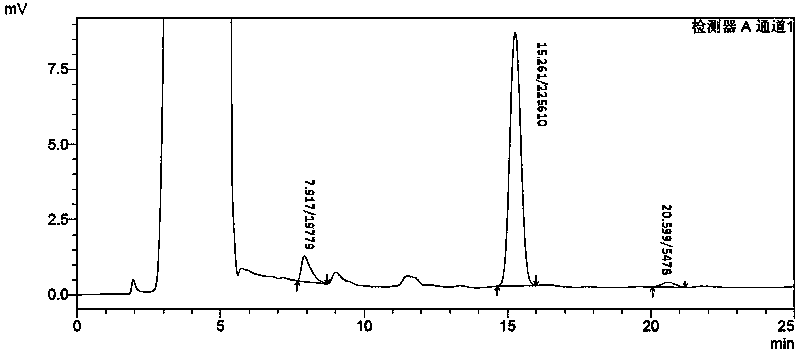
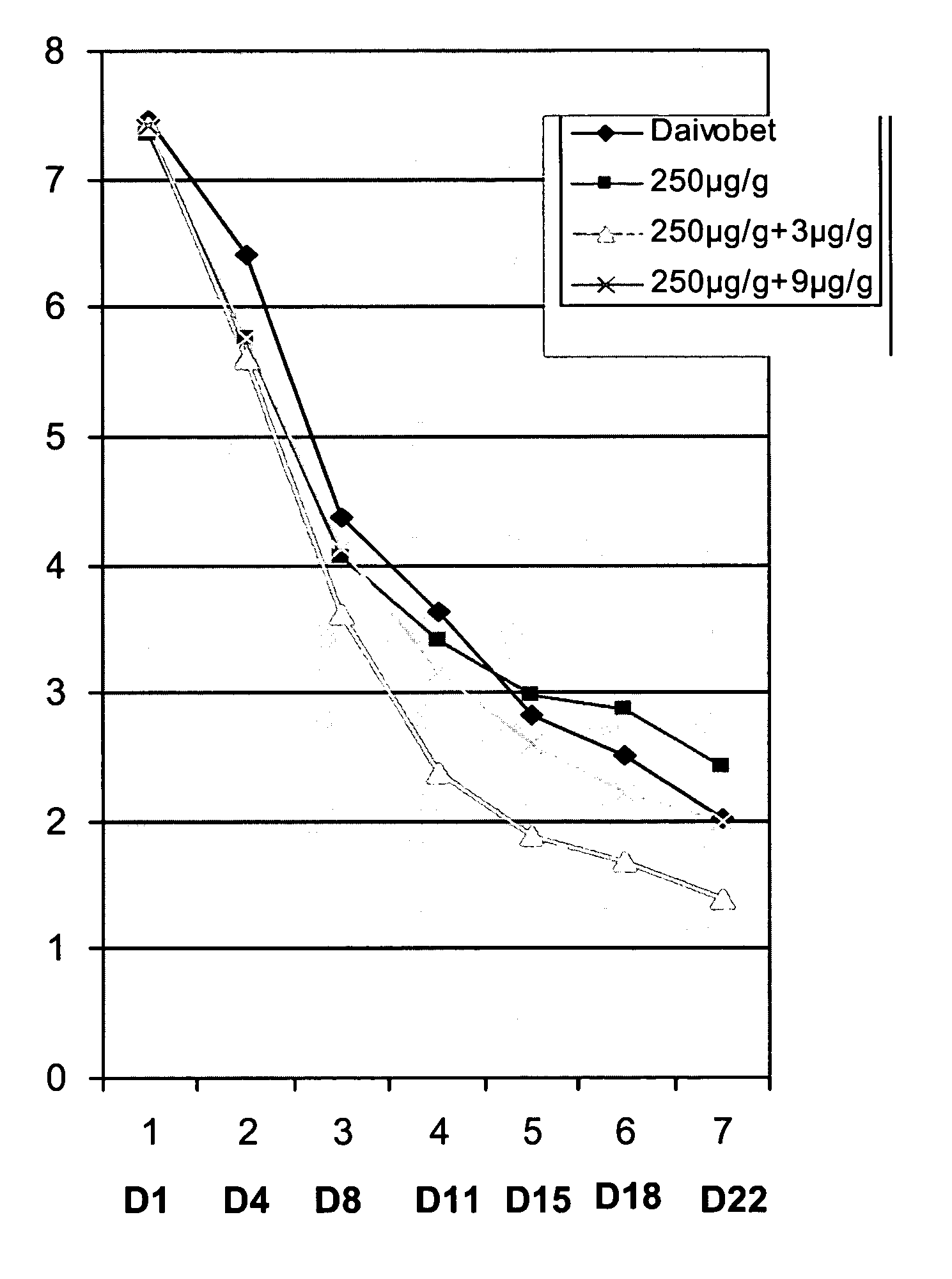
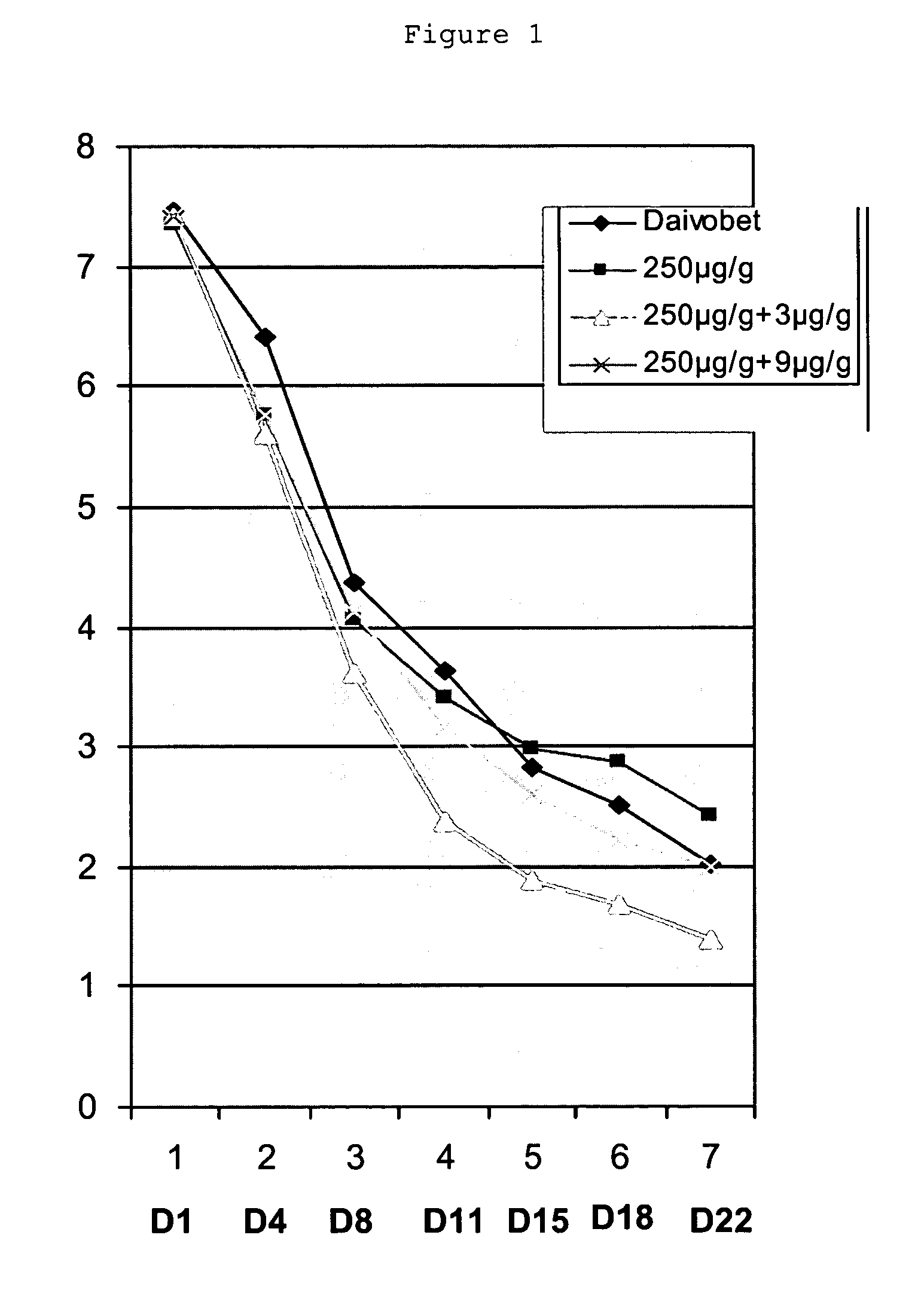
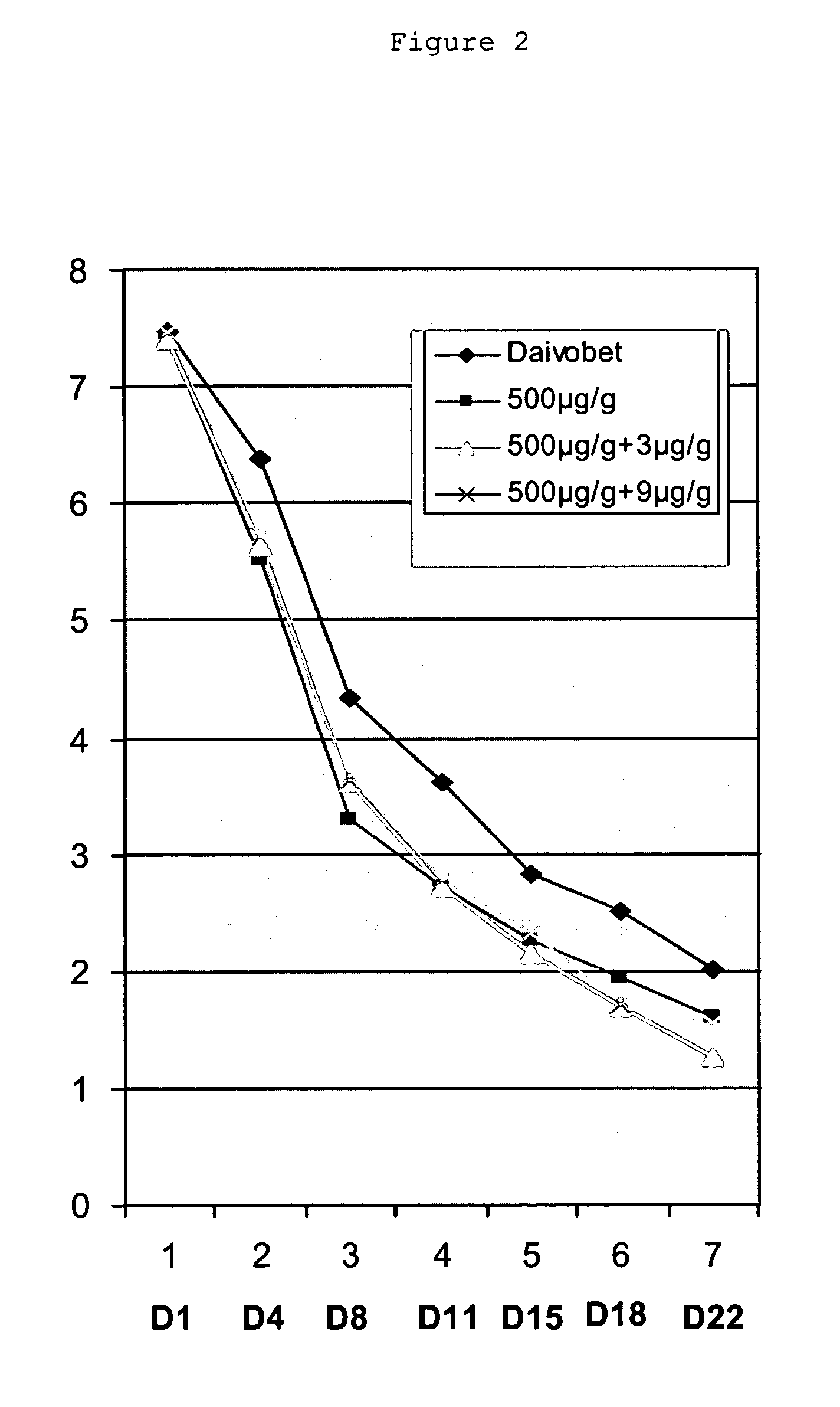
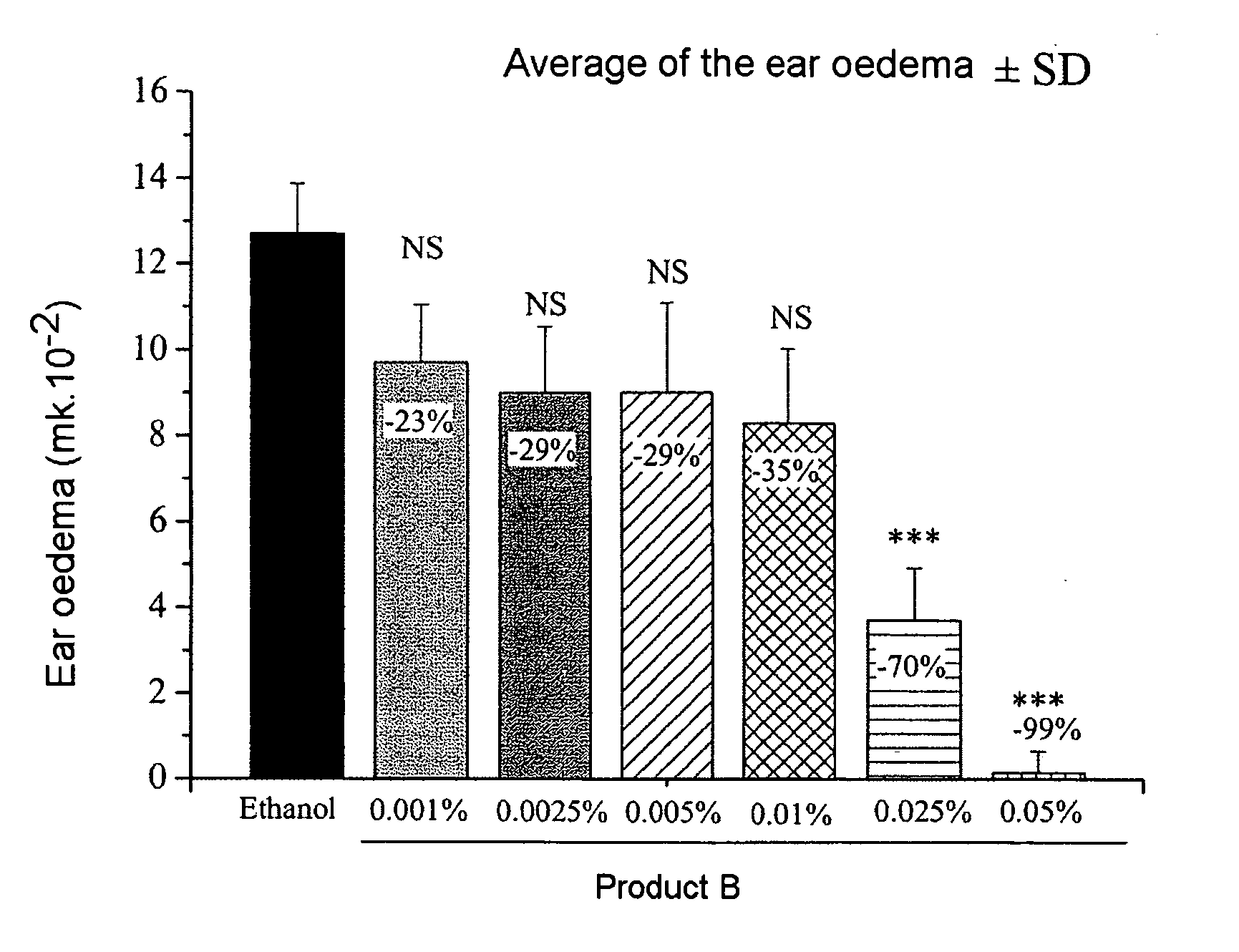
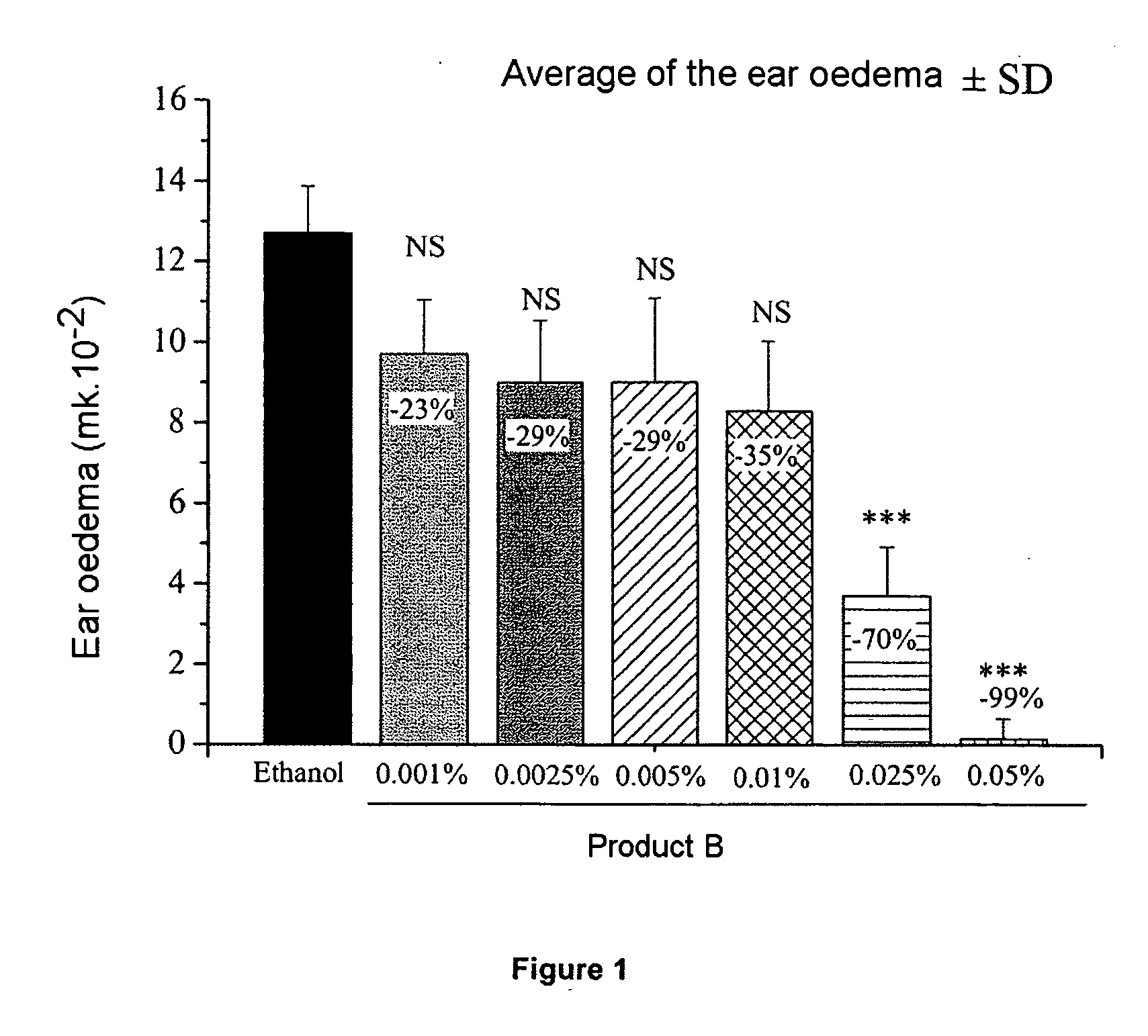
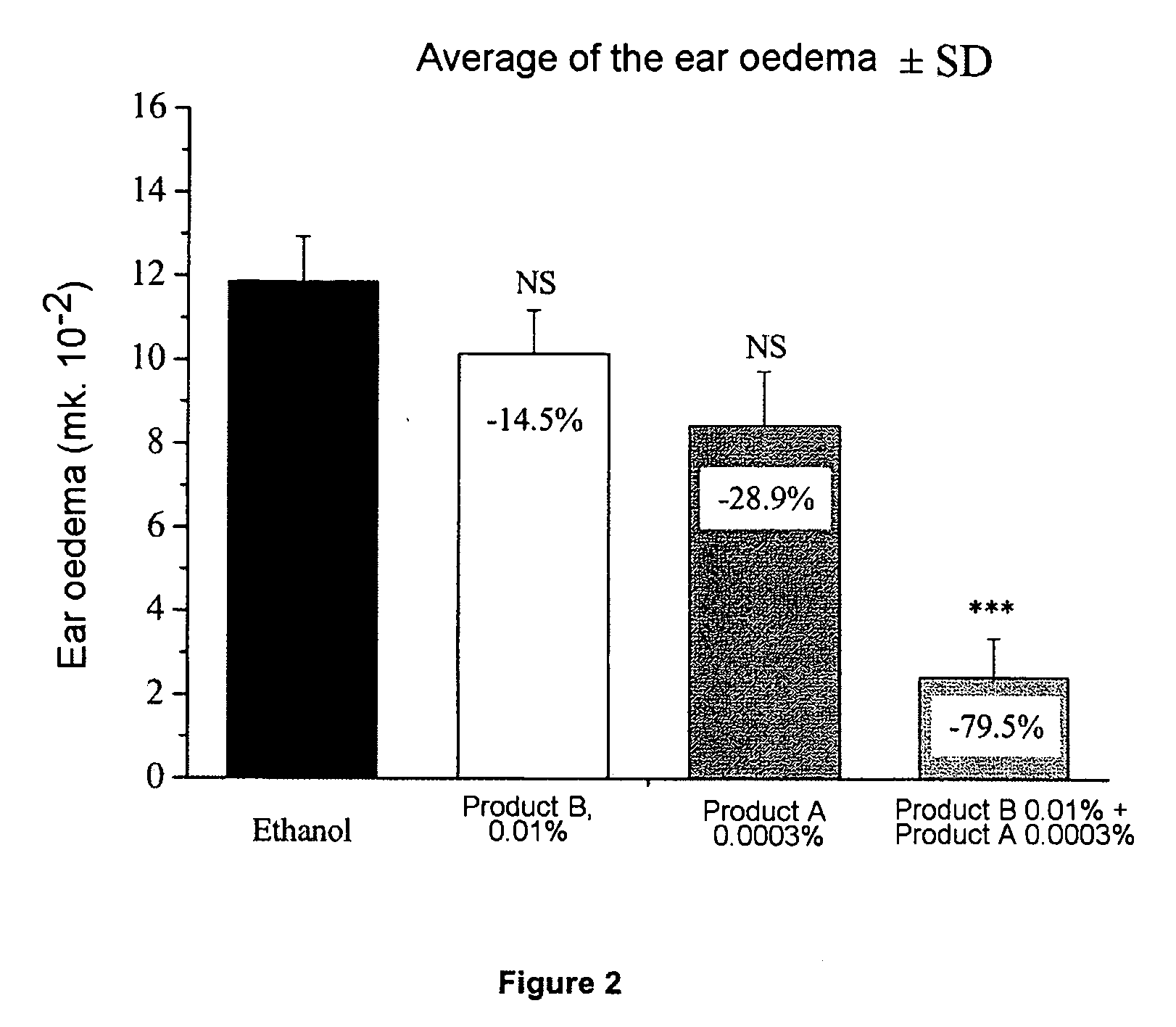
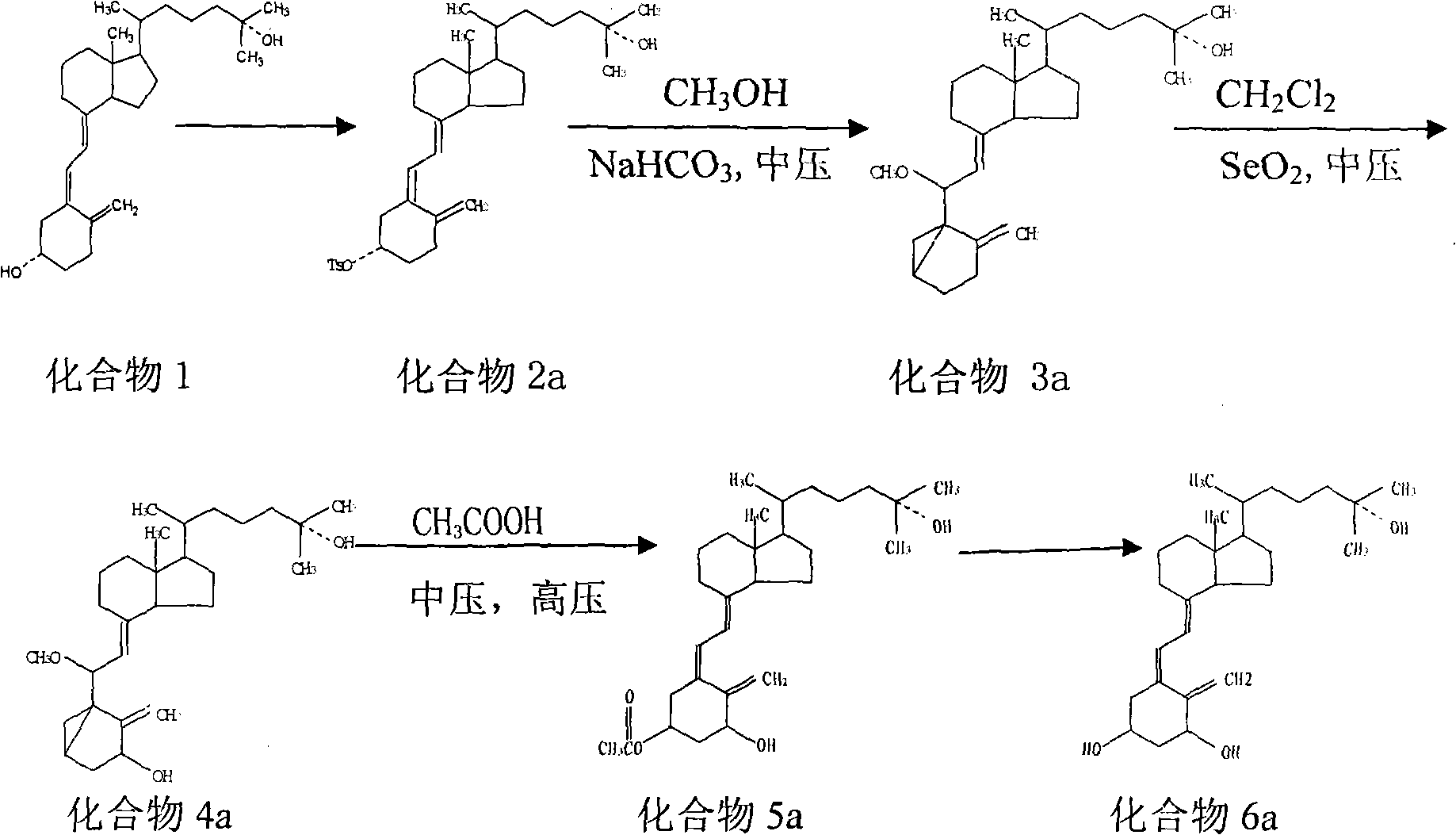
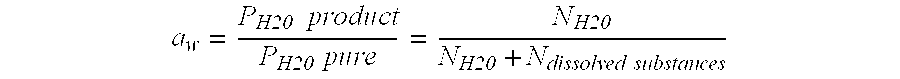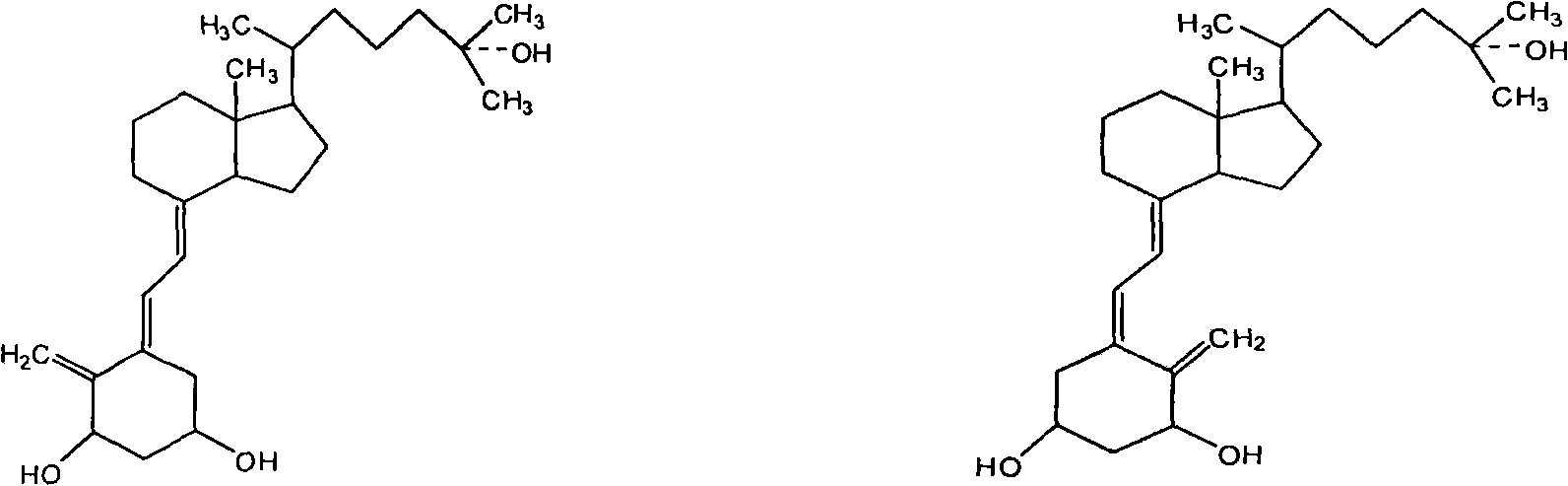Patents
Literature
