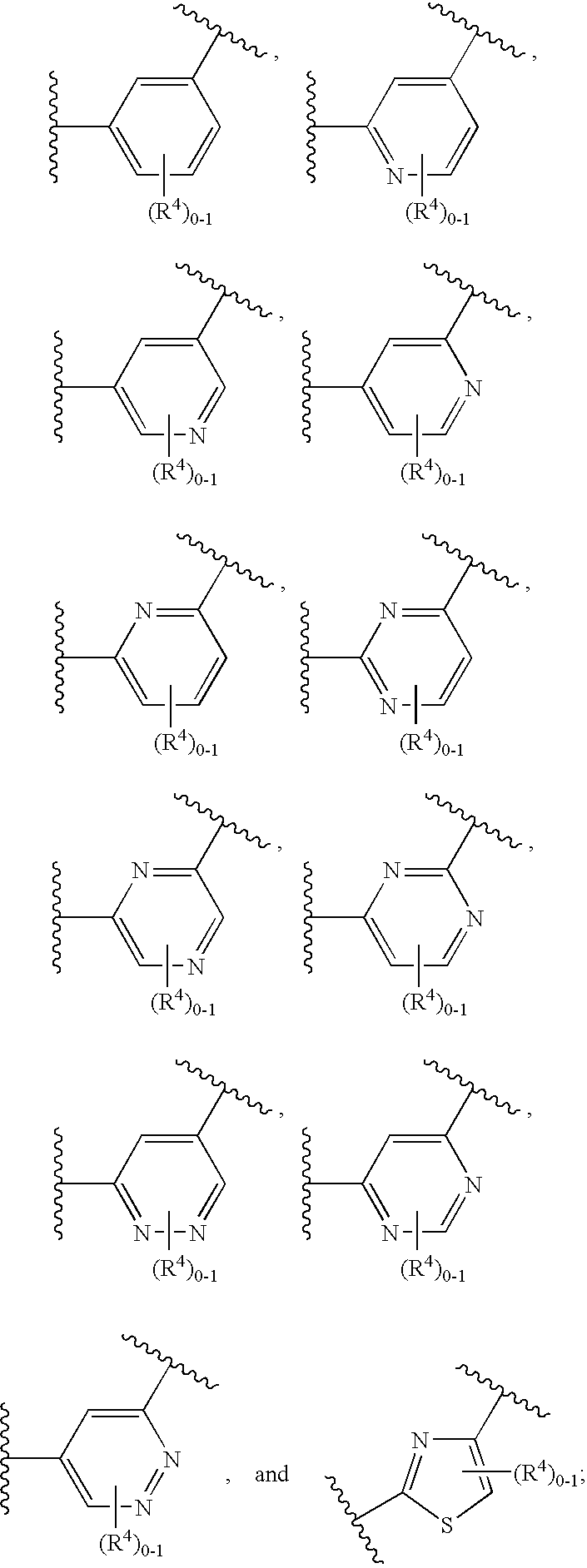
Patents
Literature
5789 results about "Asthma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A condition in which the airways (the tubes that carry air in and out of the lungs) narrow and swell causing reversible obstruction.
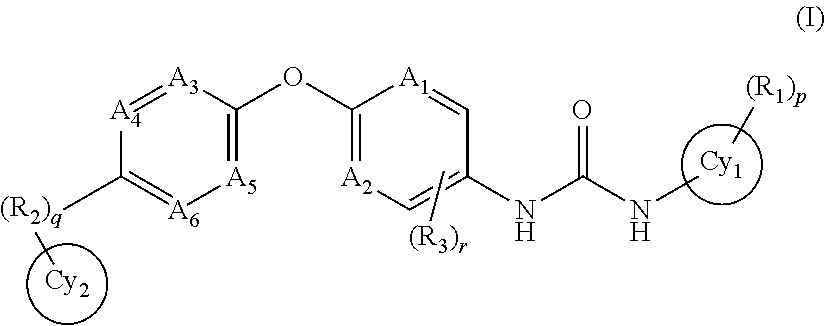
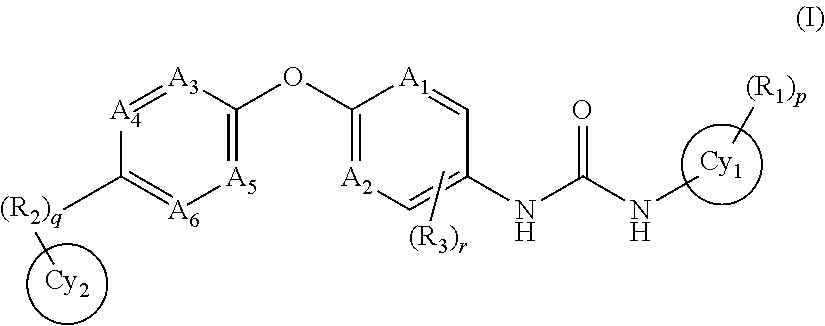
Anti-PD-L1 antibodies and uses therefor
The present invention is based, in part, on the identification of novel human anti-PD-1, PD-L1, and PD-L2 antibodies. Accordingly, the invention relates to compositions and methods for diagnosing, prognosing, and treating conditions that would benefit from modulating PD-1, PD-L1, and / or PD-L2 activity (e.g., persistent infectious diseases, autoimmune diseases, asthma, transplant rejection, inflammatory disorders and tumors) using the novel human anti-PD-1, PD-L1, and PD-L2 antibodies described herein.
Owner:DANA FARBER CANCER INST INC +2
Human Anti-pd-1, pd-l1, and pd-l2 antibodies and uses therefor
ActiveUS20110271358A1Reduced antigen binding affinityLess immunogenicAntibacterial agentsAntipyreticTransplant rejectionAutoimmune disease
The present invention is based, in part, on the identification of novel human anti-PD-1, PD-L1, and PD-L2 antibodies. Accordingly, the invention relates to compositions and methods for diagnosing, prognosing, and treating conditions that would benefit from modulating PD-1, PD-L1, and / or PD-L2 activity (e.g., persistent infectious diseases, autoimmune diseases, asthma, transplant rejection, inflammatory disorders and tumors) using the novel human anti-PD-1, PD-L1, and PD-L2 antibodies described herein.
Owner:DANA FARBER CANCER INST INC +2
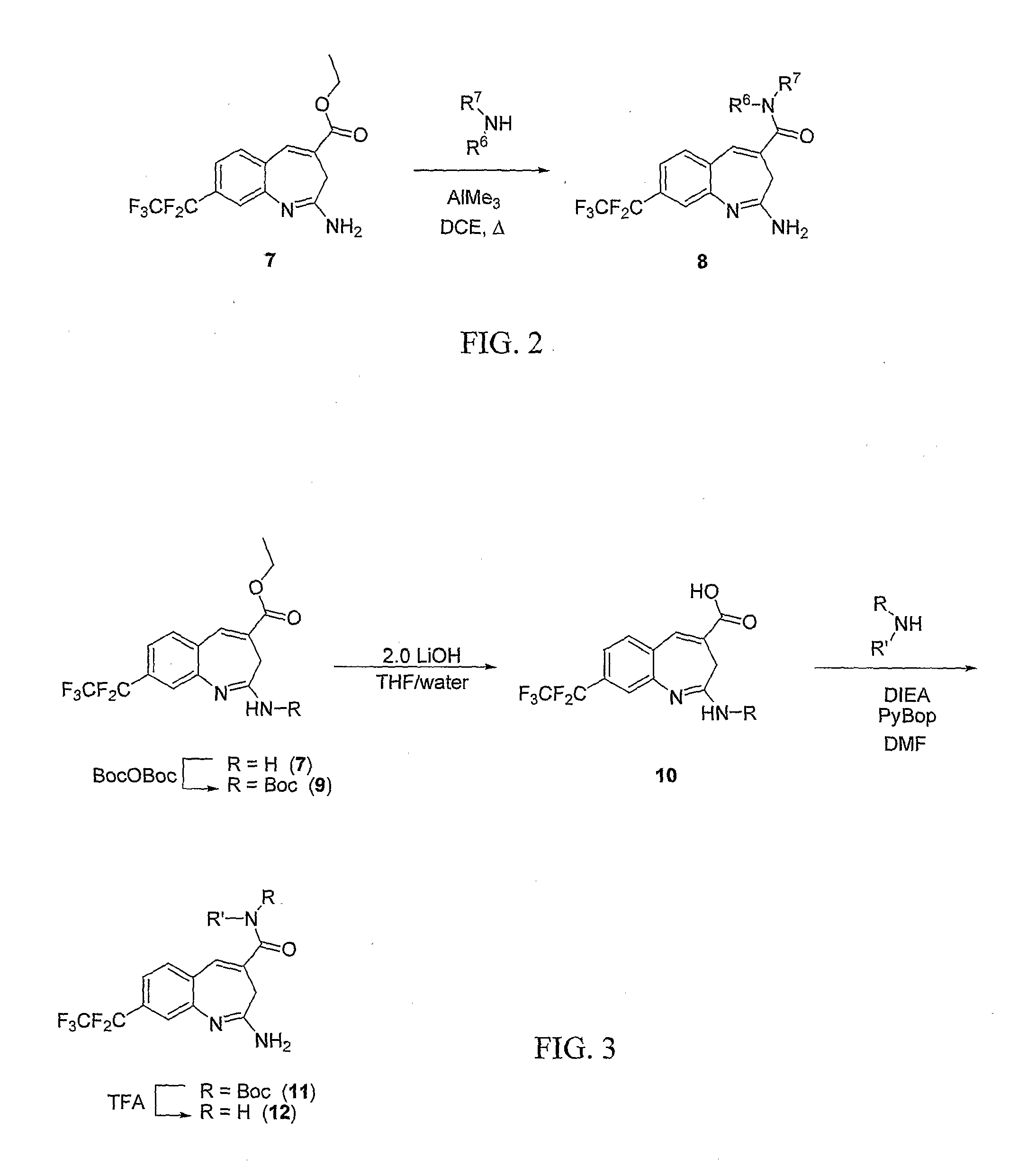
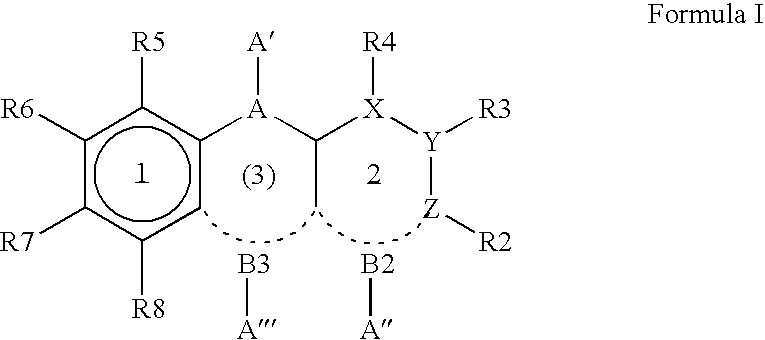
8-Substituted Benzoazepines as Toll-Like Receptor Modulators
Provided are compositions and methods useful for modulation of signaling through the Toll-like receptors TLR7 and / or TLR8. The compositions and methods have use in the treatment of autoimmunity, inflammation allergy, asthma, graft rejection, graft versus host disease, infection, sepsis, cancer and immunodeficiency.
Owner:ARRAY BIOPHARMA
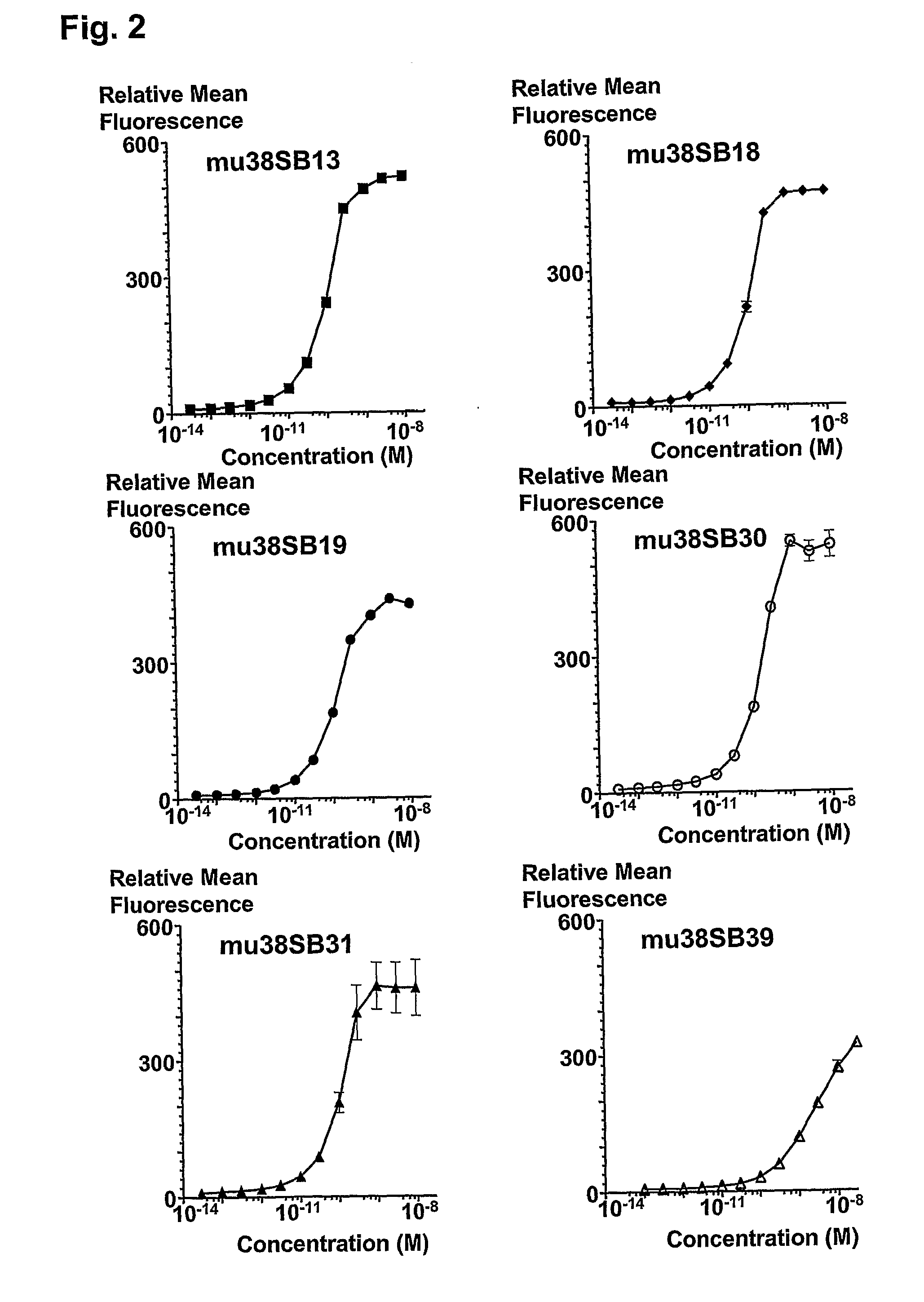
Novel Anti-cd38 antibodies for the treatment of cancer
ActiveUS20090304710A1Improve propertiesLess immunogenicSenses disorderAntipyreticComplement-dependent cytotoxicityAntibody fragments
Antibodies, humanized antibodies, resurfaced antibodies, antibody fragments, derivatized antibodies, and conjugates of same with cytotoxic agents, which specifically bind to CD38, are capable of killing CD38+ cells by apoptosis, antibody-dependent cell-mediated cytotoxicity (ADCC), and / or complement-dependent cytotoxicity (CDC). Said antibodies and fragments thereof may be used in the treatment of tumors that express CD38 protein, such as multiple myeloma, chronic lymphocytic leukemia, chronic myelogenous leukemia, acute myelogenous leukemia, or acute lymphocytic leukemia, or the treatment of autoimmune and inflammatory diseases such as systemic lupus, rheumatoid arthritis, multiple sclerosis, erythematosus, and asthma. Said derivatized antibodies may be used in the diagnosis and imaging of tumors that express elevated levels of CD38. Also provided are cytotoxic conjugates comprising a cell binding agent and a cytotoxic agent, therapeutic compositions comprising the conjugate, methods for using the conjugates in the inhibition of cell growth and the treatment of disease, and a kit comprising the cytotoxic conjugate. In particular, the cell binding agent is a monoclonal antibody, and epitope-binding fragments thereof, that recognizes and binds the CD38 protein.
