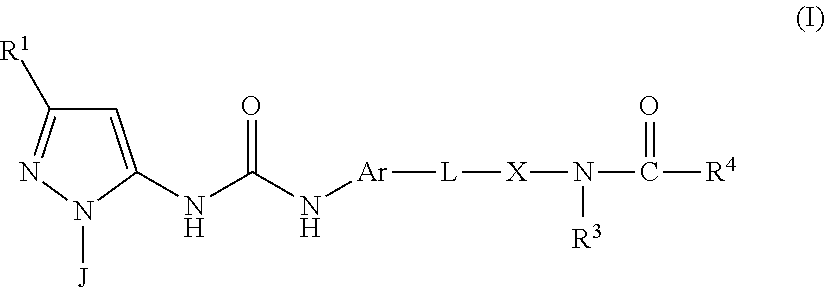Patents
Literature
392 results about "COPD" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A group of progressive lung disorders characterized by increasing breathlessness.
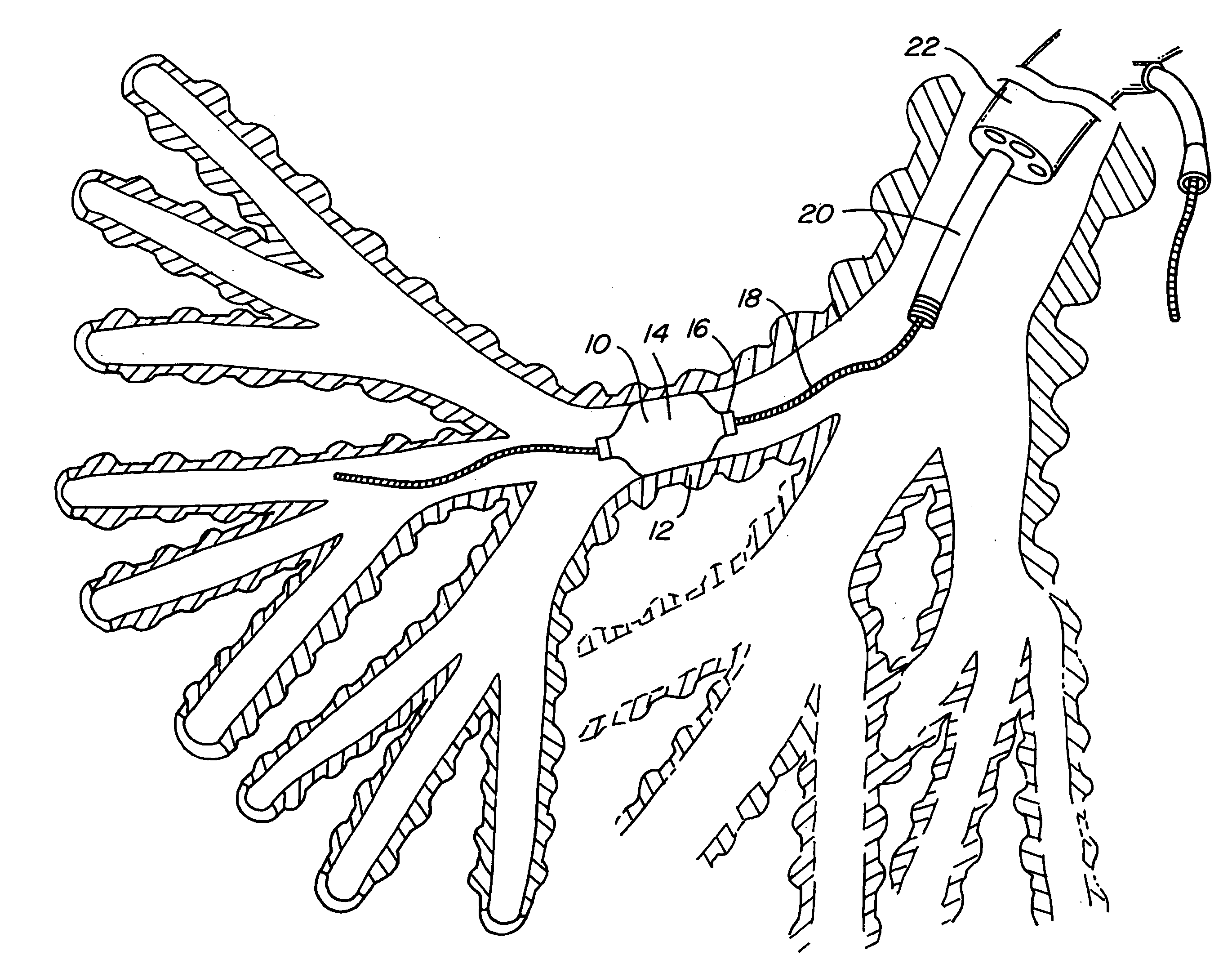
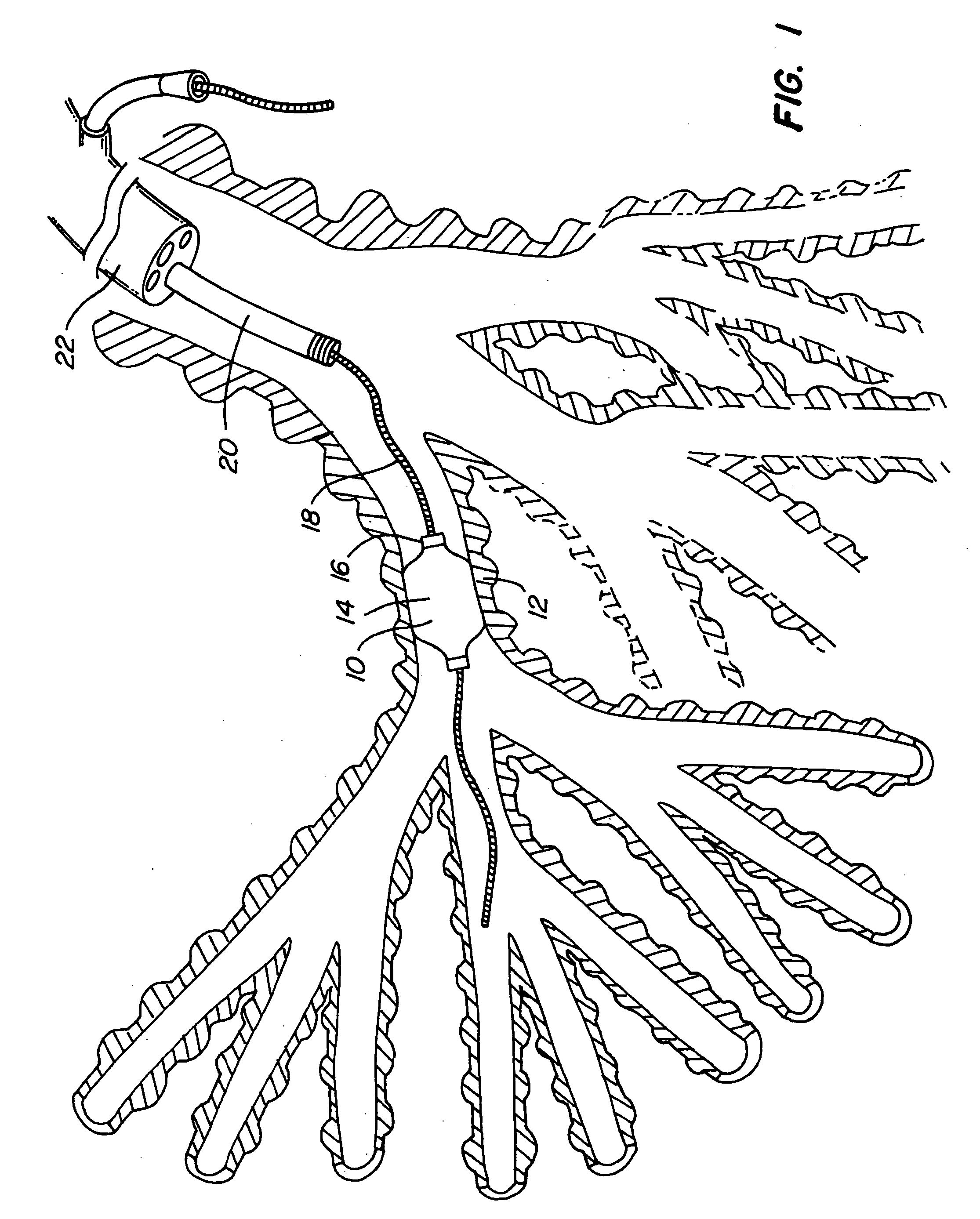
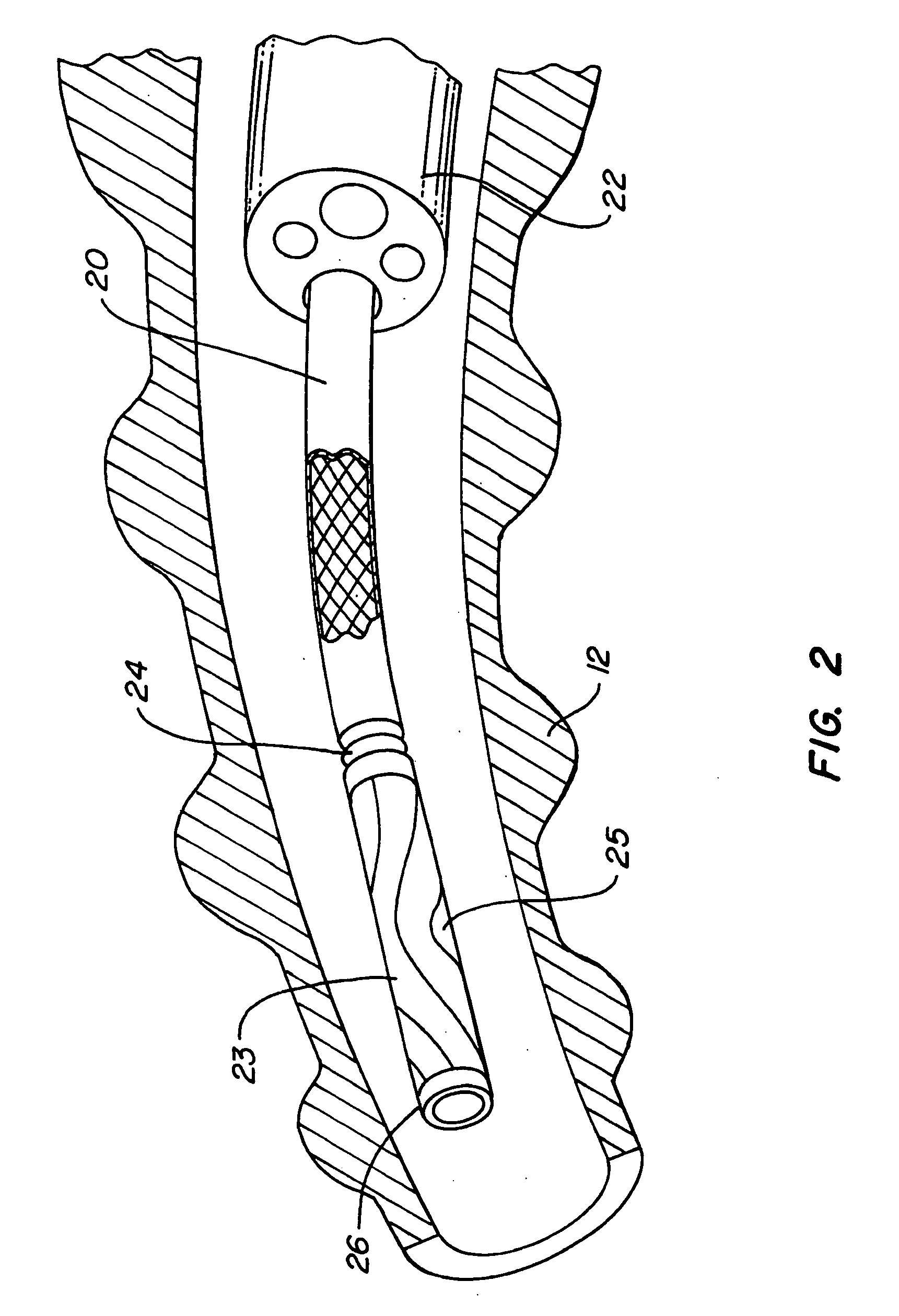
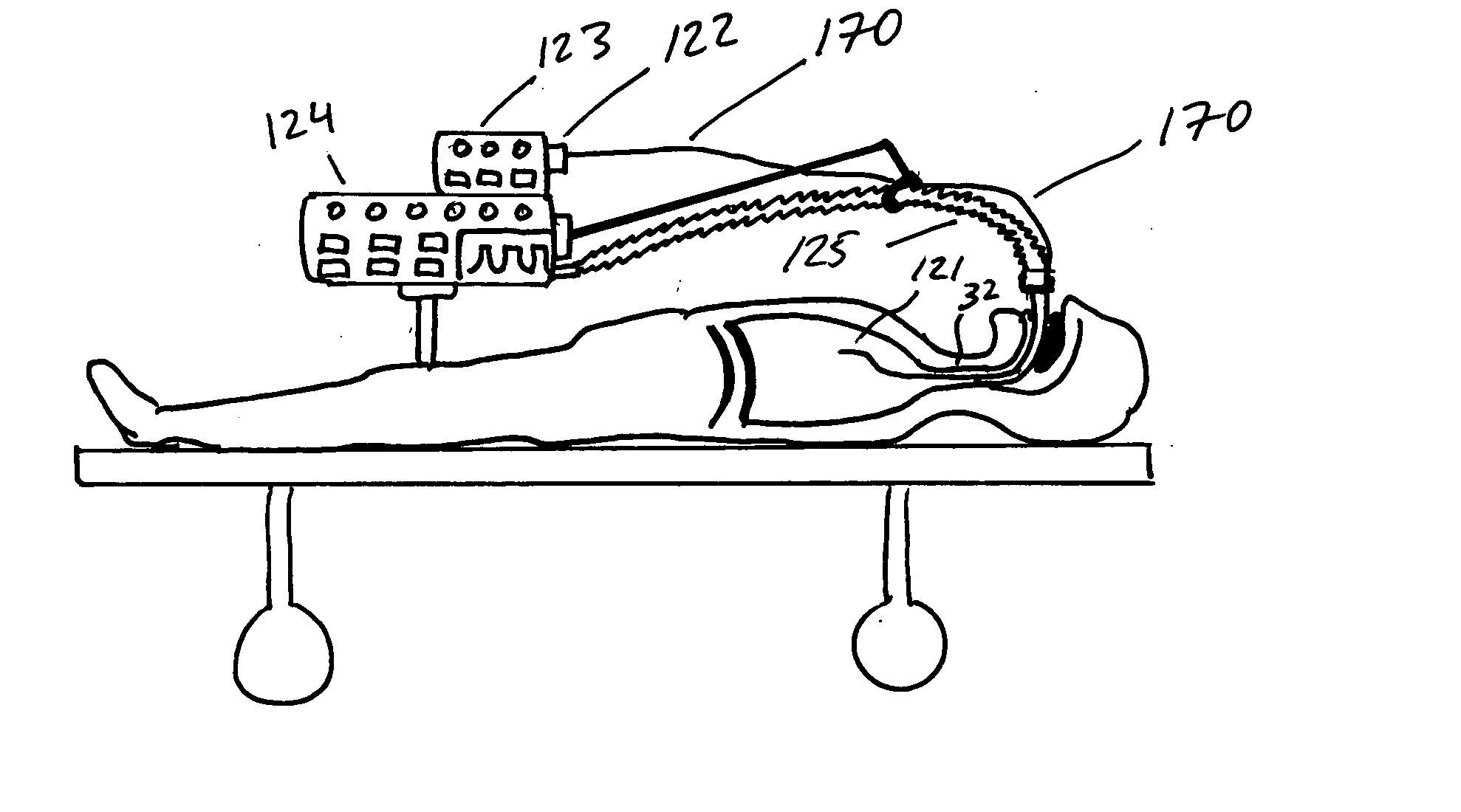
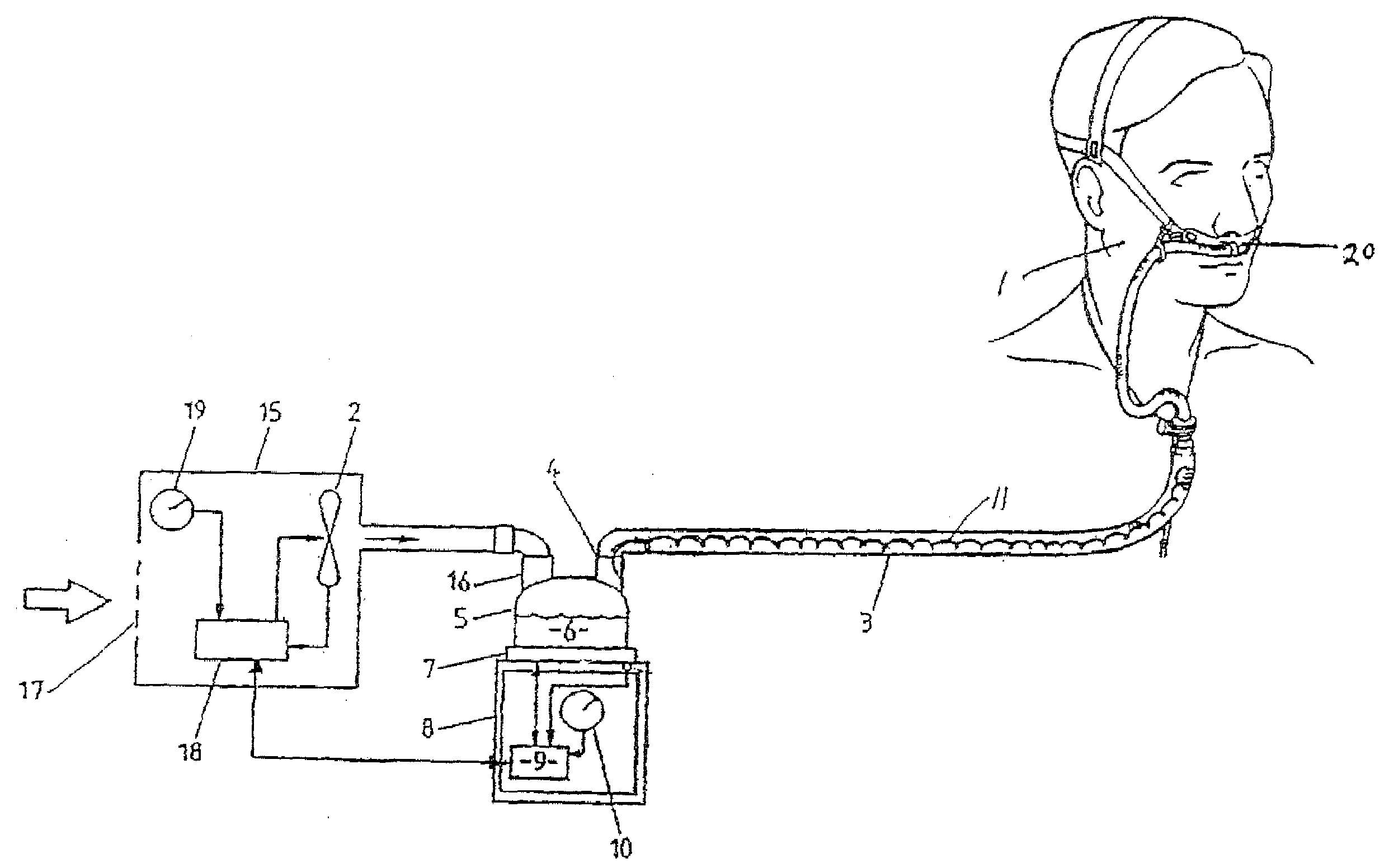
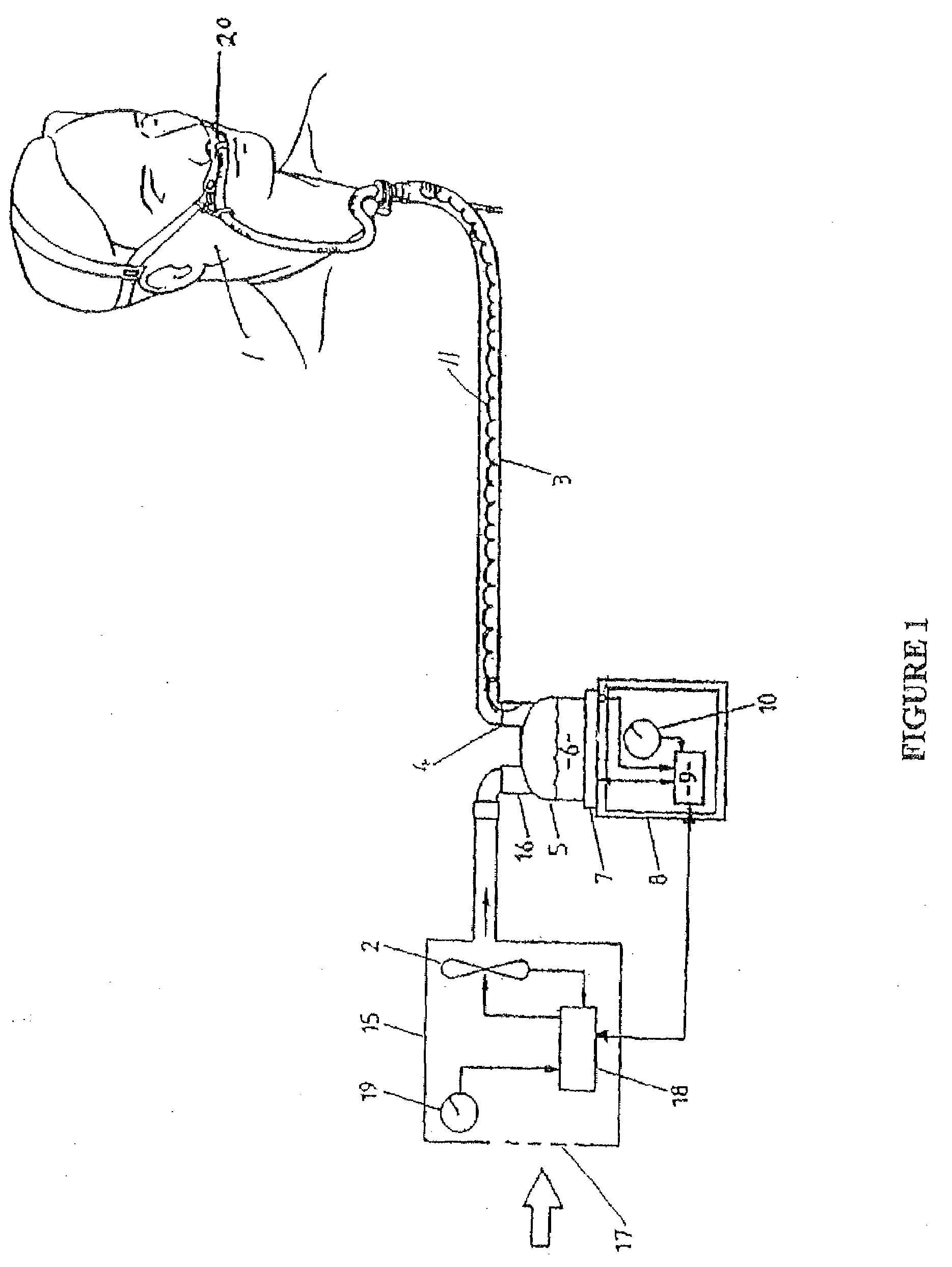
Method of treating a lung
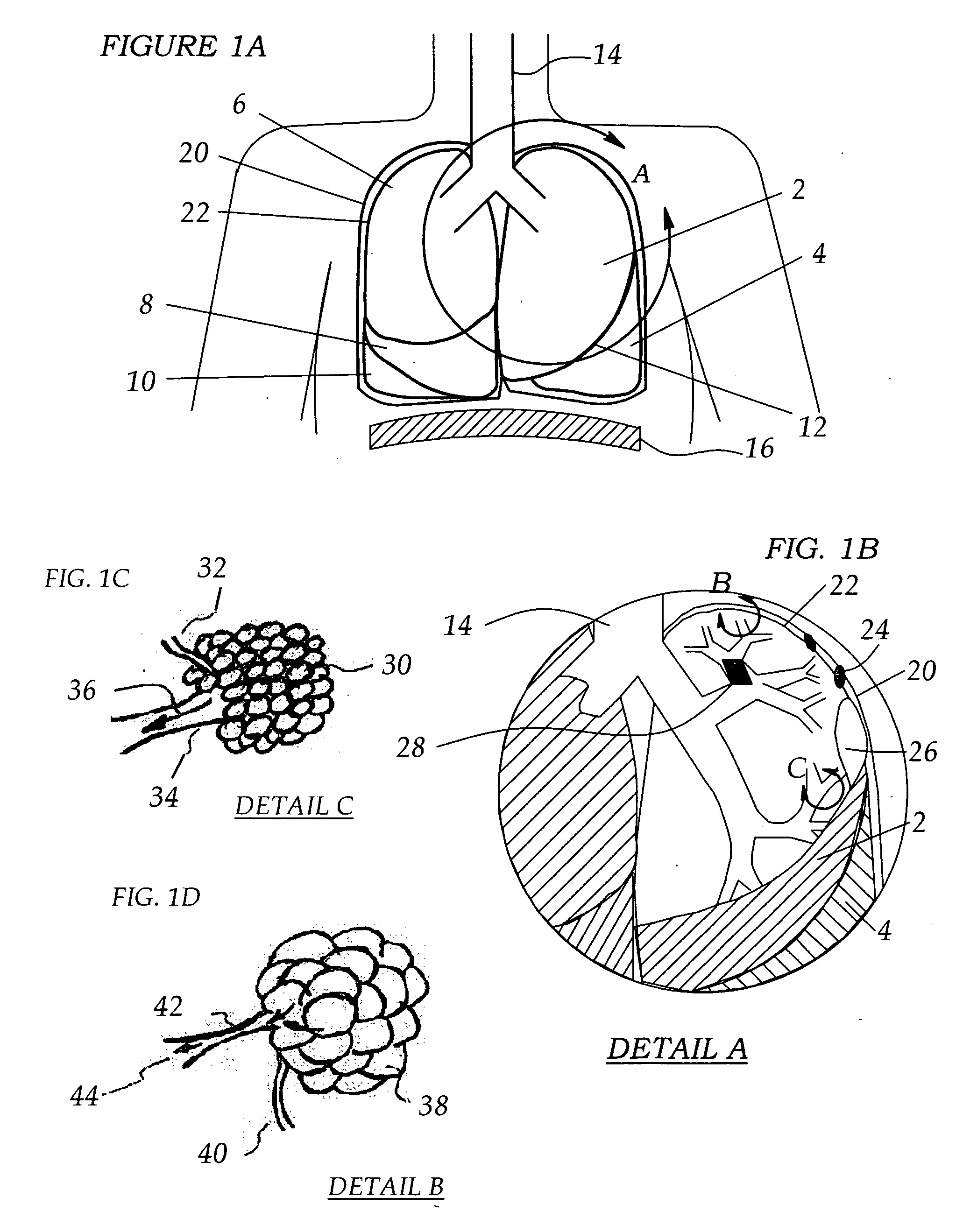
This invention provides methods for treating a lung, such as to treat a patient suffering from COPD. One aspect of the invention provides a method of treating a lung including the steps of: endotracheally delivering an expandable member (such as an inflatable member) to an air passageway of the lung with a delivery system; expanding the expandable member to make contact with a wall of the air passageway; and providing an open lumen through the expandable member to provide access to a portion of the lung distal to the expandable member. Some embodiments add the step of plugging the lumen, such as by contacting a plug with the expandable member or closing a port in the lumen. Some embodiments of the invention also include the step of reducing collateral flow around the expandable member and / or reducing collateral flow to lung regions distal to the expandable member, such as by delivering a collateral flow blocking agent, through the lumen. In some embodiments, the method includes the step of causing the portion of the lung distal to the expandable member to reduce volume, such as by compressing the lung portion.