140 results about "Calcitriol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
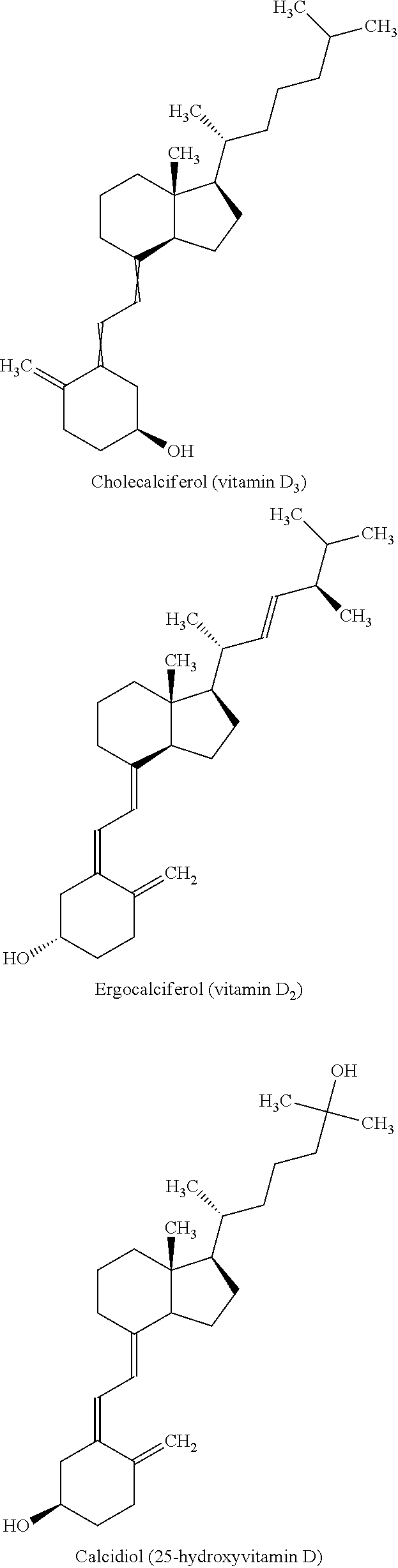
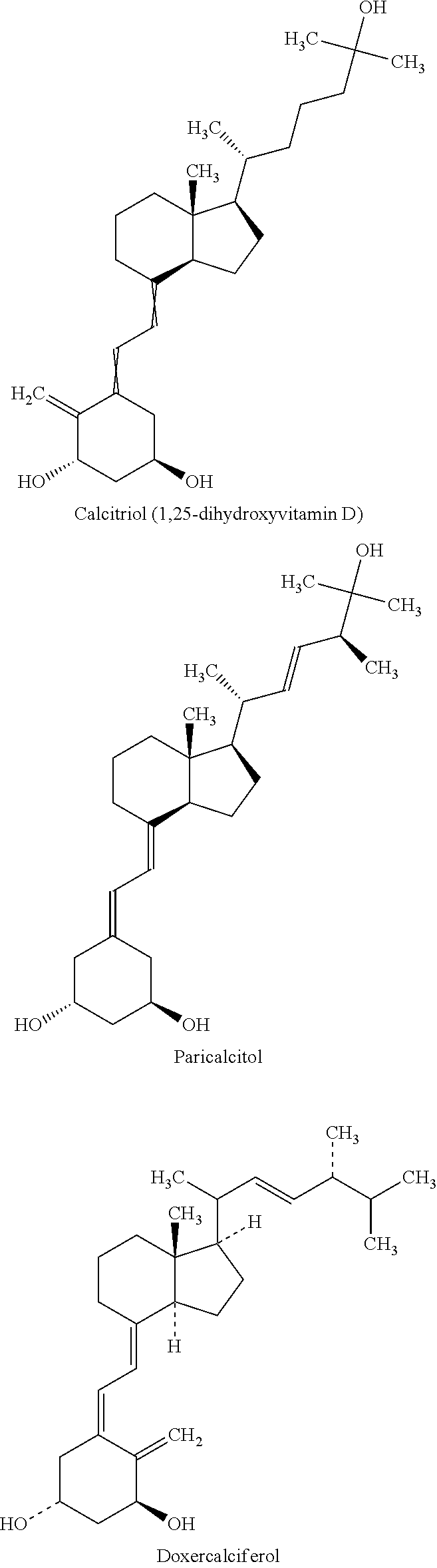
Calcitriol is a man-made active form of vitamin D. Most people get enough vitamin D from exposure to the sun and from fortified food products (e.g., dairy products, vitamins). Vitamin D helps control parathyroid hormone and the levels of certain minerals (e.g., calcium, phosphorus) that are needed for building and keeping strong bones.
