Vitamin D analog preparation and preparation method thereof
A technology for vitamins and analogs, which is applied in the preparation of pharmaceutical preparations, vitamin D analog preparations and their preparation fields, can solve problems such as the application of twin-screw extrusion technology that has not yet been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Preparation of Embodiment 1 Alfacalcidol Tablets, Small Test Process
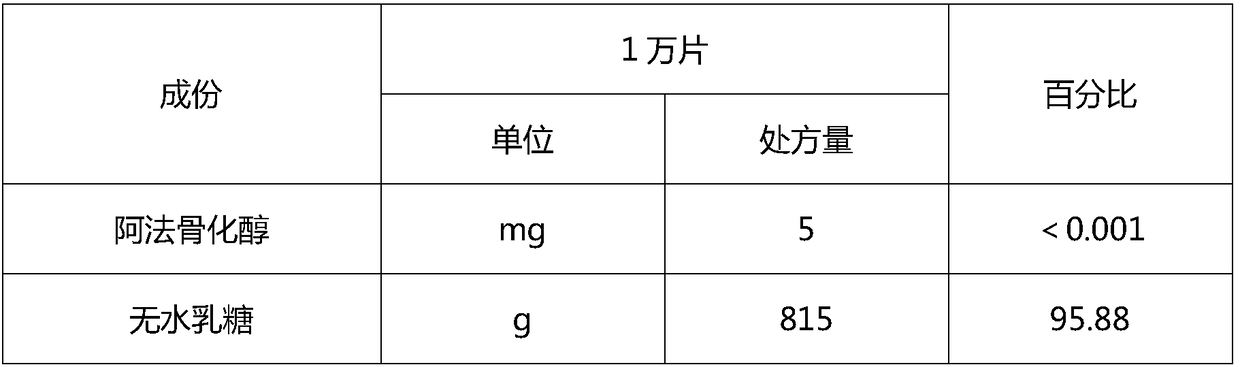
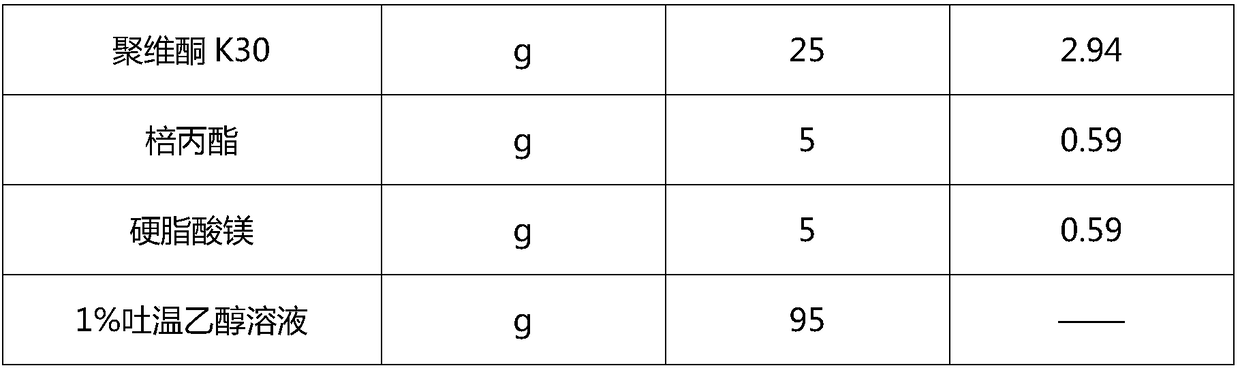
[0066] 1. Prescription
[0067]
[0068]
[0069] 2. The preparation method specifically comprises the following steps:
[0070] 1) dissolving the raw material alfacalcidol in absolute ethanol, then adding the prescribed amount of Tween and stirring to dissolve to make a solution;
[0071] 2) Weigh the prescription amount of anhydrous lactose, povidone, and propyl gallate in a wet mixing granulator, and the mixing time is greater than or equal to 5 minutes to make dry powder;
[0072] 3) Add the dry powder obtained in the above step 2) into the solid feeder, and after the solution obtained in the above step 1) is connected to the extruder through a low-pulse peristaltic pump, control the granulation temperature of the twin-screw extruder at 20-25 Between ℃, adjust the feeding speed of dry powder to 1.0-2.0kg / hr, the speed of peristaltic pump to 2rpm-4rpm, and the speed of twin-screw to 100rpm...
