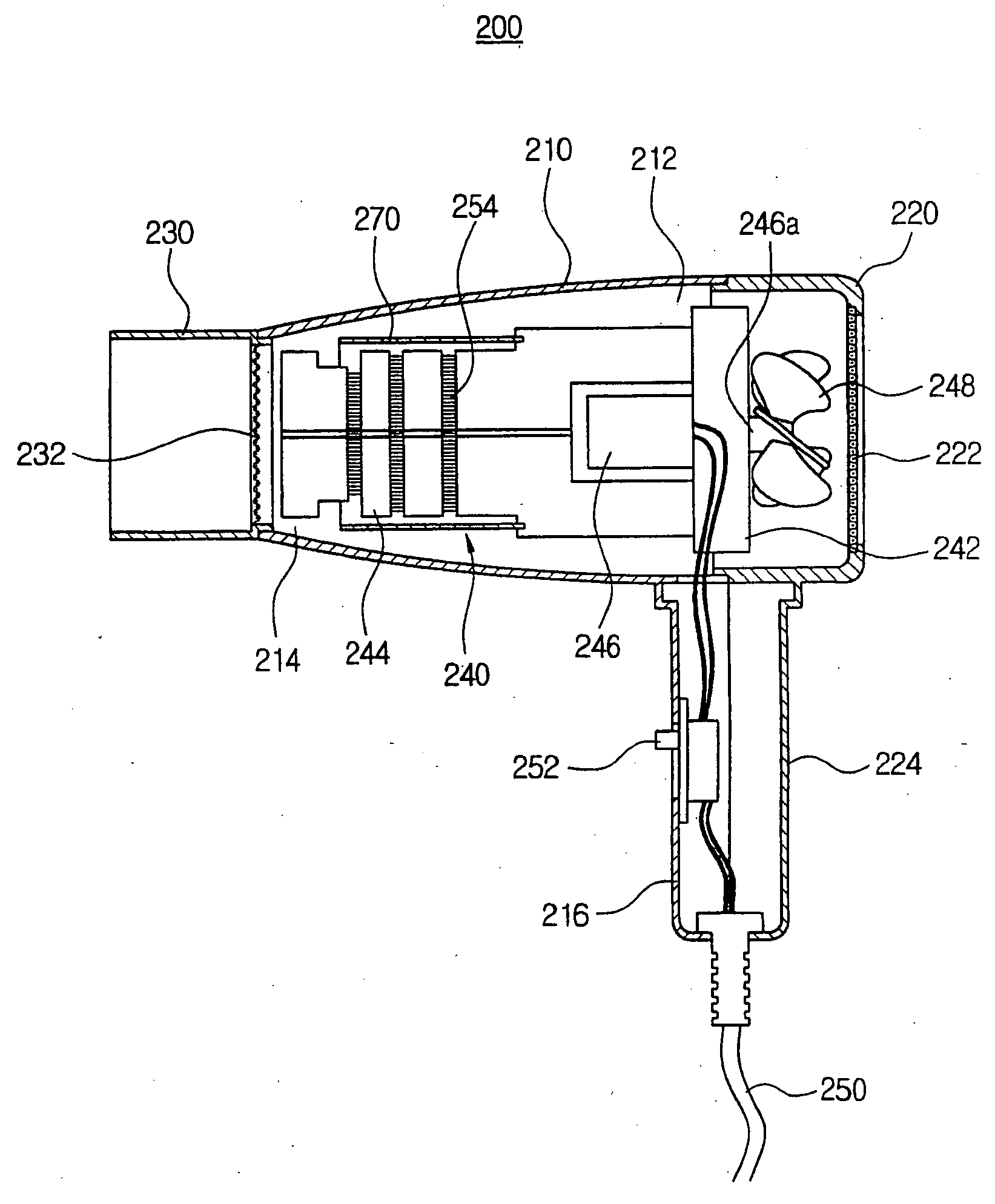
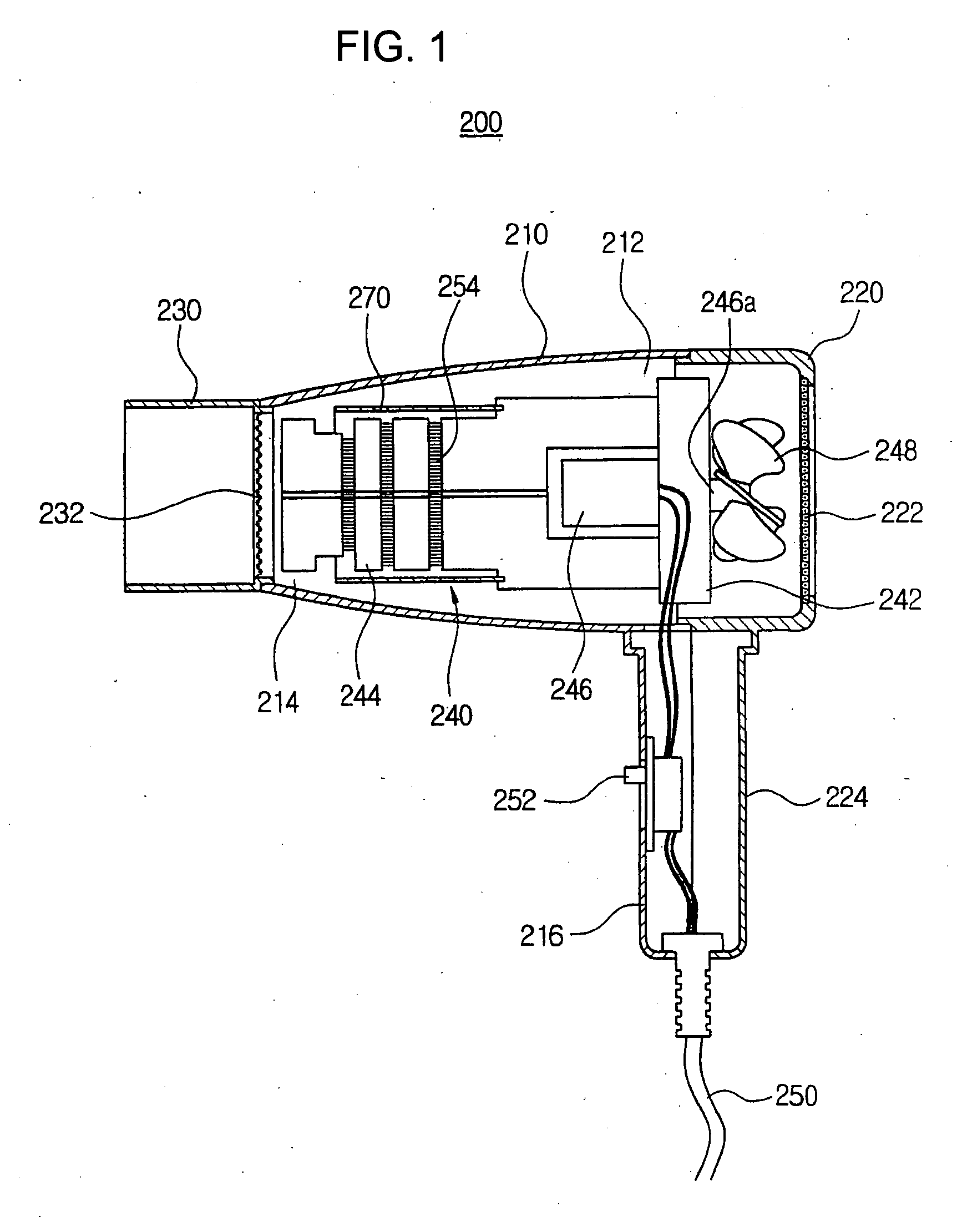
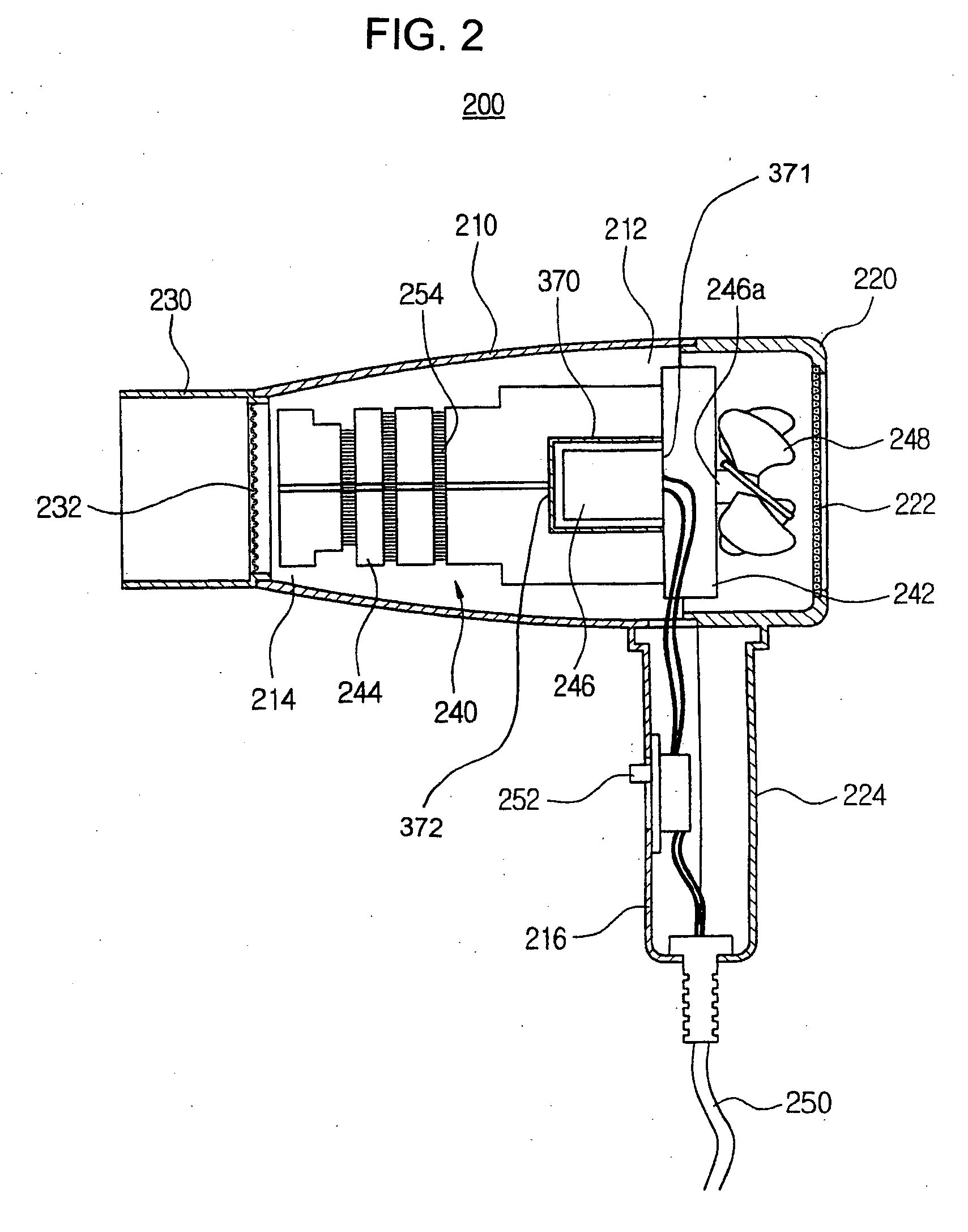
Hair dryer
InactiveUS20050108890A1Avoid spreadingDrying solid materials with heatCurling-ironsEngineeringDrive motor
Disclosed is the hair dryer. In the hair dryer, a hollow dryer housing has an inlet port, an outlet port and a front handle. An intake cover is coupled to a rear side of the dryer housing and has a rear handle protruding downwardly from the hollow dryer, for suctioning an outer air into the dryer housing. A nozzle is coupled to the outlet port of the dryer housing. A heating assembly is positioned in the dryer housing and in the intake cover, and includes a hollow assembly body, a support frame, a driving motor, a rotating shaft, and a blowing fan. A shielding member is installed along an inner side of the dryer housing, the intake cover, and the nozzle. Due to this structure, the hair dryer is capable of absorbing and shielding the magnetic field not to cause human problems such as headache, dermatitis and chronic fatigue.
Owner:PARK SU HONG
Nanoparticulate formulations of modafinil
InactiveUS20080317843A1Minimize changesQuick releaseBiocideOrganic active ingredientsSubstance abuserEnantiomer
The present invention is directed to compositions comprising a nanoparticulate modafinil compositions, or a salt(s), or an enantiomer(s), or a prodrug(s), or a polymorph(s) or derivative thereof, having improved bioavailability. The nanoparticulate modafinil composition formulation particles of the composition have an effective average particle size of less than about 2000 nm and are useful in the treatment of dyssomnias, including but not limited to, narcolepsy, chronic fatigue, eating disorders, compulsive behaviors, ADHD, addictions, substance abuse, sleepiness, nervous system diseases, conditions, syndromes, and symptoms and related diseases, conditions, and symptoms.