Sprayable compositions comprising a combination of pharmaceutical actives and an oily phase
InactiveUS20050281749A1Good acceptability and toleranceChemically stableBiocideAerosol deliveryDermatological disordersOil phase
Sprayable, anhydrous and physically / chemically stable dermatological / pharmaceutical compositions, well suited for the treatment of a variety of dermatological disorders, notably psoriasis, contain: a) a therapeutically effective amount of a solubilized corticoid, notably dissolved clobetasol propionate; b) a therapeutically effective amount of a solubilized vitamin D derivative, notably dissolved calcitriol; and c) an oily phase which comprises one or more oils; formulated into d), a sprayable and topically applicable, dermatologically / pharmaceutically acceptable vehicle therefor.
Owner:GALDERMA SA
Endometriosis treatment protocol
The endometriosis treatment protocol provides for administering to a female patient in need of treatment for endometriosis a pharmaceutical composition in a form suitable for vaginal or rectal delivery having a pharmaceutically effective amount of an aromatase inhibitor, which may be either a steroid or non-steroidal. The pharmaceutical composition may be formed as a vaginal suppository, a rectal suppository, a vaginal gel, a rectal gel, a vaginal cream or a rectal cream. The pharmaceutical composition may optionally have pharmaceutically effective amounts of progesterone and calcitriol, and may be administered in combination with an oral COX-2 inhibitor. Alternatively, the pharmaceutical composition comprises an aromatase inhibitor administered vaginally or rectally and is administered in combination with oral calcitriol and the oral COX-2 inhibitor. The aromatase inhibitor is either steroidal or non-steroidal.
Owner:SHIPPEN EUGENE R
Methods and compositions for disease treatment using inhalation
InactiveUS20120077786A1Increasing subject 's convenienceGood treatment complianceBiocideOrganic active ingredientsPulmonary edemaLung cancer
Methods and compositions for the treatment of pulmonary disease using inhalation are provided. In particular, the present disclosure provides novel methods and compositions for treating pulmonary diseases such as asthma, bronchitis, COPD, emphysema, lung cancer, pneumonia and pulmonary edema. In addition, the present disclosure provides novel methods and compositions for treating complications associated with pulmonary disease such as corticosteroid resistance and pulmonary tissue destruction. The compositions of the present disclosure comprise corticosteroid resistance agents including but not limited to vitamin D, calcitriol and equivalents thereof. The compositions of the present disclosure also comprise alveolar development and maintenance agents including but not limited to vitamin A, ATRA and equivalents thereof. The present invention provides effective administration of therapeutic agents to specific airways of the lungs by utilizing controlled site delivery.
Owner:MICRODOSE THERAPEUTX INC
Sprayable compositions comprising a combination of pharmaceutical active ingredients, an alcohol phase and an oily phase
InactiveUS20050281754A1Easy to useHigh acceptabilityCosmetic preparationsBiocideAlcoholAdditive ingredient
Sprayable, anhydrous and physically / chemically stable dermatological / pharmaceutical compositions, well suited for the treatment of a variety of dermatological disorders, notably psoriasis, contain: a) a therapeutically effective amount of a solubilized corticoid, notably dissolved clobetasol propionate; b) a therapeutically effective amount of a solubilized vitamin D derivative, notably dissolved calcitriol; and c) an alcohol phase; and d) an oily phase which comprises one or more oils; formulated into e), a sprayable and topically applicable, dermatologically / pharmaceutically acceptable vehicle therefor.
Owner:GALDERMA SA
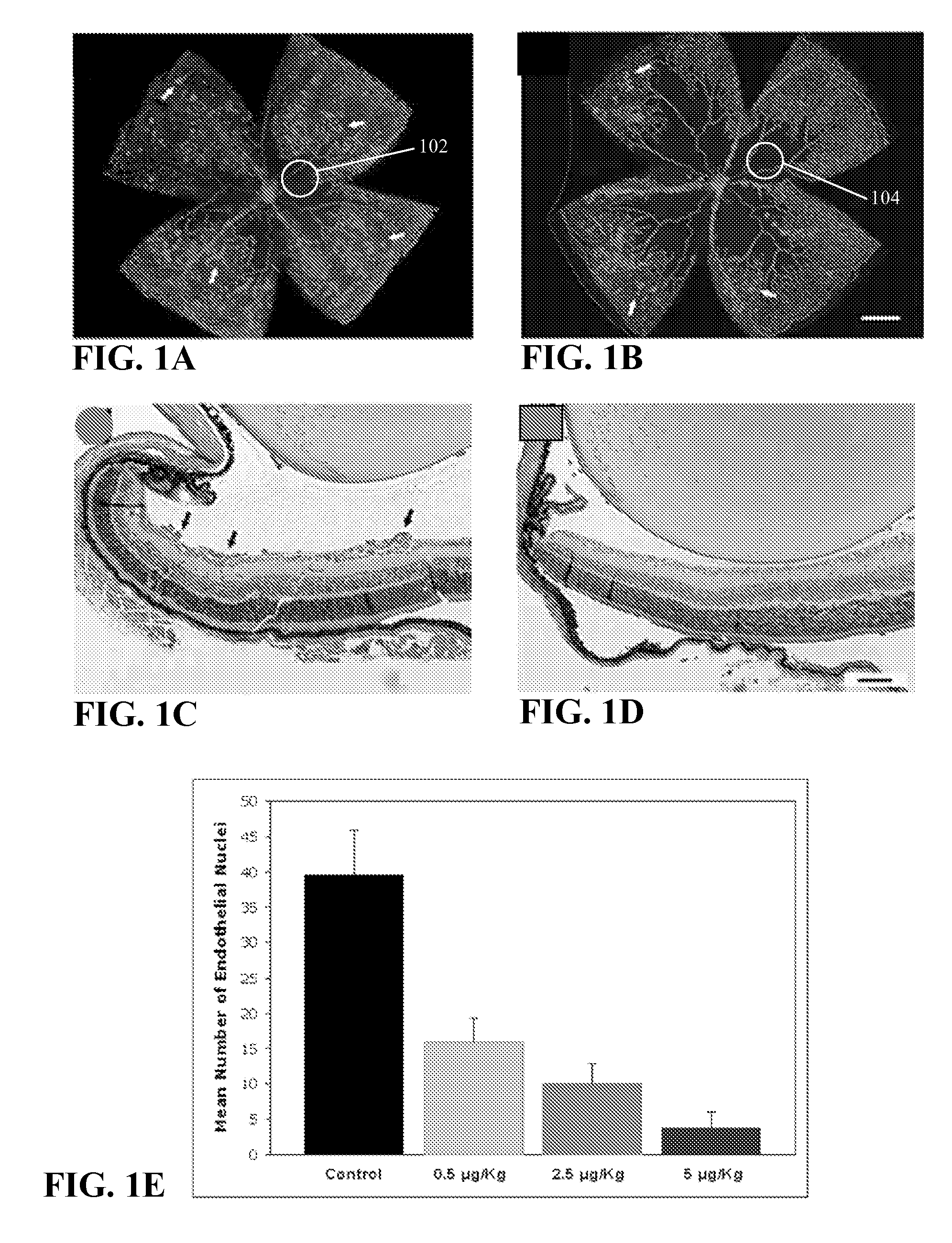
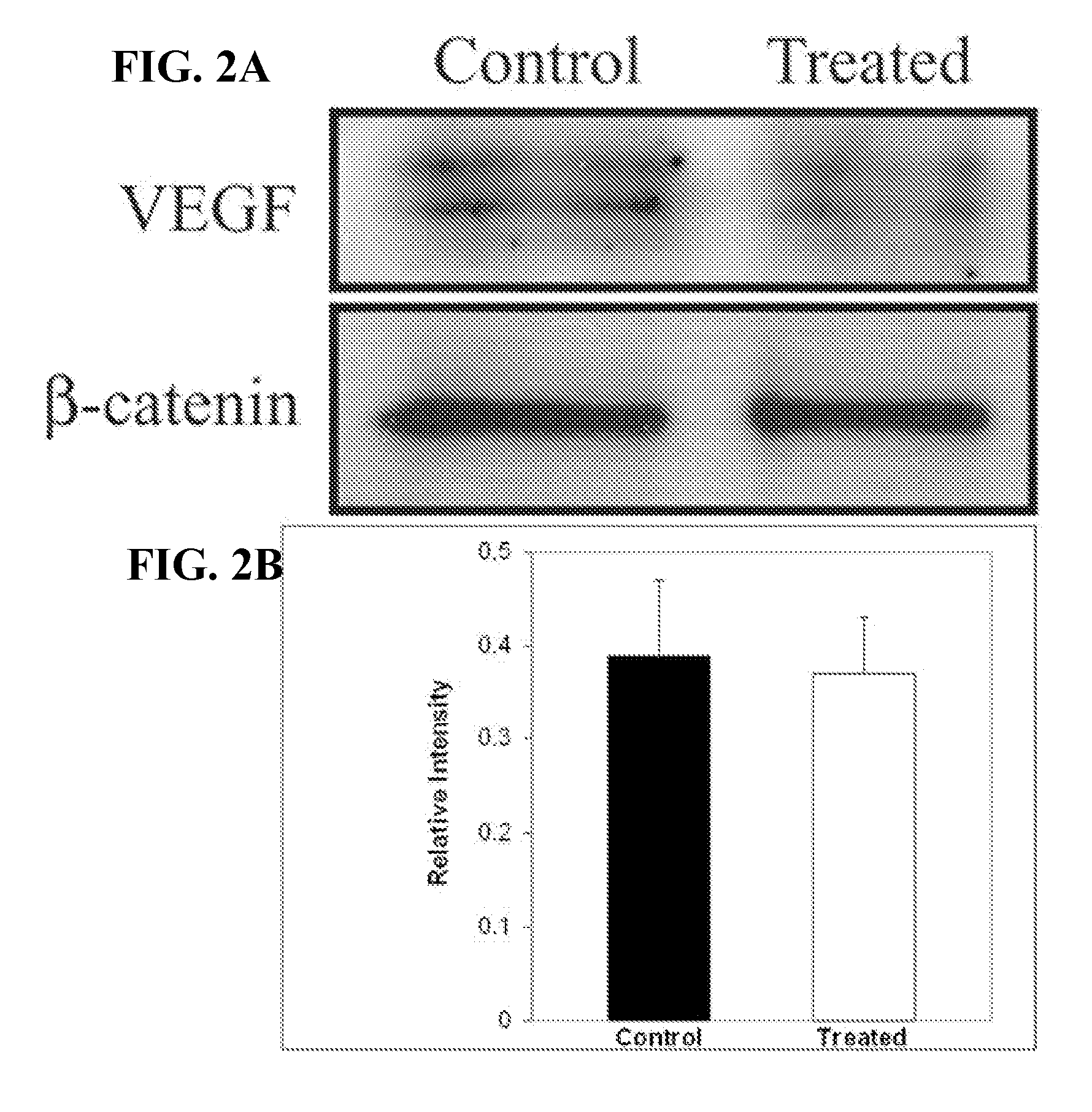
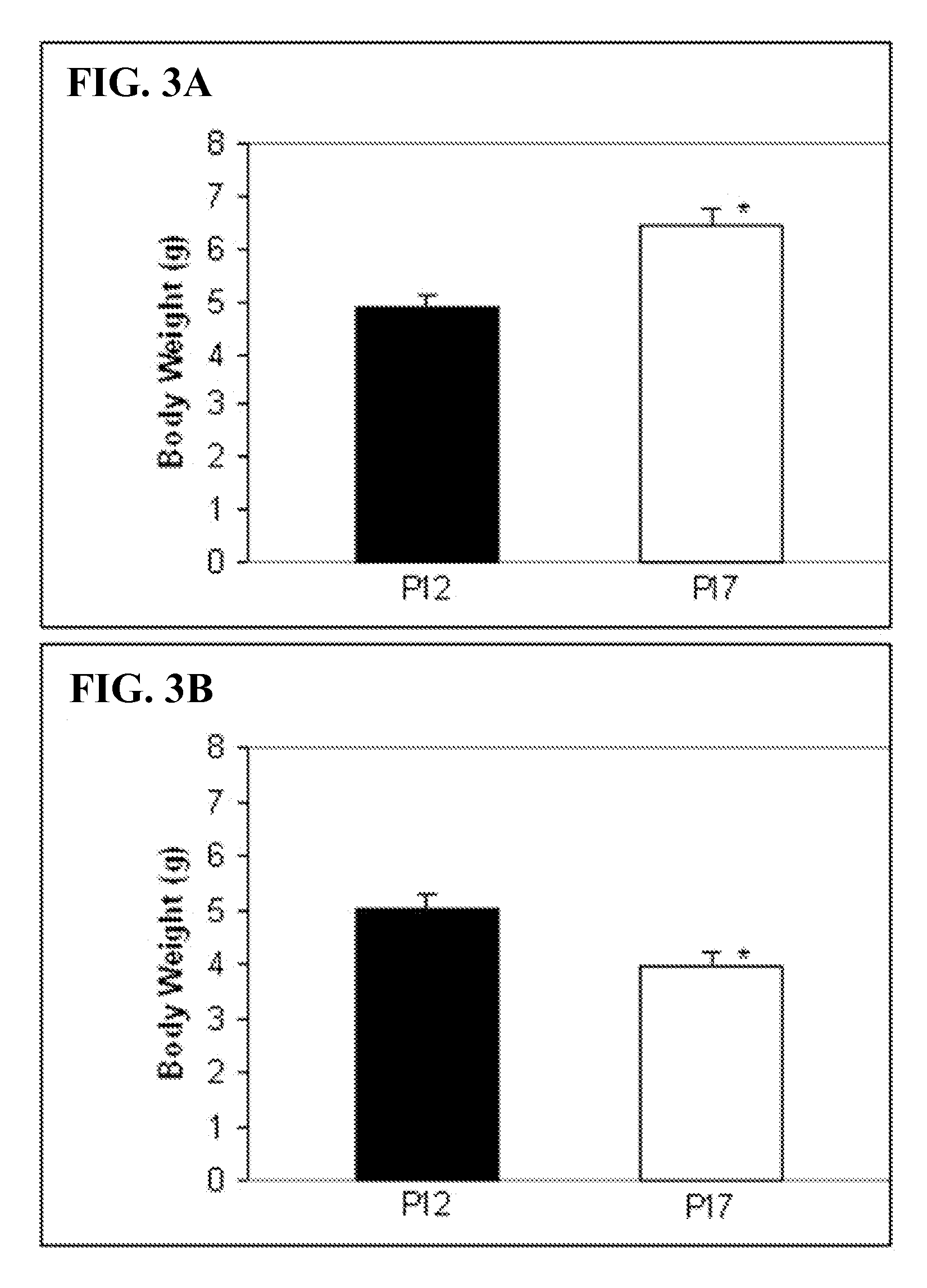
Method of using calcitriol for treating intraocular diseases associated with angiogenesis
InactiveUS20070099879A1BiocideOrganic active ingredientsDiabetic retinopathyAngiogenesis growth factor
The present invention provides a method of treating pathologies resulting from neovascular growth in the eye such as those manifested as retinopathy of prematurity, diabetic retinopathy and macular degeneration. The invention comprises the administration of an effective amount of calcitriol that is administered at doses less than toxicity and results in a significant reduction in the formation of neo-vascular growth. The invention can be used to treat existing diseases or prophylactically to treat those at risk.
Owner:WISCONSIN ALUMNI RES FOUND
Oleaginous ointments comprising two solubilized bioactive agents for the treatment of psoriasis
Topically applicable, anhydrous, hydrophobic and physically / chemically stable dermatological / pharmaceutical oleaginous ointments having effective release / penetration capacity and also having therapeutically effective amounts of calcitriol and clobetasol 17-propionate solubilized therein, are well suited for the treatment of psoriasis.
Owner:GALDERMA SA
Cosmetic/dermatological inverse emulsions containing calcitriol and clobetasol 17-propionate
InactiveUS20050281850A1Improve physical stabilityGood dispersionBiocideCosmetic preparationsEmulsionCalcitriol
Topically applicable cosmetic / dermatological compositions useful for the treatment of disorders of the skin, notably psoriasis, comprise inverse emulsions containing a glycol or water-glycol dispersed hydrophilic phase, a lipophilic continuous phase, an emulsifier having an HLB of between 2 and 7 and also having calcitriol and clobetasol 17-propionate dissolved therein.