Owner:SANOFI AVENTIS US LLC
Small molecule toll-like receptor (TLR) antagonists
The invention provides methods and compositions useful for modulating signaling through Toll-like receptors. The methods involve contacting a TLR-expressing cell with a small molecule having a core structure including at least two rings. Certain of the compounds are 4-primary amino quinolines. Many of the compounds and methods are useful specifically for inhibiting immune stimulation involving at least one of TLR9, TLR8, TLR7, and TLR3. The methods may have use in the treatment of autoimmunity, inflammation, allergy, asthma, graft rejection, graft versus host disease, infection, sepsis, cancer, and immunodeficiency.
Owner:COLEY PHARMA GMBH +1
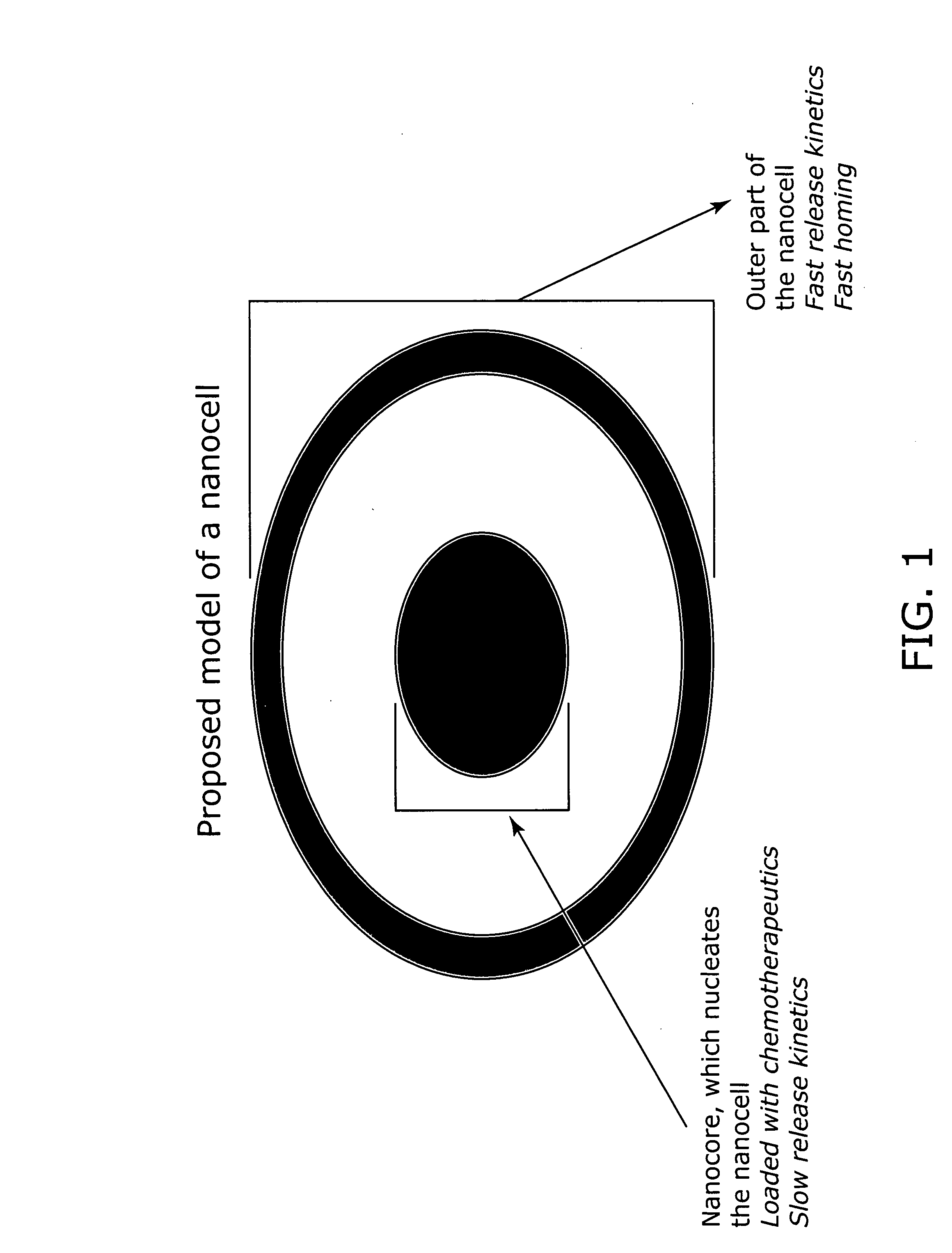
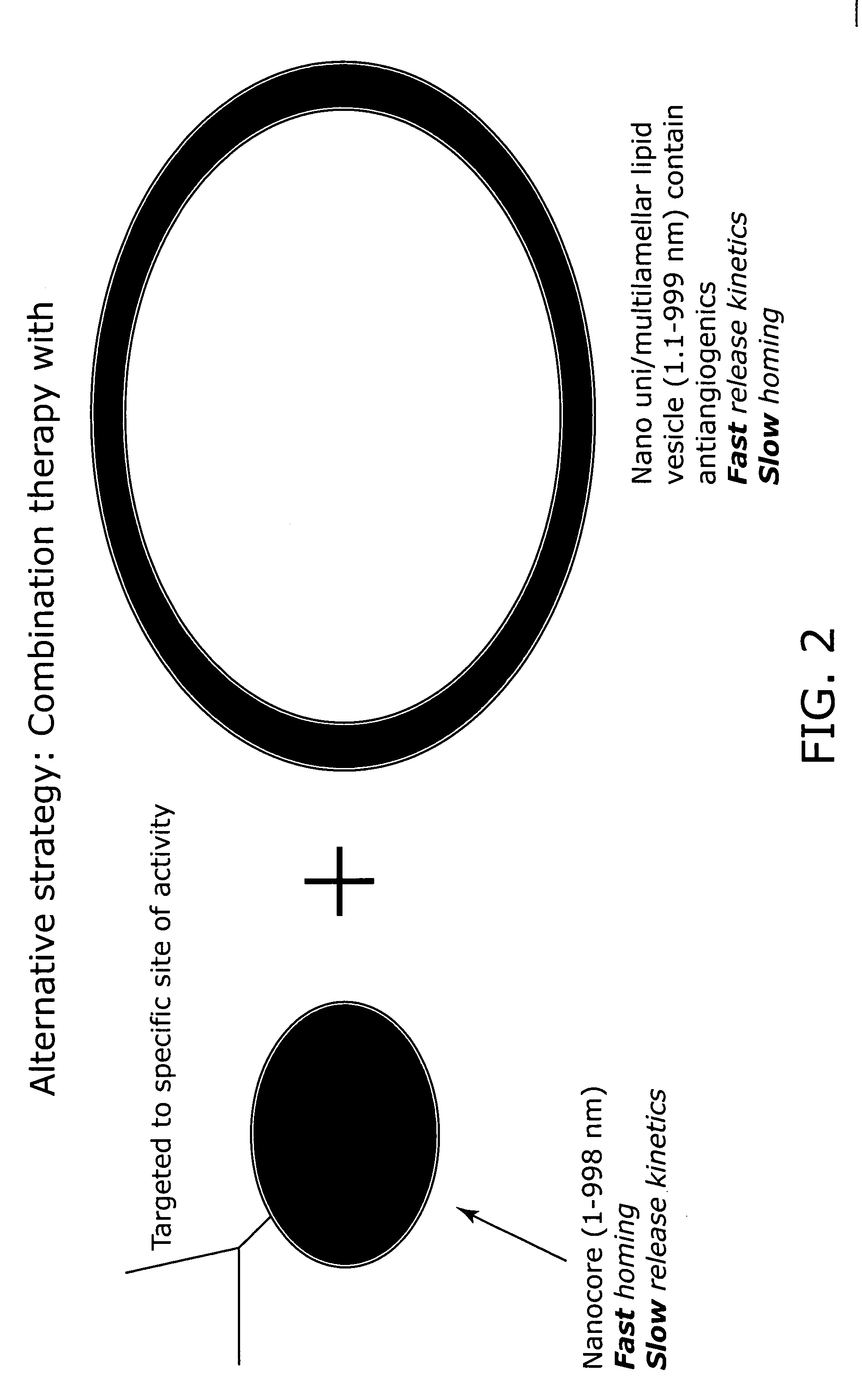
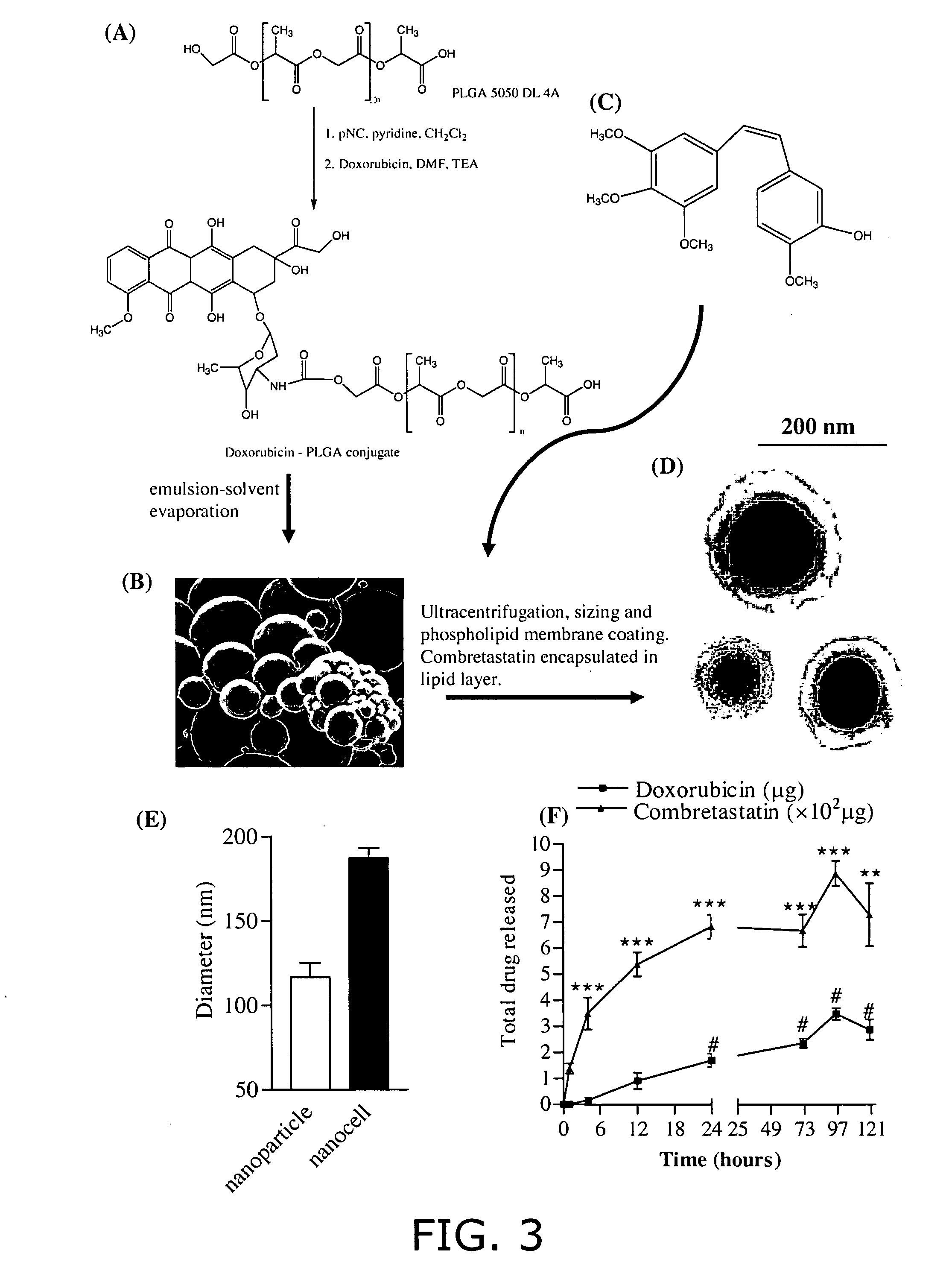
Nanocell drug delivery system
InactiveUS20050266067A1Avoid flowIncreased toxicityAntibacterial agentsOrganic active ingredientsLipid formationAntigen
Nanocells allow the sequential delivery of two different therapeutic agents with different modes of action or different pharmacokinetics. A nanocell is formed by encapsulating a nanocore with a first agent inside a lipid vesicle containing a second agent. The agent in the outer lipid compartment is released first and may exert its effect before the agent in the nanocore is released. The nanocell delivery system may be formulated in pharmaceutical composition for delivery to patients suffering from diseases such as cancer, inflammatory diseases such as asthma, autoimmune diseases such as rheumatoid arthritis, infectious diseases, and neurological diseases such as epilepsy. In treating cancer, a traditional antineoplastic agent is contained in the outer lipid vesicle of the nanocell, and an antiangiogenic agent is loaded into the nanocore. This arrangement allows the antineoplastic agent to be released first and delivered to the tumor before the tumor's blood supply is cut off by the antianiogenic agent.