Owner:ELAN PHRMA INT LTD
Oral delivery system for methylcobalamin to treat disorders
A method of treating a disorder by lollipop administering methylcobalamin, with or without folinic acid by direct delivery to the trigeminal nerve. The disorders addressed are: a) attention deficit hyperactivity disorder (ADHD); b) anxiety, depression, stress and chronic stress; c) socialization problems, mood problems, behavior problems, memory problems; d) dyslexia, depth perception problems, color viewing problems, visual and auditory processing problems, light modulation problems, night vision problems; e) speech problems such as finding words, apraxia, and articulation problems, sleep regulation problems, eye or muscle movement problems; and f) chronic fatigue problems, digestion problems, sensitivity to chemicals, viral infection, inflammatory conditions such as rheumatoid arthritis, sciatica, and fibromyalgia, asthma, irritable bowel, colitis, tinnitus, migraines, nail biting, autoimmune problems. In some embodiments, the disorders that are particularly addressed are ADHD, anxiety, stress and chronic stress, and irritable bowel. A lollipop for treating a psychological or neuro-physiological disorder and method of making thereof.
Owner:REVITAPOP
Probiotic composition at least comprising bifidobacterium bifidum w23 and capable of controlling intestinal barrier function
InactiveUS20180271919A1Improve efficiencyImprove adhesionOrganic active ingredientsNervous disorderDiseaseMigraine
The disclosure relates to the field of medicine and nutrition, more specifically, to the field of treatment and prevention of human disorders such as depression, rumination, aggression, migraine, autistic spectrum disorders (including autism and ADHD), schizophrenia, chronic fatigue, kidney disorders, metabolic syndrome or diabetes type II. The disclosure provides a pharmaceutical or food composition or a supplement comprising a multispecies probiotic composition at least comprising Bifidobacterium bifidum W23 for use in the treatment or prevention of disorders in humans and methods to use such a pharmaceutical or food composition or a supplement.
Owner:WINCLOVE HLDG BV
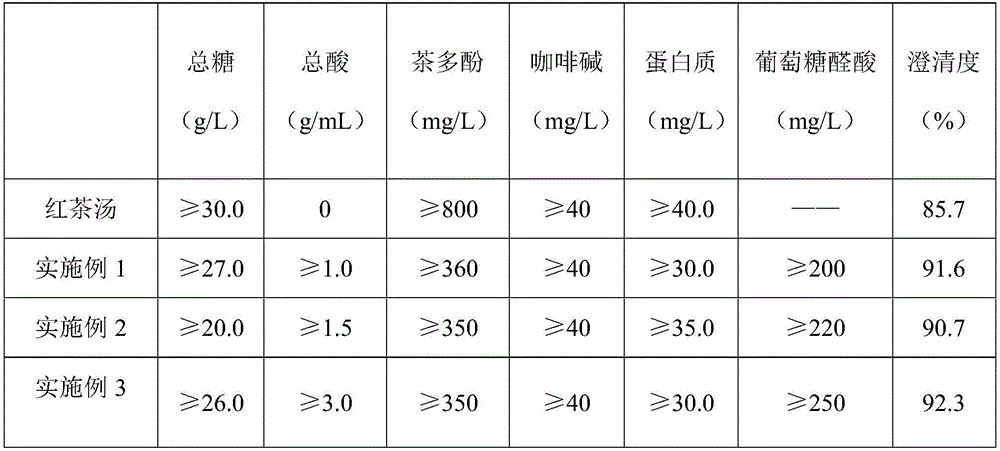
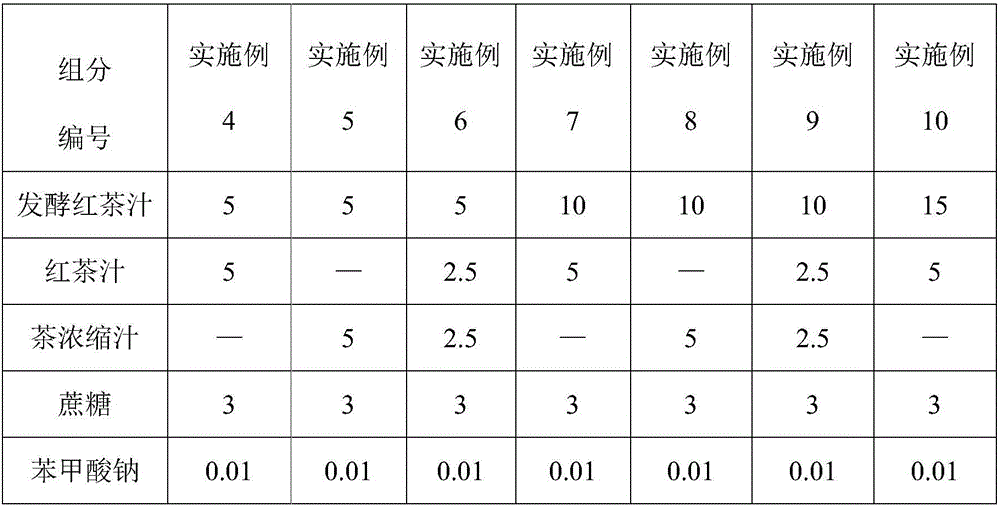
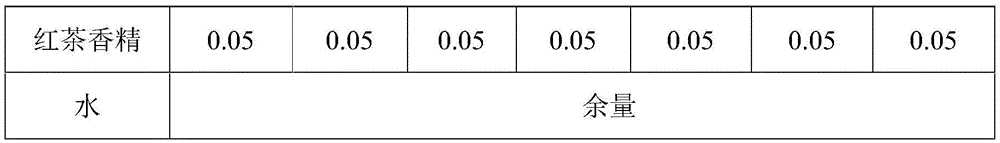
Enzymatic fermented black tea beverage and preparation method thereof
InactiveCN106173025ASolve the production processSolve the problem that product standards are not easy to controlTea extractionPreservativeBlack tea
The invention discloses an enzymatic fermented black tea beverage and a preparation method thereof. The enzymatic fermented black tea beverage comprises the following raw materials in percentage by weight: 1 to 30% of enzymatic fermented black tea juice, 1 to 10% of tea concentrated juice, 0.01 to 10% of a sweetener, 0.01 to 0.1% of a preservative, 0.05 to 0.1% of edible essence and the balance of water, and the sum of the raw materials is 100%. The enzymatic fermented black tea beverage prepared by the invention has rich nutriments and good taste, is nutritional and healthy, not only solves the problems of difficult control on the traditional fermented black tea production process and product standards and quality instability, but also greatly shortens the fermentation production cycle of fermented black tea and solves the problem of large energy consumption of the fermented black tea. The prepared fermented black tea beverage disclosed by the invention has the characteristics of clear and bright color, pure tea fragrance, mellow taste, moderate sweetness and mild sourness, and further has the healthcare functions of promoting appetite, invigorating the stomach, helping digestion, increasing appetite and the like, and the characteristics of enhancing the human immunity, regulating the body acid-base balance, improving chronic fatigue and enhancing physical constitution, thereby being superior to the traditional fermented black tea beverage.