Owner:GALDERMA SA
Pharmaceutical compositions and preparations for treatment of metabolic bone disease
The present invention relates to a pharmaceutical composition for the treatment of metabolic bone disease and the method of preparation thereof, and more particularly, to an improved pharmaceutical composition for the therapeutic treatment of metabolic disease and the method of preparation thereof, wherein said composition is prepared as a composite pharmaceutical agent which comprises calcitriol; which reduces the rate of spine fractures and increases bone density; alendronate, a bone resorption inhibitor, as two main active ingredients in an optimal mixing ratio to exert the greatest synergistic therapeutic effect; and adequate amount of other additives such as a resorption fortifier of alendronate. Thereof, the pharmaceutical composition according to the present invention can inhibit hypercalcemia caused when administered by calcitriol alone, compensate the inhibitory activity of bone remodeling caused by alendronate due to the presence of calcitriol, and improve drug compliance associated with the usual difficulty in administration as well as a side effect in esophagus, thus effectively preventing the occurence of osteoporosis.
Owner:YUYU IND
Softgel containing calcitriol and preparation method
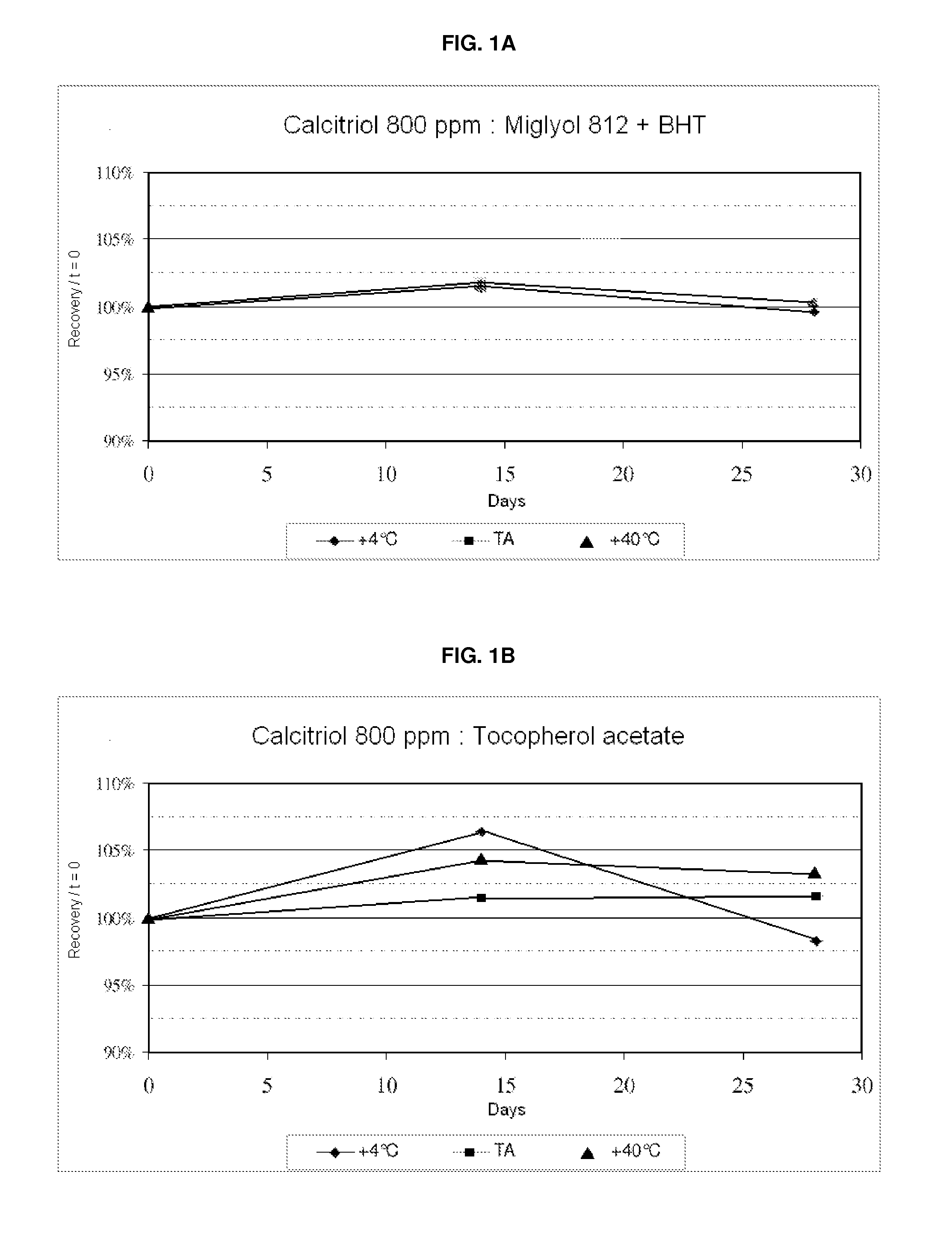
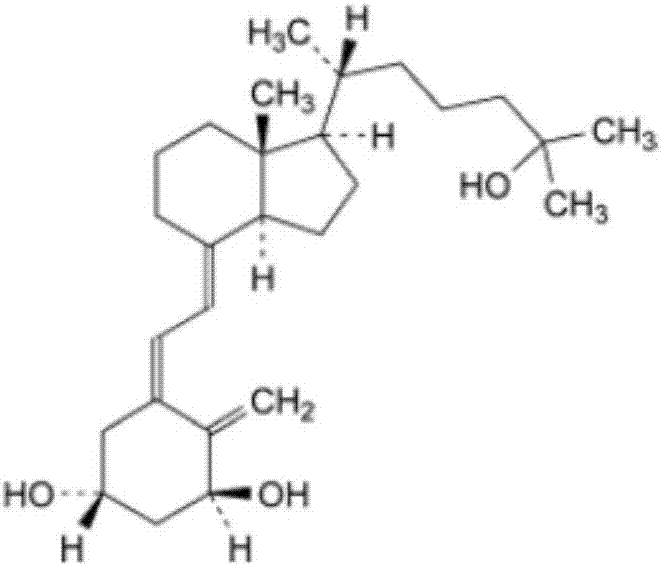
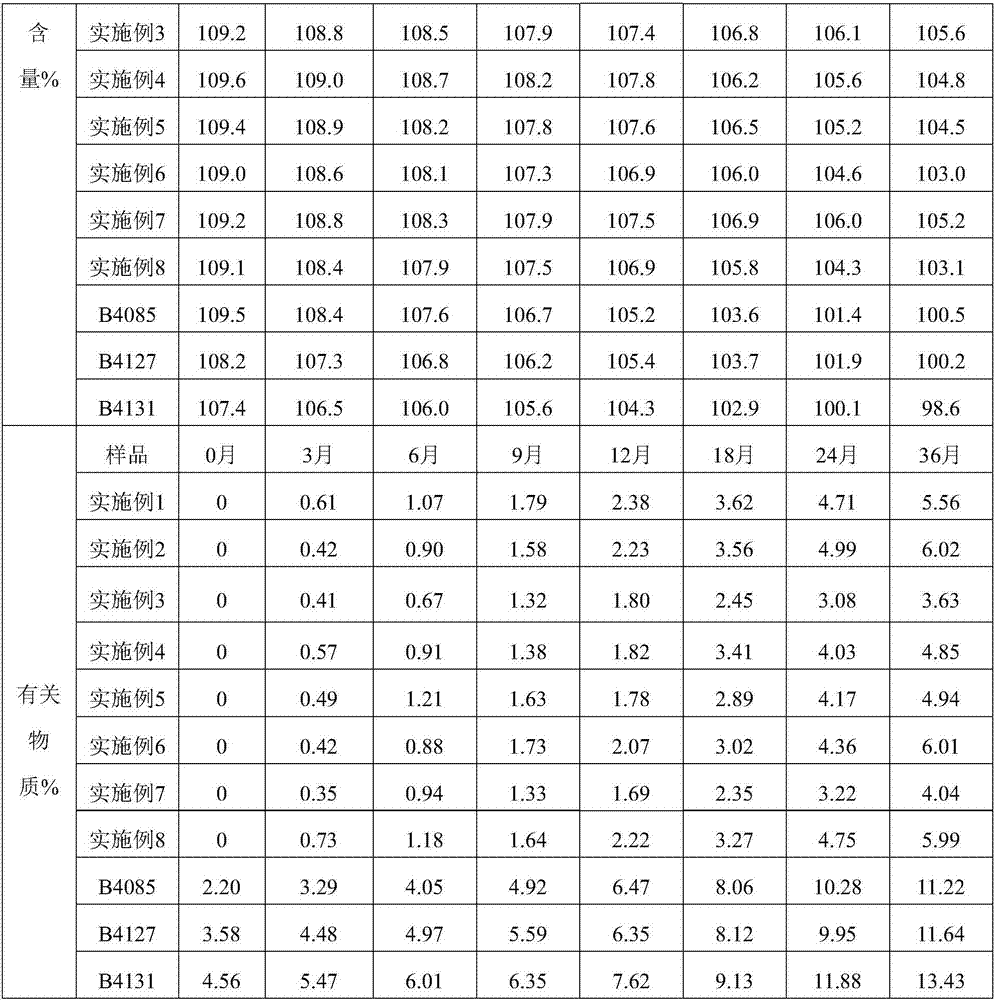
The invention relates to a softgel containing calcitriol, and the softgel comprises the following raw materials: calcitriol, middle chain triglyceride, 2,6-di-tert-butyl-4-methylphenol and butylated hydroxyanisole. The rubber of the softgel contains gelatin, glycerin, a light-screening agent and a colouring agent, and sorbitol is not contained. The preferably calcitriol amount is 0.25 mum / pill-1.00 mum / pill, middle chain triglyceride amount is 0.10g / pill-1.00g / pill, the amount of 2,6-di-tert-butyl-4-methylphenol and butylated hydroxyanisole respectively account 0.0030%-0.0090% of weight of middle chain triglyceride. The softgel containing calcitriol has the advantages of simple preparation, little impurity increase in a storage process, stable quality and high security.
Owner:SICHUAN GOWELL PHARMA
Calcitriol/clobetasol propionate compositions for the treatment of psoriasis
InactiveUS20050282792A1Good synergyOrganic active ingredientsDermatological disorderRegimenCalcitriol
Topically applicable pharmaceutical compositions useful for the treatment of psoriasis contain respective amounts of calcitriol and clobetasol propionate permitting a once-per-day effective regimen of topical application onto the part or parts of the skin affected by psoriasis.
Owner:GALDERMA SA
Vitamin D analog preparation and preparation method thereof
ActiveCN108420797AContent uniformity is not highHighly uniform dispersionOrganic active ingredientsSkeletal disorderEldecalcitolDrug product
The invention discloses a vitamin D analog preparation and a preparation method thereof. The invention aims to solve the problems that a conventional preparation method of vitamin D analog has difficulty in meeting the uniformity requirement and during the granulation process, drugs are converted and degraded easily. For the first time, a double screw extrusion technology is used to prepare a solid preparation of vitamin D analog. Various vitamin D analogs are taken as model drugs, on the basis that a preparation technology of solid preparations is deeply researched, alfacalcidol, eldecalcitol, or calcitriol is chosen to prepare the solid preparation by using a double screw extruding machine; the uniformity meets the pharmacopeia requirements; the drug stability is obviously enhanced, andthe drug quality is greatly improved.
Owner:NANJING HERON PHARM CO LTD
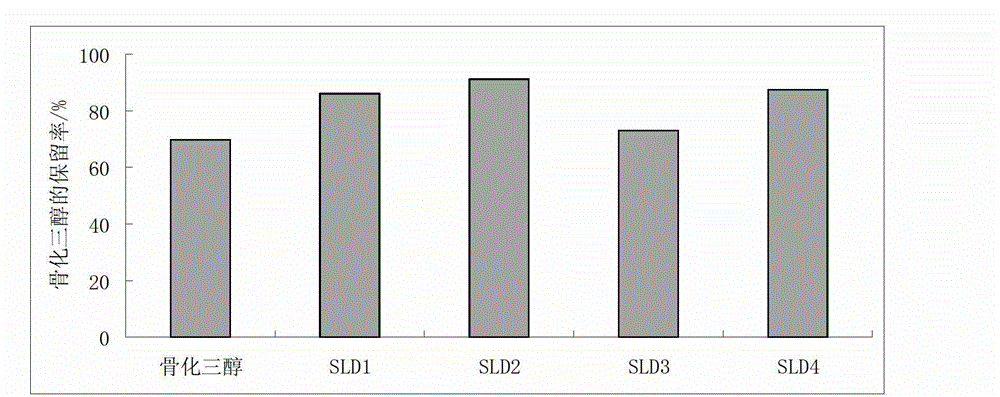
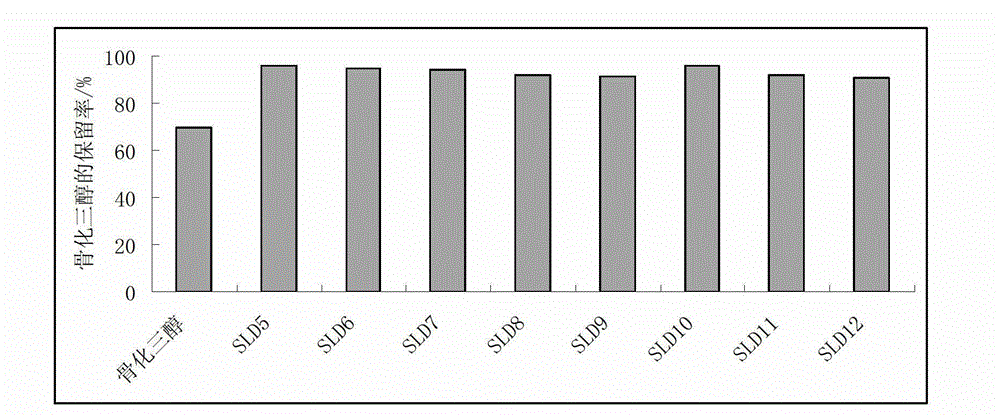
Calcitriol solid lipidic dispersion and preparation method thereof
ActiveCN102973528AImprove stabilityImprove drug stabilityOrganic active ingredientsSkeletal disorderVitamin E AcetatePolyethylene glycol
The invention discloses a calcitriol solid lipidic dispersion and a preparation method thereof. Raw materials of the calcitriol solid lipidic dispersion comprise calcitriol, one or more lipidic carriers and solid carriers, wherein a mass ratio of calcitriol to the one or more lipidic carriers is 1: (90-200000); a mass ratio of the one or more lipidic carriers to the solid carriers is (1-2): 1; and the one or more lipidic carriers are selected from caprylic triglyceride, capric triglyceride, caprylic / capric triglyceride, laurin, caprylic dilaurin, capric dilaurin, vitamin E, vitamin E succinate, vitamin E polyethylene glycol succinate and vitamin E acetate. The calcitriol solid lipidic dispersion has high drug stability and can keep a constant treatment level. The preparation method does not adopt an inorganic solvent, is suitable for industrial production and is conducive to storage and taking. An experiment proves that the calcitriol solid lipidic dispersion has good stability and dispersion uniformity satisfying requirements.