Owner:MASSACHUSETTS INST OF TECH
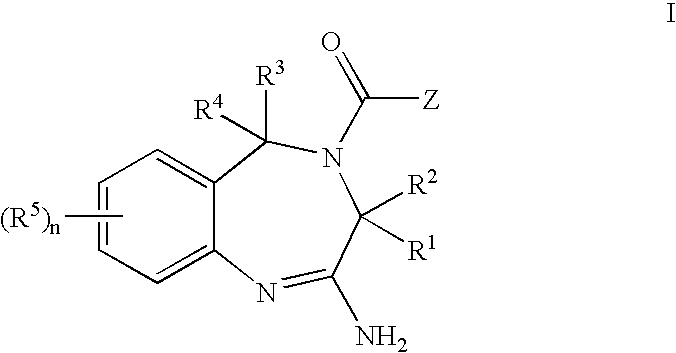
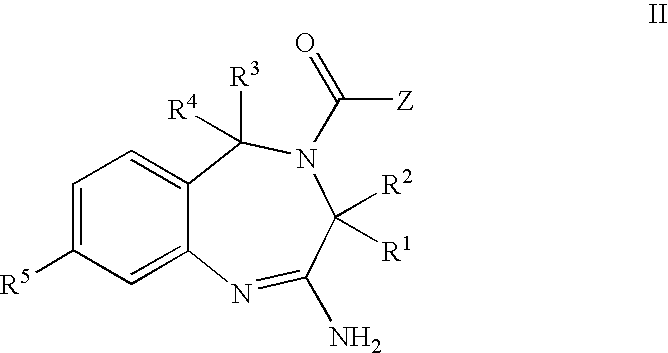
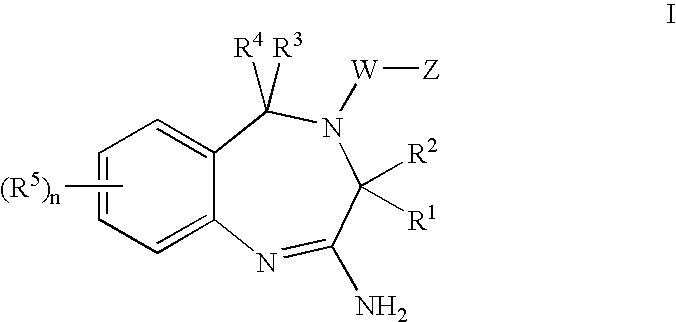
Aminodiazepines as Toll-Like Receptor Modulators
Provided are compositions and methods useful for modulation signaling through the Toll-like receptor TLR8. The compositions and methods have use in the treatment of autoimmunity, inflammation allergy, asthma, graft rejection, graft versus host disease, infection, sepsis, cancer and immunodeficiency.
Owner:ARRAY BIOPHARMA
Sterilized nanoparticulate glucocorticosteroid formulations
InactiveUS20070178051A1Readily heat sterilizedImprove the heating effectOrganic active ingredientsPowder deliveryPediatric patientMicroparticle
The invention is directed sterile to compositions of glucocorticosteroids useful in the prophylaxis and chronic treatment of asthma and other allergic and inflammatory conditions in adults and pediatric patients.
Owner:ELAN PHRMA INT LTD
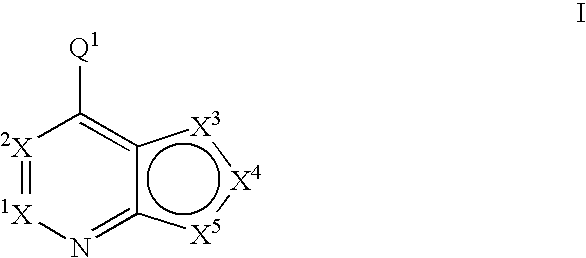
6,6-Bicyclic ring substituted heterobicyclic protein kinase inhibitors
ActiveUS20060235031A1Treatment and/or prevention of hyperproliferative diseasesBiocideSenses disorderDiseasePTK Inhibitors
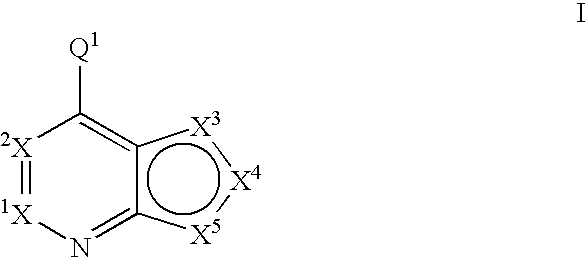
Compounds of the formula and pharmaceutically acceptable salts thereof, wherein X1, X2, X3, X4, X5, X6, X7, R1, and Q1 are defined herein, inhibit the IGF-1R enzyme and are useful for the treatment and / or prevention of hyperproliferative diseases such as cancer, inflammation, psoriasis, allergy / asthma, disease and conditions of the immune system, disease and conditions of the central nervous system.
Owner:ACERTA PHARMA BV
Method for cryospray ablation
InactiveUS20090192505A1Increase load capacityAdequate doseUltrasonic/sonic/infrasonic diagnosticsEchographic/ultrasound-imaging preparationsThoracic structureDisease
The present invention relates to methods for treating tissue in the thoracic cavity of a subject by the application of a cryogen, or using the cryogen to create an isotherm in proximity to the tissue to be treated. A wide variety of conditions may be treated using the methods of the invention including asthma, neoplastic disease and a variety of conditions characterized by inflammation in lung and chest tissue.
Owner:RESET MEDICAL
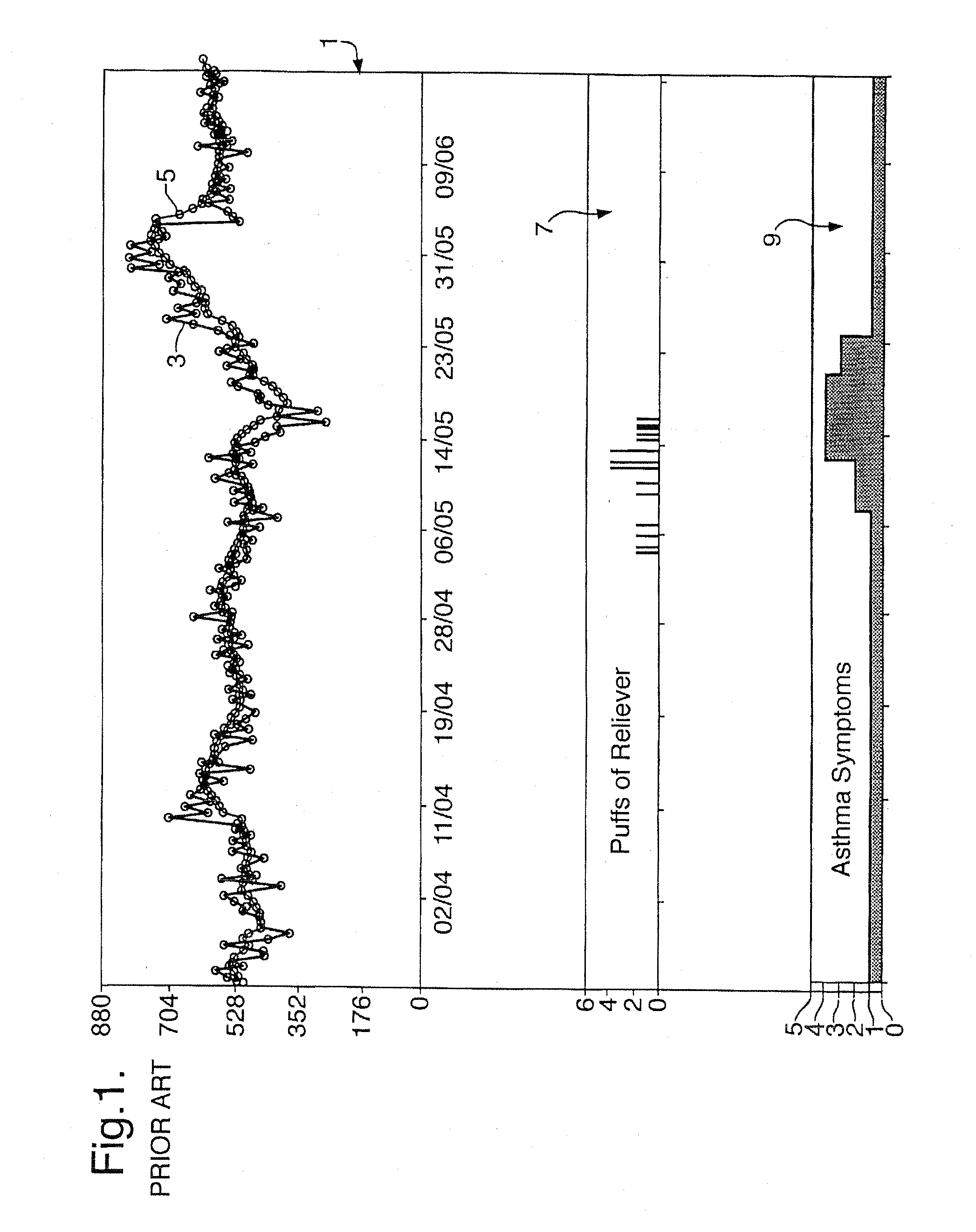
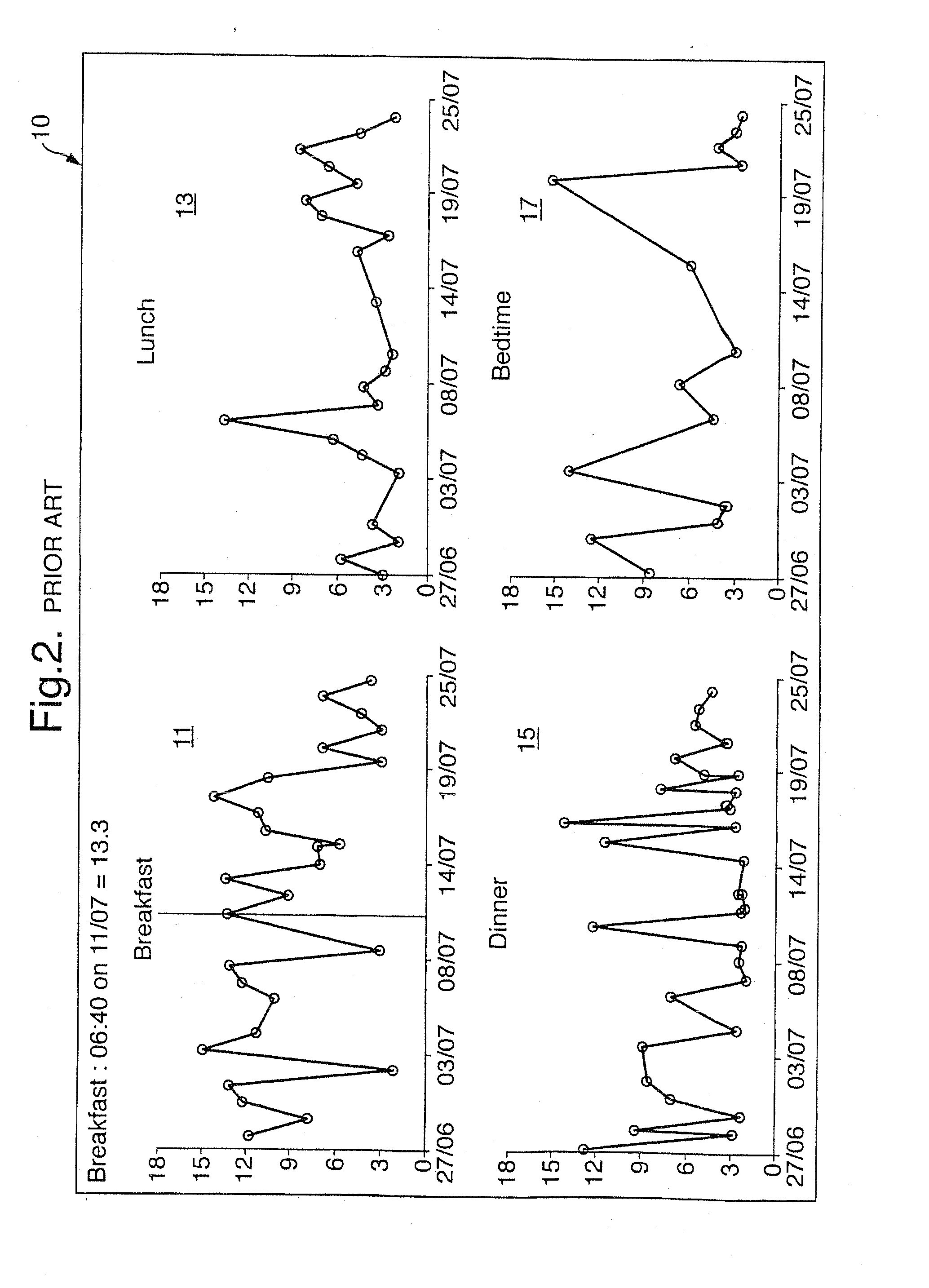
Medical Data Display
InactiveUS20100259543A1Quality is easy to controlMedical simulationDrawing from basic elementsData displayBlood Glucose Measurement
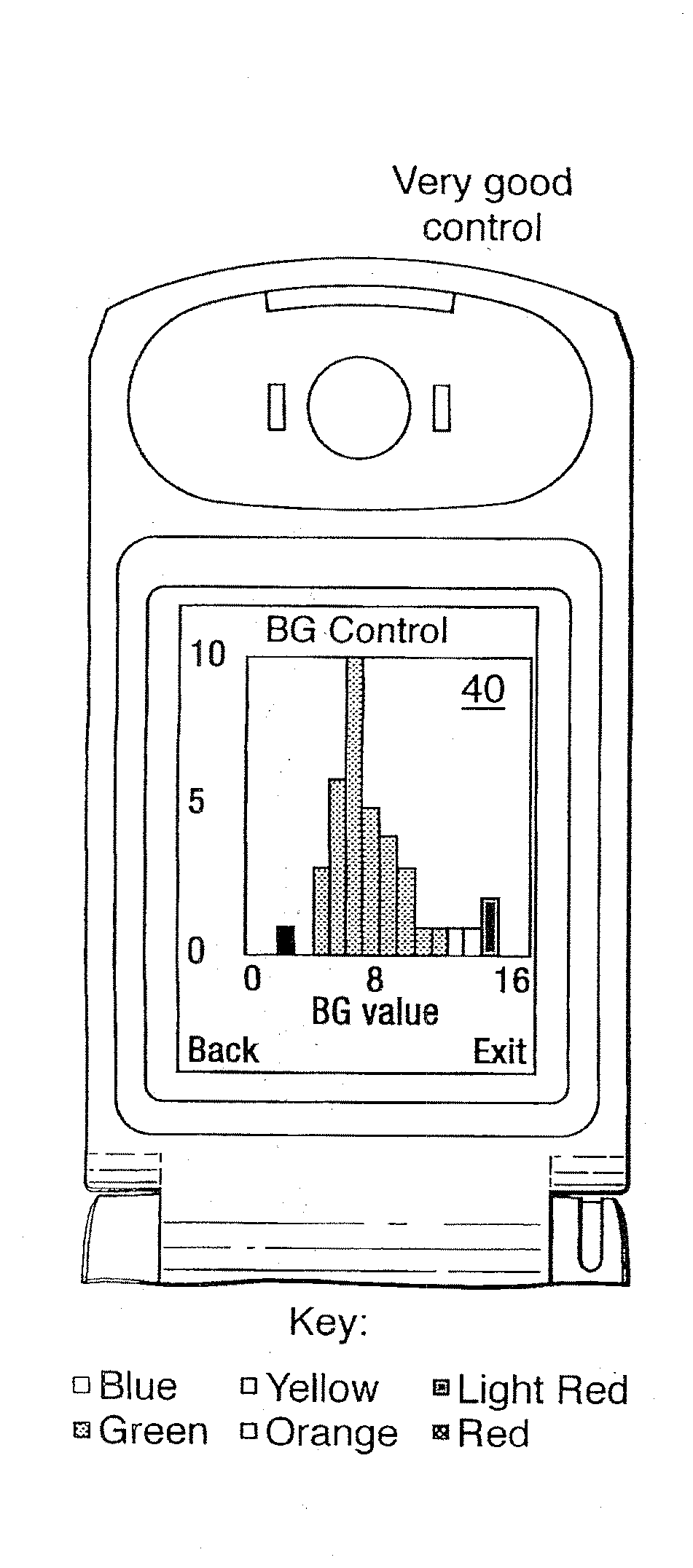
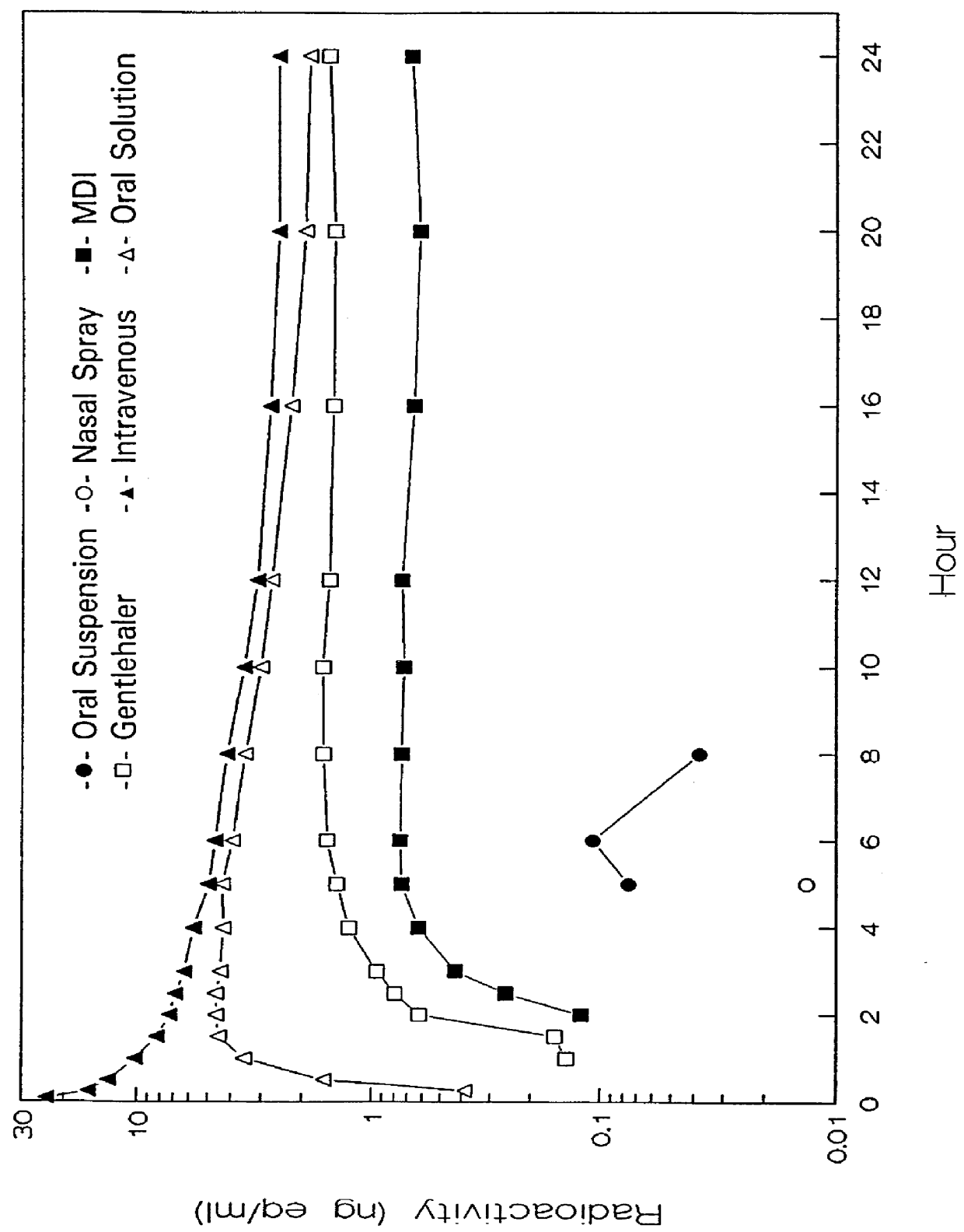