Owner:PNEUMRX
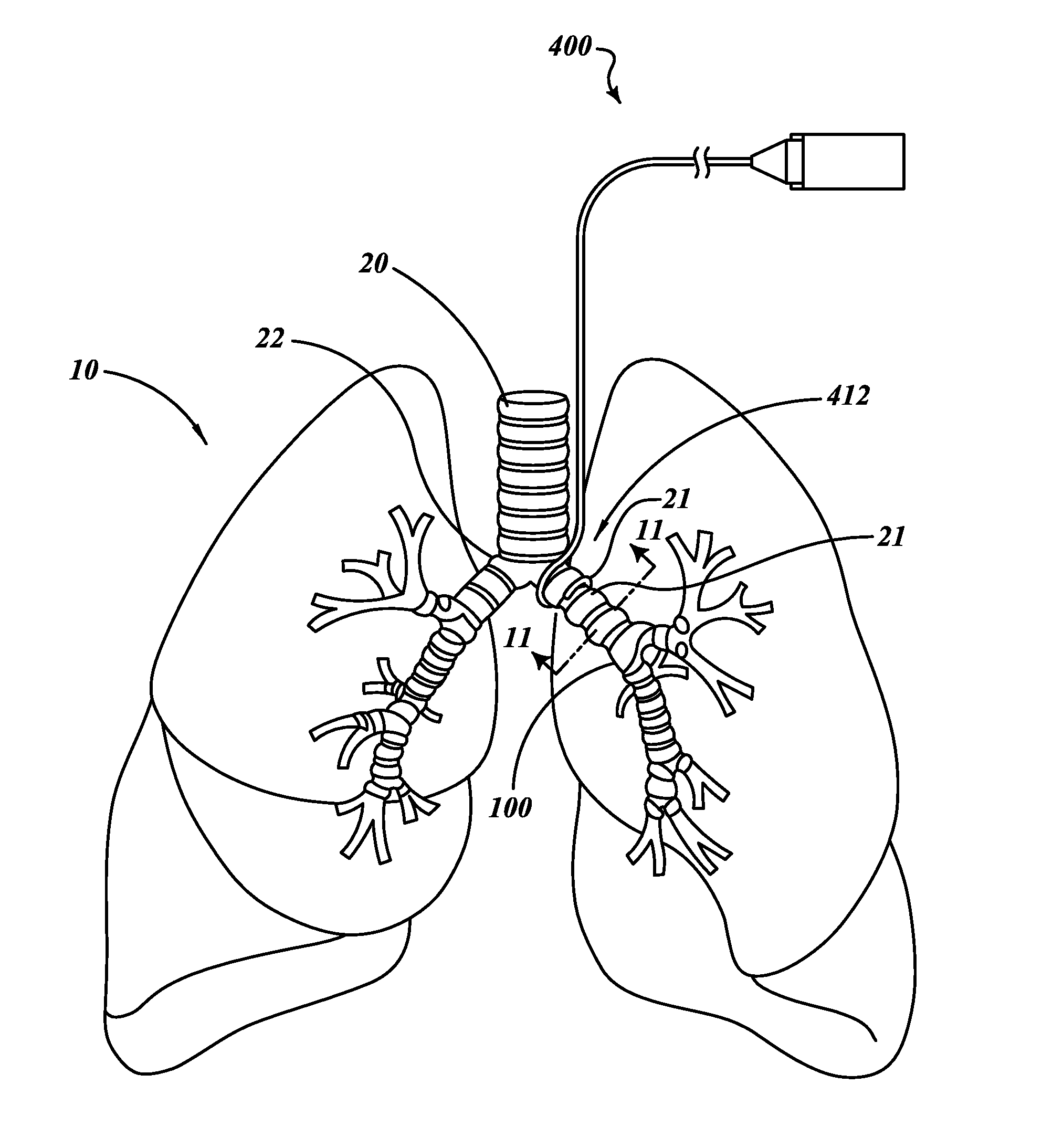
Methods, systems and devices for improving ventilation in a lung area
ActiveUS20050005936A1Facilitates of gas concentrationEasy pressure controlTracheal tubesOperating means/releasing devices for valvesDiseaseMechanical ventilation
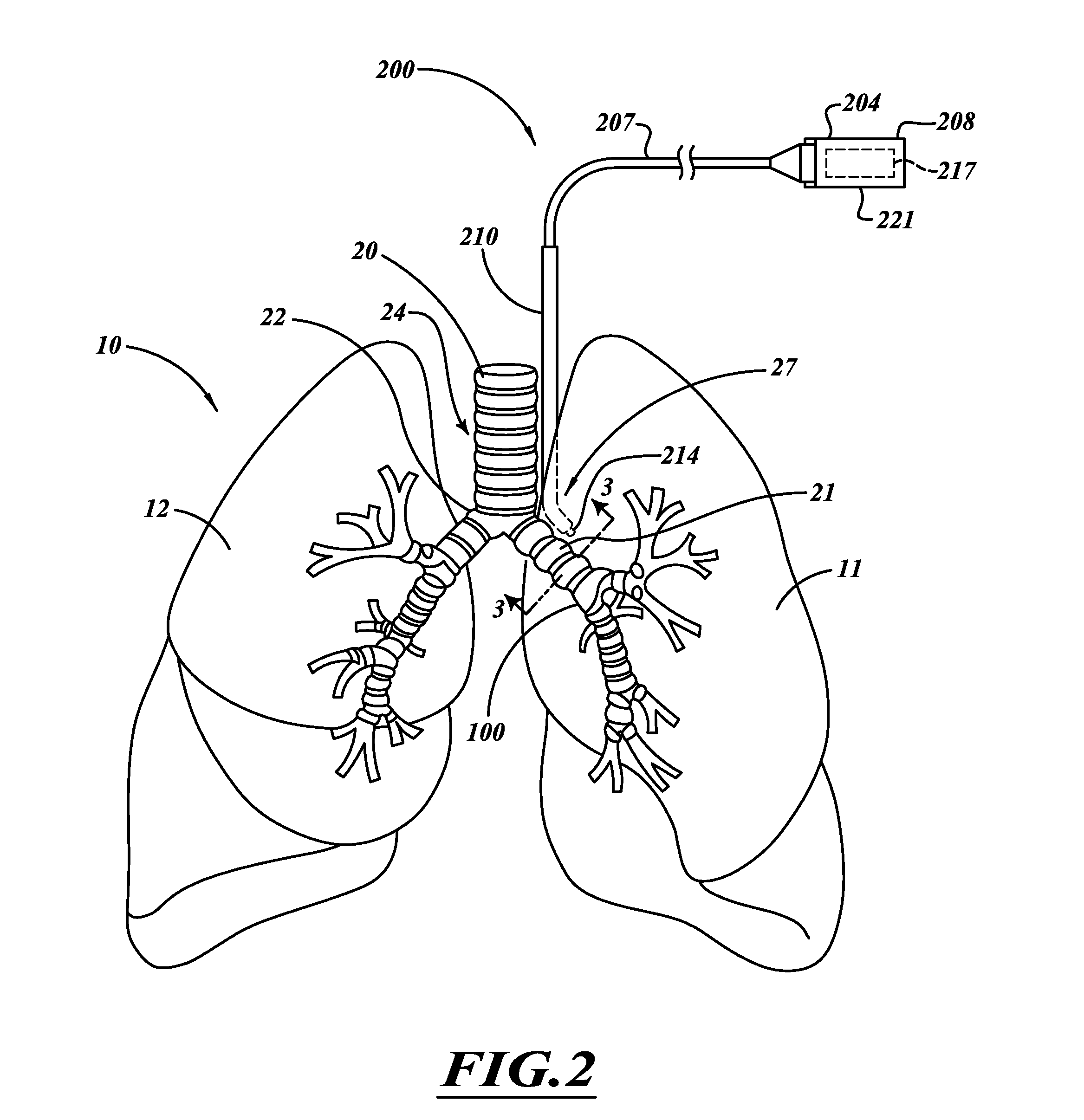
Methods, systems and devices are described for new modes of ventilation in which specific lung areas are ventilated with an indwelling trans-tracheobronchial catheter for the purpose of improving ventilation and reducing hyperinflation in that specific lung area, and for redistributing inspired air to other healthier lung areas, for treating respiratory disorders such as COPD, ARDS, SARS, CF, and TB. Trans-Tracheobronchial Segmental Ventilation (TTSV) is performed on either a naturally breathing or a mechanical ventilated patient by placing a uniquely configured indwelling catheter into a bronchus of a poorly ventilated specific lung area and providing direct ventilation to that area. The catheter can be left in place for extended periods without clinician attendance or vigilance. Ventilation includes delivery of respiratory gases, therapuetic gases or agents and evacuation of stagnant gases, mixed gases or waste fluids. Typically the catheter's distal tip is anchored without occluding the bronchus but optionally may intermittently or continuously occlude the bronchus. TTSV is optionally performed by insufflation only of the area, or by application of vacuum to the area, can include elevating or reducing the pressure in the targeted area to facilitate stagnant gas removal, or can include blocking the area to divert inspired gas to better functioning areas.
Owner:BREATHE TECHNOLOGIES INC
Breathing therapy device and method
ActiveUS20050085868A1Inhibiting respiratory driveRestricts breathingElectrotherapyElectromyographyCOPDControlled breathing
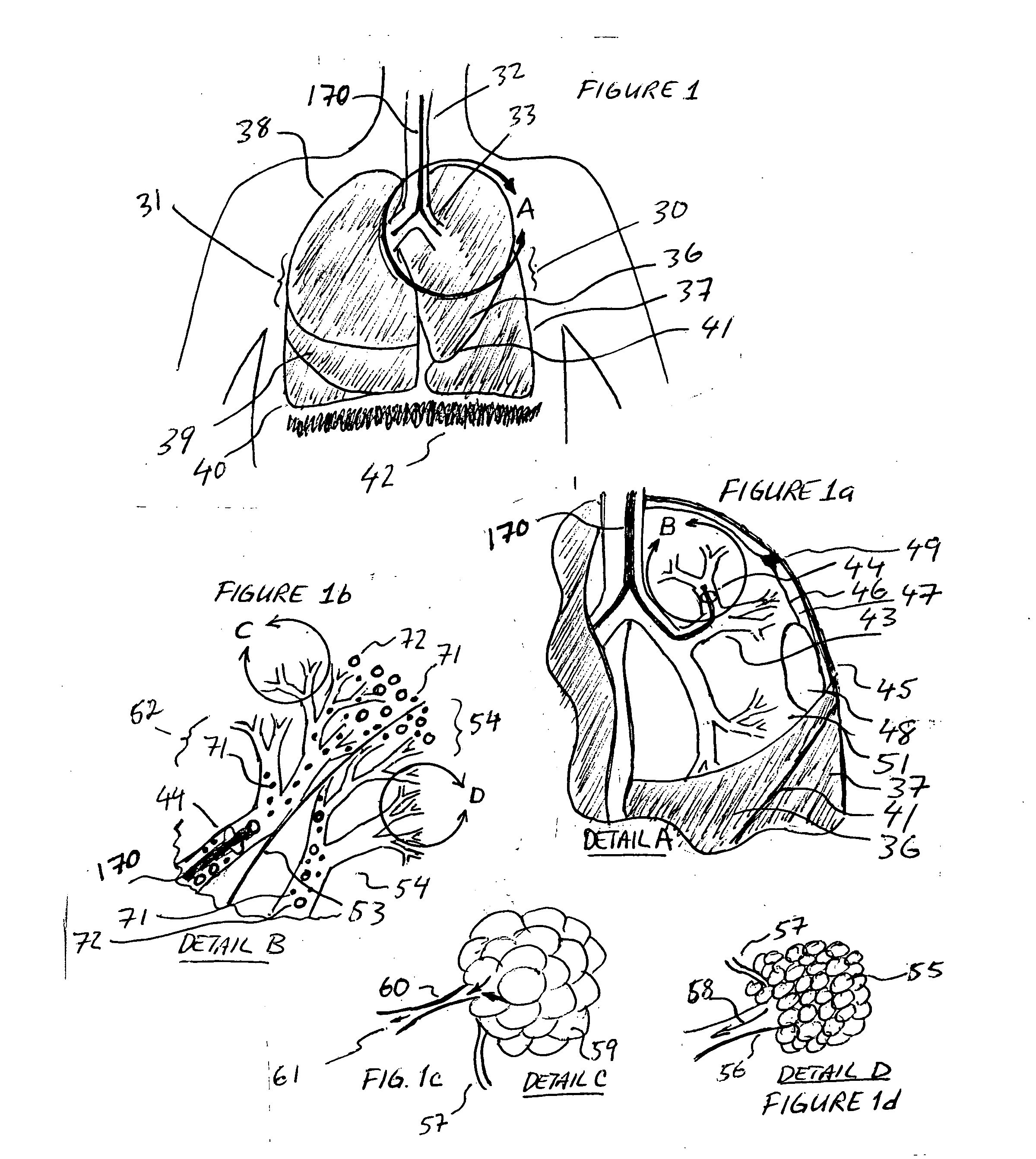
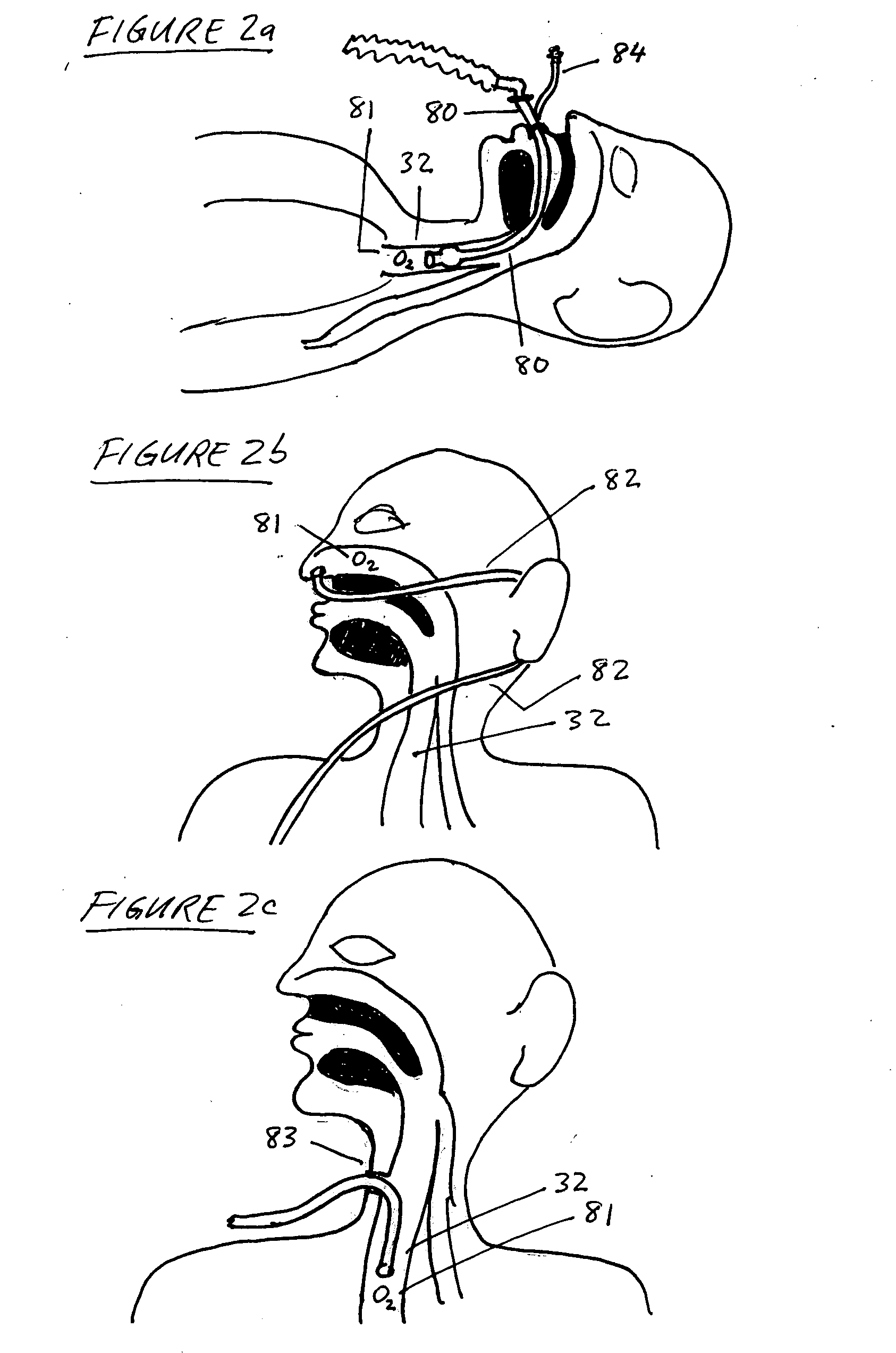
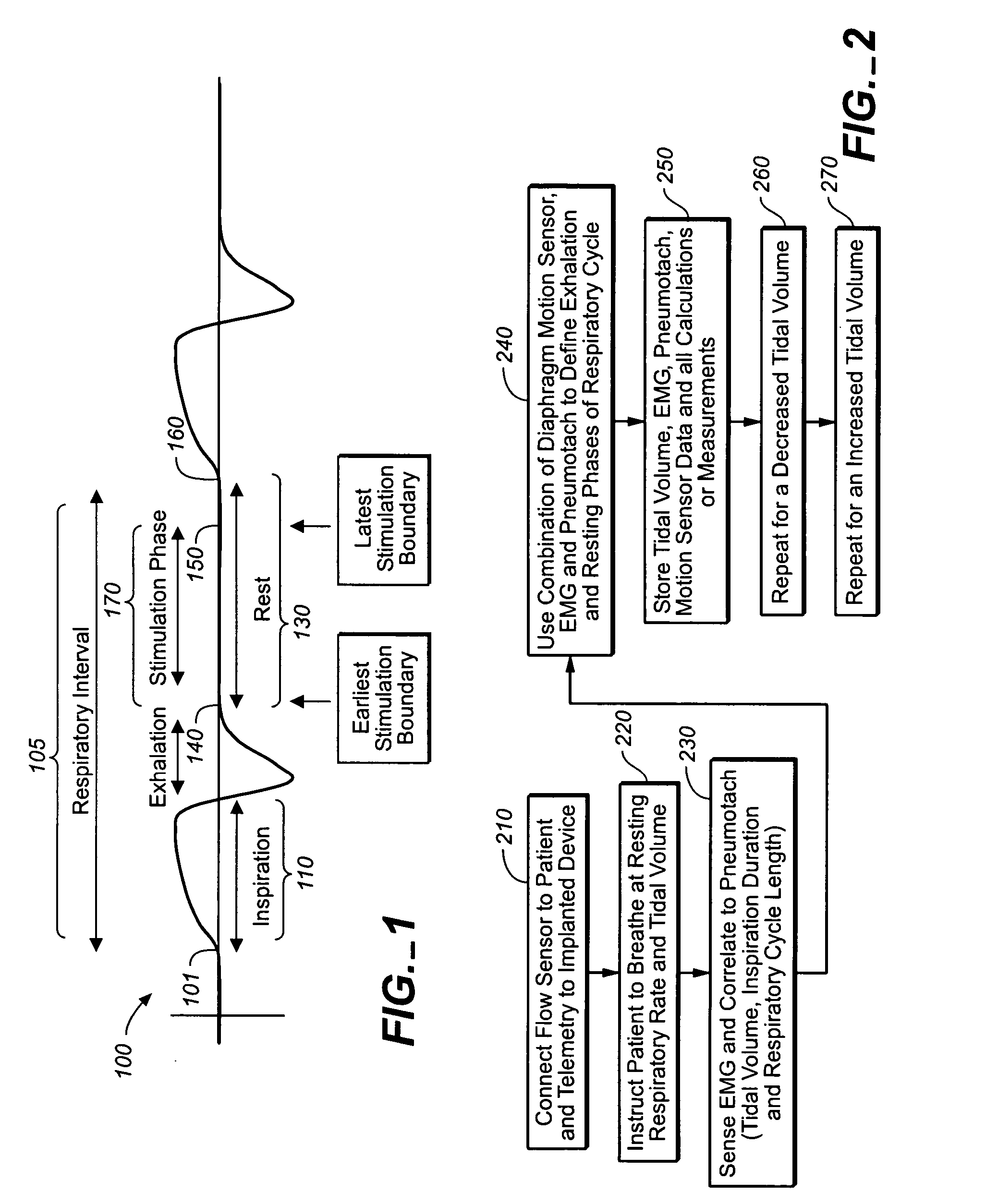
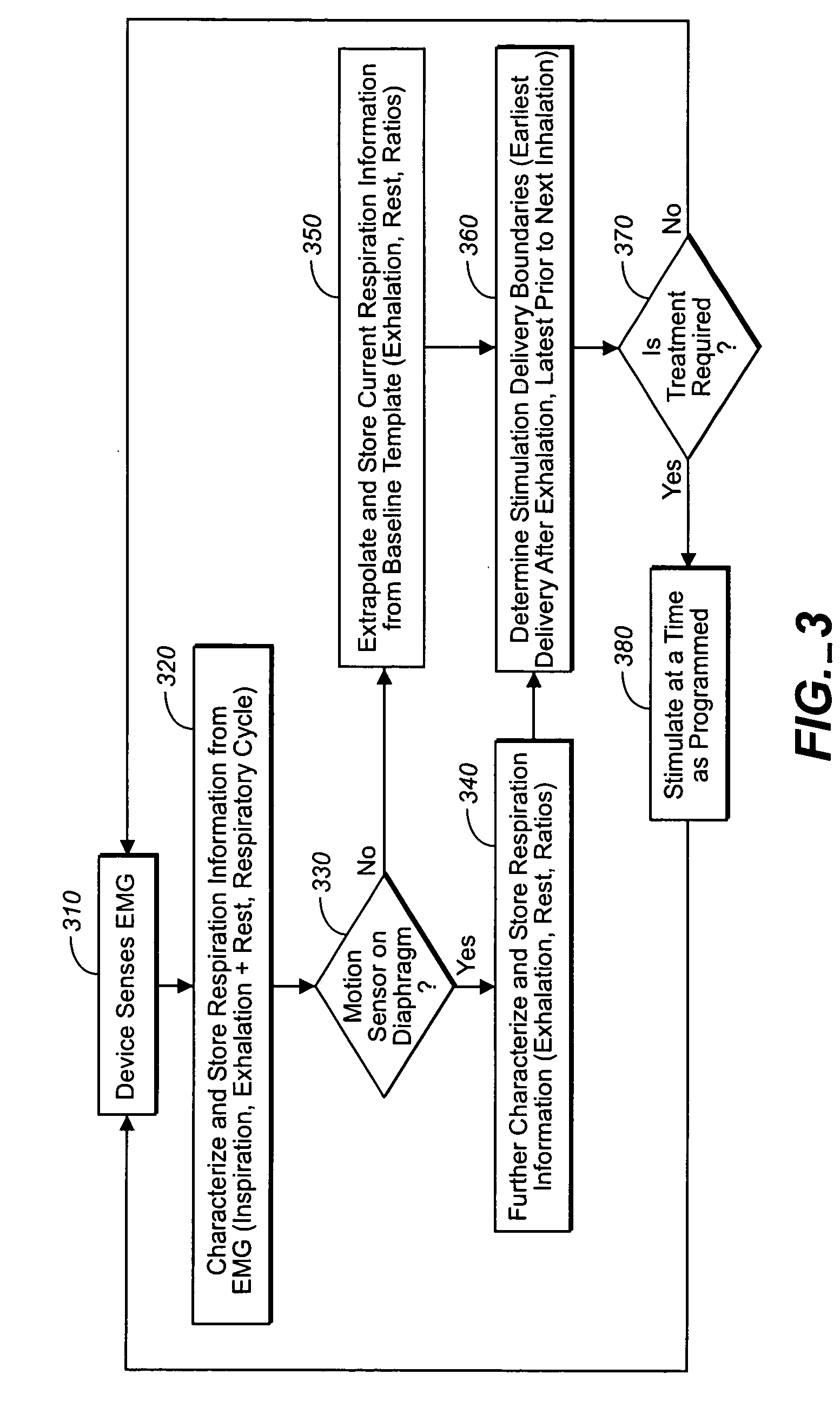
A device and method is provided for electrically stimulating the diaphragm to control breathing while inhibiting respiratory drive. A stimulation phase is identified. The stimulation phase is a period of time within the breathing cycle in which stimulation will inhibit respiratory drive. The respiratory drive inhibition may be used in a number of applications including but not limited to: improving or remodeling the heart in heart failure patients, treating apnea, chronic obstructive pulmonary disorder (COPD), and hypertension.
Owner:RMX
Compositions, formulations and kit with anti-sense oligonucleotide and anti-inflammatory steroid and/or obiquinone for treatment of respiratory and lung disesase
InactiveUS20070021360A1Decreased airwayOrganic active ingredientsBiocideDiseaseAntiendomysial antibodies
A pharmaceutical composition and formulations comprise preventative, prophylactic or therapeutic amounts of an oligo(s) anti-sense to a specific gene(s) or its corresponding mRNA(s), and a glucocorticoid and / or non-glucocorticoid steroid or a ubiquinone or their salts. The agents, composition and formulations are used for treatment of ailments associated with impaired respiration, bronchoconstriction, lung allergy(ies) or inflammation, and abnormal levels of adenosine, adenosine receptors, sensitivity to adenosine, lung surfactant and ubiquinone, such as pulmonary fibrosis, vasoconstriction, inflammation, allergies, allergic rhinitis, asthma, impeded respiration, lung pain, cystic fibrosis, bronchoconstriction, COPD, RDS, ARDS, cancer, and others. The present treatment is effectively administered by itself for conditions without known therapies, as a substitute for therapies exhibiting undesirable side effects, or in combination with other treatments, e.g. before, during and after other respiratory system therapies, radiation, chemotherapy, antibody therapy and surgery, among others. Each of the agents of this invention may be administered directly into the respiratory system so that they gain direct access to the lungs, or by other effective routes of administration. A kit comprises a delivery device, the agents and instructions for its use.
Owner:EPIGENESIS PHARMA LLC
Methods, systems and devices for improving ventilation in a lung area
ActiveUS7588033B2Effective and direct cannulationIncrease hyperinflationTracheal tubesOperating means/releasing devices for valvesDiseasePrimary bronchus
Methods, systems and devices are described for new modes of ventilation in which specific lung areas are ventilated with an indwelling trans-tracheobronchial catheter for the purpose of improving ventilation and reducing hyperinflation in that specific lung area, and for redistributing inspired air to other healthier lung areas, for treating respiratory disorders such as COPD, ARDS, SARS, CF, and TB. Trans-Tracheobronchial Segmental Ventilation (TTSV) is performed on either a naturally breathing or a mechanical ventilated patient by placing a uniquely configured indwelling catheter into a bronchus of a poorly ventilated specific lung area and providing direct ventilation to that area. The catheter can be left in place for extended periods without clinician attendance or vigilance. Ventilation includes delivery of respiratory gases, therapeutic gases or agents and evacuation of stagnant gases, mixed gases or waste fluids. Typically the catheter's distal tip is anchored without occluding the bronchus but optionally may intermittently or continuously occlude the bronchus. TTSV is optionally performed by insufflation only of the area, or by application of vacuum to the area, can include elevating or reducing the pressure in the targeted area to facilitate stagnant gas removal, or can include blocking the area to divert inspired gas to better functioning areas.
Owner:BREATHE TECHNOLOGIES INC
Non-invasive and minimally invasive denervation methods and systems for performing the same
ActiveUS20110118725A1Reduce harmReduce obstructionUltrasound therapySurgical needlesCOPDPartial denervation
A system and method can be used to denervate at least a portion of a bronchial tree. An energy emitter of an instrument is percutaneously delivered to a treatment site and outputs energy to damage nerve tissue of the bronchial tree. The denervation procedure can be performed without damaging non-targeted tissue. Minimally invasive methods can be used to open airways to improve lung function in subjects with COPD, asthma, or the like. Different sections of the bronchial tree can be denervated while leaving airways intact to reduce recovery times.