Owner:NINGBO XINUOYA MARINE BIOTECH CO LTD
Ozonized compound containing an olefin double bond
The present invention relates to an ozonized compound containing an olefin double bond, specifically to ozone addition of a compound containing an olefin double bond. According to the present invention, the obtained ozonized compound containing the olefin double bond prevents and treats infectious diseases, autoimmune diseases, ischemic diseases, retinal degenerative diseases, skin diseases, lungdiseases, kidney diseases, hematologic diseases, neurodegenerative diseases, cancer, orthopedic surgical diseases, back pains, chronic fatigue syndromes, trauma / burn and emergency surgery, surgery andorgan transplantation, and prolapse of intervertebral disc through an oral route.
Owner:NORTHWEST A & F UNIV
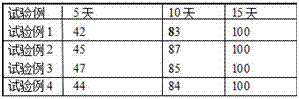
Traditional Chinese medicine composition for treating chronic fatigue syndromes and preparation method of traditional Chinese medicine composition
InactiveCN110038066AImprove Chronic Fatigue SyndromeImprove Qi Deficiency PhysiqueAntinoxious agentsPlant ingredientsCodonopsisCFS - Chronic fatigue syndrome
The invention provides a traditional Chinese medicine composition for treating chronic fatigue syndromes. The traditional Chinese medicine composition is a preparation prepared from raw drugs in partsby weight of 15-25 parts of radix astragali, 10-20 parts of radix codonopsis, 10-20 parts of bighead atractylodes rhizomes, 6-15 parts of Chinese wolfberry fruits and 6-15 parts of radix glycyrrhizaepreparata. The traditional Chinese medicine composition can effectively improve chronic fatigue syndromes and qi deficiency constitutions. The traditional Chinese medicine composition can be used forpreparing medicines for treating the chronic fatigue syndromes or the qi depression constitutions.
Owner:四川省中医药科学院中医研究所
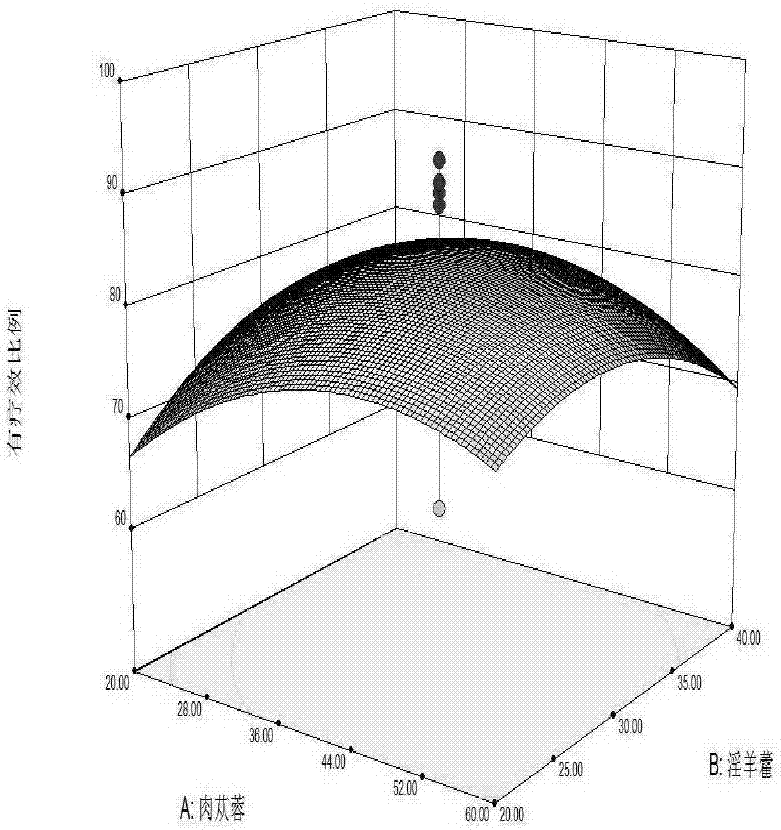
Health care wine and preparation method thereof
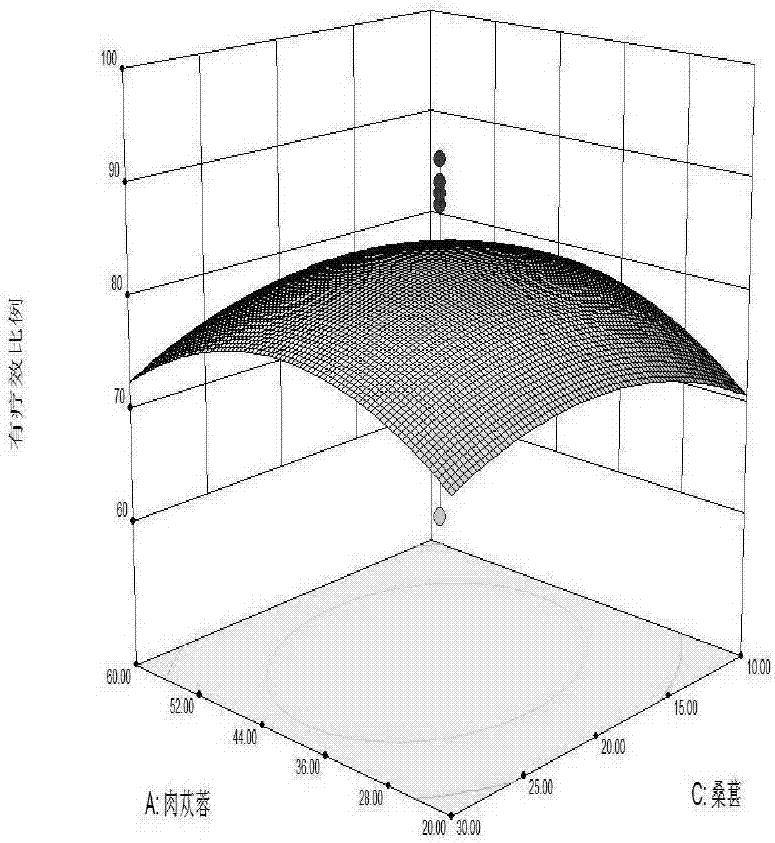
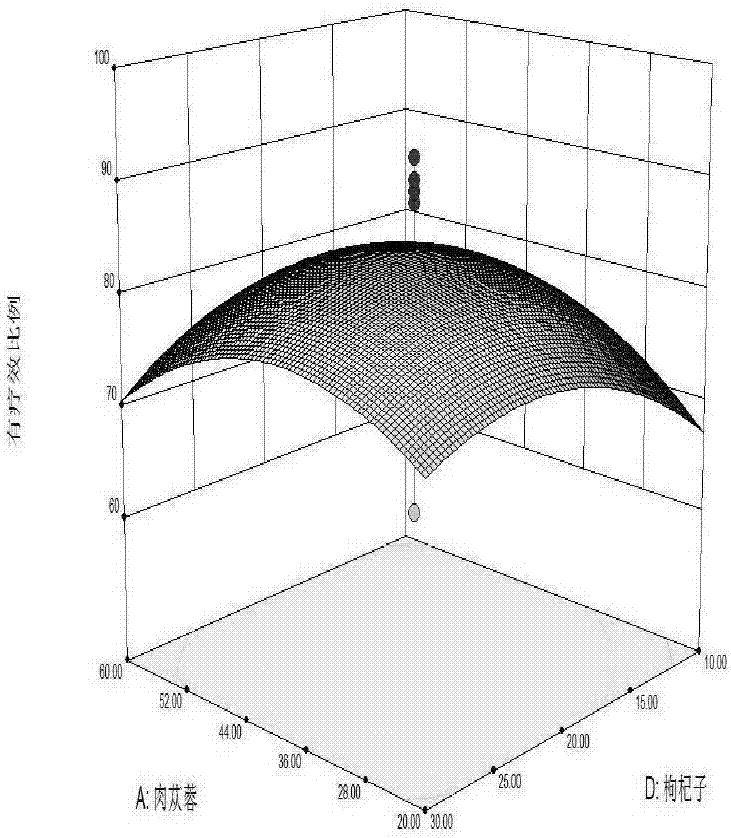
ActiveCN106929363ASolve the problem of short shelf life and unstable qualityEffective treatmentAntinoxious agentsAlcoholic beverage preparationSpleenChronic fatigue
The invention relates to a health care wine and a preparation method thereof. The wine contains 20-60 parts of cistanche, 20-40 parts of herba epimedii, 10-30 parts of mulberry, 10-30 parts of Chinese wolfberry, 10-30 parts of cornus officinalis, 10-30 parts of jujube, 1-2 parts of cervus elaphus linnaeus, and 45-65 parts of rock sugar. Soaking, filter, blending, wine storage, filling, capping and other processes are carried out to prepare the health care wine. The wine provided by the invention well solves the problems of short preservation period in traditional health care products, by preparing the materials into wine, the drug efficacy is improved, several traditional Chinese medicines in a reasonable ratio are made into the health care wine with the multiple health care effects of benefiting qi and tonifying deficiency, moistening five internal organs, harmonizing blood vessels, benefiting essence qi, supplementing essence, strengthening muscles and bones, invigorating spleen and stomach, nourishing yin and reinforcing yang, etc. The wine base is soaked, water is added to allocate the alcohol content, and rock sugar is added to blend the sugar degree, thus obtaining the product. Sensory tests show that the product is clear and transparent, has good taste, and has extensive adaptability in treatment of chronic fatigue syndromes and the health care field.
