Patents
Literature
1108 results about "Clinical research" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Clinical research is a branch of healthcare science that determines the safety and effectiveness (efficacy) of medications, devices, diagnostic products and treatment regimens intended for human use. These may be used for prevention, treatment, diagnosis or for relieving symptoms of a disease. Clinical research is different from clinical practice. In clinical practice established treatments are used, while in clinical research evidence is collected to establish a treatment.
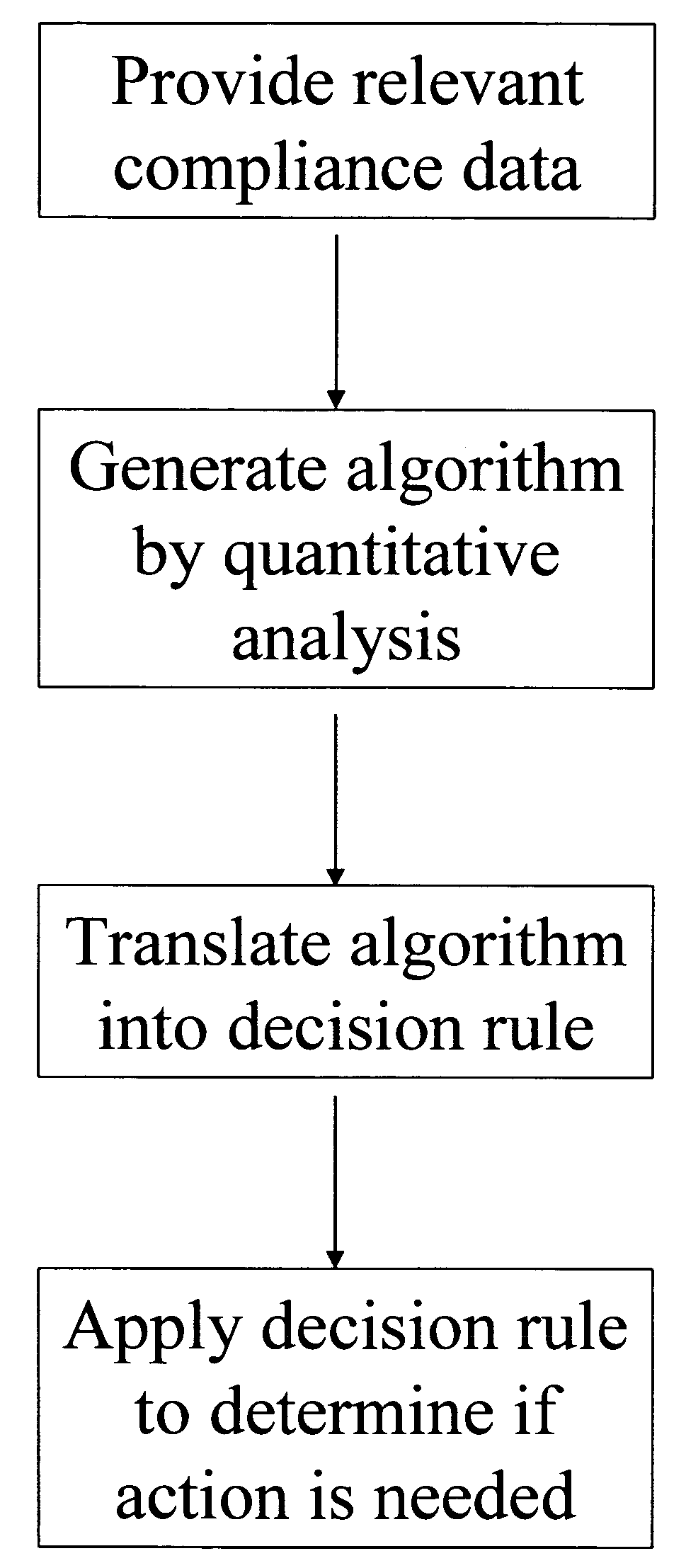
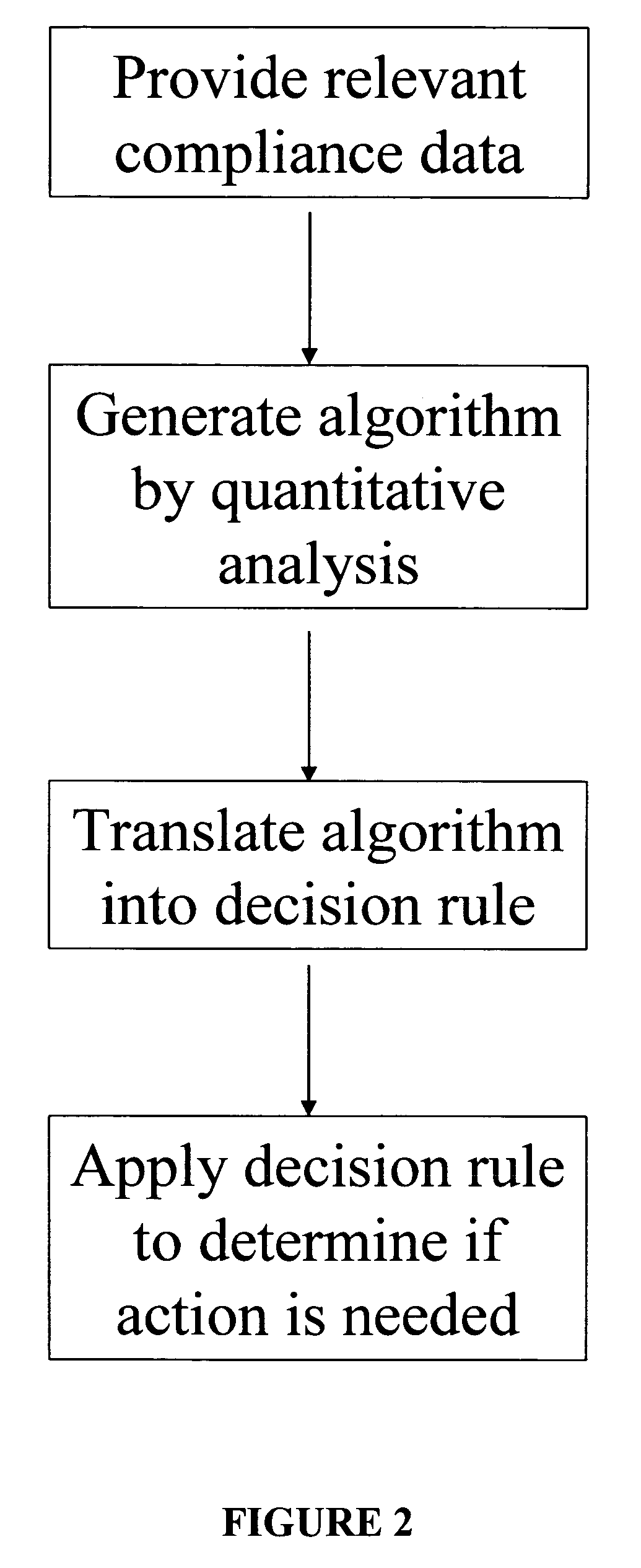
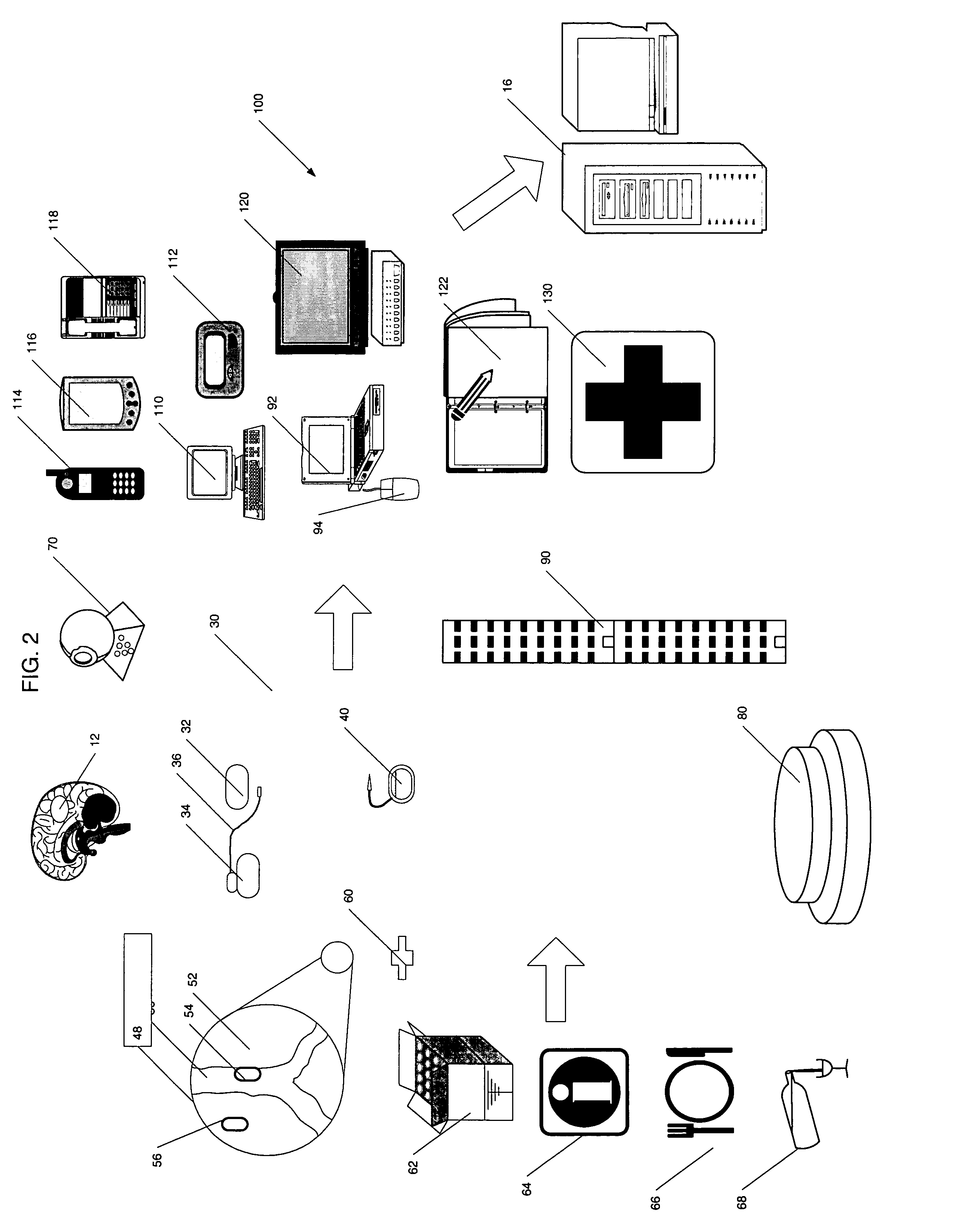
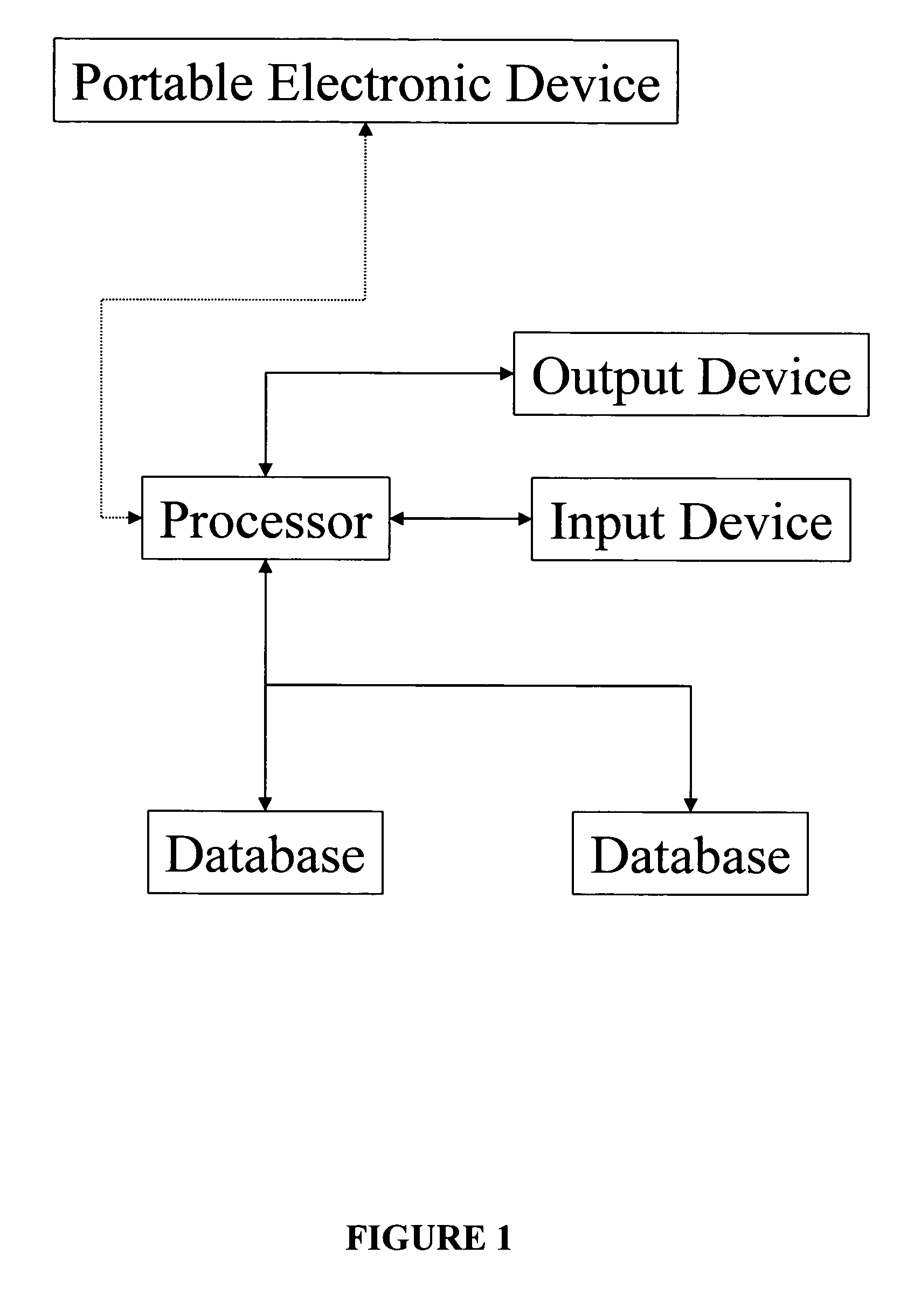
Apparatus and method for prediction and management of participant compliance in clinical research
InactiveUS7415447B2Reduce testing costsImprove statistics performanceDigital computer detailsForecastingNon complianceClinical trial
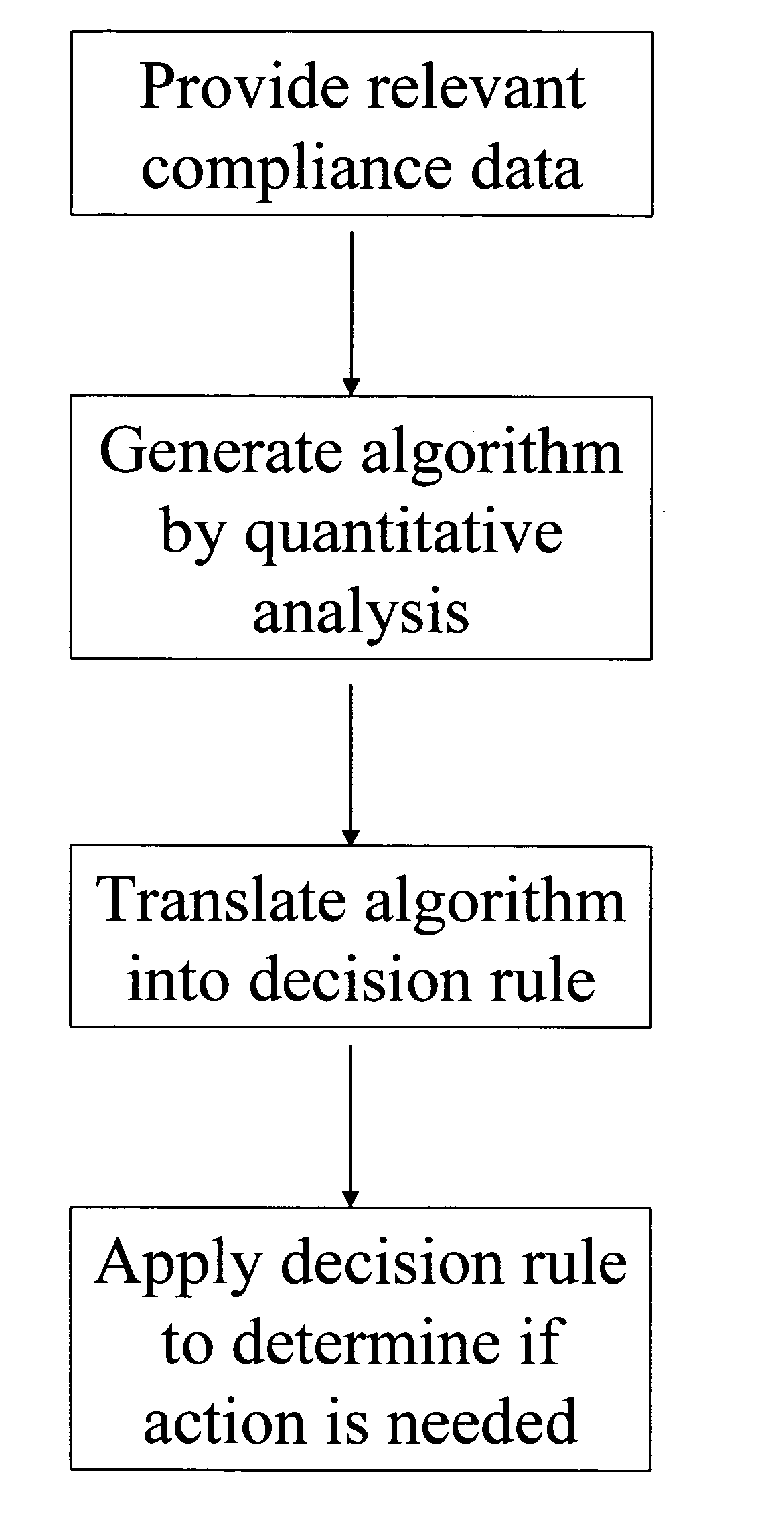
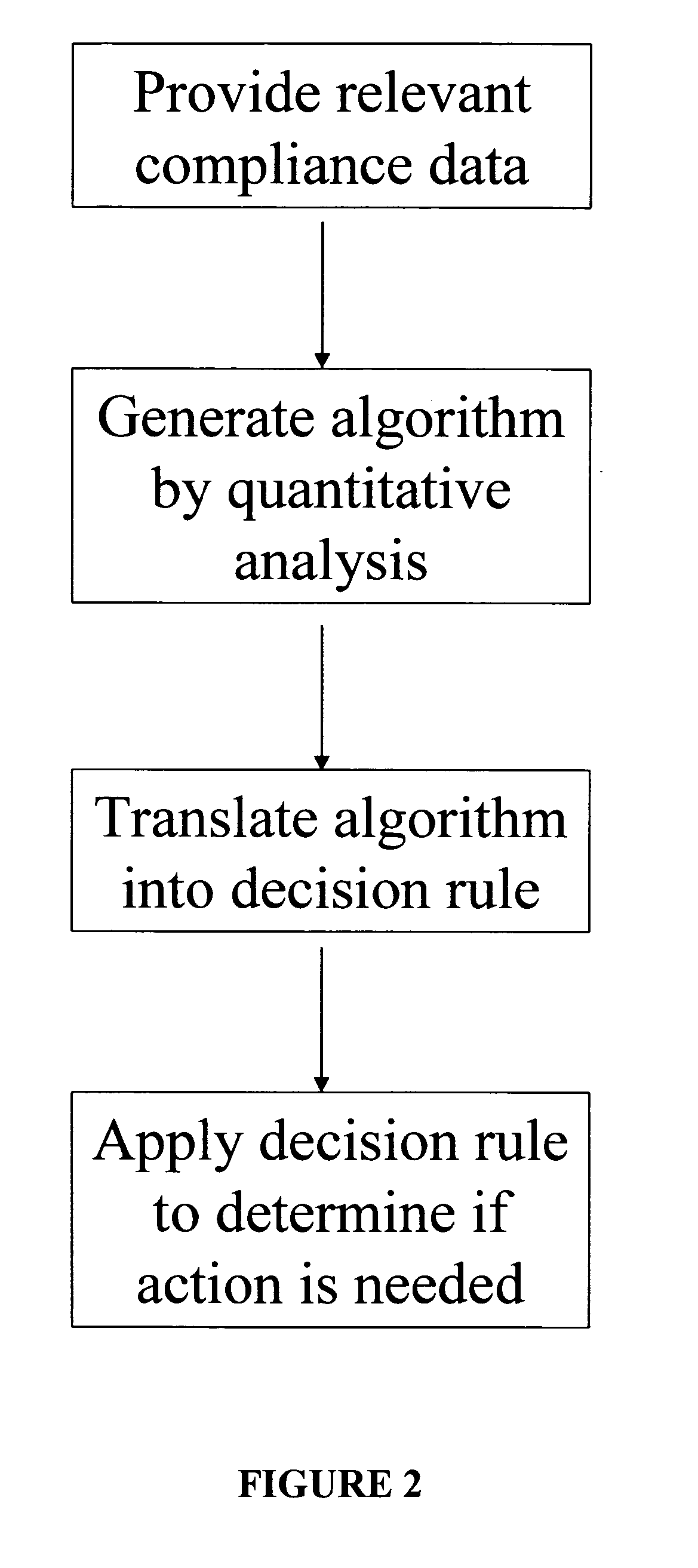
A system for developing and implementing empirically derived algorithms to generate decision rules to determine participant noncompliance and fraud with research protocols in clinical trials allows for the identification of complex patterns of variables that detect or predict participant noncompliance and fraud with research protocol, including performance and enrollment goals, in the clinical trial. The data may be used to overall predict the performance of any participant in a clinical trial, allowing selection of participants that tend to produce useful, high-quality results. The present invention can also be used to monitor participant compliance with the research protocol and goals to determine preferred actions to be performed. Optionally, the invention may provide a spectrum of noncompliance, from minor noncompliance needing only corrective feedback, to significant noncompliance requiring participant removal from the clinical trial or from future clinical trials. The algorithms and decision rules can also be domain-specific, such as detecting non-compliance or fraud among subjects in a cardiovascular drug trial, or demographically specific, such as taking into account gender, age or location, which provides for algorithms and decision rules to be optimized for the specific sample of participants being studied.