Owner:SUN YAT SEN UNIV
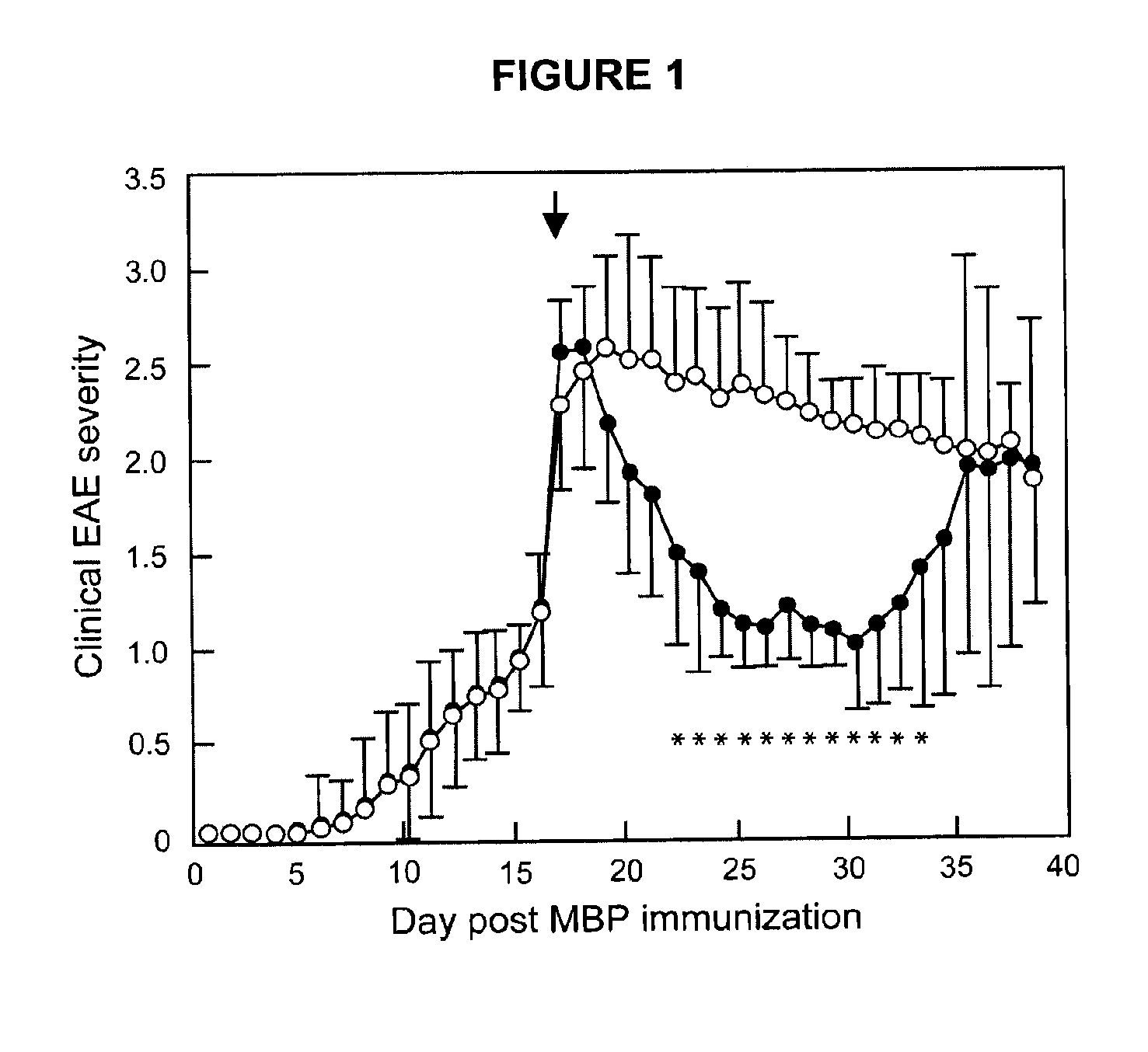
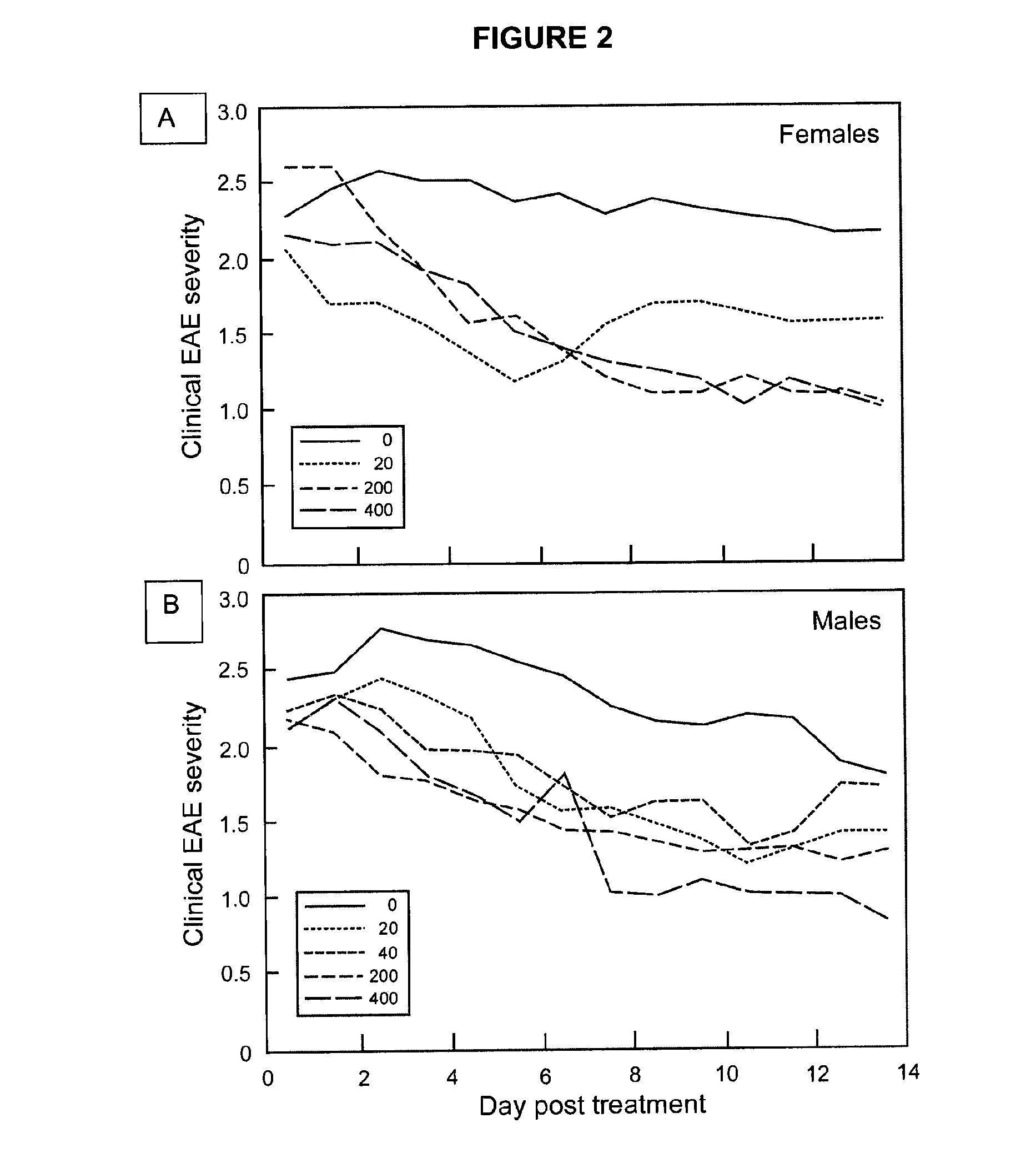
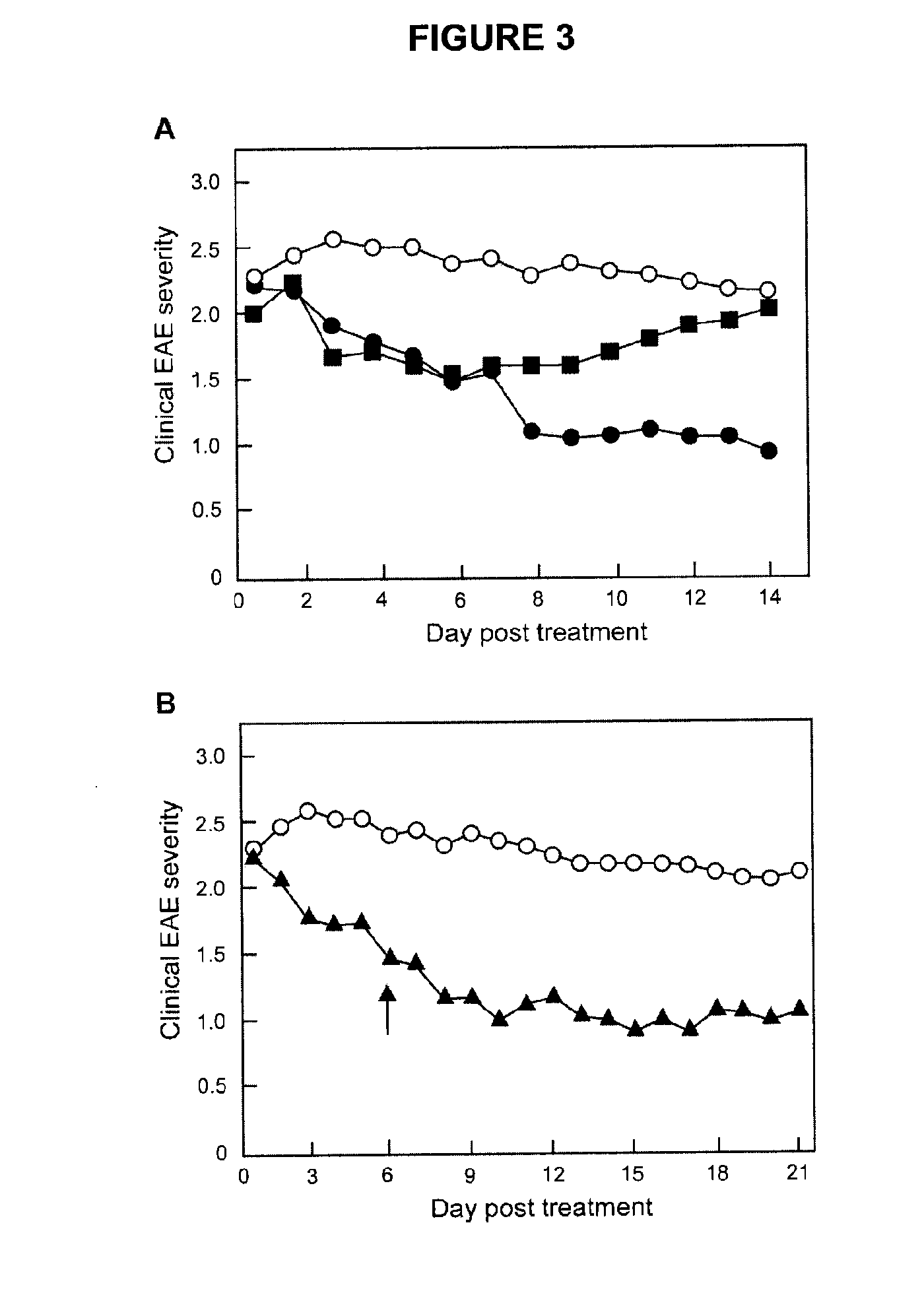
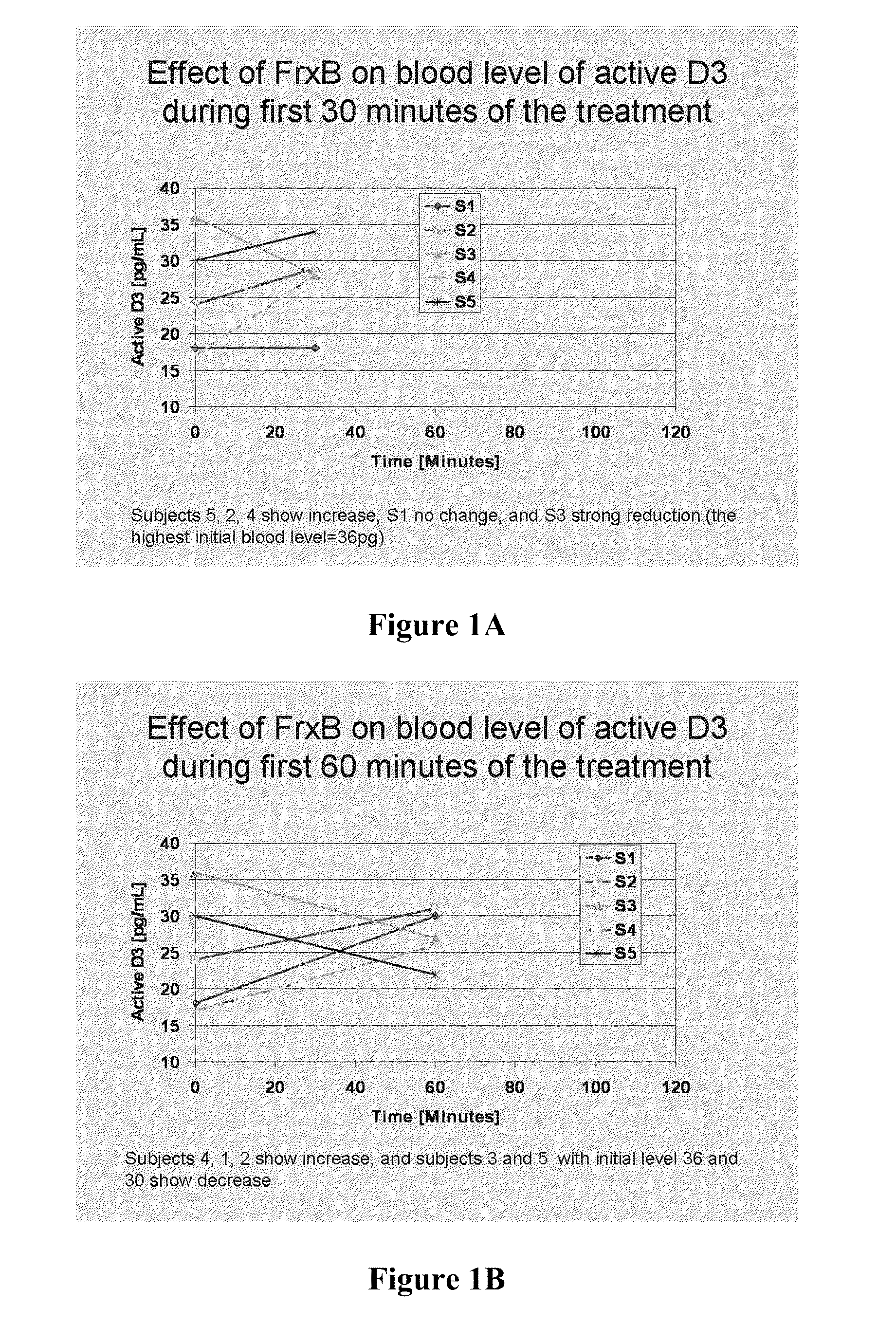
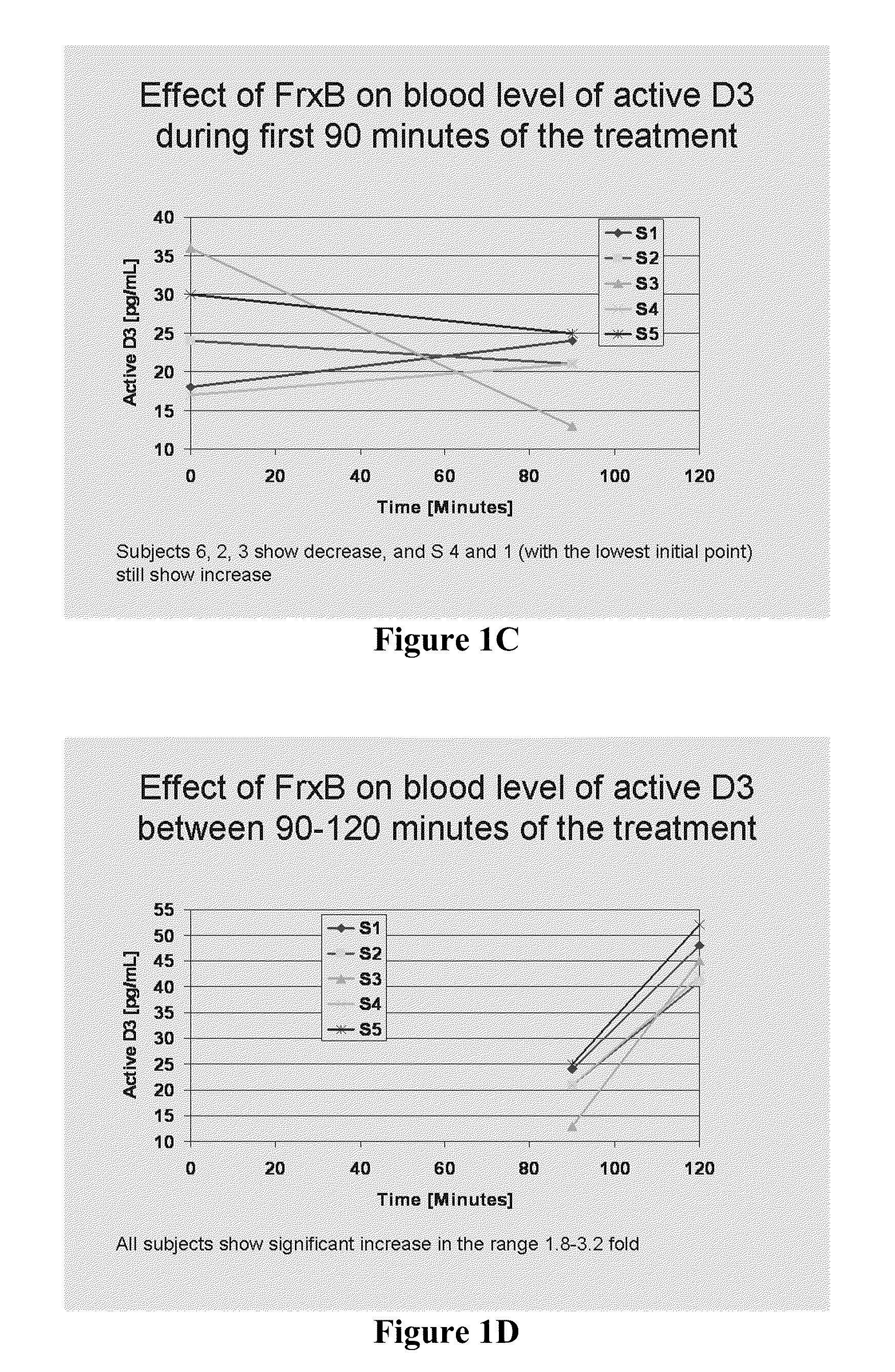
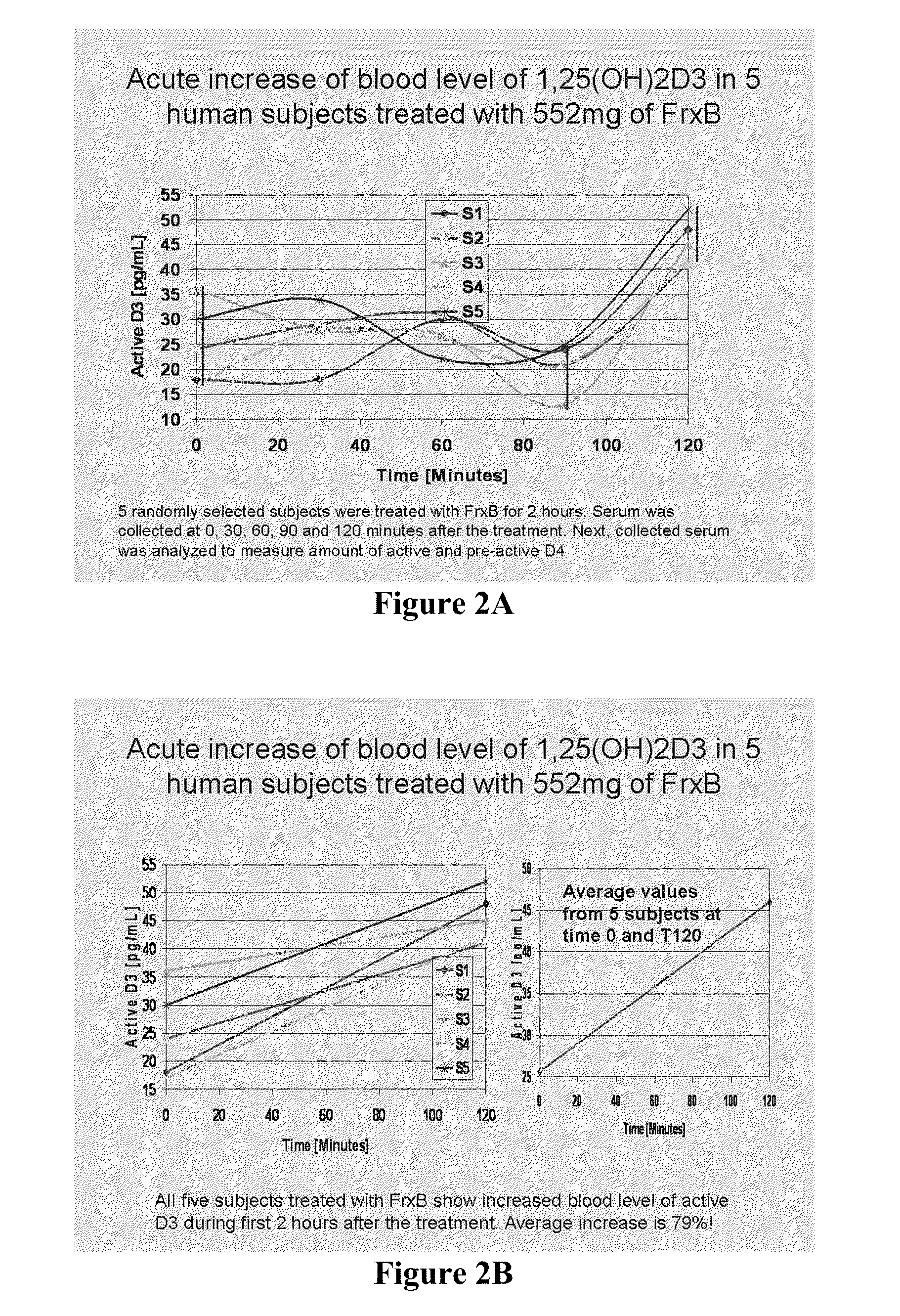
Methods of treating multiple sclerosis by administering pulse dose calcitriol
InactiveUS20090221538A1AvoidingRelieve symptomsBiocideOrganic active ingredientsPhysiologyTherapeutic treatment
Prophylactic or therapeutic treatment to inhibit the development or progress of multiple sclerosis symptoms is provided by providing intermittently administered elevated doses of calcitriol, sufficiently infrequently to avoid hypercalcemia. Such methods may include maintaining at least about a normal blood level of vitamin D3 as evidenced by a 25-(OH)D3 level of at least about 50 nmol / L.