Owner:URUMQI SHENGMING HELI HIGH TECH
Cordyceps militaris tea bag and preparation method thereof
The invention relates to a cordyceps militaris tea bag and a preparation method thereof, and belongs to the field of tea bags. Cordyceps militaris, licorice roots, Chinese wolfberry fruits, chrysanthemums, lemons, dried lily bulbs, wild jujubes, pears, dandelions, lotus leaves, mint, honeysuckle flowers, dried orange peel, haws and fructus momordicae are adopted. The preparation method comprises the following steps of performing superspeed freeze-drying by a freeze-drying technique for 1-3 seconds, and performing direct proportioning, so that people can drink the brewed cordyceps militaris tea bag; or loading dry ultrafine powder in an earthen jar, performing uniform stirring with loess at places where the cordyceps militaris grows until a paste is obtained, sealing the opening of the jar, performing natural fermentation and alcoholization, placing the treated earthen jar in natural ventilation environment, absorbing the essence of the sun and the moon in the universe, after one year, performing microwave sterilization, performing simultaneous drying, removing dampness, performing filtering, and performing loading into bags, so that the tea bag can be drunk. People drink the tea, so that the effects of preventing people from suffering from diseases and benefitting vigour are achieved, various diseases caused by chronic fatigue syndromes are relieved, and the efficacy of delaying ageing, prolonging life can be achieved.
Owner:LIAONING FUYUDA AGRI SCI & TECH
Application of n-3PUFA phosphatide in products for alleviating chronic fatigue syndromes
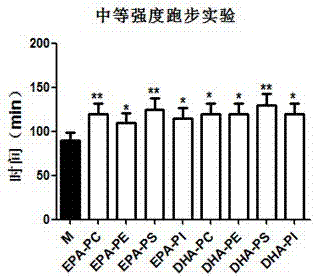
InactiveCN106880049AExtended running timeImprove fatigue toleranceOrganic active ingredientsGroup 5/15 element organic compoundsMedicineCFS - Chronic fatigue syndrome
The invention provides an application of n-3PUFA phosphatide in products for alleviating chronic fatigue syndromes. Specifically, EPA phosphatide is extracted from trepang, DHA phosphatide is extracted from squid eggs, components of the EPA phosphatide are prepared, components of the DHA phosphatide are separated, and finally weariness resistance test verification is performed on the components of the EPA phosphatide and the components of the DHA phosphatide. According to the application disclosed by the invention, the EPA phosphatide is prepared by using the trepang as a raw material, the DHA phosphatide is prepared by using the squid eggs as a raw material, then component separation and transformation are performed, and the purity of prepared PC, the purity of the prepared PE, the purity of the prepared PS and the purity of the prepared PI are all 90% or above. According to the application disclosed by the invention, animal experiments verify that the components of the prepared EPA phosphatide and the components of the prepared DHA phosphatide can greatly prolong the running time of mice in a fatigue apparatus, namely that the EPA phosphatide and the DHA phosphatide can both obviously improve the fatigue tolerance of mice suffering from chronic fatigue syndromes under moderate intensity, and the EPA phosphatide and the DHA phosphatide can effectively prevent, alleviate or treat the chronic fatigue syndromes.