A method of displaying medical data, particularly data representative of the condition of patients suffering from chronic medical conditions such as asthma, diabetes and hypertension. The display consists of two graphical elements, one of which indicates the current value of a parameter indicative of the patient's condition, this being displayed against another graphical element which represents a model of normality for that patient. The graphical element indicating the current condition may be, for example, a needle, against a scale which is constructed according to the patient-specific model of normality. This is particularly advantageous in the case of displays which have a small display area, such as mobile telephones and PDAs. Other forms of display are disclosed, such as histograms with the display being dynamically colour-coded and auto-scaled, or displays including limits which may vary. Another form of display is also disclosed which illustrates administrations of a pharmacological agent and corresponding measurements of the patient's condition, with a visual link such as colour-coding linking the administration to the corresponding condition measurement. For example several days of insulin administration dosages may be displayed alongside several days of blood glucose measurements, with the administrations colour-coded to the corresponding blood glucose measurement, to assist the patient in determining whether the insulin administration is stably controlling their condition.
Owner:E SAN LTD
Organic Compounds
InactiveUS20090186022A1Reducing required dosagingReduce potential side effectsSugar derivativesImmunoglobulins against cytokines/lymphokines/interferonsInflammatory Bowel DiseasesAtopic dermatitis
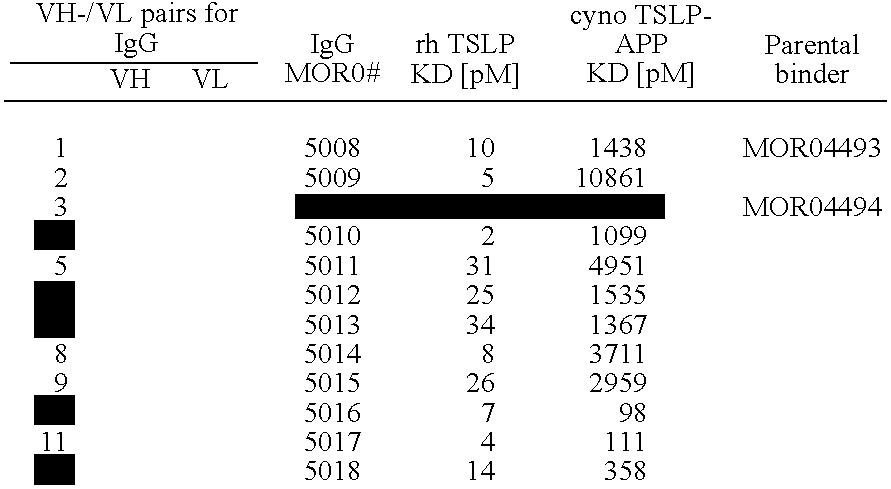
The present invention relates to human thymic stromal lymphopoietin (hTSLP) antibodies and especially those which neutralize hTSLP activity. It further relates to methods for using anti-hTSLP antibody molecules in diagnosis or treatment of hTSLP related disorders, such as asthma, atopic dermatitis, allergic rhinitis, fibrosis inflammatory bowel disease, and Hodgkin's lymphoma.
Owner:NOVARTIS AG
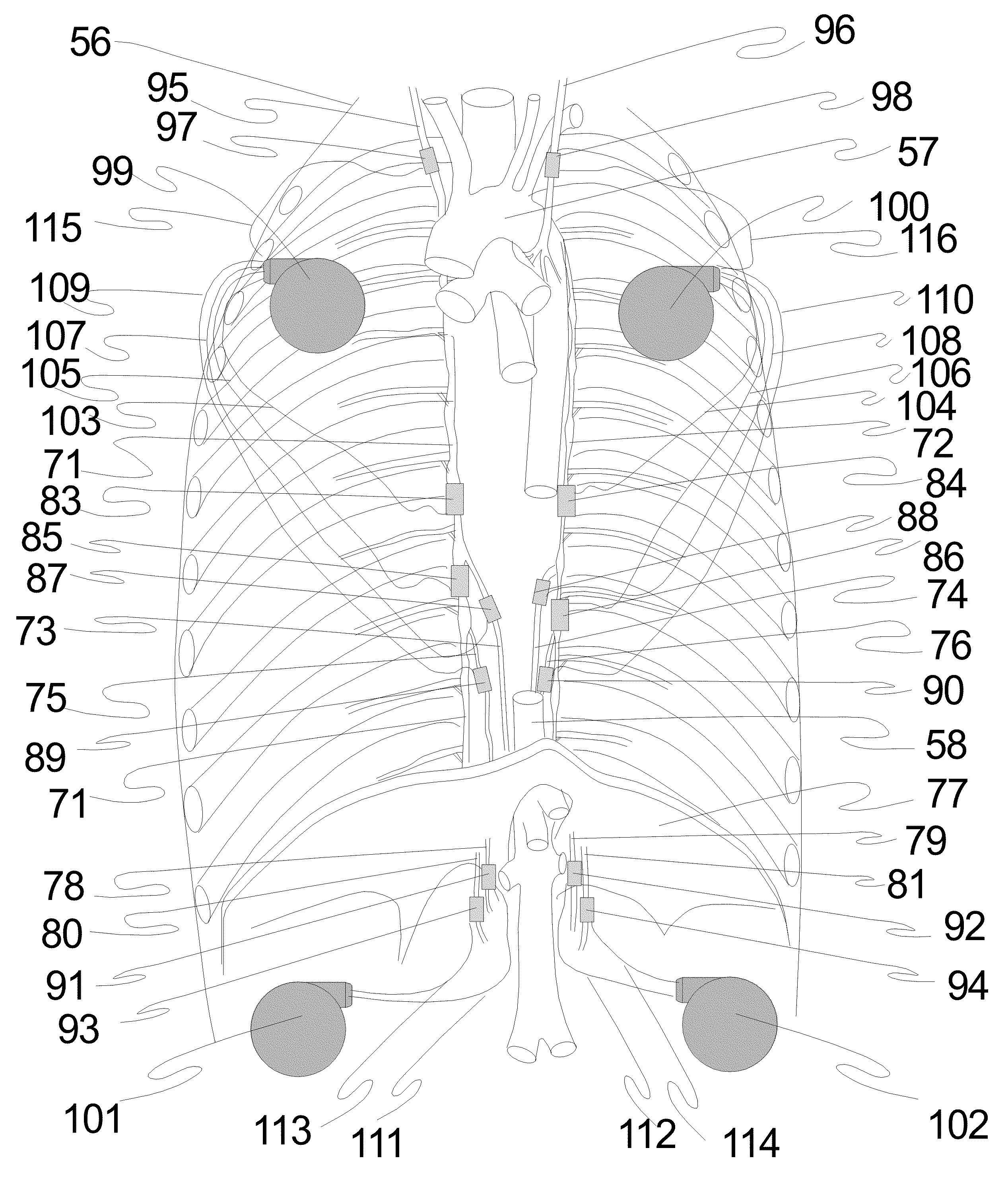
Non-invasive and minimally invasive denervation methods and systems for performing the same
ActiveUS20110118725A1Reduce harmReduce obstructionUltrasound therapySurgical needlesCOPDPartial denervation
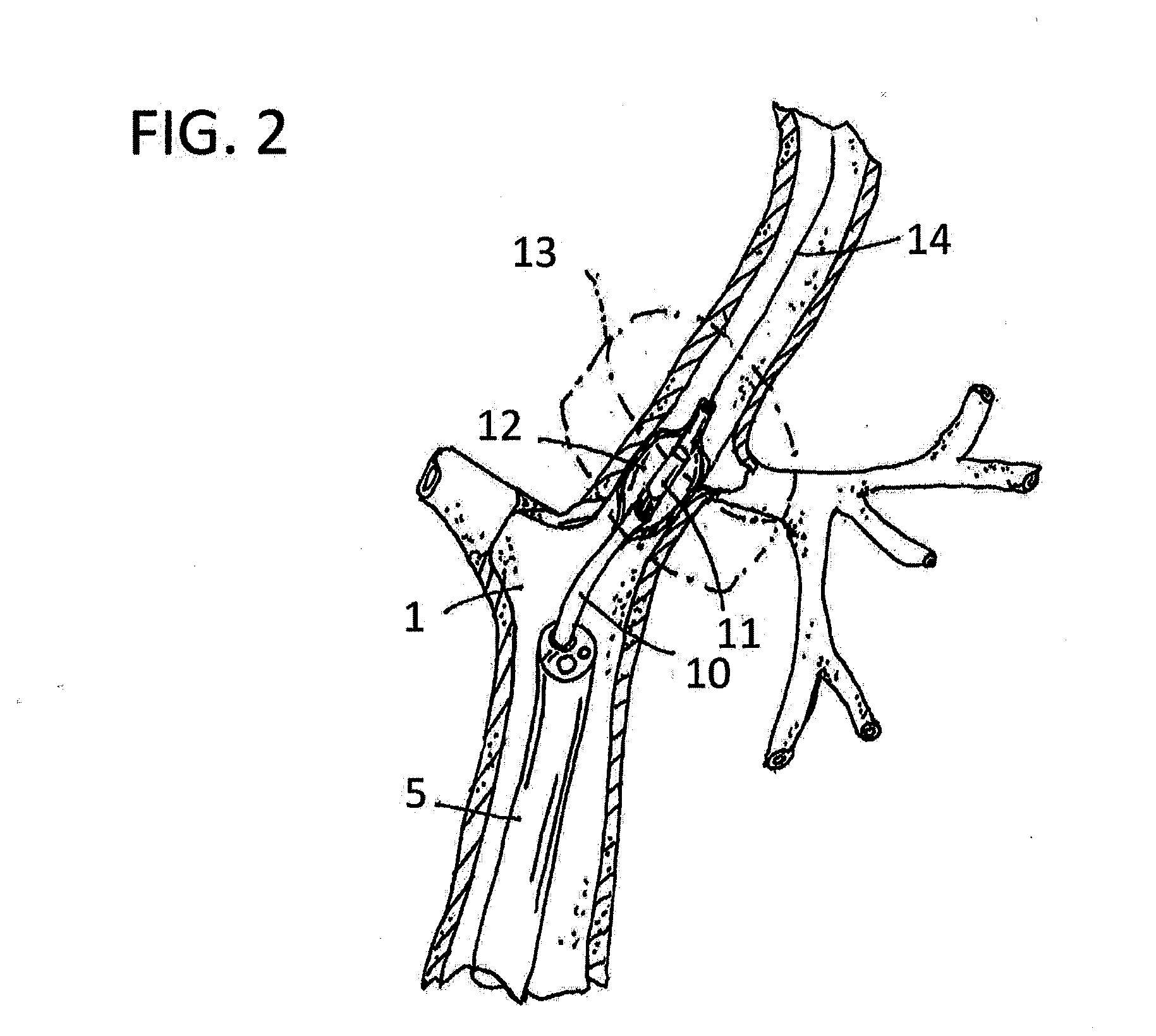
A system and method can be used to denervate at least a portion of a bronchial tree. An energy emitter of an instrument is percutaneously delivered to a treatment site and outputs energy to damage nerve tissue of the bronchial tree. The denervation procedure can be performed without damaging non-targeted tissue. Minimally invasive methods can be used to open airways to improve lung function in subjects with COPD, asthma, or the like. Different sections of the bronchial tree can be denervated while leaving airways intact to reduce recovery times.