Owner:ERESTECH
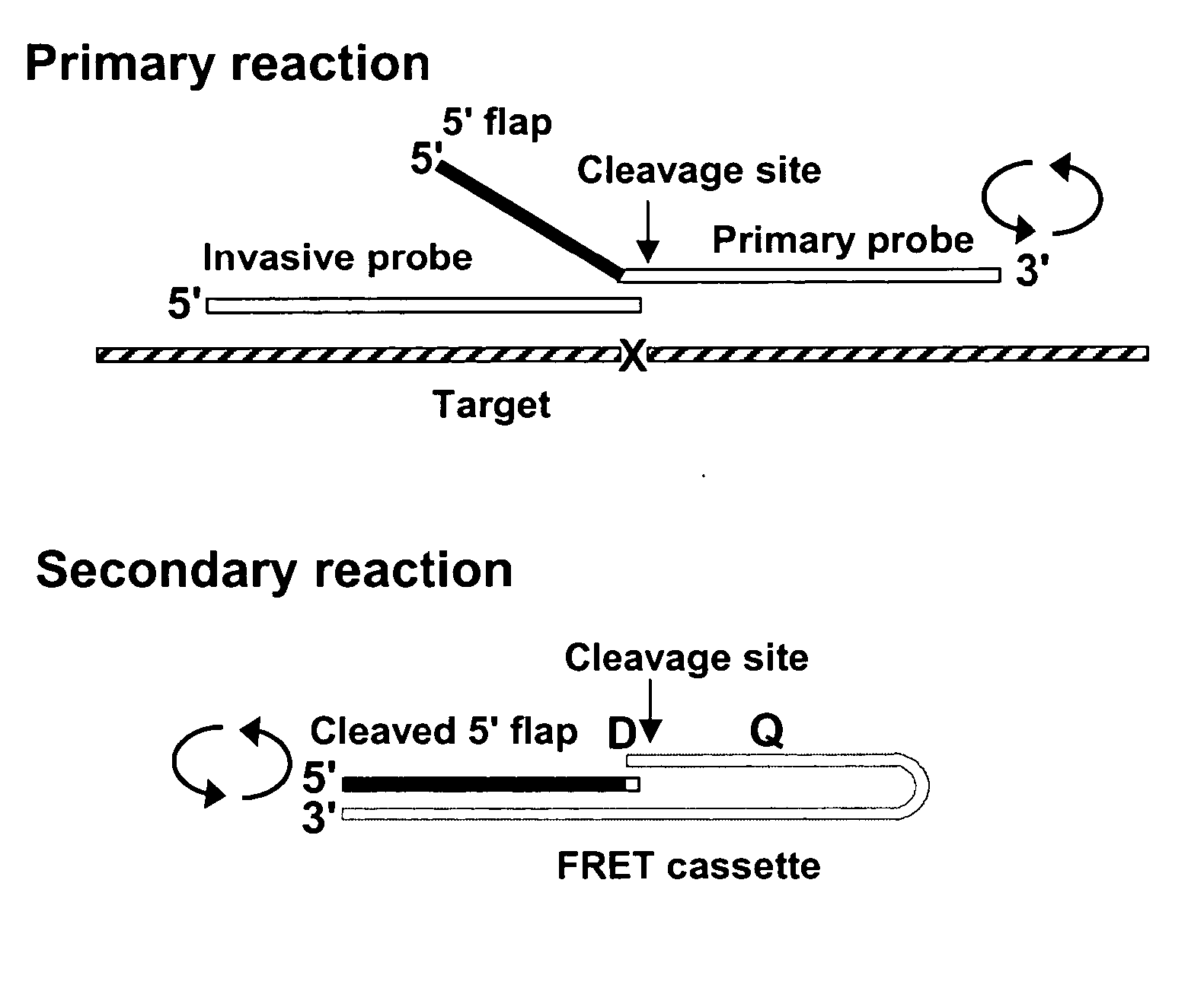
Single step detection assay
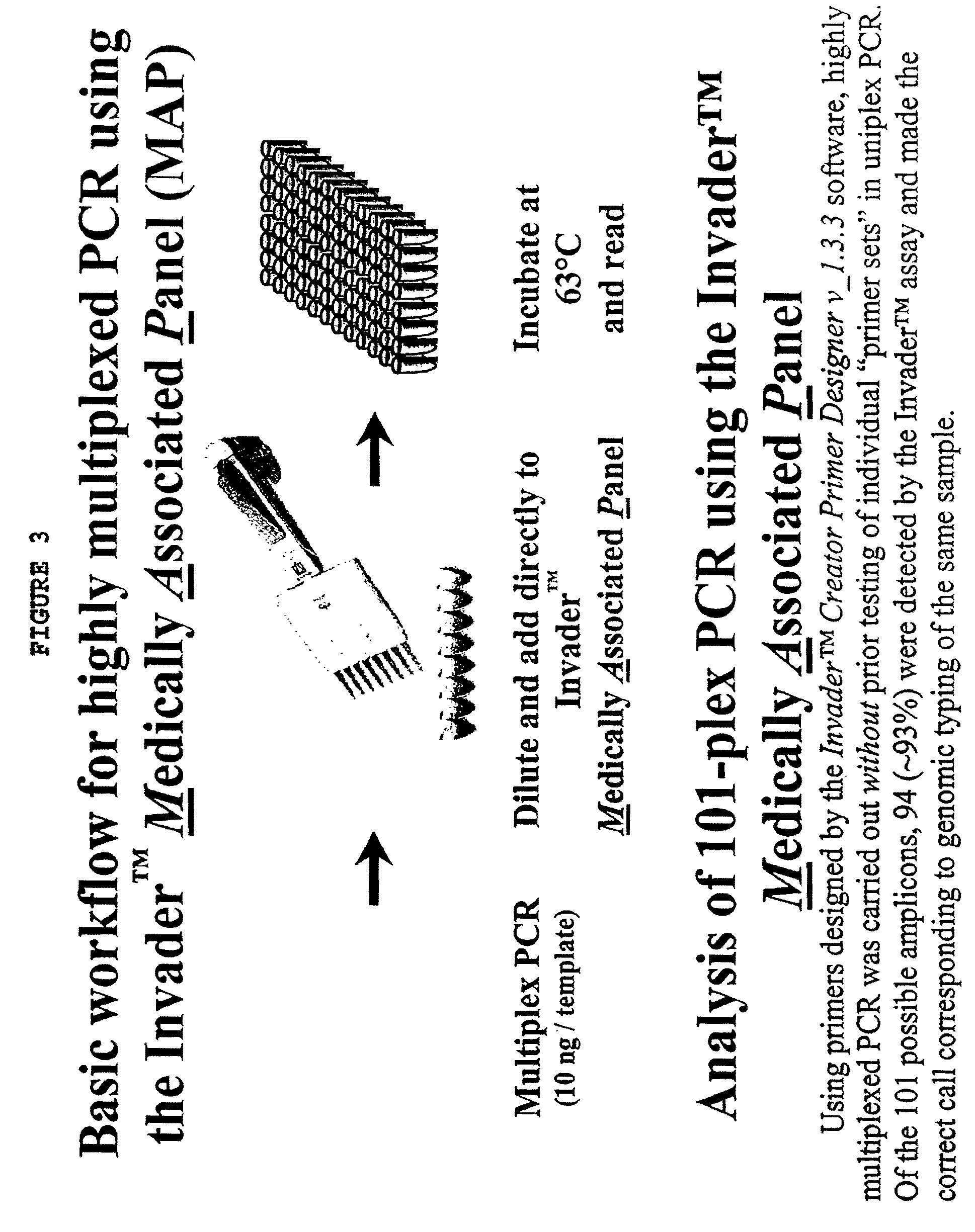
InactiveUS20060147955A1Microbiological testing/measurementFermentationNucleic acid detectionBasic research
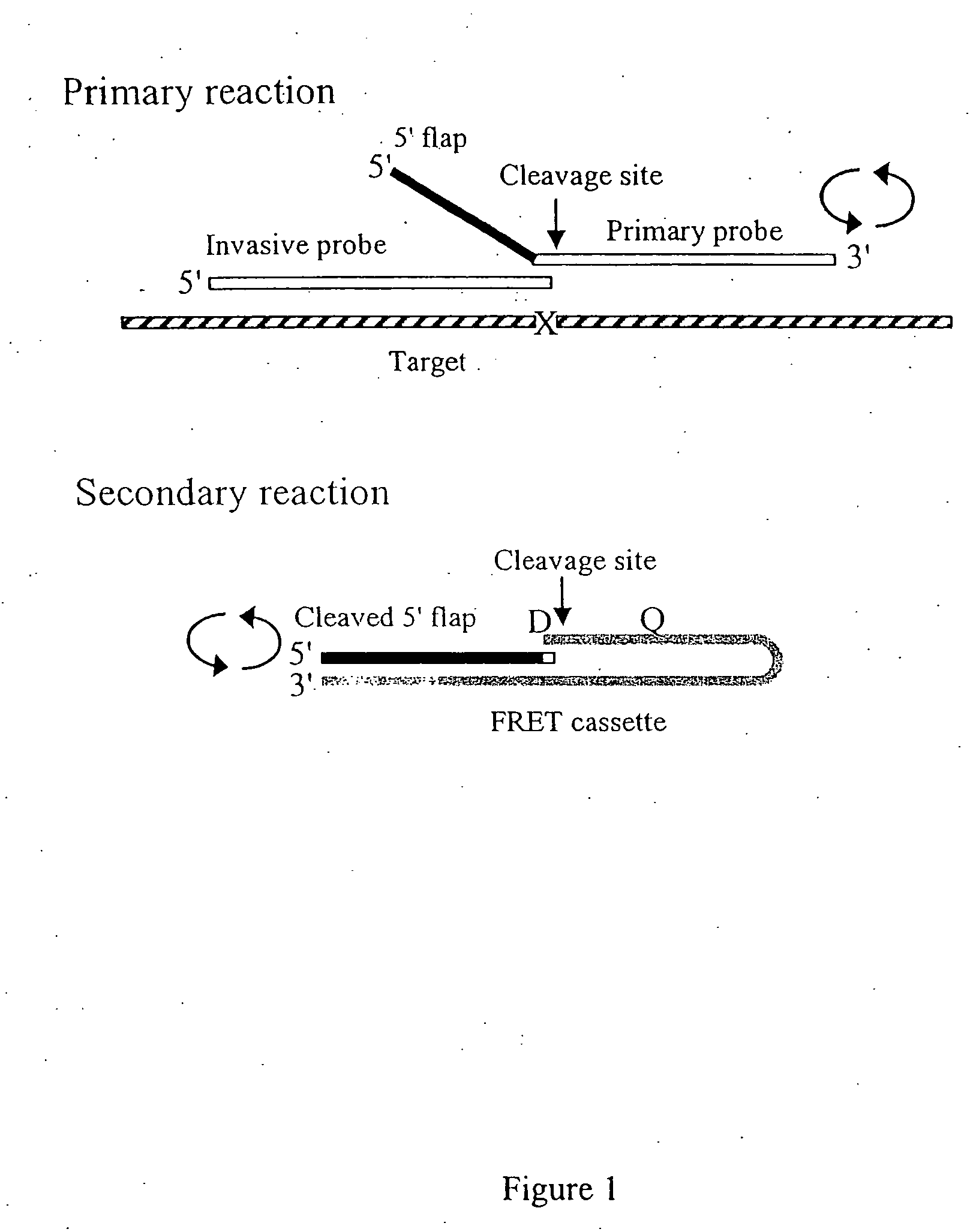
The present invention provides methods and routines for developing and optimizing nucleic acid detection assays for use in basic research, clinical research, and for the development of clinical detection assays. In particular, the present invention provides methods for designing oligonucleotide primers to be used in multiplex amplification reactions. The present invention also provides methods to optimize multiplex amplification reactions. The present invention also provides methods for combined target and signal generation assays.
Owner:THIRD WAVE TECH
Integrated data collection and analysis for clinical study
A data collection system includes remote, implantable sensors for monitoring one or more patient parameters, collecting and processing data from those sensors and utilizing that data in the performance of a clinical study of a drug or other pharmacological agent. The system assists with preparation of a protocol for a clinical trial; presentation of that protocol; assuring compliance with the protocol; and generating useful results from data collected via the system and externally for presentation to an approval forum.
Owner:MEDTRONIC INC
Apparatus and method for prediction and management of participant compliance in clinical research
InactiveUS20060184493A1Improve statistics performanceReduce testing costsDigital computer detailsForecastingCardiovascular drugNon compliance
A system for developing and implementing empirically derived algorithms to generate decision rules to determine participant noncompliance and fraud with research protocols in clinical trials allows for the identification of complex patterns of variables that detect or predict participant noncompliance and fraud with research protocol, including performance and enrollment goals, in the clinical trial. The data may be used to overall predict the performance of any participant in a clinical trial, allowing selection of participants that tend to produce useful, high-quality results. The present invention can also be used to monitor participant compliance with the research protocol and goals to determine preferred actions to be performed. Optionally, the invention may provide a spectrum of noncompliance, from minor noncompliance needing only corrective feedback, to significant noncompliance requiring participant removal from the clinical trial or from future clinical trials. The algorithms and decision rules can also be domain-specific, such as detecting non-compliance or fraud among subjects in a cardiovascular drug trial, or demographically specific, such as taking into account gender, age or location, which provides for algorithms and decision rules to be optimized for the specific sample of participants being studied.
Owner:ERESTECH
Compressed tablet formulation
This invention relates to a 50% drug loaded compressed tablet formulation for efavirenz. Efavirenz is a non-nucleoside reverse trancriptase inhibitor being studied clinically for use in the treatment of HIV infections and AIDS.
Owner:MERCK SHARP & DOHME CORP
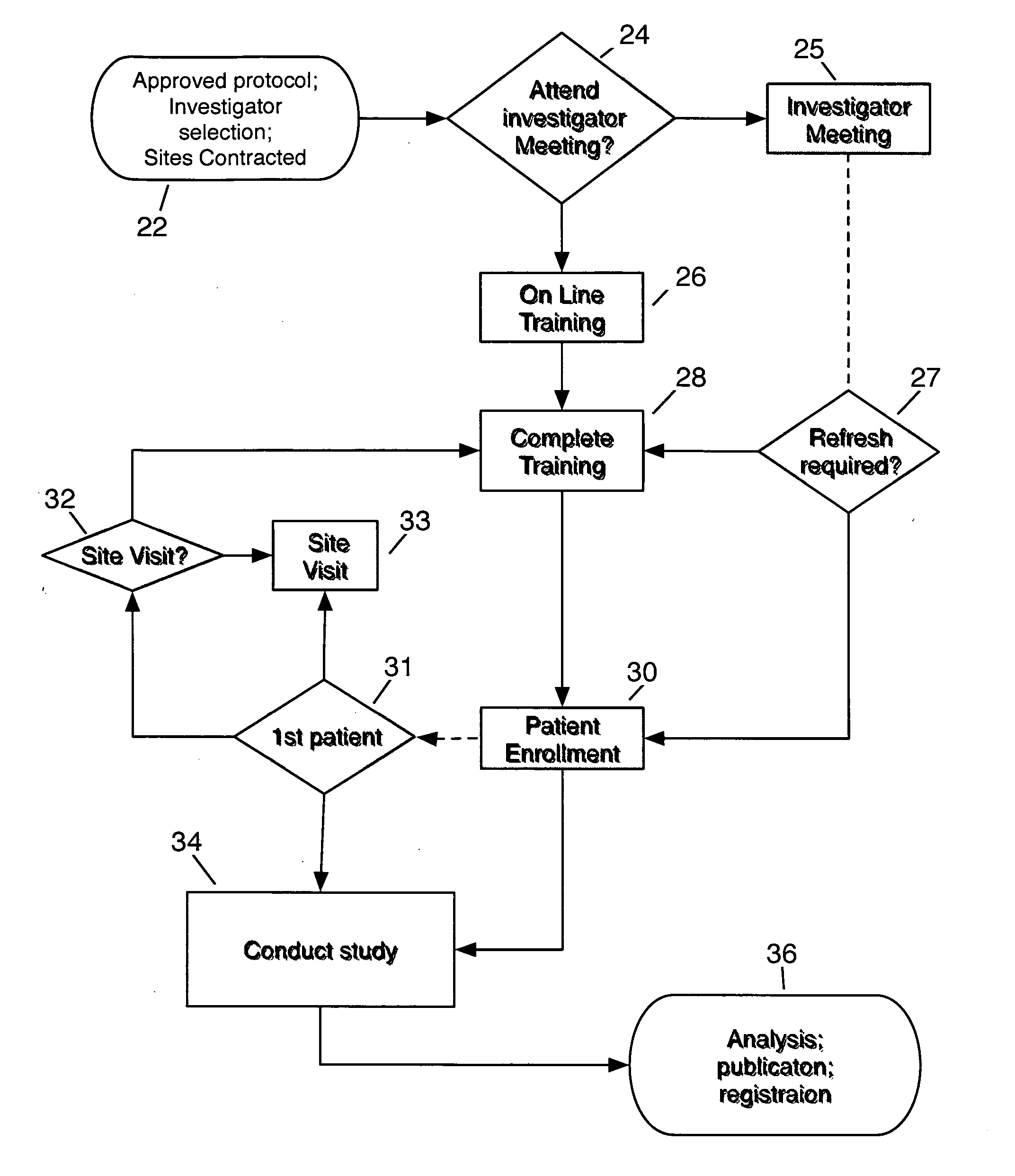
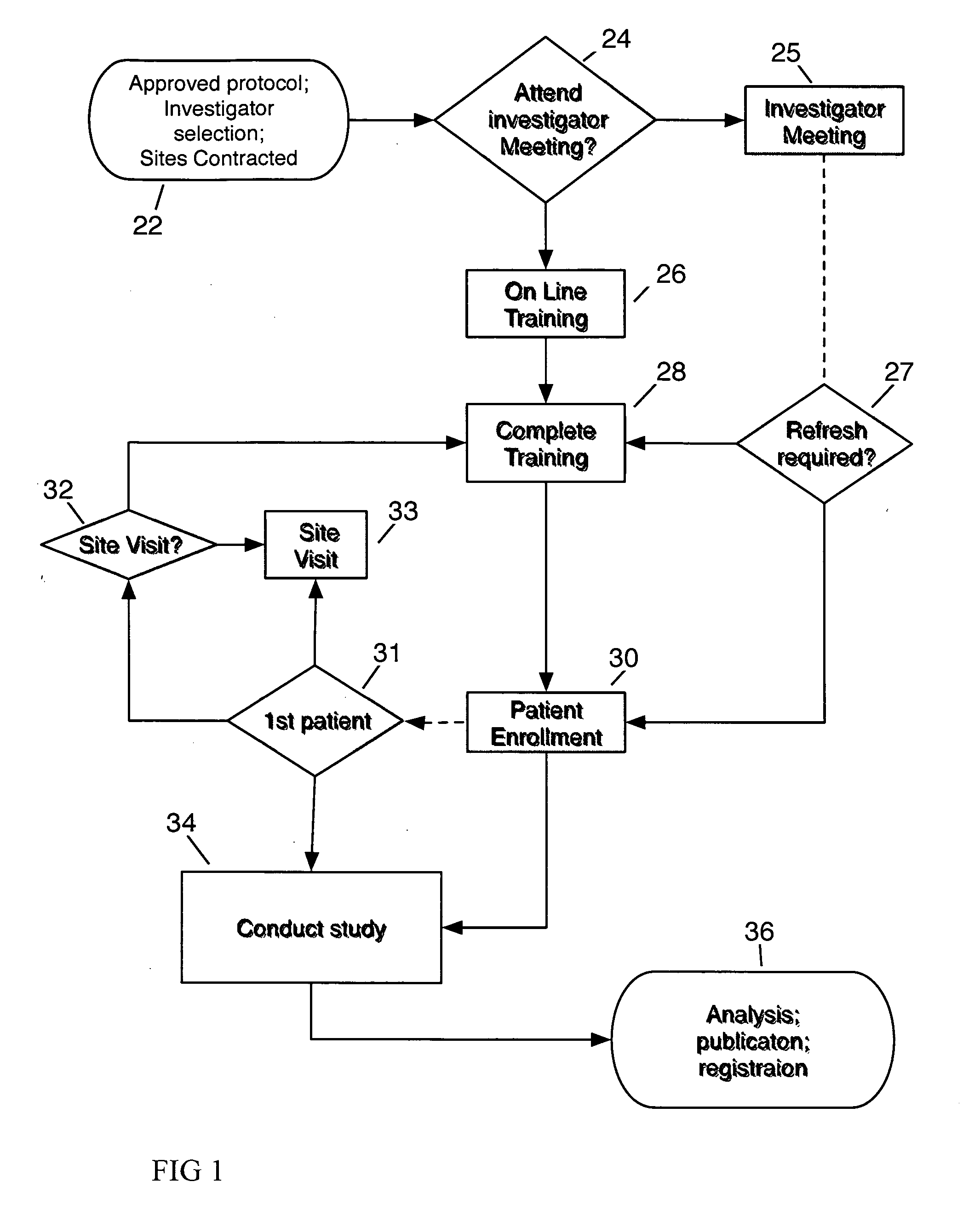
System and method for clinical trial investigator meeting delivery and training including dynamic media enrichment
InactiveUS20100136509A1Guaranteed accuracyPrevent excessive interruptionComputer-assisted medical data acquisitionTeaching apparatusInvestigator SiteInformative content
A System and Method for Delivering the substantive equivalent of an Investigator Meeting for Clinical Tπals to satisfy Investigator Site training requirements sufficient to commence patient enrollment is provided The invention further provides a means to ensure site personnel receive, review and implement Protocol Amendments in a timely and comprehensive manner The preferred embodiment includes a mechanism to track and verify actual viewing of compulsory material The invention further provides for dynamically supplementing the online presentation with content enrichment In the preferred embodiment, user questions are submitted during user viewing of online media An Administrator processes user inputs, including questions, and answers attached to the presentation at the time point at which the question / comment was generated Subsequent users / viewers are alerted to the added commentary by an icon on the display during viewing of presentation media, enabling them to benefit from questions and answers associated with the media.