Owner:NUVAIRA INC
Methods, systems and devices for desufflating a lung area
InactiveUS20050022809A1Lower the volumeOut diffusionTracheal tubesBreathing masksCOPDPositive pressure
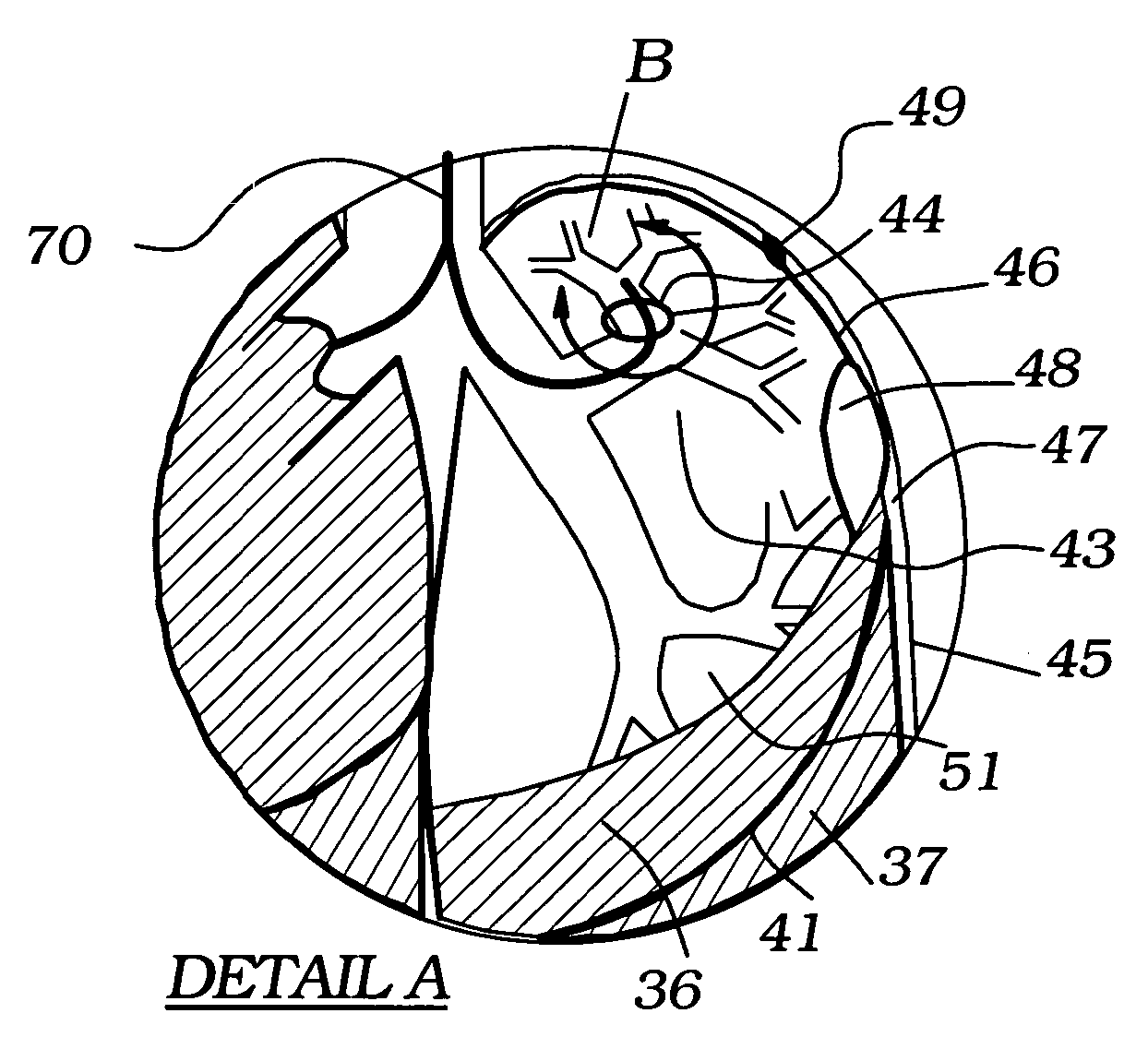
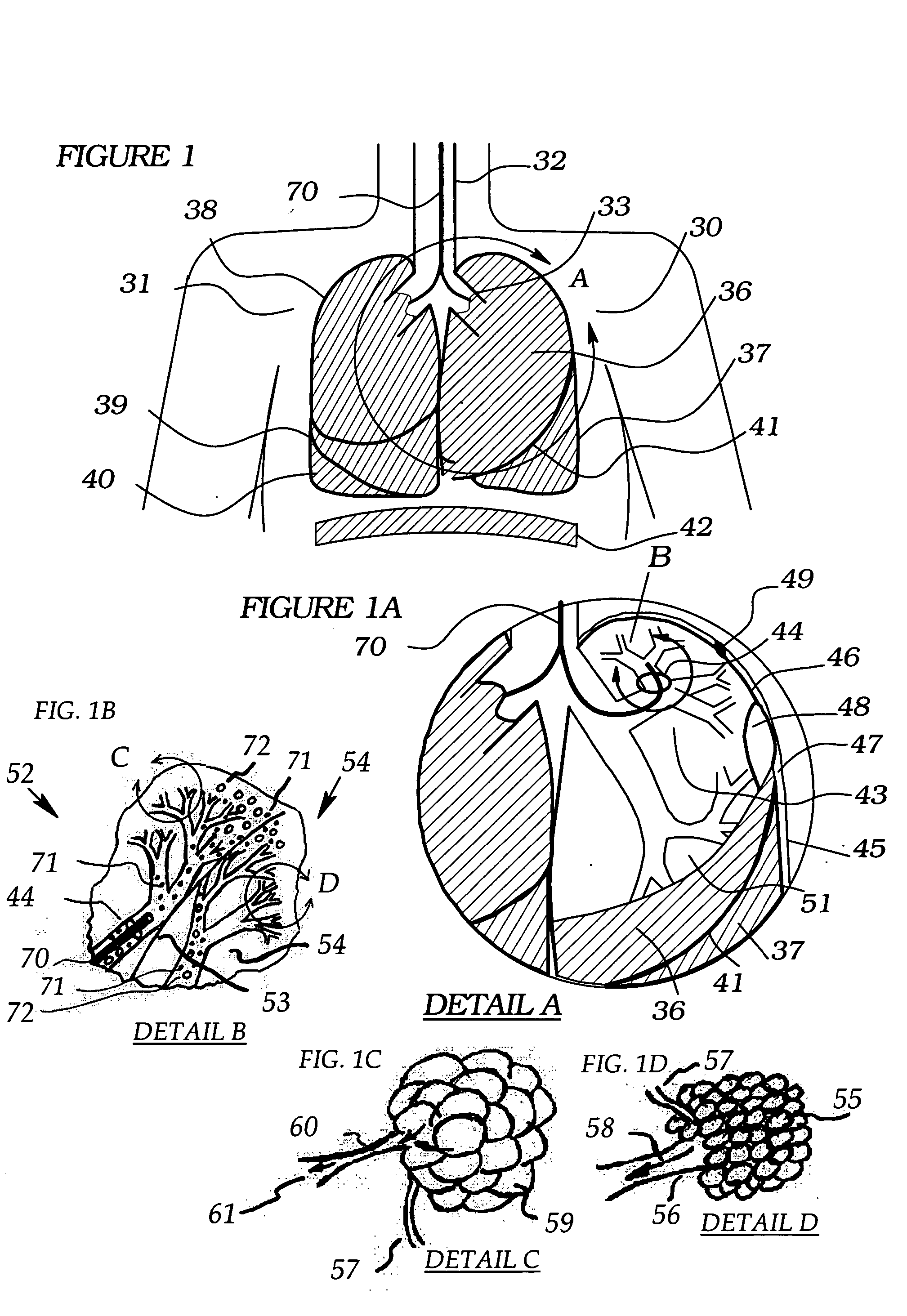
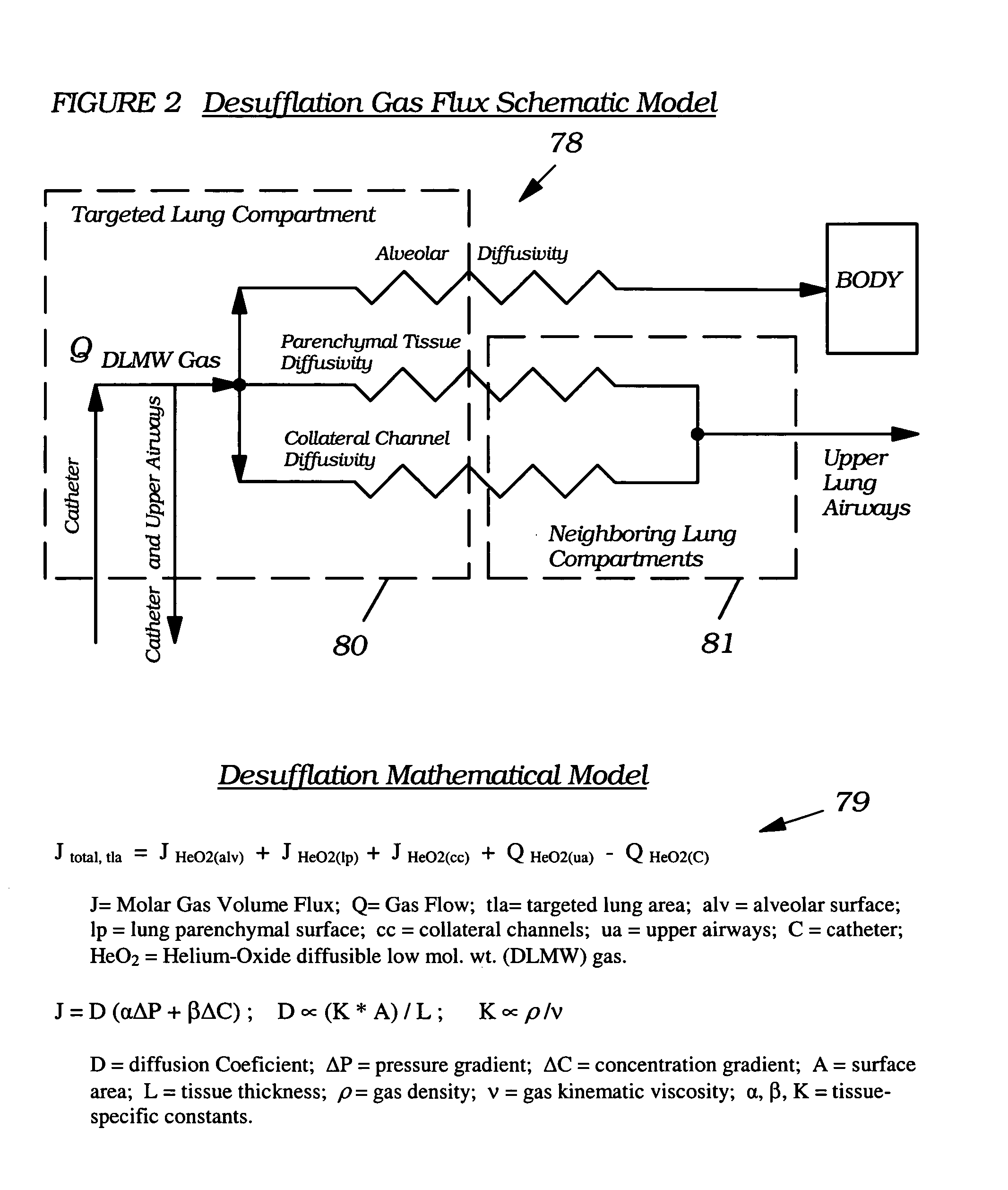
Methods, systems and devices are described for temporarily or permanently evacuating stagnating air from a diseased lung area, typically for the purpose of treating COPD. Evacuation is accomplished by displacing the stagnant CO2-rich air with a readily diffusible gas using a transluminal indwelling catheter specially configured to remain anchored in the targeted area for long term treatment without supervision. Appropriate elevated positive gas pressure in the targeted area is then regulated via the catheter and a pneumatic control unit to force under positive pressure effusion of the diffusible gas out of the area into neighboring areas while inhibiting infusion of other gases thus effecting a gradual gas volume decrease and deflation of the targeted area.
Owner:WONDKA ANTHONY DAVID
Medicament delivery devices
The invention relates to improvements in or relating to medicament delivery devices. In particular, this invention relates to a communications device which may be fitted to an electronic medicament delivery device. The invention may be particularly suitable for use with electronic medicament inhalers such as those used for the treatment of diabetes, or respiratory diseases such as asthma, COPD, cystic fibrosis, and bronchiectasis.
Owner:NEXUS6 LTD
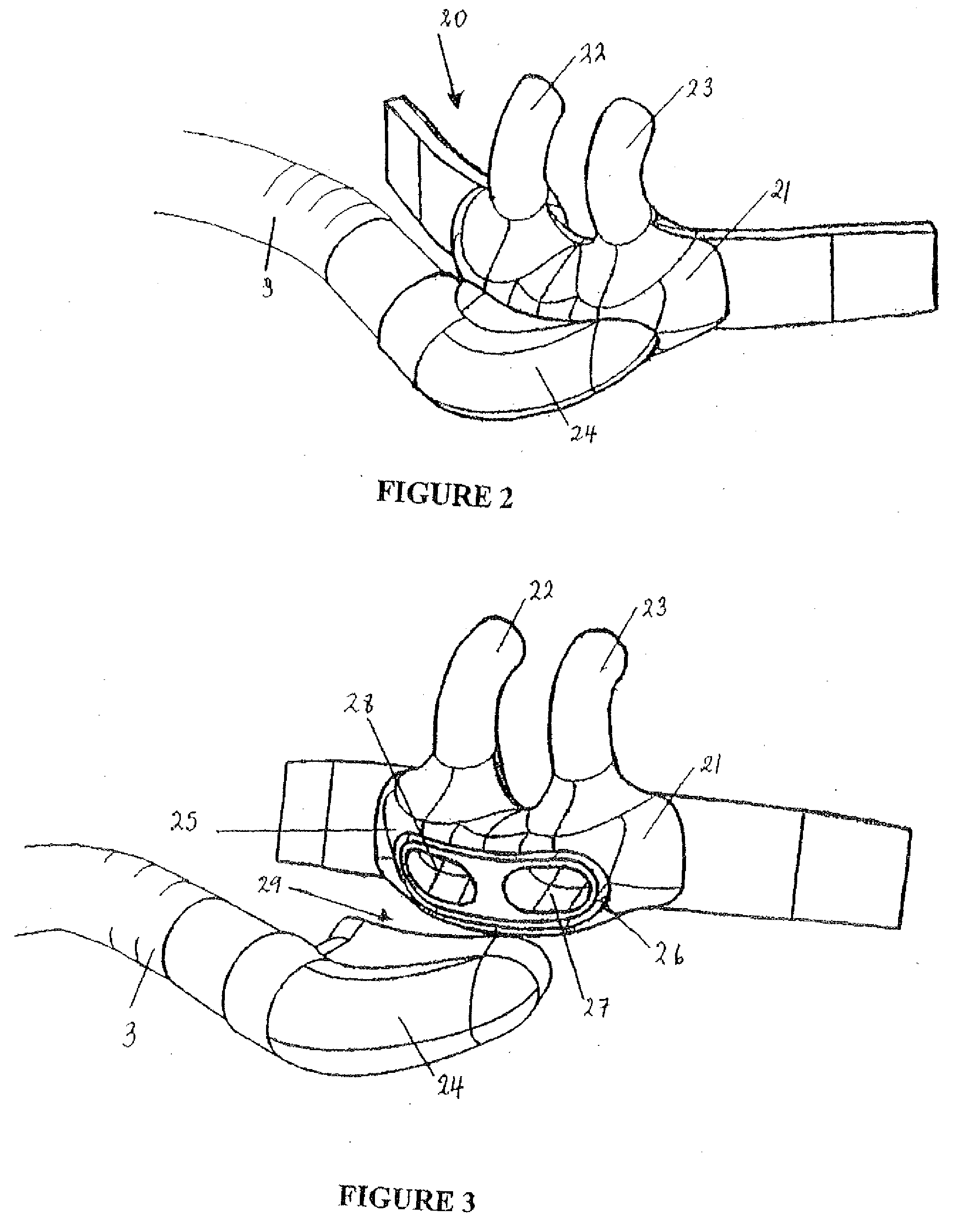
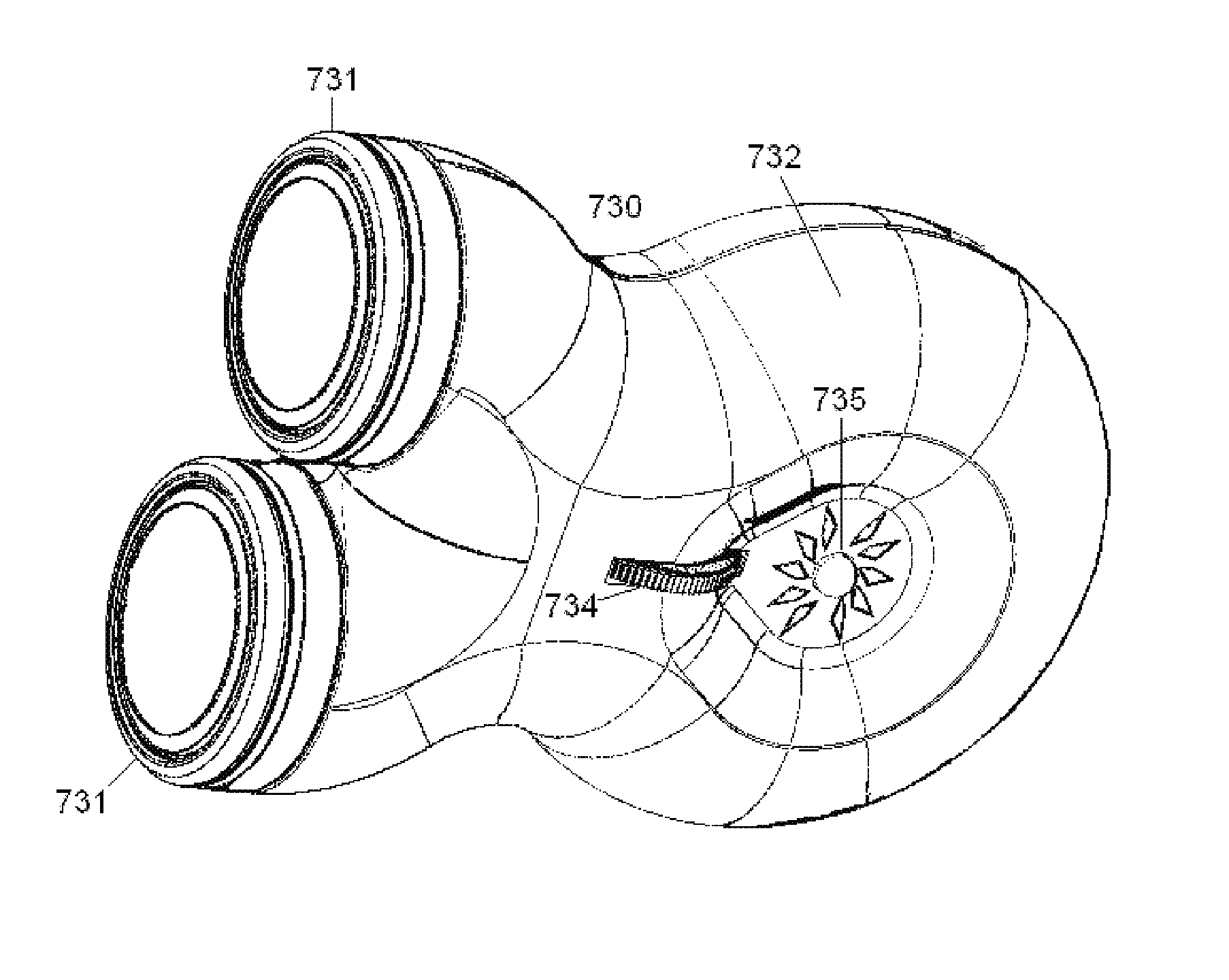
Breathing assistance apparatus
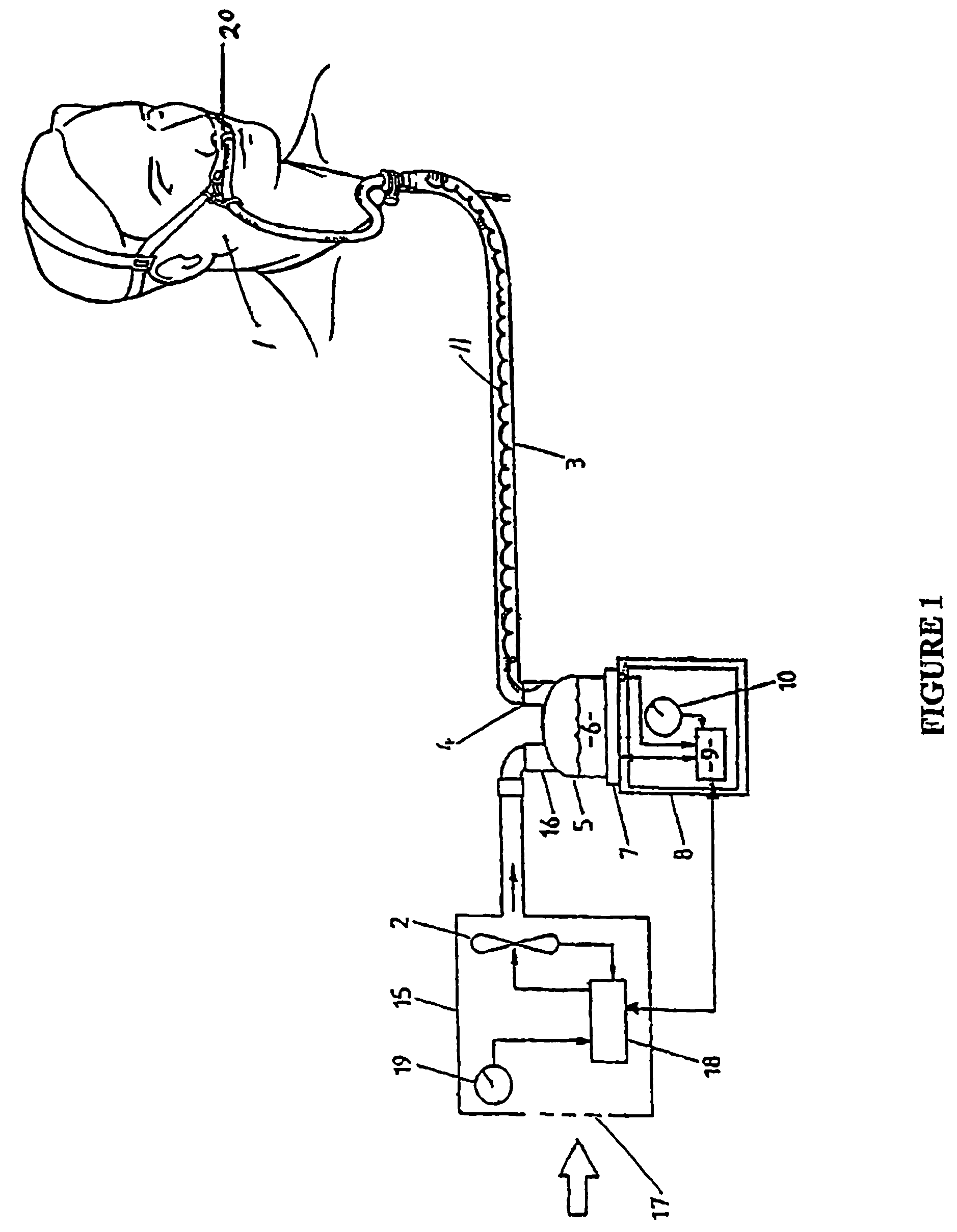
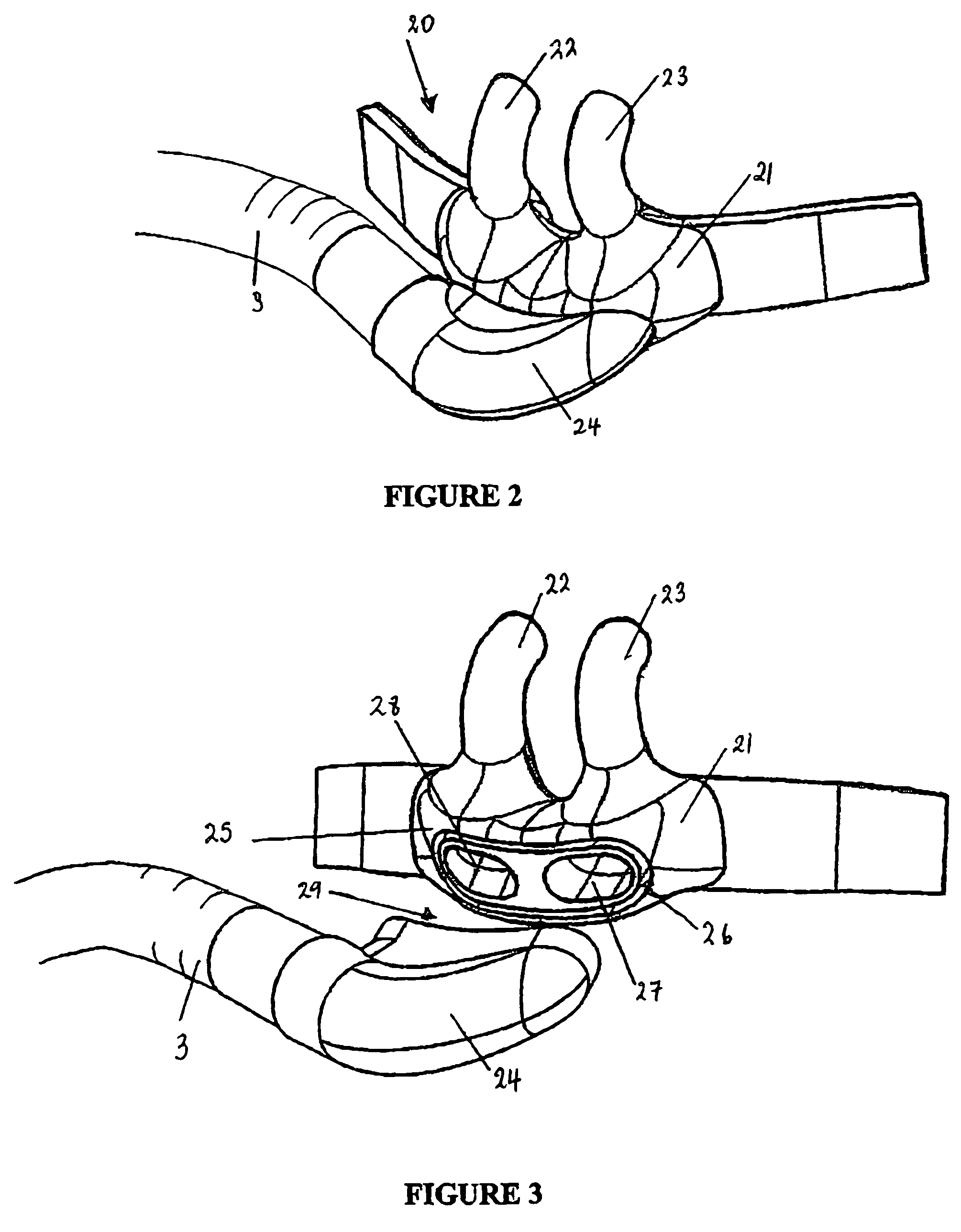
A nasal cannula assembly is disclosed having a face mount part, in use resting against a user's face, which includes at least one nasal prong capable of being fitted into a person's nares. The cannula assembly also includes a manifold part, in fluid communication with the face mount part, having a single horizontal side gases entry. In particular, this cannula assembly is for supplying heated, humidified gases to a patient suffering from COPD. A tie or lanyard is disclosed for use with a breathing assistance apparatus such as a nasal cannula, face or nasal mask or tracheostomy connector. The tie or lanyard transfers the weight of the conduits supplying gases to the breathing assistance apparatus from the breathing assistance apparatus and distributes it onto the neck of the patient.