Owner:WISCONSIN ALUMNI RES FOUND
Methods of Treating Diseased Tissue
InactiveUS20120109042A1Good effectElectrotherapyPharmaceutical delivery mechanismGeneralized psoriasisContinuous use
Two or three external therapeutic options such as clobetasol propionate spray, calcitriol ointment, and excimer laser phototherapy are used to treat generalized psoriasis with systemic safety and better efficacy than any systemic or biologic agent. Combinations of these agents, for example used in parallel and / or series for specific durations, can achieve a significant result: a PASI 75 response after only four weeks and / or a PASI 95-100 response after only six weeks.
Owner:RGT UNIV OF CALIFORNIA
Sprayable pharmaceutical compositions comprising a vitamin d derivative and an oily phase
Anhydrous sprayable physically and chemically stable pharmaceutical / dermatological compositions containing a vitamin D derivative as an active pharmaceutical ingredient, particularly calcitriol, and an oily phase are formulated into a physiologically-acceptable medium, and are useful for the treatment of a variety of conditions and afflictions, notably psoriasis.
Owner:GALDERMA SA
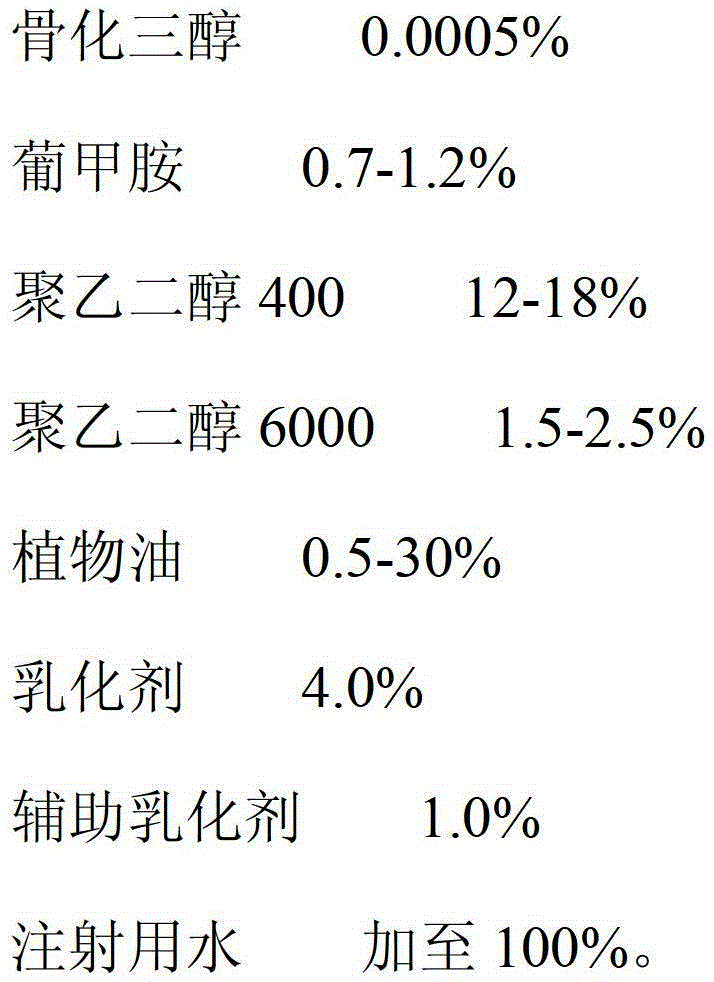
Calcitriol emulsion and preparation method thereof
ActiveCN103142478AImprove stabilityImprove bioavailabilityOrganic active ingredientsMetabolism disorderEmulsionPolyethylene glycol
The invention relates to a calcitriol emulsion and a preparation method thereof. The emulsion is prepared from the following ingredients in percentage by weight: 0.0005% of calcitriol, 0.7-1.2% of meglumine, 12-18% of polyethylene glycol 400, 1.5-2.5% of polyethylene glycol 6000, 0.5-30% of vegetable oil, 4.0% of emulsifying agent, 1.0% of coemulsifier and the balance of water for injection. According to the emulsion, the content of calcitriol is remarkably increased, so that the drug dosage is reduced; and the stability of calcitriol to light and air is improved, and the bioavailability of calcitriol is also remarkably improved.
Owner:CP PHARMA QINGDAO CO LTD
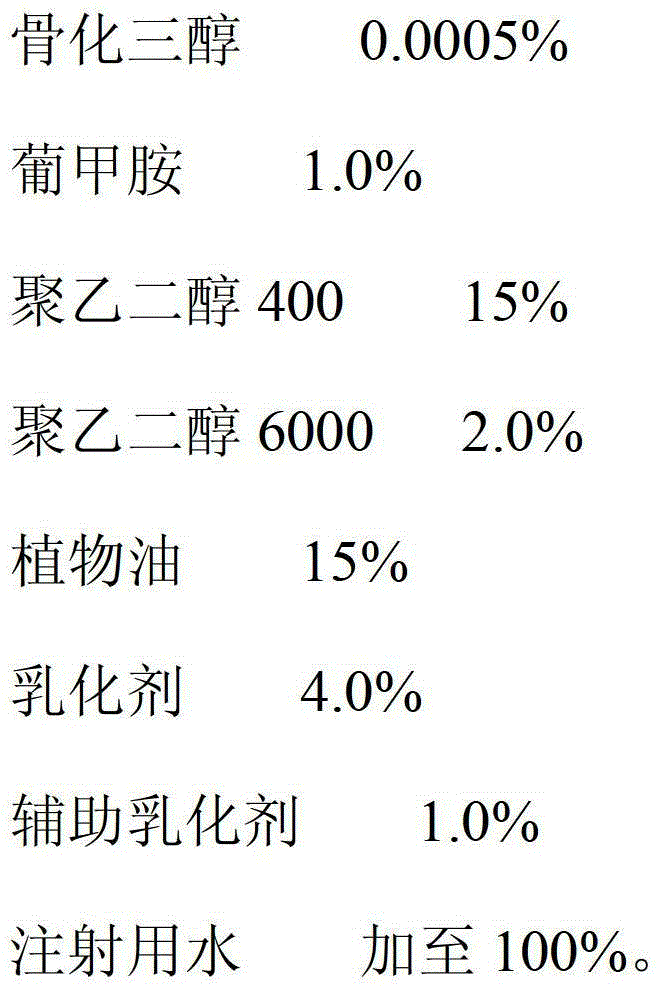
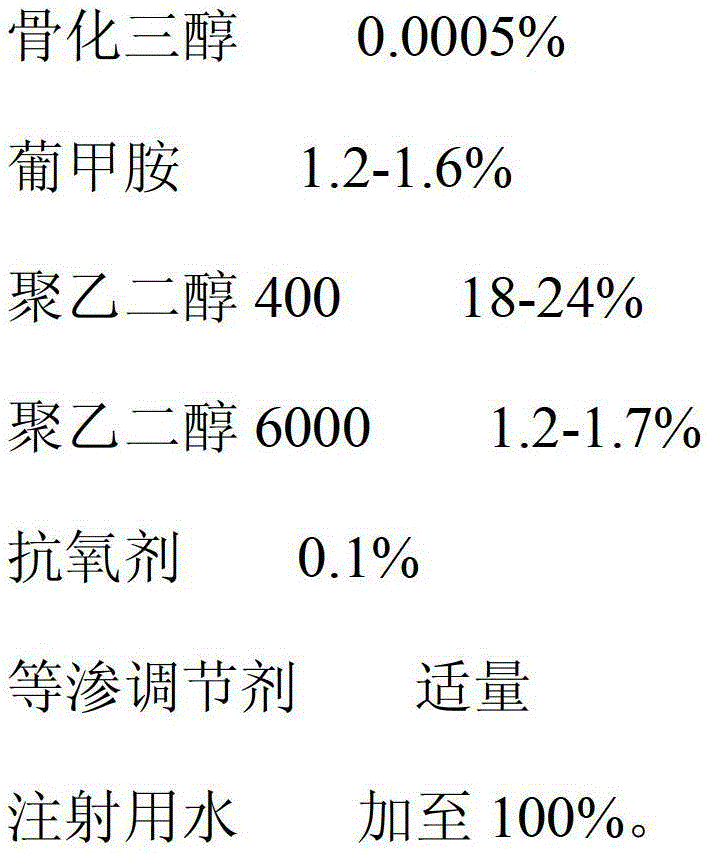
Calcitriol injection and preparation method thereof
ActiveCN103142470AImprove stabilityImprove bioavailabilityOrganic active ingredientsMetabolism disorderAdditive ingredientAntioxidant
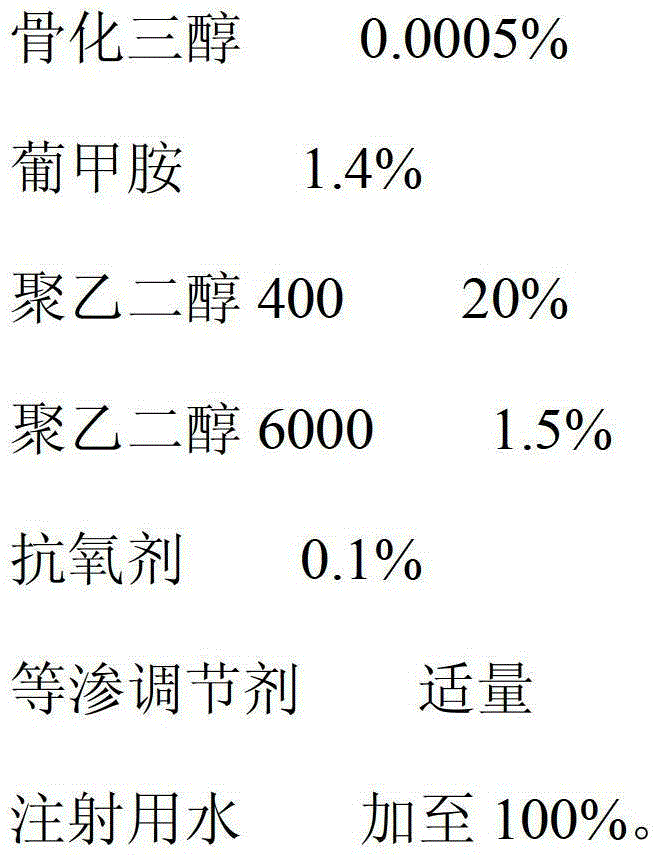
The invention relates to a calcitriol injection and a preparation method thereof. The injection is prepared from the following ingredients in percentage by weight: 0.0005% of calcitriol, 1.2-1.6% of meglumine, 18-24% of polyethylene glycol 400, 1.2-1.7% of polyethylene glycol 6000, 0.1% of antioxidant, an appropriate amount of isotonicity regulator and the balance of water for injection. According to the injection, the content of calcitriol is remarkably increased, so that the drug dosage is reduced; and the stability of calcitriol to light and air is improved, and the bioavailability of calcitriol is also remarkably improved.
Owner:CP PHARMA QINGDAO CO LTD
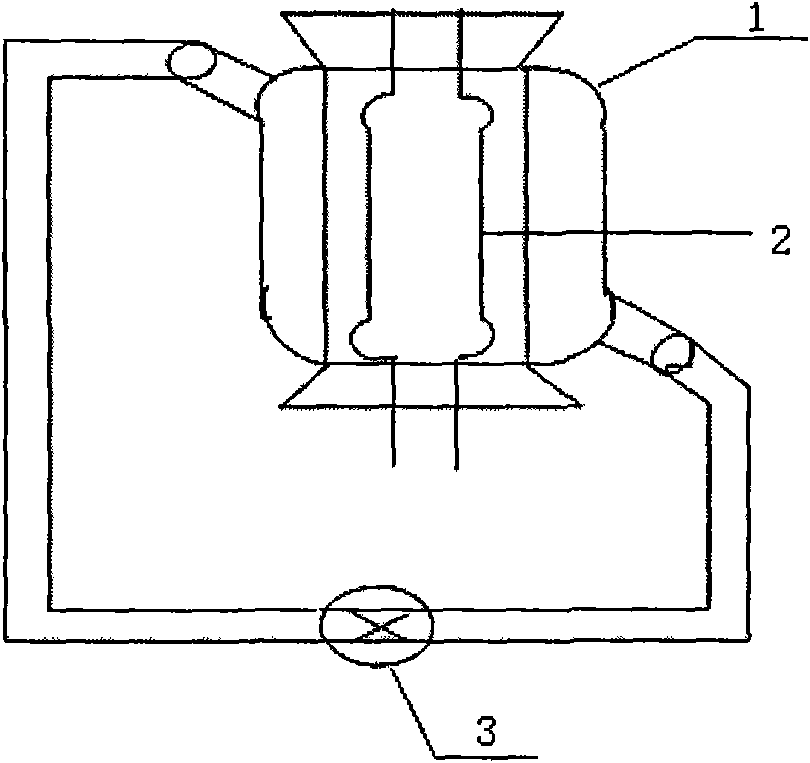
Method and device for preparing calcitriol by utilizing photochemical reaction
ActiveCN101607932ASimple structureEasy to operateOrganic chemistryChemical industryChemical reactionCooling fluid
The invention relates to a method and a device for preparing calcitriol by utilizing photochemical reaction. 5,6- trans isomer aborted as impurity in the existing production process of the calcitriol is converted into the calcitriol through the photochemical reaction, thereby the production yield of the calcitriol is increased by 50 percent, and the method for preparing the calcitriol by utilizing the photochemical reaction is carried out in a reaction device. The major structure comprises a reactor containing a clamping sleeve, a quartz glass light tube low-pressure mercury light and a low-temperature cooling liquid recycle pump, wherein the reactor is internally provided with the clamping sleeve, an inner sleeve adopts heat-resistant quartz glass, and an outer sleeve adopts the heat-resistant quartz glass the outer wall of which is plated with silver mirror; the mercury light is arranged in the inner sleeve of the reactor; reaction liquid is arranged in the outer sleeve of the reactor; and the outer sleeve of the reactor is provided with an upper outlet and a lower outlet. The preparation method has simple technical process, easy control of reaction, high product yield and strong environmental protection, and the device has simple structure, convenient operation, energy saving and good product yield.