Owner:OCEAN UNIV OF CHINA
Conic gymnadenia tuber-sealwort-radix polygonati officinalis preparation and production method thereof
InactiveCN104013817ASignificant effectDefinite curative effectAntinoxious agentsSexual disorderChronic fatigueTraditional Chinese medicine
The invention relates to the technical field of the traditional Chinese medicine and in particular relates to a conic gymnadenia tuber-sealwort-radix polygonati officinalis preparation for treating chronic fatigue and climacteric syndromes, and a production method of the preparation. The conic gymnadenia tuber-sealwort-radix polygonati officinalis preparation is prepared from such raw materials as conic gymnadenia tuber, sealwort, radix polygonati officinalis, polygonum multiflorum and fructus choerospondiatis. The conic gymnadenia tuber-sealwort-radix polygonati officinalis preparation and the conic gymnadenia tuber-sealwort-radix polygonati officinalis preparation have the advantage that all the medicinal materials are combined to take effect comprehensively so that the preparation is capable of strengthening the body of a patient, reliving fatigue, improving sleeping, enhancing memory ability, adjusting internal secretion and improving the immunity of the organism, has a remarkable curative effect on the chronic fatigue and climacteric syndromes of the patient, and is quick in taking effect and exact in curative effect without toxic and side effects; besides, the production method is capable of guaranteeing the curative effect of the conic gymnadenia tuber-sealwort-radix polygonati officinalis preparation and is simple and easy to operate and low in production cost; and the obtained preparation is convenient to carry and take.
Owner:YUANHE PHARMA CO LTD
Health-care liquor with immunoregulation function and preparation method of health-care liquor
InactiveCN106281901AThe aroma of wine and medicine is harmonious and richEntrance softAlcoholic beverage preparationImmunological disordersHuman bodyFood additive
The invention provides health-care liquor with an immunoregulation function. The health-care liquor is characterized by being prepared from components as follows: 65-73 parts of water, 15-20 parts of white liquor, 2-8 parts of a first component, 3-8 parts of a second component, 1-3 parts of a third component, 0.2-0.6 parts of a fourth component and 0-0.1 parts of a food additive. The health-care liquor has health-care functions of immunoregulation and fatigue resistance, has amber color, harmonious and strong liquor aroma and herbal fragrance, is soft when entering the mouth and has sweet and refreshing aftertaste. The scientific experiment proves that the health-care liquor has the function of two-way regulation of the immune function of the human body, also can effectively relieve fatigue and recover strength and is particularly applicable to sub-health people which work intensely for a long time, have huge mental pressure and are in chronic fatigue.
Owner:TIANJIN DARENTANG JINGWANHONG PHARMA
Degradable ceramic filled porous ultra-short implant and preparation method thereof
ActiveCN110742705AImproved equivalent elastic modulusAvoid damageDental implantsStress shieldingBone growth
The invention discloses a degradable ceramic filled porous ultra-short implant which comprises a neck portion; a body portion is connected to the lower side of the neck portion and is formed by an outer body and an inner body; the outer body is of a mutually communicating porous structure; the porous structure is filled with degradable bone cement; the inner body is a solid dense portion and is provided with inside threads; the inside threads are in matching connection with a base station; the outer body is externally provided with outside threads; and porous thread ceramic bodies are filled between the adjacent outside threads. By adopting the degradable ceramic filled porous ultra-short implant disclosed by the invention, the equivalent elastic modulus of the ultrashort implant is well improved, and the phenomena of stress shielding and chronic fatigue are reduced; by filling the degradable bone cement, the integrity of the structure during implantation of the implant can be ensured,the success rate of implantation is increased, the implantation efficiency is improved, and tissue damages are reduced; and meanwhile, by filling the porous thread ceramic bodies between the adjacentoutside threads, degradable ceramics can be degraded after implantation, bone growth is induced, the degradable bone cement is degraded, the porous structure appears, and cell growth and body fluid exchange are facilitated.
Owner:XI AN JIAOTONG UNIV
Nutrient preparation for alleviating chronic fatigue and preparation method of nutrient preparation
The invention provides a nutritional preparation for improving chronic fatigue and a preparation method thereof. The nutritional preparation is composed of maca powder, Cordyceps militaris, ginseng extract, rice bran extract, soybean polypeptide, zinc-rich yeast and zinc lactate, According to the parts by weight, 10-40 parts of maca powder, 5-30 parts of Cordyceps militaris, 1-10 parts of ginseng extract, 0.1-2 parts of rice bran extract, 1-10 parts of soybean polypeptide, 0.5-5 parts of zinc-enriched yeast 0.1-2 parts, 0.1-2 parts of zinc lactate, obtained by mixing, the nutritional preparation, each component is derived from food, safe, non-toxic and side effects, and the synergistic effect of each component, the effect of improving chronic fatigue is remarkable, its preparation method is simple, It is suitable for the needs of large-scale industrial production.
Owner:CASHESU TIANJIN PHARM TECH CO LTD
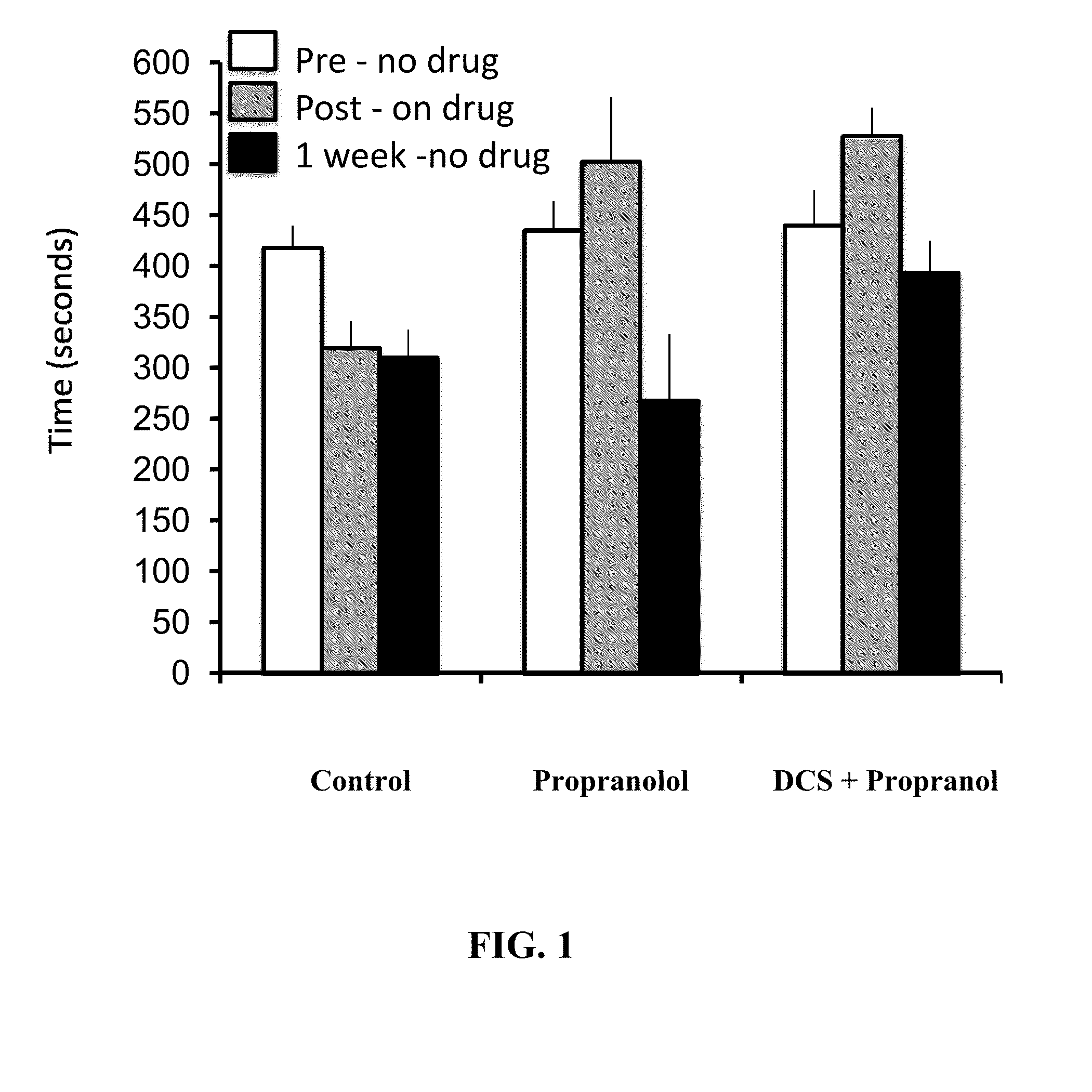
Compositions and Methods to Improve Treatment of Medical Conditions Using D-Cycloserine
The invention describes methods and compositions for alleviating medical afflictions for which anxiety may cause or exacerbate the affliction. A subject suffering from the affliction is treated with a combination of a pharmaceutical compound that enhances learning, and a second pharmaceutical recognized to be useful for treatment of the affliction, wherein D-cycloserine is the pharmaceutical compound that enhances learning. Representative afflictions include pain, mood disorders, anxiety disorders including performance anxiety, insomnia, female sexual dysfunction, chronic fatigue, autism spectrum disorders, fibromyalgia, and attention deficit-hyperactivity disorder.