Owner:FISHER & PAYKEL HEALTHCARE LTD
Methods, systems & devices for endobronchial ventilation and drug delivery
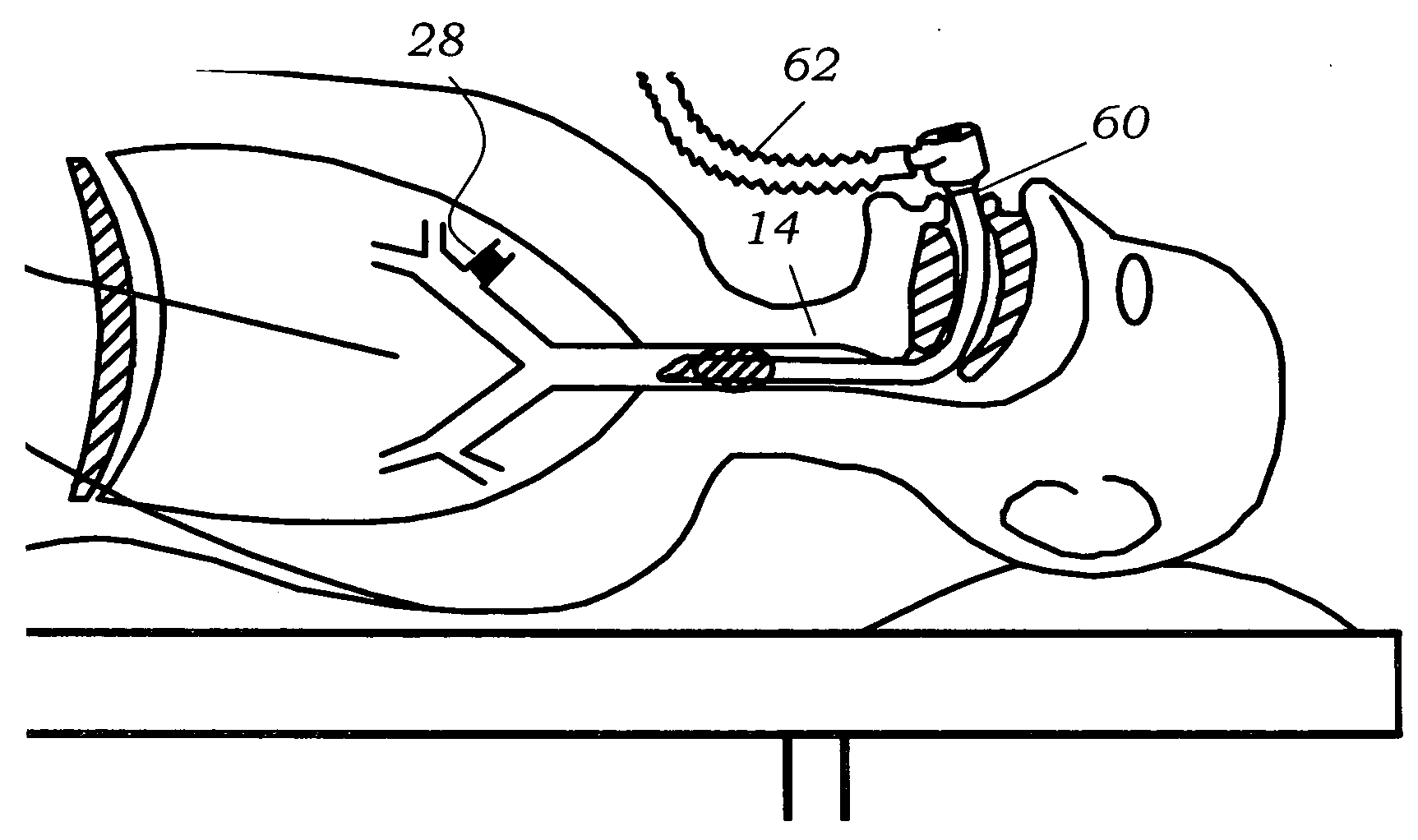
Methods, systems and devices are described for Endobronchial Ventilation using an endobronchially implanted ventilator for the purpose of treating COPD, emphysema and other lung diseases. Endobronchial drug delivery is also described using an endobronchially implanted drug pump, for therapeutic treatment of the lung or of other organs and tissues.
Owner:WONDKA ANTHONY DAVID
Apparatus and method for lung analysis
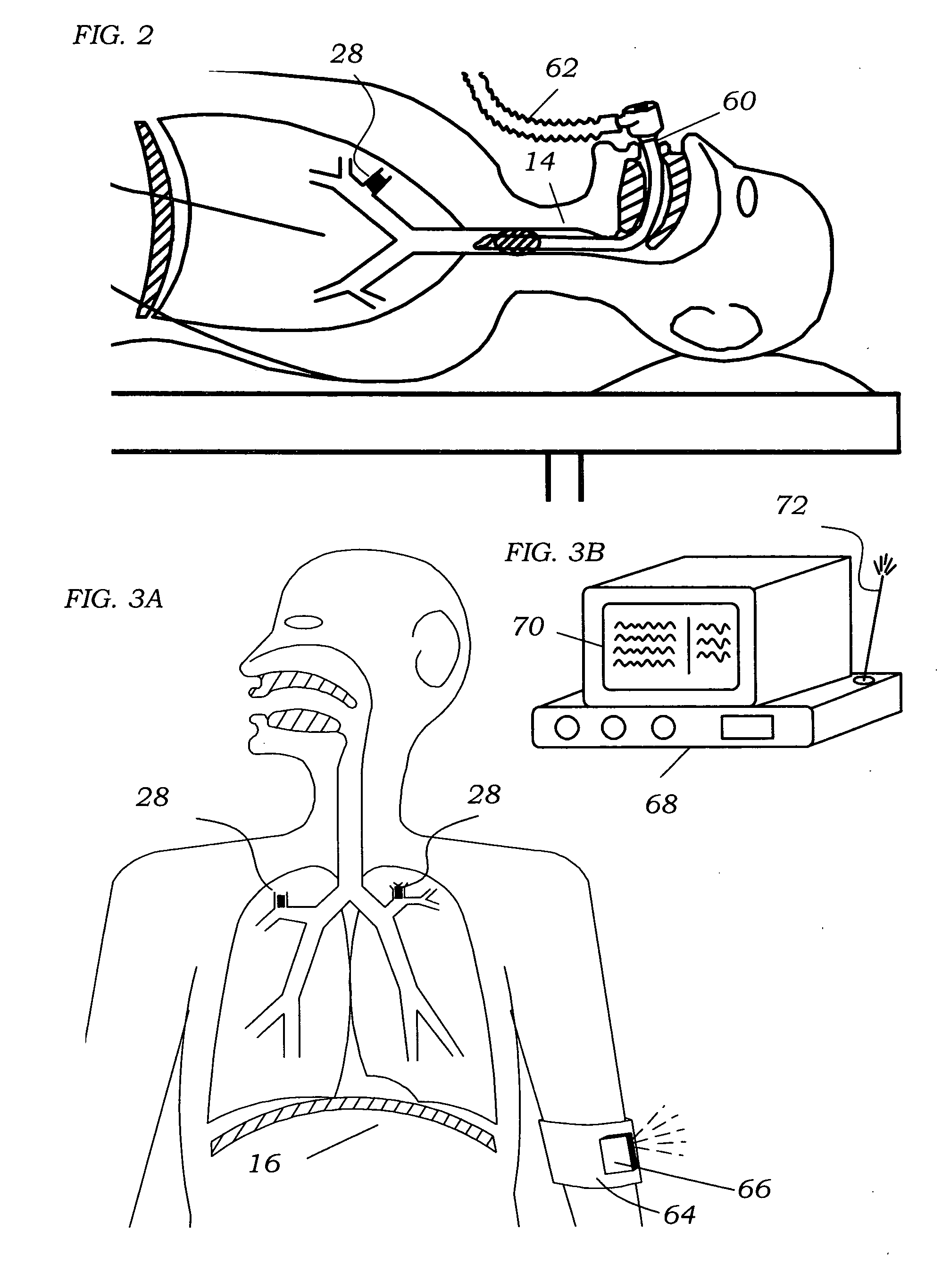
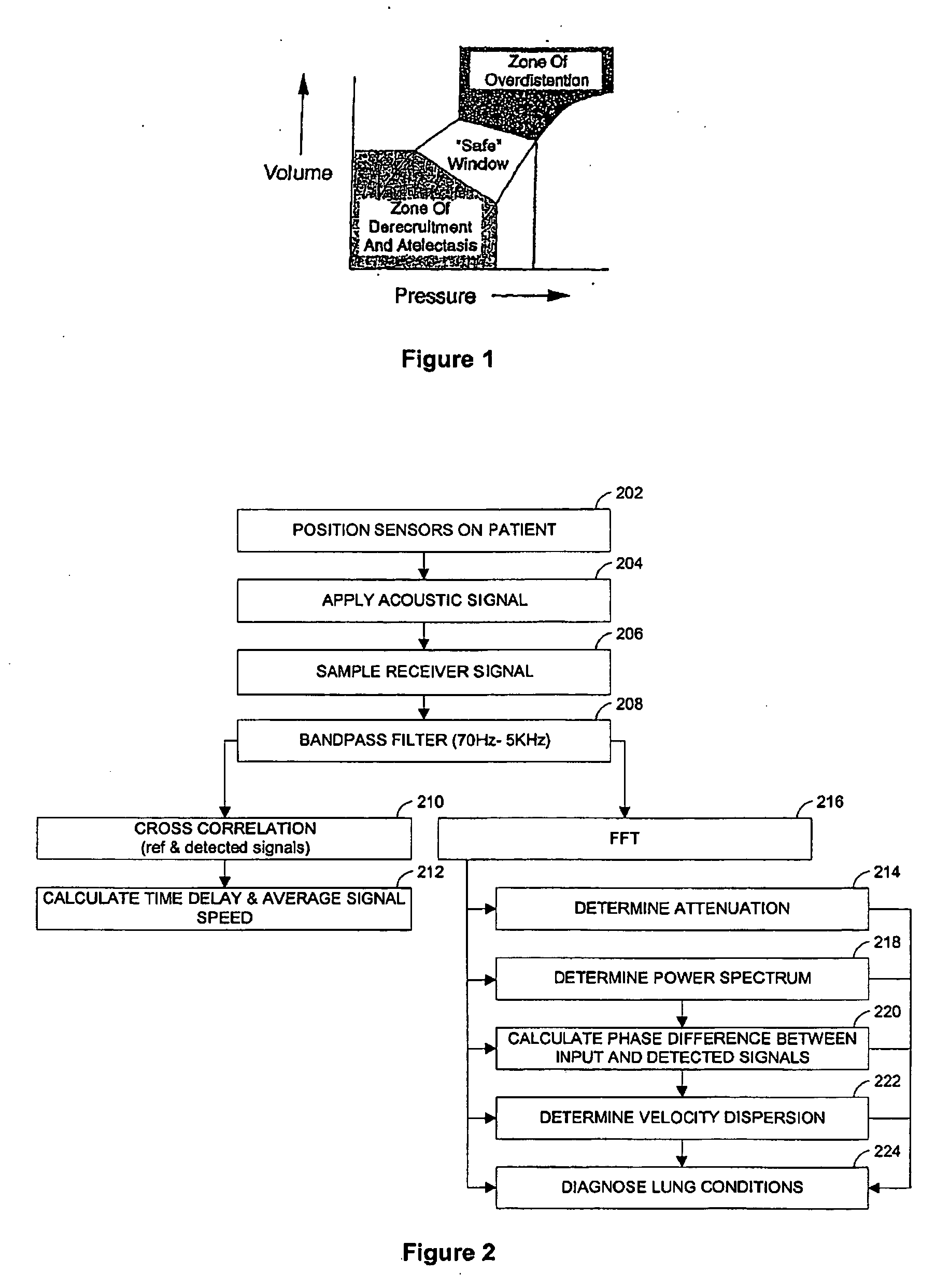
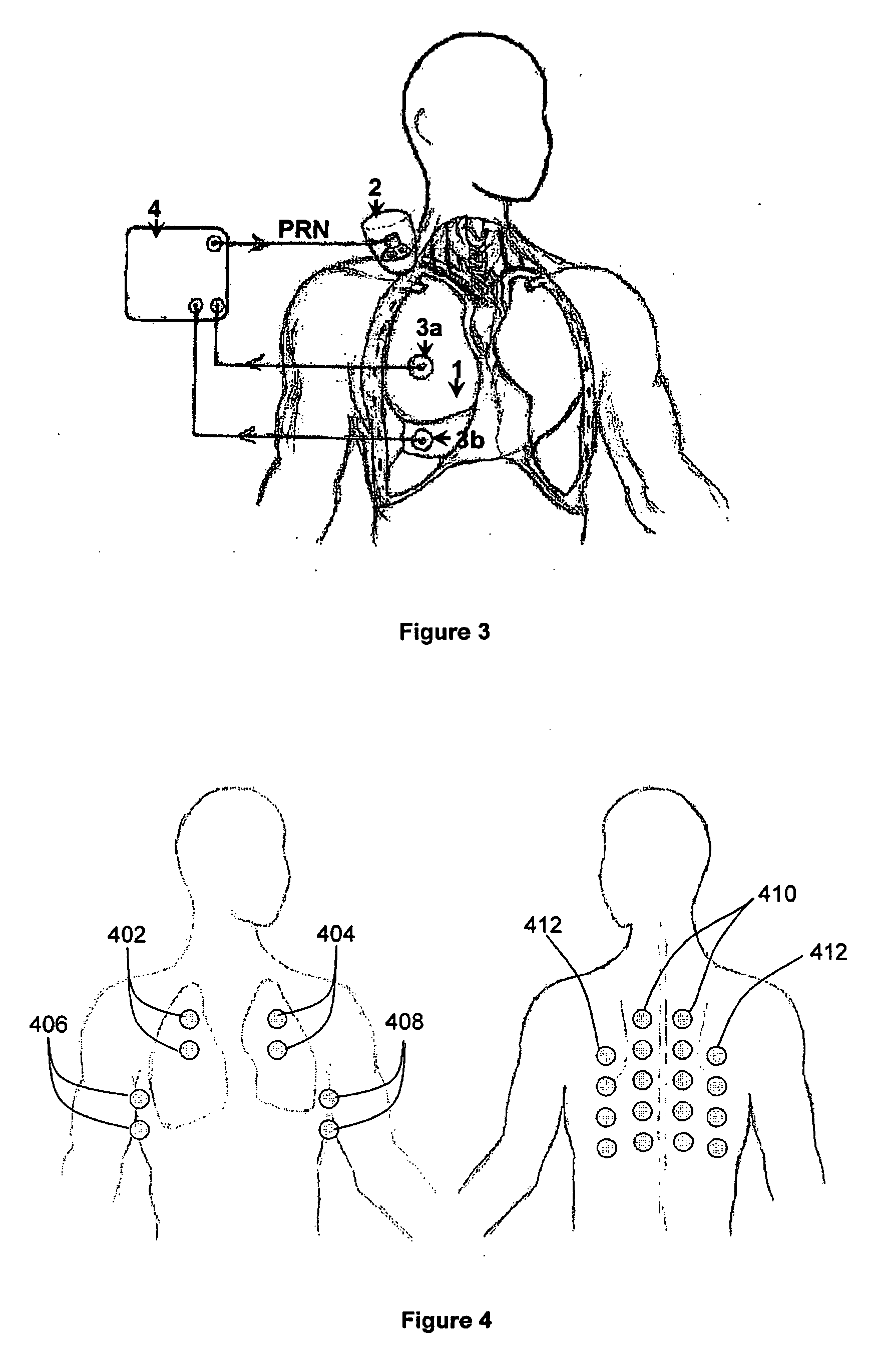
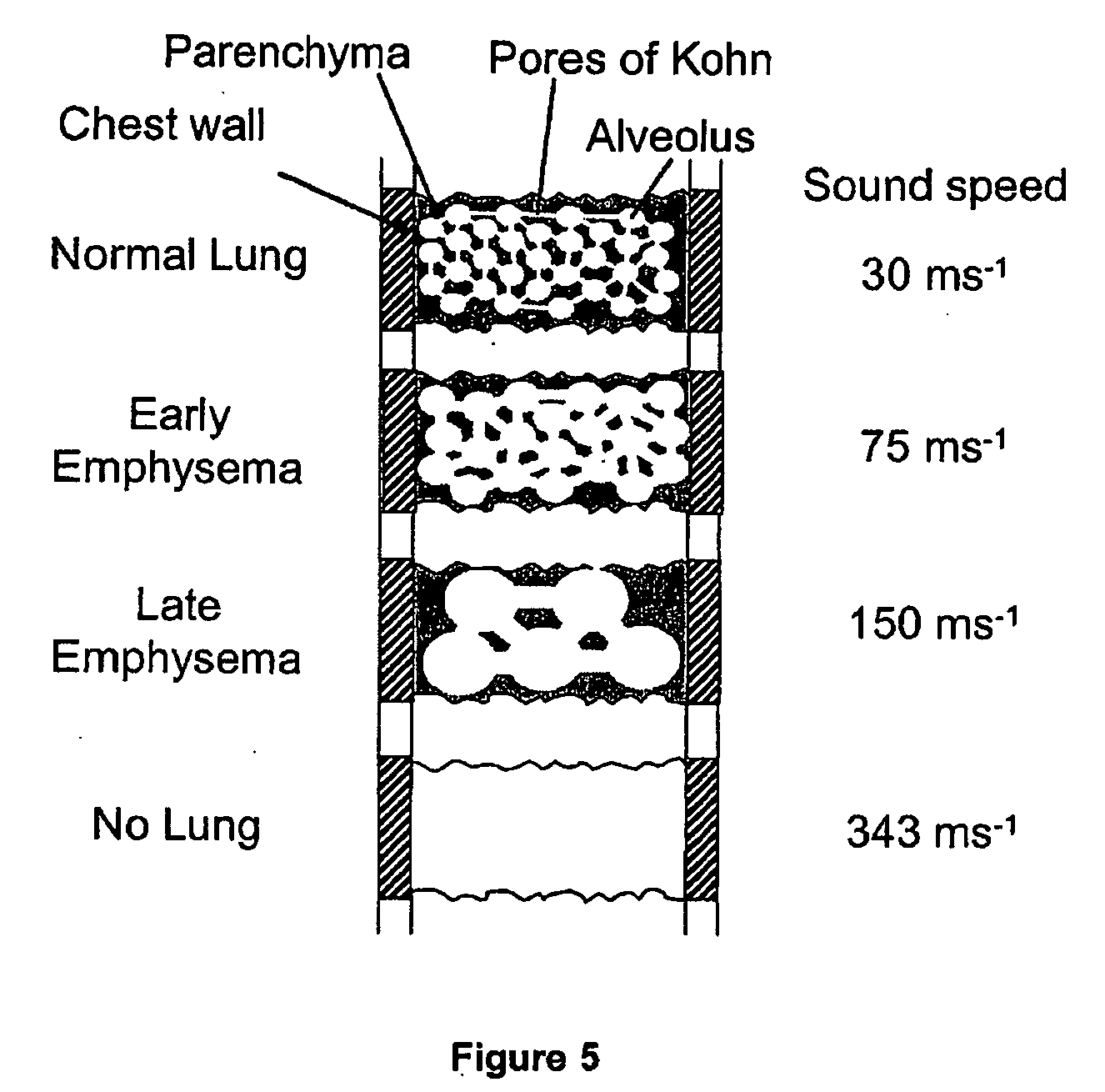
InactiveUS20060100666A1Reliable and reproducible transducer positioningEnhanced couplingOrgan movement/changes detectionHeart defibrillatorsAcoustic transmissionCOPD
An apparatus and method of detecting COPD and in particular, emphysema utilizes a change in acoustic transmission characteristics of a lung due to e.g. the appearance of fenestrae (perforations) in the