Owner:NUVAIRA INC
Use of mometasone furoate for treating airway passage and lung diseases
InactiveUS6057307AMaximize treating said rhinitisMinimize absorptionPowder deliveryBiocideDiseaseAerosolize
The administration of aerosolize particles of mometasone furoate in the form of dry powders, solutions, or aqueous suspension for treating corticosteroid-responsive diseases of the surfaces of upper and / or lower airway passages and / or lungs, e.g., allergic rhinitis and asthma is disclosed.
Owner:MERCK SHARP & DOHME CORP
Treatment of Asthma and Chronic Obstructive Pulmonary Disease With Anti-proliferate and Anti-inflammatory Drugs
InactiveUS20080175887A1Promote absorptionOrganic active ingredientsPowdered material dispensingDiseaseObstructive Pulmonary Diseases
Embodiments of the present invention provide a method for treatment of respiratory disorders such as asthma, chronic obstructive pulmonary disease, and chronic sinusitis, including cystic fibrosis, interstitial fibrosis, chronic bronchitis, emphysema, bronchopulmonary dysplasia and neoplasia. The method involves administration, preferably oral, nasal or pulmonary administration, of anti-inflammatory and anti-proliferative drugs (rapamycin or paclitaxel and their analogues).
Owner:LUTONIX INC
Antagonizing interleukin-21 receptor activity
InactiveUS20060039902A1Reduce riskSufficient amountCompounds screening/testingCompound screeningWhite blood cellFibrosis
Methods and compositions for inhibiting interleukin-21 (IL-21) / IL-21 receptor (MU-1) activity using antagonists of IL-21 or IL-21 receptor (“IL-21R” or “MU-1”), are disclosed. IL-21 / IL-21R antagonists can be used to induce immune suppression in vivo, e.g., for treating, ameliorating or preventing autoimmune or inflammatory disorders, including, e.g., inflammatory bowel disease (IBD), rheumatoid arthritis (RA), transplant / graft rejection, psoriasis, asthma, fibrosis, and systemic lupus erythematosus (SLE).
Owner:WYETH LLC
Method and apparatus for performance of thermal bronchiplasty with unfocused ultrasound
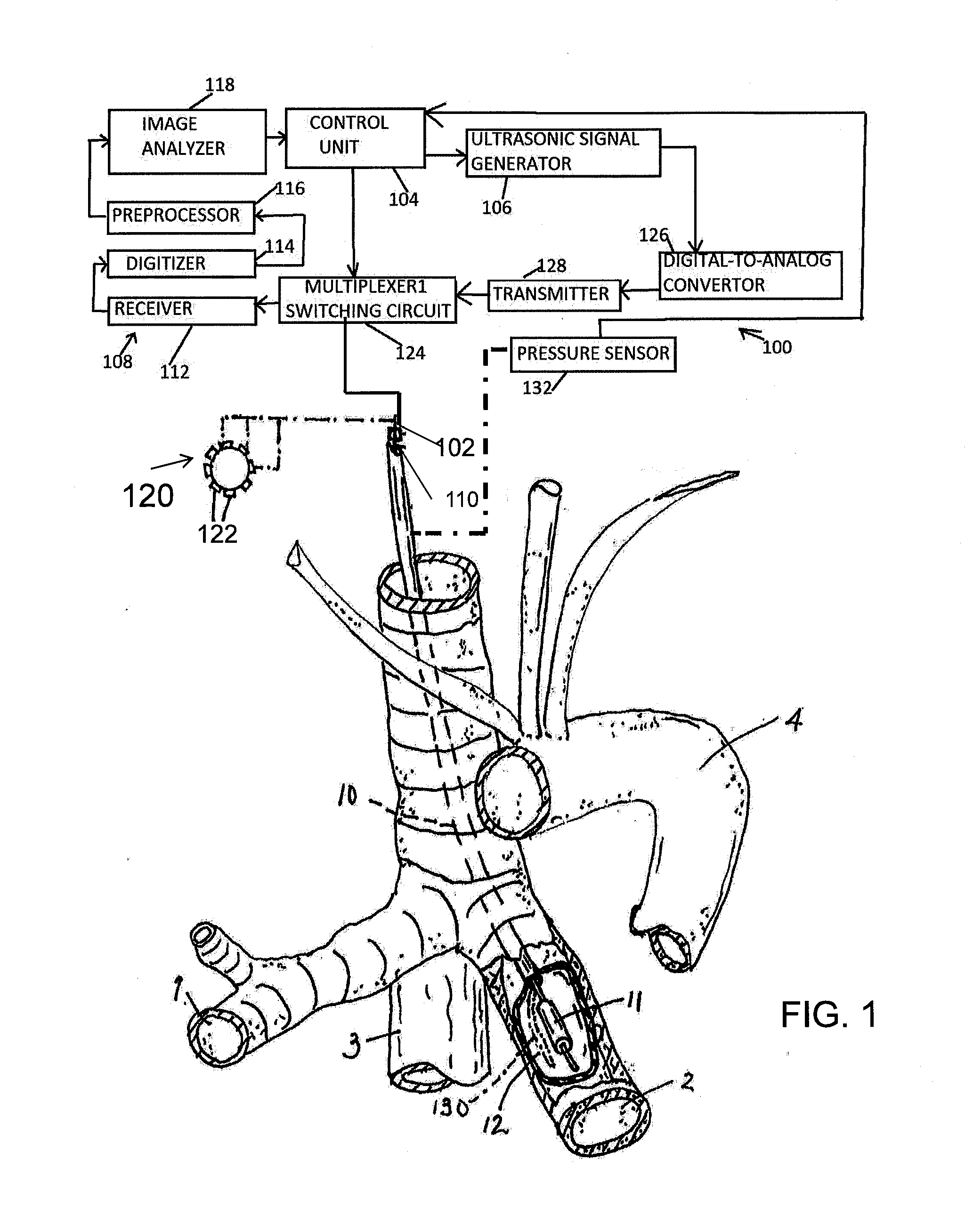
InactiveUS20160287912A1Reduce the possibilityAvoid insufficient temperatureUltrasound therapyBronchoscopesSonificationLarge target
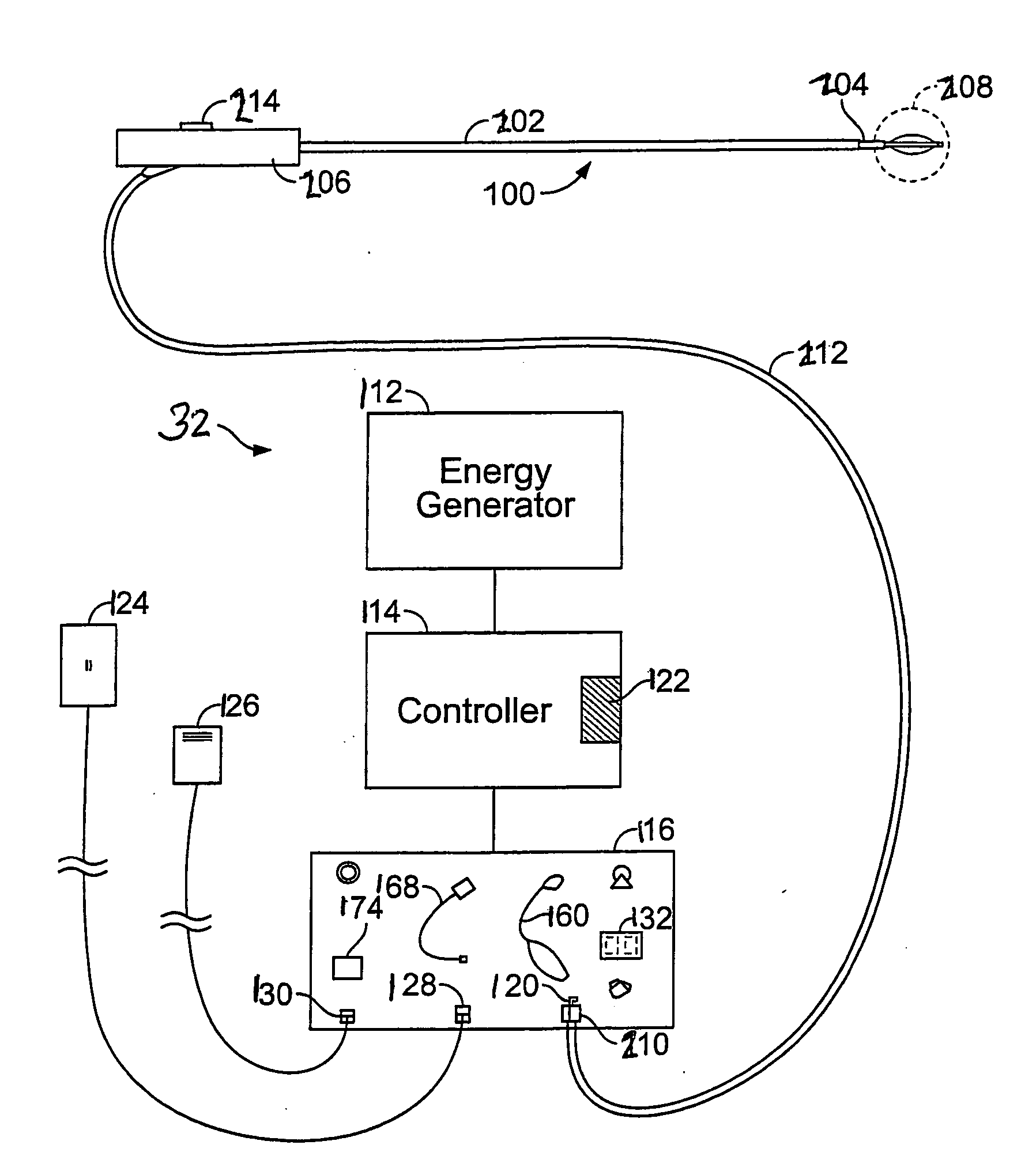
Apparatus and methods for deactivating bronchial nerves and smooth muscle extending along a bronchial branch of a mammalian subject to treat asthma and related conditions. An electromechanical transducer (11) is inserted into the bronchus as, for example, by advancing the distal end of a catheter (10) bearing the transducer into the bronchial section to be treated. The electromechanical transducer emits unfocused mechanical vibratory energy of one or more ultrasonic frequencies so as to heat tissues throughout a relatively large target region (13) as, for example, at least about 1 cm3 encompassing the bronchus to a temperature sufficient to inactivate nerves but insufficient to cause rapid ablation or necrosis of organic tissues. The treatment can be performed without locating or focusing on individual bronchial nerves.