Owner:MCDEVITT JASON P +1
Medicinal composition and use thereof
InactiveCN100418548CImprove sexual functionImprove securityPeptide/protein ingredientsGinkgophyta medical ingredientsSide effectCitrulline
A medical composition for treating sexual disfunction, impotence, immunodeficiency, chronic fatigue, and hepal and renal disfunction is prepared from ornithin and citrulline or their salts, jujube, ginkgo seed and ginseng. Its advantages are high curative effect and no toxic by-effect.
Owner:ZHEJIANG ADAMERCK BIOPHARMLABS
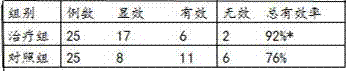
Traditional Chinese medicinal preparation capable of resisting fatigue and increasing immunity
InactiveCN102000258ALong-term useEffective Chronic Fatigue SyndromeAnthropod material medical ingredientsAntinoxious agentsSpermatorrheaAmnesia
The invention relates to traditional Chinese medicinal preparation capable of resisting fatigue and increasing immunity, which is prepared from ants, epimedium herb, south dodder seed, prepared tuber fleeceflower root, prepared manyflower solomonseal rhizome, medlar, raspberry, mrlberrt fruit, silkworm pupae and the like, has the effects of tonifying the kidney, replenishing essence, warming and tonifying kidney yang, tonifying the spleen, and tonifying qi. The preparation can be used for treating and relieving chronic fatigue syndromes, such as fatigue, bradypsychia, insomnia, amnesia, dizziness, tinnitus, and ache and weakness of waist and knee, which are caused by liver and kidney deficiency and treating sexual dysfunction syndromes, such as impotence, premature ejaculation, spermatorrhea and low sexual desire, which are caused by kidney-yang deficiency. The traditional Chinese medicinal preparation has health-care functions of resisting fatigue and increasing immunity, is safe and free from side effects and can be taken for a long time.
Owner:涂传荣
Traditional Chinese medicine composition for preventing and treating chronic fatigue syndromes and preparation method and application thereof
InactiveCN105796966ASimple processEasy to operateAntinoxious agentsNatural extract food ingredientsSide effectSmoked Plum
The invention relates to a traditional Chinese medicine composition for preventing and treating chronic fatigue syndromes and a preparation method and application thereof. The traditional Chinese medicine composition is prepared from, by weight, 10-20 parts of honeysuckle flowers, 5-15 parts of perilla leaves, 5-15 parts of tuber onion seeds, 5-15 parts of rhizoma bletillae, 5-15 parts of mint, 5-15 parts of cablin potchouli herb, 5-15 parts of chrysanthemum flowers, 10-20 parts of rhizoma phragmitis, 2-11 parts of semen sterculiae lychnophorae, 10-20 parts of rhizoma polygonati and 5-15 parts of smoked plums. The traditional Chinese medicine composition can effectively prevent and treat chronic fatigue syndromes, significantly relieve various symptoms of chronic fatigue syndrome patients, obviously improve the mental state and body comfort of patients and obviously relieve throat sore, muscle or joint sore, listlessness and fatigue, sleep disorders and other syndromes. With pure Chinese herbs adopted as raw materials, the traditional Chinese medicine composition is safe, stable, free of toxic or side effects and suitable for being taken by patients for a long term.