alveoli of the lung. The use of acoustic signals may provide good sensitivity to the existence of alveolar fenestrae, even for microscopic emphysema, and the appearance and increase in fenestrae may be determined by monitoring acoustic transmission characteristics such as, for example, an increase in acoustic signal velocity and velocity dispersion, and / or a change in attenuation. A transmitter may be located in e.g. the supra-clavicular space and receivers may be mounted on the chest. Measurements may be correlated between pairs of receivers to determine acoustic transmission profiles.
Owner:PULMOSONIX
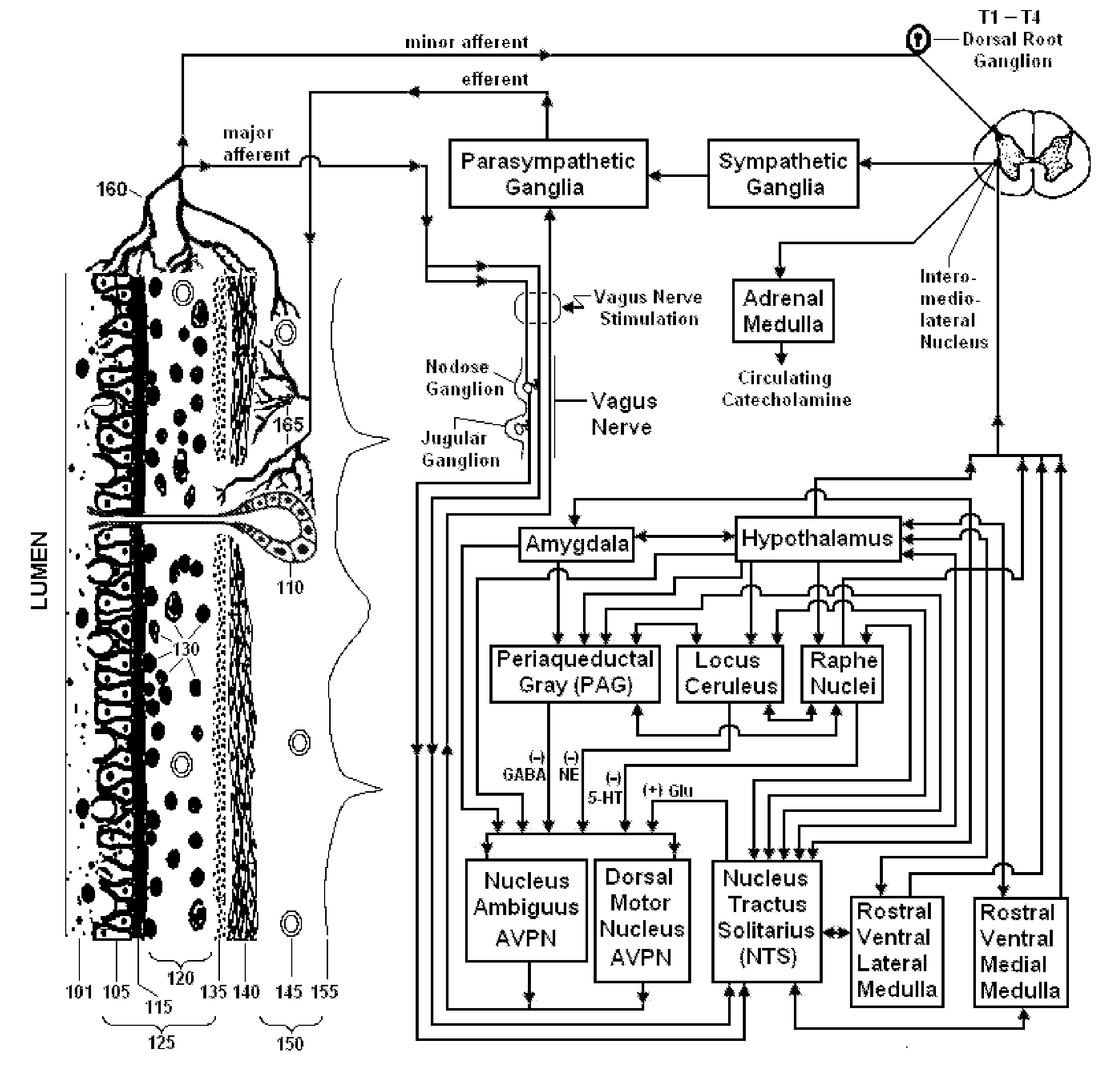
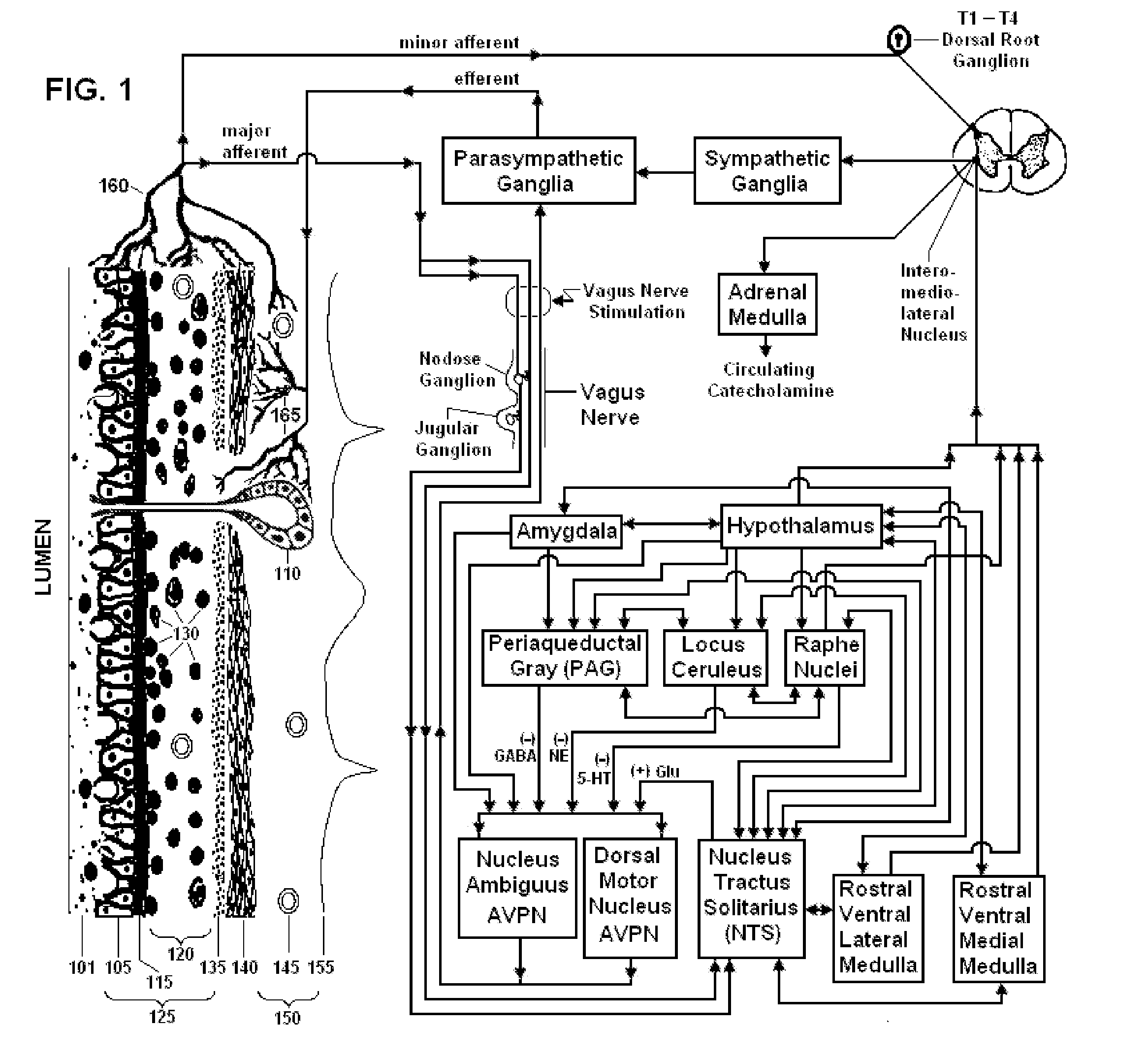
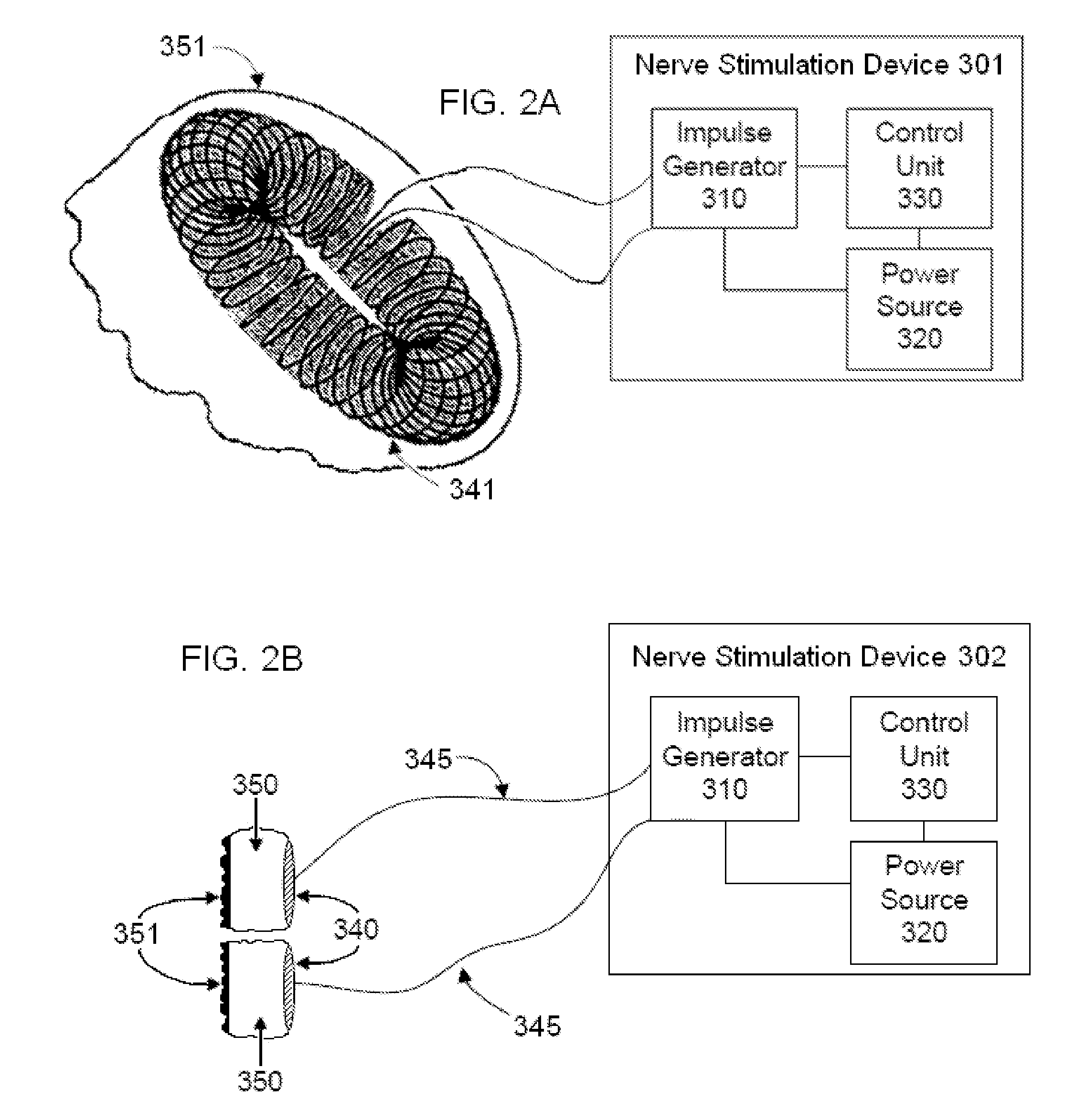
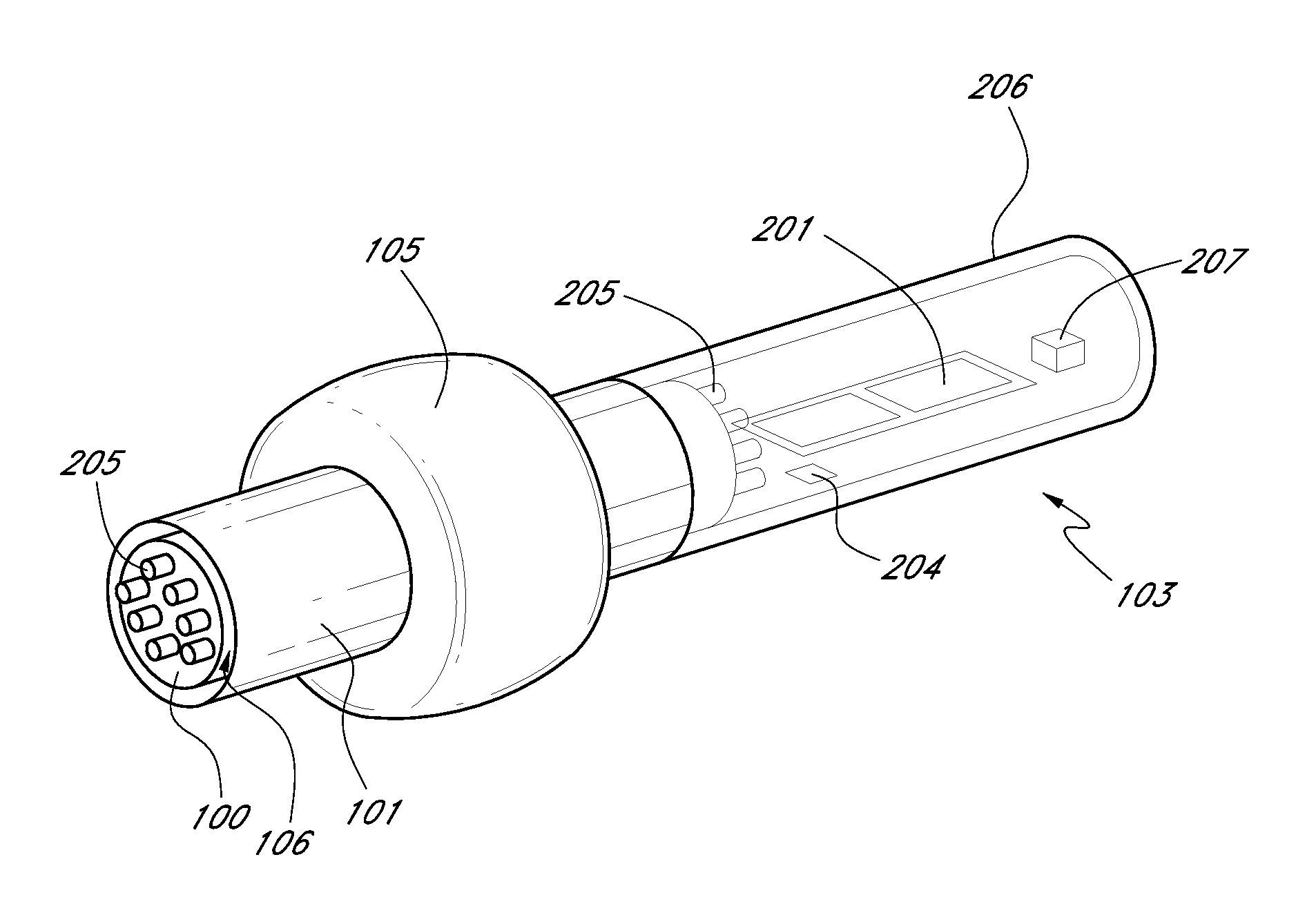
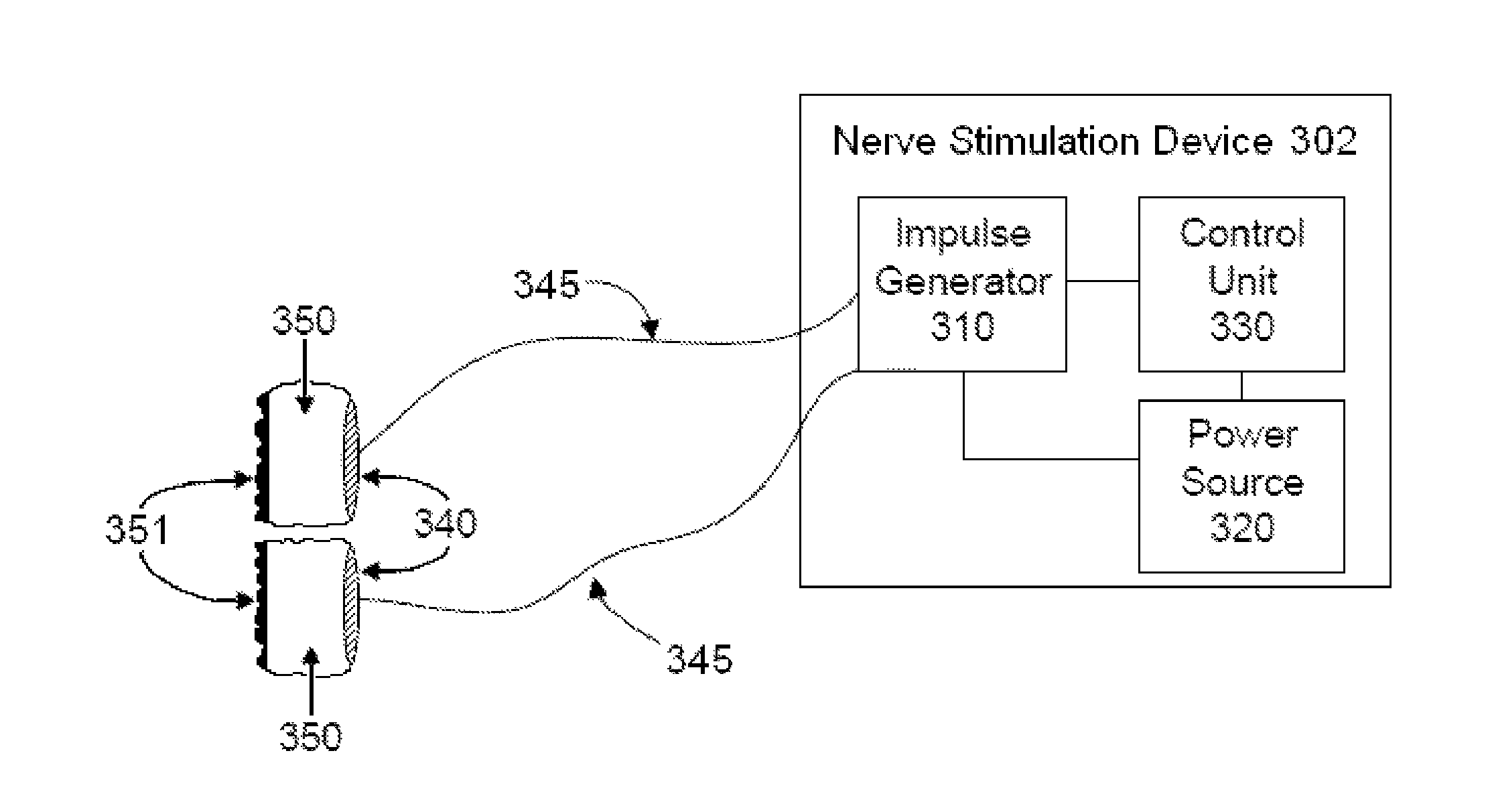
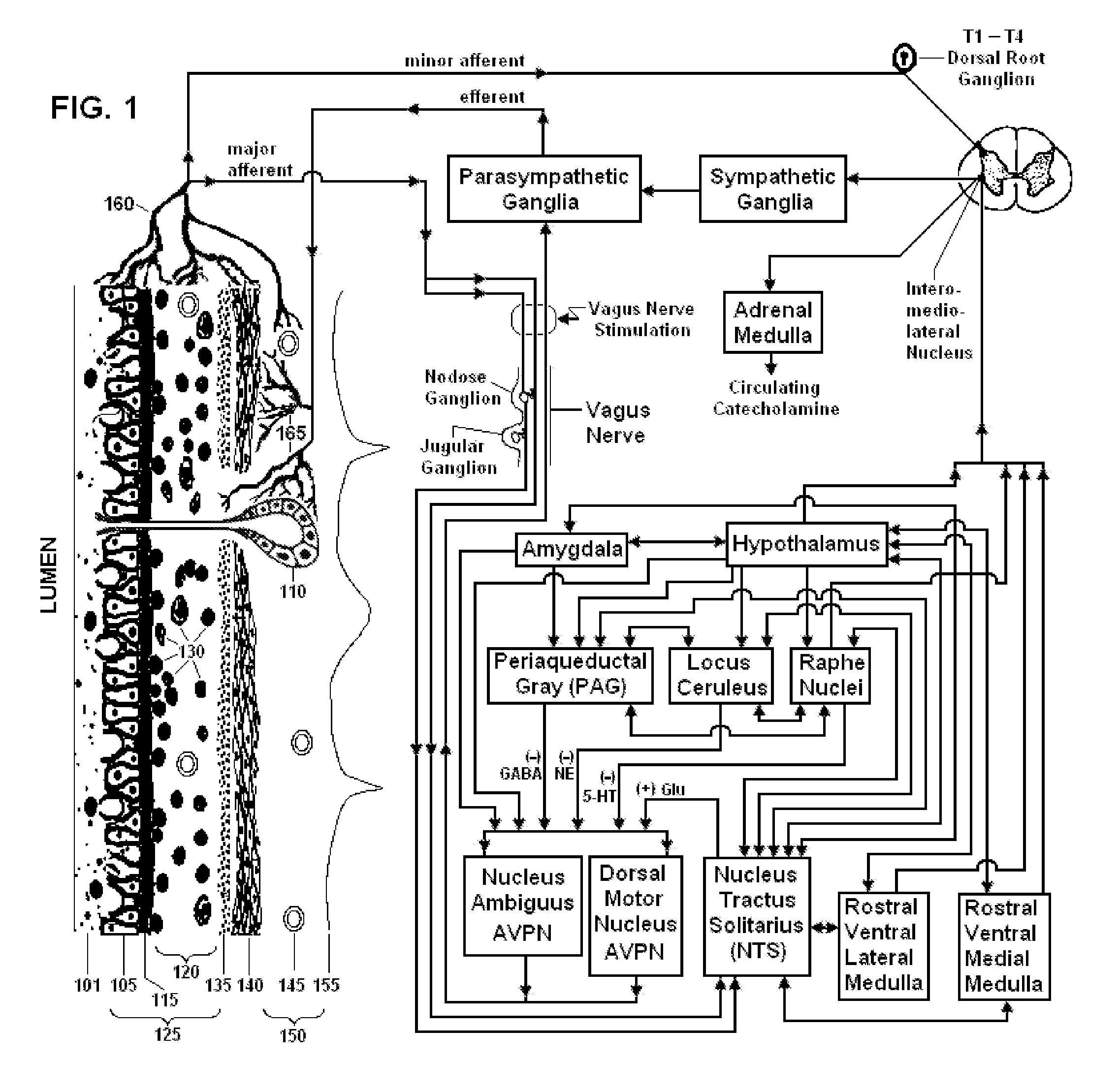
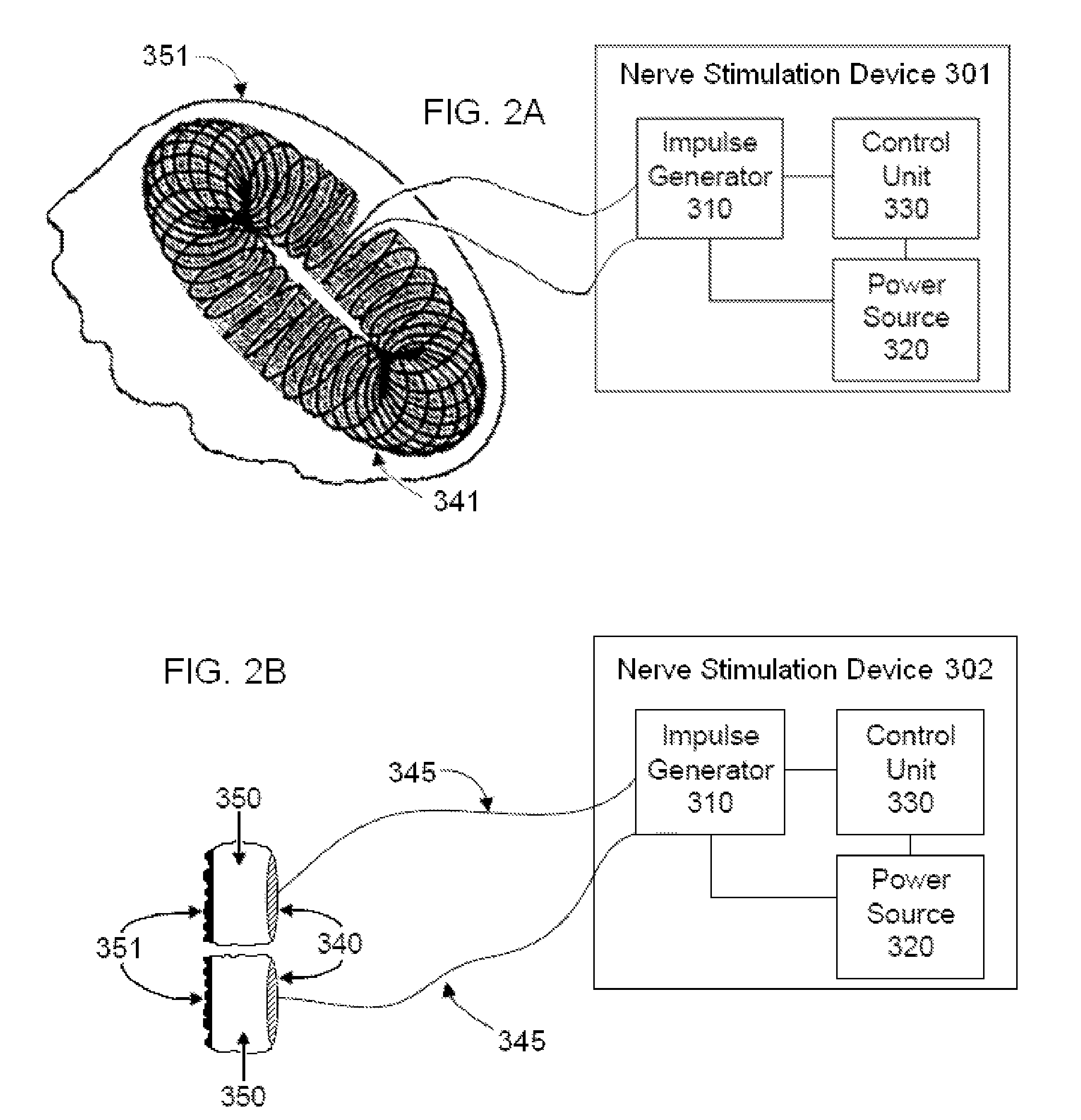
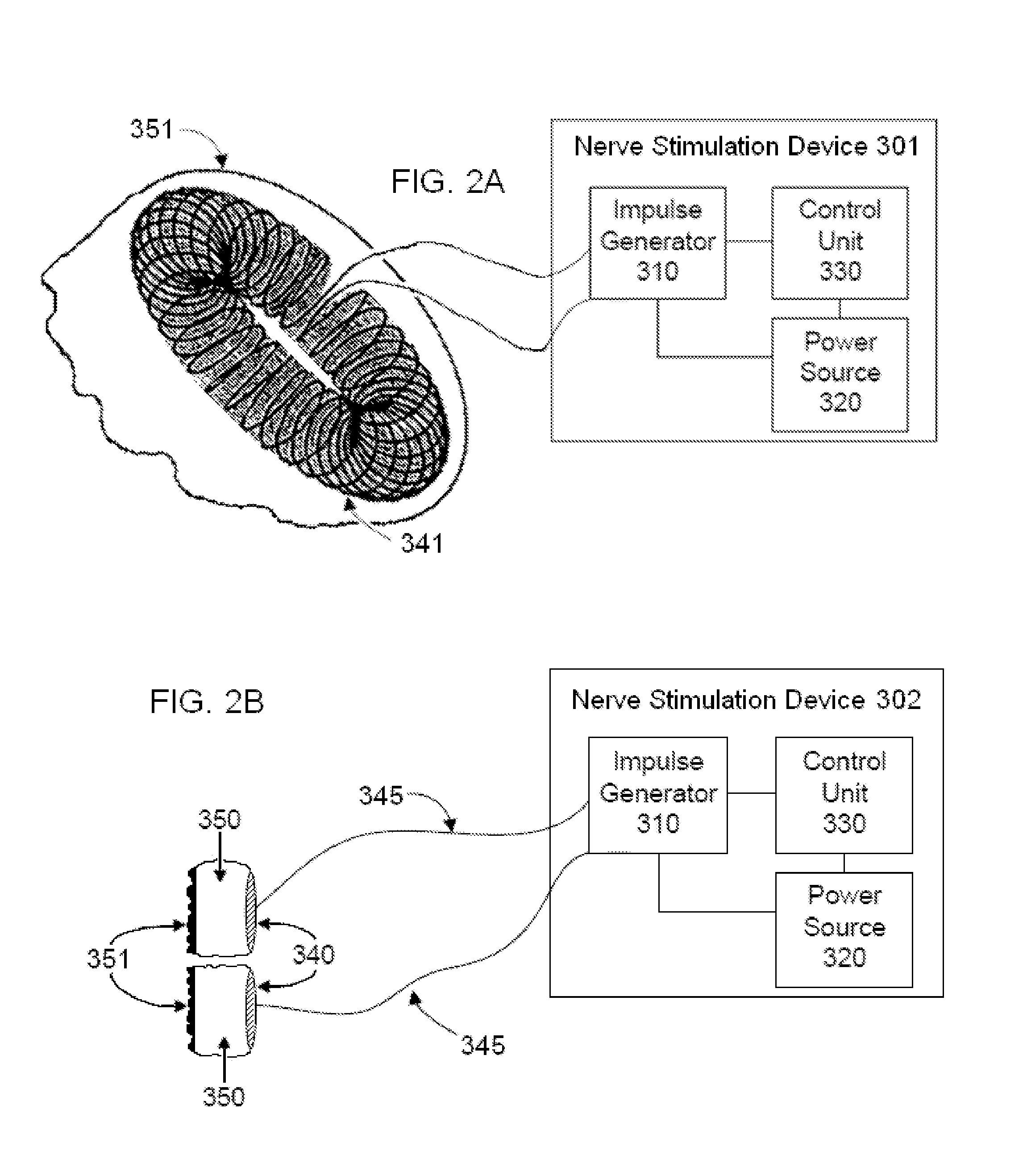
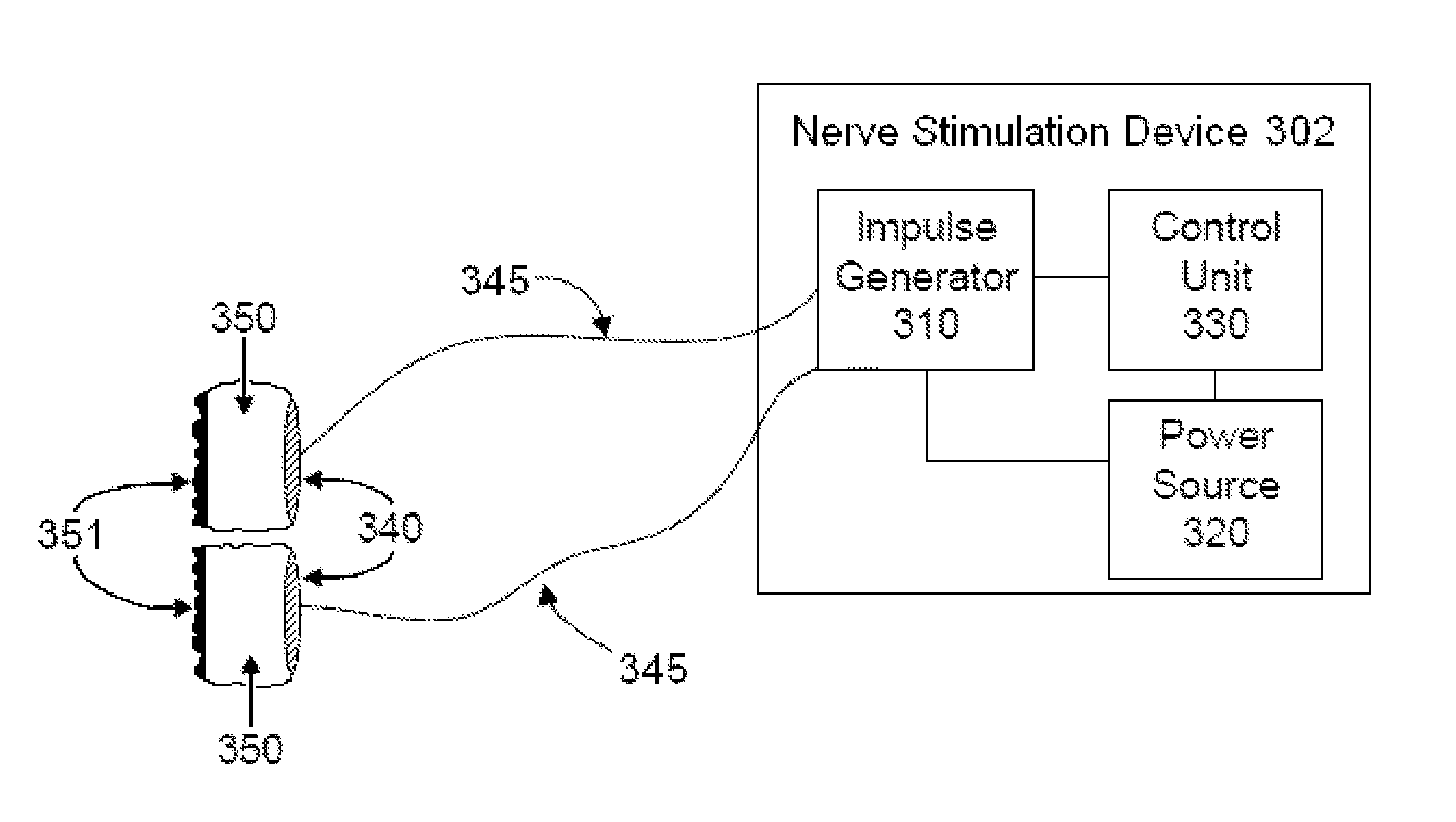
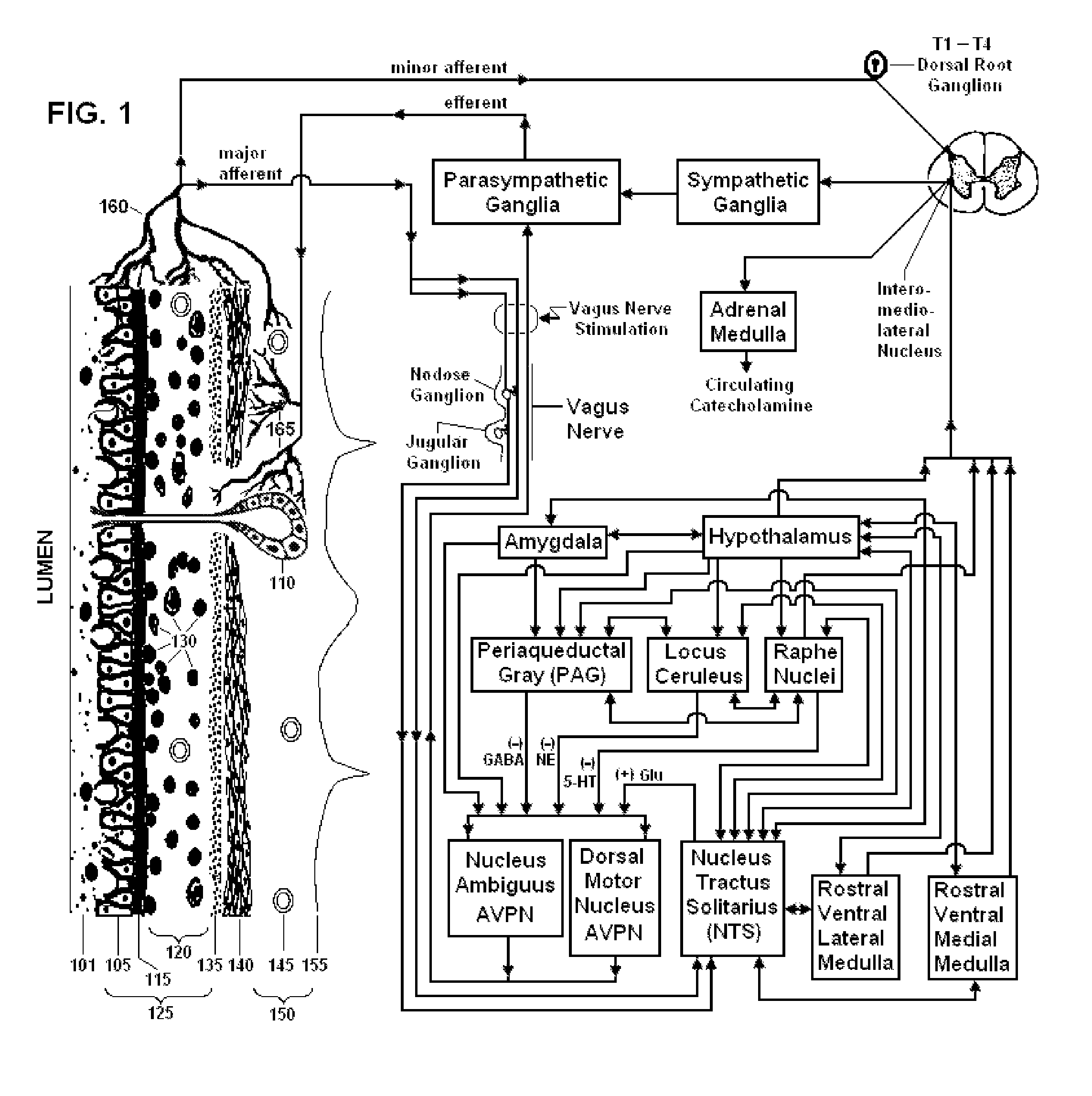
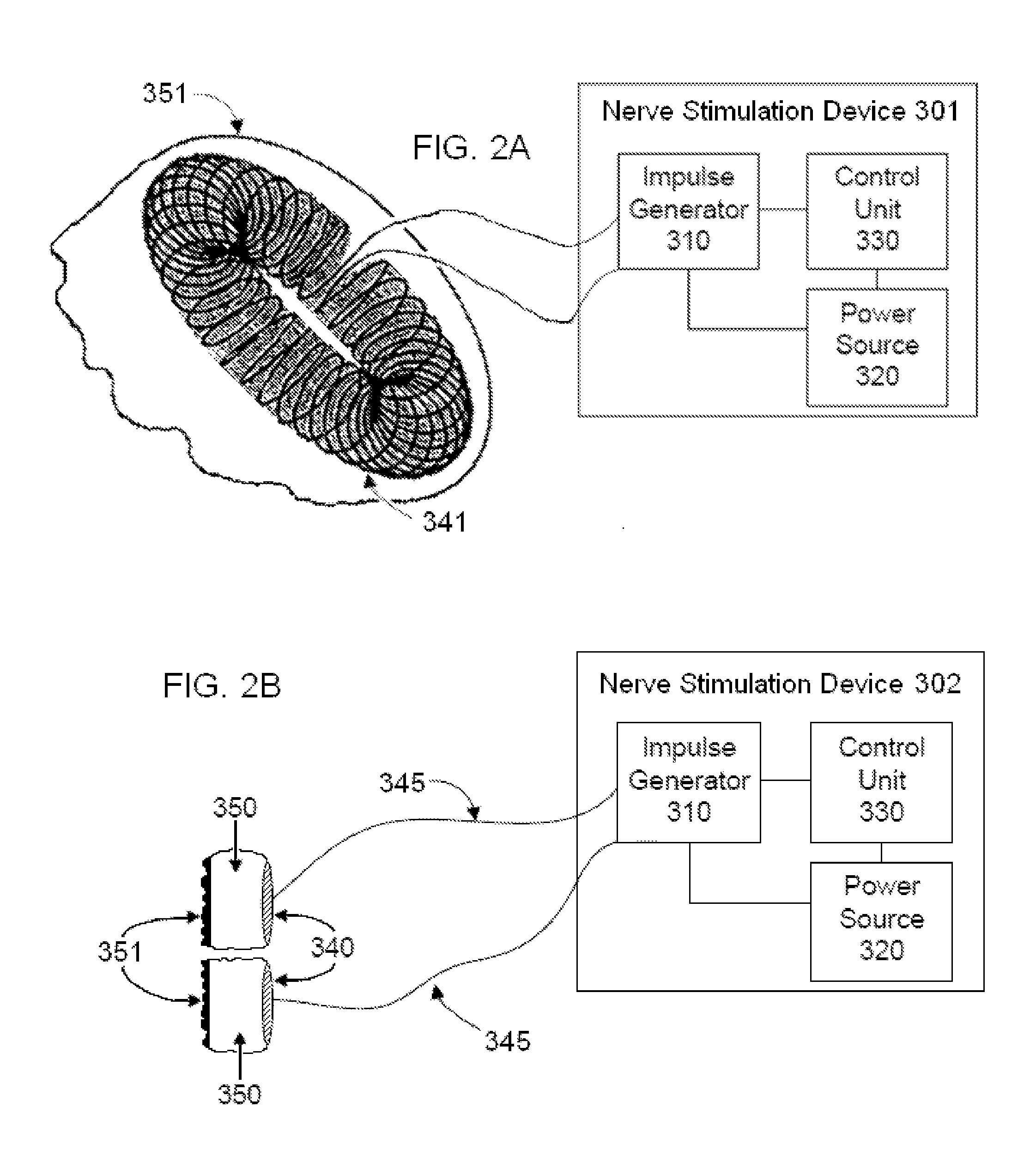
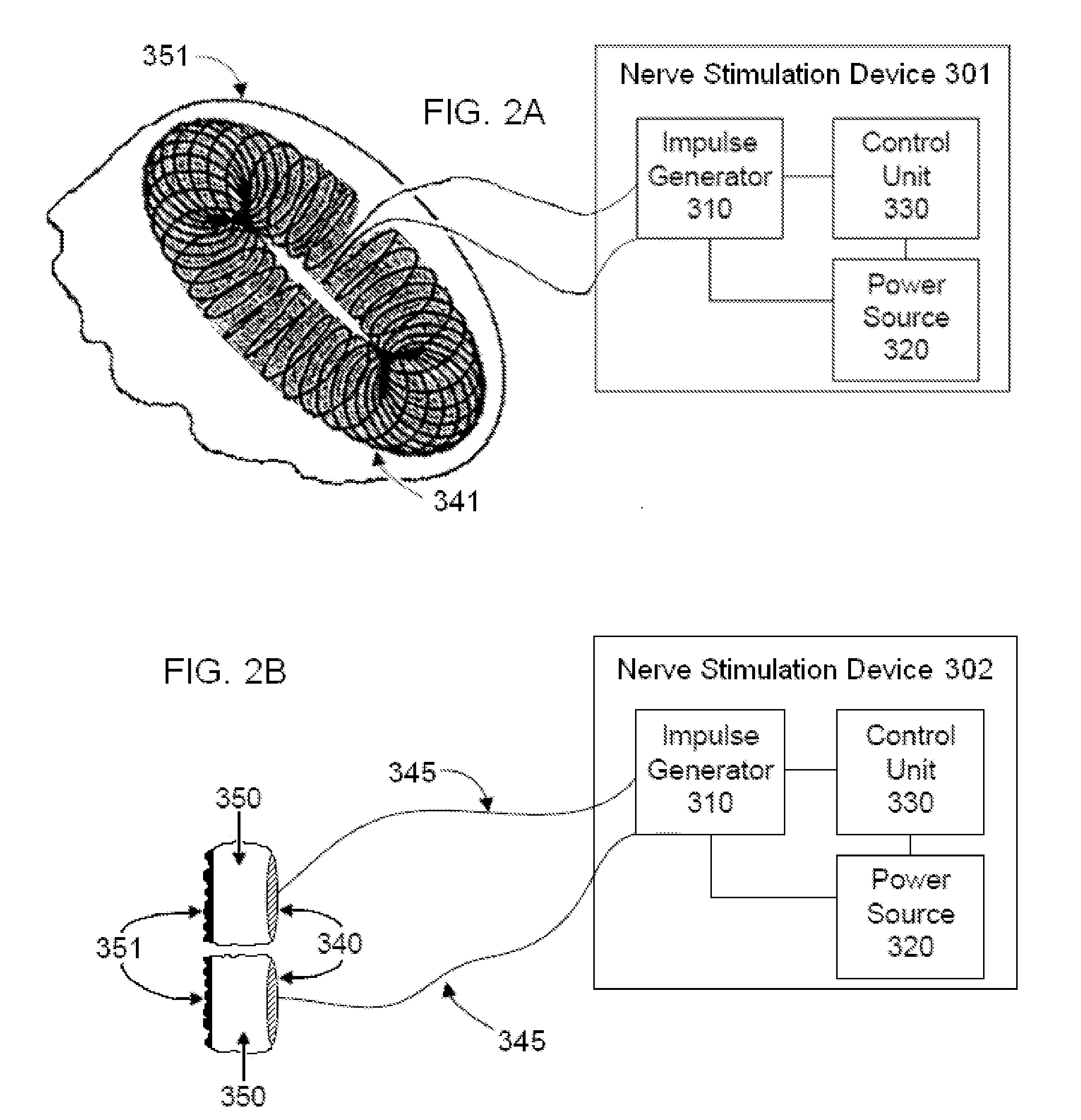