Owner:GUIDED INTERVENTIONS
Methods for treating airways
InactiveUS20060254600A1Reduce capacityReduce resistanceSurgical needlesEndoscopesRadiologyTreating Site
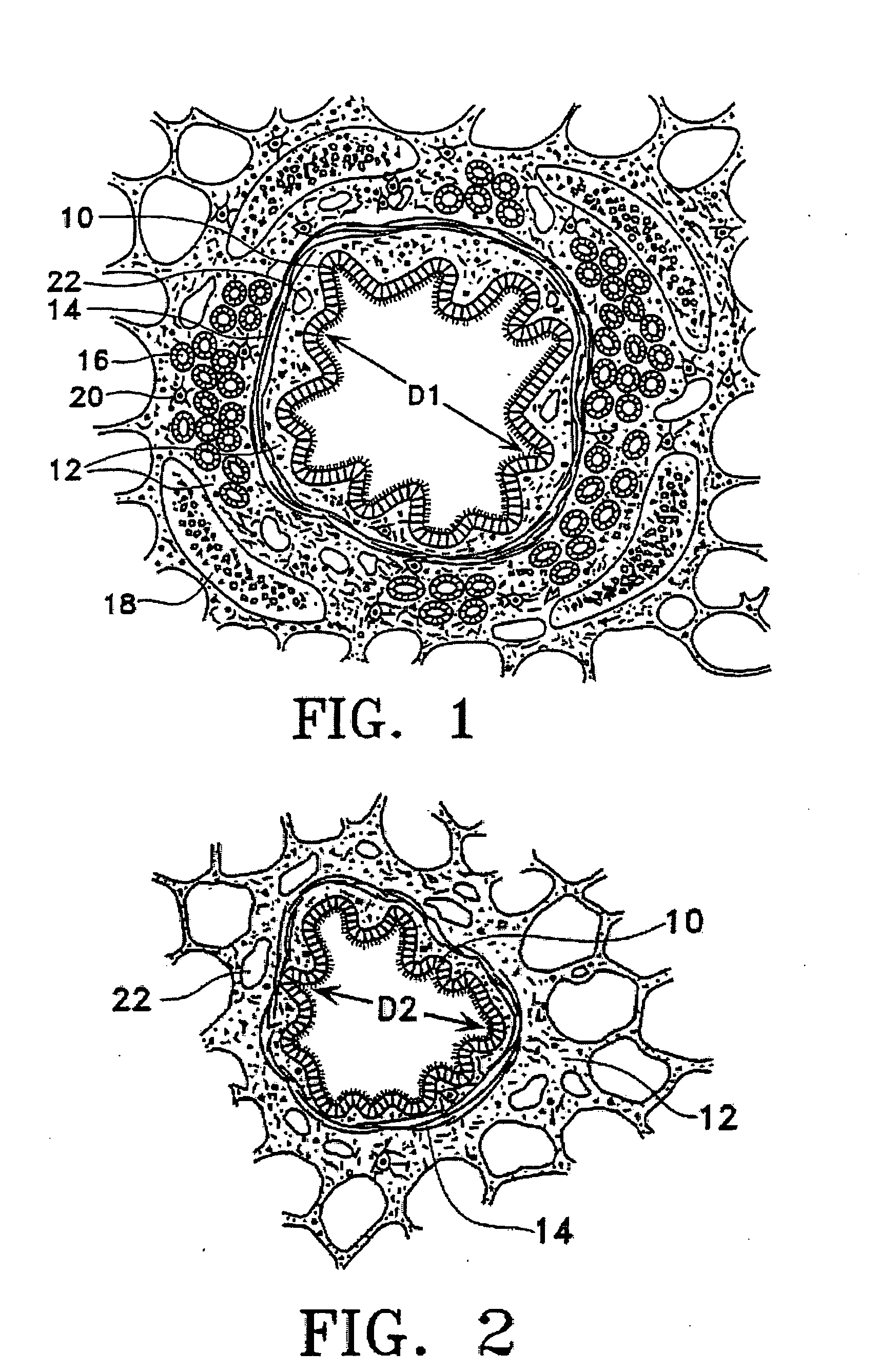
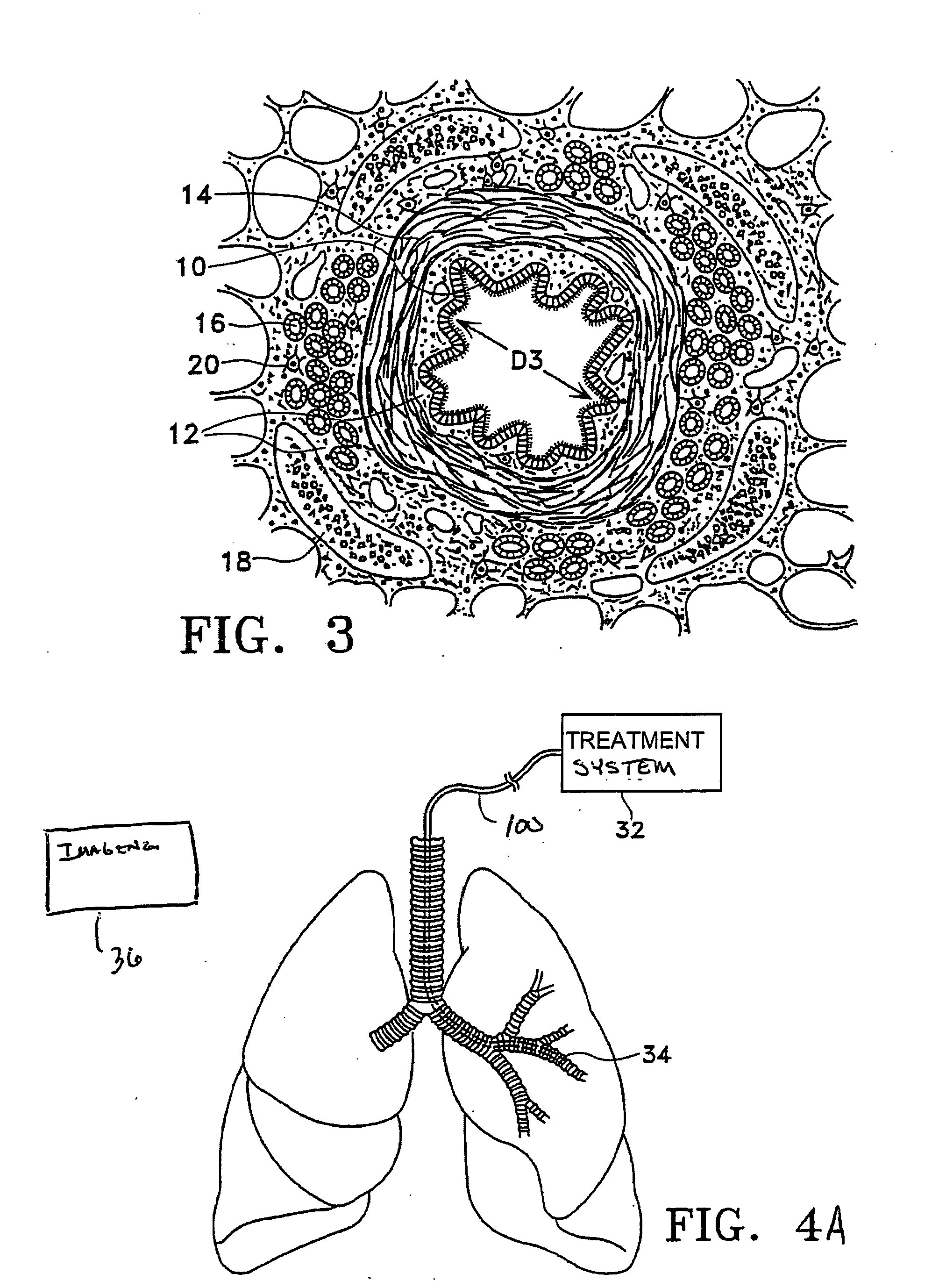
This relates to treating airways in a lung to decrease asthmatic symptoms. The also includes steps of measuring a parameter of an airway at a plurality of locations in a lung, identifying at least one treatment site from at least one of the plurality of locations based on the parameter, and applying energy to the treatment site to reduce the ability of the site to narrow.
Owner:BOSTON SCI SCIMED INC
Chinese herbal medicinal feed additive for preventing and controlling swine infectious disease
InactiveCN101695342APrevention and treatment of asthmaPrevention and treatment of contagious pleuropneumoniaFood processingAnimal feeding stuffMedicinal herbsBaical Skullcap Root
The invention provides a Chinese herbal medicinal feed additive for preventing and controlling swine infectious disease. The swine feed additive consists of 37 Chinese medicinal herbs, namely, gypsum, rehmannia root, rhinoceros horn, golden thread, cape jasmine fruit, tree peony bark, baical skullcap root, red paeony root, figwort root, common anemarrhena rhizome, forsythia suspensa , platycodon root, liquorice root, common lophatherum herb, amur corktree bark, honeysuckle flower, Chinese pulsatilla root, indigowoad root, heartleaf houttuynia herb, astragalus, szechwon tangshan root, hawkthorn fruit, medicated leaven, barley sprout, radish seed, chicken's gizzard -membrane, Chinese thorowax root, common andrographis herb, philippine violet herb, tuber fleeceflower root, massa medicata fermentata fujianensis, cyrtomium rhizome, tung leaf, tangerine peel, white paeony root, pine needle and indigowoad leaf through scientific compatibility. The feed additive is added into swine feed in the proportion; under the condition of not using any vaccine, the feed additive can effectively prevent and cure severe mixed flu symptoms, infection and other syndromes caused by swine respiratory disease, asthma, contagious pleuropneumonia, swine virus mixed flu, high swine fever, porcine circovirus, swine fever, flu, pseudorabies, salmonellosis, bacillosis, streptococcus, erysipelas, paratyphoid, eperythrozoon, toxoplasm and other multi-pathogeny and provides genuine green food for the market.
Owner:孟祥合
Trk-INHIBITING COMPOUND
ActiveUS20160000783A1Excellent kinase selectivityInhibits NGF vascular hyper permeabilityBiocideOrganic chemistryChagas diseaseBULK ACTIVE INGREDIENT
The present invention provides a drug containing a compound having Trk-inhibiting activity as an active ingredient in prophylaxis and / or therapy for Trk-related diseases such as pain, pruritus, lower urinary tract dysfunction, asthma, allergic rhinitis, inflammatory bowel disease or Chagas disease. A compound represented by the general formula (I), wherein all symbols represent the same meanings as described in the specification, a salt thereof, an N-oxide thereof, a solvate thereof or a prodrug thereof is useful as a drug component having Trk-inhibiting activity in prophylaxis and / or therapy of diseases such as pain, pruritus, lower urinary tract dysfunction, asthma, allergic rhinitis, inflammatory bowel disease or Chagas disease.
Owner:ONO PHARMA CO LTD
Toxin peptide therapeutic agents
ActiveUS20070071764A1Avoid it happening againRelieve symptomsNervous disorderAntipyreticHalf-lifeSjögren syndrome
Disclosed is a composition of matter of the formula (X1)a—(F1)d—(X2)b—(F2)e—(X3)c (I) and multimers thereof, in which F1 and F2 are half-life extending moieties, and d and e are each independently 0 or 1, provided that at least one of d and e is 1; X1, X2, and X3 are each independently -(L)f-P-(L)g-, and f and g are each independently 0 or 1; P is a toxin peptide of no more than about 80 amino acid residues in length, comprising at least two intrapeptide disulfide bonds; L is an optional linker; and a, b, and c are each independently 0 or 1, provided that at least one of a, b and c is 1. Linkage to the half-life extending moiety or moieties increases the in vivo half-life of the toxin peptide, which otherwise would be quickly degraded. A pharmaceutical composition comprises the composition and a pharmaceutically acceptable carrier. Also disclosed are a DNA encoding the inventive composition of matter, an expression vector comprising the DNA, and a host cell comprising the expression vector. Methods of treating an autoimmune disorder, such as, but not limited to, multiple sclerosis, type 1 diabetes, psoriasis, inflammatory bowel disease, contact-mediated dermatitis, rheumatoid arthritis, psoriatic arthritis, asthma, allergy, restinosis, systemic sclerosis, fibrosis, scleroderma, glomerulonephritis, Sjogren syndrome, inflammatory bone resorption, transplant rejection, graft-versus-host disease, and lupus and of preventing or mitigating a relapse of a symptom of multiple sclerosis are also disclosed.
Owner:AMGEN INC
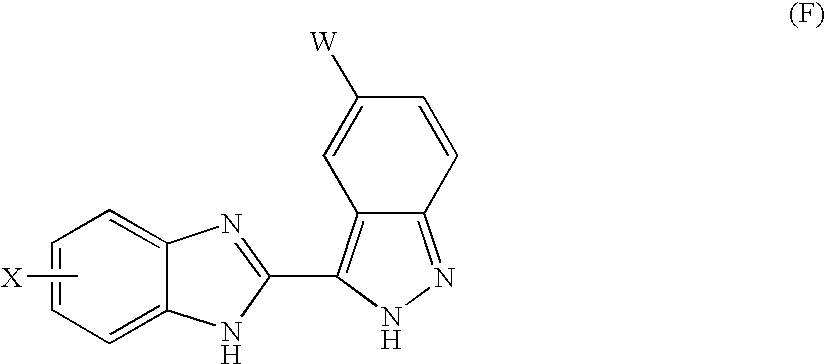
Benzimidazole derivatives and their use as KDR kinase protein inhibitors
The invention discloses and claims benzimidazole compounds of formula (I): wherein X is C—R2; Y is C—R2 or C—R3; W and Z are each C—R3; R1 is an optionally substituted aryl, heteroaryl or a saturated 5- or 6-membered monocyclic heterocyclic radical or a bicyclic heterocyclic radical; and A5 is H or alkyl; or a stereoisomer, a racemate, an enantiomer or a diastereoisomer of said compound of formula (I) or a pharmaceutically acceptable salt thereof; the use of compounds of formula (I) for the treatment of a disorder of proliferation of blood vessels, uncontrolled angiogenesis, a fibrotic disorder, a disorder of proliferation of mesangial cells, a metabolic disorder, allergy, asthma, thrombosis, a disease of the nervous system, retinopathy, psoriasis, rheumatoid arthritis, diabetes, muscle degeneration, solid tumors and cancers, pharmaceutical compositions comprising a compound of formula (I) and one or more pharmaceutically acceptable adjuvants or diluents and pharmaceutical compositions comprising a compound of formula (I) and one or more. antimitiotic agents.