Owner:MEJER ALDEN +1
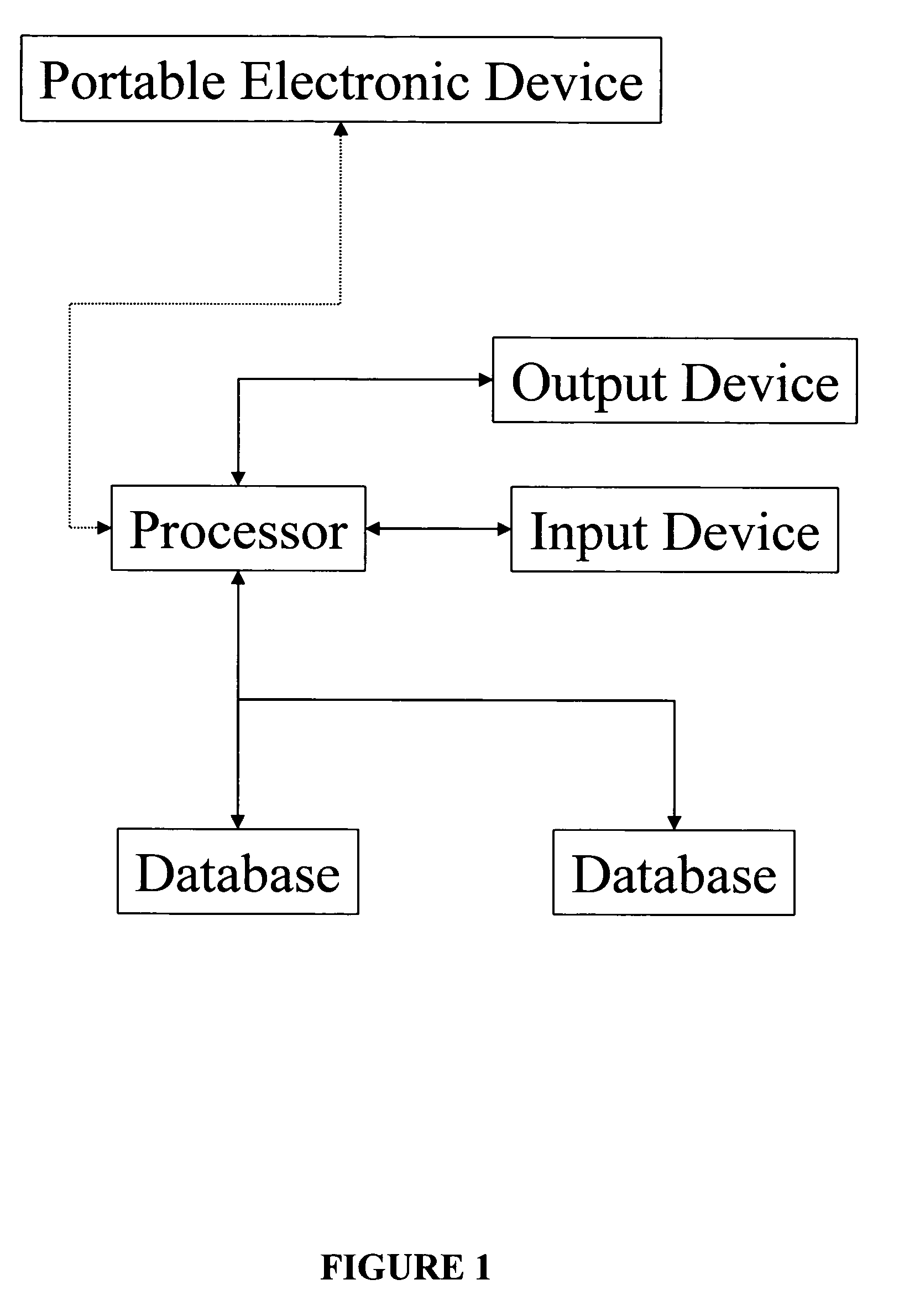
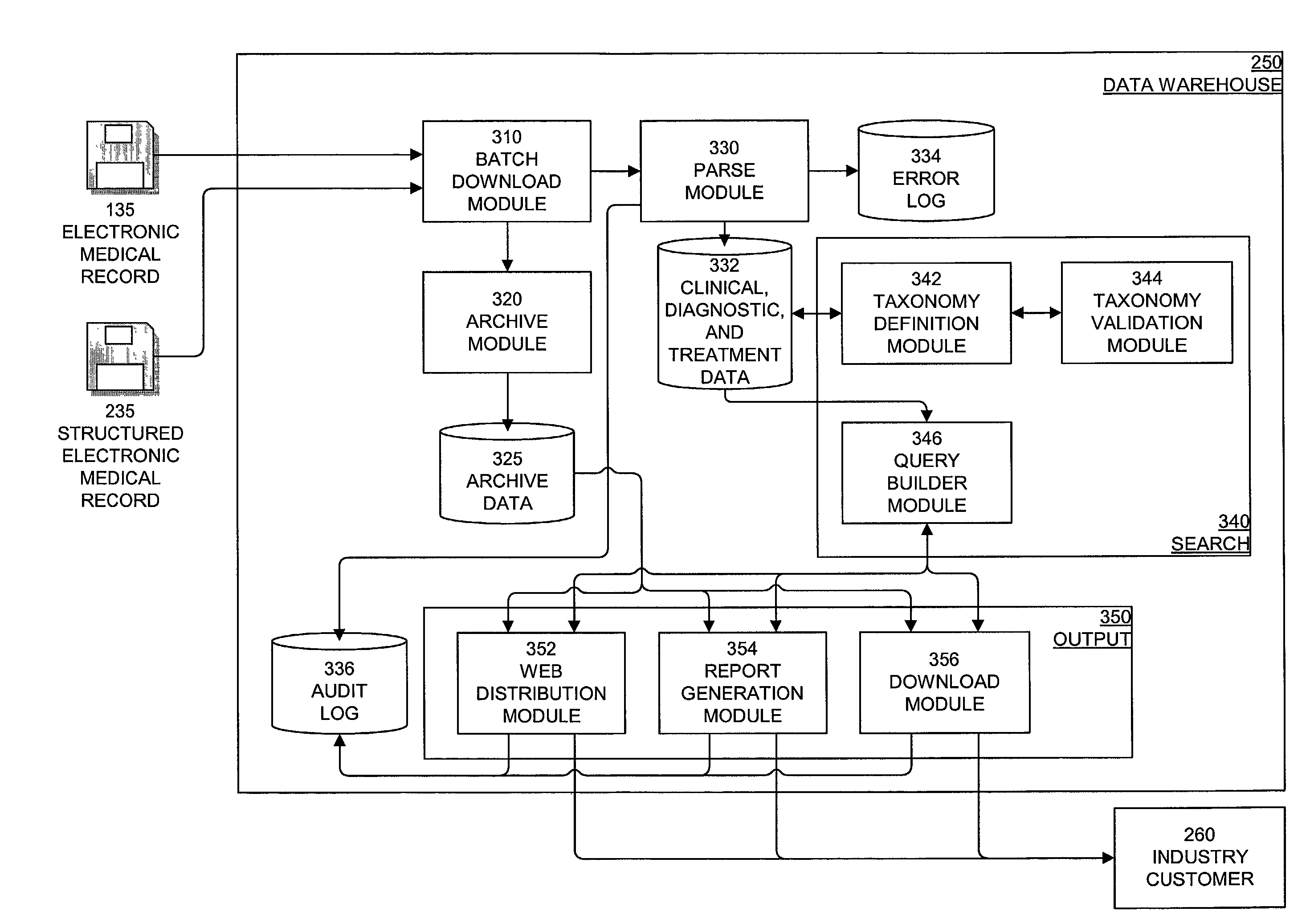
System, method, and apparatus for storing, retrieving, and integrating clinical, diagnostic, genomic, and therapeutic data
InactiveUS7529685B2Useful purposeImprove performanceMedical data miningMedical automated diagnosisClinical researchPatient data
A method, system, and computer program product for storing and retrieving patient data in a database connected to a network is disclosed. The method, system, and computer program product comprises storing clinical data in the database, extracting data from the clinical data, querying the database using a taxonomy that includes inclusive or exclusive search criterion, and receiving a result set. The method, system, and computer program product comprises creating a taxonomy that includes at least one search criterion, sending a query to the database, the query including said at least one search criteria, receiving the result set in response to the query, the result set including at least one result record, and displaying said at least one result record. The method, system, and computer program product can further include a user such as a clinical researcher, a treating physician, or a consulting physician analyzing the result set.
Owner:MD DATACOR
Efficient method and process to search structured and unstructured patient data to match patients to clinical drug/device trials
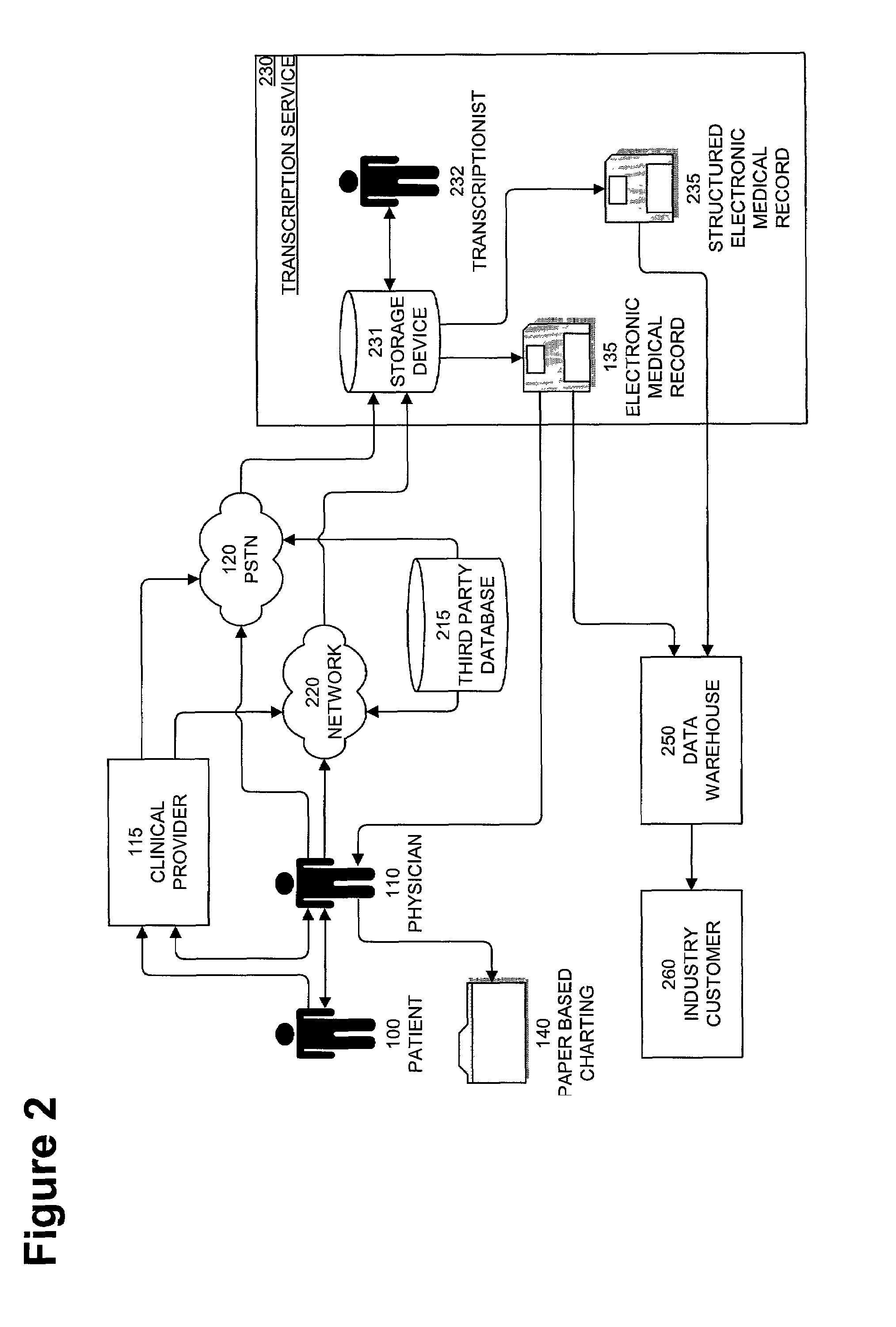
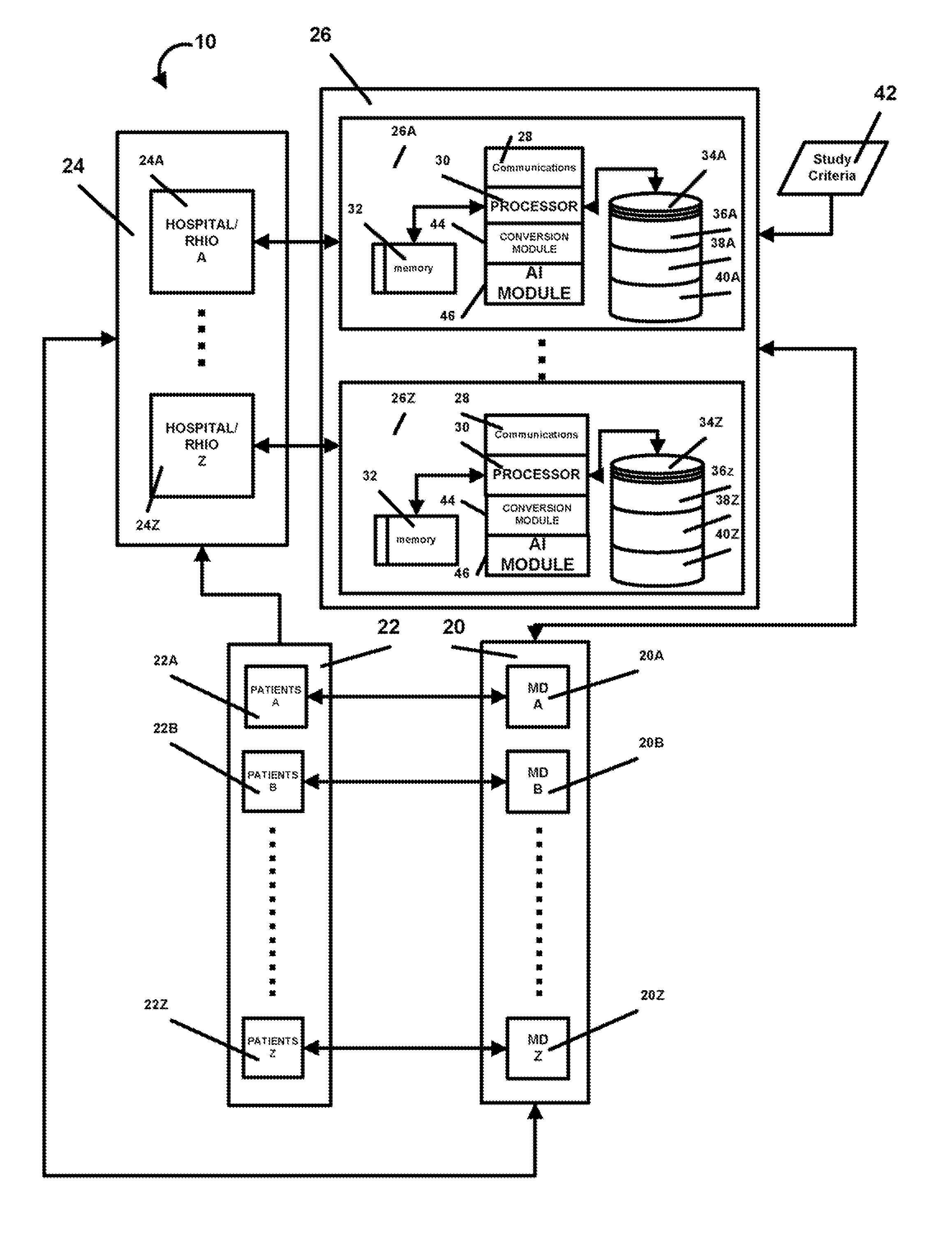
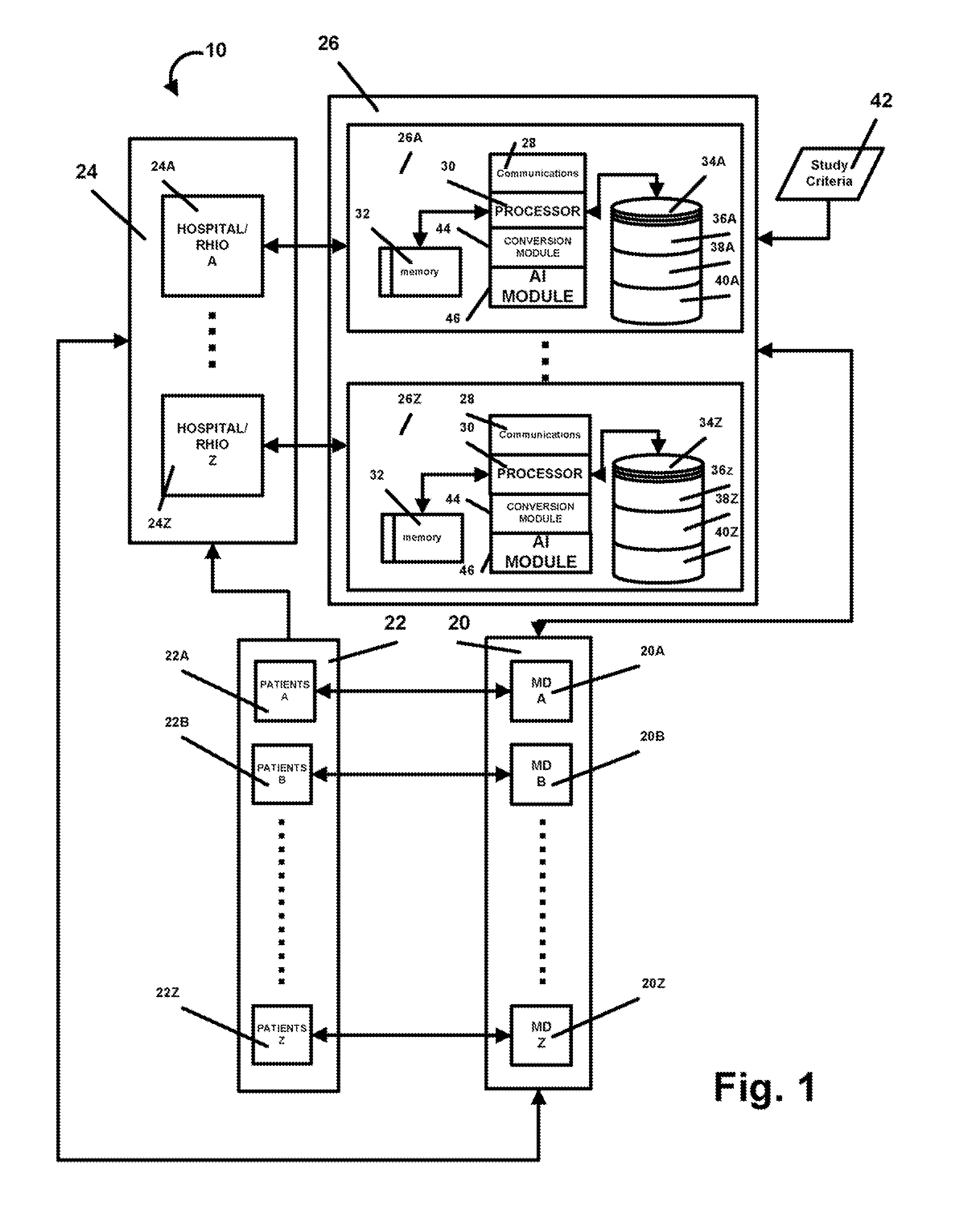
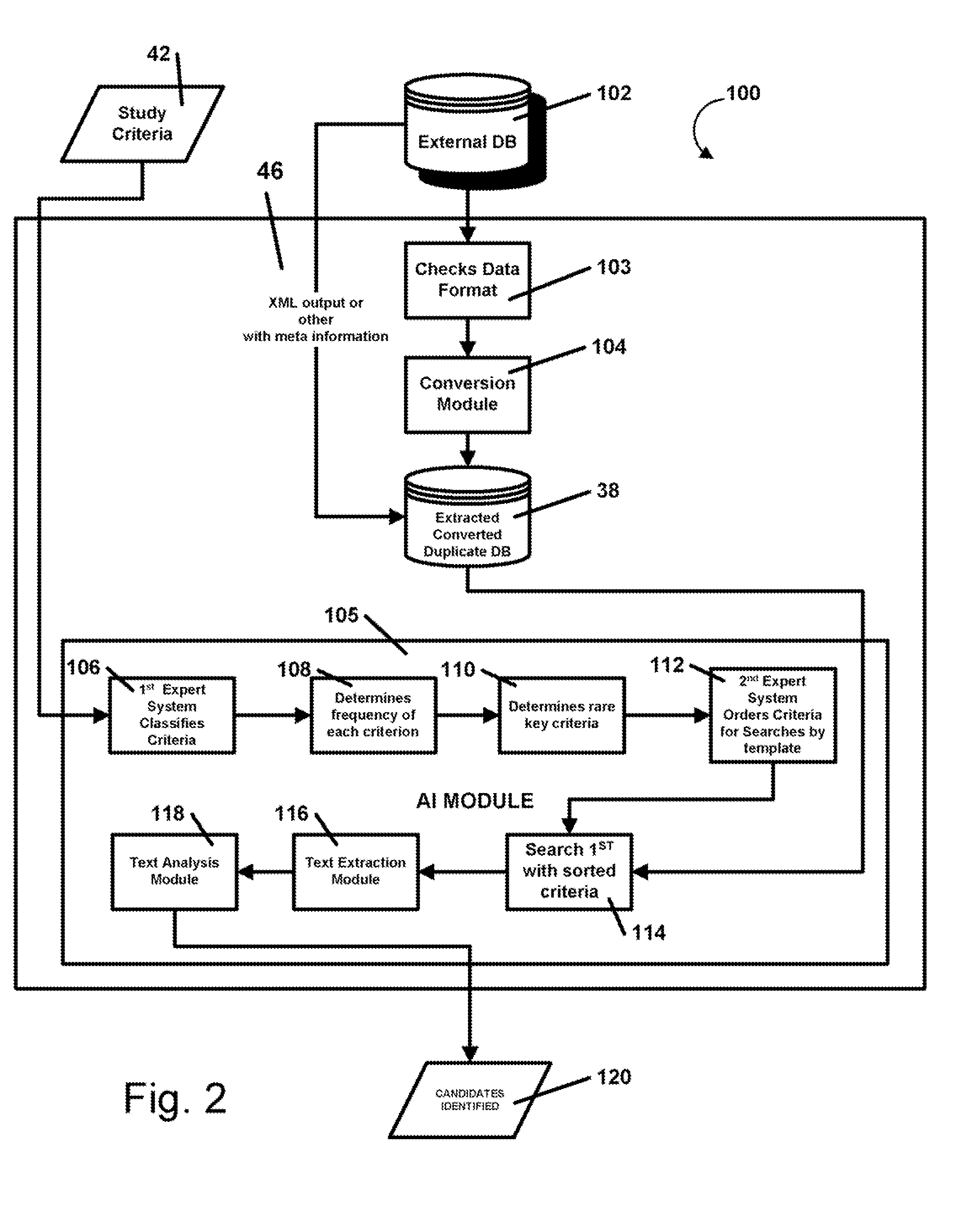
InactiveUS20080195600A1Digital data processing detailsPatient personal data managementMedical recordMedicine
A method and system that automatically matches patients to clinical drug and device trials with: a database component operative to maintain a hospital / RHIO / medical practice patient database and their corresponding medical records, and a medical practice database and their corresponding plurality of specialties, and a clinical studies database component and their corresponding plurality of clinical studies a communications component to receive changes to the database component and a processor programmed to: periodically match compatible patients and clinical studies and generate reports to matched medical practices in the medical practice database having matched patients. The processor may be programmed to more efficiently function by selecting key rare criteria first in order to search free text keywords and phrases last.