Owner:CP PHARMA QINGDAO CO LTD
Pharmaceutical compositions comprising calcitriol and a clobetasol propionate
Topically applicable pharmaceutical gel, cream, lotion, solution or ointment compositions contain synergistically effective amounts of a) calcitriol and b) clobetasol propionate, formulated into a topically applicable, pharmaceutically acceptable medium therefor, and are useful for the treatment of such dermatological afflictions or conditions as psoriasis, atopic dermatitis, contact dermatitis and seborrhoeic dermatitis.
Owner:GALDERMA SA
Preparation method of calcitriol
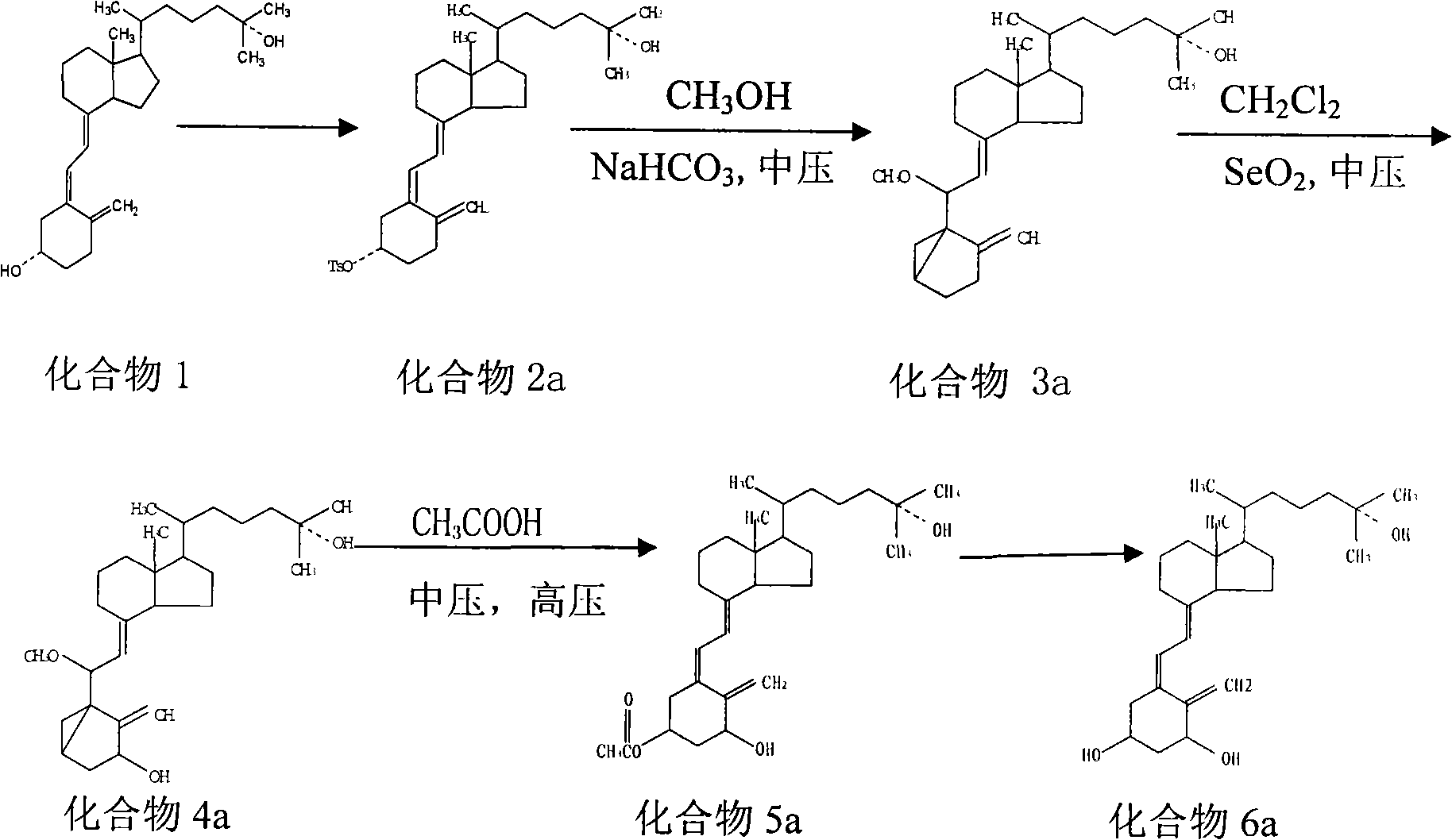
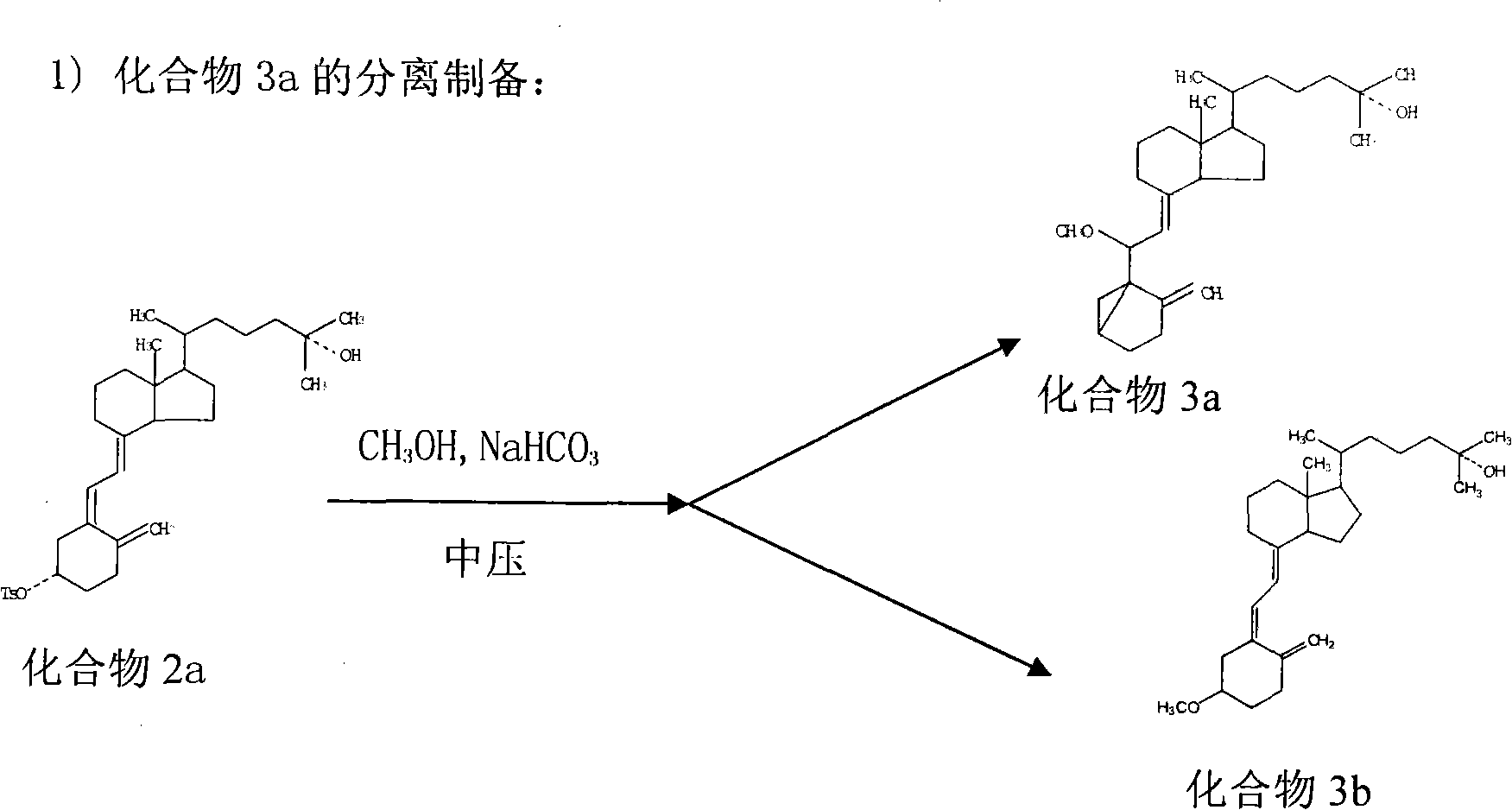
ActiveCN101607931AEfficient removalEfficient separationIon-exchange process apparatusOrganic chemistryChromatographic separationChemical reaction
The invention relates to a preparation method of calcitriol which is a compound material, belonging to the technical field of preparation of drug synthesis and organic compound synthesis. In the invention, the calcifediol is taken as raw material, a majority of produced impurities in the chemical reaction process are removed through medium-pressure liquid phase chromatography, wherein calcifediol carried out sulfonylation and ring closing on toluene, and the medium-pressure liquid phase chromatography is used for removing the impurities; products carry out oxidation reaction, medium-pressure chromatographic column separates and removes the impurities, ring opening is carried out, medium-pressure liquid phase is added for coarsely separating stereoisomeride, and at last the products separated by high-pressure liquid phase chromatography is hydrolyzed and refined to obtain the calcitriol. The invention is reasonably selected, comprehensively applies the advantages of the medium and high pressure liquid phase chromatography, has simple detaching method, can effectively remove the impurities, thoroughly separates the stereoisomeride, has high purity of the calcitriol obtained from separation, has a productive rate of 40 percent, high yield and low cost, shortens production period, is suitable for industrial scale, has application prospect, and provides a time-saving, economic and efficient method for the separation and the preparation of chiral compounds.
Owner:CP PHARMA QINGDAO CO LTD
Calcitriol dripping pill and preparation method thereof
ActiveCN101554372BImprove playbackTake effect quicklyOrganic active ingredientsSkeletal disorderDrug contentBioavailability
Owner:CP PHARMA QINGDAO CO LTD
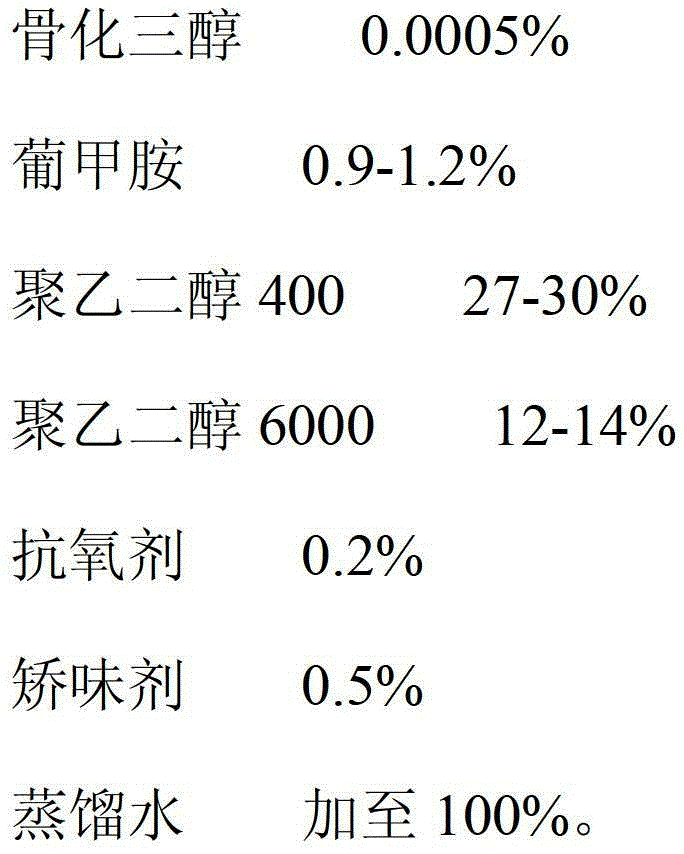
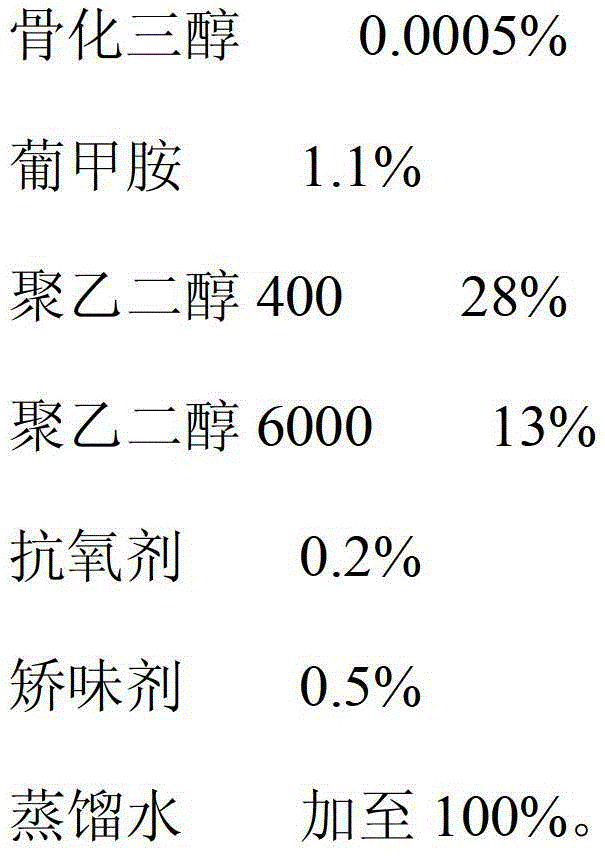
Calcitriol solution and preparation method thereof
ActiveCN103142471AIncrease contentReduce dosageOrganic active ingredientsMetabolism disorderPEG 400Distilled water
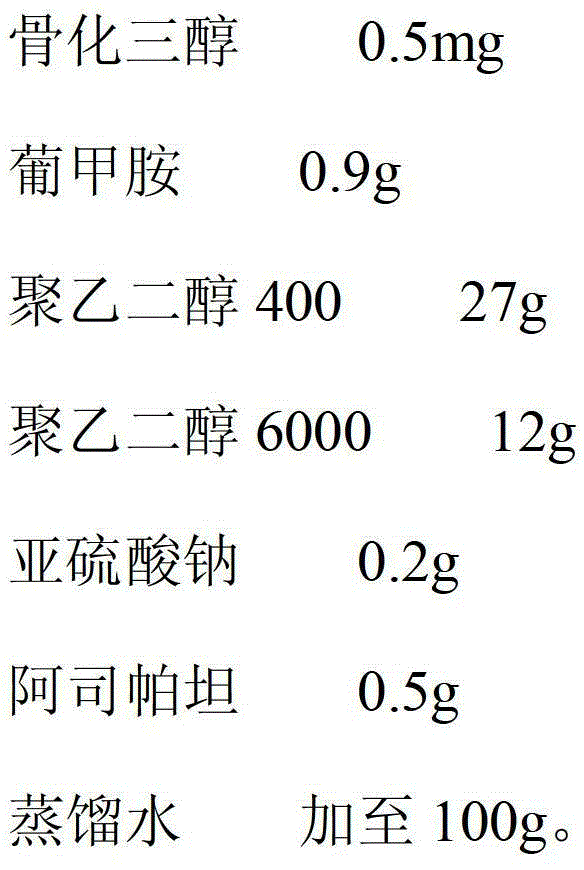
The invention relates to a calcitriol solution and a preparation method thereof. The solution is prepared from the following ingredients in percentage by weight: 0.0005% of calcitriol, 0.9-1.2% of meglumine, 27-30% of polyethylene glycol 400, 12-14% of polyethylene glycol 6000, 0.2% of antioxidant, 0.5% of flavoring agent and the balance of distilled water. Compared with the prior art, the solution has the advantages that the content of calcitriol is remarkably increased, so that the drug dosage is reduced; and the stability of calcitriol to light and air is improved, and the bioavailability of calcitriol is also remarkably improved.