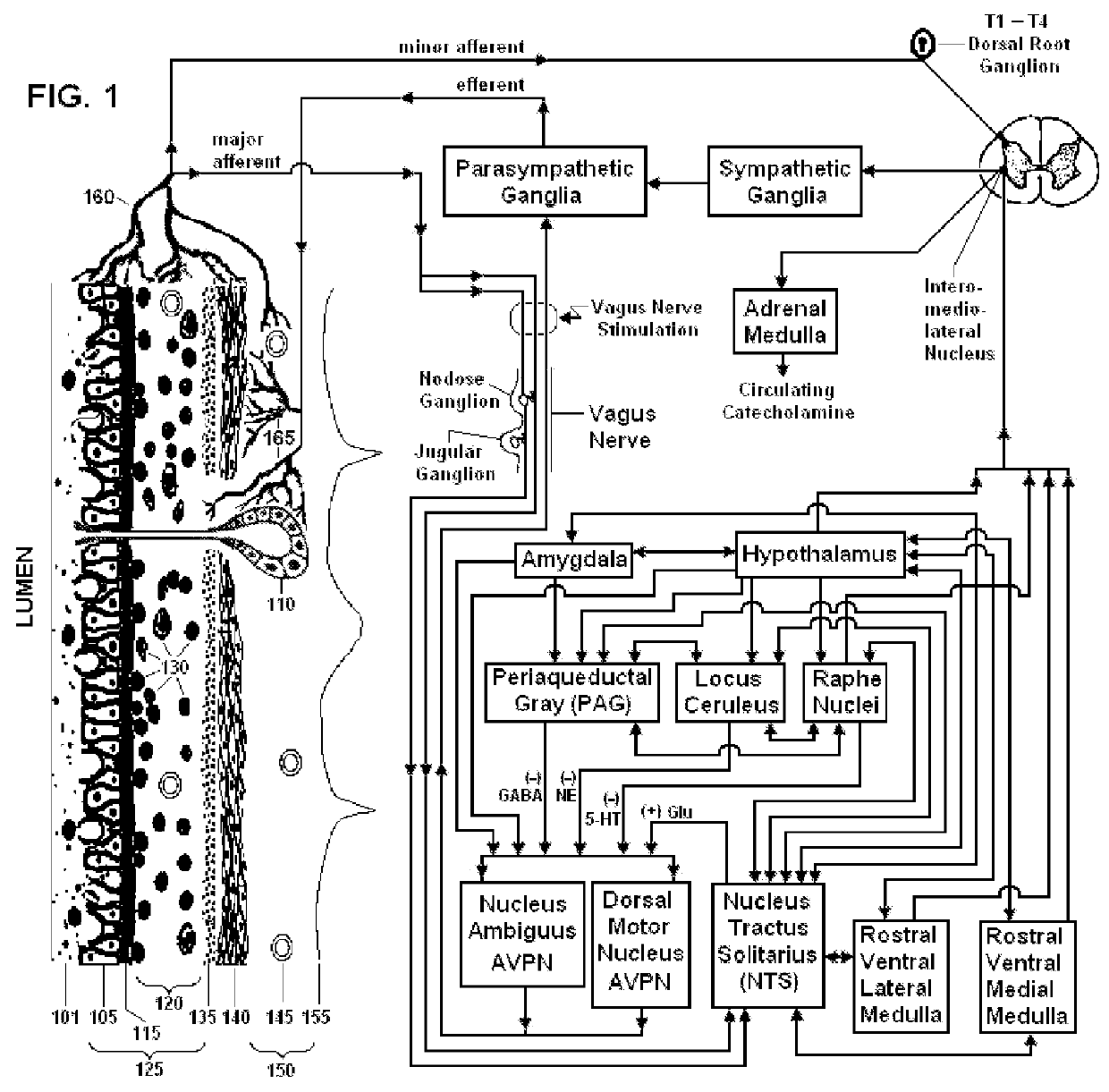
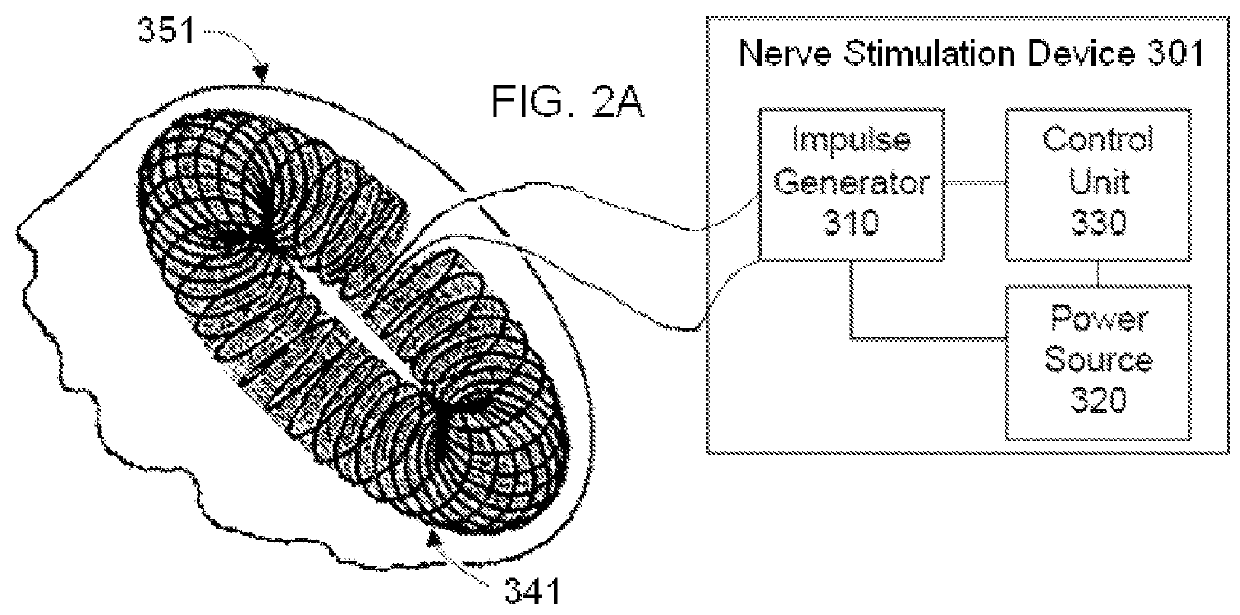
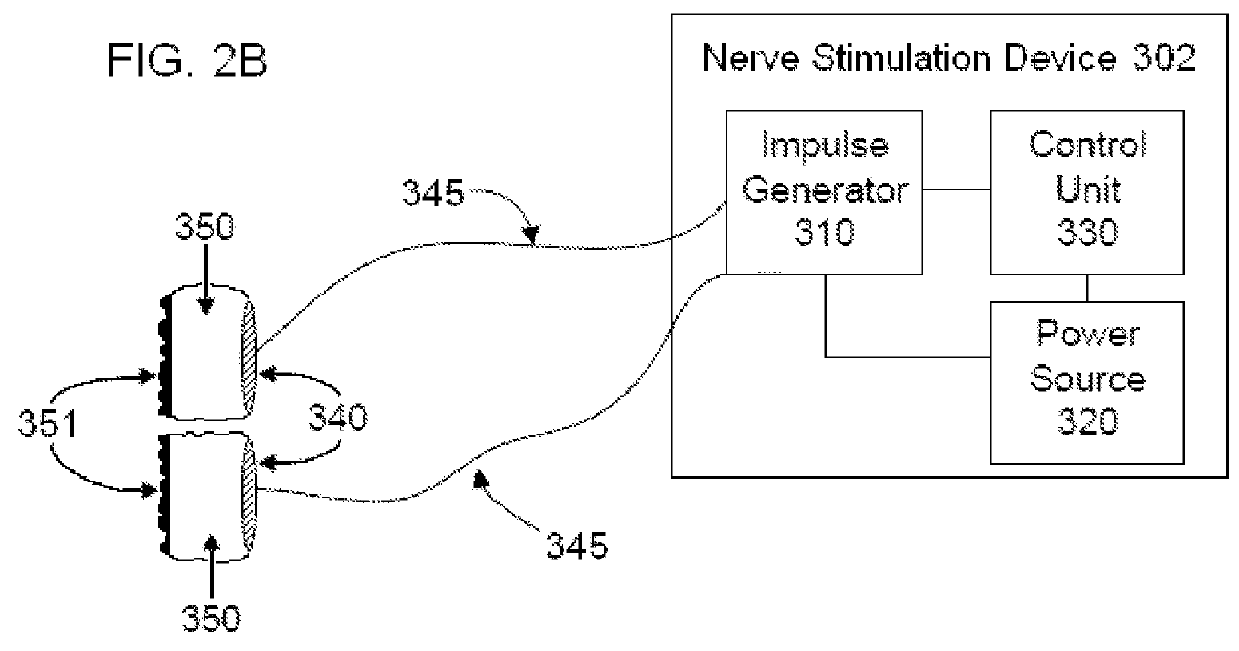
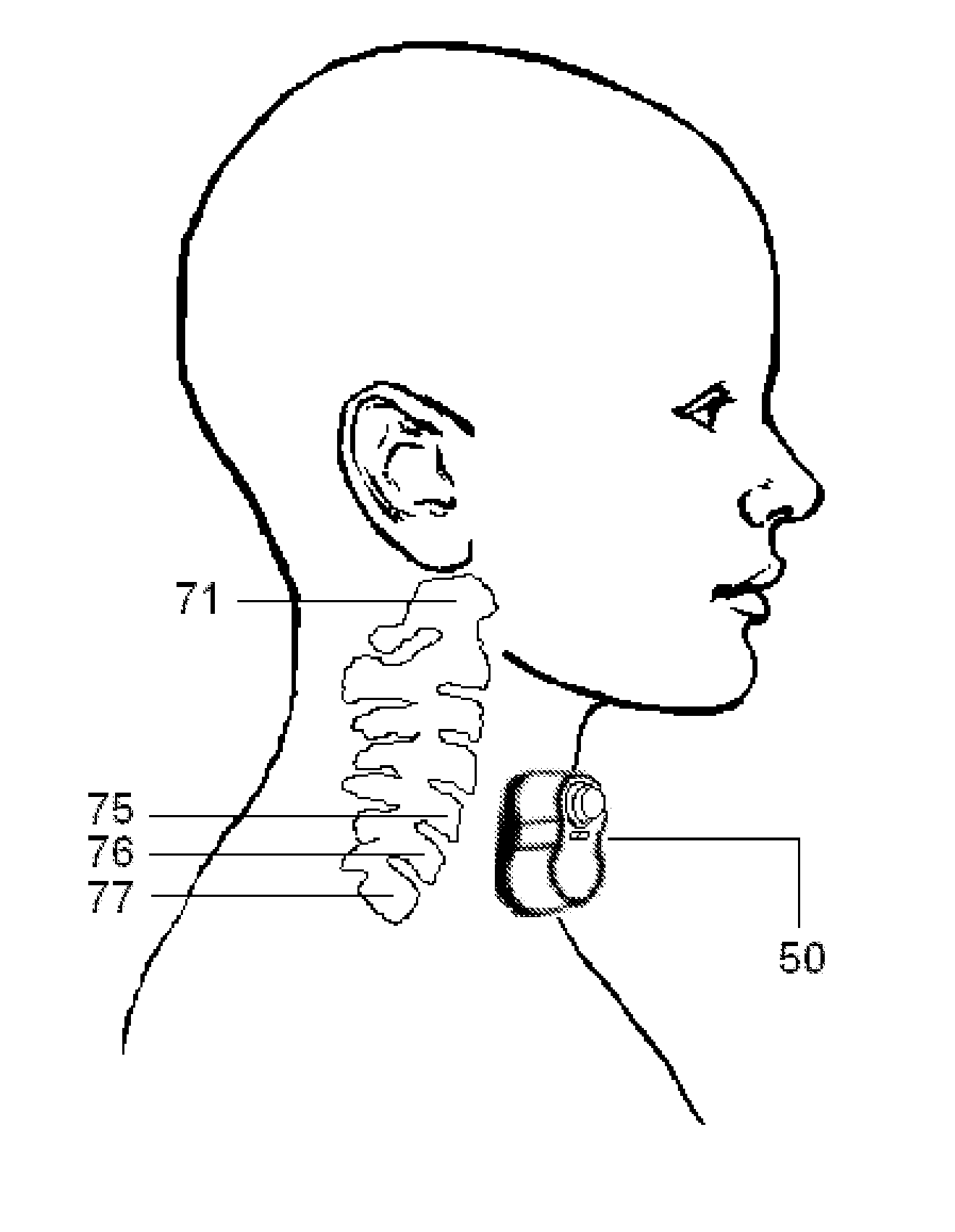
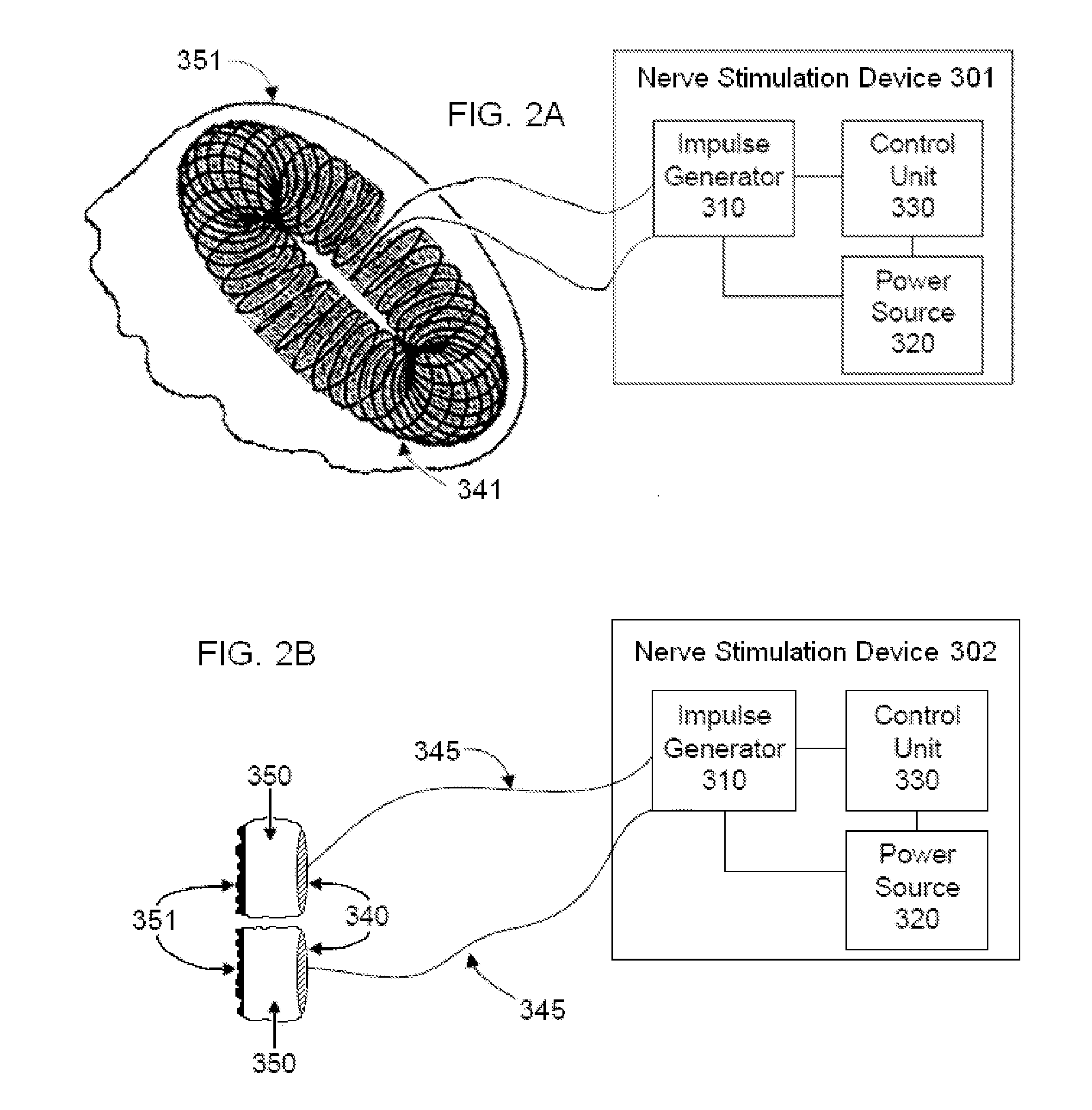
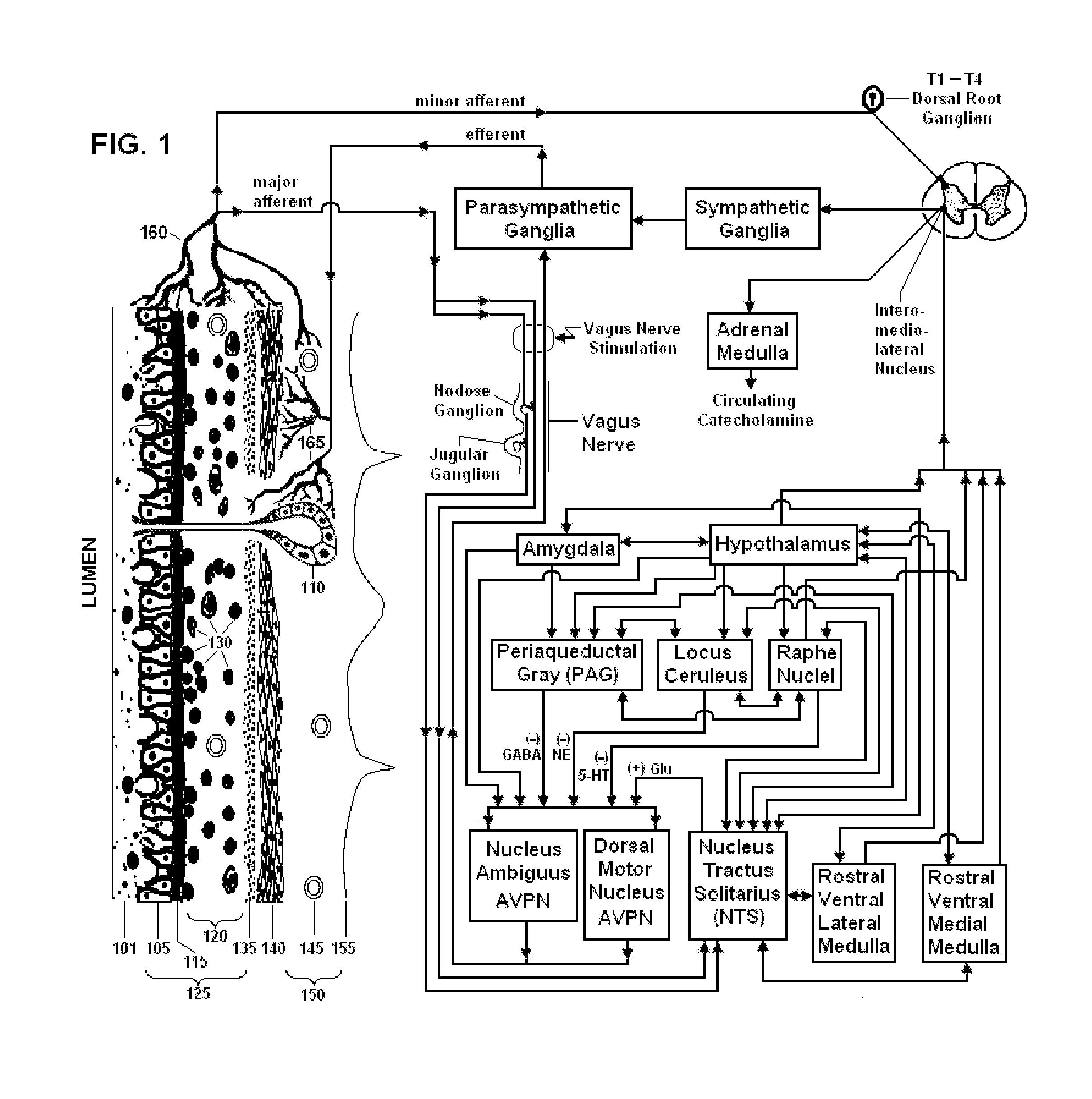
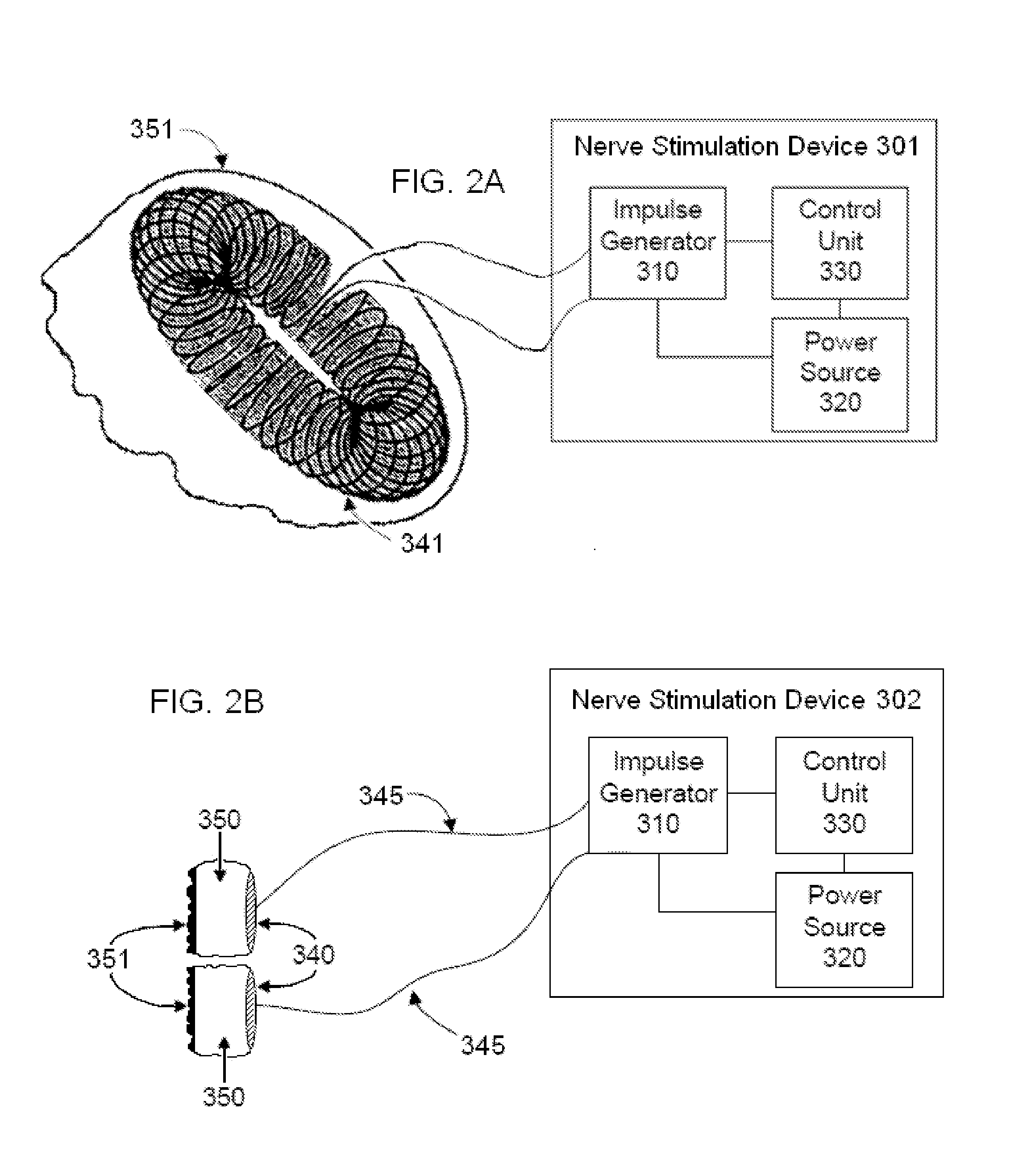
Non-invasive vagal nerve stimulation to treat disorders
ActiveUS8843210B2Excessive levelHigh activityElectrotherapyMagnetotherapy using coils/electromagnetsDiseaseNon invasive
Devices, systems and methods are disclosed for treating a variety of diseases and disorders that are primarily or at least partially driven by an imbalance in neurotransmitters in the brain, such as asthma, COPD, depression, anxiety, epilepsy, fibromyalgia, and the like. The invention involves the use of an energy source comprising magnetic and / or electrical energy that is transmitted non-invasively to, or in close proximity to, a selected nerve to temporarily stimulate, block and / or modulate the signals in the selected nerve such that neural pathways are activated to release inhibitory neurotransmitters in the patient's brain.