Owner:DEAKTER DANIEL R
Direct nucleic acid detection in bodily fluids
The present invention provides methods and routines for developing and optimizing nucleic acid detection assays for use in basic research, clinical research, and for the development of clinical detection assays. In particular, the present invention provides methods for designing oligonucleotide primers to be used in multiplex amplification reactions. The present invention also provides methods to optimize multiplex amplification reactions. The present invention also provides methods for combined target and signal generation assays.
Owner:THIRD WAVE TECH
Closed loop nerve stimulation system
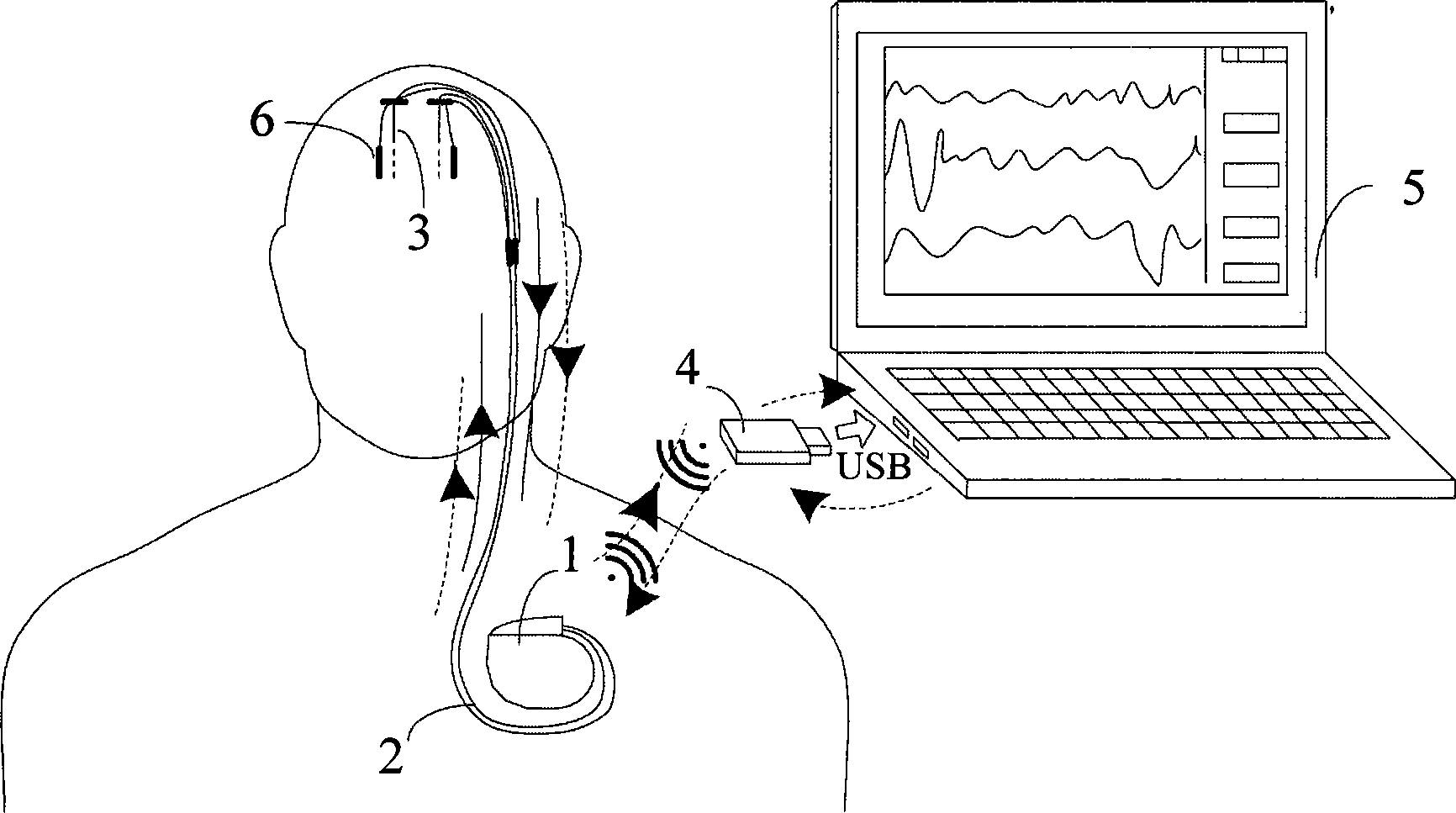
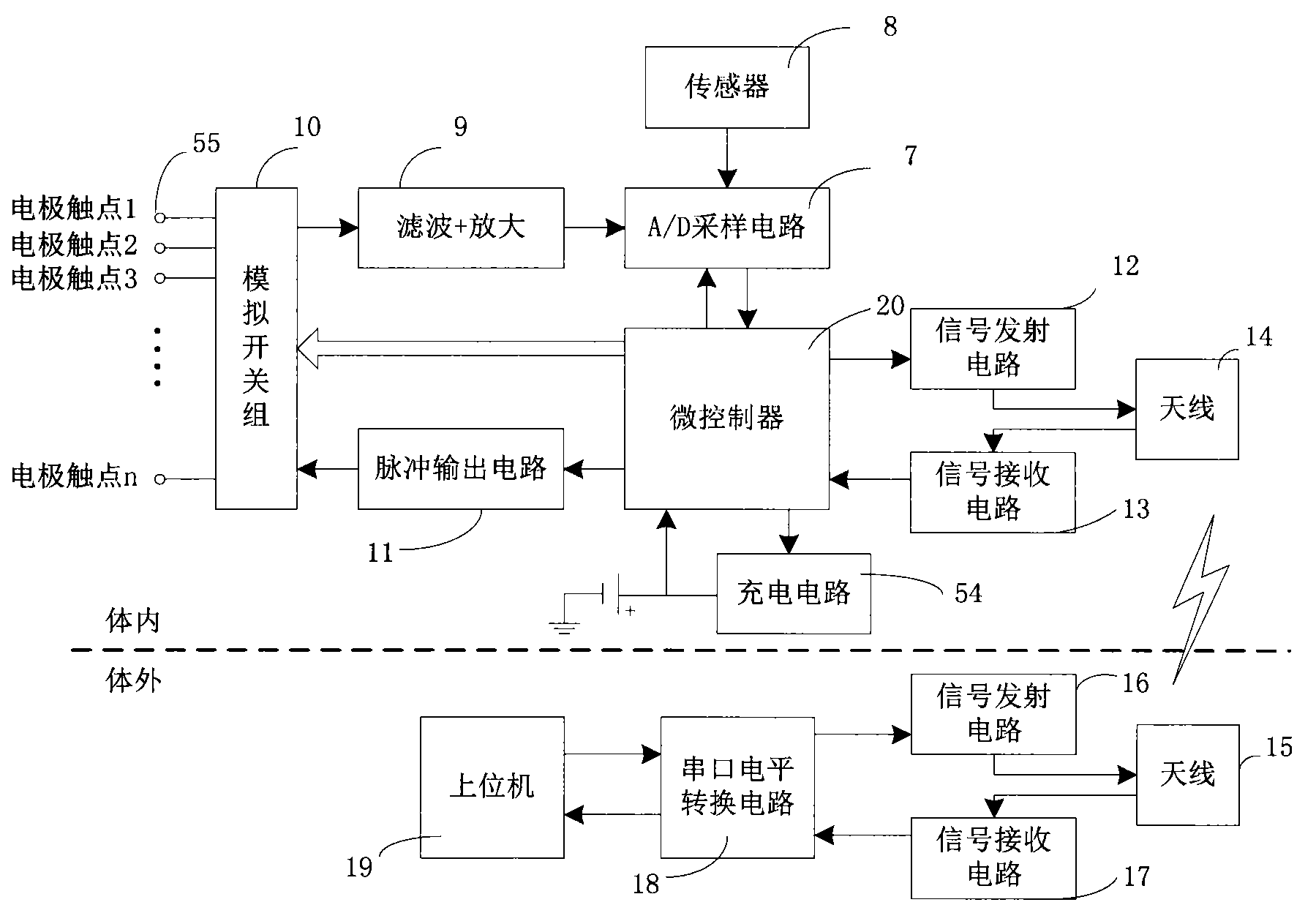
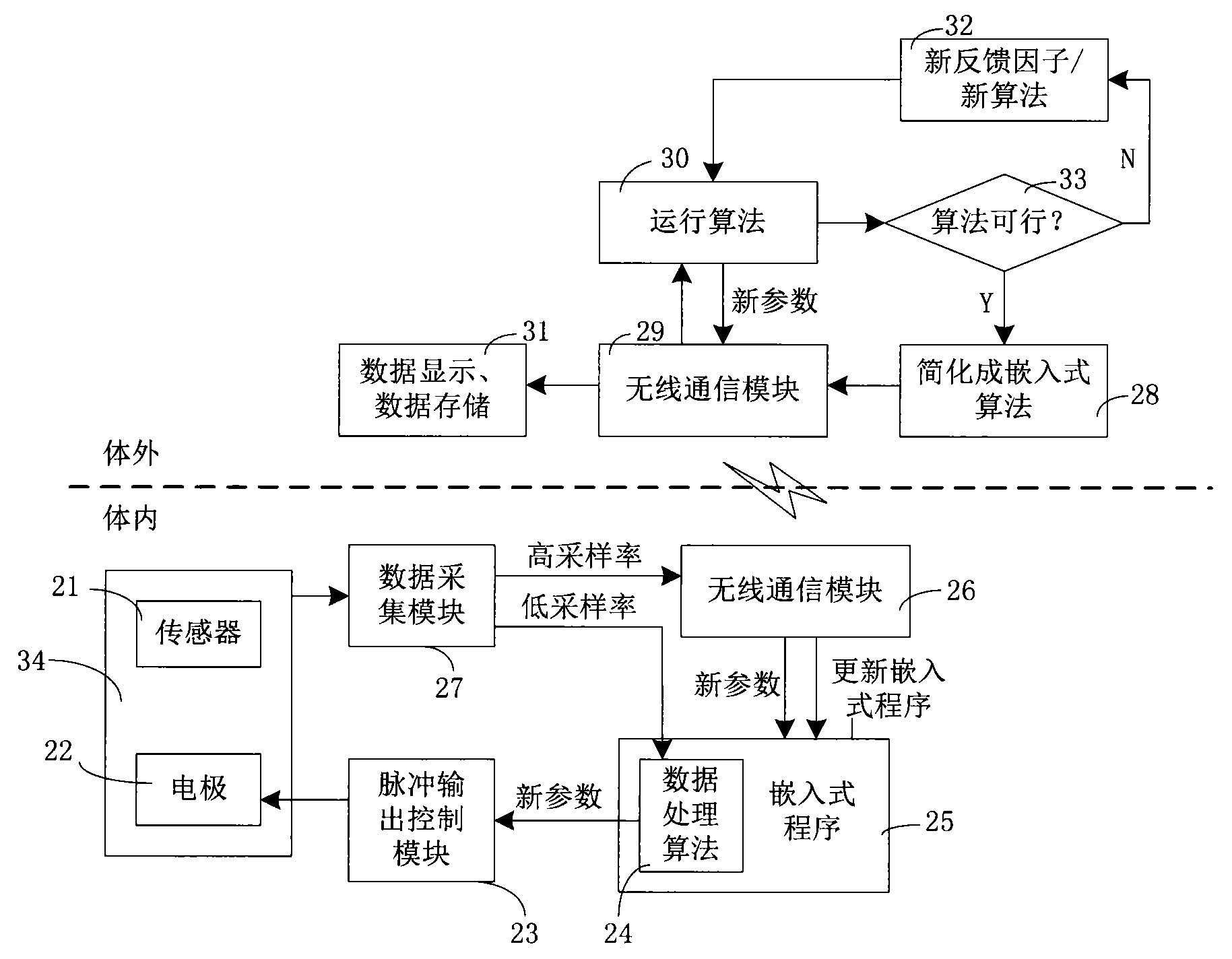
The invention belongs to the technical field of implantable medical instruments, and provides a closed loop deep brain stimulation system which can collect physiological signals of patients, adjust pulse stimulation parameters in real time and display data, store the data, design an optimization algorithm and update embedded programs in real time through an upper computer, and particularly relates to a closed loop nerve stimulation system. According to the closed loop nerve stimulation system, two closed loop work modes are provided; in one work mode, an implantable nerve stimulator carries out embedded algorithm data processing to form closed loop control; in the other work mode, the implantable nerve stimulator uploads the collected data to the upper computer through a wireless communication module, and then the upper computer carries out algorithm processing and then controls the stimulation parameters through the wireless communication module to form a closed loop. The closed loop nerve stimulation system can be used for closed loop electrical stimulation treatment of nervous system diseases or clinical research or animal research of a closed loop stimulation method and can provide a good platform for the research of the closed loop stimulation method.