Owner:CP PHARMA QINGDAO CO LTD
Calcitriol pellet and preparation method thereof
ActiveCN103142501AIncrease contentReduce dosageOrganic active ingredientsMetabolism disorderAdditive ingredientPolyethylene glycol
The invention relates to a calcitriol pellet and a preparation method thereof. The pellet is prepared from the following ingredients in percentage by weight: 0.0005% of calcitriol, 2.0-4.0% of meglumine, 1.2-1.8% of polyethylene glycol 400, 15-25% of polyethylene glycol 6000, 50.0-70.0% of filler, 5.0-10.0% of disintegrant and an appropriate amount of binder. According to the pellet, the content of calcitriol is remarkably increased, so that the drug dosage is reduced; and the stability of calcitriol to light and air is improved, and the bioavailability of calcitriol is also remarkably improved.
Owner:CP PHARMA QINGDAO CO LTD
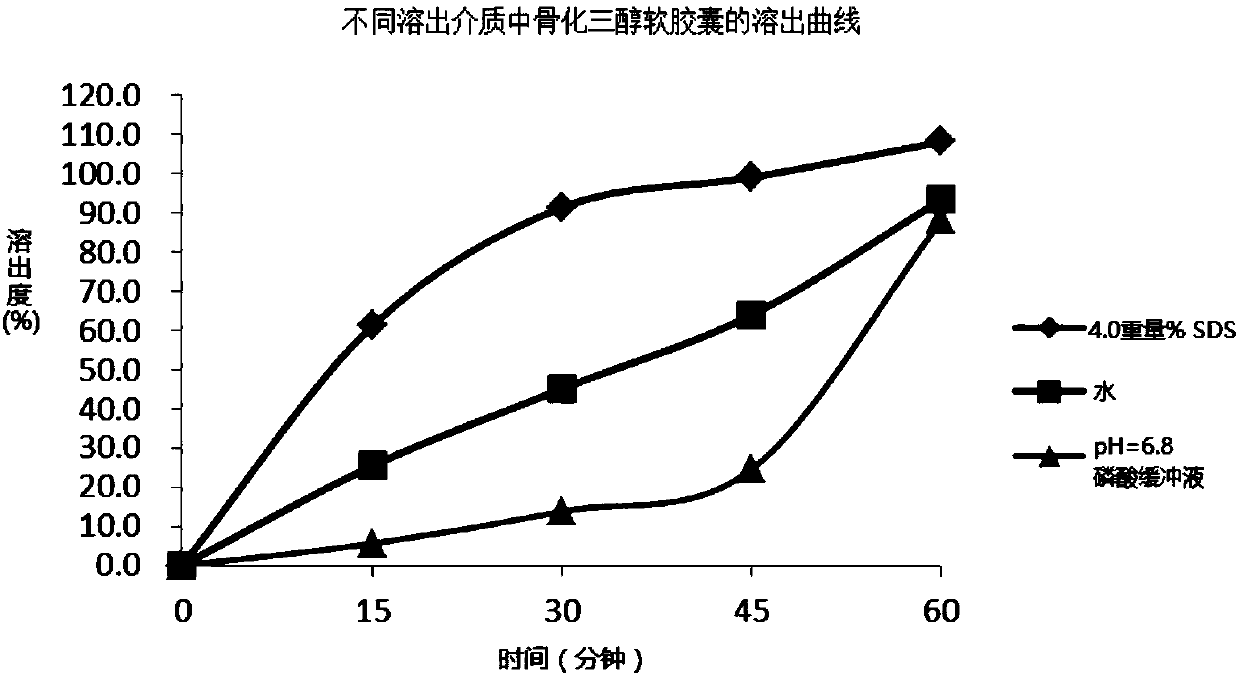
Method and system for determining dissolution rate of calcitriol soft capsules
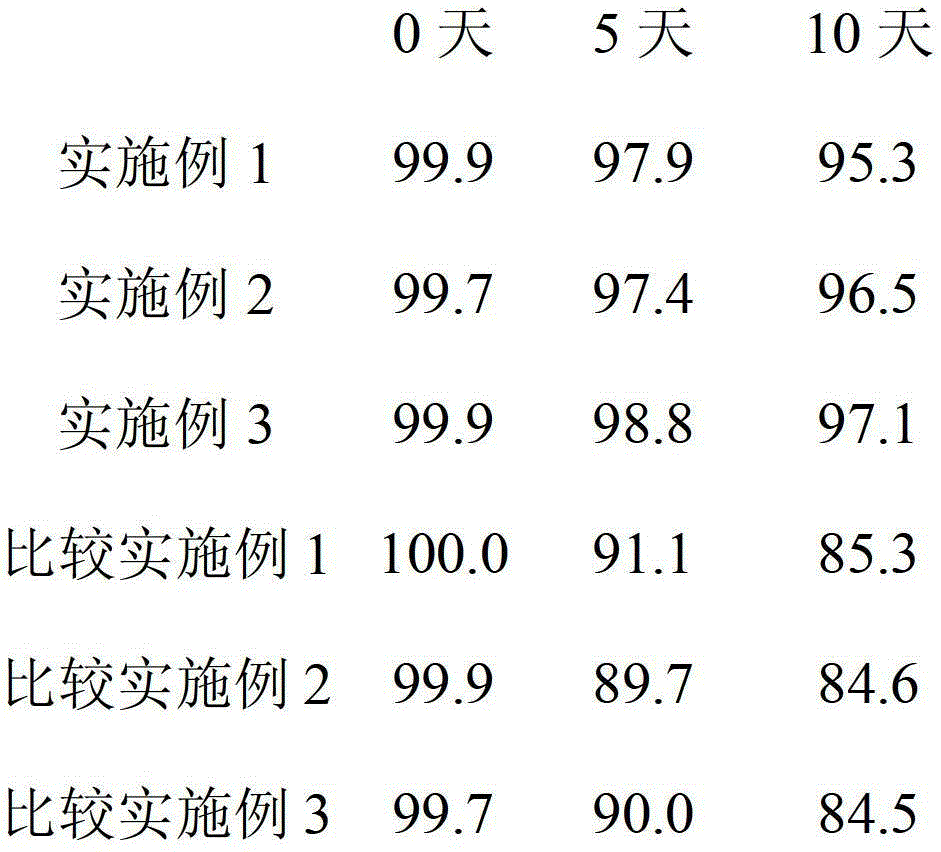
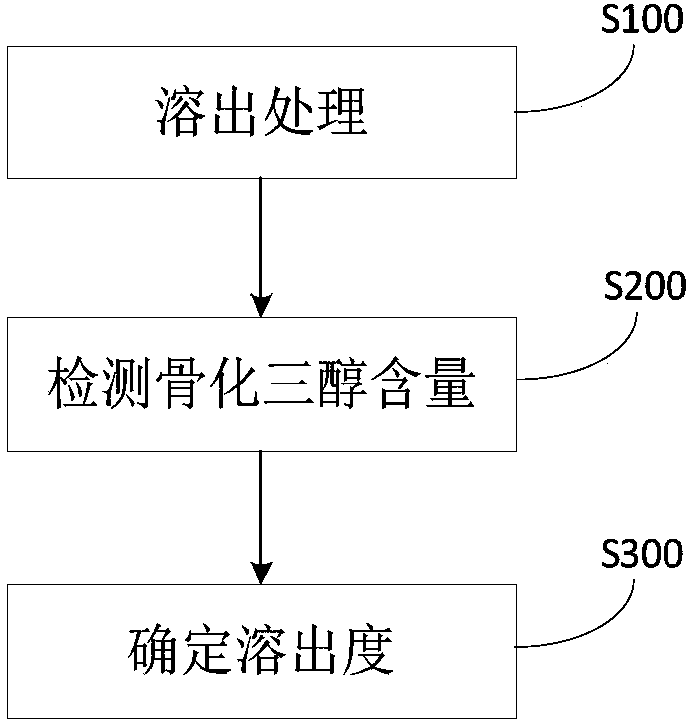
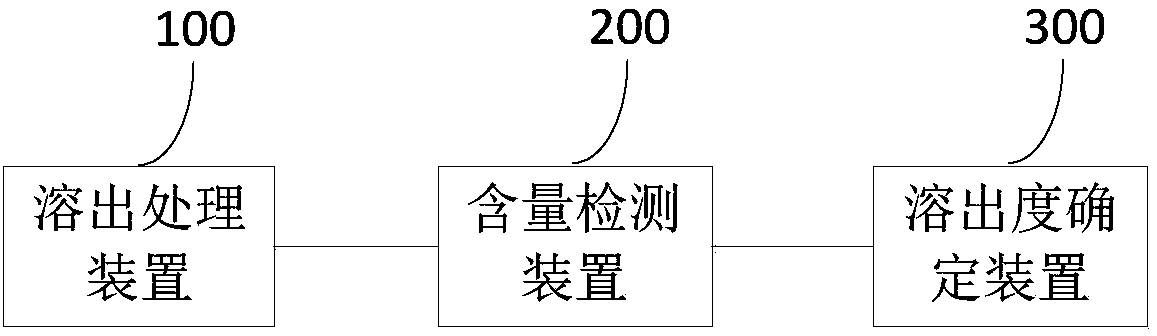
The invention discloses a method and a system for determining a dissolution rate of calcitriol soft capsules. The method comprises the following steps: (1) performing dissolution treatment on to-be-determined calcitriol soft capsules by utilizing a dissolution medium so as to conveniently obtain dissolution liquor, wherein the dissolution medium is 4.0wt% of SDS aqueous solution; (2) detecting thecontent of calcitriol in the dissolution liquor; (3) determining the dissolution rate of the calcitriol soft capsules based on the detection result of the to-be-determined calcitriol soft capsules inthe step (2). According to the method for determining the dissolution rate of the calcitriol soft capsules provided by the invention, an in-vivo dissolution behavior can be effectively simulated to determine the dissolution rate of the calcitriol soft capsules.
Owner:HUMANWELL PURACAP PHARM WUHAN CO LTD
Compositions and Methods Related to Calcitriol
ActiveUS20100256076A1Regulate immune responseReduce inflammationBiocideCarbohydrate active ingredientsOral medicationBoron containing
Contemplated compositions and methods are drawn to use of various boron-containing compounds to temporarily and transiently increase endogenous blood calcitriol concentration. The boron-containing compound is preferably a carbohydrate-boron complex having sufficient stability to achieve measurable quantities of the complex in blood upon oral administration of the complex.
Owner:VDF FUTURECEUTICALS
Calcitriol capsule type drop and preparation method thereof
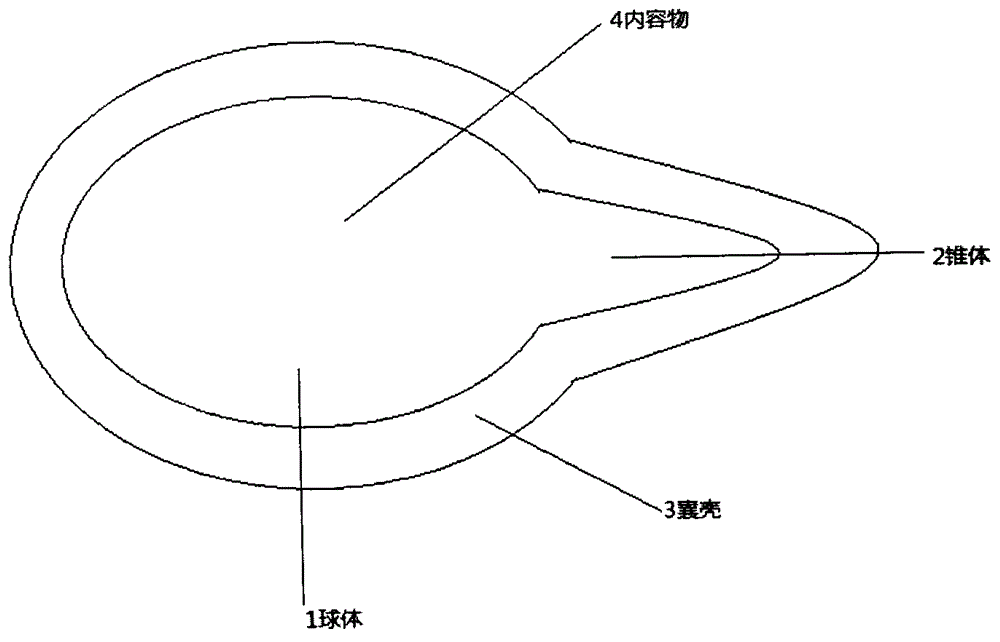
InactiveCN105362249APrevent oxidationAvoid decompositionOrganic active ingredientsSkeletal disorderMedicineSunset yellow
The present invention relates to a calcitriol capsule type drop and a preparation method thereof. The calcitriol capsule type drop comprises contents and a capsule shell, wherein the contents comprise calcitriol, butyl hydroxyanisole, butylated hydroxytoluene, and grease, and the capsule shell comprises gelatin, titanium dioxide, sunset yellow, iron oxide, water, sorbitol and glycerin. Compared with the drop in the prior art, the drop of the present invention has characteristics of high safety, easy use, strong targeting property, and the like.
Owner:HENAN TAIFENG BIOTECH CO LTD
Oral solution containing calcitriol and preparation method thereof
ActiveCN114831933AMeet clinical needsSpeed up replenishmentOrganic active ingredientsFilling using counterpressureHydroxybenzoate EthersSecondary hyperparathyroidism
The invention relates to the technical field of calcitriol, in particular to an oral solution containing calcitriol and a preparation method of the oral solution, and the oral solution containing calcitriol comprises the following components in parts by weight: 0.290-0.305 part of calcitriol; 29.7 to 30.3 parts of butyl hydroxy anisole; 29.8 to 30.2 parts of butylated hydroxytoluene (BHM); and 299987 to 300012 parts of medium chain triglycerides. The preparation method of the oral solution containing calcitriol comprises the following steps: S100, weighing: turning on a yellow light lamp in a weighing room, weighing calcitriol, medium chain triglyceride, butylated hydroxyanisole and butylated hydroxytoluene according to parts by weight, and carrying out double-person rechecking; compared with the prior art, the oral solution containing calcitriol has the following beneficial effects that the oral solution containing calcitriol can make up the blank in the field of treating secondary hyperparathyroidism of patients with moderate to severe chronic renal failure before dialysis and follow-up metabolic bone diseases and hypoparathyroidism in domestic children medication, and the clinical requirements are met.
Owner:CP PHARMA QINGDAO CO LTD
Skin cancer prevention method and product
The increased use of sunscreens, while limiting damage to the DNA, may promote cancer growth by preventing vitamin D synthesis in the skin. According to the present invention, the beneficial effects of UV radiation are obtained by incorporating vitamin D into the topical sunscreen. Application of the sunscreen prevents the harmful effects of UV radiation and the included vitamin D is activated by the skin to calcitriol for cancer prevention. Because calcitriol also promotes cellular growth and differentiation, the topical sunscreen with vitamin D may be of benefit for photoaging.
Owner:PERSON JOHN R
Dermatological compositions comprising vitamin d lipid vesicles
InactiveUS20100221350A1Reduce usageImprove comfortBiocideOrganic active ingredientsLipid formationExocytic vesicle
Dermatological / pharmaceutical compositions contain lipid vesicles dispersed in a hydrophilic phase, such vesicles including at least one vitamin D compound and particularly calcitriol, and are useful for the treatment of dermatological pathologies, notably psoriasis.
Owner:GALDERMA SA
Calcitriol liquid hard capsule, method for preparing same and application of calcitriol liquid hard capsule
InactiveCN107362151AOvercoming the problem of poor solubilityImprove stabilityOrganic active ingredientsSkeletal disorderAntioxidantHard Capsule
The invention provides a calcitriol liquid hard capsule, a method for preparing the same and application of the calcitriol liquid hard capsule. Every 1000 calcitriol liquid hard capsules comprise 0.25-0.5 mg of calcitriol, 140-170 g of oil-based matrixes and 25-40 mg of antioxidants. The method includes mixing the antioxidants and the oil-based matrixes with one another to obtain first mixtures; stirring and dissolving the first mixtures and then adding the calcitriol into the first mixtures to obtain second mixtures; continuing to stir and dissolve the second mixtures and then vacuumizing and degassing the second mixtures; filling hard capsule shells with the mixtures to obtain the calcitriol liquid hard capsule. The calcitriol liquid hard capsule, the method and the application have the advantages that the calcitriol liquid hard capsule can be effectively applied to treating post-menopause and senile osteoporosis, chronic renal failure and the like; the content uniformity of the calcitriol liquid hard capsule which is a medicine can be improved, the calcitriol liquid hard capsule is good in stability, long in quality guarantee period, high in bioavailability, low in cost and convenient to administer, and accordingly the calcitriol liquid hard capsule, the method and the application have obvious merits.
Owner:SINOPHARM CHUANKANG PHARMACEUTICAL CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com