Owner:SYNSUN HEALTH IND
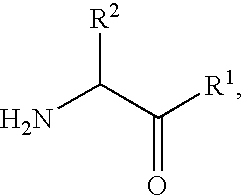
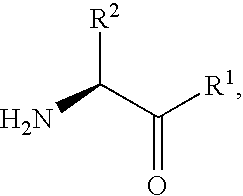
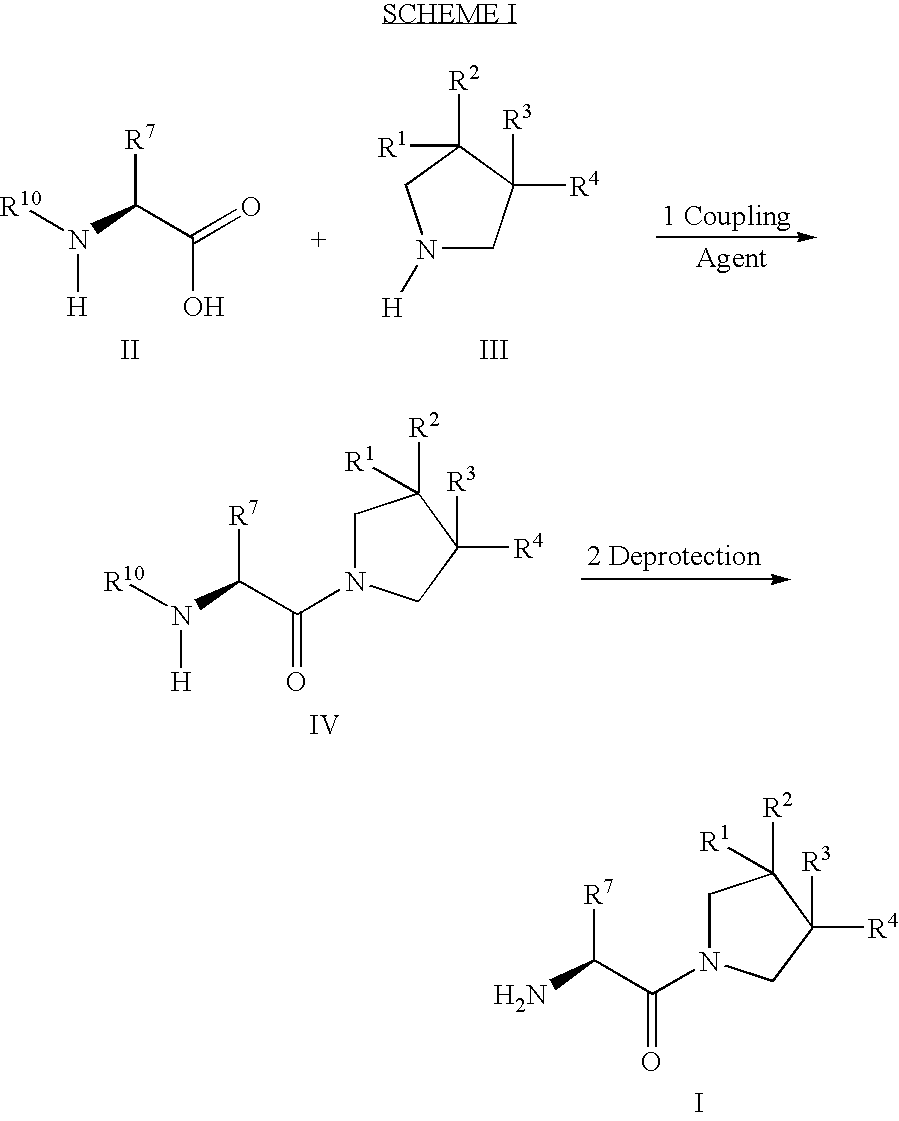
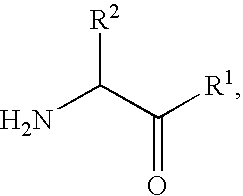
2-[bis(4-fluorophenyl)methyl]-2,7-diazaspiro[4.5]decan-10-one derivatives and related compounds as inhibitors of the human dopamine-active-transporter (DAT) protein for the treatment of e.g. attention deficit disorder (ADD)
InactiveUS20170260185A1Good choiceUseful in treatmentNervous disorderOrganic chemistrySubstance abuserImpulse control disorder
The present invention provides compounds of formula (I) and in particular 2-[bis(4-fluorophenyl)methyl]-2,7-diazaspiro[4.5]decan-10-one derivatives and related compounds as inhibitors of human dopamine-active-transporter (DAT) protein for the treatment of sexual dysfunction, affective disorders, anxiety, depression, chronic fatigue, Tourette syndrome, Angelman syndrome, attention deficit disorder (ADD), attention deficit hyperactivity disorder (ADHD), obesity, pain, obsessive-compulsive disorder, movement disorders, CNS disorders, sleep disorders, narcolepsy, conduct disorder, substance abuse (including smoking cessation), eating disorders, and impulse control disorders.
Owner:CHRONOS THERAPEUTICS
Vitality strengthening tea
InactiveCN104814195AFragrant and sweet tasteBalanced and reasonable matching ratioTea substituesAntinoxious agentsManufacturing technologyAdditive ingredient
The invention discloses vitality strengthening tea which is prepared from the following components in parts by weight: 9-10 parts of astragalus, 4-5 parts of ginsengs, 4-5 parts of bighead atractylodes rhizome, 3-4 parts of pericarpium citri reticulatae, 6-7 parts of radix ophiopogonis and 1-2 parts of raw liquorice. The invention further discloses a preparation method of the vitality strengthening tea beverage. The vitality strengthening tea disclosed by the invention is reasonable in formula and safe and reliable. Components of a prescription are improved and optimized after long-term systematic research on a qi-tonifying soup formula. By adopting the latest manufacturing technology of a scientific process, a medicinal and edible life-preserving health-care tea beverage is finished, has the effects of nourishing and strengthening and is suitable for people with poor functions of spleen and stomach and deficiency of both qi and yin. The vitality strengthening tea serves as a comprehensive anti-fatigue odd-ingredient prescription, efficacious prescription and proved prescription for preventing and treating chronic fatigue.
Owner:HEFEI SHENLING HEALTH TEA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com








![2-[bis(4-fluorophenyl)methyl]-2,7-diazaspiro[4.5]decan-10-one derivatives and related compounds as inhibitors of the human dopamine-active-transporter (DAT) protein for the treatment of e.g. attention deficit disorder (ADD) 2-[bis(4-fluorophenyl)methyl]-2,7-diazaspiro[4.5]decan-10-one derivatives and related compounds as inhibitors of the human dopamine-active-transporter (DAT) protein for the treatment of e.g. attention deficit disorder (ADD)](https://images-eureka.patsnap.com/patent_img/68a634a7-23d1-4986-b3f4-d8b07a0017a5/US20170260185A1-20170914-C00001.png)
![2-[bis(4-fluorophenyl)methyl]-2,7-diazaspiro[4.5]decan-10-one derivatives and related compounds as inhibitors of the human dopamine-active-transporter (DAT) protein for the treatment of e.g. attention deficit disorder (ADD) 2-[bis(4-fluorophenyl)methyl]-2,7-diazaspiro[4.5]decan-10-one derivatives and related compounds as inhibitors of the human dopamine-active-transporter (DAT) protein for the treatment of e.g. attention deficit disorder (ADD)](https://images-eureka.patsnap.com/patent_img/68a634a7-23d1-4986-b3f4-d8b07a0017a5/US20170260185A1-20170914-C00002.png)
![2-[bis(4-fluorophenyl)methyl]-2,7-diazaspiro[4.5]decan-10-one derivatives and related compounds as inhibitors of the human dopamine-active-transporter (DAT) protein for the treatment of e.g. attention deficit disorder (ADD) 2-[bis(4-fluorophenyl)methyl]-2,7-diazaspiro[4.5]decan-10-one derivatives and related compounds as inhibitors of the human dopamine-active-transporter (DAT) protein for the treatment of e.g. attention deficit disorder (ADD)](https://images-eureka.patsnap.com/patent_img/68a634a7-23d1-4986-b3f4-d8b07a0017a5/US20170260185A1-20170914-C00003.png)