Owner:TSINGHUA UNIV +1
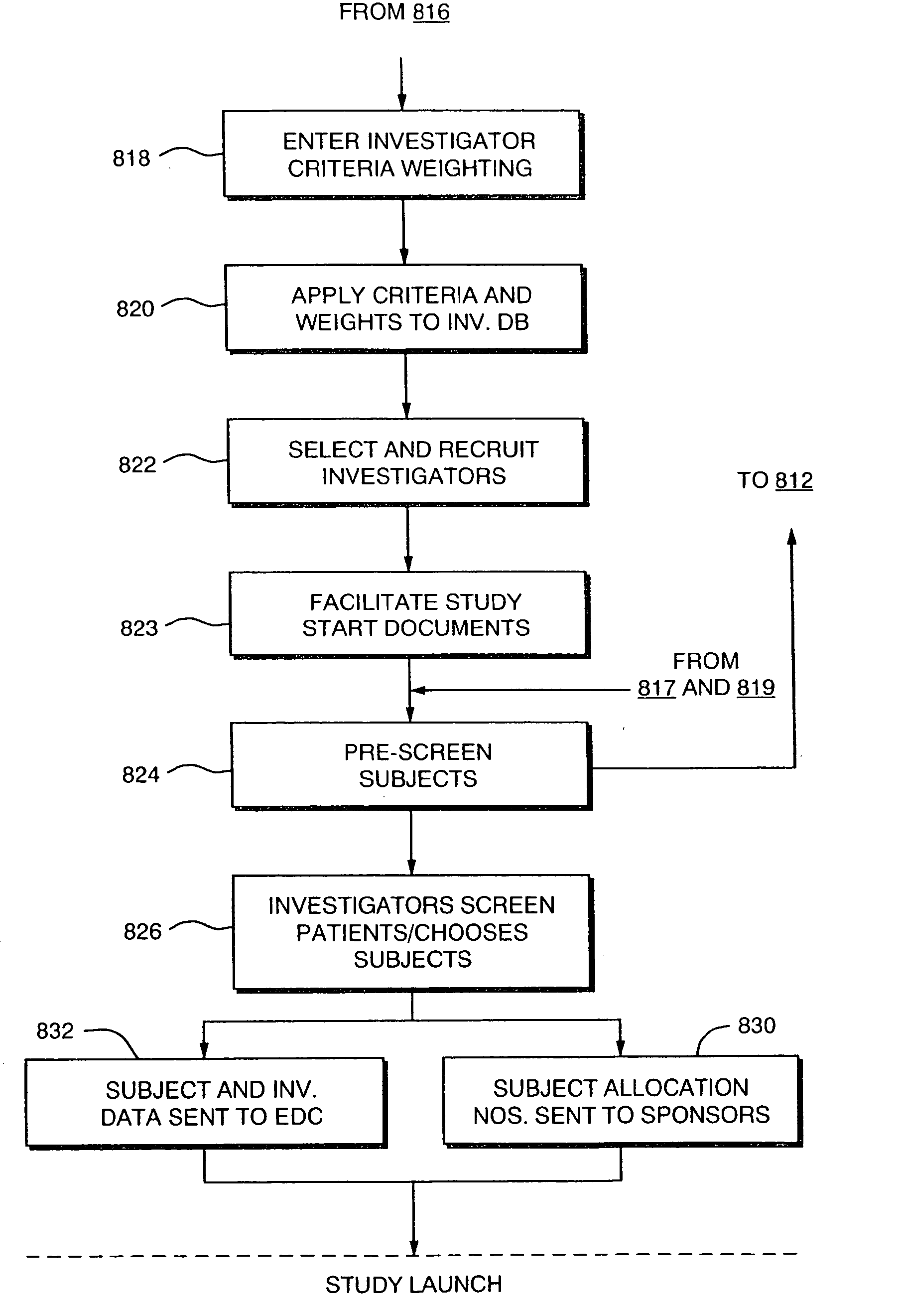
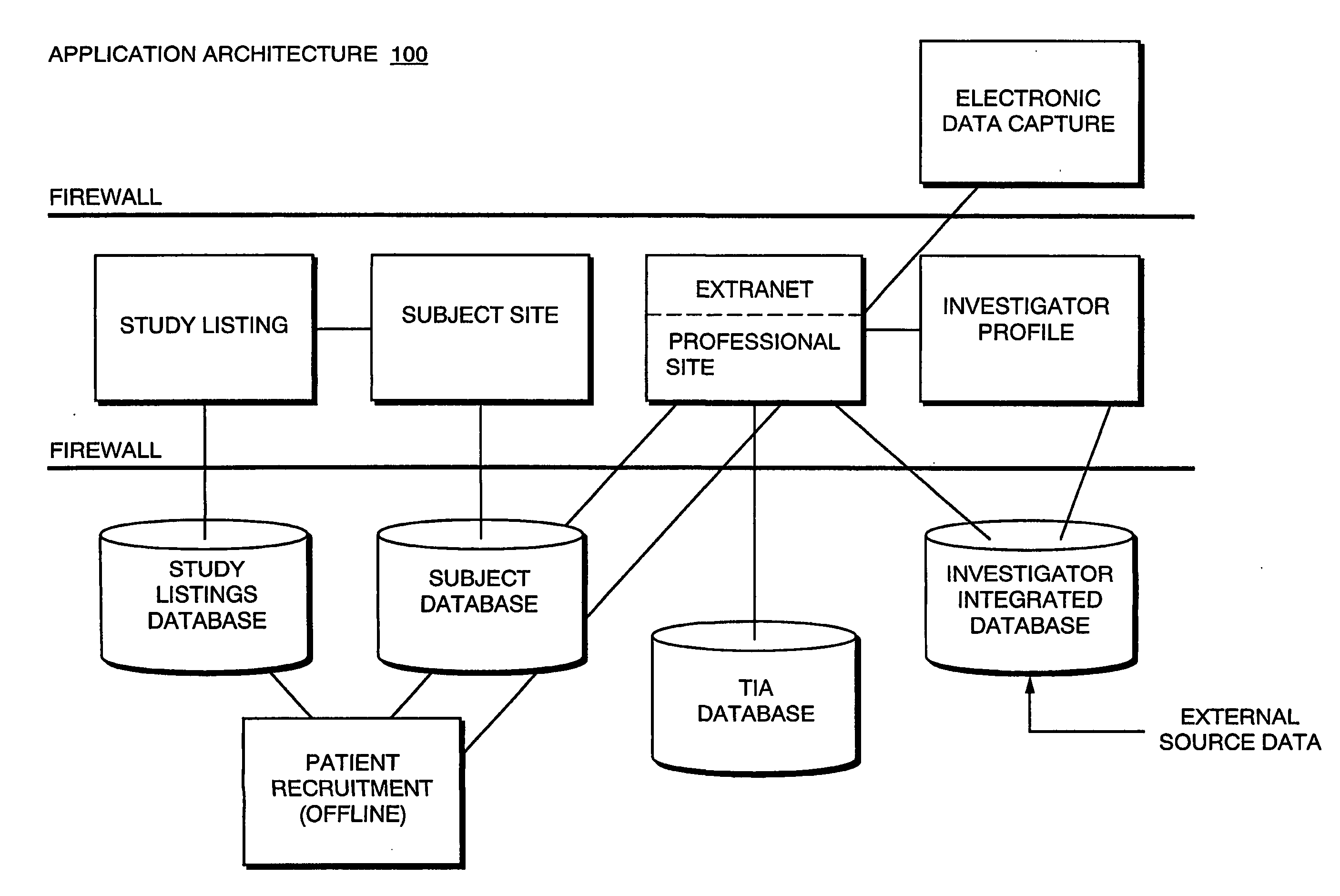
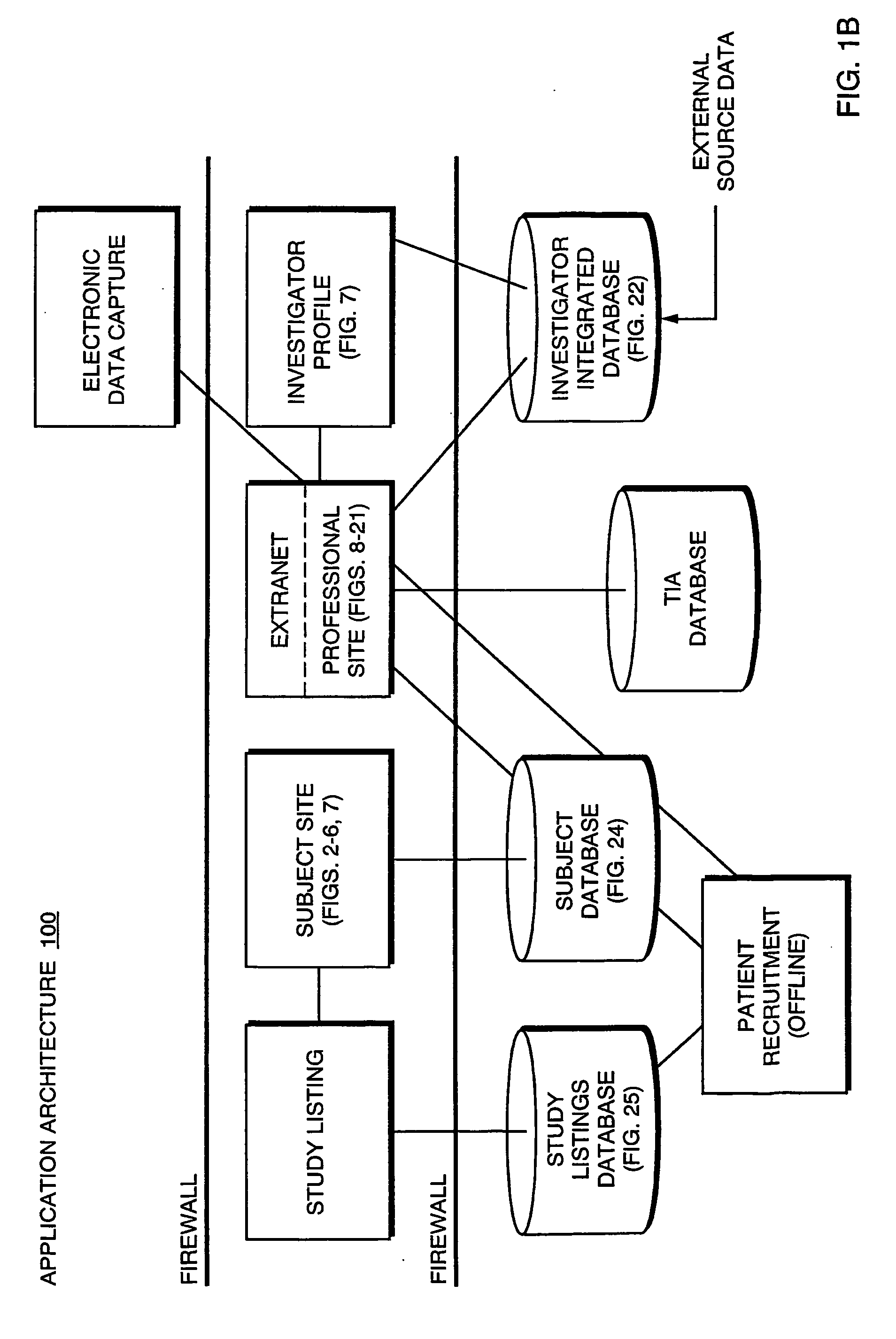
Systems and Methods for Selecting and Recruiting Investigators and Subjects for Clinical Studies
InactiveUS20080133270A1Computer-assisted medical data acquisitionOffice automationMedicineSubject matter
The present invention is directed to an integrated on-line interactive forum that promotes exchange of information among clinical study sponsors, clinical study investigators, and potential clinical study subjects. The forum includes an investigator database that contains information suitable for identification of qualified investigators for clinical studies and a subject database that contains information suitable for identification of eligible subjects for clinical studies. An extranet is coupled to the investigator database and the subject database. The extranet allows sponsors and investigators to exchange securely documents required to start a clinical study. The forum also optionally includes one or more web pages that provide information describing clinical studies to potential clinical study subjects and permit potential clinical study subjects to register for inclusion in the subject database. A therapeutic incidence area database is also optionally integrated into the forum
Owner:MICHELSON LESLIE DENNIS +2
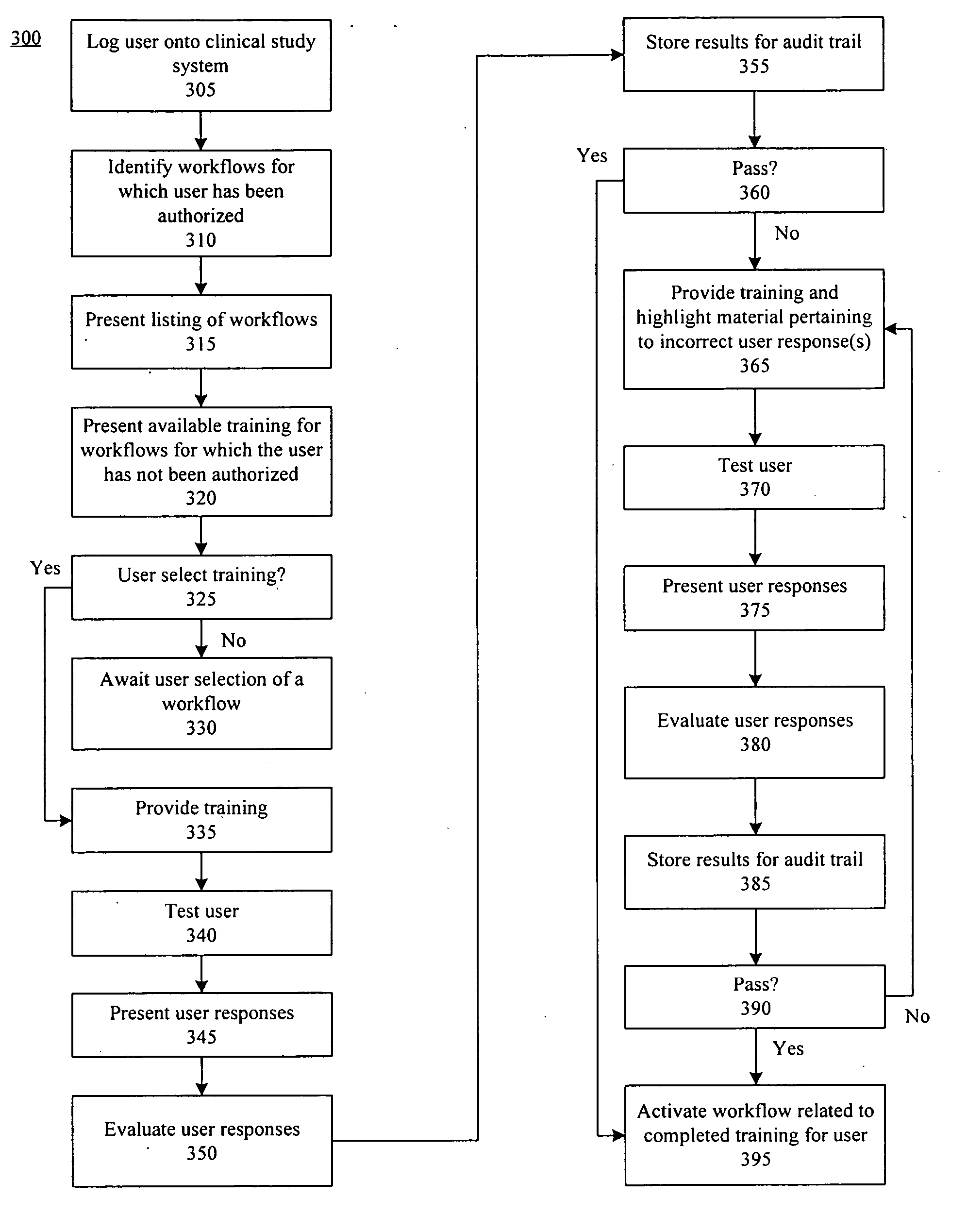
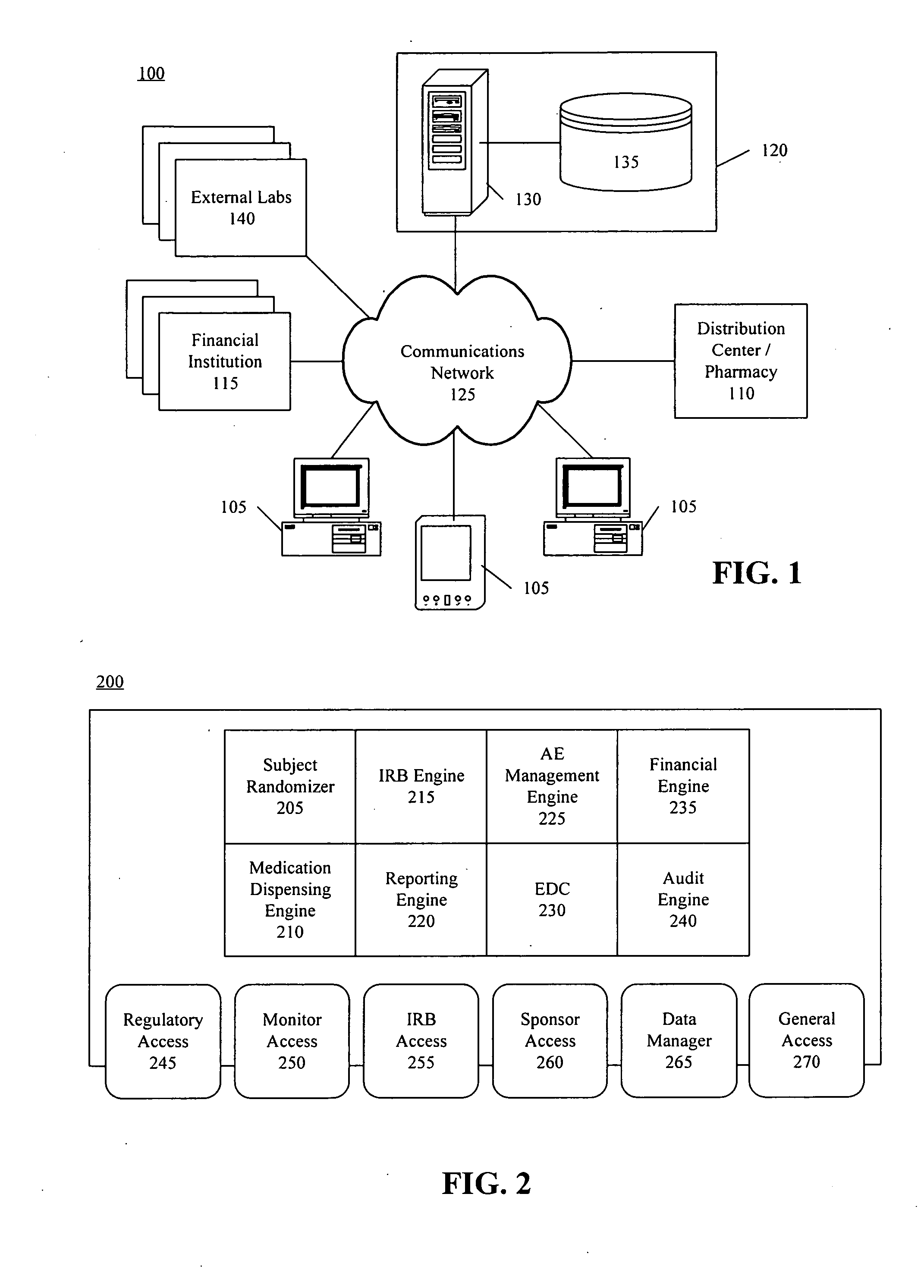
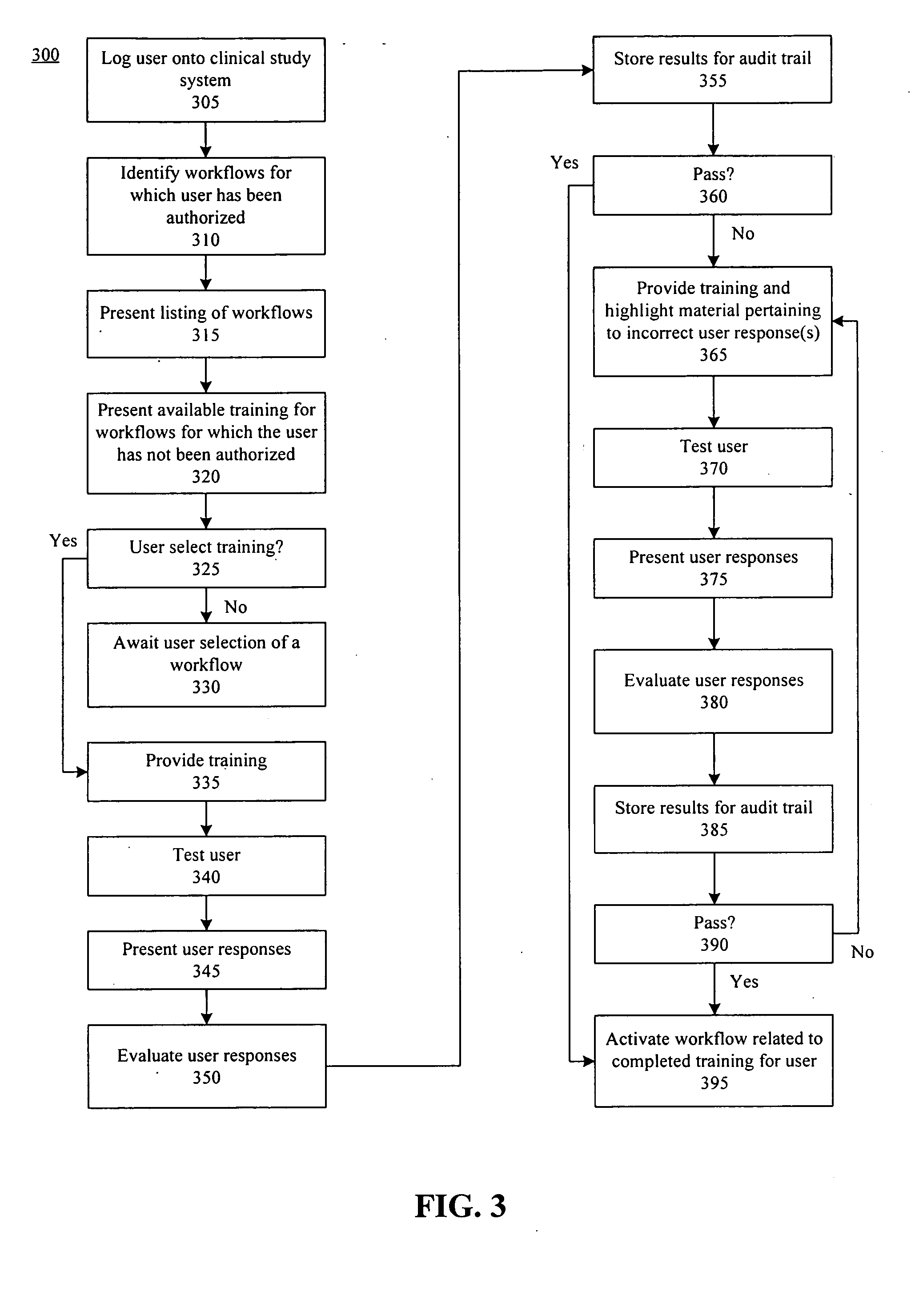
Method, system, and apparatus for clinical trial management over a communications network
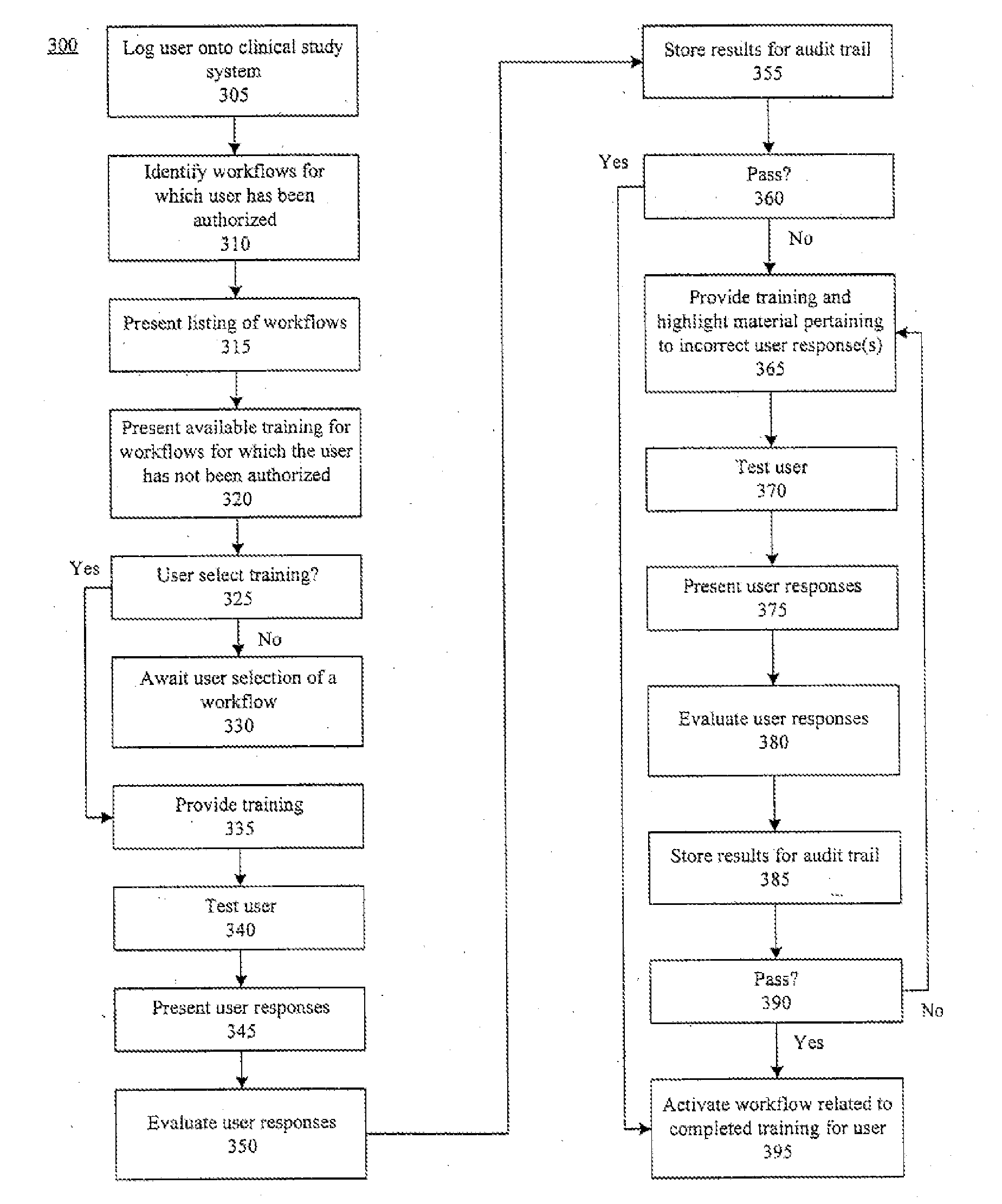
InactiveUS20050010451A1Improve securityShorten the timeData processing applicationsSurgeryPaymentMilestone
A centralized, online clinical study system configured to coordinate aspects of a clinical study can include a training module configured to evaluate whether users have completed training for selected workflows for the clinical study that are available from the clinical study system, and to provide training to registered users for the selected workflows. The system also can include a financial engine configured to determine when a participating site meets or exceeds a milestone of the clinical study and to initiate a payment to the participating site. Further, the system can include a module configured to receive and verify clinical research data and to designate the verified clinical research data as official source data for the clinical study.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
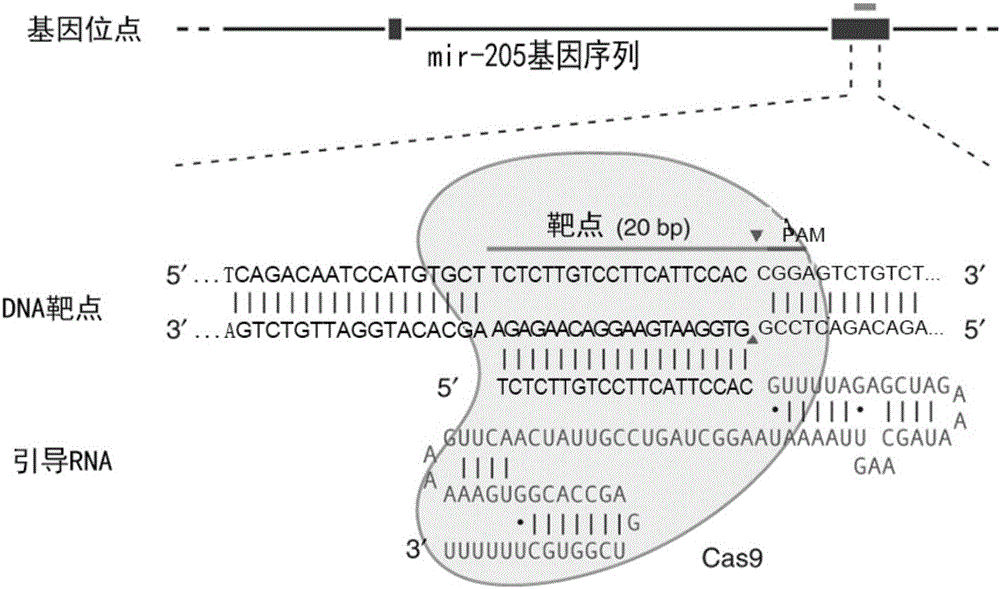
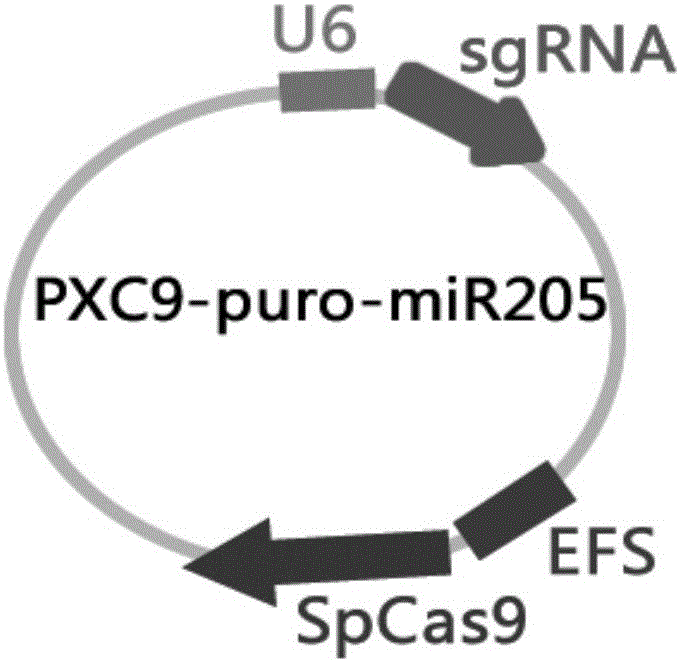
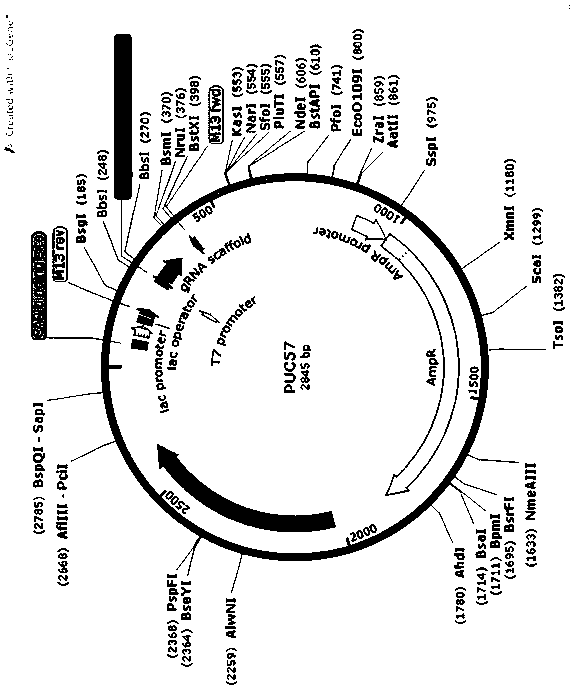
MiR-205 gene knockout kit based on CRISPR-Cas9 gene knockout technology