Owner:AVENTIS PHARMA SA (US)
Respiratory disease monitoring system
ActiveUS20110125044A1Easy translationSave them from embarrassmentStethoscopeRespiratory organ evaluationAnesthesiaAsthma
An automated system for monitoring respiratory diseases, such as asthma, provides noninvasive, multimodal monitoring of respiratory signs and symptoms that can include wheeze and cough. Some embodiments employ a mobile device, such as a cell phone, in which raw data from a microphone and an accelerometer are processed, analyzed, and stored. Data can be collected continuously. Time domain and frequency domain analyses of signals to determine, e.g., energy, duration, and spectral content of candidate sounds can be employed to discriminate symptoms of interest from background sounds and to establish significance. Accelerometer signals are analyzed to determine activity levels. Analyses of a user's symptoms and activity level prior to, during, and after an event can provide meaningful determinations of disease severity and predict future respiratory events. The system can provide a summary of data, as well as an alarm when symptom severity reaches a threshold.
Owner:UNIVERSITY OF ROCHESTER
Trk-inhibiting compound
ActiveUS9242977B2Safe prophylactic and therapeutic agentHigh selectivityNervous disorderOrganic chemistryChagas diseaseInflammatory bowel disease
An object of the present invention is to provide a drug containing a compound having Trk-inhibiting activity as an active ingredient in prophylaxis and / or therapy of diseases such as pain, pruritus, lower urinary tract dysfunction, asthma, allergic rhinitis, inflammatory bowel disease or Chagas disease. A compound represented by the general formula (I):(wherein all symbols represent the same meanings as described in the specification), a salt thereof, an N-oxide thereof, a solvate thereof or a prodrug thereof is useful as a drug component having Trk-inhibiting activity in prophylaxis and / or therapy of diseases such as pain, pruritus, lower urinary tract dysfunction, asthma, allergic rhinitis, inflammatory bowel disease or Chagas disease.
Owner:ONO PHARMA CO LTD
Expandable electode devices and methods of treating bronchial tubes
InactiveUS20070106296A1Minimal traumaAlteration of coefficientElectrotherapyDilatorsBronchial tubeObstructive Pulmonary Diseases
Methods are provided for treating collapsed bronchial tubes found in patients with chronic obstructive pulmonary diseases, such as asthma. The method includes heating the bronchial tube to cause tissue in the wall of the bronchial tube to undergo a structural transformation effective to render the wall capable of supporting a non-collapsed lumen. The procedure effectively reinforces the structural integrity of the bronchial tube wall and thereby prevents the lumen from collapsing.
Owner:BOSTON SCI SCIMED INC
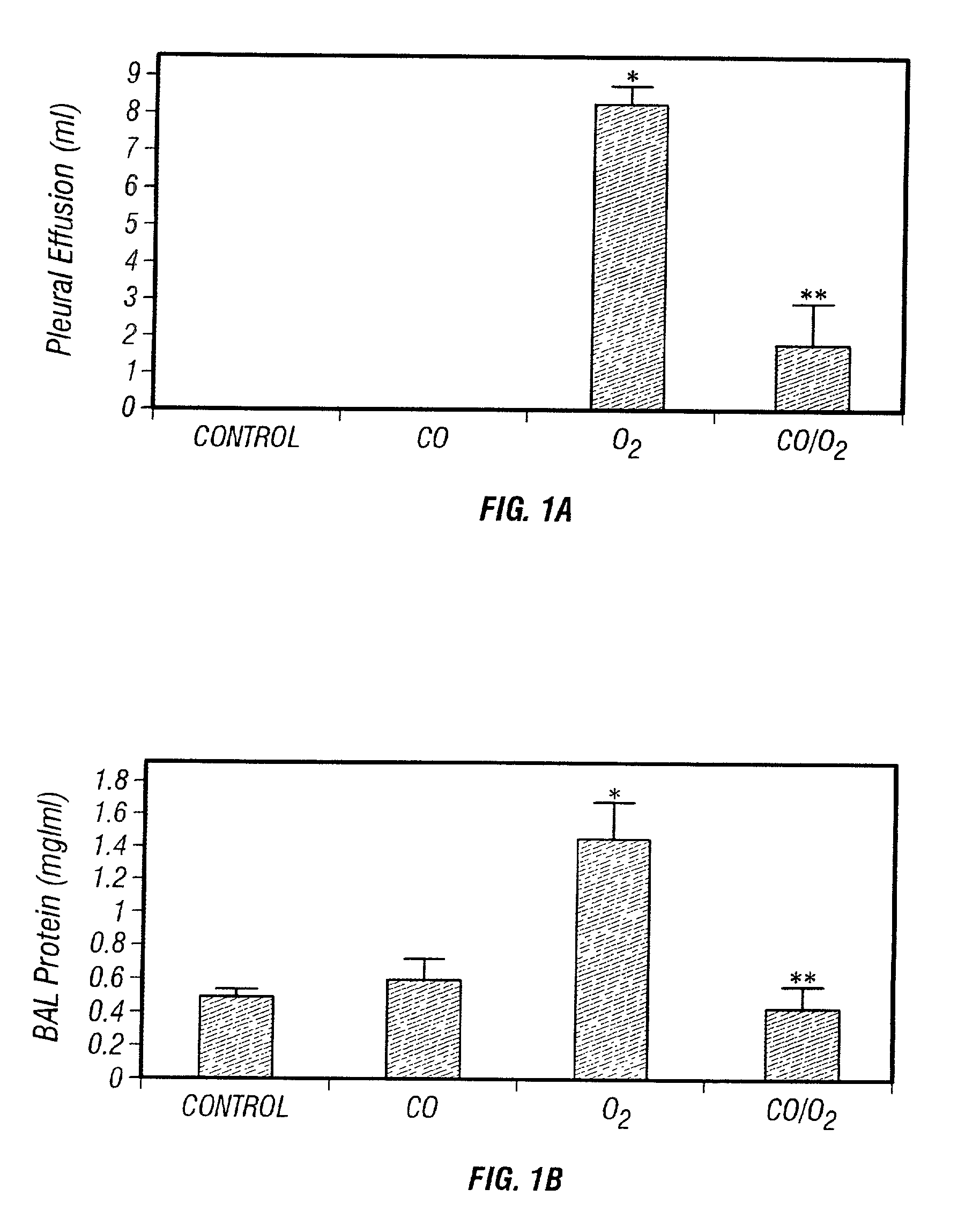
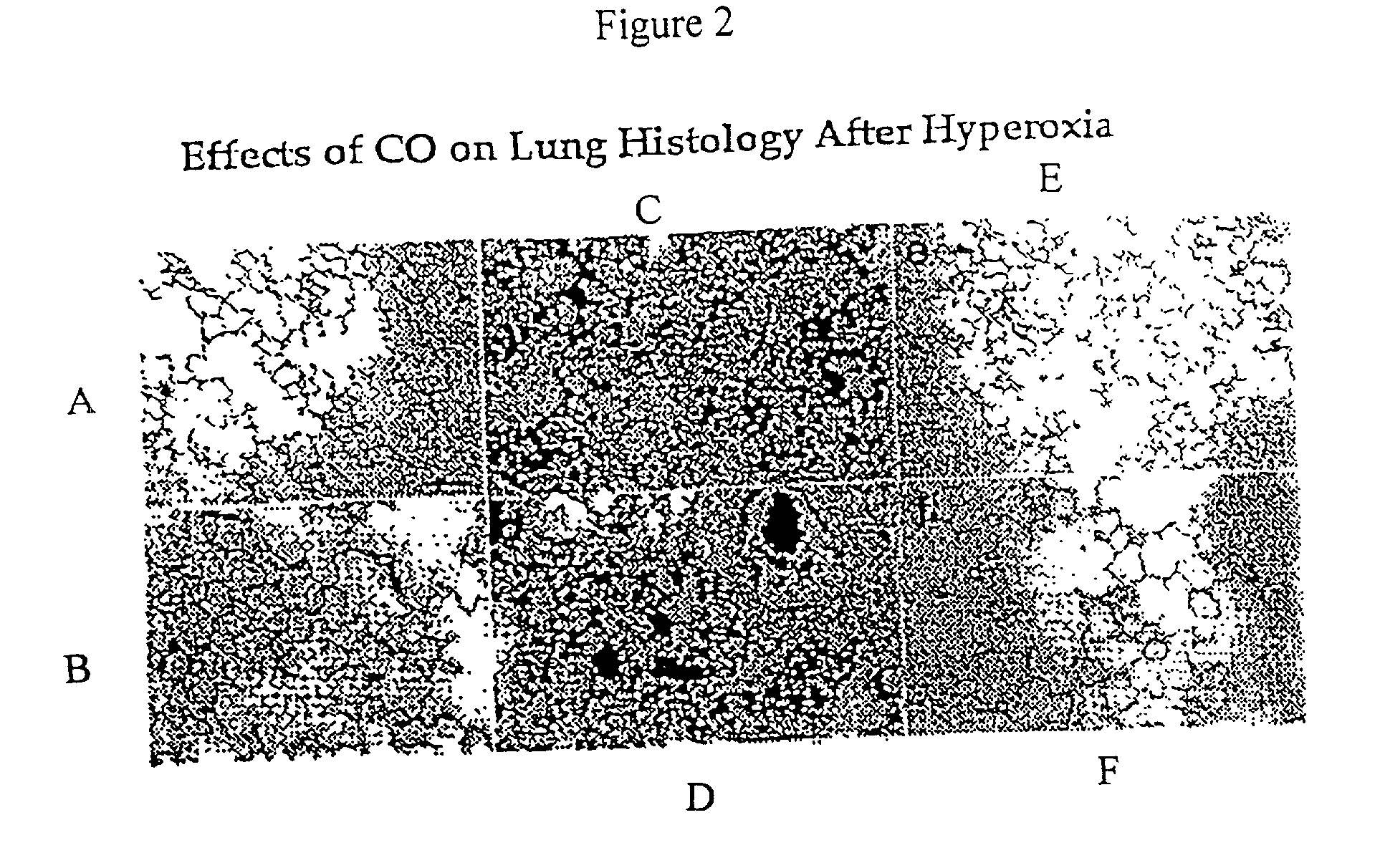
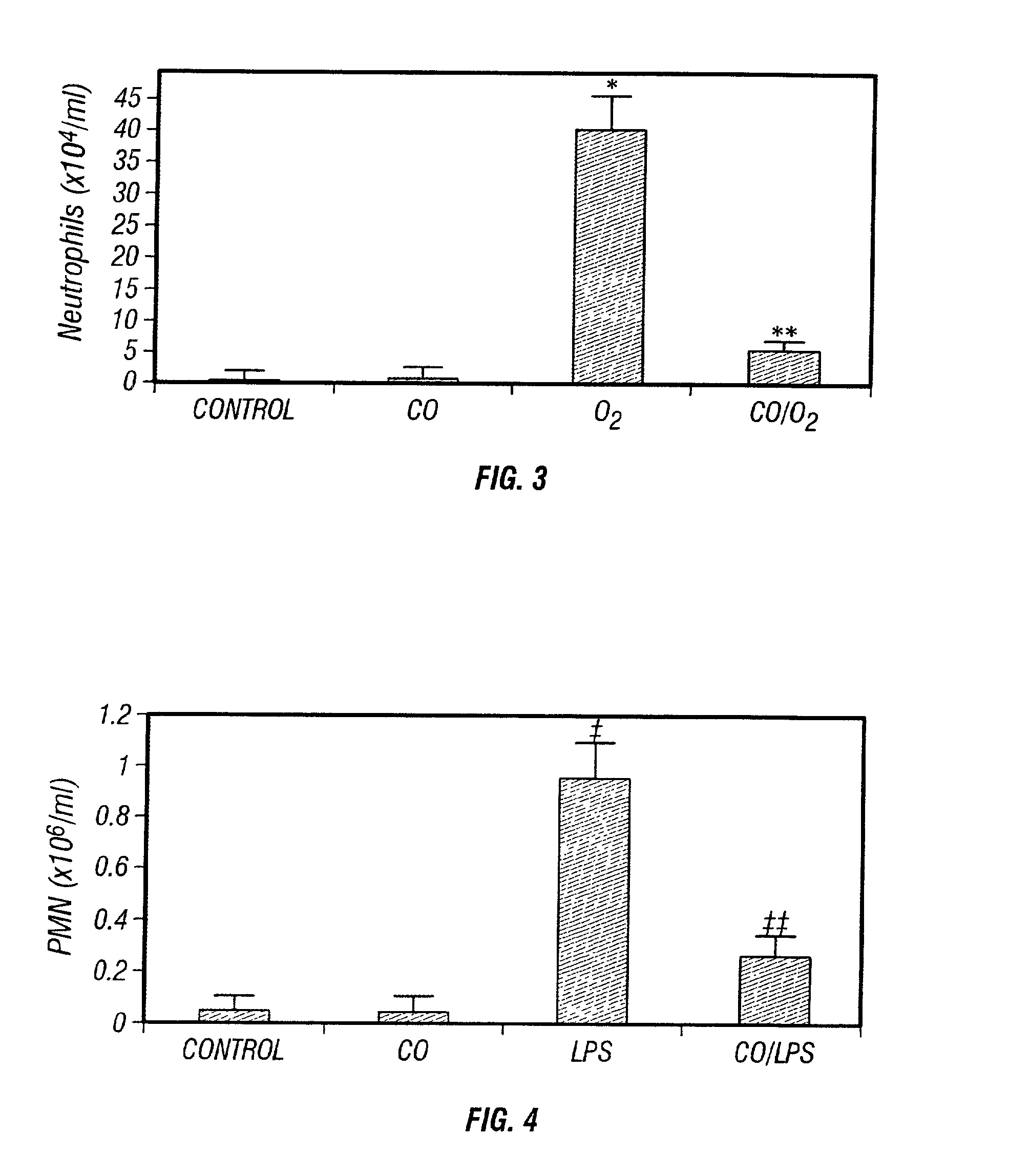
Carbon monoxide as a biomarker and therapeutic agent
InactiveUS20020155166A1Reduce concentrationBiocideInorganic active ingredientsInterstitial lung diseaseRESPIRATORY DISTRESS SYNDROME ADULT
The present invention relates to the use of carbon monoxide (CO) as a biomarker and therapeutic agent of heart, lung, liver, spleen, brain, skin and kidney diseases and other conditions and disease states including, for example, asthma, emphysema, bronchitis, adult respiratory distress syndrome, sepsis, cystic fibrosis, pneumonia, interstitial lung diseases, idiopathic pulmonary diseases, other lung diseases including primary pulmonary hypertension, secondary pulmonary hypertension, cancers, including lung, larynx and throat cancer, arthritis, wound healing, Parkinson's disease, Alzheimer's disease, peripheral vascular disease and pulmonary vascular thrombotic diseases such as pulmonary embolism. CO may be used to provide anti-inflammatory relief in patients suffering from oxidative stress and other conditions especially including sepsis and septic shock. In addition, carbon monoxide may be used as a biomarker or therapeutic agent for reducing respiratory distress in lung transplant patients and to reduce or inhibit oxidative stress and inflammation in transplant patients.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Apparatus for autonomic neuromodulation for the treatment of systemic disease
InactiveUS20100241183A1Delay is slowPreserving and prolonging effect of modulationSpinal electrodesUltrasound therapyNervous systemEfferent
A method, apparatus, and surgical technique for the modulation of autonomic function, for the purpose of treating any of several conditions and diseases, including obesity, metabolic disorders, endocrine disorders, diabetes, respiratory disease, asthma, inflammatory disease, immunological disease, infection, cancer, cardiac disease, cardiovascular disease, cerebrovascular disease, stroke, vasospasm, vascular disease, psychiatric disease, depression, affective disorders, anxiety disorders, and other conditions. This includes neural and tissue modulators, including implanted devices, used to modulate efferent and afferent autonomic neurons to influence or control autonomic or other neural function, including modulation of sympathetic and parasympathetic nervous system components as well as their combination.