Embodiment 2
[0076] Preparation of Embodiment 2 Alfacalcidol Tablets, Pilot Test Process
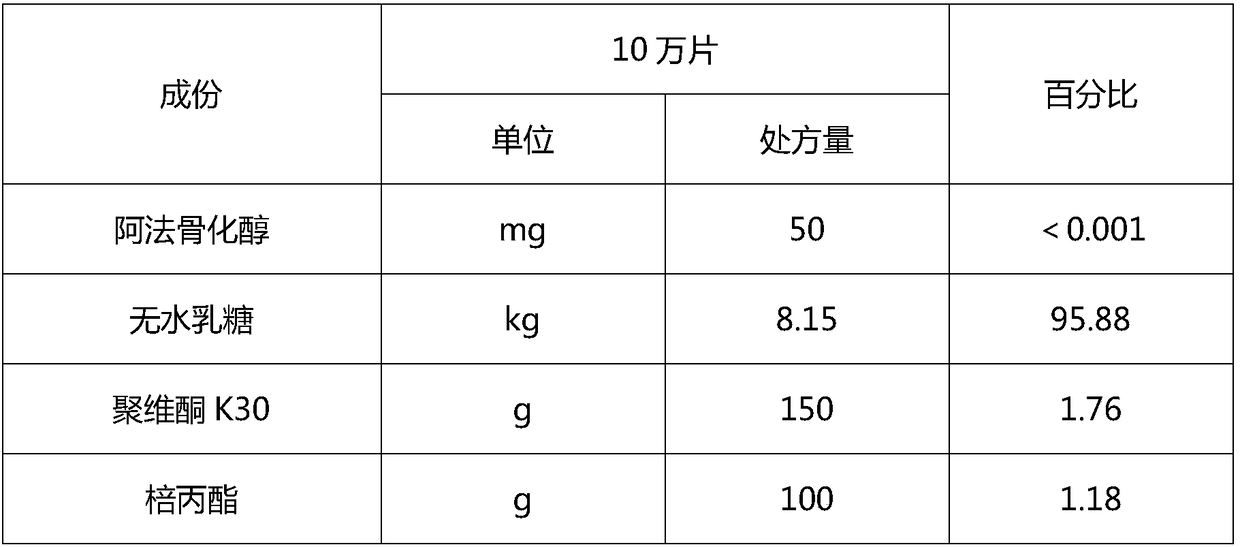
[0077] 1. Prescription
[0078]
[0079]
[0080] 2. The preparation method specifically comprises the following steps:
[0081] 1) Dissolve the raw material alfacalcidol in absolute ethanol, then add the prescribed amount of Tween and stir to dissolve to make a solution; 2) Weigh the prescribed amount of anhydrous lactose, povidone, and propyl gallate in wet In the method mixing granulator, the mixing time is greater than or equal to 5 minutes, and made into dry powder;
[0082] 3) Add the dry powder obtained in the above step 2) into the solid feeder, and after the solution obtained in the above step 1) is connected to the extruder through a low-pulse peristaltic pump, control the granulation temperature of the twin-screw extruder at 20-25 Between ℃, adjust the feeding speed of dry powder to 2.0-3.0kg / hr, the speed of peristaltic pump to 4rpm-8rpm, and the speed of twin-screw to 300rpm-400rpm...
Embodiment 3
[0086] Preparation of Embodiment 3 Alfacalcidol Tablets, Small Test Process
[0087] 1. Prescription
[0088]
[0089]
[0090] 2. The preparation method specifically comprises the following steps:
[0091] 1) dissolving the crude drug alfacalcidol in absolute ethanol to make a solution;
[0092] 2) Weigh the prescription amount of anhydrous lactose, povidone, and propyl gallate in a wet mixing granulator, and the mixing time is greater than or equal to 5 minutes to make dry powder;
[0093] 3) Add the dry powder obtained in the above step 2) into the solid feeder, and after the solution obtained in the above step 1) is connected to the extruder through a low-pulse peristaltic pump, control the granulation temperature of the twin-screw extruder at 20-25 Between ℃, adjust the feeding speed of dry powder to 1.0-2.0kg / hr, the speed of peristaltic pump to 2rpm-4rpm, and the speed of twin-screw to 100rpm-200rpm, and the above-mentioned dry powder and solution enter the twin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com