ActiveCN105112445AVector-based foreign material introductionDNA/RNA fragmentationEnzyme digestionFluorescence
The invention discloses a miR-205 gene knockout kit based on CRISPR-Cas9 gene knockout technology. According to the invention, an optimal CRISPR-Cas9 target sequence of a certain amount of miR-205 is obtained through target design software and an sgRNA single chain is synthesized in vitro; an insertion fragment is obtained through processing; then sgRNA is inoculated into a plasmid vector by using T4 ligase; and a miR-205 gene knockout cell strain is obtained through transfection of an LNCap cell strain, continuous drug screening and fluorescence detection. Heterogenous hybridization double strands are obtained by extracting DNAs of a cell, PCR amplification of miR-205, purification, denaturation of a PCR product and annealing; T7E1 enzyme digestion test is employed to determining shearing efficiency of a CRISPR-Cas9 system on miR-205; a verified optimized a miR-205 gene knockout CRISPR-Cas9 target sequence is obtained; and the kit is constructed on the basis of the target sequence and can be used for directional knockout of miR-205 genes. The kit has the characteristics of high gene knockout efficiency, fast speed, easiness and economic performance and has wide prospects in the aspects of construction of animal models and clinical research of medical science.
Owner:广州辉园苑医药科技有限公司
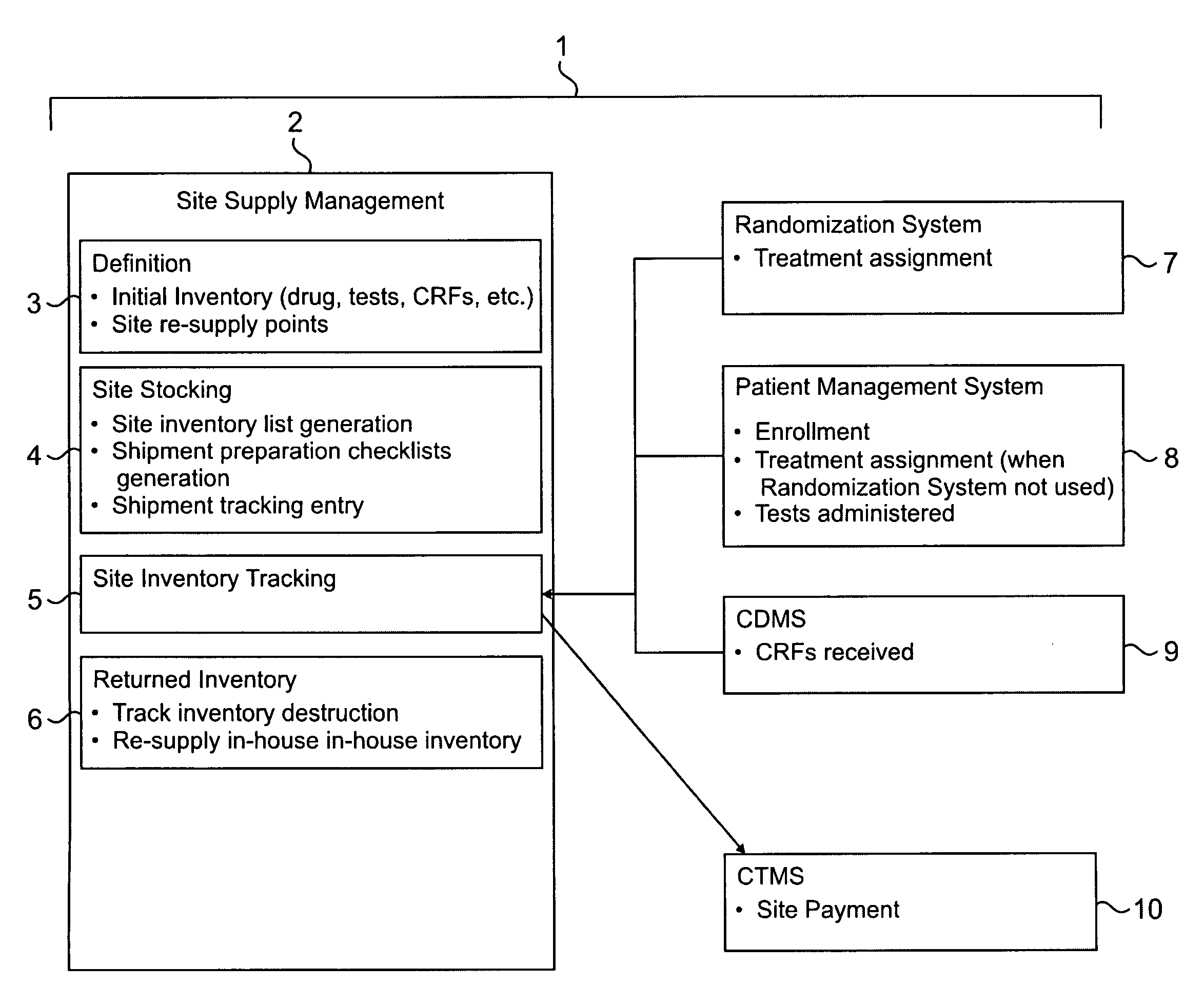
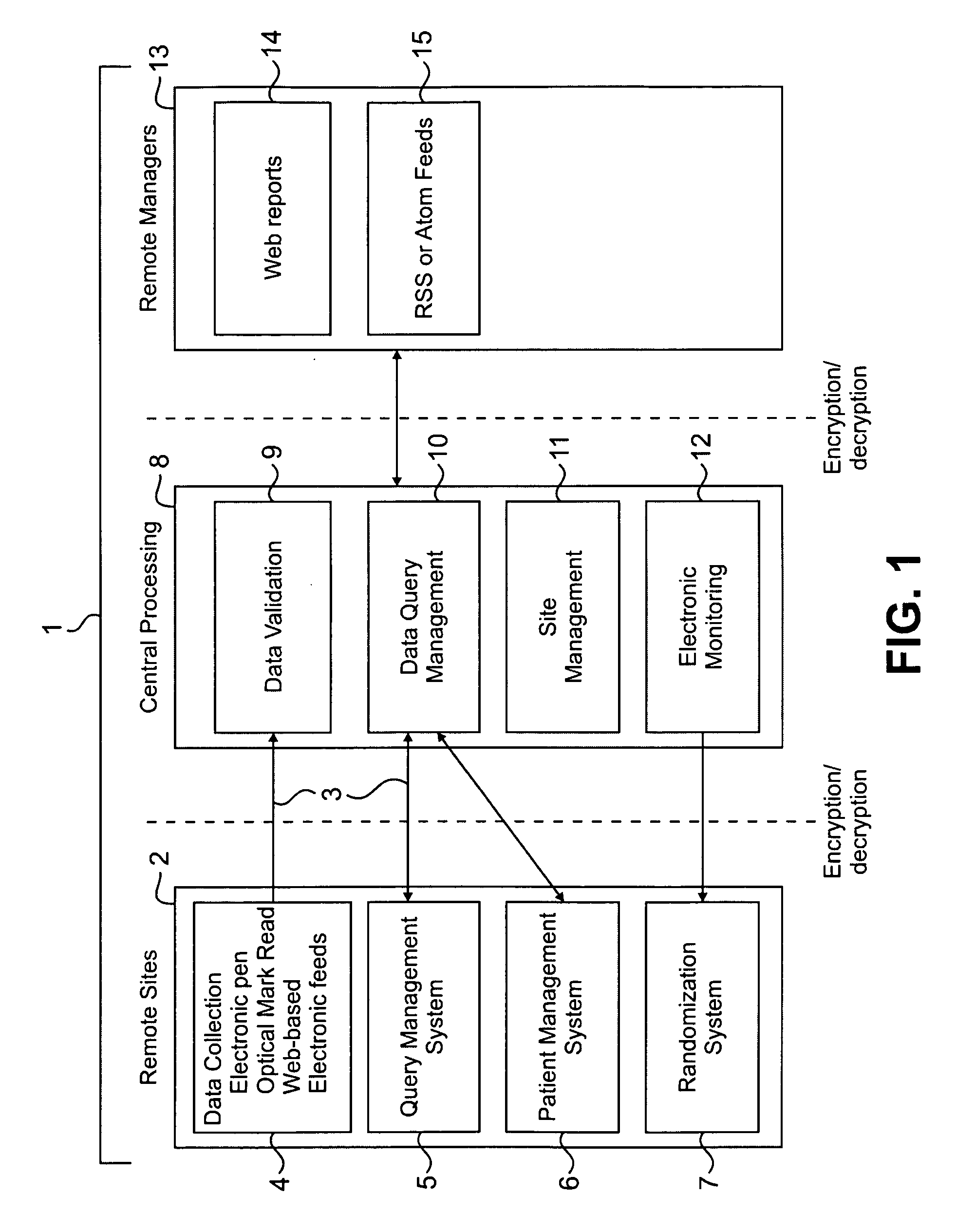
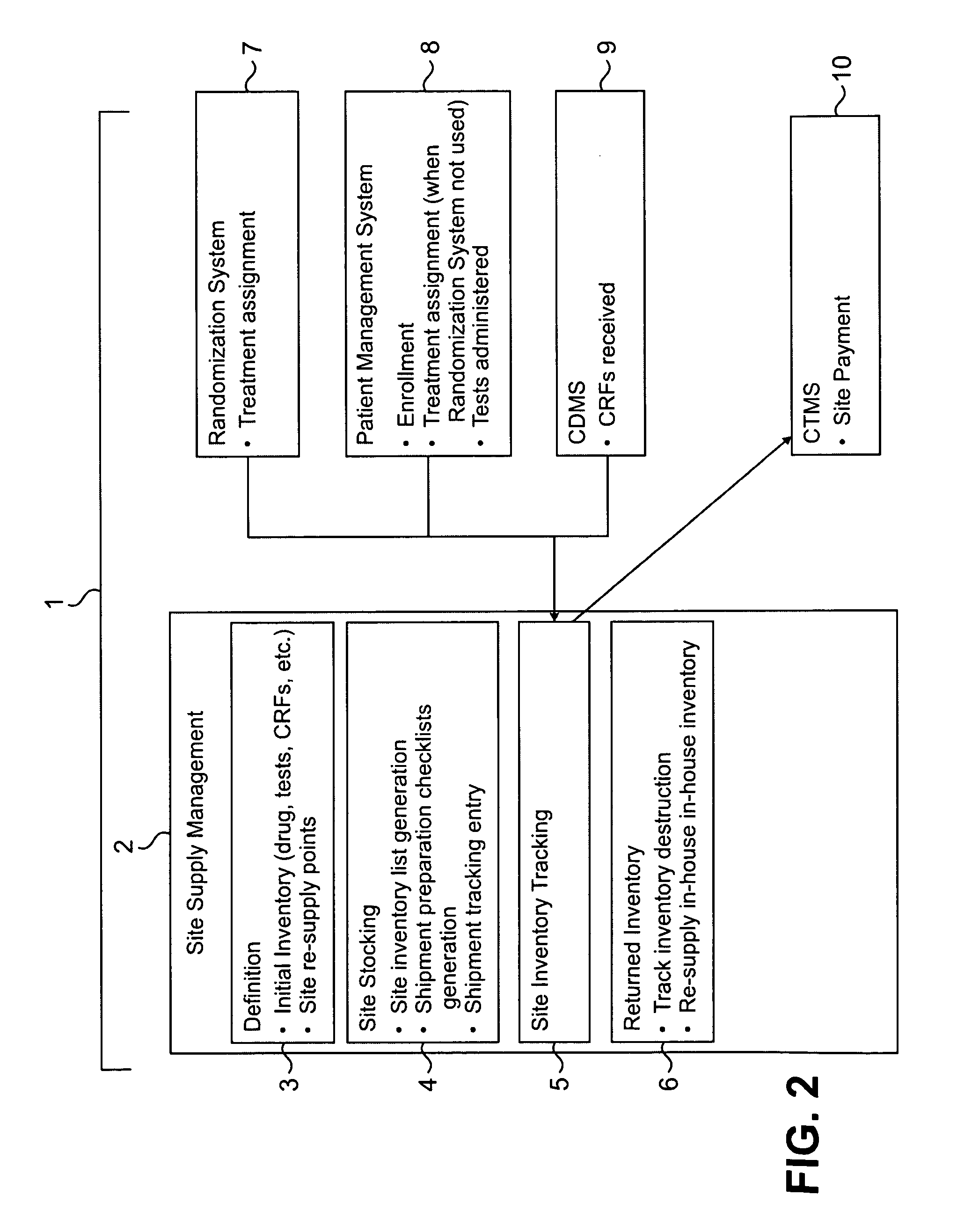
Method and system for collection, validation, and reporting of data and meta-data in conducting adaptive clinical trials
InactiveUS20080270181A1Reduce paymentHigh error rateMedical data miningDigital data processing detailsInterim analysisTreatment Arm
A method and system are described for centrally managing data in an adaptive clinical trial or other adaptive process that is conducted at a plurality of geographically remote sites. The invention includes (1) flexible means for collecting data from remote sites; (2) processing, tracking, and validating such data and meta-data at a processing location; (3) interacting between central and remote sites to manage and resolve data discrepancies (4) reporting data to managers and remote sites; and (5) facilitation of special services to clinical research such as flexible randomization of patients, patient participation eligibility verification and double-blind trials. The invention is of particular relevance to adaptive clinical trials and other applications that demand the ability to quickly collect, process, and respond to various forms of data in order to adjust actions such as randomization schedules, interim analyses, treatment arm pruning, editing subpopulations, and other adaptive measures.