Owner:DILORENZO BIOMEDICAL
Fused heterobicyclic kinase inhibitors
InactiveUS20070208053A1Low toxicityDisrupting virus life-cycleAntibacterial agentsBiocideNervous systemAllergy
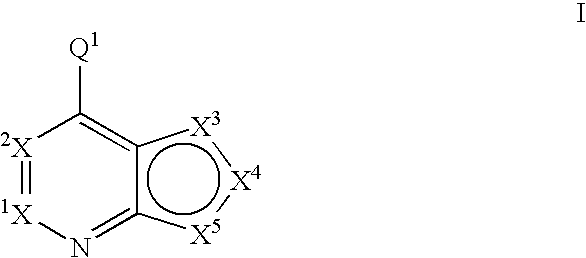
Compounds of the formula and pharmaceutically acceptable salts thereof, wherein X1, X2, X3, X4, X5, X5, X7, R1, and Q1 are defined herein, inhibit kinase enzymes and are useful for the treatment and / or prevention of hyperproliferative diseases such as cancer. The compounds are also useful in the treatment of inflammation, allergy, asthma, disease and conditions of the immune system, disease and conditions of the nervous system, cardiovascular diseases, disease and conditions of the eye, dermatological diseases, osteoporosis, diabetes, multiple sclerosis, and infections.
Owner:OSI PHARMA INC
Soluble guanylate cyclase activators
A compound having the structureuseful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, angina pectoris, thromboses, restenoses, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver in a human or animal patient.
Owner:MERCK SHARP & DOHME CORP
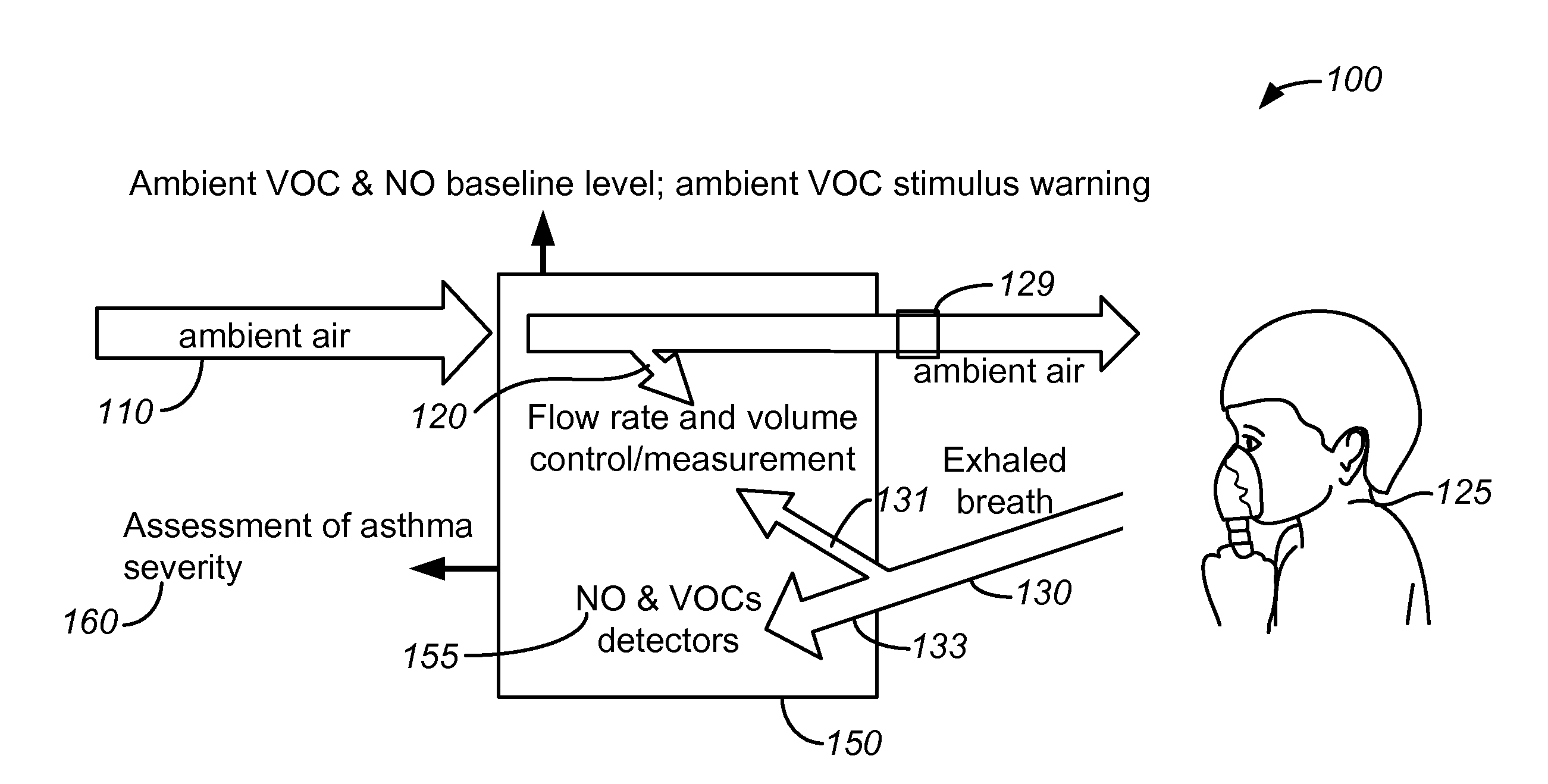
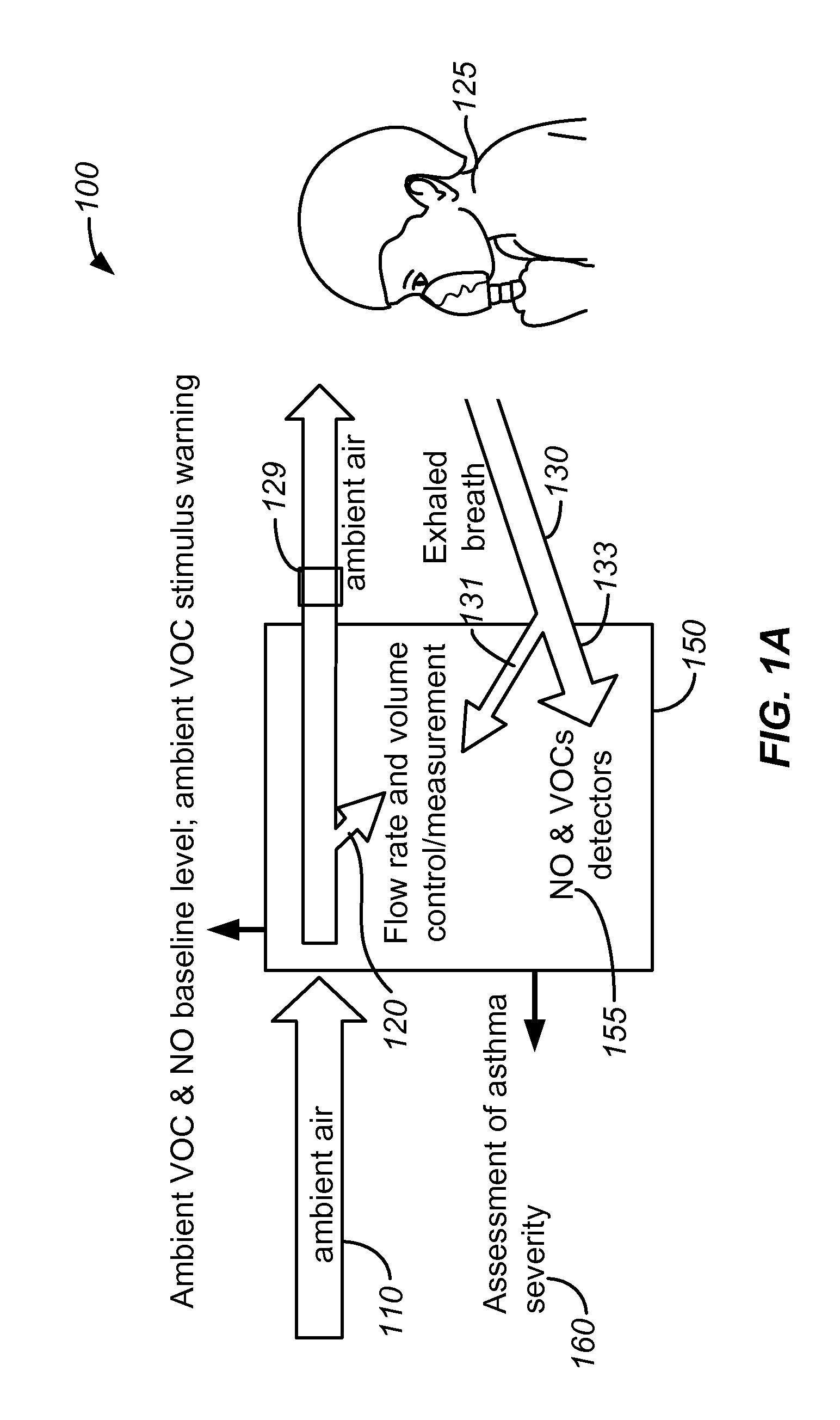
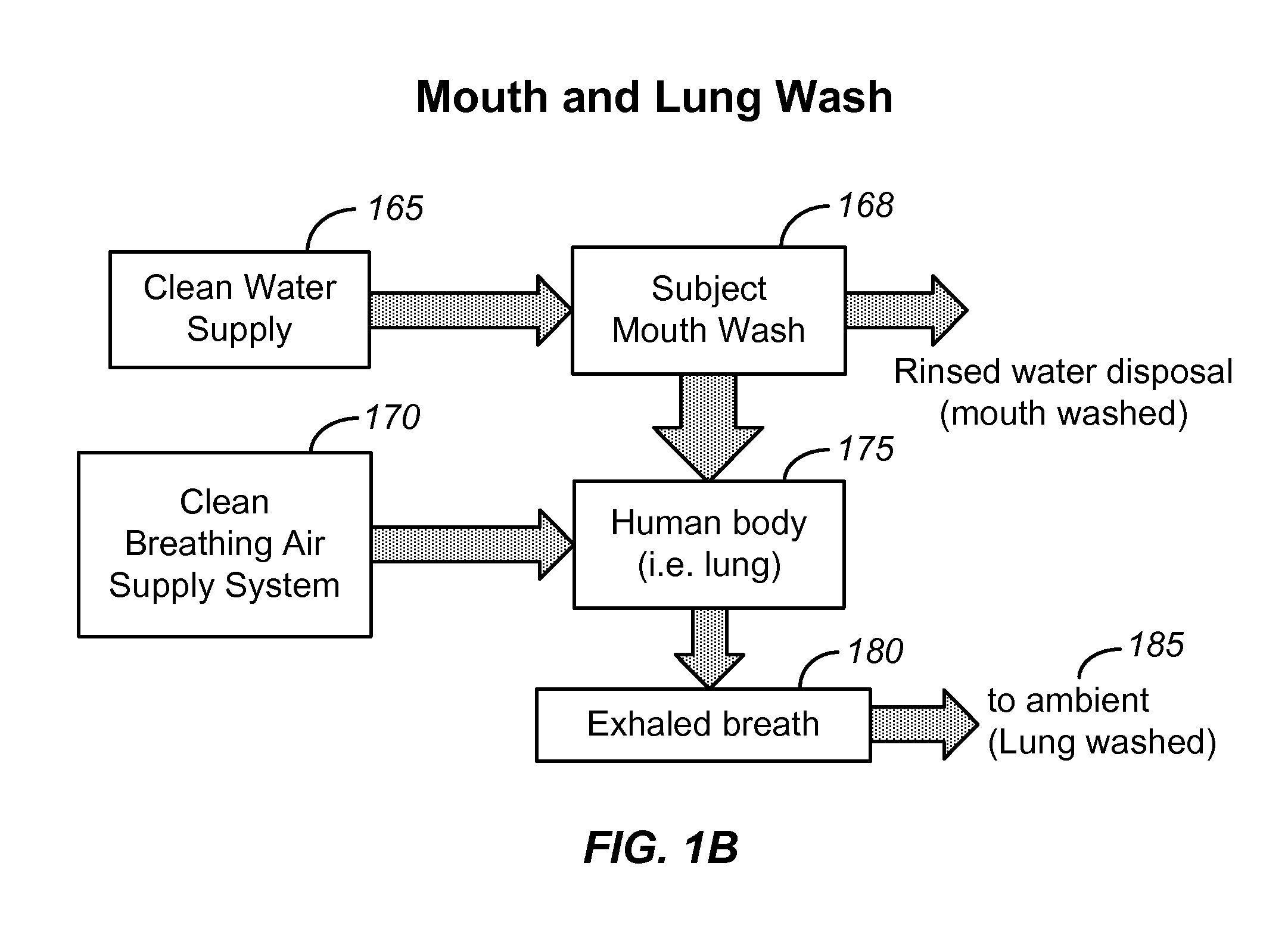
Breath analysis systems and methods for asthma, tuberculosis and lung cancer diagnostics and disease management
ActiveUS20100137733A1Efficient managementEffective controlComponent separationRespiratory organ evaluationDiseasePulmonary tuberculosis
Methods and systems are disclosed for the detecting of whether a subject has a lung disorder such as asthma, tuberculosis or lung cancer. Monitoring the subject's health and prognosis is also disclosed.
Owner:TRICORNTECH TAIWAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com