Owner:ELECTROCORE
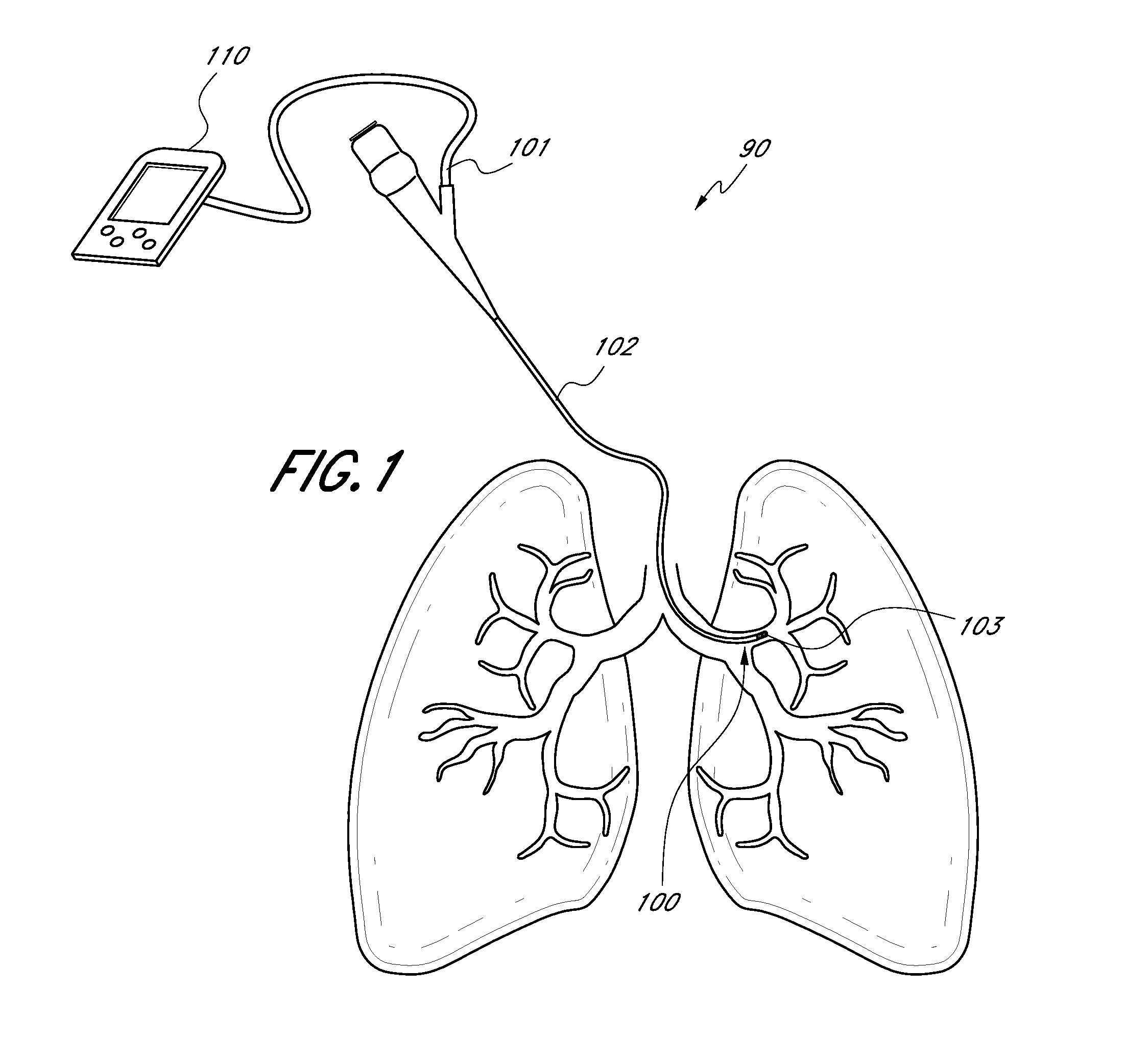
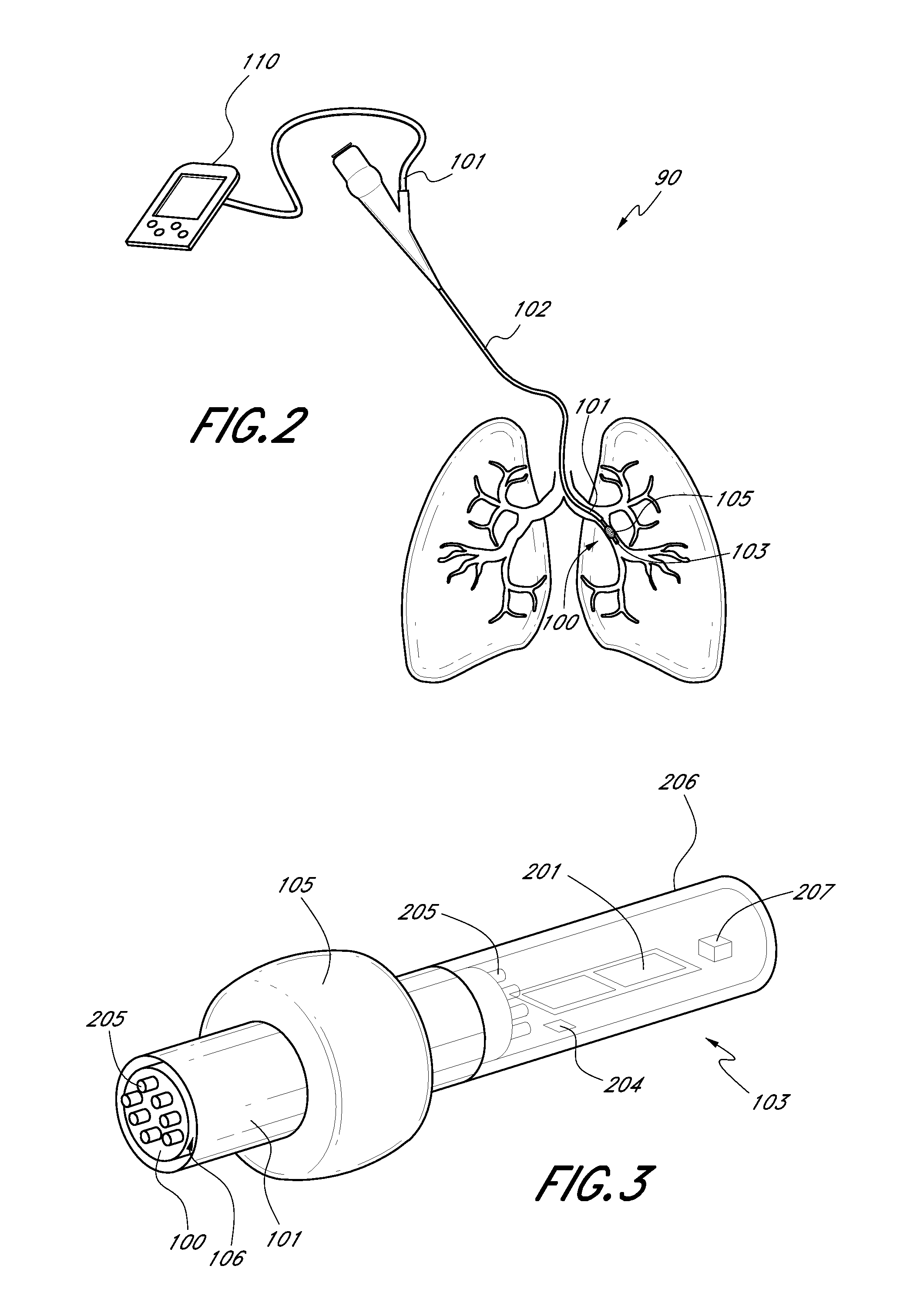
Direct lung sensor systems, methods, and apparatuses
InactiveUS20110201956A1Direct and accurate and simple and minimally invasiveAccurate healthRespiratorsBronchoscopesCOPDOptimal treatment
Devices, systems, and methods for diagnosing physiological parameters of the lungs and treating associated medical conditions are disclosed herein. In particular, certain embodiments permit detection of air flow in lung passageways, air leaks, gas concentration (in particular oxygen), and temperature measurements. Measurements obtained using the devices, systems, and methods disclosed herein may also be used to determine optimal treatment sites for medical conditions such as emphysema, COPD, or lung volume reduction.
Owner:GYRUS ACMI INC (D B A OLYMPUS SURGICAL TECH AMERICA)
Methods and compositions for disease treatment using inhalation
InactiveUS20120077786A1Increasing subject 's convenienceGood treatment complianceBiocideOrganic active ingredientsPulmonary edemaLung cancer
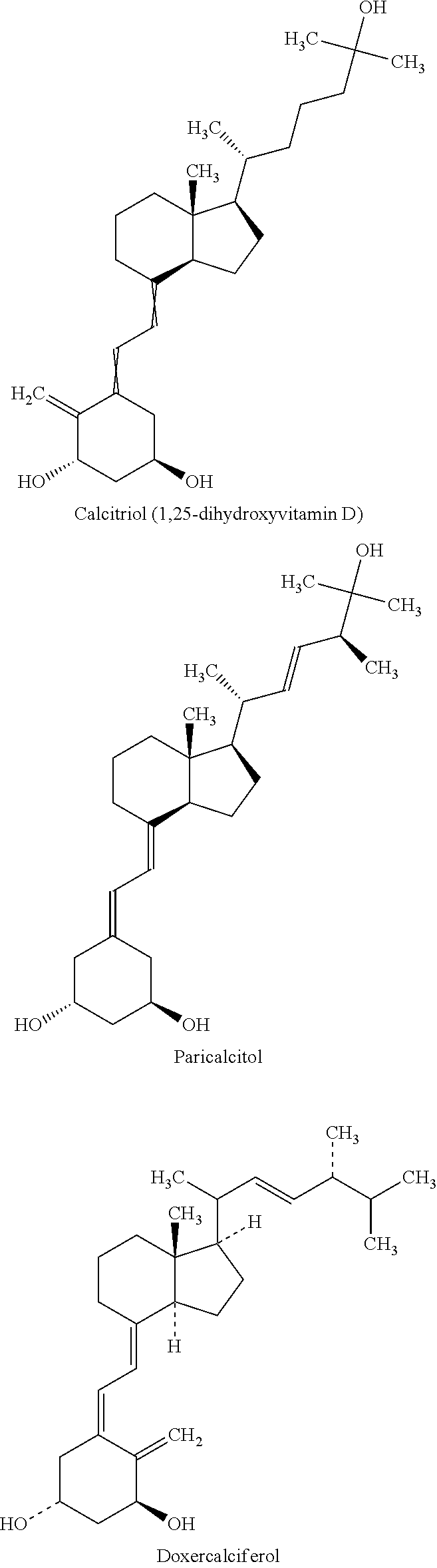
Methods and compositions for the treatment of pulmonary disease using inhalation are provided. In particular, the present disclosure provides novel methods and compositions for treating pulmonary diseases such as asthma, bronchitis, COPD, emphysema, lung cancer, pneumonia and pulmonary edema. In addition, the present disclosure provides novel methods and compositions for treating complications associated with pulmonary disease such as corticosteroid resistance and pulmonary tissue destruction. The compositions of the present disclosure comprise corticosteroid resistance agents including but not limited to vitamin D, calcitriol and equivalents thereof. The compositions of the present disclosure also comprise alveolar development and maintenance agents including but not limited to vitamin A, ATRA and equivalents thereof. The present invention provides effective administration of therapeutic agents to specific airways of the lungs by utilizing controlled site delivery.
Owner:MICRODOSE THERAPEUTX INC
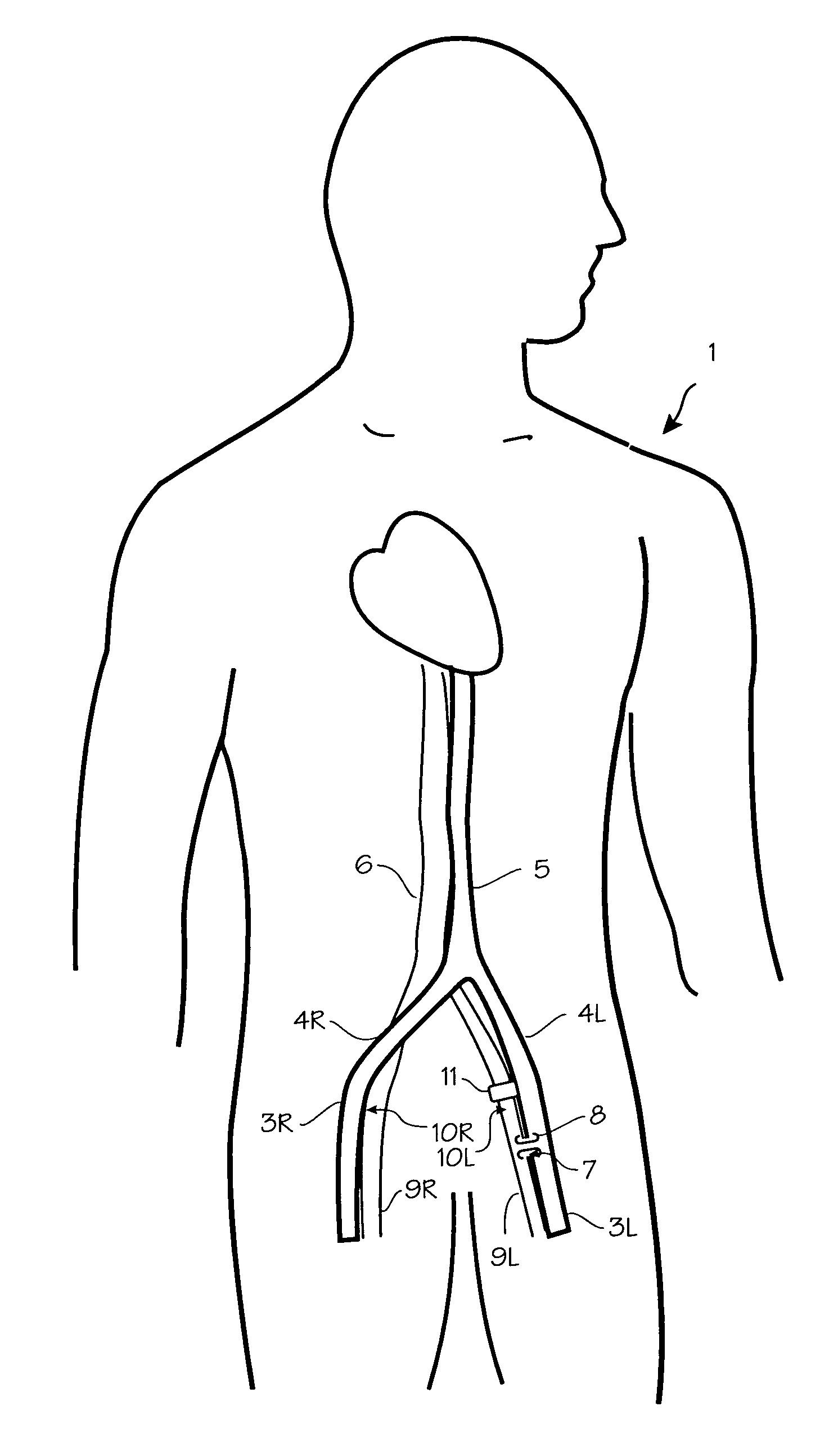
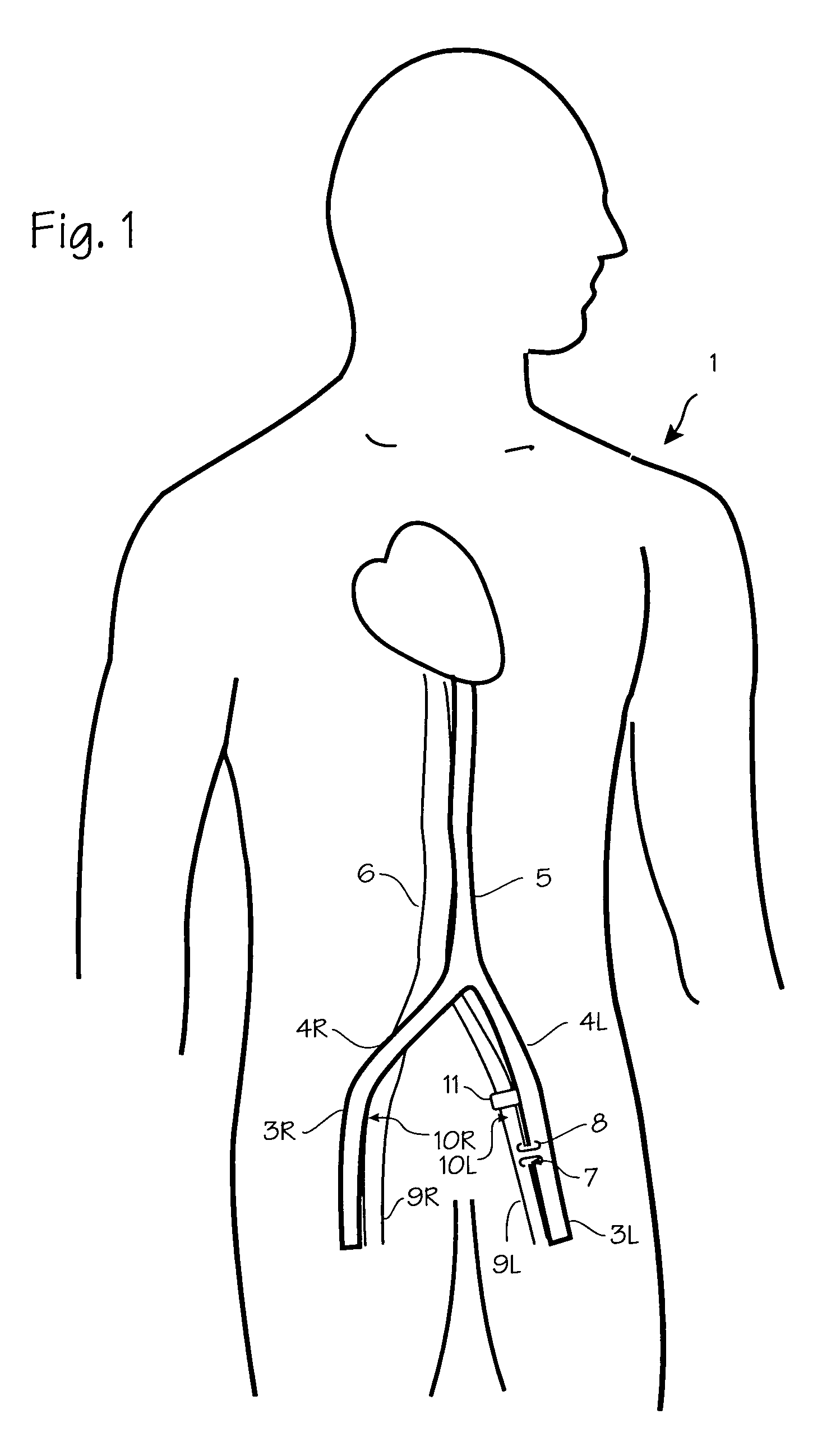
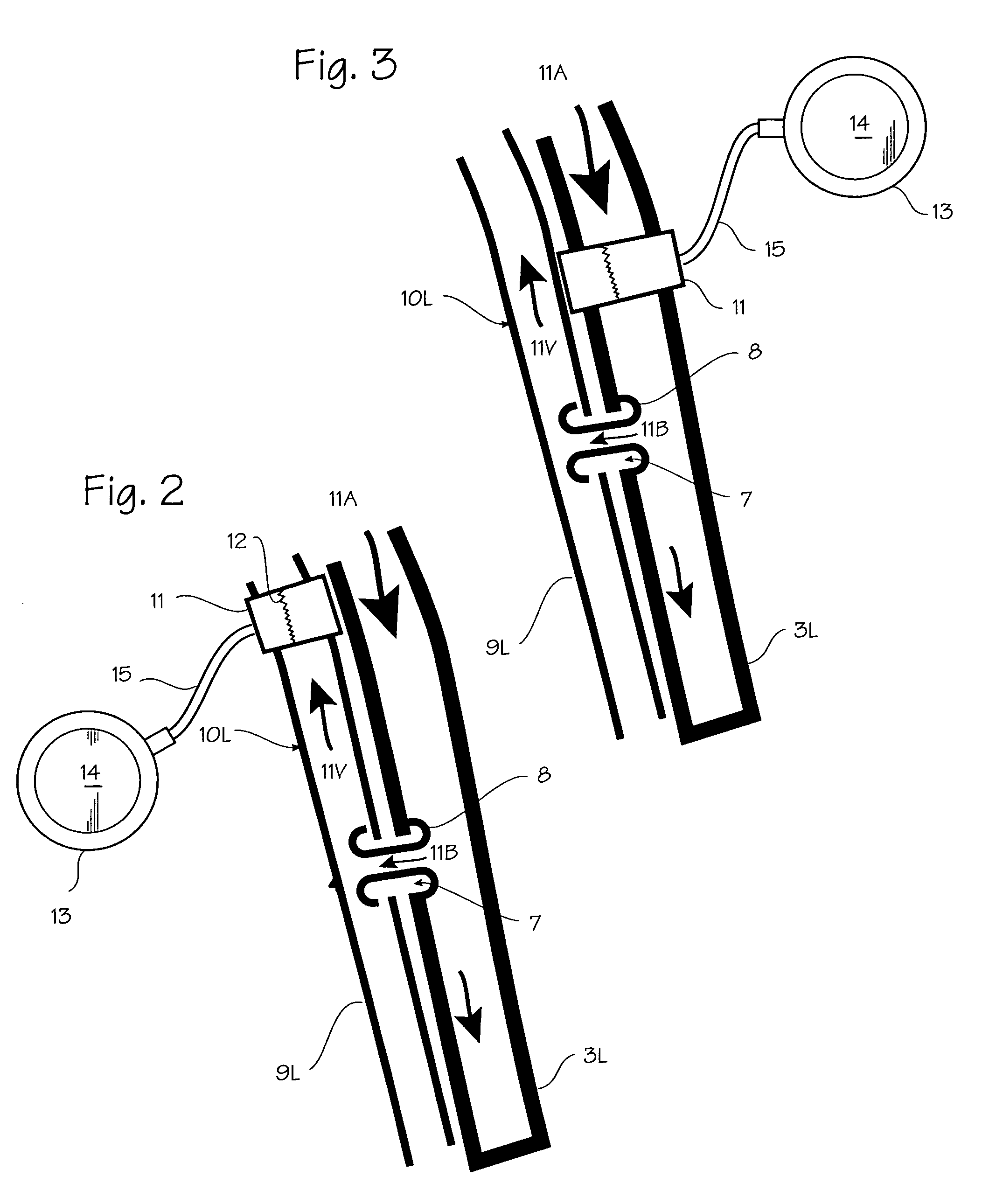
Method of treating COPD with artificial arterio-venous fistula and flow mediating systems
A method for treatment of COPD, hypertension, and left ventricular hypertrophy, and chronic hypoxia including creation of an artificial arterio-venous fistula and installation of a flow mediating device proximate the fistula. The flow mediating device is operated to limit flow as medically indicated to provide the optimum amount of bypass flow.
Owner:EDWARDS LIFESCIENCES CORP
Non-invasive vagal nerve stimulation to treat disorders
ActiveUS8918178B2Effect pressureEffect rateElectrotherapyMagnetotherapy using coils/electromagnetsDiseaseNon invasive
Owner:ELECTROCORE
Non-invasive and minimally invasive denervation methods and systems for performing the same
ActiveUS8911439B2Reduce obstructionReduce productionUltrasound therapySurgical needlesCOPDPartial denervation
A system and method can be used to denervate at least a portion of a bronchial tree. An energy emitter of an instrument is percutaneously delivered to a treatment site and outputs energy to damage nerve tissue of the bronchial tree. The denervation procedure can be performed without damaging non-targeted tissue. Minimally invasive methods can be used to open airways to improve lung function in subjects with COPD, asthma, or the like. Different sections of the bronchial tree can be denervated while leaving airways intact to reduce recovery times.
Owner:HOLAIRA INC
Non-invasive vagal nerve stimulation to treat disorders
ActiveUS8983628B2Effect pressureEffect rateMagnetotherapy using coils/electromagnetsRespiratory organ evaluationDiseaseNon invasive
Devices, systems and methods are disclosed for treating a variety of diseases and disorders that are primarily or at least partially driven by an imbalance in neurotransmitters in the brain, such as asthma, COPD, depression, anxiety, epilepsy, fibromyalgia, and the like. The invention involves the use of an energy source comprising magnetic and / or electrical energy that is transmitted non-invasively to, or in close proximity to, a selected nerve to temporarily stimulate, block and / or modulate the signals in the selected nerve such that neural pathways are activated to release inhibitory neurotransmitters in the patient's brain.