Owner:PALADIN ALICIA
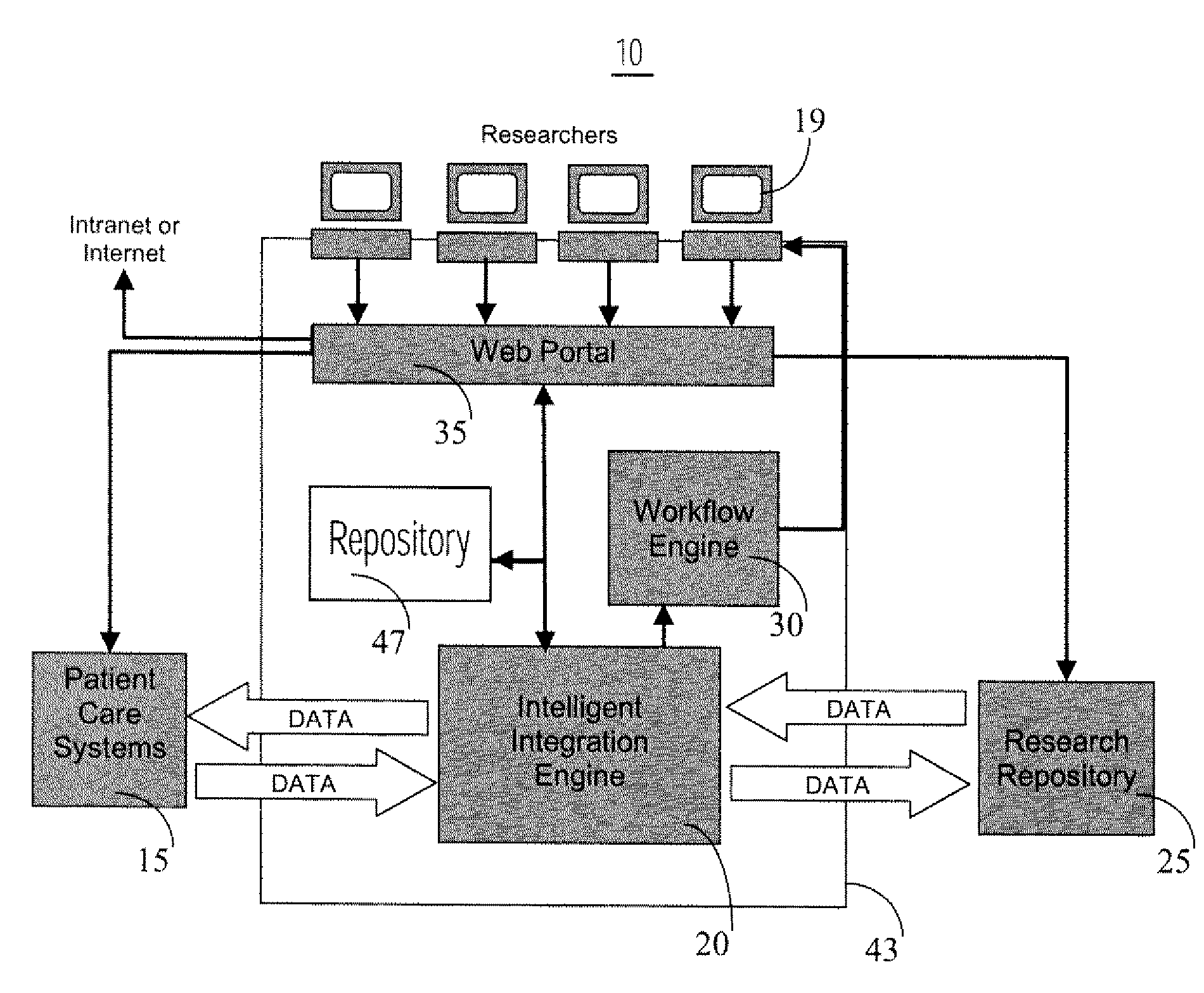
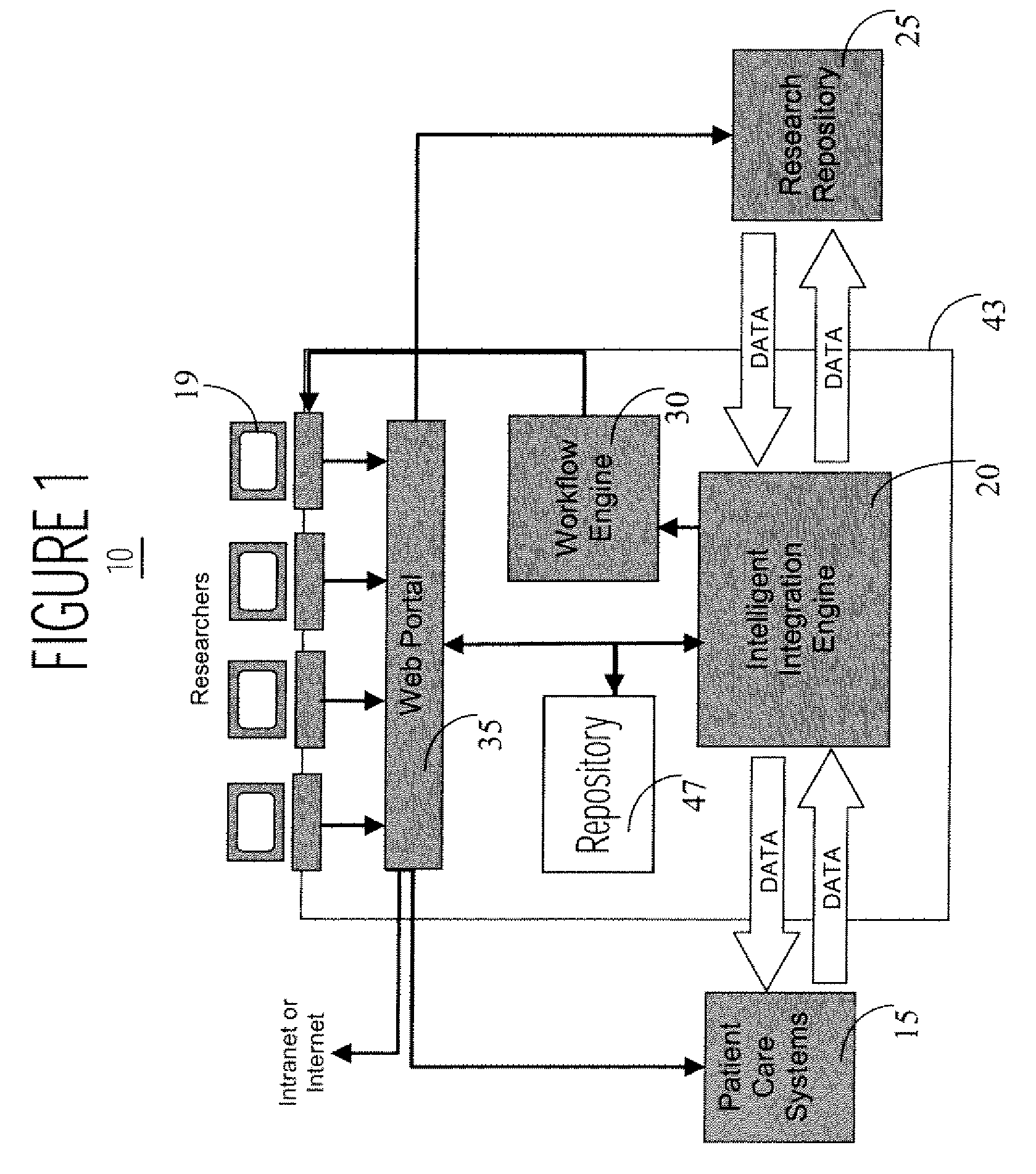
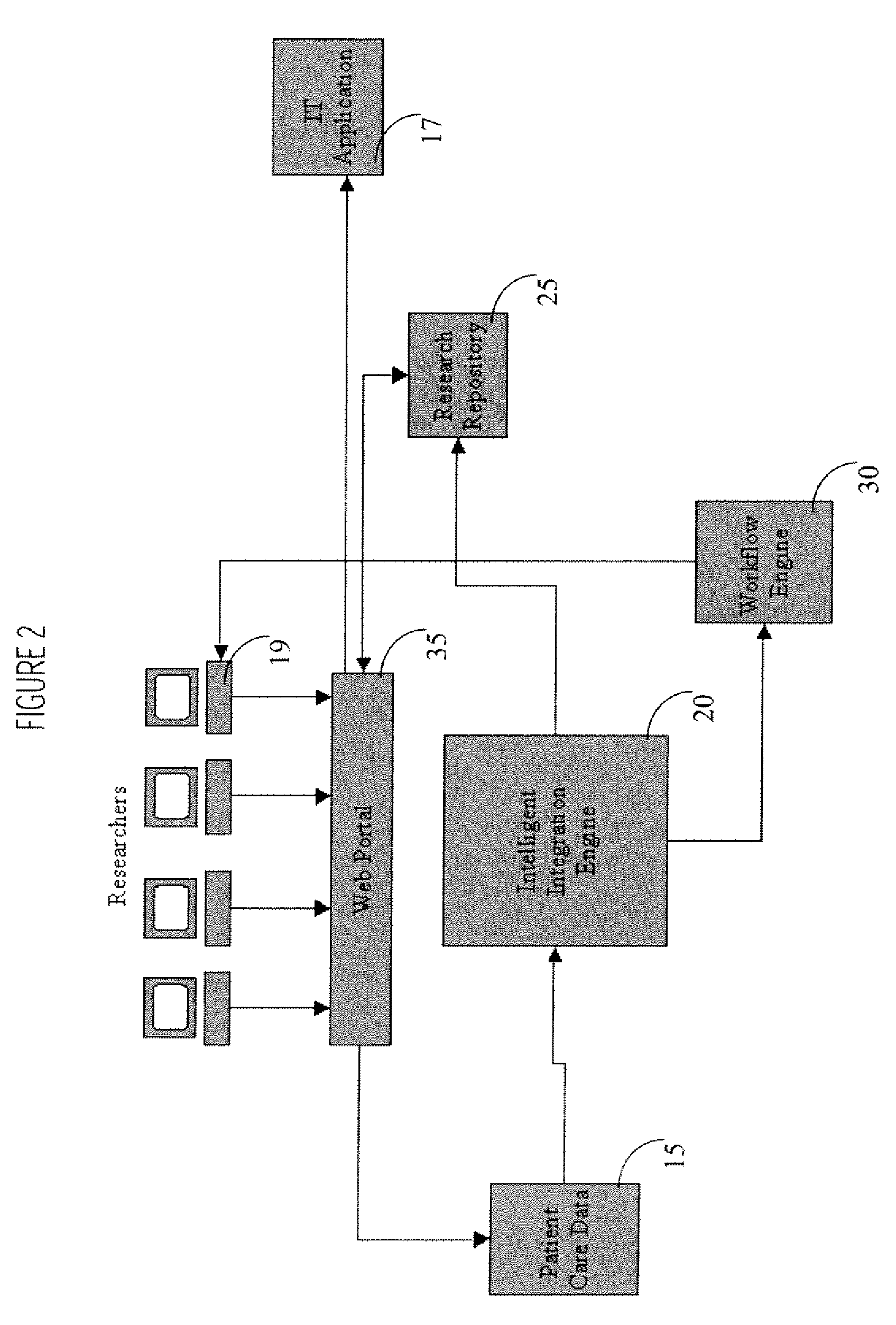
Interface Between Clinical and Research Information Systems
InactiveUS20080059241A1Data processing applicationsTelemedicineClinical trialResearch information system
A system provides a clinical trial or research process with access to, and use of information employed in patient care by facilitating access to information via a portal (e.g., a Web compatible portal) and providing automated transfer of data collected during patient care workflows for storage in clinical research information systems. An interface system enables bidirectional exchange of data between a hospital clinical information system and a clinical trial or research information system. The interface system employs at least one repository including information associating a clinical trial with patient identifiers, healthcare worker identifiers, an indicator of individual status of a patient in the clinical trial and an identifier of a site involved in the clinical trial. An interface processor provides medical information of a particular patient, acquired using a hospital clinical information system, to a clinical trial or research storage repository by using information derived from the at least one repository for automatically determining, the particular patient is enrolled in a clinical trial and the particular patient participation in the clinical trial is still current.
Owner:SIEMENS MEDICAL SOLUTIONS USA INC +1
Sgrna (ribonucleic acid) sequence for editing ccr5 gene by crispr (clustered regularly interspaced short palindromic repeats) technology and application thereof
InactiveCN107177591AImprove cutting efficiencyLow probability of off-targetOrganic active ingredientsAntiviralsClinical researchA-DNA
The present invention relates to editing the sgRNA sequence of CCR5 gene by using CRISPR technology and its application. The present invention provides an sgRNA sequence for the CCR5 gene, wherein the sgRNA sequence is selected from: CCR5-sgRNA1: AGGGCAACTAAATACATTCT, CCR5-sgRNA2: TGCCAAAAAATCAATGTGAA, CCR5-sgRNA3: AGTGGGACTTTGGAAATACA, and CCR5-sgRNA4: ATGCACAGGGTGGAACAAGA. The present invention also relates to a DNA sequence encoding the sgRNA sequence, a vector comprising the sgRNA sequence or the DNA sequence, a cell comprising the vector and uses thereof. The invention provides a safe and efficient sgRNA, which has the advantages of high cutting efficiency and low off-target probability, and has broad prospects in clinical research and application of AIDS gene therapy.
Owner:PEKING UNIV
Amplification methods and compositions
InactiveUS7790393B2Microbiological testing/measurementBuying/selling/leasing transactionsNucleic acid detectionOligonucleotide primers
The present invention provides methods and routines for developing and optimizing nucleic acid detection assays for use in basic research, clinical research, and for the development of clinical detection assays. In particular, the present invention provides methods for designing oligonucleotide primers to be used in multiplex amplification reactions. The present invention also provides methods to optimize multiplex amplification reactions.
Owner:GEN PROBE INC
Method for establishing Gadd45a knockout rabbit model by adopting knockout technology
InactiveCN107630043APredictive effectReduce the risk of research and developmentStable introduction of DNAAnimal husbandryRabbit modelPlasmid dna
The invention relates to a method for establishing a Gadd45a knockout rabbit model by adopting a knockout technology and belongs to the technical field of biotechnology. The invention aims to establish a rabbit model by knocking out GADD45a gene by utilizing a Grispr / cas9 technology and to provide the method for establishing the Gadd45a knockout rabbit model by adopting the knockout technology andfor researching the influence of the gene on animal liver. The method provided by the invention comprises the following steps: establishing sgRNA; synthesizing double-stranded DNA; linearizing p UC-57 carrier; linking p UC-57 carrier with double-stranded DNA; converting; performing monoclonal picking and plasmid DNA extraction; identifying plasmid sequence; expressing plasmid with CAS9; and performing digestion linearization for in vitro transcription. Through related detection, the invention successfully acquires the Gadd45a knockout rabbit model; the model is established for simulating theclinic pathological processes of the liver diseases, such as, fatty liver, liver cirrhosis and liver cancer after giving the corresponding alcohol stimulation, is capable of effectively forecasting the clinic application effects of new vaccine, new drugs and new diagnostic reagents, and meanwhile, and is capable of greatly reducing the risk in researching and developing the new drugs; and a basismodel is supplied for the clinical research.
Owner:JILIN UNIV
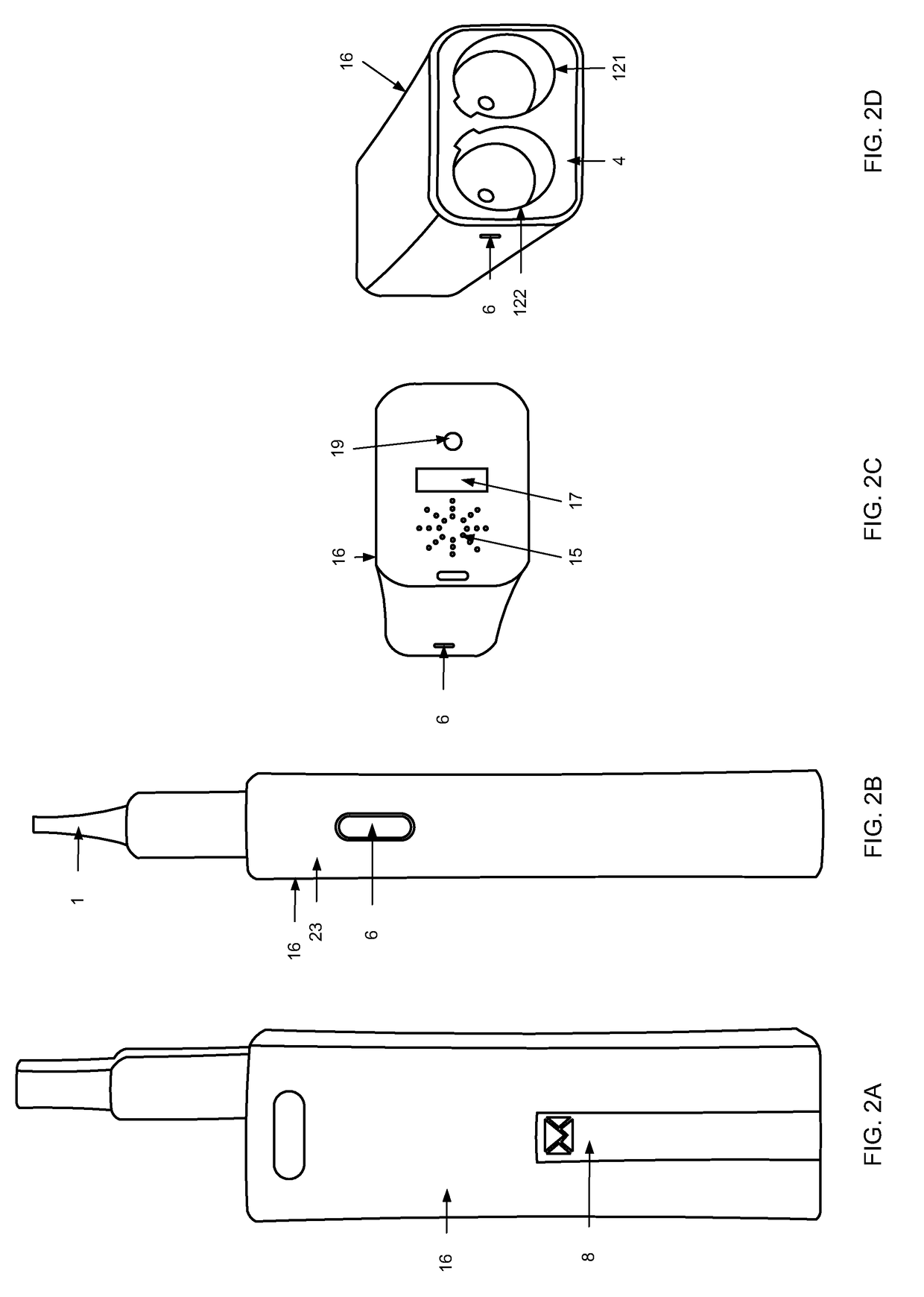
Inhalation device, system and method
A device, system, and method for vaporizing substances in a pod, utilized for inhalation by a user. The device includes components to measure the content and dosage pods containing substances, among other features. The device includes a graphical user interface that may be integrated with the device or implemented through a mobile device, which allows the user to transmit user data to a remote database and / or healthcare provider for personal and clinical data collection. The user data can be used for enhancing personal treatment or clinical research.
Owner:RESOLVE DIGITAL HEALTH INC
Health care research, management and delivery system
InactiveUS20140297311A1Facilitates audit oversightFacilitates administrationTelemedicineComputer-assisted medical data acquisitionDispensaryClinical trial
A health care management and delivery system includes a hosted environment that provides health care treatment, diagnosis, and / or management. Health care providers are linked to one another and to a central network, which is linked to patient via the hosted environment. The patient interfaces with the hosted environment, which includes hosted algorithms approved by the provider network. The patient may also have medical devices that facilitate collection of vital sign data (e.g., digital thermometer) and administration of treatment (e.g., medicine dispensary). The health care provider can license the hosted environment to deliver health care services remotely based on globally standardized protocols. The hosted environment includes all patient health records and information which can be accessed globally. The hosted environment conducts data analytics to continuously improve and add new standardized protocols. Additionally, a virtual clinical research organization (CRO) is provided, such that treating physicians and patients can participate in clinical trials and registries and have access to new medical treatments and improved safety and outcomes.
Owner:JACKSON BECKY L
Method, system, and apparatus for clinical trial management over a communications network
A centralized, online clinical study system configured to coordinate aspects of a clinical study can include a training module configured to evaluate whether users have completed training for selected workflows for the clinical study that are available from the clinical study system, and to provide training to registered users for the selected workflows. The system also can include a financial engine configured to determine when a participating site meets or exceeds a milestone of the clinical study and to initiate a payment to the participating site. Further, the system can include a module configured to receive and verify clinical research data and to designate the verified clinical research data as official source data for the clinical study.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Method for manufacturing a conductive grid for attachment to a blister package
The Med-ic™ Electronic Compliance Monitor (ECM) addresses the problem of patient non-compliance with prescribed medication. The Med-ic™ ECM provides precise information about the patient's use of blister-packaged medication in clinical research and general pharmacy settings. Using an on-board central processing unit (CPU), the Med-ic™ ECM records the time each tablet or capsule is expelled from the blister package, keeping a record for later analysis. At the time of refilling or follow-up visit, the information is downloaded to the research assistant's, physician's or pharmacist's computer where it can be displayed graphically. The data can be stored for later analysis. Production of a Med-ic™ ECM Tag involves numerous steps. These steps incorporate certain methods and technologies to accomplish their objective, the steps being detailed in the specification.
Owner:INTELLIGENT DEVICES SEZC
Serum-free medium and method for culturing mesenchymal stem cell
InactiveCN106479971AImprove stabilityIncrease success rateCulture processSkeletal/connective tissue cellsCell phenotypeSerum free media
The invention belongs to the technical field of stem cells, and particularly relates to a serum-free medium for culturing a mesenchymal stem cell. According to the serum-free medium, a basal culture medium L-DMEM or DMEM / F12 and multiple additives are combined in a customized manner for MSCs in vitro culture, so that ingredients in the culture medium are synergized; the stability and success rate for culturing MSCs in vitro can be greatly improved especially due to application of DKK-1; and the safety of cultured cells in clinical researches is improved by adopting a formula without animal-sourced ingredient. Compared with the prior art, the serum-free medium has the advantages of greatly improving the value adding efficiency of MSCs in vitro culture and maintaining cell growth form of MSCs and cell phenotype stability thereof, provides a novel efficient, stable and safety culture system to MSCs culture in clinical application, and has high scientific researching and medical application values.
Owner:深圳江淼医疗有限公司
Inhalation device, system and method
A device, system, and method for vaporizing substances in a pod or a cartridge, utilized for inhalation by a user. The device includes components to measure the content and dosage pods or cartridges containing substances, among other features. The device includes a graphical user interface that may be integrated with the device or implemented through a mobile device, which allows the user to transmit user data to a remote database and / or healthcare provider for personal and clinical data collection. The user data can be used for enhancing personal treatment or clinical research.
Owner:RESOLVE DIGITAL HEALTH INC
Stem cell freezing and storing medium and preparation method and freezing and storing method thereof
The invention discloses a stem cell freezing and storing medium and a preparation method and freezing and storing method thereof. The stem cell freezing and storing medium comprises, by weight, 3-10 parts of dimethyl sulfoxide, 2-7 parts of human serum albumin, 0.5-3 parts of mycose, 0.2-2 parts of dextran 40 and 2-6 parts of hetastarch. Cells can be frozen for a long time by the stem cell freezing and storing medium, freezing damage of the cells can be remarkably reduced, the resuscitated cells have a high degree of survival rate and adherent property, and freezing and storing effect of the cells is improved; meanwhile, the compositions of the stem cell freezing and storing medium are clear and are medical compendial injection-grade excipients without containing serum, the risk of contamination and allergen introduced by the use of heterogeneous serum is effectively prevented, the content of DMSO (dimethylsulfoxide) is low, the negative effect of the DMSO on the cells is lowered, highsecurity and good stability are achieved, requirements of CFDA (China food and drug administration) and FDA (food and drug administration) are met, and the stem cell freezing and storing medium can be directly used in human infusion and is suitable for clinical research and treatment.
Owner:广州赛隽生物科技有限公司
Systems and methods for selecting and recruiting investigators and subjects for clinical studies
The present invention is directed to an integrated on-line interactive forum that promotes exchange of information among clinical study sponsors, clinical study investigators, and potential clinical study subjects. The forum includes an investigator database that contains information suitable for identification of qualified investigators for clinical studies and a subject database that contains information suitable for identification of eligible subjects for clinical studies. An extranet is coupled to the investigator database and the subject database. The extranet allows sponsors and investigators to exchange securely documents required to start a clinical study. The forum also optionally includes one or more web pages that provide information describing clinical studies to potential clinical study subjects and permit potential clinical study subjects to register for inclusion in the subject database. A therapeutic incidence area database is also optionally integrated into the forum
Owner:INTERTRIALS COM
Preparation method of chitosan composite nerve conduit
InactiveCN101138656APromote regenerationGood tissue compatibilityCatheterTubular organ implantsSingle elementClinical research
The present invention relates to the technical field for the medical and biological material. In the recent ten years, researchers all over the world have proved that it is possible that the nerve frame can replace the antilogous transplantation in nerve defect repairing. But the chitosan tube of a single element has poor elastic property, which is easy to fracture; therefore the nerve stump is hard to be fixed in the tube. The present invention provides a preparation method for the chitosan nerve tube, which has great stretching intensity, long time maintenance in the body and capacity of biodegradation and absorption. In the present invention, the collagen protein and the acid solution of the chitosan are mixed into a composite according to a proper volume ratio. The composite liquid receives layers solidification on the model. The animal test and the clinical research discover that the present invention is capable of bridge joint between the nerve defect ends; the stretching intensity is strong and maintenance in the body is long; reproduction of the nerve is promoted. The technique of the present invention is simple with low cost, which can replace the traditional nerve joint method. The present invention has good application prospect in clinical treatment for the peripheral nerve defect.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
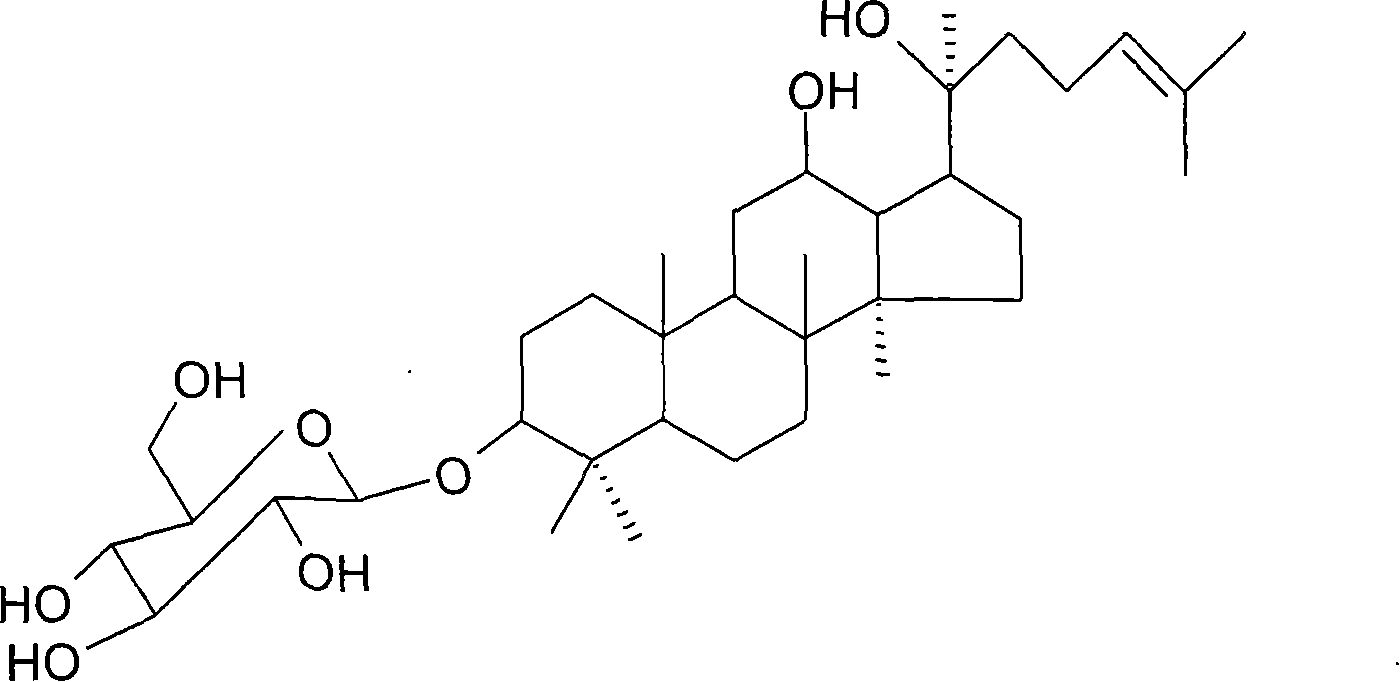
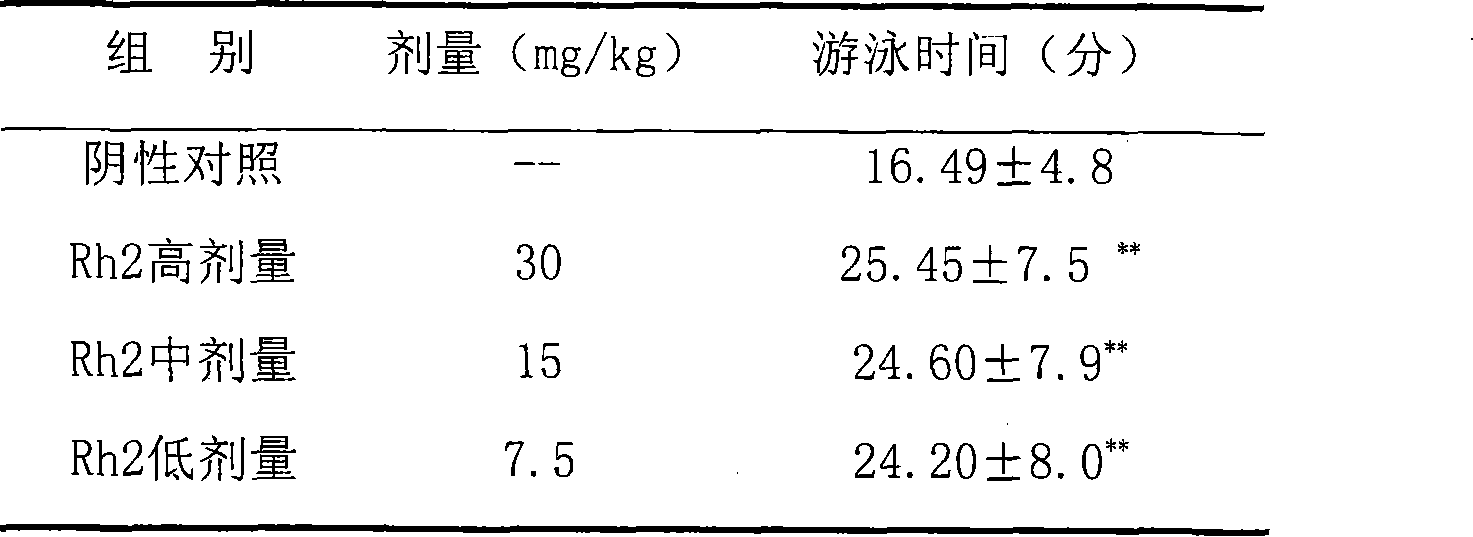
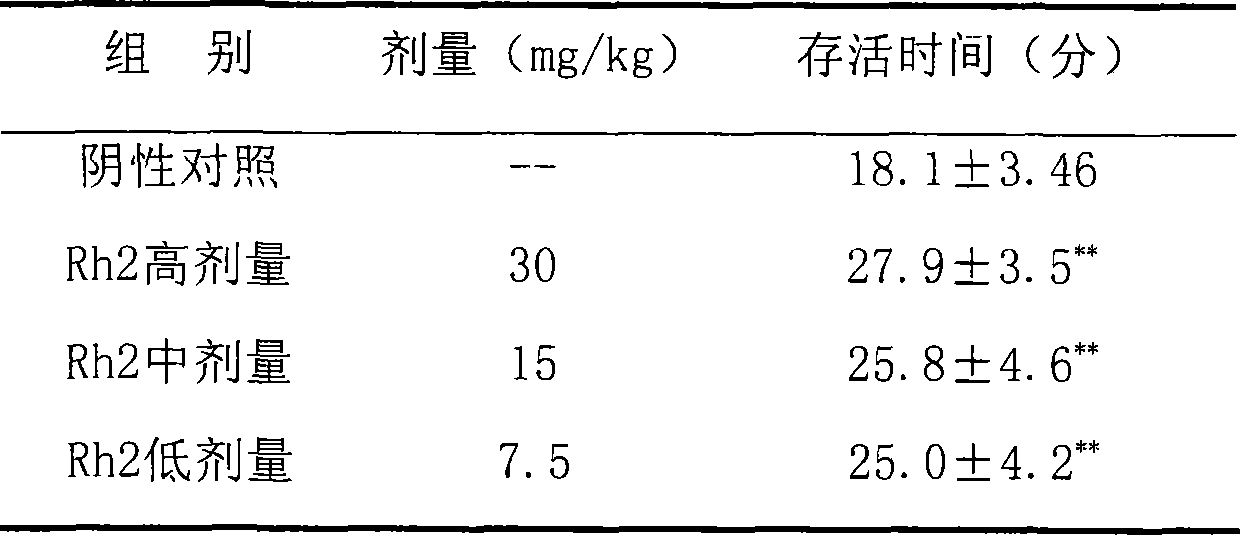
Application of 20(S)-ginsenoside Rh2 compound in preparing anti-fatigue medicament
The invention relates to application of a 20(S)-ginsenoside Rh2, namely 20(S)-protopanoxadiol-3-O-beta-D-glucopyranoside compound in the field of pharmacy. The compound can confront the swimming fatigue action of tumor-bearing animals and enhance the oxygen deficit resistance and immunologic function of the tumor-bearing animals. The safety experiment shows that the compound has high safety, does not influence the central nervous system, cardiovascular system and respiratory system of animals, and has no mutagenesis function. The clinical research shows that the compound can effectively reduce the brief fatigue index BFI score of a tumor patient, improve the functional evaluation FACTIT-F score of chronic disease treatment, improve the KPS score, and simultaneously relieve Chinese medicine symptoms so as to improve the fatigue degree of the tumor patient. The compound has high safety and good anti-fatigue function under test dose, and can be used for the treatment of fatigue, in particular cancer related fatigue.
Owner:上海药谷药业有限公司
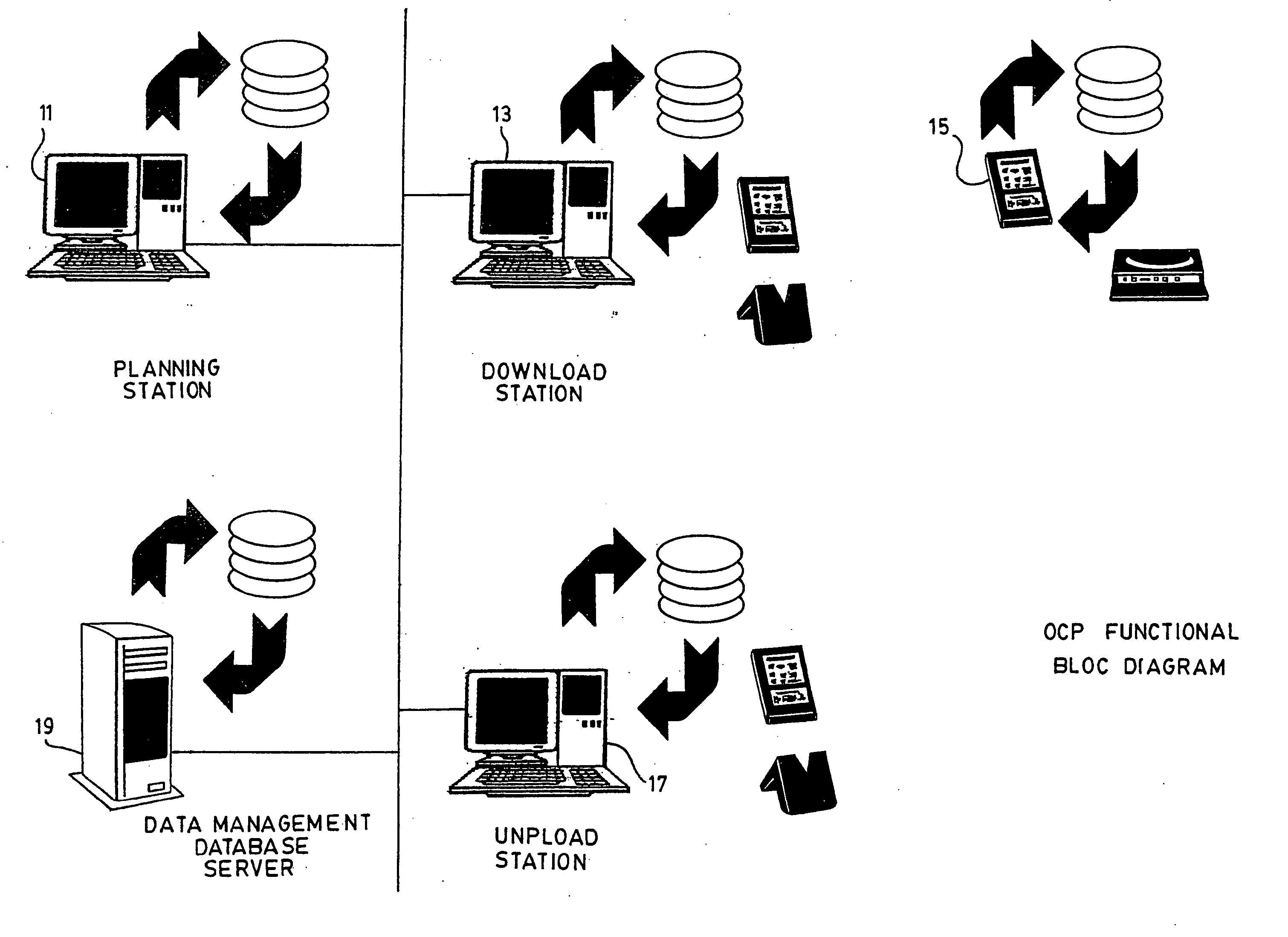
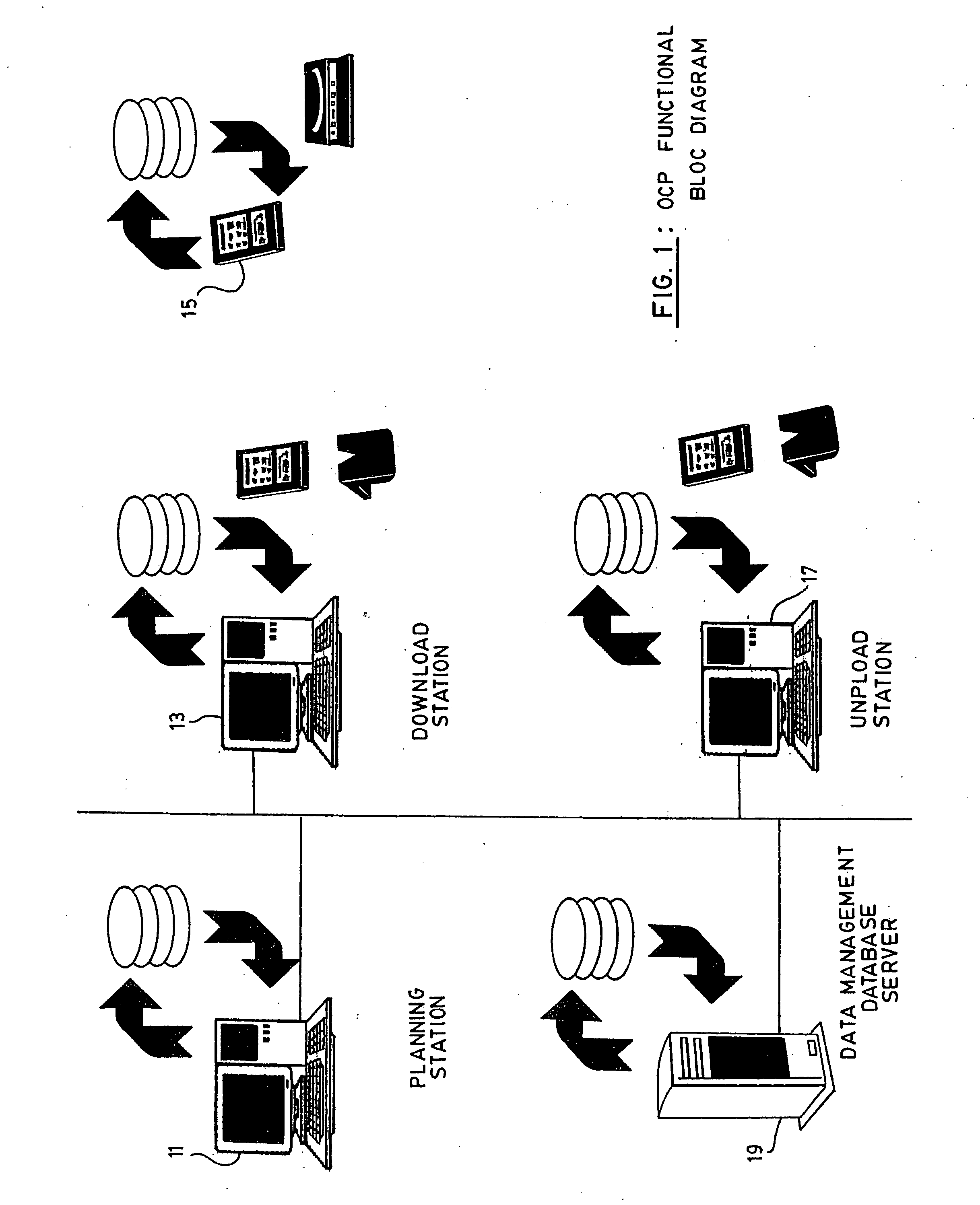
System and method for the collection of observations
InactiveUS20050108658A1Easy to handleError minimizationLocal control/monitoringComputer-assisted medical data acquisitionAviationSoftware engineering
A system for the collection of observations is described which has particular application in the clinical research and aerospace industries. The system has a planning station, for preparing a work package of observations to be collected; a download station, for downloading the work package to at least one primary capture tool, the primary capture tool being used to collect observations according to the work package autonomously; and an upload station, for uploading the observations from the at least one primary capture tool. The invention also concerns a method for collecting observations, as well as a graphical interface therefor. The invention makes use of an atomic database, which obviates the need to reprogram the database when it is to be used for a different set of observations to be collected.
Owner:MRC NETWORKS
Clinical data management system
The invention relates to a clinical data management system. The clinical data management system comprises a use working table, a medical record management module, a follow-up management module, a project management module, an integrated query module and an automatic acquisition task management module and is characterized in that the medical record management module comprises a medical record grouping sub-module, a medical record importing sub-module, a medical record browsing sub-module and a data checking sub-module, the medical record grouping sub-module is used for grouping of medical records in a system, the medical record importing sub-module is used for batch importing of the medical records, the medical record browsing sub-module is used for browsing the current basic information state of the medical records, the data checking sub-module system is used for checking user data in the system, the follow-up management module is used for collecting follow-up information, the project management module is used for management and operations on project data, and the automatic acquisition task management module is used for task management of automatic data acquisition. The clinical data management system can achieve centralized unified management of clinical research of all medical institutions and individual support for a single research project.
Owner:王任直 +14
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com