Owner:ELECTROCORE
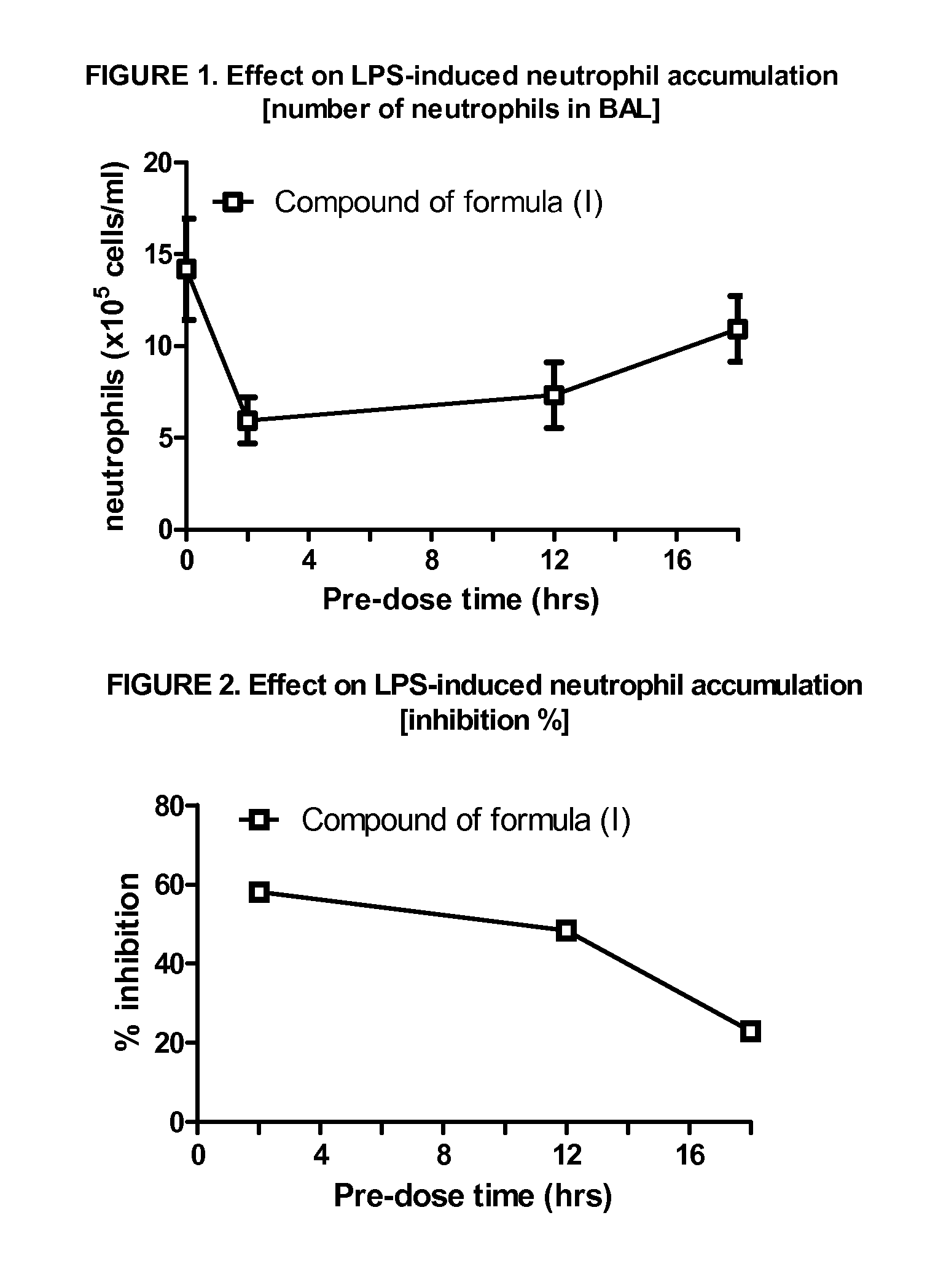
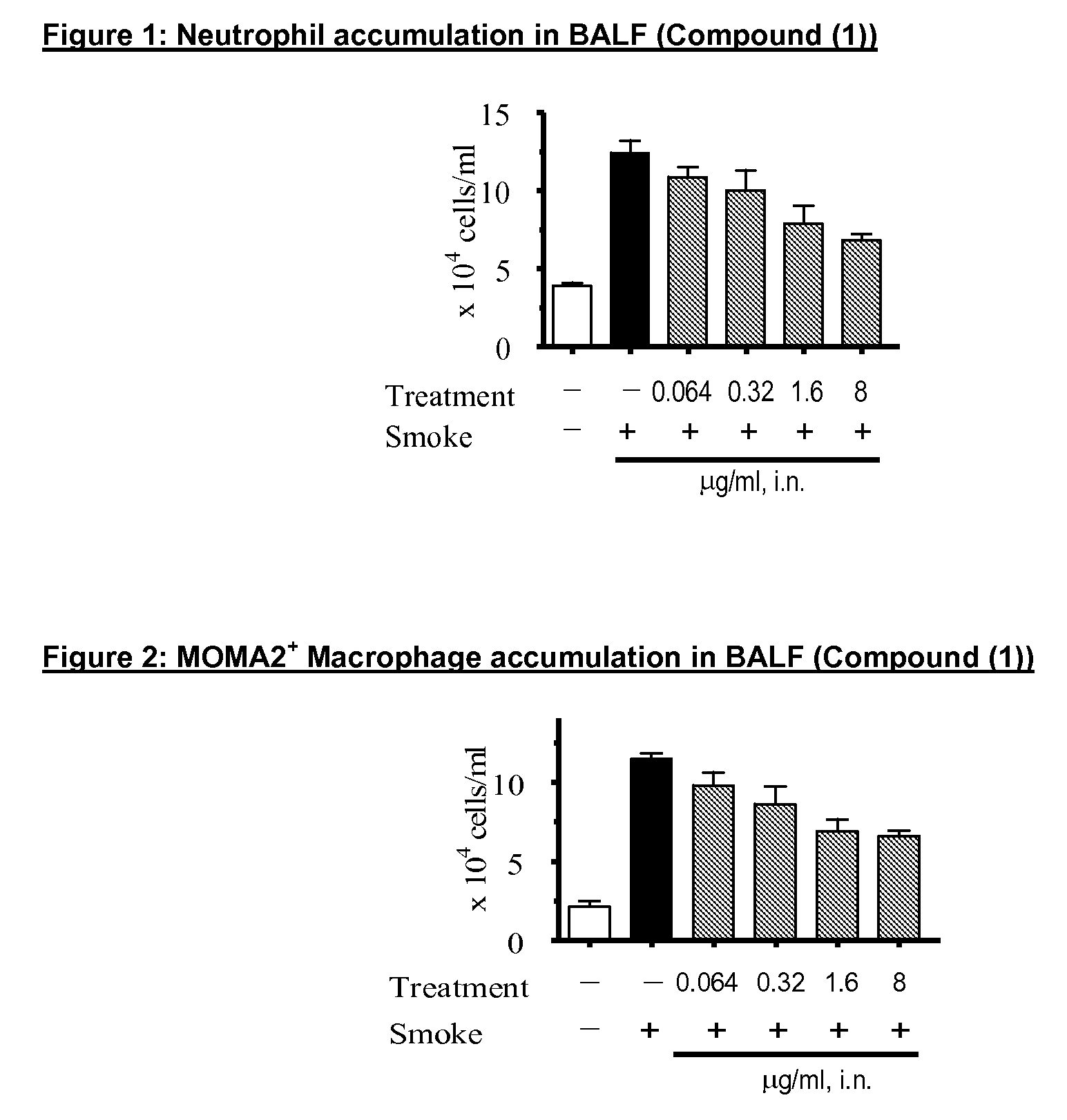
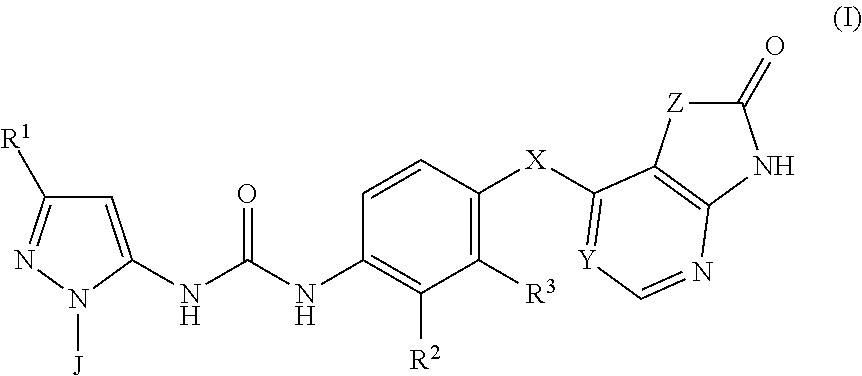
p38 MAP kinase inhibitors
There is provided a compound of formula (I) or a pharmaceutically acceptable salt or solvate thereof, including all tautomers thereof, compositions comprising the same, use of said compound and compositions for treatment, in particular for the treatment of asthma and COPD, and processes for the preparation of said compound.
Owner:RESPIVERT
Non-invasive vagal nerve stimulation to treat disorders
ActiveUS9399134B2Excessive levelHigh activityMagnetotherapy using coils/electromagnetsExternal electrodesDiseaseNon invasive
Devices, systems and methods are disclosed for treating a variety of diseases and disorders that are primarily or at least partially driven by an imbalance in neurotransmitters in the brain, such as asthma, COPD, depression, anxiety, epilepsy, fibromyalgia, and the like. The technology involves the use of an energy source comprising magnetic and / or electrical energy that is transmitted non-invasively to, or in close proximity to, a selected nerve to temporarily stimulate, block and / or modulate the signals in the selected nerve such that neural pathways are activated to release inhibitory neurotransmitters in the patient's brain.
Owner:ELECTROCORE
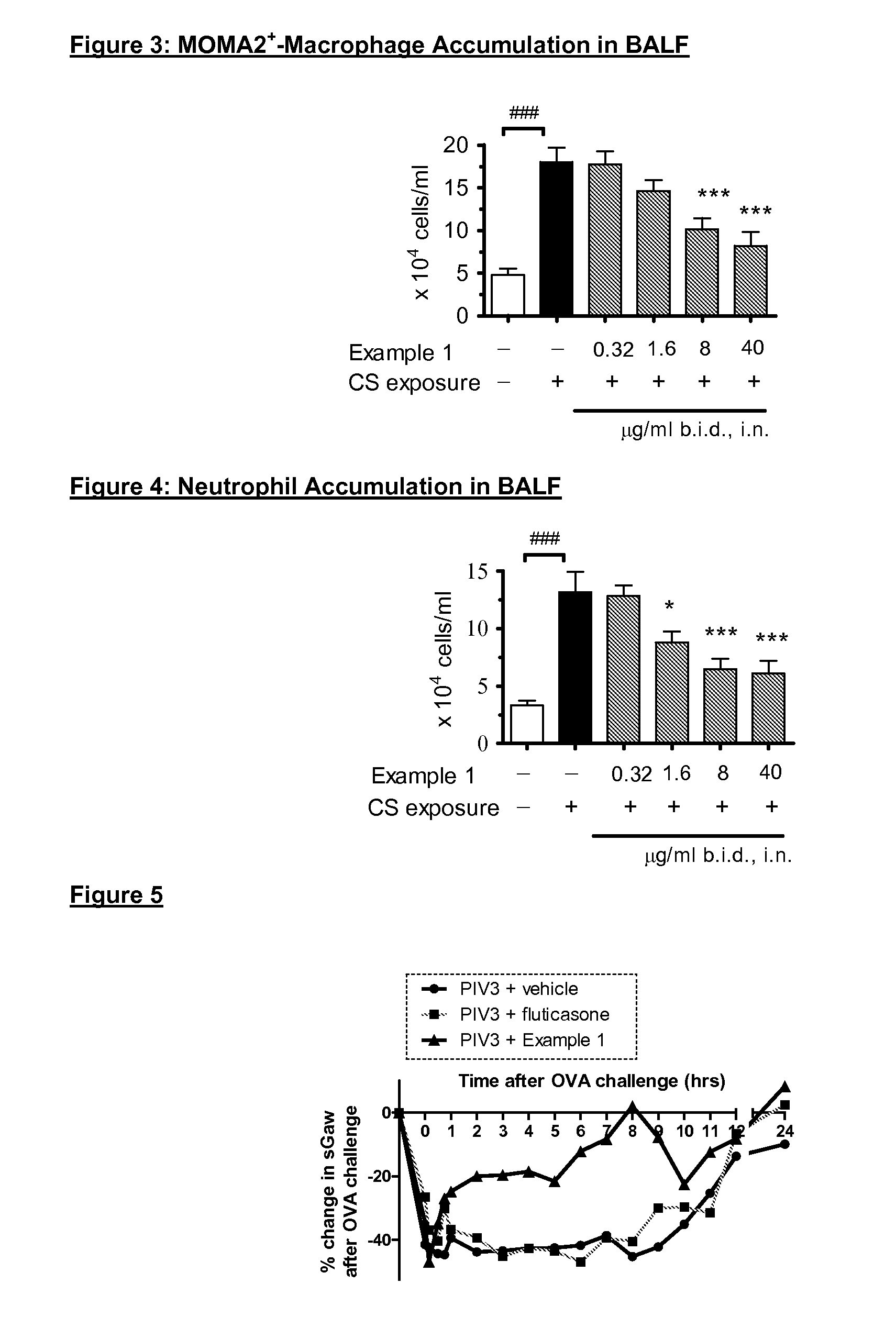
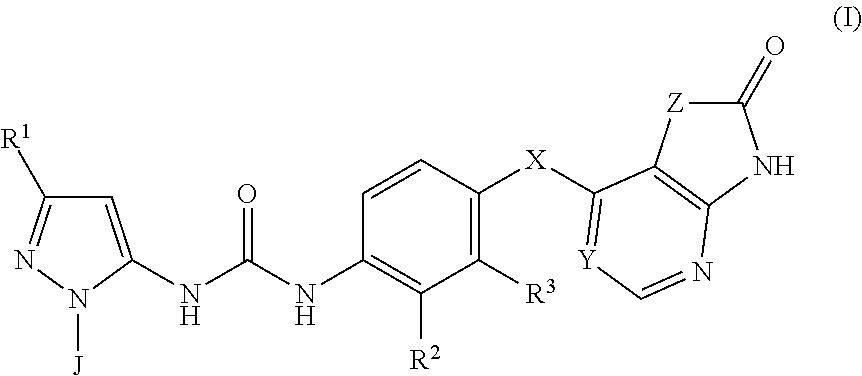
P38MAP kinase inhibitor
The present invention relates to a compound of formula (I):or a pharmaceutically acceptable salt thereof, including all stereoisomers and tautomers, which is an inhibitor of p38 mitogen-activated protein kinase enzymes (referred to herein as p38 MAP kinase inhibitors), particularly the alpha and gamma kinase sub-types thereof, and its use in therapy, including in pharmaceutical combinations, especially in the treatment of inflammatory diseases, including inflammatory diseases of the lung, such as COPD.
Owner:RESPIVERT
Non-invasive vagal nerve stimulation to treat disorders
ActiveUS20130238049A1Effect pressureEffect rateElectrotherapyMagnetotherapy using coils/electromagnetsNon invasiveBrain pathway
Devices, systems and methods are disclosed for treating a variety of diseases and disorders that are primarily or at least partially driven by an imbalance in neurotransmitters in the brain, such as asthma, COPD, depression, anxiety, epilepsy, fibromyalgia, and the like. The invention involves the use of an energy source comprising magnetic and / or electrical energy that is transmitted non-invasively to, or in close proximity to, a selected nerve to temporarily stimulate, block and / or modulate the signals in the selected nerve such that neural pathways are activated to release inhibitory neurotransmitters in the patient's brain.
Owner:ELECTROCORE
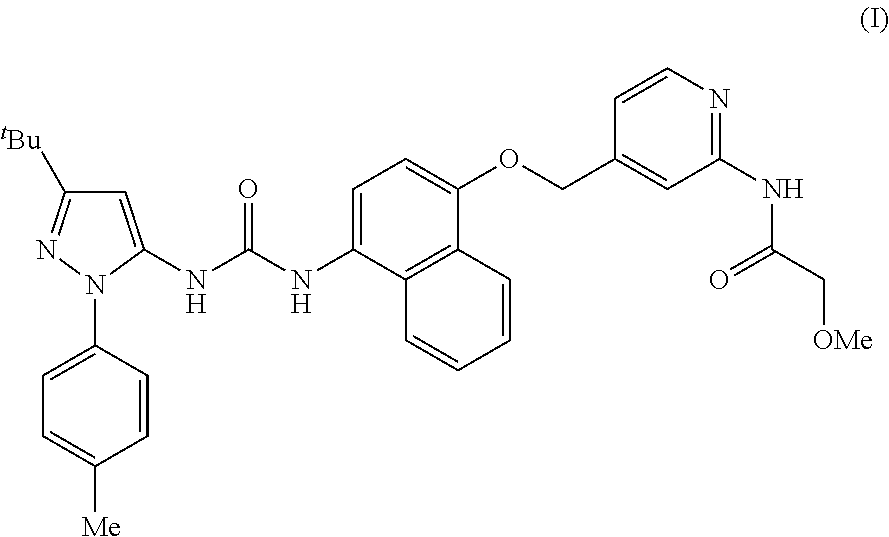
Respiratory formulations and compounds for use therein
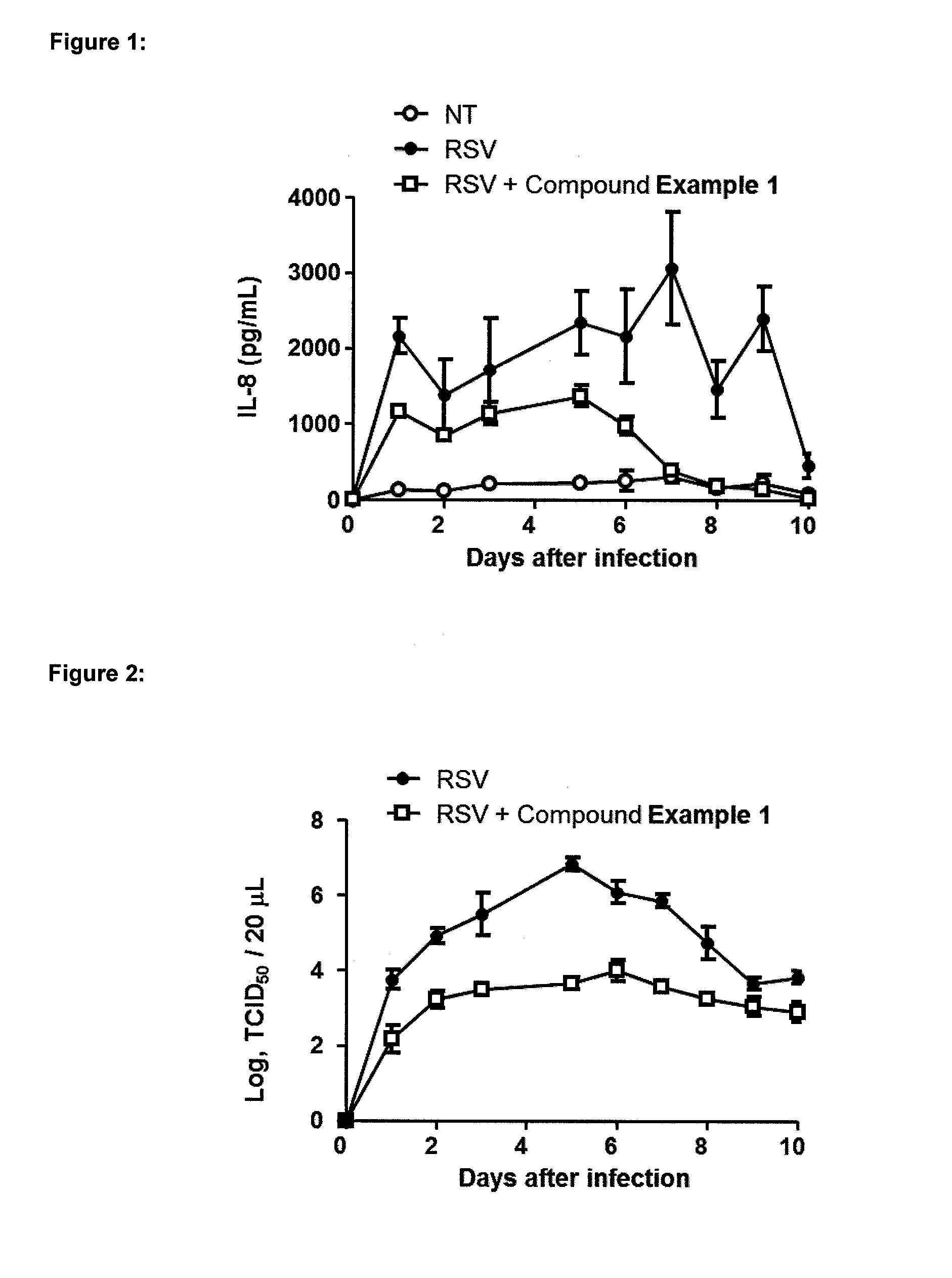
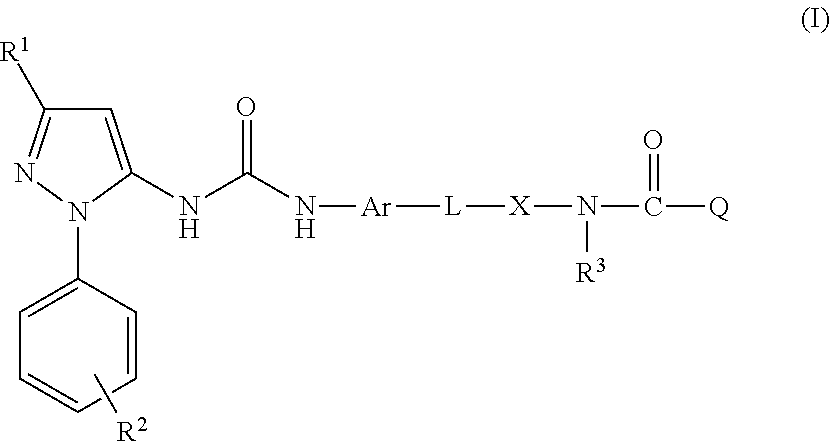
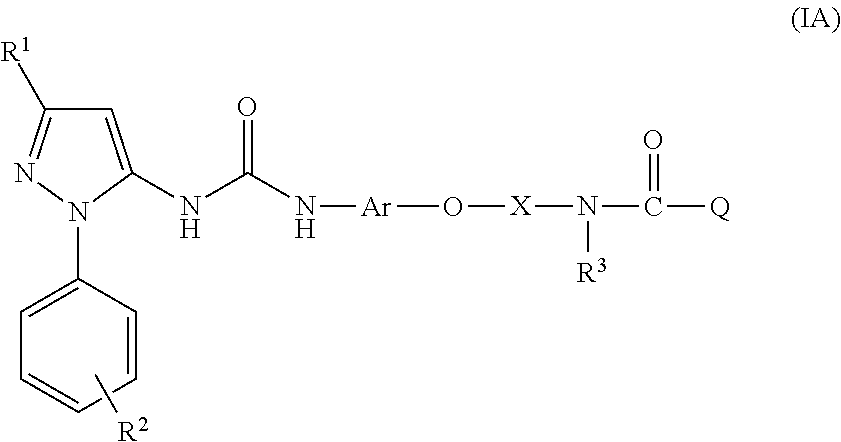
The present invention relates to respiratory formulations comprising a compound of formula (I): and use of said compounds and compositions in treatment, for example in the treatment of an inflammatory disease or a respiratory disorder, in particular an inflammatory mediated and / or virally mediated respiratory disorder such as asthma and COPD or the treatment or prevention of viral infection, for example infection by influenza virus, rhinovirus or RSV. The invention also extends to certain novel compounds of formula (I).
Owner:RESPIVERT
Ureido-pyrazole derivatives for use in the treatment of rhinovirus infections
Medicinal use The disclosure relates to compounds of formula (I) for use in the treatment or prophylaxis of respiratory syncitial virus (RSV) infection in particular viral exacerbation of a respiratory disorder such as bronchitis, asthma, COPD and / or cystic fibrosis, methods of treating or preventing RSV infection employing said compounds or pharmaceutical composition comprising the same.
Owner:RESPIVERT
P38 MAP Kinase Inhibitors
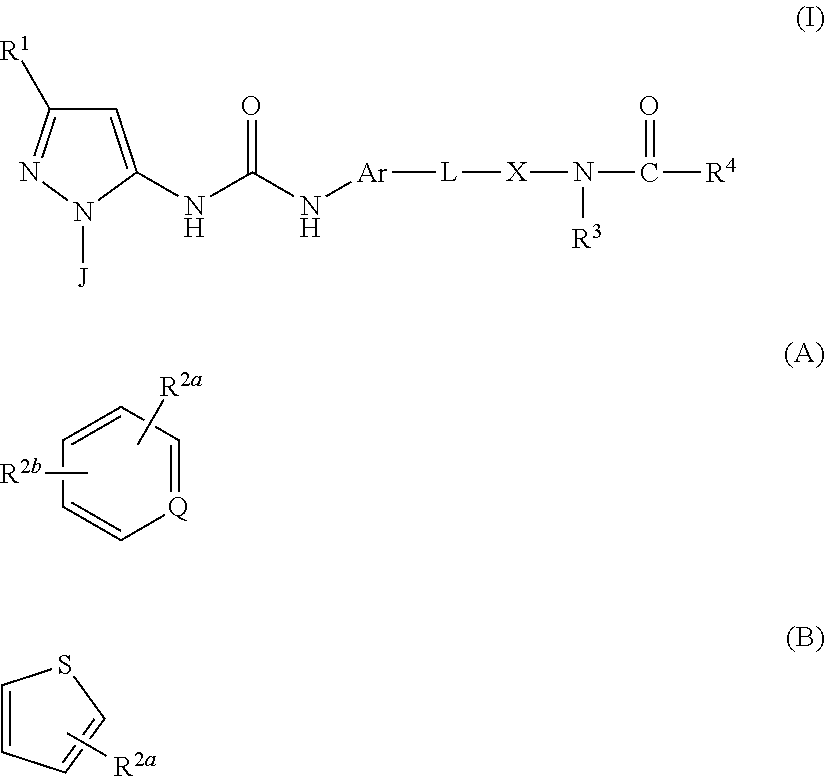
There is provided a compound of formula (I): wherein: J represents (A): or (B): compositions comprising same, processes for preparing said compounds and use thereof in treatment, particularly in the treatment of inflammatory disease, such as asthma, COPD and 15 rheumatoid arthritis.
Owner:RESPIVERT
Non-invasive vagal nerve stimulation to treat disorders
Devices, systems and methods are disclosed for treating a variety of diseases and disorders that are primarily or at least partially driven by an imbalance in neurotransmitters in the brain, such as asthma, COPD, depression, anxiety, epilepsy, fibromyalgia, and the like. The invention involves the use of an energy source comprising magnetic and / or electrical energy that is transmitted non-invasively to, or in close proximity to, a selected nerve to temporarily stimulate, block and / or modulate the signals in the selected nerve such that neural pathways are activated to release inhibitory neurotransmitters in the patient's brain.
Owner:ELECTROCORE
Nerve stimulation methods for averting imminent onset or episode of a disease
Devices, systems and methods are disclosed for treating a variety of diseases and disorders that are primarily or at least partially driven by an imbalance in neurotransmitters in the brain, such as asthma, COPD, depression, anxiety, epilepsy, fibromyalgia, and the like. The invention involves the use of an energy source comprising magnetic and / or electrical energy that is transmitted non-invasively to, or in close proximity to, a selected nerve to temporarily stimulate, block and / or modulate the signals in the selected nerve such that neural pathways are activated to release inhibitory neurotransmitters in the patient's brain.
Owner:ELECTROCORE
Breathing assistance apparatus
A nasal cannula assembly is disclosed having a face mount part, in use resting against a user's face, which includes at least one nasal prong capable of being fitted into a person's nares. The cannula assembly also includes a manifold part, in fluid communication with the face mount part, having a single horizontal side gases entry. In particular, this cannula assembly is for supplying heated, humidified gases to a patient suffering from COPD. A tie or lanyard is disclosed for use with a breathing assistance apparatus such as a nasal cannula, face or nasal mask or tracheostomy connector. The tie or lanyard transfers the weight of the conduits supplying gases to the breathing assistance apparatus from the breathing assistance apparatus and distributes it onto the neck of the patient.
Owner:FISHER & PAYKEL HEALTHCARE LTD
Non-invasive vagal nerve stimulation to treat disorders
ActiveUS20130245711A1Effect pressureEffect rateElectrotherapyMagnetotherapy using coils/electromagnetsDiseaseNon invasive
Devices, systems and methods are disclosed for treating a variety of diseases and disorders that are primarily or at least partially driven by an imbalance in neurotransmitters in the brain, such as asthma, COPD, depression, anxiety, epilepsy, fibromyalgia, and the like. The invention involves the use of an energy source comprising magnetic and / or electrical energy that is transmitted non-invasively to, or in close proximity to, a selected nerve to temporarily stimulate, block and / or modulate the signals in the selected nerve such that neural pathways are activated to release inhibitory neurotransmitters in the patient's brain.
Owner:ELECTROCORE
Fibronectin based scaffold domain proteins that bind to myostatin
ActiveUS8853154B2Inhibitory activityIncrease volumeBacteriaPeptide/protein ingredientsMyostatinFibrosis
The present invention relates to fibronectin-based scaffold domain proteins that bind to myostatin. The invention also relates to the use of these proteins in therapeutic applications to treat muscular dystrophy, cachexia, sarcopenia, osteoarthritis, osteoporosis, diabetes, obesity, COPD, chronic kidney disease, heart failure, myocardial infarction, and fibrosis. The invention further relates to cells comprising such proteins, polynucleotides encoding such proteins or fragments thereof, and to vectors comprising the polynucleotides encoding the proteins.
Owner:BRISTOL MYERS SQUIBB CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com